
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
TRIAZOPHOS
RESIDUES
Explanation
Triazophos was first evaluated in 1982 when a temporary
acceptable daily intake (ADI) was estimated on the basis of the
toxicological data available (FAO/WHO 1982)1. At that time, the
information on use patterns, residues, fate of residues and methods of
analysis was not evaluated. The following monograph presents the
evaluation of the additional information and makes recommendations for
maximum residue limits (MRLs).
RESIDUES IN FOOD AND THEIR EVALUATION
Information was available on the purity of the technical grade
active ingredient used in the formulation of triazophos pesticides.
This contains a minimum of 92 percent triazophos, together with
varying amounts of five substances derived from intermediates used in
the synthesis and with phosphorylated and unphosphorylated resins
produced in the reaction.
The Meeting held the view that none of these impurities, other
than sulfotep, were of toxicological significance.
Triazophos, a contact and stomach poison for insects and mites,
is registered in many countries of the American continents, Africa,
Asia and Europe. It is mostly marketed as a 40 percent emulsifiable
concentrate (E.C.); other registrations include 15 percent and 25
percent liquid formulations for ULV application, granules and a 30
percent wettable powder (W.P.).
It has a broad spectrum of activity and a good initial and
residual effect against a variety of insects and mites that damage
crops. It exhibits ovicidal action against summer eggs of mites and
eggs of certain insects. It is especially effective against the summer
fruit tortrix moth (Adoxophyes reticulana), citrus psyllid (Trioza
sp.) and the diamond-back moth (Plutella xylostella. Earias sp.,
Pectinophora sp., Spodoptera sp. and Bemisia sp. are also
killed.
Triazophos controls a large number of insect pests and mites that
damage agricultural, horticultural and forest crops. The main fields
of application are cotton, sugarcane, maize, potatoes, vegetables,
fruits, coffee and ornamentals.
1 See Annex 2 for FAO and WHO documentation.
The registered uses are listed in Table 1, where application
rates/concentrations, pro-harvest intervals and established MRLs are
indicated. In addition to the information on use patterns from the 15
countries listed in Table 1, the Meeting received comprehensive
details of approved use patterns in a further 11 countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Reports of 318 residue trials, together with corresponding
analytical results, were reviewed. These are summarized in Table 2,
where crops have been arranged into groups corresponding to the Codex
classification of food groups. Table 2 contains information about the
country where trials were conducted and a summary of the rate of
application, number of treatments, preharvest interval, plant part
analysed, limit of determination in mg/kg, number of analyses, number
of residues below limit of determination and residues found above this
limit.
Where the analysis was made separately on the peel and pulp of
fruit with inedible peel, the residue levels in the whole fruit have
been estimated from knowledge of the average ratio of pulp to peel.
Results of some typical trials on crops where residues were found
at levels significantly above the limit of determination have been
summarized in Table 3. From this tabulation it is possible to
determine the rate of disappearance of residues as well as the
residues remaining at the time of normal harvest. The following
observations have been made.
Cereals
Maize
No residues could be determined in any of the analyses. The limit
of determination varied from 0.005 to 0.07 mg/kg in the different
trials.
Wheat and oats
The use of triazophos on wheat and oats is of minor importance.
In two trials, residues of 0.02 mg/kg were determined using an
analytical method with a limit of determination of 0.01 mg/kg.
Fruit
Apples and pears
The results obtained are variable owing to changing application
conditions. A residue level of 0.2 mg/kg was not exceeded when 0.3 kg
a.i./ha was applied as a total of four applications at fortnightly
intervals and a pre-harvest interval of 50 days. The same applies when
an amount of 0.6 kg a.i./ha was used with a pre-harvest interval of 80
to 90 days.
Table 1. Registration Status of triazophos1
Country Dose Crop Pre-harvest MRLs Remarks
(a.i.) Interval (mg/kg)
(days)
Brazil 400-600g/ha cotton 28 0.03* limit of detection
citrus 60 0.002*
coffee 14 0.01*
Belgium 30-80g fruit culture 75 0.1 for
in 100 l water apples and
240g/ha sugarbeet 45 pears
Czechoslovakia
400g/ha potatoes 14 - aerial application
400/ha rape 14 - only before flowering
0.6g/l fruit - -
800g/ha sugarbeet and - - only before flowering
fodder beet - -
Denmark ornamental -
plants
pome fruits 2 months
Federal 0.2g/l cauliflower 35 0.2
Republic 240g/ha bushybeans 14 0.2
Germany 240g/ha field beans 21 0.2
240g/ha potatoes 14 0.05
240g/ha sugarbeet 42 0.05
600-l 200g/ha maize - 0.05
0.6g/l, fruit culture - 0.2 only one application
240-360g/ha forest - - after flowering
0.4g/l ornamental - -
plants
Table 1. (continued)
Country Dose Crop Pre-harvest MRLs Remarks
(a.i.) Interval (mg/kg)
(days)
Great 300-1 000g/ha apples 28 -
Britain pears 28 -
strawberries 28 -
raspberries 28 -
sugarbeet 28 -
maize 28 -
carrots 28 -
leaf brassicas 28 -
field beans 28 -
ornamentals 28 -
potatoes 28 -
broad beans 28 -
celery 28 -
leeks and 28 -
parsnips
cereals 28 -
root brassicas 28 -
peas 21 -
Hungary 400-1 600g/ha fruit culture 21 0.1
400-480g/ha viticulture 21 0.1
400g/ha tobacco 21 0.1
280-400g/ha soybean 21 0.1
400g/ha maize 21 0.1
280g/ha rape and 21 0.1
mustard
400g/ha vegetables 21 0.1
Indonesia 0.3-0.6g/l Chinese 14 -
cabbage
1 kg a.i./ha cotton 14 -
Table 1. (continued)
Country Dose Crop Pre-harvest MRLs Remarks
(a.i.) Interval (mg/kg)
(days)
Iran 0.6g/l pistachio nuts 14 -
Malaysia 380g-400gg.i/ha rice 14 -
The Netherlands
0.4-0.6g/l strawberry - 0.01 only before flowering
apples - 0.01
pears - 0.01 only before flowering
New Zealand 300-400g/ha fodder
brassicas 14
Poland 240g/ha field beans 28 -
240g/ha sugarbeet 42 -
120-240g/ha brassicas - -
360g/ha potatoes 14 -
Spain 0.6-0.8g/l citrus 30 -
0.6-0.8g/l fruit culture 40
0.3-0.6g/l cotton -
0.6-0.8g/l maize -
0.6-0.8g/l potatoes -
0.6-0.8g/l hazelnuts -
South Africa 5g in 100 l oranges and 56* 2.0 *if last spray at golf
water grape fruit ball size
up to 300g pro-rated
in 100 l water valencias 1* 2.0 *single spray at 60ml/
100 l water
Table 1. (continued)
Country Dose Crop Pre-harvest MRLs Remarks
(a.i.) Interval (mg/kg)
(days)
4* 2.0 *double sprays at 60ml/
100 l five weeks apart
Lemons 1* 2.0 *shortly before harvest
single spray at 60 ml/
100 l water
South Africa 5g in 100 l lemons 4* 2.0 *if second spray at
water 60ml/is applied 5
up to 300 g weeks later
in 100 l water
all citrus 70* 2.0 *when more than 285ml/
100 l used per crop
including sprays for
thrips
maize 21 0.1* *green maize
bananas 7 2.0
1 This summary includes only countries where MRLs and/or waiting periods are established.
A further group of countries have approved the use of triazophos. Those include
Bulgaria, Colombia, Ecuador, El Salvador, Egypt, Korea, Romania, Thailand, United
Kingdom and Zimbabwe.
Table 2. Triazophos Residues in Crops from Supervised Trials
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
Cereals
-Maize 1.2 1 28-68 grain 0.005 3 3
Federal 0.045-0.2 1-2 28-171 grain 0.01 3 3
Republic 1.2 1 60-69 grain 0.04 4 4
Germany 1.2 1 14 grain 0.05 1 1
0.6-1.2 1-2 61-65 grain 0.07 3 3
France 1.2 1 91 grain 0.07 1 1
-Oats 0.8 1 93 grain 0.03 1 1
Federal
Republic
Germany
-Wheat 0.4 1-3 28-30 grain 0.01 1 - 0.02
Brazil
Fruits
-Apple 0.2-0.81 2 90 whole fruit 0.01 2 - 0.01-0.1
Argentina 0.2-0.4 1-2 45-56 whole fruit 0.01 - 1 0.01-0.18
United 0.16 1-2 45-83 whole fruit 0.004 3 1 0.01-0.04
Kingdom 0.1-0.34 1 28 whole fruit 0.01 2 - 0.02-0.04
France 0.15-0.24 1 21-30 whole fruit 0.15 4 3 0.21
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-Apple
Germany 1.2 2 60 whole fruit 0.004 1 - 0.1
2.0 1 132 whole fruit 0.005 1 - 0
1.6-3.2g/tree1 74-118 whole fruit 0.005 9 - 0-0.08
1.5 1 133 whole fruit 0.009 1 1 -
1.2 1-2 60-131 whole fruit 0.01 5 4 0.02
