
CAPTAN (addendum)
First draft prepared by
J.-J. Larsen
Institute of Toxicology, National Food Agency of Denmark, Ministry of
Health, Soborg, Denmark
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Short-term toxicity
Genotoxicity
Comments
Toxicological evaluation
References
Explanation
Captan was evaluated toxicologically by the Joint Meeting in
1963, 1965, 1969, 1973, 1977, 1978, 1982, 1984, and 1990 (Annex I,
references 2, 3, 12, 20, 28, 30, 38, 42, and 59). Toxicological
monographs were prepared in 1963, 1965, and 1969 (Annex I, references
2, 4, and 13), and monograph addenda were prepared in 1973, 1977,
1978, 1982, 1984, and 1990 (Annex I, references 21, 29, 31, 39, 43,
and 61). Additional information on distribution, excretion, bio-
transformation, genotoxicity, and short-term toxicity are reviewed in
this monograph addendum.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
The tissue distribution and excretion of a single oral dose of
10 mg/kg bw [14C-cyclohexene ring]-labelled captan was studied in
five male and five female Sprague-Dawley rats (weighing 202-243 g),
obtained from Charles River, Margate, Kent, United Kingdom. Urinary
and faecal excretion of radiolabel was monitored over seven days, and
the residual concentrations of radiolabel were then measured in
selected tissues. The excretion profiles were similar for male and
female rats, the urinary route predominating in animals of each sex;
81% of the administered radioactivity was excreted in the urine and
8-9% in the faeces over 48 h, and 69-73% in the urine and 23-25% in
the faeces over 96 h. The residual concentrations in the tissue were
negligible, the highest concentrations occurring in the kidneys
(< 0.01% of the total radiolabel) after 48 h and in the blood (about
2 Ág equivalents of captan per gram) after 96 h (Trivedi, 1990a,b)
The effect of oral pretreatment with 10 mg/kg bw unlabelled
captan for 14 days before an oral dose of 10 mg/kg bw [14C-
cyclohexene ring]-labelled captan was studied in eight male and eight
female Sprague-Dawley rats (weighing 192-260 g), obtained from Charles
River, Margate, Kent, United Kingdom. The urinary and faecal excretion
of radiolabel was monitored over seven days, and the residual
concentrations of radiolabel were then measured in selected tissues.
The excretion profiles of male and female rats were similar, the
urinary route predominating: 88-91% of the administered radiolabel
in the urine and 7-9% in the faeces over 48 h. The residual
concentrations in tissues were neglible in animals of each sex, the
highest concentrations occurring in the kidneys (0.04 Ág/g). The
pretreatment thus had little or no effect on the route or rate of
elimination of radiolabelled substance (Bratt, 1990).
(b) Biotransformation
The fate of orally administed 14C-captan was studied in eight
male and eight female Simonsen albino rats, received from Simonsen
Laboratories, Gilroy, USA, weighing 185-200 g. The animals were given
100 mg/kg bw N-trichloro-[14C]-methylthio-4-cyclohexene-1,2-
dicarboximide (0.34 mCi/mmol, aqueous suspension in 1 ml of 1%
traganth gum and 005% Tween-20) by gastric intubation, and radiolabel
was determined in urine, faeces, and expired air. Within 9 h, 50% of
the dose had been excreted; the final distribution was 52% in urine,
23% in expired air, 16% in faeces, and 0.6% in tissues. The urinary
metabolites were identified as thiazolidine-2-thione-4-carboxylic acid
(19%), a salt of dithiobis(methane-sulfonic acid) (54%), and the
disulfide monoxide derivative of dithiobis(methanesulfonic acid)
(14%). 14C-Carbon dioxide was identified in expired air. Radioactive
thiazolidine-2-thione-4-carboxylic acid was detected in the urine of
rats treated simultaneously with captan and either [U-14C]cystine or
reduced 35 S-glutathione. 35 S-Dithiobis(methanesulfonic acid) was
excreted in the urine of rats treated simultaneously with sodium
35 S-sulfite and either captan or thiophosgene. The metabolism of
captan thus appears to involve evolution of thiophosgene derived from
the trichloromethylthio moiety. Thiophosgene is detoxified, at least
in part, by three mechanisms: (i) oxidation and/or hydrolysis to
carbon dioxide; (ii) reaction with a cysteine moiety to yield
thiazolidine-2-thione-4-carboxylic acid; and (iii) reaction with
sulfite to produce dithiobis(methanesulfonic acid). Degradation in the
gastrointestinal tract appears to play a major role in the metabolism
of captan (DeBaun et al., 1974).
The metabolites of captan were measured in the urine and faeces
of five male and five female Sprague-Dawley rats (weighing 192-260 g,
obtained from Charles River, Margate, Kent, United Kingdom) treated
with a single oral dose of 10 or 500 mg/kg bw [14C-cyclohexene
ring]-labelled captan. Some of the animals that received the dose of
]0 mg/kg bw were pretreated with 10 mg/kg bw unlabelled captan for 14
days. Seven urinary metabilites were identified: 3-hydroxy-4,5-
cyclohexene-1,2-dicarboximide (42%), 5-hydroxy-3,4-cyclohexene-
1,2-dicarboximide (6%), 6-hydroxy-1-amido-2-carboxy-4,5-cyclohexene
(13%), 4,5-cyclohexene-1,2-dicarboximide (11%), 1-amido-2-carboxy-
4,5-cyclohexene (7%), 4,5-dihydroxy-1,2-dicarboximide (6%), and
4,5-epoxy-1,2-dicarboximide (5%). Two unidentified metabolites
account:ed for 4 and 2%, respectively, of the urinary radiolabel.
Radiolabel remaining at the origin of the thin-layer chromatography
plates, which could not be matched to identified metabolites,
accounted for 7% of the urinary radiolabel. No significant difference
was noted between the sexes or between dosing regimes in the quantity
of urinary metabolites. Faecal extracts obtained from rats dosed with
10 and 500 mg/kg bw captan contained 7 and 43% respectively, of an
unidentified metabolite (possibly captan). 4,5-Cyclohexene-1,2-
dicarboximide accounted for an average of 35% of the faecal
metabolites. 3-Hydroxy-4,5- or 5-hydroxy-3,4-cyclohexene-1,2-
dicarboximide were major faecal metabolites, the quantities depending
on the treatment regime and sex. 4,5-Dihydroxy-1,2-dicarboximide,
1-amido-2-carboxy-4,5-cyclohexene, and polar metabolites were found in
only relatively small amounts, and 4,5-epoxy-1,2-dicarboximide was not
detected in faecal extracts (Lappin & Havell, 1990).
The dermal and oral absorption and metabolism of captan were
studied in a pilot study of two human volunteers, and the results were
compared with earlier findings in rats (Krieger & Thongsinthusak,
1993). The usefulness of thiazolidine-2-thione-4-carboxylic acid and
4,5-cyclohexene-1,2-dicarboximide as biomarkers of occupational
exposure was also evaluated. Both compouns were rapidly detected after
dermal application of 15 mg captan (purity, 99.1%) dissolved in 1 ml
chloroform (analytical reagent grade) to two healthy adult men
weighing 84 and 150 kg. The concentration of 4,5-cyclohexene-1,2-
dicarboximide in 12-h urine samples was 5-640 ppb (the lower limit was
the minimal detectable limit), and the concentrations were stable for
at least one year when the samples were stored at -20░C. Thiazolidine-
2-thione-4-carboxylic acid was also stable, but the minimal detectable
limit was 50 ppb. The same two individuals took oral doses of 0.1 or
1 mg/kg bw, urine was collected at 12-h intervals for four days, and
the samples were analysed for same two metabolites. Both were detected
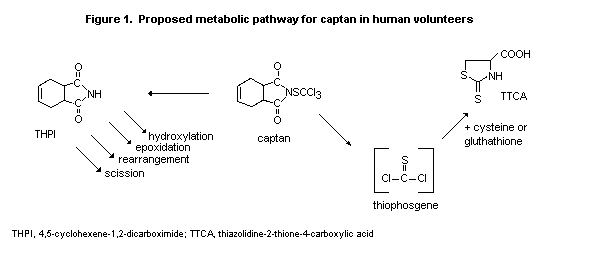
in urine within the first 24 h (Figure 1) but represented only small
percentages of the dose of captan administered: thiazolidine-2-thione-
4-carboxylic, 4-9%, and 4,5-cyclohexene-1,2-dicarboximide, 1-3%,
perhaps because there are alternative metabolic pathways. In an
earlier study in rats (DeBaun et al., 1974) given captan at much
higher oral doses (77-100 mg/kg bw), 4,5-cyclohexene-1,2-dicarboximide
and thiazolidine-2-thione-4-carboxylic acid in the urine represented
13 and 19%, respectively, of the dose of captan. In the rats,
4,5-cyclohexene-1,2-dicarboximide was further metabolized by
hydroxylation, epoxidation, rearrangement, and internal scission to
products that were not assayed in the study of humans. Although the
authors of the study in humans concluded that dermal and oral
metabolism of captan are different in man and rats, the study did not
demonstrate species differences. The significantly higher doses used
in rats, the limited number of human volunteers, and the lack of
information on other metabolites limited the conclusions that could be
drawn.
 2. Toxicological studies
(a) Short-term toxicity
Mice
Two groups of 15 male CD1 mice were fed diets containing 0 or
6000 ppm captan (purity, 89.4%) for up to 91 consecutive days. The
concentration of captan was within 4% of nominal concentration, and
the homogeneity and stability of the diet were satisfactory for the
period and conditions of storage used. The animals were observed
daily; body weights were recorded daily for the first week of the
study and weekly thereafter. Food consumption was recorded throughout
the study. Five animals per group were killed after 28, 56, and 91
days of treatment and examined post mortem. The small intestine
(duodenum, ileum, and jejunum) was removed and prepared by a 'gut
rolling' procedure, starting at the ileo-caecal junction. Sections
were stained with haematoxylin and eosin or with antibodies to
proliferating cell nuclear antigen and examined by light microscopy.
Sections were evaluated for histopathological change, for
proliferating cell nuclear antigen labelling index (percent cells in
the crypts labelled with antigen), for mean number of crypt cells, and
for the ratio of villus to crypt height.
Body-weight loss was seen among treated mice during the first
week of the study in association with significantly reduced food
consumption. This was considered to reflect the unpalatability of the
captan diets. Food consumption was similar to that of controls from
week 2 until the end of the study, and the body-weight gain of treated
mice was similar to that of controls for the remainder of the study.
The body weights, however, were significantly lower than those of
controls from week 2 until the end of the study.
