
DIAZINON
First draft prepared by
E. Bosshard
Federal Office of Public Health,
Schwerzenbach, Switzerland
EXPLANATION
Diazinon was previously evaluated by the Joint Meeting in 1963,
1965, 1966 and 1970 (Annex I, references 2, 3, 6, 14). An ADI of 0-
0.002 mg/kg bw was allocated in 1966, based on a NOAEL of 0.02 mg/kg
bw/day in human volunteers (Annex I, reference 7). The compound was
reviewed at the present Meeting on the basis of the CCPR periodic
review programme. This monograph summarizes the data received since
the previous evaluation and contains relevant data from the previous
monographs and monograph addenda.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
The fate of diazinon in various animal species was studied
after oral and topical applications using unlabelled and
radiolabelled diazinon in chicken, rats, guinea-pigs, dogs, sheep,
goats and cows. Additional studies were performed in vitro using
tissue slices or cell fractions from different tissues and various
species to investigate the biotransformation of the compound. A
short summary on the metabolism of diazinon has been published
(Miyamoto 1976).
Absorption, distribution and excretion
Oral studies
Rats
After the application of a single oral dose of 0.8 mg/rat (4
mg/kg bw) 14C-labelled diazinon (labels at 2-14C, 4-14C or ethoxy-
14C) to four male and two female Wistar rats, radioactivity was
eliminated practically completely during the 168-hours observation
period with either label. Excretion of applied radioactivity
amounted to 65-80% in urine, and 16-24% in faeces. The excretion
half-time was estimated to be 12 hours for the pyrimidine-labelled
and 7 hours for the ethoxy-labelled compound. After the application
of side-chain labelled material, about 6% of the applied dose was
eliminated as 14CO2. The absence of radioactive CO2 in the
expired air after application of ring-labelled diazinon shows that
no cleavage of the pyrimidine ring occurred (Mücke et al., 1970).
After feeding male rats during 10 days 0.1 mg/rat/day (0.5
mg/kg bw/day) of 2-14C-diazinon, 2.9% of the applied dose was found
in the essential organs 6 hours after the final application, whereas
2 days after cessation of treatment the radioactivity was below the
detection limit (< 0.1%). These results indicate that no
accumulation of diazinon or its metabolites occurs in the body
(Mücke et al., 1970; Annex I, reference 15).
In a more recent study, two groups of rats (5/sex/dose) were
treated with a single oral dose of 10 or 100 mg/kg bw 14C-diazinon.
A third group was treated daily with unlabelled diazinon at a dose
level of 10 mg/kg bw during 14 days, followed by the same dose of
14C-labelled diazinon on day 15. The elimination of the
radioactivity was monitored over a seven-day period. 14C-
elimination of radioactivity was rapid and occurred mainly via the
urinary tract. In the low-dose group, 93% of the applied
radioactivity was excreted in the urine in males and 86% in females
within 24 hours. At the high-dose level, an average of 91% and 58%
in males and females, respectively, was excreted in the first 24
hours. After preconditioning, about 90% of the 14C-dose was
excreted within 24 hours in both sexes. The total amounts
eliminated over the 7-day observation period did not differ between
the various dosing regimens. Total urinary excretion amounted to
96%, and faecal elimination to 3%. These results suggest that
complete absorption occurs following intragastric administration and
supports the hypothesis that the small amount of faecal
radioactivity found may be of biliary origin. Tissue concentrations
of 14C-diazinon and metabolites seven days after dosing were mostly
less than 0.05 ppm for low-dose and pre-conditioned animals. In
high-dose animals, residue levels in different organs varied between
0.1 and 0.4 ppm (red blood cells) with female rats showing
consistently slightly higher tissue residues than males. The 14C
in the blood was associated primarily with the cellular fraction,
suggesting that binding occurred (Capps et al., 1989; Craine
1989).
Guinea-pigs
32P-Labelled diazinon was administered orally or
subcutaneously to male guinea-pigs at a dose level of 45 mg/kg bw.
After oral dosing, 80% of the applied radioactivity was excreted in
the urine within 48 hours, whereas faecal excretion was 10% mostly
eliminated within the first day. After subcutaneous dosing, urinary
excretion was 53%, faecal excretion was minimal. Highest residues
were found in the caecum corresponding to 36% and 5% of the
radioactivity administered 16 hours after oral and subcutaneous
dosing, respectively. About 1-2% of the radioactivity was found in
the liver after 16 hours. During the 7-day observation period, over
87% of the dose was eliminated in the excreta, mainly in the urine,
indicating that the large amounts found in the caecum after oral
treatment ultimately left the body via the kidneys. The
accumulation of radioactivity in the caecum also following
subcutaneous injection indicates that this tissue may play a role in
metabolism or elimination of diazinon and/or its metabolites in
guinea-pigs (Kaplanis et al., 1962).
Hens
After oral administration of 14C-diazinon to 4 laying hens by
capsule for 7 consecutive days at daily doses of 2.8 mg/animal
corresponding to 25 ppm, 79% of the total dose applied was
eliminated in the excreta and 0.1% was found in the tissues at
sacrifice, about 24 hours after the final dose. Highest tissue
levels of 0.15 ppm were found in the kidney. Maximum residues in
eggs amounted to 0.07 ppm. The treatment caused some reduction in
body weight in most animals but had no influence on the general
health condition (Simoneaux, 1988b; Simoneaux et al., 1988;
Burgener & Seim 1988).
Dogs
In an intravenous study, 14C-ethoxy-labelled diazinon was
injected at a dose level of 0.2 mg/kg bw. After 24 hours, 58% of
the applied radioactivity was recovered in urine (Iverson et al.,
1975).
Goats
Two lactating goats were treated daily with 14C-diazinon by
capsule for four consecutive days at a dose of 150 mg/animal (4
mg/kg bw). Urinary excretion amounted to 64% of the applied dose,
whereas faeces contained an average of 10%. Highest residues of 2
ppm 14C were found in kidneys whereas the levels in the other
tissues ranged from 0.2 to 1.2 ppm. Highest levels in milk were
0.5 ppm (Pickles & Seim 1988; Simoneaux, 1988a,c; Simoneaux et al.,
1988).
Cows
A lactating cow was orally treated by capsule with a dose level
of 20 mg/kg bw of 32P-labelled diazinon. About 74% of the applied
dose was excreted in the urine within 36 hours and 6% in the faeces.
The cumulative percentage of total dose in milk 36 hours after
treatment was less than 0.08% (Robbins et al., 1957).
In summary, diazinon is rapidly and almost completely absorbed
and eliminated after oral application. Excretion occurs mainly via
the kidney. Diazinon or its metabolites do not accumulate in the
body.
Dermal studies
Rats
Groups of 4 rats were dermally treated with dose levels of 1 or
10 mg/kg bw 14C-diazinon dissolved in tetrahydrofuran. Renal
excretion ranged from 70-80% of the applied dose at both dose levels
over the 6 days observation period; elimination in faeces was
usually less than 10%. Most of the radioactivity was eliminated
within 48 hours after application. After 72 hours, less than 1% of
the applied dose was found on the skin. Highest tissue residues
were measured 8 hours after treatment varying between 0.1 and 0.4
ppm in the low-dose animals. Residues in the high-dose animals were
correspondingly higher. These results show that diazinon is easily
absorbed through the skin (Ballantine et al., 1984).
Sheep
Two sheep were dermally exposed for 3 days to diazinon at a
daily dose of 40 mg/kg bw. The sheep were sacrificed six hours
after the final application. Radiolabelled residues were observed
in all tissues at levels ranging from 2.2 to the highest value of 13
ppm in kidney. Because the study was designed to allow the
identification of metabolites in sheep tissue after topical
application of 14C-diazinon, no results were presented with respect
to elimination of the compound (Capps et al., 1990; Pickles & Seim
1990).
Biotransformation
In vitro studies
Diazinon was incubated with liver microsomes from different
avian species, rat, guinea-pig, pig, sheep and cow. Metabolites
identified included hydroxydiazinon, isohydroxydiazinon,
dehydroxydiazinon, their oxons and diazoxon. Yields and rates of
production of these metabolites varied between the different species
(Machin et al., 1975).
In vitro studies using microsomal preparations from rat liver
showed that diazinon was converted rapidly to water-soluble
metabolites (Dahm, 1970). The oxidation of diazinon by the
microsomal enzyme system fortified with NADPH or NADH occurred
through hydroxylation of the ring alkyl side chain, desulfuration,
and cleavage of the arylphosphate bound. The major metabolic
products of diazinon were hydroxydiazinon, diazoxon and
hydroxydiazoxon. Other metabolites identified were 2-isopropyl-4-
methyl-6-hydroxypyrimidine, 2-(2'-hydroxy-2'-propyl)-4-methyl-6-
hydroxypyrimidine, diethyl-phosphorothioic acid and
diethylphosphoric acid, which were all produced by the cleavage of
the arylphosphate bound (Shishido et al., 1972a). Diazoxon, the
active toxicant, formed by the oxidation of diazinon through
desulfuration was degraded mainly by hydrolysis resulting in the two
metabolites diethylphosphoric acid and 2-isopropyl-4-methyl-6-
hydroxypyrimidine (Shishido & Fukami 1972). A glutathione conjugate
S-(2-isopropyl-4-methyl-6-pyrimidinyl) glutathione was also
identified. This compound was formed by conjugation of reduced
glutathione and the pyrimidinyl moiety of diazinon with the
simultaneous cleavage of the phosphate ester bound (Shishido et
al., 1972b). In insects, diazoxon is degraded slowly by the
microsomal mixed function oxidase system, while no diazoxon-
hydrolyzing enzyme was found (Shishido & Fukami 1972; Yang et al.,
1971).
In vivo studies
Biotransformation of diazinon has been extensively studied in
numerous mammalian species and also in hens. Considerable breakdown
occurs in all species studied.
Rats
Metabolic studies in rats treated with 14C-labelled diazinon
(2-14C, 4-14C or ethoxy-14C labels) by the oral route (4 mg/kg bw)
showed that the parent compound is degraded rapidly yielding the 3
pyrimidinols, 2-isopropyl-6-methyl-4(1H)-pyrimidinone, 2-(alpha-
hydroxyisopropyl)-6-methyl-4(1H)-pyrimidinone and its beta isomer as
main urinary metabolites. A number of polar unidentified substances
was also found. In addition to small amounts of unchanged diazinon
the same metabolites were also found in faeces. Diazoxon as a
labile and transient intermediate was absent in the extracts of
urine and faeces. The absence of radiolabelled CO2 indicated that
no cleavage of the pyrimidine ring took place. The intravenous
application of these main metabolites revealed that the pyrimidinols
are further degraded yielding some unidentified polar metabolites
(Mücke et al., 1970).
In a more recent study, rats were treated with single oral
doses of 10 or 100 mg/kg bw of 14C-labelled diazinon. Another
group was preconditioned for 14 days with unlabelled diazinon and
then dosed with 10 mg/kg bw of 14C-labelled diazinon. A similar
metabolic pattern as in the above-mentioned study was found. In
urine, the 3 pyrimidinols accounted for 65% of the applied
radioactivity, whereas 15% consisted of polar non-identified
metabolites and trace amounts of diazinon (0.11%), hydroxydiazinon
(0.12%) and diazoxon (0.14%) (Capps et al., 1989).
Dogs
Metabolic studies in dogs were performed after oral and
intravenous administration of ring-labelled and ethoxy-labelled
diazinon, respectively. After i.v. application the 14C-ethoxy-
labelled diazinon, diethyl phosphoric acid (DEP) and diethyl
phosphorothioic acid (DETP) were detected in urine, whereas after
the oral dosing with the 14C-ring-labelled diazinon, the
pyrimidinols 2-isopropyl-6-methyl-4(1H)-pyrimidinone and 2-(alpha-
hydroxyisopropyl)-6-methyl-4(1H)-pyrimidinone could be identified.
In contrast to the results of an in vitro study (Shishido & Fukami
1972) no evidence was given from this dog study that the cleavage
of the ester bond was glutathione-mediated (Iverson et al., 1975).
Cows
In a metabolic study with cows treated with an oral dose of 20
mg/kg bw 32P-labelled diazinon, DEP and DETP were found as urinary
end products of diazinon metabolism (Robbins et al., 1957).
The pyrimidinols were also identified as the major metabolites
in urine and tissues of dermally treated sheep (Capps et al.,
1990) and orally treated goats and hens (Simoneaux 1988c,d;
Simoneaux et al., 1988). The respective glucuronides were also
identified in these species (Sachsse & Bathe, 1976; Simoneaux
1988c,d; Simoneaux et al., 1989; Capps et al., 1990).
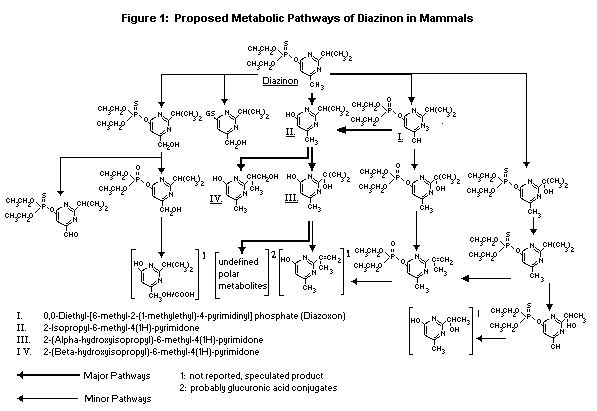
In summary, diazinon was found to be readily degraded and the
metabolites formed were mainly eliminated via the kidneys. The main
degradative pathway of diazinon in mammals includes the
oxidase/hydrolase-mediated cleavage of the ester bond leading
directly and via diazoxon to the pyrimidinol derivative 2-isopropyl-
6-methyl-4(1H)-pyrimidinone, which is further oxidized at the
isopropyl substituent resulting in the hydroxy pyrimidinols either
excreted as such or further degraded to more polar metabolites.
Metabolites with an intact pyrimidinyl phosphorus ester bond such as
hydroxydiazinon, diazoxon and its hydroxy derivative are only
transient products which are ultimately cleaved to their
corresponding pyrimidine analogs. The uncleaved products were found
only in in vitro studies (Hagenbuch & Mücke 1985).
Figure 1 presents the proposed metabolic pathway of diazinon in
mammals.
Toxicological studies
Acute toxicity studies
The results of the acute studies are summarized in Table 1.
Clinical signs of acute toxicity are consistent with those
caused by organophosphates including decrease of spontaneous
activity, sedation, dyspnea, ataxia, tremors, convulsions,
lacrimation and diarrhoea. The symptoms were reversible in
surviving animals.
The studies on the acute toxicity of diazinon were conducted
with technical grade material of 95.7-97.1% purity. Samples tested
before 1979 show a much higher acute toxicity particularly due to
the content of the highly toxic by-product tetraethyl-pyrrophosphate
(TEPP). Improvements in the manufacturing of diazinon did reduce
the content of toxic by-products (Annex I, reference 15, Ciba-Geigy
1992). WHO has classified diazinon as moderately hazardous (WHO,
1992).
Table 1. Acute toxicity of diazinon (technical material)
Species Sex Route LD50/LC50* Purity Reference
(mg/kg bw)/(mg/l) %
Mouse M,F oral 187 ? Bathe, 1972a
Rat M,F oral 422 97.1 Bathe & Gfeller, 1980
M,F oral 1250 ? Kuhn, 1989a
M,F oral 300 95.7 Piccirillo, 1978
M,F oral 1012 96.1 Schoch & Gfeller, 1985
Rat M,F dermal > 2150 ? Bathe, 1972b
Rat M,F inhalation 2.3* ? Holbert, 1989
Rabbit M,F dermal > 2020 ? Kuhn, 1989d
Irritation, sensitization
Diazinon (tech.) was evaluated for irritation potential in
rabbit eyes (Kuhn, 1989b) and skin (Kuhn, 1989c). The compound was
not considered as an irritant.
In a skin sensitization study in guinea-pigs, diazinon did not
reveal a sensitizing potential (Kuhn, 1989e).
Potentiation studies
Potentiation studies were conducted with chlordimeform,
jodfenphos, methacrifos and profenofos. No potentiation was
observed after combined application of equitoxic doses (Sachsse &
Bathe, 1975, 1976, 1977, 1978).
A marked decrease of the acute toxicity during the degradation
of diazinon was demonstrated by the studies on the pyrimidinol
metabolites 2-isopropyl-6-methyl-4(1H)-pyrimidinone and 2-(alpha-
hydroxyisopropyl)-6-methyl-4(1H)-pyrimidinone (Mücke et al., 1970;
Annex I, reference 15).
 Short-term toxicity studies
Although reported in the summaries of the following studies,
the inhibition of plasma cholinesterase was not utilized as a
criterion for the NOAEL, whereas inhibition of the erythrocyte
cholinesterase activity may be taken as an indicator of an adverse
effect of anticholinesterase pesticides. An inhibition of > 20%
compared to the control activity is regarded as significant (Dressel
et al., 1980; WHO, 1990).
Rats
Two short-term inhalation studies were conducted in rats. In a
first study groups of rats (9/sex/group) were exposed to an aerosol
of diazinon (purity 97.1%; droplet size < 1 µm 30-40%, 1-7 µm 50%)
for 6 hours a day, 5 days per week for three weeks. Only the
animals' snouts were exposed to the aerosol. The mean
concentrations were 0, 151, 245 or 559 mg/m3. The treatment did
not cause changes in the mortality rate, in haematology,
macroscopical and histopathological findings or organ weights that
were attributable to the inhalation of diazinon. Exophthalmus and
diarrhoea were observed in animals at all dose levels and tonic-
clonic muscle spasms occurred in the high-dose animals. The
symptoms were reversible. Food intake at the highest dose level was
reduced at the beginning of the treatment period. Body-weight gain
was reduced in male rats at 245 and 559 mg/m3, and in female rats
at 559 mg/m3. Plasma cholinesterase was inhibited at the
intermediate and high levels, resulting in values corresponding to
56% and 37% of the activity in control animals, respectively.
Erythrocyte cholinesterase was inhibited only at the highest dose
level (34% of the control activity). Brain cholinesterase activity
was dose-dependently reduced at all dose levels resulting in
activities of 81, 56 and 37% of the control activity in both sexes
at the low-, medium- and high-dose levels, respectively. The
cholinesterase values returned to normal at the end of the 25-day
recovery period. The NOAEL in this study was < 151 mg/m3
(corresponding to an estimated dose of 55 mg/kg bw/day), based on
lack of significant brain cholinesterase inhibition at the lowest
dose level (Zak et al., 1973).
In a more recent 21-day inhalation study, diazinon (purity 88%;
particle diameter 0.7-1.4 µm) was administered in a nose-only
exposure system in rats (10/sex/group). The intended aerosol
concentrations were 0, 0.1, 0.3, 1 or 10 mg/m3 (actual: 0, 0.05,
0.46, 1.57, 11.6 mg/m3). The animals were exposed during 6 hours a
day, 5 days per week for 3 weeks. The treatment did not adversely
affect body-weight gain, food consumption, haematological
parameters, organ weights, macroscopy and histopathological
findings. At 1 and 10 mg/m3, plasma cholinesterase activity was
reduced in females by 70% and 50% compared to control animals,
respectively. Erythrocyte cholinesterase was inhibited to 60% of
the control activity at 10 mg/m3 in both sexes, whereas the
activity of the brain cholinesterase was reduced in females only to
80% of the control activity at 1 mg/m3, and to 60% in the high-dose
animals. The increase of the relative lung weight observed in
females at 0.3 and 1 mg/m3 but not at the high dose was not
considered biologically significant. The NOAEL in this study was
0.46 mg/m3 (corresponding to an estimated dose of 0.2 mg/kg
bw/day), based on inhibition of brain cholinesterase at higher dose
levels (Hartmann, 1990).
Four groups of rats (Sprague-Dawley; Crl: VAF/Plus CD(SD)Br.;
15/sex/group) were orally treated with diazinon (purity 87.7%) at
dietary concentrations of 0, 0.5, 5, 250 or 2500 ppm for 90 days.
The treatment had no effect on mortality, water consumption,
ophthalmoscopy or urinalysis. Treatment-related occurrence of soft
faeces, hypersensitivity to touch and sound and in some male animals
aggressiveness were observed at the 2500 ppm dose level. Moreover,
hyperactivity was observed in several animals of the high-dose group
at the end of the study. Body-weight gain was reduced at 2500 ppm
by 6% in males and 13% in females compared to control animals. Food
consumption was reduced at 2500 ppm sporadically on single days in
both sexes. Haematological and biochemical investigations were
performed in the last study week. In the highest dose females,
slight reductions in haemoglobin and haematocrit values, as well as
an increase of the white blood cell count and reticulocyte count
were observed. A dose-dependent decrease in the mean serum
cholinesterase activity was observed in females and males at 5 ppm
(22% and 74%, respectively, of activity in control group), at 250
ppm (3% and 12% of the control activity, respectively) and at 2500
ppm (2% and 4% of the control activity, respectively). Erythrocyte
cholinesterase activity was reduced to 73% and 59% of control
activity at 250 and 2500 ppm in males and females, respectively. A
reduction in brain cholinesterase activity was found at 250 ppm in
females only (59%) and at 2500 ppm in both sexes resulting in
activities of 40%-50% of the control activity. An increase in
female liver and kidney weights was observed at 2500 ppm.
Microscopic evidence of centrolobular hepatocellular hypertrophy was
observed in single females at 250 ppm and in most females at 2500
ppm. The NOAEL in this study was 5 ppm, equal to 0.4 mg/kg bw/day,
based on erythrocyte and brain cholinesterase inhibition at 250 ppm
and higher (Singh et al., 1988).
Rabbits
In a dermal toxicity study, groups of albino rabbits
(5/sex/group) received dermal applications of 0, 1, 5 or 100 mg/kg
bw diazinon (purity 97.1%; 50% aqueous suspension in PEG 300), 5
days a week during a 3-week period. Because of high mortality in
males at 100 mg/kg bw (4/5 animals died between study days 3 and 6),
this dose level was reduced to 50 mg/kg bw on day 8 of the study.
In high-dose animals, signs of toxicity included anorexia, ataxia,
tremors, diarrhoea, hypoactivity, hypotonia and salivation. Body-
weight gain and food consumption tended to be higher in treated
animals compared to control animals. Serum cholinesterase was
inhibited in both sexes at 100/50 mg/kg bw resulting in values of
about 37% of the control group activity. At 5 mg/kg bw, activities
were 77% and 65% of the control activity in males and females,
respectively. At 1 mg/kg bw, inhibition was only found in females
resulting in an activity of 68%. Erythrocyte acetylcholinesterase
was inhibited only at 100/50 mg/kg bw resulting in 61% and 68% of
the control activity in males and females, respectively. Brain
acetylcholinesterase was also inhibited only at the highest dose
level of 100/50 mg/kg bw resulting in values corresponding to 72%
and 57% of the control activity in males and females, respectively.
The only change in organ weights was a decrease in kidney weight in
females at 100/50 mg/kg bw. No treatment-related macroscopic or
microscopic alterations were observed with the exception of slight
erythema at the application sites. Histologically a slight
hyperkeratosis was observed in treated skin of high-dose animals
The results of this study indicate a marked percutaneous absorption
and are thus in agreement with the results of other studies. The
NOAEL in this study was 5 mg/kg bw dermal, based on erythrocyte and
brain cholinesterase inhibition at 100/50 mg/kg bw (Ballantine et
al., 1984; Tai & Katz 1984).
Dogs
In a 90-day feeding study, diazinon (purity 87.7%) was
administered to groups of beagle dogs (4/sex/group) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
No treatment-related mortalities occurred. The treatment did not
adversely affect food consumption, ophthalmoscopy, haematology,
urinalysis, organ weights or macroscopic findings. Clinical signs
such as emesis or bloody faeces were sporadically observed in males
at 300 ppm and in females at 150 ppm. Reduced body-weight gain was
observed in females at 150 ppm and at 300 ppm in both sexes. In
males, inhibition of serum cholinesterase was observed resulting in
activities of 70%, 20% and 15% of that measured in control animals
at the end of the study at 0.5, 150 and 300 ppm, respectively.
Erythrocytes activities corresponding to 75% and 69% of control
activity were measured at 150 and 300 ppm, whereas in brain the
acetyl cholinesterase inhibition resulted in 69% and 58% of control
activity at 150 and 300 ppm, respectively. In females a reduction
in acetylcholinesterase activity was observed at 150 and 300 ppm.
The inhibition of serum and erythrocyte cholinesterase resulted in
an activity of about 18% and 70% of control activity, respectively,
at both dose levels, and brain cholinesterase inhibition resulted in
70% and 55% of control activity at 150 and 300 ppm, respectively.
Other changes of biochemical parameters consisted of a decrease in
total protein at 300 ppm in males. The only microscopic alteration
that might be compound-related was atrophy of the pancreatic acini
in one male dog of the highest dose group. The NOAEL in this study
was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on erythrocyte and
brain cholinesterase inhibition at 150 ppm and above (Barnes et
al., 1988).
Diazinon has been reported to produce pancreatic acinar lesions
in dogs at 75 mg/kg bw/day (Frick et al., 1987).
In a 52-week oral toxicity study, groups of beagle dogs
(4/sex/group) were fed diazinon (purity 87.7%) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
Due to lack of body-weight gain the dietary concentration of 300 ppm
was reduced to 225 ppm after 14 weeks of treatment (dose 300/225
ppm). Mortality was not increased by treatment and haematology,
urinalysis, gross pathology and histopathology revealed no changes
attributable to treatment. Overt clinical signs of dehydration and
emaciation became evident in one male at 300/225 ppm. The symptoms
remained even though the initial dose was reduced. Reductions in
body-weight gain were observed at 150 ppm and higher in males and at
300/225 ppm in females. However, no clear-cut dose-response
relationship was evident and the differences attained statistical
significance compared to the control group at only some observation
times. Food consumption was reduced at 150 ppm and higher, again
without a clear dose-response relationship, most probably due to
reduced palatability of the feed admixtures. Treatment-related
decreases in acetylcholinesterase activity at dose levels of 0.5 ppm
and higher were found: serum cholinesterase was reduced at 0.5 ppm
and higher in males and at 150 ppm and higher in females, resulting
in activities of about 20% of the activity in the control group at
150 and 300/225 ppm in both sexes. Erythrocyte cholinesterase
activity was also reduced at 150 and 300/225 ppm, where activities
corresponding to about 70% of the control activity were measured in
both sexes. Brain cholinesterase was inhibited at 150 ppm to 75% of
control activity in females and at 300/225 ppm to 65% and 75% of
control activity in females and males, respectively. The NOAEL in
this study was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on
erythrocyte and brain cholinesterase inhibition at 150 ppm and above
(Rudzki et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of mice (B6C3F1 Hybrid; 50 (control 25)/sex/group)
were administered diazinon (purity 98%) at 0, 100 or 200 ppm over
103 weeks. Survival, body weight, clinical signs and pathology were
investigated. The treatment had no effect on survival or body-
weight development. An increased incidence of hepatocellular
carcinomas was observed in the low-dose males only. The incidences
of hepatocellular carcinomas were 19, 43 and 21% in the control, low
and high-dose males, respectively, whereas the incidence of liver
adenomas was not increased in treated animals compared to control
animals. The mean incidences of hepatocellular carcinomas in
historical controls of this mouse strain (18 studies) were reported
to be 14% (max. 27%) for males and 3% (max. 10%) for females.
Concerning the total incidence of carcinomas and adenomas,
incidences of 29% (max. 58%) and 11% (max. 34%) in males and females
were reported (personal communication from Ciba Geigy to WHO, 1993).
Thus the incidence of hepatocellular carcinomas found in males of
the low-dose group markedly exceeded the range of historical control
incidences. Because no dose-response relationship was evident, the
result was most probably fortuitous and could not be interpreted as
providing evidence for a carcinogenic potential of diazinon in mice
(NCI, 1976).
Rats
Groups of rats (Fischer F344; 50 (control 25)/sex/group) were
fed diazinon (purity 98%) at dietary concentrations of 0, 400 or 800
ppm for 103 weeks. Survival, body weight, clinical signs and
pathology were the parameters investigated. There was no dose-
related reduction of survival and the body-weight development was
similar in control and treatment groups. Clinical signs of
hyperactivity were observed in treated males in both dose groups and
in high-dose females. The incidence of leukaemia was increased in
low-dose males (50%) compared to the incidence in the concurrent
control (20%) and high-dose males (24%). The leukaemias were of the
type usually seen in aging F344 rats. In females, the incidences of
leukaemia were also higher (12% at 400 ppm and 10% at 800 ppm)
compared to the concurrent controls (4%). Historical control
incidences of leukaemia were reported to be 48% for males (range 32-
62%) and 27% for females (range 14-15%). Because of the lack of a
dose-response relationship, the increase in tumour incidence was
considered of questionable significance and the results therefore
could not be interpreted as providing evidence for a tumorigenic
potential of diazinon in rats (NCI, 1976; NIEHS, 1991).
