
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
K.L. Hamernik
Office of Pesticide Programs, Environmental Protection Agency,
Washington DC, USA
|
Biochemical aspects: Absorption, distribution, excretion and biotransformation |
Triazophos (O,O-diethyl O-1-phenyl-1H-1,2,4-triazol-3-yl phosphorothioate) is an organophosphorus pesticide, which was most recently evaluated toxicologically by the 1993 JMPR (Annex 1, reference 68), when an ADI of 0–0.001 mg/kg bw was established. It was re-evaluated by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
Rats
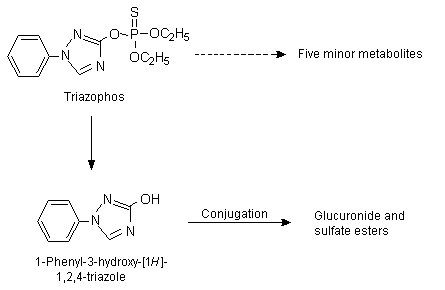
Groups of five male and five female Wistar SPF rats given a single oral dose of [14C]triazophos (labelled at the 3 position of the triazole ring) in olive oil by intubation at a dose of 2.8 mg/rat (equivalent to 15–21 mg/kg bw), excreted 76% of the administered dose in urine and 21% in faeces within 48 h. No significant difference in the rate or route of elimination was found between the sexes. Analysis of tissues from animals killed 4 days after treatment showed that 14C residues represented < 0.04% of the administered dose in kidneys, gonads, brain, muscle and skin, 0.089% in liver and 0.31% in the gastrointestinal tract. Rapid elimination of [14C]triazophos was also observed in male and female rats intubated with the radioactive compound in olive oil at a dose of 0.56 mg/rat (equivalent to 3.1–4.3 mg/kg bw per day) for 12 consecutive days, with 70–83% of the administered dose recovered in urine and 18–31% in faeces during the dosing period. Four days after the end of the 12-day treatment, the concentration of 14C residues in most of the tissues analysed (subcutaneous fat, kidney, gonads, liver, brain, muscle and skin) did not exceed 0.0008% of the administered dose. The exception was the gastrointestinal tract, which still contained 0.5% of the dose. It is not clear whether urine and faeces from the study with single and multiple doses were analysed for metabolites separately and whether the metabolic profiles were qualitatively or quantitatively similar. The main urinary metabolite was urea-14C, representing 85% of the radioactivity excreted by this route. Other metabolites detected in the urine were [3-14C]1-phenyl-1,2,4-triazol-3-ol, [3-14C]1-phenylsemicarbazide and [14C]semicarbazide, all conjugated with glucuronic acid. Each of these three metabolites accounted for 3–5% of the 14C eliminated in urine. Low concentrations of two unidentified metabolites were also found. In the faeces, unchanged [14C]triazophos (40% of the total activity in faeces) and [3-14C]1-phenyl-1,2,4-triazol-3-ol (60% of the activity) were identified (Bock & Thier, 1976). No statement of quality assurance (QA) or good laboratory practice (GLP) was provided, and the protocol had deficiencies.
The metabolic fate of triazophos was studied in 23 female Wistar (WISKf (SPF 71)) rats given triazophos labelled at the 3 position (radiochemical purity, 98%) as a single oral dose of about 5 mg/kg bw in sesame oil by gastric intubation. Twenty animals were used to investigate excretion and metabolism and the three others for blood assays. Pooled urine and faecal samples were collected after 24, 48, and 96 h, and blood samples were taken 0, 0.5, 2, 4, 6, 8, 24 and 48 h after administration. The maximal concentrations in blood were attained after about 4 h. The mean half-life of radioactivity in the blood was 3.8 h. The recovery rate of 98% after 96 h indicates that excretion was relatively complete. The predominant route of excretion was urinary, with > 90% of the administered radioactivity excreted within 48 h. Faecal elimination accounted for 4.5% of the administered dose after 48 h. The residual concentrations of radioactivity in the tissues analysed (liver, kidney, lung, heart, brain, spinal cord and retroperitoneal and subcutaneous fat) were highest in kidney and liver but were still relatively low: <0.004 ppm. Pooled urine contained three identifiable metabolites, as shown in Figure 1: 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (43% of the administered dose) and its glucuronide (36%) and sulfate conjugates (13%). The glucuronide was unstable and was apparently converted to the parent compound at room temperature. Unchanged triazophos was not detected in urine. The authors reported that the quantities of radioactivity in the faeces were too low for characterization of the chemical species (Schwalbe-Fehl & Schmidt, 1986). GLP and QA statements were provided, and the protocol was consistent with guidelines.

Figure 1. Metabolic pathway of 14C-triazophos
From Schwalbe-Fehl & Schmidt (1986)
Dogs
The metabolic fate of triazophos was examined in beagle dogs, with the same treatment and sampling regimen as for rats. Two female dogs were treated by gastric intubation with [14C]triazophos at a dose of 4.4–4.8 mg/kg bw in sesame oil. Urinary excretion predominated, representing an average of 85% of the administered dose after 24 h and 92% after 48 h. Faecal elimination accounted for 0.3% of the administered dose after 24 h and and 7.2% after 48 h. Maximal blood concentrations were attained after 2 h. After 48 h, no radioactivity was detectable in blood, and the mean half-life was 3.6 h. Residual concentrations in tissues were not determined because the animals were not killed at termination. Qualitatively, the metabolic fate of triazophos in dogs was similar to that in rats (see Figure 1). The urine contained the same three metabolites as in rats, namely 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (18% of the administered dose) and its glucuronide (60%) and sulfate (5%) conjugates. One metabolite was found only in dog urine (representing 11% of the administered dose), which was thought to be another sulfate ester conjugate of the 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole metabolite. Quantitatively, less of this metabolite was present in dog urine (18% of the administered dose) at the time of analysis than in rats (43%). The instability of the glucuronide conjugate and its potential conversion to the free metabolite were also observed in dogs, and the somewhat higher concentration of the glucuronide metabolite in the urine of dogs (60% of the administered dose) than of rats (36%) was considered not to be significant. Unchanged triazophos was not detected in urine. The faeces contained low concentrations of triazophos and the free 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole metabolite as well as five unidentified metabolites at about 0.7, 0.3 and 7.3% of the administered dose, respectively (Schwalbe-Fehl & Schmidt, 1986). GLP and QA statements were provided, and the protocol was consistent with guidelines.
The acute toxicity of triazophos is summarized in Table 1.
Table 1. Acute toxicity of triazophos
|
Species |
Strain |
Sex |
Route |
Vehicle |
LD50/LC50 |
Purity (%) |
Reference |
|
Mouse |
NMRI |
Male |
Oral |
Sesame oil |
31 |
93a |
Hollander & Weigand (1977a) |
|
Mouse |
NMRI |
Female |
Oral |
Sesame oil |
29 |
93a |
Hollander & Weigand (1977b) |
|
Mouse |
NMRI |
Male |
Oral |
Starch mucilage |
76 |
92.7a |
Hollander & Weigand (1978a) |
|
Mouse |
NMRI |
Female |
Oral |
Starch mucilage |
41 |
92.7a |
Hollander & Weigand (1978b) |
|
Mouse |
NMRI |
Male |
Intraperitoneal |
Starch mucilage |
46 |
90a |
Scholz & Weigand (1972a) |
|
Female |
37 |
||||||
|
Mouse |
NMRI |
Male |
Subcutaneous |
Sesame oil |
90 |
92.7a |
Hollander & Weigand (1977c) |
|
Mouse |
NMRI |
Female |
Subcutaneous |
Sesame oil |
68 |
92.7a |
Hollander & Weigand (1977d) |
|
Rat |
Wistar |
Female |
Oral |
Starch mucilage |
82 |
95a |
Scholz & Weigand (1967) |
|
Rat |
Wistar |
Male |
Oral |
Starch mucilage |
68 |
93a |
Hollander & Weigand (1977e) |
|
Rat |
Wistar |
Female |
Oral |
Sesame oil |
48 |
93a |
Hollander & Weigand (1977f) |
|
Rat |
Wistar |
Female |
Oral |
Starch mucilage |
64 |
93a |
Hollander & Weigand (1977g) |
|
Rat |
Wistar |
Female |
Oral |
Starch mucilage |
66 |
93 |
Scholz & Weigand (1969) |
|
Rat |
Wistar |
Female |
Oral |
Sesame oil |
57 |
93a |
Hollander & Weigand (1976a) |
|
Rat |
Wistar |
Male |
Oral |
Sesame oil |
59 |
93a |
Hollander & Weigand (1977h) |
|
Rat |
Wistar |
Male |
Intraperitoneal |
Sesame oil |
57 |
93a |
Hollander & Weigand (1977i) |
|
Rat |
Wistar |
Female |
Intraperitoneal |
Sesame oil |
61 |
93a |
Hollander & Weigand (1977j) |
|
Rat |
Wistar |
Female |
Intraperitoneal |
Sesame oil |
107 |
Not reported |
Scholz & Weigand (1968) |
|
Rat |
Wistar |
Male |
Dermal |
Undiluted |
> 2000 |
92.6b |
Diehl & Leist (1986a) |
|
Female |
1000 |
||||||
|
Rat |
Wistar |
Female |
Dermal |
Sesame oil |
1100 |
90a |
Scholz & Weigand (1972b) |
|
Rat |
Wistar |
Male |
Subcutaneous |
Sesame oil |
280 |
92.1a |
Hollander & Weigand (1977k) |
|
Rat |
Wistar |
Female |
Subcutaneous |
Sesame oil |
150 |
93a |
Hollander & Weigand (1977l) |
|
Rat |
Wistar |
Female |
Inhalation |
Undiluted aerosol |
0.56 |
931,2 |
Hollander & Weigand (1977m) |
|
Rat |
Wistar |
Male |
Inhalation |
Undiluted aerosol |
0.61 |
92.6c |
Hollander & Weigand (1987) |
|
Female |
0.45 |
||||||
|
Guinea-pig |
Pirbright white |
Male |
Oral |
Sesame oil |
26 |
88a |
Scholz & Weigand (1973a) |
|
Guinea-pig |
Pirbright white |
Female |
Oral |
Sesame oil |
35 |
88a |
Scholz & Weigand (1973b) |
|
Dog |
Beagle |
Male |
Oral |
Sesame oil |
> 800 |
93 |
Scholz & Brunk (1969) |
|
Female |
~ 500 |
|
Most reports did not contain statements of GLP or QA, but the performance was generally consistent with Subdivision F Guidelines of the Environmental Protection Agency (USA; 1982 or 1984, revised). |
|
|
a |
Information on purity obtained from sponsor |
|
b |
GLP and QA statements provided; protocol generally consistent with Subdivision F Guidelines (1982 or 1984, revised) |
|
c |
GLP and QA statements provided and protocol generally consistent with Subdivision F Guidelines (1982 or 1984, revised), but median mass aerodynamic diameter not provided and particle size distribution could not be confirmed; particle size described as being in the range 0.49 to > 15 fm |
In mice, rats, guinea-pigs, and dogs given a single oral, intraperitoneal or subcutaneous dose of triazophos, no clear difference in sensitivity was seen by sex. In rats treated by dermal or inhalation exposure, females were more sensitive than males. When the test material was administered orally, intraperitoneally or subcutaneously, mice, rats and guinea-pigs generally died within minutes or hours to several days after dosing. The clinical signs of toxicity in these species included tremor, abdominal position, muscle tremors, tonic convulsions, accelerated, laboured or jerky respiration, lachrymation, salivation, saltatory spasm, disequilibrium and hind-leg paralysis. In the few dogs tested, deaths occurred 30–54 h after oral dosing in females only, and clinical signs were seen within 1 h to 4 days or more after treatment. The clinical signs of toxicity in dogs included refusal of food, vomiting, retching, diarrhoea, hypersalivation, tremor, staggering gait, laboured respiration, miosis and disequilibrium. Deaths occurred 1–5 days after dermal treatment and within several hours to about 4 days after exposure by inhalation, with clinical signs of toxicity similar to those in rodents.
(a) Primary dermal and ocular irritation
In a patch test for skin irritation, 0.5 ml of technical-grade triazophos (purity, 92%) was applied undiluted or as a 1% or 10% dilution in sesame oil to the shaved flanks of groups of six SPF albino Himalayan rabbits (sex not specified), weighing 1.5–2 kg. Adjacent areas of intact and abraded skin were treated on each animal for 24 h. The diluent was not assessed. The exposed areas were evaluated 24, 48 and 72 h after the start of the test. Five of six animals died with 24–48 h after application of undiluted test material, with no obvious signs of toxicity; therefore, dermal irritation could not be fully evaluated. Negligible dermal irritation (slight oedema, sometimes with slight erythema) was observed in the other two groups 24 and 48 h after application, with Draize (1977) irritation scores of 0.2 and 0.44 for intact and abraded skin, respectively, in the group treated with the 10% dilution and 0.33 and 0.44 for intact and abraded skin, respectively, in the group treated with the 1% dilution. All signs of irritation had cleared by 72 h (Hollander & Weigand, 1977n). QA and GLP statements were not provided, but the protocol was consistent with Subdivision F Guidelines (1984 revision) of the Environmental Protection Agency (USA).
In a test for ocular irritation, 0.1 ml of technical-grade triazophos (purity, 92%) was applied undiluted or as a 1% or 10% dilution in sesame oil to the conjunctival sac of one eye of groups of six SPF albino Himalayan rabbits (sex not specified). The animals were evaluated for ocular reactions after 1, 7 and 24 h, after which all eyes were flushed with sodium chloride solution. Two additional examinations were made 48 and 72 h after application with fluorescein. The diluent alone was not assessed. One animal died 48 h after application of undiluted test material, with no obvious signs of toxicity. The undiluted material caused corneal redness and minor irritation of the iris, which had cleared by 24 h after instillation. Minor conjunctival redness, chemosis and discharge and slight corneal opacity were observed in the group treated with the 10% dilution; animals treated with the 1% dilution showed only the conjunctival changes, of lesser severity. The effects in these two groups had cleared by 48 h. Overall, undiluted or diluted test material was minimally irritating after ocular instillation (Hollander & Weigand, 1977n). QA and GLP statements were not provided, but the protocol was consistent with Subdivision F Guidelines (1984 revision) of the Environmental Protection Agency.
(b) Dermal sensitization
In a study of dermal sensitization with a Buehler-type closed-patch method, technical-grade triazophos (purity not specified) was applied as a 10% dilution in sesame oil BP73 for 6 h, nine times over a 3-week period to 10 male Pirbright guinea-pigs at a dose of 0.5 ml per animal to an area of close-clipped flank measuring about 30 cm2. A 10% dilution had previously been determined to cause minimal irritation. After the last application and a 14-day treatment-free period, the animals were challenged with 0.5 ml of a 5% dilution of triazophos in sesame oil, which had previously been shown not to be irritating, and the skin was examined for reactions 24 and 48 h after exposure. Although the 10% dilution caused slight reddening of the exposed area in all animals during treatment, the 5% dilution caused no reaction on challenge. Under the conditions of this study, the test material was not a dermal sensitizer (Hollander & Weigand, 1976b). QA and GLP statements were not provided; the protocol was partly consistent with Subdivision F guidelines of the Environmental Protection Agency.
Technical-grade triazophos (purity, 97.4%) was evaluated for its potential to cause dermal sensitization in female Pirbright white guinea-pigs in a Magnusson and Kligman maximization-type test. In a preliminary study to determine the tolerance of the animals to intradermal injections, dilutions of 10%, 5%, 2%, 0.4% and 0.08% triazophos in polyethylene glycol caused moderate-to-pronounced erythema and slight oedema. One animal each at 10% and 5% had to be killed owing to clinical signs of toxicity (decreased spontaneous activity, trembling, contracted flanks, squatting position, irregular respiration, increased salivation and clonic spasms); therefore, a concentration of 3% was selected for intradermal injection in the main study.
In the main study, 20 animals received two 0.1-ml intradermal injections of 50% Freund adjuvant, a 3% solution of test material in polyethylene glycol and a 3% solution of test material in 50% Freund adjuvant for induction. Ten control and five other animals received two 0.1-ml intradermal injections of 50% Freund adjuvant at two sites and polyethylene glycol at another site. Dermal examination during the week after injection showed maximal reactions of moderate erythema and slight oedema at the injection site on animals exposed to Freund adjuvent with or without test material. White–brown skin incrustation and areas of hardened or scab-covered skin were also observed. A maximal response of moderate-to-severe erythema was seen with polyethylene glycol alone or with the test material. On day 9, the 48-h dermal induction phase of the study was begun. The intradermal injection sites were first covered with a 2 × 4 cellulose patch to which 0.5 g of polyethylene glycol or a 10% dilution of the test material in polyethylene glycol had been applied, as appropriate, and then the entire application area was covered with an occlusive, impermeable bandage. The 10% dilution of triazophos was selected on the basis of the results of a preliminary study to determine a non-irritating, non-toxic concentration after a 24-h dermal application. In that study, one death with no accompanying clinical signs was reported at a concentration of 100% triazophos; the concentrations of 50% and 10% in polyethylene glycol caused no toxic or irritating response, but the 50% concentration was not used because of the possibility of irritation in the presence of Freund adjuvant. After the dermal induction phase of the main study, the subsequent 24-h dermal challenge phases on days 15–18 for the five additional animals and on days 22–25 for the control and triazophos-treated groups with 10% triazophos in polyethylene glycol caused no signs of toxicity, irritation or sensitization 24 or 48 h after the end of exposure. Under the conditions of this study, the test material was not a dermal sensitizer (Schollmeier & Leist, 1989). QA and GLP statements were provided, and the protocol was consistent with Subdivision F guidelines of the Environmental Protection Agency.
(a) Oral administration
Mice
In a preliminary study, groups of 10 male and 10 female NMRKf (SPF71) mice received diets containing technical-grade triazophos (purity, 93.1%) in sesame oil at a concentration of 0, 20, 80, 160 or 320 ppm, equal to 0, 3.1, 12, 25 and 51 mg/kg bw per day for males and 0, 3.3, 13, 28 and 57 mg/kg bw per day for females, for 13 weeks. The animals were observed for general health and deaths, food and water consumption and body-weight changes; haematological, clinical chemical and urine end-points, organ weights and macroscopic and microscopic appearance were evaluated. Although the scope of the microscopic examination was described as limited, most of the recommended tissues were evaluated. Serum, erythrocyte and brain cholinesterase activities were assayed after 13 weeks of treatment. Cholinesterase inhibition was calculated relative to the value for the appropriate concurrent control group.
There were no deaths or clinical signs of toxicity during the study. No treatment-related effects were found on general health, food or water consumption, body weight, haematological or urinary end-points or gross or microscopic appearance. Organ weight changes considered to be treatment-related were increases of 10–15% in absolute liver weights (statistically significant in females) and relative liver weights (statistically significant in both sexes) at the highest dietary concentration. Owing to variability in response among groups, the observed changes (mostly statistically significant) in clinical chemical parameters (slight increases in cholesterol, triglyceride and total lipid concentrations and gamma-glutamyl transferase activity) could not be related definitively to consumption of the test material. Serum cholinesterase activity was markedly and statistically significantly decreased, by 84–99%, in all treated groups. Statistically significant inhibition of brain cholinesterase activity, by 19% and 44% in females and 15% and 39% (latter statistically significant) in males were found at 160 and 320 ppm, respectively. These changes were considered to be treatment-related, as was statistically significant inhibition of erythrocyte cholinesterase activity in males, by 41% and 45% at 160 and 320 ppm, respectively, and in females, by 44%, 44% and 50% at 80, 160 and 320 ppm, respectively. A shallow dose–response relationship was seen for the groups at the two higher concentrations. Inhibition of erythrocyte cholinesterase activity by 18% in females at 3.3 mg/kg bw per day, although statistically significant, was considered not to be biologically significant. In a 2-year feeding study in NMRI mice, no statistically significant inhibition of erythrocyte cholinesterase activity was observed in either sex at lower doses of 0.83–0.95 mg/kg bw per day (Donaubauer, 1989). The NOAEL was 20 ppm (equal to 3.1 mg/kg bw per day) on the basis of statistically significant inhibition of erythrocyte cholinesterase activity in both sexes and inhibition of brain cholinesterase activity in females at the next highest dose (Ebert et al., 1987). QA and GLP statements were provided, and the protocol was generally consistent with Subdivision F guidelines (1982 and 1984, revised) of the Environmental Protection Agency.
Groups of 10 male and 10 female Wistar rats received diets containing technical-grade triazophos (purity, 90%) at a concentration of 0, 1, 3, 10 or 200 ppm (raised to 400 ppm in week 6), equivalent to 0, 0.05, 0.15, 0.5 and 10/20 mg/kg bw per day, for 13 weeks. The concentrations were selected on the basis of the results of a range -finding study in which body weight, food intake and whole-brain cholinesterase activity were decreased and relative kidney weights were increased at dietary concentrations of 400 and 600 ppm. The animals were observed for deaths and clinical signs of toxicity, body weight, food intake and food use efficiency; limited haematological (haemoglobin content, packed cell volume and erythrocyte and leukocyte counts) and clinical chemical (alanine and aspartate aminotransferase and alkaline phosphatase activity) tests were performed, and urine evaluations, organ weights and macroscopic and microscopic evaluation of selected tissues (lung, trachea, salivary glands, prostate, epididymides, uterus, urinary bladder, skeletal muscle, thoracic aorta, oesophagus, six levels of the gastrointestinal tract, pancreas and axillary and mesenteric lymph nodes) were assessed for control and animals at the highest dietary concentration only. Plasma and erythrocyte cholinesterase activities were assessed at weeks 3, 6 and 12 for all animals, and whole-brain cholinesterase activity was measured in pooled samples from four animals of each sex per group at the end of the study. Cholinesterase inhibition was determined relative to that of the appropriate concurrent control group.