The only macroscopic findings in treated mice killed after 28
days was pronounced thickening of the duodenal mucosa. Similar but
less pronounced effects were observed in treated mice killed after 56
or 91 days. Histopathological changes in the small intestine were
recorded at all times in treated animals. The effects consisted of
marked crypt-cell hyperplasia with concomitant atrophy of the
associated villi and were limited to approximately the first 7 cm of
the duodenum. A marked increase in the number of mitotic figures was
seen within the hyperplastic crypts. There was also increased
inflammatory cell infiltrate in the expanded lamina propria, limited
to the area of the duodenum with diffuse cell hyperplasia. The
inflammatory cells were predominately mononuclear and were most
prominent at day 28. At later times, only occasional villi were
affected by the inflammatory cell infiltrate. In addition to the
diffuse hyperplasia seen at day 28, focal crypt hyperplasia,
characterized by increased crypt profiles and focal flattening of the
villi, was present in animals killed on days 57 and 92. Again, the
effect was confined to approximately the first 7 cm of the duodenum.
The proliferating cell nuclear antigen labelling index was
increased in the crypt cells in the proximal duodenum, and the average
number of cells per duodenal crypt was increased at all times in
treated mice. The highest crypt cell number was seen at day 28, and
there was a trend for the number of cells to decrease with increasing
exposure to captan, the villus to crypt height ratio was biologically
significantly reduced in treated mice at all times. Thus, in this
study, prominent crypt-cell hyperplasia was produced within the first
28 days in the duodenum of mice fed a diet containing 6000 ppm captan.
The effect was also present at 56 or 91 days but was diminished,
probably by compensatory mechanisms (Allen, 1994).
Groups of five male and five female CD1 mice (body weights,
33.8-35.2 g), received as weanlings (29-33 days old) from Charles
River, Margate, Kent, United Kingdom, were fed diets containing 0,
400, 800, 3000, or 6000 ppm captan (purity, 89.4%) for 56 days.
Analysis of the diets showed that the achieved dietary concentrations
of captan were within 9% of target levels; the homogeneity (maximum
deviation, 6.8%) and chemical stability of captan in the diet stored
at room temperature over 24 days (87.5-100% of the initial
concentration) or over 73 days at -20░C (85.2-109% of the initial
concentration) were also satisfactory. Clinical signs, body weight,
and food consumption were monitored daily for the first week and then
weekly for the remainder of the study. At termination on day 57, the
animals were sacrificed, and the small intestine was prepared for
histopathology by a 'gut rolling' procedure, starting at the
ileo-caecal junction and rolling forward to the duodenum. Duodenal
hyperplasia was evaluated by (i) visual examination of routinely
stained sections for histopathological changes, (ii) assessment of the
mean number of cells in the crypt-cell population, (iii) measurement
of the bromodeoxyuridine labelling index (percent cells labelled with
bromodeoxyuridine as a ratio to the total number of cells in the
crypts), and (iv) measurement of the ratio of villus to crypt height.
The stomach, jejunum, and ileum were evaluated for histopathological
changes only.
The body-weight gains of mice fed 3000 or 6000 ppm were lower
than those of controls throughout the study, and there was a slight
reduction at 400 and 800 ppm, although this was confined to the first
few days. A similar pattern of change was seen in food consumption and
was consistent, at least in part, with a reduction in the palatability
of the diet containing captan. Captan induced a morphologically
discernible diffuse hyperplasia of the crypt cells, which was
localized to the first 7 cm of the duodenum after the pylorus of the
stomach, at 3000 and 6000 ppm in males and at 800, 3000, and 6000 ppm
in females. There was an increase in the crypt-cell labelling index at
3000 and 6000 ppm in animals of each sex and an increased incidence in
the numbers of cells in the crypt-cell population at dietary levels of
800 ppm and above. In addition, a decrease in the villus to crypt
height ratio was seen at 3000 and 6000 ppm in both males and females.
These changes were accompanied by mononuclear inflammatory cells in
the lamina propria. Mild crypt-cell hyperplasia in the jejunum of male
mice was seen at 3000 and 6000 ppm, and there was mild hyperplasia of
the forestomach epithelium with hypertrophy of the gastric pits of the
glandular portion of the stomach in males at 3000 ppm and in animals
of each sex at 6000 ppm. The NOAEL was 400 ppm on the basis of
pathological changes including duodenal hyperplasia (Tinston, 1995).
Rats
The effect of captan on preneoplastic enzyme-altered liver foci
was studied in groups of 15 male Fischer 344 rats, six weeks old,
given 200 mg/kg bw N-nitrosodiethylamine intraperitoneally and two
weeks later fed 4000 ppm (equivalent to 200 mg/kg bw) captan (purity,
90.5%) for six weeks and then sacrificed. The rats underwent a partial
hepatectomy at week 3. A small increase in the number of glutathione
S-transferase (placental form)positive foci was seen, but the effect
was reported to be borderline. There was no effect on mortality or
body-weight gain (Cabral et al., 1991).
(b) Genotoxicity
The potential for captan to interact with DNA was studied
in vivo in Swiss ICR-derived CD-1 mice and Osborne-Mendel rats which
received a suspension of 14C-trichlormethyl-captan (radiochemical
purity, 96-98%; specific activity, 19 mCi/mmol) by gavage. DNA was
isolated from various tissues and radiolabel was assayed by
scintillation spectrometry. Male mice receiving 1600 mg/kg bw and male
and female rats given 300 mg/kg bw of labelled captan were sacrificed
4 or 24 h after treatment, and DNA was extracted from the liver and
testis. No significant disintegration was found to be associated with
the DNA from either organ. Rats were not used in subsequent
experiments because the amount of radiolabelled material with high
specific activity was not sufficient to treat both mice and rats.
Radiolabel was associated with DNA in all of the organs from mice
that were examined (stomach, duodenum, intestine, kidney, liver,
testis) 24 h after oral administration of 156 mg/kg bw labelled captan
(50-56 mCi/mmol). No correlation was seen between the level of
captan-DNA associations and the site of tumour formation. Since
information on the spectrum and structure of the captan-induced DNA
modifications in specific tissues after administration of captan was
not available, the authors were not able to correlate the observed
associations with proposed mechanisms of carcinogenesis. Dialysis used
to investigate the nature of the captan-DNA interactions in vivo
indicated that up to 90% of the detected radioactivity represented
either non-covalently associated captan or covalent associations that
were hydrolysed non-enzymatically, releasing soluble, labelled
nucleotides. The exact nature of the remaining captan-DNA associations
was not known (Selsky & Matheson, 1981).
The effect of captan on microtubules and micro filaments was
studied in vitro in tubulin prepared from porcine brain and in
cultured mouse fibroblasts. Turbidometry at 350 nm showed that captan
caused a dose-dependent inhibition of tubulin assembly after
incubation. At equimolar concentration with tubulin (30 Ámol/litre),
captan totally inhibited microtubule formation; at lower
concentrations (5-30 Ámol/litre), captan also promoted disassembly of
preformed microtubules. These effects were confirmed by electron
microscopy. Immunofluorescent microscopy of cultured mouse fibroblasts
showed that captan also depolymerized microtubules, and the extent of
microtubule disassembly was concentration- and time-dependent. While a
3-h incubation of the cells with 10 mmol/litre captan was required to
completely disturb the microtubular structures, a 12-h incubation with
5 Ámol/litre was required to produce the same effect. Recovery of
microtubules was observed when preincubated cells were washed
extensively. The effect of captan on microtubules was considered to be
specific, since in equimolar concentrations in vitro it did not
interact with G-or F-actin isolated from rabbit muscle or with the
fluorescent actin pattern in mouse fibroblasts incubated with
10 mmol/litre captan for up to 12 h. Microfilaments typical of
untreated cells were seen in captan-treated fibroblasts. Thus, captan
interacts at equimolar concentrations with tubulin, affecting the
assembly and disassembly of microtubules in isolated tubulin and
cultured mammalian cells (Stournaras et al., 1991).
Groups of 100 male CD-1 mice were given a single dose of
890 mg/kg bw of 35 S-captan (radiochemical purity, 95%; specific
activity, 888 MBq/mmol per litre), 82 mg/kg bw of 14C-1-methyl-1-
nitrosourea (positive control), or a vehicle formulation by gavage.
Six hours after sacrifice, the stomach, jejunum, liver, and bone
marrow were sampled, and DNA was extracted from each tissue and
purified; the radiolabel associated with these DNA extracts was then
measured. Radiolabel was shown to be associated with DNA extracts
originating from all tissues of mice treated with captan or the
positive control but not from those given the vehicle. Covalent
binding indexes (micromoles of captan bound per mole of nucleotide
divided by millimoles of captan administered per kilogram body weight)
of 2.8, 38, 38, 46, and 91 were determined for DNA from bone marrow,
liver, stomach, dudenum, and jejunum, respectively. Selected tissues
were further purified in order to determine whether the radiolabel in
the extracts was associated with DNA. Radiolabel in liver extracts
from captan-treated mice, but not from positive control mice, was
found at the base of the tube, away from the main DNA fraction. In
contrast, radiolabel associated with DNA from the jejunum and duodenum
of captan-treated animals clearly separated at a higher position on
the density gradient than the main DNA fraction. Although captan was
associated with DNA in all of the organs examined, this study did not
indicate whether the association was covalent (Pritchard & Lappin,
1991).
When 14C-captan (radiochemical purity, 95-98%) was incubated at
25░C with calf thymus DNA, about 0.3% of the radiolabel was associated
with the DNA. In the absence of glutathione, this association was
present at time zero and did not increase with time, suggesting that
the radiolabel represents loosely bound, unextracted material rather
than covalently bound adducts. In the presence of glutathione, the
amount of radiolabel associated with DNA appeared to increase
initially but rapidly reached a constant level, which again was
independent of time. In both cases, the level of associated radiolabel
was dependent on the concentration of captan but was unaffected by pH.
Because of the high levels of associated radiolabel at time zero, the
lack of effect of time and pH, and the low radiochemical purity of the
captan used, it could not be concluded that captan binds to DNA to any
significant extent under these conditions. These results also preclude
identification of the DNA adducts. After incubation of 14C-captan
with each deoxynucleoside or DNA base in the presence or absence of
glutathione, no reaction was seen with captan, as measured by
high-performance liquid chromatography and scintillation counting of
the eluent.
Groups of 6-12 male CD-1 mice weighing 30-40 g (6-8 weeks old)
were each given a single dose of 3 mmol/kg bw 35 S-N-acetylcysteine,
2-oxo-[35 S]-thiazolidine-4-carboxylate, 2-thioxo-[35 S]-
thiazolidine-4-carboxylate, or 35 S-captan in vehicle by gavage;
another group of male mice was given the vehicle alone. Six hours
after treatment, the mice were killed, their livers were removed and
homogenized, and DNA extracts were examined. The study indicated no
significant covalent binding of captan to DNA, despite the very large
doses administered.
