
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 97
METHOMYL
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria 178:
Methomyl
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for Methomyl
(Health and safety guide ; no. 97)
1.Methomyl - toxicity 2.Insecticides - Carbamate
3.Environmental exposure I.Series
ISBN 92 4 151097 8 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.1.1. Primary constituent
1.1.2. Technical product
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Exposure
2.2. Uptake, metabolism, and excretion
2.3. Effects on non-target organisms in the environment
2.4. Effects on experimental animals and in vitro
test systems
2.5. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and
protection, first aid
4.2. Advice to physicians
4.2.1. Symptoms of poisoning
4.2.2. Medical treatment
4.2.3. Health surveillance advice
4.3. Explosion and fire and hazards
4.4. Storage
4.5. Transport
4.6. Spillage and disposal
4.6.1. Spillage
4.6.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluations by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Labelling, packaging, and transport
6.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
1.1.1 Primary constituent
Common name (ISO): methomyl
Chemical structure:
O
"
"
CH3-C=N-O-C-NH-CH3
'
'
S - CH3
Molecular formula: C5H10N2O2S
Relative molecular
mass: 162.2
IUPAC chemical name: S-methyl- N-(methylcarbamoyloxy)
thioacetimidate
CAS chemical name: methyl N-[[(methylamino)
carbonyl]oxy]ethanimido-thioate
CAS registry number: 16752-77-5
RTECS number: AK2975000
Synonyms: metomil, mesomil, OMS 1196
1.1.2 Technical product
Major trade names: Flytek, Golden Fly Bait, Lannate, Methomex,
Nudrin, Pillarmate
Purity: >98% w/w
Impurities: S-methyl- N-hydroxy-thioacetimidate (0.2%)
1,3-dimethylurea (0.4%)
1.2 Physical and Chemical Properties
Methomyl is a white crystalline solid with a melting point of 77°C. It
is soluble in water and in many organic solvents. It is stable at
temperatures up to 140°C; the autoignition temperature is 265°C.
Methomyl is stable in sterile distilled water at pH values of 5-7 but
decomposes increasingly with higher pH and temperature levels. Its
half-life in water at pH 9 is 30 days.
Some physical properties of methomyl are listed in Table 1.
Table 1. Physical properties of methomyl
Physical state crystalline solid
Colour white
Odour slight sulfurous
Melting point 77°C
Vapour pressure (at 25°C) 6.65 mPa
Henry's Law constant 2.1 × 10-11 atm-m3/mole
Octanol-water partition
coefficient (Kow) 1.24
Solubility:
water 54.7 g/litre
toluene 30 g/litre
isopropanol 220 g/litre
ethanol 420 g/litre
acetone 720 g/litre
methanol 1000 g/litre
1.3 Analytical Methods
The principal analytical procedure for the determination of methomyl
residues in foods, crops, and environmental solid media consists of
extraction with an organic solvent followed by solvent partition and
then, usually, a column cleanup. Water samples are mainly submitted
directly to solid phase extraction. The cleaned up samples are
analysed using HPLC or GLC methods, in some cases, after conversion to
the oxime derivative.
Methomyl as technical material and in formulations can be determined
by reverse phase HPLC followed by UV analysis.
1.4 Production and Uses
Methomyl is a monomethyl carbamate insecticide that has been in use
since 1966. It is used in many countries to control insects on fruit,
vines, hops, vegetables, grain, soyabeans, cotton, and ornamentals.
Methomyl is mainly formulated as water-soluble powders and
water-miscible liquids. These products are diluted with water and
applied using ground or aerial spraying equipment. Typical application
rates are 0.15-1.0 kg active ingredient per hectare.
2. SUMMARY AND EVALUATION
2.1 Exposure
Methomyl is photolysed rapidly in water with a half-life of 2-3 days.
It is broken down rapidly in the aquatic environment; analysis of
various water sources in the vicinity of methomyl applications has
shown non-detectable (<0.02 mg/kg) or very low concentrations of the
compound.
When applied in the field, methomyl is not very mobile in the soil and
remains mostly in the top 15-cm layer. Under these conditions, it is
broken down rapidly with a half-life of 11-30 days. Carbon dioxide is
an end product of the degradation. Very low levels of methomyl may be
found in the soil after aerial or ground application to crops at
recommended rates.
