
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 178
METHOMYL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared Dr M.L. Lithchfield, Arundel, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Methomyl
(Environmental health criteria ; 178)
1.Methomyl - toxicity 2.Insecticides, Carbamate
I.Series
ISBN 92 4 157178 0 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
1. SUMMARY
1.1. Identity, physical and chemical properties, and analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory animals
1.6. Effects on laboratory mammals and in vitro test systems
1.7. Effects on humans
1.8. Effects on non-target organisms in the laboratory and field
1.9. Evaluation of human health risks and effects on the
environment
1.10. Conclusion
2. IDENTITY PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Sample preparation
2.4.2. Analytical determination
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production processes and levels
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Water
4.1.2. Soil
4.1.3. Vegetation
4.2. Transformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.3. Interaction with other physical, chemical or biological
factors
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Soil
5.1.3. Food crops
5.1.4. Other crops
5.1.5. Dairy products
5.1.6. Animal feed
5.2. General population exposure
5.2.1. Food
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Retention and turnover
6.6. Reaction with body components
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.4.1. Skin irritation
7.4.2. Eye irritation
7.4.3. Skin sensitization
7.5. Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1. Embryotoxicity and teratogenicity
7.5.2. Reproduction studies
7.6. Mutagenicity
7.7. Carcinogenicity
7.8. Other special studies
7.8.1. Cholinesterase studies in vivo and in vitro
7.8.2. Neurotoxicity
7.8.3. Potentiation studies
7.8.4. Antidote studies
7.8.5. Other studies
7.9. Factors modifying toxicity
7.10. Mechanisms of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population
8.1.1. Accidental and suicidal poisoning
8.2. Adverse effects of occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.2.1. Algae
9.2.2. Fish
9.2.3. Other aquatic organisms
9.3. Terrestrial organisms
9.3.1. Terrestrial invertebrates
9.3.2. Birds
9.4. Field studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-15
from the National Institute of Environmental Health Sciences, National
Institutes of Health, USA, and by financial support from the European
Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope, and
the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews on
the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data, whenever
available, are preferred to animal data. Animal and in vitro
studies provide support and are used mainly to supply evidence missing
from human studies. It is mandatory that research on human subjects
is conducted in full accord with ethical principles, including the
provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation of
the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the
environment
* Conclusions and recommendations for protection of human
health and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the priority
list, the IPCS Secretariat consults with the Cooperating Organizations
and all the Participating Institutions before embarking on the
preparation of the monograph.
Procedures
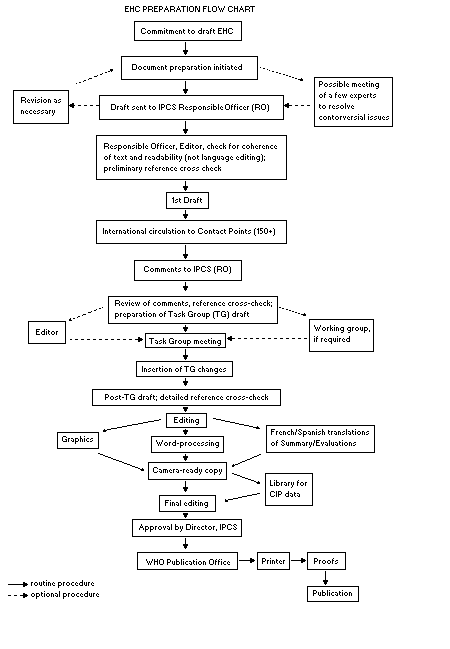
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an initial
review by a small panel of experts to determine its scientific quality
and objectivity. Once the RO finds the document acceptable as a first
draft, it is distributed, in its unedited form, to well over 150 EHC
contact points throughout the world who are asked to comment on its
completeness and accuracy and, where necessary, provide additional
material. The contact points, usually designated by governments, may
be Participating Institutions, IPCS Focal Points, or individual
scientists known for their particular expertise. Generally some four
months are allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate
in the preparation of the EHC monograph must, in addition to serving
in their personal capacity as scientists, inform the RO if at any time
a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Dept. of Human Services and Health, Canberra, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, PA, USA
Dr Jun Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr Palarp Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr Salah A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of Agriculture
Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Observers
Dr R. Gardiner, GIFAP, Brussels, Belgium (Representative of GIFAP)
Dr B. Julin, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr S.M. Kennedy, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr Ronald L. Mull, Du Pont Agricultural Products, Wilmington, DE,
United States of America (Representative of GIFAP)
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Office, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
The Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP) met in Geneva from 25 October to 3 November 1994. Dr W. Kreisel,
Executive Director, welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS on behalf of the three IPCS cooperating
organizations (UNEP/ILO/ WHO). The CAG reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methomyl.
The first draft of the monograph was prepared by
Dr M.L. Litchfield, Arundel, United Kingdom. Extensive scientific
comments were received following circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs and
these comments were incorporated into the second draft by the
Secretariat.
The fact that E.I. Du Pont de Nemours and Co. made available to
IPCS and the Core Assessment Group proprietary toxicological
information on their products is gratefully acknowledged. This
allowed the Group to make its evaluation on a more complete data base.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ADI Acceptable Daily Intake
ALC Approximate Lethal Concentration
CAS Chemical Abstracts Service
CCPR Codex Committee on Pesticide Residues
EbC50 median effective concentration for inhibition of growth
based on comparison of areas under the growth curves after
"b" hours
ECD electron capture detector
FID flame ionization detector
FSD flame photometric detector selective for sulfur
GC gas chromatography
GLC gas-liquid chromatography
GOT glutamic oxaloacetic transaminase
GPT glutamic pyruvic transaminase
HPLC high performance liquid chromatography
ISO International Organization for Standardization
IUPAC International Union of Pure and Applied Chemistry
JMPR Joint FAO/WHO Meeting on Pesticide Residues
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
MATC maximum acceptable toxicant concentration
MHTA S-methyl- N-hydroxythioacetimidate (a metabolite of
methomyl)
mPa millipascal (7.5 × 10-6 mmHg)
MRL maximum residue limit
NOEC no-observed-effect concentration
P2S N-methyl-pyridium-2-aldoxime methane-sulphonate
(antidote)
PAM pyridine-2-aldoxime methiodiode (antidote)
RTECS Registry of Toxic Effects of Chemical Substances
TEAC tetraethylammonium chloride
TOCP tri- o-cresyl phosphate
TOD total oxygen demand
UV ultraviolet
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
Methomyl is a white crystalline solid with a melting point of
77°C and a vapour pressure of 0.72 mPa (25°C). Its solubility in
water is 54.7 g/litre and its octanol/water partition coefficient
(Kow) is 1.24. It is stable in sterile water at pH 7, but is broken
down at higher pH values, the half-life being 30 days at pH 9 and
25°C.
The analytical procedure for the determination of methomyl in
different samples is extraction followed by clean-up and analysis by
HPLC or GLC. In some cases methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
1.2 Sources of human and environmental exposure
Methomyl is produced by reacting S-methyl N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. It is a carbamate insecticide used on a wide
range of crops throughout the world. Crops protected include fruit,
vines, hops, vegetables, grain, soya bean, cotton and ornamentals.
Indoor uses include the control of flies in animal houses and dairies.
The main formulations are water soluble powders and water
miscible liquids, which are diluted with water for ground or aerial
spraying of crops. Typical active ingredient rates are 0.15 to
1.0 kg/ha. The main sources of human exposure are during the
preparation and application of these products and from the ingestion
of crop residues in foodstuffs (see section 5.3.1.4).
1.3 Environmental transport, distribution and transformation
In laboratory studies, methomyl adsorbs poorly to soil. Weak
adsorption to clay minerals, particularly illite, has been
demonstrated; adsorption to soil organic matter is 50 times greater
but still relatively weak. Hardly any desorption of bound residue is
seen. With these characteristics, methomyl would be expected to be
mobile in soil.
Under natural environmental conditions, abiotic degradation of
methomyl by hydrolysis or photolysis is slow or absent.
Aerobic degradation in soil is about twice as fast as anaerobic
degradation. Reported half-lives of methomyl in soil vary from a few
days to more than 50 days; dry conditions delay breakdown. In
practice in the field most applications should lead to a half-life of
around one week.
In field conditions, methomyl does not leach to levels below 20
to 30 cm into the soil and does not contaminate ground water.
When 14C-methomyl is applied to plant leaves it is absorbed but
not translocated to other parts of the plant. When applied to the
root system it is absorbed into the plant where the principle residue
component is methomyl itself. Volatile breakdown products are CO2
and acetonitrile. The remainder of the activity is incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars. The half-life of methomyl in plant foliage is a few days.
There was no evidence for accumulation of methomyl in rainbow
trout exposed to the compound for 28 days in a flow-through system.
1.4 Environmental levels and human exposure
Methomyl levels are likely to be either very low or undetectable
(< 0.02 mg/litre) in ground water on the evidence of analyses of
various water sources after the application of the compound at
recommended rates.
Low residue levels of methomyl are present in food and other
crops at harvesting, the levels depending upon factors such as the
applied rate, time interval after the last application and the type of
crop. The residue is composed primarily of methomyl.
Residues of methomyl in dairy products are either undetectable or
very low. Lactating cows given methomyl by capsule at a rate
equivalent to 80 mg/kg in their feed for 28 days showed no detectable
residues of methomyl or the metabolite MHTA in milk or tissues
(< 0.02 mg/kg). No methomyl was detected in eggs or tissues of
laying hens given 1 or 10 mg/kg in the diet for 4 weeks.
In total diet or individual food analyses in the USA, the
concentrations of methomyl in sample surveys were either undetectable
or very low. Residue levels are further reduced by processes such as
washing, peeling and cooking.
Re-entry exposure studies, specifically for California desert
conditions, showed that, when workers returned to vineyards where
dislodgeable foliar residues had fallen to 0.1 µg/cm2, the highest
exposure occurred on the upper body and head during grape girdling and
on the upper body and hands during raisin harvesting. Harvesting and
packing table grapes resulted in the lowest exposure. Inhalation
exposure was minimal.
After methomyl was sprayed on cucumber and tomato plants, ambient
air concentrations in the greenhouse ranged up to 4.7 µg/m3 on the
day after spraying. Three and 7 days after spraying, breathing zone
methomyl concentrations ranged up to 14.5 and 0.7 µg/m3,
respectively. Hand-wash methomyl values ranged from 10 to 322 µg
per h work in a greenhouse. This indicated that dermal exposure was a
more important route of exposure than inhalation and that re-entry
intervals should be based on dermal exposure data.
1.5 Kinetics and metabolism in laboratory animals
The absorption, metabolism and excretion of methomyl after oral
administration to rats are very rapid, the processes being completed
within a few days. When rats were given radiolabelled methomyl
(5 mg/kg body weight), 54% of the dose was excreted in urine and 2-3%
in faeces within 7 days, and 34% in expired air within 5 days. After
7 days, 8-9% of the 14C dose remained in the tissues and carcass,
which was incorporated into endogenous constituents. The highest
concentration of radioactivity was in the blood (representing 2% of
the dose).
The major metabolic components in expired air of rats were carbon
dioxide and acetonitrile in the ratio of about 2:1. The major
metabolite in urine was the mercapturic acid derivative of methomyl,
which was equal to 17% of the dose. Neither methomyl nor its oxime
derivative was detected.
The proposed metabolic pathway includes displacement of the
S-methyl grouping by glutathione followed by enzymic transformation
to give the mercapturic acid derivative. Another pathway is by
hydrolysis to give MHTA, which is rapidly broken down to carbon
dioxide. A further possible route is conversion of syn-methomyl (the
insecticidal form) to its anti-isomer, which undergoes hydrolysis,
rearrangement and elimination reactions to give acetonitrile.
Methomyl is similarly metabolized in the monkey, except that the
mercapturic acid derivative is a minor component in the urine.
The penetration of 14C-methomyl was estimated to be 85% within
one hour after dermal application in acetone to mice. At that time 3%
of the dose had appeared in blood, 5% in liver and 13% had been
excreted. Within 8 h the total excretion was 54.5%.
The rapid breakdown and elimination of methomyl in the rat,
together with its lack of accumulation in tissues, are comparable to
that seen in ruminants.
Methomyl is completely broken down when cows or goats are dosed.
No methomyl or its oxime derivative was detected in milk or tissues.
It was shown that the compound was metabolized and incorporated into
natural constituents of milk and liver.
No nitrosomethomyl was detected when 14C-methomyl was incubated
under simulated stomach conditions with sodium nitrite in a cured meat
macerate.
1.6 Effects on laboratory mammals and in vitro test systems
Methomyl has high acute oral toxicity, with an oral LD50 in the
rat of 17-45 mg/kg body weight. It is also highly toxic to rats by
the inhalation route, with a 4-h LC50 of 0.26 mg/litre in aerosol
form. Dermal toxicity is very low, with the LD50 exceeding
2000 mg/kg body weight in the rabbit (intact skin) and > 1000 mg/kg
body weight in the rat (abraded skin). Signs of acute toxic action
are those expected of a cholinesterase inhibitor and include among
others profuse salivation, lacrimation, tremor and pupil constriction.
Recovery from the effects was rapid. No gross pathological effects
due to treatment were seen in the organs examined. Methomyl is not a
skin irritant or sensitizer and is a mild eye irritant.
Repeated dietary administration over longer periods did not lead
to accumulation or increase in toxic effect. Rats and dogs fed diets
containing methomyl up to 250 mg/kg and 400 mg/kg in the diet,
respectively, for 13 weeks did not show any toxic signs or mortality.
Rats fed at the 250 mg/kg level showed small decreases in body weight
gain, lower haemoglobin levels and moderate erythroid hyperplasia in
the bone marrow. The NOEL in rats was 50 mg/kg in the diet
(equivalent to 3.6 mg/kg body weight per day). Rabbits given repeated
dermal applications of methomyl at doses up to 500 mg/kg body weight
per day for 21 days showed hyperactivity and depressed plasma and
brain cholinesterase activity at the top dose. The NOAEL was 50 mg/kg
body weight per day in this study.
Long-term studies were carried out on rats at methomyl
dietary levels of 0, 50, 100 or 400 mg/kg and on mice at 0, 50, 75 or
200 mg/kg. Effects on rats at the top dose included depressed body
weight gain and lowered haemoglobin and haematocrit values. The NOEL
was 100 mg/kg in the diet, equivalent to 5 mg/kg body weight per day.
In the study in mice, an increased mortality rate and decreased
haemoglobin and red blood cell counts were seen at the two higher dose
levels. The NOEL was 50 mg/kg in the diet, equivalent to 8.7 mg/kg
body weight per day. In a 2-year toxicity study in dogs (0, 50, 100,
400 or 1000 mg/kg in the diet), clinical signs of toxicity were noted
in some animals at the top dose together with slight to moderate
anaemia. The NOEL was 100 mg/kg in the diet, equivalent to 3 mg/kg
body weight per day.
There was no evidence of treatment-related increases in tumour
incidences in 2-year studies on rats and mice, indicating that
methomyl is not carcinogenic. It was not genotoxic in bacterial or
mammalian cells in vitro and was negative in tests for primary DNA
damage in bacterial and mammalian cells in vitro and in an in vivo rat
bone marrow chromosomal study. It showed cytogenetic potential in
human lymphocytes in vitro, as shown by increases in micronuclei and
chromosome aberrations. Methomyl did not produce embryotoxic or
teratogenic effects in rats or rabbits at doses up to 400 mg/kg in the
diet or 16 mg/kg body weight per day by gavage, respectively, at which
levels toxic effects were present in the dams. In a 3-generation
reproduction study in rats at dose levels of 50 or 100 mg/kg in the
diet (equivalent to 5 or 10 mg/kg body weight per day) methomyl did
not affect fertility, gestation or lactation indices and there were no
treatment-related gross abnormalities.
Methomyl did not show delayed neurotoxicity after single or
repeated administration. Rats fed 800 mg/kg in the diet showed
significant depression of blood cholinesterase activity only in the
early stages of a 5-month study. In a 28-day dietary study, brain
cholinesterase activity was only slightly depressed at this dose
level. This indicated the rapid reversibility of methomyl-inhibited
cholinesterase activity in the animals during the feeding periods. In
vitro, human erythrocyte cholinesterase activity was six times more
sensitive to the inhibitory action of methomyl than that of the rat,
although the rates of spontaneous reactivation were similar.
Atropine was shown to be the most consistently effective antidote
for methomyl poisoning based upon the results of studies in several
species.
1.7 Effects on humans
Reports on accidental and suicidal poisonings with methomyl
provide some information on effect levels and recovery. Three out of
five victims of accidental poisoning from a contaminated meal died
within 3 h of the ingestion. It was estimated that the victims had
consumed about 12-15 mg methomyl/kg body weight. A 31-year-old woman
and her 6-year-old son, both of whom died as a result of deliberate
poisoning, showed concentrations of methomyl in the liver of 15.4 and
56.5 mg/kg, respectively. The estimated doses were 55 mg/kg body
weight for the mother and 13 mg/kg body weight for the son. Six hours
after ingesting approximately 2.25 g methomyl, a woman's blood
contained .6 mg methomyl/kg. Methomyl could not be detected 22 h
after ingestion, when the woman was recovering.
A pesticide operator, who did not take any precautions when
mixing a powdered methomyl formulation for spraying vegetables,
displayed poisoning symptoms within one hour and showed a blood
cholinesterase activity 40% of normal after 12 h, with recovery to 80%
of normal activity within 36 h. Other operators, following the
recommended precautions, did not show any symptoms or effects on red
blood cell or plasma cholinesterase activity during activities with
the aerial application of methomyl.
1.8 Effects on non-target organisms in the laboratory and field
Methomyl showed no effects on soil fungal or bacterial
populations, nitrification or dehydrogenase activity when applied at
recommended rates.
An NOEC for algal growth of 6.5 mg/litre was established for
methomyl in laboratory studies.
Methomyl is moderately to highly toxic to fish, the 96-h LC50
values being in the range of 0.5-2 mg/litre for a variety of species.
In a longer-term (21 days) study the LC50 for fingerling trout was
1.3 mg/litre methomyl when tested as a Lannate 20L (21.5% methomyl)
formulation. In an early- life-stage toxicity study over 28 days with
fathead minnows, the MATC was estimated to be > 57 and < 117 µg/litre.
In acute toxicity tests with other aquatic organisms, Daphnia
magna was one of the most susceptible species to methomyl, the 48-h
LC50 being 0.032 mg/litre. In a 21-day study on the survival,
growth and reproductive capacity of Daphnia magna, the maximum
acceptable toxicant concentration for methomyl was > 1.6 and
< 3.5 µg/litre.
Methomyl is toxic to honey-bees, the reported contact LD50
being 1.29 µg/bee and the oral LD50 0.2 µg/bee.
The acute toxicity of methomyl has been assessed in several bird
species, typical acute oral LD50 values being 10 mg/kg body weight
for pigeons and 34 mg/kg body weight for Japanese quail. It is
relatively less toxic by the dietary route, the 8-day dietary LC50
being 1100 mg/kg methomyl in the diet for bobwhite quail and
2883 mg/kg methomyl in the diet for mallard ducks. In 18-to 20-week
one-generation studies, the NOEC was 150 mg/kg methomyl in the diet in
bobwhite quail and mallard ducks.
No effects were seen on bobwhite quail when they were exposed to
serial spray applications of methomyl at recommended rates. Two
studies on wild bird populations, after methomyl was sprayed over
forest land or hop fields at recommended rates, did not reveal any
apparent changes in bird activity and caused no treatment-related
effect or mortality. Fat deposits of song birds in treated forests
were reduced relative to controls; this was considered to be an
indirect effect through reduction in insect food.
1.9 Evaluation of human health risks and effects on the environment
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The adsorption of methomyl to soil is low to moderate with hardly
any desorption. Aerobic degradation in soil (with a half-life of
around one week) is about twice as fast as anaerobic degradation.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. Absorbed
methomyl concentrations in food crops decline rapidly to about 5%
within one week.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl with LC50 values in the order of 10 to
100 µg/litre.
Fish, both freshwater and estuarine, are less sensitive, the
LC50 values ranging from 0.5 to 7 mg/litre. Given the low
persistence of methomyl and its relatively low acute toxicity to fish,
the risk is expected to be low.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil.
Methomyl is classified as highly toxic to honey-bees with a
topical LD50 of around 0.1 µg/bee.
Acute oral LD50 values for various bird species range between
10 and 40 mg/kg body weight. Dietary LC50 values (5 days) range from
1100 to 3700 mg/kg diet. Methomyl poses an acute risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
1.10 Conclusion
Considering the qualitative and quantitative characteristics of
methomyl toxicity, the Task Group concluded that 0.03 mg/kg body
weight per day will probably not cause adverse effects in humans by
any route of exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
O
"
CH3-C=N-O-C-NH-CH3
'
S - CH3
Used in the syn-isomer form
Molecular formula: C5H10N2O2S
Relative molecular mass: 162.2
ISO common name: methomyl
IUPAC chemical name: S-methyl- N-[(methyl-carbamoyl)oxy]
thio-acetimidate
CAS chemical name: methyl N[[(methyl-amino)carbonyl]
oxy] ethanimidothioate
CAS registry number: 16752-77-5
RTECS number: AK 2975000
Synonyms: metomil, mesomil, OMS 1196
Trade names Flytek (Zoecon), Golden Fly Bait
(manufacturers and (Sorex), Lannate (Du Pont), Methomex
suppliers): (Makhteshim), Methomyl (various),
Nudrin (Shell), Pillarmate (Pillar)
Technical product purity: > 98% w/w
Technical product S-methyl- N-hydroxy-thioacetimidate
impurities: (0.2%), 1,3-dimethylurea (0.4%)
2.2 Physical and chemical properties
The physical properties of methomyl are listed in Table 1.
Table 1. Physical properties of methomyl (Silveira, 1990)
Physical state crystalline solid
Colour white
Odour slight sulfurous
Melting point 77°C
Vapour pressure 0.72 mPa (at 25°C)
Henry's Law constant 2.1 × 10-11 atm-m3/mole
Octanol-water partition coefficient 1.24
(Kow)
Solubility:
water 54.7 g/litre
toluene 30 g/litre
isopropanol 220 g/litre
ethanol 420 g/litre
acetone 720 g/litre
methanol 1000 g/litre
Methomyl is stable at temperatures up to 140°C. It is not
sensitive to impact, but dusts may form explosive mixtures in air.
The autoignition temperature is 265°C. Methomyl is stable to
sunlight; it does not decompose when exposed for 120 days. It is
stable in sterile buffered water at 25°C (at pH 5 or 7 no breakdown
occurred within 30 days), but it is increasingly decomposed with
increasing pH and temperature. The half-life in water at pH 9 is 30
days. Methomyl at concentrations of 10 or 100 mg/litre in water is
decomposed by artificial sunlight with half-lives of 5.5 and 2 days,
respectively. Methomyl itself is not corrosive but aqueous solutions
may be mildly corrosive to iron (Silveira, 1990). Irradiation of
methomyl in aqueous solution at 254 nm for 10 h gave rise to
acetonitrile (40%), dimethyl disulfide (30%), acetone (15%) and
N-ethylideneme-thylamine (5%); the rest was unidentified products
(Freeman & Ndip, 1984).
2.3 Conversion factors
1 ppm = 6.62 mg/m3
1 mg/m3 = 0.151 ppm
2.4 Analytical methods
Analytical methods for the detection and determination of
methomyl in a variety of substrates are shown in Table 2. In general,
methomyl is extracted from the sample followed by clean-up and HPLC or
GLC analysis. In some cases the methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
2.4.1 Sample preparation
Solid samples are extracted with organic solvents followed by
solvent partition and then, usually, a column clean-up. Water samples
are mainly submitted directly to solid phase extraction.
2.4.2 Analytical determination
The cleaned-up samples are submitted to either HPLC or GLC
analysis, in some cases after conversion to the oxime derivative.
HPLC analysis is coupled with UV detection, sometimes after conversion
to a fluorescent derivative. GLC detection is provided by FID, FSD,
ECD or microcoulometric detectors. A GC-mass spectrometric detection
method has been described (Brodsky, 1991).
Table 2. Methods for the determination of methomyl
Sample type Sample preparation Analytical method Limit of Reference
extraction/clean-up detection
Technical methomyl Reverse phase HPLC 254 nm UV detector not applicable Du Pont (1982)
and formulations
Plant, animal or Extract (ethyl acetate), add water, GLC with 0.02 mg/kg Pease & Kirkland
soil residues evaporate, acidify, extract & discard S-microcoulometer detector (25 g sample, (1968); Leitch &
(hexane), extract (chloroform), concentrate, or flame photometric 93% recovery) Pease (1973)
derivatize by alkaline hydrolysis detector
Crop residues extract (acetonitrile), partition (hexane), HPLC, UV detector at 0.02 mg/kg Clark & Kennedy
Florisil clean-up 233 nm (10 g sample, (1990)
98% recovery)
Non-fatty matrix extract (methanol), 3-step solvent partition HPLC, post column < 0.05 mg/kg Labare (1990)
residues Celite/charcoal column clean-up, derivatization, fluorescent (150 g sample,
concentrate, filter detector at 254 nm 89% recovery)
Vegetables Homogenized (20 g sample) with HPLC/UV µg/kg range Ivie (1980)
methylene chloride. Clean-up 10 ml of the (µ Baudpac C18 column)
extract by passing through SEP-PAK silica
cartridge. Wash with 2 ml CH2Cl2. Elute
with CH2Cl2:CH3OH (1:1 v/v). Evaporate
eluate to dryness. Redissolve in 1 ml of
CH3CN:H2O(1:1 v/v)
Table 2 cont'd).
Sample type Sample preparation Analytical method Limit of Reference
extraction/clean-up detection
Body fluids derivatize by alkaline hydrolysis, extract GC/chemical ionization 0.01 mg/kg Miyazaki et al.
(ethyl acetate), concentrate, convert to mass spectroscopy (2 g sample, (1989)
trimethysilyl ether derivative 95% recovery)
Soil samples extracted with ethyl acetate; HPLC, UV detector at 0.020 mg/kg Kennedy (1989)
filtered; evaporated to 5 ml; silica gel 233 nm (5 ml sample,
clean-up used when cleaner extract 94-102%
needed for HPLC recovery)
Groundwater extract (solid phase adsorbent), elute HPLC, UV detector < 0.1 µg/litre Batelle (1991)
(acetonitrile), concentrate (1 litre sample,
53-62%
recovery)
Well water Filter, automated sample injection, HPLC, flurometric detector at 1 µg/litre Hill et al. (1984)
post-column alkaline hydrolysis and 230 nm excitation and (0.5 ml sample,
conversion to a fluorophore 418 nm emission cut-off 95% recovery)
filter
Drinking-water as above as above 0.7 µg/litre Foerst & Moye
(0.4 ml sample, (1985)
90% recovery)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Methomyl does not occur naturally in the environment.
3.2 Anthropogenic sources
3.2.1 Production processes and levels
Methomyl is produced by reacting S-methyl- N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. The unreacted MHTA is recovered and the
remaining reaction product is subjected to solvent exchange into water
followed by crystallization and centrifugation. The ensuing wet cake
is dried to give technical methomyl (Council of the European
Communities, 1991).
The worldwide production has been estimated to be less than 7000
tonnes (SRI, 1988).
There are no data available on possible releases to the
environment from production processes and transportation.
3.2.2 Uses
Methomyl was introduced as an insecticide in 1966. It is used
for the control of a large variety of insects on a wide range of crops
throughout the world. It is particularly active on many lepidopterous
insects. It acts by direct contact and following ingestion, through
the stomach. Treated crops include fruit, vines, hops, vegetables,
grain, soya beans, cotton and ornamentals. Indoor uses include the
control of flies in animal houses and dairies.
A global estimate of the amount of methomyl used annually for the
above purposes is not available. However, the annual amount used in
the USA was estimated to be approximately 1300 tonnes in 1987 and
1992. The major crops treated in that country are sweet corn, apples,
lettuce, soya beans, peanuts, tomatoes, cotton, corn, alfalfa, and
grapes, accounting for nearly 80% of the total amount used (US EPA,
1988; Gianessi & Puffer, 1992).
The main formulated products are water-soluble powders (25-90%
methomyl) and water-miscible liquids (12.5-29% methomyl). These
products are diluted with water and applied by ground or aerial spray
equipment. Typical methomyl concentrations in the spray solutions are
200-500 mg/litre. Typical active ingredient rates are 0.15-1.0 kg/ha
although higher rates, up to 2 kg/ha, may be used for some purposes.
Repeat applications, as directed on the label, may be required to
maintain control of insect infestations. Examples of crops treated
and methomyl use rates for the USA and Australia are given by FAO/WHO
(1990a,b). Methomyl formulations are compatible in use with many
other insecticides and fungicides, and combined formulations are
registered and available for use in many countries. Methomyl is often
used with one or more other products in a tank-mix. Possible
potentiation by other cholinesterase inhibitors should be considered
when assessing the safety of use of the tank-mix formulations.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Water
The transference of methomyl from greenhouse plants to soil and
to water was assessed on chrysanthemums, grown three times per annum,
with methomyl applied over a 6-month period at 1-week to 2-month
intervals at a rate of 1.4 kg/ha. The three soils investigated were
frequently irrigated and the drainage water was collected at a depth
of 0.8 m. The adsorption isotherms for the three soils indicated that
the adsorption of methomyl was weak to moderate. The concentration of
methomyl in the drainage water was undetectable (< 0.1 µg/litre) in
60% of the samples and was below 1 µg/litre in the remainder (Leistra
et al., 1984).
Methomyl was applied at a high rate of 2.2 kg/ha to a loamy sand
soil on a farm site with a 10% slope. No methomyl was detected
(< 0.01 mg/litre) at the base of the site after a total of about
80 mm of natural and artificial rain had fallen on the site over a
period of 15 days (Harvey & Pease, 1973).
14C-Methomyl was applied at a very high rate of 5 kg/ha to
cylinders (38 cm deep) containing a fine sand soil or a loamy sand
soil. No radioactivity was found in the eluate collected after the
columns had been subjected to heavy rain (Harvey & Pease, 1973).
In natural waters, pesticides can be photochemically degraded by
direct (the pesticide absorbs sunlight) and indirect (other chemicals
in the water absorb sunlight, and transfer the energy to the
pesticide) mechanisms. Current regulatory studies only address the
direct mechanism, and thus neglect an important degradative process.
This can result in unrealistically high estimates of persistence of a
pesticide in surface water.
These data would suggest that any methomyl residues present in
agricultural waters would be rapidly degraded, and that methomyl would
not be expected to have an impact on non-target aquatic organisms.
4.1.2 Soil
Batch equilibrium studies were carried out with 14C-methomyl on
two sandy loams and two silt loams. Aqueous solutions containing
0.2-6 mg methomyl/litre of were mixed with the soils and shaken for
24 h. A separate study was also undertaken on each soil using TLC.
Methomyl was shown to be weakly adsorbed on one of the silt loams
(Ka=1.4), and poorly adsorbed on the other three soils. Desorption
was related to soil organic matter content and indicated that methomyl
is not readily desorbed. In the TLC assessment the Rf values
(0.46-0.82) indicated methomyl as Class 4 (mobile) on a sandy loam and
Class 3 (intermediate mobility) on the other three soils (Priester,
1984).
A silty clay loam, a silt loam and two sandy loams were made up
as slurries and spread on TLC plates. Methomyl and a minor soil
metabolite, S-methyl- N-hydroxy-thioacetimidate (MHTA) were applied
at 1 µg/spot and the plates developed. Methomyl and its metabolite
were considered to be very mobile, with Rf values of 0.64-0.79 and
0.86-0.93, respectively, in the four soils (US EPA, 1988).
In a field study on a sandy loam in California, methomyl was
applied to cabbage by back-pack sprayers at a very high rate of
10 kg/ha (single application). Samples of the soil were taken at 0
and 6 h and then at intervals from 3 to 272 days after application.
Analysis showed that methomyl residues remained mostly in the top
15 cm of soil with deepest penetration into the 15-30 cm layer after
48 cm rainfall/irrigation. On this basis the mobility of methomyl was
considered to be low to moderate under field conditions in this soil
(Kennedy, 1989).
In another field study on a soil characterized as a loam and silt
loam, methomyl was applied to cabbage by back-pack sprayers at the
high rate of 4.4 kg/ha (single application). Soil samples were taken
at 0 and 6 h and then at intervals over a 91-day period after
application. Methomyl residues were found only in the top 15 cm of
soil after 28 cm rainfall/irrigation. On this basis methomyl was
considered to be of low mobility in these field conditions (Kennedy,
1991).
Cox et al. (1993) studied methomyl adsorption to 14 soils from
southern Spain. These varied in pH from 5.3 to 7.9, in percentage of
organic matter from 0.59 to 2.5%, in cation exchange capacity from 4.2
to 28.5, and in the percentage of clay minerals. The methomyl
concentration in soil and water components following shaking for 24 h
was measured by HPLC. Simple and multiple regression analysis was
used to evaluate which factors affected methomyl adsorption. Soils
were equilibrated with both 20 and 50 µM methomyl (high purity); there
was no significant difference between concentration at the same
soil/solution ratio, indicating poor affinity of methomyl for the
soils. Both simple and multiple regression indicated soil organic
matter, clay content and clay minerals, methomyl in soil and illite
content as the major features of soil affecting adsorption of
methomyl. Further studies examined adsorption to individual soil
components. Humic acid showed by far the highest affinity for
methomyl with Kd values 50 times greater than for clay minerals.
Montmonillonites showed a similar Kd to illite in these studies,
contrary to findings with whole soils. The authors suggest, on the
basis of maturation studies, that adsorption to clay may occur in the
interlamellar space of the minerals.
4.1.3 Vegetation
When 14C-methomyl was applied to the surface of tobacco plant
leaves the compound was absorbed in the leaf but not translocated to
other parts of the plant (Harvey & Reiser, 1973).
In a study by Fung et al. (1978), each tobacco seedling received
250 ml of water containing 500 µg methomyl/litre (equivalent to about
0.5 kg active ingredient per hectare of 17 000 plants) after
transplantation. The concentration of methomyl peaked at 15 mg/kg in
the leaves and at 2.5 mg/kg in the growing tips 2 weeks after
treatment. Subsequently, the concentration decreased, which could be
explained almost entirely by growth dilution. At 9 weeks after
transplantation, the plants were sprayed with a solution of 500 mg
methomyl/litre. Three weeks after this (i.e. 12 weeks after
transplantation), another similar application was made. Some plants
received an additional application of 250 ml of a 500 mg
methomyl/litre solution on each side of the row of plants.
Concentrations increased sharply after these treatments and dropped
afterwards: levels in the leaves of plants with both foliar
application peaked at 9 and 6 mg/kg; levels in leaves which received
foliar and root applications peaked at 18 and 11 mg/kg. Part of the
decrease after application could again be explained by growth
dilution. It appears, therefore, that translocation of methomyl from
roots to leaves can occur (Fung et al., 1978).
A sandy loam soil was treated with 14C-methomyl at a rate of
4.4 kg/ha and, 30-120 days later, cabbage, red beet and sunflower
seeds were sown and the plants grown to maturity. Thirty days after
treatment the soil contained 26% of the original methomyl whereas
after 120 days it contained only 8%. All crops, sown at 30 or 120
days, contained only very small residues of methomyl and/or
metabolites, equivalent to 0.01 mg/kg or less at harvest (Harvey,
1978).
4.2 Transformation
4.2.1 Biodegradation
In a study by Harvey (1972a), 14C-methomyl (1 mg/litre) in
river water (pH 6.3) was exposed for 8 weeks from JulySeptember in the
USA. The compound degraded with a half-life of about one week. The
initial degradation product was MHTA followed by breakdown to carbon
dioxide, which accounted for 65% of the original radioactivity after 8
weeks. The S-oxide of MHTA was also detected in small amounts. At
termination, 9% of the original activity was present in sediment and
the biological film on the walls of the container.
Laboratory studies were carried out on a non-sterile silt loam at
its natural pH of 4.7 and at an adjusted pH of 7.9; an alkaline soil
(pH 7.9) was also evaluated. All soils were treated with
14C-methomyl at a high rate equivalent to 4.4-6.1 kg/ha and the
breakdown was assessed over 42 days. Methomyl degraded (52-69%) in 42
days, carbon dioxide (31-45%) being the main decomposition product.
Small amounts of MHTA (1-2%) were present in the soil at termination.
It was shown that the 14CO2 could be reincorporated into soil
organic matter (Harvey & Pease, 1973) (J. Harvey, Jnr (1976):
supplement to "Decomposition of methomyl in soil"; personal
communication by Du Pont to IPCS, dated 28 July 1976).
Under field conditions the decomposition of methomyl was more
rapid, with a 71% loss from a silt loam soil within one month; none
was detected after one year. MHTA was present in trace amounts at 1
and 3 months but was not present at one year. Most of the residual
application was found in the top 75 mm of soil, and none was found
below 200 mm. Decomposition was rather more rapid in fine sand and
loamy sand soils (Harvey & Pease, 1973).
When applied at a concentration of 4.1 mg/kg to a microbially
active loam soil (equivalent to a very high rate of 9 kg/ha),
14C-methomyl was metabolized with a half-life of approximately
11 days. The decomposition followed first-order kinetics and the main
end product was 14CO2 (Zwick & Malik, 1990a). These results were
in agreement with the studies described above and with other aerobic
soil metabolism studies conducted on soils of high or low organic
matter content and various pHs (Harvey, 1972b; 1977a,b).
Dissipation studies of methomyl in loam soils in California and
Mississippi resulted in half-lives of 8 weeks and 5 days, respectively
(Kennedy, 1989; 1991). In addition to differences in temperature,
field moisture differences during the experiment seem largely
responsible for these differences in half-life, because adjusting
field moisture of the California soil to 75% of its capacity in the
laboratory reduced the half-life to 11 days (Kennedy, 1991). Field
moisture conditions greatly decrease the air content of the soil. In
anaerobic soils it has been shown that ferrous ions facilitate the
rapid degradation of methomyl (Bromilow et al., 1986).
Methomyl is also degraded under anaerobic soil conditions. An
alkaline soil with low organic matter content was incubated with
methomyl (4.1 mg/kg) aerobically for 14 days and then anaerobically
for 60 days. The half-life under anaerobic conditions was
approximately 14 days and 14CO2 was a major break-down product,
equivalent to 23.4% of the applied activity during the 60 days of
anaerobic incubation. Unextractable activity was 30% of the total at
7 days and 24% after 60 days of anaerobic treatment. Most of this was
associated with soil organic matter (Zwick & Malik, 1990b).
Anaerobic degradation (Eh 80-310 mV) was studied in samples of
sand, loamy sand and fine sand, taken from below the soil water table
at four locations in the Netherlands (Smelt et al., 1983). In each
case, methomyl was incubated at 10°C and when pH was between 7.4 and
7.7 the half-life was less than 0.2 day (one hour after the start of
the experiment, 38-63% of the applied dose was recovered, and after
24 h, 0.15-5% of the applied dose was recovered. When the fine sand
sample was incubated at 10°C and pH 5.0, methomyl could be detected
for 3 days, and the rate of decrease corresponded to a half-life of
7 h.
The role of microbial action was shown by perfusing two soil
samples (fine sandy loam at pH 6.1 and fine sandy clay loam at pH 5.87
with organic matter content in both of 2.1-2.3%) with methomyl
solution (6 mg/litre) with and without sodium azide (Fung & Uren,
1977). The contribution of adsorption or dissipation of methomyl from
solutions was small when compared with that of microbial
transformation. The latter amounted to 25-45% in 42 days after a lag
phase of 7-14 days. When previously perfused soil was re-exposed to
fresh methomyl solution, 60-75% was lost in 42 days without any lag
phase.
The metabolic fate of methomyl has been investigated in tobacco,
corn and cabbage (Harvey & Reiser, 1973). Tobacco was grown from
seedlings, and when the plants were 18 cm high the roots were treated
with 14C-methomyl (10 mg/litre solution). Cabbage (42 days old) and
corn (28 cm high) plants were treated with 14C-methomyl via foliar
application. Each plant was placed in a glass metabolism apparatus
for radioactivity measurement of volatile products and plant material.
Tobacco absorbed 20-25% of the available activity over a 4-week
period. One quarter of this was retained in plant tissue and the
remainder volatilized. The principal component of plant tissue
activity was methomyl. The volatile components were carbon dioxide
and acetonitrile in equal proportions. Of the applied activity, 47%
was lost from the growing shoots of young corn as volatile components
within 10 days. This was composed of CO2 and acetonitrile in the
ratio of 1:4. One week after treatment of cabbage leaves, 20% of the
activity was lost as CO2 and acetonitrile in equal proportions. The
extracts of the treated plants were investigated for the presence of
three possible metabolites, MHTA and the S-oxide and S,S-dioxide
derivatives of methomyl. There was no evidence for the presence of
these compounds. The only terminal residue specifically detected was
methomyl. The remainder of the radioactivity was incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars.
The biodegradation of methomyl was also studied in corn and
cabbage under field conditions after the application of the
radiolabelled compound. The outer leaves of cabbage contained most of
the radioactive residue of which a small proportion (3-4%) was
identified as methomyl. In corn, the outer portions contained most of
the radioactive residue with about 2 mg methomyl/kg being present
(Harvey & Reiser, 1973).
The half-life of methomyl was determined in cotton leaves sampled
during periods without rainfall after a single application at
0.75 kg/ha, the maximum label rate. The foliar half-life was
estimated to be between 0.6 and 2.2 days, with an average of 1.1 days
(Eble & Tomic, 1991). Bull (1974) applied radiolabelled
14C-methomyl to leaves of tobacco in aqueous solution. Almost half
of the applied methomyl penetrated the leaves within the first few
hours. Surface residues were largely lost within 48 h and the parent
compound was the only radioactive component of the unabsorbed dose.
The absorbed methomyl was degraded within 8 days (mostly within 48 h).
No S-oxide, S,S-dioxide or oxime derivatives were found in the plants,
the methomyl being degraded to acetonitrile and CO2. After methomyl
was applied directly to tobacco leaves, its half-life was 3-7 days
(Harvey & Reiser, 1973). Studies describing the decline of
dislodgeable foliar residues on various crops are reviewed in
section 5.3.
4.2.2 Abiotic degradation
When a 3% solution of methomyl in distilled water was stored for
168 days, 90% of the compound was recovered at the end of the period.
The remainder was recovered as MHTA (Harvey, 1967).
The hydrolysis of methomyl was studied at pH 5, 7 and 9, at
concentrations of 10 and 100 mg/litre, and at 25°C. The compound was
stable for 30 days at pH 5 and 7 but broke down at pH 9 with a
half-life of about 30 days. The hydrolysis product was MHTA
(Friedman, 1983).
The photolysis of methomyl was studied at initial concentrations
of 10 and 100 mg/litre and at pH 5 under UV light. Methomyl
photolysed rapidly at both concentrations with a half-life of 2-3 days
at 100 mg/litre. The principal photo-product was acetonitrile
(Harvey, 1983).
In a study by Swanson (1986), 14C-methomyl was applied to a
thin layer of a silt loam soil on glass plates and exposed to natural
sunlight for 30 days at 24-28°C. The compound decomposed with an
estimated half-life of 34 days. The principal decomposition product
was acetonitrile. Duplicate preparations, kept in the dark, did not
decompose.
Methomyl degraded rapidly in slightly alkaline solution (pH 8.85)
with a chlorine/methomyl ratio of 10. The degradation rate increased
with increasing temperature, increasing chlorine concentration, and
decreasing pH. The reaction rate with free chlorine was 1000-fold
faster than with chloramine. Methomyl degraded to acetic acid,
methanesulfonic acid and dichloromethylamine after forming methomyl
sulfoxide and N-chloromethomyl (Miles & Oshiro, 1990). Mason et al.
(1990) also reported that the removal of methomyl can be effectively
achieved by some disinfectants (Cl2, O3) but not by ClO2.
4.2.3 Bioaccumulation
Rainbow trout were exposed to 0.075 and 0.75 mg methomyl per
litre in a flow-through test system for up to 28 and 21 days,
respectively, and then placed in clean water (pH 7.3, total hardness
25 mg CaCO3/litre at 18°C). At the higher concentration, fish
tissue contained 0.36-0.45 mg methomyl/kg during the exposure period
and, at the lower concentration, 0.04-0.07 mg/kg. Within one day of
depuration the methomyl tissue levels fell to below 0.02 mg/kg in both
exposure groups. There was therefore no indication of bioaccumulation
of methomyl in these studies (Sleight, 1971).
4.3 Interaction with other physical, chemical or biological factors
When 14C-methomyl was incubated with a rumen microorganism
culture at a level of 1 mg/kg and at 38°C for 24 h, 90% was
metabolized to a volatile component which was identified as
acetonitrile by gas chromatography. Less than 0.1% of the total
activity was recovered as methomyl or MHTA (Belasco, 1972b).
No nitrosomethomyl was detected (< 1 µg/kg) when 14Cmethomyl
(1 mg/kg) was incubated under simulated stomach conditions (pH 2) with
sodium nitrite (16-20 mg/kg) in a macerate of cured meat for 1 or 3 h
at 37°C (Han, 1975).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In 60% of drain water samples from greenhouses containing treated
plants in an area south of The Hague, The Netherlands, methomyl could
not be detected (< 0.1 µg/litre). In the remaining 40% samples its
concentration was < 1 µg/litre (Leistra et al., 1984).
Out of 22 404 wells sampled and analysed for methomyl in the US
EPA pesticide monitoring programme, 85 showed detectable methomyl
concentrations within the range of trace to 20 µg/litre, i.e. within
the lifetime health advisory figure of 200 µg/litre (US EPA, 1991).
After a cedar swamp had been sprayed with Lannate LV formulation
(29% a.i.) at a rate of 0.28 kg methomyl/ha, no methomyl could be
detected (< 0.02 mg/litre) in surface water at any time between 1 and
58 days after treatment (Du Pont, 1978).
5.1.2 Soil
Soil samples taken from a tobacco farm in Maryland, USA, after a
single application of methomyl (Lannate 90% a.i.; 13.4 kg/ha)
contained 0.6 mg methomyl/kg at 0-15 cm depth after 8 days. At a
depth of 15-30 cm, 0.1 mg/kg was detected after 17 days. No methomyl
(< 0.02 mg/kg) was detected after 47 days at either level. Lannate
is applied as an incorporation to a depth of 10 cm (Pease, 1968).
Levels of methomyl in soil after an upland forest and a cedar
swamp had been sprayed with Lannate LV at a rate of 0.28 kg
methomyl/ha were either very low (< 0.07 mg/kg) or undetectable
(< 0.02 mg/kg). No residues were detected (< 0.02 mg/kg) at an
application of half the above rate (Du Pont, 1978).
5.1.3 Food crops
Methomyl is used as a broad spectrum insecticide on many food
crops, hence low residues may be present at harvesting. The amount of
residue at harvest depends upon factors such as the application rate,
time interval between the last application and harvest, and the type
of crop. The residue is composed mainly of methomyl itself. The
residue levels expected in food crops at harvest can be derived from
the numerous supervised trials which have been carried out on many
crops in countries worldwide (FAO/WHO, 1988a,b; 1990a,b). Pre-harvest
intervals are also set on the basis of the results of supervised
trials, e.g. 7 days for lettuce and onions in USA, 1 day for brassicas
and tomatoes in Australia (FAO/WHO, 1990a,b).
The decline of methomyl residues on food crops after application
was demonstrated by the field treatment of broccoli followed by
harvesting at intervals thereafter. The results of treatment at
0.55 kg/ha (6 applications) are shown in Table 3 (Du Pont, 1973).
Table 3. The decline of surface and absorbed methomyl residues in
broccoli (Du Pont, 1973)
Days after treatment Residue (mg/kg)
1 1.0
3 0.15
7 0.04
10 0.02
Similar examples of the decline of methomyl residues have been
shown for lettuce and cauliflower (Braun et al., 1980), celery (Braun
et al., 1982), wheat (Du Pont, 1973; FAO/WHO, 1988a,b) and tomatoes
(FAO/WHO, 1990a,b).
Treated food crops processed after harvesting generally do not
show a concentration of methomyl residues in the processed fractions.
Unwashed whole oranges were found to contain 0.96 mg/kg at harvest and
0.88 mg/kg after washing. The dried peel contained 2.8 mg/kg whereas
the pulp, juice, cold process oil and molasses contained no detectable
residue (< 0.02 mg/kg). Similarly, tomatoes analysed after harvest
contained 0.38 mg/kg while the processed fractions, wet pomace, dry
pomace, juice and puree contained < 0.02 mg/kg (Kennedy & Hay, 1991a;
Marxmiller & Hay, 1991). Mint oil produced from the distillation of
methomyl-treated plants and wine produced from treated grapes
contained no detectable residues (< 0.04 and < 0.02 mg/kg,
respectively) of methomyl (Kiigemagi et al., 1973; Brodsky, 1991).
5.1.4 Other crops
Environmental levels of methomyl on treated crops such as cotton
and tobacco may be deduced from supervised trials.
After 14 applications of methomyl at 0.5 kg/ha and two at the
high rate of 1.65 kg/ha, cotton contained 0.17 mg methomyl/kg 15 days
after the last application. Processed fractions showed < 0.02 mg/kg
in oil, 0.065 mg/kg in meal and 0.14 mg/kg in hulls (Kennedy & Hay,
1991b).
The residue levels in tobacco leaves immediately after the
application of methomyl at the recommended rate of 0.56 kg/ha were 44
and 88 mg/kg at two sites in the USA. After five days these levels
had dropped to 0.7 and 1.4 mg/kg. Approximately 96% of the methomyl
was lost during flue-curing (Leidy et al., 1977).
5.1.5 Dairy products
There are no reports of methomyl residues in dairy produce.
Groups of lactating cows (three/group) were dosed methomyl by
capsule at a rate equivalent to 8, 24 or 80 mg/kg in their feed for
28 days. Milk collected during the dosing period and tissues taken at
termination contained no detectable residues of methomyl or its
metabolite MHTA. Acetonitrile was detected in the milk of cows dosed
with methomyl at 8 mg/kg, the milk concentration of acetonitrile
reaching a plateau of 0.04-0.1 mg/kg by day 4. This component was
also present in liver (0.08 mg/kg) and kidney and muscle (0.04 mg/kg)
at this dose level. Acetamide concentrations in milk and tissue of
cows dosed with 80 mg/kg were the same as those found in control
animals. Radiolabel assessment showed that the acetamide derived from
methomyl was < 1% of the total dose (Powley, 1991). In another study,
methomyl was fed to cows at 2 or 20 mg/kg in feed and no methomyl
(< 0.02 mg/kg) was detected in milk over a 30-day period nor in meat
tissue at termination (Du Pont, 1967).
The 14C-residue in milk was equivalent to 0.13 mg/kg 4 days
after two goats had been fed 14C-methomyl at 8 mg/kg diet. Methomyl
itself could not be detected. Total 14C-residues in a range of
tissues were very low (< 0.001-0.003 mg/kg) (Osman et al., 1983).
5.1.6 Animal feed
Some indication of methomyl residues expected in those portions
of treated crops used for animal feed can be deduced from the
following studies. Methomyl concentrations were 0.35 mg/kg in forage,
0.14 mg/kg in cannery waste and < 0.02 mg/kg in kernels from sweet
corn treated with methomyl at 0.9 kg/ha and harvested 9 days after the
last of four applications (Harvey & Yates, 1967). Methomyl residues
on sweet corn forage harvested immediately after the last of nine
foliar applications at 0.5 or 1 kg/ha were 0.15-0.60 mg/kg and
0.2-0.72 mg/kg, respectively (US EPA, 1988). The residues on samples
of wheat straw taken 7 days after foliar application of 0.55 kg
methomyl/ha were < 0.02-6.5 mg/kg, and after 14-18 days they were
< 0.02-0.8 mg/kg (US EPA, 1988).
5.2 General population exposure
Information available on general population exposure is limited
and derives primarily from only one country. More complete exposure
information via various routes specific to regions and countries is
required to assess the risk of occupational exposure and intake of
residues.
5.2.1 Food
The market baskets collected for the US FDA total diet study
prior to 1991 consisted of 234 food items. Of these, a total of 72
items (69 adult foods, 2 baby foods and water) were analysed by
methodology known to be capable of determining methomyl. Methomyl was
detected only in 11 food items collected from 1987 to April 1991 (20
market basket studies): watermelon, pear, strawberries, grapes,
cantaloupe, raisins, lettuce, celery, cauliflower, cucumber, and green
sweet peppers. The levels detected in these food samples ranged from
trace to 0.630 mg/kg, well below the tolerances established by US EPA
(US FDA, 1993a).
The total number of domestic and imported food samples analysed
by methodology known to be capable of determining methomyl in US FDA
regulatory monitoring programmes during the period 1988-1992 was 7765.
Of these, methomyl residues were detected only in 112 samples. Four
samples were found to be violative: one domestic sample of strawberry
(4.53 mg/kg) and one imported cantaloupe (0.28 mg/kg) exceeded the
USA tolerances and there were two imported samples for which no USA
tolerances have been established (okra, 0.05 mg/kg and pepino,
0.46 mg/kg) (US FDA, 1993b).
Residues in foodstuffs are reduced by domestic processing such as
washing, peeling and cooking. For example, 50-90% of methomyl
residues on celery was removed by trimming (FAO/WHO, 1986). Methomyl
residues declined by 70-93% in tomatoes, peas or cabbage after 30 min
cooking in boiling water in open containers (Holt, 1971). Methomyl
was added to spinach puree to give a concentration of 50 mg/kg and
then processed in closed cans for 40 min at 122°C. No methomyl was
detected (< 0.05 mg/kg) at the end of this period (Niven, 1971).
Methomyl has not been detected in wine or mint oil prepared from
crops previously treated with methomyl (see section 5.1.3).
No methomyl could be detected (< 0.02 mg/kg) in eggs or tissues
of laying hens given 1 or 10 mg methomyl/kg diet for 4 weeks (Sherman,
1972).
5.3 Occupational exposure
A series of studies was carried out to determine worker re-entry
times after the application of methomyl to grape vines in California,
USA (Dong et al., 1992; Reeve et al., 1992). These studies were
specific to the desert conditions found in California and should not
be compared to studies on other crops or in other climates. Methomyl
was applied at different times of the growing season at 1 kg/ha and
the dislodgeable foliar residues were measured at time intervals after
application to estimate how long it would take to reach the desired
level of 0.1 µg/cm2 on the leaves. Under desert conditions with
water supplied only by furrow or drip irrigation, it was found that it
required about 5 days to reach this level in June, when grape girdling
was carried out, and about 10 days in September, at harvesting
(Powley, 1989, 1990a,b).
A worker re-entry study was undertaken to estimate exposure after
entry into vineyards when dislodgeable foliar residues had fallen to
0.1 µg methomyl/cm2 or less. Each worker wore ankle length tights
(except raisin grape harvesters) and long sleeved T-shirt, both worn
under normal work clothes. Each wore a personal air sampling pump and
two patches were attached to work hats. Sample patches were worn on
the thigh and ankle on the normal work clothes by most workers during
girdling operations. Work continued for 3-4 h. Methomyl exposure
when girdling field grapes ranged from 315-1214 µg/h with highest
values on the upper body and head. Exposure was highest to the upper
body and hands of raisin harvesters where overall daily exposure was
463-865 µg/h. Harvesting and packing table grapes resulted in the
lowest methomyl exposures of 219 and 102 µg/h, respectively.
Inhalation exposure was minimal (Merricks, 1990).
It should be emphasized that the rate of decay of methomyl in the
Californian studies described above is not representative of the
situation in grape culture in other areas of the world or for other
crops, as, due to irrigation and other cultural practices, Californian
grape vines are quite large and have lush foliage which maximizes
exposure. Methomyl does not hydrolyse readily in the hot dry
desert-like conditions and this gives rise to atypical transfer rates.
Dislodgeable foliar residue from cotton plants was the subject of
three studies in Arizona, USA (Cahill et al., 1975; Ware et al, 1978,
1980). In each case, methomyl was applied at 0.55 kg/ha and leaf
samples were taken for analysis of dislodgeable residues up to 96 h
after. In each study the methomyl residues had declined to 0.1 µg/cm2
or less within 48 h.
Dislodgeable foliar residues were determined after spraying mint
to estimate possible exposure to workers moving irrigation pipes.
After spraying methomyl at l kg/ha from the air, dislodgeable residues
were 1.5 µg/cm2 at 4 h and 0.32 µg/cm2 at 48 h, and, after
applying 2 kg/ha, residues were 2.3 µg/cm2 at 4 h and 0.6 µg/cm2 at
48 h (Kiigemagi & Deinzer, 1979).
A pilot study was undertaken in Thailand with a methomyl 90%
soluble powder formulation to assess the use of food dyes as markers
for pesticide exposure. Pesticide operators prepared and sprayed the
diluted methomyl formulation containing the dyes on low (broccoli,
chinese kale), medium (tobacco) or tall (citrus) crops with knapsack
or high pressure power sprayers. Measurement of dye content of the
outer garments showed that exposure occurred mainly to the lower body
and legs when spraying low crops and mainly to the upper body and arms
when spraying tall crops. Some correlation was shown between the
amounts of methomyl and dye deposited on the outer garments. However,
the number of participants (two per group) was too small to draw
definite conclusions, and more work needs to be done to establish
these correlations (Ambridge, 1992).
Methomyl was not detected in air samples from the working zones
of operators during normal closed transfer, mixing-loading operations.
During application, methomyl air concentrations of up to 7.5 µg/m3
were found in applicator working zones (Knaak et al., 1980).
In order to establish a post-application re-entry interval for
workers employed in greenhouse operations, methomyl dislodgeable
foliar residue data were collected from rose foliage. It was shown
that after a single high rate of application of 3.2 kg/ha it took
nearly 5 days for the dislodgeable residue to decline to the required
level of 0.1 µg/cm2. It was estimated that for the application rate
of 1 kg/ha, the highest normally used for rose treatment, a re-entry
interval of 48 h would be required (Oswald et al., 1991).
The concentration of methomyl in greenhouse air was measured
directly by an atmospheric pressure chemical ionization mass
spectrometer system. The atmosphere was monitored during spraying of
roses and for 26 h thereafter. Samples taken at head height during
spraying showed methomyl levels of about 33.1-39.7 µg/m3 (5-6 ppb).
A few hours later, at the end of the day's operations, concentrations
were about 19.9-26.5 µg/m3 (3-4 ppb). When monitoring resumed the
following morning the air concentrations were still at about the same
level indicating that methomyl, deposited in aerosol droplets on the
roses, had not fully evaporated (Williams et al., 1982).
Ambient air and breathing zone samples were analysed in four
greenhouses 1 day before and 7 days after methomyl was sprayed on
cucumber and tomato plants. Ambient air methomyl concentrations
ranged up to 4.7 µg/m3 on the first day after spraying. Three and
seven days after spraying, breathing zone methomyl concentrations
ranged up to 14.5 and 0.7 µg/m3, respectively. Hand-wash methomyl
values ranged from 10 to 322 µg/h of work in a greenhouse. The
authors considered that dermal exposure, as indicated by the hand-wash
data, was a more important factor than air exposure and that re-entry
intervals should be set according to information derived from the
former (Boleij et al., 1991).
Ambient air in a pesticide storage building was monitored over a
3-h period using high volume air samplers and absorption on XAD-4 or
XE-340 resins. Methomyl was stored in the building as a liquid
concentrate along with other pesticide formulations. The average
methomyl air concentration over the sampling period was 13.7 ng/m3.
This represents a value of 0.18 µg/m3 when converted to a 40-h
working week and can be compared with the ACGIH TLV of 2500 µg/m3
(Yeboah & Kilgore, 1984).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
The term 14C-methomyl in this section refers to
S-methyl-[1-14C]-N-[(methylcarbamoyl)oxy] thioacetimidate, unless
otherwise stated.
6.1 Absorption
The absorption of 14C-methomyl was very rapid after oral dosing
of 5 mg/kg to male or female rats. About 80% of the activity was
excreted within 24 h as metabolic products in urine and expired air.
Only 2-3% was found in faeces (Harvey et al., 1973; Hawkins et al.,
1991).
A similar pattern was seen in the cynomolgus monkey following a
single 5 mg/kg oral dose. More than 60% of the dose was eliminated in
expired air and urine within 24 h as metabolic products. Only about
3% of the dose was found in faeces over a period of 168 h after dosing
(Hawkins et al., 1992).
In an assessment of dermal penetration, 14C-methomyl was
applied in acetone to a 1 cm2 shaved area of skin of mice (7-8 weeks
old) at a rate of 1 mg/kg. The mice were then placed in metabolism
chambers and killed for radioactivity measurements at intervals up to
48 h. Within 5 min, 14C activity was detected in blood and liver.
In 60 min, 2.9% of the original 14C dose was present in blood, 5%
was in the liver, and 12.9% had been excreted (urine plus CO2 plus
faeces). Very little methomyl remained at the application site after
60 min, when penetration was estimated to be approximately 85%. The
half-life, as a measure of penetration rate, was approximately 13 min
(Shah et al, 1981).
6.2 Distribution
After 5 mg 14C-methomyl/kg had been dosed orally to five male
and five female rats, 8-9% of the initial activity was found in the
tissues and carcass 7 days later. The highest concentration of
activity was found in blood (2 mg/kg equivalents) with a distribution
of 3-4 mg/kg in red cells and 0.7-0.9 mg/kg in plasma. The
radioactivity concentrations were lower in other tissues (< 1 mg per
kg). As a proportion of the original dose, blood contained
approximately 2%, liver 0.4%, gastrointestinal tract 0.6% and other
individual tissues < 0.1% each. There was no significant difference
in distribution between males and females (Hawkins et al., 1991).
14C activity was distributed among a range of tissues after two
rats had been fed 200 mg methomyl/kg diet for 8 days and then given
5 mg 14C-methomyl/kg orally. Of the 14C dose, 9% was found in the
tissues and carcass within one day and 10% within 3 days (Harvey et
al., 1973).
When cynomolgus monkeys were given a single oral dose of 5 mg
14C-methomyl/kg, approximately 5% of the radioactivity was retained
in the tissues after 168 h. The highest concentrations of activity
were in the liver (0.7-0.9 mg/kg equivalents), fat (0.4-0.7 mg/kg
equivalents) and kidney (0.4-0.5 mg/kg equivalents). Lower
concentrations found in other tissues were generally higher than blood
levels of 0.1-0.2 mg/kg equivalents (Hawkins et al., 1992).
One hour after the dermal applications of 14C-methomyl to mice
(see section 6.1), 2.9% of the dose was present in blood, 5% in liver
and 56% in the remaining carcass. After 8 h the distribution was 6.1%
in blood, 3.3% in liver, 3.8% in the gastro-intestinal tract and
smaller amounts (< 1%) in other individual tissues. The remaining
carcass contained 15% of the original dose (Shah et al., 1981).
6.3 Metabolic transformation
In an initial investigation, two male rats were fed a diet
containing 200 mg methomyl/kg for 8 days, followed by intra-gastric
intubation of 1.2 mg 14C-methomyl (=5 mg/kg). One male rat was
treated similarly except that the 14C-methomyl was given after 19
days. Urinary and volatile metabolite identification was carried out
1 or 3 days after the 14C dose. Volatile products, trapped in
caustic soda solution or in cold traps, were identified as carbon
dioxide and acetonitrile, the latter confirmed by mass spectroscopy.
Countercurrent distribution of the urine showed that nearly all the
radioactivity was present as polar material. Methomyl, its
S,S-dioxide and MHTA were not detected. The methomyl S-oxide could
not be detected by TLC (Harvey et al., 1973).
A more detailed study (Hawkins et al., 1991), with five male and
five female rats receiving single oral doses of 14C-methomyl
(5 mg/kg), confirmed that the expired metabolites (over 120 h) were
carbon dioxide (22%) and acetonitrile (12%). The radioactive
components of the 0-24 h urine were separated by reverse phase HPLC,
ion partition chromatography and TLC. The major metabolite in urine
was identified by NMR and mass spectroscopy as the mercapturic acid
derivative of methomyl, equivalent to about 17% of the 14C-dose.
There were 10 minor components which included, on tentative
identification, acetonitrile, acetate and methomyl oxime sulfate.
Methomyl, MHTA and the anti-isomer form of methomyl were not detected.
Metabolic pathways for methomyl in the rat include the
displacement of the S-methyl moiety by glutathione and enzymic
transformation to give the mercapturic acid derivative. Another
pathway involves hydrolysis to give MHTA which is rapidly
broken down to carbon dioxide (Fig. 1).
Another proposed pathway involves the conversion of the
syn-isomer of methomyl (the insecticide form) to its anti-isomer. The
latter has been shown to produce acetonitrile as the main volatile
metabolite when given orally to rats (see section 6.4). It is
proposed that the anti-isomer hydrolyses to the anti-MHTA,
which then undergoes a Beckmann re-arrangement and elimination
reaction to form acetonitrile (Huhtanen & Dorough, 1976).
It is also likely that certain metabolic products such as
acetonitrile undergo further reactions, with the carbon components
being incorporated into natural body constituents such as fatty acids,
neutral lipids and glycerol, as shown in ruminants (see section
4.2.1).
It is probable that two of the main metabolic pathways also
operate in the monkey. When an oral dose of 14C-methomyl
(5 mg/kg body weight) was given to cynomolgus monkeys, the
major metabolites were CO2 (32-38%) and acetonitrile (4-7%) in the
expired air. These were derived, presumably, by the same processes as
described for the rat above. A combination of HPLC and TLC
characterized 18 radioactive metabolites in urine, with no metabolite
representing more than 4% of the dose. Small amounts of acetonitrile,
acetate, acetamide and MHTA sulfate were among the products found.
The mercapturic acid derivative of methomyl (a major rat urinary
metabolite) accounted for about 1% of the dose. The presence of these
minor metabolites are presumably the result of extensive metabolism of
primary metabolites (Hawkins et al., 1992).
A lactating cow was dosed twice daily by capsule for 28 days
with 14C-methomyl at a rate equivalent to 8 mg/kg in feed. Milk
samples were collected each day and selected tissues were taken within
24 h of the last dose. Radioactivity appeared in milk within one day
and reached a plateau of 0.5 mg/kg (equivalents) within 6 days. This
activity was mostly due to the reincorporation of the radiolabel into
fatty acids, lactose and other acetate derived products. No methomyl
or MHTA was detected; acetonitrile accounted for about 25% of the
radioactivity. The liver showed the greatest concentration of
radioactivity, equivalent to 9.23 mg/kg; kidney contained only
2.01 mg/kg and there were lower concentrations in fat and muscle. No
methomyl was detected in tissue; most of the radioactivity was
considered to be the result of reincorporation of the radiolabel as
acetate into natural constituents (Monson & Ryan, 1991).
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate
in the preparation of the EHC monograph must, in addition to serving
in their personal capacity as scientists, inform the RO if at any time
a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Dept. of Human Services and Health, Canberra, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, PA, USA
Dr Jun Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr Palarp Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr Salah A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of Agriculture
Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Observers
Dr R. Gardiner, GIFAP, Brussels, Belgium (Representative of GIFAP)
Dr B. Julin, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr S.M. Kennedy, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr Ronald L. Mull, Du Pont Agricultural Products, Wilmington, DE,
United States of America (Representative of GIFAP)
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Office, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
The Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP) met in Geneva from 25 October to 3 November 1994. Dr W. Kreisel,
Executive Director, welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS on behalf of the three IPCS cooperating
organizations (UNEP/ILO/ WHO). The CAG reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methomyl.
The first draft of the monograph was prepared by
Dr M.L. Litchfield, Arundel, United Kingdom. Extensive scientific
comments were received following circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs and
these comments were incorporated into the second draft by the
Secretariat.
The fact that E.I. Du Pont de Nemours and Co. made available to
IPCS and the Core Assessment Group proprietary toxicological
information on their products is gratefully acknowledged. This
allowed the Group to make its evaluation on a more complete data base.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ADI Acceptable Daily Intake
ALC Approximate Lethal Concentration
CAS Chemical Abstracts Service
CCPR Codex Committee on Pesticide Residues
EbC50 median effective concentration for inhibition of growth
based on comparison of areas under the growth curves after
"b" hours
ECD electron capture detector
FID flame ionization detector
FSD flame photometric detector selective for sulfur
GC gas chromatography
GLC gas-liquid chromatography
GOT glutamic oxaloacetic transaminase
GPT glutamic pyruvic transaminase
HPLC high performance liquid chromatography
ISO International Organization for Standardization
IUPAC International Union of Pure and Applied Chemistry
JMPR Joint FAO/WHO Meeting on Pesticide Residues
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
MATC maximum acceptable toxicant concentration
MHTA S-methyl- N-hydroxythioacetimidate (a metabolite of
methomyl)
mPa millipascal (7.5 × 10-6 mmHg)
MRL maximum residue limit
NOEC no-observed-effect concentration
P2S N-methyl-pyridium-2-aldoxime methane-sulphonate
(antidote)
PAM pyridine-2-aldoxime methiodiode (antidote)
RTECS Registry of Toxic Effects of Chemical Substances
TEAC tetraethylammonium chloride
TOCP tri- o-cresyl phosphate
TOD total oxygen demand
UV ultraviolet
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
Methomyl is a white crystalline solid with a melting point of
77°C and a vapour pressure of 0.72 mPa (25°C). Its solubility in
water is 54.7 g/litre and its octanol/water partition coefficient
(Kow) is 1.24. It is stable in sterile water at pH 7, but is broken
down at higher pH values, the half-life being 30 days at pH 9 and
25°C.
The analytical procedure for the determination of methomyl in
different samples is extraction followed by clean-up and analysis by
HPLC or GLC. In some cases methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
1.2 Sources of human and environmental exposure
Methomyl is produced by reacting S-methyl N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. It is a carbamate insecticide used on a wide
range of crops throughout the world. Crops protected include fruit,
vines, hops, vegetables, grain, soya bean, cotton and ornamentals.
Indoor uses include the control of flies in animal houses and dairies.
The main formulations are water soluble powders and water
miscible liquids, which are diluted with water for ground or aerial
spraying of crops. Typical active ingredient rates are 0.15 to
1.0 kg/ha. The main sources of human exposure are during the
preparation and application of these products and from the ingestion
of crop residues in foodstuffs (see section 5.3.1.4).
1.3 Environmental transport, distribution and transformation
In laboratory studies, methomyl adsorbs poorly to soil. Weak
adsorption to clay minerals, particularly illite, has been
demonstrated; adsorption to soil organic matter is 50 times greater
but still relatively weak. Hardly any desorption of bound residue is
seen. With these characteristics, methomyl would be expected to be
mobile in soil.
Under natural environmental conditions, abiotic degradation of
methomyl by hydrolysis or photolysis is slow or absent.
Aerobic degradation in soil is about twice as fast as anaerobic
degradation. Reported half-lives of methomyl in soil vary from a few
days to more than 50 days; dry conditions delay breakdown. In
practice in the field most applications should lead to a half-life of
around one week.
In field conditions, methomyl does not leach to levels below 20
to 30 cm into the soil and does not contaminate ground water.
When 14C-methomyl is applied to plant leaves it is absorbed but
not translocated to other parts of the plant. When applied to the
root system it is absorbed into the plant where the principle residue
component is methomyl itself. Volatile breakdown products are CO2
and acetonitrile. The remainder of the activity is incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars. The half-life of methomyl in plant foliage is a few days.
There was no evidence for accumulation of methomyl in rainbow
trout exposed to the compound for 28 days in a flow-through system.
1.4 Environmental levels and human exposure
Methomyl levels are likely to be either very low or undetectable
(< 0.02 mg/litre) in ground water on the evidence of analyses of
various water sources after the application of the compound at
recommended rates.
Low residue levels of methomyl are present in food and other
crops at harvesting, the levels depending upon factors such as the
applied rate, time interval after the last application and the type of
crop. The residue is composed primarily of methomyl.
Residues of methomyl in dairy products are either undetectable or
very low. Lactating cows given methomyl by capsule at a rate
equivalent to 80 mg/kg in their feed for 28 days showed no detectable
residues of methomyl or the metabolite MHTA in milk or tissues
(< 0.02 mg/kg). No methomyl was detected in eggs or tissues of
laying hens given 1 or 10 mg/kg in the diet for 4 weeks.
In total diet or individual food analyses in the USA, the
concentrations of methomyl in sample surveys were either undetectable
or very low. Residue levels are further reduced by processes such as
washing, peeling and cooking.
Re-entry exposure studies, specifically for California desert
conditions, showed that, when workers returned to vineyards where
dislodgeable foliar residues had fallen to 0.1 µg/cm2, the highest
exposure occurred on the upper body and head during grape girdling and
on the upper body and hands during raisin harvesting. Harvesting and
packing table grapes resulted in the lowest exposure. Inhalation
exposure was minimal.
After methomyl was sprayed on cucumber and tomato plants, ambient
air concentrations in the greenhouse ranged up to 4.7 µg/m3 on the
day after spraying. Three and 7 days after spraying, breathing zone
methomyl concentrations ranged up to 14.5 and 0.7 µg/m3,
respectively. Hand-wash methomyl values ranged from 10 to 322 µg
per h work in a greenhouse. This indicated that dermal exposure was a
more important route of exposure than inhalation and that re-entry
intervals should be based on dermal exposure data.
1.5 Kinetics and metabolism in laboratory animals
The absorption, metabolism and excretion of methomyl after oral
administration to rats are very rapid, the processes being completed
within a few days. When rats were given radiolabelled methomyl
(5 mg/kg body weight), 54% of the dose was excreted in urine and 2-3%
in faeces within 7 days, and 34% in expired air within 5 days. After
7 days, 8-9% of the 14C dose remained in the tissues and carcass,
which was incorporated into endogenous constituents. The highest
concentration of radioactivity was in the blood (representing 2% of
the dose).
The major metabolic components in expired air of rats were carbon
dioxide and acetonitrile in the ratio of about 2:1. The major
metabolite in urine was the mercapturic acid derivative of methomyl,
which was equal to 17% of the dose. Neither methomyl nor its oxime
derivative was detected.
The proposed metabolic pathway includes displacement of the
S-methyl grouping by glutathione followed by enzymic transformation
to give the mercapturic acid derivative. Another pathway is by
hydrolysis to give MHTA, which is rapidly broken down to carbon
dioxide. A further possible route is conversion of syn-methomyl (the
insecticidal form) to its anti-isomer, which undergoes hydrolysis,
rearrangement and elimination reactions to give acetonitrile.
Methomyl is similarly metabolized in the monkey, except that the
mercapturic acid derivative is a minor component in the urine.
The penetration of 14C-methomyl was estimated to be 85% within
one hour after dermal application in acetone to mice. At that time 3%
of the dose had appeared in blood, 5% in liver and 13% had been
excreted. Within 8 h the total excretion was 54.5%.
The rapid breakdown and elimination of methomyl in the rat,
together with its lack of accumulation in tissues, are comparable to
that seen in ruminants.
Methomyl is completely broken down when cows or goats are dosed.
No methomyl or its oxime derivative was detected in milk or tissues.
It was shown that the compound was metabolized and incorporated into
natural constituents of milk and liver.
No nitrosomethomyl was detected when 14C-methomyl was incubated
under simulated stomach conditions with sodium nitrite in a cured meat
macerate.
1.6 Effects on laboratory mammals and in vitro test systems
Methomyl has high acute oral toxicity, with an oral LD50 in the
rat of 17-45 mg/kg body weight. It is also highly toxic to rats by
the inhalation route, with a 4-h LC50 of 0.26 mg/litre in aerosol
form. Dermal toxicity is very low, with the LD50 exceeding
2000 mg/kg body weight in the rabbit (intact skin) and > 1000 mg/kg
body weight in the rat (abraded skin). Signs of acute toxic action
are those expected of a cholinesterase inhibitor and include among
others profuse salivation, lacrimation, tremor and pupil constriction.
Recovery from the effects was rapid. No gross pathological effects
due to treatment were seen in the organs examined. Methomyl is not a
skin irritant or sensitizer and is a mild eye irritant.
Repeated dietary administration over longer periods did not lead
to accumulation or increase in toxic effect. Rats and dogs fed diets
containing methomyl up to 250 mg/kg and 400 mg/kg in the diet,
respectively, for 13 weeks did not show any toxic signs or mortality.
Rats fed at the 250 mg/kg level showed small decreases in body weight
gain, lower haemoglobin levels and moderate erythroid hyperplasia in
the bone marrow. The NOEL in rats was 50 mg/kg in the diet
(equivalent to 3.6 mg/kg body weight per day). Rabbits given repeated
dermal applications of methomyl at doses up to 500 mg/kg body weight
per day for 21 days showed hyperactivity and depressed plasma and
brain cholinesterase activity at the top dose. The NOAEL was 50 mg/kg
body weight per day in this study.
Long-term studies were carried out on rats at methomyl
dietary levels of 0, 50, 100 or 400 mg/kg and on mice at 0, 50, 75 or
200 mg/kg. Effects on rats at the top dose included depressed body
weight gain and lowered haemoglobin and haematocrit values. The NOEL
was 100 mg/kg in the diet, equivalent to 5 mg/kg body weight per day.
In the study in mice, an increased mortality rate and decreased
haemoglobin and red blood cell counts were seen at the two higher dose
levels. The NOEL was 50 mg/kg in the diet, equivalent to 8.7 mg/kg
body weight per day. In a 2-year toxicity study in dogs (0, 50, 100,
400 or 1000 mg/kg in the diet), clinical signs of toxicity were noted
in some animals at the top dose together with slight to moderate
anaemia. The NOEL was 100 mg/kg in the diet, equivalent to 3 mg/kg
body weight per day.
There was no evidence of treatment-related increases in tumour
incidences in 2-year studies on rats and mice, indicating that
methomyl is not carcinogenic. It was not genotoxic in bacterial or
mammalian cells in vitro and was negative in tests for primary DNA
damage in bacterial and mammalian cells in vitro and in an in vivo rat
bone marrow chromosomal study. It showed cytogenetic potential in
human lymphocytes in vitro, as shown by increases in micronuclei and
chromosome aberrations. Methomyl did not produce embryotoxic or
teratogenic effects in rats or rabbits at doses up to 400 mg/kg in the
diet or 16 mg/kg body weight per day by gavage, respectively, at which
levels toxic effects were present in the dams. In a 3-generation
reproduction study in rats at dose levels of 50 or 100 mg/kg in the
diet (equivalent to 5 or 10 mg/kg body weight per day) methomyl did
not affect fertility, gestation or lactation indices and there were no
treatment-related gross abnormalities.
Methomyl did not show delayed neurotoxicity after single or
repeated administration. Rats fed 800 mg/kg in the diet showed
significant depression of blood cholinesterase activity only in the
early stages of a 5-month study. In a 28-day dietary study, brain
cholinesterase activity was only slightly depressed at this dose
level. This indicated the rapid reversibility of methomyl-inhibited
cholinesterase activity in the animals during the feeding periods. In
vitro, human erythrocyte cholinesterase activity was six times more
sensitive to the inhibitory action of methomyl than that of the rat,
although the rates of spontaneous reactivation were similar.
Atropine was shown to be the most consistently effective antidote
for methomyl poisoning based upon the results of studies in several
species.
1.7 Effects on humans
Reports on accidental and suicidal poisonings with methomyl
provide some information on effect levels and recovery. Three out of
five victims of accidental poisoning from a contaminated meal died
within 3 h of the ingestion. It was estimated that the victims had
consumed about 12-15 mg methomyl/kg body weight. A 31-year-old woman
and her 6-year-old son, both of whom died as a result of deliberate
poisoning, showed concentrations of methomyl in the liver of 15.4 and
56.5 mg/kg, respectively. The estimated doses were 55 mg/kg body
weight for the mother and 13 mg/kg body weight for the son. Six hours
after ingesting approximately 2.25 g methomyl, a woman's blood
contained .6 mg methomyl/kg. Methomyl could not be detected 22 h
after ingestion, when the woman was recovering.
A pesticide operator, who did not take any precautions when
mixing a powdered methomyl formulation for spraying vegetables,
displayed poisoning symptoms within one hour and showed a blood
cholinesterase activity 40% of normal after 12 h, with recovery to 80%
of normal activity within 36 h. Other operators, following the
recommended precautions, did not show any symptoms or effects on red
blood cell or plasma cholinesterase activity during activities with
the aerial application of methomyl.
1.8 Effects on non-target organisms in the laboratory and field
Methomyl showed no effects on soil fungal or bacterial
populations, nitrification or dehydrogenase activity when applied at
recommended rates.
An NOEC for algal growth of 6.5 mg/litre was established for
methomyl in laboratory studies.
Methomyl is moderately to highly toxic to fish, the 96-h LC50
values being in the range of 0.5-2 mg/litre for a variety of species.
In a longer-term (21 days) study the LC50 for fingerling trout was
1.3 mg/litre methomyl when tested as a Lannate 20L (21.5% methomyl)
formulation. In an early- life-stage toxicity study over 28 days with
fathead minnows, the MATC was estimated to be > 57 and < 117 µg/litre.
In acute toxicity tests with other aquatic organisms, Daphnia
magna was one of the most susceptible species to methomyl, the 48-h
LC50 being 0.032 mg/litre. In a 21-day study on the survival,
growth and reproductive capacity of Daphnia magna, the maximum
acceptable toxicant concentration for methomyl was > 1.6 and
< 3.5 µg/litre.
Methomyl is toxic to honey-bees, the reported contact LD50
being 1.29 µg/bee and the oral LD50 0.2 µg/bee.
The acute toxicity of methomyl has been assessed in several bird
species, typical acute oral LD50 values being 10 mg/kg body weight
for pigeons and 34 mg/kg body weight for Japanese quail. It is
relatively less toxic by the dietary route, the 8-day dietary LC50
being 1100 mg/kg methomyl in the diet for bobwhite quail and
2883 mg/kg methomyl in the diet for mallard ducks. In 18-to 20-week
one-generation studies, the NOEC was 150 mg/kg methomyl in the diet in
bobwhite quail and mallard ducks.
No effects were seen on bobwhite quail when they were exposed to
serial spray applications of methomyl at recommended rates. Two
studies on wild bird populations, after methomyl was sprayed over
forest land or hop fields at recommended rates, did not reveal any
apparent changes in bird activity and caused no treatment-related
effect or mortality. Fat deposits of song birds in treated forests
were reduced relative to controls; this was considered to be an
indirect effect through reduction in insect food.
1.9 Evaluation of human health risks and effects on the environment
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The adsorption of methomyl to soil is low to moderate with hardly
any desorption. Aerobic degradation in soil (with a half-life of
around one week) is about twice as fast as anaerobic degradation.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. Absorbed
methomyl concentrations in food crops decline rapidly to about 5%
within one week.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl with LC50 values in the order of 10 to
100 µg/litre.
Fish, both freshwater and estuarine, are less sensitive, the
LC50 values ranging from 0.5 to 7 mg/litre. Given the low
persistence of methomyl and its relatively low acute toxicity to fish,
the risk is expected to be low.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil.
Methomyl is classified as highly toxic to honey-bees with a
topical LD50 of around 0.1 µg/bee.
Acute oral LD50 values for various bird species range between
10 and 40 mg/kg body weight. Dietary LC50 values (5 days) range from
1100 to 3700 mg/kg diet. Methomyl poses an acute risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
1.10 Conclusion
Considering the qualitative and quantitative characteristics of
methomyl toxicity, the Task Group concluded that 0.03 mg/kg body
weight per day will probably not cause adverse effects in humans by
any route of exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
O
"
CH3-C=N-O-C-NH-CH3
'
S - CH3
Used in the syn-isomer form
Molecular formula: C5H10N2O2S
Relative molecular mass: 162.2
ISO common name: methomyl
IUPAC chemical name: S-methyl- N-[(methyl-carbamoyl)oxy]
thio-acetimidate
CAS chemical name: methyl N[[(methyl-amino)carbonyl]
oxy] ethanimidothioate
CAS registry number: 16752-77-5
RTECS number: AK 2975000
Synonyms: metomil, mesomil, OMS 1196
Trade names Flytek (Zoecon), Golden Fly Bait
(manufacturers and (Sorex), Lannate (Du Pont), Methomex
suppliers): (Makhteshim), Methomyl (various),
Nudrin (Shell), Pillarmate (Pillar)
Technical product purity: > 98% w/w
Technical product S-methyl- N-hydroxy-thioacetimidate
impurities: (0.2%), 1,3-dimethylurea (0.4%)
2.2 Physical and chemical properties
The physical properties of methomyl are listed in Table 1.
Table 1. Physical properties of methomyl (Silveira, 1990)
Physical state crystalline solid
Colour white
Odour slight sulfurous
Melting point 77°C
Vapour pressure 0.72 mPa (at 25°C)
Henry's Law constant 2.1 × 10-11 atm-m3/mole
Octanol-water partition coefficient 1.24
(Kow)
Solubility:
water 54.7 g/litre
toluene 30 g/litre
isopropanol 220 g/litre
ethanol 420 g/litre
acetone 720 g/litre
methanol 1000 g/litre
Methomyl is stable at temperatures up to 140°C. It is not
sensitive to impact, but dusts may form explosive mixtures in air.
The autoignition temperature is 265°C. Methomyl is stable to
sunlight; it does not decompose when exposed for 120 days. It is
stable in sterile buffered water at 25°C (at pH 5 or 7 no breakdown
occurred within 30 days), but it is increasingly decomposed with
increasing pH and temperature. The half-life in water at pH 9 is 30
days. Methomyl at concentrations of 10 or 100 mg/litre in water is
decomposed by artificial sunlight with half-lives of 5.5 and 2 days,
respectively. Methomyl itself is not corrosive but aqueous solutions
may be mildly corrosive to iron (Silveira, 1990). Irradiation of
methomyl in aqueous solution at 254 nm for 10 h gave rise to
acetonitrile (40%), dimethyl disulfide (30%), acetone (15%) and
N-ethylideneme-thylamine (5%); the rest was unidentified products
(Freeman & Ndip, 1984).
2.3 Conversion factors
1 ppm = 6.62 mg/m3
1 mg/m3 = 0.151 ppm
2.4 Analytical methods
Analytical methods for the detection and determination of
methomyl in a variety of substrates are shown in Table 2. In general,
methomyl is extracted from the sample followed by clean-up and HPLC or
GLC analysis. In some cases the methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
2.4.1 Sample preparation
Solid samples are extracted with organic solvents followed by
solvent partition and then, usually, a column clean-up. Water samples
are mainly submitted directly to solid phase extraction.
2.4.2 Analytical determination
The cleaned-up samples are submitted to either HPLC or GLC
analysis, in some cases after conversion to the oxime derivative.
HPLC analysis is coupled with UV detection, sometimes after conversion
to a fluorescent derivative. GLC detection is provided by FID, FSD,
ECD or microcoulometric detectors. A GC-mass spectrometric detection
method has been described (Brodsky, 1991).
Table 2. Methods for the determination of methomyl
Sample type Sample preparation Analytical method Limit of Reference
extraction/clean-up detection
Technical methomyl Reverse phase HPLC 254 nm UV detector not applicable Du Pont (1982)
and formulations
Plant, animal or Extract (ethyl acetate), add water, GLC with 0.02 mg/kg Pease & Kirkland
soil residues evaporate, acidify, extract & discard S-microcoulometer detector (25 g sample, (1968); Leitch &
(hexane), extract (chloroform), concentrate, or flame photometric 93% recovery) Pease (1973)
derivatize by alkaline hydrolysis detector
Crop residues extract (acetonitrile), partition (hexane), HPLC, UV detector at 0.02 mg/kg Clark & Kennedy
Florisil clean-up 233 nm (10 g sample, (1990)
98% recovery)
Non-fatty matrix extract (methanol), 3-step solvent partition HPLC, post column < 0.05 mg/kg Labare (1990)
residues Celite/charcoal column clean-up, derivatization, fluorescent (150 g sample,
concentrate, filter detector at 254 nm 89% recovery)
Vegetables Homogenized (20 g sample) with HPLC/UV µg/kg range Ivie (1980)
methylene chloride. Clean-up 10 ml of the (µ Baudpac C18 column)
extract by passing through SEP-PAK silica
cartridge. Wash with 2 ml CH2Cl2. Elute
with CH2Cl2:CH3OH (1:1 v/v). Evaporate
eluate to dryness. Redissolve in 1 ml of
CH3CN:H2O(1:1 v/v)
Table 2 cont'd).
Sample type Sample preparation Analytical method Limit of Reference
extraction/clean-up detection
Body fluids derivatize by alkaline hydrolysis, extract GC/chemical ionization 0.01 mg/kg Miyazaki et al.
(ethyl acetate), concentrate, convert to mass spectroscopy (2 g sample, (1989)
trimethysilyl ether derivative 95% recovery)
Soil samples extracted with ethyl acetate; HPLC, UV detector at 0.020 mg/kg Kennedy (1989)
filtered; evaporated to 5 ml; silica gel 233 nm (5 ml sample,
clean-up used when cleaner extract 94-102%
needed for HPLC recovery)
Groundwater extract (solid phase adsorbent), elute HPLC, UV detector < 0.1 µg/litre Batelle (1991)
(acetonitrile), concentrate (1 litre sample,
53-62%
recovery)
Well water Filter, automated sample injection, HPLC, flurometric detector at 1 µg/litre Hill et al. (1984)
post-column alkaline hydrolysis and 230 nm excitation and (0.5 ml sample,
conversion to a fluorophore 418 nm emission cut-off 95% recovery)
filter
Drinking-water as above as above 0.7 µg/litre Foerst & Moye
(0.4 ml sample, (1985)
90% recovery)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Methomyl does not occur naturally in the environment.
3.2 Anthropogenic sources
3.2.1 Production processes and levels
Methomyl is produced by reacting S-methyl- N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. The unreacted MHTA is recovered and the
remaining reaction product is subjected to solvent exchange into water
followed by crystallization and centrifugation. The ensuing wet cake
is dried to give technical methomyl (Council of the European
Communities, 1991).
The worldwide production has been estimated to be less than 7000
tonnes (SRI, 1988).
There are no data available on possible releases to the
environment from production processes and transportation.
3.2.2 Uses
Methomyl was introduced as an insecticide in 1966. It is used
for the control of a large variety of insects on a wide range of crops
throughout the world. It is particularly active on many lepidopterous
insects. It acts by direct contact and following ingestion, through
the stomach. Treated crops include fruit, vines, hops, vegetables,
grain, soya beans, cotton and ornamentals. Indoor uses include the
control of flies in animal houses and dairies.
A global estimate of the amount of methomyl used annually for the
above purposes is not available. However, the annual amount used in
the USA was estimated to be approximately 1300 tonnes in 1987 and
1992. The major crops treated in that country are sweet corn, apples,
lettuce, soya beans, peanuts, tomatoes, cotton, corn, alfalfa, and
grapes, accounting for nearly 80% of the total amount used (US EPA,
1988; Gianessi & Puffer, 1992).
The main formulated products are water-soluble powders (25-90%
methomyl) and water-miscible liquids (12.5-29% methomyl). These
products are diluted with water and applied by ground or aerial spray
equipment. Typical methomyl concentrations in the spray solutions are
200-500 mg/litre. Typical active ingredient rates are 0.15-1.0 kg/ha
although higher rates, up to 2 kg/ha, may be used for some purposes.
Repeat applications, as directed on the label, may be required to
maintain control of insect infestations. Examples of crops treated
and methomyl use rates for the USA and Australia are given by FAO/WHO
(1990a,b). Methomyl formulations are compatible in use with many
other insecticides and fungicides, and combined formulations are
registered and available for use in many countries. Methomyl is often
used with one or more other products in a tank-mix. Possible
potentiation by other cholinesterase inhibitors should be considered
when assessing the safety of use of the tank-mix formulations.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Water
The transference of methomyl from greenhouse plants to soil and
to water was assessed on chrysanthemums, grown three times per annum,
with methomyl applied over a 6-month period at 1-week to 2-month
intervals at a rate of 1.4 kg/ha. The three soils investigated were
frequently irrigated and the drainage water was collected at a depth
of 0.8 m. The adsorption isotherms for the three soils indicated that
the adsorption of methomyl was weak to moderate. The concentration of
methomyl in the drainage water was undetectable (< 0.1 µg/litre) in
60% of the samples and was below 1 µg/litre in the remainder (Leistra
et al., 1984).
Methomyl was applied at a high rate of 2.2 kg/ha to a loamy sand
soil on a farm site with a 10% slope. No methomyl was detected
(< 0.01 mg/litre) at the base of the site after a total of about
80 mm of natural and artificial rain had fallen on the site over a
period of 15 days (Harvey & Pease, 1973).
14C-Methomyl was applied at a very high rate of 5 kg/ha to
cylinders (38 cm deep) containing a fine sand soil or a loamy sand
soil. No radioactivity was found in the eluate collected after the
columns had been subjected to heavy rain (Harvey & Pease, 1973).
In natural waters, pesticides can be photochemically degraded by
direct (the pesticide absorbs sunlight) and indirect (other chemicals
in the water absorb sunlight, and transfer the energy to the
pesticide) mechanisms. Current regulatory studies only address the
direct mechanism, and thus neglect an important degradative process.
This can result in unrealistically high estimates of persistence of a
pesticide in surface water.
These data would suggest that any methomyl residues present in
agricultural waters would be rapidly degraded, and that methomyl would
not be expected to have an impact on non-target aquatic organisms.
4.1.2 Soil
Batch equilibrium studies were carried out with 14C-methomyl on
two sandy loams and two silt loams. Aqueous solutions containing
0.2-6 mg methomyl/litre of were mixed with the soils and shaken for
24 h. A separate study was also undertaken on each soil using TLC.
Methomyl was shown to be weakly adsorbed on one of the silt loams
(Ka=1.4), and poorly adsorbed on the other three soils. Desorption
was related to soil organic matter content and indicated that methomyl
is not readily desorbed. In the TLC assessment the Rf values
(0.46-0.82) indicated methomyl as Class 4 (mobile) on a sandy loam and
Class 3 (intermediate mobility) on the other three soils (Priester,
1984).
A silty clay loam, a silt loam and two sandy loams were made up
as slurries and spread on TLC plates. Methomyl and a minor soil
metabolite, S-methyl- N-hydroxy-thioacetimidate (MHTA) were applied
at 1 µg/spot and the plates developed. Methomyl and its metabolite
were considered to be very mobile, with Rf values of 0.64-0.79 and
0.86-0.93, respectively, in the four soils (US EPA, 1988).
In a field study on a sandy loam in California, methomyl was
applied to cabbage by back-pack sprayers at a very high rate of
10 kg/ha (single application). Samples of the soil were taken at 0
and 6 h and then at intervals from 3 to 272 days after application.
Analysis showed that methomyl residues remained mostly in the top
15 cm of soil with deepest penetration into the 15-30 cm layer after
48 cm rainfall/irrigation. On this basis the mobility of methomyl was
considered to be low to moderate under field conditions in this soil
(Kennedy, 1989).
In another field study on a soil characterized as a loam and silt
loam, methomyl was applied to cabbage by back-pack sprayers at the
high rate of 4.4 kg/ha (single application). Soil samples were taken
at 0 and 6 h and then at intervals over a 91-day period after
application. Methomyl residues were found only in the top 15 cm of
soil after 28 cm rainfall/irrigation. On this basis methomyl was
considered to be of low mobility in these field conditions (Kennedy,
1991).
Cox et al. (1993) studied methomyl adsorption to 14 soils from
southern Spain. These varied in pH from 5.3 to 7.9, in percentage of
organic matter from 0.59 to 2.5%, in cation exchange capacity from 4.2
to 28.5, and in the percentage of clay minerals. The methomyl
concentration in soil and water components following shaking for 24 h
was measured by HPLC. Simple and multiple regression analysis was
used to evaluate which factors affected methomyl adsorption. Soils
were equilibrated with both 20 and 50 µM methomyl (high purity); there
was no significant difference between concentration at the same
soil/solution ratio, indicating poor affinity of methomyl for the
soils. Both simple and multiple regression indicated soil organic
matter, clay content and clay minerals, methomyl in soil and illite
content as the major features of soil affecting adsorption of
methomyl. Further studies examined adsorption to individual soil
components. Humic acid showed by far the highest affinity for
methomyl with Kd values 50 times greater than for clay minerals.
Montmonillonites showed a similar Kd to illite in these studies,
contrary to findings with whole soils. The authors suggest, on the
basis of maturation studies, that adsorption to clay may occur in the
interlamellar space of the minerals.
4.1.3 Vegetation
When 14C-methomyl was applied to the surface of tobacco plant
leaves the compound was absorbed in the leaf but not translocated to
other parts of the plant (Harvey & Reiser, 1973).
In a study by Fung et al. (1978), each tobacco seedling received
250 ml of water containing 500 µg methomyl/litre (equivalent to about
0.5 kg active ingredient per hectare of 17 000 plants) after
transplantation. The concentration of methomyl peaked at 15 mg/kg in
the leaves and at 2.5 mg/kg in the growing tips 2 weeks after
treatment. Subsequently, the concentration decreased, which could be
explained almost entirely by growth dilution. At 9 weeks after
transplantation, the plants were sprayed with a solution of 500 mg
methomyl/litre. Three weeks after this (i.e. 12 weeks after
transplantation), another similar application was made. Some plants
received an additional application of 250 ml of a 500 mg
methomyl/litre solution on each side of the row of plants.
Concentrations increased sharply after these treatments and dropped
afterwards: levels in the leaves of plants with both foliar
application peaked at 9 and 6 mg/kg; levels in leaves which received
foliar and root applications peaked at 18 and 11 mg/kg. Part of the
decrease after application could again be explained by growth
dilution. It appears, therefore, that translocation of methomyl from
roots to leaves can occur (Fung et al., 1978).
A sandy loam soil was treated with 14C-methomyl at a rate of
4.4 kg/ha and, 30-120 days later, cabbage, red beet and sunflower
seeds were sown and the plants grown to maturity. Thirty days after
treatment the soil contained 26% of the original methomyl whereas
after 120 days it contained only 8%. All crops, sown at 30 or 120
days, contained only very small residues of methomyl and/or
metabolites, equivalent to 0.01 mg/kg or less at harvest (Harvey,
1978).
4.2 Transformation
4.2.1 Biodegradation
In a study by Harvey (1972a), 14C-methomyl (1 mg/litre) in
river water (pH 6.3) was exposed for 8 weeks from JulySeptember in the
USA. The compound degraded with a half-life of about one week. The
initial degradation product was MHTA followed by breakdown to carbon
dioxide, which accounted for 65% of the original radioactivity after 8
weeks. The S-oxide of MHTA was also detected in small amounts. At
termination, 9% of the original activity was present in sediment and
the biological film on the walls of the container.
Laboratory studies were carried out on a non-sterile silt loam at
its natural pH of 4.7 and at an adjusted pH of 7.9; an alkaline soil
(pH 7.9) was also evaluated. All soils were treated with
14C-methomyl at a high rate equivalent to 4.4-6.1 kg/ha and the
breakdown was assessed over 42 days. Methomyl degraded (52-69%) in 42
days, carbon dioxide (31-45%) being the main decomposition product.
Small amounts of MHTA (1-2%) were present in the soil at termination.
It was shown that the 14CO2 could be reincorporated into soil
organic matter (Harvey & Pease, 1973) (J. Harvey, Jnr (1976):
supplement to "Decomposition of methomyl in soil"; personal
communication by Du Pont to IPCS, dated 28 July 1976).
Under field conditions the decomposition of methomyl was more
rapid, with a 71% loss from a silt loam soil within one month; none
was detected after one year. MHTA was present in trace amounts at 1
and 3 months but was not present at one year. Most of the residual
application was found in the top 75 mm of soil, and none was found
below 200 mm. Decomposition was rather more rapid in fine sand and
loamy sand soils (Harvey & Pease, 1973).
When applied at a concentration of 4.1 mg/kg to a microbially
active loam soil (equivalent to a very high rate of 9 kg/ha),
14C-methomyl was metabolized with a half-life of approximately
11 days. The decomposition followed first-order kinetics and the main
end product was 14CO2 (Zwick & Malik, 1990a). These results were
in agreement with the studies described above and with other aerobic
soil metabolism studies conducted on soils of high or low organic
matter content and various pHs (Harvey, 1972b; 1977a,b).
Dissipation studies of methomyl in loam soils in California and
Mississippi resulted in half-lives of 8 weeks and 5 days, respectively
(Kennedy, 1989; 1991). In addition to differences in temperature,
field moisture differences during the experiment seem largely
responsible for these differences in half-life, because adjusting
field moisture of the California soil to 75% of its capacity in the
laboratory reduced the half-life to 11 days (Kennedy, 1991). Field
moisture conditions greatly decrease the air content of the soil. In
anaerobic soils it has been shown that ferrous ions facilitate the
rapid degradation of methomyl (Bromilow et al., 1986).
Methomyl is also degraded under anaerobic soil conditions. An
alkaline soil with low organic matter content was incubated with
methomyl (4.1 mg/kg) aerobically for 14 days and then anaerobically
for 60 days. The half-life under anaerobic conditions was
approximately 14 days and 14CO2 was a major break-down product,
equivalent to 23.4% of the applied activity during the 60 days of
anaerobic incubation. Unextractable activity was 30% of the total at
7 days and 24% after 60 days of anaerobic treatment. Most of this was
associated with soil organic matter (Zwick & Malik, 1990b).
Anaerobic degradation (Eh 80-310 mV) was studied in samples of
sand, loamy sand and fine sand, taken from below the soil water table
at four locations in the Netherlands (Smelt et al., 1983). In each
case, methomyl was incubated at 10°C and when pH was between 7.4 and
7.7 the half-life was less than 0.2 day (one hour after the start of
the experiment, 38-63% of the applied dose was recovered, and after
24 h, 0.15-5% of the applied dose was recovered. When the fine sand
sample was incubated at 10°C and pH 5.0, methomyl could be detected
for 3 days, and the rate of decrease corresponded to a half-life of
7 h.
The role of microbial action was shown by perfusing two soil
samples (fine sandy loam at pH 6.1 and fine sandy clay loam at pH 5.87
with organic matter content in both of 2.1-2.3%) with methomyl
solution (6 mg/litre) with and without sodium azide (Fung & Uren,
1977). The contribution of adsorption or dissipation of methomyl from
solutions was small when compared with that of microbial
transformation. The latter amounted to 25-45% in 42 days after a lag
phase of 7-14 days. When previously perfused soil was re-exposed to
fresh methomyl solution, 60-75% was lost in 42 days without any lag
phase.
The metabolic fate of methomyl has been investigated in tobacco,
corn and cabbage (Harvey & Reiser, 1973). Tobacco was grown from
seedlings, and when the plants were 18 cm high the roots were treated
with 14C-methomyl (10 mg/litre solution). Cabbage (42 days old) and
corn (28 cm high) plants were treated with 14C-methomyl via foliar
application. Each plant was placed in a glass metabolism apparatus
for radioactivity measurement of volatile products and plant material.
Tobacco absorbed 20-25% of the available activity over a 4-week
period. One quarter of this was retained in plant tissue and the
remainder volatilized. The principal component of plant tissue
activity was methomyl. The volatile components were carbon dioxide
and acetonitrile in equal proportions. Of the applied activity, 47%
was lost from the growing shoots of young corn as volatile components
within 10 days. This was composed of CO2 and acetonitrile in the
ratio of 1:4. One week after treatment of cabbage leaves, 20% of the
activity was lost as CO2 and acetonitrile in equal proportions. The
extracts of the treated plants were investigated for the presence of
three possible metabolites, MHTA and the S-oxide and S,S-dioxide
derivatives of methomyl. There was no evidence for the presence of
these compounds. The only terminal residue specifically detected was
methomyl. The remainder of the radioactivity was incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars.
The biodegradation of methomyl was also studied in corn and
cabbage under field conditions after the application of the
radiolabelled compound. The outer leaves of cabbage contained most of
the radioactive residue of which a small proportion (3-4%) was
identified as methomyl. In corn, the outer portions contained most of
the radioactive residue with about 2 mg methomyl/kg being present
(Harvey & Reiser, 1973).
The half-life of methomyl was determined in cotton leaves sampled
during periods without rainfall after a single application at
0.75 kg/ha, the maximum label rate. The foliar half-life was
estimated to be between 0.6 and 2.2 days, with an average of 1.1 days
(Eble & Tomic, 1991). Bull (1974) applied radiolabelled
14C-methomyl to leaves of tobacco in aqueous solution. Almost half
of the applied methomyl penetrated the leaves within the first few
hours. Surface residues were largely lost within 48 h and the parent
compound was the only radioactive component of the unabsorbed dose.
The absorbed methomyl was degraded within 8 days (mostly within 48 h).
No S-oxide, S,S-dioxide or oxime derivatives were found in the plants,
the methomyl being degraded to acetonitrile and CO2. After methomyl
was applied directly to tobacco leaves, its half-life was 3-7 days
(Harvey & Reiser, 1973). Studies describing the decline of
dislodgeable foliar residues on various crops are reviewed in
section 5.3.
4.2.2 Abiotic degradation
When a 3% solution of methomyl in distilled water was stored for
168 days, 90% of the compound was recovered at the end of the period.
The remainder was recovered as MHTA (Harvey, 1967).
The hydrolysis of methomyl was studied at pH 5, 7 and 9, at
concentrations of 10 and 100 mg/litre, and at 25°C. The compound was
stable for 30 days at pH 5 and 7 but broke down at pH 9 with a
half-life of about 30 days. The hydrolysis product was MHTA
(Friedman, 1983).
The photolysis of methomyl was studied at initial concentrations
of 10 and 100 mg/litre and at pH 5 under UV light. Methomyl
photolysed rapidly at both concentrations with a half-life of 2-3 days
at 100 mg/litre. The principal photo-product was acetonitrile
(Harvey, 1983).
In a study by Swanson (1986), 14C-methomyl was applied to a
thin layer of a silt loam soil on glass plates and exposed to natural
sunlight for 30 days at 24-28°C. The compound decomposed with an
estimated half-life of 34 days. The principal decomposition product
was acetonitrile. Duplicate preparations, kept in the dark, did not
decompose.
