
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
THIRAM
Chemical name
Bis(dimethylthiocarbamoyl) disulfide;
tetramethylthiuramdisulfide.
Synonyms
TMTD, thiuramyl
Empirical formula
C6H12N2S4
Structural formula
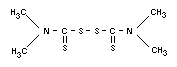 BIOLOGICAL DATA
Biochemical aspects
There are investigations on tetraethyl thiuram disulfide (TETD,
Disulfiram), a homologue of thiram. Experiments on TETD (tagged with
35S) showed that in rats 80-95% of an oral dose in absorbed,
elimination is delayed and a remarkable storage of 35S in the tissue
occurs (mainly in the adrenals, liver and spleen) (Eldjarn, 1950). The
compound was distributed in different tissues in a similar way to
carbon disulfide. The liver is important in the detoxication of the
compound. Animal tissues have been found to degrade TETD to
diethyldithiocarbamate (Harzlick & Irvine, 1921), which in turn
decomposed to carbon disulfide and diethylamine (Prickett & Johnston,
1953). Investigations on the fate of thiram in the digestive tract of
ruminants revealed a similar degradation by micro-organism, which was
not complete (Robbins & Kastelic, 1961); 4.0% of the ingested thiram
appeared in the faeces and 1.5% in the urine. The metabolic fate of
thiram absorbed into the body from the gastro-intestinal tract
probably depends to some extent on the species of animal (Robbins &
Kastelic, 1961). Thiuram disulfides can act as inhibitors of liver
enzymes (Owens, 1953). Thiram acts in vivo in a similar way to
tetraethyl thiuramdisulfide, i.e., it inhibits the acetaldehyde
oxidation enzyme systems. Some authors believe that it is the first
stage of alcohol metabolism, i.e, the oxidation to acetaldehyde that
is mainly inhibited and only to a less extent the conversion of
aldehyde into acetic acid (Hald & Jacobson, 1948; Thorn & Ludwig,
1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1500-2000 Kirchheim, 1951
Mouse Intraperitoneal 250 Hald et al., 1952
Rat Oral 780-865 Lehman, 1951
Handbook of Toxicology, 1959
Rabbit Oral 210 Lehman, 1951
Handbook of Toxicology, 1959
In man oral doses of 0.5 g of Arasan (containing 75% thiram) were
not toxic (Domingo, 1952).
Short-term studies
Fowl. 80 ppm of thiram in the diet of laying hens resulted in
misshapen or soft-shelled eggs, retardation or even cessation of
production of egg. Chicks showed retarded growth, reduced feed
efficiency and increased mortality (Johnson et al., 1955). Thiram was
toxic to chicks at 40 ppm (approximately equivalent to 6.8 mg/kg
body-weight) in the diet and goslings at less than 150 ppm, but turkey
poults tolerated 200 ppm. The symptoms of poisoning were leg
deformities, weight loss, crooked and curled toes, enlarged hocks and
some slipped tendons and spraddles (Waibel et al., 1957).
Dog. Groups of 3 male dogs were fed diets containing 10, 50 and
200 ppm of thiram for one year. No abnormalities or effects on growth
were observed and no gross or microscopic changes at any level
(Fitzhugh, 1963).
Long-term studies
Groups each of 24 rats (12 males and 12 females) were given a
diet containing 48 ppm of thiram over 3 generations. No effects on
growth, reproduction, blood picture and mortality rate were found. No
gross or histological changes were observed (van Esch, 1956). In
another experiment, 12 female and 12 male rats given 200 ppm in the
diet for 8 months showed no appreciable changes in growth and
mortality rate, and 300 ppm for 65 weeks did not give rise to specific
evidence of poisoning (Tollenaar, 1951).
Groups of 24 rats, 12 females and 12 males, were fed diets
containing 100, 300, 1000 and 2500 ppm of thiram. Rats with 300 and
1000 and 2500 ppm in the diet for 65 weeks showed weakness, ataxia and
various degrees of paralysis; also histological changes (calcification
in the brain stem and cerebellum and dystrophic changes in the leg
muscles) were found, and at 2500 ppm there was an increased mortality
rate (Lehman, 1952).
