
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
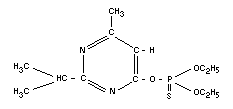
DIAZINON
Chemical names
O,O-diethyl-O-(2-isopropyl-6-methyl-4-pyrimidinyl)-
phosphorothioate; diethyl 2-isopropyl-6-methyl-4-pyrimidinyl
phosphorothionate; O, O-diethyl-O-(4-methyl-2-
isopropyl-pyrimid-6-yl)-phosphorothioate.
Empirical formula
C12H21O3N2SP
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Diazinon can be broken down to diazoxon and
tetraethylmonothiopyrophosphate which are very potent cholinesterase
inhibitors (Schrader, 1963). Experiments carried out with diazinon
labelled with 32P in cow (Robbins et al., 1957) and a goat (Vigne et
al., 1957) have shown that the 32P is rapidly eliminated in the
urine, since only a small proportion of the radioactivity can be
detected after 24 hours in the blood, faeces and milk. The urinary
elimination products in the cow have been studied by a combination of
paper chromatography and measurement of radioactivity; they largely
consist of metabolites which include diethyl thiophosphate and diethyl
phosphate. Similar results have been observed in a dog (Miller, 1963).
In experiments in which guinea-pigs received 32P-labelled
diazinon either orally or subcutaneously, it was concluded that
diazinon is efficiently absorbed through the intestine and eliminated
with the urine. The caecum might have a role in the metabolism of the
compound, even if it is injected subcutaneously (Kaplanis et al.,
1962). Technical diazinon contains 1-3% of dipyrimidyl ester which has
a slight insecticidal activity (oral LD50 to mice approximately 325
mg/kg). A by-product is diethyl thiophosphate which has no
insecticidal properties (oral LD50 to mice 750 mg/kg) (Gysin &
Margot, 1958).
Hereford cattle were sprayed weekly with 1-1.5 gallons of a spray
containing 0.0-0.1% diazinon for 16 weeks and samples of omental fat
were examined 1-14 days after the last application. Residues of
diazinon were found at 1 and 7 days but not after 14 days (Claborn et
al., 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse, male Oral 82 Bruce et al., 1955
(technical product)
Mouse Oral 77-122.5 Gasser, 1953
(as emulsion or
suspension)
Mouse Intraperitoneal 65 Klotzche, 1955
(technical product)
Rat, male Oral 100-150 Gasser, 1953
(technical product)
Rat, male Oral 108 Gaines, 1960
Rat, female Oral 76 Gaines, 1960
Guinea-pig Oral 320 Gasser, 1953
(suspension)
Rabbit Oral 143 Gasser, 1953
(suspension)
Turkey Oral 6.81 Hazleton Laboratories, 1954
Chicken Oral 40.8 Hazleton Laboratories, 1954
Goose Oral 14.7 Hazleton Laboratories, 1954
Sheep and cattle. In calves oral doses of 1 mg/kg produced
signs of toxicity and 10 mg/kg is a lethal dose. In steers and sheep
doses up to 25 and 30 mg/kg respectively produced toxic signs but not
death. Doses of 10 and 20 mg/kg respectively were non-toxic (Radeleff,
1958).
Man. One man swallowed a quantity of diazinon equivalent to 30
mg/kg body-weight without any detriment to health (Gassmann, 1957),
and another man took an amount equivalent to 250 mg/kg body-weight and
recovered after treatment (Bockel, 1957).
Short-term studies
Mouse. One batch of 10 animals was subjected to the daily oral
administration of approximately 2.2 mg/kg as a 20% emulsion; 2 of the
animals were still alive after 330 days (Gasser, 1953).
Rat. The daily oral dose of diazinon (as an emulsion) which can
be tolerated by 50% of the animals for at least 30 days is
approximately 55 mg for males and approximately 77 mg for females
(Gasser, 1953).
Two groups, each of 10 male rats, were fed for 4 weeks on diets
containing respectively 100 and 1000 ppm of technical diazinon (85%
pure). No toxic symptoms or pathological lesions were noted in the
experimental groups, except a slight inhibition of growth at the
higher concentration, i.e. 1000 ppm. A distinct inhibition of the
cholinesterase activity of the brain and particularly of the
erythrocytes was seen at the higher concentration, whereas at the
concentration of 100 ppm, only the cholinesterase activity of the
erythrocytes was inhibited to any significant extent. No significant
inhibition of the plasma cholinesterase activity was found at either
of the 2 concentrations tested (Bruce et al., 1955).
Seven groups of young rats, 5 males and 5 females, were fed for 6
months diets containing 1, 2, 4, 8 and 16 ppm of diazinon (in the form
of a freshly made solution in olive oil or in the form of oil
containing the insecticide as a residue). As compared with 2 control
groups of 10 animals each, no influence on growth-rate was found. The
cholinesterase activity of the erythrocytes was significantly
inhibited while that of the plasma remained practically unchanged. No
differences in toxicity were detected among rats given freshly-made
solutions and those given the insecticide as a residue (Mélis et al.,
1959).
Groups of male rats were fed for 16 weeks diets containing
respectively 1, 5, 25 and 125 ppm of diazinon. At the end of this
treatment, there was no significant inhibition of the plasma or brain
cholinesterase activity in the animals fed 25 ppm, whereas the
cholinesterase activity of the erythrocytes was decreased by 46%. The
concentrations of 5 and 1 ppm did not bring about any significant
changes (Edson & Noakes, 1960).
Groups of 30 rats (15 of each sex) were given 0.5, 1, 2 or 4 ppm
diazinon in their diets for 90 days. No weight or growth changes were
observed. Cholinesterase activity in the plasma was inhibited at 4 ppm
(Hazleton Laboratories, 1956).
