
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
DIAZINON
Explanation
Diazinon was evaluated at the Joint Meeting in 1965, 1966, 1967 and
1968. Since the previous evaluation (FAO/WHO, 1968), some new
experimental work has been reported on this compound. This new work is
presented and discussed in the following monograph addendum.
Information relating to the use, and to the occurrence of residues, of
the pesticide has been freshly reviewed, and that part of this
addendum headed RESIDUES IN FOOD AND THEIR EVALUATION is intended to
replace the contests of previous monographs under this heading.
IDENTITY
Stabilization of diazinon formulations appear to have eliminated toxic
condensation products of diethylphosphoric and diethylphosphorothioic
acids and, thereby, to have reduced the overall mammalian toxicity
(Geigy, 1969).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption and distribution
Following daily oral administration of diazinon to two rats for ten
days at a daily dose of 0.02 mg/kg body-weight, residue levels of less
than 1 percent of the totally applied dose were found one day after
cessation of treatment. Of the tissues examined, muscle (0.77 percent
of the total dose applied), small intestine (0.65 percent),
colon-caecum (0.76 percent), stomach-oesophagus (0.25 percent), fat
(0.23 percent) and liver (0.16 percent) had the highest values six
hours after the last application. This study precludes the
accumulation of diazinon in mammalian tissue (Mücke et al., 1970).
Excretion
Further investigations in the rat were carried out by Mücke et al.,
(1970) and these studies again confirmed that diazinon and its
metabolites are rapidly excreted mainly in the urine. The studies
indicated that no opening of the pyrimidine ring with subsequent
oxidation of the fragments of CO2 takes place. From 95 percent (in
females) to 98 percent (in males) of an oral dose of diazinon was
excreted in 168 hours, with the major quantity present in urine (69
percent in females and 80 percent in males) and faeces (24 percent in
females and 16 percent in males). The material was present in urine
and faeces as three derivatives of the pyrimidyl moiety and one polar
unidentified fraction, presumably containing several components.
The distribution and the excretion of 32P-labelled insecticide was
also followed in the dog (Millar, 1963) and in the guinea pig
(Kaplanis et al., 1962). Considerable breakdown of diazinon in these
species was confirmed. In the case of the guinea pig, the elimination
of the radioactivity ceased within 7 days, by which time more than 87
percent of the oral dose left the body with the urine.
Biotransformation
Organophosphorus insecticides are degraded in animals by cleavage of
phosphorus ester linkages. In the case of phosphorothioates, the
common routes of metabolism are the ones leading to the production of
dialkyl phosphorothioic acids and the other forming, after
denitrification, dialkyl phosphoric acids. It has long been assumed
that these metabolites were produced by hydrolytic action of
phosphatases.
It is suggested that many of the so-called phosphatase products or
hydrolysis products may actually be oxidative metabolites.
Results of in vitro studies using rat liver microsomes and reduced
pyridine nucleotide cofactors in the presence of oxygen indicate that
diazinon is oxidatively activated to the oxygen analogue diazoxon and
degraded by hydrolysis to diethylphosphorothioic acid (Nakatsugawa et
al., 1969).
Mücke et al. (1970), investigating the in vivo degradation of
diazinon in the rat, characterized urinary oxidative metabolites of
the pyrimidyl moiety following hydrolysis as *(see below) and the
**(see below) as well as the major unchanged enol,
2-isopropyl-4-methyl-6-hydroxypyrimidine.
Thin layer chromatographic analysis of the urine demonstrated the
presence of four metabolite fractions. Fractions 1, 2 and 3 were found
to be homogeneous, whereas fraction 4 contained a series of very polar
substances. Spectroscopic investigations of these metabolites revealed
that each contained the same heterocyclic moiety. This ring system has
been identified as:
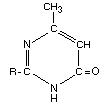 Hydrolysis of the ester bond yielding
2-isopropyl-4-methyl-6-hydroxypyrimidine and oxidation at the primary
and tertiary carbon atom of the isopropyl side chain were found as the
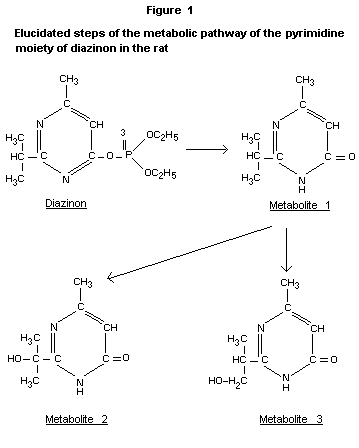
main degradative mechanisms. The structure of the main metabolites was
confirmed by independent synthesis, and the inhibitory activities on
acetyl cholinesterase were determined. The structure of the
metabolites and the metabolic pathway of the pyrimidine moiety are
shown in Figure 1.
* 2-(1-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
** 2-(2-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
The actual sequence of the degradation reactions was determined by
following the fate of 14C-labelled metabolites after intravenous
application. Metabolite 1 resulted in the same pattern of metabolites
as diazinon itself. Metabolite 2 was excreted mainly unchanged and
metabolite 3 was excreted in a completely unchanged form.
Hastie (1963) reviewed available data on the metabolism and
elimination of diazinon from animals and animal tissues. When orally
administered, diazinon is degraded by the digestive enzymes before the
lipid-soluble material can reach the fat depots. The more circuitous
route involved following dermal application allows a certain amount of
diazinon to bypass the sites of degradation, thereby reaching the fat
depots unchanged. Reabsorption into the digestive tract from these
depots is a somewhat delayed process.
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Hamster and rabbit
An examination of the teratogenic potential of diazinon was performed
using Golden Syrian hamsters and New Zealand white rabbits (Robens,
1969). Oral administration of diazinon in maize oil at a dose of 0.125
mg/kg body-weight on day 6, 7 and 8 of gestation and at 2.25 mg/kg
body-weight on day 7 or 8 produced no terata in the hamster.
Administration of diazinon to rabbits daily from day 5 to 15 of
gestation at 7 or 30 mg/kg body-weight per day induced no terata or
dose-related embryotoxic effects.
Special studies on acute toxicity of metabolites
LD50 values following acute oral administration to rats are available
for two diazinon metabolites:
2-isopropyl-4-methyl-6-hydroxypyrimidine: 2 700 mg/kg body-weight
2-(1'-hydroxyisopropyl)-4-methyl-6-hydroxypyrimidine:
5 000 mg/kg body-weight
(Mücke et al., 1970).
Hydrolysis of the ester bond yielding
2-isopropyl-4-methyl-6-hydroxypyrimidine and oxidation at the primary
and tertiary carbon atom of the isopropyl side chain were found as the
main degradative mechanisms. The structure of the main metabolites was
confirmed by independent synthesis, and the inhibitory activities on
acetyl cholinesterase were determined. The structure of the
metabolites and the metabolic pathway of the pyrimidine moiety are
shown in Figure 1.
* 2-(1-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
** 2-(2-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
The actual sequence of the degradation reactions was determined by
following the fate of 14C-labelled metabolites after intravenous
application. Metabolite 1 resulted in the same pattern of metabolites
as diazinon itself. Metabolite 2 was excreted mainly unchanged and
metabolite 3 was excreted in a completely unchanged form.
Hastie (1963) reviewed available data on the metabolism and
elimination of diazinon from animals and animal tissues. When orally
administered, diazinon is degraded by the digestive enzymes before the
lipid-soluble material can reach the fat depots. The more circuitous
route involved following dermal application allows a certain amount of
diazinon to bypass the sites of degradation, thereby reaching the fat
depots unchanged. Reabsorption into the digestive tract from these
depots is a somewhat delayed process.
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Hamster and rabbit
An examination of the teratogenic potential of diazinon was performed
using Golden Syrian hamsters and New Zealand white rabbits (Robens,
1969). Oral administration of diazinon in maize oil at a dose of 0.125
mg/kg body-weight on day 6, 7 and 8 of gestation and at 2.25 mg/kg
body-weight on day 7 or 8 produced no terata in the hamster.
Administration of diazinon to rabbits daily from day 5 to 15 of
gestation at 7 or 30 mg/kg body-weight per day induced no terata or
dose-related embryotoxic effects.
Special studies on acute toxicity of metabolites
LD50 values following acute oral administration to rats are available
for two diazinon metabolites:
2-isopropyl-4-methyl-6-hydroxypyrimidine: 2 700 mg/kg body-weight
2-(1'-hydroxyisopropyl)-4-methyl-6-hydroxypyrimidine:
5 000 mg/kg body-weight
(Mücke et al., 1970).
 Results in Table I indicate a complete loss of acetyl cholinesterase
inhibitory power. The table also indicates that a drastic reduction in
the acute toxicity of the insecticide had occurred during metabolism
(Mücke et al., 1970).
TABLE I
Properties of the main metabolites
Compound Acute LD50 Inhibition of
AChE ID50
Diazinon approx. 250 mg/kg 2.7 × 10-5M
Metabolite 1 approx. 2 700 mg/kg 10-2M
Metabolite 2 5 000 mg/kg 10-2M
Recent investigations by Mücke et al. (1970), have shown that
2-isopropyl-4-methyl-pyrimidin-6-ol is of low toxicity, having an
acute oral LD50 of approximately 2 700 mg/kg in the rat, and that
there is no detectable anticholinesterase activity. These findings
provide an answer to the doubts expressed by Ralls et al. (1966),
concerning the toxicity of 2-isopropyl-4-methyl-pyrimidin-6-ol.
No information is available on the toxicity of the metabolite found
after treatment of kale with diazinon (see "FATE OF RESIDUES. In
plants").
Acute toxicity
TABLE II
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat oral 250 (male) Gaines, 1969
Rat oral 285 (female) Gaines, 1969
Rat oral 466 Boyd & Carsky, 1969
Rat oral 293-408 (male) Edson & Noakes, 1960
Rat dermal 900 (male) Gaines, 1969
TABLE II (cont'd)
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat dermal 455 (female) Gaines, 1969
Acute toxicity of diazinon to rats is summarized in Table II.
Differences in LD50 values expressed over the past few years may be
due to the presence of impurities contaminating early diazinon
samples. It has been shown that, under certain conditions,
combinations of molecules of diethyl phosphorothioic acid and
diethylphosphoric acid can condense to produce
tetraethylpyrophosphoric esters, which may be extremely toxic.
Stabilization of diazinon formulations appear to have eliminated some
of these condensation products and reduced the acute LD50 (Geigy,
1969).
As has been demonstrated with other pesticides, the acute LD50 to
rats increased as the protein level in the diet increased or decreased
from an optimal level. Raising the protein content from 29 percent to
81 percent, or lowering the protein content to 4 percent, increased
the toxicity approximately twofold (Boyd et al., 1969).
Short-term studies
Dog and pig
Groups of pigs (three male and three female) and dogs (three male and
three female) were orally administered diazinon by capsule daily for
periods up to eight months at doses of 0, 1.25, 2.5, 5 and 10 mg/kg
body-weight/day to pigs and 0, 2.5, 5, 10 and 20 mg/kg body-weight/day
to dogs (Earl et al., 1970). In pigs, mortality and cholinergic signs
of poisoning were evident at 2.5 mg/kg/day and above. Although
significantly increased myeloid/erythroid (ME) ratios were observed,
no aplastic anaemia was evident. In dogs, mortality and cholinergic
signs of poisoning were evident above 10 mg/kg, with significantly
increased ME ratios observed in dogs dying at 20 mg/kg. No evidence of
aplastic anaemia was observed in dogs and pigs.
Long-term studies
No new information available.
COMMENTS
It has been demonstrated that diazinon is oxidized in vitro to
diazoxon and further to diethylphosphorothioic acid. Farther
degradation has been shown to occur in vivo with the urinary
metabolites, which are substantially less toxic than the parent
compound. Distribution of diazinon in the body was relatively low,
with no accumulation in tissues or organs noted. Excretion in urine
and faeces was fairly rapid. No signs of blood dyscrasias were evident
in studies with dogs and pigs. No teratogenic or embryotoxic effects
were observed in hamsters and rabbits.
Stabilization of diazinon formulations have been reported to have
eliminated potential condensation products of diethylphosphoric acid
and diethylphosphorothioic acid. Information on the long-term toxicity
effects of diazinon is still inadequate. No information is available
on residuous anticholinesterase metabolites which may occur in plants.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.1 mg/kg body-weight/day
Dog: 0.02 mg/kg body-weight/day
Monkey: 0.05 mg/kg body-weight/day
Man: 0.02 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 = 0.002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Diazinon is a broad spectrum insecticide which has found many
applications, including control of:
(a) pests of field crops, orchards and pastures;
(b) animal ectoparasites;
(c) soil-inhabiting insects;
(d) industrial, public health and domestic pests.
Numerous formulations, including emulsifiable concentrates, wettable
powders, dusts and granules, are available. Foliar pests are normally
controlled by application in spray form, but effective control of rice
stem borer and some leaf hoppers has been obtained through application
of granules. Animals are normally treated by dipping, spraying,
jetting or dusting.
In the United States, diazinon is registered for application to more
than 60 food or feed crops including most fruits, vegetables and
forages. Use on corn (maize) for control of corn root worm and corn
ear worm and to alfalfa (lucerne) for control of alfalfa weevil are
major applications. There is an increasing use on rice.
Dipping, jetting or spraying of sheep for protection against sheep
blowfly are major uses in Australia, New Zealand, South Africa and
South America. Due to its solubility in wool grease, the material can
remain on the sheep in a stable state for several weeks and thus
provide effective protection against parasite attack. Dermal
applications to sheep, cattle and goats are also used for control of
lice, ticks, horn flies and certain other insects.
Table III summarizes typical dosages and pre-harvest periods for the
various crop categories. Diazinon has been found effective in
controlling over 100 species of food crop pests such as mites, aphids,
thrips, maggots, fruit flies, worms, beetles, grasshoppers, leaf
miners, etc. Seed furrow soil treatments are used for several root
vegetable crops.
TABLE III
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Tree fruits 0.5 lb/100 gal (full coverage) 10-20
Caneberries
(raspberries,
blackberries
loganberries) 1.0 lb/acre (full coverage) 7
Citrus 0.5 lb/100 gal (full coverage) 7-21
Leafy vegetables 0.5-1.0 lb/acre 5-21
Root vegetables 0.5-1.2 lb/acre 10
Others 0.25-1.0 lb acre 0-7
TABLE III (cont'd)
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Forages & hays 0-5-1.0 lb/acre No grazing
limitations
4-10 days
before cutting
hay
Cattle & sheep 1.0-2.3 gal of 0.30-0.5% (14
spray per animal pre-slaughter
period)
Post-harvest treatments
There is no commercial post-harvest use of diazinon on crops.
Other uses
Diazinon is recommended for fly control in dairy barns and other farm
buildings as well as in food processing plants; however, this is
becoming less important because of resistance developed by flies. It
is also used in households to control carpet beetles and clothes
moths, in home gardens and in buildings to combat ants, cockroaches,
fireants, etc. Control of cockroaches is an important field of use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of diazinon on or in plants, in animal tissues, or even in
the soil, are not highly persistent. In many countries and on over
sixty crops, residues have been determined at various time intervals
after applications of varying amounts of diazinon. For example, in the
controlled experiments with apples in the U.S.A., 16 varieties were
treated in 15 states using ´ to 1 lb/100 gal (i.e., full coverage
sprays) on various spray schedules and in combination with other
pesticides and fungicides.
A summary of the numerous data (Geigy, 1956-67) obtained from the
rates of application shown in Table III is given in Table IV.
TABLE IV
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Tree fruits
Apples 0.6-0.8 14 0.1-0.4 5
Pears 0.6-0.7 14 0.1-0.3 5
Cherries 3-7 10 0.1-0.3 3
Peaches 2-6 20 0.1-0.6 3-6
Apricots 1-4 10 0.1 2
Nectarines 1-6 10 0.2 3-4
Plums & prunes 2-4 10 <0.1-0.2 2
Figs 0.5 10 <0.1 3
Nuts
Almonds )
Filberts ) 0.1 (nuts) 10 <0.1 (nuts)
Walnuts ) 60 <0.1 (kernels)
Peanuts
Citrus
Oranges 3-5 21 0.1-0.4 4
Lemons 4.0 21 0.6-0.7 6-7
Grapefruit 0.3 7 0.1-0.2 7
Caneberries and small fruit
Strawberries 0.9-1.4 5 0.2-0.4 2-4
Grapes 1-8 18 <0.1-0.3 3
Cranberries 9 7 <0.1 1
Blueberries 1-4 7 <0.1-0.3 1-2
Blackberries )
Boysenberries ) 1-5 7 <0.1-0.3 2
Loganberries )
Raspberries )
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Leafy vegetables
Cabbage 1-2.5 7 <0.1-0.7 4-6
Celery 2-9 10 <0.1-0.7 2
Cauliflower 1 5 0.4-0.5 7
Broccoli 1-2 5 <0.1-0.5 3
Lettuce 6-17 10 0.3-0.5 1-2
Spinach 5-12 10 <0.1-0.2 2
Endive 3-18 10 0.1 2
Collards 3 12 <0.1 2
Kale 10-24 12 0.1-0.2 1
Parsley 1-6 12 0.1-3.0 2
Swiss chard 2 12 <0.1 2
Turnip greens 4-15 12 <0.1 2
Brussels sprouts 0.2 10 <0.1
Root vegetables (foliar application)
Beets <0.1 0
Onions 7-13 (green) 10 (green) 0.4-0.6 (green) 2
2-3 (dry) 10 (dry) <0.1-0.3 (dry) 2
Carrots 1-2 10 0.1-0.3 2-3
Carrots (soil
application) 120 0.1
Parsnips 0.7 10 0.3 6
Radishes <0.1-0.4 10 <0.1 4
Turnips 0.5 10 0.4-0.5 21
Vegetables (others)
Peppers 0.6-0.8 5 <0.1-0.2 2-3
Cucumbers 1.0-2.5 7 <0.1 2
Green beans 1-2 7 <0.1 2
Lima beans 0.2-1.0 7 <0.1-0.2 2
Squash 0.1-0.2 3 <0.1-0.2 2
Maize (ears only) <0.1 0 <0.1 -
Peas (plus pod) <0.1 0 <0.1 -
Miscellaneous
Tomatoes 0.1-0.4 3 <0.1-0.2 2
Melons 0.1-0.7 3 <0.1-0.2 2-3
Olives 1-6 75 <0.2-0.6 ca 25
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Hops (cones) 3-11 14 0.1-0.3 3
Sorghum (grain) 0.5 7 <0.1 2
Cotton (seed) 0.1-0.2 7 <0.1
Safflower (seed) <0.1
Sunflower 80 0.1-0.2
Sugarcane (stems) <0.1 7 <0.1
Tea 7 <0.1 (manufactured)
Coffee 1 7 0.1 (green beans)
Cereal crops
Wheat 13 <0.1 (grain)
Meat
Pre-slaughter
period
Lamb (fat) 1-3 14 0.1-0.75 5
Beef (fat) 1-3 14 <0.1-0.2 3
Forages
Pre-grazing Cutting Initial Residue Half
interval time residue at life
(days) (days) in forage cutting (days)
(ppm) (ppm)
Alfalfa 0 7 12-24 0.2-0.5 2
Clover 0 7 4-14 1.0-4.0 2-3
Range/pasture
grass 0 21 9 4.0-7.2 2-3
30 (oil
formulation) 54
Pea/bean 0 4 5-10 2.0-5.0 2
Maize 0 0 12-21 - 2
General Comments
In animals
Reports from numerous trials indicate that residues of diazinon in
subcutaneous fatty tissues of sheep and cattle treated for parasite
control do not exceed 0.75 ppm one day after treatment. The residue
levels in internal fat, however, may be higher. Average residue levels
decline rapidly following treatment, but fat samples from individual
animals may exceed 0.75 ppm for 14 days. Hastie (1965) showed that
standard treatment procedures resulted in residues in sheep one day
after treatment which were in excess of 1 ppm in the fat. After three
days, residue levels had declined to 0.3-0.5 ppm. Claborn et al.
(1963) showed that cattle sprayed with diazinon contained residues in
omental fat six days after treatment, but residues were not detectable
at 14 days.
Residues of diazinon in animal fat may be influenced by the condition
of the treated animal due to the dilution factor brought about by the
total amount of fat present. Application rates also affect the residue
levels.
In plants
Residue levels in various crops have been reviewed by Bartsch (1970).
Residue analyses of pip fruit have indicated that 90 percent of
residues are in the peel of ripe fruit and only 2-4 percent in the
pulp. No residues at all were detected in the pulp and juice of citrus
fruits, since the peel serves as a barrier (Gunther et al., 1958).
Residues present in any crop are dependent upon application rates,
cultural practices and type of formulation used. Residues have
generally been more persistent in glasshouse crops than in field crops
(Bartsch, 1970). Granular formulations have given more variable
residue levels in treated crops than have liquids applied as sprays.
However, studies have indicated that residue levels are higher overall
after crop treatment with emulsions rather than granules (Maier-Bode,
1967).
After crop application of emulsions or granules, practically no
residues have been detected in tubers (potatoes, sweet potatoes and
yams) or in grain (rice and wheat). Residues in seeds of cotton,
sunflower and safflower contain only little diazinon in contrast to
the respective oil products.
Immediately after treatment of forage crops, residues may reach 150
ppm. Residue levels fall very rapidly, due in part to growth of the
forage plants, and within a period of 1-2 days are below the current
tolerances of 60 ppm for grass and 40 ppm for alfalfa (in the U.S.A.)
Due to the solubility of diazinon in lipids, high residues may be
found in olive oil, but they are below the accepted tolerance levels
of 2 ppm in Italy and 1 ppm in U.S.A., 7-8 weeks after treatment.
Carrots have been examined with particular care on account of their
dietary significance, bearing in mind that they are often eaten
uncooked, and because of their biochemical properties. Residue figures
vary widely, depending on timing of application, formulation and
method of application. All trial data reviewed by Bartsch (1970)
indicated that unless harvest is carried out within 60 days of last
treatment (soil-application), the residue level is below 0.5 ppm in
all cases.
FATE OF RESIDUES
In animals
Investigations on the metabolic fate of diazinon in mammals were
initiated mainly by the widespread use of the product as an
ectoparasiticide in ruminants. Residue studies in fat and milk of cows
(Bourne and Arthur, 1967; Claborn et al., 1963; Derbyshire and Murphy,
1962; Matthysse and Lisk, 1968) and of sheep (Harrison and Hastie,
1965; Matthysse et al., 1968) were carried out after the insecticide
had been applied regularly by spraying and dipping or by feeding the
animals on pasture treated with the insecticide. Only small amounts
of diazinon residues have been found in fat and milk, whereas the
other tissues were free of the insecticide. In two early studies in
the cow (Robbins et al., 1957) and in the goat (Vigne et al., 1957),
the rapid and complete elimination of the 32P-labelled insecticide
in urine, faeces, blood and milk was shown, demonstrating urine as the
main route of excretion.
Rai and Roan (1959) found no residues of diazinon in the milk of dairy
animals given daily oral doses of diazinon at the rates of 1.06, 5.30
and 10.60 mg/kg of body-weight over a three-week feeding period. These
administration rates are calculated to be 100, 500 and 1 000 ppm on
the basis of the grain fed, or 51, 290 and 500 ppm on the basis of hay
consumed. Steers treated with 165 and 825 ppm in daily oral doses
calculated on the basis of grain fed showed traces of diazinon in
blood, urine, muscle, liver and brain. Only in fat was a significant
residue found, being 0.23 ppm at the maximum feeding level. These
results were obtained by the use of three methods of analysis.
In plants
Practical residue determination on many occasions has shown that
diazinon does not persist long as a residue on most food crops. It
appears that the movement or metabolism of diazinon depends upon the
plant species (Coffin and McKinley, 1964; Ralls et al., 1966; Gunner
et al., 1966).
Diazinon is passed through bean plants unchanged, with rapid
translocation and emergence in bean root exudates after application of
diazinon to aerial portion of the plant (Gunner et al., 1966). Sugar
beet seedlings absorb diazinon applied to the soil (Onsager and Rusk,
1967) and is translocated in quantities to render the plant
insecticidal. This is contrary to earlier observations (Gunner, 1966),
where it was found that diazinon was absorbed by beans but not
translocated in toxic amounts.
Analysis of alfalfa (lucerne) indicated that diazinon was absorbed
from treated soil into the plant (Nelson and Hamilton, 1970). No
metabolites of diazinon were found in the alfalfa, and diazinon was
expired by the plants. These findings substantiate the results
obtained by Gunner et al. (1966) with beans.
