
CAPTAN JMPR 1973
Explanation
This fungicide was evaluated by the 1965 and 1969 FAO/WHO
Meetings on Pesticide Residues (FAO/WHO, 1965; FAO/WHO, 1969). A
temporary acceptable daily intake of 0-0.125 mg/kg was established but
further information on the metabolism, particularly of the
trichloromethylthio-moiety, and on the possible teratogenic effect in
nonhuman primates was requested. It was considered that evidence on
the effects of feeding a low-protein diet on the toxicity of captan
was desirable. Further data have been made available and are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Sixteen male and female rats received 82 mg/kg captan labelled
with 14C in the C=O group orally by gavage as an aqueous solution in
1% tragacanth/0.05% Tween-20. Two animals received 14C labelled
cis-1,2,dicarboximido-4-cyclohexene orally and others 14C-labelled
epoxide of cis-1 2,dicarboximido-4-cyclohexene by the
intraperitoneal route. Excretion of activity in urine, faeces and
expired air and activity remaining in organs were determined after
various periods of time. The results showed that captan was absorbed
well from the gastrointestinal tract and that 92% of activity was
excreted in 48 hours and 97% in 96 hours. About 85% was excreted in
urine and 12% in faeces. Excretion in expired air was <0.1%. After 48
hours the average activity in tissues had declined to 5% of the level
at 24 hours and after eight days nearly all tissues contained below an
equivalent of 1 ppm captan (Hoffman et al., 1973).
Sixteen male and female rats received 100 mg/kg captan labelled
with 14C in the trichloromethylthio-moiety and the excretion
determined after various periods of time. Four days after receiving
labelled captan orally 16% of activity was recovered from faeces, 23%
from expired air, 52% in urine and 0.6% in tissues (De Baun et al.,
1973).
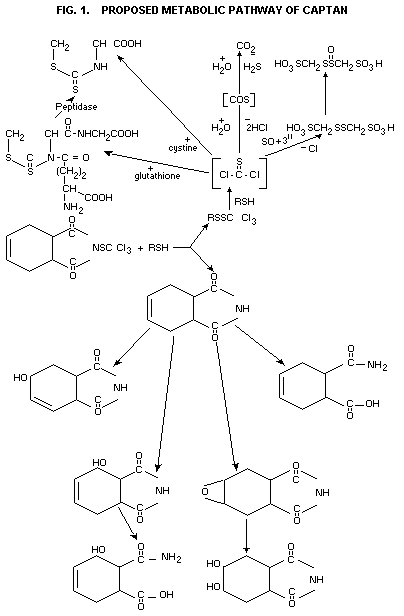
Identification of metabolites in rats receiving captan labelled
in various positions showed that the probable metabolic pathway for
captan is as shown in Fig. 1 (Hoffman et al., 1973; De Baun et al.,
1973).
Three goats received daily treatment equivalent to ingesting
diets containing 0, 20 or 100 ppm unlabelled captan for seven days and
then the same levels of captan labelled with 14C in the C=0 group for
three days. Animals were killed six or seven days after the last dose
of captan. Over 97% of the 80% of activity which could be accounted
for was excreted rapidly within 48 hours of the last dose. Activity
was divided evenly between urine and faeces. Milk contained 0.1 to
 0.2% of the administered dose. At autopsy muscle contained 0.1% of the
administered dose of which 95% was in a water soluble form; it was
calculated that muscle contained 0.01 ppm or less of captan. Other
tissues contained less than 0.02% of the dose. Urine contained no
captan while faeces contained captan or tetrahydrophthalimide (Mitoma,
1972).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse. Three groups each of 50 male and 50 female Swiss mice were
fed on diets containing 0, 3750 and 7500 ppm technical captan for 18
months. A fourth (positive control) group received diet containing 10
ppm N-nitroso diethylamine. The frequency and total number of deaths
was similar in all groups. No abnormal behaviour was noted in treated
groups. Abnormalities found in animals of the positive control group
consisted of focal hyperplasia and neoplasia in the liver, and
neoplasia of the lung and forestomach. No tumours were found in mice
of other groups. No other outstanding differences were noted between
test and control animals on gross examination at autopsy and
histopathological examination of 8-11 animals of each sex from the
groups failed to detect other lesions which could be attributed to
captan treatment (Reyna et al., 1973a).
Rat. Groups of 50 male and 50 female rats were fed for two years on
diets containing 0, 1000 and 2000 ppm technical captan. A positive
control group of 25 male and 25 female rats received diet containing
10 ppm N-nitroso diethylamine. The mortality rate was high in positive
control animals. The rate was similar in males of control and 1000 and
2000 ppm groups, but the rate in females of the test groups was
greater than in female controls. This increased mortality in
captan-treated females was not considered to be of significance, based
on the authors' experience with their strain of laboratory animal. No
abnormalities attributable to captan were found on gross examination
at autopsy. Lesions seen on histological examination of organs from 10
animals of each sex killed after two years' treatment were those
common in rats of that age and none could be attributed to ingestion
of captan. The tumour incidence was not increased by exposure to
captan. Hepatocellular hyperplasia and benign and malignant tumours of
the liver and carcinoma of the forestomach seen in positive control
rats were attributed to treatment with the nitrosamine (Reyna et al.,
1973b).
Special studies on mutagenicity
Host mediated assays were conducted in mice using Salmonella
typhimurium G.46 and Serratia marcescens a21. The animals received
a single 500 mg/kg dose of captan. Positive results were attained at
this dosage level with these two systems (Buselmaier et al., 1972).
