
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
CAPTAN
IDENTITY
Chemical name
N-(trichloromethylthio)-3a,4,7,7a-tetrahydrophthalimide (IUPAC)
Synonyms
N-(trichloromethylthio) cyclohex-4-one-1,2-dicarboxyimide
Structural formula
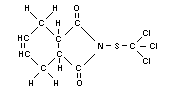 Other relevant chemical properties
The technical product is claimed to contain 96 percent captan; the
remainder consists of common salt (sodium chloride), water and
unreacted tetrahydrophthalimide. The pure crystal ins product, m.p.
178° C, is reported to have a vapour pressure of less than 1 × 10-6
torr at 25° C, but that of the technical product is stated to be much
higher. Solubility in water at normal temperatures is extremely low;
it is less than 10 percent in common organic solvents. Captan is
stable, except under alkaline conditions, at normal temperature; it
will decompose at temperatures in the region of 100° C, very slowly in
dry or rapidly in moist atmospheres (Klayder, 1963). In alkaline
solution, 1.0N NaOH, captan yields 2.8 equivalents of chloride ion.
Cooking, especially in water would lead to rapid decomposition of
residues to water-soluble products.
Formulated products include wettable powders containing 50 to 83
percent a.i. and dusts, those for field use containing a few percent
a.i. and those for seed treatment 75 Percent.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
This pesticide was evaluated for acceptable daily intake by the 1965
Joint FAO/WHO Meeting on Pesticide Residues (FAO/WHO, 1965). Since
that time additional information has become available, especially on
the metabolism and on the effect upon reproduction. Therefore, the
previously published monograph has been revised and is now reproduced
in its entirety.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Captan is rapidly decomposed in both human and rabbit blood at room
temperature. Decomposition appears to be according to first order
kinetics although some concentration dependency is evident, possibly
due to the low solubility of captan in aqueous media. At initial
concentrations of 100 µg/ml or 1/µg/ml in human blood the half-life of
captan was calculated as 0.9 minutes or 0.2 minutes respectively. In
rabbit blood at an initial concentration of 1 µg/ml, the half-life is
0.3 minutes (Crossley, 1967).
Captan, as an aqueous slurry, was administered by intragastric
intubation to female rabbits at levels of 0 mg/kg body-weight (two
rabbits) or 500 mg/kg body-weight (five rabbits). Blood was drawn
directly from the heart at intervals up to 56 hours and analysed for
captan and its metabolite tetrahydrophthalimide. No captan was
detected in the blood at any time after administration of the dose,
using an analytical method sensitive to 0.025 µg/ml. A build-up of the
metabolite tetrahydrophthalimide occurred in the blood which reached a
maximum of about 25 µg/ml 30 hours after intragastric administration
of captan. From then on it decreased to a level of 4.2 µg/ml after 56
hours; the estimated half-life being 6 hours (Crossley, 1967).
Recent information on the metabolism of captan has been inferred from
work on captafol, a compound which differs from captan only in the
nature of the chlorinated group attached to the sulphur atom (see the
monograph on captafol). Rats were fed celery which had been treated
with captafol to give levels of 60 or 600 ppm. The stomach content of
the animals was analysed at several intervals and both
tetrahydrophthalimide and tetrahydrophthalic acid were detected
(Leary, 1966).
Rats, dogs and monkeys wore fed carbon14-carbonyl labelled captafol.
The radioactivity rapidly entered the blood and tetrahydrophthalimide
was detected in the blood, faeces and urine. Tetrahydrophthalamic acid
as well as other more water soluble metabolites were the principal
radioactive compounds present in the blood and urine. Because of the
similarity in structure it has been assumed that captan would be
metabolized in a similar fashion with respect to the
tetrahydrophthalic acid portion of its molecule (Dye, 1969).
Feeding studies with captan demonstrated that captan is not stored in
the eggs or flesh of poultry nor in the tissues of pigs (Weir, 1957;
Link et al., 1956).
Effect on enzymes and other biochemical parameters
In the presence of compounds such as cysteine which contain sulfhydryl
groups, all the three chlorine atoms in captan are liberated an
chloride ions, and four sulfhydryl groups disappear for every molecule
of captan that reacts. In general captan initially reacts with two
molecules of a simple thiol to give tetrahydrophthalimide, the
disulfide derived from the thiol, thiophosgene and one chloride ion.
The thiophosgene reacts with two additional molecules of the thiol, to
give ultimately two more chloride ions, carbon disulfide and the
sulfide derived from the thiol. In the case where cysteine is the
thiol the reaction is slightly more complicated resulting in the
formation of a substituted thiazolidinethione. The presence of all
these compounds from the metabolism of captan, has been confirmed
chemically (Owens, 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
A three-generation reproduction study was conducted with rats which
received technical captan in their diet in concentrations of 0, 100,
500, and 1000 ppm. Groups of 16 female animals were used and two
litters were produced in each generation. No significant differences
were found between control and captan treated rats with respect to
fertility, gestation, viability or lactation indices, or in the
weaning weights in the first two generations. No effects were observed
in the third generation except for a slightly lowered lactation index
for the group receiving 1000 ppm of captan. Histopathological
examination of tissues from 10 pups receiving 1000 ppm in the third
generation revealed no damage (Kennedy, 1966; Kennedy, et al., 1968).
Special studies on teratogenicity
Chicken
A group of 5 male and 18 female White Leghorn chickens were fed a diet
containing 0 or 2,300 ppm (equivalent to 75 mg/kg body-weight)
technical captan for 6 weeks. The birds were observed for body-weight
effects, food consumption, behavioural reaction, and egg production.
Eggs collected during days 29 through 39 were incubated to determine
the extent of hatchability and the presence of any effects on the
chicks. No adverse effects were noted (Palazzolo, 1966).
Captan was injected in dimethylsulfoxide solution into either the yolk
or air cell of fresh fertile White Leghorn eggs in amounts sufficient
to produce concentrations of 3 to 20 ppm in the eggs. The eggs were
incubated and non-viable embryos and hatched chicks were examined for
gross abnormalities. In a total of 1,292 eggs, the incidence of
malformations was 7.8 percent compared to an incidence of less than 2
percent in solvent-injected and uninjected control eggs. Malformations
of the feet and legs followed a specific pattern. Micromelia, amelia,
and phocomelia accounted for most of the deformities (Verrett, at al.,
1969).
Hamster
Groups, each comprising 20 pregnant female hamsters ware fed diets
containing concentrations of captan sufficient to enable the animals
to receive an average daily intake of 0, 125, 250, or 1000
mg/kg/body-weight of captan from days 1 through 15 of gestation. At
day 159 all females were sacrificed, the young were surgically removed
and the foetal development and structural formation of each was
examined. The incidence of abnormal effects was not greater in any
test group than in the controls. The dose level of 1000
mg/kg/body-weight of captan resulted in a significant increase in
foetal resorption (Kennedy, at al., 1968).
Monkey
Groups of seven pregnant Rhesus monkeys were given daily oral doses of
6.35, 12.5 or 25 mg/kg body-weight of captan on days 22 through 32 of
gestation. (Thalidomide was used as a positive control at dosage
levels of 5 mg/kg/body-weight/day in six animals and at 10
mg/kg/body-weight/day in four animals). Foetuses were recovered on
approximately day 84 of gestation by Caesarian section and examined
for organ and skeletal defects. Foetal mortality occurred in three of
seven monkeys at the 25 mg/kg level. The foetal mortality in the
parent colony not fed captan was 13.2 percent on 439 conceptions.
There was no abnormality among any foetus in either of the three dose
levels of captan (Courtney, 1968).
Groups of seven pregnant monkeys (Rhesus and stumptail) were given
captan orally at levels of 10, 25 or 75 mg/kg body-weight daily on
days 21 through 34 of gestation. One abortion occurred at the 75 mg/kg
level but there were no malformations. (Thalidomide was given as a
positive control an in the previous experiment (Vondruska, 1969).
Rabbit
Four pregnant New Zealand White rabbits were given daily oral doses of
80 mg/kg bodyweight of captan during days 7 through 12 of gestation.
Captan produced no embryotoxicity in the litters of rabbits (Fabro at
al., 1965).
A group of six pregnant Dutch Belted rabbits was given 75
mg/kg/body-weight/day of technical captan, orally on days 6 through 16
of gestation. Three other groups each containing five to seven New
Zealand White rabbits were given 18.75, 37.5 or 75
mg/kg/body-weight/day of technical captan orally on days 6 through 18
of gestation. A control group and a positive control group (75 mg/kg
thalidomide) wore also maintained. No malformed foetuses occurred in
any group treated with captan. An increased incidence of foetal
resorption occurred in the New Zealand White rabbits given 75 mg/kg of
captan. Another group of nine pregnant Dutch Belted rabbits were given
75 mg/kg/body-weight/day of technical tetrahydrophthalimide, a
metabolite of captan, on days 6 through 16 of gestation. A slight rise
in the occurrence of resorption sites occurred. No skeletal
abnormality was observed (Kennedy, et al., 1968).
Groups of 9 pregnant New Zealand white rabbits were given captan at
dose-levels of 37.5, 75 or 150 mg/kg body-weight/day from days 6
through 16 of gestation. Thalidomide was used as a positive control at
levels of 75 and 150 mg/kg body-weight and produced the expected
teratological response. Captan at 75 mg/kg caused 9 malformed young
from 75 implantations of 9 pregnant does. At the dose level of 37.5
mg/kg captan produced one malformed foetus from 49 implantation sites
(McLaughlin at al., 1969).
Rat
Groups of five to ten pregnant female rats were given oral doses of 0,
50, 100, or 250 mg/kg/body-weight day of technical captan from days 6
through 15 of gestation or 0, 500, 1000 or 2000 mg/kg/body-weight/day
from days 8 through 10. Examination of 371 foetuses obtained from the
captan-treated rats revealed no significant increase in the number of
abnormalities. Three and two grossly malformed foetuses were found
from the rats treated with 1000 and 2000 mg/kg/body-weight/day of
captan respectively; compared with one in the corn oil control and
none in the lower doses of captan (Kennedy, et al., 1968).
Special studies on mutagenicity
Mouse
Single intraperitoneal injections of technical captan at doses of 0,
3.5 and 7.0 mg/kg body-weight were administered to an unspecified
number of male mice. Another group of male mice was given 100 mg/kg
body-weight of methyl methanesulfonate as a positive control. The
treated male groups were mated with three groups of untreated females
on each of three consecutive weeks post-treatment. The results
demonstrated that dominant lethal mutations were not induced by
treatment with captan (Arnold, 1967).
The mutagenic activity of captan was evaluated in bacteria, in the
heteroploid human embryo lung call line, and in a cell line derived
from the kidney of the rat-kangaroo. In bacteria, captan increased the
mutation rate both in streptomycin-dependent E. coli and a
thymine-dependent E. coli strain. Captan inhibited both growth and
mitosis of heteroploid embryonic lung cells in concentrations of less
than 5 µg/ml. In the studies with the rat-kangaroo cell line the
chromosome breaks and mitotic inhibition were proportional to the
concentration of captan over the range of 1 to 10 µg/ml (Legator, et
al., 1969).
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
captan from 7 days of age for 18 months. The dose of 215 mg/kg
body-weight was given to the mice daily by gavage from the seventh day
of age to the time of weaning at four weeks of age; thereafter, the
chemical was added to the diet in the corresponding amount of 560 ppm.
There was no significant increase in tumours in the group fed captan
compared with the controls (Innes, et al., 1969).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat oral 9,000 Elsea, 1957
Rat oral 12,500 Boyd and Krijnen, 1968
Rat oral 480 (low
protein diet)
Mouse i.p. 10 Arnold, 1967
Short-term studies
Chicken
An unspecified number of chicks were fed a diet containing 320 ppm of
captan for 28 days. In another experiment a group of 30 chicks was fed
the same dosage for 74 days along with a control group of 10 chicks.
No gross abnormalities were found in the birds in either experiment
(Ackerson and Mussehl, 1953; Link et al., 1956).
Groups of 15 hens were fed diets containing 0, 100, 1000, or 10,000
ppm of technical captan for 90 days. The hens receiving 100 and 1000
ppm of captan displayed normal food consumption, egg production, and
survival. In those receiving 10,000 ppm there was food-refusal, weight
loss, and decreased egg production. There were no gross effects
observed at autopsy in any cup. Analysis of the eggs and of the
tissues of the hens revealed no stored captan (Weir, 1957).
Dog
Groups of four dogs, each comprising two male and two female animals,
were started on a dose regime of 0, 10, 25 and 50 mg/kg body-weight of
captan. At the beginning of week 10 the dose level of the dogs
receiving 25 mg/kg was increased to 100 mg/kg. At week 18 these dose
levels were again increased to 100 mg/kg and 300 mg/kg respectively.
The captan was administered by gelatin capsule normally six days a
week for 66 weeks. Liver and kidney weights were slightly increased in
the dogs which received 300 mg/kg body-weight. There was no evidence
of systemic toxicity and no gross or histopathological changes in
tissues due to treatment at any dose-level, nor were there any
significant changes in haematological or biochemical findings
(Fogleman, 1955).
Pig
Eight half-grown pigs were fed for three months on corn treated with
captan to provide a level of 540 ppm. The treatment did not affect the
rate of food consumption, rate of growth or general health of the
animals when compared to the effect on an equal number of control
animals. There was no gross abnormality observed in any animal on the
captan diet (Batter 1953).
Four groups, comprising 10 weanling pigs each of about 14 kg in
weight, were fed rations containing 0, 420, 820, 1,680 ppm of captan
for 119 days. No gross pathological effects that could be attributed
to captan were observed (Meads and Warner, 1954).
Three pigs were fed 500 ppm and two pigs were fed 4000 ppm of captan
in the diet for 22-25 weeks. The animals displayed no abnormal
symptoms, but no further observations were made (Johnson, 1954).
Seven pigs were fed 480 ppm of captan in their diet for 14 weeks,
three other animals served as controls. The test animals displayed
normal weight gain. Gross examination of all organs revealed no
pathological changes and histopathological study of the liver and
kidneys revealed no abnormalities. Erythrocyte and lenkocyte counts
were not significantly different between test and control groups. No
residual captan (i.e. <0.1 ppm) was found in the tissues of the
animals (Link at al., 1956).
Rat
Five groups, each containing 11 male and 11 female rats, were fed
diets containing either technical or recrystallized captan at dose
levels up to 0, 5000 or 10,000 ppm for 13 weeks. All experimental
groups were started at a level of 500 ppm of captan and the dose
levels were gradually increased until the 5000 ppm level was reached
after four weeks and the 10,000 ppm level after seven weeks. Both dose
levels caused growth retardation; however, there was no difference
between comparable groups of rate receiving the technical and
recrystallized material (Gray, 1954).
Long-term studies
Rat
Groups, each containing 10 male and 10 female rats, were fed diets
containing 0, 1000 and 5000 ppm of technical captan for two years.
Another group of 20 rats received 10,000 ppm of technical captan for
24 weeks. This group was then divided in half, one half being fed
recrystallized captan for 30 weeks; the other half continued on the
technical compound for 30 weeks. The female animals in the group
receiving 1000 ppm of captan had a reduction in weight gain for the
last 16 weeks of the experiment. Female rats receiving 5000 ppm of
technical captan also displayed reduced weight gain, and both sexes on
diets containing 10,000 ppm of either technical or recrystallized
captan had marked growth depression. At autopsy, indication of
testicular atrophy was found in some animals fed 10,000 ppm. Otherwise
the organ-weight, blood picture, tumour frequency and histological
studies were not significantly different from those in controls (Weir,
1956).
A group of 30 male and 30 female rats was fed dietary levels of 1000
ppm of captan for 17 months. Body-weight gains, food consumption,
survival rate and tumour incidence were comparable to a control group
(Kay, 1961).
COMMENT
The acute short-term and long-term studies on captan provide adequate
information to determine no-effect levels. Information of the fate of
the trichloromethylthio moiety in the metabolism of captan is still
incomplete. The observation that the acute toxicity of the compound to
rats is many times higher when the animals are fed a low protein diet
is of some concern. The special studies are extensive and cover a
range of animal species. Possible teratogenic effects in rabbits and
indications of embryotoxic effects in monkeys were observed, and
therefore only a temporary acceptable daily intake was established.