Methomyl has a low octanol:water partition coefficient and has shown
no evidence for bioaccumulation in fish. It is not likely to
accumulate in the environment.
After its application to crops, methomyl is broken down on plant
foliage to give carbon dioxide and acetonitrile. The remaining residue
is incorporated into natural plant components, such as Krebs cycle
acids and sugars. Methomyl's half-life on growing foliage is 1-7 days.
Any remaining residue is composed mainly of methomyl itself, which may
be present in low concentrations in food crops at harvesting. These
residues may be reduced further during transport, storage, and
processing. Total diet and individual food analyses show undetectable
or very low methomyl residues. Thus, exposure of the general
population to methomyl is expected to be very low.
After re-entry into treated vineyards, the areas of workers' bodies
that received the highest exposure to dislodgeable foliar residues of
methomyl were the upper body and head, during grape girdling and
the upper body and hands, during raisin harvesting. Inhalation
exposure was minimal. One day after methomyl application to cucumbers
and tomatoes in green-houses, the ambient air concentration was
4.7 µg/m3. Hand wash sampling was considered to be the most reliable
indicator of operator exposure in these circumstances.
2.2 Uptake, Metabolism, and Excretion
Methomyl is absorbed very rapidly after oral administration to rats.
Distribution to tissues and the excretion of breakdown products also
occur very rapidly. The main excretory routes are expired air (as
carbon dioxide and acetonitrile in the ratio 2:1), 34% in 5 days, and
urine, 54% in 7 days. The main urinary metabolite is the mercapturic
acid derivative of methomyl. It is probable that the S-methyl group
on methomyl is displaced by glutathione followed by enzymatic
transformation to give the mercapturic acid derivative. The expired
air products are probably derived from the hydrolysis of methomyl to
its oxime followed by breakdown to carbon dioxide, and, by conversion
of methomyl to its anti-isomeric form, which undergoes hydrolysis,
rearrangement, and elimination reactions to give acetonitrile.
In mice, the penetration of 14C-methomyl through the skin was
estimated to be 85%, one hour after its dermal application in acetone.
Despite this, the dermal toxicity of methomyl is low (section 2.4),
probably because of its rapid metabolism and excretion. Total
radioactivity excreted was 54% within 8 h of application.
Methomyl is also rapidly broken down and eliminated from ruminants
after oral administration. Lactating cows, given 80 mg/kg feed for 28
days, showed no detectable residues of methomyl (<0.02 mg/kg) in milk
or tissues.
Overall, the absorption, metabolism, and excretion of methomyl is very
rapid in mammals. Much of an oral dose is eliminated within 24 h and
its half-life is a few hours. Tissue levels of methomyl are low and
less than that in blood. There is no evidence for accumulation of
methomyl in the body.
2.3 Effects on Non-target Organisms in the Environment
There is no apparent effect on soil microorganisms when methomyl is
applied at recommended rates.
Methomyl is moderately to highly toxic for fish with 96-h LC50s
mostly in the range of 0.5-2 mg/litre. In an early life stage, 28-day,
toxicity study on fathead minnows, the maximum acceptable toxicant
concentrations (MATC) were >57 and <117 µg/litre, respectively.
Daphnia magna appears to be the most susceptible of other aquatic
organisms with a 48-h LC50 of 31.7 µg/litre. The Fidler crab was one
of the least susceptible with a 96-h LC50 of 2380 µg/litre. In a
21-day study on survival, growth, and reproductive capacity in
Daphnia magna, the MATC was >1.6 and <3.5 µg/litre.
The contact and oral toxicity of methomyl is high for honey bees.
Field observations indicate that early morning or evening application
of methomyl to flowering crops is advisable, because bees are less
active and the spray has time to dry before exposure occurs.
Methomyl is toxic for birds with acute oral LD50s of 10 mg/kg body
weight for the pigeon and 34 mg/kg body weight for the Japanese quail.
It is relatively less toxic by the dietary route with 8-h LC50s of
1100 mg/kg and 2880 mg/kg for the bobwhite quail and mallard duck,
respectively. No effects were seen on birds in field studies, when
methomyl was sprayed at recommended rates from the air or using ground
equipment.