The metabolism of diazinon in plants has been shown to involve
hydrolysis of the phosphorus primidylester bond and subsequent
metabolism of the 2-isopropyl-4-methyl-6-hydroxypyrimidine to carbon
dioxide. Small amounts of diazoxon have at times been detected in
field-grown crops (Gomaa et al., 1969). The fact that diazoxon levels
are very low, if present at all, indicates oxidation is very minor or
that the oxon is hydrolysed as rapidly as it is found. Margot and
Gysin (1957) indicated loss of insecticidal activity on plants caused
by evaporation of diazinon and through hydrolysis. No metabolites of
diazinon were found.
Coffin and McKinley (1964) reported on the metabolism and persistence
of diazinon on field-sprayed lettuce. Diazinon residues decreased from
8.1 ppm to 0.3 ppm from four hours to seven days after spraying, and
detectable quantities of diazinon were present at 10 and 14 days. No
significant amount of diazoxon or other metabolites were found by the
paper chromatographic detection system.
Grasses and grains grown for forage which had been treated with
diazinon were analysed by the sulphide procedure. Maier-Bode (1963)
found diminution of residues occurred only in the uncut grasses. After
cutting and while drying to hay, little of the diazinon was lost.
Ralls et al. (1966), studied the fate of 35S-labelled diazinon on
field-grown crops and found a rapid decrease in diazinon residues. The
metabolite identified from field samples was diazoxon. Three
thin-layer chromatographic systems showed the presence of this
metabolite on spinach at 0.005 to 0.01 ppm five days after spraying.
Paper chromatography of snap bean extracts harvested seven days after
treatment showed an increase in a cholinesterase-inhibiting compound
with an Rf value corresponding to diazoxon.
Additional studies in the field revealed residue levels of diazinon
(I), diazoxon (II) and 2-isopropyl-4-methylpyrimidin-6-ol (III) made
by Ralls et al. (1967), using diazinon-32p. A spinach sample
analysed one hour after spraying contained 31.7 ppm (I), 1.5 ppm (II)
and 2.5 ppm (III). Analysis of a four-day sample gave 1.8 ppm (I),
0.34 ppm (II) and 2.5 ppm (III). Although diazinon and its oxygen
analogue dissipated rapidly, compound (III), the result of further
hydrolysis of diazoxon persisted at the same level. The mammalian
toxicity of this persistent compound was at the time not known.
Similar experiments with snap bean and tomato plants showed the same
rapid disappearance of diazinon and diazoxon to levels greatly below
0.1 ppm after four days. Less than 0.1 ppm of compound (III) was found
in all four-day samples.
Refined analytical techniques were used by Eberle and Novak (1969), to
further investigate the fate of diazinon in plants. The only
cholinesterase-inhibiting metabolite detectable at any time after
diazinon application was diazoxon. The maximum levels of diazoxon
found in apples and olive oil were 0.004 ppm and 0.007 ppm,
respectively. At harvest, fruit and vegetables in all instances
contained less than 0.002 ppm diazoxon.
The appearance and subsequent disappearance of traces of diazoxon in
the crops examined indicate that diazinon is oxidized in plants to
diazoxon which is, in turn, rapidly altered to
noncholinesterase-inhibiting products. All samples were analysed for
monothiotetraethylpyrophosphate (S-TEPP), but no residues could be
detected at the limit of 0.002 ppm. These results are at variance with
the findings of Melchiorri et al. (1964), and Siesto et al. (1964),
who earlier reported evidence of the formation of S-TEPP and TEPP. The
investigations of Eberle and Novak do, however, support the findings
of Ralls et al. (1966), in that the only cholinesterase-inhibiting
metabolite detectable at any time after diazinon application is
diazoxon.
The levels of diazinon and diazoxon reported by Eberle and Novak are
summarized in Table V.
TABLE V
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
40 (3 treatments) golden apples 0 2.8 <0.002
7 0.6 0.004
21 0.3 0.002
63 (harvest) 0.05 <0.002
40 (3 treatments) Jonathan 0 1.3 0.002
apples 28 0.2 <0.002
70 (harvest) 0.1 <0.002
50 (1 treatment) pears 49 0.01 <0.002
50 (2 treatments) 35 0.01 0.003
50 (3 treatments) 21 0.02 <0.002
50 (4 treatments) 10 0.12 0.003
TABLE V (cont'd)
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
25 (2 treatments) Langstieler 7 0.2 <0.002
cherries 28 (harvest) 0.02 <0.002
25 (2 treatments) Schauenburger 7 0.4 <0.002
cherries 28 (harvest) 0.02 <0.002
4 (2 treatments) Flakeer 29 1.3
carrots 80 (harvest) 0.2 <0.002
4 (2 treatments) Guérande 29 2.2
carrots 90 (harvest) 0.2 <0.002
1 treatment onions 125 (harvest) 0.05 <0.002
10 (1 treatment) radish 36 (harvest) <0.02 <0.002
25 Milan cabbage 0 27
7 1.7
21 0.05
63 (harvest) <0.02 <0.002
25 Blanc cabbage 0 12.5
7 1.1
63 (harvest <0.02 <0.002
In contrast to the findings of other workers, a further alteration
product, hydroxysiazinon, has been reported by Pardue et al. (1970).
This previously unidentified compound was detected during a study of
diazinon field-sprayed kale samples by a technique of enzyme
inhibition thin layer chromatography of the extracts previously
oxidized by bromine water.
The levels of hydroxydiazinon found in kale in comparison with
diazinon and diazoxon are indicated in Table VI.
TABLE VI
Residues found by GLC in diazinon-treated kale
Days after application
2 7 11 15
Diazinon (ppm) 8.8 2.9 2.0 1.6
Diazoxon (Ppm) 0.004 0.007 0.002 0.002
Alteration product of
diazinon1 (ppm) 0.18 0.05 0.03 0.03
1 Later identified as hydroxydiazinon. Quantitated using the compound
prepared by UV-irradiation of diazinon.
Pardue et al. (1970) have not offered any possible explanation for the
presence of hydroxydiazinon. Many of the cruciferous crops have waxy
cuticles which would have a tendency to hold diazinon. The effect of
UV-irradiation on diazinon could then possibly cause alteration to
hydroxydiazinon.
In soil
Literature on diazinon residues in soils has been mainly confined to
nonflooded conditions (Getzin and Rosefield, 1966; Getzin, 1967;
Gunner et al., 1966). Getzin (1967) reported that greater amounts of
the hydrolysis product were recovered from soil fumigated with
propylene oxide than from nonfumigated soil. Conversely, little 14CO2
was released from the fumigated soil treated with 14C-labelled
diazinon, while large amounts were released from nonfumigated soil. He
also suggested that the initial step in the degradation of diazinon in
nonflooded soils is hydrolysis at the heterocyclic phosphate bond
(phosphorus-oxygen-pyrimidine bond), followed by disruption of the
pyrimidine ring and the subsequent release of 14CO2. Soil
microflora appeared to play a major role in the of the degradation of
the parent molecule. Trela et al. (1968) recently observed that the
degradation of diazinon into pyrimidine and phosphorothioate
derivatives was greatly stimulated in the presence of microorganisms
isolated from diazinon-treated soil.
The degradation of diazinon in submerged neutral or alkaline soil
showed that diazinon disappeared at a faster rate from nonsterilized
soils than from sterilized soils (Sethunathan and MacRae, 1969), thus
indicating the participation of soil microflora in its degradation.
Surprisingly, only a small amount of 14CO2 was released from
nonsterilized soils treated with 14C-labelled diazinon (labelled at
the 2-position on the pyrimidine ring). This result is not in
agreement with the results on 14CO2 evolution from ring-labelled
diazinon reported for nonflooded, soils (Getzin, 1967). This
difference suggests that the fate of diazinon under submerged
conditions might be different from that in nonflooded conditions.
In the study of the persistence of diazinon (14C-labelled at the
4-position on the pyrimidine ring) in submerged soils, soil microflora
appeared to assist in its degradation into a less toxic hydrolysis
product (2-isopropyl-6-methyl-4-hydroxypyrimidine). This hydrolysis
product was, however, resistant to further degradation under submerged
conditions (Sethunathan and Yoshida, 1969).
From the foregoing, it appears that the major step in the degradation
of diazinon in flooded soil is hydrolysis, resulting in the formation
of 2-isopropyl-6-methyl-4-hydroxypyrimidine as one of the degradation
products. Under submerged conditions, where the bulk of soil
microflora is anaerobic, oxidation is negligible and the hydrolysis
product tends to accumulate and persist in large quantities without
being oxidized. The more rapid degradation of diazinon and the greater
recovery of hydrolysis product from nonsterilized soils suggest that,
in flooded soils, soil microflora play an important role - direct or
indirect - in the hydrolysis of diazinon to
2-isopropyl-6-methyl-4-hydroxypyrimidine, but not thereafter. The
accumulation of 2-isopropyl-6-methyl-4-hydroxypyrimidine in submerged
soils should not, however, pose a serious residue problem since, based
on the anticholinesterase activity of the two compounds (Margot and
Gysin, 1957), the hydrolysis product of diazinon is far less toxic
than the parent molecule. In addition, drying the soil or increasing
aeration during land preparation for the succeeding rice crop may
completely eliminate this degradation product by oxidation.
The decomposition of diazinon under nonflooded conditions, using two
soil types in conjunction with three other factors, was explored by
Bro-Rasmussen et al. (1968). These other factors considered were:
(i) activity of soil microorganisms
(ii) water content of soil
(iii) concentration of diazinon.
The disappearance rate of diazinon varied considerably with half-lives
ranging from 21 to 80 days. Results indicated that all factors
influenced the rate of diazinon degradations and that microorganisms
play an important role in the disappearance of diazinon from the soil.
In storage and processing
Residue levels of diazinon, diazoxon and
2-isopropyl-4-methyl-pyrimidin-6-ol were measured in snap beans,
spinach and tomatoes subjected to washing, blanching and pealing (for
tomatoes) under simulated commercial conditions (Ralls et al., 1967).
Diazinon on spinach at harvest 4 days after spraying was present at
1.8 ppm and diazoxon at 0.34 ppm. A spray rinse did not significantly
reduce residues. Detergent washing reduced diazinon to 0.77 ppm and
diazoxon to 0.18 ppm, and steam blanching gave a total reduction to
about 30 percent of the original residue. Only a water blanching
process significantly reduced the level of the pyrimidinol metabolite
from 2.5 ppm to 0.1 ppm. Residues at harvest (8 days) on snap beans
and tomatoes were less than 0.1 ppm. Subsequent commercially simulated
treatment appeared to have little or no effect, except possibly the
commercial peeling of tomatoes. Further studies on the reduction of
residues during food processing operations are reported by Farrow et
al. (1969).
Water washing of tomatoes (without detergent) removed 88 percent of
total residues, but caused an apparent increase of 11 percent with
spinach. A similar increase was seen with other insecticides such as
parathion. Crops such as spinach and broccoli have large surface areas
and are therefore subject to considerable leaching of water-soluble
solids during washing. Since results are expressed on a dry weight
basis, this results in an apparent increase in residue levels.
Commercial blanching operations are carried out using either hot water
or steam. Although washing was not effective in removing diazinon from
spinach, hot water blanching removed 60 percent of the residue. During
the combined canning operations it was found that there is a good
overall removal of organophosphorus residues. Thus there was a 99
percent removal of malathion residues from tomatoes, 94 percent
removal from green beans and 66 percent removal of parathion from
spinach; however, removal of parathion residues from broccoli, which
is processed by snap freezing, amounted to only 10 percent.
Diazinon is recommended in some countries for application to grapes,
and Painter et al. (1963) studied the fate of diazinon after addition
to grape must. Diazinon did not cause any measurable effect on
fermentation and was not found in any component after fermentation.
The absence of diazinon after fermentation is probably due to the
hydrolysis of the compound under the acid conditions prevailing. It is
interesting to note that several other organophosphorus compounds were
detected in finished wine when subjected to similar experimental
procedures.
In water
Gomaa et al. (1969) have considered breakdown of diazinon and diazoxon
which may occur in water. Techniques were developed to study the
hydrolysis of diazinon and diazoxon in aqueous media under varying
conditions of temperature and pH. Diazinon and diazoxon are
quantitatively hydrolysed to 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylthiophosphoric or diethylphosphoric acid.
In general, at 20°C, diazoxon hydrolysis proceeds much faster than
diazinon under comparable conditions. A large difference in rate of
hydrolysis can be detected under acidic conditions, where diazoxon is
hydrolysed 30 times faster than diazinon. The differences in rates
decrease as neutrality is approached, but increase again as the pH of
the hydrolysis solution increases.
The work of Gomaa et al., indicates that some caution should be
exercized concerning movement or application of diazinon into water.
Sethunathan and MacRae (1969), suggested that soil microflora play an
important part in the hydrolysis of diazinon in flooded soils. These
conditions are unlikely to apply to aqueous media, which may account
for the persistence of diazinon reported by Gomaa et al.
Evidence of residues in food moving in commerce or at consumption
Of some 14 800 randomly selected samples of raw agricultural products
examined by the U.S. Food and Drug Administration from June 1965
through 1966, only 32 samples showed any detectable residue of
diazinon. Total diet studies conducted during 1965 and 1966 by the
U.S. Food and Drug Administration revealed that 98 percent of the food
samples contained no detectable residues of diazinon. The remaining 2
percent contained only trace quantities. A multidetection gas
chromatographic method, using an electron capture detector and/or a
thermionic detector specific for phosphorus, was used for the
analyses. The sensitivity of the method was about 0.05 ppm (Duggan et
al., 1967).
METHODS OF RESIDUE ANALYSIS
Most of the residue data summarized in this monograph were obtained
using one or two of four different methods of analysis developed by
Geigy Chemical Company (1956-67).
A sulphide procedure was considered most accurate when spray history
was known. In this procedure, diazinon is extracted from crops with a
solvent and from the solvent with 48 percent HBr. The 48 percent HBr
treatment adds a high degree of selectivity for the determination of
diazinon. Upon boiling the acid solution, diazinon sulphur is
converted to H2S and distilled off. It is collected in zinc acetate
solution and then converted to a methylene blue complex, which is
determined spectrophotometrically. Sensitivity of the sulphide
procedure is about 0.1-0.2 ppm. Some crops, such as kale, had high
natural sulphur blanks, so these crops were analysed by a phosphate
method. Thiocarbamates such as ferbam also interfere and must be
removed by an additional cleanup step.
Some phosphorothioates are known to form relatively stable metabolic
products containing no sulphur, for which the method described above
would not be applicable, so a cholinesterase inhibition method was
utilized to validate the sulphide procedure. The method based on
determining the phosphorus of diazinon and one based on the
ultraviolet absorption properties of the pyrimidine portion of the
molecule are fraught with high blank and cleanup problems. Sensitivity
of these methods is about 0.3-0.4 ppm.
None of these four methods was of adequate sensitivity or specificity
for "total diet" samples. Such data were not possible until the GLC
methods based on electron capture and thermionic detectors were used.
Diazinon and diazoxon are detected and may be determined by the
multiresidue method of Abbott et al. (1970), which was successfully
used in the total diet studies carried out in England and Wales. The
methods of Storherr et al. (1964, 1965), using an ethyl acetate
extraction and either a sweep codistillation or celite column cleanup
with gas chromatographic detection, provide a rapid and adequately
sensitive procedure for diazinon in most food commodities.
J. R. Geigy SA has developed a gas chromatographic method which is
applied following a shakeout with 48 percent HBr. The gas
chromatographic methods are sensitive to about 0.01 ppm or better. A
number of thin layer chromatographic procedures described in the
literature will provide a confirmative test. Specific analytical
methods for the detection of diazinon have been considerably refined
in recent years. Extraction, cleanup and analytical techniques have
been reviewed by Eberle (unpublished).
An improved procedure for routine determination of diazinon residues
in fruits, vegetables, soils, etc. using various selective gas
chromatographic detector systems has been reported by Eberle and Novak
(1969). Possible metabolites with strong cholinesterase-inhibiting
activity are detected on TLC plates by fly-head cholinesterase
inhibition with a limit of detection of 0.002 ppm. The above method is
considered a marked improvement over previously described methods and
permits determination of diazinon residues in a variety of crops
together with detection of cholinesterase-inhibiting metabolites.
NATIONAL TOLERANCES
Table VII summarizes national tolerances which have been established
for diazinon.
TABLE VII
Country Tolerance (ppm) Crop
Australia 0.75 fruits, grains, vegetables
Canada 0.1 maize, peas
0.25 melons, figs, cranberries and
7 vegetables
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
Canada 0.5 beans, cucumbers, turnips
(continued) 0.75 tree fruits including citrus,
grapes, strawberries and 16
vegetables
Germany 0.5 on or in vegetables, fruits,
(Fed. Rep.) root crops, leg-wes, grapes
and hops
Hungary 0.5 food
India 0 (proposed) cereals
Italy 2 olive oil
Netherlands 0.5 on or in
(i) vegetables or parts thereof
for consumption, including
edible mushrooms and edible
roots, bulbs and tubers
(ii) edible fruits of vegetables
and fruit crops or parts
thereof
New Zealand 0.75 food
Switzerland The Swiss Intercantonal commission for
toxic materials ("Commission Intercantonale
des Toxiques") proposes a tolerance of 0.5
ppm. Federal regulations are in preparation
U.S.A 0.1 banana pulp, potatoes, sweet
potatoes
0.2 bananas
0.5 almonds, filberts, pecans and
walnuts
0.75 ca 25 fruits and 25 vegetables,
fat of meat and meat byproducts
of cattle and sheep
1 olives and olive oil
3 almond hulls
10 5 hays
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
25 bean and pea forage
40 alfalfa (fresh), clover (fresh)
and maize forage
60 pasture grasses
APPRAISAL
Diazinon was first synthesized in 1951 and introduced as an
experimental insecticide in 1952. Initial development of the product
was prompted by the appearance of DDT resistance in flies, mosquitoes
and other insects. Extensive markets were developed for the product in
the late 1950's, and major areas of use included control of pests in
maize and alfalfa (U.S.A.), control of cockroaches and other insects
in buildings, the control of sheep ectoparasites (Australasia) and
control of a variety of insects attacking fruit and vegetables (Europe
and U.S.A.).
Diazinon does not persist for lengthy periods in either plant or
animal tissues. Resistant action depends on a combination of factors
including plant or animal species, application rates, cultural
practices, climatic conditions, etc. In most instances, the half-life
on crops is two to three days, except in olives where it ranges from
12 to 25 days, depending on stage of maturity of the olive at
spraying.
In plants, diazoxon has been established as the principal
anticholinesterase metabolite, though it occurs only as an
insignificant fraction of the whole residue. This metabolite is in
turn rapidly converted to noncholinesterase-inhibiting products.
Studies with radio-labelled insecticide have shown that in plants,
water and soil as well as mammals, detoxification of the insecticide
takes place during the metabolism in all systems examined where
residues are likely to occur.
Several analytical methods are available for determining residues in
plant or animal tissue. When utilized in accordance with good
agricultural practice, the treated product may have residues up to the
proposed tolerances, but only a small portion of each food commodity
in these categories is likely to be treated. There are data showing
that a significant amount of reduction in residues will take place
during washing, preparation and processing of food for consumption. In
"total diet" samples, diazinon has seldom been found, and then only at
a low level. Residue data are available on more than 70 crops from
several different countries.
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are based on residues likely to be found at
harvest following currently approved use patterns. On commodities
other than nuts, oilseeds, olives, olive oil and fat of meat, residues
will continue to decline during storage and shipment with a probable
half-life of less than five days.
The tolerances are expressed as diazinon, since it is known that the
oxygen analogue, diazoxon, if present, does not occur at
concentrations above 0.004 ppm.
Peaches, citrus and cherries 0.7 ppm
Other fruits 0.5 ppm
Leafy vegetables 0.7 ppm
Other vegetables 0.5 ppm
Grain (wheat, barley, rice) 0.1 ppm
Nuts almonds, walnuts, filberts
pecans, groundnuts) 0.5 ppm
Oilseeds (cotton, safflower, sunflower) 0.5 ppm
Sweet maize (on kernels and cob with
husks removed) 0.7 ppm
Olives and olive oil 2 ppm
Fat of meat of cattle, sheep and pigs
FURTHER WORK OR INFORMATION
DESIRABLE
1. Reproduction studies on one rodent and one nonrodent species.
2. Toxicological information on residual anticholinesterase
metabolites of diazinon in plants.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G (1970)
Pesticides in the total diet in England and Wales 1966-67. Pest. Sci.,
1(1): 10-13
Bartsch, E. (1970) Residue data of diazinon. J. R. Geigy SA,
Switzerland (unpublished)
Bourne, J. R. and Arthur, B. W. (1967) Diazinon residues in the milk
of dairy cows. J. econ. Ent., 60: 402-405
Boyd, E. M. and Carsky, E. (1969) Kwashiorkorigenic diet and diazinon
toxicity. Acta. pharmacol. Toxicol., 27: 284-294
Boyd, E. M., Carsky, E. and Krijnen, C. J. (1969) The effects of diets
containing from 0 to 81 percent casein on the acute oral toxicity of
diazinon. Clin. Toxicol., 2: 295-302
Bro-Rasmussen, F., Noddegaard and Voldum-Clausen, K. (1968)
Degradation of diazinon in soil. K.J. Sci. Fd. Agr., 19: 278-281
Claborn, H. V., Mann, R. D., Younger, R. L. and Radeleff, R. D. (1963)
Diazinon residues in the fat of sprayed cattle. J. econ. Ent., 56(6):
858-859
Coffin, D. E. and McKinely, W. P. (1964) The metabolism and
persistence of systox, diazinon and phosdrin on field-sprayed lettuce.
J. Assoc. Off. Agr. Chem., 47(4): 632-640
Derbyshire, J. C. and Murphy, R. T. (1962) Diazinon residues in
treated silage and milk of cows fed with powdered diazinon. J. Agr.
Fd. Chem., 10(5): 384-386
Duggan, R. E., Barry, H. C. and Johnson, L. Y. (1967) Pesticide
residues in total diet samples. II. Pest. Monitor. J., 1(2): 2-12
Earl, F. L., Melveger, B. E., Reinwall, J. E., Bierbower, G. W. and
Curtis, J. M. (1970) Diazinon toxicity - comparative studies in dogs
and miniature swine. Toxicol. appl. Pharmacol. (in press)
Eberle, D. O. (1970) Analysis of diazinon. J. R. Geigy SA, Switzerland
(unpublished)
Eberle, D. O. and Novak, D. (1969a) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 228
Eberle, D. O. and Novak, D. (1969b) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 1067-1074
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food
FAO/WHO. (1968) 1968 evaluations of some pesticide residues in food.
FAO/PL:1968/M/9/1. WHO/Food Add./69-35
Farrow, R. P., Elkins, F. R., Rose, W. W., Lamb, F. C., Ralls, J. W.
and Mercer, J. W. (1969) Canning operations that reduce insecticide
levels in prepared foods and in solid food waste. Residue Reviews,
29: 73-87
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Geigy Chemical Company. (1956-67) Unpublished data and methods of
analysis in pesticide petitions, submitted to the U.S. Food and Drug
Administration
Geigy Chemical Company. (1969) Diazinon-deterioration-stabilization
and influence on toxicity. Unpublished report
Getzin, L. W. (1967) Metabolism of diazinon and zinophos in soils. J.
econ. Ent., 60: 505-508
Getzin, L. W. and Rosefield, I. (1966) Persistence of diazinon and
zinophos in soils. J. econ. Ent., 59(3): 512-516
Gomaa, H. M., Suffett, I. H. and Faust, S. D. (1969) Kinetics of
hydrolysis of diazinon and diazoxon. Residue Reviews, 29: 171-190
Gunner, H. B., Zukerman, B. M., Walker, R. W. Miller, C. W., Denbert,
K. H. and Longley, R. E. (1966) The distribution and persistence of
diazinon applied to plant and soil and its influence on soil
microflora. Plant Soil; 25: 249-264
Gunther, F. A., Ewart, W. H., Blinn, R. C., Elmer, H. S. and Wacker,
G. B. (1958) Field persistence comparisons of residues of the
insecticide diazinon in lemons and Valencia oranges and effects on
juice flavour. Agr. Fd. Chem., 6: 521
Harrison, D. L. and Hastie, B. A. (1965) Diazinon residues in the milk
of cows and fat of sheep after feeding on pasture treated with
diazinon. New Zealand J. agr. Res., 9: 1-7
Hastie, B. A. (1963) The metabolism and elimination of diazinon from
animals, animal tissues and foodstuffs. Geigy Agricultural Chemicals,
Australia (bulletin)
Hastie, B. A. (1965) Diazinon residues in sheep fat. Geigy
Agricultural Chemicals, Australia (unpublished)
Kaplanis, J. N., Louloudes, S. J. and Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65: 70-75
Maier-Bode, H. (1963) Residues of insecticides on cover crops growing
in orchards after application of organic phosphorus toxicants on the
trees. Z. Pflanzenkrankh. Pflanzenschutz, 70(80): 449-459
Maier-Bode, H. (1967) Untersuchungen über den Gehalt
landwirtschaftlicher und gärtnerischer Ernteprodukte an
Pflanzenschutzmittelrückständen. Der Ministerpräsident des Landes
Nordrhein-West., Landesamt für Forschung, Jahrbuch 1967, S. 391.