3.2g/tree 1 132 whole fruit 0.01 1 - 0.01
1.2g/tree 1 63-75 whole fruit 0.03 2 - 0-0.09
New Zealand - 1 36 whole fruit 0.01 1 - 0.01
South 0.126-0.2 1-9 28-50 whole fruit 0.01 17 - 0.01-0.3
Africa
-Citrus
South 1.3-3.3 4 49-132 peel 0.002 - 1.2-2.7
Africa 1.3-3.3 4 49-132 pulp 0.02 3 -
whole fruit 0.3-0.7
-Grapefruit
South 14.85 1-2 168-211 peel 0.002 2 0.01-0.7
Africa 14.85 1-2 168-211 pulp 0.02 2 -
whole fruit 0.02-0.25
-Lemons
South 0.61-1.69 1-6 18-58 peel 0.01 14 - 0.3-4œ8
Africa pulp 0.01 14 11 0.01
whole fruit 0.1-1.6
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-Lemons
South 1.32 2 10-14 peel 0.01 4 2.0-2.7
Africa 1.32 2 10-14 pulp 0.01 4 0.03-0.04
1.32 2 10-14 juice 0.01 8 0.02-0.03
whole fruit 0.7-0.93
-Oranges
Italy 1.9-3.8 6 99 peel 0.01 2 5.3-11.0
0.76-3.8 5-6 99-122 pulp 0.02 4 1 0.04-0.07
0.76 5 122 peel 0.02 2 5.0-5.8
whole fruit 1.35-2.8
Morocco 2.4-3.2 1 60 peel 0.002 2 1 0.002
2.4-3.2 1 60 pulp 0.002 2 2 -
1.6-3.2 1 30 peel 0.006 3 0.24-0.35
1.6-3.2 1 30 pulp 0.006 3 1 0.01-0.02
whole fruit 0.07-0.1
South 1.85 2 14 peel 0.01 4 1.3-3.5
Africa 1.85 2 14 pulp 0.01 4 0.05-0.2
1.85 2 14 juice 0.01 9 0-03-0.05
whole fruit 0.36-1.0
1.2 1 42 peel 0.02 1 1.6
1.2 1 42 pulp 0.008 1 1
whole fruit 0.4
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-Oranges
United 0.89-3.56 3 60 peel 0.01 3 5.3-17.0
States pulp 0.02 3 3 0.04-0.06
whole fruit 1.35-4.3
2.27 1 57-218 peel 0.01 2 1 0.2
2.27 1 57-218 pulp 0.01 2 2
whole fruit 0.05
-Pears
United 0.16-0.96 1 45 whole fruit 0.01 4 2 0.05-0.24
Kingdom 0.16-0.96 1 28 whole fruit 0.02 3 - 0.03-0.1
South 0.18 5 49 whole fruit 0.01 1 1
Africa
Vegetables
-Brussels sprouts
United 0.21 1 21 green plant 0.01 1 1
Kingdom 0.4-0.8 1 20-28 not stated1 0.02 8 - 0.03-0.09
-Savoy cabbage
Federal 0.8 1 35-83 not stated 0.009 8 7 0.02
Republic 0.2 1 35-87 not stated 0.02 3 2 0.02
Germany
-Summer Cabbage
United 0.01-0.04 1 54 not stated 0.13 3 3
Kingdom g/plant
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-White Cabbage
Federal 0.04-0.1 g/ 1-2 84 not stated 0.004 3
Republic plant
Germany 0.36-3.0 1 56-84 not stated 0.004 3 3
1.5-2.5 1 90 not stated 0.00 2 1 0.01
0.36-3.0 1 42-123 not stated 0.01 8 7 0.02
0.04-0.1 g/ 1-2 63-123 not stated 0.01 3 2 0.01
plant
New 0.6 1 28 not stated 0.05 1 1
Zealand
Philippines 0.07-0.28g/ha 9 21 not stated 0.004 3 2 0.06
0.45 7-11 7 not stated 0.005 3 - 0.008-0.01
United 0.6-1.2 1 21-28 not stated 0.01 3 2 0.01
Kingdom
-Carrots
Federal 3.2-4.0 1 88 not stated 0.004 2 0.01
Republic
Germany
United 1.12-2.24 1 23-115 not stated 0.01 5 0.01-0.06
Kingdom
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-Cauliflower
Federal 0.1 g/plant 1 53-64 head 0.004 3 1 0.007
Republic 1.5-3.0 1 59 head 0.004 2 2
Germany 0.8 1 35-70 head 0.006 9 9
0.016 g/plant 1 49 not stated 0.008 3 2 0.04
0.48 1 35-63 not stated 0.02 2 0.09-0.1
0.9 1 42 head 0.003 1 0.008
0.2 1 35-60 head 0.03 2 2
0.36 1 56 head 0.06 1 1
0.36 1 16-55 not stated 0.06 2 1 0.2
0.36 1 56 head 0.06 1 1
0.48 1 63 plant 0.06 1 1
-Onions
Federal 12-15 g/kg seed 1 133-142 onion 0.01 5 5
Republic leaves 0.01 4 2 0.02
Germany peel 0.01 2 2
12-20 g/kg seed 1 129-159 onion 0.02 6 4 0.02-0.03
leaves 0.04 1 1
peel 0.04 6 5 0.02
0.12-0.18 1 115-161 onion 0.03 4 4
1 leaves 0.03 4 4
12-15 g/kg seed 1 122 onion 0.2 2 2
leaves 0.05 2 2
peel 0.09 2 1 0.1
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
United 10 g/kg seed 1 98 not stated 0.02 1 0.04
Kingdom
-Peas
Federal 0.36 I 11-21 pea 0.05 4 4
Republic 0.36-1.6 1-2 14-30 pea 0.02 6 6
Germany 0.36 2 14-24 pea 0.005 2 2
0.24 1 35 pea 0.001 1 1
United 0.6 2 23 pea 0.009 4 4
Kingdom 0.34-0.67 1 21 pea 0.01 4 3 0.03
0.34 1 19 pea 0.05 1 0.08
-Potatoes
Federal 0.36-0.48 1 35-28 whole 0.001 3 0.001-0.004
Republic 0.18-0.24 2 14 whole 0.002 2 2
Germany 0.18 2 14 whole 0.005 4 4
0.48 1 35 whole 0.007 2 1 0.009
0.24 2 13-14 peel 0.007 3 3
0.24 2 13-14 pulp 0.06 3 3
0.18 2 14 pulp 0.01 1 1
Others
-Coffee
Brazil 0.4 1 6-35 green beans 0.01 4 4
-Cotton
Australia 0.9-1.8 5 57 seed surface 0.04 2 1 0.1
seed interior 0.02 2 1 0.1
0.9-1.8 5 57 fibre 0.5 2 0.5-3.2
Table 2. (continued)
Crop/ Rate of No. of Preharvest Plant Limit of No. of No. of residues Residues above
Country application treatments interval Part determination analyses below limit of limit of
(kg a.i./ha) (days) analysed (mg/kg) determination determination
-Cotton
South 0.6-1.5 1-4 25 seed surface 0.03 6 6
Africa seed interior 5 5 0.05
0.6-1.2 1-4 25 fibre 0.2 6 4 0.29-1.44
Turkey 0.6-0.8 1 48 fibre 0.02 2 0.5-1.1
1 48 seed 0.02 2 1 0.1
-Sugarbeet
Federal 0.12-1.6 1 120-175 beet 0.005 8 8
Republic 0.48 kg/100 kg 1 175-196 beet 0.05 4 4 0.05
Germany seed
5.0 1 169 beet 0.05 1 1
0.24-0.48 1-3 35-42 beet 0.01 5 4 0.01
-Tobacco
Canada 8.425 1 77-105 leaves 0.2 1 1
Philippines 0.077-0.12 5 21 leaves 0.01 6 9.4-5.5
1 Not stated whether edible part was analysed.
Table 3. Triazophos Residues in Crops from Supervised Trials
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 10 20 30 50 80 100 >120
part kg a.i./ha
Oranges
South Africa peel 1975 4 2 40 E.C. 2.91 1.5 1.2 (132)
pulp n.d. n.d.
peel 4 3.3-8.3 40 E.C. 1.6
pulp n.d.
Italy peel 1980/ 5 0.75 40 E.C. 8.9 7.0 5.6 5.8
81
pulp 0.2 0.07 0.06 0.04
peel 1980 5 0.75 40 E.C. 11.0 7.1 6.1 5.0
pulp 0.2 0.2 0.07 0.06
peel 1979 6 1.9 40 E.C. 7.6 9.5 5.3
pulp 0.07 0.02 n.d.
peel 1979 6 3.8 40 E.C. 15.0 8.7 11.0
pulp 0.06 0.05 0.07
United States peel 1973 1 2.27 40 E.C. 0.2
pulp n.d.
Morocco peel 1972 1 3.2 40 E.C. 0.002
pulp n.d.
peel 1972 1 2.4 40 E.C. 0.35
pulp 0.01
peel 1972 1 3.2 40 E.C. 0.42
pulp 0.02
Table 3. (continued)
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 0 3 7 14 21 28 42
part kg a.i./ha
Oranges
South Africa whole 1975 1 1.2 40 E.C. 0.6 0.4 0.5 0.6 0.4 0.3 0.6
peel 1978 2 1.85 40 E.C. 3.7 3.1 2.3 3.5
pulp 0.1 0.02 0.03 0.05
peel 1978 2 1.85 40 E.C. 4.6 4.9 2.5 1.7
pulp 0.2 0.04 0.1 0.2
Lemons
South Africa peel 1979 3 1.22 40 E.C. 2.4 2.1
pulp 0.01 n.d.
peel 1980 4 2.88 40 E.C. 3.0 2.0
pulp n.d. n.d.
peel 1980 6 0.61 Bait 3.3 2.6
pulp 0.01 0.01
peel 1979 3 4.32 30 W.P. 2.9 2.4
pulp n.d. n.d.
peel 1977 1 1.69 40 E.C. 1.4 1.3 1.1 0.5 0.6 0.3
pulp n.d. n.d. n.d. n.d. n.d. n.d.
peel 1978 2 1.32 40 E.C. 5.3 2.2 3.7 2.6
pulp 0.02 0.03 0.03 0.05
0 25 40 60 90 120 >120
Apples
Argentina 1980/81 2 2.7 30% W.P. 0.9 0.7 0.4 0.4 0.1
1980/81 2 2.4 1.1 0.2 0.2 0.2 0.01
Table 3. (continued)
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 0 25 40 60 90 120 >120
part kg a.i./ha
Federal 1975 1 3.0 40% E.C. 0.3 0.2 0.07 n.d.
Republic 1975 1 3.0 40% E.C. 0.3 0.3 0.09 0.07
Germany 1972 1 8g/tree 40% E.C. 1.1 0.5 0.2 0.08
1972 1 8g/tree 40% E.C. 0.05 0.02 0.06 0.009
1977 2 1.2 40% E.C. 0.2 0.06 0.02
South Africa 1979 3 0.2 30% W.P. 0.4 0.3 0.2
1979 6 0.2 30% W.P. 0.6 0.2 0.2
1979 3 0.2 40% E.C. 0.4 0.3 0.3
1981 4 0.2 30% W.P. 0.4 0.2 0.2 0.1
1980/81 4 0.2 30% W.P. 0.4 0.3 0.2 0.2
1980 6 0.27 30% W.P. 0.4 0.2 0.2
1981 5 0.18 30 W.P. 0.5 n.d. n.d. n.d.
1971 1 0.96 40 E.C. 0.05
1971 1 0.64 40 E.C. 0.24
1971 1 0.96 40 E.C. 0.11
1971 1 0.32 40 E.C. 0.03
0 7 14
Bananas
South Africa peel 2 36g/1001 40 E.C. 1.6 0.8 0.6
pulp 0.08 0.03 0.07
whole 0.58 0.3 0.25
peel 50g/1001 40 E.C. 1.9 1.0 0.1
pulp 0.09 0.1 0.09
whole 0.7 0.4 0.09
Table 3. (continued)
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 0 7 14 21 28 35 84
part kg a.i./ha
Brussels Sprouts
United 1974 1 0.8 40 E.C. 0.06
Kingdom 1971 1 0.4 40 E.C. 0.03
1974 1 0.8 40 E.C. 0.05
1974 1 0.4 40 E.C. n.d.
1975 1 0.8 40 E.C. 0.09
1974 1 0.4 40 E.C. 0.04
Savoy Cabbage
Federal 1974 1 0.8 40 E.C. 0.02 0.03 n.d. n.d. 0.02
Republic 1974 1 0.8 40 E.C. n.d. n.d. n.d. n.d.
Germany 1974 1 0.8 40 E.C. 0.4 n.d. n.d. n.d. n.d. n.d.
1974 1 0.2 40 E.C. 0.2 0.1 0.09 0.1 0.05 0.02
Summer Cabbage
United 1971 1 0.01g/plant 5% Gran n.d.
Kingdom
White Cabbage
Federal 1973 1 0.36 40 E,C. 2.2 0.07 0.04 n.d. n.d.
Republic 1973 1 0.36 40 E.C. 3.6 0.02 0.03 n.d. 0.05 n.d.
Germany
Philippines 1974 9 0.2g/l 40 E.C. 0.06 0.01 0.01 n.d.
1974 9 0.4g/l 40 E.C. 0.09 0.03 n.d. n.d.
1974 9 0.6g/l 40 E.C. 1.4 0.1 n.d. 0.06
New Zealand 1975 1 0.6 40 E.C. 4.0 0.17 0.06 n.d.
Table 3. (continued)
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 0 7 14 21 28 35 84
part kg a.i./ha
Cauliflower
Federal 1973 1 0.36 40 E.C. 0.3 n.d. n.d. n.d.