These studies provide no clear evidence for a reaction between
captan or its breakdown products and DNA bases or deoxynucleosides.
Furthermore, captan is, at best, an extremely weak genotoxin
in vivo. Any binding of captan to DNA must be extremely low and
would be impossible to measure by currently available methods. Thus,
the tumours seen in mice must arise by a mechanism other than one
involving direct interaction between captan and DNA (Provan et al.,
1992).
The genotoxicity of captan was also studied in vitro in
cultured human foreskin fibroblasts. Treatment with 0.16- 1.7
Ámol/litre captan reduced cloning ability and produced DNA
single-strand breaks and DNA-protein cross-links; an excision repair
response was elicited at 17-260 Ámol/litre. Captan also inhibited
cellular DNA synthesis at 3-17 Ámol/litre and formed stable adducts in
herring sperm DNA and human cellular DNA. Misincorporation of
nucleotides into synthetic DNA templates was significantly increased
after treatment with captan at 1-12 mmol/litre in vitro in the
presence of Escherichia coli DNA polymerase I, suggesting that DNA
adduct formation with captan could have mutagenic consequences. This
study shows that captan can interact with DNA at a number of levels
and that these interactions may provide the basis for a genotoxic
effect. The extreme cytotoxicity of the compound could, however, be
due to other cellular effects, since at the IC50 for cell killing
(about 0.8 Ámol/litre), none of the genotoxic events was seen (Snyder,
1992).
Comments
The excretion profiles of male and female rats were similar, the
urinary route predominating. The residual tissue concentrations seven
days after dosing were negligible in animals of each sex, the highest
concentrations being found in the kidneys and blood. The metabolism of
captan in rats appears to involve the evolution of thiophosgene,
derived from the trichloromethylthio moiety. Thiophosgene is
detoxified, at least in part, by three mechanisms: oxidation and/or
hydrolysis to carbon dioxide; reaction with the cysteine moiety of
glutathione to yield thiazolidine-2-thione-4-carboxylic acid; and
reaction with sulfite to produce dithiobis(methanesulfonic acid).
Degradation in the gastrointestinal tract appears to play a major role
in the metabolism of captan.
Studies on the hyperplasia induced by captan showed prominent
crypt-cell hyperplasia within 28 days in the proximal duodenum of mice
fed diets containing 6000 ppm captan for 91 days or 800, 3000, or
6000 ppm for 56 days. The NOAEL was 400 ppm, the lowest dose
administered for 56 days, equivalent to 60 mg/kg bw per day, on the
basis of duodenal hyperplasia.
Captan has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic
in vitro but not in vivo. The in-vitro responses are reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications have been
investigated. Studies of the genotoxicity of captan in mouse duodenum
indicate that it does not bind covalently to DNA (although metabolic
incorporation of the N-trichloromethylthio-carbon does occur) and
nuclear aberrations are not induced. The Meeting concluded that captan
does not present a significant genotoxic risk, owing to the presence
of an efficient detoxification mechanism in vivo.
There appear to have been no studies on the effects of captan on
glutathione levels analogous to those conducted with folpet. Dietary
studies of 4-8 weeks' duration have shown that irritation and
consequent inflammation as well as hyperplasia occur in the proximal
duodenum of mice. These data indicate that sustained proliferative
stimulation of the proximal duodenum is a consequence of oral
administration of captan. The Meeting concluded that this finding
represents an important element in the process by which captan,
which is not genotoxic in vivo, induces tumours in the mouse
gastrointestinal tract.
The information available to the present Meeting provides no
basis for altering the existing ADI for captan, which is based on an
NOAEL of 12.5 mg/kg bw per day determined in studies of reproductive
toxicity in rats and monkeys, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 400 ppm equivalent to 60 mg/kg bw per day (56-day study of
toxicity)
Rat: 250 ppm, equivalent to 12.5 mg/kg bw per day (study of
reproductive toxicity)
Dog: 100 mg/kg bw per day (66-week study of toxicity)
Monkey: 12.5 mg/kg bw per day (study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Allen, S.L. (1994) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/L/5674 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Bratt, H. (1990) Captan: Repeat dose study (10 mg/kg) in the rat.
Unpublished report No. CTL/P/2958 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, United
Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Cabral, R, Hoshiya, T., Hakol, K., Hasegawa, R. Fukushima, S. & Ito,
N. (1991) A rapid in vivo bioassay for the carcinogenicity of
pesticides. Tumori, 77, 185-188.
DeBaun, J.R., Miaullis, J.B., Knarr, J. Mihailovski, A. & Menn, J.J.
(1974) The fate of N-trichloro[14C]-methylthio-4-cyclohexene-
1,2-dicarboximide ([14C]captan) in the rat. Xenobiotica, 4,
101-119.
Krieger, R.I. & Thongsinthusak, T. (1993) Captan metabolism in humans
yields two biomarkers, tetrahydrophthalimide (THPI) and
thiazolidine-2-thione-4-carboxylic acid (TTCA) in urine.
Drug Chem. Toxicol., 16, 207-225.
Lappin, G.J. & Havell, M.L. (1990) Captan: Biotransformation study in
the rat. Unpublished report No. CTL/P/2951 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Pritchard, D.J. & Lappin, G.J. (1991) Captan: DNA binding study in the
mouse. Unpublished report No. CTL/P/3380 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Provan, W.M., Eyton-Jones, H. & Green, T. (1992). The potential of
captan to react with DNA. Unpublished report No. CTL/R/1131 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, United Kingdom. Submitted to WHO by ICI Agrochemicals,
Fernhurst, Haslemere, Surrey, United Kingdom.
Selsky, C.A. & Matheson, D.W. (1981) The association of captan with
mouse and rat deoxyribonucleic acid. Unpublished report No. T-
10435 from The in vitro Toxicology Section, Environmental Health
Center, Stauffer Chemical Company, Farmington, Connecticut, USA.
Submitted to WHO by ICI Agrochemicals, Fernhurst, Haslemere,
Surrey, United Kingdom.
Snyder, R.D. (1992) Effects of captan on DNA and DNA metabolic
processes in human diploid fibroblasts. Environ. Mol. Mutag.,
20, 127-133
Stournaras, C., Saridakis, I., Fostinis, Y & Georgoulias V. (1991)
Interaction of captan with mammalian microtubules. Cell
Biochem. Func., 9, 23-28
Tinston, D.J. (1995) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/P/4532 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ZENECA Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990a) Captan: Excretion mid tissue retension of a single
oral dose (10 mg/kg) in the rat. Unpublished report No.
CTL/P/2820 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990b) Captan: Excretion and tissue retension of a
single oral dose (500 mg/kg) in the rat. Unpublished report No.
CTL/P/2862 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
2. Toxicological studies
(a) Short-term toxicity
Mice
Two groups of 15 male CD1 mice were fed diets containing 0 or
6000 ppm captan (purity, 89.4%) for up to 91 consecutive days. The
concentration of captan was within 4% of nominal concentration, and
the homogeneity and stability of the diet were satisfactory for the
period and conditions of storage used. The animals were observed
daily; body weights were recorded daily for the first week of the
study and weekly thereafter. Food consumption was recorded throughout
the study. Five animals per group were killed after 28, 56, and 91
days of treatment and examined post mortem. The small intestine
(duodenum, ileum, and jejunum) was removed and prepared by a 'gut
rolling' procedure, starting at the ileo-caecal junction. Sections
were stained with haematoxylin and eosin or with antibodies to
proliferating cell nuclear antigen and examined by light microscopy.
Sections were evaluated for histopathological change, for
proliferating cell nuclear antigen labelling index (percent cells in
the crypts labelled with antigen), for mean number of crypt cells, and
for the ratio of villus to crypt height.
Body-weight loss was seen among treated mice during the first
week of the study in association with significantly reduced food
consumption. This was considered to reflect the unpalatability of the
captan diets. Food consumption was similar to that of controls from
week 2 until the end of the study, and the body-weight gain of treated
mice was similar to that of controls for the remainder of the study.
The body weights, however, were significantly lower than those of
controls from week 2 until the end of the study.
The only macroscopic findings in treated mice killed after 28
days was pronounced thickening of the duodenal mucosa. Similar but
less pronounced effects were observed in treated mice killed after 56
or 91 days. Histopathological changes in the small intestine were
recorded at all times in treated animals. The effects consisted of
marked crypt-cell hyperplasia with concomitant atrophy of the
associated villi and were limited to approximately the first 7 cm of
the duodenum. A marked increase in the number of mitotic figures was
seen within the hyperplastic crypts. There was also increased
inflammatory cell infiltrate in the expanded lamina propria, limited
to the area of the duodenum with diffuse cell hyperplasia. The
inflammatory cells were predominately mononuclear and were most
prominent at day 28. At later times, only occasional villi were
affected by the inflammatory cell infiltrate. In addition to the
diffuse hyperplasia seen at day 28, focal crypt hyperplasia,
characterized by increased crypt profiles and focal flattening of the
villi, was present in animals killed on days 57 and 92. Again, the
effect was confined to approximately the first 7 cm of the duodenum.
The proliferating cell nuclear antigen labelling index was
increased in the crypt cells in the proximal duodenum, and the average
number of cells per duodenal crypt was increased at all times in
treated mice. The highest crypt cell number was seen at day 28, and
there was a trend for the number of cells to decrease with increasing
exposure to captan, the villus to crypt height ratio was biologically
significantly reduced in treated mice at all times. Thus, in this
study, prominent crypt-cell hyperplasia was produced within the first
28 days in the duodenum of mice fed a diet containing 6000 ppm captan.
The effect was also present at 56 or 91 days but was diminished,
probably by compensatory mechanisms (Allen, 1994).
Groups of five male and five female CD1 mice (body weights,
33.8-35.2 g), received as weanlings (29-33 days old) from Charles
River, Margate, Kent, United Kingdom, were fed diets containing 0,
400, 800, 3000, or 6000 ppm captan (purity, 89.4%) for 56 days.
Analysis of the diets showed that the achieved dietary concentrations
of captan were within 9% of target levels; the homogeneity (maximum
deviation, 6.8%) and chemical stability of captan in the diet stored
at room temperature over 24 days (87.5-100% of the initial
concentration) or over 73 days at -20░C (85.2-109% of the initial
concentration) were also satisfactory. Clinical signs, body weight,
and food consumption were monitored daily for the first week and then
weekly for the remainder of the study. At termination on day 57, the
animals were sacrificed, and the small intestine was prepared for
histopathology by a 'gut rolling' procedure, starting at the
ileo-caecal junction and rolling forward to the duodenum. Duodenal
hyperplasia was evaluated by (i) visual examination of routinely
stained sections for histopathological changes, (ii) assessment of the
mean number of cells in the crypt-cell population, (iii) measurement
of the bromodeoxyuridine labelling index (percent cells labelled with
bromodeoxyuridine as a ratio to the total number of cells in the
crypts), and (iv) measurement of the ratio of villus to crypt height.