Captan was administered to adult male drosophila by topical
application and injection and to larvae by leg feeding. The results
showed that no mutagenic changes could be produced in these systems
(Mollet, 1973, Kramers and Knapp, 1973).
Special studies on teratogenicity
Rat. Pregnant rats received a single 3500 mg/kg bw dose of captan on
one of the days between days 8-15 of gestation. An embryotoxic effect
was seen with an increased number of post implantation deaths. No
malformations were grossly visible but haematomas occurred in various
parts of the body and anophthalmia and microphthalmia, hydrocephalus
and intracranial haematomas were seen at autopsy of the fetuses
(Mirkova, 1973).
Hamster. Groups of two to 10 pregnant hamsters were administered a
single dose of between 200 and 1000 mg captan/kg bw on days seven or
eight of gestation or dosed daily from the sixth to the tenth day with
a total of between 500 and 2500 mg captan/kg bw. They were killed and
examined on the fifteenth day of gestation. At the highest dosage
levels maternal mortality was increased and some abnormal fetuses were
produced but lower dosage levels produced no signs of teratogenic
activity (Robens, 1970).
Dog. Four groups of six female beagles were mated with a group of
six untreated males, each male being mated to one female from each of
the four test groups. The females then received control diet providing
30 or 60 mg/kg bw/day or intraperitoneal injections of 0.15 mg/kg of
6-diazo-5-oxo-alpha-norleucine (DON) on days 20, 21 and 22 of
gestation. Three females of each group were placed on control diet
after whelping, the remainder continuing to receive test material
until they were killed eight weeks after whelping.
No adult animals died during the test and the growth pattern of
females and young was unaffected by treatment. The mortality of
progeny of test groups compared favourably with that of controls.
Captan did not impair the ability of females to carry the young to
parturition or affect the weight, length and growth of young. All
progeny from females treated with DON showed cleft palates and
clubbing of the extremities but no abnormalities were found in young
from captan-treated females. No gross or histologically detectable
abnormalities or skeletal abnormalities were observed which could be
attributed to treatment with captan (Jackson et al., 1968).
Acute toxicity
Animal Route LD50 Reference
mg/kg
Mouse oral 130 Vasikidge and
Mandzegamadze, 1973
Rat oral 2650 "
Rabbit oral 740 "
Short-term studies
Mouse. Groups of mice were administered 1.15, 4.0, 5.3 or 10.6 mg
captan/kg bw daily by gavage for two months or 6.0, 11.6, 14.7 or 530
mg captan/kg bw daily by gavage for one year. Further details are not
available. The abnormalities said to occur were changes in the testes,
reduced sperm motility, prolongation of aestrus cycle, increase of
serum transaminase activity and reduction of blood prothrombin
concentration. Male and female rats were unaffected by 10 and 5 mg/kg
levels respectively and male and female mice by 2.6 and 1.0 mg/kg
levels respectively. (Vasikidge and Mandzegamadze, 1973).
Long-term studies
Mouse. (See under "Special studies on carcinogenicity").
Rat. (See under "Special study on carcinogenicity")
Comments
Extensive investigations have shown that captan is well absorbed
from the gastrointestinal trace and rapidly metabolized and eliminated
from the body. The probable metabolic pathways of both the
tetrahydrophthalimide and trichloromethyl thi-moieties have been
elucidated.
Although no further study has been carried out on the embryotoxic
or teratogenic effects in nonhuman primates it was noted that in one
experiment previously reported, and since published, effects were not
found in rhesus or stump tall monkeys which had been exposed to up to
75 mg captan/kg bw daily during days 21-34 of gestation. No effects
were seen in the progeny of dogs exposed to twice the dosage levels
which increased fetal mortality in rhesus monkeys. It was not,
however, certain that the fetuses of this species were exposed to
captan or its metabolites. Rats exposed to a single high dose of
captan during pregnancy produced young with haematomas in various
parts of the body as well as anomalies of the eye and brain. The
Meeting felt that these embryotoxic effects should be further
investigated in view of the known association between agenesis of a
particular region of the body and haematoma formation. A
teratogenicity study in hamsters showed that malformations occurred at
dosage levels causing mortality while at lower dosage levels
malformations were not observed even though fetal and maternal
morbidity was increased.
Two further long-term studies have failed to demonstrate any
carcinogenic potential in captan.
Captan gave positive results for mutagenicity in a host mediated
assay, but negative results were seen in a dominant lethal test using
mice and in a test for mutagenicity in which drosophila were exposed
to high dosage levels.
No studies are reported on the acute effects of the fungicide in
protein-deficient animals but the further extensive studies reported
allow estimation of an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Mouse: 7500 ppm in diet equivalent to 1070 mg/kg bw/day
Rat: 2000 ppm in diet equivalent to 100 mg/kg bw/day
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.1 mg/kg
FURTHER WORK OR INFORMATION
Desirable
1. Investigation of the significance of haematoma
information in the fetus in relation to fetal death and
malformation.
2. Information on the nature, level, and fate of residues
following washing, blanching, storage, and thermal processing of
treated crops.
3. Residue data obtained by the newer methods of analysis
on the main commodities for which tolerances have been
recommended.
4. Information on the fate of captan in the soil.
REFERENCES
Buselmaier, W. von, Rohrborn, G. and Propping, P.
1972 Mutagenitäts Untersuchungen mit Pestiziden in
Host-mediated Assay und mit dam Dommanten
Letaltest an der Maus, Biol. Zbl. 91: 311-325
De Baun, J. R., Miaullis, J. B., Knarr, J., Mihailovski, A.
