
THIRAM
First draft prepared by J.-J. Larsen
National Food Agency, Ministry of Health
Soborg, Denmark
EXPLANATION
Thiram, a dimethyl dithiocarbamate fungicide, was evaluated by
the Joint Meeting several times between 1963 and 1987 (Annex 1,
references 2, 4, 8, 14, 22, 28, 34, 44 and 50). A temporary ADI of
0-0.005 mg/kg bw, allocated in 1974, was extended in 1977 and 1980.
The temporary ADI was withdrawn in 1985 because of the inadequancy
of the total data base. The studies available to the 1987 Joint
Meeting were not adequate for estimating an ADI. A complete data
base on thiram has been generated since the previous evaluation, and
was evaluated at the present Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
A single oral dose of 14C-thiram was administered to male and
female Charles River CD random bred, VAF Plus (SPF) rats to
determine its absorption, excretion and final distribution.
Unlabelled thiram (purity 98.5%) and 14C-thiram (radiochemical
purity 100%) dissolved in PEG-400 solution was administered by
gavage at 125 mg/kg bw (dosage volume 4.95 ml/kg, concentration
25.24 mg/ml, specific activity 1.748 x 107 dpm/ml (± 0.006 x 107
SD)), to 5 rats/sex and at 1.9 mg/kg bw (dosage volume 4.75 ml/kg,
concentration 0.4 mg/ml, specific activity 1.721 x 107 dpm/ml (±
0.003 x 107 SD)), to 5 rats/sex (weight range 180-250 g). PEG-400
solution was administered to one rat/sex as controls. Urine and
faeces were collected for 7 days. The rats were then necropsied and
tissues were collected. Homogenates (20%) were prepared from the
tissues and faecal material and were oxidized. Radioactivity was
determined by liquid scintillation counting.
Only 32% of the administered dose was recovered, mainly from
the urine (25%). About 3% was recovered from the various organs.
Blood, bone and liver contained significant quantities of the test
material or its metabolites. Only 3% of the administered dose was
recovered in the faeces. Dose level or sex did not affect total
recovery. Approximately 70% of the administered thiram, not
recovered, may have been metabolized to CO2 or other volatiles in
the expired air or by bacterial action in the faeces or urine during
the intervals between collections (Gay, 1987).
Following 14 days pretreatment to 5 Taconic farm Sprague-Dawley
rats/sex (males 6-9 weeks old, 227-250 g; females 9-10 weeks old,
197-220 g) at a dose level of 2 mg thiram/kg bw/day, a single dose
of 14C-thiram/kg bw (radiochemical purity > 98%, specific
activity 15.5 mCi/mmol) was administered. Doses were administered as
a suspension in PEG-400 (5 ml/kg bw). The stability of labelled and
unlabelled thiram was satisfactory. The percentages of 14C-thiram-
derived radioactivity were determined in urine, faeces and expired
air at intervals up to 96 hr, and the concentrations and percentages
were determined in tissues at 96 h following dosing.
14C-Thiram was well absorbed by both sexes (>83% of the
dose) following oral administration. Radioactivity was excreted in
the urine (35-40% of the dose within 96 h), faeces (2-5%), and
expired air (41-48%). Excretion was more extensive and rapid in
urine and expired air within the first 12 h post-dosing, while the
majority of the faecal radioactivity was excreted after 24 h. The
majority of the dose (at least 83.7 and 89.6% for male and female
rats, respectively) was eliminated from the body within four days
post-dosing. Sex did not affect the extent or rate of excretion of
orally administered 14C-thiram. Trace levels of radioactivity were
detected in all tissues analyzed at 96 h after dosing. In general,
the highest concentrations were in liver, blood cells and kidneys,
and the lowest were in brain, plasma, and skeletal muscle. About 2-
3% of the dose remained in tissues after four days. The total
recovery of radioactivity averaged about 85 and 93% for males and
females, respectively (Nomeir & Markham, 1990).
The bioavailability of radioactivity from a single feeding with
a diet containing 30 ppm equal to 1.5 mg 14C-thiram/kg bw (mixture
of labelled (purity 92.4%) and non-labelled substance (purity
97.5%)) was evaluated in five male Charles River Crl:CDRBR albino
rats (body-weight 246-270 g). The specific activity of 14C-thiram
was 15 mCi/mmol. The stability of the test materials was
satisfactory. Test rats, conditioned to consume an entire day's feed
during the last hour of their dark cycle, were fed once with 14C-
thiram-containing diet and then placed in metabolism cages.
Radioactivity in the urine, faeces and expired air (carbon dioxide
and carbon disulphide) over the next 72 h and the residues in the
carcass and gastrointestinal tract at 72 hours were determined. The
expired gases contained 41% of the administered radioactivity, urine
contained 38% and carcass contained 6%. The faeces and
gastrointestinal tract accounted for 20% of the dose. The total
recovery of radioactivity averaged 105%.
It was demonstrated that 85% of the radioactivity equivalents
was absorbed into the systemic circulation of the rat. Assumptions
were made that there was no elimination of absorbed radioactivity
into the gastrointestinal tract and that all of the radioactivity in
the expired gases, urine, and carcass represented absorbed thiram
(Hiles, 1989).
Biotransformation
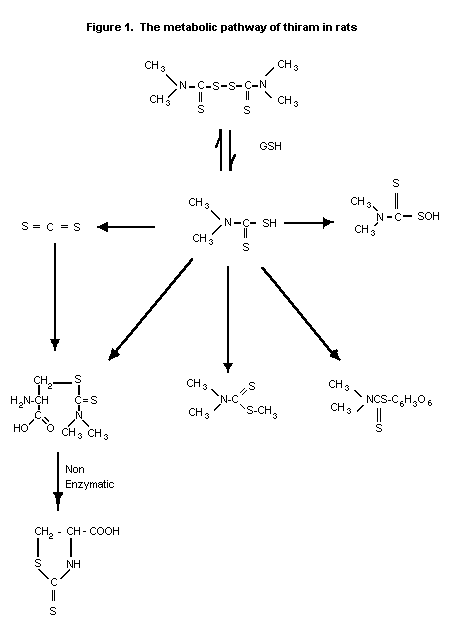
The metabolic pathway of thiram in rats is shown in Figure 1.
Male Sprague-Dawley rats (160-200 g from Southern Animal Farms)
were injected i.p. with 15, 30 or 60 mg thiram (purity 99%)/kg bw
(solvent corn oil). Following injection rats were placed
individually in a 4-litre all-glass metabolic cage. The expired air
was drawn, over a period of 5 h, through traps containing 1N NaOH (1
trap), then through ethanol-diethylamine-triethanolamine-cupric
acetate reagent (2 traps). The amount of carbon disulphide dissolved
in the reagent in the traps was determined colorimetrically using a
spectrophotometer. In a similar experiment, 60 mg thiram/kg bw was
given to rats previously treated intraperitoneally with 40 mg SKF
525-A/kg bw (1 dose 30 min earlier) or with 5 daily doses of 50 mg
phenobarbital/kg bw. In subsequent experiments, rats were treated
i.p. with 60 mg thiram/kg bw and sacrificed at 5 and 24 hour
intervals. Activities of hepatic microsomal and serum enzymes were
measured. The extent of thiram-induced liver injury was assessed by
ASAT and SDH activity.
A dose-dependent excretion of carbon disulphide (completed
within 5 h was demonstrated in the expired air (2.6, 26, and 120
nmol at doses of 15, 30, and 60 mg/kg bw, respectively). Expiration
of carbon disulphide, was increased by phenobarbital pre-treatment
and decreased by SKF 525-A pre-treatment. Activities of hepatic
microsomal and serum enzymes indicated that thiram caused a
significant loss of cytochrome P-450 and benzphetamine N-demethylase
activity and significant elevation of SDH and ASAT activity. It was
concluded that carbon disulphide is an in vivo metabolite of thiram
and may be responsible for the observed hepatotoxicity (Dalvi &
Deoras, 1986).
The volatile 14C-residues in the expired air were determined
in Charles River Sprague-Dawley rats (age 7 weeks). Following 10
days acclimatization, three rats received an oral dose of 2.1-2.5 mg
14C-thiram/kg bw (purity 98%, specific activity 20.7 mCi/mmol).
Each rat was then placed in a sealed glass metabolism cage for 96
hours. The exit gases from the metabolism cage were passed through a
scrubbing tower containing Harvey Carbon-14 Cocktail (efficient at
trapping CO2, CS2, and COS), then through a solution of
diethylamine/ethanol and finally through a solution of Viles CS2
reagent. Air flow through the metabolism cage was 3.5-4.0 l/min.
Urine was also collected and radioassayed.
An average of 61% of the radioactive dose was recovered as
volatiles. More than 99.5% of the volatiles was recovered in the
Harvey Carbon-14 Cocktail. Labelled residues in urine accounted for
25-43% of the radioactive dose. The total radioactivity recovered in
the volatiles and the urine for the three rats averaged 94% of the
dose. Results indicate that thiram is largely metabolised into
volatiles such as CO2, CS2, and COS. The study does not allow a
characterization or quantification of the three metabolites (Norris,
1989).
The identification of thiram metabolites in urine was
determined in 2 Charles River Crl:CDR(SD)BR rats/sex. The rats
(approximately 5 weeks old) were fed diets containing 50 ppm
unlabelled thiram for nine weeks followed by a single oral dose of
14C-thiram (purity 99%, specific activity 15.5 mCi/mmol). Samples
of urine were collected over the first 24 h after treatment
termination and analyzed by HPLC.
 Approximately 60% of the administered radioactivity was
recovered as expired CS2 and 30% was found in the urine. Thiram
was rapidly degraded to more polar products. Virtually no unchanged
thiram was detected in the urine. Five urinary metabolites were
detected by HPLC and were identified by mass spectrometry. The
identified metabolites were an alanine derivative of CS2 (10%); a
glucuronide conjugate of dimethyldithiocarbamate (DDC) (20%); a
thiosulfenic acid (34%); the methyl ester of DDC (6%); and an
alanine conjugate (30%). The presence of these polar conjugates
demonstrates that the metabolic pathway involved a reduction of the
disulphide bond and subsequent reactions of the thiol moiety to form
oxidative and conjugative polar products (McManus, 1991).
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of thiram
Species Sex Route LD50 LC50 References
(mg/kg bw) (mg/m3)
Mouse M oral 4000 Matthiask, 1973
Mouse F oral 2300-3800 Lee et al., 1975;
1978
Rat M oral 3700-4000 Thouin, 1985a;
Lee et al., 1975;
1978
Rat F oral 1800-1900 Thouin, 1985a;
Lee et al., 1975;
1978
Rat M&F inhalation > 100 Debets, 1985
Rabbit M&F dermal > 2000 Thouin, 1985b
Short-term toxicity studies
Mice
Ten Charles River albino mice (Crl:CD-1R(ICR)BR)/sex/group
(males 24.3-24.8 g, females 19.0-19.3 g) received in the diet 0,
300, 600, or 1200 ppm thiram (purity 97.5%) for 4 weeks equal to 0,
54, 108 or 201 mg/kg bw/day in males and 62, 118 or 241 mg/kg bw/day
in females. Stability and homogeneity of the test substance was
satisfactory. The animals were observed daily for mortality, signs
of toxicity and food consumption. Body-weights were recorded weekly.
Haematology and clinical chemistry tests were performed at sacrifice
on days 29 or 30. Gross and histopathological examinations of the
animals were carried out.
Body-weights were significantly and dose-dependently reduced in
males at all dose levels. Food consumption was significantly lower
for both sexes at all dose levels when compared with controls.
Statistically significant differences in clinical chemistry and
haematological parameters included reduced erythrocyte counts,
haemoglobin, and haematocrit for males at all dose levels; increased
platelet counts for females at 600 and 1200 ppm; reduced serum
glucose for females at 1200 ppm. Statistically significant and dose-
dependent increased organ-to-body-weight ratios of the brain,
kidneys and liver in males probably resulted from statistically
significant and dose-dependent reduced terminal body-weights. A
NOAEL could not be determined because of the 28-48% reduction in
food intake at 300 ppm (Kehoe, 1989b).
Rats
In a 13-week study, 10 Charles River rats
(Crl:CDR(SD)BR)/sex/group (males 138 ±6 g and females 125 ±4 g)
received dietary concentrations of 0, 50, 500, or 1000 ppm thiram
(purity 99.4%) equivalent to 0, 2.5, 25 or 50 mg/kg bw/day. Mean
stability assay results for diets stored frozen for 14 and 35 days
ranged from 81-99%. Diets analyzed after being in the animals room
for 7 days were 17, 80, and 88%, respectively, of the nominal
levels. Mean values for homogeneity assay results were 80, 97, and
98%, respectively, of the nominal levels of 50, 500, and 1000 ppm.
The mean±SD of determined concentration of thiram was 40 ± 1, 483 ±
8, and 976 ± 15 ppm. The animals were observed daily for mortality
and signs of toxicity. Body-weights and food consumptions were
recorded weekly and haematology and clinical chemistry were
determined at study termination. Ophthalmic observations were done
before and at termination of study. Gross and histopathological
examinations from the control and 1000 ppm group and of macroscopic
lesions, lungs, liver, and kidneys from all 50 ppm and all 500 ppm
animals was carried out at study termination.
Body weights, cumulative body-weight gains, and food
consumption were significantly reduced throughout the study for both
sexes at 500 and 1000 ppm. Changes in clinical chemistry and
haematological parameters occurred at dose levels of 500 and 1000
ppm. The changes considered to be treatment-related were reduced
erythrocyte counts, haemoglobin and haematocrit in females;
increased MCV and MCH in both sexes; increased white blood cell,
corrected white blood cell, absolute neutrophil, absolute lymphocyte
and absolute monocyte counts in females; reduced total protein and
glucose in both sexes; reduced albumin and increased urea nitrogen
and chloride in females.
At 500 and 1000 ppm animals a tendency to reduced terminal
body-weights with correspondingly reduced absolute organ weights and
increased organ to body-weight ratios were observed.
Macroscopically, the non-glandular stomach in some animals showed
areas of erosion and the mesenteric lymph nodes were diffusely red
or mottled. Microscopically, the mucosa of the nonglandular stomach
had focal areas of erosion/ulceration, mucosal hyperplasia, or both,
accompanied by some submucosal inflammation and edema. These changes
appeared to be treatment-related. The mesenteric lymph nodes were
frequently congested but otherwise normal. The NOAEL in this study
was 50 ppm, equivalent to 2.5 mg/kg bw/day (Kehoe, 1988b).
Dogs
Two acclimated beagle dogs (5 months of age and weighing 5.6 to
8.5 kg/sex/group) were fed dietary concentrations of 0, 125, 500, or
2000 ppm thiram (purity 99.4%), equivalent to 0, 3, 13 or 50 mg/kg
bw/day for 4 weeks. All animals were observed daily for toxic signs
and mortality. Body-weight and food consumption was recorded weekly.
Clinical chemistry, gross and histopathological examinations were
performed on all animals. Homogeneity assays indicated from 94-96%
(mean values) of the nominal values. There were no data on
stability, but thiram was stored under similar conditions to that in
other studies where stability was satisfactory.
One 2000 ppm one female died during week 2 and one male became
moribund and was sacrificed during week 4. Therefore, the high-dose
of 2000 ppm was reduced to 1500 ppm during weeks 3 and 4. The
observations in the female were primarily colon changes associated
with the acute death and subsequent post-mortem change. The male had
only a few incidental macroscopic and microscopic findings and,
therefore, its death was not considered compound-related. At week 4,
body-weights for both sexes at 500 ppm were approximately 15% lower
and at 2000/1500 ppm, 33 and 39% lower than controls respectively.
Food consumption during week 4 for 125, 500, and 2000/1500 ppm males
were 12%, 29%, and 92% lower than controls respectively, and for
females 13%, 49%, and 78% lower. Treatment-related changes in
clinical chemistry and haematological parameters comprised reduced
erythrocyte counts, haemoglobin and haematocrit in males given 500
or 2000/1500 ppm. One male given 2000/1500 ppm had increased ALAT,
ASAT and ALP. Slightly reduced absolute lymphocyte counts and
slightly higher platelet counts, total bilirubin and urea nitrogen
occurred in some male and female dogs given 500 or 2000 ppm. The
surviving 2000/1500 ppm dogs sacrificed at week 4 had notably
reduced terminal body-weights and correspondingly reduced absolute
organ weights. The liver of the males had hepatocellular
degeneration with sinusoidal cell proliferation and pigmentation.
There were no major macroscopic or microscopic findings in the
remaining dogs. The NOAEL for the study was determined to be 125
ppm, equivalent to 3 mg/kg bw/day, based on the lower body-weights
and changes in clinical chemistry parameters (Kehoe 1988a).
In a 13-week study on 5 month old beagle dogs, four beagle
dogs/sex/group were fed dietary concentrations of 0, 75, 250, or 500
ppm thiram (purity 97.5%), equal to 0, 2.2, 6.9 or 12 mg/kg bw/day
in males and 0, 2.3, 7.3 or 13 mg/kg bw/day in females. The mean (±
SD) body-weight at study initiation was 7.4±0.6, 7.3±1.1, 7.4±0.5,
and 7.3±0.6 kg for males receiving 0, 75, 250, and 500 ppm. and were
6.8 ± 0.9, 7.1 ± 1.4, 6.3 ± 0.5, and 6.7±0.5 kg for females. All
animals were observed daily for clinical signs, moribundity and
mortality. Body-weight and food consumption was recorded weekly.
Ophthalmic examinations were performed prior to and at the end of
the study. Clinical chemistry measurements, haematological
examinations and gross and histopathology examinations were done on
all animals. Homogeneity of diets was within ± 8% of nominal values.
Mean stability assays of diets frozen for 7 days, stored in the
animal room for 1 day and then frozen for 4 weeks, were within ± 5%
of nominal levels.
Body weights for the 500 ppm dogs were significantly reduced.
Food consumption was significantly reduced in 500 ppm males and in
250 and 500 ppm females. Treatment-related effects for clinical
chemistry and haematological parameters were reduced erythrocyte
counts, higher MCV and MCH in 75, 250, and 500 ppm dogs; reduced
haemoglobin and haematocrit in 500 ppm females; higher platelet
counts in 250 and 500 ppm males; lower total protein and albumin in
75, 250, and 500 ppm males and females; and higher cholesterol in
250 and 500 ppm males and females. The NOAEL for this study was 75
ppm equal to 2.2 and 2.3 mg/kg bw/day in males and females
respectively, based on haematological changes noted in both sexes
(Kehoe, 1989a).
Six 4 to 5 month old beagle dogs/sex/group (initial weight 4.0
to 6.8 kg) were fed dietary concentrations of 0, 30, 90, or 250 ppm
thiram (purity 97.5%), equal to 0, 0.84, 2.6, and 7.4 mg/kg bw/day
in males and 0, 0.90, 2.5, and 7.2 mg/kg bw/day in females for 52
weeks. All animals were observed daily for clinical signs and
mortality. Body weights and food consumption were recorded weekly
for weeks 1 through 16, and monthly thereafter. Ophthalmic
examinations were performed prior to and at the end of the study.
Haematology, clinical chemistry, gross and histopathology were
performed on all animals. Mean values for homogeneity assays were
83, 92, and 92% of the nominal levels of 30, 90, and 250 ppm,
respectively. Mean stability assay results for diets kept below 0 °C
for 7 days and stored in the animal room for one day were 84%, 92%
and 96% of the nominal levels of 30, 90, and 250 ppm, respectively.
No ophthalmic lesions were observed in any animals. Erythrocyte
counts were reduced in males given 250 ppm, total protein was lower
and cholesterol was higher in males given 90 or 250 ppm and in
females given 250 ppm. Albumin was reduced in both sexes given 250
ppm. Absolute liver weights in males given 90 or 250 ppm were
significantly increased. Liver-to-body-weight ratios in males given
30, 90, or 250 ppm and in females given 250 ppm were also
significantly increased, as were liver-to-brain weight ratios in
males given 250 ppm. The significant increase in liver-to-body-
weight ratios in males given 30 ppm was due to a slight increase in
absolute liver weight and a slight decrease in body weight.
Furthermore, the observation that the body weights of males given 90
ppm were increased in relation to those of the controls and those of
males given 30 ppm demonstrates, that the slight decrease in body
weights seen in the 30 ppm group was spurious. Thus, the increase in
liver-to-body-weight ratio seen in males given 30 ppm was not
considered a test material-related effect. The changes in liver
weights, in conjunction with the altered total protein, albumin, and
cholesterol levels suggest a test material-related effect on liver
function and size. These changes were probably adaptive responses to
the presence of this compound. Based on increased absolute liver
weights and altered clinical chemistry parameters in males given 90
or 250 ppm, and altered clinical chemistry parameters in females at
250 ppm, the NOAEL for male dogs was 30 ppm, equal to 0.84 mg/kg
bw/day, and the NOAEL for female dogs was 90 ppm equal to 2.5 mg/kg
bw/day (Kehoe 1991a).
Four groups of 4 beagle dogs/sex/dose were treated orally via
gelatin capsule with thiram (purity 98.7%) at doses of 0 (blank
capsule), 0.4, 4, or 40 mg/kg bw/day for 104 weeks. Body weights and
food intake were measured weekly. Water consumption, haematology,
ophthalmoscopy, clinical chemistry and gross and histopathology of
all organs were performed.
Both sexes at 40 mg/kg bw/day showed severe toxic signs
including nausea, vomiting, salivation, and clonic convulsion and
severe anaemia within the first 11 weeks of treatment and were all
subjected to unscheduled necropsy before the 29th week of treatment.
The dogs also showed ophthalmological changes such as fundal
haemorrhage, miosis, and desquamation of the retina which were
consistent with retinal lesions observed histopathologically. In the
4 mg/kg bw/day group, nausea, vomiting and salivation were common
findings in both sexes and one female showed clonic convulsion from
week 37. Parameters including haematocrit, haemoglobin, and
erythrocyte count were depressed from week 4 in both sexes treated
with 4 or 40 mg/kg bw/day, indicating anaemia. Changes in
biochemical parameters indicating liver failure were observed from
week 4 in the 40 mg/kg bw/day males and later in both sexes dosed
with 4 or 40 mg/kg bw/day. Kidney damage was detected in the
histopathological examination in two female dogs in each of the 4
and 40 mg/kg bw/day groups. Histological lesions in the central or
peripheral nervous system relating to the observed neurological
disturbances were not found. It was concluded that no sex difference
in response to treatment was demonstrated. Based on the neurological
disturbances, anaemia and the effects on the liver, the NOAEL was
0.4 mg/kg bw/day (Maita et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Four groups of 50 acclimated (24 days) Charles River Crl:CD-
1R(ICR)BR mice/sex/dose level (approximately 7 weeks of age) were
fed thiram (97.5% purity) at dietary concentrations of 0, 15, 150,
or 300 ppm (males), equal to 3, 24 or 50 mg/kg bw/day and 0, 15,
300, or 600 ppm (females), equal to 3, 57 or 112 mg/kg bw/day for 97
weeks, when the most sensitive group showed 50% mortality.
Homogeneity assays indicated from 75-84%, 95-100%, 94-103%, and 101-
103% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively. Mean stability assay results for diets frozen for 6
days and stored in the animal room for one day were 55, 90, 90, and
97% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively, demonstrating stability of varying acceptability.
Survival, clinical signs, body weights, food consumption, feed
efficiency, haematological parameters, necropsy, absolute and
relative organ weight and histopathological examination were
studied.
No substance-related oncogenic effects or adverse effects on
survival were observed in any test group. No changes attributable to
treatment were seen in any of the parameters evaluated in the 15 ppm
groups. There was no clinical indication of neurotoxicity at any
dose level. Mean body weight, weight gain, and total food
consumption were statistically significantly decreased in a dose-
dependent manner in the mid- and high-level groups beginning at
weeks 4-5. At study termination, mean weights were 7%, 15%, 14%, and
19% below control and mean total food consumption was 6%, 10%, 12%,
and 17% below control for the 150 ppm males, 300 ppm males, 300 ppm
females, and 600 ppm females, respectively. Increased frequency of
sores or reddened areas on the skin, generally on the ears,
consistent with bacterial dermatitis was noted for the 300 ppm males
and 600 ppm females. Mild but significant decreases in mean
erythrocyte count, haemoglobin, and haematocrit values were seen for
the 600 ppm females at week 97. Histopathology showed no evidence of
thiram-induced neoplasia. Non-neoplastic findings in the mid- and
high-level male and female mice consisted of retinal atrophy,
intracytoplasmic protein-like droplets in the urinary bladder
superficial transitional epithelium, and necrosis and suppurative
inflammation in the skin of the 150 and 300 ppm males and 300 and
600 ppm females; hyperkeratosis in the nonglandular stomach of the
300 ppm males and 300 and 600 ppm females; and increased pigment in
the spleen and decreased pigment in the inner adrenal cortex of the
300 and 600 ppm females.
No oncogenic effect was observed in doses up to 300 ppm, equal
to 50 mg/kg bw/day in males and 600 ppm, equal to 112 mg/kg bw/day
in females. Based on the body-weight reduction, the NOAEL for long-
term toxicity was 15 ppm, equal to 3 mg/kg bw/day (Trutter, 1992).
Rats
Four groups of 64 Jcl:Wistar rats were fed dietary
concentrations of 0, 3, 30, or 300 ppm thiram (purity 98.7%), equal
to 0, 0.1, 1.2 or 12 mg/kg bw/day for males and 0, 0.1, 1.4 or 14
mg/kg bw/day for females, for 104 weeks. Body weights, food intake,
and water intake were measured weekly up to week 52 and monthly
thereafter. Haematology, clinical chemistry, ophthalmoscopy and
gross and histopathology were performed on all rats. Test diets were
prepared twice weekly during the study. Data on homogeneity were not
available, but diets were stable up to 1 week.
Mortality of females in the 30 and 300 ppm groups was slightly
increased compared to controls during the last 8 weeks of treatment,
apparently correlated to an incidental higher occurrence of
pituitary tumours during this period. Overall incidence of pituitary
tumours in females was comparable to that in the control group.
Decreased body-weight gain and reduced food intake were observed in
both sexes at 300 ppm. Anaemia and regressive changes in the sciatic
nerve accompanied by atrophy of the calf muscle ( M. triceps surae)
were seen in the 300 ppm females. During the last 8 weeks of the
treatment period, the incidence of pituitary adenomas was increased
in females of the 30 and 300 ppm groups compared to that in
controls. Because of the small number of animals, statistical
analysis was not carried out. Statistical analysis of pituitary
tumour incidence in all animals at the end of the experiment showed
no significant differences. No evidence of carcinogenic potential
was observed. Based on mortality, anaemia, nerve degeneration and
muscle atrophy a NOAEL of 30 ppm, equal to 1.2 and 1.4 mg/kg bw/day
was determined in males and females, respectively (Miata et al.,
1991).
