
METHOMYL
EXPLANATION
Methomyl was evaluated for acceptable daily intake by the Joint
Meeting in 1978 (Annex 1, FAO/WHO, 1979a), but an ADI could not be
allocated because of a lack of data. The present toxicological
monograph summarizes the data that were submitted to the 1986 Joint
Meeting.
IDENTITY AND PROPERTIES
CHEMICAL NAME
IUPAC: S-methyl-N-[(methylcarbamoyl)oxy]thioacetimidate
CAS: Methyl N-[[(methylamino)carbonyl]oxy]ethanimid-
othioate (CAS Registry No. 16752-77-5)
SYNONYMS DPX-1179; Lannate(R); Nudrin(R); SD 14999;
WL 18236; Mesomile(R).
STRUCTURAL FORMULA
O
"
CH3 - C = N - O - C - NH - CH3
'
S - CH3
EMPIRICAL FORMULA C5H10N2O2S
MOLECULAR WEIGHT 162.23
PHYSICAL STATE AND White crystalline solid with a slight
COLOR sulfurous odor
DENSITY 1.2946 (at 24°C)
VAPOUR PRESSURE 5 × 10-5 mm Hg (25°C)
MELTING POINT 78 to 79°C
STABILITY Stable in solid form and in aqueous solutions at
pH 7.0 or less. Rapidly decomposes in alkaline
solutions and in moist soils.
SOLUBILITY Solvent g/kg at 25°C
Water 58
Methanol 1000
Acetone 730
Ethanol 420
Isopropanol 220
Toluene 30
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, biotransformation, and excretion
Each of 3 male Crl:CD(SD)BR rats was administered a single dose
of 14C-methomyl (99% pure, labelled in the 1 position, the oximino
carbon) after preconditioning by feeding a diet that contained 200 ppm
unlabeled methomyl. Two of the rats were preconditioned for 8 days
prior to being administered 14C-methomyl at a dose of 5 mg/kg b.w.
The third rat was preconditioned for 19 days prior to dosing with
14C-methomyl at 3.5 mg/kg b.w. Methomyl was rapidly absorbed and
excreted, with a minor portion (10%) retained in the body after 24
hours. Most of the radioactivity was eliminated as carbon dioxide,
acetonitrile, and polar urinary metabolites in the ratio of 1:2:1. The
urinary metabolites, which represented approximately 25% of the
administered dose, were not identified. Methomyl, the S-oxide or
S,S-dioxide of methomyl, the glucuronide or sulfate conjugates of
methomyl, or S-methyl-N-hydroxythio-acetimidate were not detected in
urine by counter-current distribution, enzymatic treatment, or
thin-layer chromatography techniques (Harvey, et al., 1973).
Syn-methomyl (the form of methomyl which is produced and sold)
was metabolized differently by the rat than was the less stable
anti-methomyl. The syn-isomer gave rise to an oxime that was
metabolized primarily to CO2, while the anti-isomer primarily
produced acetonitrile. It was shown that the syn-isomer was
partially isomerized to the anti-configuration in the rat prior to
hydrolysis of the ester linkage. This conversion was related to the
formation of acetonitrile (Huhtanen & Dorough, 1976).
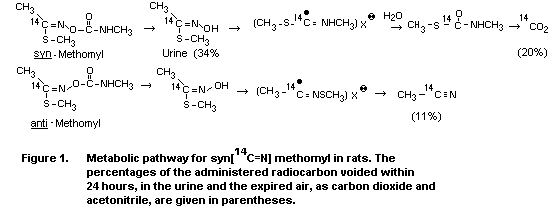
A proposed metabolic pathway for methomyl is shown in Figure 1.
For additional information on the metabolism of methomyl, see the
toxicological monograph on thiodicarb that was published after the
1985 Meeting (Annex 1, FAO/WHO, 1986c).
The dermal penetration, tissue distribution, retention, and
excretion of 14C-methomyl were compared with those of other
pesticides in ICR mice. Approximately 88% of the applied methomyl had
penetrated the skin within 6 hours of application. The penetration
half-life of 13.3 minutes (time for one-half of the applied dose to
penetrate the skin) was similar to that of other carbamates, but
faster than that of the organophosphates that were evaluated,
parathion, malathion, chlorpyrifos, and chlorpyrifos-methomyl. Within
5 to 15 minutes after application, methomyl was detectable in blood,
liver, fat, carcass, and excreta. There was a continual increase of
methomyl in the blood, and by 8 hours it had attained a higher
percentage of dose (6.1%) than the other pesticides examined
(0.3 - 2.7%) (Shah et al., 1981).
 Effects on enzymes and other biochemical parameters
Methomyl was administered in the diet to groups of Crl:CD(SD)BR
rats at levels of 0, 5, 100, 200, 400, or 800 ppm for 5 months. There
were 10 rats/sex/dose. Peripheral blood samples were taken at 4, 11,
32, 43, 60, and 79 days and enzyme activities were measured in whole
blood by the Ellman method. Significant inhibition was reported at
800 ppm in both sexes, but not at 400 ppm (Singles, 1970).
Groups of Crl:CD(SD)BR rats (8/sex/group) were fed technical
methomyl in their diets at dose levels of 0, 100, 400, or 800 ppm for
29 days. Analysis of erythrocyte, plasma, and brain cholinesterase,
determined separately, demonstrated that brain cholinesterase was more
sensitive to methomyl. Females at 100, 400, and 800 ppm demonstrated
depressed brain cholinesterase activity after 29 days compared to
controls, statistically significant at the high dose only. Erythrocyte
and plasma cholinesterase activities, although depresses in males and
females at 800 ppm, were not statistically different from controls
(Barnes, 1978).
Toxicological studies
Special studies on mutagenicity
Methomyl has been shown to be negative in a number of test
systems that have assessed the ability of this compound to cause gene
mutations, structural chromosomal defects, and direct DNA damage. A
single positive result for sister chromatid exchange (SCE) was
reported in an abstract (DeBuyst, B. & Van Larebeke, 1982); however,
this study is not acceptable for evaluation, because methods, purity
of the test substances, and actual results were not reported.
Summary results are presented in Table 1.
Special studies on reproduction
Rats
Groups of male and female Charles River CD rats (13 males/group
and 26 females/group in the F0 generation, 20 males/group and 40
females/group in the F1 generation) were fed diets containing 0, 75,
600, or 1200 ppm technical methomyl continuously over 2 generations.
Feed analyses indicated that the actual levels of methomyl in the test
diets were within acceptable limits of the targeted values. Animals
were observed twice daily for clinical signs of toxicity and body
weights and food consumption were measured weekly. During gestation,
females were weighed on day 3 and daily from days 6 through
parturition. F0 and F1 males were sacrificed after mating, and
sperm counts were performed. After birth, litter size, pup weights,
sex, and numbers of live and dead pups were determined. Pups were
examined daily during lactation for abnormalities of appearance or
behaviour, and were weighed on days 1, 4, 7, 14, and 21 of lactation.
After weaning, pups were weighed weekly. F0 and F1 parents were
subjected to complete necropsies and haematological evaluations at the
end of mating or weaning (males and females, respectively).
Randomly-selected pups from each generation (10/sex/dose) were also
necropsied.
Mean body weights were significantly decreased at most of the
measured intervals in mid- and high-dose male and female parents in
the F0 generation, and in all parental treatment groups in the F1
generation. The decrease in the F1 generation was dose-related, and
ranged from 5 - 10% in the low-dose groups to 20 - 25% in the
high-dose groups; a NOEL was not established for this finding. A
similar pattern was noted in dams during gestation, as dose-related,
statistically-significant decreases were noted in all F1 female
treatment groups, and in mid- and high-dose F0 treatment groups.
These decreases appeared to be related to decreases in food
consumption.
Table 1. Special studies on mutagenicity
Test system Test object Concentration Purity Results References
used
Ames test1 S. typhimurium 0.005, 0.05, Technical Negative2 Blevins,
TA98, TA100, 0.5, 5, et al., 1977
TA1535, 50 nM
TA1537,
& TA1538
Ames test1 S. typhimurium 1, 5, 10, 50, Technical Negative3 Simmon,
TA100, 100, 500, et al., 1977
TA1535, 1000 µg/plate
TA1537,
& TA1538
Ames test1 E. coli 1, 10, 50, Technical Negative4 Simmon
WP2 100, 500, et al., 1977
1000 µg/plate
1 Both with and without metabolic activation.
2 The nitroso- derivative of methomyl was positive at 50 nM.
3 The positive control, 4-o-tolylazo-o-toluidine at 25 µg/plate, gave the expected positive response.
4 The positive controls, AF-2 at 0.1 µg/plate and 2-aminoanthracene at 10 µg/plate, gave the expected positive responses.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
DNA repair E. coli 1 mg/disc Technical Negative5 Simmon
assay W3110, p3478 et al., 1977
B. subtilis
H17, m45
Mitotic S. cerevisiae D3 1.0, 1.5, 2.0, Technical Negative6 Simmon
recombination1 2.5, 3.0 et al., 1977
3.5% w/v
Gene mutations Chinese 1, 5, 10 mM Unknown Negative7 Wojciechowski
in vitro1 hamster et al., 1982
ovary V79
CHO/HGPRT Chinese Nonactivated: 99% Negative8 McCooey
gene hamster 10, 20, 40, et al., 1984
mutation1 ovary cells 50, 55 mM.
CHO-K1, Activated:
BH4 clone 100, 150, 200,
250, 350 mM
5 The positive control, 1-phenyl-3-dimethyltriazene at 1 mg/disc, and the negative control, chloramphenicol at
0.03 mg/disc, gave the expected responses.
6 The positive control, 0.025% 1,2,3,4-diepoxybutane, gave the expected positive response.
7 This study is not acceptable for evaluation because the purity of the test substance was not identified.
8 The positive control, 0.5 mM EMS, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
Sister In vitro Unknown Unknown Positive9 DeBuyst &
chromatid human Van Larebeke,
exchange1 lymphocytes 1982
Sex-linked Drosophila 4 & 10 ppm Technical Negative10 Valencia,
recessive melanogaster 1981
lethal
Unscheduled Primary rat 0, 1, 10, 100, 99% Negative11 Vincent
DNA hepatocytes 1000, 5000, et al., 1985
synthesis from male 10,000,
Crl:CD rats 75,000 µM
Unscheduled WI38 10-7, 10-6, Technical Negative12 Simmon
DNA fibroblasts 10-5, 10-4, et al., 1977
synthesis 10-3 M
9 This study is unacceptable for evaluation because it is in abstract form; methods and data were not reported.
10 The positive controls, EMS, EDB, TMP, and EI, gave the expected positive responses when tested at the appropriate
concentrations.
11 The positive control, 100 and 500 µM DMBA, gave the expected positive response.
12 The positive control, 10-5 M 4-nitroquinoline, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
In vivo Sprague-Dawley 0, 2, 6, 99% Negative13 Farrow
bone marrow male and female 20 mg/kg et al., 1984
cytogenetics rats
13 The positive control, 40 mg/kg cyclosphosphamide, gave the expected positive response.
At necropsy of the F0 parents, dose-related, statistically-
significant decreases in red cell count, haemoglobin concentration,
and haematocrit were noted in mid- and high-dose females; males were
not similarly affected. No statistically-significant decreases in
blood ChE activity were noted; however, mean plasma ChE activity was
decreased by 15% and 25% in high-dose F0 males and females,
respectively. Haematology/ChE results were not reported for F1
animals. Absolute and relative weights of several organs were altered
in a manner consistent with the treatment-related changes in body
weights, as absolute organ weights tended to decrease whereas relative
organ weights tended to increase, relative to the controls. No effects
of treatment on histopathological examination of F0 or F1
parents were noted.
Fertility, gestation length, and male sperm counts were not
adversely affected by treatment. However, dose-related statistically-
significant decreases in litter size and mean number of live pups
born were noted in all F1 treatment groups and in high-dose
F0 rats; a NOEL was not established for this finding. Pup survival
during lactation was significantly reduced in high-dose F1 pups
(born of F0 dams), but survivial did not appear to be affected
in F2 pups (born of F1 dams) after day 7 of lactation (a
significant decrease was noted in high-dose pups on day 4). Mean body
weights during lactation were significantly reduced in a dose-related
manner in all F1 pups; however, body weights were reduced only in
the mid- and high-dose groups of the F2 generation; a NOEL was not
established for this finding. No treatment-related histopathological
changes were noted in F1 or F2 pups (Lu, 1983).
Groups of Charles River CD rats (10 males/group and 20
females/group) were fed diets containing 0, 50, or 100 ppm methomyl
(purity unspecified) over 3 generations. F3b weanlings (10 sex/dose)
were subjected to necropsy and examined for histopathological changes.
A third group of weanlings (F3c) was fed test diets for 9 weeks and
then control diet during weeks 10 through 14 to assess the effect of
treatment on growth.
The study report indicated that no effects of treatment on
fertility, litter size, number of live pups, or pup survivial were
noted. A slight (10%) decrease in the mean body weights of male and
female pups at weaning in the F2 and F3 generations was not
considered to be related to treatment. No effects of treatment on
absolute or relative organ weights were noted. No treatment-related
changes in the gross or microscopic appearance of tissues were
reported; however, actual data were not submitted. Growth of F3c
weanlings fed 100 ppm methomyl was significantly less than that of
control rats, which was related to decreased food intake. This trend
was not reversed by removal of animals from the test diet for 4 weeks
(Kundzin & Busey, 1968).
Special studies on teratogenicity
Rats
Groups of naturally-bred Charles River (ChR-CD) female rats
(25/group) were randomly assigned to treatment groups that received
test diets containing 0, 50, 100, or 400 ppm technical methomyl
(< 99% active ingredient) and 1% Mazola corn oil over days 6 - 15 of
presumed gestation. Average doses were calculated to be 0, 4.9, 9.4,
and 34 mg/kg b.w./day, based on mean food consumption and body
weights. Control rats received rat laboratory feed with 1% corn oil.
Body weights, food consumption, and clinical signs were recorded
periodically during the gestation period. On day 21 of gestation, rats
were sacrificed with chloroform, and the ovaries and uteri were
removed and examined. The following parameters were determined: number
of corpora lutea, number of implantations, number and location of live
and dead fetuses and resorptions, body weights and crown-rump lengths
of live fetuses, and any gross fetal abnormalities. Approximately
one-half of the fetuses from each litter were cleared and stained for
skeletal examination, and the remaining fetuses were fixed and
examined for visceral anomalies.
No effects of treatment on the incidence of clinical signs were
apparent, and no deaths were noted. Mean maternal body weight was
reduced to 92 and 95% of the mean control weight on days 16 and 21,
respectively, in high-dose dams (p < 0.05 by Dunnett's LSD). Mean
feed consumption was also reduced by about 15% in high-dose dams over
days 6 - 16, but it was similar to feed consumption of control animals
over days 16 - 21 (treatment was terminated on day 16). Maternal
weight gain and feed consumption in the low- and mid-dose groups did
not appear to be significantly affected. No effects of treatment on
any maternal reproductive indices were apparent. Mean fetal body
weights and crown-rump lengths were not affected in any test groups.
No effects of treatment on the fetal or litter incidences of visceral
or skeletal anomalies were noted at doses up to and including a
maternally-toxic dose of 400 ppm (34 mg/kg b.w./day) (Rogers et
al., 1978).
Rabbits
Groups of artificially-inseminated New Zealand white (DLI:NZW)
rabbits were randomly assigned to test groups (20/group) that were
administered 0, 2, 6, or 16 mg/kg b.w./day technical methomyl (98.7%
active ingredient) over days 7 -19 of presumed gestation. The route of
administration was by gavage (5 ml/kg of deionized water), and dosages
were selected on the basis of a preliminary study which demonstrated
that a dose of 24 mg/kg b.w./day was lethal to 2/4 rabbits, and
produced significant clinical signs in the remaining 2 does. Control
animals received only deionized water. Rabbits were examined daily for
clinical signs of toxicity, and body weights were recorded
periodically. On day 29 of gestation, does were sacrificed by CO2
asphyxiation, and uteri were removed and weighed. The following
parameters were determined: number of corpora lutea, number and
placement of implantations, resorptions, and live and dead fetuses.
Fetuses were individually weighed, and live fetuses were killed and
examined for gross external, visceral, and skeletal abnormalities.
One mid-dose and 6 high-dose rabbits died as a result of
treatment, and a statistically-significant increase in the incidence
of signs related to cholinesterase inhibition (tremours, hyper-
activity, salivation, etc.) was noted in high-dose animals. Mean
body weights were increased by about 5% on day 29 in surviving
high-dose does, and mean weight gain over days 0 - 29 was increased
about 3-fold in these rabbits (p < 0.01 by Dunnett's LSD). No effects
of treatment on the number of resorptions or litter size were
apparent. Statistically-significant increases in mean fetal body
weights of 14 - 19% were noted in all dose groups, which appeared to
be treatment-related. This change was reported to be related to
increased food consumption in treated rabbits in the latter portion of
the study; however, actual food consumption data were not submitted.
No evidence of oedema was noted in treated fetuses, nor was any effect
of treatment on the incidences of fetal malformations or variations
apparent, at doses up to and including a maternally-toxic dose of
16 mg/kg b.w./day (Christian et al., 1983).
Data were combined from 2 older studies, conducted 6 months
apart, in which a total of 12 rabbits/group were treated with 0, 45 -
50, or 90 - 100 mg/kg b.w./day technical methomyl (purity 90 - 100%)
mixed into the diet at appropriate concentrations to achieve the
desired dosages. The animals were treated from days 8 - 16 of presumed
gestation. Clinical signs and food consumption were recorded daily and
body weights were measured weekly. One-half of the does in each group
were sacrificed on day 29 and subjected to caesarean necropsy, whereas
the remaining rabbits in each group were allowed to deliver normally.
One-third of the fetuses or pups from each litter were examined for
skeletal anomalies. The extent of soft tissue examinations was not
adequately described, but appeared to be restricted to gross
observations of internal organs.
One control and 1 low-dose rabbit died during the study period,
neither of which was pregnant. One other control doe delivered a
full-term litter on the second day of treatment, and was excluded. One
control doe and one low-dose doe aborted. High-dose rabbits were
reported to have consistent weight loss related to decreased food
consumption; however, actual data were not submitted. The incidences
of pregnancy, after combination of the 2 studies, were 4/12, 8/12, and
5/12 in the control, mid-dose, and high-dose groups, respectively. No
effects of treatment on maternal reproductive indices, fetal body
weights or size, or visceral/skeletal findings were noted
(Busey, 1967a).
Acute toxicity
The acute toxicity of methomyl to several animal species is
summarized in Table 2. Methomyl is highly toxic via both the oral and
inhalation routes of exposure. The severity and onset of cholinergic
signs were related to the administered dose.
Table 2. Acute toxicity of methomyl
Solvent LD50 LC50
Species Route Sex (vehicle) (mg/kg b.w.) (mg/1) Reference
Rat Oral M Peanut oil1 26 - Sherman, 1964
Oral M Peanut oil1 17 - Sherman, 1966
F 23.5
Oral M Water2 45 - Trivits, 1979
Dermal M Water1 > 1000 - Morrow, 1972
Inhalation, M Aerosol - 0.30 Foster, 1966a
4 hours
Rat Inhalation, M Vapour - 0.04 Foster, 1966b
4 hours
Inhalation, M Spray - 0.45 Hornberger,
4 hours 1967
Rabbit Oral M Acetone/ 30 - Sherman, 1968a
peanut oil1
Dermal M Water1 > 5000 - Dashiell, 1971
Dermal M&F Water1 > 1500 - Majut & Hood,
1966
Chicken Oral F Acetone/ 28 - Krauss & Stula,
peanut oil1 1967
Dog Oral M Gelatin 20 - Sherman, 1968b
capsule1
1 Technical methomyl having a purity > 98% was used.
2 Technical methomyl having a purity of 100% was used.
Short-term studies
Rats
Groups of Charles River (CD) rats, 10/sex/group, were adminis-
tered methomyl (technical, 100%) in the diet for 90 days at dose
levels of 0, 10, 50, or 250 ppm. An additional group was fed diets
that contained 125 ppm methomyl for 42 days and 500 ppm methomyl to
the end of the study. Observations included mortality, serological,
haematological, and urological examinations, plasma and erythryocyte
ChE activities, food and water consumption, body weights, and gross
and microscopic analyses. No deaths or dose-related behavioural,
clinical, haematological, serological, urological, or pathological
effects were observed. Body weights were depressed in males at 250 ppm
and at 125/500 ppm, and in females at 125/500 ppm. Sporadic decreases
in haemoglobin content or erythrocyte count were observed in test-
group males and females, but the responses were not time or dose
dependent. Moderate erythroid hyperplasia was observed in the bone
marrow of male rats fed 250 ppm. The NOEL was 50 ppm methomyl, based
on body-weight depression in males (Paynter, 1966).
