
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
TRIAZOPHOS
IDENTITY
Chemical names
O,O-diethyl O-1-phenyl-1,2,4-triazol-3-yl phosphorothioate; O,O-
diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) phosphorothioate; 1-phenyl-
1,2,4-triazolyl-3-(O,O diethyl-thionophosphate)
Synonyms
Hoe 02960
Trade name
RHostathion
Structural formula
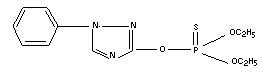 Empirical formula
C12H16N303PS
Molecular weight
313.3
Other information on identity and properties
Pure active ingredient
Density 1.247 g/cm3 at 20°C
Melting point 2 - 5°C
Vapour pressure 1 × 10-5 Torr at 38°C
1 × 10-3 Torr at 74°C
Flash point
Open 192° ± 5°C (Cleveland)
Closed 135° ± 2°C (Pensky-Martens)
Solubility at 20°C
water 35 ppm ± 5 ppm
n-hexane 0.9%
toluene >33%
ethyl acetate 100%
acetone 100%
ethanol >33%
Technical active ingredient
Physical form light yellow to dark brown liquid.
Odour characteristic of phosphoric ester
Density 1.24 g/cm3 ± 0.05 g/cm3 at 20°C
Melting point 0°C to 5°C
Solubility soluble in aromatic hydrocarbons, alcohols,
esters and ketones
Stability
Chemical stability decomposed by acids and alkalies
Thermal stability not stable in undiluted form
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Elimination and Biotransformation
Rat
In male and female rats (Wistar SPF) treated with a single oral
dose of 14C-triazophos (labelling at the 3-position of the triazole
ring) at 2.76 mg/rat or approximately 15-21 mg/kg bw, about 76% of the
administered radioactive dose was excreted in the urine and 21% in the
faeces within 48 hours of dosing. No significant sex difference in
rate and route of elimination was evident. Analyses of tissues from
these animals sacrificed four days after treatment indicated 14C
residues detected (as % of administered dose) to be invariably less
than 0.04% in kidney, gonads, brain, muscle and skin, 0.089% in liver
and 0.31% in the gastrointestinal tract. The rapid elimination of
14C-triazophos was similarly observed when male and female rats were
intubated with the radioactive compound at 0.56 mg/rat or
approximately 3.1-4.3 mg/kg bw/day for 12 consecutive days. Thus, 69.5
- 83.4% of the daily administered dose was recovered in the urine and
18.1 - 30.9% in the faeces during the dosing period. At sacrifice four
days after termination of the 12-day treatment period, none of the
tissues analyses (subcutaneous fat, kidney, gonads, liver, brain,
muscle and skin) had 14C residues greater than 0.0008% of the
administered dose. An exception was the gastrointestinal tract, which
still contained 0.5% of the given dose.
The major urinary metabolite was urea-14C, amounting to
approximately 85% of the radioactivity excreted via this route. Other
metabolites detected in the urine were 1-phenyl-1,2,4-triazol-3-ol-3-
14C, 1-phenylsemicarbazide-3-14C and semicarbazide-14C, all as
conjugates with glucuronic acid. Each of these three metabolites
accounted for 3-5% of the 14C eliminated in the urine. In the faeces,
unchanged 14C-triazophos (40% of the total activity in this route)
and 1-phenyl-l,21-4-triazol-3-ol-3-14C (60% of the activity) were
identified (Bock and Thier 1976).
Effects on Enzymes and Other Biochemical Parameters
See under Observations in Humans.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
In a standard 3-generation (2 litters/generation) reproduction
study, 10 male and 20 female newly weaned rats (Wistar derived) were
fed dietary levels of triazophos at 0, 0.3, 1.0 or 10 ppm and mated at
weeks 12 and 20 to produce two successive litters. Weanlings of the
second litters (i.e. F1b and F2b) were selected to become parents of
the next generations. F3b weanlings, 10 males and 10 females/group,
were maintained on dietary feedings for 4 weeks for a toxicity study.
Based on summary data available (no data on individual litters
provided in the report), mortality, behaviour and body weight and
pregnancy rate were unaffected in any of the parental generations.
Except for a decrease in pup weight at 10 ppm on day 20 in the F2a
litters, there were no dose- or treatment-related effects as judged by
litter size at birth, sex ratio, mortality and body weight of young
during lactation, resorption quotient (number of implantation sites/
number of young born) and gross malformations of pups at birth. The
reduced pup weight on day 20 at 10 ppm appeared to be of doubtful
significance in the light of its occurrence in only a single progeny
generation (F2a litters). In F3b weanling pups maintained on dietary
feeding for four weeks, there was no mortality or adverse effect on
body weight. Significant inhibition (21%) of plasma cholinesterase
occurred at 10 ppm in the females after 23 days. Erythrocyte
cholinesterase assayed at the same period was not significantly
depressed in the treated groups. At terminal sacrifice, there were no
significant variations from controls in brain cholinesterase activity,
organ weights and gross pathological changes. Histopathological
evaluation of selected tissues from animals of control and top dosage
groups revealed no treatment-related alterations (Til et al 1975).
Special Studies on Mutagenicity
Triazophos was tested for its mutagenic potential in a reverse
mutation assay in the presence or absence of a metabolic activation
system (S-9 homogenate of liver from rats induced with Arochlor 1254).
The tester strains used were Salmonella typhimurium TA 98,
TA 100, TA 1535 and TA 1537. At the concentrations used, 0.2 to
5 000 µg/plate, triazophos was not mutagenic to any of the tester
strains with or without the presence of the metabolic activation
preparation (Gericke 1977).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Two groups of 6 white Leghorn hens (mean weight 1.75 kg) were
intubated with a single dose of 25 mg/kg bw of triazophos. Survivors
were similarly treated again with another dose of the same magnitude
21 days later and observed for a further period of 21 days. (The acute
oral LD50 of triazophos in hens was determined to be approximately
24 mg/kg bw prior to initiation of the neurotoxicity study.) Hens in
one of these groups were treated with atropine (10 mg/kg bw) and
Toxagonin (4 mg/kg bw) by an unspecified route once before and four or
five times after the first and second dose of triazophos. The
untreated control group comprised 6 hens. One hen in each of the
treated groups died after the first dose. Following the second dose,
mortality occurred in 2 hens protected with antidotes and in 3
unprotected hens. No neurotoxic signs were noted in any of the treated
birds at any time during the study, although toxic symptoms indicative
of anticholinesterase poisoning, such as disturbed equilibrium,
salivation, lethargy and extensor spasms, were present during the
first two days after each dose of triazophos. Histopathological
examination of the spinal medulla (cervical medulla, cervical
enlargement, thoracic medulla and lumbar enlargement) and sciatic
nerve from 5 surviving hens (3 of these with antidote treatment) at
25 mg/kg bw and 1 untreated control hen surviving terminally revealed
no morphologically demonstrable changes characteristic of delayed
neurotoxic effects. One of the 5 hens at 25 mg/kg bw, however, showed
macroscopic and microscopic lesions in medulla oblongata and spinal
medulla classified with chicken gliomata, which was considered to be
independent of treatment. Positive control hens, treated with TOCP,
displayed clinical symptoms and histopathological changes typical of
delayed neurotoxicity (Kramer and Weigand 1974).
Special Studies on Skin and Eye Irritation
Results of a patch test with rabbits (SPF-albino Himalayan
strain) indicated triazophos, as a 1% or 10% dilution in sesame oil,
to be practically non-irritating to the skin. However 5 of the 6
treated rabbits died 24-48 hours after 0.5 ml of undiluted triazophos
was applied to each of the two sites (one abraded) on their clipped
skin (Hollander and Weigand 1977a).
An eye irritation study in rabbits (SPF - albino Himalayan
strain) suggested undiluted triazophos, 1% or 10% dilutions of
triazophos in sesame oil, to be of a low irritant potential to eyes. A
single dose of 0.1 ml of the undiluted material applied to one eye
was, however, fatal to 1/6 treated rabbits in 48 hours (Hollander and
Weigand 1977a).
Acute Toxicity
Toxic signs of oral poisoning similar in mice and rats, were
characterized by tremors, abdominal position and squatting. Mortality
usually occurred 10 minutes to 5 days after lethal doses. No specific
information was given on the time of onset and duration of symptoms
(Hollander and Weigand, 1977b, 1977c, 1977d; Scholz and Weigand 1969,
Hollander and Weigand 1977e). The LD50 in these two species is
reported in Table 1 for different treatment routes and vehicles.
Table 1. Acute Toxicity to Triazophos in the Mouse and Rat
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Mouse M oral 1 sesame oil 31 Hollander and Weigand
(SPF-NMRI) 1977b
F 29 Hollander and
Weigand 1977c
Rat M oral starch 68 Hollander and
(SPF-Wistar) suspension Weigand 1977d
F oral starch slurry 66 Scholz and Weigand 1969
F oral starch mucilage 64 Hollander and Weigand
1977e
Rat F i.p. oil of benne 107 Scholz and Weigand 1968
(SPF-Wistar)
Rat F dermal 2 sesame oil 1100 Weigand 1972
(24-hour
exposure)
1 In the oral studies, animals were fasted 15 to 18 hours prior to dosing.
2 Treated site was occluded during the exposure period.
Dog
In groups of generally 2 beagle dogs (mixed sex; 20-hours fasted)
treated with single doses of triazophos (93% pure) in sesame oil by
gavage, no mortality occurred at 320 mg/kg bw and below. Both dogs at
800 mg/kg bw and 1/2 dogs at 500 mg/kg bw died within 3 days of
dosing. Vomiting was observed in 2/2 dogs at both 80 and 800 mg/kg bw,
1/2 dogs at 125 mg/kg bw and 1/1 dog at 160 mg/kg bw usually 3 to 6
hours after treatment, but in none of the 2 dogs each at 50, 320 and
500 mg/kg bw. Other toxic symptoms included hypersalivation, tremors,
miosis and staggering gait. The median lethal dose appeared to be in
the vicinity of 500 mg/kg bw (Scholz and Brunk 1969).
Short-Term Studies
Rat
Groups of 10 male and 10 female weanling rats (Wistar derived)
were fed diets containing triazophos (90% pure) at 0, 1, 3, 10 or
200 ppm for 13 weeks. The highest level was raised to 400 ppm in the
6th week. Survival was 100%. Body weight, food consumption and
behaviour as well as haematology, clinical chemistry and urinalysis
parameters were not adversely affected. Activity of plasma
cholinesterase was depressed (920%) in males of top dosage group and
in females at 3 ppm and above at all sampling intervals (weeks 3, 6
and 12). The enzyme was also inhibited in females at 1 ppm by 19% and
in males at 10 ppm by 23% at week 12 only. Terminal brain
cholinesterase and erythrocyte cholinesterase assayed at the three
intervals were inhibited (>20%) only in the top dosage group (both
sexes). The magnitude of depression of plasma, erythrocyte or brain
cholinesterase was greater in females than in males at the same dosage
levels. At terminal sacrifice, there were no gross pathological
changes or organ weight variation associated with the compound. No
treatment-induced histopathological changes were observed in a variety
of tissues evaluated. The study suggested 1 ppm as a virtual no-effect
level (Til et al 1971).
Dog
Male and female purebred beagles (3 males and 3 females per
group) were fed dietary levels of triazophos at 0, 0.03, 0.1, 0.3,
1.0, 5.0 or 100 ppm for 13 weeks. There was no mortality or treatment-
induced abnormal behaviour. Body weight, food consumption and
haematological, biochemical (including kidney and liver function
tests) and urinalysis values were not affected in any dose-response
pattern. Inhibition (>20%, dose related) of plasma cholinesterase
occurred at and above 1 ppm at weeks 3 and 7 and at both 5 and 100 ppm
at week 12 also. Whole blood cholinesterase was reduced (>20%) in the
top dosage group only at weeks 3, 7 and 12. Activity of terminal brain
cholinesterase was not inhibited (>20%). At the conclusion of the
study, organ weights and gross pathological changes were comparable in
all groups. Histopathological examination of a set of over 30 tissues
from each dog of the control and the treated groups at 1 ppm and above
revealed no abnormalities related to ingestion of triazole. The no-
effect level was 0.3 ppm (Til et al 1971).
Groups of 4 male and 4 female purebred beagles were fed
triazophos (purity not specified) in their diet at 0, 0.3, 1.0 or
3.0 ppm for 2 years. The highest dietary level was increased to 6 ppm
after 5 weeks and to 200 ppm after 16 weeks. There were no treatment-
related findings with respect to mortality, appearance and behaviour
and food consumption. Growth was decreased in the top dosage group
(both sexes) at week 36 and thereafter. Periodic haematological and
biochemical studies and urinalysis during the study indicated
significant changes, confined to the top dosage group, in values of
SAP, serum albumin, SGOT and specific gravity of the urine.
Measurements of plasma and erythrocyte cholinesterase nine times over
the course of the experiment revealed dose-related inhibition (>20%)
of plasma cholinesterase at and above 1 ppm at all intervals and at
0.3 ppm at weeks 13 and 26. Erythrocyte cholinesterase was depressed
(>20%) only at the top dosage group at week 17 and thereafter. Brain
cholinesterase assayed terminally was not inhibited (>20%). Animals
sacrificed at the end of the study exhibited an increase in absolute
weight of the liver and organ/body weight ratio of the liver and the
thyroid at the top dosage level. No compound-induced histopathological
lesions were observed in a variety of tissues evaluated, including
liver and thyroid. The dietary level of 0.3 ppm was a marginal no-
effect level (Til et al 1976).