Although methomyl is likely to have high acute oral toxicity for
wildlife mammals, it is unlikely to have any significant effect on
them in normal usage, apart from a small depression of blood
cholinesterase activity.
2.4 Effects on Experimental Animals and in vitro Test Systems
Methomyl is a carbamate cholinesterase inhibitor, depressing
cholinesterase activity in the blood and brain. It causes signs
typical of anticholinesterase action, depending upon the dose,
including pupil constriction, profuse salivation, lacrimation, and
tremor. Recovery from the toxic effects is rapid in surviving animals
after acute doses, because of the rapid reversibility of
cholinesterase inhibition.
The acute oral toxicity of methomyl in the rat is high with an LD50
of 17-45 mg/kg body weight. It is also toxic for the rat via
inhalation with a 4-h LC50 of 0.26 mg/litre, in aerosol form. The
dermal toxicity of methomyl in the rabbit is low with an LD50 of
>2000 mg/kg body weight. Methomyl is not a skin irritant or
sensitizer and is only mildly irritant to the eye.
The repeated administration of methomyl does not show any accumulated
or increased toxic action. Rats showed mild to moderate toxic effects
when given methomyl at 250 mg/kg diet for 13 weeks. Rabbits given
repeated dermal applications of 500 mg/kg body weight per day for 21
days showed hyperactivity and depressed plasma and brain
cholinesterase activity. No effects were observed at 5 mg/kg body
weight per day. In long-term studies, no-observed-effect levels
(NOELs) were established at 100 mg/kg diet for the rat and the dog,
and, at 50 mg/kg for the mouse.
There was no evidence for a carcinogenic effect in 2-year rat and
mouse studies.
Methomyl was not mutagenic in several types of n vitro assay with
various end-points, or in an in vivo rat bone-marrow chromosome
study.
Neither embryotoxic nor teratogenic effects were produced in rats or
rabbits at doses of up to 400 mg/kg diet or 16 mg/kg body weight per
day (by gavage), respectively. Rat reproduction studies over 2 or 3
generations did not show any morphological effects. In a 3-generation
study, reproductive indices were not affected at a dietary level of
100 mg/kg.
Delayed neurotoxicity was not shown in any of the above studies or in
studies on the hen. Measurements of plasma, erythrocyte, and brain
cholinesterase activity during the toxicity studies showed that
cholinesterase inhibition was not increased with repeated dosing.
Atropine was the most effective antidote on the evidence of studies on
several species.
2.5 Effects on Human Beings
Reports of accidental or suicidal poisoning have shown that victims
exhibited cholinergic symptoms following acute overexposure. Survivors
recovered rapidly from the effects. In some cases, effective treatment
was provided by the administration of atropine. Analysis of tissues
and excreta and estimates of the amount ingested indicated that doses
as low as 12-15 mg/kg body weight could be lethal. Reports of
occupational effects usually indicate non-observance of recommended
safety precautions as a causal factor.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Methomyl is a monomethyl carbamate insecticide that is highly toxic by
the oral and inhalation routes. Misuse or disregard of label safety
instructions may therefore lead to severe poisoning.
Its low dermal toxicity and the application of correct handling and
use procedures ensure a low potential for occupational hazard.
Exposure of the general population is expected to be very low and
should not constitute a health hazard.
Methomyl is moderately to highly toxic for aquatic species, bees,
birds, and mammals in laboratory studies. The results of field studies
and observations indicate that methomyl does not give rise to
long-lasting adverse effects, when used as recommended. Methomyl
residue levels in the environment are very low or undetectable.
3.2 Recommendations
Continued emphasis should be given to the correct handling and use of
methomyl so that workers and users apply good work practices, hygiene
measures, and recommended safety precautions.
Observations on regularly exposed workers should be maintained.