Margot, A. and Gysin, H. (1957) Diazinon, its degradation products and
their properties. Belv. Chim. Acta., 40: 1562
Mathysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ectoparasite and fly control on dairy
cattle, with notes on safety of diazinon and ciodrin to calves. J.
econ. Ent., 61(5): 1394-1398
Melchiorri, P. Maffei F. and Siesto. A. J. (1964) Farmaco (Pavia), Ed.
Prat., 19: 610
Michel, H. O. (1949) J. lab. clin. Med., 34: 1564
Millar, K. R. (1963) Detection and distribution of 32P-labelled
diazinon in dog tissues after oral administration. New Zealand vet.
J., 11(6): 141-144
Mücke, W., Alt, K. O. and Esser, H. O. (1970) Degradation of
14C-labelled diazinon in the rat. J. Agr. Fd. Chem., 18(2): 208-212
Nakatsugawa, T., Tolman, N. M. and Dalm, P. A. (1969) Oxidative
degradation of diazinon by rat liver microsomes. Brochem. Pharmacol.,
18: 685-688
Nelson, L. L. and Hamilton, E. W. (1970) Metabolism of diazinon in
alfalfa. J. econ. Ent., 63(3): 874-878
Onsager, J. A. and Rusk, H. W. (1967) Adsorption and translocation of
diazinon and Stauffer N-2790 in sugarbeet seedlings. J. econ. Ent.,
60(2): 586-588
Painter, R. P. and Kilgore, W. W. (1963) Distribution of pesticides in
fermentation products obtained from artificially fortified grape
musts. J. Fd Sci., 28: 342-346
Pardue, J. R., Hansen, E. R., Barron, R. P. and Chen, J. J. (1970)
Diazinon residues on field-sprayed kale. Hydroxydiazinon - a new
alteration product of diazinon. J. Agr. Fd Chem., 18: 405-408
Rai, L. and Roan, C. C. (1959) Report included in Geigy Chemical
Company pesticide petition to the U.S. Food and Drug Administration
Ralls, J. W., Gilmore, D. R. and Cortes, A. (1966) Fate of radioactive
o,o-diethyl o-(2-isopropyl-4-methylpyrimidin-6-ol) phosphorothioate on
field grown experimental crops. J. Agr. Fd Chem., 14(4): 387-392
Ralls, J. W., Gilmore, D. R., Cortes. A., Schutt, S. H. and Mercer, W.
A. (1967) Residue levels of diazinon and its transformation products
on tomatoes, spinach and beans. Fd Tech., 21: 92-94
Robbins, W. E., Hopkins, T. L. and Eddy, O. W. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem., 5(7): 509-513
Robens, J. F. (1969) Teratologic studies of carbaryl, diazinon, norea
disulfiram and thiram in small laboratory animals. Toxicol. appl.
Pharmacol., 15: 152-163
Sethunathan, N. and MacRae, I. C. (1969) Persistence and
biodegradation of diazinon in submerged soil. J. Agr. Fd Chem., 17:
221
Sethunathan, N. and Yoshida, T. (1969) Fate of diazinon in submerged
soil. Accumulation of hydrolysis product. J. Agr. Fd Chem., 17(6):
1192-1195
Siesto, A. J., Maffei, F. and Melchiorri P. (1964) Estratto da
Archivio Italiano di Scienze Farmacologiche, Series 3, 13: 3
Storherr, R. W., Getz, M. E., Watts, R. R., Friedman, S. J., Erwin,
F., Giuffrida, L. and Ives, F. (1964) Identification and analyses of
five organo-phosphate pesticides. Recoveries from crops fortified at
different levels. J. Assoc. Off. Agr. Chem., 47(6): 1087-1093
Storherr, R. W. and Watts, R. R. (1965) A sweep co-distillation
cleanup method for organophosphate pesticides. J. Assoc. Off. Agr.
Chem., 48(6): 1154-1160
Vigne, J. P., Chouteau, J., Tabau, R.-L., Rancien, P. and Karamanian,
A. (1957) Sur le métabolisme d'un insecticide organo-phosphoré, le
diéthylthionophosphate de 2 isopropyl 4 méthyl 6 oxypyrimidine chez la
chèvre. Bull. Acad. Vét. Fr., 30: 85-92
Results in Table I indicate a complete loss of acetyl cholinesterase
inhibitory power. The table also indicates that a drastic reduction in
the acute toxicity of the insecticide had occurred during metabolism
(Mücke et al., 1970).
TABLE I
Properties of the main metabolites
Compound Acute LD50 Inhibition of
AChE ID50
Diazinon approx. 250 mg/kg 2.7 × 10-5M
Metabolite 1 approx. 2 700 mg/kg 10-2M
Metabolite 2 5 000 mg/kg 10-2M
Recent investigations by Mücke et al. (1970), have shown that
2-isopropyl-4-methyl-pyrimidin-6-ol is of low toxicity, having an
acute oral LD50 of approximately 2 700 mg/kg in the rat, and that
there is no detectable anticholinesterase activity. These findings
provide an answer to the doubts expressed by Ralls et al. (1966),
concerning the toxicity of 2-isopropyl-4-methyl-pyrimidin-6-ol.
No information is available on the toxicity of the metabolite found
after treatment of kale with diazinon (see "FATE OF RESIDUES. In
plants").
Acute toxicity
TABLE II
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat oral 250 (male) Gaines, 1969
Rat oral 285 (female) Gaines, 1969
Rat oral 466 Boyd & Carsky, 1969
Rat oral 293-408 (male) Edson & Noakes, 1960
Rat dermal 900 (male) Gaines, 1969
TABLE II (cont'd)
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat dermal 455 (female) Gaines, 1969
Acute toxicity of diazinon to rats is summarized in Table II.
Differences in LD50 values expressed over the past few years may be
due to the presence of impurities contaminating early diazinon
samples. It has been shown that, under certain conditions,
combinations of molecules of diethyl phosphorothioic acid and
diethylphosphoric acid can condense to produce
tetraethylpyrophosphoric esters, which may be extremely toxic.
Stabilization of diazinon formulations appear to have eliminated some
of these condensation products and reduced the acute LD50 (Geigy,
1969).
As has been demonstrated with other pesticides, the acute LD50 to
rats increased as the protein level in the diet increased or decreased
from an optimal level. Raising the protein content from 29 percent to
81 percent, or lowering the protein content to 4 percent, increased
the toxicity approximately twofold (Boyd et al., 1969).
Short-term studies
Dog and pig
Groups of pigs (three male and three female) and dogs (three male and
three female) were orally administered diazinon by capsule daily for
periods up to eight months at doses of 0, 1.25, 2.5, 5 and 10 mg/kg
body-weight/day to pigs and 0, 2.5, 5, 10 and 20 mg/kg body-weight/day
to dogs (Earl et al., 1970). In pigs, mortality and cholinergic signs
of poisoning were evident at 2.5 mg/kg/day and above. Although
significantly increased myeloid/erythroid (ME) ratios were observed,
no aplastic anaemia was evident. In dogs, mortality and cholinergic
signs of poisoning were evident above 10 mg/kg, with significantly
increased ME ratios observed in dogs dying at 20 mg/kg. No evidence of
aplastic anaemia was observed in dogs and pigs.
Long-term studies
No new information available.
COMMENTS
It has been demonstrated that diazinon is oxidized in vitro to
diazoxon and further to diethylphosphorothioic acid. Farther
degradation has been shown to occur in vivo with the urinary
metabolites, which are substantially less toxic than the parent
compound. Distribution of diazinon in the body was relatively low,
with no accumulation in tissues or organs noted. Excretion in urine
and faeces was fairly rapid. No signs of blood dyscrasias were evident
in studies with dogs and pigs. No teratogenic or embryotoxic effects
were observed in hamsters and rabbits.
Stabilization of diazinon formulations have been reported to have
eliminated potential condensation products of diethylphosphoric acid
and diethylphosphorothioic acid. Information on the long-term toxicity
effects of diazinon is still inadequate. No information is available
on residuous anticholinesterase metabolites which may occur in plants.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.1 mg/kg body-weight/day
Dog: 0.02 mg/kg body-weight/day
Monkey: 0.05 mg/kg body-weight/day
Man: 0.02 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 = 0.002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Diazinon is a broad spectrum insecticide which has found many
applications, including control of:
(a) pests of field crops, orchards and pastures;
(b) animal ectoparasites;
(c) soil-inhabiting insects;
(d) industrial, public health and domestic pests.
Numerous formulations, including emulsifiable concentrates, wettable
powders, dusts and granules, are available. Foliar pests are normally
controlled by application in spray form, but effective control of rice
stem borer and some leaf hoppers has been obtained through application
of granules. Animals are normally treated by dipping, spraying,
jetting or dusting.
In the United States, diazinon is registered for application to more
than 60 food or feed crops including most fruits, vegetables and
forages. Use on corn (maize) for control of corn root worm and corn
ear worm and to alfalfa (lucerne) for control of alfalfa weevil are
major applications. There is an increasing use on rice.
Dipping, jetting or spraying of sheep for protection against sheep
blowfly are major uses in Australia, New Zealand, South Africa and
South America. Due to its solubility in wool grease, the material can
remain on the sheep in a stable state for several weeks and thus
provide effective protection against parasite attack. Dermal
applications to sheep, cattle and goats are also used for control of
lice, ticks, horn flies and certain other insects.
Table III summarizes typical dosages and pre-harvest periods for the
various crop categories. Diazinon has been found effective in
controlling over 100 species of food crop pests such as mites, aphids,
thrips, maggots, fruit flies, worms, beetles, grasshoppers, leaf
miners, etc. Seed furrow soil treatments are used for several root
vegetable crops.
TABLE III
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Tree fruits 0.5 lb/100 gal (full coverage) 10-20
Caneberries
(raspberries,
blackberries
loganberries) 1.0 lb/acre (full coverage) 7
Citrus 0.5 lb/100 gal (full coverage) 7-21
Leafy vegetables 0.5-1.0 lb/acre 5-21
Root vegetables 0.5-1.2 lb/acre 10
Others 0.25-1.0 lb acre 0-7
TABLE III (cont'd)
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Forages & hays 0-5-1.0 lb/acre No grazing
limitations
4-10 days
before cutting
hay
Cattle & sheep 1.0-2.3 gal of 0.30-0.5% (14
spray per animal pre-slaughter
period)
Post-harvest treatments
There is no commercial post-harvest use of diazinon on crops.
Other uses
Diazinon is recommended for fly control in dairy barns and other farm
buildings as well as in food processing plants; however, this is
becoming less important because of resistance developed by flies. It
is also used in households to control carpet beetles and clothes
moths, in home gardens and in buildings to combat ants, cockroaches,
fireants, etc. Control of cockroaches is an important field of use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of diazinon on or in plants, in animal tissues, or even in
the soil, are not highly persistent. In many countries and on over
sixty crops, residues have been determined at various time intervals
after applications of varying amounts of diazinon. For example, in the
controlled experiments with apples in the U.S.A., 16 varieties were
treated in 15 states using ´ to 1 lb/100 gal (i.e., full coverage
sprays) on various spray schedules and in combination with other
pesticides and fungicides.
A summary of the numerous data (Geigy, 1956-67) obtained from the
rates of application shown in Table III is given in Table IV.
TABLE IV
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Tree fruits
Apples 0.6-0.8 14 0.1-0.4 5
Pears 0.6-0.7 14 0.1-0.3 5
Cherries 3-7 10 0.1-0.3 3
Peaches 2-6 20 0.1-0.6 3-6
Apricots 1-4 10 0.1 2
Nectarines 1-6 10 0.2 3-4
Plums & prunes 2-4 10 <0.1-0.2 2
Figs 0.5 10 <0.1 3
Nuts
Almonds )
Filberts ) 0.1 (nuts) 10 <0.1 (nuts)
Walnuts ) 60 <0.1 (kernels)
Peanuts
Citrus
Oranges 3-5 21 0.1-0.4 4
Lemons 4.0 21 0.6-0.7 6-7
Grapefruit 0.3 7 0.1-0.2 7
Caneberries and small fruit
Strawberries 0.9-1.4 5 0.2-0.4 2-4
Grapes 1-8 18 <0.1-0.3 3
Cranberries 9 7 <0.1 1
Blueberries 1-4 7 <0.1-0.3 1-2
Blackberries )
Boysenberries ) 1-5 7 <0.1-0.3 2
Loganberries )
Raspberries )
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Leafy vegetables
Cabbage 1-2.5 7 <0.1-0.7 4-6
Celery 2-9 10 <0.1-0.7 2
Cauliflower 1 5 0.4-0.5 7
Broccoli 1-2 5 <0.1-0.5 3
Lettuce 6-17 10 0.3-0.5 1-2
Spinach 5-12 10 <0.1-0.2 2
Endive 3-18 10 0.1 2
Collards 3 12 <0.1 2
Kale 10-24 12 0.1-0.2 1
Parsley 1-6 12 0.1-3.0 2
Swiss chard 2 12 <0.1 2
Turnip greens 4-15 12 <0.1 2
Brussels sprouts 0.2 10 <0.1
Root vegetables (foliar application)
Beets <0.1 0
Onions 7-13 (green) 10 (green) 0.4-0.6 (green) 2
2-3 (dry) 10 (dry) <0.1-0.3 (dry) 2
Carrots 1-2 10 0.1-0.3 2-3
Carrots (soil
application) 120 0.1
Parsnips 0.7 10 0.3 6
Radishes <0.1-0.4 10 <0.1 4
Turnips 0.5 10 0.4-0.5 21
Vegetables (others)
Peppers 0.6-0.8 5 <0.1-0.2 2-3
Cucumbers 1.0-2.5 7 <0.1 2
Green beans 1-2 7 <0.1 2
Lima beans 0.2-1.0 7 <0.1-0.2 2
Squash 0.1-0.2 3 <0.1-0.2 2
Maize (ears only) <0.1 0 <0.1 -
Peas (plus pod) <0.1 0 <0.1 -
Miscellaneous
Tomatoes 0.1-0.4 3 <0.1-0.2 2
Melons 0.1-0.7 3 <0.1-0.2 2-3
Olives 1-6 75 <0.2-0.6 ca 25
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Hops (cones) 3-11 14 0.1-0.3 3
Sorghum (grain) 0.5 7 <0.1 2
Cotton (seed) 0.1-0.2 7 <0.1
Safflower (seed) <0.1
Sunflower 80 0.1-0.2
Sugarcane (stems) <0.1 7 <0.1
Tea 7 <0.1 (manufactured)
Coffee 1 7 0.1 (green beans)
Cereal crops
Wheat 13 <0.1 (grain)
Meat
Pre-slaughter
period
Lamb (fat) 1-3 14 0.1-0.75 5
Beef (fat) 1-3 14 <0.1-0.2 3
Forages
Pre-grazing Cutting Initial Residue Half
interval time residue at life
(days) (days) in forage cutting (days)
(ppm) (ppm)
Alfalfa 0 7 12-24 0.2-0.5 2
Clover 0 7 4-14 1.0-4.0 2-3
Range/pasture
grass 0 21 9 4.0-7.2 2-3
30 (oil
formulation) 54
Pea/bean 0 4 5-10 2.0-5.0 2
Maize 0 0 12-21 - 2
General Comments
In animals
Reports from numerous trials indicate that residues of diazinon in
subcutaneous fatty tissues of sheep and cattle treated for parasite
control do not exceed 0.75 ppm one day after treatment. The residue
levels in internal fat, however, may be higher. Average residue levels
decline rapidly following treatment, but fat samples from individual
animals may exceed 0.75 ppm for 14 days. Hastie (1965) showed that
standard treatment procedures resulted in residues in sheep one day
after treatment which were in excess of 1 ppm in the fat. After three
days, residue levels had declined to 0.3-0.5 ppm. Claborn et al.
(1963) showed that cattle sprayed with diazinon contained residues in
omental fat six days after treatment, but residues were not detectable
at 14 days.
Residues of diazinon in animal fat may be influenced by the condition
of the treated animal due to the dilution factor brought about by the
total amount of fat present. Application rates also affect the residue
levels.
In plants
Residue levels in various crops have been reviewed by Bartsch (1970).
Residue analyses of pip fruit have indicated that 90 percent of
residues are in the peel of ripe fruit and only 2-4 percent in the
pulp. No residues at all were detected in the pulp and juice of citrus
fruits, since the peel serves as a barrier (Gunther et al., 1958).
Residues present in any crop are dependent upon application rates,
cultural practices and type of formulation used. Residues have
generally been more persistent in glasshouse crops than in field crops
(Bartsch, 1970). Granular formulations have given more variable
residue levels in treated crops than have liquids applied as sprays.
However, studies have indicated that residue levels are higher overall
after crop treatment with emulsions rather than granules (Maier-Bode,
1967).
After crop application of emulsions or granules, practically no
residues have been detected in tubers (potatoes, sweet potatoes and
yams) or in grain (rice and wheat). Residues in seeds of cotton,
sunflower and safflower contain only little diazinon in contrast to
the respective oil products.
Immediately after treatment of forage crops, residues may reach 150
ppm. Residue levels fall very rapidly, due in part to growth of the
forage plants, and within a period of 1-2 days are below the current
tolerances of 60 ppm for grass and 40 ppm for alfalfa (in the U.S.A.)
Due to the solubility of diazinon in lipids, high residues may be
found in olive oil, but they are below the accepted tolerance levels
of 2 ppm in Italy and 1 ppm in U.S.A., 7-8 weeks after treatment.
Carrots have been examined with particular care on account of their
dietary significance, bearing in mind that they are often eaten
uncooked, and because of their biochemical properties. Residue figures
vary widely, depending on timing of application, formulation and
method of application. All trial data reviewed by Bartsch (1970)
indicated that unless harvest is carried out within 60 days of last
treatment (soil-application), the residue level is below 0.5 ppm in
all cases.
FATE OF RESIDUES
In animals
Investigations on the metabolic fate of diazinon in mammals were
initiated mainly by the widespread use of the product as an
ectoparasiticide in ruminants. Residue studies in fat and milk of cows
(Bourne and Arthur, 1967; Claborn et al., 1963; Derbyshire and Murphy,
1962; Matthysse and Lisk, 1968) and of sheep (Harrison and Hastie,
1965; Matthysse et al., 1968) were carried out after the insecticide
had been applied regularly by spraying and dipping or by feeding the
animals on pasture treated with the insecticide. Only small amounts
of diazinon residues have been found in fat and milk, whereas the
other tissues were free of the insecticide. In two early studies in
the cow (Robbins et al., 1957) and in the goat (Vigne et al., 1957),
the rapid and complete elimination of the 32P-labelled insecticide
in urine, faeces, blood and milk was shown, demonstrating urine as the
main route of excretion.
Rai and Roan (1959) found no residues of diazinon in the milk of dairy
animals given daily oral doses of diazinon at the rates of 1.06, 5.30
and 10.60 mg/kg of body-weight over a three-week feeding period. These
administration rates are calculated to be 100, 500 and 1 000 ppm on
the basis of the grain fed, or 51, 290 and 500 ppm on the basis of hay
consumed. Steers treated with 165 and 825 ppm in daily oral doses
calculated on the basis of grain fed showed traces of diazinon in
blood, urine, muscle, liver and brain. Only in fat was a significant
residue found, being 0.23 ppm at the maximum feeding level. These
results were obtained by the use of three methods of analysis.
In plants
Practical residue determination on many occasions has shown that
diazinon does not persist long as a residue on most food crops. It
appears that the movement or metabolism of diazinon depends upon the
plant species (Coffin and McKinley, 1964; Ralls et al., 1966; Gunner
et al., 1966).
Diazinon is passed through bean plants unchanged, with rapid
translocation and emergence in bean root exudates after application of
diazinon to aerial portion of the plant (Gunner et al., 1966). Sugar
beet seedlings absorb diazinon applied to the soil (Onsager and Rusk,
1967) and is translocated in quantities to render the plant
insecticidal. This is contrary to earlier observations (Gunner, 1966),
where it was found that diazinon was absorbed by beans but not
translocated in toxic amounts.
Analysis of alfalfa (lucerne) indicated that diazinon was absorbed
from treated soil into the plant (Nelson and Hamilton, 1970). No
metabolites of diazinon were found in the alfalfa, and diazinon was
expired by the plants. These findings substantiate the results
obtained by Gunner et al. (1966) with beans.
The metabolism of diazinon in plants has been shown to involve
hydrolysis of the phosphorus primidylester bond and subsequent
metabolism of the 2-isopropyl-4-methyl-6-hydroxypyrimidine to carbon
dioxide. Small amounts of diazoxon have at times been detected in
field-grown crops (Gomaa et al., 1969). The fact that diazoxon levels
are very low, if present at all, indicates oxidation is very minor or
that the oxon is hydrolysed as rapidly as it is found. Margot and
Gysin (1957) indicated loss of insecticidal activity on plants caused
by evaporation of diazinon and through hydrolysis. No metabolites of
diazinon were found.
Coffin and McKinley (1964) reported on the metabolism and persistence
of diazinon on field-sprayed lettuce. Diazinon residues decreased from
8.1 ppm to 0.3 ppm from four hours to seven days after spraying, and
detectable quantities of diazinon were present at 10 and 14 days. No
significant amount of diazoxon or other metabolites were found by the
paper chromatographic detection system.
Grasses and grains grown for forage which had been treated with
diazinon were analysed by the sulphide procedure. Maier-Bode (1963)
found diminution of residues occurred only in the uncut grasses. After
cutting and while drying to hay, little of the diazinon was lost.
Ralls et al. (1966), studied the fate of 35S-labelled diazinon on
field-grown crops and found a rapid decrease in diazinon residues. The
metabolite identified from field samples was diazoxon. Three
thin-layer chromatographic systems showed the presence of this
metabolite on spinach at 0.005 to 0.01 ppm five days after spraying.
Paper chromatography of snap bean extracts harvested seven days after
treatment showed an increase in a cholinesterase-inhibiting compound
with an Rf value corresponding to diazoxon.
Additional studies in the field revealed residue levels of diazinon
(I), diazoxon (II) and 2-isopropyl-4-methylpyrimidin-6-ol (III) made
by Ralls et al. (1967), using diazinon-32p. A spinach sample
analysed one hour after spraying contained 31.7 ppm (I), 1.5 ppm (II)
and 2.5 ppm (III). Analysis of a four-day sample gave 1.8 ppm (I),
0.34 ppm (II) and 2.5 ppm (III). Although diazinon and its oxygen
analogue dissipated rapidly, compound (III), the result of further
hydrolysis of diazoxon persisted at the same level. The mammalian
toxicity of this persistent compound was at the time not known.
Similar experiments with snap bean and tomato plants showed the same
rapid disappearance of diazinon and diazoxon to levels greatly below
0.1 ppm after four days. Less than 0.1 ppm of compound (III) was found
in all four-day samples.
Refined analytical techniques were used by Eberle and Novak (1969), to
further investigate the fate of diazinon in plants. The only
cholinesterase-inhibiting metabolite detectable at any time after
diazinon application was diazoxon. The maximum levels of diazoxon
found in apples and olive oil were 0.004 ppm and 0.007 ppm,
respectively. At harvest, fruit and vegetables in all instances
contained less than 0.002 ppm diazoxon.
The appearance and subsequent disappearance of traces of diazoxon in
the crops examined indicate that diazinon is oxidized in plants to
diazoxon which is, in turn, rapidly altered to
noncholinesterase-inhibiting products. All samples were analysed for
monothiotetraethylpyrophosphate (S-TEPP), but no residues could be
detected at the limit of 0.002 ppm. These results are at variance with
the findings of Melchiorri et al. (1964), and Siesto et al. (1964),
who earlier reported evidence of the formation of S-TEPP and TEPP. The
investigations of Eberle and Novak do, however, support the findings
of Ralls et al. (1966), in that the only cholinesterase-inhibiting
metabolite detectable at any time after diazinon application is
diazoxon.