Long-Term Studies
Rat
Groups of 30 male and 30 female rats (Wistar derived) were fed
dietary levels of triazophos (of unspecified purity) at 0, 0.3, 1.0,
3.0, 10 or 200 ppm for 2 years. (The 0.3 ppm and 10 ppm groups
were discarded at week 28 "because the plasma and erythrocyte
cholinesterase was not inhibited in the 0.3 and 1 ppm groups and
decreased in the 3 and 10 ppm groups".) Mortality, appearance and
general behaviour and body weight were not affected by treatment.
Between 23-70% males and 43-87% females of all groups, including the
control, survived the duration of the study. (Over 50% males and at
least 70% females per control and treated group were still alive at 96
weeks.) Food consumption was slightly reduced at 200 ppm in males
during the first year and in females after 47 weeks. Plasma
cholinesterase was inhibited (>20%), in a dose-dependent pattern, at
10 ppm at weeks 4 (females only), 13 and 26 (both sexes) and at 3 ppm
in females at weeks 52 and 102. Animals at 200 ppm exhibited marked
depression (>20%) of plasma and erythrocyte cholinesterase (both
sexes) at all intervals and of terminal brain cholinesterase (females
only). No compound-related effects were observed on haematological,
biochemical and urine parameters measured periodically up to five
times over the course of the study. At the conclusion of the
experiment, there were some variations in organ/body weight ratio of
several selected organs but they did not follow a dose-response trend.
Histopathological evaluation of over 20 organs plus all grossly
abnormal tissues from each animal, initially limited to 20 males and
20 females of the control and 200 ppm groups (comprising terminal
survivors supplemented with animals sacrificed in extremis or dying
toward the end of the study) and later extended to all animals of the
intermediate dosage groups, showed no alterations attributable to
inclusion of triazophos in the diet. There were no dose- or compound-
related increases in incidence of any particular type of tumour. Based
on the data, 1 ppm was the no-effect level (Til and Leegwater 1975;
Birkvens and Beems 1979).
Observations in Humans
In a preliminary study, a male volunteer (80 kg) was given
triazophos orally for 4 consecutive mornings. The dose was 1 mg or
0.0125 mg/kg body weight/day on day 1 and 5 mg (0.0625 mg/kg body
weight/day) from days 2 through 4. Depression (=20%) of plasma
cholinesterase, initially observed after the third dose and more
pronounced after the fourth dose, was still evident 3 days after
treatment withdrawal. No inhibition (<20%) of erythrocyte
cholinesterase was noted at any time during the study. Headache was
the only complaint reported, which began to occur after the second
dose and persisted through the day following the last dose (Leegwater
1971).
Two male and two female volunteers were orally doses with
0.0125 mg/kg bw/day of triazophos for 5 days. Compared to pre-
treatment levels there was no depression (<20%) of cholinesterase in
plasma or erythrocyte measured 5 hours after the second and fourth
doses. Two days later, the same 2 male volunteers were given 2
additional courses of triazophos with a rest period of 2 days between
the courses. The first course comprised 5 daily doses at 0.03 mg/kg
bw/day and the second course 5 daily doses at 0.05 mg/kg bw/day.
Activity of plasma cholinesterase, slightly reduced (17-18%) in both
subjects after 4 doses in the first course and after 3 doses in the
second course, was significantly inhibited (>30%) after 4 days in
the second course. Complete recovery of the enzyme was not apparent
until 16 days following completion of the second course. Erythrocyte
cholinesterase was not adversely affected at any time during the
experiment (Leegwater 1972).
Healthy adult volunteers (2 males and 3 females, aged 18-23
years) were given 0.0125 mg triazophos/kg bw/day orally, 5 days/week
for 3 consecutive weeks in a pilot study. All 5 subjects completed the
study but one female missed one dose during the first dosing week.
There was no significant change in the activity of plasma or
erythrocyte cholinesterase at any time during the dosing period, as
compared to that in the one-week, pre-exposure 'run-in' period. The
only symptom reported was headache experienced by one female subject
each morning about 1 1/2 hours after dosing on the first 3 days of the
first treatment week. Medical examination and routine haematology,
biochemistry and urinalysis conducted before and 13 days after the
dosing period revealed no significant changes attributable to
triazophos, although one female volunteer (the same one complaining of
headache) showed a tendency toward anaemia (Cooper et al 1973a). In
a second pilot study, 3 male and 2 female healthy adult subjects were
given 0.025 mg triazophos/kg bw/day, 5 times weekly for 3 weeks.
Reduction of plasma cholinesterase (11-17%), which appeared to be
related to duration of treatment, was stated to attain statistical
significance in weeks 2 and 3 of treatment and during the first week
of withdrawal, Erythrocyte cholinesterase was not adversely affected
at any time during the study (Cooper et al 1973b).
Twenty-five healthy adult subjects (13 males and 12 females) were
administered 0.0125 mg triazophos/kg bw/day, 5 days/week for 3
consecutive weeks. Medical examination and routine haematology,
biochemistry and urinalysis were conducted prior to the study and at
the end of a 4-week post-dosing follow-up period. Plasma and
erythrocyte cholinesterase was assayed prior to the study and at least
three times weekly throughout the dosing and follow-up periods. All 25
subjects completed the study. Two males and 1 female were withdrawn
from treatment during the second dosing week owing to progressive
inhibition of plasma cholinesterase to less than 80% of the pre-dose
levels, but dosing resumed for these 3 during treatment week 3. Two
other subjects missed 1 or 2 doses, respectively, due to diarrhoea and
sickness. (Based on the statement that "other subjects who had to
unavoidably miss blood sampling (taken prior to the daily dose, i.e.
approximately 24 hours after the previous dose) on occasions during
the three dosing weeks were given their daily doses to take at the
appropriate time on the following day", it would appear that
additional subjects also might have missed an unspecified number of
doses during the treatment period.) During the first dosing week, 50%
of the female subjects reported urinary frequency with some degree of
urinary urgency. Other complaints, isolated in incidence, included
tiredness and lethargy, nausea and diarrhoea. There were some
significant differences between pre- and post-treatment values in
blood pressure, pulse rate and some laboratory parameters but the
post-treatment values were within the limits normally considered
acceptable to the testing laboratory. A comparison of the mean plasma
cholinesterase activity (of all 25 subjects) obtained before the test
with that at each dosing and follow-up week indicated no statistically
significant difference. However, a total of 6 individuals (including
the 3 withdrawn from treatment during the second week) exhibited a
depression (> 20%) of plasma cholinesterase, mainly during the second
and third week of dosing when their own pre-treatment levels were used
as the basis of comparison. Recovery of the enzyme to pre-exposure
levels were more or less complete in the majority of subjects by the
end of the fourth follow-up week. No significant compound-inducted
inhibition of erythrocyte cholinesterase was noted during the study.
The data suggested 0.0125 mg/kg body weight as the minimum effect
level with respect to plasma cholinesterase (Cracknell et al 1973).
COMMENTS
Triazophos was evaluated for the first time by the present
Meeting.
In rats, triazophos, an organophosphorous insecticide, is rapidly
eliminated predominantly in the urine and also in the faeces. It is
readily degraded, with urea being the major urinary metabolite.
Neither triazophos nor its metabolites appear to accumulate to any
significant extent in the tissues. Metabolism data in mammalian
species other than the rat are not available.
Oral LD50 values of triazophos ranged from approximately
30 mg/kg bw in mice, 65 mg/kg bw in rats to about 500 mg/kg bw in
dogs, suggesting a significant species variation in sensitivity to
acute toxicity of the compound. A significant sex difference was not
evident.
Summary data provided for a 3-generation reproduction study in
rats suggested 10 ppm as a no-effect level on reproduction. There were
no teratogenicity studies. An in vitro microbial assay and a delayed
neurotoxicity study in hens were negative. Nevertheless, the
possibility exists that the dosage level used (equivalent to an oral
LD50) in the hen study might be too low to permit an adequate testing
of the compound for its delayed neurotoxic potential. Studies designed
specifically to evaluate the carcinogenic activity of triazophos are
not available, although a 2-year feeding study in rats indicated no
increase in incidence of any particular type of tumour.
No-effect levels had been defined in 13-week and 2-year feeding
studies in rats and dogs, with plasma cholinesterase being
demonstrated to be the most sensitive indicator of biological
activity. Observations in humans suggested a significant individual
variation to the effect of triazophos on plasma cholinesterase.
Despite establishment of no-effect levels in two mammalian
species, the Meeting could only allocate a temporary ADI since
additional data, as listed below, are required to complete the
toxicological data base.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 0.3 ppm in the diet, equivalent to 0.008 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. A carcinogenicity study.
2. Teratogenicity study in at least one mammalian species.
3. Metabolism studies in additional mammalian species to explain
species differences in acute toxicity.
4. Additional mutagenicity studies.
Desirable
1. An appropriate delayed neurotoxicity study in hens.
2. Data on individual litters for the currently available three-
generation reproduction study in rats.
3. Acute oral toxicity data on any metabolite of triazophos that is
found in food crops.
REFERENCES
Bock, R. and Thier, W. Metabolism and fate of triazophos in rats.
1976 Pesticide Science 7: 307-314.
Berkvens, J.M. and Beems, R.B. Chronic (two-year) toxicity study with
1979 Hoe 2960 in rats. Part 3. Microscopic pathological
examination of intermediate dose animals. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Cooper, A.J., Haynes, G., Street, A.E. and Rudd, C.J. A pilot study to
1973a observe the in-vivo effects of Hoe-2960 on the
cholinesterase activity in man administered 0.0125 mg/kg day
p.o. Report from Huntingdon Research Centre, England.
Submitted to the World Health Organization by Hoechst.
(Unpublished)
1973b A pilot study to observe the in vivo effects of Hoe-2960 on
the cholinesterase activity in man administered 0.025
mg/kg/day p.o. Report from Huntingdon Research Centre,
England, Submitted to the World Health Organization by
Hoechst. (Unpublished)
Cracknell, D.D., Hawkes, G., Street, A.E. and Rudd, C.J. The in-vivo
1973 effects of Hoe-2960 on the cholinesterase activity in man
administered 0.0125 mg/kg/day. Report from Huntingdon
Research Centre, England. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Gericke, D. Test for mutagenicity in bacterial strains in the absence
1977 and presence of a liver preparation. Hoe-02960 01 AS 101.
Report from Hoechst. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Hollander and Weigand. Hoe-02960 01 AS 101 Irritance to the rabbit
1977a skin and eye mucosa. Report from Hoechst, Submitted to the
World Health Organization by Hoechst. (Unpublished)
1977b Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-NMRI
mouse (vehicle: sesame oil). Report from Hoechst submitted
to the World Health Organization by Hoechst. (Unpublished)
1977c Hoe-02960 01 AS 101 Acute oral toxicity to the female
SPF-NMRI mouse (vehicle: sesame oil). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977d Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-
Wistar rat (vehicle: starch suspension). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977e Akute orale toxizitat von Hoe 02960 01 AS 101 and weiblichen
SPF-Wistar ratten (vehikel: starkeschleim). Report from
Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Kramer and Weigand, Test of triazophos (Hoe-02960) for neurotoxicity
1974 in hens. Report from Farbeverke Hoechst AG submitted to the
World Health Organization by Hoechst. (Unpublished)
Leegwater, D.C. Preliminary study on the in-vivo effect of Hoe-2960 in
1971 man. Report from Central Institute for Nutrition and Food
Research, Zeist. Submitted to the World Health Organization
by Hoechst. (Unpublished)
1972 In-vivo study on the effect of Hoe-2960/Op 22 on the blood
cholinesterase of man. Report from Central Institute for
Nutrition and Food Research, Zeist. Submitted to the World
Health Organization by Hoechst. (Unpublished)
Scholz and Weigand. Triazophos-determination of the acute
1968 intraperitoneal toxicity. Report from Hoechst submitted to
the World Health Organization by Hoechst. (Unpublished)
1969 W12712 = Hoe-02960. 1-phenyl-1,2,4-triazolyl-3-(O,O-diethyl
thionophosphate) (Op. Techn. No. 3, Content 93%). Report
from Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Scholz and Brunk. W12712 = Hoe 2960. Acute oral toxicity in dogs.
1969 Report from Hoechst submitted to the World Health
Organization by Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Seinen, W. Subchronic (90-day) toxicity
1971a study with Hoe-2960 Op. 22 in albino rats. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Til, H.P., Seinen, W., Leegwater, D.C. and van der Meulen, H.C.
1971b Subchronic toxicity study with Hoe 2960, Op.22 in beagle
dogs. Report from Central Institute for Nutrition and Food
Research, Zeist, submitted to the World Health Organization
by Hoechst. (Unpublished)
Til, H.P., Hendriksen, C.F.M. and Leegwater D.C. Multigeneration study
1975 with Hoe-2960 in rats. Report from Central Institute for
Nutrition and Food Research, Zeist, submitted to the World
Health Organization by Farbeverke Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Kollen, C.H. Long-term (two year)
1976 toxicity study with Hoe 2960 in dogs. Report from Central
Institute for Nutrition and Food Research Zeist, submitted
to the World Health Organization by Hoechst. (Unpublished)
Til, H.P. and Leegwater, D.C. Chronic (two year) toxicity study with
1975 Hoe-2960 in rats. Part I. Results of clinical haematological
biochemical and organ weight measurements. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Weigand. Testing of dermal toxicity in female SPF-Wistar rats of our
1972 own breed, after a single treatment. Report (No. A 00092)
from Hoechst, submitted to the World Health Organization by
Hoechst. (Unpublished)
Empirical formula
C12H16N303PS
Molecular weight
313.3
Other information on identity and properties
Pure active ingredient
Density 1.247 g/cm3 at 20°C
Melting point 2 - 5°C
Vapour pressure 1 × 10-5 Torr at 38°C
1 × 10-3 Torr at 74°C
Flash point
Open 192° ± 5°C (Cleveland)
Closed 135° ± 2°C (Pensky-Martens)
Solubility at 20°C
water 35 ppm ± 5 ppm
n-hexane 0.9%
toluene >33%
ethyl acetate 100%
acetone 100%
ethanol >33%
Technical active ingredient
Physical form light yellow to dark brown liquid.