However, the biochemical requirements referred to in the previous
monograph on captan (FAO/WHO, 1965) have now been partially fulfilled
and therefore it was considered justified to establish a slightly
higher acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 100 mg/kg body-weight/day
Monkey: 12.5 mg/kg body-weight/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captan is a non-systemic fungicide with no insecticidal or acaricidal
activity. According to a survey (Kirby, 1969) of world-wide fungicide
use on top fruit, captan was first choice for control of scab on
apple, pear and peach, bitter rot and black rot on apple, sooty blotch
and leaf spot on pear, brown rot on cherry, peach and plum, and leaf
spot on sweet cherry. Other fruits on which its use is recommended
include apricots and grapes, and it is only now being superseded for
control of botrytis (gray mould) on strawberries. Captan is approved
in the U.K. for the control of stem rot of tomato. It is also used on
citrus and many vegetables.
In the U.K. in 1967, captan was used on about 11,000 hectares of
apples and 2,500 hectares of pears, with a small usage on plum, cherry
and peach. Probably about 1000 hectares of apples received captan for
control of storage rots, i.e. were sprayed in August-September. The
rate of use is 0.1 percent a.i. high volume, or about 3 kg a.i. per
ha. West Germany recommends 2 kg a.i. per ha for control of apple scab
or vine downy mildew.
Captan is of no value for control of rusts or powdery mildews.
Post-harvest treatments
Captan is used for rot control in stored potatoes and as a dip for
fruit and vegetables. It is also used as a pre-packing spray for
packing boxes. Prunes dipped in suspensions of the 50 percent w.p. at
0.12, 0.24 and 0.48 percent a.i. bore 3.6, 4.8 and 11 ppm and 2.3, 6.1
and 11.1 ppm captan before and after dehydration, respectively (Archer
and Corbin, 1969).
Other uses
Captan is used as a seed dressing, particularly on peas. It has been
proposed for ringworm control on animals; milk obtained 24 hours after
a cow had been sprayed contained less captan than could be detected
(<1 ppm) (Hansen, 1953). Captan is also used on turf and ornamentals,
especially roses, and as a soil fungicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Interval Rate Residue
Crop days % a.i. ppm
Apple 1 0.12 4 to 36
42 0.12 0.3 to 0.5
94 0.12 0.2
Apricot (fresh) 0 0.12 16 to 17.5
7 0.12 11
21 0.12 6 to 9
42 0.12 4 to 5
Apricot (dried) 7 0.12 8
40 0.12 <1
Cherry 0 0.24 10 to 53
7 0.24 6
14 0.24 4
0 0.12 4 to 22
7 0.12 3
14 0.12 1
Citrus 1 0.12 9 to 13
Fig 0 0.12 1.7
2 0.12 0.2
Peach 0 0.12 2 to 6
5 0.12 3
14 0.12 8
0 0.24 3 to 7
3 0.24 8 to 11
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Peach 14 0.24 1 to 12
Pear 1 0.12 1 to 28
9 0.12 8 to 12
Plum (Post-harvest) 1.2 to 4.8 4 to 11 (green)
do. do. 3 to 11 (dehydrated)
Sweet potato 0 0.24 2
0 0.48 25
Hops 119 0.24 0.1 to 1.5
Rhubarb 0 0.12 6 to 14
Grape 1 0.12 12 to 34
57 0.12 4
1 4.6 kg per ha 0.1 to 0.4
90 4.6 kg per ha 0.1 to 0.5
Strawberry 0 5.75 kg per ha 6.5
2 do. 3 to 7
5 do. 3 to 5
8 do. 1 to 5
Blueberry 0 0.24 0.8 to 1.6
Cranberry 0 4.6 kg per ha 4 to 7
83 do. 1.5 to 2.5
Raspberry 7 2.9 kg per ha 0.7
12 do. 11
Raisin (before wash) 0 1.15 kg per ha 27 to 35
(after wash) 0 do. 1 to 2
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Cucumber 1 0.12 5.6 to 6.3
7 0.12 0.3 to 2.5
Lettuce 0 0.18 0.2 to 6
5 0.18 1
10 0.18 0.7 to 5
Green beans 0 0.24 2 to 7
Pepper 1 0.12 6 to 8
7 0.12 1 to 9
Spinach 3 0.12 75
10 0.12 15
Tomato 0 0.24 6 to 12
3 0.24 10
20 0.24 <1
Further information on residues is available from the literature. One
late-season application of captan at 0.1 percent to apples and pears
gave deposits of about 1 ppm, falling to undetectable levels at
harvest (Martin and Pickard, 1955). Strawberries sprayed five times,
from early bloom to one day before picking, with 1.5 kg per ha captan
on each occasion bore 6.0 ppm captan; fruit picked three days later
bore 4.7 ppm and fruit picked eight days after the final spray bore
2.9 ppm; fruit from plots sprayed with 2.2 kg per ha in another year
bore similar but smaller residues (Fahey et al. 1962). Strawberries
sprayed twice in early bloom with captan at 0.4 percent a.i. (9.2 kg
a.i. per ha) had 6 ppm on the first fruits to ripen and less than 2
ppm on fruits picked eight days later (Sillibourne 1966). In Finland,
0.15% a.i. sprayed on to lettuce led to 57 ppm after three days:
washing reduced this to 2 ppm (State Inst. Agric. Chem., Helsinki,
1969).
FATE OF RESIDUES
Captan does not appear to undergo any chemical change on plant
surfaces, and only a very small amount appears to enter plant tissues.
Potatoes dipped in a captan slurry had 29 ppm on the skin and only 0.3
ppm in the pulp seven days later; no residue could be detected in the
pulp after baking or boiling (Chevron Chemical Company, Pesticide
Petition No. 15, 1955, direct communication).
Residues of up to 8 ppm on Valencia oranges were reduced by the normal
commercial washing procedure to 0.2 ppm. When 10 ppm captan was added
to washed oranges before processing to pulp, no captan could be
detected in the pulp or molasses (Chevron, 1959).
METHODS OF RESIDUE ANALYSIS
The method of Kittleson (1952) for the calorimetric determination of
captan residues forms the basis of the recommended procedure
(Association of Official Agricultural Chemists, 1965) which is
suitable for regulatory purposes at the present time. The cleaned-up
extract of fruit or green vegetables is heated with resorcinol and the
optical density of the resultant solution in acetic acid is measured
at 425 nm. Ospenson et al. (1964) have reviewed the uses of this
procedure, which is inaccurate below about 0.5 ppm of captan, and a
similar but less sensitive method using pyridine as the colour-forming
reagent.
More recently, interest has been shown in chromatographic methods for
captan. Kilgore et al. (1967) proposed a gas chromatographic method
for the examination of apricots, peaches, tomatoes and cottonseed.
Using a silicone column with electron-capture detection, the detection
limit was about 0.01 ppm with recoveries averaging 92 percent; under
similar chromatographic conditions captan was clearly separated from
difolatan (Kilgore and White, 1967). Several stationary phases were
studied by Bevenue and Ogata 1968) who found the cyanosilicone GE
XE-60 most suitable for captan residue analysis since it gave the best
separation from folpet. These three allied compounds, captan, folpet
and difolatan, were examined by Pomerantz and Ross (1968) who devised
gas and thin-layer chromatographic systems for the separation,
identification and determination of the parent compounds and some of
their possible degradation products. Archer and Corbin (1969) have
suggested a thin-layer chromatographic procedure for detecting captan
residues in prune fruits and blossoms. These chromatographic methods
should form the basis of a suitable regulatory procedure for the
determination of residues of captan in fruit, and it is recommended
that such a method should be established.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Benelux Tree fruits, vegetables 15
Canada Tree fruits, vegetables, nuts, 40
small fruits, root crops,
melons, leafy vegetables
Germany (Fed. Rep.) Fruits 15
United States of Beetgreens, cherry, lettuce, 100
America spinach
Stone fruits, grapes, mangoes 50
celery, leeks, onions (green),
shallots
Apple, pear, avocado, small 25
fruits, cucurbits, onions
(dry), tomato, garlic, egg
plant
Beet (roots), cottonseed, 12 2
vegetables
Beans (dry and succulent), 25 (interim)
citrus pineapple potato
Almond (kernels) 2 (interim)
Almond (hulls) 100 (interim)
APPRAISAL
Captan is a non-systemic fungicide that has attained very wide use on
fruit, vegetables, ornamentals, turf and seeds; it is probably
recommended for more diseases on deciduous top fruit than any other
fungicide. It provides no control of powdery mildews and little of
rusts. The technical product is stated to contain 95 percent captan,
the main impurities being water, common salt, and unreacted
tetrahydrophthalimide.
Solubility in water is very low and residues remain as superficial
deposits; these are partly removed by washing with water, especially
from non-waxy crops such as strawberry. Dry captan is stable to heat
and U.V. radiation, but aqueous suspensions are quickly decomposed at
100° C or in alkaline media. Hydrogen sulphide is evolved and all
other breakdown products remain in solution. The instability of the
-N-S-bond leads to release of tetrahydrophthalimide and deamination
products. Cooking and other processing methods lead to rapid
decomposition of captan residues. Persistence on crop surfaces is
relatively long.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples and cherries 40 ppm
Pears 30 ppm
Apricots 20 ppm
Citrus, peaches, plums, rhubarb,
tomatoes 15 ppm
Strawberries, cranberries,
raspberries, cucumbers,
lettuce, green beans, peppers 10 ppm
Raisins (dried vine fruits) 5 ppm
Insufficient data were available to enable tolerances to be suggested
for blueberries, figs, hops, sweet potatoes or spinach.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Further teratogenicity studies in non-human primates.
2. Further studies on metabolism especially on the
trichloromethylthiomoiety.
3. Residue data for other crops, including blueberries, figs, hops,
sweet potatoes and spinach.
4. Residue data from countries other than the U.S.A.
DESIRABLE
1. The effects of feeding a low protein diet on the chronic toxicity
of captan.
2. The development and evaluation of a GLC method, distinguishing
captan from captafol and folpet, suitable for regulatory purposes.
REFERENCES
Ackerson, G.W. and Mussehl, F.E. (1953) Toxicity of treated seed corn
in rations for chicks. Unpub. rept. Departments of Biochemistry.
Nutrition and Poultry Husbandry, University of Nebraska
Archer, T.E. and Corbin, J.B. (1969) Detection of captan residues in
prune fruits and blossoms by thin-layer chromatography. Bull. envir.
Contam. Toxicol. 4:55-63
Arnold, D. (1967) Mutagenic study on captan. Swiss white mice. Unpub.
rept. from Industrial Bio-test Laboratories submitted to California
Chemical Company
Association of Official Agricultural Chemists. (1965) Official methods
of analysis of the Association of Official Agricultural Chemists. 10th
ed. Washington D.C. 390:24.111
Batte, E.G. (1953) A study of the effect of feeding Orthocide(R)
treated seed to hogs. Unpub. rept. submitted by Stauffer Chemical
Company
Bevenue, A. and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by Chromatography. J. Chromatog. 36:529-31
Boyd, E.M. and Krijnen, C.J, (1968) Toxicity of captan and
protein-deficient diet. J. clin, Pharmacol. 8:225-34
Chevron. (1959) Captan residues an processed citrus by-products.
Chevron Report November 1959
Courtney, K.D. (1968) Teratological investigation of captan, imidan
and thalidomide in Macaca mulatta. Unpub. rept. of the Bionetics
Research Laboratories, submitted to Stauffer Chemical Company
Crossley, J. (1967) The stability of captan in blood. Unpub. rept.
prepared and submitted by Chevron Chemical Company
Dye, D.F. (1969) Difolatan(R). Unpub. summary report prepared and
submitted by Chevron Chemical Company
Elsea, J.R. (1957) Captan technical 91%, pure DDT. Acute oral
administration; acute potentiation. Unpub. rept. of Hazleton
Laboratories submitted to Stauffer Chemical Company and to California
Spray-Chemical Company (cited by Lehman, 1965)
Fabro, S., Smith, R.L. and Williams, R.T. (1965) Embryotoxic activity
of some pesticides and drugs related to phthalimide Fd. Cosmet.
Toxicol. 31 587-90
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., and Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. econ.
Ent. 55: 179-84
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Fogleman, R.W. (1955) Technical captan, recrystallized captan. Chronic
oral administration - dogs. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Gray, E.H. (1954) Technical captan, recrystallized captan. Subacute
feeding-comparative study. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Hansen, J. (1953) California Spray-Chemical Corporation (unpublished).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst. 42: 1102-14
Johnson, D.F. (1954) A toxicity test of
n-trichloromethylthiotetrahydrophthalimide. Southwestern Vet. 8:
55-57
Kay, J.H. and Calandra, J.C. (1961) Chronic oral toxicity of captan.
Albino rate. Addendum report to Ortho Division, California Chemical
Company prepared by Industrial Bio-test Laboratories Inc.
Kennedy, G. (1966) Three generation reproduction study on captan
- albino rats. Unpub. rept. of Industrial Bio-test Laboratories
submitted to Chevron Chemical Company
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet, and Difolotan.
Toxicol. appl. Pharmacol. 13:420-30
Kilgore, W.W. and White, E.R. (1967) Determination of difolatan
residues in fruits by electron-capture gas chromatography. J. Agr. Fd
Chem. 15: 1118-20
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determination of captan residues. J. Agr. Fd Chem. 151 1035-37
Kirby, A.H.M. Fungicides for deciduous top fruit: a survey in 1968. Wd
Rev. Pest 1969 Control, 8: 45-58
Kittleson, A.R. (1952) Colorimetric determination of
N-trichloromethylthiotetrahydro-phthalimide. Analyt. Chem. 24: 1173-75
Klayder, T.J. (1963) Captan in green vegetables. J. Assn Off, Anal.
Chem. 46: 241-3
Leary, J.B. (1966) Difolatan: shay rat tests, Unpub. rept. of Chevron
Chemical Company (cited by Crossley, 1967)
Legator, M. S., Kelley, F.J., Green, S. and Oswald, E.J. (1969)
Mutagenic effects of captan. Annals W.Y. Acad. Sci. 160: 344-51
Lehman, A.J. (1965) "Summaries of Pesticide Toxicity", Assoc. Food and
Drug Offic., Topeka, Kansas, page 96
Link, R.P., Smith, J.C. and Morrill, C.O. (1956) Toxicity studies on
captan-treated corn in pigs and chickens. J. Amer. vet. Med. Ass. 128:
614-16
Martin, J.T. and Pickard, J.A. (1955) Spray application problems: XIII
determination of captan deposits; progress report. Ann. Rap. Long
Ashton Roe. Stn for 1954, 83-9.
McLaughlin, J.P., Reynaldo, E.P., Lamar, J.K. and Marlaic, J.P. (1969)
Teratology studies in rabbits with captan, folpet, and thalidomide.
Toxicol. appl. Pharmacol. 14:641
Meade, R.J. and Warner, D.R. (1954) The effect of seed disinfectants,
Orthocide and Arasan, upon the performance of growing fattening swine.
Unpub. rept. from Nebraska Agricultural Experiment Station, College of
Agriculture, University of Nebraska. submitted by Stauffer Chemical
Company
Ospenson, J.N., Pack, D.E., Kohn, G.K., Burchfield, H.P. and Storrs,
E.E. (1964) in Analytical Methods for Pesticides, Plant Growth
Regulators and Food Additives. Edited by O. Zweig. Volume III.
Academic Press, PP. 11-25
Owens, R.G. (1969) Metabolism of fungicides and related compounds.
Annal. N.Y. Acad. Sci. 100: 114-32
Palazzalo, R.J. (1966) Chicken reproduction study in captan. Unpub.
rept. of Industrial Bio-test Laboratories, submitted to Chevron
Chemical Company
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds, thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51: 1058-62
Sillibourne, J.M. (1956) Chemical control of grey mould on
strawberries; deposits of fungicides on Royal Sovereign in relation to
harvest residues. Rep. E. Malling Res. Stn for 1965, 183-91
State Institute Agric. Chem., (1969) Helsinki. Investigations on
pesticide residues, 1968, P.19
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compound in the developing chicken embryo. Annals N.Y. Acad. Sci.,
160: 334-43
Vondruska, J.F. (1969) Teratological investigation of captan in Macaca
mulatta (Rhesus monkey) and Macaca arctoides (stumptailed macque).