1973 and Menn, J. J. The metabolic rate of captan
(trichloromethylthio-14C) in the rat. Unpublished
report of Stauffer Agricultural Research Center
submitted by Stauffer Chemical Co.
Hoffman, L. J., De Baun, J. R., Knarr, J. and Menn, J. J.
1973 Metabolism of N-(trichloromethylthio)-1,2-
dicarboximido 14C-4-cyclohexene (captan) in the
rat. Unpublished report of Stauffer Agricultural
Research Center submitted by Stauffer Chemical Co.
Jackson, G., Kodras. R. and Fancher, O. E. Progeny study of
1968 captan (SX-114) in beagle dogs. IBT No. J5438.
Unpublished report of Ind. Bio-Test Labs submitted
by Chevron Chemical Co.
Kramers, P. G. N. and Knapp, A. G. A. C. Mutagenicity tests
1973 with captan and folpet in Drosophila melanogaster.
Mutation Research, 21: 149-154
Mitoma, C. Pilot study to determine the nature and magnitude
1972 of the residues from ingestion of captan in a
ruminating animal. Unpublished report of Stanford
Research Institute submitted by Chevron Chemical
Co. and Stauffer Chemical Co.
Mirkova, E. The effect of captan on the Embryogenesis of
1973 albino rats. Unpublished report, Research
Institute of Hygiene and Occupational Health,
Sofia
Mollet, P. Untersuchungen Über Mutagenität und Toxizität
1973 von captan bei Drosophila. Mutation Research,
21: 137-138
Reyna, M.S. Kennedy, G.L. and Keplinger, M. L. 18-month
1973a carcinogenic study with captan technical in Swiss
white mice. Unpublished report of Ind. Bio-Test
Labs submitted by Chevron Chemical Co.
Reyna, M. S., Kennedy, G. L. and Keplinger, M. L. Two-year
1973b carcinogenic study with captan technical in albino
rats. Unpublished report of Ind. Bio-Test Labs
submitted by Chevron Chemical Co.
Robens, J. F. Teratogenic activity of several phthalimide
1970 derivatives in the golden hamster. Toxicol. Appl.
Pharmacol, 16: 24-34
Vasikidge, V. I. and Mandzegamadze, P. N. Captan toxicity and
1973 its hygienic standardization in foodstuffs.
Unpublished report
0.2% of the administered dose. At autopsy muscle contained 0.1% of the
administered dose of which 95% was in a water soluble form; it was
calculated that muscle contained 0.01 ppm or less of captan. Other
tissues contained less than 0.02% of the dose. Urine contained no
captan while faeces contained captan or tetrahydrophthalimide (Mitoma,
1972).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse. Three groups each of 50 male and 50 female Swiss mice were
fed on diets containing 0, 3750 and 7500 ppm technical captan for 18
months. A fourth (positive control) group received diet containing 10
ppm N-nitroso diethylamine. The frequency and total number of deaths
was similar in all groups. No abnormal behaviour was noted in treated
groups. Abnormalities found in animals of the positive control group
consisted of focal hyperplasia and neoplasia in the liver, and
neoplasia of the lung and forestomach. No tumours were found in mice
of other groups. No other outstanding differences were noted between
test and control animals on gross examination at autopsy and
histopathological examination of 8-11 animals of each sex from the
groups failed to detect other lesions which could be attributed to
captan treatment (Reyna et al., 1973a).
Rat. Groups of 50 male and 50 female rats were fed for two years on
diets containing 0, 1000 and 2000 ppm technical captan. A positive
control group of 25 male and 25 female rats received diet containing
10 ppm N-nitroso diethylamine. The mortality rate was high in positive
control animals. The rate was similar in males of control and 1000 and
2000 ppm groups, but the rate in females of the test groups was
greater than in female controls. This increased mortality in
captan-treated females was not considered to be of significance, based
on the authors' experience with their strain of laboratory animal. No
abnormalities attributable to captan were found on gross examination
at autopsy. Lesions seen on histological examination of organs from 10
animals of each sex killed after two years' treatment were those
common in rats of that age and none could be attributed to ingestion
of captan. The tumour incidence was not increased by exposure to
captan. Hepatocellular hyperplasia and benign and malignant tumours of
the liver and carcinoma of the forestomach seen in positive control
rats were attributed to treatment with the nitrosamine (Reyna et al.,
1973b).
Special studies on mutagenicity
Host mediated assays were conducted in mice using Salmonella
typhimurium G.46 and Serratia marcescens a21. The animals received
a single 500 mg/kg dose of captan. Positive results were attained at
this dosage level with these two systems (Buselmaier et al., 1972).
Captan was administered to adult male drosophila by topical
application and injection and to larvae by leg feeding. The results
showed that no mutagenic changes could be produced in these systems
(Mollet, 1973, Kramers and Knapp, 1973).
Special studies on teratogenicity
Rat. Pregnant rats received a single 3500 mg/kg bw dose of captan on
one of the days between days 8-15 of gestation. An embryotoxic effect
was seen with an increased number of post implantation deaths. No
malformations were grossly visible but haematomas occurred in various
parts of the body and anophthalmia and microphthalmia, hydrocephalus
and intracranial haematomas were seen at autopsy of the fetuses
(Mirkova, 1973).