Odour characteristic of phosphoric ester
Density 1.24 g/cm3 ± 0.05 g/cm3 at 20°C
Melting point 0°C to 5°C
Solubility soluble in aromatic hydrocarbons, alcohols,
esters and ketones
Stability
Chemical stability decomposed by acids and alkalies
Thermal stability not stable in undiluted form
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Elimination and Biotransformation
Rat
In male and female rats (Wistar SPF) treated with a single oral
dose of 14C-triazophos (labelling at the 3-position of the triazole
ring) at 2.76 mg/rat or approximately 15-21 mg/kg bw, about 76% of the
administered radioactive dose was excreted in the urine and 21% in the
faeces within 48 hours of dosing. No significant sex difference in
rate and route of elimination was evident. Analyses of tissues from
these animals sacrificed four days after treatment indicated 14C
residues detected (as % of administered dose) to be invariably less
than 0.04% in kidney, gonads, brain, muscle and skin, 0.089% in liver
and 0.31% in the gastrointestinal tract. The rapid elimination of
14C-triazophos was similarly observed when male and female rats were
intubated with the radioactive compound at 0.56 mg/rat or
approximately 3.1-4.3 mg/kg bw/day for 12 consecutive days. Thus, 69.5
- 83.4% of the daily administered dose was recovered in the urine and
18.1 - 30.9% in the faeces during the dosing period. At sacrifice four
days after termination of the 12-day treatment period, none of the
tissues analyses (subcutaneous fat, kidney, gonads, liver, brain,
muscle and skin) had 14C residues greater than 0.0008% of the
administered dose. An exception was the gastrointestinal tract, which
still contained 0.5% of the given dose.
The major urinary metabolite was urea-14C, amounting to
approximately 85% of the radioactivity excreted via this route. Other
metabolites detected in the urine were 1-phenyl-1,2,4-triazol-3-ol-3-
14C, 1-phenylsemicarbazide-3-14C and semicarbazide-14C, all as
conjugates with glucuronic acid. Each of these three metabolites
accounted for 3-5% of the 14C eliminated in the urine. In the faeces,
unchanged 14C-triazophos (40% of the total activity in this route)
and 1-phenyl-l,21-4-triazol-3-ol-3-14C (60% of the activity) were
identified (Bock and Thier 1976).
Effects on Enzymes and Other Biochemical Parameters
See under Observations in Humans.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
In a standard 3-generation (2 litters/generation) reproduction
study, 10 male and 20 female newly weaned rats (Wistar derived) were
fed dietary levels of triazophos at 0, 0.3, 1.0 or 10 ppm and mated at
weeks 12 and 20 to produce two successive litters. Weanlings of the
second litters (i.e. F1b and F2b) were selected to become parents of
the next generations. F3b weanlings, 10 males and 10 females/group,
were maintained on dietary feedings for 4 weeks for a toxicity study.
Based on summary data available (no data on individual litters
provided in the report), mortality, behaviour and body weight and
pregnancy rate were unaffected in any of the parental generations.
Except for a decrease in pup weight at 10 ppm on day 20 in the F2a
litters, there were no dose- or treatment-related effects as judged by
litter size at birth, sex ratio, mortality and body weight of young
during lactation, resorption quotient (number of implantation sites/
number of young born) and gross malformations of pups at birth. The
reduced pup weight on day 20 at 10 ppm appeared to be of doubtful
significance in the light of its occurrence in only a single progeny
generation (F2a litters). In F3b weanling pups maintained on dietary
feeding for four weeks, there was no mortality or adverse effect on
body weight. Significant inhibition (21%) of plasma cholinesterase
occurred at 10 ppm in the females after 23 days. Erythrocyte
cholinesterase assayed at the same period was not significantly
depressed in the treated groups. At terminal sacrifice, there were no
significant variations from controls in brain cholinesterase activity,
organ weights and gross pathological changes. Histopathological
evaluation of selected tissues from animals of control and top dosage
groups revealed no treatment-related alterations (Til et al 1975).
Special Studies on Mutagenicity
Triazophos was tested for its mutagenic potential in a reverse
mutation assay in the presence or absence of a metabolic activation
system (S-9 homogenate of liver from rats induced with Arochlor 1254).
The tester strains used were Salmonella typhimurium TA 98,
TA 100, TA 1535 and TA 1537. At the concentrations used, 0.2 to
5 000 µg/plate, triazophos was not mutagenic to any of the tester
strains with or without the presence of the metabolic activation
preparation (Gericke 1977).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Two groups of 6 white Leghorn hens (mean weight 1.75 kg) were
intubated with a single dose of 25 mg/kg bw of triazophos. Survivors
were similarly treated again with another dose of the same magnitude
21 days later and observed for a further period of 21 days. (The acute
oral LD50 of triazophos in hens was determined to be approximately
24 mg/kg bw prior to initiation of the neurotoxicity study.) Hens in
one of these groups were treated with atropine (10 mg/kg bw) and
Toxagonin (4 mg/kg bw) by an unspecified route once before and four or
five times after the first and second dose of triazophos. The
untreated control group comprised 6 hens. One hen in each of the
treated groups died after the first dose. Following the second dose,
mortality occurred in 2 hens protected with antidotes and in 3
unprotected hens. No neurotoxic signs were noted in any of the treated
birds at any time during the study, although toxic symptoms indicative
of anticholinesterase poisoning, such as disturbed equilibrium,
salivation, lethargy and extensor spasms, were present during the
first two days after each dose of triazophos. Histopathological
examination of the spinal medulla (cervical medulla, cervical
enlargement, thoracic medulla and lumbar enlargement) and sciatic
nerve from 5 surviving hens (3 of these with antidote treatment) at
25 mg/kg bw and 1 untreated control hen surviving terminally revealed
no morphologically demonstrable changes characteristic of delayed
neurotoxic effects. One of the 5 hens at 25 mg/kg bw, however, showed
macroscopic and microscopic lesions in medulla oblongata and spinal
medulla classified with chicken gliomata, which was considered to be
independent of treatment. Positive control hens, treated with TOCP,
displayed clinical symptoms and histopathological changes typical of
delayed neurotoxicity (Kramer and Weigand 1974).
Special Studies on Skin and Eye Irritation
Results of a patch test with rabbits (SPF-albino Himalayan
strain) indicated triazophos, as a 1% or 10% dilution in sesame oil,
to be practically non-irritating to the skin. However 5 of the 6
treated rabbits died 24-48 hours after 0.5 ml of undiluted triazophos
was applied to each of the two sites (one abraded) on their clipped
skin (Hollander and Weigand 1977a).
An eye irritation study in rabbits (SPF - albino Himalayan
strain) suggested undiluted triazophos, 1% or 10% dilutions of
triazophos in sesame oil, to be of a low irritant potential to eyes. A
single dose of 0.1 ml of the undiluted material applied to one eye
was, however, fatal to 1/6 treated rabbits in 48 hours (Hollander and
Weigand 1977a).
Acute Toxicity
Toxic signs of oral poisoning similar in mice and rats, were
characterized by tremors, abdominal position and squatting. Mortality
usually occurred 10 minutes to 5 days after lethal doses. No specific
information was given on the time of onset and duration of symptoms
(Hollander and Weigand, 1977b, 1977c, 1977d; Scholz and Weigand 1969,
Hollander and Weigand 1977e). The LD50 in these two species is
reported in Table 1 for different treatment routes and vehicles.
Table 1. Acute Toxicity to Triazophos in the Mouse and Rat
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Mouse M oral 1 sesame oil 31 Hollander and Weigand
(SPF-NMRI) 1977b
F 29 Hollander and
Weigand 1977c
Rat M oral starch 68 Hollander and
(SPF-Wistar) suspension Weigand 1977d
F oral starch slurry 66 Scholz and Weigand 1969
F oral starch mucilage 64 Hollander and Weigand
1977e
Rat F i.p. oil of benne 107 Scholz and Weigand 1968
(SPF-Wistar)
Rat F dermal 2 sesame oil 1100 Weigand 1972
(24-hour
exposure)
1 In the oral studies, animals were fasted 15 to 18 hours prior to dosing.
2 Treated site was occluded during the exposure period.
Dog
In groups of generally 2 beagle dogs (mixed sex; 20-hours fasted)
treated with single doses of triazophos (93% pure) in sesame oil by
gavage, no mortality occurred at 320 mg/kg bw and below. Both dogs at
800 mg/kg bw and 1/2 dogs at 500 mg/kg bw died within 3 days of
dosing. Vomiting was observed in 2/2 dogs at both 80 and 800 mg/kg bw,
1/2 dogs at 125 mg/kg bw and 1/1 dog at 160 mg/kg bw usually 3 to 6
hours after treatment, but in none of the 2 dogs each at 50, 320 and
500 mg/kg bw. Other toxic symptoms included hypersalivation, tremors,
miosis and staggering gait. The median lethal dose appeared to be in
the vicinity of 500 mg/kg bw (Scholz and Brunk 1969).
Short-Term Studies
Rat
Groups of 10 male and 10 female weanling rats (Wistar derived)
were fed diets containing triazophos (90% pure) at 0, 1, 3, 10 or
200 ppm for 13 weeks. The highest level was raised to 400 ppm in the
6th week. Survival was 100%. Body weight, food consumption and
behaviour as well as haematology, clinical chemistry and urinalysis
parameters were not adversely affected. Activity of plasma
cholinesterase was depressed (920%) in males of top dosage group and
in females at 3 ppm and above at all sampling intervals (weeks 3, 6
and 12). The enzyme was also inhibited in females at 1 ppm by 19% and
in males at 10 ppm by 23% at week 12 only. Terminal brain
cholinesterase and erythrocyte cholinesterase assayed at the three
intervals were inhibited (>20%) only in the top dosage group (both
sexes). The magnitude of depression of plasma, erythrocyte or brain
cholinesterase was greater in females than in males at the same dosage
levels. At terminal sacrifice, there were no gross pathological
changes or organ weight variation associated with the compound. No
treatment-induced histopathological changes were observed in a variety
of tissues evaluated. The study suggested 1 ppm as a virtual no-effect
level (Til et al 1971).
Dog
Male and female purebred beagles (3 males and 3 females per
group) were fed dietary levels of triazophos at 0, 0.03, 0.1, 0.3,
1.0, 5.0 or 100 ppm for 13 weeks. There was no mortality or treatment-
induced abnormal behaviour. Body weight, food consumption and
haematological, biochemical (including kidney and liver function
tests) and urinalysis values were not affected in any dose-response
pattern. Inhibition (>20%, dose related) of plasma cholinesterase
occurred at and above 1 ppm at weeks 3 and 7 and at both 5 and 100 ppm
at week 12 also. Whole blood cholinesterase was reduced (>20%) in the
top dosage group only at weeks 3, 7 and 12. Activity of terminal brain
cholinesterase was not inhibited (>20%). At the conclusion of the
study, organ weights and gross pathological changes were comparable in
all groups. Histopathological examination of a set of over 30 tissues
from each dog of the control and the treated groups at 1 ppm and above
revealed no abnormalities related to ingestion of triazole. The no-
effect level was 0.3 ppm (Til et al 1971).
Groups of 4 male and 4 female purebred beagles were fed
triazophos (purity not specified) in their diet at 0, 0.3, 1.0 or
3.0 ppm for 2 years. The highest dietary level was increased to 6 ppm
after 5 weeks and to 200 ppm after 16 weeks. There were no treatment-
related findings with respect to mortality, appearance and behaviour
and food consumption. Growth was decreased in the top dosage group
(both sexes) at week 36 and thereafter. Periodic haematological and
biochemical studies and urinalysis during the study indicated
significant changes, confined to the top dosage group, in values of
SAP, serum albumin, SGOT and specific gravity of the urine.
Measurements of plasma and erythrocyte cholinesterase nine times over
the course of the experiment revealed dose-related inhibition (>20%)
of plasma cholinesterase at and above 1 ppm at all intervals and at
0.3 ppm at weeks 13 and 26. Erythrocyte cholinesterase was depressed
(>20%) only at the top dosage group at week 17 and thereafter. Brain
cholinesterase assayed terminally was not inhibited (>20%). Animals
sacrificed at the end of the study exhibited an increase in absolute
weight of the liver and organ/body weight ratio of the liver and the
thyroid at the top dosage level. No compound-induced histopathological
lesions were observed in a variety of tissues evaluated, including
liver and thyroid. The dietary level of 0.3 ppm was a marginal no-
effect level (Til et al 1976).