The stomach, jejunum, and ileum were evaluated for histopathological
changes only.
The body-weight gains of mice fed 3000 or 6000 ppm were lower
than those of controls throughout the study, and there was a slight
reduction at 400 and 800 ppm, although this was confined to the first
few days. A similar pattern of change was seen in food consumption and
was consistent, at least in part, with a reduction in the palatability
of the diet containing captan. Captan induced a morphologically
discernible diffuse hyperplasia of the crypt cells, which was
localized to the first 7 cm of the duodenum after the pylorus of the
stomach, at 3000 and 6000 ppm in males and at 800, 3000, and 6000 ppm
in females. There was an increase in the crypt-cell labelling index at
3000 and 6000 ppm in animals of each sex and an increased incidence in
the numbers of cells in the crypt-cell population at dietary levels of
800 ppm and above. In addition, a decrease in the villus to crypt
height ratio was seen at 3000 and 6000 ppm in both males and females.
These changes were accompanied by mononuclear inflammatory cells in
the lamina propria. Mild crypt-cell hyperplasia in the jejunum of male
mice was seen at 3000 and 6000 ppm, and there was mild hyperplasia of
the forestomach epithelium with hypertrophy of the gastric pits of the
glandular portion of the stomach in males at 3000 ppm and in animals
of each sex at 6000 ppm. The NOAEL was 400 ppm on the basis of
pathological changes including duodenal hyperplasia (Tinston, 1995).
Rats
The effect of captan on preneoplastic enzyme-altered liver foci
was studied in groups of 15 male Fischer 344 rats, six weeks old,
given 200 mg/kg bw N-nitrosodiethylamine intraperitoneally and two
weeks later fed 4000 ppm (equivalent to 200 mg/kg bw) captan (purity,
90.5%) for six weeks and then sacrificed. The rats underwent a partial
hepatectomy at week 3. A small increase in the number of glutathione
S-transferase (placental form)positive foci was seen, but the effect
was reported to be borderline. There was no effect on mortality or
body-weight gain (Cabral et al., 1991).
(b) Genotoxicity
The potential for captan to interact with DNA was studied
in vivo in Swiss ICR-derived CD-1 mice and Osborne-Mendel rats which
received a suspension of 14C-trichlormethyl-captan (radiochemical
purity, 96-98%; specific activity, 19 mCi/mmol) by gavage. DNA was
isolated from various tissues and radiolabel was assayed by
scintillation spectrometry. Male mice receiving 1600 mg/kg bw and male
and female rats given 300 mg/kg bw of labelled captan were sacrificed
4 or 24 h after treatment, and DNA was extracted from the liver and
testis. No significant disintegration was found to be associated with
the DNA from either organ. Rats were not used in subsequent
experiments because the amount of radiolabelled material with high
specific activity was not sufficient to treat both mice and rats.
Radiolabel was associated with DNA in all of the organs from mice
that were examined (stomach, duodenum, intestine, kidney, liver,
testis) 24 h after oral administration of 156 mg/kg bw labelled captan
(50-56 mCi/mmol). No correlation was seen between the level of
captan-DNA associations and the site of tumour formation. Since
information on the spectrum and structure of the captan-induced DNA
modifications in specific tissues after administration of captan was
not available, the authors were not able to correlate the observed
associations with proposed mechanisms of carcinogenesis. Dialysis used
to investigate the nature of the captan-DNA interactions in vivo
indicated that up to 90% of the detected radioactivity represented
either non-covalently associated captan or covalent associations that
were hydrolysed non-enzymatically, releasing soluble, labelled
nucleotides. The exact nature of the remaining captan-DNA associations
was not known (Selsky & Matheson, 1981).
The effect of captan on microtubules and micro filaments was
studied in vitro in tubulin prepared from porcine brain and in
cultured mouse fibroblasts. Turbidometry at 350 nm showed that captan
caused a dose-dependent inhibition of tubulin assembly after
incubation. At equimolar concentration with tubulin (30 Ámol/litre),
captan totally inhibited microtubule formation; at lower
concentrations (5-30 Ámol/litre), captan also promoted disassembly of
preformed microtubules. These effects were confirmed by electron
microscopy. Immunofluorescent microscopy of cultured mouse fibroblasts
showed that captan also depolymerized microtubules, and the extent of
microtubule disassembly was concentration- and time-dependent. While a
3-h incubation of the cells with 10 mmol/litre captan was required to
completely disturb the microtubular structures, a 12-h incubation with
5 Ámol/litre was required to produce the same effect. Recovery of
microtubules was observed when preincubated cells were washed
extensively. The effect of captan on microtubules was considered to be
specific, since in equimolar concentrations in vitro it did not
interact with G-or F-actin isolated from rabbit muscle or with the
fluorescent actin pattern in mouse fibroblasts incubated with
10 mmol/litre captan for up to 12 h. Microfilaments typical of
untreated cells were seen in captan-treated fibroblasts. Thus, captan
interacts at equimolar concentrations with tubulin, affecting the
assembly and disassembly of microtubules in isolated tubulin and
cultured mammalian cells (Stournaras et al., 1991).
Groups of 100 male CD-1 mice were given a single dose of
890 mg/kg bw of 35 S-captan (radiochemical purity, 95%; specific
activity, 888 MBq/mmol per litre), 82 mg/kg bw of 14C-1-methyl-1-
nitrosourea (positive control), or a vehicle formulation by gavage.
Six hours after sacrifice, the stomach, jejunum, liver, and bone
marrow were sampled, and DNA was extracted from each tissue and
purified; the radiolabel associated with these DNA extracts was then
measured. Radiolabel was shown to be associated with DNA extracts
originating from all tissues of mice treated with captan or the
positive control but not from those given the vehicle. Covalent
binding indexes (micromoles of captan bound per mole of nucleotide
divided by millimoles of captan administered per kilogram body weight)
of 2.8, 38, 38, 46, and 91 were determined for DNA from bone marrow,
liver, stomach, dudenum, and jejunum, respectively. Selected tissues
were further purified in order to determine whether the radiolabel in
the extracts was associated with DNA. Radiolabel in liver extracts
from captan-treated mice, but not from positive control mice, was
found at the base of the tube, away from the main DNA fraction. In
contrast, radiolabel associated with DNA from the jejunum and duodenum
of captan-treated animals clearly separated at a higher position on
the density gradient than the main DNA fraction. Although captan was
associated with DNA in all of the organs examined, this study did not
indicate whether the association was covalent (Pritchard & Lappin,
1991).
When 14C-captan (radiochemical purity, 95-98%) was incubated at
25░C with calf thymus DNA, about 0.3% of the radiolabel was associated
with the DNA. In the absence of glutathione, this association was
present at time zero and did not increase with time, suggesting that
the radiolabel represents loosely bound, unextracted material rather
than covalently bound adducts. In the presence of glutathione, the
amount of radiolabel associated with DNA appeared to increase
initially but rapidly reached a constant level, which again was
independent of time. In both cases, the level of associated radiolabel
was dependent on the concentration of captan but was unaffected by pH.
Because of the high levels of associated radiolabel at time zero, the
lack of effect of time and pH, and the low radiochemical purity of the
captan used, it could not be concluded that captan binds to DNA to any
significant extent under these conditions. These results also preclude
identification of the DNA adducts. After incubation of 14C-captan
with each deoxynucleoside or DNA base in the presence or absence of
glutathione, no reaction was seen with captan, as measured by
high-performance liquid chromatography and scintillation counting of
the eluent.
Groups of 6-12 male CD-1 mice weighing 30-40 g (6-8 weeks old)
were each given a single dose of 3 mmol/kg bw 35 S-N-acetylcysteine,
2-oxo-[35 S]-thiazolidine-4-carboxylate, 2-thioxo-[35 S]-
thiazolidine-4-carboxylate, or 35 S-captan in vehicle by gavage;
another group of male mice was given the vehicle alone. Six hours
after treatment, the mice were killed, their livers were removed and
homogenized, and DNA extracts were examined. The study indicated no
significant covalent binding of captan to DNA, despite the very large
doses administered.
These studies provide no clear evidence for a reaction between
captan or its breakdown products and DNA bases or deoxynucleosides.
Furthermore, captan is, at best, an extremely weak genotoxin
in vivo. Any binding of captan to DNA must be extremely low and
would be impossible to measure by currently available methods. Thus,
the tumours seen in mice must arise by a mechanism other than one
involving direct interaction between captan and DNA (Provan et al.,
1992).
The genotoxicity of captan was also studied in vitro in
cultured human foreskin fibroblasts. Treatment with 0.16- 1.7
Ámol/litre captan reduced cloning ability and produced DNA
single-strand breaks and DNA-protein cross-links; an excision repair
response was elicited at 17-260 Ámol/litre. Captan also inhibited
cellular DNA synthesis at 3-17 Ámol/litre and formed stable adducts in
herring sperm DNA and human cellular DNA. Misincorporation of
nucleotides into synthetic DNA templates was significantly increased
after treatment with captan at 1-12 mmol/litre in vitro in the
presence of Escherichia coli DNA polymerase I, suggesting that DNA
adduct formation with captan could have mutagenic consequences. This
study shows that captan can interact with DNA at a number of levels
and that these interactions may provide the basis for a genotoxic
effect. The extreme cytotoxicity of the compound could, however, be
due to other cellular effects, since at the IC50 for cell killing
(about 0.8 Ámol/litre), none of the genotoxic events was seen (Snyder,
1992).
Comments
The excretion profiles of male and female rats were similar, the
urinary route predominating. The residual tissue concentrations seven
days after dosing were negligible in animals of each sex, the highest
concentrations being found in the kidneys and blood. The metabolism of
captan in rats appears to involve the evolution of thiophosgene,
derived from the trichloromethylthio moiety. Thiophosgene is
detoxified, at least in part, by three mechanisms: oxidation and/or
hydrolysis to carbon dioxide; reaction with the cysteine moiety of
glutathione to yield thiazolidine-2-thione-4-carboxylic acid; and
reaction with sulfite to produce dithiobis(methanesulfonic acid).
Degradation in the gastrointestinal tract appears to play a major role
in the metabolism of captan.
Studies on the hyperplasia induced by captan showed prominent
crypt-cell hyperplasia within 28 days in the proximal duodenum of mice
fed diets containing 6000 ppm captan for 91 days or 800, 3000, or
6000 ppm for 56 days. The NOAEL was 400 ppm, the lowest dose
administered for 56 days, equivalent to 60 mg/kg bw per day, on the
basis of duodenal hyperplasia.