There were no deaths or signs of toxicity during the study. Body weights were generally similar among groups, although some slight, statistically significant elevations occurred in females at 200/400 ppm up to week 6 and in females at 10 ppm from week 10 on. Slight increases in food consumption were also noted in these two groups. The food use efficiency of males at 200/400 ppm was somewhat lower than that of other groups of males. No toxicologically relevant findings were noted in the evaluations of urine, clinical chemistry or organ weights. The haematological assessment showed no remarkable changes. A substantial, statistically significant decrease in eosinophil count in females at 200/400 ppm was not seen in males and was considered not to be related to treatment. The data on cholinesterase activity were not evaluated statistically. Plasma and erythrocyte cholinesterase activities were fairly consistent at weeks 3, 6 and 12. At week 12, plasma cholinesterase activity was inhibited by 23% and > 77% for males at 10 ppm and 200/400 ppm, respectively, and by 27%, 62% and > 96% for females at 3, 10 and 200/400 ppm, respectively. This effect was considered to be biologically relevant, as was inhibition of erythrocyte cholinesterase activity by 69% in males at 200/400 ppm and by 15% and 69% in females at 10 and 200/400 ppm, respectively. Also considered to be biologically relevant was the strong inhibition of brain cholinesterase activity by 44% and 87% in males and females at 200/400 ppm, respectively. No treatment-related gross or microscopic findings were found; however, all the animals examined had histopathological evidence of chronic respiratory disease, and a number of animals in the control group and at the highest dietary concentration had urinary bladder parasitic infestation. The NOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity at the next highest dose. There was no QA or GLP statement, but the protocol was partly consistent with Subdivision F guidelines (1982 and 1984, revised) of the Environmental Protection Agency (Til et al., 1971).
Groups of 10 male and 10 female SPF Wistar (KFM-Han) outbred rats were given diets containing technical-grade triazophos (purity, 92.6%) at a concentration of 0, 1, 20 or 400 ppm, equal to 0, 0.07, 1.5 and 31 mg/kg bw per day for males and 0, 0.08, 1.6 and 36 mg/kg bw per day for females, for 13 weeks. An additional 10 animals of each sex given 0, 20 and 400 ppm were allowed to recover for 4 weeks. The animals were observed for viability, deaths, clinical signs of toxicity, food consumption and body weight; an ophthalmological examination, haematological, clinical chemical and urine end-points, organ weights and gross and microscopic appearance were assessed. Plasma and erythrocyte cholinesterase activities were measured after 13 weeks of treatment and after the recovery period. Brain cholinesterase activity was assayed in what were described as one-half brain sections at the end of both the main study and the recovery phase. Inhibition of cholinesterase activity was determined relative to the value for the appropriate concurrent control group.
During treatment, no deaths and no clinical signs of toxicity were observed. Food consumption was occasionally higher in males and often higher in females at the highest dietary concentration than in groups at lower concentrations. Otherwise, the food consumption was similar among groups. No differences in body weight were observed among groups. The ophthalmoscopic examination was reported to show unremarkable results. Some generally slight but statistically significant haematological and clinical chemical changes were noted in animals at the highest concentration at 13 weeks, which were considered by the authors to be treatment-related because they were not observed at the end of the recovery period. However, these changes were not associated with pathological findings and were generally within the range of values considered normal for untreated Wistar/Han rats aged 13–18 weeks. The haematological changes included decreased erythrocyte count (females), haemoglobin (both sexes), mean corpuscular haemoglobin concentration (both sexes), mean corpuscular volume (females), mean corpuscular haemoglobin (females) and prothrombin time (females) and increased leukocyte (both sexes) and platelet (males) counts. The clinical chemical changes included decreased urea and possibly glucose concentrations (both sexes), increased P, K and total protein concentrations (one or both sexes) and increased total cholesterol and high-density lipoprotein phospholipid levels (females). At week 13, plasma cholinesterase activity was statistically significantly inhibited in females at 20 and 400 ppm by 66% and 85%, respectively, and erythrocyte cholinesterase activity was statistically significantly inhibited by 41% and 45% in the same groups (poor dose–response relationship); brain cholinesterase activity was statistically significantly inhibited by 35% in females at 400 ppm only. In males at week 13, statistically significant inhibition of 24% and 30% (poor dose–response relationship) was seen in plasma cholinesterase activity at 20 and 400 ppm, while no significant inhibition was found in erythrocytes. Brain cholinesterase activity was statistically significantly inhibited by 12% in males at 400 ppm. These findings were all considered by the authors to be treatment-related. The results of urine analysis were unremarkable. There were no obvious treatment-related changes in organ weights or in their macro- or microscopic appearance. All treatment-related changes in the main study were considered to have reversed by the end of the recovery period. The NOAEL was 1 ppm, equal to 0.08 mg/kg bw per day, on the basis of statistically significant inhibition of erythrocyte cholinesterase of 41% at the next highest dose. QA and GLP statements were provided, and the protocol was consistent with Subdivison F guidelines (1982 revision) of the Environmental Protection Agency (Tennekes et al., 1987).
In a study to examine the recovery of cholinesterase activity in blood and brain of rodents, groups of 10 male and 10 female Wistar (CIVO colony) rats received diets containing triazophos (purity, 88%) at a concentration of 0, 3, 10 or 200 ppm, equivalent to 0, 0.15, 0.5 and 10 mg/kg bw per day (calculated with a conversion factor of 0.05), for 48 weeks. The dosing period was followed by a treatment-free recovery period of 7 weeks. The animals were monitored for general condition, deaths and behavioural and body-weight changes. Plasma and erythrocyte cholinesterase activities were assessed in all animals at weeks 33 and 43 and during the recovery period on days 3, 7, 14, 28 and 49. The study was terminated at the end of the recovery period, and brain cholinesterase activity was measured in pooled homogenates of the right hemispheres of five animals of each sex per group. Cholinesterase inhibition was calculated relative to the value for the appropriate concurrent control group.
One male at 200 ppm died during week 16 of the study. As the cause of death was not discussed, it may have been related to treatment, as substantial cholinesterase inhibition was observed at this dose. No clinical signs of toxicity or behavioural changes were noted The body weights of males at the two higher dietary concentrations were often increased relative to those of the control group, and the increases were sometimes statistically significant from week 44 on into the recovery period. The body weights of females at 10 ppm were also often increased when compared with the control group. At treatment week 43, statistically significant inhibition of plasma cholinesterase activity was observed at 10 and 200 ppm in males (22% and 80%, respectively) and females (61% and 93%, respectively). Similar inhibition was noted at week 33. Plasma cholinesterase activity returned to levels that were not statistically significantly different from those of the appropriate control group by day 3 after treatment in males and day 7 after treatment in females. At treatment week 43, erythrocyte cholinesterase activity was statistically significantly inhibited by 15% and 73% in males and 20% and 73% in females at 10 and 200 ppm, respectively. Similar levels of inhibition were observed at treatment week 33, except that none was found in males at 10 ppm. Erythrocyte cholinesterase activity returned to levels that were not statistically significantly different from those of the appropriate control group by day 14 after treatment for females at 10 ppm, by day 28 after treatment for males at 10 ppm and by day 49 after treatment for animals at 200 ppm. Brain cholinesterase activity was not inhibited at any dose, but it was measured only after the full 49-day recovery period. A mammary tumour in one female at 10 ppm was not attributed to treatment. The NOAEL was 3 ppm, equivalent to 0.15 mg/kg bw per day, on the basis of statistically significant inhibition of erythrocyte cholinesterase activity in both sexes during treatment and part of the recovery phase of the study at the next higher dose. No QA or GLP statements were provided. This study was not conducted according to a particular guideline, but the protocol was reasonable for the intent of the study (Til & Leegwater, 1974).
Dogs
Technical-grade triazophos (purity, 92.6%) was included in the diet of four male and four female beagles at a concentration of 0.3 ppm and in that of groups of six males and six females at a concentration of 0, 9 or 270/180 ppm, for 13 weeks. The highest concentration was reduced from 270 ppm to 180 ppm from day 33 until termination. After 13 weeks of treatment, two animals of each sex at 0 and 9 ppm were continued without treatment for a 4-week recovery period. The concentrations were equal to intakes of 0, 0.01, 0.28 and 6 mg/kg bw per day for males and 0, 0.01, 0.3 and 6.5 mg/kg bw per day for females. The animals were observed for deaths, general health and clinical signs and underwent basic auditory tests, ophthalmic and neurological examinations and assessments of food and water consumption. Body weight, haematological, clinical chemical and urine end-points, organ weights and macroscopic and microscopic appearance were assessed. Plasma and erythrocyte cholinesterase activities were measured before treatment, at weeks 4 and 13 of the main study and at the end of the recovery period. Brain cholinesterase activity in a section of cerebellum was assayed at the end of both the main study and the recovery phase. Inhibition of cholinesterase activity was calculated relative to the value for the appropriate concurrent control group.
All animals of each sex at 270/180 ppm showed clinical signs throughout the main study. Diarrhoea and vomiting began in the first 2 weeks of treatment, and salivation was a frequent finding from week 5 onwards, as was decreased motility. Tremor was noted in one male. Occasional instances of vomiting, diarrhoea and salivation in the other groups did not appear to be treatment-related. Two males and one female found when moribund were killed on days 42, 81 and 55, respectively, due to conditions considered to be related to treatment, as all of them manifested clinical signs of toxicity, decreased food consumption and body-weight loss. Decreased food consumption and body weights were noted only in animals at 270/180 ppm. The results of basic hearing tests and ophthalmic and neurological examinations were reported to be normal. Males and females at 270/180 ppm showed slight decreases in haemoglobin concentration, and males showed a decreased erythrocyte count and erythrocyte volume fraction relative to control values at week 13. Alanine aminotransferase and alkaline phosphatase activities were notably elevated in animals of each sex at 270/180 ppm at weeks 4 and 13, as were gamma-glutamyl transferase and ornithine carbamyl transferase activities at weeks 4 and 13 for males and week 4 for females at this dietary concentration. Other clinical chemical changes seen during the main study in one or both sexes at 270/180 ppm in weeks 4 and/or 13 included slight-to-moderate decrease in high-density lipoprotein cholesterol and phospholipids, slight decreases in Ca, Na, K, total protein and glucose concentrations and slight changes in the protein electrophoretic pattern (albumin and globulin fractions).
Plasma cholinesterase activity was statistically significantly inhibited, by 71–91%, in males and females at 9 and 270/180 ppm in both week 4 and week 13. While inhibition by 23% in females at 0.3 ppm in week 4 was statistically significant, inhibition by 19% in these females in week 13 was not, nor was inhibition by 16–17% in males at this dietary concentration at either time. Erythrocyte cholinesterase activity was statistically significantly inhibited at weeks 4 and 13 in males and females at 270/180 ppm (by 92–95%) and 9 ppm (29–40%). Inhibition by 7–17% in animals at 0.3 ppm was not statistically significant. Brain cholinesterase activity, measured at termination, was statistically significantly inhibited by 10% in males at 270/180 ppm. Inhibition by 9% in females at 270/180 ppm was not statistically significant. No inhibition was observed in other groups.
The results of urine analysis were unremarkable. Although the liver:body weight ratio was slightly but statistically significantly increased in females at 270/180 ppm, no statistically significant changes were observed in absolute liver weight or in liver weight relative to brain weight nor in liver weight parameters in males. The macroscopic findings attributed to treatment were duodenal wall thickening in four males and one female at 270/180 ppm, jejunal wall thickening in one male and effects ascribed to poor general condition of animals at 270/180 ppm, such as ascites and small spleen or thymus. Histopathological examination showed the following effects definitively attributed to treatment: hypertrophy of the duodenal wall in all animals at 270/180 ppm and degenerative or inflammatory lesions in the zygomatic gland of these males. Testicular maturation arrest, reduced thyroidal secretion, thyroid atrophy, bone-marrow atrophy and adrenal cortical vacuolation found in animals at 270/180 ppm were considered to be related to the poor general health of this group. These findings were generally not reflected in changes in organ weights relative to the control group. Papillary muscle mineralization of the left ventricle of the heart was considered by the pathologist as possibly related to treatment, as it is not a common spontaneous finding. While it was observed in two males at 270/180 ppm, however, there was no dose–response relationship in females. At the end of the recovery period, the only findings attributed to treatment were decreased erythrocyte cholinesterase activity by 25% and 52% in males and females at 9 ppm, respectively (two animals of each sex per group and no measurements made at other dietary concentrations). The authors concluded that the NOAEL was < 0.3 ppm (equal to 0.01 mg/kg bw per day) on the basis of inhibition of blood cholinesterase activity by 7–23%. Plasma cholinesterase activity was statistically significantly decreased only in females at 0.3 ppm and only at 4 weeks and not at 13 weeks. At 4 weeks, the inhibition was relatively small (23%). Erythrocyte cholinesterase activity was not statistically significantly inhibited in animals of either sex at 0.3 ppm. If inhibition of plasma cholinesterase activity is discounted, the NOAEL was 0.3 ppm, equal to 0.01 mg/kg bw per day, on the basis of statistically significant inhibition of erythrocyte cholinesterase activity at the end of treatment at the next highest dose. Statements of compliance with QA and GLP were provided, and the protocol was consistent with Subdivision F guidelines (November 1982) of the Environmental Protection Agency (Block et al., 1988).
Groups of male and female pure-bred beagles received diets containing technical-grade triazophos (purity, 92.6%) at a concentration of 0, 0.2, 0.4, 4 or 80 ppm, equal to intakes of 0, 0.007, 0.012, 0.13 and 2.4 mg/kg bw per day for males and 0, 0.006, 0.012, 0.14 and 2.7 mg/kg bw per day for females, for 52 weeks. Six animals of each sex received 0 and 80 ppm, and there were four animals of each sex in the other three groups. The animals were observed for deaths and clinical signs, food and water consumption and body weight; basic veterinary auditory and ophthalmic examinations and haematological, clinical chemical and urine tests were conducted; organs were weighed and were examined macroscopically and microscopically. Plasma and erythrocyte cholinesterase activities were measured before treatment and in weeks 14, 26 and 52; brain cholinesterase activity (in a section of cerebellum) was assayed at the end of the study. Inhibition of cholinesterase activity was calculated relative to the value for the appropriate concurrent control group.
One female at 80 ppm was killed on day 76, and the treatment of a second female in this group was stopped on day 106 because of persistent diarrhoea and poor condition accompanied by marked inhibition of plasma and erythrocyte cholinesterase activity. These effects were considered likely to be treatment-related. Frequent or persistent diarrhoea occurred in many animals at 80 ppm, and these animals had a slightly higher incidence of vomiting than other groups. Although diarrhoea was found in all groups, it generally was not frequent or persistent at dietary concentrations < 4 ppm. Diarrhoea was relatively frequent in one female at 4 ppm (about 53 occurrences) and was persistent in one male at this dietary concentration (e.g. about 138 occurrences immediately after week 1, associated with dehydration, emaciation and decreased body weight). The possibility that the findings in the male were related to treatment could not be dismissed. As moderate-to-severe bronchopneumonia affected some animals at 0.2 and 0.4 ppm, the higher frequency of diarrhoea (about 30 occurrences) in one male at 0.4 ppm with severe bronchopneumonia could not readily be attributed to treatment in the absence of other related findings. One male at 0.2 ppm had a general non-treatment-related wasting condition not associated with diarrhoea or vomiting. Decreased body weight and/or food consumption were seen in some animals at 80 ppm. The food consumption of one female at 4 ppm was slightly decreased towards the end of the study. The results of auditory, ophthalmic and urine analyses were unremarkable. Minor alterations in some clinical chemical parameters at 80 ppm could not clearly be related to treatment at lower doses. There were no statistically significant changes in organ weights and no significant macroscopic or histopathological findings in the dog that died during the study or in other animals. Similar patterns and levels of inhibition of plasma and erythrocyte cholinesterase activity were noted in weeks 14, 26 and 52. At 52 weeks, statistically significant inhibition of plasma cholinesterase activity, by 22%, 74% and 89% was seen in males at 0.4, 4 and 80 ppm, respectively, and statistically significant inhibition, by 68% and 85%, was seen in females at 4 and 80 ppm, respectively. Erythrocyte cholinesterase activity was statistically significantly inhibited by 87–92% in males and females at 80 ppm and was marginally inhibited in males at 4 ppm, with statistically significant decreases of 32% at 26 weeks and 24% and 29% at weeks 52 and 14, respectively. Brain cholinesterase activity was not inhibited at any dose at 52 weeks. The NOAEL was 0.4 ppm, equal to 0.012 mg/kg bw per day, on the basis of findings of persistent diarrhoea associated with dehydration, emaciation and decreased body weight in one male, for which a relationship to treatment could not be dismissed, and group mean inhibition of erythrocyte cholinesterase activity of 24–32% at the next highest dose. GLP and QA statements were provided, and the protocol was consistent with Subdivision F guidelines (1984 revision) of the Environmental Protection Agency (Allen et al., 1989).
Rhesus monkeys
Groups of one male and one female rhesus monkeys received triazophos (purity not stated) suspended in 2% starch slurry by gavage at a constant volume of 5 ml/kg bw for 22 consecutive days at a dose of 0.025 or 0.05 mg/kg bw per day. The animals were monitored for general condition, behaviour and food and water consumption. Body weights were measured at the beginning of the study, on the day after the last treatment and 9 or 11 days after treatment. Blood samples for measurement of serum and erythrocyte cholinesterase activity, by both the Ellman et al. (1961) and Michel methods, were taken before treatment, 24 h after the administration of each dose and twice each week after the final dose until day 15 after treatment for animals at the lower dose and day 43 after treatment for those at the higher dose. Brain cholinesterase activity was not assayed.
Treatment had no apparent effect on behaviour or general condition, and no clinical signs of toxicity were observed. Food consumption and body weights were within physiological limits during the study, and there were no treatment-related changes. Although data on food consumption were not shown, it was stated that the animals at the lower dose and the female at the higher dose ate the expected amounts of feed, while the male at the higher dose ate 40–45% less food than anticipated until after treatment stopped. The body weight of this male at the end of the treatment phase and 9 and 11 days after treatment was similar to that at the beginning of the study. The body weight of the female at the higher dose increased by about 4% during treatment and changed little after treatment. Decreases of 2.6% and 15% in body weight were reported during treatment for the male and female at the lower dose, respectively, when compared with the values at study initiation. Relative to the initial values, the body weight of the male on treatment day 9 or 11 was decreased by 4.7%, while that of the female was slightly increased. When the data on serum cholinesterase activity were examined by time, the overall pattern of results with the two methods of measurement were similar. At 0.05 mg/kg bw per day, serum cholinesterase activity was inhibited by 25–35% in both sexes 24 h after the first dose, and, depending on the method used and fluctuations in readings due to variability, the inhibition reached a plateau of 50–67% in the male and 62–73% in the female after 5–8 days of treatment. After treatment, the enzyme activity took 18–22 days to approach pre-treatment values. More variation in serum cholinesterase activity over time was noted at the lower dose, apparently more so with the Ellman et al. (1961) method during treatment. At this dose, the serum cholinesterase activity of the female was slightly decreased (by 15%) 24 h after the first dose, with no apparent decrease in the male. Enzyme activity decreased during treatment, and the inhibition may have approached a plateau of 60% in the female and a low of 30% in the male near or at the end of treatment. After treatment, the enzyme activity approached the pre-treatment level in the female by day 15 and was achieved in the male by about day 8. Within the levels of variability of the assay methods, erythrocyte cholinesterase activity did not appear to be inhibited at either dose. The NOAEL was 0.05 mg/kg bw per day, the highest dose tested, since no inhibition of erythrocyte cholinesterase activity or other effects were observed. No statements of compliance with QA or GLP were provided. Although the protocol did not meet a particular guideline, it was satisfactory for the intention of study (Scholz & Baeder, 1971).
(b) Dermal application
Rats
Technical-grade triazophos (purity, 97.6%) in a sesame oil was applied to the shaved skin of the back on an area equivalent to about 10% of the total body surface area of 15 male and 15 female Wistar (KFM-Han) outbred SPF rats at a dose of 0, 0.5, 5 or 50 mg/kg bw per day for 6 h/day, 5 days per week, for a total of 22–23 applications over 30–31 days (main study phase). Five animals from each group were continued for 28–29 days for a treatment-free recovery period. The animals were observed for deaths, clinical signs of toxicity, food consumption and body weight; ophthalmic, haematological and clinical chemical examinations were conducted; organs were weighed, and were examined macroscopically and microscopically. Plasma, erythrocyte and brain (sample described as one-half section) cholinesterase activities were measured at the end of both the treatment and the recovery period. Inhibition of cholinesterase activity was calculated relative to the value for the appropriate concurrent control group.