Long-Term Studies
Rat
Groups of 30 male and 30 female rats (Wistar derived) were fed
dietary levels of triazophos (of unspecified purity) at 0, 0.3, 1.0,
3.0, 10 or 200 ppm for 2 years. (The 0.3 ppm and 10 ppm groups
were discarded at week 28 "because the plasma and erythrocyte
cholinesterase was not inhibited in the 0.3 and 1 ppm groups and
decreased in the 3 and 10 ppm groups".) Mortality, appearance and
general behaviour and body weight were not affected by treatment.
Between 23-70% males and 43-87% females of all groups, including the
control, survived the duration of the study. (Over 50% males and at
least 70% females per control and treated group were still alive at 96
weeks.) Food consumption was slightly reduced at 200 ppm in males
during the first year and in females after 47 weeks. Plasma
cholinesterase was inhibited (>20%), in a dose-dependent pattern, at
10 ppm at weeks 4 (females only), 13 and 26 (both sexes) and at 3 ppm
in females at weeks 52 and 102. Animals at 200 ppm exhibited marked
depression (>20%) of plasma and erythrocyte cholinesterase (both
sexes) at all intervals and of terminal brain cholinesterase (females
only). No compound-related effects were observed on haematological,
biochemical and urine parameters measured periodically up to five
times over the course of the study. At the conclusion of the
experiment, there were some variations in organ/body weight ratio of
several selected organs but they did not follow a dose-response trend.
Histopathological evaluation of over 20 organs plus all grossly
abnormal tissues from each animal, initially limited to 20 males and
20 females of the control and 200 ppm groups (comprising terminal
survivors supplemented with animals sacrificed in extremis or dying
toward the end of the study) and later extended to all animals of the
intermediate dosage groups, showed no alterations attributable to
inclusion of triazophos in the diet. There were no dose- or compound-
related increases in incidence of any particular type of tumour. Based
on the data, 1 ppm was the no-effect level (Til and Leegwater 1975;
Birkvens and Beems 1979).
Observations in Humans
In a preliminary study, a male volunteer (80 kg) was given
triazophos orally for 4 consecutive mornings. The dose was 1 mg or
0.0125 mg/kg body weight/day on day 1 and 5 mg (0.0625 mg/kg body
weight/day) from days 2 through 4. Depression (=20%) of plasma
cholinesterase, initially observed after the third dose and more
pronounced after the fourth dose, was still evident 3 days after
treatment withdrawal. No inhibition (<20%) of erythrocyte
cholinesterase was noted at any time during the study. Headache was
the only complaint reported, which began to occur after the second
dose and persisted through the day following the last dose (Leegwater
1971).
Two male and two female volunteers were orally doses with
0.0125 mg/kg bw/day of triazophos for 5 days. Compared to pre-
treatment levels there was no depression (<20%) of cholinesterase in
plasma or erythrocyte measured 5 hours after the second and fourth
doses. Two days later, the same 2 male volunteers were given 2
additional courses of triazophos with a rest period of 2 days between
the courses. The first course comprised 5 daily doses at 0.03 mg/kg
bw/day and the second course 5 daily doses at 0.05 mg/kg bw/day.
Activity of plasma cholinesterase, slightly reduced (17-18%) in both
subjects after 4 doses in the first course and after 3 doses in the
second course, was significantly inhibited (>30%) after 4 days in
the second course. Complete recovery of the enzyme was not apparent
until 16 days following completion of the second course. Erythrocyte
cholinesterase was not adversely affected at any time during the
experiment (Leegwater 1972).
Healthy adult volunteers (2 males and 3 females, aged 18-23
years) were given 0.0125 mg triazophos/kg bw/day orally, 5 days/week
for 3 consecutive weeks in a pilot study. All 5 subjects completed the
study but one female missed one dose during the first dosing week.
There was no significant change in the activity of plasma or
erythrocyte cholinesterase at any time during the dosing period, as
compared to that in the one-week, pre-exposure 'run-in' period. The
only symptom reported was headache experienced by one female subject
each morning about 1 1/2 hours after dosing on the first 3 days of the
first treatment week. Medical examination and routine haematology,
biochemistry and urinalysis conducted before and 13 days after the
dosing period revealed no significant changes attributable to
triazophos, although one female volunteer (the same one complaining of
headache) showed a tendency toward anaemia (Cooper et al 1973a). In
a second pilot study, 3 male and 2 female healthy adult subjects were
given 0.025 mg triazophos/kg bw/day, 5 times weekly for 3 weeks.
Reduction of plasma cholinesterase (11-17%), which appeared to be
related to duration of treatment, was stated to attain statistical
significance in weeks 2 and 3 of treatment and during the first week
of withdrawal, Erythrocyte cholinesterase was not adversely affected
at any time during the study (Cooper et al 1973b).
Twenty-five healthy adult subjects (13 males and 12 females) were
administered 0.0125 mg triazophos/kg bw/day, 5 days/week for 3
consecutive weeks. Medical examination and routine haematology,
biochemistry and urinalysis were conducted prior to the study and at
the end of a 4-week post-dosing follow-up period. Plasma and
erythrocyte cholinesterase was assayed prior to the study and at least
three times weekly throughout the dosing and follow-up periods. All 25
subjects completed the study. Two males and 1 female were withdrawn
from treatment during the second dosing week owing to progressive
inhibition of plasma cholinesterase to less than 80% of the pre-dose
levels, but dosing resumed for these 3 during treatment week 3. Two
other subjects missed 1 or 2 doses, respectively, due to diarrhoea and
sickness. (Based on the statement that "other subjects who had to
unavoidably miss blood sampling (taken prior to the daily dose, i.e.
approximately 24 hours after the previous dose) on occasions during
the three dosing weeks were given their daily doses to take at the
appropriate time on the following day", it would appear that
additional subjects also might have missed an unspecified number of
doses during the treatment period.) During the first dosing week, 50%
of the female subjects reported urinary frequency with some degree of
urinary urgency. Other complaints, isolated in incidence, included
tiredness and lethargy, nausea and diarrhoea. There were some
significant differences between pre- and post-treatment values in
blood pressure, pulse rate and some laboratory parameters but the
post-treatment values were within the limits normally considered
acceptable to the testing laboratory. A comparison of the mean plasma
cholinesterase activity (of all 25 subjects) obtained before the test
with that at each dosing and follow-up week indicated no statistically
significant difference. However, a total of 6 individuals (including
the 3 withdrawn from treatment during the second week) exhibited a
depression (> 20%) of plasma cholinesterase, mainly during the second
and third week of dosing when their own pre-treatment levels were used
as the basis of comparison. Recovery of the enzyme to pre-exposure
levels were more or less complete in the majority of subjects by the
end of the fourth follow-up week. No significant compound-inducted
inhibition of erythrocyte cholinesterase was noted during the study.
The data suggested 0.0125 mg/kg body weight as the minimum effect
level with respect to plasma cholinesterase (Cracknell et al 1973).
COMMENTS
Triazophos was evaluated for the first time by the present
Meeting.
In rats, triazophos, an organophosphorous insecticide, is rapidly
eliminated predominantly in the urine and also in the faeces. It is
readily degraded, with urea being the major urinary metabolite.
Neither triazophos nor its metabolites appear to accumulate to any
significant extent in the tissues. Metabolism data in mammalian
species other than the rat are not available.
Oral LD50 values of triazophos ranged from approximately
30 mg/kg bw in mice, 65 mg/kg bw in rats to about 500 mg/kg bw in
dogs, suggesting a significant species variation in sensitivity to
acute toxicity of the compound. A significant sex difference was not
evident.
Summary data provided for a 3-generation reproduction study in
rats suggested 10 ppm as a no-effect level on reproduction. There were
no teratogenicity studies. An in vitro microbial assay and a delayed
neurotoxicity study in hens were negative. Nevertheless, the
possibility exists that the dosage level used (equivalent to an oral
LD50) in the hen study might be too low to permit an adequate testing
of the compound for its delayed neurotoxic potential. Studies designed
specifically to evaluate the carcinogenic activity of triazophos are
not available, although a 2-year feeding study in rats indicated no
increase in incidence of any particular type of tumour.
No-effect levels had been defined in 13-week and 2-year feeding
studies in rats and dogs, with plasma cholinesterase being
demonstrated to be the most sensitive indicator of biological
activity. Observations in humans suggested a significant individual
variation to the effect of triazophos on plasma cholinesterase.
Despite establishment of no-effect levels in two mammalian
species, the Meeting could only allocate a temporary ADI since
additional data, as listed below, are required to complete the
toxicological data base.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 0.3 ppm in the diet, equivalent to 0.008 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. A carcinogenicity study.
2. Teratogenicity study in at least one mammalian species.
3. Metabolism studies in additional mammalian species to explain
species differences in acute toxicity.
4. Additional mutagenicity studies.
Desirable
1. An appropriate delayed neurotoxicity study in hens.
2. Data on individual litters for the currently available three-
generation reproduction study in rats.
3. Acute oral toxicity data on any metabolite of triazophos that is
found in food crops.
REFERENCES
Bock, R. and Thier, W. Metabolism and fate of triazophos in rats.
1976 Pesticide Science 7: 307-314.
Berkvens, J.M. and Beems, R.B. Chronic (two-year) toxicity study with
1979 Hoe 2960 in rats. Part 3. Microscopic pathological
examination of intermediate dose animals. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Cooper, A.J., Haynes, G., Street, A.E. and Rudd, C.J. A pilot study to
1973a observe the in-vivo effects of Hoe-2960 on the
cholinesterase activity in man administered 0.0125 mg/kg day
p.o. Report from Huntingdon Research Centre, England.
Submitted to the World Health Organization by Hoechst.
(Unpublished)
1973b A pilot study to observe the in vivo effects of Hoe-2960 on
the cholinesterase activity in man administered 0.025
mg/kg/day p.o. Report from Huntingdon Research Centre,
England, Submitted to the World Health Organization by
Hoechst. (Unpublished)
Cracknell, D.D., Hawkes, G., Street, A.E. and Rudd, C.J. The in-vivo
1973 effects of Hoe-2960 on the cholinesterase activity in man
administered 0.0125 mg/kg/day. Report from Huntingdon
Research Centre, England. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Gericke, D. Test for mutagenicity in bacterial strains in the absence
1977 and presence of a liver preparation. Hoe-02960 01 AS 101.
Report from Hoechst. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Hollander and Weigand. Hoe-02960 01 AS 101 Irritance to the rabbit
1977a skin and eye mucosa. Report from Hoechst, Submitted to the
World Health Organization by Hoechst. (Unpublished)
1977b Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-NMRI
mouse (vehicle: sesame oil). Report from Hoechst submitted
to the World Health Organization by Hoechst. (Unpublished)
1977c Hoe-02960 01 AS 101 Acute oral toxicity to the female
SPF-NMRI mouse (vehicle: sesame oil). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977d Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-
Wistar rat (vehicle: starch suspension). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977e Akute orale toxizitat von Hoe 02960 01 AS 101 and weiblichen
SPF-Wistar ratten (vehikel: starkeschleim). Report from
Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Kramer and Weigand, Test of triazophos (Hoe-02960) for neurotoxicity
1974 in hens. Report from Farbeverke Hoechst AG submitted to the
World Health Organization by Hoechst. (Unpublished)
Leegwater, D.C. Preliminary study on the in-vivo effect of Hoe-2960 in
1971 man. Report from Central Institute for Nutrition and Food
Research, Zeist. Submitted to the World Health Organization
by Hoechst. (Unpublished)
1972 In-vivo study on the effect of Hoe-2960/Op 22 on the blood
cholinesterase of man. Report from Central Institute for
Nutrition and Food Research, Zeist. Submitted to the World
Health Organization by Hoechst. (Unpublished)
Scholz and Weigand. Triazophos-determination of the acute
1968 intraperitoneal toxicity. Report from Hoechst submitted to
the World Health Organization by Hoechst. (Unpublished)
1969 W12712 = Hoe-02960. 1-phenyl-1,2,4-triazolyl-3-(O,O-diethyl
thionophosphate) (Op. Techn. No. 3, Content 93%). Report
from Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Scholz and Brunk. W12712 = Hoe 2960. Acute oral toxicity in dogs.
1969 Report from Hoechst submitted to the World Health
Organization by Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Seinen, W. Subchronic (90-day) toxicity
1971a study with Hoe-2960 Op. 22 in albino rats. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Til, H.P., Seinen, W., Leegwater, D.C. and van der Meulen, H.C.
1971b Subchronic toxicity study with Hoe 2960, Op.22 in beagle
dogs. Report from Central Institute for Nutrition and Food
Research, Zeist, submitted to the World Health Organization
by Hoechst. (Unpublished)
Til, H.P., Hendriksen, C.F.M. and Leegwater D.C. Multigeneration study
1975 with Hoe-2960 in rats. Report from Central Institute for
Nutrition and Food Research, Zeist, submitted to the World
Health Organization by Farbeverke Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Kollen, C.H. Long-term (two year)
1976 toxicity study with Hoe 2960 in dogs. Report from Central
Institute for Nutrition and Food Research Zeist, submitted
to the World Health Organization by Hoechst. (Unpublished)
Til, H.P. and Leegwater, D.C. Chronic (two year) toxicity study with
1975 Hoe-2960 in rats. Part I. Results of clinical haematological
biochemical and organ weight measurements. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Weigand. Testing of dermal toxicity in female SPF-Wistar rats of our
1972 own breed, after a single treatment. Report (No. A 00092)
from Hoechst, submitted to the World Health Organization by
Hoechst. (Unpublished)
 Empirical formula
C12H16N303PS
Molecular weight
313.3
Other information on identity and properties
Pure active ingredient
Density 1.247 g/cm3 at 20°C
Melting point 2 - 5°C
Vapour pressure 1 × 10-5 Torr at 38°C
1 × 10-3 Torr at 74°C
Flash point
Open 192° ± 5°C (Cleveland)
Closed 135° ± 2°C (Pensky-Martens)
Solubility at 20°C
water 35 ppm ± 5 ppm
n-hexane 0.9%
toluene >33%
ethyl acetate 100%
acetone 100%
ethanol >33%
Technical active ingredient
Physical form light yellow to dark brown liquid.