Unpub. rept. of Industrial Bio-Test Laboratories---submitted to
Chevron Chemical Company
Weir, R.J. (1956) Technical captan, recrystallized captan. Chronic
feeding - rats. Final report. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to Chevron Chemical Company
Weir, R.J. (1957) Technical captan, captan technical 91%. Subacute
feeding poultry. Unpub. rept. of Hazleton Laboratories submitted to
Stauffer Chemical Company and to Chevron Chemical Company
Other relevant chemical properties
The technical product is claimed to contain 96 percent captan; the
remainder consists of common salt (sodium chloride), water and
unreacted tetrahydrophthalimide. The pure crystal ins product, m.p.
178° C, is reported to have a vapour pressure of less than 1 × 10-6
torr at 25° C, but that of the technical product is stated to be much
higher. Solubility in water at normal temperatures is extremely low;
it is less than 10 percent in common organic solvents. Captan is
stable, except under alkaline conditions, at normal temperature; it
will decompose at temperatures in the region of 100° C, very slowly in
dry or rapidly in moist atmospheres (Klayder, 1963). In alkaline
solution, 1.0N NaOH, captan yields 2.8 equivalents of chloride ion.
Cooking, especially in water would lead to rapid decomposition of
residues to water-soluble products.
Formulated products include wettable powders containing 50 to 83
percent a.i. and dusts, those for field use containing a few percent
a.i. and those for seed treatment 75 Percent.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
This pesticide was evaluated for acceptable daily intake by the 1965
Joint FAO/WHO Meeting on Pesticide Residues (FAO/WHO, 1965). Since
that time additional information has become available, especially on
the metabolism and on the effect upon reproduction. Therefore, the
previously published monograph has been revised and is now reproduced
in its entirety.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Captan is rapidly decomposed in both human and rabbit blood at room
temperature. Decomposition appears to be according to first order
kinetics although some concentration dependency is evident, possibly
due to the low solubility of captan in aqueous media. At initial
concentrations of 100 µg/ml or 1/µg/ml in human blood the half-life of
captan was calculated as 0.9 minutes or 0.2 minutes respectively. In
rabbit blood at an initial concentration of 1 µg/ml, the half-life is
0.3 minutes (Crossley, 1967).
Captan, as an aqueous slurry, was administered by intragastric
intubation to female rabbits at levels of 0 mg/kg body-weight (two
rabbits) or 500 mg/kg body-weight (five rabbits). Blood was drawn
directly from the heart at intervals up to 56 hours and analysed for
captan and its metabolite tetrahydrophthalimide. No captan was
detected in the blood at any time after administration of the dose,
using an analytical method sensitive to 0.025 µg/ml. A build-up of the
metabolite tetrahydrophthalimide occurred in the blood which reached a
maximum of about 25 µg/ml 30 hours after intragastric administration
of captan. From then on it decreased to a level of 4.2 µg/ml after 56
hours; the estimated half-life being 6 hours (Crossley, 1967).
Recent information on the metabolism of captan has been inferred from
work on captafol, a compound which differs from captan only in the
nature of the chlorinated group attached to the sulphur atom (see the
monograph on captafol). Rats were fed celery which had been treated
with captafol to give levels of 60 or 600 ppm. The stomach content of
the animals was analysed at several intervals and both
tetrahydrophthalimide and tetrahydrophthalic acid were detected
(Leary, 1966).
Rats, dogs and monkeys wore fed carbon14-carbonyl labelled captafol.
The radioactivity rapidly entered the blood and tetrahydrophthalimide
was detected in the blood, faeces and urine. Tetrahydrophthalamic acid
as well as other more water soluble metabolites were the principal
radioactive compounds present in the blood and urine. Because of the
similarity in structure it has been assumed that captan would be
metabolized in a similar fashion with respect to the
tetrahydrophthalic acid portion of its molecule (Dye, 1969).
Feeding studies with captan demonstrated that captan is not stored in
the eggs or flesh of poultry nor in the tissues of pigs (Weir, 1957;
Link et al., 1956).
Effect on enzymes and other biochemical parameters
In the presence of compounds such as cysteine which contain sulfhydryl
groups, all the three chlorine atoms in captan are liberated an
chloride ions, and four sulfhydryl groups disappear for every molecule
of captan that reacts. In general captan initially reacts with two
molecules of a simple thiol to give tetrahydrophthalimide, the
disulfide derived from the thiol, thiophosgene and one chloride ion.
The thiophosgene reacts with two additional molecules of the thiol, to
give ultimately two more chloride ions, carbon disulfide and the
sulfide derived from the thiol. In the case where cysteine is the
thiol the reaction is slightly more complicated resulting in the
formation of a substituted thiazolidinethione. The presence of all
these compounds from the metabolism of captan, has been confirmed
chemically (Owens, 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
A three-generation reproduction study was conducted with rats which
received technical captan in their diet in concentrations of 0, 100,
500, and 1000 ppm. Groups of 16 female animals were used and two
litters were produced in each generation. No significant differences
were found between control and captan treated rats with respect to
fertility, gestation, viability or lactation indices, or in the
weaning weights in the first two generations. No effects were observed
in the third generation except for a slightly lowered lactation index
for the group receiving 1000 ppm of captan. Histopathological
examination of tissues from 10 pups receiving 1000 ppm in the third
generation revealed no damage (Kennedy, 1966; Kennedy, et al., 1968).
Special studies on teratogenicity
Chicken
A group of 5 male and 18 female White Leghorn chickens were fed a diet
containing 0 or 2,300 ppm (equivalent to 75 mg/kg body-weight)
technical captan for 6 weeks. The birds were observed for body-weight
effects, food consumption, behavioural reaction, and egg production.
Eggs collected during days 29 through 39 were incubated to determine
the extent of hatchability and the presence of any effects on the
chicks. No adverse effects were noted (Palazzolo, 1966).
Captan was injected in dimethylsulfoxide solution into either the yolk
or air cell of fresh fertile White Leghorn eggs in amounts sufficient
to produce concentrations of 3 to 20 ppm in the eggs. The eggs were
incubated and non-viable embryos and hatched chicks were examined for
gross abnormalities. In a total of 1,292 eggs, the incidence of
malformations was 7.8 percent compared to an incidence of less than 2
percent in solvent-injected and uninjected control eggs. Malformations
of the feet and legs followed a specific pattern. Micromelia, amelia,
and phocomelia accounted for most of the deformities (Verrett, at al.,
1969).
Hamster
Groups, each comprising 20 pregnant female hamsters ware fed diets
containing concentrations of captan sufficient to enable the animals
to receive an average daily intake of 0, 125, 250, or 1000
mg/kg/body-weight of captan from days 1 through 15 of gestation. At
day 159 all females were sacrificed, the young were surgically removed
and the foetal development and structural formation of each was
examined. The incidence of abnormal effects was not greater in any
test group than in the controls. The dose level of 1000
mg/kg/body-weight of captan resulted in a significant increase in
foetal resorption (Kennedy, at al., 1968).
Monkey
Groups of seven pregnant Rhesus monkeys were given daily oral doses of
6.35, 12.5 or 25 mg/kg body-weight of captan on days 22 through 32 of
gestation. (Thalidomide was used as a positive control at dosage
levels of 5 mg/kg/body-weight/day in six animals and at 10
mg/kg/body-weight/day in four animals). Foetuses were recovered on
approximately day 84 of gestation by Caesarian section and examined
for organ and skeletal defects. Foetal mortality occurred in three of
seven monkeys at the 25 mg/kg level. The foetal mortality in the
parent colony not fed captan was 13.2 percent on 439 conceptions.
There was no abnormality among any foetus in either of the three dose
levels of captan (Courtney, 1968).
Groups of seven pregnant monkeys (Rhesus and stumptail) were given
captan orally at levels of 10, 25 or 75 mg/kg body-weight daily on
days 21 through 34 of gestation. One abortion occurred at the 75 mg/kg
level but there were no malformations. (Thalidomide was given as a
positive control an in the previous experiment (Vondruska, 1969).
Rabbit
Four pregnant New Zealand White rabbits were given daily oral doses of
80 mg/kg bodyweight of captan during days 7 through 12 of gestation.
Captan produced no embryotoxicity in the litters of rabbits (Fabro at
al., 1965).
A group of six pregnant Dutch Belted rabbits was given 75
mg/kg/body-weight/day of technical captan, orally on days 6 through 16
of gestation. Three other groups each containing five to seven New
Zealand White rabbits were given 18.75, 37.5 or 75
mg/kg/body-weight/day of technical captan orally on days 6 through 18
of gestation. A control group and a positive control group (75 mg/kg
thalidomide) wore also maintained. No malformed foetuses occurred in
any group treated with captan. An increased incidence of foetal
resorption occurred in the New Zealand White rabbits given 75 mg/kg of
captan. Another group of nine pregnant Dutch Belted rabbits were given
75 mg/kg/body-weight/day of technical tetrahydrophthalimide, a
metabolite of captan, on days 6 through 16 of gestation. A slight rise
in the occurrence of resorption sites occurred. No skeletal
abnormality was observed (Kennedy, et al., 1968).
Groups of 9 pregnant New Zealand white rabbits were given captan at
dose-levels of 37.5, 75 or 150 mg/kg body-weight/day from days 6
through 16 of gestation. Thalidomide was used as a positive control at
levels of 75 and 150 mg/kg body-weight and produced the expected
teratological response. Captan at 75 mg/kg caused 9 malformed young
from 75 implantations of 9 pregnant does. At the dose level of 37.5
mg/kg captan produced one malformed foetus from 49 implantation sites
(McLaughlin at al., 1969).
Rat
Groups of five to ten pregnant female rats were given oral doses of 0,
50, 100, or 250 mg/kg/body-weight day of technical captan from days 6
through 15 of gestation or 0, 500, 1000 or 2000 mg/kg/body-weight/day
from days 8 through 10. Examination of 371 foetuses obtained from the
captan-treated rats revealed no significant increase in the number of
abnormalities. Three and two grossly malformed foetuses were found
from the rats treated with 1000 and 2000 mg/kg/body-weight/day of
captan respectively; compared with one in the corn oil control and
none in the lower doses of captan (Kennedy, et al., 1968).
Special studies on mutagenicity
Mouse
Single intraperitoneal injections of technical captan at doses of 0,
3.5 and 7.0 mg/kg body-weight were administered to an unspecified
number of male mice. Another group of male mice was given 100 mg/kg
body-weight of methyl methanesulfonate as a positive control. The
treated male groups were mated with three groups of untreated females
on each of three consecutive weeks post-treatment. The results
demonstrated that dominant lethal mutations were not induced by
treatment with captan (Arnold, 1967).
The mutagenic activity of captan was evaluated in bacteria, in the
heteroploid human embryo lung call line, and in a cell line derived
from the kidney of the rat-kangaroo. In bacteria, captan increased the
mutation rate both in streptomycin-dependent E. coli and a
thymine-dependent E. coli strain. Captan inhibited both growth and
mitosis of heteroploid embryonic lung cells in concentrations of less
than 5 µg/ml. In the studies with the rat-kangaroo cell line the
chromosome breaks and mitotic inhibition were proportional to the
concentration of captan over the range of 1 to 10 µg/ml (Legator, et
al., 1969).
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
captan from 7 days of age for 18 months. The dose of 215 mg/kg
body-weight was given to the mice daily by gavage from the seventh day
of age to the time of weaning at four weeks of age; thereafter, the
chemical was added to the diet in the corresponding amount of 560 ppm.
There was no significant increase in tumours in the group fed captan
compared with the controls (Innes, et al., 1969).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat oral 9,000 Elsea, 1957
Rat oral 12,500 Boyd and Krijnen, 1968
Rat oral 480 (low
protein diet)
Mouse i.p. 10 Arnold, 1967
Short-term studies
Chicken
An unspecified number of chicks were fed a diet containing 320 ppm of
captan for 28 days. In another experiment a group of 30 chicks was fed
the same dosage for 74 days along with a control group of 10 chicks.
No gross abnormalities were found in the birds in either experiment
(Ackerson and Mussehl, 1953; Link et al., 1956).
Groups of 15 hens were fed diets containing 0, 100, 1000, or 10,000
ppm of technical captan for 90 days. The hens receiving 100 and 1000
ppm of captan displayed normal food consumption, egg production, and
survival. In those receiving 10,000 ppm there was food-refusal, weight
loss, and decreased egg production. There were no gross effects
observed at autopsy in any cup. Analysis of the eggs and of the
tissues of the hens revealed no stored captan (Weir, 1957).
Dog
Groups of four dogs, each comprising two male and two female animals,
were started on a dose regime of 0, 10, 25 and 50 mg/kg body-weight of
captan. At the beginning of week 10 the dose level of the dogs
receiving 25 mg/kg was increased to 100 mg/kg. At week 18 these dose
levels were again increased to 100 mg/kg and 300 mg/kg respectively.
The captan was administered by gelatin capsule normally six days a
week for 66 weeks. Liver and kidney weights were slightly increased in
the dogs which received 300 mg/kg body-weight. There was no evidence
of systemic toxicity and no gross or histopathological changes in
tissues due to treatment at any dose-level, nor were there any
significant changes in haematological or biochemical findings
(Fogleman, 1955).
Pig
Eight half-grown pigs were fed for three months on corn treated with
captan to provide a level of 540 ppm. The treatment did not affect the
rate of food consumption, rate of growth or general health of the
animals when compared to the effect on an equal number of control
animals. There was no gross abnormality observed in any animal on the
captan diet (Batter 1953).
Four groups, comprising 10 weanling pigs each of about 14 kg in
weight, were fed rations containing 0, 420, 820, 1,680 ppm of captan
for 119 days. No gross pathological effects that could be attributed
to captan were observed (Meads and Warner, 1954).
Three pigs were fed 500 ppm and two pigs were fed 4000 ppm of captan
in the diet for 22-25 weeks. The animals displayed no abnormal
symptoms, but no further observations were made (Johnson, 1954).
Seven pigs were fed 480 ppm of captan in their diet for 14 weeks,
three other animals served as controls. The test animals displayed
normal weight gain. Gross examination of all organs revealed no
pathological changes and histopathological study of the liver and
kidneys revealed no abnormalities. Erythrocyte and lenkocyte counts
were not significantly different between test and control groups. No
residual captan (i.e. <0.1 ppm) was found in the tissues of the
animals (Link at al., 1956).
Rat
Five groups, each containing 11 male and 11 female rats, were fed
diets containing either technical or recrystallized captan at dose
levels up to 0, 5000 or 10,000 ppm for 13 weeks. All experimental
groups were started at a level of 500 ppm of captan and the dose
levels were gradually increased until the 5000 ppm level was reached
after four weeks and the 10,000 ppm level after seven weeks. Both dose
levels caused growth retardation; however, there was no difference
between comparable groups of rate receiving the technical and
recrystallized material (Gray, 1954).
Long-term studies
Rat
Groups, each containing 10 male and 10 female rats, were fed diets
containing 0, 1000 and 5000 ppm of technical captan for two years.
Another group of 20 rats received 10,000 ppm of technical captan for
24 weeks. This group was then divided in half, one half being fed
recrystallized captan for 30 weeks; the other half continued on the
technical compound for 30 weeks. The female animals in the group
receiving 1000 ppm of captan had a reduction in weight gain for the
last 16 weeks of the experiment. Female rats receiving 5000 ppm of
technical captan also displayed reduced weight gain, and both sexes on
diets containing 10,000 ppm of either technical or recrystallized
captan had marked growth depression. At autopsy, indication of
testicular atrophy was found in some animals fed 10,000 ppm. Otherwise
the organ-weight, blood picture, tumour frequency and histological
studies were not significantly different from those in controls (Weir,
1956).
A group of 30 male and 30 female rats was fed dietary levels of 1000
ppm of captan for 17 months. Body-weight gains, food consumption,
survival rate and tumour incidence were comparable to a control group
(Kay, 1961).
COMMENT
The acute short-term and long-term studies on captan provide adequate
information to determine no-effect levels. Information of the fate of
the trichloromethylthio moiety in the metabolism of captan is still
incomplete. The observation that the acute toxicity of the compound to
rats is many times higher when the animals are fed a low protein diet
is of some concern. The special studies are extensive and cover a
range of animal species. Possible teratogenic effects in rabbits and
indications of embryotoxic effects in monkeys were observed, and
therefore only a temporary acceptable daily intake was established.