Four groups of sixty 36-day old Charles River Crl:CDR(SD)BR
rats/sex/group (initial weights 125-161 g for males and 115-193 g
for females) were fed dietary concentrations of 0, 30, 150, or 300
ppm thiram (purity 97.5%), equal to 0, 1.5, 7.3 or 15 mg/kg bw/day
in males and 0, 1.8, 8.9 or 19 mg/kg bw/day in females, for 104
weeks. Mean values for stability assays of diets kept below 0 °C for
6 days, animal room for one day, then below 0°C for 30 days were
15.5 ppm (52%), 123 ppm (82%), and 260 ppm (87%) for nominal
contents of 30, 150, and 300 ppm, respectively. The values for
homogeneity assay results ranged from 21.8-24.2 (73-81%), 128-138
(85-92%), and 255-276 ppm (85-92%) for nominal contents of 30, 150,
and 300 ppm, respectively. Cumulative means for the routine diet
analysis for dose confirmation were 16.3 (54%), 119(79%), and 262
ppm (87%) and the assay results ranged from 4.41-27.5 ppm, 81.8-151
ppm, and 179-320 ppm of the nominal dose levels of 30, 150, and 300
ppm, respectively, through 104 weeks. Data from a parallel study
with 14C-thiram stored under similar conditions (Hiles, 1989)
showed, that animals on a diet containing 30 ppm could be expected
to receive a systemic exposure to thiram and/or thiram-derived
materials approximately equal to the amount added to the diet, even
though the expected level could not be verified analytically.
Therefore, these data were considered to demonstrate that the
anticipated levels of thiram were achieved in the test diets. The
animals were observed daily for clinical signs and mortality. Body-
weight and food consumption were recorded weekly and haematology,
clinical chemistry, and urine analysis parameters were evaluated
during the study and at study termination. Ophthalmic observations
were done before and at termination of the study and gross and
histopathology examinations were carried out at study termination.
Clinical signs possibly test material-related for males
included swollen nose, soft faeces and opaque eyes. Soft faeces were
possibly test material-related at 150 and 300 ppm for the females.
Body-weight and cumulative body-weight gains in both sexes were
statistically significantly lower than those of the controls at 150
and 300 ppm. Food consumption was statistically significantly lower
than that in the controls for both sexes given 30, 150, or 300 ppm.
Reduced erythrocyte counts, haemoglobin, haematocrit and higher mean
corpuscular volume and mean corpuscular haemoglobin were observed in
females given 150 or 300 ppm. Statistically significant positive
trend analyses for hepatocellular adenomas (both sexes) and thyroid
C-cell adenomas (terminal sacrifice males and terminal plus
unscheduled sacrificed females) were reported but individual group
comparisons with controls were statistically insignificant. There
was no increase in the incidence of thyroid C-cell carcinomas.
Extramedullary haematopoiesis in the liver appeared to be increased
in males at 150 or 300 ppm and in females at 300 ppm. The biological
significance of this finding is unclear. Steatosis/fatty
infiltration of the pancreas (not uncommon in old SD rats) appeared
to be increased for males and females given 150 or 300 ppm. Also in
the pancreas, multifocal acinar atrophy was more common for males
given 150 or 300 ppm. The incidence of bile duct hyperplasia showed
a statistically significant positive trend in females, but was
statistically significantly increased only at 300 ppm. There were no
histopathological findings recorded that suggested test material-
related neurotoxicity.
Based on statistically significant decreases in body weights
and cumulative body-weight gains for males and females given 150 or
300 ppm, effects on haematological parameters in females given 150
or 300 ppm, and histopathological changes in males and females given
150 or 300 ppm, the NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg
bw/day in males and females, respectively. Thiram was not found to
be carcinogenic at doses up to 15 and 19 mg/kg bw/day in males and
females, respectively (Kehoe, 1991b).
Three groups of 50 five-week old Charles River SPF F344 rats,
initial mean body-weight of about 200 g for males and about 150 g
for females, were fed dietary concentrations of 0, 500 or 1000 ppm,
equal to 0, 18 or 39 mg/kg bw/day in males and 0, 20 or 42 mg/kg
bw/day in females for 104 weeks. Body weights and food consumption
were measured at regular intervals and haematology and clinical
chemistry were evaluated during the study and at study termination.
Gross and histopathology were performed. Homogeneity data and
stability data were not given.
Reduced body-weight gain and food consumption were seen at 500
and 1000 ppm in both sexes but especially in females of the high-
dose group. In males reduced liver function was observed.
Mononuclear cell leukaemia was found with an incidence of 20%, 8%,
and 4% in males and 29%, 12%, and 4% in females for 0, 500, and 1000
ppm animals, respectively. No tumour induction related to the
treatment was observed. It was concluded that thiram had no
carcinogenic effect at doses up to 39 and 42 mg/kg bw/day in males
and females, respectively (Hasegawa et al., 1988).
Reproduction studies
In a two-generation reproduction study, thiram (purity 97.6%)
was administered to Charles River Crl:CD VAF/Plus rats in dietary
concentrations of 0, 30, 60 or 180 ppm equal to 0, 1.5, 2.9 or 8.9
mg/kg bw/day in the F0 males and 0, 2.3, 4.6 or 14 mg/kg bw/day in
F0 females, and 0, 1.8, 3.8 or 11 mg/kg bw/day in the F1 males
and 0, 2.4, 5.1 or 16 mg/kg bw/day in the F1 females. Homogeneity
of thiram was satisfactory in the mixed diets. The test compound was
stable kept frozen for 7 days and reasonably stable (losses of 18%,
16%, and 9% in the 30, 60, and 180 ppm diet, respectively) kept at
room temperature for 12 hours. The mean ± SD percent of target ppm
found in all analyzed and administered 30, 60, and 180 ppm diets was
93 ± 6.3, 94 ± 6.6, and 98 ± 5.2%, respectively.The F0 and F1
parental generation consisted of 26 males and 26 females per group.
F0 animals were treated beginning at 63 days of age for 81 days
prior to the initial mating (F1a). A second litter (F1b) was
produced after a rest period of 16 days following weaning of the
F1a litter. Since conception rates were poor across all groups,
including the controls, in the F1b litter, an F1c litter was
also produced. The F1 parents were selected from the F1c
litters. These animals were treated beginning at 22 days of age for
a minimum of 84 days prior to their initial (F2a) mating. The F1
parents were mated twice to produce theF2a and F2b litters. All
parental animals, and pups were observed daily for mortality and
overt toxicity. Detailed clinical observations were recorded at
least once a week. Reproductive and litter parameters assessed
included male and female fertility indices, events at parturition,
gestation length, litter size, numbers of viable and stillborn pups,
and offspring survival and growth during lactation. All parental
animals, and all F1c weanlings not selected to remain on study and
all F2b weanlings were subjected to a gross necropsy. The F1a,
F1b, and the F2a offspring were euthanized and discarded
following weaning.
Mean maternal body weights and mean food consumption of the
F0 females were reduced at 60 and 180 ppm during the F1a
gestation period. Reductions in mean maternal body-weight and/or
food consumption were also observed at 180 ppm during the F1b and
F1c gestation periods and the F1a, F1b, and F1c lactation
periods. Similar reductions were observed for the F1 females at
180 ppm during the F2a and F2b gestation and lactation periods.
Reductions in mean weekly food consumption of the F0 males and
females were observed at 60 and 180 ppm. Mean offspring body weights
were consistently reduced to a significant degree in all litters
across both generations at the 180 ppm level. Apart from a
consistent statistically significant reduction in body weights in
the top dosed offspring during the F1b and F1c gestation periods
and the F1a, F1b, and F1c lactation periods, no substance-
related changes were found in the reproductive parameters or in the
parameters for post-natal development.
Based on the findings of parenteral systemic toxicity, the
NOAEL was 30 ppm, equal to 1.5 and 2.3 mg/kg bw/day in males and
females, respectively. With respect to filial systemic toxicity, the
NOAEL was 60 ppm, equal to 3.8 and 5.1 mg/kg bw/day in males and
females, respectively. With respect to reproduction and post-natal
development, the NOAEL was greater than 180 ppm equal to 8.9 and 14
mg/kg bw/day in males and females, respectively (York, 1991).
Special studies on genotoxicity
Data are shown in Table 2.
Special studies on teratogenicity
In a preliminary oral teratology study, groups of 6 Charles
River CD strain (Sprague-Dawley origin) rats (196-229 g) were
administered dose levels of 0, 5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 10 ml/kg bw) by gavage from day 6 through day 15 of
gestation. Control rats received the vehicle, 0.5% (W/V) CMC and
0.5% (W/V) Tween 80 in distilled water. Homogeneity and stability of
thiram in the suspensions were satisfactory (mean concentrations ±
SD of the 0.5, 1.0, 2.0, 4.0, 8.0, and 16 mg/ml thiram suspensions
were 0.5 ± 0.004, 0.97 ± 0.003, 1.96 ± 0.05, 3.94 ± 0.12, and 8.06 ±
0.25, and 16.6 ± 0.2 mg/ml, respectively). All females were killed
on day 20 of gestation for examination of uterine contents.
Table 2. Results of genotoxicity assays on thiram
Concentration
Test system Test object of thiram Purity Results Reference
In vitro
Ames test S. typhimurium 98.7% Poth (1990)
(±S9) TA 1535} +(±S9)
TA 100} 1-100 µg/plate (-S9) +(±S9)
TA 1537} 1-1000 µg/plate (+S9) -(±S9)
TA 1538} -(±S9)
TA 98 {10-1000 µg/plate (-S9) -(±S9)
{1-1000 µg/plate (+S9)
Gene mutation V79 Chinese 1-10 µg/ml (-S9) 100% -(±S9) Debets & Enninga
(±S9) hamster 10-56 µg/ml (+S9) (1986)
cells (HPRT-
locus)
Chromosome Chinese 0.003-0.023 µg/ml (-S9) 99.8% -(±S9) Putman (1987a)
aberrations hamster 0.2-1.5 µg/ml (+S9)
(±S9) ovary cells
-
DNA repair Primary rat 0.03-10 µg/ml 100% - Weterings (1985)
(test) hepatocytes
SCE induction human 5-25 µg/ml 98.6% + Perocco et al. (1989)
(with and without lymphocytes
S-9 mix)
Table 2 (cont'd)
Concentration
Test system Test object of thiram Purity Results Reference
In vivo
Mouse spot test mouse (NMRI) 75, 750 mg/kg 98.7% - Völkner (1991)
bw per os
Micronucleus test mouse (CD-1) 38-377 mg/kg 99.8% - Putman (1987b)
bw (i.p.)
Germ cell mouse (NMRI) 75-750 mg/kg 99.7% - Völkner (1990)
cytogenetic assay bw per os
Germ cell mouse (Swiss total doses n.g. (+) Prasad et al. (1987)
cytogenic assay albino) 80-320 mg/kg
(gavage)
n.g.: not given
A dose-related loss of body weight was seen at 20, 40, and 80
mg/kg bw/day. Treatment at 40 and 80 mg/kg bw/day produced a dose-
related increase in the total number of early and late resorptions
and a consequent increase in post-implantation loss and reduction in
viable litter size. It was concluded that the highest dose to be
used in a main teratology study in the rat should not exceed 40
mg/kg bw/day (Tesh et al. 1986).
Four groups of 25 female Charles River CD SD rats (193-236 g,
9-11 weeks old) were administered dose levels of 0 (vehicle), 7.5,
15 or 30 mg (99.0-99.8% purity) thiram/kg bw/day by gavage on day 6
through 15 of gestation. Pre-pairing acclimatization was for 5 days.
Test solutions (dosage volume, 10 ml/kg bw) were prepared in a 0.5%
w/v CMC and 0.5% w/v Tween 80 vehicle. All females were killed on
day 20 of gestation for examination of their uterine contents.
Analyses showed that the concentrations of test substance in the
test mixtures were 0.62-0.75 mg/ml, 1.47-1.49 mg/ml, and 2.88-2.91
mg/ml of intended concentrations of 0.75, 1.5, and 3.0 mg/ml,
respectively. The test substance was stable at room temperature
throughout the study. The concentration of tetramethylthiuram
monosulphide was less than 0.01%.
With the exception of a dosage-related incidence in the number
of females showing areas of hair loss on head, neck, back, and/or
limbs, the general condition of females was comparable in all
groups. At 15 and 30 mg/kg bw/day a transient, dose-related, loss of
body-weight was observed. At 7.5 mg/kg bw/day the rate of weight
gain was slightly, but significantly, reduced during the treatment
period, but subsequent weight gain was similar to that of the
controls. There were no adverse effects upon implantation or upon
fetal survival, but fetal and placental weights were significantly
reduced at 30 mg/kg bw/day. Placental weights were also slightly,
but significantly, reduced in the 7.5 and 15 mg/kg bw/day animals,
and foetal weight was slightly reduced at 15 mg/kg bw/day, but these
values were within historical control ranges. At 30 mg/kg bw/day
there was evidence of fetal immaturity e.g., reduced skeletal
ossification and increased incidence of space between the body wall
and organs, and there was a slightly increased incidence of
subcutaneous oedema. At 15 and 30 mg/kg bw/day the incidence of 13th
ribs of reduced size was slightly increased, but was not related to
dosage. Three foetuses with diaphragmatic hernia were observed, two
at 7.5 and one at 30 mg/kg bw/day. The NOAEL for fetal toxicity was
7.5 mg/kg bw/day. Reduced maternal body weight and placental weight
precluded the establishment of a NOAEL for maternal toxicity. The
toxic effects (immaturity and increased incidence of 13th ribs of
reduced size) at higher doses were considered a result of maternal
toxicity (Tesh et al., 1988a).
Rabbits
In a preliminary oral teratology study female New Zeeland white
rabbits (18-24 months old 3.4-4.5 kg) were artificially inseminated.
The day of insemination was designated day 0 of gestation. The
animals were allowed a minimum of three weeks acclimatization before
dosing. Thiram (purity 99.1%) was administered by gavage to 4
females per group from days 6-19, inclusive, of gestation at dosages
of 0 (vehicle), 1, 3, 5, 7.5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 5 ml/kg bw). Control animals received the vehicle
(the vehicle was 0.5% w/v aqueous CMC mucilage containing 0.5% w/v
Tween 80). Stability and concentration (89-106% of nominal) of the
test compound at room temperature was ensured by formulation of
fresh test solutions each day. On day 29 of gestation, the animals
were killed and their uterine contents examined.
Females receiving 10, 20, 40, or 80 mg/kg bw/day exhibited
marked body-weight loss during the treatment period. Eight females
(one on 20 mg/kg bw/day, three on 40 mg/kg bw/day and all four on 80
mg/kg bw/day) died or were killed in extremis. A further two deaths
(one control and one on 40 mg/kg bw/day) occurred but were not
attributed to treatment. Females receiving doses between 1 and 7.5
mg/kg bw/day showed slight reductions in their rate of body-weight
gains with some indication of a dosage relationship. Treatment at 20
mg/kg bw/day was associated with total litter resorption in 2
females and a marked increase in post-implantation loss in the one
female that carried a live litter to term. All 10 mg/kg bw/day
females carried their litters to term, but there was a slight
increase in post-implantation loss compared with the controls. At
7.5 mg/kg bw/day one female showed total litter loss, but in the
surviving two females post-implantation loss was similar to that of
the controls. It was concluded that dose-levels of thiram for use in
a main teratology study in the rabbit should not exceed 5 mg/kg
bw/day to prevent effect on survival in utero (Tesh et al.,
1987).
New Zeeland white rabbits 16-24 weeks of age and 3.7-4.8 kg
body-weight, were artificially inseminated. The day of insemination
was designated day 0 of gestation. The animals were allowed a
minimum of three weeks acclimatization before dosing. Thiram (purity
99.5%) was administered by gavage from day 6-19 of gestation
inclusive, at doses of 0, 1.0, 2.5 or 5.0 mg/kg bw/day (volume-
dosage 5.0 ml/kg bw). The vehicle was 0.5% (W/V) aqueous CMC
mucilage containing 0.5% (W/V) Tween 80. Stability and concentration
of the test compound at room temperature were satisfactory. The
concentration of tetramethylthiuram monosulphide was less than
0.03%. The number of control animals was 18+14 (initial controls +
additional controls from a parallel study due to unusually poor
pattern of body-weight gain, in part, to the performance of two
females). The number of test animals per group were 15, 18, and 20
for the 1.0, 2.5, and 5.0 mg/kg bw/day dose group, respectively. All
females were killed on Day 29 of gestation for examination. After
removal of the reproductive tract the following was recorded: number
of corpora lutea per ovary; number of implantation sites, number of
resorption sites; number and distribution of live and dead foetuses
in each uterine horn; weights and abnormalities of individual
fetuses and placentae, thorough examination including skeletal
examination of all fetuses.
The general condition of control and treated females was
essentially similar throughout the investigation. Eight females died
or were killed in extremis during the study (one in each of groups 1
and 2, two in group 3, and four in group 4). Necropsy findings
revealed evidence of respiratory or gastrointestinal tract disorder,
or accidental tracheal intubation. No evidence was apparent of any
involvement of thiram. The body-weight gain of females receiving 5.0
mg/kg bw/day was slightly but statistically significantly reduced.
Females receiving 1.0 or 2.5 mg/kg bw/day remained unaffected by
treatment with thiram. One control female and one 5.0 mg/kg bw/day
female aborted during the study. In addition, one female in each of
the groups receiving 2.5 or 5.0 mg/kg bw/day exhibited total litter
resorption. Litter parameters were unaffected by treatment with
thiram. Survival, growth and morphological development in utero
were essentially unaffected by treatment. The NOAEL for maternal
toxicity was 2.5 mg/kg bw/day and the NOAEL for embryofetal toxicity
was 5.0 mg/kg bw/day. There was no evidence of teratogenic potential
(Tesh et al., 1988b).
Five-month old New Zeeland white SPF female rabbits were
acclimatized and artificially inseminated. Groups of 20 inseminated
rabbits were dosed once daily by oral gavage with 0, 1.0, 5.0, or 10
mg/kg bw/day (dose volume 3.0 ml/kg bw) thiram solved in 0.5% Tween
80 and 0.5% low-viscosity CMC on gestation days 7 through 19.
Homogeneity mean values ranged from 107-108% of target and the
relative standard deviations were < 4.5%. Stability at room
temperature for 10 days indicated 95-99% of the initial prepared
concentrations. Caesarean sections were performed on all surviving
females on gestation day 29, followed by teratologic examination of
the fetuses. Individual fetuses were weighed, sexed, tagged and
examined for external malformations and variations. Each fetus was
dissected internally, sexed and examined for visceral malformations
and variations. The head was examined using multiple coronal slices.
Skeletal examination was carried out after Alizarin Red S staining.
A single death observed in the 10 mg/kg bw/day group was
attributed to gavage injury from dosing and two deaths in the
control group were attributed to pneumonia. No treatment-related
adverse findings were observed with regard to clinical signs or
necropsy findings of animals surviving the scheduled euthanasia.
Statistically significant increases in body-weight gain and food
consumption were observed in the treated groups when compared with
the control values. These increases were not considered to be
adverse effects of the test article. No treatment-related maternal
effects were observed at cesarean section examination, and no
adverse effects on fetal development were observed at the
teratological examination. The NOAELs for maternal toxicity,
embryofetal toxicity and teratogenicity were higher than 10 mg/kg
bw/day (York, 1992).
Special studies for neurotoxicity
Data from special neurotoxicity testing of thiram were not
available. Some endpoints related to neurotoxicity have been
investigated in the standard toxicity testing procedures reported
above.
Observations in humans
Workers, surgeons and other personnel using rubber gloves might
get contact dermatitis due to the presence of thiram in the rubber
(Lisi et al., 1987). Oral exposure to thiram might lead to dermal
sensitization to the substance (Goitre et al., 1981).
COMMENTS
Following oral administration to rats, thiram was well absorbed
(>83%) and eliminated via the expired air (41-48%), urine (25-40%),
and faeces (2-5%). About 3% was recovered in various organs. The
majority of the dose (84-90%) was eliminated within four days after
dosing.
The metabolism of thiram was studied in rats. During the first
five hours after administration, a dose-dependent formation of
carbon disulphide was demonstrated in the expired air. Metabolites
detected in urine included polar oxidation products and conjugates.
The acute oral toxicity of thiram is low in mice and rats. The
World Health Organization has classified thiram as slightly
hazardous (WHO, 1992).
A 13-week dietary study in rats at levels of 0, 50, 500 or 1000
ppm resulted in changes in haematological and serum biochemical
parameters and gastric irritation at 500 and 1000 ppm. The NOAEL was
50 ppm, equivalent to 2.5 mg/kg bw/day.
Beagle dogs received thiram as a dietary admixture at levels of
0, 75, 250 or 500 ppm for 13 weeks or at levels of 0, 30, 90 or 250
ppm for 52 weeks. The NOAELs were 75 ppm (equal to 2.2 and 2.3 mg/kg
bw/day in males and females, respectively, for the 13-week study)
and 30 ppm (equal to 0.84 mg/kg bw/day) in males and 90 ppm (equal
to 2.5 mg/kg bw/day) in females in the 52-week study on the basis of
changes in body weight, increased absolute and relative liver
weights, and changes in haematological and serum biochemical
parameters. In another study, dogs received thiram in gelatin
capsules at doses of 0, 0.4, 4 or 40 mg/kg bw/day 7 days/week for
104 weeks. Nausea, vomiting and salivation, ophthalmological
effects, convulsions, changes in haematological parameters, and
renal changes were observed at 4 and 40 mg/kg bw/day. On the basis
of the described effects, the NOAEL was 0.4 mg/kg bw/day. Since
thiram was administered in capsules in this experiment and
significantly less information was available on the study conditions
compared to those in the former two experiments with dietary
administrations of thiram this NOAEL was not used as the basis for
the estimation of an ADI.
In a 97-week oncogenicity study in mice using dietary thiram
concentrations of 0, 15, 150 or 300/600 ppm, the effects included
dose-dependent decreases of food consumption and body weight gain
and changes in haematological parameters. Non-neoplastic findings
included retinal atrophy, changes in the urinary bladder and in the
skin, hyperkeratosis in the non-glandular stomach, and increased
pigmentation in the spleen. Thiram was not carcinogenic in mice. The
NOAEL for long-term toxicity in male and female mice was 15 ppm,
equal to 3 mg/kg bw/day.
In a two-year toxicity study in rats at dietary concentrations
of 0, 3, 30 or 300 ppm, the NOAEL was 30 ppm, equal to 1.2 and 1.4
mg/kg bw/day in males and females, respectively. It was based on
lower red blood cell count, haemoglobin and haematocrit levels and
degenerative changes of the sciatic nerve accompanied by atrophy of
the gastrocnemius muscle at 300 ppm.
In a second two-year long-term toxicity/carcinogenicity study
in rats at dietary concentrations of 0, 30, 150 or 300 ppm, dose-
dependent lower erythrocyte counts, haemoglobin and haematocrit
levels were observed. Based on these haematological changes, the
NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg bw/day in males and
females, respectively.
In a 2-year carcinogenicity study in rats at dietary
concentrations of 0, 500, or 1000 ppm (equal to 39 and 42 mg/kg
bw/day in males and females, respectively) there was no evidence of
carcinogenicity. The Meeting concluded that thiram was not
carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 30, 60 or 180 ppm, no adverse effects on
reproduction were observed. The NOAEL for reproductive effects was
> 180 ppm (equal to > 8.9 and > 14 mg/kg bw/day in males and
females, respectively). The NOAEL for systemic toxicity was 30 ppm
(equal to 1.5 and 2.3 mg/kg bw/day in males and females,
respectively). This NOAEL was based upon reduction in body-weight
and/or food consumption in both parental and offspring animals.
An oral teratogenicity study was performed in rats at gavage
dose levels of 0, 7.5, 15 or 30 mg/kg bw/day. A NOAEL for maternal
toxicity was not determined due to a dose-dependent decrease in body
weight gain and placental weight at all dose levels. Teratogenicity
was not observed.
In an oral teratogenicity study in rabbits at gavage doses of
0, 1.0, 2.5 or 5.0 mg/kg bw/day, a NOAEL of 2.5 mg/kg bw/day for
maternal toxicity was based on a dose-dependent reduction of body-
weight gain. Teratogenicity was not observed.
An oral teratogenicity study was carried out in rabbits at
gavage dose levels of 0, 1.0, 5.0 or 10 mg/kg bw/day. The NOAEL for
maternal toxicity was higher than 10 mg/kg bw/day. Teratogenicity
was not observed.
Thiram was mutagenic in the Ames test but not in mammalian
cells in vitro. Since thiram was not mutagenic in vivo, the
Meeting concluded that it did not present a genotoxic hazard for
humans.
The central and peripheral nervous systems have been recognized
as a possible target for thiram toxicity. The neurotoxicity may be
related to the thiram metabolite carbon disulphide.
An ADI was allocated, based on the 1-year study in dogs and the
2-year studies in rats, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 15 ppm, equal to 3 mg/kg bw/day (97-week study)
Rat: 30 ppm, equal to 1.2 mg/kg bw/day (two-year study)
30 ppm, equal to 1.5 mg/kg bw/day (two-generation
reproduction study)
Rabbit: 2.5 mg/kg bw/day (teratology study, maternal
toxicity)
Dog: 30 ppm in the diet, equal to 0.84 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Clarification of the potential for neurotoxicity of
thiram.
2. Observations in humans.
REFERENCES
Beauchamp, R.O., Bus, J.S., Popp, J.A., Boreiko, C.J. & Golberg, L.
(1983) A critical review of the literature on the carbon disulphide
toxicology. CRD Crit. Rev. Toxicol., 11: 169-272.
Dalvi R.R. (1987) Dose-dependent liver toxicity of thiram
administered intraperitoneally to rats. J. Environ. Biol., 8: 25-
31.
Dalvi, R.R. & Deoras, D.P. (1986) Metabolism of a dithiocarbamate
fungicide thiram to carbon disulphide in the rat and its hepatotoxic
implications. Acta Pharmacol. Toxicol., 58: 38-42.
Debets, F.M.H. (1985) Evaluation of the acute inhalation toxicity of
TMTD technical in the rat. Unpublished report no.: 0113/232 from
NOTOX v.o.f. s-Hertogenbosch, the Netherlands. Submitted to WHO by
UCB.
Debets, F.M.H. & Enninga, I.C. (1986) Evaluation of the mutagenic
activity of TMTD technical in an in vitro mammalian cell gene
mutation test with V79 Chinese hamster cells. Unpublished report No.