Republic 1973 1 0.36 40 E.C. 2.1 0.2 n.d. n.d. n.d.
Germany 1974 1 0.48 40 E.C. 0.4 0.4 0.2 0.2 0.1 0.09
7 21 28 35 49 63 84
Carrots
Federal 1972 1 4.0 40 E.C. 0.04 0.18 0.06
Republic 1972 1 3.2 40 E.C. 0.04 0.07 0.02
Germany
Peas
United pea 1974 1 0.34 40 E.C. 0.008
Kingdom pod 0.5
pea 1972 1 0.67 40 E.C. n.d.
pod 0.04
pea 1972 2 0.6 40 E.C. n.d.
pod 0.4
Table 3. (continued)
Application Residues (mg/kg) at intervals (days) after application
Crop/Country Plant Year No. rate formulation 7 21 28 35 49 63 84
part kg a.i./ha
Cottonseed
Australia 1970 5 0.9 40 E.C. 0.35 0.2
1970 5 0.9 40 E.C. 0.1 n.d.
Cottonseed
South Africa 1970 4 0.6 40 E.C. 0.2 0.05
1970 1 0.6 40 E.C. 0.05 n.d.
1970 3 0.6 40 E.C. 0.1 n.d. n.d.
1970 3 1.2 40 E.C. 0.07 n.d. n.d.
0 3 7 14 21
Tobacco
Philippines 1977 5 0.3 40 E.C. 27.6 13.3 12.7 8.2 6.9
1977 5 0.4 40 E.C. 25.2 13.8 8.6 11.4 7.5
1977 5 0.48 40 E.C. 27.5 15.5 18.7 16.1 9.4
1977 5 0.39 40 E.C. 36.2 8.5 15.7 6.4 8.3
1 n.d. = not detected.
Citrus
One to six applications of triazophos to various species of
citrus showed residue levels in the pulp of less than 0.1 mg/kg. Only
in one out of 100 trials was a residue of 0.2 mg/kg determined. The
fruit juices showed residue levels well below 0.05 mg/kg. The residue
level in the peel was significantly higher, ranging up to 5.0 mg/kg.
Studies in South Africa have shown that when citrus fruit is small and
immature triazophos does penetrate into the pulp, but this residue is
completely degraded within 30 days and before the fruit is harvested
(see Table 4).
Vegetables
The vegetable samples analysed showed few or no residues above
the limit of determination.
Carrots
In the course of investigations into the control of carrot fly,
Suett (1975) showed that triazophos is readily taken up by carrots
soon after spraying and that uptake continues during active growth.
Following incorporation into the soil of 2 kg/ha of triazophos, the
residue level in the carrots 6 mo. later ranged from 0.01 to 0.1
mg/kg. The method and rate of application, carrot cultivar, soil type
and age of carrots at harvest affected the uptake of residues. During
storage, the residue level may increase due to loss of moisture from
the carrot. The 6-8 percent of the carrot removed during peeling
contains 75-90 percent of the total residues present. Cooking further
reduces the concentration of residues,
Potatoes
None of the trials showed residue levels above 0.01 mg/kg.
Sugarbeet
No residues could be found in most of the trials. In some cases,
0.01 to 0.05 mg/kg was determined.
Cotton
The highest residue level determined on cotton seed was 0.1
mg/kg.
Tobacco
In six trials conducted in the Philippines, a maximal residue
level of 9.4 mg/kg was determined following five applications. A
further trial carried out in Canada showed residues of 0.2 mg/kg
following a single application.
Table 4. Triazophos Residues in Oranges from Supervised Trials
Spray Residue (mg/kg) at interval (days) after treatment
concentration O 11 21 32 41 50 57
PULP
0.025% a.i. n.d. 1.4 0.3 n.d. n.d. n.d. n.d.
n.d. 0.6 0.2 n.d. n.d. n.d. n.d.
n.d. 0.2 0.08 n.d. n.d. n.d. n.d.
n.d. 0.08 0.02 n.d. n.d. n.d. n.d.
0.04% a.i. n.d. 0.08 0.12 n.d. n.d. n.d. n.d.
n.d. 0.05 0.3 n.d. n.d. n.d. n.d.
n.d. 0.012 0.08 n.d. n.d. n.d. n.d.
n.d. 0.07 0.02 n.d. n.d. n.d. n.d.
0.08% a.i. n.d. 0.01 0.01 n.d. n.d. n.d. n.d.
n.d. 0.01 0.01 n.d. n.d. n.d. 0.01
n.d. 0.01 0.02 n.d. n.d. n.d. 0.01
n.d. 0.01 0.02 n.d. n.d. n.d. 0.01
PEEL
0.025% a.i. 1.2 2.1 1.3 1.5 1.2 1.9 1.7
60 ml/100 l 1.1 1.7 1.7 1.7 1.6 1.1 1.5
0.81 1.5 0.9 1.2 1.2 1.1 0.9
0.83 1.5 1.2 2.4 1.5 1.0 1.0
0.04% a.i. 1.5 3.0 2.7 3.2 2.9 1.7 2.1
100 ml/100 l 1.7 2.5 2.2 3.8 1.8 1.7 2.9
1.4 2.8 2.4 3.2 2.1 1.7 2.5
1.8 2.7 4.1 3.3 3.8 2.6 2.7
0.08% a.i, 2.6 4.3 3.8 3.7 3.3 3.9 3.8
200 ml/100 l 3.9 7.3 6.5 3.2 3.3 4.3 6.3
4.0 5.8 5.3 3.4 3.6 6.1 4.9
3.7 5.5 5.0 4.9 3.7 5.4 5.1
Coffee
No residues could be determined in the green coffee beans.
FATE OF RESIDUES
In Animals
No information was available on the distribution, excretion,
storage or fate of triazophos in livestock. The 1982 Meeting evaluated
information on its absorption, distribution, elimination and
biotransformation in rats.
In Plants
The disappearance of residues from the plant surface is mainly
caused by the evaporation of the unchanged active ingredient, if
washing off by rain is excluded. This could be verified by trials with
14C-labelled active ingredient on cotton, beans and rice (Thier
et al. 1971; Gorbach 1970; Thier et al. 1973; Bock et al. 1975).
The residual half-life on cotton and rice was of the order of two to
three weeks and, was four weeks on beans.
Unchanged triazophos represents the only toxicologically
significant residue at harvest and it can readily be determined by the
usual means of residue analysis applicable to this substance (Thier
et al. 1979). At the time of harvest the degradation product
1-phenyl-3-hydroxy-l,2,4-triazole always represents a small fraction
of the total residue. Only traces of the P=O analogue of triazophos,
1-phenyl-3-(O,O-diethyl-phosphoryl) 1,2,4-triazole, are detectable on
the plant surface, as confirmed by model trials with 14C-labelled
triazophos in cotton, beans and rice (Gorbach 1970; Thier et al.
1971; Thier et al. 1973). Thus, for example, on cotton plants four
weeks after the final application in the greenhouse the residue of
unchanged triazophos amounted to 4 percent of the applied
radioactivity, 0.1 percent corresponded to 1-phenyl-3-hydroxy-1,
2,4-triazole and 0.04 percent to the P=O-analogue (Thier et al.
1973). The latter was the only ChE inhibiting metabolite found. In
routine residue samples of apples, grapes and oranges, ChE
inhibition was measured using a Technicon Auto-Analyser (Thier
et al. 1974). After deduction of the ChE inhibition
attributable to the intact active ingredient present, the
remaining ChE inhibition totalled in the region of only 0.1 ppb,
if attributed solely to the P=O analogue of triazophos.
The uptake of triazophos and the translocation from the treated
spot into other parts of the plant is different for the individual
plant species.
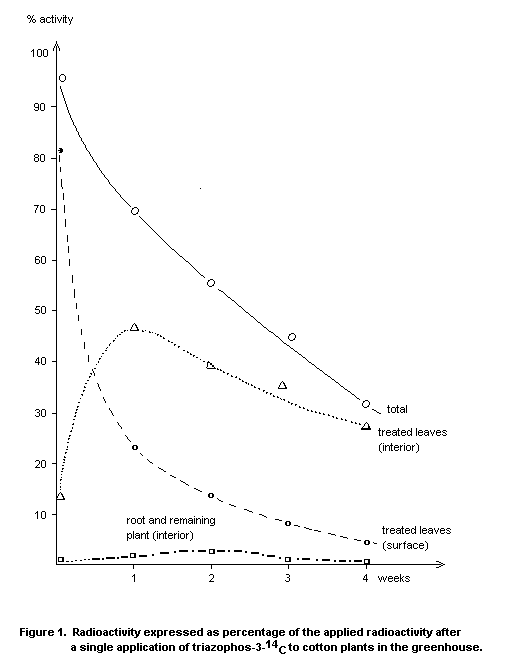
Cotton
The persistence of triazophos on cotton plants was longer in the
greenhouse than in the field. The substance penetrated rather quickly
into deeper layers of the treated leaves but was not translocated in
significant amounts into other parts of the plant. In the greenhouse,
27 percent of the applied radioactivity was still present in the
interior of the treated leaves after 4 weeks. The amount of intact
triazophos in these leaves was 25 mg/kg. The P=O analogue of
triazophos occurred only temporarily in the interior of treated
leaves, the maximum residue level amounting to 0.5 mg/kg (Figure 1).
Only slight residual radioactivity, or none at all, was
detectable in the new shoots. Any radioactive residues found in the
cotton fibre and seeds must have reached these parts by means of the
biological transport of triazophos and its metabolites, since only the
leaves were treated individually in the greenhouse trials and
contamination of the bolls was avoided.
It was also found that far less triazophos was absorbed via the
roots from moist soil than from a hydroponic culture, the ratio being
about 1 : 15 (Thier et al. 1973).
Fifteen weeks after initial application, ca. 0.025 percent of the
total radio activity applied directly to the plants was still present
in the cotton fibre. The residues consisted of unchanged triazophos
(0.02 mg/kg) 1-phenyl-3-hydroxy-l,2,4-triazole (0.01 mg/kg) and an
unidentified compound (0.01 mg/kg). In the seeds, 0.3 mg/kg triazophos
and 0.1 mg/kg 1-phenyl-3-hydroxy-l,2,4-triazole were found.
The results of field applications in Spain demonstrated that only
minor residues of triazophos and its metabolites are likely to be
present in cotton fibre and seeds if the last application takes place
before opening of the bolls (Table 3). The levels of the residues in
the cotton fibre and seeds were ca. 0.1 mg/kg unchanged triazophos and
0.1 mg/kg 1-phenyl-3-hydroxy-l,2,4-triazole; 0.1 mg/kg of an
unidentified metabolite without ChE inhibiting properties, assumed to
be a des-ethyl derivative of triazophos was also found. The identity
of the intact active ingredient and the known metabolites was
confirmed by comparison with control samples fortified with the
labelled compounds (Thier et al. 1973).