Odour characteristic of phosphoric ester
Density 1.24 g/cm3 ± 0.05 g/cm3 at 20°C
Melting point 0°C to 5°C
Solubility soluble in aromatic hydrocarbons, alcohols,
esters and ketones
Stability
Chemical stability decomposed by acids and alkalies
Thermal stability not stable in undiluted form
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Elimination and Biotransformation
Rat
In male and female rats (Wistar SPF) treated with a single oral
dose of 14C-triazophos (labelling at the 3-position of the triazole
ring) at 2.76 mg/rat or approximately 15-21 mg/kg bw, about 76% of the
administered radioactive dose was excreted in the urine and 21% in the
faeces within 48 hours of dosing. No significant sex difference in
rate and route of elimination was evident. Analyses of tissues from
these animals sacrificed four days after treatment indicated 14C
residues detected (as % of administered dose) to be invariably less
than 0.04% in kidney, gonads, brain, muscle and skin, 0.089% in liver
and 0.31% in the gastrointestinal tract. The rapid elimination of
14C-triazophos was similarly observed when male and female rats were
intubated with the radioactive compound at 0.56 mg/rat or
approximately 3.1-4.3 mg/kg bw/day for 12 consecutive days. Thus, 69.5
- 83.4% of the daily administered dose was recovered in the urine and
18.1 - 30.9% in the faeces during the dosing period. At sacrifice four
days after termination of the 12-day treatment period, none of the
tissues analyses (subcutaneous fat, kidney, gonads, liver, brain,
muscle and skin) had 14C residues greater than 0.0008% of the
administered dose. An exception was the gastrointestinal tract, which
still contained 0.5% of the given dose.
The major urinary metabolite was urea-14C, amounting to
approximately 85% of the radioactivity excreted via this route. Other
metabolites detected in the urine were 1-phenyl-1,2,4-triazol-3-ol-3-
14C, 1-phenylsemicarbazide-3-14C and semicarbazide-14C, all as
conjugates with glucuronic acid. Each of these three metabolites
accounted for 3-5% of the 14C eliminated in the urine. In the faeces,
unchanged 14C-triazophos (40% of the total activity in this route)
and 1-phenyl-l,21-4-triazol-3-ol-3-14C (60% of the activity) were
identified (Bock and Thier 1976).
Effects on Enzymes and Other Biochemical Parameters
See under Observations in Humans.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
In a standard 3-generation (2 litters/generation) reproduction
study, 10 male and 20 female newly weaned rats (Wistar derived) were
fed dietary levels of triazophos at 0, 0.3, 1.0 or 10 ppm and mated at
weeks 12 and 20 to produce two successive litters. Weanlings of the
second litters (i.e. F1b and F2b) were selected to become parents of
the next generations. F3b weanlings, 10 males and 10 females/group,
were maintained on dietary feedings for 4 weeks for a toxicity study.
Based on summary data available (no data on individual litters
provided in the report), mortality, behaviour and body weight and
pregnancy rate were unaffected in any of the parental generations.
Except for a decrease in pup weight at 10 ppm on day 20 in the F2a
litters, there were no dose- or treatment-related effects as judged by
litter size at birth, sex ratio, mortality and body weight of young
during lactation, resorption quotient (number of implantation sites/
number of young born) and gross malformations of pups at birth. The
reduced pup weight on day 20 at 10 ppm appeared to be of doubtful
significance in the light of its occurrence in only a single progeny
generation (F2a litters). In F3b weanling pups maintained on dietary
feeding for four weeks, there was no mortality or adverse effect on
body weight. Significant inhibition (21%) of plasma cholinesterase
occurred at 10 ppm in the females after 23 days. Erythrocyte
cholinesterase assayed at the same period was not significantly
depressed in the treated groups. At terminal sacrifice, there were no
significant variations from controls in brain cholinesterase activity,
organ weights and gross pathological changes. Histopathological
evaluation of selected tissues from animals of control and top dosage
groups revealed no treatment-related alterations (Til et al 1975).
Special Studies on Mutagenicity
Triazophos was tested for its mutagenic potential in a reverse
mutation assay in the presence or absence of a metabolic activation
system (S-9 homogenate of liver from rats induced with Arochlor 1254).
The tester strains used were Salmonella typhimurium TA 98,
TA 100, TA 1535 and TA 1537. At the concentrations used, 0.2 to
5 000 µg/plate, triazophos was not mutagenic to any of the tester
strains with or without the presence of the metabolic activation
preparation (Gericke 1977).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Two groups of 6 white Leghorn hens (mean weight 1.75 kg) were
intubated with a single dose of 25 mg/kg bw of triazophos. Survivors
were similarly treated again with another dose of the same magnitude
21 days later and observed for a further period of 21 days. (The acute
oral LD50 of triazophos in hens was determined to be approximately
24 mg/kg bw prior to initiation of the neurotoxicity study.) Hens in
one of these groups were treated with atropine (10 mg/kg bw) and
Toxagonin (4 mg/kg bw) by an unspecified route once before and four or
five times after the first and second dose of triazophos. The
untreated control group comprised 6 hens. One hen in each of the
treated groups died after the first dose. Following the second dose,
mortality occurred in 2 hens protected with antidotes and in 3
unprotected hens. No neurotoxic signs were noted in any of the treated
birds at any time during the study, although toxic symptoms indicative
of anticholinesterase poisoning, such as disturbed equilibrium,
salivation, lethargy and extensor spasms, were present during the
first two days after each dose of triazophos. Histopathological
examination of the spinal medulla (cervical medulla, cervical
enlargement, thoracic medulla and lumbar enlargement) and sciatic
nerve from 5 surviving hens (3 of these with antidote treatment) at
25 mg/kg bw and 1 untreated control hen surviving terminally revealed
no morphologically demonstrable changes characteristic of delayed
neurotoxic effects. One of the 5 hens at 25 mg/kg bw, however, showed
macroscopic and microscopic lesions in medulla oblongata and spinal
medulla classified with chicken gliomata, which was considered to be
independent of treatment. Positive control hens, treated with TOCP,
displayed clinical symptoms and histopathological changes typical of
delayed neurotoxicity (Kramer and Weigand 1974).
Special Studies on Skin and Eye Irritation
Results of a patch test with rabbits (SPF-albino Himalayan
strain) indicated triazophos, as a 1% or 10% dilution in sesame oil,
to be practically non-irritating to the skin. However 5 of the 6
treated rabbits died 24-48 hours after 0.5 ml of undiluted triazophos
was applied to each of the two sites (one abraded) on their clipped
skin (Hollander and Weigand 1977a).
An eye irritation study in rabbits (SPF - albino Himalayan
strain) suggested undiluted triazophos, 1% or 10% dilutions of
triazophos in sesame oil, to be of a low irritant potential to eyes. A
single dose of 0.1 ml of the undiluted material applied to one eye
was, however, fatal to 1/6 treated rabbits in 48 hours (Hollander and
Weigand 1977a).
Acute Toxicity
Toxic signs of oral poisoning similar in mice and rats, were
characterized by tremors, abdominal position and squatting. Mortality
usually occurred 10 minutes to 5 days after lethal doses. No specific
information was given on the time of onset and duration of symptoms
(Hollander and Weigand, 1977b, 1977c, 1977d; Scholz and Weigand 1969,
Hollander and Weigand 1977e). The LD50 in these two species is
reported in Table 1 for different treatment routes and vehicles.
Table 1. Acute Toxicity to Triazophos in the Mouse and Rat
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Mouse M oral 1 sesame oil 31 Hollander and Weigand
(SPF-NMRI) 1977b
F 29 Hollander and
Weigand 1977c
Rat M oral starch 68 Hollander and
(SPF-Wistar) suspension Weigand 1977d
F oral starch slurry 66 Scholz and Weigand 1969
F oral starch mucilage 64 Hollander and Weigand
1977e
Rat F i.p. oil of benne 107 Scholz and Weigand 1968
(SPF-Wistar)
Rat F dermal 2 sesame oil 1100 Weigand 1972
(24-hour
exposure)
1 In the oral studies, animals were fasted 15 to 18 hours prior to dosing.
2 Treated site was occluded during the exposure period.
Dog
In groups of generally 2 beagle dogs (mixed sex; 20-hours fasted)
treated with single doses of triazophos (93% pure) in sesame oil by
gavage, no mortality occurred at 320 mg/kg bw and below. Both dogs at
800 mg/kg bw and 1/2 dogs at 500 mg/kg bw died within 3 days of
dosing. Vomiting was observed in 2/2 dogs at both 80 and 800 mg/kg bw,
1/2 dogs at 125 mg/kg bw and 1/1 dog at 160 mg/kg bw usually 3 to 6
hours after treatment, but in none of the 2 dogs each at 50, 320 and
500 mg/kg bw. Other toxic symptoms included hypersalivation, tremors,
miosis and staggering gait. The median lethal dose appeared to be in
the vicinity of 500 mg/kg bw (Scholz and Brunk 1969).
Short-Term Studies
Rat
Groups of 10 male and 10 female weanling rats (Wistar derived)
were fed diets containing triazophos (90% pure) at 0, 1, 3, 10 or
200 ppm for 13 weeks. The highest level was raised to 400 ppm in the
6th week. Survival was 100%. Body weight, food consumption and
behaviour as well as haematology, clinical chemistry and urinalysis
parameters were not adversely affected. Activity of plasma
cholinesterase was depressed (920%) in males of top dosage group and
in females at 3 ppm and above at all sampling intervals (weeks 3, 6
and 12). The enzyme was also inhibited in females at 1 ppm by 19% and
in males at 10 ppm by 23% at week 12 only. Terminal brain
cholinesterase and erythrocyte cholinesterase assayed at the three
intervals were inhibited (>20%) only in the top dosage group (both
sexes). The magnitude of depression of plasma, erythrocyte or brain
cholinesterase was greater in females than in males at the same dosage
levels. At terminal sacrifice, there were no gross pathological
changes or organ weight variation associated with the compound. No
treatment-induced histopathological changes were observed in a variety
of tissues evaluated. The study suggested 1 ppm as a virtual no-effect
level (Til et al 1971).
Dog
Male and female purebred beagles (3 males and 3 females per
group) were fed dietary levels of triazophos at 0, 0.03, 0.1, 0.3,
1.0, 5.0 or 100 ppm for 13 weeks. There was no mortality or treatment-
induced abnormal behaviour. Body weight, food consumption and
haematological, biochemical (including kidney and liver function
tests) and urinalysis values were not affected in any dose-response
pattern. Inhibition (>20%, dose related) of plasma cholinesterase
occurred at and above 1 ppm at weeks 3 and 7 and at both 5 and 100 ppm
at week 12 also. Whole blood cholinesterase was reduced (>20%) in the
top dosage group only at weeks 3, 7 and 12. Activity of terminal brain
cholinesterase was not inhibited (>20%). At the conclusion of the
study, organ weights and gross pathological changes were comparable in
all groups. Histopathological examination of a set of over 30 tissues
from each dog of the control and the treated groups at 1 ppm and above
revealed no abnormalities related to ingestion of triazole. The no-
effect level was 0.3 ppm (Til et al 1971).
Groups of 4 male and 4 female purebred beagles were fed
triazophos (purity not specified) in their diet at 0, 0.3, 1.0 or
3.0 ppm for 2 years. The highest dietary level was increased to 6 ppm
after 5 weeks and to 200 ppm after 16 weeks. There were no treatment-
related findings with respect to mortality, appearance and behaviour
and food consumption. Growth was decreased in the top dosage group
(both sexes) at week 36 and thereafter. Periodic haematological and
biochemical studies and urinalysis during the study indicated
significant changes, confined to the top dosage group, in values of
SAP, serum albumin, SGOT and specific gravity of the urine.
Measurements of plasma and erythrocyte cholinesterase nine times over
the course of the experiment revealed dose-related inhibition (>20%)
of plasma cholinesterase at and above 1 ppm at all intervals and at
0.3 ppm at weeks 13 and 26. Erythrocyte cholinesterase was depressed
(>20%) only at the top dosage group at week 17 and thereafter. Brain
cholinesterase assayed terminally was not inhibited (>20%). Animals
sacrificed at the end of the study exhibited an increase in absolute
weight of the liver and organ/body weight ratio of the liver and the
thyroid at the top dosage level. No compound-induced histopathological
lesions were observed in a variety of tissues evaluated, including
liver and thyroid. The dietary level of 0.3 ppm was a marginal no-
effect level (Til et al 1976).
Long-Term Studies
Rat
Groups of 30 male and 30 female rats (Wistar derived) were fed
dietary levels of triazophos (of unspecified purity) at 0, 0.3, 1.0,
3.0, 10 or 200 ppm for 2 years. (The 0.3 ppm and 10 ppm groups
were discarded at week 28 "because the plasma and erythrocyte
cholinesterase was not inhibited in the 0.3 and 1 ppm groups and
decreased in the 3 and 10 ppm groups".) Mortality, appearance and
general behaviour and body weight were not affected by treatment.