Captan has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic
in vitro but not in vivo. The in-vitro responses are reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications have been
investigated. Studies of the genotoxicity of captan in mouse duodenum
indicate that it does not bind covalently to DNA (although metabolic
incorporation of the N-trichloromethylthio-carbon does occur) and
nuclear aberrations are not induced. The Meeting concluded that captan
does not present a significant genotoxic risk, owing to the presence
of an efficient detoxification mechanism in vivo.
There appear to have been no studies on the effects of captan on
glutathione levels analogous to those conducted with folpet. Dietary
studies of 4-8 weeks' duration have shown that irritation and
consequent inflammation as well as hyperplasia occur in the proximal
duodenum of mice. These data indicate that sustained proliferative
stimulation of the proximal duodenum is a consequence of oral
administration of captan. The Meeting concluded that this finding
represents an important element in the process by which captan,
which is not genotoxic in vivo, induces tumours in the mouse
gastrointestinal tract.
The information available to the present Meeting provides no
basis for altering the existing ADI for captan, which is based on an
NOAEL of 12.5 mg/kg bw per day determined in studies of reproductive
toxicity in rats and monkeys, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 400 ppm equivalent to 60 mg/kg bw per day (56-day study of
toxicity)
Rat: 250 ppm, equivalent to 12.5 mg/kg bw per day (study of
reproductive toxicity)
Dog: 100 mg/kg bw per day (66-week study of toxicity)
Monkey: 12.5 mg/kg bw per day (study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Allen, S.L. (1994) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/L/5674 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Bratt, H. (1990) Captan: Repeat dose study (10 mg/kg) in the rat.
Unpublished report No. CTL/P/2958 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, United
Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Cabral, R, Hoshiya, T., Hakol, K., Hasegawa, R. Fukushima, S. & Ito,
N. (1991) A rapid in vivo bioassay for the carcinogenicity of
pesticides. Tumori, 77, 185-188.
DeBaun, J.R., Miaullis, J.B., Knarr, J. Mihailovski, A. & Menn, J.J.
(1974) The fate of N-trichloro[14C]-methylthio-4-cyclohexene-
1,2-dicarboximide ([14C]captan) in the rat. Xenobiotica, 4,
101-119.
Krieger, R.I. & Thongsinthusak, T. (1993) Captan metabolism in humans
yields two biomarkers, tetrahydrophthalimide (THPI) and
thiazolidine-2-thione-4-carboxylic acid (TTCA) in urine.
Drug Chem. Toxicol., 16, 207-225.
Lappin, G.J. & Havell, M.L. (1990) Captan: Biotransformation study in
the rat. Unpublished report No. CTL/P/2951 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Pritchard, D.J. & Lappin, G.J. (1991) Captan: DNA binding study in the
mouse. Unpublished report No. CTL/P/3380 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Provan, W.M., Eyton-Jones, H. & Green, T. (1992). The potential of
captan to react with DNA. Unpublished report No. CTL/R/1131 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, United Kingdom. Submitted to WHO by ICI Agrochemicals,
Fernhurst, Haslemere, Surrey, United Kingdom.
Selsky, C.A. & Matheson, D.W. (1981) The association of captan with
mouse and rat deoxyribonucleic acid. Unpublished report No. T-
10435 from The in vitro Toxicology Section, Environmental Health
Center, Stauffer Chemical Company, Farmington, Connecticut, USA.
Submitted to WHO by ICI Agrochemicals, Fernhurst, Haslemere,
Surrey, United Kingdom.
Snyder, R.D. (1992) Effects of captan on DNA and DNA metabolic
processes in human diploid fibroblasts. Environ. Mol. Mutag.,
20, 127-133
Stournaras, C., Saridakis, I., Fostinis, Y & Georgoulias V. (1991)
Interaction of captan with mammalian microtubules. Cell
Biochem. Func., 9, 23-28
Tinston, D.J. (1995) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/P/4532 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ZENECA Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990a) Captan: Excretion mid tissue retension of a single
oral dose (10 mg/kg) in the rat. Unpublished report No.
CTL/P/2820 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990b) Captan: Excretion and tissue retension of a
single oral dose (500 mg/kg) in the rat. Unpublished report No.
CTL/P/2862 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
 2. Toxicological studies
(a) Short-term toxicity
Mice
Two groups of 15 male CD1 mice were fed diets containing 0 or
6000 ppm captan (purity, 89.4%) for up to 91 consecutive days. The
concentration of captan was within 4% of nominal concentration, and
the homogeneity and stability of the diet were satisfactory for the
period and conditions of storage used. The animals were observed
daily; body weights were recorded daily for the first week of the
study and weekly thereafter. Food consumption was recorded throughout
the study. Five animals per group were killed after 28, 56, and 91
days of treatment and examined post mortem. The small intestine
(duodenum, ileum, and jejunum) was removed and prepared by a 'gut
rolling' procedure, starting at the ileo-caecal junction. Sections
were stained with haematoxylin and eosin or with antibodies to
proliferating cell nuclear antigen and examined by light microscopy.
Sections were evaluated for histopathological change, for
proliferating cell nuclear antigen labelling index (percent cells in
the crypts labelled with antigen), for mean number of crypt cells, and
for the ratio of villus to crypt height.
Body-weight loss was seen among treated mice during the first
week of the study in association with significantly reduced food
consumption. This was considered to reflect the unpalatability of the
captan diets. Food consumption was similar to that of controls from
week 2 until the end of the study, and the body-weight gain of treated
mice was similar to that of controls for the remainder of the study.
The body weights, however, were significantly lower than those of
controls from week 2 until the end of the study.
The only macroscopic findings in treated mice killed after 28
days was pronounced thickening of the duodenal mucosa. Similar but
less pronounced effects were observed in treated mice killed after 56
or 91 days. Histopathological changes in the small intestine were
recorded at all times in treated animals. The effects consisted of
marked crypt-cell hyperplasia with concomitant atrophy of the
associated villi and were limited to approximately the first 7 cm of
the duodenum. A marked increase in the number of mitotic figures was
seen within the hyperplastic crypts. There was also increased
inflammatory cell infiltrate in the expanded lamina propria, limited
to the area of the duodenum with diffuse cell hyperplasia. The
inflammatory cells were predominately mononuclear and were most
prominent at day 28. At later times, only occasional villi were
affected by the inflammatory cell infiltrate. In addition to the
diffuse hyperplasia seen at day 28, focal crypt hyperplasia,
characterized by increased crypt profiles and focal flattening of the
villi, was present in animals killed on days 57 and 92. Again, the
effect was confined to approximately the first 7 cm of the duodenum.
The proliferating cell nuclear antigen labelling index was
increased in the crypt cells in the proximal duodenum, and the average
number of cells per duodenal crypt was increased at all times in
treated mice. The highest crypt cell number was seen at day 28, and
there was a trend for the number of cells to decrease with increasing
exposure to captan, the villus to crypt height ratio was biologically
significantly reduced in treated mice at all times. Thus, in this
study, prominent crypt-cell hyperplasia was produced within the first
28 days in the duodenum of mice fed a diet containing 6000 ppm captan.
The effect was also present at 56 or 91 days but was diminished,
probably by compensatory mechanisms (Allen, 1994).
Groups of five male and five female CD1 mice (body weights,
33.8-35.2 g), received as weanlings (29-33 days old) from Charles
River, Margate, Kent, United Kingdom, were fed diets containing 0,
400, 800, 3000, or 6000 ppm captan (purity, 89.4%) for 56 days.
Analysis of the diets showed that the achieved dietary concentrations
of captan were within 9% of target levels; the homogeneity (maximum
deviation, 6.8%) and chemical stability of captan in the diet stored
at room temperature over 24 days (87.5-100% of the initial
concentration) or over 73 days at -20░C (85.2-109% of the initial
concentration) were also satisfactory. Clinical signs, body weight,
and food consumption were monitored daily for the first week and then
weekly for the remainder of the study. At termination on day 57, the
animals were sacrificed, and the small intestine was prepared for
histopathology by a 'gut rolling' procedure, starting at the
ileo-caecal junction and rolling forward to the duodenum. Duodenal
hyperplasia was evaluated by (i) visual examination of routinely
stained sections for histopathological changes, (ii) assessment of the
mean number of cells in the crypt-cell population, (iii) measurement
of the bromodeoxyuridine labelling index (percent cells labelled with
bromodeoxyuridine as a ratio to the total number of cells in the
crypts), and (iv) measurement of the ratio of villus to crypt height.
The stomach, jejunum, and ileum were evaluated for histopathological
changes only.
The body-weight gains of mice fed 3000 or 6000 ppm were lower
than those of controls throughout the study, and there was a slight
reduction at 400 and 800 ppm, although this was confined to the first
few days. A similar pattern of change was seen in food consumption and
was consistent, at least in part, with a reduction in the palatability
of the diet containing captan. Captan induced a morphologically
discernible diffuse hyperplasia of the crypt cells, which was
localized to the first 7 cm of the duodenum after the pylorus of the
stomach, at 3000 and 6000 ppm in males and at 800, 3000, and 6000 ppm
in females. There was an increase in the crypt-cell labelling index at
3000 and 6000 ppm in animals of each sex and an increased incidence in
the numbers of cells in the crypt-cell population at dietary levels of
800 ppm and above. In addition, a decrease in the villus to crypt
height ratio was seen at 3000 and 6000 ppm in both males and females.
These changes were accompanied by mononuclear inflammatory cells in
the lamina propria. Mild crypt-cell hyperplasia in the jejunum of male
mice was seen at 3000 and 6000 ppm, and there was mild hyperplasia of
the forestomach epithelium with hypertrophy of the gastric pits of the
glandular portion of the stomach in males at 3000 ppm and in animals
of each sex at 6000 ppm. The NOAEL was 400 ppm on the basis of
pathological changes including duodenal hyperplasia (Tinston, 1995).
Rats
The effect of captan on preneoplastic enzyme-altered liver foci
was studied in groups of 15 male Fischer 344 rats, six weeks old,
given 200 mg/kg bw N-nitrosodiethylamine intraperitoneally and two
weeks later fed 4000 ppm (equivalent to 200 mg/kg bw) captan (purity,
90.5%) for six weeks and then sacrificed. The rats underwent a partial
hepatectomy at week 3. A small increase in the number of glutathione
S-transferase (placental form)positive foci was seen, but the effect
was reported to be borderline. There was no effect on mortality or
body-weight gain (Cabral et al., 1991).