Groups of 20 young rats were placed on diets containing 100, 300
and 500 ppm thiram for 2 years (Bär, 1959). Small reductions in the
growth rate were seen at all concentrations. At concentrations of 300
and 500 ppm an increased mortality rate was seen, while at 500 ppm
convulsions, thyroid hyperplasia (Griepentrog, 1962) and calcification
in the cerebellum, hypothalamus and medulla oblongata were observed
(Griepentrog, 1961).
Comments on experimental studies reported
There are long-term studies in rats, but no other species has
been studied. Dose levels of 100 and 300 ppm in the diet of the rat
had a questionable effect on the thyroid but at 48 ppm no changes were
seen. Chicks appear to be especially susceptible to thiram. Before
these results can be taken into account, confirmation is needed with
detailed information; and because long-term reproduction studies with
3 generations of rats did not show any abnormalities in the litters,
the rat was selected for evaluation.
It is known that TMTD in a dose of 0.5 to 1.5 g per day can be
taken by man for many weeks without ill-effect unless alcohol is
consumed.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight per day.
Dog. 5 mg/kg body-weight.
(Note. An effect was found in the chick at 6.8 mg/kg
body-weight.)
Estimate of acceptable daily intake for man
0-0.025 mg/kg body-weight.
Further work desirable
Biochemical studies. Extension of the experiments in chicken.
REFERENCES
Bär, F. (1959) International Union of Pure and Applied Chemistry
Congress, München, September
Domingo, A. F. (1952) Rev. Med. vet. (Caracas), 11, 335
Eldjarn, L. (1950) Scand. J. Clin. Lab. Invest., 2, 198
van Esch, G. J. (1956) Versl. Volksgezondh., 166
Fitzhugh, O. G. (1963) Personal communication
Griepentrog, F. (1961) Arch. Psychiat. Nervenkr., 202, 412
Griepentrog, F. (1962) Beitr. Path. Anat., 126, 243
Hald, J. & Jacobson, E. (1948) Acta pharmacol. (Kbh.), 4, 305
Hald, J., Jacobson, E. & Larsen, V. (1952) Acta pharmacol. (Kbh.),
8, 329
Handbook of Toxicology (1959) vol. 3, Saunders, Philadelphia
Hanzlick, P. J. & Irvine, A. (1921) J. Pharmacol. exp. Ther., 17,
349
Johnson, E. L. Waibel, P. E. & Pomeroy, B. S. (1955) Proc Amer. vet.
med. Ass., 92, 322
Kirchheim, D. (1951) Naunyn-Schmiedeberg's Arch. exp. Path.
Pharmak., 214, 59
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S. 16, 47-53,126-32
Owens, R. G. (1953) Contr, Boyce Thompson Inst., 17, 221
Prickett, C. S. & Johnston, C. D. (1953) Biochim. biophys. Acta,
12, 542
Robbins, R. C. & Kastelic, J. (1961) J. Agric. Food Chem., 9, 256
Thorn & Ludwig (1962) The dithiocarbamates and related compounds,
Elsevier Mongraphs
Tollenaar, F. D. (1951) Neth. Milk and Dairy J., 5, 46
Waibel, P. E., Johnson, E. L. & Pomeroy, B. S. (1957) Poultry Sci.,
36, 697
BIOLOGICAL DATA
Biochemical aspects
There are investigations on tetraethyl thiuram disulfide (TETD,
Disulfiram), a homologue of thiram. Experiments on TETD (tagged with
35S) showed that in rats 80-95% of an oral dose in absorbed,
elimination is delayed and a remarkable storage of 35S in the tissue
occurs (mainly in the adrenals, liver and spleen) (Eldjarn, 1950). The
compound was distributed in different tissues in a similar way to
carbon disulfide. The liver is important in the detoxication of the
compound. Animal tissues have been found to degrade TETD to
diethyldithiocarbamate (Harzlick & Irvine, 1921), which in turn
decomposed to carbon disulfide and diethylamine (Prickett & Johnston,
1953). Investigations on the fate of thiram in the digestive tract of
ruminants revealed a similar degradation by micro-organism, which was
not complete (Robbins & Kastelic, 1961); 4.0% of the ingested thiram
appeared in the faeces and 1.5% in the urine. The metabolic fate of
thiram absorbed into the body from the gastro-intestinal tract
probably depends to some extent on the species of animal (Robbins &
Kastelic, 1961). Thiuram disulfides can act as inhibitors of liver
enzymes (Owens, 1953). Thiram acts in vivo in a similar way to
tetraethyl thiuramdisulfide, i.e., it inhibits the acetaldehyde
oxidation enzyme systems. Some authors believe that it is the first
stage of alcohol metabolism, i.e, the oxidation to acetaldehyde that
is mainly inhibited and only to a less extent the conversion of
aldehyde into acetic acid (Hald & Jacobson, 1948; Thorn & Ludwig,
1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1500-2000 Kirchheim, 1951
Mouse Intraperitoneal 250 Hald et al., 1952
Rat Oral 780-865 Lehman, 1951
Handbook of Toxicology, 1959
Rabbit Oral 210 Lehman, 1951
Handbook of Toxicology, 1959
In man oral doses of 0.5 g of Arasan (containing 75% thiram) were
not toxic (Domingo, 1952).