Unnecessary contamination of the environment should be avoided by
following appropriate disposal practices. The insecticide must be
applied at recommended rates in field use and, where necessary, any
special precautions to avoid environmental overexposure should be
observed.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Methomyl is a monomethyl carbamate insecticide. The acute toxicity of
technical methomyl by the oral and inhalation routes is high. The oral
toxicity of formulations for laboratory mammals is lower. Both
technical and formulated methomyl products can be hazardous for
humans, if incorrectly handled or misused. Poisoned individuals may
show symptoms typical of anti-cholinesterase action and their onset
may be rapid in cases of over-exposure. Although the dermal toxicity
of methomyl is low and it is not a skin irritant or sensitizer,
adequate precautions should be taken to prevent skin and eye
contamination or to remove any such contamination quickly.
The human health hazards associated with methomyl exposure together
with preventive and protective measures and first aid recommendations
are shown in Table 2.
4.2 Advice to Physicians
4.2.1 Symptoms of poisoning
Methomyl directly inhibits cholinesterase activity, therefore signs of
poisoning can arise very quickly, sometimes within minutes. Early
symptoms include malaise, muscle weakness, dizziness, and sweating.
Headache, salivation, nausea, vomiting, abdominal pain, and diarrhoea
are often prominent. Constriction of pupils (miosis), blurred vision,
and muscle twitching are other symptoms reported. Dyspnoea,
bronchospasm, and chest tightness, leading to pulmonary oedema in the
more severe cases, can also occur. Severe neurological indications
including convulsions and coma are less commonly observed than with
organophosphate poisoning.
The effects of poisoning are likely to be of shorter duration than
those of organophosphorus toxicity. Because blood cholinesterase
activity can return to normal within a few hours of exposure, its
measurement may not be a helpful indicator of poisoning and recovery.
However, it may be of some use if measured within an hour or so of a
severe poisoning, provided that a rapid assay method is used that
takes into account the rapid enzyme reactivation, which can occur in
blood samples as well as in vivo. A normal blood cholinesterase
activity value does not disprove the diagnosis of symptoms and
treatment of a suspected poisoning should proceed immediately.
4.2.2 Medical treatment
For each case, the appropriate emergency first aid (as shown in
Table 2) should be applied. In the case of a swallowed dose, when the
patient is conscious, induce vomiting or perform gastric lavage and
administer activated charcoal. If the patient has difficulty in
breathing or is unconscious, give oxygen and begin cardiopulmonary
resuscitation (CPR) as needed.
Administer atropine sulfate intravenously or intramuscularly as soon
as possible.
N.B. The use of oximes, e.g., pralidoxime (2-PAM), is not recommended
for carbamate poisonings.
Initially, administer 1-2 mg atropine sulfate intravenously or
intra-muscularly to adults. Repeat dosage at 10 to 30-min intervals
until symptoms are relieved and full atropinization is achieved. For
severe intoxication, initially administer 2-4 mg atropine sulfate
intravenously or intramuscularly and give repeated 2 mg doses every
3-10 min until signs of cholinesterase inhibition disappear. For mild
intoxication of children (<13 years), administer atropine sulfate at
0.05 mg/kg body weight and repeat every 15-30 min until atropinization
is achieved. Maintain a mild degree of atropinization until the
patient recovers by administering 0.02 mg/kg body weight as repeated
doses.
Observe the patient for at least 24 h to ensure that cholinergic
symptoms do not recur.
If the insecticide consists of methomyl in combination with an
organophosphorus pesticide and the poisoning is due partly, or wholly,
to the organophosphate, then the use of oximes, e.g., 2-PAM is
recommended.