NOTOX 0174/EV 1 from NOTOX C.V. DD 's-Hertogenbosch, Netherlands.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Gay, M.H. (1987) Rat metabolism of 14C thiramTM, single dose
study. Unpublished report No. 87003B from Biotek, Inc.,
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Goitre, M., Bedollo, P.G., & Cane, D. (1981) Allergic dermatitis and
oral challenge to tetramethylthiuram disulphide. Contact Derm., 7:
272-273.
Hasegawa, R., Takahashi, M., Furukawa, F., Toyoda, K., Sato, H.,
Junejang, J. & Hayashi, Y. (1988) Carcinogenicity study of
tetramethylthiuram disulphide (thiram) in F334 rats. Toxicology,
51: 155-165.
Hiles, R.A. (1989) Bioavailability study in male rats with a 14C-
thiram-treated diet. Unpublished report No. HLA 6111-131 from
Hazleton Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988a) Four-Week range-finding study with thiram in
dogs. Unpublished report No. HLA 6111-109 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988b) Thirteen-Week toxicity study with thiram in
rats. Unpublished report No. HLA 6111-110 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1989a) 13-Week toxicity study with thiram in dogs.
Unpublished report No. HLA 6111-121 from Hazleton Laboratories,
Inc., Wisconsin, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Kehoe, D.F. (1989b) 4-Week dietary range-finding study with thiram
in mice. Unpublished report No. HLA 6111-127 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991a) 52-Week dietary chronic toxicity study with
thiram in dogs. Unpublished report No. HLA 6111-112 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991b) 104-Week combined chronic toxicity and
carcinogenicity study with thiram in rats. Unpublished report No.
HLA 6111-113 from Hazleton Laboratories, Inc., Wisconsin, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.C. et al. (1975) Toxicological evaluation of ferric
dimethyldithiocarbamate (ferbam) and dithiocarbamate (thiram) with
acute toxicity of manganese and zinc ethylenebisdithiocarbamates
(maneb and zinc). Final report, MRI project no.: 36123-B. Submitted
to WHO by UCB.
Lee, C-C. & Peters, P.J. (1976) Neurotoxicity and behavioral effects
of thiram in rats. Environmental Health Perspectives, 17: 35-43.
Lee, C.C. et al. (1978) Oral toxicity of ferric
dimethyldithiocarbamate (ferbam) and tetramedthylthiuram disulfide
(thiram) in rodents. J. toxicol. environ. health, 4, 93-106.
Submitted to WHO by UCB.
Lisi, P., Caraffini, S. & Assalve, D. (1987) Irritation and
sensitization potential of pesticides. Contact Derm., 17: 212-218.
Maita, K., Tsuda, S. & Shirasu, Y. (1991) Chronic toxicity studies
with thiram in wistar rats and beagle dogs. Fundamental and Applied
Toxicol., 16: 667-686.
McManus, J.P. (1991) Metabolism of [14C] thiram in the rat:
Urinary metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Middlebury, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Matthiasck, L. (1973) Uber den Einfluss von L-cystein auf die
Teratogenese durch Thiram (TMTD) bei NMRI-Mausen. Arch. Toxikol. 30,
251-262. Submitted to WHO by UCB.
Musacchio, J.M., Goldstein, M., Anagnoste, B. Poch, G., & Kopin L.J
(1966) Inhibition of dopamine beta-hydroxylase by disulfiram in
vivo. J. Pharmacol. Exp. Ther., 152: 56-61.
Nomeir, A.A. & Markham, P. (1990) Disposition and metabolism of
thiram in rats after pretreatment with thiram for 14 days.
Unpublished report No. ADL 65492 from Arthur D. Little, Inc.
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Norris, K.J. (1989) Determination of volatile 14C-residues from
rats orally administered 14C-thiram. Unpublished report No. 1113A
from Analytical Development Corp., Colorado Springs, USA. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Perocco, P., Santucci, M.A., Campani, A.G. & Forti, G.C. (1989)
Toxic and DNA-damaging activities of the fungicides mancozeb and
thiram (TMTD) on human lymphocytes in vitro. Teratogenesis,
carcinogenesis, and mutagenesis., 9: 75-81.
Poth, A. (1990) Salmonella typhimurium reverse mutation assay with
thiram. Unpublished report No. CCR 175116 from CCR Cytotest Cell
Research GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Prasad, H., Pushpavathi, K., Rita, P. & Reddy, P.P. (1987) The
effect of thiram on the germ cells of male mice. Fd. Chem. Toxic.
25: 709-711.
Putman, D.L. (1987a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. Unpublished report No. T5558.337 from Microbiological
associates, Inc., Maryland, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Putman, D.L. (1987b): Micronucleus cytogenetic assay in mice.
Unpublished report No. T5558.122 from Microbiological associates,
Inc., Maryland, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R. & Higgins, C. (1986)
Thiram: Effects of oral administration upon pregnancy in the rat,
preliminary teratology study. Unpublished report No. 86/TRK001/704
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W. & Crisp, V.C. (1987) Thiram: Preliminary
teratology study in the rabbit. Unpublished report No. 87/TRK003/122
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Higgins, C., Wilby,
O.K. & Tesh, S.A. (1988a) Teratology study in the rat. Unpublished
report No. 87/TRK002/179 from Life Science Research, Suffolk,
England. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W., Crisp, V.C., Wilby, O.K. & Tesh, S.A.
(1988b) Thiram: Teratology study in the rabbit. Unpublished report
No. 87/TRK004/541 from Life Science Research, Suffolk, England.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Thouin, M.H. (1985a) Evaluation of the acute oral toxicity of TMTD
technical in the rat. Unpublished report no.: 0174/238 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Thouin, M.H. (1985b) Evaluation of the acute oral toxicity of TMTD
technical in the rabbit. Unpublished report no.: 0113/211 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Trutter, J.A. (1992) Oncogenicity study in mice with thiram.
Unpublished report No. 798-223 from Hazleton Laboratories, Inc.,
9200 Leesburg Turnpike, Vienna, Virginia 22182, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. (1985) Evaluation of the DNA repair inducing
ability of TMTD technical in a primary culture of rat hepatocytes.
Unpublished report No. NOTOX 0174/ER156 from NOTOX C.V. DD 's-
Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1990) Mouse germ-cell cytogenetic assay with thiram.
Unpublished report No. CCR 175127 from CCR Cytotest Cell Research
GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1991) Mutation assay in somatic cells of the mouse
(mouse spot test) with thiram. Unpublished report No. CCR 200902
from CCR Cytotest Cell Research GmbH & Co. KG, Rossdorf, Germany.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
York, R.G. (1991) Two-generation reproduction study in rats.
Unpublished report No. 399-104 from International Research and
Development Corporation, Michigan, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
York, R.G. (1992) Development toxicity study in New Zealand
white rabbits. Unpublished report No. 399-121 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Approximately 60% of the administered radioactivity was
recovered as expired CS2 and 30% was found in the urine. Thiram
was rapidly degraded to more polar products. Virtually no unchanged
thiram was detected in the urine. Five urinary metabolites were
detected by HPLC and were identified by mass spectrometry. The
identified metabolites were an alanine derivative of CS2 (10%); a
glucuronide conjugate of dimethyldithiocarbamate (DDC) (20%); a
thiosulfenic acid (34%); the methyl ester of DDC (6%); and an
alanine conjugate (30%). The presence of these polar conjugates
demonstrates that the metabolic pathway involved a reduction of the
disulphide bond and subsequent reactions of the thiol moiety to form
oxidative and conjugative polar products (McManus, 1991).
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of thiram
Species Sex Route LD50 LC50 References
(mg/kg bw) (mg/m3)
Mouse M oral 4000 Matthiask, 1973
Mouse F oral 2300-3800 Lee et al., 1975;
1978
Rat M oral 3700-4000 Thouin, 1985a;
Lee et al., 1975;
1978
Rat F oral 1800-1900 Thouin, 1985a;
Lee et al., 1975;
1978
Rat M&F inhalation > 100 Debets, 1985
Rabbit M&F dermal > 2000 Thouin, 1985b
Short-term toxicity studies
Mice
Ten Charles River albino mice (Crl:CD-1R(ICR)BR)/sex/group
(males 24.3-24.8 g, females 19.0-19.3 g) received in the diet 0,
300, 600, or 1200 ppm thiram (purity 97.5%) for 4 weeks equal to 0,
54, 108 or 201 mg/kg bw/day in males and 62, 118 or 241 mg/kg bw/day
in females. Stability and homogeneity of the test substance was
satisfactory. The animals were observed daily for mortality, signs
of toxicity and food consumption. Body-weights were recorded weekly.
Haematology and clinical chemistry tests were performed at sacrifice
on days 29 or 30. Gross and histopathological examinations of the
animals were carried out.
Body-weights were significantly and dose-dependently reduced in
males at all dose levels. Food consumption was significantly lower
for both sexes at all dose levels when compared with controls.
Statistically significant differences in clinical chemistry and
haematological parameters included reduced erythrocyte counts,
haemoglobin, and haematocrit for males at all dose levels; increased
platelet counts for females at 600 and 1200 ppm; reduced serum
glucose for females at 1200 ppm. Statistically significant and dose-
dependent increased organ-to-body-weight ratios of the brain,
kidneys and liver in males probably resulted from statistically
significant and dose-dependent reduced terminal body-weights. A
NOAEL could not be determined because of the 28-48% reduction in
food intake at 300 ppm (Kehoe, 1989b).
Rats
In a 13-week study, 10 Charles River rats
(Crl:CDR(SD)BR)/sex/group (males 138 ±6 g and females 125 ±4 g)
received dietary concentrations of 0, 50, 500, or 1000 ppm thiram
(purity 99.4%) equivalent to 0, 2.5, 25 or 50 mg/kg bw/day. Mean
stability assay results for diets stored frozen for 14 and 35 days
ranged from 81-99%. Diets analyzed after being in the animals room
for 7 days were 17, 80, and 88%, respectively, of the nominal
levels. Mean values for homogeneity assay results were 80, 97, and
98%, respectively, of the nominal levels of 50, 500, and 1000 ppm.
The mean±SD of determined concentration of thiram was 40 ± 1, 483 ±
8, and 976 ± 15 ppm. The animals were observed daily for mortality
and signs of toxicity. Body-weights and food consumptions were
recorded weekly and haematology and clinical chemistry were
determined at study termination. Ophthalmic observations were done
before and at termination of study. Gross and histopathological
examinations from the control and 1000 ppm group and of macroscopic
lesions, lungs, liver, and kidneys from all 50 ppm and all 500 ppm
animals was carried out at study termination.
Body weights, cumulative body-weight gains, and food
consumption were significantly reduced throughout the study for both
sexes at 500 and 1000 ppm. Changes in clinical chemistry and
haematological parameters occurred at dose levels of 500 and 1000
ppm. The changes considered to be treatment-related were reduced
erythrocyte counts, haemoglobin and haematocrit in females;
increased MCV and MCH in both sexes; increased white blood cell,
corrected white blood cell, absolute neutrophil, absolute lymphocyte
and absolute monocyte counts in females; reduced total protein and
glucose in both sexes; reduced albumin and increased urea nitrogen
and chloride in females.
At 500 and 1000 ppm animals a tendency to reduced terminal
body-weights with correspondingly reduced absolute organ weights and
increased organ to body-weight ratios were observed.
Macroscopically, the non-glandular stomach in some animals showed
areas of erosion and the mesenteric lymph nodes were diffusely red
or mottled. Microscopically, the mucosa of the nonglandular stomach
had focal areas of erosion/ulceration, mucosal hyperplasia, or both,
accompanied by some submucosal inflammation and edema. These changes
appeared to be treatment-related. The mesenteric lymph nodes were
frequently congested but otherwise normal. The NOAEL in this study
was 50 ppm, equivalent to 2.5 mg/kg bw/day (Kehoe, 1988b).
Dogs
Two acclimated beagle dogs (5 months of age and weighing 5.6 to
8.5 kg/sex/group) were fed dietary concentrations of 0, 125, 500, or
2000 ppm thiram (purity 99.4%), equivalent to 0, 3, 13 or 50 mg/kg
bw/day for 4 weeks. All animals were observed daily for toxic signs
and mortality. Body-weight and food consumption was recorded weekly.
Clinical chemistry, gross and histopathological examinations were
performed on all animals. Homogeneity assays indicated from 94-96%
(mean values) of the nominal values. There were no data on
stability, but thiram was stored under similar conditions to that in
other studies where stability was satisfactory.
One 2000 ppm one female died during week 2 and one male became
moribund and was sacrificed during week 4. Therefore, the high-dose
of 2000 ppm was reduced to 1500 ppm during weeks 3 and 4. The
observations in the female were primarily colon changes associated
with the acute death and subsequent post-mortem change. The male had
only a few incidental macroscopic and microscopic findings and,
therefore, its death was not considered compound-related. At week 4,
body-weights for both sexes at 500 ppm were approximately 15% lower
and at 2000/1500 ppm, 33 and 39% lower than controls respectively.
Food consumption during week 4 for 125, 500, and 2000/1500 ppm males
were 12%, 29%, and 92% lower than controls respectively, and for
females 13%, 49%, and 78% lower. Treatment-related changes in
clinical chemistry and haematological parameters comprised reduced
erythrocyte counts, haemoglobin and haematocrit in males given 500
or 2000/1500 ppm. One male given 2000/1500 ppm had increased ALAT,
ASAT and ALP. Slightly reduced absolute lymphocyte counts and
slightly higher platelet counts, total bilirubin and urea nitrogen
occurred in some male and female dogs given 500 or 2000 ppm. The
surviving 2000/1500 ppm dogs sacrificed at week 4 had notably
reduced terminal body-weights and correspondingly reduced absolute
organ weights. The liver of the males had hepatocellular
degeneration with sinusoidal cell proliferation and pigmentation.
There were no major macroscopic or microscopic findings in the
remaining dogs. The NOAEL for the study was determined to be 125
ppm, equivalent to 3 mg/kg bw/day, based on the lower body-weights
and changes in clinical chemistry parameters (Kehoe 1988a).
In a 13-week study on 5 month old beagle dogs, four beagle
dogs/sex/group were fed dietary concentrations of 0, 75, 250, or 500
ppm thiram (purity 97.5%), equal to 0, 2.2, 6.9 or 12 mg/kg bw/day
in males and 0, 2.3, 7.3 or 13 mg/kg bw/day in females. The mean (±
SD) body-weight at study initiation was 7.4±0.6, 7.3±1.1, 7.4±0.5,
and 7.3±0.6 kg for males receiving 0, 75, 250, and 500 ppm. and were
6.8 ± 0.9, 7.1 ± 1.4, 6.3 ± 0.5, and 6.7±0.5 kg for females. All
animals were observed daily for clinical signs, moribundity and
mortality. Body-weight and food consumption was recorded weekly.
Ophthalmic examinations were performed prior to and at the end of
the study. Clinical chemistry measurements, haematological
examinations and gross and histopathology examinations were done on
all animals. Homogeneity of diets was within ± 8% of nominal values.
Mean stability assays of diets frozen for 7 days, stored in the
animal room for 1 day and then frozen for 4 weeks, were within ± 5%
of nominal levels.
Body weights for the 500 ppm dogs were significantly reduced.
Food consumption was significantly reduced in 500 ppm males and in
250 and 500 ppm females. Treatment-related effects for clinical
chemistry and haematological parameters were reduced erythrocyte
counts, higher MCV and MCH in 75, 250, and 500 ppm dogs; reduced
haemoglobin and haematocrit in 500 ppm females; higher platelet
counts in 250 and 500 ppm males; lower total protein and albumin in
75, 250, and 500 ppm males and females; and higher cholesterol in
250 and 500 ppm males and females. The NOAEL for this study was 75
ppm equal to 2.2 and 2.3 mg/kg bw/day in males and females
respectively, based on haematological changes noted in both sexes
(Kehoe, 1989a).
Six 4 to 5 month old beagle dogs/sex/group (initial weight 4.0
to 6.8 kg) were fed dietary concentrations of 0, 30, 90, or 250 ppm
thiram (purity 97.5%), equal to 0, 0.84, 2.6, and 7.4 mg/kg bw/day
in males and 0, 0.90, 2.5, and 7.2 mg/kg bw/day in females for 52
weeks. All animals were observed daily for clinical signs and
mortality. Body weights and food consumption were recorded weekly
for weeks 1 through 16, and monthly thereafter. Ophthalmic
examinations were performed prior to and at the end of the study.
Haematology, clinical chemistry, gross and histopathology were
performed on all animals. Mean values for homogeneity assays were
83, 92, and 92% of the nominal levels of 30, 90, and 250 ppm,
respectively. Mean stability assay results for diets kept below 0 °C
for 7 days and stored in the animal room for one day were 84%, 92%
and 96% of the nominal levels of 30, 90, and 250 ppm, respectively.
No ophthalmic lesions were observed in any animals. Erythrocyte
counts were reduced in males given 250 ppm, total protein was lower
and cholesterol was higher in males given 90 or 250 ppm and in
females given 250 ppm. Albumin was reduced in both sexes given 250
ppm. Absolute liver weights in males given 90 or 250 ppm were
significantly increased. Liver-to-body-weight ratios in males given
30, 90, or 250 ppm and in females given 250 ppm were also
significantly increased, as were liver-to-brain weight ratios in
males given 250 ppm. The significant increase in liver-to-body-
weight ratios in males given 30 ppm was due to a slight increase in
absolute liver weight and a slight decrease in body weight.
Furthermore, the observation that the body weights of males given 90
ppm were increased in relation to those of the controls and those of
males given 30 ppm demonstrates, that the slight decrease in body
weights seen in the 30 ppm group was spurious. Thus, the increase in
liver-to-body-weight ratio seen in males given 30 ppm was not
considered a test material-related effect. The changes in liver
weights, in conjunction with the altered total protein, albumin, and
cholesterol levels suggest a test material-related effect on liver
function and size. These changes were probably adaptive responses to
the presence of this compound. Based on increased absolute liver
weights and altered clinical chemistry parameters in males given 90
or 250 ppm, and altered clinical chemistry parameters in females at
250 ppm, the NOAEL for male dogs was 30 ppm, equal to 0.84 mg/kg
bw/day, and the NOAEL for female dogs was 90 ppm equal to 2.5 mg/kg
bw/day (Kehoe 1991a).
Four groups of 4 beagle dogs/sex/dose were treated orally via
gelatin capsule with thiram (purity 98.7%) at doses of 0 (blank
capsule), 0.4, 4, or 40 mg/kg bw/day for 104 weeks. Body weights and
food intake were measured weekly. Water consumption, haematology,
ophthalmoscopy, clinical chemistry and gross and histopathology of
all organs were performed.
Both sexes at 40 mg/kg bw/day showed severe toxic signs
including nausea, vomiting, salivation, and clonic convulsion and
severe anaemia within the first 11 weeks of treatment and were all
subjected to unscheduled necropsy before the 29th week of treatment.
The dogs also showed ophthalmological changes such as fundal
haemorrhage, miosis, and desquamation of the retina which were
consistent with retinal lesions observed histopathologically. In the
4 mg/kg bw/day group, nausea, vomiting and salivation were common
findings in both sexes and one female showed clonic convulsion from
week 37. Parameters including haematocrit, haemoglobin, and
erythrocyte count were depressed from week 4 in both sexes treated
with 4 or 40 mg/kg bw/day, indicating anaemia. Changes in
biochemical parameters indicating liver failure were observed from
week 4 in the 40 mg/kg bw/day males and later in both sexes dosed
with 4 or 40 mg/kg bw/day. Kidney damage was detected in the
histopathological examination in two female dogs in each of the 4
and 40 mg/kg bw/day groups. Histological lesions in the central or
peripheral nervous system relating to the observed neurological
disturbances were not found. It was concluded that no sex difference
in response to treatment was demonstrated. Based on the neurological
disturbances, anaemia and the effects on the liver, the NOAEL was
0.4 mg/kg bw/day (Maita et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Four groups of 50 acclimated (24 days) Charles River Crl:CD-
1R(ICR)BR mice/sex/dose level (approximately 7 weeks of age) were
fed thiram (97.5% purity) at dietary concentrations of 0, 15, 150,
or 300 ppm (males), equal to 3, 24 or 50 mg/kg bw/day and 0, 15,
300, or 600 ppm (females), equal to 3, 57 or 112 mg/kg bw/day for 97
weeks, when the most sensitive group showed 50% mortality.
Homogeneity assays indicated from 75-84%, 95-100%, 94-103%, and 101-
103% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively. Mean stability assay results for diets frozen for 6
days and stored in the animal room for one day were 55, 90, 90, and
97% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively, demonstrating stability of varying acceptability.
Survival, clinical signs, body weights, food consumption, feed
efficiency, haematological parameters, necropsy, absolute and
relative organ weight and histopathological examination were
studied.
No substance-related oncogenic effects or adverse effects on
survival were observed in any test group. No changes attributable to
treatment were seen in any of the parameters evaluated in the 15 ppm
groups. There was no clinical indication of neurotoxicity at any
dose level. Mean body weight, weight gain, and total food
consumption were statistically significantly decreased in a dose-
dependent manner in the mid- and high-level groups beginning at
weeks 4-5. At study termination, mean weights were 7%, 15%, 14%, and
19% below control and mean total food consumption was 6%, 10%, 12%,
and 17% below control for the 150 ppm males, 300 ppm males, 300 ppm
females, and 600 ppm females, respectively. Increased frequency of
sores or reddened areas on the skin, generally on the ears,
consistent with bacterial dermatitis was noted for the 300 ppm males
and 600 ppm females. Mild but significant decreases in mean
erythrocyte count, haemoglobin, and haematocrit values were seen for
the 600 ppm females at week 97. Histopathology showed no evidence of
thiram-induced neoplasia. Non-neoplastic findings in the mid- and
high-level male and female mice consisted of retinal atrophy,
intracytoplasmic protein-like droplets in the urinary bladder
superficial transitional epithelium, and necrosis and suppurative
inflammation in the skin of the 150 and 300 ppm males and 300 and
600 ppm females; hyperkeratosis in the nonglandular stomach of the
300 ppm males and 300 and 600 ppm females; and increased pigment in
the spleen and decreased pigment in the inner adrenal cortex of the
300 and 600 ppm females.
No oncogenic effect was observed in doses up to 300 ppm, equal
to 50 mg/kg bw/day in males and 600 ppm, equal to 112 mg/kg bw/day
in females. Based on the body-weight reduction, the NOAEL for long-
term toxicity was 15 ppm, equal to 3 mg/kg bw/day (Trutter, 1992).
Rats
Four groups of 64 Jcl:Wistar rats were fed dietary
concentrations of 0, 3, 30, or 300 ppm thiram (purity 98.7%), equal
to 0, 0.1, 1.2 or 12 mg/kg bw/day for males and 0, 0.1, 1.4 or 14
mg/kg bw/day for females, for 104 weeks. Body weights, food intake,
and water intake were measured weekly up to week 52 and monthly
thereafter. Haematology, clinical chemistry, ophthalmoscopy and
gross and histopathology were performed on all rats. Test diets were
prepared twice weekly during the study. Data on homogeneity were not
available, but diets were stable up to 1 week.
Mortality of females in the 30 and 300 ppm groups was slightly
increased compared to controls during the last 8 weeks of treatment,
apparently correlated to an incidental higher occurrence of
pituitary tumours during this period. Overall incidence of pituitary
tumours in females was comparable to that in the control group.
Decreased body-weight gain and reduced food intake were observed in
both sexes at 300 ppm. Anaemia and regressive changes in the sciatic
nerve accompanied by atrophy of the calf muscle ( M. triceps surae)
were seen in the 300 ppm females. During the last 8 weeks of the
treatment period, the incidence of pituitary adenomas was increased
in females of the 30 and 300 ppm groups compared to that in
controls. Because of the small number of animals, statistical
analysis was not carried out. Statistical analysis of pituitary
tumour incidence in all animals at the end of the experiment showed
no significant differences. No evidence of carcinogenic potential
was observed. Based on mortality, anaemia, nerve degeneration and
muscle atrophy a NOAEL of 30 ppm, equal to 1.2 and 1.4 mg/kg bw/day
was determined in males and females, respectively (Miata et al.,
1991).
Four groups of sixty 36-day old Charles River Crl:CDR(SD)BR
rats/sex/group (initial weights 125-161 g for males and 115-193 g
for females) were fed dietary concentrations of 0, 30, 150, or 300
ppm thiram (purity 97.5%), equal to 0, 1.5, 7.3 or 15 mg/kg bw/day
in males and 0, 1.8, 8.9 or 19 mg/kg bw/day in females, for 104
weeks. Mean values for stability assays of diets kept below 0 °C for
6 days, animal room for one day, then below 0°C for 30 days were
15.5 ppm (52%), 123 ppm (82%), and 260 ppm (87%) for nominal
contents of 30, 150, and 300 ppm, respectively. The values for
homogeneity assay results ranged from 21.8-24.2 (73-81%), 128-138
(85-92%), and 255-276 ppm (85-92%) for nominal contents of 30, 150,
and 300 ppm, respectively. Cumulative means for the routine diet
analysis for dose confirmation were 16.3 (54%), 119(79%), and 262
ppm (87%) and the assay results ranged from 4.41-27.5 ppm, 81.8-151
ppm, and 179-320 ppm of the nominal dose levels of 30, 150, and 300
ppm, respectively, through 104 weeks. Data from a parallel study
with 14C-thiram stored under similar conditions (Hiles, 1989)
showed, that animals on a diet containing 30 ppm could be expected
to receive a systemic exposure to thiram and/or thiram-derived
materials approximately equal to the amount added to the diet, even
though the expected level could not be verified analytically.
Therefore, these data were considered to demonstrate that the
anticipated levels of thiram were achieved in the test diets. The
animals were observed daily for clinical signs and mortality. Body-
weight and food consumption were recorded weekly and haematology,
clinical chemistry, and urine analysis parameters were evaluated
during the study and at study termination. Ophthalmic observations
were done before and at termination of the study and gross and
histopathology examinations were carried out at study termination.
Clinical signs possibly test material-related for males
included swollen nose, soft faeces and opaque eyes. Soft faeces were
possibly test material-related at 150 and 300 ppm for the females.
Body-weight and cumulative body-weight gains in both sexes were
statistically significantly lower than those of the controls at 150
and 300 ppm. Food consumption was statistically significantly lower
than that in the controls for both sexes given 30, 150, or 300 ppm.
Reduced erythrocyte counts, haemoglobin, haematocrit and higher mean
corpuscular volume and mean corpuscular haemoglobin were observed in
females given 150 or 300 ppm. Statistically significant positive
trend analyses for hepatocellular adenomas (both sexes) and thyroid
C-cell adenomas (terminal sacrifice males and terminal plus
unscheduled sacrificed females) were reported but individual group
comparisons with controls were statistically insignificant. There
was no increase in the incidence of thyroid C-cell carcinomas.