Dog. Six dogs, 2 males and 4 females, received, on 6 days per
week for 43 weeks, oral doses of diazinon as a water-dispersible
powder in capsules. In animals receiving doses of up to 6.5 mg/kg
body-weight (on the average of 4.3 mg/kg), no visible symptoms were
noted, but as compared with 2 controls, 1 male and 1 female, there was
a very distinct inhibition of the cholinesterase activity of the plasm
and erythrocytes. With doses of 9.3 mg/kg body-weight or above, toxic
effects were observed in addition to inhibition of cholinesterase
activity (loss of appetite, slight loss of weight, excitation or
depression, trembling) (Bruce et al., 1955).
Three groups of 2 dogs each, one male and one female, received
for 90 days diets containing 0.25, 0.75 and 75 ppm of diazinon and
were compared with 5 controls. The erythrocyte cholinesterase activity
was inhibited only at the concentration of 75 ppm whereas inhibition
of plasm cholinesterase activity was also observed at the
concentration of 0.75 ppm. However, this inhibition was small and the
activity returned to normal after an additional period of 6 weeks,
despite continuation of the treatment. At the concentration of 0.25
ppm no significant inhibition of blood cholinesterase activity was
observed (Williams et al., 1959).
Groups of 2 male and 2 female dogs received diazinon as daily
oral doses of either 0.02, 0.04 or 0.08 mg/kg/day for 31 days.
Inhibition of plasma cholinesterase, was obvious in the latter group
after 3 days. In the group receiving 0.04 mg/kg/day only a moderate
decrease was observed (Hazleton Laboratories, 1956).
Monkeys. In an experiment which is still in progress, groups of
3 males and 3 female monkeys were given daily oral doses of 0.05, 0.5,
5 or 10 mg/kg of diazinon. Doses of 10 mg/kg/day for 3 weeks produced
general signs of sickness. Plasm cholinesterase activity decreased at
doses of 0.5-1 mg/kg/day or more. Red blood cells cholinesterase
decreased with doses of 5 mg/kg/day or more (Geigy, 1964).
Cattle. Cows were given orally 1.06, 5.3 or 10.6 mg/kg/day for
3 weeks and steers received 1.06 or 5.3 mg/kg/day for 2 weeks. Blood
cholinesterase was inhibited in all groups. No diazinon residues were
found in the cows' milk. In steers diazinon was found in the fat
tissue (Rai & Roan, 1960).
Fifteen calves were given 10, 25, 40 or 80 ppm of diazinon in the
diet, starting at 1 week of age for 14 weeks. Blood cholinesterase
inhibition was obvious in all the groups, with a dose-response
relationship. However, it did not appear before weaning. One calf
given 40 ppm died after 12 weeks of treatment, and the death was
considered "indirectly" related to the latter. In animals killed after
the end of the treatment no pathological changes were found (Geigy,
1963).
Long-term studies
Rat. Two groups of 40 weanling rats each, 20 males and 20
females, and 1 group of 26 male rats were fed for 72 weeks on diets
containing 10, 100 and 1000 ppm of diazinon incorporated as a 25%
water-dispersible powder. At the concentration of 1000 ppm some
inhibition of growth was observed, while at all concentrations some of
the animals developed respiratory troubles and skin lesions. These
symptoms, nevertheless, cannot be regarded as significant, since they
also appeared in some of the controls. The authors carried out
autopsies on a number of the animals, but did not find any macroscopic
or histological lesions. Nor did they find any significant difference
in food consumption and mortality rate as between the treated and
control batches (Bruce et al., 1955).
Comments on experimental studies reported
The information concerning the long-term toxicity effects of
diazinon is inadequate. On the other hand, the influence of the
repeated ingestion of diazinon on the blood cholinesterase activity of
the rat and the dog has been well studied.
EVALUATION
Level causing no significant toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.10 mg/kg/day.
Dog: 0.02 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0002 mg/kg/day.
Comment and further work required
The acceptable daily intake figure for diazinon arrived at by
applying the customary safety factor to the no-effect level in the
most sensitive species (i.e. the dog), is much lower than any other
acceptable daily intake figure for organo-phosphorus compounds in the
present series, thus suggesting a high toxicity of this compound. This
did not seem to be borne out by user experience in the field but the
Committee emphasized the lack of definite information on the toxicity
of diazinon to man.
REFERENCES
Bockel, P. (1957) Dtsch. med. Wschr., 1230
Bruce, R. B., Howard, J. W. & Elsea, J. R. (1955) J. Agric. Food
Chem., 3, 1017
Claborn, H. V., Mann, H. D., Younger, R. L., & Radeleff, R. D. (1963)
J. Econ. Ent., 56, 858
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gasser, R. (1953) Ztschr. f. Naturforsch., 8b, 225
Gassmann, R. (1957) Praxis, 46 (18) 393; (19), 416
Geigy (1963) Unpublished data
Geigy (1964) Unpublished data
Gysin, H. & Margot, A. (1958) J. Agr. Food Chem., 6, 900
Hazleton Laboratories (1954) Unpublished data
Hazleton Laboratories (1956) Unpublished data
Kaplanis, J. N., Louloudes, S. J. & Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65, 70
Klotzche, C. (1955) Arzneimittel-Forsch., 5, 436
Mélis, R., Montanelli, P. & Mélis, G. (1959) Ann. Sanità pubbl.,
20, 5
Miller, C. R. (1963) N.Z. Vet. J., 11 (Received from Geigy
Agricultural Chemicals (1964))
Radeleff, R. D. (1958) Advanc. vet. Sci., 4, 265
Rai, L. & Roan, C. C. (1960) Submitted P. P. 232
Robbins, W. E., Hopkins, T. L. & Eddy, G. W. (1957) J. Agr. Food
Chem., 5, 509
Schrader, J. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
Vigne, J. P., Chouteau, J., Tabau, R. L., Rancien, P. & Karamanian, A.