The levels of diazinon and diazoxon reported by Eberle and Novak are
summarized in Table V.
TABLE V
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
40 (3 treatments) golden apples 0 2.8 <0.002
7 0.6 0.004
21 0.3 0.002
63 (harvest) 0.05 <0.002
40 (3 treatments) Jonathan 0 1.3 0.002
apples 28 0.2 <0.002
70 (harvest) 0.1 <0.002
50 (1 treatment) pears 49 0.01 <0.002
50 (2 treatments) 35 0.01 0.003
50 (3 treatments) 21 0.02 <0.002
50 (4 treatments) 10 0.12 0.003
TABLE V (cont'd)
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
25 (2 treatments) Langstieler 7 0.2 <0.002
cherries 28 (harvest) 0.02 <0.002
25 (2 treatments) Schauenburger 7 0.4 <0.002
cherries 28 (harvest) 0.02 <0.002
4 (2 treatments) Flakeer 29 1.3
carrots 80 (harvest) 0.2 <0.002
4 (2 treatments) Guérande 29 2.2
carrots 90 (harvest) 0.2 <0.002
1 treatment onions 125 (harvest) 0.05 <0.002
10 (1 treatment) radish 36 (harvest) <0.02 <0.002
25 Milan cabbage 0 27
7 1.7
21 0.05
63 (harvest) <0.02 <0.002
25 Blanc cabbage 0 12.5
7 1.1
63 (harvest <0.02 <0.002
In contrast to the findings of other workers, a further alteration
product, hydroxysiazinon, has been reported by Pardue et al. (1970).
This previously unidentified compound was detected during a study of
diazinon field-sprayed kale samples by a technique of enzyme
inhibition thin layer chromatography of the extracts previously
oxidized by bromine water.
The levels of hydroxydiazinon found in kale in comparison with
diazinon and diazoxon are indicated in Table VI.
TABLE VI
Residues found by GLC in diazinon-treated kale
Days after application
2 7 11 15
Diazinon (ppm) 8.8 2.9 2.0 1.6
Diazoxon (Ppm) 0.004 0.007 0.002 0.002
Alteration product of
diazinon1 (ppm) 0.18 0.05 0.03 0.03
1 Later identified as hydroxydiazinon. Quantitated using the compound
prepared by UV-irradiation of diazinon.
Pardue et al. (1970) have not offered any possible explanation for the
presence of hydroxydiazinon. Many of the cruciferous crops have waxy
cuticles which would have a tendency to hold diazinon. The effect of
UV-irradiation on diazinon could then possibly cause alteration to
hydroxydiazinon.
In soil
Literature on diazinon residues in soils has been mainly confined to
nonflooded conditions (Getzin and Rosefield, 1966; Getzin, 1967;
Gunner et al., 1966). Getzin (1967) reported that greater amounts of
the hydrolysis product were recovered from soil fumigated with
propylene oxide than from nonfumigated soil. Conversely, little 14CO2
was released from the fumigated soil treated with 14C-labelled
diazinon, while large amounts were released from nonfumigated soil. He
also suggested that the initial step in the degradation of diazinon in
nonflooded soils is hydrolysis at the heterocyclic phosphate bond
(phosphorus-oxygen-pyrimidine bond), followed by disruption of the
pyrimidine ring and the subsequent release of 14CO2. Soil
microflora appeared to play a major role in the of the degradation of
the parent molecule. Trela et al. (1968) recently observed that the
degradation of diazinon into pyrimidine and phosphorothioate
derivatives was greatly stimulated in the presence of microorganisms
isolated from diazinon-treated soil.
The degradation of diazinon in submerged neutral or alkaline soil
showed that diazinon disappeared at a faster rate from nonsterilized
soils than from sterilized soils (Sethunathan and MacRae, 1969), thus
indicating the participation of soil microflora in its degradation.
Surprisingly, only a small amount of 14CO2 was released from
nonsterilized soils treated with 14C-labelled diazinon (labelled at
the 2-position on the pyrimidine ring). This result is not in
agreement with the results on 14CO2 evolution from ring-labelled
diazinon reported for nonflooded, soils (Getzin, 1967). This
difference suggests that the fate of diazinon under submerged
conditions might be different from that in nonflooded conditions.
In the study of the persistence of diazinon (14C-labelled at the
4-position on the pyrimidine ring) in submerged soils, soil microflora
appeared to assist in its degradation into a less toxic hydrolysis
product (2-isopropyl-6-methyl-4-hydroxypyrimidine). This hydrolysis
product was, however, resistant to further degradation under submerged
conditions (Sethunathan and Yoshida, 1969).
From the foregoing, it appears that the major step in the degradation
of diazinon in flooded soil is hydrolysis, resulting in the formation
of 2-isopropyl-6-methyl-4-hydroxypyrimidine as one of the degradation
products. Under submerged conditions, where the bulk of soil
microflora is anaerobic, oxidation is negligible and the hydrolysis
product tends to accumulate and persist in large quantities without
being oxidized. The more rapid degradation of diazinon and the greater
recovery of hydrolysis product from nonsterilized soils suggest that,
in flooded soils, soil microflora play an important role - direct or
indirect - in the hydrolysis of diazinon to
2-isopropyl-6-methyl-4-hydroxypyrimidine, but not thereafter. The
accumulation of 2-isopropyl-6-methyl-4-hydroxypyrimidine in submerged
soils should not, however, pose a serious residue problem since, based
on the anticholinesterase activity of the two compounds (Margot and
Gysin, 1957), the hydrolysis product of diazinon is far less toxic
than the parent molecule. In addition, drying the soil or increasing
aeration during land preparation for the succeeding rice crop may
completely eliminate this degradation product by oxidation.
The decomposition of diazinon under nonflooded conditions, using two
soil types in conjunction with three other factors, was explored by
Bro-Rasmussen et al. (1968). These other factors considered were:
(i) activity of soil microorganisms
(ii) water content of soil
(iii) concentration of diazinon.
The disappearance rate of diazinon varied considerably with half-lives
ranging from 21 to 80 days. Results indicated that all factors
influenced the rate of diazinon degradations and that microorganisms
play an important role in the disappearance of diazinon from the soil.
In storage and processing
Residue levels of diazinon, diazoxon and
2-isopropyl-4-methyl-pyrimidin-6-ol were measured in snap beans,
spinach and tomatoes subjected to washing, blanching and pealing (for
tomatoes) under simulated commercial conditions (Ralls et al., 1967).
Diazinon on spinach at harvest 4 days after spraying was present at
1.8 ppm and diazoxon at 0.34 ppm. A spray rinse did not significantly
reduce residues. Detergent washing reduced diazinon to 0.77 ppm and
diazoxon to 0.18 ppm, and steam blanching gave a total reduction to
about 30 percent of the original residue. Only a water blanching
process significantly reduced the level of the pyrimidinol metabolite
from 2.5 ppm to 0.1 ppm. Residues at harvest (8 days) on snap beans
and tomatoes were less than 0.1 ppm. Subsequent commercially simulated
treatment appeared to have little or no effect, except possibly the
commercial peeling of tomatoes. Further studies on the reduction of
residues during food processing operations are reported by Farrow et
al. (1969).
Water washing of tomatoes (without detergent) removed 88 percent of
total residues, but caused an apparent increase of 11 percent with
spinach. A similar increase was seen with other insecticides such as
parathion. Crops such as spinach and broccoli have large surface areas
and are therefore subject to considerable leaching of water-soluble
solids during washing. Since results are expressed on a dry weight
basis, this results in an apparent increase in residue levels.
Commercial blanching operations are carried out using either hot water
or steam. Although washing was not effective in removing diazinon from
spinach, hot water blanching removed 60 percent of the residue. During
the combined canning operations it was found that there is a good
overall removal of organophosphorus residues. Thus there was a 99
percent removal of malathion residues from tomatoes, 94 percent
removal from green beans and 66 percent removal of parathion from
spinach; however, removal of parathion residues from broccoli, which
is processed by snap freezing, amounted to only 10 percent.
Diazinon is recommended in some countries for application to grapes,
and Painter et al. (1963) studied the fate of diazinon after addition
to grape must. Diazinon did not cause any measurable effect on
fermentation and was not found in any component after fermentation.
The absence of diazinon after fermentation is probably due to the
hydrolysis of the compound under the acid conditions prevailing. It is
interesting to note that several other organophosphorus compounds were
detected in finished wine when subjected to similar experimental
procedures.
In water
Gomaa et al. (1969) have considered breakdown of diazinon and diazoxon
which may occur in water. Techniques were developed to study the
hydrolysis of diazinon and diazoxon in aqueous media under varying
conditions of temperature and pH. Diazinon and diazoxon are
quantitatively hydrolysed to 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylthiophosphoric or diethylphosphoric acid.
In general, at 20°C, diazoxon hydrolysis proceeds much faster than
diazinon under comparable conditions. A large difference in rate of
hydrolysis can be detected under acidic conditions, where diazoxon is
hydrolysed 30 times faster than diazinon. The differences in rates
decrease as neutrality is approached, but increase again as the pH of
the hydrolysis solution increases.
The work of Gomaa et al., indicates that some caution should be
exercized concerning movement or application of diazinon into water.
Sethunathan and MacRae (1969), suggested that soil microflora play an
important part in the hydrolysis of diazinon in flooded soils. These
conditions are unlikely to apply to aqueous media, which may account
for the persistence of diazinon reported by Gomaa et al.
Evidence of residues in food moving in commerce or at consumption
Of some 14 800 randomly selected samples of raw agricultural products
examined by the U.S. Food and Drug Administration from June 1965
through 1966, only 32 samples showed any detectable residue of
diazinon. Total diet studies conducted during 1965 and 1966 by the
U.S. Food and Drug Administration revealed that 98 percent of the food
samples contained no detectable residues of diazinon. The remaining 2
percent contained only trace quantities. A multidetection gas
chromatographic method, using an electron capture detector and/or a
thermionic detector specific for phosphorus, was used for the
analyses. The sensitivity of the method was about 0.05 ppm (Duggan et
al., 1967).
METHODS OF RESIDUE ANALYSIS
Most of the residue data summarized in this monograph were obtained
using one or two of four different methods of analysis developed by
Geigy Chemical Company (1956-67).
A sulphide procedure was considered most accurate when spray history
was known. In this procedure, diazinon is extracted from crops with a
solvent and from the solvent with 48 percent HBr. The 48 percent HBr
treatment adds a high degree of selectivity for the determination of
diazinon. Upon boiling the acid solution, diazinon sulphur is
converted to H2S and distilled off. It is collected in zinc acetate
solution and then converted to a methylene blue complex, which is
determined spectrophotometrically. Sensitivity of the sulphide
procedure is about 0.1-0.2 ppm. Some crops, such as kale, had high
natural sulphur blanks, so these crops were analysed by a phosphate
method. Thiocarbamates such as ferbam also interfere and must be
removed by an additional cleanup step.
Some phosphorothioates are known to form relatively stable metabolic
products containing no sulphur, for which the method described above
would not be applicable, so a cholinesterase inhibition method was
utilized to validate the sulphide procedure. The method based on
determining the phosphorus of diazinon and one based on the
ultraviolet absorption properties of the pyrimidine portion of the
molecule are fraught with high blank and cleanup problems. Sensitivity
of these methods is about 0.3-0.4 ppm.
None of these four methods was of adequate sensitivity or specificity
for "total diet" samples. Such data were not possible until the GLC
methods based on electron capture and thermionic detectors were used.
Diazinon and diazoxon are detected and may be determined by the
multiresidue method of Abbott et al. (1970), which was successfully
used in the total diet studies carried out in England and Wales. The
methods of Storherr et al. (1964, 1965), using an ethyl acetate
extraction and either a sweep codistillation or celite column cleanup
with gas chromatographic detection, provide a rapid and adequately
sensitive procedure for diazinon in most food commodities.
J. R. Geigy SA has developed a gas chromatographic method which is
applied following a shakeout with 48 percent HBr. The gas
chromatographic methods are sensitive to about 0.01 ppm or better. A
number of thin layer chromatographic procedures described in the
literature will provide a confirmative test. Specific analytical
methods for the detection of diazinon have been considerably refined
in recent years. Extraction, cleanup and analytical techniques have
been reviewed by Eberle (unpublished).
An improved procedure for routine determination of diazinon residues
in fruits, vegetables, soils, etc. using various selective gas
chromatographic detector systems has been reported by Eberle and Novak
(1969). Possible metabolites with strong cholinesterase-inhibiting
activity are detected on TLC plates by fly-head cholinesterase
inhibition with a limit of detection of 0.002 ppm. The above method is
considered a marked improvement over previously described methods and
permits determination of diazinon residues in a variety of crops
together with detection of cholinesterase-inhibiting metabolites.
NATIONAL TOLERANCES
Table VII summarizes national tolerances which have been established
for diazinon.
TABLE VII
Country Tolerance (ppm) Crop
Australia 0.75 fruits, grains, vegetables
Canada 0.1 maize, peas
0.25 melons, figs, cranberries and
7 vegetables
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
Canada 0.5 beans, cucumbers, turnips
(continued) 0.75 tree fruits including citrus,
grapes, strawberries and 16
vegetables
Germany 0.5 on or in vegetables, fruits,
(Fed. Rep.) root crops, leg-wes, grapes
and hops
Hungary 0.5 food
India 0 (proposed) cereals
Italy 2 olive oil
Netherlands 0.5 on or in
(i) vegetables or parts thereof
for consumption, including
edible mushrooms and edible
roots, bulbs and tubers
(ii) edible fruits of vegetables
and fruit crops or parts
thereof
New Zealand 0.75 food
Switzerland The Swiss Intercantonal commission for
toxic materials ("Commission Intercantonale
des Toxiques") proposes a tolerance of 0.5
ppm. Federal regulations are in preparation
U.S.A 0.1 banana pulp, potatoes, sweet
potatoes
0.2 bananas
0.5 almonds, filberts, pecans and
walnuts
0.75 ca 25 fruits and 25 vegetables,
fat of meat and meat byproducts
of cattle and sheep
1 olives and olive oil
3 almond hulls
10 5 hays
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
25 bean and pea forage
40 alfalfa (fresh), clover (fresh)
and maize forage
60 pasture grasses
APPRAISAL
Diazinon was first synthesized in 1951 and introduced as an
experimental insecticide in 1952. Initial development of the product
was prompted by the appearance of DDT resistance in flies, mosquitoes
and other insects. Extensive markets were developed for the product in
the late 1950's, and major areas of use included control of pests in
maize and alfalfa (U.S.A.), control of cockroaches and other insects
in buildings, the control of sheep ectoparasites (Australasia) and
control of a variety of insects attacking fruit and vegetables (Europe
and U.S.A.).
Diazinon does not persist for lengthy periods in either plant or
animal tissues. Resistant action depends on a combination of factors
including plant or animal species, application rates, cultural
practices, climatic conditions, etc. In most instances, the half-life
on crops is two to three days, except in olives where it ranges from
12 to 25 days, depending on stage of maturity of the olive at
spraying.
In plants, diazoxon has been established as the principal
anticholinesterase metabolite, though it occurs only as an
insignificant fraction of the whole residue. This metabolite is in
turn rapidly converted to noncholinesterase-inhibiting products.
Studies with radio-labelled insecticide have shown that in plants,
water and soil as well as mammals, detoxification of the insecticide
takes place during the metabolism in all systems examined where
residues are likely to occur.
Several analytical methods are available for determining residues in
plant or animal tissue. When utilized in accordance with good
agricultural practice, the treated product may have residues up to the
proposed tolerances, but only a small portion of each food commodity
in these categories is likely to be treated. There are data showing
that a significant amount of reduction in residues will take place
during washing, preparation and processing of food for consumption. In
"total diet" samples, diazinon has seldom been found, and then only at
a low level. Residue data are available on more than 70 crops from
several different countries.
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are based on residues likely to be found at
harvest following currently approved use patterns. On commodities
other than nuts, oilseeds, olives, olive oil and fat of meat, residues
will continue to decline during storage and shipment with a probable
half-life of less than five days.
The tolerances are expressed as diazinon, since it is known that the
oxygen analogue, diazoxon, if present, does not occur at
concentrations above 0.004 ppm.
Peaches, citrus and cherries 0.7 ppm
Other fruits 0.5 ppm
Leafy vegetables 0.7 ppm
Other vegetables 0.5 ppm
Grain (wheat, barley, rice) 0.1 ppm
Nuts almonds, walnuts, filberts
pecans, groundnuts) 0.5 ppm
Oilseeds (cotton, safflower, sunflower) 0.5 ppm
Sweet maize (on kernels and cob with
husks removed) 0.7 ppm
Olives and olive oil 2 ppm
Fat of meat of cattle, sheep and pigs
FURTHER WORK OR INFORMATION
DESIRABLE
1. Reproduction studies on one rodent and one nonrodent species.
2. Toxicological information on residual anticholinesterase
metabolites of diazinon in plants.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G (1970)
Pesticides in the total diet in England and Wales 1966-67. Pest. Sci.,
1(1): 10-13
Bartsch, E. (1970) Residue data of diazinon. J. R. Geigy SA,
Switzerland (unpublished)
Bourne, J. R. and Arthur, B. W. (1967) Diazinon residues in the milk
of dairy cows. J. econ. Ent., 60: 402-405
Boyd, E. M. and Carsky, E. (1969) Kwashiorkorigenic diet and diazinon
toxicity. Acta. pharmacol. Toxicol., 27: 284-294
Boyd, E. M., Carsky, E. and Krijnen, C. J. (1969) The effects of diets
containing from 0 to 81 percent casein on the acute oral toxicity of
diazinon. Clin. Toxicol., 2: 295-302
Bro-Rasmussen, F., Noddegaard and Voldum-Clausen, K. (1968)
Degradation of diazinon in soil. K.J. Sci. Fd. Agr., 19: 278-281
Claborn, H. V., Mann, R. D., Younger, R. L. and Radeleff, R. D. (1963)
Diazinon residues in the fat of sprayed cattle. J. econ. Ent., 56(6):
858-859
Coffin, D. E. and McKinely, W. P. (1964) The metabolism and
persistence of systox, diazinon and phosdrin on field-sprayed lettuce.
J. Assoc. Off. Agr. Chem., 47(4): 632-640
Derbyshire, J. C. and Murphy, R. T. (1962) Diazinon residues in
treated silage and milk of cows fed with powdered diazinon. J. Agr.
Fd. Chem., 10(5): 384-386
Duggan, R. E., Barry, H. C. and Johnson, L. Y. (1967) Pesticide
residues in total diet samples. II. Pest. Monitor. J., 1(2): 2-12
Earl, F. L., Melveger, B. E., Reinwall, J. E., Bierbower, G. W. and
Curtis, J. M. (1970) Diazinon toxicity - comparative studies in dogs
and miniature swine. Toxicol. appl. Pharmacol. (in press)
Eberle, D. O. (1970) Analysis of diazinon. J. R. Geigy SA, Switzerland
(unpublished)
Eberle, D. O. and Novak, D. (1969a) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 228
Eberle, D. O. and Novak, D. (1969b) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 1067-1074
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food
FAO/WHO. (1968) 1968 evaluations of some pesticide residues in food.
FAO/PL:1968/M/9/1. WHO/Food Add./69-35
Farrow, R. P., Elkins, F. R., Rose, W. W., Lamb, F. C., Ralls, J. W.
and Mercer, J. W. (1969) Canning operations that reduce insecticide
levels in prepared foods and in solid food waste. Residue Reviews,
29: 73-87
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Geigy Chemical Company. (1956-67) Unpublished data and methods of
analysis in pesticide petitions, submitted to the U.S. Food and Drug
Administration
Geigy Chemical Company. (1969) Diazinon-deterioration-stabilization
and influence on toxicity. Unpublished report
Getzin, L. W. (1967) Metabolism of diazinon and zinophos in soils. J.
econ. Ent., 60: 505-508
Getzin, L. W. and Rosefield, I. (1966) Persistence of diazinon and
zinophos in soils. J. econ. Ent., 59(3): 512-516
Gomaa, H. M., Suffett, I. H. and Faust, S. D. (1969) Kinetics of
hydrolysis of diazinon and diazoxon. Residue Reviews, 29: 171-190
Gunner, H. B., Zukerman, B. M., Walker, R. W. Miller, C. W., Denbert,
K. H. and Longley, R. E. (1966) The distribution and persistence of
diazinon applied to plant and soil and its influence on soil
microflora. Plant Soil; 25: 249-264
Gunther, F. A., Ewart, W. H., Blinn, R. C., Elmer, H. S. and Wacker,
G. B. (1958) Field persistence comparisons of residues of the
insecticide diazinon in lemons and Valencia oranges and effects on
juice flavour. Agr. Fd. Chem., 6: 521
Harrison, D. L. and Hastie, B. A. (1965) Diazinon residues in the milk
of cows and fat of sheep after feeding on pasture treated with
diazinon. New Zealand J. agr. Res., 9: 1-7
Hastie, B. A. (1963) The metabolism and elimination of diazinon from
animals, animal tissues and foodstuffs. Geigy Agricultural Chemicals,
Australia (bulletin)
Hastie, B. A. (1965) Diazinon residues in sheep fat. Geigy
Agricultural Chemicals, Australia (unpublished)
Kaplanis, J. N., Louloudes, S. J. and Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65: 70-75
Maier-Bode, H. (1963) Residues of insecticides on cover crops growing
in orchards after application of organic phosphorus toxicants on the
trees. Z. Pflanzenkrankh. Pflanzenschutz, 70(80): 449-459
Maier-Bode, H. (1967) Untersuchungen über den Gehalt
landwirtschaftlicher und gärtnerischer Ernteprodukte an
Pflanzenschutzmittelrückständen. Der Ministerpräsident des Landes
Nordrhein-West., Landesamt für Forschung, Jahrbuch 1967, S. 391.
Margot, A. and Gysin, H. (1957) Diazinon, its degradation products and
their properties. Belv. Chim. Acta., 40: 1562
Mathysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ectoparasite and fly control on dairy
cattle, with notes on safety of diazinon and ciodrin to calves. J.
econ. Ent., 61(5): 1394-1398
Melchiorri, P. Maffei F. and Siesto. A. J. (1964) Farmaco (Pavia), Ed.
Prat., 19: 610
Michel, H. O. (1949) J. lab. clin. Med., 34: 1564
Millar, K. R. (1963) Detection and distribution of 32P-labelled
diazinon in dog tissues after oral administration. New Zealand vet.
J., 11(6): 141-144
Mücke, W., Alt, K. O. and Esser, H. O. (1970) Degradation of
14C-labelled diazinon in the rat. J. Agr. Fd. Chem., 18(2): 208-212
Nakatsugawa, T., Tolman, N. M. and Dalm, P. A. (1969) Oxidative
degradation of diazinon by rat liver microsomes. Brochem. Pharmacol.,
18: 685-688
Nelson, L. L. and Hamilton, E. W. (1970) Metabolism of diazinon in
alfalfa. J. econ. Ent., 63(3): 874-878
Onsager, J. A. and Rusk, H. W. (1967) Adsorption and translocation of
diazinon and Stauffer N-2790 in sugarbeet seedlings. J. econ. Ent.,
60(2): 586-588
Painter, R. P. and Kilgore, W. W. (1963) Distribution of pesticides in
fermentation products obtained from artificially fortified grape
musts. J. Fd Sci., 28: 342-346
Pardue, J. R., Hansen, E. R., Barron, R. P. and Chen, J. J. (1970)
Diazinon residues on field-sprayed kale. Hydroxydiazinon - a new
alteration product of diazinon. J. Agr. Fd Chem., 18: 405-408
Rai, L. and Roan, C. C. (1959) Report included in Geigy Chemical
Company pesticide petition to the U.S. Food and Drug Administration
Ralls, J. W., Gilmore, D. R. and Cortes, A. (1966) Fate of radioactive
o,o-diethyl o-(2-isopropyl-4-methylpyrimidin-6-ol) phosphorothioate on
field grown experimental crops. J. Agr. Fd Chem., 14(4): 387-392
Ralls, J. W., Gilmore, D. R., Cortes. A., Schutt, S. H. and Mercer, W.
A. (1967) Residue levels of diazinon and its transformation products
on tomatoes, spinach and beans. Fd Tech., 21: 92-94
Robbins, W. E., Hopkins, T. L. and Eddy, O. W. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem., 5(7): 509-513
Robens, J. F. (1969) Teratologic studies of carbaryl, diazinon, norea
disulfiram and thiram in small laboratory animals. Toxicol. appl.