Extramedullary haematopoiesis in the liver appeared to be increased
in males at 150 or 300 ppm and in females at 300 ppm. The biological
significance of this finding is unclear. Steatosis/fatty
infiltration of the pancreas (not uncommon in old SD rats) appeared
to be increased for males and females given 150 or 300 ppm. Also in
the pancreas, multifocal acinar atrophy was more common for males
given 150 or 300 ppm. The incidence of bile duct hyperplasia showed
a statistically significant positive trend in females, but was
statistically significantly increased only at 300 ppm. There were no
histopathological findings recorded that suggested test material-
related neurotoxicity.
Based on statistically significant decreases in body weights
and cumulative body-weight gains for males and females given 150 or
300 ppm, effects on haematological parameters in females given 150
or 300 ppm, and histopathological changes in males and females given
150 or 300 ppm, the NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg
bw/day in males and females, respectively. Thiram was not found to
be carcinogenic at doses up to 15 and 19 mg/kg bw/day in males and
females, respectively (Kehoe, 1991b).
Three groups of 50 five-week old Charles River SPF F344 rats,
initial mean body-weight of about 200 g for males and about 150 g
for females, were fed dietary concentrations of 0, 500 or 1000 ppm,
equal to 0, 18 or 39 mg/kg bw/day in males and 0, 20 or 42 mg/kg
bw/day in females for 104 weeks. Body weights and food consumption
were measured at regular intervals and haematology and clinical
chemistry were evaluated during the study and at study termination.
Gross and histopathology were performed. Homogeneity data and
stability data were not given.
Reduced body-weight gain and food consumption were seen at 500
and 1000 ppm in both sexes but especially in females of the high-
dose group. In males reduced liver function was observed.
Mononuclear cell leukaemia was found with an incidence of 20%, 8%,
and 4% in males and 29%, 12%, and 4% in females for 0, 500, and 1000
ppm animals, respectively. No tumour induction related to the
treatment was observed. It was concluded that thiram had no
carcinogenic effect at doses up to 39 and 42 mg/kg bw/day in males
and females, respectively (Hasegawa et al., 1988).
Reproduction studies
In a two-generation reproduction study, thiram (purity 97.6%)
was administered to Charles River Crl:CD VAF/Plus rats in dietary
concentrations of 0, 30, 60 or 180 ppm equal to 0, 1.5, 2.9 or 8.9
mg/kg bw/day in the F0 males and 0, 2.3, 4.6 or 14 mg/kg bw/day in
F0 females, and 0, 1.8, 3.8 or 11 mg/kg bw/day in the F1 males
and 0, 2.4, 5.1 or 16 mg/kg bw/day in the F1 females. Homogeneity
of thiram was satisfactory in the mixed diets. The test compound was
stable kept frozen for 7 days and reasonably stable (losses of 18%,
16%, and 9% in the 30, 60, and 180 ppm diet, respectively) kept at
room temperature for 12 hours. The mean ± SD percent of target ppm
found in all analyzed and administered 30, 60, and 180 ppm diets was
93 ± 6.3, 94 ± 6.6, and 98 ± 5.2%, respectively.The F0 and F1
parental generation consisted of 26 males and 26 females per group.
F0 animals were treated beginning at 63 days of age for 81 days
prior to the initial mating (F1a). A second litter (F1b) was
produced after a rest period of 16 days following weaning of the
F1a litter. Since conception rates were poor across all groups,
including the controls, in the F1b litter, an F1c litter was
also produced. The F1 parents were selected from the F1c
litters. These animals were treated beginning at 22 days of age for
a minimum of 84 days prior to their initial (F2a) mating. The F1
parents were mated twice to produce theF2a and F2b litters. All
parental animals, and pups were observed daily for mortality and
overt toxicity. Detailed clinical observations were recorded at
least once a week. Reproductive and litter parameters assessed
included male and female fertility indices, events at parturition,
gestation length, litter size, numbers of viable and stillborn pups,
and offspring survival and growth during lactation. All parental
animals, and all F1c weanlings not selected to remain on study and
all F2b weanlings were subjected to a gross necropsy. The F1a,
F1b, and the F2a offspring were euthanized and discarded
following weaning.
Mean maternal body weights and mean food consumption of the
F0 females were reduced at 60 and 180 ppm during the F1a
gestation period. Reductions in mean maternal body-weight and/or
food consumption were also observed at 180 ppm during the F1b and
F1c gestation periods and the F1a, F1b, and F1c lactation
periods. Similar reductions were observed for the F1 females at
180 ppm during the F2a and F2b gestation and lactation periods.
Reductions in mean weekly food consumption of the F0 males and
females were observed at 60 and 180 ppm. Mean offspring body weights
were consistently reduced to a significant degree in all litters
across both generations at the 180 ppm level. Apart from a
consistent statistically significant reduction in body weights in
the top dosed offspring during the F1b and F1c gestation periods
and the F1a, F1b, and F1c lactation periods, no substance-
related changes were found in the reproductive parameters or in the
parameters for post-natal development.
Based on the findings of parenteral systemic toxicity, the
NOAEL was 30 ppm, equal to 1.5 and 2.3 mg/kg bw/day in males and
females, respectively. With respect to filial systemic toxicity, the
NOAEL was 60 ppm, equal to 3.8 and 5.1 mg/kg bw/day in males and
females, respectively. With respect to reproduction and post-natal
development, the NOAEL was greater than 180 ppm equal to 8.9 and 14
mg/kg bw/day in males and females, respectively (York, 1991).
Special studies on genotoxicity
Data are shown in Table 2.
Special studies on teratogenicity
In a preliminary oral teratology study, groups of 6 Charles
River CD strain (Sprague-Dawley origin) rats (196-229 g) were
administered dose levels of 0, 5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 10 ml/kg bw) by gavage from day 6 through day 15 of
gestation. Control rats received the vehicle, 0.5% (W/V) CMC and
0.5% (W/V) Tween 80 in distilled water. Homogeneity and stability of
thiram in the suspensions were satisfactory (mean concentrations ±
SD of the 0.5, 1.0, 2.0, 4.0, 8.0, and 16 mg/ml thiram suspensions
were 0.5 ± 0.004, 0.97 ± 0.003, 1.96 ± 0.05, 3.94 ± 0.12, and 8.06 ±
0.25, and 16.6 ± 0.2 mg/ml, respectively). All females were killed
on day 20 of gestation for examination of uterine contents.
Table 2. Results of genotoxicity assays on thiram
Concentration
Test system Test object of thiram Purity Results Reference
In vitro
Ames test S. typhimurium 98.7% Poth (1990)
(±S9) TA 1535} +(±S9)
TA 100} 1-100 µg/plate (-S9) +(±S9)
TA 1537} 1-1000 µg/plate (+S9) -(±S9)
TA 1538} -(±S9)
TA 98 {10-1000 µg/plate (-S9) -(±S9)
{1-1000 µg/plate (+S9)
Gene mutation V79 Chinese 1-10 µg/ml (-S9) 100% -(±S9) Debets & Enninga
(±S9) hamster 10-56 µg/ml (+S9) (1986)
cells (HPRT-
locus)
Chromosome Chinese 0.003-0.023 µg/ml (-S9) 99.8% -(±S9) Putman (1987a)
aberrations hamster 0.2-1.5 µg/ml (+S9)
(±S9) ovary cells
-
DNA repair Primary rat 0.03-10 µg/ml 100% - Weterings (1985)
(test) hepatocytes
SCE induction human 5-25 µg/ml 98.6% + Perocco et al. (1989)
(with and without lymphocytes
S-9 mix)
Table 2 (cont'd)
Concentration
Test system Test object of thiram Purity Results Reference
In vivo
Mouse spot test mouse (NMRI) 75, 750 mg/kg 98.7% - Völkner (1991)
bw per os
Micronucleus test mouse (CD-1) 38-377 mg/kg 99.8% - Putman (1987b)
bw (i.p.)
Germ cell mouse (NMRI) 75-750 mg/kg 99.7% - Völkner (1990)
cytogenetic assay bw per os
Germ cell mouse (Swiss total doses n.g. (+) Prasad et al. (1987)
cytogenic assay albino) 80-320 mg/kg
(gavage)
n.g.: not given
A dose-related loss of body weight was seen at 20, 40, and 80
mg/kg bw/day. Treatment at 40 and 80 mg/kg bw/day produced a dose-
related increase in the total number of early and late resorptions
and a consequent increase in post-implantation loss and reduction in
viable litter size. It was concluded that the highest dose to be
used in a main teratology study in the rat should not exceed 40
mg/kg bw/day (Tesh et al. 1986).
Four groups of 25 female Charles River CD SD rats (193-236 g,
9-11 weeks old) were administered dose levels of 0 (vehicle), 7.5,
15 or 30 mg (99.0-99.8% purity) thiram/kg bw/day by gavage on day 6
through 15 of gestation. Pre-pairing acclimatization was for 5 days.
Test solutions (dosage volume, 10 ml/kg bw) were prepared in a 0.5%
w/v CMC and 0.5% w/v Tween 80 vehicle. All females were killed on
day 20 of gestation for examination of their uterine contents.
Analyses showed that the concentrations of test substance in the
test mixtures were 0.62-0.75 mg/ml, 1.47-1.49 mg/ml, and 2.88-2.91
mg/ml of intended concentrations of 0.75, 1.5, and 3.0 mg/ml,
respectively. The test substance was stable at room temperature
throughout the study. The concentration of tetramethylthiuram
monosulphide was less than 0.01%.
With the exception of a dosage-related incidence in the number
of females showing areas of hair loss on head, neck, back, and/or
limbs, the general condition of females was comparable in all
groups. At 15 and 30 mg/kg bw/day a transient, dose-related, loss of
body-weight was observed. At 7.5 mg/kg bw/day the rate of weight
gain was slightly, but significantly, reduced during the treatment
period, but subsequent weight gain was similar to that of the
controls. There were no adverse effects upon implantation or upon
fetal survival, but fetal and placental weights were significantly
reduced at 30 mg/kg bw/day. Placental weights were also slightly,
but significantly, reduced in the 7.5 and 15 mg/kg bw/day animals,
and foetal weight was slightly reduced at 15 mg/kg bw/day, but these
values were within historical control ranges. At 30 mg/kg bw/day
there was evidence of fetal immaturity e.g., reduced skeletal
ossification and increased incidence of space between the body wall
and organs, and there was a slightly increased incidence of
subcutaneous oedema. At 15 and 30 mg/kg bw/day the incidence of 13th
ribs of reduced size was slightly increased, but was not related to
dosage. Three foetuses with diaphragmatic hernia were observed, two
at 7.5 and one at 30 mg/kg bw/day. The NOAEL for fetal toxicity was
7.5 mg/kg bw/day. Reduced maternal body weight and placental weight
precluded the establishment of a NOAEL for maternal toxicity. The
toxic effects (immaturity and increased incidence of 13th ribs of
reduced size) at higher doses were considered a result of maternal
toxicity (Tesh et al., 1988a).
Rabbits
In a preliminary oral teratology study female New Zeeland white
rabbits (18-24 months old 3.4-4.5 kg) were artificially inseminated.
The day of insemination was designated day 0 of gestation. The
animals were allowed a minimum of three weeks acclimatization before
dosing. Thiram (purity 99.1%) was administered by gavage to 4
females per group from days 6-19, inclusive, of gestation at dosages
of 0 (vehicle), 1, 3, 5, 7.5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 5 ml/kg bw). Control animals received the vehicle
(the vehicle was 0.5% w/v aqueous CMC mucilage containing 0.5% w/v
Tween 80). Stability and concentration (89-106% of nominal) of the
test compound at room temperature was ensured by formulation of
fresh test solutions each day. On day 29 of gestation, the animals
were killed and their uterine contents examined.
Females receiving 10, 20, 40, or 80 mg/kg bw/day exhibited
marked body-weight loss during the treatment period. Eight females
(one on 20 mg/kg bw/day, three on 40 mg/kg bw/day and all four on 80
mg/kg bw/day) died or were killed in extremis. A further two deaths
(one control and one on 40 mg/kg bw/day) occurred but were not
attributed to treatment. Females receiving doses between 1 and 7.5
mg/kg bw/day showed slight reductions in their rate of body-weight
gains with some indication of a dosage relationship. Treatment at 20
mg/kg bw/day was associated with total litter resorption in 2
females and a marked increase in post-implantation loss in the one
female that carried a live litter to term. All 10 mg/kg bw/day
females carried their litters to term, but there was a slight
increase in post-implantation loss compared with the controls. At
7.5 mg/kg bw/day one female showed total litter loss, but in the
surviving two females post-implantation loss was similar to that of
the controls. It was concluded that dose-levels of thiram for use in
a main teratology study in the rabbit should not exceed 5 mg/kg
bw/day to prevent effect on survival in utero (Tesh et al.,
1987).
New Zeeland white rabbits 16-24 weeks of age and 3.7-4.8 kg
body-weight, were artificially inseminated. The day of insemination
was designated day 0 of gestation. The animals were allowed a
minimum of three weeks acclimatization before dosing. Thiram (purity
99.5%) was administered by gavage from day 6-19 of gestation
inclusive, at doses of 0, 1.0, 2.5 or 5.0 mg/kg bw/day (volume-
dosage 5.0 ml/kg bw). The vehicle was 0.5% (W/V) aqueous CMC
mucilage containing 0.5% (W/V) Tween 80. Stability and concentration
of the test compound at room temperature were satisfactory. The
concentration of tetramethylthiuram monosulphide was less than
0.03%. The number of control animals was 18+14 (initial controls +
additional controls from a parallel study due to unusually poor
pattern of body-weight gain, in part, to the performance of two
females). The number of test animals per group were 15, 18, and 20
for the 1.0, 2.5, and 5.0 mg/kg bw/day dose group, respectively. All
females were killed on Day 29 of gestation for examination. After
removal of the reproductive tract the following was recorded: number
of corpora lutea per ovary; number of implantation sites, number of
resorption sites; number and distribution of live and dead foetuses
in each uterine horn; weights and abnormalities of individual
fetuses and placentae, thorough examination including skeletal
examination of all fetuses.
The general condition of control and treated females was
essentially similar throughout the investigation. Eight females died
or were killed in extremis during the study (one in each of groups 1
and 2, two in group 3, and four in group 4). Necropsy findings
revealed evidence of respiratory or gastrointestinal tract disorder,
or accidental tracheal intubation. No evidence was apparent of any
involvement of thiram. The body-weight gain of females receiving 5.0
mg/kg bw/day was slightly but statistically significantly reduced.
Females receiving 1.0 or 2.5 mg/kg bw/day remained unaffected by
treatment with thiram. One control female and one 5.0 mg/kg bw/day
female aborted during the study. In addition, one female in each of
the groups receiving 2.5 or 5.0 mg/kg bw/day exhibited total litter
resorption. Litter parameters were unaffected by treatment with
thiram. Survival, growth and morphological development in utero
were essentially unaffected by treatment. The NOAEL for maternal
toxicity was 2.5 mg/kg bw/day and the NOAEL for embryofetal toxicity
was 5.0 mg/kg bw/day. There was no evidence of teratogenic potential
(Tesh et al., 1988b).
Five-month old New Zeeland white SPF female rabbits were
acclimatized and artificially inseminated. Groups of 20 inseminated
rabbits were dosed once daily by oral gavage with 0, 1.0, 5.0, or 10
mg/kg bw/day (dose volume 3.0 ml/kg bw) thiram solved in 0.5% Tween
80 and 0.5% low-viscosity CMC on gestation days 7 through 19.
Homogeneity mean values ranged from 107-108% of target and the
relative standard deviations were < 4.5%. Stability at room
temperature for 10 days indicated 95-99% of the initial prepared
concentrations. Caesarean sections were performed on all surviving
females on gestation day 29, followed by teratologic examination of
the fetuses. Individual fetuses were weighed, sexed, tagged and
examined for external malformations and variations. Each fetus was
dissected internally, sexed and examined for visceral malformations
and variations. The head was examined using multiple coronal slices.
Skeletal examination was carried out after Alizarin Red S staining.
A single death observed in the 10 mg/kg bw/day group was
attributed to gavage injury from dosing and two deaths in the
control group were attributed to pneumonia. No treatment-related
adverse findings were observed with regard to clinical signs or
necropsy findings of animals surviving the scheduled euthanasia.
Statistically significant increases in body-weight gain and food
consumption were observed in the treated groups when compared with
the control values. These increases were not considered to be
adverse effects of the test article. No treatment-related maternal
effects were observed at cesarean section examination, and no
adverse effects on fetal development were observed at the
teratological examination. The NOAELs for maternal toxicity,
embryofetal toxicity and teratogenicity were higher than 10 mg/kg
bw/day (York, 1992).
Special studies for neurotoxicity
Data from special neurotoxicity testing of thiram were not
available. Some endpoints related to neurotoxicity have been
investigated in the standard toxicity testing procedures reported
above.
Observations in humans
Workers, surgeons and other personnel using rubber gloves might
get contact dermatitis due to the presence of thiram in the rubber
(Lisi et al., 1987). Oral exposure to thiram might lead to dermal
sensitization to the substance (Goitre et al., 1981).
COMMENTS
Following oral administration to rats, thiram was well absorbed
(>83%) and eliminated via the expired air (41-48%), urine (25-40%),
and faeces (2-5%). About 3% was recovered in various organs. The
majority of the dose (84-90%) was eliminated within four days after
dosing.
The metabolism of thiram was studied in rats. During the first
five hours after administration, a dose-dependent formation of
carbon disulphide was demonstrated in the expired air. Metabolites
detected in urine included polar oxidation products and conjugates.
The acute oral toxicity of thiram is low in mice and rats. The
World Health Organization has classified thiram as slightly
hazardous (WHO, 1992).
A 13-week dietary study in rats at levels of 0, 50, 500 or 1000
ppm resulted in changes in haematological and serum biochemical
parameters and gastric irritation at 500 and 1000 ppm. The NOAEL was
50 ppm, equivalent to 2.5 mg/kg bw/day.
Beagle dogs received thiram as a dietary admixture at levels of
0, 75, 250 or 500 ppm for 13 weeks or at levels of 0, 30, 90 or 250
ppm for 52 weeks. The NOAELs were 75 ppm (equal to 2.2 and 2.3 mg/kg
bw/day in males and females, respectively, for the 13-week study)
and 30 ppm (equal to 0.84 mg/kg bw/day) in males and 90 ppm (equal
to 2.5 mg/kg bw/day) in females in the 52-week study on the basis of
changes in body weight, increased absolute and relative liver
weights, and changes in haematological and serum biochemical
parameters. In another study, dogs received thiram in gelatin
capsules at doses of 0, 0.4, 4 or 40 mg/kg bw/day 7 days/week for
104 weeks. Nausea, vomiting and salivation, ophthalmological
effects, convulsions, changes in haematological parameters, and
renal changes were observed at 4 and 40 mg/kg bw/day. On the basis
of the described effects, the NOAEL was 0.4 mg/kg bw/day. Since
thiram was administered in capsules in this experiment and
significantly less information was available on the study conditions
compared to those in the former two experiments with dietary
administrations of thiram this NOAEL was not used as the basis for
the estimation of an ADI.
In a 97-week oncogenicity study in mice using dietary thiram
concentrations of 0, 15, 150 or 300/600 ppm, the effects included
dose-dependent decreases of food consumption and body weight gain
and changes in haematological parameters. Non-neoplastic findings
included retinal atrophy, changes in the urinary bladder and in the
skin, hyperkeratosis in the non-glandular stomach, and increased
pigmentation in the spleen. Thiram was not carcinogenic in mice. The
NOAEL for long-term toxicity in male and female mice was 15 ppm,
equal to 3 mg/kg bw/day.
In a two-year toxicity study in rats at dietary concentrations
of 0, 3, 30 or 300 ppm, the NOAEL was 30 ppm, equal to 1.2 and 1.4
mg/kg bw/day in males and females, respectively. It was based on
lower red blood cell count, haemoglobin and haematocrit levels and
degenerative changes of the sciatic nerve accompanied by atrophy of
the gastrocnemius muscle at 300 ppm.
In a second two-year long-term toxicity/carcinogenicity study
in rats at dietary concentrations of 0, 30, 150 or 300 ppm, dose-
dependent lower erythrocyte counts, haemoglobin and haematocrit
levels were observed. Based on these haematological changes, the
NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg bw/day in males and
females, respectively.
In a 2-year carcinogenicity study in rats at dietary
concentrations of 0, 500, or 1000 ppm (equal to 39 and 42 mg/kg
bw/day in males and females, respectively) there was no evidence of
carcinogenicity. The Meeting concluded that thiram was not
carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 30, 60 or 180 ppm, no adverse effects on
reproduction were observed. The NOAEL for reproductive effects was
> 180 ppm (equal to > 8.9 and > 14 mg/kg bw/day in males and
females, respectively). The NOAEL for systemic toxicity was 30 ppm
(equal to 1.5 and 2.3 mg/kg bw/day in males and females,
respectively). This NOAEL was based upon reduction in body-weight
and/or food consumption in both parental and offspring animals.
An oral teratogenicity study was performed in rats at gavage
dose levels of 0, 7.5, 15 or 30 mg/kg bw/day. A NOAEL for maternal
toxicity was not determined due to a dose-dependent decrease in body
weight gain and placental weight at all dose levels. Teratogenicity
was not observed.
In an oral teratogenicity study in rabbits at gavage doses of
0, 1.0, 2.5 or 5.0 mg/kg bw/day, a NOAEL of 2.5 mg/kg bw/day for
maternal toxicity was based on a dose-dependent reduction of body-
weight gain. Teratogenicity was not observed.
An oral teratogenicity study was carried out in rabbits at
gavage dose levels of 0, 1.0, 5.0 or 10 mg/kg bw/day. The NOAEL for
maternal toxicity was higher than 10 mg/kg bw/day. Teratogenicity
was not observed.
Thiram was mutagenic in the Ames test but not in mammalian
cells in vitro. Since thiram was not mutagenic in vivo, the
Meeting concluded that it did not present a genotoxic hazard for
humans.
The central and peripheral nervous systems have been recognized
as a possible target for thiram toxicity. The neurotoxicity may be
related to the thiram metabolite carbon disulphide.
An ADI was allocated, based on the 1-year study in dogs and the
2-year studies in rats, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 15 ppm, equal to 3 mg/kg bw/day (97-week study)
Rat: 30 ppm, equal to 1.2 mg/kg bw/day (two-year study)
30 ppm, equal to 1.5 mg/kg bw/day (two-generation
reproduction study)
Rabbit: 2.5 mg/kg bw/day (teratology study, maternal
toxicity)
Dog: 30 ppm in the diet, equal to 0.84 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Clarification of the potential for neurotoxicity of
thiram.
2. Observations in humans.
REFERENCES
Beauchamp, R.O., Bus, J.S., Popp, J.A., Boreiko, C.J. & Golberg, L.
(1983) A critical review of the literature on the carbon disulphide
toxicology. CRD Crit. Rev. Toxicol., 11: 169-272.
Dalvi R.R. (1987) Dose-dependent liver toxicity of thiram
administered intraperitoneally to rats. J. Environ. Biol., 8: 25-
31.
Dalvi, R.R. & Deoras, D.P. (1986) Metabolism of a dithiocarbamate
fungicide thiram to carbon disulphide in the rat and its hepatotoxic
implications. Acta Pharmacol. Toxicol., 58: 38-42.
Debets, F.M.H. (1985) Evaluation of the acute inhalation toxicity of
TMTD technical in the rat. Unpublished report no.: 0113/232 from
NOTOX v.o.f. s-Hertogenbosch, the Netherlands. Submitted to WHO by
UCB.
Debets, F.M.H. & Enninga, I.C. (1986) Evaluation of the mutagenic
activity of TMTD technical in an in vitro mammalian cell gene
mutation test with V79 Chinese hamster cells. Unpublished report No.
NOTOX 0174/EV 1 from NOTOX C.V. DD 's-Hertogenbosch, Netherlands.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Gay, M.H. (1987) Rat metabolism of 14C thiramTM, single dose
study. Unpublished report No. 87003B from Biotek, Inc.,
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Goitre, M., Bedollo, P.G., & Cane, D. (1981) Allergic dermatitis and
oral challenge to tetramethylthiuram disulphide. Contact Derm., 7:
272-273.
Hasegawa, R., Takahashi, M., Furukawa, F., Toyoda, K., Sato, H.,
Junejang, J. & Hayashi, Y. (1988) Carcinogenicity study of
tetramethylthiuram disulphide (thiram) in F334 rats. Toxicology,
51: 155-165.
Hiles, R.A. (1989) Bioavailability study in male rats with a 14C-
thiram-treated diet. Unpublished report No. HLA 6111-131 from
Hazleton Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988a) Four-Week range-finding study with thiram in
dogs. Unpublished report No. HLA 6111-109 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988b) Thirteen-Week toxicity study with thiram in
rats. Unpublished report No. HLA 6111-110 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1989a) 13-Week toxicity study with thiram in dogs.
Unpublished report No. HLA 6111-121 from Hazleton Laboratories,
Inc., Wisconsin, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Kehoe, D.F. (1989b) 4-Week dietary range-finding study with thiram
in mice. Unpublished report No. HLA 6111-127 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991a) 52-Week dietary chronic toxicity study with
thiram in dogs. Unpublished report No. HLA 6111-112 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991b) 104-Week combined chronic toxicity and
carcinogenicity study with thiram in rats. Unpublished report No.
HLA 6111-113 from Hazleton Laboratories, Inc., Wisconsin, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.C. et al. (1975) Toxicological evaluation of ferric
dimethyldithiocarbamate (ferbam) and dithiocarbamate (thiram) with
acute toxicity of manganese and zinc ethylenebisdithiocarbamates
(maneb and zinc). Final report, MRI project no.: 36123-B. Submitted
to WHO by UCB.
Lee, C-C. & Peters, P.J. (1976) Neurotoxicity and behavioral effects
of thiram in rats. Environmental Health Perspectives, 17: 35-43.
Lee, C.C. et al. (1978) Oral toxicity of ferric
dimethyldithiocarbamate (ferbam) and tetramedthylthiuram disulfide
(thiram) in rodents. J. toxicol. environ. health, 4, 93-106.
Submitted to WHO by UCB.
Lisi, P., Caraffini, S. & Assalve, D. (1987) Irritation and
sensitization potential of pesticides. Contact Derm., 17: 212-218.