Between 23-70% males and 43-87% females of all groups, including the
control, survived the duration of the study. (Over 50% males and at
least 70% females per control and treated group were still alive at 96
weeks.) Food consumption was slightly reduced at 200 ppm in males
during the first year and in females after 47 weeks. Plasma
cholinesterase was inhibited (>20%), in a dose-dependent pattern, at
10 ppm at weeks 4 (females only), 13 and 26 (both sexes) and at 3 ppm
in females at weeks 52 and 102. Animals at 200 ppm exhibited marked
depression (>20%) of plasma and erythrocyte cholinesterase (both
sexes) at all intervals and of terminal brain cholinesterase (females
only). No compound-related effects were observed on haematological,
biochemical and urine parameters measured periodically up to five
times over the course of the study. At the conclusion of the
experiment, there were some variations in organ/body weight ratio of
several selected organs but they did not follow a dose-response trend.
Histopathological evaluation of over 20 organs plus all grossly
abnormal tissues from each animal, initially limited to 20 males and
20 females of the control and 200 ppm groups (comprising terminal
survivors supplemented with animals sacrificed in extremis or dying
toward the end of the study) and later extended to all animals of the
intermediate dosage groups, showed no alterations attributable to
inclusion of triazophos in the diet. There were no dose- or compound-
related increases in incidence of any particular type of tumour. Based
on the data, 1 ppm was the no-effect level (Til and Leegwater 1975;
Birkvens and Beems 1979).
Observations in Humans
In a preliminary study, a male volunteer (80 kg) was given
triazophos orally for 4 consecutive mornings. The dose was 1 mg or
0.0125 mg/kg body weight/day on day 1 and 5 mg (0.0625 mg/kg body
weight/day) from days 2 through 4. Depression (=20%) of plasma
cholinesterase, initially observed after the third dose and more
pronounced after the fourth dose, was still evident 3 days after
treatment withdrawal. No inhibition (<20%) of erythrocyte
cholinesterase was noted at any time during the study. Headache was
the only complaint reported, which began to occur after the second
dose and persisted through the day following the last dose (Leegwater
1971).
Two male and two female volunteers were orally doses with
0.0125 mg/kg bw/day of triazophos for 5 days. Compared to pre-
treatment levels there was no depression (<20%) of cholinesterase in
plasma or erythrocyte measured 5 hours after the second and fourth
doses. Two days later, the same 2 male volunteers were given 2
additional courses of triazophos with a rest period of 2 days between
the courses. The first course comprised 5 daily doses at 0.03 mg/kg
bw/day and the second course 5 daily doses at 0.05 mg/kg bw/day.
Activity of plasma cholinesterase, slightly reduced (17-18%) in both
subjects after 4 doses in the first course and after 3 doses in the
second course, was significantly inhibited (>30%) after 4 days in
the second course. Complete recovery of the enzyme was not apparent
until 16 days following completion of the second course. Erythrocyte
cholinesterase was not adversely affected at any time during the
experiment (Leegwater 1972).
Healthy adult volunteers (2 males and 3 females, aged 18-23
years) were given 0.0125 mg triazophos/kg bw/day orally, 5 days/week
for 3 consecutive weeks in a pilot study. All 5 subjects completed the
study but one female missed one dose during the first dosing week.
There was no significant change in the activity of plasma or
erythrocyte cholinesterase at any time during the dosing period, as
compared to that in the one-week, pre-exposure 'run-in' period. The
only symptom reported was headache experienced by one female subject
each morning about 1 1/2 hours after dosing on the first 3 days of the
first treatment week. Medical examination and routine haematology,
biochemistry and urinalysis conducted before and 13 days after the
dosing period revealed no significant changes attributable to
triazophos, although one female volunteer (the same one complaining of
headache) showed a tendency toward anaemia (Cooper et al 1973a). In
a second pilot study, 3 male and 2 female healthy adult subjects were
given 0.025 mg triazophos/kg bw/day, 5 times weekly for 3 weeks.
Reduction of plasma cholinesterase (11-17%), which appeared to be
related to duration of treatment, was stated to attain statistical
significance in weeks 2 and 3 of treatment and during the first week
of withdrawal, Erythrocyte cholinesterase was not adversely affected
at any time during the study (Cooper et al 1973b).
Twenty-five healthy adult subjects (13 males and 12 females) were
administered 0.0125 mg triazophos/kg bw/day, 5 days/week for 3
consecutive weeks. Medical examination and routine haematology,
biochemistry and urinalysis were conducted prior to the study and at
the end of a 4-week post-dosing follow-up period. Plasma and
erythrocyte cholinesterase was assayed prior to the study and at least
three times weekly throughout the dosing and follow-up periods. All 25
subjects completed the study. Two males and 1 female were withdrawn
from treatment during the second dosing week owing to progressive
inhibition of plasma cholinesterase to less than 80% of the pre-dose
levels, but dosing resumed for these 3 during treatment week 3. Two
other subjects missed 1 or 2 doses, respectively, due to diarrhoea and
sickness. (Based on the statement that "other subjects who had to
unavoidably miss blood sampling (taken prior to the daily dose, i.e.
approximately 24 hours after the previous dose) on occasions during
the three dosing weeks were given their daily doses to take at the
appropriate time on the following day", it would appear that
additional subjects also might have missed an unspecified number of
doses during the treatment period.) During the first dosing week, 50%
of the female subjects reported urinary frequency with some degree of
urinary urgency. Other complaints, isolated in incidence, included
tiredness and lethargy, nausea and diarrhoea. There were some
significant differences between pre- and post-treatment values in
blood pressure, pulse rate and some laboratory parameters but the
post-treatment values were within the limits normally considered
acceptable to the testing laboratory. A comparison of the mean plasma
cholinesterase activity (of all 25 subjects) obtained before the test
with that at each dosing and follow-up week indicated no statistically
significant difference. However, a total of 6 individuals (including
the 3 withdrawn from treatment during the second week) exhibited a
depression (> 20%) of plasma cholinesterase, mainly during the second
and third week of dosing when their own pre-treatment levels were used
as the basis of comparison. Recovery of the enzyme to pre-exposure
levels were more or less complete in the majority of subjects by the
end of the fourth follow-up week. No significant compound-inducted
inhibition of erythrocyte cholinesterase was noted during the study.
The data suggested 0.0125 mg/kg body weight as the minimum effect
level with respect to plasma cholinesterase (Cracknell et al 1973).
COMMENTS
Triazophos was evaluated for the first time by the present
Meeting.
In rats, triazophos, an organophosphorous insecticide, is rapidly
eliminated predominantly in the urine and also in the faeces. It is
readily degraded, with urea being the major urinary metabolite.
Neither triazophos nor its metabolites appear to accumulate to any
significant extent in the tissues. Metabolism data in mammalian
species other than the rat are not available.
Oral LD50 values of triazophos ranged from approximately
30 mg/kg bw in mice, 65 mg/kg bw in rats to about 500 mg/kg bw in
dogs, suggesting a significant species variation in sensitivity to
acute toxicity of the compound. A significant sex difference was not
evident.
Summary data provided for a 3-generation reproduction study in
rats suggested 10 ppm as a no-effect level on reproduction. There were
no teratogenicity studies. An in vitro microbial assay and a delayed
neurotoxicity study in hens were negative. Nevertheless, the
possibility exists that the dosage level used (equivalent to an oral
LD50) in the hen study might be too low to permit an adequate testing
of the compound for its delayed neurotoxic potential. Studies designed
specifically to evaluate the carcinogenic activity of triazophos are
not available, although a 2-year feeding study in rats indicated no
increase in incidence of any particular type of tumour.
No-effect levels had been defined in 13-week and 2-year feeding
studies in rats and dogs, with plasma cholinesterase being
demonstrated to be the most sensitive indicator of biological
activity. Observations in humans suggested a significant individual
variation to the effect of triazophos on plasma cholinesterase.
Despite establishment of no-effect levels in two mammalian
species, the Meeting could only allocate a temporary ADI since
additional data, as listed below, are required to complete the
toxicological data base.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 0.3 ppm in the diet, equivalent to 0.008 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. A carcinogenicity study.
2. Teratogenicity study in at least one mammalian species.
3. Metabolism studies in additional mammalian species to explain
species differences in acute toxicity.
4. Additional mutagenicity studies.
Desirable
1. An appropriate delayed neurotoxicity study in hens.
2. Data on individual litters for the currently available three-
generation reproduction study in rats.
3. Acute oral toxicity data on any metabolite of triazophos that is
found in food crops.
REFERENCES
Bock, R. and Thier, W. Metabolism and fate of triazophos in rats.
1976 Pesticide Science 7: 307-314.
Berkvens, J.M. and Beems, R.B. Chronic (two-year) toxicity study with
1979 Hoe 2960 in rats. Part 3. Microscopic pathological
examination of intermediate dose animals. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Cooper, A.J., Haynes, G., Street, A.E. and Rudd, C.J. A pilot study to
1973a observe the in-vivo effects of Hoe-2960 on the
cholinesterase activity in man administered 0.0125 mg/kg day
p.o. Report from Huntingdon Research Centre, England.
Submitted to the World Health Organization by Hoechst.
(Unpublished)
1973b A pilot study to observe the in vivo effects of Hoe-2960 on
the cholinesterase activity in man administered 0.025
mg/kg/day p.o. Report from Huntingdon Research Centre,
England, Submitted to the World Health Organization by
Hoechst. (Unpublished)
Cracknell, D.D., Hawkes, G., Street, A.E. and Rudd, C.J. The in-vivo
1973 effects of Hoe-2960 on the cholinesterase activity in man
administered 0.0125 mg/kg/day. Report from Huntingdon
Research Centre, England. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Gericke, D. Test for mutagenicity in bacterial strains in the absence
1977 and presence of a liver preparation. Hoe-02960 01 AS 101.
Report from Hoechst. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Hollander and Weigand. Hoe-02960 01 AS 101 Irritance to the rabbit
1977a skin and eye mucosa. Report from Hoechst, Submitted to the
World Health Organization by Hoechst. (Unpublished)
1977b Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-NMRI
mouse (vehicle: sesame oil). Report from Hoechst submitted
to the World Health Organization by Hoechst. (Unpublished)
1977c Hoe-02960 01 AS 101 Acute oral toxicity to the female
SPF-NMRI mouse (vehicle: sesame oil). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977d Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-
Wistar rat (vehicle: starch suspension). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977e Akute orale toxizitat von Hoe 02960 01 AS 101 and weiblichen
SPF-Wistar ratten (vehikel: starkeschleim). Report from
Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Kramer and Weigand, Test of triazophos (Hoe-02960) for neurotoxicity
1974 in hens. Report from Farbeverke Hoechst AG submitted to the
World Health Organization by Hoechst. (Unpublished)
Leegwater, D.C. Preliminary study on the in-vivo effect of Hoe-2960 in
1971 man. Report from Central Institute for Nutrition and Food
Research, Zeist. Submitted to the World Health Organization
by Hoechst. (Unpublished)
1972 In-vivo study on the effect of Hoe-2960/Op 22 on the blood
cholinesterase of man. Report from Central Institute for
Nutrition and Food Research, Zeist. Submitted to the World
Health Organization by Hoechst. (Unpublished)
Scholz and Weigand. Triazophos-determination of the acute
1968 intraperitoneal toxicity. Report from Hoechst submitted to
the World Health Organization by Hoechst. (Unpublished)
1969 W12712 = Hoe-02960. 1-phenyl-1,2,4-triazolyl-3-(O,O-diethyl
thionophosphate) (Op. Techn. No. 3, Content 93%). Report
from Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Scholz and Brunk. W12712 = Hoe 2960. Acute oral toxicity in dogs.
1969 Report from Hoechst submitted to the World Health
Organization by Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Seinen, W. Subchronic (90-day) toxicity
1971a study with Hoe-2960 Op. 22 in albino rats. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Til, H.P., Seinen, W., Leegwater, D.C. and van der Meulen, H.C.
1971b Subchronic toxicity study with Hoe 2960, Op.22 in beagle
dogs. Report from Central Institute for Nutrition and Food
Research, Zeist, submitted to the World Health Organization
by Hoechst. (Unpublished)
Til, H.P., Hendriksen, C.F.M. and Leegwater D.C. Multigeneration study
1975 with Hoe-2960 in rats. Report from Central Institute for
Nutrition and Food Research, Zeist, submitted to the World
Health Organization by Farbeverke Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Kollen, C.H. Long-term (two year)
1976 toxicity study with Hoe 2960 in dogs. Report from Central
Institute for Nutrition and Food Research Zeist, submitted
to the World Health Organization by Hoechst. (Unpublished)
Til, H.P. and Leegwater, D.C. Chronic (two year) toxicity study with
1975 Hoe-2960 in rats. Part I. Results of clinical haematological
biochemical and organ weight measurements. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Weigand. Testing of dermal toxicity in female SPF-Wistar rats of our
1972 own breed, after a single treatment. Report (No. A 00092)
from Hoechst, submitted to the World Health Organization by
Hoechst. (Unpublished)
Empirical formula
C12H16N303PS
Molecular weight
313.3
Other information on identity and properties
Pure active ingredient
Density 1.247 g/cm3 at 20°C
Melting point 2 - 5°C
Vapour pressure 1 × 10-5 Torr at 38°C
1 × 10-3 Torr at 74°C
Flash point
Open 192° ± 5°C (Cleveland)
Closed 135° ± 2°C (Pensky-Martens)
Solubility at 20°C
water 35 ppm ± 5 ppm
n-hexane 0.9%
toluene >33%
ethyl acetate 100%
acetone 100%
ethanol >33%
Technical active ingredient
Physical form light yellow to dark brown liquid.