Pharmacol., 15: 152-163
Sethunathan, N. and MacRae, I. C. (1969) Persistence and
biodegradation of diazinon in submerged soil. J. Agr. Fd Chem., 17:
221
Sethunathan, N. and Yoshida, T. (1969) Fate of diazinon in submerged
soil. Accumulation of hydrolysis product. J. Agr. Fd Chem., 17(6):
1192-1195
Siesto, A. J., Maffei, F. and Melchiorri P. (1964) Estratto da
Archivio Italiano di Scienze Farmacologiche, Series 3, 13: 3
Storherr, R. W., Getz, M. E., Watts, R. R., Friedman, S. J., Erwin,
F., Giuffrida, L. and Ives, F. (1964) Identification and analyses of
five organo-phosphate pesticides. Recoveries from crops fortified at
different levels. J. Assoc. Off. Agr. Chem., 47(6): 1087-1093
Storherr, R. W. and Watts, R. R. (1965) A sweep co-distillation
cleanup method for organophosphate pesticides. J. Assoc. Off. Agr.
Chem., 48(6): 1154-1160
Vigne, J. P., Chouteau, J., Tabau, R.-L., Rancien, P. and Karamanian,
A. (1957) Sur le métabolisme d'un insecticide organo-phosphoré, le
diéthylthionophosphate de 2 isopropyl 4 méthyl 6 oxypyrimidine chez la
chèvre. Bull. Acad. Vét. Fr., 30: 85-92
 Hydrolysis of the ester bond yielding
2-isopropyl-4-methyl-6-hydroxypyrimidine and oxidation at the primary
and tertiary carbon atom of the isopropyl side chain were found as the
main degradative mechanisms. The structure of the main metabolites was
confirmed by independent synthesis, and the inhibitory activities on
acetyl cholinesterase were determined. The structure of the
metabolites and the metabolic pathway of the pyrimidine moiety are
shown in Figure 1.
* 2-(1-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
** 2-(2-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
The actual sequence of the degradation reactions was determined by
following the fate of 14C-labelled metabolites after intravenous
application. Metabolite 1 resulted in the same pattern of metabolites
as diazinon itself. Metabolite 2 was excreted mainly unchanged and
metabolite 3 was excreted in a completely unchanged form.
Hastie (1963) reviewed available data on the metabolism and
elimination of diazinon from animals and animal tissues. When orally
administered, diazinon is degraded by the digestive enzymes before the
lipid-soluble material can reach the fat depots. The more circuitous
route involved following dermal application allows a certain amount of
diazinon to bypass the sites of degradation, thereby reaching the fat
depots unchanged. Reabsorption into the digestive tract from these
depots is a somewhat delayed process.
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Hamster and rabbit
An examination of the teratogenic potential of diazinon was performed
using Golden Syrian hamsters and New Zealand white rabbits (Robens,
1969). Oral administration of diazinon in maize oil at a dose of 0.125
mg/kg body-weight on day 6, 7 and 8 of gestation and at 2.25 mg/kg
body-weight on day 7 or 8 produced no terata in the hamster.
Administration of diazinon to rabbits daily from day 5 to 15 of
gestation at 7 or 30 mg/kg body-weight per day induced no terata or
dose-related embryotoxic effects.
Special studies on acute toxicity of metabolites
LD50 values following acute oral administration to rats are available
for two diazinon metabolites:
2-isopropyl-4-methyl-6-hydroxypyrimidine: 2 700 mg/kg body-weight
2-(1'-hydroxyisopropyl)-4-methyl-6-hydroxypyrimidine:
5 000 mg/kg body-weight
(Mücke et al., 1970).
Hydrolysis of the ester bond yielding
2-isopropyl-4-methyl-6-hydroxypyrimidine and oxidation at the primary
and tertiary carbon atom of the isopropyl side chain were found as the
main degradative mechanisms. The structure of the main metabolites was
confirmed by independent synthesis, and the inhibitory activities on
acetyl cholinesterase were determined. The structure of the
metabolites and the metabolic pathway of the pyrimidine moiety are
shown in Figure 1.
* 2-(1-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
** 2-(2-hydroxy-2-propyl)-4-methyl-6-hydroxypyrimidine
The actual sequence of the degradation reactions was determined by
following the fate of 14C-labelled metabolites after intravenous
application. Metabolite 1 resulted in the same pattern of metabolites
as diazinon itself. Metabolite 2 was excreted mainly unchanged and
metabolite 3 was excreted in a completely unchanged form.
Hastie (1963) reviewed available data on the metabolism and
elimination of diazinon from animals and animal tissues. When orally
administered, diazinon is degraded by the digestive enzymes before the
lipid-soluble material can reach the fat depots. The more circuitous
route involved following dermal application allows a certain amount of
diazinon to bypass the sites of degradation, thereby reaching the fat
depots unchanged. Reabsorption into the digestive tract from these
depots is a somewhat delayed process.
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Hamster and rabbit
An examination of the teratogenic potential of diazinon was performed
using Golden Syrian hamsters and New Zealand white rabbits (Robens,
1969). Oral administration of diazinon in maize oil at a dose of 0.125
mg/kg body-weight on day 6, 7 and 8 of gestation and at 2.25 mg/kg
body-weight on day 7 or 8 produced no terata in the hamster.
Administration of diazinon to rabbits daily from day 5 to 15 of
gestation at 7 or 30 mg/kg body-weight per day induced no terata or
dose-related embryotoxic effects.
Special studies on acute toxicity of metabolites
LD50 values following acute oral administration to rats are available
for two diazinon metabolites:
2-isopropyl-4-methyl-6-hydroxypyrimidine: 2 700 mg/kg body-weight
2-(1'-hydroxyisopropyl)-4-methyl-6-hydroxypyrimidine:
5 000 mg/kg body-weight
(Mücke et al., 1970).
 Results in Table I indicate a complete loss of acetyl cholinesterase
inhibitory power. The table also indicates that a drastic reduction in
the acute toxicity of the insecticide had occurred during metabolism
(Mücke et al., 1970).
TABLE I
Properties of the main metabolites
Compound Acute LD50 Inhibition of
AChE ID50
Diazinon approx. 250 mg/kg 2.7 × 10-5M
Metabolite 1 approx. 2 700 mg/kg 10-2M
Metabolite 2 5 000 mg/kg 10-2M
Recent investigations by Mücke et al. (1970), have shown that
2-isopropyl-4-methyl-pyrimidin-6-ol is of low toxicity, having an
acute oral LD50 of approximately 2 700 mg/kg in the rat, and that
there is no detectable anticholinesterase activity. These findings
provide an answer to the doubts expressed by Ralls et al. (1966),
concerning the toxicity of 2-isopropyl-4-methyl-pyrimidin-6-ol.
No information is available on the toxicity of the metabolite found
after treatment of kale with diazinon (see "FATE OF RESIDUES. In
plants").
Acute toxicity
TABLE II
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat oral 250 (male) Gaines, 1969
Rat oral 285 (female) Gaines, 1969
Rat oral 466 Boyd & Carsky, 1969
Rat oral 293-408 (male) Edson & Noakes, 1960
Rat dermal 900 (male) Gaines, 1969
TABLE II (cont'd)
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat dermal 455 (female) Gaines, 1969
Acute toxicity of diazinon to rats is summarized in Table II.
Differences in LD50 values expressed over the past few years may be
due to the presence of impurities contaminating early diazinon
samples. It has been shown that, under certain conditions,
combinations of molecules of diethyl phosphorothioic acid and
diethylphosphoric acid can condense to produce
tetraethylpyrophosphoric esters, which may be extremely toxic.
Stabilization of diazinon formulations appear to have eliminated some
of these condensation products and reduced the acute LD50 (Geigy,
1969).
As has been demonstrated with other pesticides, the acute LD50 to
rats increased as the protein level in the diet increased or decreased
from an optimal level. Raising the protein content from 29 percent to
81 percent, or lowering the protein content to 4 percent, increased
the toxicity approximately twofold (Boyd et al., 1969).
Short-term studies
Dog and pig
Groups of pigs (three male and three female) and dogs (three male and
three female) were orally administered diazinon by capsule daily for
periods up to eight months at doses of 0, 1.25, 2.5, 5 and 10 mg/kg
body-weight/day to pigs and 0, 2.5, 5, 10 and 20 mg/kg body-weight/day
to dogs (Earl et al., 1970). In pigs, mortality and cholinergic signs
of poisoning were evident at 2.5 mg/kg/day and above. Although
significantly increased myeloid/erythroid (ME) ratios were observed,
no aplastic anaemia was evident. In dogs, mortality and cholinergic
signs of poisoning were evident above 10 mg/kg, with significantly
increased ME ratios observed in dogs dying at 20 mg/kg. No evidence of
aplastic anaemia was observed in dogs and pigs.
Long-term studies
No new information available.
COMMENTS
It has been demonstrated that diazinon is oxidized in vitro to
diazoxon and further to diethylphosphorothioic acid. Farther
degradation has been shown to occur in vivo with the urinary
metabolites, which are substantially less toxic than the parent
compound. Distribution of diazinon in the body was relatively low,
with no accumulation in tissues or organs noted. Excretion in urine
and faeces was fairly rapid. No signs of blood dyscrasias were evident
in studies with dogs and pigs. No teratogenic or embryotoxic effects
were observed in hamsters and rabbits.
Stabilization of diazinon formulations have been reported to have
eliminated potential condensation products of diethylphosphoric acid
and diethylphosphorothioic acid. Information on the long-term toxicity
effects of diazinon is still inadequate. No information is available
on residuous anticholinesterase metabolites which may occur in plants.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.1 mg/kg body-weight/day
Dog: 0.02 mg/kg body-weight/day
Monkey: 0.05 mg/kg body-weight/day
Man: 0.02 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 = 0.002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Diazinon is a broad spectrum insecticide which has found many
applications, including control of:
(a) pests of field crops, orchards and pastures;
(b) animal ectoparasites;
(c) soil-inhabiting insects;
(d) industrial, public health and domestic pests.
Numerous formulations, including emulsifiable concentrates, wettable
powders, dusts and granules, are available. Foliar pests are normally
controlled by application in spray form, but effective control of rice
stem borer and some leaf hoppers has been obtained through application
of granules. Animals are normally treated by dipping, spraying,
jetting or dusting.
In the United States, diazinon is registered for application to more
than 60 food or feed crops including most fruits, vegetables and
forages. Use on corn (maize) for control of corn root worm and corn
ear worm and to alfalfa (lucerne) for control of alfalfa weevil are
major applications. There is an increasing use on rice.
Dipping, jetting or spraying of sheep for protection against sheep
blowfly are major uses in Australia, New Zealand, South Africa and
South America. Due to its solubility in wool grease, the material can
remain on the sheep in a stable state for several weeks and thus
provide effective protection against parasite attack. Dermal
applications to sheep, cattle and goats are also used for control of
lice, ticks, horn flies and certain other insects.
Table III summarizes typical dosages and pre-harvest periods for the
various crop categories. Diazinon has been found effective in
controlling over 100 species of food crop pests such as mites, aphids,
thrips, maggots, fruit flies, worms, beetles, grasshoppers, leaf
miners, etc. Seed furrow soil treatments are used for several root
vegetable crops.
TABLE III
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Tree fruits 0.5 lb/100 gal (full coverage) 10-20
Caneberries
(raspberries,
blackberries
loganberries) 1.0 lb/acre (full coverage) 7
Citrus 0.5 lb/100 gal (full coverage) 7-21
Leafy vegetables 0.5-1.0 lb/acre 5-21
Root vegetables 0.5-1.2 lb/acre 10
Others 0.25-1.0 lb acre 0-7
TABLE III (cont'd)
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Forages & hays 0-5-1.0 lb/acre No grazing
limitations
4-10 days
before cutting
hay
Cattle & sheep 1.0-2.3 gal of 0.30-0.5% (14
spray per animal pre-slaughter
period)
Post-harvest treatments
There is no commercial post-harvest use of diazinon on crops.
Other uses
Diazinon is recommended for fly control in dairy barns and other farm
buildings as well as in food processing plants; however, this is
becoming less important because of resistance developed by flies. It
is also used in households to control carpet beetles and clothes
moths, in home gardens and in buildings to combat ants, cockroaches,
fireants, etc. Control of cockroaches is an important field of use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of diazinon on or in plants, in animal tissues, or even in
the soil, are not highly persistent. In many countries and on over
sixty crops, residues have been determined at various time intervals
after applications of varying amounts of diazinon. For example, in the
controlled experiments with apples in the U.S.A., 16 varieties were
treated in 15 states using ´ to 1 lb/100 gal (i.e., full coverage
sprays) on various spray schedules and in combination with other
pesticides and fungicides.
A summary of the numerous data (Geigy, 1956-67) obtained from the
rates of application shown in Table III is given in Table IV.
TABLE IV
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Tree fruits
Apples 0.6-0.8 14 0.1-0.4 5
Pears 0.6-0.7 14 0.1-0.3 5
Cherries 3-7 10 0.1-0.3 3
Peaches 2-6 20 0.1-0.6 3-6
Apricots 1-4 10 0.1 2
Nectarines 1-6 10 0.2 3-4
Plums & prunes 2-4 10 <0.1-0.2 2
Figs 0.5 10 <0.1 3
Nuts
Almonds )
Filberts ) 0.1 (nuts) 10 <0.1 (nuts)
Walnuts ) 60 <0.1 (kernels)
Peanuts
Citrus
Oranges 3-5 21 0.1-0.4 4
Lemons 4.0 21 0.6-0.7 6-7
Grapefruit 0.3 7 0.1-0.2 7
Caneberries and small fruit
Strawberries 0.9-1.4 5 0.2-0.4 2-4
Grapes 1-8 18 <0.1-0.3 3
Cranberries 9 7 <0.1 1
Blueberries 1-4 7 <0.1-0.3 1-2
Blackberries )
Boysenberries ) 1-5 7 <0.1-0.3 2
Loganberries )
Raspberries )
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Leafy vegetables
Cabbage 1-2.5 7 <0.1-0.7 4-6
Celery 2-9 10 <0.1-0.7 2
Cauliflower 1 5 0.4-0.5 7
Broccoli 1-2 5 <0.1-0.5 3
Lettuce 6-17 10 0.3-0.5 1-2
Spinach 5-12 10 <0.1-0.2 2
Endive 3-18 10 0.1 2
Collards 3 12 <0.1 2
Kale 10-24 12 0.1-0.2 1
Parsley 1-6 12 0.1-3.0 2
Swiss chard 2 12 <0.1 2
Turnip greens 4-15 12 <0.1 2
Brussels sprouts 0.2 10 <0.1
Root vegetables (foliar application)
Beets <0.1 0
Onions 7-13 (green) 10 (green) 0.4-0.6 (green) 2
2-3 (dry) 10 (dry) <0.1-0.3 (dry) 2
Carrots 1-2 10 0.1-0.3 2-3
Carrots (soil
application) 120 0.1
Parsnips 0.7 10 0.3 6
Radishes <0.1-0.4 10 <0.1 4
Turnips 0.5 10 0.4-0.5 21
Vegetables (others)
Peppers 0.6-0.8 5 <0.1-0.2 2-3
Cucumbers 1.0-2.5 7 <0.1 2
Green beans 1-2 7 <0.1 2
Lima beans 0.2-1.0 7 <0.1-0.2 2
Squash 0.1-0.2 3 <0.1-0.2 2
Maize (ears only) <0.1 0 <0.1 -
Peas (plus pod) <0.1 0 <0.1 -
Miscellaneous
Tomatoes 0.1-0.4 3 <0.1-0.2 2
Melons 0.1-0.7 3 <0.1-0.2 2-3
Olives 1-6 75 <0.2-0.6 ca 25
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Hops (cones) 3-11 14 0.1-0.3 3
Sorghum (grain) 0.5 7 <0.1 2
Cotton (seed) 0.1-0.2 7 <0.1
Safflower (seed) <0.1
Sunflower 80 0.1-0.2
Sugarcane (stems) <0.1 7 <0.1
Tea 7 <0.1 (manufactured)
Coffee 1 7 0.1 (green beans)
Cereal crops
Wheat 13 <0.1 (grain)
Meat
Pre-slaughter
period
Lamb (fat) 1-3 14 0.1-0.75 5
Beef (fat) 1-3 14 <0.1-0.2 3
Forages
Pre-grazing Cutting Initial Residue Half
interval time residue at life
(days) (days) in forage cutting (days)
(ppm) (ppm)
Alfalfa 0 7 12-24 0.2-0.5 2
Clover 0 7 4-14 1.0-4.0 2-3
Range/pasture
grass 0 21 9 4.0-7.2 2-3
30 (oil
formulation) 54
Pea/bean 0 4 5-10 2.0-5.0 2
Maize 0 0 12-21 - 2
General Comments
In animals
Reports from numerous trials indicate that residues of diazinon in
subcutaneous fatty tissues of sheep and cattle treated for parasite
control do not exceed 0.75 ppm one day after treatment. The residue
levels in internal fat, however, may be higher. Average residue levels
decline rapidly following treatment, but fat samples from individual
animals may exceed 0.75 ppm for 14 days. Hastie (1965) showed that
standard treatment procedures resulted in residues in sheep one day
after treatment which were in excess of 1 ppm in the fat. After three
days, residue levels had declined to 0.3-0.5 ppm. Claborn et al.
(1963) showed that cattle sprayed with diazinon contained residues in
omental fat six days after treatment, but residues were not detectable
at 14 days.
Residues of diazinon in animal fat may be influenced by the condition
of the treated animal due to the dilution factor brought about by the
total amount of fat present. Application rates also affect the residue
levels.
In plants
Residue levels in various crops have been reviewed by Bartsch (1970).
Residue analyses of pip fruit have indicated that 90 percent of
residues are in the peel of ripe fruit and only 2-4 percent in the
pulp. No residues at all were detected in the pulp and juice of citrus
fruits, since the peel serves as a barrier (Gunther et al., 1958).
Residues present in any crop are dependent upon application rates,
cultural practices and type of formulation used. Residues have
generally been more persistent in glasshouse crops than in field crops
(Bartsch, 1970). Granular formulations have given more variable
residue levels in treated crops than have liquids applied as sprays.
However, studies have indicated that residue levels are higher overall
after crop treatment with emulsions rather than granules (Maier-Bode,
1967).
After crop application of emulsions or granules, practically no
residues have been detected in tubers (potatoes, sweet potatoes and
yams) or in grain (rice and wheat). Residues in seeds of cotton,
sunflower and safflower contain only little diazinon in contrast to
the respective oil products.
Immediately after treatment of forage crops, residues may reach 150
ppm. Residue levels fall very rapidly, due in part to growth of the
forage plants, and within a period of 1-2 days are below the current
tolerances of 60 ppm for grass and 40 ppm for alfalfa (in the U.S.A.)
Due to the solubility of diazinon in lipids, high residues may be
found in olive oil, but they are below the accepted tolerance levels
of 2 ppm in Italy and 1 ppm in U.S.A., 7-8 weeks after treatment.
Carrots have been examined with particular care on account of their
dietary significance, bearing in mind that they are often eaten
uncooked, and because of their biochemical properties. Residue figures
vary widely, depending on timing of application, formulation and
method of application. All trial data reviewed by Bartsch (1970)
indicated that unless harvest is carried out within 60 days of last
treatment (soil-application), the residue level is below 0.5 ppm in
all cases.
FATE OF RESIDUES
In animals
Investigations on the metabolic fate of diazinon in mammals were
initiated mainly by the widespread use of the product as an
ectoparasiticide in ruminants. Residue studies in fat and milk of cows
(Bourne and Arthur, 1967; Claborn et al., 1963; Derbyshire and Murphy,
1962; Matthysse and Lisk, 1968) and of sheep (Harrison and Hastie,
1965; Matthysse et al., 1968) were carried out after the insecticide
had been applied regularly by spraying and dipping or by feeding the
animals on pasture treated with the insecticide. Only small amounts
of diazinon residues have been found in fat and milk, whereas the
other tissues were free of the insecticide. In two early studies in
the cow (Robbins et al., 1957) and in the goat (Vigne et al., 1957),
the rapid and complete elimination of the 32P-labelled insecticide
in urine, faeces, blood and milk was shown, demonstrating urine as the
main route of excretion.
Rai and Roan (1959) found no residues of diazinon in the milk of dairy
animals given daily oral doses of diazinon at the rates of 1.06, 5.30
and 10.60 mg/kg of body-weight over a three-week feeding period. These
administration rates are calculated to be 100, 500 and 1 000 ppm on
the basis of the grain fed, or 51, 290 and 500 ppm on the basis of hay
consumed. Steers treated with 165 and 825 ppm in daily oral doses
calculated on the basis of grain fed showed traces of diazinon in
blood, urine, muscle, liver and brain. Only in fat was a significant
residue found, being 0.23 ppm at the maximum feeding level. These
results were obtained by the use of three methods of analysis.
In plants
Practical residue determination on many occasions has shown that
diazinon does not persist long as a residue on most food crops. It
appears that the movement or metabolism of diazinon depends upon the
plant species (Coffin and McKinley, 1964; Ralls et al., 1966; Gunner
et al., 1966).
Diazinon is passed through bean plants unchanged, with rapid
translocation and emergence in bean root exudates after application of
diazinon to aerial portion of the plant (Gunner et al., 1966). Sugar
beet seedlings absorb diazinon applied to the soil (Onsager and Rusk,
1967) and is translocated in quantities to render the plant
insecticidal. This is contrary to earlier observations (Gunner, 1966),
where it was found that diazinon was absorbed by beans but not
translocated in toxic amounts.
Analysis of alfalfa (lucerne) indicated that diazinon was absorbed
from treated soil into the plant (Nelson and Hamilton, 1970). No
metabolites of diazinon were found in the alfalfa, and diazinon was
expired by the plants. These findings substantiate the results
obtained by Gunner et al. (1966) with beans.
The metabolism of diazinon in plants has been shown to involve
hydrolysis of the phosphorus primidylester bond and subsequent
metabolism of the 2-isopropyl-4-methyl-6-hydroxypyrimidine to carbon
dioxide. Small amounts of diazoxon have at times been detected in
field-grown crops (Gomaa et al., 1969). The fact that diazoxon levels
are very low, if present at all, indicates oxidation is very minor or
that the oxon is hydrolysed as rapidly as it is found. Margot and
Gysin (1957) indicated loss of insecticidal activity on plants caused
by evaporation of diazinon and through hydrolysis. No metabolites of
diazinon were found.
Coffin and McKinley (1964) reported on the metabolism and persistence
of diazinon on field-sprayed lettuce. Diazinon residues decreased from
8.1 ppm to 0.3 ppm from four hours to seven days after spraying, and
detectable quantities of diazinon were present at 10 and 14 days. No
significant amount of diazoxon or other metabolites were found by the
paper chromatographic detection system.
Grasses and grains grown for forage which had been treated with
diazinon were analysed by the sulphide procedure. Maier-Bode (1963)
found diminution of residues occurred only in the uncut grasses. After
cutting and while drying to hay, little of the diazinon was lost.
Ralls et al. (1966), studied the fate of 35S-labelled diazinon on
field-grown crops and found a rapid decrease in diazinon residues. The
metabolite identified from field samples was diazoxon. Three
thin-layer chromatographic systems showed the presence of this
metabolite on spinach at 0.005 to 0.01 ppm five days after spraying.
Paper chromatography of snap bean extracts harvested seven days after
treatment showed an increase in a cholinesterase-inhibiting compound
with an Rf value corresponding to diazoxon.
Additional studies in the field revealed residue levels of diazinon
(I), diazoxon (II) and 2-isopropyl-4-methylpyrimidin-6-ol (III) made
by Ralls et al. (1967), using diazinon-32p. A spinach sample
analysed one hour after spraying contained 31.7 ppm (I), 1.5 ppm (II)
and 2.5 ppm (III). Analysis of a four-day sample gave 1.8 ppm (I),
0.34 ppm (II) and 2.5 ppm (III). Although diazinon and its oxygen
analogue dissipated rapidly, compound (III), the result of further
hydrolysis of diazoxon persisted at the same level. The mammalian
toxicity of this persistent compound was at the time not known.
Similar experiments with snap bean and tomato plants showed the same
rapid disappearance of diazinon and diazoxon to levels greatly below
0.1 ppm after four days. Less than 0.1 ppm of compound (III) was found
in all four-day samples.