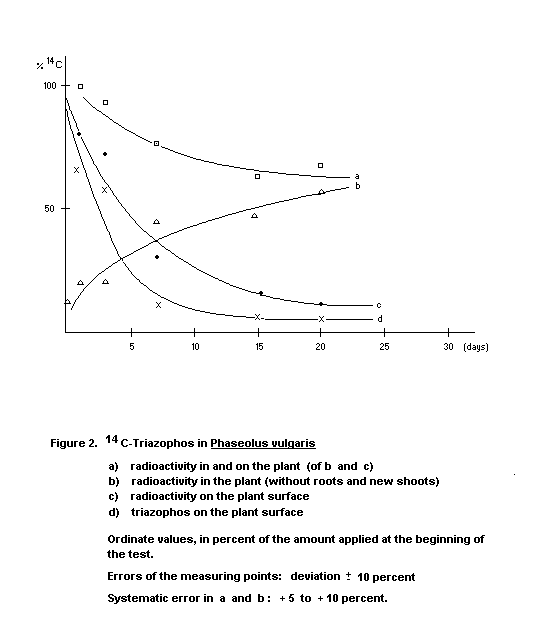
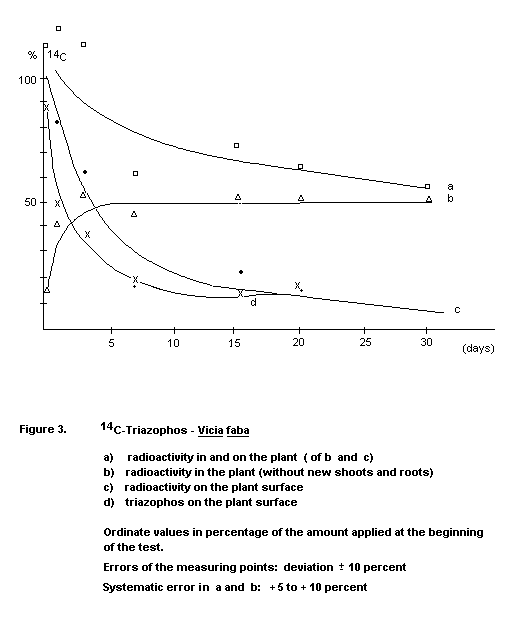
Beans
In broad beans (Vicia faba) and dwarf beans (Phaseolus
vulgaris), triazophos was absorbed by the plants, then metabolized
and transported to the roots and new shoots (Figures 2 and 3). About
40 percent of the active ingredient (ca. 20 mg/kg) applied evaporated
within 20 to 30 days; 45 to 55 percent was degraded and remained in
the plant. About 4 to 10 percent was retained as parent compound in
and on the plant (Gorbach 1970).
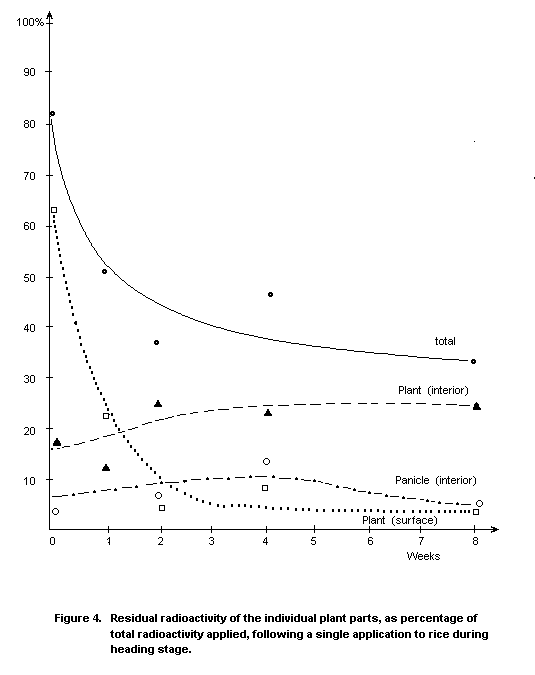
 Rice
Transport into the rice grain occurs only to a very low extent
(Figure 4). As in cotton, triazophos is less readily taken up from wet
soil than from a hydroponic culture; the ratio is about 1 : 30.
Greenhouse and field trials showed that only negligible residues
of triazophos are likely to occur in rice grain, although
contamination is possible during threshing. An unidentified metabolite
found at low levels in the grain was not a ChE inhibitor in vitro.
Rice straw and husks contained residues of triazophos and its
metabolites; this should be considered when using the straw as fodder
for livestock (Thier et al. 1971).
In Soil
The degradation capability of different soils was found by model
trials with nine soil types, one of them tropical (from a Philippine
rice field) and eight from different regions of the Federal Republic
of Germany (Bock et al. 1975). Two of these were standard soils;
they were used for trials for determination of degradation rates and
formation of metabolites after different periods of time. In addition,
14CO2-production from 14C-labelled active ingredient was
investigated.
The sandy soil showed a good mineralization capacity, as
demonstrated by rapidly increasing CO2 production which rose to 18
percent after 42 days (no figs. included) (Martens 1974). Eighteen
days after application, less than 50 percent of the applied amount was
still detectable as parent compound. After the eighth day, only one
metabolite, representing 2-4 percent of the initial radioactivity, was
found but could not be identified. In the less active sandy-loam soil
the half-life was 87 days, with slower CO2 production (Martens 1974).
Of the remaining seven soils, four degraded the compound more
rapidly. After 12 weeks, only 3-8 percent of the initial radioactivity
was recovered; the other three soils still contained 22-28% of the
active ingredient. In nearly all of these seven soils, three unknown
compounds and traces of hydroxy triazole (1 percent) occurred
simultaneously as metabolites in individual amounts of 1-6 percent of
the initial radioactivity.
The leaching behaviour of triazophos was examined according to a
regulation of the Biologische Bundesanstalt f. Land-und
Forstwirtschaft. Contamination of the water table is not likely to
occur as no triazophos was detected in the seepage water under drastic
experimental conditions (Kelker & Thier 1973).
In the field, a loamy and a sandy soil were sprayed individually
with labelled triazophos at concentrations of 0.48 and 0.96 kg/ha.
After 90 days, only slight radioactivity (1.8 to 4.3 percent of that
applied) was detectable in the upper 10 cm of the treated plots, the
radioactivity in loamy soil being slightly higher than that in the
Rice
Transport into the rice grain occurs only to a very low extent
(Figure 4). As in cotton, triazophos is less readily taken up from wet
soil than from a hydroponic culture; the ratio is about 1 : 30.
Greenhouse and field trials showed that only negligible residues
of triazophos are likely to occur in rice grain, although
contamination is possible during threshing. An unidentified metabolite
found at low levels in the grain was not a ChE inhibitor in vitro.
Rice straw and husks contained residues of triazophos and its
metabolites; this should be considered when using the straw as fodder
for livestock (Thier et al. 1971).
In Soil
The degradation capability of different soils was found by model
trials with nine soil types, one of them tropical (from a Philippine
rice field) and eight from different regions of the Federal Republic
of Germany (Bock et al. 1975). Two of these were standard soils;
they were used for trials for determination of degradation rates and
formation of metabolites after different periods of time. In addition,
14CO2-production from 14C-labelled active ingredient was
investigated.
The sandy soil showed a good mineralization capacity, as
demonstrated by rapidly increasing CO2 production which rose to 18
percent after 42 days (no figs. included) (Martens 1974). Eighteen
days after application, less than 50 percent of the applied amount was
still detectable as parent compound. After the eighth day, only one
metabolite, representing 2-4 percent of the initial radioactivity, was
found but could not be identified. In the less active sandy-loam soil
the half-life was 87 days, with slower CO2 production (Martens 1974).
Of the remaining seven soils, four degraded the compound more
rapidly. After 12 weeks, only 3-8 percent of the initial radioactivity
was recovered; the other three soils still contained 22-28% of the
active ingredient. In nearly all of these seven soils, three unknown
compounds and traces of hydroxy triazole (1 percent) occurred
simultaneously as metabolites in individual amounts of 1-6 percent of
the initial radioactivity.
The leaching behaviour of triazophos was examined according to a
regulation of the Biologische Bundesanstalt f. Land-und
Forstwirtschaft. Contamination of the water table is not likely to
occur as no triazophos was detected in the seepage water under drastic
experimental conditions (Kelker & Thier 1973).
In the field, a loamy and a sandy soil were sprayed individually
with labelled triazophos at concentrations of 0.48 and 0.96 kg/ha.
After 90 days, only slight radioactivity (1.8 to 4.3 percent of that
applied) was detectable in the upper 10 cm of the treated plots, the
radioactivity in loamy soil being slightly higher than that in the
 sandy soil. The lower soil layers (10-20 cm), as well as the untreated
neighbouring plots, exhibited little or no radioactivity (Bock et
al. 1975).
A loamy-sandy soil treated with different formulations of
triazophos showed low residues in the upper layer (0-10 cm) and very
low or no residues in deeper layers (10-30 cm) after 29 to 141 days
(Kelker & Gorbach 1974).
Triazophos was shown to have no effect, at concentrations up to
100 mg/kg, on the rate of nitrogen mineralization in the soil, on the
process of fixation of atmospheric nitrogen and on CO2 production in
a loess soil with high organic matter content. Furthermore, it had no
inhibiting effect on the isolated nitrogen-fixing enzyme nitrogenase
from Azotobacter vinelandii cells (Neven et al. 1975). In
addition, neither bacterial nor fungal growth nor oxygen uptake by the
soils were inhibited (Tu 1980).
In a study that measured the effects of 32 separate pesticides on
the enzyme activity of an organic soil, it was found that triazophos
inhibited dehydrogenase after one week and that the inhibition was
more pronounced after two weeks of incubation, but was not as
pronounced as that occurring merely as a result of heating the soil,
which apparently releases soil organic matter. Triazophos inhibited
the action of microflora involved in soil decomposition of OP
compounds. Triazophos was the only pesticide of those tested that did
not show some inhibition of urease at 10 mg/kg after one week of
incubation. Following treatment with other pesticides, the urease
activity returned to normal after two weeks. The author observed that
the inhibition of soil enzymes is only transitory (Tu 1981).
In Processing
A study was made to determine the fate of triazophos residues in
cottonseed subjected to normal processing. Triazophos was applied to
cotton in Arizona when the balls were 80 percent mature. Three rates
were applied (0.4, 0.6 and 1.1 kg/ha) by aircraft to separate plots
and samples of cotton were collected 73 days later. The cotton was
ginned and the cottonseed processed. The three fractions of the seed
were analysed separately and the crude oil likewise after refining,
bleaching and hydrogenating.
The bulk of the residues was in the crude oil, which was
significantly affected by the refining process. The concentration of
residue was reduced by bleaching and completely eliminated by
hydrogenation. No residues were carried over into soapstock. The
amount of triazophos remaining in those fractions used for annual
feeds (hulls and cottonseed meal) was apparently quite low.
In extensive experiments conducted in Taiwan Province of China in
1966 (Talekar et al. 1977), Chinese cabbage plants were sprayed at
weekly intervals until harvest. Residues were monitored 0, 1, 3 and 7
days after the last spraying. Triazophos persisted longer than all
other organophosphorus and carbamate insecticides tested. It was found
that the bulk of the residues were in the four outer leaf layers. A
simple washing in cold water removed 50 percent of the triazophos
residues from the leaves. There was a further decrease of about 50
percent when the leaves were boiled in water for 20 min. (after
allowing for the loss of about 60 percent of the water in the fresh
leaves).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
There are no reports of triazophos residues being found in any
published total diet studies. The Swedish government reported that
triazophos was found only 14 times in routine analyses of 3 028
samples of fruit and vegetables conducted in the 28 months ended 30
April 1983. All of these samples were of imported commodities. The
results are given in Table 5 (Sweden 1983).