Odour characteristic of phosphoric ester
Density 1.24 g/cm3 ± 0.05 g/cm3 at 20°C
Melting point 0°C to 5°C
Solubility soluble in aromatic hydrocarbons, alcohols,
esters and ketones
Stability
Chemical stability decomposed by acids and alkalies
Thermal stability not stable in undiluted form
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Elimination and Biotransformation
Rat
In male and female rats (Wistar SPF) treated with a single oral
dose of 14C-triazophos (labelling at the 3-position of the triazole
ring) at 2.76 mg/rat or approximately 15-21 mg/kg bw, about 76% of the
administered radioactive dose was excreted in the urine and 21% in the
faeces within 48 hours of dosing. No significant sex difference in
rate and route of elimination was evident. Analyses of tissues from
these animals sacrificed four days after treatment indicated 14C
residues detected (as % of administered dose) to be invariably less
than 0.04% in kidney, gonads, brain, muscle and skin, 0.089% in liver
and 0.31% in the gastrointestinal tract. The rapid elimination of
14C-triazophos was similarly observed when male and female rats were
intubated with the radioactive compound at 0.56 mg/rat or
approximately 3.1-4.3 mg/kg bw/day for 12 consecutive days. Thus, 69.5
- 83.4% of the daily administered dose was recovered in the urine and
18.1 - 30.9% in the faeces during the dosing period. At sacrifice four
days after termination of the 12-day treatment period, none of the
tissues analyses (subcutaneous fat, kidney, gonads, liver, brain,
muscle and skin) had 14C residues greater than 0.0008% of the
administered dose. An exception was the gastrointestinal tract, which
still contained 0.5% of the given dose.
The major urinary metabolite was urea-14C, amounting to
approximately 85% of the radioactivity excreted via this route. Other
metabolites detected in the urine were 1-phenyl-1,2,4-triazol-3-ol-3-
14C, 1-phenylsemicarbazide-3-14C and semicarbazide-14C, all as
conjugates with glucuronic acid. Each of these three metabolites
accounted for 3-5% of the 14C eliminated in the urine. In the faeces,
unchanged 14C-triazophos (40% of the total activity in this route)
and 1-phenyl-l,21-4-triazol-3-ol-3-14C (60% of the activity) were
identified (Bock and Thier 1976).
Effects on Enzymes and Other Biochemical Parameters
See under Observations in Humans.
TOXICOLOGICAL STUDIES
Special Study on Reproduction
In a standard 3-generation (2 litters/generation) reproduction
study, 10 male and 20 female newly weaned rats (Wistar derived) were
fed dietary levels of triazophos at 0, 0.3, 1.0 or 10 ppm and mated at
weeks 12 and 20 to produce two successive litters. Weanlings of the
second litters (i.e. F1b and F2b) were selected to become parents of
the next generations. F3b weanlings, 10 males and 10 females/group,
were maintained on dietary feedings for 4 weeks for a toxicity study.
Based on summary data available (no data on individual litters
provided in the report), mortality, behaviour and body weight and
pregnancy rate were unaffected in any of the parental generations.
Except for a decrease in pup weight at 10 ppm on day 20 in the F2a
litters, there were no dose- or treatment-related effects as judged by
litter size at birth, sex ratio, mortality and body weight of young
during lactation, resorption quotient (number of implantation sites/
number of young born) and gross malformations of pups at birth. The
reduced pup weight on day 20 at 10 ppm appeared to be of doubtful
significance in the light of its occurrence in only a single progeny
generation (F2a litters). In F3b weanling pups maintained on dietary
feeding for four weeks, there was no mortality or adverse effect on
body weight. Significant inhibition (21%) of plasma cholinesterase
occurred at 10 ppm in the females after 23 days. Erythrocyte
cholinesterase assayed at the same period was not significantly
depressed in the treated groups. At terminal sacrifice, there were no
significant variations from controls in brain cholinesterase activity,
organ weights and gross pathological changes. Histopathological
evaluation of selected tissues from animals of control and top dosage
groups revealed no treatment-related alterations (Til et al 1975).
Special Studies on Mutagenicity
Triazophos was tested for its mutagenic potential in a reverse
mutation assay in the presence or absence of a metabolic activation
system (S-9 homogenate of liver from rats induced with Arochlor 1254).
The tester strains used were Salmonella typhimurium TA 98,
TA 100, TA 1535 and TA 1537. At the concentrations used, 0.2 to
5 000 µg/plate, triazophos was not mutagenic to any of the tester
strains with or without the presence of the metabolic activation
preparation (Gericke 1977).
Special Studies on Carcinogenicity
See under Long-Term Studies.
Special Studies on Neurotoxicity
Hen
Two groups of 6 white Leghorn hens (mean weight 1.75 kg) were
intubated with a single dose of 25 mg/kg bw of triazophos. Survivors
were similarly treated again with another dose of the same magnitude
21 days later and observed for a further period of 21 days. (The acute
oral LD50 of triazophos in hens was determined to be approximately
24 mg/kg bw prior to initiation of the neurotoxicity study.) Hens in
one of these groups were treated with atropine (10 mg/kg bw) and
Toxagonin (4 mg/kg bw) by an unspecified route once before and four or
five times after the first and second dose of triazophos. The
untreated control group comprised 6 hens. One hen in each of the
treated groups died after the first dose. Following the second dose,
mortality occurred in 2 hens protected with antidotes and in 3
unprotected hens. No neurotoxic signs were noted in any of the treated
birds at any time during the study, although toxic symptoms indicative
of anticholinesterase poisoning, such as disturbed equilibrium,
salivation, lethargy and extensor spasms, were present during the
first two days after each dose of triazophos. Histopathological
examination of the spinal medulla (cervical medulla, cervical
enlargement, thoracic medulla and lumbar enlargement) and sciatic
nerve from 5 surviving hens (3 of these with antidote treatment) at
25 mg/kg bw and 1 untreated control hen surviving terminally revealed
no morphologically demonstrable changes characteristic of delayed
neurotoxic effects. One of the 5 hens at 25 mg/kg bw, however, showed
macroscopic and microscopic lesions in medulla oblongata and spinal
medulla classified with chicken gliomata, which was considered to be
independent of treatment. Positive control hens, treated with TOCP,
displayed clinical symptoms and histopathological changes typical of
delayed neurotoxicity (Kramer and Weigand 1974).
Special Studies on Skin and Eye Irritation
Results of a patch test with rabbits (SPF-albino Himalayan
strain) indicated triazophos, as a 1% or 10% dilution in sesame oil,
to be practically non-irritating to the skin. However 5 of the 6
treated rabbits died 24-48 hours after 0.5 ml of undiluted triazophos
was applied to each of the two sites (one abraded) on their clipped
skin (Hollander and Weigand 1977a).
An eye irritation study in rabbits (SPF - albino Himalayan
strain) suggested undiluted triazophos, 1% or 10% dilutions of
triazophos in sesame oil, to be of a low irritant potential to eyes. A
single dose of 0.1 ml of the undiluted material applied to one eye
was, however, fatal to 1/6 treated rabbits in 48 hours (Hollander and
Weigand 1977a).
Acute Toxicity
Toxic signs of oral poisoning similar in mice and rats, were
characterized by tremors, abdominal position and squatting. Mortality
usually occurred 10 minutes to 5 days after lethal doses. No specific
information was given on the time of onset and duration of symptoms
(Hollander and Weigand, 1977b, 1977c, 1977d; Scholz and Weigand 1969,
Hollander and Weigand 1977e). The LD50 in these two species is
reported in Table 1 for different treatment routes and vehicles.
Table 1. Acute Toxicity to Triazophos in the Mouse and Rat
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Mouse M oral 1 sesame oil 31 Hollander and Weigand
(SPF-NMRI) 1977b
F 29 Hollander and
Weigand 1977c
Rat M oral starch 68 Hollander and
(SPF-Wistar) suspension Weigand 1977d
F oral starch slurry 66 Scholz and Weigand 1969
F oral starch mucilage 64 Hollander and Weigand
1977e
Rat F i.p. oil of benne 107 Scholz and Weigand 1968
(SPF-Wistar)
Rat F dermal 2 sesame oil 1100 Weigand 1972
(24-hour
exposure)
1 In the oral studies, animals were fasted 15 to 18 hours prior to dosing.
2 Treated site was occluded during the exposure period.
Dog
In groups of generally 2 beagle dogs (mixed sex; 20-hours fasted)
treated with single doses of triazophos (93% pure) in sesame oil by
gavage, no mortality occurred at 320 mg/kg bw and below. Both dogs at
800 mg/kg bw and 1/2 dogs at 500 mg/kg bw died within 3 days of
dosing. Vomiting was observed in 2/2 dogs at both 80 and 800 mg/kg bw,
1/2 dogs at 125 mg/kg bw and 1/1 dog at 160 mg/kg bw usually 3 to 6
hours after treatment, but in none of the 2 dogs each at 50, 320 and
500 mg/kg bw. Other toxic symptoms included hypersalivation, tremors,
miosis and staggering gait. The median lethal dose appeared to be in
the vicinity of 500 mg/kg bw (Scholz and Brunk 1969).
Short-Term Studies
Rat
Groups of 10 male and 10 female weanling rats (Wistar derived)
were fed diets containing triazophos (90% pure) at 0, 1, 3, 10 or
200 ppm for 13 weeks. The highest level was raised to 400 ppm in the
6th week. Survival was 100%. Body weight, food consumption and
behaviour as well as haematology, clinical chemistry and urinalysis
parameters were not adversely affected. Activity of plasma
cholinesterase was depressed (920%) in males of top dosage group and
in females at 3 ppm and above at all sampling intervals (weeks 3, 6
and 12). The enzyme was also inhibited in females at 1 ppm by 19% and
in males at 10 ppm by 23% at week 12 only. Terminal brain
cholinesterase and erythrocyte cholinesterase assayed at the three
intervals were inhibited (>20%) only in the top dosage group (both
sexes). The magnitude of depression of plasma, erythrocyte or brain
cholinesterase was greater in females than in males at the same dosage
levels. At terminal sacrifice, there were no gross pathological
changes or organ weight variation associated with the compound. No
treatment-induced histopathological changes were observed in a variety
of tissues evaluated. The study suggested 1 ppm as a virtual no-effect
level (Til et al 1971).
Dog
Male and female purebred beagles (3 males and 3 females per
group) were fed dietary levels of triazophos at 0, 0.03, 0.1, 0.3,
1.0, 5.0 or 100 ppm for 13 weeks. There was no mortality or treatment-
induced abnormal behaviour. Body weight, food consumption and
haematological, biochemical (including kidney and liver function
tests) and urinalysis values were not affected in any dose-response
pattern. Inhibition (>20%, dose related) of plasma cholinesterase
occurred at and above 1 ppm at weeks 3 and 7 and at both 5 and 100 ppm
at week 12 also. Whole blood cholinesterase was reduced (>20%) in the
top dosage group only at weeks 3, 7 and 12. Activity of terminal brain
cholinesterase was not inhibited (>20%). At the conclusion of the
study, organ weights and gross pathological changes were comparable in
all groups. Histopathological examination of a set of over 30 tissues
from each dog of the control and the treated groups at 1 ppm and above
revealed no abnormalities related to ingestion of triazole. The no-
effect level was 0.3 ppm (Til et al 1971).
Groups of 4 male and 4 female purebred beagles were fed
triazophos (purity not specified) in their diet at 0, 0.3, 1.0 or
3.0 ppm for 2 years. The highest dietary level was increased to 6 ppm
after 5 weeks and to 200 ppm after 16 weeks. There were no treatment-
related findings with respect to mortality, appearance and behaviour
and food consumption. Growth was decreased in the top dosage group
(both sexes) at week 36 and thereafter. Periodic haematological and
biochemical studies and urinalysis during the study indicated
significant changes, confined to the top dosage group, in values of
SAP, serum albumin, SGOT and specific gravity of the urine.
Measurements of plasma and erythrocyte cholinesterase nine times over
the course of the experiment revealed dose-related inhibition (>20%)
of plasma cholinesterase at and above 1 ppm at all intervals and at
0.3 ppm at weeks 13 and 26. Erythrocyte cholinesterase was depressed
(>20%) only at the top dosage group at week 17 and thereafter. Brain
cholinesterase assayed terminally was not inhibited (>20%). Animals
sacrificed at the end of the study exhibited an increase in absolute
weight of the liver and organ/body weight ratio of the liver and the
thyroid at the top dosage level. No compound-induced histopathological
lesions were observed in a variety of tissues evaluated, including
liver and thyroid. The dietary level of 0.3 ppm was a marginal no-
effect level (Til et al 1976).
Long-Term Studies
Rat
Groups of 30 male and 30 female rats (Wistar derived) were fed
dietary levels of triazophos (of unspecified purity) at 0, 0.3, 1.0,
3.0, 10 or 200 ppm for 2 years. (The 0.3 ppm and 10 ppm groups
were discarded at week 28 "because the plasma and erythrocyte
cholinesterase was not inhibited in the 0.3 and 1 ppm groups and
decreased in the 3 and 10 ppm groups".) Mortality, appearance and
general behaviour and body weight were not affected by treatment.