However, the biochemical requirements referred to in the previous
monograph on captan (FAO/WHO, 1965) have now been partially fulfilled
and therefore it was considered justified to establish a slightly
higher acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 100 mg/kg body-weight/day
Monkey: 12.5 mg/kg body-weight/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captan is a non-systemic fungicide with no insecticidal or acaricidal
activity. According to a survey (Kirby, 1969) of world-wide fungicide
use on top fruit, captan was first choice for control of scab on
apple, pear and peach, bitter rot and black rot on apple, sooty blotch
and leaf spot on pear, brown rot on cherry, peach and plum, and leaf
spot on sweet cherry. Other fruits on which its use is recommended
include apricots and grapes, and it is only now being superseded for
control of botrytis (gray mould) on strawberries. Captan is approved
in the U.K. for the control of stem rot of tomato. It is also used on
citrus and many vegetables.
In the U.K. in 1967, captan was used on about 11,000 hectares of
apples and 2,500 hectares of pears, with a small usage on plum, cherry
and peach. Probably about 1000 hectares of apples received captan for
control of storage rots, i.e. were sprayed in August-September. The
rate of use is 0.1 percent a.i. high volume, or about 3 kg a.i. per
ha. West Germany recommends 2 kg a.i. per ha for control of apple scab
or vine downy mildew.
Captan is of no value for control of rusts or powdery mildews.
Post-harvest treatments
Captan is used for rot control in stored potatoes and as a dip for
fruit and vegetables. It is also used as a pre-packing spray for
packing boxes. Prunes dipped in suspensions of the 50 percent w.p. at
0.12, 0.24 and 0.48 percent a.i. bore 3.6, 4.8 and 11 ppm and 2.3, 6.1
and 11.1 ppm captan before and after dehydration, respectively (Archer
and Corbin, 1969).
Other uses
Captan is used as a seed dressing, particularly on peas. It has been
proposed for ringworm control on animals; milk obtained 24 hours after
a cow had been sprayed contained less captan than could be detected
(<1 ppm) (Hansen, 1953). Captan is also used on turf and ornamentals,
especially roses, and as a soil fungicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Interval Rate Residue
Crop days % a.i. ppm
Apple 1 0.12 4 to 36
42 0.12 0.3 to 0.5
94 0.12 0.2
Apricot (fresh) 0 0.12 16 to 17.5
7 0.12 11
21 0.12 6 to 9
42 0.12 4 to 5
Apricot (dried) 7 0.12 8
40 0.12 <1
Cherry 0 0.24 10 to 53
7 0.24 6
14 0.24 4
0 0.12 4 to 22
7 0.12 3
14 0.12 1
Citrus 1 0.12 9 to 13
Fig 0 0.12 1.7
2 0.12 0.2
Peach 0 0.12 2 to 6
5 0.12 3
14 0.12 8
0 0.24 3 to 7
3 0.24 8 to 11
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Peach 14 0.24 1 to 12
Pear 1 0.12 1 to 28
9 0.12 8 to 12
Plum (Post-harvest) 1.2 to 4.8 4 to 11 (green)
do. do. 3 to 11 (dehydrated)
Sweet potato 0 0.24 2
0 0.48 25
Hops 119 0.24 0.1 to 1.5
Rhubarb 0 0.12 6 to 14
Grape 1 0.12 12 to 34
57 0.12 4
1 4.6 kg per ha 0.1 to 0.4
90 4.6 kg per ha 0.1 to 0.5
Strawberry 0 5.75 kg per ha 6.5
2 do. 3 to 7
5 do. 3 to 5
8 do. 1 to 5
Blueberry 0 0.24 0.8 to 1.6
Cranberry 0 4.6 kg per ha 4 to 7
83 do. 1.5 to 2.5
Raspberry 7 2.9 kg per ha 0.7
12 do. 11
Raisin (before wash) 0 1.15 kg per ha 27 to 35
(after wash) 0 do. 1 to 2
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Cucumber 1 0.12 5.6 to 6.3
7 0.12 0.3 to 2.5
Lettuce 0 0.18 0.2 to 6
5 0.18 1
10 0.18 0.7 to 5
Green beans 0 0.24 2 to 7
Pepper 1 0.12 6 to 8
7 0.12 1 to 9
Spinach 3 0.12 75
10 0.12 15
Tomato 0 0.24 6 to 12
3 0.24 10
20 0.24 <1
Further information on residues is available from the literature. One
late-season application of captan at 0.1 percent to apples and pears
gave deposits of about 1 ppm, falling to undetectable levels at
harvest (Martin and Pickard, 1955). Strawberries sprayed five times,
from early bloom to one day before picking, with 1.5 kg per ha captan
on each occasion bore 6.0 ppm captan; fruit picked three days later
bore 4.7 ppm and fruit picked eight days after the final spray bore
2.9 ppm; fruit from plots sprayed with 2.2 kg per ha in another year
bore similar but smaller residues (Fahey et al. 1962). Strawberries
sprayed twice in early bloom with captan at 0.4 percent a.i. (9.2 kg
a.i. per ha) had 6 ppm on the first fruits to ripen and less than 2
ppm on fruits picked eight days later (Sillibourne 1966). In Finland,
0.15% a.i. sprayed on to lettuce led to 57 ppm after three days:
washing reduced this to 2 ppm (State Inst. Agric. Chem., Helsinki,
1969).
FATE OF RESIDUES
Captan does not appear to undergo any chemical change on plant
surfaces, and only a very small amount appears to enter plant tissues.
Potatoes dipped in a captan slurry had 29 ppm on the skin and only 0.3
ppm in the pulp seven days later; no residue could be detected in the
pulp after baking or boiling (Chevron Chemical Company, Pesticide
Petition No. 15, 1955, direct communication).
Residues of up to 8 ppm on Valencia oranges were reduced by the normal
commercial washing procedure to 0.2 ppm. When 10 ppm captan was added
to washed oranges before processing to pulp, no captan could be
detected in the pulp or molasses (Chevron, 1959).
METHODS OF RESIDUE ANALYSIS
The method of Kittleson (1952) for the calorimetric determination of
captan residues forms the basis of the recommended procedure
(Association of Official Agricultural Chemists, 1965) which is
suitable for regulatory purposes at the present time. The cleaned-up
extract of fruit or green vegetables is heated with resorcinol and the
optical density of the resultant solution in acetic acid is measured
at 425 nm. Ospenson et al. (1964) have reviewed the uses of this
procedure, which is inaccurate below about 0.5 ppm of captan, and a
similar but less sensitive method using pyridine as the colour-forming
reagent.
More recently, interest has been shown in chromatographic methods for
captan. Kilgore et al. (1967) proposed a gas chromatographic method
for the examination of apricots, peaches, tomatoes and cottonseed.
Using a silicone column with electron-capture detection, the detection
limit was about 0.01 ppm with recoveries averaging 92 percent; under
similar chromatographic conditions captan was clearly separated from
difolatan (Kilgore and White, 1967). Several stationary phases were
studied by Bevenue and Ogata 1968) who found the cyanosilicone GE
XE-60 most suitable for captan residue analysis since it gave the best
separation from folpet. These three allied compounds, captan, folpet
and difolatan, were examined by Pomerantz and Ross (1968) who devised
gas and thin-layer chromatographic systems for the separation,
identification and determination of the parent compounds and some of
their possible degradation products. Archer and Corbin (1969) have
suggested a thin-layer chromatographic procedure for detecting captan
residues in prune fruits and blossoms. These chromatographic methods
should form the basis of a suitable regulatory procedure for the
determination of residues of captan in fruit, and it is recommended
that such a method should be established.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Benelux Tree fruits, vegetables 15
Canada Tree fruits, vegetables, nuts, 40
small fruits, root crops,
melons, leafy vegetables
Germany (Fed. Rep.) Fruits 15
United States of Beetgreens, cherry, lettuce, 100
America spinach
Stone fruits, grapes, mangoes 50
celery, leeks, onions (green),
shallots
Apple, pear, avocado, small 25
fruits, cucurbits, onions
(dry), tomato, garlic, egg
plant
Beet (roots), cottonseed, 12 2
vegetables
Beans (dry and succulent), 25 (interim)
citrus pineapple potato
Almond (kernels) 2 (interim)
Almond (hulls) 100 (interim)
APPRAISAL
Captan is a non-systemic fungicide that has attained very wide use on
fruit, vegetables, ornamentals, turf and seeds; it is probably
recommended for more diseases on deciduous top fruit than any other
fungicide. It provides no control of powdery mildews and little of
rusts. The technical product is stated to contain 95 percent captan,
the main impurities being water, common salt, and unreacted
tetrahydrophthalimide.
Solubility in water is very low and residues remain as superficial
deposits; these are partly removed by washing with water, especially
from non-waxy crops such as strawberry. Dry captan is stable to heat
and U.V. radiation, but aqueous suspensions are quickly decomposed at
100° C or in alkaline media. Hydrogen sulphide is evolved and all
other breakdown products remain in solution. The instability of the
-N-S-bond leads to release of tetrahydrophthalimide and deamination
products. Cooking and other processing methods lead to rapid
decomposition of captan residues. Persistence on crop surfaces is
relatively long.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples and cherries 40 ppm
Pears 30 ppm
Apricots 20 ppm
Citrus, peaches, plums, rhubarb,
tomatoes 15 ppm
Strawberries, cranberries,
raspberries, cucumbers,
lettuce, green beans, peppers 10 ppm
Raisins (dried vine fruits) 5 ppm
Insufficient data were available to enable tolerances to be suggested
for blueberries, figs, hops, sweet potatoes or spinach.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Further teratogenicity studies in non-human primates.
2. Further studies on metabolism especially on the
trichloromethylthiomoiety.
3. Residue data for other crops, including blueberries, figs, hops,
sweet potatoes and spinach.
4. Residue data from countries other than the U.S.A.
DESIRABLE
1. The effects of feeding a low protein diet on the chronic toxicity
of captan.
2. The development and evaluation of a GLC method, distinguishing
captan from captafol and folpet, suitable for regulatory purposes.
REFERENCES
Ackerson, G.W. and Mussehl, F.E. (1953) Toxicity of treated seed corn
in rations for chicks. Unpub. rept. Departments of Biochemistry.
Nutrition and Poultry Husbandry, University of Nebraska
Archer, T.E. and Corbin, J.B. (1969) Detection of captan residues in
prune fruits and blossoms by thin-layer chromatography. Bull. envir.
Contam. Toxicol. 4:55-63
Arnold, D. (1967) Mutagenic study on captan. Swiss white mice. Unpub.
rept. from Industrial Bio-test Laboratories submitted to California
Chemical Company
Association of Official Agricultural Chemists. (1965) Official methods
of analysis of the Association of Official Agricultural Chemists. 10th
ed. Washington D.C. 390:24.111
Batte, E.G. (1953) A study of the effect of feeding Orthocide(R)
treated seed to hogs. Unpub. rept. submitted by Stauffer Chemical
Company
Bevenue, A. and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by Chromatography. J. Chromatog. 36:529-31
Boyd, E.M. and Krijnen, C.J, (1968) Toxicity of captan and
protein-deficient diet. J. clin, Pharmacol. 8:225-34
Chevron. (1959) Captan residues an processed citrus by-products.
Chevron Report November 1959
Courtney, K.D. (1968) Teratological investigation of captan, imidan
and thalidomide in Macaca mulatta. Unpub. rept. of the Bionetics
Research Laboratories, submitted to Stauffer Chemical Company
Crossley, J. (1967) The stability of captan in blood. Unpub. rept.
prepared and submitted by Chevron Chemical Company
Dye, D.F. (1969) Difolatan(R). Unpub. summary report prepared and
submitted by Chevron Chemical Company
Elsea, J.R. (1957) Captan technical 91%, pure DDT. Acute oral
administration; acute potentiation. Unpub. rept. of Hazleton
Laboratories submitted to Stauffer Chemical Company and to California
Spray-Chemical Company (cited by Lehman, 1965)
Fabro, S., Smith, R.L. and Williams, R.T. (1965) Embryotoxic activity
of some pesticides and drugs related to phthalimide Fd. Cosmet.
Toxicol. 31 587-90
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., and Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. econ.
Ent. 55: 179-84
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Fogleman, R.W. (1955) Technical captan, recrystallized captan. Chronic
oral administration - dogs. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Gray, E.H. (1954) Technical captan, recrystallized captan. Subacute
feeding-comparative study. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Hansen, J. (1953) California Spray-Chemical Corporation (unpublished).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst. 42: 1102-14
Johnson, D.F. (1954) A toxicity test of
n-trichloromethylthiotetrahydrophthalimide. Southwestern Vet. 8:
55-57
Kay, J.H. and Calandra, J.C. (1961) Chronic oral toxicity of captan.
Albino rate. Addendum report to Ortho Division, California Chemical
Company prepared by Industrial Bio-test Laboratories Inc.
Kennedy, G. (1966) Three generation reproduction study on captan
- albino rats. Unpub. rept. of Industrial Bio-test Laboratories
submitted to Chevron Chemical Company
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet, and Difolotan.
Toxicol. appl. Pharmacol. 13:420-30
Kilgore, W.W. and White, E.R. (1967) Determination of difolatan
residues in fruits by electron-capture gas chromatography. J. Agr. Fd
Chem. 15: 1118-20
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determination of captan residues. J. Agr. Fd Chem. 151 1035-37
Kirby, A.H.M. Fungicides for deciduous top fruit: a survey in 1968. Wd
Rev. Pest 1969 Control, 8: 45-58
Kittleson, A.R. (1952) Colorimetric determination of
N-trichloromethylthiotetrahydro-phthalimide. Analyt. Chem. 24: 1173-75
Klayder, T.J. (1963) Captan in green vegetables. J. Assn Off, Anal.
Chem. 46: 241-3
Leary, J.B. (1966) Difolatan: shay rat tests, Unpub. rept. of Chevron
Chemical Company (cited by Crossley, 1967)
Legator, M. S., Kelley, F.J., Green, S. and Oswald, E.J. (1969)
Mutagenic effects of captan. Annals W.Y. Acad. Sci. 160: 344-51
Lehman, A.J. (1965) "Summaries of Pesticide Toxicity", Assoc. Food and
Drug Offic., Topeka, Kansas, page 96
Link, R.P., Smith, J.C. and Morrill, C.O. (1956) Toxicity studies on
captan-treated corn in pigs and chickens. J. Amer. vet. Med. Ass. 128:
614-16
Martin, J.T. and Pickard, J.A. (1955) Spray application problems: XIII
determination of captan deposits; progress report. Ann. Rap. Long
Ashton Roe. Stn for 1954, 83-9.
McLaughlin, J.P., Reynaldo, E.P., Lamar, J.K. and Marlaic, J.P. (1969)
Teratology studies in rabbits with captan, folpet, and thalidomide.
Toxicol. appl. Pharmacol. 14:641
Meade, R.J. and Warner, D.R. (1954) The effect of seed disinfectants,
Orthocide and Arasan, upon the performance of growing fattening swine.
Unpub. rept. from Nebraska Agricultural Experiment Station, College of
Agriculture, University of Nebraska. submitted by Stauffer Chemical
Company
Ospenson, J.N., Pack, D.E., Kohn, G.K., Burchfield, H.P. and Storrs,
E.E. (1964) in Analytical Methods for Pesticides, Plant Growth
Regulators and Food Additives. Edited by O. Zweig. Volume III.
Academic Press, PP. 11-25
Owens, R.G. (1969) Metabolism of fungicides and related compounds.
Annal. N.Y. Acad. Sci. 100: 114-32
Palazzalo, R.J. (1966) Chicken reproduction study in captan. Unpub.
rept. of Industrial Bio-test Laboratories, submitted to Chevron
Chemical Company
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds, thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51: 1058-62
Sillibourne, J.M. (1956) Chemical control of grey mould on
strawberries; deposits of fungicides on Royal Sovereign in relation to
harvest residues. Rep. E. Malling Res. Stn for 1965, 183-91
State Institute Agric. Chem., (1969) Helsinki. Investigations on
pesticide residues, 1968, P.19
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compound in the developing chicken embryo. Annals N.Y. Acad. Sci.,
160: 334-43
Vondruska, J.F. (1969) Teratological investigation of captan in Macaca
mulatta (Rhesus monkey) and Macaca arctoides (stumptailed macque).
Unpub. rept. of Industrial Bio-Test Laboratories---submitted to
Chevron Chemical Company
Weir, R.J. (1956) Technical captan, recrystallized captan. Chronic
feeding - rats. Final report. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to Chevron Chemical Company
Weir, R.J. (1957) Technical captan, captan technical 91%. Subacute
feeding poultry. Unpub. rept. of Hazleton Laboratories submitted to
Stauffer Chemical Company and to Chevron Chemical Company
 Other relevant chemical properties
The technical product is claimed to contain 96 percent captan; the
remainder consists of common salt (sodium chloride), water and
unreacted tetrahydrophthalimide. The pure crystal ins product, m.p.