One control male died at the end of the main study; there were no deaths among treated animals, and no clinical signs of toxicity were observed. No treatment-related differences were found in food consumption, body weights or ophthalmic or haematological end-points. Slight, statistically significant changes in clinical chemical parameters attributed to treatment by the authors included increased glucose and urea concentrations in males at the two higher doses and females at the highest dose, and increased alanine aminotransferase activity and decreased triglyceride concentrations in males at the highest dose. At the end of the main study, plasma cholinesterase activity was statistically significantly inhibited by 19% and 38% in males at the intermediate and highest doses, respectively, and by 18%, 65% and 86% in females at the three doses, respectively. Erythrocyte cholinesterase activity was statistically significantly inhibited at the two higher doses, by 20% and 84% in males and by 50% and 91% in females. Statistically significant inhibition of brain cholinesterase activity, by 26% and 46%, was observed in males and females at the highest dose. The changes in organ weights ascribed to treatment by the authors consisted of slight, sometimes statistically significant increases in absolute and relative adrenal weights in females at the two higher doses, although the dose–response relationship was relatively flat. Macroscopic examination showed evidence of dermal irritation characterized by acanthosis, hypergranulosis and hyperkeratosis in some animals in all four groups, which was attributed to mechanical injury at the application site. No gross or microscopic findings were attributed to treatment. Some of the adrenal weight changes and dermal irritation persisted to the end of the recovery phase, while other changes seen in the main study had generally resolved. The NOAEL was 0.5 mg/kg bw per day on the basis of inhibition of erythrocyte cholinesterase activity, statistically significant increases in glucose and urea concentrations in males and increased adrenal weights in females at higher doses. A statement of QA and compliance with GLP was provided. The protocol was consistent with Subdivision F guidelines (November 1982 and revised, 1984) of the Environmental Protection Agency (Thevenaz et al., 1988).
(c) Inhalation
Rats
Technical-grade triazophos (purity, 97.6%) was administered to groups of 15 male and 15 female Wistar (KFM-Han) outbred rats by nose-only inhalation for 6 h/day, 5 days per week, for a total of 23 exposures over 28 days at nominal doses of 0, 1, 5 and 25 mg/m3, corresponding to mean analytical concentrations of 0, 1, 4.9 and 27 mg/m3. Ten of the 15 animals of each sex per group were killed at the end of the 28-day exposure, and the remaining five animals of each sex per group were followed for an additional 4-week treatment-free recovery phase. The animals were observed for viability, deaths, clinical signs of toxicity, food consumption and body weight; ophthalmic, haematological and clinical chemical examinations were conducted; organs were weighed and were examined grossly and microscopically. Plasma, erythrocyte and brain (one-half brain) cholinesterase activities were measured at the end of the exposure and recovery phases. Inhibition of cholinesterase activity was calculated relative to the value for the appropriate concurrent control group. Although the overall mean analytical concentrations of test material to which the animals were exposed were generally close to the nominal concentrations, variation was found in the individual samples taken during the study, in which the percentages of the targeted dose achieved in the treated groups ranged from 56% to 190%. Although the mass median aerodynamic diameter and geometric standard deviation did not appear to have been calculated, the median particle size for the two lower doses was generally between 1.1 fm and 1.6 fm. Of the overall particle size of the aerosol sampled, approximately 91–96% was < 3 fm, with a standard deviation of 0.7–3.3%.
One female at the highest dose died on day 21 during exposure, reportedly due to pulmonary haemorrhage of undetermined cause, associated with circulatory failure. The pathologist considered that the death was not related to treatment; the animal showed no clinical signs of toxicity. No signs of toxicity were observed in other animals, and there were no effects on food consumption or body weight or on ophthalmic end-points. There were no changes in haematological or clinical chemical end-points (other than cholinesterase activity) or changes in organ weights obviously related to treatment. Plasma, erythrocyte and brain cholinesterase activity was statistically significantly inhibited in males at the highest dose, by 22%, 73% and 22%, respectively. In females at the two higher doses, plasma cholinesterase activity was statistically significantly inhibited, by 60% and 83%, respectively, and erythrocyte cholinesterase activity was statistically significantly inhibited by 28% and 82%, respectively. There was no statistically significant decrease in cholinesterase activity during the recovery period. Brain cholinesterase activity was not inhibited in females. There were no remarkable gross or microscopic findings during exposure. During the recovery phase, one female at the intermediate dose was found to have an osteochondroma, a rare benign neoplasm, but the pathologist considered this to be an incidental lesion unlikely to have been induced by the test material during the short exposure. The recovery phase was otherwise unremarkable. The NOAEL was 1 mg/m3 on the basis of statistically significant inhibition of erythrocyte cholinesterase activity at higher doses. A statement of QA and of compliance with GLP were provided. The protocol was generally consistent with OECD guidelines (1981) (Bernstein et al., 1987).
Mice
Groups of 60 male and 60 female NMRI mice received diets containing technical-grade triazophos (purity, 93.1–95.9%) dissolved in sesame oil DAB 7 at a concentration of 0, 6, 30 or 150 ppm for up to 24 months, equal to intakes of 0, 0.83, 4.2 and 20 mg/kg bw per day for males and 0, 0.95, 4.9 and 24 mg/kg bw per day for females. Ten animals of each sex per group were scheduled for interim sacrifice at 12 months and the remaining 50 of each sex per group for sacrifice at 24 months. The doses were selected on the basis of the results of a preliminary 90-day study in NMRI mice. The animals were observed for general health, deaths, food and water consumption and body weight; haematological and clinical chemical end-points were determined; organs were weighed, and were examined macroscopically and microscopically. Serum, erythrocyte and brain (right hemisphere) cholinesterase activities were assayed at 12 and 24 months of treatment. Inhibition of cholinesterase activity was calculated relative to the value for the appropriate concurrent control group.
No treatment-related effects were evident in behaviour or general health, body weight, food consumption, haematological parameters, organ weights or macroscopic appearance. Slightly increased cholesterol concentrations observed in males at 30 and 150 ppm at 24 months were not statistically significant; however, a 34% decrease in alkaline phosphatase activity in males at 150 ppm was. This decrease could not be related to treatment in the absence of other findings in males and was not observed in females. Treatment-related inhibition of serum cholinesterase activity was observed in all treated groups at both 12 and 24 months. At 24 months, serum cholinesterase activity was inhibited by 58%, 80% and 93% in males and 65%, 86% and 94% in females at the three dietary concentrations, respectively. The decreases were statistically significant in all groups except males at 6 ppm. Similar levels of inhibition were seen at 12 months. Erythrocyte cholinesterase activity was statistically significantly inhibited in males at 150 ppm, by 46% at 12 months and by 41% at 24 months. In females at 30 and 150 ppm, erythrocyte cholinesterase activity was statistically significantly inhibited by 34% and 54% at 12 months and 33% and 48% at 24 months. Brain cholinesterase activity was statistically significantly inhibited by 43% in females at 150 ppm. These decreases in enzyme activity were considered to be treatment-related. The mortality rate was similar in males and females during the first year of the study, but the rate increased slightly, although not statistically significantly, in males and females at 150 ppm during the second year. The overall mortality rates at 0, 6, 30 and 150 ppm were 48%, 50%, 50% and 62% for males and 46%, 52%, 54% and 62% for females. It was reported that most of the deaths among females in the second year of the study were due to malignant lymphoma. Microscopic examination showed a slight increase in the incidence of a non-neoplastic lesion described as diffuse hypertrophy of the liver in females at 150 ppm at 24 months, the incidences per number of animals examined being 5/48, 5/48, 7/48 and 11/48 at 0, 6, 30 and 150 ppm, respectively. The lesion was not observed at the time of interim sacrifice at 12 months. The increase was not statistically significant, and there was no microscopic evidence of progression to neoplasia in the liver or changes in liver weight to suggest a relationship to treatment. The pathologist suggested that this lesion was associated with a finding in the livers of some animals consistent with incidental viral hepatitis. Males at 150 ppm had a lower incidence of diffuse liver hypertrophy than controls and other treated groups.
Malignant lymphoma, described as a tumour of the haemolymphoreticular system, was observed with increasing frequency in females and to a lesser extent in males at 150 ppm (Table 2). When Peto analyses for trend were performed, including animals killed at 12 and 24 months and any intercurrent deaths, a p value of 0.14 was found for males and a p value of 0.025 for females. A re-analysis (Pallen & Lautraite, 2002a) of the incidence of this tumour was performed with unadjusted tests, as the survival analysis showed no difference between treated and control groups of males and females. Only the data from the 24-month sacrifice were used, as addition of data from the interim sacrifice added only one more tumour in both the female control group and that at 150 ppm and only slightly changed the total numbers of tumours and added more tumours in the male control group and that at 6 ppm. Therefore, the outcomes of analyses with and without the data from the interim sacrifice would be expected to be similar. The Cochran-Armitage test (one-sided) did not show a significant dose-related trend in the incidence of malignant lymphomas for males (p = 0.097) or females (p = 0.14), and pair-wise comparisons of each treated group with the control group by the Fisher exact test (one-sided) did not show statistically significant differences at the 5% level of significance. In addition, data on controls in 10 24-month studies conducted in the same laboratory between 1985 and 1995 in the same strain of mouse showed total and mean incidences of malignant lymphoma of 23% and 23%, respectively (range, 13–36%), for males and 39% and 40%, respectively (range, 10–60%) for females. The incidences of malignant lymphoma in treated male and female mice in this study, calculated with and without the data from the interim sacrifice, fell within this range.
Table 2. Incidences of malignant lymphoma in mice given diets containing triazophos
|
Sex |
Dose (ppm) |
At interim sacrifice |
At terminal sacrifice |
Total incidence |
||||
|
No. examined |
Incidence |
No. examined |
Incidence |
No. |
% |
|||
|
No. |
% |
|||||||
|
Male |
0 |
9 |
2 |
49 |
11 |
22 |
13 |
22 |
|
6 |
10 |
2 |
48 |
13 |
27 |
15 |
26 |
|
|
30 |
10 |
0 |
50 |
16 |
32 |
16 |
27 |
|
|
150 |
10 |
1 |
50 |
17 |
34 |
18 |
30 |
|
|
Female |
0 |
10 |
1 |
50 |
22 |
44 |
23 |
38 |
|
6 |
10 |
0 |
49 |
25 |
51 |
25 |
42 |
|
|
30 |
10 |
0 |
50 |
23 |
46 |
23 |
38 |
|
|
150 |
10 |
1 |
50 |
29 |
58 |
30 |
50 |
|
From Donaubauer (1989); study conducted 1986–88
Figures include intercurrent deaths
A slight increase in the incidence of another neoplastic lesion, uterine stromal-cell sarcoma, was seen in females at 30 and 150 ppm. The incidences per number of animals examined at 24 months, including intercurrent deaths, were 1/49 (2%), 0/49 (0%), 3/49 (6%) and 3/47 (6%) at 0, 6, 30 and 150 ppm. Tumours of this type were not found in animals killed at the interim sacrifice. A Peto analysis for trend resulted in a p value of 0.052, at the 5% level of significance. Data on controls in the same laboratory used in a re-analysis for this tumour type showed a total incidence of 1% and a mean incidence of 1.6%, with a range of 0.0–6.0%. The incidences in this study fell within that range. Subsequent re-analysis of the data (Pallen & Lautraite, 2002a) with unadjusted statistical tests did not indicate a statistically significant dose-related trend (p = 0.079; Cochran-Armitage trend test, one-sided) in the incidence of uterine stromal-cell sarcoma. A pair-wise comparison of the data for mice at 30 and 150 ppm with the control group by the Fisher exact test (one-sided) also showed no statistically significant difference at the 5% level of significance. The re-analysis and the incidence data for other controls for both malignant lymphomas and the uterine stromal-cell sarcomas support the conclusion that these tumours were incidental and not related to treatment. Other microscopic findings in the study showed no obvious relationship to treatment. The NOAEL for systemic effects was 60 ppm, equal to 0.83 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity at higher doses. There was no evidence of carcinogenicity in this study. This study complied with QA and GLP and was consistent with Subdivision F guidelines (1984 revision) of the Environmental Protection Agency (Donaubauer, 1989).
Rats
Groups of 80 Wistar rats (KFM-Han) outbred SPF rats received diets containing technical-grade triazophos (purity, 92.6%) at a concentration of 0, 3, 27 or 240 ppm, equal to intakes of 0, 0.15, 1.3 and 12 mg/kg bw per day for males and 0, 0.18, 1.6 and 15 mg/kg bw per day for females. The animals were housed five per cage. Of the 30 animals of each sex per group assigned to determine chronic toxicity, 10 of each sex per group were killed after 52 weeks of treatment, and the remaining 20 were killed after 104 weeks. The 50 animals of each sex per group assigned for determination of carcinogenicity were killed after 118 weeks on the test diets. The animals were observed for viability, deaths, clinical signs, food and water consumption and body weight; organs were weighed and were examined macroscopically and microscopically. Animals used to determine toxicity also underwent routine auditory and ophthalmic tests and measurement of haematological, clinical chemical and urine parameters. Histopathological examinations were performed on all tissues designated in the protocol from all rats and on all tissues found suspect on necropsy. Plasma and erythrocyte cholinesterase activities were measured in animals designated for determination of chronic toxicity in weeks 27, 52/53, 79/80 and 105, and brain cholinesterase activity in ‘a section of brain tissue’ was measured in weeks 52/53 and week 105. Inhibition of cholinesterase activity was calculated relative to the values for concurrent controls.
Survival was similar in all groups, with rates of 47%, 51%, 47% and 50% for males and 49%, 46%, 44% and 50% for females at 0, 3, 27 and 240 ppm. No clinical signs of toxicity obviously related to treatment were found, and there were no apparent effects on body weight or food consumption. Females at 240 ppm tended to weigh slightly more and eat slightly more food during the study than the control group, although their water consumption was lower than that of controls at times, particularly between weeks 4 and 42. The results of the ophthalmic and auditory tests and urine analysis were unremarkable. Slight alterations in haematololgical parameters were found mostly during study weeks 50/51 and 77 in one or both sexes at 240 ppm, including slight decreases in erythrocyte volume fraction, erythrocyte count and haemoglobin and a slight increase in reticulocyte count. These were considered to be treatment-related but compensatory changes. Slight alterations in clinical chemical parameters were found, mostly between 25 and 77 weeks, in one or both sexes, mostly at 240 ppm but sometimes at 27 ppm, which included slightly increased cholesterol, total lipid and P concentrations and slightly decreased glucose and total protein concentrations. These may have been treatment-related but appeared to be compensatory and not toxicologically significant. None of the changes in organ weights was clearly related to treatment. Plasma and erythrocyte cholinesterase activities were consistently statistically significantly inhibited at all times in males and females at 27 and 240 ppm. The plasma enzyme activity was decreased in males by 21–51% at 27 ppm and 54–67% at 240 ppm and in females by 54–67% at 27 ppm and 71–78% at 240 ppm. Similarly, erythrocyte cholinesterase activity was inhibited in males by 50–73% at 27 ppm and 73–89% at 240 ppm and in females by 48–64% at 27 ppm and 81–90% at 240 ppm. Brain cholinesterase activity was statistically significantly inhibited only in females at 240 ppm, by 28% at week 52/53 and 21% at week 105. It is not clear whether the tissue sample selected for measurement of brain cholinesterase activity was representative or whether the sampling was consistent, as the study report described the sample simply as a section of brain tissue.
Macroscopic examination showed that males at 240 ppm had a higher incidence of nodular changes in the pancreas than the control group. Microscopic examination revealed a non-neoplastic lesion described as mostly moderate to marked focal or multifocal pancreatic acinar-cell hyperplasia in all groups of treated males, the incidence increasing with dose (Table 3). The lesion was not found in females. A statistical analysis (Pallen & Lautraite, 2002b) of the incidence of this lesion in males was performed with unadjusted tests, as the survival analysis showed no difference between treated and control groups. The analysis included males from the 52- and 104-week sacrifices of the chronic toxicity phases, those killed at 118 weeks at the end of the carcinogenicity phase and any intercurrent deaths. The Cochran-Armitage test, one-sided, including and excluding the highest dietary concentration, showed a significant dose-related trend in the incidence of pancreatic acinar-cell hyperplasia in males at p = 0.0001 and p = 0.0088, respectively. Pair-wise comparison of each treated group and the control group by the Fisher exact test (one-sided) showed significant differences for the groups at 27 and 240 ppm, at p = 0.029 and p = 0.0017, but a nonsignificant difference for the group at 3 ppm (p = 0.50). Data for controls in studies conducted in the same testing facility between 1982 and 2000 in a total of 2438 male Wistar rats showed a total incidence of 10% and a mean incidence of 9.8% (range, 0–31%) for pancreatic acinar-cell hyperplasia. Data for controls in three studies performed between 1988 and 1991 in the testing facility, in which 230 male Wistar rats were examined, showed a total incidence of 10% and a mean incidence of 8.1% (range, 0–23%) for this pancreatic lesion. Although the incidence of pancreatic acinar hyperplasia in males in the present study was within the range, the incidence at 240 ppm (11%) was greater than the total and mean incidences, there was a dose–response relationship with a significant positive trend, and the pair-wise comparison was significant at p < 0.05 for animals at 27 and 240 ppm. Therefore, a relationship between the occurrence of the lesion and treatment at these concentrations cannot be dismissed. No treatment-related neoplastic findings were observed in the pancreas of males or females in this study.
Table 3. Incidences of pancreatic acinar-cell hyperplasia in rats given diets containing triazophos
|
Sex |
Dose (ppm) |
Chronic toxicity phase |
Carcinogenicity phase (118 weeks) |
Total |
||||||
|
52 weeks |
104 weeks |
No. examined |
Incidence |
No. examined |
Incidence |
|||||
|
No. examined |
Incidence |
No. examined |
Incidence |
No. |
% |
|||||
|
Male |
0 |
10 |
0 |
20 |
0 |
48 |
0 |
78 |
0 |
0 |
|
3 |
10 |
0 |
20 |
0 |
49 |
1 |
79 |
1 |
1.3 |
|
|
27 |
10 |
0 |
20 |
2 |
48 |
3 |
78 |
5 |
6.4 |
|
|
240 |
10 |
0 |
20 |
3 |
50 |
6 |
80 |
9 |
11 |
|
|
Female |
0 |
10 |
0 |
20 |
0 |
50 |
0 |
80 |
0 |
0 |
|
3 |
10 |
0 |
20 |
0 |
50 |
0 |
80 |
0 |
0 |
|
|
27 |
10 |
0 |
18 |
0 |
50 |
0 |
78 |
0 |
0 |
|
|
240 |
10 |
0 |
20 |
0 |
49 |
0 |
79 |
0 |
0 |
|
From Tennekes et al. (1990); study conducted 1986–89
Figures include intercurrent deaths
Another non-neoplastic lesion frequently found in males and females was spinal cord radiculoneuropathy. In males, the incidence of this lesion was greater in all treated groups than that in the control group, the incidences per number of animals examined being 23/80 (29%), 37/80 (46%), 40/79 (51%) and 39/79 (49%) at 0, 3, 27 and 240 ppm. However, the dose–response relationship was poor. Data on 1929 other control male Wistar rats in studies performed in the same testing facility between 1982 and 2000 indicated that the incidence of 29% in the control group in the present study was higher than that in other controls, which had a total and a mean incidence of 18%; however, the data on previous controls showed that the occurrence of this lesion was variable in both male (range, 0–99%) and female (range, 0–100%) Wistar rats. Therefore, no clear-cut relationship of this lesion to treatment was indicated.
An increased incidence of mammary gland fibroadenomas was seen, particularly in females at 240 ppm, relative to the control group, the incidences per number of animals examined being 22/80 (28%), 19/80 (24%), 24/80 (30%) and 28/80 (35%) at 0, 3, 27 and 240 ppm, respectively. A Peto analysis for trend presented in the original report, which included animals that were killed or died intercurrently, showed a p value of 0.054. A re-analysis (Pallen & Lautraite, 2002b) of the incidence of this tumor was conducted with unadjusted tests, as the survival analysis showed no difference between treated and control groups of females. Neither a significant trend (p = 0.1133) nor significant pairwise comparisons (p = 0.43 at 27 ppm and p = 0.20 at 240 ppm) were found with the Cochran-Armitage trend test (one-sided) and the Fisher exact test (one-sided), respectively. The incidence at the high dose (35%) was slightly greater than the total or mean incidence of 30% seen in control female Wistar rats in studies performed in the same testing facility between 1982 and 2000, in which 2506 animals were examined, and was slightly greater than the total incidence of 25% and the mean incidence of 27% found in three studies performed between 1988 and 1991. However, the incidence was within the ranges of 6–60% seen in studies performed between 1982 and 2000 and 15–38% seen in three studies done between 1988 and 1991. There was no dose–response relationship, as the incidence of this lesion was increased only in the group at 240 ppm. Therefore, a definitive relationship to treatment was not supported.
A similar statistical re-analysis (Pallen & Lautraite, 2002b) was performed for haemangiomas, which were found at higher incidence in females at 240 ppm than in controls. The incidences were 5/80 (6.3%), 4/80 (5%), 4/80 (5%) and 10/80 (12%) at 0, 3, 27 and 240 ppm, respectively. The re-analysis showed no significant trend (p = 0.088) and a nonsignificant pair-wise comparison at 240 ppm (p = 0.14). Therefore, a clear relationship to treatment was not supported. No data on the incidence of this lesion in previous controls were available. Males showed no increase in the incidence of haemangiomas relative to controls. Other macroscopic and microscopic findings in this study were not clearly related to treatment. The NOAEL for systemic toxicity was 3 ppm, equal to 0.15 mg/kg bw per day, on the basis of the increased incidence of pancreatic acinar-cell hyperplasia in males and decreased erythrocyte cholinesterase activity in both sexes at higher doses. There was no evidence of carcinogenicity in this study. This study was compliant with QA and GLP and consistent with Subdivision F guidelines (1984) of the Environmental Protection Agency (Tennekes et al., 1990).