(1957) Bull. Acad. vét. Fr., 30, 84
Williams, M. W. Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol. Appl.
Pharmacol., 1, 1
BIOLOGICAL DATA
Biochemical aspects
Diazinon can be broken down to diazoxon and
tetraethylmonothiopyrophosphate which are very potent cholinesterase
inhibitors (Schrader, 1963). Experiments carried out with diazinon
labelled with 32P in cow (Robbins et al., 1957) and a goat (Vigne et
al., 1957) have shown that the 32P is rapidly eliminated in the
urine, since only a small proportion of the radioactivity can be
detected after 24 hours in the blood, faeces and milk. The urinary
elimination products in the cow have been studied by a combination of
paper chromatography and measurement of radioactivity; they largely
consist of metabolites which include diethyl thiophosphate and diethyl
phosphate. Similar results have been observed in a dog (Miller, 1963).
In experiments in which guinea-pigs received 32P-labelled
diazinon either orally or subcutaneously, it was concluded that
diazinon is efficiently absorbed through the intestine and eliminated
with the urine. The caecum might have a role in the metabolism of the
compound, even if it is injected subcutaneously (Kaplanis et al.,
1962). Technical diazinon contains 1-3% of dipyrimidyl ester which has
a slight insecticidal activity (oral LD50 to mice approximately 325
mg/kg). A by-product is diethyl thiophosphate which has no
insecticidal properties (oral LD50 to mice 750 mg/kg) (Gysin &
Margot, 1958).
Hereford cattle were sprayed weekly with 1-1.5 gallons of a spray
containing 0.0-0.1% diazinon for 16 weeks and samples of omental fat
were examined 1-14 days after the last application. Residues of
diazinon were found at 1 and 7 days but not after 14 days (Claborn et
al., 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse, male Oral 82 Bruce et al., 1955
(technical product)
Mouse Oral 77-122.5 Gasser, 1953
(as emulsion or
suspension)
Mouse Intraperitoneal 65 Klotzche, 1955
(technical product)
Rat, male Oral 100-150 Gasser, 1953
(technical product)
Rat, male Oral 108 Gaines, 1960
Rat, female Oral 76 Gaines, 1960
Guinea-pig Oral 320 Gasser, 1953
(suspension)
Rabbit Oral 143 Gasser, 1953
(suspension)
Turkey Oral 6.81 Hazleton Laboratories, 1954
Chicken Oral 40.8 Hazleton Laboratories, 1954
Goose Oral 14.7 Hazleton Laboratories, 1954
Sheep and cattle. In calves oral doses of 1 mg/kg produced
signs of toxicity and 10 mg/kg is a lethal dose. In steers and sheep
doses up to 25 and 30 mg/kg respectively produced toxic signs but not
death. Doses of 10 and 20 mg/kg respectively were non-toxic (Radeleff,
1958).
Man. One man swallowed a quantity of diazinon equivalent to 30
mg/kg body-weight without any detriment to health (Gassmann, 1957),
and another man took an amount equivalent to 250 mg/kg body-weight and
recovered after treatment (Bockel, 1957).
Short-term studies
Mouse. One batch of 10 animals was subjected to the daily oral
administration of approximately 2.2 mg/kg as a 20% emulsion; 2 of the
animals were still alive after 330 days (Gasser, 1953).
Rat. The daily oral dose of diazinon (as an emulsion) which can
be tolerated by 50% of the animals for at least 30 days is
approximately 55 mg for males and approximately 77 mg for females
(Gasser, 1953).
Two groups, each of 10 male rats, were fed for 4 weeks on diets
containing respectively 100 and 1000 ppm of technical diazinon (85%
pure). No toxic symptoms or pathological lesions were noted in the
experimental groups, except a slight inhibition of growth at the
higher concentration, i.e. 1000 ppm. A distinct inhibition of the
cholinesterase activity of the brain and particularly of the
erythrocytes was seen at the higher concentration, whereas at the
concentration of 100 ppm, only the cholinesterase activity of the
erythrocytes was inhibited to any significant extent. No significant
inhibition of the plasma cholinesterase activity was found at either
of the 2 concentrations tested (Bruce et al., 1955).
Seven groups of young rats, 5 males and 5 females, were fed for 6
months diets containing 1, 2, 4, 8 and 16 ppm of diazinon (in the form
of a freshly made solution in olive oil or in the form of oil
containing the insecticide as a residue). As compared with 2 control
groups of 10 animals each, no influence on growth-rate was found. The
cholinesterase activity of the erythrocytes was significantly
inhibited while that of the plasma remained practically unchanged. No
differences in toxicity were detected among rats given freshly-made
solutions and those given the insecticide as a residue (Mélis et al.,
1959).
Groups of male rats were fed for 16 weeks diets containing
respectively 1, 5, 25 and 125 ppm of diazinon. At the end of this
treatment, there was no significant inhibition of the plasma or brain
cholinesterase activity in the animals fed 25 ppm, whereas the
cholinesterase activity of the erythrocytes was decreased by 46%. The
concentrations of 5 and 1 ppm did not bring about any significant
changes (Edson & Noakes, 1960).
Groups of 30 rats (15 of each sex) were given 0.5, 1, 2 or 4 ppm
diazinon in their diets for 90 days. No weight or growth changes were
observed. Cholinesterase activity in the plasma was inhibited at 4 ppm
(Hazleton Laboratories, 1956).