Rabbits
Methomyl (90% soluble concentrate) was administered dermally to
New Zealand albino rabbits (5/sex/dose) at 0 or 200 mg/kg b.w. to both
intact and abraded skin areas. Applications were 5 days a week for 21
days. There were no toxic symptoms noted in animals with intact skin.
There were signs of ChE inhibition and 2 deaths in animals with
abraded skin, although ChE activity was comparable to controls. There
was no evidence of cumulative toxicity or of compound-related effects
on organ weights or histopathology (Busey, 1967b).
Dogs
Groups of 11- to 13-month old beagle dogs (4/sex/group) were fed
diets that contained methomyl (97.5% methomyl, 2.5% HiSil 233) in corn
oil at dosages of 0, 50, 100, or 400 ppm for 3 months. Mean daily
intakes corresponded to 0, 1.44, 3.18, and 14.7 mg/kg b.w./day for
males and 0, 1.45, 3.01, and 12.5 mg/kg b.w./day for females, respect-
ively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, and haemato-
logical, clinical chemical, and urological effects. Cholinesterase
activities were not determined. Organs were weighed and gross necropsy
and histopathology were performed at terminal sacrifice. No compound-
related effects were reported. However, a NOEL for this study could
not be determined because blood cholinesterase activities were not
determined and the animals were older than is recommended by currently
acceptable international guidelines (i.e. OECD test methods) for
conducting short-term non-rodent feeding studies (preferably they
should be 4-6 months of age, but not older than 9 months) (Sherman
et al., 1967).
Long-term studies
Mice
Groups of 80 male and 80 female albino weanling CD-1 mice (6
weeks old) were administered methomyl (technical, purity 99%) in the
diet for 104 weeks at initial dose levels of 10, 50, 100, or 800 ppm.
Due to high mortality, the high dose was reduced to 400 ppm at week 28
and then to 200 ppm at week 39. The mid-dose level was also reduced at
week 39 to 75 ppm. Mean dietary intakes were 8.7, 15.4, and 93.5 mg/kg
b.w./day for males and 10.6, 19.1, and 118 mg/kg b.w./day for females,
respectively.
All animals were observed routinely for clinical signs,
mortality, moribundity, body-weight changes, and food consumption.
Haematological evaluations were performed periodically throughout the
study. Selected organs were weighed, including the brain, thymus,
lungs, heart, spleen, liver, kidneys, testes (w/epididymides),
adrenals, and pituitary. Complete gross and microscopic analyses of
tissues were performed on each animal.
Body-weight gain and food consumption of animals in the treated
groups were equivalent to controls. Mortality was increased among mid-
and high-dose animals throughout the study. Mortality in the low-dose
males was initially increased, in comparison to controls, but was
comparable at the termination of the study. Survival was greater than
50% in all groups at 11 months and approximately 25 - 30% at 2 years.
Red cell mass was apparently decreased during the first 26 weeks
at the mid- and high-dose levels, as evidenced by lower haemoglobin
levels, RBC counts, and haematocrit. These effects were reversed when
the amount of methomyl in the diet was reduced to 75 and 200 ppm in
the mid- and high-dose levels, respectively.
Necropsy and pathological examinations were unremarkable and
there were no compound-related histological changes. A substantial
amount of autolysis occurred in animals found dead prior to term,
which precluded any meaningful analysis of treatment-related effects
in these early deaths. There were no compound-related increases in
neoplastic changes at doses up to and including 200 ppm for 104 weeks.
The NOEL was 50 ppm, based on adverse effects on red cell mass at
higher dose levels (Serota et al., 1981).
Rats
Groups of Charles River rats (35 males and 35 females/group; 70
males and 70 females used as controls) were administered methomyl in
the diet at dose levels of 0, 50, 100, 200, or 400 ppm for 22 months.
These diets corresponded to 0, 2.4, 4.8, 9.6, and 20 mg/kg b.w./day
for males and 0, 2.8, 5.8, 11, and 24 mg/kg for females, respectively.
Body weights, food consumption, appearance, and behaviour were
recorded weekly from weeks 1 through 26 and monthly thereafter to week
96. Clinical chemistry observations included BUN, SGPT, SAP, glucose,
plasma, red blood cell, and brain cholinesterases, and, at the
conclusion of the study, bone-marrow differential counts. The
cholinesterase assays were performed using a delta pH method. This
method is considered inadequate for evaluating cholinesterase
inhibition by carbamates and the data are therefore unreliable.
Haematology determinations included haematocrit, haemoglobin, red
blood cell counts, and total and differential leukocyte counts;
urinalysis included pH, specific gravity, sugar, protein, bilirubin,
occult blood, and microscopic examination of the sediment. At 12
months, 5 rats of each sex in each group were sacrificed. At 22
months, because of the presence of respiratory disease and high
mortality, all surviving animals were sacrificed. Terminal body
weights and gross and microscopic examinations were performed. Organ
weights were obtained for the following tissues: brain, heart, liver,
spleen, kidneys, and testes. Organ and organ-to-body-weight ratios
were recorded and calculated. Microscopic examinations were performed
on the following tissues from rats in the control and 400 ppm groups:
brain, thyroid, heart, liver, spleen, kidneys, adrenals, stomach,
small intestine, large intestine, testes, bone marrow, muscle, and
sciatic nerve. The liver, kidneys, and spleen from rats in the other
groups were examined.
Growth and food consumption, measured only over the first year,
were reduced at 400 ppm. At 200 ppm, growth of males was reduced when
compared with control values. Haematology, blood chemistry, and
urinalysis values were similar in treated and control groups, although
there was a dose-related trend toward decreased haemoglobin in female
treatment groups. This was significant at 18 and 22 months in females
in the 200 and 400 ppm groups. Males were not similarly affected.
Compound-related increases in the incidence and severity of
extramedullary haematopoiesis were observed in the spleens of females
fed 200 or 400 ppm methomyl.
Gross and microscopic examinations were performed at 12 and 22
months. Increased testes, adrenal, liver, and brain weights were
observed at 400 ppm. At 200 and 400 ppm increased thyroid weights in
females were observed. Macroscopic changes in the kidneys, including
enlargement, discoloration, and texture changes were not reflected in
weight differences. Microscopic changes were evident in the kidneys of
both males and females at 400 ppm. The histopathological changes were
characterized by tubular hypertrophy and vacuolation of the epithelial
cells of the proximal convoluted tubules. At 200 and 400 ppm,
extramedullary haematopoiesis was noted in the spleen of females. No
microscopic changes were evident in other tissues, including those in
which weight increases were noted. There was no evidence of oncogenic
potential at any dose level. The 100 ppm dose level could be
considered the NOEL in this study, based on macro- and microscopic
changes in the thyroid, spleen, and kidneys, as well as on body-weight
changes. However, since cholinesterase activity was not measured by an
appropriate and sensitive method for such a potent ChE inhibitor, an
accurate NOEL for this study could not be determined (Busey, 1968a).
In a chronic feeding/oncogenicity study, ChR-CD rats were
administered methomyl (> 99% pure) in the diet for 24 months. Groups
of 80 male and 80 female rats were fed diets containing 0, 50, 100, or
400 ppm methomyl. These concentrations were equal to 0, 2.4, 4.8, and
20 mg/kg b.w./day for males and 0, 2.3, 6.3, and 26 mg/kg b.w./day for
females, respectively. Food consumption and body weights were
measured, and haematological, clinical chemistry, and urological
evaluations were conducted routinely throughout the study. Erythrocyte
and brain acetytcholinesterase activities were measured by the Ellman
method. After 12 months, 10 rats/sex/treatment group were sacrificed
and necropsied, selected organs were weighed, and selected tissues/
organs were examined histologically. At the end of the study all rats
were sacrificed and examined in a similar manner. Tissues from rats
found dead or killed in extremis were examined histologically.
Body-weight gains of males and females in the 400 ppm group were
depressed for the first 1 to 1.5 years, but they recovered thereafter
and were comparable to control body-weight gains by 24 months.
Significantly decreased haemoglobin, RBC counts, and haematocrits were
evident in females in the 400 ppm group. Significant differences were
not observed between control and test-group animals with regard to
erythrocyte or brain cholinesterase activities. However, due to a
possible effect of storage of blood samples prior to measurement, the
potential effect of the test compound on cholinesterases could have
been undiscovered due to fast spontaneous reactivation of carbamylated
enzyme.
Relative testes weights of high-dose males were significantly
greater than those of controls, but there were no observed
pathological changes. Female rats fed > 100 ppm methomyl had
increased relative liver weights, but no associated histopathological
alterations were identified. Female rats fed 400 ppm methomyl also had
increased relative spleen weights, but no noticeable pathological
tissue responses. The incidence of bone marrow hyperplasia, focal
hyperplasia in the adrenal medulla, and focal degeneration/angiectasis
in the adrenal cortex were increased in male rats fed 400 ppm
methomyl. There were no other significant pathological changes and no
oncogenic potential was evident at any of the doses administered in
this study. A NOEL of 100 ppm was estimated based on body-weight
changes and on the haematologic response at 400 ppm (Kaplan et al.,
1981).
Dogs
Groups of young adult (> 12 months of age) beagles (4 male and 4
females/group) were administered methomyl in the diet at dose levels
of 0, 50, 100, 400, or 1000 ppm for 2 years. Methomyl was incorporated
into a ground dry dog meal. Diet mixtures were prepared weekly. Daily
observations were made of behaviour and appearance, and body-weight
and food-consumption data were recorded weekly. Clinical chemistry
studies were performed initially and at 3, 6, 12, 18, and 24 months.
Determinations were made of blood sugar, BUN, SAP, SGPT, SGOT, and
prothrombin time. Serum electrolytes, total protein, and albumin were
determined at 18 and 24 months. Haematology examinations included the
following: haematocrit and haemoglobin concentration, red blood cell
count, total and differential leukocyte count, and bone marrow
differential count at the termination of the study. Urinalysis
included: appearance, pH, specific gravity, sugar, acetone, protein,
bilirubin, occult blood, and microscopic examination of the sediment.
At 24 months, urinary sodium, potassium, chloride, glucose, protein,
and phosphorous were determined. Plasma and erythrocyte cholinesterase
activities were measured by the monometric method at the ninth week in
animals in the control and 1000 ppm groups and during the thirteenth
week in animals in the 1000 ppm group. At the end of 1 year, 1
dog/sex/group was sacrificed and the following organ weights recorded
after gross examination: brain, thyroid, heart, liver, spleen,
kidneys, adrenals, and testes. Organ-to-body weight ratios were
calculated. At 24 months the remaining dogs were sacrificed and the
following tissues were examined from each dog: brain, pituitary,
thyroid, thymus, lungs, heart, liver, spleen, kidneys, adrenals,
stomach, pancreas, duodenum, jejunum, ileum, colon, mesenteric lymph
node, urinary bladder, ovary, uterus, skin, bone, bone marrow, muscle,
sciatic nerve, and testes. The same organs were weighed as after 1
year.
Two out of 4 females in the 1000 ppm group died within 8 weeks of
the beginning of the study. Signs of poisoning included tremours,
salivation, and incoordination, which were observed only once in males
at 1000 ppm. Haematology studies in 1 high-dose male indicated severe
anaemia and moderate leukopenia (decreased haematocrit, haemoglobin,
RBC count and platelet count; increased reticulocyte count; and
changes in the differential count). This condition improved when the
dog was placed on a control diet at 84 weeks, but worsened when
1000 ppm methomyl was replaced in the diet at the ninety-fifth week.
Enlarged spleen and kidneys were noted at sacrifice (24 months). The
remaining high-dose males and 2/4 high-dose females demonstrated
similar haematologic abnormalities at 3 months, but recovered to
normal by 24 months. Growth and food consumption were unaffected by
treatment; however, all dogs were young adults at the start of the
test and therefore were already beyond the critical growth months for
this species. This is considered a deficiency in this study. Clinical
chemistry and urinalyses were comparable between treated groups and
controls. Since plasma and erythrocyte cholinesterase activities were
assayed by a method considered inadequate for carbamate inhibition,
these data are unreliable for estimating the cholinesterase inhibiting
properties of methomyl in dogs.
At 24 months, gross pathology examinations showed liver and
spleen enlargement in the male that was severely anaemic. Kidney
weights were increased in males at 1000 ppm and in 1 male at 400 ppm.
Microscopic examination showed alterations in the spleen
(extramedullary haematopoiesis) and kidneys (pigment in the cytoplasm
and swelling of the epithelial cells of the proximal convoluted
tubules in males and females), liver (bile duct proliferation) and
bone marrow (increased activity in both erythroid and myeloid series)
at 1000 ppm. At 400 ppm similar kidney abnormalities were present only
in males. Alterations were not observed in the lower-dose groups. A
no-effect level of 100 ppm (equal to 3.1 mg/kg b.w./day) was
tentatively estimated, based on the adverse haematologic effects and
on kidney and spleen morphologic changes at > 400 ppm. However, in
the absence of adequate data for adverse effects on cholinesterase
activity an accurate estimate of a NOEL cannot be determined
(Busey, 1968b).
Observations in humans
Epidemiological studies of poisonings from the ingestion of
contaminated food indicate that a single oral dose as low as
12-15 mg/kg b.w. can be fatal in humans (Liddle et al., 1979;
Araki et al., 1982).
An assessment of workers who routinely handled methomyl in a
factory indicated that workers were occasionally inadvertently exposed
and required hospitalization and treatment for symptoms of
cholinesterase inhibition, presumably due to methomyl intoxication.
Blood cholinesterase levels were normal for these workers, however,
and no quantitation of dose was presented. The methodology and timing
of ChE measurements were questionable (Morse et al., 1979).
COMMENTS
Methomyl is rapidly absorbed from the gastrointestinal tract or
through the skin, and is rapidly metabolized and eliminated. The
metabolic fate of methomyl depends on the isomeric configuration, as
the syn form (the technical product) is primarily degraded to CO2,
whereas the anti form is degraded to acetonitrile. Some conversion
of syn-methomyl to anti-methomyl apparently occurs in rats.
The acute toxicity of methomyl is due to inhibition of
acetylcholinesterase. The oral LD50 in experimental animals ranges
from 20 to 45 mg/kg b.w.; atropine is an antidote to these acute toxic
effects.
Long-term feeding studies in mice and rats do not suggest any
evidence of oncogenicity for methomyl. Toxic effects included anaemia
(mice, rats, and dogs), decreased weight gain (rats), and
histopathological changes in the kidney (dogs). The no-observed-effect
levels for chronic toxicity, calculated from dietary intake, were;
8.7 mg/kg b.w./day in mice, 4.8 mg/kg b.w./day in rats, and 3.1 mg/kg
b.w./day in dogs.
The available chronic toxicity data are somewhat limited by the
lack of adequate determinations of cholinesterase activity. However,
the effects on cholinesterases are reversible. In order to estimate
the rate of spontaneous reactivation in man, the submission of the
results of additional in vitro studies is required. These studies
should provide data on the inhibition and reactivation rate constants
for the effect of methomyl on human plasma and erythrocyte
cholinesterases.
Available epidemiological data for humans indicate that a dose of
12 - 15 mg/kg b.w. methomyl, ingested as a bolus with food, can be
lethal owing to suppression of cholinesterase activity. This value is
similar to the acute lethal doses identified in animal studies.
No evidence of teratogenicity or embryotoxicity was apparent in
rats or rabbits. The available rat reproduction studies, when
considered together, demonstrate a NOEL of 50 ppm (equivalent to
2.5 mg/kg b.w./day), based on decreases in body-weight gain and food
consumption in a study reported in 1968, and on slight decreases in
litter size at 75 ppm in a study reported in 1983. No other evidence
of effects on fertility or other reproductive parameters was noted.
No evidence of mutagenicity was noted in a number of genotoxicity
studies.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
NOELs for chronic oral toxicity are established as;
Mouse: 50 ppm, equal to 8.7 mg/kg b.w./day.
Rat: 50 ppm, equivalent to 2.5 mg/kg b.w./day.
Dog: 100 ppm, equal to 3.1 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0 - 0.01 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF A FULL ADI IS
IMPRACTICABLE, TO BE SUBMITTED TO WHO BY 1988:
Additional in vitro studies in human blood to demonstrate the
time-course of plasma and red blood cell cholinesterase inhibition and
reactivation.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Further observations in humans.
REFERENCES
Araki, M., Yonemitsu, K., Kambe, T., Idaka, D., Tsunenari, S., Kanda,
1982 M., & Kambara, T. Forensic toxicological investigations on
fatal cases of carbamate pesticide methomyl (Lannate(R))
poisoning. Nippon Hoigaku Zasshi, 36: 584 - 588.
Barnes, J.R. Cholinesterase tests with methomyl. Unpublished report
1978 No. HLR-280-78 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Blevins, R.D. Lee, M., & Regan, J.D. Mutagenicity screening of five
1977 methyl 1977 carbamate insecticides and their nitrosos
derivatives using mutants of Salmonella typhimurium LT2.
Mutat. Res., 56: 1 - 6.
Busey, W.M. Teratology study in rabbits with "Lannate(R)" methomyl
1967a insecticide. Unpublished Report No. HLO-257-67 from Hazleton
Laboratories, Inc., Falls Church, VA., USA. Submitted to WHO
by E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Busey, W.M. Repeated dermal study in rabbits - "Lannate(R)" methomyl
1967b insecticide (90% methomyl). Unpublished Report
No. HLO-254-67 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Busey, W.M. 22-Month dietary feeding study in rats with "Lannate(R)"
1968a methomyl insecticide. Unpublished Report No. 201-164 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Busey, W.M. Two year dietary administration in dogs with "Lannate(R)"
1968b methomyl insecticide. Unpublished Report No. 201-165 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Christian, M.S., Hoberman, A.M., & Feussner, E.L. Embryo-fetal
1983 toxicity and teratogenicity study of methomyl in the rabbit.
Unpublished report No. HLO-331-83 from Argus Research
Laboratories, Inc., Horsham, PA, USA. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Dashiell, O. Acute skin absorption LD50 test in rabbits using technical
1971 methomyl (> 98% methomyl). Unpublished report No.
HLR-340-71 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
DeBuyst, B. & Van Larebeke, N. Induction of sister-chromatid exchanges
1982 in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242.
Farrow, M.G., Cortina, T., & Padilla-Nash, H. In vivo bone marrow
1984 chromosome study in rats [with] H No. 15,000 - Final Report.
Unpublished Report No. HLO-63-84 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966a methomyl (> 98% methomyl) progress report. Unpublished
report No. HLR-73-66 from Haskell Laboratory for Toxicology
and Industrial Medicine, E.I. du Pont de Nemours and Co.,
Inc. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966b methomyl (> 98% methomyl). Unpublished report
No. HLR-214-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Harvey, J., Jelinek, A.G., & Sherman, H. Metabolism of methomyl in the
1973 rat. J. Agric. Fd. Chem., 21: 769 - 775.
Hornberger, C.S. Acute inhalation toxicity of aqueous spray mist.
1967 Unpublished report No. HLR-171-67 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
1976 reactions in the metabolism of methomyl in rats.
Pest. Biochem and Physiol., 6: 571 - 583.
Kaplan, A.M., Trochimowicz, H.J., & Barba, C. Long-term feeding study
1981 in rats with S-methomyl-N-(methylcarbomoyloxy)
thioacetimidate (methomyl; INX-1179). Unpublished report
No. HLR-235-81 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Kraus, W. & Stula, E.F. Acute oral LD50 and delayed neurotoxicity
1967 (paralysis) tests in hens using technical methomyl (> 98%
methomyl). Unpublished report No. HLR-161-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Kundzin, M. & Busey, W.M. Three-generation reproduction study with
1968 Lannate(R) 1968 methomyl insecticide. Unpublished Report
No. MRO 888-1 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Liddle, J.A., Kimbrough, R.D., Needham, L.L., Cline, R.E., Smrek,
1979 A.L., Yert, L.W., Bayse, D.D., Ellington, A.C., & Dennis,
P.A. A fatal episode of accidental methomyl poisoning.
Clin. Toxicol., 15; 159 - 167.
Lu, C.C. Nudrin(R) two-generation reproduction study in rats.
1983 Unpublished report no. WRC RIR-275 from Westhollow Research
Center, Shell Development Co., Houston, TX, USA. Submitted
to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Majhut, J. & Hood, D. Acute skin absorption, approx, lethal dose (ALD)
1966 test in rabbits using 55 and 26% formulations of (technical)
methomyl in aqueous solutions. Unpublished report
No. HLR-216-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M., & Hemingway, R.E.
1984 CHO/HGPRT assay for gene mutations using technical methomyl.
Unpublished report No. HLR-556-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Morrow, R.W. Acute skin absorption test (ALD) in rabbits using
1972 technical methomyl and a 25% methomyl formulation
(Lannate(R) 25W). Unpublished report No. HLR-438-72 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Morse, D.L., Baker, E.L., Kimbrough, R.D., & Wisseman, C.L.