The results of tests for genotoxicity performed with triazophos are summarized in Table 4.
Table 4. Results of tests for genotoxicity with triazophos
|
Test system |
Test object |
Concentration |
Purity |
Results |
Reference |
|
In vitro |
|||||
|
Reverse gene mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
0.2–5000 fg/plate in DMSO or ethanol, ± S9 |
92% |
Negative |
Gericke & Wagner (1977) |
|
Forward gene mutation |
S. pombe |
1000–4000 fl/l, ± S9a |
92.4% |
Negative |
Fumero & Mondino (1980a) |
|
Chromosomal aberration |
Human lymphocytes |
0.92–920 fl/l, –S9; 0.8–800 fl/l, +S9 in DMSOb |
92.4% |
Negative |
Fumero & Mondino (1981) |
|
Gene conversion |
S. cerevisiae |
1000–4000 fl/l, ± S9a |
92.4% |
Negative |
Fumero & Mondino (1980b) |
|
In vivo |
|||||
|
Micronucleus formation |
Mouse |
0.2–20 mg/kg bw in sesame oil, administered twice at interval of 24 h |
92.4% |
Negativec |
Mayer et al. (1980) |
|
Sex-linked recessive lethal mutation |
Adult D. melanogaster MRA strain |
Ingestion or injection of 0–30 ppm in dimethyl sulfoxide:water:sucrose |
Not reported |
Positive after ingestion |
Velazquez et al. (1990) |
|
Sex chromosome non-disjunction |
D. melanogaster ring-X strain |
0–1 ppm in dimethyl sulfoxide:water:sucrose |
Not reported |
Weakly positive |
Velazquez et al. (1990) |
|
Sex-chromosome loss |
Adult D. melanogaster ring-X strain |
Ingestion or injection |
Not reported |
Negative |
Velazquez et al. (1990) |
|
S9, 9000 × g supernatant fraction from rodent liver |
|
|
a |
No vehicle; material introduced into test system |
|
b |
Cytotoxic at highest dose tested with and without S9 activation. |
|
c |
Generally acceptable: highest dose tested (20 mg/kg bw, twice) reported to be maximum tolerated dose, but no cytotoxic effect was seen on the target cells. |
The results of most of the tests were negative; however, in one acceptably performed study in Drosophila melanogaster in vivo (Velazquez et al., 1990), triazophos caused point mutations in a test for sex-linked recessive lethal mutations in an insecticide-resistant strain (MRA) treated by ingestion. In a second study, triazophos gave weakly positive results for sex chromosome non-disjunction in male germ cells of ring-X strain insecta after exposure during the third-instar larval phase.
Rats
In a preliminary one-generation study of reproductive toxicity, groups of 10 male and 10 female Wistar/HAN (Kfm: WIST, outbred, SPF quality) rats received diets containing technical-grade triazophos (purity, 92.6%) at a concentration of 0, 1, 20 or 400 ppm during a 3-week pre-mating period and throughout mating, gestation and lactation. Triazophos was similarly administered for 1 week after weaning on day 21 post partum to F1 pups that were not used for determination of organ weights. The concentrations corresponded to estimated intakes in the pre-mating period of 0, 0.08–0.1, 2 and 32–41 mg/kg bw per day for males and 0, 0.09–0.1, 2 and 32–42 mg/kg bw per day for females; estimated intakes in the gestation period to be 0, 0.08–0.1, 2 and 37–40 mg/kg bw per day for females; estimated intakes post mating to be 0, 0.06–0.07, 1 and 26–30 mg/kg bw per day for males; and estimated intakes in the lactation period to be 0, 0.13–0.21, 3–4 and 71–75 mg/kg bw per day for females. On day 4 post partum, the litters were randomly reduced to eight pups. The parental adults were killed shortly after weaning, on day 21 post partum for the two pups of each sex per dose selected for determination of organ weights and on day 28 for the remaining pups after weighing. The parental animals were observed for deaths, clinical signs of toxicity, body weight and food consumption; reproductive performance was assessed, including mating, fertility, duration of gestation, implantation rate, post-implantation loss and litter size and loss up to day 21 post partum; and organs were weighed and examined macroscopically. Offspring were assessed for viability, abnormal behaviour in nesting and nursing, clinical signs of toxicity, external signs of gross anomalies, litter sex ratio, body weight, weights of selected organs and food consumption after weaning. No animals were examined for histopathological changes. Cholinesterase activity was not measured.
Treatment-related effects were observed only at 400 ppm. The parental effects included the deaths of three females during lactation, which were attributed to treatment. These and other females at 400 ppm showed clinical signs of toxicity including exophthalmus, aggressive behaviour, tremor, ataxia, emaciation, dyspnoea and squealing during lactation and piloerection during gestation. The clinical signs followed a period of increased intake, especially during lactation, although food consumption declined as the signs appeared. Decreases in body weight were noted in dams at 400 ppm relative to controls, during this time and earlier in the study. Statistically significant changes in organ weights in females at 400 ppm (decreased absolute and relative (to brain) weights of the liver and spleen and slight increases in the weights of the brain, lung and kidney relative to body weight) were attributed to statistically significant decreases in terminal body weight. Reproductive effects noted in the group at 400 ppm included a slight increase in post-implantation loss associated with a slight decrease in the number of live pups at the first observation time post partum on or around day 0, a statistically significant decrease in the number of live pups on day 4 and a statistically significant increase in pup loss on days 4–21 post partum, due in part to pups found dead or killed after parental deaths. The body weights of pups at 400 ppm were statistically significantly decreased during lactation. The absolute weights of the brain, liver, kidney, spleen, testes, ovary and uterus in male and female pups were decreased relative to controls, and in most cases the decreases were statistically significant. These and other changes in relative organ weights of pups were attributed to the relatively lower body weights of this group. The weights of pup brains relative to body weights were somewhat higher than those of controls. Additional treatment-related effects were reported in the group at 400 ppm on days 21–28 post partum: two of two male pups and two of six female pups died, and food consumption and body weights were decreased relative to the control group on day 28 post partum. An increase in post-implantation loss and decreased litter size post partum in the group at 20 ppm was associated with the total loss of one litter due to the failure of one dam in that group to rear her pups, and was not attributed to treatment. No treatment-related anomalies, malformations or abnormalities were seen on macroscopic examination of pups or parents. Despite the limited study design and parameters examined, the NOAEL for systemic toxicity in parental animals, for reproductive effects and for effects in offspring was 20 ppm, equal to 1 mg/kg bw per day. QA and GLP statements were provided. The study was not performed to meet a specific guideline as it was a preliminary study (Suter et al., 1989a).
In a two-generation study of reproductive toxicity, groups of 25 male and 25 female Wistar/HAN (Kfm: Wist, outbred, SPF quality) rats received diets containing technical-grade triazophos (purity, 92.6%) at a concentration of 0, 3, 27 or 240 ppm during pre-mating, mating, gestation and lactation, equal to intakes for the F0 generation of 0, 0.2–0.3, 1–3 and 12–25 mg/kg bw per day and for the F1 generation of 0, 0.1–0.4, 1–4 and 12–35 mg/kg bw per day. The study generally followed a standard protocol. F0 adults were dosed for 70 days and F1 pups selected for breeding of the F2 generation were dosed for 125 days. F0 and F1 breeder animals were placed on treated feed when they were about 8 weeks and 4 weeks of age, respectively. The F1 and F2 litters were reduced to eight pups per litter on day 4 post partum and weaned on day 21 post partum, at which time the study was terminated and tissues from reproductive and endocrine organs, glands and structures, brain, liver, kidneys and spleen were taken for weighing and microscopic evaluation. The parental animals were observed for deaths, clinical signs of toxicity, body weight and food consumption; reproductive performance was assessed, including mating and fertility, duration of gestation, implantation rate and post-implantation loss and litter size and loss, up to day 21 post partum; organs were weighed and examined macroscopically. The offspring were assessed for viability (daily from day 1 post partum), abnormal behaviour in nesting and nursing, clinical signs of toxicity, external signs of gross malformations and/or anomalies, litter sex ratio, body weight (on days 1, 4, 7, 14 and 21 of lactation), and organ weights in one male and one female pup from each F1 and F2 litter. Histopathological evaluation was conducted of the adrenal glands, cervix, coagulating glands, epididymides, kidneys, liver, ovaries, pituitary gland, prostate, seminal vesicles, spleen, testes, thymus, thyroid gland, uterus, vagina, and all gross lesions from F0 and F1 controls and at 240 ppm that had been selected for mating, from any animals in these groups that did not perform during mating, from one male and one female pup from each F2 litter (those selected for determination of organ weights) and from animals that died intercurrently or with gross lesions. Cholinesterase activity was not measured in this study.
Treatment-related effects were observed only in animals at 240 ppm. Four rats died during the study, all in the F1 generation: one male at 3 ppm died before treatment, one female at 240 ppm died 21 days post coitum, and two females at 240 ppm died during the lactation period. It is unlikely that the death of the male at 3 ppm was treatment-related, as no other relevant findings were made in this animal or in others in the group. The deaths among females at 240 ppm might have been related to treatment, as these animals showed clinical signs of toxicity; one female showed histopathological signs consistent with circulatory failure. Persistent aggressive behaviour in both sexes of the F0 generation during the pre-mating period was observed, with recurring instances of exophthalmia, ataxia, tremor and sometimes dyspnoea in F0 females during lactation, in F1 pups from day 18 post partum and continuing into the pre-mating period of F1 generation breeder males and females, and in adult F1 females during the lactation period. The results of observations for clinical signs in F2 pups were not reported. Body weight and food consumption were statistically significantly decreased in F0 females during lactation, in F1 parental females during most or all of the observation periods and in F1 parental males during pre-mating.
Treatment had no effect on F0 generation mating performance or fertility, duration of gestation, implantation rate, post-implantation loss or postnatal loss on days 0–4 post partum. Slightly more dead pups (five) were found at parturition in the group at 240 ppm compared with the controls (two) and those at 3 ppm (none) and 27 ppm (none), but the numbers of live pups at this time were similar in all groups. Loss of F1 litters on days 4–21 post partum after litter size adjustment was increased in the group at 240 ppm (23%) compared with the control group (1.6%) and that at 3 ppm (1.6%), and this effect was considered to be related to treatment. An increase was also seen at 27 ppm (6.3%), but this was considered unlikely to be due to treatment because 5 of the 12 lost pups in this group were in the same litter and no effects on this parameter were seen in the F1 generation. The mating performance, fertility and duration of gestation of the F1 generation did not appear to be affected by treatment. There was a slight decrease in the number of implantation sites and an increase in post-implantation loss in the group at 240 ppm relative to the control group, which may have been treatment-related. A decrease in the number of living F2 pups, an increase in the number of dead pups at the time of parturition, an increase in F2 postnatal loss on days 0–4 post partum and an increase in pup loss on days 4–21 after culling in the group at 240 ppm relative to control group values were ascribed to treatment. Two dams at 240 ppm lost entire F2 litters during lactation. One case was due to maternal death, which subsequent killing of the pups; the other was apparently due to cannibalization. Statistically significant decreases in F1 pup body weights during the entire 21-day lactation period and in F2 pup body weights on days 4, 7, 14 and 21 post partum were ascribed to treatment. Treatment had no effect on the sex ratio in either generation.
Statistically significant changes in absolute or relative (to body and/or brain weight) organ weight noted in F0 or F1 parents and offspring at 240 ppm were attributed by the authors to the decreased body weights of these animals. The changes noted included decreased absolute and relative seminal vesicle weight, increased absolute and relative kidney weight and increased relative liver weight in F0 males; decreased absolute and relative liver weight, spleen weight and pituitary weight and increased brain weight in F0 females; decreased absolute seminal vesicle weight in F1 males; decreased absolute and relative spleen and ovarian weights, decreased liver weight and increased kidney weight in F1 females, and decreased absolute and relative liver weights and absolute kidney and testis weights in male F1 pups. The absolute brain weights were decreased by 3–4% in male and female F1 pups but were increased relative to body weight by about 10%. Increases in relative brain weight of 13–15% were also reported for F2 pups of each sex. F2 pups also showed decreases in absolute and relative liver weights in males and kidney weight in females. The absolute and relative weights of the pituitary were decreased in females, but the dose–response relationship was poor, and no correlative histopathological findings were observed. Macroscopic and microscopic examinations did not reveal any treatment-related findings in parental animals or offspring. The finding in two male F1 pups at 240 ppm of agenesis of the epididymides or of the left testis and left epididymis were considered to be incidental. The NOAEL for parental and reproductive toxicity was 27 ppm, equal to 1 mg/kg bw per day. GLP and QA statements were provided, and the protocol was generally consistent with Subdivision F (1984) guidelines of the Environmental Protection Agency (Suter et al., 1989b).
Rats
In a study of developmental toxicity, groups of 20–23 Wistar (Hoe: WISKf (SPF71)) rats received diets containing technical-grade triazophos (purity, 89.4%) at a concentration of 0, 10, 50 or 250 ppm, equal to intakes of 0, 0.87, 4.2 and 22 mg/kg bw per day, on days 7–16 of gestation. Analysis of the diet for the content of the test material and its homogeneity and stability in the feed were not reported. The basis of selection of the doses for the study was not entirely clear but was reported to be oral LD50 values for female Wistar rats. The study was terminated on day 21 of gestation. The day of detection of vaginal sperm was considered to be day 1 of gestation. Only gravid females were evaluated in the analysis, consisting of 20/20, 20/22, 20/23 and 20/20 at 0, 3, 10 and 30 ppm, respectively. The adult gravid animals were observed for behaviour, general condition, deaths, premature delivery, abortion, food consumption and body-weight changes and underwent terminal necropsy. Corpora lutea were not counted. The litters and fetuses were assessed for fetal viability, sex and crown-to-rump length, number of resorptions, placental and fetal weights and general appearance and externally recognizable anomalies. Approximately half the fetuses were examined for skeletal anomalies by staining with Alizarin S and stereoscopic microscope examination, and the remainder were examined for visceral anomalies by the Wilson technique. Cholinesterase activity was not measured.
There were no premature births or abortions and no treatment-related maternal effects on mortality rate, behaviour, general condition, food consumption or body weight. At necropsy, placental weights and the numbers of implantations and resorption sites per dam were found to be unaffected by treatment. No association with treatment was found on fetal viability, sex ratio, body weight, crown-to-rump length or external, visceral or skeletal abnormalities. Owing to the lack of information on the test material content of the diet and clear information on the basis for dose selection, it is uncertain whether the dams were sufficiently challenged. The NOAEL for both maternal and developmental effects was greater than or equal to the nominal concentration of 250 ppm, equal to a nominal dose of 22 mg/kg bw per day, the highest dose tested. QA and GLP statements were not provided. Although the protocol was mainly consistent with Subdivision F guidelines (1982 and 1984, revised) of the Environmental Protection Agency, the study had notable deficiencies (Baeder et al., 1976).
Rabbits
In a preliminary study, technical-grade triazophos (purity, 92.1%) in sesame oil was administered by gavage to groups of four female New Zealand white rabbits at a dose of 0, 1, 3, 10 or 30 mg/kg bw per day on days 6–19 of gestation. The study was terminated on day 29 of gestation. As the results of this study were inconclusive, two additional groups of four females were similarly exposed to the test material at a dose of 5 or 10 mg/kg bw per day. For comparison, the results of exposure to a vehicle of distilled water were reported for four female control rabbits from a concurrent study of embryotoxicity (although these were not considered here). All groups of adult females were observed for general condition, signs of toxicity, deaths, premature delivery, abortion, total litter loss, corpora lutea count, food and water consumption, body and placental weight; the results of terminal necropsy were assessed. Litters and fetuses were assessed for numbers of viable young (total and per sex), implantations, early, late and total resorptions, pre- and post-implantation losses, mean fetal weights and morphological abnormalities in an external (apparently visual) examination and an internal examination of the neck and thoracic and abdominal cavities (with low-power magnification if necessary). Cholinesterase activity was not measured.
The general health status of a number of the adult animals was poor. Six adult females died or were killed in extremis during the study: two at 30 mg/kg bw per day, one in each of the groups at 10 mg/kg bw per day, one at 3 mg/kg bw per day and two given sesame oil. Necropsy indicated that the deaths were due to disorders or infection, alone or in combination, of the respiratory, gastrointestinal or urinary tracts. After dosing, two animals at 30 mg/kg bw per day had laboured breathing, slight muscular tremors, ataxia and incontinence, and one animal in the second group given 10 mg/kg bw per day had an increased respiration rate that may have been treatment-related. As two of these animals (one at 30 mg/kg bw per day and one at 10 mg/kg bw per day) were killed in extremis, a contribution of treatment to the overall condition of these animals cannot be ruled out. Although there were few animals for evaluation of body-weight changes or food consumption at some times in some groups, notable decreases in body weight (by 0.25–0.5 kg) were observed on days 6–12 of gestation among does at 30 mg/kg bw per day and in both groups at 10 mg/kg bw per day, with some but not complete recovery after treatment. Smaller decreases, with recovery after treatment, were noted at 3 mg/kg bw per day (0.09 kg) and 5 mg/kg bw per day (0.03 kg). Food consumption varied and was difficult to evaluate, although it appeared to be decreased in does at the highest dose, with no recovery. All the does except one control female became pregnant. One control delivered prematurely on day 8 of gestation; one doe in the first group given 10 mg/kg bw per day and one at 30 mg/kg bw per day aborted on days 28 and 27, respectively, and one doe in the second group given 10 mg/kg bw per day lost the entire litter (classified as early resorption). A relationship to treatment at 10 and 30 mg/kg bw per day could not be excluded, although the animal at the highest dose had evidence of a possible gastrointestinal disorder at necropsy. No definitive relationship to treatment could be found for differences among groups in litter parameters, but the evaluation was limited by the small numbers of litters available for comparison. Although pre- and post-implantation losses of 70% and 33%, respectively, were seen at the highest dose, similar losses were also found in the sesame oil control group. No treatment-related morphological changes in fetuses were observed. QA and GLP statements were not provided, and the study was not performed to meet a specific regulatory guideline (Tesh et al., 1985a).
In a study of developmental toxicity, technical-grade triazophos (purity, 92.1%) in sesame oil was administered by gavage to groups of 18 female New Zealand white rabbits at a dose of 0, 2, 4 or 8 mg/kg bw per day on days 6–19 of gestation. The study was terminated on day 29 of gestation. All adult females were evaluated for general condition, signs of toxicity, deaths, premature delivery, abortions, corpora lutea count, litter loss, food and water consumption and body-weight changes; findings at terminal necropsy were assessed. Litters and fetuses were assessed for the numbers of viable young (total and per sex), implantations, early, late and total resorptions, pre- and post-implantation losses, placental and fetal weights; fetuses were evaluated for morphological abnormalities in an external (apparently visual) examination, an internal examination of the neck and thoracic and abdominal cavities and a skeletal examination (with a modification of the Dawson alizarin staining technique). Cholinesterase activity was not measured.
Five adult females died or were killed in extremis during the study: two at the lowest dose, one at the intermediate dose and two at the highest dose. Necropsy indicated that the deaths were due to disorders of the respiratory and gastrointestinal tracts and, in the case of one female at the lowest dose, to tracheal damage during gavage. No clinical signs of toxicity were observed. During days 6–20 of gestation, treated animals gained somewhat less weight than the control group (0.2 kg). The group at the highest dose, which gained 0.07 kg, lagged behind those at the lowest (0.1 kg) and intermediate doses (0.12 kg) during this period. After dosing, the control group and those at the two lower doses gained about the same amount of weight (0.3–0.34 kg), while those at the highest dose gained slightly less weight (0.28 kg). None of the changes in body weight in the treated groups was statistically significant relative to the control group. Food consumption was slightly lower during days 6–12 of gestation for does at the highest dose (140 ± 50 g/rabbit/ per day) relative to that of the control group (150 ± 40 g/rabbit per day) and the other groups (the data were not analysed statistically). All the does except one at the highest dose became pregnant. One at this dose aborted on day 22 of gestation, and necropsy showed evidence of a gastrointestinal tract disorder and kidney enlargement. Another female at this dose with no clear evidence of illness resorbed the entire litter from one implant (classified as early resorption), although the pre-implantation loss of this female was high (83%). A relationship to treatment could not be dismissed in either case. There were no obvious treatment-related differences among groups in litter parameters. No treatment-related fetal external, visceral or skeletal abnormalities were found by the methods used. The NOAEL for maternal toxicity was 4 mg/kg bw per day on the basis of slight decreases in body weight and food consumption at the next highest dose. The dose-ranging study (Tesh et al., 1985a) had shown suspected treatment-related toxicity at doses >10 mg/kg bw per day, comprising decreases in maternal body weight and food consumption and clinical signs, and a possible contribution of treatment to the morbidity and mortality, although non-treatment-related illness was a confounding factor. The NOAEL for developmental toxicity was 4 mg/kg bw per day on the basis of effects on pregnancy at the next highest dose and at doses > 10 mg/kg bw per day in the dose-ranging study for which a relationship to treatment could not be dismissed. A QA statement was provided, but a GLP statement was not. The report did not state that the study had been performed to meet a regulatory guideline. Although the protocol was generally consistent with Subdivision F guidelines of the Environmental Protection Agency, the study had some deficiencies (Tesh et al., 1985b).