Table 2. Human health hazards, preventive and protective measures, and first aid
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: readily absorbed following Avoid exposure
ingestion or inhalation, or
through the skin; if absorbed may
cause cholinesterase inhibition
poisoning: weakness, headache,
vomiting, diarrhoea, excessive
sweating, salivation, pinpoint
pupils, muscular twitching; in
severe cases, dyspnoea, broncospasm,
and chest tightness
SKIN: extensive contamination may Wear clothing covering most of the Remove and wash contaminated clothing; wash
cause poisoning body, protective gloves, and apron contaminated skin with plenty of soap and
water; obtain medical attention, if necessary
EYES: pupil constriction, mild Wear safety goggles or face shield Flush eyes with clean water for at least 15 min;
irritation obtain medical attention if effects persist
INHALATION: overexposure may Avoid breathing dust or aerosol, use Move into fresh air immediately; use oxygen
cause poisoning adequate ventilation, use recommended or artificial respiration, if needed; obtain
respiratory equipment, if required medical attention
INGESTION: an unlikely occupational Wash hands before eating, drinking,
hazard using the toilet, and after work
Accidental or intentional ingestion Keep the insecticide out of reach of Obtain medical attention immediately; if
may rapidly lead to severe children and under lock and key breathing has stopped, apply artificial
poisoning respiration; induce vomiting, if person is
conscious
4.2.3 Health surveillance advice
Unlike most organophosphorus compounds, the use of blood
cholinesterase activity monitoring is of limited value for the
assessment of occupational exposure to methomyl and many other
N-methylcarbamates. This is because of the fast reversal of enzyme
inhibition in vivo and the difficulty of submitting blood samples
quickly enough for instantaneous assay. It may be useful, however, to
help in differential diagnosis in eliminating poisoning with an
anticholinesterase compound.
4.3 Explosion and Fire Hazards
Technical methomyl is not sensitive to impact. Its lower explosion
limit is 0.269 g/litre and its autoignition temperature is 265°C.
Dusts may form an explosive mixture in air. Hazardous gases and
vapours may be produced under fire conditions including sulfur oxides,
HCN, and methylisocyanate. Liquid formulations may be combustible and
heating releases vapours that are ignitable.
In case of fire, evacuate personnel to a safe area. Use water squirts,
dry powder, foam, or dry ice to control/extinguish fire. Warn
fire-fighting personnel to wear full protective equipment, including
self-contained breathing apparatus. Prevent contamination of local
water sources.
4.4 Storage
Keep containers tightly closed in dry, cool, well-ventilated areas set
aside for pesticide storage. Do not store them near foodstuffs or
animal feed. Keep products out of reach of children and unauthorized
personnel. Liquid formulations should be kept at a temperature above
0°C.
4.5 Transport
Comply with any local regulations regarding the movement of hazardous
goods. Do not load into transport units containing foodstuffs or
animal feed. Ensure that containers are undamaged and sound and that
labels are securely fixed and intact before transportation.
4.6 Spillage and Disposal
4.6.1 Spillage
Check any fire and explosion hazards and safety precautions before
clean up. Wear appropriate personal protective equipment. Evacuate
personnel and thoroughly ventilate the area. Keep upwind of the
spillage and remove sources of heat, sparks, flame, impact, friction,
and electricity. Use sawdust, sand, or other absorbent material to
help clean up; transfer to a clean, dry, empty container and label it.
Contain any liquid spillage with a barrier of soil, sand, or other
suitable, available material and prevent it from reaching drainage
systems and waterways. If any product remains in crevices, treat with
sodium hydroxide solution, allow to stand for 4 h, then flush out with
water (caution: sodium hydroxide causes skin burns and eye
damage).
4.6.2 Disposal
Disposal must comply with any local or government legislation. Methods
recommended are those described by FAO and GIFAP. Ensure that disposal
does not contaminate drinking-water and food or animal feed and that
there is no danger of run-off or seepage into drainage systems or
watercourses.
Contaminated absorbents, containers, and surplus product should be
burnt in an efficient high temperature incinerator (950-1200°C)
equipped with an effluent gas scrubber. Alternatively, bury in an
approved dump or landfill, or, burn, if allowed by the local
authority.
After agricultural use, excess pesticide, spray mixture, or rinsate
must be used up by further application according to label
instructions. If it is not possible to dispose of the concentrate or
spray mixture in this way, they may be decomposed by the addition of
sodium hydroxide solution (after appropriate dilution of concentrate).
Allow to stand for 4 h, above pH 10, and then incinerate, or, after
neutralization to about pH 7, dispose of according to local
regulations (caution: sodium hydroxide causes skin burns
and eye damage).
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Methomyl is moderately to highly toxic for aquatic organisms. Its
acute toxicity for birds is high, but dietary toxicity is
comparatively low. Methomyl is broken down rapidly in the environment
and does not bioaccumulate. Only very low levels have been found in
soil, water, and plant foliage. It is unlikely that methomyl will pose
a threat to aquatic or bird life at recommended rates of usage.