1979 Propanilchloracne and methomyl toxicity in workers of a
pesticide manufacturing plant. Clin. Toxicol.,
15: 13 - 21.
Paynter, O. Three month dietary administration in rats using
1966 insecticide 1179 (technical methomyl, > 98% purity).
Unpublished Report No. MRO-848 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Rogers, A.S., Culik, R., Kaplan, A.M., & Aftosmis, J.G. Oral
1978 teratogenic study in rats with Lannate(R) (INX-1179).
Unpublished report No. HLR-498-78 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Serota, D.G., Machotka, S.V., Hasting, T.F., Alsaker, R.D., &
1981 Fezio, W.L. 104-Week chronic toxicity and carcinogenicity
study in mice. Unpublished Report No. HLO-253-81 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Shah, P.V., Monroe, R.J., & Guthrie, F.E. Comparative rates of dermal
1981 penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59: 414 - 423.
Sherman, H. Acute oral (approx. lethal dose - ALD) and 10-dose oral
1966 subacute toxicity test in rats using technical methomyl
(> 98% methomyl). Unpublished report No. HLR-100-64 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Sherman, H. Acute oral LD50 test in rats using technical methomyl
1966 (> 98% methomyl). Unpublished report No. HLR-210-66 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Sherman, H., Barnes, J.R., Stula, E.F., & Zapp, J.A. Three-month
1967 feeding study on dogs with S-methyl-N-(methylcarbomoyloxy)
thioacetimidate (Lannate(R) methomyl insecticide;
INX-1179). Unpublished report No. HLR-168-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in rabbits using
1968a technical methomyl (> 98%). Unpublished report
No. HLR-250-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in dogs using
1968b technical methomyl (> 98%). Unpublished report
No. HLR-279-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Simmon, V.F.; Mitchell, A.D., & Jorgenson, T.A. Evaluation of selected
1977 pesticides as chemical mutagens: in vitro and in vivo
studies. Unpublished EPA report No. EPA-600/1-77-028 from
U.S. Environmental Protection Agency, Health Effects
Research Laboratory, Research Triangle Park, N.C., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Singles, G.H. INX-1179 (methomyl) and cholinesterase activity.
1970 Unpublished report No. HLR-186-70 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Trivits, R. Acute oral LD50 test in rats using technical methomyl.
1979 Unpublished report No. HLR-497-79 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Valencia, R. Mutagenesis screening of pesticides in Drosophila.
1981 Unpublished EPA report No. 600/1/81/017. Prepared by Warf
Institute, Inc. for the U.S. Environmental Protection
Agency. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Vincent, D.R., Arce, G.T., & Sarrif, A.M. Assessment of methomyl
1985 (INX-1179-255) in the in vitro unscheduled DNA synthesis
assay in primary rat hepatocytes. Unpublished report
No. HLR-149-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Wojciechowski, J.P., Kaur, P., & Sabharwal, P.S. Induction of ouabain
1982 resistance in V-79 cells by four carbamate pesticides.
Env. Research, 29: 481 - 53.
Effects on enzymes and other biochemical parameters
Methomyl was administered in the diet to groups of Crl:CD(SD)BR
rats at levels of 0, 5, 100, 200, 400, or 800 ppm for 5 months. There
were 10 rats/sex/dose. Peripheral blood samples were taken at 4, 11,
32, 43, 60, and 79 days and enzyme activities were measured in whole
blood by the Ellman method. Significant inhibition was reported at
800 ppm in both sexes, but not at 400 ppm (Singles, 1970).
Groups of Crl:CD(SD)BR rats (8/sex/group) were fed technical
methomyl in their diets at dose levels of 0, 100, 400, or 800 ppm for
29 days. Analysis of erythrocyte, plasma, and brain cholinesterase,
determined separately, demonstrated that brain cholinesterase was more
sensitive to methomyl. Females at 100, 400, and 800 ppm demonstrated
depressed brain cholinesterase activity after 29 days compared to
controls, statistically significant at the high dose only. Erythrocyte
and plasma cholinesterase activities, although depresses in males and
females at 800 ppm, were not statistically different from controls
(Barnes, 1978).
Toxicological studies
Special studies on mutagenicity
Methomyl has been shown to be negative in a number of test
systems that have assessed the ability of this compound to cause gene
mutations, structural chromosomal defects, and direct DNA damage. A
single positive result for sister chromatid exchange (SCE) was
reported in an abstract (DeBuyst, B. & Van Larebeke, 1982); however,
this study is not acceptable for evaluation, because methods, purity
of the test substances, and actual results were not reported.
Summary results are presented in Table 1.
Special studies on reproduction
Rats
Groups of male and female Charles River CD rats (13 males/group
and 26 females/group in the F0 generation, 20 males/group and 40
females/group in the F1 generation) were fed diets containing 0, 75,
600, or 1200 ppm technical methomyl continuously over 2 generations.
Feed analyses indicated that the actual levels of methomyl in the test
diets were within acceptable limits of the targeted values. Animals
were observed twice daily for clinical signs of toxicity and body
weights and food consumption were measured weekly. During gestation,
females were weighed on day 3 and daily from days 6 through
parturition. F0 and F1 males were sacrificed after mating, and
sperm counts were performed. After birth, litter size, pup weights,
sex, and numbers of live and dead pups were determined. Pups were
examined daily during lactation for abnormalities of appearance or
behaviour, and were weighed on days 1, 4, 7, 14, and 21 of lactation.
After weaning, pups were weighed weekly. F0 and F1 parents were
subjected to complete necropsies and haematological evaluations at the
end of mating or weaning (males and females, respectively).
Randomly-selected pups from each generation (10/sex/dose) were also
necropsied.
Mean body weights were significantly decreased at most of the
measured intervals in mid- and high-dose male and female parents in
the F0 generation, and in all parental treatment groups in the F1
generation. The decrease in the F1 generation was dose-related, and
ranged from 5 - 10% in the low-dose groups to 20 - 25% in the
high-dose groups; a NOEL was not established for this finding. A
similar pattern was noted in dams during gestation, as dose-related,
statistically-significant decreases were noted in all F1 female
treatment groups, and in mid- and high-dose F0 treatment groups.
These decreases appeared to be related to decreases in food
consumption.
Table 1. Special studies on mutagenicity
Test system Test object Concentration Purity Results References
used
Ames test1 S. typhimurium 0.005, 0.05, Technical Negative2 Blevins,
TA98, TA100, 0.5, 5, et al., 1977
TA1535, 50 nM
TA1537,
& TA1538
Ames test1 S. typhimurium 1, 5, 10, 50, Technical Negative3 Simmon,
TA100, 100, 500, et al., 1977
TA1535, 1000 µg/plate
TA1537,
& TA1538
Ames test1 E. coli 1, 10, 50, Technical Negative4 Simmon
WP2 100, 500, et al., 1977
1000 µg/plate
1 Both with and without metabolic activation.
2 The nitroso- derivative of methomyl was positive at 50 nM.
3 The positive control, 4-o-tolylazo-o-toluidine at 25 µg/plate, gave the expected positive response.
4 The positive controls, AF-2 at 0.1 µg/plate and 2-aminoanthracene at 10 µg/plate, gave the expected positive responses.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
DNA repair E. coli 1 mg/disc Technical Negative5 Simmon
assay W3110, p3478 et al., 1977
B. subtilis
H17, m45
Mitotic S. cerevisiae D3 1.0, 1.5, 2.0, Technical Negative6 Simmon
recombination1 2.5, 3.0 et al., 1977
3.5% w/v
Gene mutations Chinese 1, 5, 10 mM Unknown Negative7 Wojciechowski
in vitro1 hamster et al., 1982
ovary V79
CHO/HGPRT Chinese Nonactivated: 99% Negative8 McCooey
gene hamster 10, 20, 40, et al., 1984
mutation1 ovary cells 50, 55 mM.
CHO-K1, Activated:
BH4 clone 100, 150, 200,
250, 350 mM
5 The positive control, 1-phenyl-3-dimethyltriazene at 1 mg/disc, and the negative control, chloramphenicol at
0.03 mg/disc, gave the expected responses.
6 The positive control, 0.025% 1,2,3,4-diepoxybutane, gave the expected positive response.
7 This study is not acceptable for evaluation because the purity of the test substance was not identified.
8 The positive control, 0.5 mM EMS, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
Sister In vitro Unknown Unknown Positive9 DeBuyst &
chromatid human Van Larebeke,
exchange1 lymphocytes 1982
Sex-linked Drosophila 4 & 10 ppm Technical Negative10 Valencia,
recessive melanogaster 1981
lethal
Unscheduled Primary rat 0, 1, 10, 100, 99% Negative11 Vincent
DNA hepatocytes 1000, 5000, et al., 1985
synthesis from male 10,000,
Crl:CD rats 75,000 µM
Unscheduled WI38 10-7, 10-6, Technical Negative12 Simmon
DNA fibroblasts 10-5, 10-4, et al., 1977
synthesis 10-3 M
9 This study is unacceptable for evaluation because it is in abstract form; methods and data were not reported.
10 The positive controls, EMS, EDB, TMP, and EI, gave the expected positive responses when tested at the appropriate
concentrations.
11 The positive control, 100 and 500 µM DMBA, gave the expected positive response.
12 The positive control, 10-5 M 4-nitroquinoline, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
In vivo Sprague-Dawley 0, 2, 6, 99% Negative13 Farrow
bone marrow male and female 20 mg/kg et al., 1984
cytogenetics rats
13 The positive control, 40 mg/kg cyclosphosphamide, gave the expected positive response.
At necropsy of the F0 parents, dose-related, statistically-
significant decreases in red cell count, haemoglobin concentration,
and haematocrit were noted in mid- and high-dose females; males were
not similarly affected. No statistically-significant decreases in
blood ChE activity were noted; however, mean plasma ChE activity was
decreased by 15% and 25% in high-dose F0 males and females,
respectively. Haematology/ChE results were not reported for F1
animals. Absolute and relative weights of several organs were altered
in a manner consistent with the treatment-related changes in body
weights, as absolute organ weights tended to decrease whereas relative
organ weights tended to increase, relative to the controls. No effects
of treatment on histopathological examination of F0 or F1
parents were noted.
Fertility, gestation length, and male sperm counts were not
adversely affected by treatment. However, dose-related statistically-
significant decreases in litter size and mean number of live pups
born were noted in all F1 treatment groups and in high-dose
F0 rats; a NOEL was not established for this finding. Pup survival
during lactation was significantly reduced in high-dose F1 pups
(born of F0 dams), but survivial did not appear to be affected
in F2 pups (born of F1 dams) after day 7 of lactation (a
significant decrease was noted in high-dose pups on day 4). Mean body
weights during lactation were significantly reduced in a dose-related
manner in all F1 pups; however, body weights were reduced only in
the mid- and high-dose groups of the F2 generation; a NOEL was not
established for this finding. No treatment-related histopathological
changes were noted in F1 or F2 pups (Lu, 1983).
Groups of Charles River CD rats (10 males/group and 20
females/group) were fed diets containing 0, 50, or 100 ppm methomyl
(purity unspecified) over 3 generations. F3b weanlings (10 sex/dose)
were subjected to necropsy and examined for histopathological changes.
A third group of weanlings (F3c) was fed test diets for 9 weeks and
then control diet during weeks 10 through 14 to assess the effect of
treatment on growth.
The study report indicated that no effects of treatment on
fertility, litter size, number of live pups, or pup survivial were
noted. A slight (10%) decrease in the mean body weights of male and
female pups at weaning in the F2 and F3 generations was not
considered to be related to treatment. No effects of treatment on
absolute or relative organ weights were noted. No treatment-related
changes in the gross or microscopic appearance of tissues were
reported; however, actual data were not submitted. Growth of F3c
weanlings fed 100 ppm methomyl was significantly less than that of
control rats, which was related to decreased food intake. This trend
was not reversed by removal of animals from the test diet for 4 weeks
(Kundzin & Busey, 1968).
Special studies on teratogenicity
Rats
Groups of naturally-bred Charles River (ChR-CD) female rats
(25/group) were randomly assigned to treatment groups that received
test diets containing 0, 50, 100, or 400 ppm technical methomyl
(< 99% active ingredient) and 1% Mazola corn oil over days 6 - 15 of
presumed gestation. Average doses were calculated to be 0, 4.9, 9.4,
and 34 mg/kg b.w./day, based on mean food consumption and body
weights. Control rats received rat laboratory feed with 1% corn oil.
Body weights, food consumption, and clinical signs were recorded
periodically during the gestation period. On day 21 of gestation, rats
were sacrificed with chloroform, and the ovaries and uteri were
removed and examined. The following parameters were determined: number
of corpora lutea, number of implantations, number and location of live
and dead fetuses and resorptions, body weights and crown-rump lengths
of live fetuses, and any gross fetal abnormalities. Approximately
one-half of the fetuses from each litter were cleared and stained for
skeletal examination, and the remaining fetuses were fixed and
examined for visceral anomalies.
No effects of treatment on the incidence of clinical signs were
apparent, and no deaths were noted. Mean maternal body weight was
reduced to 92 and 95% of the mean control weight on days 16 and 21,
respectively, in high-dose dams (p < 0.05 by Dunnett's LSD). Mean
feed consumption was also reduced by about 15% in high-dose dams over
days 6 - 16, but it was similar to feed consumption of control animals
over days 16 - 21 (treatment was terminated on day 16). Maternal
weight gain and feed consumption in the low- and mid-dose groups did
not appear to be significantly affected. No effects of treatment on
any maternal reproductive indices were apparent. Mean fetal body
weights and crown-rump lengths were not affected in any test groups.
No effects of treatment on the fetal or litter incidences of visceral
or skeletal anomalies were noted at doses up to and including a
maternally-toxic dose of 400 ppm (34 mg/kg b.w./day) (Rogers et
al., 1978).
Rabbits
Groups of artificially-inseminated New Zealand white (DLI:NZW)
rabbits were randomly assigned to test groups (20/group) that were
administered 0, 2, 6, or 16 mg/kg b.w./day technical methomyl (98.7%
active ingredient) over days 7 -19 of presumed gestation. The route of
administration was by gavage (5 ml/kg of deionized water), and dosages
were selected on the basis of a preliminary study which demonstrated
that a dose of 24 mg/kg b.w./day was lethal to 2/4 rabbits, and
produced significant clinical signs in the remaining 2 does. Control
animals received only deionized water. Rabbits were examined daily for
clinical signs of toxicity, and body weights were recorded
periodically. On day 29 of gestation, does were sacrificed by CO2
asphyxiation, and uteri were removed and weighed. The following
parameters were determined: number of corpora lutea, number and
placement of implantations, resorptions, and live and dead fetuses.
Fetuses were individually weighed, and live fetuses were killed and
examined for gross external, visceral, and skeletal abnormalities.
One mid-dose and 6 high-dose rabbits died as a result of
treatment, and a statistically-significant increase in the incidence
of signs related to cholinesterase inhibition (tremours, hyper-
activity, salivation, etc.) was noted in high-dose animals. Mean
body weights were increased by about 5% on day 29 in surviving
high-dose does, and mean weight gain over days 0 - 29 was increased
about 3-fold in these rabbits (p < 0.01 by Dunnett's LSD). No effects
of treatment on the number of resorptions or litter size were
apparent. Statistically-significant increases in mean fetal body
weights of 14 - 19% were noted in all dose groups, which appeared to
be treatment-related. This change was reported to be related to
increased food consumption in treated rabbits in the latter portion of
the study; however, actual food consumption data were not submitted.
No evidence of oedema was noted in treated fetuses, nor was any effect
of treatment on the incidences of fetal malformations or variations
apparent, at doses up to and including a maternally-toxic dose of
16 mg/kg b.w./day (Christian et al., 1983).
Data were combined from 2 older studies, conducted 6 months
apart, in which a total of 12 rabbits/group were treated with 0, 45 -
50, or 90 - 100 mg/kg b.w./day technical methomyl (purity 90 - 100%)
mixed into the diet at appropriate concentrations to achieve the
desired dosages. The animals were treated from days 8 - 16 of presumed
gestation. Clinical signs and food consumption were recorded daily and
body weights were measured weekly. One-half of the does in each group
were sacrificed on day 29 and subjected to caesarean necropsy, whereas
the remaining rabbits in each group were allowed to deliver normally.
One-third of the fetuses or pups from each litter were examined for
skeletal anomalies. The extent of soft tissue examinations was not
adequately described, but appeared to be restricted to gross
observations of internal organs.
One control and 1 low-dose rabbit died during the study period,
neither of which was pregnant. One other control doe delivered a
full-term litter on the second day of treatment, and was excluded. One
control doe and one low-dose doe aborted. High-dose rabbits were
reported to have consistent weight loss related to decreased food
consumption; however, actual data were not submitted. The incidences
of pregnancy, after combination of the 2 studies, were 4/12, 8/12, and
5/12 in the control, mid-dose, and high-dose groups, respectively. No
effects of treatment on maternal reproductive indices, fetal body
weights or size, or visceral/skeletal findings were noted
(Busey, 1967a).
Acute toxicity
The acute toxicity of methomyl to several animal species is
summarized in Table 2. Methomyl is highly toxic via both the oral and
inhalation routes of exposure. The severity and onset of cholinergic
signs were related to the administered dose.
Table 2. Acute toxicity of methomyl
Solvent LD50 LC50
Species Route Sex (vehicle) (mg/kg b.w.) (mg/1) Reference
Rat Oral M Peanut oil1 26 - Sherman, 1964
Oral M Peanut oil1 17 - Sherman, 1966
F 23.5
Oral M Water2 45 - Trivits, 1979
Dermal M Water1 > 1000 - Morrow, 1972
Inhalation, M Aerosol - 0.30 Foster, 1966a
4 hours
Rat Inhalation, M Vapour - 0.04 Foster, 1966b
4 hours
Inhalation, M Spray - 0.45 Hornberger,
4 hours 1967
Rabbit Oral M Acetone/ 30 - Sherman, 1968a
peanut oil1
Dermal M Water1 > 5000 - Dashiell, 1971
Dermal M&F Water1 > 1500 - Majut & Hood,
1966
Chicken Oral F Acetone/ 28 - Krauss & Stula,
peanut oil1 1967
Dog Oral M Gelatin 20 - Sherman, 1968b
capsule1
1 Technical methomyl having a purity > 98% was used.
2 Technical methomyl having a purity of 100% was used.
Short-term studies
Rats
Groups of Charles River (CD) rats, 10/sex/group, were adminis-
tered methomyl (technical, 100%) in the diet for 90 days at dose
levels of 0, 10, 50, or 250 ppm. An additional group was fed diets
that contained 125 ppm methomyl for 42 days and 500 ppm methomyl to
the end of the study. Observations included mortality, serological,
haematological, and urological examinations, plasma and erythryocyte
ChE activities, food and water consumption, body weights, and gross
and microscopic analyses. No deaths or dose-related behavioural,
clinical, haematological, serological, urological, or pathological
effects were observed. Body weights were depressed in males at 250 ppm
and at 125/500 ppm, and in females at 125/500 ppm. Sporadic decreases
in haemoglobin content or erythrocyte count were observed in test-
group males and females, but the responses were not time or dose
dependent. Moderate erythroid hyperplasia was observed in the bone
marrow of male rats fed 250 ppm. The NOEL was 50 ppm methomyl, based
on body-weight depression in males (Paynter, 1966).
Rabbits
Methomyl (90% soluble concentrate) was administered dermally to
New Zealand albino rabbits (5/sex/dose) at 0 or 200 mg/kg b.w. to both
intact and abraded skin areas. Applications were 5 days a week for 21
days. There were no toxic symptoms noted in animals with intact skin.
There were signs of ChE inhibition and 2 deaths in animals with
abraded skin, although ChE activity was comparable to controls. There
was no evidence of cumulative toxicity or of compound-related effects
on organ weights or histopathology (Busey, 1967b).
Dogs
Groups of 11- to 13-month old beagle dogs (4/sex/group) were fed
diets that contained methomyl (97.5% methomyl, 2.5% HiSil 233) in corn
oil at dosages of 0, 50, 100, or 400 ppm for 3 months. Mean daily
intakes corresponded to 0, 1.44, 3.18, and 14.7 mg/kg b.w./day for
males and 0, 1.45, 3.01, and 12.5 mg/kg b.w./day for females, respect-
ively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, and haemato-
logical, clinical chemical, and urological effects. Cholinesterase
activities were not determined. Organs were weighed and gross necropsy
and histopathology were performed at terminal sacrifice. No compound-
related effects were reported. However, a NOEL for this study could
not be determined because blood cholinesterase activities were not
determined and the animals were older than is recommended by currently
acceptable international guidelines (i.e. OECD test methods) for
conducting short-term non-rodent feeding studies (preferably they
should be 4-6 months of age, but not older than 9 months) (Sherman
et al., 1967).