A number of studies were performed in hens to evaluate the potential of triazophos to induce delayed polyneuropathy. These included three studies in which single doses of triazophos of 12, 25 and 50 mg/kg bw were given by gavage, with or without pharmacological protection, with challenge 21 days after the initial dose; a 20-day feeding study with measurement of neuropathy target esterase (NTE), a 3-month feeding study, a study in which single doses of up to 10 mg/kg bw were given by gavage without pharmacological protection and various preliminary studies for dose selection.
Groups of six white Leghorn hens were given technical-grade triazophos (purity, 88%) in sesame oil by intubation at a dose of 25 mg/kg bw. This dose was selected on the basis of the results of a preliminary study in which the mortality rates in groups of three hens given a single dose of 12, 25, or 50 mg/kg bw by intubation were 0/3, 2/3 and 3/3, respectively, the birds dying within 2 h of dosing and showing clinical signs of toxicity, including disturbed equilibrium, laboured breathing and diarrhoea. The main study included an untreated control group, two groups given triazophos and a group treated with 500 mg/kg bw tri-ortho-cresyl phophate (TOCP) as a positive control. The birds in one of the two groups given triazophos were pretreated by intraperitoneal injection with atropine (1%, 10 mg/kg bw) and obidoxime chloride (0.4%, 4 mg/kg bw) before treatment with triazophos, and were treated five additional times during the next 21 days with the atropine and obidoxime chloride combination as an antidote to cholinergic toxicity. The other group of hens was treated with 25 mg/kg bw triazophos alone. After the 21-day observation period, surviving birds given triazophos were dosed once again with 25 mg/kg bw alone or after pretreatment with the atropine–obidoxime chloride combination, followed in the latter group by four additional antidote treatments. TOCP was not administered again. Birds given triazophos and the control groups were observed for another 21 days before the end of the study. During the study, the birds were observed daily for deaths and clinical signs of toxicity, and body weights were measured. At the end of the study, the birds were anaesthetized and perfused with 8% formalin before macroscopic examination. Segments of the spinal medulla (cervical medulla, cervical enlargement, thoracic medulla and lumbar enlargement) and nerves of the brachial and lumbosacral plexus (sciatic nerve) were examined microscopically after formalin fixation, Paraplast embedding and staining with haemotoxylin and eosin. Tibial nerve does not appear to have been sampled. Tissues were also examined after periodic acid–Schiff and medullary sheath staining. Neither cholinesterase activity nor NTE activity was measured.
During the first 21-day observation period, one hen given triazophos plus antidote died 1 h after dosing, and one hen given triazophos alone died several hours after dosing. The clinical signs of toxicity seen up to day 3 after dosing, which were allayed by antidote treatment, were similar in the two groups and included heavy salivation, rapid breathing, disturbance of equilibrium, extensor spasms and lying on the side and in a crouched position. The signs that began in the TOCP-treated group on day 12 after dosing included disturbed equilibrium and evidence of paralysis, followed in subsequent days by decreased food consumption and birds found in a squatting position. One TOCP-treated bird was killed on day 21 after dosing because of poor condition. After the second dose of triazophos, two more birds died within the next 24 h in the group given the antidote combination and three more birds died in the group given triazophos alone. Clinical signs of toxicity similar to those seen after the first dose were observed. Two more hens in the positive control group died or were killed 35–37 days after dosing, and most of the remaining birds in this group continued to manifest clinical signs consistent with delayed neurotoxicity. Decreases in body weight were seen in some birds given triazophos or TOCP at various times during the study. The condition of the untreated control group was unremarkable throughout the study. Owing to deaths, fewer than six birds per group were available for macroscopic and microscopic examination, with three hens given triazophos plus antidote, two given triazophos alone and five given TOCP. Only one untreated hen was examined microscopically. The one bird with macroscopic findings (unilateral swelling in the lumbar region of the spinal medulla), which had received triazophos alone, showed microscopic evidence of what was described as non-treatment-related chicken gliomata (reported as focal neuroglial proliferation with inflammatory perivascular infiltrates in the medulla oblongata and numerous regions of the spinal medulla but most marked in the lumbar region). No microscopic lesions were found in the other triazophos-treated birds or in the untreated control group. The microscopic findings in TOCP-treated birds were generally consistent with delayed neurotoxicity, including axon swelling accompanied by focal glial-cell proliferation and isolated foci of demyelination, varying in incidence and distribution through the spinal medulla but without observable changes in the peripheral nerves. Under the conditions of the study, with apparently limited peripheral nerve sampling for histopathological examination, triazophos did not show acute delayed neurotoxic potential. Neither a QA nor a GLP statement was provided. The protocol followed the guidelines available at the time (proposed toxicology guidelines in the USA, 1972) (Kramer & Weigand, 1974), but the study had some deficiencies by current standards.
Technical-grade triazophos (purity, 92.6%) was tested for acute delayed neurotoxicity potential in white Leghorn hens. In a preliminary study for dose selection, the LD50 in hens for technical-grade triaziphos dissolved in sesame oil was estimated by probit analysis to be 7.5 mg/kg bw, with a 95% confidence interval of 4.8–10 mg/kg bw. When the test material was administered as a single dose by gastric intubation to groups of five birds at a dose of 3.2, 6.3, 8.0, 12, 16, 25 or 50 mg/kg bw, the mortality rates were 0/5, 2/5, 2/5, 5/5, 5/5, 5/5 and 5/5, respectively, all deaths occurring 0.5–4 h after dosing. The clinical signs of toxicity observed, mainly within several hours after dosing, included tonic spasms, salivation, lachrymation, convulsive wing-flapping and terminal orthotonos. The findings of a macroscopic examination conducted on birds that died on test or on those that survived a 14-day observation period were not reported. In another preliminary study, treatment with 10 mg/kg bw atropine sulfate alone or in combination with 75 mg/kg bw pyridine-2-aldoxime methoiodide protected against cholinergic toxicity, so that the birds could be challenged with a higher dose of triazophos.
Technical-grade triazophos was administered as a single dose by gastric intubation to groups of five hens at a dose of 12, 25 or 50 mg/kg bw. Single doses of atropine and pyridine-2-aldoxime methoiodide were administered by intraperitoneal intubation just before dosing with triazophos and as needed after dosing, depending on the cholinergic symptoms. The mortality rates in these groups were 1/5, 1/5 and 2/5, all deaths occurring the evening after dosing when antidote could not be administered. The clinical signs of toxicity observed, almost all reverting within the first 4 days after dosing, included increased swallowing, miosis, narrowed palpebral slits, negative tail reflex, prone position, ataxic or uncoordinated gait, standing on hocks, reduced spontaneous activity, paresis of legs, disequilibrium, diarrhoea, salivation, trembling and some decreases in body weight. Macroscopic examination of birds that died on test and those that survived a 15-day observation period showed no obvious indications of nerve damage. The protective LD50 based on these results was estimated to be 50 mg/kg bw.
In the main study, technical-grade triazophos emulsified in sesame oil was administered as a single dose of 50 mg/kg bw by gastric intubation to 20 fasted hens. Two additional groups of six hens were similarly dosed with 500 mg/kg bw of TOCP or 1 ml/kg bw of sesame oil. Shortly before and after dosing, single doses of atropine sulfate (10 mg/kg bw) and pyridine-2-aldoxime methoiodide (75 mg/kg bw) were administered to all triazophos-treated birds by intraperitoneal intubation and as needed as the study progressed, as an antidote to cholinergic symptoms. After a 21-day observation period, surviving birds given triazophos and the vehicle control group were challenged and observed for an additional 21 days before the end of the study on day 43. Antidote therapy was administered as before to the group given triazophos. TOCP was not administered again, and the birds given TOCP were killed on day 23. During the study, the birds were observed daily for deaths and clinical signs of toxicity; a graded evaluation of ataxia was conducted daily and twice weekly outside the cage as part of a forced motor activity examination; body weights and food consumption were measured, and all birds surviving the second day of the study were examined macroscopically and microscopically after perfusion in situ with an 8% formalin solution. Haematoxylin and eosin and luxol cresyl violet–haematoxylin stains and the periodic acid–Schiff reaction were used to evaluate tissues. Full details of the tissue preparation were not reported. The tissues examined were the cerebrum, cerebellum, brain stem, medulla oblongata, cervical, thoracic and lumbosacral spinal cord and sciatic and tibial nerves. Neither cholinesterase nor NTE activity was measured.
Birds given the vehicle alone survived the study with no unusual clinical findings, and they tended to gain weight as the study progressed. With the exception of one bird with a minimal (grade 1) glial-cell nodule in the cerebral cortex on microscopic examination, no macroscopic or microscopic alterations were noted in this group, and the assessment for ataxia was negative. No intercurrent deaths occurred in the positive control group. The birds had typical delayed neurotoxic effects, including locomotor deficits starting on days 8–14 after dosing, progressing to moderate to severe ataxia in most and paralysis in some by termination on day 23. Although no macroscopic histopathological findings were reported, correlative microscopic lesions were found, particularly in the spinal cord. The lesions included plaque-shaped decomposition products in myelin sheaths of minimal to moderate severity in the lumbar and/or thoracic spinal cord of all birds and in the sciatic nerve of one bird. Other findings among birds in this group included cerebral cortex glial-cell nodules (2/6) and perivascular (round) cell infiltrates in the cerebrum (2/6) and/or spinal cord (2/6) or peripheral nerve (2/6) of minimal to slight severity. Nine hens given triazophos groups died during the first 2 days after dosing (histopathology was not performed on these birds), and six more died the day after the second application, with cholinergic symptoms (specific clinical signs were not reported, although antidotes were administered). One hen was killed on day 38 of the study after it continued to lose weight. Four birds survived to termination. As a group, birds treated with triazophos tended to have decreased body weight after dosing, with some recovery, and to eat less food than the vehicle control group. Of the seven birds that died on day 23 or were killed on day 38, none showed macroscopic histological lesions of the nervous system or motor deficits; on microscopic examination, however, one bird had ballooning of myelin sheaths, with plaqued-shaped decomposition products of minimal severity (grade 1) in the lumbar spinal cord, and another had glial-cell nodules of minimal severity in the cerebral cortex. Of the birds that survived until day 43 (the end of the study), none showed macroscopic lesions, but one bird had slight ataxia (grade 1) from day 35 until the end of the study, accompanied by microscopic lesions of minimal severity, described as plaque-shaped decomposition products in isolated dilated myelin sheaths in the thoracic spinal cord. Similar lesions of somewhat greater severity (slight, grade 2) were found in the lumbar spinal cord of another bird, which also showed continuing, progressive ataxia from day 28, worsening to moderate severity by the end of the study. A third bird had cerebral cortex glial-cell nodules and a perivascular-cell infiltrate of minimal to slight severity, with no obvious motor deficits. Taken together, these findings provide evidence of delayed neurotoxicity, even in the small number of birds examined. QA and GLP statements were provided. The study protocol was similar to that recommended in the Subdivision F guidelines (October 1982) of the Environmental Protection Agency (Ebert & Mayer, 1988).
Technical-grade triazophos (purity, 96.8%) was tested for its potential to cause acute delayed neurotoxicity in white Leghorn hens at doses chosen on the basis of the results of the study described above (Ebert & Mayer, 1988). The test material emulsified in sesame oil was administered as a single dose of 12 mg/kg bw by gastric intubation to 15 fasted hens. Two additional groups of six hens were similarly dosed with TOCP at 500 mg/kg bw as positive controls or with sesame oil at 1 ml/kg bw. Shortly before and after dosing, single doses of atropine sulfate (10 mg/kg bw) and pyridine-2-aldoxime methoiodide were injected into the peritoneum, as needed, as an antidote to cholinergic symptoms. After a 21-day observation period, birds given triazophos or the vehicle were challenged and observed for an additional 21 days before the end of the study on day 43. Antidote was then administered again to the group given triazophos. TOCP was not administered again, and the TOCP-treated birds were killed on day 21 or 23. During the study, the birds were observed daily for deaths and clinical signs of toxicity; a graded evaluation of ataxia was conducted daily and twice weekly outside the cage, as part of a forced motor activity examination; body weights and food consumption were measured, and all birds were evaluated macroscopically and microscopically after perfusion in situ with a 4% formalin solution. Haematoxylin and eosin, cresyl violet-Luxol fast blue and Azan stains were used in a routine evaluation of paraplast-embedded brain, spinal cord and peripheral nerves, and segments of the right and left tibial nerve were stained with Oil red (frozen sections) or Sudan black (for multiple fibre teasing) for additional analysis. The tissues examined were cerebrum, mesencephalon (with quadrigeminal plate), cerebellum with rhombencephalon, pons and part of the medulla oblongata, cervical, thoracic and lumbosacral spinal cord and proximal and distal bilateral segments of the sciatic nerve (lumbosacral plexus) and the tibial nerve. Ultrathin sections of the left sciatic nerve fixed in gluteraldehyde and osmium tetroxide, embedded in Epon and stained with Azur II, were also prepared. Neither cholinesterase nor NTE activity was measured.
All birds in the vehicle control group survived the study with no unusual clinical findings; the birds tended to gain weight as the study progressed. There were no macroscopic findings, and the assessment for ataxia was negative. Four of six birds had no histopathological lesions, one of six had minimal (grade 1) plaque-type decomposition with lipid often transformed in the frozen section of the right tibial nerve, and another bird had perivascular round or round-cell infiltrates of minimal severity in the cerebral hemisphere, cerebellum and medulla and lumbosacral spinal cord. None of the birds in the positive control group died. Signs of ataxia began on days 8–12 in all six birds and increased to severe levels or paralysis as the study progressed. There were no macroscopic pathological findings. Microscopic evaluation revealed minimal-to-severe lesions considered to be consistent with delayed neuropathy in all birds, such as torpedo-like axonal swellings in the thoracic spinal cord and plaque-shaped or globular decomposition of the fibres, often with lipid transformation in the peripheral nerves, and proliferation of Schwann cells and myelinophagocytosis. Indications of fibre decomposition were seen in the tibial nerve in most birds with all four methods of tissue preparation used; however, there was no strict correlation between the occurrence or severity of neuropathological findings in the tissues of a given bird or in a given degree of motor disturbance. The absence of lesions in the lumbar spinal cord was considered to be due to the area sampled. All the triazophos-treated hens survived to the end of the study, and most had some clinical signs during the first 1–3 days after dosing, such as standing on hocks, reduced spontaneous activity, ruffled feathers and ataxic gait. Although their body-weight gain was generally similar to the average for the control group, they appeared to eat less food on a week-to-week basis. The assessment for ataxia was negative in all birds, and no macroscopic lesions were found. The histopathological examination showed no obvious lesions in seven of the 15 hens, but another seven had perivascular round-cell infiltrates in the cerebral hemisphere, which was of slight severity (grade 2) in several birds and of minimal severity (grade 1) in the rest. This incidence was higher than in the vehicle control group (1/6, minimal severity) and the TOCP-treated group (2/6, slight-to-severe). The pathologist considered that infiltrates of this type were spontaneous and noted that they had frequently been seen in control groups in other studies and did not correlate with typical lesions found after organophosphate poisoning. One triazophos-treated bird with cerebral infiltrates also had glial-cell nodules and/or perivascular round or round-cell infiltrates of slight or unspecified severity in the thoracic spinal cord and the sciatic nerve. Another bird with cerebral infiltrate had plaque-type decomposition of the isolated fibres of a single axon of minimal severity (grade 1) in the teased fibre preparation only of the right tibial nerve, which was considered by the pathologist to be artefactual. Single nerve fibre decomposition was also seen in the frozen section of the tibial nerve of one hen given the vehicle alone. No conclusive indications of delayed neurotoxicity were found under the conditions of this study. Although one triazophos-treated bird had some single fibre plaque-type decomposition, the grade was minimal, an artefactual cause could not be excluded, the bird showed no signs of delayed ataxia and fibre decomposition in this nerve was also noted in one control bird. Neverthless, NTE activity was not measured in this study, and the toxicological significance of the increased incidence of cerebral hemisphere perivascular round-cell infiltrates in triazophos-treated birds relative to those given the vehicle or TOCP was not clearly established. QA and GLP statements were provided, and the study protocol was similar to that recommended in Subdivision F guidelines (October 1982) of the Environmental Protection Agency (Ebert, 1989).
In a range-finding study of delayed neurotoxicity with measurement of NTE, groups of five white Leghorn hens (SPF quality) received diets containing technical-grade triazophos (purity, 96.8%) at a concentrations of 0, 50, 100, 150 or 200 ppm for 20 days, equivalent to overall average nominal intakes of 0, 3.2, 7.7, 10 and 11 mg/kg bw per day. The day after the last treatment, three hens per group were necropsied, and NTE activity was measured in brain and spinal cord of the remaining two hens in each group (these birds were not necropsied). No positive control group was used. Doses were selected on the basis of the results of a study in which an LD50 value of 7.4 mg/kg bw was obtained. The birds were monitored for viability, deaths, food and relative food consumption and body-weight changes. A graded evaluation of ataxia (forced motor activity for behavioural abnormalities, locomotor ataxia and paralysis) was performed daily in the cage and twice weekly outside of the cage. The birds that were necropsied were first perfused with a neutral buffered 4% formaldehye solution; however, although tissues were taken from the brain, spinal cord and peripheral nerves, they were not examined histopathologically. Cholinesterase inhibition was not measured.
There were no deaths or clinical signs of toxicity. All groups of birds, including the control group, lost weight during the study, with decreases at the end of the study relative to values at the start of the study of –9.2% to –17%; these were not statistically significant. Notable (but not statistically significant) intra- and intergroup variation in food consumption and relative food consumption was observed throughout the study due to food wastage. In addition, notable variation was observed among groups in week-to-week food consumption and relative food consumption, with wide variation in food intake, such that dosing was not constant over time. The calculated overall nominal intakes of the test substance were thus expressed as averages for the study. There was little difference in intake by the groups at the two higher dietary concentrations (10 and 11 mg/kg bw per day, respectively). The results of the forced motor activity assessment were negative, and no lesions were found at necropsy. No inhibition of NTE activity was found in the brain, and the values in the spinal cord (93%, 83%, 85% and 89% of control at 50, 100, 150 and 200 ppm) were not clearly different from the values for the control group; however, the absence of NTE measurements for a positive control group precluded evaluation of the assay. Under the conditions of the study, there was no evidence of delayed neurotoxicity, and there were no effects on any of the parameters assessed. The study was only preliminary, however, with a duration of only 20 days, no delay after treatment to allow for possible development of delayed polyneuropathy, no histopathological assessment, no measurement of cholinesterase activity and assessment of only two birds for NTE activity. In addition, owing to food wastage and the cyclical eating habits of the birds, the intake of the test material was variable and difficult to assess. The NOAEL was > 11 mg/kg bw per day, the highest dose tested. A QA statement and a statement of compliance with GLP were provided. The study did not follow exactly the guidelines of the Environmental Protection Agency for the inclusion of NTE assays, but this was a range-finding study (Ullman et al., 1991a).
Groups of 10 domestic white Leghorn hens (SPF quality) received diets containing technical-grade triazophos (purity, 96.8%) at a concentration of 0, 50, 110 or 250 ppm for 3 months. Owing to food wastage and a natural tendency for weekly cycles of high and low food consumption, the intakes of the test material were variable and only average daily intakes could be estimated (except for the control group). These were 0, 3.9, 9.6 and 15 mg/kg bw per day at 0, 50, 110 or 250 ppm, respectively. No concurrent positive control group was included. A study with TOCP conducted at about the same time was used for comparison, although this was limited, as the route of administration was different (Ullmann et al., 1991b). In that study, TOCP was administered by gavage to groups of 10 hens at a dose of 0, 10 or 20 mg/kg bw per day for 3 months and at 50 mg/kg bw per day for only 28 days, stopped because of overt symptoms of neurotoxicity associated with ataxia of delayed onset. NTE and plasma cholinesterase were assayed at week 12 for hens at doses of 0–20 mg/kg bw per day, and NTE alone was assessed at week 4 for hens at 50 mg/kg bw day. Only birds given 0, 10 and 20 mg/kg bw per day were examined histologically. In the study in which triazophos was given in the diet, the birds were monitored for viability, deaths, clinical signs of toxicity, food consumption and body-weight changes. Graded forced motor activity was assessed daily in the cage and twice weekly out of the cage with respect to locomotor ability, ataxia, behavioural abnormalities and paralysis. Plasma cholinesterase activity was measured in all birds before treatment and in weeks 7 and 13. At the end of the study, all birds were necropsied, perfused with neutral buffered 4% formaldehyde solution and examined histologically. A four-tier system for neuropathological lesions of the spinal cord and peripheral nerves was used on 5-µm sections of the cerebrum, cerebellum, medulla oblongata, upper cervical bulb, mid-thoracic and lumbo-sacral region and right and left sciatic and tibial nerves fixed in 4% buffered formaldehyde, embedded in paraffin and stained with haematoxylin and eosin, Bodian silver stain for axons and Luxol fast blue for myelin sheaths. NTE activity was not measured.