However, direct, or unnecessary, contamination of streams, rivers,
ponds, lakes, and other natural water-courses should be avoided.
Follow disposal advice outlined in section 4.6.2.
Methomyl is acutely toxic for bees. Do not spray during periods when
bees are foraging; apply methomyl in the early morning or evening when
bees are less active and the spray has time to dry.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluations by International Bodies
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) evaluated
methomyl at its meetings in 1975, 1976, 1977, 1978, 1986, 1987, 1988,
1989, 1990, and 1991. In 1989, an acceptable daily intake (ADI) of
0-0.03 mg/kg body weight was established. See the table on pp. 24-25
for Codex maximum residue limits (MRL).
Methomyl is listed in Class IB, "Highly Hazardous" category, in the
WHO Recommended Classification of Pesticides by Hazard on the basis of
its acute oral LD50.
6.2 Exposure Limit Values
Some exposure limit values for methomyl are given in the table on
pp. 24-25.
6.3 Specific Restrictions
Methomyl has been approved for use as a pesticide in many countries.
In some countries, specific uses are defined as well as limitations
and precautions, particularly for products with a high concentration
of the active ingredient.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies methomyl in:
-Hazard Class 6.1: poisonous substance
-Packing Group II: a substance presenting a
relatively medium risk of
poisoning in transport
The label should be as follows:
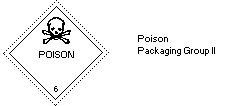 The European Economic Community legislation requires labelling of
methomyl as a dangerous substance using the symbol:
The European Economic Community legislation requires labelling of
methomyl as a dangerous substance using the symbol:
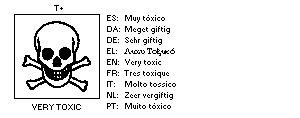 The label must read:
Very toxic by inhalation and if swallowed.
6.5 Waste Disposal
In the USA, methomyl waste is regarded as acutely hazardous. Improper
disposal of excess pesticide, spray mixture, or rinsate is a violation
of Federal law. If the wastes cannot be disposed of according to the
label instruction, contact the appropriate Pesticide or Environmental
Control Agency or Hazardous Waste representative for guidance. Do not
contaminate water, food, or animal feed during storage or disposal.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Exposure limit values
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace United Long-term exposure (8-h TWA) 2.5 mg/m3 1993
Kingdom
USA/ACGIH Threshold limit value (TLV)
- Time-weighted average (TWA) 2.5 mg/m3 1977
USA/OSHA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 2.5 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.03 mg/kg 1989
residues body weight
FAO/WHO Maximum residue limit (MRL) 1991
(methomyl + oxime as methomyl)
- meats, milks 0.02 mg/kg
- cottonseed, peanuts, potatoes, soyabeans, 0.1 mg/kg
beans (dry), sugar beet, sweet corn
- cucumbers, egg plants, melons, onion 0.2 mg/kg
bulbs, pineapples, sorghum, squashes
- barley, oats, onions (Welsh), peas 0.5 mg/kg
(shelled), tomatoes, wheat
FOOD (contd)
Medium Specification Country/ Exposure limit description Value Effective
organization date
- citrus, peppers, sorghum, forage 1 mg/kg
- asparagus, cauliflowers, celery, 2 mg/kg
common beans (pods and/or immature
seed), mint hay, pome fruits
- barley straw and fodder (dry), cabbage 5 mg/kg
(head), grapes, kale, lettuce (head),
nectarines, oat straw & fodder (dry),
peaches, peanut forage (green), peas,
spinach, wheat straw and fodder (dry)
- alfalfa forage, green hops (dry), pea 10 mg/kg
vines (green) soyabean forage (green)
WATER Drinking- Australia "Standard" maximum residue level 0.06 mg/litre 1987
USA/EPA Health advisory, longer term 0.2 mg/litre 1992
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1990) International code of conduct on the distribution and use
of pesticides (Amended version). Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed., Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - a
general introduction. Geneva, World Health Organization.
IPCS (1995) Environmental Health Criteria 178: Methomyl, Geneva, World
Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Plestina R (1984) Prevention, diagnosis, and treatment of insecticide
poisoning, Geneva, World Health Organisation (unpublished document
WHO/VBC/84.889).
