
PIRIMICARB JMPR 1976
IDENTITY
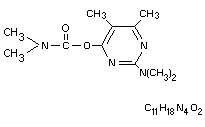
Chemical name
2-dimethylamino-5,6-dimethylpyrimidin-4-yl
dimethylcarbamate.
Synonyms
PPO62, APHOX(R), PIRIMOR(R), FERNOS(R).
Structural Formula
 Other information on identity and properties
Primicarb is a colourless, crystalline solid. It melts at
90.5°C.
Vapour pressure (torr):
1.58 x 10-5 at 25°C
3.0 x 10-5 at 30°C
4.4. x 10-5 at 35°C
1.8 x 10-5 at 65°C
Solubility (g per 100 ml at 25°C).
Water 0.27
Methanol 23
Ethanol 25
Acetone 40
Xylene 29
Chloroform 32
Pirimicarb is stable at temperatures up to 37°C for at least
two years.
Pirimicarb forms stable salts with both organic and
inorganic acids. It is decomposed by prolonged boiling with acids
or alkalis to 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine
and dimethylamine. The hydroxypyrimidine is stable in boiling
acid and alkaline solutions and forms stable salts with both
acids and bases.
Purity
Not less than 95% pure, usually 98%. Impurities contain a
maximum of 1.3% of other pyrimidine carbamates. Formulated
products include a 50% ai. dispersible grain, 25% and 50%
dispersible powders, a 10% ai smoke generator, a 5% ai ultra-low
volume formulation and a 5% ai emulsifiable concentrate.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption Distribution and Excretion
Pirimicarb is rapidly absorbed, distributed, metabolized and
excreted in mammals. In studies with dogs and rats administered
subacute doses of 14C-labelled pirimicarb (ring or carbonyl
position), excretion was rapid. In dogs, within one day 74-86% of
the administered dose of ring labelled pirimicarb was eliminated,
primarily in the urine. Over the following 3 days, minor
quantities were released. In total, from 86 to 94% of the
administered ring labelled dose was recovered (79-88% in urine,
6-7% in feces). With carbonyl (C=0) labelled pirimicarb, only
15-26% of the dose administered to dogs was recovered,
predominantly in urine. The unrecovered product (14CO2) was
believed to be eliminated rapidly in expired air as suggested by
studies where rats were administered 14C-carbonyl-pirimicarb by
Other information on identity and properties
Primicarb is a colourless, crystalline solid. It melts at
90.5°C.
Vapour pressure (torr):
1.58 x 10-5 at 25°C
3.0 x 10-5 at 30°C
4.4. x 10-5 at 35°C
1.8 x 10-5 at 65°C
Solubility (g per 100 ml at 25°C).
Water 0.27
Methanol 23
Ethanol 25
Acetone 40
Xylene 29
Chloroform 32
Pirimicarb is stable at temperatures up to 37°C for at least
two years.
Pirimicarb forms stable salts with both organic and
inorganic acids. It is decomposed by prolonged boiling with acids
or alkalis to 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine
and dimethylamine. The hydroxypyrimidine is stable in boiling
acid and alkaline solutions and forms stable salts with both
acids and bases.
Purity
Not less than 95% pure, usually 98%. Impurities contain a
maximum of 1.3% of other pyrimidine carbamates. Formulated
products include a 50% ai. dispersible grain, 25% and 50%
dispersible powders, a 10% ai smoke generator, a 5% ai ultra-low
volume formulation and a 5% ai emulsifiable concentrate.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption Distribution and Excretion
Pirimicarb is rapidly absorbed, distributed, metabolized and
excreted in mammals. In studies with dogs and rats administered
subacute doses of 14C-labelled pirimicarb (ring or carbonyl
position), excretion was rapid. In dogs, within one day 74-86% of
the administered dose of ring labelled pirimicarb was eliminated,
primarily in the urine. Over the following 3 days, minor
quantities were released. In total, from 86 to 94% of the
administered ring labelled dose was recovered (79-88% in urine,
6-7% in feces). With carbonyl (C=0) labelled pirimicarb, only
15-26% of the dose administered to dogs was recovered,
predominantly in urine. The unrecovered product (14CO2) was
believed to be eliminated rapidly in expired air as suggested by
studies where rats were administered 14C-carbonyl-pirimicarb by
 oral gavage or intraperitoneal injection. Over 50% of the
administered dose was expired as 14CO2 within 5 hours following
administration by either route (Fletcher et al., 1971e).