Short-term studies
Fowl. 80 ppm of thiram in the diet of laying hens resulted in
misshapen or soft-shelled eggs, retardation or even cessation of
production of egg. Chicks showed retarded growth, reduced feed
efficiency and increased mortality (Johnson et al., 1955). Thiram was
toxic to chicks at 40 ppm (approximately equivalent to 6.8 mg/kg
body-weight) in the diet and goslings at less than 150 ppm, but turkey
poults tolerated 200 ppm. The symptoms of poisoning were leg
deformities, weight loss, crooked and curled toes, enlarged hocks and
some slipped tendons and spraddles (Waibel et al., 1957).
Dog. Groups of 3 male dogs were fed diets containing 10, 50 and
200 ppm of thiram for one year. No abnormalities or effects on growth
were observed and no gross or microscopic changes at any level
(Fitzhugh, 1963).
Long-term studies
Groups each of 24 rats (12 males and 12 females) were given a
diet containing 48 ppm of thiram over 3 generations. No effects on
growth, reproduction, blood picture and mortality rate were found. No
gross or histological changes were observed (van Esch, 1956). In
another experiment, 12 female and 12 male rats given 200 ppm in the
diet for 8 months showed no appreciable changes in growth and
mortality rate, and 300 ppm for 65 weeks did not give rise to specific
evidence of poisoning (Tollenaar, 1951).
Groups of 24 rats, 12 females and 12 males, were fed diets
containing 100, 300, 1000 and 2500 ppm of thiram. Rats with 300 and
1000 and 2500 ppm in the diet for 65 weeks showed weakness, ataxia and
various degrees of paralysis; also histological changes (calcification
in the brain stem and cerebellum and dystrophic changes in the leg
muscles) were found, and at 2500 ppm there was an increased mortality
rate (Lehman, 1952).
Groups of 20 young rats were placed on diets containing 100, 300
and 500 ppm thiram for 2 years (Bär, 1959). Small reductions in the
growth rate were seen at all concentrations. At concentrations of 300
and 500 ppm an increased mortality rate was seen, while at 500 ppm
convulsions, thyroid hyperplasia (Griepentrog, 1962) and calcification
in the cerebellum, hypothalamus and medulla oblongata were observed
(Griepentrog, 1961).
Comments on experimental studies reported
There are long-term studies in rats, but no other species has
been studied. Dose levels of 100 and 300 ppm in the diet of the rat
had a questionable effect on the thyroid but at 48 ppm no changes were
seen. Chicks appear to be especially susceptible to thiram. Before
these results can be taken into account, confirmation is needed with
detailed information; and because long-term reproduction studies with
3 generations of rats did not show any abnormalities in the litters,
the rat was selected for evaluation.
It is known that TMTD in a dose of 0.5 to 1.5 g per day can be
taken by man for many weeks without ill-effect unless alcohol is
consumed.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight per day.
Dog. 5 mg/kg body-weight.