Hamster. Groups of two to 10 pregnant hamsters were administered a
single dose of between 200 and 1000 mg captan/kg bw on days seven or
eight of gestation or dosed daily from the sixth to the tenth day with
a total of between 500 and 2500 mg captan/kg bw. They were killed and
examined on the fifteenth day of gestation. At the highest dosage
levels maternal mortality was increased and some abnormal fetuses were
produced but lower dosage levels produced no signs of teratogenic
activity (Robens, 1970).
Dog. Four groups of six female beagles were mated with a group of
six untreated males, each male being mated to one female from each of
the four test groups. The females then received control diet providing
30 or 60 mg/kg bw/day or intraperitoneal injections of 0.15 mg/kg of
6-diazo-5-oxo-alpha-norleucine (DON) on days 20, 21 and 22 of
gestation. Three females of each group were placed on control diet
after whelping, the remainder continuing to receive test material
until they were killed eight weeks after whelping.
No adult animals died during the test and the growth pattern of
females and young was unaffected by treatment. The mortality of
progeny of test groups compared favourably with that of controls.
Captan did not impair the ability of females to carry the young to
parturition or affect the weight, length and growth of young. All
progeny from females treated with DON showed cleft palates and
clubbing of the extremities but no abnormalities were found in young
from captan-treated females. No gross or histologically detectable
abnormalities or skeletal abnormalities were observed which could be
attributed to treatment with captan (Jackson et al., 1968).
Acute toxicity
Animal Route LD50 Reference
mg/kg
Mouse oral 130 Vasikidge and
Mandzegamadze, 1973
Rat oral 2650 "
Rabbit oral 740 "
Short-term studies
Mouse. Groups of mice were administered 1.15, 4.0, 5.3 or 10.6 mg
captan/kg bw daily by gavage for two months or 6.0, 11.6, 14.7 or 530
mg captan/kg bw daily by gavage for one year. Further details are not
available. The abnormalities said to occur were changes in the testes,
reduced sperm motility, prolongation of aestrus cycle, increase of
serum transaminase activity and reduction of blood prothrombin
concentration. Male and female rats were unaffected by 10 and 5 mg/kg
levels respectively and male and female mice by 2.6 and 1.0 mg/kg
levels respectively. (Vasikidge and Mandzegamadze, 1973).
Long-term studies
Mouse. (See under "Special studies on carcinogenicity").
Rat. (See under "Special study on carcinogenicity")
Comments
Extensive investigations have shown that captan is well absorbed
from the gastrointestinal trace and rapidly metabolized and eliminated
from the body. The probable metabolic pathways of both the
tetrahydrophthalimide and trichloromethyl thi-moieties have been
elucidated.
Although no further study has been carried out on the embryotoxic
or teratogenic effects in nonhuman primates it was noted that in one
experiment previously reported, and since published, effects were not
found in rhesus or stump tall monkeys which had been exposed to up to
75 mg captan/kg bw daily during days 21-34 of gestation. No effects
were seen in the progeny of dogs exposed to twice the dosage levels
which increased fetal mortality in rhesus monkeys. It was not,
however, certain that the fetuses of this species were exposed to
captan or its metabolites. Rats exposed to a single high dose of
captan during pregnancy produced young with haematomas in various
parts of the body as well as anomalies of the eye and brain. The
Meeting felt that these embryotoxic effects should be further
investigated in view of the known association between agenesis of a
particular region of the body and haematoma formation. A
teratogenicity study in hamsters showed that malformations occurred at
dosage levels causing mortality while at lower dosage levels
malformations were not observed even though fetal and maternal
morbidity was increased.
Two further long-term studies have failed to demonstrate any
carcinogenic potential in captan.
Captan gave positive results for mutagenicity in a host mediated
assay, but negative results were seen in a dominant lethal test using
mice and in a test for mutagenicity in which drosophila were exposed
to high dosage levels.
No studies are reported on the acute effects of the fungicide in
protein-deficient animals but the further extensive studies reported
allow estimation of an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Mouse: 7500 ppm in diet equivalent to 1070 mg/kg bw/day
Rat: 2000 ppm in diet equivalent to 100 mg/kg bw/day
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.1 mg/kg
FURTHER WORK OR INFORMATION
Desirable
1. Investigation of the significance of haematoma
information in the fetus in relation to fetal death and
malformation.
2. Information on the nature, level, and fate of residues
following washing, blanching, storage, and thermal processing of
treated crops.
3. Residue data obtained by the newer methods of analysis
on the main commodities for which tolerances have been
recommended.
4. Information on the fate of captan in the soil.
REFERENCES
Buselmaier, W. von, Rohrborn, G. and Propping, P.
1972 Mutagenitäts Untersuchungen mit Pestiziden in
Host-mediated Assay und mit dam Dommanten
Letaltest an der Maus, Biol. Zbl. 91: 311-325
De Baun, J. R., Miaullis, J. B., Knarr, J., Mihailovski, A.
1973 and Menn, J. J. The metabolic rate of captan
(trichloromethylthio-14C) in the rat. Unpublished
report of Stauffer Agricultural Research Center
submitted by Stauffer Chemical Co.
Hoffman, L. J., De Baun, J. R., Knarr, J. and Menn, J. J.
1973 Metabolism of N-(trichloromethylthio)-1,2-
dicarboximido 14C-4-cyclohexene (captan) in the
rat. Unpublished report of Stauffer Agricultural
Research Center submitted by Stauffer Chemical Co.
Jackson, G., Kodras. R. and Fancher, O. E. Progeny study of
1968 captan (SX-114) in beagle dogs. IBT No. J5438.
Unpublished report of Ind. Bio-Test Labs submitted
by Chevron Chemical Co.