Long-term studies
Mice
Groups of 80 male and 80 female albino weanling CD-1 mice (6
weeks old) were administered methomyl (technical, purity 99%) in the
diet for 104 weeks at initial dose levels of 10, 50, 100, or 800 ppm.
Due to high mortality, the high dose was reduced to 400 ppm at week 28
and then to 200 ppm at week 39. The mid-dose level was also reduced at
week 39 to 75 ppm. Mean dietary intakes were 8.7, 15.4, and 93.5 mg/kg
b.w./day for males and 10.6, 19.1, and 118 mg/kg b.w./day for females,
respectively.
All animals were observed routinely for clinical signs,
mortality, moribundity, body-weight changes, and food consumption.
Haematological evaluations were performed periodically throughout the
study. Selected organs were weighed, including the brain, thymus,
lungs, heart, spleen, liver, kidneys, testes (w/epididymides),
adrenals, and pituitary. Complete gross and microscopic analyses of
tissues were performed on each animal.
Body-weight gain and food consumption of animals in the treated
groups were equivalent to controls. Mortality was increased among mid-
and high-dose animals throughout the study. Mortality in the low-dose
males was initially increased, in comparison to controls, but was
comparable at the termination of the study. Survival was greater than
50% in all groups at 11 months and approximately 25 - 30% at 2 years.
Red cell mass was apparently decreased during the first 26 weeks
at the mid- and high-dose levels, as evidenced by lower haemoglobin
levels, RBC counts, and haematocrit. These effects were reversed when
the amount of methomyl in the diet was reduced to 75 and 200 ppm in
the mid- and high-dose levels, respectively.
Necropsy and pathological examinations were unremarkable and
there were no compound-related histological changes. A substantial
amount of autolysis occurred in animals found dead prior to term,
which precluded any meaningful analysis of treatment-related effects
in these early deaths. There were no compound-related increases in
neoplastic changes at doses up to and including 200 ppm for 104 weeks.
The NOEL was 50 ppm, based on adverse effects on red cell mass at
higher dose levels (Serota et al., 1981).
Rats
Groups of Charles River rats (35 males and 35 females/group; 70
males and 70 females used as controls) were administered methomyl in
the diet at dose levels of 0, 50, 100, 200, or 400 ppm for 22 months.
These diets corresponded to 0, 2.4, 4.8, 9.6, and 20 mg/kg b.w./day
for males and 0, 2.8, 5.8, 11, and 24 mg/kg for females, respectively.
Body weights, food consumption, appearance, and behaviour were
recorded weekly from weeks 1 through 26 and monthly thereafter to week
96. Clinical chemistry observations included BUN, SGPT, SAP, glucose,
plasma, red blood cell, and brain cholinesterases, and, at the
conclusion of the study, bone-marrow differential counts. The
cholinesterase assays were performed using a delta pH method. This
method is considered inadequate for evaluating cholinesterase
inhibition by carbamates and the data are therefore unreliable.
Haematology determinations included haematocrit, haemoglobin, red
blood cell counts, and total and differential leukocyte counts;
urinalysis included pH, specific gravity, sugar, protein, bilirubin,
occult blood, and microscopic examination of the sediment. At 12
months, 5 rats of each sex in each group were sacrificed. At 22
months, because of the presence of respiratory disease and high
mortality, all surviving animals were sacrificed. Terminal body
weights and gross and microscopic examinations were performed. Organ
weights were obtained for the following tissues: brain, heart, liver,
spleen, kidneys, and testes. Organ and organ-to-body-weight ratios
were recorded and calculated. Microscopic examinations were performed
on the following tissues from rats in the control and 400 ppm groups:
brain, thyroid, heart, liver, spleen, kidneys, adrenals, stomach,
small intestine, large intestine, testes, bone marrow, muscle, and
sciatic nerve. The liver, kidneys, and spleen from rats in the other
groups were examined.
Growth and food consumption, measured only over the first year,
were reduced at 400 ppm. At 200 ppm, growth of males was reduced when
compared with control values. Haematology, blood chemistry, and
urinalysis values were similar in treated and control groups, although
there was a dose-related trend toward decreased haemoglobin in female
treatment groups. This was significant at 18 and 22 months in females
in the 200 and 400 ppm groups. Males were not similarly affected.
Compound-related increases in the incidence and severity of
extramedullary haematopoiesis were observed in the spleens of females
fed 200 or 400 ppm methomyl.
Gross and microscopic examinations were performed at 12 and 22
months. Increased testes, adrenal, liver, and brain weights were
observed at 400 ppm. At 200 and 400 ppm increased thyroid weights in
females were observed. Macroscopic changes in the kidneys, including
enlargement, discoloration, and texture changes were not reflected in
weight differences. Microscopic changes were evident in the kidneys of
both males and females at 400 ppm. The histopathological changes were
characterized by tubular hypertrophy and vacuolation of the epithelial
cells of the proximal convoluted tubules. At 200 and 400 ppm,
extramedullary haematopoiesis was noted in the spleen of females. No
microscopic changes were evident in other tissues, including those in
which weight increases were noted. There was no evidence of oncogenic
potential at any dose level. The 100 ppm dose level could be
considered the NOEL in this study, based on macro- and microscopic
changes in the thyroid, spleen, and kidneys, as well as on body-weight
changes. However, since cholinesterase activity was not measured by an
appropriate and sensitive method for such a potent ChE inhibitor, an
accurate NOEL for this study could not be determined (Busey, 1968a).
In a chronic feeding/oncogenicity study, ChR-CD rats were
administered methomyl (> 99% pure) in the diet for 24 months. Groups
of 80 male and 80 female rats were fed diets containing 0, 50, 100, or
400 ppm methomyl. These concentrations were equal to 0, 2.4, 4.8, and
20 mg/kg b.w./day for males and 0, 2.3, 6.3, and 26 mg/kg b.w./day for
females, respectively. Food consumption and body weights were
measured, and haematological, clinical chemistry, and urological
evaluations were conducted routinely throughout the study. Erythrocyte
and brain acetytcholinesterase activities were measured by the Ellman
method. After 12 months, 10 rats/sex/treatment group were sacrificed
and necropsied, selected organs were weighed, and selected tissues/
organs were examined histologically. At the end of the study all rats
were sacrificed and examined in a similar manner. Tissues from rats
found dead or killed in extremis were examined histologically.
Body-weight gains of males and females in the 400 ppm group were
depressed for the first 1 to 1.5 years, but they recovered thereafter
and were comparable to control body-weight gains by 24 months.
Significantly decreased haemoglobin, RBC counts, and haematocrits were
evident in females in the 400 ppm group. Significant differences were
not observed between control and test-group animals with regard to
erythrocyte or brain cholinesterase activities. However, due to a
possible effect of storage of blood samples prior to measurement, the
potential effect of the test compound on cholinesterases could have
been undiscovered due to fast spontaneous reactivation of carbamylated
enzyme.
Relative testes weights of high-dose males were significantly
greater than those of controls, but there were no observed
pathological changes. Female rats fed > 100 ppm methomyl had
increased relative liver weights, but no associated histopathological
alterations were identified. Female rats fed 400 ppm methomyl also had
increased relative spleen weights, but no noticeable pathological
tissue responses. The incidence of bone marrow hyperplasia, focal
hyperplasia in the adrenal medulla, and focal degeneration/angiectasis
in the adrenal cortex were increased in male rats fed 400 ppm
methomyl. There were no other significant pathological changes and no
oncogenic potential was evident at any of the doses administered in
this study. A NOEL of 100 ppm was estimated based on body-weight
changes and on the haematologic response at 400 ppm (Kaplan et al.,
1981).
Dogs
Groups of young adult (> 12 months of age) beagles (4 male and 4
females/group) were administered methomyl in the diet at dose levels
of 0, 50, 100, 400, or 1000 ppm for 2 years. Methomyl was incorporated
into a ground dry dog meal. Diet mixtures were prepared weekly. Daily
observations were made of behaviour and appearance, and body-weight
and food-consumption data were recorded weekly. Clinical chemistry
studies were performed initially and at 3, 6, 12, 18, and 24 months.
Determinations were made of blood sugar, BUN, SAP, SGPT, SGOT, and
prothrombin time. Serum electrolytes, total protein, and albumin were
determined at 18 and 24 months. Haematology examinations included the
following: haematocrit and haemoglobin concentration, red blood cell
count, total and differential leukocyte count, and bone marrow
differential count at the termination of the study. Urinalysis
included: appearance, pH, specific gravity, sugar, acetone, protein,
bilirubin, occult blood, and microscopic examination of the sediment.
At 24 months, urinary sodium, potassium, chloride, glucose, protein,
and phosphorous were determined. Plasma and erythrocyte cholinesterase
activities were measured by the monometric method at the ninth week in
animals in the control and 1000 ppm groups and during the thirteenth
week in animals in the 1000 ppm group. At the end of 1 year, 1
dog/sex/group was sacrificed and the following organ weights recorded
after gross examination: brain, thyroid, heart, liver, spleen,
kidneys, adrenals, and testes. Organ-to-body weight ratios were
calculated. At 24 months the remaining dogs were sacrificed and the
following tissues were examined from each dog: brain, pituitary,
thyroid, thymus, lungs, heart, liver, spleen, kidneys, adrenals,
stomach, pancreas, duodenum, jejunum, ileum, colon, mesenteric lymph
node, urinary bladder, ovary, uterus, skin, bone, bone marrow, muscle,
sciatic nerve, and testes. The same organs were weighed as after 1
year.
Two out of 4 females in the 1000 ppm group died within 8 weeks of
the beginning of the study. Signs of poisoning included tremours,
salivation, and incoordination, which were observed only once in males
at 1000 ppm. Haematology studies in 1 high-dose male indicated severe
anaemia and moderate leukopenia (decreased haematocrit, haemoglobin,
RBC count and platelet count; increased reticulocyte count; and
changes in the differential count). This condition improved when the
dog was placed on a control diet at 84 weeks, but worsened when
1000 ppm methomyl was replaced in the diet at the ninety-fifth week.
Enlarged spleen and kidneys were noted at sacrifice (24 months). The
remaining high-dose males and 2/4 high-dose females demonstrated
similar haematologic abnormalities at 3 months, but recovered to
normal by 24 months. Growth and food consumption were unaffected by
treatment; however, all dogs were young adults at the start of the
test and therefore were already beyond the critical growth months for
this species. This is considered a deficiency in this study. Clinical
chemistry and urinalyses were comparable between treated groups and
controls. Since plasma and erythrocyte cholinesterase activities were
assayed by a method considered inadequate for carbamate inhibition,
these data are unreliable for estimating the cholinesterase inhibiting
properties of methomyl in dogs.
At 24 months, gross pathology examinations showed liver and
spleen enlargement in the male that was severely anaemic. Kidney
weights were increased in males at 1000 ppm and in 1 male at 400 ppm.
Microscopic examination showed alterations in the spleen
(extramedullary haematopoiesis) and kidneys (pigment in the cytoplasm
and swelling of the epithelial cells of the proximal convoluted
tubules in males and females), liver (bile duct proliferation) and
bone marrow (increased activity in both erythroid and myeloid series)
at 1000 ppm. At 400 ppm similar kidney abnormalities were present only
in males. Alterations were not observed in the lower-dose groups. A
no-effect level of 100 ppm (equal to 3.1 mg/kg b.w./day) was
tentatively estimated, based on the adverse haematologic effects and
on kidney and spleen morphologic changes at > 400 ppm. However, in
the absence of adequate data for adverse effects on cholinesterase
activity an accurate estimate of a NOEL cannot be determined
(Busey, 1968b).
Observations in humans
Epidemiological studies of poisonings from the ingestion of
contaminated food indicate that a single oral dose as low as
12-15 mg/kg b.w. can be fatal in humans (Liddle et al., 1979;
Araki et al., 1982).
An assessment of workers who routinely handled methomyl in a
factory indicated that workers were occasionally inadvertently exposed
and required hospitalization and treatment for symptoms of
cholinesterase inhibition, presumably due to methomyl intoxication.
Blood cholinesterase levels were normal for these workers, however,
and no quantitation of dose was presented. The methodology and timing
of ChE measurements were questionable (Morse et al., 1979).
COMMENTS
Methomyl is rapidly absorbed from the gastrointestinal tract or
through the skin, and is rapidly metabolized and eliminated. The
metabolic fate of methomyl depends on the isomeric configuration, as
the syn form (the technical product) is primarily degraded to CO2,
whereas the anti form is degraded to acetonitrile. Some conversion
of syn-methomyl to anti-methomyl apparently occurs in rats.
The acute toxicity of methomyl is due to inhibition of
acetylcholinesterase. The oral LD50 in experimental animals ranges
from 20 to 45 mg/kg b.w.; atropine is an antidote to these acute toxic
effects.
Long-term feeding studies in mice and rats do not suggest any
evidence of oncogenicity for methomyl. Toxic effects included anaemia
(mice, rats, and dogs), decreased weight gain (rats), and
histopathological changes in the kidney (dogs). The no-observed-effect
levels for chronic toxicity, calculated from dietary intake, were;
8.7 mg/kg b.w./day in mice, 4.8 mg/kg b.w./day in rats, and 3.1 mg/kg
b.w./day in dogs.
The available chronic toxicity data are somewhat limited by the
lack of adequate determinations of cholinesterase activity. However,
the effects on cholinesterases are reversible. In order to estimate
the rate of spontaneous reactivation in man, the submission of the
results of additional in vitro studies is required. These studies
should provide data on the inhibition and reactivation rate constants
for the effect of methomyl on human plasma and erythrocyte
cholinesterases.
Available epidemiological data for humans indicate that a dose of
12 - 15 mg/kg b.w. methomyl, ingested as a bolus with food, can be
lethal owing to suppression of cholinesterase activity. This value is
similar to the acute lethal doses identified in animal studies.
No evidence of teratogenicity or embryotoxicity was apparent in
rats or rabbits. The available rat reproduction studies, when
considered together, demonstrate a NOEL of 50 ppm (equivalent to
2.5 mg/kg b.w./day), based on decreases in body-weight gain and food
consumption in a study reported in 1968, and on slight decreases in
litter size at 75 ppm in a study reported in 1983. No other evidence
of effects on fertility or other reproductive parameters was noted.
No evidence of mutagenicity was noted in a number of genotoxicity
studies.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
NOELs for chronic oral toxicity are established as;
Mouse: 50 ppm, equal to 8.7 mg/kg b.w./day.
Rat: 50 ppm, equivalent to 2.5 mg/kg b.w./day.
Dog: 100 ppm, equal to 3.1 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0 - 0.01 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF A FULL ADI IS
IMPRACTICABLE, TO BE SUBMITTED TO WHO BY 1988:
Additional in vitro studies in human blood to demonstrate the
time-course of plasma and red blood cell cholinesterase inhibition and
reactivation.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Further observations in humans.
REFERENCES
Araki, M., Yonemitsu, K., Kambe, T., Idaka, D., Tsunenari, S., Kanda,
1982 M., & Kambara, T. Forensic toxicological investigations on
fatal cases of carbamate pesticide methomyl (Lannate(R))
poisoning. Nippon Hoigaku Zasshi, 36: 584 - 588.
Barnes, J.R. Cholinesterase tests with methomyl. Unpublished report
1978 No. HLR-280-78 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Blevins, R.D. Lee, M., & Regan, J.D. Mutagenicity screening of five
1977 methyl 1977 carbamate insecticides and their nitrosos
derivatives using mutants of Salmonella typhimurium LT2.
Mutat. Res., 56: 1 - 6.
Busey, W.M. Teratology study in rabbits with "Lannate(R)" methomyl
1967a insecticide. Unpublished Report No. HLO-257-67 from Hazleton
Laboratories, Inc., Falls Church, VA., USA. Submitted to WHO
by E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Busey, W.M. Repeated dermal study in rabbits - "Lannate(R)" methomyl
1967b insecticide (90% methomyl). Unpublished Report
No. HLO-254-67 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Busey, W.M. 22-Month dietary feeding study in rats with "Lannate(R)"
1968a methomyl insecticide. Unpublished Report No. 201-164 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Busey, W.M. Two year dietary administration in dogs with "Lannate(R)"
1968b methomyl insecticide. Unpublished Report No. 201-165 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Christian, M.S., Hoberman, A.M., & Feussner, E.L. Embryo-fetal
1983 toxicity and teratogenicity study of methomyl in the rabbit.
Unpublished report No. HLO-331-83 from Argus Research
Laboratories, Inc., Horsham, PA, USA. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Dashiell, O. Acute skin absorption LD50 test in rabbits using technical
1971 methomyl (> 98% methomyl). Unpublished report No.
HLR-340-71 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
DeBuyst, B. & Van Larebeke, N. Induction of sister-chromatid exchanges
1982 in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242.
Farrow, M.G., Cortina, T., & Padilla-Nash, H. In vivo bone marrow
1984 chromosome study in rats [with] H No. 15,000 - Final Report.
Unpublished Report No. HLO-63-84 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966a methomyl (> 98% methomyl) progress report. Unpublished
report No. HLR-73-66 from Haskell Laboratory for Toxicology
and Industrial Medicine, E.I. du Pont de Nemours and Co.,
Inc. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966b methomyl (> 98% methomyl). Unpublished report
No. HLR-214-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Harvey, J., Jelinek, A.G., & Sherman, H. Metabolism of methomyl in the
1973 rat. J. Agric. Fd. Chem., 21: 769 - 775.
Hornberger, C.S. Acute inhalation toxicity of aqueous spray mist.
1967 Unpublished report No. HLR-171-67 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
1976 reactions in the metabolism of methomyl in rats.
Pest. Biochem and Physiol., 6: 571 - 583.
Kaplan, A.M., Trochimowicz, H.J., & Barba, C. Long-term feeding study
1981 in rats with S-methomyl-N-(methylcarbomoyloxy)
thioacetimidate (methomyl; INX-1179). Unpublished report
No. HLR-235-81 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Kraus, W. & Stula, E.F. Acute oral LD50 and delayed neurotoxicity
1967 (paralysis) tests in hens using technical methomyl (> 98%
methomyl). Unpublished report No. HLR-161-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Kundzin, M. & Busey, W.M. Three-generation reproduction study with
1968 Lannate(R) 1968 methomyl insecticide. Unpublished Report
No. MRO 888-1 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Liddle, J.A., Kimbrough, R.D., Needham, L.L., Cline, R.E., Smrek,
1979 A.L., Yert, L.W., Bayse, D.D., Ellington, A.C., & Dennis,
P.A. A fatal episode of accidental methomyl poisoning.
Clin. Toxicol., 15; 159 - 167.
Lu, C.C. Nudrin(R) two-generation reproduction study in rats.
1983 Unpublished report no. WRC RIR-275 from Westhollow Research
Center, Shell Development Co., Houston, TX, USA. Submitted
to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Majhut, J. & Hood, D. Acute skin absorption, approx, lethal dose (ALD)
1966 test in rabbits using 55 and 26% formulations of (technical)
methomyl in aqueous solutions. Unpublished report
No. HLR-216-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M., & Hemingway, R.E.
1984 CHO/HGPRT assay for gene mutations using technical methomyl.
Unpublished report No. HLR-556-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Morrow, R.W. Acute skin absorption test (ALD) in rabbits using
1972 technical methomyl and a 25% methomyl formulation
(Lannate(R) 25W). Unpublished report No. HLR-438-72 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Morse, D.L., Baker, E.L., Kimbrough, R.D., & Wisseman, C.L.
1979 Propanilchloracne and methomyl toxicity in workers of a
pesticide manufacturing plant. Clin. Toxicol.,
15: 13 - 21.
Paynter, O. Three month dietary administration in rats using
1966 insecticide 1179 (technical methomyl, > 98% purity).
Unpublished Report No. MRO-848 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Rogers, A.S., Culik, R., Kaplan, A.M., & Aftosmis, J.G. Oral
1978 teratogenic study in rats with Lannate(R) (INX-1179).
Unpublished report No. HLR-498-78 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Serota, D.G., Machotka, S.V., Hasting, T.F., Alsaker, R.D., &
1981 Fezio, W.L. 104-Week chronic toxicity and carcinogenicity
study in mice. Unpublished Report No. HLO-253-81 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Shah, P.V., Monroe, R.J., & Guthrie, F.E. Comparative rates of dermal
1981 penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59: 414 - 423.