(Note. An effect was found in the chick at 6.8 mg/kg
body-weight.)
Estimate of acceptable daily intake for man
0-0.025 mg/kg body-weight.
Further work desirable
Biochemical studies. Extension of the experiments in chicken.
REFERENCES
Bär, F. (1959) International Union of Pure and Applied Chemistry
Congress, München, September
Domingo, A. F. (1952) Rev. Med. vet. (Caracas), 11, 335
Eldjarn, L. (1950) Scand. J. Clin. Lab. Invest., 2, 198
van Esch, G. J. (1956) Versl. Volksgezondh., 166
Fitzhugh, O. G. (1963) Personal communication
Griepentrog, F. (1961) Arch. Psychiat. Nervenkr., 202, 412
Griepentrog, F. (1962) Beitr. Path. Anat., 126, 243
Hald, J. & Jacobson, E. (1948) Acta pharmacol. (Kbh.), 4, 305
Hald, J., Jacobson, E. & Larsen, V. (1952) Acta pharmacol. (Kbh.),
8, 329
Handbook of Toxicology (1959) vol. 3, Saunders, Philadelphia
Hanzlick, P. J. & Irvine, A. (1921) J. Pharmacol. exp. Ther., 17,
349
Johnson, E. L. Waibel, P. E. & Pomeroy, B. S. (1955) Proc Amer. vet.
med. Ass., 92, 322
Kirchheim, D. (1951) Naunyn-Schmiedeberg's Arch. exp. Path.
Pharmak., 214, 59
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S. 16, 47-53,126-32
Owens, R. G. (1953) Contr, Boyce Thompson Inst., 17, 221
Prickett, C. S. & Johnston, C. D. (1953) Biochim. biophys. Acta,
12, 542
Robbins, R. C. & Kastelic, J. (1961) J. Agric. Food Chem., 9, 256
Thorn & Ludwig (1962) The dithiocarbamates and related compounds,
Elsevier Mongraphs
Tollenaar, F. D. (1951) Neth. Milk and Dairy J., 5, 46
Waibel, P. E., Johnson, E. L. & Pomeroy, B. S. (1957) Poultry Sci.,
36, 697
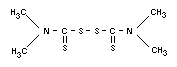 BIOLOGICAL DATA
Biochemical aspects
There are investigations on tetraethyl thiuram disulfide (TETD,
Disulfiram), a homologue of thiram. Experiments on TETD (tagged with
35S) showed that in rats 80-95% of an oral dose in absorbed,
elimination is delayed and a remarkable storage of 35S in the tissue
occurs (mainly in the adrenals, liver and spleen) (Eldjarn, 1950). The
compound was distributed in different tissues in a similar way to
carbon disulfide. The liver is important in the detoxication of the
compound. Animal tissues have been found to degrade TETD to
diethyldithiocarbamate (Harzlick & Irvine, 1921), which in turn
decomposed to carbon disulfide and diethylamine (Prickett & Johnston,
1953). Investigations on the fate of thiram in the digestive tract of
ruminants revealed a similar degradation by micro-organism, which was
not complete (Robbins & Kastelic, 1961); 4.0% of the ingested thiram
appeared in the faeces and 1.5% in the urine. The metabolic fate of
thiram absorbed into the body from the gastro-intestinal tract
probably depends to some extent on the species of animal (Robbins &
Kastelic, 1961). Thiuram disulfides can act as inhibitors of liver
enzymes (Owens, 1953). Thiram acts in vivo in a similar way to
tetraethyl thiuramdisulfide, i.e., it inhibits the acetaldehyde
oxidation enzyme systems. Some authors believe that it is the first
stage of alcohol metabolism, i.e, the oxidation to acetaldehyde that
is mainly inhibited and only to a less extent the conversion of
aldehyde into acetic acid (Hald & Jacobson, 1948; Thorn & Ludwig,
1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1500-2000 Kirchheim, 1951
Mouse Intraperitoneal 250 Hald et al., 1952
Rat Oral 780-865 Lehman, 1951
Handbook of Toxicology, 1959
Rabbit Oral 210 Lehman, 1951
Handbook of Toxicology, 1959
In man oral doses of 0.5 g of Arasan (containing 75% thiram) were
not toxic (Domingo, 1952).