Methomyl degraded rapidly in slightly alkaline solution (pH 8.85)
with a chlorine/methomyl ratio of 10. The degradation rate increased
with increasing temperature, increasing chlorine concentration, and
decreasing pH. The reaction rate with free chlorine was 1000-fold
faster than with chloramine. Methomyl degraded to acetic acid,
methanesulfonic acid and dichloromethylamine after forming methomyl
sulfoxide and N-chloromethomyl (Miles & Oshiro, 1990). Mason et al.
(1990) also reported that the removal of methomyl can be effectively
achieved by some disinfectants (Cl2, O3) but not by ClO2.
4.2.3 Bioaccumulation
Rainbow trout were exposed to 0.075 and 0.75 mg methomyl per
litre in a flow-through test system for up to 28 and 21 days,
respectively, and then placed in clean water (pH 7.3, total hardness
25 mg CaCO3/litre at 18°C). At the higher concentration, fish
tissue contained 0.36-0.45 mg methomyl/kg during the exposure period
and, at the lower concentration, 0.04-0.07 mg/kg. Within one day of
depuration the methomyl tissue levels fell to below 0.02 mg/kg in both
exposure groups. There was therefore no indication of bioaccumulation
of methomyl in these studies (Sleight, 1971).
4.3 Interaction with other physical, chemical or biological factors
When 14C-methomyl was incubated with a rumen microorganism
culture at a level of 1 mg/kg and at 38°C for 24 h, 90% was
metabolized to a volatile component which was identified as
acetonitrile by gas chromatography. Less than 0.1% of the total
activity was recovered as methomyl or MHTA (Belasco, 1972b).
No nitrosomethomyl was detected (< 1 µg/kg) when 14Cmethomyl
(1 mg/kg) was incubated under simulated stomach conditions (pH 2) with
sodium nitrite (16-20 mg/kg) in a macerate of cured meat for 1 or 3 h
at 37°C (Han, 1975).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In 60% of drain water samples from greenhouses containing treated
plants in an area south of The Hague, The Netherlands, methomyl could
not be detected (< 0.1 µg/litre). In the remaining 40% samples its
concentration was < 1 µg/litre (Leistra et al., 1984).
Out of 22 404 wells sampled and analysed for methomyl in the US
EPA pesticide monitoring programme, 85 showed detectable methomyl
concentrations within the range of trace to 20 µg/litre, i.e. within
the lifetime health advisory figure of 200 µg/litre (US EPA, 1991).
After a cedar swamp had been sprayed with Lannate LV formulation
(29% a.i.) at a rate of 0.28 kg methomyl/ha, no methomyl could be
detected (< 0.02 mg/litre) in surface water at any time between 1 and
58 days after treatment (Du Pont, 1978).
5.1.2 Soil
Soil samples taken from a tobacco farm in Maryland, USA, after a
single application of methomyl (Lannate 90% a.i.; 13.4 kg/ha)
contained 0.6 mg methomyl/kg at 0-15 cm depth after 8 days. At a
depth of 15-30 cm, 0.1 mg/kg was detected after 17 days. No methomyl
(< 0.02 mg/kg) was detected after 47 days at either level. Lannate
is applied as an incorporation to a depth of 10 cm (Pease, 1968).
Levels of methomyl in soil after an upland forest and a cedar
swamp had been sprayed with Lannate LV at a rate of 0.28 kg
methomyl/ha were either very low (< 0.07 mg/kg) or undetectable
(< 0.02 mg/kg). No residues were detected (< 0.02 mg/kg) at an
application of half the above rate (Du Pont, 1978).
5.1.3 Food crops
Methomyl is used as a broad spectrum insecticide on many food
crops, hence low residues may be present at harvesting. The amount of
residue at harvest depends upon factors such as the application rate,
time interval between the last application and harvest, and the type
of crop. The residue is composed mainly of methomyl itself. The
residue levels expected in food crops at harvest can be derived from
the numerous supervised trials which have been carried out on many
crops in countries worldwide (FAO/WHO, 1988a,b; 1990a,b). Pre-harvest
intervals are also set on the basis of the results of supervised
trials, e.g. 7 days for lettuce and onions in USA, 1 day for brassicas
and tomatoes in Australia (FAO/WHO, 1990a,b).
The decline of methomyl residues on food crops after application
was demonstrated by the field treatment of broccoli followed by
harvesting at intervals thereafter. The results of treatment at
0.55 kg/ha (6 applications) are shown in Table 3 (Du Pont, 1973).
Table 3. The decline of surface and absorbed methomyl residues in
broccoli (Du Pont, 1973)
Days after treatment Residue (mg/kg)
1 1.0
3 0.15
7 0.04
10 0.02
Similar examples of the decline of methomyl residues have been
shown for lettuce and cauliflower (Braun et al., 1980), celery (Braun
et al., 1982), wheat (Du Pont, 1973; FAO/WHO, 1988a,b) and tomatoes
(FAO/WHO, 1990a,b).
Treated food crops processed after harvesting generally do not
show a concentration of methomyl residues in the processed fractions.
Unwashed whole oranges were found to contain 0.96 mg/kg at harvest and
0.88 mg/kg after washing. The dried peel contained 2.8 mg/kg whereas
the pulp, juice, cold process oil and molasses contained no detectable
residue (< 0.02 mg/kg). Similarly, tomatoes analysed after harvest
contained 0.38 mg/kg while the processed fractions, wet pomace, dry
pomace, juice and puree contained < 0.02 mg/kg (Kennedy & Hay, 1991a;
Marxmiller & Hay, 1991). Mint oil produced from the distillation of
methomyl-treated plants and wine produced from treated grapes
contained no detectable residues (< 0.04 and < 0.02 mg/kg,
respectively) of methomyl (Kiigemagi et al., 1973; Brodsky, 1991).
5.1.4 Other crops
Environmental levels of methomyl on treated crops such as cotton
and tobacco may be deduced from supervised trials.
After 14 applications of methomyl at 0.5 kg/ha and two at the
high rate of 1.65 kg/ha, cotton contained 0.17 mg methomyl/kg 15 days
after the last application. Processed fractions showed < 0.02 mg/kg
in oil, 0.065 mg/kg in meal and 0.14 mg/kg in hulls (Kennedy & Hay,
1991b).
The residue levels in tobacco leaves immediately after the
application of methomyl at the recommended rate of 0.56 kg/ha were 44
and 88 mg/kg at two sites in the USA. After five days these levels
had dropped to 0.7 and 1.4 mg/kg. Approximately 96% of the methomyl
was lost during flue-curing (Leidy et al., 1977).
5.1.5 Dairy products
There are no reports of methomyl residues in dairy produce.
Groups of lactating cows (three/group) were dosed methomyl by
capsule at a rate equivalent to 8, 24 or 80 mg/kg in their feed for
28 days. Milk collected during the dosing period and tissues taken at
termination contained no detectable residues of methomyl or its
metabolite MHTA. Acetonitrile was detected in the milk of cows dosed
with methomyl at 8 mg/kg, the milk concentration of acetonitrile
reaching a plateau of 0.04-0.1 mg/kg by day 4. This component was
also present in liver (0.08 mg/kg) and kidney and muscle (0.04 mg/kg)
at this dose level. Acetamide concentrations in milk and tissue of
cows dosed with 80 mg/kg were the same as those found in control
animals. Radiolabel assessment showed that the acetamide derived from
methomyl was < 1% of the total dose (Powley, 1991). In another study,
methomyl was fed to cows at 2 or 20 mg/kg in feed and no methomyl
(< 0.02 mg/kg) was detected in milk over a 30-day period nor in meat
tissue at termination (Du Pont, 1967).
The 14C-residue in milk was equivalent to 0.13 mg/kg 4 days
after two goats had been fed 14C-methomyl at 8 mg/kg diet. Methomyl
itself could not be detected. Total 14C-residues in a range of
tissues were very low (< 0.001-0.003 mg/kg) (Osman et al., 1983).
5.1.6 Animal feed
Some indication of methomyl residues expected in those portions
of treated crops used for animal feed can be deduced from the
following studies. Methomyl concentrations were 0.35 mg/kg in forage,
0.14 mg/kg in cannery waste and < 0.02 mg/kg in kernels from sweet
corn treated with methomyl at 0.9 kg/ha and harvested 9 days after the
last of four applications (Harvey & Yates, 1967). Methomyl residues
on sweet corn forage harvested immediately after the last of nine
foliar applications at 0.5 or 1 kg/ha were 0.15-0.60 mg/kg and
0.2-0.72 mg/kg, respectively (US EPA, 1988). The residues on samples
of wheat straw taken 7 days after foliar application of 0.55 kg
methomyl/ha were < 0.02-6.5 mg/kg, and after 14-18 days they were
< 0.02-0.8 mg/kg (US EPA, 1988).
5.2 General population exposure
Information available on general population exposure is limited
and derives primarily from only one country. More complete exposure
information via various routes specific to regions and countries is
required to assess the risk of occupational exposure and intake of
residues.
5.2.1 Food
The market baskets collected for the US FDA total diet study
prior to 1991 consisted of 234 food items. Of these, a total of 72
items (69 adult foods, 2 baby foods and water) were analysed by
methodology known to be capable of determining methomyl. Methomyl was
detected only in 11 food items collected from 1987 to April 1991 (20
market basket studies): watermelon, pear, strawberries, grapes,
cantaloupe, raisins, lettuce, celery, cauliflower, cucumber, and green
sweet peppers. The levels detected in these food samples ranged from
trace to 0.630 mg/kg, well below the tolerances established by US EPA
(US FDA, 1993a).
The total number of domestic and imported food samples analysed
by methodology known to be capable of determining methomyl in US FDA
regulatory monitoring programmes during the period 1988-1992 was 7765.
Of these, methomyl residues were detected only in 112 samples. Four
samples were found to be violative: one domestic sample of strawberry
(4.53 mg/kg) and one imported cantaloupe (0.28 mg/kg) exceeded the
USA tolerances and there were two imported samples for which no USA
tolerances have been established (okra, 0.05 mg/kg and pepino,
0.46 mg/kg) (US FDA, 1993b).
Residues in foodstuffs are reduced by domestic processing such as
washing, peeling and cooking. For example, 50-90% of methomyl
residues on celery was removed by trimming (FAO/WHO, 1986). Methomyl
residues declined by 70-93% in tomatoes, peas or cabbage after 30 min
cooking in boiling water in open containers (Holt, 1971). Methomyl
was added to spinach puree to give a concentration of 50 mg/kg and
then processed in closed cans for 40 min at 122°C. No methomyl was
detected (< 0.05 mg/kg) at the end of this period (Niven, 1971).
Methomyl has not been detected in wine or mint oil prepared from
crops previously treated with methomyl (see section 5.1.3).
No methomyl could be detected (< 0.02 mg/kg) in eggs or tissues
of laying hens given 1 or 10 mg methomyl/kg diet for 4 weeks (Sherman,
1972).
5.3 Occupational exposure
A series of studies was carried out to determine worker re-entry
times after the application of methomyl to grape vines in California,
USA (Dong et al., 1992; Reeve et al., 1992). These studies were
specific to the desert conditions found in California and should not
be compared to studies on other crops or in other climates. Methomyl
was applied at different times of the growing season at 1 kg/ha and
the dislodgeable foliar residues were measured at time intervals after
application to estimate how long it would take to reach the desired
level of 0.1 µg/cm2 on the leaves. Under desert conditions with
water supplied only by furrow or drip irrigation, it was found that it
required about 5 days to reach this level in June, when grape girdling
was carried out, and about 10 days in September, at harvesting
(Powley, 1989, 1990a,b).
A worker re-entry study was undertaken to estimate exposure after
entry into vineyards when dislodgeable foliar residues had fallen to
0.1 µg methomyl/cm2 or less. Each worker wore ankle length tights
(except raisin grape harvesters) and long sleeved T-shirt, both worn
under normal work clothes. Each wore a personal air sampling pump and
two patches were attached to work hats. Sample patches were worn on
the thigh and ankle on the normal work clothes by most workers during
girdling operations. Work continued for 3-4 h. Methomyl exposure
when girdling field grapes ranged from 315-1214 µg/h with highest
values on the upper body and head. Exposure was highest to the upper
body and hands of raisin harvesters where overall daily exposure was
463-865 µg/h. Harvesting and packing table grapes resulted in the
lowest methomyl exposures of 219 and 102 µg/h, respectively.
Inhalation exposure was minimal (Merricks, 1990).
It should be emphasized that the rate of decay of methomyl in the
Californian studies described above is not representative of the
situation in grape culture in other areas of the world or for other
crops, as, due to irrigation and other cultural practices, Californian
grape vines are quite large and have lush foliage which maximizes
exposure. Methomyl does not hydrolyse readily in the hot dry
desert-like conditions and this gives rise to atypical transfer rates.
Dislodgeable foliar residue from cotton plants was the subject of
three studies in Arizona, USA (Cahill et al., 1975; Ware et al, 1978,
1980). In each case, methomyl was applied at 0.55 kg/ha and leaf
samples were taken for analysis of dislodgeable residues up to 96 h
after. In each study the methomyl residues had declined to 0.1 µg/cm2
or less within 48 h.
Dislodgeable foliar residues were determined after spraying mint
to estimate possible exposure to workers moving irrigation pipes.
After spraying methomyl at l kg/ha from the air, dislodgeable residues
were 1.5 µg/cm2 at 4 h and 0.32 µg/cm2 at 48 h, and, after
applying 2 kg/ha, residues were 2.3 µg/cm2 at 4 h and 0.6 µg/cm2 at
48 h (Kiigemagi & Deinzer, 1979).
A pilot study was undertaken in Thailand with a methomyl 90%
soluble powder formulation to assess the use of food dyes as markers
for pesticide exposure. Pesticide operators prepared and sprayed the
diluted methomyl formulation containing the dyes on low (broccoli,
chinese kale), medium (tobacco) or tall (citrus) crops with knapsack
or high pressure power sprayers. Measurement of dye content of the
outer garments showed that exposure occurred mainly to the lower body
and legs when spraying low crops and mainly to the upper body and arms
when spraying tall crops. Some correlation was shown between the
amounts of methomyl and dye deposited on the outer garments. However,
the number of participants (two per group) was too small to draw
definite conclusions, and more work needs to be done to establish
these correlations (Ambridge, 1992).
Methomyl was not detected in air samples from the working zones
of operators during normal closed transfer, mixing-loading operations.
During application, methomyl air concentrations of up to 7.5 µg/m3
were found in applicator working zones (Knaak et al., 1980).
In order to establish a post-application re-entry interval for
workers employed in greenhouse operations, methomyl dislodgeable
foliar residue data were collected from rose foliage. It was shown
that after a single high rate of application of 3.2 kg/ha it took
nearly 5 days for the dislodgeable residue to decline to the required
level of 0.1 µg/cm2. It was estimated that for the application rate
of 1 kg/ha, the highest normally used for rose treatment, a re-entry
interval of 48 h would be required (Oswald et al., 1991).
The concentration of methomyl in greenhouse air was measured
directly by an atmospheric pressure chemical ionization mass
spectrometer system. The atmosphere was monitored during spraying of
roses and for 26 h thereafter. Samples taken at head height during
spraying showed methomyl levels of about 33.1-39.7 µg/m3 (5-6 ppb).
A few hours later, at the end of the day's operations, concentrations
were about 19.9-26.5 µg/m3 (3-4 ppb). When monitoring resumed the
following morning the air concentrations were still at about the same
level indicating that methomyl, deposited in aerosol droplets on the
roses, had not fully evaporated (Williams et al., 1982).
Ambient air and breathing zone samples were analysed in four
greenhouses 1 day before and 7 days after methomyl was sprayed on
cucumber and tomato plants. Ambient air methomyl concentrations
ranged up to 4.7 µg/m3 on the first day after spraying. Three and
seven days after spraying, breathing zone methomyl concentrations
ranged up to 14.5 and 0.7 µg/m3, respectively. Hand-wash methomyl
values ranged from 10 to 322 µg/h of work in a greenhouse. The
authors considered that dermal exposure, as indicated by the hand-wash
data, was a more important factor than air exposure and that re-entry
intervals should be set according to information derived from the
former (Boleij et al., 1991).
Ambient air in a pesticide storage building was monitored over a
3-h period using high volume air samplers and absorption on XAD-4 or
XE-340 resins. Methomyl was stored in the building as a liquid
concentrate along with other pesticide formulations. The average
methomyl air concentration over the sampling period was 13.7 ng/m3.
This represents a value of 0.18 µg/m3 when converted to a 40-h
working week and can be compared with the ACGIH TLV of 2500 µg/m3
(Yeboah & Kilgore, 1984).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
The term 14C-methomyl in this section refers to
S-methyl-[1-14C]-N-[(methylcarbamoyl)oxy] thioacetimidate, unless
otherwise stated.
6.1 Absorption
The absorption of 14C-methomyl was very rapid after oral dosing
of 5 mg/kg to male or female rats. About 80% of the activity was
excreted within 24 h as metabolic products in urine and expired air.
Only 2-3% was found in faeces (Harvey et al., 1973; Hawkins et al.,
1991).
A similar pattern was seen in the cynomolgus monkey following a
single 5 mg/kg oral dose. More than 60% of the dose was eliminated in
expired air and urine within 24 h as metabolic products. Only about
3% of the dose was found in faeces over a period of 168 h after dosing
(Hawkins et al., 1992).
In an assessment of dermal penetration, 14C-methomyl was
applied in acetone to a 1 cm2 shaved area of skin of mice (7-8 weeks
old) at a rate of 1 mg/kg. The mice were then placed in metabolism
chambers and killed for radioactivity measurements at intervals up to
48 h. Within 5 min, 14C activity was detected in blood and liver.
In 60 min, 2.9% of the original 14C dose was present in blood, 5%
was in the liver, and 12.9% had been excreted (urine plus CO2 plus
faeces). Very little methomyl remained at the application site after
60 min, when penetration was estimated to be approximately 85%. The
half-life, as a measure of penetration rate, was approximately 13 min
(Shah et al, 1981).
6.2 Distribution
After 5 mg 14C-methomyl/kg had been dosed orally to five male
and five female rats, 8-9% of the initial activity was found in the
tissues and carcass 7 days later. The highest concentration of
activity was found in blood (2 mg/kg equivalents) with a distribution
of 3-4 mg/kg in red cells and 0.7-0.9 mg/kg in plasma. The
radioactivity concentrations were lower in other tissues (< 1 mg per
kg). As a proportion of the original dose, blood contained
approximately 2%, liver 0.4%, gastrointestinal tract 0.6% and other
individual tissues < 0.1% each. There was no significant difference
in distribution between males and females (Hawkins et al., 1991).
14C activity was distributed among a range of tissues after two
rats had been fed 200 mg methomyl/kg diet for 8 days and then given
5 mg 14C-methomyl/kg orally. Of the 14C dose, 9% was found in the
tissues and carcass within one day and 10% within 3 days (Harvey et
al., 1973).
When cynomolgus monkeys were given a single oral dose of 5 mg
14C-methomyl/kg, approximately 5% of the radioactivity was retained
in the tissues after 168 h. The highest concentrations of activity
were in the liver (0.7-0.9 mg/kg equivalents), fat (0.4-0.7 mg/kg
equivalents) and kidney (0.4-0.5 mg/kg equivalents). Lower
concentrations found in other tissues were generally higher than blood
levels of 0.1-0.2 mg/kg equivalents (Hawkins et al., 1992).
One hour after the dermal applications of 14C-methomyl to mice
(see section 6.1), 2.9% of the dose was present in blood, 5% in liver
and 56% in the remaining carcass. After 8 h the distribution was 6.1%
in blood, 3.3% in liver, 3.8% in the gastro-intestinal tract and
smaller amounts (< 1%) in other individual tissues. The remaining
carcass contained 15% of the original dose (Shah et al., 1981).
6.3 Metabolic transformation
In an initial investigation, two male rats were fed a diet
containing 200 mg methomyl/kg for 8 days, followed by intra-gastric
intubation of 1.2 mg 14C-methomyl (=5 mg/kg). One male rat was
treated similarly except that the 14C-methomyl was given after 19
days. Urinary and volatile metabolite identification was carried out
1 or 3 days after the 14C dose. Volatile products, trapped in
caustic soda solution or in cold traps, were identified as carbon
dioxide and acetonitrile, the latter confirmed by mass spectroscopy.
Countercurrent distribution of the urine showed that nearly all the
radioactivity was present as polar material. Methomyl, its
S,S-dioxide and MHTA were not detected. The methomyl S-oxide could
not be detected by TLC (Harvey et al., 1973).
A more detailed study (Hawkins et al., 1991), with five male and
five female rats receiving single oral doses of 14C-methomyl
(5 mg/kg), confirmed that the expired metabolites (over 120 h) were
carbon dioxide (22%) and acetonitrile (12%). The radioactive
components of the 0-24 h urine were separated by reverse phase HPLC,
ion partition chromatography and TLC. The major metabolite in urine
was identified by NMR and mass spectroscopy as the mercapturic acid
derivative of methomyl, equivalent to about 17% of the 14C-dose.
There were 10 minor components which included, on tentative
identification, acetonitrile, acetate and methomyl oxime sulfate.
Methomyl, MHTA and the anti-isomer form of methomyl were not detected.
Metabolic pathways for methomyl in the rat include the
displacement of the S-methyl moiety by glutathione and enzymic
transformation to give the mercapturic acid derivative. Another
pathway involves hydrolysis to give MHTA which is rapidly
broken down to carbon dioxide (Fig. 1).
Another proposed pathway involves the conversion of the
syn-isomer of methomyl (the insecticide form) to its anti-isomer. The
latter has been shown to produce acetonitrile as the main volatile
metabolite when given orally to rats (see section 6.4). It is
proposed that the anti-isomer hydrolyses to the anti-MHTA,
which then undergoes a Beckmann re-arrangement and elimination
reaction to form acetonitrile (Huhtanen & Dorough, 1976).
It is also likely that certain metabolic products such as
acetonitrile undergo further reactions, with the carbon components
being incorporated into natural body constituents such as fatty acids,
neutral lipids and glycerol, as shown in ruminants (see section
4.2.1).
It is probable that two of the main metabolic pathways also
operate in the monkey. When an oral dose of 14C-methomyl
(5 mg/kg body weight) was given to cynomolgus monkeys, the
major metabolites were CO2 (32-38%) and acetonitrile (4-7%) in the
expired air. These were derived, presumably, by the same processes as
described for the rat above. A combination of HPLC and TLC
characterized 18 radioactive metabolites in urine, with no metabolite
representing more than 4% of the dose. Small amounts of acetonitrile,
acetate, acetamide and MHTA sulfate were among the products found.
The mercapturic acid derivative of methomyl (a major rat urinary
metabolite) accounted for about 1% of the dose. The presence of these
minor metabolites are presumably the result of extensive metabolism of
primary metabolites (Hawkins et al., 1992).
A lactating cow was dosed twice daily by capsule for 28 days
with 14C-methomyl at a rate equivalent to 8 mg/kg in feed. Milk
samples were collected each day and selected tissues were taken within
24 h of the last dose. Radioactivity appeared in milk within one day
and reached a plateau of 0.5 mg/kg (equivalents) within 6 days. This
activity was mostly due to the reincorporation of the radiolabel into
fatty acids, lactose and other acetate derived products. No methomyl
or MHTA was detected; acetonitrile accounted for about 25% of the
radioactivity. The liver showed the greatest concentration of
radioactivity, equivalent to 9.23 mg/kg; kidney contained only
2.01 mg/kg and there were lower concentrations in fat and muscle. No
methomyl was detected in tissue; most of the radioactivity was
considered to be the result of reincorporation of the radiolabel as
acetate into natural constituents (Monson & Ryan, 1991).
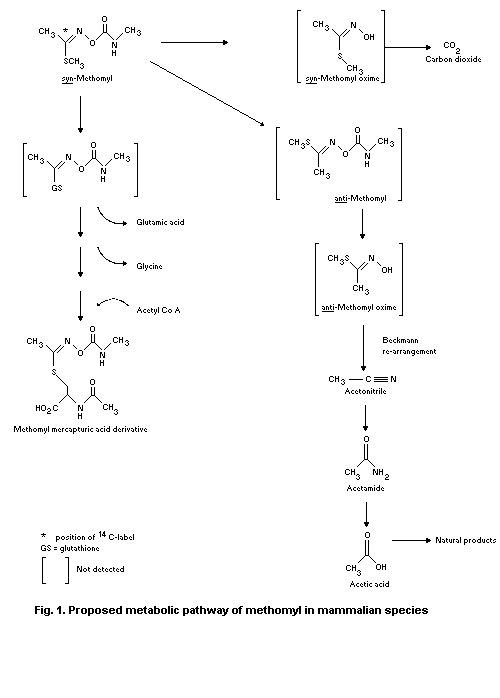 A lactating goat was given 14C-methomyl by capsule, twice a
day, for 10 days at a dose rate equivalent to 20 mg/kg in feed. Milk,
blood, urine and faeces were sampled daily and selected tissues taken
within one day of the last dose. No methomyl or the metabolite MHTA
was detected in any of the samples. Approximately 16% and 7% of the
activity was excreted in urine and faeces, respectively, and about 8%
appeared in the milk and 17% in expired air. The milk activity
reached a plateau after 3 days and was equivalent to approximately
2 mg/kg. At this time the lactose component contained about 11-13% of
the milk activity. Hexane extracts, containing the triglyceride
components, contained 26-37% of the milk activity and the casein
component 8-9%. This indicates that methomyl had been completely
broken down and incorporated into natural constituents of milk.
Acetonitrile was identified as a volatile metabolite in milk and blood
(Harvey, 1980).
The examination of the liver fractions showed that the
radio-activity derived from methomyl was found in glycerol,
glycerol-3-phosphate, fatty acids, neutral lipids and insoluble
protein. This indicates a metabolic pathway via acetonitrile and
acetate into the natural occurring constituents in the liver. The
breakdown of methomyl and distribution of metabolic products in the
liver was shown to be similar in the cow (Monson, 1989).
Acetonitrile, CO2 and reincorporation products derived from
acetate found after the application of methomyl to plants (section
4.2.1) are similar to those identified in the above animal studies.
The proposed metabolic pathway for methomyl in animals is shown
in Fig. 1.
6.4 Elimination and excretion
Rat and monkey studies show that methomyl is very rapidly
metabolized and eliminated, the processes being largely completed
within 24 h.
Rats fed 200 mg methomyl/kg in diet and then given 5 mg
14C-methomyl/kg orally (see section 6.3 for detail) showed a 50%
elimination of 14C in expired air in 3 days in the form of carbon
dioxide and acetonitrile in the proportion of 1:2. Urinary excretion
of 14C was 27% in one day (Harvey et al, 1973).
In a more detailed study, where male and female rats were given
5 mg 14C-methomyl/kg orally (see section 6.3), approximately 53% of
the radioactivity was excreted in urine over 7 days, 45% of the dose
being excreted in the first 6 h. Faecal excretion contributed only
2-3% over 7 days. The other major path of elimination (over 5 days)
was via expired air as carbon dioxide (22% of dose) and acetonitrile
(12% of dose). Of this, 18% of the dose was expired as CO2 within
6 h and 10% as acetonitrile in 24 h. Overall, most of the
radiolabelled dose (80%) had been eliminated in 24 h with an estimated
half-life of 5 h. There was no obvious difference in the amount or
rate of excretion between males and females. The single oral dose
given to these animals (5 mg/kg) produced mild clinical signs of
cholinesterase inhibition which disappeared within 2 h of dosing
(Hawkins et al., 1991).
When methomyl was radiolabelled on the carbonyl group, the
elimination of 14CO2 was very rapid and equivalent to about 85% of
the oral dose in male and female rats. When the labelling was at the
- 14C=N group, the overall elimination in expired air in 24 h was
30% in the form of CO2 and acetonitrile (in the proportion of 2:1).
When 14C-MHTA was administered in the same way the expired component
was mainly 14CO2, equivalent to 22% of the dose. The anti-isomer
of methomyl mainly produced acetonitrile in the expired air,
equivalent to 28% of the dose given orally to rats. Rats given the
anti-MHTA by intraperitoneal injection produced ten times more
acetonitrile than those given the syn-MHTA. The urine from rats
treated orally with 14C-methomyl or MHTA contained 25-35% of the
radioactivity over a 24-h period (Huhtanen & Dorough, 1976).
In monkeys given 5 mg 14C-methomyl/kg orally, approximately 32%
of the dose was excreted in urine in 7 days, with 34% as CO2 and 5%
as acetonitrile in the expired air. Most of this was excreted in the
first 24 h. Faecal excretion amounted to only 3-4% (Hawkins et al.,
1992).
After dermal application of 14C-methomyl to mice (see section
6.1), the total excretion (urine plus CO2 plus faeces), as a
proportion of the applied dose, was 0.2% in 15 min, 12.9% in 60 min
and 54.5% in 480 min (Shah et al., 1981).
6.5 Retention and turnover
The absorption, metabolism and excretion of methomyl in the rat
are very rapid. No methomyl can be detected within the tissues or
excretory products within a few hours of dosing. Most of the dose is
eliminated within 24 h with an estimated half-life of 5 h (Hawkins et
al., 1991). Metabolic products, mainly in urine and expired air, are
also eliminated rapidly; tissue concentrations are very small and
lower than blood levels. There is no evidence for accumulation in
tissues. A similar picture exists for the metabolism of methomyl in
ruminant species.
6.6 Reaction with body components
Methomyl is a potent direct inhibitor of acetylcholinesterase in
both insects and mammals. The carbamylated enzymes undergo rapid
spontaneous reactivation (see section 7.8.1).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The results of the single exposure of technical methomyl by the
oral, dermal and inhalation routes in various species are shown in
Table 4.
Methomyl is very toxic by the oral route. In the rat, the signs
of toxicity are those expected from a cholinesterase inhibitor and
include profuse salivation, lacrimation, tremor, abnormal posture,
pupil constriction, diarrhoea and prostration. At lethal doses the
rats died within hours. Survivors began to recover within several
hours and had fully recovered within days. No compound-related
changes were seen in organs subjected to histopathological
examination.
The acute dermal toxicity of methomyl is very low. No deaths
occurred in rats or rabbits at the doses shown in Table 4. The
compound has high toxicity by the inhalation route in the rat, with
affected animals showing typical signs of cholinesterase inhibition.
No target organ effect was seen upon histopathological examination.
An LD50 of approximately 8 mg/kg was obtained when methomyl was
injected intraperitoneally in rats (Dashiell, 1972).
The acute oral toxicity of methomyl formulations is in proportion
to the amount of a.i. present and, therefore, they are less toxic than
methomyl itself (Table 5). The same pattern is seen with the results
of acute inhalation studies. The signs of toxicity in the oral and
inhalation studies are those shown by the active ingredient. The
dermal toxicity of the formulations is very low.
Ocular toxicity was shown by a solid formulation containing 92.4%
methomyl when 10 mg was introduced into rabbits' eyes. Typical
anticholinesterase effects were seen up to 20 min after treatment,
including pupillary constriction of the treated eyes, incoordination,
tremors and profuse salivation. All of the effects had disappeared by
the next day (Sarver, 1991g).
7.2 Short-term exposure
Six male rats were given methomyl in peanut oil by gavage at a
dose of 5.1 mg/kg body weight per day, 5 times per week for 2 weeks.
The signs of toxicity were the same as those exhibited in acute oral
studies (section 7.1) but they regressed during the second week of
dosing. All animals survived, and there were no compound-related
histopathological changes (Kaplan & Sherman, 1977).
Table 4. Acute toxicity of technical grade methomyl
Species Sex Route Vehicle Testa Result Reference
Rat M oral peanut oil LD50 17 mg/kg Sherman (1966)
Rat F oral peanut oil LD50 23.5 mg/kg Sherman (1968a)
Rat M oral water LD50 45 mg/kg Trivits (1979)
Rat M oral water LD50 34 mg/kg Sarver (1991a)
Rat F oral water LD50 30 mg/kg Sarver (1991a)
Rat M dermal water LD50 abraded skin > 1000 mg/kg Morrow (1972)
Rat M inhalation aerosol 4-h ALC 0.30 mg/litre Foster (1966a)
Rat M inhalation vapour 4-h ALC 0.04 mg/litre Foster (1966b)
Rat M inhalation spray 4-h LC50 0.45 mg/litre Hornberger
(1967)
Rat M/F inhalation aerosol 4-h LC50 0.258 mg/litre Panepinto
(1991a)
Rabbit M oral acetone/ ALD 30 mg/kg Sherman (1968c)
peanut oil
Table 4. (cont'd).
Species Sex Route Vehicle Testa Result Reference
Rabbit M/F dermal water LD50 intact skin > 2000 mg/kg Sarver (1991b)
Dog M oral capsule ALD 20 mg/kg Sherman (1968d)
Guinea-pig M oral acetone/ ALD 15 mg/kg Kaplan &
peanut oil Sherman (1977)
Monkey M/F oral water ALD 40 mg/kg Kaplan &
Sherman (1977)
a ALC = approximate lethal concentration; ALD = approximate lethal dose
Table 5. Acute toxicity of some methomyl formulations
Formulation Species Route Vehicle LD50 (mg/kg) or LC50 Reference
(% a.i.) (mg/litre)a
Lannate 40 SP rat oral water 61 (male) Sarver (1992a)
(41.6%) 73 (female)
Lannate 20 L rat oral methanol 129 Lheritier (1991a)
(21.5%)
Lannate 12.5 L rat oral water 208 Sarver (1991c)
(12.7%)
Lannate 40 SP rabbit dermal (intact skin) water > 2000 Sarver (1992b)
(41.6%)
Lannate 20 L rat dermal (intact skin) methanol > 4000 Lheritier (1991b)
(21.5%)
Lannate 40 SP rat inhalation (4 h) aerosol 0.66 Kelly (1992)
Lannate 20 L rat inhalation (4 h) aerosol 1.3 Jackson et al. (1991)
(21.5%)
Lannate 12.5 L rat inhalation (4 h) aerosol 3.7 Panepinto (1991b)
(12.7%)
a Results are for both sexes, unless stated otherwise
Groups of 10 male and 10 female rats were fed diets containing 0,
10, 50, 125/500 (125 mg/kg for 6 weeks, 500 mg/kg for remaining
period) or 250 mg methomyl/kg for 13 weeks. Assessments included
haematological, biochemical and histopathological examinations. There
were no mortalities nor clinical signs due to treatment in any group.
A slight decrease in body weight was seen in males at the two higher
dose levels and in females at 125/500 mg/kg. This was accompanied by
lower food consumption. The only change attributed to methomyl on
histopathological examination was moderate erythroid hyperplasia in
the bone marrow of males fed 250 mg/kg. Lower haemoglobin values in
the males of this group at two months could be linked to this effect.
Histopathological examination of animals in the 125/500 mg/kg group
did not reveal any changes due to treatment. The noobserved-effect
level based upon body weight change was considered to be 50 mg/kg
diet, equivalent to 3.56 mg methomyl/kg body weight per day (Paynter,
1966; Busey, 1966).
When groups of four male and four female one-year-old dogs were
fed diets containing 0, 50, 100 or 400 mg methomyl/kg for 3 months, no
effects were seen in a range of clinical, haematological, biochemical
and histopathological assessments (Kaplan & Sherman, 1977).
A dose of 200 mg methomyl/kg body weight was applied as a 5%
aqueous solution once a day 5 times per week for 3 weeks to the intact
or abraded skin of five male, five female rabbits. Each dose was
occluded for 6 h and then the site washed with water. There were no
mortalities in animals with intact skin; clinical signs included nasal
discharge, wheezing and diarrhoea. With abraded skin, signs of
toxicity, including laboured respiration, salivation and tremors,
appeared within one hour of dosing, and three animals died, one after
the first application and the other after the eighth application. No
treatment-related changes were seen upon histopathological examination
(Kaplan & Sherman, 1977).
Groups of rabbits were treated dermally (intact clipped skin)
with doses of 0 (10 males, 10 females), 5, 50 (5 males 5 females) or
500 (10 males, 10 females) mg methomyl/kg body weight per day for 21
days. The doses were applied in water each day and occluded for 6 h.
Half the males and females in the control and highest dose groups were
killed at the end of the dosing regime, together with all animals in
the other groups. The remaining animals in the control and highest
dose groups were allowed to recover over a 14-day period and then
killed. The only change noted clinically was a greater incidence of
hyperactivity at 500 mg/kg. There were no effects on body weight
gain, haematology, blood biochemistry, organ weights or organ
histopathology. Plasma cholinesterase activity was depressed to 36%
and 55% of normal at day 21 in highest dose males and females,
respectively. Brain cholinesterase activity was depressed to 48% and
68%, respectively, in males and females at this dose, but red cell
cholinesterase activity, although slightly decreased, was considered
to be biologically insignificantly affected. In males, at 50 mg/kg,
the plasma cholinesterase activity was depressed to 77% and there was
no inhibition of red blood cell cholinesterase activity of either sex.
All the depressed cholinesterase activities had returned to control
values at the end of the 14-day recovery period. The
no-observed-effect level, based upon changes in plasma cholinesterase
activity, was 5 mg/kg body weight per day in males and 50 mg/kg in
female rabbits (Brock, 1989).
Formulations of methomyl have also been tested by repeated dermal
application to rabbits at doses equivalent to 50 or 100 mg
methomyl/kg. No effects were seen after ten daily doses of Lannate L
(25.5% methomyl). Ten daily doses of Lannate LV (30% methomyl)
produced mild skin irritation at the higher dose level. Plasma
cholinesterase activity was somewhat depressed at both dose levels but
returned to normal over a 14-day recovery period (McAlack, 1973,
Edwards, 1980).
A Lannate dust containing 45% methomyl was the subject of a
3-month inhalation study in male rats. Exposure was at 14.8 mg/m3
for 4 h/day, 5 days/week; the mass median dynamic diameter of the dust
was 4.4 µm. There were no effects on body weight, organ weight,
histopathology or red cell cholinesterase activity. Plasma
cholinesterase activity was depressed to about 29% of the
pre-treatment activity in samples taken 4 h after the last exposure
(Ta'Naka et al., 1987).
7.3 Long-term exposure
Groups of 35 male and 35 female rats were fed diets containing
methomyl at 0 (2 groups), 50, 100, 200 or 400 mg/kg for 22 months.
Five males and 5 females per group were killed at 12 months for gross
and histopathological examination. Assessments were made throughout
the study for clinical condition, body weight, food consumption,
haematology, blood and urine biochemistry, organ weight and
histopathology. Growth of males at the highest dose was significantly
lower than that of controls over the first year. Growth of males at
200 mg/kg and females at 400 mg/kg was also lower at this time, but
not significantly. Food consumption over the first 26 weeks was
significantly lower than that of controls for the males fed 200 and
400 mg/kg. At 18 and 22 months there was a trend towards lower
haemoglobin values in treated female groups. Compound-related
increases in the incidence and severity of extra medullary
haematopoiesis were observed in the spleen of females fed 200 or
400 mg/kg. Compound-related changes were also seen in the kidneys of
both sexes at 400 mg/kg, characterized by vacuolization of epithelial
cells and hypertrophy of the proximal convoluted tubules. On the
basis of the body weight and haematopoietic findings in this study,
the long-term no-observed-effect level for methomyl in rats was
considered to be 100 mg/kg diet (Kaplan & Sherman, 1977).
In a 2-year study on rats, methomyl was fed in the diet to groups
of 80 males and 80 females at levels of 0, 50, 100 or 400 mg/kg. The
animals were observed and weighed regularly, and haematological and
blood and urine biochemical measurements were made at 3, 6, 12, 18 and
24 months. Red cell cholinesterase activity was assayed in additional
groups at 1, 2 and 4 weeks and 3, 6, 12 and 20 months. Brain
cholinesterase activity was assayed at 12 and 20 months. Extensive
histopathological examination was performed on animals killed at
scheduled intervals, on animals dying and on animals killed at the end
of the study.
There was a significant reduction in body weight gain in both
sexes at 400 mg/kg over the first 12 months but not during the
subsequent 12 months. There were no clinical signs attributable to
treatment. Survival rate was the same among the groups and there was
an acceptable number of animals at termination (40-50%). Erythrocyte
counts, haemoglobin values and haematocrits were significantly lower
in females at 400 mg/kg. There were no compound-related changes for
red cell or brain cholinesterase activity nor for other biochemical
parameters. Organ weights were unaffected by treatment. The incidence
of bone marrow hyperplasia, focal hyperplasia in the adrenal medulla
and focal degeneration in the adrenal cortex was slightly increased in
males at 400 mg/kg. The no-observed-effect level in the rat was
there-fore confirmed as 100 mg methomyl/kg diet, equivalent to about
5 mg/kg body weight per day (Kaplan, 1981).
A comprehensive long-term (2 years) study on the mouse was
initiated with 80 males and 80 females per group at levels of 0, 50,
100 and 800 mg methomyl/kg diet. Because of early high mortality, the
level of the highest dose group was reduced to 400 mg/kg at week 28
and then to 200 mg/kg at week 39. The level of the mid-dose group was
also reduced to 75 mg/kg at week 39. Regular clinical observations
and body weight and food consumption measurements were undertaken.
Haematological analysis was carried out at 4, 13, 26, 52, 78 and 104
weeks. Organ weights were measured and an extensive histopathological
examination carried out on all animals dying or killed. The mortality
rate was higher than that of controls at the highest and medium dose
levels throughout the study, and was slightly higher, in male mice
only, at the low dose level in the latter stage of the study. There
were no clinical findings due to treatment and no effects on body
weight gain or food consumption. Some effects were noted on red cell
parameters such as haemoglobin values and red cell counts during the
first 26 weeks at the medium and highest dose levels. However, these
were not apparent during the remainder of the study, after these dose
levels had been decreased. No effects were observed on organ weights
or microscopic findings. The no-observed-effect level was considered
to be 50 mg/kg in diet, equivalent to approximately 10 mg/kg body
weight per day (Serota et al, 1981).
A 2-year dog study was initiated with dietary levels of methomyl
of 0, 50, 100, 400 and 1000 mg/kg with four males and four females per
group. After one year, one male and one female from each group was
killed for interim examination. Clinical condition was regularly
observed and routine measurements made on body weight, food
consumption, and haematological and bio-chemical parameters. After 2
years the surviving animals were killed, organs were weighed and a
histopathological examination was undertaken.
No clinical signs due to treatment were seen in animals at 50,
100 and 400 mg/kg. One highest dose female died after 8 weeks and its
replacement also died within 18 days after showing compound-related
toxic signs. Two males at this dose level exhibited tremors,
salivation and incoordination during week 13. Slight to moderate
anaemia was noted in the highest dose animals at 13 weeks and this
persisted in one of the males. No effects were seen on body weight or
biochemical parameters. Histopathological effects were noted in the
kidneys and spleens of animals at 400 and 1000 mg/kg. Changes in
kidneys were characterized by increased pigmentation and epithelial
swelling, and in the spleen as extra-medullary haematopoiesis and
increased pigmentation. A minimal to slight increase in bile duct
proliferation was seen in livers of dogs at 1000 mg/kg, and bone
marrow activity was also slightly increased in animals at this dose
level. The no-observed-effect level was therefore 100 mg/kg diet,
equivalent to about 3 mg methomyl/kg body weight per day (Kaplan &
Sherman, 1977).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
The skin irritation potential of methomyl was evaluated in six
male rabbits with 0.5 g of the test material, moistened with deionized
water, applied to the back of each animal. Each site was covered with
a semi-occlusive dressing and exposure continued for 4 h. The skin
was examined and evaluated 1, 24, 48 and 72 h after the end of the
exposure period. No dermal irritation was seen in any of the animals
during the study and, therefore, methomyl is classified as
non-irritant to skin (Sarver, 1991d).
Methomyl formulations were also evaluated for skin irritation
potential using the same test method as described above. The
substances tested were a solid formulation containing 92.4% methomyl
(Sarver, 1991e) and two liquid formulations containing approximately
20% and 12.7% methomyl (Clement, 1987a; Sarver, 1991f). None of the
formulations irritated rabbit skin.
In a test on 10 guinea-pigs, methomyl was applied as a 60% paste
in propylene glycol or as a 26% solution in Cellosolve and the 24-h
reaction was noted as being mildly irritating (Kaplan & Sherman,
1977).
7.4.2 Eye irritation
Methomyl, either as 10 mg dry powder or 0.1 ml of a 10% solution
in propylene glycol, was tested in rabbits' eyes (2 per group). One
of each pair was washed after 20 sec and examination of each eye made
at 1, 2, 3, 4 and 6 h after treatment and for up to 6 days thereafter.
Only mild conjunctivitis was seen on the day of treatment but no
corneal injury was noted (Kaplan & Sherman, 1977).
A solid formulation containing 92.4% methomyl was tested in the
eyes of six male rabbits. A quantity of 10 mg was introduced into one
eye of each rabbit and evaluations of the reaction were undertaken
after 1, 24, 48 and 72 h. Pupillary constriction was evident after
one hour but was not present the next day (see also section 7.1).
Only very mild conjunctival chemosis and redness was seen at 24 h
after treatment and the formulation was considered to be non-irritant
(Sarver, 1991g).
Two liquid formulations, containing approximately 20% and 12.7%
methomyl, respectively, were tested for eye irritancy using the method
described in the previous paragraph. In both cases, the formulation
produced ocular irritation in the form of conjunctival redness and
chemosis, iritis and corneal opacity within one hour and were
classified as irritant. These effects had disappeared after 21 days.
(Clement, 1987b; Sarver, 1991h).
7.4.3 Skin sensitization
In a test to evaluate the skin sensitization potential of
methomyl, the material was applied as a paste to the abraded skin of
five guinea-pigs 3 times a week for 3 weeks; five other guinea-pigs
received four intradermal injections of 0.1 ml of a 1% solution. The
animals were then challenged after a 2-week rest period. Methomyl was
shown not to be a skin sensitizer in this test (Kaplan & Sherman,
1977).
A closed-patch repeated insult dermal sensitization evaluation
(Beuhler test) was undertaken in guinea-pigs with technical methomyl.
The test compound (300 mg), moistened with water, was applied to the
intact skin of each of 10 males and 10 females for 6 h once a week for
3 weeks. Two weeks after the last induction treatment, the animals
were challenged with another 300 mg methomyl for 6 h. No erythema was
observed in either the induction or challenge phases, and therefore
methomyl did not produce delayed contact sensitivity in the
guinea-pig. Positive control animals treated with DNCB produced the
expected sensitization responses (Armondi, 1991a).
A solid formulation containing 94.2% methomyl, subjected to the
evaluation described in the previous paragraph, also failed to produce
delayed contact hypersensitivity in guinea-pigs (Armondi, 1991b). The
same result was obtained with a liquid formulation containing 21.5%
methomyl when given at 0.5 ml per application (Mercier, 1991).
Another liquid formulation containing 12.7% methomyl was tested in the
Magnusson-Kligman guinea-pig sensitization maximization procedure and
was found to be a moderate skin sensitizer under the conditions of the
test (Armondi, 1992).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Embryotoxicity and teratogenicity
Teratogenicity studies have been undertaken in the rat and
rabbit.
Pregnant rats (25 per group) were fed 0, 50, 100 or 400 mg
methomyl/kg diet during days 6-15 of gestation. The dams were
observed and weighed regularly and then killed on day 21 of gestation.
At that time the uterus was removed, and the number of corpora lutea,
implantation sites, live and dead fetuses, resorptions and weight of
live fetuses were recorded. About one half of the fetuses from each
litter were taken for skeletal examination and the remainder for soft
tissue examination. The pregnant rats at 400 mg/kg had significantly
lower body weight and ate less food than those in the control group.
There were no clinical signs of toxicity and all rats survived the
test period. Parameters of pregnancy and fetal development, such as
the number of litters with partial or total resorptions and fetal
weight, were not affected in the test groups compared to controls.
There were no treatment-related abnormalities in the fetuses.
Methomyl was therefore not embryotoxic or teratogenic in the rat at
levels up to 400 mg/kg diet, equivalent to 34 mg/kg body weight per
day (Rogers et al., 1978).
Groups of 20 pregnant rabbits were given 0, 2, 6 or 16 mg
methomyl/kg body weight per day by gavage on days 7-19 of gestation.
The animals were observed and weighed regularly and killed on day 29.
The uteruses were then examined for pregnancy, number of
implantations, early and late resorptions and live or dead fetuses.
Delivered pups were weighed and examined for visceral or skeletal
abnormality. One pregnant rabbit at 6 mg/kg died, probably due to
gavage error. At 16 mg/kg there were seven deaths during the test
period due to the compound. Clinical signs at the highest dose level
included tremors, hyperactivity, excessive salivation, aggression and
convulsions. The no-observed-effect level for maternal toxicity was
therefore 6 mg/kg body weight per day. There was no effect on
pregnancy rate or on the average numbers of corpora lutea,
implantations, resorptions or live fetuses per dam in treated groups
compared to controls. There were no gross external visceral or
skeletal variations due to treatment. Methomyl is therefore not
embryotoxic or teratogenic to the rabbit at doses up to 16 mg/kg, at
which dose maternal toxicity occurred (Christian et al., 1983).
In another study, pregnant rabbits (12 per group) were fed diets
containing up to 100 mg methomyl/kg. The pregnancy rate was low in
all groups, including controls. No effects were noted on a range of
parameters including reproductive indices, fetal body weight or
visceral and skeletal abnormalities. However, due to experimental
inadequacies the results are difficult to assess (FAO/WHO, 1987).
7.5.2 Reproduction studies
A three-generation (two litters per generation) rat study was
undertaken to test the potential reproductive effects of methomyl (Lu,
1983). The compound was fed at levels of 0, 50 or 100 mg/kg diet to 10
males and 20 females for approximately 90 days post weaning prior to
first mating to produce the F1a litter. Test diets were fed through
to subsequent re-mating to produce the F1b litter. Ten males and 20
females per group were then selected from the F1b litter and
continued on their respective diets for approximately 90 days to
produce F2a and then F2b litters. The same procedure was used to
produce subsequent litters of the next generation. Records were kept
of all matings, number of pregnancies, number of young in each litter
born alive or dead, the body weight of offspring at weaning, and the
respective reproduction and lactation indices. Ten males and 10
female offspring were selected from each group of the F3b litter for
histopathological examination. Methomyl did not affect the
reproductive performance of rats in this study. There were no effects
on fertility, gestation or lactation and no compound-related
abnormalities on gross examination. No treatment related changes were
seen upon histopathological examination of tissues from the offspring
in the F3b litter (Kaplan & Sherman, 1977).
A two-generation rat study was also carried out using high
dietary levels of methomyl (600 and 1200 mg/kg) as well as a lower
level of 75 mg/kg. The F0 parents (13 males, 26 females) were mated
after 100 days treatment and the F1 parents (20 males, 40 females)
after a minimum of 120 days. Spermatogenesis (based on sperm count)
was measured on adult males in both generations after breeding, in
addition to the standard assessments of reproductive performance.
Histopathological examination was undertaken on a selection of tissues
from parents and pups in both generations. Body weight depression in
parents, seen at all or some stages during treatment at 600 and
1200 mg/kg in both generations and at 75 mg/kg in the F1 generation,
was probably related to lower food intake. Reduced red blood cell
counts were seen in females at the highest dose level, together with a
small (15-25%) depression in plasma cholinesterase activity in both
sexes at this level. Decreases in litter size and live births were
shown for the highest dose F0 group and for all F1 groups for
which a clear no-observed-effect level was not established. Survival
during lactation in F1 pups (from F0 parents) was reduced at the
highest dose level, as was survival in highest dose F2 pups during
early lactation up to day 4. Pup body weight was slightly reduced at
75 mg/kg, significantly reduced at the two higher levels in the F1
generations and reduced at the 600 and 1200 mg/kg levels in the F2
generation. No histopathological changes due to treatment were seen
in any of the tissues examined in parents or offspring. No effects on
the reproductive parameters of fertility indices, gestation length or
male sperm counts were seen at dose levels up to and including 1200 mg
methomyl/kg in diet (Lu, 1983).
7.6 Mutagenicity
Numerous in vitro mutagenicity assays, to various end-points,
have been carried out on methomyl by several investigators (Table 6).
Methomyl did not show mutagenicity or cause primary DNA damage in
bacterial or mammalian cells in vitro. It showed cytogenetic
potential in human lymphocytes in vitro as indicated by an increase
in micronuclei and chromosomal aberrations.
In an in vivo study, the clastogenic potential of methomyl was
assessed by its ability to cause numerical and structural chromosomal
aberrations in rat bone marrow cells. Single doses of methomyl were
given by gavage to groups of 15 male and 15 female rats at doses of 2,
6 or 20 mg/kg body weight; five males and five females were killed at
6, 24 and 48 h after dosing and the bone marrow cells harvested. These
were examined for chromatid and chromosome breaks and for gaps and
other aberrations. The results showed that there was no significant
increase in the frequency of chromosomal aberrations and no
significant differences between the chromosome numbers and the mitotic
indices of the dosed groups and the controls (Farrow et al, 1984).
Table 6. Summary of results of in vitro mutagenicity assays and related end-point studies on methomyl
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
Prokaryotes
Point mutation Salmonella typhimurium - 50 nM negative Blevins et al. (1977a)
(5 strains)a
Point mutation S. typhimurium (5 strains) + and - up to 5000 µg/plate negative Moriya et al. (1983)
Escherichia coli (WP2hcr) + and - negative
Point mutation S. typhimurium (5 strains) + and - up to 1 mg/plate negative Waters et al. 1982
Point mutation E. coli, WP2uvr A + and - up to 1 mg/plate negative Waters et al. 1982
DNA damage E. coli pol A - 1 mg/disc negative Waters et al. 1982
W3100, p 3478
DNA damage Bacillus subtil recM45 - 1 mg/disc negative Waters et al. 1982
Yeast
DNA damage Saccharomyces cerevisiae + and - 1-3.5% negative Waters et al. 1982
D3
Mammalian cells
DNA damage Human lung fibroblast WI-38 + and - 10-7-10-3M negative Waters et al. 1982
DNA damage Rat hepatocytes (UDS) - 1 µM-75 mM negative Vincent (1985)
Table 6 (cont'd).
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
DNA damage Human skin cell DNA - 10-5M negative Blevins et al. (1977b)
Gene mutation Chinese hamster V79 cells + and - 1-10 mM negative Wojciechowski et al. (1982)
Gene mutation Chinese hamster ovary - 10-55 mM negative McCooey et al. (1984)
(CHO) cells, HGPRT + 100-350 mM
Chromosome Human lymphocytesb NA 0.02-0.54 mM positive Bonatti et al. (1994)
aberrations (whole blood culture)
Sister-chromatid Human lymphocytesb NA 0.02-0.54 mM negative Bonatti et al. (1994)
exchange (whole blood culture)
Micronuclei Human lymphocytesb NA 0.01-0.09 mM positive Bonatti et al. (1994)
(whole blood culture)
DNA single strand Human lymphocytesb NA 0.06-2 mM negative Bonatti et al. (1994)
breaks (whole blood culture)
DNA oxidative Human lymphocytesb NA 0.25 - 1 mM negative Bonatti et al (1994)
damage (whole blood culture)
a Plate incorporation and spot tests
b The metabolic activity of the cell cultures was demonstrated by the positive control response
c NA = not applicable
Methomyl was tested in the sex-linked recessive lethal assay with
Drosophila melanogaster at concentrations of 4 and 10 mg per litre
in the test medium. No increase in mutation rate was observed in this
assay (Waters et al., 1982).
A formulation (Lannate 20) containing 20% methomyl, but of
otherwise unstated composition, caused an increase in the number of
sex-linked recessive lethals, but with no observed translocations.
The same formulation increased the incidence of abnormal sperm and
frequency of chromosomal aberrations in mice (Hemavathy &
Krishnamurthy, 1987a,b).
7.7 Carcinogenicity
The carcinogenic potential of methomyl was assessed in the
long-term rat and mouse studies already described in section 7.3.
In the earliest study, groups of 35 male and 35 female rats were
fed 0 (2 groups), 50, 100, 200 or 400 mg methomyl/kg diet for 22
months. No differences were seen in the incidence of neoplastic
lesions between control and treated groups (Kaplan & Sherman, 1977).
In the more recent rat study, groups of 80 males and 80 females
were fed 0, 50, 100 or 400 mg methomyl/kg diet for 2 years. The
mortality rates were similar in all groups and there was an overall
survival of 40-50% by the end of the study. More than 30 tissues/rat
were examined microscopically from animals killed or dying during the
study and at interim and terminal sacrifice. There was no indication
of any differences between groups in the appearance of any individual
tumour nor were there any differences between groups for the number of
tumour-bearing rats or those with benign or malignant tumours (Kaplan,
1981).
The 2-year mouse study was initiated at dietary levels of 0, 50,
100 and 800 mg/kg with groups of 80 males and 80 females. By week 39
the highest dose level had been reduced to 200 mg/kg and the 100 mg/kg
level to 75 mg/kg (see section 7.3 for details). Mortality was higher
in the highest dose male and female groups than in controls throughout
the study and was also somewhat higher in the mid-dose group.
Adequate numbers of mice survived to the termination of the study
(30-50% for female groups and 38-68% for male groups).
Histopathological examination on more than 30 tissues per mouse was
undertaken on animals killed or dying during the study and at terminal
sacrifice. There were no differences between groups for the numbers
of tumour-bearing mice or those with benign or malignant tumours
(Serota et al., 1981).
Methomyl did not transform hamster embryo cells in a
trans-placental host-mediated assay, nor did the cultured cells from
this assay induce tumours when injected subcutaneously into young
adult nude mice (Quarles et al., 1979).
7.8 Other special studies
7.8.1 Cholinesterase studies in vivo and in vitro
Due attention must be given to the measurement of cholinesterase
activity in blood and brain samples because of the fast spontaneous
reactivation of the methomyl-inhibited enzyme. It is important to
analyse samples in the minimum possible time after sampling and to use
an analytical method which requires the minimum of sample dilution and
shortest assay time. If these criteria are not followed then
cholinesterase inhibition due to methomyl can be misinterpreted.
In groups of rats fed 0, 50, 100, 200, 400 or 800 mg methomyl per
kg diet for 79 days, only those fed the highest dose showed blood
acetylcholinesterase activity inhibition as assayed by the Ellman
method. Males showed blood cholinesterase activity inhibition of
25-40% early in the study (by 11 days) while females showed this
inhibition towards the end of the study period (Singles, 1970a). In
an extension of this study, the blood acetyl-cholinesterase activity
of rats fed 400 or 800 mg/kg was assayed by the Ellman method and a pH
stat method after 5 months of feeding. In neither group was there an
effect on blood acetyl-cholinesterase activity (Singles, 1970b).
Methomyl was fed to groups of rats in the diet at levels of 0,
100, 400 or 800 mg/kg, and blood was taken for red cell and plasma
acetylcholinesterase activity assay at 1, 7, 14, 21 and 28 days.
Brain acetylcholinesterase activity was also measured on rats at 14
and 29 days. All assays were undertaken by the Ellman method. There
were small depressions of acetylcholinesterase activity (up to 20%) in
red cells and plasma of males and females at 800 mg/kg. Brain
acetylcholinesterase activity was also slightly depressed (<17%) at
this dose level. There were no consistent findings of
acetylcholinesterase inhibition at the two lower levels of 100 and
400 mg/kg (Barnes, 1978).
Lannate, containing 90% methomyl, was given to 14 male rats at
41 mg/kg body weight by oral intubation each day for 8 successive
days. The animals were killed 24 h after the last dose and blood was
taken for the assay of serum cholinesterase activity by a colorimetric
method. The activity was depressed by 40% compared to controls
(Borady et al., 1983).
Cholinesterase activity in plasma and red cells of rats was
assayed after the dermal application of methomyl. Four groups of rats
were treated dermally with 200 mg methomyl per rat and blood samples
were collected 2, 4, 6 and 24 h after treatment. Plasma
cholinesterase activity was markedly reduced at each time interval
compared to controls (22-50% of control value) while red cell
cholinesterase activity was less affected (80-91% of control value).
Groups of rats were also treated dermally with 25 or 100 mg per rat
and blood was collected at 24 and 72 h after treatment. Plasma
cholinesterase activity was depressed at both levels and at both time
intervals, but red cell acetylcholinesterase was unaffected (Henry,
1981).
Lannate powder, containing 45% methomyl, was the subject of
inhalation studies using a single 4-h exposure to 9.9 mg/m3 or
repeated exposures to 14.8 mg/m3 (see section 7.2 for details).
Plasma cholinesterase activity was markedly depressed (by 50%) for 4 h
after the single exposure, but showed recovery almost to the control
value within 20 h. Red cell cholinesterase activity was not
inhibited. Plasma cholinesterase activity was less affected (29%
depression) 4 h after the last of the repeat exposures than after the
single exposure (Ta'Naka et al., 1987).
The I50 value (the molar concentration of inhibitor giving 50%
reduction in enzyme activity in given experimental conditions) for
methomyl was determined in vitro for human and rat red blood cell
acetylcholinesterase activity using a modified Ellman method. Human
acetylcholinesterase was approximately six times more sensitive to
methomyl's action than rat acetylcholinesterase (I50 values of
0.265 × 10-5 mol/litre and 1.56 × 10-5 mol/litre, respect-ively).
Regeneration studies using a gel filtration method showed that
methomyl-inhibited rat acetylcholinesterase regenerates at a faster
rate than the human enzyme, with half-lives of 26.6 and 38.0 min,
respectively (Carakostas, 1987).
7.8.2 Neurotoxicity
Hens given a single oral (LD50) dose of 28 mg methomyl/kg
either died within 10 min or survived. Survivors showed toxic signs
of lacrimation and salivation, but no evidence of wing or leg
paralysis. After a 21-day recovery period, no abnormalities were
observed upon histopathological examination of the sciatic nerve.
Doses much higher than the oral LD50 (up to 200 mg/kg) were given to
hens without causing mortality after they had been pre-treated with
atropine sulfate subcutaneously at 10 mg/kg. Wing or leg paralysis
was not observed and there were no histopathological abnormalities in
the sciatic nerve after a 21-day recovery period. Birds treated with
tri- o-cresyl phosphate (TOCP) at 500 mg/kg showed the leg paralysis
and sciatic nerve degeneration expected of a positive control
substance (Krauss & Stula, 1967).
7.8.3 Potentiation studies
Potentiating properties were determined by administering one-half
the oral LD50 of methomyl to rats, followed immediately by one-half
the LD50 of another anticholinesterase pesticide, and then recording
the resultant mortality over 14 days. Out of 18 pesticides tested,
only Sevin and Ronnel showed the ability to increase mortality
indicative of a potentiation effect (Sherman, 1967). In another study,
the inhibition of cholinesterase activity in rat blood after the oral
administration of methomyl alone or in combination with another
anticholinesterase pesticide was used to measure potentiating effects.
Methomyl in combination with methyl parathion or phosdrin was shown to
be potentiating, while antagonism occurred when it was combined with
dimethoate (Henry, 1975).
Rats (10 per sex and per group) were given methomyl at 200 mg/kg
diet and ethanol as a 10% aqueous solution, either separately or
together, for a period of 12 weeks. There was some evidence of
increased effects as a result of the combined administration resulting
in decreased body weights from weeks 2 (females) and 4 (males) and
increased relative organ weights for adrenals (males) and kidneys
(females). In male rats, the combination of the two compounds
increased hepatic triglycerides and free fatty acids and decreased
brain acetylcholinesterase activity more than that expected from
either compound alone (Antal et al., 1979). Ethanol administered in
the diet to rats, at a level equivalent to 25% of the calorie intake,
did not potentiate erythrocyte acetylcholinesterase inhibition by
methomyl coadministration at 200 mg/kg in the diet (Bracy et al.,
1979).
In female rats only, the combination of methomyl and caffeine
retarded growth, increased the relative weight of kidneys, spleen,
adrenals, liver and heart, depressed glucose-6-phosphate dehydrogenase
activity, and increased aniline hydroxylase and glucose-6-phosphatase
activity (Bedö & Cieleszky, 1980).
7.8.4 Antidote studies
The antidotal properties of several agents against the toxicity
of methomyl have been examined in a number of species.
Potential antidotes were administered within one minute after
oral lethal doses of methomyl (30 or 60 mg/kg) were given to rats. Of
the agents used, atropine sulfate given intraperitoneally as a single
dose of 50 mg/kg proved to be the most effective antidote. Other
agents used which were less effective or ineffective were
pyridine-2-aldoxime methiodide (PAM) and tetra-ethylammonium chloride
(TEAC) (Sherman, 1968a). Rats were also used in a study of the
effectiveness of obidoxime or N-methyl-pyridinium-2-aldoxime
methane-sulfonate (P2S) as antidotes alone or in combination with
atropine sulfate. Methomyl was injected subcutaneously as solutions
of logarithmically graded concentrations (1 ml/kg body weight), and at
the onset of signs of toxicity atropine sulfate (17.4 mg/kg), P2S
(50 mg/kg) and obidoxime (90 mg/kg) were injected subcutaneously
either alone or in a combination. Lethality was reduced by atropine
sulfate or P2S but obidoxime was ineffective. Atropine sulfate in
combination with P2S also was effective (Natoff & Reiff, 1973).
Mice fed a lethal dose of 100 mg methomyl/kg in pellets and then
given TEAC by injection 10 min later survived, and resumed normal
activity within another 10 min (Andrews & Miskus, 1968). Atropine
sulfate (50 mg/kg intraperitoneal) was shown to have antidotal
activity in guinea-pigs given methomyl orally at lethal single doses
of 15-60 mg/kg (Sherman, 1968b). Dogs given a single oral dose of 10
or 20 mg methomyl/kg by capsule were protected against the toxic
effects if atropine sulfate was given very quickly, i.e. within a few
minutes. The antidote was more effective when given intravenously
than when given intramuscularly or orally (Sherman, 1968c).
The antidotal effectiveness of atropine administered
intravenously or orally at 1 or 10 mg/kg and hexamethonium or TEAC
given intravenously at 10 mg/kg was evaluated in rhesus monkeys dosed
with 20 or 40 mg methomyl/kg orally. Atropine at 1 mg/kg, given
intravenously or orally, was effective as an antidote for the
sublethal dose of 20 mg methomyl/kg. Atropine at 10 mg/kg orally,
administered immediately after the appearance of toxic signs, was an
effective antidote for the lethal dose of 40 mg methomyl/kg. Recovery
occurred within 2-5 h of the methomyl dose. TEAC at 10 mg/kg
(intravenous) also prevented the death of one monkey after a lethal
dose of methomyl, although recovery took longer than after atropine.
Hexamethonium did not appear to be very effective (Teeters, 1968).
7.8.5 Other studies
Lannate (90% methomyl) was given to 14 male rats at the high dose
level of 41 mg/kg by intubation each day for 8 days and the animals
were killed 24 h after the last dose for hepatic and serum assays.
Liver weight was not increased, nor was there histopathological
evidence of fatty deposit, but total lipids including cholesterol and
phospholipids were increased. The treatment also increased the level
of serum triglycerides, phospholipids and free fatty acids and
increased the activity of GOT, GPT and alkaline phosphatase enzymes
(Borady et al., 1983). Lung triglyceride, cholesterol and
phospholipid contents were not affected when rats were exposed to
Lannate (45% methomyl) dust at 14.8 mg/m3 by inhalation for 4 h/day,
5 days/week for 3 months (Ta'Naka et al, 1987).
Single oral doses of 2 or 10 mg methomyl/kg increased pancreatic
chymotrypsin, lipase and amylase activities in male and female rats.
Rats fed 100, 400 or 800 mg/kg diet for 10 days showed elevated serum
cholesterol in females only. Hepatic aminopyrine demethylase and
aniline hydroxylase activities were increased in female rats at 400
and 800 mg/kg. When rats were given 100 or 200 mg/kg diet, females
fed 200 mg/kg showed elevation of total serum lipids and cholesterol.
The females also showed decreased hepatic glucose-6-phosphatase
activity and vitamin A at this level (Bedö & Cieleszky, 1980).
The effect of methomyl on a number of serum constituents was
studied in rats given oral doses of 6.8 mg/kg per day, 6 days/week for
4 weeks. Increases were seen over the 4-week period for serum
glucose, cholesterol, GOT and GPT, while decreases were seen for serum
total protein albumin, globulin and cholinesterase activity. However,
since control values did not seem to be available for the 4-week
period, it was difficult to evaluate the significance of the results
for methomyl (Saleh, 1990a,b,c).
7.9 Factors modifying toxicity
It has been shown that synthesized nitrosomethomyl is mutagenic
in vitro and is capable of producing stomach tumours in rats
(Blevins et al., 1977a,b; Lijinsky & Schmahl, 1978). However, when
methomyl is incubated with nitrite and macerated meat under simulated
stomach conditions, there is no evidence that nitrosomethomyl is
formed (see section 4.3).
7.10 Mechanisms of toxicity - mode of action
As a member of the carbamate class of compounds, methomyl has a
well-known mode of action via inhibition of the enzyme
acetylcholinesterase at nerve junctions. A detailed description of
the mechanism of action of carbamates on cholinesterases is given in
Environmental Health Criteria 64: Carbamate pesticides: a general
introduction (IPCS, 1986). Studies with methomyl show that the onset
of toxic action is rapid and that, in common with many other
carbamates, the toxic effect is rapidly reversible. Because of this,
it is important to use appropriate assay methods when measuring
cholinesterase activities during toxicity studies. Failure to allow
for the rapid reversibility of the action can lead to underestimation
of enzyme inhibition.
The acute toxic action of methomyl is characterized by signs of
poisoning typical of anticholinesterase action, i.e. lacrimation,
profuse salivation, tremor and pupil constriction. Surviving animals
quickly show signs of recovery, often within hours. The toxic action
can be countered by the anticholinergic antidote atropine sulfate.
The potency of methomyl is greatest when given by the oral or
inhalation routes of administration. It has very low acute toxicity
when given dermally, presumably because during the slower absorption
phase there is time for recovery from toxic action and thus the effect
is never fully exerted.
8. EFFECTS ON HUMANS
8.1 General population
8.1.1 Accidental and suicidal poisoning
Five Jamaican fishermen prepared a meal to which they
accidentally added methomyl instead of salt. Within minutes of eating
the meal, three of the men were badly affected, twitching, trembling
and frothing at the mouth, and died within 3 h. One of the other two
also showed toxic symptoms, while the other was unaffected. Both
survivors were given atropine and the symptomatic patient recovered
within 2 h after treatment. Postmortem examination of the dead men
revealed highly congested stomach lining, lungs, trachea and bronchi.
Analysis showed that part of the meal (roti) contained about 1%
methomyl. It was estimated that the victims had consumed about
12-15 mg methomyl/kg (Liddle et al., 1979).
A 31-year-old woman committed suicide, using a methomyl
preparation, together with her three children, one of whom (a
9-year-old son) survived. Postmortem examination showed congestion of
the stomach mucous membranes and the lungs. Analysis of organs of the
mother and a 6-year-old son showed highest methomyl concentrations in
the liver (15.4 and 56.5 mg/kg, respectively). Large amounts of
methomyl were present in the stomach contents and it was estimated
that the doses were 55 mg/kg for the mother and 13 mg/kg for the son
(Araki et al., 1982).
A woman attempted suicide by ingesting about 2.25 g methomyl.
After 6 h, methomyl was present in the blood at a concentration of
1.61 mg/kg and in the urine at 10.9 mg/litre; at 15 h the levels were
0.04 mg/kg and 0.25 mg/litre, respectively, and at 22 h methomyl could
not be detected (Noda, 1984).
Symptoms and treatment were described for 11 patients who had
suffered methomyl poisoning in Spain over a 5-year period.
Intoxication was accidental in six cases and suicidal in the other
five. The time interval between exposure and admission for treatment
averaged 2.8 h. All of the subjects showed cholinergic symptoms;
plasma cholinesterase activity was normal in four cases and moderately
reduced in the others. Treatments applied included gastric lavage,
washing the skin, administration of activated charcoal and small doses
of atropine, according to the symptoms involved. All the patients
recovered within 24-48 h (Martinez-Chuecos et al., 1990).
A blood level of 0.57 mg methomyl/litre was measured in a pilot
who had died as a result of a crash while spraying a solution of
methomyl and chlorothalonil in methanol. Analysis was not undertaken
for chlorothalonil and methanol (Driskell et al., 1991).
A 79-year-old man and his 73-year-old wife attempted suicide by
ingesting methomyl powder. The woman died within 19 h but the husband
survived after treatment by gastrolavage, followed by administration
of atropine sulfate. The serum methomyl concentration in the woman
was 44 mg/kg 1 h after ingestion and 0.2 mg/kg in the blood at
autopsy. The methomyl blood concentration of the man was
0.01-0.1 mg/kg 28 h after ingestion (Miyazaki et al., 1989).
8.2 Adverse effects of occupational exposure
Pesticide operators were reported to be affected after handling
powder formulations of methomyl. In one case, an operator mixed a
powder formulation and sprayed vegetables without taking any special
precautions. He displayed poisoning symptoms within 1 h. His blood
cholinesterase activity had decreased to 40% of normal after 12 h but
had recovered to within 80% of normal after 36 h. Other operators, a
mixer-loader, pilot and markers, using recommended safety precautions,
did not show any effects on their red cell or plasma cholinesterase
activities after handling liquid formulations and subsequent aerial
application of methomyl (Simpson & Bermingham, 1977).
In a survey of agricultural applicators in California, USA, in
1982-1989, methomyl was reported to be involved in 129 out of 5371
exposure-related illnesses due to pesticides. For the period
1982-1985 and for the same area, methomyl was considered responsible
for 47 illnesses out of 238 reported cases. Exposure was described as
either short term (< 3 days), longer term (> 3 days) or accidental
(Brown et al., 1989). In 1986, in a summary of reported
pesticide-related illnesses and injuries in California, methomyl was
identified as the probable causative agent in 7 out of 1065 confirmed
occupational cases. These cases were described as "..having some
likelihood of being pesticide related" (Edmiston & Maddy, 1987).
An investigation of workers in a pesticide formulation plant
revealed that 11 out of 102 workers had been hospitalized due to
work-related illness. Most frequent were symptoms of exposure to
methomyl and methaemoglobinaemia due to 3,4-dichloroaniline (Morse et
al., 1979).
Significant T-wave changes (decreased height, inversion, and
leftward deviation of T-wave axis) were shown in 10 out of 22 spraymen
applying methomyl for five days under field conditions. These changes
reverted to pre-exposure levels within one week. Sufficient
information was not available to evaluate the significance of these
findings for operator exposure, and further studies are required to
fully understand their significance (Saiyed et al., 1992).
Two case reports of allergic contact dermatitis with exposure to
methomyl have been described. In the first case, a 26-year-old woman
had gradually worsening itchy hand eczema for six months when working
in a plant nursery, pollinating, pricking out and potting plants
sprayed with methomyl (Lannate) solution. After she avoided touching
the sprayed plants or used polyvinyl gloves she became free of eczema.
She also reacted positively to a 1% aqueous Lannate solution,
indicating that Lannate was a cause of the hand eczema. The second
patient had already had hand eczema for 14 years with recent
worsening. As soon as she changed her job and no longer had contact
with Lannate her eczema disappeared (Bruynzeel, 1991).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effect of methomyl on sewage microorganisms was assessed
indirectly by monitoring total oxygen demand (TOD) over a 24-h period.
A mixture of activated sludge, phosphate buffer and a simulated waste
consisting of Bactopeptone and meat extract was incubated with
methomyl at concentrations of 0, 1, 5, 10, 50 and 90 mg/litre. Only
at 90 mg/litre was there a slight effect on TOD (<10%), indicative of
a minor effect on microorganism activity (Belasco, 1972a).
A fine sand and two silt loam soils were treated with 18 mg
methomyl/kg, and the soil fungi and bacteria populations counted after
11 days. The effect on total microbial activity was determined by
measuring the CO2 evolved periodically over 56 days. Neither soil
microorganism populations nor CO2 production was affected by the
high level of methomyl in the soils, when compared to controls
(Peeples, 1977).
When methomyl was incorporated into a silt loam sand at 0.5 mg/kg
together with ammonium sulfate, no effect on nitrification occurred
over a 6-week period. This level of incorporation represented the
recommended field treatment of 0.56 kg/ha. At ten times this level of
incorporation there was some delay (8-9 days) in reaching the 50%
nitrification level, but by 4 weeks the level was the same as that in
the control soil (Han, 1972).
Methomyl, in the form of Lannate 20L (20% a.i.), was incorporated
into a sandy and a loamy soil at rates of 1.87 and 18.7 mg a.i./kg and
incubated at 20°C for 28 days. Dehydrogenase activity, measured at 14
and 28 days, was unaffected by either rate of incorporation in both
soils. Ammonia nitrogen and nitrate nitrogen declined by about the
same extent in both soils over the 28-day period. This could have
been due to stimulation of nitrogen assimilation by the product
(Danneberg, 1991).
9.2 Aquatic organisms
9.2.1 Algae
The effect of methomyl on algal growth was assessed using
Selenastrum capricornutum as test species. The organism was cultured
under continuous illumination at 24°C with methomyl at concentrations
of 6.25-100 mg/litre for 120 h. Algal growth was monitored at
intervals over the exposure period by measuring the absorbency of
light at 665 nm. The EbC50, (median effective concentration for
inhibition of growth based on a comparison of areas under the growth
curves after "b" hours) after 72 and 120 h was 60 mg/litre. The
ErC50 (median effective concentration for growth inhibition based
on a comparison of maximum growth rates from "x" to "y" hours) from
24-48 h was 140 mg/litre (extrapolated value). The no-observed-effect
concentration was 6.25 mg/litre (Douglas & Handley, 1988).
A similar study was carried out using Lannate 20L (20% methomyl)
as test substance at concentrations of 6.25-200 mg/litre. The EbC50
(72 h) was 68 mg/litre, EbC50 (120 h) was 116 mg/litre and the ErC50
(0-24 h) was 67 mg/litre. A no-observed-effect concentration of
6.25 mg/litre was established (Douglas & Halls, 1991).
9.2.2 Fish
The acute toxicities (96-h LC50, etc.) for several species of
fish are shown in Table 7. The 96-h LC50 values lie mostly within
the range of 0.5-2 mg/litre. The most resistant species tested were
the cutthroat trout with a 96-h LC50 of 4-7 mg/litre. Generally,
within a species, the higher the water temperature the higher the
acute toxicity. Results for some methomyl formulations are shown in
Table 8. These show lower acute toxicity, corresponding to the lower
methomyl content. There are few descriptions of toxic signs in
affected fish. In an acute study with carp and tilapia, the fish sank
to the bottom of the tank and moved sluggishly, then became highly
excited 10 min before death (El-Refai et al., 1976). Shrimps became
lethargic before dying (Bentley, 1973; Sleight, 1973).
Two longer-term studies have been undertaken with methomyl and a
formulation. This included a 21-day flow-through study on fingerling
rainbow trout with Lannate 20L (21.5% methomyl) at 13°C. The
concentrations tested were 0.63, 1.3, 2.5, 5.0, 10 and 20 mg/litre,
equivalent to 0.14-4.3 mg methomyl/litre. No effects on fish length
or weight were noted throughout the study. Some increase in mortality
occurred, from day 12 onwards, in the 5 mg/litre group, and greater
dose-related increases were seen at the two higher concentrations
throughout most of the study. Surviving fish at these concentrations
showed increasing incidence of one or more of the signs of
discoloration, lying on the tank bottom, gasping, bloated stomach,
loss of equilibrium. The 21-day LC50 was calculated to be
6.1 mg/litre formulation (equiv. 1.3 mg methomyl/litre) and the
no-observed-effect concentration was 2.5 mg/litre formulation (Baer,
1991c).
Table 7. The acute toxicity of methomyl to fish (static test)
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Atlantic salmon (Salmo salar) 12 1.12 USDI (1978)
(0.48 g) 17 0.56 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) 12 2.00 USDI (1978)
22 0.86 USDI (1978)
23 2.00 Carter & Graves (1973)
22 1.00 USDI (1975)
Brook trout (Salvelinus fontinalis) 12 1.50 USDI (1978)
Carp (Cyprinus carpio) (5 cm) 25 2.8 Yoshida & Nishiuchi (1972)
Channel catfish (Ictalurus punctatus) 22 0.53 USDI (1978)
(1.8 g) 26 0.92 Carter & Graves (1973)
Cutthroat trout (Salmo clarkie) 10 6.8 USDI (1978)
10 4.05 USDI (1975)
Fathead minow (Pimephales promelas) - 2.8 Mayer & Ellersieck (1986)
(0.75 g)
Japanese goldfish (Carassius auratus) 25 2.7 Yoshida & Nishiuchi
(4 cm) (1972)
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi (1972)
Table 7. (cont'd).
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi (1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
Table 8. The acute toxicity of some methomyl formulations to fish (static test)
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Bluegill sunfish (Lepomis macrochirus) 24% kg a.i. - 0.7 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) Lannate 20 L 21.5 5.1 Baer (1991a)
Bluegill sunfish (Lepomis macrochirus) Lannate 90% WDP - 2.1 USDI (1978)
Carp (Cyprinus carpio) Lannate 25 WP 22 4.7 - Ökolimna (1980)
12.5
Carp (Cyprinus carpio) (1.75 g) Lannate 24 EC 22-25 2.96 El-Refai et al. (1976)
Carp (Cyprinus carpio) (31.5 g) Lannate 24 EC 1.21 El-Refai et al. (1976)
Rainbow trout (Salmo gairdneri) Lannate 20 L 12.6 18 Baer (1991b)
Lannate 90% WDP 13 3.4 McCain (1971)
Tilapia (Tilapia nilocita) (1.5 g) Lannate 24EC 22-25 0.92 El-Refai et al. (1976)
Tilapia (Tilapia nilocita) (13.8 g) 0.88
Channel catfish (Ictaurus punctalus) 24% liquid form - 0.3 USDI (1978)
Channel catfish (Ictaurus punctalus) 29% liquid form 0.3 USDI (1978)
Brook trout (Salvelinus fontinalis) 24% liquid form - 2.2 USDI (1978)
Table 8 (cont'd).
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi
(1972)
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi
(1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
In an early life-stage toxicity study, fathead minnow embryos and
larvae were exposed for 28 days to methomyl at concentrations of
27-491 µg/litre. The water was changed at the rate of 10 aquarium
volumes each 24 h and was maintained at a temperature of 25°C. Embryo
hatch and larval survival and growth was evaluated. The percentage
embryo hatch was not affected at any concentration. Larval survival
was significantly reduced at concentrations down to 117 µg/litre and
growth reduced at 243 and 491 µg/litre. The maximum acceptable
toxicant concentration was estimated to be >57 µg/litre and
<117 µg/litre (Driscoll & Muska, 1982).
9.2.3 Other aquatic organisms
The acute toxicity results for a variety of other aquatic
organisms are shown in Table 9. Daphnia magna appears to be one of
the most susceptible species to the acute toxic action of methomyl,
and the Eastern oyster the least susceptible. This difference was
further emphasized in two other studies. The 48-h EC50 for the
Lannate 20L formulation (21.5% methomyl) was 0.033 mg/litre
(equiv.0.007 mg methomyl/litre) for Daphnia magna neonates (Baer,
1991d). The 96-h EC50 for the Eastern oyster was > 140 mg/litre,
as measured by the effect of methomyl on shell deposition in a
flow-through test at 21.2 to 23.7°C (Ward & Boeri, 1991).
The effect of methomyl or its formulation on Daphnia magna was
studied in longer-term tests on survival, growth and reproductive
capacity. A study was initiated with < 24-h-old daphnids exposed to
measured methomyl concentrations of 0.7, 1.0, 1.6, 3.5, 7.5 or
13.8 µg/litre. The test was conducted over a 21-day period at 20°C
under semi-static conditions with 2-day renewal of the test
solutions. Methomyl was found to be stable in these solutions for up
to 72 h. Daphnid survival and growth were not affected at any
concentration. The number of young produced and number of young per
adult were reduced at 3.5, 7.5 and 13.8 µg/litre. There was some
delay in the first day of reproduction at all levels, but this was
considered to be biologically significant only at 3.5 µg/litre or
more, where the number of young was affected. The maximum acceptable
toxicant concentration was estimated to be between 1.6 and
3.5 µg/litre (Brittelli, 1982).
In another 21-day study, Daphnia magna were exposed to Lannate
20 (21.5% methomyl) at nominal concentrations of 0.63, 1.5, 3.4, 8.
18, 43, 100 or 232 µg/litre. The test conditions and parameters
measured were as described above. At 232 µg/litre all daphnids died
early in the study, but there was no effect on survival at the lower
concentrations nor was growth affected. The 21-day EC50, based upon
adult survival, was estimated to be 160 µg/litre (equivalent to 26 µg
methomyl/litre). The total young produced and number of young per
adult were decreased at 3.4 µg/litre but not at 8 µg/litre. The
21-day no-observed-effect concentration was therefore considered to be
8 µg/litre, equivalent to 2.1 µg methomyl/litre (Baer, 1991e).
Table 9. The acute toxicity of methomyl to other aquatic organisms (static test)
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Midge (Chironomus plumosus) mature 95% technical 22 0.088 (EC50) USDI (1978)
24% conc 0.032 USDI (1978)
Eastern oyster (Crassostrea embryo/ technical 20 4.0 (EC50) Ward & Boeri (1990)
virginica) larvae
Fiddler crab (Uca pugilator) 20 mm Lannate L 21 2.38 Bentley (1973)
(24%)
Grass shrimp (Palaemonetes 18 mm Lannate 90 21 0.049 Sleight (1973)
vulgaris
18 mm Lannate L 21 0.130 Bentley (1973)
(24%)
Gammarus pseudolimnaeus mature technical 17 0.92 USDI (1978)
Mud crab (Neopanope texana) 15 mm Lannate 90 21 0.41 Sleight (1973)
Mysid shrimp (Mysidopsis bahia) 10 day 99% technical 21.5 0.22 Ward & Boeri (1989)
Pink shrimp (Panaeus duorarum) 55 mm Lannate 90 21 0.019 Sleight (1973)
Stonefly (Pteronarcys dorsarta) naiad technical 7 0.034 USDI (1975)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Stonefly (Pheronarcella badia) Year Class 1 99% technical 0.069 Mayer & Ellersieck (1986)
Stonefly (Pheronarcella badia) Year Class 1 24% a.i. 0.06 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 95% technical 0.034 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 24% a.i. 0.92 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 95% a.i. 0.343 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 24% a.i. 0.029 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 99% technical 0.92 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 0.72 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 1.05 Mayer & Ellersieck (1986)
(flowthrough)
Water flea (Daphnia magna) neonate 95% technical 18 0.032 Goodman (1978)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Water flea (Daphnia magna) 1st instar 95% technical 21 0.009 (EC50) USDI (1978)
Water flea (Daphnia magna) - 24% conc 0.0076 USDI (1978)
Water flea (Daphnia magna) - Lannate 20L 20.4 0.033 Baer (1991e)
(21.5)
Water flea (Daphnia magna) 1st instar 95% technical 0.088 Mayer & Ellersieck (1986)
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The acute toxicity of a liquid (Lannate 20L) and a solid (Lannate
25WP) methomyl formulation to earthworms (Eisenia foetida andrei) was
determined over a 14-day period. The formulations were mixed with
artificial soil (70% industrial sand, 20% kaolite and 10% moss peat)
at six concentrations of 0-500 mg/kg (Lannate 20L) and 0-1000 mg/kg
(Lannate 25WP), with 40 earthworms per concentration. The 7-day
LC50 values were estimated to be 165 mg/kg and 147 mg/kg,
respectively, and the 14-day LC50 values 102 mg/kg and 87 mg/kg,
respectively (Armstrong et al., 1991; Caley et al., 1991).
Laboratory tests were undertaken to determine the broadcast
dosage of methomyl, as a 90% SP, required to kill 50% of earthworms
(night crawlers, Lumbricus terrestris L). The test substance was
mixed with moistened potting soil at six rates from 0 to 35 kg/ha with
40 worms per rate. The estimated rate causing 50% mortality was
11.4 kg methomyl/ha (Ruppel & Laughlin, 1977).
The biochemical changes in experimental snails, Eubania
vermiculata (Müller), were studied after treatment with 0.2%
methomyl in bran bait (w/w) for periods of 1, 3, 5, 7 and 10 days.
There was a significant reduction in total soluble proteins, lipids
and glycogenic content, and significant increase of glutamic
oxalocetic transaminase, glutamic pyruvic transaminase and
catalase activities (El-Wakil & Radwan, 1991).
A bran methomyl bait (0.5% w/w) was tested for its molluscicidal
activity on the white garden snail (Theba psiana) and compared with
that of four other oxime carbamate pesticides (aldicarb, aldoxycarb,
oxamyl and thiofanox). Methomyl had the most potent molluscicidal
activity. The time for 50% mortality of snails (LT50) for methomyl,
oxamyl, aldoxycarb, aldicarb and thiofanox was 2.31, 3.97, 4.69, 5.77
and 6.67 days, respectively. The activities of acetylcholine
esterase, acid phosphatase and alkaline phosphatase were inhibited by
the pesticides in line with their potency. GOT and GPT activities
were significantly increased by methomyl (Radwan et al., 1992).
Juvenile (4-week-old) laboratory reared earthworms
(Allolobophora caliginosa) were kept individually in soil treated
with methomyl for 7 days. Relative toxicity, i.e. concentration in
soil (mg/kg) causing zero growth, compared to the standard,
carbofuran, (0.10) was 0.54 (Martin, 1986).
The acute toxicity of methomyl to bees has been reported by
several investigators using different types of test. In one such test
groups of 20 bees were treated individually with a range of doses, the
methomyl being applied to the thorax of each bee in a 1 µl acetone
solution. The mortality was determined after 48 h and the LD50 was
calculated as 0.1 µg/bee (Meade, 1984). The acute toxicity of
methomyl by topical application to the honey-bee (Apis mellifera) was
compared to that of the Western Yellow jacket (Vespula pensylvanica)
after the pleural application of a 1 µl solution in acetone to each
insect. Methomyl was less toxic to the honey-bee, with a 48-h LD50
of 12 µg/g, than to the Yellow jacket (0.9 µg/g) (Johansen & Davis,
1972). A contact LD50 of 1.29 µg per bee has been reported by
Atkins et al., (1976) and an oral LD50 of 0.2 µg/bee by Clinch &
Ross (1970).
The speed of action of methomyl on honey-bees was assessed in a
special laboratory trial. A commercial formulation (90% DP) was
diluted in 33% sucrose solution to give concentrations equal to 1.5, 2
or 4 x LD90. These were fed to bees and the effects observed as two
stages; the first was characterized by very fast and erratic movement
and the second by the bees being unable to walk or fly. The time
intervals to reach each stage were recorded (20 bees), as were the
times taken for 50% and 90% bees to be affected. Methomyl was the
fastest acting of 10 pesticides tested, taking about 3-5 min to give a
50% effect level. It was suggested that this type of information was
needed when interpreting the results of field trials (Clinch & Ross,
1970).
In a tent trial, a methomyl formulation (35% methomyl) was
applied to Phacelia at a 0.3% concentration in the evening after bees
had finished flying and then rewetted early next morning prior to the
start of flight. Observations were made later that day and during the
following two days. Fewer bees visited the trial tent during this day
than visited the control tent. Large numbers of bees were found dead
in front of the hive and at the trial tent edges (about one order of
magnitude higher) on the first day. On the second day about three
times the number of dead bees were found when compared to the control.
Fewer bees were found dead when the trial was repeated with the spray
deposit left dry (Stute, 1983).
Methomyl was among 400 pesticides assessed in honey-bee
laboratory and field studies by the University of California over 20
years. The compound was placed in the highly toxic category, i.e.
severe losses should be expected if the pesticide is used when bees
are present at treatment or within a day thereafter. It was shown
that the contact LD50 of 1.29 µg/bee can be converted directly to
1.29 kg/ha, this being the application rate expected to cause a 50%
mortality among foraging bees in a treated field crop, at the time of
application or shortly thereafter. Several recommendations were
included to help minimize bee losses when spraying with insecticides,
including early morning or night applications, since there is less
risk to honey-bees at these times (Atkins et al., 1976).
9.3.2 Birds
The results for methomyl in acute oral and short-term dietary
studies in birds are shown in Tables 10 and 11. In an acute toxicity
study on the bobwhite quail, a dose of 5.62 mg/kg produced lethargy,
reduced reaction to external stimuli, loss of coordination and lower
limb weakness within 30 min of dosing. The birds recovered within 2 h.
The effects increased at higher doses and included salivation and wing
droop, but recovery in survivors was rapid, within hours to one day.
There were no mortalities at 10 mg/kg or less (Beavers, 1983).
Chickens given methomyl by capsule at 25 or 50 mg/kg showed muscle
tremor, ataxia, salivation, convulsions and death within 30 min
(Palmer & Schlinke, 1978).
In a one-generation study, 20-week-old bobwhite quail, 16 male
and 16 female per group, were fed diets containing 0, 50, 150 or
500 mg methomyl/kg for 20 weeks in their first breeding season.
Assessments included adult clinical health, weight gain and food
consumption and the reproductive parameters for numbers of eggs laid,
development of eggs, viability of embryos, percent hatchability,
offspring survival and eggshell thickness. There were no signs of
toxicity in the adults or of treatment-related mortality. There was a
slight decrease in body weight gain in males at 500 mg/kg up to week
8. No effects on reproductive parameters were seen at the two lower
dietary levels. No treatment-related effects were observed for egg
shell thickness at methomyl concentrations of 50, 150 or 500 mg/kg.
Mean thicknesses of bobwhite quail eggs were 0.221 (± 0.02), 0.216
(± 0.01) and 0.214 (± 0.01) mm, respectively. Treatment groups did
not differ significantly from the control (0.212 ± 0.02 mm). A small
but biologically significant reduction in the numbers of eggs laid per
hen and a subsequent reduction in the numbers of offspring were seen
at 500 mg/kg. A clear no-observed-effect concentration of 150 mg
methomyl/kg diet was therefore determined (Beavers et al., 1991a).
A one-generation study of similar design and the same dietary
concentrations was undertaken with the mallard duck for a period of 18
weeks. There were no signs of toxicity or treatment-related
mortalities in the adults. No effect was seen on body weight at the
two lower levels but a decrease in weight gain occurred in hens fed
500 mg/kg over the last 10 weeks of the study. Egg shell thickness
was not affected by methomyl. Mean shell thicknesses of eggs from
exposed ducks were 0.378 (±0.03), 0.379 (±0.07) and 0.370 (±0.03) mm
for 50, 150 and 500 mg/kg, respectively. No significant differences
from the control (0.378 ± 0.03 mm) were detected. A slight reduction
in the percentage of viable embryos was observed at 500 mg/kg. A
clear no-observed-effect concentration was therefore established at
150 mg/kg (Beavers et al., 1991b).
Table 10. Acute toxicity studies on birds
Species Test Acute oral LD50 Reference
(mg/kg)
Bobwhite quail (Colinus virginianus) intubation, suspended in corn oil 24.2 Beavers (1983)
Japanese quail (Coturnix japonica) intubation, suspended in 34 Smith (1982)
carboxymethylcellulose
Chicken (White Leghorn-Cornish) capsule minimum toxic dose Palmer & Schlinke (1978)
25
Mallard duck (Anas platyrhynchos) 15.9 Tucker & Crabtree (1970)
Pheasant (Phasianus colchicus) 15.4 Tucker & Crabtree (1970)
Common pigeon (Columba livia) in polyethylene glycol 10.0 Schafer (1975)a
Common grackle (Quiscalus quiscala) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) in polyethylene glycol 31.6 Schafer (1975)a
House sparrow (Passer domesticus) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) gavage in propylene glycol 42 Schafer (1972)
Redwing (Agelaius phoeniceus) gavage in propylene glycol 10 Schafer (1972)
a Schafer EW Jr (1975) Avian toxicity tests - letter to Dr HJ Thome, April 28 1975 (unpublished report submitted to WHO by Du Pont)
Table 11. Dietary toxicity studies on birds
Species 8-day Test Dietary LC50 Reference
(5 days feeding, 3 days (mg/kg)
observation)
Bobwhite quail (Colinus virginianus) 4 concentrations 1100 Heath et al. (1972)
Japanese quail (Coturnix japonica) 6 concentrations 3124 Heath et al. (1972)
Mallard duck (Anas platyrhynchas) 6 concentrations 2883 Heath et al. (1972)
Bobwhite quail 7 concentrations 3680 Busey (1967)
Pekin duck 7 concentrations 1890 Busey (1967)
Methomyl did not give any indication of a teratogenic effect in a
chick embryo test when it was injected into the yolk (Proctor et al.,
1976).
9.4 Field studies
Bobwhite quails, 6 males and 6 females, were exposed to sprays of
Lannate formulations, equivalent to a field application of 1 kg
methomyl/ha, once every 48 h for a total of four applications. The
birds were killed 14 days after the last application. There were no
observable effects due to exposure and no treatment-related changes in
tissues on gross examination (Aftosmis, 1973a,b).
A simulated field trial was carried out on two groups of bobwhite
quail (three males and three females per group). One group was fed
prior to treatment, the other group was fed 12 h after treatment. The
treatment consisted of spraying methomyl over the test area at the
rate of 1.1 kg/ha. In all, six sprayings were made, each 5 days
apart, with a 15-day observation period after the last application.
Some weight loss occurred in the group fed prior to treatment;
otherwise there were no observable effects on surviving birds
compared to controls and no changes due to treatment upon gross
examination of tissues (Hinkle & Cameron, 1980).
In 1978, 400 ha of Maine (USA) forest land was treated with the
formulation Lannate LV at the rate of 0.28 kg methomyl/ha to control
spruce budworm. Monitoring of more than 30 species of songbirds was
undertaken over 6-day census periods pre-spray, immediately post-spray
and 2 weeks post-spray. The census was carried out firstly by
individual bird counts and then by territorial counts. Searches were
made for dead birds. Spray deposit cards were used to ensure that the
insecticide was present in the census plots. A concurrent sampling
and analytical programme determined the residue levels of methomyl in
trees and other plants, leaf litter, soil and water. The maximum
level detected was 15 mg/kg in foliage from the top third of the
trees. Underbrush had initial residues of 6 mg/kg, low levels were
found in leaf litter, negligible amounts in soil and none in water.
These levels dissipated rapidly apart from leaf litter where low
levels lasted 34-61 days. The study revealed no evidence of changes
in the activity levels among songbirds. No dead or intoxicated birds
were found and there was no disruption of nesting birds (Brown, 1978).
A study of brain cholinesterase activity was undertaken on wild
mice (Mus musculus) trapped over a period of 3 days after a soya
bean field had been sprayed with Lannate 1.8 L at 0.5 kg methomyl/ha.
Some inhibition of brain cholinesterase activity occurred at a level
of about 10% over the study period indicating the possibility of a
small effect (Montz et al., 1983).
A census of birds was undertaken before and after spraying a
field of hops in Kent, United Kingdom, with 0.56 kg methomyl per ha.
Records were made of birds seen, feeding in the hop field, singing and
nesting. Monitoring was carried out 1, 3 and 10 days after spraying.
Principal species observed feeding upon the sprayed area included
blackbird, song thrush, various tits and finches, robin, wren and
hedge sparrow. There was no apparent difference in feeding habits
before or after spraying and no unhealthy or dead birds were found
over the total observation period (Orpin, 1971).
Methomyl (90% SP), applied at 3.4 kg/ha pasture, produced
decreases in population and biomass of three species of earthworms of
28.5% and 14%, respectively. The compound was considered to be the
least active of the seven pesticides tested (Tomlin & Gore, 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The proposed toxicological criteria for setting guidance values
are presented in Table 12.
10.2 Evaluation of effects on the environment
Adsorption of methomyl to soil is low to moderate with hardly any
desorption. Aerobic degradation in soil (with a half-life of around
one week) is about twice as fast as anaerobic degradation. A relative
increase in soil organic matter delays degradation.
Table 12. Proposed toxicological criteria for setting guidance values
Exposure scenario Relevant route/effect Result/remarks
Short-term oral, acute, several species including human highly toxic; rat LD50 = 17 mg/kg body weight;
(1-7 days) LOELa= 5 mg/kg body weight
eye, irritation, rabbit mild irritant
inhalation, acute, rat highly toxic; LC50 = 0.26 mg/litre (4-h aerosol);
NOELb = 0.14 mg/litre
dermal, acute, rat and rabbit low toxicity; intact skin - LD50 > 2000 mg/kg body weight;
NOELc = 2000 mg/kg body weight in the rabbit
Medium-term repeat dermal, rabbit 21-day study; no toxicologically significant effects at
(1-26 weeks) 50 mg/kg body weight per day
repeat oral, rat 13-week study; NOEL = 3.6 mg/kg body weight per day
maternal oral, rabbit teratology study; NOEL = 6 mg/kg body weight per day
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body weight per day
a mild cholinergic signs of toxicity in guinea-pig
b no-observed-effect level for clinical signs and death
c no clinical symptoms noted except signs of skin irritation
Despite the above adsorption characteristics, leaching of
methomyl in soil to levels deeper than 20 to 30 cm has not been
observed. The concentrations of methomyl in both surface and well
water are below the limit of detection. Methomyl is degraded rapidly
with a half-life of about one week in water and sediment. In sterile
water, methomyl is stable for at least 30 days at normal environmental
pH.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. In contrast,
when applied to soil, uptake through the roots occurs readily with
rapid translocation to the leaves. Adsorbed foliar residues degrade
with a half-life in the order of 4 days. Absorbed methomyl
concentrations in food crops decline rapidly to about 5% within one
week; this may be due to growth dilution.
Bioaccumulation by rainbow trout did not occur in a flow-through
study. Depuration occurred within one day of transfer to clean water.
Trout were discoloured when exposed to levels between 0.075 and
0.75 mg/litre, an effect that disappeared within 5 days, the time
depending on original exposure concentration.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil; nitrification can be
delayed at applications 10 times higher. An aquatic green alga showed
a NOEC for growth of 6.25 mg/litre.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl, the LC50s being of the order of 10 to
100 µg/litre. MATC values from two 21-day Daphnia studies were
estimated to be around 2 µg/litre. Kills of aquatic invertebrates are
expected following overspray.
Fish, both freshwater and estuarine, are less sensitive, the
LC50s ranging from 0.5 to 7 mg/litre. Two longer-term studies gave
an NOEC of 0.5 mg/litre for lethality of fingerling rainbow trout and
an MATC of > 0.06 and > 0.12 mg litre for survival of embryo larval
stages of fathead minnow. Given the low persistence of methomyl and
its relatively low acute toxicity to fish, the risk is expected to be
low.
Laboratory tests in artificial soil with methomyl formulations
(25% WP) established a 14-day LC50 of 90-100 mg formulation/kg for
earthworms. It was estimated that 11 kg a.i./ha of methomyl would
kill 50% of earthworms. A field application of 3 kg a.i./ha caused
reduced earthworm population (28%) and biomass (14%). The TER for
earthworms is around 40 indicating low risk.
Methomyl is classified as highly toxic to honey-bees, the topical
LD50 being approximately 0.1 µg/bee. Field (tent) trials showed
dead bees both at the treatment tent and hive when residues from
spraying the previous day were wetted. Less effect was seen with dry
residues. Methomyl has not been implicated in bee incidents in the
field; this probably reflects advice to restrict spraying times to
protect bees.
Acute oral LD50s for various bird species range between 10 and
40 mg/kg body weight. Dietary LC50s (5 days) range from 1100 to
3700 mg/kg diet. Methomyl poses an acute oral risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds. An example of a toxicity exposure ratio
for birds and fish is shown in Table 13.
The NOEC for reproduction was established at 150 mg/kg diet for
both the bobwhite quail and mallard duck. Field studies following
spraying of methomyl formulations in forests showed no mortality of
songbirds and no changes in feeding behaviour or general activity.
Reduced fat deposits of songbirds reflect reduced insect prey. It was
not considered that methomyl poses a threat to birds after recommended
applications.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
Table 13. Toxicity exposure ratios for birds, fish and aquatic invertebrates based on application rates of 2.5 kg a.i./ha
methomyl to soybeans (worst case)
Risk category LC50 (mg/litre or mg/kg diet) Estimated exposure Toxicity/exposure ratio (TER)c
(mg/litre or mg/kg diet)a,b
Acute bird 10 50-364 02-0.027
Acute fish (stream) 0.5 0.2 2.5
Acute fish (pond) 0.5 0.04 12.3
Acute aquatic invertebrate 0.009 0.2 0.045
(stream)
Acute aquatic invertebrate 0.009 0.04 0.225
(pond)
a Estimated environmental concentration in the terrestrial environment (for bird exposure) is based on the stated application rate
and the assumption of deposition on short grass using the US EPA monogram
b Aquatic exposure concentrations were taken from the STREAM model based on a single application and estimated run-off into water;
no direct overspray is included
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate likely exposure to toxic concentrations
by organisms in the different risk categories
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
Considering the toxicological characteristics of methomyl, both
qualitatively and quantitatively, it is concluded, on the basis of the
no-observed-effect level of 3 mg/kg body weight per day in the 2-year
toxicity study on dogs and applying a 100-fold uncertainty factor,
that 0.03 mg/kg body weight per day will probably not cause adverse
effects in humans by any route of exposure.
12. FURTHER RESEARCH
No further studies were thought to be necessary.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has discussed and
evaluated methomyl on several occasions since 1975. Residue aspects
were discussed in 1975, 1976, 1977, 1978, 1986, 1987, 1988, 1989, 1990
and 1991 (FAO/WHO, 1976, 1977, 1978, 1979, 1986, 1987, 1988a,b,
1990a,b,c, 1991). Toxicological evaluations took place in 1978, 1986
and 1989, when an Acceptable Daily Intake (ADI) of 0-0.03 mg/kg body
weight was established (FAO/WHO, 1979, 1987, 1990a,b).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for methomyl in various commodities
(FAO/WHO, 1993).
It should be noted that the 1992 CCPR meeting decided to combine
MRLs for thiodicarb and methomyl into a single list. In the cases of
different MRLs the higher limit would prevail.
Methomyl is listed in Class IB ("Highly Hazardous") in the WHO
Recommended Classification of Pesticides by Hazard and
Guidelines to Classification (1994-1995) on the basis of its rat acute
oral LD50 value of 17 mg/kg body weight (IPCS, 1994).