Diazinon (purity 87.7%) was administered as feed admixture to
groups of rats (Sprague-Dawley; 30-40/sex/dose) at concentrations of
0, 0.1, 1.5, 125 or 250 ppm (adjusted for purity) for up to 98-99
weeks. An additional vehicle control group was included in this
study. The animals of this dose group were treated with epoxidized
soybean oil. Up to 10 rats/sex/group were used for the interim
sacrifice after at least 52 weeks of treatment. Another number of
up to 10 rats/sex from the control and high-dose group were placed
on a four-week recovery period and sacrificed thereafter. The study
was terminated after 98-99 weeks because of decreased survivability
in the 0.1 ppm male group only, which was unrelated to treatment and
associated with age-related changes (e.g., senile nephropathy and/or
pituitary adenoma, both considered to be the result of senescence in
this rat strain). This earlier termination was not considered to
have affected the quality or integrity of the study because a
sufficient number of animals were at risk in the other treatment
groups for the development of tumours. The treatment did not cause
increased mortality, and did not affect ophthalmological or
haematological findings, urinalysis or organ weights. Body-weight
gain was increased in males at dose levels of 0.1 ppm and higher and
in some instances in females at 125 ppm and higher compared to the
untreated control animals. Because the body-weight gain in the
vehicle control group also showed an increase compared to the
untreated controls, the increases in body-weight may reflect an
increased palatability of the feed admixtures containing the
epoxidized soybean oil. In fact the increases in mean body-weight
gain generally coincided with increases in mean food consumption in
these dose groups. Decreases in serum cholinesterase activities
were observed at concentrations of 1.5 ppm and higher in both sexes,
resulting in values of about 50% of the control activity at the end
of the study. A dose-dependent reduction of erythrocyte
cholinesterase activity was observed at 125 and 250 ppm resulting in
activities of 80% and 75% of the control activity in males and
females, respectively, at either dose level at the end of the study.
Brain cholinesterase activity was also inhibited to 76% and 71% of
the control activity in males and females, respectively, at 125 ppm,
and to 58% and 52% of the control activity at 250 ppm in males and
females, respectively. Similar inhibition was observed after the
first year of the study. A slight reduction of less than 9% was
still found after the 4-week recovery period in erythrocyte and
brain cholinesterase activity at 250 ppm. Gross and microscopic
pathology examinations did not give evidence of any compound-related
lesions. The NOAEL in this study was 1.5 ppm, equal to 0.07 mg/kg
bw/day, based on inhibition of erythrocyte and brain cholinesterase
at 125 ppm and above (Kirchner et al., 1991).
Reproduction studies
Rats
Diazinon (purity 94.9%) was administered in the diet to groups
of rats (Sprague-Dawley CRCD; 30/sex/group) over two parental
generations (F0, F1) and up to weaning of the F2 pups at
concentrations of 0, 10, 100 or 500 ppm. Treatment-related clinical
symptoms and deaths were observed in a few high-dose females. They
consisted of tremors (3/30) and dystocia (2/30) followed by death or
sacrifice in the F0 generation. In the F1 generation, a few high-
dose females showed tremors as only compound-related signs. Food
consumption was increased in females of the 500 ppm group in the F0
generation, whereas in the F1 generation, a dose-related reduction
of food consumption was observed at 100 and 500 ppm in males only.
Body-weight gain was lower in F0 females at 500 ppm during
gestation. In the F1 generation, reduced body-weight gain was
observed at 100 and 500 ppm in males and at 500 ppm in females.
There were no treatment-related effects on mating behaviour and the
reproductive parameters (including mating, fertility, gestation
indices, number of viable pups, and number of stillborn pups) were
comparable in control and treatment groups in the F0 generation and
at the low and intermediate dose level of the F1 generation. At
500 ppm, a greater proportion of females showed a prolonged
gestation duration at both generations. A decrease in the number of
pregnancies and viable pups as well as reduced fertility and mating
indices were observed in high-dose females of the F1 generation. A
dose-related reduction in survival of F1 pups was observed at 100
ppm and 500 ppm and in F2 at 500 ppm. Weights of F0 pups were
reduced in the 100 and 500 ppm groups, and in F2 pups at 500 ppm.
No treatment-related malformations were found in the pups. The
NOAEL in this study was 10 ppm, equivalent to 0.5 mg/kg bw/day,
based on reduced parental body-weight gain and reduced viability of
pups and pup weights at 100 ppm and higher (Giknis 1989).
Special studies on embryotoxicity/teratogenicity
Mice
Groups of mice (6 females/dose) were given oral daily doses of
0, 0.18 or 9 mg/kg bw diazinon throughout gestation. Treated
animals gained less weight during gestation compared to control
animals. Weight gain of pups born to mothers receiving 9 mg/kg
bw/day was reduced. Daily testing for physiological and behavioural
development of the pups revealed some evidence of retarded
development among the offspring of high-dose animals (e.g.,
retardation of eye and ear opening). Measures of endurance and
coordination also gave some evidence of impairment (e.g., increased
rod cling endurance in both groups, reduced rotarod endurance,
impaired running performance in a maze inclined plane test).
Impaired reactions were sometimes observed at both dose levels but
without a clear dose-effect relationship. Examination of brain
tissue of only 8 of a total number of 132 offspring at the high-dose
level revealed morphological abnormalities in the forebrain. The
relationship of these findings to the observed behavioural changes
is unknown (Spyker & Avery 1977). The evidence for morphological
effects of diazinon on the developing brain was considered
insufficient by an expert (Krinke, 1991).
Rats
In a teratogenicity study in Sprague-Dawley rats at dose levels
of 0, 15, 50 or 100 mg/kg bw/day administered orally on days 6
through 15 of gestation, dams at the 100 mg/kg bw/day dose level
showed a marked decrease in food consumption correlating with weight
loss at the beginning of the treatment period. Skeletal assessment
showed a slightly higher incidence of incomplete ossification at
different sites in the fetuses at 100 mg/kg bw/day. Visceral
examination revealed a dystopia cordis in association with
hypoplasia of lungs in 1/105 fetuses at 100 mg/kg bw/day. This
anomaly has been reported to occur spontaneously in control animals.
Because of the single occurrence this anomaly was not considered to
be due to a direct action of diazinon but to be secondary to
maternal toxicity (Fritz, 1974).
In a later study, groups of rats (Charles River Crl. COBS
CD(SD)(BR); 27 females/group) were treated with doses of diazinon
(purity 97.4%) at 0, 10, 20 or 100 mg/kg bw/day by gavage during
gestational days 6 through 15. The treatment had no effect on the
mortality of the dams. During the first half of the dosing period,
the dams lost weight and food consumption was reduced at 100 mg/kg
bw/day. No compound-related clinical signs and no abnormal gross
pathological findings were observed in the dams. At 100 mg/kg
bw/day some reproductive parameters differed from the control values
but no statistical significance was achieved (e.g., increase in
number of resorptions, percent pre- and post-implantation loss,
reduction in number of live fetuses). On gross observation, 3/262
(1%) fetuses from 3 litters showed external malformations (single
occurrences of a umbilical hernia, filament tail, sublingual
extraneous soft tissue), whereas in the concurrent controls no
similar effects were observed. Umbilical hernia and tail
abnormalities however are reported to be spontaneous malformations
observed routinely in this rat strain. Concerning skeletal
variations, an increased incidence in rudimentary ribs (T-14) was
observed in all treatment groups attaining statistical significance
in the high-dose group (5% versus 0%). Because of the single
occurrences of the different malformations observed and because the
malformations also occur in control animals they were considered to
be a consequence of the marked maternotoxicity at this dose level,
and not due to a teratogenic effect of the test compound. The
increased incidence of the skeletal variation observed at the top
dose level was considered to be related to maternotoxicity at this
dose level. The NOAEL in this study was 20 mg/kg bw/day based on
maternotoxicity and fetotoxicity at the higher dose level (Infurna &
Arthur, 1985).
Rabbits
Diazinon (purity 89.2%) was administered to groups of pregnant
rabbits (New Zeeland white; 18-22 females/group) by gavage at dose
levels of 0, 7, 25 or 100 mg/kg bw/day from days 6-18 of gestation.
An increase in maternal mortality was observed at 100 mg/kg bw/day
(41% vs 0% in all other groups). Overt clinical signs of maternal
toxicity at 100 mg/kg bw/day included tremors, convulsions,
hypoactivity and anorexia. Reduced body-weight gain was found at
the highest dose level. The treatment did not influence the number
of corpora lutea, number of implantation sites, live fetuses per
litter or fetal weight. The incidence of visceral and skeletal
malformations and variations showed no differences between the
groups that could be attributed to treatment. The study therefore
gave no evidence for an embryotoxic or teratogenic activity of
diazinon. The NOAEL in this study was 25 mg/kg bw/day based on
materno-toxicity at the highest dose level (Harris & Holson, 1981).
Chickens
The teratogenic effects of some anticholinesterase insecticides
(organophosphates and methylcarbamates) on chicken embryos have been
known for some time. In a first study, eggs were injected with
diazinon to explore some of the biochemical variables that might be
involved in producing micromelia, beak and feather deformities (type
I signs) in avian embryos that are known to be caused by OP
insecticides. The results of this study confirm the assumption that
a tryptophan deficiency (tryptophan plays an important role in the
developing embryo) may cause the abnormalities observed (Kushaba-
Rugaaju & Kitos, 1985).
Another study was conducted to investigate the involvement of
cholinergic functions in OP-induced teratogenesis in chick embryos
by examining the effects of diazinon on the developmental pattern of
AChE and ChAT activities in hindlimb, wing and brain. The results
of this study confirmed that type II teratogenesis (wry neck, short
neck, arthrogryposis and muscular hypoplasia of legs) may be
mediated via cholinergic nicotinic receptors. Cholinergic
dysfunction including decreased activity of AChE and ChAT was not
correlated with diazinon-induced type I teratogenesis (micromelia,
abnormal feathering) (Misawa et al., 1981).
Cattle
As part of a survey to determine the causes of abortion in
dairy cattle in Wisconsin, the potential role of pesticides was
examined. Diazinon in the form of wettable powder was orally
administered to two pregnant cows at a daily dose level of 6.6 mg/kg
bw. The continuous treatment started in the 5th and 6th month of
pregnancy (abortion commonly affects cows in the 5th to 8th month of
pregnancy) and was maintained until parturition. No diazinon
residues were detected in the tissues examined (fat, liver, kidney)
nor in milk. The treatment failed to reveal any abortions (Macklin
& Ribelin 1971).
Special studies on genotoxicity
Diazinon has been adequately tested in a series of genotoxicity
assays. The results are summarized in Table 2.
Special study of effects on the pancreas
A study was conducted using a canine model to investigate the
induction of pancreatic ductal hypertension following cholinesterase
inhibitor intoxication known to be an important triggering mechanism
in the pathogenesis of acute and chronic pancreatitis. Diazinon was
intravenously administered (25 mg/kg bw) to the pancreatic ampulla
of dogs and the tissue cholinesterase activity of the canine
pancreatic sphincters was determined. Two enzymes are responsible
for the total cholinesterase activity of whole blood: a membrane-
bound AChE associated with the erythrocyte membrane, and a soluble
enzyme, pseudocholinesterase or butyrylcholinesterase (BChE) in the
serum. In tissues, which also contain the two forms of
cholinesterase, AChE is important in the regulation of neuromuscular
activity and parasympathetic ganglion transmission, while the role
of BChE is unknown. The observation of the negative correlation
between serum BChE activity and intraductal pancreatic pressure
supports the hypothesis that the pancreatic ductal hypertension
which occurs following cholinesterase inhibitor intoxication is due
to a selective reduction in pancreatic BChE activity (Dressel et
al., 1980).
The induction of acute pancreatitis by OPs found in dogs was
confirmed in guinea-pigs but not in cats. These results may reflect
species-related differences in the distribution of pancreatic BChE
(Frick et al., 1987).
Special studies on antidotes
Previous reports with respect to the usefulness of PAM and
other oxine reactivators against this organophosphate have been
published (Sanderson & Edson, 1959; Wills, 1959). A recent study
was undertaken to provide information on the antidotal effectiveness
of pyridine-2-aldoxime methochloride (2-PAM) against diazinon
poisoning in animals. The administration of atropine (16 mg/ kg bw;
i.m.) or 2-PAM (30 mg/kg bw; i.v.) alone 10 minutes after poisoning
rats with doses of 235 mg/kg bw corresponding to the approximate
oral LD50 or higher provided little or no protection. Best
protection was achieved when the oxime was given orally in
conjunction with atropine (i.m.) or followed by a subsequent dose of
2-PAM orally or i.v. Administration of 2-PAM to diazinon-poisoned
rabbits (1600 mg/kg bw) resulted in reactivation of inhibited blood
ChE activity concurrent with a decrease in signs of poisoning.
Within 2 hours, however, the animals were again weak and ataxic and
blood ChE showed renewed inhibition. The authors suggested that
effective therapy of diazinon intoxication requires repeated doses
of oxime to maintain effective antidote levels in the body (Harris
et al., 1969). In dogs and guinea-pigs, pretreatment with
atropine protected the animals against diazinon-induced pancreatitis
(Frick et al., 1987).
Special study on delayed neurotoxicity
Hens
An oral dose of 28 mg/kg bw/day of diazinon technical (87%
purity) was administered to a group of 18 hens (the target dose of
13 mg/kg bw/day as the approximate LD50 was doubled due to a
preparation error). The schedule to protect the hens from acute
cholinergic effects of diazinon consisted of a 10 mg/kg bw atropine
pretreatment (i.m.). At the time of diazinon dosing, 2-PAM at an
i.m. dose of 50 mg/kg bw was administered. Post-treatment consisted
of concurrent doses of atropine and 2-PAM one and five hours
following dosing. Since there were no neurotoxic responses in the
test group in the three weeks following the treatment they were
again treated with 13 mg/kg bw of diazinon on day 21. One test
group hen was found dead on day 5 after the first dosing, and one
hen six hours after the second treatment. One hen exhibited a
slight unsteadiness in walking only on day 41. No neurotoxic signs
became apparent during the three-week observation period after the
second treatment. Histopathological examination revealed no lesions
in the brain, spinal cord or peripheral nerves in diazinon-treated
animals, whereas animals of the positive control (TOCP treatment)
showed multiple lesions (axonal degeneration) consistent with
peripheral neuropathy (Jenkins, 1988).
Observations in humans
Numerous reports of intentional and accidental intake of
diazinon and other OPs have been published describing the clinical
manifestations of organophosphate toxicity and the usefulness of
erythrocyte and serum cholinesterase activity assays for diagnosis
as well as the efficacy of supportive and specific therapies
(Kabrawala et al., 1965; Payot, 1966; Banerjee, 1967; Gupta &
Patel, 1968; Zwiener & Ginsburg, 1988).
A case of acute diazinon poisoning complicated by pericarditis
and pneumonia followed by recovery has been reported. The
intoxication caused the occurrence of cyanosis, tracheobronchial
congestion, pulmonary edema and pneumonia. The treatment included
the intramuscular injection of atropine (Banerjee, 1967).
Another complication reported following accidental ingestion of
diazinon consisted of severe pancreatitis and a pseudocyst (Dressel
et al., 1979). The induction of pancreatitis was reproduced in
dogs treated intravenously with a dose of 5 mg/kg bw diazinon.
Pancreatitis may be the result of hypersecretion and ductal
obstruction (Dressel et al., 1980).
Table 2. Results of genotoxicity assays on diazinon
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
In vitro
Ames test S. typhimurium 313-5000 µg/0.1 ml 88.0% negative negative Geleick & Arni, 1990
TA 98, 100, 1535, 1537 DMSO
E. coli WP2uvrA
Ames test S. typhimurium in DMSO >50-1000 ? negative negative Marshall et al., 1976
TA 1535, 1536, 1537, 1538 µg/plate
Mouse lymphoma Mouse lymphoma cell 1. 6 to 60 µg/ml with 97.2% negative negative Dollenmeier & Müller,
assay L5178Y/TK+/- activation 1986
2. 12 to 120 µg/ml
without activation
Mouse lymphoma Mouse lymphoma cell up to 100 µg/ml ? positive positive McGregor et al., 1989
assay L5178Y TK+/-
Sister chromatid Chinese hamster cells 10, 20 and 40 µg/ml 99.2% negative Chen et al., 1981
exchange study (V79) 99.2% in DMSO
Chromosomal Chinese hamster lung 0.1 mg/ml ? cytotoxic positive Matsuoka et al., 1979
aberration test cells
Sister chromatid Human lymphoid cells 0.02, 0.2, 2.0 and ? negative negative1 Sobti et al., 1982
exchange study (LAZ-007) 200 µg/ml
in Ethanol (< 0.1%)
Sister chromatid Human lymphoid cells 12.5, 25, 50, 100 and 87.5% negative negative Strasser & Arni, 1988
exchange study (CCL-156) 200 µg/ml in DMSO
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Whole blood human 1. 0.0668 to 2000 µg/ml 88.0% equivocal equivocal2 Murli & Haworth, 1990a
exchange study lymphocytes in DMSO (ħ activation)
2. 0.668 to 20 µg/ml
(non-activation)
3. 2-66.8 µg/ml
(activated)
Autoradiographic Rat hepatocytes 1.1 to 120 µg/ml in 88.0% negative Hertner & Arni, 1990
DNA repair test DMSO
In vivo
Micronucleus test Mouse 1. 120 mg/kg bw oral 87.5% negative Ceresa, 1988
in vivo 2. 30, 60, and
120 mg/kg bw oral
Dominant lethal Mouse oral dose of 14 and 45 95% negative Fritz, 1975
study mg/kg bw
Chromosome studies Mouse a) daily oral doses of ? negative a) Hool & Müller, 1981c
in male germinal a) spermatogonia 10.5, 21 and 63 mg/kg b) Hool & Müller, 1981b
epithelium b) spermatocytes bw for five days, or
b) 5 treatments with
the same doses over
a period of 10 days
Sister chromatid Bone marrow cells single oral doses of 88.0% negative Murli & Haworth, 1990b
exchange study of mice 10, 50 and 100
in vivo mg/kg bw
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Chinese hamster bone oral gavage of 6.5 ? negative Hool & Müller, 1981a
exchange study marrow cell to 26 mg/kg bw
Nucleus anomaly Chinese hamster bone oral gavage of ? negative Hool & Müller, 1981d
test marrow cells 6.5, 13 and 26 mg/kg
bw/day for two days
1 Cyotoxicity at a concentration of 20 µg/ml. The increased frequency of sister chromatid exchanges at 20 µg/ml with
metabolic activation did not show a doubling of the control frequency and was therefore not considered positive.
2 Two independent trials: Cytotoxicity at concentrations of 66.8 µg/ml (with activation) respectively. Increases in the SCE
frequency (just doubling) observed only in the first assay without metabolic activation at dose levels of 0.668 µg/ml to
20 µg/ml, but without a dose-relationship. Increases observed after activation in both trials did not show doubling and again
no dose-response. Thus the results of these studies are not considered positive.
A study of 60 poisoning cases with diazinon has been reported.
The most common clinical manifestations were vomiting, giddiness,
constricted pupils and signs of bronchoconstriction with pulmonary
congestion. The amount of poison ingested varied from 4 ml to 15 ml
with an average of 7.5 ml (% active ingredient not specified in
publication). This was ingested by 55 patients with suicidal
intention, whereas in 5 patients the compound was taken
accidentally. Atropine injections were administered repeatedly (up
to a total dose of 22.4 g over 42 hours). Five patients died in
spite of intensive atropine therapy initiated more than 8 hours
after ingestion. Other cases who had ingested the same amount of
poison as the fatal cases received treatment within three hours of
ingestion. These findings emphasize the importance of early
treatment in diazinon poisoning (Gupta & Patel, 1968). In addition,
these results are in agreement with an earlier observation that the
combination of diazinon with cholinesterase occurs in two stages, an
early reversible stage and a late irreversible stage (Grob, 1956).
In another study, cases of organophosphate and carbamate
poisoning in 37 infants and children were reported, only 5 of which
were associated with diazinon. Miosis, excessive salivation, muscle
weakness, respiratory distress, lethargy and tachycardia were the
most common clinical findings. Erythrocyte cholinesterase
activities were determined from 24 patients showing erythrocyte
cholinesterase activities that were less than 50% of the lower limit
of the normal range. There were no differences between the
erythrocyte and serum cholinesterase activity in 20/24 patients from
whom both tests were obtained, whereas in the remaining 4 cases
either the erythrocyte or the serum activity was decreased. A
combined atropine/pralidoxime therapy is recommended in case of
organophosphate toxicity. Although the recommended dose for
atropine in infants is 0.01-0.02 mg/kg bw, the dose is generally
insufficient for treatment of signs and symptoms secondary to
organophosphate poisoning (Zwiener & Ginsburg, 1988).
Neurobehavioural effects of short-term, low-level exposure to
diazinon were investigated in 99 pest control workers. A computer
assisted neuro-behavioural test battery (including attention,
vigilance, hand-eye coordination, visual perception, verbal ability)
was used before and after their work shift. The diazinon metabolite
diethyl-thiophosphate (DETP) was measured in urine samples collected
from 46 diazinon applicators and 56 non-applicators. Post-shift
DETP values were 24 and 3 ppb for applicators and non-applicators,
respectively. The study failed to demonstrate any behavioral
effects of diazinon under the conditions of this study (Maizlish et
al., 1987).
The results of the following old study have been reported in an
earlier monograph (Annex I, reference 7). Because of certain
inaccuracies in that monograph, the study was reviewed again and
summarized below.
A subacute oral toxicity study was conducted in four male
volunteers. Diazinon (different batches, purity 95.4-95.7%) was
administered in capsules at dose levels of 2-2.5 mg per day per
person, equal to a dose of 0.025 mg/kg bw/day. The test period
lasted 42 days with an interruption in treatment from the 5th to
10th day in two volunteers and for 34 consecutive days in the other
two volunteers. Plasma cholinesterase activity was markedly
depressed the first 6 days of treatment in 2/4 subjects (no plasma
cholinesterase activity measurable). Therefore treatment was
interrupted for 6 days to enable recovery. Subsequent treatment at
the same dose level did not reveal inhibitory effects on plasma
cholinesterase. The fluctuations observed during the treatment
period were similar to those observed during the pre-test period.
In no instance was the erythrocyte acetylcholinesterase depressed
compared to the pre-test values. Other parameters investigated
included haematology and blood chemistry, urinalyses and
symptomatology. No changes were observed that could be attributed
to the treatment. The NOAEL in this study was 0.025 mg/kg bw/day
based on transitory depression of plasma cholinesterase activity as
the only effect observed at this dose level (Payot, 1966).
COMMENTS
Following oral administration to rats, diazinon was almost
completely absorbed and eliminated, mainly in the urine.
The main degradative pathway includes the oxidase/hydrolase-
mediated cleavage of the ester bond leading to the pyrimidinol
derivative 2-isopropyl-6-methyl-4(1H)-pyrimidinone, which is further
oxidized to more polar metabolites.
Diazinon has moderate acute oral toxicity to mice and rats.
The clinical signs observed were consistent with cholinesterase
inhibition and included sedation, tremors, convulsions and ataxia.
It has been classified by WHO as moderately hazardous.
In an oral 90-day feeding study in rats at dietary
concentrations of 0, 0.5, 5, 250 or 2500 ppm, the NOAEL was 5 ppm
(equal to 0.4 mg/kg bw/day), based on erythrocyte and brain
cholinesterase inhibition at 250 ppm and higher.
In short-term studies in dogs, diazinon was administered at
dietary concentrations of 0, 0.1, 0.5, 150 or 300 ppm for either 90
days or 52 weeks. In both studies, the NOAEL was 0.5 ppm, equal to
0.02 mg/kg bw/day based on erythrocyte and brain cholinesterase
inhibition at 150 ppm and above.
In a carcinogenicity study in mice, diazinon was administered
at dietary concentrations of 0, 100 or 200 ppm over 103 weeks.
There was no evidence of carcinogenicity.
In a carcinogenicity study in rats diazinon was administered at
dietary concentrations of 0, 400 or 800 ppm for 103 weeks. There
was no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study, rats were
maintained on a diet containing diazinon at concentrations of 0,
0.1, 1.5, 125 or 250 ppm for up to 99 weeks. The NOAEL was 1.5 ppm,
equal to 0.07 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase at 125 ppm and above. There was no evidence of
carcinogenicity.
A multigeneration study was conducted in rats using dietary
concentrations of 0, 10, 100 or 500 ppm. The NOAEL was 10 ppm
(equivalent to 0.5 mg/kg bw/day), based on a reduction in parental
body-weight gain in the F1 generation and a reduced survival rate
and reduced body weight of F1 pups at 100 ppm.
In a teratogenicity study in rats, diazinon was orally
administered at dose levels of 0, 10, 20 or 100 mg/kg bw/day.
Maternal toxicity, indicated by weight loss correlating with reduced
food consumption, became evident at 100 mg/kg bw/day. Effects on
the fetuses at this dose level consisted of retarded ossification
and an increased incidence of rudimentary ribs. The NOAEL was 20
mg/kg bw/day based on maternotoxicity and fetotoxicity. There was
no evidence of teratogenicity.
A teratogenicity study in rabbits conducted with oral dose
levels of 0, 7, 25 or 100 mg/kg bw/day revealed clinical signs of
maternotoxicity, increased mortality and reduced body-weight gain at
100 mg/kg bw/day. The NOAEL was 25 mg/kg bw/day. There was no
evidence of teratogenicity.
A neurotoxicity study performed with hens treated at dose
levels of 13 or 28 mg/kg bw/day (protected by atropine pre-
treatment) did not reveal evidence of delayed neurotoxicity.
Diazinon has been adequately tested in a series of genotoxicity
assays. Chromosomal aberrations were induced in cultured mammalian
cells, but there were no other indications of genotoxicity. The
Meeting concluded that diazinon was not genotoxic.
Diazinon was evaluated in four human male volunteers who
received 0.025 mg/kg bw/day of diazinon in capsules for 34-36 days.
There were no consistent treatment-related effects on plasma or
erythrocyte cholinesterase activity, blood chemistry or urinalysis.
No clinical effects were reported. The NOAEL was 0.025 mg/kg
bw/day.
The ADI of 0-0.002 mg/kg bw, which was based on the NOAEL of
0.025 mg/kg bw/day in the study in humans using a 10-fold safety
factor, was maintained.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm, equal to 0.4 mg/kg bw/day (90-day study)
1.5 ppm, equal to 0.07 mg/kg bw/day (99-week study)
10 ppm, equivalent to 0.5 mg/kg bw/day
(reproduction study)
20 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 25 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 0.5 ppm, equal to 0.02 mg/kg bw/day (one-year study)
Human: 0.025 mg/kg bw/day (34-36-day study)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ballantine, L., Marco, G.J. & Williams, S.C. (1984) Percutaneous
absorption of 2 delta - 14C - Diazinon in Rats. Numbers ABR-84011,
302950. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Banerjee, D. (1967) Pericarditis in acute diazinon poisoning. A case
report. Armed Forces Med. J. (India), 23: 187-190.
Barnes, T.B., Arthur, A.T. & Hazelette, J.R. (1988) Diazinon (MG-8):
90-Day Oral Toxicity Study in Dogs. Number 882012. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Bathe, R. (1972a) Acute oral LD50 of technical diazinon in the
mouse. Number Siss 1679. Unpublished Report Prepared by Ciba-Geigy
Ltd., Sisseln, Switzerland.
Bathe, R. (1972b) Acute dermal LD50 of technical diazinon (G-24480)
in the rat. Number Siss 1679. Unpublished Report Prepared by Ciba-
Geigy Ltd., Sisseln, Switzerland.
Bathe, R. & Gfeller, W. (1980) Acute oral LD50 in the rat of
technical G 24480. Number 800478. Unpublished Report Prepared by
Ciba-Geigy Ltd., Sisseln, Switzerland.
Burgener, J. & Seim, V. (1988) Biological report for the metabolism
of 14C-diazinon in Laying Hens Dosed at 25 ppm. Numbers BIOL-88006,
302213. Unpublished Report Prepared by Ciba-Geigy Corp., Vero Beach,
Florida.
Capps, T., Brown, K., Lai, K. & Tweedy, B. (1989) Characterization
and identification of diazinon metabolites in rats. Numbers ABR-
88164, 302211 and 302925. Unpublished Report Prepared by Ciba-Geigy
Corp., Greensboro, NC.
Capps, T. M., Summer, D., Bar, H.P. & Carlin, T.J. (1990)
Characterization and identification of major metabolites in tissues
of sheep treated dermally with 14C-diazinon. Numbers ABR-90014,
302925. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Ceresa, C. (1988) G 24480: Micronucleus test (mouse). Number 871696.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H.H., Hsueh, J.L., Sirianni, S.K. & Huang, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutation Research, 88: 307-316.
Ciba-Geigy 1992: Diazinon Data Summaries. Working Paper for the WHO
Expert group on Pesticide Residues. Biological Data and
Toxicological Evaluation. June 23.
Craine, E.M. (1989) Study of 14C-diazinon disposition in the rat
(biological phase and analytical phase I). Numbers WIL-82030,
302925. Unpublished Report Prepared by WIL Research Laboratories
Inc., Ashland, OH and Ciba-Geigy Corp., Greensboro, NC.
Dahm, P.A. (1970) Some aspects of the metabolism of parathion and
diazinon. In: Biochemical Toxicology of Insecticides. ed. by
O'Brien, R.D. and Yamamoto, I., Academic Press London.
Dollenmeier, P. & Müller, D. (1986) G 24480 techn: L 5178Y/T+/-
mouse lymphoma mutagenicity test. Number 840396. Unpublished Report
Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Dressel, Th. D., Goodale, R.L., Borner, J.W. & Etani, S. (1980) A
study of the cholinesterase of the canine pancreatic sphincters and
the relationship between reduced butyrylcholinesterase activity and
pancreatic ductal hypertension. Annals of Surgery, 192/5: 614-619.
Dressel, T.D., Goodale, K.L., Arneson, M.A. & Borner, J.W. (1979)
Pancreatitis as a complication of anticholinesterase insecticide
intoxication. Annals of Surgery, 189: 199-204.
Frick, T.W., Dalo, S., O'Leary, J.F., Runge, W., Borner, J.W.,
Baraniewski, H., Dressel, T., Shearen, J.G. & Goodale, K.L., (1987)
Effects of insecticide, diazinon, on pancreas of dog, cat and
guinea-pig. J. Environ. Path. Toxicol. Oncol. 7(4): 1-11.