(b) Genotoxicity
The potential for captan to interact with DNA was studied
in vivo in Swiss ICR-derived CD-1 mice and Osborne-Mendel rats which
received a suspension of 14C-trichlormethyl-captan (radiochemical
purity, 96-98%; specific activity, 19 mCi/mmol) by gavage. DNA was
isolated from various tissues and radiolabel was assayed by
scintillation spectrometry. Male mice receiving 1600 mg/kg bw and male
and female rats given 300 mg/kg bw of labelled captan were sacrificed
4 or 24 h after treatment, and DNA was extracted from the liver and
testis. No significant disintegration was found to be associated with
the DNA from either organ. Rats were not used in subsequent
experiments because the amount of radiolabelled material with high
specific activity was not sufficient to treat both mice and rats.
Radiolabel was associated with DNA in all of the organs from mice
that were examined (stomach, duodenum, intestine, kidney, liver,
testis) 24 h after oral administration of 156 mg/kg bw labelled captan
(50-56 mCi/mmol). No correlation was seen between the level of
captan-DNA associations and the site of tumour formation. Since
information on the spectrum and structure of the captan-induced DNA
modifications in specific tissues after administration of captan was
not available, the authors were not able to correlate the observed
associations with proposed mechanisms of carcinogenesis. Dialysis used
to investigate the nature of the captan-DNA interactions in vivo
indicated that up to 90% of the detected radioactivity represented
either non-covalently associated captan or covalent associations that
were hydrolysed non-enzymatically, releasing soluble, labelled
nucleotides. The exact nature of the remaining captan-DNA associations
was not known (Selsky & Matheson, 1981).
The effect of captan on microtubules and micro filaments was
studied in vitro in tubulin prepared from porcine brain and in
cultured mouse fibroblasts. Turbidometry at 350 nm showed that captan
caused a dose-dependent inhibition of tubulin assembly after
incubation. At equimolar concentration with tubulin (30 Ámol/litre),
captan totally inhibited microtubule formation; at lower
concentrations (5-30 Ámol/litre), captan also promoted disassembly of
preformed microtubules. These effects were confirmed by electron
microscopy. Immunofluorescent microscopy of cultured mouse fibroblasts
showed that captan also depolymerized microtubules, and the extent of
microtubule disassembly was concentration- and time-dependent. While a
3-h incubation of the cells with 10 mmol/litre captan was required to
completely disturb the microtubular structures, a 12-h incubation with
5 Ámol/litre was required to produce the same effect. Recovery of
microtubules was observed when preincubated cells were washed
extensively. The effect of captan on microtubules was considered to be
specific, since in equimolar concentrations in vitro it did not
interact with G-or F-actin isolated from rabbit muscle or with the
fluorescent actin pattern in mouse fibroblasts incubated with
10 mmol/litre captan for up to 12 h. Microfilaments typical of
untreated cells were seen in captan-treated fibroblasts. Thus, captan
interacts at equimolar concentrations with tubulin, affecting the
assembly and disassembly of microtubules in isolated tubulin and
cultured mammalian cells (Stournaras et al., 1991).
Groups of 100 male CD-1 mice were given a single dose of
890 mg/kg bw of 35 S-captan (radiochemical purity, 95%; specific
activity, 888 MBq/mmol per litre), 82 mg/kg bw of 14C-1-methyl-1-
nitrosourea (positive control), or a vehicle formulation by gavage.
Six hours after sacrifice, the stomach, jejunum, liver, and bone
marrow were sampled, and DNA was extracted from each tissue and
purified; the radiolabel associated with these DNA extracts was then
measured. Radiolabel was shown to be associated with DNA extracts
originating from all tissues of mice treated with captan or the
positive control but not from those given the vehicle. Covalent
binding indexes (micromoles of captan bound per mole of nucleotide
divided by millimoles of captan administered per kilogram body weight)
of 2.8, 38, 38, 46, and 91 were determined for DNA from bone marrow,
liver, stomach, dudenum, and jejunum, respectively. Selected tissues
were further purified in order to determine whether the radiolabel in
the extracts was associated with DNA. Radiolabel in liver extracts
from captan-treated mice, but not from positive control mice, was
found at the base of the tube, away from the main DNA fraction. In
contrast, radiolabel associated with DNA from the jejunum and duodenum
of captan-treated animals clearly separated at a higher position on
the density gradient than the main DNA fraction. Although captan was
associated with DNA in all of the organs examined, this study did not
indicate whether the association was covalent (Pritchard & Lappin,
1991).
When 14C-captan (radiochemical purity, 95-98%) was incubated at
25░C with calf thymus DNA, about 0.3% of the radiolabel was associated
with the DNA. In the absence of glutathione, this association was
present at time zero and did not increase with time, suggesting that
the radiolabel represents loosely bound, unextracted material rather
than covalently bound adducts. In the presence of glutathione, the
amount of radiolabel associated with DNA appeared to increase
initially but rapidly reached a constant level, which again was
independent of time. In both cases, the level of associated radiolabel
was dependent on the concentration of captan but was unaffected by pH.
Because of the high levels of associated radiolabel at time zero, the
lack of effect of time and pH, and the low radiochemical purity of the
captan used, it could not be concluded that captan binds to DNA to any
significant extent under these conditions. These results also preclude
identification of the DNA adducts. After incubation of 14C-captan
with each deoxynucleoside or DNA base in the presence or absence of
glutathione, no reaction was seen with captan, as measured by
high-performance liquid chromatography and scintillation counting of
the eluent.
Groups of 6-12 male CD-1 mice weighing 30-40 g (6-8 weeks old)
were each given a single dose of 3 mmol/kg bw 35 S-N-acetylcysteine,
2-oxo-[35 S]-thiazolidine-4-carboxylate, 2-thioxo-[35 S]-
thiazolidine-4-carboxylate, or 35 S-captan in vehicle by gavage;
another group of male mice was given the vehicle alone. Six hours
after treatment, the mice were killed, their livers were removed and
homogenized, and DNA extracts were examined. The study indicated no
significant covalent binding of captan to DNA, despite the very large
doses administered.
These studies provide no clear evidence for a reaction between
captan or its breakdown products and DNA bases or deoxynucleosides.
Furthermore, captan is, at best, an extremely weak genotoxin
in vivo. Any binding of captan to DNA must be extremely low and
would be impossible to measure by currently available methods. Thus,
the tumours seen in mice must arise by a mechanism other than one
involving direct interaction between captan and DNA (Provan et al.,
1992).
The genotoxicity of captan was also studied in vitro in
cultured human foreskin fibroblasts. Treatment with 0.16- 1.7
Ámol/litre captan reduced cloning ability and produced DNA
single-strand breaks and DNA-protein cross-links; an excision repair
response was elicited at 17-260 Ámol/litre. Captan also inhibited
cellular DNA synthesis at 3-17 Ámol/litre and formed stable adducts in
herring sperm DNA and human cellular DNA. Misincorporation of
nucleotides into synthetic DNA templates was significantly increased
after treatment with captan at 1-12 mmol/litre in vitro in the
presence of Escherichia coli DNA polymerase I, suggesting that DNA
adduct formation with captan could have mutagenic consequences. This
study shows that captan can interact with DNA at a number of levels
and that these interactions may provide the basis for a genotoxic
effect. The extreme cytotoxicity of the compound could, however, be
due to other cellular effects, since at the IC50 for cell killing
(about 0.8 Ámol/litre), none of the genotoxic events was seen (Snyder,
1992).
Comments
The excretion profiles of male and female rats were similar, the
urinary route predominating. The residual tissue concentrations seven
days after dosing were negligible in animals of each sex, the highest
concentrations being found in the kidneys and blood. The metabolism of
captan in rats appears to involve the evolution of thiophosgene,
derived from the trichloromethylthio moiety. Thiophosgene is
detoxified, at least in part, by three mechanisms: oxidation and/or
hydrolysis to carbon dioxide; reaction with the cysteine moiety of
glutathione to yield thiazolidine-2-thione-4-carboxylic acid; and
reaction with sulfite to produce dithiobis(methanesulfonic acid).
Degradation in the gastrointestinal tract appears to play a major role
in the metabolism of captan.
Studies on the hyperplasia induced by captan showed prominent
crypt-cell hyperplasia within 28 days in the proximal duodenum of mice
fed diets containing 6000 ppm captan for 91 days or 800, 3000, or
6000 ppm for 56 days. The NOAEL was 400 ppm, the lowest dose
administered for 56 days, equivalent to 60 mg/kg bw per day, on the
basis of duodenal hyperplasia.
Captan has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic
in vitro but not in vivo. The in-vitro responses are reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications have been
investigated. Studies of the genotoxicity of captan in mouse duodenum
indicate that it does not bind covalently to DNA (although metabolic
incorporation of the N-trichloromethylthio-carbon does occur) and
nuclear aberrations are not induced. The Meeting concluded that captan
does not present a significant genotoxic risk, owing to the presence
of an efficient detoxification mechanism in vivo.
There appear to have been no studies on the effects of captan on
glutathione levels analogous to those conducted with folpet. Dietary
studies of 4-8 weeks' duration have shown that irritation and
consequent inflammation as well as hyperplasia occur in the proximal
duodenum of mice. These data indicate that sustained proliferative
stimulation of the proximal duodenum is a consequence of oral
administration of captan. The Meeting concluded that this finding
represents an important element in the process by which captan,
which is not genotoxic in vivo, induces tumours in the mouse
gastrointestinal tract.
The information available to the present Meeting provides no
basis for altering the existing ADI for captan, which is based on an
NOAEL of 12.5 mg/kg bw per day determined in studies of reproductive
toxicity in rats and monkeys, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 400 ppm equivalent to 60 mg/kg bw per day (56-day study of
toxicity)
Rat: 250 ppm, equivalent to 12.5 mg/kg bw per day (study of
reproductive toxicity)
Dog: 100 mg/kg bw per day (66-week study of toxicity)
Monkey: 12.5 mg/kg bw per day (study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Allen, S.L. (1994) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/L/5674 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Bratt, H. (1990) Captan: Repeat dose study (10 mg/kg) in the rat.
Unpublished report No. CTL/P/2958 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, United
Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Cabral, R, Hoshiya, T., Hakol, K., Hasegawa, R. Fukushima, S. & Ito,
N. (1991) A rapid in vivo bioassay for the carcinogenicity of
pesticides. Tumori, 77, 185-188.
DeBaun, J.R., Miaullis, J.B., Knarr, J. Mihailovski, A. & Menn, J.J.
(1974) The fate of N-trichloro[14C]-methylthio-4-cyclohexene-
1,2-dicarboximide ([14C]captan) in the rat. Xenobiotica, 4,
101-119.
Krieger, R.I. & Thongsinthusak, T. (1993) Captan metabolism in humans
yields two biomarkers, tetrahydrophthalimide (THPI) and
thiazolidine-2-thione-4-carboxylic acid (TTCA) in urine.