The time-weighted average (TWA) adopted by the American
Conference of Governmental Industrial Chemists (ACGIH) is 2.5 mg/m3
(ACGIH, 1994-1995).
REFERENCES
* Report submitted to WHO by E.I. Du Pont de Nemours and Co.,
Wilmington, Delaware, USA
Aftosmis JG (1973a) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamine acid, S-methyl ester (25% a.i.) (Lannate L
methomyl insecticide). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-354-73*).
Aftosmis JG (1973b) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamic acid, S-methyl ester (30% a.i.) (non-flammable
Lannate L methomyl insecticide), Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-606-73*).
Ambridge EM (1992) Report on field trials to assess operator
contamination during application of two different pesticides to crops
of varying heights in Thailand, November/December 1991 (NRI Contract
No. 00195) (Unpublished report*).
ACGIH (1994-1995) Threshold limit values for chemical substances in
the work environment. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, p 28.
Andrews TL & Miskus RP (1968) Tetraethylammonium chloride as an
antidote for certain insecticides in mice. Science, 159: 1367-1368.
Antal M, Bedö M, Constantinovits G, Nagy K, & Szépvölgyi J (1979)
Studies on the interaction of methomyl and ethanol in rats. Food
Cosmet Toxicol, 17: 333-338.
Araki M, Yonemitsu K, Kambe T, Idaka D, Tsunenari S, Kanda M, &
Kambara T (1982), [Forensic toxicological investigations in fatal
cases of carbamate pesticide methomyl (LannateR) poisoning.] Nippon
Hoigaku Zasshi, 36: 584-588 (in Japanese).
Armondi S (1991a) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-394 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-345-91*).
Armondi S (1991b) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-425 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report HLO 346-91*).
Armondi S (1992) Closed-patch repeated insult dermal sensitization
study (maximization method) with DPX-X1179-424 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-659-91*).
Armstrong K, Caley CY, Hall BE, & Knight B (1991) Lannate 20L:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inveresk Research International Ltd (Unpublished report
No. 8546*).
Atkins EL, Anderson LD, Kellum DD, & Neuman KW (1976) Protecting honey
bees from pesticides. Berkeley, California, University of California,
Division of Agricultural Sciences, 15 pp (Leaflet 2883).
Baer KN (1991a) Static, acute, 96-hour LC50 of DPX-X1179-423 to
bluegill sunfish (Lepomis macrochirus). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-30-91*).
Baer KN (1991b) Static, acute, 96-hour LC50 of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-29-91*).
Baer KN (1991c) Flow-through, 21-day toxicity of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-34-91*).
Baer KN (1991d) Static, acute, 48-hour EC50 of DPX-X1179-423
(LannateR 20L) to Daphnia magna. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-150-91*).
Baer KN (1991e) Chronic toxicity of DPX-X1179-423 (LannateR 20L) to
Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-292-91*).
Barnes JR (1978) Cholinesterase tests with methomyl. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-280-78*).
Battelle (1991) Method for determination of oxamyl and methomyl in
groundwater. Columbus, Ohio, Battelle Institute (Unpublished report
No. AMR-1392-89*).
Beavers JB (1983) An acute oral toxicity study in the Bobwhite with
H-15,000. Easton, Maryland, Wildlife International Ltd (Unpublished
report No. HLO-464-83*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991a) A one-generation
reproduction study with the Northern Bobwhite (Colinus virginianus).
Easton, Maryland, Wildlife International Ltd (Unpublished report
No. HLO-337-91*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991b) A one-generation
reproduction study with the mallard (Anas platyrhynchos). Easton,
Maryland, Wildlife International Ltd (Unpublished report
No. HLO-336-91*).
Bedö M & Cieleszky V (1990) Nutritional toxicology in the evaluation
of pesticides. Bibl Nutr Dieta, 29: 20-31.
Belasco IJ (1972a) Effect of methomyl on the activity of sewage
microorganisms (Unpublished report No. ML/ME15*).
Belasco IJ (1972b) Methomyl: Incubation with rumen microorganisms
(Unpublished report No. ML/ME26*).
Bentley RE (1973) Acute toxicity of H-8385 to grass shrimp
(Palaemonetes vulgaris) and fiddler crab (Uca pugilator). Wareham,
Massachusetts, Bionomics Inc. (Unpublished report No. HLO-504-73*).
Blevins RD, Lee M, & Regan JD (1977a) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56: 1-6.
Blevins RD, Lijinsky W, & Regan JD (1977b) Nitrosated methylcarbamate
insecticides: Effect on the DNA of human cells. Mutat Res, 44: 1-7.
Boeri RL & Ward TJ (1989) Static acute toxicity of methomyl to the
sheepshead minnow, Cyprinodon variegatus. Hampton, New Hampshire,
Resource Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-700-89*).
Boleij JSM, Kromhout H, Fleuren M, Tieleman W, & Verstappen G (1991)
Re-entry after methomyl application in greenhouses. Appl Occup Environ
Hyg, 6(8): 672-676.
Bonatti S, Bolognesi C, Degan P, & Abbondandolo A (1994) Genotoxic
effects of the carbamate insecticide methomyl. 1. In vitro studies
with pure compound and the technical formulation Lannate 25. Environ
Mol Mutagen, 23: 306-311.
Borady AMA, Mikhail TH, Awadallah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23: 33-44.
Boulton JJK, Boyce CBC, Jewess PJ, & Jones RF (1971) Comparative
properties of N-acetyl derivatives of oxime N-methylcarbamates & aryl
N-methyl carbamates as insecticides and acetylcholinesterase
inhibitors. Pestic Sci, 2: 10-15.
Bracy OL, Doyle RS, Kennedy M, McNally SM, Weed JD, & Thorne BM (1979)
Effects of methomyl and ethanol on behaviour in the Sprague-Dawley
rat. Pharmacol Biochem Behav, 10: 21-25.
Braun HE, Ritcey GM, Frank R, McEwen FL, & Ripley BD (1980)
Dissipation rates of insecticides in six minor vegetable crops grown
on organic soils in Ontario, Canada. Pestic Sci, 11: 605-616.
Braun HE, Ritcey GM, Ripley BD, McEwen FL, & Frank R (1982) Studies of
the disappearance of nine pesticides on celery and lettuce grown on
muck soils in Ontario 1977-1980. Pestic Sci, 13: 119-128.
Brittelli MR (1982) Chronic toxicity of methomyl to Daphnia magna.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-46-82*).
Brock WJ (1989) Repeated dose dermal toxicity: 21-day study with
DPX-X1179-394 (methomyl) in rabbits. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-387-89*).
Brodsky J (1991) Determination of residues of methomyl in grapes, wine
& wine processing fractions by GC-MS following treatment with
"Lannate", season 1990, France. Frankfurt, Germany, Battelle-Europe
(Unpublished report No. BE-A-11-91-10-BF*).
Bromilow RH, Briggs G, Williams MR, Smelt JH, Tuinstra LGMT, & Traag
WA (1986) The role of ferrous ions in the rapid degradation of oxamyl,
methomyl and aldicarb in anaerobic soils. Pestic Sci, 17: 535-547.
Brown HL (1978) The effects of Lannate LV on singing male songbirds in
Maine in 1978 (Unpublished report No. ML/FW-12 report*).
Brown SK, Ames RG, & Mengle DC (1989) Occupational illnesses from
cholinesterase-inhibiting pesticides among agricultural applicators in
California, 1982-1985. Arch Environ Health, 44: 34-39.
Bruynzeel DP (1991) Contact sensitivity to Lannate(R). Contact
Dermatitis, 25: 60-61.
Bull DL (1974) Fate of methomyl on cotton. Environ Entomol,
3: 723-724.
Busey WM (1966) Three-month dietary administration - Rats: Insecticide
1179. Supplement to final report. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-848*).
Busey WM (1967) Acute aqueous exposure - Goldfish, Bluegill, and
Rainbow trout. Acute dietary administration - Peking ducks & Bobwhite
quail: Insecticide 1179. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-888-1*).
Cahill WP, Estesen B, & Ware GW (1975) Foliage residues of
insecticides on cotton. Bull Environ Contam Toxicol, 13: 334-337.
Caley CY, Cameron BD, Hall BE, & Knight B (1991) Lannate 25 WP:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inverest Research International Ltd (Unpublished report
No. 8455*).
Carakostas MC (1987) Inhibition and regeneration kinetics for human
and rat acetyl-cholinesterase exposed to methomyl in vitro. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-379-88*).
Carbonnel E, Puig M, Xamena N, Creus A, & Marco R. (1990) Sister
chromatid exchange in lymphocytes of agricultural workers exposed to
pesticides. Mutagenesis, 5(4): 403-405.
Carter FL & Graves JB (1973) Measuring effects of insecticides on
aquatic animals. La Agric, 16: 14-15.
Christian MS, Hoberman AM, & Fuessner EL (1983) Embryo-fetal toxicity
and teratogenicity study of methomyl in the rabbit. Horsham,
Pennsylvania, Argus Research Laboratories Inc. (Unpublished report
No. HLO-331-83*).
Clark S & Kennedy SM (1990) Analytical method for the quantification
of methomyl in grapes. Sacramento, California, Morse Laboratories
(Unpublished report No. AMR-1806-90*).
Clement C (1987a) Test to evaluate the acute cutaneous primary
irritation and corrosivity in the rabbit. L'Arbresle, France, Hazleton
France, 16 pp (Unpublished report No. 702408*).
Clement C (1987b) Test to evaluate acute ocular irritation and
corrosivity in the rabbit. L'Arbresle, France, Hazleton France, 26 pp
(Unpublished report No. 703313*).
Clinch PG & Ross IGM (1970) Laboratory assessment of the speed of
action on honey bees of orally dosed insecticides. N Z J Agric Res,
13: 717-725.
Council of the European Communities (1991) Council directive of 15
July 1991 concerning the placing of plant protection products on the
market (91/414/EEC). Off J Eur Communities, L230: Part II.
Cox L, Hermosin MC, & Comejo J (1993) Adsorption of methomyl by soils
of southern Spain and soil components. Chemosphere, 27: 837-849.
Danneberg G (1991) Investigation of the effects of Lannate 20L on the
activity of the microflora of soil. Frankfurt, Germany, Battelle
Institute (Unpublished report No. BE-S-11-91-01-DEH-01*).
Dashiell OL (1972) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-518-72*).
Debuyst B & Van Larebeke N (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat Res, 113: 242-243.
Dong MH, Krieger RI, & Ross JH (1992) Calculated re-entry interval for
table rape harvesters working in California vineyards treated with
methomyl. Bull Environ Contam Toxicol, 49: 708-714.
Douglas MT & Halls RWS (1991) The algistatic activity of Lannate 20L.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPC-16(f) 91399*).
Douglas MT & Handley JW (1988) The algistatic activity of methomyl
Tech. (DPX-X1179-00620-06). Huntingdon, United Kingdom, Huntingdon
Research Centre (Unpublished report No. DPT-171(j) 871676*).
Driscoll RR & Muska CF (1982) Early life stage toxicity of methomyl to
fathead minnow. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-528-82*).
Driskell WJ, Groce DF, Hill RH Jr, & Birky MM (1991) Methomyl in the
blood of a pilot who crashed during aerial spraying. J Anal Toxicol,
15: 339-340.
Du Pont (1967) Methomyl - Livestock feeding studies: Milk and meat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Industrial and
Biochemicals Department (Unpublished report No. ML/ME27*).
Du Pont (1973) Petition for residue tolerance - methomyl: Broccoli,
brussel sprouts, cauliflower, spinach, celery. Pesticide Petition 4F
1448, Section D, 1973. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Biochemicals Department (Unpublished report*).
Du Pont (1978) Ecosystem residue study: Spruce/fir forest and cedar
swamp, Princeton, Maine 1978. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. ML/PC-25*).
Du Pont (1982) LannateR insecticide formulations and technical
methomyl - Determination of methomyl (INX-1179) - Reversed-phase
liquid chromatography (RPLC) assay method (Method No. L30.4662/(E).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report [Second issue]*).
Eble JE & Tomic DM (1991) Foliar half-life of methomyl in cotton
leaves. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. AMR-1871-90*).
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards DF (1980) 10 Day subacute skin absorption test on rabbits.
Mewark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-166-80*).
El-Refai A, Fahmy FA, Mahmound FA, Abdel-Lateef, & Imam AKE (1976)
Toxicity of three insecticides to two species of fish. Int Pest
Control, Nov/Dec: 4-8.
El-Wakil HB & Radwan MA (1991) Biochemical studies on the terrestrial
snail Eubania vermiculata (Müller) treated with some pesticides. J
Environ Sci Health, B26(5/6): 479-489.
FAO (1985) Guidelines for the disposal of waste pesticide containers
on the farm. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1976) Pesticide residues in food. Report of the 1975 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. Geneva, World Health
Organization (WHO Technical Report Series No. 592; FAO Plant
Production and Protection Series No. 1).
FAO/WHO (1977) 1976 Evaluations of some pesticide residues in food:
The monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Series
No. 8; AGP:1976/M/14).
FAO/WHO (1978) Pesticide residues in food. 1977 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10 Sup).
FAO/WHO (1979) Pesticide residues in food. 1978 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 15 Sup).
FAO/WHO (1986) Pesticide residues in food - 1986. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78).
FAO/WHO (1987) Pesticide residues in food - 1986. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78/2).
FAO/WHO (1988a) Pesticide residues in food - 1987. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 86/1).
FAO/WHO (1988b) Pesticide residues in food - 1988. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 93/1).
FAO/WHO (1990a) Pesticide residues in food - 1989. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100).
FAO/WHO (1990b) Pesticide residues in food - 1989. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100/2).
FAO/WHO (1990c) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 103/1).
FAO/WHO (1991) Pesticide residues in food - 1991. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper No. 111).
FAO/WHO (1993) Codex Alimentarius - Volume two: Pesticide residues in
food. Rome, Food and Agriculture Organization.
Farrow MG, Cortina T, & Padilla-Nash H (1984) In vivo bone marrow
chromosome study in rats, H15,000. Final Report. Vienna, Virginia,
Hazleton Biotechnologies Corporation (Unpublished report
No. HLO-63-84*).
Fayez V & Baig MRE (1991) Short-term toxicity of methomyl in rats.
Chemosphere, 23(3): 375-382.
Foerst DL & Moye HA (1985) Aldicarb and related compounds in drinking
water via direct aqueous injection HPLC with post column
derivatisation. Cincinnati, Ohio, US Environmental Protection Agency
(EPA/600/D-85/051).
Fossi MC, Leonzio C, Massi A, Lari L, & Casini S (1992) Serum esterase
inhibition in birds: A nondestructive biomarker to assess
organophosphorus and carbamate contamination. Arch Environ Contam
Toxicol, 23: 99-104.
Foster GV (1966a) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl) progress report. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-73-66*).
Foster GV (1966b) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-214-66*).
Friedman PL (1983) Hydrolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. AMR-109-83*).
Freeman PK & Ndip EMN (1984) Photochemistry of oxime carbamates 2.
Phototransformations of methomyl. J Agric Food Chem, 32(4): 877-881.
Fung KH & Uren NC (1977) Microbial transformation of S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate (methomyl) in soils. J Agric
Food Chem, 25(4): 966-969.
Fung KH, Luke RKJ, & Uren NC (1978) Concentrations of methomyl in
Australian tobacco plants following transplant, foliar and soil
treatments. Tob Sci, 22: 24-26.
Gianessi LP & Puffer CA (1992) Insecticide used in US crop production.
Resources for the Future. Washington, DC.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Agrochemical Manufacturers.
Goodman NC (1978) 48-Hour LC50 to Daphnia magnia. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-165-78*).
Gordon M & Richter ED (1991) Hazards associated with aerial spraying
of organophosphate insecticides in Israel. Rev Environ Health,
9(4): 229-238.
Gupta RC, Goad JT, & Kadel WL (1992) Characteristic changes in LDH and
its isoenzymes as biomarkers under the influence of acute methomyl
toxicity. Fed Am Soc Exp Biol J, 6(4): A1307.
Han JCY (1972) Evaluation of possible effects of methomyl on
nitrifying bacteria in soil (Unpublished report ML/ME16*).
Han JCY (1975) Absence of nitroso formation from [14C] methomyl and
sodium nitrite under simulated stomach conditions. J Agric Food Chem,
23: 892-896.
Harvey J Jr (1967) Exposure of S-methyl N-[(methyl carbomoyl)oxy]
thioacetimidate in sunlight, water and soil (Unpublished report
No. ML/ME-10*).
Harvey J Jr (1972a) Decomposition of 14C-methomyl in aerated river
water exposed to sunlight (Unpublished report No. ML/ME-13*).
Harvey J Jr (1972b) Decomposition of 14C-methomyl in a high organic
matter soil (Unpublished report No. ML/ME-18*).
Harvey J Jr (1977a) Decomposition of 14C-methomyl in a sandy loam
soil in the greenhouse (Unpublished report No. ML/ME-19*).
Harvey J Jr (1977b) Degradation of 14C-methomyl in Flanagan silt
loam in biometer flasks (Unpublished report No. ML/ME-20*).
Harvey J Jr (1978) Crop rotation study with 14C-methomyl in the
greenhouse. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Biochemicals Department (Unpublished report No. ML/ME-22*).
Harvey J Jr (1980) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report No. AMR-22-80*).
Harvey J Jr (1983) Photolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Chemicals
Department (Unpublished report No. AMR-121-83*).
Harvey J Jr & Pease HL (1973) Decomposition of methomyl in soil. J
Agric Food Chem, 21(5): 784-786.
Harvey J Jr & Reiser RW (1973) Metabolism of methomyl in tobacco, corn
and cabbage. J Agric Food Chem, 21: 775-783.
Harvey J Jr & Yates RA (1967) Metabolism of methomyl in the corn
plant. I. Plant growth chamber - Carbon 14 studies (Unpublished report
No. ML/ME2*).
Harvey J Jr, Jelinek AG, & Sherman H (1973) Metabolism of methomyl in
the rat. J Agric Food Chem, 21(5): 769-775.
Hashimoto Y & Fukami J (1969) Toxicity of orally and topically applied
pesticide ingredients to Carp Cyprinus carpio Linné. Botuy Kagaku,
34: 63-66.
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1991) The metabolism of
[1-14C] methomyl in rats. Huntingdon, United Kingdom, Huntingdon
Research Centre and Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products (Unpublished report No. AMR-1584-90*).
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1992) The metabolism of
[1-14C] methomyl in male cynomolgus monkeys. Huntingdon, United
Kingdom, Huntingdon Research Centre (Unpublished report
No. AMR-1902-90*).
Heath RG, Spann JW, Hill EF, & Kreitzer JF (1972) Comparative dietary
toxicities of pesticides to birds. Washington, DC, US Department of
the Interior, Fish and Wildlife Service (Special Scientific
Report-Wildlife No. 152).
Hemavathy KC & Krishnamurthy NB (1987a) Mutagenicity studies in
Drosophila melanogaster with Lannate 20. Mutat Res, 191: 41-43.
Hemavathy KC & Krishnamurthy NB (1987b) Evaluation of Lannate 20, a
carbamate pesticide in the germ cells of male rice. Environ Res,
42: 362-365.
Henry NW III (1975) LannateR L methomyl insecticide potentiation
studies. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-278-75*).
Henry JE (1981) Acute dermal methomyl-cholinesterase response study in
male rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-303-81*).
Hill KM, Hollowell RH, & Dal Cortivo LA (1984) Determination of
N-methylcarbamate pesticides in well water by liquid chromatography
with postcolumn fluorescence derivatization. Anal Chem, 56: 2465-2468.
Hinkle S & Cameron JT (1980) Simulated field trial in Bobwhite quail,
H-13 099. Final report. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished report No. HLO-97-80*).
Holt RF (1971) LannateR residue cooking studies. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Hornberger CS (1967) Acute inhalation toxicity of aqueous spray mist.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-171-67*).
Hosling GC (1969) Dietary administration - Cotornix Quail: INX-1179
(Unpublished report No. HLO-238-69*).
Huhtanen K & Dorough HW (1976) Isomerization and Beckmann
rearrangement reactions in the metabolism of methomyl in rats. Pestic
Biochem Physiol, 6: 571-583.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - A
general introduction. Geneva, World Health Organization.
IPCS (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization (Unpublished document WHO/PCS/94.2).
Ivie KF (1980) High performance liquid chromatography (HPLC) in
pesticide residue analysis. In: Zweig G & Sherman J ed. Updated
general techniques and additional pesticides. New York, London,
Academic Press, pp 55-58.
Jackson GC, Hardy CJ, Gregson RL, Offer JM, & Gopinath C (1991)
Lannate 20L: Acute inhalation toxicity in rats 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPT-247/91521*).
Johansen CA & Davis HG (1972) Toxicity of nine insecticides to the
Western yellowjacket. J Econ Entomol, 65: 40-42.
Kaplan AM (1981) Long-term feeding study in rats with S-methyl
N-[(methylcaramoyl)oxy] thioacetimidate (methomyl, INX-1179). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-235-81*).
Kaplan AM & Sherman H (1977) Toxicity studies with methyl
N-[[(methylamino) carbonyl]oxy]-ethanimidothioate. Toxicol Appl
Pharmacol, 40: 1-17.
Kelly DP (1992) Acute inhalation toxicity study with DPX-X1179-440 in
rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-326-92*).
Kennedy SM (1989) Field soil dissipation of Lannate(R) L insecticide.
Sacramento, California, Morse Laboratories Inc. (Unpublished report
No. AMR-1215-88*).
Kennedy CM (1991) Field soil dissipation of Lannate(R) L insecticide
- A 1991 study. Sacramento, California, Morse Laboratories Inc. and
Lincoln, Nebraska, Harris Environmental Technologies Inc. (Unpublished
report No. AMR-1921-91*).
Kennedy CM & Hay RJ (1991a) Magnitude of residues of methomyl
insecticide in citrus and its processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1361-89*).
Kennedy CM & Hay RJ (1991b) Magnitude of residues of methomyl
insecticide in cottonseed and its processed fractions. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1355-89*).
Kiigemagi U & Deinzer ML (1979) Dislodgeable and total residues of
methomyl on mint foliage. Bull Environ Contam Toxicol, 22: 517-521.
Kiigemagi U, Wellman D, Cooley EJ, & Terriere LC (1973) Residues of
the insecticides phorate and methomyl in mint hay and oil. Pestic Sci,
4: 89-99.
Knaak JE, Jackson T, Fredrickson AS, Rivera L, Maddy KT, & Akesson NB
(1980) Safety effectiveness of closed transfer, mixing, loading and
application equipment in preventing exposure to pesticides. Arch
Environ Contam Toxicol, 9: 231.
Krauss WC & Stula EF (1967) Oral LD50 and delayed paralysis tests
(Hens). Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-161-67*).
Labare A (1990) Testing of DPX-X1179 through FDA multi-residue
protocols A-E. Beltsville, Maryland, Biospherics Inc. (Unpublished
report No. AMR-1405-89*).
Leidy RB, Domanski JJ, Haire PL, & Sheets TJ (1977) Effects of
weathering and flue-curing on methomyl residues on tobacco. Arch
Environ Contam Toxicol, 5: 199-206.
Leistra M, Dekker A, & Van Der Burg AMM (1984) Computed and measured
leaching of the insecticide methomyl from greenhouse soils into water
courses. Water Air Soil Pollut, 23: 155-167.
Leitch RE & Pease HL (1973) LannateR-methomyl. In: Sherma J & Zweig
G ed. Analytical methods for pesticides and plant growth regulators,
7th ed. New York, London, Academic Press, pp 331-338.
Lheritier M (1991a) Test to evaluate the acute toxicity following a
single oral administration (LD50) in the rat. L'Arbresle, France,
Hazleton France (Unpublished report No. 104367*).
Lheritier M (1991b) Test to evaluate the acute toxicity following a
single cutaneous application (limit test) in the rat. L'Arbresle,
France, Hazleton France (Unpublished report No. 106393*).
Liddle JA, Kimbrough RD, Needham LL, Cline RE, Smrek AL, Yert LW, &
Bayse DD (1979) A fatal episode of accidental methomyl poisoning. Clin
Toxicol, 15(2): 159-167.
Lijinsky W & Schmahl D (1978) Carcinogenicity of N-nitroso derivatives
of N-methylcarbamate insecticides in rats. Ecotoxicol Environ Saf,
2: 413-419.
Lu CC (1983) NudrinR: Two-generation reproduction study in rats
(Protocol No. RA-274). Houston, Texas, Shell Development Co.,
Westhollow Research Center (Unpublished report*).
McAlack JW (1973) Ten-day subacute exposure of rabbit skin to LannateR
L insecticide. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report HLR 24-73*).
McCain JC (1971), Acute fish toxicity study - static freshwater:
Lannate, MR-581, H-6854. Falls Church, Virginia, Hazleton Laboratories
Inc. (Unpublished report No. HLO-71-71*).
McConnel R, Pacheco Anton AF, & Magnotti R (1990) Crop duster aviation
mechanics: High risk for pesticide poisoning. Am J Public Health,
80(10): 1236-1239.
McCooey KT, Chromey NC, Sarrif AM & Hemingway RE (1984), CHO/HGPRT
assay for gene mutation. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-556-83*).
Macieira OJD & Hebling Beraldo MJA (1989) Laboratory toxicity of
insecticides to workers to Trigona spinipes. J Apic Res, 28(1): 3-6.
Martin NA (1986) Toxicity of pesticides to Allolobophora caliginosa
(Oligochaeta: Lumbricidae). N Z J Agric Res, 29: 699-706.
Martinez-Chuecos J, Molinero-Somolinos F, Solé-Violan J, & Rubio-Sanz
R (1990) Management of methomyl poisoning. Hum Exp Toxicol,
9: 251-254.
Marxmiller RL & Hay RJ (1991) Magnitude of residues of methomyl
insecticide in tomatoes and their processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1360-69*).
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res,
24(1): 11-21.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Meade AB (1984) Methomyl toxicity to honey bee. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. METH/ECO 9*).
Mercier O (1991) Test to evaluate the sensitising potential by topical
applications in the Guinea-pig. "The Buehler Test". L'Arbresle,
France, Hazleton France (Unpublished report No. 106348*).
Merricks DL (1990) Lannate insecticide - Field worker exposure study
in grape girdling and harvesting operations. Frederick, Maryland,
Agrisearch Incorporated, Sacramento, California, Morse Laboratories
Inc. and Fresno, California, Siemer and Associates Inc. (Unpublished
report No. AMR-1442-89*).
Miles CJ & Oshiro WC (1990) Degradation of methomyl in chlorinated
water. Environ Toxicol Chem, 9: 535-540.
Miyazaki T, Yashiki M, Kojima T, Chikasue F, Ochiai A, & Hidani Y
(1989) Fatal and non-fatal methomyl intoxication in an attempted
double suicide. Forensic Sci Int, 42: 263-270.
Monson KD (1989) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-22-80*).
Monson KD & Ryan DL (1991) [14C] Methomyl cow metabolism study.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-1675-90*).
Montz WE, Scanlon PF, & Kirkpatrick RL (1983) Effects of field
application of the anti-cholinesterase insecticide methomyl on brain
acetylcholinesterase activities in wild Mus musculus. Bull Environ
Contam Toxicol, 31: 158-163.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrow RW (1972) Acute skin absorption study on rats using technical
methomyl and a 25% methomyl formulation (Lannate(R) 25W). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-438-72*).
Morse DL, Baker EL, Kimbrough RD, & Wiseman CL (1979)
Propanil-chloracne and methomyl toxicity in workers of a pesticide
manufacturing plant. Clin Toxicol, 15(1): 13-21.
Natoff IL & Reiff B (1973) Effect of oximes on the acute toxicity of
anticholinesterase carbamates. Toxicol Appl Pharmacol, 25: 569-575.
Niven CF Jr (1971) Thermal destruction of Lannate in spinach
processing. Walnut Creek, California, Del Monte Corporation Research
Center (Unpublished report*).
Noda J (1984) [Determination of methomyl by using chemical ionization
mass fragmentography. A case report of methomyl poisoning and the
animal experiment of its poisoning.] Nippon Hoigaku Zasshi, 38: 71-82
(in Japanese).
Ökolimna (1980) [Fish toxicity, carps, Lannate 25 WP.] Burgwedel,
Germany, Ökolimna (Unpublished report*) (in German).
Orpin R (1971) Methomyl - Study of effects on wild life. Glaston,
United Kingdom, Farm Protection Ltd (Unpublished report*).
Osman AZ, Hazza A, Nagwa I, & Awad TM (1983) Fate and metabolism of
the insecticide 14C-Lannate in farm animals. Isot Rad Res,
15(2): 111-120.
Oswald T, Adams J & Hicks SC (1991), LannateR insecticide -
Dislodgeable foliar residue study in Rose Greenhouse operations.
Fresno, California, Siemer and Associates Inc. (Unpublished report
No. AMR-1909-90*).
Owens CB, Owens EW, & Zahn D (1978) The extent of exposure of migrant
workers to pesticide and pesticide residues (Abstract). Int J
Chronobil, 5(2): 428-429.
Palmer JS & Schlinke JC (1978) Preliminary toxicological evaluations
of six pesticide compounds in cattle, sheep and chickens (Unpublished
report METH/TOX5*).
Panepinto AS (1991a) Acute inhalation toxicity study with
DPX-X1179-427 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-678-91*).
Panepinto AS (1991b) Acute inhalation toxicity study with
DPX-X1179-424 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-560-91*).
Paynter OE (1966) Three-month dietary administration - Rats.
Insecticide 1179. Falls Church, Virginia, Hazleton Laboratories Inc.
(Unpublished report*).
Pease HL (1968) Methomyl residue analysis - Soils. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Pease HL & Kirkland JJ (1968) Determination of methomyl residues using
microcolourmetric gas chromatography. J Agric Food Chem, 16: 554-557.
Peeples JL (1977) Effect of methomyl on soil microorganisms
(Unpublished report No. ML/ME21*).
Powley CR (1989) Methomyl foliar dislodgeable residues on grapes in
California. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. BMP/DFR-189*).
Powley CR (1990a) LannateR insecticide: Dislodgeable foliar residue
study in grape girdling and harvesting operations. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1445-89*).
Powley CR (1990b) LannateR insecticide: Dislodgeable foliar residue
study on grapes grown in California. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-1515-89*).
Powley CR (1991) Determination of possible bioaccumulation of
14C-methomyl in lactating dairy cows. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-898-87*).
Priester TM (1984) Batch equilibrium (adsorption/desorption) and soil
thin-layer chromatography studies with methomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. AMR-174-84*).
Proctor NH, Moscioni AD, & Casida JE (1976) Chicken embryo NAD levels
lowered by teratogenic organophosphorus and methylcarbamate
insecticides. Biochem Pharmacol, 25: 757-762.
Quarles JM, Sega MW, Schenley CK, & Lijinsky W (1979) Transformation
of hamster fetal cells by nitrosated pesticides in a transplacental
assay. Cancer Res, 39: 4525-4533.
Radwan MA, El-Wakil HB, & Osman KA (1992) Toxicity and biochemical
impact of certain oxime carbamate pesticides against terrestrial
snail, Theba psiana (Müller). J Environ Sci Health, B27: 759-773.
Reeve MW, O'Connell LP, Bissell S, & Ross J (1992) Characterization of
methomyl dissipation on grape foliage. Bull Environ Contam Toxicol,
49: 105-111.
Rogers AS, Culik R, Kaplan AM, & Aftosmis JG (1978) Oral teratogenic
study in rats with Lannate (INX-1179). Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-498-78*).
Ruppel RF & Laughlin CW (1977) Toxicity of some soil pesticides to
earthworms. J Kansas Entomol Soc, 50: 113-118.
Saiyed HN, Sadhu HG, Bhatnagar VK, Dewan A, Venkaiah K, & Kashyap SK
(1992) Cardiac toxicity following short-term exposure to methomyl in
spraymen and rabbits. Hum Exp Toxicol, 11(2): 93-97.
Saleh F (1990a) Metabolic effects of the carbamate insecticide
(methomyl) on rats. I. Levels of glucose, lipids and cholesterol in
serum after administration of the insecticide. Egypt J Physiol Sci,
14(1-2): 45-54.
Saleh F (1990b) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in serum cholinesterase and
transaminases following treatment of the insecticide. Egypt J Physiol
Sci, 14(1-2): 55-64.
Saleh F (1990c) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in some blood biochemical indices in
the rats poisoned with the insecticide. Egypt J Physiol Sci,
14(1-2): 65-74.
Sarver JW (1991a) Acute oral toxicity study with DPX-X1179-394 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-661-91*).
Sarver JW (1991b) Acute dermal toxicity study of DPX-X1179-394 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-455-91*).
Sarver JW (1991c) Acute oral toxicity study with DPX-X1179-424 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-171-91*).
Sarver JW (1991d) Primary dermal irritation study with DPX-X1179-394
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-165-91*).
Sarver JW (1991e) Primary dermal irritation study with DPX-X1179-425
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-166-91*).
Sarver JW (1991f) Primary dermal irritation study with DPX-X1179-424
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-162-91*).
Sarver JW (1991g) Primary eye irritation study with DPX-X1179-425 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-280-91*).
Sarver JW (1991h) Primary eye irritation study with DPX-X1179-424 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-279-91*).
Sarver JW (1992a) Acute oral toxicity study with DPX-X1179-439 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-858-91*).
Sarver JW (1992b) Acute dermal toxicity study of DPX-X1179-439 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-825-91*).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Serota DG, Machotka SV, Hastings TF, Alsaker RD, & Lane Fezio W (1981)
104-Week chronic toxicity and carcinogenicity study in mice. Methomyl;
H-11,135. Vienna, Virginia, Hazleton Laboratories America Inc.
(Unpublished report No. HLO-253-81*).
Shah PV, Monroe RJ, & Guthrie FE (1981) Comparative rates of dermal
penetration of insecticides in mice. Toxicol Appl Pharmacol,
59: 414-423.
Sherman H (1966) Acute oral LD50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-210-66*).
Sherman H (1967) Acute oral potentiation studies with S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate [INX-1179). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-160-67*).
Sherman H (1968a) Antidote studies with rats. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-251-68*).
Sherman H (1968b) Acute oral and antidote tests with guinea pigs.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-252-68*).
Sherman H (1968c) Acute oral toxicity and antidote tests in rabbits
using technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-250-68*).
Sherman H (1968d) Acute oral toxicity and antidote tests in dogs using
technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-279-68*).
Sherman H (1972) Chicken & egg study. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-55-72*).
Silveira EJ (1990) Technical methomyl - physical and chemical
characteristics. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products Department (Unpublished report
No. AMR-1753-90*).
Simpson GR & Bermingham S (1977) Poisoning by carbamate pesticides.
Med J Aust, 2: 148-149.
Singles GH (1970a) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-186-70*).
Singles GH (1970b) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-327-70*).
Sleight BH (1971) Continuous exposure of rainbow trout (Salmo
gairdneri) to LannateR in water. Wareham, Massachusetts, Bionomics
Inc. (Unpublished report*).
Sleight BH (1973) Acute toxicity of H-7946 to grass shrimp
(Palaemonetes vulgaris), pink shrimp (Penaeus duorarum) and mud
crab (Neopanope texana). Wareham, Massachusetts, Bionomics Inc.
(Unpublished report No. HLO-186-73*).
Smelt JH, Dekker A, Leistra M, & Houx NWH (1983) Conversion of four
carbamoyloximes in soil samples from above and below the soil water
table. Pestic Sci, 14: 173-181.
Smith LW (1982) Wildlife toxicity studies with methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. ML/FW20*
SRI International (1988) Pesticides and intermediates, Supplement C.
Menlo Park, California, SRI International (Report No. 171C).
Stute K (1983) Results of the registration trials on bee toxicity -
1983. Celle, Germany, Du Pont de Nemours (Germany) GmbH (Unpublished
report No. METH/ECO14*).
Swanson MB (1986) Photodegradation of [1-14C] methomyl in soil.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products Department (Unpublished report No. AMR-611-86*).
Ta'Naka I, Igisu H, Haratake J, Cho S, Mori K, Fujishiro K, Inoue N,
Horie A, & Akiyama T (1987) Cumulative toxicity potential of methomyl
aerosol by repeated inhalation. Am Ind Hyg Assoc J, 48(4): 330-334.
Teeters WR (1968) Lannate antidotal study - monkeys. Falls Church,
Virginia, Hazleton Laboratories Inc. (Unpublished report
No. 201-219*).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a fungicide
on the numbers and biomass of earthworms in pasture. Bull Environ
Contam Toxicol, 12(4): 487-492.
Trivits R (1979) Acute oral LD50 test in rats using technical
methomyl. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-496-79*).
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 74-75 (Resource Publication No. 84).
USDI (1975) Acute toxicity studies. Cutthroat trout and stonefly
larvae. Columbia, Missouri, US Department of the Interior, Fish and
Wildlife Service, Columbia National Fishery Research Laboratory
(Unpublished report No. ML/FW-1*).
USDI (1978) Methomyl - Summary of acute toxicity. Columbia, Missouri,
US Department of the Interior, Fish and Wildlife Service, Columbia
National Fishery Research Laboratory (Unpublished report
No. ML/FW-3*).
USEPA (1988) Methomyl science chapters - June 1988: Parts I and II.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances.
USEPA (1991) Pesticides in ground water database. Washington, DC, US
Environmental Protection Agency, Pesticides and Toxic Substances.
US FDA (1993a) Food and Drug Administration total diet study: 20
market basket studies, October 1986 - April 1991: Findings of methomyl
in food collected for total diet study. Washington, DC, US Food and
Drug Administration.
US FDA (1993b) FDA-Regulatory monitoring data: FY 1988-1992 - Findings
of methomyl in samples of domestic and imported foods. Washington, DC,
US Food and Drug Administration.
US FDA (1993c) FDA-Regulatory monitoring data: FY 1988-1992 - Counts
of samples of domestic and imported foods and feeds analyzed by
methodology known to be capable of determining methomyl. Washington,
DC, US Food and Drug Administration.
Vincent DR (1985) Assessment of methomyl (INX-1179-255) in the
in vitro unscheduled DNA synthesis assay in primary rat hepatocytes.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-149-85*).
Ward TJ & Boeri RL (1989) Static acute toxicity of methomyl to the
mysid, Mysidopsis bahia. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-675-89*).
Ward TJ & Boeri RL (1990) Static acute toxicity of methomyl to bivalve
mollusc embryos and larvae. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-422-90*).
Ward TJ & Boeri RL (1991) Acute flow-through mollusc shell deposition
test with DPX-X1179-394 (methomyl). Hampton, New Hampshire, Resource
Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-710-91*).
Ware GW, Estesen B, & Cahill WP (1978) Dislodgeable insecticide
residues on cotton (1975). Bull Environ Contam Toxicol, 20: 17-19.
Ware GW, Estesen BJ, & Buck NA (1980) Dislodgeable insecticide
residues on cotton foliage: Acephate, AC222705, EPN, fenvalerate,
methomyl, methyl parathion, permethrin and thiodicarb. Bull Environ
Contam Toxicol, 25: 608-615.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. In: Fleck RA & Hollaender A ed. Genetic toxicology: An
agricultural perspective. New York, London, Plenum Press, pp 275-324.
Williams DT, Denley HV, Lane DA, & Quan ESK (1982) Real time
monitoring of methomyl air levels during and after spraying in a
greenhouse. Am Ind Hyg Assoc J, 43: 190-195.
Wojciechowski JP, Kaur P, & Sabharwal PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Yeboah PO & Kilgore WW (1984) Analysis of airborne pesticides in a
commercial pesticide storage building. Bull Environ Contam Toxicol,
32: 629-634.
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Jpn Bull Agric Chem Insp Stn, 12: 122-128.
Zwick TC & Malik N (1990a) Aerobic metabolism of [1-14C] methomyl in
Madera, California soil. Columbus, Ohio, Battelle Memorial Institute,
Environmental Sciences Department (Unpublished report
No. AMR-1543-89*).
Zwick TC & Malik N (1990b) Anaerobic metabolism of [1-14C] methomyl
in Madera, California soil. Columbus, Ohio, Battelle Memorial
Institute, Environmental Sciences Department (Unpublished report
No. AMR-1544-89*).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le méthomyl est un solide cristallin blanc dont le point de
fusion est de 77°C et la tension de vapeur de 0,72 mPa à 25°C. Sa
solubilité dans l'eau est de 54,7 g/litre et son coefficient de
partage octanol/eau (Kow) est de 1,24. Il est stable dans l'eau
stérile à pH 7 mais il se décompose à pH plus élevé, sa demi-vie étant
de 30 jours à pH 9 et 25°C.
Le dosage du méthomyl dans divers échantillons consiste en une
extraction suivie d'une purification, l'analyse finale s'effectuant
par chromatographie en phase liquide à haute performance ou
chromatographie gaz/liquide. Dans certains cas, avant le dosage
proprement dit, on transforme le méthomyl en oxime ou en fluorophore
(post-colonne).
2. Sources d'exposition humaine et environnementale
Le méthomyl est préparé en faisant réagir le
N-hydroxythioacétimidate de S-méthyle (MHTA) en solution dans le
chlorure de méthylène, sur l'isocyanate de méthyle gazeux à 30-50°C.
Il s'agit d'un carbamate à propriété insecticide que l'on utilise
partout dans le monde sur toutes sortes de cultures. Il sert
notamment à protéger les fruits, les vignes, le houblon, les légumes,
les céréales, le soja, le coton et les plantes ornementales. A
l'intérieur des bâtiments, on l'utilise aussi pour détruire les
mouches dans les animaleries et les laiteries.
Il est principalement présenté sous forme de poudre hydro-soluble
ou encore de liquide miscible à l'eau que l'on dilue pour le
traitement des cultures au sol ou en pulvérisations aériennes. Les
doses d'emploi vont habituellement de 0,15 à 1,0 kg de matière active
par hectare. C'est essentiellement au cours de la préparation et de
l'épandage de ces produits ou par suite de l'ingestion de résidus
subsistant sur des cultures vivrières qu'il peut y avoir exposition
humaine (voir section 5.3.1.4).
3. Transport, distribution et transformation dans l'environnement
Les études de laboratoire montrent que le méthomyl est faiblement
adsorbé aux particules du sol. On a ainsi montré que l'adsorption
était faible sur les minéraux argileux en particulier l'illite; sur
les matières organiques du sol, elle est multipliée par 50 tout en
restant encore relativement faible. Une fois adsorbés, les résidus ne
se désorbent pratiquement pas. Du fait de ses propriétés, on peut
penser que le méthomyl doit être doté d'une certaine mobilité dans le
sol.
Dans l'environnement naturel, la décomposition abiotique du
méthomyl par hydrolyse ou photolyse est lente ou nulle.
Dans le sol, la décomposition aérobie est environ deux fois plus
rapide que la décomposition anaérobie. On a fait état de valeurs de
la demi-vie dans le sol qui vont de quelques jours à plus de 50 jours;
la sécheresse retarde la décomposition. Dans la pratique, on peut
considérer que dans la plupart des cas, on aura après épandage, une
demi-vie d'environ une semaine.
Dans les conditions de plein champ, le méthomyl ne pénètre pas
dans le sol, par lessivage, à une profondeur de plus de 20-30 cm et il
n'y a pas de contamination des eaux souterraines.
Lorsqu'on épand du méthomyl marqué au 14C sur les feuilles de
végétaux, il y absorption, mais le produit n'est pas transporté dans
d'autres parties de la plante. Lorsqu'on l'applique sur le système
radiculaire, le composé est fixé par la plante, le principal résidu
étant le méthomyl lui-même. Les produits de décomposition volatils du
méthomyl sont le CO2 et l'acétonitrile. Le reste de la fraction
radioactive se retrouve dans les constituants naturels de la plante
tels que les lipides ainsi que les acides et les sucres du cycle de
Krebs. Dans le feuillage, la demi-vie du méthomyl est de l'ordre de
quelques jours.
Après exposition de truites arc-en-ciel à ce composé pendant 28
jours dans un système à courant d'eau, on n'a trouvé aucun signe
d'accumulation.
4. Concentrations dans l'environnement et exposition humaine
Si l'on en croit les analyses effectuées sur diverses sources
d'eau après épandage du composé aux doses recommandées, il est
probable que les concentrations de méthomyl dans les eaux souterraines
sont soit très faibles, soit inférieures à la limite de détection
(< 0,02 mg/litre).
De faibles résidus de méthomyl se retrouvent sur les cultures,
notamment vivrières, lors de la récolte, les quantités dépendant de
facteurs tels que la dose d'emploi, le laps de temps écoulé depuis le
dernier épandage et le type de culture. Ces résidus sont
essentiellement constitués du produit initial.
Dans les produits laitiers, les résidus de méthomyl sont soit
inférieurs à la limite de détection, soit très faibles. Après
administration de méthomyl à des vaches laitières sous forme de
capsules, pendant 28 jours à une dose équivalant à 80 mg/kg de
nourriture, on n'a pas retrouvé de résidus décelables de ce composé ou
de son métabolite (le MHTA) dans la lait ou les tissus de ces animaux
(< 0,02 mg/kg). Aucune trace de méthomyl n'a été non plus décelée
dans les oeufs ou dans les tissus de poules pondeuses qui en avaient
reçu 1 ou 10 mg/kg dans leur nourriture pendant quatre semaines.
Des analyses effectuées par sondage aux Etats-Unis sur la ration
totale ou sur certains types d'aliments, ont montré que la
concentration de méthomyl était soit très faible, soit inférieure à la
limite de détection. Par ailleurs, un certain nombre d'opérations
tels que le lavage, l'épluchage ou la cuisson, réduisent encore la
teneur en résidus.
Des études effectuées lors du retour des ouvriers agricoles sur
les vignobles après épandage, notamment dans les conditions du désert
de Californie, ont montré que pour un résidu foliaire mobile tombé à
0,1 µg/cm2, l'exposition était maximale au niveau du torse et de la
tête lorsque les ouvriers agricoles pratiquaient l'incision annulaire,
le torse et les mains étant les plus touchés lors de la cueillette du
raisin. En ce qui concerne le raisin de table, c'est lors de la
cueillette et de l'emballage que l'exposition était la plus faible.
L'exposition par inhalation était minime.
Après pulvérisation de méthomyl sur des plants de concombres et
de tomates, on a mesuré dans l'air de la serre qui les abritait, des
concentrations d'insecticide allant jusqu'à 4,7 µg/m3 dans la
journée qui a suivi l'épandage. Trois, puis sept jours après ce
traitement, les concentrations de méthomyl dans la zone de
respiration allaient respectivement jusqu'à 14,5 ou 0,7 µg/m3. Dans
l'eau de lavage des mains des jardiniers d'une serre, on a retrouvé
des quantités de méthomyl allant de 10 à 300 µg/heure de travail.
Cela montre que l'exposition cutanée est une voie d'exposition plus
importante que l'inhalation et que la durée de l'attente à observer
avant de retourner sur les lieux doit être basée sur les données
d'exposition cutanée.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après administration à des rats par voie orale, le méthomyl est
rapidement absorbé, métabolisé et excrété, l'ensemble du processus
étant achevé en quelques jours. Une semaine après avoir administré à
des rats du méthomyl radio-marqué à raison de 5 mg/kg de poids
corporel, on a constaté que 54% de la dose était excrétés dans
l'urine, 2 à 3% dans les matières fécales et 34% dans l'air expiré (en
l'espace de cinq jours). Au bout de sept jours, il restait dans les
tissus et la carcasse 8 à 9% de la radioactivité, le carbone-14 étant
incorporé dans les constituants endogènes. C'est dans le sang que la
fraction radioactive était la plus importante (2% de la dose).
Chez ces rats, les principaux métabolites présents dans l'air
expiré étaient du dioxyde de carbone et l'acétonitrile dans le rapport
d'environ 2:1. Dans les urines, le principal métabolite était
constitué par un dérivé mercapturique du méthomyl, à hauteur de 17% de
la dose. On n'a pas décelé la présence de méthomyl ni de son oxime.
On pense que la métabolisation du méthomyl s'effectue selon le
schéma suivant: déplacement du groupe S-méthyle par le glutathion,
puis transformation enzymatique en dérivé de l'acide mercapturique.
Il existe une autre voie métabolique, à savoir l'hydrolyse en MHTA qui
est ensuite rapidement décomposé pour donner du dioxyde de carbone. Il
pourrait y avoir aussi conversion du syn-méthomyl (qui est la forme
insecticide) en isomère anti, lequel serait ensuite décomposé en
acétonitrile par des réactions d'hydrolyse, de transposition et
d'élimination. Chez le singe, le métabolisme est analogue, à cela
près que le dérivé de l'acide mercapturique ne constitue qu'un
métabolite urinaire mineur.
Une heure après application cutanée à des souris d'une solution
acétonique de méthomyl marqué au 14C, on a constaté que le taux de
pénétration était de l'ordre de 85%. A ce moment, 3% de la dose
étaient présents dans le sang, 5% dans le foie et 13% avaient été
excrétés. Au bout de huit heures, l'excrétion totale était de 54,5%.
La décomposition et l'élimination rapides du méthomyl chez le rat
ainsi que son absence d'accumulation tissulaire, est comparable à ce
que l'on observe chez les ruminants.
Du méthomyl administré à des vaches et à des chèvres a subi une
dégradation totale. Ni le composé initial, ni son oxime n'ont été
retrouvés dans le lait ou les tissus. On a montré que le méthomyl
était métabolisé et incorporé dans les constituants naturels du lait
et du foie.
Après avoir fait incuber du méthomyl marqué au 14C avec du
nitrite de sodium dans un macérat de viande séchée, dans des
conditions simulant le milieu gastrique, on n'a pas mis en évidence la
formation de nitrosométhomyl.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
Le méthomyl présente une forte toxicité aiguë par voie orale, sa
DL50 chez le rat allant de 17 à 45 mg/kg de poids corporel. Il
présente également une forte toxicité inhalatoire chez cet animal, la
CL50 à 4 heures étant de 0,26 mg/litre lorsque le composé est inhalé
sous forme d'aérosol. La toxicité cutanée est très faible, la DL50
dépassant 2000 mg/kg de poids corporel chez le lapin (peau intacte) et
1000 mg/kg de poids corporel chez le rat (peau abrasée). Les signes
d'intoxication aiguë sont ceux que l'on peut attendre d'un inhibiteur
de la cholinestérase et consistent, entre autres, en une
hypersalivation, une lacrimation, des tremblements et un myosis. On a
constaté que les animaux récupéraient rapidement et l'examen de leurs
organes n'a pas révélé la présence de lésions anatomopathologies
visibles qui soient imputables au traitement. Le méthomyl ne provoque
pas d'irritation ou d'hypersensibilisation cutanée et il est
légèrement irritant pour la muqueuse oculaire.
L'administration répétée de méthomyl dans la nourriture pendant
des périodes prolongées n'a pas conduit à une accumulation ou à un
accroissement des effets toxiques. Des rats et des chiens à qui on
avait administré une nourriture contenant jusqu'à 250 mg/kg et
400 mg/kg respectivement de méthomyl, pendant 13 semaines, n'ont
présenté ni signes d'intoxication ni surmortalité. Chez des rats qui
en avaient reçu dans leur nourriture à la dose de 250 mg/kg, on a
observé une légère diminution du gain de poids, une réduction du taux
d'hémoglobine et une hyperplasie modérée de la lignée érythroblastique
au niveau de la moelle osseuse. Chez le rat, la dose sans effets
observables était de 50 mg/kg de nourriture (soit l'équivalent d'une
dose quotidienne de 3,6 mg/kg de poids corporel). Des lapins, qui
avaient subi des applications cutanées répétées de méthomyl à des
doses quotidiennes allant jusqu'à 500 mg/kg de poids corporel, pendant
21 jours, ont présenté une hyperactivité ainsi qu'une réduction de
l'activité cholinestérasique plasmatique et cérébrale à la dose la
plus élevée. La dose sans effets nocifs observables était égale,
selon cette étude, à 50 mg/kg de poids corporel par jour.
Au cours d'études à long terme, des rats ont reçu du méthomyl
dans leur nourriture à raison de 0, 50, 100 ou 400 mg/kg et des souris
en ont reçu de la même manière, 0, 50, 75 ou 200 mg/kg. Chez les
rats, les effets observés à la dose la plus élevée consistaient en une
réduction du gain de poids ainsi qu'en une diminution du taux
d'hémoglobine et de la valeur de l'hématocrite. La dose sans effets
observables était de 100 mg/kg de nourriture, soit l'équivalent d'une
dose quotidienne de 5 mg/kg de poids corporel. Chez les souris, on a
constaté aux deux doses les plus élevées, une augmentation du taux de
mortalité ainsi qu'une diminution du taux d'hémoglobine et du nombre
de globules rouges. La dose sans effets observables a été évaluée à
50 mg/kg de nourriture, soit l'équivalent d'une dose quotidienne de
8,7 mg/kg de poids corporel. Lors d'une étude toxicologique de deux
ans chez des chiens (doses de 0, 50, 100, 400 ou 1000 mg/kg de
nourriture), on a observé des signes d'intoxication chez certains
animaux à la dose la plus forte, avec une anémie légère à modérée. La
dose sans effets observables était de 100 mg/kg de nourriture, soit
l'équivalent d'une dose quotidienne de 3 mg/kg de poids corporel.
Des études de deux ans sur des rats et des souris n'ont révélé
aucun signe d'accroissement de l'incidence des tumeurs qui soient
imputables à l'administration de méthomyl, ce qui indique que le
méthomyl n'est pas cancérogène. Il se s'est pas révélé non plus
génotoxique lors d'épreuves in vitro sur des cellules bactériennes
ou mammaliennes et la recherche de lésions primaires de l'ADN sur des
cellules bactériennes ou mammaliennes in vitro ou encore
d'aberrations chromosomiques médullaires lors d'épreuves in vivo
chez le rat, a donné des résultats négatifs. On a cependant observé
la possibilité d'effets cytogénétiques sur des lymphocytes humains
in vivo, attestée par l'augmentation du nombre de micronoyaux et
d'aberrations chromosomiques. Chez des rats et des lapins qui en
avaient reçu par gavage des doses allant jusqu'à 400 mg/kg de
nourriture (soit une dose quotidienne de 16 mg/kg de poids corporel),
le méthomyl n'a pas produit non plus d'effets embryotoxiques ou
tératogènes, alors qu'à ces doses, des effets toxiques pouvaient être
constatés chez les femelles gravides. Lors d'une étude de
reproduction portant sur trois générations de rats, qui avaient reçu
50 ou 100 mg de méthomyl/kg de nourriture (soit l'équivalent quotidien
de 50 ou 10 mg/kg de poids corporel), on n'a pas constaté d'effets sur
la fécondité, la gestation ou la lactation qui soient imputables au
méthomyl et aucune anomalie visible résultant de ce traitement n'a été
observée.
Après administration unique ou répétée de méthomyl, on n'a pas
observé de neurotoxicité retardée. Des rats qui en avaient reçu dans
leur nourriture à raison de 800 mg/kg, n'ont présenté une diminution
sensible de leur activité cholinestérasique sanguine qu'au début d'une
étude de cinq mois. Une étude d'alimentation de 28 jours n'a révélé
qu'une légère diminution de l'activité cholinestérasique cérébrale à
cette dose. Il y a donc eu réversibilité rapide de l'effet
anticholinestérasique du méthomyl chez les animaux au cours de la
période d'expérimentation. In vitro, l'activité cholinestérasique
érythrocytaire humaine s'est révélée six fois plus sensible à l'action
inhibitrice du méthomyl que celle du rat, mais la réactivation
spontanée s'est produite sensiblement à la même vitesse.
Des études menées sur diverses espèces ont montré que l'atropine
est le meilleur antidote d'une intoxication par le méthomyl.
7. Effets sur l'homme
La lecture des rapports sur les intoxications accidentelles et
les suicides par empoisonnement avec du méthomyl éclaire quelque peu
sur la gravité des effets et les possibilités de récupération. Sur
cinq victimes d'une intoxication accidentelle due à la consommation
d'aliments contaminés, trois sont décédées dans les trois heures
suivant le repas. On estime que les victimes avaient ingéré environ
12 à 15 mg de méthomyl/kg de poids corporel. Une femme âgée de 31 ans
et son fils de six ans, qui étaient tous les deux décédés par suite
d'un empoisonnement volontaire, présentaient des concentrations
hépatiques de méthomyl respectivement égales à 15,4 et 56,5 mg/kg.
Les doses ont été estimées à 55 mg/kg de poids corporel pour la mère
et 13 mg/kg de poids corporel pour l'enfant. Chez une femme qui avait
ingéré environ 2,25 g de méthomyl, on a retrouvé dans son sang six
heures plus tard une quantité de méthomyl égale à 1,6 mg/kg.
Vingt-deux heures après l'ingestion, alors que la patiente se
remettait, on n'a plus retrouvé de méthomyl.
Un travailleur chargé d'épandre des pesticides, qui n'avait pas
pris la moindre précaution alors qu'il préparait une bouillie à base
de méthomyl en poudre pour traiter des légumes, a présenté des
symptômes d'intoxication au bout d'une heure avec réduction à 40% de
la normale de son activité cholinestérasique sanguine au bout de 12
heures, la récupération à 80% de la normale intervenant 36 heures plus
tard. D'autres travailleurs, qui avaient pris les précautions
d'usage, n'ont présenté aucun symptôme ou effet sur l'activité de leur
cholinestérase érythrocytaire ou plasmatique lors de l'épandage de
méthomyl par voie aérienne.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
Le méthomyl n'a eu aucun effet sur des populations de champignons
ou de bactéries terricoles, et en particulier sur leur action
nitrifiante ou sur l'activité de la déshydrogénase, lorsqu'il était
appliqué aux doses recommandées.
Des études en laboratoire ont permis de fixer à 6,5 mg/litre la
concentration sans effets observables sur la croissance des algues.
Le méthomyl est moyennement à fortement toxique pour les
poissons, les valeurs de CL50 à 96 heures se situant, pour diverses
espèces, dans l'intervalle 0,5-2 mg/litre. Une étude à long terme (21
jours) a montré que la CL50 pour des alevins de truite exposés à du
Lannate 20L (21,5% de méthomyl), était égale à 1,3 mg de méthomyl par
litre. Lors d'une étude toxicologique portant sur de jeunes
cyprinidés de l'espèce Pimephales promelas, la MATC (concentration
maximale acceptable de substance toxique) a été trouvée comprise entre
57 et 177 µg/litre.
Des études de toxicité aiguë portant sur d'autres organismes
aquatiques ont montré que Daphnia magna était l'espèce la plus
sensible au méthomyl, la CL50 à 48 heures étant de 0,032 mg/litre.
Lors d'une étude de 21 jours au cours de laquelle on a étudié la
survie, la croissance et la capacité de reproduction de Daphnia magna,
on a constaté que la concentration maximale acceptable de substance
toxique (MATC) pour le méthomyl était comprise entre 1,6 et
3,5 µg/litre.
Le méthomyl est toxique pour les abeilles, la DL50 par contact
étant de 1,29 µg/insecte et la DL50 par voie orale, de
0,2 µg/insecte.
On a évalué la toxicité aiguë du méthomyl chez plusieurs espèces
d'oiseaux, les valeurs caractéristiques de DL50 aiguë par voie orale
se situant à 10 mg/kg de poids corporel chez les pigeons et à 34 mg/kg
de poids corporel chez la caille japonaise. Il est relativement moins
toxique lorsqu'il est mêlé à la nourriture, la CL50 à 8 jours étant
dans ce cas de 1100 mg/kg de méthomyl (mêlé à la nourriture) pour le
colin de Virginie et de 2883 mg de méthomyl/kg de nourriture pour le
colvert. Lors d'études qui ont duré de 18 à 20 semaines et ont porté
sur une génération, on a évalué à 150 mg/kg de méthomyl dans la
nourriture, la concentration sans effets nocifs observables pour le
colin de Virginie et le colvert.
Aucun effet n'a été observé chez des colins de Virginie qui
avaient été exposés à une série de pulvérisations de méthomyl aux
doses recommandées. Après un épandage de méthomyl sur une zone
forestière et des champs de houblon, aux doses recommandées, on a
effectué deux études sur des populations aviaires sauvages. Elles
n'ont pas révélé de modification de l'activité des oiseaux et n'ont
provoqué aucun effet, en particulier aucun effet létal. Par rapport
aux témoins, on a constaté qu'il y avait réduction des dépôts
graisseux chez les oiseaux chanteurs des forêts traitées; on estime
qu'il s'agit là d'un effet indirect dû à la réduction des populations
d'insectes dont se nourrissent ces oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Le méthomyl est un carbamate qui inhibe la cholinestérase et dont
le mode d'action toxique est bien connu. Il présente une toxicité
particulièrement élevée lorsqu'il est absorbé par voie orale ou par
inhalation, ainsi que le montrent les études sur l'animal, mais sa
toxicité par voie cutanée est faible. Chez l'animal, les signes
d'intoxication aiguë sont caractéristiques d'une inhibition de la
cholinestérase. Cette intoxication est rapidement réversible, avec
une disparition rapide des symptômes et une désinhibition également
rapide des cholinestérases sanguine et cérébrale. Cette prompte
récupération est due au fait que l'inhibition par le méthomyl de la
cholinestérase est rapidement réversible, et d'ailleurs facilitée par
la vitesse d'excrétion élevée de ce composé. Ce que l'on sait des
intoxications humaines accidentelles ou volontaires montre que la
toxicité aiguë du méthomyl est du même ordre chez l'homme que chez
l'animal de laboratoire.
Comme l'action du méthomyl est rapidement réversible pendant la
période d'administration, il a rarement été possible d'observer des
signes d'intoxication aiguë et d'inhibition de la cholinestérase
sanguine au cours des études où on l'administrait mêlé à la
nourriture. Les observations les plus régulièrement rapportées à
l'occasion d'études à long terme consistent, aux doses les plus
élevées dans l'alimentation, en une réduction du gain de poids chez
les rongeurs et une diminution des paramètres érythrocytaires chez les
rongeurs et les chiens. Trois études de longue durée chez des
rongeurs n'ont pas permis de mettre en évidence d'activité
cancérogène. Le composé ne s'est pas non plus montré génotoxique lors
d'épreuves in vitro portant sur divers paramètres biotoxicologiques,
néanmoins il y a une possibilité d'effets cytogénétiques sur les
lymphocytes humains. Une étude chromosomique effectuée in vivo sur
la moelle osseuse de rats s'est également révélée négative.
Chacune des études à long terme sur l'animal a permis, à partir
de la réduction du gain de poids et des paramètres érythrocytaires,
d'obtenir une valeur de la dose sans effets nocifs observables. Elle
se situait à 5 mg/kg de poids corporel par jour chez le rat, à
8,7 mg/kg de poids corporel par jour chez la souris et à 3 mg/kg de
poids corporel par jour chez le chien. Faute d'une différenciation
marquée des effets toxiques entre les différentes espèces, il ressort
de ces études que la dose sans effets nocifs observables chez le
chien, c'est-à-dire 3 mg/kg de poids corporel par jour, peut être
utilisée pour évaluer le risque chez l'homme.
Le méthomyl est faiblement à modérément adsorbé aux particules du
sol, et ne s'en désorbe pratiquement pas. Dans le sol, il se
décompose en aérobiose (avec une demi-vie d'environ une semaine)
environ deux fois plus vite qu'en anaérobiose.
Une fois déposé sur les feuilles de végétaux, le méthomyl est
rapidement absorbé à hauteur de 50% de la dose - les 50% restant étant
adsorbés - et rien n'indique qu'il y ait transport à l'intérieur de la
plante. La concentration du méthomyl absorbé par les cultures
vivrières tombe rapidement à environ 5% de sa valeur initiale en
l'espace d'une semaine.
Plusieurs invertébrés aquatiques, et en particulier les daphnies,
sont très sensibles au méthomyl avec des valeurs de la CL50 de
l'ordre de 10 à 100 µg/litre.
Les poissons, qu'il s'agisse d'espèces d'eau douce ou d'espèces
estuarielles, y sont moins sensibles, puisque les valeurs de la CL50
s'étagent entre 0,5 et 7 mg/litre. Etant donné la faible persistance
du méthomyl et sa toxicité aiguë relativement faible pour les
poissons, le risque encouru par ces derniers est relativement faible.
Aux doses d'emploi recommandées, le méthomyl n'a pas d'effets
nocifs sur l'activité microbienne terricole en climat tempéré.
Le méthomyl est classé comme hautement toxique pour les abeilles
avec une DL50 topique d'environ 0,1 µg/insecte.
Les valeurs de la DL50 aiguë par voie orale varient entre 10 et
40 mg/kg de poids corporel chez diverses espèces d'oiseaux. Les
valeurs de CL50 à cinq jours par voie alimentaire vont de 1100 à
3700 mg/kg de nourriture. Il y a risque d'intoxication aiguë pour les
oiseaux, en particulier lorsque le méthomyl est sous forme de
granulés; son absorption à partir de nourriture contaminée ne devrait
cependant pas être mortelle pour les oiseaux.
La forte toxicité aiguë du méthomyl pour les mammifères de
laboratoire permet de conclure qu'il est tout aussi dangereux pour les
mammifères sauvages.
10. Conclusion
Compte tenu des aspects qualitatifs et quantitatifs de la
toxicité du méthomyl, le groupe de travail a conclu qu'une dose
quotidienne de 0,03 mg/kg de poids corporel ne devrait probablement
pas causer d'effets nocifs chez l'homme, quel que soit le mode
d'exposition.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El metomilo es un sólido cristalino blanco con un punto de fusión
de 77°C y una presión de vapor de 0,72 mPa (25°C). Tiene una
solubilidad en agua de 54,7 g/litro y su coeficiente de reparto
octanol/agua (Kow) es de 1,24. Es estable en agua estéril a pH 7,
pero se descompone a pH más elevado, con una semivida de 30 días a pH
9 y 25°C.
El procedimiento analítico para la determinación del metomilo en
muestras diferentes es la extracción seguida de limpieza y análisis
mediante cromatografía líquida de alto rendimiento o cromatografía
gas-líquido. En algunos casos, el metomilo se convierte en su
derivado oxima o en un derivado fluoróforo (después de la columna)
antes de su determinación analítica.
2. Fuentes de exposición humana y ambiental
El metomilo de produce haciendo reaccionar el S-metil- N-
hidroxitioacetimidato (MHTA) en cloruro de metileno con isocianato de
metilo gaseoso a 30-50°C. Es un insecticida de tipo carbamato
utilizado en una gran diversidad de cultivos en todo el mundo. Entre
los cultivos protegidos figuran frutales, vides, lúpulo, hortalizas,
cereales, soja, algodón y plantas ornamentales. En espacios cerrados
se utiliza en establos o vaquerías para luchar contra las moscas.
Las formaciones principales son polvos hidrosolubles y líquidos
hidromiscibles, que se diluyen en agua para el rociado superficial o
aéreo de los cultivos. Las proporciones normales del principio activo
son de 0,15-1,0 kg/ha. La exposición humana ocurre principalmente
durante la preparación y aplicación de estos productos y por ingestión
de residuos que quedan en los alimentos cosechados (véase la
sección 5.3.1.4).
3. Transporte, distribución y transformación en el medio ambiente
En estudios de laboratorio se ha observado que el metomilo se
adsorbe poco al suelo. Se ha demostrado una adsorción débil a los
minerales arcillosos, sobre todo la ilita; la adsorción a la materia
orgánica del suelo es 50 veces mayor, pero sigue siendo relativamente
escasa. Prácticamente no hay desorción del residuo adsorbido. Estas
características llevan a prever que el metomilo tendrá movilidad en el
suelo.
En condiciones medioambientales naturales, su degradación
abiótica por hidrólisis o fotólisis es lenta o no se produce.
La degradación aerobia en el suelo es alrededor de dos veces más
rápida que la anaerobia. Las semividas notificadas para el metomilo
en el suelo varían entre unos días y más de 50. Las condiciones secas
retrasan la degradación. En la práctica, la mayor parte de las
aplicaciones en el campo deberían dar lugar a una semivida de
alrededor de una semana.
En condiciones de campo, el metomilo no sufre lixiviación en el
suelo a profundidades superiores a 20-30 cm y no contamina las aguas
subterráneas.
El 14C-metomilo aplicado a las hojas de las plantas es
absorbido, pero no transportado a otras partes de la planta. Se si lo
aplica en el sistema radicular, la planta lo absorbe y el componente
principal del residuo que queda es el propio metomilo. Los productos
volátiles derivados de su descomposición son CO2 y acetonitrilo. El
resto de la actividad se incorpora a los componentes naturales de la
planta, tales como lípidos, ácidos del ciclo de Krebs y azúcares. La
semivida del metomilo en el follaje de la planta es de unos pocos
días.
No se han encontrado indicios de acumulación del metomilo en
truchas irisadas expuestas al compuesto durante 28 días en un sistema
de flujo continuo.
4. Niveles medioambientales y exposición humana
Los análisis de diversas fuentes de agua tras la aplicación de
las dosis recomendadas del compuesto indican que los niveles de
metomilo en las aguas subterráneas probablemente serán muy bajos o
inferiores al límite de detección (< 0,02 mg/litro).
En los cultivos de productos alimenticios y de otro tipo se
observan niveles bajos de residuos de metomilo en el momento de la
recolección; su concentración depende de diversos factores, como la
cantidad aplicada, el periodo transcurrido desde la última aplicación
y el tipo de cultivo. Los residuos están formados básicamente por
metomilo.
Los residuos de metomilo en los productos lácteos no son
detectables o son muy bajos. No se observaron residuos detectables de
metomilo ni del metabolito MHTA en la leche ni en los tejidos
(< 0,02 mg/kg) de vacas lactantes que habían recibido en el pienso
cápsulas de metomilo en una concentración equivalente a 80 mg/kg
durante 28 días. No se detectó la presencia de metomilo en los huevos
ni en los tejidos de gallinas ponedoras a las que se habían
administrado cantidades de 1 ó 10 mg/kg en los alimentos durante
cuatro semanas.
En los Estados Unidos de América se hicieron análisis de
regímenes completos de alimentación y de determinados alimentos; en
los estudios de muestreo correspondientes, las concentraciones de
metomilo resultaron inferiores al límite de detección o muy bajas.
Los niveles de residuos se reducen aún más por efecto de procesos
tales como el lavado, pelado y guisado de los alimentos.
En estudios sobre la exposición de quienes regresan a zonas
tratadas, en particular en las condiciones del desierto de California,
se ha observado que los trabajadores que volvieron a los viñedos
cuando los residuos foliares que podían desprenderse se habían
reducido a 0,1 µg/cm2, sufrieron la mayor exposición en la parte
superior del cuerpo y en la cabeza durante el atado de los racimos y
en la parte superior del cuerpo y en las manos durante la vendimia.
La recogida y el embalado de las uvas de mesa dieron lugar a la
exposición más baja. La exposición por inhalación fue mínima.
Tras haberse rociado con metomilo plantas de pepino y tomate, las
concentraciones en el aire del invernadero llegaban a 4,7 µg/m3 al
día siguiente del rociado. Tres y siete días después del rociado, las
concentraciones de metomilo en la zona de respiración ascendían a
14,5 y 0,7 µg/m3, respectivamente. Los valores del metomilo en el
agua de lavarse las manos oscilaban entre 10 y 322 µg por hora de
trabajo en un invernadero. Esto indicaba que la vía de exposición
cutánea era más importante que la inhalación y que los intervalos
previos al regreso al lugar tratado deberían basarse en los datos
sobre la exposición cutánea.
5. Cinética y metabolismo en animales de laboratorio
La absorción, el metabolismo y la excreción del metomilo tras la
administración oral a ratas es muy rápida, completándose el proceso en
unos pocos días. En un plazo de siete días después de la
administración a ratas de 5 mg/kg de peso corporal de metomilo
radiomarcado, el 54% se excretó en la orina, el 2-3% en las heces y el
34% en el aire expirado (en cinco días). Después de siete días, en
los tejidos y en el esqueleto quedaba el 8-9% de la dosis de 14C,
que se había incorporado a los constituyentes endógenos. La mayor
concentración de radiactividad se detectó en la sangre (equivalente al
2% de la dosis).
Los componentes metabólicos principales en el aire expirado por
las ratas eran el anhídrido carbónico y el acetonitrilo, en una
proporción de 2:1. El principal metabolito en la orina fue el
derivado mercaptúrico del metomilo, que era igual al 17% de la dosis.
No se detectó metomilo ni su derivado oxima.
La vía metabólica propuesta comprende el desplazamiento del grupo
S-metilo por el glutatión, seguido de una transformación enzimática
que da lugar al derivado mercaptúrico. Otra vía es la hidrólisis, por
la que se produce MHTA, que se descompone rápidamente hasta dar
anhídrido carbónico. Otra vía posible es la conversión del
sin-metomilo (la forma insecticida) en su anti-isómero, que sufre
reacciones de hidrólisis, recomposición y eliminación hasta dar
acetonitrilo. El metomilo se metaboliza de forma semejante en el
mono, salvo que el derivado mercaptúrico es un componente secundario
en la orina.
La penetración del 14C-metomilo una hora después de haber sido
aplicado a ratones por vía cutánea en una solución de acetona se
estimó en un 85%. Para entonces, el 3% de la dosis se hallaba presente
en la sangre, el 5% en el hígado y el 13% se había excretado. En un
plazo de 8 horas la excreción total era del 54,5%.
La descomposición y eliminación rápidas del metomilo en la rata,
junto con su falta de acumulación en los tejidos, son comparables a lo
observado en rumiantes.
El metomilo sufre una descomposición completa en las vacas y
cabras que han recibido una dosis. No se detectaron metomilo ni su
derivado oxima en la leche ni en los tejidos de estos animales. Se
puso de manifiesto que el producto se metabolizaba e incorporaba a los
componentes naturales de la leche y del hígado.
No se detectó la presencia de nitrosometomilo después de haberse
incubado 14C-metomilo con nitrito sódico en un macerado de carne
curada, en condiciones que simulaban las del estómago.
6. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
El metomilo administrado por vía oral tiene una elevada toxicidad
aguda, con una DL50 oral de 17-45 mg/kg de peso corporal en la rata.
También es muy tóxico para la rata por inhalación; en aerosol, la
CL50 a las 4 horas es de 0,26 mg/litro. La toxicidad cutánea es muy
baja; la DL50 es de más de 2000 mg/kg de peso corporal en conejos
(piel intacta) y > 1000 mg/kg de peso corporal en ratas (piel
raspada). Los signos de toxicidad aguda son los que cabe esperar de
un inhibidor de la colinesterasa, entre otros salivación profusa,
lacrimación, temblor y contracción pupilar. La recuperación de estos
efectos fue rápida. En los órganos examinados no se observaron
efectos patológicos graves. El metomilo no irrita ni sensibiliza la
piel, pero irrita levemente los ojos.
La administración repetida de metomilo con los alimentos durante
periodos más largos no produjo aumento de los efectos tóxicos ni
acumulación. Las ratas y perros cuya alimentación contenía metomilo
en cantidades de hasta 250 mg/kg y 400 mg/kg, respectivamente, durante
13 semanas no mostraron signos de toxicidad ni acusaron mortalidad.
Las ratas que recibieron con los alimentos 250 mg/kg mostraron una
pequeña disminución en el aumento del peso corporal, niveles de
hemoglobina más bajos e hiperplasia eritroidea moderada de la médula
ósea. El NOEL en ratas fue de 50 mg/kg en los alimentos (equivalentes
a 3,6 mg/kg de peso corporal por día). Los conejos que recibieron
aplicaciones cutáneas repetidas de metomilo en dosis de hasta
500 mg/kg de peso corporal por día durante 21 días mostraron
hiperactividad y una reducción de la actividad colinesterásica
plasmática y cerebral con la dosis más elevada. El NOAEL en este
estudio fue de 50 mg/kg de peso corporal por día.
Se realizaron estudios de larga duración en ratas que recibían
metomilo en los alimentos en concentraciones de 0, 50, 100 ó 400 mg/kg
y en ratones que recibían 0, 50, 75 ó 200 mg/kg. Los efectos de la
dosis más alta en las ratas fueron una disminución del aumento de peso
y una reducción de la concentración de hemoglobina y del valor
hematócrito. El NOEL fue de 100 mg/kg de alimentos, equivalente a
5 mg/kg de peso corporal por día. En el estudio en ratones, se
observó aumento de la tasa de mortalidad y una reducción de la
hemoglobina y del número de eritrocitos en los dos niveles de dosis
más elevados. El NOEL fue de 50 mg/kg de alimentos, equivalente a
8,7 mg/kg de peso corporal por día. En un estudio de dos años sobre
toxicidad en perros (0, 50, 100, 400 ó 1000 mg/kg de alimentos) con la
dosis más elevada se detectaron síntomas de toxicidad en algunos
animales, junto con anemias de ligeras a moderadas. El NOEL fue de
100 mg/kg de alimentos, equivalentes a 3 mg/kg de peso corporal por
día.
En estudios de dos años en ratas y ratones no se encontró ningún
indicio de aumento de la incidencia de tumores relacionado con el
tratamiento, signo de que el metomilo no es carcinógeno. No fue
genotóxico en célula de bacterias o de mamíferos in vitro y las
pruebas realizadas para determinar la presencia de lesiones primarias
del ADN en células de bacterias y de mamíferos in vitro dieron
resultados negativos; un estudio cromosómico in vivo de médula ósea
de rata también dio resultados negativos. El potencial citogenético
del metomilo en linfocitos humanos in vitro se puso de manifiesto
mediante un aumento del número de micronúcleos y de aberraciones
cromosómicas. El metomilo no produjo efectos embriotóxicos ni
teratogénicos en ratas ni en conejos en dosis de hasta 400 mg/kg en
los alimentos y de 16 mg/kg de peso corporal por día administrados por
sonda, respectivamente; a esos niveles produjo efectos tóxicos en las
madres. En un estudio de reproducción de tres generaciones en ratas
con dosis de 50 ó 100 mg/kg de alimentos (equivalentes a 5 ó 10 mg/kg
de peso corporal por día), el metomilo no tuvo efectos sobre los
índices de fecundidad, gestación o lactación y no se produjeron
anomalías graves relacionadas con el tratamiento.
No se observó neurotoxicidad retardada después de la
administración de una sola dosis o de una serie de dosis de metomilo.
En un estudio de cinco meses en el que se suministraron a ratas
800 mg/kg de alimentos se observó una reducción importante de la
actividad de la colinesterasa sanguínea sólo en las fases iniciales.
En un estudio de 28 días, la administración de esa misma dosis con los
alimentos dio lugar a una reducción ligera solamente de la actividad
de la colinesterasa cerebral. Esto indica que la inhibición de la
actividad de la colinesterasa por el metomilo es reversible
rápidamente en los animales durante los periodos de ingestión.
In vitro, la actividad de la eritrocito colinesterasa humana fue
seis veces más sensible a la acción inhibidora del metomilo que la de
la rata, aunque los índices de reactivación espontánea fueron
semejantes.
Los resultados obtenidos en estudios realizados con varias
especies mostraron que la atropina es el antídoto de eficacia más
general contra la intoxicación por metomilo.
7. Efectos en el ser humano
Las notificaciones de intoxicaciones accidentales y suicidas con
metomilo proporcionan alguna información sobre los niveles de efectos
y la recuperación. Tres de cinco víctimas accidentales de
intoxicación por una comida contaminada murieron antes de que
transcurrieran tres horas desde la ingestión. Se estimó que las
víctimas habían consumido alrededor de 12-15 mg de metomilo/kg de peso
corporal. Una mujer de 31 años y su hijo de seis, que murieron a
causa de un envenenamiento deliberado, tenían en el hígado
concentraciones de metomilo de 15,4 y 56,5 mg/kg, respectivamente.
Las dosis estimadas fueron de 55 mg/kg de peso corporal para la madre
y de 13 mg/kg de peso corporal para el hijo. Seis horas después de
haber ingerido unos 2,25 g de metomilo, la sangre de una mujer
contenía 1,6 mg de metomilo/kg. A las 22 horas de la ingestión no se
podía detectar la presencia de metomilo y la mujer se estaba
recuperando.
Un operario que manipulaba plaguicidas había mezclado, sin tomar
precauciones, una formulación de metomilo en polvo para el rociado de
hortalizas; antes de una hora manifestó síntomas de intoxicación y
después de 12 horas la actividad de colinesterasa sanguínea se había
reducido al 40% de la normal; a las 36 horas la recuperación llegaba
al 80% de la actividad normal. Otros operarios que habían tomado las
precauciones recomendadas no acusaron ningún síntoma ni efectos en la
actividad de la colinesterasa eritrocitaria y plasmática durante
actividades de aplicación aérea de metomilo.
8. Efectos en organismos no destinatarios en el laboratorio y sobre
el terreno
No se observaron efectos en poblaciones de hongos y bacterias del
suelo, en particular en su acción nitrificante y en la actividad de la
deshidrogenasa después de haberse aplicado metomilo en las dosis
recomendadas.
En estudios de laboratorio se determinó una NOEC de
6,5 mg/litro para el crecimiento de las algas.
El metomilo tiene una toxicidad entre moderada y alta para los
peces, con una CL50 a las 96 horas de 0,5 a 2 mg/litro para una
serie de especies. En un estudio de mayor duración (21 días),
utilizando la formulación Lannate 20L (21,5% de metomilo), la CL50
para los alevines de trucha fue de 1,3 mg/litro. En un estudio de
toxicidad de 28 días durante la primera fase de la vida de piscardos
(Pimaphales promelas), la máxima concentración intoxicante aceptable
(MATC) estimada fue de > 57 y < 117 µg/litro.
En pruebas de toxicidad aguda con otros organismos acuáticos,
Daphnia magna fue una de las especies más vulnerables al metomilo, con
una CL50 de 0,032 mg/litro a las 48 horas. En un estudio de 21 días
sobre la capacidad de supervivencia, crecimiento y reproducción de
Daphnia magna, la máxima concentración intoxicante aceptable para el
metomilo fue de > 1,6 y < 3,5 µg/litro.
El metomilo es tóxico para las abejas melíferas; se ha notificado
una DL50 por contacto de 1,29 µg/abeja y una DL50 oral de
0,2 µg/abeja.
Se ha evaluado la toxicidad aguda del metomilo en varias especies
de aves; los valores característicos de la DL50 oral aguda son de
10 mg/kg de peso corporal en palomas y de 34 mg/kg de peso corporal en
la codorniz japonesa. El metomilo es relativamente menos tóxicos si
se ingiere con los alimentos; en ese caso, la CL50 en ocho días es
de 1100 mg/kg de metomilo en los alimentos para la codorniz Colinus
virginianus y de 2883 mg/kg de metomilo en los alimentos para el
pato silvestre. En estudios de una generación realizados durante 18 a
20 semanas, la NOEC fue de 150 mg/kg de metomilo en los alimentos para
la codorniz Colinus virginianus y para el pato silvestre.
No se observaron efectos sobre Colinus virginianus tras su
exposición a una serie de aplicaciones de metomilo por rociado en las
dosis recomendadas. En dos estudios sobre poblaciones de aves
silvestres, después de haberse rociado con metomilo tierras forestales
y campos de lúpulo en las dosis recomendadas, no se observó ningún
cambio manifiesto en la actividad de las aves ni se produjeron efectos
ni mortalidad relacionados con el tratamiento. Los depósitos de grasa
de las aves canoras de los bosques tratados se redujeron en
comparación con el grupo testigo; se consideró que se trataba de un
efecto indirecto de la disminución de los insectos que les sirven de
alimento.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
El metomilo es un inhibidor de la carbamato colinesterasa
mediante un mecanismo bien conocido de acción tóxica. En estudios
realizados en animales se ha observado una toxicidad aguda
particularmente alta por vía oral y respiratoria, pero la toxicidad
por vía cutánea es baja. Los signos de toxicidad aguda en animales
son los característicos de los inhibidores de la colinesterasa. La
reversibilidad de la acción tóxica aguda es rápida; los supervivientes
se recuperan con rapidez de los signos tóxicos y de la inhibición de
la colinesterasa en la sangre y el cerebro. La pronta remisión de los
efectos tóxicos se debe a la rápida reversibilidad de la inhibición de
la colinesterasa, reversibilidad favorecida por la eliminación rápida
del metomilo del organismo. Los datos sobre intoxicaciones humanas
accidentales e intencionales muestran que el nivel de toxicidad aguda
del metomilo en el ser humano es semejante al observado en los
animales de laboratorio.
Habida cuenta de la reversibilidad rápida de la acción del
metomilo durante los periodos de ingestión, en los estudios realizados
raramente se observaron síntomas de toxicidad aguda e inhibición de la
colinesterasa sanguínea. Los resultados más constantes en los
estudios de mayor duración con niveles de alimentación más elevados
fueron una reducción del aumento del peso corporal en roedores y una
disminución del número de glóbulos rojos en roedores y perros. No se
observaron indicios de carcinogenicidad potencial en tres estudios de
larga duración realizados en roedores. El compuesto dio resultados
negativos en las pruebas de genotoxicidad in vitro en las que se
investigaron varios puntos finales, en cambio mostró potencial
citogenético en linfocitos humanos. El resultado de un estudio
cromosómico realizado in vivo sobre médula ósea de rata fue
negativo.
Se identificaron los NOEL en cada uno de los estudios de larga
duración realizados con animales, teniendo en cuenta la reducción del
aumento del peso corporal y el número de eritrocitos. Los NOEL
resultaron ser de 5 mg/kg de peso corporal por día en ratas, 8,7 mg/kg
de peso corporal por día en ratones y 3 mg/kg de peso corporal por día
en perros. Como esos estudios no revelaron diferencias acentuadas
entre especies en cuanto a los efectos tóxicos, se debería utilizar el
NOEL en el perro, que es de 3 mg/kg de peso corporal por día, a
efectos de la estimación del riesgo en el ser humano.
La adsorción del metomilo al suelo es de baja a moderada, y
prácticamente no hay desorción. La degradación aerobia en el suelo
(con una semivida de alrededor de una semana) es aproximadamente dos
veces más rápida que la degradación anaerobia.
La aplicación de metomilo a hojas de plantas da lugar a una
absorción rápida de casi la mitad de la cantidad aplicada (la otra
mitad se adsorbe) y no hay indicios de transporte. La concentración
del metomilo absorbido en los cultivos de productos alimenticios
disminuye rápidamente hasta alrededor del 5% en una semana.
Varios invertebrados acuáticos, en particular los dáfnidos, son
muy sensibles al metomilo, con CL50 del orden de 10 a 100 µg/litro.
Los peces, tanto de agua dulce como estuarinos, son menos
sensibles, oscilando la CL50 entre 0,5 y 7 mg/litro. Dada la escasa
persistencia del metomilo y su toxicidad aguda relativamente baja para
los peces, se supone que el riesgo es bajo.
En las dosis de aplicación recomendadas, el metomilo no menoscaba
la actividad microbiana en suelos templados.
Este producto está clasificado como muy tóxico para las abejas
melíferas y su DL50 tópica es de aproximadamente 0,1 µg/abeja.
La DL50 aguda por vía oral para varias especies de aves oscila
entre 10 y 40 mg/kg de peso corporal. Los valores de la CL50 en la
alimentación (cinco días) varían entre 1100 y 3700 mg/kg de alimentos.
El metomilo, sobre todo en forma de gránulos, constituye un riesgo
agudo para las aves, pero no se prevé que la ingestión de alimentos
contaminados con metomilo pueda causarles la muerte.
La elevada toxicidad aguda del metomilo para los mamíferos de
laboratorio indica que existe un peligro semejante para los mamíferos
silvestres.
10. Conclusiones
Teniendo en cuenta las características cualitativas y
cuantitativas de la toxicidad del metomilo, el Grupo de Trabajo llegó
a la conclusión de que 0,03 mg/kg de peso corporal al día
probablemente no causarían efectos adversos en el ser humano por
ninguna vía de exposición.
A lactating goat was given 14C-methomyl by capsule, twice a
day, for 10 days at a dose rate equivalent to 20 mg/kg in feed. Milk,
blood, urine and faeces were sampled daily and selected tissues taken
within one day of the last dose. No methomyl or the metabolite MHTA
was detected in any of the samples. Approximately 16% and 7% of the
activity was excreted in urine and faeces, respectively, and about 8%
appeared in the milk and 17% in expired air. The milk activity
reached a plateau after 3 days and was equivalent to approximately
2 mg/kg. At this time the lactose component contained about 11-13% of
the milk activity. Hexane extracts, containing the triglyceride
components, contained 26-37% of the milk activity and the casein
component 8-9%. This indicates that methomyl had been completely
broken down and incorporated into natural constituents of milk.
Acetonitrile was identified as a volatile metabolite in milk and blood
(Harvey, 1980).
The examination of the liver fractions showed that the
radio-activity derived from methomyl was found in glycerol,
glycerol-3-phosphate, fatty acids, neutral lipids and insoluble
protein. This indicates a metabolic pathway via acetonitrile and
acetate into the natural occurring constituents in the liver. The
breakdown of methomyl and distribution of metabolic products in the
liver was shown to be similar in the cow (Monson, 1989).
Acetonitrile, CO2 and reincorporation products derived from
acetate found after the application of methomyl to plants (section
4.2.1) are similar to those identified in the above animal studies.
The proposed metabolic pathway for methomyl in animals is shown
in Fig. 1.
6.4 Elimination and excretion
Rat and monkey studies show that methomyl is very rapidly
metabolized and eliminated, the processes being largely completed
within 24 h.
Rats fed 200 mg methomyl/kg in diet and then given 5 mg
14C-methomyl/kg orally (see section 6.3 for detail) showed a 50%
elimination of 14C in expired air in 3 days in the form of carbon
dioxide and acetonitrile in the proportion of 1:2. Urinary excretion
of 14C was 27% in one day (Harvey et al, 1973).
In a more detailed study, where male and female rats were given
5 mg 14C-methomyl/kg orally (see section 6.3), approximately 53% of
the radioactivity was excreted in urine over 7 days, 45% of the dose
being excreted in the first 6 h. Faecal excretion contributed only
2-3% over 7 days. The other major path of elimination (over 5 days)
was via expired air as carbon dioxide (22% of dose) and acetonitrile
(12% of dose). Of this, 18% of the dose was expired as CO2 within
6 h and 10% as acetonitrile in 24 h. Overall, most of the
radiolabelled dose (80%) had been eliminated in 24 h with an estimated
half-life of 5 h. There was no obvious difference in the amount or
rate of excretion between males and females. The single oral dose
given to these animals (5 mg/kg) produced mild clinical signs of
cholinesterase inhibition which disappeared within 2 h of dosing
(Hawkins et al., 1991).
When methomyl was radiolabelled on the carbonyl group, the
elimination of 14CO2 was very rapid and equivalent to about 85% of
the oral dose in male and female rats. When the labelling was at the
- 14C=N group, the overall elimination in expired air in 24 h was
30% in the form of CO2 and acetonitrile (in the proportion of 2:1).
When 14C-MHTA was administered in the same way the expired component
was mainly 14CO2, equivalent to 22% of the dose. The anti-isomer
of methomyl mainly produced acetonitrile in the expired air,
equivalent to 28% of the dose given orally to rats. Rats given the
anti-MHTA by intraperitoneal injection produced ten times more
acetonitrile than those given the syn-MHTA. The urine from rats
treated orally with 14C-methomyl or MHTA contained 25-35% of the
radioactivity over a 24-h period (Huhtanen & Dorough, 1976).
In monkeys given 5 mg 14C-methomyl/kg orally, approximately 32%
of the dose was excreted in urine in 7 days, with 34% as CO2 and 5%
as acetonitrile in the expired air. Most of this was excreted in the
first 24 h. Faecal excretion amounted to only 3-4% (Hawkins et al.,
1992).
After dermal application of 14C-methomyl to mice (see section
6.1), the total excretion (urine plus CO2 plus faeces), as a
proportion of the applied dose, was 0.2% in 15 min, 12.9% in 60 min
and 54.5% in 480 min (Shah et al., 1981).
6.5 Retention and turnover
The absorption, metabolism and excretion of methomyl in the rat
are very rapid. No methomyl can be detected within the tissues or
excretory products within a few hours of dosing. Most of the dose is
eliminated within 24 h with an estimated half-life of 5 h (Hawkins et
al., 1991). Metabolic products, mainly in urine and expired air, are
also eliminated rapidly; tissue concentrations are very small and
lower than blood levels. There is no evidence for accumulation in
tissues. A similar picture exists for the metabolism of methomyl in
ruminant species.
6.6 Reaction with body components
Methomyl is a potent direct inhibitor of acetylcholinesterase in
both insects and mammals. The carbamylated enzymes undergo rapid
spontaneous reactivation (see section 7.8.1).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The results of the single exposure of technical methomyl by the
oral, dermal and inhalation routes in various species are shown in
Table 4.
Methomyl is very toxic by the oral route. In the rat, the signs
of toxicity are those expected from a cholinesterase inhibitor and
include profuse salivation, lacrimation, tremor, abnormal posture,
pupil constriction, diarrhoea and prostration. At lethal doses the
rats died within hours. Survivors began to recover within several
hours and had fully recovered within days. No compound-related
changes were seen in organs subjected to histopathological
examination.
The acute dermal toxicity of methomyl is very low. No deaths
occurred in rats or rabbits at the doses shown in Table 4. The
compound has high toxicity by the inhalation route in the rat, with
affected animals showing typical signs of cholinesterase inhibition.
No target organ effect was seen upon histopathological examination.
An LD50 of approximately 8 mg/kg was obtained when methomyl was
injected intraperitoneally in rats (Dashiell, 1972).
The acute oral toxicity of methomyl formulations is in proportion
to the amount of a.i. present and, therefore, they are less toxic than
methomyl itself (Table 5). The same pattern is seen with the results
of acute inhalation studies. The signs of toxicity in the oral and
inhalation studies are those shown by the active ingredient. The
dermal toxicity of the formulations is very low.
Ocular toxicity was shown by a solid formulation containing 92.4%
methomyl when 10 mg was introduced into rabbits' eyes. Typical
anticholinesterase effects were seen up to 20 min after treatment,
including pupillary constriction of the treated eyes, incoordination,
tremors and profuse salivation. All of the effects had disappeared by
the next day (Sarver, 1991g).
7.2 Short-term exposure
Six male rats were given methomyl in peanut oil by gavage at a
dose of 5.1 mg/kg body weight per day, 5 times per week for 2 weeks.
The signs of toxicity were the same as those exhibited in acute oral
studies (section 7.1) but they regressed during the second week of
dosing. All animals survived, and there were no compound-related
histopathological changes (Kaplan & Sherman, 1977).
Table 4. Acute toxicity of technical grade methomyl
Species Sex Route Vehicle Testa Result Reference
Rat M oral peanut oil LD50 17 mg/kg Sherman (1966)
Rat F oral peanut oil LD50 23.5 mg/kg Sherman (1968a)
Rat M oral water LD50 45 mg/kg Trivits (1979)
Rat M oral water LD50 34 mg/kg Sarver (1991a)
Rat F oral water LD50 30 mg/kg Sarver (1991a)
Rat M dermal water LD50 abraded skin > 1000 mg/kg Morrow (1972)
Rat M inhalation aerosol 4-h ALC 0.30 mg/litre Foster (1966a)
Rat M inhalation vapour 4-h ALC 0.04 mg/litre Foster (1966b)
Rat M inhalation spray 4-h LC50 0.45 mg/litre Hornberger
(1967)
Rat M/F inhalation aerosol 4-h LC50 0.258 mg/litre Panepinto
(1991a)
Rabbit M oral acetone/ ALD 30 mg/kg Sherman (1968c)
peanut oil
Table 4. (cont'd).
Species Sex Route Vehicle Testa Result Reference
Rabbit M/F dermal water LD50 intact skin > 2000 mg/kg Sarver (1991b)
Dog M oral capsule ALD 20 mg/kg Sherman (1968d)
Guinea-pig M oral acetone/ ALD 15 mg/kg Kaplan &
peanut oil Sherman (1977)
Monkey M/F oral water ALD 40 mg/kg Kaplan &
Sherman (1977)
a ALC = approximate lethal concentration; ALD = approximate lethal dose
Table 5. Acute toxicity of some methomyl formulations
Formulation Species Route Vehicle LD50 (mg/kg) or LC50 Reference
(% a.i.) (mg/litre)a
Lannate 40 SP rat oral water 61 (male) Sarver (1992a)
(41.6%) 73 (female)
Lannate 20 L rat oral methanol 129 Lheritier (1991a)
(21.5%)
Lannate 12.5 L rat oral water 208 Sarver (1991c)
(12.7%)
Lannate 40 SP rabbit dermal (intact skin) water > 2000 Sarver (1992b)
(41.6%)
Lannate 20 L rat dermal (intact skin) methanol > 4000 Lheritier (1991b)
(21.5%)
Lannate 40 SP rat inhalation (4 h) aerosol 0.66 Kelly (1992)
Lannate 20 L rat inhalation (4 h) aerosol 1.3 Jackson et al. (1991)
(21.5%)
Lannate 12.5 L rat inhalation (4 h) aerosol 3.7 Panepinto (1991b)
(12.7%)
a Results are for both sexes, unless stated otherwise
Groups of 10 male and 10 female rats were fed diets containing 0,
10, 50, 125/500 (125 mg/kg for 6 weeks, 500 mg/kg for remaining
period) or 250 mg methomyl/kg for 13 weeks. Assessments included
haematological, biochemical and histopathological examinations. There
were no mortalities nor clinical signs due to treatment in any group.
A slight decrease in body weight was seen in males at the two higher
dose levels and in females at 125/500 mg/kg. This was accompanied by
lower food consumption. The only change attributed to methomyl on
histopathological examination was moderate erythroid hyperplasia in
the bone marrow of males fed 250 mg/kg. Lower haemoglobin values in
the males of this group at two months could be linked to this effect.
Histopathological examination of animals in the 125/500 mg/kg group
did not reveal any changes due to treatment. The noobserved-effect
level based upon body weight change was considered to be 50 mg/kg
diet, equivalent to 3.56 mg methomyl/kg body weight per day (Paynter,
1966; Busey, 1966).
When groups of four male and four female one-year-old dogs were
fed diets containing 0, 50, 100 or 400 mg methomyl/kg for 3 months, no
effects were seen in a range of clinical, haematological, biochemical
and histopathological assessments (Kaplan & Sherman, 1977).
A dose of 200 mg methomyl/kg body weight was applied as a 5%
aqueous solution once a day 5 times per week for 3 weeks to the intact
or abraded skin of five male, five female rabbits. Each dose was
occluded for 6 h and then the site washed with water. There were no
mortalities in animals with intact skin; clinical signs included nasal
discharge, wheezing and diarrhoea. With abraded skin, signs of
toxicity, including laboured respiration, salivation and tremors,
appeared within one hour of dosing, and three animals died, one after
the first application and the other after the eighth application. No
treatment-related changes were seen upon histopathological examination
(Kaplan & Sherman, 1977).
Groups of rabbits were treated dermally (intact clipped skin)
with doses of 0 (10 males, 10 females), 5, 50 (5 males 5 females) or
500 (10 males, 10 females) mg methomyl/kg body weight per day for 21
days. The doses were applied in water each day and occluded for 6 h.
Half the males and females in the control and highest dose groups were
killed at the end of the dosing regime, together with all animals in
the other groups. The remaining animals in the control and highest
dose groups were allowed to recover over a 14-day period and then
killed. The only change noted clinically was a greater incidence of
hyperactivity at 500 mg/kg. There were no effects on body weight
gain, haematology, blood biochemistry, organ weights or organ
histopathology. Plasma cholinesterase activity was depressed to 36%
and 55% of normal at day 21 in highest dose males and females,
respectively. Brain cholinesterase activity was depressed to 48% and
68%, respectively, in males and females at this dose, but red cell
cholinesterase activity, although slightly decreased, was considered
to be biologically insignificantly affected. In males, at 50 mg/kg,
the plasma cholinesterase activity was depressed to 77% and there was
no inhibition of red blood cell cholinesterase activity of either sex.
All the depressed cholinesterase activities had returned to control
values at the end of the 14-day recovery period. The
no-observed-effect level, based upon changes in plasma cholinesterase
activity, was 5 mg/kg body weight per day in males and 50 mg/kg in
female rabbits (Brock, 1989).
Formulations of methomyl have also been tested by repeated dermal
application to rabbits at doses equivalent to 50 or 100 mg
methomyl/kg. No effects were seen after ten daily doses of Lannate L
(25.5% methomyl). Ten daily doses of Lannate LV (30% methomyl)
produced mild skin irritation at the higher dose level. Plasma
cholinesterase activity was somewhat depressed at both dose levels but
returned to normal over a 14-day recovery period (McAlack, 1973,
Edwards, 1980).
A Lannate dust containing 45% methomyl was the subject of a
3-month inhalation study in male rats. Exposure was at 14.8 mg/m3
for 4 h/day, 5 days/week; the mass median dynamic diameter of the dust
was 4.4 µm. There were no effects on body weight, organ weight,
histopathology or red cell cholinesterase activity. Plasma
cholinesterase activity was depressed to about 29% of the
pre-treatment activity in samples taken 4 h after the last exposure
(Ta'Naka et al., 1987).
7.3 Long-term exposure
Groups of 35 male and 35 female rats were fed diets containing
methomyl at 0 (2 groups), 50, 100, 200 or 400 mg/kg for 22 months.
Five males and 5 females per group were killed at 12 months for gross
and histopathological examination. Assessments were made throughout
the study for clinical condition, body weight, food consumption,
haematology, blood and urine biochemistry, organ weight and
histopathology. Growth of males at the highest dose was significantly
lower than that of controls over the first year. Growth of males at
200 mg/kg and females at 400 mg/kg was also lower at this time, but
not significantly. Food consumption over the first 26 weeks was
significantly lower than that of controls for the males fed 200 and
400 mg/kg. At 18 and 22 months there was a trend towards lower
haemoglobin values in treated female groups. Compound-related
increases in the incidence and severity of extra medullary
haematopoiesis were observed in the spleen of females fed 200 or
400 mg/kg. Compound-related changes were also seen in the kidneys of
both sexes at 400 mg/kg, characterized by vacuolization of epithelial
cells and hypertrophy of the proximal convoluted tubules. On the
basis of the body weight and haematopoietic findings in this study,
the long-term no-observed-effect level for methomyl in rats was
considered to be 100 mg/kg diet (Kaplan & Sherman, 1977).
In a 2-year study on rats, methomyl was fed in the diet to groups
of 80 males and 80 females at levels of 0, 50, 100 or 400 mg/kg. The
animals were observed and weighed regularly, and haematological and
blood and urine biochemical measurements were made at 3, 6, 12, 18 and
24 months. Red cell cholinesterase activity was assayed in additional
groups at 1, 2 and 4 weeks and 3, 6, 12 and 20 months. Brain
cholinesterase activity was assayed at 12 and 20 months. Extensive
histopathological examination was performed on animals killed at
scheduled intervals, on animals dying and on animals killed at the end
of the study.
There was a significant reduction in body weight gain in both
sexes at 400 mg/kg over the first 12 months but not during the
subsequent 12 months. There were no clinical signs attributable to
treatment. Survival rate was the same among the groups and there was
an acceptable number of animals at termination (40-50%). Erythrocyte
counts, haemoglobin values and haematocrits were significantly lower
in females at 400 mg/kg. There were no compound-related changes for
red cell or brain cholinesterase activity nor for other biochemical
parameters. Organ weights were unaffected by treatment. The incidence
of bone marrow hyperplasia, focal hyperplasia in the adrenal medulla
and focal degeneration in the adrenal cortex was slightly increased in
males at 400 mg/kg. The no-observed-effect level in the rat was
there-fore confirmed as 100 mg methomyl/kg diet, equivalent to about
5 mg/kg body weight per day (Kaplan, 1981).
A comprehensive long-term (2 years) study on the mouse was
initiated with 80 males and 80 females per group at levels of 0, 50,
100 and 800 mg methomyl/kg diet. Because of early high mortality, the
level of the highest dose group was reduced to 400 mg/kg at week 28
and then to 200 mg/kg at week 39. The level of the mid-dose group was
also reduced to 75 mg/kg at week 39. Regular clinical observations
and body weight and food consumption measurements were undertaken.
Haematological analysis was carried out at 4, 13, 26, 52, 78 and 104
weeks. Organ weights were measured and an extensive histopathological
examination carried out on all animals dying or killed. The mortality
rate was higher than that of controls at the highest and medium dose
levels throughout the study, and was slightly higher, in male mice
only, at the low dose level in the latter stage of the study. There
were no clinical findings due to treatment and no effects on body
weight gain or food consumption. Some effects were noted on red cell
parameters such as haemoglobin values and red cell counts during the
first 26 weeks at the medium and highest dose levels. However, these
were not apparent during the remainder of the study, after these dose
levels had been decreased. No effects were observed on organ weights
or microscopic findings. The no-observed-effect level was considered
to be 50 mg/kg in diet, equivalent to approximately 10 mg/kg body
weight per day (Serota et al, 1981).
A 2-year dog study was initiated with dietary levels of methomyl
of 0, 50, 100, 400 and 1000 mg/kg with four males and four females per
group. After one year, one male and one female from each group was
killed for interim examination. Clinical condition was regularly
observed and routine measurements made on body weight, food
consumption, and haematological and bio-chemical parameters. After 2
years the surviving animals were killed, organs were weighed and a
histopathological examination was undertaken.
No clinical signs due to treatment were seen in animals at 50,
100 and 400 mg/kg. One highest dose female died after 8 weeks and its
replacement also died within 18 days after showing compound-related
toxic signs. Two males at this dose level exhibited tremors,
salivation and incoordination during week 13. Slight to moderate
anaemia was noted in the highest dose animals at 13 weeks and this
persisted in one of the males. No effects were seen on body weight or
biochemical parameters. Histopathological effects were noted in the
kidneys and spleens of animals at 400 and 1000 mg/kg. Changes in
kidneys were characterized by increased pigmentation and epithelial
swelling, and in the spleen as extra-medullary haematopoiesis and
increased pigmentation. A minimal to slight increase in bile duct
proliferation was seen in livers of dogs at 1000 mg/kg, and bone
marrow activity was also slightly increased in animals at this dose
level. The no-observed-effect level was therefore 100 mg/kg diet,
equivalent to about 3 mg methomyl/kg body weight per day (Kaplan &
Sherman, 1977).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
The skin irritation potential of methomyl was evaluated in six
male rabbits with 0.5 g of the test material, moistened with deionized
water, applied to the back of each animal. Each site was covered with
a semi-occlusive dressing and exposure continued for 4 h. The skin
was examined and evaluated 1, 24, 48 and 72 h after the end of the
exposure period. No dermal irritation was seen in any of the animals
during the study and, therefore, methomyl is classified as
non-irritant to skin (Sarver, 1991d).