Fritz, H. (1974) G 24480 (Diazinon Techn.): Reproduction study - rat
segment ii: (test for teratogenic on embryotoxic effects).
Unpublished report prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Fritz, H. (1975) G 24480 (Diazinon Techn.): Dominant lethal study
mouse. Number 327507. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Geleick, D. & Arni, P. (1990) Salmonella and Escherichia/liver-
microsome test. Number 891346. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Giknis, M.L.A. (1989) Diazinon techn.: A two-generation reproductive
study in albino rats. Numbers MIN-852218, 88128. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Grob, D. (1956) The manifestations and treatment of poisoning due to
nerve gas and other organic phosphate anticholinesterase compounds.
A.M.A. Arch. Intern. Med. 98: 221.
Gupta, O.P. & Patel, D.D. (1968) Diazinon poisoning. A study of
sixty cases. J. Assoc. Physicians of India, 16: 457-463.
Hagenbuch, J.P. & Mücke, W. (1985) The fate of diazinon in mammals.
Biochemistry Product Evaluation 1/85. Unpublished Report Prepared by
Ciba-Geigy Ltd., Basle, Switzerland.
Harris, S. B. & Holson, J.F. (1981) A teratology study of diazinon
in New Zealand white rabbits. Number 281005. Unpublished Report
Prepared by Science Applications Inc., La Jolla, CA.
Harris, L.W., Fleisher, J.H., Innerebner, T.A., Cliff, W. & Sim,
V.M. (1969) The effects of atropine-oxime therapy on cholinesterase
activity and the survival of animals poisoned with 0,0-diethyl-0-(2-
isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate. Toxicol. Appl.
Pharmacol. 15: 216-224.
Hartmann, H.R. (1990) G 24480: 21-Day repeated exposure inhalation
toxicity in the rat (nose-only exposure). Number 891205. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Arni, P. (1990) G 24480: Autoradiographic DNA repair
test on rat hepatocytes, Number 891345. Unpublished Report Prepared
by Ciba-Geigy Ltd., Basle, Switzerland.
Holbert, M.S. (1989) Diazinon MG8 FL 880045: acute inhalation
toxicity study in rats. Number 5947-89. Unpublished Report Prepared
by Stillmeadow Inc., Houston, Texas.
Hool, G. & Müller, D. (1981a) G 24480: Sister-chromatid exchange
study Chinese hamster. Number 801504. Unpublished Report Including
Supplement to the Report (dated October 13, 1981) Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981b) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatocytes. Number 801502. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981c) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatogonia. Number 801501. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981d) G 24480: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster. Number 801503.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Infurna, R.N. & Arthur, A.T. (1985) A teratology study of diazinon
technical in Charles River rats. Numbers MIN-82296, 52-83.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Iverson, F., Grant, D.L. & Lacroix, J. (1975) Diazinon metabolism in
the dog. Bull. Env. Cont. Toxicol. 13/5: 611-618.
Jenkins, L.J. (1988) Acute delayed neurotoxicity of diazinon mg-8 in
domestic fowl. Number 5152-87. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kabrawala, V.N., Shah, R.M. & Oza, G.G. (1965) Diazinon poisoning. A
study of 25 cases. The Indian Practitioner, 18: October, 711-717.
Kaplanis, J.N., Louloudes, S.J. & Roan, C.C. (1962) The
distribution and excretion of 32p-labelled diazinon in guinea-pigs.
Transactions of the Kansas Academy of Science, 65/1: 70-75.
Kirchner, F.K., McCormick, G.C & Arthur, A.T. (1991) G 24480 Techn.:
One/two-year oral toxicity study in rats. Number 882018. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Krinke, G.J. (1991) Comment on paper: neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice by Spyker
and Avery. J. Toxicol. Environm. Health, 3: 989-1002 (1977).
Letter dated December 17 (1991) prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Kuhn, J.O. (1989a) G 24480 tech. (Diazinon MG 8 FL 880045): Acute
oral toxicity study in rats. Number 5942-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989b) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
eye irritation study in rabbits. Number 5944-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989c) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
dermal irritation study in rabbits. Number 5945-89. Unpublished
Report Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989d) Diazinon MG8 FL 880045: Acute dermal toxicity
study in rabbits. Number 5943-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989e) Diazinon MG8 FL 880045: Dermal sensitization
study in guinea-pigs. Number 5946-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kushaba-Rugaaju, S. & Kitos, P.A. (1985) Effects of diazinon on
nucleotide and amino acid contents of chick embryos -teratogenic
considerations. Biochemical Pharmacology, 34(11): 1937-1943.
Machin, A.F., Rogers, H., Cross, A.J., Quick, M.P., Howells, L.C. &
Janes, N.F. (1975) Metabolic aspects of the toxicology of diazinon.
I hepatic metabolism in the sheep, cow, pig, guinea-pig, rat,
turkey, chicken and duck. Pestic. Sci. 6: 461-473.
Macklin, A.W. & Ribelin, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc. (JAVMA), 159/12:
1743-1748.
Maizlish, N., Schenker, M., Weisskopf, C., Seiber, J., & Samuels S.
(1987) A behavioral evaluation of pest control workers with short-
term, low-level exposure to the organophosphate diazinon. Am. J.
Ind. Med. 12: 153-172.
Marshall, Th. C. Dorough, H.W. & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agric. Food Chem. 24/3: 560-563.
Matsuda, A., Hayashi, M. & Ishidate, M. (1979) Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutation Research, 66: 277-290.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 Coded Chemicals.
Environm. Mol. Mutagen, 12: 85-154.
Misawa, M., Doull, J., Kitos,P.A. & Uyeki, F.M. (1981) Teratogenic
effects of cholinergic insecticides in chick embryos I. Diazinon
treatment on acetylcholinesterase and choline acetyltransferase
activities. Toxicol. Appl. Pharmacol. 57: 20-29.
Miyamoto, J. (1976) Terminal residues: evaluation of diazinon. J.
Assoc. Offic. Anal. Chem. 59: 878-880.
Mücke, W., Alt, K.O. & Esser, H.O. (1970) Degradation of 14C-
labelled diazinon in the rat. Agric. Food. Chem. 18/2: 208-212.
Murli, H. & Haworth S.R. (1990b) Mutagenicity test on diazinon MG 8:
in vivo sister chromatid exchange assay. Number HLA-12226-0-458,
TX 900093. Unpublished Report Prepared by Hazleton Laboratories
America Inc., Kensington, Maryland.
Murli, H. & Haworth, S.R. (1990a) Mutagenicity test on diazinon MG 8
in an in vitro cytogenetic assay measuring sister chromatid
exchange frequencies in cultured whole blood human lymphocytes.
Number HLA-12226-0-448, TX 900093. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
NCI (1976) Bioassay of diazinon for possible carcinogenicity. Nov.
1976. Carcinogenesis Testing Program National Cancer Institute
(NCI). U.S. Department of Health, Education and Welfare. DHEW
Publication No. (NIH) 79-1392.
NIEHS (National Institute of Environmental Health Services) (1991)
Toxicology data management system tumor incidence in control animals
by route and vehicle of administration F/344N rats. Prepared for
NIEHS by Analytical Sciences Inc., Durham, NC, USA.
Payot, P.H. (1966) Subacute oral toxicity study on diazinon AS-
humans. Unpublished Report dated October, Prepared by Geigy, Basle,
Switzerland.
Piccirillo, V.J. (1978) Acute oral toxicity study in rats with
diazinon technical. Number 483-143. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
Pickles, M. & Seim, V. (1988) Biological report for the metabolism
of 2-pyrimidinyl-14c-diazinon in a lactating goat. Number BIOL-
88004, 302212. Unpublished Report Prepared by Ciba-Geigy Corp., Vero
Beach, Florida.
Pickles, M. & Seim, V. (1990) Biological report for the metabolism
of 14C-diazinon on sheep. Numbers: BIOL-89014, 302925. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Robbins, W.E., Eddy, G.W. & Hopkins, Th.L. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem. 5/7: 509-513.
Rudzki, M.W., Arthur, A.T. & McCormick, G.C. (1991) Diazinon (MG-8):
52-week oral toxicity study in dogs. Number 882014. Unpublished
Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sachsse, K. & Bathe, R. (1975) Potentiation study: C8514
(chlordimeform) versus 3 insecticides, G 24 480 (Diazinon), CGA 12
223 and CGA 15324 in the rat. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1976) Potentiation study: C9491
(Jodfenphos) versus 2 Insecticides, G 24480 (Diazinon), and C 177
(Dichlorvos) in the rat. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977) CGA 15342 versus 2 insecticides, GS
13005 (Methidathion) and G 24480 (Diazinon) in rat. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1978) Potentiation study CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (Phosphamidon), GS 13005
(Methidathion), G 24480 (Diazinon) CGA 15324 and Malathion. Number
404478 Siss 6526. Unpublished Report Prepared by Ciba-Geigy Ltd.,
Basle, Switzerland.
Sanderson, D.M. & Edson, E.F. (1959) Oxime therapy in poisoning by
six organophosphorus insecticides in the rat. J. Pharm. Pharmacol.
11: 721-728.
Schoch, M. & Gfeller, W. (1985) G 24480 techn. Acute oral LD50 in
the rat. Number 850864. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland
Shishido, T., Usui, K. & Fukami, J. (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pest.
Biochem. Physiol. 2: 27-38.
Shishido, T., Usui, K., Sato, M. & Fukami, J. (1972b) Enzymatic
conjugation of diazinon with glutathione in rat and American
cockroach. Pest. Bioch. Physiol. 2: 51-63.
Shishido, T. & Fukami, J. (1972) Enzymatic hydrolysis of diazoxon by
rat tissue homogenates. Pest. Bioch. Physiol. 2: 39-50.
Simoneaux, B., Ballantine, L., Brown, K. & Lai, K. (1988) Metabolite
identification in hens and goats treated with 14C-diazinon. Number
ABR-88135. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B., Brown, K. & Lai, K. Summer, D. (1989) Supplemental
report on the nature of residues of diazinon in hens. Numbers ABR-
89040, 302213. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988a) Disposition of 14C-diazinon in goats.
Number ABR-88117. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988b) Disposition of 14C-diazinon in chickens.
Number ABR-88116. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988c) Characterization of 14C-diazinon
metabolites in goats. Number ABR-88118. Unpublished Report Prepared
by Ciba-Geigy Corp., Greensboro, NC.
Simoneaux, B.J. (1988d) Characterization of 14C-diazinon
metabolites in chickens. Number ABR-88119. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Singh, A.R., Arthur, A.T. & McCormick, G.C. (1988) Diazinon (MG-8)
13-week oral feeding study in rats. Numbers MIN-882011, 88083.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sobti, R.C., Krishan, A. & Pfaffenburger, C.D. (1982) Cytokinetic
and cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutation Research,
102: 89-102.
Spyker, J.M. & Avery, D.L. (1977) Neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice. J.
Toxicol. Environm. Health, 3: 989-1002.
Strasser, F. & Arni, P. (1988) G 24480: Sister chromatid exchange
test on human lymphocytes cell line CCL 156 in vitro. Number
871697. Unpublished Report Prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Tai, Ch. & Katz, R. (1984) Diazinon Techn.: 21-day dermal toxicity
study in rabbits. Number 842007. Unpublished Report Prepared by
Ciba-Geigy Corp. Greensboro, NC.
WHO (1990) Principles for the toxicological assessment of pesticide
residues in food. Environmental Health Criteria 104, Geneva.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wills, J.H. (1959) Recent studies of organic phosphate poisoning.
Federation Proc. 18: 1020-1025
Yang, S.H.R., Hodgson, E. & Dautermann, W.C. (1971) Metabolism in
vitro of diazinon and diazoxon in rat liver. J. Agr. Food Chem.
19/1: 10-13.
Zak, F., Hess, R., Luetkemeier, H. & Sachsse, K. (1973) 21-day
inhalation study on the rat with technical diazinon. Number Siss
1679. Unpublished Report Prepared by Ciba-Geigy Sisseln,
Switzerland.
Zwiener, R.J. & Ginsburg, C.M. (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81/1: 121-126.
Short-term toxicity studies
Although reported in the summaries of the following studies,
the inhibition of plasma cholinesterase was not utilized as a
criterion for the NOAEL, whereas inhibition of the erythrocyte
cholinesterase activity may be taken as an indicator of an adverse
effect of anticholinesterase pesticides. An inhibition of > 20%
compared to the control activity is regarded as significant (Dressel
et al., 1980; WHO, 1990).
Rats
Two short-term inhalation studies were conducted in rats. In a
first study groups of rats (9/sex/group) were exposed to an aerosol
of diazinon (purity 97.1%; droplet size < 1 µm 30-40%, 1-7 µm 50%)
for 6 hours a day, 5 days per week for three weeks. Only the
animals' snouts were exposed to the aerosol. The mean
concentrations were 0, 151, 245 or 559 mg/m3. The treatment did
not cause changes in the mortality rate, in haematology,
macroscopical and histopathological findings or organ weights that
were attributable to the inhalation of diazinon. Exophthalmus and
diarrhoea were observed in animals at all dose levels and tonic-
clonic muscle spasms occurred in the high-dose animals. The
symptoms were reversible. Food intake at the highest dose level was
reduced at the beginning of the treatment period. Body-weight gain
was reduced in male rats at 245 and 559 mg/m3, and in female rats
at 559 mg/m3. Plasma cholinesterase was inhibited at the
intermediate and high levels, resulting in values corresponding to
56% and 37% of the activity in control animals, respectively.
Erythrocyte cholinesterase was inhibited only at the highest dose
level (34% of the control activity). Brain cholinesterase activity
was dose-dependently reduced at all dose levels resulting in
activities of 81, 56 and 37% of the control activity in both sexes
at the low-, medium- and high-dose levels, respectively. The
cholinesterase values returned to normal at the end of the 25-day
recovery period. The NOAEL in this study was < 151 mg/m3
(corresponding to an estimated dose of 55 mg/kg bw/day), based on
lack of significant brain cholinesterase inhibition at the lowest
dose level (Zak et al., 1973).
In a more recent 21-day inhalation study, diazinon (purity 88%;
particle diameter 0.7-1.4 µm) was administered in a nose-only
exposure system in rats (10/sex/group). The intended aerosol
concentrations were 0, 0.1, 0.3, 1 or 10 mg/m3 (actual: 0, 0.05,
0.46, 1.57, 11.6 mg/m3). The animals were exposed during 6 hours a
day, 5 days per week for 3 weeks. The treatment did not adversely
affect body-weight gain, food consumption, haematological
parameters, organ weights, macroscopy and histopathological
findings. At 1 and 10 mg/m3, plasma cholinesterase activity was
reduced in females by 70% and 50% compared to control animals,
respectively. Erythrocyte cholinesterase was inhibited to 60% of
the control activity at 10 mg/m3 in both sexes, whereas the
activity of the brain cholinesterase was reduced in females only to
80% of the control activity at 1 mg/m3, and to 60% in the high-dose
animals. The increase of the relative lung weight observed in
females at 0.3 and 1 mg/m3 but not at the high dose was not
considered biologically significant. The NOAEL in this study was
0.46 mg/m3 (corresponding to an estimated dose of 0.2 mg/kg
bw/day), based on inhibition of brain cholinesterase at higher dose
levels (Hartmann, 1990).
Four groups of rats (Sprague-Dawley; Crl: VAF/Plus CD(SD)Br.;
15/sex/group) were orally treated with diazinon (purity 87.7%) at
dietary concentrations of 0, 0.5, 5, 250 or 2500 ppm for 90 days.
The treatment had no effect on mortality, water consumption,
ophthalmoscopy or urinalysis. Treatment-related occurrence of soft
faeces, hypersensitivity to touch and sound and in some male animals
aggressiveness were observed at the 2500 ppm dose level. Moreover,
hyperactivity was observed in several animals of the high-dose group
at the end of the study. Body-weight gain was reduced at 2500 ppm
by 6% in males and 13% in females compared to control animals. Food
consumption was reduced at 2500 ppm sporadically on single days in
both sexes. Haematological and biochemical investigations were
performed in the last study week. In the highest dose females,
slight reductions in haemoglobin and haematocrit values, as well as
an increase of the white blood cell count and reticulocyte count
were observed. A dose-dependent decrease in the mean serum
cholinesterase activity was observed in females and males at 5 ppm
(22% and 74%, respectively, of activity in control group), at 250
ppm (3% and 12% of the control activity, respectively) and at 2500
ppm (2% and 4% of the control activity, respectively). Erythrocyte
cholinesterase activity was reduced to 73% and 59% of control
activity at 250 and 2500 ppm in males and females, respectively. A
reduction in brain cholinesterase activity was found at 250 ppm in
females only (59%) and at 2500 ppm in both sexes resulting in
activities of 40%-50% of the control activity. An increase in
female liver and kidney weights was observed at 2500 ppm.
Microscopic evidence of centrolobular hepatocellular hypertrophy was
observed in single females at 250 ppm and in most females at 2500
ppm. The NOAEL in this study was 5 ppm, equal to 0.4 mg/kg bw/day,
based on erythrocyte and brain cholinesterase inhibition at 250 ppm
and higher (Singh et al., 1988).
Rabbits
In a dermal toxicity study, groups of albino rabbits
(5/sex/group) received dermal applications of 0, 1, 5 or 100 mg/kg
bw diazinon (purity 97.1%; 50% aqueous suspension in PEG 300), 5
days a week during a 3-week period. Because of high mortality in
males at 100 mg/kg bw (4/5 animals died between study days 3 and 6),
this dose level was reduced to 50 mg/kg bw on day 8 of the study.
In high-dose animals, signs of toxicity included anorexia, ataxia,
tremors, diarrhoea, hypoactivity, hypotonia and salivation. Body-
weight gain and food consumption tended to be higher in treated
animals compared to control animals. Serum cholinesterase was
inhibited in both sexes at 100/50 mg/kg bw resulting in values of
about 37% of the control group activity. At 5 mg/kg bw, activities
were 77% and 65% of the control activity in males and females,
respectively. At 1 mg/kg bw, inhibition was only found in females
resulting in an activity of 68%. Erythrocyte acetylcholinesterase
was inhibited only at 100/50 mg/kg bw resulting in 61% and 68% of
the control activity in males and females, respectively. Brain
acetylcholinesterase was also inhibited only at the highest dose
level of 100/50 mg/kg bw resulting in values corresponding to 72%
and 57% of the control activity in males and females, respectively.
The only change in organ weights was a decrease in kidney weight in
females at 100/50 mg/kg bw. No treatment-related macroscopic or
microscopic alterations were observed with the exception of slight
erythema at the application sites. Histologically a slight
hyperkeratosis was observed in treated skin of high-dose animals
The results of this study indicate a marked percutaneous absorption
and are thus in agreement with the results of other studies. The
NOAEL in this study was 5 mg/kg bw dermal, based on erythrocyte and
brain cholinesterase inhibition at 100/50 mg/kg bw (Ballantine et
al., 1984; Tai & Katz 1984).
Dogs
In a 90-day feeding study, diazinon (purity 87.7%) was
administered to groups of beagle dogs (4/sex/group) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
No treatment-related mortalities occurred. The treatment did not
adversely affect food consumption, ophthalmoscopy, haematology,
urinalysis, organ weights or macroscopic findings. Clinical signs
such as emesis or bloody faeces were sporadically observed in males
at 300 ppm and in females at 150 ppm. Reduced body-weight gain was
observed in females at 150 ppm and at 300 ppm in both sexes. In
males, inhibition of serum cholinesterase was observed resulting in
activities of 70%, 20% and 15% of that measured in control animals
at the end of the study at 0.5, 150 and 300 ppm, respectively.
Erythrocytes activities corresponding to 75% and 69% of control
activity were measured at 150 and 300 ppm, whereas in brain the
acetyl cholinesterase inhibition resulted in 69% and 58% of control
activity at 150 and 300 ppm, respectively. In females a reduction
in acetylcholinesterase activity was observed at 150 and 300 ppm.
The inhibition of serum and erythrocyte cholinesterase resulted in
an activity of about 18% and 70% of control activity, respectively,
at both dose levels, and brain cholinesterase inhibition resulted in
70% and 55% of control activity at 150 and 300 ppm, respectively.
Other changes of biochemical parameters consisted of a decrease in
total protein at 300 ppm in males. The only microscopic alteration
that might be compound-related was atrophy of the pancreatic acini
in one male dog of the highest dose group. The NOAEL in this study
was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on erythrocyte and
brain cholinesterase inhibition at 150 ppm and above (Barnes et
al., 1988).
Diazinon has been reported to produce pancreatic acinar lesions
in dogs at 75 mg/kg bw/day (Frick et al., 1987).
In a 52-week oral toxicity study, groups of beagle dogs
(4/sex/group) were fed diazinon (purity 87.7%) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
Due to lack of body-weight gain the dietary concentration of 300 ppm
was reduced to 225 ppm after 14 weeks of treatment (dose 300/225
ppm). Mortality was not increased by treatment and haematology,
urinalysis, gross pathology and histopathology revealed no changes
attributable to treatment. Overt clinical signs of dehydration and
emaciation became evident in one male at 300/225 ppm. The symptoms
remained even though the initial dose was reduced. Reductions in
body-weight gain were observed at 150 ppm and higher in males and at
300/225 ppm in females. However, no clear-cut dose-response
relationship was evident and the differences attained statistical
significance compared to the control group at only some observation
times. Food consumption was reduced at 150 ppm and higher, again
without a clear dose-response relationship, most probably due to
reduced palatability of the feed admixtures. Treatment-related
decreases in acetylcholinesterase activity at dose levels of 0.5 ppm
and higher were found: serum cholinesterase was reduced at 0.5 ppm
and higher in males and at 150 ppm and higher in females, resulting
in activities of about 20% of the activity in the control group at
150 and 300/225 ppm in both sexes. Erythrocyte cholinesterase
activity was also reduced at 150 and 300/225 ppm, where activities
corresponding to about 70% of the control activity were measured in
both sexes. Brain cholinesterase was inhibited at 150 ppm to 75% of
control activity in females and at 300/225 ppm to 65% and 75% of
control activity in females and males, respectively. The NOAEL in
this study was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on
erythrocyte and brain cholinesterase inhibition at 150 ppm and above
(Rudzki et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of mice (B6C3F1 Hybrid; 50 (control 25)/sex/group)
were administered diazinon (purity 98%) at 0, 100 or 200 ppm over
103 weeks. Survival, body weight, clinical signs and pathology were
investigated. The treatment had no effect on survival or body-
weight development. An increased incidence of hepatocellular
carcinomas was observed in the low-dose males only. The incidences
of hepatocellular carcinomas were 19, 43 and 21% in the control, low
and high-dose males, respectively, whereas the incidence of liver
adenomas was not increased in treated animals compared to control
animals. The mean incidences of hepatocellular carcinomas in
historical controls of this mouse strain (18 studies) were reported
to be 14% (max. 27%) for males and 3% (max. 10%) for females.
Concerning the total incidence of carcinomas and adenomas,
incidences of 29% (max. 58%) and 11% (max. 34%) in males and females
were reported (personal communication from Ciba Geigy to WHO, 1993).
Thus the incidence of hepatocellular carcinomas found in males of
the low-dose group markedly exceeded the range of historical control
incidences. Because no dose-response relationship was evident, the
result was most probably fortuitous and could not be interpreted as
providing evidence for a carcinogenic potential of diazinon in mice
(NCI, 1976).
Rats
Groups of rats (Fischer F344; 50 (control 25)/sex/group) were
fed diazinon (purity 98%) at dietary concentrations of 0, 400 or 800
ppm for 103 weeks. Survival, body weight, clinical signs and
pathology were the parameters investigated. There was no dose-
related reduction of survival and the body-weight development was
similar in control and treatment groups. Clinical signs of
hyperactivity were observed in treated males in both dose groups and
in high-dose females. The incidence of leukaemia was increased in
low-dose males (50%) compared to the incidence in the concurrent
control (20%) and high-dose males (24%). The leukaemias were of the
type usually seen in aging F344 rats. In females, the incidences of
leukaemia were also higher (12% at 400 ppm and 10% at 800 ppm)
compared to the concurrent controls (4%). Historical control
incidences of leukaemia were reported to be 48% for males (range 32-
62%) and 27% for females (range 14-15%). Because of the lack of a
dose-response relationship, the increase in tumour incidence was
considered of questionable significance and the results therefore
could not be interpreted as providing evidence for a tumorigenic
potential of diazinon in rats (NCI, 1976; NIEHS, 1991).
Diazinon (purity 87.7%) was administered as feed admixture to
groups of rats (Sprague-Dawley; 30-40/sex/dose) at concentrations of
0, 0.1, 1.5, 125 or 250 ppm (adjusted for purity) for up to 98-99
weeks. An additional vehicle control group was included in this
study. The animals of this dose group were treated with epoxidized
soybean oil. Up to 10 rats/sex/group were used for the interim
sacrifice after at least 52 weeks of treatment. Another number of
up to 10 rats/sex from the control and high-dose group were placed
on a four-week recovery period and sacrificed thereafter. The study
was terminated after 98-99 weeks because of decreased survivability
in the 0.1 ppm male group only, which was unrelated to treatment and
associated with age-related changes (e.g., senile nephropathy and/or
pituitary adenoma, both considered to be the result of senescence in
this rat strain). This earlier termination was not considered to
have affected the quality or integrity of the study because a
sufficient number of animals were at risk in the other treatment
groups for the development of tumours. The treatment did not cause
increased mortality, and did not affect ophthalmological or
haematological findings, urinalysis or organ weights. Body-weight
gain was increased in males at dose levels of 0.1 ppm and higher and
in some instances in females at 125 ppm and higher compared to the
untreated control animals. Because the body-weight gain in the
vehicle control group also showed an increase compared to the
untreated controls, the increases in body-weight may reflect an
increased palatability of the feed admixtures containing the
epoxidized soybean oil. In fact the increases in mean body-weight
gain generally coincided with increases in mean food consumption in
these dose groups. Decreases in serum cholinesterase activities
were observed at concentrations of 1.5 ppm and higher in both sexes,
resulting in values of about 50% of the control activity at the end
of the study. A dose-dependent reduction of erythrocyte
cholinesterase activity was observed at 125 and 250 ppm resulting in
activities of 80% and 75% of the control activity in males and
females, respectively, at either dose level at the end of the study.
Brain cholinesterase activity was also inhibited to 76% and 71% of
the control activity in males and females, respectively, at 125 ppm,
and to 58% and 52% of the control activity at 250 ppm in males and
females, respectively. Similar inhibition was observed after the
first year of the study. A slight reduction of less than 9% was
still found after the 4-week recovery period in erythrocyte and
brain cholinesterase activity at 250 ppm. Gross and microscopic
pathology examinations did not give evidence of any compound-related
lesions. The NOAEL in this study was 1.5 ppm, equal to 0.07 mg/kg
bw/day, based on inhibition of erythrocyte and brain cholinesterase
at 125 ppm and above (Kirchner et al., 1991).
Reproduction studies
Rats
Diazinon (purity 94.9%) was administered in the diet to groups
of rats (Sprague-Dawley CRCD; 30/sex/group) over two parental
generations (F0, F1) and up to weaning of the F2 pups at
concentrations of 0, 10, 100 or 500 ppm. Treatment-related clinical
symptoms and deaths were observed in a few high-dose females. They
consisted of tremors (3/30) and dystocia (2/30) followed by death or
sacrifice in the F0 generation. In the F1 generation, a few high-
dose females showed tremors as only compound-related signs. Food
consumption was increased in females of the 500 ppm group in the F0
generation, whereas in the F1 generation, a dose-related reduction
of food consumption was observed at 100 and 500 ppm in males only.
Body-weight gain was lower in F0 females at 500 ppm during
gestation. In the F1 generation, reduced body-weight gain was
observed at 100 and 500 ppm in males and at 500 ppm in females.
There were no treatment-related effects on mating behaviour and the
reproductive parameters (including mating, fertility, gestation
indices, number of viable pups, and number of stillborn pups) were
comparable in control and treatment groups in the F0 generation and
at the low and intermediate dose level of the F1 generation. At
500 ppm, a greater proportion of females showed a prolonged
gestation duration at both generations. A decrease in the number of
pregnancies and viable pups as well as reduced fertility and mating
indices were observed in high-dose females of the F1 generation. A
dose-related reduction in survival of F1 pups was observed at 100
ppm and 500 ppm and in F2 at 500 ppm. Weights of F0 pups were
reduced in the 100 and 500 ppm groups, and in F2 pups at 500 ppm.
No treatment-related malformations were found in the pups. The
NOAEL in this study was 10 ppm, equivalent to 0.5 mg/kg bw/day,
based on reduced parental body-weight gain and reduced viability of
pups and pup weights at 100 ppm and higher (Giknis 1989).
Special studies on embryotoxicity/teratogenicity
Mice
Groups of mice (6 females/dose) were given oral daily doses of
0, 0.18 or 9 mg/kg bw diazinon throughout gestation. Treated
animals gained less weight during gestation compared to control
animals. Weight gain of pups born to mothers receiving 9 mg/kg
bw/day was reduced. Daily testing for physiological and behavioural
development of the pups revealed some evidence of retarded
development among the offspring of high-dose animals (e.g.,
retardation of eye and ear opening). Measures of endurance and
coordination also gave some evidence of impairment (e.g., increased
rod cling endurance in both groups, reduced rotarod endurance,
impaired running performance in a maze inclined plane test).
Impaired reactions were sometimes observed at both dose levels but
without a clear dose-effect relationship. Examination of brain
tissue of only 8 of a total number of 132 offspring at the high-dose
level revealed morphological abnormalities in the forebrain. The
relationship of these findings to the observed behavioural changes
is unknown (Spyker & Avery 1977). The evidence for morphological
effects of diazinon on the developing brain was considered
insufficient by an expert (Krinke, 1991).