Between 23-70% males and 43-87% females of all groups, including the
control, survived the duration of the study. (Over 50% males and at
least 70% females per control and treated group were still alive at 96
weeks.) Food consumption was slightly reduced at 200 ppm in males
during the first year and in females after 47 weeks. Plasma
cholinesterase was inhibited (>20%), in a dose-dependent pattern, at
10 ppm at weeks 4 (females only), 13 and 26 (both sexes) and at 3 ppm
in females at weeks 52 and 102. Animals at 200 ppm exhibited marked
depression (>20%) of plasma and erythrocyte cholinesterase (both
sexes) at all intervals and of terminal brain cholinesterase (females
only). No compound-related effects were observed on haematological,
biochemical and urine parameters measured periodically up to five
times over the course of the study. At the conclusion of the
experiment, there were some variations in organ/body weight ratio of
several selected organs but they did not follow a dose-response trend.
Histopathological evaluation of over 20 organs plus all grossly
abnormal tissues from each animal, initially limited to 20 males and
20 females of the control and 200 ppm groups (comprising terminal
survivors supplemented with animals sacrificed in extremis or dying
toward the end of the study) and later extended to all animals of the
intermediate dosage groups, showed no alterations attributable to
inclusion of triazophos in the diet. There were no dose- or compound-
related increases in incidence of any particular type of tumour. Based
on the data, 1 ppm was the no-effect level (Til and Leegwater 1975;
Birkvens and Beems 1979).
Observations in Humans
In a preliminary study, a male volunteer (80 kg) was given
triazophos orally for 4 consecutive mornings. The dose was 1 mg or
0.0125 mg/kg body weight/day on day 1 and 5 mg (0.0625 mg/kg body
weight/day) from days 2 through 4. Depression (=20%) of plasma
cholinesterase, initially observed after the third dose and more
pronounced after the fourth dose, was still evident 3 days after
treatment withdrawal. No inhibition (<20%) of erythrocyte
cholinesterase was noted at any time during the study. Headache was
the only complaint reported, which began to occur after the second
dose and persisted through the day following the last dose (Leegwater
1971).
Two male and two female volunteers were orally doses with
0.0125 mg/kg bw/day of triazophos for 5 days. Compared to pre-
treatment levels there was no depression (<20%) of cholinesterase in
plasma or erythrocyte measured 5 hours after the second and fourth
doses. Two days later, the same 2 male volunteers were given 2
additional courses of triazophos with a rest period of 2 days between
the courses. The first course comprised 5 daily doses at 0.03 mg/kg
bw/day and the second course 5 daily doses at 0.05 mg/kg bw/day.
Activity of plasma cholinesterase, slightly reduced (17-18%) in both
subjects after 4 doses in the first course and after 3 doses in the
second course, was significantly inhibited (>30%) after 4 days in
the second course. Complete recovery of the enzyme was not apparent
until 16 days following completion of the second course. Erythrocyte
cholinesterase was not adversely affected at any time during the
experiment (Leegwater 1972).
Healthy adult volunteers (2 males and 3 females, aged 18-23
years) were given 0.0125 mg triazophos/kg bw/day orally, 5 days/week
for 3 consecutive weeks in a pilot study. All 5 subjects completed the
study but one female missed one dose during the first dosing week.
There was no significant change in the activity of plasma or
erythrocyte cholinesterase at any time during the dosing period, as
compared to that in the one-week, pre-exposure 'run-in' period. The
only symptom reported was headache experienced by one female subject
each morning about 1 1/2 hours after dosing on the first 3 days of the
first treatment week. Medical examination and routine haematology,
biochemistry and urinalysis conducted before and 13 days after the
dosing period revealed no significant changes attributable to
triazophos, although one female volunteer (the same one complaining of
headache) showed a tendency toward anaemia (Cooper et al 1973a). In
a second pilot study, 3 male and 2 female healthy adult subjects were
given 0.025 mg triazophos/kg bw/day, 5 times weekly for 3 weeks.
Reduction of plasma cholinesterase (11-17%), which appeared to be
related to duration of treatment, was stated to attain statistical
significance in weeks 2 and 3 of treatment and during the first week
of withdrawal, Erythrocyte cholinesterase was not adversely affected
at any time during the study (Cooper et al 1973b).
Twenty-five healthy adult subjects (13 males and 12 females) were
administered 0.0125 mg triazophos/kg bw/day, 5 days/week for 3
consecutive weeks. Medical examination and routine haematology,
biochemistry and urinalysis were conducted prior to the study and at
the end of a 4-week post-dosing follow-up period. Plasma and
erythrocyte cholinesterase was assayed prior to the study and at least
three times weekly throughout the dosing and follow-up periods. All 25
subjects completed the study. Two males and 1 female were withdrawn
from treatment during the second dosing week owing to progressive
inhibition of plasma cholinesterase to less than 80% of the pre-dose
levels, but dosing resumed for these 3 during treatment week 3. Two
other subjects missed 1 or 2 doses, respectively, due to diarrhoea and
sickness. (Based on the statement that "other subjects who had to
unavoidably miss blood sampling (taken prior to the daily dose, i.e.
approximately 24 hours after the previous dose) on occasions during
the three dosing weeks were given their daily doses to take at the
appropriate time on the following day", it would appear that
additional subjects also might have missed an unspecified number of
doses during the treatment period.) During the first dosing week, 50%
of the female subjects reported urinary frequency with some degree of
urinary urgency. Other complaints, isolated in incidence, included
tiredness and lethargy, nausea and diarrhoea. There were some
significant differences between pre- and post-treatment values in
blood pressure, pulse rate and some laboratory parameters but the
post-treatment values were within the limits normally considered
acceptable to the testing laboratory. A comparison of the mean plasma
cholinesterase activity (of all 25 subjects) obtained before the test
with that at each dosing and follow-up week indicated no statistically
significant difference. However, a total of 6 individuals (including
the 3 withdrawn from treatment during the second week) exhibited a
depression (> 20%) of plasma cholinesterase, mainly during the second
and third week of dosing when their own pre-treatment levels were used
as the basis of comparison. Recovery of the enzyme to pre-exposure
levels were more or less complete in the majority of subjects by the
end of the fourth follow-up week. No significant compound-inducted
inhibition of erythrocyte cholinesterase was noted during the study.
The data suggested 0.0125 mg/kg body weight as the minimum effect
level with respect to plasma cholinesterase (Cracknell et al 1973).
COMMENTS
Triazophos was evaluated for the first time by the present
Meeting.
In rats, triazophos, an organophosphorous insecticide, is rapidly
eliminated predominantly in the urine and also in the faeces. It is
readily degraded, with urea being the major urinary metabolite.
Neither triazophos nor its metabolites appear to accumulate to any
significant extent in the tissues. Metabolism data in mammalian
species other than the rat are not available.
Oral LD50 values of triazophos ranged from approximately
30 mg/kg bw in mice, 65 mg/kg bw in rats to about 500 mg/kg bw in
dogs, suggesting a significant species variation in sensitivity to
acute toxicity of the compound. A significant sex difference was not
evident.
Summary data provided for a 3-generation reproduction study in
rats suggested 10 ppm as a no-effect level on reproduction. There were
no teratogenicity studies. An in vitro microbial assay and a delayed
neurotoxicity study in hens were negative. Nevertheless, the
possibility exists that the dosage level used (equivalent to an oral
LD50) in the hen study might be too low to permit an adequate testing
of the compound for its delayed neurotoxic potential. Studies designed
specifically to evaluate the carcinogenic activity of triazophos are
not available, although a 2-year feeding study in rats indicated no
increase in incidence of any particular type of tumour.
No-effect levels had been defined in 13-week and 2-year feeding
studies in rats and dogs, with plasma cholinesterase being
demonstrated to be the most sensitive indicator of biological
activity. Observations in humans suggested a significant individual
variation to the effect of triazophos on plasma cholinesterase.
Despite establishment of no-effect levels in two mammalian
species, the Meeting could only allocate a temporary ADI since
additional data, as listed below, are required to complete the
toxicological data base.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 ppm in the diet, equivalent to 0.05 mg/kg bw
Dog : 0.3 ppm in the diet, equivalent to 0.008 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0002 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. A carcinogenicity study.
2. Teratogenicity study in at least one mammalian species.
3. Metabolism studies in additional mammalian species to explain
species differences in acute toxicity.
4. Additional mutagenicity studies.
Desirable
1. An appropriate delayed neurotoxicity study in hens.
2. Data on individual litters for the currently available three-
generation reproduction study in rats.
3. Acute oral toxicity data on any metabolite of triazophos that is
found in food crops.
REFERENCES
Bock, R. and Thier, W. Metabolism and fate of triazophos in rats.
1976 Pesticide Science 7: 307-314.
Berkvens, J.M. and Beems, R.B. Chronic (two-year) toxicity study with
1979 Hoe 2960 in rats. Part 3. Microscopic pathological
examination of intermediate dose animals. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Cooper, A.J., Haynes, G., Street, A.E. and Rudd, C.J. A pilot study to
1973a observe the in-vivo effects of Hoe-2960 on the
cholinesterase activity in man administered 0.0125 mg/kg day
p.o. Report from Huntingdon Research Centre, England.
Submitted to the World Health Organization by Hoechst.
(Unpublished)
1973b A pilot study to observe the in vivo effects of Hoe-2960 on
the cholinesterase activity in man administered 0.025
mg/kg/day p.o. Report from Huntingdon Research Centre,
England, Submitted to the World Health Organization by
Hoechst. (Unpublished)
Cracknell, D.D., Hawkes, G., Street, A.E. and Rudd, C.J. The in-vivo
1973 effects of Hoe-2960 on the cholinesterase activity in man
administered 0.0125 mg/kg/day. Report from Huntingdon
Research Centre, England. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Gericke, D. Test for mutagenicity in bacterial strains in the absence
1977 and presence of a liver preparation. Hoe-02960 01 AS 101.
Report from Hoechst. Submitted to the World Health
Organization by Hoechst. (Unpublished)
Hollander and Weigand. Hoe-02960 01 AS 101 Irritance to the rabbit
1977a skin and eye mucosa. Report from Hoechst, Submitted to the
World Health Organization by Hoechst. (Unpublished)
1977b Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-NMRI
mouse (vehicle: sesame oil). Report from Hoechst submitted
to the World Health Organization by Hoechst. (Unpublished)
1977c Hoe-02960 01 AS 101 Acute oral toxicity to the female
SPF-NMRI mouse (vehicle: sesame oil). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977d Hoe-02960 01 AS 101 Acute oral toxicity to the male SPF-
Wistar rat (vehicle: starch suspension). Report from Hoechst
submitted to the World Health Organization by Hoechst.
(Unpublished)
1977e Akute orale toxizitat von Hoe 02960 01 AS 101 and weiblichen
SPF-Wistar ratten (vehikel: starkeschleim). Report from
Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Kramer and Weigand, Test of triazophos (Hoe-02960) for neurotoxicity
1974 in hens. Report from Farbeverke Hoechst AG submitted to the
World Health Organization by Hoechst. (Unpublished)
Leegwater, D.C. Preliminary study on the in-vivo effect of Hoe-2960 in
1971 man. Report from Central Institute for Nutrition and Food
Research, Zeist. Submitted to the World Health Organization
by Hoechst. (Unpublished)
1972 In-vivo study on the effect of Hoe-2960/Op 22 on the blood
cholinesterase of man. Report from Central Institute for
Nutrition and Food Research, Zeist. Submitted to the World
Health Organization by Hoechst. (Unpublished)
Scholz and Weigand. Triazophos-determination of the acute
1968 intraperitoneal toxicity. Report from Hoechst submitted to
the World Health Organization by Hoechst. (Unpublished)
1969 W12712 = Hoe-02960. 1-phenyl-1,2,4-triazolyl-3-(O,O-diethyl
thionophosphate) (Op. Techn. No. 3, Content 93%). Report
from Hoechst submitted to the World Health Organization by
Hoechst. (Unpublished)
Scholz and Brunk. W12712 = Hoe 2960. Acute oral toxicity in dogs.
1969 Report from Hoechst submitted to the World Health
Organization by Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Seinen, W. Subchronic (90-day) toxicity
1971a study with Hoe-2960 Op. 22 in albino rats. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Til, H.P., Seinen, W., Leegwater, D.C. and van der Meulen, H.C.
1971b Subchronic toxicity study with Hoe 2960, Op.22 in beagle
dogs. Report from Central Institute for Nutrition and Food
Research, Zeist, submitted to the World Health Organization
by Hoechst. (Unpublished)
Til, H.P., Hendriksen, C.F.M. and Leegwater D.C. Multigeneration study
1975 with Hoe-2960 in rats. Report from Central Institute for
Nutrition and Food Research, Zeist, submitted to the World
Health Organization by Farbeverke Hoechst. (Unpublished)
Til, H.P., Leegwater, D.C. and Kollen, C.H. Long-term (two year)
1976 toxicity study with Hoe 2960 in dogs. Report from Central
Institute for Nutrition and Food Research Zeist, submitted
to the World Health Organization by Hoechst. (Unpublished)
Til, H.P. and Leegwater, D.C. Chronic (two year) toxicity study with
1975 Hoe-2960 in rats. Part I. Results of clinical haematological
biochemical and organ weight measurements. Report from
Central Institute for Nutrition and Food Research, Zeist,
submitted to the World Health Organization by Hoechst.
(Unpublished)
Weigand. Testing of dermal toxicity in female SPF-Wistar rats of our
1972 own breed, after a single treatment. Report (No. A 00092)
from Hoechst, submitted to the World Health Organization by
Hoechst. (Unpublished)