Methomyl formulations were also evaluated for skin irritation
potential using the same test method as described above. The
substances tested were a solid formulation containing 92.4% methomyl
(Sarver, 1991e) and two liquid formulations containing approximately
20% and 12.7% methomyl (Clement, 1987a; Sarver, 1991f). None of the
formulations irritated rabbit skin.
In a test on 10 guinea-pigs, methomyl was applied as a 60% paste
in propylene glycol or as a 26% solution in Cellosolve and the 24-h
reaction was noted as being mildly irritating (Kaplan & Sherman,
1977).
7.4.2 Eye irritation
Methomyl, either as 10 mg dry powder or 0.1 ml of a 10% solution
in propylene glycol, was tested in rabbits' eyes (2 per group). One
of each pair was washed after 20 sec and examination of each eye made
at 1, 2, 3, 4 and 6 h after treatment and for up to 6 days thereafter.
Only mild conjunctivitis was seen on the day of treatment but no
corneal injury was noted (Kaplan & Sherman, 1977).
A solid formulation containing 92.4% methomyl was tested in the
eyes of six male rabbits. A quantity of 10 mg was introduced into one
eye of each rabbit and evaluations of the reaction were undertaken
after 1, 24, 48 and 72 h. Pupillary constriction was evident after
one hour but was not present the next day (see also section 7.1).
Only very mild conjunctival chemosis and redness was seen at 24 h
after treatment and the formulation was considered to be non-irritant
(Sarver, 1991g).
Two liquid formulations, containing approximately 20% and 12.7%
methomyl, respectively, were tested for eye irritancy using the method
described in the previous paragraph. In both cases, the formulation
produced ocular irritation in the form of conjunctival redness and
chemosis, iritis and corneal opacity within one hour and were
classified as irritant. These effects had disappeared after 21 days.
(Clement, 1987b; Sarver, 1991h).
7.4.3 Skin sensitization
In a test to evaluate the skin sensitization potential of
methomyl, the material was applied as a paste to the abraded skin of
five guinea-pigs 3 times a week for 3 weeks; five other guinea-pigs
received four intradermal injections of 0.1 ml of a 1% solution. The
animals were then challenged after a 2-week rest period. Methomyl was
shown not to be a skin sensitizer in this test (Kaplan & Sherman,
1977).
A closed-patch repeated insult dermal sensitization evaluation
(Beuhler test) was undertaken in guinea-pigs with technical methomyl.
The test compound (300 mg), moistened with water, was applied to the
intact skin of each of 10 males and 10 females for 6 h once a week for
3 weeks. Two weeks after the last induction treatment, the animals
were challenged with another 300 mg methomyl for 6 h. No erythema was
observed in either the induction or challenge phases, and therefore
methomyl did not produce delayed contact sensitivity in the
guinea-pig. Positive control animals treated with DNCB produced the
expected sensitization responses (Armondi, 1991a).
A solid formulation containing 94.2% methomyl, subjected to the
evaluation described in the previous paragraph, also failed to produce
delayed contact hypersensitivity in guinea-pigs (Armondi, 1991b). The
same result was obtained with a liquid formulation containing 21.5%
methomyl when given at 0.5 ml per application (Mercier, 1991).
Another liquid formulation containing 12.7% methomyl was tested in the
Magnusson-Kligman guinea-pig sensitization maximization procedure and
was found to be a moderate skin sensitizer under the conditions of the
test (Armondi, 1992).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Embryotoxicity and teratogenicity
Teratogenicity studies have been undertaken in the rat and
rabbit.
Pregnant rats (25 per group) were fed 0, 50, 100 or 400 mg
methomyl/kg diet during days 6-15 of gestation. The dams were
observed and weighed regularly and then killed on day 21 of gestation.
At that time the uterus was removed, and the number of corpora lutea,
implantation sites, live and dead fetuses, resorptions and weight of
live fetuses were recorded. About one half of the fetuses from each
litter were taken for skeletal examination and the remainder for soft
tissue examination. The pregnant rats at 400 mg/kg had significantly
lower body weight and ate less food than those in the control group.
There were no clinical signs of toxicity and all rats survived the
test period. Parameters of pregnancy and fetal development, such as
the number of litters with partial or total resorptions and fetal
weight, were not affected in the test groups compared to controls.
There were no treatment-related abnormalities in the fetuses.
Methomyl was therefore not embryotoxic or teratogenic in the rat at
levels up to 400 mg/kg diet, equivalent to 34 mg/kg body weight per
day (Rogers et al., 1978).
Groups of 20 pregnant rabbits were given 0, 2, 6 or 16 mg
methomyl/kg body weight per day by gavage on days 7-19 of gestation.
The animals were observed and weighed regularly and killed on day 29.
The uteruses were then examined for pregnancy, number of
implantations, early and late resorptions and live or dead fetuses.
Delivered pups were weighed and examined for visceral or skeletal
abnormality. One pregnant rabbit at 6 mg/kg died, probably due to
gavage error. At 16 mg/kg there were seven deaths during the test
period due to the compound. Clinical signs at the highest dose level
included tremors, hyperactivity, excessive salivation, aggression and
convulsions. The no-observed-effect level for maternal toxicity was
therefore 6 mg/kg body weight per day. There was no effect on
pregnancy rate or on the average numbers of corpora lutea,
implantations, resorptions or live fetuses per dam in treated groups
compared to controls. There were no gross external visceral or
skeletal variations due to treatment. Methomyl is therefore not
embryotoxic or teratogenic to the rabbit at doses up to 16 mg/kg, at
which dose maternal toxicity occurred (Christian et al., 1983).
In another study, pregnant rabbits (12 per group) were fed diets
containing up to 100 mg methomyl/kg. The pregnancy rate was low in
all groups, including controls. No effects were noted on a range of
parameters including reproductive indices, fetal body weight or
visceral and skeletal abnormalities. However, due to experimental
inadequacies the results are difficult to assess (FAO/WHO, 1987).
7.5.2 Reproduction studies
A three-generation (two litters per generation) rat study was
undertaken to test the potential reproductive effects of methomyl (Lu,
1983). The compound was fed at levels of 0, 50 or 100 mg/kg diet to 10
males and 20 females for approximately 90 days post weaning prior to
first mating to produce the F1a litter. Test diets were fed through
to subsequent re-mating to produce the F1b litter. Ten males and 20
females per group were then selected from the F1b litter and
continued on their respective diets for approximately 90 days to
produce F2a and then F2b litters. The same procedure was used to
produce subsequent litters of the next generation. Records were kept
of all matings, number of pregnancies, number of young in each litter
born alive or dead, the body weight of offspring at weaning, and the
respective reproduction and lactation indices. Ten males and 10
female offspring were selected from each group of the F3b litter for
histopathological examination. Methomyl did not affect the
reproductive performance of rats in this study. There were no effects
on fertility, gestation or lactation and no compound-related
abnormalities on gross examination. No treatment related changes were
seen upon histopathological examination of tissues from the offspring
in the F3b litter (Kaplan & Sherman, 1977).
A two-generation rat study was also carried out using high
dietary levels of methomyl (600 and 1200 mg/kg) as well as a lower
level of 75 mg/kg. The F0 parents (13 males, 26 females) were mated
after 100 days treatment and the F1 parents (20 males, 40 females)
after a minimum of 120 days. Spermatogenesis (based on sperm count)
was measured on adult males in both generations after breeding, in
addition to the standard assessments of reproductive performance.
Histopathological examination was undertaken on a selection of tissues
from parents and pups in both generations. Body weight depression in
parents, seen at all or some stages during treatment at 600 and
1200 mg/kg in both generations and at 75 mg/kg in the F1 generation,
was probably related to lower food intake. Reduced red blood cell
counts were seen in females at the highest dose level, together with a
small (15-25%) depression in plasma cholinesterase activity in both
sexes at this level. Decreases in litter size and live births were
shown for the highest dose F0 group and for all F1 groups for
which a clear no-observed-effect level was not established. Survival
during lactation in F1 pups (from F0 parents) was reduced at the
highest dose level, as was survival in highest dose F2 pups during
early lactation up to day 4. Pup body weight was slightly reduced at
75 mg/kg, significantly reduced at the two higher levels in the F1
generations and reduced at the 600 and 1200 mg/kg levels in the F2
generation. No histopathological changes due to treatment were seen
in any of the tissues examined in parents or offspring. No effects on
the reproductive parameters of fertility indices, gestation length or
male sperm counts were seen at dose levels up to and including 1200 mg
methomyl/kg in diet (Lu, 1983).
7.6 Mutagenicity
Numerous in vitro mutagenicity assays, to various end-points,
have been carried out on methomyl by several investigators (Table 6).
Methomyl did not show mutagenicity or cause primary DNA damage in
bacterial or mammalian cells in vitro. It showed cytogenetic
potential in human lymphocytes in vitro as indicated by an increase
in micronuclei and chromosomal aberrations.
In an in vivo study, the clastogenic potential of methomyl was
assessed by its ability to cause numerical and structural chromosomal
aberrations in rat bone marrow cells. Single doses of methomyl were
given by gavage to groups of 15 male and 15 female rats at doses of 2,
6 or 20 mg/kg body weight; five males and five females were killed at
6, 24 and 48 h after dosing and the bone marrow cells harvested. These
were examined for chromatid and chromosome breaks and for gaps and
other aberrations. The results showed that there was no significant
increase in the frequency of chromosomal aberrations and no
significant differences between the chromosome numbers and the mitotic
indices of the dosed groups and the controls (Farrow et al, 1984).
Table 6. Summary of results of in vitro mutagenicity assays and related end-point studies on methomyl
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
Prokaryotes
Point mutation Salmonella typhimurium - 50 nM negative Blevins et al. (1977a)
(5 strains)a
Point mutation S. typhimurium (5 strains) + and - up to 5000 µg/plate negative Moriya et al. (1983)
Escherichia coli (WP2hcr) + and - negative
Point mutation S. typhimurium (5 strains) + and - up to 1 mg/plate negative Waters et al. 1982
Point mutation E. coli, WP2uvr A + and - up to 1 mg/plate negative Waters et al. 1982
DNA damage E. coli pol A - 1 mg/disc negative Waters et al. 1982
W3100, p 3478
DNA damage Bacillus subtil recM45 - 1 mg/disc negative Waters et al. 1982
Yeast
DNA damage Saccharomyces cerevisiae + and - 1-3.5% negative Waters et al. 1982
D3
Mammalian cells
DNA damage Human lung fibroblast WI-38 + and - 10-7-10-3M negative Waters et al. 1982
DNA damage Rat hepatocytes (UDS) - 1 µM-75 mM negative Vincent (1985)
Table 6 (cont'd).
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
DNA damage Human skin cell DNA - 10-5M negative Blevins et al. (1977b)
Gene mutation Chinese hamster V79 cells + and - 1-10 mM negative Wojciechowski et al. (1982)
Gene mutation Chinese hamster ovary - 10-55 mM negative McCooey et al. (1984)
(CHO) cells, HGPRT + 100-350 mM
Chromosome Human lymphocytesb NA 0.02-0.54 mM positive Bonatti et al. (1994)
aberrations (whole blood culture)
Sister-chromatid Human lymphocytesb NA 0.02-0.54 mM negative Bonatti et al. (1994)
exchange (whole blood culture)
Micronuclei Human lymphocytesb NA 0.01-0.09 mM positive Bonatti et al. (1994)
(whole blood culture)
DNA single strand Human lymphocytesb NA 0.06-2 mM negative Bonatti et al. (1994)
breaks (whole blood culture)
DNA oxidative Human lymphocytesb NA 0.25 - 1 mM negative Bonatti et al (1994)
damage (whole blood culture)
a Plate incorporation and spot tests
b The metabolic activity of the cell cultures was demonstrated by the positive control response
c NA = not applicable
Methomyl was tested in the sex-linked recessive lethal assay with
Drosophila melanogaster at concentrations of 4 and 10 mg per litre
in the test medium. No increase in mutation rate was observed in this
assay (Waters et al., 1982).
A formulation (Lannate 20) containing 20% methomyl, but of
otherwise unstated composition, caused an increase in the number of
sex-linked recessive lethals, but with no observed translocations.
The same formulation increased the incidence of abnormal sperm and
frequency of chromosomal aberrations in mice (Hemavathy &
Krishnamurthy, 1987a,b).
7.7 Carcinogenicity
The carcinogenic potential of methomyl was assessed in the
long-term rat and mouse studies already described in section 7.3.
In the earliest study, groups of 35 male and 35 female rats were
fed 0 (2 groups), 50, 100, 200 or 400 mg methomyl/kg diet for 22
months. No differences were seen in the incidence of neoplastic
lesions between control and treated groups (Kaplan & Sherman, 1977).
In the more recent rat study, groups of 80 males and 80 females
were fed 0, 50, 100 or 400 mg methomyl/kg diet for 2 years. The
mortality rates were similar in all groups and there was an overall
survival of 40-50% by the end of the study. More than 30 tissues/rat
were examined microscopically from animals killed or dying during the
study and at interim and terminal sacrifice. There was no indication
of any differences between groups in the appearance of any individual
tumour nor were there any differences between groups for the number of
tumour-bearing rats or those with benign or malignant tumours (Kaplan,
1981).
The 2-year mouse study was initiated at dietary levels of 0, 50,
100 and 800 mg/kg with groups of 80 males and 80 females. By week 39
the highest dose level had been reduced to 200 mg/kg and the 100 mg/kg
level to 75 mg/kg (see section 7.3 for details). Mortality was higher
in the highest dose male and female groups than in controls throughout
the study and was also somewhat higher in the mid-dose group.
Adequate numbers of mice survived to the termination of the study
(30-50% for female groups and 38-68% for male groups).
Histopathological examination on more than 30 tissues per mouse was
undertaken on animals killed or dying during the study and at terminal
sacrifice. There were no differences between groups for the numbers
of tumour-bearing mice or those with benign or malignant tumours
(Serota et al., 1981).
Methomyl did not transform hamster embryo cells in a
trans-placental host-mediated assay, nor did the cultured cells from
this assay induce tumours when injected subcutaneously into young
adult nude mice (Quarles et al., 1979).
7.8 Other special studies
7.8.1 Cholinesterase studies in vivo and in vitro
Due attention must be given to the measurement of cholinesterase
activity in blood and brain samples because of the fast spontaneous
reactivation of the methomyl-inhibited enzyme. It is important to
analyse samples in the minimum possible time after sampling and to use
an analytical method which requires the minimum of sample dilution and
shortest assay time. If these criteria are not followed then
cholinesterase inhibition due to methomyl can be misinterpreted.
In groups of rats fed 0, 50, 100, 200, 400 or 800 mg methomyl per
kg diet for 79 days, only those fed the highest dose showed blood
acetylcholinesterase activity inhibition as assayed by the Ellman
method. Males showed blood cholinesterase activity inhibition of
25-40% early in the study (by 11 days) while females showed this
inhibition towards the end of the study period (Singles, 1970a). In
an extension of this study, the blood acetyl-cholinesterase activity
of rats fed 400 or 800 mg/kg was assayed by the Ellman method and a pH
stat method after 5 months of feeding. In neither group was there an
effect on blood acetyl-cholinesterase activity (Singles, 1970b).
Methomyl was fed to groups of rats in the diet at levels of 0,
100, 400 or 800 mg/kg, and blood was taken for red cell and plasma
acetylcholinesterase activity assay at 1, 7, 14, 21 and 28 days.
Brain acetylcholinesterase activity was also measured on rats at 14
and 29 days. All assays were undertaken by the Ellman method. There
were small depressions of acetylcholinesterase activity (up to 20%) in
red cells and plasma of males and females at 800 mg/kg. Brain
acetylcholinesterase activity was also slightly depressed (<17%) at
this dose level. There were no consistent findings of
acetylcholinesterase inhibition at the two lower levels of 100 and
400 mg/kg (Barnes, 1978).
Lannate, containing 90% methomyl, was given to 14 male rats at
41 mg/kg body weight by oral intubation each day for 8 successive
days. The animals were killed 24 h after the last dose and blood was
taken for the assay of serum cholinesterase activity by a colorimetric
method. The activity was depressed by 40% compared to controls
(Borady et al., 1983).
Cholinesterase activity in plasma and red cells of rats was
assayed after the dermal application of methomyl. Four groups of rats
were treated dermally with 200 mg methomyl per rat and blood samples
were collected 2, 4, 6 and 24 h after treatment. Plasma
cholinesterase activity was markedly reduced at each time interval
compared to controls (22-50% of control value) while red cell
cholinesterase activity was less affected (80-91% of control value).
Groups of rats were also treated dermally with 25 or 100 mg per rat
and blood was collected at 24 and 72 h after treatment. Plasma
cholinesterase activity was depressed at both levels and at both time
intervals, but red cell acetylcholinesterase was unaffected (Henry,
1981).
Lannate powder, containing 45% methomyl, was the subject of
inhalation studies using a single 4-h exposure to 9.9 mg/m3 or
repeated exposures to 14.8 mg/m3 (see section 7.2 for details).
Plasma cholinesterase activity was markedly depressed (by 50%) for 4 h
after the single exposure, but showed recovery almost to the control
value within 20 h. Red cell cholinesterase activity was not
inhibited. Plasma cholinesterase activity was less affected (29%
depression) 4 h after the last of the repeat exposures than after the
single exposure (Ta'Naka et al., 1987).
The I50 value (the molar concentration of inhibitor giving 50%
reduction in enzyme activity in given experimental conditions) for
methomyl was determined in vitro for human and rat red blood cell
acetylcholinesterase activity using a modified Ellman method. Human
acetylcholinesterase was approximately six times more sensitive to
methomyl's action than rat acetylcholinesterase (I50 values of
0.265 × 10-5 mol/litre and 1.56 × 10-5 mol/litre, respect-ively).
Regeneration studies using a gel filtration method showed that
methomyl-inhibited rat acetylcholinesterase regenerates at a faster
rate than the human enzyme, with half-lives of 26.6 and 38.0 min,
respectively (Carakostas, 1987).
7.8.2 Neurotoxicity
Hens given a single oral (LD50) dose of 28 mg methomyl/kg
either died within 10 min or survived. Survivors showed toxic signs
of lacrimation and salivation, but no evidence of wing or leg
paralysis. After a 21-day recovery period, no abnormalities were
observed upon histopathological examination of the sciatic nerve.
Doses much higher than the oral LD50 (up to 200 mg/kg) were given to
hens without causing mortality after they had been pre-treated with
atropine sulfate subcutaneously at 10 mg/kg. Wing or leg paralysis
was not observed and there were no histopathological abnormalities in
the sciatic nerve after a 21-day recovery period. Birds treated with
tri- o-cresyl phosphate (TOCP) at 500 mg/kg showed the leg paralysis
and sciatic nerve degeneration expected of a positive control
substance (Krauss & Stula, 1967).
7.8.3 Potentiation studies
Potentiating properties were determined by administering one-half
the oral LD50 of methomyl to rats, followed immediately by one-half
the LD50 of another anticholinesterase pesticide, and then recording
the resultant mortality over 14 days. Out of 18 pesticides tested,
only Sevin and Ronnel showed the ability to increase mortality
indicative of a potentiation effect (Sherman, 1967). In another study,
the inhibition of cholinesterase activity in rat blood after the oral
administration of methomyl alone or in combination with another
anticholinesterase pesticide was used to measure potentiating effects.
Methomyl in combination with methyl parathion or phosdrin was shown to
be potentiating, while antagonism occurred when it was combined with
dimethoate (Henry, 1975).
Rats (10 per sex and per group) were given methomyl at 200 mg/kg
diet and ethanol as a 10% aqueous solution, either separately or
together, for a period of 12 weeks. There was some evidence of
increased effects as a result of the combined administration resulting
in decreased body weights from weeks 2 (females) and 4 (males) and
increased relative organ weights for adrenals (males) and kidneys
(females). In male rats, the combination of the two compounds
increased hepatic triglycerides and free fatty acids and decreased
brain acetylcholinesterase activity more than that expected from
either compound alone (Antal et al., 1979). Ethanol administered in
the diet to rats, at a level equivalent to 25% of the calorie intake,
did not potentiate erythrocyte acetylcholinesterase inhibition by
methomyl coadministration at 200 mg/kg in the diet (Bracy et al.,
1979).
In female rats only, the combination of methomyl and caffeine
retarded growth, increased the relative weight of kidneys, spleen,
adrenals, liver and heart, depressed glucose-6-phosphate dehydrogenase
activity, and increased aniline hydroxylase and glucose-6-phosphatase
activity (Bedö & Cieleszky, 1980).
7.8.4 Antidote studies
The antidotal properties of several agents against the toxicity
of methomyl have been examined in a number of species.
Potential antidotes were administered within one minute after
oral lethal doses of methomyl (30 or 60 mg/kg) were given to rats. Of
the agents used, atropine sulfate given intraperitoneally as a single
dose of 50 mg/kg proved to be the most effective antidote. Other
agents used which were less effective or ineffective were
pyridine-2-aldoxime methiodide (PAM) and tetra-ethylammonium chloride
(TEAC) (Sherman, 1968a). Rats were also used in a study of the
effectiveness of obidoxime or N-methyl-pyridinium-2-aldoxime
methane-sulfonate (P2S) as antidotes alone or in combination with
atropine sulfate. Methomyl was injected subcutaneously as solutions
of logarithmically graded concentrations (1 ml/kg body weight), and at
the onset of signs of toxicity atropine sulfate (17.4 mg/kg), P2S
(50 mg/kg) and obidoxime (90 mg/kg) were injected subcutaneously
either alone or in a combination. Lethality was reduced by atropine
sulfate or P2S but obidoxime was ineffective. Atropine sulfate in
combination with P2S also was effective (Natoff & Reiff, 1973).
Mice fed a lethal dose of 100 mg methomyl/kg in pellets and then
given TEAC by injection 10 min later survived, and resumed normal
activity within another 10 min (Andrews & Miskus, 1968). Atropine
sulfate (50 mg/kg intraperitoneal) was shown to have antidotal
activity in guinea-pigs given methomyl orally at lethal single doses
of 15-60 mg/kg (Sherman, 1968b). Dogs given a single oral dose of 10
or 20 mg methomyl/kg by capsule were protected against the toxic
effects if atropine sulfate was given very quickly, i.e. within a few
minutes. The antidote was more effective when given intravenously
than when given intramuscularly or orally (Sherman, 1968c).
The antidotal effectiveness of atropine administered
intravenously or orally at 1 or 10 mg/kg and hexamethonium or TEAC
given intravenously at 10 mg/kg was evaluated in rhesus monkeys dosed
with 20 or 40 mg methomyl/kg orally. Atropine at 1 mg/kg, given
intravenously or orally, was effective as an antidote for the
sublethal dose of 20 mg methomyl/kg. Atropine at 10 mg/kg orally,
administered immediately after the appearance of toxic signs, was an
effective antidote for the lethal dose of 40 mg methomyl/kg. Recovery
occurred within 2-5 h of the methomyl dose. TEAC at 10 mg/kg
(intravenous) also prevented the death of one monkey after a lethal
dose of methomyl, although recovery took longer than after atropine.
Hexamethonium did not appear to be very effective (Teeters, 1968).
7.8.5 Other studies
Lannate (90% methomyl) was given to 14 male rats at the high dose
level of 41 mg/kg by intubation each day for 8 days and the animals
were killed 24 h after the last dose for hepatic and serum assays.
Liver weight was not increased, nor was there histopathological
evidence of fatty deposit, but total lipids including cholesterol and
phospholipids were increased. The treatment also increased the level
of serum triglycerides, phospholipids and free fatty acids and
increased the activity of GOT, GPT and alkaline phosphatase enzymes
(Borady et al., 1983). Lung triglyceride, cholesterol and
phospholipid contents were not affected when rats were exposed to
Lannate (45% methomyl) dust at 14.8 mg/m3 by inhalation for 4 h/day,
5 days/week for 3 months (Ta'Naka et al, 1987).
Single oral doses of 2 or 10 mg methomyl/kg increased pancreatic
chymotrypsin, lipase and amylase activities in male and female rats.
Rats fed 100, 400 or 800 mg/kg diet for 10 days showed elevated serum
cholesterol in females only. Hepatic aminopyrine demethylase and
aniline hydroxylase activities were increased in female rats at 400
and 800 mg/kg. When rats were given 100 or 200 mg/kg diet, females
fed 200 mg/kg showed elevation of total serum lipids and cholesterol.
The females also showed decreased hepatic glucose-6-phosphatase
activity and vitamin A at this level (Bedö & Cieleszky, 1980).
The effect of methomyl on a number of serum constituents was
studied in rats given oral doses of 6.8 mg/kg per day, 6 days/week for
4 weeks. Increases were seen over the 4-week period for serum
glucose, cholesterol, GOT and GPT, while decreases were seen for serum
total protein albumin, globulin and cholinesterase activity. However,
since control values did not seem to be available for the 4-week
period, it was difficult to evaluate the significance of the results
for methomyl (Saleh, 1990a,b,c).
7.9 Factors modifying toxicity
It has been shown that synthesized nitrosomethomyl is mutagenic
in vitro and is capable of producing stomach tumours in rats
(Blevins et al., 1977a,b; Lijinsky & Schmahl, 1978). However, when
methomyl is incubated with nitrite and macerated meat under simulated
stomach conditions, there is no evidence that nitrosomethomyl is
formed (see section 4.3).
7.10 Mechanisms of toxicity - mode of action
As a member of the carbamate class of compounds, methomyl has a
well-known mode of action via inhibition of the enzyme
acetylcholinesterase at nerve junctions. A detailed description of
the mechanism of action of carbamates on cholinesterases is given in
Environmental Health Criteria 64: Carbamate pesticides: a general
introduction (IPCS, 1986). Studies with methomyl show that the onset
of toxic action is rapid and that, in common with many other
carbamates, the toxic effect is rapidly reversible. Because of this,
it is important to use appropriate assay methods when measuring
cholinesterase activities during toxicity studies. Failure to allow
for the rapid reversibility of the action can lead to underestimation
of enzyme inhibition.
The acute toxic action of methomyl is characterized by signs of
poisoning typical of anticholinesterase action, i.e. lacrimation,
profuse salivation, tremor and pupil constriction. Surviving animals
quickly show signs of recovery, often within hours. The toxic action
can be countered by the anticholinergic antidote atropine sulfate.
The potency of methomyl is greatest when given by the oral or
inhalation routes of administration. It has very low acute toxicity
when given dermally, presumably because during the slower absorption
phase there is time for recovery from toxic action and thus the effect
is never fully exerted.
8. EFFECTS ON HUMANS
8.1 General population
8.1.1 Accidental and suicidal poisoning
Five Jamaican fishermen prepared a meal to which they
accidentally added methomyl instead of salt. Within minutes of eating
the meal, three of the men were badly affected, twitching, trembling
and frothing at the mouth, and died within 3 h. One of the other two
also showed toxic symptoms, while the other was unaffected. Both
survivors were given atropine and the symptomatic patient recovered
within 2 h after treatment. Postmortem examination of the dead men
revealed highly congested stomach lining, lungs, trachea and bronchi.
Analysis showed that part of the meal (roti) contained about 1%
methomyl. It was estimated that the victims had consumed about
12-15 mg methomyl/kg (Liddle et al., 1979).
A 31-year-old woman committed suicide, using a methomyl
preparation, together with her three children, one of whom (a
9-year-old son) survived. Postmortem examination showed congestion of
the stomach mucous membranes and the lungs. Analysis of organs of the
mother and a 6-year-old son showed highest methomyl concentrations in
the liver (15.4 and 56.5 mg/kg, respectively). Large amounts of
methomyl were present in the stomach contents and it was estimated
that the doses were 55 mg/kg for the mother and 13 mg/kg for the son
(Araki et al., 1982).
A woman attempted suicide by ingesting about 2.25 g methomyl.
After 6 h, methomyl was present in the blood at a concentration of
1.61 mg/kg and in the urine at 10.9 mg/litre; at 15 h the levels were
0.04 mg/kg and 0.25 mg/litre, respectively, and at 22 h methomyl could
not be detected (Noda, 1984).
Symptoms and treatment were described for 11 patients who had
suffered methomyl poisoning in Spain over a 5-year period.
Intoxication was accidental in six cases and suicidal in the other
five. The time interval between exposure and admission for treatment
averaged 2.8 h. All of the subjects showed cholinergic symptoms;
plasma cholinesterase activity was normal in four cases and moderately
reduced in the others. Treatments applied included gastric lavage,
washing the skin, administration of activated charcoal and small doses
of atropine, according to the symptoms involved. All the patients
recovered within 24-48 h (Martinez-Chuecos et al., 1990).
A blood level of 0.57 mg methomyl/litre was measured in a pilot
who had died as a result of a crash while spraying a solution of
methomyl and chlorothalonil in methanol. Analysis was not undertaken
for chlorothalonil and methanol (Driskell et al., 1991).
A 79-year-old man and his 73-year-old wife attempted suicide by
ingesting methomyl powder. The woman died within 19 h but the husband
survived after treatment by gastrolavage, followed by administration
of atropine sulfate. The serum methomyl concentration in the woman
was 44 mg/kg 1 h after ingestion and 0.2 mg/kg in the blood at
autopsy. The methomyl blood concentration of the man was
0.01-0.1 mg/kg 28 h after ingestion (Miyazaki et al., 1989).
8.2 Adverse effects of occupational exposure
Pesticide operators were reported to be affected after handling
powder formulations of methomyl. In one case, an operator mixed a
powder formulation and sprayed vegetables without taking any special
precautions. He displayed poisoning symptoms within 1 h. His blood
cholinesterase activity had decreased to 40% of normal after 12 h but
had recovered to within 80% of normal after 36 h. Other operators, a
mixer-loader, pilot and markers, using recommended safety precautions,
did not show any effects on their red cell or plasma cholinesterase
activities after handling liquid formulations and subsequent aerial
application of methomyl (Simpson & Bermingham, 1977).
In a survey of agricultural applicators in California, USA, in
1982-1989, methomyl was reported to be involved in 129 out of 5371
exposure-related illnesses due to pesticides. For the period
1982-1985 and for the same area, methomyl was considered responsible
for 47 illnesses out of 238 reported cases. Exposure was described as
either short term (< 3 days), longer term (> 3 days) or accidental
(Brown et al., 1989). In 1986, in a summary of reported
pesticide-related illnesses and injuries in California, methomyl was
identified as the probable causative agent in 7 out of 1065 confirmed
occupational cases. These cases were described as "..having some
likelihood of being pesticide related" (Edmiston & Maddy, 1987).
An investigation of workers in a pesticide formulation plant
revealed that 11 out of 102 workers had been hospitalized due to
work-related illness. Most frequent were symptoms of exposure to
methomyl and methaemoglobinaemia due to 3,4-dichloroaniline (Morse et
al., 1979).
Significant T-wave changes (decreased height, inversion, and
leftward deviation of T-wave axis) were shown in 10 out of 22 spraymen
applying methomyl for five days under field conditions. These changes
reverted to pre-exposure levels within one week. Sufficient
information was not available to evaluate the significance of these
findings for operator exposure, and further studies are required to
fully understand their significance (Saiyed et al., 1992).
Two case reports of allergic contact dermatitis with exposure to
methomyl have been described. In the first case, a 26-year-old woman
had gradually worsening itchy hand eczema for six months when working
in a plant nursery, pollinating, pricking out and potting plants
sprayed with methomyl (Lannate) solution. After she avoided touching
the sprayed plants or used polyvinyl gloves she became free of eczema.
She also reacted positively to a 1% aqueous Lannate solution,
indicating that Lannate was a cause of the hand eczema. The second
patient had already had hand eczema for 14 years with recent
worsening. As soon as she changed her job and no longer had contact
with Lannate her eczema disappeared (Bruynzeel, 1991).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effect of methomyl on sewage microorganisms was assessed
indirectly by monitoring total oxygen demand (TOD) over a 24-h period.
A mixture of activated sludge, phosphate buffer and a simulated waste
consisting of Bactopeptone and meat extract was incubated with
methomyl at concentrations of 0, 1, 5, 10, 50 and 90 mg/litre. Only
at 90 mg/litre was there a slight effect on TOD (<10%), indicative of
a minor effect on microorganism activity (Belasco, 1972a).
A fine sand and two silt loam soils were treated with 18 mg
methomyl/kg, and the soil fungi and bacteria populations counted after
11 days. The effect on total microbial activity was determined by
measuring the CO2 evolved periodically over 56 days. Neither soil
microorganism populations nor CO2 production was affected by the
high level of methomyl in the soils, when compared to controls
(Peeples, 1977).
When methomyl was incorporated into a silt loam sand at 0.5 mg/kg
together with ammonium sulfate, no effect on nitrification occurred
over a 6-week period. This level of incorporation represented the
recommended field treatment of 0.56 kg/ha. At ten times this level of
incorporation there was some delay (8-9 days) in reaching the 50%
nitrification level, but by 4 weeks the level was the same as that in
the control soil (Han, 1972).
Methomyl, in the form of Lannate 20L (20% a.i.), was incorporated
into a sandy and a loamy soil at rates of 1.87 and 18.7 mg a.i./kg and
incubated at 20°C for 28 days. Dehydrogenase activity, measured at 14
and 28 days, was unaffected by either rate of incorporation in both
soils. Ammonia nitrogen and nitrate nitrogen declined by about the
same extent in both soils over the 28-day period. This could have
been due to stimulation of nitrogen assimilation by the product
(Danneberg, 1991).
9.2 Aquatic organisms
9.2.1 Algae
The effect of methomyl on algal growth was assessed using
Selenastrum capricornutum as test species. The organism was cultured
under continuous illumination at 24°C with methomyl at concentrations
of 6.25-100 mg/litre for 120 h. Algal growth was monitored at
intervals over the exposure period by measuring the absorbency of
light at 665 nm. The EbC50, (median effective concentration for
inhibition of growth based on a comparison of areas under the growth
curves after "b" hours) after 72 and 120 h was 60 mg/litre. The
ErC50 (median effective concentration for growth inhibition based
on a comparison of maximum growth rates from "x" to "y" hours) from
24-48 h was 140 mg/litre (extrapolated value). The no-observed-effect
concentration was 6.25 mg/litre (Douglas & Handley, 1988).
A similar study was carried out using Lannate 20L (20% methomyl)
as test substance at concentrations of 6.25-200 mg/litre. The EbC50
(72 h) was 68 mg/litre, EbC50 (120 h) was 116 mg/litre and the ErC50
(0-24 h) was 67 mg/litre. A no-observed-effect concentration of
6.25 mg/litre was established (Douglas & Halls, 1991).
9.2.2 Fish
The acute toxicities (96-h LC50, etc.) for several species of
fish are shown in Table 7. The 96-h LC50 values lie mostly within
the range of 0.5-2 mg/litre. The most resistant species tested were
the cutthroat trout with a 96-h LC50 of 4-7 mg/litre. Generally,
within a species, the higher the water temperature the higher the
acute toxicity. Results for some methomyl formulations are shown in
Table 8. These show lower acute toxicity, corresponding to the lower
methomyl content. There are few descriptions of toxic signs in
affected fish. In an acute study with carp and tilapia, the fish sank
to the bottom of the tank and moved sluggishly, then became highly
excited 10 min before death (El-Refai et al., 1976). Shrimps became
lethargic before dying (Bentley, 1973; Sleight, 1973).
Two longer-term studies have been undertaken with methomyl and a
formulation. This included a 21-day flow-through study on fingerling
rainbow trout with Lannate 20L (21.5% methomyl) at 13°C. The
concentrations tested were 0.63, 1.3, 2.5, 5.0, 10 and 20 mg/litre,
equivalent to 0.14-4.3 mg methomyl/litre. No effects on fish length
or weight were noted throughout the study. Some increase in mortality
occurred, from day 12 onwards, in the 5 mg/litre group, and greater
dose-related increases were seen at the two higher concentrations
throughout most of the study. Surviving fish at these concentrations
showed increasing incidence of one or more of the signs of
discoloration, lying on the tank bottom, gasping, bloated stomach,
loss of equilibrium. The 21-day LC50 was calculated to be
6.1 mg/litre formulation (equiv. 1.3 mg methomyl/litre) and the
no-observed-effect concentration was 2.5 mg/litre formulation (Baer,
1991c).
Table 7. The acute toxicity of methomyl to fish (static test)
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Atlantic salmon (Salmo salar) 12 1.12 USDI (1978)
(0.48 g) 17 0.56 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) 12 2.00 USDI (1978)
22 0.86 USDI (1978)
23 2.00 Carter & Graves (1973)
22 1.00 USDI (1975)
Brook trout (Salvelinus fontinalis) 12 1.50 USDI (1978)
Carp (Cyprinus carpio) (5 cm) 25 2.8 Yoshida & Nishiuchi (1972)
Channel catfish (Ictalurus punctatus) 22 0.53 USDI (1978)
(1.8 g) 26 0.92 Carter & Graves (1973)
Cutthroat trout (Salmo clarkie) 10 6.8 USDI (1978)
10 4.05 USDI (1975)
Fathead minow (Pimephales promelas) - 2.8 Mayer & Ellersieck (1986)
(0.75 g)
Japanese goldfish (Carassius auratus) 25 2.7 Yoshida & Nishiuchi
(4 cm) (1972)
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi (1972)
Table 7. (cont'd).
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi (1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
Table 8. The acute toxicity of some methomyl formulations to fish (static test)
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Bluegill sunfish (Lepomis macrochirus) 24% kg a.i. - 0.7 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) Lannate 20 L 21.5 5.1 Baer (1991a)
Bluegill sunfish (Lepomis macrochirus) Lannate 90% WDP - 2.1 USDI (1978)
Carp (Cyprinus carpio) Lannate 25 WP 22 4.7 - Ökolimna (1980)
12.5
Carp (Cyprinus carpio) (1.75 g) Lannate 24 EC 22-25 2.96 El-Refai et al. (1976)
Carp (Cyprinus carpio) (31.5 g) Lannate 24 EC 1.21 El-Refai et al. (1976)
Rainbow trout (Salmo gairdneri) Lannate 20 L 12.6 18 Baer (1991b)
Lannate 90% WDP 13 3.4 McCain (1971)
Tilapia (Tilapia nilocita) (1.5 g) Lannate 24EC 22-25 0.92 El-Refai et al. (1976)
Tilapia (Tilapia nilocita) (13.8 g) 0.88
Channel catfish (Ictaurus punctalus) 24% liquid form - 0.3 USDI (1978)
Channel catfish (Ictaurus punctalus) 29% liquid form 0.3 USDI (1978)
Brook trout (Salvelinus fontinalis) 24% liquid form - 2.2 USDI (1978)
Table 8 (cont'd).
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi
(1972)
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi
(1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
In an early life-stage toxicity study, fathead minnow embryos and
larvae were exposed for 28 days to methomyl at concentrations of
27-491 µg/litre. The water was changed at the rate of 10 aquarium
volumes each 24 h and was maintained at a temperature of 25°C. Embryo
hatch and larval survival and growth was evaluated. The percentage
embryo hatch was not affected at any concentration. Larval survival
was significantly reduced at concentrations down to 117 µg/litre and
growth reduced at 243 and 491 µg/litre. The maximum acceptable
toxicant concentration was estimated to be >57 µg/litre and
<117 µg/litre (Driscoll & Muska, 1982).
9.2.3 Other aquatic organisms
The acute toxicity results for a variety of other aquatic
organisms are shown in Table 9. Daphnia magna appears to be one of
the most susceptible species to the acute toxic action of methomyl,
and the Eastern oyster the least susceptible. This difference was
further emphasized in two other studies. The 48-h EC50 for the
Lannate 20L formulation (21.5% methomyl) was 0.033 mg/litre
(equiv.0.007 mg methomyl/litre) for Daphnia magna neonates (Baer,
1991d). The 96-h EC50 for the Eastern oyster was > 140 mg/litre,
as measured by the effect of methomyl on shell deposition in a
flow-through test at 21.2 to 23.7°C (Ward & Boeri, 1991).
The effect of methomyl or its formulation on Daphnia magna was
studied in longer-term tests on survival, growth and reproductive
capacity. A study was initiated with < 24-h-old daphnids exposed to
measured methomyl concentrations of 0.7, 1.0, 1.6, 3.5, 7.5 or
13.8 µg/litre. The test was conducted over a 21-day period at 20°C
under semi-static conditions with 2-day renewal of the test
solutions. Methomyl was found to be stable in these solutions for up
to 72 h. Daphnid survival and growth were not affected at any
concentration. The number of young produced and number of young per
adult were reduced at 3.5, 7.5 and 13.8 µg/litre. There was some
delay in the first day of reproduction at all levels, but this was
considered to be biologically significant only at 3.5 µg/litre or
more, where the number of young was affected. The maximum acceptable
toxicant concentration was estimated to be between 1.6 and
3.5 µg/litre (Brittelli, 1982).
In another 21-day study, Daphnia magna were exposed to Lannate
20 (21.5% methomyl) at nominal concentrations of 0.63, 1.5, 3.4, 8.
18, 43, 100 or 232 µg/litre. The test conditions and parameters
measured were as described above. At 232 µg/litre all daphnids died
early in the study, but there was no effect on survival at the lower
concentrations nor was growth affected. The 21-day EC50, based upon
adult survival, was estimated to be 160 µg/litre (equivalent to 26 µg
methomyl/litre). The total young produced and number of young per
adult were decreased at 3.4 µg/litre but not at 8 µg/litre. The
21-day no-observed-effect concentration was therefore considered to be
8 µg/litre, equivalent to 2.1 µg methomyl/litre (Baer, 1991e).
Table 9. The acute toxicity of methomyl to other aquatic organisms (static test)
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Midge (Chironomus plumosus) mature 95% technical 22 0.088 (EC50) USDI (1978)
24% conc 0.032 USDI (1978)
Eastern oyster (Crassostrea embryo/ technical 20 4.0 (EC50) Ward & Boeri (1990)
virginica) larvae
Fiddler crab (Uca pugilator) 20 mm Lannate L 21 2.38 Bentley (1973)
(24%)
Grass shrimp (Palaemonetes 18 mm Lannate 90 21 0.049 Sleight (1973)
vulgaris
18 mm Lannate L 21 0.130 Bentley (1973)
(24%)
Gammarus pseudolimnaeus mature technical 17 0.92 USDI (1978)
Mud crab (Neopanope texana) 15 mm Lannate 90 21 0.41 Sleight (1973)
Mysid shrimp (Mysidopsis bahia) 10 day 99% technical 21.5 0.22 Ward & Boeri (1989)
Pink shrimp (Panaeus duorarum) 55 mm Lannate 90 21 0.019 Sleight (1973)
Stonefly (Pteronarcys dorsarta) naiad technical 7 0.034 USDI (1975)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Stonefly (Pheronarcella badia) Year Class 1 99% technical 0.069 Mayer & Ellersieck (1986)
Stonefly (Pheronarcella badia) Year Class 1 24% a.i. 0.06 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 95% technical 0.034 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 24% a.i. 0.92 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 95% a.i. 0.343 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 24% a.i. 0.029 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 99% technical 0.92 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 0.72 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 1.05 Mayer & Ellersieck (1986)
(flowthrough)
Water flea (Daphnia magna) neonate 95% technical 18 0.032 Goodman (1978)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Water flea (Daphnia magna) 1st instar 95% technical 21 0.009 (EC50) USDI (1978)
Water flea (Daphnia magna) - 24% conc 0.0076 USDI (1978)
Water flea (Daphnia magna) - Lannate 20L 20.4 0.033 Baer (1991e)
(21.5)
Water flea (Daphnia magna) 1st instar 95% technical 0.088 Mayer & Ellersieck (1986)
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The acute toxicity of a liquid (Lannate 20L) and a solid (Lannate
25WP) methomyl formulation to earthworms (Eisenia foetida andrei) was
determined over a 14-day period. The formulations were mixed with
artificial soil (70% industrial sand, 20% kaolite and 10% moss peat)
at six concentrations of 0-500 mg/kg (Lannate 20L) and 0-1000 mg/kg
(Lannate 25WP), with 40 earthworms per concentration. The 7-day
LC50 values were estimated to be 165 mg/kg and 147 mg/kg,
respectively, and the 14-day LC50 values 102 mg/kg and 87 mg/kg,
respectively (Armstrong et al., 1991; Caley et al., 1991).
Laboratory tests were undertaken to determine the broadcast
dosage of methomyl, as a 90% SP, required to kill 50% of earthworms
(night crawlers, Lumbricus terrestris L). The test substance was
mixed with moistened potting soil at six rates from 0 to 35 kg/ha with
40 worms per rate. The estimated rate causing 50% mortality was
11.4 kg methomyl/ha (Ruppel & Laughlin, 1977).
The biochemical changes in experimental snails, Eubania
vermiculata (Müller), were studied after treatment with 0.2%
methomyl in bran bait (w/w) for periods of 1, 3, 5, 7 and 10 days.
There was a significant reduction in total soluble proteins, lipids
and glycogenic content, and significant increase of glutamic
oxalocetic transaminase, glutamic pyruvic transaminase and
catalase activities (El-Wakil & Radwan, 1991).
A bran methomyl bait (0.5% w/w) was tested for its molluscicidal
activity on the white garden snail (Theba psiana) and compared with
that of four other oxime carbamate pesticides (aldicarb, aldoxycarb,
oxamyl and thiofanox). Methomyl had the most potent molluscicidal
activity. The time for 50% mortality of snails (LT50) for methomyl,
oxamyl, aldoxycarb, aldicarb and thiofanox was 2.31, 3.97, 4.69, 5.77
and 6.67 days, respectively. The activities of acetylcholine
esterase, acid phosphatase and alkaline phosphatase were inhibited by
the pesticides in line with their potency. GOT and GPT activities
were significantly increased by methomyl (Radwan et al., 1992).
Juvenile (4-week-old) laboratory reared earthworms
(Allolobophora caliginosa) were kept individually in soil treated
with methomyl for 7 days. Relative toxicity, i.e. concentration in
soil (mg/kg) causing zero growth, compared to the standard,
carbofuran, (0.10) was 0.54 (Martin, 1986).
The acute toxicity of methomyl to bees has been reported by
several investigators using different types of test. In one such test
groups of 20 bees were treated individually with a range of doses, the
methomyl being applied to the thorax of each bee in a 1 µl acetone
solution. The mortality was determined after 48 h and the LD50 was
calculated as 0.1 µg/bee (Meade, 1984). The acute toxicity of
methomyl by topical application to the honey-bee (Apis mellifera) was
compared to that of the Western Yellow jacket (Vespula pensylvanica)
after the pleural application of a 1 µl solution in acetone to each
insect. Methomyl was less toxic to the honey-bee, with a 48-h LD50
of 12 µg/g, than to the Yellow jacket (0.9 µg/g) (Johansen & Davis,
1972). A contact LD50 of 1.29 µg per bee has been reported by
Atkins et al., (1976) and an oral LD50 of 0.2 µg/bee by Clinch &
Ross (1970).
The speed of action of methomyl on honey-bees was assessed in a
special laboratory trial. A commercial formulation (90% DP) was
diluted in 33% sucrose solution to give concentrations equal to 1.5, 2
or 4 x LD90. These were fed to bees and the effects observed as two
stages; the first was characterized by very fast and erratic movement
and the second by the bees being unable to walk or fly. The time
intervals to reach each stage were recorded (20 bees), as were the
times taken for 50% and 90% bees to be affected. Methomyl was the
fastest acting of 10 pesticides tested, taking about 3-5 min to give a
50% effect level. It was suggested that this type of information was
needed when interpreting the results of field trials (Clinch & Ross,
1970).
In a tent trial, a methomyl formulation (35% methomyl) was
applied to Phacelia at a 0.3% concentration in the evening after bees
had finished flying and then rewetted early next morning prior to the
start of flight. Observations were made later that day and during the
following two days. Fewer bees visited the trial tent during this day
than visited the control tent. Large numbers of bees were found dead
in front of the hive and at the trial tent edges (about one order of
magnitude higher) on the first day. On the second day about three
times the number of dead bees were found when compared to the control.
Fewer bees were found dead when the trial was repeated with the spray
deposit left dry (Stute, 1983).
Methomyl was among 400 pesticides assessed in honey-bee
laboratory and field studies by the University of California over 20
years. The compound was placed in the highly toxic category, i.e.
severe losses should be expected if the pesticide is used when bees
are present at treatment or within a day thereafter. It was shown
that the contact LD50 of 1.29 µg/bee can be converted directly to
1.29 kg/ha, this being the application rate expected to cause a 50%
mortality among foraging bees in a treated field crop, at the time of
application or shortly thereafter. Several recommendations were
included to help minimize bee losses when spraying with insecticides,
including early morning or night applications, since there is less
risk to honey-bees at these times (Atkins et al., 1976).
9.3.2 Birds
The results for methomyl in acute oral and short-term dietary
studies in birds are shown in Tables 10 and 11. In an acute toxicity
study on the bobwhite quail, a dose of 5.62 mg/kg produced lethargy,
reduced reaction to external stimuli, loss of coordination and lower
limb weakness within 30 min of dosing. The birds recovered within 2 h.
The effects increased at higher doses and included salivation and wing
droop, but recovery in survivors was rapid, within hours to one day.
There were no mortalities at 10 mg/kg or less (Beavers, 1983).
Chickens given methomyl by capsule at 25 or 50 mg/kg showed muscle
tremor, ataxia, salivation, convulsions and death within 30 min
(Palmer & Schlinke, 1978).
In a one-generation study, 20-week-old bobwhite quail, 16 male
and 16 female per group, were fed diets containing 0, 50, 150 or
500 mg methomyl/kg for 20 weeks in their first breeding season.
Assessments included adult clinical health, weight gain and food
consumption and the reproductive parameters for numbers of eggs laid,
development of eggs, viability of embryos, percent hatchability,
offspring survival and eggshell thickness. There were no signs of
toxicity in the adults or of treatment-related mortality. There was a
slight decrease in body weight gain in males at 500 mg/kg up to week
8. No effects on reproductive parameters were seen at the two lower
dietary levels. No treatment-related effects were observed for egg
shell thickness at methomyl concentrations of 50, 150 or 500 mg/kg.
Mean thicknesses of bobwhite quail eggs were 0.221 (± 0.02), 0.216
(± 0.01) and 0.214 (± 0.01) mm, respectively. Treatment groups did
not differ significantly from the control (0.212 ± 0.02 mm). A small
but biologically significant reduction in the numbers of eggs laid per
hen and a subsequent reduction in the numbers of offspring were seen
at 500 mg/kg. A clear no-observed-effect concentration of 150 mg
methomyl/kg diet was therefore determined (Beavers et al., 1991a).
A one-generation study of similar design and the same dietary
concentrations was undertaken with the mallard duck for a period of 18
weeks. There were no signs of toxicity or treatment-related
mortalities in the adults. No effect was seen on body weight at the
two lower levels but a decrease in weight gain occurred in hens fed
500 mg/kg over the last 10 weeks of the study. Egg shell thickness
was not affected by methomyl. Mean shell thicknesses of eggs from
exposed ducks were 0.378 (±0.03), 0.379 (±0.07) and 0.370 (±0.03) mm
for 50, 150 and 500 mg/kg, respectively. No significant differences
from the control (0.378 ± 0.03 mm) were detected. A slight reduction
in the percentage of viable embryos was observed at 500 mg/kg. A
clear no-observed-effect concentration was therefore established at
150 mg/kg (Beavers et al., 1991b).
Table 10. Acute toxicity studies on birds
Species Test Acute oral LD50 Reference
(mg/kg)
Bobwhite quail (Colinus virginianus) intubation, suspended in corn oil 24.2 Beavers (1983)
Japanese quail (Coturnix japonica) intubation, suspended in 34 Smith (1982)
carboxymethylcellulose
Chicken (White Leghorn-Cornish) capsule minimum toxic dose Palmer & Schlinke (1978)
25
Mallard duck (Anas platyrhynchos) 15.9 Tucker & Crabtree (1970)
Pheasant (Phasianus colchicus) 15.4 Tucker & Crabtree (1970)
Common pigeon (Columba livia) in polyethylene glycol 10.0 Schafer (1975)a
Common grackle (Quiscalus quiscala) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) in polyethylene glycol 31.6 Schafer (1975)a
House sparrow (Passer domesticus) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) gavage in propylene glycol 42 Schafer (1972)
Redwing (Agelaius phoeniceus) gavage in propylene glycol 10 Schafer (1972)
a Schafer EW Jr (1975) Avian toxicity tests - letter to Dr HJ Thome, April 28 1975 (unpublished report submitted to WHO by Du Pont)
Table 11. Dietary toxicity studies on birds
Species 8-day Test Dietary LC50 Reference
(5 days feeding, 3 days (mg/kg)
observation)
Bobwhite quail (Colinus virginianus) 4 concentrations 1100 Heath et al. (1972)
Japanese quail (Coturnix japonica) 6 concentrations 3124 Heath et al. (1972)
Mallard duck (Anas platyrhynchas) 6 concentrations 2883 Heath et al. (1972)
Bobwhite quail 7 concentrations 3680 Busey (1967)
Pekin duck 7 concentrations 1890 Busey (1967)
Methomyl did not give any indication of a teratogenic effect in a
chick embryo test when it was injected into the yolk (Proctor et al.,
1976).
9.4 Field studies
Bobwhite quails, 6 males and 6 females, were exposed to sprays of
Lannate formulations, equivalent to a field application of 1 kg
methomyl/ha, once every 48 h for a total of four applications. The
birds were killed 14 days after the last application. There were no
observable effects due to exposure and no treatment-related changes in
tissues on gross examination (Aftosmis, 1973a,b).
A simulated field trial was carried out on two groups of bobwhite
quail (three males and three females per group). One group was fed
prior to treatment, the other group was fed 12 h after treatment. The
treatment consisted of spraying methomyl over the test area at the
rate of 1.1 kg/ha. In all, six sprayings were made, each 5 days
apart, with a 15-day observation period after the last application.
Some weight loss occurred in the group fed prior to treatment;
otherwise there were no observable effects on surviving birds
compared to controls and no changes due to treatment upon gross
examination of tissues (Hinkle & Cameron, 1980).
In 1978, 400 ha of Maine (USA) forest land was treated with the
formulation Lannate LV at the rate of 0.28 kg methomyl/ha to control
spruce budworm. Monitoring of more than 30 species of songbirds was
undertaken over 6-day census periods pre-spray, immediately post-spray
and 2 weeks post-spray. The census was carried out firstly by
individual bird counts and then by territorial counts. Searches were
made for dead birds. Spray deposit cards were used to ensure that the
insecticide was present in the census plots. A concurrent sampling
and analytical programme determined the residue levels of methomyl in
trees and other plants, leaf litter, soil and water. The maximum
level detected was 15 mg/kg in foliage from the top third of the
trees. Underbrush had initial residues of 6 mg/kg, low levels were
found in leaf litter, negligible amounts in soil and none in water.
These levels dissipated rapidly apart from leaf litter where low
levels lasted 34-61 days. The study revealed no evidence of changes
in the activity levels among songbirds. No dead or intoxicated birds
were found and there was no disruption of nesting birds (Brown, 1978).
A study of brain cholinesterase activity was undertaken on wild
mice (Mus musculus) trapped over a period of 3 days after a soya
bean field had been sprayed with Lannate 1.8 L at 0.5 kg methomyl/ha.
Some inhibition of brain cholinesterase activity occurred at a level
of about 10% over the study period indicating the possibility of a
small effect (Montz et al., 1983).
A census of birds was undertaken before and after spraying a
field of hops in Kent, United Kingdom, with 0.56 kg methomyl per ha.
Records were made of birds seen, feeding in the hop field, singing and
nesting. Monitoring was carried out 1, 3 and 10 days after spraying.
Principal species observed feeding upon the sprayed area included
blackbird, song thrush, various tits and finches, robin, wren and
hedge sparrow. There was no apparent difference in feeding habits
before or after spraying and no unhealthy or dead birds were found
over the total observation period (Orpin, 1971).
Methomyl (90% SP), applied at 3.4 kg/ha pasture, produced
decreases in population and biomass of three species of earthworms of
28.5% and 14%, respectively. The compound was considered to be the
least active of the seven pesticides tested (Tomlin & Gore, 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The proposed toxicological criteria for setting guidance values
are presented in Table 12.
10.2 Evaluation of effects on the environment
Adsorption of methomyl to soil is low to moderate with hardly any
desorption. Aerobic degradation in soil (with a half-life of around
one week) is about twice as fast as anaerobic degradation. A relative
increase in soil organic matter delays degradation.
Table 12. Proposed toxicological criteria for setting guidance values
Exposure scenario Relevant route/effect Result/remarks
Short-term oral, acute, several species including human highly toxic; rat LD50 = 17 mg/kg body weight;
(1-7 days) LOELa= 5 mg/kg body weight
eye, irritation, rabbit mild irritant
inhalation, acute, rat highly toxic; LC50 = 0.26 mg/litre (4-h aerosol);
NOELb = 0.14 mg/litre
dermal, acute, rat and rabbit low toxicity; intact skin - LD50 > 2000 mg/kg body weight;
NOELc = 2000 mg/kg body weight in the rabbit
Medium-term repeat dermal, rabbit 21-day study; no toxicologically significant effects at
(1-26 weeks) 50 mg/kg body weight per day
repeat oral, rat 13-week study; NOEL = 3.6 mg/kg body weight per day
maternal oral, rabbit teratology study; NOEL = 6 mg/kg body weight per day
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body weight per day
a mild cholinergic signs of toxicity in guinea-pig
b no-observed-effect level for clinical signs and death
c no clinical symptoms noted except signs of skin irritation
Despite the above adsorption characteristics, leaching of
methomyl in soil to levels deeper than 20 to 30 cm has not been
observed. The concentrations of methomyl in both surface and well
water are below the limit of detection. Methomyl is degraded rapidly
with a half-life of about one week in water and sediment. In sterile
water, methomyl is stable for at least 30 days at normal environmental
pH.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. In contrast,
when applied to soil, uptake through the roots occurs readily with
rapid translocation to the leaves. Adsorbed foliar residues degrade
with a half-life in the order of 4 days. Absorbed methomyl
concentrations in food crops decline rapidly to about 5% within one
week; this may be due to growth dilution.
Bioaccumulation by rainbow trout did not occur in a flow-through
study. Depuration occurred within one day of transfer to clean water.
Trout were discoloured when exposed to levels between 0.075 and
0.75 mg/litre, an effect that disappeared within 5 days, the time
depending on original exposure concentration.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil; nitrification can be
delayed at applications 10 times higher. An aquatic green alga showed
a NOEC for growth of 6.25 mg/litre.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl, the LC50s being of the order of 10 to
100 µg/litre. MATC values from two 21-day Daphnia studies were
estimated to be around 2 µg/litre. Kills of aquatic invertebrates are
expected following overspray.
Fish, both freshwater and estuarine, are less sensitive, the
LC50s ranging from 0.5 to 7 mg/litre. Two longer-term studies gave
an NOEC of 0.5 mg/litre for lethality of fingerling rainbow trout and
an MATC of > 0.06 and > 0.12 mg litre for survival of embryo larval
stages of fathead minnow. Given the low persistence of methomyl and
its relatively low acute toxicity to fish, the risk is expected to be
low.
Laboratory tests in artificial soil with methomyl formulations
(25% WP) established a 14-day LC50 of 90-100 mg formulation/kg for
earthworms. It was estimated that 11 kg a.i./ha of methomyl would
kill 50% of earthworms. A field application of 3 kg a.i./ha caused
reduced earthworm population (28%) and biomass (14%). The TER for
earthworms is around 40 indicating low risk.
Methomyl is classified as highly toxic to honey-bees, the topical
LD50 being approximately 0.1 µg/bee. Field (tent) trials showed
dead bees both at the treatment tent and hive when residues from
spraying the previous day were wetted. Less effect was seen with dry
residues. Methomyl has not been implicated in bee incidents in the
field; this probably reflects advice to restrict spraying times to
protect bees.
Acute oral LD50s for various bird species range between 10 and
40 mg/kg body weight. Dietary LC50s (5 days) range from 1100 to
3700 mg/kg diet. Methomyl poses an acute oral risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds. An example of a toxicity exposure ratio
for birds and fish is shown in Table 13.
The NOEC for reproduction was established at 150 mg/kg diet for
both the bobwhite quail and mallard duck. Field studies following
spraying of methomyl formulations in forests showed no mortality of
songbirds and no changes in feeding behaviour or general activity.
Reduced fat deposits of songbirds reflect reduced insect prey. It was
not considered that methomyl poses a threat to birds after recommended
applications.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
Table 13. Toxicity exposure ratios for birds, fish and aquatic invertebrates based on application rates of 2.5 kg a.i./ha
methomyl to soybeans (worst case)
Risk category LC50 (mg/litre or mg/kg diet) Estimated exposure Toxicity/exposure ratio (TER)c
(mg/litre or mg/kg diet)a,b
Acute bird 10 50-364 02-0.027
Acute fish (stream) 0.5 0.2 2.5
Acute fish (pond) 0.5 0.04 12.3
Acute aquatic invertebrate 0.009 0.2 0.045
(stream)
Acute aquatic invertebrate 0.009 0.04 0.225
(pond)
a Estimated environmental concentration in the terrestrial environment (for bird exposure) is based on the stated application rate
and the assumption of deposition on short grass using the US EPA monogram
b Aquatic exposure concentrations were taken from the STREAM model based on a single application and estimated run-off into water;
no direct overspray is included
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate likely exposure to toxic concentrations
by organisms in the different risk categories
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
Considering the toxicological characteristics of methomyl, both
qualitatively and quantitatively, it is concluded, on the basis of the
no-observed-effect level of 3 mg/kg body weight per day in the 2-year
toxicity study on dogs and applying a 100-fold uncertainty factor,
that 0.03 mg/kg body weight per day will probably not cause adverse
effects in humans by any route of exposure.
12. FURTHER RESEARCH
No further studies were thought to be necessary.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has discussed and
evaluated methomyl on several occasions since 1975. Residue aspects
were discussed in 1975, 1976, 1977, 1978, 1986, 1987, 1988, 1989, 1990
and 1991 (FAO/WHO, 1976, 1977, 1978, 1979, 1986, 1987, 1988a,b,
1990a,b,c, 1991). Toxicological evaluations took place in 1978, 1986
and 1989, when an Acceptable Daily Intake (ADI) of 0-0.03 mg/kg body
weight was established (FAO/WHO, 1979, 1987, 1990a,b).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for methomyl in various commodities
(FAO/WHO, 1993).
It should be noted that the 1992 CCPR meeting decided to combine
MRLs for thiodicarb and methomyl into a single list. In the cases of
different MRLs the higher limit would prevail.
Methomyl is listed in Class IB ("Highly Hazardous") in the WHO
Recommended Classification of Pesticides by Hazard and
Guidelines to Classification (1994-1995) on the basis of its rat acute
oral LD50 value of 17 mg/kg body weight (IPCS, 1994).
The time-weighted average (TWA) adopted by the American
Conference of Governmental Industrial Chemists (ACGIH) is 2.5 mg/m3
(ACGIH, 1994-1995).
REFERENCES
* Report submitted to WHO by E.I. Du Pont de Nemours and Co.,
Wilmington, Delaware, USA
Aftosmis JG (1973a) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamine acid, S-methyl ester (25% a.i.) (Lannate L
methomyl insecticide). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-354-73*).
Aftosmis JG (1973b) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamic acid, S-methyl ester (30% a.i.) (non-flammable
Lannate L methomyl insecticide), Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-606-73*).
Ambridge EM (1992) Report on field trials to assess operator
contamination during application of two different pesticides to crops
of varying heights in Thailand, November/December 1991 (NRI Contract
No. 00195) (Unpublished report*).
ACGIH (1994-1995) Threshold limit values for chemical substances in
the work environment. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, p 28.
Andrews TL & Miskus RP (1968) Tetraethylammonium chloride as an
antidote for certain insecticides in mice. Science, 159: 1367-1368.
Antal M, Bedö M, Constantinovits G, Nagy K, & Szépvölgyi J (1979)
Studies on the interaction of methomyl and ethanol in rats. Food
Cosmet Toxicol, 17: 333-338.
Araki M, Yonemitsu K, Kambe T, Idaka D, Tsunenari S, Kanda M, &
Kambara T (1982), [Forensic toxicological investigations in fatal
cases of carbamate pesticide methomyl (LannateR) poisoning.] Nippon
Hoigaku Zasshi, 36: 584-588 (in Japanese).
Armondi S (1991a) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-394 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-345-91*).
Armondi S (1991b) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-425 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report HLO 346-91*).
Armondi S (1992) Closed-patch repeated insult dermal sensitization
study (maximization method) with DPX-X1179-424 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-659-91*).
Armstrong K, Caley CY, Hall BE, & Knight B (1991) Lannate 20L:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inveresk Research International Ltd (Unpublished report
No. 8546*).
Atkins EL, Anderson LD, Kellum DD, & Neuman KW (1976) Protecting honey
bees from pesticides. Berkeley, California, University of California,
Division of Agricultural Sciences, 15 pp (Leaflet 2883).
Baer KN (1991a) Static, acute, 96-hour LC50 of DPX-X1179-423 to
bluegill sunfish (Lepomis macrochirus). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-30-91*).
Baer KN (1991b) Static, acute, 96-hour LC50 of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-29-91*).
Baer KN (1991c) Flow-through, 21-day toxicity of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-34-91*).
Baer KN (1991d) Static, acute, 48-hour EC50 of DPX-X1179-423
(LannateR 20L) to Daphnia magna. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-150-91*).
Baer KN (1991e) Chronic toxicity of DPX-X1179-423 (LannateR 20L) to
Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-292-91*).
Barnes JR (1978) Cholinesterase tests with methomyl. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-280-78*).
Battelle (1991) Method for determination of oxamyl and methomyl in
groundwater. Columbus, Ohio, Battelle Institute (Unpublished report
No. AMR-1392-89*).
Beavers JB (1983) An acute oral toxicity study in the Bobwhite with
H-15,000. Easton, Maryland, Wildlife International Ltd (Unpublished
report No. HLO-464-83*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991a) A one-generation
reproduction study with the Northern Bobwhite (Colinus virginianus).
Easton, Maryland, Wildlife International Ltd (Unpublished report
No. HLO-337-91*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991b) A one-generation
reproduction study with the mallard (Anas platyrhynchos). Easton,
Maryland, Wildlife International Ltd (Unpublished report
No. HLO-336-91*).
Bedö M & Cieleszky V (1990) Nutritional toxicology in the evaluation
of pesticides. Bibl Nutr Dieta, 29: 20-31.
Belasco IJ (1972a) Effect of methomyl on the activity of sewage
microorganisms (Unpublished report No. ML/ME15*).
Belasco IJ (1972b) Methomyl: Incubation with rumen microorganisms
(Unpublished report No. ML/ME26*).
Bentley RE (1973) Acute toxicity of H-8385 to grass shrimp
(Palaemonetes vulgaris) and fiddler crab (Uca pugilator). Wareham,
Massachusetts, Bionomics Inc. (Unpublished report No. HLO-504-73*).
Blevins RD, Lee M, & Regan JD (1977a) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56: 1-6.
Blevins RD, Lijinsky W, & Regan JD (1977b) Nitrosated methylcarbamate
insecticides: Effect on the DNA of human cells. Mutat Res, 44: 1-7.
Boeri RL & Ward TJ (1989) Static acute toxicity of methomyl to the
sheepshead minnow, Cyprinodon variegatus. Hampton, New Hampshire,
Resource Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-700-89*).
Boleij JSM, Kromhout H, Fleuren M, Tieleman W, & Verstappen G (1991)
Re-entry after methomyl application in greenhouses. Appl Occup Environ
Hyg, 6(8): 672-676.
Bonatti S, Bolognesi C, Degan P, & Abbondandolo A (1994) Genotoxic
effects of the carbamate insecticide methomyl. 1. In vitro studies
with pure compound and the technical formulation Lannate 25. Environ
Mol Mutagen, 23: 306-311.
Borady AMA, Mikhail TH, Awadallah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23: 33-44.
Boulton JJK, Boyce CBC, Jewess PJ, & Jones RF (1971) Comparative
properties of N-acetyl derivatives of oxime N-methylcarbamates & aryl
N-methyl carbamates as insecticides and acetylcholinesterase
inhibitors. Pestic Sci, 2: 10-15.
Bracy OL, Doyle RS, Kennedy M, McNally SM, Weed JD, & Thorne BM (1979)
Effects of methomyl and ethanol on behaviour in the Sprague-Dawley
rat. Pharmacol Biochem Behav, 10: 21-25.
Braun HE, Ritcey GM, Frank R, McEwen FL, & Ripley BD (1980)
Dissipation rates of insecticides in six minor vegetable crops grown
on organic soils in Ontario, Canada. Pestic Sci, 11: 605-616.
Braun HE, Ritcey GM, Ripley BD, McEwen FL, & Frank R (1982) Studies of
the disappearance of nine pesticides on celery and lettuce grown on
muck soils in Ontario 1977-1980. Pestic Sci, 13: 119-128.
Brittelli MR (1982) Chronic toxicity of methomyl to Daphnia magna.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-46-82*).
Brock WJ (1989) Repeated dose dermal toxicity: 21-day study with
DPX-X1179-394 (methomyl) in rabbits. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-387-89*).
Brodsky J (1991) Determination of residues of methomyl in grapes, wine
& wine processing fractions by GC-MS following treatment with
"Lannate", season 1990, France. Frankfurt, Germany, Battelle-Europe
(Unpublished report No. BE-A-11-91-10-BF*).
Bromilow RH, Briggs G, Williams MR, Smelt JH, Tuinstra LGMT, & Traag
WA (1986) The role of ferrous ions in the rapid degradation of oxamyl,
methomyl and aldicarb in anaerobic soils. Pestic Sci, 17: 535-547.
Brown HL (1978) The effects of Lannate LV on singing male songbirds in
Maine in 1978 (Unpublished report No. ML/FW-12 report*).
Brown SK, Ames RG, & Mengle DC (1989) Occupational illnesses from
cholinesterase-inhibiting pesticides among agricultural applicators in
California, 1982-1985. Arch Environ Health, 44: 34-39.
Bruynzeel DP (1991) Contact sensitivity to Lannate(R). Contact
Dermatitis, 25: 60-61.
Bull DL (1974) Fate of methomyl on cotton. Environ Entomol,
3: 723-724.
Busey WM (1966) Three-month dietary administration - Rats: Insecticide
1179. Supplement to final report. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-848*).
Busey WM (1967) Acute aqueous exposure - Goldfish, Bluegill, and
Rainbow trout. Acute dietary administration - Peking ducks & Bobwhite
quail: Insecticide 1179. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-888-1*).
Cahill WP, Estesen B, & Ware GW (1975) Foliage residues of
insecticides on cotton. Bull Environ Contam Toxicol, 13: 334-337.
Caley CY, Cameron BD, Hall BE, & Knight B (1991) Lannate 25 WP:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inverest Research International Ltd (Unpublished report
No. 8455*).
Carakostas MC (1987) Inhibition and regeneration kinetics for human
and rat acetyl-cholinesterase exposed to methomyl in vitro. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-379-88*).
Carbonnel E, Puig M, Xamena N, Creus A, & Marco R. (1990) Sister
chromatid exchange in lymphocytes of agricultural workers exposed to
pesticides. Mutagenesis, 5(4): 403-405.
Carter FL & Graves JB (1973) Measuring effects of insecticides on
aquatic animals. La Agric, 16: 14-15.
Christian MS, Hoberman AM, & Fuessner EL (1983) Embryo-fetal toxicity
and teratogenicity study of methomyl in the rabbit. Horsham,
Pennsylvania, Argus Research Laboratories Inc. (Unpublished report
No. HLO-331-83*).
Clark S & Kennedy SM (1990) Analytical method for the quantification
of methomyl in grapes. Sacramento, California, Morse Laboratories
(Unpublished report No. AMR-1806-90*).
Clement C (1987a) Test to evaluate the acute cutaneous primary
irritation and corrosivity in the rabbit. L'Arbresle, France, Hazleton
France, 16 pp (Unpublished report No. 702408*).
Clement C (1987b) Test to evaluate acute ocular irritation and
corrosivity in the rabbit. L'Arbresle, France, Hazleton France, 26 pp
(Unpublished report No. 703313*).
Clinch PG & Ross IGM (1970) Laboratory assessment of the speed of
action on honey bees of orally dosed insecticides. N Z J Agric Res,
13: 717-725.
Council of the European Communities (1991) Council directive of 15
July 1991 concerning the placing of plant protection products on the
market (91/414/EEC). Off J Eur Communities, L230: Part II.
Cox L, Hermosin MC, & Comejo J (1993) Adsorption of methomyl by soils
of southern Spain and soil components. Chemosphere, 27: 837-849.
Danneberg G (1991) Investigation of the effects of Lannate 20L on the
activity of the microflora of soil. Frankfurt, Germany, Battelle
Institute (Unpublished report No. BE-S-11-91-01-DEH-01*).
Dashiell OL (1972) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-518-72*).
Debuyst B & Van Larebeke N (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat Res, 113: 242-243.
Dong MH, Krieger RI, & Ross JH (1992) Calculated re-entry interval for
table rape harvesters working in California vineyards treated with
methomyl. Bull Environ Contam Toxicol, 49: 708-714.
Douglas MT & Halls RWS (1991) The algistatic activity of Lannate 20L.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPC-16(f) 91399*).
Douglas MT & Handley JW (1988) The algistatic activity of methomyl
Tech. (DPX-X1179-00620-06). Huntingdon, United Kingdom, Huntingdon
Research Centre (Unpublished report No. DPT-171(j) 871676*).
Driscoll RR & Muska CF (1982) Early life stage toxicity of methomyl to
fathead minnow. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-528-82*).
Driskell WJ, Groce DF, Hill RH Jr, & Birky MM (1991) Methomyl in the
blood of a pilot who crashed during aerial spraying. J Anal Toxicol,
15: 339-340.
Du Pont (1967) Methomyl - Livestock feeding studies: Milk and meat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Industrial and
Biochemicals Department (Unpublished report No. ML/ME27*).
Du Pont (1973) Petition for residue tolerance - methomyl: Broccoli,
brussel sprouts, cauliflower, spinach, celery. Pesticide Petition 4F
1448, Section D, 1973. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Biochemicals Department (Unpublished report*).
Du Pont (1978) Ecosystem residue study: Spruce/fir forest and cedar
swamp, Princeton, Maine 1978. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. ML/PC-25*).
Du Pont (1982) LannateR insecticide formulations and technical
methomyl - Determination of methomyl (INX-1179) - Reversed-phase
liquid chromatography (RPLC) assay method (Method No. L30.4662/(E).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report [Second issue]*).
Eble JE & Tomic DM (1991) Foliar half-life of methomyl in cotton
leaves. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. AMR-1871-90*).
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards DF (1980) 10 Day subacute skin absorption test on rabbits.
Mewark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-166-80*).
El-Refai A, Fahmy FA, Mahmound FA, Abdel-Lateef, & Imam AKE (1976)
Toxicity of three insecticides to two species of fish. Int Pest
Control, Nov/Dec: 4-8.
El-Wakil HB & Radwan MA (1991) Biochemical studies on the terrestrial
snail Eubania vermiculata (Müller) treated with some pesticides. J
Environ Sci Health, B26(5/6): 479-489.
FAO (1985) Guidelines for the disposal of waste pesticide containers
on the farm. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1976) Pesticide residues in food. Report of the 1975 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. Geneva, World Health
Organization (WHO Technical Report Series No. 592; FAO Plant
Production and Protection Series No. 1).
FAO/WHO (1977) 1976 Evaluations of some pesticide residues in food:
The monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Series
No. 8; AGP:1976/M/14).
FAO/WHO (1978) Pesticide residues in food. 1977 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10 Sup).
FAO/WHO (1979) Pesticide residues in food. 1978 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 15 Sup).
FAO/WHO (1986) Pesticide residues in food - 1986. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78).
FAO/WHO (1987) Pesticide residues in food - 1986. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78/2).
FAO/WHO (1988a) Pesticide residues in food - 1987. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 86/1).
FAO/WHO (1988b) Pesticide residues in food - 1988. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 93/1).
FAO/WHO (1990a) Pesticide residues in food - 1989. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100).
FAO/WHO (1990b) Pesticide residues in food - 1989. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100/2).
FAO/WHO (1990c) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 103/1).
FAO/WHO (1991) Pesticide residues in food - 1991. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper No. 111).
FAO/WHO (1993) Codex Alimentarius - Volume two: Pesticide residues in
food. Rome, Food and Agriculture Organization.
Farrow MG, Cortina T, & Padilla-Nash H (1984) In vivo bone marrow
chromosome study in rats, H15,000. Final Report. Vienna, Virginia,
Hazleton Biotechnologies Corporation (Unpublished report
No. HLO-63-84*).
Fayez V & Baig MRE (1991) Short-term toxicity of methomyl in rats.
Chemosphere, 23(3): 375-382.
Foerst DL & Moye HA (1985) Aldicarb and related compounds in drinking
water via direct aqueous injection HPLC with post column
derivatisation. Cincinnati, Ohio, US Environmental Protection Agency
(EPA/600/D-85/051).
Fossi MC, Leonzio C, Massi A, Lari L, & Casini S (1992) Serum esterase
inhibition in birds: A nondestructive biomarker to assess
organophosphorus and carbamate contamination. Arch Environ Contam
Toxicol, 23: 99-104.
Foster GV (1966a) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl) progress report. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-73-66*).
Foster GV (1966b) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-214-66*).
Friedman PL (1983) Hydrolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. AMR-109-83*).
Freeman PK & Ndip EMN (1984) Photochemistry of oxime carbamates 2.
Phototransformations of methomyl. J Agric Food Chem, 32(4): 877-881.
Fung KH & Uren NC (1977) Microbial transformation of S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate (methomyl) in soils. J Agric
Food Chem, 25(4): 966-969.
Fung KH, Luke RKJ, & Uren NC (1978) Concentrations of methomyl in
Australian tobacco plants following transplant, foliar and soil
treatments. Tob Sci, 22: 24-26.
Gianessi LP & Puffer CA (1992) Insecticide used in US crop production.
Resources for the Future. Washington, DC.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Agrochemical Manufacturers.
Goodman NC (1978) 48-Hour LC50 to Daphnia magnia. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-165-78*).
Gordon M & Richter ED (1991) Hazards associated with aerial spraying
of organophosphate insecticides in Israel. Rev Environ Health,
9(4): 229-238.
Gupta RC, Goad JT, & Kadel WL (1992) Characteristic changes in LDH and
its isoenzymes as biomarkers under the influence of acute methomyl
toxicity. Fed Am Soc Exp Biol J, 6(4): A1307.
Han JCY (1972) Evaluation of possible effects of methomyl on
nitrifying bacteria in soil (Unpublished report ML/ME16*).
Han JCY (1975) Absence of nitroso formation from [14C] methomyl and
sodium nitrite under simulated stomach conditions. J Agric Food Chem,
23: 892-896.
Harvey J Jr (1967) Exposure of S-methyl N-[(methyl carbomoyl)oxy]
thioacetimidate in sunlight, water and soil (Unpublished report
No. ML/ME-10*).
Harvey J Jr (1972a) Decomposition of 14C-methomyl in aerated river
water exposed to sunlight (Unpublished report No. ML/ME-13*).
Harvey J Jr (1972b) Decomposition of 14C-methomyl in a high organic
matter soil (Unpublished report No. ML/ME-18*).
Harvey J Jr (1977a) Decomposition of 14C-methomyl in a sandy loam
soil in the greenhouse (Unpublished report No. ML/ME-19*).
Harvey J Jr (1977b) Degradation of 14C-methomyl in Flanagan silt
loam in biometer flasks (Unpublished report No. ML/ME-20*).
Harvey J Jr (1978) Crop rotation study with 14C-methomyl in the
greenhouse. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Biochemicals Department (Unpublished report No. ML/ME-22*).
Harvey J Jr (1980) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report No. AMR-22-80*).
Harvey J Jr (1983) Photolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Chemicals
Department (Unpublished report No. AMR-121-83*).
Harvey J Jr & Pease HL (1973) Decomposition of methomyl in soil. J
Agric Food Chem, 21(5): 784-786.
Harvey J Jr & Reiser RW (1973) Metabolism of methomyl in tobacco, corn
and cabbage. J Agric Food Chem, 21: 775-783.
Harvey J Jr & Yates RA (1967) Metabolism of methomyl in the corn
plant. I. Plant growth chamber - Carbon 14 studies (Unpublished report
No. ML/ME2*).
Harvey J Jr, Jelinek AG, & Sherman H (1973) Metabolism of methomyl in
the rat. J Agric Food Chem, 21(5): 769-775.
Hashimoto Y & Fukami J (1969) Toxicity of orally and topically applied
pesticide ingredients to Carp Cyprinus carpio Linné. Botuy Kagaku,
34: 63-66.
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1991) The metabolism of
[1-14C] methomyl in rats. Huntingdon, United Kingdom, Huntingdon
Research Centre and Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products (Unpublished report No. AMR-1584-90*).
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1992) The metabolism of
[1-14C] methomyl in male cynomolgus monkeys. Huntingdon, United
Kingdom, Huntingdon Research Centre (Unpublished report
No. AMR-1902-90*).
Heath RG, Spann JW, Hill EF, & Kreitzer JF (1972) Comparative dietary
toxicities of pesticides to birds. Washington, DC, US Department of
the Interior, Fish and Wildlife Service (Special Scientific
Report-Wildlife No. 152).
Hemavathy KC & Krishnamurthy NB (1987a) Mutagenicity studies in
Drosophila melanogaster with Lannate 20. Mutat Res, 191: 41-43.
Hemavathy KC & Krishnamurthy NB (1987b) Evaluation of Lannate 20, a
carbamate pesticide in the germ cells of male rice. Environ Res,
42: 362-365.
Henry NW III (1975) LannateR L methomyl insecticide potentiation
studies. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-278-75*).
Henry JE (1981) Acute dermal methomyl-cholinesterase response study in
male rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-303-81*).
Hill KM, Hollowell RH, & Dal Cortivo LA (1984) Determination of
N-methylcarbamate pesticides in well water by liquid chromatography
with postcolumn fluorescence derivatization. Anal Chem, 56: 2465-2468.
Hinkle S & Cameron JT (1980) Simulated field trial in Bobwhite quail,
H-13 099. Final report. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished report No. HLO-97-80*).
Holt RF (1971) LannateR residue cooking studies. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Hornberger CS (1967) Acute inhalation toxicity of aqueous spray mist.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-171-67*).
Hosling GC (1969) Dietary administration - Cotornix Quail: INX-1179
(Unpublished report No. HLO-238-69*).
Huhtanen K & Dorough HW (1976) Isomerization and Beckmann
rearrangement reactions in the metabolism of methomyl in rats. Pestic
Biochem Physiol, 6: 571-583.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - A
general introduction. Geneva, World Health Organization.
IPCS (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization (Unpublished document WHO/PCS/94.2).
Ivie KF (1980) High performance liquid chromatography (HPLC) in
pesticide residue analysis. In: Zweig G & Sherman J ed. Updated
general techniques and additional pesticides. New York, London,
Academic Press, pp 55-58.
Jackson GC, Hardy CJ, Gregson RL, Offer JM, & Gopinath C (1991)
Lannate 20L: Acute inhalation toxicity in rats 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPT-247/91521*).
Johansen CA & Davis HG (1972) Toxicity of nine insecticides to the
Western yellowjacket. J Econ Entomol, 65: 40-42.
Kaplan AM (1981) Long-term feeding study in rats with S-methyl
N-[(methylcaramoyl)oxy] thioacetimidate (methomyl, INX-1179). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-235-81*).
Kaplan AM & Sherman H (1977) Toxicity studies with methyl
N-[[(methylamino) carbonyl]oxy]-ethanimidothioate. Toxicol Appl
Pharmacol, 40: 1-17.
Kelly DP (1992) Acute inhalation toxicity study with DPX-X1179-440 in
rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-326-92*).
Kennedy SM (1989) Field soil dissipation of Lannate(R) L insecticide.
Sacramento, California, Morse Laboratories Inc. (Unpublished report
No. AMR-1215-88*).
Kennedy CM (1991) Field soil dissipation of Lannate(R) L insecticide
- A 1991 study. Sacramento, California, Morse Laboratories Inc. and
Lincoln, Nebraska, Harris Environmental Technologies Inc. (Unpublished
report No. AMR-1921-91*).
Kennedy CM & Hay RJ (1991a) Magnitude of residues of methomyl
insecticide in citrus and its processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1361-89*).
Kennedy CM & Hay RJ (1991b) Magnitude of residues of methomyl
insecticide in cottonseed and its processed fractions. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1355-89*).
Kiigemagi U & Deinzer ML (1979) Dislodgeable and total residues of
methomyl on mint foliage. Bull Environ Contam Toxicol, 22: 517-521.
Kiigemagi U, Wellman D, Cooley EJ, & Terriere LC (1973) Residues of
the insecticides phorate and methomyl in mint hay and oil. Pestic Sci,
4: 89-99.
Knaak JE, Jackson T, Fredrickson AS, Rivera L, Maddy KT, & Akesson NB
(1980) Safety effectiveness of closed transfer, mixing, loading and
application equipment in preventing exposure to pesticides. Arch
Environ Contam Toxicol, 9: 231.
Krauss WC & Stula EF (1967) Oral LD50 and delayed paralysis tests
(Hens). Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-161-67*).
Labare A (1990) Testing of DPX-X1179 through FDA multi-residue
protocols A-E. Beltsville, Maryland, Biospherics Inc. (Unpublished
report No. AMR-1405-89*).
Leidy RB, Domanski JJ, Haire PL, & Sheets TJ (1977) Effects of
weathering and flue-curing on methomyl residues on tobacco. Arch
Environ Contam Toxicol, 5: 199-206.
Leistra M, Dekker A, & Van Der Burg AMM (1984) Computed and measured
leaching of the insecticide methomyl from greenhouse soils into water
courses. Water Air Soil Pollut, 23: 155-167.
Leitch RE & Pease HL (1973) LannateR-methomyl. In: Sherma J & Zweig
G ed. Analytical methods for pesticides and plant growth regulators,
7th ed. New York, London, Academic Press, pp 331-338.
Lheritier M (1991a) Test to evaluate the acute toxicity following a
single oral administration (LD50) in the rat. L'Arbresle, France,
Hazleton France (Unpublished report No. 104367*).
Lheritier M (1991b) Test to evaluate the acute toxicity following a
single cutaneous application (limit test) in the rat. L'Arbresle,
France, Hazleton France (Unpublished report No. 106393*).
Liddle JA, Kimbrough RD, Needham LL, Cline RE, Smrek AL, Yert LW, &
Bayse DD (1979) A fatal episode of accidental methomyl poisoning. Clin
Toxicol, 15(2): 159-167.
Lijinsky W & Schmahl D (1978) Carcinogenicity of N-nitroso derivatives
of N-methylcarbamate insecticides in rats. Ecotoxicol Environ Saf,
2: 413-419.
Lu CC (1983) NudrinR: Two-generation reproduction study in rats
(Protocol No. RA-274). Houston, Texas, Shell Development Co.,
Westhollow Research Center (Unpublished report*).
McAlack JW (1973) Ten-day subacute exposure of rabbit skin to LannateR
L insecticide. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report HLR 24-73*).
McCain JC (1971), Acute fish toxicity study - static freshwater:
Lannate, MR-581, H-6854. Falls Church, Virginia, Hazleton Laboratories
Inc. (Unpublished report No. HLO-71-71*).
McConnel R, Pacheco Anton AF, & Magnotti R (1990) Crop duster aviation
mechanics: High risk for pesticide poisoning. Am J Public Health,
80(10): 1236-1239.
McCooey KT, Chromey NC, Sarrif AM & Hemingway RE (1984), CHO/HGPRT
assay for gene mutation. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-556-83*).
Macieira OJD & Hebling Beraldo MJA (1989) Laboratory toxicity of
insecticides to workers to Trigona spinipes. J Apic Res, 28(1): 3-6.
Martin NA (1986) Toxicity of pesticides to Allolobophora caliginosa
(Oligochaeta: Lumbricidae). N Z J Agric Res, 29: 699-706.
Martinez-Chuecos J, Molinero-Somolinos F, Solé-Violan J, & Rubio-Sanz
R (1990) Management of methomyl poisoning. Hum Exp Toxicol,
9: 251-254.
Marxmiller RL & Hay RJ (1991) Magnitude of residues of methomyl
insecticide in tomatoes and their processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1360-69*).
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res,
24(1): 11-21.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Meade AB (1984) Methomyl toxicity to honey bee. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. METH/ECO 9*).
Mercier O (1991) Test to evaluate the sensitising potential by topical
applications in the Guinea-pig. "The Buehler Test". L'Arbresle,
France, Hazleton France (Unpublished report No. 106348*).
Merricks DL (1990) Lannate insecticide - Field worker exposure study
in grape girdling and harvesting operations. Frederick, Maryland,
Agrisearch Incorporated, Sacramento, California, Morse Laboratories
Inc. and Fresno, California, Siemer and Associates Inc. (Unpublished
report No. AMR-1442-89*).
Miles CJ & Oshiro WC (1990) Degradation of methomyl in chlorinated
water. Environ Toxicol Chem, 9: 535-540.
Miyazaki T, Yashiki M, Kojima T, Chikasue F, Ochiai A, & Hidani Y
(1989) Fatal and non-fatal methomyl intoxication in an attempted
double suicide. Forensic Sci Int, 42: 263-270.
Monson KD (1989) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-22-80*).
Monson KD & Ryan DL (1991) [14C] Methomyl cow metabolism study.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-1675-90*).
Montz WE, Scanlon PF, & Kirkpatrick RL (1983) Effects of field
application of the anti-cholinesterase insecticide methomyl on brain
acetylcholinesterase activities in wild Mus musculus. Bull Environ
Contam Toxicol, 31: 158-163.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrow RW (1972) Acute skin absorption study on rats using technical
methomyl and a 25% methomyl formulation (Lannate(R) 25W). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-438-72*).
Morse DL, Baker EL, Kimbrough RD, & Wiseman CL (1979)
Propanil-chloracne and methomyl toxicity in workers of a pesticide
manufacturing plant. Clin Toxicol, 15(1): 13-21.
Natoff IL & Reiff B (1973) Effect of oximes on the acute toxicity of
anticholinesterase carbamates. Toxicol Appl Pharmacol, 25: 569-575.
Niven CF Jr (1971) Thermal destruction of Lannate in spinach
processing. Walnut Creek, California, Del Monte Corporation Research
Center (Unpublished report*).
Noda J (1984) [Determination of methomyl by using chemical ionization
mass fragmentography. A case report of methomyl poisoning and the
animal experiment of its poisoning.] Nippon Hoigaku Zasshi, 38: 71-82
(in Japanese).
Ökolimna (1980) [Fish toxicity, carps, Lannate 25 WP.] Burgwedel,
Germany, Ökolimna (Unpublished report*) (in German).
Orpin R (1971) Methomyl - Study of effects on wild life. Glaston,
United Kingdom, Farm Protection Ltd (Unpublished report*).
Osman AZ, Hazza A, Nagwa I, & Awad TM (1983) Fate and metabolism of
the insecticide 14C-Lannate in farm animals. Isot Rad Res,
15(2): 111-120.
Oswald T, Adams J & Hicks SC (1991), LannateR insecticide -
Dislodgeable foliar residue study in Rose Greenhouse operations.
Fresno, California, Siemer and Associates Inc. (Unpublished report
No. AMR-1909-90*).
Owens CB, Owens EW, & Zahn D (1978) The extent of exposure of migrant
workers to pesticide and pesticide residues (Abstract). Int J
Chronobil, 5(2): 428-429.
Palmer JS & Schlinke JC (1978) Preliminary toxicological evaluations
of six pesticide compounds in cattle, sheep and chickens (Unpublished
report METH/TOX5*).
Panepinto AS (1991a) Acute inhalation toxicity study with
DPX-X1179-427 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-678-91*).
Panepinto AS (1991b) Acute inhalation toxicity study with
DPX-X1179-424 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-560-91*).
Paynter OE (1966) Three-month dietary administration - Rats.
Insecticide 1179. Falls Church, Virginia, Hazleton Laboratories Inc.
(Unpublished report*).
Pease HL (1968) Methomyl residue analysis - Soils. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Pease HL & Kirkland JJ (1968) Determination of methomyl residues using
microcolourmetric gas chromatography. J Agric Food Chem, 16: 554-557.
Peeples JL (1977) Effect of methomyl on soil microorganisms
(Unpublished report No. ML/ME21*).
Powley CR (1989) Methomyl foliar dislodgeable residues on grapes in
California. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. BMP/DFR-189*).
Powley CR (1990a) LannateR insecticide: Dislodgeable foliar residue
study in grape girdling and harvesting operations. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1445-89*).
Powley CR (1990b) LannateR insecticide: Dislodgeable foliar residue
study on grapes grown in California. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-1515-89*).
Powley CR (1991) Determination of possible bioaccumulation of
14C-methomyl in lactating dairy cows. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-898-87*).
Priester TM (1984) Batch equilibrium (adsorption/desorption) and soil
thin-layer chromatography studies with methomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. AMR-174-84*).
Proctor NH, Moscioni AD, & Casida JE (1976) Chicken embryo NAD levels
lowered by teratogenic organophosphorus and methylcarbamate
insecticides. Biochem Pharmacol, 25: 757-762.
Quarles JM, Sega MW, Schenley CK, & Lijinsky W (1979) Transformation
of hamster fetal cells by nitrosated pesticides in a transplacental
assay. Cancer Res, 39: 4525-4533.
Radwan MA, El-Wakil HB, & Osman KA (1992) Toxicity and biochemical
impact of certain oxime carbamate pesticides against terrestrial
snail, Theba psiana (Müller). J Environ Sci Health, B27: 759-773.
Reeve MW, O'Connell LP, Bissell S, & Ross J (1992) Characterization of
methomyl dissipation on grape foliage. Bull Environ Contam Toxicol,
49: 105-111.
Rogers AS, Culik R, Kaplan AM, & Aftosmis JG (1978) Oral teratogenic
study in rats with Lannate (INX-1179). Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-498-78*).
Ruppel RF & Laughlin CW (1977) Toxicity of some soil pesticides to
earthworms. J Kansas Entomol Soc, 50: 113-118.
Saiyed HN, Sadhu HG, Bhatnagar VK, Dewan A, Venkaiah K, & Kashyap SK
(1992) Cardiac toxicity following short-term exposure to methomyl in
spraymen and rabbits. Hum Exp Toxicol, 11(2): 93-97.
Saleh F (1990a) Metabolic effects of the carbamate insecticide
(methomyl) on rats. I. Levels of glucose, lipids and cholesterol in
serum after administration of the insecticide. Egypt J Physiol Sci,
14(1-2): 45-54.
Saleh F (1990b) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in serum cholinesterase and
transaminases following treatment of the insecticide. Egypt J Physiol
Sci, 14(1-2): 55-64.
Saleh F (1990c) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in some blood biochemical indices in
the rats poisoned with the insecticide. Egypt J Physiol Sci,
14(1-2): 65-74.
Sarver JW (1991a) Acute oral toxicity study with DPX-X1179-394 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-661-91*).
Sarver JW (1991b) Acute dermal toxicity study of DPX-X1179-394 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-455-91*).
Sarver JW (1991c) Acute oral toxicity study with DPX-X1179-424 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-171-91*).
Sarver JW (1991d) Primary dermal irritation study with DPX-X1179-394
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-165-91*).
Sarver JW (1991e) Primary dermal irritation study with DPX-X1179-425
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-166-91*).
Sarver JW (1991f) Primary dermal irritation study with DPX-X1179-424
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-162-91*).
Sarver JW (1991g) Primary eye irritation study with DPX-X1179-425 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-280-91*).
Sarver JW (1991h) Primary eye irritation study with DPX-X1179-424 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-279-91*).
Sarver JW (1992a) Acute oral toxicity study with DPX-X1179-439 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-858-91*).
Sarver JW (1992b) Acute dermal toxicity study of DPX-X1179-439 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-825-91*).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Serota DG, Machotka SV, Hastings TF, Alsaker RD, & Lane Fezio W (1981)
104-Week chronic toxicity and carcinogenicity study in mice. Methomyl;
H-11,135. Vienna, Virginia, Hazleton Laboratories America Inc.
(Unpublished report No. HLO-253-81*).
Shah PV, Monroe RJ, & Guthrie FE (1981) Comparative rates of dermal
penetration of insecticides in mice. Toxicol Appl Pharmacol,
59: 414-423.
Sherman H (1966) Acute oral LD50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-210-66*).
Sherman H (1967) Acute oral potentiation studies with S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate [INX-1179). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-160-67*).
Sherman H (1968a) Antidote studies with rats. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-251-68*).
Sherman H (1968b) Acute oral and antidote tests with guinea pigs.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-252-68*).
Sherman H (1968c) Acute oral toxicity and antidote tests in rabbits
using technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-250-68*).
Sherman H (1968d) Acute oral toxicity and antidote tests in dogs using
technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-279-68*).
Sherman H (1972) Chicken & egg study. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-55-72*).
Silveira EJ (1990) Technical methomyl - physical and chemical
characteristics. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products Department (Unpublished report
No. AMR-1753-90*).
Simpson GR & Bermingham S (1977) Poisoning by carbamate pesticides.
Med J Aust, 2: 148-149.
Singles GH (1970a) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-186-70*).
Singles GH (1970b) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-327-70*).
Sleight BH (1971) Continuous exposure of rainbow trout (Salmo
gairdneri) to LannateR in water. Wareham, Massachusetts, Bionomics
Inc. (Unpublished report*).
Sleight BH (1973) Acute toxicity of H-7946 to grass shrimp
(Palaemonetes vulgaris), pink shrimp (Penaeus duorarum) and mud
crab (Neopanope texana). Wareham, Massachusetts, Bionomics Inc.
(Unpublished report No. HLO-186-73*).
Smelt JH, Dekker A, Leistra M, & Houx NWH (1983) Conversion of four
carbamoyloximes in soil samples from above and below the soil water
table. Pestic Sci, 14: 173-181.
Smith LW (1982) Wildlife toxicity studies with methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. ML/FW20*
SRI International (1988) Pesticides and intermediates, Supplement C.
Menlo Park, California, SRI International (Report No. 171C).
Stute K (1983) Results of the registration trials on bee toxicity -
1983. Celle, Germany, Du Pont de Nemours (Germany) GmbH (Unpublished
report No. METH/ECO14*).
Swanson MB (1986) Photodegradation of [1-14C] methomyl in soil.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products Department (Unpublished report No. AMR-611-86*).
Ta'Naka I, Igisu H, Haratake J, Cho S, Mori K, Fujishiro K, Inoue N,
Horie A, & Akiyama T (1987) Cumulative toxicity potential of methomyl
aerosol by repeated inhalation. Am Ind Hyg Assoc J, 48(4): 330-334.
Teeters WR (1968) Lannate antidotal study - monkeys. Falls Church,
Virginia, Hazleton Laboratories Inc. (Unpublished report
No. 201-219*).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a fungicide
on the numbers and biomass of earthworms in pasture. Bull Environ
Contam Toxicol, 12(4): 487-492.
Trivits R (1979) Acute oral LD50 test in rats using technical
methomyl. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-496-79*).
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 74-75 (Resource Publication No. 84).
USDI (1975) Acute toxicity studies. Cutthroat trout and stonefly
larvae. Columbia, Missouri, US Department of the Interior, Fish and
Wildlife Service, Columbia National Fishery Research Laboratory
(Unpublished report No. ML/FW-1*).
USDI (1978) Methomyl - Summary of acute toxicity. Columbia, Missouri,
US Department of the Interior, Fish and Wildlife Service, Columbia
National Fishery Research Laboratory (Unpublished report
No. ML/FW-3*).
USEPA (1988) Methomyl science chapters - June 1988: Parts I and II.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances.
USEPA (1991) Pesticides in ground water database. Washington, DC, US
Environmental Protection Agency, Pesticides and Toxic Substances.
US FDA (1993a) Food and Drug Administration total diet study: 20
market basket studies, October 1986 - April 1991: Findings of methomyl
in food collected for total diet study. Washington, DC, US Food and
Drug Administration.
US FDA (1993b) FDA-Regulatory monitoring data: FY 1988-1992 - Findings
of methomyl in samples of domestic and imported foods. Washington, DC,
US Food and Drug Administration.
US FDA (1993c) FDA-Regulatory monitoring data: FY 1988-1992 - Counts
of samples of domestic and imported foods and feeds analyzed by
methodology known to be capable of determining methomyl. Washington,
DC, US Food and Drug Administration.
Vincent DR (1985) Assessment of methomyl (INX-1179-255) in the
in vitro unscheduled DNA synthesis assay in primary rat hepatocytes.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-149-85*).
Ward TJ & Boeri RL (1989) Static acute toxicity of methomyl to the
mysid, Mysidopsis bahia. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-675-89*).
Ward TJ & Boeri RL (1990) Static acute toxicity of methomyl to bivalve
mollusc embryos and larvae. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-422-90*).
Ward TJ & Boeri RL (1991) Acute flow-through mollusc shell deposition
test with DPX-X1179-394 (methomyl). Hampton, New Hampshire, Resource
Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-710-91*).
Ware GW, Estesen B, & Cahill WP (1978) Dislodgeable insecticide
residues on cotton (1975). Bull Environ Contam Toxicol, 20: 17-19.
Ware GW, Estesen BJ, & Buck NA (1980) Dislodgeable insecticide
residues on cotton foliage: Acephate, AC222705, EPN, fenvalerate,
methomyl, methyl parathion, permethrin and thiodicarb. Bull Environ
Contam Toxicol, 25: 608-615.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. In: Fleck RA & Hollaender A ed. Genetic toxicology: An
agricultural perspective. New York, London, Plenum Press, pp 275-324.
Williams DT, Denley HV, Lane DA, & Quan ESK (1982) Real time
monitoring of methomyl air levels during and after spraying in a
greenhouse. Am Ind Hyg Assoc J, 43: 190-195.
Wojciechowski JP, Kaur P, & Sabharwal PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Yeboah PO & Kilgore WW (1984) Analysis of airborne pesticides in a
commercial pesticide storage building. Bull Environ Contam Toxicol,
32: 629-634.
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Jpn Bull Agric Chem Insp Stn, 12: 122-128.
Zwick TC & Malik N (1990a) Aerobic metabolism of [1-14C] methomyl in
Madera, California soil. Columbus, Ohio, Battelle Memorial Institute,
Environmental Sciences Department (Unpublished report
No. AMR-1543-89*).
Zwick TC & Malik N (1990b) Anaerobic metabolism of [1-14C] methomyl
in Madera, California soil. Columbus, Ohio, Battelle Memorial
Institute, Environmental Sciences Department (Unpublished report
No. AMR-1544-89*).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le méthomyl est un solide cristallin blanc dont le point de
fusion est de 77°C et la tension de vapeur de 0,72 mPa à 25°C. Sa
solubilité dans l'eau est de 54,7 g/litre et son coefficient de
partage octanol/eau (Kow) est de 1,24. Il est stable dans l'eau
stérile à pH 7 mais il se décompose à pH plus élevé, sa demi-vie étant
de 30 jours à pH 9 et 25°C.
Le dosage du méthomyl dans divers échantillons consiste en une
extraction suivie d'une purification, l'analyse finale s'effectuant
par chromatographie en phase liquide à haute performance ou
chromatographie gaz/liquide. Dans certains cas, avant le dosage
proprement dit, on transforme le méthomyl en oxime ou en fluorophore
(post-colonne).
2. Sources d'exposition humaine et environnementale
Le méthomyl est préparé en faisant réagir le
N-hydroxythioacétimidate de S-méthyle (MHTA) en solution dans le
chlorure de méthylène, sur l'isocyanate de méthyle gazeux à 30-50°C.
Il s'agit d'un carbamate à propriété insecticide que l'on utilise
partout dans le monde sur toutes sortes de cultures. Il sert
notamment à protéger les fruits, les vignes, le houblon, les légumes,
les céréales, le soja, le coton et les plantes ornementales. A
l'intérieur des bâtiments, on l'utilise aussi pour détruire les
mouches dans les animaleries et les laiteries.
Il est principalement présenté sous forme de poudre hydro-soluble
ou encore de liquide miscible à l'eau que l'on dilue pour le
traitement des cultures au sol ou en pulvérisations aériennes. Les
doses d'emploi vont habituellement de 0,15 à 1,0 kg de matière active
par hectare. C'est essentiellement au cours de la préparation et de
l'épandage de ces produits ou par suite de l'ingestion de résidus
subsistant sur des cultures vivrières qu'il peut y avoir exposition
humaine (voir section 5.3.1.4).
3. Transport, distribution et transformation dans l'environnement
Les études de laboratoire montrent que le méthomyl est faiblement
adsorbé aux particules du sol. On a ainsi montré que l'adsorption
était faible sur les minéraux argileux en particulier l'illite; sur
les matières organiques du sol, elle est multipliée par 50 tout en
restant encore relativement faible. Une fois adsorbés, les résidus ne
se désorbent pratiquement pas. Du fait de ses propriétés, on peut
penser que le méthomyl doit être doté d'une certaine mobilité dans le
sol.
Dans l'environnement naturel, la décomposition abiotique du
méthomyl par hydrolyse ou photolyse est lente ou nulle.
Dans le sol, la décomposition aérobie est environ deux fois plus
rapide que la décomposition anaérobie. On a fait état de valeurs de
la demi-vie dans le sol qui vont de quelques jours à plus de 50 jours;
la sécheresse retarde la décomposition. Dans la pratique, on peut
considérer que dans la plupart des cas, on aura après épandage, une
demi-vie d'environ une semaine.