One control bird died on day 21 of an unidentified cause after losing weight; it showed no symptoms or remarkable pathological findings. One bird at 250 ppm died on day 76 after losing weight rapidly over the previous few weeks and presenting with what were described as symptoms of delayed neurotoxicity. Food consumption varied greatly; overall, however, the group at 250 ppm ate much less (perhaps about half as much ) than the other groups, and the differences were often statistically significant compared with controls. Owing to the variation in food consumption, the actual doses received by the birds were difficult to determine. While the control group had an overall weight gain of 9%, the birds at 50, 110 and 250 ppm showed decreases in body-weight gain of 4%, 6%, 8% and 30%. Restless, excited behaviour was observed relatively consistently in all birds from week 4. Two birds with symptoms consistent with delayed neurotoxicity, one of which died, showed sedated behaviour towards the end of the study. Plasma cholinesterase activity was statistically significantly inhibited by 80–95% in all triazophos-treated groups, at both 7 and 13 weeks. Except for one bird at 50 ppm that was considered to have slightly positive outcomes (grade 2 or 1) on days 83 and 84, no effects were seen in the forced motor activity test at 50 or 110 ppm. A grade 1 score in this test was seen in one bird at 250 ppm on day 71, which progressed to a score of 8 (bird unable to stand) over the next 5 days, at which point it died. Histopathological examination showed that this bird had some disruption, fragmentation and distortion of a few axons, with minimal changes in the myelin sheath (grade 2) in cervical, thoracic and lumbar spinal cord sections and grade 1 findings in a tibial nerve section. A second bird at 250 ppm also had positive forced motor activity scores from day 71 (grade 1), then none for several days, grade 1 score again on day 78, none for several days, then a grade 2 score (slight incoordination and occasional stumbling or wing-drooping especially after exertion), after which no positive response was seen until the end of the study. The histopathological appearance of this bird was notable, as it had not only grade 2 lesions in the cervical and thoracic spinal cord but also a grade 3 lesion (more axons involved and more disruption of the myelin sheath than in grade 2) in the lumbar spine, reported to be similar to that seen with TOCP.
In the study with TOCP, lesions said to be typical of TOCP neurotoxicity were found only in the group at 20 mg/kg bw per day and only in the spinal cord (up to grade 3) and peripheral nerve (up to grade 2). No ataxia was found in this group, but NTE activity was statistically significantly inhibited by 77% in brain and by 70% in spinal cord, while plasma cholinesterase activity was statistically significantly inhibited by 50%. The histopathological findings seen at 10 mg/kg per day TOCP were considered by the pathologist not to be treatment-related. NTE activity was statistically significantly inhibited by 63% in brain and by 50% in the spinal column, with statistically significant inhibition of plasma cholinesterase activity by 28%. Birds given 50 mg/kg bw per day TOCP were not examined histologically. The inhibition of NTE activity could only be estimated, at 93% in brain and 87% in spinal cord, as there was no value for concurrent controls, since dosing with TOCP in this group was started later than for the other groups. Plasma cholinesterase activity was estimated to be inhibited by 47%.
In the study conducted with trazophos in the diet, the control group appeared to have a higher incidence of lesions than in other studies, even in those in which hens from the same source were used. Almost all the control birds were reported to have grade 1 lesions in spinal or peripheral tissues, while grade 2 findings were relatively rare. A similar pattern of scores and frequency and distribution of lesions was reported for birds at 50 ppm, and a slight increase was found in grade 2 scores. At 250 ppm, the frequency of grade 2 scores was higher, and a grade 3 score was observed in one bird with delayed motor deficits. No clear histopathological effect was seen at 110 ppm. At 250 ppm, therefore, treatment-related neuropathological changes were observed in the spinal cord and peripheral nerve, and two birds in this group had motor symptoms characteristic of delayed neurotoxicity. The NOAEL was 110 ppm on the basis of neurotoxicity (a relative increase in the frequency of higher grade histopathological lesions and delayed motor function deficits associated with the death of one bird) at 250 ppm. The corresponding intake of triazophos at 110 ppm, of 9.6 mg/kg bw per day, is only an estimate because of food wastage and may not be reliable. The possibility that the findings in this study were an indication of treatment-related delayed neurotoxicity could not be dismissed. QA and GLP statements were provided. The study protocol approximated a guideline (Ullmann et al., 1991c), but had deficiencies.
In a preliminary study to determine plasma and brain cholinesterase activities as a basis for dose selection in a subsequent study, a single dose of technical-grade triazophos (purity, 94.4%) in corn oil was administered at a dose of 5 or 10 mg/kg bw by gavage to groups of two domestic white Leghorn hens free from viral diseases and medication and with no abnormalities of gait. A third group of two hens was similarly dosed at 750 mg/kg bw with TOCP in the same vehicle as a positive control. Another control group of five hens was left untreated. Plasma cholinesterase activity was assessed in the triazophos- and TOCP-treated groups, with butyrylthiocholine iodide as the substrate, 90 min before treatment and 6 and 48 h after dosing. Brain cholinesterase activity (sample site not specified), with acetylthiocholine iodide as the substrate, was measured 48 h (day 3) after dosing in triazophos- and TOCP-treated groups and 10 days after dosing in the untreated birds. Inhibition of plasma cholinesterase activity was calculated from individual values before and after treatment, and inhibition of brain cholinesterase activity in each treated group was calculated relative to the values for the untreated control group. Birds were observed for deaths, clinical signs of toxicity and body-weight changes.
There were no deaths in any group. Triazophos- and TOCP-treated birds lost weight during the study; the changes in body weight in untreated birds were not reported. The clinical signs of toxicity in birds given triazophos at the lower dose were excitement, restlessness, fright, sedation, and diarrhoea; at the higher dose, ataxia, dyspnoea and miosis were also seen. Birds given TOCP showed fright and diarrhoea. At the lower dose of triazophos, plasma cholinesterase activity was inhibited by 80–86% 6 h after dosing, and plasma and brain cholinesterase activities were inhibited by 58–65% and 0–38%, respectively, 48 h after dosing. At the higher dose of triazophos, plasma cholinesterase activity was inhibited by 84–90% 6 h after treatment, and plasma and brain cholinesterase activities were decreased by 50–69% and 28–35%, respectively, 48 h after treatment. TOCP inhibited plasma cholinesterase activity by 54–73% at 6 h and 74–84% at 48 h and brain cholinesterase activity by 26–35% 48 h after treatment. A NOAEL for inhibition of brain and plasma cholinesterase activity was not identified. QA and GLP statements were not provided for this preliminary study. The protocol was reasonable for the intent of the study (Mahl, 1992a).
In a preliminary study to estimate a lethal dose range for testing for acute delayed neurotoxicity, technical-grade triazophos (purity, 94.4%) in corn oil was administered by gavage at a single dose of 10, 30, 50 or 70 mg/kg bw to groups of two domestic white Leghorn hens (SPF quality). Groups of two hens received TOCP in corn oil at 750 or 1000 mg/kg bw as positive controls. The birds were observed for 21 days after dosing. During the study, body-weight changes were monitored, and the birds were observed for deaths, viability, neurobehavioural changes and clinical signs of toxicity. A neurological examination was conducted daily for behavioural irregularities, abnormal body carriage, forced motor activity (ataxia) and paralysis, sedation, pupil diameter, lachrymation and salivation, and observations outside the cage were made on days 8, 11 and/or12, 14 and/or 15, 16, 20 and 22. All birds were necropsied at the time of death or at the end of the study on day 22 after dosing. They were not examined histologically.
The mortality rates were 0%, 50%, 50% and 100%, respectively, at 10, 30, 50 and 70 mg/kg bw. All deaths occurred within 1 h of dosing. There were no deaths at the lower dose of TOCP, but both birds given 1000 mg/kg bw were killed for humane reasons on day 20 because of treatment-related effects (primarily severe ataxia). Clinical changes (including sedation, ataxia, tonic and clonic spasms, dyspnoea and salivation) in birds treated with triazophos began at doses > 50 mg/kg bw shortly after dosing. Sedation and ataxia continued to be observed in survivors at 30 and 50 mg/kg bw for up to 2 days. Ataxia was observed in the group given the lower dose of TOCP on day 15 and in that given the higher dose on day 12, and the severity increased during the observation period. The birds given the higher dose of TOCP also exhibited restlessness and agitated behaviour during this time. Some decrease in body weight was seen at various times in all triazophos- and TOCP-treated groups of birds that survived past the day of dosing. At necropsy, triazophos-treated hens that died during the study were found to have cardiac dilatation, but the lungs were not collapsed. No macroscopic effects were observed in birds that died intercurrently or were killed at the end of the study. The LD50 was calculated by logit estimation to be 33 mg/kg bw. QA and GLP statements were not provided. Although the study was not conducted to meet a specific guideline, the protocol was generally consistent with that recommended in Subdivision F guidelines (March 1991) of the Environmental Protection Agency (Mahl, 1992b).
In a study to assess delayed neurotoxicity in birds that were not receiving pharmacological protection from the cholinergic effects of treatment, groups of 15 white Leghorn hens received technical-grade triazophos (purity, 94.7%) in corn oil as a single dose of 0, 2.5, 5 or 10 mg/kg bw by gavage. An additional group of 15 hens was similarly dosed with TOCP at 750 mg/kg bw as a positive control. The doses were based on the results of previous studies. Nine birds from each group were selected for measurement of brain cholinesterase activity and brain and spinal cord NTE activity, with one-half brain for each assay; three birds per group were tested at 48 h and 10 and 22 or 23 days after dosing, and the remaining six birds per group were subjected to histopathological examination after a 21- or 22-day observation period. Birds that died or were killed in extremis were also examined microscopically. Plasma cholinesterase activity was assessed in all birds before treatment, 24 and 48 h after dosing and in any remaining birds of the original 15 per group 8, 10 and 22 or 23 days after dosing. All birds were observed for viability, deaths and body-weight changes, and daily for clinical signs of toxicity, behavioural or neurological abnormalities, locomotor disturbances and paralysis, including a graded neurological examination and forced motor activity (ataxia) assessment outside the cage on days 5, 8, 11, 14, 17, 21, 22 and 23. The report stated that food consumption was not determined because the hens wasted food. At the end of the study, all birds were necropsied and examined macroscopically. After whole-bird perfusion with 4% neutral phosphate-buffered formaldehyde, a histopathological examination with a four-tier graded system of scoring for neuropathological lesions of the spinal cord and peripheral nerves based on the method of Prentice & Roberts (1983) was performed on 4-µm sections of the cerebrum, cerebellum, medulla oblongata, cervical, thoracic and lumbar spinal cord and right and left sciatic and tibial nerves fixed in 4% buffered formaldehyde, embedded in paraffin, and stained with haematoxylin and eosin, Luxol fast blue and Nissel and silver impregnation.
One hen in the vehicle control group died of unknown causes on day12, two birds given TOCP were killed with severe paralysis on day 2, and two birds at 10 mg/kg bw triazophos died 2 and 18 h after dosing, possibly of causes related to cholinergic toxicity. The two birds at 10 mg/kg bw died too early in the study for a meaningful histopathologial evaluation. Birds given the vehicle alone occasionally showed restless, excited or frightened behaviour, and birds given the lowest dose of triazophos also showed frightened behaviour occasionally. Statistically significant decreases in body weight were observed at 10 mg/kg bw triazophos and with TOCP. Clinical findings attributable to treatment, which resolved within a few days to a week, were seen at 5 mg/kg bw triazophos, including ataxia and sedation in addition to an increased incidence of frightened behaviour. At 10 mg/kg bw triazophos, dyspnoea, abnormal posture, ataxia and diarrhoea occurred, in addition to sedation and frightened, restless and excited behaviour. Frightened, restless or excited behaviour was occasionally seen in these two groups after the first week. Vehicle control and triazophos-treated birds showed no clinical manifestations of delayed neurotoxicity or signs in the assessment of forced motor activity. Birds given TOCP showed frightened, restless or excited behaviour at various times during the study and some diarrhoea early on. Ataxia consistent with delayed neuropathy was first seen in all surviving birds in this group on day 14 or 15, which increased in severity from slight or minimal to moderately severe or severe with time. Plasma cholinesterase activity was statistically significantly inhibited relative to concurrent controls, by 77%, 27%, 37% and 61%, respectively, for the group given TOCP and those given the three doses of triazophos 24 h after dosing and by 75%, 20%, 31% and 36% in the same groups 48 h after dosing. After 8 days, the enzyme activity in these groups had largely recovered, although that of the group given TOCP was still statistically significantly inhibited by 24%. No statistically significant inhibition of brain cholinesterase activity was observed in any group, including that given TOCP, at any time at which it was measured. NTE activity in brain and spinal cord was statistically significantly inhibited by 81–82% in the group given TOCP at 48 h and by 24% and 50%, respectively, after 10 days; statistically nonsignificant inhibition by 17–20% was seen after 21 days. In triazophos-treated birds, NTE activity in spinal cord was not inhibited at any dose or time. Brain NTE activity was statistically significantly increased by 40–50% in all triazophos-treated groups at 48 h, but this was ascribed by the authors to a low value in concurrent controls at this time. Macroscopic examination revealed no remarkable changes. Light microscopy showede evidence of neurotoxicity in all six TOCP-treated birds. All birds had slight (grade 2) myelin breakdown in the cerebellum (tractus spinocerebellaris), and four of the six had minimal-to-slight (grade 1–2) single axon degeneration. Lesions described as axonal degeneration characterized by ballooning fragmentation or a powdered appearance with silver impregnation, digestion chambers or minimal mononuclear infiltration were observed at grades of minimal to slight in one or more spinal cord areas in all birds, in the sciatic nerve (mostly the right nerve) of five birds at grades of minimal to slight and in the tibial nerve of all six birds with minimal to moderate severity (grade 1–3). In triazophos-treated birds, lesions described as degeneration of single axons of minimal (grade 1) severity were observed in the thoracic spinal cord and/or tibial or sciatic nerve of two birds at the lowest dose, in the lumbar spinal cord of one bird at the intermediate dose and in the thoracic or cervical spinal cord of three birds and in the tibial nerve of another at the hghest dose. The same lesion was observed in the lumbar spinal cord or tibial nerve of two birds in the vehicle control group, which also had some mononuclear infiltrate. None of the findings in triazophos-treated hens was considered by the pathologist to be treatment-related. Under the conditions of this study, there was no evidence that triazophos caused acute delayed neurotoxicity. QA and GLP statements were provided, and the study was conducted in accordance with guidelines (1991 addendum) of the Environmental Protection Agency (Mahl, 1992c).
The histopathological findings in the study in which triazophos was given to hens for 3 months in the diet (Ullmann et al., 1991b) and in the study in which TOCP was given for 3 months by gavage (Ullman et al., 1991c) were re-evaluated by a group that included the original consultant pathologists and pathologists representing the sponsor of the chemical at that time and the laboratory where the studies were conducted. The re-evaluation included slides for hens given TOCP at 50 mg/kg bw per day, which had not been available at the time of the original analysis. These slides were considered to illustrate the Wallerian degeneration thought to be characteristic of organophosphates better than the slides from hens given the lower dose of TOCP (20 mg/kg bw per day). Members of the group, separately and together, compared the slides for birds at the higher dose of triazophos and for controls with those from the study with TOCP and concluded that the findings with triazophos did not represent lesions characteristic of delayed neuropathy, especially when compared with the lesions seen after 50 mg/kg bw per day TOCP, which represented an extreme response to treatment. The group considered that the histopathological scores for the group given the higher dietary concentration of triazophos (250 ppm) were slightly increased in incidence and severity, chiefly in the spinal cord, when compared with concurrent controls but that the lesions observed were of a type seen in both control and treated groups and were not comparable in severity and incidence to those seen with TOCP. The absence of references in the literature indicating a relationship between exposure to triazophosphates and delayed neurotoxicity was mentioned in support of this conclusion.
In addition, the group apparently considered previously unavailable data from other unspecified studies with triazophos, which purportedly provided evidence of lack of delayed neurotoxic potential for the chemical. The nature and source of this supplementary information was not clear. Comments in the report on the possible nature of the lesions in the study with triazophos included the opinions that they might represent normal variation in background lesions, that some findings were preparation artefacts due to large cross-cut fibres and that the lesions might be indicative of spontaneous neuropathy since mononuclear cell infiltrates were sometimes observed. The cause of the motor deficits of delayed onset and the associated death of hens at the highest dietary concentration of triazophos were not specifically discussed. Although the re-evaluation report cited the lack of NTE activity in the 20-day study at all concentrations tested up to 200 ppm, this preliminary study with no post-treatment observation period showed no effects on any parameter investigated, included evaluation of only two birds per group for NTE activity, did not include measurement of blood cholinesterase activity as an indication of absorption or brain cholinesterase activity, included no histopathological evaluation and had no positive control group to substantiate the sensitivity of the NTE assay. A subsequent study with relatively low single doses of triazophos showed no inhibition of NTE or brain cholinesterase activity, while the concurrent TOCP positive control did; however, this study did not include multiple doses and doses only up to 10 mg/kg bw per day were used.
In two studies, female Wistar or Glaxo rats were treated by gavage with technical-grade triazophos (purity not specified) in arachis oil at a single dose of 87 mg/kg bw or triazophos (purity, 94.1%) in 2% starch mucilage at a single dose of 10 mg/kg bw. The two doses were estimated to be at or above the LD50. Various potential antidotes or antidote combinations were then administered intraperitoneally 1.5–10 min after treatment to investigate which decreased the mortality rate during an observation period of 72 h or 14 days. Further doses of the antidotes were given if clinical signs of toxicity reappeared after temporary amelioration. Of the substances tested, combination therapy with atropine sulfate and pyridine-2-aldoxim methoiodide or atropine sulfate and obidoxime chloride was more effective than no treatment or treatment with atropine sulfate alone or atropine methylnitrate (reported to have primarily peripheral activity) alone. No QA or GLP statement was provided. The studies were not performed to meet a specific guideline, but the protocol was reasonable for the purposes intended (Cohen, 1971; Ehling, 1993).
Several studies were conducted with 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole, a metabolite of triazophos. All were consistent with guidelines of the Environmental Protection Agency or the OECD and were compliant with QA and GLP.
(i) Acute toxicity
The LD50 of technical-grade 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (purity, 99.9%) in starch mucilage given orally to male and female Wistar rats was > 5000 mg/kg bw. There were no deaths. Clinical signs starting about 10 min after treatment and ending by day 3 after dosing included decreased spontaneous activity, high-legged gait, contracted flanks and squatting position (Diehl & Leist, 1986b).
(ii) Primary ocular irritation
1-Phenyl-3-hydroxy-(1H)-1,2,4-triazole technical (purity, 99.2%) was placed at a dose of 100 mg into the conjunctival sac of three female New Zealand white rabbits for 24 h, and then washed out. The rabbits showed injected conjunctival blood vessels and a clear–colourless ocular discharge starting soon after treatment and clearing by 72 h after dosing. The substance was considered to be minimally irritating to the eye. No clinical signs of toxicity were observed (Hammerl, 1997).
(iii) Mutagenicity
Technical-grade 1-phenyl-3-hydroxy-(1H)-1,2,4-triazole (purity, 99.2–99.3%) suspended in dimethyl sulfoxide was tested for mutagenic potential in vitro in three studies with and without a metabolic activation system. In a test for reverse mutation in Salmonella typhimurium strains TA100, TA1535, TA1537 and TA98, the substance was mutagenic only in TA98 in the presence of metabolic activation. It did not induce gene mutation at the Hprt locus or cause chromosomal aberrations in Chinese hamster lung V79 cells (Muller, 1997, 1998; Stammberger & Graser, 1999).
Four preliminary or pilot studies were performed with triazophos in humans with a view to conducting a larger study.
In the first preliminary study, triazophos (purity, 90%) initially prepared in ethanol and then diluted with tap water to provide single nominal doses of 0.012 and 0.062 mg/kg bw per day in a dose volume of approximately 100 ml was administered sequentially to one 41-year-old man orally. The lower dose was administered on day 1 and the higher dose on days 2, 3 and 4, followed by a 7-day recovery period. Blood samples for determination of plasma and erythrocyte cholinesterase activities by a modification of the Ellman technique (Ellman et al., 1961) were taken from the finger tip before dosing on all treatment days, 0.5, 1 and 2 h after dosing on days 1 and 2, and 2 and 5 h after dosing on days 3 and 4. Samples were also taken on recovery days 3 and 7. No apparent change in plasma cholinesterase activity was found on days 1 and 2, but decreases estimated (because of an uncertain baseline) at 20% and up to 34% were observed on days 3 and 4, respectively. Some residual depression by 21% and 14% was found on days 3 and 7 of the recovery period, respectively. Treatment had no apparent effect on erythrocyte cholinesterase activity. The man experienced headache for 3 days from day 2 of dosing to day 1 after dosing was stopped, which might have been related to treatment. Under the conditions of this study, an NOAEL could not be identified. No GLP or QA statement was provided for this preliminary study (Leegwater, 1971).