Sherman, H. Acute oral (approx. lethal dose - ALD) and 10-dose oral
1966 subacute toxicity test in rats using technical methomyl
(> 98% methomyl). Unpublished report No. HLR-100-64 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Sherman, H. Acute oral LD50 test in rats using technical methomyl
1966 (> 98% methomyl). Unpublished report No. HLR-210-66 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Sherman, H., Barnes, J.R., Stula, E.F., & Zapp, J.A. Three-month
1967 feeding study on dogs with S-methyl-N-(methylcarbomoyloxy)
thioacetimidate (Lannate(R) methomyl insecticide;
INX-1179). Unpublished report No. HLR-168-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in rabbits using
1968a technical methomyl (> 98%). Unpublished report
No. HLR-250-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in dogs using
1968b technical methomyl (> 98%). Unpublished report
No. HLR-279-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Simmon, V.F.; Mitchell, A.D., & Jorgenson, T.A. Evaluation of selected
1977 pesticides as chemical mutagens: in vitro and in vivo
studies. Unpublished EPA report No. EPA-600/1-77-028 from
U.S. Environmental Protection Agency, Health Effects
Research Laboratory, Research Triangle Park, N.C., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Singles, G.H. INX-1179 (methomyl) and cholinesterase activity.
1970 Unpublished report No. HLR-186-70 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Trivits, R. Acute oral LD50 test in rats using technical methomyl.
1979 Unpublished report No. HLR-497-79 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Valencia, R. Mutagenesis screening of pesticides in Drosophila.
1981 Unpublished EPA report No. 600/1/81/017. Prepared by Warf
Institute, Inc. for the U.S. Environmental Protection
Agency. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Vincent, D.R., Arce, G.T., & Sarrif, A.M. Assessment of methomyl
1985 (INX-1179-255) in the in vitro unscheduled DNA synthesis
assay in primary rat hepatocytes. Unpublished report
No. HLR-149-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Wojciechowski, J.P., Kaur, P., & Sabharwal, P.S. Induction of ouabain
1982 resistance in V-79 cells by four carbamate pesticides.
Env. Research, 29: 481 - 53.
 Effects on enzymes and other biochemical parameters
Methomyl was administered in the diet to groups of Crl:CD(SD)BR
rats at levels of 0, 5, 100, 200, 400, or 800 ppm for 5 months. There
were 10 rats/sex/dose. Peripheral blood samples were taken at 4, 11,
32, 43, 60, and 79 days and enzyme activities were measured in whole
blood by the Ellman method. Significant inhibition was reported at
800 ppm in both sexes, but not at 400 ppm (Singles, 1970).
Groups of Crl:CD(SD)BR rats (8/sex/group) were fed technical
methomyl in their diets at dose levels of 0, 100, 400, or 800 ppm for
29 days. Analysis of erythrocyte, plasma, and brain cholinesterase,
determined separately, demonstrated that brain cholinesterase was more
sensitive to methomyl. Females at 100, 400, and 800 ppm demonstrated
depressed brain cholinesterase activity after 29 days compared to
controls, statistically significant at the high dose only. Erythrocyte
and plasma cholinesterase activities, although depresses in males and
females at 800 ppm, were not statistically different from controls
(Barnes, 1978).
Toxicological studies
Special studies on mutagenicity
Methomyl has been shown to be negative in a number of test
systems that have assessed the ability of this compound to cause gene
mutations, structural chromosomal defects, and direct DNA damage. A
single positive result for sister chromatid exchange (SCE) was
reported in an abstract (DeBuyst, B. & Van Larebeke, 1982); however,
this study is not acceptable for evaluation, because methods, purity
of the test substances, and actual results were not reported.
Summary results are presented in Table 1.
Special studies on reproduction
Rats
Groups of male and female Charles River CD rats (13 males/group
and 26 females/group in the F0 generation, 20 males/group and 40
females/group in the F1 generation) were fed diets containing 0, 75,
600, or 1200 ppm technical methomyl continuously over 2 generations.
Feed analyses indicated that the actual levels of methomyl in the test
diets were within acceptable limits of the targeted values. Animals
were observed twice daily for clinical signs of toxicity and body
weights and food consumption were measured weekly. During gestation,
females were weighed on day 3 and daily from days 6 through
parturition. F0 and F1 males were sacrificed after mating, and
sperm counts were performed. After birth, litter size, pup weights,
sex, and numbers of live and dead pups were determined. Pups were
examined daily during lactation for abnormalities of appearance or
behaviour, and were weighed on days 1, 4, 7, 14, and 21 of lactation.
After weaning, pups were weighed weekly. F0 and F1 parents were
subjected to complete necropsies and haematological evaluations at the
end of mating or weaning (males and females, respectively).
Randomly-selected pups from each generation (10/sex/dose) were also
necropsied.
Mean body weights were significantly decreased at most of the
measured intervals in mid- and high-dose male and female parents in
the F0 generation, and in all parental treatment groups in the F1
generation. The decrease in the F1 generation was dose-related, and
ranged from 5 - 10% in the low-dose groups to 20 - 25% in the
high-dose groups; a NOEL was not established for this finding. A
similar pattern was noted in dams during gestation, as dose-related,
statistically-significant decreases were noted in all F1 female
treatment groups, and in mid- and high-dose F0 treatment groups.
These decreases appeared to be related to decreases in food
consumption.
Table 1. Special studies on mutagenicity
Test system Test object Concentration Purity Results References
used
Ames test1 S. typhimurium 0.005, 0.05, Technical Negative2 Blevins,
TA98, TA100, 0.5, 5, et al., 1977
TA1535, 50 nM
TA1537,
& TA1538
Ames test1 S. typhimurium 1, 5, 10, 50, Technical Negative3 Simmon,
TA100, 100, 500, et al., 1977
TA1535, 1000 µg/plate
TA1537,
& TA1538
Ames test1 E. coli 1, 10, 50, Technical Negative4 Simmon
WP2 100, 500, et al., 1977
1000 µg/plate
1 Both with and without metabolic activation.
2 The nitroso- derivative of methomyl was positive at 50 nM.
3 The positive control, 4-o-tolylazo-o-toluidine at 25 µg/plate, gave the expected positive response.
4 The positive controls, AF-2 at 0.1 µg/plate and 2-aminoanthracene at 10 µg/plate, gave the expected positive responses.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
DNA repair E. coli 1 mg/disc Technical Negative5 Simmon
assay W3110, p3478 et al., 1977
B. subtilis
H17, m45
Mitotic S. cerevisiae D3 1.0, 1.5, 2.0, Technical Negative6 Simmon
recombination1 2.5, 3.0 et al., 1977
3.5% w/v
Gene mutations Chinese 1, 5, 10 mM Unknown Negative7 Wojciechowski
in vitro1 hamster et al., 1982
ovary V79
CHO/HGPRT Chinese Nonactivated: 99% Negative8 McCooey
gene hamster 10, 20, 40, et al., 1984
mutation1 ovary cells 50, 55 mM.
CHO-K1, Activated:
BH4 clone 100, 150, 200,
250, 350 mM
5 The positive control, 1-phenyl-3-dimethyltriazene at 1 mg/disc, and the negative control, chloramphenicol at
0.03 mg/disc, gave the expected responses.
6 The positive control, 0.025% 1,2,3,4-diepoxybutane, gave the expected positive response.
7 This study is not acceptable for evaluation because the purity of the test substance was not identified.
8 The positive control, 0.5 mM EMS, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
Sister In vitro Unknown Unknown Positive9 DeBuyst &
chromatid human Van Larebeke,
exchange1 lymphocytes 1982
Sex-linked Drosophila 4 & 10 ppm Technical Negative10 Valencia,
recessive melanogaster 1981
lethal
Unscheduled Primary rat 0, 1, 10, 100, 99% Negative11 Vincent
DNA hepatocytes 1000, 5000, et al., 1985
synthesis from male 10,000,
Crl:CD rats 75,000 µM
Unscheduled WI38 10-7, 10-6, Technical Negative12 Simmon
DNA fibroblasts 10-5, 10-4, et al., 1977
synthesis 10-3 M
9 This study is unacceptable for evaluation because it is in abstract form; methods and data were not reported.
10 The positive controls, EMS, EDB, TMP, and EI, gave the expected positive responses when tested at the appropriate
concentrations.
11 The positive control, 100 and 500 µM DMBA, gave the expected positive response.
12 The positive control, 10-5 M 4-nitroquinoline, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
In vivo Sprague-Dawley 0, 2, 6, 99% Negative13 Farrow
bone marrow male and female 20 mg/kg et al., 1984
cytogenetics rats
13 The positive control, 40 mg/kg cyclosphosphamide, gave the expected positive response.
At necropsy of the F0 parents, dose-related, statistically-
significant decreases in red cell count, haemoglobin concentration,
and haematocrit were noted in mid- and high-dose females; males were
not similarly affected. No statistically-significant decreases in
blood ChE activity were noted; however, mean plasma ChE activity was
decreased by 15% and 25% in high-dose F0 males and females,
respectively. Haematology/ChE results were not reported for F1
animals. Absolute and relative weights of several organs were altered
in a manner consistent with the treatment-related changes in body
weights, as absolute organ weights tended to decrease whereas relative
organ weights tended to increase, relative to the controls. No effects
of treatment on histopathological examination of F0 or F1
parents were noted.
Fertility, gestation length, and male sperm counts were not
adversely affected by treatment. However, dose-related statistically-
significant decreases in litter size and mean number of live pups
born were noted in all F1 treatment groups and in high-dose
F0 rats; a NOEL was not established for this finding. Pup survival
during lactation was significantly reduced in high-dose F1 pups
(born of F0 dams), but survivial did not appear to be affected
in F2 pups (born of F1 dams) after day 7 of lactation (a
significant decrease was noted in high-dose pups on day 4). Mean body
weights during lactation were significantly reduced in a dose-related
manner in all F1 pups; however, body weights were reduced only in
the mid- and high-dose groups of the F2 generation; a NOEL was not
established for this finding. No treatment-related histopathological
changes were noted in F1 or F2 pups (Lu, 1983).
Groups of Charles River CD rats (10 males/group and 20
females/group) were fed diets containing 0, 50, or 100 ppm methomyl
(purity unspecified) over 3 generations. F3b weanlings (10 sex/dose)
were subjected to necropsy and examined for histopathological changes.
A third group of weanlings (F3c) was fed test diets for 9 weeks and
then control diet during weeks 10 through 14 to assess the effect of
treatment on growth.
The study report indicated that no effects of treatment on
fertility, litter size, number of live pups, or pup survivial were
noted. A slight (10%) decrease in the mean body weights of male and
female pups at weaning in the F2 and F3 generations was not
considered to be related to treatment. No effects of treatment on
absolute or relative organ weights were noted. No treatment-related
changes in the gross or microscopic appearance of tissues were
reported; however, actual data were not submitted. Growth of F3c
weanlings fed 100 ppm methomyl was significantly less than that of
control rats, which was related to decreased food intake. This trend
was not reversed by removal of animals from the test diet for 4 weeks
(Kundzin & Busey, 1968).
Special studies on teratogenicity
Rats
Groups of naturally-bred Charles River (ChR-CD) female rats
(25/group) were randomly assigned to treatment groups that received
test diets containing 0, 50, 100, or 400 ppm technical methomyl
(< 99% active ingredient) and 1% Mazola corn oil over days 6 - 15 of
presumed gestation. Average doses were calculated to be 0, 4.9, 9.4,
and 34 mg/kg b.w./day, based on mean food consumption and body
weights. Control rats received rat laboratory feed with 1% corn oil.
Body weights, food consumption, and clinical signs were recorded
periodically during the gestation period. On day 21 of gestation, rats
were sacrificed with chloroform, and the ovaries and uteri were
removed and examined. The following parameters were determined: number
of corpora lutea, number of implantations, number and location of live
and dead fetuses and resorptions, body weights and crown-rump lengths
of live fetuses, and any gross fetal abnormalities. Approximately
one-half of the fetuses from each litter were cleared and stained for
skeletal examination, and the remaining fetuses were fixed and
examined for visceral anomalies.
No effects of treatment on the incidence of clinical signs were
apparent, and no deaths were noted. Mean maternal body weight was
reduced to 92 and 95% of the mean control weight on days 16 and 21,
respectively, in high-dose dams (p < 0.05 by Dunnett's LSD). Mean
feed consumption was also reduced by about 15% in high-dose dams over
days 6 - 16, but it was similar to feed consumption of control animals
over days 16 - 21 (treatment was terminated on day 16). Maternal
weight gain and feed consumption in the low- and mid-dose groups did
not appear to be significantly affected. No effects of treatment on
any maternal reproductive indices were apparent. Mean fetal body
weights and crown-rump lengths were not affected in any test groups.
No effects of treatment on the fetal or litter incidences of visceral
or skeletal anomalies were noted at doses up to and including a
maternally-toxic dose of 400 ppm (34 mg/kg b.w./day) (Rogers et
al., 1978).
Rabbits
Groups of artificially-inseminated New Zealand white (DLI:NZW)
rabbits were randomly assigned to test groups (20/group) that were
administered 0, 2, 6, or 16 mg/kg b.w./day technical methomyl (98.7%
active ingredient) over days 7 -19 of presumed gestation. The route of
administration was by gavage (5 ml/kg of deionized water), and dosages
were selected on the basis of a preliminary study which demonstrated
that a dose of 24 mg/kg b.w./day was lethal to 2/4 rabbits, and
produced significant clinical signs in the remaining 2 does. Control
animals received only deionized water. Rabbits were examined daily for
clinical signs of toxicity, and body weights were recorded
periodically. On day 29 of gestation, does were sacrificed by CO2
asphyxiation, and uteri were removed and weighed. The following
parameters were determined: number of corpora lutea, number and
placement of implantations, resorptions, and live and dead fetuses.
Fetuses were individually weighed, and live fetuses were killed and
examined for gross external, visceral, and skeletal abnormalities.
One mid-dose and 6 high-dose rabbits died as a result of
treatment, and a statistically-significant increase in the incidence
of signs related to cholinesterase inhibition (tremours, hyper-
activity, salivation, etc.) was noted in high-dose animals. Mean
body weights were increased by about 5% on day 29 in surviving
high-dose does, and mean weight gain over days 0 - 29 was increased
about 3-fold in these rabbits (p < 0.01 by Dunnett's LSD). No effects
of treatment on the number of resorptions or litter size were
apparent. Statistically-significant increases in mean fetal body
weights of 14 - 19% were noted in all dose groups, which appeared to
be treatment-related. This change was reported to be related to
increased food consumption in treated rabbits in the latter portion of
the study; however, actual food consumption data were not submitted.
No evidence of oedema was noted in treated fetuses, nor was any effect
of treatment on the incidences of fetal malformations or variations
apparent, at doses up to and including a maternally-toxic dose of
16 mg/kg b.w./day (Christian et al., 1983).
Data were combined from 2 older studies, conducted 6 months
apart, in which a total of 12 rabbits/group were treated with 0, 45 -
50, or 90 - 100 mg/kg b.w./day technical methomyl (purity 90 - 100%)
mixed into the diet at appropriate concentrations to achieve the
desired dosages. The animals were treated from days 8 - 16 of presumed
gestation. Clinical signs and food consumption were recorded daily and
body weights were measured weekly. One-half of the does in each group
were sacrificed on day 29 and subjected to caesarean necropsy, whereas
the remaining rabbits in each group were allowed to deliver normally.
One-third of the fetuses or pups from each litter were examined for
skeletal anomalies. The extent of soft tissue examinations was not
adequately described, but appeared to be restricted to gross
observations of internal organs.
One control and 1 low-dose rabbit died during the study period,
neither of which was pregnant. One other control doe delivered a
full-term litter on the second day of treatment, and was excluded. One
control doe and one low-dose doe aborted. High-dose rabbits were
reported to have consistent weight loss related to decreased food
consumption; however, actual data were not submitted. The incidences
of pregnancy, after combination of the 2 studies, were 4/12, 8/12, and
5/12 in the control, mid-dose, and high-dose groups, respectively. No
effects of treatment on maternal reproductive indices, fetal body
weights or size, or visceral/skeletal findings were noted
(Busey, 1967a).
Acute toxicity
The acute toxicity of methomyl to several animal species is
summarized in Table 2. Methomyl is highly toxic via both the oral and
inhalation routes of exposure. The severity and onset of cholinergic
signs were related to the administered dose.
Table 2. Acute toxicity of methomyl
Solvent LD50 LC50
Species Route Sex (vehicle) (mg/kg b.w.) (mg/1) Reference
Rat Oral M Peanut oil1 26 - Sherman, 1964
Oral M Peanut oil1 17 - Sherman, 1966
F 23.5
Oral M Water2 45 - Trivits, 1979
Dermal M Water1 > 1000 - Morrow, 1972
Inhalation, M Aerosol - 0.30 Foster, 1966a
4 hours
Rat Inhalation, M Vapour - 0.04 Foster, 1966b
4 hours
Inhalation, M Spray - 0.45 Hornberger,
4 hours 1967
Rabbit Oral M Acetone/ 30 - Sherman, 1968a
peanut oil1
Dermal M Water1 > 5000 - Dashiell, 1971
Dermal M&F Water1 > 1500 - Majut & Hood,
1966
Chicken Oral F Acetone/ 28 - Krauss & Stula,
peanut oil1 1967
Dog Oral M Gelatin 20 - Sherman, 1968b
capsule1
1 Technical methomyl having a purity > 98% was used.
2 Technical methomyl having a purity of 100% was used.
Short-term studies
Rats
Groups of Charles River (CD) rats, 10/sex/group, were adminis-
tered methomyl (technical, 100%) in the diet for 90 days at dose
levels of 0, 10, 50, or 250 ppm. An additional group was fed diets
that contained 125 ppm methomyl for 42 days and 500 ppm methomyl to
the end of the study. Observations included mortality, serological,
haematological, and urological examinations, plasma and erythryocyte
ChE activities, food and water consumption, body weights, and gross
and microscopic analyses. No deaths or dose-related behavioural,
clinical, haematological, serological, urological, or pathological
effects were observed. Body weights were depressed in males at 250 ppm
and at 125/500 ppm, and in females at 125/500 ppm. Sporadic decreases
in haemoglobin content or erythrocyte count were observed in test-
group males and females, but the responses were not time or dose
dependent. Moderate erythroid hyperplasia was observed in the bone
marrow of male rats fed 250 ppm. The NOEL was 50 ppm methomyl, based
on body-weight depression in males (Paynter, 1966).
Rabbits
Methomyl (90% soluble concentrate) was administered dermally to
New Zealand albino rabbits (5/sex/dose) at 0 or 200 mg/kg b.w. to both
intact and abraded skin areas. Applications were 5 days a week for 21
days. There were no toxic symptoms noted in animals with intact skin.
There were signs of ChE inhibition and 2 deaths in animals with
abraded skin, although ChE activity was comparable to controls. There
was no evidence of cumulative toxicity or of compound-related effects
on organ weights or histopathology (Busey, 1967b).
Dogs
Groups of 11- to 13-month old beagle dogs (4/sex/group) were fed
diets that contained methomyl (97.5% methomyl, 2.5% HiSil 233) in corn
oil at dosages of 0, 50, 100, or 400 ppm for 3 months. Mean daily
intakes corresponded to 0, 1.44, 3.18, and 14.7 mg/kg b.w./day for
males and 0, 1.45, 3.01, and 12.5 mg/kg b.w./day for females, respect-
ively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, and haemato-
logical, clinical chemical, and urological effects. Cholinesterase
activities were not determined. Organs were weighed and gross necropsy
and histopathology were performed at terminal sacrifice. No compound-
related effects were reported. However, a NOEL for this study could
not be determined because blood cholinesterase activities were not
determined and the animals were older than is recommended by currently
acceptable international guidelines (i.e. OECD test methods) for
conducting short-term non-rodent feeding studies (preferably they
should be 4-6 months of age, but not older than 9 months) (Sherman
et al., 1967).
Long-term studies
Mice
Groups of 80 male and 80 female albino weanling CD-1 mice (6
weeks old) were administered methomyl (technical, purity 99%) in the
diet for 104 weeks at initial dose levels of 10, 50, 100, or 800 ppm.
Due to high mortality, the high dose was reduced to 400 ppm at week 28
and then to 200 ppm at week 39. The mid-dose level was also reduced at
week 39 to 75 ppm. Mean dietary intakes were 8.7, 15.4, and 93.5 mg/kg
b.w./day for males and 10.6, 19.1, and 118 mg/kg b.w./day for females,
respectively.
All animals were observed routinely for clinical signs,
mortality, moribundity, body-weight changes, and food consumption.
Haematological evaluations were performed periodically throughout the
study. Selected organs were weighed, including the brain, thymus,
lungs, heart, spleen, liver, kidneys, testes (w/epididymides),
adrenals, and pituitary. Complete gross and microscopic analyses of
tissues were performed on each animal.
Body-weight gain and food consumption of animals in the treated
groups were equivalent to controls. Mortality was increased among mid-
and high-dose animals throughout the study. Mortality in the low-dose
males was initially increased, in comparison to controls, but was
comparable at the termination of the study. Survival was greater than
50% in all groups at 11 months and approximately 25 - 30% at 2 years.