Kramers, P. G. N. and Knapp, A. G. A. C. Mutagenicity tests
1973 with captan and folpet in Drosophila melanogaster.
Mutation Research, 21: 149-154
Mitoma, C. Pilot study to determine the nature and magnitude
1972 of the residues from ingestion of captan in a
ruminating animal. Unpublished report of Stanford
Research Institute submitted by Chevron Chemical
Co. and Stauffer Chemical Co.
Mirkova, E. The effect of captan on the Embryogenesis of
1973 albino rats. Unpublished report, Research
Institute of Hygiene and Occupational Health,
Sofia
Mollet, P. Untersuchungen Über Mutagenität und Toxizität
1973 von captan bei Drosophila. Mutation Research,
21: 137-138
Reyna, M.S. Kennedy, G.L. and Keplinger, M. L. 18-month
1973a carcinogenic study with captan technical in Swiss
white mice. Unpublished report of Ind. Bio-Test
Labs submitted by Chevron Chemical Co.
Reyna, M. S., Kennedy, G. L. and Keplinger, M. L. Two-year
1973b carcinogenic study with captan technical in albino
rats. Unpublished report of Ind. Bio-Test Labs
submitted by Chevron Chemical Co.
Robens, J. F. Teratogenic activity of several phthalimide
1970 derivatives in the golden hamster. Toxicol. Appl.
Pharmacol, 16: 24-34
Vasikidge, V. I. and Mandzegamadze, P. N. Captan toxicity and
1973 its hygienic standardization in foodstuffs.
Unpublished report
 0.2% of the administered dose. At autopsy muscle contained 0.1% of the
administered dose of which 95% was in a water soluble form; it was
calculated that muscle contained 0.01 ppm or less of captan. Other
tissues contained less than 0.02% of the dose. Urine contained no
captan while faeces contained captan or tetrahydrophthalimide (Mitoma,
1972).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse. Three groups each of 50 male and 50 female Swiss mice were
fed on diets containing 0, 3750 and 7500 ppm technical captan for 18
months. A fourth (positive control) group received diet containing 10
ppm N-nitroso diethylamine. The frequency and total number of deaths
was similar in all groups. No abnormal behaviour was noted in treated
groups. Abnormalities found in animals of the positive control group
consisted of focal hyperplasia and neoplasia in the liver, and
neoplasia of the lung and forestomach. No tumours were found in mice
of other groups. No other outstanding differences were noted between
test and control animals on gross examination at autopsy and
histopathological examination of 8-11 animals of each sex from the
groups failed to detect other lesions which could be attributed to
captan treatment (Reyna et al., 1973a).
Rat. Groups of 50 male and 50 female rats were fed for two years on
diets containing 0, 1000 and 2000 ppm technical captan. A positive
control group of 25 male and 25 female rats received diet containing
10 ppm N-nitroso diethylamine. The mortality rate was high in positive
control animals. The rate was similar in males of control and 1000 and
2000 ppm groups, but the rate in females of the test groups was
greater than in female controls. This increased mortality in
captan-treated females was not considered to be of significance, based
on the authors' experience with their strain of laboratory animal. No
abnormalities attributable to captan were found on gross examination
at autopsy. Lesions seen on histological examination of organs from 10
animals of each sex killed after two years' treatment were those
common in rats of that age and none could be attributed to ingestion
of captan. The tumour incidence was not increased by exposure to
captan. Hepatocellular hyperplasia and benign and malignant tumours of
the liver and carcinoma of the forestomach seen in positive control
rats were attributed to treatment with the nitrosamine (Reyna et al.,
1973b).
Special studies on mutagenicity
Host mediated assays were conducted in mice using Salmonella
typhimurium G.46 and Serratia marcescens a21. The animals received
a single 500 mg/kg dose of captan. Positive results were attained at
this dosage level with these two systems (Buselmaier et al., 1972).
Captan was administered to adult male drosophila by topical
application and injection and to larvae by leg feeding. The results
showed that no mutagenic changes could be produced in these systems
(Mollet, 1973, Kramers and Knapp, 1973).
Special studies on teratogenicity
Rat. Pregnant rats received a single 3500 mg/kg bw dose of captan on
one of the days between days 8-15 of gestation. An embryotoxic effect
was seen with an increased number of post implantation deaths. No
malformations were grossly visible but haematomas occurred in various
parts of the body and anophthalmia and microphthalmia, hydrocephalus
and intracranial haematomas were seen at autopsy of the fetuses
(Mirkova, 1973).
Hamster. Groups of two to 10 pregnant hamsters were administered a
single dose of between 200 and 1000 mg captan/kg bw on days seven or
eight of gestation or dosed daily from the sixth to the tenth day with
a total of between 500 and 2500 mg captan/kg bw. They were killed and
examined on the fifteenth day of gestation. At the highest dosage
levels maternal mortality was increased and some abnormal fetuses were
produced but lower dosage levels produced no signs of teratogenic
activity (Robens, 1970).
Dog. Four groups of six female beagles were mated with a group of
six untreated males, each male being mated to one female from each of
the four test groups. The females then received control diet providing
30 or 60 mg/kg bw/day or intraperitoneal injections of 0.15 mg/kg of
6-diazo-5-oxo-alpha-norleucine (DON) on days 20, 21 and 22 of
gestation. Three females of each group were placed on control diet
after whelping, the remainder continuing to receive test material
until they were killed eight weeks after whelping.
No adult animals died during the test and the growth pattern of
females and young was unaffected by treatment. The mortality of
progeny of test groups compared favourably with that of controls.