Dog. Six dogs, 2 males and 4 females, received, on 6 days per
week for 43 weeks, oral doses of diazinon as a water-dispersible
powder in capsules. In animals receiving doses of up to 6.5 mg/kg
body-weight (on the average of 4.3 mg/kg), no visible symptoms were
noted, but as compared with 2 controls, 1 male and 1 female, there was
a very distinct inhibition of the cholinesterase activity of the plasm
and erythrocytes. With doses of 9.3 mg/kg body-weight or above, toxic
effects were observed in addition to inhibition of cholinesterase
activity (loss of appetite, slight loss of weight, excitation or
depression, trembling) (Bruce et al., 1955).
Three groups of 2 dogs each, one male and one female, received
for 90 days diets containing 0.25, 0.75 and 75 ppm of diazinon and
were compared with 5 controls. The erythrocyte cholinesterase activity
was inhibited only at the concentration of 75 ppm whereas inhibition
of plasm cholinesterase activity was also observed at the
concentration of 0.75 ppm. However, this inhibition was small and the
activity returned to normal after an additional period of 6 weeks,
despite continuation of the treatment. At the concentration of 0.25
ppm no significant inhibition of blood cholinesterase activity was
observed (Williams et al., 1959).
Groups of 2 male and 2 female dogs received diazinon as daily
oral doses of either 0.02, 0.04 or 0.08 mg/kg/day for 31 days.
Inhibition of plasma cholinesterase, was obvious in the latter group
after 3 days. In the group receiving 0.04 mg/kg/day only a moderate
decrease was observed (Hazleton Laboratories, 1956).
Monkeys. In an experiment which is still in progress, groups of
3 males and 3 female monkeys were given daily oral doses of 0.05, 0.5,
5 or 10 mg/kg of diazinon. Doses of 10 mg/kg/day for 3 weeks produced
general signs of sickness. Plasm cholinesterase activity decreased at
doses of 0.5-1 mg/kg/day or more. Red blood cells cholinesterase
decreased with doses of 5 mg/kg/day or more (Geigy, 1964).
Cattle. Cows were given orally 1.06, 5.3 or 10.6 mg/kg/day for
3 weeks and steers received 1.06 or 5.3 mg/kg/day for 2 weeks. Blood
cholinesterase was inhibited in all groups. No diazinon residues were
found in the cows' milk. In steers diazinon was found in the fat
tissue (Rai & Roan, 1960).
Fifteen calves were given 10, 25, 40 or 80 ppm of diazinon in the
diet, starting at 1 week of age for 14 weeks. Blood cholinesterase
inhibition was obvious in all the groups, with a dose-response
relationship. However, it did not appear before weaning. One calf
given 40 ppm died after 12 weeks of treatment, and the death was
considered "indirectly" related to the latter. In animals killed after
the end of the treatment no pathological changes were found (Geigy,
1963).
Long-term studies
Rat. Two groups of 40 weanling rats each, 20 males and 20
females, and 1 group of 26 male rats were fed for 72 weeks on diets
containing 10, 100 and 1000 ppm of diazinon incorporated as a 25%
water-dispersible powder. At the concentration of 1000 ppm some
inhibition of growth was observed, while at all concentrations some of
the animals developed respiratory troubles and skin lesions. These
symptoms, nevertheless, cannot be regarded as significant, since they
also appeared in some of the controls. The authors carried out
autopsies on a number of the animals, but did not find any macroscopic
or histological lesions. Nor did they find any significant difference
in food consumption and mortality rate as between the treated and
control batches (Bruce et al., 1955).
Comments on experimental studies reported
The information concerning the long-term toxicity effects of
diazinon is inadequate. On the other hand, the influence of the
repeated ingestion of diazinon on the blood cholinesterase activity of
the rat and the dog has been well studied.
EVALUATION
Level causing no significant toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.10 mg/kg/day.
Dog: 0.02 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0002 mg/kg/day.
Comment and further work required
The acceptable daily intake figure for diazinon arrived at by
applying the customary safety factor to the no-effect level in the
most sensitive species (i.e. the dog), is much lower than any other
acceptable daily intake figure for organo-phosphorus compounds in the
present series, thus suggesting a high toxicity of this compound. This
did not seem to be borne out by user experience in the field but the
Committee emphasized the lack of definite information on the toxicity
of diazinon to man.
REFERENCES
Bockel, P. (1957) Dtsch. med. Wschr., 1230
Bruce, R. B., Howard, J. W. & Elsea, J. R. (1955) J. Agric. Food
Chem., 3, 1017
Claborn, H. V., Mann, H. D., Younger, R. L., & Radeleff, R. D. (1963)
J. Econ. Ent., 56, 858
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gasser, R. (1953) Ztschr. f. Naturforsch., 8b, 225
Gassmann, R. (1957) Praxis, 46 (18) 393; (19), 416
Geigy (1963) Unpublished data
Geigy (1964) Unpublished data
Gysin, H. & Margot, A. (1958) J. Agr. Food Chem., 6, 900
Hazleton Laboratories (1954) Unpublished data
Hazleton Laboratories (1956) Unpublished data
Kaplanis, J. N., Louloudes, S. J. & Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65, 70
Klotzche, C. (1955) Arzneimittel-Forsch., 5, 436
Mélis, R., Montanelli, P. & Mélis, G. (1959) Ann. Sanità pubbl.,
20, 5
Miller, C. R. (1963) N.Z. Vet. J., 11 (Received from Geigy
Agricultural Chemicals (1964))
Radeleff, R. D. (1958) Advanc. vet. Sci., 4, 265
Rai, L. & Roan, C. C. (1960) Submitted P. P. 232
Robbins, W. E., Hopkins, T. L. & Eddy, G. W. (1957) J. Agr. Food
Chem., 5, 509
Schrader, J. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
Vigne, J. P., Chouteau, J., Tabau, R. L., Rancien, P. & Karamanian, A.