METHODS OF RESIDUE ANALYSIS
A standard method for determination of triazophos in biological
material was developed at Hoechst AG (Thier et al. 1979). The active
ingredient is extracted with acetone from the sample material with
homogenization in an Ultraturrax apparatus. The acetone extract is
diluted with aqueous NaCl solution and extracted with methylene
chloride. The combined methylene chloride extracts are dried in a
sodium sulphate column, concentrated and the residue dissolved in
ethyl acetate. If purification of the extracts of biological material
is required, this is done by column chromatography on polystyrene gel
with ethyl acetate as the mobile phase.
The active ingredient is determined by gas chromatography using a
flame-photometric detector. The recovery rate is 80-100 percent after
addition of 0.01-1.0 mg/kg of active ingredient to the untreated
control samples. The limit of detection is about 0.005 mg/kg.
NATIONAL MAXIMUM RESIDUE LIMITS
For existing maximum residue limits in countries where triazophos
is registered see Table 1. Sweden has established an MRL for fruit and
vegetables of 0.3 mg/kg.
APPRAISAL
This organophosphorus insecticide was evaluated in 1982, when a
temporary ADI was estimated. The uses, residues resulting from such
uses and the fate of residues were not considered at that time.
Table 5. Triazophos Residues found in Imported Food, Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.07 0.07-0.18 0.19-0.34 >0.34 (mg/kg)
Apple Sweden 286 286
Import 917 912 3 2 2.0
Mandarin Import 292 291 1 0.27
Orange Import 622 618 3 1 0.35
Pepper Import 206 205 1 0.63
(sweet)
Tomato Sweden 205 205
Import 500 497 3 0.22
1 Total number of samples analysed: 8 654; Period: 81-01-01 to 83-04-30.
Triazophos is a contact and stomach poison for insects and mites
with a broad spectrum of effect against pests of many crops. It
exhibits ovicidal action against summer eggs of mites and some insects
and activity against free-living nematodes. The main fields of
commercial use are cotton, sugarcane, maize, potatoes, vegetables,
fruits (especially citrus), coffee and tobacco.
Triazophos has been registered in many countries since 1970/71
and the Meeting received comprehensive information on use patterns in
18 countries where MRL's and withholding periods have been
established. Authority to use triazophos extends to more than 30
countries. The application rate ranges from 40 g to 2 kg/ha.
The Meeting received reports of 318 residue trials together with
corresponding analytical results. These revealed that, owing to the
relatively low rate of application, the initial deposits are generally
less than 2 mg/kg. The residual half-life under rainfree conditions
ranges from two to four weeks, depending on the crop, with a
considerable reduction following rain. This is because the major
proportion of the deposit remains on the surface.
Though triazophos penetrates plant surfaces, it is not
translocated to any measurable extent. The cholinesterase-inhibiting
residues at harvest consist almost exclusively of the parent compound,
the P = 0 analogue being either absent or, if present, occurring at
extremely low concentrations.
No information was available on the fate of triazophos residues
in livestock but, from studies carried out on rats, it appears likely
that triazophos and its degradation products are rapidly excreted in
urine and faeces and that little or none is retained in body tissues
or fat.
Triazophos has been shown not to leach from soil, not to affect
biological processes in soil and to degrade at a moderately rapid rate
in soils typical of several parts of the world. Triazophos is not
taken up from soil by plants, although it may be absorbed from
hydroponic solution.
Information was available on the effect of processing and
refining on residues in cottonseed and cottonseed oil, together with
one study from Taiwan Province of China on Chinese cabbage, where it
was shown that 50 percent of the triazophos residue is removed by
water washing and 50 percent of the remainder is destroyed by cooking.
Triazophos residues in cottonseed oil were destroyed when the oil was
hydrogenated.
Triazophos residues can be extracted from plant materials with
acetone and, after transfer to methylene chloride, can be determined
by gas chromatography, using a flamephotometric detector. The method
gives food recoveries over the range 0.01-1.0 mg/kg with a limit of
determination of 0.005 mg/kg.
National MRLs have been established in a few countries.
RECOMMENDATIONS
The following MRL's are recommended for triazophos, determined
and expressed as triazophos only. Metabolites are not included.
Commodity MRL Preharvest interval (days)
(mg/kg) on which MRL is based
bananas 2 7
Brussel's sprouts 0.1 21
cabbage 0.1 21
carrots 0.1 30
citrus fruit 2 60
coffee 0.05 14
cotton seed 0.1 28
cereal grains 0.05 40
onions 0.1 30
pome:fruit 0.2 50
peas 0.1 14
potatoes 0.05 14
sugarbeet 0.05 45
FURTHER WORK OR INFORMATION
Desirable
Information on the fate of triazophos in
livestock.
REFERENCES - RESIDUES
Bock, K.-D., Bock, R., Fischer, H., Gorbach, S. & Thier, W.G.
1975 Triazophos (active substance in the sales
product Hostathion(R)). Environmental impact,
degradation on/in plants, in soil and in
warmblooded animals. Environmental Quality and
Safety, Suppl. vol. III, p. 833-839. Georg
Thieme Publishers, Stuttgart.
Gorbach, S. Degradation and distribution of triazophos in and on bean
1970 plants. Hoechst AG - Research report no.
01-Z53-0174-70.
Kelker, H. & Thier, W. Seepage behaviour of triazophos. Hoechst AG,
1973 Reports to the Biologische Bundesanstalt fuer
Land- und Forstwirtschaft according to
Memorandum No. 37.
Kelker, W. & Gorbach, S. Trial for determination of triazophos
1974 degradation in field soil. Hoechst AG-Internal
report.
Martens, R. The residual behaviour of triazophos in the soil.
1974 Institute for Soil Biology. Research Institute
for Agriculture., Braunschweig. (Unpublished)
Neven, H., Vlassak, K. & Heremans, K.A.H. Invloed van ipuron,
1975 dinosebacetaat en triazophos op enkele
biologische processen in de bodem. Meded. Fac.
Land-bouwwetensch., 40(2,II) : 1221-30.
Suett, D.L. Insecticide residues and carrot fly control. Agricultural
1975 Department Advisory Service, Quarterly Review
No. 19 125-138, Ministry of Agriculture,
Fisheries and Food, U.K.
Sweden. Triazophos analyses in imported fruit and vegetables. Data
1983 submitted to FAO.
Talekar, N.S., Sun, L.T., Lee, E.M., Chen, J.S., Lee, T.M. & Lu, S.
1977 Residual behaviour of several insecticides on
Chinese Cabbage. J. Econ. Entomology, 70(6) :
689-692.
Thier, W., Gorbach, S., Brommer, U. & Fischer, H. Distribution and
1971 metabolism of triazophos-3-14C and triazophos-
5-14C, respectively, with regard to the
triazole-component in and on rice under
different test conditions. Hoechst AG-Research
report no. 01-Z53-0193-71.
Thier, W. Gorbach, S. & Fischer, H. Model experiments on the
1973 distribution and metabolism of triazophos in
and on cotton plants under different test
conditions. English translation of publication
in Z. Anal. Chem., 267 : 181-86.
Thier, W., Schulze, E.F., Fischer, H. & Lohfink, G. In vitro
1974 examinations of residue samples originating
from field trials with triazophos (apples,
oranges, grapes) for determination of ChE-
inhibiting substances, using the
Auto-Analyser. Hoechst AG-Research report
no. 01-253-225-74.
Thier, W., Losse, K. & Merz, H.D. Method of analysis for determination
1979 of HOE 02960 OI (triazophos) in biological
material. Hoechst AG-Internal report AL 17/79.
Tu, C.M. Influence of pesticides and some of the oxidized analogues
1980 on microbial populations, nitrification and
respiration activities in soil. Bull. Environ.
Contam. Toxicol., 24 : 13-19.
Tu, C.M. Effects of some pesticides on enzyme activities in an
1981 organic soil. Bull. Environ. Contam. Toxicol.,
27 : 109-114.
sandy soil. The lower soil layers (10-20 cm), as well as the untreated
neighbouring plots, exhibited little or no radioactivity (Bock et
al. 1975).
A loamy-sandy soil treated with different formulations of
triazophos showed low residues in the upper layer (0-10 cm) and very
low or no residues in deeper layers (10-30 cm) after 29 to 141 days
(Kelker & Gorbach 1974).
Triazophos was shown to have no effect, at concentrations up to
100 mg/kg, on the rate of nitrogen mineralization in the soil, on the
process of fixation of atmospheric nitrogen and on CO2 production in
a loess soil with high organic matter content. Furthermore, it had no
inhibiting effect on the isolated nitrogen-fixing enzyme nitrogenase
from Azotobacter vinelandii cells (Neven et al. 1975). In
addition, neither bacterial nor fungal growth nor oxygen uptake by the
soils were inhibited (Tu 1980).
In a study that measured the effects of 32 separate pesticides on
the enzyme activity of an organic soil, it was found that triazophos
inhibited dehydrogenase after one week and that the inhibition was
more pronounced after two weeks of incubation, but was not as
pronounced as that occurring merely as a result of heating the soil,
which apparently releases soil organic matter. Triazophos inhibited
the action of microflora involved in soil decomposition of OP
compounds. Triazophos was the only pesticide of those tested that did
not show some inhibition of urease at 10 mg/kg after one week of
incubation. Following treatment with other pesticides, the urease
activity returned to normal after two weeks. The author observed that
the inhibition of soil enzymes is only transitory (Tu 1981).
In Processing
A study was made to determine the fate of triazophos residues in
cottonseed subjected to normal processing. Triazophos was applied to
cotton in Arizona when the balls were 80 percent mature. Three rates
were applied (0.4, 0.6 and 1.1 kg/ha) by aircraft to separate plots
and samples of cotton were collected 73 days later. The cotton was
ginned and the cottonseed processed. The three fractions of the seed
were analysed separately and the crude oil likewise after refining,
bleaching and hydrogenating.
The bulk of the residues was in the crude oil, which was
significantly affected by the refining process. The concentration of
residue was reduced by bleaching and completely eliminated by
hydrogenation. No residues were carried over into soapstock. The
amount of triazophos remaining in those fractions used for annual
feeds (hulls and cottonseed meal) was apparently quite low.
In extensive experiments conducted in Taiwan Province of China in
1966 (Talekar et al. 1977), Chinese cabbage plants were sprayed at
weekly intervals until harvest. Residues were monitored 0, 1, 3 and 7
days after the last spraying. Triazophos persisted longer than all
other organophosphorus and carbamate insecticides tested. It was found
that the bulk of the residues were in the four outer leaf layers. A
simple washing in cold water removed 50 percent of the triazophos
residues from the leaves. There was a further decrease of about 50
percent when the leaves were boiled in water for 20 min. (after
allowing for the loss of about 60 percent of the water in the fresh
leaves).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
There are no reports of triazophos residues being found in any
published total diet studies. The Swedish government reported that
triazophos was found only 14 times in routine analyses of 3 028
samples of fruit and vegetables conducted in the 28 months ended 30
April 1983. All of these samples were of imported commodities. The
results are given in Table 5 (Sweden 1983).
METHODS OF RESIDUE ANALYSIS
A standard method for determination of triazophos in biological
material was developed at Hoechst AG (Thier et al. 1979). The active
ingredient is extracted with acetone from the sample material with
homogenization in an Ultraturrax apparatus. The acetone extract is
diluted with aqueous NaCl solution and extracted with methylene
chloride. The combined methylene chloride extracts are dried in a
sodium sulphate column, concentrated and the residue dissolved in
ethyl acetate. If purification of the extracts of biological material
is required, this is done by column chromatography on polystyrene gel
with ethyl acetate as the mobile phase.