Dans les conditions de plein champ, le méthomyl ne pénètre pas
dans le sol, par lessivage, à une profondeur de plus de 20-30 cm et il
n'y a pas de contamination des eaux souterraines.
Lorsqu'on épand du méthomyl marqué au 14C sur les feuilles de
végétaux, il y absorption, mais le produit n'est pas transporté dans
d'autres parties de la plante. Lorsqu'on l'applique sur le système
radiculaire, le composé est fixé par la plante, le principal résidu
étant le méthomyl lui-même. Les produits de décomposition volatils du
méthomyl sont le CO2 et l'acétonitrile. Le reste de la fraction
radioactive se retrouve dans les constituants naturels de la plante
tels que les lipides ainsi que les acides et les sucres du cycle de
Krebs. Dans le feuillage, la demi-vie du méthomyl est de l'ordre de
quelques jours.
Après exposition de truites arc-en-ciel à ce composé pendant 28
jours dans un système à courant d'eau, on n'a trouvé aucun signe
d'accumulation.
4. Concentrations dans l'environnement et exposition humaine
Si l'on en croit les analyses effectuées sur diverses sources
d'eau après épandage du composé aux doses recommandées, il est
probable que les concentrations de méthomyl dans les eaux souterraines
sont soit très faibles, soit inférieures à la limite de détection
(< 0,02 mg/litre).
De faibles résidus de méthomyl se retrouvent sur les cultures,
notamment vivrières, lors de la récolte, les quantités dépendant de
facteurs tels que la dose d'emploi, le laps de temps écoulé depuis le
dernier épandage et le type de culture. Ces résidus sont
essentiellement constitués du produit initial.
Dans les produits laitiers, les résidus de méthomyl sont soit
inférieurs à la limite de détection, soit très faibles. Après
administration de méthomyl à des vaches laitières sous forme de
capsules, pendant 28 jours à une dose équivalant à 80 mg/kg de
nourriture, on n'a pas retrouvé de résidus décelables de ce composé ou
de son métabolite (le MHTA) dans la lait ou les tissus de ces animaux
(< 0,02 mg/kg). Aucune trace de méthomyl n'a été non plus décelée
dans les oeufs ou dans les tissus de poules pondeuses qui en avaient
reçu 1 ou 10 mg/kg dans leur nourriture pendant quatre semaines.
Des analyses effectuées par sondage aux Etats-Unis sur la ration
totale ou sur certains types d'aliments, ont montré que la
concentration de méthomyl était soit très faible, soit inférieure à la
limite de détection. Par ailleurs, un certain nombre d'opérations
tels que le lavage, l'épluchage ou la cuisson, réduisent encore la
teneur en résidus.
Des études effectuées lors du retour des ouvriers agricoles sur
les vignobles après épandage, notamment dans les conditions du désert
de Californie, ont montré que pour un résidu foliaire mobile tombé à
0,1 µg/cm2, l'exposition était maximale au niveau du torse et de la
tête lorsque les ouvriers agricoles pratiquaient l'incision annulaire,
le torse et les mains étant les plus touchés lors de la cueillette du
raisin. En ce qui concerne le raisin de table, c'est lors de la
cueillette et de l'emballage que l'exposition était la plus faible.
L'exposition par inhalation était minime.
Après pulvérisation de méthomyl sur des plants de concombres et
de tomates, on a mesuré dans l'air de la serre qui les abritait, des
concentrations d'insecticide allant jusqu'à 4,7 µg/m3 dans la
journée qui a suivi l'épandage. Trois, puis sept jours après ce
traitement, les concentrations de méthomyl dans la zone de
respiration allaient respectivement jusqu'à 14,5 ou 0,7 µg/m3. Dans
l'eau de lavage des mains des jardiniers d'une serre, on a retrouvé
des quantités de méthomyl allant de 10 à 300 µg/heure de travail.
Cela montre que l'exposition cutanée est une voie d'exposition plus
importante que l'inhalation et que la durée de l'attente à observer
avant de retourner sur les lieux doit être basée sur les données
d'exposition cutanée.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après administration à des rats par voie orale, le méthomyl est
rapidement absorbé, métabolisé et excrété, l'ensemble du processus
étant achevé en quelques jours. Une semaine après avoir administré à
des rats du méthomyl radio-marqué à raison de 5 mg/kg de poids
corporel, on a constaté que 54% de la dose était excrétés dans
l'urine, 2 à 3% dans les matières fécales et 34% dans l'air expiré (en
l'espace de cinq jours). Au bout de sept jours, il restait dans les
tissus et la carcasse 8 à 9% de la radioactivité, le carbone-14 étant
incorporé dans les constituants endogènes. C'est dans le sang que la
fraction radioactive était la plus importante (2% de la dose).
Chez ces rats, les principaux métabolites présents dans l'air
expiré étaient du dioxyde de carbone et l'acétonitrile dans le rapport
d'environ 2:1. Dans les urines, le principal métabolite était
constitué par un dérivé mercapturique du méthomyl, à hauteur de 17% de
la dose. On n'a pas décelé la présence de méthomyl ni de son oxime.
On pense que la métabolisation du méthomyl s'effectue selon le
schéma suivant: déplacement du groupe S-méthyle par le glutathion,
puis transformation enzymatique en dérivé de l'acide mercapturique.
Il existe une autre voie métabolique, à savoir l'hydrolyse en MHTA qui
est ensuite rapidement décomposé pour donner du dioxyde de carbone. Il
pourrait y avoir aussi conversion du syn-méthomyl (qui est la forme
insecticide) en isomère anti, lequel serait ensuite décomposé en
acétonitrile par des réactions d'hydrolyse, de transposition et
d'élimination. Chez le singe, le métabolisme est analogue, à cela
près que le dérivé de l'acide mercapturique ne constitue qu'un
métabolite urinaire mineur.
Une heure après application cutanée à des souris d'une solution
acétonique de méthomyl marqué au 14C, on a constaté que le taux de
pénétration était de l'ordre de 85%. A ce moment, 3% de la dose
étaient présents dans le sang, 5% dans le foie et 13% avaient été
excrétés. Au bout de huit heures, l'excrétion totale était de 54,5%.
La décomposition et l'élimination rapides du méthomyl chez le rat
ainsi que son absence d'accumulation tissulaire, est comparable à ce
que l'on observe chez les ruminants.
Du méthomyl administré à des vaches et à des chèvres a subi une
dégradation totale. Ni le composé initial, ni son oxime n'ont été
retrouvés dans le lait ou les tissus. On a montré que le méthomyl
était métabolisé et incorporé dans les constituants naturels du lait
et du foie.
Après avoir fait incuber du méthomyl marqué au 14C avec du
nitrite de sodium dans un macérat de viande séchée, dans des
conditions simulant le milieu gastrique, on n'a pas mis en évidence la
formation de nitrosométhomyl.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
Le méthomyl présente une forte toxicité aiguë par voie orale, sa
DL50 chez le rat allant de 17 à 45 mg/kg de poids corporel. Il
présente également une forte toxicité inhalatoire chez cet animal, la
CL50 à 4 heures étant de 0,26 mg/litre lorsque le composé est inhalé
sous forme d'aérosol. La toxicité cutanée est très faible, la DL50
dépassant 2000 mg/kg de poids corporel chez le lapin (peau intacte) et
1000 mg/kg de poids corporel chez le rat (peau abrasée). Les signes
d'intoxication aiguë sont ceux que l'on peut attendre d'un inhibiteur
de la cholinestérase et consistent, entre autres, en une
hypersalivation, une lacrimation, des tremblements et un myosis. On a
constaté que les animaux récupéraient rapidement et l'examen de leurs
organes n'a pas révélé la présence de lésions anatomopathologies
visibles qui soient imputables au traitement. Le méthomyl ne provoque
pas d'irritation ou d'hypersensibilisation cutanée et il est
légèrement irritant pour la muqueuse oculaire.
L'administration répétée de méthomyl dans la nourriture pendant
des périodes prolongées n'a pas conduit à une accumulation ou à un
accroissement des effets toxiques. Des rats et des chiens à qui on
avait administré une nourriture contenant jusqu'à 250 mg/kg et
400 mg/kg respectivement de méthomyl, pendant 13 semaines, n'ont
présenté ni signes d'intoxication ni surmortalité. Chez des rats qui
en avaient reçu dans leur nourriture à la dose de 250 mg/kg, on a
observé une légère diminution du gain de poids, une réduction du taux
d'hémoglobine et une hyperplasie modérée de la lignée érythroblastique
au niveau de la moelle osseuse. Chez le rat, la dose sans effets
observables était de 50 mg/kg de nourriture (soit l'équivalent d'une
dose quotidienne de 3,6 mg/kg de poids corporel). Des lapins, qui
avaient subi des applications cutanées répétées de méthomyl à des
doses quotidiennes allant jusqu'à 500 mg/kg de poids corporel, pendant
21 jours, ont présenté une hyperactivité ainsi qu'une réduction de
l'activité cholinestérasique plasmatique et cérébrale à la dose la
plus élevée. La dose sans effets nocifs observables était égale,
selon cette étude, à 50 mg/kg de poids corporel par jour.
Au cours d'études à long terme, des rats ont reçu du méthomyl
dans leur nourriture à raison de 0, 50, 100 ou 400 mg/kg et des souris
en ont reçu de la même manière, 0, 50, 75 ou 200 mg/kg. Chez les
rats, les effets observés à la dose la plus élevée consistaient en une
réduction du gain de poids ainsi qu'en une diminution du taux
d'hémoglobine et de la valeur de l'hématocrite. La dose sans effets
observables était de 100 mg/kg de nourriture, soit l'équivalent d'une
dose quotidienne de 5 mg/kg de poids corporel. Chez les souris, on a
constaté aux deux doses les plus élevées, une augmentation du taux de
mortalité ainsi qu'une diminution du taux d'hémoglobine et du nombre
de globules rouges. La dose sans effets observables a été évaluée à
50 mg/kg de nourriture, soit l'équivalent d'une dose quotidienne de
8,7 mg/kg de poids corporel. Lors d'une étude toxicologique de deux
ans chez des chiens (doses de 0, 50, 100, 400 ou 1000 mg/kg de
nourriture), on a observé des signes d'intoxication chez certains
animaux à la dose la plus forte, avec une anémie légère à modérée. La
dose sans effets observables était de 100 mg/kg de nourriture, soit
l'équivalent d'une dose quotidienne de 3 mg/kg de poids corporel.
Des études de deux ans sur des rats et des souris n'ont révélé
aucun signe d'accroissement de l'incidence des tumeurs qui soient
imputables à l'administration de méthomyl, ce qui indique que le
méthomyl n'est pas cancérogène. Il se s'est pas révélé non plus
génotoxique lors d'épreuves in vitro sur des cellules bactériennes
ou mammaliennes et la recherche de lésions primaires de l'ADN sur des
cellules bactériennes ou mammaliennes in vitro ou encore
d'aberrations chromosomiques médullaires lors d'épreuves in vivo
chez le rat, a donné des résultats négatifs. On a cependant observé
la possibilité d'effets cytogénétiques sur des lymphocytes humains
in vivo, attestée par l'augmentation du nombre de micronoyaux et
d'aberrations chromosomiques. Chez des rats et des lapins qui en
avaient reçu par gavage des doses allant jusqu'à 400 mg/kg de
nourriture (soit une dose quotidienne de 16 mg/kg de poids corporel),
le méthomyl n'a pas produit non plus d'effets embryotoxiques ou
tératogènes, alors qu'à ces doses, des effets toxiques pouvaient être
constatés chez les femelles gravides. Lors d'une étude de
reproduction portant sur trois générations de rats, qui avaient reçu
50 ou 100 mg de méthomyl/kg de nourriture (soit l'équivalent quotidien
de 50 ou 10 mg/kg de poids corporel), on n'a pas constaté d'effets sur
la fécondité, la gestation ou la lactation qui soient imputables au
méthomyl et aucune anomalie visible résultant de ce traitement n'a été
observée.
Après administration unique ou répétée de méthomyl, on n'a pas
observé de neurotoxicité retardée. Des rats qui en avaient reçu dans
leur nourriture à raison de 800 mg/kg, n'ont présenté une diminution
sensible de leur activité cholinestérasique sanguine qu'au début d'une
étude de cinq mois. Une étude d'alimentation de 28 jours n'a révélé
qu'une légère diminution de l'activité cholinestérasique cérébrale à
cette dose. Il y a donc eu réversibilité rapide de l'effet
anticholinestérasique du méthomyl chez les animaux au cours de la
période d'expérimentation. In vitro, l'activité cholinestérasique
érythrocytaire humaine s'est révélée six fois plus sensible à l'action
inhibitrice du méthomyl que celle du rat, mais la réactivation
spontanée s'est produite sensiblement à la même vitesse.
Des études menées sur diverses espèces ont montré que l'atropine
est le meilleur antidote d'une intoxication par le méthomyl.
7. Effets sur l'homme
La lecture des rapports sur les intoxications accidentelles et
les suicides par empoisonnement avec du méthomyl éclaire quelque peu
sur la gravité des effets et les possibilités de récupération. Sur
cinq victimes d'une intoxication accidentelle due à la consommation
d'aliments contaminés, trois sont décédées dans les trois heures
suivant le repas. On estime que les victimes avaient ingéré environ
12 à 15 mg de méthomyl/kg de poids corporel. Une femme âgée de 31 ans
et son fils de six ans, qui étaient tous les deux décédés par suite
d'un empoisonnement volontaire, présentaient des concentrations
hépatiques de méthomyl respectivement égales à 15,4 et 56,5 mg/kg.
Les doses ont été estimées à 55 mg/kg de poids corporel pour la mère
et 13 mg/kg de poids corporel pour l'enfant. Chez une femme qui avait
ingéré environ 2,25 g de méthomyl, on a retrouvé dans son sang six
heures plus tard une quantité de méthomyl égale à 1,6 mg/kg.
Vingt-deux heures après l'ingestion, alors que la patiente se
remettait, on n'a plus retrouvé de méthomyl.
Un travailleur chargé d'épandre des pesticides, qui n'avait pas
pris la moindre précaution alors qu'il préparait une bouillie à base
de méthomyl en poudre pour traiter des légumes, a présenté des
symptômes d'intoxication au bout d'une heure avec réduction à 40% de
la normale de son activité cholinestérasique sanguine au bout de 12
heures, la récupération à 80% de la normale intervenant 36 heures plus
tard. D'autres travailleurs, qui avaient pris les précautions
d'usage, n'ont présenté aucun symptôme ou effet sur l'activité de leur
cholinestérase érythrocytaire ou plasmatique lors de l'épandage de
méthomyl par voie aérienne.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
Le méthomyl n'a eu aucun effet sur des populations de champignons
ou de bactéries terricoles, et en particulier sur leur action
nitrifiante ou sur l'activité de la déshydrogénase, lorsqu'il était
appliqué aux doses recommandées.
Des études en laboratoire ont permis de fixer à 6,5 mg/litre la
concentration sans effets observables sur la croissance des algues.
Le méthomyl est moyennement à fortement toxique pour les
poissons, les valeurs de CL50 à 96 heures se situant, pour diverses
espèces, dans l'intervalle 0,5-2 mg/litre. Une étude à long terme (21
jours) a montré que la CL50 pour des alevins de truite exposés à du
Lannate 20L (21,5% de méthomyl), était égale à 1,3 mg de méthomyl par
litre. Lors d'une étude toxicologique portant sur de jeunes
cyprinidés de l'espèce Pimephales promelas, la MATC (concentration
maximale acceptable de substance toxique) a été trouvée comprise entre
57 et 177 µg/litre.
Des études de toxicité aiguë portant sur d'autres organismes
aquatiques ont montré que Daphnia magna était l'espèce la plus
sensible au méthomyl, la CL50 à 48 heures étant de 0,032 mg/litre.
Lors d'une étude de 21 jours au cours de laquelle on a étudié la
survie, la croissance et la capacité de reproduction de Daphnia magna,
on a constaté que la concentration maximale acceptable de substance
toxique (MATC) pour le méthomyl était comprise entre 1,6 et
3,5 µg/litre.
Le méthomyl est toxique pour les abeilles, la DL50 par contact
étant de 1,29 µg/insecte et la DL50 par voie orale, de
0,2 µg/insecte.
On a évalué la toxicité aiguë du méthomyl chez plusieurs espèces
d'oiseaux, les valeurs caractéristiques de DL50 aiguë par voie orale
se situant à 10 mg/kg de poids corporel chez les pigeons et à 34 mg/kg
de poids corporel chez la caille japonaise. Il est relativement moins
toxique lorsqu'il est mêlé à la nourriture, la CL50 à 8 jours étant
dans ce cas de 1100 mg/kg de méthomyl (mêlé à la nourriture) pour le
colin de Virginie et de 2883 mg de méthomyl/kg de nourriture pour le
colvert. Lors d'études qui ont duré de 18 à 20 semaines et ont porté
sur une génération, on a évalué à 150 mg/kg de méthomyl dans la
nourriture, la concentration sans effets nocifs observables pour le
colin de Virginie et le colvert.
Aucun effet n'a été observé chez des colins de Virginie qui
avaient été exposés à une série de pulvérisations de méthomyl aux
doses recommandées. Après un épandage de méthomyl sur une zone
forestière et des champs de houblon, aux doses recommandées, on a
effectué deux études sur des populations aviaires sauvages. Elles
n'ont pas révélé de modification de l'activité des oiseaux et n'ont
provoqué aucun effet, en particulier aucun effet létal. Par rapport
aux témoins, on a constaté qu'il y avait réduction des dépôts
graisseux chez les oiseaux chanteurs des forêts traitées; on estime
qu'il s'agit là d'un effet indirect dû à la réduction des populations
d'insectes dont se nourrissent ces oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Le méthomyl est un carbamate qui inhibe la cholinestérase et dont
le mode d'action toxique est bien connu. Il présente une toxicité
particulièrement élevée lorsqu'il est absorbé par voie orale ou par
inhalation, ainsi que le montrent les études sur l'animal, mais sa
toxicité par voie cutanée est faible. Chez l'animal, les signes
d'intoxication aiguë sont caractéristiques d'une inhibition de la
cholinestérase. Cette intoxication est rapidement réversible, avec
une disparition rapide des symptômes et une désinhibition également
rapide des cholinestérases sanguine et cérébrale. Cette prompte
récupération est due au fait que l'inhibition par le méthomyl de la
cholinestérase est rapidement réversible, et d'ailleurs facilitée par
la vitesse d'excrétion élevée de ce composé. Ce que l'on sait des
intoxications humaines accidentelles ou volontaires montre que la
toxicité aiguë du méthomyl est du même ordre chez l'homme que chez
l'animal de laboratoire.
Comme l'action du méthomyl est rapidement réversible pendant la
période d'administration, il a rarement été possible d'observer des
signes d'intoxication aiguë et d'inhibition de la cholinestérase
sanguine au cours des études où on l'administrait mêlé à la
nourriture. Les observations les plus régulièrement rapportées à
l'occasion d'études à long terme consistent, aux doses les plus
élevées dans l'alimentation, en une réduction du gain de poids chez
les rongeurs et une diminution des paramètres érythrocytaires chez les
rongeurs et les chiens. Trois études de longue durée chez des
rongeurs n'ont pas permis de mettre en évidence d'activité
cancérogène. Le composé ne s'est pas non plus montré génotoxique lors
d'épreuves in vitro portant sur divers paramètres biotoxicologiques,
néanmoins il y a une possibilité d'effets cytogénétiques sur les
lymphocytes humains. Une étude chromosomique effectuée in vivo sur
la moelle osseuse de rats s'est également révélée négative.
Chacune des études à long terme sur l'animal a permis, à partir
de la réduction du gain de poids et des paramètres érythrocytaires,
d'obtenir une valeur de la dose sans effets nocifs observables. Elle
se situait à 5 mg/kg de poids corporel par jour chez le rat, à
8,7 mg/kg de poids corporel par jour chez la souris et à 3 mg/kg de
poids corporel par jour chez le chien. Faute d'une différenciation
marquée des effets toxiques entre les différentes espèces, il ressort
de ces études que la dose sans effets nocifs observables chez le
chien, c'est-à-dire 3 mg/kg de poids corporel par jour, peut être
utilisée pour évaluer le risque chez l'homme.
Le méthomyl est faiblement à modérément adsorbé aux particules du
sol, et ne s'en désorbe pratiquement pas. Dans le sol, il se
décompose en aérobiose (avec une demi-vie d'environ une semaine)
environ deux fois plus vite qu'en anaérobiose.
Une fois déposé sur les feuilles de végétaux, le méthomyl est
rapidement absorbé à hauteur de 50% de la dose - les 50% restant étant
adsorbés - et rien n'indique qu'il y ait transport à l'intérieur de la
plante. La concentration du méthomyl absorbé par les cultures
vivrières tombe rapidement à environ 5% de sa valeur initiale en
l'espace d'une semaine.
Plusieurs invertébrés aquatiques, et en particulier les daphnies,
sont très sensibles au méthomyl avec des valeurs de la CL50 de
l'ordre de 10 à 100 µg/litre.
Les poissons, qu'il s'agisse d'espèces d'eau douce ou d'espèces
estuarielles, y sont moins sensibles, puisque les valeurs de la CL50
s'étagent entre 0,5 et 7 mg/litre. Etant donné la faible persistance
du méthomyl et sa toxicité aiguë relativement faible pour les
poissons, le risque encouru par ces derniers est relativement faible.
Aux doses d'emploi recommandées, le méthomyl n'a pas d'effets
nocifs sur l'activité microbienne terricole en climat tempéré.
Le méthomyl est classé comme hautement toxique pour les abeilles
avec une DL50 topique d'environ 0,1 µg/insecte.
Les valeurs de la DL50 aiguë par voie orale varient entre 10 et
40 mg/kg de poids corporel chez diverses espèces d'oiseaux. Les
valeurs de CL50 à cinq jours par voie alimentaire vont de 1100 à
3700 mg/kg de nourriture. Il y a risque d'intoxication aiguë pour les
oiseaux, en particulier lorsque le méthomyl est sous forme de
granulés; son absorption à partir de nourriture contaminée ne devrait
cependant pas être mortelle pour les oiseaux.
La forte toxicité aiguë du méthomyl pour les mammifères de
laboratoire permet de conclure qu'il est tout aussi dangereux pour les
mammifères sauvages.
10. Conclusion
Compte tenu des aspects qualitatifs et quantitatifs de la
toxicité du méthomyl, le groupe de travail a conclu qu'une dose
quotidienne de 0,03 mg/kg de poids corporel ne devrait probablement
pas causer d'effets nocifs chez l'homme, quel que soit le mode
d'exposition.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El metomilo es un sólido cristalino blanco con un punto de fusión
de 77°C y una presión de vapor de 0,72 mPa (25°C). Tiene una
solubilidad en agua de 54,7 g/litro y su coeficiente de reparto
octanol/agua (Kow) es de 1,24. Es estable en agua estéril a pH 7,
pero se descompone a pH más elevado, con una semivida de 30 días a pH
9 y 25°C.
El procedimiento analítico para la determinación del metomilo en
muestras diferentes es la extracción seguida de limpieza y análisis
mediante cromatografía líquida de alto rendimiento o cromatografía
gas-líquido. En algunos casos, el metomilo se convierte en su
derivado oxima o en un derivado fluoróforo (después de la columna)
antes de su determinación analítica.
2. Fuentes de exposición humana y ambiental
El metomilo de produce haciendo reaccionar el S-metil- N-
hidroxitioacetimidato (MHTA) en cloruro de metileno con isocianato de
metilo gaseoso a 30-50°C. Es un insecticida de tipo carbamato
utilizado en una gran diversidad de cultivos en todo el mundo. Entre
los cultivos protegidos figuran frutales, vides, lúpulo, hortalizas,
cereales, soja, algodón y plantas ornamentales. En espacios cerrados
se utiliza en establos o vaquerías para luchar contra las moscas.
Las formaciones principales son polvos hidrosolubles y líquidos
hidromiscibles, que se diluyen en agua para el rociado superficial o
aéreo de los cultivos. Las proporciones normales del principio activo
son de 0,15-1,0 kg/ha. La exposición humana ocurre principalmente
durante la preparación y aplicación de estos productos y por ingestión
de residuos que quedan en los alimentos cosechados (véase la
sección 5.3.1.4).
3. Transporte, distribución y transformación en el medio ambiente
En estudios de laboratorio se ha observado que el metomilo se
adsorbe poco al suelo. Se ha demostrado una adsorción débil a los
minerales arcillosos, sobre todo la ilita; la adsorción a la materia
orgánica del suelo es 50 veces mayor, pero sigue siendo relativamente
escasa. Prácticamente no hay desorción del residuo adsorbido. Estas
características llevan a prever que el metomilo tendrá movilidad en el
suelo.
En condiciones medioambientales naturales, su degradación
abiótica por hidrólisis o fotólisis es lenta o no se produce.
La degradación aerobia en el suelo es alrededor de dos veces más
rápida que la anaerobia. Las semividas notificadas para el metomilo
en el suelo varían entre unos días y más de 50. Las condiciones secas
retrasan la degradación. En la práctica, la mayor parte de las
aplicaciones en el campo deberían dar lugar a una semivida de
alrededor de una semana.
En condiciones de campo, el metomilo no sufre lixiviación en el
suelo a profundidades superiores a 20-30 cm y no contamina las aguas
subterráneas.
El 14C-metomilo aplicado a las hojas de las plantas es
absorbido, pero no transportado a otras partes de la planta. Se si lo
aplica en el sistema radicular, la planta lo absorbe y el componente
principal del residuo que queda es el propio metomilo. Los productos
volátiles derivados de su descomposición son CO2 y acetonitrilo. El
resto de la actividad se incorpora a los componentes naturales de la
planta, tales como lípidos, ácidos del ciclo de Krebs y azúcares. La
semivida del metomilo en el follaje de la planta es de unos pocos
días.
No se han encontrado indicios de acumulación del metomilo en
truchas irisadas expuestas al compuesto durante 28 días en un sistema
de flujo continuo.
4. Niveles medioambientales y exposición humana
Los análisis de diversas fuentes de agua tras la aplicación de
las dosis recomendadas del compuesto indican que los niveles de
metomilo en las aguas subterráneas probablemente serán muy bajos o
inferiores al límite de detección (< 0,02 mg/litro).
En los cultivos de productos alimenticios y de otro tipo se
observan niveles bajos de residuos de metomilo en el momento de la
recolección; su concentración depende de diversos factores, como la
cantidad aplicada, el periodo transcurrido desde la última aplicación
y el tipo de cultivo. Los residuos están formados básicamente por
metomilo.
Los residuos de metomilo en los productos lácteos no son
detectables o son muy bajos. No se observaron residuos detectables de
metomilo ni del metabolito MHTA en la leche ni en los tejidos
(< 0,02 mg/kg) de vacas lactantes que habían recibido en el pienso
cápsulas de metomilo en una concentración equivalente a 80 mg/kg
durante 28 días. No se detectó la presencia de metomilo en los huevos
ni en los tejidos de gallinas ponedoras a las que se habían
administrado cantidades de 1 ó 10 mg/kg en los alimentos durante
cuatro semanas.
En los Estados Unidos de América se hicieron análisis de
regímenes completos de alimentación y de determinados alimentos; en
los estudios de muestreo correspondientes, las concentraciones de
metomilo resultaron inferiores al límite de detección o muy bajas.
Los niveles de residuos se reducen aún más por efecto de procesos
tales como el lavado, pelado y guisado de los alimentos.
En estudios sobre la exposición de quienes regresan a zonas
tratadas, en particular en las condiciones del desierto de California,
se ha observado que los trabajadores que volvieron a los viñedos
cuando los residuos foliares que podían desprenderse se habían
reducido a 0,1 µg/cm2, sufrieron la mayor exposición en la parte
superior del cuerpo y en la cabeza durante el atado de los racimos y
en la parte superior del cuerpo y en las manos durante la vendimia.
La recogida y el embalado de las uvas de mesa dieron lugar a la
exposición más baja. La exposición por inhalación fue mínima.
Tras haberse rociado con metomilo plantas de pepino y tomate, las
concentraciones en el aire del invernadero llegaban a 4,7 µg/m3 al
día siguiente del rociado. Tres y siete días después del rociado, las
concentraciones de metomilo en la zona de respiración ascendían a
14,5 y 0,7 µg/m3, respectivamente. Los valores del metomilo en el
agua de lavarse las manos oscilaban entre 10 y 322 µg por hora de
trabajo en un invernadero. Esto indicaba que la vía de exposición
cutánea era más importante que la inhalación y que los intervalos
previos al regreso al lugar tratado deberían basarse en los datos
sobre la exposición cutánea.
5. Cinética y metabolismo en animales de laboratorio
La absorción, el metabolismo y la excreción del metomilo tras la
administración oral a ratas es muy rápida, completándose el proceso en
unos pocos días. En un plazo de siete días después de la
administración a ratas de 5 mg/kg de peso corporal de metomilo
radiomarcado, el 54% se excretó en la orina, el 2-3% en las heces y el
34% en el aire expirado (en cinco días). Después de siete días, en
los tejidos y en el esqueleto quedaba el 8-9% de la dosis de 14C,
que se había incorporado a los constituyentes endógenos. La mayor
concentración de radiactividad se detectó en la sangre (equivalente al
2% de la dosis).
Los componentes metabólicos principales en el aire expirado por
las ratas eran el anhídrido carbónico y el acetonitrilo, en una
proporción de 2:1. El principal metabolito en la orina fue el
derivado mercaptúrico del metomilo, que era igual al 17% de la dosis.
No se detectó metomilo ni su derivado oxima.
La vía metabólica propuesta comprende el desplazamiento del grupo
S-metilo por el glutatión, seguido de una transformación enzimática
que da lugar al derivado mercaptúrico. Otra vía es la hidrólisis, por
la que se produce MHTA, que se descompone rápidamente hasta dar
anhídrido carbónico. Otra vía posible es la conversión del
sin-metomilo (la forma insecticida) en su anti-isómero, que sufre
reacciones de hidrólisis, recomposición y eliminación hasta dar
acetonitrilo. El metomilo se metaboliza de forma semejante en el
mono, salvo que el derivado mercaptúrico es un componente secundario
en la orina.
La penetración del 14C-metomilo una hora después de haber sido
aplicado a ratones por vía cutánea en una solución de acetona se
estimó en un 85%. Para entonces, el 3% de la dosis se hallaba presente
en la sangre, el 5% en el hígado y el 13% se había excretado. En un
plazo de 8 horas la excreción total era del 54,5%.
La descomposición y eliminación rápidas del metomilo en la rata,
junto con su falta de acumulación en los tejidos, son comparables a lo
observado en rumiantes.
El metomilo sufre una descomposición completa en las vacas y
cabras que han recibido una dosis. No se detectaron metomilo ni su
derivado oxima en la leche ni en los tejidos de estos animales. Se
puso de manifiesto que el producto se metabolizaba e incorporaba a los
componentes naturales de la leche y del hígado.
No se detectó la presencia de nitrosometomilo después de haberse
incubado 14C-metomilo con nitrito sódico en un macerado de carne
curada, en condiciones que simulaban las del estómago.
6. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
El metomilo administrado por vía oral tiene una elevada toxicidad
aguda, con una DL50 oral de 17-45 mg/kg de peso corporal en la rata.
También es muy tóxico para la rata por inhalación; en aerosol, la
CL50 a las 4 horas es de 0,26 mg/litro. La toxicidad cutánea es muy
baja; la DL50 es de más de 2000 mg/kg de peso corporal en conejos
(piel intacta) y > 1000 mg/kg de peso corporal en ratas (piel
raspada). Los signos de toxicidad aguda son los que cabe esperar de
un inhibidor de la colinesterasa, entre otros salivación profusa,
lacrimación, temblor y contracción pupilar. La recuperación de estos
efectos fue rápida. En los órganos examinados no se observaron
efectos patológicos graves. El metomilo no irrita ni sensibiliza la
piel, pero irrita levemente los ojos.
La administración repetida de metomilo con los alimentos durante
periodos más largos no produjo aumento de los efectos tóxicos ni
acumulación. Las ratas y perros cuya alimentación contenía metomilo
en cantidades de hasta 250 mg/kg y 400 mg/kg, respectivamente, durante
13 semanas no mostraron signos de toxicidad ni acusaron mortalidad.
Las ratas que recibieron con los alimentos 250 mg/kg mostraron una
pequeña disminución en el aumento del peso corporal, niveles de
hemoglobina más bajos e hiperplasia eritroidea moderada de la médula
ósea. El NOEL en ratas fue de 50 mg/kg en los alimentos (equivalentes
a 3,6 mg/kg de peso corporal por día). Los conejos que recibieron
aplicaciones cutáneas repetidas de metomilo en dosis de hasta
500 mg/kg de peso corporal por día durante 21 días mostraron
hiperactividad y una reducción de la actividad colinesterásica
plasmática y cerebral con la dosis más elevada. El NOAEL en este
estudio fue de 50 mg/kg de peso corporal por día.
Se realizaron estudios de larga duración en ratas que recibían
metomilo en los alimentos en concentraciones de 0, 50, 100 ó 400 mg/kg
y en ratones que recibían 0, 50, 75 ó 200 mg/kg. Los efectos de la
dosis más alta en las ratas fueron una disminución del aumento de peso
y una reducción de la concentración de hemoglobina y del valor
hematócrito. El NOEL fue de 100 mg/kg de alimentos, equivalente a
5 mg/kg de peso corporal por día. En el estudio en ratones, se
observó aumento de la tasa de mortalidad y una reducción de la
hemoglobina y del número de eritrocitos en los dos niveles de dosis
más elevados. El NOEL fue de 50 mg/kg de alimentos, equivalente a
8,7 mg/kg de peso corporal por día. En un estudio de dos años sobre
toxicidad en perros (0, 50, 100, 400 ó 1000 mg/kg de alimentos) con la
dosis más elevada se detectaron síntomas de toxicidad en algunos
animales, junto con anemias de ligeras a moderadas. El NOEL fue de
100 mg/kg de alimentos, equivalentes a 3 mg/kg de peso corporal por
día.
En estudios de dos años en ratas y ratones no se encontró ningún
indicio de aumento de la incidencia de tumores relacionado con el
tratamiento, signo de que el metomilo no es carcinógeno. No fue
genotóxico en célula de bacterias o de mamíferos in vitro y las
pruebas realizadas para determinar la presencia de lesiones primarias
del ADN en células de bacterias y de mamíferos in vitro dieron
resultados negativos; un estudio cromosómico in vivo de médula ósea
de rata también dio resultados negativos. El potencial citogenético
del metomilo en linfocitos humanos in vitro se puso de manifiesto
mediante un aumento del número de micronúcleos y de aberraciones
cromosómicas. El metomilo no produjo efectos embriotóxicos ni
teratogénicos en ratas ni en conejos en dosis de hasta 400 mg/kg en
los alimentos y de 16 mg/kg de peso corporal por día administrados por
sonda, respectivamente; a esos niveles produjo efectos tóxicos en las
madres. En un estudio de reproducción de tres generaciones en ratas
con dosis de 50 ó 100 mg/kg de alimentos (equivalentes a 5 ó 10 mg/kg
de peso corporal por día), el metomilo no tuvo efectos sobre los
índices de fecundidad, gestación o lactación y no se produjeron
anomalías graves relacionadas con el tratamiento.
No se observó neurotoxicidad retardada después de la
administración de una sola dosis o de una serie de dosis de metomilo.
En un estudio de cinco meses en el que se suministraron a ratas
800 mg/kg de alimentos se observó una reducción importante de la
actividad de la colinesterasa sanguínea sólo en las fases iniciales.
En un estudio de 28 días, la administración de esa misma dosis con los
alimentos dio lugar a una reducción ligera solamente de la actividad
de la colinesterasa cerebral. Esto indica que la inhibición de la
actividad de la colinesterasa por el metomilo es reversible
rápidamente en los animales durante los periodos de ingestión.
In vitro, la actividad de la eritrocito colinesterasa humana fue
seis veces más sensible a la acción inhibidora del metomilo que la de
la rata, aunque los índices de reactivación espontánea fueron
semejantes.
Los resultados obtenidos en estudios realizados con varias
especies mostraron que la atropina es el antídoto de eficacia más
general contra la intoxicación por metomilo.
7. Efectos en el ser humano
Las notificaciones de intoxicaciones accidentales y suicidas con
metomilo proporcionan alguna información sobre los niveles de efectos
y la recuperación. Tres de cinco víctimas accidentales de
intoxicación por una comida contaminada murieron antes de que
transcurrieran tres horas desde la ingestión. Se estimó que las
víctimas habían consumido alrededor de 12-15 mg de metomilo/kg de peso
corporal. Una mujer de 31 años y su hijo de seis, que murieron a
causa de un envenenamiento deliberado, tenían en el hígado
concentraciones de metomilo de 15,4 y 56,5 mg/kg, respectivamente.
Las dosis estimadas fueron de 55 mg/kg de peso corporal para la madre
y de 13 mg/kg de peso corporal para el hijo. Seis horas después de
haber ingerido unos 2,25 g de metomilo, la sangre de una mujer
contenía 1,6 mg de metomilo/kg. A las 22 horas de la ingestión no se
podía detectar la presencia de metomilo y la mujer se estaba
recuperando.
Un operario que manipulaba plaguicidas había mezclado, sin tomar
precauciones, una formulación de metomilo en polvo para el rociado de
hortalizas; antes de una hora manifestó síntomas de intoxicación y
después de 12 horas la actividad de colinesterasa sanguínea se había
reducido al 40% de la normal; a las 36 horas la recuperación llegaba
al 80% de la actividad normal. Otros operarios que habían tomado las
precauciones recomendadas no acusaron ningún síntoma ni efectos en la
actividad de la colinesterasa eritrocitaria y plasmática durante
actividades de aplicación aérea de metomilo.
8. Efectos en organismos no destinatarios en el laboratorio y sobre
el terreno
No se observaron efectos en poblaciones de hongos y bacterias del
suelo, en particular en su acción nitrificante y en la actividad de la
deshidrogenasa después de haberse aplicado metomilo en las dosis
recomendadas.
En estudios de laboratorio se determinó una NOEC de
6,5 mg/litro para el crecimiento de las algas.
El metomilo tiene una toxicidad entre moderada y alta para los
peces, con una CL50 a las 96 horas de 0,5 a 2 mg/litro para una
serie de especies. En un estudio de mayor duración (21 días),
utilizando la formulación Lannate 20L (21,5% de metomilo), la CL50
para los alevines de trucha fue de 1,3 mg/litro. En un estudio de
toxicidad de 28 días durante la primera fase de la vida de piscardos
(Pimaphales promelas), la máxima concentración intoxicante aceptable
(MATC) estimada fue de > 57 y < 117 µg/litro.
En pruebas de toxicidad aguda con otros organismos acuáticos,
Daphnia magna fue una de las especies más vulnerables al metomilo, con
una CL50 de 0,032 mg/litro a las 48 horas. En un estudio de 21 días
sobre la capacidad de supervivencia, crecimiento y reproducción de
Daphnia magna, la máxima concentración intoxicante aceptable para el
metomilo fue de > 1,6 y < 3,5 µg/litro.
El metomilo es tóxico para las abejas melíferas; se ha notificado
una DL50 por contacto de 1,29 µg/abeja y una DL50 oral de
0,2 µg/abeja.
Se ha evaluado la toxicidad aguda del metomilo en varias especies
de aves; los valores característicos de la DL50 oral aguda son de
10 mg/kg de peso corporal en palomas y de 34 mg/kg de peso corporal en
la codorniz japonesa. El metomilo es relativamente menos tóxicos si
se ingiere con los alimentos; en ese caso, la CL50 en ocho días es
de 1100 mg/kg de metomilo en los alimentos para la codorniz Colinus
virginianus y de 2883 mg/kg de metomilo en los alimentos para el
pato silvestre. En estudios de una generación realizados durante 18 a
20 semanas, la NOEC fue de 150 mg/kg de metomilo en los alimentos para
la codorniz Colinus virginianus y para el pato silvestre.
No se observaron efectos sobre Colinus virginianus tras su
exposición a una serie de aplicaciones de metomilo por rociado en las
dosis recomendadas. En dos estudios sobre poblaciones de aves
silvestres, después de haberse rociado con metomilo tierras forestales
y campos de lúpulo en las dosis recomendadas, no se observó ningún
cambio manifiesto en la actividad de las aves ni se produjeron efectos
ni mortalidad relacionados con el tratamiento. Los depósitos de grasa
de las aves canoras de los bosques tratados se redujeron en
comparación con el grupo testigo; se consideró que se trataba de un
efecto indirecto de la disminución de los insectos que les sirven de
alimento.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
El metomilo es un inhibidor de la carbamato colinesterasa
mediante un mecanismo bien conocido de acción tóxica. En estudios
realizados en animales se ha observado una toxicidad aguda
particularmente alta por vía oral y respiratoria, pero la toxicidad
por vía cutánea es baja. Los signos de toxicidad aguda en animales
son los característicos de los inhibidores de la colinesterasa. La
reversibilidad de la acción tóxica aguda es rápida; los supervivientes
se recuperan con rapidez de los signos tóxicos y de la inhibición de
la colinesterasa en la sangre y el cerebro. La pronta remisión de los
efectos tóxicos se debe a la rápida reversibilidad de la inhibición de
la colinesterasa, reversibilidad favorecida por la eliminación rápida
del metomilo del organismo. Los datos sobre intoxicaciones humanas
accidentales e intencionales muestran que el nivel de toxicidad aguda
del metomilo en el ser humano es semejante al observado en los
animales de laboratorio.
Habida cuenta de la reversibilidad rápida de la acción del
metomilo durante los periodos de ingestión, en los estudios realizados
raramente se observaron síntomas de toxicidad aguda e inhibición de la
colinesterasa sanguínea. Los resultados más constantes en los
estudios de mayor duración con niveles de alimentación más elevados
fueron una reducción del aumento del peso corporal en roedores y una
disminución del número de glóbulos rojos en roedores y perros. No se
observaron indicios de carcinogenicidad potencial en tres estudios de
larga duración realizados en roedores. El compuesto dio resultados
negativos en las pruebas de genotoxicidad in vitro en las que se
investigaron varios puntos finales, en cambio mostró potencial
citogenético en linfocitos humanos. El resultado de un estudio
cromosómico realizado in vivo sobre médula ósea de rata fue
negativo.
Se identificaron los NOEL en cada uno de los estudios de larga
duración realizados con animales, teniendo en cuenta la reducción del
aumento del peso corporal y el número de eritrocitos. Los NOEL
resultaron ser de 5 mg/kg de peso corporal por día en ratas, 8,7 mg/kg
de peso corporal por día en ratones y 3 mg/kg de peso corporal por día
en perros. Como esos estudios no revelaron diferencias acentuadas
entre especies en cuanto a los efectos tóxicos, se debería utilizar el
NOEL en el perro, que es de 3 mg/kg de peso corporal por día, a
efectos de la estimación del riesgo en el ser humano.
La adsorción del metomilo al suelo es de baja a moderada, y
prácticamente no hay desorción. La degradación aerobia en el suelo
(con una semivida de alrededor de una semana) es aproximadamente dos
veces más rápida que la degradación anaerobia.
La aplicación de metomilo a hojas de plantas da lugar a una
absorción rápida de casi la mitad de la cantidad aplicada (la otra
mitad se adsorbe) y no hay indicios de transporte. La concentración
del metomilo absorbido en los cultivos de productos alimenticios
disminuye rápidamente hasta alrededor del 5% en una semana.
Varios invertebrados acuáticos, en particular los dáfnidos, son
muy sensibles al metomilo, con CL50 del orden de 10 a 100 µg/litro.
Los peces, tanto de agua dulce como estuarinos, son menos
sensibles, oscilando la CL50 entre 0,5 y 7 mg/litro. Dada la escasa
persistencia del metomilo y su toxicidad aguda relativamente baja para
los peces, se supone que el riesgo es bajo.
En las dosis de aplicación recomendadas, el metomilo no menoscaba
la actividad microbiana en suelos templados.
Este producto está clasificado como muy tóxico para las abejas
melíferas y su DL50 tópica es de aproximadamente 0,1 µg/abeja.
La DL50 aguda por vía oral para varias especies de aves oscila
entre 10 y 40 mg/kg de peso corporal. Los valores de la CL50 en la
alimentación (cinco días) varían entre 1100 y 3700 mg/kg de alimentos.
El metomilo, sobre todo en forma de gránulos, constituye un riesgo
agudo para las aves, pero no se prevé que la ingestión de alimentos
contaminados con metomilo pueda causarles la muerte.
La elevada toxicidad aguda del metomilo para los mamíferos de
laboratorio indica que existe un peligro semejante para los mamíferos
silvestres.
10. Conclusiones
Teniendo en cuenta las características cualitativas y
cuantitativas de la toxicidad del metomilo, el Grupo de Trabajo llegó
a la conclusión de que 0,03 mg/kg de peso corporal al día
probablemente no causarían efectos adversos en el ser humano por
ninguna vía de exposición.
 The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate
in the preparation of the EHC monograph must, in addition to serving
in their personal capacity as scientists, inform the RO if at any time
a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Dept. of Human Services and Health, Canberra, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, PA, USA
Dr Jun Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr Palarp Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr Salah A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of Agriculture
Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Observers
Dr R. Gardiner, GIFAP, Brussels, Belgium (Representative of GIFAP)
Dr B. Julin, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr S.M. Kennedy, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr Ronald L. Mull, Du Pont Agricultural Products, Wilmington, DE,
United States of America (Representative of GIFAP)
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Office, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
The Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP) met in Geneva from 25 October to 3 November 1994. Dr W. Kreisel,
Executive Director, welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS on behalf of the three IPCS cooperating
organizations (UNEP/ILO/ WHO). The CAG reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methomyl.
The first draft of the monograph was prepared by
Dr M.L. Litchfield, Arundel, United Kingdom. Extensive scientific
comments were received following circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs and
these comments were incorporated into the second draft by the
Secretariat.
The fact that E.I. Du Pont de Nemours and Co. made available to
IPCS and the Core Assessment Group proprietary toxicological
information on their products is gratefully acknowledged. This
allowed the Group to make its evaluation on a more complete data base.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ADI Acceptable Daily Intake
ALC Approximate Lethal Concentration
CAS Chemical Abstracts Service
CCPR Codex Committee on Pesticide Residues
EbC50 median effective concentration for inhibition of growth
based on comparison of areas under the growth curves after
"b" hours
ECD electron capture detector
FID flame ionization detector
FSD flame photometric detector selective for sulfur
GC gas chromatography
GLC gas-liquid chromatography
GOT glutamic oxaloacetic transaminase
GPT glutamic pyruvic transaminase
HPLC high performance liquid chromatography
ISO International Organization for Standardization
IUPAC International Union of Pure and Applied Chemistry
JMPR Joint FAO/WHO Meeting on Pesticide Residues
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
MATC maximum acceptable toxicant concentration
MHTA S-methyl- N-hydroxythioacetimidate (a metabolite of
methomyl)
mPa millipascal (7.5 × 10-6 mmHg)
MRL maximum residue limit
NOEC no-observed-effect concentration
P2S N-methyl-pyridium-2-aldoxime methane-sulphonate
(antidote)
PAM pyridine-2-aldoxime methiodiode (antidote)
RTECS Registry of Toxic Effects of Chemical Substances
TEAC tetraethylammonium chloride
TOCP tri- o-cresyl phosphate
TOD total oxygen demand
UV ultraviolet
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
Methomyl is a white crystalline solid with a melting point of
77°C and a vapour pressure of 0.72 mPa (25°C). Its solubility in
water is 54.7 g/litre and its octanol/water partition coefficient
(Kow) is 1.24. It is stable in sterile water at pH 7, but is broken
down at higher pH values, the half-life being 30 days at pH 9 and
25°C.
The analytical procedure for the determination of methomyl in
different samples is extraction followed by clean-up and analysis by
HPLC or GLC. In some cases methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
1.2 Sources of human and environmental exposure
Methomyl is produced by reacting S-methyl N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. It is a carbamate insecticide used on a wide
range of crops throughout the world. Crops protected include fruit,
vines, hops, vegetables, grain, soya bean, cotton and ornamentals.
Indoor uses include the control of flies in animal houses and dairies.
The main formulations are water soluble powders and water
miscible liquids, which are diluted with water for ground or aerial
spraying of crops. Typical active ingredient rates are 0.15 to
1.0 kg/ha. The main sources of human exposure are during the
preparation and application of these products and from the ingestion
of crop residues in foodstuffs (see section 5.3.1.4).
1.3 Environmental transport, distribution and transformation
In laboratory studies, methomyl adsorbs poorly to soil. Weak
adsorption to clay minerals, particularly illite, has been
demonstrated; adsorption to soil organic matter is 50 times greater
but still relatively weak. Hardly any desorption of bound residue is
seen. With these characteristics, methomyl would be expected to be
mobile in soil.
Under natural environmental conditions, abiotic degradation of
methomyl by hydrolysis or photolysis is slow or absent.
Aerobic degradation in soil is about twice as fast as anaerobic
degradation. Reported half-lives of methomyl in soil vary from a few
days to more than 50 days; dry conditions delay breakdown. In
practice in the field most applications should lead to a half-life of
around one week.
In field conditions, methomyl does not leach to levels below 20
to 30 cm into the soil and does not contaminate ground water.
When 14C-methomyl is applied to plant leaves it is absorbed but
not translocated to other parts of the plant. When applied to the
root system it is absorbed into the plant where the principle residue
component is methomyl itself. Volatile breakdown products are CO2
and acetonitrile. The remainder of the activity is incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars. The half-life of methomyl in plant foliage is a few days.
There was no evidence for accumulation of methomyl in rainbow
trout exposed to the compound for 28 days in a flow-through system.
1.4 Environmental levels and human exposure
Methomyl levels are likely to be either very low or undetectable
(< 0.02 mg/litre) in ground water on the evidence of analyses of
various water sources after the application of the compound at
recommended rates.
Low residue levels of methomyl are present in food and other
crops at harvesting, the levels depending upon factors such as the
applied rate, time interval after the last application and the type of
crop. The residue is composed primarily of methomyl.
Residues of methomyl in dairy products are either undetectable or
very low. Lactating cows given methomyl by capsule at a rate
equivalent to 80 mg/kg in their feed for 28 days showed no detectable
residues of methomyl or the metabolite MHTA in milk or tissues
(< 0.02 mg/kg). No methomyl was detected in eggs or tissues of
laying hens given 1 or 10 mg/kg in the diet for 4 weeks.
In total diet or individual food analyses in the USA, the
concentrations of methomyl in sample surveys were either undetectable
or very low. Residue levels are further reduced by processes such as
washing, peeling and cooking.
Re-entry exposure studies, specifically for California desert
conditions, showed that, when workers returned to vineyards where
dislodgeable foliar residues had fallen to 0.1 µg/cm2, the highest
exposure occurred on the upper body and head during grape girdling and
on the upper body and hands during raisin harvesting. Harvesting and
packing table grapes resulted in the lowest exposure. Inhalation
exposure was minimal.
After methomyl was sprayed on cucumber and tomato plants, ambient
air concentrations in the greenhouse ranged up to 4.7 µg/m3 on the
day after spraying. Three and 7 days after spraying, breathing zone
methomyl concentrations ranged up to 14.5 and 0.7 µg/m3,
respectively. Hand-wash methomyl values ranged from 10 to 322 µg
per h work in a greenhouse. This indicated that dermal exposure was a
more important route of exposure than inhalation and that re-entry
intervals should be based on dermal exposure data.
1.5 Kinetics and metabolism in laboratory animals
The absorption, metabolism and excretion of methomyl after oral
administration to rats are very rapid, the processes being completed
within a few days. When rats were given radiolabelled methomyl
(5 mg/kg body weight), 54% of the dose was excreted in urine and 2-3%
in faeces within 7 days, and 34% in expired air within 5 days. After
7 days, 8-9% of the 14C dose remained in the tissues and carcass,
which was incorporated into endogenous constituents. The highest
concentration of radioactivity was in the blood (representing 2% of
the dose).
The major metabolic components in expired air of rats were carbon
dioxide and acetonitrile in the ratio of about 2:1. The major
metabolite in urine was the mercapturic acid derivative of methomyl,
which was equal to 17% of the dose. Neither methomyl nor its oxime
derivative was detected.
The proposed metabolic pathway includes displacement of the
S-methyl grouping by glutathione followed by enzymic transformation
to give the mercapturic acid derivative. Another pathway is by
hydrolysis to give MHTA, which is rapidly broken down to carbon
dioxide. A further possible route is conversion of syn-methomyl (the
insecticidal form) to its anti-isomer, which undergoes hydrolysis,
rearrangement and elimination reactions to give acetonitrile.
Methomyl is similarly metabolized in the monkey, except that the
mercapturic acid derivative is a minor component in the urine.
The penetration of 14C-methomyl was estimated to be 85% within
one hour after dermal application in acetone to mice. At that time 3%
of the dose had appeared in blood, 5% in liver and 13% had been
excreted. Within 8 h the total excretion was 54.5%.
The rapid breakdown and elimination of methomyl in the rat,
together with its lack of accumulation in tissues, are comparable to
that seen in ruminants.
Methomyl is completely broken down when cows or goats are dosed.
No methomyl or its oxime derivative was detected in milk or tissues.
It was shown that the compound was metabolized and incorporated into
natural constituents of milk and liver.
No nitrosomethomyl was detected when 14C-methomyl was incubated
under simulated stomach conditions with sodium nitrite in a cured meat
macerate.
1.6 Effects on laboratory mammals and in vitro test systems
Methomyl has high acute oral toxicity, with an oral LD50 in the
rat of 17-45 mg/kg body weight. It is also highly toxic to rats by
the inhalation route, with a 4-h LC50 of 0.26 mg/litre in aerosol
form. Dermal toxicity is very low, with the LD50 exceeding
2000 mg/kg body weight in the rabbit (intact skin) and > 1000 mg/kg
body weight in the rat (abraded skin). Signs of acute toxic action
are those expected of a cholinesterase inhibitor and include among
others profuse salivation, lacrimation, tremor and pupil constriction.
Recovery from the effects was rapid. No gross pathological effects
due to treatment were seen in the organs examined. Methomyl is not a
skin irritant or sensitizer and is a mild eye irritant.
Repeated dietary administration over longer periods did not lead
to accumulation or increase in toxic effect. Rats and dogs fed diets
containing methomyl up to 250 mg/kg and 400 mg/kg in the diet,
respectively, for 13 weeks did not show any toxic signs or mortality.
Rats fed at the 250 mg/kg level showed small decreases in body weight
gain, lower haemoglobin levels and moderate erythroid hyperplasia in
the bone marrow. The NOEL in rats was 50 mg/kg in the diet
(equivalent to 3.6 mg/kg body weight per day). Rabbits given repeated
dermal applications of methomyl at doses up to 500 mg/kg body weight
per day for 21 days showed hyperactivity and depressed plasma and
brain cholinesterase activity at the top dose. The NOAEL was 50 mg/kg
body weight per day in this study.
Long-term studies were carried out on rats at methomyl
dietary levels of 0, 50, 100 or 400 mg/kg and on mice at 0, 50, 75 or
200 mg/kg. Effects on rats at the top dose included depressed body
weight gain and lowered haemoglobin and haematocrit values. The NOEL
was 100 mg/kg in the diet, equivalent to 5 mg/kg body weight per day.
In the study in mice, an increased mortality rate and decreased
haemoglobin and red blood cell counts were seen at the two higher dose
levels. The NOEL was 50 mg/kg in the diet, equivalent to 8.7 mg/kg
body weight per day. In a 2-year toxicity study in dogs (0, 50, 100,
400 or 1000 mg/kg in the diet), clinical signs of toxicity were noted
in some animals at the top dose together with slight to moderate
anaemia. The NOEL was 100 mg/kg in the diet, equivalent to 3 mg/kg
body weight per day.
There was no evidence of treatment-related increases in tumour
incidences in 2-year studies on rats and mice, indicating that
methomyl is not carcinogenic. It was not genotoxic in bacterial or
mammalian cells in vitro and was negative in tests for primary DNA
damage in bacterial and mammalian cells in vitro and in an in vivo rat
bone marrow chromosomal study. It showed cytogenetic potential in
human lymphocytes in vitro, as shown by increases in micronuclei and
chromosome aberrations. Methomyl did not produce embryotoxic or
teratogenic effects in rats or rabbits at doses up to 400 mg/kg in the
diet or 16 mg/kg body weight per day by gavage, respectively, at which
levels toxic effects were present in the dams. In a 3-generation
reproduction study in rats at dose levels of 50 or 100 mg/kg in the
diet (equivalent to 5 or 10 mg/kg body weight per day) methomyl did
not affect fertility, gestation or lactation indices and there were no
treatment-related gross abnormalities.
Methomyl did not show delayed neurotoxicity after single or
repeated administration. Rats fed 800 mg/kg in the diet showed
significant depression of blood cholinesterase activity only in the
early stages of a 5-month study. In a 28-day dietary study, brain
cholinesterase activity was only slightly depressed at this dose
level. This indicated the rapid reversibility of methomyl-inhibited
cholinesterase activity in the animals during the feeding periods. In
vitro, human erythrocyte cholinesterase activity was six times more
sensitive to the inhibitory action of methomyl than that of the rat,
although the rates of spontaneous reactivation were similar.
Atropine was shown to be the most consistently effective antidote
for methomyl poisoning based upon the results of studies in several
species.
1.7 Effects on humans
Reports on accidental and suicidal poisonings with methomyl
provide some information on effect levels and recovery. Three out of
five victims of accidental poisoning from a contaminated meal died
within 3 h of the ingestion. It was estimated that the victims had
consumed about 12-15 mg methomyl/kg body weight. A 31-year-old woman
and her 6-year-old son, both of whom died as a result of deliberate
poisoning, showed concentrations of methomyl in the liver of 15.4 and
56.5 mg/kg, respectively. The estimated doses were 55 mg/kg body
weight for the mother and 13 mg/kg body weight for the son. Six hours
after ingesting approximately 2.25 g methomyl, a woman's blood
contained .6 mg methomyl/kg. Methomyl could not be detected 22 h
after ingestion, when the woman was recovering.
A pesticide operator, who did not take any precautions when
mixing a powdered methomyl formulation for spraying vegetables,
displayed poisoning symptoms within one hour and showed a blood
cholinesterase activity 40% of normal after 12 h, with recovery to 80%
of normal activity within 36 h. Other operators, following the
recommended precautions, did not show any symptoms or effects on red
blood cell or plasma cholinesterase activity during activities with
the aerial application of methomyl.
1.8 Effects on non-target organisms in the laboratory and field
Methomyl showed no effects on soil fungal or bacterial
populations, nitrification or dehydrogenase activity when applied at
recommended rates.
An NOEC for algal growth of 6.5 mg/litre was established for
methomyl in laboratory studies.
Methomyl is moderately to highly toxic to fish, the 96-h LC50
values being in the range of 0.5-2 mg/litre for a variety of species.
In a longer-term (21 days) study the LC50 for fingerling trout was
1.3 mg/litre methomyl when tested as a Lannate 20L (21.5% methomyl)
formulation. In an early- life-stage toxicity study over 28 days with
fathead minnows, the MATC was estimated to be > 57 and < 117 µg/litre.
In acute toxicity tests with other aquatic organisms, Daphnia
magna was one of the most susceptible species to methomyl, the 48-h
LC50 being 0.032 mg/litre. In a 21-day study on the survival,
growth and reproductive capacity of Daphnia magna, the maximum
acceptable toxicant concentration for methomyl was > 1.6 and
< 3.5 µg/litre.
Methomyl is toxic to honey-bees, the reported contact LD50
being 1.29 µg/bee and the oral LD50 0.2 µg/bee.
The acute toxicity of methomyl has been assessed in several bird
species, typical acute oral LD50 values being 10 mg/kg body weight
for pigeons and 34 mg/kg body weight for Japanese quail. It is
relatively less toxic by the dietary route, the 8-day dietary LC50
being 1100 mg/kg methomyl in the diet for bobwhite quail and
2883 mg/kg methomyl in the diet for mallard ducks. In 18-to 20-week
one-generation studies, the NOEC was 150 mg/kg methomyl in the diet in
bobwhite quail and mallard ducks.
No effects were seen on bobwhite quail when they were exposed to
serial spray applications of methomyl at recommended rates. Two
studies on wild bird populations, after methomyl was sprayed over
forest land or hop fields at recommended rates, did not reveal any
apparent changes in bird activity and caused no treatment-related
effect or mortality. Fat deposits of song birds in treated forests
were reduced relative to controls; this was considered to be an
indirect effect through reduction in insect food.
1.9 Evaluation of human health risks and effects on the environment
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The adsorption of methomyl to soil is low to moderate with hardly
any desorption. Aerobic degradation in soil (with a half-life of
around one week) is about twice as fast as anaerobic degradation.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. Absorbed
methomyl concentrations in food crops decline rapidly to about 5%
within one week.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl with LC50 values in the order of 10 to
100 µg/litre.
Fish, both freshwater and estuarine, are less sensitive, the
LC50 values ranging from 0.5 to 7 mg/litre. Given the low
persistence of methomyl and its relatively low acute toxicity to fish,
the risk is expected to be low.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil.
Methomyl is classified as highly toxic to honey-bees with a
topical LD50 of around 0.1 µg/bee.
Acute oral LD50 values for various bird species range between
10 and 40 mg/kg body weight. Dietary LC50 values (5 days) range from
1100 to 3700 mg/kg diet. Methomyl poses an acute risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
1.10 Conclusion
Considering the qualitative and quantitative characteristics of
methomyl toxicity, the Task Group concluded that 0.03 mg/kg body
weight per day will probably not cause adverse effects in humans by
any route of exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
O
"
CH3-C=N-O-C-NH-CH3
'
S - CH3
Used in the syn-isomer form
Molecular formula: C5H10N2O2S
Relative molecular mass: 162.2
ISO common name: methomyl
IUPAC chemical name: S-methyl- N-[(methyl-carbamoyl)oxy]
thio-acetimidate
CAS chemical name: methyl N[[(methyl-amino)carbonyl]
oxy] ethanimidothioate
CAS registry number:
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate
in the preparation of the EHC monograph must, in addition to serving
in their personal capacity as scientists, inform the RO if at any time
a conflict of interest, whether actual or potential, could be
perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are
briefed before each meeting on their role and responsibility in
ensuring that these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Dept. of Human Services and Health, Canberra, Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, PA, USA
Dr Jun Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr Palarp Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr Salah A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of Agriculture
Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist, The
Netherlands
Observers
Dr R. Gardiner, GIFAP, Brussels, Belgium (Representative of GIFAP)
Dr B. Julin, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr S.M. Kennedy, Du Pont de Nemours (Belgium), Brussels, Belgium
(Representative of GIFAP)
Dr Ronald L. Mull, Du Pont Agricultural Products, Wilmington, DE,
United States of America (Representative of GIFAP)
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Office, Geneva, Switzerland
Dr R. Plestina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR METHOMYL
The Core Assessment Group (CAG) of the Joint Meeting on Pesticides
(JMP) met in Geneva from 25 October to 3 November 1994. Dr W. Kreisel,
Executive Director, welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS on behalf of the three IPCS cooperating
organizations (UNEP/ILO/ WHO). The CAG reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methomyl.
The first draft of the monograph was prepared by
Dr M.L. Litchfield, Arundel, United Kingdom. Extensive scientific
comments were received following circulation of the first draft to the
IPCS contact points for Environmental Health Criteria monographs and
these comments were incorporated into the second draft by the
Secretariat.
The fact that E.I. Du Pont de Nemours and Co. made available to
IPCS and the Core Assessment Group proprietary toxicological
information on their products is gratefully acknowledged. This
allowed the Group to make its evaluation on a more complete data base.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively. The efforts of all who helped in the
preparation and finalization of the monograph are gratefully
acknowledged.
ABBREVIATIONS
ADI Acceptable Daily Intake
ALC Approximate Lethal Concentration
CAS Chemical Abstracts Service
CCPR Codex Committee on Pesticide Residues
EbC50 median effective concentration for inhibition of growth
based on comparison of areas under the growth curves after
"b" hours
ECD electron capture detector
FID flame ionization detector
FSD flame photometric detector selective for sulfur
GC gas chromatography
GLC gas-liquid chromatography
GOT glutamic oxaloacetic transaminase
GPT glutamic pyruvic transaminase
HPLC high performance liquid chromatography
ISO International Organization for Standardization
IUPAC International Union of Pure and Applied Chemistry
JMPR Joint FAO/WHO Meeting on Pesticide Residues
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
MATC maximum acceptable toxicant concentration
MHTA S-methyl- N-hydroxythioacetimidate (a metabolite of
methomyl)
mPa millipascal (7.5 × 10-6 mmHg)
MRL maximum residue limit
NOEC no-observed-effect concentration
P2S N-methyl-pyridium-2-aldoxime methane-sulphonate
(antidote)
PAM pyridine-2-aldoxime methiodiode (antidote)
RTECS Registry of Toxic Effects of Chemical Substances
TEAC tetraethylammonium chloride
TOCP tri- o-cresyl phosphate
TOD total oxygen demand
UV ultraviolet
1. Summary
1.1 Identity, physical and chemical properties, and analytical
methods
Methomyl is a white crystalline solid with a melting point of
77°C and a vapour pressure of 0.72 mPa (25°C). Its solubility in
water is 54.7 g/litre and its octanol/water partition coefficient
(Kow) is 1.24. It is stable in sterile water at pH 7, but is broken
down at higher pH values, the half-life being 30 days at pH 9 and
25°C.
The analytical procedure for the determination of methomyl in
different samples is extraction followed by clean-up and analysis by
HPLC or GLC. In some cases methomyl is converted to its oxime
derivative or a fluorophore derivative (post-column) prior to
analytical determination.
1.2 Sources of human and environmental exposure
Methomyl is produced by reacting S-methyl N-hydroxythio-
acetimidate (MHTA) in methylene chloride with gaseous methyl
isocyanate at 30-50°C. It is a carbamate insecticide used on a wide
range of crops throughout the world. Crops protected include fruit,
vines, hops, vegetables, grain, soya bean, cotton and ornamentals.
Indoor uses include the control of flies in animal houses and dairies.
The main formulations are water soluble powders and water
miscible liquids, which are diluted with water for ground or aerial
spraying of crops. Typical active ingredient rates are 0.15 to
1.0 kg/ha. The main sources of human exposure are during the
preparation and application of these products and from the ingestion
of crop residues in foodstuffs (see section 5.3.1.4).
1.3 Environmental transport, distribution and transformation
In laboratory studies, methomyl adsorbs poorly to soil. Weak
adsorption to clay minerals, particularly illite, has been
demonstrated; adsorption to soil organic matter is 50 times greater
but still relatively weak. Hardly any desorption of bound residue is
seen. With these characteristics, methomyl would be expected to be
mobile in soil.
Under natural environmental conditions, abiotic degradation of
methomyl by hydrolysis or photolysis is slow or absent.
Aerobic degradation in soil is about twice as fast as anaerobic
degradation. Reported half-lives of methomyl in soil vary from a few
days to more than 50 days; dry conditions delay breakdown. In
practice in the field most applications should lead to a half-life of
around one week.
In field conditions, methomyl does not leach to levels below 20
to 30 cm into the soil and does not contaminate ground water.
When 14C-methomyl is applied to plant leaves it is absorbed but
not translocated to other parts of the plant. When applied to the
root system it is absorbed into the plant where the principle residue
component is methomyl itself. Volatile breakdown products are CO2
and acetonitrile. The remainder of the activity is incorporated into
natural plant components such as lipids and Krebs cycle acids and
sugars. The half-life of methomyl in plant foliage is a few days.
There was no evidence for accumulation of methomyl in rainbow
trout exposed to the compound for 28 days in a flow-through system.
1.4 Environmental levels and human exposure
Methomyl levels are likely to be either very low or undetectable
(< 0.02 mg/litre) in ground water on the evidence of analyses of
various water sources after the application of the compound at
recommended rates.
Low residue levels of methomyl are present in food and other
crops at harvesting, the levels depending upon factors such as the
applied rate, time interval after the last application and the type of
crop. The residue is composed primarily of methomyl.
Residues of methomyl in dairy products are either undetectable or
very low. Lactating cows given methomyl by capsule at a rate
equivalent to 80 mg/kg in their feed for 28 days showed no detectable
residues of methomyl or the metabolite MHTA in milk or tissues
(< 0.02 mg/kg). No methomyl was detected in eggs or tissues of
laying hens given 1 or 10 mg/kg in the diet for 4 weeks.
In total diet or individual food analyses in the USA, the
concentrations of methomyl in sample surveys were either undetectable
or very low. Residue levels are further reduced by processes such as
washing, peeling and cooking.
Re-entry exposure studies, specifically for California desert
conditions, showed that, when workers returned to vineyards where
dislodgeable foliar residues had fallen to 0.1 µg/cm2, the highest
exposure occurred on the upper body and head during grape girdling and
on the upper body and hands during raisin harvesting. Harvesting and
packing table grapes resulted in the lowest exposure. Inhalation
exposure was minimal.
After methomyl was sprayed on cucumber and tomato plants, ambient
air concentrations in the greenhouse ranged up to 4.7 µg/m3 on the
day after spraying. Three and 7 days after spraying, breathing zone
methomyl concentrations ranged up to 14.5 and 0.7 µg/m3,
respectively. Hand-wash methomyl values ranged from 10 to 322 µg
per h work in a greenhouse. This indicated that dermal exposure was a
more important route of exposure than inhalation and that re-entry
intervals should be based on dermal exposure data.
1.5 Kinetics and metabolism in laboratory animals
The absorption, metabolism and excretion of methomyl after oral
administration to rats are very rapid, the processes being completed
within a few days. When rats were given radiolabelled methomyl
(5 mg/kg body weight), 54% of the dose was excreted in urine and 2-3%
in faeces within 7 days, and 34% in expired air within 5 days. After
7 days, 8-9% of the 14C dose remained in the tissues and carcass,
which was incorporated into endogenous constituents. The highest
concentration of radioactivity was in the blood (representing 2% of
the dose).
The major metabolic components in expired air of rats were carbon
dioxide and acetonitrile in the ratio of about 2:1. The major
metabolite in urine was the mercapturic acid derivative of methomyl,
which was equal to 17% of the dose. Neither methomyl nor its oxime
derivative was detected.
The proposed metabolic pathway includes displacement of the
S-methyl grouping by glutathione followed by enzymic transformation
to give the mercapturic acid derivative. Another pathway is by
hydrolysis to give MHTA, which is rapidly broken down to carbon
dioxide. A further possible route is conversion of syn-methomyl (the
insecticidal form) to its anti-isomer, which undergoes hydrolysis,
rearrangement and elimination reactions to give acetonitrile.
Methomyl is similarly metabolized in the monkey, except that the
mercapturic acid derivative is a minor component in the urine.
The penetration of 14C-methomyl was estimated to be 85% within
one hour after dermal application in acetone to mice. At that time 3%
of the dose had appeared in blood, 5% in liver and 13% had been
excreted. Within 8 h the total excretion was 54.5%.
The rapid breakdown and elimination of methomyl in the rat,
together with its lack of accumulation in tissues, are comparable to
that seen in ruminants.
Methomyl is completely broken down when cows or goats are dosed.
No methomyl or its oxime derivative was detected in milk or tissues.
It was shown that the compound was metabolized and incorporated into
natural constituents of milk and liver.
No nitrosomethomyl was detected when 14C-methomyl was incubated
under simulated stomach conditions with sodium nitrite in a cured meat
macerate.
1.6 Effects on laboratory mammals and in vitro test systems
Methomyl has high acute oral toxicity, with an oral LD50 in the
rat of 17-45 mg/kg body weight. It is also highly toxic to rats by
the inhalation route, with a 4-h LC50 of 0.26 mg/litre in aerosol
form. Dermal toxicity is very low, with the LD50 exceeding
2000 mg/kg body weight in the rabbit (intact skin) and > 1000 mg/kg
body weight in the rat (abraded skin). Signs of acute toxic action
are those expected of a cholinesterase inhibitor and include among
others profuse salivation, lacrimation, tremor and pupil constriction.
Recovery from the effects was rapid. No gross pathological effects
due to treatment were seen in the organs examined. Methomyl is not a
skin irritant or sensitizer and is a mild eye irritant.
Repeated dietary administration over longer periods did not lead
to accumulation or increase in toxic effect. Rats and dogs fed diets
containing methomyl up to 250 mg/kg and 400 mg/kg in the diet,
respectively, for 13 weeks did not show any toxic signs or mortality.
Rats fed at the 250 mg/kg level showed small decreases in body weight
gain, lower haemoglobin levels and moderate erythroid hyperplasia in
the bone marrow. The NOEL in rats was 50 mg/kg in the diet
(equivalent to 3.6 mg/kg body weight per day). Rabbits given repeated
dermal applications of methomyl at doses up to 500 mg/kg body weight
per day for 21 days showed hyperactivity and depressed plasma and
brain cholinesterase activity at the top dose. The NOAEL was 50 mg/kg
body weight per day in this study.
Long-term studies were carried out on rats at methomyl
dietary levels of 0, 50, 100 or 400 mg/kg and on mice at 0, 50, 75 or
200 mg/kg. Effects on rats at the top dose included depressed body
weight gain and lowered haemoglobin and haematocrit values. The NOEL
was 100 mg/kg in the diet, equivalent to 5 mg/kg body weight per day.
In the study in mice, an increased mortality rate and decreased
haemoglobin and red blood cell counts were seen at the two higher dose
levels. The NOEL was 50 mg/kg in the diet, equivalent to 8.7 mg/kg
body weight per day. In a 2-year toxicity study in dogs (0, 50, 100,
400 or 1000 mg/kg in the diet), clinical signs of toxicity were noted
in some animals at the top dose together with slight to moderate
anaemia. The NOEL was 100 mg/kg in the diet, equivalent to 3 mg/kg
body weight per day.
There was no evidence of treatment-related increases in tumour
incidences in 2-year studies on rats and mice, indicating that
methomyl is not carcinogenic. It was not genotoxic in bacterial or
mammalian cells in vitro and was negative in tests for primary DNA
damage in bacterial and mammalian cells in vitro and in an in vivo rat
bone marrow chromosomal study. It showed cytogenetic potential in
human lymphocytes in vitro, as shown by increases in micronuclei and
chromosome aberrations. Methomyl did not produce embryotoxic or
teratogenic effects in rats or rabbits at doses up to 400 mg/kg in the
diet or 16 mg/kg body weight per day by gavage, respectively, at which
levels toxic effects were present in the dams. In a 3-generation
reproduction study in rats at dose levels of 50 or 100 mg/kg in the
diet (equivalent to 5 or 10 mg/kg body weight per day) methomyl did
not affect fertility, gestation or lactation indices and there were no
treatment-related gross abnormalities.
Methomyl did not show delayed neurotoxicity after single or
repeated administration. Rats fed 800 mg/kg in the diet showed
significant depression of blood cholinesterase activity only in the
early stages of a 5-month study. In a 28-day dietary study, brain
cholinesterase activity was only slightly depressed at this dose
level. This indicated the rapid reversibility of methomyl-inhibited
cholinesterase activity in the animals during the feeding periods. In
vitro, human erythrocyte cholinesterase activity was six times more
sensitive to the inhibitory action of methomyl than that of the rat,
although the rates of spontaneous reactivation were similar.
Atropine was shown to be the most consistently effective antidote
for methomyl poisoning based upon the results of studies in several
species.
1.7 Effects on humans
Reports on accidental and suicidal poisonings with methomyl
provide some information on effect levels and recovery. Three out of
five victims of accidental poisoning from a contaminated meal died
within 3 h of the ingestion. It was estimated that the victims had
consumed about 12-15 mg methomyl/kg body weight. A 31-year-old woman
and her 6-year-old son, both of whom died as a result of deliberate
poisoning, showed concentrations of methomyl in the liver of 15.4 and
56.5 mg/kg, respectively. The estimated doses were 55 mg/kg body
weight for the mother and 13 mg/kg body weight for the son. Six hours
after ingesting approximately 2.25 g methomyl, a woman's blood
contained .6 mg methomyl/kg. Methomyl could not be detected 22 h
after ingestion, when the woman was recovering.
A pesticide operator, who did not take any precautions when
mixing a powdered methomyl formulation for spraying vegetables,
displayed poisoning symptoms within one hour and showed a blood
cholinesterase activity 40% of normal after 12 h, with recovery to 80%
of normal activity within 36 h. Other operators, following the
recommended precautions, did not show any symptoms or effects on red
blood cell or plasma cholinesterase activity during activities with
the aerial application of methomyl.
1.8 Effects on non-target organisms in the laboratory and field
Methomyl showed no effects on soil fungal or bacterial
populations, nitrification or dehydrogenase activity when applied at
recommended rates.
An NOEC for algal growth of 6.5 mg/litre was established for
methomyl in laboratory studies.
Methomyl is moderately to highly toxic to fish, the 96-h LC50
values being in the range of 0.5-2 mg/litre for a variety of species.
In a longer-term (21 days) study the LC50 for fingerling trout was
1.3 mg/litre methomyl when tested as a Lannate 20L (21.5% methomyl)
formulation. In an early- life-stage toxicity study over 28 days with
fathead minnows, the MATC was estimated to be > 57 and < 117 µg/litre.
In acute toxicity tests with other aquatic organisms, Daphnia
magna was one of the most susceptible species to methomyl, the 48-h
LC50 being 0.032 mg/litre. In a 21-day study on the survival,
growth and reproductive capacity of Daphnia magna, the maximum
acceptable toxicant concentration for methomyl was > 1.6 and
< 3.5 µg/litre.
Methomyl is toxic to honey-bees, the reported contact LD50
being 1.29 µg/bee and the oral LD50 0.2 µg/bee.
The acute toxicity of methomyl has been assessed in several bird
species, typical acute oral LD50 values being 10 mg/kg body weight
for pigeons and 34 mg/kg body weight for Japanese quail. It is
relatively less toxic by the dietary route, the 8-day dietary LC50
being 1100 mg/kg methomyl in the diet for bobwhite quail and
2883 mg/kg methomyl in the diet for mallard ducks. In 18-to 20-week
one-generation studies, the NOEC was 150 mg/kg methomyl in the diet in
bobwhite quail and mallard ducks.
No effects were seen on bobwhite quail when they were exposed to
serial spray applications of methomyl at recommended rates. Two
studies on wild bird populations, after methomyl was sprayed over
forest land or hop fields at recommended rates, did not reveal any
apparent changes in bird activity and caused no treatment-related
effect or mortality. Fat deposits of song birds in treated forests
were reduced relative to controls; this was considered to be an
indirect effect through reduction in insect food.
1.9 Evaluation of human health risks and effects on the environment
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The adsorption of methomyl to soil is low to moderate with hardly
any desorption. Aerobic degradation in soil (with a half-life of
around one week) is about twice as fast as anaerobic degradation.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. Absorbed
methomyl concentrations in food crops decline rapidly to about 5%
within one week.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl with LC50 values in the order of 10 to
100 µg/litre.
Fish, both freshwater and estuarine, are less sensitive, the
LC50 values ranging from 0.5 to 7 mg/litre. Given the low
persistence of methomyl and its relatively low acute toxicity to fish,
the risk is expected to be low.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil.
Methomyl is classified as highly toxic to honey-bees with a
topical LD50 of around 0.1 µg/bee.
Acute oral LD50 values for various bird species range between
10 and 40 mg/kg body weight. Dietary LC50 values (5 days) range from
1100 to 3700 mg/kg diet. Methomyl poses an acute risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
1.10 Conclusion
Considering the qualitative and quantitative characteristics of
methomyl toxicity, the Task Group concluded that 0.03 mg/kg body
weight per day will probably not cause adverse effects in humans by
any route of exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
O
"
CH3-C=N-O-C-NH-CH3
'
S - CH3
Used in the syn-isomer form
Molecular formula: C5H10N2O2S
Relative molecular mass: 162.2
ISO common name: methomyl
IUPAC chemical name: S-methyl- N-[(methyl-carbamoyl)oxy]
thio-acetimidate
CAS chemical name: methyl N[[(methyl-amino)carbonyl]
oxy] ethanimidothioate
CAS registry number:  A lactating goat was given 14C-methomyl by capsule, twice a
day, for 10 days at a dose rate equivalent to 20 mg/kg in feed. Milk,
blood, urine and faeces were sampled daily and selected tissues taken
within one day of the last dose. No methomyl or the metabolite MHTA
was detected in any of the samples. Approximately 16% and 7% of the
activity was excreted in urine and faeces, respectively, and about 8%
appeared in the milk and 17% in expired air. The milk activity
reached a plateau after 3 days and was equivalent to approximately
2 mg/kg. At this time the lactose component contained about 11-13% of
the milk activity. Hexane extracts, containing the triglyceride
components, contained 26-37% of the milk activity and the casein
component 8-9%. This indicates that methomyl had been completely
broken down and incorporated into natural constituents of milk.
Acetonitrile was identified as a volatile metabolite in milk and blood
(Harvey, 1980).
The examination of the liver fractions showed that the
radio-activity derived from methomyl was found in glycerol,
glycerol-3-phosphate, fatty acids, neutral lipids and insoluble
protein. This indicates a metabolic pathway via acetonitrile and
acetate into the natural occurring constituents in the liver. The
breakdown of methomyl and distribution of metabolic products in the
liver was shown to be similar in the cow (Monson, 1989).
Acetonitrile, CO2 and reincorporation products derived from
acetate found after the application of methomyl to plants (section
4.2.1) are similar to those identified in the above animal studies.
The proposed metabolic pathway for methomyl in animals is shown
in Fig. 1.
6.4 Elimination and excretion
Rat and monkey studies show that methomyl is very rapidly
metabolized and eliminated, the processes being largely completed
within 24 h.
Rats fed 200 mg methomyl/kg in diet and then given 5 mg
14C-methomyl/kg orally (see section 6.3 for detail) showed a 50%
elimination of 14C in expired air in 3 days in the form of carbon
dioxide and acetonitrile in the proportion of 1:2. Urinary excretion
of 14C was 27% in one day (Harvey et al, 1973).
In a more detailed study, where male and female rats were given
5 mg 14C-methomyl/kg orally (see section 6.3), approximately 53% of
the radioactivity was excreted in urine over 7 days, 45% of the dose
being excreted in the first 6 h. Faecal excretion contributed only
2-3% over 7 days. The other major path of elimination (over 5 days)
was via expired air as carbon dioxide (22% of dose) and acetonitrile
(12% of dose). Of this, 18% of the dose was expired as CO2 within
6 h and 10% as acetonitrile in 24 h. Overall, most of the
radiolabelled dose (80%) had been eliminated in 24 h with an estimated
half-life of 5 h. There was no obvious difference in the amount or
rate of excretion between males and females. The single oral dose
given to these animals (5 mg/kg) produced mild clinical signs of
cholinesterase inhibition which disappeared within 2 h of dosing
(Hawkins et al., 1991).
When methomyl was radiolabelled on the carbonyl group, the
elimination of 14CO2 was very rapid and equivalent to about 85% of
the oral dose in male and female rats. When the labelling was at the
- 14C=N group, the overall elimination in expired air in 24 h was
30% in the form of CO2 and acetonitrile (in the proportion of 2:1).
When 14C-MHTA was administered in the same way the expired component
was mainly 14CO2, equivalent to 22% of the dose. The anti-isomer
of methomyl mainly produced acetonitrile in the expired air,
equivalent to 28% of the dose given orally to rats. Rats given the
anti-MHTA by intraperitoneal injection produced ten times more
acetonitrile than those given the syn-MHTA. The urine from rats
treated orally with 14C-methomyl or MHTA contained 25-35% of the
radioactivity over a 24-h period (Huhtanen & Dorough, 1976).
In monkeys given 5 mg 14C-methomyl/kg orally, approximately 32%
of the dose was excreted in urine in 7 days, with 34% as CO2 and 5%
as acetonitrile in the expired air. Most of this was excreted in the
first 24 h. Faecal excretion amounted to only 3-4% (Hawkins et al.,
1992).
After dermal application of 14C-methomyl to mice (see section
6.1), the total excretion (urine plus CO2 plus faeces), as a
proportion of the applied dose, was 0.2% in 15 min, 12.9% in 60 min
and 54.5% in 480 min (Shah et al., 1981).
6.5 Retention and turnover
The absorption, metabolism and excretion of methomyl in the rat
are very rapid. No methomyl can be detected within the tissues or
excretory products within a few hours of dosing. Most of the dose is
eliminated within 24 h with an estimated half-life of 5 h (Hawkins et
al., 1991). Metabolic products, mainly in urine and expired air, are
also eliminated rapidly; tissue concentrations are very small and
lower than blood levels. There is no evidence for accumulation in
tissues. A similar picture exists for the metabolism of methomyl in
ruminant species.
6.6 Reaction with body components
Methomyl is a potent direct inhibitor of acetylcholinesterase in
both insects and mammals. The carbamylated enzymes undergo rapid
spontaneous reactivation (see section 7.8.1).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The results of the single exposure of technical methomyl by the
oral, dermal and inhalation routes in various species are shown in
Table 4.
Methomyl is very toxic by the oral route. In the rat, the signs
of toxicity are those expected from a cholinesterase inhibitor and
include profuse salivation, lacrimation, tremor, abnormal posture,
pupil constriction, diarrhoea and prostration. At lethal doses the
rats died within hours. Survivors began to recover within several
hours and had fully recovered within days. No compound-related
changes were seen in organs subjected to histopathological
examination.
The acute dermal toxicity of methomyl is very low. No deaths
occurred in rats or rabbits at the doses shown in Table 4. The
compound has high toxicity by the inhalation route in the rat, with
affected animals showing typical signs of cholinesterase inhibition.
No target organ effect was seen upon histopathological examination.
An LD50 of approximately 8 mg/kg was obtained when methomyl was
injected intraperitoneally in rats (Dashiell, 1972).
The acute oral toxicity of methomyl formulations is in proportion
to the amount of a.i. present and, therefore, they are less toxic than
methomyl itself (Table 5). The same pattern is seen with the results
of acute inhalation studies. The signs of toxicity in the oral and
inhalation studies are those shown by the active ingredient. The
dermal toxicity of the formulations is very low.
Ocular toxicity was shown by a solid formulation containing 92.4%
methomyl when 10 mg was introduced into rabbits' eyes. Typical
anticholinesterase effects were seen up to 20 min after treatment,
including pupillary constriction of the treated eyes, incoordination,
tremors and profuse salivation. All of the effects had disappeared by
the next day (Sarver, 1991g).
7.2 Short-term exposure
Six male rats were given methomyl in peanut oil by gavage at a
dose of 5.1 mg/kg body weight per day, 5 times per week for 2 weeks.
The signs of toxicity were the same as those exhibited in acute oral
studies (section 7.1) but they regressed during the second week of
dosing. All animals survived, and there were no compound-related
histopathological changes (Kaplan & Sherman, 1977).
Table 4. Acute toxicity of technical grade methomyl
Species Sex Route Vehicle Testa Result Reference
Rat M oral peanut oil LD50 17 mg/kg Sherman (1966)
Rat F oral peanut oil LD50 23.5 mg/kg Sherman (1968a)
Rat M oral water LD50 45 mg/kg Trivits (1979)
Rat M oral water LD50 34 mg/kg Sarver (1991a)
Rat F oral water LD50 30 mg/kg Sarver (1991a)
Rat M dermal water LD50 abraded skin > 1000 mg/kg Morrow (1972)
Rat M inhalation aerosol 4-h ALC 0.30 mg/litre Foster (1966a)
Rat M inhalation vapour 4-h ALC 0.04 mg/litre Foster (1966b)
Rat M inhalation spray 4-h LC50 0.45 mg/litre Hornberger
(1967)
Rat M/F inhalation aerosol 4-h LC50 0.258 mg/litre Panepinto
(1991a)
Rabbit M oral acetone/ ALD 30 mg/kg Sherman (1968c)
peanut oil
Table 4. (cont'd).
Species Sex Route Vehicle Testa Result Reference
Rabbit M/F dermal water LD50 intact skin > 2000 mg/kg Sarver (1991b)
Dog M oral capsule ALD 20 mg/kg Sherman (1968d)
Guinea-pig M oral acetone/ ALD 15 mg/kg Kaplan &
peanut oil Sherman (1977)
Monkey M/F oral water ALD 40 mg/kg Kaplan &
Sherman (1977)
a ALC = approximate lethal concentration; ALD = approximate lethal dose
Table 5. Acute toxicity of some methomyl formulations
Formulation Species Route Vehicle LD50 (mg/kg) or LC50 Reference
(% a.i.) (mg/litre)a
Lannate 40 SP rat oral water 61 (male) Sarver (1992a)
(41.6%) 73 (female)
Lannate 20 L rat oral methanol 129 Lheritier (1991a)
(21.5%)
Lannate 12.5 L rat oral water 208 Sarver (1991c)
(12.7%)
Lannate 40 SP rabbit dermal (intact skin) water > 2000 Sarver (1992b)
(41.6%)
Lannate 20 L rat dermal (intact skin) methanol > 4000 Lheritier (1991b)
(21.5%)
Lannate 40 SP rat inhalation (4 h) aerosol 0.66 Kelly (1992)
Lannate 20 L rat inhalation (4 h) aerosol 1.3 Jackson et al. (1991)
(21.5%)
Lannate 12.5 L rat inhalation (4 h) aerosol 3.7 Panepinto (1991b)
(12.7%)
a Results are for both sexes, unless stated otherwise
Groups of 10 male and 10 female rats were fed diets containing 0,
10, 50, 125/500 (125 mg/kg for 6 weeks, 500 mg/kg for remaining
period) or 250 mg methomyl/kg for 13 weeks. Assessments included
haematological, biochemical and histopathological examinations. There
were no mortalities nor clinical signs due to treatment in any group.
A slight decrease in body weight was seen in males at the two higher
dose levels and in females at 125/500 mg/kg. This was accompanied by
lower food consumption. The only change attributed to methomyl on
histopathological examination was moderate erythroid hyperplasia in
the bone marrow of males fed 250 mg/kg. Lower haemoglobin values in
the males of this group at two months could be linked to this effect.
Histopathological examination of animals in the 125/500 mg/kg group
did not reveal any changes due to treatment. The noobserved-effect
level based upon body weight change was considered to be 50 mg/kg
diet, equivalent to 3.56 mg methomyl/kg body weight per day (Paynter,
1966; Busey, 1966).
When groups of four male and four female one-year-old dogs were
fed diets containing 0, 50, 100 or 400 mg methomyl/kg for 3 months, no
effects were seen in a range of clinical, haematological, biochemical
and histopathological assessments (Kaplan & Sherman, 1977).
A dose of 200 mg methomyl/kg body weight was applied as a 5%
aqueous solution once a day 5 times per week for 3 weeks to the intact
or abraded skin of five male, five female rabbits. Each dose was
occluded for 6 h and then the site washed with water. There were no
mortalities in animals with intact skin; clinical signs included nasal
discharge, wheezing and diarrhoea. With abraded skin, signs of
toxicity, including laboured respiration, salivation and tremors,
appeared within one hour of dosing, and three animals died, one after
the first application and the other after the eighth application. No
treatment-related changes were seen upon histopathological examination
(Kaplan & Sherman, 1977).
Groups of rabbits were treated dermally (intact clipped skin)
with doses of 0 (10 males, 10 females), 5, 50 (5 males 5 females) or
500 (10 males, 10 females) mg methomyl/kg body weight per day for 21
days. The doses were applied in water each day and occluded for 6 h.
Half the males and females in the control and highest dose groups were
killed at the end of the dosing regime, together with all animals in
the other groups. The remaining animals in the control and highest
dose groups were allowed to recover over a 14-day period and then
killed. The only change noted clinically was a greater incidence of
hyperactivity at 500 mg/kg. There were no effects on body weight
gain, haematology, blood biochemistry, organ weights or organ
histopathology. Plasma cholinesterase activity was depressed to 36%
and 55% of normal at day 21 in highest dose males and females,
respectively. Brain cholinesterase activity was depressed to 48% and
68%, respectively, in males and females at this dose, but red cell
cholinesterase activity, although slightly decreased, was considered
to be biologically insignificantly affected. In males, at 50 mg/kg,
the plasma cholinesterase activity was depressed to 77% and there was
no inhibition of red blood cell cholinesterase activity of either sex.
All the depressed cholinesterase activities had returned to control
values at the end of the 14-day recovery period. The
no-observed-effect level, based upon changes in plasma cholinesterase
activity, was 5 mg/kg body weight per day in males and 50 mg/kg in
female rabbits (Brock, 1989).
Formulations of methomyl have also been tested by repeated dermal
application to rabbits at doses equivalent to 50 or 100 mg
methomyl/kg. No effects were seen after ten daily doses of Lannate L
(25.5% methomyl). Ten daily doses of Lannate LV (30% methomyl)
produced mild skin irritation at the higher dose level. Plasma
cholinesterase activity was somewhat depressed at both dose levels but
returned to normal over a 14-day recovery period (McAlack, 1973,
Edwards, 1980).
A Lannate dust containing 45% methomyl was the subject of a
3-month inhalation study in male rats. Exposure was at 14.8 mg/m3
for 4 h/day, 5 days/week; the mass median dynamic diameter of the dust
was 4.4 µm. There were no effects on body weight, organ weight,
histopathology or red cell cholinesterase activity. Plasma
cholinesterase activity was depressed to about 29% of the
pre-treatment activity in samples taken 4 h after the last exposure
(Ta'Naka et al., 1987).
7.3 Long-term exposure
Groups of 35 male and 35 female rats were fed diets containing
methomyl at 0 (2 groups), 50, 100, 200 or 400 mg/kg for 22 months.
Five males and 5 females per group were killed at 12 months for gross
and histopathological examination. Assessments were made throughout
the study for clinical condition, body weight, food consumption,
haematology, blood and urine biochemistry, organ weight and
histopathology. Growth of males at the highest dose was significantly
lower than that of controls over the first year. Growth of males at
200 mg/kg and females at 400 mg/kg was also lower at this time, but
not significantly. Food consumption over the first 26 weeks was
significantly lower than that of controls for the males fed 200 and
400 mg/kg. At 18 and 22 months there was a trend towards lower
haemoglobin values in treated female groups. Compound-related
increases in the incidence and severity of extra medullary
haematopoiesis were observed in the spleen of females fed 200 or
400 mg/kg. Compound-related changes were also seen in the kidneys of
both sexes at 400 mg/kg, characterized by vacuolization of epithelial
cells and hypertrophy of the proximal convoluted tubules. On the
basis of the body weight and haematopoietic findings in this study,
the long-term no-observed-effect level for methomyl in rats was
considered to be 100 mg/kg diet (Kaplan & Sherman, 1977).
In a 2-year study on rats, methomyl was fed in the diet to groups
of 80 males and 80 females at levels of 0, 50, 100 or 400 mg/kg. The
animals were observed and weighed regularly, and haematological and
blood and urine biochemical measurements were made at 3, 6, 12, 18 and
24 months. Red cell cholinesterase activity was assayed in additional
groups at 1, 2 and 4 weeks and 3, 6, 12 and 20 months. Brain
cholinesterase activity was assayed at 12 and 20 months. Extensive
histopathological examination was performed on animals killed at
scheduled intervals, on animals dying and on animals killed at the end
of the study.
There was a significant reduction in body weight gain in both
sexes at 400 mg/kg over the first 12 months but not during the
subsequent 12 months. There were no clinical signs attributable to
treatment. Survival rate was the same among the groups and there was
an acceptable number of animals at termination (40-50%). Erythrocyte
counts, haemoglobin values and haematocrits were significantly lower
in females at 400 mg/kg. There were no compound-related changes for
red cell or brain cholinesterase activity nor for other biochemical
parameters. Organ weights were unaffected by treatment. The incidence
of bone marrow hyperplasia, focal hyperplasia in the adrenal medulla
and focal degeneration in the adrenal cortex was slightly increased in
males at 400 mg/kg. The no-observed-effect level in the rat was
there-fore confirmed as 100 mg methomyl/kg diet, equivalent to about
5 mg/kg body weight per day (Kaplan, 1981).
A comprehensive long-term (2 years) study on the mouse was
initiated with 80 males and 80 females per group at levels of 0, 50,
100 and 800 mg methomyl/kg diet. Because of early high mortality, the
level of the highest dose group was reduced to 400 mg/kg at week 28
and then to 200 mg/kg at week 39. The level of the mid-dose group was
also reduced to 75 mg/kg at week 39. Regular clinical observations
and body weight and food consumption measurements were undertaken.
Haematological analysis was carried out at 4, 13, 26, 52, 78 and 104
weeks. Organ weights were measured and an extensive histopathological
examination carried out on all animals dying or killed. The mortality
rate was higher than that of controls at the highest and medium dose
levels throughout the study, and was slightly higher, in male mice
only, at the low dose level in the latter stage of the study. There
were no clinical findings due to treatment and no effects on body
weight gain or food consumption. Some effects were noted on red cell
parameters such as haemoglobin values and red cell counts during the
first 26 weeks at the medium and highest dose levels. However, these
were not apparent during the remainder of the study, after these dose
levels had been decreased. No effects were observed on organ weights
or microscopic findings. The no-observed-effect level was considered
to be 50 mg/kg in diet, equivalent to approximately 10 mg/kg body
weight per day (Serota et al, 1981).
A 2-year dog study was initiated with dietary levels of methomyl
of 0, 50, 100, 400 and 1000 mg/kg with four males and four females per
group. After one year, one male and one female from each group was
killed for interim examination. Clinical condition was regularly
observed and routine measurements made on body weight, food
consumption, and haematological and bio-chemical parameters. After 2
years the surviving animals were killed, organs were weighed and a
histopathological examination was undertaken.
No clinical signs due to treatment were seen in animals at 50,
100 and 400 mg/kg. One highest dose female died after 8 weeks and its
replacement also died within 18 days after showing compound-related
toxic signs. Two males at this dose level exhibited tremors,
salivation and incoordination during week 13. Slight to moderate
anaemia was noted in the highest dose animals at 13 weeks and this
persisted in one of the males. No effects were seen on body weight or
biochemical parameters. Histopathological effects were noted in the
kidneys and spleens of animals at 400 and 1000 mg/kg. Changes in
kidneys were characterized by increased pigmentation and epithelial
swelling, and in the spleen as extra-medullary haematopoiesis and
increased pigmentation. A minimal to slight increase in bile duct
proliferation was seen in livers of dogs at 1000 mg/kg, and bone
marrow activity was also slightly increased in animals at this dose
level. The no-observed-effect level was therefore 100 mg/kg diet,
equivalent to about 3 mg methomyl/kg body weight per day (Kaplan &
Sherman, 1977).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
The skin irritation potential of methomyl was evaluated in six
male rabbits with 0.5 g of the test material, moistened with deionized
water, applied to the back of each animal. Each site was covered with
a semi-occlusive dressing and exposure continued for 4 h. The skin
was examined and evaluated 1, 24, 48 and 72 h after the end of the
exposure period. No dermal irritation was seen in any of the animals
during the study and, therefore, methomyl is classified as
non-irritant to skin (Sarver, 1991d).
Methomyl formulations were also evaluated for skin irritation
potential using the same test method as described above. The
substances tested were a solid formulation containing 92.4% methomyl
(Sarver, 1991e) and two liquid formulations containing approximately
20% and 12.7% methomyl (Clement, 1987a; Sarver, 1991f). None of the
formulations irritated rabbit skin.
In a test on 10 guinea-pigs, methomyl was applied as a 60% paste
in propylene glycol or as a 26% solution in Cellosolve and the 24-h
reaction was noted as being mildly irritating (Kaplan & Sherman,
1977).
7.4.2 Eye irritation
Methomyl, either as 10 mg dry powder or 0.1 ml of a 10% solution
in propylene glycol, was tested in rabbits' eyes (2 per group). One
of each pair was washed after 20 sec and examination of each eye made
at 1, 2, 3, 4 and 6 h after treatment and for up to 6 days thereafter.
Only mild conjunctivitis was seen on the day of treatment but no
corneal injury was noted (Kaplan & Sherman, 1977).
A solid formulation containing 92.4% methomyl was tested in the
eyes of six male rabbits. A quantity of 10 mg was introduced into one
eye of each rabbit and evaluations of the reaction were undertaken
after 1, 24, 48 and 72 h. Pupillary constriction was evident after
one hour but was not present the next day (see also section 7.1).
Only very mild conjunctival chemosis and redness was seen at 24 h
after treatment and the formulation was considered to be non-irritant
(Sarver, 1991g).
Two liquid formulations, containing approximately 20% and 12.7%
methomyl, respectively, were tested for eye irritancy using the method
described in the previous paragraph. In both cases, the formulation
produced ocular irritation in the form of conjunctival redness and
chemosis, iritis and corneal opacity within one hour and were
classified as irritant. These effects had disappeared after 21 days.
(Clement, 1987b; Sarver, 1991h).
7.4.3 Skin sensitization
In a test to evaluate the skin sensitization potential of
methomyl, the material was applied as a paste to the abraded skin of
five guinea-pigs 3 times a week for 3 weeks; five other guinea-pigs
received four intradermal injections of 0.1 ml of a 1% solution. The
animals were then challenged after a 2-week rest period. Methomyl was
shown not to be a skin sensitizer in this test (Kaplan & Sherman,
1977).
A closed-patch repeated insult dermal sensitization evaluation
(Beuhler test) was undertaken in guinea-pigs with technical methomyl.
The test compound (300 mg), moistened with water, was applied to the
intact skin of each of 10 males and 10 females for 6 h once a week for
3 weeks. Two weeks after the last induction treatment, the animals
were challenged with another 300 mg methomyl for 6 h. No erythema was
observed in either the induction or challenge phases, and therefore
methomyl did not produce delayed contact sensitivity in the
guinea-pig. Positive control animals treated with DNCB produced the
expected sensitization responses (Armondi, 1991a).
A solid formulation containing 94.2% methomyl, subjected to the
evaluation described in the previous paragraph, also failed to produce
delayed contact hypersensitivity in guinea-pigs (Armondi, 1991b). The
same result was obtained with a liquid formulation containing 21.5%
methomyl when given at 0.5 ml per application (Mercier, 1991).
Another liquid formulation containing 12.7% methomyl was tested in the
Magnusson-Kligman guinea-pig sensitization maximization procedure and
was found to be a moderate skin sensitizer under the conditions of the
test (Armondi, 1992).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Embryotoxicity and teratogenicity
Teratogenicity studies have been undertaken in the rat and
rabbit.
Pregnant rats (25 per group) were fed 0, 50, 100 or 400 mg
methomyl/kg diet during days 6-15 of gestation. The dams were
observed and weighed regularly and then killed on day 21 of gestation.
At that time the uterus was removed, and the number of corpora lutea,
implantation sites, live and dead fetuses, resorptions and weight of
live fetuses were recorded. About one half of the fetuses from each
litter were taken for skeletal examination and the remainder for soft
tissue examination. The pregnant rats at 400 mg/kg had significantly
lower body weight and ate less food than those in the control group.
There were no clinical signs of toxicity and all rats survived the
test period. Parameters of pregnancy and fetal development, such as
the number of litters with partial or total resorptions and fetal
weight, were not affected in the test groups compared to controls.
There were no treatment-related abnormalities in the fetuses.
Methomyl was therefore not embryotoxic or teratogenic in the rat at
levels up to 400 mg/kg diet, equivalent to 34 mg/kg body weight per
day (Rogers et al., 1978).
Groups of 20 pregnant rabbits were given 0, 2, 6 or 16 mg
methomyl/kg body weight per day by gavage on days 7-19 of gestation.
The animals were observed and weighed regularly and killed on day 29.
The uteruses were then examined for pregnancy, number of
implantations, early and late resorptions and live or dead fetuses.
Delivered pups were weighed and examined for visceral or skeletal
abnormality. One pregnant rabbit at 6 mg/kg died, probably due to
gavage error. At 16 mg/kg there were seven deaths during the test
period due to the compound. Clinical signs at the highest dose level
included tremors, hyperactivity, excessive salivation, aggression and
convulsions. The no-observed-effect level for maternal toxicity was
therefore 6 mg/kg body weight per day. There was no effect on
pregnancy rate or on the average numbers of corpora lutea,
implantations, resorptions or live fetuses per dam in treated groups
compared to controls. There were no gross external visceral or
skeletal variations due to treatment. Methomyl is therefore not
embryotoxic or teratogenic to the rabbit at doses up to 16 mg/kg, at
which dose maternal toxicity occurred (Christian et al., 1983).
In another study, pregnant rabbits (12 per group) were fed diets
containing up to 100 mg methomyl/kg. The pregnancy rate was low in
all groups, including controls. No effects were noted on a range of
parameters including reproductive indices, fetal body weight or
visceral and skeletal abnormalities. However, due to experimental
inadequacies the results are difficult to assess (FAO/WHO, 1987).
7.5.2 Reproduction studies
A three-generation (two litters per generation) rat study was
undertaken to test the potential reproductive effects of methomyl (Lu,
1983). The compound was fed at levels of 0, 50 or 100 mg/kg diet to 10
males and 20 females for approximately 90 days post weaning prior to
first mating to produce the F1a litter. Test diets were fed through
to subsequent re-mating to produce the F1b litter. Ten males and 20
females per group were then selected from the F1b litter and
continued on their respective diets for approximately 90 days to
produce F2a and then F2b litters. The same procedure was used to
produce subsequent litters of the next generation. Records were kept
of all matings, number of pregnancies, number of young in each litter
born alive or dead, the body weight of offspring at weaning, and the
respective reproduction and lactation indices. Ten males and 10
female offspring were selected from each group of the F3b litter for
histopathological examination. Methomyl did not affect the
reproductive performance of rats in this study. There were no effects
on fertility, gestation or lactation and no compound-related
abnormalities on gross examination. No treatment related changes were
seen upon histopathological examination of tissues from the offspring
in the F3b litter (Kaplan & Sherman, 1977).
A two-generation rat study was also carried out using high
dietary levels of methomyl (600 and 1200 mg/kg) as well as a lower
level of 75 mg/kg. The F0 parents (13 males, 26 females) were mated
after 100 days treatment and the F1 parents (20 males, 40 females)
after a minimum of 120 days. Spermatogenesis (based on sperm count)
was measured on adult males in both generations after breeding, in
addition to the standard assessments of reproductive performance.