(1957) Bull. Acad. vét. Fr., 30, 84
Williams, M. W. Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol. Appl.
Pharmacol., 1, 1
 BIOLOGICAL DATA
Biochemical aspects
Diazinon can be broken down to diazoxon and
tetraethylmonothiopyrophosphate which are very potent cholinesterase
inhibitors (Schrader, 1963). Experiments carried out with diazinon
labelled with 32P in cow (Robbins et al., 1957) and a goat (Vigne et
al., 1957) have shown that the 32P is rapidly eliminated in the
urine, since only a small proportion of the radioactivity can be
detected after 24 hours in the blood, faeces and milk. The urinary
elimination products in the cow have been studied by a combination of
paper chromatography and measurement of radioactivity; they largely
consist of metabolites which include diethyl thiophosphate and diethyl
phosphate. Similar results have been observed in a dog (Miller, 1963).
In experiments in which guinea-pigs received 32P-labelled
diazinon either orally or subcutaneously, it was concluded that
diazinon is efficiently absorbed through the intestine and eliminated
with the urine. The caecum might have a role in the metabolism of the
compound, even if it is injected subcutaneously (Kaplanis et al.,
1962). Technical diazinon contains 1-3% of dipyrimidyl ester which has
a slight insecticidal activity (oral LD50 to mice approximately 325
mg/kg). A by-product is diethyl thiophosphate which has no
insecticidal properties (oral LD50 to mice 750 mg/kg) (Gysin &
Margot, 1958).
Hereford cattle were sprayed weekly with 1-1.5 gallons of a spray
containing 0.0-0.1% diazinon for 16 weeks and samples of omental fat
were examined 1-14 days after the last application. Residues of
diazinon were found at 1 and 7 days but not after 14 days (Claborn et
al., 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse, male Oral 82 Bruce et al., 1955
(technical product)
Mouse Oral 77-122.5 Gasser, 1953
(as emulsion or
suspension)
Mouse Intraperitoneal 65 Klotzche, 1955
(technical product)
Rat, male Oral 100-150 Gasser, 1953
(technical product)
Rat, male Oral 108 Gaines, 1960
Rat, female Oral 76 Gaines, 1960
Guinea-pig Oral 320 Gasser, 1953
(suspension)
Rabbit Oral 143 Gasser, 1953
(suspension)
Turkey Oral 6.81 Hazleton Laboratories, 1954
Chicken Oral 40.8 Hazleton Laboratories, 1954
Goose Oral 14.7 Hazleton Laboratories, 1954
Sheep and cattle. In calves oral doses of 1 mg/kg produced
signs of toxicity and 10 mg/kg is a lethal dose. In steers and sheep
doses up to 25 and 30 mg/kg respectively produced toxic signs but not
death. Doses of 10 and 20 mg/kg respectively were non-toxic (Radeleff,
1958).
Man. One man swallowed a quantity of diazinon equivalent to 30
mg/kg body-weight without any detriment to health (Gassmann, 1957),
and another man took an amount equivalent to 250 mg/kg body-weight and
recovered after treatment (Bockel, 1957).
Short-term studies
Mouse. One batch of 10 animals was subjected to the daily oral
administration of approximately 2.2 mg/kg as a 20% emulsion; 2 of the
animals were still alive after 330 days (Gasser, 1953).
Rat. The daily oral dose of diazinon (as an emulsion) which can
be tolerated by 50% of the animals for at least 30 days is
approximately 55 mg for males and approximately 77 mg for females
(Gasser, 1953).
Two groups, each of 10 male rats, were fed for 4 weeks on diets
containing respectively 100 and 1000 ppm of technical diazinon (85%
pure). No toxic symptoms or pathological lesions were noted in the
experimental groups, except a slight inhibition of growth at the
higher concentration, i.e. 1000 ppm. A distinct inhibition of the
cholinesterase activity of the brain and particularly of the
erythrocytes was seen at the higher concentration, whereas at the
concentration of 100 ppm, only the cholinesterase activity of the
erythrocytes was inhibited to any significant extent. No significant
inhibition of the plasma cholinesterase activity was found at either
of the 2 concentrations tested (Bruce et al., 1955).
Seven groups of young rats, 5 males and 5 females, were fed for 6
months diets containing 1, 2, 4, 8 and 16 ppm of diazinon (in the form
of a freshly made solution in olive oil or in the form of oil
containing the insecticide as a residue). As compared with 2 control
groups of 10 animals each, no influence on growth-rate was found. The
cholinesterase activity of the erythrocytes was significantly
inhibited while that of the plasma remained practically unchanged. No
differences in toxicity were detected among rats given freshly-made
solutions and those given the insecticide as a residue (Mélis et al.,
1959).
Groups of male rats were fed for 16 weeks diets containing
respectively 1, 5, 25 and 125 ppm of diazinon. At the end of this
treatment, there was no significant inhibition of the plasma or brain
cholinesterase activity in the animals fed 25 ppm, whereas the
cholinesterase activity of the erythrocytes was decreased by 46%. The
concentrations of 5 and 1 ppm did not bring about any significant
changes (Edson & Noakes, 1960).
Groups of 30 rats (15 of each sex) were given 0.5, 1, 2 or 4 ppm
diazinon in their diets for 90 days. No weight or growth changes were
observed. Cholinesterase activity in the plasma was inhibited at 4 ppm
(Hazleton Laboratories, 1956).
Dog. Six dogs, 2 males and 4 females, received, on 6 days per
week for 43 weeks, oral doses of diazinon as a water-dispersible
powder in capsules. In animals receiving doses of up to 6.5 mg/kg
body-weight (on the average of 4.3 mg/kg), no visible symptoms were
noted, but as compared with 2 controls, 1 male and 1 female, there was
a very distinct inhibition of the cholinesterase activity of the plasm
and erythrocytes. With doses of 9.3 mg/kg body-weight or above, toxic
effects were observed in addition to inhibition of cholinesterase
activity (loss of appetite, slight loss of weight, excitation or
depression, trembling) (Bruce et al., 1955).