In a second preliminary study, triazophos (purity, 90%) was initially dissolved in ethanol and then diluted with water to provide a single nominal doses of 0.012, 0.03 or 0.05 mg/kg bw per day and given to two male and two female volunteers, aged 40–50 years, for 5 consecutive days. After a 2-day rest, the men were given the intermediate dose for 5 days, and then, after another 2-day rest, were given the highest dose for an additional 5 days. They were then allowed to recover for 2 weeks. Plasma and erythrocyte cholinesterase activities were measured in blood taken from the finger tip twice before dosing, on days 2 and 4 of each treatment regimen and twice during the recovery period. A modification of the Ellman et al. (1961) method was used. It was not reported whether subjects were asked about any reactions to treatment. Cholinesterase activity was not determined in one woman on one of the days before treatment and was not assessed in men on day 2 of administration of the lowest dose. In comparison with pre-treatment values, treatment with 0.012 mg/kg per day on day 4 had no apparent effect on plasma cholinesterase activity in either sex, and the baseline activity was similar to pre-treatment values in men at the intermediate dose. On days 2 and 4 of treatment with 0.03 mg/kg bw per day, plasma cholinesterase activity in men was decreased by 11–17%. After the rest period, plasma cholinesterase activity was still somewhat depressed and was further decreased by day 4 of treatment with 0.05 mg/kg bw per day, by about 40% relative to pre-treatment values. The values had not completely returned to baseline by the end of the recovery period. Treatment had no apparent effect on erythrocyte cholinesterase activity in either sex. The NOAEL for inhibition of plasma cholinesterase activity was 0.012 mg/kg bw per day and that for inhibition of erythrocyte cholinesterase activity was a 0.05 mg/kg bw. No GLP or QA statements were provided for this preliminary study (Leegwater, 1972).
In a third preliminary study, technical-grade triazophos (purity, 90%), initially prepared in 100% ethanol and stored as a dilution in 100% or 30% ethanol before dosing, was administered orally over 3 weeks at a nominal dose of 0.012 mg/kg bw per day in 150 ml of lime juice to two male and three female healthy volunteers aged 18–23 years from the staff of the laboratory where the study was performed. Lime juice without the test material was administered to participants for about 10 days before dosing. The volunteers were treated on the 5 days of the work week, with a break on weekends. They were observed for overt signs of toxicity during dosing and during an additional treatment-free week. A medical history and medical interview were taken before study initiation and at the end of the study. A physical examination and blood and urine analyses were conducted before treatment and about 13 days after treatment had stopped. Plasma and erythrocyte cholinesterase activities were measured. During treatment, blood samples were taken 24 h after the previous day’s dose, and cholinesterase activity was measured four times before dosing, two or three times during each week of treatment and three times in the week after treatment. All five participants completed the study.
Treatment had no apparent effect on either plasma or erythrocyte cholinesterase activity, although there was intra- and interindividual variation in the readings. No overt reactions were noted and no changes in health status occurred during the study, although one woman had headache 1.5 h after dosing on the first 3 days, which could not be attributed definitively to treatment. This woman was described as having a tendency towards anaemia, which was corroborated in haemoglobin readings before and after dosing. Some individuals commented that the taste of the lime juice changed at a certain point, corresponding to the initiation of dosing with the test material. Under the conditions of the study, treatment had no effects. No GLP or QA statements were provided for this preliminary study (Cooper & Street, 1973).
In a fourth preliminary study, similar to that described above, technical-grade triazophos (purity not specified), initially prepared in 100% ethanol and stored as a dilution in 100% or 30% ethanol before dosing, was administered over 3 weeks at a nominal dose of 0.025 mg/kg bw per day in 150 ml of lime juice to three male and two female healthy volunteers, 21–25 years of age, from the staff of the laboratory where the study was performed. Lime juice without the test material was administered to participants for about 10 days before dosing. The subjects were treated during the 5 days of the work week, with a break on weekends. They were observed for overt signs of toxicity. After dosing, they were observed for an additional treatment-free week. A medical history and medical interview were taken before the start and at the end of the study. A physical examination and blood and urine analyses were conducted before treatment and about 13 days after treatment had stopped. Plasma and erythrocyte cholinesterase activities were measured. During treatment, blood samples were taken 24 h after the previous day’s dose, and cholinesterase activity was measured four times before dosing, two or three times during each week of treatment and three times in the week after treatment. All five participants completed the study.
No overt reactions were noted, and no changes in health status were found during the study. Some individuals commented that the taste of the lime juice changed at a time corresponding to the beginning of dosing with the test material. Although there was some inter- and intra-individual variation in cholinesterase activity and the sample size was small, a t test on the data for both sexes showed a significant decrease in plasma cholinesterase activity between the period before dosing, weeks 2 and 3 of dosing and the recovery period. A comparison of means for individuals before dosing and values at the beginning or the middle of the recovery period also showed a relative decrease in plasma cholinesterase activity during the study (by 13–28%) for individuals of each sex. A similar comparison for inhibition of erythrocyte cholinesterase activity showed a slight relative decrease (by 9%) in women, although the authors did not find a statistically significant difference in erythrocyte cholinesterase activity at any time. The small sample size and data variation complicated analysis and interpretation of the date, such that an effect on erythrocyte cholinesterase activity could not be established definitively. The NOAEL for inhibition of plasma cholinesterase activity was < 0.025 mg/kg bw per day, and the NOAEL for inhibition of erythrocyte cholinesterase activity was 0.025 mg/kg bw per day. No GLP or QA statements were provided for this preliminary study (Cooper et al., 1973).
In a larger study, triazophos (purity, 88%), initially prepared in and stored as a dilution in 30% ethanol before dosing, was administered over 3 weeks at a nominal dose of 0.0125 mg/kg bw per day in 150 ml of lime juice to volunteers from the staff of the laboratory at which the study was performed. Lime juice without the test material was given to participants for about 1 week before dosing. The volunteers, described as healthy, were 13 men aged 17–59 years (average, 26 years) and 12 women aged 16–51 years (average, 28 years). They were given the test material only during the five days of the work week, with a break on weekends. They were observed for overt signs of toxicity. After dosing, they were observed for an additional 4 weeks. A medical history was taken before study initiation, and a physical examination and blood and urine analyses were conducted before treatment and after the end of the treatment-free recovery period. Subjects were requested to inform the investigators if they felt unwell. Plasma and erythrocyte cholinesterase activities were measured. Blood samples for measurement of cholinesterase activity were taken three times before dosing, three times during weeks 1 and 2 of dosing, four times during week 3 of dosing and three times during each week of the treatment-free period. A 20% depression in individual plasma or erythrocyte cholinesterase activity relative to the mean value for the individual before dosing was considered grounds for discontinuing dosing. All 25 subjects completed the study.
A slight but statistically significant decrease in group mean blood pressure from that before dosing in women and minor alterations in group mean values in either sex for some haematological and clinical chemical parameters were within normal limits for the laboratory and considered not to be related to treatment. About 50% of the women reported urinating more often, with some feeling of urgency during the first week of dosing, which was attributed to psychosocial causes. One man reported feeling tired and listless during the second week of dosing. Although this individual’s plasma cholinesterase activity fell to a limit of concern at the end of the week, the attending physician opted to continue dosing. The plasma cholinesterase activity of this individual did not decrease further during the rest of the study. Another man reporting feeling tired and listless during the second and third weeks of dosing and also had headache, sore throat and soreness in the nose and ear. Two women had diarrhoea or diarrhoea and vomiting during the second week of dosing, causing absence from work. Another man was absent due to gastrointestinal upset during week 4 after dosing. The authors mentioned that gastrointestinal viral-type infections that had affected workers who were not participating in the study might have been a factor in some of the symptoms noted. The plasma cholinesterase activity of two men and one woman dropped rapidly during the first week of treatment, such that dosing was stopped for 6 days. It was re-commenced during treatment week 3, when the enzyme activity rose to levels considered sufficient. Decreased plasma cholinesterase activity at the end of dosing relative to the mean before dosing, by about 20%, was noted in some individuals, whereas little change in plasma cholinesterase activity was seen in others. The activity of some individuals never completely returned to the values before treatment.
Erythrocyte cholinesterase activity was not affected, and no statistically significant changes were observed in cholinesterase activity with the methods used. Evaluation of the symptoms reported suggested that they could generally be explained by non-treatment-related factors. Thus, the urinary urgency and frequency in women, reported only during the first week of dosing, could have had a psychosocial basis; the headache, sore throat and soreness in the nose and ear in one man who also felt tired and listless were consistent with a viral or other type of infection; gastrointestinal symptoms occurred in one man four weeks after dosing was stopped; diarrhoea or vomiting in two women during the second week of dosing did not persist into the third week of dosing; and another man reported feeling tired and listless only during the second week of dosing. Although plasma cholinesterase activity was inhibited in some individuals, the Meeting considered this to be a marker of exposure only and not an adverse effect. The Meeting considered that inhibition of erythrocyte cholinesterase activity is a surrogate for potential effects of organophosphorus compounds on the peripheral nervous system. Since evidence from studies in experimental animals indicates that triazophos acts predominantly peripherally, the absence of inhibition of erythrocyte cholinesterase activity in this study indicates that the clinical findings were not related to treatment. The absence of inhibition of erythrocyte cholinesterase activity in the any of the preliminary studies, lasting up to 3 weeks at oral doses of triazophos > 0.012 mg/kg bw per day, and the lack of any clear-cut treatment-related findings in the two preliminary studies of 3 weeks’ duration in which several male and female subjects were given oral doses of 0.012 and 0.025 mg/kg bw per day, also supports this conclusion. The NOAEL was 0.0125 mg/kg bw per day (Cracknell et al., 1973). No GLP or QA statements were provided, and the protocol followed no specific guideline.
In studies in which a single oral dose of [14C]triazophos was administered by gavage to rats or dogs, absorption was rapid and essentially complete. Most of the radiolabel was excreted within 48–96 h, with a half-life in blood in both species of about 3.5 h. It was excreted primarily in urine. The metabolism in rats and dogs was qualitatively similar, and there did not appear to be a sex difference. Little residual radioactivity was found in the tissues that were analysed. Triazophos was metabolized to 1-phenyl-3-hydroxy-[1H]-1,2,4-triazole, and this compound and its glucuronide and sulfate ester conjugates were the predominant compounds in urine, representing most of the administered dose. In a 12-day study with repeated doses, triazophos showed little potential for bioaccumulation. By analogy with other phosphorothioate organophosphorus compounds, triazophos is probably metabolically activated to the oxon, which inhibits acetylcholinesterase activity.
In mice, rats and guinea-pigs, the acute oral LD50 values ranged from 26 to 82 mg/kg bw, while dogs had higher values: about 500 mg/kg bw in females and > 800 mg/kg bw in males. In studies of acute dermal and inhalation exposure in rats, females were more sensitive than males; the acute dermal LD50 ranged from 1000 to > 2000 mg/kg bw and the acute inhalation (4 h, nose-only) LC50 ranged from 0.45 to 0.61 mg/l. Deaths occurred within minutes to several days after oral administration. The clinical signs included tremor, tonic convulsions, accelerated, laboured or jerky respiration, lachrymation, salivation, saltatory spasms, disequilibrium, hind-limb paralysis, vomiting or retching, diarrhoea and miosis, depending on the species. WHO has classified triazophos as ‘highly hazardous’ (WHO, 2000).
In rabbits, triazophos was not irritating to the eyes or skin; however, dermal and, to a lesser extent, ocular treatment with undiluted material caused some deaths. Triazophos was not a dermal sensitizer in guinea-pigs in either a Buehler or Magnusson and Kligman maximization test.
The most sensitive effect observed after treatment with triazophos is inhibition of erythrocyte cholinesterase activity. In both short- and long-term studies in several species treated orally, cholinesterase was preferentially inhibited peripherally and inhibition of brain cholinesterase activity occurred at doses higher than those at which clinical signs were observed. Hence, inhibition of erythrocyte cholinesterase activity was considered to be an appropriate surrogate for potential effects on the peripheral nervous system.
Systemic effects other than inhibition of cholinesterase activity, if any occurred, were observed in short-term studies in rodents only at the highest doses tested and generally consisted of slight perturbations of haematological and clinical chemical parameters. Other than one unexplained death in a 43-week study in rats, there were no deaths and no clinical signs in these studies. The NOAELs for inhibition of erythrocyte cholinesterase activity were between 1 and 20 ppm (0.08–3 mg/kg bw per day), depending on dose spacing. In a 13-week study in dogs, effects other than inhibition of erythrocyte cholinesterase activity were found only at the highest dose and included moribundity, clinical signs consistent with cholinergic toxicity (such as salivation, diarrhoea, vomiting and tremor), decreased food consumption and loss of body weight, haematological and clinical chemical changes in one or both sexes, hypertrophy of the duodenal wall in all animals and degenerative or inflammatory lesions in the zygomatic gland in males. The NOAEL in this study was 0.3 ppm (equal to 0.01 mg/kg bw per day) on the basis of inhibition of erythrocyte cholinesterase activity.
In a 52-week study in dogs, some animals were reported to have had bronchopneumonia and other signs of illness, which may have confounded interpretation of the results. Persistent diarrhoea was seen in many animals at the highest dose and in one male at the intermediate dose; the possibility that these findings were related to treatment could not be dismissed. Decreased erythrocyte cholinesterase activity (24–32%) was found in males at the intermediate dose. The NOAEL was 0.4 ppm, equal to 0.012 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity.
No effects other than inhibition of cholinesterase activity were found in a long-term study of toxicity in mice. The NOAEL was 6 ppm, equal to 0.83 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity.
In a 2-year study of toxicity in rats, erythrocyte cholinesterase activity was significantly inhibited by > 20% at the two higher doses. Slight perturbations in haematological and clinical chemical parameters seen in one or both sexes at these doses were considered to be related to treatment but to represent only compensatory changes. An increased incidence of pancreatic acinar-cell hyperplasia in males at the two higher doses may have been related to treatment. The NOAEL was 3 ppm, equal to 0.15 mg/kg bw per day.
In long-term studies of toxicity and carcinogenicity in mice and rats, triazophos induced no significant or consistent increase in the incidence of any tumour type. The Meeting concluded that triazophos is not carcinogenic in mice or rats.
The genotoxic potential of triazophos was assessed in an adequate range of tests in vitro and in a test for micronucleus formation in mice in vivo. The Meeting concluded that triazophos is unlikely to pose a genotoxic hazard in vivo.
On the basis of the absence of carcinogenic effects in mice and rats and the overall weight of evidence from the genotoxicity studies, the Meeting concluded that triazophos is unlikely to pose a carcinogenic risk to humans.
In a study of reproductive toxicity in rats, effects on parental animals and pups were observed only at 240 ppm (equal to 12 mg/kg bw per day), the highest dose tested. Clinical signs of toxicity, aggressive behaviour and decreased body weight and food consumption were seen in parents of both generations, and some treatment-related deaths were found among the F1 parents. The only effects on reproduction were some pup losses and decreases in pup body weights during the lactation period. The NOAEL for parental toxicity and reproductive toxicity was 27 ppm, equal to 1 mg/kg bw per day.
The studies of developmental toxicity in rats and rabbits were difficult to interpret owing to the choice of dose and/or the occurrence of illness unrelated to treatment. In rabbits, the combined NOAEL for maternal toxicity in a dose range-finding study and the main study was 4 mg/kg bw per day, on the basis of slight decreases in body weight and food consumption and clinical signs of toxicity at higher doses. The NOAEL for developmental toxicity was 4 mg/kg bw per day on the basis of possible effects on pregnancy outcome at higher doses. No malformations were observed at the highest dose tested in the main study, in rabbits (8 mg/kg bw per day) or in rats (22 mg/kg bw per day).
Several studies were conducted in which single doses were given by gavage to assess the potential of triazophos to induce delayed polyneuropathy in hens. Doses of triazophos of up to 12 mg/kg bw, given with pharmacological protection against cholinergic effects, and rechallenge with triazophos after 3 weeks did not result in behavioural or morphological signs of delayed polyneuropathy. At 50 mg/kg bw, there was some evidence of atypical neuropathology in the spinal cord, and delayed motor activity was observed in two animals. Brain cholinesterase activity and neuropathy target esterase activity were not affected at an oral dose of 10 mg/kg bw without antidotal protection. The potential of triazophos to induce delayed polyneuropathy after repeated oral administration was assessed in hens at a dietary concentration of 0, 50, 110 or 250 ppm. Food intake was variable because of wastage and cyclical eating habits. The results were compared with those seen with tri-ortho-cresyl phosphate in a separate study. At the highest dose of triazophos, delayed motor activity and atypical neuropathological findings in the spinal cord and periphery were reported. The histopathological findings were not typical of the classical Wallerian degeneration associated with organophosphorus-induced delayed polyneuropathy, nor could it be ascertained if the clinical signs were due to inhibition of cholinesterase activity. Neuropathy target esterase was not measured in the main study, but, when it was assessed in a separate 20-day feeding study, no inhibition was observed at doses up to 200 ppm (equivalent to 10 mg/kg bw per day). Although a few animals in the main study showed signs consistent with delayed polyneuropathy, these might well have been due to prolonged inhibition of cholinesterase activity and/or an increase in the frequency of spontaneous lesions in the nervous system due to weight loss or disease. The Meeting concluded that there was no concern for induction of delayed polyneuropathy by triazophos at doses that could be achieved in the human diet.
Several studies were conducted in which volunteers were given triazophos at doses of 0.0125–0.0625 mg/kg bw for up to 3 weeks. In the main study, conducted according to the standards of the time, triazophos administered for 3 weeks, 5 days per week, at a dose of 0.0125 mg/kg bw per day, had no effect on erythrocyte cholinesterase activity. Although signs and symptoms consistent with inhibition of cholinesterase activity were reported by some individuals, these were attributed to non-treatment-related causes, such as gastrointestinal viral-type infections or psychosocial interactions in the absence of inhibition of erythrocyte cholinesterase activity. The NOAEL was 0.0125 mg/kg bw per day, the only dose tested.
The major metabolite of triazophos, 1-phenyl-3-hydroxy-[1H]-1,2,4-triazole, was of low acute oral toxicity in rats (LD50, > 5000 mg/kg bw) and was minimally irritating to the eyes of rabbits. The weight of the evidence from studies of genotoxicity suggested that this metabolite is of no genotoxic concern.
No cases of human poisoning were found in the literature, and no adverse affects were reported among workers at the sponsor’s triazophos manufacturing plant.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of triazophos to fetuses, infants and children.
The Meeting established an ADI of 0–0.001 mg/kg bw on the basis of the NOAEL of 0.0125 mg/kg bw per day, the only dose tested, in the 3-week study in volunteers, in which no effects on erythrocyte cholinesterase activity or clinical signs were observed, and a safety factor of 10. The data on humans were used because the relevant effects in animals were also linked to inhibition of cholinesterase activity. The duration of administration in the study in volunteers was considered to be sufficient to permit maximal inhibition of cholinesterase activity. The ADI was considered to be sufficiently protective against any neurotoxic effect of the chemical, including delayed polyneuropathy.