Histopathological examination was undertaken on a selection of tissues
from parents and pups in both generations. Body weight depression in
parents, seen at all or some stages during treatment at 600 and
1200 mg/kg in both generations and at 75 mg/kg in the F1 generation,
was probably related to lower food intake. Reduced red blood cell
counts were seen in females at the highest dose level, together with a
small (15-25%) depression in plasma cholinesterase activity in both
sexes at this level. Decreases in litter size and live births were
shown for the highest dose F0 group and for all F1 groups for
which a clear no-observed-effect level was not established. Survival
during lactation in F1 pups (from F0 parents) was reduced at the
highest dose level, as was survival in highest dose F2 pups during
early lactation up to day 4. Pup body weight was slightly reduced at
75 mg/kg, significantly reduced at the two higher levels in the F1
generations and reduced at the 600 and 1200 mg/kg levels in the F2
generation. No histopathological changes due to treatment were seen
in any of the tissues examined in parents or offspring. No effects on
the reproductive parameters of fertility indices, gestation length or
male sperm counts were seen at dose levels up to and including 1200 mg
methomyl/kg in diet (Lu, 1983).
7.6 Mutagenicity
Numerous in vitro mutagenicity assays, to various end-points,
have been carried out on methomyl by several investigators (Table 6).
Methomyl did not show mutagenicity or cause primary DNA damage in
bacterial or mammalian cells in vitro. It showed cytogenetic
potential in human lymphocytes in vitro as indicated by an increase
in micronuclei and chromosomal aberrations.
In an in vivo study, the clastogenic potential of methomyl was
assessed by its ability to cause numerical and structural chromosomal
aberrations in rat bone marrow cells. Single doses of methomyl were
given by gavage to groups of 15 male and 15 female rats at doses of 2,
6 or 20 mg/kg body weight; five males and five females were killed at
6, 24 and 48 h after dosing and the bone marrow cells harvested. These
were examined for chromatid and chromosome breaks and for gaps and
other aberrations. The results showed that there was no significant
increase in the frequency of chromosomal aberrations and no
significant differences between the chromosome numbers and the mitotic
indices of the dosed groups and the controls (Farrow et al, 1984).
Table 6. Summary of results of in vitro mutagenicity assays and related end-point studies on methomyl
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
Prokaryotes
Point mutation Salmonella typhimurium - 50 nM negative Blevins et al. (1977a)
(5 strains)a
Point mutation S. typhimurium (5 strains) + and - up to 5000 µg/plate negative Moriya et al. (1983)
Escherichia coli (WP2hcr) + and - negative
Point mutation S. typhimurium (5 strains) + and - up to 1 mg/plate negative Waters et al. 1982
Point mutation E. coli, WP2uvr A + and - up to 1 mg/plate negative Waters et al. 1982
DNA damage E. coli pol A - 1 mg/disc negative Waters et al. 1982
W3100, p 3478
DNA damage Bacillus subtil recM45 - 1 mg/disc negative Waters et al. 1982
Yeast
DNA damage Saccharomyces cerevisiae + and - 1-3.5% negative Waters et al. 1982
D3
Mammalian cells
DNA damage Human lung fibroblast WI-38 + and - 10-7-10-3M negative Waters et al. 1982
DNA damage Rat hepatocytes (UDS) - 1 µM-75 mM negative Vincent (1985)
Table 6 (cont'd).
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
DNA damage Human skin cell DNA - 10-5M negative Blevins et al. (1977b)
Gene mutation Chinese hamster V79 cells + and - 1-10 mM negative Wojciechowski et al. (1982)
Gene mutation Chinese hamster ovary - 10-55 mM negative McCooey et al. (1984)
(CHO) cells, HGPRT + 100-350 mM
Chromosome Human lymphocytesb NA 0.02-0.54 mM positive Bonatti et al. (1994)
aberrations (whole blood culture)
Sister-chromatid Human lymphocytesb NA 0.02-0.54 mM negative Bonatti et al. (1994)
exchange (whole blood culture)
Micronuclei Human lymphocytesb NA 0.01-0.09 mM positive Bonatti et al. (1994)
(whole blood culture)
DNA single strand Human lymphocytesb NA 0.06-2 mM negative Bonatti et al. (1994)
breaks (whole blood culture)
DNA oxidative Human lymphocytesb NA 0.25 - 1 mM negative Bonatti et al (1994)
damage (whole blood culture)
a Plate incorporation and spot tests
b The metabolic activity of the cell cultures was demonstrated by the positive control response
c NA = not applicable
Methomyl was tested in the sex-linked recessive lethal assay with
Drosophila melanogaster at concentrations of 4 and 10 mg per litre
in the test medium. No increase in mutation rate was observed in this
assay (Waters et al., 1982).
A formulation (Lannate 20) containing 20% methomyl, but of
otherwise unstated composition, caused an increase in the number of
sex-linked recessive lethals, but with no observed translocations.
The same formulation increased the incidence of abnormal sperm and
frequency of chromosomal aberrations in mice (Hemavathy &
Krishnamurthy, 1987a,b).
7.7 Carcinogenicity
The carcinogenic potential of methomyl was assessed in the
long-term rat and mouse studies already described in section 7.3.
In the earliest study, groups of 35 male and 35 female rats were
fed 0 (2 groups), 50, 100, 200 or 400 mg methomyl/kg diet for 22
months. No differences were seen in the incidence of neoplastic
lesions between control and treated groups (Kaplan & Sherman, 1977).
In the more recent rat study, groups of 80 males and 80 females
were fed 0, 50, 100 or 400 mg methomyl/kg diet for 2 years. The
mortality rates were similar in all groups and there was an overall
survival of 40-50% by the end of the study. More than 30 tissues/rat
were examined microscopically from animals killed or dying during the
study and at interim and terminal sacrifice. There was no indication
of any differences between groups in the appearance of any individual
tumour nor were there any differences between groups for the number of
tumour-bearing rats or those with benign or malignant tumours (Kaplan,
1981).
The 2-year mouse study was initiated at dietary levels of 0, 50,
100 and 800 mg/kg with groups of 80 males and 80 females. By week 39
the highest dose level had been reduced to 200 mg/kg and the 100 mg/kg
level to 75 mg/kg (see section 7.3 for details). Mortality was higher
in the highest dose male and female groups than in controls throughout
the study and was also somewhat higher in the mid-dose group.
Adequate numbers of mice survived to the termination of the study
(30-50% for female groups and 38-68% for male groups).
Histopathological examination on more than 30 tissues per mouse was
undertaken on animals killed or dying during the study and at terminal
sacrifice. There were no differences between groups for the numbers
of tumour-bearing mice or those with benign or malignant tumours
(Serota et al., 1981).
Methomyl did not transform hamster embryo cells in a
trans-placental host-mediated assay, nor did the cultured cells from
this assay induce tumours when injected subcutaneously into young
adult nude mice (Quarles et al., 1979).
7.8 Other special studies
7.8.1 Cholinesterase studies in vivo and in vitro
Due attention must be given to the measurement of cholinesterase
activity in blood and brain samples because of the fast spontaneous
reactivation of the methomyl-inhibited enzyme. It is important to
analyse samples in the minimum possible time after sampling and to use
an analytical method which requires the minimum of sample dilution and
shortest assay time. If these criteria are not followed then
cholinesterase inhibition due to methomyl can be misinterpreted.
In groups of rats fed 0, 50, 100, 200, 400 or 800 mg methomyl per
kg diet for 79 days, only those fed the highest dose showed blood
acetylcholinesterase activity inhibition as assayed by the Ellman
method. Males showed blood cholinesterase activity inhibition of
25-40% early in the study (by 11 days) while females showed this
inhibition towards the end of the study period (Singles, 1970a). In
an extension of this study, the blood acetyl-cholinesterase activity
of rats fed 400 or 800 mg/kg was assayed by the Ellman method and a pH
stat method after 5 months of feeding. In neither group was there an
effect on blood acetyl-cholinesterase activity (Singles, 1970b).
Methomyl was fed to groups of rats in the diet at levels of 0,
100, 400 or 800 mg/kg, and blood was taken for red cell and plasma
acetylcholinesterase activity assay at 1, 7, 14, 21 and 28 days.
Brain acetylcholinesterase activity was also measured on rats at 14
and 29 days. All assays were undertaken by the Ellman method. There
were small depressions of acetylcholinesterase activity (up to 20%) in
red cells and plasma of males and females at 800 mg/kg. Brain
acetylcholinesterase activity was also slightly depressed (<17%) at
this dose level. There were no consistent findings of
acetylcholinesterase inhibition at the two lower levels of 100 and
400 mg/kg (Barnes, 1978).
Lannate, containing 90% methomyl, was given to 14 male rats at
41 mg/kg body weight by oral intubation each day for 8 successive
days. The animals were killed 24 h after the last dose and blood was
taken for the assay of serum cholinesterase activity by a colorimetric
method. The activity was depressed by 40% compared to controls
(Borady et al., 1983).
Cholinesterase activity in plasma and red cells of rats was
assayed after the dermal application of methomyl. Four groups of rats
were treated dermally with 200 mg methomyl per rat and blood samples
were collected 2, 4, 6 and 24 h after treatment. Plasma
cholinesterase activity was markedly reduced at each time interval
compared to controls (22-50% of control value) while red cell
cholinesterase activity was less affected (80-91% of control value).
Groups of rats were also treated dermally with 25 or 100 mg per rat
and blood was collected at 24 and 72 h after treatment. Plasma
cholinesterase activity was depressed at both levels and at both time
intervals, but red cell acetylcholinesterase was unaffected (Henry,
1981).
Lannate powder, containing 45% methomyl, was the subject of
inhalation studies using a single 4-h exposure to 9.9 mg/m3 or
repeated exposures to 14.8 mg/m3 (see section 7.2 for details).
Plasma cholinesterase activity was markedly depressed (by 50%) for 4 h
after the single exposure, but showed recovery almost to the control
value within 20 h. Red cell cholinesterase activity was not
inhibited. Plasma cholinesterase activity was less affected (29%
depression) 4 h after the last of the repeat exposures than after the
single exposure (Ta'Naka et al., 1987).
The I50 value (the molar concentration of inhibitor giving 50%
reduction in enzyme activity in given experimental conditions) for
methomyl was determined in vitro for human and rat red blood cell
acetylcholinesterase activity using a modified Ellman method. Human
acetylcholinesterase was approximately six times more sensitive to
methomyl's action than rat acetylcholinesterase (I50 values of
0.265 × 10-5 mol/litre and 1.56 × 10-5 mol/litre, respect-ively).
Regeneration studies using a gel filtration method showed that
methomyl-inhibited rat acetylcholinesterase regenerates at a faster
rate than the human enzyme, with half-lives of 26.6 and 38.0 min,
respectively (Carakostas, 1987).
7.8.2 Neurotoxicity
Hens given a single oral (LD50) dose of 28 mg methomyl/kg
either died within 10 min or survived. Survivors showed toxic signs
of lacrimation and salivation, but no evidence of wing or leg
paralysis. After a 21-day recovery period, no abnormalities were
observed upon histopathological examination of the sciatic nerve.
Doses much higher than the oral LD50 (up to 200 mg/kg) were given to
hens without causing mortality after they had been pre-treated with
atropine sulfate subcutaneously at 10 mg/kg. Wing or leg paralysis
was not observed and there were no histopathological abnormalities in
the sciatic nerve after a 21-day recovery period. Birds treated with
tri- o-cresyl phosphate (TOCP) at 500 mg/kg showed the leg paralysis
and sciatic nerve degeneration expected of a positive control
substance (Krauss & Stula, 1967).
7.8.3 Potentiation studies
Potentiating properties were determined by administering one-half
the oral LD50 of methomyl to rats, followed immediately by one-half
the LD50 of another anticholinesterase pesticide, and then recording
the resultant mortality over 14 days. Out of 18 pesticides tested,
only Sevin and Ronnel showed the ability to increase mortality
indicative of a potentiation effect (Sherman, 1967). In another study,
the inhibition of cholinesterase activity in rat blood after the oral
administration of methomyl alone or in combination with another
anticholinesterase pesticide was used to measure potentiating effects.
Methomyl in combination with methyl parathion or phosdrin was shown to
be potentiating, while antagonism occurred when it was combined with
dimethoate (Henry, 1975).
Rats (10 per sex and per group) were given methomyl at 200 mg/kg
diet and ethanol as a 10% aqueous solution, either separately or
together, for a period of 12 weeks. There was some evidence of
increased effects as a result of the combined administration resulting
in decreased body weights from weeks 2 (females) and 4 (males) and
increased relative organ weights for adrenals (males) and kidneys
(females). In male rats, the combination of the two compounds
increased hepatic triglycerides and free fatty acids and decreased
brain acetylcholinesterase activity more than that expected from
either compound alone (Antal et al., 1979). Ethanol administered in
the diet to rats, at a level equivalent to 25% of the calorie intake,
did not potentiate erythrocyte acetylcholinesterase inhibition by
methomyl coadministration at 200 mg/kg in the diet (Bracy et al.,
1979).
In female rats only, the combination of methomyl and caffeine
retarded growth, increased the relative weight of kidneys, spleen,
adrenals, liver and heart, depressed glucose-6-phosphate dehydrogenase
activity, and increased aniline hydroxylase and glucose-6-phosphatase
activity (Bedö & Cieleszky, 1980).
7.8.4 Antidote studies
The antidotal properties of several agents against the toxicity
of methomyl have been examined in a number of species.
Potential antidotes were administered within one minute after
oral lethal doses of methomyl (30 or 60 mg/kg) were given to rats. Of
the agents used, atropine sulfate given intraperitoneally as a single
dose of 50 mg/kg proved to be the most effective antidote. Other
agents used which were less effective or ineffective were
pyridine-2-aldoxime methiodide (PAM) and tetra-ethylammonium chloride
(TEAC) (Sherman, 1968a). Rats were also used in a study of the
effectiveness of obidoxime or N-methyl-pyridinium-2-aldoxime
methane-sulfonate (P2S) as antidotes alone or in combination with
atropine sulfate. Methomyl was injected subcutaneously as solutions
of logarithmically graded concentrations (1 ml/kg body weight), and at
the onset of signs of toxicity atropine sulfate (17.4 mg/kg), P2S
(50 mg/kg) and obidoxime (90 mg/kg) were injected subcutaneously
either alone or in a combination. Lethality was reduced by atropine
sulfate or P2S but obidoxime was ineffective. Atropine sulfate in
combination with P2S also was effective (Natoff & Reiff, 1973).
Mice fed a lethal dose of 100 mg methomyl/kg in pellets and then
given TEAC by injection 10 min later survived, and resumed normal
activity within another 10 min (Andrews & Miskus, 1968). Atropine
sulfate (50 mg/kg intraperitoneal) was shown to have antidotal
activity in guinea-pigs given methomyl orally at lethal single doses
of 15-60 mg/kg (Sherman, 1968b). Dogs given a single oral dose of 10
or 20 mg methomyl/kg by capsule were protected against the toxic
effects if atropine sulfate was given very quickly, i.e. within a few
minutes. The antidote was more effective when given intravenously
than when given intramuscularly or orally (Sherman, 1968c).
The antidotal effectiveness of atropine administered
intravenously or orally at 1 or 10 mg/kg and hexamethonium or TEAC
given intravenously at 10 mg/kg was evaluated in rhesus monkeys dosed
with 20 or 40 mg methomyl/kg orally. Atropine at 1 mg/kg, given
intravenously or orally, was effective as an antidote for the
sublethal dose of 20 mg methomyl/kg. Atropine at 10 mg/kg orally,
administered immediately after the appearance of toxic signs, was an
effective antidote for the lethal dose of 40 mg methomyl/kg. Recovery
occurred within 2-5 h of the methomyl dose. TEAC at 10 mg/kg
(intravenous) also prevented the death of one monkey after a lethal
dose of methomyl, although recovery took longer than after atropine.
Hexamethonium did not appear to be very effective (Teeters, 1968).
7.8.5 Other studies
Lannate (90% methomyl) was given to 14 male rats at the high dose
level of 41 mg/kg by intubation each day for 8 days and the animals
were killed 24 h after the last dose for hepatic and serum assays.
Liver weight was not increased, nor was there histopathological
evidence of fatty deposit, but total lipids including cholesterol and
phospholipids were increased. The treatment also increased the level
of serum triglycerides, phospholipids and free fatty acids and
increased the activity of GOT, GPT and alkaline phosphatase enzymes
(Borady et al., 1983). Lung triglyceride, cholesterol and
phospholipid contents were not affected when rats were exposed to
Lannate (45% methomyl) dust at 14.8 mg/m3 by inhalation for 4 h/day,
5 days/week for 3 months (Ta'Naka et al, 1987).
Single oral doses of 2 or 10 mg methomyl/kg increased pancreatic
chymotrypsin, lipase and amylase activities in male and female rats.
Rats fed 100, 400 or 800 mg/kg diet for 10 days showed elevated serum
cholesterol in females only. Hepatic aminopyrine demethylase and
aniline hydroxylase activities were increased in female rats at 400
and 800 mg/kg. When rats were given 100 or 200 mg/kg diet, females
fed 200 mg/kg showed elevation of total serum lipids and cholesterol.
The females also showed decreased hepatic glucose-6-phosphatase
activity and vitamin A at this level (Bedö & Cieleszky, 1980).
The effect of methomyl on a number of serum constituents was
studied in rats given oral doses of 6.8 mg/kg per day, 6 days/week for
4 weeks. Increases were seen over the 4-week period for serum
glucose, cholesterol, GOT and GPT, while decreases were seen for serum
total protein albumin, globulin and cholinesterase activity. However,
since control values did not seem to be available for the 4-week
period, it was difficult to evaluate the significance of the results
for methomyl (Saleh, 1990a,b,c).
7.9 Factors modifying toxicity
It has been shown that synthesized nitrosomethomyl is mutagenic
in vitro and is capable of producing stomach tumours in rats
(Blevins et al., 1977a,b; Lijinsky & Schmahl, 1978). However, when
methomyl is incubated with nitrite and macerated meat under simulated
stomach conditions, there is no evidence that nitrosomethomyl is
formed (see section 4.3).
7.10 Mechanisms of toxicity - mode of action
As a member of the carbamate class of compounds, methomyl has a
well-known mode of action via inhibition of the enzyme
acetylcholinesterase at nerve junctions. A detailed description of
the mechanism of action of carbamates on cholinesterases is given in
Environmental Health Criteria 64: Carbamate pesticides: a general
introduction (IPCS, 1986). Studies with methomyl show that the onset
of toxic action is rapid and that, in common with many other
carbamates, the toxic effect is rapidly reversible. Because of this,
it is important to use appropriate assay methods when measuring
cholinesterase activities during toxicity studies. Failure to allow
for the rapid reversibility of the action can lead to underestimation
of enzyme inhibition.
The acute toxic action of methomyl is characterized by signs of
poisoning typical of anticholinesterase action, i.e. lacrimation,
profuse salivation, tremor and pupil constriction. Surviving animals
quickly show signs of recovery, often within hours. The toxic action
can be countered by the anticholinergic antidote atropine sulfate.
The potency of methomyl is greatest when given by the oral or
inhalation routes of administration. It has very low acute toxicity
when given dermally, presumably because during the slower absorption
phase there is time for recovery from toxic action and thus the effect
is never fully exerted.
8. EFFECTS ON HUMANS
8.1 General population
8.1.1 Accidental and suicidal poisoning
Five Jamaican fishermen prepared a meal to which they
accidentally added methomyl instead of salt. Within minutes of eating
the meal, three of the men were badly affected, twitching, trembling
and frothing at the mouth, and died within 3 h. One of the other two
also showed toxic symptoms, while the other was unaffected. Both
survivors were given atropine and the symptomatic patient recovered
within 2 h after treatment. Postmortem examination of the dead men
revealed highly congested stomach lining, lungs, trachea and bronchi.
Analysis showed that part of the meal (roti) contained about 1%
methomyl. It was estimated that the victims had consumed about
12-15 mg methomyl/kg (Liddle et al., 1979).
A 31-year-old woman committed suicide, using a methomyl
preparation, together with her three children, one of whom (a
9-year-old son) survived. Postmortem examination showed congestion of
the stomach mucous membranes and the lungs. Analysis of organs of the
mother and a 6-year-old son showed highest methomyl concentrations in
the liver (15.4 and 56.5 mg/kg, respectively). Large amounts of
methomyl were present in the stomach contents and it was estimated
that the doses were 55 mg/kg for the mother and 13 mg/kg for the son
(Araki et al., 1982).
A woman attempted suicide by ingesting about 2.25 g methomyl.
After 6 h, methomyl was present in the blood at a concentration of
1.61 mg/kg and in the urine at 10.9 mg/litre; at 15 h the levels were
0.04 mg/kg and 0.25 mg/litre, respectively, and at 22 h methomyl could
not be detected (Noda, 1984).
Symptoms and treatment were described for 11 patients who had
suffered methomyl poisoning in Spain over a 5-year period.
Intoxication was accidental in six cases and suicidal in the other
five. The time interval between exposure and admission for treatment
averaged 2.8 h. All of the subjects showed cholinergic symptoms;
plasma cholinesterase activity was normal in four cases and moderately
reduced in the others. Treatments applied included gastric lavage,
washing the skin, administration of activated charcoal and small doses
of atropine, according to the symptoms involved. All the patients
recovered within 24-48 h (Martinez-Chuecos et al., 1990).
A blood level of 0.57 mg methomyl/litre was measured in a pilot
who had died as a result of a crash while spraying a solution of
methomyl and chlorothalonil in methanol. Analysis was not undertaken
for chlorothalonil and methanol (Driskell et al., 1991).
A 79-year-old man and his 73-year-old wife attempted suicide by
ingesting methomyl powder. The woman died within 19 h but the husband
survived after treatment by gastrolavage, followed by administration
of atropine sulfate. The serum methomyl concentration in the woman
was 44 mg/kg 1 h after ingestion and 0.2 mg/kg in the blood at
autopsy. The methomyl blood concentration of the man was
0.01-0.1 mg/kg 28 h after ingestion (Miyazaki et al., 1989).
8.2 Adverse effects of occupational exposure
Pesticide operators were reported to be affected after handling
powder formulations of methomyl. In one case, an operator mixed a
powder formulation and sprayed vegetables without taking any special
precautions. He displayed poisoning symptoms within 1 h. His blood
cholinesterase activity had decreased to 40% of normal after 12 h but
had recovered to within 80% of normal after 36 h. Other operators, a
mixer-loader, pilot and markers, using recommended safety precautions,
did not show any effects on their red cell or plasma cholinesterase
activities after handling liquid formulations and subsequent aerial
application of methomyl (Simpson & Bermingham, 1977).
In a survey of agricultural applicators in California, USA, in
1982-1989, methomyl was reported to be involved in 129 out of 5371
exposure-related illnesses due to pesticides. For the period
1982-1985 and for the same area, methomyl was considered responsible
for 47 illnesses out of 238 reported cases. Exposure was described as
either short term (< 3 days), longer term (> 3 days) or accidental
(Brown et al., 1989). In 1986, in a summary of reported
pesticide-related illnesses and injuries in California, methomyl was
identified as the probable causative agent in 7 out of 1065 confirmed
occupational cases. These cases were described as "..having some
likelihood of being pesticide related" (Edmiston & Maddy, 1987).
An investigation of workers in a pesticide formulation plant
revealed that 11 out of 102 workers had been hospitalized due to
work-related illness. Most frequent were symptoms of exposure to
methomyl and methaemoglobinaemia due to 3,4-dichloroaniline (Morse et
al., 1979).
Significant T-wave changes (decreased height, inversion, and
leftward deviation of T-wave axis) were shown in 10 out of 22 spraymen
applying methomyl for five days under field conditions. These changes
reverted to pre-exposure levels within one week. Sufficient
information was not available to evaluate the significance of these
findings for operator exposure, and further studies are required to
fully understand their significance (Saiyed et al., 1992).
Two case reports of allergic contact dermatitis with exposure to
methomyl have been described. In the first case, a 26-year-old woman
had gradually worsening itchy hand eczema for six months when working
in a plant nursery, pollinating, pricking out and potting plants
sprayed with methomyl (Lannate) solution. After she avoided touching
the sprayed plants or used polyvinyl gloves she became free of eczema.
She also reacted positively to a 1% aqueous Lannate solution,
indicating that Lannate was a cause of the hand eczema. The second
patient had already had hand eczema for 14 years with recent
worsening. As soon as she changed her job and no longer had contact
with Lannate her eczema disappeared (Bruynzeel, 1991).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effect of methomyl on sewage microorganisms was assessed
indirectly by monitoring total oxygen demand (TOD) over a 24-h period.
A mixture of activated sludge, phosphate buffer and a simulated waste
consisting of Bactopeptone and meat extract was incubated with
methomyl at concentrations of 0, 1, 5, 10, 50 and 90 mg/litre. Only
at 90 mg/litre was there a slight effect on TOD (<10%), indicative of
a minor effect on microorganism activity (Belasco, 1972a).
A fine sand and two silt loam soils were treated with 18 mg
methomyl/kg, and the soil fungi and bacteria populations counted after
11 days. The effect on total microbial activity was determined by
measuring the CO2 evolved periodically over 56 days. Neither soil
microorganism populations nor CO2 production was affected by the
high level of methomyl in the soils, when compared to controls
(Peeples, 1977).
When methomyl was incorporated into a silt loam sand at 0.5 mg/kg
together with ammonium sulfate, no effect on nitrification occurred
over a 6-week period. This level of incorporation represented the
recommended field treatment of 0.56 kg/ha. At ten times this level of
incorporation there was some delay (8-9 days) in reaching the 50%
nitrification level, but by 4 weeks the level was the same as that in
the control soil (Han, 1972).
Methomyl, in the form of Lannate 20L (20% a.i.), was incorporated
into a sandy and a loamy soil at rates of 1.87 and 18.7 mg a.i./kg and
incubated at 20°C for 28 days. Dehydrogenase activity, measured at 14
and 28 days, was unaffected by either rate of incorporation in both
soils. Ammonia nitrogen and nitrate nitrogen declined by about the
same extent in both soils over the 28-day period. This could have
been due to stimulation of nitrogen assimilation by the product
(Danneberg, 1991).
9.2 Aquatic organisms
9.2.1 Algae
The effect of methomyl on algal growth was assessed using
Selenastrum capricornutum as test species. The organism was cultured
under continuous illumination at 24°C with methomyl at concentrations
of 6.25-100 mg/litre for 120 h. Algal growth was monitored at
intervals over the exposure period by measuring the absorbency of
light at 665 nm. The EbC50, (median effective concentration for
inhibition of growth based on a comparison of areas under the growth
curves after "b" hours) after 72 and 120 h was 60 mg/litre. The
ErC50 (median effective concentration for growth inhibition based
on a comparison of maximum growth rates from "x" to "y" hours) from
24-48 h was 140 mg/litre (extrapolated value). The no-observed-effect
concentration was 6.25 mg/litre (Douglas & Handley, 1988).
A similar study was carried out using Lannate 20L (20% methomyl)
as test substance at concentrations of 6.25-200 mg/litre. The EbC50
(72 h) was 68 mg/litre, EbC50 (120 h) was 116 mg/litre and the ErC50
(0-24 h) was 67 mg/litre. A no-observed-effect concentration of
6.25 mg/litre was established (Douglas & Halls, 1991).
9.2.2 Fish
The acute toxicities (96-h LC50, etc.) for several species of
fish are shown in Table 7. The 96-h LC50 values lie mostly within
the range of 0.5-2 mg/litre. The most resistant species tested were
the cutthroat trout with a 96-h LC50 of 4-7 mg/litre. Generally,
within a species, the higher the water temperature the higher the
acute toxicity. Results for some methomyl formulations are shown in
Table 8. These show lower acute toxicity, corresponding to the lower
methomyl content. There are few descriptions of toxic signs in
affected fish. In an acute study with carp and tilapia, the fish sank
to the bottom of the tank and moved sluggishly, then became highly
excited 10 min before death (El-Refai et al., 1976). Shrimps became
lethargic before dying (Bentley, 1973; Sleight, 1973).
Two longer-term studies have been undertaken with methomyl and a
formulation. This included a 21-day flow-through study on fingerling
rainbow trout with Lannate 20L (21.5% methomyl) at 13°C. The
concentrations tested were 0.63, 1.3, 2.5, 5.0, 10 and 20 mg/litre,
equivalent to 0.14-4.3 mg methomyl/litre. No effects on fish length
or weight were noted throughout the study. Some increase in mortality
occurred, from day 12 onwards, in the 5 mg/litre group, and greater
dose-related increases were seen at the two higher concentrations
throughout most of the study. Surviving fish at these concentrations
showed increasing incidence of one or more of the signs of
discoloration, lying on the tank bottom, gasping, bloated stomach,
loss of equilibrium. The 21-day LC50 was calculated to be
6.1 mg/litre formulation (equiv. 1.3 mg methomyl/litre) and the
no-observed-effect concentration was 2.5 mg/litre formulation (Baer,
1991c).
Table 7. The acute toxicity of methomyl to fish (static test)
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Atlantic salmon (Salmo salar) 12 1.12 USDI (1978)
(0.48 g) 17 0.56 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) 12 2.00 USDI (1978)
22 0.86 USDI (1978)
23 2.00 Carter & Graves (1973)
22 1.00 USDI (1975)
Brook trout (Salvelinus fontinalis) 12 1.50 USDI (1978)
Carp (Cyprinus carpio) (5 cm) 25 2.8 Yoshida & Nishiuchi (1972)
Channel catfish (Ictalurus punctatus) 22 0.53 USDI (1978)
(1.8 g) 26 0.92 Carter & Graves (1973)
Cutthroat trout (Salmo clarkie) 10 6.8 USDI (1978)
10 4.05 USDI (1975)
Fathead minow (Pimephales promelas) - 2.8 Mayer & Ellersieck (1986)
(0.75 g)
Japanese goldfish (Carassius auratus) 25 2.7 Yoshida & Nishiuchi
(4 cm) (1972)
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi (1972)
Table 7. (cont'd).
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi (1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
Table 8. The acute toxicity of some methomyl formulations to fish (static test)
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Bluegill sunfish (Lepomis macrochirus) 24% kg a.i. - 0.7 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) Lannate 20 L 21.5 5.1 Baer (1991a)
Bluegill sunfish (Lepomis macrochirus) Lannate 90% WDP - 2.1 USDI (1978)
Carp (Cyprinus carpio) Lannate 25 WP 22 4.7 - Ökolimna (1980)
12.5
Carp (Cyprinus carpio) (1.75 g) Lannate 24 EC 22-25 2.96 El-Refai et al. (1976)
Carp (Cyprinus carpio) (31.5 g) Lannate 24 EC 1.21 El-Refai et al. (1976)
Rainbow trout (Salmo gairdneri) Lannate 20 L 12.6 18 Baer (1991b)
Lannate 90% WDP 13 3.4 McCain (1971)
Tilapia (Tilapia nilocita) (1.5 g) Lannate 24EC 22-25 0.92 El-Refai et al. (1976)
Tilapia (Tilapia nilocita) (13.8 g) 0.88
Channel catfish (Ictaurus punctalus) 24% liquid form - 0.3 USDI (1978)
Channel catfish (Ictaurus punctalus) 29% liquid form 0.3 USDI (1978)
Brook trout (Salvelinus fontinalis) 24% liquid form - 2.2 USDI (1978)
Table 8 (cont'd).
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi
(1972)
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi
(1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
In an early life-stage toxicity study, fathead minnow embryos and
larvae were exposed for 28 days to methomyl at concentrations of
27-491 µg/litre. The water was changed at the rate of 10 aquarium
volumes each 24 h and was maintained at a temperature of 25°C. Embryo
hatch and larval survival and growth was evaluated. The percentage
embryo hatch was not affected at any concentration. Larval survival
was significantly reduced at concentrations down to 117 µg/litre and
growth reduced at 243 and 491 µg/litre. The maximum acceptable
toxicant concentration was estimated to be >57 µg/litre and
<117 µg/litre (Driscoll & Muska, 1982).
9.2.3 Other aquatic organisms
The acute toxicity results for a variety of other aquatic
organisms are shown in Table 9. Daphnia magna appears to be one of
the most susceptible species to the acute toxic action of methomyl,
and the Eastern oyster the least susceptible. This difference was
further emphasized in two other studies. The 48-h EC50 for the
Lannate 20L formulation (21.5% methomyl) was 0.033 mg/litre
(equiv.0.007 mg methomyl/litre) for Daphnia magna neonates (Baer,
1991d). The 96-h EC50 for the Eastern oyster was > 140 mg/litre,
as measured by the effect of methomyl on shell deposition in a
flow-through test at 21.2 to 23.7°C (Ward & Boeri, 1991).
The effect of methomyl or its formulation on Daphnia magna was
studied in longer-term tests on survival, growth and reproductive
capacity. A study was initiated with < 24-h-old daphnids exposed to
measured methomyl concentrations of 0.7, 1.0, 1.6, 3.5, 7.5 or
13.8 µg/litre. The test was conducted over a 21-day period at 20°C
under semi-static conditions with 2-day renewal of the test
solutions. Methomyl was found to be stable in these solutions for up
to 72 h. Daphnid survival and growth were not affected at any
concentration. The number of young produced and number of young per
adult were reduced at 3.5, 7.5 and 13.8 µg/litre. There was some
delay in the first day of reproduction at all levels, but this was
considered to be biologically significant only at 3.5 µg/litre or
more, where the number of young was affected. The maximum acceptable
toxicant concentration was estimated to be between 1.6 and
3.5 µg/litre (Brittelli, 1982).
In another 21-day study, Daphnia magna were exposed to Lannate
20 (21.5% methomyl) at nominal concentrations of 0.63, 1.5, 3.4, 8.
18, 43, 100 or 232 µg/litre. The test conditions and parameters
measured were as described above. At 232 µg/litre all daphnids died
early in the study, but there was no effect on survival at the lower
concentrations nor was growth affected. The 21-day EC50, based upon
adult survival, was estimated to be 160 µg/litre (equivalent to 26 µg
methomyl/litre). The total young produced and number of young per
adult were decreased at 3.4 µg/litre but not at 8 µg/litre. The
21-day no-observed-effect concentration was therefore considered to be
8 µg/litre, equivalent to 2.1 µg methomyl/litre (Baer, 1991e).
Table 9. The acute toxicity of methomyl to other aquatic organisms (static test)
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Midge (Chironomus plumosus) mature 95% technical 22 0.088 (EC50) USDI (1978)
24% conc 0.032 USDI (1978)
Eastern oyster (Crassostrea embryo/ technical 20 4.0 (EC50) Ward & Boeri (1990)
virginica) larvae
Fiddler crab (Uca pugilator) 20 mm Lannate L 21 2.38 Bentley (1973)
(24%)
Grass shrimp (Palaemonetes 18 mm Lannate 90 21 0.049 Sleight (1973)
vulgaris
18 mm Lannate L 21 0.130 Bentley (1973)
(24%)
Gammarus pseudolimnaeus mature technical 17 0.92 USDI (1978)
Mud crab (Neopanope texana) 15 mm Lannate 90 21 0.41 Sleight (1973)
Mysid shrimp (Mysidopsis bahia) 10 day 99% technical 21.5 0.22 Ward & Boeri (1989)
Pink shrimp (Panaeus duorarum) 55 mm Lannate 90 21 0.019 Sleight (1973)
Stonefly (Pteronarcys dorsarta) naiad technical 7 0.034 USDI (1975)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Stonefly (Pheronarcella badia) Year Class 1 99% technical 0.069 Mayer & Ellersieck (1986)
Stonefly (Pheronarcella badia) Year Class 1 24% a.i. 0.06 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 95% technical 0.034 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 24% a.i. 0.92 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 95% a.i. 0.343 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 24% a.i. 0.029 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 99% technical 0.92 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 0.72 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 1.05 Mayer & Ellersieck (1986)
(flowthrough)
Water flea (Daphnia magna) neonate 95% technical 18 0.032 Goodman (1978)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Water flea (Daphnia magna) 1st instar 95% technical 21 0.009 (EC50) USDI (1978)
Water flea (Daphnia magna) - 24% conc 0.0076 USDI (1978)
Water flea (Daphnia magna) - Lannate 20L 20.4 0.033 Baer (1991e)
(21.5)
Water flea (Daphnia magna) 1st instar 95% technical 0.088 Mayer & Ellersieck (1986)
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The acute toxicity of a liquid (Lannate 20L) and a solid (Lannate
25WP) methomyl formulation to earthworms (Eisenia foetida andrei) was
determined over a 14-day period. The formulations were mixed with
artificial soil (70% industrial sand, 20% kaolite and 10% moss peat)
at six concentrations of 0-500 mg/kg (Lannate 20L) and 0-1000 mg/kg
(Lannate 25WP), with 40 earthworms per concentration. The 7-day
LC50 values were estimated to be 165 mg/kg and 147 mg/kg,
respectively, and the 14-day LC50 values 102 mg/kg and 87 mg/kg,
respectively (Armstrong et al., 1991; Caley et al., 1991).
Laboratory tests were undertaken to determine the broadcast
dosage of methomyl, as a 90% SP, required to kill 50% of earthworms
(night crawlers, Lumbricus terrestris L). The test substance was
mixed with moistened potting soil at six rates from 0 to 35 kg/ha with
40 worms per rate. The estimated rate causing 50% mortality was
11.4 kg methomyl/ha (Ruppel & Laughlin, 1977).
The biochemical changes in experimental snails, Eubania
vermiculata (Müller), were studied after treatment with 0.2%
methomyl in bran bait (w/w) for periods of 1, 3, 5, 7 and 10 days.
There was a significant reduction in total soluble proteins, lipids
and glycogenic content, and significant increase of glutamic
oxalocetic transaminase, glutamic pyruvic transaminase and
catalase activities (El-Wakil & Radwan, 1991).
A bran methomyl bait (0.5% w/w) was tested for its molluscicidal
activity on the white garden snail (Theba psiana) and compared with
that of four other oxime carbamate pesticides (aldicarb, aldoxycarb,
oxamyl and thiofanox). Methomyl had the most potent molluscicidal
activity. The time for 50% mortality of snails (LT50) for methomyl,
oxamyl, aldoxycarb, aldicarb and thiofanox was 2.31, 3.97, 4.69, 5.77
and 6.67 days, respectively. The activities of acetylcholine
esterase, acid phosphatase and alkaline phosphatase were inhibited by
the pesticides in line with their potency. GOT and GPT activities
were significantly increased by methomyl (Radwan et al., 1992).
Juvenile (4-week-old) laboratory reared earthworms
(Allolobophora caliginosa) were kept individually in soil treated
with methomyl for 7 days. Relative toxicity, i.e. concentration in
soil (mg/kg) causing zero growth, compared to the standard,
carbofuran, (0.10) was 0.54 (Martin, 1986).
The acute toxicity of methomyl to bees has been reported by
several investigators using different types of test. In one such test
groups of 20 bees were treated individually with a range of doses, the
methomyl being applied to the thorax of each bee in a 1 µl acetone
solution. The mortality was determined after 48 h and the LD50 was
calculated as 0.1 µg/bee (Meade, 1984). The acute toxicity of
methomyl by topical application to the honey-bee (Apis mellifera) was
compared to that of the Western Yellow jacket (Vespula pensylvanica)
after the pleural application of a 1 µl solution in acetone to each
insect. Methomyl was less toxic to the honey-bee, with a 48-h LD50
of 12 µg/g, than to the Yellow jacket (0.9 µg/g) (Johansen & Davis,
1972). A contact LD50 of 1.29 µg per bee has been reported by
Atkins et al., (1976) and an oral LD50 of 0.2 µg/bee by Clinch &
Ross (1970).
The speed of action of methomyl on honey-bees was assessed in a
special laboratory trial. A commercial formulation (90% DP) was
diluted in 33% sucrose solution to give concentrations equal to 1.5, 2
or 4 x LD90. These were fed to bees and the effects observed as two
stages; the first was characterized by very fast and erratic movement
and the second by the bees being unable to walk or fly. The time
intervals to reach each stage were recorded (20 bees), as were the
times taken for 50% and 90% bees to be affected. Methomyl was the
fastest acting of 10 pesticides tested, taking about 3-5 min to give a
50% effect level. It was suggested that this type of information was
needed when interpreting the results of field trials (Clinch & Ross,
1970).
In a tent trial, a methomyl formulation (35% methomyl) was
applied to Phacelia at a 0.3% concentration in the evening after bees
had finished flying and then rewetted early next morning prior to the
start of flight. Observations were made later that day and during the
following two days. Fewer bees visited the trial tent during this day
than visited the control tent. Large numbers of bees were found dead
in front of the hive and at the trial tent edges (about one order of
magnitude higher) on the first day. On the second day about three
times the number of dead bees were found when compared to the control.
Fewer bees were found dead when the trial was repeated with the spray
deposit left dry (Stute, 1983).
Methomyl was among 400 pesticides assessed in honey-bee
laboratory and field studies by the University of California over 20
years. The compound was placed in the highly toxic category, i.e.
severe losses should be expected if the pesticide is used when bees
are present at treatment or within a day thereafter. It was shown
that the contact LD50 of 1.29 µg/bee can be converted directly to
1.29 kg/ha, this being the application rate expected to cause a 50%
mortality among foraging bees in a treated field crop, at the time of
application or shortly thereafter. Several recommendations were
included to help minimize bee losses when spraying with insecticides,
including early morning or night applications, since there is less
risk to honey-bees at these times (Atkins et al., 1976).
9.3.2 Birds
The results for methomyl in acute oral and short-term dietary
studies in birds are shown in Tables 10 and 11. In an acute toxicity
study on the bobwhite quail, a dose of 5.62 mg/kg produced lethargy,
reduced reaction to external stimuli, loss of coordination and lower
limb weakness within 30 min of dosing. The birds recovered within 2 h.
The effects increased at higher doses and included salivation and wing
droop, but recovery in survivors was rapid, within hours to one day.
There were no mortalities at 10 mg/kg or less (Beavers, 1983).
Chickens given methomyl by capsule at 25 or 50 mg/kg showed muscle
tremor, ataxia, salivation, convulsions and death within 30 min
(Palmer & Schlinke, 1978).
In a one-generation study, 20-week-old bobwhite quail, 16 male
and 16 female per group, were fed diets containing 0, 50, 150 or
500 mg methomyl/kg for 20 weeks in their first breeding season.
Assessments included adult clinical health, weight gain and food
consumption and the reproductive parameters for numbers of eggs laid,
development of eggs, viability of embryos, percent hatchability,
offspring survival and eggshell thickness. There were no signs of
toxicity in the adults or of treatment-related mortality. There was a
slight decrease in body weight gain in males at 500 mg/kg up to week
8. No effects on reproductive parameters were seen at the two lower
dietary levels. No treatment-related effects were observed for egg
shell thickness at methomyl concentrations of 50, 150 or 500 mg/kg.
Mean thicknesses of bobwhite quail eggs were 0.221 (± 0.02), 0.216
(± 0.01) and 0.214 (± 0.01) mm, respectively. Treatment groups did
not differ significantly from the control (0.212 ± 0.02 mm). A small
but biologically significant reduction in the numbers of eggs laid per
hen and a subsequent reduction in the numbers of offspring were seen
at 500 mg/kg. A clear no-observed-effect concentration of 150 mg
methomyl/kg diet was therefore determined (Beavers et al., 1991a).
A one-generation study of similar design and the same dietary
concentrations was undertaken with the mallard duck for a period of 18
weeks. There were no signs of toxicity or treatment-related
mortalities in the adults. No effect was seen on body weight at the
two lower levels but a decrease in weight gain occurred in hens fed
500 mg/kg over the last 10 weeks of the study. Egg shell thickness
was not affected by methomyl. Mean shell thicknesses of eggs from
exposed ducks were 0.378 (±0.03), 0.379 (±0.07) and 0.370 (±0.03) mm
for 50, 150 and 500 mg/kg, respectively. No significant differences
from the control (0.378 ± 0.03 mm) were detected. A slight reduction
in the percentage of viable embryos was observed at 500 mg/kg. A
clear no-observed-effect concentration was therefore established at
150 mg/kg (Beavers et al., 1991b).
Table 10. Acute toxicity studies on birds
Species Test Acute oral LD50 Reference
(mg/kg)
Bobwhite quail (Colinus virginianus) intubation, suspended in corn oil 24.2 Beavers (1983)
Japanese quail (Coturnix japonica) intubation, suspended in 34 Smith (1982)
carboxymethylcellulose
Chicken (White Leghorn-Cornish) capsule minimum toxic dose Palmer & Schlinke (1978)
25
Mallard duck (Anas platyrhynchos) 15.9 Tucker & Crabtree (1970)
Pheasant (Phasianus colchicus) 15.4 Tucker & Crabtree (1970)
Common pigeon (Columba livia) in polyethylene glycol 10.0 Schafer (1975)a
Common grackle (Quiscalus quiscala) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) in polyethylene glycol 31.6 Schafer (1975)a
House sparrow (Passer domesticus) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) gavage in propylene glycol 42 Schafer (1972)
Redwing (Agelaius phoeniceus) gavage in propylene glycol 10 Schafer (1972)
a Schafer EW Jr (1975) Avian toxicity tests - letter to Dr HJ Thome, April 28 1975 (unpublished report submitted to WHO by Du Pont)
Table 11. Dietary toxicity studies on birds
Species 8-day Test Dietary LC50 Reference
(5 days feeding, 3 days (mg/kg)
observation)
Bobwhite quail (Colinus virginianus) 4 concentrations 1100 Heath et al. (1972)
Japanese quail (Coturnix japonica) 6 concentrations 3124 Heath et al. (1972)
Mallard duck (Anas platyrhynchas) 6 concentrations 2883 Heath et al. (1972)
Bobwhite quail 7 concentrations 3680 Busey (1967)
Pekin duck 7 concentrations 1890 Busey (1967)
Methomyl did not give any indication of a teratogenic effect in a
chick embryo test when it was injected into the yolk (Proctor et al.,
1976).
9.4 Field studies
Bobwhite quails, 6 males and 6 females, were exposed to sprays of
Lannate formulations, equivalent to a field application of 1 kg
methomyl/ha, once every 48 h for a total of four applications. The
birds were killed 14 days after the last application. There were no
observable effects due to exposure and no treatment-related changes in
tissues on gross examination (Aftosmis, 1973a,b).
A simulated field trial was carried out on two groups of bobwhite
quail (three males and three females per group). One group was fed
prior to treatment, the other group was fed 12 h after treatment. The
treatment consisted of spraying methomyl over the test area at the
rate of 1.1 kg/ha. In all, six sprayings were made, each 5 days
apart, with a 15-day observation period after the last application.
Some weight loss occurred in the group fed prior to treatment;
otherwise there were no observable effects on surviving birds
compared to controls and no changes due to treatment upon gross
examination of tissues (Hinkle & Cameron, 1980).
In 1978, 400 ha of Maine (USA) forest land was treated with the
formulation Lannate LV at the rate of 0.28 kg methomyl/ha to control
spruce budworm. Monitoring of more than 30 species of songbirds was
undertaken over 6-day census periods pre-spray, immediately post-spray
and 2 weeks post-spray. The census was carried out firstly by
individual bird counts and then by territorial counts. Searches were
made for dead birds. Spray deposit cards were used to ensure that the
insecticide was present in the census plots. A concurrent sampling
and analytical programme determined the residue levels of methomyl in
trees and other plants, leaf litter, soil and water. The maximum
level detected was 15 mg/kg in foliage from the top third of the
trees. Underbrush had initial residues of 6 mg/kg, low levels were
found in leaf litter, negligible amounts in soil and none in water.
These levels dissipated rapidly apart from leaf litter where low
levels lasted 34-61 days. The study revealed no evidence of changes
in the activity levels among songbirds. No dead or intoxicated birds
were found and there was no disruption of nesting birds (Brown, 1978).
A study of brain cholinesterase activity was undertaken on wild
mice (Mus musculus) trapped over a period of 3 days after a soya
bean field had been sprayed with Lannate 1.8 L at 0.5 kg methomyl/ha.
Some inhibition of brain cholinesterase activity occurred at a level
of about 10% over the study period indicating the possibility of a
small effect (Montz et al., 1983).
A census of birds was undertaken before and after spraying a
field of hops in Kent, United Kingdom, with 0.56 kg methomyl per ha.
Records were made of birds seen, feeding in the hop field, singing and
nesting. Monitoring was carried out 1, 3 and 10 days after spraying.
Principal species observed feeding upon the sprayed area included
blackbird, song thrush, various tits and finches, robin, wren and
hedge sparrow. There was no apparent difference in feeding habits
before or after spraying and no unhealthy or dead birds were found
over the total observation period (Orpin, 1971).
Methomyl (90% SP), applied at 3.4 kg/ha pasture, produced
decreases in population and biomass of three species of earthworms of
28.5% and 14%, respectively. The compound was considered to be the
least active of the seven pesticides tested (Tomlin & Gore, 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The proposed toxicological criteria for setting guidance values
are presented in Table 12.
10.2 Evaluation of effects on the environment
Adsorption of methomyl to soil is low to moderate with hardly any
desorption. Aerobic degradation in soil (with a half-life of around
one week) is about twice as fast as anaerobic degradation. A relative
increase in soil organic matter delays degradation.
Table 12. Proposed toxicological criteria for setting guidance values
Exposure scenario Relevant route/effect Result/remarks
Short-term oral, acute, several species including human highly toxic; rat LD50 = 17 mg/kg body weight;
(1-7 days) LOELa= 5 mg/kg body weight
eye, irritation, rabbit mild irritant
inhalation, acute, rat highly toxic; LC50 = 0.26 mg/litre (4-h aerosol);
NOELb = 0.14 mg/litre
dermal, acute, rat and rabbit low toxicity; intact skin - LD50 > 2000 mg/kg body weight;
NOELc = 2000 mg/kg body weight in the rabbit
Medium-term repeat dermal, rabbit 21-day study; no toxicologically significant effects at
(1-26 weeks) 50 mg/kg body weight per day
repeat oral, rat 13-week study; NOEL = 3.6 mg/kg body weight per day
maternal oral, rabbit teratology study; NOEL = 6 mg/kg body weight per day
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body weight per day
a mild cholinergic signs of toxicity in guinea-pig
b no-observed-effect level for clinical signs and death
c no clinical symptoms noted except signs of skin irritation
Despite the above adsorption characteristics, leaching of
methomyl in soil to levels deeper than 20 to 30 cm has not been
observed. The concentrations of methomyl in both surface and well
water are below the limit of detection. Methomyl is degraded rapidly
with a half-life of about one week in water and sediment. In sterile
water, methomyl is stable for at least 30 days at normal environmental
pH.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. In contrast,
when applied to soil, uptake through the roots occurs readily with
rapid translocation to the leaves. Adsorbed foliar residues degrade
with a half-life in the order of 4 days. Absorbed methomyl
concentrations in food crops decline rapidly to about 5% within one
week; this may be due to growth dilution.
Bioaccumulation by rainbow trout did not occur in a flow-through
study. Depuration occurred within one day of transfer to clean water.
Trout were discoloured when exposed to levels between 0.075 and
0.75 mg/litre, an effect that disappeared within 5 days, the time
depending on original exposure concentration.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil; nitrification can be
delayed at applications 10 times higher. An aquatic green alga showed
a NOEC for growth of 6.25 mg/litre.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl, the LC50s being of the order of 10 to
100 µg/litre. MATC values from two 21-day Daphnia studies were
estimated to be around 2 µg/litre. Kills of aquatic invertebrates are
expected following overspray.
Fish, both freshwater and estuarine, are less sensitive, the
LC50s ranging from 0.5 to 7 mg/litre. Two longer-term studies gave
an NOEC of 0.5 mg/litre for lethality of fingerling rainbow trout and
an MATC of > 0.06 and > 0.12 mg litre for survival of embryo larval
stages of fathead minnow. Given the low persistence of methomyl and
its relatively low acute toxicity to fish, the risk is expected to be
low.
Laboratory tests in artificial soil with methomyl formulations
(25% WP) established a 14-day LC50 of 90-100 mg formulation/kg for
earthworms. It was estimated that 11 kg a.i./ha of methomyl would
kill 50% of earthworms. A field application of 3 kg a.i./ha caused
reduced earthworm population (28%) and biomass (14%). The TER for
earthworms is around 40 indicating low risk.
Methomyl is classified as highly toxic to honey-bees, the topical
LD50 being approximately 0.1 µg/bee. Field (tent) trials showed
dead bees both at the treatment tent and hive when residues from
spraying the previous day were wetted. Less effect was seen with dry
residues. Methomyl has not been implicated in bee incidents in the
field; this probably reflects advice to restrict spraying times to
protect bees.
Acute oral LD50s for various bird species range between 10 and
40 mg/kg body weight. Dietary LC50s (5 days) range from 1100 to
3700 mg/kg diet. Methomyl poses an acute oral risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds. An example of a toxicity exposure ratio
for birds and fish is shown in Table 13.
The NOEC for reproduction was established at 150 mg/kg diet for
both the bobwhite quail and mallard duck. Field studies following
spraying of methomyl formulations in forests showed no mortality of
songbirds and no changes in feeding behaviour or general activity.
Reduced fat deposits of songbirds reflect reduced insect prey. It was
not considered that methomyl poses a threat to birds after recommended
applications.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
Table 13. Toxicity exposure ratios for birds, fish and aquatic invertebrates based on application rates of 2.5 kg a.i./ha
methomyl to soybeans (worst case)
Risk category LC50 (mg/litre or mg/kg diet) Estimated exposure Toxicity/exposure ratio (TER)c
(mg/litre or mg/kg diet)a,b
Acute bird 10 50-364 02-0.027
Acute fish (stream) 0.5 0.2 2.5
Acute fish (pond) 0.5 0.04 12.3
Acute aquatic invertebrate 0.009 0.2 0.045
(stream)
Acute aquatic invertebrate 0.009 0.04 0.225
(pond)
a Estimated environmental concentration in the terrestrial environment (for bird exposure) is based on the stated application rate
and the assumption of deposition on short grass using the US EPA monogram
b Aquatic exposure concentrations were taken from the STREAM model based on a single application and estimated run-off into water;
no direct overspray is included
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate likely exposure to toxic concentrations
by organisms in the different risk categories
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
Considering the toxicological characteristics of methomyl, both
qualitatively and quantitatively, it is concluded, on the basis of the
no-observed-effect level of 3 mg/kg body weight per day in the 2-year
toxicity study on dogs and applying a 100-fold uncertainty factor,
that 0.03 mg/kg body weight per day will probably not cause adverse
effects in humans by any route of exposure.
12. FURTHER RESEARCH
No further studies were thought to be necessary.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has discussed and
evaluated methomyl on several occasions since 1975. Residue aspects
were discussed in 1975, 1976, 1977, 1978, 1986, 1987, 1988, 1989, 1990
and 1991 (FAO/WHO, 1976, 1977, 1978, 1979, 1986, 1987, 1988a,b,
1990a,b,c, 1991). Toxicological evaluations took place in 1978, 1986
and 1989, when an Acceptable Daily Intake (ADI) of 0-0.03 mg/kg body
weight was established (FAO/WHO, 1979, 1987, 1990a,b).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for methomyl in various commodities
(FAO/WHO, 1993).
It should be noted that the 1992 CCPR meeting decided to combine
MRLs for thiodicarb and methomyl into a single list. In the cases of
different MRLs the higher limit would prevail.
Methomyl is listed in Class IB ("Highly Hazardous") in the WHO
Recommended Classification of Pesticides by Hazard and
Guidelines to Classification (1994-1995) on the basis of its rat acute
oral LD50 value of 17 mg/kg body weight (IPCS, 1994).
The time-weighted average (TWA) adopted by the American
Conference of Governmental Industrial Chemists (ACGIH) is 2.5 mg/m3
(ACGIH, 1994-1995).
REFERENCES
* Report submitted to WHO by E.I. Du Pont de Nemours and Co.,
Wilmington, Delaware, USA
Aftosmis JG (1973a) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamine acid, S-methyl ester (25% a.i.) (Lannate L
methomyl insecticide). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-354-73*).
Aftosmis JG (1973b) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamic acid, S-methyl ester (30% a.i.) (non-flammable
Lannate L methomyl insecticide), Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-606-73*).
Ambridge EM (1992) Report on field trials to assess operator
contamination during application of two different pesticides to crops
of varying heights in Thailand, November/December 1991 (NRI Contract
No. 00195) (Unpublished report*).
ACGIH (1994-1995) Threshold limit values for chemical substances in
the work environment. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, p 28.
Andrews TL & Miskus RP (1968) Tetraethylammonium chloride as an
antidote for certain insecticides in mice. Science, 159: 1367-1368.
Antal M, Bedö M, Constantinovits G, Nagy K, & Szépvölgyi J (1979)
Studies on the interaction of methomyl and ethanol in rats. Food
Cosmet Toxicol, 17: 333-338.
Araki M, Yonemitsu K, Kambe T, Idaka D, Tsunenari S, Kanda M, &
Kambara T (1982), [Forensic toxicological investigations in fatal
cases of carbamate pesticide methomyl (LannateR) poisoning.] Nippon
Hoigaku Zasshi, 36: 584-588 (in Japanese).
Armondi S (1991a) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-394 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-345-91*).
Armondi S (1991b) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-425 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report HLO 346-91*).
Armondi S (1992) Closed-patch repeated insult dermal sensitization
study (maximization method) with DPX-X1179-424 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-659-91*).
Armstrong K, Caley CY, Hall BE, & Knight B (1991) Lannate 20L:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inveresk Research International Ltd (Unpublished report
No. 8546*).
Atkins EL, Anderson LD, Kellum DD, & Neuman KW (1976) Protecting honey
bees from pesticides. Berkeley, California, University of California,
Division of Agricultural Sciences, 15 pp (Leaflet 2883).
Baer KN (1991a) Static, acute, 96-hour LC50 of DPX-X1179-423 to
bluegill sunfish (Lepomis macrochirus). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-30-91*).
Baer KN (1991b) Static, acute, 96-hour LC50 of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-29-91*).
Baer KN (1991c) Flow-through, 21-day toxicity of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-34-91*).
Baer KN (1991d) Static, acute, 48-hour EC50 of DPX-X1179-423
(LannateR 20L) to Daphnia magna. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-150-91*).
Baer KN (1991e) Chronic toxicity of DPX-X1179-423 (LannateR 20L) to
Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-292-91*).
Barnes JR (1978) Cholinesterase tests with methomyl. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-280-78*).
Battelle (1991) Method for determination of oxamyl and methomyl in
groundwater. Columbus, Ohio, Battelle Institute (Unpublished report
No. AMR-1392-89*).
Beavers JB (1983) An acute oral toxicity study in the Bobwhite with
H-15,000. Easton, Maryland, Wildlife International Ltd (Unpublished
report No. HLO-464-83*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991a) A one-generation
reproduction study with the Northern Bobwhite (Colinus virginianus).
Easton, Maryland, Wildlife International Ltd (Unpublished report
No. HLO-337-91*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991b) A one-generation
reproduction study with the mallard (Anas platyrhynchos). Easton,
Maryland, Wildlife International Ltd (Unpublished report
No. HLO-336-91*).
Bedö M & Cieleszky V (1990) Nutritional toxicology in the evaluation
of pesticides. Bibl Nutr Dieta, 29: 20-31.
Belasco IJ (1972a) Effect of methomyl on the activity of sewage
microorganisms (Unpublished report No. ML/ME15*).
Belasco IJ (1972b) Methomyl: Incubation with rumen microorganisms
(Unpublished report No. ML/ME26*).
Bentley RE (1973) Acute toxicity of H-8385 to grass shrimp
(Palaemonetes vulgaris) and fiddler crab (Uca pugilator). Wareham,
Massachusetts, Bionomics Inc. (Unpublished report No. HLO-504-73*).
Blevins RD, Lee M, & Regan JD (1977a) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56: 1-6.
Blevins RD, Lijinsky W, & Regan JD (1977b) Nitrosated methylcarbamate
insecticides: Effect on the DNA of human cells. Mutat Res, 44: 1-7.
Boeri RL & Ward TJ (1989) Static acute toxicity of methomyl to the
sheepshead minnow, Cyprinodon variegatus. Hampton, New Hampshire,
Resource Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-700-89*).
Boleij JSM, Kromhout H, Fleuren M, Tieleman W, & Verstappen G (1991)
Re-entry after methomyl application in greenhouses. Appl Occup Environ
Hyg, 6(8): 672-676.
Bonatti S, Bolognesi C, Degan P, & Abbondandolo A (1994) Genotoxic
effects of the carbamate insecticide methomyl. 1. In vitro studies
with pure compound and the technical formulation Lannate 25. Environ
Mol Mutagen, 23: 306-311.
Borady AMA, Mikhail TH, Awadallah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23: 33-44.
Boulton JJK, Boyce CBC, Jewess PJ, & Jones RF (1971) Comparative
properties of N-acetyl derivatives of oxime N-methylcarbamates & aryl
N-methyl carbamates as insecticides and acetylcholinesterase
inhibitors. Pestic Sci, 2: 10-15.
Bracy OL, Doyle RS, Kennedy M, McNally SM, Weed JD, & Thorne BM (1979)
Effects of methomyl and ethanol on behaviour in the Sprague-Dawley
rat. Pharmacol Biochem Behav, 10: 21-25.
Braun HE, Ritcey GM, Frank R, McEwen FL, & Ripley BD (1980)
Dissipation rates of insecticides in six minor vegetable crops grown
on organic soils in Ontario, Canada. Pestic Sci, 11: 605-616.
Braun HE, Ritcey GM, Ripley BD, McEwen FL, & Frank R (1982) Studies of
the disappearance of nine pesticides on celery and lettuce grown on
muck soils in Ontario 1977-1980. Pestic Sci, 13: 119-128.
Brittelli MR (1982) Chronic toxicity of methomyl to Daphnia magna.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-46-82*).
Brock WJ (1989) Repeated dose dermal toxicity: 21-day study with
DPX-X1179-394 (methomyl) in rabbits. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-387-89*).
Brodsky J (1991) Determination of residues of methomyl in grapes, wine
& wine processing fractions by GC-MS following treatment with
"Lannate", season 1990, France. Frankfurt, Germany, Battelle-Europe
(Unpublished report No. BE-A-11-91-10-BF*).
Bromilow RH, Briggs G, Williams MR, Smelt JH, Tuinstra LGMT, & Traag
WA (1986) The role of ferrous ions in the rapid degradation of oxamyl,
methomyl and aldicarb in anaerobic soils. Pestic Sci, 17: 535-547.
Brown HL (1978) The effects of Lannate LV on singing male songbirds in
Maine in 1978 (Unpublished report No. ML/FW-12 report*).
Brown SK, Ames RG, & Mengle DC (1989) Occupational illnesses from
cholinesterase-inhibiting pesticides among agricultural applicators in
California, 1982-1985. Arch Environ Health, 44: 34-39.
Bruynzeel DP (1991) Contact sensitivity to Lannate(R). Contact
Dermatitis, 25: 60-61.
Bull DL (1974) Fate of methomyl on cotton. Environ Entomol,
3: 723-724.
Busey WM (1966) Three-month dietary administration - Rats: Insecticide
1179. Supplement to final report. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-848*).
Busey WM (1967) Acute aqueous exposure - Goldfish, Bluegill, and
Rainbow trout. Acute dietary administration - Peking ducks & Bobwhite
quail: Insecticide 1179. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-888-1*).
Cahill WP, Estesen B, & Ware GW (1975) Foliage residues of
insecticides on cotton. Bull Environ Contam Toxicol, 13: 334-337.
Caley CY, Cameron BD, Hall BE, & Knight B (1991) Lannate 25 WP:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inverest Research International Ltd (Unpublished report
No. 8455*).
Carakostas MC (1987) Inhibition and regeneration kinetics for human
and rat acetyl-cholinesterase exposed to methomyl in vitro. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-379-88*).
Carbonnel E, Puig M, Xamena N, Creus A, & Marco R. (1990) Sister
chromatid exchange in lymphocytes of agricultural workers exposed to
pesticides. Mutagenesis, 5(4): 403-405.
Carter FL & Graves JB (1973) Measuring effects of insecticides on
aquatic animals. La Agric, 16: 14-15.
Christian MS, Hoberman AM, & Fuessner EL (1983) Embryo-fetal toxicity
and teratogenicity study of methomyl in the rabbit. Horsham,
Pennsylvania, Argus Research Laboratories Inc. (Unpublished report
No. HLO-331-83*).
Clark S & Kennedy SM (1990) Analytical method for the quantification
of methomyl in grapes. Sacramento, California, Morse Laboratories
(Unpublished report No. AMR-1806-90*).
Clement C (1987a) Test to evaluate the acute cutaneous primary
irritation and corrosivity in the rabbit. L'Arbresle, France, Hazleton
France, 16 pp (Unpublished report No. 702408*).
Clement C (1987b) Test to evaluate acute ocular irritation and
corrosivity in the rabbit. L'Arbresle, France, Hazleton France, 26 pp
(Unpublished report No. 703313*).
Clinch PG & Ross IGM (1970) Laboratory assessment of the speed of
action on honey bees of orally dosed insecticides. N Z J Agric Res,
13: 717-725.
Council of the European Communities (1991) Council directive of 15
July 1991 concerning the placing of plant protection products on the
market (91/414/EEC). Off J Eur Communities, L230: Part II.
Cox L, Hermosin MC, & Comejo J (1993) Adsorption of methomyl by soils
of southern Spain and soil components. Chemosphere, 27: 837-849.
Danneberg G (1991) Investigation of the effects of Lannate 20L on the
activity of the microflora of soil. Frankfurt, Germany, Battelle
Institute (Unpublished report No. BE-S-11-91-01-DEH-01*).
Dashiell OL (1972) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-518-72*).
Debuyst B & Van Larebeke N (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat Res, 113: 242-243.
Dong MH, Krieger RI, & Ross JH (1992) Calculated re-entry interval for
table rape harvesters working in California vineyards treated with
methomyl. Bull Environ Contam Toxicol, 49: 708-714.
Douglas MT & Halls RWS (1991) The algistatic activity of Lannate 20L.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPC-16(f) 91399*).
Douglas MT & Handley JW (1988) The algistatic activity of methomyl
Tech. (DPX-X1179-00620-06). Huntingdon, United Kingdom, Huntingdon
Research Centre (Unpublished report No. DPT-171(j) 871676*).
Driscoll RR & Muska CF (1982) Early life stage toxicity of methomyl to
fathead minnow. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-528-82*).
Driskell WJ, Groce DF, Hill RH Jr, & Birky MM (1991) Methomyl in the
blood of a pilot who crashed during aerial spraying. J Anal Toxicol,
15: 339-340.
Du Pont (1967) Methomyl - Livestock feeding studies: Milk and meat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Industrial and
Biochemicals Department (Unpublished report No. ML/ME27*).
Du Pont (1973) Petition for residue tolerance - methomyl: Broccoli,
brussel sprouts, cauliflower, spinach, celery. Pesticide Petition 4F
1448, Section D, 1973. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Biochemicals Department (Unpublished report*).
Du Pont (1978) Ecosystem residue study: Spruce/fir forest and cedar
swamp, Princeton, Maine 1978. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. ML/PC-25*).
Du Pont (1982) LannateR insecticide formulations and technical
methomyl - Determination of methomyl (INX-1179) - Reversed-phase
liquid chromatography (RPLC) assay method (Method No. L30.4662/(E).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report [Second issue]*).
Eble JE & Tomic DM (1991) Foliar half-life of methomyl in cotton
leaves. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. AMR-1871-90*).
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards DF (1980) 10 Day subacute skin absorption test on rabbits.
Mewark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-166-80*).
El-Refai A, Fahmy FA, Mahmound FA, Abdel-Lateef, & Imam AKE (1976)
Toxicity of three insecticides to two species of fish. Int Pest
Control, Nov/Dec: 4-8.
El-Wakil HB & Radwan MA (1991) Biochemical studies on the terrestrial
snail Eubania vermiculata (Müller) treated with some pesticides. J
Environ Sci Health, B26(5/6): 479-489.
FAO (1985) Guidelines for the disposal of waste pesticide containers
on the farm. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1976) Pesticide residues in food. Report of the 1975 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. Geneva, World Health
Organization (WHO Technical Report Series No. 592; FAO Plant
Production and Protection Series No. 1).
FAO/WHO (1977) 1976 Evaluations of some pesticide residues in food:
The monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Series
No. 8; AGP:1976/M/14).
FAO/WHO (1978) Pesticide residues in food. 1977 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10 Sup).
FAO/WHO (1979) Pesticide residues in food. 1978 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 15 Sup).
FAO/WHO (1986) Pesticide residues in food - 1986. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78).
FAO/WHO (1987) Pesticide residues in food - 1986. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78/2).
FAO/WHO (1988a) Pesticide residues in food - 1987. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 86/1).
FAO/WHO (1988b) Pesticide residues in food - 1988. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 93/1).
FAO/WHO (1990a) Pesticide residues in food - 1989. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100).
FAO/WHO (1990b) Pesticide residues in food - 1989. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100/2).
FAO/WHO (1990c) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 103/1).
FAO/WHO (1991) Pesticide residues in food - 1991. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper No. 111).
FAO/WHO (1993) Codex Alimentarius - Volume two: Pesticide residues in
food. Rome, Food and Agriculture Organization.
Farrow MG, Cortina T, & Padilla-Nash H (1984) In vivo bone marrow
chromosome study in rats, H15,000. Final Report. Vienna, Virginia,
Hazleton Biotechnologies Corporation (Unpublished report
No. HLO-63-84*).
Fayez V & Baig MRE (1991) Short-term toxicity of methomyl in rats.
Chemosphere, 23(3): 375-382.
Foerst DL & Moye HA (1985) Aldicarb and related compounds in drinking
water via direct aqueous injection HPLC with post column
derivatisation. Cincinnati, Ohio, US Environmental Protection Agency
(EPA/600/D-85/051).
Fossi MC, Leonzio C, Massi A, Lari L, & Casini S (1992) Serum esterase
inhibition in birds: A nondestructive biomarker to assess
organophosphorus and carbamate contamination. Arch Environ Contam
Toxicol, 23: 99-104.
Foster GV (1966a) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl) progress report. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-73-66*).
Foster GV (1966b) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-214-66*).
Friedman PL (1983) Hydrolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. AMR-109-83*).
Freeman PK & Ndip EMN (1984) Photochemistry of oxime carbamates 2.
Phototransformations of methomyl. J Agric Food Chem, 32(4): 877-881.
Fung KH & Uren NC (1977) Microbial transformation of S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate (methomyl) in soils. J Agric
Food Chem, 25(4): 966-969.
Fung KH, Luke RKJ, & Uren NC (1978) Concentrations of methomyl in
Australian tobacco plants following transplant, foliar and soil
treatments. Tob Sci, 22: 24-26.
Gianessi LP & Puffer CA (1992) Insecticide used in US crop production.
Resources for the Future. Washington, DC.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Agrochemical Manufacturers.
Goodman NC (1978) 48-Hour LC50 to Daphnia magnia. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-165-78*).
Gordon M & Richter ED (1991) Hazards associated with aerial spraying
of organophosphate insecticides in Israel. Rev Environ Health,
9(4): 229-238.
Gupta RC, Goad JT, & Kadel WL (1992) Characteristic changes in LDH and
its isoenzymes as biomarkers under the influence of acute methomyl
toxicity. Fed Am Soc Exp Biol J, 6(4): A1307.
Han JCY (1972) Evaluation of possible effects of methomyl on
nitrifying bacteria in soil (Unpublished report ML/ME16*).
Han JCY (1975) Absence of nitroso formation from [14C] methomyl and
sodium nitrite under simulated stomach conditions. J Agric Food Chem,
23: 892-896.
Harvey J Jr (1967) Exposure of S-methyl N-[(methyl carbomoyl)oxy]
thioacetimidate in sunlight, water and soil (Unpublished report
No. ML/ME-10*).
Harvey J Jr (1972a) Decomposition of 14C-methomyl in aerated river
water exposed to sunlight (Unpublished report No. ML/ME-13*).
Harvey J Jr (1972b) Decomposition of 14C-methomyl in a high organic
matter soil (Unpublished report No. ML/ME-18*).
Harvey J Jr (1977a) Decomposition of 14C-methomyl in a sandy loam
soil in the greenhouse (Unpublished report No. ML/ME-19*).
Harvey J Jr (1977b) Degradation of 14C-methomyl in Flanagan silt
loam in biometer flasks (Unpublished report No. ML/ME-20*).
Harvey J Jr (1978) Crop rotation study with 14C-methomyl in the
greenhouse. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Biochemicals Department (Unpublished report No. ML/ME-22*).
Harvey J Jr (1980) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report No. AMR-22-80*).
Harvey J Jr (1983) Photolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Chemicals
Department (Unpublished report No. AMR-121-83*).
Harvey J Jr & Pease HL (1973) Decomposition of methomyl in soil. J
Agric Food Chem, 21(5): 784-786.
Harvey J Jr & Reiser RW (1973) Metabolism of methomyl in tobacco, corn
and cabbage. J Agric Food Chem, 21: 775-783.
Harvey J Jr & Yates RA (1967) Metabolism of methomyl in the corn
plant. I. Plant growth chamber - Carbon 14 studies (Unpublished report
No. ML/ME2*).
Harvey J Jr, Jelinek AG, & Sherman H (1973) Metabolism of methomyl in
the rat. J Agric Food Chem, 21(5): 769-775.
Hashimoto Y & Fukami J (1969) Toxicity of orally and topically applied
pesticide ingredients to Carp Cyprinus carpio Linné. Botuy Kagaku,
34: 63-66.
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1991) The metabolism of
[1-14C] methomyl in rats. Huntingdon, United Kingdom, Huntingdon
Research Centre and Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products (Unpublished report No. AMR-1584-90*).
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1992) The metabolism of
[1-14C] methomyl in male cynomolgus monkeys. Huntingdon, United
Kingdom, Huntingdon Research Centre (Unpublished report
No. AMR-1902-90*).
Heath RG, Spann JW, Hill EF, & Kreitzer JF (1972) Comparative dietary
toxicities of pesticides to birds. Washington, DC, US Department of
the Interior, Fish and Wildlife Service (Special Scientific
Report-Wildlife No. 152).
Hemavathy KC & Krishnamurthy NB (1987a) Mutagenicity studies in
Drosophila melanogaster with Lannate 20. Mutat Res, 191: 41-43.
Hemavathy KC & Krishnamurthy NB (1987b) Evaluation of Lannate 20, a
carbamate pesticide in the germ cells of male rice. Environ Res,
42: 362-365.
Henry NW III (1975) LannateR L methomyl insecticide potentiation
studies. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-278-75*).
Henry JE (1981) Acute dermal methomyl-cholinesterase response study in
male rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-303-81*).
Hill KM, Hollowell RH, & Dal Cortivo LA (1984) Determination of
N-methylcarbamate pesticides in well water by liquid chromatography
with postcolumn fluorescence derivatization. Anal Chem, 56: 2465-2468.
Hinkle S & Cameron JT (1980) Simulated field trial in Bobwhite quail,
H-13 099. Final report. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished report No. HLO-97-80*).
Holt RF (1971) LannateR residue cooking studies. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Hornberger CS (1967) Acute inhalation toxicity of aqueous spray mist.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-171-67*).
Hosling GC (1969) Dietary administration - Cotornix Quail: INX-1179
(Unpublished report No. HLO-238-69*).
Huhtanen K & Dorough HW (1976) Isomerization and Beckmann
rearrangement reactions in the metabolism of methomyl in rats. Pestic
Biochem Physiol, 6: 571-583.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - A
general introduction. Geneva, World Health Organization.
IPCS (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization (Unpublished document WHO/PCS/94.2).
Ivie KF (1980) High performance liquid chromatography (HPLC) in
pesticide residue analysis. In: Zweig G & Sherman J ed. Updated
general techniques and additional pesticides. New York, London,
Academic Press, pp 55-58.
Jackson GC, Hardy CJ, Gregson RL, Offer JM, & Gopinath C (1991)
Lannate 20L: Acute inhalation toxicity in rats 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPT-247/91521*).
Johansen CA & Davis HG (1972) Toxicity of nine insecticides to the
Western yellowjacket. J Econ Entomol, 65: 40-42.
Kaplan AM (1981) Long-term feeding study in rats with S-methyl
N-[(methylcaramoyl)oxy] thioacetimidate (methomyl, INX-1179). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-235-81*).
Kaplan AM & Sherman H (1977) Toxicity studies with methyl
N-[[(methylamino) carbonyl]oxy]-ethanimidothioate. Toxicol Appl
Pharmacol, 40: 1-17.
Kelly DP (1992) Acute inhalation toxicity study with DPX-X1179-440 in
rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-326-92*).
Kennedy SM (1989) Field soil dissipation of Lannate(R) L insecticide.
Sacramento, California, Morse Laboratories Inc. (Unpublished report
No. AMR-1215-88*).
Kennedy CM (1991) Field soil dissipation of Lannate(R) L insecticide
- A 1991 study. Sacramento, California, Morse Laboratories Inc. and
Lincoln, Nebraska, Harris Environmental Technologies Inc. (Unpublished
report No. AMR-1921-91*).
Kennedy CM & Hay RJ (1991a) Magnitude of residues of methomyl
insecticide in citrus and its processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1361-89*).
Kennedy CM & Hay RJ (1991b) Magnitude of residues of methomyl
insecticide in cottonseed and its processed fractions. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1355-89*).
Kiigemagi U & Deinzer ML (1979) Dislodgeable and total residues of
methomyl on mint foliage. Bull Environ Contam Toxicol, 22: 517-521.
Kiigemagi U, Wellman D, Cooley EJ, & Terriere LC (1973) Residues of
the insecticides phorate and methomyl in mint hay and oil. Pestic Sci,
4: 89-99.
Knaak JE, Jackson T, Fredrickson AS, Rivera L, Maddy KT, & Akesson NB
(1980) Safety effectiveness of closed transfer, mixing, loading and
application equipment in preventing exposure to pesticides. Arch
Environ Contam Toxicol, 9: 231.
Krauss WC & Stula EF (1967) Oral LD50 and delayed paralysis tests
(Hens). Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-161-67*).
Labare A (1990) Testing of DPX-X1179 through FDA multi-residue
protocols A-E. Beltsville, Maryland, Biospherics Inc. (Unpublished
report No. AMR-1405-89*).
Leidy RB, Domanski JJ, Haire PL, & Sheets TJ (1977) Effects of
weathering and flue-curing on methomyl residues on tobacco. Arch
Environ Contam Toxicol, 5: 199-206.
Leistra M, Dekker A, & Van Der Burg AMM (1984) Computed and measured
leaching of the insecticide methomyl from greenhouse soils into water
courses. Water Air Soil Pollut, 23: 155-167.
Leitch RE & Pease HL (1973) LannateR-methomyl. In: Sherma J & Zweig
G ed. Analytical methods for pesticides and plant growth regulators,
7th ed. New York, London, Academic Press, pp 331-338.
Lheritier M (1991a) Test to evaluate the acute toxicity following a
single oral administration (LD50) in the rat. L'Arbresle, France,
Hazleton France (Unpublished report No. 104367*).
Lheritier M (1991b) Test to evaluate the acute toxicity following a
single cutaneous application (limit test) in the rat. L'Arbresle,
France, Hazleton France (Unpublished report No. 106393*).
Liddle JA, Kimbrough RD, Needham LL, Cline RE, Smrek AL, Yert LW, &
Bayse DD (1979) A fatal episode of accidental methomyl poisoning. Clin
Toxicol, 15(2): 159-167.
Lijinsky W & Schmahl D (1978) Carcinogenicity of N-nitroso derivatives
of N-methylcarbamate insecticides in rats. Ecotoxicol Environ Saf,
2: 413-419.
Lu CC (1983) NudrinR: Two-generation reproduction study in rats
(Protocol No. RA-274). Houston, Texas, Shell Development Co.,
Westhollow Research Center (Unpublished report*).
McAlack JW (1973) Ten-day subacute exposure of rabbit skin to LannateR
L insecticide. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report HLR 24-73*).
McCain JC (1971), Acute fish toxicity study - static freshwater:
Lannate, MR-581, H-6854. Falls Church, Virginia, Hazleton Laboratories
Inc. (Unpublished report No. HLO-71-71*).
McConnel R, Pacheco Anton AF, & Magnotti R (1990) Crop duster aviation
mechanics: High risk for pesticide poisoning. Am J Public Health,
80(10): 1236-1239.
McCooey KT, Chromey NC, Sarrif AM & Hemingway RE (1984), CHO/HGPRT
assay for gene mutation. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-556-83*).
Macieira OJD & Hebling Beraldo MJA (1989) Laboratory toxicity of
insecticides to workers to Trigona spinipes. J Apic Res, 28(1): 3-6.
Martin NA (1986) Toxicity of pesticides to Allolobophora caliginosa
(Oligochaeta: Lumbricidae). N Z J Agric Res, 29: 699-706.
Martinez-Chuecos J, Molinero-Somolinos F, Solé-Violan J, & Rubio-Sanz
R (1990) Management of methomyl poisoning. Hum Exp Toxicol,
9: 251-254.
Marxmiller RL & Hay RJ (1991) Magnitude of residues of methomyl
insecticide in tomatoes and their processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1360-69*).
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res,
24(1): 11-21.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Meade AB (1984) Methomyl toxicity to honey bee. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. METH/ECO 9*).
Mercier O (1991) Test to evaluate the sensitising potential by topical
applications in the Guinea-pig. "The Buehler Test". L'Arbresle,
France, Hazleton France (Unpublished report No. 106348*).
Merricks DL (1990) Lannate insecticide - Field worker exposure study
in grape girdling and harvesting operations. Frederick, Maryland,
Agrisearch Incorporated, Sacramento, California, Morse Laboratories
Inc. and Fresno, California, Siemer and Associates Inc. (Unpublished
report No. AMR-1442-89*).
Miles CJ & Oshiro WC (1990) Degradation of methomyl in chlorinated
water. Environ Toxicol Chem, 9: 535-540.
Miyazaki T, Yashiki M, Kojima T, Chikasue F, Ochiai A, & Hidani Y
(1989) Fatal and non-fatal methomyl intoxication in an attempted
double suicide. Forensic Sci Int, 42: 263-270.
Monson KD (1989) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-22-80*).
Monson KD & Ryan DL (1991) [14C] Methomyl cow metabolism study.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-1675-90*).
Montz WE, Scanlon PF, & Kirkpatrick RL (1983) Effects of field
application of the anti-cholinesterase insecticide methomyl on brain
acetylcholinesterase activities in wild Mus musculus. Bull Environ
Contam Toxicol, 31: 158-163.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrow RW (1972) Acute skin absorption study on rats using technical
methomyl and a 25% methomyl formulation (Lannate(R) 25W). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-438-72*).
Morse DL, Baker EL, Kimbrough RD, & Wiseman CL (1979)
Propanil-chloracne and methomyl toxicity in workers of a pesticide
manufacturing plant. Clin Toxicol, 15(1): 13-21.
Natoff IL & Reiff B (1973) Effect of oximes on the acute toxicity of
anticholinesterase carbamates. Toxicol Appl Pharmacol, 25: 569-575.
Niven CF Jr (1971) Thermal destruction of Lannate in spinach
processing. Walnut Creek, California, Del Monte Corporation Research
Center (Unpublished report*).
Noda J (1984) [Determination of methomyl by using chemical ionization
mass fragmentography. A case report of methomyl poisoning and the
animal experiment of its poisoning.] Nippon Hoigaku Zasshi, 38: 71-82
(in Japanese).
Ökolimna (1980) [Fish toxicity, carps, Lannate 25 WP.] Burgwedel,
Germany, Ökolimna (Unpublished report*) (in German).
Orpin R (1971) Methomyl - Study of effects on wild life. Glaston,
United Kingdom, Farm Protection Ltd (Unpublished report*).
Osman AZ, Hazza A, Nagwa I, & Awad TM (1983) Fate and metabolism of
the insecticide 14C-Lannate in farm animals. Isot Rad Res,
15(2): 111-120.
Oswald T, Adams J & Hicks SC (1991), LannateR insecticide -
Dislodgeable foliar residue study in Rose Greenhouse operations.
Fresno, California, Siemer and Associates Inc. (Unpublished report
No. AMR-1909-90*).
Owens CB, Owens EW, & Zahn D (1978) The extent of exposure of migrant
workers to pesticide and pesticide residues (Abstract). Int J
Chronobil, 5(2): 428-429.
Palmer JS & Schlinke JC (1978) Preliminary toxicological evaluations
of six pesticide compounds in cattle, sheep and chickens (Unpublished
report METH/TOX5*).
Panepinto AS (1991a) Acute inhalation toxicity study with
DPX-X1179-427 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-678-91*).
Panepinto AS (1991b) Acute inhalation toxicity study with
DPX-X1179-424 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-560-91*).
Paynter OE (1966) Three-month dietary administration - Rats.
Insecticide 1179. Falls Church, Virginia, Hazleton Laboratories Inc.
(Unpublished report*).
Pease HL (1968) Methomyl residue analysis - Soils. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Pease HL & Kirkland JJ (1968) Determination of methomyl residues using
microcolourmetric gas chromatography. J Agric Food Chem, 16: 554-557.
Peeples JL (1977) Effect of methomyl on soil microorganisms
(Unpublished report No. ML/ME21*).
Powley CR (1989) Methomyl foliar dislodgeable residues on grapes in
California. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. BMP/DFR-189*).
Powley CR (1990a) LannateR insecticide: Dislodgeable foliar residue
study in grape girdling and harvesting operations. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1445-89*).
Powley CR (1990b) LannateR insecticide: Dislodgeable foliar residue
study on grapes grown in California. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-1515-89*).
Powley CR (1991) Determination of possible bioaccumulation of
14C-methomyl in lactating dairy cows. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-898-87*).
Priester TM (1984) Batch equilibrium (adsorption/desorption) and soil
thin-layer chromatography studies with methomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. AMR-174-84*).
Proctor NH, Moscioni AD, & Casida JE (1976) Chicken embryo NAD levels
lowered by teratogenic organophosphorus and methylcarbamate
insecticides. Biochem Pharmacol, 25: 757-762.
Quarles JM, Sega MW, Schenley CK, & Lijinsky W (1979) Transformation
of hamster fetal cells by nitrosated pesticides in a transplacental
assay. Cancer Res, 39: 4525-4533.
Radwan MA, El-Wakil HB, & Osman KA (1992) Toxicity and biochemical
impact of certain oxime carbamate pesticides against terrestrial
snail, Theba psiana (Müller). J Environ Sci Health, B27: 759-773.
Reeve MW, O'Connell LP, Bissell S, & Ross J (1992) Characterization of
methomyl dissipation on grape foliage. Bull Environ Contam Toxicol,
49: 105-111.
Rogers AS, Culik R, Kaplan AM, & Aftosmis JG (1978) Oral teratogenic
study in rats with Lannate (INX-1179). Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-498-78*).
Ruppel RF & Laughlin CW (1977) Toxicity of some soil pesticides to
earthworms. J Kansas Entomol Soc, 50: 113-118.
Saiyed HN, Sadhu HG, Bhatnagar VK, Dewan A, Venkaiah K, & Kashyap SK
(1992) Cardiac toxicity following short-term exposure to methomyl in
spraymen and rabbits. Hum Exp Toxicol, 11(2): 93-97.
Saleh F (1990a) Metabolic effects of the carbamate insecticide
(methomyl) on rats. I. Levels of glucose, lipids and cholesterol in
serum after administration of the insecticide. Egypt J Physiol Sci,
14(1-2): 45-54.
Saleh F (1990b) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in serum cholinesterase and
transaminases following treatment of the insecticide. Egypt J Physiol
Sci, 14(1-2): 55-64.
Saleh F (1990c) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in some blood biochemical indices in
the rats poisoned with the insecticide. Egypt J Physiol Sci,
14(1-2): 65-74.
Sarver JW (1991a) Acute oral toxicity study with DPX-X1179-394 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-661-91*).
Sarver JW (1991b) Acute dermal toxicity study of DPX-X1179-394 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-455-91*).
Sarver JW (1991c) Acute oral toxicity study with DPX-X1179-424 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-171-91*).
Sarver JW (1991d) Primary dermal irritation study with DPX-X1179-394
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-165-91*).
Sarver JW (1991e) Primary dermal irritation study with DPX-X1179-425
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-166-91*).
Sarver JW (1991f) Primary dermal irritation study with DPX-X1179-424
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-162-91*).
Sarver JW (1991g) Primary eye irritation study with DPX-X1179-425 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-280-91*).
Sarver JW (1991h) Primary eye irritation study with DPX-X1179-424 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-279-91*).
Sarver JW (1992a) Acute oral toxicity study with DPX-X1179-439 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-858-91*).
Sarver JW (1992b) Acute dermal toxicity study of DPX-X1179-439 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-825-91*).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Serota DG, Machotka SV, Hastings TF, Alsaker RD, & Lane Fezio W (1981)
104-Week chronic toxicity and carcinogenicity study in mice. Methomyl;
H-11,135. Vienna, Virginia, Hazleton Laboratories America Inc.
(Unpublished report No. HLO-253-81*).
Shah PV, Monroe RJ, & Guthrie FE (1981) Comparative rates of dermal
penetration of insecticides in mice. Toxicol Appl Pharmacol,
59: 414-423.
Sherman H (1966) Acute oral LD50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-210-66*).
Sherman H (1967) Acute oral potentiation studies with S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate [INX-1179). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-160-67*).
Sherman H (1968a) Antidote studies with rats. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-251-68*).
Sherman H (1968b) Acute oral and antidote tests with guinea pigs.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-252-68*).
Sherman H (1968c) Acute oral toxicity and antidote tests in rabbits
using technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-250-68*).
Sherman H (1968d) Acute oral toxicity and antidote tests in dogs using
technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-279-68*).
Sherman H (1972) Chicken & egg study. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-55-72*).
Silveira EJ (1990) Technical methomyl - physical and chemical
characteristics. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products Department (Unpublished report
No. AMR-1753-90*).
Simpson GR & Bermingham S (1977) Poisoning by carbamate pesticides.
Med J Aust, 2: 148-149.
Singles GH (1970a) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-186-70*).
Singles GH (1970b) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-327-70*).
Sleight BH (1971) Continuous exposure of rainbow trout (Salmo
gairdneri) to LannateR in water. Wareham, Massachusetts, Bionomics
Inc. (Unpublished report*).
Sleight BH (1973) Acute toxicity of H-7946 to grass shrimp
(Palaemonetes vulgaris), pink shrimp (Penaeus duorarum) and mud
crab (Neopanope texana). Wareham, Massachusetts, Bionomics Inc.
(Unpublished report No. HLO-186-73*).
Smelt JH, Dekker A, Leistra M, & Houx NWH (1983) Conversion of four
carbamoyloximes in soil samples from above and below the soil water
table. Pestic Sci, 14: 173-181.
Smith LW (1982) Wildlife toxicity studies with methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. ML/FW20*
SRI International (1988) Pesticides and intermediates, Supplement C.
Menlo Park, California, SRI International (Report No. 171C).
Stute K (1983) Results of the registration trials on bee toxicity -
1983. Celle, Germany, Du Pont de Nemours (Germany) GmbH (Unpublished
report No. METH/ECO14*).
Swanson MB (1986) Photodegradation of [1-14C] methomyl in soil.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products Department (Unpublished report No. AMR-611-86*).
Ta'Naka I, Igisu H, Haratake J, Cho S, Mori K, Fujishiro K, Inoue N,
Horie A, & Akiyama T (1987) Cumulative toxicity potential of methomyl
aerosol by repeated inhalation. Am Ind Hyg Assoc J, 48(4): 330-334.
Teeters WR (1968) Lannate antidotal study - monkeys. Falls Church,
Virginia, Hazleton Laboratories Inc. (Unpublished report
No. 201-219*).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a fungicide
on the numbers and biomass of earthworms in pasture. Bull Environ
Contam Toxicol, 12(4): 487-492.
Trivits R (1979) Acute oral LD50 test in rats using technical
methomyl. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-496-79*).
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 74-75 (Resource Publication No. 84).
USDI (1975) Acute toxicity studies. Cutthroat trout and stonefly
larvae. Columbia, Missouri, US Department of the Interior, Fish and
Wildlife Service, Columbia National Fishery Research Laboratory
(Unpublished report No. ML/FW-1*).
USDI (1978) Methomyl - Summary of acute toxicity. Columbia, Missouri,
US Department of the Interior, Fish and Wildlife Service, Columbia
National Fishery Research Laboratory (Unpublished report
No. ML/FW-3*).
USEPA (1988) Methomyl science chapters - June 1988: Parts I and II.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances.
USEPA (1991) Pesticides in ground water database. Washington, DC, US
Environmental Protection Agency, Pesticides and Toxic Substances.
US FDA (1993a) Food and Drug Administration total diet study: 20
market basket studies, October 1986 - April 1991: Findings of methomyl
in food collected for total diet study. Washington, DC, US Food and
Drug Administration.
US FDA (1993b) FDA-Regulatory monitoring data: FY 1988-1992 - Findings
of methomyl in samples of domestic and imported foods. Washington, DC,
US Food and Drug Administration.
US FDA (1993c) FDA-Regulatory monitoring data: FY 1988-1992 - Counts
of samples of domestic and imported foods and feeds analyzed by
methodology known to be capable of determining methomyl. Washington,
DC, US Food and Drug Administration.
Vincent DR (1985) Assessment of methomyl (INX-1179-255) in the
in vitro unscheduled DNA synthesis assay in primary rat hepatocytes.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-149-85*).
Ward TJ & Boeri RL (1989) Static acute toxicity of methomyl to the
mysid, Mysidopsis bahia. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-675-89*).
Ward TJ & Boeri RL (1990) Static acute toxicity of methomyl to bivalve
mollusc embryos and larvae. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-422-90*).
Ward TJ & Boeri RL (1991) Acute flow-through mollusc shell deposition
test with DPX-X1179-394 (methomyl). Hampton, New Hampshire, Resource
Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-710-91*).
Ware GW, Estesen B, & Cahill WP (1978) Dislodgeable insecticide
residues on cotton (1975). Bull Environ Contam Toxicol, 20: 17-19.
Ware GW, Estesen BJ, & Buck NA (1980) Dislodgeable insecticide
residues on cotton foliage: Acephate, AC222705, EPN, fenvalerate,
methomyl, methyl parathion, permethrin and thiodicarb. Bull Environ
Contam Toxicol, 25: 608-615.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. In: Fleck RA & Hollaender A ed. Genetic toxicology: An
agricultural perspective. New York, London, Plenum Press, pp 275-324.
Williams DT, Denley HV, Lane DA, & Quan ESK (1982) Real time
monitoring of methomyl air levels during and after spraying in a
greenhouse. Am Ind Hyg Assoc J, 43: 190-195.
Wojciechowski JP, Kaur P, & Sabharwal PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Yeboah PO & Kilgore WW (1984) Analysis of airborne pesticides in a
commercial pesticide storage building. Bull Environ Contam Toxicol,
32: 629-634.
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Jpn Bull Agric Chem Insp Stn, 12: 122-128.
Zwick TC & Malik N (1990a) Aerobic metabolism of [1-14C] methomyl in
Madera, California soil. Columbus, Ohio, Battelle Memorial Institute,
Environmental Sciences Department (Unpublished report
No. AMR-1543-89*).
Zwick TC & Malik N (1990b) Anaerobic metabolism of [1-14C] methomyl
in Madera, California soil. Columbus, Ohio, Battelle Memorial
Institute, Environmental Sciences Department (Unpublished report
No. AMR-1544-89*).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le méthomyl est un solide cristallin blanc dont le point de
fusion est de 77°C et la tension de vapeur de 0,72 mPa à 25°C. Sa
solubilité dans l'eau est de 54,7 g/litre et son coefficient de
partage octanol/eau (Kow) est de 1,24. Il est stable dans l'eau
stérile à pH 7 mais il se décompose à pH plus élevé, sa demi-vie étant
de 30 jours à pH 9 et 25°C.
Le dosage du méthomyl dans divers échantillons consiste en une
extraction suivie d'une purification, l'analyse finale s'effectuant
par chromatographie en phase liquide à haute performance ou
chromatographie gaz/liquide. Dans certains cas, avant le dosage
proprement dit, on transforme le méthomyl en oxime ou en fluorophore
(post-colonne).
2. Sources d'exposition humaine et environnementale
Le méthomyl est préparé en faisant réagir le
N-hydroxythioacétimidate de S-méthyle (MHTA) en solution dans le
chlorure de méthylène, sur l'isocyanate de méthyle gazeux à 30-50°C.
Il s'agit d'un carbamate à propriété insecticide que l'on utilise
partout dans le monde sur toutes sortes de cultures. Il sert
notamment à protéger les fruits, les vignes, le houblon, les légumes,
les céréales, le soja, le coton et les plantes ornementales. A
l'intérieur des bâtiments, on l'utilise aussi pour détruire les
mouches dans les animaleries et les laiteries.
Il est principalement présenté sous forme de poudre hydro-soluble
ou encore de liquide miscible à l'eau que l'on dilue pour le
traitement des cultures au sol ou en pulvérisations aériennes. Les
doses d'emploi vont habituellement de 0,15 à 1,0 kg de matière active
par hectare. C'est essentiellement au cours de la préparation et de
l'épandage de ces produits ou par suite de l'ingestion de résidus
subsistant sur des cultures vivrières qu'il peut y avoir exposition
humaine (voir section 5.3.1.4).
3. Transport, distribution et transformation dans l'environnement
Les études de laboratoire montrent que le méthomyl est faiblement
adsorbé aux particules du sol. On a ainsi montré que l'adsorption
était faible sur les minéraux argileux en particulier l'illite; sur
les matières organiques du sol, elle est multipliée par 50 tout en
restant encore relativement faible. Une fois adsorbés, les résidus ne
se désorbent pratiquement pas. Du fait de ses propriétés, on peut
penser que le méthomyl doit être doté d'une certaine mobilité dans le
sol.
Dans l'environnement naturel, la décomposition abiotique du
méthomyl par hydrolyse ou photolyse est lente ou nulle.
Dans le sol, la décomposition aérobie est environ deux fois plus
rapide que la décomposition anaérobie. On a fait état de valeurs de
la demi-vie dans le sol qui vont de quelques jours à plus de 50 jours;
la sécheresse retarde la décomposition. Dans la pratique, on peut
considérer que dans la plupart des cas, on aura après épandage, une
demi-vie d'environ une semaine.
Dans les conditions de plein champ, le méthomyl ne pénètre pas
dans le sol, par lessivage, à une profondeur de plus de 20-30 cm et il
n'y a pas de contamination des eaux souterraines.
Lorsqu'on épand du méthomyl marqué au 14C sur les feuilles de
végétaux, il y absorption, mais le produit n'est pas transporté dans
d'autres parties de la plante. Lorsqu'on l'applique sur le système
radiculaire, le composé est fixé par la plante, le principal résidu
étant le méthomyl lui-même. Les produits de décomposition volatils du
méthomyl sont le CO2 et l'acétonitrile. Le reste de la fraction
radioactive se retrouve dans les constituants naturels de la plante
tels que les lipides ainsi que les acides et les sucres du cycle de
Krebs. Dans le feuillage, la demi-vie du méthomyl est de l'ordre de
quelques jours.
Après exposition de truites arc-en-ciel à ce composé pendant 28
jours dans un système à courant d'eau, on n'a trouvé aucun signe
d'accumulation.
4. Concentrations dans l'environnement et exposition humaine
Si l'on en croit les analyses effectuées sur diverses sources
d'eau après épandage du composé aux doses recommandées, il est
probable que les concentrations de méthomyl dans les eaux souterraines
sont soit très faibles, soit inférieures à la limite de détection
(< 0,02 mg/litre).
De faibles résidus de méthomyl se retrouvent sur les cultures,
notamment vivrières, lors de la récolte, les quantités dépendant de
facteurs tels que la dose d'emploi, le laps de temps écoulé depuis le
dernier épandage et le type de culture. Ces résidus sont
essentiellement constitués du produit initial.
Dans les produits laitiers, les résidus de méthomyl sont soit
inférieurs à la limite de détection, soit très faibles. Après
administration de méthomyl à des vaches laitières sous forme de
capsules, pendant 28 jours à une dose équivalant à 80 mg/kg de
nourriture, on n'a pas retrouvé de résidus décelables de ce composé ou
de son métabolite (le MHTA) dans la lait ou les tissus de ces animaux
(< 0,02 mg/kg). Aucune trace de méthomyl n'a été non plus décelée
dans les oeufs ou dans les tissus de poules pondeuses qui en avaient
reçu 1 ou 10 mg/kg dans leur nourriture pendant quatre semaines.
Des analyses effectuées par sondage aux Etats-Unis sur la ration
totale ou sur certains types d'aliments, ont montré que la
concentration de méthomyl était soit très faible, soit inférieure à la
limite de détection. Par ailleurs, un certain nombre d'opérations
tels que le lavage, l'épluchage ou la cuisson, réduisent encore la
teneur en résidus.
Des études effectuées lors du retour des ouvriers agricoles sur
les vignobles après épandage, notamment dans les conditions du désert
de Californie, ont montré que pour un résidu foliaire mobile tombé à
0,1 µg/cm2, l'exposition était maximale au niveau du torse et de la
tête lorsque les ouvriers agricoles pratiquaient l'incision annulaire,
le torse et les mains étant les plus touchés lors de la cueillette du
raisin. En ce qui concerne le raisin de table, c'est lors de la
cueillette et de l'emballage que l'exposition était la plus faible.
L'exposition par inhalation était minime.
Après pulvérisation de méthomyl sur des plants de concombres et
de tomates, on a mesuré dans l'air de la serre qui les abritait, des
concentrations d'insecticide allant jusqu'à 4,7 µg/m3 dans la
journée qui a suivi l'épandage. Trois, puis sept jours après ce
traitement, les concentrations de méthomyl dans la zone de
respiration allaient respectivement jusqu'à 14,5 ou 0,7 µg/m3. Dans
l'eau de lavage des mains des jardiniers d'une serre, on a retrouvé
des quantités de méthomyl allant de 10 à 300 µg/heure de travail.
Cela montre que l'exposition cutanée est une voie d'exposition plus
importante que l'inhalation et que la durée de l'attente à observer
avant de retourner sur les lieux doit être basée sur les données
d'exposition cutanée.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après administration à des rats par voie orale, le méthomyl est
rapidement absorbé, métabolisé et excrété, l'ensemble du processus
étant achevé en quelques jours. Une semaine après avoir administré à
des rats du méthomyl radio-marqué à raison de 5 mg/kg de poids
corporel, on a constaté que 54% de la dose était excrétés dans
l'urine, 2 à 3% dans les matières fécales et 34% dans l'air expiré (en
l'espace de cinq jours). Au bout de sept jours, il restait dans les
tissus et la carcasse 8 à 9% de la radioactivité, le carbone-14 étant
incorporé dans les constituants endogènes. C'est dans le sang que la
fraction radioactive était la plus importante (2% de la dose).
Chez ces rats, les principaux métabolites présents dans l'air
expiré étaient du dioxyde de carbone et l'acétonitrile dans le rapport
d'environ 2:1. Dans les urines, le principal métabolite était
constitué par un dérivé mercapturique du méthomyl, à hauteur de 17% de
la dose. On n'a pas décelé la présence de méthomyl ni de son oxime.
On pense que la métabolisation du méthomyl s'effectue selon le
schéma suivant: déplacement du groupe S-méthyle par le glutathion,
puis transformation enzymatique en dérivé de l'acide mercapturique.
Il existe une autre voie métabolique, à savoir l'hydrolyse en MHTA qui
est ensuite rapidement décomposé pour donner du dioxyde de carbone. Il
pourrait y avoir aussi conversion du syn-méthomyl (qui est la forme
insecticide) en isomère anti, lequel serait ensuite décomposé en
acétonitrile par des réactions d'hydrolyse, de transposition et
d'élimination. Chez le singe, le métabolisme est analogue, à cela
près que le dérivé de l'acide mercapturique ne constitue qu'un
métabolite urinaire mineur.
Une heure après application cutanée à des souris d'une solution
acétonique de méthomyl marqué au 14C, on a constaté que le taux de
pénétration était de l'ordre de 85%. A ce moment, 3% de la dose
étaient présents dans le sang, 5% dans le foie et 13% avaient été
excrétés. Au bout de huit heures, l'excrétion totale était de 54,5%.
La décomposition et l'élimination rapides du méthomyl chez le rat
ainsi que son absence d'accumulation tissulaire, est comparable à ce
que l'on observe chez les ruminants.
Du méthomyl administré à des vaches et à des chèvres a subi une
dégradation totale. Ni le composé initial, ni son oxime n'ont été
retrouvés dans le lait ou les tissus. On a montré que le méthomyl
était métabolisé et incorporé dans les constituants naturels du lait
et du foie.
Après avoir fait incuber du méthomyl marqué au 14C avec du
nitrite de sodium dans un macérat de viande séchée, dans des
conditions simulant le milieu gastrique, on n'a pas mis en évidence la
formation de nitrosométhomyl.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
Le méthomyl présente une forte toxicité aiguë par voie orale, sa
DL50 chez le rat allant de 17 à 45 mg/kg de poids corporel. Il
présente également une forte toxicité inhalatoire chez cet animal, la
CL50 à 4 heures étant de 0,26 mg/litre lorsque le composé est inhalé
sous forme d'aérosol. La toxicité cutanée est très faible, la DL50
dépassant 2000 mg/kg de poids corporel chez le lapin (peau intacte) et
1000 mg/kg de poids corporel chez le rat (peau abrasée). Les signes
d'intoxication aiguë sont ceux que l'on peut attendre d'un inhibiteur
de la cholinestérase et consistent, entre autres, en une
hypersalivation, une lacrimation, des tremblements et un myosis. On a
constaté que les animaux récupéraient rapidement et l'examen de leurs
organes n'a pas révélé la présence de lésions anatomopathologies
visibles qui soient imputables au traitement. Le méthomyl ne provoque
pas d'irritation ou d'hypersensibilisation cutanée et il est
légèrement irritant pour la muqueuse oculaire.