United Nations (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification, 1994-95. Geneva, World Health
Organization (unpublished document WHO/PCS/94.2)
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
The label must read:
Very toxic by inhalation and if swallowed.
6.5 Waste Disposal
In the USA, methomyl waste is regarded as acutely hazardous. Improper
disposal of excess pesticide, spray mixture, or rinsate is a violation
of Federal law. If the wastes cannot be disposed of according to the
label instruction, contact the appropriate Pesticide or Environmental
Control Agency or Hazardous Waste representative for guidance. Do not
contaminate water, food, or animal feed during storage or disposal.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Exposure limit values
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace United Long-term exposure (8-h TWA) 2.5 mg/m3 1993
Kingdom
USA/ACGIH Threshold limit value (TLV)
- Time-weighted average (TWA) 2.5 mg/m3 1977
USA/OSHA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 2.5 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.03 mg/kg 1989
residues body weight
FAO/WHO Maximum residue limit (MRL) 1991
(methomyl + oxime as methomyl)
- meats, milks 0.02 mg/kg
- cottonseed, peanuts, potatoes, soyabeans, 0.1 mg/kg
beans (dry), sugar beet, sweet corn
- cucumbers, egg plants, melons, onion 0.2 mg/kg
bulbs, pineapples, sorghum, squashes
- barley, oats, onions (Welsh), peas 0.5 mg/kg
(shelled), tomatoes, wheat
FOOD (contd)
Medium Specification Country/ Exposure limit description Value Effective
organization date
- citrus, peppers, sorghum, forage 1 mg/kg
- asparagus, cauliflowers, celery, 2 mg/kg
common beans (pods and/or immature
seed), mint hay, pome fruits
- barley straw and fodder (dry), cabbage 5 mg/kg
(head), grapes, kale, lettuce (head),
nectarines, oat straw & fodder (dry),
peaches, peanut forage (green), peas,
spinach, wheat straw and fodder (dry)
- alfalfa forage, green hops (dry), pea 10 mg/kg
vines (green) soyabean forage (green)
WATER Drinking- Australia "Standard" maximum residue level 0.06 mg/litre 1987
USA/EPA Health advisory, longer term 0.2 mg/litre 1992
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1990) International code of conduct on the distribution and use
of pesticides (Amended version). Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed., Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - a
general introduction. Geneva, World Health Organization.
IPCS (1995) Environmental Health Criteria 178: Methomyl, Geneva, World
Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Plestina R (1984) Prevention, diagnosis, and treatment of insecticide
poisoning, Geneva, World Health Organisation (unpublished document
WHO/VBC/84.889).
United Nations (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification, 1994-95. Geneva, World Health
Organization (unpublished document WHO/PCS/94.2)
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
 The European Economic Community legislation requires labelling of
methomyl as a dangerous substance using the symbol:
The European Economic Community legislation requires labelling of
methomyl as a dangerous substance using the symbol:
 The label must read:
Very toxic by inhalation and if swallowed.
6.5 Waste Disposal
In the USA, methomyl waste is regarded as acutely hazardous. Improper
disposal of excess pesticide, spray mixture, or rinsate is a violation
of Federal law. If the wastes cannot be disposed of according to the
label instruction, contact the appropriate Pesticide or Environmental
Control Agency or Hazardous Waste representative for guidance. Do not
contaminate water, food, or animal feed during storage or disposal.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Exposure limit values
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace United Long-term exposure (8-h TWA) 2.5 mg/m3 1993
Kingdom
USA/ACGIH Threshold limit value (TLV)
- Time-weighted average (TWA) 2.5 mg/m3 1977
USA/OSHA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 2.5 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.03 mg/kg 1989
residues body weight
FAO/WHO Maximum residue limit (MRL) 1991
(methomyl + oxime as methomyl)
- meats, milks 0.02 mg/kg
- cottonseed, peanuts, potatoes, soyabeans, 0.1 mg/kg
beans (dry), sugar beet, sweet corn
- cucumbers, egg plants, melons, onion 0.2 mg/kg
bulbs, pineapples, sorghum, squashes
- barley, oats, onions (Welsh), peas 0.5 mg/kg
(shelled), tomatoes, wheat
FOOD (contd)
Medium Specification Country/ Exposure limit description Value Effective
organization date
- citrus, peppers, sorghum, forage 1 mg/kg
- asparagus, cauliflowers, celery, 2 mg/kg
common beans (pods and/or immature
seed), mint hay, pome fruits
- barley straw and fodder (dry), cabbage 5 mg/kg
(head), grapes, kale, lettuce (head),
nectarines, oat straw & fodder (dry),
peaches, peanut forage (green), peas,
spinach, wheat straw and fodder (dry)
- alfalfa forage, green hops (dry), pea 10 mg/kg
vines (green) soyabean forage (green)
WATER Drinking- Australia "Standard" maximum residue level 0.06 mg/litre 1987
USA/EPA Health advisory, longer term 0.2 mg/litre 1992
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1990) International code of conduct on the distribution and use
of pesticides (Amended version). Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed., Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - a
general introduction. Geneva, World Health Organization.