Rats
In a teratogenicity study in Sprague-Dawley rats at dose levels
of 0, 15, 50 or 100 mg/kg bw/day administered orally on days 6
through 15 of gestation, dams at the 100 mg/kg bw/day dose level
showed a marked decrease in food consumption correlating with weight
loss at the beginning of the treatment period. Skeletal assessment
showed a slightly higher incidence of incomplete ossification at
different sites in the fetuses at 100 mg/kg bw/day. Visceral
examination revealed a dystopia cordis in association with
hypoplasia of lungs in 1/105 fetuses at 100 mg/kg bw/day. This
anomaly has been reported to occur spontaneously in control animals.
Because of the single occurrence this anomaly was not considered to
be due to a direct action of diazinon but to be secondary to
maternal toxicity (Fritz, 1974).
In a later study, groups of rats (Charles River Crl. COBS
CD(SD)(BR); 27 females/group) were treated with doses of diazinon
(purity 97.4%) at 0, 10, 20 or 100 mg/kg bw/day by gavage during
gestational days 6 through 15. The treatment had no effect on the
mortality of the dams. During the first half of the dosing period,
the dams lost weight and food consumption was reduced at 100 mg/kg
bw/day. No compound-related clinical signs and no abnormal gross
pathological findings were observed in the dams. At 100 mg/kg
bw/day some reproductive parameters differed from the control values
but no statistical significance was achieved (e.g., increase in
number of resorptions, percent pre- and post-implantation loss,
reduction in number of live fetuses). On gross observation, 3/262
(1%) fetuses from 3 litters showed external malformations (single
occurrences of a umbilical hernia, filament tail, sublingual
extraneous soft tissue), whereas in the concurrent controls no
similar effects were observed. Umbilical hernia and tail
abnormalities however are reported to be spontaneous malformations
observed routinely in this rat strain. Concerning skeletal
variations, an increased incidence in rudimentary ribs (T-14) was
observed in all treatment groups attaining statistical significance
in the high-dose group (5% versus 0%). Because of the single
occurrences of the different malformations observed and because the
malformations also occur in control animals they were considered to
be a consequence of the marked maternotoxicity at this dose level,
and not due to a teratogenic effect of the test compound. The
increased incidence of the skeletal variation observed at the top
dose level was considered to be related to maternotoxicity at this
dose level. The NOAEL in this study was 20 mg/kg bw/day based on
maternotoxicity and fetotoxicity at the higher dose level (Infurna &
Arthur, 1985).
Rabbits
Diazinon (purity 89.2%) was administered to groups of pregnant
rabbits (New Zeeland white; 18-22 females/group) by gavage at dose
levels of 0, 7, 25 or 100 mg/kg bw/day from days 6-18 of gestation.
An increase in maternal mortality was observed at 100 mg/kg bw/day
(41% vs 0% in all other groups). Overt clinical signs of maternal
toxicity at 100 mg/kg bw/day included tremors, convulsions,
hypoactivity and anorexia. Reduced body-weight gain was found at
the highest dose level. The treatment did not influence the number
of corpora lutea, number of implantation sites, live fetuses per
litter or fetal weight. The incidence of visceral and skeletal
malformations and variations showed no differences between the
groups that could be attributed to treatment. The study therefore
gave no evidence for an embryotoxic or teratogenic activity of
diazinon. The NOAEL in this study was 25 mg/kg bw/day based on
materno-toxicity at the highest dose level (Harris & Holson, 1981).
Chickens
The teratogenic effects of some anticholinesterase insecticides
(organophosphates and methylcarbamates) on chicken embryos have been
known for some time. In a first study, eggs were injected with
diazinon to explore some of the biochemical variables that might be
involved in producing micromelia, beak and feather deformities (type
I signs) in avian embryos that are known to be caused by OP
insecticides. The results of this study confirm the assumption that
a tryptophan deficiency (tryptophan plays an important role in the
developing embryo) may cause the abnormalities observed (Kushaba-
Rugaaju & Kitos, 1985).
Another study was conducted to investigate the involvement of
cholinergic functions in OP-induced teratogenesis in chick embryos
by examining the effects of diazinon on the developmental pattern of
AChE and ChAT activities in hindlimb, wing and brain. The results
of this study confirmed that type II teratogenesis (wry neck, short
neck, arthrogryposis and muscular hypoplasia of legs) may be
mediated via cholinergic nicotinic receptors. Cholinergic
dysfunction including decreased activity of AChE and ChAT was not
correlated with diazinon-induced type I teratogenesis (micromelia,
abnormal feathering) (Misawa et al., 1981).
Cattle
As part of a survey to determine the causes of abortion in
dairy cattle in Wisconsin, the potential role of pesticides was
examined. Diazinon in the form of wettable powder was orally
administered to two pregnant cows at a daily dose level of 6.6 mg/kg
bw. The continuous treatment started in the 5th and 6th month of
pregnancy (abortion commonly affects cows in the 5th to 8th month of
pregnancy) and was maintained until parturition. No diazinon
residues were detected in the tissues examined (fat, liver, kidney)
nor in milk. The treatment failed to reveal any abortions (Macklin
& Ribelin 1971).
Special studies on genotoxicity
Diazinon has been adequately tested in a series of genotoxicity
assays. The results are summarized in Table 2.
Special study of effects on the pancreas
A study was conducted using a canine model to investigate the
induction of pancreatic ductal hypertension following cholinesterase
inhibitor intoxication known to be an important triggering mechanism
in the pathogenesis of acute and chronic pancreatitis. Diazinon was
intravenously administered (25 mg/kg bw) to the pancreatic ampulla
of dogs and the tissue cholinesterase activity of the canine
pancreatic sphincters was determined. Two enzymes are responsible
for the total cholinesterase activity of whole blood: a membrane-
bound AChE associated with the erythrocyte membrane, and a soluble
enzyme, pseudocholinesterase or butyrylcholinesterase (BChE) in the
serum. In tissues, which also contain the two forms of
cholinesterase, AChE is important in the regulation of neuromuscular
activity and parasympathetic ganglion transmission, while the role
of BChE is unknown. The observation of the negative correlation
between serum BChE activity and intraductal pancreatic pressure
supports the hypothesis that the pancreatic ductal hypertension
which occurs following cholinesterase inhibitor intoxication is due
to a selective reduction in pancreatic BChE activity (Dressel et
al., 1980).
The induction of acute pancreatitis by OPs found in dogs was
confirmed in guinea-pigs but not in cats. These results may reflect
species-related differences in the distribution of pancreatic BChE
(Frick et al., 1987).
Special studies on antidotes
Previous reports with respect to the usefulness of PAM and
other oxine reactivators against this organophosphate have been
published (Sanderson & Edson, 1959; Wills, 1959). A recent study
was undertaken to provide information on the antidotal effectiveness
of pyridine-2-aldoxime methochloride (2-PAM) against diazinon
poisoning in animals. The administration of atropine (16 mg/ kg bw;
i.m.) or 2-PAM (30 mg/kg bw; i.v.) alone 10 minutes after poisoning
rats with doses of 235 mg/kg bw corresponding to the approximate
oral LD50 or higher provided little or no protection. Best
protection was achieved when the oxime was given orally in
conjunction with atropine (i.m.) or followed by a subsequent dose of
2-PAM orally or i.v. Administration of 2-PAM to diazinon-poisoned
rabbits (1600 mg/kg bw) resulted in reactivation of inhibited blood
ChE activity concurrent with a decrease in signs of poisoning.
Within 2 hours, however, the animals were again weak and ataxic and
blood ChE showed renewed inhibition. The authors suggested that
effective therapy of diazinon intoxication requires repeated doses
of oxime to maintain effective antidote levels in the body (Harris
et al., 1969). In dogs and guinea-pigs, pretreatment with
atropine protected the animals against diazinon-induced pancreatitis
(Frick et al., 1987).
Special study on delayed neurotoxicity
Hens
An oral dose of 28 mg/kg bw/day of diazinon technical (87%
purity) was administered to a group of 18 hens (the target dose of
13 mg/kg bw/day as the approximate LD50 was doubled due to a
preparation error). The schedule to protect the hens from acute
cholinergic effects of diazinon consisted of a 10 mg/kg bw atropine
pretreatment (i.m.). At the time of diazinon dosing, 2-PAM at an
i.m. dose of 50 mg/kg bw was administered. Post-treatment consisted
of concurrent doses of atropine and 2-PAM one and five hours
following dosing. Since there were no neurotoxic responses in the
test group in the three weeks following the treatment they were
again treated with 13 mg/kg bw of diazinon on day 21. One test
group hen was found dead on day 5 after the first dosing, and one
hen six hours after the second treatment. One hen exhibited a
slight unsteadiness in walking only on day 41. No neurotoxic signs
became apparent during the three-week observation period after the
second treatment. Histopathological examination revealed no lesions
in the brain, spinal cord or peripheral nerves in diazinon-treated
animals, whereas animals of the positive control (TOCP treatment)
showed multiple lesions (axonal degeneration) consistent with
peripheral neuropathy (Jenkins, 1988).
Observations in humans
Numerous reports of intentional and accidental intake of
diazinon and other OPs have been published describing the clinical
manifestations of organophosphate toxicity and the usefulness of
erythrocyte and serum cholinesterase activity assays for diagnosis
as well as the efficacy of supportive and specific therapies
(Kabrawala et al., 1965; Payot, 1966; Banerjee, 1967; Gupta &
Patel, 1968; Zwiener & Ginsburg, 1988).
A case of acute diazinon poisoning complicated by pericarditis
and pneumonia followed by recovery has been reported. The
intoxication caused the occurrence of cyanosis, tracheobronchial
congestion, pulmonary edema and pneumonia. The treatment included
the intramuscular injection of atropine (Banerjee, 1967).
Another complication reported following accidental ingestion of
diazinon consisted of severe pancreatitis and a pseudocyst (Dressel
et al., 1979). The induction of pancreatitis was reproduced in
dogs treated intravenously with a dose of 5 mg/kg bw diazinon.
Pancreatitis may be the result of hypersecretion and ductal
obstruction (Dressel et al., 1980).
Table 2. Results of genotoxicity assays on diazinon
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
In vitro
Ames test S. typhimurium 313-5000 µg/0.1 ml 88.0% negative negative Geleick & Arni, 1990
TA 98, 100, 1535, 1537 DMSO
E. coli WP2uvrA
Ames test S. typhimurium in DMSO >50-1000 ? negative negative Marshall et al., 1976
TA 1535, 1536, 1537, 1538 µg/plate
Mouse lymphoma Mouse lymphoma cell 1. 6 to 60 µg/ml with 97.2% negative negative Dollenmeier & Müller,
assay L5178Y/TK+/- activation 1986
2. 12 to 120 µg/ml
without activation
Mouse lymphoma Mouse lymphoma cell up to 100 µg/ml ? positive positive McGregor et al., 1989
assay L5178Y TK+/-
Sister chromatid Chinese hamster cells 10, 20 and 40 µg/ml 99.2% negative Chen et al., 1981
exchange study (V79) 99.2% in DMSO
Chromosomal Chinese hamster lung 0.1 mg/ml ? cytotoxic positive Matsuoka et al., 1979
aberration test cells
Sister chromatid Human lymphoid cells 0.02, 0.2, 2.0 and ? negative negative1 Sobti et al., 1982
exchange study (LAZ-007) 200 µg/ml
in Ethanol (< 0.1%)
Sister chromatid Human lymphoid cells 12.5, 25, 50, 100 and 87.5% negative negative Strasser & Arni, 1988
exchange study (CCL-156) 200 µg/ml in DMSO
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Whole blood human 1. 0.0668 to 2000 µg/ml 88.0% equivocal equivocal2 Murli & Haworth, 1990a
exchange study lymphocytes in DMSO (ħ activation)
2. 0.668 to 20 µg/ml
(non-activation)
3. 2-66.8 µg/ml
(activated)
Autoradiographic Rat hepatocytes 1.1 to 120 µg/ml in 88.0% negative Hertner & Arni, 1990
DNA repair test DMSO
In vivo
Micronucleus test Mouse 1. 120 mg/kg bw oral 87.5% negative Ceresa, 1988
in vivo 2. 30, 60, and
120 mg/kg bw oral
Dominant lethal Mouse oral dose of 14 and 45 95% negative Fritz, 1975
study mg/kg bw
Chromosome studies Mouse a) daily oral doses of ? negative a) Hool & Müller, 1981c
in male germinal a) spermatogonia 10.5, 21 and 63 mg/kg b) Hool & Müller, 1981b
epithelium b) spermatocytes bw for five days, or
b) 5 treatments with
the same doses over
a period of 10 days
Sister chromatid Bone marrow cells single oral doses of 88.0% negative Murli & Haworth, 1990b
exchange study of mice 10, 50 and 100
in vivo mg/kg bw
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Chinese hamster bone oral gavage of 6.5 ? negative Hool & Müller, 1981a
exchange study marrow cell to 26 mg/kg bw
Nucleus anomaly Chinese hamster bone oral gavage of ? negative Hool & Müller, 1981d
test marrow cells 6.5, 13 and 26 mg/kg
bw/day for two days
1 Cyotoxicity at a concentration of 20 µg/ml. The increased frequency of sister chromatid exchanges at 20 µg/ml with
metabolic activation did not show a doubling of the control frequency and was therefore not considered positive.
2 Two independent trials: Cytotoxicity at concentrations of 66.8 µg/ml (with activation) respectively. Increases in the SCE
frequency (just doubling) observed only in the first assay without metabolic activation at dose levels of 0.668 µg/ml to
20 µg/ml, but without a dose-relationship. Increases observed after activation in both trials did not show doubling and again
no dose-response. Thus the results of these studies are not considered positive.
A study of 60 poisoning cases with diazinon has been reported.
The most common clinical manifestations were vomiting, giddiness,
constricted pupils and signs of bronchoconstriction with pulmonary
congestion. The amount of poison ingested varied from 4 ml to 15 ml
with an average of 7.5 ml (% active ingredient not specified in
publication). This was ingested by 55 patients with suicidal
intention, whereas in 5 patients the compound was taken
accidentally. Atropine injections were administered repeatedly (up
to a total dose of 22.4 g over 42 hours). Five patients died in
spite of intensive atropine therapy initiated more than 8 hours
after ingestion. Other cases who had ingested the same amount of
poison as the fatal cases received treatment within three hours of
ingestion. These findings emphasize the importance of early
treatment in diazinon poisoning (Gupta & Patel, 1968). In addition,
these results are in agreement with an earlier observation that the
combination of diazinon with cholinesterase occurs in two stages, an
early reversible stage and a late irreversible stage (Grob, 1956).
In another study, cases of organophosphate and carbamate
poisoning in 37 infants and children were reported, only 5 of which
were associated with diazinon. Miosis, excessive salivation, muscle
weakness, respiratory distress, lethargy and tachycardia were the
most common clinical findings. Erythrocyte cholinesterase
activities were determined from 24 patients showing erythrocyte
cholinesterase activities that were less than 50% of the lower limit
of the normal range. There were no differences between the
erythrocyte and serum cholinesterase activity in 20/24 patients from
whom both tests were obtained, whereas in the remaining 4 cases
either the erythrocyte or the serum activity was decreased. A
combined atropine/pralidoxime therapy is recommended in case of
organophosphate toxicity. Although the recommended dose for
atropine in infants is 0.01-0.02 mg/kg bw, the dose is generally
insufficient for treatment of signs and symptoms secondary to
organophosphate poisoning (Zwiener & Ginsburg, 1988).
Neurobehavioural effects of short-term, low-level exposure to
diazinon were investigated in 99 pest control workers. A computer
assisted neuro-behavioural test battery (including attention,
vigilance, hand-eye coordination, visual perception, verbal ability)
was used before and after their work shift. The diazinon metabolite
diethyl-thiophosphate (DETP) was measured in urine samples collected
from 46 diazinon applicators and 56 non-applicators. Post-shift
DETP values were 24 and 3 ppb for applicators and non-applicators,
respectively. The study failed to demonstrate any behavioral
effects of diazinon under the conditions of this study (Maizlish et
al., 1987).
The results of the following old study have been reported in an
earlier monograph (Annex I, reference 7). Because of certain
inaccuracies in that monograph, the study was reviewed again and
summarized below.
A subacute oral toxicity study was conducted in four male
volunteers. Diazinon (different batches, purity 95.4-95.7%) was
administered in capsules at dose levels of 2-2.5 mg per day per
person, equal to a dose of 0.025 mg/kg bw/day. The test period
lasted 42 days with an interruption in treatment from the 5th to
10th day in two volunteers and for 34 consecutive days in the other
two volunteers. Plasma cholinesterase activity was markedly
depressed the first 6 days of treatment in 2/4 subjects (no plasma
cholinesterase activity measurable). Therefore treatment was
interrupted for 6 days to enable recovery. Subsequent treatment at
the same dose level did not reveal inhibitory effects on plasma
cholinesterase. The fluctuations observed during the treatment
period were similar to those observed during the pre-test period.
In no instance was the erythrocyte acetylcholinesterase depressed
compared to the pre-test values. Other parameters investigated
included haematology and blood chemistry, urinalyses and
symptomatology. No changes were observed that could be attributed
to the treatment. The NOAEL in this study was 0.025 mg/kg bw/day
based on transitory depression of plasma cholinesterase activity as
the only effect observed at this dose level (Payot, 1966).
COMMENTS
Following oral administration to rats, diazinon was almost
completely absorbed and eliminated, mainly in the urine.
The main degradative pathway includes the oxidase/hydrolase-
mediated cleavage of the ester bond leading to the pyrimidinol
derivative 2-isopropyl-6-methyl-4(1H)-pyrimidinone, which is further
oxidized to more polar metabolites.
Diazinon has moderate acute oral toxicity to mice and rats.
The clinical signs observed were consistent with cholinesterase
inhibition and included sedation, tremors, convulsions and ataxia.
It has been classified by WHO as moderately hazardous.
In an oral 90-day feeding study in rats at dietary
concentrations of 0, 0.5, 5, 250 or 2500 ppm, the NOAEL was 5 ppm
(equal to 0.4 mg/kg bw/day), based on erythrocyte and brain
cholinesterase inhibition at 250 ppm and higher.
In short-term studies in dogs, diazinon was administered at
dietary concentrations of 0, 0.1, 0.5, 150 or 300 ppm for either 90
days or 52 weeks. In both studies, the NOAEL was 0.5 ppm, equal to
0.02 mg/kg bw/day based on erythrocyte and brain cholinesterase
inhibition at 150 ppm and above.
In a carcinogenicity study in mice, diazinon was administered
at dietary concentrations of 0, 100 or 200 ppm over 103 weeks.
There was no evidence of carcinogenicity.
In a carcinogenicity study in rats diazinon was administered at
dietary concentrations of 0, 400 or 800 ppm for 103 weeks. There
was no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study, rats were
maintained on a diet containing diazinon at concentrations of 0,
0.1, 1.5, 125 or 250 ppm for up to 99 weeks. The NOAEL was 1.5 ppm,
equal to 0.07 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase at 125 ppm and above. There was no evidence of
carcinogenicity.
A multigeneration study was conducted in rats using dietary
concentrations of 0, 10, 100 or 500 ppm. The NOAEL was 10 ppm
(equivalent to 0.5 mg/kg bw/day), based on a reduction in parental
body-weight gain in the F1 generation and a reduced survival rate
and reduced body weight of F1 pups at 100 ppm.
In a teratogenicity study in rats, diazinon was orally
administered at dose levels of 0, 10, 20 or 100 mg/kg bw/day.
Maternal toxicity, indicated by weight loss correlating with reduced
food consumption, became evident at 100 mg/kg bw/day. Effects on
the fetuses at this dose level consisted of retarded ossification
and an increased incidence of rudimentary ribs. The NOAEL was 20
mg/kg bw/day based on maternotoxicity and fetotoxicity. There was
no evidence of teratogenicity.
A teratogenicity study in rabbits conducted with oral dose
levels of 0, 7, 25 or 100 mg/kg bw/day revealed clinical signs of
maternotoxicity, increased mortality and reduced body-weight gain at
100 mg/kg bw/day. The NOAEL was 25 mg/kg bw/day. There was no
evidence of teratogenicity.
A neurotoxicity study performed with hens treated at dose
levels of 13 or 28 mg/kg bw/day (protected by atropine pre-
treatment) did not reveal evidence of delayed neurotoxicity.
Diazinon has been adequately tested in a series of genotoxicity
assays. Chromosomal aberrations were induced in cultured mammalian
cells, but there were no other indications of genotoxicity. The
Meeting concluded that diazinon was not genotoxic.
Diazinon was evaluated in four human male volunteers who
received 0.025 mg/kg bw/day of diazinon in capsules for 34-36 days.
There were no consistent treatment-related effects on plasma or
erythrocyte cholinesterase activity, blood chemistry or urinalysis.
No clinical effects were reported. The NOAEL was 0.025 mg/kg
bw/day.
The ADI of 0-0.002 mg/kg bw, which was based on the NOAEL of
0.025 mg/kg bw/day in the study in humans using a 10-fold safety
factor, was maintained.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm, equal to 0.4 mg/kg bw/day (90-day study)
1.5 ppm, equal to 0.07 mg/kg bw/day (99-week study)
10 ppm, equivalent to 0.5 mg/kg bw/day
(reproduction study)
20 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 25 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 0.5 ppm, equal to 0.02 mg/kg bw/day (one-year study)
Human: 0.025 mg/kg bw/day (34-36-day study)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ballantine, L., Marco, G.J. & Williams, S.C. (1984) Percutaneous
absorption of 2 delta - 14C - Diazinon in Rats. Numbers ABR-84011,
302950. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Banerjee, D. (1967) Pericarditis in acute diazinon poisoning. A case
report. Armed Forces Med. J. (India), 23: 187-190.
Barnes, T.B., Arthur, A.T. & Hazelette, J.R. (1988) Diazinon (MG-8):
90-Day Oral Toxicity Study in Dogs. Number 882012. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Bathe, R. (1972a) Acute oral LD50 of technical diazinon in the
mouse. Number Siss 1679. Unpublished Report Prepared by Ciba-Geigy
Ltd., Sisseln, Switzerland.
Bathe, R. (1972b) Acute dermal LD50 of technical diazinon (G-24480)
in the rat. Number Siss 1679. Unpublished Report Prepared by Ciba-
Geigy Ltd., Sisseln, Switzerland.
Bathe, R. & Gfeller, W. (1980) Acute oral LD50 in the rat of
technical G 24480. Number 800478. Unpublished Report Prepared by
Ciba-Geigy Ltd., Sisseln, Switzerland.
Burgener, J. & Seim, V. (1988) Biological report for the metabolism
of 14C-diazinon in Laying Hens Dosed at 25 ppm. Numbers BIOL-88006,
302213. Unpublished Report Prepared by Ciba-Geigy Corp., Vero Beach,
Florida.
Capps, T., Brown, K., Lai, K. & Tweedy, B. (1989) Characterization
and identification of diazinon metabolites in rats. Numbers ABR-
88164, 302211 and 302925. Unpublished Report Prepared by Ciba-Geigy
Corp., Greensboro, NC.
Capps, T. M., Summer, D., Bar, H.P. & Carlin, T.J. (1990)
Characterization and identification of major metabolites in tissues
of sheep treated dermally with 14C-diazinon. Numbers ABR-90014,
302925. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Ceresa, C. (1988) G 24480: Micronucleus test (mouse). Number 871696.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H.H., Hsueh, J.L., Sirianni, S.K. & Huang, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutation Research, 88: 307-316.
Ciba-Geigy 1992: Diazinon Data Summaries. Working Paper for the WHO
Expert group on Pesticide Residues. Biological Data and
Toxicological Evaluation. June 23.
Craine, E.M. (1989) Study of 14C-diazinon disposition in the rat
(biological phase and analytical phase I). Numbers WIL-82030,
302925. Unpublished Report Prepared by WIL Research Laboratories
Inc., Ashland, OH and Ciba-Geigy Corp., Greensboro, NC.
Dahm, P.A. (1970) Some aspects of the metabolism of parathion and
diazinon. In: Biochemical Toxicology of Insecticides. ed. by
O'Brien, R.D. and Yamamoto, I., Academic Press London.
Dollenmeier, P. & Müller, D. (1986) G 24480 techn: L 5178Y/T+/-
mouse lymphoma mutagenicity test. Number 840396. Unpublished Report
Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Dressel, Th. D., Goodale, R.L., Borner, J.W. & Etani, S. (1980) A
study of the cholinesterase of the canine pancreatic sphincters and
the relationship between reduced butyrylcholinesterase activity and
pancreatic ductal hypertension. Annals of Surgery, 192/5: 614-619.
Dressel, T.D., Goodale, K.L., Arneson, M.A. & Borner, J.W. (1979)
Pancreatitis as a complication of anticholinesterase insecticide
intoxication. Annals of Surgery, 189: 199-204.
Frick, T.W., Dalo, S., O'Leary, J.F., Runge, W., Borner, J.W.,
Baraniewski, H., Dressel, T., Shearen, J.G. & Goodale, K.L., (1987)
Effects of insecticide, diazinon, on pancreas of dog, cat and
guinea-pig. J. Environ. Path. Toxicol. Oncol. 7(4): 1-11.
Fritz, H. (1974) G 24480 (Diazinon Techn.): Reproduction study - rat
segment ii: (test for teratogenic on embryotoxic effects).
Unpublished report prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Fritz, H. (1975) G 24480 (Diazinon Techn.): Dominant lethal study
mouse. Number 327507. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Geleick, D. & Arni, P. (1990) Salmonella and Escherichia/liver-
microsome test. Number 891346. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Giknis, M.L.A. (1989) Diazinon techn.: A two-generation reproductive
study in albino rats. Numbers MIN-852218, 88128. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Grob, D. (1956) The manifestations and treatment of poisoning due to
nerve gas and other organic phosphate anticholinesterase compounds.
A.M.A. Arch. Intern. Med. 98: 221.
Gupta, O.P. & Patel, D.D. (1968) Diazinon poisoning. A study of
sixty cases. J. Assoc. Physicians of India, 16: 457-463.
Hagenbuch, J.P. & Mücke, W. (1985) The fate of diazinon in mammals.
Biochemistry Product Evaluation 1/85. Unpublished Report Prepared by
Ciba-Geigy Ltd., Basle, Switzerland.
Harris, S. B. & Holson, J.F. (1981) A teratology study of diazinon
in New Zealand white rabbits. Number 281005. Unpublished Report
Prepared by Science Applications Inc., La Jolla, CA.
Harris, L.W., Fleisher, J.H., Innerebner, T.A., Cliff, W. & Sim,
V.M. (1969) The effects of atropine-oxime therapy on cholinesterase
activity and the survival of animals poisoned with 0,0-diethyl-0-(2-
isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate. Toxicol. Appl.
Pharmacol. 15: 216-224.
Hartmann, H.R. (1990) G 24480: 21-Day repeated exposure inhalation
toxicity in the rat (nose-only exposure). Number 891205. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Arni, P. (1990) G 24480: Autoradiographic DNA repair
test on rat hepatocytes, Number 891345. Unpublished Report Prepared
by Ciba-Geigy Ltd., Basle, Switzerland.
Holbert, M.S. (1989) Diazinon MG8 FL 880045: acute inhalation
toxicity study in rats. Number 5947-89. Unpublished Report Prepared
by Stillmeadow Inc., Houston, Texas.
Hool, G. & Müller, D. (1981a) G 24480: Sister-chromatid exchange
study Chinese hamster. Number 801504. Unpublished Report Including
Supplement to the Report (dated October 13, 1981) Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981b) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatocytes. Number 801502. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981c) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatogonia. Number 801501. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981d) G 24480: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster. Number 801503.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Infurna, R.N. & Arthur, A.T. (1985) A teratology study of diazinon
technical in Charles River rats. Numbers MIN-82296, 52-83.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Iverson, F., Grant, D.L. & Lacroix, J. (1975) Diazinon metabolism in
the dog. Bull. Env. Cont. Toxicol. 13/5: 611-618.
Jenkins, L.J. (1988) Acute delayed neurotoxicity of diazinon mg-8 in
domestic fowl. Number 5152-87. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kabrawala, V.N., Shah, R.M. & Oza, G.G. (1965) Diazinon poisoning. A
study of 25 cases. The Indian Practitioner, 18: October, 711-717.
Kaplanis, J.N., Louloudes, S.J. & Roan, C.C. (1962) The
distribution and excretion of 32p-labelled diazinon in guinea-pigs.
Transactions of the Kansas Academy of Science, 65/1: 70-75.
Kirchner, F.K., McCormick, G.C & Arthur, A.T. (1991) G 24480 Techn.:
One/two-year oral toxicity study in rats. Number 882018. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Krinke, G.J. (1991) Comment on paper: neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice by Spyker
and Avery. J. Toxicol. Environm. Health, 3: 989-1002 (1977).
Letter dated December 17 (1991) prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Kuhn, J.O. (1989a) G 24480 tech. (Diazinon MG 8 FL 880045): Acute
oral toxicity study in rats. Number 5942-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989b) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
eye irritation study in rabbits. Number 5944-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989c) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
dermal irritation study in rabbits. Number 5945-89. Unpublished
Report Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989d) Diazinon MG8 FL 880045: Acute dermal toxicity
study in rabbits. Number 5943-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989e) Diazinon MG8 FL 880045: Dermal sensitization
study in guinea-pigs. Number 5946-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kushaba-Rugaaju, S. & Kitos, P.A. (1985) Effects of diazinon on
nucleotide and amino acid contents of chick embryos -teratogenic
considerations. Biochemical Pharmacology, 34(11): 1937-1943.
Machin, A.F., Rogers, H., Cross, A.J., Quick, M.P., Howells, L.C. &
Janes, N.F. (1975) Metabolic aspects of the toxicology of diazinon.