Red cell mass was apparently decreased during the first 26 weeks
at the mid- and high-dose levels, as evidenced by lower haemoglobin
levels, RBC counts, and haematocrit. These effects were reversed when
the amount of methomyl in the diet was reduced to 75 and 200 ppm in
the mid- and high-dose levels, respectively.
Necropsy and pathological examinations were unremarkable and
there were no compound-related histological changes. A substantial
amount of autolysis occurred in animals found dead prior to term,
which precluded any meaningful analysis of treatment-related effects
in these early deaths. There were no compound-related increases in
neoplastic changes at doses up to and including 200 ppm for 104 weeks.
The NOEL was 50 ppm, based on adverse effects on red cell mass at
higher dose levels (Serota et al., 1981).
Rats
Groups of Charles River rats (35 males and 35 females/group; 70
males and 70 females used as controls) were administered methomyl in
the diet at dose levels of 0, 50, 100, 200, or 400 ppm for 22 months.
These diets corresponded to 0, 2.4, 4.8, 9.6, and 20 mg/kg b.w./day
for males and 0, 2.8, 5.8, 11, and 24 mg/kg for females, respectively.
Body weights, food consumption, appearance, and behaviour were
recorded weekly from weeks 1 through 26 and monthly thereafter to week
96. Clinical chemistry observations included BUN, SGPT, SAP, glucose,
plasma, red blood cell, and brain cholinesterases, and, at the
conclusion of the study, bone-marrow differential counts. The
cholinesterase assays were performed using a delta pH method. This
method is considered inadequate for evaluating cholinesterase
inhibition by carbamates and the data are therefore unreliable.
Haematology determinations included haematocrit, haemoglobin, red
blood cell counts, and total and differential leukocyte counts;
urinalysis included pH, specific gravity, sugar, protein, bilirubin,
occult blood, and microscopic examination of the sediment. At 12
months, 5 rats of each sex in each group were sacrificed. At 22
months, because of the presence of respiratory disease and high
mortality, all surviving animals were sacrificed. Terminal body
weights and gross and microscopic examinations were performed. Organ
weights were obtained for the following tissues: brain, heart, liver,
spleen, kidneys, and testes. Organ and organ-to-body-weight ratios
were recorded and calculated. Microscopic examinations were performed
on the following tissues from rats in the control and 400 ppm groups:
brain, thyroid, heart, liver, spleen, kidneys, adrenals, stomach,
small intestine, large intestine, testes, bone marrow, muscle, and
sciatic nerve. The liver, kidneys, and spleen from rats in the other
groups were examined.
Growth and food consumption, measured only over the first year,
were reduced at 400 ppm. At 200 ppm, growth of males was reduced when
compared with control values. Haematology, blood chemistry, and
urinalysis values were similar in treated and control groups, although
there was a dose-related trend toward decreased haemoglobin in female
treatment groups. This was significant at 18 and 22 months in females
in the 200 and 400 ppm groups. Males were not similarly affected.
Compound-related increases in the incidence and severity of
extramedullary haematopoiesis were observed in the spleens of females
fed 200 or 400 ppm methomyl.
Gross and microscopic examinations were performed at 12 and 22
months. Increased testes, adrenal, liver, and brain weights were
observed at 400 ppm. At 200 and 400 ppm increased thyroid weights in
females were observed. Macroscopic changes in the kidneys, including
enlargement, discoloration, and texture changes were not reflected in
weight differences. Microscopic changes were evident in the kidneys of
both males and females at 400 ppm. The histopathological changes were
characterized by tubular hypertrophy and vacuolation of the epithelial
cells of the proximal convoluted tubules. At 200 and 400 ppm,
extramedullary haematopoiesis was noted in the spleen of females. No
microscopic changes were evident in other tissues, including those in
which weight increases were noted. There was no evidence of oncogenic
potential at any dose level. The 100 ppm dose level could be
considered the NOEL in this study, based on macro- and microscopic
changes in the thyroid, spleen, and kidneys, as well as on body-weight
changes. However, since cholinesterase activity was not measured by an
appropriate and sensitive method for such a potent ChE inhibitor, an
accurate NOEL for this study could not be determined (Busey, 1968a).
In a chronic feeding/oncogenicity study, ChR-CD rats were
administered methomyl (> 99% pure) in the diet for 24 months. Groups
of 80 male and 80 female rats were fed diets containing 0, 50, 100, or
400 ppm methomyl. These concentrations were equal to 0, 2.4, 4.8, and
20 mg/kg b.w./day for males and 0, 2.3, 6.3, and 26 mg/kg b.w./day for
females, respectively. Food consumption and body weights were
measured, and haematological, clinical chemistry, and urological
evaluations were conducted routinely throughout the study. Erythrocyte
and brain acetytcholinesterase activities were measured by the Ellman
method. After 12 months, 10 rats/sex/treatment group were sacrificed
and necropsied, selected organs were weighed, and selected tissues/
organs were examined histologically. At the end of the study all rats
were sacrificed and examined in a similar manner. Tissues from rats
found dead or killed in extremis were examined histologically.
Body-weight gains of males and females in the 400 ppm group were
depressed for the first 1 to 1.5 years, but they recovered thereafter
and were comparable to control body-weight gains by 24 months.
Significantly decreased haemoglobin, RBC counts, and haematocrits were
evident in females in the 400 ppm group. Significant differences were
not observed between control and test-group animals with regard to
erythrocyte or brain cholinesterase activities. However, due to a
possible effect of storage of blood samples prior to measurement, the
potential effect of the test compound on cholinesterases could have
been undiscovered due to fast spontaneous reactivation of carbamylated
enzyme.
Relative testes weights of high-dose males were significantly
greater than those of controls, but there were no observed
pathological changes. Female rats fed > 100 ppm methomyl had
increased relative liver weights, but no associated histopathological
alterations were identified. Female rats fed 400 ppm methomyl also had
increased relative spleen weights, but no noticeable pathological
tissue responses. The incidence of bone marrow hyperplasia, focal
hyperplasia in the adrenal medulla, and focal degeneration/angiectasis
in the adrenal cortex were increased in male rats fed 400 ppm
methomyl. There were no other significant pathological changes and no
oncogenic potential was evident at any of the doses administered in
this study. A NOEL of 100 ppm was estimated based on body-weight
changes and on the haematologic response at 400 ppm (Kaplan et al.,
1981).
Dogs
Groups of young adult (> 12 months of age) beagles (4 male and 4
females/group) were administered methomyl in the diet at dose levels
of 0, 50, 100, 400, or 1000 ppm for 2 years. Methomyl was incorporated
into a ground dry dog meal. Diet mixtures were prepared weekly. Daily
observations were made of behaviour and appearance, and body-weight
and food-consumption data were recorded weekly. Clinical chemistry
studies were performed initially and at 3, 6, 12, 18, and 24 months.
Determinations were made of blood sugar, BUN, SAP, SGPT, SGOT, and
prothrombin time. Serum electrolytes, total protein, and albumin were
determined at 18 and 24 months. Haematology examinations included the
following: haematocrit and haemoglobin concentration, red blood cell
count, total and differential leukocyte count, and bone marrow
differential count at the termination of the study. Urinalysis
included: appearance, pH, specific gravity, sugar, acetone, protein,
bilirubin, occult blood, and microscopic examination of the sediment.
At 24 months, urinary sodium, potassium, chloride, glucose, protein,
and phosphorous were determined. Plasma and erythrocyte cholinesterase
activities were measured by the monometric method at the ninth week in
animals in the control and 1000 ppm groups and during the thirteenth
week in animals in the 1000 ppm group. At the end of 1 year, 1
dog/sex/group was sacrificed and the following organ weights recorded
after gross examination: brain, thyroid, heart, liver, spleen,
kidneys, adrenals, and testes. Organ-to-body weight ratios were
calculated. At 24 months the remaining dogs were sacrificed and the
following tissues were examined from each dog: brain, pituitary,
thyroid, thymus, lungs, heart, liver, spleen, kidneys, adrenals,
stomach, pancreas, duodenum, jejunum, ileum, colon, mesenteric lymph
node, urinary bladder, ovary, uterus, skin, bone, bone marrow, muscle,
sciatic nerve, and testes. The same organs were weighed as after 1
year.
Two out of 4 females in the 1000 ppm group died within 8 weeks of
the beginning of the study. Signs of poisoning included tremours,
salivation, and incoordination, which were observed only once in males
at 1000 ppm. Haematology studies in 1 high-dose male indicated severe
anaemia and moderate leukopenia (decreased haematocrit, haemoglobin,
RBC count and platelet count; increased reticulocyte count; and
changes in the differential count). This condition improved when the
dog was placed on a control diet at 84 weeks, but worsened when
1000 ppm methomyl was replaced in the diet at the ninety-fifth week.
Enlarged spleen and kidneys were noted at sacrifice (24 months). The
remaining high-dose males and 2/4 high-dose females demonstrated
similar haematologic abnormalities at 3 months, but recovered to
normal by 24 months. Growth and food consumption were unaffected by
treatment; however, all dogs were young adults at the start of the
test and therefore were already beyond the critical growth months for
this species. This is considered a deficiency in this study. Clinical
chemistry and urinalyses were comparable between treated groups and
controls. Since plasma and erythrocyte cholinesterase activities were
assayed by a method considered inadequate for carbamate inhibition,
these data are unreliable for estimating the cholinesterase inhibiting
properties of methomyl in dogs.
At 24 months, gross pathology examinations showed liver and
spleen enlargement in the male that was severely anaemic. Kidney
weights were increased in males at 1000 ppm and in 1 male at 400 ppm.
Microscopic examination showed alterations in the spleen
(extramedullary haematopoiesis) and kidneys (pigment in the cytoplasm
and swelling of the epithelial cells of the proximal convoluted
tubules in males and females), liver (bile duct proliferation) and
bone marrow (increased activity in both erythroid and myeloid series)
at 1000 ppm. At 400 ppm similar kidney abnormalities were present only
in males. Alterations were not observed in the lower-dose groups. A
no-effect level of 100 ppm (equal to 3.1 mg/kg b.w./day) was
tentatively estimated, based on the adverse haematologic effects and
on kidney and spleen morphologic changes at > 400 ppm. However, in
the absence of adequate data for adverse effects on cholinesterase
activity an accurate estimate of a NOEL cannot be determined
(Busey, 1968b).
Observations in humans
Epidemiological studies of poisonings from the ingestion of
contaminated food indicate that a single oral dose as low as
12-15 mg/kg b.w. can be fatal in humans (Liddle et al., 1979;
Araki et al., 1982).
An assessment of workers who routinely handled methomyl in a
factory indicated that workers were occasionally inadvertently exposed
and required hospitalization and treatment for symptoms of
cholinesterase inhibition, presumably due to methomyl intoxication.
Blood cholinesterase levels were normal for these workers, however,
and no quantitation of dose was presented. The methodology and timing
of ChE measurements were questionable (Morse et al., 1979).
COMMENTS
Methomyl is rapidly absorbed from the gastrointestinal tract or
through the skin, and is rapidly metabolized and eliminated. The
metabolic fate of methomyl depends on the isomeric configuration, as
the syn form (the technical product) is primarily degraded to CO2,
whereas the anti form is degraded to acetonitrile. Some conversion
of syn-methomyl to anti-methomyl apparently occurs in rats.
The acute toxicity of methomyl is due to inhibition of
acetylcholinesterase. The oral LD50 in experimental animals ranges
from 20 to 45 mg/kg b.w.; atropine is an antidote to these acute toxic
effects.
Long-term feeding studies in mice and rats do not suggest any
evidence of oncogenicity for methomyl. Toxic effects included anaemia
(mice, rats, and dogs), decreased weight gain (rats), and
histopathological changes in the kidney (dogs). The no-observed-effect
levels for chronic toxicity, calculated from dietary intake, were;
8.7 mg/kg b.w./day in mice, 4.8 mg/kg b.w./day in rats, and 3.1 mg/kg
b.w./day in dogs.
The available chronic toxicity data are somewhat limited by the
lack of adequate determinations of cholinesterase activity. However,
the effects on cholinesterases are reversible. In order to estimate
the rate of spontaneous reactivation in man, the submission of the
results of additional in vitro studies is required. These studies
should provide data on the inhibition and reactivation rate constants
for the effect of methomyl on human plasma and erythrocyte
cholinesterases.
Available epidemiological data for humans indicate that a dose of
12 - 15 mg/kg b.w. methomyl, ingested as a bolus with food, can be
lethal owing to suppression of cholinesterase activity. This value is
similar to the acute lethal doses identified in animal studies.
No evidence of teratogenicity or embryotoxicity was apparent in
rats or rabbits. The available rat reproduction studies, when
considered together, demonstrate a NOEL of 50 ppm (equivalent to
2.5 mg/kg b.w./day), based on decreases in body-weight gain and food
consumption in a study reported in 1968, and on slight decreases in
litter size at 75 ppm in a study reported in 1983. No other evidence
of effects on fertility or other reproductive parameters was noted.
No evidence of mutagenicity was noted in a number of genotoxicity
studies.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
NOELs for chronic oral toxicity are established as;
Mouse: 50 ppm, equal to 8.7 mg/kg b.w./day.
Rat: 50 ppm, equivalent to 2.5 mg/kg b.w./day.
Dog: 100 ppm, equal to 3.1 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0 - 0.01 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF A FULL ADI IS
IMPRACTICABLE, TO BE SUBMITTED TO WHO BY 1988:
Additional in vitro studies in human blood to demonstrate the
time-course of plasma and red blood cell cholinesterase inhibition and
reactivation.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Further observations in humans.
REFERENCES
Araki, M., Yonemitsu, K., Kambe, T., Idaka, D., Tsunenari, S., Kanda,
1982 M., & Kambara, T. Forensic toxicological investigations on
fatal cases of carbamate pesticide methomyl (Lannate(R))
poisoning. Nippon Hoigaku Zasshi, 36: 584 - 588.
Barnes, J.R. Cholinesterase tests with methomyl. Unpublished report
1978 No. HLR-280-78 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Blevins, R.D. Lee, M., & Regan, J.D. Mutagenicity screening of five
1977 methyl 1977 carbamate insecticides and their nitrosos
derivatives using mutants of Salmonella typhimurium LT2.
Mutat. Res., 56: 1 - 6.
Busey, W.M. Teratology study in rabbits with "Lannate(R)" methomyl
1967a insecticide. Unpublished Report No. HLO-257-67 from Hazleton
Laboratories, Inc., Falls Church, VA., USA. Submitted to WHO
by E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Busey, W.M. Repeated dermal study in rabbits - "Lannate(R)" methomyl
1967b insecticide (90% methomyl). Unpublished Report
No. HLO-254-67 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Busey, W.M. 22-Month dietary feeding study in rats with "Lannate(R)"
1968a methomyl insecticide. Unpublished Report No. 201-164 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Busey, W.M. Two year dietary administration in dogs with "Lannate(R)"
1968b methomyl insecticide. Unpublished Report No. 201-165 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Christian, M.S., Hoberman, A.M., & Feussner, E.L. Embryo-fetal
1983 toxicity and teratogenicity study of methomyl in the rabbit.
Unpublished report No. HLO-331-83 from Argus Research
Laboratories, Inc., Horsham, PA, USA. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Dashiell, O. Acute skin absorption LD50 test in rabbits using technical
1971 methomyl (> 98% methomyl). Unpublished report No.
HLR-340-71 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
DeBuyst, B. & Van Larebeke, N. Induction of sister-chromatid exchanges
1982 in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242.
Farrow, M.G., Cortina, T., & Padilla-Nash, H. In vivo bone marrow
1984 chromosome study in rats [with] H No. 15,000 - Final Report.
Unpublished Report No. HLO-63-84 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966a methomyl (> 98% methomyl) progress report. Unpublished
report No. HLR-73-66 from Haskell Laboratory for Toxicology
and Industrial Medicine, E.I. du Pont de Nemours and Co.,
Inc. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966b methomyl (> 98% methomyl). Unpublished report
No. HLR-214-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Harvey, J., Jelinek, A.G., & Sherman, H. Metabolism of methomyl in the
1973 rat. J. Agric. Fd. Chem., 21: 769 - 775.
Hornberger, C.S. Acute inhalation toxicity of aqueous spray mist.
1967 Unpublished report No. HLR-171-67 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
1976 reactions in the metabolism of methomyl in rats.
Pest. Biochem and Physiol., 6: 571 - 583.
Kaplan, A.M., Trochimowicz, H.J., & Barba, C. Long-term feeding study
1981 in rats with S-methomyl-N-(methylcarbomoyloxy)
thioacetimidate (methomyl; INX-1179). Unpublished report
No. HLR-235-81 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Kraus, W. & Stula, E.F. Acute oral LD50 and delayed neurotoxicity
1967 (paralysis) tests in hens using technical methomyl (> 98%
methomyl). Unpublished report No. HLR-161-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Kundzin, M. & Busey, W.M. Three-generation reproduction study with
1968 Lannate(R) 1968 methomyl insecticide. Unpublished Report
No. MRO 888-1 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Liddle, J.A., Kimbrough, R.D., Needham, L.L., Cline, R.E., Smrek,
1979 A.L., Yert, L.W., Bayse, D.D., Ellington, A.C., & Dennis,
P.A. A fatal episode of accidental methomyl poisoning.
Clin. Toxicol., 15; 159 - 167.
Lu, C.C. Nudrin(R) two-generation reproduction study in rats.
1983 Unpublished report no. WRC RIR-275 from Westhollow Research
Center, Shell Development Co., Houston, TX, USA. Submitted
to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Majhut, J. & Hood, D. Acute skin absorption, approx, lethal dose (ALD)
1966 test in rabbits using 55 and 26% formulations of (technical)
methomyl in aqueous solutions. Unpublished report
No. HLR-216-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M., & Hemingway, R.E.
1984 CHO/HGPRT assay for gene mutations using technical methomyl.
Unpublished report No. HLR-556-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Morrow, R.W. Acute skin absorption test (ALD) in rabbits using
1972 technical methomyl and a 25% methomyl formulation
(Lannate(R) 25W). Unpublished report No. HLR-438-72 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Morse, D.L., Baker, E.L., Kimbrough, R.D., & Wisseman, C.L.
1979 Propanilchloracne and methomyl toxicity in workers of a
pesticide manufacturing plant. Clin. Toxicol.,
15: 13 - 21.
Paynter, O. Three month dietary administration in rats using
1966 insecticide 1179 (technical methomyl, > 98% purity).
Unpublished Report No. MRO-848 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Rogers, A.S., Culik, R., Kaplan, A.M., & Aftosmis, J.G. Oral
1978 teratogenic study in rats with Lannate(R) (INX-1179).
Unpublished report No. HLR-498-78 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Serota, D.G., Machotka, S.V., Hasting, T.F., Alsaker, R.D., &
1981 Fezio, W.L. 104-Week chronic toxicity and carcinogenicity
study in mice. Unpublished Report No. HLO-253-81 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Shah, P.V., Monroe, R.J., & Guthrie, F.E. Comparative rates of dermal
1981 penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59: 414 - 423.
Sherman, H. Acute oral (approx. lethal dose - ALD) and 10-dose oral
1966 subacute toxicity test in rats using technical methomyl
(> 98% methomyl). Unpublished report No. HLR-100-64 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Sherman, H. Acute oral LD50 test in rats using technical methomyl
1966 (> 98% methomyl). Unpublished report No. HLR-210-66 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Sherman, H., Barnes, J.R., Stula, E.F., & Zapp, J.A. Three-month
1967 feeding study on dogs with S-methyl-N-(methylcarbomoyloxy)
thioacetimidate (Lannate(R) methomyl insecticide;
INX-1179). Unpublished report No. HLR-168-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in rabbits using
1968a technical methomyl (> 98%). Unpublished report
No. HLR-250-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in dogs using
1968b technical methomyl (> 98%). Unpublished report
No. HLR-279-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Simmon, V.F.; Mitchell, A.D., & Jorgenson, T.A. Evaluation of selected
1977 pesticides as chemical mutagens: in vitro and in vivo
studies. Unpublished EPA report No. EPA-600/1-77-028 from
U.S. Environmental Protection Agency, Health Effects
Research Laboratory, Research Triangle Park, N.C., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Singles, G.H. INX-1179 (methomyl) and cholinesterase activity.
1970 Unpublished report No. HLR-186-70 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Trivits, R. Acute oral LD50 test in rats using technical methomyl.
1979 Unpublished report No. HLR-497-79 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Valencia, R. Mutagenesis screening of pesticides in Drosophila.
1981 Unpublished EPA report No. 600/1/81/017. Prepared by Warf
Institute, Inc. for the U.S. Environmental Protection
Agency. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Vincent, D.R., Arce, G.T., & Sarrif, A.M. Assessment of methomyl
1985 (INX-1179-255) in the in vitro unscheduled DNA synthesis
assay in primary rat hepatocytes. Unpublished report
No. HLR-149-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Wojciechowski, J.P., Kaur, P., & Sabharwal, P.S. Induction of ouabain
1982 resistance in V-79 cells by four carbamate pesticides.