178° C, is reported to have a vapour pressure of less than 1 × 10-6
torr at 25° C, but that of the technical product is stated to be much
higher. Solubility in water at normal temperatures is extremely low;
it is less than 10 percent in common organic solvents. Captan is
stable, except under alkaline conditions, at normal temperature; it
will decompose at temperatures in the region of 100° C, very slowly in
dry or rapidly in moist atmospheres (Klayder, 1963). In alkaline
solution, 1.0N NaOH, captan yields 2.8 equivalents of chloride ion.
Cooking, especially in water would lead to rapid decomposition of
residues to water-soluble products.
Formulated products include wettable powders containing 50 to 83
percent a.i. and dusts, those for field use containing a few percent
a.i. and those for seed treatment 75 Percent.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
This pesticide was evaluated for acceptable daily intake by the 1965
Joint FAO/WHO Meeting on Pesticide Residues (FAO/WHO, 1965). Since
that time additional information has become available, especially on
the metabolism and on the effect upon reproduction. Therefore, the
previously published monograph has been revised and is now reproduced
in its entirety.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Captan is rapidly decomposed in both human and rabbit blood at room
temperature. Decomposition appears to be according to first order
kinetics although some concentration dependency is evident, possibly
due to the low solubility of captan in aqueous media. At initial
concentrations of 100 µg/ml or 1/µg/ml in human blood the half-life of
captan was calculated as 0.9 minutes or 0.2 minutes respectively. In
rabbit blood at an initial concentration of 1 µg/ml, the half-life is
0.3 minutes (Crossley, 1967).
Captan, as an aqueous slurry, was administered by intragastric
intubation to female rabbits at levels of 0 mg/kg body-weight (two
rabbits) or 500 mg/kg body-weight (five rabbits). Blood was drawn
directly from the heart at intervals up to 56 hours and analysed for
captan and its metabolite tetrahydrophthalimide. No captan was
detected in the blood at any time after administration of the dose,
using an analytical method sensitive to 0.025 µg/ml. A build-up of the
metabolite tetrahydrophthalimide occurred in the blood which reached a
maximum of about 25 µg/ml 30 hours after intragastric administration
of captan. From then on it decreased to a level of 4.2 µg/ml after 56
hours; the estimated half-life being 6 hours (Crossley, 1967).
Recent information on the metabolism of captan has been inferred from
work on captafol, a compound which differs from captan only in the
nature of the chlorinated group attached to the sulphur atom (see the
monograph on captafol). Rats were fed celery which had been treated
with captafol to give levels of 60 or 600 ppm. The stomach content of
the animals was analysed at several intervals and both
tetrahydrophthalimide and tetrahydrophthalic acid were detected
(Leary, 1966).
Rats, dogs and monkeys wore fed carbon14-carbonyl labelled captafol.
The radioactivity rapidly entered the blood and tetrahydrophthalimide
was detected in the blood, faeces and urine. Tetrahydrophthalamic acid
as well as other more water soluble metabolites were the principal
radioactive compounds present in the blood and urine. Because of the
similarity in structure it has been assumed that captan would be
metabolized in a similar fashion with respect to the
tetrahydrophthalic acid portion of its molecule (Dye, 1969).
Feeding studies with captan demonstrated that captan is not stored in
the eggs or flesh of poultry nor in the tissues of pigs (Weir, 1957;
Link et al., 1956).
Effect on enzymes and other biochemical parameters
In the presence of compounds such as cysteine which contain sulfhydryl
groups, all the three chlorine atoms in captan are liberated an
chloride ions, and four sulfhydryl groups disappear for every molecule
of captan that reacts. In general captan initially reacts with two
molecules of a simple thiol to give tetrahydrophthalimide, the
disulfide derived from the thiol, thiophosgene and one chloride ion.
The thiophosgene reacts with two additional molecules of the thiol, to
give ultimately two more chloride ions, carbon disulfide and the
sulfide derived from the thiol. In the case where cysteine is the
thiol the reaction is slightly more complicated resulting in the
formation of a substituted thiazolidinethione. The presence of all
these compounds from the metabolism of captan, has been confirmed
chemically (Owens, 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
A three-generation reproduction study was conducted with rats which
received technical captan in their diet in concentrations of 0, 100,
500, and 1000 ppm. Groups of 16 female animals were used and two
litters were produced in each generation. No significant differences
were found between control and captan treated rats with respect to
fertility, gestation, viability or lactation indices, or in the
weaning weights in the first two generations. No effects were observed
in the third generation except for a slightly lowered lactation index
for the group receiving 1000 ppm of captan. Histopathological
examination of tissues from 10 pups receiving 1000 ppm in the third
generation revealed no damage (Kennedy, 1966; Kennedy, et al., 1968).
Special studies on teratogenicity
Chicken
A group of 5 male and 18 female White Leghorn chickens were fed a diet
containing 0 or 2,300 ppm (equivalent to 75 mg/kg body-weight)
technical captan for 6 weeks. The birds were observed for body-weight
effects, food consumption, behavioural reaction, and egg production.
Eggs collected during days 29 through 39 were incubated to determine
the extent of hatchability and the presence of any effects on the
chicks. No adverse effects were noted (Palazzolo, 1966).
Captan was injected in dimethylsulfoxide solution into either the yolk
or air cell of fresh fertile White Leghorn eggs in amounts sufficient
to produce concentrations of 3 to 20 ppm in the eggs. The eggs were
incubated and non-viable embryos and hatched chicks were examined for
gross abnormalities. In a total of 1,292 eggs, the incidence of
malformations was 7.8 percent compared to an incidence of less than 2
percent in solvent-injected and uninjected control eggs. Malformations
of the feet and legs followed a specific pattern. Micromelia, amelia,
and phocomelia accounted for most of the deformities (Verrett, at al.,
1969).
Hamster
Groups, each comprising 20 pregnant female hamsters ware fed diets
containing concentrations of captan sufficient to enable the animals
to receive an average daily intake of 0, 125, 250, or 1000
mg/kg/body-weight of captan from days 1 through 15 of gestation. At
day 159 all females were sacrificed, the young were surgically removed
and the foetal development and structural formation of each was
examined. The incidence of abnormal effects was not greater in any
test group than in the controls. The dose level of 1000
mg/kg/body-weight of captan resulted in a significant increase in
foetal resorption (Kennedy, at al., 1968).
Monkey
Groups of seven pregnant Rhesus monkeys were given daily oral doses of
6.35, 12.5 or 25 mg/kg body-weight of captan on days 22 through 32 of
gestation. (Thalidomide was used as a positive control at dosage
levels of 5 mg/kg/body-weight/day in six animals and at 10
mg/kg/body-weight/day in four animals). Foetuses were recovered on
approximately day 84 of gestation by Caesarian section and examined
for organ and skeletal defects. Foetal mortality occurred in three of
seven monkeys at the 25 mg/kg level. The foetal mortality in the
parent colony not fed captan was 13.2 percent on 439 conceptions.
There was no abnormality among any foetus in either of the three dose
levels of captan (Courtney, 1968).
Groups of seven pregnant monkeys (Rhesus and stumptail) were given
captan orally at levels of 10, 25 or 75 mg/kg body-weight daily on
days 21 through 34 of gestation. One abortion occurred at the 75 mg/kg
level but there were no malformations. (Thalidomide was given as a
positive control an in the previous experiment (Vondruska, 1969).
Rabbit
Four pregnant New Zealand White rabbits were given daily oral doses of
80 mg/kg bodyweight of captan during days 7 through 12 of gestation.
Captan produced no embryotoxicity in the litters of rabbits (Fabro at
al., 1965).
A group of six pregnant Dutch Belted rabbits was given 75
mg/kg/body-weight/day of technical captan, orally on days 6 through 16
of gestation. Three other groups each containing five to seven New
Zealand White rabbits were given 18.75, 37.5 or 75
mg/kg/body-weight/day of technical captan orally on days 6 through 18
of gestation. A control group and a positive control group (75 mg/kg
thalidomide) wore also maintained. No malformed foetuses occurred in
any group treated with captan. An increased incidence of foetal
resorption occurred in the New Zealand White rabbits given 75 mg/kg of
captan. Another group of nine pregnant Dutch Belted rabbits were given
75 mg/kg/body-weight/day of technical tetrahydrophthalimide, a
metabolite of captan, on days 6 through 16 of gestation. A slight rise
in the occurrence of resorption sites occurred. No skeletal
abnormality was observed (Kennedy, et al., 1968).
Groups of 9 pregnant New Zealand white rabbits were given captan at
dose-levels of 37.5, 75 or 150 mg/kg body-weight/day from days 6
through 16 of gestation. Thalidomide was used as a positive control at
levels of 75 and 150 mg/kg body-weight and produced the expected
teratological response. Captan at 75 mg/kg caused 9 malformed young
from 75 implantations of 9 pregnant does. At the dose level of 37.5
mg/kg captan produced one malformed foetus from 49 implantation sites
(McLaughlin at al., 1969).
Rat
Groups of five to ten pregnant female rats were given oral doses of 0,
50, 100, or 250 mg/kg/body-weight day of technical captan from days 6
through 15 of gestation or 0, 500, 1000 or 2000 mg/kg/body-weight/day
from days 8 through 10. Examination of 371 foetuses obtained from the
captan-treated rats revealed no significant increase in the number of
abnormalities. Three and two grossly malformed foetuses were found
from the rats treated with 1000 and 2000 mg/kg/body-weight/day of
captan respectively; compared with one in the corn oil control and
none in the lower doses of captan (Kennedy, et al., 1968).
Special studies on mutagenicity
Mouse
Single intraperitoneal injections of technical captan at doses of 0,
3.5 and 7.0 mg/kg body-weight were administered to an unspecified
number of male mice. Another group of male mice was given 100 mg/kg
body-weight of methyl methanesulfonate as a positive control. The
treated male groups were mated with three groups of untreated females
on each of three consecutive weeks post-treatment. The results
demonstrated that dominant lethal mutations were not induced by
treatment with captan (Arnold, 1967).
The mutagenic activity of captan was evaluated in bacteria, in the
heteroploid human embryo lung call line, and in a cell line derived
from the kidney of the rat-kangaroo. In bacteria, captan increased the
mutation rate both in streptomycin-dependent E. coli and a
thymine-dependent E. coli strain. Captan inhibited both growth and
mitosis of heteroploid embryonic lung cells in concentrations of less
than 5 µg/ml. In the studies with the rat-kangaroo cell line the
chromosome breaks and mitotic inhibition were proportional to the
concentration of captan over the range of 1 to 10 µg/ml (Legator, et
al., 1969).
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
captan from 7 days of age for 18 months. The dose of 215 mg/kg
body-weight was given to the mice daily by gavage from the seventh day
of age to the time of weaning at four weeks of age; thereafter, the
chemical was added to the diet in the corresponding amount of 560 ppm.
There was no significant increase in tumours in the group fed captan
compared with the controls (Innes, et al., 1969).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat oral 9,000 Elsea, 1957
Rat oral 12,500 Boyd and Krijnen, 1968
Rat oral 480 (low
protein diet)
Mouse i.p. 10 Arnold, 1967
Short-term studies
Chicken
An unspecified number of chicks were fed a diet containing 320 ppm of
captan for 28 days. In another experiment a group of 30 chicks was fed
the same dosage for 74 days along with a control group of 10 chicks.
No gross abnormalities were found in the birds in either experiment
(Ackerson and Mussehl, 1953; Link et al., 1956).
Groups of 15 hens were fed diets containing 0, 100, 1000, or 10,000
ppm of technical captan for 90 days. The hens receiving 100 and 1000
ppm of captan displayed normal food consumption, egg production, and
survival. In those receiving 10,000 ppm there was food-refusal, weight
loss, and decreased egg production. There were no gross effects
observed at autopsy in any cup. Analysis of the eggs and of the
tissues of the hens revealed no stored captan (Weir, 1957).
Dog
Groups of four dogs, each comprising two male and two female animals,
were started on a dose regime of 0, 10, 25 and 50 mg/kg body-weight of
captan. At the beginning of week 10 the dose level of the dogs
receiving 25 mg/kg was increased to 100 mg/kg. At week 18 these dose
levels were again increased to 100 mg/kg and 300 mg/kg respectively.
The captan was administered by gelatin capsule normally six days a
week for 66 weeks. Liver and kidney weights were slightly increased in
the dogs which received 300 mg/kg body-weight. There was no evidence
of systemic toxicity and no gross or histopathological changes in
tissues due to treatment at any dose-level, nor were there any
significant changes in haematological or biochemical findings
(Fogleman, 1955).
Pig
Eight half-grown pigs were fed for three months on corn treated with
captan to provide a level of 540 ppm. The treatment did not affect the
rate of food consumption, rate of growth or general health of the
animals when compared to the effect on an equal number of control
animals. There was no gross abnormality observed in any animal on the
captan diet (Batter 1953).
Four groups, comprising 10 weanling pigs each of about 14 kg in
weight, were fed rations containing 0, 420, 820, 1,680 ppm of captan
for 119 days. No gross pathological effects that could be attributed
to captan were observed (Meads and Warner, 1954).
Three pigs were fed 500 ppm and two pigs were fed 4000 ppm of captan
in the diet for 22-25 weeks. The animals displayed no abnormal
symptoms, but no further observations were made (Johnson, 1954).
Seven pigs were fed 480 ppm of captan in their diet for 14 weeks,
three other animals served as controls. The test animals displayed
normal weight gain. Gross examination of all organs revealed no
pathological changes and histopathological study of the liver and
kidneys revealed no abnormalities. Erythrocyte and lenkocyte counts
were not significantly different between test and control groups. No
residual captan (i.e. <0.1 ppm) was found in the tissues of the
animals (Link at al., 1956).
Rat
Five groups, each containing 11 male and 11 female rats, were fed
diets containing either technical or recrystallized captan at dose
levels up to 0, 5000 or 10,000 ppm for 13 weeks. All experimental
groups were started at a level of 500 ppm of captan and the dose
levels were gradually increased until the 5000 ppm level was reached
after four weeks and the 10,000 ppm level after seven weeks. Both dose
levels caused growth retardation; however, there was no difference
between comparable groups of rate receiving the technical and
recrystallized material (Gray, 1954).
Long-term studies
Rat
Groups, each containing 10 male and 10 female rats, were fed diets
containing 0, 1000 and 5000 ppm of technical captan for two years.
Another group of 20 rats received 10,000 ppm of technical captan for
24 weeks. This group was then divided in half, one half being fed
recrystallized captan for 30 weeks; the other half continued on the
technical compound for 30 weeks. The female animals in the group
receiving 1000 ppm of captan had a reduction in weight gain for the
last 16 weeks of the experiment. Female rats receiving 5000 ppm of
technical captan also displayed reduced weight gain, and both sexes on
diets containing 10,000 ppm of either technical or recrystallized
captan had marked growth depression. At autopsy, indication of
testicular atrophy was found in some animals fed 10,000 ppm. Otherwise
the organ-weight, blood picture, tumour frequency and histological
studies were not significantly different from those in controls (Weir,
1956).
A group of 30 male and 30 female rats was fed dietary levels of 1000
ppm of captan for 17 months. Body-weight gains, food consumption,
survival rate and tumour incidence were comparable to a control group
(Kay, 1961).
COMMENT
The acute short-term and long-term studies on captan provide adequate
information to determine no-effect levels. Information of the fate of
the trichloromethylthio moiety in the metabolism of captan is still
incomplete. The observation that the acute toxicity of the compound to
rats is many times higher when the animals are fed a low protein diet
is of some concern. The special studies are extensive and cover a
range of animal species. Possible teratogenic effects in rabbits and
indications of embryotoxic effects in monkeys were observed, and
therefore only a temporary acceptable daily intake was established.
However, the biochemical requirements referred to in the previous
monograph on captan (FAO/WHO, 1965) have now been partially fulfilled
and therefore it was considered justified to establish a slightly
higher acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 100 mg/kg body-weight/day
Monkey: 12.5 mg/kg body-weight/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captan is a non-systemic fungicide with no insecticidal or acaricidal
activity. According to a survey (Kirby, 1969) of world-wide fungicide
use on top fruit, captan was first choice for control of scab on
apple, pear and peach, bitter rot and black rot on apple, sooty blotch
and leaf spot on pear, brown rot on cherry, peach and plum, and leaf
spot on sweet cherry. Other fruits on which its use is recommended
include apricots and grapes, and it is only now being superseded for
control of botrytis (gray mould) on strawberries. Captan is approved
in the U.K. for the control of stem rot of tomato. It is also used on
citrus and many vegetables.