I hepatic metabolism in the sheep, cow, pig, guinea-pig, rat,
turkey, chicken and duck. Pestic. Sci. 6: 461-473.
Macklin, A.W. & Ribelin, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc. (JAVMA), 159/12:
1743-1748.
Maizlish, N., Schenker, M., Weisskopf, C., Seiber, J., & Samuels S.
(1987) A behavioral evaluation of pest control workers with short-
term, low-level exposure to the organophosphate diazinon. Am. J.
Ind. Med. 12: 153-172.
Marshall, Th. C. Dorough, H.W. & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agric. Food Chem. 24/3: 560-563.
Matsuda, A., Hayashi, M. & Ishidate, M. (1979) Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutation Research, 66: 277-290.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 Coded Chemicals.
Environm. Mol. Mutagen, 12: 85-154.
Misawa, M., Doull, J., Kitos,P.A. & Uyeki, F.M. (1981) Teratogenic
effects of cholinergic insecticides in chick embryos I. Diazinon
treatment on acetylcholinesterase and choline acetyltransferase
activities. Toxicol. Appl. Pharmacol. 57: 20-29.
Miyamoto, J. (1976) Terminal residues: evaluation of diazinon. J.
Assoc. Offic. Anal. Chem. 59: 878-880.
Mücke, W., Alt, K.O. & Esser, H.O. (1970) Degradation of 14C-
labelled diazinon in the rat. Agric. Food. Chem. 18/2: 208-212.
Murli, H. & Haworth S.R. (1990b) Mutagenicity test on diazinon MG 8:
in vivo sister chromatid exchange assay. Number HLA-12226-0-458,
TX 900093. Unpublished Report Prepared by Hazleton Laboratories
America Inc., Kensington, Maryland.
Murli, H. & Haworth, S.R. (1990a) Mutagenicity test on diazinon MG 8
in an in vitro cytogenetic assay measuring sister chromatid
exchange frequencies in cultured whole blood human lymphocytes.
Number HLA-12226-0-448, TX 900093. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
NCI (1976) Bioassay of diazinon for possible carcinogenicity. Nov.
1976. Carcinogenesis Testing Program National Cancer Institute
(NCI). U.S. Department of Health, Education and Welfare. DHEW
Publication No. (NIH) 79-1392.
NIEHS (National Institute of Environmental Health Services) (1991)
Toxicology data management system tumor incidence in control animals
by route and vehicle of administration F/344N rats. Prepared for
NIEHS by Analytical Sciences Inc., Durham, NC, USA.
Payot, P.H. (1966) Subacute oral toxicity study on diazinon AS-
humans. Unpublished Report dated October, Prepared by Geigy, Basle,
Switzerland.
Piccirillo, V.J. (1978) Acute oral toxicity study in rats with
diazinon technical. Number 483-143. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
Pickles, M. & Seim, V. (1988) Biological report for the metabolism
of 2-pyrimidinyl-14c-diazinon in a lactating goat. Number BIOL-
88004, 302212. Unpublished Report Prepared by Ciba-Geigy Corp., Vero
Beach, Florida.
Pickles, M. & Seim, V. (1990) Biological report for the metabolism
of 14C-diazinon on sheep. Numbers: BIOL-89014, 302925. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Robbins, W.E., Eddy, G.W. & Hopkins, Th.L. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem. 5/7: 509-513.
Rudzki, M.W., Arthur, A.T. & McCormick, G.C. (1991) Diazinon (MG-8):
52-week oral toxicity study in dogs. Number 882014. Unpublished
Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sachsse, K. & Bathe, R. (1975) Potentiation study: C8514
(chlordimeform) versus 3 insecticides, G 24 480 (Diazinon), CGA 12
223 and CGA 15324 in the rat. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1976) Potentiation study: C9491
(Jodfenphos) versus 2 Insecticides, G 24480 (Diazinon), and C 177
(Dichlorvos) in the rat. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977) CGA 15342 versus 2 insecticides, GS
13005 (Methidathion) and G 24480 (Diazinon) in rat. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1978) Potentiation study CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (Phosphamidon), GS 13005
(Methidathion), G 24480 (Diazinon) CGA 15324 and Malathion. Number
404478 Siss 6526. Unpublished Report Prepared by Ciba-Geigy Ltd.,
Basle, Switzerland.
Sanderson, D.M. & Edson, E.F. (1959) Oxime therapy in poisoning by
six organophosphorus insecticides in the rat. J. Pharm. Pharmacol.
11: 721-728.
Schoch, M. & Gfeller, W. (1985) G 24480 techn. Acute oral LD50 in
the rat. Number 850864. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland
Shishido, T., Usui, K. & Fukami, J. (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pest.
Biochem. Physiol. 2: 27-38.
Shishido, T., Usui, K., Sato, M. & Fukami, J. (1972b) Enzymatic
conjugation of diazinon with glutathione in rat and American
cockroach. Pest. Bioch. Physiol. 2: 51-63.
Shishido, T. & Fukami, J. (1972) Enzymatic hydrolysis of diazoxon by
rat tissue homogenates. Pest. Bioch. Physiol. 2: 39-50.
Simoneaux, B., Ballantine, L., Brown, K. & Lai, K. (1988) Metabolite
identification in hens and goats treated with 14C-diazinon. Number
ABR-88135. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B., Brown, K. & Lai, K. Summer, D. (1989) Supplemental
report on the nature of residues of diazinon in hens. Numbers ABR-
89040, 302213. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988a) Disposition of 14C-diazinon in goats.
Number ABR-88117. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988b) Disposition of 14C-diazinon in chickens.
Number ABR-88116. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988c) Characterization of 14C-diazinon
metabolites in goats. Number ABR-88118. Unpublished Report Prepared
by Ciba-Geigy Corp., Greensboro, NC.
Simoneaux, B.J. (1988d) Characterization of 14C-diazinon
metabolites in chickens. Number ABR-88119. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Singh, A.R., Arthur, A.T. & McCormick, G.C. (1988) Diazinon (MG-8)
13-week oral feeding study in rats. Numbers MIN-882011, 88083.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sobti, R.C., Krishan, A. & Pfaffenburger, C.D. (1982) Cytokinetic
and cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutation Research,
102: 89-102.
Spyker, J.M. & Avery, D.L. (1977) Neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice. J.
Toxicol. Environm. Health, 3: 989-1002.
Strasser, F. & Arni, P. (1988) G 24480: Sister chromatid exchange
test on human lymphocytes cell line CCL 156 in vitro. Number
871697. Unpublished Report Prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Tai, Ch. & Katz, R. (1984) Diazinon Techn.: 21-day dermal toxicity
study in rabbits. Number 842007. Unpublished Report Prepared by
Ciba-Geigy Corp. Greensboro, NC.
WHO (1990) Principles for the toxicological assessment of pesticide
residues in food. Environmental Health Criteria 104, Geneva.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wills, J.H. (1959) Recent studies of organic phosphate poisoning.
Federation Proc. 18: 1020-1025
Yang, S.H.R., Hodgson, E. & Dautermann, W.C. (1971) Metabolism in
vitro of diazinon and diazoxon in rat liver. J. Agr. Food Chem.
19/1: 10-13.
Zak, F., Hess, R., Luetkemeier, H. & Sachsse, K. (1973) 21-day
inhalation study on the rat with technical diazinon. Number Siss
1679. Unpublished Report Prepared by Ciba-Geigy Sisseln,
Switzerland.
Zwiener, R.J. & Ginsburg, C.M. (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81/1: 121-126.
 Short-term toxicity studies
Although reported in the summaries of the following studies,
the inhibition of plasma cholinesterase was not utilized as a
criterion for the NOAEL, whereas inhibition of the erythrocyte
cholinesterase activity may be taken as an indicator of an adverse
effect of anticholinesterase pesticides. An inhibition of > 20%
compared to the control activity is regarded as significant (Dressel
et al., 1980; WHO, 1990).
Rats
Two short-term inhalation studies were conducted in rats. In a
first study groups of rats (9/sex/group) were exposed to an aerosol
of diazinon (purity 97.1%; droplet size < 1 µm 30-40%, 1-7 µm 50%)
for 6 hours a day, 5 days per week for three weeks. Only the
animals' snouts were exposed to the aerosol. The mean
concentrations were 0, 151, 245 or 559 mg/m3. The treatment did
not cause changes in the mortality rate, in haematology,
macroscopical and histopathological findings or organ weights that
were attributable to the inhalation of diazinon. Exophthalmus and
diarrhoea were observed in animals at all dose levels and tonic-
clonic muscle spasms occurred in the high-dose animals. The
symptoms were reversible. Food intake at the highest dose level was
reduced at the beginning of the treatment period. Body-weight gain
was reduced in male rats at 245 and 559 mg/m3, and in female rats
at 559 mg/m3. Plasma cholinesterase was inhibited at the
intermediate and high levels, resulting in values corresponding to
56% and 37% of the activity in control animals, respectively.
Erythrocyte cholinesterase was inhibited only at the highest dose
level (34% of the control activity). Brain cholinesterase activity
was dose-dependently reduced at all dose levels resulting in
activities of 81, 56 and 37% of the control activity in both sexes
at the low-, medium- and high-dose levels, respectively. The
cholinesterase values returned to normal at the end of the 25-day
recovery period. The NOAEL in this study was < 151 mg/m3
(corresponding to an estimated dose of 55 mg/kg bw/day), based on
lack of significant brain cholinesterase inhibition at the lowest
dose level (Zak et al., 1973).
In a more recent 21-day inhalation study, diazinon (purity 88%;
particle diameter 0.7-1.4 µm) was administered in a nose-only
exposure system in rats (10/sex/group). The intended aerosol
concentrations were 0, 0.1, 0.3, 1 or 10 mg/m3 (actual: 0, 0.05,
0.46, 1.57, 11.6 mg/m3). The animals were exposed during 6 hours a
day, 5 days per week for 3 weeks. The treatment did not adversely
affect body-weight gain, food consumption, haematological
parameters, organ weights, macroscopy and histopathological
findings. At 1 and 10 mg/m3, plasma cholinesterase activity was
reduced in females by 70% and 50% compared to control animals,
respectively. Erythrocyte cholinesterase was inhibited to 60% of
the control activity at 10 mg/m3 in both sexes, whereas the
activity of the brain cholinesterase was reduced in females only to
80% of the control activity at 1 mg/m3, and to 60% in the high-dose
animals. The increase of the relative lung weight observed in
females at 0.3 and 1 mg/m3 but not at the high dose was not
considered biologically significant. The NOAEL in this study was
0.46 mg/m3 (corresponding to an estimated dose of 0.2 mg/kg
bw/day), based on inhibition of brain cholinesterase at higher dose
levels (Hartmann, 1990).
Four groups of rats (Sprague-Dawley; Crl: VAF/Plus CD(SD)Br.;
15/sex/group) were orally treated with diazinon (purity 87.7%) at
dietary concentrations of 0, 0.5, 5, 250 or 2500 ppm for 90 days.
The treatment had no effect on mortality, water consumption,
ophthalmoscopy or urinalysis. Treatment-related occurrence of soft
faeces, hypersensitivity to touch and sound and in some male animals
aggressiveness were observed at the 2500 ppm dose level. Moreover,
hyperactivity was observed in several animals of the high-dose group
at the end of the study. Body-weight gain was reduced at 2500 ppm
by 6% in males and 13% in females compared to control animals. Food
consumption was reduced at 2500 ppm sporadically on single days in
both sexes. Haematological and biochemical investigations were
performed in the last study week. In the highest dose females,
slight reductions in haemoglobin and haematocrit values, as well as
an increase of the white blood cell count and reticulocyte count
were observed. A dose-dependent decrease in the mean serum
cholinesterase activity was observed in females and males at 5 ppm
(22% and 74%, respectively, of activity in control group), at 250
ppm (3% and 12% of the control activity, respectively) and at 2500
ppm (2% and 4% of the control activity, respectively). Erythrocyte
cholinesterase activity was reduced to 73% and 59% of control
activity at 250 and 2500 ppm in males and females, respectively. A
reduction in brain cholinesterase activity was found at 250 ppm in
females only (59%) and at 2500 ppm in both sexes resulting in
activities of 40%-50% of the control activity. An increase in
female liver and kidney weights was observed at 2500 ppm.
Microscopic evidence of centrolobular hepatocellular hypertrophy was
observed in single females at 250 ppm and in most females at 2500
ppm. The NOAEL in this study was 5 ppm, equal to 0.4 mg/kg bw/day,
based on erythrocyte and brain cholinesterase inhibition at 250 ppm
and higher (Singh et al., 1988).
Rabbits
In a dermal toxicity study, groups of albino rabbits
(5/sex/group) received dermal applications of 0, 1, 5 or 100 mg/kg
bw diazinon (purity 97.1%; 50% aqueous suspension in PEG 300), 5
days a week during a 3-week period. Because of high mortality in
males at 100 mg/kg bw (4/5 animals died between study days 3 and 6),
this dose level was reduced to 50 mg/kg bw on day 8 of the study.
In high-dose animals, signs of toxicity included anorexia, ataxia,
tremors, diarrhoea, hypoactivity, hypotonia and salivation. Body-
weight gain and food consumption tended to be higher in treated
animals compared to control animals. Serum cholinesterase was
inhibited in both sexes at 100/50 mg/kg bw resulting in values of
about 37% of the control group activity. At 5 mg/kg bw, activities
were 77% and 65% of the control activity in males and females,
respectively. At 1 mg/kg bw, inhibition was only found in females
resulting in an activity of 68%. Erythrocyte acetylcholinesterase
was inhibited only at 100/50 mg/kg bw resulting in 61% and 68% of
the control activity in males and females, respectively. Brain
acetylcholinesterase was also inhibited only at the highest dose
level of 100/50 mg/kg bw resulting in values corresponding to 72%
and 57% of the control activity in males and females, respectively.
The only change in organ weights was a decrease in kidney weight in
females at 100/50 mg/kg bw. No treatment-related macroscopic or
microscopic alterations were observed with the exception of slight
erythema at the application sites. Histologically a slight
hyperkeratosis was observed in treated skin of high-dose animals
The results of this study indicate a marked percutaneous absorption
and are thus in agreement with the results of other studies. The
NOAEL in this study was 5 mg/kg bw dermal, based on erythrocyte and
brain cholinesterase inhibition at 100/50 mg/kg bw (Ballantine et
al., 1984; Tai & Katz 1984).
Dogs
In a 90-day feeding study, diazinon (purity 87.7%) was
administered to groups of beagle dogs (4/sex/group) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
No treatment-related mortalities occurred. The treatment did not
adversely affect food consumption, ophthalmoscopy, haematology,
urinalysis, organ weights or macroscopic findings. Clinical signs
such as emesis or bloody faeces were sporadically observed in males
at 300 ppm and in females at 150 ppm. Reduced body-weight gain was
observed in females at 150 ppm and at 300 ppm in both sexes. In
males, inhibition of serum cholinesterase was observed resulting in
activities of 70%, 20% and 15% of that measured in control animals
at the end of the study at 0.5, 150 and 300 ppm, respectively.
Erythrocytes activities corresponding to 75% and 69% of control
activity were measured at 150 and 300 ppm, whereas in brain the
acetyl cholinesterase inhibition resulted in 69% and 58% of control
activity at 150 and 300 ppm, respectively. In females a reduction
in acetylcholinesterase activity was observed at 150 and 300 ppm.
The inhibition of serum and erythrocyte cholinesterase resulted in
an activity of about 18% and 70% of control activity, respectively,
at both dose levels, and brain cholinesterase inhibition resulted in
70% and 55% of control activity at 150 and 300 ppm, respectively.
Other changes of biochemical parameters consisted of a decrease in
total protein at 300 ppm in males. The only microscopic alteration
that might be compound-related was atrophy of the pancreatic acini
in one male dog of the highest dose group. The NOAEL in this study
was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on erythrocyte and
brain cholinesterase inhibition at 150 ppm and above (Barnes et
al., 1988).
Diazinon has been reported to produce pancreatic acinar lesions
in dogs at 75 mg/kg bw/day (Frick et al., 1987).
In a 52-week oral toxicity study, groups of beagle dogs
(4/sex/group) were fed diazinon (purity 87.7%) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
Due to lack of body-weight gain the dietary concentration of 300 ppm
was reduced to 225 ppm after 14 weeks of treatment (dose 300/225
ppm). Mortality was not increased by treatment and haematology,
urinalysis, gross pathology and histopathology revealed no changes
attributable to treatment. Overt clinical signs of dehydration and
emaciation became evident in one male at 300/225 ppm. The symptoms
remained even though the initial dose was reduced. Reductions in
body-weight gain were observed at 150 ppm and higher in males and at
300/225 ppm in females. However, no clear-cut dose-response
relationship was evident and the differences attained statistical
significance compared to the control group at only some observation
times. Food consumption was reduced at 150 ppm and higher, again
without a clear dose-response relationship, most probably due to
reduced palatability of the feed admixtures. Treatment-related
decreases in acetylcholinesterase activity at dose levels of 0.5 ppm
and higher were found: serum cholinesterase was reduced at 0.5 ppm
and higher in males and at 150 ppm and higher in females, resulting
in activities of about 20% of the activity in the control group at
150 and 300/225 ppm in both sexes. Erythrocyte cholinesterase
activity was also reduced at 150 and 300/225 ppm, where activities
corresponding to about 70% of the control activity were measured in
both sexes. Brain cholinesterase was inhibited at 150 ppm to 75% of
control activity in females and at 300/225 ppm to 65% and 75% of
control activity in females and males, respectively. The NOAEL in
this study was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on
erythrocyte and brain cholinesterase inhibition at 150 ppm and above
(Rudzki et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of mice (B6C3F1 Hybrid; 50 (control 25)/sex/group)
were administered diazinon (purity 98%) at 0, 100 or 200 ppm over
103 weeks. Survival, body weight, clinical signs and pathology were
investigated. The treatment had no effect on survival or body-
weight development. An increased incidence of hepatocellular
carcinomas was observed in the low-dose males only. The incidences
of hepatocellular carcinomas were 19, 43 and 21% in the control, low
and high-dose males, respectively, whereas the incidence of liver
adenomas was not increased in treated animals compared to control
animals. The mean incidences of hepatocellular carcinomas in
historical controls of this mouse strain (18 studies) were reported
to be 14% (max. 27%) for males and 3% (max. 10%) for females.
Concerning the total incidence of carcinomas and adenomas,
incidences of 29% (max. 58%) and 11% (max. 34%) in males and females
were reported (personal communication from Ciba Geigy to WHO, 1993).
Thus the incidence of hepatocellular carcinomas found in males of
the low-dose group markedly exceeded the range of historical control
incidences. Because no dose-response relationship was evident, the
result was most probably fortuitous and could not be interpreted as
providing evidence for a carcinogenic potential of diazinon in mice
(NCI, 1976).
Rats
Groups of rats (Fischer F344; 50 (control 25)/sex/group) were
fed diazinon (purity 98%) at dietary concentrations of 0, 400 or 800
ppm for 103 weeks. Survival, body weight, clinical signs and
pathology were the parameters investigated. There was no dose-
related reduction of survival and the body-weight development was
similar in control and treatment groups. Clinical signs of
hyperactivity were observed in treated males in both dose groups and
in high-dose females. The incidence of leukaemia was increased in
low-dose males (50%) compared to the incidence in the concurrent
control (20%) and high-dose males (24%). The leukaemias were of the
type usually seen in aging F344 rats. In females, the incidences of
leukaemia were also higher (12% at 400 ppm and 10% at 800 ppm)
compared to the concurrent controls (4%). Historical control
incidences of leukaemia were reported to be 48% for males (range 32-
62%) and 27% for females (range 14-15%). Because of the lack of a
dose-response relationship, the increase in tumour incidence was
considered of questionable significance and the results therefore
could not be interpreted as providing evidence for a tumorigenic
potential of diazinon in rats (NCI, 1976; NIEHS, 1991).
Diazinon (purity 87.7%) was administered as feed admixture to
groups of rats (Sprague-Dawley; 30-40/sex/dose) at concentrations of
0, 0.1, 1.5, 125 or 250 ppm (adjusted for purity) for up to 98-99
weeks. An additional vehicle control group was included in this
study. The animals of this dose group were treated with epoxidized
soybean oil. Up to 10 rats/sex/group were used for the interim
sacrifice after at least 52 weeks of treatment. Another number of
up to 10 rats/sex from the control and high-dose group were placed
on a four-week recovery period and sacrificed thereafter. The study
was terminated after 98-99 weeks because of decreased survivability
in the 0.1 ppm male group only, which was unrelated to treatment and
associated with age-related changes (e.g., senile nephropathy and/or
pituitary adenoma, both considered to be the result of senescence in
this rat strain). This earlier termination was not considered to
have affected the quality or integrity of the study because a
sufficient number of animals were at risk in the other treatment
groups for the development of tumours. The treatment did not cause
increased mortality, and did not affect ophthalmological or
haematological findings, urinalysis or organ weights. Body-weight
gain was increased in males at dose levels of 0.1 ppm and higher and
in some instances in females at 125 ppm and higher compared to the
untreated control animals. Because the body-weight gain in the
vehicle control group also showed an increase compared to the
untreated controls, the increases in body-weight may reflect an
increased palatability of the feed admixtures containing the
epoxidized soybean oil. In fact the increases in mean body-weight
gain generally coincided with increases in mean food consumption in
these dose groups. Decreases in serum cholinesterase activities
were observed at concentrations of 1.5 ppm and higher in both sexes,
resulting in values of about 50% of the control activity at the end
of the study. A dose-dependent reduction of erythrocyte
cholinesterase activity was observed at 125 and 250 ppm resulting in
activities of 80% and 75% of the control activity in males and
females, respectively, at either dose level at the end of the study.
Brain cholinesterase activity was also inhibited to 76% and 71% of
the control activity in males and females, respectively, at 125 ppm,
and to 58% and 52% of the control activity at 250 ppm in males and
females, respectively. Similar inhibition was observed after the
first year of the study. A slight reduction of less than 9% was
still found after the 4-week recovery period in erythrocyte and
brain cholinesterase activity at 250 ppm. Gross and microscopic
pathology examinations did not give evidence of any compound-related
lesions. The NOAEL in this study was 1.5 ppm, equal to 0.07 mg/kg
bw/day, based on inhibition of erythrocyte and brain cholinesterase
at 125 ppm and above (Kirchner et al., 1991).
Reproduction studies
Rats
Diazinon (purity 94.9%) was administered in the diet to groups
of rats (Sprague-Dawley CRCD; 30/sex/group) over two parental
generations (F0, F1) and up to weaning of the F2 pups at
concentrations of 0, 10, 100 or 500 ppm. Treatment-related clinical
symptoms and deaths were observed in a few high-dose females. They
consisted of tremors (3/30) and dystocia (2/30) followed by death or
sacrifice in the F0 generation. In the F1 generation, a few high-
dose females showed tremors as only compound-related signs. Food
consumption was increased in females of the 500 ppm group in the F0
generation, whereas in the F1 generation, a dose-related reduction
of food consumption was observed at 100 and 500 ppm in males only.
Body-weight gain was lower in F0 females at 500 ppm during
gestation. In the F1 generation, reduced body-weight gain was
observed at 100 and 500 ppm in males and at 500 ppm in females.
There were no treatment-related effects on mating behaviour and the
reproductive parameters (including mating, fertility, gestation
indices, number of viable pups, and number of stillborn pups) were
comparable in control and treatment groups in the F0 generation and
at the low and intermediate dose level of the F1 generation. At
500 ppm, a greater proportion of females showed a prolonged
gestation duration at both generations. A decrease in the number of
pregnancies and viable pups as well as reduced fertility and mating
indices were observed in high-dose females of the F1 generation. A
dose-related reduction in survival of F1 pups was observed at 100
ppm and 500 ppm and in F2 at 500 ppm. Weights of F0 pups were
reduced in the 100 and 500 ppm groups, and in F2 pups at 500 ppm.
No treatment-related malformations were found in the pups. The
NOAEL in this study was 10 ppm, equivalent to 0.5 mg/kg bw/day,
based on reduced parental body-weight gain and reduced viability of
pups and pup weights at 100 ppm and higher (Giknis 1989).
Special studies on embryotoxicity/teratogenicity
Mice
Groups of mice (6 females/dose) were given oral daily doses of
0, 0.18 or 9 mg/kg bw diazinon throughout gestation. Treated
animals gained less weight during gestation compared to control
animals. Weight gain of pups born to mothers receiving 9 mg/kg
bw/day was reduced. Daily testing for physiological and behavioural
development of the pups revealed some evidence of retarded
development among the offspring of high-dose animals (e.g.,
retardation of eye and ear opening). Measures of endurance and
coordination also gave some evidence of impairment (e.g., increased
rod cling endurance in both groups, reduced rotarod endurance,
impaired running performance in a maze inclined plane test).
Impaired reactions were sometimes observed at both dose levels but
without a clear dose-effect relationship. Examination of brain
tissue of only 8 of a total number of 132 offspring at the high-dose
level revealed morphological abnormalities in the forebrain. The
relationship of these findings to the observed behavioural changes
is unknown (Spyker & Avery 1977). The evidence for morphological
effects of diazinon on the developing brain was considered
insufficient by an expert (Krinke, 1991).
Rats
In a teratogenicity study in Sprague-Dawley rats at dose levels
of 0, 15, 50 or 100 mg/kg bw/day administered orally on days 6
through 15 of gestation, dams at the 100 mg/kg bw/day dose level
showed a marked decrease in food consumption correlating with weight
loss at the beginning of the treatment period. Skeletal assessment
showed a slightly higher incidence of incomplete ossification at
different sites in the fetuses at 100 mg/kg bw/day. Visceral
examination revealed a dystopia cordis in association with
hypoplasia of lungs in 1/105 fetuses at 100 mg/kg bw/day. This
anomaly has been reported to occur spontaneously in control animals.
Because of the single occurrence this anomaly was not considered to
be due to a direct action of diazinon but to be secondary to
maternal toxicity (Fritz, 1974).
In a later study, groups of rats (Charles River Crl. COBS
CD(SD)(BR); 27 females/group) were treated with doses of diazinon
(purity 97.4%) at 0, 10, 20 or 100 mg/kg bw/day by gavage during
gestational days 6 through 15. The treatment had no effect on the
mortality of the dams. During the first half of the dosing period,
the dams lost weight and food consumption was reduced at 100 mg/kg
bw/day. No compound-related clinical signs and no abnormal gross
pathological findings were observed in the dams. At 100 mg/kg
bw/day some reproductive parameters differed from the control values
but no statistical significance was achieved (e.g., increase in
number of resorptions, percent pre- and post-implantation loss,
reduction in number of live fetuses). On gross observation, 3/262
(1%) fetuses from 3 litters showed external malformations (single
occurrences of a umbilical hernia, filament tail, sublingual
extraneous soft tissue), whereas in the concurrent controls no
similar effects were observed. Umbilical hernia and tail
abnormalities however are reported to be spontaneous malformations
observed routinely in this rat strain. Concerning skeletal
variations, an increased incidence in rudimentary ribs (T-14) was
observed in all treatment groups attaining statistical significance
in the high-dose group (5% versus 0%). Because of the single
occurrences of the different malformations observed and because the
malformations also occur in control animals they were considered to
be a consequence of the marked maternotoxicity at this dose level,
and not due to a teratogenic effect of the test compound. The
increased incidence of the skeletal variation observed at the top
dose level was considered to be related to maternotoxicity at this
dose level. The NOAEL in this study was 20 mg/kg bw/day based on
maternotoxicity and fetotoxicity at the higher dose level (Infurna &
Arthur, 1985).
Rabbits
Diazinon (purity 89.2%) was administered to groups of pregnant
rabbits (New Zeeland white; 18-22 females/group) by gavage at dose
levels of 0, 7, 25 or 100 mg/kg bw/day from days 6-18 of gestation.
An increase in maternal mortality was observed at 100 mg/kg bw/day
(41% vs 0% in all other groups). Overt clinical signs of maternal
toxicity at 100 mg/kg bw/day included tremors, convulsions,
hypoactivity and anorexia. Reduced body-weight gain was found at
the highest dose level. The treatment did not influence the number
of corpora lutea, number of implantation sites, live fetuses per
litter or fetal weight. The incidence of visceral and skeletal
malformations and variations showed no differences between the
groups that could be attributed to treatment. The study therefore
gave no evidence for an embryotoxic or teratogenic activity of
diazinon. The NOAEL in this study was 25 mg/kg bw/day based on
materno-toxicity at the highest dose level (Harris & Holson, 1981).
Chickens
The teratogenic effects of some anticholinesterase insecticides
(organophosphates and methylcarbamates) on chicken embryos have been
known for some time. In a first study, eggs were injected with
diazinon to explore some of the biochemical variables that might be
involved in producing micromelia, beak and feather deformities (type
I signs) in avian embryos that are known to be caused by OP
insecticides. The results of this study confirm the assumption that
a tryptophan deficiency (tryptophan plays an important role in the
developing embryo) may cause the abnormalities observed (Kushaba-
Rugaaju & Kitos, 1985).
Another study was conducted to investigate the involvement of
cholinergic functions in OP-induced teratogenesis in chick embryos
by examining the effects of diazinon on the developmental pattern of
AChE and ChAT activities in hindlimb, wing and brain. The results
of this study confirmed that type II teratogenesis (wry neck, short
neck, arthrogryposis and muscular hypoplasia of legs) may be
mediated via cholinergic nicotinic receptors. Cholinergic
dysfunction including decreased activity of AChE and ChAT was not
correlated with diazinon-induced type I teratogenesis (micromelia,
abnormal feathering) (Misawa et al., 1981).