Maita, K., Tsuda, S. & Shirasu, Y. (1991) Chronic toxicity studies
with thiram in wistar rats and beagle dogs. Fundamental and Applied
Toxicol., 16: 667-686.
McManus, J.P. (1991) Metabolism of [14C] thiram in the rat:
Urinary metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Middlebury, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Matthiasck, L. (1973) Uber den Einfluss von L-cystein auf die
Teratogenese durch Thiram (TMTD) bei NMRI-Mausen. Arch. Toxikol. 30,
251-262. Submitted to WHO by UCB.
Musacchio, J.M., Goldstein, M., Anagnoste, B. Poch, G., & Kopin L.J
(1966) Inhibition of dopamine beta-hydroxylase by disulfiram in
vivo. J. Pharmacol. Exp. Ther., 152: 56-61.
Nomeir, A.A. & Markham, P. (1990) Disposition and metabolism of
thiram in rats after pretreatment with thiram for 14 days.
Unpublished report No. ADL 65492 from Arthur D. Little, Inc.
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Norris, K.J. (1989) Determination of volatile 14C-residues from
rats orally administered 14C-thiram. Unpublished report No. 1113A
from Analytical Development Corp., Colorado Springs, USA. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Perocco, P., Santucci, M.A., Campani, A.G. & Forti, G.C. (1989)
Toxic and DNA-damaging activities of the fungicides mancozeb and
thiram (TMTD) on human lymphocytes in vitro. Teratogenesis,
carcinogenesis, and mutagenesis., 9: 75-81.
Poth, A. (1990) Salmonella typhimurium reverse mutation assay with
thiram. Unpublished report No. CCR 175116 from CCR Cytotest Cell
Research GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Prasad, H., Pushpavathi, K., Rita, P. & Reddy, P.P. (1987) The
effect of thiram on the germ cells of male mice. Fd. Chem. Toxic.
25: 709-711.
Putman, D.L. (1987a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. Unpublished report No. T5558.337 from Microbiological
associates, Inc., Maryland, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Putman, D.L. (1987b): Micronucleus cytogenetic assay in mice.
Unpublished report No. T5558.122 from Microbiological associates,
Inc., Maryland, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R. & Higgins, C. (1986)
Thiram: Effects of oral administration upon pregnancy in the rat,
preliminary teratology study. Unpublished report No. 86/TRK001/704
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W. & Crisp, V.C. (1987) Thiram: Preliminary
teratology study in the rabbit. Unpublished report No. 87/TRK003/122
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Higgins, C., Wilby,
O.K. & Tesh, S.A. (1988a) Teratology study in the rat. Unpublished
report No. 87/TRK002/179 from Life Science Research, Suffolk,
England. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W., Crisp, V.C., Wilby, O.K. & Tesh, S.A.
(1988b) Thiram: Teratology study in the rabbit. Unpublished report
No. 87/TRK004/541 from Life Science Research, Suffolk, England.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Thouin, M.H. (1985a) Evaluation of the acute oral toxicity of TMTD
technical in the rat. Unpublished report no.: 0174/238 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Thouin, M.H. (1985b) Evaluation of the acute oral toxicity of TMTD
technical in the rabbit. Unpublished report no.: 0113/211 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Trutter, J.A. (1992) Oncogenicity study in mice with thiram.
Unpublished report No. 798-223 from Hazleton Laboratories, Inc.,
9200 Leesburg Turnpike, Vienna, Virginia 22182, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. (1985) Evaluation of the DNA repair inducing
ability of TMTD technical in a primary culture of rat hepatocytes.
Unpublished report No. NOTOX 0174/ER156 from NOTOX C.V. DD 's-
Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1990) Mouse germ-cell cytogenetic assay with thiram.
Unpublished report No. CCR 175127 from CCR Cytotest Cell Research
GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1991) Mutation assay in somatic cells of the mouse
(mouse spot test) with thiram. Unpublished report No. CCR 200902
from CCR Cytotest Cell Research GmbH & Co. KG, Rossdorf, Germany.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
York, R.G. (1991) Two-generation reproduction study in rats.
Unpublished report No. 399-104 from International Research and
Development Corporation, Michigan, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
York, R.G. (1992) Development toxicity study in New Zealand
white rabbits. Unpublished report No. 399-121 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
 Approximately 60% of the administered radioactivity was
recovered as expired CS2 and 30% was found in the urine. Thiram
was rapidly degraded to more polar products. Virtually no unchanged
thiram was detected in the urine. Five urinary metabolites were
detected by HPLC and were identified by mass spectrometry. The
identified metabolites were an alanine derivative of CS2 (10%); a
glucuronide conjugate of dimethyldithiocarbamate (DDC) (20%); a
thiosulfenic acid (34%); the methyl ester of DDC (6%); and an
alanine conjugate (30%). The presence of these polar conjugates
demonstrates that the metabolic pathway involved a reduction of the
disulphide bond and subsequent reactions of the thiol moiety to form
oxidative and conjugative polar products (McManus, 1991).
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of thiram
Species Sex Route LD50 LC50 References
(mg/kg bw) (mg/m3)
Mouse M oral 4000 Matthiask, 1973
Mouse F oral 2300-3800 Lee et al., 1975;
1978
Rat M oral 3700-4000 Thouin, 1985a;
Lee et al., 1975;
1978
Rat F oral 1800-1900 Thouin, 1985a;
Lee et al., 1975;
1978
Rat M&F inhalation > 100 Debets, 1985
Rabbit M&F dermal > 2000 Thouin, 1985b
Short-term toxicity studies
Mice
Ten Charles River albino mice (Crl:CD-1R(ICR)BR)/sex/group
(males 24.3-24.8 g, females 19.0-19.3 g) received in the diet 0,
300, 600, or 1200 ppm thiram (purity 97.5%) for 4 weeks equal to 0,
54, 108 or 201 mg/kg bw/day in males and 62, 118 or 241 mg/kg bw/day
in females. Stability and homogeneity of the test substance was
satisfactory. The animals were observed daily for mortality, signs
of toxicity and food consumption. Body-weights were recorded weekly.
Haematology and clinical chemistry tests were performed at sacrifice
on days 29 or 30. Gross and histopathological examinations of the
animals were carried out.
Body-weights were significantly and dose-dependently reduced in
males at all dose levels. Food consumption was significantly lower
for both sexes at all dose levels when compared with controls.
Statistically significant differences in clinical chemistry and
haematological parameters included reduced erythrocyte counts,
haemoglobin, and haematocrit for males at all dose levels; increased
platelet counts for females at 600 and 1200 ppm; reduced serum
glucose for females at 1200 ppm. Statistically significant and dose-
dependent increased organ-to-body-weight ratios of the brain,
kidneys and liver in males probably resulted from statistically
significant and dose-dependent reduced terminal body-weights. A
NOAEL could not be determined because of the 28-48% reduction in
food intake at 300 ppm (Kehoe, 1989b).
Rats
In a 13-week study, 10 Charles River rats
(Crl:CDR(SD)BR)/sex/group (males 138 ±6 g and females 125 ±4 g)
received dietary concentrations of 0, 50, 500, or 1000 ppm thiram
(purity 99.4%) equivalent to 0, 2.5, 25 or 50 mg/kg bw/day. Mean
stability assay results for diets stored frozen for 14 and 35 days
ranged from 81-99%. Diets analyzed after being in the animals room
for 7 days were 17, 80, and 88%, respectively, of the nominal
levels. Mean values for homogeneity assay results were 80, 97, and
98%, respectively, of the nominal levels of 50, 500, and 1000 ppm.
The mean±SD of determined concentration of thiram was 40 ± 1, 483 ±
8, and 976 ± 15 ppm. The animals were observed daily for mortality
and signs of toxicity. Body-weights and food consumptions were
recorded weekly and haematology and clinical chemistry were
determined at study termination. Ophthalmic observations were done
before and at termination of study. Gross and histopathological
examinations from the control and 1000 ppm group and of macroscopic
lesions, lungs, liver, and kidneys from all 50 ppm and all 500 ppm
animals was carried out at study termination.
Body weights, cumulative body-weight gains, and food
consumption were significantly reduced throughout the study for both
sexes at 500 and 1000 ppm. Changes in clinical chemistry and
haematological parameters occurred at dose levels of 500 and 1000
ppm. The changes considered to be treatment-related were reduced
erythrocyte counts, haemoglobin and haematocrit in females;
increased MCV and MCH in both sexes; increased white blood cell,
corrected white blood cell, absolute neutrophil, absolute lymphocyte
and absolute monocyte counts in females; reduced total protein and
glucose in both sexes; reduced albumin and increased urea nitrogen
and chloride in females.
At 500 and 1000 ppm animals a tendency to reduced terminal
body-weights with correspondingly reduced absolute organ weights and
increased organ to body-weight ratios were observed.
Macroscopically, the non-glandular stomach in some animals showed
areas of erosion and the mesenteric lymph nodes were diffusely red
or mottled. Microscopically, the mucosa of the nonglandular stomach
had focal areas of erosion/ulceration, mucosal hyperplasia, or both,
accompanied by some submucosal inflammation and edema. These changes
appeared to be treatment-related. The mesenteric lymph nodes were
frequently congested but otherwise normal. The NOAEL in this study
was 50 ppm, equivalent to 2.5 mg/kg bw/day (Kehoe, 1988b).
Dogs
Two acclimated beagle dogs (5 months of age and weighing 5.6 to
8.5 kg/sex/group) were fed dietary concentrations of 0, 125, 500, or
2000 ppm thiram (purity 99.4%), equivalent to 0, 3, 13 or 50 mg/kg
bw/day for 4 weeks. All animals were observed daily for toxic signs
and mortality. Body-weight and food consumption was recorded weekly.
Clinical chemistry, gross and histopathological examinations were
performed on all animals. Homogeneity assays indicated from 94-96%
(mean values) of the nominal values. There were no data on
stability, but thiram was stored under similar conditions to that in
other studies where stability was satisfactory.
One 2000 ppm one female died during week 2 and one male became
moribund and was sacrificed during week 4. Therefore, the high-dose
of 2000 ppm was reduced to 1500 ppm during weeks 3 and 4. The
observations in the female were primarily colon changes associated
with the acute death and subsequent post-mortem change. The male had
only a few incidental macroscopic and microscopic findings and,
therefore, its death was not considered compound-related. At week 4,
body-weights for both sexes at 500 ppm were approximately 15% lower
and at 2000/1500 ppm, 33 and 39% lower than controls respectively.
Food consumption during week 4 for 125, 500, and 2000/1500 ppm males
were 12%, 29%, and 92% lower than controls respectively, and for
females 13%, 49%, and 78% lower. Treatment-related changes in
clinical chemistry and haematological parameters comprised reduced
erythrocyte counts, haemoglobin and haematocrit in males given 500
or 2000/1500 ppm. One male given 2000/1500 ppm had increased ALAT,
ASAT and ALP. Slightly reduced absolute lymphocyte counts and
slightly higher platelet counts, total bilirubin and urea nitrogen
occurred in some male and female dogs given 500 or 2000 ppm. The
surviving 2000/1500 ppm dogs sacrificed at week 4 had notably
reduced terminal body-weights and correspondingly reduced absolute
organ weights. The liver of the males had hepatocellular
degeneration with sinusoidal cell proliferation and pigmentation.
There were no major macroscopic or microscopic findings in the
remaining dogs. The NOAEL for the study was determined to be 125
ppm, equivalent to 3 mg/kg bw/day, based on the lower body-weights
and changes in clinical chemistry parameters (Kehoe 1988a).
In a 13-week study on 5 month old beagle dogs, four beagle
dogs/sex/group were fed dietary concentrations of 0, 75, 250, or 500
ppm thiram (purity 97.5%), equal to 0, 2.2, 6.9 or 12 mg/kg bw/day
in males and 0, 2.3, 7.3 or 13 mg/kg bw/day in females. The mean (±
SD) body-weight at study initiation was 7.4±0.6, 7.3±1.1, 7.4±0.5,
and 7.3±0.6 kg for males receiving 0, 75, 250, and 500 ppm. and were
6.8 ± 0.9, 7.1 ± 1.4, 6.3 ± 0.5, and 6.7±0.5 kg for females. All
animals were observed daily for clinical signs, moribundity and
mortality. Body-weight and food consumption was recorded weekly.
Ophthalmic examinations were performed prior to and at the end of
the study. Clinical chemistry measurements, haematological
examinations and gross and histopathology examinations were done on
all animals. Homogeneity of diets was within ± 8% of nominal values.
Mean stability assays of diets frozen for 7 days, stored in the
animal room for 1 day and then frozen for 4 weeks, were within ± 5%
of nominal levels.
Body weights for the 500 ppm dogs were significantly reduced.
Food consumption was significantly reduced in 500 ppm males and in
250 and 500 ppm females. Treatment-related effects for clinical
chemistry and haematological parameters were reduced erythrocyte
counts, higher MCV and MCH in 75, 250, and 500 ppm dogs; reduced
haemoglobin and haematocrit in 500 ppm females; higher platelet
counts in 250 and 500 ppm males; lower total protein and albumin in
75, 250, and 500 ppm males and females; and higher cholesterol in
250 and 500 ppm males and females. The NOAEL for this study was 75
ppm equal to 2.2 and 2.3 mg/kg bw/day in males and females
respectively, based on haematological changes noted in both sexes
(Kehoe, 1989a).
Six 4 to 5 month old beagle dogs/sex/group (initial weight 4.0
to 6.8 kg) were fed dietary concentrations of 0, 30, 90, or 250 ppm
thiram (purity 97.5%), equal to 0, 0.84, 2.6, and 7.4 mg/kg bw/day
in males and 0, 0.90, 2.5, and 7.2 mg/kg bw/day in females for 52
weeks. All animals were observed daily for clinical signs and
mortality. Body weights and food consumption were recorded weekly
for weeks 1 through 16, and monthly thereafter. Ophthalmic
examinations were performed prior to and at the end of the study.
Haematology, clinical chemistry, gross and histopathology were
performed on all animals. Mean values for homogeneity assays were
83, 92, and 92% of the nominal levels of 30, 90, and 250 ppm,
respectively. Mean stability assay results for diets kept below 0 °C
for 7 days and stored in the animal room for one day were 84%, 92%
and 96% of the nominal levels of 30, 90, and 250 ppm, respectively.
No ophthalmic lesions were observed in any animals. Erythrocyte
counts were reduced in males given 250 ppm, total protein was lower
and cholesterol was higher in males given 90 or 250 ppm and in
females given 250 ppm. Albumin was reduced in both sexes given 250
ppm. Absolute liver weights in males given 90 or 250 ppm were
significantly increased. Liver-to-body-weight ratios in males given
30, 90, or 250 ppm and in females given 250 ppm were also
significantly increased, as were liver-to-brain weight ratios in
males given 250 ppm. The significant increase in liver-to-body-
weight ratios in males given 30 ppm was due to a slight increase in
absolute liver weight and a slight decrease in body weight.
Furthermore, the observation that the body weights of males given 90
ppm were increased in relation to those of the controls and those of
males given 30 ppm demonstrates, that the slight decrease in body
weights seen in the 30 ppm group was spurious. Thus, the increase in
liver-to-body-weight ratio seen in males given 30 ppm was not
considered a test material-related effect. The changes in liver
weights, in conjunction with the altered total protein, albumin, and
cholesterol levels suggest a test material-related effect on liver
function and size. These changes were probably adaptive responses to
the presence of this compound. Based on increased absolute liver
weights and altered clinical chemistry parameters in males given 90
or 250 ppm, and altered clinical chemistry parameters in females at
250 ppm, the NOAEL for male dogs was 30 ppm, equal to 0.84 mg/kg
bw/day, and the NOAEL for female dogs was 90 ppm equal to 2.5 mg/kg
bw/day (Kehoe 1991a).
Four groups of 4 beagle dogs/sex/dose were treated orally via
gelatin capsule with thiram (purity 98.7%) at doses of 0 (blank
capsule), 0.4, 4, or 40 mg/kg bw/day for 104 weeks. Body weights and
food intake were measured weekly. Water consumption, haematology,
ophthalmoscopy, clinical chemistry and gross and histopathology of
all organs were performed.
Both sexes at 40 mg/kg bw/day showed severe toxic signs
including nausea, vomiting, salivation, and clonic convulsion and
severe anaemia within the first 11 weeks of treatment and were all
subjected to unscheduled necropsy before the 29th week of treatment.
The dogs also showed ophthalmological changes such as fundal
haemorrhage, miosis, and desquamation of the retina which were
consistent with retinal lesions observed histopathologically. In the
4 mg/kg bw/day group, nausea, vomiting and salivation were common
findings in both sexes and one female showed clonic convulsion from
week 37. Parameters including haematocrit, haemoglobin, and
erythrocyte count were depressed from week 4 in both sexes treated
with 4 or 40 mg/kg bw/day, indicating anaemia. Changes in
biochemical parameters indicating liver failure were observed from
week 4 in the 40 mg/kg bw/day males and later in both sexes dosed
with 4 or 40 mg/kg bw/day. Kidney damage was detected in the
histopathological examination in two female dogs in each of the 4
and 40 mg/kg bw/day groups. Histological lesions in the central or
peripheral nervous system relating to the observed neurological
disturbances were not found. It was concluded that no sex difference
in response to treatment was demonstrated. Based on the neurological
disturbances, anaemia and the effects on the liver, the NOAEL was
0.4 mg/kg bw/day (Maita et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Four groups of 50 acclimated (24 days) Charles River Crl:CD-
1R(ICR)BR mice/sex/dose level (approximately 7 weeks of age) were
fed thiram (97.5% purity) at dietary concentrations of 0, 15, 150,
or 300 ppm (males), equal to 3, 24 or 50 mg/kg bw/day and 0, 15,
300, or 600 ppm (females), equal to 3, 57 or 112 mg/kg bw/day for 97
weeks, when the most sensitive group showed 50% mortality.
Homogeneity assays indicated from 75-84%, 95-100%, 94-103%, and 101-
103% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively. Mean stability assay results for diets frozen for 6
days and stored in the animal room for one day were 55, 90, 90, and
97% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively, demonstrating stability of varying acceptability.
Survival, clinical signs, body weights, food consumption, feed
efficiency, haematological parameters, necropsy, absolute and
relative organ weight and histopathological examination were
studied.
No substance-related oncogenic effects or adverse effects on
survival were observed in any test group. No changes attributable to
treatment were seen in any of the parameters evaluated in the 15 ppm
groups. There was no clinical indication of neurotoxicity at any
dose level. Mean body weight, weight gain, and total food
consumption were statistically significantly decreased in a dose-
dependent manner in the mid- and high-level groups beginning at
weeks 4-5. At study termination, mean weights were 7%, 15%, 14%, and
19% below control and mean total food consumption was 6%, 10%, 12%,
and 17% below control for the 150 ppm males, 300 ppm males, 300 ppm
females, and 600 ppm females, respectively. Increased frequency of
sores or reddened areas on the skin, generally on the ears,
consistent with bacterial dermatitis was noted for the 300 ppm males
and 600 ppm females. Mild but significant decreases in mean
erythrocyte count, haemoglobin, and haematocrit values were seen for
the 600 ppm females at week 97. Histopathology showed no evidence of
thiram-induced neoplasia. Non-neoplastic findings in the mid- and
high-level male and female mice consisted of retinal atrophy,
intracytoplasmic protein-like droplets in the urinary bladder
superficial transitional epithelium, and necrosis and suppurative
inflammation in the skin of the 150 and 300 ppm males and 300 and
600 ppm females; hyperkeratosis in the nonglandular stomach of the
300 ppm males and 300 and 600 ppm females; and increased pigment in
the spleen and decreased pigment in the inner adrenal cortex of the
300 and 600 ppm females.
No oncogenic effect was observed in doses up to 300 ppm, equal
to 50 mg/kg bw/day in males and 600 ppm, equal to 112 mg/kg bw/day
in females. Based on the body-weight reduction, the NOAEL for long-
term toxicity was 15 ppm, equal to 3 mg/kg bw/day (Trutter, 1992).
Rats
Four groups of 64 Jcl:Wistar rats were fed dietary
concentrations of 0, 3, 30, or 300 ppm thiram (purity 98.7%), equal
to 0, 0.1, 1.2 or 12 mg/kg bw/day for males and 0, 0.1, 1.4 or 14
mg/kg bw/day for females, for 104 weeks. Body weights, food intake,
and water intake were measured weekly up to week 52 and monthly
thereafter. Haematology, clinical chemistry, ophthalmoscopy and
gross and histopathology were performed on all rats. Test diets were
prepared twice weekly during the study. Data on homogeneity were not
available, but diets were stable up to 1 week.
Mortality of females in the 30 and 300 ppm groups was slightly
increased compared to controls during the last 8 weeks of treatment,
apparently correlated to an incidental higher occurrence of
pituitary tumours during this period. Overall incidence of pituitary
tumours in females was comparable to that in the control group.
Decreased body-weight gain and reduced food intake were observed in
both sexes at 300 ppm. Anaemia and regressive changes in the sciatic
nerve accompanied by atrophy of the calf muscle ( M. triceps surae)
were seen in the 300 ppm females. During the last 8 weeks of the
treatment period, the incidence of pituitary adenomas was increased
in females of the 30 and 300 ppm groups compared to that in
controls. Because of the small number of animals, statistical
analysis was not carried out. Statistical analysis of pituitary
tumour incidence in all animals at the end of the experiment showed
no significant differences. No evidence of carcinogenic potential
was observed. Based on mortality, anaemia, nerve degeneration and
muscle atrophy a NOAEL of 30 ppm, equal to 1.2 and 1.4 mg/kg bw/day
was determined in males and females, respectively (Miata et al.,
1991).
Four groups of sixty 36-day old Charles River Crl:CDR(SD)BR
rats/sex/group (initial weights 125-161 g for males and 115-193 g
for females) were fed dietary concentrations of 0, 30, 150, or 300
ppm thiram (purity 97.5%), equal to 0, 1.5, 7.3 or 15 mg/kg bw/day
in males and 0, 1.8, 8.9 or 19 mg/kg bw/day in females, for 104
weeks. Mean values for stability assays of diets kept below 0 °C for
6 days, animal room for one day, then below 0°C for 30 days were
15.5 ppm (52%), 123 ppm (82%), and 260 ppm (87%) for nominal
contents of 30, 150, and 300 ppm, respectively. The values for
homogeneity assay results ranged from 21.8-24.2 (73-81%), 128-138
(85-92%), and 255-276 ppm (85-92%) for nominal contents of 30, 150,
and 300 ppm, respectively. Cumulative means for the routine diet
analysis for dose confirmation were 16.3 (54%), 119(79%), and 262
ppm (87%) and the assay results ranged from 4.41-27.5 ppm, 81.8-151
ppm, and 179-320 ppm of the nominal dose levels of 30, 150, and 300
ppm, respectively, through 104 weeks. Data from a parallel study
with 14C-thiram stored under similar conditions (Hiles, 1989)
showed, that animals on a diet containing 30 ppm could be expected
to receive a systemic exposure to thiram and/or thiram-derived
materials approximately equal to the amount added to the diet, even
though the expected level could not be verified analytically.
Therefore, these data were considered to demonstrate that the
anticipated levels of thiram were achieved in the test diets. The
animals were observed daily for clinical signs and mortality. Body-
weight and food consumption were recorded weekly and haematology,
clinical chemistry, and urine analysis parameters were evaluated
during the study and at study termination. Ophthalmic observations
were done before and at termination of the study and gross and
histopathology examinations were carried out at study termination.
Clinical signs possibly test material-related for males
included swollen nose, soft faeces and opaque eyes. Soft faeces were
possibly test material-related at 150 and 300 ppm for the females.
Body-weight and cumulative body-weight gains in both sexes were
statistically significantly lower than those of the controls at 150
and 300 ppm. Food consumption was statistically significantly lower
than that in the controls for both sexes given 30, 150, or 300 ppm.
Reduced erythrocyte counts, haemoglobin, haematocrit and higher mean
corpuscular volume and mean corpuscular haemoglobin were observed in
females given 150 or 300 ppm. Statistically significant positive
trend analyses for hepatocellular adenomas (both sexes) and thyroid
C-cell adenomas (terminal sacrifice males and terminal plus
unscheduled sacrificed females) were reported but individual group
comparisons with controls were statistically insignificant. There
was no increase in the incidence of thyroid C-cell carcinomas.
Extramedullary haematopoiesis in the liver appeared to be increased
in males at 150 or 300 ppm and in females at 300 ppm. The biological
significance of this finding is unclear. Steatosis/fatty
infiltration of the pancreas (not uncommon in old SD rats) appeared
to be increased for males and females given 150 or 300 ppm. Also in
the pancreas, multifocal acinar atrophy was more common for males
given 150 or 300 ppm. The incidence of bile duct hyperplasia showed
a statistically significant positive trend in females, but was
statistically significantly increased only at 300 ppm. There were no
histopathological findings recorded that suggested test material-
related neurotoxicity.
Based on statistically significant decreases in body weights
and cumulative body-weight gains for males and females given 150 or
300 ppm, effects on haematological parameters in females given 150
or 300 ppm, and histopathological changes in males and females given
150 or 300 ppm, the NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg
bw/day in males and females, respectively. Thiram was not found to
be carcinogenic at doses up to 15 and 19 mg/kg bw/day in males and
females, respectively (Kehoe, 1991b).
Three groups of 50 five-week old Charles River SPF F344 rats,
initial mean body-weight of about 200 g for males and about 150 g
for females, were fed dietary concentrations of 0, 500 or 1000 ppm,
equal to 0, 18 or 39 mg/kg bw/day in males and 0, 20 or 42 mg/kg
bw/day in females for 104 weeks. Body weights and food consumption
were measured at regular intervals and haematology and clinical
chemistry were evaluated during the study and at study termination.
Gross and histopathology were performed. Homogeneity data and
stability data were not given.
Reduced body-weight gain and food consumption were seen at 500
and 1000 ppm in both sexes but especially in females of the high-
dose group. In males reduced liver function was observed.
Mononuclear cell leukaemia was found with an incidence of 20%, 8%,
and 4% in males and 29%, 12%, and 4% in females for 0, 500, and 1000
ppm animals, respectively. No tumour induction related to the
treatment was observed. It was concluded that thiram had no
carcinogenic effect at doses up to 39 and 42 mg/kg bw/day in males
and females, respectively (Hasegawa et al., 1988).