The active ingredient is determined by gas chromatography using a
flame-photometric detector. The recovery rate is 80-100 percent after
addition of 0.01-1.0 mg/kg of active ingredient to the untreated
control samples. The limit of detection is about 0.005 mg/kg.
NATIONAL MAXIMUM RESIDUE LIMITS
For existing maximum residue limits in countries where triazophos
is registered see Table 1. Sweden has established an MRL for fruit and
vegetables of 0.3 mg/kg.
APPRAISAL
This organophosphorus insecticide was evaluated in 1982, when a
temporary ADI was estimated. The uses, residues resulting from such
uses and the fate of residues were not considered at that time.
Table 5. Triazophos Residues found in Imported Food, Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.07 0.07-0.18 0.19-0.34 >0.34 (mg/kg)
Apple Sweden 286 286
Import 917 912 3 2 2.0
Mandarin Import 292 291 1 0.27
Orange Import 622 618 3 1 0.35
Pepper Import 206 205 1 0.63
(sweet)
Tomato Sweden 205 205
Import 500 497 3 0.22
1 Total number of samples analysed: 8 654; Period: 81-01-01 to 83-04-30.
Triazophos is a contact and stomach poison for insects and mites
with a broad spectrum of effect against pests of many crops. It
exhibits ovicidal action against summer eggs of mites and some insects
and activity against free-living nematodes. The main fields of
commercial use are cotton, sugarcane, maize, potatoes, vegetables,
fruits (especially citrus), coffee and tobacco.
Triazophos has been registered in many countries since 1970/71
and the Meeting received comprehensive information on use patterns in
18 countries where MRL's and withholding periods have been
established. Authority to use triazophos extends to more than 30
countries. The application rate ranges from 40 g to 2 kg/ha.
The Meeting received reports of 318 residue trials together with
corresponding analytical results. These revealed that, owing to the
relatively low rate of application, the initial deposits are generally
less than 2 mg/kg. The residual half-life under rainfree conditions
ranges from two to four weeks, depending on the crop, with a
considerable reduction following rain. This is because the major
proportion of the deposit remains on the surface.
Though triazophos penetrates plant surfaces, it is not
translocated to any measurable extent. The cholinesterase-inhibiting
residues at harvest consist almost exclusively of the parent compound,
the P = 0 analogue being either absent or, if present, occurring at
extremely low concentrations.
No information was available on the fate of triazophos residues
in livestock but, from studies carried out on rats, it appears likely
that triazophos and its degradation products are rapidly excreted in
urine and faeces and that little or none is retained in body tissues
or fat.
Triazophos has been shown not to leach from soil, not to affect
biological processes in soil and to degrade at a moderately rapid rate
in soils typical of several parts of the world. Triazophos is not
taken up from soil by plants, although it may be absorbed from
hydroponic solution.
Information was available on the effect of processing and
refining on residues in cottonseed and cottonseed oil, together with
one study from Taiwan Province of China on Chinese cabbage, where it
was shown that 50 percent of the triazophos residue is removed by
water washing and 50 percent of the remainder is destroyed by cooking.
Triazophos residues in cottonseed oil were destroyed when the oil was
hydrogenated.
Triazophos residues can be extracted from plant materials with
acetone and, after transfer to methylene chloride, can be determined
by gas chromatography, using a flamephotometric detector. The method
gives food recoveries over the range 0.01-1.0 mg/kg with a limit of
determination of 0.005 mg/kg.
National MRLs have been established in a few countries.
RECOMMENDATIONS
The following MRL's are recommended for triazophos, determined
and expressed as triazophos only. Metabolites are not included.
Commodity MRL Preharvest interval (days)
(mg/kg) on which MRL is based
bananas 2 7
Brussel's sprouts 0.1 21
cabbage 0.1 21
carrots 0.1 30
citrus fruit 2 60
coffee 0.05 14
cotton seed 0.1 28
cereal grains 0.05 40
onions 0.1 30
pome:fruit 0.2 50
peas 0.1 14
potatoes 0.05 14
sugarbeet 0.05 45
FURTHER WORK OR INFORMATION
Desirable
Information on the fate of triazophos in
livestock.
REFERENCES - RESIDUES
Bock, K.-D., Bock, R., Fischer, H., Gorbach, S. & Thier, W.G.
1975 Triazophos (active substance in the sales
product Hostathion(R)). Environmental impact,
degradation on/in plants, in soil and in
warmblooded animals. Environmental Quality and
Safety, Suppl. vol. III, p. 833-839. Georg
Thieme Publishers, Stuttgart.
Gorbach, S. Degradation and distribution of triazophos in and on bean
1970 plants. Hoechst AG - Research report no.
01-Z53-0174-70.
Kelker, H. & Thier, W. Seepage behaviour of triazophos. Hoechst AG,
1973 Reports to the Biologische Bundesanstalt fuer
Land- und Forstwirtschaft according to
Memorandum No. 37.
Kelker, W. & Gorbach, S. Trial for determination of triazophos
1974 degradation in field soil. Hoechst AG-Internal
report.
Martens, R. The residual behaviour of triazophos in the soil.
1974 Institute for Soil Biology. Research Institute
for Agriculture., Braunschweig. (Unpublished)
Neven, H., Vlassak, K. & Heremans, K.A.H. Invloed van ipuron,
1975 dinosebacetaat en triazophos op enkele
biologische processen in de bodem. Meded. Fac.
Land-bouwwetensch., 40(2,II) : 1221-30.
Suett, D.L. Insecticide residues and carrot fly control. Agricultural
1975 Department Advisory Service, Quarterly Review
No. 19 125-138, Ministry of Agriculture,
Fisheries and Food, U.K.
Sweden. Triazophos analyses in imported fruit and vegetables. Data
1983 submitted to FAO.
Talekar, N.S., Sun, L.T., Lee, E.M., Chen, J.S., Lee, T.M. & Lu, S.
1977 Residual behaviour of several insecticides on
Chinese Cabbage. J. Econ. Entomology, 70(6) :
689-692.
Thier, W., Gorbach, S., Brommer, U. & Fischer, H. Distribution and
1971 metabolism of triazophos-3-14C and triazophos-
5-14C, respectively, with regard to the
triazole-component in and on rice under
different test conditions. Hoechst AG-Research
report no. 01-Z53-0193-71.
Thier, W. Gorbach, S. & Fischer, H. Model experiments on the
1973 distribution and metabolism of triazophos in
and on cotton plants under different test
conditions. English translation of publication
in Z. Anal. Chem., 267 : 181-86.
Thier, W., Schulze, E.F., Fischer, H. & Lohfink, G. In vitro
1974 examinations of residue samples originating
from field trials with triazophos (apples,
oranges, grapes) for determination of ChE-
inhibiting substances, using the
Auto-Analyser. Hoechst AG-Research report
no. 01-253-225-74.
Thier, W., Losse, K. & Merz, H.D. Method of analysis for determination
1979 of HOE 02960 OI (triazophos) in biological
material. Hoechst AG-Internal report AL 17/79.
Tu, C.M. Influence of pesticides and some of the oxidized analogues
1980 on microbial populations, nitrification and
respiration activities in soil. Bull. Environ.
Contam. Toxicol., 24 : 13-19.
Tu, C.M. Effects of some pesticides on enzyme activities in an
1981 organic soil. Bull. Environ. Contam. Toxicol.,
27 : 109-114.


 Rice
Transport into the rice grain occurs only to a very low extent
(Figure 4). As in cotton, triazophos is less readily taken up from wet
soil than from a hydroponic culture; the ratio is about 1 : 30.
Greenhouse and field trials showed that only negligible residues
of triazophos are likely to occur in rice grain, although
contamination is possible during threshing. An unidentified metabolite
found at low levels in the grain was not a ChE inhibitor in vitro.
Rice straw and husks contained residues of triazophos and its
metabolites; this should be considered when using the straw as fodder
for livestock (Thier et al. 1971).
In Soil
The degradation capability of different soils was found by model
trials with nine soil types, one of them tropical (from a Philippine
rice field) and eight from different regions of the Federal Republic
of Germany (Bock et al. 1975). Two of these were standard soils;
they were used for trials for determination of degradation rates and
formation of metabolites after different periods of time. In addition,
14CO2-production from 14C-labelled active ingredient was
investigated.
The sandy soil showed a good mineralization capacity, as
demonstrated by rapidly increasing CO2 production which rose to 18
percent after 42 days (no figs. included) (Martens 1974). Eighteen
days after application, less than 50 percent of the applied amount was
still detectable as parent compound. After the eighth day, only one
metabolite, representing 2-4 percent of the initial radioactivity, was
found but could not be identified. In the less active sandy-loam soil
the half-life was 87 days, with slower CO2 production (Martens 1974).
Of the remaining seven soils, four degraded the compound more
rapidly. After 12 weeks, only 3-8 percent of the initial radioactivity
was recovered; the other three soils still contained 22-28% of the
active ingredient. In nearly all of these seven soils, three unknown
compounds and traces of hydroxy triazole (1 percent) occurred
simultaneously as metabolites in individual amounts of 1-6 percent of
the initial radioactivity.
The leaching behaviour of triazophos was examined according to a
regulation of the Biologische Bundesanstalt f. Land-und
Forstwirtschaft. Contamination of the water table is not likely to
occur as no triazophos was detected in the seepage water under drastic
experimental conditions (Kelker & Thier 1973).
In the field, a loamy and a sandy soil were sprayed individually
with labelled triazophos at concentrations of 0.48 and 0.96 kg/ha.
After 90 days, only slight radioactivity (1.8 to 4.3 percent of that
applied) was detectable in the upper 10 cm of the treated plots, the
radioactivity in loamy soil being slightly higher than that in the
Rice
Transport into the rice grain occurs only to a very low extent
(Figure 4). As in cotton, triazophos is less readily taken up from wet
soil than from a hydroponic culture; the ratio is about 1 : 30.
Greenhouse and field trials showed that only negligible residues
of triazophos are likely to occur in rice grain, although
contamination is possible during threshing. An unidentified metabolite
found at low levels in the grain was not a ChE inhibitor in vitro.
Rice straw and husks contained residues of triazophos and its
metabolites; this should be considered when using the straw as fodder
for livestock (Thier et al. 1971).
In Soil
The degradation capability of different soils was found by model
trials with nine soil types, one of them tropical (from a Philippine
rice field) and eight from different regions of the Federal Republic
of Germany (Bock et al. 1975). Two of these were standard soils;
they were used for trials for determination of degradation rates and
formation of metabolites after different periods of time. In addition,
14CO2-production from 14C-labelled active ingredient was
investigated.
The sandy soil showed a good mineralization capacity, as
demonstrated by rapidly increasing CO2 production which rose to 18
percent after 42 days (no figs. included) (Martens 1974). Eighteen
days after application, less than 50 percent of the applied amount was
still detectable as parent compound. After the eighth day, only one
metabolite, representing 2-4 percent of the initial radioactivity, was
found but could not be identified. In the less active sandy-loam soil
the half-life was 87 days, with slower CO2 production (Martens 1974).
Of the remaining seven soils, four degraded the compound more
rapidly. After 12 weeks, only 3-8 percent of the initial radioactivity
was recovered; the other three soils still contained 22-28% of the
active ingredient. In nearly all of these seven soils, three unknown
compounds and traces of hydroxy triazole (1 percent) occurred
simultaneously as metabolites in individual amounts of 1-6 percent of
the initial radioactivity.