Env. Research, 29: 481 - 53.
Effects on enzymes and other biochemical parameters
Methomyl was administered in the diet to groups of Crl:CD(SD)BR
rats at levels of 0, 5, 100, 200, 400, or 800 ppm for 5 months. There
were 10 rats/sex/dose. Peripheral blood samples were taken at 4, 11,
32, 43, 60, and 79 days and enzyme activities were measured in whole
blood by the Ellman method. Significant inhibition was reported at
800 ppm in both sexes, but not at 400 ppm (Singles, 1970).
Groups of Crl:CD(SD)BR rats (8/sex/group) were fed technical
methomyl in their diets at dose levels of 0, 100, 400, or 800 ppm for
29 days. Analysis of erythrocyte, plasma, and brain cholinesterase,
determined separately, demonstrated that brain cholinesterase was more
sensitive to methomyl. Females at 100, 400, and 800 ppm demonstrated
depressed brain cholinesterase activity after 29 days compared to
controls, statistically significant at the high dose only. Erythrocyte
and plasma cholinesterase activities, although depresses in males and
females at 800 ppm, were not statistically different from controls
(Barnes, 1978).
Toxicological studies
Special studies on mutagenicity
Methomyl has been shown to be negative in a number of test
systems that have assessed the ability of this compound to cause gene
mutations, structural chromosomal defects, and direct DNA damage. A
single positive result for sister chromatid exchange (SCE) was
reported in an abstract (DeBuyst, B. & Van Larebeke, 1982); however,
this study is not acceptable for evaluation, because methods, purity
of the test substances, and actual results were not reported.
Summary results are presented in Table 1.
Special studies on reproduction
Rats
Groups of male and female Charles River CD rats (13 males/group
and 26 females/group in the F0 generation, 20 males/group and 40
females/group in the F1 generation) were fed diets containing 0, 75,
600, or 1200 ppm technical methomyl continuously over 2 generations.
Feed analyses indicated that the actual levels of methomyl in the test
diets were within acceptable limits of the targeted values. Animals
were observed twice daily for clinical signs of toxicity and body
weights and food consumption were measured weekly. During gestation,
females were weighed on day 3 and daily from days 6 through
parturition. F0 and F1 males were sacrificed after mating, and
sperm counts were performed. After birth, litter size, pup weights,
sex, and numbers of live and dead pups were determined. Pups were
examined daily during lactation for abnormalities of appearance or
behaviour, and were weighed on days 1, 4, 7, 14, and 21 of lactation.
After weaning, pups were weighed weekly. F0 and F1 parents were
subjected to complete necropsies and haematological evaluations at the
end of mating or weaning (males and females, respectively).
Randomly-selected pups from each generation (10/sex/dose) were also
necropsied.
Mean body weights were significantly decreased at most of the
measured intervals in mid- and high-dose male and female parents in
the F0 generation, and in all parental treatment groups in the F1
generation. The decrease in the F1 generation was dose-related, and
ranged from 5 - 10% in the low-dose groups to 20 - 25% in the
high-dose groups; a NOEL was not established for this finding. A
similar pattern was noted in dams during gestation, as dose-related,
statistically-significant decreases were noted in all F1 female
treatment groups, and in mid- and high-dose F0 treatment groups.
These decreases appeared to be related to decreases in food
consumption.
Table 1. Special studies on mutagenicity
Test system Test object Concentration Purity Results References
used
Ames test1 S. typhimurium 0.005, 0.05, Technical Negative2 Blevins,
TA98, TA100, 0.5, 5, et al., 1977
TA1535, 50 nM
TA1537,
& TA1538
Ames test1 S. typhimurium 1, 5, 10, 50, Technical Negative3 Simmon,
TA100, 100, 500, et al., 1977
TA1535, 1000 µg/plate
TA1537,
& TA1538
Ames test1 E. coli 1, 10, 50, Technical Negative4 Simmon
WP2 100, 500, et al., 1977
1000 µg/plate
1 Both with and without metabolic activation.
2 The nitroso- derivative of methomyl was positive at 50 nM.
3 The positive control, 4-o-tolylazo-o-toluidine at 25 µg/plate, gave the expected positive response.
4 The positive controls, AF-2 at 0.1 µg/plate and 2-aminoanthracene at 10 µg/plate, gave the expected positive responses.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
DNA repair E. coli 1 mg/disc Technical Negative5 Simmon
assay W3110, p3478 et al., 1977
B. subtilis
H17, m45
Mitotic S. cerevisiae D3 1.0, 1.5, 2.0, Technical Negative6 Simmon
recombination1 2.5, 3.0 et al., 1977
3.5% w/v
Gene mutations Chinese 1, 5, 10 mM Unknown Negative7 Wojciechowski
in vitro1 hamster et al., 1982
ovary V79
CHO/HGPRT Chinese Nonactivated: 99% Negative8 McCooey
gene hamster 10, 20, 40, et al., 1984
mutation1 ovary cells 50, 55 mM.
CHO-K1, Activated:
BH4 clone 100, 150, 200,
250, 350 mM
5 The positive control, 1-phenyl-3-dimethyltriazene at 1 mg/disc, and the negative control, chloramphenicol at
0.03 mg/disc, gave the expected responses.
6 The positive control, 0.025% 1,2,3,4-diepoxybutane, gave the expected positive response.
7 This study is not acceptable for evaluation because the purity of the test substance was not identified.
8 The positive control, 0.5 mM EMS, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
Sister In vitro Unknown Unknown Positive9 DeBuyst &
chromatid human Van Larebeke,
exchange1 lymphocytes 1982
Sex-linked Drosophila 4 & 10 ppm Technical Negative10 Valencia,
recessive melanogaster 1981
lethal
Unscheduled Primary rat 0, 1, 10, 100, 99% Negative11 Vincent
DNA hepatocytes 1000, 5000, et al., 1985
synthesis from male 10,000,
Crl:CD rats 75,000 µM
Unscheduled WI38 10-7, 10-6, Technical Negative12 Simmon
DNA fibroblasts 10-5, 10-4, et al., 1977
synthesis 10-3 M
9 This study is unacceptable for evaluation because it is in abstract form; methods and data were not reported.
10 The positive controls, EMS, EDB, TMP, and EI, gave the expected positive responses when tested at the appropriate
concentrations.
11 The positive control, 100 and 500 µM DMBA, gave the expected positive response.
12 The positive control, 10-5 M 4-nitroquinoline, gave the expected positive response.
Table 1. (cont'd).
Test system Test object Concentration Purity Results References
used
In vivo Sprague-Dawley 0, 2, 6, 99% Negative13 Farrow
bone marrow male and female 20 mg/kg et al., 1984
cytogenetics rats
13 The positive control, 40 mg/kg cyclosphosphamide, gave the expected positive response.
At necropsy of the F0 parents, dose-related, statistically-
significant decreases in red cell count, haemoglobin concentration,
and haematocrit were noted in mid- and high-dose females; males were
not similarly affected. No statistically-significant decreases in
blood ChE activity were noted; however, mean plasma ChE activity was
decreased by 15% and 25% in high-dose F0 males and females,
respectively. Haematology/ChE results were not reported for F1
animals. Absolute and relative weights of several organs were altered
in a manner consistent with the treatment-related changes in body
weights, as absolute organ weights tended to decrease whereas relative
organ weights tended to increase, relative to the controls. No effects
of treatment on histopathological examination of F0 or F1
parents were noted.
Fertility, gestation length, and male sperm counts were not
adversely affected by treatment. However, dose-related statistically-
significant decreases in litter size and mean number of live pups
born were noted in all F1 treatment groups and in high-dose
F0 rats; a NOEL was not established for this finding. Pup survival
during lactation was significantly reduced in high-dose F1 pups
(born of F0 dams), but survivial did not appear to be affected
in F2 pups (born of F1 dams) after day 7 of lactation (a
significant decrease was noted in high-dose pups on day 4). Mean body
weights during lactation were significantly reduced in a dose-related
manner in all F1 pups; however, body weights were reduced only in
the mid- and high-dose groups of the F2 generation; a NOEL was not
established for this finding. No treatment-related histopathological
changes were noted in F1 or F2 pups (Lu, 1983).
Groups of Charles River CD rats (10 males/group and 20
females/group) were fed diets containing 0, 50, or 100 ppm methomyl
(purity unspecified) over 3 generations. F3b weanlings (10 sex/dose)
were subjected to necropsy and examined for histopathological changes.
A third group of weanlings (F3c) was fed test diets for 9 weeks and
then control diet during weeks 10 through 14 to assess the effect of
treatment on growth.
The study report indicated that no effects of treatment on
fertility, litter size, number of live pups, or pup survivial were
noted. A slight (10%) decrease in the mean body weights of male and
female pups at weaning in the F2 and F3 generations was not
considered to be related to treatment. No effects of treatment on
absolute or relative organ weights were noted. No treatment-related
changes in the gross or microscopic appearance of tissues were
reported; however, actual data were not submitted. Growth of F3c
weanlings fed 100 ppm methomyl was significantly less than that of
control rats, which was related to decreased food intake. This trend
was not reversed by removal of animals from the test diet for 4 weeks
(Kundzin & Busey, 1968).
Special studies on teratogenicity
Rats
Groups of naturally-bred Charles River (ChR-CD) female rats
(25/group) were randomly assigned to treatment groups that received
test diets containing 0, 50, 100, or 400 ppm technical methomyl
(< 99% active ingredient) and 1% Mazola corn oil over days 6 - 15 of
presumed gestation. Average doses were calculated to be 0, 4.9, 9.4,
and 34 mg/kg b.w./day, based on mean food consumption and body
weights. Control rats received rat laboratory feed with 1% corn oil.
Body weights, food consumption, and clinical signs were recorded
periodically during the gestation period. On day 21 of gestation, rats
were sacrificed with chloroform, and the ovaries and uteri were
removed and examined. The following parameters were determined: number
of corpora lutea, number of implantations, number and location of live
and dead fetuses and resorptions, body weights and crown-rump lengths
of live fetuses, and any gross fetal abnormalities. Approximately
one-half of the fetuses from each litter were cleared and stained for
skeletal examination, and the remaining fetuses were fixed and
examined for visceral anomalies.
No effects of treatment on the incidence of clinical signs were
apparent, and no deaths were noted. Mean maternal body weight was
reduced to 92 and 95% of the mean control weight on days 16 and 21,
respectively, in high-dose dams (p < 0.05 by Dunnett's LSD). Mean
feed consumption was also reduced by about 15% in high-dose dams over
days 6 - 16, but it was similar to feed consumption of control animals
over days 16 - 21 (treatment was terminated on day 16). Maternal
weight gain and feed consumption in the low- and mid-dose groups did
not appear to be significantly affected. No effects of treatment on
any maternal reproductive indices were apparent. Mean fetal body
weights and crown-rump lengths were not affected in any test groups.
No effects of treatment on the fetal or litter incidences of visceral
or skeletal anomalies were noted at doses up to and including a
maternally-toxic dose of 400 ppm (34 mg/kg b.w./day) (Rogers et
al., 1978).
Rabbits
Groups of artificially-inseminated New Zealand white (DLI:NZW)
rabbits were randomly assigned to test groups (20/group) that were
administered 0, 2, 6, or 16 mg/kg b.w./day technical methomyl (98.7%
active ingredient) over days 7 -19 of presumed gestation. The route of
administration was by gavage (5 ml/kg of deionized water), and dosages
were selected on the basis of a preliminary study which demonstrated
that a dose of 24 mg/kg b.w./day was lethal to 2/4 rabbits, and
produced significant clinical signs in the remaining 2 does. Control
animals received only deionized water. Rabbits were examined daily for
clinical signs of toxicity, and body weights were recorded
periodically. On day 29 of gestation, does were sacrificed by CO2
asphyxiation, and uteri were removed and weighed. The following
parameters were determined: number of corpora lutea, number and
placement of implantations, resorptions, and live and dead fetuses.
Fetuses were individually weighed, and live fetuses were killed and
examined for gross external, visceral, and skeletal abnormalities.
One mid-dose and 6 high-dose rabbits died as a result of
treatment, and a statistically-significant increase in the incidence
of signs related to cholinesterase inhibition (tremours, hyper-
activity, salivation, etc.) was noted in high-dose animals. Mean
body weights were increased by about 5% on day 29 in surviving
high-dose does, and mean weight gain over days 0 - 29 was increased
about 3-fold in these rabbits (p < 0.01 by Dunnett's LSD). No effects
of treatment on the number of resorptions or litter size were
apparent. Statistically-significant increases in mean fetal body
weights of 14 - 19% were noted in all dose groups, which appeared to
be treatment-related. This change was reported to be related to
increased food consumption in treated rabbits in the latter portion of
the study; however, actual food consumption data were not submitted.
No evidence of oedema was noted in treated fetuses, nor was any effect
of treatment on the incidences of fetal malformations or variations
apparent, at doses up to and including a maternally-toxic dose of
16 mg/kg b.w./day (Christian et al., 1983).
Data were combined from 2 older studies, conducted 6 months
apart, in which a total of 12 rabbits/group were treated with 0, 45 -
50, or 90 - 100 mg/kg b.w./day technical methomyl (purity 90 - 100%)
mixed into the diet at appropriate concentrations to achieve the
desired dosages. The animals were treated from days 8 - 16 of presumed
gestation. Clinical signs and food consumption were recorded daily and
body weights were measured weekly. One-half of the does in each group
were sacrificed on day 29 and subjected to caesarean necropsy, whereas
the remaining rabbits in each group were allowed to deliver normally.
One-third of the fetuses or pups from each litter were examined for
skeletal anomalies. The extent of soft tissue examinations was not
adequately described, but appeared to be restricted to gross
observations of internal organs.
One control and 1 low-dose rabbit died during the study period,
neither of which was pregnant. One other control doe delivered a
full-term litter on the second day of treatment, and was excluded. One
control doe and one low-dose doe aborted. High-dose rabbits were
reported to have consistent weight loss related to decreased food
consumption; however, actual data were not submitted. The incidences
of pregnancy, after combination of the 2 studies, were 4/12, 8/12, and
5/12 in the control, mid-dose, and high-dose groups, respectively. No
effects of treatment on maternal reproductive indices, fetal body
weights or size, or visceral/skeletal findings were noted
(Busey, 1967a).
Acute toxicity
The acute toxicity of methomyl to several animal species is
summarized in Table 2. Methomyl is highly toxic via both the oral and
inhalation routes of exposure. The severity and onset of cholinergic
signs were related to the administered dose.
Table 2. Acute toxicity of methomyl
Solvent LD50 LC50
Species Route Sex (vehicle) (mg/kg b.w.) (mg/1) Reference
Rat Oral M Peanut oil1 26 - Sherman, 1964
Oral M Peanut oil1 17 - Sherman, 1966
F 23.5
Oral M Water2 45 - Trivits, 1979
Dermal M Water1 > 1000 - Morrow, 1972
Inhalation, M Aerosol - 0.30 Foster, 1966a
4 hours
Rat Inhalation, M Vapour - 0.04 Foster, 1966b
4 hours
Inhalation, M Spray - 0.45 Hornberger,
4 hours 1967
Rabbit Oral M Acetone/ 30 - Sherman, 1968a
peanut oil1
Dermal M Water1 > 5000 - Dashiell, 1971
Dermal M&F Water1 > 1500 - Majut & Hood,
1966
Chicken Oral F Acetone/ 28 - Krauss & Stula,
peanut oil1 1967
Dog Oral M Gelatin 20 - Sherman, 1968b
capsule1
1 Technical methomyl having a purity > 98% was used.
2 Technical methomyl having a purity of 100% was used.
Short-term studies
Rats
Groups of Charles River (CD) rats, 10/sex/group, were adminis-
tered methomyl (technical, 100%) in the diet for 90 days at dose
levels of 0, 10, 50, or 250 ppm. An additional group was fed diets
that contained 125 ppm methomyl for 42 days and 500 ppm methomyl to
the end of the study. Observations included mortality, serological,
haematological, and urological examinations, plasma and erythryocyte
ChE activities, food and water consumption, body weights, and gross
and microscopic analyses. No deaths or dose-related behavioural,
clinical, haematological, serological, urological, or pathological
effects were observed. Body weights were depressed in males at 250 ppm
and at 125/500 ppm, and in females at 125/500 ppm. Sporadic decreases
in haemoglobin content or erythrocyte count were observed in test-
group males and females, but the responses were not time or dose
dependent. Moderate erythroid hyperplasia was observed in the bone
marrow of male rats fed 250 ppm. The NOEL was 50 ppm methomyl, based
on body-weight depression in males (Paynter, 1966).
Rabbits
Methomyl (90% soluble concentrate) was administered dermally to
New Zealand albino rabbits (5/sex/dose) at 0 or 200 mg/kg b.w. to both
intact and abraded skin areas. Applications were 5 days a week for 21
days. There were no toxic symptoms noted in animals with intact skin.
There were signs of ChE inhibition and 2 deaths in animals with
abraded skin, although ChE activity was comparable to controls. There
was no evidence of cumulative toxicity or of compound-related effects
on organ weights or histopathology (Busey, 1967b).
Dogs
Groups of 11- to 13-month old beagle dogs (4/sex/group) were fed
diets that contained methomyl (97.5% methomyl, 2.5% HiSil 233) in corn
oil at dosages of 0, 50, 100, or 400 ppm for 3 months. Mean daily
intakes corresponded to 0, 1.44, 3.18, and 14.7 mg/kg b.w./day for
males and 0, 1.45, 3.01, and 12.5 mg/kg b.w./day for females, respect-
ively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, and haemato-
logical, clinical chemical, and urological effects. Cholinesterase
activities were not determined. Organs were weighed and gross necropsy
and histopathology were performed at terminal sacrifice. No compound-
related effects were reported. However, a NOEL for this study could
not be determined because blood cholinesterase activities were not
determined and the animals were older than is recommended by currently
acceptable international guidelines (i.e. OECD test methods) for
conducting short-term non-rodent feeding studies (preferably they
should be 4-6 months of age, but not older than 9 months) (Sherman
et al., 1967).
Long-term studies
Mice
Groups of 80 male and 80 female albino weanling CD-1 mice (6
weeks old) were administered methomyl (technical, purity 99%) in the
diet for 104 weeks at initial dose levels of 10, 50, 100, or 800 ppm.
Due to high mortality, the high dose was reduced to 400 ppm at week 28
and then to 200 ppm at week 39. The mid-dose level was also reduced at
week 39 to 75 ppm. Mean dietary intakes were 8.7, 15.4, and 93.5 mg/kg
b.w./day for males and 10.6, 19.1, and 118 mg/kg b.w./day for females,
respectively.
All animals were observed routinely for clinical signs,
mortality, moribundity, body-weight changes, and food consumption.
Haematological evaluations were performed periodically throughout the
study. Selected organs were weighed, including the brain, thymus,
lungs, heart, spleen, liver, kidneys, testes (w/epididymides),
adrenals, and pituitary. Complete gross and microscopic analyses of
tissues were performed on each animal.
Body-weight gain and food consumption of animals in the treated
groups were equivalent to controls. Mortality was increased among mid-
and high-dose animals throughout the study. Mortality in the low-dose
males was initially increased, in comparison to controls, but was
comparable at the termination of the study. Survival was greater than
50% in all groups at 11 months and approximately 25 - 30% at 2 years.
Red cell mass was apparently decreased during the first 26 weeks
at the mid- and high-dose levels, as evidenced by lower haemoglobin
levels, RBC counts, and haematocrit. These effects were reversed when
the amount of methomyl in the diet was reduced to 75 and 200 ppm in
the mid- and high-dose levels, respectively.
Necropsy and pathological examinations were unremarkable and
there were no compound-related histological changes. A substantial
amount of autolysis occurred in animals found dead prior to term,
which precluded any meaningful analysis of treatment-related effects
in these early deaths. There were no compound-related increases in
neoplastic changes at doses up to and including 200 ppm for 104 weeks.
The NOEL was 50 ppm, based on adverse effects on red cell mass at
higher dose levels (Serota et al., 1981).
Rats
Groups of Charles River rats (35 males and 35 females/group; 70
males and 70 females used as controls) were administered methomyl in
the diet at dose levels of 0, 50, 100, 200, or 400 ppm for 22 months.
These diets corresponded to 0, 2.4, 4.8, 9.6, and 20 mg/kg b.w./day
for males and 0, 2.8, 5.8, 11, and 24 mg/kg for females, respectively.
Body weights, food consumption, appearance, and behaviour were
recorded weekly from weeks 1 through 26 and monthly thereafter to week
96. Clinical chemistry observations included BUN, SGPT, SAP, glucose,
plasma, red blood cell, and brain cholinesterases, and, at the
conclusion of the study, bone-marrow differential counts. The
cholinesterase assays were performed using a delta pH method. This
method is considered inadequate for evaluating cholinesterase
inhibition by carbamates and the data are therefore unreliable.