Refined analytical techniques were used by Eberle and Novak (1969), to
further investigate the fate of diazinon in plants. The only
cholinesterase-inhibiting metabolite detectable at any time after
diazinon application was diazoxon. The maximum levels of diazoxon
found in apples and olive oil were 0.004 ppm and 0.007 ppm,
respectively. At harvest, fruit and vegetables in all instances
contained less than 0.002 ppm diazoxon.
The appearance and subsequent disappearance of traces of diazoxon in
the crops examined indicate that diazinon is oxidized in plants to
diazoxon which is, in turn, rapidly altered to
noncholinesterase-inhibiting products. All samples were analysed for
monothiotetraethylpyrophosphate (S-TEPP), but no residues could be
detected at the limit of 0.002 ppm. These results are at variance with
the findings of Melchiorri et al. (1964), and Siesto et al. (1964),
who earlier reported evidence of the formation of S-TEPP and TEPP. The
investigations of Eberle and Novak do, however, support the findings
of Ralls et al. (1966), in that the only cholinesterase-inhibiting
metabolite detectable at any time after diazinon application is
diazoxon.
The levels of diazinon and diazoxon reported by Eberle and Novak are
summarized in Table V.
TABLE V
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
40 (3 treatments) golden apples 0 2.8 <0.002
7 0.6 0.004
21 0.3 0.002
63 (harvest) 0.05 <0.002
40 (3 treatments) Jonathan 0 1.3 0.002
apples 28 0.2 <0.002
70 (harvest) 0.1 <0.002
50 (1 treatment) pears 49 0.01 <0.002
50 (2 treatments) 35 0.01 0.003
50 (3 treatments) 21 0.02 <0.002
50 (4 treatments) 10 0.12 0.003
TABLE V (cont'd)
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
25 (2 treatments) Langstieler 7 0.2 <0.002
cherries 28 (harvest) 0.02 <0.002
25 (2 treatments) Schauenburger 7 0.4 <0.002
cherries 28 (harvest) 0.02 <0.002
4 (2 treatments) Flakeer 29 1.3
carrots 80 (harvest) 0.2 <0.002
4 (2 treatments) Guérande 29 2.2
carrots 90 (harvest) 0.2 <0.002
1 treatment onions 125 (harvest) 0.05 <0.002
10 (1 treatment) radish 36 (harvest) <0.02 <0.002
25 Milan cabbage 0 27
7 1.7
21 0.05
63 (harvest) <0.02 <0.002
25 Blanc cabbage 0 12.5
7 1.1
63 (harvest <0.02 <0.002
In contrast to the findings of other workers, a further alteration
product, hydroxysiazinon, has been reported by Pardue et al. (1970).
This previously unidentified compound was detected during a study of
diazinon field-sprayed kale samples by a technique of enzyme
inhibition thin layer chromatography of the extracts previously
oxidized by bromine water.
The levels of hydroxydiazinon found in kale in comparison with
diazinon and diazoxon are indicated in Table VI.
TABLE VI
Residues found by GLC in diazinon-treated kale
Days after application
2 7 11 15
Diazinon (ppm) 8.8 2.9 2.0 1.6
Diazoxon (Ppm) 0.004 0.007 0.002 0.002
Alteration product of
diazinon1 (ppm) 0.18 0.05 0.03 0.03
1 Later identified as hydroxydiazinon. Quantitated using the compound
prepared by UV-irradiation of diazinon.
Pardue et al. (1970) have not offered any possible explanation for the
presence of hydroxydiazinon. Many of the cruciferous crops have waxy
cuticles which would have a tendency to hold diazinon. The effect of
UV-irradiation on diazinon could then possibly cause alteration to
hydroxydiazinon.
In soil
Literature on diazinon residues in soils has been mainly confined to
nonflooded conditions (Getzin and Rosefield, 1966; Getzin, 1967;
Gunner et al., 1966). Getzin (1967) reported that greater amounts of
the hydrolysis product were recovered from soil fumigated with
propylene oxide than from nonfumigated soil. Conversely, little 14CO2
was released from the fumigated soil treated with 14C-labelled
diazinon, while large amounts were released from nonfumigated soil. He
also suggested that the initial step in the degradation of diazinon in
nonflooded soils is hydrolysis at the heterocyclic phosphate bond
(phosphorus-oxygen-pyrimidine bond), followed by disruption of the
pyrimidine ring and the subsequent release of 14CO2. Soil
microflora appeared to play a major role in the of the degradation of
the parent molecule. Trela et al. (1968) recently observed that the
degradation of diazinon into pyrimidine and phosphorothioate
derivatives was greatly stimulated in the presence of microorganisms
isolated from diazinon-treated soil.
The degradation of diazinon in submerged neutral or alkaline soil
showed that diazinon disappeared at a faster rate from nonsterilized
soils than from sterilized soils (Sethunathan and MacRae, 1969), thus
indicating the participation of soil microflora in its degradation.
Surprisingly, only a small amount of 14CO2 was released from
nonsterilized soils treated with 14C-labelled diazinon (labelled at
the 2-position on the pyrimidine ring). This result is not in
agreement with the results on 14CO2 evolution from ring-labelled
diazinon reported for nonflooded, soils (Getzin, 1967). This
difference suggests that the fate of diazinon under submerged
conditions might be different from that in nonflooded conditions.
In the study of the persistence of diazinon (14C-labelled at the
4-position on the pyrimidine ring) in submerged soils, soil microflora
appeared to assist in its degradation into a less toxic hydrolysis
product (2-isopropyl-6-methyl-4-hydroxypyrimidine). This hydrolysis
product was, however, resistant to further degradation under submerged
conditions (Sethunathan and Yoshida, 1969).
From the foregoing, it appears that the major step in the degradation
of diazinon in flooded soil is hydrolysis, resulting in the formation
of 2-isopropyl-6-methyl-4-hydroxypyrimidine as one of the degradation
products. Under submerged conditions, where the bulk of soil
microflora is anaerobic, oxidation is negligible and the hydrolysis
product tends to accumulate and persist in large quantities without
being oxidized. The more rapid degradation of diazinon and the greater
recovery of hydrolysis product from nonsterilized soils suggest that,
in flooded soils, soil microflora play an important role - direct or
indirect - in the hydrolysis of diazinon to
2-isopropyl-6-methyl-4-hydroxypyrimidine, but not thereafter. The
accumulation of 2-isopropyl-6-methyl-4-hydroxypyrimidine in submerged
soils should not, however, pose a serious residue problem since, based
on the anticholinesterase activity of the two compounds (Margot and
Gysin, 1957), the hydrolysis product of diazinon is far less toxic
than the parent molecule. In addition, drying the soil or increasing
aeration during land preparation for the succeeding rice crop may
completely eliminate this degradation product by oxidation.
The decomposition of diazinon under nonflooded conditions, using two
soil types in conjunction with three other factors, was explored by
Bro-Rasmussen et al. (1968). These other factors considered were:
(i) activity of soil microorganisms
(ii) water content of soil
(iii) concentration of diazinon.
The disappearance rate of diazinon varied considerably with half-lives
ranging from 21 to 80 days. Results indicated that all factors
influenced the rate of diazinon degradations and that microorganisms
play an important role in the disappearance of diazinon from the soil.
In storage and processing
Residue levels of diazinon, diazoxon and
2-isopropyl-4-methyl-pyrimidin-6-ol were measured in snap beans,
spinach and tomatoes subjected to washing, blanching and pealing (for
tomatoes) under simulated commercial conditions (Ralls et al., 1967).
Diazinon on spinach at harvest 4 days after spraying was present at
1.8 ppm and diazoxon at 0.34 ppm. A spray rinse did not significantly
reduce residues. Detergent washing reduced diazinon to 0.77 ppm and
diazoxon to 0.18 ppm, and steam blanching gave a total reduction to
about 30 percent of the original residue. Only a water blanching
process significantly reduced the level of the pyrimidinol metabolite
from 2.5 ppm to 0.1 ppm. Residues at harvest (8 days) on snap beans
and tomatoes were less than 0.1 ppm. Subsequent commercially simulated
treatment appeared to have little or no effect, except possibly the
commercial peeling of tomatoes. Further studies on the reduction of
residues during food processing operations are reported by Farrow et
al. (1969).
Water washing of tomatoes (without detergent) removed 88 percent of
total residues, but caused an apparent increase of 11 percent with
spinach. A similar increase was seen with other insecticides such as
parathion. Crops such as spinach and broccoli have large surface areas
and are therefore subject to considerable leaching of water-soluble
solids during washing. Since results are expressed on a dry weight
basis, this results in an apparent increase in residue levels.
Commercial blanching operations are carried out using either hot water
or steam. Although washing was not effective in removing diazinon from
spinach, hot water blanching removed 60 percent of the residue. During
the combined canning operations it was found that there is a good
overall removal of organophosphorus residues. Thus there was a 99
percent removal of malathion residues from tomatoes, 94 percent
removal from green beans and 66 percent removal of parathion from
spinach; however, removal of parathion residues from broccoli, which
is processed by snap freezing, amounted to only 10 percent.
Diazinon is recommended in some countries for application to grapes,
and Painter et al. (1963) studied the fate of diazinon after addition
to grape must. Diazinon did not cause any measurable effect on
fermentation and was not found in any component after fermentation.
The absence of diazinon after fermentation is probably due to the
hydrolysis of the compound under the acid conditions prevailing. It is
interesting to note that several other organophosphorus compounds were
detected in finished wine when subjected to similar experimental
procedures.
In water
Gomaa et al. (1969) have considered breakdown of diazinon and diazoxon
which may occur in water. Techniques were developed to study the
hydrolysis of diazinon and diazoxon in aqueous media under varying
conditions of temperature and pH. Diazinon and diazoxon are
quantitatively hydrolysed to 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylthiophosphoric or diethylphosphoric acid.
In general, at 20°C, diazoxon hydrolysis proceeds much faster than
diazinon under comparable conditions. A large difference in rate of
hydrolysis can be detected under acidic conditions, where diazoxon is
hydrolysed 30 times faster than diazinon. The differences in rates
decrease as neutrality is approached, but increase again as the pH of
the hydrolysis solution increases.
The work of Gomaa et al., indicates that some caution should be
exercized concerning movement or application of diazinon into water.
Sethunathan and MacRae (1969), suggested that soil microflora play an
important part in the hydrolysis of diazinon in flooded soils. These
conditions are unlikely to apply to aqueous media, which may account
for the persistence of diazinon reported by Gomaa et al.
Evidence of residues in food moving in commerce or at consumption
Of some 14 800 randomly selected samples of raw agricultural products
examined by the U.S. Food and Drug Administration from June 1965
through 1966, only 32 samples showed any detectable residue of
diazinon. Total diet studies conducted during 1965 and 1966 by the
U.S. Food and Drug Administration revealed that 98 percent of the food
samples contained no detectable residues of diazinon. The remaining 2
percent contained only trace quantities. A multidetection gas
chromatographic method, using an electron capture detector and/or a
thermionic detector specific for phosphorus, was used for the
analyses. The sensitivity of the method was about 0.05 ppm (Duggan et
al., 1967).
METHODS OF RESIDUE ANALYSIS
Most of the residue data summarized in this monograph were obtained
using one or two of four different methods of analysis developed by
Geigy Chemical Company (1956-67).
A sulphide procedure was considered most accurate when spray history
was known. In this procedure, diazinon is extracted from crops with a
solvent and from the solvent with 48 percent HBr. The 48 percent HBr
treatment adds a high degree of selectivity for the determination of
diazinon. Upon boiling the acid solution, diazinon sulphur is
converted to H2S and distilled off. It is collected in zinc acetate
solution and then converted to a methylene blue complex, which is
determined spectrophotometrically. Sensitivity of the sulphide
procedure is about 0.1-0.2 ppm. Some crops, such as kale, had high
natural sulphur blanks, so these crops were analysed by a phosphate
method. Thiocarbamates such as ferbam also interfere and must be
removed by an additional cleanup step.
Some phosphorothioates are known to form relatively stable metabolic
products containing no sulphur, for which the method described above
would not be applicable, so a cholinesterase inhibition method was
utilized to validate the sulphide procedure. The method based on
determining the phosphorus of diazinon and one based on the
ultraviolet absorption properties of the pyrimidine portion of the
molecule are fraught with high blank and cleanup problems. Sensitivity
of these methods is about 0.3-0.4 ppm.
None of these four methods was of adequate sensitivity or specificity
for "total diet" samples. Such data were not possible until the GLC
methods based on electron capture and thermionic detectors were used.
Diazinon and diazoxon are detected and may be determined by the
multiresidue method of Abbott et al. (1970), which was successfully
used in the total diet studies carried out in England and Wales. The
methods of Storherr et al. (1964, 1965), using an ethyl acetate
extraction and either a sweep codistillation or celite column cleanup
with gas chromatographic detection, provide a rapid and adequately
sensitive procedure for diazinon in most food commodities.
J. R. Geigy SA has developed a gas chromatographic method which is
applied following a shakeout with 48 percent HBr. The gas
chromatographic methods are sensitive to about 0.01 ppm or better. A
number of thin layer chromatographic procedures described in the
literature will provide a confirmative test. Specific analytical
methods for the detection of diazinon have been considerably refined
in recent years. Extraction, cleanup and analytical techniques have
been reviewed by Eberle (unpublished).
An improved procedure for routine determination of diazinon residues
in fruits, vegetables, soils, etc. using various selective gas
chromatographic detector systems has been reported by Eberle and Novak
(1969). Possible metabolites with strong cholinesterase-inhibiting
activity are detected on TLC plates by fly-head cholinesterase
inhibition with a limit of detection of 0.002 ppm. The above method is
considered a marked improvement over previously described methods and
permits determination of diazinon residues in a variety of crops
together with detection of cholinesterase-inhibiting metabolites.
NATIONAL TOLERANCES
Table VII summarizes national tolerances which have been established
for diazinon.
TABLE VII
Country Tolerance (ppm) Crop
Australia 0.75 fruits, grains, vegetables
Canada 0.1 maize, peas
0.25 melons, figs, cranberries and
7 vegetables
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
Canada 0.5 beans, cucumbers, turnips
(continued) 0.75 tree fruits including citrus,
grapes, strawberries and 16
vegetables
Germany 0.5 on or in vegetables, fruits,
(Fed. Rep.) root crops, leg-wes, grapes
and hops
Hungary 0.5 food
India 0 (proposed) cereals
Italy 2 olive oil
Netherlands 0.5 on or in
(i) vegetables or parts thereof
for consumption, including
edible mushrooms and edible
roots, bulbs and tubers
(ii) edible fruits of vegetables
and fruit crops or parts
thereof
New Zealand 0.75 food
Switzerland The Swiss Intercantonal commission for
toxic materials ("Commission Intercantonale
des Toxiques") proposes a tolerance of 0.5
ppm. Federal regulations are in preparation
U.S.A 0.1 banana pulp, potatoes, sweet
potatoes
0.2 bananas
0.5 almonds, filberts, pecans and
walnuts
0.75 ca 25 fruits and 25 vegetables,
fat of meat and meat byproducts
of cattle and sheep
1 olives and olive oil
3 almond hulls
10 5 hays
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
25 bean and pea forage
40 alfalfa (fresh), clover (fresh)
and maize forage
60 pasture grasses
APPRAISAL
Diazinon was first synthesized in 1951 and introduced as an
experimental insecticide in 1952. Initial development of the product
was prompted by the appearance of DDT resistance in flies, mosquitoes
and other insects. Extensive markets were developed for the product in
the late 1950's, and major areas of use included control of pests in
maize and alfalfa (U.S.A.), control of cockroaches and other insects
in buildings, the control of sheep ectoparasites (Australasia) and
control of a variety of insects attacking fruit and vegetables (Europe
and U.S.A.).
Diazinon does not persist for lengthy periods in either plant or
animal tissues. Resistant action depends on a combination of factors
including plant or animal species, application rates, cultural
practices, climatic conditions, etc. In most instances, the half-life
on crops is two to three days, except in olives where it ranges from
12 to 25 days, depending on stage of maturity of the olive at
spraying.
In plants, diazoxon has been established as the principal
anticholinesterase metabolite, though it occurs only as an
insignificant fraction of the whole residue. This metabolite is in
turn rapidly converted to noncholinesterase-inhibiting products.
Studies with radio-labelled insecticide have shown that in plants,
water and soil as well as mammals, detoxification of the insecticide
takes place during the metabolism in all systems examined where
residues are likely to occur.
Several analytical methods are available for determining residues in
plant or animal tissue. When utilized in accordance with good
agricultural practice, the treated product may have residues up to the
proposed tolerances, but only a small portion of each food commodity
in these categories is likely to be treated. There are data showing
that a significant amount of reduction in residues will take place
during washing, preparation and processing of food for consumption. In
"total diet" samples, diazinon has seldom been found, and then only at
a low level. Residue data are available on more than 70 crops from
several different countries.
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are based on residues likely to be found at
harvest following currently approved use patterns. On commodities
other than nuts, oilseeds, olives, olive oil and fat of meat, residues
will continue to decline during storage and shipment with a probable
half-life of less than five days.
The tolerances are expressed as diazinon, since it is known that the
oxygen analogue, diazoxon, if present, does not occur at
concentrations above 0.004 ppm.
Peaches, citrus and cherries 0.7 ppm
Other fruits 0.5 ppm
Leafy vegetables 0.7 ppm
Other vegetables 0.5 ppm
Grain (wheat, barley, rice) 0.1 ppm
Nuts almonds, walnuts, filberts
pecans, groundnuts) 0.5 ppm
Oilseeds (cotton, safflower, sunflower) 0.5 ppm
Sweet maize (on kernels and cob with
husks removed) 0.7 ppm
Olives and olive oil 2 ppm
Fat of meat of cattle, sheep and pigs
FURTHER WORK OR INFORMATION
DESIRABLE
1. Reproduction studies on one rodent and one nonrodent species.
2. Toxicological information on residual anticholinesterase
metabolites of diazinon in plants.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G (1970)
Pesticides in the total diet in England and Wales 1966-67. Pest. Sci.,
1(1): 10-13
Bartsch, E. (1970) Residue data of diazinon. J. R. Geigy SA,
Switzerland (unpublished)
Bourne, J. R. and Arthur, B. W. (1967) Diazinon residues in the milk
of dairy cows. J. econ. Ent., 60: 402-405
Boyd, E. M. and Carsky, E. (1969) Kwashiorkorigenic diet and diazinon
toxicity. Acta. pharmacol. Toxicol., 27: 284-294
Boyd, E. M., Carsky, E. and Krijnen, C. J. (1969) The effects of diets
containing from 0 to 81 percent casein on the acute oral toxicity of
diazinon. Clin. Toxicol., 2: 295-302
Bro-Rasmussen, F., Noddegaard and Voldum-Clausen, K. (1968)
Degradation of diazinon in soil. K.J. Sci. Fd. Agr., 19: 278-281
Claborn, H. V., Mann, R. D., Younger, R. L. and Radeleff, R. D. (1963)
Diazinon residues in the fat of sprayed cattle. J. econ. Ent., 56(6):
858-859
Coffin, D. E. and McKinely, W. P. (1964) The metabolism and
persistence of systox, diazinon and phosdrin on field-sprayed lettuce.
J. Assoc. Off. Agr. Chem., 47(4): 632-640
Derbyshire, J. C. and Murphy, R. T. (1962) Diazinon residues in
treated silage and milk of cows fed with powdered diazinon. J. Agr.
Fd. Chem., 10(5): 384-386
Duggan, R. E., Barry, H. C. and Johnson, L. Y. (1967) Pesticide
residues in total diet samples. II. Pest. Monitor. J., 1(2): 2-12
Earl, F. L., Melveger, B. E., Reinwall, J. E., Bierbower, G. W. and
Curtis, J. M. (1970) Diazinon toxicity - comparative studies in dogs
and miniature swine. Toxicol. appl. Pharmacol. (in press)
Eberle, D. O. (1970) Analysis of diazinon. J. R. Geigy SA, Switzerland
(unpublished)
Eberle, D. O. and Novak, D. (1969a) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 228
Eberle, D. O. and Novak, D. (1969b) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 1067-1074
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food
FAO/WHO. (1968) 1968 evaluations of some pesticide residues in food.
FAO/PL:1968/M/9/1. WHO/Food Add./69-35
Farrow, R. P., Elkins, F. R., Rose, W. W., Lamb, F. C., Ralls, J. W.
and Mercer, J. W. (1969) Canning operations that reduce insecticide
levels in prepared foods and in solid food waste. Residue Reviews,
29: 73-87
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Geigy Chemical Company. (1956-67) Unpublished data and methods of
analysis in pesticide petitions, submitted to the U.S. Food and Drug
Administration
Geigy Chemical Company. (1969) Diazinon-deterioration-stabilization
and influence on toxicity. Unpublished report
Getzin, L. W. (1967) Metabolism of diazinon and zinophos in soils. J.
econ. Ent., 60: 505-508
Getzin, L. W. and Rosefield, I. (1966) Persistence of diazinon and
zinophos in soils. J. econ. Ent., 59(3): 512-516
Gomaa, H. M., Suffett, I. H. and Faust, S. D. (1969) Kinetics of
hydrolysis of diazinon and diazoxon. Residue Reviews, 29: 171-190
Gunner, H. B., Zukerman, B. M., Walker, R. W. Miller, C. W., Denbert,
K. H. and Longley, R. E. (1966) The distribution and persistence of
diazinon applied to plant and soil and its influence on soil
microflora. Plant Soil; 25: 249-264
Gunther, F. A., Ewart, W. H., Blinn, R. C., Elmer, H. S. and Wacker,
G. B. (1958) Field persistence comparisons of residues of the
insecticide diazinon in lemons and Valencia oranges and effects on
juice flavour. Agr. Fd. Chem., 6: 521
Harrison, D. L. and Hastie, B. A. (1965) Diazinon residues in the milk
of cows and fat of sheep after feeding on pasture treated with
diazinon. New Zealand J. agr. Res., 9: 1-7
Hastie, B. A. (1963) The metabolism and elimination of diazinon from
animals, animal tissues and foodstuffs. Geigy Agricultural Chemicals,
Australia (bulletin)
Hastie, B. A. (1965) Diazinon residues in sheep fat. Geigy
Agricultural Chemicals, Australia (unpublished)
Kaplanis, J. N., Louloudes, S. J. and Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65: 70-75
Maier-Bode, H. (1963) Residues of insecticides on cover crops growing
in orchards after application of organic phosphorus toxicants on the
trees. Z. Pflanzenkrankh. Pflanzenschutz, 70(80): 449-459
Maier-Bode, H. (1967) Untersuchungen über den Gehalt
landwirtschaftlicher und gärtnerischer Ernteprodukte an
Pflanzenschutzmittelrückständen. Der Ministerpräsident des Landes
Nordrhein-West., Landesamt für Forschung, Jahrbuch 1967, S. 391.
Margot, A. and Gysin, H. (1957) Diazinon, its degradation products and
their properties. Belv. Chim. Acta., 40: 1562
Mathysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ectoparasite and fly control on dairy
cattle, with notes on safety of diazinon and ciodrin to calves. J.
econ. Ent., 61(5): 1394-1398
Melchiorri, P. Maffei F. and Siesto. A. J. (1964) Farmaco (Pavia), Ed.
Prat., 19: 610
Michel, H. O. (1949) J. lab. clin. Med., 34: 1564
Millar, K. R. (1963) Detection and distribution of 32P-labelled
diazinon in dog tissues after oral administration. New Zealand vet.
J., 11(6): 141-144
Mücke, W., Alt, K. O. and Esser, H. O. (1970) Degradation of
14C-labelled diazinon in the rat. J. Agr. Fd. Chem., 18(2): 208-212
Nakatsugawa, T., Tolman, N. M. and Dalm, P. A. (1969) Oxidative
degradation of diazinon by rat liver microsomes. Brochem. Pharmacol.,
18: 685-688
Nelson, L. L. and Hamilton, E. W. (1970) Metabolism of diazinon in
alfalfa. J. econ. Ent., 63(3): 874-878
Onsager, J. A. and Rusk, H. W. (1967) Adsorption and translocation of
diazinon and Stauffer N-2790 in sugarbeet seedlings. J. econ. Ent.,
60(2): 586-588
Painter, R. P. and Kilgore, W. W. (1963) Distribution of pesticides in
fermentation products obtained from artificially fortified grape
musts. J. Fd Sci., 28: 342-346
Pardue, J. R., Hansen, E. R., Barron, R. P. and Chen, J. J. (1970)
Diazinon residues on field-sprayed kale. Hydroxydiazinon - a new
alteration product of diazinon. J. Agr. Fd Chem., 18: 405-408
Rai, L. and Roan, C. C. (1959) Report included in Geigy Chemical
Company pesticide petition to the U.S. Food and Drug Administration
Ralls, J. W., Gilmore, D. R. and Cortes, A. (1966) Fate of radioactive
o,o-diethyl o-(2-isopropyl-4-methylpyrimidin-6-ol) phosphorothioate on
field grown experimental crops. J. Agr. Fd Chem., 14(4): 387-392
Ralls, J. W., Gilmore, D. R., Cortes. A., Schutt, S. H. and Mercer, W.