Reproduction studies
In a two-generation reproduction study, thiram (purity 97.6%)
was administered to Charles River Crl:CD VAF/Plus rats in dietary
concentrations of 0, 30, 60 or 180 ppm equal to 0, 1.5, 2.9 or 8.9
mg/kg bw/day in the F0 males and 0, 2.3, 4.6 or 14 mg/kg bw/day in
F0 females, and 0, 1.8, 3.8 or 11 mg/kg bw/day in the F1 males
and 0, 2.4, 5.1 or 16 mg/kg bw/day in the F1 females. Homogeneity
of thiram was satisfactory in the mixed diets. The test compound was
stable kept frozen for 7 days and reasonably stable (losses of 18%,
16%, and 9% in the 30, 60, and 180 ppm diet, respectively) kept at
room temperature for 12 hours. The mean ± SD percent of target ppm
found in all analyzed and administered 30, 60, and 180 ppm diets was
93 ± 6.3, 94 ± 6.6, and 98 ± 5.2%, respectively.The F0 and F1
parental generation consisted of 26 males and 26 females per group.
F0 animals were treated beginning at 63 days of age for 81 days
prior to the initial mating (F1a). A second litter (F1b) was
produced after a rest period of 16 days following weaning of the
F1a litter. Since conception rates were poor across all groups,
including the controls, in the F1b litter, an F1c litter was
also produced. The F1 parents were selected from the F1c
litters. These animals were treated beginning at 22 days of age for
a minimum of 84 days prior to their initial (F2a) mating. The F1
parents were mated twice to produce theF2a and F2b litters. All
parental animals, and pups were observed daily for mortality and
overt toxicity. Detailed clinical observations were recorded at
least once a week. Reproductive and litter parameters assessed
included male and female fertility indices, events at parturition,
gestation length, litter size, numbers of viable and stillborn pups,
and offspring survival and growth during lactation. All parental
animals, and all F1c weanlings not selected to remain on study and
all F2b weanlings were subjected to a gross necropsy. The F1a,
F1b, and the F2a offspring were euthanized and discarded
following weaning.
Mean maternal body weights and mean food consumption of the
F0 females were reduced at 60 and 180 ppm during the F1a
gestation period. Reductions in mean maternal body-weight and/or
food consumption were also observed at 180 ppm during the F1b and
F1c gestation periods and the F1a, F1b, and F1c lactation
periods. Similar reductions were observed for the F1 females at
180 ppm during the F2a and F2b gestation and lactation periods.
Reductions in mean weekly food consumption of the F0 males and
females were observed at 60 and 180 ppm. Mean offspring body weights
were consistently reduced to a significant degree in all litters
across both generations at the 180 ppm level. Apart from a
consistent statistically significant reduction in body weights in
the top dosed offspring during the F1b and F1c gestation periods
and the F1a, F1b, and F1c lactation periods, no substance-
related changes were found in the reproductive parameters or in the
parameters for post-natal development.
Based on the findings of parenteral systemic toxicity, the
NOAEL was 30 ppm, equal to 1.5 and 2.3 mg/kg bw/day in males and
females, respectively. With respect to filial systemic toxicity, the
NOAEL was 60 ppm, equal to 3.8 and 5.1 mg/kg bw/day in males and
females, respectively. With respect to reproduction and post-natal
development, the NOAEL was greater than 180 ppm equal to 8.9 and 14
mg/kg bw/day in males and females, respectively (York, 1991).
Special studies on genotoxicity
Data are shown in Table 2.
Special studies on teratogenicity
In a preliminary oral teratology study, groups of 6 Charles
River CD strain (Sprague-Dawley origin) rats (196-229 g) were
administered dose levels of 0, 5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 10 ml/kg bw) by gavage from day 6 through day 15 of
gestation. Control rats received the vehicle, 0.5% (W/V) CMC and
0.5% (W/V) Tween 80 in distilled water. Homogeneity and stability of
thiram in the suspensions were satisfactory (mean concentrations ±
SD of the 0.5, 1.0, 2.0, 4.0, 8.0, and 16 mg/ml thiram suspensions
were 0.5 ± 0.004, 0.97 ± 0.003, 1.96 ± 0.05, 3.94 ± 0.12, and 8.06 ±
0.25, and 16.6 ± 0.2 mg/ml, respectively). All females were killed
on day 20 of gestation for examination of uterine contents.
Table 2. Results of genotoxicity assays on thiram
Concentration
Test system Test object of thiram Purity Results Reference
In vitro
Ames test S. typhimurium 98.7% Poth (1990)
(±S9) TA 1535} +(±S9)
TA 100} 1-100 µg/plate (-S9) +(±S9)
TA 1537} 1-1000 µg/plate (+S9) -(±S9)
TA 1538} -(±S9)
TA 98 {10-1000 µg/plate (-S9) -(±S9)
{1-1000 µg/plate (+S9)
Gene mutation V79 Chinese 1-10 µg/ml (-S9) 100% -(±S9) Debets & Enninga
(±S9) hamster 10-56 µg/ml (+S9) (1986)
cells (HPRT-
locus)
Chromosome Chinese 0.003-0.023 µg/ml (-S9) 99.8% -(±S9) Putman (1987a)
aberrations hamster 0.2-1.5 µg/ml (+S9)
(±S9) ovary cells
-
DNA repair Primary rat 0.03-10 µg/ml 100% - Weterings (1985)
(test) hepatocytes
SCE induction human 5-25 µg/ml 98.6% + Perocco et al. (1989)
(with and without lymphocytes
S-9 mix)
Table 2 (cont'd)
Concentration
Test system Test object of thiram Purity Results Reference
In vivo
Mouse spot test mouse (NMRI) 75, 750 mg/kg 98.7% - Völkner (1991)
bw per os
Micronucleus test mouse (CD-1) 38-377 mg/kg 99.8% - Putman (1987b)
bw (i.p.)
Germ cell mouse (NMRI) 75-750 mg/kg 99.7% - Völkner (1990)
cytogenetic assay bw per os
Germ cell mouse (Swiss total doses n.g. (+) Prasad et al. (1987)
cytogenic assay albino) 80-320 mg/kg
(gavage)
n.g.: not given
A dose-related loss of body weight was seen at 20, 40, and 80
mg/kg bw/day. Treatment at 40 and 80 mg/kg bw/day produced a dose-
related increase in the total number of early and late resorptions
and a consequent increase in post-implantation loss and reduction in
viable litter size. It was concluded that the highest dose to be
used in a main teratology study in the rat should not exceed 40
mg/kg bw/day (Tesh et al. 1986).
Four groups of 25 female Charles River CD SD rats (193-236 g,
9-11 weeks old) were administered dose levels of 0 (vehicle), 7.5,
15 or 30 mg (99.0-99.8% purity) thiram/kg bw/day by gavage on day 6
through 15 of gestation. Pre-pairing acclimatization was for 5 days.
Test solutions (dosage volume, 10 ml/kg bw) were prepared in a 0.5%
w/v CMC and 0.5% w/v Tween 80 vehicle. All females were killed on
day 20 of gestation for examination of their uterine contents.
Analyses showed that the concentrations of test substance in the
test mixtures were 0.62-0.75 mg/ml, 1.47-1.49 mg/ml, and 2.88-2.91
mg/ml of intended concentrations of 0.75, 1.5, and 3.0 mg/ml,
respectively. The test substance was stable at room temperature
throughout the study. The concentration of tetramethylthiuram
monosulphide was less than 0.01%.
With the exception of a dosage-related incidence in the number
of females showing areas of hair loss on head, neck, back, and/or
limbs, the general condition of females was comparable in all
groups. At 15 and 30 mg/kg bw/day a transient, dose-related, loss of
body-weight was observed. At 7.5 mg/kg bw/day the rate of weight
gain was slightly, but significantly, reduced during the treatment
period, but subsequent weight gain was similar to that of the
controls. There were no adverse effects upon implantation or upon
fetal survival, but fetal and placental weights were significantly
reduced at 30 mg/kg bw/day. Placental weights were also slightly,
but significantly, reduced in the 7.5 and 15 mg/kg bw/day animals,
and foetal weight was slightly reduced at 15 mg/kg bw/day, but these
values were within historical control ranges. At 30 mg/kg bw/day
there was evidence of fetal immaturity e.g., reduced skeletal
ossification and increased incidence of space between the body wall
and organs, and there was a slightly increased incidence of
subcutaneous oedema. At 15 and 30 mg/kg bw/day the incidence of 13th
ribs of reduced size was slightly increased, but was not related to
dosage. Three foetuses with diaphragmatic hernia were observed, two
at 7.5 and one at 30 mg/kg bw/day. The NOAEL for fetal toxicity was
7.5 mg/kg bw/day. Reduced maternal body weight and placental weight
precluded the establishment of a NOAEL for maternal toxicity. The
toxic effects (immaturity and increased incidence of 13th ribs of
reduced size) at higher doses were considered a result of maternal
toxicity (Tesh et al., 1988a).
Rabbits
In a preliminary oral teratology study female New Zeeland white
rabbits (18-24 months old 3.4-4.5 kg) were artificially inseminated.
The day of insemination was designated day 0 of gestation. The
animals were allowed a minimum of three weeks acclimatization before
dosing. Thiram (purity 99.1%) was administered by gavage to 4
females per group from days 6-19, inclusive, of gestation at dosages
of 0 (vehicle), 1, 3, 5, 7.5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 5 ml/kg bw). Control animals received the vehicle
(the vehicle was 0.5% w/v aqueous CMC mucilage containing 0.5% w/v
Tween 80). Stability and concentration (89-106% of nominal) of the
test compound at room temperature was ensured by formulation of
fresh test solutions each day. On day 29 of gestation, the animals
were killed and their uterine contents examined.
Females receiving 10, 20, 40, or 80 mg/kg bw/day exhibited
marked body-weight loss during the treatment period. Eight females
(one on 20 mg/kg bw/day, three on 40 mg/kg bw/day and all four on 80
mg/kg bw/day) died or were killed in extremis. A further two deaths
(one control and one on 40 mg/kg bw/day) occurred but were not
attributed to treatment. Females receiving doses between 1 and 7.5
mg/kg bw/day showed slight reductions in their rate of body-weight
gains with some indication of a dosage relationship. Treatment at 20
mg/kg bw/day was associated with total litter resorption in 2
females and a marked increase in post-implantation loss in the one
female that carried a live litter to term. All 10 mg/kg bw/day
females carried their litters to term, but there was a slight
increase in post-implantation loss compared with the controls. At
7.5 mg/kg bw/day one female showed total litter loss, but in the
surviving two females post-implantation loss was similar to that of
the controls. It was concluded that dose-levels of thiram for use in
a main teratology study in the rabbit should not exceed 5 mg/kg
bw/day to prevent effect on survival in utero (Tesh et al.,
1987).
New Zeeland white rabbits 16-24 weeks of age and 3.7-4.8 kg
body-weight, were artificially inseminated. The day of insemination
was designated day 0 of gestation. The animals were allowed a
minimum of three weeks acclimatization before dosing. Thiram (purity
99.5%) was administered by gavage from day 6-19 of gestation
inclusive, at doses of 0, 1.0, 2.5 or 5.0 mg/kg bw/day (volume-
dosage 5.0 ml/kg bw). The vehicle was 0.5% (W/V) aqueous CMC
mucilage containing 0.5% (W/V) Tween 80. Stability and concentration
of the test compound at room temperature were satisfactory. The
concentration of tetramethylthiuram monosulphide was less than
0.03%. The number of control animals was 18+14 (initial controls +
additional controls from a parallel study due to unusually poor
pattern of body-weight gain, in part, to the performance of two
females). The number of test animals per group were 15, 18, and 20
for the 1.0, 2.5, and 5.0 mg/kg bw/day dose group, respectively. All
females were killed on Day 29 of gestation for examination. After
removal of the reproductive tract the following was recorded: number
of corpora lutea per ovary; number of implantation sites, number of
resorption sites; number and distribution of live and dead foetuses
in each uterine horn; weights and abnormalities of individual
fetuses and placentae, thorough examination including skeletal
examination of all fetuses.
The general condition of control and treated females was
essentially similar throughout the investigation. Eight females died
or were killed in extremis during the study (one in each of groups 1
and 2, two in group 3, and four in group 4). Necropsy findings
revealed evidence of respiratory or gastrointestinal tract disorder,
or accidental tracheal intubation. No evidence was apparent of any
involvement of thiram. The body-weight gain of females receiving 5.0
mg/kg bw/day was slightly but statistically significantly reduced.
Females receiving 1.0 or 2.5 mg/kg bw/day remained unaffected by
treatment with thiram. One control female and one 5.0 mg/kg bw/day
female aborted during the study. In addition, one female in each of
the groups receiving 2.5 or 5.0 mg/kg bw/day exhibited total litter
resorption. Litter parameters were unaffected by treatment with
thiram. Survival, growth and morphological development in utero
were essentially unaffected by treatment. The NOAEL for maternal
toxicity was 2.5 mg/kg bw/day and the NOAEL for embryofetal toxicity
was 5.0 mg/kg bw/day. There was no evidence of teratogenic potential
(Tesh et al., 1988b).
Five-month old New Zeeland white SPF female rabbits were
acclimatized and artificially inseminated. Groups of 20 inseminated
rabbits were dosed once daily by oral gavage with 0, 1.0, 5.0, or 10
mg/kg bw/day (dose volume 3.0 ml/kg bw) thiram solved in 0.5% Tween
80 and 0.5% low-viscosity CMC on gestation days 7 through 19.
Homogeneity mean values ranged from 107-108% of target and the
relative standard deviations were < 4.5%. Stability at room
temperature for 10 days indicated 95-99% of the initial prepared
concentrations. Caesarean sections were performed on all surviving
females on gestation day 29, followed by teratologic examination of
the fetuses. Individual fetuses were weighed, sexed, tagged and
examined for external malformations and variations. Each fetus was
dissected internally, sexed and examined for visceral malformations
and variations. The head was examined using multiple coronal slices.
Skeletal examination was carried out after Alizarin Red S staining.
A single death observed in the 10 mg/kg bw/day group was
attributed to gavage injury from dosing and two deaths in the
control group were attributed to pneumonia. No treatment-related
adverse findings were observed with regard to clinical signs or
necropsy findings of animals surviving the scheduled euthanasia.
Statistically significant increases in body-weight gain and food
consumption were observed in the treated groups when compared with
the control values. These increases were not considered to be
adverse effects of the test article. No treatment-related maternal
effects were observed at cesarean section examination, and no
adverse effects on fetal development were observed at the
teratological examination. The NOAELs for maternal toxicity,
embryofetal toxicity and teratogenicity were higher than 10 mg/kg
bw/day (York, 1992).
Special studies for neurotoxicity
Data from special neurotoxicity testing of thiram were not
available. Some endpoints related to neurotoxicity have been
investigated in the standard toxicity testing procedures reported
above.
Observations in humans
Workers, surgeons and other personnel using rubber gloves might
get contact dermatitis due to the presence of thiram in the rubber
(Lisi et al., 1987). Oral exposure to thiram might lead to dermal
sensitization to the substance (Goitre et al., 1981).
COMMENTS
Following oral administration to rats, thiram was well absorbed
(>83%) and eliminated via the expired air (41-48%), urine (25-40%),
and faeces (2-5%). About 3% was recovered in various organs. The
majority of the dose (84-90%) was eliminated within four days after
dosing.
The metabolism of thiram was studied in rats. During the first
five hours after administration, a dose-dependent formation of
carbon disulphide was demonstrated in the expired air. Metabolites
detected in urine included polar oxidation products and conjugates.
The acute oral toxicity of thiram is low in mice and rats. The
World Health Organization has classified thiram as slightly
hazardous (WHO, 1992).
A 13-week dietary study in rats at levels of 0, 50, 500 or 1000
ppm resulted in changes in haematological and serum biochemical
parameters and gastric irritation at 500 and 1000 ppm. The NOAEL was
50 ppm, equivalent to 2.5 mg/kg bw/day.
Beagle dogs received thiram as a dietary admixture at levels of
0, 75, 250 or 500 ppm for 13 weeks or at levels of 0, 30, 90 or 250
ppm for 52 weeks. The NOAELs were 75 ppm (equal to 2.2 and 2.3 mg/kg
bw/day in males and females, respectively, for the 13-week study)
and 30 ppm (equal to 0.84 mg/kg bw/day) in males and 90 ppm (equal
to 2.5 mg/kg bw/day) in females in the 52-week study on the basis of
changes in body weight, increased absolute and relative liver
weights, and changes in haematological and serum biochemical
parameters. In another study, dogs received thiram in gelatin
capsules at doses of 0, 0.4, 4 or 40 mg/kg bw/day 7 days/week for
104 weeks. Nausea, vomiting and salivation, ophthalmological
effects, convulsions, changes in haematological parameters, and
renal changes were observed at 4 and 40 mg/kg bw/day. On the basis
of the described effects, the NOAEL was 0.4 mg/kg bw/day. Since
thiram was administered in capsules in this experiment and
significantly less information was available on the study conditions
compared to those in the former two experiments with dietary
administrations of thiram this NOAEL was not used as the basis for
the estimation of an ADI.
In a 97-week oncogenicity study in mice using dietary thiram
concentrations of 0, 15, 150 or 300/600 ppm, the effects included
dose-dependent decreases of food consumption and body weight gain
and changes in haematological parameters. Non-neoplastic findings
included retinal atrophy, changes in the urinary bladder and in the
skin, hyperkeratosis in the non-glandular stomach, and increased
pigmentation in the spleen. Thiram was not carcinogenic in mice. The
NOAEL for long-term toxicity in male and female mice was 15 ppm,
equal to 3 mg/kg bw/day.
In a two-year toxicity study in rats at dietary concentrations
of 0, 3, 30 or 300 ppm, the NOAEL was 30 ppm, equal to 1.2 and 1.4
mg/kg bw/day in males and females, respectively. It was based on
lower red blood cell count, haemoglobin and haematocrit levels and
degenerative changes of the sciatic nerve accompanied by atrophy of
the gastrocnemius muscle at 300 ppm.
In a second two-year long-term toxicity/carcinogenicity study
in rats at dietary concentrations of 0, 30, 150 or 300 ppm, dose-
dependent lower erythrocyte counts, haemoglobin and haematocrit
levels were observed. Based on these haematological changes, the
NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg bw/day in males and
females, respectively.
In a 2-year carcinogenicity study in rats at dietary
concentrations of 0, 500, or 1000 ppm (equal to 39 and 42 mg/kg
bw/day in males and females, respectively) there was no evidence of
carcinogenicity. The Meeting concluded that thiram was not
carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 30, 60 or 180 ppm, no adverse effects on
reproduction were observed. The NOAEL for reproductive effects was
> 180 ppm (equal to > 8.9 and > 14 mg/kg bw/day in males and
females, respectively). The NOAEL for systemic toxicity was 30 ppm
(equal to 1.5 and 2.3 mg/kg bw/day in males and females,
respectively). This NOAEL was based upon reduction in body-weight
and/or food consumption in both parental and offspring animals.
An oral teratogenicity study was performed in rats at gavage
dose levels of 0, 7.5, 15 or 30 mg/kg bw/day. A NOAEL for maternal
toxicity was not determined due to a dose-dependent decrease in body
weight gain and placental weight at all dose levels. Teratogenicity
was not observed.
In an oral teratogenicity study in rabbits at gavage doses of
0, 1.0, 2.5 or 5.0 mg/kg bw/day, a NOAEL of 2.5 mg/kg bw/day for
maternal toxicity was based on a dose-dependent reduction of body-
weight gain. Teratogenicity was not observed.
An oral teratogenicity study was carried out in rabbits at
gavage dose levels of 0, 1.0, 5.0 or 10 mg/kg bw/day. The NOAEL for
maternal toxicity was higher than 10 mg/kg bw/day. Teratogenicity
was not observed.
Thiram was mutagenic in the Ames test but not in mammalian
cells in vitro. Since thiram was not mutagenic in vivo, the
Meeting concluded that it did not present a genotoxic hazard for
humans.
The central and peripheral nervous systems have been recognized
as a possible target for thiram toxicity. The neurotoxicity may be
related to the thiram metabolite carbon disulphide.
An ADI was allocated, based on the 1-year study in dogs and the
2-year studies in rats, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 15 ppm, equal to 3 mg/kg bw/day (97-week study)
Rat: 30 ppm, equal to 1.2 mg/kg bw/day (two-year study)
30 ppm, equal to 1.5 mg/kg bw/day (two-generation
reproduction study)
Rabbit: 2.5 mg/kg bw/day (teratology study, maternal
toxicity)
Dog: 30 ppm in the diet, equal to 0.84 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Clarification of the potential for neurotoxicity of
thiram.
2. Observations in humans.
REFERENCES
Beauchamp, R.O., Bus, J.S., Popp, J.A., Boreiko, C.J. & Golberg, L.
(1983) A critical review of the literature on the carbon disulphide
toxicology. CRD Crit. Rev. Toxicol., 11: 169-272.
Dalvi R.R. (1987) Dose-dependent liver toxicity of thiram
administered intraperitoneally to rats. J. Environ. Biol., 8: 25-
31.
Dalvi, R.R. & Deoras, D.P. (1986) Metabolism of a dithiocarbamate
fungicide thiram to carbon disulphide in the rat and its hepatotoxic
implications. Acta Pharmacol. Toxicol., 58: 38-42.
Debets, F.M.H. (1985) Evaluation of the acute inhalation toxicity of
TMTD technical in the rat. Unpublished report no.: 0113/232 from
NOTOX v.o.f. s-Hertogenbosch, the Netherlands. Submitted to WHO by
UCB.
Debets, F.M.H. & Enninga, I.C. (1986) Evaluation of the mutagenic
activity of TMTD technical in an in vitro mammalian cell gene
mutation test with V79 Chinese hamster cells. Unpublished report No.
NOTOX 0174/EV 1 from NOTOX C.V. DD 's-Hertogenbosch, Netherlands.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Gay, M.H. (1987) Rat metabolism of 14C thiramTM, single dose
study. Unpublished report No. 87003B from Biotek, Inc.,
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Goitre, M., Bedollo, P.G., & Cane, D. (1981) Allergic dermatitis and
oral challenge to tetramethylthiuram disulphide. Contact Derm., 7:
272-273.
Hasegawa, R., Takahashi, M., Furukawa, F., Toyoda, K., Sato, H.,
Junejang, J. & Hayashi, Y. (1988) Carcinogenicity study of
tetramethylthiuram disulphide (thiram) in F334 rats. Toxicology,
51: 155-165.
Hiles, R.A. (1989) Bioavailability study in male rats with a 14C-
thiram-treated diet. Unpublished report No. HLA 6111-131 from
Hazleton Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988a) Four-Week range-finding study with thiram in
dogs. Unpublished report No. HLA 6111-109 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988b) Thirteen-Week toxicity study with thiram in
rats. Unpublished report No. HLA 6111-110 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1989a) 13-Week toxicity study with thiram in dogs.
Unpublished report No. HLA 6111-121 from Hazleton Laboratories,
Inc., Wisconsin, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Kehoe, D.F. (1989b) 4-Week dietary range-finding study with thiram
in mice. Unpublished report No. HLA 6111-127 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991a) 52-Week dietary chronic toxicity study with
thiram in dogs. Unpublished report No. HLA 6111-112 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991b) 104-Week combined chronic toxicity and
carcinogenicity study with thiram in rats. Unpublished report No.
HLA 6111-113 from Hazleton Laboratories, Inc., Wisconsin, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.C. et al. (1975) Toxicological evaluation of ferric
dimethyldithiocarbamate (ferbam) and dithiocarbamate (thiram) with
acute toxicity of manganese and zinc ethylenebisdithiocarbamates
(maneb and zinc). Final report, MRI project no.: 36123-B. Submitted
to WHO by UCB.
Lee, C-C. & Peters, P.J. (1976) Neurotoxicity and behavioral effects
of thiram in rats. Environmental Health Perspectives, 17: 35-43.
Lee, C.C. et al. (1978) Oral toxicity of ferric
dimethyldithiocarbamate (ferbam) and tetramedthylthiuram disulfide
(thiram) in rodents. J. toxicol. environ. health, 4, 93-106.
Submitted to WHO by UCB.
Lisi, P., Caraffini, S. & Assalve, D. (1987) Irritation and
sensitization potential of pesticides. Contact Derm., 17: 212-218.
Maita, K., Tsuda, S. & Shirasu, Y. (1991) Chronic toxicity studies
with thiram in wistar rats and beagle dogs. Fundamental and Applied
Toxicol., 16: 667-686.
McManus, J.P. (1991) Metabolism of [14C] thiram in the rat:
Urinary metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Middlebury, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Matthiasck, L. (1973) Uber den Einfluss von L-cystein auf die
Teratogenese durch Thiram (TMTD) bei NMRI-Mausen. Arch. Toxikol. 30,
251-262. Submitted to WHO by UCB.
Musacchio, J.M., Goldstein, M., Anagnoste, B. Poch, G., & Kopin L.J
(1966) Inhibition of dopamine beta-hydroxylase by disulfiram in
vivo. J. Pharmacol. Exp. Ther., 152: 56-61.
Nomeir, A.A. & Markham, P. (1990) Disposition and metabolism of
thiram in rats after pretreatment with thiram for 14 days.
Unpublished report No. ADL 65492 from Arthur D. Little, Inc.
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Norris, K.J. (1989) Determination of volatile 14C-residues from
rats orally administered 14C-thiram. Unpublished report No. 1113A
from Analytical Development Corp., Colorado Springs, USA. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Perocco, P., Santucci, M.A., Campani, A.G. & Forti, G.C. (1989)
Toxic and DNA-damaging activities of the fungicides mancozeb and
thiram (TMTD) on human lymphocytes in vitro. Teratogenesis,
carcinogenesis, and mutagenesis., 9: 75-81.
Poth, A. (1990) Salmonella typhimurium reverse mutation assay with
thiram. Unpublished report No. CCR 175116 from CCR Cytotest Cell
Research GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Prasad, H., Pushpavathi, K., Rita, P. & Reddy, P.P. (1987) The
effect of thiram on the germ cells of male mice. Fd. Chem. Toxic.
25: 709-711.
Putman, D.L. (1987a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. Unpublished report No. T5558.337 from Microbiological
associates, Inc., Maryland, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Putman, D.L. (1987b): Micronucleus cytogenetic assay in mice.
Unpublished report No. T5558.122 from Microbiological associates,
Inc., Maryland, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R. & Higgins, C. (1986)
Thiram: Effects of oral administration upon pregnancy in the rat,
preliminary teratology study. Unpublished report No. 86/TRK001/704
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W. & Crisp, V.C. (1987) Thiram: Preliminary
teratology study in the rabbit. Unpublished report No. 87/TRK003/122
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Higgins, C., Wilby,
O.K. & Tesh, S.A. (1988a) Teratology study in the rat. Unpublished
report No. 87/TRK002/179 from Life Science Research, Suffolk,
England. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W., Crisp, V.C., Wilby, O.K. & Tesh, S.A.