IPCS (1995) Environmental Health Criteria 178: Methomyl, Geneva, World
Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Plestina R (1984) Prevention, diagnosis, and treatment of insecticide
poisoning, Geneva, World Health Organisation (unpublished document
WHO/VBC/84.889).
United Nations (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification, 1994-95. Geneva, World Health
Organization (unpublished document WHO/PCS/94.2)
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
The label must read:
Very toxic by inhalation and if swallowed.
6.5 Waste Disposal
In the USA, methomyl waste is regarded as acutely hazardous. Improper
disposal of excess pesticide, spray mixture, or rinsate is a violation
of Federal law. If the wastes cannot be disposed of according to the
label instruction, contact the appropriate Pesticide or Environmental
Control Agency or Hazardous Waste representative for guidance. Do not
contaminate water, food, or animal feed during storage or disposal.
CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Exposure limit values
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace United Long-term exposure (8-h TWA) 2.5 mg/m3 1993
Kingdom
USA/ACGIH Threshold limit value (TLV)
- Time-weighted average (TWA) 2.5 mg/m3 1977
USA/OSHA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 2.5 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.03 mg/kg 1989
residues body weight
FAO/WHO Maximum residue limit (MRL) 1991
(methomyl + oxime as methomyl)
- meats, milks 0.02 mg/kg
- cottonseed, peanuts, potatoes, soyabeans, 0.1 mg/kg
beans (dry), sugar beet, sweet corn
- cucumbers, egg plants, melons, onion 0.2 mg/kg
bulbs, pineapples, sorghum, squashes
- barley, oats, onions (Welsh), peas 0.5 mg/kg
(shelled), tomatoes, wheat
FOOD (contd)
Medium Specification Country/ Exposure limit description Value Effective
organization date
- citrus, peppers, sorghum, forage 1 mg/kg
- asparagus, cauliflowers, celery, 2 mg/kg
common beans (pods and/or immature
seed), mint hay, pome fruits
- barley straw and fodder (dry), cabbage 5 mg/kg
(head), grapes, kale, lettuce (head),
nectarines, oat straw & fodder (dry),
peaches, peanut forage (green), peas,
spinach, wheat straw and fodder (dry)
- alfalfa forage, green hops (dry), pea 10 mg/kg
vines (green) soyabean forage (green)
WATER Drinking- Australia "Standard" maximum residue level 0.06 mg/litre 1987
USA/EPA Health advisory, longer term 0.2 mg/litre 1992
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1990) International code of conduct on the distribution and use
of pesticides (Amended version). Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed., Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides,
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IPCS (1986) Environmental Health Criteria 64: Carbamate pesticides - a
general introduction. Geneva, World Health Organization.
IPCS (1995) Environmental Health Criteria 178: Methomyl, Geneva, World
Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Plestina R (1984) Prevention, diagnosis, and treatment of insecticide
poisoning, Geneva, World Health Organisation (unpublished document
WHO/VBC/84.889).
United Nations (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification, 1994-95. Geneva, World Health
Organization (unpublished document WHO/PCS/94.2)
Worthing CR & Hance RJ, ed. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, The British Crop Protection Council.