Further studies on the absorption, tissue distribution and
excretion in rats following oral or ip injection again suggested
the same pattern of rapid absorption, degradation of the
carbamate ester to CO2 and minor (15%) elimination of
radioactivity in the urine (Daniel and Bratt, 1968). There was
essentially no storage in the body of animals (rats) sacrificed 8
days after treatment. Measurement of abdominal fat from rats
orally administered pirimicarb (50 mg/IC9) and sacrificed 24
hours after treatment (either a single application or up to 4
daily treatments) showed no accumulation of pirimicarb in adipose
tissue (Fletcher and Bratt, 1972).
Pirimicarb administered to a lactating cow at a dose of 1
mg/kg was rapidly excreted in the urine (96%) and feces (4%). A
trace of material (<0.3% of the recovered dose) was observed in
milk with a maximum occurring within one hour of treatment
(Hemingway et al., 1976). Pirimicarb is not expected to
accumulate in the body following repeated exposures (Fletcher and
Bratt, 1972).
Biotransformation
Qualitative identification of the urinary metabolites from
rats and dogs administered pirimicarb by oral gavage or
intraperitoneal injection showed the same series of products. The
major metabolites were identified as the hydroxy pyrimidine
(pyrimidinol) derived from pirimicarb with modifications of the
alkyl substituents of the aromatic moiety. A proposed metabolic
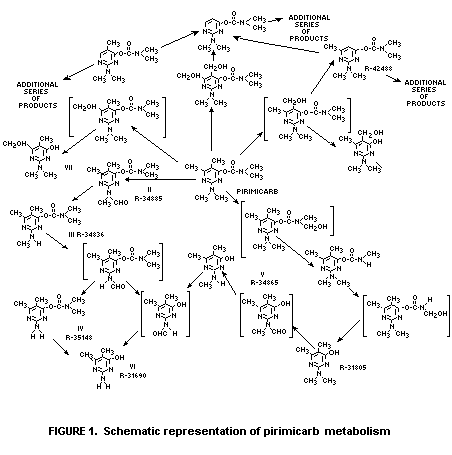
scheme for the degradation of pirimicarb is seen in Figure 1
(only a few of the many compounds proposed have been isolated).
As expected oxidative and hydrolytic mechanisms act on various
components of the molecule, namely the carbonyl ester, the
N(CH3)2 of both the ring and carbamate moiety and the CH3
groups of the 5 and 6 positions of the pyrimidine nucleus.
Quantitative determination of isolated metabolites from the urine
of rats, dogs and a lactating cow show essentially the same
pattern of metabolism. The following four metabolites were
recovered from urine:
Metabolite % of dose administered
Rat Dog Cow
2-dimethylamino-5,6-dimethyl 16.3 6.4 10
-4-hydrozypyrimidine (I)
Metabolite % of dose administered
Rat Dog Cow
2-methylamino-5,6-dimethyl 40.9 20.7 41
-4-hydroxypyrimidine (V)
2-amino-5,6-dimethyl 12.9 16.5 21
-4-hydroxypyrimidine (VI)
2-dimethylamino-6-hydroxymethyl 5.7 1.8 -
-5-methyl-4-hydroxyprimidine
(VII)
The major metabolites in milk of a lactating cow were also I, V and VI
above in a similar ratio. Conjugation of hydroxypyrimidines was not
evident in these studies. There were few aglycones recovered following
treatment of urinary products with glucuronidase and chromatographic
characterization of organic soluble products. It was assumed that the
metabolites predominated in the tautomeric pyrimidone configuration,
oral gavage or intraperitoneal injection. Over 50% of the
administered dose was expired as 14CO2 within 5 hours following
administration by either route (Fletcher et al., 1971e).
Further studies on the absorption, tissue distribution and
excretion in rats following oral or ip injection again suggested
the same pattern of rapid absorption, degradation of the
carbamate ester to CO2 and minor (15%) elimination of
radioactivity in the urine (Daniel and Bratt, 1968). There was
essentially no storage in the body of animals (rats) sacrificed 8
days after treatment. Measurement of abdominal fat from rats
orally administered pirimicarb (50 mg/IC9) and sacrificed 24
hours after treatment (either a single application or up to 4
daily treatments) showed no accumulation of pirimicarb in adipose
tissue (Fletcher and Bratt, 1972).