Drug Chem. Toxicol., 16, 207-225.
Lappin, G.J. & Havell, M.L. (1990) Captan: Biotransformation study in
the rat. Unpublished report No. CTL/P/2951 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Pritchard, D.J. & Lappin, G.J. (1991) Captan: DNA binding study in the
mouse. Unpublished report No. CTL/P/3380 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Provan, W.M., Eyton-Jones, H. & Green, T. (1992). The potential of
captan to react with DNA. Unpublished report No. CTL/R/1131 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, United Kingdom. Submitted to WHO by ICI Agrochemicals,
Fernhurst, Haslemere, Surrey, United Kingdom.
Selsky, C.A. & Matheson, D.W. (1981) The association of captan with
mouse and rat deoxyribonucleic acid. Unpublished report No. T-
10435 from The in vitro Toxicology Section, Environmental Health
Center, Stauffer Chemical Company, Farmington, Connecticut, USA.
Submitted to WHO by ICI Agrochemicals, Fernhurst, Haslemere,
Surrey, United Kingdom.
Snyder, R.D. (1992) Effects of captan on DNA and DNA metabolic
processes in human diploid fibroblasts. Environ. Mol. Mutag.,
20, 127-133
Stournaras, C., Saridakis, I., Fostinis, Y & Georgoulias V. (1991)
Interaction of captan with mammalian microtubules. Cell
Biochem. Func., 9, 23-28
Tinston, D.J. (1995) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/P/4532 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ZENECA Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990a) Captan: Excretion mid tissue retension of a single
oral dose (10 mg/kg) in the rat. Unpublished report No.
CTL/P/2820 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990b) Captan: Excretion and tissue retension of a
single oral dose (500 mg/kg) in the rat. Unpublished report No.
CTL/P/2862 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
2. Toxicological studies
(a) Short-term toxicity
Mice
Two groups of 15 male CD1 mice were fed diets containing 0 or
6000 ppm captan (purity, 89.4%) for up to 91 consecutive days. The
concentration of captan was within 4% of nominal concentration, and
the homogeneity and stability of the diet were satisfactory for the
period and conditions of storage used. The animals were observed
daily; body weights were recorded daily for the first week of the
study and weekly thereafter. Food consumption was recorded throughout
the study. Five animals per group were killed after 28, 56, and 91
days of treatment and examined post mortem. The small intestine
(duodenum, ileum, and jejunum) was removed and prepared by a 'gut
rolling' procedure, starting at the ileo-caecal junction. Sections
were stained with haematoxylin and eosin or with antibodies to
proliferating cell nuclear antigen and examined by light microscopy.
Sections were evaluated for histopathological change, for
proliferating cell nuclear antigen labelling index (percent cells in
the crypts labelled with antigen), for mean number of crypt cells, and
for the ratio of villus to crypt height.
Body-weight loss was seen among treated mice during the first
week of the study in association with significantly reduced food
consumption. This was considered to reflect the unpalatability of the
captan diets. Food consumption was similar to that of controls from
week 2 until the end of the study, and the body-weight gain of treated
mice was similar to that of controls for the remainder of the study.
The body weights, however, were significantly lower than those of
controls from week 2 until the end of the study.
The only macroscopic findings in treated mice killed after 28
days was pronounced thickening of the duodenal mucosa. Similar but
less pronounced effects were observed in treated mice killed after 56
or 91 days. Histopathological changes in the small intestine were
recorded at all times in treated animals. The effects consisted of
marked crypt-cell hyperplasia with concomitant atrophy of the
associated villi and were limited to approximately the first 7 cm of
the duodenum. A marked increase in the number of mitotic figures was
seen within the hyperplastic crypts. There was also increased
inflammatory cell infiltrate in the expanded lamina propria, limited
to the area of the duodenum with diffuse cell hyperplasia. The
inflammatory cells were predominately mononuclear and were most
prominent at day 28. At later times, only occasional villi were
affected by the inflammatory cell infiltrate. In addition to the
diffuse hyperplasia seen at day 28, focal crypt hyperplasia,
characterized by increased crypt profiles and focal flattening of the
villi, was present in animals killed on days 57 and 92. Again, the
effect was confined to approximately the first 7 cm of the duodenum.
The proliferating cell nuclear antigen labelling index was
increased in the crypt cells in the proximal duodenum, and the average
number of cells per duodenal crypt was increased at all times in
treated mice. The highest crypt cell number was seen at day 28, and
there was a trend for the number of cells to decrease with increasing
exposure to captan, the villus to crypt height ratio was biologically
significantly reduced in treated mice at all times. Thus, in this
study, prominent crypt-cell hyperplasia was produced within the first
28 days in the duodenum of mice fed a diet containing 6000 ppm captan.
The effect was also present at 56 or 91 days but was diminished,
probably by compensatory mechanisms (Allen, 1994).
Groups of five male and five female CD1 mice (body weights,
33.8-35.2 g), received as weanlings (29-33 days old) from Charles
River, Margate, Kent, United Kingdom, were fed diets containing 0,
400, 800, 3000, or 6000 ppm captan (purity, 89.4%) for 56 days.
Analysis of the diets showed that the achieved dietary concentrations
of captan were within 9% of target levels; the homogeneity (maximum
deviation, 6.8%) and chemical stability of captan in the diet stored
at room temperature over 24 days (87.5-100% of the initial
concentration) or over 73 days at -20░C (85.2-109% of the initial
concentration) were also satisfactory. Clinical signs, body weight,
and food consumption were monitored daily for the first week and then
weekly for the remainder of the study. At termination on day 57, the
animals were sacrificed, and the small intestine was prepared for
histopathology by a 'gut rolling' procedure, starting at the
ileo-caecal junction and rolling forward to the duodenum. Duodenal
hyperplasia was evaluated by (i) visual examination of routinely
stained sections for histopathological changes, (ii) assessment of the
mean number of cells in the crypt-cell population, (iii) measurement
of the bromodeoxyuridine labelling index (percent cells labelled with
bromodeoxyuridine as a ratio to the total number of cells in the
crypts), and (iv) measurement of the ratio of villus to crypt height.
The stomach, jejunum, and ileum were evaluated for histopathological
changes only.
The body-weight gains of mice fed 3000 or 6000 ppm were lower
than those of controls throughout the study, and there was a slight
reduction at 400 and 800 ppm, although this was confined to the first
few days. A similar pattern of change was seen in food consumption and
was consistent, at least in part, with a reduction in the palatability
of the diet containing captan. Captan induced a morphologically
discernible diffuse hyperplasia of the crypt cells, which was
localized to the first 7 cm of the duodenum after the pylorus of the
stomach, at 3000 and 6000 ppm in males and at 800, 3000, and 6000 ppm
in females. There was an increase in the crypt-cell labelling index at
3000 and 6000 ppm in animals of each sex and an increased incidence in
the numbers of cells in the crypt-cell population at dietary levels of
800 ppm and above. In addition, a decrease in the villus to crypt
height ratio was seen at 3000 and 6000 ppm in both males and females.
These changes were accompanied by mononuclear inflammatory cells in
the lamina propria. Mild crypt-cell hyperplasia in the jejunum of male
mice was seen at 3000 and 6000 ppm, and there was mild hyperplasia of
the forestomach epithelium with hypertrophy of the gastric pits of the
glandular portion of the stomach in males at 3000 ppm and in animals
of each sex at 6000 ppm. The NOAEL was 400 ppm on the basis of
pathological changes including duodenal hyperplasia (Tinston, 1995).
Rats
The effect of captan on preneoplastic enzyme-altered liver foci
was studied in groups of 15 male Fischer 344 rats, six weeks old,
given 200 mg/kg bw N-nitrosodiethylamine intraperitoneally and two
weeks later fed 4000 ppm (equivalent to 200 mg/kg bw) captan (purity,
90.5%) for six weeks and then sacrificed. The rats underwent a partial
hepatectomy at week 3. A small increase in the number of glutathione
S-transferase (placental form)positive foci was seen, but the effect
was reported to be borderline. There was no effect on mortality or
body-weight gain (Cabral et al., 1991).
(b) Genotoxicity
The potential for captan to interact with DNA was studied
in vivo in Swiss ICR-derived CD-1 mice and Osborne-Mendel rats which
received a suspension of 14C-trichlormethyl-captan (radiochemical
purity, 96-98%; specific activity, 19 mCi/mmol) by gavage. DNA was
isolated from various tissues and radiolabel was assayed by
scintillation spectrometry. Male mice receiving 1600 mg/kg bw and male
and female rats given 300 mg/kg bw of labelled captan were sacrificed
4 or 24 h after treatment, and DNA was extracted from the liver and
testis. No significant disintegration was found to be associated with
the DNA from either organ. Rats were not used in subsequent
experiments because the amount of radiolabelled material with high
specific activity was not sufficient to treat both mice and rats.
Radiolabel was associated with DNA in all of the organs from mice
that were examined (stomach, duodenum, intestine, kidney, liver,
testis) 24 h after oral administration of 156 mg/kg bw labelled captan
(50-56 mCi/mmol). No correlation was seen between the level of
captan-DNA associations and the site of tumour formation. Since
information on the spectrum and structure of the captan-induced DNA
modifications in specific tissues after administration of captan was
not available, the authors were not able to correlate the observed
associations with proposed mechanisms of carcinogenesis. Dialysis used
to investigate the nature of the captan-DNA interactions in vivo
indicated that up to 90% of the detected radioactivity represented
either non-covalently associated captan or covalent associations that
were hydrolysed non-enzymatically, releasing soluble, labelled
nucleotides. The exact nature of the remaining captan-DNA associations
was not known (Selsky & Matheson, 1981).
The effect of captan on microtubules and micro filaments was
studied in vitro in tubulin prepared from porcine brain and in
cultured mouse fibroblasts. Turbidometry at 350 nm showed that captan
caused a dose-dependent inhibition of tubulin assembly after
incubation. At equimolar concentration with tubulin (30 Ámol/litre),
captan totally inhibited microtubule formation; at lower
concentrations (5-30 Ámol/litre), captan also promoted disassembly of
preformed microtubules. These effects were confirmed by electron
microscopy. Immunofluorescent microscopy of cultured mouse fibroblasts
showed that captan also depolymerized microtubules, and the extent of
microtubule disassembly was concentration- and time-dependent. While a
3-h incubation of the cells with 10 mmol/litre captan was required to
completely disturb the microtubular structures, a 12-h incubation with
5 Ámol/litre was required to produce the same effect. Recovery of
microtubules was observed when preincubated cells were washed
extensively. The effect of captan on microtubules was considered to be
specific, since in equimolar concentrations in vitro it did not
interact with G-or F-actin isolated from rabbit muscle or with the
fluorescent actin pattern in mouse fibroblasts incubated with
10 mmol/litre captan for up to 12 h. Microfilaments typical of
untreated cells were seen in captan-treated fibroblasts. Thus, captan
interacts at equimolar concentrations with tubulin, affecting the
assembly and disassembly of microtubules in isolated tubulin and
cultured mammalian cells (Stournaras et al., 1991).