Cattle
As part of a survey to determine the causes of abortion in
dairy cattle in Wisconsin, the potential role of pesticides was
examined. Diazinon in the form of wettable powder was orally
administered to two pregnant cows at a daily dose level of 6.6 mg/kg
bw. The continuous treatment started in the 5th and 6th month of
pregnancy (abortion commonly affects cows in the 5th to 8th month of
pregnancy) and was maintained until parturition. No diazinon
residues were detected in the tissues examined (fat, liver, kidney)
nor in milk. The treatment failed to reveal any abortions (Macklin
& Ribelin 1971).
Special studies on genotoxicity
Diazinon has been adequately tested in a series of genotoxicity
assays. The results are summarized in Table 2.
Special study of effects on the pancreas
A study was conducted using a canine model to investigate the
induction of pancreatic ductal hypertension following cholinesterase
inhibitor intoxication known to be an important triggering mechanism
in the pathogenesis of acute and chronic pancreatitis. Diazinon was
intravenously administered (25 mg/kg bw) to the pancreatic ampulla
of dogs and the tissue cholinesterase activity of the canine
pancreatic sphincters was determined. Two enzymes are responsible
for the total cholinesterase activity of whole blood: a membrane-
bound AChE associated with the erythrocyte membrane, and a soluble
enzyme, pseudocholinesterase or butyrylcholinesterase (BChE) in the
serum. In tissues, which also contain the two forms of
cholinesterase, AChE is important in the regulation of neuromuscular
activity and parasympathetic ganglion transmission, while the role
of BChE is unknown. The observation of the negative correlation
between serum BChE activity and intraductal pancreatic pressure
supports the hypothesis that the pancreatic ductal hypertension
which occurs following cholinesterase inhibitor intoxication is due
to a selective reduction in pancreatic BChE activity (Dressel et
al., 1980).
The induction of acute pancreatitis by OPs found in dogs was
confirmed in guinea-pigs but not in cats. These results may reflect
species-related differences in the distribution of pancreatic BChE
(Frick et al., 1987).
Special studies on antidotes
Previous reports with respect to the usefulness of PAM and
other oxine reactivators against this organophosphate have been
published (Sanderson & Edson, 1959; Wills, 1959). A recent study
was undertaken to provide information on the antidotal effectiveness
of pyridine-2-aldoxime methochloride (2-PAM) against diazinon
poisoning in animals. The administration of atropine (16 mg/ kg bw;
i.m.) or 2-PAM (30 mg/kg bw; i.v.) alone 10 minutes after poisoning
rats with doses of 235 mg/kg bw corresponding to the approximate
oral LD50 or higher provided little or no protection. Best
protection was achieved when the oxime was given orally in
conjunction with atropine (i.m.) or followed by a subsequent dose of
2-PAM orally or i.v. Administration of 2-PAM to diazinon-poisoned
rabbits (1600 mg/kg bw) resulted in reactivation of inhibited blood
ChE activity concurrent with a decrease in signs of poisoning.
Within 2 hours, however, the animals were again weak and ataxic and
blood ChE showed renewed inhibition. The authors suggested that
effective therapy of diazinon intoxication requires repeated doses
of oxime to maintain effective antidote levels in the body (Harris
et al., 1969). In dogs and guinea-pigs, pretreatment with
atropine protected the animals against diazinon-induced pancreatitis
(Frick et al., 1987).
Special study on delayed neurotoxicity
Hens
An oral dose of 28 mg/kg bw/day of diazinon technical (87%
purity) was administered to a group of 18 hens (the target dose of
13 mg/kg bw/day as the approximate LD50 was doubled due to a
preparation error). The schedule to protect the hens from acute
cholinergic effects of diazinon consisted of a 10 mg/kg bw atropine
pretreatment (i.m.). At the time of diazinon dosing, 2-PAM at an
i.m. dose of 50 mg/kg bw was administered. Post-treatment consisted
of concurrent doses of atropine and 2-PAM one and five hours
following dosing. Since there were no neurotoxic responses in the
test group in the three weeks following the treatment they were
again treated with 13 mg/kg bw of diazinon on day 21. One test
group hen was found dead on day 5 after the first dosing, and one
hen six hours after the second treatment. One hen exhibited a
slight unsteadiness in walking only on day 41. No neurotoxic signs
became apparent during the three-week observation period after the
second treatment. Histopathological examination revealed no lesions
in the brain, spinal cord or peripheral nerves in diazinon-treated
animals, whereas animals of the positive control (TOCP treatment)
showed multiple lesions (axonal degeneration) consistent with
peripheral neuropathy (Jenkins, 1988).
Observations in humans
Numerous reports of intentional and accidental intake of
diazinon and other OPs have been published describing the clinical
manifestations of organophosphate toxicity and the usefulness of
erythrocyte and serum cholinesterase activity assays for diagnosis
as well as the efficacy of supportive and specific therapies
(Kabrawala et al., 1965; Payot, 1966; Banerjee, 1967; Gupta &
Patel, 1968; Zwiener & Ginsburg, 1988).
A case of acute diazinon poisoning complicated by pericarditis
and pneumonia followed by recovery has been reported. The
intoxication caused the occurrence of cyanosis, tracheobronchial
congestion, pulmonary edema and pneumonia. The treatment included
the intramuscular injection of atropine (Banerjee, 1967).
Another complication reported following accidental ingestion of
diazinon consisted of severe pancreatitis and a pseudocyst (Dressel
et al., 1979). The induction of pancreatitis was reproduced in
dogs treated intravenously with a dose of 5 mg/kg bw diazinon.
Pancreatitis may be the result of hypersecretion and ductal
obstruction (Dressel et al., 1980).
Table 2. Results of genotoxicity assays on diazinon
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
In vitro
Ames test S. typhimurium 313-5000 µg/0.1 ml 88.0% negative negative Geleick & Arni, 1990
TA 98, 100, 1535, 1537 DMSO
E. coli WP2uvrA
Ames test S. typhimurium in DMSO >50-1000 ? negative negative Marshall et al., 1976
TA 1535, 1536, 1537, 1538 µg/plate
Mouse lymphoma Mouse lymphoma cell 1. 6 to 60 µg/ml with 97.2% negative negative Dollenmeier & Müller,
assay L5178Y/TK+/- activation 1986
2. 12 to 120 µg/ml
without activation
Mouse lymphoma Mouse lymphoma cell up to 100 µg/ml ? positive positive McGregor et al., 1989
assay L5178Y TK+/-
Sister chromatid Chinese hamster cells 10, 20 and 40 µg/ml 99.2% negative Chen et al., 1981
exchange study (V79) 99.2% in DMSO
Chromosomal Chinese hamster lung 0.1 mg/ml ? cytotoxic positive Matsuoka et al., 1979
aberration test cells
Sister chromatid Human lymphoid cells 0.02, 0.2, 2.0 and ? negative negative1 Sobti et al., 1982
exchange study (LAZ-007) 200 µg/ml
in Ethanol (< 0.1%)
Sister chromatid Human lymphoid cells 12.5, 25, 50, 100 and 87.5% negative negative Strasser & Arni, 1988
exchange study (CCL-156) 200 µg/ml in DMSO
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Whole blood human 1. 0.0668 to 2000 µg/ml 88.0% equivocal equivocal2 Murli & Haworth, 1990a
exchange study lymphocytes in DMSO (ħ activation)
2. 0.668 to 20 µg/ml
(non-activation)
3. 2-66.8 µg/ml
(activated)
Autoradiographic Rat hepatocytes 1.1 to 120 µg/ml in 88.0% negative Hertner & Arni, 1990
DNA repair test DMSO
In vivo
Micronucleus test Mouse 1. 120 mg/kg bw oral 87.5% negative Ceresa, 1988
in vivo 2. 30, 60, and
120 mg/kg bw oral
Dominant lethal Mouse oral dose of 14 and 45 95% negative Fritz, 1975
study mg/kg bw
Chromosome studies Mouse a) daily oral doses of ? negative a) Hool & Müller, 1981c
in male germinal a) spermatogonia 10.5, 21 and 63 mg/kg b) Hool & Müller, 1981b
epithelium b) spermatocytes bw for five days, or
b) 5 treatments with
the same doses over
a period of 10 days
Sister chromatid Bone marrow cells single oral doses of 88.0% negative Murli & Haworth, 1990b
exchange study of mice 10, 50 and 100
in vivo mg/kg bw
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Chinese hamster bone oral gavage of 6.5 ? negative Hool & Müller, 1981a
exchange study marrow cell to 26 mg/kg bw
Nucleus anomaly Chinese hamster bone oral gavage of ? negative Hool & Müller, 1981d
test marrow cells 6.5, 13 and 26 mg/kg
bw/day for two days
1 Cyotoxicity at a concentration of 20 µg/ml. The increased frequency of sister chromatid exchanges at 20 µg/ml with
metabolic activation did not show a doubling of the control frequency and was therefore not considered positive.
2 Two independent trials: Cytotoxicity at concentrations of 66.8 µg/ml (with activation) respectively. Increases in the SCE
frequency (just doubling) observed only in the first assay without metabolic activation at dose levels of 0.668 µg/ml to
20 µg/ml, but without a dose-relationship. Increases observed after activation in both trials did not show doubling and again
no dose-response. Thus the results of these studies are not considered positive.
A study of 60 poisoning cases with diazinon has been reported.
The most common clinical manifestations were vomiting, giddiness,
constricted pupils and signs of bronchoconstriction with pulmonary
congestion. The amount of poison ingested varied from 4 ml to 15 ml
with an average of 7.5 ml (% active ingredient not specified in
publication). This was ingested by 55 patients with suicidal
intention, whereas in 5 patients the compound was taken
accidentally. Atropine injections were administered repeatedly (up
to a total dose of 22.4 g over 42 hours). Five patients died in
spite of intensive atropine therapy initiated more than 8 hours
after ingestion. Other cases who had ingested the same amount of
poison as the fatal cases received treatment within three hours of
ingestion. These findings emphasize the importance of early
treatment in diazinon poisoning (Gupta & Patel, 1968). In addition,
these results are in agreement with an earlier observation that the
combination of diazinon with cholinesterase occurs in two stages, an
early reversible stage and a late irreversible stage (Grob, 1956).
In another study, cases of organophosphate and carbamate
poisoning in 37 infants and children were reported, only 5 of which
were associated with diazinon. Miosis, excessive salivation, muscle
weakness, respiratory distress, lethargy and tachycardia were the
most common clinical findings. Erythrocyte cholinesterase
activities were determined from 24 patients showing erythrocyte
cholinesterase activities that were less than 50% of the lower limit
of the normal range. There were no differences between the
erythrocyte and serum cholinesterase activity in 20/24 patients from
whom both tests were obtained, whereas in the remaining 4 cases
either the erythrocyte or the serum activity was decreased. A
combined atropine/pralidoxime therapy is recommended in case of
organophosphate toxicity. Although the recommended dose for
atropine in infants is 0.01-0.02 mg/kg bw, the dose is generally
insufficient for treatment of signs and symptoms secondary to
organophosphate poisoning (Zwiener & Ginsburg, 1988).
Neurobehavioural effects of short-term, low-level exposure to
diazinon were investigated in 99 pest control workers. A computer
assisted neuro-behavioural test battery (including attention,
vigilance, hand-eye coordination, visual perception, verbal ability)
was used before and after their work shift. The diazinon metabolite
diethyl-thiophosphate (DETP) was measured in urine samples collected
from 46 diazinon applicators and 56 non-applicators. Post-shift
DETP values were 24 and 3 ppb for applicators and non-applicators,
respectively. The study failed to demonstrate any behavioral
effects of diazinon under the conditions of this study (Maizlish et
al., 1987).
The results of the following old study have been reported in an
earlier monograph (Annex I, reference 7). Because of certain
inaccuracies in that monograph, the study was reviewed again and
summarized below.
A subacute oral toxicity study was conducted in four male
volunteers. Diazinon (different batches, purity 95.4-95.7%) was
administered in capsules at dose levels of 2-2.5 mg per day per
person, equal to a dose of 0.025 mg/kg bw/day. The test period
lasted 42 days with an interruption in treatment from the 5th to
10th day in two volunteers and for 34 consecutive days in the other
two volunteers. Plasma cholinesterase activity was markedly
depressed the first 6 days of treatment in 2/4 subjects (no plasma
cholinesterase activity measurable). Therefore treatment was
interrupted for 6 days to enable recovery. Subsequent treatment at
the same dose level did not reveal inhibitory effects on plasma
cholinesterase. The fluctuations observed during the treatment
period were similar to those observed during the pre-test period.
In no instance was the erythrocyte acetylcholinesterase depressed
compared to the pre-test values. Other parameters investigated
included haematology and blood chemistry, urinalyses and
symptomatology. No changes were observed that could be attributed
to the treatment. The NOAEL in this study was 0.025 mg/kg bw/day
based on transitory depression of plasma cholinesterase activity as
the only effect observed at this dose level (Payot, 1966).
COMMENTS
Following oral administration to rats, diazinon was almost
completely absorbed and eliminated, mainly in the urine.
The main degradative pathway includes the oxidase/hydrolase-
mediated cleavage of the ester bond leading to the pyrimidinol
derivative 2-isopropyl-6-methyl-4(1H)-pyrimidinone, which is further
oxidized to more polar metabolites.
Diazinon has moderate acute oral toxicity to mice and rats.
The clinical signs observed were consistent with cholinesterase
inhibition and included sedation, tremors, convulsions and ataxia.
It has been classified by WHO as moderately hazardous.
In an oral 90-day feeding study in rats at dietary
concentrations of 0, 0.5, 5, 250 or 2500 ppm, the NOAEL was 5 ppm
(equal to 0.4 mg/kg bw/day), based on erythrocyte and brain
cholinesterase inhibition at 250 ppm and higher.
In short-term studies in dogs, diazinon was administered at
dietary concentrations of 0, 0.1, 0.5, 150 or 300 ppm for either 90
days or 52 weeks. In both studies, the NOAEL was 0.5 ppm, equal to
0.02 mg/kg bw/day based on erythrocyte and brain cholinesterase
inhibition at 150 ppm and above.
In a carcinogenicity study in mice, diazinon was administered
at dietary concentrations of 0, 100 or 200 ppm over 103 weeks.
There was no evidence of carcinogenicity.
In a carcinogenicity study in rats diazinon was administered at
dietary concentrations of 0, 400 or 800 ppm for 103 weeks. There
was no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study, rats were
maintained on a diet containing diazinon at concentrations of 0,
0.1, 1.5, 125 or 250 ppm for up to 99 weeks. The NOAEL was 1.5 ppm,
equal to 0.07 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase at 125 ppm and above. There was no evidence of
carcinogenicity.
A multigeneration study was conducted in rats using dietary
concentrations of 0, 10, 100 or 500 ppm. The NOAEL was 10 ppm
(equivalent to 0.5 mg/kg bw/day), based on a reduction in parental
body-weight gain in the F1 generation and a reduced survival rate
and reduced body weight of F1 pups at 100 ppm.
In a teratogenicity study in rats, diazinon was orally
administered at dose levels of 0, 10, 20 or 100 mg/kg bw/day.
Maternal toxicity, indicated by weight loss correlating with reduced
food consumption, became evident at 100 mg/kg bw/day. Effects on
the fetuses at this dose level consisted of retarded ossification
and an increased incidence of rudimentary ribs. The NOAEL was 20
mg/kg bw/day based on maternotoxicity and fetotoxicity. There was
no evidence of teratogenicity.
A teratogenicity study in rabbits conducted with oral dose
levels of 0, 7, 25 or 100 mg/kg bw/day revealed clinical signs of
maternotoxicity, increased mortality and reduced body-weight gain at
100 mg/kg bw/day. The NOAEL was 25 mg/kg bw/day. There was no
evidence of teratogenicity.
A neurotoxicity study performed with hens treated at dose
levels of 13 or 28 mg/kg bw/day (protected by atropine pre-
treatment) did not reveal evidence of delayed neurotoxicity.
Diazinon has been adequately tested in a series of genotoxicity
assays. Chromosomal aberrations were induced in cultured mammalian
cells, but there were no other indications of genotoxicity. The
Meeting concluded that diazinon was not genotoxic.
Diazinon was evaluated in four human male volunteers who
received 0.025 mg/kg bw/day of diazinon in capsules for 34-36 days.
There were no consistent treatment-related effects on plasma or
erythrocyte cholinesterase activity, blood chemistry or urinalysis.
No clinical effects were reported. The NOAEL was 0.025 mg/kg
bw/day.
The ADI of 0-0.002 mg/kg bw, which was based on the NOAEL of
0.025 mg/kg bw/day in the study in humans using a 10-fold safety
factor, was maintained.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm, equal to 0.4 mg/kg bw/day (90-day study)
1.5 ppm, equal to 0.07 mg/kg bw/day (99-week study)
10 ppm, equivalent to 0.5 mg/kg bw/day
(reproduction study)
20 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 25 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 0.5 ppm, equal to 0.02 mg/kg bw/day (one-year study)
Human: 0.025 mg/kg bw/day (34-36-day study)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ballantine, L., Marco, G.J. & Williams, S.C. (1984) Percutaneous
absorption of 2 delta - 14C - Diazinon in Rats. Numbers ABR-84011,
302950. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Banerjee, D. (1967) Pericarditis in acute diazinon poisoning. A case
report. Armed Forces Med. J. (India), 23: 187-190.
Barnes, T.B., Arthur, A.T. & Hazelette, J.R. (1988) Diazinon (MG-8):
90-Day Oral Toxicity Study in Dogs. Number 882012. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Bathe, R. (1972a) Acute oral LD50 of technical diazinon in the
mouse. Number Siss 1679. Unpublished Report Prepared by Ciba-Geigy
Ltd., Sisseln, Switzerland.
Bathe, R. (1972b) Acute dermal LD50 of technical diazinon (G-24480)
in the rat. Number Siss 1679. Unpublished Report Prepared by Ciba-
Geigy Ltd., Sisseln, Switzerland.
Bathe, R. & Gfeller, W. (1980) Acute oral LD50 in the rat of
technical G 24480. Number 800478. Unpublished Report Prepared by
Ciba-Geigy Ltd., Sisseln, Switzerland.
Burgener, J. & Seim, V. (1988) Biological report for the metabolism
of 14C-diazinon in Laying Hens Dosed at 25 ppm. Numbers BIOL-88006,
302213. Unpublished Report Prepared by Ciba-Geigy Corp., Vero Beach,
Florida.
Capps, T., Brown, K., Lai, K. & Tweedy, B. (1989) Characterization
and identification of diazinon metabolites in rats. Numbers ABR-
88164, 302211 and 302925. Unpublished Report Prepared by Ciba-Geigy
Corp., Greensboro, NC.
Capps, T. M., Summer, D., Bar, H.P. & Carlin, T.J. (1990)
Characterization and identification of major metabolites in tissues
of sheep treated dermally with 14C-diazinon. Numbers ABR-90014,
302925. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Ceresa, C. (1988) G 24480: Micronucleus test (mouse). Number 871696.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H.H., Hsueh, J.L., Sirianni, S.K. & Huang, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutation Research, 88: 307-316.
Ciba-Geigy 1992: Diazinon Data Summaries. Working Paper for the WHO
Expert group on Pesticide Residues. Biological Data and
Toxicological Evaluation. June 23.
Craine, E.M. (1989) Study of 14C-diazinon disposition in the rat
(biological phase and analytical phase I). Numbers WIL-82030,
302925. Unpublished Report Prepared by WIL Research Laboratories
Inc., Ashland, OH and Ciba-Geigy Corp., Greensboro, NC.
Dahm, P.A. (1970) Some aspects of the metabolism of parathion and
diazinon. In: Biochemical Toxicology of Insecticides. ed. by
O'Brien, R.D. and Yamamoto, I., Academic Press London.
Dollenmeier, P. & Müller, D. (1986) G 24480 techn: L 5178Y/T+/-
mouse lymphoma mutagenicity test. Number 840396. Unpublished Report
Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Dressel, Th. D., Goodale, R.L., Borner, J.W. & Etani, S. (1980) A
study of the cholinesterase of the canine pancreatic sphincters and
the relationship between reduced butyrylcholinesterase activity and
pancreatic ductal hypertension. Annals of Surgery, 192/5: 614-619.
Dressel, T.D., Goodale, K.L., Arneson, M.A. & Borner, J.W. (1979)
Pancreatitis as a complication of anticholinesterase insecticide
intoxication. Annals of Surgery, 189: 199-204.
Frick, T.W., Dalo, S., O'Leary, J.F., Runge, W., Borner, J.W.,
Baraniewski, H., Dressel, T., Shearen, J.G. & Goodale, K.L., (1987)
Effects of insecticide, diazinon, on pancreas of dog, cat and
guinea-pig. J. Environ. Path. Toxicol. Oncol. 7(4): 1-11.
Fritz, H. (1974) G 24480 (Diazinon Techn.): Reproduction study - rat
segment ii: (test for teratogenic on embryotoxic effects).
Unpublished report prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Fritz, H. (1975) G 24480 (Diazinon Techn.): Dominant lethal study
mouse. Number 327507. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Geleick, D. & Arni, P. (1990) Salmonella and Escherichia/liver-
microsome test. Number 891346. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Giknis, M.L.A. (1989) Diazinon techn.: A two-generation reproductive
study in albino rats. Numbers MIN-852218, 88128. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Grob, D. (1956) The manifestations and treatment of poisoning due to
nerve gas and other organic phosphate anticholinesterase compounds.
A.M.A. Arch. Intern. Med. 98: 221.
Gupta, O.P. & Patel, D.D. (1968) Diazinon poisoning. A study of
sixty cases. J. Assoc. Physicians of India, 16: 457-463.
Hagenbuch, J.P. & Mücke, W. (1985) The fate of diazinon in mammals.
Biochemistry Product Evaluation 1/85. Unpublished Report Prepared by
Ciba-Geigy Ltd., Basle, Switzerland.
Harris, S. B. & Holson, J.F. (1981) A teratology study of diazinon
in New Zealand white rabbits. Number 281005. Unpublished Report
Prepared by Science Applications Inc., La Jolla, CA.
Harris, L.W., Fleisher, J.H., Innerebner, T.A., Cliff, W. & Sim,
V.M. (1969) The effects of atropine-oxime therapy on cholinesterase
activity and the survival of animals poisoned with 0,0-diethyl-0-(2-
isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate. Toxicol. Appl.
Pharmacol. 15: 216-224.
Hartmann, H.R. (1990) G 24480: 21-Day repeated exposure inhalation
toxicity in the rat (nose-only exposure). Number 891205. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Arni, P. (1990) G 24480: Autoradiographic DNA repair
test on rat hepatocytes, Number 891345. Unpublished Report Prepared
by Ciba-Geigy Ltd., Basle, Switzerland.
Holbert, M.S. (1989) Diazinon MG8 FL 880045: acute inhalation
toxicity study in rats. Number 5947-89. Unpublished Report Prepared
by Stillmeadow Inc., Houston, Texas.
Hool, G. & Müller, D. (1981a) G 24480: Sister-chromatid exchange
study Chinese hamster. Number 801504. Unpublished Report Including
Supplement to the Report (dated October 13, 1981) Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981b) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatocytes. Number 801502. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981c) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatogonia. Number 801501. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981d) G 24480: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster. Number 801503.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Infurna, R.N. & Arthur, A.T. (1985) A teratology study of diazinon
technical in Charles River rats. Numbers MIN-82296, 52-83.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Iverson, F., Grant, D.L. & Lacroix, J. (1975) Diazinon metabolism in
the dog. Bull. Env. Cont. Toxicol. 13/5: 611-618.
Jenkins, L.J. (1988) Acute delayed neurotoxicity of diazinon mg-8 in
domestic fowl. Number 5152-87. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kabrawala, V.N., Shah, R.M. & Oza, G.G. (1965) Diazinon poisoning. A
study of 25 cases. The Indian Practitioner, 18: October, 711-717.
Kaplanis, J.N., Louloudes, S.J. & Roan, C.C. (1962) The
distribution and excretion of 32p-labelled diazinon in guinea-pigs.
Transactions of the Kansas Academy of Science, 65/1: 70-75.
Kirchner, F.K., McCormick, G.C & Arthur, A.T. (1991) G 24480 Techn.:
One/two-year oral toxicity study in rats. Number 882018. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Krinke, G.J. (1991) Comment on paper: neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice by Spyker
and Avery. J. Toxicol. Environm. Health, 3: 989-1002 (1977).
Letter dated December 17 (1991) prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Kuhn, J.O. (1989a) G 24480 tech. (Diazinon MG 8 FL 880045): Acute
oral toxicity study in rats. Number 5942-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989b) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
eye irritation study in rabbits. Number 5944-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989c) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
dermal irritation study in rabbits. Number 5945-89. Unpublished
Report Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989d) Diazinon MG8 FL 880045: Acute dermal toxicity
study in rabbits. Number 5943-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989e) Diazinon MG8 FL 880045: Dermal sensitization
study in guinea-pigs. Number 5946-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kushaba-Rugaaju, S. & Kitos, P.A. (1985) Effects of diazinon on
nucleotide and amino acid contents of chick embryos -teratogenic
considerations. Biochemical Pharmacology, 34(11): 1937-1943.
Machin, A.F., Rogers, H., Cross, A.J., Quick, M.P., Howells, L.C. &
Janes, N.F. (1975) Metabolic aspects of the toxicology of diazinon.
I hepatic metabolism in the sheep, cow, pig, guinea-pig, rat,
turkey, chicken and duck. Pestic. Sci. 6: 461-473.
Macklin, A.W. & Ribelin, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc. (JAVMA), 159/12:
1743-1748.
Maizlish, N., Schenker, M., Weisskopf, C., Seiber, J., & Samuels S.
(1987) A behavioral evaluation of pest control workers with short-
term, low-level exposure to the organophosphate diazinon. Am. J.
Ind. Med. 12: 153-172.
Marshall, Th. C. Dorough, H.W. & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agric. Food Chem. 24/3: 560-563.
Matsuda, A., Hayashi, M. & Ishidate, M. (1979) Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutation Research, 66: 277-290.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 Coded Chemicals.
Environm. Mol. Mutagen, 12: 85-154.
Misawa, M., Doull, J., Kitos,P.A. & Uyeki, F.M. (1981) Teratogenic
effects of cholinergic insecticides in chick embryos I. Diazinon
treatment on acetylcholinesterase and choline acetyltransferase
activities. Toxicol. Appl. Pharmacol. 57: 20-29.
Miyamoto, J. (1976) Terminal residues: evaluation of diazinon. J.
Assoc. Offic. Anal. Chem. 59: 878-880.
Mücke, W., Alt, K.O. & Esser, H.O. (1970) Degradation of 14C-
labelled diazinon in the rat. Agric. Food. Chem. 18/2: 208-212.
Murli, H. & Haworth S.R. (1990b) Mutagenicity test on diazinon MG 8:
in vivo sister chromatid exchange assay. Number HLA-12226-0-458,
TX 900093. Unpublished Report Prepared by Hazleton Laboratories
America Inc., Kensington, Maryland.
Murli, H. & Haworth, S.R. (1990a) Mutagenicity test on diazinon MG 8
in an in vitro cytogenetic assay measuring sister chromatid
exchange frequencies in cultured whole blood human lymphocytes.
Number HLA-12226-0-448, TX 900093. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
NCI (1976) Bioassay of diazinon for possible carcinogenicity. Nov.
1976. Carcinogenesis Testing Program National Cancer Institute
(NCI). U.S. Department of Health, Education and Welfare. DHEW
Publication No. (NIH) 79-1392.
NIEHS (National Institute of Environmental Health Services) (1991)
Toxicology data management system tumor incidence in control animals
by route and vehicle of administration F/344N rats. Prepared for
NIEHS by Analytical Sciences Inc., Durham, NC, USA.
Payot, P.H. (1966) Subacute oral toxicity study on diazinon AS-
humans. Unpublished Report dated October, Prepared by Geigy, Basle,
Switzerland.
Piccirillo, V.J. (1978) Acute oral toxicity study in rats with
diazinon technical. Number 483-143. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
Pickles, M. & Seim, V. (1988) Biological report for the metabolism
of 2-pyrimidinyl-14c-diazinon in a lactating goat. Number BIOL-
88004, 302212. Unpublished Report Prepared by Ciba-Geigy Corp., Vero
Beach, Florida.
Pickles, M. & Seim, V. (1990) Biological report for the metabolism
of 14C-diazinon on sheep. Numbers: BIOL-89014, 302925. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Robbins, W.E., Eddy, G.W. & Hopkins, Th.L. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem. 5/7: 509-513.
Rudzki, M.W., Arthur, A.T. & McCormick, G.C. (1991) Diazinon (MG-8):
52-week oral toxicity study in dogs. Number 882014. Unpublished
Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sachsse, K. & Bathe, R. (1975) Potentiation study: C8514
(chlordimeform) versus 3 insecticides, G 24 480 (Diazinon), CGA 12
223 and CGA 15324 in the rat. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1976) Potentiation study: C9491
(Jodfenphos) versus 2 Insecticides, G 24480 (Diazinon), and C 177
(Dichlorvos) in the rat. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977) CGA 15342 versus 2 insecticides, GS
13005 (Methidathion) and G 24480 (Diazinon) in rat. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1978) Potentiation study CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (Phosphamidon), GS 13005
(Methidathion), G 24480 (Diazinon) CGA 15324 and Malathion. Number
404478 Siss 6526. Unpublished Report Prepared by Ciba-Geigy Ltd.,
Basle, Switzerland.
Sanderson, D.M. & Edson, E.F. (1959) Oxime therapy in poisoning by
six organophosphorus insecticides in the rat. J. Pharm. Pharmacol.
11: 721-728.
Schoch, M. & Gfeller, W. (1985) G 24480 techn. Acute oral LD50 in
the rat. Number 850864. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland
Shishido, T., Usui, K. & Fukami, J. (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pest.
Biochem. Physiol. 2: 27-38.