L'administration répétée de méthomyl dans la nourriture pendant
des périodes prolongées n'a pas conduit à une accumulation ou à un
accroissement des effets toxiques. Des rats et des chiens à qui on
avait administré une nourriture contenant jusqu'à 250 mg/kg et
400 mg/kg respectivement de méthomyl, pendant 13 semaines, n'ont
présenté ni signes d'intoxication ni surmortalité. Chez des rats qui
en avaient reçu dans leur nourriture à la dose de 250 mg/kg, on a
observé une légère diminution du gain de poids, une réduction du taux
d'hémoglobine et une hyperplasie modérée de la lignée érythroblastique
au niveau de la moelle osseuse. Chez le rat, la dose sans effets
observables était de 50 mg/kg de nourriture (soit l'équivalent d'une
dose quotidienne de 3,6 mg/kg de poids corporel). Des lapins, qui
avaient subi des applications cutanées répétées de méthomyl à des
doses quotidiennes allant jusqu'à 500 mg/kg de poids corporel, pendant
21 jours, ont présenté une hyperactivité ainsi qu'une réduction de
l'activité cholinestérasique plasmatique et cérébrale à la dose la
plus élevée. La dose sans effets nocifs observables était égale,
selon cette étude, à 50 mg/kg de poids corporel par jour.
Au cours d'études à long terme, des rats ont reçu du méthomyl
dans leur nourriture à raison de 0, 50, 100 ou 400 mg/kg et des souris
en ont reçu de la même manière, 0, 50, 75 ou 200 mg/kg. Chez les
rats, les effets observés à la dose la plus élevée consistaient en une
réduction du gain de poids ainsi qu'en une diminution du taux
d'hémoglobine et de la valeur de l'hématocrite. La dose sans effets
observables était de 100 mg/kg de nourriture, soit l'équivalent d'une
dose quotidienne de 5 mg/kg de poids corporel. Chez les souris, on a
constaté aux deux doses les plus élevées, une augmentation du taux de
mortalité ainsi qu'une diminution du taux d'hémoglobine et du nombre
de globules rouges. La dose sans effets observables a été évaluée à
50 mg/kg de nourriture, soit l'équivalent d'une dose quotidienne de
8,7 mg/kg de poids corporel. Lors d'une étude toxicologique de deux
ans chez des chiens (doses de 0, 50, 100, 400 ou 1000 mg/kg de
nourriture), on a observé des signes d'intoxication chez certains
animaux à la dose la plus forte, avec une anémie légère à modérée. La
dose sans effets observables était de 100 mg/kg de nourriture, soit
l'équivalent d'une dose quotidienne de 3 mg/kg de poids corporel.
Des études de deux ans sur des rats et des souris n'ont révélé
aucun signe d'accroissement de l'incidence des tumeurs qui soient
imputables à l'administration de méthomyl, ce qui indique que le
méthomyl n'est pas cancérogène. Il se s'est pas révélé non plus
génotoxique lors d'épreuves in vitro sur des cellules bactériennes
ou mammaliennes et la recherche de lésions primaires de l'ADN sur des
cellules bactériennes ou mammaliennes in vitro ou encore
d'aberrations chromosomiques médullaires lors d'épreuves in vivo
chez le rat, a donné des résultats négatifs. On a cependant observé
la possibilité d'effets cytogénétiques sur des lymphocytes humains
in vivo, attestée par l'augmentation du nombre de micronoyaux et
d'aberrations chromosomiques. Chez des rats et des lapins qui en
avaient reçu par gavage des doses allant jusqu'à 400 mg/kg de
nourriture (soit une dose quotidienne de 16 mg/kg de poids corporel),
le méthomyl n'a pas produit non plus d'effets embryotoxiques ou
tératogènes, alors qu'à ces doses, des effets toxiques pouvaient être
constatés chez les femelles gravides. Lors d'une étude de
reproduction portant sur trois générations de rats, qui avaient reçu
50 ou 100 mg de méthomyl/kg de nourriture (soit l'équivalent quotidien
de 50 ou 10 mg/kg de poids corporel), on n'a pas constaté d'effets sur
la fécondité, la gestation ou la lactation qui soient imputables au
méthomyl et aucune anomalie visible résultant de ce traitement n'a été
observée.
Après administration unique ou répétée de méthomyl, on n'a pas
observé de neurotoxicité retardée. Des rats qui en avaient reçu dans
leur nourriture à raison de 800 mg/kg, n'ont présenté une diminution
sensible de leur activité cholinestérasique sanguine qu'au début d'une
étude de cinq mois. Une étude d'alimentation de 28 jours n'a révélé
qu'une légère diminution de l'activité cholinestérasique cérébrale à
cette dose. Il y a donc eu réversibilité rapide de l'effet
anticholinestérasique du méthomyl chez les animaux au cours de la
période d'expérimentation. In vitro, l'activité cholinestérasique
érythrocytaire humaine s'est révélée six fois plus sensible à l'action
inhibitrice du méthomyl que celle du rat, mais la réactivation
spontanée s'est produite sensiblement à la même vitesse.
Des études menées sur diverses espèces ont montré que l'atropine
est le meilleur antidote d'une intoxication par le méthomyl.
7. Effets sur l'homme
La lecture des rapports sur les intoxications accidentelles et
les suicides par empoisonnement avec du méthomyl éclaire quelque peu
sur la gravité des effets et les possibilités de récupération. Sur
cinq victimes d'une intoxication accidentelle due à la consommation
d'aliments contaminés, trois sont décédées dans les trois heures
suivant le repas. On estime que les victimes avaient ingéré environ
12 à 15 mg de méthomyl/kg de poids corporel. Une femme âgée de 31 ans
et son fils de six ans, qui étaient tous les deux décédés par suite
d'un empoisonnement volontaire, présentaient des concentrations
hépatiques de méthomyl respectivement égales à 15,4 et 56,5 mg/kg.
Les doses ont été estimées à 55 mg/kg de poids corporel pour la mère
et 13 mg/kg de poids corporel pour l'enfant. Chez une femme qui avait
ingéré environ 2,25 g de méthomyl, on a retrouvé dans son sang six
heures plus tard une quantité de méthomyl égale à 1,6 mg/kg.
Vingt-deux heures après l'ingestion, alors que la patiente se
remettait, on n'a plus retrouvé de méthomyl.
Un travailleur chargé d'épandre des pesticides, qui n'avait pas
pris la moindre précaution alors qu'il préparait une bouillie à base
de méthomyl en poudre pour traiter des légumes, a présenté des
symptômes d'intoxication au bout d'une heure avec réduction à 40% de
la normale de son activité cholinestérasique sanguine au bout de 12
heures, la récupération à 80% de la normale intervenant 36 heures plus
tard. D'autres travailleurs, qui avaient pris les précautions
d'usage, n'ont présenté aucun symptôme ou effet sur l'activité de leur
cholinestérase érythrocytaire ou plasmatique lors de l'épandage de
méthomyl par voie aérienne.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
Le méthomyl n'a eu aucun effet sur des populations de champignons
ou de bactéries terricoles, et en particulier sur leur action
nitrifiante ou sur l'activité de la déshydrogénase, lorsqu'il était
appliqué aux doses recommandées.
Des études en laboratoire ont permis de fixer à 6,5 mg/litre la
concentration sans effets observables sur la croissance des algues.
Le méthomyl est moyennement à fortement toxique pour les
poissons, les valeurs de CL50 à 96 heures se situant, pour diverses
espèces, dans l'intervalle 0,5-2 mg/litre. Une étude à long terme (21
jours) a montré que la CL50 pour des alevins de truite exposés à du
Lannate 20L (21,5% de méthomyl), était égale à 1,3 mg de méthomyl par
litre. Lors d'une étude toxicologique portant sur de jeunes
cyprinidés de l'espèce Pimephales promelas, la MATC (concentration
maximale acceptable de substance toxique) a été trouvée comprise entre
57 et 177 µg/litre.
Des études de toxicité aiguë portant sur d'autres organismes
aquatiques ont montré que Daphnia magna était l'espèce la plus
sensible au méthomyl, la CL50 à 48 heures étant de 0,032 mg/litre.
Lors d'une étude de 21 jours au cours de laquelle on a étudié la
survie, la croissance et la capacité de reproduction de Daphnia magna,
on a constaté que la concentration maximale acceptable de substance
toxique (MATC) pour le méthomyl était comprise entre 1,6 et
3,5 µg/litre.
Le méthomyl est toxique pour les abeilles, la DL50 par contact
étant de 1,29 µg/insecte et la DL50 par voie orale, de
0,2 µg/insecte.
On a évalué la toxicité aiguë du méthomyl chez plusieurs espèces
d'oiseaux, les valeurs caractéristiques de DL50 aiguë par voie orale
se situant à 10 mg/kg de poids corporel chez les pigeons et à 34 mg/kg
de poids corporel chez la caille japonaise. Il est relativement moins
toxique lorsqu'il est mêlé à la nourriture, la CL50 à 8 jours étant
dans ce cas de 1100 mg/kg de méthomyl (mêlé à la nourriture) pour le
colin de Virginie et de 2883 mg de méthomyl/kg de nourriture pour le
colvert. Lors d'études qui ont duré de 18 à 20 semaines et ont porté
sur une génération, on a évalué à 150 mg/kg de méthomyl dans la
nourriture, la concentration sans effets nocifs observables pour le
colin de Virginie et le colvert.
Aucun effet n'a été observé chez des colins de Virginie qui
avaient été exposés à une série de pulvérisations de méthomyl aux
doses recommandées. Après un épandage de méthomyl sur une zone
forestière et des champs de houblon, aux doses recommandées, on a
effectué deux études sur des populations aviaires sauvages. Elles
n'ont pas révélé de modification de l'activité des oiseaux et n'ont
provoqué aucun effet, en particulier aucun effet létal. Par rapport
aux témoins, on a constaté qu'il y avait réduction des dépôts
graisseux chez les oiseaux chanteurs des forêts traitées; on estime
qu'il s'agit là d'un effet indirect dû à la réduction des populations
d'insectes dont se nourrissent ces oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Le méthomyl est un carbamate qui inhibe la cholinestérase et dont
le mode d'action toxique est bien connu. Il présente une toxicité
particulièrement élevée lorsqu'il est absorbé par voie orale ou par
inhalation, ainsi que le montrent les études sur l'animal, mais sa
toxicité par voie cutanée est faible. Chez l'animal, les signes
d'intoxication aiguë sont caractéristiques d'une inhibition de la
cholinestérase. Cette intoxication est rapidement réversible, avec
une disparition rapide des symptômes et une désinhibition également
rapide des cholinestérases sanguine et cérébrale. Cette prompte
récupération est due au fait que l'inhibition par le méthomyl de la
cholinestérase est rapidement réversible, et d'ailleurs facilitée par
la vitesse d'excrétion élevée de ce composé. Ce que l'on sait des
intoxications humaines accidentelles ou volontaires montre que la
toxicité aiguë du méthomyl est du même ordre chez l'homme que chez
l'animal de laboratoire.
Comme l'action du méthomyl est rapidement réversible pendant la
période d'administration, il a rarement été possible d'observer des
signes d'intoxication aiguë et d'inhibition de la cholinestérase
sanguine au cours des études où on l'administrait mêlé à la
nourriture. Les observations les plus régulièrement rapportées à
l'occasion d'études à long terme consistent, aux doses les plus
élevées dans l'alimentation, en une réduction du gain de poids chez
les rongeurs et une diminution des paramètres érythrocytaires chez les
rongeurs et les chiens. Trois études de longue durée chez des
rongeurs n'ont pas permis de mettre en évidence d'activité
cancérogène. Le composé ne s'est pas non plus montré génotoxique lors
d'épreuves in vitro portant sur divers paramètres biotoxicologiques,
néanmoins il y a une possibilité d'effets cytogénétiques sur les
lymphocytes humains. Une étude chromosomique effectuée in vivo sur
la moelle osseuse de rats s'est également révélée négative.
Chacune des études à long terme sur l'animal a permis, à partir
de la réduction du gain de poids et des paramètres érythrocytaires,
d'obtenir une valeur de la dose sans effets nocifs observables. Elle
se situait à 5 mg/kg de poids corporel par jour chez le rat, à
8,7 mg/kg de poids corporel par jour chez la souris et à 3 mg/kg de
poids corporel par jour chez le chien. Faute d'une différenciation
marquée des effets toxiques entre les différentes espèces, il ressort
de ces études que la dose sans effets nocifs observables chez le
chien, c'est-à-dire 3 mg/kg de poids corporel par jour, peut être
utilisée pour évaluer le risque chez l'homme.
Le méthomyl est faiblement à modérément adsorbé aux particules du
sol, et ne s'en désorbe pratiquement pas. Dans le sol, il se
décompose en aérobiose (avec une demi-vie d'environ une semaine)
environ deux fois plus vite qu'en anaérobiose.
Une fois déposé sur les feuilles de végétaux, le méthomyl est
rapidement absorbé à hauteur de 50% de la dose - les 50% restant étant
adsorbés - et rien n'indique qu'il y ait transport à l'intérieur de la
plante. La concentration du méthomyl absorbé par les cultures
vivrières tombe rapidement à environ 5% de sa valeur initiale en
l'espace d'une semaine.
Plusieurs invertébrés aquatiques, et en particulier les daphnies,
sont très sensibles au méthomyl avec des valeurs de la CL50 de
l'ordre de 10 à 100 µg/litre.
Les poissons, qu'il s'agisse d'espèces d'eau douce ou d'espèces
estuarielles, y sont moins sensibles, puisque les valeurs de la CL50
s'étagent entre 0,5 et 7 mg/litre. Etant donné la faible persistance
du méthomyl et sa toxicité aiguë relativement faible pour les
poissons, le risque encouru par ces derniers est relativement faible.
Aux doses d'emploi recommandées, le méthomyl n'a pas d'effets
nocifs sur l'activité microbienne terricole en climat tempéré.
Le méthomyl est classé comme hautement toxique pour les abeilles
avec une DL50 topique d'environ 0,1 µg/insecte.
Les valeurs de la DL50 aiguë par voie orale varient entre 10 et
40 mg/kg de poids corporel chez diverses espèces d'oiseaux. Les
valeurs de CL50 à cinq jours par voie alimentaire vont de 1100 à
3700 mg/kg de nourriture. Il y a risque d'intoxication aiguë pour les
oiseaux, en particulier lorsque le méthomyl est sous forme de
granulés; son absorption à partir de nourriture contaminée ne devrait
cependant pas être mortelle pour les oiseaux.
La forte toxicité aiguë du méthomyl pour les mammifères de
laboratoire permet de conclure qu'il est tout aussi dangereux pour les
mammifères sauvages.
10. Conclusion
Compte tenu des aspects qualitatifs et quantitatifs de la
toxicité du méthomyl, le groupe de travail a conclu qu'une dose
quotidienne de 0,03 mg/kg de poids corporel ne devrait probablement
pas causer d'effets nocifs chez l'homme, quel que soit le mode
d'exposition.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El metomilo es un sólido cristalino blanco con un punto de fusión
de 77°C y una presión de vapor de 0,72 mPa (25°C). Tiene una
solubilidad en agua de 54,7 g/litro y su coeficiente de reparto
octanol/agua (Kow) es de 1,24. Es estable en agua estéril a pH 7,
pero se descompone a pH más elevado, con una semivida de 30 días a pH
9 y 25°C.
El procedimiento analítico para la determinación del metomilo en
muestras diferentes es la extracción seguida de limpieza y análisis
mediante cromatografía líquida de alto rendimiento o cromatografía
gas-líquido. En algunos casos, el metomilo se convierte en su
derivado oxima o en un derivado fluoróforo (después de la columna)
antes de su determinación analítica.
2. Fuentes de exposición humana y ambiental
El metomilo de produce haciendo reaccionar el S-metil- N-
hidroxitioacetimidato (MHTA) en cloruro de metileno con isocianato de
metilo gaseoso a 30-50°C. Es un insecticida de tipo carbamato
utilizado en una gran diversidad de cultivos en todo el mundo. Entre
los cultivos protegidos figuran frutales, vides, lúpulo, hortalizas,
cereales, soja, algodón y plantas ornamentales. En espacios cerrados
se utiliza en establos o vaquerías para luchar contra las moscas.
Las formaciones principales son polvos hidrosolubles y líquidos
hidromiscibles, que se diluyen en agua para el rociado superficial o
aéreo de los cultivos. Las proporciones normales del principio activo
son de 0,15-1,0 kg/ha. La exposición humana ocurre principalmente
durante la preparación y aplicación de estos productos y por ingestión
de residuos que quedan en los alimentos cosechados (véase la
sección 5.3.1.4).
3. Transporte, distribución y transformación en el medio ambiente
En estudios de laboratorio se ha observado que el metomilo se
adsorbe poco al suelo. Se ha demostrado una adsorción débil a los
minerales arcillosos, sobre todo la ilita; la adsorción a la materia
orgánica del suelo es 50 veces mayor, pero sigue siendo relativamente
escasa. Prácticamente no hay desorción del residuo adsorbido. Estas
características llevan a prever que el metomilo tendrá movilidad en el
suelo.
En condiciones medioambientales naturales, su degradación
abiótica por hidrólisis o fotólisis es lenta o no se produce.
La degradación aerobia en el suelo es alrededor de dos veces más
rápida que la anaerobia. Las semividas notificadas para el metomilo
en el suelo varían entre unos días y más de 50. Las condiciones secas
retrasan la degradación. En la práctica, la mayor parte de las
aplicaciones en el campo deberían dar lugar a una semivida de
alrededor de una semana.
En condiciones de campo, el metomilo no sufre lixiviación en el
suelo a profundidades superiores a 20-30 cm y no contamina las aguas
subterráneas.
El 14C-metomilo aplicado a las hojas de las plantas es
absorbido, pero no transportado a otras partes de la planta. Se si lo
aplica en el sistema radicular, la planta lo absorbe y el componente
principal del residuo que queda es el propio metomilo. Los productos
volátiles derivados de su descomposición son CO2 y acetonitrilo. El
resto de la actividad se incorpora a los componentes naturales de la
planta, tales como lípidos, ácidos del ciclo de Krebs y azúcares. La
semivida del metomilo en el follaje de la planta es de unos pocos
días.
No se han encontrado indicios de acumulación del metomilo en
truchas irisadas expuestas al compuesto durante 28 días en un sistema
de flujo continuo.
4. Niveles medioambientales y exposición humana
Los análisis de diversas fuentes de agua tras la aplicación de
las dosis recomendadas del compuesto indican que los niveles de
metomilo en las aguas subterráneas probablemente serán muy bajos o
inferiores al límite de detección (< 0,02 mg/litro).
En los cultivos de productos alimenticios y de otro tipo se
observan niveles bajos de residuos de metomilo en el momento de la
recolección; su concentración depende de diversos factores, como la
cantidad aplicada, el periodo transcurrido desde la última aplicación
y el tipo de cultivo. Los residuos están formados básicamente por
metomilo.
Los residuos de metomilo en los productos lácteos no son
detectables o son muy bajos. No se observaron residuos detectables de
metomilo ni del metabolito MHTA en la leche ni en los tejidos
(< 0,02 mg/kg) de vacas lactantes que habían recibido en el pienso
cápsulas de metomilo en una concentración equivalente a 80 mg/kg
durante 28 días. No se detectó la presencia de metomilo en los huevos
ni en los tejidos de gallinas ponedoras a las que se habían
administrado cantidades de 1 ó 10 mg/kg en los alimentos durante
cuatro semanas.
En los Estados Unidos de América se hicieron análisis de
regímenes completos de alimentación y de determinados alimentos; en
los estudios de muestreo correspondientes, las concentraciones de
metomilo resultaron inferiores al límite de detección o muy bajas.
Los niveles de residuos se reducen aún más por efecto de procesos
tales como el lavado, pelado y guisado de los alimentos.
En estudios sobre la exposición de quienes regresan a zonas
tratadas, en particular en las condiciones del desierto de California,
se ha observado que los trabajadores que volvieron a los viñedos
cuando los residuos foliares que podían desprenderse se habían
reducido a 0,1 µg/cm2, sufrieron la mayor exposición en la parte
superior del cuerpo y en la cabeza durante el atado de los racimos y
en la parte superior del cuerpo y en las manos durante la vendimia.
La recogida y el embalado de las uvas de mesa dieron lugar a la
exposición más baja. La exposición por inhalación fue mínima.
Tras haberse rociado con metomilo plantas de pepino y tomate, las
concentraciones en el aire del invernadero llegaban a 4,7 µg/m3 al
día siguiente del rociado. Tres y siete días después del rociado, las
concentraciones de metomilo en la zona de respiración ascendían a
14,5 y 0,7 µg/m3, respectivamente. Los valores del metomilo en el
agua de lavarse las manos oscilaban entre 10 y 322 µg por hora de
trabajo en un invernadero. Esto indicaba que la vía de exposición
cutánea era más importante que la inhalación y que los intervalos
previos al regreso al lugar tratado deberían basarse en los datos
sobre la exposición cutánea.
5. Cinética y metabolismo en animales de laboratorio
La absorción, el metabolismo y la excreción del metomilo tras la
administración oral a ratas es muy rápida, completándose el proceso en
unos pocos días. En un plazo de siete días después de la
administración a ratas de 5 mg/kg de peso corporal de metomilo
radiomarcado, el 54% se excretó en la orina, el 2-3% en las heces y el
34% en el aire expirado (en cinco días). Después de siete días, en
los tejidos y en el esqueleto quedaba el 8-9% de la dosis de 14C,
que se había incorporado a los constituyentes endógenos. La mayor
concentración de radiactividad se detectó en la sangre (equivalente al
2% de la dosis).
Los componentes metabólicos principales en el aire expirado por
las ratas eran el anhídrido carbónico y el acetonitrilo, en una
proporción de 2:1. El principal metabolito en la orina fue el
derivado mercaptúrico del metomilo, que era igual al 17% de la dosis.
No se detectó metomilo ni su derivado oxima.
La vía metabólica propuesta comprende el desplazamiento del grupo
S-metilo por el glutatión, seguido de una transformación enzimática
que da lugar al derivado mercaptúrico. Otra vía es la hidrólisis, por
la que se produce MHTA, que se descompone rápidamente hasta dar
anhídrido carbónico. Otra vía posible es la conversión del
sin-metomilo (la forma insecticida) en su anti-isómero, que sufre
reacciones de hidrólisis, recomposición y eliminación hasta dar
acetonitrilo. El metomilo se metaboliza de forma semejante en el
mono, salvo que el derivado mercaptúrico es un componente secundario
en la orina.
La penetración del 14C-metomilo una hora después de haber sido
aplicado a ratones por vía cutánea en una solución de acetona se
estimó en un 85%. Para entonces, el 3% de la dosis se hallaba presente
en la sangre, el 5% en el hígado y el 13% se había excretado. En un
plazo de 8 horas la excreción total era del 54,5%.
La descomposición y eliminación rápidas del metomilo en la rata,
junto con su falta de acumulación en los tejidos, son comparables a lo
observado en rumiantes.
El metomilo sufre una descomposición completa en las vacas y
cabras que han recibido una dosis. No se detectaron metomilo ni su
derivado oxima en la leche ni en los tejidos de estos animales. Se
puso de manifiesto que el producto se metabolizaba e incorporaba a los
componentes naturales de la leche y del hígado.
No se detectó la presencia de nitrosometomilo después de haberse
incubado 14C-metomilo con nitrito sódico en un macerado de carne
curada, en condiciones que simulaban las del estómago.
6. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
El metomilo administrado por vía oral tiene una elevada toxicidad
aguda, con una DL50 oral de 17-45 mg/kg de peso corporal en la rata.
También es muy tóxico para la rata por inhalación; en aerosol, la
CL50 a las 4 horas es de 0,26 mg/litro. La toxicidad cutánea es muy
baja; la DL50 es de más de 2000 mg/kg de peso corporal en conejos
(piel intacta) y > 1000 mg/kg de peso corporal en ratas (piel
raspada). Los signos de toxicidad aguda son los que cabe esperar de
un inhibidor de la colinesterasa, entre otros salivación profusa,
lacrimación, temblor y contracción pupilar. La recuperación de estos
efectos fue rápida. En los órganos examinados no se observaron
efectos patológicos graves. El metomilo no irrita ni sensibiliza la
piel, pero irrita levemente los ojos.
La administración repetida de metomilo con los alimentos durante
periodos más largos no produjo aumento de los efectos tóxicos ni
acumulación. Las ratas y perros cuya alimentación contenía metomilo
en cantidades de hasta 250 mg/kg y 400 mg/kg, respectivamente, durante
13 semanas no mostraron signos de toxicidad ni acusaron mortalidad.
Las ratas que recibieron con los alimentos 250 mg/kg mostraron una
pequeña disminución en el aumento del peso corporal, niveles de
hemoglobina más bajos e hiperplasia eritroidea moderada de la médula
ósea. El NOEL en ratas fue de 50 mg/kg en los alimentos (equivalentes
a 3,6 mg/kg de peso corporal por día). Los conejos que recibieron
aplicaciones cutáneas repetidas de metomilo en dosis de hasta
500 mg/kg de peso corporal por día durante 21 días mostraron
hiperactividad y una reducción de la actividad colinesterásica
plasmática y cerebral con la dosis más elevada. El NOAEL en este
estudio fue de 50 mg/kg de peso corporal por día.
Se realizaron estudios de larga duración en ratas que recibían
metomilo en los alimentos en concentraciones de 0, 50, 100 ó 400 mg/kg
y en ratones que recibían 0, 50, 75 ó 200 mg/kg. Los efectos de la
dosis más alta en las ratas fueron una disminución del aumento de peso
y una reducción de la concentración de hemoglobina y del valor
hematócrito. El NOEL fue de 100 mg/kg de alimentos, equivalente a
5 mg/kg de peso corporal por día. En el estudio en ratones, se
observó aumento de la tasa de mortalidad y una reducción de la
hemoglobina y del número de eritrocitos en los dos niveles de dosis
más elevados. El NOEL fue de 50 mg/kg de alimentos, equivalente a
8,7 mg/kg de peso corporal por día. En un estudio de dos años sobre
toxicidad en perros (0, 50, 100, 400 ó 1000 mg/kg de alimentos) con la
dosis más elevada se detectaron síntomas de toxicidad en algunos
animales, junto con anemias de ligeras a moderadas. El NOEL fue de
100 mg/kg de alimentos, equivalentes a 3 mg/kg de peso corporal por
día.
En estudios de dos años en ratas y ratones no se encontró ningún
indicio de aumento de la incidencia de tumores relacionado con el
tratamiento, signo de que el metomilo no es carcinógeno. No fue
genotóxico en célula de bacterias o de mamíferos in vitro y las
pruebas realizadas para determinar la presencia de lesiones primarias
del ADN en células de bacterias y de mamíferos in vitro dieron
resultados negativos; un estudio cromosómico in vivo de médula ósea
de rata también dio resultados negativos. El potencial citogenético
del metomilo en linfocitos humanos in vitro se puso de manifiesto
mediante un aumento del número de micronúcleos y de aberraciones
cromosómicas. El metomilo no produjo efectos embriotóxicos ni
teratogénicos en ratas ni en conejos en dosis de hasta 400 mg/kg en
los alimentos y de 16 mg/kg de peso corporal por día administrados por
sonda, respectivamente; a esos niveles produjo efectos tóxicos en las
madres. En un estudio de reproducción de tres generaciones en ratas
con dosis de 50 ó 100 mg/kg de alimentos (equivalentes a 5 ó 10 mg/kg
de peso corporal por día), el metomilo no tuvo efectos sobre los
índices de fecundidad, gestación o lactación y no se produjeron
anomalías graves relacionadas con el tratamiento.
No se observó neurotoxicidad retardada después de la
administración de una sola dosis o de una serie de dosis de metomilo.
En un estudio de cinco meses en el que se suministraron a ratas
800 mg/kg de alimentos se observó una reducción importante de la
actividad de la colinesterasa sanguínea sólo en las fases iniciales.
En un estudio de 28 días, la administración de esa misma dosis con los
alimentos dio lugar a una reducción ligera solamente de la actividad
de la colinesterasa cerebral. Esto indica que la inhibición de la
actividad de la colinesterasa por el metomilo es reversible
rápidamente en los animales durante los periodos de ingestión.
In vitro, la actividad de la eritrocito colinesterasa humana fue
seis veces más sensible a la acción inhibidora del metomilo que la de
la rata, aunque los índices de reactivación espontánea fueron
semejantes.
Los resultados obtenidos en estudios realizados con varias
especies mostraron que la atropina es el antídoto de eficacia más
general contra la intoxicación por metomilo.
7. Efectos en el ser humano
Las notificaciones de intoxicaciones accidentales y suicidas con
metomilo proporcionan alguna información sobre los niveles de efectos
y la recuperación. Tres de cinco víctimas accidentales de
intoxicación por una comida contaminada murieron antes de que
transcurrieran tres horas desde la ingestión. Se estimó que las
víctimas habían consumido alrededor de 12-15 mg de metomilo/kg de peso
corporal. Una mujer de 31 años y su hijo de seis, que murieron a
causa de un envenenamiento deliberado, tenían en el hígado
concentraciones de metomilo de 15,4 y 56,5 mg/kg, respectivamente.
Las dosis estimadas fueron de 55 mg/kg de peso corporal para la madre
y de 13 mg/kg de peso corporal para el hijo. Seis horas después de
haber ingerido unos 2,25 g de metomilo, la sangre de una mujer
contenía 1,6 mg de metomilo/kg. A las 22 horas de la ingestión no se
podía detectar la presencia de metomilo y la mujer se estaba
recuperando.
Un operario que manipulaba plaguicidas había mezclado, sin tomar
precauciones, una formulación de metomilo en polvo para el rociado de
hortalizas; antes de una hora manifestó síntomas de intoxicación y
después de 12 horas la actividad de colinesterasa sanguínea se había
reducido al 40% de la normal; a las 36 horas la recuperación llegaba
al 80% de la actividad normal. Otros operarios que habían tomado las
precauciones recomendadas no acusaron ningún síntoma ni efectos en la
actividad de la colinesterasa eritrocitaria y plasmática durante
actividades de aplicación aérea de metomilo.
8. Efectos en organismos no destinatarios en el laboratorio y sobre
el terreno
No se observaron efectos en poblaciones de hongos y bacterias del
suelo, en particular en su acción nitrificante y en la actividad de la
deshidrogenasa después de haberse aplicado metomilo en las dosis
recomendadas.
En estudios de laboratorio se determinó una NOEC de
6,5 mg/litro para el crecimiento de las algas.
El metomilo tiene una toxicidad entre moderada y alta para los
peces, con una CL50 a las 96 horas de 0,5 a 2 mg/litro para una
serie de especies. En un estudio de mayor duración (21 días),
utilizando la formulación Lannate 20L (21,5% de metomilo), la CL50
para los alevines de trucha fue de 1,3 mg/litro. En un estudio de
toxicidad de 28 días durante la primera fase de la vida de piscardos
(Pimaphales promelas), la máxima concentración intoxicante aceptable
(MATC) estimada fue de > 57 y < 117 µg/litro.
En pruebas de toxicidad aguda con otros organismos acuáticos,
Daphnia magna fue una de las especies más vulnerables al metomilo, con
una CL50 de 0,032 mg/litro a las 48 horas. En un estudio de 21 días
sobre la capacidad de supervivencia, crecimiento y reproducción de
Daphnia magna, la máxima concentración intoxicante aceptable para el
metomilo fue de > 1,6 y < 3,5 µg/litro.
El metomilo es tóxico para las abejas melíferas; se ha notificado
una DL50 por contacto de 1,29 µg/abeja y una DL50 oral de
0,2 µg/abeja.
Se ha evaluado la toxicidad aguda del metomilo en varias especies
de aves; los valores característicos de la DL50 oral aguda son de
10 mg/kg de peso corporal en palomas y de 34 mg/kg de peso corporal en
la codorniz japonesa. El metomilo es relativamente menos tóxicos si
se ingiere con los alimentos; en ese caso, la CL50 en ocho días es
de 1100 mg/kg de metomilo en los alimentos para la codorniz Colinus
virginianus y de 2883 mg/kg de metomilo en los alimentos para el
pato silvestre. En estudios de una generación realizados durante 18 a
20 semanas, la NOEC fue de 150 mg/kg de metomilo en los alimentos para
la codorniz Colinus virginianus y para el pato silvestre.
No se observaron efectos sobre Colinus virginianus tras su
exposición a una serie de aplicaciones de metomilo por rociado en las
dosis recomendadas. En dos estudios sobre poblaciones de aves
silvestres, después de haberse rociado con metomilo tierras forestales
y campos de lúpulo en las dosis recomendadas, no se observó ningún
cambio manifiesto en la actividad de las aves ni se produjeron efectos
ni mortalidad relacionados con el tratamiento. Los depósitos de grasa
de las aves canoras de los bosques tratados se redujeron en
comparación con el grupo testigo; se consideró que se trataba de un
efecto indirecto de la disminución de los insectos que les sirven de
alimento.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
El metomilo es un inhibidor de la carbamato colinesterasa
mediante un mecanismo bien conocido de acción tóxica. En estudios
realizados en animales se ha observado una toxicidad aguda
particularmente alta por vía oral y respiratoria, pero la toxicidad
por vía cutánea es baja. Los signos de toxicidad aguda en animales
son los característicos de los inhibidores de la colinesterasa. La
reversibilidad de la acción tóxica aguda es rápida; los supervivientes
se recuperan con rapidez de los signos tóxicos y de la inhibición de
la colinesterasa en la sangre y el cerebro. La pronta remisión de los
efectos tóxicos se debe a la rápida reversibilidad de la inhibición de
la colinesterasa, reversibilidad favorecida por la eliminación rápida
del metomilo del organismo. Los datos sobre intoxicaciones humanas
accidentales e intencionales muestran que el nivel de toxicidad aguda
del metomilo en el ser humano es semejante al observado en los
animales de laboratorio.
Habida cuenta de la reversibilidad rápida de la acción del
metomilo durante los periodos de ingestión, en los estudios realizados
raramente se observaron síntomas de toxicidad aguda e inhibición de la
colinesterasa sanguínea. Los resultados más constantes en los
estudios de mayor duración con niveles de alimentación más elevados
fueron una reducción del aumento del peso corporal en roedores y una
disminución del número de glóbulos rojos en roedores y perros. No se
observaron indicios de carcinogenicidad potencial en tres estudios de
larga duración realizados en roedores. El compuesto dio resultados
negativos en las pruebas de genotoxicidad in vitro en las que se
investigaron varios puntos finales, en cambio mostró potencial
citogenético en linfocitos humanos. El resultado de un estudio
cromosómico realizado in vivo sobre médula ósea de rata fue
negativo.
Se identificaron los NOEL en cada uno de los estudios de larga
duración realizados con animales, teniendo en cuenta la reducción del
aumento del peso corporal y el número de eritrocitos. Los NOEL
resultaron ser de 5 mg/kg de peso corporal por día en ratas, 8,7 mg/kg
de peso corporal por día en ratones y 3 mg/kg de peso corporal por día
en perros. Como esos estudios no revelaron diferencias acentuadas
entre especies en cuanto a los efectos tóxicos, se debería utilizar el
NOEL en el perro, que es de 3 mg/kg de peso corporal por día, a
efectos de la estimación del riesgo en el ser humano.
La adsorción del metomilo al suelo es de baja a moderada, y
prácticamente no hay desorción. La degradación aerobia en el suelo
(con una semivida de alrededor de una semana) es aproximadamente dos
veces más rápida que la degradación anaerobia.
La aplicación de metomilo a hojas de plantas da lugar a una
absorción rápida de casi la mitad de la cantidad aplicada (la otra
mitad se adsorbe) y no hay indicios de transporte. La concentración
del metomilo absorbido en los cultivos de productos alimenticios
disminuye rápidamente hasta alrededor del 5% en una semana.
Varios invertebrados acuáticos, en particular los dáfnidos, son
muy sensibles al metomilo, con CL50 del orden de 10 a 100 µg/litro.
Los peces, tanto de agua dulce como estuarinos, son menos
sensibles, oscilando la CL50 entre 0,5 y 7 mg/litro. Dada la escasa
persistencia del metomilo y su toxicidad aguda relativamente baja para
los peces, se supone que el riesgo es bajo.
En las dosis de aplicación recomendadas, el metomilo no menoscaba
la actividad microbiana en suelos templados.
Este producto está clasificado como muy tóxico para las abejas
melíferas y su DL50 tópica es de aproximadamente 0,1 µg/abeja.
La DL50 aguda por vía oral para varias especies de aves oscila
entre 10 y 40 mg/kg de peso corporal. Los valores de la CL50 en la
alimentación (cinco días) varían entre 1100 y 3700 mg/kg de alimentos.
El metomilo, sobre todo en forma de gránulos, constituye un riesgo
agudo para las aves, pero no se prevé que la ingestión de alimentos
contaminados con metomilo pueda causarles la muerte.
La elevada toxicidad aguda del metomilo para los mamíferos de
laboratorio indica que existe un peligro semejante para los mamíferos
silvestres.
10. Conclusiones
Teniendo en cuenta las características cualitativas y
cuantitativas de la toxicidad del metomilo, el Grupo de Trabajo llegó
a la conclusión de que 0,03 mg/kg de peso corporal al día
probablemente no causarían efectos adversos en el ser humano por
ninguna vía de exposición.
A lactating goat was given 14C-methomyl by capsule, twice a
day, for 10 days at a dose rate equivalent to 20 mg/kg in feed. Milk,
blood, urine and faeces were sampled daily and selected tissues taken
within one day of the last dose. No methomyl or the metabolite MHTA
was detected in any of the samples. Approximately 16% and 7% of the
activity was excreted in urine and faeces, respectively, and about 8%
appeared in the milk and 17% in expired air. The milk activity
reached a plateau after 3 days and was equivalent to approximately
2 mg/kg. At this time the lactose component contained about 11-13% of
the milk activity. Hexane extracts, containing the triglyceride
components, contained 26-37% of the milk activity and the casein
component 8-9%. This indicates that methomyl had been completely
broken down and incorporated into natural constituents of milk.
Acetonitrile was identified as a volatile metabolite in milk and blood
(Harvey, 1980).
The examination of the liver fractions showed that the
radio-activity derived from methomyl was found in glycerol,
glycerol-3-phosphate, fatty acids, neutral lipids and insoluble
protein. This indicates a metabolic pathway via acetonitrile and
acetate into the natural occurring constituents in the liver. The
breakdown of methomyl and distribution of metabolic products in the
liver was shown to be similar in the cow (Monson, 1989).
Acetonitrile, CO2 and reincorporation products derived from
acetate found after the application of methomyl to plants (section
4.2.1) are similar to those identified in the above animal studies.
The proposed metabolic pathway for methomyl in animals is shown
in Fig. 1.
6.4 Elimination and excretion
Rat and monkey studies show that methomyl is very rapidly
metabolized and eliminated, the processes being largely completed
within 24 h.
Rats fed 200 mg methomyl/kg in diet and then given 5 mg
14C-methomyl/kg orally (see section 6.3 for detail) showed a 50%
elimination of 14C in expired air in 3 days in the form of carbon
dioxide and acetonitrile in the proportion of 1:2. Urinary excretion
of 14C was 27% in one day (Harvey et al, 1973).
In a more detailed study, where male and female rats were given
5 mg 14C-methomyl/kg orally (see section 6.3), approximately 53% of
the radioactivity was excreted in urine over 7 days, 45% of the dose
being excreted in the first 6 h. Faecal excretion contributed only
2-3% over 7 days. The other major path of elimination (over 5 days)
was via expired air as carbon dioxide (22% of dose) and acetonitrile
(12% of dose). Of this, 18% of the dose was expired as CO2 within
6 h and 10% as acetonitrile in 24 h. Overall, most of the
radiolabelled dose (80%) had been eliminated in 24 h with an estimated
half-life of 5 h. There was no obvious difference in the amount or
rate of excretion between males and females. The single oral dose
given to these animals (5 mg/kg) produced mild clinical signs of
cholinesterase inhibition which disappeared within 2 h of dosing
(Hawkins et al., 1991).
When methomyl was radiolabelled on the carbonyl group, the
elimination of 14CO2 was very rapid and equivalent to about 85% of
the oral dose in male and female rats. When the labelling was at the
- 14C=N group, the overall elimination in expired air in 24 h was
30% in the form of CO2 and acetonitrile (in the proportion of 2:1).
When 14C-MHTA was administered in the same way the expired component
was mainly 14CO2, equivalent to 22% of the dose. The anti-isomer
of methomyl mainly produced acetonitrile in the expired air,
equivalent to 28% of the dose given orally to rats. Rats given the
anti-MHTA by intraperitoneal injection produced ten times more
acetonitrile than those given the syn-MHTA. The urine from rats
treated orally with 14C-methomyl or MHTA contained 25-35% of the
radioactivity over a 24-h period (Huhtanen & Dorough, 1976).
In monkeys given 5 mg 14C-methomyl/kg orally, approximately 32%
of the dose was excreted in urine in 7 days, with 34% as CO2 and 5%
as acetonitrile in the expired air. Most of this was excreted in the
first 24 h. Faecal excretion amounted to only 3-4% (Hawkins et al.,
1992).
After dermal application of 14C-methomyl to mice (see section
6.1), the total excretion (urine plus CO2 plus faeces), as a
proportion of the applied dose, was 0.2% in 15 min, 12.9% in 60 min
and 54.5% in 480 min (Shah et al., 1981).
6.5 Retention and turnover
The absorption, metabolism and excretion of methomyl in the rat
are very rapid. No methomyl can be detected within the tissues or
excretory products within a few hours of dosing. Most of the dose is
eliminated within 24 h with an estimated half-life of 5 h (Hawkins et
al., 1991). Metabolic products, mainly in urine and expired air, are
also eliminated rapidly; tissue concentrations are very small and
lower than blood levels. There is no evidence for accumulation in
tissues. A similar picture exists for the metabolism of methomyl in
ruminant species.
6.6 Reaction with body components
Methomyl is a potent direct inhibitor of acetylcholinesterase in
both insects and mammals. The carbamylated enzymes undergo rapid
spontaneous reactivation (see section 7.8.1).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The results of the single exposure of technical methomyl by the
oral, dermal and inhalation routes in various species are shown in
Table 4.
Methomyl is very toxic by the oral route. In the rat, the signs
of toxicity are those expected from a cholinesterase inhibitor and
include profuse salivation, lacrimation, tremor, abnormal posture,
pupil constriction, diarrhoea and prostration. At lethal doses the
rats died within hours. Survivors began to recover within several
hours and had fully recovered within days. No compound-related
changes were seen in organs subjected to histopathological
examination.
The acute dermal toxicity of methomyl is very low. No deaths
occurred in rats or rabbits at the doses shown in Table 4. The
compound has high toxicity by the inhalation route in the rat, with
affected animals showing typical signs of cholinesterase inhibition.
No target organ effect was seen upon histopathological examination.
An LD50 of approximately 8 mg/kg was obtained when methomyl was
injected intraperitoneally in rats (Dashiell, 1972).
The acute oral toxicity of methomyl formulations is in proportion
to the amount of a.i. present and, therefore, they are less toxic than
methomyl itself (Table 5). The same pattern is seen with the results
of acute inhalation studies. The signs of toxicity in the oral and
inhalation studies are those shown by the active ingredient. The
dermal toxicity of the formulations is very low.
Ocular toxicity was shown by a solid formulation containing 92.4%
methomyl when 10 mg was introduced into rabbits' eyes. Typical
anticholinesterase effects were seen up to 20 min after treatment,
including pupillary constriction of the treated eyes, incoordination,
tremors and profuse salivation. All of the effects had disappeared by
the next day (Sarver, 1991g).
7.2 Short-term exposure
Six male rats were given methomyl in peanut oil by gavage at a
dose of 5.1 mg/kg body weight per day, 5 times per week for 2 weeks.
The signs of toxicity were the same as those exhibited in acute oral
studies (section 7.1) but they regressed during the second week of
dosing. All animals survived, and there were no compound-related
histopathological changes (Kaplan & Sherman, 1977).
Table 4. Acute toxicity of technical grade methomyl
Species Sex Route Vehicle Testa Result Reference
Rat M oral peanut oil LD50 17 mg/kg Sherman (1966)
Rat F oral peanut oil LD50 23.5 mg/kg Sherman (1968a)
Rat M oral water LD50 45 mg/kg Trivits (1979)
Rat M oral water LD50 34 mg/kg Sarver (1991a)
Rat F oral water LD50 30 mg/kg Sarver (1991a)
Rat M dermal water LD50 abraded skin > 1000 mg/kg Morrow (1972)
Rat M inhalation aerosol 4-h ALC 0.30 mg/litre Foster (1966a)
Rat M inhalation vapour 4-h ALC 0.04 mg/litre Foster (1966b)
Rat M inhalation spray 4-h LC50 0.45 mg/litre Hornberger
(1967)
Rat M/F inhalation aerosol 4-h LC50 0.258 mg/litre Panepinto
(1991a)
Rabbit M oral acetone/ ALD 30 mg/kg Sherman (1968c)
peanut oil
Table 4. (cont'd).
Species Sex Route Vehicle Testa Result Reference
Rabbit M/F dermal water LD50 intact skin > 2000 mg/kg Sarver (1991b)
Dog M oral capsule ALD 20 mg/kg Sherman (1968d)
Guinea-pig M oral acetone/ ALD 15 mg/kg Kaplan &
peanut oil Sherman (1977)
Monkey M/F oral water ALD 40 mg/kg Kaplan &
Sherman (1977)
a ALC = approximate lethal concentration; ALD = approximate lethal dose
Table 5. Acute toxicity of some methomyl formulations
Formulation Species Route Vehicle LD50 (mg/kg) or LC50 Reference
(% a.i.) (mg/litre)a
Lannate 40 SP rat oral water 61 (male) Sarver (1992a)
(41.6%) 73 (female)
Lannate 20 L rat oral methanol 129 Lheritier (1991a)
(21.5%)
Lannate 12.5 L rat oral water 208 Sarver (1991c)
(12.7%)
Lannate 40 SP rabbit dermal (intact skin) water > 2000 Sarver (1992b)
(41.6%)
Lannate 20 L rat dermal (intact skin) methanol > 4000 Lheritier (1991b)
(21.5%)
Lannate 40 SP rat inhalation (4 h) aerosol 0.66 Kelly (1992)
Lannate 20 L rat inhalation (4 h) aerosol 1.3 Jackson et al. (1991)
(21.5%)
Lannate 12.5 L rat inhalation (4 h) aerosol 3.7 Panepinto (1991b)
(12.7%)
a Results are for both sexes, unless stated otherwise
Groups of 10 male and 10 female rats were fed diets containing 0,
10, 50, 125/500 (125 mg/kg for 6 weeks, 500 mg/kg for remaining
period) or 250 mg methomyl/kg for 13 weeks. Assessments included
haematological, biochemical and histopathological examinations. There
were no mortalities nor clinical signs due to treatment in any group.
A slight decrease in body weight was seen in males at the two higher
dose levels and in females at 125/500 mg/kg. This was accompanied by
lower food consumption. The only change attributed to methomyl on
histopathological examination was moderate erythroid hyperplasia in
the bone marrow of males fed 250 mg/kg. Lower haemoglobin values in
the males of this group at two months could be linked to this effect.
Histopathological examination of animals in the 125/500 mg/kg group
did not reveal any changes due to treatment. The noobserved-effect
level based upon body weight change was considered to be 50 mg/kg
diet, equivalent to 3.56 mg methomyl/kg body weight per day (Paynter,
1966; Busey, 1966).
When groups of four male and four female one-year-old dogs were
fed diets containing 0, 50, 100 or 400 mg methomyl/kg for 3 months, no
effects were seen in a range of clinical, haematological, biochemical
and histopathological assessments (Kaplan & Sherman, 1977).
A dose of 200 mg methomyl/kg body weight was applied as a 5%
aqueous solution once a day 5 times per week for 3 weeks to the intact
or abraded skin of five male, five female rabbits. Each dose was
occluded for 6 h and then the site washed with water. There were no
mortalities in animals with intact skin; clinical signs included nasal
discharge, wheezing and diarrhoea. With abraded skin, signs of
toxicity, including laboured respiration, salivation and tremors,
appeared within one hour of dosing, and three animals died, one after
the first application and the other after the eighth application. No
treatment-related changes were seen upon histopathological examination
(Kaplan & Sherman, 1977).
Groups of rabbits were treated dermally (intact clipped skin)
with doses of 0 (10 males, 10 females), 5, 50 (5 males 5 females) or
500 (10 males, 10 females) mg methomyl/kg body weight per day for 21
days. The doses were applied in water each day and occluded for 6 h.
Half the males and females in the control and highest dose groups were
killed at the end of the dosing regime, together with all animals in
the other groups. The remaining animals in the control and highest
dose groups were allowed to recover over a 14-day period and then
killed. The only change noted clinically was a greater incidence of
hyperactivity at 500 mg/kg. There were no effects on body weight
gain, haematology, blood biochemistry, organ weights or organ
histopathology. Plasma cholinesterase activity was depressed to 36%
and 55% of normal at day 21 in highest dose males and females,
respectively. Brain cholinesterase activity was depressed to 48% and
68%, respectively, in males and females at this dose, but red cell
cholinesterase activity, although slightly decreased, was considered
to be biologically insignificantly affected. In males, at 50 mg/kg,
the plasma cholinesterase activity was depressed to 77% and there was
no inhibition of red blood cell cholinesterase activity of either sex.
All the depressed cholinesterase activities had returned to control
values at the end of the 14-day recovery period. The
no-observed-effect level, based upon changes in plasma cholinesterase
activity, was 5 mg/kg body weight per day in males and 50 mg/kg in
female rabbits (Brock, 1989).
Formulations of methomyl have also been tested by repeated dermal
application to rabbits at doses equivalent to 50 or 100 mg
methomyl/kg. No effects were seen after ten daily doses of Lannate L
(25.5% methomyl). Ten daily doses of Lannate LV (30% methomyl)
produced mild skin irritation at the higher dose level. Plasma
cholinesterase activity was somewhat depressed at both dose levels but
returned to normal over a 14-day recovery period (McAlack, 1973,
Edwards, 1980).
A Lannate dust containing 45% methomyl was the subject of a
3-month inhalation study in male rats. Exposure was at 14.8 mg/m3
for 4 h/day, 5 days/week; the mass median dynamic diameter of the dust
was 4.4 µm. There were no effects on body weight, organ weight,
histopathology or red cell cholinesterase activity. Plasma
cholinesterase activity was depressed to about 29% of the
pre-treatment activity in samples taken 4 h after the last exposure
(Ta'Naka et al., 1987).
7.3 Long-term exposure
Groups of 35 male and 35 female rats were fed diets containing
methomyl at 0 (2 groups), 50, 100, 200 or 400 mg/kg for 22 months.
Five males and 5 females per group were killed at 12 months for gross
and histopathological examination. Assessments were made throughout
the study for clinical condition, body weight, food consumption,
haematology, blood and urine biochemistry, organ weight and
histopathology. Growth of males at the highest dose was significantly
lower than that of controls over the first year. Growth of males at
200 mg/kg and females at 400 mg/kg was also lower at this time, but
not significantly. Food consumption over the first 26 weeks was
significantly lower than that of controls for the males fed 200 and
400 mg/kg. At 18 and 22 months there was a trend towards lower
haemoglobin values in treated female groups. Compound-related
increases in the incidence and severity of extra medullary
haematopoiesis were observed in the spleen of females fed 200 or
400 mg/kg. Compound-related changes were also seen in the kidneys of
both sexes at 400 mg/kg, characterized by vacuolization of epithelial
cells and hypertrophy of the proximal convoluted tubules. On the
basis of the body weight and haematopoietic findings in this study,
the long-term no-observed-effect level for methomyl in rats was
considered to be 100 mg/kg diet (Kaplan & Sherman, 1977).
In a 2-year study on rats, methomyl was fed in the diet to groups
of 80 males and 80 females at levels of 0, 50, 100 or 400 mg/kg. The
animals were observed and weighed regularly, and haematological and
blood and urine biochemical measurements were made at 3, 6, 12, 18 and
24 months. Red cell cholinesterase activity was assayed in additional
groups at 1, 2 and 4 weeks and 3, 6, 12 and 20 months. Brain
cholinesterase activity was assayed at 12 and 20 months. Extensive
histopathological examination was performed on animals killed at
scheduled intervals, on animals dying and on animals killed at the end
of the study.
There was a significant reduction in body weight gain in both
sexes at 400 mg/kg over the first 12 months but not during the
subsequent 12 months. There were no clinical signs attributable to
treatment. Survival rate was the same among the groups and there was
an acceptable number of animals at termination (40-50%). Erythrocyte
counts, haemoglobin values and haematocrits were significantly lower
in females at 400 mg/kg. There were no compound-related changes for
red cell or brain cholinesterase activity nor for other biochemical
parameters. Organ weights were unaffected by treatment. The incidence
of bone marrow hyperplasia, focal hyperplasia in the adrenal medulla
and focal degeneration in the adrenal cortex was slightly increased in
males at 400 mg/kg. The no-observed-effect level in the rat was
there-fore confirmed as 100 mg methomyl/kg diet, equivalent to about
5 mg/kg body weight per day (Kaplan, 1981).
A comprehensive long-term (2 years) study on the mouse was
initiated with 80 males and 80 females per group at levels of 0, 50,
100 and 800 mg methomyl/kg diet. Because of early high mortality, the
level of the highest dose group was reduced to 400 mg/kg at week 28
and then to 200 mg/kg at week 39. The level of the mid-dose group was
also reduced to 75 mg/kg at week 39. Regular clinical observations
and body weight and food consumption measurements were undertaken.
Haematological analysis was carried out at 4, 13, 26, 52, 78 and 104
weeks. Organ weights were measured and an extensive histopathological
examination carried out on all animals dying or killed. The mortality
rate was higher than that of controls at the highest and medium dose
levels throughout the study, and was slightly higher, in male mice
only, at the low dose level in the latter stage of the study. There
were no clinical findings due to treatment and no effects on body
weight gain or food consumption. Some effects were noted on red cell
parameters such as haemoglobin values and red cell counts during the
first 26 weeks at the medium and highest dose levels. However, these
were not apparent during the remainder of the study, after these dose
levels had been decreased. No effects were observed on organ weights
or microscopic findings. The no-observed-effect level was considered
to be 50 mg/kg in diet, equivalent to approximately 10 mg/kg body
weight per day (Serota et al, 1981).
A 2-year dog study was initiated with dietary levels of methomyl
of 0, 50, 100, 400 and 1000 mg/kg with four males and four females per
group. After one year, one male and one female from each group was
killed for interim examination. Clinical condition was regularly
observed and routine measurements made on body weight, food
consumption, and haematological and bio-chemical parameters. After 2
years the surviving animals were killed, organs were weighed and a
histopathological examination was undertaken.
No clinical signs due to treatment were seen in animals at 50,
100 and 400 mg/kg. One highest dose female died after 8 weeks and its
replacement also died within 18 days after showing compound-related
toxic signs. Two males at this dose level exhibited tremors,
salivation and incoordination during week 13. Slight to moderate
anaemia was noted in the highest dose animals at 13 weeks and this
persisted in one of the males. No effects were seen on body weight or
biochemical parameters. Histopathological effects were noted in the
kidneys and spleens of animals at 400 and 1000 mg/kg. Changes in
kidneys were characterized by increased pigmentation and epithelial
swelling, and in the spleen as extra-medullary haematopoiesis and
increased pigmentation. A minimal to slight increase in bile duct
proliferation was seen in livers of dogs at 1000 mg/kg, and bone
marrow activity was also slightly increased in animals at this dose
level. The no-observed-effect level was therefore 100 mg/kg diet,
equivalent to about 3 mg methomyl/kg body weight per day (Kaplan &
Sherman, 1977).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
The skin irritation potential of methomyl was evaluated in six
male rabbits with 0.5 g of the test material, moistened with deionized
water, applied to the back of each animal. Each site was covered with
a semi-occlusive dressing and exposure continued for 4 h. The skin
was examined and evaluated 1, 24, 48 and 72 h after the end of the
exposure period. No dermal irritation was seen in any of the animals
during the study and, therefore, methomyl is classified as
non-irritant to skin (Sarver, 1991d).
Methomyl formulations were also evaluated for skin irritation
potential using the same test method as described above. The
substances tested were a solid formulation containing 92.4% methomyl
(Sarver, 1991e) and two liquid formulations containing approximately
20% and 12.7% methomyl (Clement, 1987a; Sarver, 1991f). None of the
formulations irritated rabbit skin.
In a test on 10 guinea-pigs, methomyl was applied as a 60% paste
in propylene glycol or as a 26% solution in Cellosolve and the 24-h
reaction was noted as being mildly irritating (Kaplan & Sherman,
1977).
7.4.2 Eye irritation
Methomyl, either as 10 mg dry powder or 0.1 ml of a 10% solution
in propylene glycol, was tested in rabbits' eyes (2 per group). One
of each pair was washed after 20 sec and examination of each eye made
at 1, 2, 3, 4 and 6 h after treatment and for up to 6 days thereafter.
Only mild conjunctivitis was seen on the day of treatment but no
corneal injury was noted (Kaplan & Sherman, 1977).
A solid formulation containing 92.4% methomyl was tested in the
eyes of six male rabbits. A quantity of 10 mg was introduced into one
eye of each rabbit and evaluations of the reaction were undertaken
after 1, 24, 48 and 72 h. Pupillary constriction was evident after
one hour but was not present the next day (see also section 7.1).
Only very mild conjunctival chemosis and redness was seen at 24 h
after treatment and the formulation was considered to be non-irritant
(Sarver, 1991g).
Two liquid formulations, containing approximately 20% and 12.7%
methomyl, respectively, were tested for eye irritancy using the method
described in the previous paragraph. In both cases, the formulation
produced ocular irritation in the form of conjunctival redness and
chemosis, iritis and corneal opacity within one hour and were
classified as irritant. These effects had disappeared after 21 days.
(Clement, 1987b; Sarver, 1991h).
7.4.3 Skin sensitization
In a test to evaluate the skin sensitization potential of
methomyl, the material was applied as a paste to the abraded skin of
five guinea-pigs 3 times a week for 3 weeks; five other guinea-pigs
received four intradermal injections of 0.1 ml of a 1% solution. The
animals were then challenged after a 2-week rest period. Methomyl was
shown not to be a skin sensitizer in this test (Kaplan & Sherman,
1977).
A closed-patch repeated insult dermal sensitization evaluation
(Beuhler test) was undertaken in guinea-pigs with technical methomyl.
The test compound (300 mg), moistened with water, was applied to the
intact skin of each of 10 males and 10 females for 6 h once a week for
3 weeks. Two weeks after the last induction treatment, the animals
were challenged with another 300 mg methomyl for 6 h. No erythema was
observed in either the induction or challenge phases, and therefore
methomyl did not produce delayed contact sensitivity in the
guinea-pig. Positive control animals treated with DNCB produced the
expected sensitization responses (Armondi, 1991a).
A solid formulation containing 94.2% methomyl, subjected to the
evaluation described in the previous paragraph, also failed to produce
delayed contact hypersensitivity in guinea-pigs (Armondi, 1991b). The
same result was obtained with a liquid formulation containing 21.5%
methomyl when given at 0.5 ml per application (Mercier, 1991).
Another liquid formulation containing 12.7% methomyl was tested in the
Magnusson-Kligman guinea-pig sensitization maximization procedure and
was found to be a moderate skin sensitizer under the conditions of the
test (Armondi, 1992).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
7.5.1 Embryotoxicity and teratogenicity
Teratogenicity studies have been undertaken in the rat and
rabbit.
Pregnant rats (25 per group) were fed 0, 50, 100 or 400 mg
methomyl/kg diet during days 6-15 of gestation. The dams were
observed and weighed regularly and then killed on day 21 of gestation.
At that time the uterus was removed, and the number of corpora lutea,
implantation sites, live and dead fetuses, resorptions and weight of
live fetuses were recorded. About one half of the fetuses from each
litter were taken for skeletal examination and the remainder for soft
tissue examination. The pregnant rats at 400 mg/kg had significantly
lower body weight and ate less food than those in the control group.
There were no clinical signs of toxicity and all rats survived the
test period. Parameters of pregnancy and fetal development, such as
the number of litters with partial or total resorptions and fetal
weight, were not affected in the test groups compared to controls.
There were no treatment-related abnormalities in the fetuses.
Methomyl was therefore not embryotoxic or teratogenic in the rat at
levels up to 400 mg/kg diet, equivalent to 34 mg/kg body weight per
day (Rogers et al., 1978).
Groups of 20 pregnant rabbits were given 0, 2, 6 or 16 mg
methomyl/kg body weight per day by gavage on days 7-19 of gestation.
The animals were observed and weighed regularly and killed on day 29.
The uteruses were then examined for pregnancy, number of
implantations, early and late resorptions and live or dead fetuses.
Delivered pups were weighed and examined for visceral or skeletal
abnormality. One pregnant rabbit at 6 mg/kg died, probably due to
gavage error. At 16 mg/kg there were seven deaths during the test
period due to the compound. Clinical signs at the highest dose level
included tremors, hyperactivity, excessive salivation, aggression and
convulsions. The no-observed-effect level for maternal toxicity was
therefore 6 mg/kg body weight per day. There was no effect on
pregnancy rate or on the average numbers of corpora lutea,
implantations, resorptions or live fetuses per dam in treated groups
compared to controls. There were no gross external visceral or
skeletal variations due to treatment. Methomyl is therefore not
embryotoxic or teratogenic to the rabbit at doses up to 16 mg/kg, at
which dose maternal toxicity occurred (Christian et al., 1983).
In another study, pregnant rabbits (12 per group) were fed diets
containing up to 100 mg methomyl/kg. The pregnancy rate was low in
all groups, including controls. No effects were noted on a range of
parameters including reproductive indices, fetal body weight or
visceral and skeletal abnormalities. However, due to experimental
inadequacies the results are difficult to assess (FAO/WHO, 1987).
7.5.2 Reproduction studies
A three-generation (two litters per generation) rat study was
undertaken to test the potential reproductive effects of methomyl (Lu,
1983). The compound was fed at levels of 0, 50 or 100 mg/kg diet to 10
males and 20 females for approximately 90 days post weaning prior to
first mating to produce the F1a litter. Test diets were fed through
to subsequent re-mating to produce the F1b litter. Ten males and 20
females per group were then selected from the F1b litter and
continued on their respective diets for approximately 90 days to
produce F2a and then F2b litters. The same procedure was used to
produce subsequent litters of the next generation. Records were kept
of all matings, number of pregnancies, number of young in each litter
born alive or dead, the body weight of offspring at weaning, and the
respective reproduction and lactation indices. Ten males and 10
female offspring were selected from each group of the F3b litter for
histopathological examination. Methomyl did not affect the
reproductive performance of rats in this study. There were no effects
on fertility, gestation or lactation and no compound-related
abnormalities on gross examination. No treatment related changes were
seen upon histopathological examination of tissues from the offspring
in the F3b litter (Kaplan & Sherman, 1977).
A two-generation rat study was also carried out using high
dietary levels of methomyl (600 and 1200 mg/kg) as well as a lower
level of 75 mg/kg. The F0 parents (13 males, 26 females) were mated
after 100 days treatment and the F1 parents (20 males, 40 females)
after a minimum of 120 days. Spermatogenesis (based on sperm count)
was measured on adult males in both generations after breeding, in
addition to the standard assessments of reproductive performance.
Histopathological examination was undertaken on a selection of tissues
from parents and pups in both generations. Body weight depression in
parents, seen at all or some stages during treatment at 600 and
1200 mg/kg in both generations and at 75 mg/kg in the F1 generation,
was probably related to lower food intake. Reduced red blood cell
counts were seen in females at the highest dose level, together with a
small (15-25%) depression in plasma cholinesterase activity in both
sexes at this level. Decreases in litter size and live births were
shown for the highest dose F0 group and for all F1 groups for
which a clear no-observed-effect level was not established. Survival
during lactation in F1 pups (from F0 parents) was reduced at the
highest dose level, as was survival in highest dose F2 pups during
early lactation up to day 4. Pup body weight was slightly reduced at
75 mg/kg, significantly reduced at the two higher levels in the F1
generations and reduced at the 600 and 1200 mg/kg levels in the F2
generation. No histopathological changes due to treatment were seen
in any of the tissues examined in parents or offspring. No effects on
the reproductive parameters of fertility indices, gestation length or
male sperm counts were seen at dose levels up to and including 1200 mg
methomyl/kg in diet (Lu, 1983).
7.6 Mutagenicity
Numerous in vitro mutagenicity assays, to various end-points,
have been carried out on methomyl by several investigators (Table 6).
Methomyl did not show mutagenicity or cause primary DNA damage in
bacterial or mammalian cells in vitro. It showed cytogenetic
potential in human lymphocytes in vitro as indicated by an increase
in micronuclei and chromosomal aberrations.
In an in vivo study, the clastogenic potential of methomyl was
assessed by its ability to cause numerical and structural chromosomal
aberrations in rat bone marrow cells. Single doses of methomyl were
given by gavage to groups of 15 male and 15 female rats at doses of 2,
6 or 20 mg/kg body weight; five males and five females were killed at
6, 24 and 48 h after dosing and the bone marrow cells harvested. These
were examined for chromatid and chromosome breaks and for gaps and
other aberrations. The results showed that there was no significant
increase in the frequency of chromosomal aberrations and no
significant differences between the chromosome numbers and the mitotic
indices of the dosed groups and the controls (Farrow et al, 1984).
Table 6. Summary of results of in vitro mutagenicity assays and related end-point studies on methomyl
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
Prokaryotes
Point mutation Salmonella typhimurium - 50 nM negative Blevins et al. (1977a)
(5 strains)a
Point mutation S. typhimurium (5 strains) + and - up to 5000 µg/plate negative Moriya et al. (1983)
Escherichia coli (WP2hcr) + and - negative
Point mutation S. typhimurium (5 strains) + and - up to 1 mg/plate negative Waters et al. 1982
Point mutation E. coli, WP2uvr A + and - up to 1 mg/plate negative Waters et al. 1982
DNA damage E. coli pol A - 1 mg/disc negative Waters et al. 1982
W3100, p 3478
DNA damage Bacillus subtil recM45 - 1 mg/disc negative Waters et al. 1982
Yeast
DNA damage Saccharomyces cerevisiae + and - 1-3.5% negative Waters et al. 1982
D3
Mammalian cells
DNA damage Human lung fibroblast WI-38 + and - 10-7-10-3M negative Waters et al. 1982
DNA damage Rat hepatocytes (UDS) - 1 µM-75 mM negative Vincent (1985)
Table 6 (cont'd).
Test Organism Activation + Dose range Mutagenic Reference
and/or -c potential
DNA damage Human skin cell DNA - 10-5M negative Blevins et al. (1977b)
Gene mutation Chinese hamster V79 cells + and - 1-10 mM negative Wojciechowski et al. (1982)
Gene mutation Chinese hamster ovary - 10-55 mM negative McCooey et al. (1984)
(CHO) cells, HGPRT + 100-350 mM
Chromosome Human lymphocytesb NA 0.02-0.54 mM positive Bonatti et al. (1994)
aberrations (whole blood culture)
Sister-chromatid Human lymphocytesb NA 0.02-0.54 mM negative Bonatti et al. (1994)
exchange (whole blood culture)
Micronuclei Human lymphocytesb NA 0.01-0.09 mM positive Bonatti et al. (1994)
(whole blood culture)
DNA single strand Human lymphocytesb NA 0.06-2 mM negative Bonatti et al. (1994)
breaks (whole blood culture)
DNA oxidative Human lymphocytesb NA 0.25 - 1 mM negative Bonatti et al (1994)
damage (whole blood culture)
a Plate incorporation and spot tests
b The metabolic activity of the cell cultures was demonstrated by the positive control response
c NA = not applicable
Methomyl was tested in the sex-linked recessive lethal assay with
Drosophila melanogaster at concentrations of 4 and 10 mg per litre
in the test medium. No increase in mutation rate was observed in this
assay (Waters et al., 1982).
A formulation (Lannate 20) containing 20% methomyl, but of
otherwise unstated composition, caused an increase in the number of
sex-linked recessive lethals, but with no observed translocations.
The same formulation increased the incidence of abnormal sperm and
frequency of chromosomal aberrations in mice (Hemavathy &
Krishnamurthy, 1987a,b).
7.7 Carcinogenicity
The carcinogenic potential of methomyl was assessed in the
long-term rat and mouse studies already described in section 7.3.
In the earliest study, groups of 35 male and 35 female rats were
fed 0 (2 groups), 50, 100, 200 or 400 mg methomyl/kg diet for 22
months. No differences were seen in the incidence of neoplastic
lesions between control and treated groups (Kaplan & Sherman, 1977).
In the more recent rat study, groups of 80 males and 80 females
were fed 0, 50, 100 or 400 mg methomyl/kg diet for 2 years. The
mortality rates were similar in all groups and there was an overall
survival of 40-50% by the end of the study. More than 30 tissues/rat
were examined microscopically from animals killed or dying during the
study and at interim and terminal sacrifice. There was no indication
of any differences between groups in the appearance of any individual
tumour nor were there any differences between groups for the number of
tumour-bearing rats or those with benign or malignant tumours (Kaplan,
1981).
The 2-year mouse study was initiated at dietary levels of 0, 50,
100 and 800 mg/kg with groups of 80 males and 80 females. By week 39
the highest dose level had been reduced to 200 mg/kg and the 100 mg/kg
level to 75 mg/kg (see section 7.3 for details). Mortality was higher
in the highest dose male and female groups than in controls throughout
the study and was also somewhat higher in the mid-dose group.
Adequate numbers of mice survived to the termination of the study
(30-50% for female groups and 38-68% for male groups).
Histopathological examination on more than 30 tissues per mouse was
undertaken on animals killed or dying during the study and at terminal
sacrifice. There were no differences between groups for the numbers
of tumour-bearing mice or those with benign or malignant tumours
(Serota et al., 1981).
Methomyl did not transform hamster embryo cells in a
trans-placental host-mediated assay, nor did the cultured cells from
this assay induce tumours when injected subcutaneously into young
adult nude mice (Quarles et al., 1979).
7.8 Other special studies
7.8.1 Cholinesterase studies in vivo and in vitro
Due attention must be given to the measurement of cholinesterase
activity in blood and brain samples because of the fast spontaneous
reactivation of the methomyl-inhibited enzyme. It is important to
analyse samples in the minimum possible time after sampling and to use
an analytical method which requires the minimum of sample dilution and
shortest assay time. If these criteria are not followed then
cholinesterase inhibition due to methomyl can be misinterpreted.
In groups of rats fed 0, 50, 100, 200, 400 or 800 mg methomyl per
kg diet for 79 days, only those fed the highest dose showed blood
acetylcholinesterase activity inhibition as assayed by the Ellman
method. Males showed blood cholinesterase activity inhibition of
25-40% early in the study (by 11 days) while females showed this
inhibition towards the end of the study period (Singles, 1970a). In
an extension of this study, the blood acetyl-cholinesterase activity
of rats fed 400 or 800 mg/kg was assayed by the Ellman method and a pH
stat method after 5 months of feeding. In neither group was there an
effect on blood acetyl-cholinesterase activity (Singles, 1970b).
Methomyl was fed to groups of rats in the diet at levels of 0,
100, 400 or 800 mg/kg, and blood was taken for red cell and plasma
acetylcholinesterase activity assay at 1, 7, 14, 21 and 28 days.
Brain acetylcholinesterase activity was also measured on rats at 14
and 29 days. All assays were undertaken by the Ellman method. There
were small depressions of acetylcholinesterase activity (up to 20%) in
red cells and plasma of males and females at 800 mg/kg. Brain
acetylcholinesterase activity was also slightly depressed (<17%) at
this dose level. There were no consistent findings of
acetylcholinesterase inhibition at the two lower levels of 100 and
400 mg/kg (Barnes, 1978).
Lannate, containing 90% methomyl, was given to 14 male rats at
41 mg/kg body weight by oral intubation each day for 8 successive
days. The animals were killed 24 h after the last dose and blood was
taken for the assay of serum cholinesterase activity by a colorimetric
method. The activity was depressed by 40% compared to controls
(Borady et al., 1983).
Cholinesterase activity in plasma and red cells of rats was
assayed after the dermal application of methomyl. Four groups of rats
were treated dermally with 200 mg methomyl per rat and blood samples
were collected 2, 4, 6 and 24 h after treatment. Plasma
cholinesterase activity was markedly reduced at each time interval
compared to controls (22-50% of control value) while red cell
cholinesterase activity was less affected (80-91% of control value).
Groups of rats were also treated dermally with 25 or 100 mg per rat
and blood was collected at 24 and 72 h after treatment. Plasma
cholinesterase activity was depressed at both levels and at both time
intervals, but red cell acetylcholinesterase was unaffected (Henry,
1981).
Lannate powder, containing 45% methomyl, was the subject of
inhalation studies using a single 4-h exposure to 9.9 mg/m3 or
repeated exposures to 14.8 mg/m3 (see section 7.2 for details).
Plasma cholinesterase activity was markedly depressed (by 50%) for 4 h
after the single exposure, but showed recovery almost to the control
value within 20 h. Red cell cholinesterase activity was not
inhibited. Plasma cholinesterase activity was less affected (29%
depression) 4 h after the last of the repeat exposures than after the
single exposure (Ta'Naka et al., 1987).
The I50 value (the molar concentration of inhibitor giving 50%
reduction in enzyme activity in given experimental conditions) for
methomyl was determined in vitro for human and rat red blood cell
acetylcholinesterase activity using a modified Ellman method. Human
acetylcholinesterase was approximately six times more sensitive to
methomyl's action than rat acetylcholinesterase (I50 values of
0.265 × 10-5 mol/litre and 1.56 × 10-5 mol/litre, respect-ively).
Regeneration studies using a gel filtration method showed that
methomyl-inhibited rat acetylcholinesterase regenerates at a faster
rate than the human enzyme, with half-lives of 26.6 and 38.0 min,
respectively (Carakostas, 1987).
7.8.2 Neurotoxicity
Hens given a single oral (LD50) dose of 28 mg methomyl/kg
either died within 10 min or survived. Survivors showed toxic signs
of lacrimation and salivation, but no evidence of wing or leg
paralysis. After a 21-day recovery period, no abnormalities were
observed upon histopathological examination of the sciatic nerve.
Doses much higher than the oral LD50 (up to 200 mg/kg) were given to
hens without causing mortality after they had been pre-treated with
atropine sulfate subcutaneously at 10 mg/kg. Wing or leg paralysis
was not observed and there were no histopathological abnormalities in
the sciatic nerve after a 21-day recovery period. Birds treated with
tri- o-cresyl phosphate (TOCP) at 500 mg/kg showed the leg paralysis
and sciatic nerve degeneration expected of a positive control
substance (Krauss & Stula, 1967).
7.8.3 Potentiation studies
Potentiating properties were determined by administering one-half
the oral LD50 of methomyl to rats, followed immediately by one-half
the LD50 of another anticholinesterase pesticide, and then recording
the resultant mortality over 14 days. Out of 18 pesticides tested,
only Sevin and Ronnel showed the ability to increase mortality
indicative of a potentiation effect (Sherman, 1967). In another study,
the inhibition of cholinesterase activity in rat blood after the oral
administration of methomyl alone or in combination with another
anticholinesterase pesticide was used to measure potentiating effects.
Methomyl in combination with methyl parathion or phosdrin was shown to
be potentiating, while antagonism occurred when it was combined with
dimethoate (Henry, 1975).
Rats (10 per sex and per group) were given methomyl at 200 mg/kg
diet and ethanol as a 10% aqueous solution, either separately or
together, for a period of 12 weeks. There was some evidence of
increased effects as a result of the combined administration resulting
in decreased body weights from weeks 2 (females) and 4 (males) and
increased relative organ weights for adrenals (males) and kidneys
(females). In male rats, the combination of the two compounds
increased hepatic triglycerides and free fatty acids and decreased
brain acetylcholinesterase activity more than that expected from
either compound alone (Antal et al., 1979). Ethanol administered in
the diet to rats, at a level equivalent to 25% of the calorie intake,
did not potentiate erythrocyte acetylcholinesterase inhibition by
methomyl coadministration at 200 mg/kg in the diet (Bracy et al.,
1979).
In female rats only, the combination of methomyl and caffeine
retarded growth, increased the relative weight of kidneys, spleen,
adrenals, liver and heart, depressed glucose-6-phosphate dehydrogenase
activity, and increased aniline hydroxylase and glucose-6-phosphatase
activity (Bedö & Cieleszky, 1980).
7.8.4 Antidote studies
The antidotal properties of several agents against the toxicity
of methomyl have been examined in a number of species.
Potential antidotes were administered within one minute after
oral lethal doses of methomyl (30 or 60 mg/kg) were given to rats. Of
the agents used, atropine sulfate given intraperitoneally as a single
dose of 50 mg/kg proved to be the most effective antidote. Other
agents used which were less effective or ineffective were
pyridine-2-aldoxime methiodide (PAM) and tetra-ethylammonium chloride
(TEAC) (Sherman, 1968a). Rats were also used in a study of the
effectiveness of obidoxime or N-methyl-pyridinium-2-aldoxime
methane-sulfonate (P2S) as antidotes alone or in combination with
atropine sulfate. Methomyl was injected subcutaneously as solutions
of logarithmically graded concentrations (1 ml/kg body weight), and at
the onset of signs of toxicity atropine sulfate (17.4 mg/kg), P2S
(50 mg/kg) and obidoxime (90 mg/kg) were injected subcutaneously
either alone or in a combination. Lethality was reduced by atropine
sulfate or P2S but obidoxime was ineffective. Atropine sulfate in
combination with P2S also was effective (Natoff & Reiff, 1973).
Mice fed a lethal dose of 100 mg methomyl/kg in pellets and then
given TEAC by injection 10 min later survived, and resumed normal
activity within another 10 min (Andrews & Miskus, 1968). Atropine
sulfate (50 mg/kg intraperitoneal) was shown to have antidotal
activity in guinea-pigs given methomyl orally at lethal single doses
of 15-60 mg/kg (Sherman, 1968b). Dogs given a single oral dose of 10
or 20 mg methomyl/kg by capsule were protected against the toxic
effects if atropine sulfate was given very quickly, i.e. within a few
minutes. The antidote was more effective when given intravenously
than when given intramuscularly or orally (Sherman, 1968c).
The antidotal effectiveness of atropine administered
intravenously or orally at 1 or 10 mg/kg and hexamethonium or TEAC
given intravenously at 10 mg/kg was evaluated in rhesus monkeys dosed
with 20 or 40 mg methomyl/kg orally. Atropine at 1 mg/kg, given
intravenously or orally, was effective as an antidote for the
sublethal dose of 20 mg methomyl/kg. Atropine at 10 mg/kg orally,
administered immediately after the appearance of toxic signs, was an
effective antidote for the lethal dose of 40 mg methomyl/kg. Recovery
occurred within 2-5 h of the methomyl dose. TEAC at 10 mg/kg
(intravenous) also prevented the death of one monkey after a lethal
dose of methomyl, although recovery took longer than after atropine.
Hexamethonium did not appear to be very effective (Teeters, 1968).
7.8.5 Other studies
Lannate (90% methomyl) was given to 14 male rats at the high dose
level of 41 mg/kg by intubation each day for 8 days and the animals
were killed 24 h after the last dose for hepatic and serum assays.
Liver weight was not increased, nor was there histopathological
evidence of fatty deposit, but total lipids including cholesterol and
phospholipids were increased. The treatment also increased the level
of serum triglycerides, phospholipids and free fatty acids and
increased the activity of GOT, GPT and alkaline phosphatase enzymes
(Borady et al., 1983). Lung triglyceride, cholesterol and
phospholipid contents were not affected when rats were exposed to
Lannate (45% methomyl) dust at 14.8 mg/m3 by inhalation for 4 h/day,
5 days/week for 3 months (Ta'Naka et al, 1987).
Single oral doses of 2 or 10 mg methomyl/kg increased pancreatic
chymotrypsin, lipase and amylase activities in male and female rats.
Rats fed 100, 400 or 800 mg/kg diet for 10 days showed elevated serum
cholesterol in females only. Hepatic aminopyrine demethylase and
aniline hydroxylase activities were increased in female rats at 400
and 800 mg/kg. When rats were given 100 or 200 mg/kg diet, females
fed 200 mg/kg showed elevation of total serum lipids and cholesterol.
The females also showed decreased hepatic glucose-6-phosphatase
activity and vitamin A at this level (Bedö & Cieleszky, 1980).
The effect of methomyl on a number of serum constituents was
studied in rats given oral doses of 6.8 mg/kg per day, 6 days/week for
4 weeks. Increases were seen over the 4-week period for serum
glucose, cholesterol, GOT and GPT, while decreases were seen for serum
total protein albumin, globulin and cholinesterase activity. However,
since control values did not seem to be available for the 4-week
period, it was difficult to evaluate the significance of the results
for methomyl (Saleh, 1990a,b,c).
7.9 Factors modifying toxicity
It has been shown that synthesized nitrosomethomyl is mutagenic
in vitro and is capable of producing stomach tumours in rats
(Blevins et al., 1977a,b; Lijinsky & Schmahl, 1978). However, when
methomyl is incubated with nitrite and macerated meat under simulated
stomach conditions, there is no evidence that nitrosomethomyl is
formed (see section 4.3).
7.10 Mechanisms of toxicity - mode of action
As a member of the carbamate class of compounds, methomyl has a
well-known mode of action via inhibition of the enzyme
acetylcholinesterase at nerve junctions. A detailed description of
the mechanism of action of carbamates on cholinesterases is given in
Environmental Health Criteria 64: Carbamate pesticides: a general
introduction (IPCS, 1986). Studies with methomyl show that the onset
of toxic action is rapid and that, in common with many other
carbamates, the toxic effect is rapidly reversible. Because of this,
it is important to use appropriate assay methods when measuring
cholinesterase activities during toxicity studies. Failure to allow
for the rapid reversibility of the action can lead to underestimation
of enzyme inhibition.
The acute toxic action of methomyl is characterized by signs of
poisoning typical of anticholinesterase action, i.e. lacrimation,
profuse salivation, tremor and pupil constriction. Surviving animals
quickly show signs of recovery, often within hours. The toxic action
can be countered by the anticholinergic antidote atropine sulfate.
The potency of methomyl is greatest when given by the oral or
inhalation routes of administration. It has very low acute toxicity
when given dermally, presumably because during the slower absorption
phase there is time for recovery from toxic action and thus the effect
is never fully exerted.
8. EFFECTS ON HUMANS
8.1 General population
8.1.1 Accidental and suicidal poisoning
Five Jamaican fishermen prepared a meal to which they
accidentally added methomyl instead of salt. Within minutes of eating
the meal, three of the men were badly affected, twitching, trembling
and frothing at the mouth, and died within 3 h. One of the other two
also showed toxic symptoms, while the other was unaffected. Both
survivors were given atropine and the symptomatic patient recovered
within 2 h after treatment. Postmortem examination of the dead men
revealed highly congested stomach lining, lungs, trachea and bronchi.
Analysis showed that part of the meal (roti) contained about 1%
methomyl. It was estimated that the victims had consumed about
12-15 mg methomyl/kg (Liddle et al., 1979).
A 31-year-old woman committed suicide, using a methomyl
preparation, together with her three children, one of whom (a
9-year-old son) survived. Postmortem examination showed congestion of
the stomach mucous membranes and the lungs. Analysis of organs of the
mother and a 6-year-old son showed highest methomyl concentrations in
the liver (15.4 and 56.5 mg/kg, respectively). Large amounts of
methomyl were present in the stomach contents and it was estimated
that the doses were 55 mg/kg for the mother and 13 mg/kg for the son
(Araki et al., 1982).
A woman attempted suicide by ingesting about 2.25 g methomyl.
After 6 h, methomyl was present in the blood at a concentration of
1.61 mg/kg and in the urine at 10.9 mg/litre; at 15 h the levels were
0.04 mg/kg and 0.25 mg/litre, respectively, and at 22 h methomyl could
not be detected (Noda, 1984).
Symptoms and treatment were described for 11 patients who had
suffered methomyl poisoning in Spain over a 5-year period.
Intoxication was accidental in six cases and suicidal in the other
five. The time interval between exposure and admission for treatment
averaged 2.8 h. All of the subjects showed cholinergic symptoms;
plasma cholinesterase activity was normal in four cases and moderately
reduced in the others. Treatments applied included gastric lavage,
washing the skin, administration of activated charcoal and small doses
of atropine, according to the symptoms involved. All the patients
recovered within 24-48 h (Martinez-Chuecos et al., 1990).
A blood level of 0.57 mg methomyl/litre was measured in a pilot
who had died as a result of a crash while spraying a solution of
methomyl and chlorothalonil in methanol. Analysis was not undertaken
for chlorothalonil and methanol (Driskell et al., 1991).
A 79-year-old man and his 73-year-old wife attempted suicide by
ingesting methomyl powder. The woman died within 19 h but the husband
survived after treatment by gastrolavage, followed by administration
of atropine sulfate. The serum methomyl concentration in the woman
was 44 mg/kg 1 h after ingestion and 0.2 mg/kg in the blood at
autopsy. The methomyl blood concentration of the man was
0.01-0.1 mg/kg 28 h after ingestion (Miyazaki et al., 1989).
8.2 Adverse effects of occupational exposure
Pesticide operators were reported to be affected after handling
powder formulations of methomyl. In one case, an operator mixed a
powder formulation and sprayed vegetables without taking any special
precautions. He displayed poisoning symptoms within 1 h. His blood
cholinesterase activity had decreased to 40% of normal after 12 h but
had recovered to within 80% of normal after 36 h. Other operators, a
mixer-loader, pilot and markers, using recommended safety precautions,
did not show any effects on their red cell or plasma cholinesterase
activities after handling liquid formulations and subsequent aerial
application of methomyl (Simpson & Bermingham, 1977).
In a survey of agricultural applicators in California, USA, in
1982-1989, methomyl was reported to be involved in 129 out of 5371
exposure-related illnesses due to pesticides. For the period
1982-1985 and for the same area, methomyl was considered responsible
for 47 illnesses out of 238 reported cases. Exposure was described as
either short term (< 3 days), longer term (> 3 days) or accidental
(Brown et al., 1989). In 1986, in a summary of reported
pesticide-related illnesses and injuries in California, methomyl was
identified as the probable causative agent in 7 out of 1065 confirmed
occupational cases. These cases were described as "..having some
likelihood of being pesticide related" (Edmiston & Maddy, 1987).
An investigation of workers in a pesticide formulation plant
revealed that 11 out of 102 workers had been hospitalized due to
work-related illness. Most frequent were symptoms of exposure to
methomyl and methaemoglobinaemia due to 3,4-dichloroaniline (Morse et
al., 1979).
Significant T-wave changes (decreased height, inversion, and
leftward deviation of T-wave axis) were shown in 10 out of 22 spraymen
applying methomyl for five days under field conditions. These changes
reverted to pre-exposure levels within one week. Sufficient
information was not available to evaluate the significance of these
findings for operator exposure, and further studies are required to
fully understand their significance (Saiyed et al., 1992).
Two case reports of allergic contact dermatitis with exposure to
methomyl have been described. In the first case, a 26-year-old woman
had gradually worsening itchy hand eczema for six months when working
in a plant nursery, pollinating, pricking out and potting plants
sprayed with methomyl (Lannate) solution. After she avoided touching
the sprayed plants or used polyvinyl gloves she became free of eczema.
She also reacted positively to a 1% aqueous Lannate solution,
indicating that Lannate was a cause of the hand eczema. The second
patient had already had hand eczema for 14 years with recent
worsening. As soon as she changed her job and no longer had contact
with Lannate her eczema disappeared (Bruynzeel, 1991).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
The effect of methomyl on sewage microorganisms was assessed
indirectly by monitoring total oxygen demand (TOD) over a 24-h period.
A mixture of activated sludge, phosphate buffer and a simulated waste
consisting of Bactopeptone and meat extract was incubated with
methomyl at concentrations of 0, 1, 5, 10, 50 and 90 mg/litre. Only
at 90 mg/litre was there a slight effect on TOD (<10%), indicative of
a minor effect on microorganism activity (Belasco, 1972a).
A fine sand and two silt loam soils were treated with 18 mg
methomyl/kg, and the soil fungi and bacteria populations counted after
11 days. The effect on total microbial activity was determined by
measuring the CO2 evolved periodically over 56 days. Neither soil
microorganism populations nor CO2 production was affected by the
high level of methomyl in the soils, when compared to controls
(Peeples, 1977).
When methomyl was incorporated into a silt loam sand at 0.5 mg/kg
together with ammonium sulfate, no effect on nitrification occurred
over a 6-week period. This level of incorporation represented the
recommended field treatment of 0.56 kg/ha. At ten times this level of
incorporation there was some delay (8-9 days) in reaching the 50%
nitrification level, but by 4 weeks the level was the same as that in
the control soil (Han, 1972).
Methomyl, in the form of Lannate 20L (20% a.i.), was incorporated
into a sandy and a loamy soil at rates of 1.87 and 18.7 mg a.i./kg and
incubated at 20°C for 28 days. Dehydrogenase activity, measured at 14
and 28 days, was unaffected by either rate of incorporation in both
soils. Ammonia nitrogen and nitrate nitrogen declined by about the
same extent in both soils over the 28-day period. This could have
been due to stimulation of nitrogen assimilation by the product
(Danneberg, 1991).
9.2 Aquatic organisms
9.2.1 Algae
The effect of methomyl on algal growth was assessed using
Selenastrum capricornutum as test species. The organism was cultured
under continuous illumination at 24°C with methomyl at concentrations
of 6.25-100 mg/litre for 120 h. Algal growth was monitored at
intervals over the exposure period by measuring the absorbency of
light at 665 nm. The EbC50, (median effective concentration for
inhibition of growth based on a comparison of areas under the growth
curves after "b" hours) after 72 and 120 h was 60 mg/litre. The
ErC50 (median effective concentration for growth inhibition based
on a comparison of maximum growth rates from "x" to "y" hours) from
24-48 h was 140 mg/litre (extrapolated value). The no-observed-effect
concentration was 6.25 mg/litre (Douglas & Handley, 1988).
A similar study was carried out using Lannate 20L (20% methomyl)
as test substance at concentrations of 6.25-200 mg/litre. The EbC50
(72 h) was 68 mg/litre, EbC50 (120 h) was 116 mg/litre and the ErC50
(0-24 h) was 67 mg/litre. A no-observed-effect concentration of
6.25 mg/litre was established (Douglas & Halls, 1991).
9.2.2 Fish
The acute toxicities (96-h LC50, etc.) for several species of
fish are shown in Table 7. The 96-h LC50 values lie mostly within
the range of 0.5-2 mg/litre. The most resistant species tested were
the cutthroat trout with a 96-h LC50 of 4-7 mg/litre. Generally,
within a species, the higher the water temperature the higher the
acute toxicity. Results for some methomyl formulations are shown in
Table 8. These show lower acute toxicity, corresponding to the lower
methomyl content. There are few descriptions of toxic signs in
affected fish. In an acute study with carp and tilapia, the fish sank
to the bottom of the tank and moved sluggishly, then became highly
excited 10 min before death (El-Refai et al., 1976). Shrimps became
lethargic before dying (Bentley, 1973; Sleight, 1973).
Two longer-term studies have been undertaken with methomyl and a
formulation. This included a 21-day flow-through study on fingerling
rainbow trout with Lannate 20L (21.5% methomyl) at 13°C. The
concentrations tested were 0.63, 1.3, 2.5, 5.0, 10 and 20 mg/litre,
equivalent to 0.14-4.3 mg methomyl/litre. No effects on fish length
or weight were noted throughout the study. Some increase in mortality
occurred, from day 12 onwards, in the 5 mg/litre group, and greater
dose-related increases were seen at the two higher concentrations
throughout most of the study. Surviving fish at these concentrations
showed increasing incidence of one or more of the signs of
discoloration, lying on the tank bottom, gasping, bloated stomach,
loss of equilibrium. The 21-day LC50 was calculated to be
6.1 mg/litre formulation (equiv. 1.3 mg methomyl/litre) and the
no-observed-effect concentration was 2.5 mg/litre formulation (Baer,
1991c).
Table 7. The acute toxicity of methomyl to fish (static test)
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Atlantic salmon (Salmo salar) 12 1.12 USDI (1978)
(0.48 g) 17 0.56 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) 12 2.00 USDI (1978)
22 0.86 USDI (1978)
23 2.00 Carter & Graves (1973)
22 1.00 USDI (1975)
Brook trout (Salvelinus fontinalis) 12 1.50 USDI (1978)
Carp (Cyprinus carpio) (5 cm) 25 2.8 Yoshida & Nishiuchi (1972)
Channel catfish (Ictalurus punctatus) 22 0.53 USDI (1978)
(1.8 g) 26 0.92 Carter & Graves (1973)
Cutthroat trout (Salmo clarkie) 10 6.8 USDI (1978)
10 4.05 USDI (1975)
Fathead minow (Pimephales promelas) - 2.8 Mayer & Ellersieck (1986)
(0.75 g)
Japanese goldfish (Carassius auratus) 25 2.7 Yoshida & Nishiuchi
(4 cm) (1972)
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi (1972)
Table 7. (cont'd).
LC50 (mg/litre)
Species Temperature (°C) 96 h 48 h 24 h Reference
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi (1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
Table 8. The acute toxicity of some methomyl formulations to fish (static test)
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Bluegill sunfish (Lepomis macrochirus) 24% kg a.i. - 0.7 USDI (1978)
Bluegill sunfish (Lepomis macrochirus) Lannate 20 L 21.5 5.1 Baer (1991a)
Bluegill sunfish (Lepomis macrochirus) Lannate 90% WDP - 2.1 USDI (1978)
Carp (Cyprinus carpio) Lannate 25 WP 22 4.7 - Ökolimna (1980)
12.5
Carp (Cyprinus carpio) (1.75 g) Lannate 24 EC 22-25 2.96 El-Refai et al. (1976)
Carp (Cyprinus carpio) (31.5 g) Lannate 24 EC 1.21 El-Refai et al. (1976)
Rainbow trout (Salmo gairdneri) Lannate 20 L 12.6 18 Baer (1991b)
Lannate 90% WDP 13 3.4 McCain (1971)
Tilapia (Tilapia nilocita) (1.5 g) Lannate 24EC 22-25 0.92 El-Refai et al. (1976)
Tilapia (Tilapia nilocita) (13.8 g) 0.88
Channel catfish (Ictaurus punctalus) 24% liquid form - 0.3 USDI (1978)
Channel catfish (Ictaurus punctalus) 29% liquid form 0.3 USDI (1978)
Brook trout (Salvelinus fontinalis) 24% liquid form - 2.2 USDI (1978)
Table 8 (cont'd).
LC50 (mg/litre)
Species Formulation Temperature (°C) 96 h 48 h Reference
Killifish (Oryzias latipes) (2.5 cm) 25 0.87 Yoshida & Nishiuchi
(1972)
Largemouth bass (Micropterus 22 1.25 USDI (1978)
salmoides) (3 g)
Loach (10 cm) 25 1.5 Yoshida & Nishiuchi
(1972)
Rainbow trout (Salmo gairdneri) 7 2.00 USDI (1978)
12 1.60 USDI (1978)
17 0.86 Mayer & Ellersieck (1986)
Sheepshead minnow (Cyprinodon 21.6 1.16 Boeri & Ward (1989)
variegatus)
In an early life-stage toxicity study, fathead minnow embryos and
larvae were exposed for 28 days to methomyl at concentrations of
27-491 µg/litre. The water was changed at the rate of 10 aquarium
volumes each 24 h and was maintained at a temperature of 25°C. Embryo
hatch and larval survival and growth was evaluated. The percentage
embryo hatch was not affected at any concentration. Larval survival
was significantly reduced at concentrations down to 117 µg/litre and
growth reduced at 243 and 491 µg/litre. The maximum acceptable
toxicant concentration was estimated to be >57 µg/litre and
<117 µg/litre (Driscoll & Muska, 1982).
9.2.3 Other aquatic organisms
The acute toxicity results for a variety of other aquatic
organisms are shown in Table 9. Daphnia magna appears to be one of
the most susceptible species to the acute toxic action of methomyl,
and the Eastern oyster the least susceptible. This difference was
further emphasized in two other studies. The 48-h EC50 for the
Lannate 20L formulation (21.5% methomyl) was 0.033 mg/litre
(equiv.0.007 mg methomyl/litre) for Daphnia magna neonates (Baer,
1991d). The 96-h EC50 for the Eastern oyster was > 140 mg/litre,
as measured by the effect of methomyl on shell deposition in a
flow-through test at 21.2 to 23.7°C (Ward & Boeri, 1991).
The effect of methomyl or its formulation on Daphnia magna was
studied in longer-term tests on survival, growth and reproductive
capacity. A study was initiated with < 24-h-old daphnids exposed to
measured methomyl concentrations of 0.7, 1.0, 1.6, 3.5, 7.5 or
13.8 µg/litre. The test was conducted over a 21-day period at 20°C
under semi-static conditions with 2-day renewal of the test
solutions. Methomyl was found to be stable in these solutions for up
to 72 h. Daphnid survival and growth were not affected at any
concentration. The number of young produced and number of young per
adult were reduced at 3.5, 7.5 and 13.8 µg/litre. There was some
delay in the first day of reproduction at all levels, but this was
considered to be biologically significant only at 3.5 µg/litre or
more, where the number of young was affected. The maximum acceptable
toxicant concentration was estimated to be between 1.6 and
3.5 µg/litre (Brittelli, 1982).
In another 21-day study, Daphnia magna were exposed to Lannate
20 (21.5% methomyl) at nominal concentrations of 0.63, 1.5, 3.4, 8.
18, 43, 100 or 232 µg/litre. The test conditions and parameters
measured were as described above. At 232 µg/litre all daphnids died
early in the study, but there was no effect on survival at the lower
concentrations nor was growth affected. The 21-day EC50, based upon
adult survival, was estimated to be 160 µg/litre (equivalent to 26 µg
methomyl/litre). The total young produced and number of young per
adult were decreased at 3.4 µg/litre but not at 8 µg/litre. The
21-day no-observed-effect concentration was therefore considered to be
8 µg/litre, equivalent to 2.1 µg methomyl/litre (Baer, 1991e).
Table 9. The acute toxicity of methomyl to other aquatic organisms (static test)
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Midge (Chironomus plumosus) mature 95% technical 22 0.088 (EC50) USDI (1978)
24% conc 0.032 USDI (1978)
Eastern oyster (Crassostrea embryo/ technical 20 4.0 (EC50) Ward & Boeri (1990)
virginica) larvae
Fiddler crab (Uca pugilator) 20 mm Lannate L 21 2.38 Bentley (1973)
(24%)
Grass shrimp (Palaemonetes 18 mm Lannate 90 21 0.049 Sleight (1973)
vulgaris
18 mm Lannate L 21 0.130 Bentley (1973)
(24%)
Gammarus pseudolimnaeus mature technical 17 0.92 USDI (1978)
Mud crab (Neopanope texana) 15 mm Lannate 90 21 0.41 Sleight (1973)
Mysid shrimp (Mysidopsis bahia) 10 day 99% technical 21.5 0.22 Ward & Boeri (1989)
Pink shrimp (Panaeus duorarum) 55 mm Lannate 90 21 0.019 Sleight (1973)
Stonefly (Pteronarcys dorsarta) naiad technical 7 0.034 USDI (1975)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Stonefly (Pheronarcella badia) Year Class 1 99% technical 0.069 Mayer & Ellersieck (1986)
Stonefly (Pheronarcella badia) Year Class 1 24% a.i. 0.06 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 95% technical 0.034 Mayer & Ellersieck (1986)
Stonefly (Slewala sp.) Year Class 1 24% a.i. 0.92 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 95% a.i. 0.343 Mayer & Ellersieck (1986)
Stonefly (Isogenus sp.) Year Class 1 24% a.i. 0.029 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 99% technical 0.92 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 0.72 Mayer & Ellersieck (1986)
Scud (Gammarus pseudolimneus) adult 24% a.i. 1.05 Mayer & Ellersieck (1986)
(flowthrough)
Water flea (Daphnia magna) neonate 95% technical 18 0.032 Goodman (1978)
Table 9. (cont'd).
LC50 (mg/litre)
Species Stage Methomyl Temperature (°C) 48 h 96 h Reference
product
Water flea (Daphnia magna) 1st instar 95% technical 21 0.009 (EC50) USDI (1978)
Water flea (Daphnia magna) - 24% conc 0.0076 USDI (1978)
Water flea (Daphnia magna) - Lannate 20L 20.4 0.033 Baer (1991e)
(21.5)
Water flea (Daphnia magna) 1st instar 95% technical 0.088 Mayer & Ellersieck (1986)
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The acute toxicity of a liquid (Lannate 20L) and a solid (Lannate
25WP) methomyl formulation to earthworms (Eisenia foetida andrei) was
determined over a 14-day period. The formulations were mixed with
artificial soil (70% industrial sand, 20% kaolite and 10% moss peat)
at six concentrations of 0-500 mg/kg (Lannate 20L) and 0-1000 mg/kg
(Lannate 25WP), with 40 earthworms per concentration. The 7-day
LC50 values were estimated to be 165 mg/kg and 147 mg/kg,
respectively, and the 14-day LC50 values 102 mg/kg and 87 mg/kg,
respectively (Armstrong et al., 1991; Caley et al., 1991).
Laboratory tests were undertaken to determine the broadcast
dosage of methomyl, as a 90% SP, required to kill 50% of earthworms
(night crawlers, Lumbricus terrestris L). The test substance was
mixed with moistened potting soil at six rates from 0 to 35 kg/ha with
40 worms per rate. The estimated rate causing 50% mortality was
11.4 kg methomyl/ha (Ruppel & Laughlin, 1977).
The biochemical changes in experimental snails, Eubania
vermiculata (Müller), were studied after treatment with 0.2%
methomyl in bran bait (w/w) for periods of 1, 3, 5, 7 and 10 days.
There was a significant reduction in total soluble proteins, lipids
and glycogenic content, and significant increase of glutamic
oxalocetic transaminase, glutamic pyruvic transaminase and
catalase activities (El-Wakil & Radwan, 1991).
A bran methomyl bait (0.5% w/w) was tested for its molluscicidal
activity on the white garden snail (Theba psiana) and compared with
that of four other oxime carbamate pesticides (aldicarb, aldoxycarb,
oxamyl and thiofanox). Methomyl had the most potent molluscicidal
activity. The time for 50% mortality of snails (LT50) for methomyl,
oxamyl, aldoxycarb, aldicarb and thiofanox was 2.31, 3.97, 4.69, 5.77
and 6.67 days, respectively. The activities of acetylcholine
esterase, acid phosphatase and alkaline phosphatase were inhibited by
the pesticides in line with their potency. GOT and GPT activities
were significantly increased by methomyl (Radwan et al., 1992).
Juvenile (4-week-old) laboratory reared earthworms
(Allolobophora caliginosa) were kept individually in soil treated
with methomyl for 7 days. Relative toxicity, i.e. concentration in
soil (mg/kg) causing zero growth, compared to the standard,
carbofuran, (0.10) was 0.54 (Martin, 1986).
The acute toxicity of methomyl to bees has been reported by
several investigators using different types of test. In one such test
groups of 20 bees were treated individually with a range of doses, the
methomyl being applied to the thorax of each bee in a 1 µl acetone
solution. The mortality was determined after 48 h and the LD50 was
calculated as 0.1 µg/bee (Meade, 1984). The acute toxicity of
methomyl by topical application to the honey-bee (Apis mellifera) was
compared to that of the Western Yellow jacket (Vespula pensylvanica)
after the pleural application of a 1 µl solution in acetone to each
insect. Methomyl was less toxic to the honey-bee, with a 48-h LD50
of 12 µg/g, than to the Yellow jacket (0.9 µg/g) (Johansen & Davis,
1972). A contact LD50 of 1.29 µg per bee has been reported by
Atkins et al., (1976) and an oral LD50 of 0.2 µg/bee by Clinch &
Ross (1970).
The speed of action of methomyl on honey-bees was assessed in a
special laboratory trial. A commercial formulation (90% DP) was
diluted in 33% sucrose solution to give concentrations equal to 1.5, 2
or 4 x LD90. These were fed to bees and the effects observed as two
stages; the first was characterized by very fast and erratic movement
and the second by the bees being unable to walk or fly. The time
intervals to reach each stage were recorded (20 bees), as were the
times taken for 50% and 90% bees to be affected. Methomyl was the
fastest acting of 10 pesticides tested, taking about 3-5 min to give a
50% effect level. It was suggested that this type of information was
needed when interpreting the results of field trials (Clinch & Ross,
1970).
In a tent trial, a methomyl formulation (35% methomyl) was
applied to Phacelia at a 0.3% concentration in the evening after bees
had finished flying and then rewetted early next morning prior to the
start of flight. Observations were made later that day and during the
following two days. Fewer bees visited the trial tent during this day
than visited the control tent. Large numbers of bees were found dead
in front of the hive and at the trial tent edges (about one order of
magnitude higher) on the first day. On the second day about three
times the number of dead bees were found when compared to the control.
Fewer bees were found dead when the trial was repeated with the spray
deposit left dry (Stute, 1983).
Methomyl was among 400 pesticides assessed in honey-bee
laboratory and field studies by the University of California over 20
years. The compound was placed in the highly toxic category, i.e.
severe losses should be expected if the pesticide is used when bees
are present at treatment or within a day thereafter. It was shown
that the contact LD50 of 1.29 µg/bee can be converted directly to
1.29 kg/ha, this being the application rate expected to cause a 50%
mortality among foraging bees in a treated field crop, at the time of
application or shortly thereafter. Several recommendations were
included to help minimize bee losses when spraying with insecticides,
including early morning or night applications, since there is less
risk to honey-bees at these times (Atkins et al., 1976).
9.3.2 Birds
The results for methomyl in acute oral and short-term dietary
studies in birds are shown in Tables 10 and 11. In an acute toxicity
study on the bobwhite quail, a dose of 5.62 mg/kg produced lethargy,
reduced reaction to external stimuli, loss of coordination and lower
limb weakness within 30 min of dosing. The birds recovered within 2 h.