Captan did not impair the ability of females to carry the young to
parturition or affect the weight, length and growth of young. All
progeny from females treated with DON showed cleft palates and
clubbing of the extremities but no abnormalities were found in young
from captan-treated females. No gross or histologically detectable
abnormalities or skeletal abnormalities were observed which could be
attributed to treatment with captan (Jackson et al., 1968).
Acute toxicity
Animal Route LD50 Reference
mg/kg
Mouse oral 130 Vasikidge and
Mandzegamadze, 1973
Rat oral 2650 "
Rabbit oral 740 "
Short-term studies
Mouse. Groups of mice were administered 1.15, 4.0, 5.3 or 10.6 mg
captan/kg bw daily by gavage for two months or 6.0, 11.6, 14.7 or 530
mg captan/kg bw daily by gavage for one year. Further details are not
available. The abnormalities said to occur were changes in the testes,
reduced sperm motility, prolongation of aestrus cycle, increase of
serum transaminase activity and reduction of blood prothrombin
concentration. Male and female rats were unaffected by 10 and 5 mg/kg
levels respectively and male and female mice by 2.6 and 1.0 mg/kg
levels respectively. (Vasikidge and Mandzegamadze, 1973).
Long-term studies
Mouse. (See under "Special studies on carcinogenicity").
Rat. (See under "Special study on carcinogenicity")
Comments
Extensive investigations have shown that captan is well absorbed
from the gastrointestinal trace and rapidly metabolized and eliminated
from the body. The probable metabolic pathways of both the
tetrahydrophthalimide and trichloromethyl thi-moieties have been
elucidated.
Although no further study has been carried out on the embryotoxic
or teratogenic effects in nonhuman primates it was noted that in one
experiment previously reported, and since published, effects were not
found in rhesus or stump tall monkeys which had been exposed to up to
75 mg captan/kg bw daily during days 21-34 of gestation. No effects
were seen in the progeny of dogs exposed to twice the dosage levels
which increased fetal mortality in rhesus monkeys. It was not,
however, certain that the fetuses of this species were exposed to
captan or its metabolites. Rats exposed to a single high dose of
captan during pregnancy produced young with haematomas in various
parts of the body as well as anomalies of the eye and brain. The
Meeting felt that these embryotoxic effects should be further
investigated in view of the known association between agenesis of a
particular region of the body and haematoma formation. A
teratogenicity study in hamsters showed that malformations occurred at
dosage levels causing mortality while at lower dosage levels
malformations were not observed even though fetal and maternal
morbidity was increased.
Two further long-term studies have failed to demonstrate any
carcinogenic potential in captan.
Captan gave positive results for mutagenicity in a host mediated
assay, but negative results were seen in a dominant lethal test using
mice and in a test for mutagenicity in which drosophila were exposed
to high dosage levels.
No studies are reported on the acute effects of the fungicide in
protein-deficient animals but the further extensive studies reported
allow estimation of an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Mouse: 7500 ppm in diet equivalent to 1070 mg/kg bw/day
Rat: 2000 ppm in diet equivalent to 100 mg/kg bw/day
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.1 mg/kg
FURTHER WORK OR INFORMATION
Desirable
1. Investigation of the significance of haematoma
information in the fetus in relation to fetal death and
malformation.
2. Information on the nature, level, and fate of residues
following washing, blanching, storage, and thermal processing of
treated crops.
3. Residue data obtained by the newer methods of analysis
on the main commodities for which tolerances have been
recommended.
4. Information on the fate of captan in the soil.
REFERENCES
Buselmaier, W. von, Rohrborn, G. and Propping, P.
1972 Mutagenitäts Untersuchungen mit Pestiziden in
Host-mediated Assay und mit dam Dommanten
Letaltest an der Maus, Biol. Zbl. 91: 311-325
De Baun, J. R., Miaullis, J. B., Knarr, J., Mihailovski, A.
1973 and Menn, J. J. The metabolic rate of captan
(trichloromethylthio-14C) in the rat. Unpublished
report of Stauffer Agricultural Research Center
submitted by Stauffer Chemical Co.
Hoffman, L. J., De Baun, J. R., Knarr, J. and Menn, J. J.
1973 Metabolism of N-(trichloromethylthio)-1,2-
dicarboximido 14C-4-cyclohexene (captan) in the
rat. Unpublished report of Stauffer Agricultural
Research Center submitted by Stauffer Chemical Co.
Jackson, G., Kodras. R. and Fancher, O. E. Progeny study of
1968 captan (SX-114) in beagle dogs. IBT No. J5438.
Unpublished report of Ind. Bio-Test Labs submitted
by Chevron Chemical Co.
Kramers, P. G. N. and Knapp, A. G. A. C. Mutagenicity tests
1973 with captan and folpet in Drosophila melanogaster.
Mutation Research, 21: 149-154
Mitoma, C. Pilot study to determine the nature and magnitude
1972 of the residues from ingestion of captan in a
ruminating animal. Unpublished report of Stanford
Research Institute submitted by Chevron Chemical
Co. and Stauffer Chemical Co.
Mirkova, E. The effect of captan on the Embryogenesis of
1973 albino rats. Unpublished report, Research
Institute of Hygiene and Occupational Health,
Sofia
Mollet, P. Untersuchungen Über Mutagenität und Toxizität
1973 von captan bei Drosophila. Mutation Research,
21: 137-138
Reyna, M.S. Kennedy, G.L. and Keplinger, M. L. 18-month
1973a carcinogenic study with captan technical in Swiss
white mice. Unpublished report of Ind. Bio-Test
Labs submitted by Chevron Chemical Co.