In the U.K. in 1967, captan was used on about 11,000 hectares of
apples and 2,500 hectares of pears, with a small usage on plum, cherry
and peach. Probably about 1000 hectares of apples received captan for
control of storage rots, i.e. were sprayed in August-September. The
rate of use is 0.1 percent a.i. high volume, or about 3 kg a.i. per
ha. West Germany recommends 2 kg a.i. per ha for control of apple scab
or vine downy mildew.
Captan is of no value for control of rusts or powdery mildews.
Post-harvest treatments
Captan is used for rot control in stored potatoes and as a dip for
fruit and vegetables. It is also used as a pre-packing spray for
packing boxes. Prunes dipped in suspensions of the 50 percent w.p. at
0.12, 0.24 and 0.48 percent a.i. bore 3.6, 4.8 and 11 ppm and 2.3, 6.1
and 11.1 ppm captan before and after dehydration, respectively (Archer
and Corbin, 1969).
Other uses
Captan is used as a seed dressing, particularly on peas. It has been
proposed for ringworm control on animals; milk obtained 24 hours after
a cow had been sprayed contained less captan than could be detected
(<1 ppm) (Hansen, 1953). Captan is also used on turf and ornamentals,
especially roses, and as a soil fungicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Interval Rate Residue
Crop days % a.i. ppm
Apple 1 0.12 4 to 36
42 0.12 0.3 to 0.5
94 0.12 0.2
Apricot (fresh) 0 0.12 16 to 17.5
7 0.12 11
21 0.12 6 to 9
42 0.12 4 to 5
Apricot (dried) 7 0.12 8
40 0.12 <1
Cherry 0 0.24 10 to 53
7 0.24 6
14 0.24 4
0 0.12 4 to 22
7 0.12 3
14 0.12 1
Citrus 1 0.12 9 to 13
Fig 0 0.12 1.7
2 0.12 0.2
Peach 0 0.12 2 to 6
5 0.12 3
14 0.12 8
0 0.24 3 to 7
3 0.24 8 to 11
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Peach 14 0.24 1 to 12
Pear 1 0.12 1 to 28
9 0.12 8 to 12
Plum (Post-harvest) 1.2 to 4.8 4 to 11 (green)
do. do. 3 to 11 (dehydrated)
Sweet potato 0 0.24 2
0 0.48 25
Hops 119 0.24 0.1 to 1.5
Rhubarb 0 0.12 6 to 14
Grape 1 0.12 12 to 34
57 0.12 4
1 4.6 kg per ha 0.1 to 0.4
90 4.6 kg per ha 0.1 to 0.5
Strawberry 0 5.75 kg per ha 6.5
2 do. 3 to 7
5 do. 3 to 5
8 do. 1 to 5
Blueberry 0 0.24 0.8 to 1.6
Cranberry 0 4.6 kg per ha 4 to 7
83 do. 1.5 to 2.5
Raspberry 7 2.9 kg per ha 0.7
12 do. 11
Raisin (before wash) 0 1.15 kg per ha 27 to 35
(after wash) 0 do. 1 to 2
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Cucumber 1 0.12 5.6 to 6.3
7 0.12 0.3 to 2.5
Lettuce 0 0.18 0.2 to 6
5 0.18 1
10 0.18 0.7 to 5
Green beans 0 0.24 2 to 7
Pepper 1 0.12 6 to 8
7 0.12 1 to 9
Spinach 3 0.12 75
10 0.12 15
Tomato 0 0.24 6 to 12
3 0.24 10
20 0.24 <1
Further information on residues is available from the literature. One
late-season application of captan at 0.1 percent to apples and pears
gave deposits of about 1 ppm, falling to undetectable levels at
harvest (Martin and Pickard, 1955). Strawberries sprayed five times,
from early bloom to one day before picking, with 1.5 kg per ha captan
on each occasion bore 6.0 ppm captan; fruit picked three days later
bore 4.7 ppm and fruit picked eight days after the final spray bore
2.9 ppm; fruit from plots sprayed with 2.2 kg per ha in another year
bore similar but smaller residues (Fahey et al. 1962). Strawberries
sprayed twice in early bloom with captan at 0.4 percent a.i. (9.2 kg
a.i. per ha) had 6 ppm on the first fruits to ripen and less than 2
ppm on fruits picked eight days later (Sillibourne 1966). In Finland,
0.15% a.i. sprayed on to lettuce led to 57 ppm after three days:
washing reduced this to 2 ppm (State Inst. Agric. Chem., Helsinki,
1969).
FATE OF RESIDUES
Captan does not appear to undergo any chemical change on plant
surfaces, and only a very small amount appears to enter plant tissues.
Potatoes dipped in a captan slurry had 29 ppm on the skin and only 0.3
ppm in the pulp seven days later; no residue could be detected in the
pulp after baking or boiling (Chevron Chemical Company, Pesticide
Petition No. 15, 1955, direct communication).
Residues of up to 8 ppm on Valencia oranges were reduced by the normal
commercial washing procedure to 0.2 ppm. When 10 ppm captan was added
to washed oranges before processing to pulp, no captan could be
detected in the pulp or molasses (Chevron, 1959).
METHODS OF RESIDUE ANALYSIS
The method of Kittleson (1952) for the calorimetric determination of
captan residues forms the basis of the recommended procedure
(Association of Official Agricultural Chemists, 1965) which is
suitable for regulatory purposes at the present time. The cleaned-up
extract of fruit or green vegetables is heated with resorcinol and the
optical density of the resultant solution in acetic acid is measured
at 425 nm. Ospenson et al. (1964) have reviewed the uses of this
procedure, which is inaccurate below about 0.5 ppm of captan, and a
similar but less sensitive method using pyridine as the colour-forming
reagent.
More recently, interest has been shown in chromatographic methods for
captan. Kilgore et al. (1967) proposed a gas chromatographic method
for the examination of apricots, peaches, tomatoes and cottonseed.
Using a silicone column with electron-capture detection, the detection
limit was about 0.01 ppm with recoveries averaging 92 percent; under
similar chromatographic conditions captan was clearly separated from
difolatan (Kilgore and White, 1967). Several stationary phases were
studied by Bevenue and Ogata 1968) who found the cyanosilicone GE
XE-60 most suitable for captan residue analysis since it gave the best
separation from folpet. These three allied compounds, captan, folpet
and difolatan, were examined by Pomerantz and Ross (1968) who devised
gas and thin-layer chromatographic systems for the separation,
identification and determination of the parent compounds and some of
their possible degradation products. Archer and Corbin (1969) have
suggested a thin-layer chromatographic procedure for detecting captan
residues in prune fruits and blossoms. These chromatographic methods
should form the basis of a suitable regulatory procedure for the
determination of residues of captan in fruit, and it is recommended
that such a method should be established.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Benelux Tree fruits, vegetables 15
Canada Tree fruits, vegetables, nuts, 40
small fruits, root crops,
melons, leafy vegetables
Germany (Fed. Rep.) Fruits 15
United States of Beetgreens, cherry, lettuce, 100
America spinach
Stone fruits, grapes, mangoes 50
celery, leeks, onions (green),
shallots
Apple, pear, avocado, small 25
fruits, cucurbits, onions
(dry), tomato, garlic, egg
plant
Beet (roots), cottonseed, 12 2
vegetables
Beans (dry and succulent), 25 (interim)
citrus pineapple potato
Almond (kernels) 2 (interim)
Almond (hulls) 100 (interim)
APPRAISAL
Captan is a non-systemic fungicide that has attained very wide use on
fruit, vegetables, ornamentals, turf and seeds; it is probably
recommended for more diseases on deciduous top fruit than any other
fungicide. It provides no control of powdery mildews and little of
rusts. The technical product is stated to contain 95 percent captan,
the main impurities being water, common salt, and unreacted
tetrahydrophthalimide.
Solubility in water is very low and residues remain as superficial
deposits; these are partly removed by washing with water, especially
from non-waxy crops such as strawberry. Dry captan is stable to heat
and U.V. radiation, but aqueous suspensions are quickly decomposed at
100° C or in alkaline media. Hydrogen sulphide is evolved and all
other breakdown products remain in solution. The instability of the
-N-S-bond leads to release of tetrahydrophthalimide and deamination
products. Cooking and other processing methods lead to rapid
decomposition of captan residues. Persistence on crop surfaces is
relatively long.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples and cherries 40 ppm
Pears 30 ppm
Apricots 20 ppm
Citrus, peaches, plums, rhubarb,
tomatoes 15 ppm
Strawberries, cranberries,
raspberries, cucumbers,
lettuce, green beans, peppers 10 ppm
Raisins (dried vine fruits) 5 ppm
Insufficient data were available to enable tolerances to be suggested
for blueberries, figs, hops, sweet potatoes or spinach.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Further teratogenicity studies in non-human primates.
2. Further studies on metabolism especially on the
trichloromethylthiomoiety.
3. Residue data for other crops, including blueberries, figs, hops,
sweet potatoes and spinach.
4. Residue data from countries other than the U.S.A.
DESIRABLE
1. The effects of feeding a low protein diet on the chronic toxicity
of captan.
2. The development and evaluation of a GLC method, distinguishing
captan from captafol and folpet, suitable for regulatory purposes.
REFERENCES
Ackerson, G.W. and Mussehl, F.E. (1953) Toxicity of treated seed corn
in rations for chicks. Unpub. rept. Departments of Biochemistry.
Nutrition and Poultry Husbandry, University of Nebraska
Archer, T.E. and Corbin, J.B. (1969) Detection of captan residues in
prune fruits and blossoms by thin-layer chromatography. Bull. envir.
Contam. Toxicol. 4:55-63
Arnold, D. (1967) Mutagenic study on captan. Swiss white mice. Unpub.
rept. from Industrial Bio-test Laboratories submitted to California
Chemical Company
Association of Official Agricultural Chemists. (1965) Official methods
of analysis of the Association of Official Agricultural Chemists. 10th
ed. Washington D.C. 390:24.111
Batte, E.G. (1953) A study of the effect of feeding Orthocide(R)
treated seed to hogs. Unpub. rept. submitted by Stauffer Chemical
Company
Bevenue, A. and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by Chromatography. J. Chromatog. 36:529-31
Boyd, E.M. and Krijnen, C.J, (1968) Toxicity of captan and
protein-deficient diet. J. clin, Pharmacol. 8:225-34
Chevron. (1959) Captan residues an processed citrus by-products.
Chevron Report November 1959
Courtney, K.D. (1968) Teratological investigation of captan, imidan
and thalidomide in Macaca mulatta. Unpub. rept. of the Bionetics
Research Laboratories, submitted to Stauffer Chemical Company
Crossley, J. (1967) The stability of captan in blood. Unpub. rept.
prepared and submitted by Chevron Chemical Company
Dye, D.F. (1969) Difolatan(R). Unpub. summary report prepared and
submitted by Chevron Chemical Company
Elsea, J.R. (1957) Captan technical 91%, pure DDT. Acute oral
administration; acute potentiation. Unpub. rept. of Hazleton
Laboratories submitted to Stauffer Chemical Company and to California
Spray-Chemical Company (cited by Lehman, 1965)
Fabro, S., Smith, R.L. and Williams, R.T. (1965) Embryotoxic activity
of some pesticides and drugs related to phthalimide Fd. Cosmet.
Toxicol. 31 587-90
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., and Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. econ.
Ent. 55: 179-84
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Fogleman, R.W. (1955) Technical captan, recrystallized captan. Chronic
oral administration - dogs. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Gray, E.H. (1954) Technical captan, recrystallized captan. Subacute
feeding-comparative study. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Hansen, J. (1953) California Spray-Chemical Corporation (unpublished).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst. 42: 1102-14
Johnson, D.F. (1954) A toxicity test of
n-trichloromethylthiotetrahydrophthalimide. Southwestern Vet. 8:
55-57
Kay, J.H. and Calandra, J.C. (1961) Chronic oral toxicity of captan.
Albino rate. Addendum report to Ortho Division, California Chemical
Company prepared by Industrial Bio-test Laboratories Inc.
Kennedy, G. (1966) Three generation reproduction study on captan
- albino rats. Unpub. rept. of Industrial Bio-test Laboratories
submitted to Chevron Chemical Company
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet, and Difolotan.
Toxicol. appl. Pharmacol. 13:420-30
Kilgore, W.W. and White, E.R. (1967) Determination of difolatan
residues in fruits by electron-capture gas chromatography. J. Agr. Fd
Chem. 15: 1118-20
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determination of captan residues. J. Agr. Fd Chem. 151 1035-37
Kirby, A.H.M. Fungicides for deciduous top fruit: a survey in 1968. Wd
Rev. Pest 1969 Control, 8: 45-58
Kittleson, A.R. (1952) Colorimetric determination of
N-trichloromethylthiotetrahydro-phthalimide. Analyt. Chem. 24: 1173-75
Klayder, T.J. (1963) Captan in green vegetables. J. Assn Off, Anal.
Chem. 46: 241-3
Leary, J.B. (1966) Difolatan: shay rat tests, Unpub. rept. of Chevron
Chemical Company (cited by Crossley, 1967)
Legator, M. S., Kelley, F.J., Green, S. and Oswald, E.J. (1969)
Mutagenic effects of captan. Annals W.Y. Acad. Sci. 160: 344-51
Lehman, A.J. (1965) "Summaries of Pesticide Toxicity", Assoc. Food and
Drug Offic., Topeka, Kansas, page 96
Link, R.P., Smith, J.C. and Morrill, C.O. (1956) Toxicity studies on
captan-treated corn in pigs and chickens. J. Amer. vet. Med. Ass. 128:
614-16
Martin, J.T. and Pickard, J.A. (1955) Spray application problems: XIII
determination of captan deposits; progress report. Ann. Rap. Long
Ashton Roe. Stn for 1954, 83-9.
McLaughlin, J.P., Reynaldo, E.P., Lamar, J.K. and Marlaic, J.P. (1969)
Teratology studies in rabbits with captan, folpet, and thalidomide.
Toxicol. appl. Pharmacol. 14:641
Meade, R.J. and Warner, D.R. (1954) The effect of seed disinfectants,
Orthocide and Arasan, upon the performance of growing fattening swine.
Unpub. rept. from Nebraska Agricultural Experiment Station, College of
Agriculture, University of Nebraska. submitted by Stauffer Chemical
Company
Ospenson, J.N., Pack, D.E., Kohn, G.K., Burchfield, H.P. and Storrs,
E.E. (1964) in Analytical Methods for Pesticides, Plant Growth
Regulators and Food Additives. Edited by O. Zweig. Volume III.
Academic Press, PP. 11-25
Owens, R.G. (1969) Metabolism of fungicides and related compounds.
Annal. N.Y. Acad. Sci. 100: 114-32
Palazzalo, R.J. (1966) Chicken reproduction study in captan. Unpub.
rept. of Industrial Bio-test Laboratories, submitted to Chevron
Chemical Company
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds, thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51: 1058-62
Sillibourne, J.M. (1956) Chemical control of grey mould on
strawberries; deposits of fungicides on Royal Sovereign in relation to
harvest residues. Rep. E. Malling Res. Stn for 1965, 183-91
State Institute Agric. Chem., (1969) Helsinki. Investigations on
pesticide residues, 1968, P.19
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compound in the developing chicken embryo. Annals N.Y. Acad. Sci.,
160: 334-43
Vondruska, J.F. (1969) Teratological investigation of captan in Macaca
mulatta (Rhesus monkey) and Macaca arctoides (stumptailed macque).
Unpub. rept. of Industrial Bio-Test Laboratories---submitted to
Chevron Chemical Company
Weir, R.J. (1956) Technical captan, recrystallized captan. Chronic
feeding - rats. Final report. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to Chevron Chemical Company
Weir, R.J. (1957) Technical captan, captan technical 91%. Subacute
feeding poultry. Unpub. rept. of Hazleton Laboratories submitted to
Stauffer Chemical Company and to Chevron Chemical Company
Other relevant chemical properties
The technical product is claimed to contain 96 percent captan; the
remainder consists of common salt (sodium chloride), water and
unreacted tetrahydrophthalimide. The pure crystal ins product, m.p.