The effects increased at higher doses and included salivation and wing
droop, but recovery in survivors was rapid, within hours to one day.
There were no mortalities at 10 mg/kg or less (Beavers, 1983).
Chickens given methomyl by capsule at 25 or 50 mg/kg showed muscle
tremor, ataxia, salivation, convulsions and death within 30 min
(Palmer & Schlinke, 1978).
In a one-generation study, 20-week-old bobwhite quail, 16 male
and 16 female per group, were fed diets containing 0, 50, 150 or
500 mg methomyl/kg for 20 weeks in their first breeding season.
Assessments included adult clinical health, weight gain and food
consumption and the reproductive parameters for numbers of eggs laid,
development of eggs, viability of embryos, percent hatchability,
offspring survival and eggshell thickness. There were no signs of
toxicity in the adults or of treatment-related mortality. There was a
slight decrease in body weight gain in males at 500 mg/kg up to week
8. No effects on reproductive parameters were seen at the two lower
dietary levels. No treatment-related effects were observed for egg
shell thickness at methomyl concentrations of 50, 150 or 500 mg/kg.
Mean thicknesses of bobwhite quail eggs were 0.221 (± 0.02), 0.216
(± 0.01) and 0.214 (± 0.01) mm, respectively. Treatment groups did
not differ significantly from the control (0.212 ± 0.02 mm). A small
but biologically significant reduction in the numbers of eggs laid per
hen and a subsequent reduction in the numbers of offspring were seen
at 500 mg/kg. A clear no-observed-effect concentration of 150 mg
methomyl/kg diet was therefore determined (Beavers et al., 1991a).
A one-generation study of similar design and the same dietary
concentrations was undertaken with the mallard duck for a period of 18
weeks. There were no signs of toxicity or treatment-related
mortalities in the adults. No effect was seen on body weight at the
two lower levels but a decrease in weight gain occurred in hens fed
500 mg/kg over the last 10 weeks of the study. Egg shell thickness
was not affected by methomyl. Mean shell thicknesses of eggs from
exposed ducks were 0.378 (±0.03), 0.379 (±0.07) and 0.370 (±0.03) mm
for 50, 150 and 500 mg/kg, respectively. No significant differences
from the control (0.378 ± 0.03 mm) were detected. A slight reduction
in the percentage of viable embryos was observed at 500 mg/kg. A
clear no-observed-effect concentration was therefore established at
150 mg/kg (Beavers et al., 1991b).
Table 10. Acute toxicity studies on birds
Species Test Acute oral LD50 Reference
(mg/kg)
Bobwhite quail (Colinus virginianus) intubation, suspended in corn oil 24.2 Beavers (1983)
Japanese quail (Coturnix japonica) intubation, suspended in 34 Smith (1982)
carboxymethylcellulose
Chicken (White Leghorn-Cornish) capsule minimum toxic dose Palmer & Schlinke (1978)
25
Mallard duck (Anas platyrhynchos) 15.9 Tucker & Crabtree (1970)
Pheasant (Phasianus colchicus) 15.4 Tucker & Crabtree (1970)
Common pigeon (Columba livia) in polyethylene glycol 10.0 Schafer (1975)a
Common grackle (Quiscalus quiscala) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) in polyethylene glycol 31.6 Schafer (1975)a
House sparrow (Passer domesticus) in polyethylene glycol 13.3 Schafer (1975)a
Starling (Sturnus vulgaris) gavage in propylene glycol 42 Schafer (1972)
Redwing (Agelaius phoeniceus) gavage in propylene glycol 10 Schafer (1972)
a Schafer EW Jr (1975) Avian toxicity tests - letter to Dr HJ Thome, April 28 1975 (unpublished report submitted to WHO by Du Pont)
Table 11. Dietary toxicity studies on birds
Species 8-day Test Dietary LC50 Reference
(5 days feeding, 3 days (mg/kg)
observation)
Bobwhite quail (Colinus virginianus) 4 concentrations 1100 Heath et al. (1972)
Japanese quail (Coturnix japonica) 6 concentrations 3124 Heath et al. (1972)
Mallard duck (Anas platyrhynchas) 6 concentrations 2883 Heath et al. (1972)
Bobwhite quail 7 concentrations 3680 Busey (1967)
Pekin duck 7 concentrations 1890 Busey (1967)
Methomyl did not give any indication of a teratogenic effect in a
chick embryo test when it was injected into the yolk (Proctor et al.,
1976).
9.4 Field studies
Bobwhite quails, 6 males and 6 females, were exposed to sprays of
Lannate formulations, equivalent to a field application of 1 kg
methomyl/ha, once every 48 h for a total of four applications. The
birds were killed 14 days after the last application. There were no
observable effects due to exposure and no treatment-related changes in
tissues on gross examination (Aftosmis, 1973a,b).
A simulated field trial was carried out on two groups of bobwhite
quail (three males and three females per group). One group was fed
prior to treatment, the other group was fed 12 h after treatment. The
treatment consisted of spraying methomyl over the test area at the
rate of 1.1 kg/ha. In all, six sprayings were made, each 5 days
apart, with a 15-day observation period after the last application.
Some weight loss occurred in the group fed prior to treatment;
otherwise there were no observable effects on surviving birds
compared to controls and no changes due to treatment upon gross
examination of tissues (Hinkle & Cameron, 1980).
In 1978, 400 ha of Maine (USA) forest land was treated with the
formulation Lannate LV at the rate of 0.28 kg methomyl/ha to control
spruce budworm. Monitoring of more than 30 species of songbirds was
undertaken over 6-day census periods pre-spray, immediately post-spray
and 2 weeks post-spray. The census was carried out firstly by
individual bird counts and then by territorial counts. Searches were
made for dead birds. Spray deposit cards were used to ensure that the
insecticide was present in the census plots. A concurrent sampling
and analytical programme determined the residue levels of methomyl in
trees and other plants, leaf litter, soil and water. The maximum
level detected was 15 mg/kg in foliage from the top third of the
trees. Underbrush had initial residues of 6 mg/kg, low levels were
found in leaf litter, negligible amounts in soil and none in water.
These levels dissipated rapidly apart from leaf litter where low
levels lasted 34-61 days. The study revealed no evidence of changes
in the activity levels among songbirds. No dead or intoxicated birds
were found and there was no disruption of nesting birds (Brown, 1978).
A study of brain cholinesterase activity was undertaken on wild
mice (Mus musculus) trapped over a period of 3 days after a soya
bean field had been sprayed with Lannate 1.8 L at 0.5 kg methomyl/ha.
Some inhibition of brain cholinesterase activity occurred at a level
of about 10% over the study period indicating the possibility of a
small effect (Montz et al., 1983).
A census of birds was undertaken before and after spraying a
field of hops in Kent, United Kingdom, with 0.56 kg methomyl per ha.
Records were made of birds seen, feeding in the hop field, singing and
nesting. Monitoring was carried out 1, 3 and 10 days after spraying.
Principal species observed feeding upon the sprayed area included
blackbird, song thrush, various tits and finches, robin, wren and
hedge sparrow. There was no apparent difference in feeding habits
before or after spraying and no unhealthy or dead birds were found
over the total observation period (Orpin, 1971).
Methomyl (90% SP), applied at 3.4 kg/ha pasture, produced
decreases in population and biomass of three species of earthworms of
28.5% and 14%, respectively. The compound was considered to be the
least active of the seven pesticides tested (Tomlin & Gore, 1974).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Methomyl is a carbamate cholinesterase inhibitor with a
well-known mechanism of toxic action. It is particularly toxic by the
acute oral and inhalation routes in animal studies, but it has low
dermal toxicity. Acute toxic signs in animals are typical of those of
a cholinesterase inhibitor. The reversibility of acute toxic action
is rapid, with survivors showing quick recovery from toxic signs and
reversal of cholinesterase inhibition in the blood and brain. The
quick recovery from toxic effects is due to the rapid reversibility of
methomyl-inhibited cholinesterase, which is facilitated by the rapid
clearance of the compound from the body. Data from accidental and
intentional human poisonings show that the level of acute methomyl
toxicity in humans is similar to that found in laboratory animals.
Because of the rapid reversibility of the action of methomyl
during periods of feeding, acute toxic signs and blood cholinesterase
inhibition were rarely seen in dietary studies. The most consistent
findings in longer-term studies at the higher dietary levels were
decreases in body weight gain in rodents and reduced red blood cell
indices in rodents and dogs. There was no evidence for carcinogenic
potential from three long-term studies in rodents. The compound was
negative in in vitro genotoxicity tests that investigated several
end-points, but methomyl showed cytogenetic potential in human
lymphocytes. It was negative in an in vivo rat bone marrow
chromosomal study.
NOELs were identified in each of the long-term animal studies,
based upon depression of body weight gain and red blood cell indices.
These were 5 mg/kg body weight per day in rats, 8.7 mg/kg body weight
per day in mice and 3 mg/kg body weight per day in dogs. In the
absence of any marked species differences in toxic effect in these
studies, the NOEL in the dog of 3 mg/kg body weight per day should be
used for the purpose of human risk estimation.
The proposed toxicological criteria for setting guidance values
are presented in Table 12.
10.2 Evaluation of effects on the environment
Adsorption of methomyl to soil is low to moderate with hardly any
desorption. Aerobic degradation in soil (with a half-life of around
one week) is about twice as fast as anaerobic degradation. A relative
increase in soil organic matter delays degradation.
Table 12. Proposed toxicological criteria for setting guidance values
Exposure scenario Relevant route/effect Result/remarks
Short-term oral, acute, several species including human highly toxic; rat LD50 = 17 mg/kg body weight;
(1-7 days) LOELa= 5 mg/kg body weight
eye, irritation, rabbit mild irritant
inhalation, acute, rat highly toxic; LC50 = 0.26 mg/litre (4-h aerosol);
NOELb = 0.14 mg/litre
dermal, acute, rat and rabbit low toxicity; intact skin - LD50 > 2000 mg/kg body weight;
NOELc = 2000 mg/kg body weight in the rabbit
Medium-term repeat dermal, rabbit 21-day study; no toxicologically significant effects at
(1-26 weeks) 50 mg/kg body weight per day
repeat oral, rat 13-week study; NOEL = 3.6 mg/kg body weight per day
maternal oral, rabbit teratology study; NOEL = 6 mg/kg body weight per day
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body weight per day
a mild cholinergic signs of toxicity in guinea-pig
b no-observed-effect level for clinical signs and death
c no clinical symptoms noted except signs of skin irritation
Despite the above adsorption characteristics, leaching of
methomyl in soil to levels deeper than 20 to 30 cm has not been
observed. The concentrations of methomyl in both surface and well
water are below the limit of detection. Methomyl is degraded rapidly
with a half-life of about one week in water and sediment. In sterile
water, methomyl is stable for at least 30 days at normal environmental
pH.
Application of methomyl to plant leaves results in rapid
absorption of about half the amount applied (the other half being
adsorbed), and there is no indication of translocation. In contrast,
when applied to soil, uptake through the roots occurs readily with
rapid translocation to the leaves. Adsorbed foliar residues degrade
with a half-life in the order of 4 days. Absorbed methomyl
concentrations in food crops decline rapidly to about 5% within one
week; this may be due to growth dilution.
Bioaccumulation by rainbow trout did not occur in a flow-through
study. Depuration occurred within one day of transfer to clean water.
Trout were discoloured when exposed to levels between 0.075 and
0.75 mg/litre, an effect that disappeared within 5 days, the time
depending on original exposure concentration.
At recommended application rates, methomyl does not adversely
affect microbial activity in temperate soil; nitrification can be
delayed at applications 10 times higher. An aquatic green alga showed
a NOEC for growth of 6.25 mg/litre.
Several aquatic invertebrates, and particularly daphnids, are
very sensitive to methomyl, the LC50s being of the order of 10 to
100 µg/litre. MATC values from two 21-day Daphnia studies were
estimated to be around 2 µg/litre. Kills of aquatic invertebrates are
expected following overspray.
Fish, both freshwater and estuarine, are less sensitive, the
LC50s ranging from 0.5 to 7 mg/litre. Two longer-term studies gave
an NOEC of 0.5 mg/litre for lethality of fingerling rainbow trout and
an MATC of > 0.06 and > 0.12 mg litre for survival of embryo larval
stages of fathead minnow. Given the low persistence of methomyl and
its relatively low acute toxicity to fish, the risk is expected to be
low.
Laboratory tests in artificial soil with methomyl formulations
(25% WP) established a 14-day LC50 of 90-100 mg formulation/kg for
earthworms. It was estimated that 11 kg a.i./ha of methomyl would
kill 50% of earthworms. A field application of 3 kg a.i./ha caused
reduced earthworm population (28%) and biomass (14%). The TER for
earthworms is around 40 indicating low risk.
Methomyl is classified as highly toxic to honey-bees, the topical
LD50 being approximately 0.1 µg/bee. Field (tent) trials showed
dead bees both at the treatment tent and hive when residues from
spraying the previous day were wetted. Less effect was seen with dry
residues. Methomyl has not been implicated in bee incidents in the
field; this probably reflects advice to restrict spraying times to
protect bees.
Acute oral LD50s for various bird species range between 10 and
40 mg/kg body weight. Dietary LC50s (5 days) range from 1100 to
3700 mg/kg diet. Methomyl poses an acute oral risk to birds,
particularly from granules; dietary intake from contaminated food is
not expected to kill birds. An example of a toxicity exposure ratio
for birds and fish is shown in Table 13.
The NOEC for reproduction was established at 150 mg/kg diet for
both the bobwhite quail and mallard duck. Field studies following
spraying of methomyl formulations in forests showed no mortality of
songbirds and no changes in feeding behaviour or general activity.
Reduced fat deposits of songbirds reflect reduced insect prey. It was
not considered that methomyl poses a threat to birds after recommended
applications.
The high acute toxicity of methomyl to laboratory mammals
indicates a similar hazard to wild mammals.
Table 13. Toxicity exposure ratios for birds, fish and aquatic invertebrates based on application rates of 2.5 kg a.i./ha
methomyl to soybeans (worst case)
Risk category LC50 (mg/litre or mg/kg diet) Estimated exposure Toxicity/exposure ratio (TER)c
(mg/litre or mg/kg diet)a,b
Acute bird 10 50-364 02-0.027
Acute fish (stream) 0.5 0.2 2.5
Acute fish (pond) 0.5 0.04 12.3
Acute aquatic invertebrate 0.009 0.2 0.045
(stream)
Acute aquatic invertebrate 0.009 0.04 0.225
(pond)
a Estimated environmental concentration in the terrestrial environment (for bird exposure) is based on the stated application rate
and the assumption of deposition on short grass using the US EPA monogram
b Aquatic exposure concentrations were taken from the STREAM model based on a single application and estimated run-off into water;
no direct overspray is included
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate likely exposure to toxic concentrations
by organisms in the different risk categories
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
Considering the toxicological characteristics of methomyl, both
qualitatively and quantitatively, it is concluded, on the basis of the
no-observed-effect level of 3 mg/kg body weight per day in the 2-year
toxicity study on dogs and applying a 100-fold uncertainty factor,
that 0.03 mg/kg body weight per day will probably not cause adverse
effects in humans by any route of exposure.
12. FURTHER RESEARCH
No further studies were thought to be necessary.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues has discussed and
evaluated methomyl on several occasions since 1975. Residue aspects
were discussed in 1975, 1976, 1977, 1978, 1986, 1987, 1988, 1989, 1990
and 1991 (FAO/WHO, 1976, 1977, 1978, 1979, 1986, 1987, 1988a,b,
1990a,b,c, 1991). Toxicological evaluations took place in 1978, 1986
and 1989, when an Acceptable Daily Intake (ADI) of 0-0.03 mg/kg body
weight was established (FAO/WHO, 1979, 1987, 1990a,b).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for methomyl in various commodities
(FAO/WHO, 1993).
It should be noted that the 1992 CCPR meeting decided to combine
MRLs for thiodicarb and methomyl into a single list. In the cases of
different MRLs the higher limit would prevail.
Methomyl is listed in Class IB ("Highly Hazardous") in the WHO
Recommended Classification of Pesticides by Hazard and
Guidelines to Classification (1994-1995) on the basis of its rat acute
oral LD50 value of 17 mg/kg body weight (IPCS, 1994).
The time-weighted average (TWA) adopted by the American
Conference of Governmental Industrial Chemists (ACGIH) is 2.5 mg/m3
(ACGIH, 1994-1995).
REFERENCES
* Report submitted to WHO by E.I. Du Pont de Nemours and Co.,
Wilmington, Delaware, USA
Aftosmis JG (1973a) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamine acid, S-methyl ester (25% a.i.) (Lannate L
methomyl insecticide). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-354-73*).
Aftosmis JG (1973b) Carbamic acid, methyl ester with oxime function of
thiolacetohydroxamic acid, S-methyl ester (30% a.i.) (non-flammable
Lannate L methomyl insecticide), Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-606-73*).
Ambridge EM (1992) Report on field trials to assess operator
contamination during application of two different pesticides to crops
of varying heights in Thailand, November/December 1991 (NRI Contract
No. 00195) (Unpublished report*).
ACGIH (1994-1995) Threshold limit values for chemical substances in
the work environment. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, p 28.
Andrews TL & Miskus RP (1968) Tetraethylammonium chloride as an
antidote for certain insecticides in mice. Science, 159: 1367-1368.
Antal M, Bedö M, Constantinovits G, Nagy K, & Szépvölgyi J (1979)
Studies on the interaction of methomyl and ethanol in rats. Food
Cosmet Toxicol, 17: 333-338.
Araki M, Yonemitsu K, Kambe T, Idaka D, Tsunenari S, Kanda M, &
Kambara T (1982), [Forensic toxicological investigations in fatal
cases of carbamate pesticide methomyl (LannateR) poisoning.] Nippon
Hoigaku Zasshi, 36: 584-588 (in Japanese).
Armondi S (1991a) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-394 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-345-91*).
Armondi S (1991b) Closed-patch repeated insult dermal sensitization
study (Buehler method) with DPX-X1179-425 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report HLO 346-91*).
Armondi S (1992) Closed-patch repeated insult dermal sensitization
study (maximization method) with DPX-X1179-424 in Guinea pigs. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLO-659-91*).
Armstrong K, Caley CY, Hall BE, & Knight B (1991) Lannate 20L:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inveresk Research International Ltd (Unpublished report
No. 8546*).
Atkins EL, Anderson LD, Kellum DD, & Neuman KW (1976) Protecting honey
bees from pesticides. Berkeley, California, University of California,
Division of Agricultural Sciences, 15 pp (Leaflet 2883).
Baer KN (1991a) Static, acute, 96-hour LC50 of DPX-X1179-423 to
bluegill sunfish (Lepomis macrochirus). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-30-91*).
Baer KN (1991b) Static, acute, 96-hour LC50 of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-29-91*).
Baer KN (1991c) Flow-through, 21-day toxicity of DPX-X1179-423
(LannateR 20L) to rainbow trout (Oncorhynchus mykiss). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-34-91*).
Baer KN (1991d) Static, acute, 48-hour EC50 of DPX-X1179-423
(LannateR 20L) to Daphnia magna. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-150-91*).
Baer KN (1991e) Chronic toxicity of DPX-X1179-423 (LannateR 20L) to
Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-292-91*).
Barnes JR (1978) Cholinesterase tests with methomyl. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-280-78*).
Battelle (1991) Method for determination of oxamyl and methomyl in
groundwater. Columbus, Ohio, Battelle Institute (Unpublished report
No. AMR-1392-89*).
Beavers JB (1983) An acute oral toxicity study in the Bobwhite with
H-15,000. Easton, Maryland, Wildlife International Ltd (Unpublished
report No. HLO-464-83*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991a) A one-generation
reproduction study with the Northern Bobwhite (Colinus virginianus).
Easton, Maryland, Wildlife International Ltd (Unpublished report
No. HLO-337-91*).
Beavers JB, Hawrot R, Lynn SP, & Jaber M (1991b) A one-generation
reproduction study with the mallard (Anas platyrhynchos). Easton,
Maryland, Wildlife International Ltd (Unpublished report
No. HLO-336-91*).
Bedö M & Cieleszky V (1990) Nutritional toxicology in the evaluation
of pesticides. Bibl Nutr Dieta, 29: 20-31.
Belasco IJ (1972a) Effect of methomyl on the activity of sewage
microorganisms (Unpublished report No. ML/ME15*).
Belasco IJ (1972b) Methomyl: Incubation with rumen microorganisms
(Unpublished report No. ML/ME26*).
Bentley RE (1973) Acute toxicity of H-8385 to grass shrimp
(Palaemonetes vulgaris) and fiddler crab (Uca pugilator). Wareham,
Massachusetts, Bionomics Inc. (Unpublished report No. HLO-504-73*).
Blevins RD, Lee M, & Regan JD (1977a) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56: 1-6.
Blevins RD, Lijinsky W, & Regan JD (1977b) Nitrosated methylcarbamate
insecticides: Effect on the DNA of human cells. Mutat Res, 44: 1-7.
Boeri RL & Ward TJ (1989) Static acute toxicity of methomyl to the
sheepshead minnow, Cyprinodon variegatus. Hampton, New Hampshire,
Resource Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-700-89*).
Boleij JSM, Kromhout H, Fleuren M, Tieleman W, & Verstappen G (1991)
Re-entry after methomyl application in greenhouses. Appl Occup Environ
Hyg, 6(8): 672-676.
Bonatti S, Bolognesi C, Degan P, & Abbondandolo A (1994) Genotoxic
effects of the carbamate insecticide methomyl. 1. In vitro studies
with pure compound and the technical formulation Lannate 25. Environ
Mol Mutagen, 23: 306-311.
Borady AMA, Mikhail TH, Awadallah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23: 33-44.
Boulton JJK, Boyce CBC, Jewess PJ, & Jones RF (1971) Comparative
properties of N-acetyl derivatives of oxime N-methylcarbamates & aryl
N-methyl carbamates as insecticides and acetylcholinesterase
inhibitors. Pestic Sci, 2: 10-15.
Bracy OL, Doyle RS, Kennedy M, McNally SM, Weed JD, & Thorne BM (1979)
Effects of methomyl and ethanol on behaviour in the Sprague-Dawley
rat. Pharmacol Biochem Behav, 10: 21-25.
Braun HE, Ritcey GM, Frank R, McEwen FL, & Ripley BD (1980)
Dissipation rates of insecticides in six minor vegetable crops grown
on organic soils in Ontario, Canada. Pestic Sci, 11: 605-616.
Braun HE, Ritcey GM, Ripley BD, McEwen FL, & Frank R (1982) Studies of
the disappearance of nine pesticides on celery and lettuce grown on
muck soils in Ontario 1977-1980. Pestic Sci, 13: 119-128.
Brittelli MR (1982) Chronic toxicity of methomyl to Daphnia magna.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-46-82*).
Brock WJ (1989) Repeated dose dermal toxicity: 21-day study with
DPX-X1179-394 (methomyl) in rabbits. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-387-89*).
Brodsky J (1991) Determination of residues of methomyl in grapes, wine
& wine processing fractions by GC-MS following treatment with
"Lannate", season 1990, France. Frankfurt, Germany, Battelle-Europe
(Unpublished report No. BE-A-11-91-10-BF*).
Bromilow RH, Briggs G, Williams MR, Smelt JH, Tuinstra LGMT, & Traag
WA (1986) The role of ferrous ions in the rapid degradation of oxamyl,
methomyl and aldicarb in anaerobic soils. Pestic Sci, 17: 535-547.
Brown HL (1978) The effects of Lannate LV on singing male songbirds in
Maine in 1978 (Unpublished report No. ML/FW-12 report*).
Brown SK, Ames RG, & Mengle DC (1989) Occupational illnesses from
cholinesterase-inhibiting pesticides among agricultural applicators in
California, 1982-1985. Arch Environ Health, 44: 34-39.
Bruynzeel DP (1991) Contact sensitivity to Lannate(R). Contact
Dermatitis, 25: 60-61.
Bull DL (1974) Fate of methomyl on cotton. Environ Entomol,
3: 723-724.
Busey WM (1966) Three-month dietary administration - Rats: Insecticide
1179. Supplement to final report. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-848*).
Busey WM (1967) Acute aqueous exposure - Goldfish, Bluegill, and
Rainbow trout. Acute dietary administration - Peking ducks & Bobwhite
quail: Insecticide 1179. Falls Church, Virginia, Hazleton
Laboratories Inc. (Unpublished report No. MRO-888-1*).
Cahill WP, Estesen B, & Ware GW (1975) Foliage residues of
insecticides on cotton. Bull Environ Contam Toxicol, 13: 334-337.
Caley CY, Cameron BD, Hall BE, & Knight B (1991) Lannate 25 WP:
Determination of acute toxicity (LC50) in earthworms. Tranent,
Scotland, Inverest Research International Ltd (Unpublished report
No. 8455*).
Carakostas MC (1987) Inhibition and regeneration kinetics for human
and rat acetyl-cholinesterase exposed to methomyl in vitro. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-379-88*).
Carbonnel E, Puig M, Xamena N, Creus A, & Marco R. (1990) Sister
chromatid exchange in lymphocytes of agricultural workers exposed to
pesticides. Mutagenesis, 5(4): 403-405.
Carter FL & Graves JB (1973) Measuring effects of insecticides on
aquatic animals. La Agric, 16: 14-15.
Christian MS, Hoberman AM, & Fuessner EL (1983) Embryo-fetal toxicity
and teratogenicity study of methomyl in the rabbit. Horsham,
Pennsylvania, Argus Research Laboratories Inc. (Unpublished report
No. HLO-331-83*).
Clark S & Kennedy SM (1990) Analytical method for the quantification
of methomyl in grapes. Sacramento, California, Morse Laboratories
(Unpublished report No. AMR-1806-90*).
Clement C (1987a) Test to evaluate the acute cutaneous primary
irritation and corrosivity in the rabbit. L'Arbresle, France, Hazleton
France, 16 pp (Unpublished report No. 702408*).
Clement C (1987b) Test to evaluate acute ocular irritation and
corrosivity in the rabbit. L'Arbresle, France, Hazleton France, 26 pp
(Unpublished report No. 703313*).
Clinch PG & Ross IGM (1970) Laboratory assessment of the speed of
action on honey bees of orally dosed insecticides. N Z J Agric Res,
13: 717-725.
Council of the European Communities (1991) Council directive of 15
July 1991 concerning the placing of plant protection products on the
market (91/414/EEC). Off J Eur Communities, L230: Part II.
Cox L, Hermosin MC, & Comejo J (1993) Adsorption of methomyl by soils
of southern Spain and soil components. Chemosphere, 27: 837-849.
Danneberg G (1991) Investigation of the effects of Lannate 20L on the
activity of the microflora of soil. Frankfurt, Germany, Battelle
Institute (Unpublished report No. BE-S-11-91-01-DEH-01*).
Dashiell OL (1972) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-518-72*).
Debuyst B & Van Larebeke N (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat Res, 113: 242-243.
Dong MH, Krieger RI, & Ross JH (1992) Calculated re-entry interval for
table rape harvesters working in California vineyards treated with
methomyl. Bull Environ Contam Toxicol, 49: 708-714.
Douglas MT & Halls RWS (1991) The algistatic activity of Lannate 20L.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPC-16(f) 91399*).
Douglas MT & Handley JW (1988) The algistatic activity of methomyl
Tech. (DPX-X1179-00620-06). Huntingdon, United Kingdom, Huntingdon
Research Centre (Unpublished report No. DPT-171(j) 871676*).
Driscoll RR & Muska CF (1982) Early life stage toxicity of methomyl to
fathead minnow. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-528-82*).
Driskell WJ, Groce DF, Hill RH Jr, & Birky MM (1991) Methomyl in the
blood of a pilot who crashed during aerial spraying. J Anal Toxicol,
15: 339-340.
Du Pont (1967) Methomyl - Livestock feeding studies: Milk and meat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Industrial and
Biochemicals Department (Unpublished report No. ML/ME27*).
Du Pont (1973) Petition for residue tolerance - methomyl: Broccoli,
brussel sprouts, cauliflower, spinach, celery. Pesticide Petition 4F
1448, Section D, 1973. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Biochemicals Department (Unpublished report*).
Du Pont (1978) Ecosystem residue study: Spruce/fir forest and cedar
swamp, Princeton, Maine 1978. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. ML/PC-25*).
Du Pont (1982) LannateR insecticide formulations and technical
methomyl - Determination of methomyl (INX-1179) - Reversed-phase
liquid chromatography (RPLC) assay method (Method No. L30.4662/(E).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report [Second issue]*).
Eble JE & Tomic DM (1991) Foliar half-life of methomyl in cotton
leaves. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. AMR-1871-90*).
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards DF (1980) 10 Day subacute skin absorption test on rabbits.
Mewark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-166-80*).
El-Refai A, Fahmy FA, Mahmound FA, Abdel-Lateef, & Imam AKE (1976)
Toxicity of three insecticides to two species of fish. Int Pest
Control, Nov/Dec: 4-8.
El-Wakil HB & Radwan MA (1991) Biochemical studies on the terrestrial
snail Eubania vermiculata (Müller) treated with some pesticides. J
Environ Sci Health, B26(5/6): 479-489.
FAO (1985) Guidelines for the disposal of waste pesticide containers
on the farm. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1976) Pesticide residues in food. Report of the 1975 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. Geneva, World Health
Organization (WHO Technical Report Series No. 592; FAO Plant
Production and Protection Series No. 1).
FAO/WHO (1977) 1976 Evaluations of some pesticide residues in food:
The monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Series
No. 8; AGP:1976/M/14).
FAO/WHO (1978) Pesticide residues in food. 1977 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10 Sup).
FAO/WHO (1979) Pesticide residues in food. 1978 Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 15 Sup).
FAO/WHO (1986) Pesticide residues in food - 1986. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78).
FAO/WHO (1987) Pesticide residues in food - 1986. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 78/2).
FAO/WHO (1988a) Pesticide residues in food - 1987. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 86/1).
FAO/WHO (1988b) Pesticide residues in food - 1988. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 93/1).
FAO/WHO (1990a) Pesticide residues in food - 1989. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100).
FAO/WHO (1990b) Pesticide residues in food - 1989. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 100/2).
FAO/WHO (1990c) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 103/1).
FAO/WHO (1991) Pesticide residues in food - 1991. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper No. 111).
FAO/WHO (1993) Codex Alimentarius - Volume two: Pesticide residues in
food. Rome, Food and Agriculture Organization.
Farrow MG, Cortina T, & Padilla-Nash H (1984) In vivo bone marrow
chromosome study in rats, H15,000. Final Report. Vienna, Virginia,
Hazleton Biotechnologies Corporation (Unpublished report
No. HLO-63-84*).
Fayez V & Baig MRE (1991) Short-term toxicity of methomyl in rats.
Chemosphere, 23(3): 375-382.
Foerst DL & Moye HA (1985) Aldicarb and related compounds in drinking
water via direct aqueous injection HPLC with post column
derivatisation. Cincinnati, Ohio, US Environmental Protection Agency
(EPA/600/D-85/051).
Fossi MC, Leonzio C, Massi A, Lari L, & Casini S (1992) Serum esterase
inhibition in birds: A nondestructive biomarker to assess
organophosphorus and carbamate contamination. Arch Environ Contam
Toxicol, 23: 99-104.
Foster GV (1966a) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl) progress report. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-73-66*).
Foster GV (1966b) Acute inhalation LC50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-214-66*).
Friedman PL (1983) Hydrolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. AMR-109-83*).
Freeman PK & Ndip EMN (1984) Photochemistry of oxime carbamates 2.
Phototransformations of methomyl. J Agric Food Chem, 32(4): 877-881.
Fung KH & Uren NC (1977) Microbial transformation of S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate (methomyl) in soils. J Agric
Food Chem, 25(4): 966-969.
Fung KH, Luke RKJ, & Uren NC (1978) Concentrations of methomyl in
Australian tobacco plants following transplant, foliar and soil
treatments. Tob Sci, 22: 24-26.
Gianessi LP & Puffer CA (1992) Insecticide used in US crop production.
Resources for the Future. Washington, DC.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Agrochemical Manufacturers.
Goodman NC (1978) 48-Hour LC50 to Daphnia magnia. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-165-78*).
Gordon M & Richter ED (1991) Hazards associated with aerial spraying
of organophosphate insecticides in Israel. Rev Environ Health,
9(4): 229-238.
Gupta RC, Goad JT, & Kadel WL (1992) Characteristic changes in LDH and
its isoenzymes as biomarkers under the influence of acute methomyl
toxicity. Fed Am Soc Exp Biol J, 6(4): A1307.
Han JCY (1972) Evaluation of possible effects of methomyl on
nitrifying bacteria in soil (Unpublished report ML/ME16*).
Han JCY (1975) Absence of nitroso formation from [14C] methomyl and
sodium nitrite under simulated stomach conditions. J Agric Food Chem,
23: 892-896.
Harvey J Jr (1967) Exposure of S-methyl N-[(methyl carbomoyl)oxy]
thioacetimidate in sunlight, water and soil (Unpublished report
No. ML/ME-10*).
Harvey J Jr (1972a) Decomposition of 14C-methomyl in aerated river
water exposed to sunlight (Unpublished report No. ML/ME-13*).
Harvey J Jr (1972b) Decomposition of 14C-methomyl in a high organic
matter soil (Unpublished report No. ML/ME-18*).
Harvey J Jr (1977a) Decomposition of 14C-methomyl in a sandy loam
soil in the greenhouse (Unpublished report No. ML/ME-19*).
Harvey J Jr (1977b) Degradation of 14C-methomyl in Flanagan silt
loam in biometer flasks (Unpublished report No. ML/ME-20*).
Harvey J Jr (1978) Crop rotation study with 14C-methomyl in the
greenhouse. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Biochemicals Department (Unpublished report No. ML/ME-22*).
Harvey J Jr (1980) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Biochemicals
Department (Unpublished report No. AMR-22-80*).
Harvey J Jr (1983) Photolysis of [1-14C] methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Chemicals
Department (Unpublished report No. AMR-121-83*).
Harvey J Jr & Pease HL (1973) Decomposition of methomyl in soil. J
Agric Food Chem, 21(5): 784-786.
Harvey J Jr & Reiser RW (1973) Metabolism of methomyl in tobacco, corn
and cabbage. J Agric Food Chem, 21: 775-783.
Harvey J Jr & Yates RA (1967) Metabolism of methomyl in the corn
plant. I. Plant growth chamber - Carbon 14 studies (Unpublished report
No. ML/ME2*).
Harvey J Jr, Jelinek AG, & Sherman H (1973) Metabolism of methomyl in
the rat. J Agric Food Chem, 21(5): 769-775.
Hashimoto Y & Fukami J (1969) Toxicity of orally and topically applied
pesticide ingredients to Carp Cyprinus carpio Linné. Botuy Kagaku,
34: 63-66.
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1991) The metabolism of
[1-14C] methomyl in rats. Huntingdon, United Kingdom, Huntingdon
Research Centre and Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products (Unpublished report No. AMR-1584-90*).
Hawkins DR, Mayo BC, Pollard AD, & Haynes LM (1992) The metabolism of
[1-14C] methomyl in male cynomolgus monkeys. Huntingdon, United
Kingdom, Huntingdon Research Centre (Unpublished report
No. AMR-1902-90*).
Heath RG, Spann JW, Hill EF, & Kreitzer JF (1972) Comparative dietary
toxicities of pesticides to birds. Washington, DC, US Department of
the Interior, Fish and Wildlife Service (Special Scientific
Report-Wildlife No. 152).
Hemavathy KC & Krishnamurthy NB (1987a) Mutagenicity studies in
Drosophila melanogaster with Lannate 20. Mutat Res, 191: 41-43.
Hemavathy KC & Krishnamurthy NB (1987b) Evaluation of Lannate 20, a
carbamate pesticide in the germ cells of male rice. Environ Res,
42: 362-365.
Henry NW III (1975) LannateR L methomyl insecticide potentiation
studies. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-278-75*).
Henry JE (1981) Acute dermal methomyl-cholinesterase response study in
male rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-303-81*).
Hill KM, Hollowell RH, & Dal Cortivo LA (1984) Determination of
N-methylcarbamate pesticides in well water by liquid chromatography
with postcolumn fluorescence derivatization. Anal Chem, 56: 2465-2468.
Hinkle S & Cameron JT (1980) Simulated field trial in Bobwhite quail,
H-13 099. Final report. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished report No. HLO-97-80*).
Holt RF (1971) LannateR residue cooking studies. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Hornberger CS (1967) Acute inhalation toxicity of aqueous spray mist.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-171-67*).
Hosling GC (1969) Dietary administration - Cotornix Quail: INX-1179
(Unpublished report No. HLO-238-69*).
Huhtanen K & Dorough HW (1976) Isomerization and Beckmann
rearrangement reactions in the metabolism of methomyl in rats. Pestic
Biochem Physiol, 6: 571-583.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - A
general introduction. Geneva, World Health Organization.
IPCS (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization (Unpublished document WHO/PCS/94.2).
Ivie KF (1980) High performance liquid chromatography (HPLC) in
pesticide residue analysis. In: Zweig G & Sherman J ed. Updated
general techniques and additional pesticides. New York, London,
Academic Press, pp 55-58.
Jackson GC, Hardy CJ, Gregson RL, Offer JM, & Gopinath C (1991)
Lannate 20L: Acute inhalation toxicity in rats 4-hour exposure.
Huntingdon, United Kingdom, Huntingdon Research Centre (Unpublished
report No. DPT-247/91521*).
Johansen CA & Davis HG (1972) Toxicity of nine insecticides to the
Western yellowjacket. J Econ Entomol, 65: 40-42.
Kaplan AM (1981) Long-term feeding study in rats with S-methyl
N-[(methylcaramoyl)oxy] thioacetimidate (methomyl, INX-1179). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-235-81*).
Kaplan AM & Sherman H (1977) Toxicity studies with methyl
N-[[(methylamino) carbonyl]oxy]-ethanimidothioate. Toxicol Appl
Pharmacol, 40: 1-17.
Kelly DP (1992) Acute inhalation toxicity study with DPX-X1179-440 in
rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-326-92*).
Kennedy SM (1989) Field soil dissipation of Lannate(R) L insecticide.
Sacramento, California, Morse Laboratories Inc. (Unpublished report
No. AMR-1215-88*).
Kennedy CM (1991) Field soil dissipation of Lannate(R) L insecticide
- A 1991 study. Sacramento, California, Morse Laboratories Inc. and
Lincoln, Nebraska, Harris Environmental Technologies Inc. (Unpublished
report No. AMR-1921-91*).
Kennedy CM & Hay RJ (1991a) Magnitude of residues of methomyl
insecticide in citrus and its processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1361-89*).
Kennedy CM & Hay RJ (1991b) Magnitude of residues of methomyl
insecticide in cottonseed and its processed fractions. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1355-89*).
Kiigemagi U & Deinzer ML (1979) Dislodgeable and total residues of
methomyl on mint foliage. Bull Environ Contam Toxicol, 22: 517-521.
Kiigemagi U, Wellman D, Cooley EJ, & Terriere LC (1973) Residues of
the insecticides phorate and methomyl in mint hay and oil. Pestic Sci,
4: 89-99.
Knaak JE, Jackson T, Fredrickson AS, Rivera L, Maddy KT, & Akesson NB
(1980) Safety effectiveness of closed transfer, mixing, loading and
application equipment in preventing exposure to pesticides. Arch
Environ Contam Toxicol, 9: 231.
Krauss WC & Stula EF (1967) Oral LD50 and delayed paralysis tests
(Hens). Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-161-67*).
Labare A (1990) Testing of DPX-X1179 through FDA multi-residue
protocols A-E. Beltsville, Maryland, Biospherics Inc. (Unpublished
report No. AMR-1405-89*).
Leidy RB, Domanski JJ, Haire PL, & Sheets TJ (1977) Effects of
weathering and flue-curing on methomyl residues on tobacco. Arch
Environ Contam Toxicol, 5: 199-206.
Leistra M, Dekker A, & Van Der Burg AMM (1984) Computed and measured
leaching of the insecticide methomyl from greenhouse soils into water
courses. Water Air Soil Pollut, 23: 155-167.
Leitch RE & Pease HL (1973) LannateR-methomyl. In: Sherma J & Zweig
G ed. Analytical methods for pesticides and plant growth regulators,
7th ed. New York, London, Academic Press, pp 331-338.
Lheritier M (1991a) Test to evaluate the acute toxicity following a
single oral administration (LD50) in the rat. L'Arbresle, France,
Hazleton France (Unpublished report No. 104367*).
Lheritier M (1991b) Test to evaluate the acute toxicity following a
single cutaneous application (limit test) in the rat. L'Arbresle,
France, Hazleton France (Unpublished report No. 106393*).
Liddle JA, Kimbrough RD, Needham LL, Cline RE, Smrek AL, Yert LW, &
Bayse DD (1979) A fatal episode of accidental methomyl poisoning. Clin
Toxicol, 15(2): 159-167.
Lijinsky W & Schmahl D (1978) Carcinogenicity of N-nitroso derivatives
of N-methylcarbamate insecticides in rats. Ecotoxicol Environ Saf,
2: 413-419.
Lu CC (1983) NudrinR: Two-generation reproduction study in rats
(Protocol No. RA-274). Houston, Texas, Shell Development Co.,
Westhollow Research Center (Unpublished report*).
McAlack JW (1973) Ten-day subacute exposure of rabbit skin to LannateR
L insecticide. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report HLR 24-73*).
McCain JC (1971), Acute fish toxicity study - static freshwater:
Lannate, MR-581, H-6854. Falls Church, Virginia, Hazleton Laboratories
Inc. (Unpublished report No. HLO-71-71*).
McConnel R, Pacheco Anton AF, & Magnotti R (1990) Crop duster aviation
mechanics: High risk for pesticide poisoning. Am J Public Health,
80(10): 1236-1239.
McCooey KT, Chromey NC, Sarrif AM & Hemingway RE (1984), CHO/HGPRT
assay for gene mutation. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-556-83*).
Macieira OJD & Hebling Beraldo MJA (1989) Laboratory toxicity of
insecticides to workers to Trigona spinipes. J Apic Res, 28(1): 3-6.
Martin NA (1986) Toxicity of pesticides to Allolobophora caliginosa
(Oligochaeta: Lumbricidae). N Z J Agric Res, 29: 699-706.
Martinez-Chuecos J, Molinero-Somolinos F, Solé-Violan J, & Rubio-Sanz
R (1990) Management of methomyl poisoning. Hum Exp Toxicol,
9: 251-254.
Marxmiller RL & Hay RJ (1991) Magnitude of residues of methomyl
insecticide in tomatoes and their processed fractions. Wilington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1360-69*).
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res,
24(1): 11-21.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication No. 160).
Meade AB (1984) Methomyl toxicity to honey bee. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. METH/ECO 9*).
Mercier O (1991) Test to evaluate the sensitising potential by topical
applications in the Guinea-pig. "The Buehler Test". L'Arbresle,
France, Hazleton France (Unpublished report No. 106348*).
Merricks DL (1990) Lannate insecticide - Field worker exposure study
in grape girdling and harvesting operations. Frederick, Maryland,
Agrisearch Incorporated, Sacramento, California, Morse Laboratories
Inc. and Fresno, California, Siemer and Associates Inc. (Unpublished
report No. AMR-1442-89*).
Miles CJ & Oshiro WC (1990) Degradation of methomyl in chlorinated
water. Environ Toxicol Chem, 9: 535-540.
Miyazaki T, Yashiki M, Kojima T, Chikasue F, Ochiai A, & Hidani Y
(1989) Fatal and non-fatal methomyl intoxication in an attempted
double suicide. Forensic Sci Int, 42: 263-270.
Monson KD (1989) Metabolism of 14C-methomyl in the lactating goat.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-22-80*).
Monson KD & Ryan DL (1991) [14C] Methomyl cow metabolism study.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products (Unpublished report No. AMR-1675-90*).
Montz WE, Scanlon PF, & Kirkpatrick RL (1983) Effects of field
application of the anti-cholinesterase insecticide methomyl on brain
acetylcholinesterase activities in wild Mus musculus. Bull Environ
Contam Toxicol, 31: 158-163.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrow RW (1972) Acute skin absorption study on rats using technical
methomyl and a 25% methomyl formulation (Lannate(R) 25W). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-438-72*).
Morse DL, Baker EL, Kimbrough RD, & Wiseman CL (1979)
Propanil-chloracne and methomyl toxicity in workers of a pesticide
manufacturing plant. Clin Toxicol, 15(1): 13-21.
Natoff IL & Reiff B (1973) Effect of oximes on the acute toxicity of
anticholinesterase carbamates. Toxicol Appl Pharmacol, 25: 569-575.
Niven CF Jr (1971) Thermal destruction of Lannate in spinach
processing. Walnut Creek, California, Del Monte Corporation Research
Center (Unpublished report*).
Noda J (1984) [Determination of methomyl by using chemical ionization
mass fragmentography. A case report of methomyl poisoning and the
animal experiment of its poisoning.] Nippon Hoigaku Zasshi, 38: 71-82
(in Japanese).
Ökolimna (1980) [Fish toxicity, carps, Lannate 25 WP.] Burgwedel,
Germany, Ökolimna (Unpublished report*) (in German).
Orpin R (1971) Methomyl - Study of effects on wild life. Glaston,
United Kingdom, Farm Protection Ltd (Unpublished report*).
Osman AZ, Hazza A, Nagwa I, & Awad TM (1983) Fate and metabolism of
the insecticide 14C-Lannate in farm animals. Isot Rad Res,
15(2): 111-120.
Oswald T, Adams J & Hicks SC (1991), LannateR insecticide -
Dislodgeable foliar residue study in Rose Greenhouse operations.
Fresno, California, Siemer and Associates Inc. (Unpublished report
No. AMR-1909-90*).
Owens CB, Owens EW, & Zahn D (1978) The extent of exposure of migrant
workers to pesticide and pesticide residues (Abstract). Int J
Chronobil, 5(2): 428-429.
Palmer JS & Schlinke JC (1978) Preliminary toxicological evaluations
of six pesticide compounds in cattle, sheep and chickens (Unpublished
report METH/TOX5*).
Panepinto AS (1991a) Acute inhalation toxicity study with
DPX-X1179-427 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-678-91*).
Panepinto AS (1991b) Acute inhalation toxicity study with
DPX-X1179-424 in rats. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Haskell Laboratory (Unpublished report No. HLR-560-91*).
Paynter OE (1966) Three-month dietary administration - Rats.
Insecticide 1179. Falls Church, Virginia, Hazleton Laboratories Inc.
(Unpublished report*).
Pease HL (1968) Methomyl residue analysis - Soils. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Industrial and Biochemicals
Department (Unpublished report*).
Pease HL & Kirkland JJ (1968) Determination of methomyl residues using
microcolourmetric gas chromatography. J Agric Food Chem, 16: 554-557.
Peeples JL (1977) Effect of methomyl on soil microorganisms
(Unpublished report No. ML/ME21*).
Powley CR (1989) Methomyl foliar dislodgeable residues on grapes in
California. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Agricultural Products (Unpublished report No. BMP/DFR-189*).
Powley CR (1990a) LannateR insecticide: Dislodgeable foliar residue
study in grape girdling and harvesting operations. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Agricultural Products
(Unpublished report No. AMR-1445-89*).
Powley CR (1990b) LannateR insecticide: Dislodgeable foliar residue
study on grapes grown in California. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-1515-89*).
Powley CR (1991) Determination of possible bioaccumulation of
14C-methomyl in lactating dairy cows. Wilmington, Delaware, E.I. Du
Pont de Nemours and Co., Agricultural Products (Unpublished report
No. AMR-898-87*).
Priester TM (1984) Batch equilibrium (adsorption/desorption) and soil
thin-layer chromatography studies with methomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Agricultural Chemicals Department
(Unpublished report No. AMR-174-84*).
Proctor NH, Moscioni AD, & Casida JE (1976) Chicken embryo NAD levels
lowered by teratogenic organophosphorus and methylcarbamate
insecticides. Biochem Pharmacol, 25: 757-762.
Quarles JM, Sega MW, Schenley CK, & Lijinsky W (1979) Transformation
of hamster fetal cells by nitrosated pesticides in a transplacental
assay. Cancer Res, 39: 4525-4533.
Radwan MA, El-Wakil HB, & Osman KA (1992) Toxicity and biochemical
impact of certain oxime carbamate pesticides against terrestrial
snail, Theba psiana (Müller). J Environ Sci Health, B27: 759-773.
Reeve MW, O'Connell LP, Bissell S, & Ross J (1992) Characterization of
methomyl dissipation on grape foliage. Bull Environ Contam Toxicol,
49: 105-111.
Rogers AS, Culik R, Kaplan AM, & Aftosmis JG (1978) Oral teratogenic
study in rats with Lannate (INX-1179). Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-498-78*).
Ruppel RF & Laughlin CW (1977) Toxicity of some soil pesticides to
earthworms. J Kansas Entomol Soc, 50: 113-118.
Saiyed HN, Sadhu HG, Bhatnagar VK, Dewan A, Venkaiah K, & Kashyap SK
(1992) Cardiac toxicity following short-term exposure to methomyl in
spraymen and rabbits. Hum Exp Toxicol, 11(2): 93-97.
Saleh F (1990a) Metabolic effects of the carbamate insecticide
(methomyl) on rats. I. Levels of glucose, lipids and cholesterol in
serum after administration of the insecticide. Egypt J Physiol Sci,
14(1-2): 45-54.
Saleh F (1990b) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in serum cholinesterase and
transaminases following treatment of the insecticide. Egypt J Physiol
Sci, 14(1-2): 55-64.
Saleh F (1990c) Metabolic effects of the carbamate insecticide
(methomyl) on rats. II. Changes in some blood biochemical indices in
the rats poisoned with the insecticide. Egypt J Physiol Sci,
14(1-2): 65-74.
Sarver JW (1991a) Acute oral toxicity study with DPX-X1179-394 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-661-91*).
Sarver JW (1991b) Acute dermal toxicity study of DPX-X1179-394 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-455-91*).
Sarver JW (1991c) Acute oral toxicity study with DPX-X1179-424 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-171-91*).
Sarver JW (1991d) Primary dermal irritation study with DPX-X1179-394
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-165-91*).
Sarver JW (1991e) Primary dermal irritation study with DPX-X1179-425
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-166-91*).
Sarver JW (1991f) Primary dermal irritation study with DPX-X1179-424
in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-162-91*).
Sarver JW (1991g) Primary eye irritation study with DPX-X1179-425 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-280-91*).
Sarver JW (1991h) Primary eye irritation study with DPX-X1179-424 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-279-91*).
Sarver JW (1992a) Acute oral toxicity study with DPX-X1179-439 in male
and female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Haskell Laboratory (Unpublished report No. HLR-858-91*).
Sarver JW (1992b) Acute dermal toxicity study of DPX-X1179-439 in
rabbits. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-825-91*).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Serota DG, Machotka SV, Hastings TF, Alsaker RD, & Lane Fezio W (1981)
104-Week chronic toxicity and carcinogenicity study in mice. Methomyl;
H-11,135. Vienna, Virginia, Hazleton Laboratories America Inc.
(Unpublished report No. HLO-253-81*).
Shah PV, Monroe RJ, & Guthrie FE (1981) Comparative rates of dermal
penetration of insecticides in mice. Toxicol Appl Pharmacol,
59: 414-423.
Sherman H (1966) Acute oral LD50 test in rats using technical
methomyl (>98% methomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-210-66*).
Sherman H (1967) Acute oral potentiation studies with S-methyl
N-[(methylcarbamoyl)oxy] thioacetimidate [INX-1179). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Haskell Laboratory (Unpublished
report No. HLR-160-67*).
Sherman H (1968a) Antidote studies with rats. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-251-68*).
Sherman H (1968b) Acute oral and antidote tests with guinea pigs.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-252-68*).
Sherman H (1968c) Acute oral toxicity and antidote tests in rabbits
using technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-250-68*).
Sherman H (1968d) Acute oral toxicity and antidote tests in dogs using
technical methomyl (>98%). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Haskell Laboratory (Unpublished report No. HLR-279-68*).
Sherman H (1972) Chicken & egg study. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Haskell Laboratory (Unpublished report
No. HLR-55-72*).
Silveira EJ (1990) Technical methomyl - physical and chemical
characteristics. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Agricultural Products Department (Unpublished report
No. AMR-1753-90*).
Simpson GR & Bermingham S (1977) Poisoning by carbamate pesticides.
Med J Aust, 2: 148-149.
Singles GH (1970a) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-186-70*).
Singles GH (1970b) INX-1179 and cholinesterase activity. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-327-70*).
Sleight BH (1971) Continuous exposure of rainbow trout (Salmo
gairdneri) to LannateR in water. Wareham, Massachusetts, Bionomics
Inc. (Unpublished report*).
Sleight BH (1973) Acute toxicity of H-7946 to grass shrimp
(Palaemonetes vulgaris), pink shrimp (Penaeus duorarum) and mud
crab (Neopanope texana). Wareham, Massachusetts, Bionomics Inc.
(Unpublished report No. HLO-186-73*).
Smelt JH, Dekker A, Leistra M, & Houx NWH (1983) Conversion of four
carbamoyloximes in soil samples from above and below the soil water
table. Pestic Sci, 14: 173-181.
Smith LW (1982) Wildlife toxicity studies with methomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Biochemicals Department
(Unpublished report No. ML/FW20*
SRI International (1988) Pesticides and intermediates, Supplement C.
Menlo Park, California, SRI International (Report No. 171C).
Stute K (1983) Results of the registration trials on bee toxicity -
1983. Celle, Germany, Du Pont de Nemours (Germany) GmbH (Unpublished
report No. METH/ECO14*).
Swanson MB (1986) Photodegradation of [1-14C] methomyl in soil.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Agricultural
Products Department (Unpublished report No. AMR-611-86*).
Ta'Naka I, Igisu H, Haratake J, Cho S, Mori K, Fujishiro K, Inoue N,
Horie A, & Akiyama T (1987) Cumulative toxicity potential of methomyl
aerosol by repeated inhalation. Am Ind Hyg Assoc J, 48(4): 330-334.
Teeters WR (1968) Lannate antidotal study - monkeys. Falls Church,
Virginia, Hazleton Laboratories Inc. (Unpublished report
No. 201-219*).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a fungicide
on the numbers and biomass of earthworms in pasture. Bull Environ
Contam Toxicol, 12(4): 487-492.
Trivits R (1979) Acute oral LD50 test in rats using technical
methomyl. Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell
Laboratory (Unpublished report No. HLR-496-79*).
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 74-75 (Resource Publication No. 84).
USDI (1975) Acute toxicity studies. Cutthroat trout and stonefly
larvae. Columbia, Missouri, US Department of the Interior, Fish and
Wildlife Service, Columbia National Fishery Research Laboratory
(Unpublished report No. ML/FW-1*).
USDI (1978) Methomyl - Summary of acute toxicity. Columbia, Missouri,
US Department of the Interior, Fish and Wildlife Service, Columbia
National Fishery Research Laboratory (Unpublished report
No. ML/FW-3*).
USEPA (1988) Methomyl science chapters - June 1988: Parts I and II.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances.
USEPA (1991) Pesticides in ground water database. Washington, DC, US
Environmental Protection Agency, Pesticides and Toxic Substances.
US FDA (1993a) Food and Drug Administration total diet study: 20
market basket studies, October 1986 - April 1991: Findings of methomyl
in food collected for total diet study. Washington, DC, US Food and
Drug Administration.
US FDA (1993b) FDA-Regulatory monitoring data: FY 1988-1992 - Findings
of methomyl in samples of domestic and imported foods. Washington, DC,
US Food and Drug Administration.
US FDA (1993c) FDA-Regulatory monitoring data: FY 1988-1992 - Counts
of samples of domestic and imported foods and feeds analyzed by
methodology known to be capable of determining methomyl. Washington,
DC, US Food and Drug Administration.
Vincent DR (1985) Assessment of methomyl (INX-1179-255) in the
in vitro unscheduled DNA synthesis assay in primary rat hepatocytes.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Haskell Laboratory
(Unpublished report No. HLR-149-85*).
Ward TJ & Boeri RL (1989) Static acute toxicity of methomyl to the
mysid, Mysidopsis bahia. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-675-89*).
Ward TJ & Boeri RL (1990) Static acute toxicity of methomyl to bivalve
mollusc embryos and larvae. Hampton, New Hampshire, Resource Analysts
Inc., EnviroSystems Division (Unpublished report No. HLO-422-90*).
Ward TJ & Boeri RL (1991) Acute flow-through mollusc shell deposition
test with DPX-X1179-394 (methomyl). Hampton, New Hampshire, Resource
Analysts Inc., EnviroSystems Division (Unpublished report
No. HLO-710-91*).
Ware GW, Estesen B, & Cahill WP (1978) Dislodgeable insecticide
residues on cotton (1975). Bull Environ Contam Toxicol, 20: 17-19.
Ware GW, Estesen BJ, & Buck NA (1980) Dislodgeable insecticide
residues on cotton foliage: Acephate, AC222705, EPN, fenvalerate,
methomyl, methyl parathion, permethrin and thiodicarb. Bull Environ
Contam Toxicol, 25: 608-615.
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. In: Fleck RA & Hollaender A ed. Genetic toxicology: An
agricultural perspective. New York, London, Plenum Press, pp 275-324.
Williams DT, Denley HV, Lane DA, & Quan ESK (1982) Real time
monitoring of methomyl air levels during and after spraying in a
greenhouse. Am Ind Hyg Assoc J, 43: 190-195.
Wojciechowski JP, Kaur P, & Sabharwal PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Yeboah PO & Kilgore WW (1984) Analysis of airborne pesticides in a
commercial pesticide storage building. Bull Environ Contam Toxicol,
32: 629-634.
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Jpn Bull Agric Chem Insp Stn, 12: 122-128.
Zwick TC & Malik N (1990a) Aerobic metabolism of [1-14C] methomyl in
Madera, California soil. Columbus, Ohio, Battelle Memorial Institute,
Environmental Sciences Department (Unpublished report
No. AMR-1543-89*).
Zwick TC & Malik N (1990b) Anaerobic metabolism of [1-14C] methomyl
in Madera, California soil. Columbus, Ohio, Battelle Memorial
Institute, Environmental Sciences Department (Unpublished report
No. AMR-1544-89*).
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le méthomyl est un solide cristallin blanc dont le point de
fusion est de 77°C et la tension de vapeur de 0,72 mPa à 25°C. Sa
solubilité dans l'eau est de 54,7 g/litre et son coefficient de
partage octanol/eau (Kow) est de 1,24. Il est stable dans l'eau
stérile à pH 7 mais il se décompose à pH plus élevé, sa demi-vie étant
de 30 jours à pH 9 et 25°C.
Le dosage du méthomyl dans divers échantillons consiste en une
extraction suivie d'une purification, l'analyse finale s'effectuant
par chromatographie en phase liquide à haute performance ou
chromatographie gaz/liquide. Dans certains cas, avant le dosage
proprement dit, on transforme le méthomyl en oxime ou en fluorophore
(post-colonne).
2. Sources d'exposition humaine et environnementale
Le méthomyl est préparé en faisant réagir le
N-hydroxythioacétimidate de S-méthyle (MHTA) en solution dans le
chlorure de méthylène, sur l'isocyanate de méthyle gazeux à 30-50°C.
Il s'agit d'un carbamate à propriété insecticide que l'on utilise
partout dans le monde sur toutes sortes de cultures. Il sert
notamment à protéger les fruits, les vignes, le houblon, les légumes,
les céréales, le soja, le coton et les plantes ornementales. A
l'intérieur des bâtiments, on l'utilise aussi pour détruire les
mouches dans les animaleries et les laiteries.
Il est principalement présenté sous forme de poudre hydro-soluble
ou encore de liquide miscible à l'eau que l'on dilue pour le
traitement des cultures au sol ou en pulvérisations aériennes. Les
doses d'emploi vont habituellement de 0,15 à 1,0 kg de matière active
par hectare. C'est essentiellement au cours de la préparation et de
l'épandage de ces produits ou par suite de l'ingestion de résidus
subsistant sur des cultures vivrières qu'il peut y avoir exposition
humaine (voir section 5.3.1.4).
3. Transport, distribution et transformation dans l'environnement
Les études de laboratoire montrent que le méthomyl est faiblement
adsorbé aux particules du sol. On a ainsi montré que l'adsorption
était faible sur les minéraux argileux en particulier l'illite; sur
les matières organiques du sol, elle est multipliée par 50 tout en
restant encore relativement faible. Une fois adsorbés, les résidus ne
se désorbent pratiquement pas. Du fait de ses propriétés, on peut
penser que le méthomyl doit être doté d'une certaine mobilité dans le
sol.
Dans l'environnement naturel, la décomposition abiotique du
méthomyl par hydrolyse ou photolyse est lente ou nulle.
Dans le sol, la décomposition aérobie est environ deux fois plus
rapide que la décomposition anaérobie. On a fait état de valeurs de
la demi-vie dans le sol qui vont de quelques jours à plus de 50 jours;
la sécheresse retarde la décomposition. Dans la pratique, on peut
considérer que dans la plupart des cas, on aura après épandage, une
demi-vie d'environ une semaine.
Dans les conditions de plein champ, le méthomyl ne pénètre pas
dans le sol, par lessivage, à une profondeur de plus de 20-30 cm et il
n'y a pas de contamination des eaux souterraines.
Lorsqu'on épand du méthomyl marqué au 14C sur les feuilles de
végétaux, il y absorption, mais le produit n'est pas transporté dans
d'autres parties de la plante. Lorsqu'on l'applique sur le système
radiculaire, le composé est fixé par la plante, le principal résidu
étant le méthomyl lui-même. Les produits de décomposition volatils du
méthomyl sont le CO2 et l'acétonitrile. Le reste de la fraction
radioactive se retrouve dans les constituants naturels de la plante
tels que les lipides ainsi que les acides et les sucres du cycle de
Krebs. Dans le feuillage, la demi-vie du méthomyl est de l'ordre de
quelques jours.
Après exposition de truites arc-en-ciel à ce composé pendant 28
jours dans un système à courant d'eau, on n'a trouvé aucun signe
d'accumulation.
4. Concentrations dans l'environnement et exposition humaine
Si l'on en croit les analyses effectuées sur diverses sources
d'eau après épandage du composé aux doses recommandées, il est
probable que les concentrations de méthomyl dans les eaux souterraines
sont soit très faibles, soit inférieures à la limite de détection
(< 0,02 mg/litre).
De faibles résidus de méthomyl se retrouvent sur les cultures,
notamment vivrières, lors de la récolte, les quantités dépendant de
facteurs tels que la dose d'emploi, le laps de temps écoulé depuis le
dernier épandage et le type de culture. Ces résidus sont
essentiellement constitués du produit initial.
Dans les produits laitiers, les résidus de méthomyl sont soit
inférieurs à la limite de détection, soit très faibles. Après
administration de méthomyl à des vaches laitières sous forme de
capsules, pendant 28 jours à une dose équivalant à 80 mg/kg de
nourriture, on n'a pas retrouvé de résidus décelables de ce composé ou
de son métabolite (le MHTA) dans la lait ou les tissus de ces animaux
(< 0,02 mg/kg). Aucune trace de méthomyl n'a été non plus décelée
dans les oeufs ou dans les tissus de poules pondeuses qui en avaient
reçu 1 ou 10 mg/kg dans leur nourriture pendant quatre semaines.
Des analyses effectuées par sondage aux Etats-Unis sur la ration
totale ou sur certains types d'aliments, ont montré que la
concentration de méthomyl était soit très faible, soit inférieure à la
limite de détection. Par ailleurs, un certain nombre d'opérations
tels que le lavage, l'épluchage ou la cuisson, réduisent encore la
teneur en résidus.
Des études effectuées lors du retour des ouvriers agricoles sur
les vignobles après épandage, notamment dans les conditions du désert
de Californie, ont montré que pour un résidu foliaire mobile tombé à
0,1 µg/cm2, l'exposition était maximale au niveau du torse et de la
tête lorsque les ouvriers agricoles pratiquaient l'incision annulaire,
le torse et les mains étant les plus touchés lors de la cueillette du
raisin. En ce qui concerne le raisin de table, c'est lors de la
cueillette et de l'emballage que l'exposition était la plus faible.
L'exposition par inhalation était minime.
Après pulvérisation de méthomyl sur des plants de concombres et
de tomates, on a mesuré dans l'air de la serre qui les abritait, des
concentrations d'insecticide allant jusqu'à 4,7 µg/m3 dans la
journée qui a suivi l'épandage. Trois, puis sept jours après ce
traitement, les concentrations de méthomyl dans la zone de
respiration allaient respectivement jusqu'à 14,5 ou 0,7 µg/m3. Dans
l'eau de lavage des mains des jardiniers d'une serre, on a retrouvé
des quantités de méthomyl allant de 10 à 300 µg/heure de travail.
Cela montre que l'exposition cutanée est une voie d'exposition plus
importante que l'inhalation et que la durée de l'attente à observer
avant de retourner sur les lieux doit être basée sur les données
d'exposition cutanée.
5. Cinétique et métabolisme chez les animaux de laboratoire
Après administration à des rats par voie orale, le méthomyl est
rapidement absorbé, métabolisé et excrété, l'ensemble du processus
étant achevé en quelques jours. Une semaine après avoir administré à
des rats du méthomyl radio-marqué à raison de 5 mg/kg de poids
corporel, on a constaté que 54% de la dose était excrétés dans
l'urine, 2 à 3% dans les matières fécales et 34% dans l'air expiré (en
l'espace de cinq jours). Au bout de sept jours, il restait dans les
tissus et la carcasse 8 à 9% de la radioactivité, le carbone-14 étant
incorporé dans les constituants endogènes. C'est dans le sang que la
fraction radioactive était la plus importante (2% de la dose).
Chez ces rats, les principaux métabolites présents dans l'air
expiré étaient du dioxyde de carbone et l'acétonitrile dans le rapport
d'environ 2:1. Dans les urines, le principal métabolite était
constitué par un dérivé mercapturique du méthomyl, à hauteur de 17% de
la dose. On n'a pas décelé la présence de méthomyl ni de son oxime.
On pense que la métabolisation du méthomyl s'effectue selon le
schéma suivant: déplacement du groupe S-méthyle par le glutathion,
puis transformation enzymatique en dérivé de l'acide mercapturique.
Il existe une autre voie métabolique, à savoir l'hydrolyse en MHTA qui
est ensuite rapidement décomposé pour donner du dioxyde de carbone. Il
pourrait y avoir aussi conversion du syn-méthomyl (qui est la forme
insecticide) en isomère anti, lequel serait ensuite décomposé en
acétonitrile par des réactions d'hydrolyse, de transposition et
d'élimination. Chez le singe, le métabolisme est analogue, à cela
près que le dérivé de l'acide mercapturique ne constitue qu'un
métabolite urinaire mineur.
Une heure après application cutanée à des souris d'une solution
acétonique de méthomyl marqué au 14C, on a constaté que le taux de
pénétration était de l'ordre de 85%. A ce moment, 3% de la dose
étaient présents dans le sang, 5% dans le foie et 13% avaient été
excrétés. Au bout de huit heures, l'excrétion totale était de 54,5%.
La décomposition et l'élimination rapides du méthomyl chez le rat
ainsi que son absence d'accumulation tissulaire, est comparable à ce
que l'on observe chez les ruminants.
Du méthomyl administré à des vaches et à des chèvres a subi une
dégradation totale. Ni le composé initial, ni son oxime n'ont été
retrouvés dans le lait ou les tissus. On a montré que le méthomyl
était métabolisé et incorporé dans les constituants naturels du lait
et du foie.
Après avoir fait incuber du méthomyl marqué au 14C avec du
nitrite de sodium dans un macérat de viande séchée, dans des
conditions simulant le milieu gastrique, on n'a pas mis en évidence la
formation de nitrosométhomyl.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
Le méthomyl présente une forte toxicité aiguë par voie orale, sa
DL50 chez le rat allant de 17 à 45 mg/kg de poids corporel. Il
présente également une forte toxicité inhalatoire chez cet animal, la
CL50 à 4 heures étant de 0,26 mg/litre lorsque le composé est inhalé
sous forme d'aérosol. La toxicité cutanée est très faible, la DL50
dépassant 2000 mg/kg de poids corporel chez le lapin (peau intacte) et
1000 mg/kg de poids corporel chez le rat (peau abrasée). Les signes
d'intoxication aiguë sont ceux que l'on peut attendre d'un inhibiteur
de la cholinestérase et consistent, entre autres, en une
hypersalivation, une lacrimation, des tremblements et un myosis. On a
constaté que les animaux récupéraient rapidement et l'examen de leurs
organes n'a pas révélé la présence de lésions anatomopathologies
visibles qui soient imputables au traitement. Le méthomyl ne provoque
pas d'irritation ou d'hypersensibilisation cutanée et il est
légèrement irritant pour la muqueuse oculaire.
L'administration répétée de méthomyl dans la nourriture pendant
des périodes prolongées n'a pas conduit à une accumulation ou à un
accroissement des effets toxiques. Des rats et des chiens à qui on
avait administré une nourriture contenant jusqu'à 250 mg/kg et
400 mg/kg respectivement de méthomyl, pendant 13 semaines, n'ont
présenté ni signes d'intoxication ni surmortalité. Chez des rats qui
en avaient reçu dans leur nourriture à la dose de 250 mg/kg, on a
observé une légère diminution du gain de poids, une réduction du taux
d'hémoglobine et une hyperplasie modérée de la lignée érythroblastique
au niveau de la moelle osseuse. Chez le rat, la dose sans effets
observables était de 50 mg/kg de nourriture (soit l'équivalent d'une
dose quotidienne de 3,6 mg/kg de poids corporel). Des lapins, qui
avaient subi des applications cutanées répétées de méthomyl à des
doses quotidiennes allant jusqu'à 500 mg/kg de poids corporel, pendant
21 jours, ont présenté une hyperactivité ainsi qu'une réduction de
l'activité cholinestérasique plasmatique et cérébrale à la dose la
plus élevée. La dose sans effets nocifs observables était égale,
selon cette étude, à 50 mg/kg de poids corporel par jour.
Au cours d'études à long terme, des rats ont reçu du méthomyl
dans leur nourriture à raison de 0, 50, 100 ou 400 mg/kg et des souris
en ont reçu de la même manière, 0, 50, 75 ou 200 mg/kg. Chez les
rats, les effets observés à la dose la plus élevée consistaient en une
réduction du gain de poids ainsi qu'en une diminution du taux
d'hémoglobine et de la valeur de l'hématocrite. La dose sans effets
observables était de 100 mg/kg de nourriture, soit l'équivalent d'une
dose quotidienne de 5 mg/kg de poids corporel. Chez les souris, on a
constaté aux deux doses les plus élevées, une augmentation du taux de
mortalité ainsi qu'une diminution du taux d'hémoglobine et du nombre
de globules rouges. La dose sans effets observables a été évaluée à
50 mg/kg de nourriture, soit l'équivalent d'une dose quotidienne de
8,7 mg/kg de poids corporel. Lors d'une étude toxicologique de deux
ans chez des chiens (doses de 0, 50, 100, 400 ou 1000 mg/kg de
nourriture), on a observé des signes d'intoxication chez certains
animaux à la dose la plus forte, avec une anémie légère à modérée. La
dose sans effets observables était de 100 mg/kg de nourriture, soit
l'équivalent d'une dose quotidienne de 3 mg/kg de poids corporel.
Des études de deux ans sur des rats et des souris n'ont révélé
aucun signe d'accroissement de l'incidence des tumeurs qui soient
imputables à l'administration de méthomyl, ce qui indique que le
méthomyl n'est pas cancérogène. Il se s'est pas révélé non plus
génotoxique lors d'épreuves in vitro sur des cellules bactériennes
ou mammaliennes et la recherche de lésions primaires de l'ADN sur des
cellules bactériennes ou mammaliennes in vitro ou encore
d'aberrations chromosomiques médullaires lors d'épreuves in vivo
chez le rat, a donné des résultats négatifs. On a cependant observé
la possibilité d'effets cytogénétiques sur des lymphocytes humains
in vivo, attestée par l'augmentation du nombre de micronoyaux et
d'aberrations chromosomiques. Chez des rats et des lapins qui en
avaient reçu par gavage des doses allant jusqu'à 400 mg/kg de
nourriture (soit une dose quotidienne de 16 mg/kg de poids corporel),
le méthomyl n'a pas produit non plus d'effets embryotoxiques ou
tératogènes, alors qu'à ces doses, des effets toxiques pouvaient être
constatés chez les femelles gravides. Lors d'une étude de
reproduction portant sur trois générations de rats, qui avaient reçu
50 ou 100 mg de méthomyl/kg de nourriture (soit l'équivalent quotidien
de 50 ou 10 mg/kg de poids corporel), on n'a pas constaté d'effets sur
la fécondité, la gestation ou la lactation qui soient imputables au
méthomyl et aucune anomalie visible résultant de ce traitement n'a été
observée.
Après administration unique ou répétée de méthomyl, on n'a pas
observé de neurotoxicité retardée. Des rats qui en avaient reçu dans
leur nourriture à raison de 800 mg/kg, n'ont présenté une diminution
sensible de leur activité cholinestérasique sanguine qu'au début d'une
étude de cinq mois. Une étude d'alimentation de 28 jours n'a révélé
qu'une légère diminution de l'activité cholinestérasique cérébrale à
cette dose. Il y a donc eu réversibilité rapide de l'effet
anticholinestérasique du méthomyl chez les animaux au cours de la
période d'expérimentation. In vitro, l'activité cholinestérasique
érythrocytaire humaine s'est révélée six fois plus sensible à l'action
inhibitrice du méthomyl que celle du rat, mais la réactivation
spontanée s'est produite sensiblement à la même vitesse.
Des études menées sur diverses espèces ont montré que l'atropine
est le meilleur antidote d'une intoxication par le méthomyl.
7. Effets sur l'homme
La lecture des rapports sur les intoxications accidentelles et
les suicides par empoisonnement avec du méthomyl éclaire quelque peu
sur la gravité des effets et les possibilités de récupération. Sur
cinq victimes d'une intoxication accidentelle due à la consommation
d'aliments contaminés, trois sont décédées dans les trois heures
suivant le repas. On estime que les victimes avaient ingéré environ
12 à 15 mg de méthomyl/kg de poids corporel. Une femme âgée de 31 ans
et son fils de six ans, qui étaient tous les deux décédés par suite
d'un empoisonnement volontaire, présentaient des concentrations
hépatiques de méthomyl respectivement égales à 15,4 et 56,5 mg/kg.
Les doses ont été estimées à 55 mg/kg de poids corporel pour la mère
et 13 mg/kg de poids corporel pour l'enfant. Chez une femme qui avait
ingéré environ 2,25 g de méthomyl, on a retrouvé dans son sang six
heures plus tard une quantité de méthomyl égale à 1,6 mg/kg.
Vingt-deux heures après l'ingestion, alors que la patiente se
remettait, on n'a plus retrouvé de méthomyl.
Un travailleur chargé d'épandre des pesticides, qui n'avait pas
pris la moindre précaution alors qu'il préparait une bouillie à base
de méthomyl en poudre pour traiter des légumes, a présenté des
symptômes d'intoxication au bout d'une heure avec réduction à 40% de
la normale de son activité cholinestérasique sanguine au bout de 12
heures, la récupération à 80% de la normale intervenant 36 heures plus
tard. D'autres travailleurs, qui avaient pris les précautions
d'usage, n'ont présenté aucun symptôme ou effet sur l'activité de leur
cholinestérase érythrocytaire ou plasmatique lors de l'épandage de
méthomyl par voie aérienne.
8. Effets sur les organismes non visés au laboratoire et dans leur
milieu naturel
Le méthomyl n'a eu aucun effet sur des populations de champignons
ou de bactéries terricoles, et en particulier sur leur action
nitrifiante ou sur l'activité de la déshydrogénase, lorsqu'il était
appliqué aux doses recommandées.
Des études en laboratoire ont permis de fixer à 6,5 mg/litre la
concentration sans effets observables sur la croissance des algues.
Le méthomyl est moyennement à fortement toxique pour les
poissons, les valeurs de CL50 à 96 heures se situant, pour diverses
espèces, dans l'intervalle 0,5-2 mg/litre. Une étude à long terme (21
jours) a montré que la CL50 pour des alevins de truite exposés à du
Lannate 20L (21,5% de méthomyl), était égale à 1,3 mg de méthomyl par
litre. Lors d'une étude toxicologique portant sur de jeunes
cyprinidés de l'espèce Pimephales promelas, la MATC (concentration
maximale acceptable de substance toxique) a été trouvée comprise entre
57 et 177 µg/litre.
Des études de toxicité aiguë portant sur d'autres organismes
aquatiques ont montré que Daphnia magna était l'espèce la plus
sensible au méthomyl, la CL50 à 48 heures étant de 0,032 mg/litre.
Lors d'une étude de 21 jours au cours de laquelle on a étudié la
survie, la croissance et la capacité de reproduction de Daphnia magna,
on a constaté que la concentration maximale acceptable de substance
toxique (MATC) pour le méthomyl était comprise entre 1,6 et
3,5 µg/litre.
Le méthomyl est toxique pour les abeilles, la DL50 par contact
étant de 1,29 µg/insecte et la DL50 par voie orale, de
0,2 µg/insecte.
On a évalué la toxicité aiguë du méthomyl chez plusieurs espèces
d'oiseaux, les valeurs caractéristiques de DL50 aiguë par voie orale
se situant à 10 mg/kg de poids corporel chez les pigeons et à 34 mg/kg
de poids corporel chez la caille japonaise. Il est relativement moins
toxique lorsqu'il est mêlé à la nourriture, la CL50 à 8 jours étant
dans ce cas de 1100 mg/kg de méthomyl (mêlé à la nourriture) pour le
colin de Virginie et de 2883 mg de méthomyl/kg de nourriture pour le
colvert. Lors d'études qui ont duré de 18 à 20 semaines et ont porté
sur une génération, on a évalué à 150 mg/kg de méthomyl dans la
nourriture, la concentration sans effets nocifs observables pour le
colin de Virginie et le colvert.
Aucun effet n'a été observé chez des colins de Virginie qui
avaient été exposés à une série de pulvérisations de méthomyl aux
doses recommandées. Après un épandage de méthomyl sur une zone
forestière et des champs de houblon, aux doses recommandées, on a
effectué deux études sur des populations aviaires sauvages. Elles
n'ont pas révélé de modification de l'activité des oiseaux et n'ont
provoqué aucun effet, en particulier aucun effet létal. Par rapport
aux témoins, on a constaté qu'il y avait réduction des dépôts
graisseux chez les oiseaux chanteurs des forêts traitées; on estime
qu'il s'agit là d'un effet indirect dû à la réduction des populations
d'insectes dont se nourrissent ces oiseaux.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
Le méthomyl est un carbamate qui inhibe la cholinestérase et dont
le mode d'action toxique est bien connu. Il présente une toxicité
particulièrement élevée lorsqu'il est absorbé par voie orale ou par
inhalation, ainsi que le montrent les études sur l'animal, mais sa
toxicité par voie cutanée est faible. Chez l'animal, les signes
d'intoxication aiguë sont caractéristiques d'une inhibition de la
cholinestérase. Cette intoxication est rapidement réversible, avec
une disparition rapide des symptômes et une désinhibition également
rapide des cholinestérases sanguine et cérébrale. Cette prompte
récupération est due au fait que l'inhibition par le méthomyl de la
cholinestérase est rapidement réversible, et d'ailleurs facilitée par
la vitesse d'excrétion élevée de ce composé. Ce que l'on sait des
intoxications humaines accidentelles ou volontaires montre que la
toxicité aiguë du méthomyl est du même ordre chez l'homme que chez
l'animal de laboratoire.
Comme l'action du méthomyl est rapidement réversible pendant la
période d'administration, il a rarement été possible d'observer des
signes d'intoxication aiguë et d'inhibition de la cholinestérase
sanguine au cours des études où on l'administrait mêlé à la
nourriture. Les observations les plus régulièrement rapportées à
l'occasion d'études à long terme consistent, aux doses les plus
élevées dans l'alimentation, en une réduction du gain de poids chez
les rongeurs et une diminution des paramètres érythrocytaires chez les
rongeurs et les chiens. Trois études de longue durée chez des
rongeurs n'ont pas permis de mettre en évidence d'activité
cancérogène. Le composé ne s'est pas non plus montré génotoxique lors
d'épreuves in vitro portant sur divers paramètres biotoxicologiques,
néanmoins il y a une possibilité d'effets cytogénétiques sur les
lymphocytes humains. Une étude chromosomique effectuée in vivo sur
la moelle osseuse de rats s'est également révélée négative.
Chacune des études à long terme sur l'animal a permis, à partir
de la réduction du gain de poids et des paramètres érythrocytaires,
d'obtenir une valeur de la dose sans effets nocifs observables. Elle
se situait à 5 mg/kg de poids corporel par jour chez le rat, à
8,7 mg/kg de poids corporel par jour chez la souris et à 3 mg/kg de
poids corporel par jour chez le chien. Faute d'une différenciation
marquée des effets toxiques entre les différentes espèces, il ressort
de ces études que la dose sans effets nocifs observables chez le
chien, c'est-à-dire 3 mg/kg de poids corporel par jour, peut être
utilisée pour évaluer le risque chez l'homme.
Le méthomyl est faiblement à modérément adsorbé aux particules du
sol, et ne s'en désorbe pratiquement pas. Dans le sol, il se
décompose en aérobiose (avec une demi-vie d'environ une semaine)
environ deux fois plus vite qu'en anaérobiose.
Une fois déposé sur les feuilles de végétaux, le méthomyl est
rapidement absorbé à hauteur de 50% de la dose - les 50% restant étant
adsorbés - et rien n'indique qu'il y ait transport à l'intérieur de la
plante. La concentration du méthomyl absorbé par les cultures
vivrières tombe rapidement à environ 5% de sa valeur initiale en
l'espace d'une semaine.
Plusieurs invertébrés aquatiques, et en particulier les daphnies,
sont très sensibles au méthomyl avec des valeurs de la CL50 de
l'ordre de 10 à 100 µg/litre.
Les poissons, qu'il s'agisse d'espèces d'eau douce ou d'espèces
estuarielles, y sont moins sensibles, puisque les valeurs de la CL50
s'étagent entre 0,5 et 7 mg/litre. Etant donné la faible persistance
du méthomyl et sa toxicité aiguë relativement faible pour les
poissons, le risque encouru par ces derniers est relativement faible.
Aux doses d'emploi recommandées, le méthomyl n'a pas d'effets
nocifs sur l'activité microbienne terricole en climat tempéré.
Le méthomyl est classé comme hautement toxique pour les abeilles
avec une DL50 topique d'environ 0,1 µg/insecte.
Les valeurs de la DL50 aiguë par voie orale varient entre 10 et
40 mg/kg de poids corporel chez diverses espèces d'oiseaux. Les
valeurs de CL50 à cinq jours par voie alimentaire vont de 1100 à
3700 mg/kg de nourriture. Il y a risque d'intoxication aiguë pour les
oiseaux, en particulier lorsque le méthomyl est sous forme de
granulés; son absorption à partir de nourriture contaminée ne devrait
cependant pas être mortelle pour les oiseaux.
La forte toxicité aiguë du méthomyl pour les mammifères de
laboratoire permet de conclure qu'il est tout aussi dangereux pour les
mammifères sauvages.
10. Conclusion
Compte tenu des aspects qualitatifs et quantitatifs de la
toxicité du méthomyl, le groupe de travail a conclu qu'une dose
quotidienne de 0,03 mg/kg de poids corporel ne devrait probablement
pas causer d'effets nocifs chez l'homme, quel que soit le mode
d'exposition.
Resumen
1. Identidad, propiedades físicas y químicas y métodos analíticos
El metomilo es un sólido cristalino blanco con un punto de fusión
de 77°C y una presión de vapor de 0,72 mPa (25°C). Tiene una
solubilidad en agua de 54,7 g/litro y su coeficiente de reparto
octanol/agua (Kow) es de 1,24. Es estable en agua estéril a pH 7,
pero se descompone a pH más elevado, con una semivida de 30 días a pH
9 y 25°C.
El procedimiento analítico para la determinación del metomilo en
muestras diferentes es la extracción seguida de limpieza y análisis
mediante cromatografía líquida de alto rendimiento o cromatografía
gas-líquido. En algunos casos, el metomilo se convierte en su
derivado oxima o en un derivado fluoróforo (después de la columna)
antes de su determinación analítica.
2. Fuentes de exposición humana y ambiental
El metomilo de produce haciendo reaccionar el S-metil- N-
hidroxitioacetimidato (MHTA) en cloruro de metileno con isocianato de
metilo gaseoso a 30-50°C. Es un insecticida de tipo carbamato
utilizado en una gran diversidad de cultivos en todo el mundo. Entre
los cultivos protegidos figuran frutales, vides, lúpulo, hortalizas,
cereales, soja, algodón y plantas ornamentales. En espacios cerrados
se utiliza en establos o vaquerías para luchar contra las moscas.
Las formaciones principales son polvos hidrosolubles y líquidos
hidromiscibles, que se diluyen en agua para el rociado superficial o
aéreo de los cultivos. Las proporciones normales del principio activo
son de 0,15-1,0 kg/ha. La exposición humana ocurre principalmente
durante la preparación y aplicación de estos productos y por ingestión
de residuos que quedan en los alimentos cosechados (véase la
sección 5.3.1.4).
3. Transporte, distribución y transformación en el medio ambiente
En estudios de laboratorio se ha observado que el metomilo se
adsorbe poco al suelo. Se ha demostrado una adsorción débil a los
minerales arcillosos, sobre todo la ilita; la adsorción a la materia
orgánica del suelo es 50 veces mayor, pero sigue siendo relativamente
escasa. Prácticamente no hay desorción del residuo adsorbido. Estas
características llevan a prever que el metomilo tendrá movilidad en el
suelo.
En condiciones medioambientales naturales, su degradación
abiótica por hidrólisis o fotólisis es lenta o no se produce.
La degradación aerobia en el suelo es alrededor de dos veces más
rápida que la anaerobia. Las semividas notificadas para el metomilo
en el suelo varían entre unos días y más de 50. Las condiciones secas
retrasan la degradación. En la práctica, la mayor parte de las
aplicaciones en el campo deberían dar lugar a una semivida de
alrededor de una semana.
En condiciones de campo, el metomilo no sufre lixiviación en el
suelo a profundidades superiores a 20-30 cm y no contamina las aguas
subterráneas.
El 14C-metomilo aplicado a las hojas de las plantas es
absorbido, pero no transportado a otras partes de la planta. Se si lo
aplica en el sistema radicular, la planta lo absorbe y el componente
principal del residuo que queda es el propio metomilo. Los productos
volátiles derivados de su descomposición son CO2 y acetonitrilo. El
resto de la actividad se incorpora a los componentes naturales de la
planta, tales como lípidos, ácidos del ciclo de Krebs y azúcares. La
semivida del metomilo en el follaje de la planta es de unos pocos
días.
No se han encontrado indicios de acumulación del metomilo en
truchas irisadas expuestas al compuesto durante 28 días en un sistema
de flujo continuo.
4. Niveles medioambientales y exposición humana
Los análisis de diversas fuentes de agua tras la aplicación de
las dosis recomendadas del compuesto indican que los niveles de
metomilo en las aguas subterráneas probablemente serán muy bajos o
inferiores al límite de detección (< 0,02 mg/litro).
En los cultivos de productos alimenticios y de otro tipo se
observan niveles bajos de residuos de metomilo en el momento de la
recolección; su concentración depende de diversos factores, como la
cantidad aplicada, el periodo transcurrido desde la última aplicación
y el tipo de cultivo. Los residuos están formados básicamente por
metomilo.
Los residuos de metomilo en los productos lácteos no son
detectables o son muy bajos. No se observaron residuos detectables de
metomilo ni del metabolito MHTA en la leche ni en los tejidos
(< 0,02 mg/kg) de vacas lactantes que habían recibido en el pienso
cápsulas de metomilo en una concentración equivalente a 80 mg/kg
durante 28 días. No se detectó la presencia de metomilo en los huevos
ni en los tejidos de gallinas ponedoras a las que se habían
administrado cantidades de 1 ó 10 mg/kg en los alimentos durante
cuatro semanas.
En los Estados Unidos de América se hicieron análisis de
regímenes completos de alimentación y de determinados alimentos; en
los estudios de muestreo correspondientes, las concentraciones de
metomilo resultaron inferiores al límite de detección o muy bajas.
Los niveles de residuos se reducen aún más por efecto de procesos
tales como el lavado, pelado y guisado de los alimentos.
En estudios sobre la exposición de quienes regresan a zonas
tratadas, en particular en las condiciones del desierto de California,
se ha observado que los trabajadores que volvieron a los viñedos
cuando los residuos foliares que podían desprenderse se habían
reducido a 0,1 µg/cm2, sufrieron la mayor exposición en la parte
superior del cuerpo y en la cabeza durante el atado de los racimos y
en la parte superior del cuerpo y en las manos durante la vendimia.
La recogida y el embalado de las uvas de mesa dieron lugar a la
exposición más baja. La exposición por inhalación fue mínima.
Tras haberse rociado con metomilo plantas de pepino y tomate, las
concentraciones en el aire del invernadero llegaban a 4,7 µg/m3 al
día siguiente del rociado. Tres y siete días después del rociado, las
concentraciones de metomilo en la zona de respiración ascendían a
14,5 y 0,7 µg/m3, respectivamente. Los valores del metomilo en el
agua de lavarse las manos oscilaban entre 10 y 322 µg por hora de
trabajo en un invernadero. Esto indicaba que la vía de exposición
cutánea era más importante que la inhalación y que los intervalos
previos al regreso al lugar tratado deberían basarse en los datos
sobre la exposición cutánea.
5. Cinética y metabolismo en animales de laboratorio
La absorción, el metabolismo y la excreción del metomilo tras la
administración oral a ratas es muy rápida, completándose el proceso en
unos pocos días. En un plazo de siete días después de la
administración a ratas de 5 mg/kg de peso corporal de metomilo
radiomarcado, el 54% se excretó en la orina, el 2-3% en las heces y el
34% en el aire expirado (en cinco días). Después de siete días, en
los tejidos y en el esqueleto quedaba el 8-9% de la dosis de 14C,
que se había incorporado a los constituyentes endógenos. La mayor
concentración de radiactividad se detectó en la sangre (equivalente al
2% de la dosis).
Los componentes metabólicos principales en el aire expirado por
las ratas eran el anhídrido carbónico y el acetonitrilo, en una
proporción de 2:1. El principal metabolito en la orina fue el
derivado mercaptúrico del metomilo, que era igual al 17% de la dosis.
No se detectó metomilo ni su derivado oxima.
La vía metabólica propuesta comprende el desplazamiento del grupo
S-metilo por el glutatión, seguido de una transformación enzimática
que da lugar al derivado mercaptúrico. Otra vía es la hidrólisis, por
la que se produce MHTA, que se descompone rápidamente hasta dar
anhídrido carbónico. Otra vía posible es la conversión del
sin-metomilo (la forma insecticida) en su anti-isómero, que sufre
reacciones de hidrólisis, recomposición y eliminación hasta dar
acetonitrilo. El metomilo se metaboliza de forma semejante en el
mono, salvo que el derivado mercaptúrico es un componente secundario
en la orina.
La penetración del 14C-metomilo una hora después de haber sido
aplicado a ratones por vía cutánea en una solución de acetona se
estimó en un 85%. Para entonces, el 3% de la dosis se hallaba presente
en la sangre, el 5% en el hígado y el 13% se había excretado. En un
plazo de 8 horas la excreción total era del 54,5%.
La descomposición y eliminación rápidas del metomilo en la rata,
junto con su falta de acumulación en los tejidos, son comparables a lo
observado en rumiantes.
El metomilo sufre una descomposición completa en las vacas y
cabras que han recibido una dosis. No se detectaron metomilo ni su
derivado oxima en la leche ni en los tejidos de estos animales. Se
puso de manifiesto que el producto se metabolizaba e incorporaba a los
componentes naturales de la leche y del hígado.
No se detectó la presencia de nitrosometomilo después de haberse
incubado 14C-metomilo con nitrito sódico en un macerado de carne
curada, en condiciones que simulaban las del estómago.
6. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
El metomilo administrado por vía oral tiene una elevada toxicidad
aguda, con una DL50 oral de 17-45 mg/kg de peso corporal en la rata.
También es muy tóxico para la rata por inhalación; en aerosol, la
CL50 a las 4 horas es de 0,26 mg/litro. La toxicidad cutánea es muy
baja; la DL50 es de más de 2000 mg/kg de peso corporal en conejos
(piel intacta) y > 1000 mg/kg de peso corporal en ratas (piel
raspada). Los signos de toxicidad aguda son los que cabe esperar de
un inhibidor de la colinesterasa, entre otros salivación profusa,
lacrimación, temblor y contracción pupilar. La recuperación de estos
efectos fue rápida. En los órganos examinados no se observaron
efectos patológicos graves. El metomilo no irrita ni sensibiliza la
piel, pero irrita levemente los ojos.
La administración repetida de metomilo con los alimentos durante
periodos más largos no produjo aumento de los efectos tóxicos ni
acumulación. Las ratas y perros cuya alimentación contenía metomilo
en cantidades de hasta 250 mg/kg y 400 mg/kg, respectivamente, durante
13 semanas no mostraron signos de toxicidad ni acusaron mortalidad.
Las ratas que recibieron con los alimentos 250 mg/kg mostraron una
pequeña disminución en el aumento del peso corporal, niveles de
hemoglobina más bajos e hiperplasia eritroidea moderada de la médula
ósea. El NOEL en ratas fue de 50 mg/kg en los alimentos (equivalentes
a 3,6 mg/kg de peso corporal por día). Los conejos que recibieron
aplicaciones cutáneas repetidas de metomilo en dosis de hasta
500 mg/kg de peso corporal por día durante 21 días mostraron
hiperactividad y una reducción de la actividad colinesterásica
plasmática y cerebral con la dosis más elevada. El NOAEL en este
estudio fue de 50 mg/kg de peso corporal por día.
Se realizaron estudios de larga duración en ratas que recibían
metomilo en los alimentos en concentraciones de 0, 50, 100 ó 400 mg/kg
y en ratones que recibían 0, 50, 75 ó 200 mg/kg. Los efectos de la
dosis más alta en las ratas fueron una disminución del aumento de peso
y una reducción de la concentración de hemoglobina y del valor
hematócrito. El NOEL fue de 100 mg/kg de alimentos, equivalente a
5 mg/kg de peso corporal por día. En el estudio en ratones, se
observó aumento de la tasa de mortalidad y una reducción de la
hemoglobina y del número de eritrocitos en los dos niveles de dosis
más elevados. El NOEL fue de 50 mg/kg de alimentos, equivalente a
8,7 mg/kg de peso corporal por día. En un estudio de dos años sobre
toxicidad en perros (0, 50, 100, 400 ó 1000 mg/kg de alimentos) con la
dosis más elevada se detectaron síntomas de toxicidad en algunos
animales, junto con anemias de ligeras a moderadas. El NOEL fue de
100 mg/kg de alimentos, equivalentes a 3 mg/kg de peso corporal por
día.
En estudios de dos años en ratas y ratones no se encontró ningún
indicio de aumento de la incidencia de tumores relacionado con el
tratamiento, signo de que el metomilo no es carcinógeno. No fue
genotóxico en célula de bacterias o de mamíferos in vitro y las
pruebas realizadas para determinar la presencia de lesiones primarias
del ADN en células de bacterias y de mamíferos in vitro dieron
resultados negativos; un estudio cromosómico in vivo de médula ósea
de rata también dio resultados negativos. El potencial citogenético
del metomilo en linfocitos humanos in vitro se puso de manifiesto
mediante un aumento del número de micronúcleos y de aberraciones
cromosómicas. El metomilo no produjo efectos embriotóxicos ni
teratogénicos en ratas ni en conejos en dosis de hasta 400 mg/kg en
los alimentos y de 16 mg/kg de peso corporal por día administrados por
sonda, respectivamente; a esos niveles produjo efectos tóxicos en las
madres. En un estudio de reproducción de tres generaciones en ratas
con dosis de 50 ó 100 mg/kg de alimentos (equivalentes a 5 ó 10 mg/kg
de peso corporal por día), el metomilo no tuvo efectos sobre los
índices de fecundidad, gestación o lactación y no se produjeron
anomalías graves relacionadas con el tratamiento.
No se observó neurotoxicidad retardada después de la
administración de una sola dosis o de una serie de dosis de metomilo.
En un estudio de cinco meses en el que se suministraron a ratas
800 mg/kg de alimentos se observó una reducción importante de la
actividad de la colinesterasa sanguínea sólo en las fases iniciales.
En un estudio de 28 días, la administración de esa misma dosis con los
alimentos dio lugar a una reducción ligera solamente de la actividad
de la colinesterasa cerebral. Esto indica que la inhibición de la
actividad de la colinesterasa por el metomilo es reversible
rápidamente en los animales durante los periodos de ingestión.
In vitro, la actividad de la eritrocito colinesterasa humana fue
seis veces más sensible a la acción inhibidora del metomilo que la de
la rata, aunque los índices de reactivación espontánea fueron
semejantes.
Los resultados obtenidos en estudios realizados con varias
especies mostraron que la atropina es el antídoto de eficacia más
general contra la intoxicación por metomilo.
7. Efectos en el ser humano
Las notificaciones de intoxicaciones accidentales y suicidas con
metomilo proporcionan alguna información sobre los niveles de efectos
y la recuperación. Tres de cinco víctimas accidentales de
intoxicación por una comida contaminada murieron antes de que
transcurrieran tres horas desde la ingestión. Se estimó que las
víctimas habían consumido alrededor de 12-15 mg de metomilo/kg de peso
corporal. Una mujer de 31 años y su hijo de seis, que murieron a
causa de un envenenamiento deliberado, tenían en el hígado
concentraciones de metomilo de 15,4 y 56,5 mg/kg, respectivamente.
Las dosis estimadas fueron de 55 mg/kg de peso corporal para la madre
y de 13 mg/kg de peso corporal para el hijo. Seis horas después de
haber ingerido unos 2,25 g de metomilo, la sangre de una mujer
contenía 1,6 mg de metomilo/kg. A las 22 horas de la ingestión no se
podía detectar la presencia de metomilo y la mujer se estaba
recuperando.
Un operario que manipulaba plaguicidas había mezclado, sin tomar
precauciones, una formulación de metomilo en polvo para el rociado de
hortalizas; antes de una hora manifestó síntomas de intoxicación y
después de 12 horas la actividad de colinesterasa sanguínea se había
reducido al 40% de la normal; a las 36 horas la recuperación llegaba
al 80% de la actividad normal. Otros operarios que habían tomado las
precauciones recomendadas no acusaron ningún síntoma ni efectos en la
actividad de la colinesterasa eritrocitaria y plasmática durante
actividades de aplicación aérea de metomilo.
8. Efectos en organismos no destinatarios en el laboratorio y sobre
el terreno
No se observaron efectos en poblaciones de hongos y bacterias del
suelo, en particular en su acción nitrificante y en la actividad de la
deshidrogenasa después de haberse aplicado metomilo en las dosis
recomendadas.
En estudios de laboratorio se determinó una NOEC de
6,5 mg/litro para el crecimiento de las algas.
El metomilo tiene una toxicidad entre moderada y alta para los
peces, con una CL50 a las 96 horas de 0,5 a 2 mg/litro para una
serie de especies. En un estudio de mayor duración (21 días),
utilizando la formulación Lannate 20L (21,5% de metomilo), la CL50
para los alevines de trucha fue de 1,3 mg/litro. En un estudio de
toxicidad de 28 días durante la primera fase de la vida de piscardos
(Pimaphales promelas), la máxima concentración intoxicante aceptable
(MATC) estimada fue de > 57 y < 117 µg/litro.
En pruebas de toxicidad aguda con otros organismos acuáticos,
Daphnia magna fue una de las especies más vulnerables al metomilo, con
una CL50 de 0,032 mg/litro a las 48 horas. En un estudio de 21 días
sobre la capacidad de supervivencia, crecimiento y reproducción de
Daphnia magna, la máxima concentración intoxicante aceptable para el
metomilo fue de > 1,6 y < 3,5 µg/litro.
El metomilo es tóxico para las abejas melíferas; se ha notificado
una DL50 por contacto de 1,29 µg/abeja y una DL50 oral de
0,2 µg/abeja.
Se ha evaluado la toxicidad aguda del metomilo en varias especies
de aves; los valores característicos de la DL50 oral aguda son de
10 mg/kg de peso corporal en palomas y de 34 mg/kg de peso corporal en
la codorniz japonesa. El metomilo es relativamente menos tóxicos si
se ingiere con los alimentos; en ese caso, la CL50 en ocho días es
de 1100 mg/kg de metomilo en los alimentos para la codorniz Colinus
virginianus y de 2883 mg/kg de metomilo en los alimentos para el
pato silvestre. En estudios de una generación realizados durante 18 a
20 semanas, la NOEC fue de 150 mg/kg de metomilo en los alimentos para
la codorniz Colinus virginianus y para el pato silvestre.
No se observaron efectos sobre Colinus virginianus tras su
exposición a una serie de aplicaciones de metomilo por rociado en las
dosis recomendadas. En dos estudios sobre poblaciones de aves
silvestres, después de haberse rociado con metomilo tierras forestales
y campos de lúpulo en las dosis recomendadas, no se observó ningún
cambio manifiesto en la actividad de las aves ni se produjeron efectos
ni mortalidad relacionados con el tratamiento. Los depósitos de grasa
de las aves canoras de los bosques tratados se redujeron en
comparación con el grupo testigo; se consideró que se trataba de un
efecto indirecto de la disminución de los insectos que les sirven de
alimento.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
El metomilo es un inhibidor de la carbamato colinesterasa
mediante un mecanismo bien conocido de acción tóxica. En estudios
realizados en animales se ha observado una toxicidad aguda
particularmente alta por vía oral y respiratoria, pero la toxicidad
por vía cutánea es baja. Los signos de toxicidad aguda en animales
son los característicos de los inhibidores de la colinesterasa. La
reversibilidad de la acción tóxica aguda es rápida; los supervivientes
se recuperan con rapidez de los signos tóxicos y de la inhibición de
la colinesterasa en la sangre y el cerebro. La pronta remisión de los
efectos tóxicos se debe a la rápida reversibilidad de la inhibición de
la colinesterasa, reversibilidad favorecida por la eliminación rápida
del metomilo del organismo. Los datos sobre intoxicaciones humanas
accidentales e intencionales muestran que el nivel de toxicidad aguda
del metomilo en el ser humano es semejante al observado en los
animales de laboratorio.
Habida cuenta de la reversibilidad rápida de la acción del
metomilo durante los periodos de ingestión, en los estudios realizados
raramente se observaron síntomas de toxicidad aguda e inhibición de la
colinesterasa sanguínea. Los resultados más constantes en los
estudios de mayor duración con niveles de alimentación más elevados
fueron una reducción del aumento del peso corporal en roedores y una
disminución del número de glóbulos rojos en roedores y perros. No se
observaron indicios de carcinogenicidad potencial en tres estudios de
larga duración realizados en roedores. El compuesto dio resultados
negativos en las pruebas de genotoxicidad in vitro en las que se
investigaron varios puntos finales, en cambio mostró potencial
citogenético en linfocitos humanos. El resultado de un estudio
cromosómico realizado in vivo sobre médula ósea de rata fue
negativo.
Se identificaron los NOEL en cada uno de los estudios de larga
duración realizados con animales, teniendo en cuenta la reducción del
aumento del peso corporal y el número de eritrocitos. Los NOEL
resultaron ser de 5 mg/kg de peso corporal por día en ratas, 8,7 mg/kg
de peso corporal por día en ratones y 3 mg/kg de peso corporal por día
en perros. Como esos estudios no revelaron diferencias acentuadas
entre especies en cuanto a los efectos tóxicos, se debería utilizar el
NOEL en el perro, que es de 3 mg/kg de peso corporal por día, a
efectos de la estimación del riesgo en el ser humano.
La adsorción del metomilo al suelo es de baja a moderada, y
prácticamente no hay desorción. La degradación aerobia en el suelo
(con una semivida de alrededor de una semana) es aproximadamente dos
veces más rápida que la degradación anaerobia.
La aplicación de metomilo a hojas de plantas da lugar a una
absorción rápida de casi la mitad de la cantidad aplicada (la otra
mitad se adsorbe) y no hay indicios de transporte. La concentración
del metomilo absorbido en los cultivos de productos alimenticios
disminuye rápidamente hasta alrededor del 5% en una semana.
Varios invertebrados acuáticos, en particular los dáfnidos, son
muy sensibles al metomilo, con CL50 del orden de 10 a 100 µg/litro.
Los peces, tanto de agua dulce como estuarinos, son menos
sensibles, oscilando la CL50 entre 0,5 y 7 mg/litro. Dada la escasa
persistencia del metomilo y su toxicidad aguda relativamente baja para
los peces, se supone que el riesgo es bajo.
En las dosis de aplicación recomendadas, el metomilo no menoscaba
la actividad microbiana en suelos templados.
Este producto está clasificado como muy tóxico para las abejas
melíferas y su DL50 tópica es de aproximadamente 0,1 µg/abeja.
La DL50 aguda por vía oral para varias especies de aves oscila
entre 10 y 40 mg/kg de peso corporal. Los valores de la CL50 en la
alimentación (cinco días) varían entre 1100 y 3700 mg/kg de alimentos.
El metomilo, sobre todo en forma de gránulos, constituye un riesgo
agudo para las aves, pero no se prevé que la ingestión de alimentos
contaminados con metomilo pueda causarles la muerte.
La elevada toxicidad aguda del metomilo para los mamíferos de
laboratorio indica que existe un peligro semejante para los mamíferos
silvestres.
10. Conclusiones
Teniendo en cuenta las características cualitativas y
cuantitativas de la toxicidad del metomilo, el Grupo de Trabajo llegó
a la conclusión de que 0,03 mg/kg de peso corporal al día
probablemente no causarían efectos adversos en el ser humano por
ninguna vía de exposición.