Groups of 100 male CD-1 mice were given a single dose of
890 mg/kg bw of 35 S-captan (radiochemical purity, 95%; specific
activity, 888 MBq/mmol per litre), 82 mg/kg bw of 14C-1-methyl-1-
nitrosourea (positive control), or a vehicle formulation by gavage.
Six hours after sacrifice, the stomach, jejunum, liver, and bone
marrow were sampled, and DNA was extracted from each tissue and
purified; the radiolabel associated with these DNA extracts was then
measured. Radiolabel was shown to be associated with DNA extracts
originating from all tissues of mice treated with captan or the
positive control but not from those given the vehicle. Covalent
binding indexes (micromoles of captan bound per mole of nucleotide
divided by millimoles of captan administered per kilogram body weight)
of 2.8, 38, 38, 46, and 91 were determined for DNA from bone marrow,
liver, stomach, dudenum, and jejunum, respectively. Selected tissues
were further purified in order to determine whether the radiolabel in
the extracts was associated with DNA. Radiolabel in liver extracts
from captan-treated mice, but not from positive control mice, was
found at the base of the tube, away from the main DNA fraction. In
contrast, radiolabel associated with DNA from the jejunum and duodenum
of captan-treated animals clearly separated at a higher position on
the density gradient than the main DNA fraction. Although captan was
associated with DNA in all of the organs examined, this study did not
indicate whether the association was covalent (Pritchard & Lappin,
1991).
When 14C-captan (radiochemical purity, 95-98%) was incubated at
25░C with calf thymus DNA, about 0.3% of the radiolabel was associated
with the DNA. In the absence of glutathione, this association was
present at time zero and did not increase with time, suggesting that
the radiolabel represents loosely bound, unextracted material rather
than covalently bound adducts. In the presence of glutathione, the
amount of radiolabel associated with DNA appeared to increase
initially but rapidly reached a constant level, which again was
independent of time. In both cases, the level of associated radiolabel
was dependent on the concentration of captan but was unaffected by pH.
Because of the high levels of associated radiolabel at time zero, the
lack of effect of time and pH, and the low radiochemical purity of the
captan used, it could not be concluded that captan binds to DNA to any
significant extent under these conditions. These results also preclude
identification of the DNA adducts. After incubation of 14C-captan
with each deoxynucleoside or DNA base in the presence or absence of
glutathione, no reaction was seen with captan, as measured by
high-performance liquid chromatography and scintillation counting of
the eluent.
Groups of 6-12 male CD-1 mice weighing 30-40 g (6-8 weeks old)
were each given a single dose of 3 mmol/kg bw 35 S-N-acetylcysteine,
2-oxo-[35 S]-thiazolidine-4-carboxylate, 2-thioxo-[35 S]-
thiazolidine-4-carboxylate, or 35 S-captan in vehicle by gavage;
another group of male mice was given the vehicle alone. Six hours
after treatment, the mice were killed, their livers were removed and
homogenized, and DNA extracts were examined. The study indicated no
significant covalent binding of captan to DNA, despite the very large
doses administered.
These studies provide no clear evidence for a reaction between
captan or its breakdown products and DNA bases or deoxynucleosides.
Furthermore, captan is, at best, an extremely weak genotoxin
in vivo. Any binding of captan to DNA must be extremely low and
would be impossible to measure by currently available methods. Thus,
the tumours seen in mice must arise by a mechanism other than one
involving direct interaction between captan and DNA (Provan et al.,
1992).
The genotoxicity of captan was also studied in vitro in
cultured human foreskin fibroblasts. Treatment with 0.16- 1.7
Ámol/litre captan reduced cloning ability and produced DNA
single-strand breaks and DNA-protein cross-links; an excision repair
response was elicited at 17-260 Ámol/litre. Captan also inhibited
cellular DNA synthesis at 3-17 Ámol/litre and formed stable adducts in
herring sperm DNA and human cellular DNA. Misincorporation of
nucleotides into synthetic DNA templates was significantly increased
after treatment with captan at 1-12 mmol/litre in vitro in the
presence of Escherichia coli DNA polymerase I, suggesting that DNA
adduct formation with captan could have mutagenic consequences. This
study shows that captan can interact with DNA at a number of levels
and that these interactions may provide the basis for a genotoxic
effect. The extreme cytotoxicity of the compound could, however, be
due to other cellular effects, since at the IC50 for cell killing
(about 0.8 Ámol/litre), none of the genotoxic events was seen (Snyder,
1992).
Comments
The excretion profiles of male and female rats were similar, the
urinary route predominating. The residual tissue concentrations seven
days after dosing were negligible in animals of each sex, the highest
concentrations being found in the kidneys and blood. The metabolism of
captan in rats appears to involve the evolution of thiophosgene,
derived from the trichloromethylthio moiety. Thiophosgene is
detoxified, at least in part, by three mechanisms: oxidation and/or
hydrolysis to carbon dioxide; reaction with the cysteine moiety of
glutathione to yield thiazolidine-2-thione-4-carboxylic acid; and
reaction with sulfite to produce dithiobis(methanesulfonic acid).
Degradation in the gastrointestinal tract appears to play a major role
in the metabolism of captan.
Studies on the hyperplasia induced by captan showed prominent
crypt-cell hyperplasia within 28 days in the proximal duodenum of mice
fed diets containing 6000 ppm captan for 91 days or 800, 3000, or
6000 ppm for 56 days. The NOAEL was 400 ppm, the lowest dose
administered for 56 days, equivalent to 60 mg/kg bw per day, on the
basis of duodenal hyperplasia.
Captan has been adequately tested for genotoxicity in a range of
assays, which demonstrate that it is mutagenic and clastogenic
in vitro but not in vivo. The in-vitro responses are reduced or
abolished by the presence of liver homogenates, serum, glutathione, or
cysteine whenever these experimental modifications have been
investigated. Studies of the genotoxicity of captan in mouse duodenum
indicate that it does not bind covalently to DNA (although metabolic
incorporation of the N-trichloromethylthio-carbon does occur) and
nuclear aberrations are not induced. The Meeting concluded that captan
does not present a significant genotoxic risk, owing to the presence
of an efficient detoxification mechanism in vivo.
There appear to have been no studies on the effects of captan on
glutathione levels analogous to those conducted with folpet. Dietary
studies of 4-8 weeks' duration have shown that irritation and
consequent inflammation as well as hyperplasia occur in the proximal
duodenum of mice. These data indicate that sustained proliferative
stimulation of the proximal duodenum is a consequence of oral
administration of captan. The Meeting concluded that this finding
represents an important element in the process by which captan,
which is not genotoxic in vivo, induces tumours in the mouse
gastrointestinal tract.
The information available to the present Meeting provides no
basis for altering the existing ADI for captan, which is based on an
NOAEL of 12.5 mg/kg bw per day determined in studies of reproductive
toxicity in rats and monkeys, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 400 ppm equivalent to 60 mg/kg bw per day (56-day study of
toxicity)
Rat: 250 ppm, equivalent to 12.5 mg/kg bw per day (study of
reproductive toxicity)
Dog: 100 mg/kg bw per day (66-week study of toxicity)
Monkey: 12.5 mg/kg bw per day (study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Allen, S.L. (1994) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/L/5674 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Bratt, H. (1990) Captan: Repeat dose study (10 mg/kg) in the rat.
Unpublished report No. CTL/P/2958 from ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, United
Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Cabral, R, Hoshiya, T., Hakol, K., Hasegawa, R. Fukushima, S. & Ito,
N. (1991) A rapid in vivo bioassay for the carcinogenicity of
pesticides. Tumori, 77, 185-188.
DeBaun, J.R., Miaullis, J.B., Knarr, J. Mihailovski, A. & Menn, J.J.
(1974) The fate of N-trichloro[14C]-methylthio-4-cyclohexene-
1,2-dicarboximide ([14C]captan) in the rat. Xenobiotica, 4,
101-119.
Krieger, R.I. & Thongsinthusak, T. (1993) Captan metabolism in humans
yields two biomarkers, tetrahydrophthalimide (THPI) and
thiazolidine-2-thione-4-carboxylic acid (TTCA) in urine.
Drug Chem. Toxicol., 16, 207-225.
Lappin, G.J. & Havell, M.L. (1990) Captan: Biotransformation study in
the rat. Unpublished report No. CTL/P/2951 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Pritchard, D.J. & Lappin, G.J. (1991) Captan: DNA binding study in the
mouse. Unpublished report No. CTL/P/3380 from ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ICI Agrochemicals, Fernhurst,
Haslemere, Surrey, United Kingdom.
Provan, W.M., Eyton-Jones, H. & Green, T. (1992). The potential of
captan to react with DNA. Unpublished report No. CTL/R/1131 from
ICI Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, United Kingdom. Submitted to WHO by ICI Agrochemicals,
Fernhurst, Haslemere, Surrey, United Kingdom.
Selsky, C.A. & Matheson, D.W. (1981) The association of captan with
mouse and rat deoxyribonucleic acid. Unpublished report No. T-
10435 from The in vitro Toxicology Section, Environmental Health
Center, Stauffer Chemical Company, Farmington, Connecticut, USA.
Submitted to WHO by ICI Agrochemicals, Fernhurst, Haslemere,
Surrey, United Kingdom.
Snyder, R.D. (1992) Effects of captan on DNA and DNA metabolic
processes in human diploid fibroblasts. Environ. Mol. Mutag.,
20, 127-133
Stournaras, C., Saridakis, I., Fostinis, Y & Georgoulias V. (1991)
Interaction of captan with mammalian microtubules. Cell
Biochem. Func., 9, 23-28
Tinston, D.J. (1995) Captan: Investigation of duodenal hyperplasia in
mice. Unpublished report No. CTL/P/4532 from Zeneca Central
Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire,
United Kingdom. Submitted to WHO by ZENECA Agrochemicals,
Fenhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990a) Captan: Excretion mid tissue retension of a single
oral dose (10 mg/kg) in the rat. Unpublished report No.
CTL/P/2820 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
Trivedi, S. (1990b) Captan: Excretion and tissue retension of a
single oral dose (500 mg/kg) in the rat. Unpublished report No.
CTL/P/2862 from ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, United Kingdom. Submitted to WHO by ICI
Agrochemicals, Fernhurst, Haslemere, Surrey, United Kingdom.