Shishido, T., Usui, K., Sato, M. & Fukami, J. (1972b) Enzymatic
conjugation of diazinon with glutathione in rat and American
cockroach. Pest. Bioch. Physiol. 2: 51-63.
Shishido, T. & Fukami, J. (1972) Enzymatic hydrolysis of diazoxon by
rat tissue homogenates. Pest. Bioch. Physiol. 2: 39-50.
Simoneaux, B., Ballantine, L., Brown, K. & Lai, K. (1988) Metabolite
identification in hens and goats treated with 14C-diazinon. Number
ABR-88135. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B., Brown, K. & Lai, K. Summer, D. (1989) Supplemental
report on the nature of residues of diazinon in hens. Numbers ABR-
89040, 302213. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988a) Disposition of 14C-diazinon in goats.
Number ABR-88117. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988b) Disposition of 14C-diazinon in chickens.
Number ABR-88116. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988c) Characterization of 14C-diazinon
metabolites in goats. Number ABR-88118. Unpublished Report Prepared
by Ciba-Geigy Corp., Greensboro, NC.
Simoneaux, B.J. (1988d) Characterization of 14C-diazinon
metabolites in chickens. Number ABR-88119. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Singh, A.R., Arthur, A.T. & McCormick, G.C. (1988) Diazinon (MG-8)
13-week oral feeding study in rats. Numbers MIN-882011, 88083.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sobti, R.C., Krishan, A. & Pfaffenburger, C.D. (1982) Cytokinetic
and cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutation Research,
102: 89-102.
Spyker, J.M. & Avery, D.L. (1977) Neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice. J.
Toxicol. Environm. Health, 3: 989-1002.
Strasser, F. & Arni, P. (1988) G 24480: Sister chromatid exchange
test on human lymphocytes cell line CCL 156 in vitro. Number
871697. Unpublished Report Prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Tai, Ch. & Katz, R. (1984) Diazinon Techn.: 21-day dermal toxicity
study in rabbits. Number 842007. Unpublished Report Prepared by
Ciba-Geigy Corp. Greensboro, NC.
WHO (1990) Principles for the toxicological assessment of pesticide
residues in food. Environmental Health Criteria 104, Geneva.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wills, J.H. (1959) Recent studies of organic phosphate poisoning.
Federation Proc. 18: 1020-1025
Yang, S.H.R., Hodgson, E. & Dautermann, W.C. (1971) Metabolism in
vitro of diazinon and diazoxon in rat liver. J. Agr. Food Chem.
19/1: 10-13.
Zak, F., Hess, R., Luetkemeier, H. & Sachsse, K. (1973) 21-day
inhalation study on the rat with technical diazinon. Number Siss
1679. Unpublished Report Prepared by Ciba-Geigy Sisseln,
Switzerland.
Zwiener, R.J. & Ginsburg, C.M. (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81/1: 121-126.
Short-term toxicity studies
Although reported in the summaries of the following studies,
the inhibition of plasma cholinesterase was not utilized as a
criterion for the NOAEL, whereas inhibition of the erythrocyte
cholinesterase activity may be taken as an indicator of an adverse
effect of anticholinesterase pesticides. An inhibition of > 20%
compared to the control activity is regarded as significant (Dressel
et al., 1980; WHO, 1990).
Rats
Two short-term inhalation studies were conducted in rats. In a
first study groups of rats (9/sex/group) were exposed to an aerosol
of diazinon (purity 97.1%; droplet size < 1 µm 30-40%, 1-7 µm 50%)
for 6 hours a day, 5 days per week for three weeks. Only the
animals' snouts were exposed to the aerosol. The mean
concentrations were 0, 151, 245 or 559 mg/m3. The treatment did
not cause changes in the mortality rate, in haematology,
macroscopical and histopathological findings or organ weights that
were attributable to the inhalation of diazinon. Exophthalmus and
diarrhoea were observed in animals at all dose levels and tonic-
clonic muscle spasms occurred in the high-dose animals. The
symptoms were reversible. Food intake at the highest dose level was
reduced at the beginning of the treatment period. Body-weight gain
was reduced in male rats at 245 and 559 mg/m3, and in female rats
at 559 mg/m3. Plasma cholinesterase was inhibited at the
intermediate and high levels, resulting in values corresponding to
56% and 37% of the activity in control animals, respectively.
Erythrocyte cholinesterase was inhibited only at the highest dose
level (34% of the control activity). Brain cholinesterase activity
was dose-dependently reduced at all dose levels resulting in
activities of 81, 56 and 37% of the control activity in both sexes
at the low-, medium- and high-dose levels, respectively. The
cholinesterase values returned to normal at the end of the 25-day
recovery period. The NOAEL in this study was < 151 mg/m3
(corresponding to an estimated dose of 55 mg/kg bw/day), based on
lack of significant brain cholinesterase inhibition at the lowest
dose level (Zak et al., 1973).
In a more recent 21-day inhalation study, diazinon (purity 88%;
particle diameter 0.7-1.4 µm) was administered in a nose-only
exposure system in rats (10/sex/group). The intended aerosol
concentrations were 0, 0.1, 0.3, 1 or 10 mg/m3 (actual: 0, 0.05,
0.46, 1.57, 11.6 mg/m3). The animals were exposed during 6 hours a
day, 5 days per week for 3 weeks. The treatment did not adversely
affect body-weight gain, food consumption, haematological
parameters, organ weights, macroscopy and histopathological
findings. At 1 and 10 mg/m3, plasma cholinesterase activity was
reduced in females by 70% and 50% compared to control animals,
respectively. Erythrocyte cholinesterase was inhibited to 60% of
the control activity at 10 mg/m3 in both sexes, whereas the
activity of the brain cholinesterase was reduced in females only to
80% of the control activity at 1 mg/m3, and to 60% in the high-dose
animals. The increase of the relative lung weight observed in
females at 0.3 and 1 mg/m3 but not at the high dose was not
considered biologically significant. The NOAEL in this study was
0.46 mg/m3 (corresponding to an estimated dose of 0.2 mg/kg
bw/day), based on inhibition of brain cholinesterase at higher dose
levels (Hartmann, 1990).
Four groups of rats (Sprague-Dawley; Crl: VAF/Plus CD(SD)Br.;
15/sex/group) were orally treated with diazinon (purity 87.7%) at
dietary concentrations of 0, 0.5, 5, 250 or 2500 ppm for 90 days.
The treatment had no effect on mortality, water consumption,
ophthalmoscopy or urinalysis. Treatment-related occurrence of soft
faeces, hypersensitivity to touch and sound and in some male animals
aggressiveness were observed at the 2500 ppm dose level. Moreover,
hyperactivity was observed in several animals of the high-dose group
at the end of the study. Body-weight gain was reduced at 2500 ppm
by 6% in males and 13% in females compared to control animals. Food
consumption was reduced at 2500 ppm sporadically on single days in
both sexes. Haematological and biochemical investigations were
performed in the last study week. In the highest dose females,
slight reductions in haemoglobin and haematocrit values, as well as
an increase of the white blood cell count and reticulocyte count
were observed. A dose-dependent decrease in the mean serum
cholinesterase activity was observed in females and males at 5 ppm
(22% and 74%, respectively, of activity in control group), at 250
ppm (3% and 12% of the control activity, respectively) and at 2500
ppm (2% and 4% of the control activity, respectively). Erythrocyte
cholinesterase activity was reduced to 73% and 59% of control
activity at 250 and 2500 ppm in males and females, respectively. A
reduction in brain cholinesterase activity was found at 250 ppm in
females only (59%) and at 2500 ppm in both sexes resulting in
activities of 40%-50% of the control activity. An increase in
female liver and kidney weights was observed at 2500 ppm.
Microscopic evidence of centrolobular hepatocellular hypertrophy was
observed in single females at 250 ppm and in most females at 2500
ppm. The NOAEL in this study was 5 ppm, equal to 0.4 mg/kg bw/day,
based on erythrocyte and brain cholinesterase inhibition at 250 ppm
and higher (Singh et al., 1988).
Rabbits
In a dermal toxicity study, groups of albino rabbits
(5/sex/group) received dermal applications of 0, 1, 5 or 100 mg/kg
bw diazinon (purity 97.1%; 50% aqueous suspension in PEG 300), 5
days a week during a 3-week period. Because of high mortality in
males at 100 mg/kg bw (4/5 animals died between study days 3 and 6),
this dose level was reduced to 50 mg/kg bw on day 8 of the study.
In high-dose animals, signs of toxicity included anorexia, ataxia,
tremors, diarrhoea, hypoactivity, hypotonia and salivation. Body-
weight gain and food consumption tended to be higher in treated
animals compared to control animals. Serum cholinesterase was
inhibited in both sexes at 100/50 mg/kg bw resulting in values of
about 37% of the control group activity. At 5 mg/kg bw, activities
were 77% and 65% of the control activity in males and females,
respectively. At 1 mg/kg bw, inhibition was only found in females
resulting in an activity of 68%. Erythrocyte acetylcholinesterase
was inhibited only at 100/50 mg/kg bw resulting in 61% and 68% of
the control activity in males and females, respectively. Brain
acetylcholinesterase was also inhibited only at the highest dose
level of 100/50 mg/kg bw resulting in values corresponding to 72%
and 57% of the control activity in males and females, respectively.
The only change in organ weights was a decrease in kidney weight in
females at 100/50 mg/kg bw. No treatment-related macroscopic or
microscopic alterations were observed with the exception of slight
erythema at the application sites. Histologically a slight
hyperkeratosis was observed in treated skin of high-dose animals
The results of this study indicate a marked percutaneous absorption
and are thus in agreement with the results of other studies. The
NOAEL in this study was 5 mg/kg bw dermal, based on erythrocyte and
brain cholinesterase inhibition at 100/50 mg/kg bw (Ballantine et
al., 1984; Tai & Katz 1984).
Dogs
In a 90-day feeding study, diazinon (purity 87.7%) was
administered to groups of beagle dogs (4/sex/group) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
No treatment-related mortalities occurred. The treatment did not
adversely affect food consumption, ophthalmoscopy, haematology,
urinalysis, organ weights or macroscopic findings. Clinical signs
such as emesis or bloody faeces were sporadically observed in males
at 300 ppm and in females at 150 ppm. Reduced body-weight gain was
observed in females at 150 ppm and at 300 ppm in both sexes. In
males, inhibition of serum cholinesterase was observed resulting in
activities of 70%, 20% and 15% of that measured in control animals
at the end of the study at 0.5, 150 and 300 ppm, respectively.
Erythrocytes activities corresponding to 75% and 69% of control
activity were measured at 150 and 300 ppm, whereas in brain the
acetyl cholinesterase inhibition resulted in 69% and 58% of control
activity at 150 and 300 ppm, respectively. In females a reduction
in acetylcholinesterase activity was observed at 150 and 300 ppm.
The inhibition of serum and erythrocyte cholinesterase resulted in
an activity of about 18% and 70% of control activity, respectively,
at both dose levels, and brain cholinesterase inhibition resulted in
70% and 55% of control activity at 150 and 300 ppm, respectively.
Other changes of biochemical parameters consisted of a decrease in
total protein at 300 ppm in males. The only microscopic alteration
that might be compound-related was atrophy of the pancreatic acini
in one male dog of the highest dose group. The NOAEL in this study
was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on erythrocyte and
brain cholinesterase inhibition at 150 ppm and above (Barnes et
al., 1988).
Diazinon has been reported to produce pancreatic acinar lesions
in dogs at 75 mg/kg bw/day (Frick et al., 1987).
In a 52-week oral toxicity study, groups of beagle dogs
(4/sex/group) were fed diazinon (purity 87.7%) at dietary
concentrations of 0, 0.1, 0.5, 150 or 300 ppm (adjusted for purity).
Due to lack of body-weight gain the dietary concentration of 300 ppm
was reduced to 225 ppm after 14 weeks of treatment (dose 300/225
ppm). Mortality was not increased by treatment and haematology,
urinalysis, gross pathology and histopathology revealed no changes
attributable to treatment. Overt clinical signs of dehydration and
emaciation became evident in one male at 300/225 ppm. The symptoms
remained even though the initial dose was reduced. Reductions in
body-weight gain were observed at 150 ppm and higher in males and at
300/225 ppm in females. However, no clear-cut dose-response
relationship was evident and the differences attained statistical
significance compared to the control group at only some observation
times. Food consumption was reduced at 150 ppm and higher, again
without a clear dose-response relationship, most probably due to
reduced palatability of the feed admixtures. Treatment-related
decreases in acetylcholinesterase activity at dose levels of 0.5 ppm
and higher were found: serum cholinesterase was reduced at 0.5 ppm
and higher in males and at 150 ppm and higher in females, resulting
in activities of about 20% of the activity in the control group at
150 and 300/225 ppm in both sexes. Erythrocyte cholinesterase
activity was also reduced at 150 and 300/225 ppm, where activities
corresponding to about 70% of the control activity were measured in
both sexes. Brain cholinesterase was inhibited at 150 ppm to 75% of
control activity in females and at 300/225 ppm to 65% and 75% of
control activity in females and males, respectively. The NOAEL in
this study was 0.5 ppm, equal to 0.02 mg/kg bw/day, based on
erythrocyte and brain cholinesterase inhibition at 150 ppm and above
(Rudzki et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of mice (B6C3F1 Hybrid; 50 (control 25)/sex/group)
were administered diazinon (purity 98%) at 0, 100 or 200 ppm over
103 weeks. Survival, body weight, clinical signs and pathology were
investigated. The treatment had no effect on survival or body-
weight development. An increased incidence of hepatocellular
carcinomas was observed in the low-dose males only. The incidences
of hepatocellular carcinomas were 19, 43 and 21% in the control, low
and high-dose males, respectively, whereas the incidence of liver
adenomas was not increased in treated animals compared to control
animals. The mean incidences of hepatocellular carcinomas in
historical controls of this mouse strain (18 studies) were reported
to be 14% (max. 27%) for males and 3% (max. 10%) for females.
Concerning the total incidence of carcinomas and adenomas,
incidences of 29% (max. 58%) and 11% (max. 34%) in males and females
were reported (personal communication from Ciba Geigy to WHO, 1993).
Thus the incidence of hepatocellular carcinomas found in males of
the low-dose group markedly exceeded the range of historical control
incidences. Because no dose-response relationship was evident, the
result was most probably fortuitous and could not be interpreted as
providing evidence for a carcinogenic potential of diazinon in mice
(NCI, 1976).
Rats
Groups of rats (Fischer F344; 50 (control 25)/sex/group) were
fed diazinon (purity 98%) at dietary concentrations of 0, 400 or 800
ppm for 103 weeks. Survival, body weight, clinical signs and
pathology were the parameters investigated. There was no dose-
related reduction of survival and the body-weight development was
similar in control and treatment groups. Clinical signs of
hyperactivity were observed in treated males in both dose groups and
in high-dose females. The incidence of leukaemia was increased in
low-dose males (50%) compared to the incidence in the concurrent
control (20%) and high-dose males (24%). The leukaemias were of the
type usually seen in aging F344 rats. In females, the incidences of
leukaemia were also higher (12% at 400 ppm and 10% at 800 ppm)
compared to the concurrent controls (4%). Historical control
incidences of leukaemia were reported to be 48% for males (range 32-
62%) and 27% for females (range 14-15%). Because of the lack of a
dose-response relationship, the increase in tumour incidence was
considered of questionable significance and the results therefore
could not be interpreted as providing evidence for a tumorigenic
potential of diazinon in rats (NCI, 1976; NIEHS, 1991).
Diazinon (purity 87.7%) was administered as feed admixture to
groups of rats (Sprague-Dawley; 30-40/sex/dose) at concentrations of
0, 0.1, 1.5, 125 or 250 ppm (adjusted for purity) for up to 98-99
weeks. An additional vehicle control group was included in this
study. The animals of this dose group were treated with epoxidized
soybean oil. Up to 10 rats/sex/group were used for the interim
sacrifice after at least 52 weeks of treatment. Another number of
up to 10 rats/sex from the control and high-dose group were placed
on a four-week recovery period and sacrificed thereafter. The study
was terminated after 98-99 weeks because of decreased survivability
in the 0.1 ppm male group only, which was unrelated to treatment and
associated with age-related changes (e.g., senile nephropathy and/or
pituitary adenoma, both considered to be the result of senescence in
this rat strain). This earlier termination was not considered to
have affected the quality or integrity of the study because a
sufficient number of animals were at risk in the other treatment
groups for the development of tumours. The treatment did not cause
increased mortality, and did not affect ophthalmological or
haematological findings, urinalysis or organ weights. Body-weight
gain was increased in males at dose levels of 0.1 ppm and higher and
in some instances in females at 125 ppm and higher compared to the
untreated control animals. Because the body-weight gain in the
vehicle control group also showed an increase compared to the
untreated controls, the increases in body-weight may reflect an
increased palatability of the feed admixtures containing the
epoxidized soybean oil. In fact the increases in mean body-weight
gain generally coincided with increases in mean food consumption in
these dose groups. Decreases in serum cholinesterase activities
were observed at concentrations of 1.5 ppm and higher in both sexes,
resulting in values of about 50% of the control activity at the end
of the study. A dose-dependent reduction of erythrocyte
cholinesterase activity was observed at 125 and 250 ppm resulting in
activities of 80% and 75% of the control activity in males and
females, respectively, at either dose level at the end of the study.
Brain cholinesterase activity was also inhibited to 76% and 71% of
the control activity in males and females, respectively, at 125 ppm,
and to 58% and 52% of the control activity at 250 ppm in males and
females, respectively. Similar inhibition was observed after the
first year of the study. A slight reduction of less than 9% was
still found after the 4-week recovery period in erythrocyte and
brain cholinesterase activity at 250 ppm. Gross and microscopic
pathology examinations did not give evidence of any compound-related
lesions. The NOAEL in this study was 1.5 ppm, equal to 0.07 mg/kg
bw/day, based on inhibition of erythrocyte and brain cholinesterase
at 125 ppm and above (Kirchner et al., 1991).
Reproduction studies
Rats
Diazinon (purity 94.9%) was administered in the diet to groups
of rats (Sprague-Dawley CRCD; 30/sex/group) over two parental
generations (F0, F1) and up to weaning of the F2 pups at
concentrations of 0, 10, 100 or 500 ppm. Treatment-related clinical
symptoms and deaths were observed in a few high-dose females. They
consisted of tremors (3/30) and dystocia (2/30) followed by death or
sacrifice in the F0 generation. In the F1 generation, a few high-
dose females showed tremors as only compound-related signs. Food
consumption was increased in females of the 500 ppm group in the F0
generation, whereas in the F1 generation, a dose-related reduction
of food consumption was observed at 100 and 500 ppm in males only.
Body-weight gain was lower in F0 females at 500 ppm during
gestation. In the F1 generation, reduced body-weight gain was
observed at 100 and 500 ppm in males and at 500 ppm in females.
There were no treatment-related effects on mating behaviour and the
reproductive parameters (including mating, fertility, gestation
indices, number of viable pups, and number of stillborn pups) were
comparable in control and treatment groups in the F0 generation and
at the low and intermediate dose level of the F1 generation. At
500 ppm, a greater proportion of females showed a prolonged
gestation duration at both generations. A decrease in the number of
pregnancies and viable pups as well as reduced fertility and mating
indices were observed in high-dose females of the F1 generation. A
dose-related reduction in survival of F1 pups was observed at 100
ppm and 500 ppm and in F2 at 500 ppm. Weights of F0 pups were
reduced in the 100 and 500 ppm groups, and in F2 pups at 500 ppm.
No treatment-related malformations were found in the pups. The
NOAEL in this study was 10 ppm, equivalent to 0.5 mg/kg bw/day,
based on reduced parental body-weight gain and reduced viability of
pups and pup weights at 100 ppm and higher (Giknis 1989).
Special studies on embryotoxicity/teratogenicity
Mice
Groups of mice (6 females/dose) were given oral daily doses of
0, 0.18 or 9 mg/kg bw diazinon throughout gestation. Treated
animals gained less weight during gestation compared to control
animals. Weight gain of pups born to mothers receiving 9 mg/kg
bw/day was reduced. Daily testing for physiological and behavioural
development of the pups revealed some evidence of retarded
development among the offspring of high-dose animals (e.g.,
retardation of eye and ear opening). Measures of endurance and
coordination also gave some evidence of impairment (e.g., increased
rod cling endurance in both groups, reduced rotarod endurance,
impaired running performance in a maze inclined plane test).
Impaired reactions were sometimes observed at both dose levels but
without a clear dose-effect relationship. Examination of brain
tissue of only 8 of a total number of 132 offspring at the high-dose
level revealed morphological abnormalities in the forebrain. The
relationship of these findings to the observed behavioural changes
is unknown (Spyker & Avery 1977). The evidence for morphological
effects of diazinon on the developing brain was considered
insufficient by an expert (Krinke, 1991).
Rats
In a teratogenicity study in Sprague-Dawley rats at dose levels
of 0, 15, 50 or 100 mg/kg bw/day administered orally on days 6
through 15 of gestation, dams at the 100 mg/kg bw/day dose level
showed a marked decrease in food consumption correlating with weight
loss at the beginning of the treatment period. Skeletal assessment
showed a slightly higher incidence of incomplete ossification at
different sites in the fetuses at 100 mg/kg bw/day. Visceral
examination revealed a dystopia cordis in association with
hypoplasia of lungs in 1/105 fetuses at 100 mg/kg bw/day. This
anomaly has been reported to occur spontaneously in control animals.
Because of the single occurrence this anomaly was not considered to
be due to a direct action of diazinon but to be secondary to
maternal toxicity (Fritz, 1974).
In a later study, groups of rats (Charles River Crl. COBS
CD(SD)(BR); 27 females/group) were treated with doses of diazinon
(purity 97.4%) at 0, 10, 20 or 100 mg/kg bw/day by gavage during
gestational days 6 through 15. The treatment had no effect on the
mortality of the dams. During the first half of the dosing period,
the dams lost weight and food consumption was reduced at 100 mg/kg
bw/day. No compound-related clinical signs and no abnormal gross
pathological findings were observed in the dams. At 100 mg/kg
bw/day some reproductive parameters differed from the control values
but no statistical significance was achieved (e.g., increase in
number of resorptions, percent pre- and post-implantation loss,
reduction in number of live fetuses). On gross observation, 3/262
(1%) fetuses from 3 litters showed external malformations (single
occurrences of a umbilical hernia, filament tail, sublingual
extraneous soft tissue), whereas in the concurrent controls no
similar effects were observed. Umbilical hernia and tail
abnormalities however are reported to be spontaneous malformations
observed routinely in this rat strain. Concerning skeletal
variations, an increased incidence in rudimentary ribs (T-14) was
observed in all treatment groups attaining statistical significance
in the high-dose group (5% versus 0%). Because of the single
occurrences of the different malformations observed and because the
malformations also occur in control animals they were considered to
be a consequence of the marked maternotoxicity at this dose level,
and not due to a teratogenic effect of the test compound. The
increased incidence of the skeletal variation observed at the top
dose level was considered to be related to maternotoxicity at this
dose level. The NOAEL in this study was 20 mg/kg bw/day based on
maternotoxicity and fetotoxicity at the higher dose level (Infurna &
Arthur, 1985).
Rabbits
Diazinon (purity 89.2%) was administered to groups of pregnant
rabbits (New Zeeland white; 18-22 females/group) by gavage at dose
levels of 0, 7, 25 or 100 mg/kg bw/day from days 6-18 of gestation.
An increase in maternal mortality was observed at 100 mg/kg bw/day
(41% vs 0% in all other groups). Overt clinical signs of maternal
toxicity at 100 mg/kg bw/day included tremors, convulsions,
hypoactivity and anorexia. Reduced body-weight gain was found at
the highest dose level. The treatment did not influence the number
of corpora lutea, number of implantation sites, live fetuses per
litter or fetal weight. The incidence of visceral and skeletal
malformations and variations showed no differences between the
groups that could be attributed to treatment. The study therefore
gave no evidence for an embryotoxic or teratogenic activity of
diazinon. The NOAEL in this study was 25 mg/kg bw/day based on
materno-toxicity at the highest dose level (Harris & Holson, 1981).
Chickens
The teratogenic effects of some anticholinesterase insecticides
(organophosphates and methylcarbamates) on chicken embryos have been
known for some time. In a first study, eggs were injected with
diazinon to explore some of the biochemical variables that might be
involved in producing micromelia, beak and feather deformities (type
I signs) in avian embryos that are known to be caused by OP
insecticides. The results of this study confirm the assumption that
a tryptophan deficiency (tryptophan plays an important role in the
developing embryo) may cause the abnormalities observed (Kushaba-
Rugaaju & Kitos, 1985).
Another study was conducted to investigate the involvement of
cholinergic functions in OP-induced teratogenesis in chick embryos
by examining the effects of diazinon on the developmental pattern of
AChE and ChAT activities in hindlimb, wing and brain. The results
of this study confirmed that type II teratogenesis (wry neck, short
neck, arthrogryposis and muscular hypoplasia of legs) may be
mediated via cholinergic nicotinic receptors. Cholinergic
dysfunction including decreased activity of AChE and ChAT was not
correlated with diazinon-induced type I teratogenesis (micromelia,
abnormal feathering) (Misawa et al., 1981).
Cattle
As part of a survey to determine the causes of abortion in
dairy cattle in Wisconsin, the potential role of pesticides was
examined. Diazinon in the form of wettable powder was orally
administered to two pregnant cows at a daily dose level of 6.6 mg/kg
bw. The continuous treatment started in the 5th and 6th month of
pregnancy (abortion commonly affects cows in the 5th to 8th month of
pregnancy) and was maintained until parturition. No diazinon
residues were detected in the tissues examined (fat, liver, kidney)
nor in milk. The treatment failed to reveal any abortions (Macklin
& Ribelin 1971).
Special studies on genotoxicity
Diazinon has been adequately tested in a series of genotoxicity
assays. The results are summarized in Table 2.
Special study of effects on the pancreas
A study was conducted using a canine model to investigate the
induction of pancreatic ductal hypertension following cholinesterase
inhibitor intoxication known to be an important triggering mechanism
in the pathogenesis of acute and chronic pancreatitis. Diazinon was
intravenously administered (25 mg/kg bw) to the pancreatic ampulla
of dogs and the tissue cholinesterase activity of the canine
pancreatic sphincters was determined. Two enzymes are responsible
for the total cholinesterase activity of whole blood: a membrane-
bound AChE associated with the erythrocyte membrane, and a soluble
enzyme, pseudocholinesterase or butyrylcholinesterase (BChE) in the
serum. In tissues, which also contain the two forms of
cholinesterase, AChE is important in the regulation of neuromuscular
activity and parasympathetic ganglion transmission, while the role
of BChE is unknown. The observation of the negative correlation
between serum BChE activity and intraductal pancreatic pressure
supports the hypothesis that the pancreatic ductal hypertension
which occurs following cholinesterase inhibitor intoxication is due
to a selective reduction in pancreatic BChE activity (Dressel et
al., 1980).
The induction of acute pancreatitis by OPs found in dogs was
confirmed in guinea-pigs but not in cats. These results may reflect
species-related differences in the distribution of pancreatic BChE
(Frick et al., 1987).
Special studies on antidotes
Previous reports with respect to the usefulness of PAM and
other oxine reactivators against this organophosphate have been
published (Sanderson & Edson, 1959; Wills, 1959). A recent study
was undertaken to provide information on the antidotal effectiveness
of pyridine-2-aldoxime methochloride (2-PAM) against diazinon
poisoning in animals. The administration of atropine (16 mg/ kg bw;
i.m.) or 2-PAM (30 mg/kg bw; i.v.) alone 10 minutes after poisoning
rats with doses of 235 mg/kg bw corresponding to the approximate
oral LD50 or higher provided little or no protection. Best
protection was achieved when the oxime was given orally in
conjunction with atropine (i.m.) or followed by a subsequent dose of
2-PAM orally or i.v. Administration of 2-PAM to diazinon-poisoned
rabbits (1600 mg/kg bw) resulted in reactivation of inhibited blood
ChE activity concurrent with a decrease in signs of poisoning.
Within 2 hours, however, the animals were again weak and ataxic and
blood ChE showed renewed inhibition. The authors suggested that
effective therapy of diazinon intoxication requires repeated doses
of oxime to maintain effective antidote levels in the body (Harris
et al., 1969). In dogs and guinea-pigs, pretreatment with
atropine protected the animals against diazinon-induced pancreatitis
(Frick et al., 1987).
Special study on delayed neurotoxicity
Hens
An oral dose of 28 mg/kg bw/day of diazinon technical (87%
purity) was administered to a group of 18 hens (the target dose of
13 mg/kg bw/day as the approximate LD50 was doubled due to a
preparation error). The schedule to protect the hens from acute
cholinergic effects of diazinon consisted of a 10 mg/kg bw atropine
pretreatment (i.m.). At the time of diazinon dosing, 2-PAM at an
i.m. dose of 50 mg/kg bw was administered. Post-treatment consisted
of concurrent doses of atropine and 2-PAM one and five hours
following dosing. Since there were no neurotoxic responses in the
test group in the three weeks following the treatment they were
again treated with 13 mg/kg bw of diazinon on day 21. One test
group hen was found dead on day 5 after the first dosing, and one
hen six hours after the second treatment. One hen exhibited a
slight unsteadiness in walking only on day 41. No neurotoxic signs
became apparent during the three-week observation period after the
second treatment. Histopathological examination revealed no lesions
in the brain, spinal cord or peripheral nerves in diazinon-treated
animals, whereas animals of the positive control (TOCP treatment)
showed multiple lesions (axonal degeneration) consistent with
peripheral neuropathy (Jenkins, 1988).