Short-term studies
Fowl. 80 ppm of thiram in the diet of laying hens resulted in
misshapen or soft-shelled eggs, retardation or even cessation of
production of egg. Chicks showed retarded growth, reduced feed
efficiency and increased mortality (Johnson et al., 1955). Thiram was
toxic to chicks at 40 ppm (approximately equivalent to 6.8 mg/kg
body-weight) in the diet and goslings at less than 150 ppm, but turkey
poults tolerated 200 ppm. The symptoms of poisoning were leg
deformities, weight loss, crooked and curled toes, enlarged hocks and
some slipped tendons and spraddles (Waibel et al., 1957).
Dog. Groups of 3 male dogs were fed diets containing 10, 50 and
200 ppm of thiram for one year. No abnormalities or effects on growth
were observed and no gross or microscopic changes at any level
(Fitzhugh, 1963).
Long-term studies
Groups each of 24 rats (12 males and 12 females) were given a
diet containing 48 ppm of thiram over 3 generations. No effects on
growth, reproduction, blood picture and mortality rate were found. No
gross or histological changes were observed (van Esch, 1956). In
another experiment, 12 female and 12 male rats given 200 ppm in the
diet for 8 months showed no appreciable changes in growth and
mortality rate, and 300 ppm for 65 weeks did not give rise to specific
evidence of poisoning (Tollenaar, 1951).
Groups of 24 rats, 12 females and 12 males, were fed diets
containing 100, 300, 1000 and 2500 ppm of thiram. Rats with 300 and
1000 and 2500 ppm in the diet for 65 weeks showed weakness, ataxia and
various degrees of paralysis; also histological changes (calcification
in the brain stem and cerebellum and dystrophic changes in the leg
muscles) were found, and at 2500 ppm there was an increased mortality
rate (Lehman, 1952).
Groups of 20 young rats were placed on diets containing 100, 300
and 500 ppm thiram for 2 years (Bär, 1959). Small reductions in the
growth rate were seen at all concentrations. At concentrations of 300
and 500 ppm an increased mortality rate was seen, while at 500 ppm
convulsions, thyroid hyperplasia (Griepentrog, 1962) and calcification
in the cerebellum, hypothalamus and medulla oblongata were observed
(Griepentrog, 1961).
Comments on experimental studies reported
There are long-term studies in rats, but no other species has
been studied. Dose levels of 100 and 300 ppm in the diet of the rat
had a questionable effect on the thyroid but at 48 ppm no changes were
seen. Chicks appear to be especially susceptible to thiram. Before
these results can be taken into account, confirmation is needed with
detailed information; and because long-term reproduction studies with
3 generations of rats did not show any abnormalities in the litters,
the rat was selected for evaluation.
It is known that TMTD in a dose of 0.5 to 1.5 g per day can be
taken by man for many weeks without ill-effect unless alcohol is
consumed.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight per day.
Dog. 5 mg/kg body-weight.
(Note. An effect was found in the chick at 6.8 mg/kg
body-weight.)
Estimate of acceptable daily intake for man
0-0.025 mg/kg body-weight.
Further work desirable
Biochemical studies. Extension of the experiments in chicken.
REFERENCES
Bär, F. (1959) International Union of Pure and Applied Chemistry
Congress, München, September
Domingo, A. F. (1952) Rev. Med. vet. (Caracas), 11, 335
Eldjarn, L. (1950) Scand. J. Clin. Lab. Invest., 2, 198
van Esch, G. J. (1956) Versl. Volksgezondh., 166
Fitzhugh, O. G. (1963) Personal communication
Griepentrog, F. (1961) Arch. Psychiat. Nervenkr., 202, 412
Griepentrog, F. (1962) Beitr. Path. Anat., 126, 243
Hald, J. & Jacobson, E. (1948) Acta pharmacol. (Kbh.), 4, 305
Hald, J., Jacobson, E. & Larsen, V. (1952) Acta pharmacol. (Kbh.),
8, 329
Handbook of Toxicology (1959) vol. 3, Saunders, Philadelphia
Hanzlick, P. J. & Irvine, A. (1921) J. Pharmacol. exp. Ther., 17,
349
Johnson, E. L. Waibel, P. E. & Pomeroy, B. S. (1955) Proc Amer. vet.
med. Ass., 92, 322
Kirchheim, D. (1951) Naunyn-Schmiedeberg's Arch. exp. Path.