Three groups of 2 dogs each, one male and one female, received
for 90 days diets containing 0.25, 0.75 and 75 ppm of diazinon and
were compared with 5 controls. The erythrocyte cholinesterase activity
was inhibited only at the concentration of 75 ppm whereas inhibition
of plasm cholinesterase activity was also observed at the
concentration of 0.75 ppm. However, this inhibition was small and the
activity returned to normal after an additional period of 6 weeks,
despite continuation of the treatment. At the concentration of 0.25
ppm no significant inhibition of blood cholinesterase activity was
observed (Williams et al., 1959).
Groups of 2 male and 2 female dogs received diazinon as daily
oral doses of either 0.02, 0.04 or 0.08 mg/kg/day for 31 days.
Inhibition of plasma cholinesterase, was obvious in the latter group
after 3 days. In the group receiving 0.04 mg/kg/day only a moderate
decrease was observed (Hazleton Laboratories, 1956).
Monkeys. In an experiment which is still in progress, groups of
3 males and 3 female monkeys were given daily oral doses of 0.05, 0.5,
5 or 10 mg/kg of diazinon. Doses of 10 mg/kg/day for 3 weeks produced
general signs of sickness. Plasm cholinesterase activity decreased at
doses of 0.5-1 mg/kg/day or more. Red blood cells cholinesterase
decreased with doses of 5 mg/kg/day or more (Geigy, 1964).
Cattle. Cows were given orally 1.06, 5.3 or 10.6 mg/kg/day for
3 weeks and steers received 1.06 or 5.3 mg/kg/day for 2 weeks. Blood
cholinesterase was inhibited in all groups. No diazinon residues were
found in the cows' milk. In steers diazinon was found in the fat
tissue (Rai & Roan, 1960).
Fifteen calves were given 10, 25, 40 or 80 ppm of diazinon in the
diet, starting at 1 week of age for 14 weeks. Blood cholinesterase
inhibition was obvious in all the groups, with a dose-response
relationship. However, it did not appear before weaning. One calf
given 40 ppm died after 12 weeks of treatment, and the death was
considered "indirectly" related to the latter. In animals killed after
the end of the treatment no pathological changes were found (Geigy,
1963).
Long-term studies
Rat. Two groups of 40 weanling rats each, 20 males and 20
females, and 1 group of 26 male rats were fed for 72 weeks on diets
containing 10, 100 and 1000 ppm of diazinon incorporated as a 25%
water-dispersible powder. At the concentration of 1000 ppm some
inhibition of growth was observed, while at all concentrations some of
the animals developed respiratory troubles and skin lesions. These
symptoms, nevertheless, cannot be regarded as significant, since they
also appeared in some of the controls. The authors carried out
autopsies on a number of the animals, but did not find any macroscopic
or histological lesions. Nor did they find any significant difference
in food consumption and mortality rate as between the treated and
control batches (Bruce et al., 1955).
Comments on experimental studies reported
The information concerning the long-term toxicity effects of
diazinon is inadequate. On the other hand, the influence of the
repeated ingestion of diazinon on the blood cholinesterase activity of
the rat and the dog has been well studied.
EVALUATION
Level causing no significant toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.10 mg/kg/day.
Dog: 0.02 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0002 mg/kg/day.
Comment and further work required
The acceptable daily intake figure for diazinon arrived at by
applying the customary safety factor to the no-effect level in the
most sensitive species (i.e. the dog), is much lower than any other
acceptable daily intake figure for organo-phosphorus compounds in the
present series, thus suggesting a high toxicity of this compound. This
did not seem to be borne out by user experience in the field but the
Committee emphasized the lack of definite information on the toxicity
of diazinon to man.
REFERENCES
Bockel, P. (1957) Dtsch. med. Wschr., 1230
Bruce, R. B., Howard, J. W. & Elsea, J. R. (1955) J. Agric. Food
Chem., 3, 1017
Claborn, H. V., Mann, H. D., Younger, R. L., & Radeleff, R. D. (1963)
J. Econ. Ent., 56, 858
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gasser, R. (1953) Ztschr. f. Naturforsch., 8b, 225
Gassmann, R. (1957) Praxis, 46 (18) 393; (19), 416
Geigy (1963) Unpublished data
Geigy (1964) Unpublished data
Gysin, H. & Margot, A. (1958) J. Agr. Food Chem., 6, 900
Hazleton Laboratories (1954) Unpublished data
Hazleton Laboratories (1956) Unpublished data
Kaplanis, J. N., Louloudes, S. J. & Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65, 70
Klotzche, C. (1955) Arzneimittel-Forsch., 5, 436
Mélis, R., Montanelli, P. & Mélis, G. (1959) Ann. Sanità pubbl.,
20, 5
Miller, C. R. (1963) N.Z. Vet. J., 11 (Received from Geigy
Agricultural Chemicals (1964))
Radeleff, R. D. (1958) Advanc. vet. Sci., 4, 265
Rai, L. & Roan, C. C. (1960) Submitted P. P. 232
Robbins, W. E., Hopkins, T. L. & Eddy, G. W. (1957) J. Agr. Food
Chem., 5, 509
Schrader, J. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
Vigne, J. P., Chouteau, J., Tabau, R. L., Rancien, P. & Karamanian, A.
(1957) Bull. Acad. vét. Fr., 30, 84
Williams, M. W. Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol. Appl.