A. (1967) Residue levels of diazinon and its transformation products
on tomatoes, spinach and beans. Fd Tech., 21: 92-94
Robbins, W. E., Hopkins, T. L. and Eddy, O. W. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem., 5(7): 509-513
Robens, J. F. (1969) Teratologic studies of carbaryl, diazinon, norea
disulfiram and thiram in small laboratory animals. Toxicol. appl.
Pharmacol., 15: 152-163
Sethunathan, N. and MacRae, I. C. (1969) Persistence and
biodegradation of diazinon in submerged soil. J. Agr. Fd Chem., 17:
221
Sethunathan, N. and Yoshida, T. (1969) Fate of diazinon in submerged
soil. Accumulation of hydrolysis product. J. Agr. Fd Chem., 17(6):
1192-1195
Siesto, A. J., Maffei, F. and Melchiorri P. (1964) Estratto da
Archivio Italiano di Scienze Farmacologiche, Series 3, 13: 3
Storherr, R. W., Getz, M. E., Watts, R. R., Friedman, S. J., Erwin,
F., Giuffrida, L. and Ives, F. (1964) Identification and analyses of
five organo-phosphate pesticides. Recoveries from crops fortified at
different levels. J. Assoc. Off. Agr. Chem., 47(6): 1087-1093
Storherr, R. W. and Watts, R. R. (1965) A sweep co-distillation
cleanup method for organophosphate pesticides. J. Assoc. Off. Agr.
Chem., 48(6): 1154-1160
Vigne, J. P., Chouteau, J., Tabau, R.-L., Rancien, P. and Karamanian,
A. (1957) Sur le métabolisme d'un insecticide organo-phosphoré, le
diéthylthionophosphate de 2 isopropyl 4 méthyl 6 oxypyrimidine chez la
chèvre. Bull. Acad. Vét. Fr., 30: 85-92
Results in Table I indicate a complete loss of acetyl cholinesterase
inhibitory power. The table also indicates that a drastic reduction in
the acute toxicity of the insecticide had occurred during metabolism
(Mücke et al., 1970).
TABLE I
Properties of the main metabolites
Compound Acute LD50 Inhibition of
AChE ID50
Diazinon approx. 250 mg/kg 2.7 × 10-5M
Metabolite 1 approx. 2 700 mg/kg 10-2M
Metabolite 2 5 000 mg/kg 10-2M
Recent investigations by Mücke et al. (1970), have shown that
2-isopropyl-4-methyl-pyrimidin-6-ol is of low toxicity, having an
acute oral LD50 of approximately 2 700 mg/kg in the rat, and that
there is no detectable anticholinesterase activity. These findings
provide an answer to the doubts expressed by Ralls et al. (1966),
concerning the toxicity of 2-isopropyl-4-methyl-pyrimidin-6-ol.
No information is available on the toxicity of the metabolite found
after treatment of kale with diazinon (see "FATE OF RESIDUES. In
plants").
Acute toxicity
TABLE II
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat oral 250 (male) Gaines, 1969
Rat oral 285 (female) Gaines, 1969
Rat oral 466 Boyd & Carsky, 1969
Rat oral 293-408 (male) Edson & Noakes, 1960
Rat dermal 900 (male) Gaines, 1969
TABLE II (cont'd)
Acute toxicity to the rat
(various workers)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat dermal 455 (female) Gaines, 1969
Acute toxicity of diazinon to rats is summarized in Table II.
Differences in LD50 values expressed over the past few years may be
due to the presence of impurities contaminating early diazinon
samples. It has been shown that, under certain conditions,
combinations of molecules of diethyl phosphorothioic acid and
diethylphosphoric acid can condense to produce
tetraethylpyrophosphoric esters, which may be extremely toxic.
Stabilization of diazinon formulations appear to have eliminated some
of these condensation products and reduced the acute LD50 (Geigy,
1969).
As has been demonstrated with other pesticides, the acute LD50 to
rats increased as the protein level in the diet increased or decreased
from an optimal level. Raising the protein content from 29 percent to
81 percent, or lowering the protein content to 4 percent, increased
the toxicity approximately twofold (Boyd et al., 1969).
Short-term studies
Dog and pig
Groups of pigs (three male and three female) and dogs (three male and
three female) were orally administered diazinon by capsule daily for
periods up to eight months at doses of 0, 1.25, 2.5, 5 and 10 mg/kg
body-weight/day to pigs and 0, 2.5, 5, 10 and 20 mg/kg body-weight/day
to dogs (Earl et al., 1970). In pigs, mortality and cholinergic signs
of poisoning were evident at 2.5 mg/kg/day and above. Although
significantly increased myeloid/erythroid (ME) ratios were observed,
no aplastic anaemia was evident. In dogs, mortality and cholinergic
signs of poisoning were evident above 10 mg/kg, with significantly
increased ME ratios observed in dogs dying at 20 mg/kg. No evidence of
aplastic anaemia was observed in dogs and pigs.
Long-term studies
No new information available.
COMMENTS
It has been demonstrated that diazinon is oxidized in vitro to
diazoxon and further to diethylphosphorothioic acid. Farther
degradation has been shown to occur in vivo with the urinary
metabolites, which are substantially less toxic than the parent
compound. Distribution of diazinon in the body was relatively low,
with no accumulation in tissues or organs noted. Excretion in urine
and faeces was fairly rapid. No signs of blood dyscrasias were evident
in studies with dogs and pigs. No teratogenic or embryotoxic effects
were observed in hamsters and rabbits.
Stabilization of diazinon formulations have been reported to have
eliminated potential condensation products of diethylphosphoric acid
and diethylphosphorothioic acid. Information on the long-term toxicity
effects of diazinon is still inadequate. No information is available
on residuous anticholinesterase metabolites which may occur in plants.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.1 mg/kg body-weight/day
Dog: 0.02 mg/kg body-weight/day
Monkey: 0.05 mg/kg body-weight/day
Man: 0.02 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 = 0.002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Diazinon is a broad spectrum insecticide which has found many
applications, including control of:
(a) pests of field crops, orchards and pastures;
(b) animal ectoparasites;
(c) soil-inhabiting insects;
(d) industrial, public health and domestic pests.
Numerous formulations, including emulsifiable concentrates, wettable
powders, dusts and granules, are available. Foliar pests are normally
controlled by application in spray form, but effective control of rice
stem borer and some leaf hoppers has been obtained through application
of granules. Animals are normally treated by dipping, spraying,
jetting or dusting.
In the United States, diazinon is registered for application to more
than 60 food or feed crops including most fruits, vegetables and
forages. Use on corn (maize) for control of corn root worm and corn
ear worm and to alfalfa (lucerne) for control of alfalfa weevil are
major applications. There is an increasing use on rice.
Dipping, jetting or spraying of sheep for protection against sheep
blowfly are major uses in Australia, New Zealand, South Africa and
South America. Due to its solubility in wool grease, the material can
remain on the sheep in a stable state for several weeks and thus
provide effective protection against parasite attack. Dermal
applications to sheep, cattle and goats are also used for control of
lice, ticks, horn flies and certain other insects.
Table III summarizes typical dosages and pre-harvest periods for the
various crop categories. Diazinon has been found effective in
controlling over 100 species of food crop pests such as mites, aphids,
thrips, maggots, fruit flies, worms, beetles, grasshoppers, leaf
miners, etc. Seed furrow soil treatments are used for several root
vegetable crops.
TABLE III
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Tree fruits 0.5 lb/100 gal (full coverage) 10-20
Caneberries
(raspberries,
blackberries
loganberries) 1.0 lb/acre (full coverage) 7
Citrus 0.5 lb/100 gal (full coverage) 7-21
Leafy vegetables 0.5-1.0 lb/acre 5-21
Root vegetables 0.5-1.2 lb/acre 10
Others 0.25-1.0 lb acre 0-7
TABLE III (cont'd)
Diazinon dosages and pre-harvest periods
Crop Actual dosage Pre-harvest
period (days)
Forages & hays 0-5-1.0 lb/acre No grazing
limitations
4-10 days
before cutting
hay
Cattle & sheep 1.0-2.3 gal of 0.30-0.5% (14
spray per animal pre-slaughter
period)
Post-harvest treatments
There is no commercial post-harvest use of diazinon on crops.
Other uses
Diazinon is recommended for fly control in dairy barns and other farm
buildings as well as in food processing plants; however, this is
becoming less important because of resistance developed by flies. It
is also used in households to control carpet beetles and clothes
moths, in home gardens and in buildings to combat ants, cockroaches,
fireants, etc. Control of cockroaches is an important field of use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of diazinon on or in plants, in animal tissues, or even in
the soil, are not highly persistent. In many countries and on over
sixty crops, residues have been determined at various time intervals
after applications of varying amounts of diazinon. For example, in the
controlled experiments with apples in the U.S.A., 16 varieties were
treated in 15 states using ´ to 1 lb/100 gal (i.e., full coverage
sprays) on various spray schedules and in combination with other
pesticides and fungicides.
A summary of the numerous data (Geigy, 1956-67) obtained from the
rates of application shown in Table III is given in Table IV.
TABLE IV
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Tree fruits
Apples 0.6-0.8 14 0.1-0.4 5
Pears 0.6-0.7 14 0.1-0.3 5
Cherries 3-7 10 0.1-0.3 3
Peaches 2-6 20 0.1-0.6 3-6
Apricots 1-4 10 0.1 2
Nectarines 1-6 10 0.2 3-4
Plums & prunes 2-4 10 <0.1-0.2 2
Figs 0.5 10 <0.1 3
Nuts
Almonds )
Filberts ) 0.1 (nuts) 10 <0.1 (nuts)
Walnuts ) 60 <0.1 (kernels)
Peanuts
Citrus
Oranges 3-5 21 0.1-0.4 4
Lemons 4.0 21 0.6-0.7 6-7
Grapefruit 0.3 7 0.1-0.2 7
Caneberries and small fruit
Strawberries 0.9-1.4 5 0.2-0.4 2-4
Grapes 1-8 18 <0.1-0.3 3
Cranberries 9 7 <0.1 1
Blueberries 1-4 7 <0.1-0.3 1-2
Blackberries )
Boysenberries ) 1-5 7 <0.1-0.3 2
Loganberries )
Raspberries )
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Leafy vegetables
Cabbage 1-2.5 7 <0.1-0.7 4-6
Celery 2-9 10 <0.1-0.7 2
Cauliflower 1 5 0.4-0.5 7
Broccoli 1-2 5 <0.1-0.5 3
Lettuce 6-17 10 0.3-0.5 1-2
Spinach 5-12 10 <0.1-0.2 2
Endive 3-18 10 0.1 2
Collards 3 12 <0.1 2
Kale 10-24 12 0.1-0.2 1
Parsley 1-6 12 0.1-3.0 2
Swiss chard 2 12 <0.1 2
Turnip greens 4-15 12 <0.1 2
Brussels sprouts 0.2 10 <0.1
Root vegetables (foliar application)
Beets <0.1 0
Onions 7-13 (green) 10 (green) 0.4-0.6 (green) 2
2-3 (dry) 10 (dry) <0.1-0.3 (dry) 2
Carrots 1-2 10 0.1-0.3 2-3
Carrots (soil
application) 120 0.1
Parsnips 0.7 10 0.3 6
Radishes <0.1-0.4 10 <0.1 4
Turnips 0.5 10 0.4-0.5 21
Vegetables (others)
Peppers 0.6-0.8 5 <0.1-0.2 2-3
Cucumbers 1.0-2.5 7 <0.1 2
Green beans 1-2 7 <0.1 2
Lima beans 0.2-1.0 7 <0.1-0.2 2
Squash 0.1-0.2 3 <0.1-0.2 2
Maize (ears only) <0.1 0 <0.1 -
Peas (plus pod) <0.1 0 <0.1 -
Miscellaneous
Tomatoes 0.1-0.4 3 <0.1-0.2 2
Melons 0.1-0.7 3 <0.1-0.2 2-3
Olives 1-6 75 <0.2-0.6 ca 25
TABLE IV (cont'd)
Diazinon residues at different application rates
Typical Residues at
Crops initial Pre-harvest pre-harvest Estimated
residues period period half-life
(ppm) (days) indicated (ppm) (days)
Hops (cones) 3-11 14 0.1-0.3 3
Sorghum (grain) 0.5 7 <0.1 2
Cotton (seed) 0.1-0.2 7 <0.1
Safflower (seed) <0.1
Sunflower 80 0.1-0.2
Sugarcane (stems) <0.1 7 <0.1
Tea 7 <0.1 (manufactured)
Coffee 1 7 0.1 (green beans)
Cereal crops
Wheat 13 <0.1 (grain)
Meat
Pre-slaughter
period
Lamb (fat) 1-3 14 0.1-0.75 5
Beef (fat) 1-3 14 <0.1-0.2 3
Forages
Pre-grazing Cutting Initial Residue Half
interval time residue at life
(days) (days) in forage cutting (days)
(ppm) (ppm)
Alfalfa 0 7 12-24 0.2-0.5 2
Clover 0 7 4-14 1.0-4.0 2-3
Range/pasture
grass 0 21 9 4.0-7.2 2-3
30 (oil
formulation) 54
Pea/bean 0 4 5-10 2.0-5.0 2
Maize 0 0 12-21 - 2
General Comments
In animals
Reports from numerous trials indicate that residues of diazinon in
subcutaneous fatty tissues of sheep and cattle treated for parasite
control do not exceed 0.75 ppm one day after treatment. The residue
levels in internal fat, however, may be higher. Average residue levels
decline rapidly following treatment, but fat samples from individual
animals may exceed 0.75 ppm for 14 days. Hastie (1965) showed that
standard treatment procedures resulted in residues in sheep one day
after treatment which were in excess of 1 ppm in the fat. After three
days, residue levels had declined to 0.3-0.5 ppm. Claborn et al.
(1963) showed that cattle sprayed with diazinon contained residues in
omental fat six days after treatment, but residues were not detectable
at 14 days.
Residues of diazinon in animal fat may be influenced by the condition
of the treated animal due to the dilution factor brought about by the
total amount of fat present. Application rates also affect the residue
levels.
In plants
Residue levels in various crops have been reviewed by Bartsch (1970).
Residue analyses of pip fruit have indicated that 90 percent of
residues are in the peel of ripe fruit and only 2-4 percent in the
pulp. No residues at all were detected in the pulp and juice of citrus
fruits, since the peel serves as a barrier (Gunther et al., 1958).
Residues present in any crop are dependent upon application rates,
cultural practices and type of formulation used. Residues have
generally been more persistent in glasshouse crops than in field crops
(Bartsch, 1970). Granular formulations have given more variable
residue levels in treated crops than have liquids applied as sprays.
However, studies have indicated that residue levels are higher overall
after crop treatment with emulsions rather than granules (Maier-Bode,
1967).
After crop application of emulsions or granules, practically no
residues have been detected in tubers (potatoes, sweet potatoes and
yams) or in grain (rice and wheat). Residues in seeds of cotton,
sunflower and safflower contain only little diazinon in contrast to
the respective oil products.
Immediately after treatment of forage crops, residues may reach 150
ppm. Residue levels fall very rapidly, due in part to growth of the
forage plants, and within a period of 1-2 days are below the current
tolerances of 60 ppm for grass and 40 ppm for alfalfa (in the U.S.A.)
Due to the solubility of diazinon in lipids, high residues may be
found in olive oil, but they are below the accepted tolerance levels
of 2 ppm in Italy and 1 ppm in U.S.A., 7-8 weeks after treatment.
Carrots have been examined with particular care on account of their
dietary significance, bearing in mind that they are often eaten
uncooked, and because of their biochemical properties. Residue figures
vary widely, depending on timing of application, formulation and
method of application. All trial data reviewed by Bartsch (1970)
indicated that unless harvest is carried out within 60 days of last
treatment (soil-application), the residue level is below 0.5 ppm in
all cases.
FATE OF RESIDUES
In animals
Investigations on the metabolic fate of diazinon in mammals were
initiated mainly by the widespread use of the product as an
ectoparasiticide in ruminants. Residue studies in fat and milk of cows
(Bourne and Arthur, 1967; Claborn et al., 1963; Derbyshire and Murphy,
1962; Matthysse and Lisk, 1968) and of sheep (Harrison and Hastie,
1965; Matthysse et al., 1968) were carried out after the insecticide
had been applied regularly by spraying and dipping or by feeding the
animals on pasture treated with the insecticide. Only small amounts
of diazinon residues have been found in fat and milk, whereas the
other tissues were free of the insecticide. In two early studies in
the cow (Robbins et al., 1957) and in the goat (Vigne et al., 1957),
the rapid and complete elimination of the 32P-labelled insecticide
in urine, faeces, blood and milk was shown, demonstrating urine as the
main route of excretion.
Rai and Roan (1959) found no residues of diazinon in the milk of dairy
animals given daily oral doses of diazinon at the rates of 1.06, 5.30
and 10.60 mg/kg of body-weight over a three-week feeding period. These
administration rates are calculated to be 100, 500 and 1 000 ppm on
the basis of the grain fed, or 51, 290 and 500 ppm on the basis of hay
consumed. Steers treated with 165 and 825 ppm in daily oral doses
calculated on the basis of grain fed showed traces of diazinon in
blood, urine, muscle, liver and brain. Only in fat was a significant
residue found, being 0.23 ppm at the maximum feeding level. These
results were obtained by the use of three methods of analysis.
In plants
Practical residue determination on many occasions has shown that
diazinon does not persist long as a residue on most food crops. It
appears that the movement or metabolism of diazinon depends upon the
plant species (Coffin and McKinley, 1964; Ralls et al., 1966; Gunner
et al., 1966).
Diazinon is passed through bean plants unchanged, with rapid
translocation and emergence in bean root exudates after application of
diazinon to aerial portion of the plant (Gunner et al., 1966). Sugar
beet seedlings absorb diazinon applied to the soil (Onsager and Rusk,
1967) and is translocated in quantities to render the plant
insecticidal. This is contrary to earlier observations (Gunner, 1966),
where it was found that diazinon was absorbed by beans but not
translocated in toxic amounts.
Analysis of alfalfa (lucerne) indicated that diazinon was absorbed
from treated soil into the plant (Nelson and Hamilton, 1970). No
metabolites of diazinon were found in the alfalfa, and diazinon was
expired by the plants. These findings substantiate the results
obtained by Gunner et al. (1966) with beans.
The metabolism of diazinon in plants has been shown to involve
hydrolysis of the phosphorus primidylester bond and subsequent
metabolism of the 2-isopropyl-4-methyl-6-hydroxypyrimidine to carbon
dioxide. Small amounts of diazoxon have at times been detected in
field-grown crops (Gomaa et al., 1969). The fact that diazoxon levels
are very low, if present at all, indicates oxidation is very minor or
that the oxon is hydrolysed as rapidly as it is found. Margot and
Gysin (1957) indicated loss of insecticidal activity on plants caused
by evaporation of diazinon and through hydrolysis. No metabolites of
diazinon were found.
Coffin and McKinley (1964) reported on the metabolism and persistence
of diazinon on field-sprayed lettuce. Diazinon residues decreased from
8.1 ppm to 0.3 ppm from four hours to seven days after spraying, and
detectable quantities of diazinon were present at 10 and 14 days. No
significant amount of diazoxon or other metabolites were found by the
paper chromatographic detection system.
Grasses and grains grown for forage which had been treated with
diazinon were analysed by the sulphide procedure. Maier-Bode (1963)
found diminution of residues occurred only in the uncut grasses. After
cutting and while drying to hay, little of the diazinon was lost.
Ralls et al. (1966), studied the fate of 35S-labelled diazinon on
field-grown crops and found a rapid decrease in diazinon residues. The
metabolite identified from field samples was diazoxon. Three
thin-layer chromatographic systems showed the presence of this
metabolite on spinach at 0.005 to 0.01 ppm five days after spraying.
Paper chromatography of snap bean extracts harvested seven days after
treatment showed an increase in a cholinesterase-inhibiting compound
with an Rf value corresponding to diazoxon.
Additional studies in the field revealed residue levels of diazinon
(I), diazoxon (II) and 2-isopropyl-4-methylpyrimidin-6-ol (III) made
by Ralls et al. (1967), using diazinon-32p. A spinach sample
analysed one hour after spraying contained 31.7 ppm (I), 1.5 ppm (II)
and 2.5 ppm (III). Analysis of a four-day sample gave 1.8 ppm (I),
0.34 ppm (II) and 2.5 ppm (III). Although diazinon and its oxygen
analogue dissipated rapidly, compound (III), the result of further
hydrolysis of diazoxon persisted at the same level. The mammalian
toxicity of this persistent compound was at the time not known.
Similar experiments with snap bean and tomato plants showed the same
rapid disappearance of diazinon and diazoxon to levels greatly below
0.1 ppm after four days. Less than 0.1 ppm of compound (III) was found
in all four-day samples.
Refined analytical techniques were used by Eberle and Novak (1969), to
further investigate the fate of diazinon in plants. The only
cholinesterase-inhibiting metabolite detectable at any time after
diazinon application was diazoxon. The maximum levels of diazoxon
found in apples and olive oil were 0.004 ppm and 0.007 ppm,
respectively. At harvest, fruit and vegetables in all instances
contained less than 0.002 ppm diazoxon.
The appearance and subsequent disappearance of traces of diazoxon in
the crops examined indicate that diazinon is oxidized in plants to
diazoxon which is, in turn, rapidly altered to
noncholinesterase-inhibiting products. All samples were analysed for
monothiotetraethylpyrophosphate (S-TEPP), but no residues could be
detected at the limit of 0.002 ppm. These results are at variance with
the findings of Melchiorri et al. (1964), and Siesto et al. (1964),
who earlier reported evidence of the formation of S-TEPP and TEPP. The
investigations of Eberle and Novak do, however, support the findings
of Ralls et al. (1966), in that the only cholinesterase-inhibiting
metabolite detectable at any time after diazinon application is
diazoxon.
The levels of diazinon and diazoxon reported by Eberle and Novak are
summarized in Table V.
TABLE V
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
40 (3 treatments) golden apples 0 2.8 <0.002
7 0.6 0.004
21 0.3 0.002
63 (harvest) 0.05 <0.002
40 (3 treatments) Jonathan 0 1.3 0.002
apples 28 0.2 <0.002
70 (harvest) 0.1 <0.002
50 (1 treatment) pears 49 0.01 <0.002
50 (2 treatments) 35 0.01 0.003
50 (3 treatments) 21 0.02 <0.002
50 (4 treatments) 10 0.12 0.003
TABLE V (cont'd)
Residues of diazinon and diazoxon on vegetables and fruit
Application rate Crop Days after Residues found (ppm)
(g ai/100 l) last diazinon diazoxon
treatment
25 (2 treatments) Langstieler 7 0.2 <0.002
cherries 28 (harvest) 0.02 <0.002
25 (2 treatments) Schauenburger 7 0.4 <0.002
cherries 28 (harvest) 0.02 <0.002
4 (2 treatments) Flakeer 29 1.3
carrots 80 (harvest) 0.2 <0.002
4 (2 treatments) Guérande 29 2.2
carrots 90 (harvest) 0.2 <0.002
1 treatment onions 125 (harvest) 0.05 <0.002
10 (1 treatment) radish 36 (harvest) <0.02 <0.002
25 Milan cabbage 0 27
7 1.7
21 0.05
63 (harvest) <0.02 <0.002
25 Blanc cabbage 0 12.5
7 1.1
63 (harvest <0.02 <0.002
In contrast to the findings of other workers, a further alteration
product, hydroxysiazinon, has been reported by Pardue et al. (1970).
This previously unidentified compound was detected during a study of
diazinon field-sprayed kale samples by a technique of enzyme
inhibition thin layer chromatography of the extracts previously
oxidized by bromine water.
The levels of hydroxydiazinon found in kale in comparison with
diazinon and diazoxon are indicated in Table VI.