(1988b) Thiram: Teratology study in the rabbit. Unpublished report
No. 87/TRK004/541 from Life Science Research, Suffolk, England.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Thouin, M.H. (1985a) Evaluation of the acute oral toxicity of TMTD
technical in the rat. Unpublished report no.: 0174/238 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Thouin, M.H. (1985b) Evaluation of the acute oral toxicity of TMTD
technical in the rabbit. Unpublished report no.: 0113/211 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Trutter, J.A. (1992) Oncogenicity study in mice with thiram.
Unpublished report No. 798-223 from Hazleton Laboratories, Inc.,
9200 Leesburg Turnpike, Vienna, Virginia 22182, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. (1985) Evaluation of the DNA repair inducing
ability of TMTD technical in a primary culture of rat hepatocytes.
Unpublished report No. NOTOX 0174/ER156 from NOTOX C.V. DD 's-
Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1990) Mouse germ-cell cytogenetic assay with thiram.
Unpublished report No. CCR 175127 from CCR Cytotest Cell Research
GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1991) Mutation assay in somatic cells of the mouse
(mouse spot test) with thiram. Unpublished report No. CCR 200902
from CCR Cytotest Cell Research GmbH & Co. KG, Rossdorf, Germany.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
York, R.G. (1991) Two-generation reproduction study in rats.
Unpublished report No. 399-104 from International Research and
Development Corporation, Michigan, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
York, R.G. (1992) Development toxicity study in New Zealand
white rabbits. Unpublished report No. 399-121 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Approximately 60% of the administered radioactivity was
recovered as expired CS2 and 30% was found in the urine. Thiram
was rapidly degraded to more polar products. Virtually no unchanged
thiram was detected in the urine. Five urinary metabolites were
detected by HPLC and were identified by mass spectrometry. The
identified metabolites were an alanine derivative of CS2 (10%); a
glucuronide conjugate of dimethyldithiocarbamate (DDC) (20%); a
thiosulfenic acid (34%); the methyl ester of DDC (6%); and an
alanine conjugate (30%). The presence of these polar conjugates
demonstrates that the metabolic pathway involved a reduction of the
disulphide bond and subsequent reactions of the thiol moiety to form
oxidative and conjugative polar products (McManus, 1991).
Toxicological studies
Acute toxicity studies
Table 1. Acute toxicity of thiram
Species Sex Route LD50 LC50 References
(mg/kg bw) (mg/m3)
Mouse M oral 4000 Matthiask, 1973
Mouse F oral 2300-3800 Lee et al., 1975;
1978
Rat M oral 3700-4000 Thouin, 1985a;
Lee et al., 1975;
1978
Rat F oral 1800-1900 Thouin, 1985a;
Lee et al., 1975;
1978
Rat M&F inhalation > 100 Debets, 1985
Rabbit M&F dermal > 2000 Thouin, 1985b
Short-term toxicity studies
Mice
Ten Charles River albino mice (Crl:CD-1R(ICR)BR)/sex/group
(males 24.3-24.8 g, females 19.0-19.3 g) received in the diet 0,
300, 600, or 1200 ppm thiram (purity 97.5%) for 4 weeks equal to 0,
54, 108 or 201 mg/kg bw/day in males and 62, 118 or 241 mg/kg bw/day
in females. Stability and homogeneity of the test substance was
satisfactory. The animals were observed daily for mortality, signs
of toxicity and food consumption. Body-weights were recorded weekly.
Haematology and clinical chemistry tests were performed at sacrifice
on days 29 or 30. Gross and histopathological examinations of the
animals were carried out.
Body-weights were significantly and dose-dependently reduced in
males at all dose levels. Food consumption was significantly lower
for both sexes at all dose levels when compared with controls.
Statistically significant differences in clinical chemistry and
haematological parameters included reduced erythrocyte counts,
haemoglobin, and haematocrit for males at all dose levels; increased
platelet counts for females at 600 and 1200 ppm; reduced serum
glucose for females at 1200 ppm. Statistically significant and dose-
dependent increased organ-to-body-weight ratios of the brain,
kidneys and liver in males probably resulted from statistically
significant and dose-dependent reduced terminal body-weights. A
NOAEL could not be determined because of the 28-48% reduction in
food intake at 300 ppm (Kehoe, 1989b).
Rats
In a 13-week study, 10 Charles River rats
(Crl:CDR(SD)BR)/sex/group (males 138 ±6 g and females 125 ±4 g)
received dietary concentrations of 0, 50, 500, or 1000 ppm thiram
(purity 99.4%) equivalent to 0, 2.5, 25 or 50 mg/kg bw/day. Mean
stability assay results for diets stored frozen for 14 and 35 days
ranged from 81-99%. Diets analyzed after being in the animals room
for 7 days were 17, 80, and 88%, respectively, of the nominal
levels. Mean values for homogeneity assay results were 80, 97, and
98%, respectively, of the nominal levels of 50, 500, and 1000 ppm.
The mean±SD of determined concentration of thiram was 40 ± 1, 483 ±
8, and 976 ± 15 ppm. The animals were observed daily for mortality
and signs of toxicity. Body-weights and food consumptions were
recorded weekly and haematology and clinical chemistry were
determined at study termination. Ophthalmic observations were done
before and at termination of study. Gross and histopathological
examinations from the control and 1000 ppm group and of macroscopic
lesions, lungs, liver, and kidneys from all 50 ppm and all 500 ppm
animals was carried out at study termination.
Body weights, cumulative body-weight gains, and food
consumption were significantly reduced throughout the study for both
sexes at 500 and 1000 ppm. Changes in clinical chemistry and
haematological parameters occurred at dose levels of 500 and 1000
ppm. The changes considered to be treatment-related were reduced
erythrocyte counts, haemoglobin and haematocrit in females;
increased MCV and MCH in both sexes; increased white blood cell,
corrected white blood cell, absolute neutrophil, absolute lymphocyte
and absolute monocyte counts in females; reduced total protein and
glucose in both sexes; reduced albumin and increased urea nitrogen
and chloride in females.
At 500 and 1000 ppm animals a tendency to reduced terminal
body-weights with correspondingly reduced absolute organ weights and
increased organ to body-weight ratios were observed.
Macroscopically, the non-glandular stomach in some animals showed
areas of erosion and the mesenteric lymph nodes were diffusely red
or mottled. Microscopically, the mucosa of the nonglandular stomach
had focal areas of erosion/ulceration, mucosal hyperplasia, or both,
accompanied by some submucosal inflammation and edema. These changes
appeared to be treatment-related. The mesenteric lymph nodes were
frequently congested but otherwise normal. The NOAEL in this study
was 50 ppm, equivalent to 2.5 mg/kg bw/day (Kehoe, 1988b).
Dogs
Two acclimated beagle dogs (5 months of age and weighing 5.6 to
8.5 kg/sex/group) were fed dietary concentrations of 0, 125, 500, or
2000 ppm thiram (purity 99.4%), equivalent to 0, 3, 13 or 50 mg/kg
bw/day for 4 weeks. All animals were observed daily for toxic signs
and mortality. Body-weight and food consumption was recorded weekly.
Clinical chemistry, gross and histopathological examinations were
performed on all animals. Homogeneity assays indicated from 94-96%
(mean values) of the nominal values. There were no data on
stability, but thiram was stored under similar conditions to that in
other studies where stability was satisfactory.
One 2000 ppm one female died during week 2 and one male became
moribund and was sacrificed during week 4. Therefore, the high-dose
of 2000 ppm was reduced to 1500 ppm during weeks 3 and 4. The
observations in the female were primarily colon changes associated
with the acute death and subsequent post-mortem change. The male had
only a few incidental macroscopic and microscopic findings and,
therefore, its death was not considered compound-related. At week 4,
body-weights for both sexes at 500 ppm were approximately 15% lower
and at 2000/1500 ppm, 33 and 39% lower than controls respectively.
Food consumption during week 4 for 125, 500, and 2000/1500 ppm males
were 12%, 29%, and 92% lower than controls respectively, and for
females 13%, 49%, and 78% lower. Treatment-related changes in
clinical chemistry and haematological parameters comprised reduced
erythrocyte counts, haemoglobin and haematocrit in males given 500
or 2000/1500 ppm. One male given 2000/1500 ppm had increased ALAT,
ASAT and ALP. Slightly reduced absolute lymphocyte counts and
slightly higher platelet counts, total bilirubin and urea nitrogen
occurred in some male and female dogs given 500 or 2000 ppm. The
surviving 2000/1500 ppm dogs sacrificed at week 4 had notably
reduced terminal body-weights and correspondingly reduced absolute
organ weights. The liver of the males had hepatocellular
degeneration with sinusoidal cell proliferation and pigmentation.
There were no major macroscopic or microscopic findings in the
remaining dogs. The NOAEL for the study was determined to be 125
ppm, equivalent to 3 mg/kg bw/day, based on the lower body-weights
and changes in clinical chemistry parameters (Kehoe 1988a).
In a 13-week study on 5 month old beagle dogs, four beagle
dogs/sex/group were fed dietary concentrations of 0, 75, 250, or 500
ppm thiram (purity 97.5%), equal to 0, 2.2, 6.9 or 12 mg/kg bw/day
in males and 0, 2.3, 7.3 or 13 mg/kg bw/day in females. The mean (±
SD) body-weight at study initiation was 7.4±0.6, 7.3±1.1, 7.4±0.5,
and 7.3±0.6 kg for males receiving 0, 75, 250, and 500 ppm. and were
6.8 ± 0.9, 7.1 ± 1.4, 6.3 ± 0.5, and 6.7±0.5 kg for females. All
animals were observed daily for clinical signs, moribundity and
mortality. Body-weight and food consumption was recorded weekly.
Ophthalmic examinations were performed prior to and at the end of
the study. Clinical chemistry measurements, haematological
examinations and gross and histopathology examinations were done on
all animals. Homogeneity of diets was within ± 8% of nominal values.
Mean stability assays of diets frozen for 7 days, stored in the
animal room for 1 day and then frozen for 4 weeks, were within ± 5%
of nominal levels.
Body weights for the 500 ppm dogs were significantly reduced.
Food consumption was significantly reduced in 500 ppm males and in
250 and 500 ppm females. Treatment-related effects for clinical
chemistry and haematological parameters were reduced erythrocyte
counts, higher MCV and MCH in 75, 250, and 500 ppm dogs; reduced
haemoglobin and haematocrit in 500 ppm females; higher platelet
counts in 250 and 500 ppm males; lower total protein and albumin in
75, 250, and 500 ppm males and females; and higher cholesterol in
250 and 500 ppm males and females. The NOAEL for this study was 75
ppm equal to 2.2 and 2.3 mg/kg bw/day in males and females
respectively, based on haematological changes noted in both sexes
(Kehoe, 1989a).
Six 4 to 5 month old beagle dogs/sex/group (initial weight 4.0
to 6.8 kg) were fed dietary concentrations of 0, 30, 90, or 250 ppm
thiram (purity 97.5%), equal to 0, 0.84, 2.6, and 7.4 mg/kg bw/day
in males and 0, 0.90, 2.5, and 7.2 mg/kg bw/day in females for 52
weeks. All animals were observed daily for clinical signs and
mortality. Body weights and food consumption were recorded weekly
for weeks 1 through 16, and monthly thereafter. Ophthalmic
examinations were performed prior to and at the end of the study.
Haematology, clinical chemistry, gross and histopathology were
performed on all animals. Mean values for homogeneity assays were
83, 92, and 92% of the nominal levels of 30, 90, and 250 ppm,
respectively. Mean stability assay results for diets kept below 0 °C
for 7 days and stored in the animal room for one day were 84%, 92%
and 96% of the nominal levels of 30, 90, and 250 ppm, respectively.
No ophthalmic lesions were observed in any animals. Erythrocyte
counts were reduced in males given 250 ppm, total protein was lower
and cholesterol was higher in males given 90 or 250 ppm and in
females given 250 ppm. Albumin was reduced in both sexes given 250
ppm. Absolute liver weights in males given 90 or 250 ppm were
significantly increased. Liver-to-body-weight ratios in males given
30, 90, or 250 ppm and in females given 250 ppm were also
significantly increased, as were liver-to-brain weight ratios in
males given 250 ppm. The significant increase in liver-to-body-
weight ratios in males given 30 ppm was due to a slight increase in
absolute liver weight and a slight decrease in body weight.
Furthermore, the observation that the body weights of males given 90
ppm were increased in relation to those of the controls and those of
males given 30 ppm demonstrates, that the slight decrease in body
weights seen in the 30 ppm group was spurious. Thus, the increase in
liver-to-body-weight ratio seen in males given 30 ppm was not
considered a test material-related effect. The changes in liver
weights, in conjunction with the altered total protein, albumin, and
cholesterol levels suggest a test material-related effect on liver
function and size. These changes were probably adaptive responses to
the presence of this compound. Based on increased absolute liver
weights and altered clinical chemistry parameters in males given 90
or 250 ppm, and altered clinical chemistry parameters in females at
250 ppm, the NOAEL for male dogs was 30 ppm, equal to 0.84 mg/kg
bw/day, and the NOAEL for female dogs was 90 ppm equal to 2.5 mg/kg
bw/day (Kehoe 1991a).
Four groups of 4 beagle dogs/sex/dose were treated orally via
gelatin capsule with thiram (purity 98.7%) at doses of 0 (blank
capsule), 0.4, 4, or 40 mg/kg bw/day for 104 weeks. Body weights and
food intake were measured weekly. Water consumption, haematology,
ophthalmoscopy, clinical chemistry and gross and histopathology of
all organs were performed.
Both sexes at 40 mg/kg bw/day showed severe toxic signs
including nausea, vomiting, salivation, and clonic convulsion and
severe anaemia within the first 11 weeks of treatment and were all
subjected to unscheduled necropsy before the 29th week of treatment.
The dogs also showed ophthalmological changes such as fundal
haemorrhage, miosis, and desquamation of the retina which were
consistent with retinal lesions observed histopathologically. In the
4 mg/kg bw/day group, nausea, vomiting and salivation were common
findings in both sexes and one female showed clonic convulsion from
week 37. Parameters including haematocrit, haemoglobin, and
erythrocyte count were depressed from week 4 in both sexes treated
with 4 or 40 mg/kg bw/day, indicating anaemia. Changes in
biochemical parameters indicating liver failure were observed from
week 4 in the 40 mg/kg bw/day males and later in both sexes dosed
with 4 or 40 mg/kg bw/day. Kidney damage was detected in the
histopathological examination in two female dogs in each of the 4
and 40 mg/kg bw/day groups. Histological lesions in the central or
peripheral nervous system relating to the observed neurological
disturbances were not found. It was concluded that no sex difference
in response to treatment was demonstrated. Based on the neurological
disturbances, anaemia and the effects on the liver, the NOAEL was
0.4 mg/kg bw/day (Maita et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Four groups of 50 acclimated (24 days) Charles River Crl:CD-
1R(ICR)BR mice/sex/dose level (approximately 7 weeks of age) were
fed thiram (97.5% purity) at dietary concentrations of 0, 15, 150,
or 300 ppm (males), equal to 3, 24 or 50 mg/kg bw/day and 0, 15,
300, or 600 ppm (females), equal to 3, 57 or 112 mg/kg bw/day for 97
weeks, when the most sensitive group showed 50% mortality.
Homogeneity assays indicated from 75-84%, 95-100%, 94-103%, and 101-
103% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively. Mean stability assay results for diets frozen for 6
days and stored in the animal room for one day were 55, 90, 90, and
97% of the nominal levels at 15, 150, 300, and 600 ppm,
respectively, demonstrating stability of varying acceptability.
Survival, clinical signs, body weights, food consumption, feed
efficiency, haematological parameters, necropsy, absolute and
relative organ weight and histopathological examination were
studied.
No substance-related oncogenic effects or adverse effects on
survival were observed in any test group. No changes attributable to
treatment were seen in any of the parameters evaluated in the 15 ppm
groups. There was no clinical indication of neurotoxicity at any
dose level. Mean body weight, weight gain, and total food
consumption were statistically significantly decreased in a dose-
dependent manner in the mid- and high-level groups beginning at
weeks 4-5. At study termination, mean weights were 7%, 15%, 14%, and
19% below control and mean total food consumption was 6%, 10%, 12%,
and 17% below control for the 150 ppm males, 300 ppm males, 300 ppm
females, and 600 ppm females, respectively. Increased frequency of
sores or reddened areas on the skin, generally on the ears,
consistent with bacterial dermatitis was noted for the 300 ppm males
and 600 ppm females. Mild but significant decreases in mean
erythrocyte count, haemoglobin, and haematocrit values were seen for
the 600 ppm females at week 97. Histopathology showed no evidence of
thiram-induced neoplasia. Non-neoplastic findings in the mid- and
high-level male and female mice consisted of retinal atrophy,
intracytoplasmic protein-like droplets in the urinary bladder
superficial transitional epithelium, and necrosis and suppurative
inflammation in the skin of the 150 and 300 ppm males and 300 and
600 ppm females; hyperkeratosis in the nonglandular stomach of the
300 ppm males and 300 and 600 ppm females; and increased pigment in
the spleen and decreased pigment in the inner adrenal cortex of the
300 and 600 ppm females.
No oncogenic effect was observed in doses up to 300 ppm, equal
to 50 mg/kg bw/day in males and 600 ppm, equal to 112 mg/kg bw/day
in females. Based on the body-weight reduction, the NOAEL for long-
term toxicity was 15 ppm, equal to 3 mg/kg bw/day (Trutter, 1992).
Rats
Four groups of 64 Jcl:Wistar rats were fed dietary
concentrations of 0, 3, 30, or 300 ppm thiram (purity 98.7%), equal
to 0, 0.1, 1.2 or 12 mg/kg bw/day for males and 0, 0.1, 1.4 or 14
mg/kg bw/day for females, for 104 weeks. Body weights, food intake,
and water intake were measured weekly up to week 52 and monthly
thereafter. Haematology, clinical chemistry, ophthalmoscopy and
gross and histopathology were performed on all rats. Test diets were
prepared twice weekly during the study. Data on homogeneity were not
available, but diets were stable up to 1 week.
Mortality of females in the 30 and 300 ppm groups was slightly
increased compared to controls during the last 8 weeks of treatment,
apparently correlated to an incidental higher occurrence of
pituitary tumours during this period. Overall incidence of pituitary
tumours in females was comparable to that in the control group.
Decreased body-weight gain and reduced food intake were observed in
both sexes at 300 ppm. Anaemia and regressive changes in the sciatic
nerve accompanied by atrophy of the calf muscle ( M. triceps surae)
were seen in the 300 ppm females. During the last 8 weeks of the
treatment period, the incidence of pituitary adenomas was increased
in females of the 30 and 300 ppm groups compared to that in
controls. Because of the small number of animals, statistical
analysis was not carried out. Statistical analysis of pituitary
tumour incidence in all animals at the end of the experiment showed
no significant differences. No evidence of carcinogenic potential
was observed. Based on mortality, anaemia, nerve degeneration and
muscle atrophy a NOAEL of 30 ppm, equal to 1.2 and 1.4 mg/kg bw/day
was determined in males and females, respectively (Miata et al.,
1991).
Four groups of sixty 36-day old Charles River Crl:CDR(SD)BR
rats/sex/group (initial weights 125-161 g for males and 115-193 g
for females) were fed dietary concentrations of 0, 30, 150, or 300
ppm thiram (purity 97.5%), equal to 0, 1.5, 7.3 or 15 mg/kg bw/day
in males and 0, 1.8, 8.9 or 19 mg/kg bw/day in females, for 104
weeks. Mean values for stability assays of diets kept below 0 °C for
6 days, animal room for one day, then below 0°C for 30 days were
15.5 ppm (52%), 123 ppm (82%), and 260 ppm (87%) for nominal
contents of 30, 150, and 300 ppm, respectively. The values for
homogeneity assay results ranged from 21.8-24.2 (73-81%), 128-138
(85-92%), and 255-276 ppm (85-92%) for nominal contents of 30, 150,
and 300 ppm, respectively. Cumulative means for the routine diet
analysis for dose confirmation were 16.3 (54%), 119(79%), and 262
ppm (87%) and the assay results ranged from 4.41-27.5 ppm, 81.8-151
ppm, and 179-320 ppm of the nominal dose levels of 30, 150, and 300
ppm, respectively, through 104 weeks. Data from a parallel study
with 14C-thiram stored under similar conditions (Hiles, 1989)
showed, that animals on a diet containing 30 ppm could be expected
to receive a systemic exposure to thiram and/or thiram-derived
materials approximately equal to the amount added to the diet, even
though the expected level could not be verified analytically.
Therefore, these data were considered to demonstrate that the
anticipated levels of thiram were achieved in the test diets. The
animals were observed daily for clinical signs and mortality. Body-
weight and food consumption were recorded weekly and haematology,
clinical chemistry, and urine analysis parameters were evaluated
during the study and at study termination. Ophthalmic observations
were done before and at termination of the study and gross and
histopathology examinations were carried out at study termination.
Clinical signs possibly test material-related for males
included swollen nose, soft faeces and opaque eyes. Soft faeces were
possibly test material-related at 150 and 300 ppm for the females.
Body-weight and cumulative body-weight gains in both sexes were
statistically significantly lower than those of the controls at 150
and 300 ppm. Food consumption was statistically significantly lower
than that in the controls for both sexes given 30, 150, or 300 ppm.
Reduced erythrocyte counts, haemoglobin, haematocrit and higher mean
corpuscular volume and mean corpuscular haemoglobin were observed in
females given 150 or 300 ppm. Statistically significant positive
trend analyses for hepatocellular adenomas (both sexes) and thyroid
C-cell adenomas (terminal sacrifice males and terminal plus
unscheduled sacrificed females) were reported but individual group
comparisons with controls were statistically insignificant. There
was no increase in the incidence of thyroid C-cell carcinomas.
Extramedullary haematopoiesis in the liver appeared to be increased
in males at 150 or 300 ppm and in females at 300 ppm. The biological
significance of this finding is unclear. Steatosis/fatty
infiltration of the pancreas (not uncommon in old SD rats) appeared
to be increased for males and females given 150 or 300 ppm. Also in
the pancreas, multifocal acinar atrophy was more common for males
given 150 or 300 ppm. The incidence of bile duct hyperplasia showed
a statistically significant positive trend in females, but was
statistically significantly increased only at 300 ppm. There were no
histopathological findings recorded that suggested test material-
related neurotoxicity.
Based on statistically significant decreases in body weights
and cumulative body-weight gains for males and females given 150 or
300 ppm, effects on haematological parameters in females given 150
or 300 ppm, and histopathological changes in males and females given
150 or 300 ppm, the NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg
bw/day in males and females, respectively. Thiram was not found to
be carcinogenic at doses up to 15 and 19 mg/kg bw/day in males and
females, respectively (Kehoe, 1991b).
Three groups of 50 five-week old Charles River SPF F344 rats,
initial mean body-weight of about 200 g for males and about 150 g
for females, were fed dietary concentrations of 0, 500 or 1000 ppm,
equal to 0, 18 or 39 mg/kg bw/day in males and 0, 20 or 42 mg/kg
bw/day in females for 104 weeks. Body weights and food consumption
were measured at regular intervals and haematology and clinical
chemistry were evaluated during the study and at study termination.
Gross and histopathology were performed. Homogeneity data and
stability data were not given.
Reduced body-weight gain and food consumption were seen at 500
and 1000 ppm in both sexes but especially in females of the high-
dose group. In males reduced liver function was observed.
Mononuclear cell leukaemia was found with an incidence of 20%, 8%,
and 4% in males and 29%, 12%, and 4% in females for 0, 500, and 1000
ppm animals, respectively. No tumour induction related to the
treatment was observed. It was concluded that thiram had no
carcinogenic effect at doses up to 39 and 42 mg/kg bw/day in males
and females, respectively (Hasegawa et al., 1988).
Reproduction studies
In a two-generation reproduction study, thiram (purity 97.6%)
was administered to Charles River Crl:CD VAF/Plus rats in dietary
concentrations of 0, 30, 60 or 180 ppm equal to 0, 1.5, 2.9 or 8.9
mg/kg bw/day in the F0 males and 0, 2.3, 4.6 or 14 mg/kg bw/day in
F0 females, and 0, 1.8, 3.8 or 11 mg/kg bw/day in the F1 males
and 0, 2.4, 5.1 or 16 mg/kg bw/day in the F1 females. Homogeneity
of thiram was satisfactory in the mixed diets. The test compound was
stable kept frozen for 7 days and reasonably stable (losses of 18%,
16%, and 9% in the 30, 60, and 180 ppm diet, respectively) kept at
room temperature for 12 hours. The mean ± SD percent of target ppm
found in all analyzed and administered 30, 60, and 180 ppm diets was
93 ± 6.3, 94 ± 6.6, and 98 ± 5.2%, respectively.The F0 and F1
parental generation consisted of 26 males and 26 females per group.
F0 animals were treated beginning at 63 days of age for 81 days
prior to the initial mating (F1a). A second litter (F1b) was
produced after a rest period of 16 days following weaning of the
F1a litter. Since conception rates were poor across all groups,
including the controls, in the F1b litter, an F1c litter was
also produced. The F1 parents were selected from the F1c
litters. These animals were treated beginning at 22 days of age for
a minimum of 84 days prior to their initial (F2a) mating. The F1
parents were mated twice to produce theF2a and F2b litters. All
parental animals, and pups were observed daily for mortality and
overt toxicity. Detailed clinical observations were recorded at
least once a week. Reproductive and litter parameters assessed
included male and female fertility indices, events at parturition,
gestation length, litter size, numbers of viable and stillborn pups,
and offspring survival and growth during lactation. All parental
animals, and all F1c weanlings not selected to remain on study and
all F2b weanlings were subjected to a gross necropsy. The F1a,
F1b, and the F2a offspring were euthanized and discarded
following weaning.
Mean maternal body weights and mean food consumption of the
F0 females were reduced at 60 and 180 ppm during the F1a
gestation period. Reductions in mean maternal body-weight and/or
food consumption were also observed at 180 ppm during the F1b and
F1c gestation periods and the F1a, F1b, and F1c lactation
periods. Similar reductions were observed for the F1 females at
180 ppm during the F2a and F2b gestation and lactation periods.
Reductions in mean weekly food consumption of the F0 males and
females were observed at 60 and 180 ppm. Mean offspring body weights
were consistently reduced to a significant degree in all litters
across both generations at the 180 ppm level. Apart from a
consistent statistically significant reduction in body weights in
the top dosed offspring during the F1b and F1c gestation periods
and the F1a, F1b, and F1c lactation periods, no substance-
related changes were found in the reproductive parameters or in the
parameters for post-natal development.