Pharmacol., 1, 1
BIOLOGICAL DATA
Biochemical aspects
Diazinon can be broken down to diazoxon and
tetraethylmonothiopyrophosphate which are very potent cholinesterase
inhibitors (Schrader, 1963). Experiments carried out with diazinon
labelled with 32P in cow (Robbins et al., 1957) and a goat (Vigne et
al., 1957) have shown that the 32P is rapidly eliminated in the
urine, since only a small proportion of the radioactivity can be
detected after 24 hours in the blood, faeces and milk. The urinary
elimination products in the cow have been studied by a combination of
paper chromatography and measurement of radioactivity; they largely
consist of metabolites which include diethyl thiophosphate and diethyl
phosphate. Similar results have been observed in a dog (Miller, 1963).
In experiments in which guinea-pigs received 32P-labelled
diazinon either orally or subcutaneously, it was concluded that
diazinon is efficiently absorbed through the intestine and eliminated
with the urine. The caecum might have a role in the metabolism of the
compound, even if it is injected subcutaneously (Kaplanis et al.,
1962). Technical diazinon contains 1-3% of dipyrimidyl ester which has
a slight insecticidal activity (oral LD50 to mice approximately 325
mg/kg). A by-product is diethyl thiophosphate which has no
insecticidal properties (oral LD50 to mice 750 mg/kg) (Gysin &
Margot, 1958).
Hereford cattle were sprayed weekly with 1-1.5 gallons of a spray
containing 0.0-0.1% diazinon for 16 weeks and samples of omental fat
were examined 1-14 days after the last application. Residues of
diazinon were found at 1 and 7 days but not after 14 days (Claborn et
al., 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse, male Oral 82 Bruce et al., 1955
(technical product)
Mouse Oral 77-122.5 Gasser, 1953
(as emulsion or
suspension)
Mouse Intraperitoneal 65 Klotzche, 1955
(technical product)
Rat, male Oral 100-150 Gasser, 1953
(technical product)
Rat, male Oral 108 Gaines, 1960
Rat, female Oral 76 Gaines, 1960
Guinea-pig Oral 320 Gasser, 1953
(suspension)
Rabbit Oral 143 Gasser, 1953
(suspension)
Turkey Oral 6.81 Hazleton Laboratories, 1954
Chicken Oral 40.8 Hazleton Laboratories, 1954
Goose Oral 14.7 Hazleton Laboratories, 1954
Sheep and cattle. In calves oral doses of 1 mg/kg produced
signs of toxicity and 10 mg/kg is a lethal dose. In steers and sheep
doses up to 25 and 30 mg/kg respectively produced toxic signs but not
death. Doses of 10 and 20 mg/kg respectively were non-toxic (Radeleff,
1958).
Man. One man swallowed a quantity of diazinon equivalent to 30
mg/kg body-weight without any detriment to health (Gassmann, 1957),
and another man took an amount equivalent to 250 mg/kg body-weight and
recovered after treatment (Bockel, 1957).
Short-term studies
Mouse. One batch of 10 animals was subjected to the daily oral
administration of approximately 2.2 mg/kg as a 20% emulsion; 2 of the
animals were still alive after 330 days (Gasser, 1953).
Rat. The daily oral dose of diazinon (as an emulsion) which can
be tolerated by 50% of the animals for at least 30 days is
approximately 55 mg for males and approximately 77 mg for females
(Gasser, 1953).
Two groups, each of 10 male rats, were fed for 4 weeks on diets
containing respectively 100 and 1000 ppm of technical diazinon (85%
pure). No toxic symptoms or pathological lesions were noted in the
experimental groups, except a slight inhibition of growth at the
higher concentration, i.e. 1000 ppm. A distinct inhibition of the
cholinesterase activity of the brain and particularly of the
erythrocytes was seen at the higher concentration, whereas at the
concentration of 100 ppm, only the cholinesterase activity of the
erythrocytes was inhibited to any significant extent. No significant
inhibition of the plasma cholinesterase activity was found at either
of the 2 concentrations tested (Bruce et al., 1955).
Seven groups of young rats, 5 males and 5 females, were fed for 6
months diets containing 1, 2, 4, 8 and 16 ppm of diazinon (in the form
of a freshly made solution in olive oil or in the form of oil
containing the insecticide as a residue). As compared with 2 control
groups of 10 animals each, no influence on growth-rate was found. The
cholinesterase activity of the erythrocytes was significantly
inhibited while that of the plasma remained practically unchanged. No
differences in toxicity were detected among rats given freshly-made
solutions and those given the insecticide as a residue (Mélis et al.,
1959).
Groups of male rats were fed for 16 weeks diets containing
respectively 1, 5, 25 and 125 ppm of diazinon. At the end of this
treatment, there was no significant inhibition of the plasma or brain
cholinesterase activity in the animals fed 25 ppm, whereas the
cholinesterase activity of the erythrocytes was decreased by 46%. The
concentrations of 5 and 1 ppm did not bring about any significant
changes (Edson & Noakes, 1960).
Groups of 30 rats (15 of each sex) were given 0.5, 1, 2 or 4 ppm
diazinon in their diets for 90 days. No weight or growth changes were
observed. Cholinesterase activity in the plasma was inhibited at 4 ppm
(Hazleton Laboratories, 1956).
Dog. Six dogs, 2 males and 4 females, received, on 6 days per
week for 43 weeks, oral doses of diazinon as a water-dispersible
powder in capsules. In animals receiving doses of up to 6.5 mg/kg
body-weight (on the average of 4.3 mg/kg), no visible symptoms were
noted, but as compared with 2 controls, 1 male and 1 female, there was
a very distinct inhibition of the cholinesterase activity of the plasm
and erythrocytes. With doses of 9.3 mg/kg body-weight or above, toxic
effects were observed in addition to inhibition of cholinesterase
activity (loss of appetite, slight loss of weight, excitation or
depression, trembling) (Bruce et al., 1955).