The Meeting established an acute RfD of 0.001 mg/kg bw on the basis of the NOAEL of 0.0125 mg/kg bw per day in the 3-week study in humans and a safety factor of 10.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya |
Inhibition of erythrocyte cholinesterase |
6 ppm, equal to |
30 ppm, equal to |
|
Carcinogenicity |
150 ppm, equal to |
– |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Inhibition of erythrocyte cholinesterase activity and toxicity |
3 ppm, equal to |
27 ppm, equal to |
|
Carcinogenicity |
240 ppm, equal to |
– |
||
|
Multigeneration study of reproductive toxicitya |
Parental and offspring toxicity |
27 ppm, equal to |
240 ppm, equal to |
|
|
Developmental toxicitya |
Maternal toxicity |
250 ppm, equal to |
– |
|
|
Embryo- and fetotoxicity |
250 ppm, equal to |
– |
||
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
4 mg/kg bw per day |
8 mg/kg bw per day |
|
Embryo- and fetotoxicity |
4 mg/kg bw per day |
8 mg/kg bw per day |
||
|
Dog |
1-year study of toxicitya |
Inhibition of erythrocyte cholinesterase activity and toxicity |
0.4 ppm, equal to |
4 ppm, equal to |
|
Human |
3-week studyd |
Inhibition of erythrocyte cholinesterase activity and toxicity |
0.0125 mg/kg bw per daye |
– |
a Dietary administration
b Highest dose tested
c Gavage
d Oral administration
e Only dose tested
Estimate of acceptable daily intake for humans
0–0.001 mg/kg bw
Estimate of acute reference dose
0.001 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid, essentially complete |
|
Distribution |
Extensive |
|
Potential for accumulation |
Little |
|
Rate and extent of excretion |
Rapid, almost complete within 48–96 h; mainly in urine; half-life in blood, 3.8 h in rats, 3.6 h in dogs |
|
Metabolism in animals |
Cleavage to 1-phenyl-3-hydroxy-[1H]-1,2,4-triazole followed by conjugation with glucuronide and sulfate |
|
Toxicologically significant compounds |
Parent and oxon |
|
Acute toxicity |
|
|
Rat, LD50, oral |
48–82 mg/kg bw |
|
Rat, LD50, dermal |
1000 mg/kg bw |
|
Rat, LC50, inhalation (nose-only) |
0.45–0.61 mg/l |
|
Skin irritation |
Negligible, but undiluted material caused some deaths |
|
Eye irritation |
Minimally irritating, but undiluted material caused some deaths |
|
Skin sensitization |
Negative in Buehler and in Magnusson and Kligman tests |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Inhibition of erythrocyte (but not brain) cholinesterase activity and clinical signs of toxicity at higher doses in animals but not in humans |
|
Lowest relevant oral NOAEL |
0.012 mg/kg per day (1-year study in dogs); 0.0125 mg/kg bw per day (humans) |
|
Genotoxicity |
Unlikely to pose a genotoxic risk in vivo |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Inhibition of erythrocyte cholinesterase activity |
|
Lowest relevant NOAEL |
0.15 mg/kg bw per day (rats) |
|
Carcinogenicity |
Not carcinogenic |
|
Reproductive toxicity |
|
|
Target/critical effect for reproductive toxicity |
Reduced pup viability and weight |
|
Lowest relevant NOAEL for reproductive toxicity |
1 mg/kg bw per day |
|
Target/critical effect for developmental toxicity |
Effects on pregnancy outcome |
|
Lowest relevant NOAEL for developmental toxicity |
4 mg/kg bw per day |
|
Neurotoxicity |
|
|
Delayed neuropathy |
No concern for delayed polyneuropathy at doses relevant to human dietary intake |
|
Medical data |
No human poisoning cases found in the literature, and no adverse affects were reported at the sponsor’s triazophos manufacturing plant. |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.001 mg/kg bw |
3-week, humans |
10 |
|
Acute RfD |
0.001 mg/kg bw |
3-week, humans |
10 |
Allen, T.R., Frei, T., Lütkemeier, H., Vogel, W., Terrier, C.H., Vogel, O. & Armstrong, J. (1989) Chronic oral toxicity, 52-week feeding study in beagle dogs. Unpublished report No. 071548 from RCC, Itingen, Switzerland, 5 July 1989. Aventis document A41631. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Baeder, C., Weigand, W. & Kramer, M. (1976) Report on an embryotoxicity study of triazophos in Wistar rats after oral administration in the feed. Unpublished report No. 76.0436 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 17 September 1976. Aventis document A10854. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Bernstein, D.M., Luetkemeier, H., Vogel, W. & Schlotke, B. (1987) Subacute (28-day) repeated dose inhalation toxicity study in rats. Unpublished report No. 084723 from RCC, Itingen, Switzerland, 16 November 1987. Aventis document A37375. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Block, I., Frei, T.H., Luetkemeier, H., Vogel, W. & Terrier, C.H. (1988) Subchronic oral toxicity 13-week feeding study in beagle dogs. Unpublished report No. 071651 from RCC, Itingen, Switzerland, 22 February 1988. Aventis document A38079. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Bock, R. & Thier, W. (1976) Metabolism and fate of triazophos in rats. Pestic. Sci., 7, 307–314.
Cohen, E.M. (1971) Therapeutic effects of various antidotes against intoxication with compound Hoe 02960 in rats. Unpublished report No. 29.184 from TNO, Rijswijk, Netherlands, 28 September 1971. Aventis document A00104. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Cooper AJ & Street AE (1973) A pilot study to observe the in vivo effects of Hoe 02960 on the cholinesterase activity in man administered 0.0125 mg/kg/day p.o. Unpublished report No. 5565A/72/961 from Huntingdon Research Centre, Huntingdon, England, 23 July 1973. Aventis document A00404. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Cooper, A.J., Haynes, G., Street, A.E. & Rudd, C. (1973) A pilot study to observe the in vivo effects of Hoe 02960 on the cholinesterase activity in man administered 0.025 mg/kg/day p.o. Unpublished report No. 5565B/72/961 from Huntingdon Research Centre, Huntingdon, England, 23 July 1973. Aventis document A00955. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Cracknell, D.D., Hawkes, G. & Street, A.E. (1973) The in vivo effects of Hoe 02960 on the cholinesterase activity in man administered 0.0125 mg/kg/day. Unpublished report No. HAST/73885 from Huntingdon Research Centre, Huntingdon, England, 10 December 1973. Aventis document A00928. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Diehl, K.-H. & Leist, K.-H. (1986a) Hoe 014622—Technical substance. Testing for acute dermal toxicity in the male and female Wistar rat. Unpublished report No. 86.1423 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 13 November 1986. Aventis document A43292. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Diehl, K.-H. & Leist, K.-H. (1986b) Testing for acute dermal toxicity in the male and female Wistar rat. Unpublished report No. 86.0771 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 04 July 1986. Aventis document A35526. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Donaubauer, H.H. (1989) Combined chronic toxicity and carcinogenicity in mice—24 month feeding study. Unpublished report No. 89.0926 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 20 June 1989. Aventis document A41713. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Draize, J.H. (1977) Dermal and eye toxicity tests. In: Principles and Procedures for Evaluating the Toxicity of Household Substances, Washington DC: National Academy of Sciences, pp. 48–49.
Ebert, E. (1989) Testing for acute delayed neurotoxicity in white Leghorn hens. Unpublished report No. 89.1304 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 16 October 1989. Aventis document A42064. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ebert, E. & Mayer, D. (1988) Testing for acute delayed neurotoxicity in white Leghorn hens. Unpublished report No. 88.1767 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 21 December 1988. Aventis document A41235. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ebert, E., Leist, K.-H., Mayer, D. & Langer, K.-H. (1987) [Subchronic range finding test (13-week feeding study) in NMRI mice.] Unpublished report No. 87.0358 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 25 March 1987. Aventis document A35695. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany (in German).
Ehling, L. (1993) Triazophos, substance technical (code: Hoe 002960 00 ZD93 0002); effects of antidotes tested on female Wistar rats. Unpublished report No. 93.0616 from Hoechst AG, Pharma Development Central Toxicology, Frankfurt am Main, Germany, 15 September 1993. Aventis document A51355. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ellman, G.L., Courtney, K.D., Andres, V. & Featherstone, R.M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7, 88–95.
Fumero, S. & Mondino, A. (1980a) Study of the mutagenic activity in vitro of the compound Hoe 02960 with Schizosaccharomyces pombe. Unpublished report from Istituto di Ricerche Biomediche ‘Antoine Marxer’, SpA, Italy, 24 June 1980. Aventis document A21618. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Fumero, S. & Mondino, A. (1980b) Study of the mutagenic activity in vitro of the compound Hoe 02960 with Saccharomyces cervisiae. Gene conversion DNA repair test. Unpublished report from Istituto di Ricerche Biomediche ‘Antoine Marxer’, SpA, Italy, 24 June 1980. Aventis document A21617. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Fumero, S. & Mondino, A. (1981) In vitro study of chromosome aberration induced by the test article Hoe 02960 OH AS204 in cultured humane lymphocytes. Unpublished report from Istituto di Ricerche Biomediche ‘Antoine Marxer’, SpA, Italy, 10 August 1981. Aventis document A23175. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Gericke, D. & Wagner, W.H. (1977) Test for mutagenicity in bacteria strains in the absence and presence of a liver preparation. Unpublished report No. 77.0012 from Hoechst AG, Cancer Research, Frankfurt am Main, Germany, 2 March 1977. Aventis document A13798. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hammerl, R. (1997) AE F014622; substance, technical testing for primary eye irritation in the rabbit. Unpublished report No. 97.0317 from Hoechst AG, Marion Roussel Preclinical Development Germany, Drug Safety, Frankfurt am Main, Germany, 4 July 1986. Aventis document A59500. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1976a) Acute oral toxicity of Hoe 92060 O I AS 101 (active ingredient techn.) to the female SPF-Wistar rat. Unpublished report No. 379/76 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 31 August 1976. Aventis document A27319. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1976b) Test for sensitizing properties of Hoe 02960 OI AS001 in the guinea pig. Unpublished report No. 216/76 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 26 May 1976. Aventis document A11641. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977a) Acute oral toxicity to the male SPF-NMRI mouse. Unpublished report No. 362/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 15 April 1977. Aventis document A12522. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977b) Acute oral toxicity to the female SPF-NMRI mouse. Unpublished report No. 363/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 15 April 1977. Aventis document A12523. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977c) Acute subcutaneous toxicity of Hoe 02960 O I AS101 to the male SPF-NMRI mouse. Unpublished report No. 368/77 from Hoechst AG, Pharma Research Toxikology Frankfurt am Main, Germany, 18 April 1977. Aventis document A28908. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977d) Acute subcutaneous toxicity of Hoe 02960 O I AS101 to the female NMRI-mouse. Unpublished report No. 369/77 from Hoechst AG, Pharma Research Toxikology Frankfurt am Main, Germany, 18 April 1977. Aventis document A30613. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977e) Acute oral toxicity to the male SPF-Wistar rat. Unpublished report No. 77.0360 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 15 April 1977. Aventis document A17875. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977f) Acute oral toxicity of Hoe 02960 0 I AS101 to the female SPF-Wistar rat. Unpublished report No. 359/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 14 April 1977. Aventis document A28912. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977g) Acute oral toxicity to the female SPF-Wistar rat. Unpublished eport No. 77.0361 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 15 April 1977. Aventis document A28911. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977h) Acute oral toxicity of Hoe 02960 0 I AS101 to the male SPF-Wistar rat. Unpublished report No. 358/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 14 April 1977. Aventis document A28913. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977i) Testing for acute intraperitoneal toxicity in the male SPF Wistar rats. Unpublished report No. 77.0364 from Hoechst AG, Pharma Development, Frankfurt am Main, Germany, 15 April 1977. Aventis document A30614. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977j) Testing for acute intraperitoneal toxicity in the male SPF Wistar rats. Unpublished report No. 77.0365 from Hoechst AG, Pharma Development, Frankfurt am Main, Germany, 18 April 1977. Aventis document A30615. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977k) Acute subcutaneous toxicity of Hoe 02960 O I AS101 to the male SPF-Wistar rat. Unpublished report No. 366/77 from Hoechst AG, Pharma Research Toxikology Frankfurt am Main, Germany, 6 June 1977. Aventis document A28910. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977l) Acute subcutaneous toxicity of Hoe 02960 O I AS101 to the female SPF-Wistar rat. Unpublished report No. 367/77 from Hoechst AG, Pharma Research Toxikology Frankfurt am Main, Germany, 6 June 1977. Aventis document A28909. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977m) Acute inhalation toxicity of the aerosol of Hoe 02960 OI AS101 to the female SPF-Wistar rat, 4-h LC50. Unpublished report No. 112/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 16 February 1977. Aventis document A17873. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1977n) Hoe 02960 O I AS101—Irritance to the rabbit skin and eye mucosa. Unpublished report No. 251/77 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 24 March 1977. Aventis document A12483. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1978a) Hoe 02960 I AS 101. Acute oral toxicity to the male SPF-NMRI mouse. Unpublished report No. 135/87 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 21 August 1987. Aventis document A18274. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1978b) Hoe 02960 I AS 101. Acute oral toxicity to the female SPF-NMRI mouse. Unpublished report No. 136/87 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 21 August 1987. Aventis document A18273. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Hollander, H. & Weigand, W. (1987) Triazophos—Substance technical (Code: Hoe 002960 OI ZD93 0002) Testing for acute aerosol inhalation toxicity in the male and female SPF Wistar rat; 4-hour LC50. Unpublished report No. 86.1570 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 12 January 1987. Aventis document A43287. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Kramer, M. & Weigand, W. (1974) Test of triazophos (Hoe 02960) for neurotoxicity in hens. Unpublished report from Farbwerke Hoechst AG, Frankfurt am Main, Germany, 1 February 1974. Aventis document A01142. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Leegwater, D.C. (1971) Preliminary study on the in vivo effect of Hoe 02960 in man. Unpublished report from CIVO/TNO, Zeist, Netherlands, 23 April 1971. Aventis document A00107. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Leegwater, D.C. (1972) In vivo study on the effect of Hoe 02960/ Op 22 on the blood cholinesterase of man. Unpublished report No. R3678 from CIVO/TNO, Zeist, Netherlands, January 1972. Aventis document A00109. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Mahl, A. (1992a) Determination of plasma and brain cholinesetrase activity following single oral application in laying hens in a preliminary study. Unpublished report No. 319792 from RCC Itingen, Switzerland, 14 April 1992. Aventis document A47796. Amendment to document A47658. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Mahl, A. (1992b) Preliminary acute oral toxicity in laying hens. Unpublished report No. 319781 from RCC Itingen, Switzerland, 8 April 1992. Aventis document A47658. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Mahl, A. (1992c) Delayed neurotoxicity study following single oral application with triazophos substance technical in laying hens. Unpublished report No. 316293 from RCC Itingen, Switzerland, 29 April 1992. Aventis document A48482 (report) and A48669 (amendment to report). Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Mayer, D., Weigand, W. & Kramer, M. (1980) Test report on the mutagenicity of Hoe 02960—Active ingredient after oral administration to NMRI mice—Micronucleus test. Unpublished report No. 381/80 from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 8 July 1980. Aventis document A24931. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Muller, W. (1997) AE F014622 bacterial reverse mutation test. Unpublished report No. 97.0440 from Hoechst AG, Marion Roussel Preclinical Development, Germany, Drug Safety, Frankfurt am Main, Germany, 23 July 1986. Aventis document A59501. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Muller, W. (1998) AE F014622 in vitro Chinese hamster lung V79 cell HPRT mutation test. Unpublished report No. 98.0122 from Hoechst AG, Marion Roussel Preclinical Development, Germany, Drug Safety, Frankfurt am Main, Germany, 13 May 1998. Aventis document C000048. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany
Pallen, C. & Lautraite, S. (2002a) Position paper: Triazophos combined toxicity and carcinogenicity study in mice—Complementary statistical analysis. Unpublished report from Bayer CropScience, Sophia Antipolis Cedex, France. Submitted to WHO by Bayer CropScience, Sophia Antipolis Cedex, France.
Pallen, C. & Lautraite, S. (2002b) Position paper: Triazophos combined toxicity and carcinogenicity study in rat—Complementary statistical analysis. Unpublished report from Bayer CropScience, Sophia Antipolis Cedex, France. Submitted to WHO by Bayer CropScience, Sophia Antipolis Cedex, France.
Schollmeier, U. & Leist, K.H. (1989) Triazophos; substance technical (code: Hoe 002960 00 ZD93 0002). Testing for sensitising properties in the Pirbright-white guinea pig in a maximisation test. Unpublished report No. 98.1492 from Hoechst AG, Pharma Research Toxicology and Pathology, Frankfurt am Main, Germany, 1 December 1989. Aventis document A42256. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Baeder, C. (1971) Report on a 22-day feeding trial with Hoe 2960 an rhesus monkeys. Unpublished report from Farbwerke Hoechst AG, Frankfurt am Main, Germany, 4 November 1971. Aventis document A00933. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Brunk, R. (1969) Acute oral toxicity in the dog. Unpublished report from Farbwerke Hoechst AG, Pharma Research, Frankfurt am Main, Germany, 30 April 1969. Aventis document A00093. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1967a) Acute oral toxicity in rats. Unpublished report from Farbwerke Hoechst AG, Frankfurt am Main, Germany, 30 June, 1967. Aventis document A00090. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1968) Determination of the acute intraperitoneal toxicity. Unpublished report from Farbwerke Hoechst AG, Frankfurt am Main, Germany, 13 November, 1968. Aventis document A00531. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1969) Acute oral toxicity in rats. Unpublished report from Hoechst AG, Frankfurt am Main, Germany, 9 May 1969. Aventis document A05926. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1972a) HOE 029600—1-phenyl-1-2.4-triazolyl-3-(0.0-diethyl-thionophosphate), batch 22. Toxicological examination. Acute intraperitoneal toxicity in NMRI mice; acute dermal toxicity to female Wistar rats. Unpublished report from Farbwerke Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 21 April 1972. Aventis document A01868. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1972b) HOE 2960—1-phenyl-3-(0.0-diethyl-phosphoryl)-1,2,4-triazole, batch 22. Testing of dermal toxicity in female SPF-Wistar rats. Unpublished report from Farbwerke Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 21 April 1972. Aventis document A00092. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1973a) Acute oral toxicity of triazophos Hoe 02960 O I to the male Perbright guinea pig. Unpublished report from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 6 November 1973. Aventis document A29550. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Scholz, J. & Weigand, W. (1973b) Acute oral toxicity of triazophos Hoe 02960 O I to the female Perbright guinea pig. Unpublished report from Hoechst AG, Pharma Research Toxicology, Frankfurt am Main, Germany, 5 November 1973. Aventis document A29549. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Schwalbe-Fehl, M. & Schmidt, E. (1986) Hoe 002960-14-C, triazophos, comparative metabolism study in rats and dogs. Unpublished report No. CM048/85 from Hoechst AG, Frankfurt am Main, Germany, 4 February 1986. Aventis document A32754. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Stammberger, I. & Graser, H. (1999) AE F014622 substance technical. In vitro Chinese hamster lung V79 cells chromosome aberration assay test Unpublished report No. 98.0122 from Hoechst AG, Marion Roussel Preclinical Development, Germany, Drug Safety, Frankfurt am Main, Germany, 29 January, 1999. Aventis document C002772. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany
Suter, P., Vogel, W. & Terrier, C.H. (1989a) Preliminary study to the two-generation reproduction study in the rat. Unpublished report No. 071550 from RCC Itingen, Switzerland, 8 August 1989. Aventis document A41632. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Suter, P., Vogel, W., Armstrong, J.M. & Terrier, C.H. (1989b) Two-generation reproduction study in the rat. Unpublished report No. 071561 from RCC Itingen, Switzerland, 27 June 1989. Aventis document A41694. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Tennekes, H., Horst, K., Luetkemeier, H., Vogel, W., Schlotke, B. & Terrier, C.H. (1987) Subchronic oral toxicity, 13 week feeding study in rats. Unpublished report No. 071818 from RCC Itingen, Switzerland, 10 December 1987. Aventis document A37398. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Tennekes, H., Janiak, T., Probst, D., Luetkemeier, H., Vogel, O., Westen, H., Biedermann, K. & Heusner, W. (1990) Chronic toxicity/oncogenicity feeding study in rats. Unpublished report No. 071537 from RCC Itingen, Switzerland, 24 December 1990. Aventis document A44716. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Tesh, J.M., Ross, F.W. & Wightman, T.J. (1985a) Triazophos: Active ingredient technical—Embryotoxicity study in rabbits—Dose range finding study. Unpublished report No. 84/HAG091/303 from LSR, Eye, Suffolk, England, 12 March 1985. Aventis document A31189. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Tesh, J.M., Ross, F.W. & Wightman, T.J. (1985b) Triazophos: Active ingredient technical—Embryotoxicity study in rabbits. Unpublished report No. 84/HAG092/549 from LSR, Eye, Suffolk, England, 7 February 1985. Aventis document A30890. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Thevenaz, P.H., Luetkemeier, H., Vogel, W., Schlotke, B., Vogel, W. & Terrier, C.H. (1988) Subacute (28-day) repeated dose dermal toxicity study in rats. Unpublished report No. 084734 from RCC Itingen, Switzerland, 5 February 1988. Aventis document A37386. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Til, H.P. & Leegwater, D.C. (1974) Investigation of the recovery of cholinesterase inhibition induced by Hoe 2960 in rats. Unpublished report from CIVO/TNO No. R4505 Zeist, Netherlands 16 October 1974. Aventis document A09008. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Til, H.P., Leegwater, D.C. & Seinen, W. (1971) Subchronic (90-day) toxicity study with Hoe2960 Op. 22 in albino rats. Unpublished report from CIVO/TNO No. R3654 Zeist, Netherlands, 24 December 1971. Aventis document A00094. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ullmann, L., Sacher, R., Porricello, T., Janiak, T.H., Luetkemeier, H., Biedermann, K., Vogel, O. & Terrier, C.H. (1991a) Range finding study with NTE (neuropathy target esterase) determination; 20-day delayed neurotoxicity (feeding) study with triazophos-substance technical in the hen. Unpublished report No. 234303 from RCC Itingen, Switzerland, 21 January 1991. Aventis document A45134. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ullmann, L., Sacher, R., Porricello, T., Janiak, T.H., Luetkemeier, H., Biedermann, K., Vogel, O. & Terrier, C.H. (1991b) Positive control experiment. 3-month subchronic delayed neurotoxicity (gavage) study with tri-ortho-cresyl phosphate in the hen. Unpublished report No. 234325 from RCC Itingen, Switzerland, 12 April 1991. Aventis document A45671. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Ullmann, L., Sacher, R., Porricello, T., Janiak, T.H., Luetkemeier, H., Biedermann, K., Vogel, O. & Terrier, C.H. (1991c) 3-month subchronic delayed neurotoxicity (feeding) study with triazophos—Substance technical in the hen. Unpublished report No. 234314 from RCC Itingen, Switzerland, 9 April 1991. Aventis document A45976. Submitted to WHO by Aventis CropScience, Frankfurt am Main, Germany.
Velazquez, A., Xamena, N., Creus, A. & Marcos, R. (1990) Mutagenic evaluation of the organophosphorus insecticides methyl parathion and triazophos in Drosophila melanogaster. J. Toxicol. Environ. Health, 31, 313–325.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 2000–2202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
See Also:
Toxicological Abbreviations
Triazophos (Pesticide residues in food: 1982 evaluations)
Triazophos (Pesticide residues in food: 1983 evaluations)
Triazophos (Pesticide residues in food: 1986 evaluations Part II Toxicology)
Triazophos (Pesticide residues in food: 1991 evaluations Part II Toxicology)
Triazophos (Pesticide residues in food: 1993 evaluations Part II Toxicology)