Reyna, M. S., Kennedy, G. L. and Keplinger, M. L. Two-year
1973b carcinogenic study with captan technical in albino
rats. Unpublished report of Ind. Bio-Test Labs
submitted by Chevron Chemical Co.
Robens, J. F. Teratogenic activity of several phthalimide
1970 derivatives in the golden hamster. Toxicol. Appl.
Pharmacol, 16: 24-34
Vasikidge, V. I. and Mandzegamadze, P. N. Captan toxicity and
1973 its hygienic standardization in foodstuffs.
Unpublished report
0.2% of the administered dose. At autopsy muscle contained 0.1% of the
administered dose of which 95% was in a water soluble form; it was
calculated that muscle contained 0.01 ppm or less of captan. Other
tissues contained less than 0.02% of the dose. Urine contained no
captan while faeces contained captan or tetrahydrophthalimide (Mitoma,
1972).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse. Three groups each of 50 male and 50 female Swiss mice were
fed on diets containing 0, 3750 and 7500 ppm technical captan for 18
months. A fourth (positive control) group received diet containing 10
ppm N-nitroso diethylamine. The frequency and total number of deaths
was similar in all groups. No abnormal behaviour was noted in treated
groups. Abnormalities found in animals of the positive control group
consisted of focal hyperplasia and neoplasia in the liver, and
neoplasia of the lung and forestomach. No tumours were found in mice
of other groups. No other outstanding differences were noted between
test and control animals on gross examination at autopsy and
histopathological examination of 8-11 animals of each sex from the
groups failed to detect other lesions which could be attributed to
captan treatment (Reyna et al., 1973a).
Rat. Groups of 50 male and 50 female rats were fed for two years on
diets containing 0, 1000 and 2000 ppm technical captan. A positive
control group of 25 male and 25 female rats received diet containing
10 ppm N-nitroso diethylamine. The mortality rate was high in positive
control animals. The rate was similar in males of control and 1000 and
2000 ppm groups, but the rate in females of the test groups was
greater than in female controls. This increased mortality in
captan-treated females was not considered to be of significance, based
on the authors' experience with their strain of laboratory animal. No
abnormalities attributable to captan were found on gross examination
at autopsy. Lesions seen on histological examination of organs from 10
animals of each sex killed after two years' treatment were those
common in rats of that age and none could be attributed to ingestion
of captan. The tumour incidence was not increased by exposure to
captan. Hepatocellular hyperplasia and benign and malignant tumours of
the liver and carcinoma of the forestomach seen in positive control
rats were attributed to treatment with the nitrosamine (Reyna et al.,
1973b).
Special studies on mutagenicity
Host mediated assays were conducted in mice using Salmonella
typhimurium G.46 and Serratia marcescens a21. The animals received
a single 500 mg/kg dose of captan. Positive results were attained at
this dosage level with these two systems (Buselmaier et al., 1972).
Captan was administered to adult male drosophila by topical
application and injection and to larvae by leg feeding. The results
showed that no mutagenic changes could be produced in these systems
(Mollet, 1973, Kramers and Knapp, 1973).
Special studies on teratogenicity
Rat. Pregnant rats received a single 3500 mg/kg bw dose of captan on
one of the days between days 8-15 of gestation. An embryotoxic effect
was seen with an increased number of post implantation deaths. No
malformations were grossly visible but haematomas occurred in various
parts of the body and anophthalmia and microphthalmia, hydrocephalus
and intracranial haematomas were seen at autopsy of the fetuses
(Mirkova, 1973).
Hamster. Groups of two to 10 pregnant hamsters were administered a
single dose of between 200 and 1000 mg captan/kg bw on days seven or
eight of gestation or dosed daily from the sixth to the tenth day with
a total of between 500 and 2500 mg captan/kg bw. They were killed and
examined on the fifteenth day of gestation. At the highest dosage
levels maternal mortality was increased and some abnormal fetuses were
produced but lower dosage levels produced no signs of teratogenic
activity (Robens, 1970).
Dog. Four groups of six female beagles were mated with a group of
six untreated males, each male being mated to one female from each of
the four test groups. The females then received control diet providing
30 or 60 mg/kg bw/day or intraperitoneal injections of 0.15 mg/kg of
6-diazo-5-oxo-alpha-norleucine (DON) on days 20, 21 and 22 of
gestation. Three females of each group were placed on control diet
after whelping, the remainder continuing to receive test material
until they were killed eight weeks after whelping.
No adult animals died during the test and the growth pattern of
females and young was unaffected by treatment. The mortality of
progeny of test groups compared favourably with that of controls.
Captan did not impair the ability of females to carry the young to
parturition or affect the weight, length and growth of young. All
progeny from females treated with DON showed cleft palates and
clubbing of the extremities but no abnormalities were found in young
from captan-treated females. No gross or histologically detectable
abnormalities or skeletal abnormalities were observed which could be
attributed to treatment with captan (Jackson et al., 1968).
Acute toxicity
Animal Route LD50 Reference
mg/kg
Mouse oral 130 Vasikidge and
Mandzegamadze, 1973
Rat oral 2650 "
Rabbit oral 740 "
Short-term studies
Mouse. Groups of mice were administered 1.15, 4.0, 5.3 or 10.6 mg
captan/kg bw daily by gavage for two months or 6.0, 11.6, 14.7 or 530
mg captan/kg bw daily by gavage for one year. Further details are not
available. The abnormalities said to occur were changes in the testes,
reduced sperm motility, prolongation of aestrus cycle, increase of
serum transaminase activity and reduction of blood prothrombin
concentration. Male and female rats were unaffected by 10 and 5 mg/kg
levels respectively and male and female mice by 2.6 and 1.0 mg/kg
levels respectively. (Vasikidge and Mandzegamadze, 1973).