178° C, is reported to have a vapour pressure of less than 1 × 10-6
torr at 25° C, but that of the technical product is stated to be much
higher. Solubility in water at normal temperatures is extremely low;
it is less than 10 percent in common organic solvents. Captan is
stable, except under alkaline conditions, at normal temperature; it
will decompose at temperatures in the region of 100° C, very slowly in
dry or rapidly in moist atmospheres (Klayder, 1963). In alkaline
solution, 1.0N NaOH, captan yields 2.8 equivalents of chloride ion.
Cooking, especially in water would lead to rapid decomposition of
residues to water-soluble products.
Formulated products include wettable powders containing 50 to 83
percent a.i. and dusts, those for field use containing a few percent
a.i. and those for seed treatment 75 Percent.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
This pesticide was evaluated for acceptable daily intake by the 1965
Joint FAO/WHO Meeting on Pesticide Residues (FAO/WHO, 1965). Since
that time additional information has become available, especially on
the metabolism and on the effect upon reproduction. Therefore, the
previously published monograph has been revised and is now reproduced
in its entirety.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Captan is rapidly decomposed in both human and rabbit blood at room
temperature. Decomposition appears to be according to first order
kinetics although some concentration dependency is evident, possibly
due to the low solubility of captan in aqueous media. At initial
concentrations of 100 µg/ml or 1/µg/ml in human blood the half-life of
captan was calculated as 0.9 minutes or 0.2 minutes respectively. In
rabbit blood at an initial concentration of 1 µg/ml, the half-life is
0.3 minutes (Crossley, 1967).
Captan, as an aqueous slurry, was administered by intragastric
intubation to female rabbits at levels of 0 mg/kg body-weight (two
rabbits) or 500 mg/kg body-weight (five rabbits). Blood was drawn
directly from the heart at intervals up to 56 hours and analysed for
captan and its metabolite tetrahydrophthalimide. No captan was
detected in the blood at any time after administration of the dose,
using an analytical method sensitive to 0.025 µg/ml. A build-up of the
metabolite tetrahydrophthalimide occurred in the blood which reached a
maximum of about 25 µg/ml 30 hours after intragastric administration
of captan. From then on it decreased to a level of 4.2 µg/ml after 56
hours; the estimated half-life being 6 hours (Crossley, 1967).
Recent information on the metabolism of captan has been inferred from
work on captafol, a compound which differs from captan only in the
nature of the chlorinated group attached to the sulphur atom (see the
monograph on captafol). Rats were fed celery which had been treated
with captafol to give levels of 60 or 600 ppm. The stomach content of
the animals was analysed at several intervals and both
tetrahydrophthalimide and tetrahydrophthalic acid were detected
(Leary, 1966).
Rats, dogs and monkeys wore fed carbon14-carbonyl labelled captafol.
The radioactivity rapidly entered the blood and tetrahydrophthalimide
was detected in the blood, faeces and urine. Tetrahydrophthalamic acid
as well as other more water soluble metabolites were the principal
radioactive compounds present in the blood and urine. Because of the
similarity in structure it has been assumed that captan would be
metabolized in a similar fashion with respect to the
tetrahydrophthalic acid portion of its molecule (Dye, 1969).
Feeding studies with captan demonstrated that captan is not stored in
the eggs or flesh of poultry nor in the tissues of pigs (Weir, 1957;
Link et al., 1956).
Effect on enzymes and other biochemical parameters
In the presence of compounds such as cysteine which contain sulfhydryl
groups, all the three chlorine atoms in captan are liberated an
chloride ions, and four sulfhydryl groups disappear for every molecule
of captan that reacts. In general captan initially reacts with two
molecules of a simple thiol to give tetrahydrophthalimide, the
disulfide derived from the thiol, thiophosgene and one chloride ion.
The thiophosgene reacts with two additional molecules of the thiol, to
give ultimately two more chloride ions, carbon disulfide and the
sulfide derived from the thiol. In the case where cysteine is the
thiol the reaction is slightly more complicated resulting in the
formation of a substituted thiazolidinethione. The presence of all
these compounds from the metabolism of captan, has been confirmed
chemically (Owens, 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
A three-generation reproduction study was conducted with rats which
received technical captan in their diet in concentrations of 0, 100,
500, and 1000 ppm. Groups of 16 female animals were used and two
litters were produced in each generation. No significant differences
were found between control and captan treated rats with respect to
fertility, gestation, viability or lactation indices, or in the
weaning weights in the first two generations. No effects were observed
in the third generation except for a slightly lowered lactation index
for the group receiving 1000 ppm of captan. Histopathological
examination of tissues from 10 pups receiving 1000 ppm in the third
generation revealed no damage (Kennedy, 1966; Kennedy, et al., 1968).
Special studies on teratogenicity
Chicken
A group of 5 male and 18 female White Leghorn chickens were fed a diet
containing 0 or 2,300 ppm (equivalent to 75 mg/kg body-weight)
technical captan for 6 weeks. The birds were observed for body-weight
effects, food consumption, behavioural reaction, and egg production.
Eggs collected during days 29 through 39 were incubated to determine
the extent of hatchability and the presence of any effects on the
chicks. No adverse effects were noted (Palazzolo, 1966).
Captan was injected in dimethylsulfoxide solution into either the yolk
or air cell of fresh fertile White Leghorn eggs in amounts sufficient
to produce concentrations of 3 to 20 ppm in the eggs. The eggs were
incubated and non-viable embryos and hatched chicks were examined for
gross abnormalities. In a total of 1,292 eggs, the incidence of
malformations was 7.8 percent compared to an incidence of less than 2
percent in solvent-injected and uninjected control eggs. Malformations
of the feet and legs followed a specific pattern. Micromelia, amelia,
and phocomelia accounted for most of the deformities (Verrett, at al.,
1969).
Hamster
Groups, each comprising 20 pregnant female hamsters ware fed diets
containing concentrations of captan sufficient to enable the animals
to receive an average daily intake of 0, 125, 250, or 1000
mg/kg/body-weight of captan from days 1 through 15 of gestation. At
day 159 all females were sacrificed, the young were surgically removed
and the foetal development and structural formation of each was
examined. The incidence of abnormal effects was not greater in any
test group than in the controls. The dose level of 1000
mg/kg/body-weight of captan resulted in a significant increase in
foetal resorption (Kennedy, at al., 1968).
Monkey
Groups of seven pregnant Rhesus monkeys were given daily oral doses of
6.35, 12.5 or 25 mg/kg body-weight of captan on days 22 through 32 of
gestation. (Thalidomide was used as a positive control at dosage
levels of 5 mg/kg/body-weight/day in six animals and at 10
mg/kg/body-weight/day in four animals). Foetuses were recovered on
approximately day 84 of gestation by Caesarian section and examined
for organ and skeletal defects. Foetal mortality occurred in three of
seven monkeys at the 25 mg/kg level. The foetal mortality in the
parent colony not fed captan was 13.2 percent on 439 conceptions.
There was no abnormality among any foetus in either of the three dose
levels of captan (Courtney, 1968).
Groups of seven pregnant monkeys (Rhesus and stumptail) were given
captan orally at levels of 10, 25 or 75 mg/kg body-weight daily on
days 21 through 34 of gestation. One abortion occurred at the 75 mg/kg
level but there were no malformations. (Thalidomide was given as a
positive control an in the previous experiment (Vondruska, 1969).
Rabbit
Four pregnant New Zealand White rabbits were given daily oral doses of
80 mg/kg bodyweight of captan during days 7 through 12 of gestation.
Captan produced no embryotoxicity in the litters of rabbits (Fabro at
al., 1965).
A group of six pregnant Dutch Belted rabbits was given 75
mg/kg/body-weight/day of technical captan, orally on days 6 through 16
of gestation. Three other groups each containing five to seven New
Zealand White rabbits were given 18.75, 37.5 or 75
mg/kg/body-weight/day of technical captan orally on days 6 through 18
of gestation. A control group and a positive control group (75 mg/kg
thalidomide) wore also maintained. No malformed foetuses occurred in
any group treated with captan. An increased incidence of foetal
resorption occurred in the New Zealand White rabbits given 75 mg/kg of
captan. Another group of nine pregnant Dutch Belted rabbits were given
75 mg/kg/body-weight/day of technical tetrahydrophthalimide, a
metabolite of captan, on days 6 through 16 of gestation. A slight rise
in the occurrence of resorption sites occurred. No skeletal
abnormality was observed (Kennedy, et al., 1968).
Groups of 9 pregnant New Zealand white rabbits were given captan at
dose-levels of 37.5, 75 or 150 mg/kg body-weight/day from days 6
through 16 of gestation. Thalidomide was used as a positive control at
levels of 75 and 150 mg/kg body-weight and produced the expected
teratological response. Captan at 75 mg/kg caused 9 malformed young
from 75 implantations of 9 pregnant does. At the dose level of 37.5
mg/kg captan produced one malformed foetus from 49 implantation sites
(McLaughlin at al., 1969).
Rat
Groups of five to ten pregnant female rats were given oral doses of 0,
50, 100, or 250 mg/kg/body-weight day of technical captan from days 6
through 15 of gestation or 0, 500, 1000 or 2000 mg/kg/body-weight/day
from days 8 through 10. Examination of 371 foetuses obtained from the
captan-treated rats revealed no significant increase in the number of
abnormalities. Three and two grossly malformed foetuses were found
from the rats treated with 1000 and 2000 mg/kg/body-weight/day of
captan respectively; compared with one in the corn oil control and
none in the lower doses of captan (Kennedy, et al., 1968).
Special studies on mutagenicity
Mouse
Single intraperitoneal injections of technical captan at doses of 0,
3.5 and 7.0 mg/kg body-weight were administered to an unspecified
number of male mice. Another group of male mice was given 100 mg/kg
body-weight of methyl methanesulfonate as a positive control. The
treated male groups were mated with three groups of untreated females
on each of three consecutive weeks post-treatment. The results
demonstrated that dominant lethal mutations were not induced by
treatment with captan (Arnold, 1967).
The mutagenic activity of captan was evaluated in bacteria, in the
heteroploid human embryo lung call line, and in a cell line derived
from the kidney of the rat-kangaroo. In bacteria, captan increased the
mutation rate both in streptomycin-dependent E. coli and a
thymine-dependent E. coli strain. Captan inhibited both growth and
mitosis of heteroploid embryonic lung cells in concentrations of less
than 5 µg/ml. In the studies with the rat-kangaroo cell line the
chromosome breaks and mitotic inhibition were proportional to the
concentration of captan over the range of 1 to 10 µg/ml (Legator, et
al., 1969).
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
captan from 7 days of age for 18 months. The dose of 215 mg/kg
body-weight was given to the mice daily by gavage from the seventh day
of age to the time of weaning at four weeks of age; thereafter, the
chemical was added to the diet in the corresponding amount of 560 ppm.
There was no significant increase in tumours in the group fed captan
compared with the controls (Innes, et al., 1969).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat oral 9,000 Elsea, 1957
Rat oral 12,500 Boyd and Krijnen, 1968
Rat oral 480 (low
protein diet)
Mouse i.p. 10 Arnold, 1967
Short-term studies
Chicken
An unspecified number of chicks were fed a diet containing 320 ppm of
captan for 28 days. In another experiment a group of 30 chicks was fed
the same dosage for 74 days along with a control group of 10 chicks.
No gross abnormalities were found in the birds in either experiment
(Ackerson and Mussehl, 1953; Link et al., 1956).
Groups of 15 hens were fed diets containing 0, 100, 1000, or 10,000
ppm of technical captan for 90 days. The hens receiving 100 and 1000
ppm of captan displayed normal food consumption, egg production, and
survival. In those receiving 10,000 ppm there was food-refusal, weight
loss, and decreased egg production. There were no gross effects
observed at autopsy in any cup. Analysis of the eggs and of the
tissues of the hens revealed no stored captan (Weir, 1957).
Dog
Groups of four dogs, each comprising two male and two female animals,
were started on a dose regime of 0, 10, 25 and 50 mg/kg body-weight of
captan. At the beginning of week 10 the dose level of the dogs
receiving 25 mg/kg was increased to 100 mg/kg. At week 18 these dose
levels were again increased to 100 mg/kg and 300 mg/kg respectively.
The captan was administered by gelatin capsule normally six days a
week for 66 weeks. Liver and kidney weights were slightly increased in
the dogs which received 300 mg/kg body-weight. There was no evidence
of systemic toxicity and no gross or histopathological changes in
tissues due to treatment at any dose-level, nor were there any
significant changes in haematological or biochemical findings
(Fogleman, 1955).
Pig
Eight half-grown pigs were fed for three months on corn treated with
captan to provide a level of 540 ppm. The treatment did not affect the
rate of food consumption, rate of growth or general health of the
animals when compared to the effect on an equal number of control
animals. There was no gross abnormality observed in any animal on the
captan diet (Batter 1953).
Four groups, comprising 10 weanling pigs each of about 14 kg in
weight, were fed rations containing 0, 420, 820, 1,680 ppm of captan
for 119 days. No gross pathological effects that could be attributed
to captan were observed (Meads and Warner, 1954).
Three pigs were fed 500 ppm and two pigs were fed 4000 ppm of captan
in the diet for 22-25 weeks. The animals displayed no abnormal
symptoms, but no further observations were made (Johnson, 1954).
Seven pigs were fed 480 ppm of captan in their diet for 14 weeks,
three other animals served as controls. The test animals displayed
normal weight gain. Gross examination of all organs revealed no
pathological changes and histopathological study of the liver and
kidneys revealed no abnormalities. Erythrocyte and lenkocyte counts
were not significantly different between test and control groups. No
residual captan (i.e. <0.1 ppm) was found in the tissues of the
animals (Link at al., 1956).
Rat
Five groups, each containing 11 male and 11 female rats, were fed
diets containing either technical or recrystallized captan at dose
levels up to 0, 5000 or 10,000 ppm for 13 weeks. All experimental
groups were started at a level of 500 ppm of captan and the dose
levels were gradually increased until the 5000 ppm level was reached
after four weeks and the 10,000 ppm level after seven weeks. Both dose
levels caused growth retardation; however, there was no difference
between comparable groups of rate receiving the technical and
recrystallized material (Gray, 1954).
Long-term studies
Rat
Groups, each containing 10 male and 10 female rats, were fed diets
containing 0, 1000 and 5000 ppm of technical captan for two years.
Another group of 20 rats received 10,000 ppm of technical captan for
24 weeks. This group was then divided in half, one half being fed
recrystallized captan for 30 weeks; the other half continued on the
technical compound for 30 weeks. The female animals in the group
receiving 1000 ppm of captan had a reduction in weight gain for the
last 16 weeks of the experiment. Female rats receiving 5000 ppm of
technical captan also displayed reduced weight gain, and both sexes on
diets containing 10,000 ppm of either technical or recrystallized
captan had marked growth depression. At autopsy, indication of
testicular atrophy was found in some animals fed 10,000 ppm. Otherwise
the organ-weight, blood picture, tumour frequency and histological
studies were not significantly different from those in controls (Weir,
1956).
A group of 30 male and 30 female rats was fed dietary levels of 1000
ppm of captan for 17 months. Body-weight gains, food consumption,
survival rate and tumour incidence were comparable to a control group
(Kay, 1961).
COMMENT
The acute short-term and long-term studies on captan provide adequate
information to determine no-effect levels. Information of the fate of
the trichloromethylthio moiety in the metabolism of captan is still
incomplete. The observation that the acute toxicity of the compound to
rats is many times higher when the animals are fed a low protein diet
is of some concern. The special studies are extensive and cover a
range of animal species. Possible teratogenic effects in rabbits and
indications of embryotoxic effects in monkeys were observed, and
therefore only a temporary acceptable daily intake was established.
However, the biochemical requirements referred to in the previous
monograph on captan (FAO/WHO, 1965) have now been partially fulfilled
and therefore it was considered justified to establish a slightly
higher acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 100 mg/kg body-weight/day
Monkey: 12.5 mg/kg body-weight/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.125 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captan is a non-systemic fungicide with no insecticidal or acaricidal
activity. According to a survey (Kirby, 1969) of world-wide fungicide
use on top fruit, captan was first choice for control of scab on
apple, pear and peach, bitter rot and black rot on apple, sooty blotch
and leaf spot on pear, brown rot on cherry, peach and plum, and leaf
spot on sweet cherry. Other fruits on which its use is recommended
include apricots and grapes, and it is only now being superseded for
control of botrytis (gray mould) on strawberries. Captan is approved
in the U.K. for the control of stem rot of tomato. It is also used on
citrus and many vegetables.