Pharmak., 214, 59
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S. 16, 47-53,126-32
Owens, R. G. (1953) Contr, Boyce Thompson Inst., 17, 221
Prickett, C. S. & Johnston, C. D. (1953) Biochim. biophys. Acta,
12, 542
Robbins, R. C. & Kastelic, J. (1961) J. Agric. Food Chem., 9, 256
Thorn & Ludwig (1962) The dithiocarbamates and related compounds,
Elsevier Mongraphs
Tollenaar, F. D. (1951) Neth. Milk and Dairy J., 5, 46
Waibel, P. E., Johnson, E. L. & Pomeroy, B. S. (1957) Poultry Sci.,
36, 697
BIOLOGICAL DATA
Biochemical aspects
There are investigations on tetraethyl thiuram disulfide (TETD,
Disulfiram), a homologue of thiram. Experiments on TETD (tagged with
35S) showed that in rats 80-95% of an oral dose in absorbed,
elimination is delayed and a remarkable storage of 35S in the tissue
occurs (mainly in the adrenals, liver and spleen) (Eldjarn, 1950). The
compound was distributed in different tissues in a similar way to
carbon disulfide. The liver is important in the detoxication of the
compound. Animal tissues have been found to degrade TETD to
diethyldithiocarbamate (Harzlick & Irvine, 1921), which in turn
decomposed to carbon disulfide and diethylamine (Prickett & Johnston,
1953). Investigations on the fate of thiram in the digestive tract of
ruminants revealed a similar degradation by micro-organism, which was
not complete (Robbins & Kastelic, 1961); 4.0% of the ingested thiram
appeared in the faeces and 1.5% in the urine. The metabolic fate of
thiram absorbed into the body from the gastro-intestinal tract
probably depends to some extent on the species of animal (Robbins &
Kastelic, 1961). Thiuram disulfides can act as inhibitors of liver
enzymes (Owens, 1953). Thiram acts in vivo in a similar way to
tetraethyl thiuramdisulfide, i.e., it inhibits the acetaldehyde
oxidation enzyme systems. Some authors believe that it is the first
stage of alcohol metabolism, i.e, the oxidation to acetaldehyde that
is mainly inhibited and only to a less extent the conversion of
aldehyde into acetic acid (Hald & Jacobson, 1948; Thorn & Ludwig,
1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1500-2000 Kirchheim, 1951
Mouse Intraperitoneal 250 Hald et al., 1952
Rat Oral 780-865 Lehman, 1951
Handbook of Toxicology, 1959
Rabbit Oral 210 Lehman, 1951
Handbook of Toxicology, 1959
In man oral doses of 0.5 g of Arasan (containing 75% thiram) were
not toxic (Domingo, 1952).
Short-term studies
Fowl. 80 ppm of thiram in the diet of laying hens resulted in
misshapen or soft-shelled eggs, retardation or even cessation of
production of egg. Chicks showed retarded growth, reduced feed
efficiency and increased mortality (Johnson et al., 1955). Thiram was
toxic to chicks at 40 ppm (approximately equivalent to 6.8 mg/kg
body-weight) in the diet and goslings at less than 150 ppm, but turkey
poults tolerated 200 ppm. The symptoms of poisoning were leg
deformities, weight loss, crooked and curled toes, enlarged hocks and
some slipped tendons and spraddles (Waibel et al., 1957).
Dog. Groups of 3 male dogs were fed diets containing 10, 50 and
200 ppm of thiram for one year. No abnormalities or effects on growth
were observed and no gross or microscopic changes at any level
(Fitzhugh, 1963).