The leaching behaviour of triazophos was examined according to a
regulation of the Biologische Bundesanstalt f. Land-und
Forstwirtschaft. Contamination of the water table is not likely to
occur as no triazophos was detected in the seepage water under drastic
experimental conditions (Kelker & Thier 1973).
In the field, a loamy and a sandy soil were sprayed individually
with labelled triazophos at concentrations of 0.48 and 0.96 kg/ha.
After 90 days, only slight radioactivity (1.8 to 4.3 percent of that
applied) was detectable in the upper 10 cm of the treated plots, the
radioactivity in loamy soil being slightly higher than that in the
 sandy soil. The lower soil layers (10-20 cm), as well as the untreated
neighbouring plots, exhibited little or no radioactivity (Bock et
al. 1975).
A loamy-sandy soil treated with different formulations of
triazophos showed low residues in the upper layer (0-10 cm) and very
low or no residues in deeper layers (10-30 cm) after 29 to 141 days
(Kelker & Gorbach 1974).
Triazophos was shown to have no effect, at concentrations up to
100 mg/kg, on the rate of nitrogen mineralization in the soil, on the
process of fixation of atmospheric nitrogen and on CO2 production in
a loess soil with high organic matter content. Furthermore, it had no
inhibiting effect on the isolated nitrogen-fixing enzyme nitrogenase
from Azotobacter vinelandii cells (Neven et al. 1975). In
addition, neither bacterial nor fungal growth nor oxygen uptake by the
soils were inhibited (Tu 1980).
In a study that measured the effects of 32 separate pesticides on
the enzyme activity of an organic soil, it was found that triazophos
inhibited dehydrogenase after one week and that the inhibition was
more pronounced after two weeks of incubation, but was not as
pronounced as that occurring merely as a result of heating the soil,
which apparently releases soil organic matter. Triazophos inhibited
the action of microflora involved in soil decomposition of OP
compounds. Triazophos was the only pesticide of those tested that did
not show some inhibition of urease at 10 mg/kg after one week of
incubation. Following treatment with other pesticides, the urease
activity returned to normal after two weeks. The author observed that
the inhibition of soil enzymes is only transitory (Tu 1981).
In Processing
A study was made to determine the fate of triazophos residues in
cottonseed subjected to normal processing. Triazophos was applied to
cotton in Arizona when the balls were 80 percent mature. Three rates
were applied (0.4, 0.6 and 1.1 kg/ha) by aircraft to separate plots
and samples of cotton were collected 73 days later. The cotton was
ginned and the cottonseed processed. The three fractions of the seed
were analysed separately and the crude oil likewise after refining,
bleaching and hydrogenating.
The bulk of the residues was in the crude oil, which was
significantly affected by the refining process. The concentration of
residue was reduced by bleaching and completely eliminated by
hydrogenation. No residues were carried over into soapstock. The
amount of triazophos remaining in those fractions used for annual
feeds (hulls and cottonseed meal) was apparently quite low.
In extensive experiments conducted in Taiwan Province of China in
1966 (Talekar et al. 1977), Chinese cabbage plants were sprayed at
weekly intervals until harvest. Residues were monitored 0, 1, 3 and 7
days after the last spraying. Triazophos persisted longer than all
other organophosphorus and carbamate insecticides tested. It was found
that the bulk of the residues were in the four outer leaf layers. A
simple washing in cold water removed 50 percent of the triazophos
residues from the leaves. There was a further decrease of about 50
percent when the leaves were boiled in water for 20 min. (after
allowing for the loss of about 60 percent of the water in the fresh
leaves).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
There are no reports of triazophos residues being found in any
published total diet studies. The Swedish government reported that
triazophos was found only 14 times in routine analyses of 3 028
samples of fruit and vegetables conducted in the 28 months ended 30
April 1983. All of these samples were of imported commodities. The
results are given in Table 5 (Sweden 1983).
METHODS OF RESIDUE ANALYSIS
A standard method for determination of triazophos in biological
material was developed at Hoechst AG (Thier et al. 1979). The active
ingredient is extracted with acetone from the sample material with
homogenization in an Ultraturrax apparatus. The acetone extract is
diluted with aqueous NaCl solution and extracted with methylene
chloride. The combined methylene chloride extracts are dried in a
sodium sulphate column, concentrated and the residue dissolved in
ethyl acetate. If purification of the extracts of biological material
is required, this is done by column chromatography on polystyrene gel
with ethyl acetate as the mobile phase.
The active ingredient is determined by gas chromatography using a
flame-photometric detector. The recovery rate is 80-100 percent after
addition of 0.01-1.0 mg/kg of active ingredient to the untreated
control samples. The limit of detection is about 0.005 mg/kg.
NATIONAL MAXIMUM RESIDUE LIMITS
For existing maximum residue limits in countries where triazophos
is registered see Table 1. Sweden has established an MRL for fruit and
vegetables of 0.3 mg/kg.
APPRAISAL
This organophosphorus insecticide was evaluated in 1982, when a
temporary ADI was estimated. The uses, residues resulting from such
uses and the fate of residues were not considered at that time.
Table 5. Triazophos Residues found in Imported Food, Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.07 0.07-0.18 0.19-0.34 >0.34 (mg/kg)
Apple Sweden 286 286
Import 917 912 3 2 2.0
Mandarin Import 292 291 1 0.27
Orange Import 622 618 3 1 0.35
Pepper Import 206 205 1 0.63
(sweet)
Tomato Sweden 205 205
Import 500 497 3 0.22
1 Total number of samples analysed: 8 654; Period: 81-01-01 to 83-04-30.
Triazophos is a contact and stomach poison for insects and mites
with a broad spectrum of effect against pests of many crops. It
exhibits ovicidal action against summer eggs of mites and some insects
and activity against free-living nematodes. The main fields of
commercial use are cotton, sugarcane, maize, potatoes, vegetables,
fruits (especially citrus), coffee and tobacco.
Triazophos has been registered in many countries since 1970/71
and the Meeting received comprehensive information on use patterns in
18 countries where MRL's and withholding periods have been
established. Authority to use triazophos extends to more than 30
countries. The application rate ranges from 40 g to 2 kg/ha.
The Meeting received reports of 318 residue trials together with
corresponding analytical results. These revealed that, owing to the
relatively low rate of application, the initial deposits are generally
less than 2 mg/kg. The residual half-life under rainfree conditions
ranges from two to four weeks, depending on the crop, with a
considerable reduction following rain. This is because the major
proportion of the deposit remains on the surface.
Though triazophos penetrates plant surfaces, it is not
translocated to any measurable extent. The cholinesterase-inhibiting
residues at harvest consist almost exclusively of the parent compound,
the P = 0 analogue being either absent or, if present, occurring at
extremely low concentrations.
No information was available on the fate of triazophos residues
in livestock but, from studies carried out on rats, it appears likely
that triazophos and its degradation products are rapidly excreted in
urine and faeces and that little or none is retained in body tissues
or fat.
Triazophos has been shown not to leach from soil, not to affect
biological processes in soil and to degrade at a moderately rapid rate
in soils typical of several parts of the world. Triazophos is not
taken up from soil by plants, although it may be absorbed from
hydroponic solution.
Information was available on the effect of processing and
refining on residues in cottonseed and cottonseed oil, together with
one study from Taiwan Province of China on Chinese cabbage, where it
was shown that 50 percent of the triazophos residue is removed by
water washing and 50 percent of the remainder is destroyed by cooking.
Triazophos residues in cottonseed oil were destroyed when the oil was
hydrogenated.
Triazophos residues can be extracted from plant materials with
acetone and, after transfer to methylene chloride, can be determined
by gas chromatography, using a flamephotometric detector. The method
gives food recoveries over the range 0.01-1.0 mg/kg with a limit of
determination of 0.005 mg/kg.
National MRLs have been established in a few countries.
RECOMMENDATIONS
The following MRL's are recommended for triazophos, determined
and expressed as triazophos only. Metabolites are not included.
Commodity MRL Preharvest interval (days)
(mg/kg) on which MRL is based
bananas 2 7
Brussel's sprouts 0.1 21
cabbage 0.1 21
carrots 0.1 30
citrus fruit 2 60
coffee 0.05 14
cotton seed 0.1 28
cereal grains 0.05 40
onions 0.1 30
pome:fruit 0.2 50
peas 0.1 14
potatoes 0.05 14
sugarbeet 0.05 45
FURTHER WORK OR INFORMATION
Desirable
Information on the fate of triazophos in
livestock.
REFERENCES - RESIDUES
Bock, K.-D., Bock, R., Fischer, H., Gorbach, S. & Thier, W.G.
1975 Triazophos (active substance in the sales
product Hostathion(R)). Environmental impact,
degradation on/in plants, in soil and in
warmblooded animals. Environmental Quality and
Safety, Suppl. vol. III, p. 833-839. Georg
Thieme Publishers, Stuttgart.
Gorbach, S. Degradation and distribution of triazophos in and on bean
1970 plants. Hoechst AG - Research report no.
01-Z53-0174-70.
Kelker, H. & Thier, W. Seepage behaviour of triazophos. Hoechst AG,
1973 Reports to the Biologische Bundesanstalt fuer
Land- und Forstwirtschaft according to
Memorandum No. 37.
Kelker, W. & Gorbach, S. Trial for determination of triazophos
1974 degradation in field soil. Hoechst AG-Internal
report.
Martens, R. The residual behaviour of triazophos in the soil.
1974 Institute for Soil Biology. Research Institute
for Agriculture., Braunschweig. (Unpublished)
Neven, H., Vlassak, K. & Heremans, K.A.H. Invloed van ipuron,
1975 dinosebacetaat en triazophos op enkele
biologische processen in de bodem. Meded. Fac.
Land-bouwwetensch., 40(2,II) : 1221-30.
Suett, D.L. Insecticide residues and carrot fly control. Agricultural
1975 Department Advisory Service, Quarterly Review
No. 19 125-138, Ministry of Agriculture,
Fisheries and Food, U.K.
Sweden. Triazophos analyses in imported fruit and vegetables. Data
1983 submitted to FAO.
Talekar, N.S., Sun, L.T., Lee, E.M., Chen, J.S., Lee, T.M. & Lu, S.
1977 Residual behaviour of several insecticides on
Chinese Cabbage. J. Econ. Entomology, 70(6) :
689-692.
Thier, W., Gorbach, S., Brommer, U. & Fischer, H. Distribution and
1971 metabolism of triazophos-3-14C and triazophos-
5-14C, respectively, with regard to the
triazole-component in and on rice under
different test conditions. Hoechst AG-Research
report no. 01-Z53-0193-71.
Thier, W. Gorbach, S. & Fischer, H. Model experiments on the
1973 distribution and metabolism of triazophos in
and on cotton plants under different test
conditions. English translation of publication
in Z. Anal. Chem., 267 : 181-86.
Thier, W., Schulze, E.F., Fischer, H. & Lohfink, G. In vitro
1974 examinations of residue samples originating
from field trials with triazophos (apples,
oranges, grapes) for determination of ChE-
inhibiting substances, using the
Auto-Analyser. Hoechst AG-Research report
no. 01-253-225-74.
Thier, W., Losse, K. & Merz, H.D. Method of analysis for determination
1979 of HOE 02960 OI (triazophos) in biological
material. Hoechst AG-Internal report AL 17/79.