Long-term studies
Mouse. (See under "Special studies on carcinogenicity").
Rat. (See under "Special study on carcinogenicity")
Comments
Extensive investigations have shown that captan is well absorbed
from the gastrointestinal trace and rapidly metabolized and eliminated
from the body. The probable metabolic pathways of both the
tetrahydrophthalimide and trichloromethyl thi-moieties have been
elucidated.
Although no further study has been carried out on the embryotoxic
or teratogenic effects in nonhuman primates it was noted that in one
experiment previously reported, and since published, effects were not
found in rhesus or stump tall monkeys which had been exposed to up to
75 mg captan/kg bw daily during days 21-34 of gestation. No effects
were seen in the progeny of dogs exposed to twice the dosage levels
which increased fetal mortality in rhesus monkeys. It was not,
however, certain that the fetuses of this species were exposed to
captan or its metabolites. Rats exposed to a single high dose of
captan during pregnancy produced young with haematomas in various
parts of the body as well as anomalies of the eye and brain. The
Meeting felt that these embryotoxic effects should be further
investigated in view of the known association between agenesis of a
particular region of the body and haematoma formation. A
teratogenicity study in hamsters showed that malformations occurred at
dosage levels causing mortality while at lower dosage levels
malformations were not observed even though fetal and maternal
morbidity was increased.
Two further long-term studies have failed to demonstrate any
carcinogenic potential in captan.
Captan gave positive results for mutagenicity in a host mediated
assay, but negative results were seen in a dominant lethal test using
mice and in a test for mutagenicity in which drosophila were exposed
to high dosage levels.
No studies are reported on the acute effects of the fungicide in
protein-deficient animals but the further extensive studies reported
allow estimation of an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Mouse: 7500 ppm in diet equivalent to 1070 mg/kg bw/day
Rat: 2000 ppm in diet equivalent to 100 mg/kg bw/day
Dog: 100 mg/kg bw/day
Monkey: 12.5 mg/kg bw/day
Estimate of acceptable daily intake for man
0-0.1 mg/kg
FURTHER WORK OR INFORMATION
Desirable
1. Investigation of the significance of haematoma
information in the fetus in relation to fetal death and
malformation.
2. Information on the nature, level, and fate of residues
following washing, blanching, storage, and thermal processing of
treated crops.
3. Residue data obtained by the newer methods of analysis
on the main commodities for which tolerances have been
recommended.
4. Information on the fate of captan in the soil.
REFERENCES
Buselmaier, W. von, Rohrborn, G. and Propping, P.
1972 Mutagenitäts Untersuchungen mit Pestiziden in
Host-mediated Assay und mit dam Dommanten
Letaltest an der Maus, Biol. Zbl. 91: 311-325
De Baun, J. R., Miaullis, J. B., Knarr, J., Mihailovski, A.
1973 and Menn, J. J. The metabolic rate of captan
(trichloromethylthio-14C) in the rat. Unpublished
report of Stauffer Agricultural Research Center
submitted by Stauffer Chemical Co.
Hoffman, L. J., De Baun, J. R., Knarr, J. and Menn, J. J.
1973 Metabolism of N-(trichloromethylthio)-1,2-
dicarboximido 14C-4-cyclohexene (captan) in the
rat. Unpublished report of Stauffer Agricultural
Research Center submitted by Stauffer Chemical Co.
Jackson, G., Kodras. R. and Fancher, O. E. Progeny study of
1968 captan (SX-114) in beagle dogs. IBT No. J5438.
Unpublished report of Ind. Bio-Test Labs submitted
by Chevron Chemical Co.
Kramers, P. G. N. and Knapp, A. G. A. C. Mutagenicity tests
1973 with captan and folpet in Drosophila melanogaster.
Mutation Research, 21: 149-154
Mitoma, C. Pilot study to determine the nature and magnitude
1972 of the residues from ingestion of captan in a
ruminating animal. Unpublished report of Stanford
Research Institute submitted by Chevron Chemical
Co. and Stauffer Chemical Co.
Mirkova, E. The effect of captan on the Embryogenesis of
1973 albino rats. Unpublished report, Research
Institute of Hygiene and Occupational Health,
Sofia
Mollet, P. Untersuchungen Über Mutagenität und Toxizität
1973 von captan bei Drosophila. Mutation Research,
21: 137-138
Reyna, M.S. Kennedy, G.L. and Keplinger, M. L. 18-month
1973a carcinogenic study with captan technical in Swiss
white mice. Unpublished report of Ind. Bio-Test
Labs submitted by Chevron Chemical Co.
Reyna, M. S., Kennedy, G. L. and Keplinger, M. L. Two-year
1973b carcinogenic study with captan technical in albino
rats. Unpublished report of Ind. Bio-Test Labs
submitted by Chevron Chemical Co.
Robens, J. F. Teratogenic activity of several phthalimide
1970 derivatives in the golden hamster. Toxicol. Appl.
Pharmacol, 16: 24-34
Vasikidge, V. I. and Mandzegamadze, P. N. Captan toxicity and
1973 its hygienic standardization in foodstuffs.
Unpublished report