Long-term studies
Groups each of 24 rats (12 males and 12 females) were given a
diet containing 48 ppm of thiram over 3 generations. No effects on
growth, reproduction, blood picture and mortality rate were found. No
gross or histological changes were observed (van Esch, 1956). In
another experiment, 12 female and 12 male rats given 200 ppm in the
diet for 8 months showed no appreciable changes in growth and
mortality rate, and 300 ppm for 65 weeks did not give rise to specific
evidence of poisoning (Tollenaar, 1951).
Groups of 24 rats, 12 females and 12 males, were fed diets
containing 100, 300, 1000 and 2500 ppm of thiram. Rats with 300 and
1000 and 2500 ppm in the diet for 65 weeks showed weakness, ataxia and
various degrees of paralysis; also histological changes (calcification
in the brain stem and cerebellum and dystrophic changes in the leg
muscles) were found, and at 2500 ppm there was an increased mortality
rate (Lehman, 1952).
Groups of 20 young rats were placed on diets containing 100, 300
and 500 ppm thiram for 2 years (Bär, 1959). Small reductions in the
growth rate were seen at all concentrations. At concentrations of 300
and 500 ppm an increased mortality rate was seen, while at 500 ppm
convulsions, thyroid hyperplasia (Griepentrog, 1962) and calcification
in the cerebellum, hypothalamus and medulla oblongata were observed
(Griepentrog, 1961).
Comments on experimental studies reported
There are long-term studies in rats, but no other species has
been studied. Dose levels of 100 and 300 ppm in the diet of the rat
had a questionable effect on the thyroid but at 48 ppm no changes were
seen. Chicks appear to be especially susceptible to thiram. Before
these results can be taken into account, confirmation is needed with
detailed information; and because long-term reproduction studies with
3 generations of rats did not show any abnormalities in the litters,
the rat was selected for evaluation.
It is known that TMTD in a dose of 0.5 to 1.5 g per day can be
taken by man for many weeks without ill-effect unless alcohol is
consumed.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight per day.
Dog. 5 mg/kg body-weight.
(Note. An effect was found in the chick at 6.8 mg/kg
body-weight.)
Estimate of acceptable daily intake for man
0-0.025 mg/kg body-weight.
Further work desirable
Biochemical studies. Extension of the experiments in chicken.
REFERENCES
Bär, F. (1959) International Union of Pure and Applied Chemistry
Congress, München, September
Domingo, A. F. (1952) Rev. Med. vet. (Caracas), 11, 335
Eldjarn, L. (1950) Scand. J. Clin. Lab. Invest., 2, 198
van Esch, G. J. (1956) Versl. Volksgezondh., 166
Fitzhugh, O. G. (1963) Personal communication
Griepentrog, F. (1961) Arch. Psychiat. Nervenkr., 202, 412
Griepentrog, F. (1962) Beitr. Path. Anat., 126, 243
Hald, J. & Jacobson, E. (1948) Acta pharmacol. (Kbh.), 4, 305
Hald, J., Jacobson, E. & Larsen, V. (1952) Acta pharmacol. (Kbh.),
8, 329
Handbook of Toxicology (1959) vol. 3, Saunders, Philadelphia
Hanzlick, P. J. & Irvine, A. (1921) J. Pharmacol. exp. Ther., 17,
349
Johnson, E. L. Waibel, P. E. & Pomeroy, B. S. (1955) Proc Amer. vet.
med. Ass., 92, 322
Kirchheim, D. (1951) Naunyn-Schmiedeberg's Arch. exp. Path.
Pharmak., 214, 59
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S. 16, 47-53,126-32
Owens, R. G. (1953) Contr, Boyce Thompson Inst., 17, 221
Prickett, C. S. & Johnston, C. D. (1953) Biochim. biophys. Acta,
12, 542
Robbins, R. C. & Kastelic, J. (1961) J. Agric. Food Chem., 9, 256
Thorn & Ludwig (1962) The dithiocarbamates and related compounds,
Elsevier Mongraphs
Tollenaar, F. D. (1951) Neth. Milk and Dairy J., 5, 46
Waibel, P. E., Johnson, E. L. & Pomeroy, B. S. (1957) Poultry Sci.,
36, 697