Haematology determinations included haematocrit, haemoglobin, red
blood cell counts, and total and differential leukocyte counts;
urinalysis included pH, specific gravity, sugar, protein, bilirubin,
occult blood, and microscopic examination of the sediment. At 12
months, 5 rats of each sex in each group were sacrificed. At 22
months, because of the presence of respiratory disease and high
mortality, all surviving animals were sacrificed. Terminal body
weights and gross and microscopic examinations were performed. Organ
weights were obtained for the following tissues: brain, heart, liver,
spleen, kidneys, and testes. Organ and organ-to-body-weight ratios
were recorded and calculated. Microscopic examinations were performed
on the following tissues from rats in the control and 400 ppm groups:
brain, thyroid, heart, liver, spleen, kidneys, adrenals, stomach,
small intestine, large intestine, testes, bone marrow, muscle, and
sciatic nerve. The liver, kidneys, and spleen from rats in the other
groups were examined.
Growth and food consumption, measured only over the first year,
were reduced at 400 ppm. At 200 ppm, growth of males was reduced when
compared with control values. Haematology, blood chemistry, and
urinalysis values were similar in treated and control groups, although
there was a dose-related trend toward decreased haemoglobin in female
treatment groups. This was significant at 18 and 22 months in females
in the 200 and 400 ppm groups. Males were not similarly affected.
Compound-related increases in the incidence and severity of
extramedullary haematopoiesis were observed in the spleens of females
fed 200 or 400 ppm methomyl.
Gross and microscopic examinations were performed at 12 and 22
months. Increased testes, adrenal, liver, and brain weights were
observed at 400 ppm. At 200 and 400 ppm increased thyroid weights in
females were observed. Macroscopic changes in the kidneys, including
enlargement, discoloration, and texture changes were not reflected in
weight differences. Microscopic changes were evident in the kidneys of
both males and females at 400 ppm. The histopathological changes were
characterized by tubular hypertrophy and vacuolation of the epithelial
cells of the proximal convoluted tubules. At 200 and 400 ppm,
extramedullary haematopoiesis was noted in the spleen of females. No
microscopic changes were evident in other tissues, including those in
which weight increases were noted. There was no evidence of oncogenic
potential at any dose level. The 100 ppm dose level could be
considered the NOEL in this study, based on macro- and microscopic
changes in the thyroid, spleen, and kidneys, as well as on body-weight
changes. However, since cholinesterase activity was not measured by an
appropriate and sensitive method for such a potent ChE inhibitor, an
accurate NOEL for this study could not be determined (Busey, 1968a).
In a chronic feeding/oncogenicity study, ChR-CD rats were
administered methomyl (> 99% pure) in the diet for 24 months. Groups
of 80 male and 80 female rats were fed diets containing 0, 50, 100, or
400 ppm methomyl. These concentrations were equal to 0, 2.4, 4.8, and
20 mg/kg b.w./day for males and 0, 2.3, 6.3, and 26 mg/kg b.w./day for
females, respectively. Food consumption and body weights were
measured, and haematological, clinical chemistry, and urological
evaluations were conducted routinely throughout the study. Erythrocyte
and brain acetytcholinesterase activities were measured by the Ellman
method. After 12 months, 10 rats/sex/treatment group were sacrificed
and necropsied, selected organs were weighed, and selected tissues/
organs were examined histologically. At the end of the study all rats
were sacrificed and examined in a similar manner. Tissues from rats
found dead or killed in extremis were examined histologically.
Body-weight gains of males and females in the 400 ppm group were
depressed for the first 1 to 1.5 years, but they recovered thereafter
and were comparable to control body-weight gains by 24 months.
Significantly decreased haemoglobin, RBC counts, and haematocrits were
evident in females in the 400 ppm group. Significant differences were
not observed between control and test-group animals with regard to
erythrocyte or brain cholinesterase activities. However, due to a
possible effect of storage of blood samples prior to measurement, the
potential effect of the test compound on cholinesterases could have
been undiscovered due to fast spontaneous reactivation of carbamylated
enzyme.
Relative testes weights of high-dose males were significantly
greater than those of controls, but there were no observed
pathological changes. Female rats fed > 100 ppm methomyl had
increased relative liver weights, but no associated histopathological
alterations were identified. Female rats fed 400 ppm methomyl also had
increased relative spleen weights, but no noticeable pathological
tissue responses. The incidence of bone marrow hyperplasia, focal
hyperplasia in the adrenal medulla, and focal degeneration/angiectasis
in the adrenal cortex were increased in male rats fed 400 ppm
methomyl. There were no other significant pathological changes and no
oncogenic potential was evident at any of the doses administered in
this study. A NOEL of 100 ppm was estimated based on body-weight
changes and on the haematologic response at 400 ppm (Kaplan et al.,
1981).
Dogs
Groups of young adult (> 12 months of age) beagles (4 male and 4
females/group) were administered methomyl in the diet at dose levels
of 0, 50, 100, 400, or 1000 ppm for 2 years. Methomyl was incorporated
into a ground dry dog meal. Diet mixtures were prepared weekly. Daily
observations were made of behaviour and appearance, and body-weight
and food-consumption data were recorded weekly. Clinical chemistry
studies were performed initially and at 3, 6, 12, 18, and 24 months.
Determinations were made of blood sugar, BUN, SAP, SGPT, SGOT, and
prothrombin time. Serum electrolytes, total protein, and albumin were
determined at 18 and 24 months. Haematology examinations included the
following: haematocrit and haemoglobin concentration, red blood cell
count, total and differential leukocyte count, and bone marrow
differential count at the termination of the study. Urinalysis
included: appearance, pH, specific gravity, sugar, acetone, protein,
bilirubin, occult blood, and microscopic examination of the sediment.
At 24 months, urinary sodium, potassium, chloride, glucose, protein,
and phosphorous were determined. Plasma and erythrocyte cholinesterase
activities were measured by the monometric method at the ninth week in
animals in the control and 1000 ppm groups and during the thirteenth
week in animals in the 1000 ppm group. At the end of 1 year, 1
dog/sex/group was sacrificed and the following organ weights recorded
after gross examination: brain, thyroid, heart, liver, spleen,
kidneys, adrenals, and testes. Organ-to-body weight ratios were
calculated. At 24 months the remaining dogs were sacrificed and the
following tissues were examined from each dog: brain, pituitary,
thyroid, thymus, lungs, heart, liver, spleen, kidneys, adrenals,
stomach, pancreas, duodenum, jejunum, ileum, colon, mesenteric lymph
node, urinary bladder, ovary, uterus, skin, bone, bone marrow, muscle,
sciatic nerve, and testes. The same organs were weighed as after 1
year.
Two out of 4 females in the 1000 ppm group died within 8 weeks of
the beginning of the study. Signs of poisoning included tremours,
salivation, and incoordination, which were observed only once in males
at 1000 ppm. Haematology studies in 1 high-dose male indicated severe
anaemia and moderate leukopenia (decreased haematocrit, haemoglobin,
RBC count and platelet count; increased reticulocyte count; and
changes in the differential count). This condition improved when the
dog was placed on a control diet at 84 weeks, but worsened when
1000 ppm methomyl was replaced in the diet at the ninety-fifth week.
Enlarged spleen and kidneys were noted at sacrifice (24 months). The
remaining high-dose males and 2/4 high-dose females demonstrated
similar haematologic abnormalities at 3 months, but recovered to
normal by 24 months. Growth and food consumption were unaffected by
treatment; however, all dogs were young adults at the start of the
test and therefore were already beyond the critical growth months for
this species. This is considered a deficiency in this study. Clinical
chemistry and urinalyses were comparable between treated groups and
controls. Since plasma and erythrocyte cholinesterase activities were
assayed by a method considered inadequate for carbamate inhibition,
these data are unreliable for estimating the cholinesterase inhibiting
properties of methomyl in dogs.
At 24 months, gross pathology examinations showed liver and
spleen enlargement in the male that was severely anaemic. Kidney
weights were increased in males at 1000 ppm and in 1 male at 400 ppm.
Microscopic examination showed alterations in the spleen
(extramedullary haematopoiesis) and kidneys (pigment in the cytoplasm
and swelling of the epithelial cells of the proximal convoluted
tubules in males and females), liver (bile duct proliferation) and
bone marrow (increased activity in both erythroid and myeloid series)
at 1000 ppm. At 400 ppm similar kidney abnormalities were present only
in males. Alterations were not observed in the lower-dose groups. A
no-effect level of 100 ppm (equal to 3.1 mg/kg b.w./day) was
tentatively estimated, based on the adverse haematologic effects and
on kidney and spleen morphologic changes at > 400 ppm. However, in
the absence of adequate data for adverse effects on cholinesterase
activity an accurate estimate of a NOEL cannot be determined
(Busey, 1968b).
Observations in humans
Epidemiological studies of poisonings from the ingestion of
contaminated food indicate that a single oral dose as low as
12-15 mg/kg b.w. can be fatal in humans (Liddle et al., 1979;
Araki et al., 1982).
An assessment of workers who routinely handled methomyl in a
factory indicated that workers were occasionally inadvertently exposed
and required hospitalization and treatment for symptoms of
cholinesterase inhibition, presumably due to methomyl intoxication.
Blood cholinesterase levels were normal for these workers, however,
and no quantitation of dose was presented. The methodology and timing
of ChE measurements were questionable (Morse et al., 1979).
COMMENTS
Methomyl is rapidly absorbed from the gastrointestinal tract or
through the skin, and is rapidly metabolized and eliminated. The
metabolic fate of methomyl depends on the isomeric configuration, as
the syn form (the technical product) is primarily degraded to CO2,
whereas the anti form is degraded to acetonitrile. Some conversion
of syn-methomyl to anti-methomyl apparently occurs in rats.
The acute toxicity of methomyl is due to inhibition of
acetylcholinesterase. The oral LD50 in experimental animals ranges
from 20 to 45 mg/kg b.w.; atropine is an antidote to these acute toxic
effects.
Long-term feeding studies in mice and rats do not suggest any
evidence of oncogenicity for methomyl. Toxic effects included anaemia
(mice, rats, and dogs), decreased weight gain (rats), and
histopathological changes in the kidney (dogs). The no-observed-effect
levels for chronic toxicity, calculated from dietary intake, were;
8.7 mg/kg b.w./day in mice, 4.8 mg/kg b.w./day in rats, and 3.1 mg/kg
b.w./day in dogs.
The available chronic toxicity data are somewhat limited by the
lack of adequate determinations of cholinesterase activity. However,
the effects on cholinesterases are reversible. In order to estimate
the rate of spontaneous reactivation in man, the submission of the
results of additional in vitro studies is required. These studies
should provide data on the inhibition and reactivation rate constants
for the effect of methomyl on human plasma and erythrocyte
cholinesterases.
Available epidemiological data for humans indicate that a dose of
12 - 15 mg/kg b.w. methomyl, ingested as a bolus with food, can be
lethal owing to suppression of cholinesterase activity. This value is
similar to the acute lethal doses identified in animal studies.
No evidence of teratogenicity or embryotoxicity was apparent in
rats or rabbits. The available rat reproduction studies, when
considered together, demonstrate a NOEL of 50 ppm (equivalent to
2.5 mg/kg b.w./day), based on decreases in body-weight gain and food
consumption in a study reported in 1968, and on slight decreases in
litter size at 75 ppm in a study reported in 1983. No other evidence
of effects on fertility or other reproductive parameters was noted.
No evidence of mutagenicity was noted in a number of genotoxicity
studies.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
NOELs for chronic oral toxicity are established as;
Mouse: 50 ppm, equal to 8.7 mg/kg b.w./day.
Rat: 50 ppm, equivalent to 2.5 mg/kg b.w./day.
Dog: 100 ppm, equal to 3.1 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0 - 0.01 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF A FULL ADI IS
IMPRACTICABLE, TO BE SUBMITTED TO WHO BY 1988:
Additional in vitro studies in human blood to demonstrate the
time-course of plasma and red blood cell cholinesterase inhibition and
reactivation.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
Further observations in humans.
REFERENCES
Araki, M., Yonemitsu, K., Kambe, T., Idaka, D., Tsunenari, S., Kanda,
1982 M., & Kambara, T. Forensic toxicological investigations on
fatal cases of carbamate pesticide methomyl (Lannate(R))
poisoning. Nippon Hoigaku Zasshi, 36: 584 - 588.
Barnes, J.R. Cholinesterase tests with methomyl. Unpublished report
1978 No. HLR-280-78 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Blevins, R.D. Lee, M., & Regan, J.D. Mutagenicity screening of five
1977 methyl 1977 carbamate insecticides and their nitrosos
derivatives using mutants of Salmonella typhimurium LT2.
Mutat. Res., 56: 1 - 6.
Busey, W.M. Teratology study in rabbits with "Lannate(R)" methomyl
1967a insecticide. Unpublished Report No. HLO-257-67 from Hazleton
Laboratories, Inc., Falls Church, VA., USA. Submitted to WHO
by E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Busey, W.M. Repeated dermal study in rabbits - "Lannate(R)" methomyl
1967b insecticide (90% methomyl). Unpublished Report
No. HLO-254-67 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Busey, W.M. 22-Month dietary feeding study in rats with "Lannate(R)"
1968a methomyl insecticide. Unpublished Report No. 201-164 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Busey, W.M. Two year dietary administration in dogs with "Lannate(R)"
1968b methomyl insecticide. Unpublished Report No. 201-165 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Christian, M.S., Hoberman, A.M., & Feussner, E.L. Embryo-fetal
1983 toxicity and teratogenicity study of methomyl in the rabbit.
Unpublished report No. HLO-331-83 from Argus Research
Laboratories, Inc., Horsham, PA, USA. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Dashiell, O. Acute skin absorption LD50 test in rabbits using technical
1971 methomyl (> 98% methomyl). Unpublished report No.
HLR-340-71 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
DeBuyst, B. & Van Larebeke, N. Induction of sister-chromatid exchanges
1982 in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242.
Farrow, M.G., Cortina, T., & Padilla-Nash, H. In vivo bone marrow
1984 chromosome study in rats [with] H No. 15,000 - Final Report.
Unpublished Report No. HLO-63-84 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966a methomyl (> 98% methomyl) progress report. Unpublished
report No. HLR-73-66 from Haskell Laboratory for Toxicology
and Industrial Medicine, E.I. du Pont de Nemours and Co.,
Inc. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Foster, G.V. Acute inhalation LC50 test in rats using technical
1966b methomyl (> 98% methomyl). Unpublished report
No. HLR-214-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Harvey, J., Jelinek, A.G., & Sherman, H. Metabolism of methomyl in the
1973 rat. J. Agric. Fd. Chem., 21: 769 - 775.
Hornberger, C.S. Acute inhalation toxicity of aqueous spray mist.
1967 Unpublished report No. HLR-171-67 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
1976 reactions in the metabolism of methomyl in rats.
Pest. Biochem and Physiol., 6: 571 - 583.
Kaplan, A.M., Trochimowicz, H.J., & Barba, C. Long-term feeding study
1981 in rats with S-methomyl-N-(methylcarbomoyloxy)
thioacetimidate (methomyl; INX-1179). Unpublished report
No. HLR-235-81 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Kraus, W. & Stula, E.F. Acute oral LD50 and delayed neurotoxicity
1967 (paralysis) tests in hens using technical methomyl (> 98%
methomyl). Unpublished report No. HLR-161-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Kundzin, M. & Busey, W.M. Three-generation reproduction study with
1968 Lannate(R) 1968 methomyl insecticide. Unpublished Report
No. MRO 888-1 from Hazleton Laboratories, Inc., Falls
Church, VA., USA. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Liddle, J.A., Kimbrough, R.D., Needham, L.L., Cline, R.E., Smrek,
1979 A.L., Yert, L.W., Bayse, D.D., Ellington, A.C., & Dennis,
P.A. A fatal episode of accidental methomyl poisoning.
Clin. Toxicol., 15; 159 - 167.
Lu, C.C. Nudrin(R) two-generation reproduction study in rats.
1983 Unpublished report no. WRC RIR-275 from Westhollow Research
Center, Shell Development Co., Houston, TX, USA. Submitted
to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Majhut, J. & Hood, D. Acute skin absorption, approx, lethal dose (ALD)
1966 test in rabbits using 55 and 26% formulations of (technical)
methomyl in aqueous solutions. Unpublished report
No. HLR-216-66 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M., & Hemingway, R.E.
1984 CHO/HGPRT assay for gene mutations using technical methomyl.
Unpublished report No. HLR-556-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Morrow, R.W. Acute skin absorption test (ALD) in rabbits using
1972 technical methomyl and a 25% methomyl formulation
(Lannate(R) 25W). Unpublished report No. HLR-438-72 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Morse, D.L., Baker, E.L., Kimbrough, R.D., & Wisseman, C.L.
1979 Propanilchloracne and methomyl toxicity in workers of a
pesticide manufacturing plant. Clin. Toxicol.,
15: 13 - 21.
Paynter, O. Three month dietary administration in rats using
1966 insecticide 1179 (technical methomyl, > 98% purity).
Unpublished Report No. MRO-848 from Hazleton Laboratories,
Inc., Falls Church, VA., USA. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Rogers, A.S., Culik, R., Kaplan, A.M., & Aftosmis, J.G. Oral
1978 teratogenic study in rats with Lannate(R) (INX-1179).
Unpublished report No. HLR-498-78 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Serota, D.G., Machotka, S.V., Hasting, T.F., Alsaker, R.D., &
1981 Fezio, W.L. 104-Week chronic toxicity and carcinogenicity
study in mice. Unpublished Report No. HLO-253-81 from
Hazleton Laboratories, Inc., Falls Church, VA., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Shah, P.V., Monroe, R.J., & Guthrie, F.E. Comparative rates of dermal
1981 penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59: 414 - 423.
Sherman, H. Acute oral (approx. lethal dose - ALD) and 10-dose oral
1966 subacute toxicity test in rats using technical methomyl
(> 98% methomyl). Unpublished report No. HLR-100-64 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Sherman, H. Acute oral LD50 test in rats using technical methomyl
1966 (> 98% methomyl). Unpublished report No. HLR-210-66 from
Haskell Laboratory for Toxicology and Industrial Medicine,
E.I. du Pont de Nemours and Co., Inc. Submitted to WHO by
E.I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Sherman, H., Barnes, J.R., Stula, E.F., & Zapp, J.A. Three-month
1967 feeding study on dogs with S-methyl-N-(methylcarbomoyloxy)
thioacetimidate (Lannate(R) methomyl insecticide;
INX-1179). Unpublished report No. HLR-168-67 from Haskell
Laboratory for Toxicology and Industrial Medicine, E.I. du
Pont de Nemours and Co., Inc. Submitted to WHO by E.I. du
Pont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in rabbits using
1968a technical methomyl (> 98%). Unpublished report
No. HLR-250-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Sherman, H. Acute oral toxicity and antidote tests in dogs using
1968b technical methomyl (> 98%). Unpublished report
No. HLR-279-68 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Simmon, V.F.; Mitchell, A.D., & Jorgenson, T.A. Evaluation of selected
1977 pesticides as chemical mutagens: in vitro and in vivo
studies. Unpublished EPA report No. EPA-600/1-77-028 from
U.S. Environmental Protection Agency, Health Effects
Research Laboratory, Research Triangle Park, N.C., USA.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Singles, G.H. INX-1179 (methomyl) and cholinesterase activity.
1970 Unpublished report No. HLR-186-70 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Trivits, R. Acute oral LD50 test in rats using technical methomyl.
1979 Unpublished report No. HLR-497-79 from Haskell Laboratory
for Toxicology and Industrial Medicine, E.I. du Pont de
Nemours and Co., Inc. Submitted to WHO by E.I. du Pont de
Nemours and Co., Inc., Wilmington, Delaware, USA.
Valencia, R. Mutagenesis screening of pesticides in Drosophila.
1981 Unpublished EPA report No. 600/1/81/017. Prepared by Warf
Institute, Inc. for the U.S. Environmental Protection
Agency. Submitted to WHO by E.I. du Pont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Vincent, D.R., Arce, G.T., & Sarrif, A.M. Assessment of methomyl
1985 (INX-1179-255) in the in vitro unscheduled DNA synthesis
assay in primary rat hepatocytes. Unpublished report
No. HLR-149-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, E.I. du Pont de Nemours and Co., Inc.
Submitted to WHO by E.I. du Pont de Nemours and Co., Inc.,
Wilmington, Delaware, USA.
Wojciechowski, J.P., Kaur, P., & Sabharwal, P.S. Induction of ouabain
1982 resistance in V-79 cells by four carbamate pesticides.
Env. Research, 29: 481 - 53.