Three groups of 2 dogs each, one male and one female, received
for 90 days diets containing 0.25, 0.75 and 75 ppm of diazinon and
were compared with 5 controls. The erythrocyte cholinesterase activity
was inhibited only at the concentration of 75 ppm whereas inhibition
of plasm cholinesterase activity was also observed at the
concentration of 0.75 ppm. However, this inhibition was small and the
activity returned to normal after an additional period of 6 weeks,
despite continuation of the treatment. At the concentration of 0.25
ppm no significant inhibition of blood cholinesterase activity was
observed (Williams et al., 1959).
Groups of 2 male and 2 female dogs received diazinon as daily
oral doses of either 0.02, 0.04 or 0.08 mg/kg/day for 31 days.
Inhibition of plasma cholinesterase, was obvious in the latter group
after 3 days. In the group receiving 0.04 mg/kg/day only a moderate
decrease was observed (Hazleton Laboratories, 1956).
Monkeys. In an experiment which is still in progress, groups of
3 males and 3 female monkeys were given daily oral doses of 0.05, 0.5,
5 or 10 mg/kg of diazinon. Doses of 10 mg/kg/day for 3 weeks produced
general signs of sickness. Plasm cholinesterase activity decreased at
doses of 0.5-1 mg/kg/day or more. Red blood cells cholinesterase
decreased with doses of 5 mg/kg/day or more (Geigy, 1964).
Cattle. Cows were given orally 1.06, 5.3 or 10.6 mg/kg/day for
3 weeks and steers received 1.06 or 5.3 mg/kg/day for 2 weeks. Blood
cholinesterase was inhibited in all groups. No diazinon residues were
found in the cows' milk. In steers diazinon was found in the fat
tissue (Rai & Roan, 1960).
Fifteen calves were given 10, 25, 40 or 80 ppm of diazinon in the
diet, starting at 1 week of age for 14 weeks. Blood cholinesterase
inhibition was obvious in all the groups, with a dose-response
relationship. However, it did not appear before weaning. One calf
given 40 ppm died after 12 weeks of treatment, and the death was
considered "indirectly" related to the latter. In animals killed after
the end of the treatment no pathological changes were found (Geigy,
1963).
Long-term studies
Rat. Two groups of 40 weanling rats each, 20 males and 20
females, and 1 group of 26 male rats were fed for 72 weeks on diets
containing 10, 100 and 1000 ppm of diazinon incorporated as a 25%
water-dispersible powder. At the concentration of 1000 ppm some
inhibition of growth was observed, while at all concentrations some of
the animals developed respiratory troubles and skin lesions. These
symptoms, nevertheless, cannot be regarded as significant, since they
also appeared in some of the controls. The authors carried out
autopsies on a number of the animals, but did not find any macroscopic
or histological lesions. Nor did they find any significant difference
in food consumption and mortality rate as between the treated and
control batches (Bruce et al., 1955).
Comments on experimental studies reported
The information concerning the long-term toxicity effects of
diazinon is inadequate. On the other hand, the influence of the
repeated ingestion of diazinon on the blood cholinesterase activity of
the rat and the dog has been well studied.
EVALUATION
Level causing no significant toxicological effect
Rat: 2 ppm in the diet, equivalent to 0.10 mg/kg/day.
Dog: 0.02 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0002 mg/kg/day.
Comment and further work required
The acceptable daily intake figure for diazinon arrived at by
applying the customary safety factor to the no-effect level in the
most sensitive species (i.e. the dog), is much lower than any other
acceptable daily intake figure for organo-phosphorus compounds in the
present series, thus suggesting a high toxicity of this compound. This
did not seem to be borne out by user experience in the field but the
Committee emphasized the lack of definite information on the toxicity
of diazinon to man.
REFERENCES
Bockel, P. (1957) Dtsch. med. Wschr., 1230
Bruce, R. B., Howard, J. W. & Elsea, J. R. (1955) J. Agric. Food
Chem., 3, 1017
Claborn, H. V., Mann, H. D., Younger, R. L., & Radeleff, R. D. (1963)
J. Econ. Ent., 56, 858
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gasser, R. (1953) Ztschr. f. Naturforsch., 8b, 225
Gassmann, R. (1957) Praxis, 46 (18) 393; (19), 416
Geigy (1963) Unpublished data
Geigy (1964) Unpublished data
Gysin, H. & Margot, A. (1958) J. Agr. Food Chem., 6, 900
Hazleton Laboratories (1954) Unpublished data
Hazleton Laboratories (1956) Unpublished data
Kaplanis, J. N., Louloudes, S. J. & Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65, 70
Klotzche, C. (1955) Arzneimittel-Forsch., 5, 436
Mélis, R., Montanelli, P. & Mélis, G. (1959) Ann. Sanità pubbl.,
20, 5
Miller, C. R. (1963) N.Z. Vet. J., 11 (Received from Geigy
Agricultural Chemicals (1964))
Radeleff, R. D. (1958) Advanc. vet. Sci., 4, 265
Rai, L. & Roan, C. C. (1960) Submitted P. P. 232
Robbins, W. E., Hopkins, T. L. & Eddy, G. W. (1957) J. Agr. Food
Chem., 5, 509
Schrader, J. (1963) Die Entwicklung neuer insektizider
Phosphorsäure-Ester, Verlag Chemie Weinheim
Vigne, J. P., Chouteau, J., Tabau, R. L., Rancien, P. & Karamanian, A.
(1957) Bull. Acad. vét. Fr., 30, 84
Williams, M. W. Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol. Appl.
Pharmacol., 1, 1