Observations in humans
Numerous reports of intentional and accidental intake of
diazinon and other OPs have been published describing the clinical
manifestations of organophosphate toxicity and the usefulness of
erythrocyte and serum cholinesterase activity assays for diagnosis
as well as the efficacy of supportive and specific therapies
(Kabrawala et al., 1965; Payot, 1966; Banerjee, 1967; Gupta &
Patel, 1968; Zwiener & Ginsburg, 1988).
A case of acute diazinon poisoning complicated by pericarditis
and pneumonia followed by recovery has been reported. The
intoxication caused the occurrence of cyanosis, tracheobronchial
congestion, pulmonary edema and pneumonia. The treatment included
the intramuscular injection of atropine (Banerjee, 1967).
Another complication reported following accidental ingestion of
diazinon consisted of severe pancreatitis and a pseudocyst (Dressel
et al., 1979). The induction of pancreatitis was reproduced in
dogs treated intravenously with a dose of 5 mg/kg bw diazinon.
Pancreatitis may be the result of hypersecretion and ductal
obstruction (Dressel et al., 1980).
Table 2. Results of genotoxicity assays on diazinon
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
In vitro
Ames test S. typhimurium 313-5000 µg/0.1 ml 88.0% negative negative Geleick & Arni, 1990
TA 98, 100, 1535, 1537 DMSO
E. coli WP2uvrA
Ames test S. typhimurium in DMSO >50-1000 ? negative negative Marshall et al., 1976
TA 1535, 1536, 1537, 1538 µg/plate
Mouse lymphoma Mouse lymphoma cell 1. 6 to 60 µg/ml with 97.2% negative negative Dollenmeier & Müller,
assay L5178Y/TK+/- activation 1986
2. 12 to 120 µg/ml
without activation
Mouse lymphoma Mouse lymphoma cell up to 100 µg/ml ? positive positive McGregor et al., 1989
assay L5178Y TK+/-
Sister chromatid Chinese hamster cells 10, 20 and 40 µg/ml 99.2% negative Chen et al., 1981
exchange study (V79) 99.2% in DMSO
Chromosomal Chinese hamster lung 0.1 mg/ml ? cytotoxic positive Matsuoka et al., 1979
aberration test cells
Sister chromatid Human lymphoid cells 0.02, 0.2, 2.0 and ? negative negative1 Sobti et al., 1982
exchange study (LAZ-007) 200 µg/ml
in Ethanol (< 0.1%)
Sister chromatid Human lymphoid cells 12.5, 25, 50, 100 and 87.5% negative negative Strasser & Arni, 1988
exchange study (CCL-156) 200 µg/ml in DMSO
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Whole blood human 1. 0.0668 to 2000 µg/ml 88.0% equivocal equivocal2 Murli & Haworth, 1990a
exchange study lymphocytes in DMSO (ħ activation)
2. 0.668 to 20 µg/ml
(non-activation)
3. 2-66.8 µg/ml
(activated)
Autoradiographic Rat hepatocytes 1.1 to 120 µg/ml in 88.0% negative Hertner & Arni, 1990
DNA repair test DMSO
In vivo
Micronucleus test Mouse 1. 120 mg/kg bw oral 87.5% negative Ceresa, 1988
in vivo 2. 30, 60, and
120 mg/kg bw oral
Dominant lethal Mouse oral dose of 14 and 45 95% negative Fritz, 1975
study mg/kg bw
Chromosome studies Mouse a) daily oral doses of ? negative a) Hool & Müller, 1981c
in male germinal a) spermatogonia 10.5, 21 and 63 mg/kg b) Hool & Müller, 1981b
epithelium b) spermatocytes bw for five days, or
b) 5 treatments with
the same doses over
a period of 10 days
Sister chromatid Bone marrow cells single oral doses of 88.0% negative Murli & Haworth, 1990b
exchange study of mice 10, 50 and 100
in vivo mg/kg bw
Table 2 (contd)
Test system Test object Concentration/dose Purity Results Reference
Non-activated Activated
Sister chromatid Chinese hamster bone oral gavage of 6.5 ? negative Hool & Müller, 1981a
exchange study marrow cell to 26 mg/kg bw
Nucleus anomaly Chinese hamster bone oral gavage of ? negative Hool & Müller, 1981d
test marrow cells 6.5, 13 and 26 mg/kg
bw/day for two days
1 Cyotoxicity at a concentration of 20 µg/ml. The increased frequency of sister chromatid exchanges at 20 µg/ml with
metabolic activation did not show a doubling of the control frequency and was therefore not considered positive.
2 Two independent trials: Cytotoxicity at concentrations of 66.8 µg/ml (with activation) respectively. Increases in the SCE
frequency (just doubling) observed only in the first assay without metabolic activation at dose levels of 0.668 µg/ml to
20 µg/ml, but without a dose-relationship. Increases observed after activation in both trials did not show doubling and again
no dose-response. Thus the results of these studies are not considered positive.
A study of 60 poisoning cases with diazinon has been reported.
The most common clinical manifestations were vomiting, giddiness,
constricted pupils and signs of bronchoconstriction with pulmonary
congestion. The amount of poison ingested varied from 4 ml to 15 ml
with an average of 7.5 ml (% active ingredient not specified in
publication). This was ingested by 55 patients with suicidal
intention, whereas in 5 patients the compound was taken
accidentally. Atropine injections were administered repeatedly (up
to a total dose of 22.4 g over 42 hours). Five patients died in
spite of intensive atropine therapy initiated more than 8 hours
after ingestion. Other cases who had ingested the same amount of
poison as the fatal cases received treatment within three hours of
ingestion. These findings emphasize the importance of early
treatment in diazinon poisoning (Gupta & Patel, 1968). In addition,
these results are in agreement with an earlier observation that the
combination of diazinon with cholinesterase occurs in two stages, an
early reversible stage and a late irreversible stage (Grob, 1956).
In another study, cases of organophosphate and carbamate
poisoning in 37 infants and children were reported, only 5 of which
were associated with diazinon. Miosis, excessive salivation, muscle
weakness, respiratory distress, lethargy and tachycardia were the
most common clinical findings. Erythrocyte cholinesterase
activities were determined from 24 patients showing erythrocyte
cholinesterase activities that were less than 50% of the lower limit
of the normal range. There were no differences between the
erythrocyte and serum cholinesterase activity in 20/24 patients from
whom both tests were obtained, whereas in the remaining 4 cases
either the erythrocyte or the serum activity was decreased. A
combined atropine/pralidoxime therapy is recommended in case of
organophosphate toxicity. Although the recommended dose for
atropine in infants is 0.01-0.02 mg/kg bw, the dose is generally
insufficient for treatment of signs and symptoms secondary to
organophosphate poisoning (Zwiener & Ginsburg, 1988).
Neurobehavioural effects of short-term, low-level exposure to
diazinon were investigated in 99 pest control workers. A computer
assisted neuro-behavioural test battery (including attention,
vigilance, hand-eye coordination, visual perception, verbal ability)
was used before and after their work shift. The diazinon metabolite
diethyl-thiophosphate (DETP) was measured in urine samples collected
from 46 diazinon applicators and 56 non-applicators. Post-shift
DETP values were 24 and 3 ppb for applicators and non-applicators,
respectively. The study failed to demonstrate any behavioral
effects of diazinon under the conditions of this study (Maizlish et
al., 1987).
The results of the following old study have been reported in an
earlier monograph (Annex I, reference 7). Because of certain
inaccuracies in that monograph, the study was reviewed again and
summarized below.
A subacute oral toxicity study was conducted in four male
volunteers. Diazinon (different batches, purity 95.4-95.7%) was
administered in capsules at dose levels of 2-2.5 mg per day per
person, equal to a dose of 0.025 mg/kg bw/day. The test period
lasted 42 days with an interruption in treatment from the 5th to
10th day in two volunteers and for 34 consecutive days in the other
two volunteers. Plasma cholinesterase activity was markedly
depressed the first 6 days of treatment in 2/4 subjects (no plasma
cholinesterase activity measurable). Therefore treatment was
interrupted for 6 days to enable recovery. Subsequent treatment at
the same dose level did not reveal inhibitory effects on plasma
cholinesterase. The fluctuations observed during the treatment
period were similar to those observed during the pre-test period.
In no instance was the erythrocyte acetylcholinesterase depressed
compared to the pre-test values. Other parameters investigated
included haematology and blood chemistry, urinalyses and
symptomatology. No changes were observed that could be attributed
to the treatment. The NOAEL in this study was 0.025 mg/kg bw/day
based on transitory depression of plasma cholinesterase activity as
the only effect observed at this dose level (Payot, 1966).
COMMENTS
Following oral administration to rats, diazinon was almost
completely absorbed and eliminated, mainly in the urine.
The main degradative pathway includes the oxidase/hydrolase-
mediated cleavage of the ester bond leading to the pyrimidinol
derivative 2-isopropyl-6-methyl-4(1H)-pyrimidinone, which is further
oxidized to more polar metabolites.
Diazinon has moderate acute oral toxicity to mice and rats.
The clinical signs observed were consistent with cholinesterase
inhibition and included sedation, tremors, convulsions and ataxia.
It has been classified by WHO as moderately hazardous.
In an oral 90-day feeding study in rats at dietary
concentrations of 0, 0.5, 5, 250 or 2500 ppm, the NOAEL was 5 ppm
(equal to 0.4 mg/kg bw/day), based on erythrocyte and brain
cholinesterase inhibition at 250 ppm and higher.
In short-term studies in dogs, diazinon was administered at
dietary concentrations of 0, 0.1, 0.5, 150 or 300 ppm for either 90
days or 52 weeks. In both studies, the NOAEL was 0.5 ppm, equal to
0.02 mg/kg bw/day based on erythrocyte and brain cholinesterase
inhibition at 150 ppm and above.
In a carcinogenicity study in mice, diazinon was administered
at dietary concentrations of 0, 100 or 200 ppm over 103 weeks.
There was no evidence of carcinogenicity.
In a carcinogenicity study in rats diazinon was administered at
dietary concentrations of 0, 400 or 800 ppm for 103 weeks. There
was no evidence of carcinogenicity.
In a long-term toxicity/carcinogenicity study, rats were
maintained on a diet containing diazinon at concentrations of 0,
0.1, 1.5, 125 or 250 ppm for up to 99 weeks. The NOAEL was 1.5 ppm,
equal to 0.07 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase at 125 ppm and above. There was no evidence of
carcinogenicity.
A multigeneration study was conducted in rats using dietary
concentrations of 0, 10, 100 or 500 ppm. The NOAEL was 10 ppm
(equivalent to 0.5 mg/kg bw/day), based on a reduction in parental
body-weight gain in the F1 generation and a reduced survival rate
and reduced body weight of F1 pups at 100 ppm.
In a teratogenicity study in rats, diazinon was orally
administered at dose levels of 0, 10, 20 or 100 mg/kg bw/day.
Maternal toxicity, indicated by weight loss correlating with reduced
food consumption, became evident at 100 mg/kg bw/day. Effects on
the fetuses at this dose level consisted of retarded ossification
and an increased incidence of rudimentary ribs. The NOAEL was 20
mg/kg bw/day based on maternotoxicity and fetotoxicity. There was
no evidence of teratogenicity.
A teratogenicity study in rabbits conducted with oral dose
levels of 0, 7, 25 or 100 mg/kg bw/day revealed clinical signs of
maternotoxicity, increased mortality and reduced body-weight gain at
100 mg/kg bw/day. The NOAEL was 25 mg/kg bw/day. There was no
evidence of teratogenicity.
A neurotoxicity study performed with hens treated at dose
levels of 13 or 28 mg/kg bw/day (protected by atropine pre-
treatment) did not reveal evidence of delayed neurotoxicity.
Diazinon has been adequately tested in a series of genotoxicity
assays. Chromosomal aberrations were induced in cultured mammalian
cells, but there were no other indications of genotoxicity. The
Meeting concluded that diazinon was not genotoxic.
Diazinon was evaluated in four human male volunteers who
received 0.025 mg/kg bw/day of diazinon in capsules for 34-36 days.
There were no consistent treatment-related effects on plasma or
erythrocyte cholinesterase activity, blood chemistry or urinalysis.
No clinical effects were reported. The NOAEL was 0.025 mg/kg
bw/day.
The ADI of 0-0.002 mg/kg bw, which was based on the NOAEL of
0.025 mg/kg bw/day in the study in humans using a 10-fold safety
factor, was maintained.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm, equal to 0.4 mg/kg bw/day (90-day study)
1.5 ppm, equal to 0.07 mg/kg bw/day (99-week study)
10 ppm, equivalent to 0.5 mg/kg bw/day
(reproduction study)
20 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Rabbit: 25 mg/kg bw/day (maternotoxicity in teratogenicity
study)
Dog: 0.5 ppm, equal to 0.02 mg/kg bw/day (one-year study)
Human: 0.025 mg/kg bw/day (34-36-day study)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ballantine, L., Marco, G.J. & Williams, S.C. (1984) Percutaneous
absorption of 2 delta - 14C - Diazinon in Rats. Numbers ABR-84011,
302950. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Banerjee, D. (1967) Pericarditis in acute diazinon poisoning. A case
report. Armed Forces Med. J. (India), 23: 187-190.
Barnes, T.B., Arthur, A.T. & Hazelette, J.R. (1988) Diazinon (MG-8):
90-Day Oral Toxicity Study in Dogs. Number 882012. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Bathe, R. (1972a) Acute oral LD50 of technical diazinon in the
mouse. Number Siss 1679. Unpublished Report Prepared by Ciba-Geigy
Ltd., Sisseln, Switzerland.
Bathe, R. (1972b) Acute dermal LD50 of technical diazinon (G-24480)
in the rat. Number Siss 1679. Unpublished Report Prepared by Ciba-
Geigy Ltd., Sisseln, Switzerland.
Bathe, R. & Gfeller, W. (1980) Acute oral LD50 in the rat of
technical G 24480. Number 800478. Unpublished Report Prepared by
Ciba-Geigy Ltd., Sisseln, Switzerland.
Burgener, J. & Seim, V. (1988) Biological report for the metabolism
of 14C-diazinon in Laying Hens Dosed at 25 ppm. Numbers BIOL-88006,
302213. Unpublished Report Prepared by Ciba-Geigy Corp., Vero Beach,
Florida.
Capps, T., Brown, K., Lai, K. & Tweedy, B. (1989) Characterization
and identification of diazinon metabolites in rats. Numbers ABR-
88164, 302211 and 302925. Unpublished Report Prepared by Ciba-Geigy
Corp., Greensboro, NC.
Capps, T. M., Summer, D., Bar, H.P. & Carlin, T.J. (1990)
Characterization and identification of major metabolites in tissues
of sheep treated dermally with 14C-diazinon. Numbers ABR-90014,
302925. Unpublished Report Prepared by Ciba-Geigy Corp., Greensboro,
NC.
Ceresa, C. (1988) G 24480: Micronucleus test (mouse). Number 871696.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H.H., Hsueh, J.L., Sirianni, S.K. & Huang, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutation Research, 88: 307-316.
Ciba-Geigy 1992: Diazinon Data Summaries. Working Paper for the WHO
Expert group on Pesticide Residues. Biological Data and
Toxicological Evaluation. June 23.
Craine, E.M. (1989) Study of 14C-diazinon disposition in the rat
(biological phase and analytical phase I). Numbers WIL-82030,
302925. Unpublished Report Prepared by WIL Research Laboratories
Inc., Ashland, OH and Ciba-Geigy Corp., Greensboro, NC.
Dahm, P.A. (1970) Some aspects of the metabolism of parathion and
diazinon. In: Biochemical Toxicology of Insecticides. ed. by
O'Brien, R.D. and Yamamoto, I., Academic Press London.
Dollenmeier, P. & Müller, D. (1986) G 24480 techn: L 5178Y/T+/-
mouse lymphoma mutagenicity test. Number 840396. Unpublished Report
Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Dressel, Th. D., Goodale, R.L., Borner, J.W. & Etani, S. (1980) A
study of the cholinesterase of the canine pancreatic sphincters and
the relationship between reduced butyrylcholinesterase activity and
pancreatic ductal hypertension. Annals of Surgery, 192/5: 614-619.
Dressel, T.D., Goodale, K.L., Arneson, M.A. & Borner, J.W. (1979)
Pancreatitis as a complication of anticholinesterase insecticide
intoxication. Annals of Surgery, 189: 199-204.
Frick, T.W., Dalo, S., O'Leary, J.F., Runge, W., Borner, J.W.,
Baraniewski, H., Dressel, T., Shearen, J.G. & Goodale, K.L., (1987)
Effects of insecticide, diazinon, on pancreas of dog, cat and
guinea-pig. J. Environ. Path. Toxicol. Oncol. 7(4): 1-11.
Fritz, H. (1974) G 24480 (Diazinon Techn.): Reproduction study - rat
segment ii: (test for teratogenic on embryotoxic effects).
Unpublished report prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Fritz, H. (1975) G 24480 (Diazinon Techn.): Dominant lethal study
mouse. Number 327507. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Geleick, D. & Arni, P. (1990) Salmonella and Escherichia/liver-
microsome test. Number 891346. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Giknis, M.L.A. (1989) Diazinon techn.: A two-generation reproductive
study in albino rats. Numbers MIN-852218, 88128. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Grob, D. (1956) The manifestations and treatment of poisoning due to
nerve gas and other organic phosphate anticholinesterase compounds.
A.M.A. Arch. Intern. Med. 98: 221.
Gupta, O.P. & Patel, D.D. (1968) Diazinon poisoning. A study of
sixty cases. J. Assoc. Physicians of India, 16: 457-463.
Hagenbuch, J.P. & Mücke, W. (1985) The fate of diazinon in mammals.
Biochemistry Product Evaluation 1/85. Unpublished Report Prepared by
Ciba-Geigy Ltd., Basle, Switzerland.
Harris, S. B. & Holson, J.F. (1981) A teratology study of diazinon
in New Zealand white rabbits. Number 281005. Unpublished Report
Prepared by Science Applications Inc., La Jolla, CA.
Harris, L.W., Fleisher, J.H., Innerebner, T.A., Cliff, W. & Sim,
V.M. (1969) The effects of atropine-oxime therapy on cholinesterase
activity and the survival of animals poisoned with 0,0-diethyl-0-(2-
isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate. Toxicol. Appl.
Pharmacol. 15: 216-224.
Hartmann, H.R. (1990) G 24480: 21-Day repeated exposure inhalation
toxicity in the rat (nose-only exposure). Number 891205. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, Th. & Arni, P. (1990) G 24480: Autoradiographic DNA repair
test on rat hepatocytes, Number 891345. Unpublished Report Prepared
by Ciba-Geigy Ltd., Basle, Switzerland.
Holbert, M.S. (1989) Diazinon MG8 FL 880045: acute inhalation
toxicity study in rats. Number 5947-89. Unpublished Report Prepared
by Stillmeadow Inc., Houston, Texas.
Hool, G. & Müller, D. (1981a) G 24480: Sister-chromatid exchange
study Chinese hamster. Number 801504. Unpublished Report Including
Supplement to the Report (dated October 13, 1981) Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981b) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatocytes. Number 801502. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981c) G 24480: Chromosome studies in male
germinal epithelium mouse. Test for mutagenic effects on
spermatogonia. Number 801501. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Müller, D. (1981d) G 24480: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster. Number 801503.
Unpublished Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Infurna, R.N. & Arthur, A.T. (1985) A teratology study of diazinon
technical in Charles River rats. Numbers MIN-82296, 52-83.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Iverson, F., Grant, D.L. & Lacroix, J. (1975) Diazinon metabolism in
the dog. Bull. Env. Cont. Toxicol. 13/5: 611-618.
Jenkins, L.J. (1988) Acute delayed neurotoxicity of diazinon mg-8 in
domestic fowl. Number 5152-87. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kabrawala, V.N., Shah, R.M. & Oza, G.G. (1965) Diazinon poisoning. A
study of 25 cases. The Indian Practitioner, 18: October, 711-717.
Kaplanis, J.N., Louloudes, S.J. & Roan, C.C. (1962) The
distribution and excretion of 32p-labelled diazinon in guinea-pigs.
Transactions of the Kansas Academy of Science, 65/1: 70-75.
Kirchner, F.K., McCormick, G.C & Arthur, A.T. (1991) G 24480 Techn.:
One/two-year oral toxicity study in rats. Number 882018. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Krinke, G.J. (1991) Comment on paper: neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice by Spyker
and Avery. J. Toxicol. Environm. Health, 3: 989-1002 (1977).
Letter dated December 17 (1991) prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Kuhn, J.O. (1989a) G 24480 tech. (Diazinon MG 8 FL 880045): Acute
oral toxicity study in rats. Number 5942-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989b) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
eye irritation study in rabbits. Number 5944-89. Unpublished Report
Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989c) G 24480 tech. (Diazinon MG 8 FL 880045): Primary
dermal irritation study in rabbits. Number 5945-89. Unpublished
Report Prepared by Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989d) Diazinon MG8 FL 880045: Acute dermal toxicity
study in rabbits. Number 5943-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kuhn, J.O. (1989e) Diazinon MG8 FL 880045: Dermal sensitization
study in guinea-pigs. Number 5946-89. Unpublished Report Prepared by
Stillmeadow Inc., Houston, Texas.
Kushaba-Rugaaju, S. & Kitos, P.A. (1985) Effects of diazinon on
nucleotide and amino acid contents of chick embryos -teratogenic
considerations. Biochemical Pharmacology, 34(11): 1937-1943.
Machin, A.F., Rogers, H., Cross, A.J., Quick, M.P., Howells, L.C. &
Janes, N.F. (1975) Metabolic aspects of the toxicology of diazinon.
I hepatic metabolism in the sheep, cow, pig, guinea-pig, rat,
turkey, chicken and duck. Pestic. Sci. 6: 461-473.
Macklin, A.W. & Ribelin, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc. (JAVMA), 159/12:
1743-1748.
Maizlish, N., Schenker, M., Weisskopf, C., Seiber, J., & Samuels S.
(1987) A behavioral evaluation of pest control workers with short-
term, low-level exposure to the organophosphate diazinon. Am. J.
Ind. Med. 12: 153-172.
Marshall, Th. C. Dorough, H.W. & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agric. Food Chem. 24/3: 560-563.
Matsuda, A., Hayashi, M. & Ishidate, M. (1979) Chromosomal
aberration tests on 29 chemicals combined with S9 mix in vitro.
Mutation Research, 66: 277-290.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 Coded Chemicals.
Environm. Mol. Mutagen, 12: 85-154.
Misawa, M., Doull, J., Kitos,P.A. & Uyeki, F.M. (1981) Teratogenic
effects of cholinergic insecticides in chick embryos I. Diazinon
treatment on acetylcholinesterase and choline acetyltransferase
activities. Toxicol. Appl. Pharmacol. 57: 20-29.
Miyamoto, J. (1976) Terminal residues: evaluation of diazinon. J.
Assoc. Offic. Anal. Chem. 59: 878-880.
Mücke, W., Alt, K.O. & Esser, H.O. (1970) Degradation of 14C-
labelled diazinon in the rat. Agric. Food. Chem. 18/2: 208-212.
Murli, H. & Haworth S.R. (1990b) Mutagenicity test on diazinon MG 8:
in vivo sister chromatid exchange assay. Number HLA-12226-0-458,
TX 900093. Unpublished Report Prepared by Hazleton Laboratories
America Inc., Kensington, Maryland.
Murli, H. & Haworth, S.R. (1990a) Mutagenicity test on diazinon MG 8
in an in vitro cytogenetic assay measuring sister chromatid
exchange frequencies in cultured whole blood human lymphocytes.
Number HLA-12226-0-448, TX 900093. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
NCI (1976) Bioassay of diazinon for possible carcinogenicity. Nov.
1976. Carcinogenesis Testing Program National Cancer Institute
(NCI). U.S. Department of Health, Education and Welfare. DHEW
Publication No. (NIH) 79-1392.
NIEHS (National Institute of Environmental Health Services) (1991)
Toxicology data management system tumor incidence in control animals
by route and vehicle of administration F/344N rats. Prepared for
NIEHS by Analytical Sciences Inc., Durham, NC, USA.
Payot, P.H. (1966) Subacute oral toxicity study on diazinon AS-
humans. Unpublished Report dated October, Prepared by Geigy, Basle,
Switzerland.
Piccirillo, V.J. (1978) Acute oral toxicity study in rats with
diazinon technical. Number 483-143. Unpublished Report Prepared by
Hazleton Laboratories America Inc., Kensington, Maryland.
Pickles, M. & Seim, V. (1988) Biological report for the metabolism
of 2-pyrimidinyl-14c-diazinon in a lactating goat. Number BIOL-
88004, 302212. Unpublished Report Prepared by Ciba-Geigy Corp., Vero
Beach, Florida.
Pickles, M. & Seim, V. (1990) Biological report for the metabolism
of 14C-diazinon on sheep. Numbers: BIOL-89014, 302925. Unpublished
Report Prepared by Ciba-Geigy Corp., Greensboro, NC.
Robbins, W.E., Eddy, G.W. & Hopkins, Th.L. (1957) Metabolism and
excretion of phosphorus-32-labelled diazinon in a cow. J. Agr. Food
Chem. 5/7: 509-513.
Rudzki, M.W., Arthur, A.T. & McCormick, G.C. (1991) Diazinon (MG-8):
52-week oral toxicity study in dogs. Number 882014. Unpublished
Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sachsse, K. & Bathe, R. (1975) Potentiation study: C8514
(chlordimeform) versus 3 insecticides, G 24 480 (Diazinon), CGA 12
223 and CGA 15324 in the rat. Unpublished Report Prepared by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1976) Potentiation study: C9491
(Jodfenphos) versus 2 Insecticides, G 24480 (Diazinon), and C 177
(Dichlorvos) in the rat. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977) CGA 15342 versus 2 insecticides, GS
13005 (Methidathion) and G 24480 (Diazinon) in rat. Unpublished
Report Prepared by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1978) Potentiation study CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (Phosphamidon), GS 13005
(Methidathion), G 24480 (Diazinon) CGA 15324 and Malathion. Number
404478 Siss 6526. Unpublished Report Prepared by Ciba-Geigy Ltd.,
Basle, Switzerland.
Sanderson, D.M. & Edson, E.F. (1959) Oxime therapy in poisoning by
six organophosphorus insecticides in the rat. J. Pharm. Pharmacol.
11: 721-728.
Schoch, M. & Gfeller, W. (1985) G 24480 techn. Acute oral LD50 in
the rat. Number 850864. Unpublished Report Prepared by Ciba-Geigy
Ltd., Basle, Switzerland
Shishido, T., Usui, K. & Fukami, J. (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pest.
Biochem. Physiol. 2: 27-38.
Shishido, T., Usui, K., Sato, M. & Fukami, J. (1972b) Enzymatic
conjugation of diazinon with glutathione in rat and American
cockroach. Pest. Bioch. Physiol. 2: 51-63.
Shishido, T. & Fukami, J. (1972) Enzymatic hydrolysis of diazoxon by
rat tissue homogenates. Pest. Bioch. Physiol. 2: 39-50.
Simoneaux, B., Ballantine, L., Brown, K. & Lai, K. (1988) Metabolite
identification in hens and goats treated with 14C-diazinon. Number
ABR-88135. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B., Brown, K. & Lai, K. Summer, D. (1989) Supplemental
report on the nature of residues of diazinon in hens. Numbers ABR-
89040, 302213. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988a) Disposition of 14C-diazinon in goats.
Number ABR-88117. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988b) Disposition of 14C-diazinon in chickens.
Number ABR-88116. Unpublished Report Prepared by Ciba-Geigy Corp.,
Greensboro, NC.
Simoneaux, B.J. (1988c) Characterization of 14C-diazinon
metabolites in goats. Number ABR-88118. Unpublished Report Prepared
by Ciba-Geigy Corp., Greensboro, NC.
Simoneaux, B.J. (1988d) Characterization of 14C-diazinon
metabolites in chickens. Number ABR-88119. Unpublished Report
Prepared by Ciba-Geigy Corp., Greensboro, NC.
Singh, A.R., Arthur, A.T. & McCormick, G.C. (1988) Diazinon (MG-8)
13-week oral feeding study in rats. Numbers MIN-882011, 88083.
Unpublished Report Prepared by Ciba-Geigy Corp., Summit, NJ.
Sobti, R.C., Krishan, A. & Pfaffenburger, C.D. (1982) Cytokinetic
and cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutation Research,
102: 89-102.
Spyker, J.M. & Avery, D.L. (1977) Neurobehavioral effects of
prenatal exposure to the organophosphate diazinon in mice. J.
Toxicol. Environm. Health, 3: 989-1002.
Strasser, F. & Arni, P. (1988) G 24480: Sister chromatid exchange
test on human lymphocytes cell line CCL 156 in vitro. Number
871697. Unpublished Report Prepared by Ciba-Geigy Ltd., Basle,
Switzerland.
Tai, Ch. & Katz, R. (1984) Diazinon Techn.: 21-day dermal toxicity
study in rabbits. Number 842007. Unpublished Report Prepared by
Ciba-Geigy Corp. Greensboro, NC.
WHO (1990) Principles for the toxicological assessment of pesticide
residues in food. Environmental Health Criteria 104, Geneva.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wills, J.H. (1959) Recent studies of organic phosphate poisoning.
Federation Proc. 18: 1020-1025
Yang, S.H.R., Hodgson, E. & Dautermann, W.C. (1971) Metabolism in
vitro of diazinon and diazoxon in rat liver. J. Agr. Food Chem.
19/1: 10-13.
Zak, F., Hess, R., Luetkemeier, H. & Sachsse, K. (1973) 21-day
inhalation study on the rat with technical diazinon. Number Siss
1679. Unpublished Report Prepared by Ciba-Geigy Sisseln,
Switzerland.
Zwiener, R.J. & Ginsburg, C.M. (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81/1: 121-126.