Based on the findings of parenteral systemic toxicity, the
NOAEL was 30 ppm, equal to 1.5 and 2.3 mg/kg bw/day in males and
females, respectively. With respect to filial systemic toxicity, the
NOAEL was 60 ppm, equal to 3.8 and 5.1 mg/kg bw/day in males and
females, respectively. With respect to reproduction and post-natal
development, the NOAEL was greater than 180 ppm equal to 8.9 and 14
mg/kg bw/day in males and females, respectively (York, 1991).
Special studies on genotoxicity
Data are shown in Table 2.
Special studies on teratogenicity
In a preliminary oral teratology study, groups of 6 Charles
River CD strain (Sprague-Dawley origin) rats (196-229 g) were
administered dose levels of 0, 5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 10 ml/kg bw) by gavage from day 6 through day 15 of
gestation. Control rats received the vehicle, 0.5% (W/V) CMC and
0.5% (W/V) Tween 80 in distilled water. Homogeneity and stability of
thiram in the suspensions were satisfactory (mean concentrations ±
SD of the 0.5, 1.0, 2.0, 4.0, 8.0, and 16 mg/ml thiram suspensions
were 0.5 ± 0.004, 0.97 ± 0.003, 1.96 ± 0.05, 3.94 ± 0.12, and 8.06 ±
0.25, and 16.6 ± 0.2 mg/ml, respectively). All females were killed
on day 20 of gestation for examination of uterine contents.
Table 2. Results of genotoxicity assays on thiram
Concentration
Test system Test object of thiram Purity Results Reference
In vitro
Ames test S. typhimurium 98.7% Poth (1990)
(±S9) TA 1535} +(±S9)
TA 100} 1-100 µg/plate (-S9) +(±S9)
TA 1537} 1-1000 µg/plate (+S9) -(±S9)
TA 1538} -(±S9)
TA 98 {10-1000 µg/plate (-S9) -(±S9)
{1-1000 µg/plate (+S9)
Gene mutation V79 Chinese 1-10 µg/ml (-S9) 100% -(±S9) Debets & Enninga
(±S9) hamster 10-56 µg/ml (+S9) (1986)
cells (HPRT-
locus)
Chromosome Chinese 0.003-0.023 µg/ml (-S9) 99.8% -(±S9) Putman (1987a)
aberrations hamster 0.2-1.5 µg/ml (+S9)
(±S9) ovary cells
-
DNA repair Primary rat 0.03-10 µg/ml 100% - Weterings (1985)
(test) hepatocytes
SCE induction human 5-25 µg/ml 98.6% + Perocco et al. (1989)
(with and without lymphocytes
S-9 mix)
Table 2 (cont'd)
Concentration
Test system Test object of thiram Purity Results Reference
In vivo
Mouse spot test mouse (NMRI) 75, 750 mg/kg 98.7% - Völkner (1991)
bw per os
Micronucleus test mouse (CD-1) 38-377 mg/kg 99.8% - Putman (1987b)
bw (i.p.)
Germ cell mouse (NMRI) 75-750 mg/kg 99.7% - Völkner (1990)
cytogenetic assay bw per os
Germ cell mouse (Swiss total doses n.g. (+) Prasad et al. (1987)
cytogenic assay albino) 80-320 mg/kg
(gavage)
n.g.: not given
A dose-related loss of body weight was seen at 20, 40, and 80
mg/kg bw/day. Treatment at 40 and 80 mg/kg bw/day produced a dose-
related increase in the total number of early and late resorptions
and a consequent increase in post-implantation loss and reduction in
viable litter size. It was concluded that the highest dose to be
used in a main teratology study in the rat should not exceed 40
mg/kg bw/day (Tesh et al. 1986).
Four groups of 25 female Charles River CD SD rats (193-236 g,
9-11 weeks old) were administered dose levels of 0 (vehicle), 7.5,
15 or 30 mg (99.0-99.8% purity) thiram/kg bw/day by gavage on day 6
through 15 of gestation. Pre-pairing acclimatization was for 5 days.
Test solutions (dosage volume, 10 ml/kg bw) were prepared in a 0.5%
w/v CMC and 0.5% w/v Tween 80 vehicle. All females were killed on
day 20 of gestation for examination of their uterine contents.
Analyses showed that the concentrations of test substance in the
test mixtures were 0.62-0.75 mg/ml, 1.47-1.49 mg/ml, and 2.88-2.91
mg/ml of intended concentrations of 0.75, 1.5, and 3.0 mg/ml,
respectively. The test substance was stable at room temperature
throughout the study. The concentration of tetramethylthiuram
monosulphide was less than 0.01%.
With the exception of a dosage-related incidence in the number
of females showing areas of hair loss on head, neck, back, and/or
limbs, the general condition of females was comparable in all
groups. At 15 and 30 mg/kg bw/day a transient, dose-related, loss of
body-weight was observed. At 7.5 mg/kg bw/day the rate of weight
gain was slightly, but significantly, reduced during the treatment
period, but subsequent weight gain was similar to that of the
controls. There were no adverse effects upon implantation or upon
fetal survival, but fetal and placental weights were significantly
reduced at 30 mg/kg bw/day. Placental weights were also slightly,
but significantly, reduced in the 7.5 and 15 mg/kg bw/day animals,
and foetal weight was slightly reduced at 15 mg/kg bw/day, but these
values were within historical control ranges. At 30 mg/kg bw/day
there was evidence of fetal immaturity e.g., reduced skeletal
ossification and increased incidence of space between the body wall
and organs, and there was a slightly increased incidence of
subcutaneous oedema. At 15 and 30 mg/kg bw/day the incidence of 13th
ribs of reduced size was slightly increased, but was not related to
dosage. Three foetuses with diaphragmatic hernia were observed, two
at 7.5 and one at 30 mg/kg bw/day. The NOAEL for fetal toxicity was
7.5 mg/kg bw/day. Reduced maternal body weight and placental weight
precluded the establishment of a NOAEL for maternal toxicity. The
toxic effects (immaturity and increased incidence of 13th ribs of
reduced size) at higher doses were considered a result of maternal
toxicity (Tesh et al., 1988a).
Rabbits
In a preliminary oral teratology study female New Zeeland white
rabbits (18-24 months old 3.4-4.5 kg) were artificially inseminated.
The day of insemination was designated day 0 of gestation. The
animals were allowed a minimum of three weeks acclimatization before
dosing. Thiram (purity 99.1%) was administered by gavage to 4
females per group from days 6-19, inclusive, of gestation at dosages
of 0 (vehicle), 1, 3, 5, 7.5, 10, 20, 40, or 80 mg/kg bw/day
(volume-dosage 5 ml/kg bw). Control animals received the vehicle
(the vehicle was 0.5% w/v aqueous CMC mucilage containing 0.5% w/v
Tween 80). Stability and concentration (89-106% of nominal) of the
test compound at room temperature was ensured by formulation of
fresh test solutions each day. On day 29 of gestation, the animals
were killed and their uterine contents examined.
Females receiving 10, 20, 40, or 80 mg/kg bw/day exhibited
marked body-weight loss during the treatment period. Eight females
(one on 20 mg/kg bw/day, three on 40 mg/kg bw/day and all four on 80
mg/kg bw/day) died or were killed in extremis. A further two deaths
(one control and one on 40 mg/kg bw/day) occurred but were not
attributed to treatment. Females receiving doses between 1 and 7.5
mg/kg bw/day showed slight reductions in their rate of body-weight
gains with some indication of a dosage relationship. Treatment at 20
mg/kg bw/day was associated with total litter resorption in 2
females and a marked increase in post-implantation loss in the one
female that carried a live litter to term. All 10 mg/kg bw/day
females carried their litters to term, but there was a slight
increase in post-implantation loss compared with the controls. At
7.5 mg/kg bw/day one female showed total litter loss, but in the
surviving two females post-implantation loss was similar to that of
the controls. It was concluded that dose-levels of thiram for use in
a main teratology study in the rabbit should not exceed 5 mg/kg
bw/day to prevent effect on survival in utero (Tesh et al.,
1987).
New Zeeland white rabbits 16-24 weeks of age and 3.7-4.8 kg
body-weight, were artificially inseminated. The day of insemination
was designated day 0 of gestation. The animals were allowed a
minimum of three weeks acclimatization before dosing. Thiram (purity
99.5%) was administered by gavage from day 6-19 of gestation
inclusive, at doses of 0, 1.0, 2.5 or 5.0 mg/kg bw/day (volume-
dosage 5.0 ml/kg bw). The vehicle was 0.5% (W/V) aqueous CMC
mucilage containing 0.5% (W/V) Tween 80. Stability and concentration
of the test compound at room temperature were satisfactory. The
concentration of tetramethylthiuram monosulphide was less than
0.03%. The number of control animals was 18+14 (initial controls +
additional controls from a parallel study due to unusually poor
pattern of body-weight gain, in part, to the performance of two
females). The number of test animals per group were 15, 18, and 20
for the 1.0, 2.5, and 5.0 mg/kg bw/day dose group, respectively. All
females were killed on Day 29 of gestation for examination. After
removal of the reproductive tract the following was recorded: number
of corpora lutea per ovary; number of implantation sites, number of
resorption sites; number and distribution of live and dead foetuses
in each uterine horn; weights and abnormalities of individual
fetuses and placentae, thorough examination including skeletal
examination of all fetuses.
The general condition of control and treated females was
essentially similar throughout the investigation. Eight females died
or were killed in extremis during the study (one in each of groups 1
and 2, two in group 3, and four in group 4). Necropsy findings
revealed evidence of respiratory or gastrointestinal tract disorder,
or accidental tracheal intubation. No evidence was apparent of any
involvement of thiram. The body-weight gain of females receiving 5.0
mg/kg bw/day was slightly but statistically significantly reduced.
Females receiving 1.0 or 2.5 mg/kg bw/day remained unaffected by
treatment with thiram. One control female and one 5.0 mg/kg bw/day
female aborted during the study. In addition, one female in each of
the groups receiving 2.5 or 5.0 mg/kg bw/day exhibited total litter
resorption. Litter parameters were unaffected by treatment with
thiram. Survival, growth and morphological development in utero
were essentially unaffected by treatment. The NOAEL for maternal
toxicity was 2.5 mg/kg bw/day and the NOAEL for embryofetal toxicity
was 5.0 mg/kg bw/day. There was no evidence of teratogenic potential
(Tesh et al., 1988b).
Five-month old New Zeeland white SPF female rabbits were
acclimatized and artificially inseminated. Groups of 20 inseminated
rabbits were dosed once daily by oral gavage with 0, 1.0, 5.0, or 10
mg/kg bw/day (dose volume 3.0 ml/kg bw) thiram solved in 0.5% Tween
80 and 0.5% low-viscosity CMC on gestation days 7 through 19.
Homogeneity mean values ranged from 107-108% of target and the
relative standard deviations were < 4.5%. Stability at room
temperature for 10 days indicated 95-99% of the initial prepared
concentrations. Caesarean sections were performed on all surviving
females on gestation day 29, followed by teratologic examination of
the fetuses. Individual fetuses were weighed, sexed, tagged and
examined for external malformations and variations. Each fetus was
dissected internally, sexed and examined for visceral malformations
and variations. The head was examined using multiple coronal slices.
Skeletal examination was carried out after Alizarin Red S staining.
A single death observed in the 10 mg/kg bw/day group was
attributed to gavage injury from dosing and two deaths in the
control group were attributed to pneumonia. No treatment-related
adverse findings were observed with regard to clinical signs or
necropsy findings of animals surviving the scheduled euthanasia.
Statistically significant increases in body-weight gain and food
consumption were observed in the treated groups when compared with
the control values. These increases were not considered to be
adverse effects of the test article. No treatment-related maternal
effects were observed at cesarean section examination, and no
adverse effects on fetal development were observed at the
teratological examination. The NOAELs for maternal toxicity,
embryofetal toxicity and teratogenicity were higher than 10 mg/kg
bw/day (York, 1992).
Special studies for neurotoxicity
Data from special neurotoxicity testing of thiram were not
available. Some endpoints related to neurotoxicity have been
investigated in the standard toxicity testing procedures reported
above.
Observations in humans
Workers, surgeons and other personnel using rubber gloves might
get contact dermatitis due to the presence of thiram in the rubber
(Lisi et al., 1987). Oral exposure to thiram might lead to dermal
sensitization to the substance (Goitre et al., 1981).
COMMENTS
Following oral administration to rats, thiram was well absorbed
(>83%) and eliminated via the expired air (41-48%), urine (25-40%),
and faeces (2-5%). About 3% was recovered in various organs. The
majority of the dose (84-90%) was eliminated within four days after
dosing.
The metabolism of thiram was studied in rats. During the first
five hours after administration, a dose-dependent formation of
carbon disulphide was demonstrated in the expired air. Metabolites
detected in urine included polar oxidation products and conjugates.
The acute oral toxicity of thiram is low in mice and rats. The
World Health Organization has classified thiram as slightly
hazardous (WHO, 1992).
A 13-week dietary study in rats at levels of 0, 50, 500 or 1000
ppm resulted in changes in haematological and serum biochemical
parameters and gastric irritation at 500 and 1000 ppm. The NOAEL was
50 ppm, equivalent to 2.5 mg/kg bw/day.
Beagle dogs received thiram as a dietary admixture at levels of
0, 75, 250 or 500 ppm for 13 weeks or at levels of 0, 30, 90 or 250
ppm for 52 weeks. The NOAELs were 75 ppm (equal to 2.2 and 2.3 mg/kg
bw/day in males and females, respectively, for the 13-week study)
and 30 ppm (equal to 0.84 mg/kg bw/day) in males and 90 ppm (equal
to 2.5 mg/kg bw/day) in females in the 52-week study on the basis of
changes in body weight, increased absolute and relative liver
weights, and changes in haematological and serum biochemical
parameters. In another study, dogs received thiram in gelatin
capsules at doses of 0, 0.4, 4 or 40 mg/kg bw/day 7 days/week for
104 weeks. Nausea, vomiting and salivation, ophthalmological
effects, convulsions, changes in haematological parameters, and
renal changes were observed at 4 and 40 mg/kg bw/day. On the basis
of the described effects, the NOAEL was 0.4 mg/kg bw/day. Since
thiram was administered in capsules in this experiment and
significantly less information was available on the study conditions
compared to those in the former two experiments with dietary
administrations of thiram this NOAEL was not used as the basis for
the estimation of an ADI.
In a 97-week oncogenicity study in mice using dietary thiram
concentrations of 0, 15, 150 or 300/600 ppm, the effects included
dose-dependent decreases of food consumption and body weight gain
and changes in haematological parameters. Non-neoplastic findings
included retinal atrophy, changes in the urinary bladder and in the
skin, hyperkeratosis in the non-glandular stomach, and increased
pigmentation in the spleen. Thiram was not carcinogenic in mice. The
NOAEL for long-term toxicity in male and female mice was 15 ppm,
equal to 3 mg/kg bw/day.
In a two-year toxicity study in rats at dietary concentrations
of 0, 3, 30 or 300 ppm, the NOAEL was 30 ppm, equal to 1.2 and 1.4
mg/kg bw/day in males and females, respectively. It was based on
lower red blood cell count, haemoglobin and haematocrit levels and
degenerative changes of the sciatic nerve accompanied by atrophy of
the gastrocnemius muscle at 300 ppm.
In a second two-year long-term toxicity/carcinogenicity study
in rats at dietary concentrations of 0, 30, 150 or 300 ppm, dose-
dependent lower erythrocyte counts, haemoglobin and haematocrit
levels were observed. Based on these haematological changes, the
NOAEL was 30 ppm, equal to 1.5 and 1.8 mg/kg bw/day in males and
females, respectively.
In a 2-year carcinogenicity study in rats at dietary
concentrations of 0, 500, or 1000 ppm (equal to 39 and 42 mg/kg
bw/day in males and females, respectively) there was no evidence of
carcinogenicity. The Meeting concluded that thiram was not
carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 30, 60 or 180 ppm, no adverse effects on
reproduction were observed. The NOAEL for reproductive effects was
> 180 ppm (equal to > 8.9 and > 14 mg/kg bw/day in males and
females, respectively). The NOAEL for systemic toxicity was 30 ppm
(equal to 1.5 and 2.3 mg/kg bw/day in males and females,
respectively). This NOAEL was based upon reduction in body-weight
and/or food consumption in both parental and offspring animals.
An oral teratogenicity study was performed in rats at gavage
dose levels of 0, 7.5, 15 or 30 mg/kg bw/day. A NOAEL for maternal
toxicity was not determined due to a dose-dependent decrease in body
weight gain and placental weight at all dose levels. Teratogenicity
was not observed.
In an oral teratogenicity study in rabbits at gavage doses of
0, 1.0, 2.5 or 5.0 mg/kg bw/day, a NOAEL of 2.5 mg/kg bw/day for
maternal toxicity was based on a dose-dependent reduction of body-
weight gain. Teratogenicity was not observed.
An oral teratogenicity study was carried out in rabbits at
gavage dose levels of 0, 1.0, 5.0 or 10 mg/kg bw/day. The NOAEL for
maternal toxicity was higher than 10 mg/kg bw/day. Teratogenicity
was not observed.
Thiram was mutagenic in the Ames test but not in mammalian
cells in vitro. Since thiram was not mutagenic in vivo, the
Meeting concluded that it did not present a genotoxic hazard for
humans.
The central and peripheral nervous systems have been recognized
as a possible target for thiram toxicity. The neurotoxicity may be
related to the thiram metabolite carbon disulphide.
An ADI was allocated, based on the 1-year study in dogs and the
2-year studies in rats, using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 15 ppm, equal to 3 mg/kg bw/day (97-week study)
Rat: 30 ppm, equal to 1.2 mg/kg bw/day (two-year study)
30 ppm, equal to 1.5 mg/kg bw/day (two-generation
reproduction study)
Rabbit: 2.5 mg/kg bw/day (teratology study, maternal
toxicity)
Dog: 30 ppm in the diet, equal to 0.84 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
1. Clarification of the potential for neurotoxicity of
thiram.
2. Observations in humans.
REFERENCES
Beauchamp, R.O., Bus, J.S., Popp, J.A., Boreiko, C.J. & Golberg, L.
(1983) A critical review of the literature on the carbon disulphide
toxicology. CRD Crit. Rev. Toxicol., 11: 169-272.
Dalvi R.R. (1987) Dose-dependent liver toxicity of thiram
administered intraperitoneally to rats. J. Environ. Biol., 8: 25-
31.
Dalvi, R.R. & Deoras, D.P. (1986) Metabolism of a dithiocarbamate
fungicide thiram to carbon disulphide in the rat and its hepatotoxic
implications. Acta Pharmacol. Toxicol., 58: 38-42.
Debets, F.M.H. (1985) Evaluation of the acute inhalation toxicity of
TMTD technical in the rat. Unpublished report no.: 0113/232 from
NOTOX v.o.f. s-Hertogenbosch, the Netherlands. Submitted to WHO by
UCB.
Debets, F.M.H. & Enninga, I.C. (1986) Evaluation of the mutagenic
activity of TMTD technical in an in vitro mammalian cell gene
mutation test with V79 Chinese hamster cells. Unpublished report No.
NOTOX 0174/EV 1 from NOTOX C.V. DD 's-Hertogenbosch, Netherlands.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Gay, M.H. (1987) Rat metabolism of 14C thiramTM, single dose
study. Unpublished report No. 87003B from Biotek, Inc.,
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Goitre, M., Bedollo, P.G., & Cane, D. (1981) Allergic dermatitis and
oral challenge to tetramethylthiuram disulphide. Contact Derm., 7:
272-273.
Hasegawa, R., Takahashi, M., Furukawa, F., Toyoda, K., Sato, H.,
Junejang, J. & Hayashi, Y. (1988) Carcinogenicity study of
tetramethylthiuram disulphide (thiram) in F334 rats. Toxicology,
51: 155-165.
Hiles, R.A. (1989) Bioavailability study in male rats with a 14C-
thiram-treated diet. Unpublished report No. HLA 6111-131 from
Hazleton Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988a) Four-Week range-finding study with thiram in
dogs. Unpublished report No. HLA 6111-109 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1988b) Thirteen-Week toxicity study with thiram in
rats. Unpublished report No. HLA 6111-110 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1989a) 13-Week toxicity study with thiram in dogs.
Unpublished report No. HLA 6111-121 from Hazleton Laboratories,
Inc., Wisconsin, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Kehoe, D.F. (1989b) 4-Week dietary range-finding study with thiram
in mice. Unpublished report No. HLA 6111-127 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991a) 52-Week dietary chronic toxicity study with
thiram in dogs. Unpublished report No. HLA 6111-112 from Hazleton
Laboratories, Inc., Wisconsin, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Kehoe, D.F. (1991b) 104-Week combined chronic toxicity and
carcinogenicity study with thiram in rats. Unpublished report No.
HLA 6111-113 from Hazleton Laboratories, Inc., Wisconsin, USA.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Lee, C.C. et al. (1975) Toxicological evaluation of ferric
dimethyldithiocarbamate (ferbam) and dithiocarbamate (thiram) with
acute toxicity of manganese and zinc ethylenebisdithiocarbamates
(maneb and zinc). Final report, MRI project no.: 36123-B. Submitted
to WHO by UCB.
Lee, C-C. & Peters, P.J. (1976) Neurotoxicity and behavioral effects
of thiram in rats. Environmental Health Perspectives, 17: 35-43.
Lee, C.C. et al. (1978) Oral toxicity of ferric
dimethyldithiocarbamate (ferbam) and tetramedthylthiuram disulfide
(thiram) in rodents. J. toxicol. environ. health, 4, 93-106.
Submitted to WHO by UCB.
Lisi, P., Caraffini, S. & Assalve, D. (1987) Irritation and
sensitization potential of pesticides. Contact Derm., 17: 212-218.
Maita, K., Tsuda, S. & Shirasu, Y. (1991) Chronic toxicity studies
with thiram in wistar rats and beagle dogs. Fundamental and Applied
Toxicol., 16: 667-686.
McManus, J.P. (1991) Metabolism of [14C] thiram in the rat:
Urinary metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Middlebury, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Matthiasck, L. (1973) Uber den Einfluss von L-cystein auf die
Teratogenese durch Thiram (TMTD) bei NMRI-Mausen. Arch. Toxikol. 30,
251-262. Submitted to WHO by UCB.
Musacchio, J.M., Goldstein, M., Anagnoste, B. Poch, G., & Kopin L.J
(1966) Inhibition of dopamine beta-hydroxylase by disulfiram in
vivo. J. Pharmacol. Exp. Ther., 152: 56-61.
Nomeir, A.A. & Markham, P. (1990) Disposition and metabolism of
thiram in rats after pretreatment with thiram for 14 days.
Unpublished report No. ADL 65492 from Arthur D. Little, Inc.
Massachusetts, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Norris, K.J. (1989) Determination of volatile 14C-residues from
rats orally administered 14C-thiram. Unpublished report No. 1113A
from Analytical Development Corp., Colorado Springs, USA. Submitted
to WHO by UCB Chemicals, Brussels, Belgium.
Perocco, P., Santucci, M.A., Campani, A.G. & Forti, G.C. (1989)
Toxic and DNA-damaging activities of the fungicides mancozeb and
thiram (TMTD) on human lymphocytes in vitro. Teratogenesis,
carcinogenesis, and mutagenesis., 9: 75-81.
Poth, A. (1990) Salmonella typhimurium reverse mutation assay with
thiram. Unpublished report No. CCR 175116 from CCR Cytotest Cell
Research GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
Prasad, H., Pushpavathi, K., Rita, P. & Reddy, P.P. (1987) The
effect of thiram on the germ cells of male mice. Fd. Chem. Toxic.
25: 709-711.
Putman, D.L. (1987a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. Unpublished report No. T5558.337 from Microbiological
associates, Inc., Maryland, USA. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Putman, D.L. (1987b): Micronucleus cytogenetic assay in mice.
Unpublished report No. T5558.122 from Microbiological associates,
Inc., Maryland, USA. Submitted to WHO by UCB Chemicals, Brussels,
Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R. & Higgins, C. (1986)
Thiram: Effects of oral administration upon pregnancy in the rat,
preliminary teratology study. Unpublished report No. 86/TRK001/704
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W. & Crisp, V.C. (1987) Thiram: Preliminary
teratology study in the rabbit. Unpublished report No. 87/TRK003/122
from Life Science Research, Suffolk, England. Submitted to WHO by
UCB Chemicals, Brussels, Belgium.
Tesh, J.M., McAnulty, P.A., Willoughby, C.R., Higgins, C., Wilby,
O.K. & Tesh, S.A. (1988a) Teratology study in the rat. Unpublished
report No. 87/TRK002/179 from Life Science Research, Suffolk,
England. Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Tesh, J.M., Ross, F.W., Crisp, V.C., Wilby, O.K. & Tesh, S.A.
(1988b) Thiram: Teratology study in the rabbit. Unpublished report
No. 87/TRK004/541 from Life Science Research, Suffolk, England.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
Thouin, M.H. (1985a) Evaluation of the acute oral toxicity of TMTD
technical in the rat. Unpublished report no.: 0174/238 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Thouin, M.H. (1985b) Evaluation of the acute oral toxicity of TMTD
technical in the rabbit. Unpublished report no.: 0113/211 fron NOTOX
v.o.f., s-Hertogenbosch, the Netherlands. Submitted to WHO by UCB.
Trutter, J.A. (1992) Oncogenicity study in mice with thiram.
Unpublished report No. 798-223 from Hazleton Laboratories, Inc.,
9200 Leesburg Turnpike, Vienna, Virginia 22182, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
Weterings, P.J.J.M. (1985) Evaluation of the DNA repair inducing
ability of TMTD technical in a primary culture of rat hepatocytes.
Unpublished report No. NOTOX 0174/ER156 from NOTOX C.V. DD 's-
Hertogenbosch, Netherlands. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1990) Mouse germ-cell cytogenetic assay with thiram.
Unpublished report No. CCR 175127 from CCR Cytotest Cell Research
GmbH & Co. KG, Rossdorf, Germany. Submitted to WHO by UCB Chemicals,
Brussels, Belgium.
Völkner, W. (1991) Mutation assay in somatic cells of the mouse
(mouse spot test) with thiram. Unpublished report No. CCR 200902
from CCR Cytotest Cell Research GmbH & Co. KG, Rossdorf, Germany.
Submitted to WHO by UCB Chemicals, Brussels, Belgium.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
York, R.G. (1991) Two-generation reproduction study in rats.
Unpublished report No. 399-104 from International Research and
Development Corporation, Michigan, USA. Submitted to WHO by UCB
Chemicals, Brussels, Belgium.
York, R.G. (1992) Development toxicity study in New Zealand
white rabbits. Unpublished report No. 399-121 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by UCB Chemicals, Brussels, Belgium.