TABLE VI
Residues found by GLC in diazinon-treated kale
Days after application
2 7 11 15
Diazinon (ppm) 8.8 2.9 2.0 1.6
Diazoxon (Ppm) 0.004 0.007 0.002 0.002
Alteration product of
diazinon1 (ppm) 0.18 0.05 0.03 0.03
1 Later identified as hydroxydiazinon. Quantitated using the compound
prepared by UV-irradiation of diazinon.
Pardue et al. (1970) have not offered any possible explanation for the
presence of hydroxydiazinon. Many of the cruciferous crops have waxy
cuticles which would have a tendency to hold diazinon. The effect of
UV-irradiation on diazinon could then possibly cause alteration to
hydroxydiazinon.
In soil
Literature on diazinon residues in soils has been mainly confined to
nonflooded conditions (Getzin and Rosefield, 1966; Getzin, 1967;
Gunner et al., 1966). Getzin (1967) reported that greater amounts of
the hydrolysis product were recovered from soil fumigated with
propylene oxide than from nonfumigated soil. Conversely, little 14CO2
was released from the fumigated soil treated with 14C-labelled
diazinon, while large amounts were released from nonfumigated soil. He
also suggested that the initial step in the degradation of diazinon in
nonflooded soils is hydrolysis at the heterocyclic phosphate bond
(phosphorus-oxygen-pyrimidine bond), followed by disruption of the
pyrimidine ring and the subsequent release of 14CO2. Soil
microflora appeared to play a major role in the of the degradation of
the parent molecule. Trela et al. (1968) recently observed that the
degradation of diazinon into pyrimidine and phosphorothioate
derivatives was greatly stimulated in the presence of microorganisms
isolated from diazinon-treated soil.
The degradation of diazinon in submerged neutral or alkaline soil
showed that diazinon disappeared at a faster rate from nonsterilized
soils than from sterilized soils (Sethunathan and MacRae, 1969), thus
indicating the participation of soil microflora in its degradation.
Surprisingly, only a small amount of 14CO2 was released from
nonsterilized soils treated with 14C-labelled diazinon (labelled at
the 2-position on the pyrimidine ring). This result is not in
agreement with the results on 14CO2 evolution from ring-labelled
diazinon reported for nonflooded, soils (Getzin, 1967). This
difference suggests that the fate of diazinon under submerged
conditions might be different from that in nonflooded conditions.
In the study of the persistence of diazinon (14C-labelled at the
4-position on the pyrimidine ring) in submerged soils, soil microflora
appeared to assist in its degradation into a less toxic hydrolysis
product (2-isopropyl-6-methyl-4-hydroxypyrimidine). This hydrolysis
product was, however, resistant to further degradation under submerged
conditions (Sethunathan and Yoshida, 1969).
From the foregoing, it appears that the major step in the degradation
of diazinon in flooded soil is hydrolysis, resulting in the formation
of 2-isopropyl-6-methyl-4-hydroxypyrimidine as one of the degradation
products. Under submerged conditions, where the bulk of soil
microflora is anaerobic, oxidation is negligible and the hydrolysis
product tends to accumulate and persist in large quantities without
being oxidized. The more rapid degradation of diazinon and the greater
recovery of hydrolysis product from nonsterilized soils suggest that,
in flooded soils, soil microflora play an important role - direct or
indirect - in the hydrolysis of diazinon to
2-isopropyl-6-methyl-4-hydroxypyrimidine, but not thereafter. The
accumulation of 2-isopropyl-6-methyl-4-hydroxypyrimidine in submerged
soils should not, however, pose a serious residue problem since, based
on the anticholinesterase activity of the two compounds (Margot and
Gysin, 1957), the hydrolysis product of diazinon is far less toxic
than the parent molecule. In addition, drying the soil or increasing
aeration during land preparation for the succeeding rice crop may
completely eliminate this degradation product by oxidation.
The decomposition of diazinon under nonflooded conditions, using two
soil types in conjunction with three other factors, was explored by
Bro-Rasmussen et al. (1968). These other factors considered were:
(i) activity of soil microorganisms
(ii) water content of soil
(iii) concentration of diazinon.
The disappearance rate of diazinon varied considerably with half-lives
ranging from 21 to 80 days. Results indicated that all factors
influenced the rate of diazinon degradations and that microorganisms
play an important role in the disappearance of diazinon from the soil.
In storage and processing
Residue levels of diazinon, diazoxon and
2-isopropyl-4-methyl-pyrimidin-6-ol were measured in snap beans,
spinach and tomatoes subjected to washing, blanching and pealing (for
tomatoes) under simulated commercial conditions (Ralls et al., 1967).
Diazinon on spinach at harvest 4 days after spraying was present at
1.8 ppm and diazoxon at 0.34 ppm. A spray rinse did not significantly
reduce residues. Detergent washing reduced diazinon to 0.77 ppm and
diazoxon to 0.18 ppm, and steam blanching gave a total reduction to
about 30 percent of the original residue. Only a water blanching
process significantly reduced the level of the pyrimidinol metabolite
from 2.5 ppm to 0.1 ppm. Residues at harvest (8 days) on snap beans
and tomatoes were less than 0.1 ppm. Subsequent commercially simulated
treatment appeared to have little or no effect, except possibly the
commercial peeling of tomatoes. Further studies on the reduction of
residues during food processing operations are reported by Farrow et
al. (1969).
Water washing of tomatoes (without detergent) removed 88 percent of
total residues, but caused an apparent increase of 11 percent with
spinach. A similar increase was seen with other insecticides such as
parathion. Crops such as spinach and broccoli have large surface areas
and are therefore subject to considerable leaching of water-soluble
solids during washing. Since results are expressed on a dry weight
basis, this results in an apparent increase in residue levels.
Commercial blanching operations are carried out using either hot water
or steam. Although washing was not effective in removing diazinon from
spinach, hot water blanching removed 60 percent of the residue. During
the combined canning operations it was found that there is a good
overall removal of organophosphorus residues. Thus there was a 99
percent removal of malathion residues from tomatoes, 94 percent
removal from green beans and 66 percent removal of parathion from
spinach; however, removal of parathion residues from broccoli, which
is processed by snap freezing, amounted to only 10 percent.
Diazinon is recommended in some countries for application to grapes,
and Painter et al. (1963) studied the fate of diazinon after addition
to grape must. Diazinon did not cause any measurable effect on
fermentation and was not found in any component after fermentation.
The absence of diazinon after fermentation is probably due to the
hydrolysis of the compound under the acid conditions prevailing. It is
interesting to note that several other organophosphorus compounds were
detected in finished wine when subjected to similar experimental
procedures.
In water
Gomaa et al. (1969) have considered breakdown of diazinon and diazoxon
which may occur in water. Techniques were developed to study the
hydrolysis of diazinon and diazoxon in aqueous media under varying
conditions of temperature and pH. Diazinon and diazoxon are
quantitatively hydrolysed to 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylthiophosphoric or diethylphosphoric acid.
In general, at 20°C, diazoxon hydrolysis proceeds much faster than
diazinon under comparable conditions. A large difference in rate of
hydrolysis can be detected under acidic conditions, where diazoxon is
hydrolysed 30 times faster than diazinon. The differences in rates
decrease as neutrality is approached, but increase again as the pH of
the hydrolysis solution increases.
The work of Gomaa et al., indicates that some caution should be
exercized concerning movement or application of diazinon into water.
Sethunathan and MacRae (1969), suggested that soil microflora play an
important part in the hydrolysis of diazinon in flooded soils. These
conditions are unlikely to apply to aqueous media, which may account
for the persistence of diazinon reported by Gomaa et al.
Evidence of residues in food moving in commerce or at consumption
Of some 14 800 randomly selected samples of raw agricultural products
examined by the U.S. Food and Drug Administration from June 1965
through 1966, only 32 samples showed any detectable residue of
diazinon. Total diet studies conducted during 1965 and 1966 by the
U.S. Food and Drug Administration revealed that 98 percent of the food
samples contained no detectable residues of diazinon. The remaining 2
percent contained only trace quantities. A multidetection gas
chromatographic method, using an electron capture detector and/or a
thermionic detector specific for phosphorus, was used for the
analyses. The sensitivity of the method was about 0.05 ppm (Duggan et
al., 1967).
METHODS OF RESIDUE ANALYSIS
Most of the residue data summarized in this monograph were obtained
using one or two of four different methods of analysis developed by
Geigy Chemical Company (1956-67).
A sulphide procedure was considered most accurate when spray history
was known. In this procedure, diazinon is extracted from crops with a
solvent and from the solvent with 48 percent HBr. The 48 percent HBr
treatment adds a high degree of selectivity for the determination of
diazinon. Upon boiling the acid solution, diazinon sulphur is
converted to H2S and distilled off. It is collected in zinc acetate
solution and then converted to a methylene blue complex, which is
determined spectrophotometrically. Sensitivity of the sulphide
procedure is about 0.1-0.2 ppm. Some crops, such as kale, had high
natural sulphur blanks, so these crops were analysed by a phosphate
method. Thiocarbamates such as ferbam also interfere and must be
removed by an additional cleanup step.
Some phosphorothioates are known to form relatively stable metabolic
products containing no sulphur, for which the method described above
would not be applicable, so a cholinesterase inhibition method was
utilized to validate the sulphide procedure. The method based on
determining the phosphorus of diazinon and one based on the
ultraviolet absorption properties of the pyrimidine portion of the
molecule are fraught with high blank and cleanup problems. Sensitivity
of these methods is about 0.3-0.4 ppm.
None of these four methods was of adequate sensitivity or specificity
for "total diet" samples. Such data were not possible until the GLC
methods based on electron capture and thermionic detectors were used.
Diazinon and diazoxon are detected and may be determined by the
multiresidue method of Abbott et al. (1970), which was successfully
used in the total diet studies carried out in England and Wales. The
methods of Storherr et al. (1964, 1965), using an ethyl acetate
extraction and either a sweep codistillation or celite column cleanup
with gas chromatographic detection, provide a rapid and adequately
sensitive procedure for diazinon in most food commodities.
J. R. Geigy SA has developed a gas chromatographic method which is
applied following a shakeout with 48 percent HBr. The gas
chromatographic methods are sensitive to about 0.01 ppm or better. A
number of thin layer chromatographic procedures described in the
literature will provide a confirmative test. Specific analytical
methods for the detection of diazinon have been considerably refined
in recent years. Extraction, cleanup and analytical techniques have
been reviewed by Eberle (unpublished).
An improved procedure for routine determination of diazinon residues
in fruits, vegetables, soils, etc. using various selective gas
chromatographic detector systems has been reported by Eberle and Novak
(1969). Possible metabolites with strong cholinesterase-inhibiting
activity are detected on TLC plates by fly-head cholinesterase
inhibition with a limit of detection of 0.002 ppm. The above method is
considered a marked improvement over previously described methods and
permits determination of diazinon residues in a variety of crops
together with detection of cholinesterase-inhibiting metabolites.
NATIONAL TOLERANCES
Table VII summarizes national tolerances which have been established
for diazinon.
TABLE VII
Country Tolerance (ppm) Crop
Australia 0.75 fruits, grains, vegetables
Canada 0.1 maize, peas
0.25 melons, figs, cranberries and
7 vegetables
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
Canada 0.5 beans, cucumbers, turnips
(continued) 0.75 tree fruits including citrus,
grapes, strawberries and 16
vegetables
Germany 0.5 on or in vegetables, fruits,
(Fed. Rep.) root crops, leg-wes, grapes
and hops
Hungary 0.5 food
India 0 (proposed) cereals
Italy 2 olive oil
Netherlands 0.5 on or in
(i) vegetables or parts thereof
for consumption, including
edible mushrooms and edible
roots, bulbs and tubers
(ii) edible fruits of vegetables
and fruit crops or parts
thereof
New Zealand 0.75 food
Switzerland The Swiss Intercantonal commission for
toxic materials ("Commission Intercantonale
des Toxiques") proposes a tolerance of 0.5
ppm. Federal regulations are in preparation
U.S.A 0.1 banana pulp, potatoes, sweet
potatoes
0.2 bananas
0.5 almonds, filberts, pecans and
walnuts
0.75 ca 25 fruits and 25 vegetables,
fat of meat and meat byproducts
of cattle and sheep
1 olives and olive oil
3 almond hulls
10 5 hays
TABLE VII (cont'd)
Country Tolerance (ppm) Crop
25 bean and pea forage
40 alfalfa (fresh), clover (fresh)
and maize forage
60 pasture grasses
APPRAISAL
Diazinon was first synthesized in 1951 and introduced as an
experimental insecticide in 1952. Initial development of the product
was prompted by the appearance of DDT resistance in flies, mosquitoes
and other insects. Extensive markets were developed for the product in
the late 1950's, and major areas of use included control of pests in
maize and alfalfa (U.S.A.), control of cockroaches and other insects
in buildings, the control of sheep ectoparasites (Australasia) and
control of a variety of insects attacking fruit and vegetables (Europe
and U.S.A.).
Diazinon does not persist for lengthy periods in either plant or
animal tissues. Resistant action depends on a combination of factors
including plant or animal species, application rates, cultural
practices, climatic conditions, etc. In most instances, the half-life
on crops is two to three days, except in olives where it ranges from
12 to 25 days, depending on stage of maturity of the olive at
spraying.
In plants, diazoxon has been established as the principal
anticholinesterase metabolite, though it occurs only as an
insignificant fraction of the whole residue. This metabolite is in
turn rapidly converted to noncholinesterase-inhibiting products.
Studies with radio-labelled insecticide have shown that in plants,
water and soil as well as mammals, detoxification of the insecticide
takes place during the metabolism in all systems examined where
residues are likely to occur.
Several analytical methods are available for determining residues in
plant or animal tissue. When utilized in accordance with good
agricultural practice, the treated product may have residues up to the
proposed tolerances, but only a small portion of each food commodity
in these categories is likely to be treated. There are data showing
that a significant amount of reduction in residues will take place
during washing, preparation and processing of food for consumption. In
"total diet" samples, diazinon has seldom been found, and then only at
a low level. Residue data are available on more than 70 crops from
several different countries.
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are based on residues likely to be found at
harvest following currently approved use patterns. On commodities
other than nuts, oilseeds, olives, olive oil and fat of meat, residues
will continue to decline during storage and shipment with a probable
half-life of less than five days.
The tolerances are expressed as diazinon, since it is known that the
oxygen analogue, diazoxon, if present, does not occur at
concentrations above 0.004 ppm.
Peaches, citrus and cherries 0.7 ppm
Other fruits 0.5 ppm
Leafy vegetables 0.7 ppm
Other vegetables 0.5 ppm
Grain (wheat, barley, rice) 0.1 ppm
Nuts almonds, walnuts, filberts
pecans, groundnuts) 0.5 ppm
Oilseeds (cotton, safflower, sunflower) 0.5 ppm
Sweet maize (on kernels and cob with
husks removed) 0.7 ppm
Olives and olive oil 2 ppm
Fat of meat of cattle, sheep and pigs
FURTHER WORK OR INFORMATION
DESIRABLE
1. Reproduction studies on one rodent and one nonrodent species.
2. Toxicological information on residual anticholinesterase
metabolites of diazinon in plants.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G (1970)
Pesticides in the total diet in England and Wales 1966-67. Pest. Sci.,
1(1): 10-13
Bartsch, E. (1970) Residue data of diazinon. J. R. Geigy SA,
Switzerland (unpublished)
Bourne, J. R. and Arthur, B. W. (1967) Diazinon residues in the milk
of dairy cows. J. econ. Ent., 60: 402-405
Boyd, E. M. and Carsky, E. (1969) Kwashiorkorigenic diet and diazinon
toxicity. Acta. pharmacol. Toxicol., 27: 284-294
Boyd, E. M., Carsky, E. and Krijnen, C. J. (1969) The effects of diets
containing from 0 to 81 percent casein on the acute oral toxicity of
diazinon. Clin. Toxicol., 2: 295-302
Bro-Rasmussen, F., Noddegaard and Voldum-Clausen, K. (1968)
Degradation of diazinon in soil. K.J. Sci. Fd. Agr., 19: 278-281
Claborn, H. V., Mann, R. D., Younger, R. L. and Radeleff, R. D. (1963)
Diazinon residues in the fat of sprayed cattle. J. econ. Ent., 56(6):
858-859
Coffin, D. E. and McKinely, W. P. (1964) The metabolism and
persistence of systox, diazinon and phosdrin on field-sprayed lettuce.
J. Assoc. Off. Agr. Chem., 47(4): 632-640
Derbyshire, J. C. and Murphy, R. T. (1962) Diazinon residues in
treated silage and milk of cows fed with powdered diazinon. J. Agr.
Fd. Chem., 10(5): 384-386
Duggan, R. E., Barry, H. C. and Johnson, L. Y. (1967) Pesticide
residues in total diet samples. II. Pest. Monitor. J., 1(2): 2-12
Earl, F. L., Melveger, B. E., Reinwall, J. E., Bierbower, G. W. and
Curtis, J. M. (1970) Diazinon toxicity - comparative studies in dogs
and miniature swine. Toxicol. appl. Pharmacol. (in press)
Eberle, D. O. (1970) Analysis of diazinon. J. R. Geigy SA, Switzerland
(unpublished)
Eberle, D. O. and Novak, D. (1969a) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 228
Eberle, D. O. and Novak, D. (1969b) Fate of diazinon in field sprayed
agricultural crops, soil and olive oil. J. Assoc. Off. Anal. Chem.,
52: 1067-1074
Edson, E. F. and Noakes, D. N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. appl. Pharmacol.,
2: 523-539
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food
FAO/WHO. (1968) 1968 evaluations of some pesticide residues in food.
FAO/PL:1968/M/9/1. WHO/Food Add./69-35
Farrow, R. P., Elkins, F. R., Rose, W. W., Lamb, F. C., Ralls, J. W.
and Mercer, J. W. (1969) Canning operations that reduce insecticide
levels in prepared foods and in solid food waste. Residue Reviews,
29: 73-87
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14: 515-534
Geigy Chemical Company. (1956-67) Unpublished data and methods of
analysis in pesticide petitions, submitted to the U.S. Food and Drug
Administration
Geigy Chemical Company. (1969) Diazinon-deterioration-stabilization
and influence on toxicity. Unpublished report
Getzin, L. W. (1967) Metabolism of diazinon and zinophos in soils. J.
econ. Ent., 60: 505-508
Getzin, L. W. and Rosefield, I. (1966) Persistence of diazinon and
zinophos in soils. J. econ. Ent., 59(3): 512-516
Gomaa, H. M., Suffett, I. H. and Faust, S. D. (1969) Kinetics of
hydrolysis of diazinon and diazoxon. Residue Reviews, 29: 171-190
Gunner, H. B., Zukerman, B. M., Walker, R. W. Miller, C. W., Denbert,
K. H. and Longley, R. E. (1966) The distribution and persistence of
diazinon applied to plant and soil and its influence on soil
microflora. Plant Soil; 25: 249-264
Gunther, F. A., Ewart, W. H., Blinn, R. C., Elmer, H. S. and Wacker,
G. B. (1958) Field persistence comparisons of residues of the
insecticide diazinon in lemons and Valencia oranges and effects on
juice flavour. Agr. Fd. Chem., 6: 521
Harrison, D. L. and Hastie, B. A. (1965) Diazinon residues in the milk
of cows and fat of sheep after feeding on pasture treated with
diazinon. New Zealand J. agr. Res., 9: 1-7
Hastie, B. A. (1963) The metabolism and elimination of diazinon from
animals, animal tissues and foodstuffs. Geigy Agricultural Chemicals,
Australia (bulletin)
Hastie, B. A. (1965) Diazinon residues in sheep fat. Geigy
Agricultural Chemicals, Australia (unpublished)
Kaplanis, J. N., Louloudes, S. J. and Roan, C. C. (1962) Trans. Kansas
Acad. Sci., 65: 70-75
Maier-Bode, H. (1963) Residues of insecticides on cover crops growing
in orchards after application of organic phosphorus toxicants on the
trees. Z. Pflanzenkrankh. Pflanzenschutz, 70(80): 449-459
Maier-Bode, H. (1967) Untersuchungen über den Gehalt
landwirtschaftlicher und gärtnerischer Ernteprodukte an
Pflanzenschutzmittelrückständen. Der Ministerpräsident des Landes
Nordrhein-West., Landesamt für Forschung, Jahrbuch 1967, S. 391.
Margot, A. and Gysin, H. (1957) Diazinon, its degradation products and
their properties. Belv. Chim. Acta., 40: 1562
Mathysse, J. G. and Lisk, D. (1968) Residues of diazinon, coumaphos,
ciodrin, methoxychlor and rotenone in cow's milk from treatments
similar to those used for ectoparasite and fly control on dairy
cattle, with notes on safety of diazinon and ciodrin to calves. J.
econ. Ent., 61(5): 1394-1398
Melchiorri, P. Maffei F. and Siesto. A. J. (1964) Farmaco (Pavia), Ed.
Prat., 19: 610
Michel, H. O. (1949) J. lab. clin. Med., 34: 1564
Millar, K. R. (1963) Detection and distribution of 32P-labelled
diazinon in dog tissues after oral administration. New Zealand vet.
J., 11(6): 141-144
Mücke, W., Alt, K. O. and Esser, H. O. (1970) Degradation of
14C-labelled diazinon in the rat. J. Agr. Fd. Chem., 18(2): 208-212
Nakatsugawa, T., Tolman, N. M. and Dalm, P. A. (1969) Oxidative
degradation of diazinon by rat liver microsomes. Brochem. Pharmacol.,
18: 685-688
Nelson, L. L. and Hamilton, E. W. (1970) Metabolism of diazinon in
alfalfa. J. econ. Ent., 63(3): 874-878
Onsager, J. A. and Rusk, H. W. (1967) Adsorption and translocation of
diazinon and Stauffer N-2790 in sugarbeet seedlings. J. econ. Ent.,
60(2): 586-588
Painter, R. P. and Kilgore, W. W. (1963) Distribution of pesticides in
fermentation products obtained from artificially fortified grape
musts. J. Fd Sci., 28: 342-346
Pardue, J. R., Hansen, E. R., Barron, R. P. and Chen, J. J. (1970)
Diazinon residues on field-sprayed kale. Hydroxydiazinon - a new
alteration product of diazinon. J. Agr. Fd Chem., 18: 405-408
Rai, L. and Roan, C. C. (1959) Report included in Geigy Chemical
Company pesticide petition to the U.S. Food and Drug Administration
Ralls, J. W., Gilmore, D. R. and Cortes, A. (1966) Fate of radioactive
o,o-diethyl o-(2-isopropyl-4-methylpyrimidin-6-ol) phosphorothioate on
field grown experimental crops. J. Agr. Fd Chem., 14(4): 387-392
Ralls, J. W., Gilmore, D. R., Cortes. A., Schutt, S. H. and Mercer, W.
A. (1967) Residue levels of diazinon and its transformation products
on tomatoes, spinach and beans. Fd Tech., 21: 92-94
Robbins, W. E., Hopkins, T. L. and Eddy, O. W. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem., 5(7): 509-513
Robens, J. F. (1969) Teratologic studies of carbaryl, diazinon, norea
disulfiram and thiram in small laboratory animals. Toxicol. appl.
Pharmacol., 15: 152-163
Sethunathan, N. and MacRae, I. C. (1969) Persistence and
biodegradation of diazinon in submerged soil. J. Agr. Fd Chem., 17:
221
Sethunathan, N. and Yoshida, T. (1969) Fate of diazinon in submerged
soil. Accumulation of hydrolysis product. J. Agr. Fd Chem., 17(6):
1192-1195
Siesto, A. J., Maffei, F. and Melchiorri P. (1964) Estratto da
Archivio Italiano di Scienze Farmacologiche, Series 3, 13: 3
Storherr, R. W., Getz, M. E., Watts, R. R., Friedman, S. J., Erwin,
F., Giuffrida, L. and Ives, F. (1964) Identification and analyses of
five organo-phosphate pesticides. Recoveries from crops fortified at
different levels. J. Assoc. Off. Agr. Chem., 47(6): 1087-1093
Storherr, R. W. and Watts, R. R. (1965) A sweep co-distillation
cleanup method for organophosphate pesticides. J. Assoc. Off. Agr.
Chem., 48(6): 1154-1160
Vigne, J. P., Chouteau, J., Tabau, R.-L., Rancien, P. and Karamanian,
A. (1957) Sur le métabolisme d'un insecticide organo-phosphoré, le
diéthylthionophosphate de 2 isopropyl 4 méthyl 6 oxypyrimidine chez la
chèvre. Bull. Acad. Vét. Fr., 30: 85-92