Pirimicarb administered to a lactating cow at a dose of 1
mg/kg was rapidly excreted in the urine (96%) and feces (4%). A
trace of material (<0.3% of the recovered dose) was observed in
milk with a maximum occurring within one hour of treatment
(Hemingway et al., 1976). Pirimicarb is not expected to
accumulate in the body following repeated exposures (Fletcher and
Bratt, 1972).
Biotransformation
Qualitative identification of the urinary metabolites from
rats and dogs administered pirimicarb by oral gavage or
intraperitoneal injection showed the same series of products. The
major metabolites were identified as the hydroxy pyrimidine
(pyrimidinol) derived from pirimicarb with modifications of the
alkyl substituents of the aromatic moiety. A proposed metabolic
scheme for the degradation of pirimicarb is seen in Figure 1
(only a few of the many compounds proposed have been isolated).
As expected oxidative and hydrolytic mechanisms act on various
components of the molecule, namely the carbonyl ester, the
N(CH3)2 of both the ring and carbamate moiety and the CH3
groups of the 5 and 6 positions of the pyrimidine nucleus.
Quantitative determination of isolated metabolites from the urine
of rats, dogs and a lactating cow show essentially the same
pattern of metabolism. The following four metabolites were
recovered from urine:
Metabolite % of dose administered
Rat Dog Cow
2-dimethylamino-5,6-dimethyl 16.3 6.4 10
-4-hydrozypyrimidine (I)
Metabolite % of dose administered
Rat Dog Cow
2-methylamino-5,6-dimethyl 40.9 20.7 41
-4-hydroxypyrimidine (V)
2-amino-5,6-dimethyl 12.9 16.5 21
-4-hydroxypyrimidine (VI)
2-dimethylamino-6-hydroxymethyl 5.7 1.8 -
-5-methyl-4-hydroxyprimidine
(VII)
The major metabolites in milk of a lactating cow were also I, V and VI
above in a similar ratio. Conjugation of hydroxypyrimidines was not
evident in these studies. There were few aglycones recovered following
treatment of urinary products with glucuronidase and chromatographic
characterization of organic soluble products. It was assumed that the
metabolites predominated in the tautomeric pyrimidone configuration,
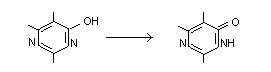 restricting conjugation. Small quantities of the hydroxypyrimidine
were recovered as conjugates but the major products were passed as
unconjugated metabolites in the urine of rats and dogs (Fletcher et
al., 1971e).
Two products were identified as degradation products in plants
which were not found in dog or rat urine, the 2-formyl (methylamino)
(R-34885,II) and the 2-methylamino (demethyl-pirimicarb, R34836, III)
derivatives containing the intact carbamate. While these were isolated
from plants and not animals, the probability exists that they are
formed in mammals as transitory products since the carbamate is labile
and subject to rapid cleavage. The identification of the 2-methylamino
(V) and the 2-amibo (VI) hydropyrimidine derivates of pirimicarb in
rat and dog urine suggest the same sequence of metabolism and
breakdown in animals as plants with quantitative differences in the
final products.
Effects on Enzymes and other biochemical parameters
Pirimicarb is an anticholinesterase agent and, as other
N-methyl-and N,N-dimethylcarbamates, elicits a parasympathomimetic
response in certain animals. Evaluation of reversible cholinesterase
inhibition by carbamate esters is difficult. Using an automated delta
pH method the following I50 values for some pirimicarb metabolites
were obtained (Table 1).
TABLE 1. Inhibition of cholinesterase by Pirimicarb metabolites
Metabolite I50
Molarity X 106
2-Methylamino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (III) 4.54
2-amino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (IV) 4.34
2-dimethylamino-5,6-dimethyl
-4-hydroxypyrimidine (I) 14.9 X 103
2-methylamino-5,6-dimethyl
-4-hydroxypyrimidine (V) 4.25 X 103
(Fletcher et al., 1971e)
Using a female cat, a time course study of cholinesterase
depression was run following acute poisoning by intravenous injection
of a dose of 20 mg/kg. Rapid in vivo depression of pretreatment
plasma cholinesterase activity was obtained which persisted for more
than 4´ hours, long after clinical signs of poisoning had stopped.
These results are summarized in Table 2.