In the U.K. in 1967, captan was used on about 11,000 hectares of
apples and 2,500 hectares of pears, with a small usage on plum, cherry
and peach. Probably about 1000 hectares of apples received captan for
control of storage rots, i.e. were sprayed in August-September. The
rate of use is 0.1 percent a.i. high volume, or about 3 kg a.i. per
ha. West Germany recommends 2 kg a.i. per ha for control of apple scab
or vine downy mildew.
Captan is of no value for control of rusts or powdery mildews.
Post-harvest treatments
Captan is used for rot control in stored potatoes and as a dip for
fruit and vegetables. It is also used as a pre-packing spray for
packing boxes. Prunes dipped in suspensions of the 50 percent w.p. at
0.12, 0.24 and 0.48 percent a.i. bore 3.6, 4.8 and 11 ppm and 2.3, 6.1
and 11.1 ppm captan before and after dehydration, respectively (Archer
and Corbin, 1969).
Other uses
Captan is used as a seed dressing, particularly on peas. It has been
proposed for ringworm control on animals; milk obtained 24 hours after
a cow had been sprayed contained less captan than could be detected
(<1 ppm) (Hansen, 1953). Captan is also used on turf and ornamentals,
especially roses, and as a soil fungicide.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Interval Rate Residue
Crop days % a.i. ppm
Apple 1 0.12 4 to 36
42 0.12 0.3 to 0.5
94 0.12 0.2
Apricot (fresh) 0 0.12 16 to 17.5
7 0.12 11
21 0.12 6 to 9
42 0.12 4 to 5
Apricot (dried) 7 0.12 8
40 0.12 <1
Cherry 0 0.24 10 to 53
7 0.24 6
14 0.24 4
0 0.12 4 to 22
7 0.12 3
14 0.12 1
Citrus 1 0.12 9 to 13
Fig 0 0.12 1.7
2 0.12 0.2
Peach 0 0.12 2 to 6
5 0.12 3
14 0.12 8
0 0.24 3 to 7
3 0.24 8 to 11
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Peach 14 0.24 1 to 12
Pear 1 0.12 1 to 28
9 0.12 8 to 12
Plum (Post-harvest) 1.2 to 4.8 4 to 11 (green)
do. do. 3 to 11 (dehydrated)
Sweet potato 0 0.24 2
0 0.48 25
Hops 119 0.24 0.1 to 1.5
Rhubarb 0 0.12 6 to 14
Grape 1 0.12 12 to 34
57 0.12 4
1 4.6 kg per ha 0.1 to 0.4
90 4.6 kg per ha 0.1 to 0.5
Strawberry 0 5.75 kg per ha 6.5
2 do. 3 to 7
5 do. 3 to 5
8 do. 1 to 5
Blueberry 0 0.24 0.8 to 1.6
Cranberry 0 4.6 kg per ha 4 to 7
83 do. 1.5 to 2.5
Raspberry 7 2.9 kg per ha 0.7
12 do. 11
Raisin (before wash) 0 1.15 kg per ha 27 to 35
(after wash) 0 do. 1 to 2
(cont'd)
Interval Rate Residue
Crop days % a.i. ppm
Cucumber 1 0.12 5.6 to 6.3
7 0.12 0.3 to 2.5
Lettuce 0 0.18 0.2 to 6
5 0.18 1
10 0.18 0.7 to 5
Green beans 0 0.24 2 to 7
Pepper 1 0.12 6 to 8
7 0.12 1 to 9
Spinach 3 0.12 75
10 0.12 15
Tomato 0 0.24 6 to 12
3 0.24 10
20 0.24 <1
Further information on residues is available from the literature. One
late-season application of captan at 0.1 percent to apples and pears
gave deposits of about 1 ppm, falling to undetectable levels at
harvest (Martin and Pickard, 1955). Strawberries sprayed five times,
from early bloom to one day before picking, with 1.5 kg per ha captan
on each occasion bore 6.0 ppm captan; fruit picked three days later
bore 4.7 ppm and fruit picked eight days after the final spray bore
2.9 ppm; fruit from plots sprayed with 2.2 kg per ha in another year
bore similar but smaller residues (Fahey et al. 1962). Strawberries
sprayed twice in early bloom with captan at 0.4 percent a.i. (9.2 kg
a.i. per ha) had 6 ppm on the first fruits to ripen and less than 2
ppm on fruits picked eight days later (Sillibourne 1966). In Finland,
0.15% a.i. sprayed on to lettuce led to 57 ppm after three days:
washing reduced this to 2 ppm (State Inst. Agric. Chem., Helsinki,
1969).
FATE OF RESIDUES
Captan does not appear to undergo any chemical change on plant
surfaces, and only a very small amount appears to enter plant tissues.
Potatoes dipped in a captan slurry had 29 ppm on the skin and only 0.3
ppm in the pulp seven days later; no residue could be detected in the
pulp after baking or boiling (Chevron Chemical Company, Pesticide
Petition No. 15, 1955, direct communication).
Residues of up to 8 ppm on Valencia oranges were reduced by the normal
commercial washing procedure to 0.2 ppm. When 10 ppm captan was added
to washed oranges before processing to pulp, no captan could be
detected in the pulp or molasses (Chevron, 1959).
METHODS OF RESIDUE ANALYSIS
The method of Kittleson (1952) for the calorimetric determination of
captan residues forms the basis of the recommended procedure
(Association of Official Agricultural Chemists, 1965) which is
suitable for regulatory purposes at the present time. The cleaned-up
extract of fruit or green vegetables is heated with resorcinol and the
optical density of the resultant solution in acetic acid is measured
at 425 nm. Ospenson et al. (1964) have reviewed the uses of this
procedure, which is inaccurate below about 0.5 ppm of captan, and a
similar but less sensitive method using pyridine as the colour-forming
reagent.
More recently, interest has been shown in chromatographic methods for
captan. Kilgore et al. (1967) proposed a gas chromatographic method
for the examination of apricots, peaches, tomatoes and cottonseed.
Using a silicone column with electron-capture detection, the detection
limit was about 0.01 ppm with recoveries averaging 92 percent; under
similar chromatographic conditions captan was clearly separated from
difolatan (Kilgore and White, 1967). Several stationary phases were
studied by Bevenue and Ogata 1968) who found the cyanosilicone GE
XE-60 most suitable for captan residue analysis since it gave the best
separation from folpet. These three allied compounds, captan, folpet
and difolatan, were examined by Pomerantz and Ross (1968) who devised
gas and thin-layer chromatographic systems for the separation,
identification and determination of the parent compounds and some of
their possible degradation products. Archer and Corbin (1969) have
suggested a thin-layer chromatographic procedure for detecting captan
residues in prune fruits and blossoms. These chromatographic methods
should form the basis of a suitable regulatory procedure for the
determination of residues of captan in fruit, and it is recommended
that such a method should be established.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Benelux Tree fruits, vegetables 15
Canada Tree fruits, vegetables, nuts, 40
small fruits, root crops,
melons, leafy vegetables
Germany (Fed. Rep.) Fruits 15
United States of Beetgreens, cherry, lettuce, 100
America spinach
Stone fruits, grapes, mangoes 50
celery, leeks, onions (green),
shallots
Apple, pear, avocado, small 25
fruits, cucurbits, onions
(dry), tomato, garlic, egg
plant
Beet (roots), cottonseed, 12 2
vegetables
Beans (dry and succulent), 25 (interim)
citrus pineapple potato
Almond (kernels) 2 (interim)
Almond (hulls) 100 (interim)
APPRAISAL
Captan is a non-systemic fungicide that has attained very wide use on
fruit, vegetables, ornamentals, turf and seeds; it is probably
recommended for more diseases on deciduous top fruit than any other
fungicide. It provides no control of powdery mildews and little of
rusts. The technical product is stated to contain 95 percent captan,
the main impurities being water, common salt, and unreacted
tetrahydrophthalimide.
Solubility in water is very low and residues remain as superficial
deposits; these are partly removed by washing with water, especially
from non-waxy crops such as strawberry. Dry captan is stable to heat
and U.V. radiation, but aqueous suspensions are quickly decomposed at
100° C or in alkaline media. Hydrogen sulphide is evolved and all
other breakdown products remain in solution. The instability of the
-N-S-bond leads to release of tetrahydrophthalimide and deamination
products. Cooking and other processing methods lead to rapid
decomposition of captan residues. Persistence on crop surfaces is
relatively long.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples and cherries 40 ppm
Pears 30 ppm
Apricots 20 ppm
Citrus, peaches, plums, rhubarb,
tomatoes 15 ppm
Strawberries, cranberries,
raspberries, cucumbers,
lettuce, green beans, peppers 10 ppm
Raisins (dried vine fruits) 5 ppm
Insufficient data were available to enable tolerances to be suggested
for blueberries, figs, hops, sweet potatoes or spinach.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Further teratogenicity studies in non-human primates.
2. Further studies on metabolism especially on the
trichloromethylthiomoiety.
3. Residue data for other crops, including blueberries, figs, hops,
sweet potatoes and spinach.
4. Residue data from countries other than the U.S.A.
DESIRABLE
1. The effects of feeding a low protein diet on the chronic toxicity
of captan.
2. The development and evaluation of a GLC method, distinguishing
captan from captafol and folpet, suitable for regulatory purposes.
REFERENCES
Ackerson, G.W. and Mussehl, F.E. (1953) Toxicity of treated seed corn
in rations for chicks. Unpub. rept. Departments of Biochemistry.
Nutrition and Poultry Husbandry, University of Nebraska
Archer, T.E. and Corbin, J.B. (1969) Detection of captan residues in
prune fruits and blossoms by thin-layer chromatography. Bull. envir.
Contam. Toxicol. 4:55-63
Arnold, D. (1967) Mutagenic study on captan. Swiss white mice. Unpub.
rept. from Industrial Bio-test Laboratories submitted to California
Chemical Company
Association of Official Agricultural Chemists. (1965) Official methods
of analysis of the Association of Official Agricultural Chemists. 10th
ed. Washington D.C. 390:24.111
Batte, E.G. (1953) A study of the effect of feeding Orthocide(R)
treated seed to hogs. Unpub. rept. submitted by Stauffer Chemical
Company
Bevenue, A. and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by Chromatography. J. Chromatog. 36:529-31
Boyd, E.M. and Krijnen, C.J, (1968) Toxicity of captan and
protein-deficient diet. J. clin, Pharmacol. 8:225-34
Chevron. (1959) Captan residues an processed citrus by-products.
Chevron Report November 1959
Courtney, K.D. (1968) Teratological investigation of captan, imidan
and thalidomide in Macaca mulatta. Unpub. rept. of the Bionetics
Research Laboratories, submitted to Stauffer Chemical Company
Crossley, J. (1967) The stability of captan in blood. Unpub. rept.
prepared and submitted by Chevron Chemical Company
Dye, D.F. (1969) Difolatan(R). Unpub. summary report prepared and
submitted by Chevron Chemical Company
Elsea, J.R. (1957) Captan technical 91%, pure DDT. Acute oral
administration; acute potentiation. Unpub. rept. of Hazleton
Laboratories submitted to Stauffer Chemical Company and to California
Spray-Chemical Company (cited by Lehman, 1965)
Fabro, S., Smith, R.L. and Williams, R.T. (1965) Embryotoxic activity
of some pesticides and drugs related to phthalimide Fd. Cosmet.
Toxicol. 31 587-90
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., and Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. econ.
Ent. 55: 179-84
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
Fogleman, R.W. (1955) Technical captan, recrystallized captan. Chronic
oral administration - dogs. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Gray, E.H. (1954) Technical captan, recrystallized captan. Subacute
feeding-comparative study. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to California
Spray-Chemical Company
Hansen, J. (1953) California Spray-Chemical Corporation (unpublished).
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Nat. Cancer Inst. 42: 1102-14
Johnson, D.F. (1954) A toxicity test of
n-trichloromethylthiotetrahydrophthalimide. Southwestern Vet. 8:
55-57
Kay, J.H. and Calandra, J.C. (1961) Chronic oral toxicity of captan.
Albino rate. Addendum report to Ortho Division, California Chemical
Company prepared by Industrial Bio-test Laboratories Inc.
Kennedy, G. (1966) Three generation reproduction study on captan
- albino rats. Unpub. rept. of Industrial Bio-test Laboratories
submitted to Chevron Chemical Company
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet, and Difolotan.
Toxicol. appl. Pharmacol. 13:420-30
Kilgore, W.W. and White, E.R. (1967) Determination of difolatan
residues in fruits by electron-capture gas chromatography. J. Agr. Fd
Chem. 15: 1118-20
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determination of captan residues. J. Agr. Fd Chem. 151 1035-37
Kirby, A.H.M. Fungicides for deciduous top fruit: a survey in 1968. Wd
Rev. Pest 1969 Control, 8: 45-58
Kittleson, A.R. (1952) Colorimetric determination of
N-trichloromethylthiotetrahydro-phthalimide. Analyt. Chem. 24: 1173-75
Klayder, T.J. (1963) Captan in green vegetables. J. Assn Off, Anal.
Chem. 46: 241-3
Leary, J.B. (1966) Difolatan: shay rat tests, Unpub. rept. of Chevron
Chemical Company (cited by Crossley, 1967)
Legator, M. S., Kelley, F.J., Green, S. and Oswald, E.J. (1969)
Mutagenic effects of captan. Annals W.Y. Acad. Sci. 160: 344-51
Lehman, A.J. (1965) "Summaries of Pesticide Toxicity", Assoc. Food and
Drug Offic., Topeka, Kansas, page 96
Link, R.P., Smith, J.C. and Morrill, C.O. (1956) Toxicity studies on
captan-treated corn in pigs and chickens. J. Amer. vet. Med. Ass. 128:
614-16
Martin, J.T. and Pickard, J.A. (1955) Spray application problems: XIII
determination of captan deposits; progress report. Ann. Rap. Long
Ashton Roe. Stn for 1954, 83-9.
McLaughlin, J.P., Reynaldo, E.P., Lamar, J.K. and Marlaic, J.P. (1969)
Teratology studies in rabbits with captan, folpet, and thalidomide.
Toxicol. appl. Pharmacol. 14:641
Meade, R.J. and Warner, D.R. (1954) The effect of seed disinfectants,
Orthocide and Arasan, upon the performance of growing fattening swine.
Unpub. rept. from Nebraska Agricultural Experiment Station, College of
Agriculture, University of Nebraska. submitted by Stauffer Chemical
Company
Ospenson, J.N., Pack, D.E., Kohn, G.K., Burchfield, H.P. and Storrs,
E.E. (1964) in Analytical Methods for Pesticides, Plant Growth
Regulators and Food Additives. Edited by O. Zweig. Volume III.
Academic Press, PP. 11-25
Owens, R.G. (1969) Metabolism of fungicides and related compounds.
Annal. N.Y. Acad. Sci. 100: 114-32
Palazzalo, R.J. (1966) Chicken reproduction study in captan. Unpub.
rept. of Industrial Bio-test Laboratories, submitted to Chevron
Chemical Company
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds, thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51: 1058-62
Sillibourne, J.M. (1956) Chemical control of grey mould on
strawberries; deposits of fungicides on Royal Sovereign in relation to
harvest residues. Rep. E. Malling Res. Stn for 1965, 183-91
State Institute Agric. Chem., (1969) Helsinki. Investigations on
pesticide residues, 1968, P.19
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compound in the developing chicken embryo. Annals N.Y. Acad. Sci.,
160: 334-43
Vondruska, J.F. (1969) Teratological investigation of captan in Macaca
mulatta (Rhesus monkey) and Macaca arctoides (stumptailed macque).
Unpub. rept. of Industrial Bio-Test Laboratories---submitted to
Chevron Chemical Company
Weir, R.J. (1956) Technical captan, recrystallized captan. Chronic
feeding - rats. Final report. Unpub. rept. of Hazleton Laboratories
submitted to Stauffer Chemical Company and to Chevron Chemical Company
Weir, R.J. (1957) Technical captan, captan technical 91%. Subacute
feeding poultry. Unpub. rept. of Hazleton Laboratories submitted to
Stauffer Chemical Company and to Chevron Chemical Company