Tu, C.M. Influence of pesticides and some of the oxidized analogues
1980 on microbial populations, nitrification and
respiration activities in soil. Bull. Environ.
Contam. Toxicol., 24 : 13-19.
Tu, C.M. Effects of some pesticides on enzyme activities in an
1981 organic soil. Bull. Environ. Contam. Toxicol.,
27 : 109-114.
sandy soil. The lower soil layers (10-20 cm), as well as the untreated
neighbouring plots, exhibited little or no radioactivity (Bock et
al. 1975).
A loamy-sandy soil treated with different formulations of
triazophos showed low residues in the upper layer (0-10 cm) and very
low or no residues in deeper layers (10-30 cm) after 29 to 141 days
(Kelker & Gorbach 1974).
Triazophos was shown to have no effect, at concentrations up to
100 mg/kg, on the rate of nitrogen mineralization in the soil, on the
process of fixation of atmospheric nitrogen and on CO2 production in
a loess soil with high organic matter content. Furthermore, it had no
inhibiting effect on the isolated nitrogen-fixing enzyme nitrogenase
from Azotobacter vinelandii cells (Neven et al. 1975). In
addition, neither bacterial nor fungal growth nor oxygen uptake by the
soils were inhibited (Tu 1980).
In a study that measured the effects of 32 separate pesticides on
the enzyme activity of an organic soil, it was found that triazophos
inhibited dehydrogenase after one week and that the inhibition was
more pronounced after two weeks of incubation, but was not as
pronounced as that occurring merely as a result of heating the soil,
which apparently releases soil organic matter. Triazophos inhibited
the action of microflora involved in soil decomposition of OP
compounds. Triazophos was the only pesticide of those tested that did
not show some inhibition of urease at 10 mg/kg after one week of
incubation. Following treatment with other pesticides, the urease
activity returned to normal after two weeks. The author observed that
the inhibition of soil enzymes is only transitory (Tu 1981).
In Processing
A study was made to determine the fate of triazophos residues in
cottonseed subjected to normal processing. Triazophos was applied to
cotton in Arizona when the balls were 80 percent mature. Three rates
were applied (0.4, 0.6 and 1.1 kg/ha) by aircraft to separate plots
and samples of cotton were collected 73 days later. The cotton was
ginned and the cottonseed processed. The three fractions of the seed
were analysed separately and the crude oil likewise after refining,
bleaching and hydrogenating.
The bulk of the residues was in the crude oil, which was
significantly affected by the refining process. The concentration of
residue was reduced by bleaching and completely eliminated by
hydrogenation. No residues were carried over into soapstock. The
amount of triazophos remaining in those fractions used for annual
feeds (hulls and cottonseed meal) was apparently quite low.
In extensive experiments conducted in Taiwan Province of China in
1966 (Talekar et al. 1977), Chinese cabbage plants were sprayed at
weekly intervals until harvest. Residues were monitored 0, 1, 3 and 7
days after the last spraying. Triazophos persisted longer than all
other organophosphorus and carbamate insecticides tested. It was found
that the bulk of the residues were in the four outer leaf layers. A
simple washing in cold water removed 50 percent of the triazophos
residues from the leaves. There was a further decrease of about 50
percent when the leaves were boiled in water for 20 min. (after
allowing for the loss of about 60 percent of the water in the fresh
leaves).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
There are no reports of triazophos residues being found in any
published total diet studies. The Swedish government reported that
triazophos was found only 14 times in routine analyses of 3 028
samples of fruit and vegetables conducted in the 28 months ended 30
April 1983. All of these samples were of imported commodities. The
results are given in Table 5 (Sweden 1983).
METHODS OF RESIDUE ANALYSIS
A standard method for determination of triazophos in biological
material was developed at Hoechst AG (Thier et al. 1979). The active
ingredient is extracted with acetone from the sample material with
homogenization in an Ultraturrax apparatus. The acetone extract is
diluted with aqueous NaCl solution and extracted with methylene
chloride. The combined methylene chloride extracts are dried in a
sodium sulphate column, concentrated and the residue dissolved in
ethyl acetate. If purification of the extracts of biological material
is required, this is done by column chromatography on polystyrene gel
with ethyl acetate as the mobile phase.
The active ingredient is determined by gas chromatography using a
flame-photometric detector. The recovery rate is 80-100 percent after
addition of 0.01-1.0 mg/kg of active ingredient to the untreated
control samples. The limit of detection is about 0.005 mg/kg.
NATIONAL MAXIMUM RESIDUE LIMITS
For existing maximum residue limits in countries where triazophos
is registered see Table 1. Sweden has established an MRL for fruit and
vegetables of 0.3 mg/kg.
APPRAISAL
This organophosphorus insecticide was evaluated in 1982, when a
temporary ADI was estimated. The uses, residues resulting from such
uses and the fate of residues were not considered at that time.
Table 5. Triazophos Residues found in Imported Food, Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.07 0.07-0.18 0.19-0.34 >0.34 (mg/kg)
Apple Sweden 286 286
Import 917 912 3 2 2.0
Mandarin Import 292 291 1 0.27
Orange Import 622 618 3 1 0.35
Pepper Import 206 205 1 0.63
(sweet)
Tomato Sweden 205 205
Import 500 497 3 0.22
1 Total number of samples analysed: 8 654; Period: 81-01-01 to 83-04-30.
Triazophos is a contact and stomach poison for insects and mites
with a broad spectrum of effect against pests of many crops. It
exhibits ovicidal action against summer eggs of mites and some insects
and activity against free-living nematodes. The main fields of
commercial use are cotton, sugarcane, maize, potatoes, vegetables,
fruits (especially citrus), coffee and tobacco.
Triazophos has been registered in many countries since 1970/71
and the Meeting received comprehensive information on use patterns in
18 countries where MRL's and withholding periods have been
established. Authority to use triazophos extends to more than 30
countries. The application rate ranges from 40 g to 2 kg/ha.
The Meeting received reports of 318 residue trials together with
corresponding analytical results. These revealed that, owing to the
relatively low rate of application, the initial deposits are generally
less than 2 mg/kg. The residual half-life under rainfree conditions
ranges from two to four weeks, depending on the crop, with a
considerable reduction following rain. This is because the major
proportion of the deposit remains on the surface.
Though triazophos penetrates plant surfaces, it is not
translocated to any measurable extent. The cholinesterase-inhibiting
residues at harvest consist almost exclusively of the parent compound,
the P = 0 analogue being either absent or, if present, occurring at
extremely low concentrations.
No information was available on the fate of triazophos residues
in livestock but, from studies carried out on rats, it appears likely
that triazophos and its degradation products are rapidly excreted in
urine and faeces and that little or none is retained in body tissues
or fat.
Triazophos has been shown not to leach from soil, not to affect
biological processes in soil and to degrade at a moderately rapid rate
in soils typical of several parts of the world. Triazophos is not
taken up from soil by plants, although it may be absorbed from
hydroponic solution.
Information was available on the effect of processing and
refining on residues in cottonseed and cottonseed oil, together with
one study from Taiwan Province of China on Chinese cabbage, where it
was shown that 50 percent of the triazophos residue is removed by
water washing and 50 percent of the remainder is destroyed by cooking.
Triazophos residues in cottonseed oil were destroyed when the oil was
hydrogenated.
Triazophos residues can be extracted from plant materials with
acetone and, after transfer to methylene chloride, can be determined
by gas chromatography, using a flamephotometric detector. The method
gives food recoveries over the range 0.01-1.0 mg/kg with a limit of
determination of 0.005 mg/kg.
National MRLs have been established in a few countries.
RECOMMENDATIONS
The following MRL's are recommended for triazophos, determined
and expressed as triazophos only. Metabolites are not included.
Commodity MRL Preharvest interval (days)
(mg/kg) on which MRL is based
bananas 2 7
Brussel's sprouts 0.1 21
cabbage 0.1 21
carrots 0.1 30
citrus fruit 2 60
coffee 0.05 14
cotton seed 0.1 28
cereal grains 0.05 40
onions 0.1 30
pome:fruit 0.2 50
peas 0.1 14
potatoes 0.05 14
sugarbeet 0.05 45
FURTHER WORK OR INFORMATION
Desirable
Information on the fate of triazophos in
livestock.
REFERENCES - RESIDUES
Bock, K.-D., Bock, R., Fischer, H., Gorbach, S. & Thier, W.G.
1975 Triazophos (active substance in the sales
product Hostathion(R)). Environmental impact,
degradation on/in plants, in soil and in
warmblooded animals. Environmental Quality and
Safety, Suppl. vol. III, p. 833-839. Georg
Thieme Publishers, Stuttgart.
Gorbach, S. Degradation and distribution of triazophos in and on bean
1970 plants. Hoechst AG - Research report no.
01-Z53-0174-70.
Kelker, H. & Thier, W. Seepage behaviour of triazophos. Hoechst AG,
1973 Reports to the Biologische Bundesanstalt fuer
Land- und Forstwirtschaft according to
Memorandum No. 37.
Kelker, W. & Gorbach, S. Trial for determination of triazophos
1974 degradation in field soil. Hoechst AG-Internal
report.
Martens, R. The residual behaviour of triazophos in the soil.
1974 Institute for Soil Biology. Research Institute
for Agriculture., Braunschweig. (Unpublished)
Neven, H., Vlassak, K. & Heremans, K.A.H. Invloed van ipuron,
1975 dinosebacetaat en triazophos op enkele
biologische processen in de bodem. Meded. Fac.
Land-bouwwetensch., 40(2,II) : 1221-30.
Suett, D.L. Insecticide residues and carrot fly control. Agricultural
1975 Department Advisory Service, Quarterly Review
No. 19 125-138, Ministry of Agriculture,
Fisheries and Food, U.K.
Sweden. Triazophos analyses in imported fruit and vegetables. Data
1983 submitted to FAO.
Talekar, N.S., Sun, L.T., Lee, E.M., Chen, J.S., Lee, T.M. & Lu, S.
1977 Residual behaviour of several insecticides on
Chinese Cabbage. J. Econ. Entomology, 70(6) :
689-692.
Thier, W., Gorbach, S., Brommer, U. & Fischer, H. Distribution and
1971 metabolism of triazophos-3-14C and triazophos-
5-14C, respectively, with regard to the
triazole-component in and on rice under
different test conditions. Hoechst AG-Research
report no. 01-Z53-0193-71.
Thier, W. Gorbach, S. & Fischer, H. Model experiments on the
1973 distribution and metabolism of triazophos in
and on cotton plants under different test
conditions. English translation of publication
in Z. Anal. Chem., 267 : 181-86.
Thier, W., Schulze, E.F., Fischer, H. & Lohfink, G. In vitro
1974 examinations of residue samples originating
from field trials with triazophos (apples,
oranges, grapes) for determination of ChE-
inhibiting substances, using the
Auto-Analyser. Hoechst AG-Research report
no. 01-253-225-74.
Thier, W., Losse, K. & Merz, H.D. Method of analysis for determination
1979 of HOE 02960 OI (triazophos) in biological
material. Hoechst AG-Internal report AL 17/79.
Tu, C.M. Influence of pesticides and some of the oxidized analogues
1980 on microbial populations, nitrification and
respiration activities in soil. Bull. Environ.
Contam. Toxicol., 24 : 13-19.
Tu, C.M. Effects of some pesticides on enzyme activities in an
1981 organic soil. Bull. Environ. Contam. Toxicol.,
27 : 109-114.