TABLE 2. Symptoms and plasma cholinesterase depression in a cat
poisoned with pirimicarb
Time Signs of Poisoning Plasma Cholinesterase
(% Inhibition)
5 min. salivation, trembling 72
15 min. salivation, fibrillation 72
30 min. reduced fibrillation 73
1 hr. none 72
2 hr. 75
4´ hr. 73
(Clark, 1967b)
An attempt was made to resolve questions raised on the
reliability of the routine automated procedure used for the
determination of blood cholinesterase activity. The original method
used extended substrate incubation times and large dilutions. Both of
these factors are known to be responsible for enhancing spontaneous
reactivation of the carbamoylated enzyme. In an attempt to demonstrate
that the N,N-dimethylcarbamoylated cholinesterase is sufficiently
stable to be measured by techniques not normally employed, a shortened
incubation time (5.5 min. instead of 23 min) and reduced dilution were
used. Acetylcholine concentrations were not changed. With these
modifications a comparison of the method used routinely, a radiometric
(14C-acetylcholine) method and a modified automated delta Ph method
was made to evaluate cholinesterase depression in vivo in rats.
Rats were orally administered pirimicarb at 30 mg/kg and blood
removed after 1 hour. Dogs were administered pirimicarb at 30 mg/kg
dose and blood was sampled at one hour after treatment.
Cholinesterase depression was similar in all methods of assay
employed. In rats, male and female, plasma measured 50% inhibition by
all methods. In dogs, a comparison of the radiometric
14C-acetylcholine method with the automated delta Ph method showed no
inhibition of plasma cholinesterase at 4 mg/kg. In addition, plasma
cholinesterase from treated animals was found to be stable over a 24
hour interval at 4-5°C with no loss in inhibition spontaneously
regenerated activity).
From the foregoing study, it was concluded that a stable
carbamoylated enzyme analogous to a phosphorylated enzyme may be
formed with pirimicarb and the reduced activity can successfully be
measured by an automated delta Ph method. It was concluded that the
K3 step was slow and a stable intermediate was formed. The conclusion
of this study is that the method used in most studies to measure
cholinesterase activity did not underestimate the inhibition and give
misleading results (Litchfield, 1968).
In vitro studies in part confirmed these conclusions
especially with respect to pseudocholinesterase enzymes. The reaction
of pirimicarb and horse serum cholinesterase yielded a stable N,N-
dimethylcarbamoylated enzyme having a k3 of 5 X 10-4min-1.
However, with acetylcholinesterase (eel or RBC) the k3 is higher (3.7
X 10-2 min-1 and 0.9 X 10-2 min-1 respectively) and the spontaneous
reactivity is much greater. Thus, the use of plasma or serum
cholinesterase to assay pirimicarb inhibition would show adequate
values for the interaction of enzyme and inhibitor while the use of
RBC as other-acetylcholinesterase sources would be expected to give
misleading values especially where extended assay times or large
dilutions were a factor (Main, A.R. personal communication).
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of rats (12 male and 24 female rats/group) were fed
pirimicarb in the diet at 0,250 and 750 ppm and mated to begin a
3-generation, 2-litter per generation standard reproduction study. At
the conclusion of the study, tissues from 10 rats of each sex from the
F3b generation were examined microscopically. Four offspring were
observed to be malformed at birth (3 litter mates of the second
generation F2a - 250 ppm and one 750 ppm F2a fetus). Reduced body
weight of the parents (specifically females but often males as well)
was noted at both treatment levels. Growth of pups prior to weaning
was not affected by pirimicarb in the diet although a reduced growth
was seen thereafter in all three generations. The basic reproduction
indices normally measured (fertility, gestation, lactation and
viability) were not affected. Evaluation of parturition data suggests
no effects on the ability to maintain pregnancy. No adverse effects on
reproduction were observed with pirimicarb. Because malformations were
observed in only 4 fetuses of 2665 treated it was concluded that these
were not as a result of pirimicarb in the diet (Fletcher and Sotheran,
1971).
Special studies on carcinogenesis
Groups of mice (50 of each sex per group; SPF, Alderly Park
strain) were fed pirimicarb in the diet at levels of 0, 300 and 1500
ppm for 80 weeks. Mortality was reported to be a result of endemic
respiratory disease. The clinical condition of the mice at autopsy
confirmed a high incidence of respiratory disease. A slight body
weight reduction was noted only at the high dose level. No effects
were noted on food consumption or on gross and microscopic analysis of
most tissues and organs. The incidence of pulmonary tumors was
significantly higher in both treated groups than controls used in the
study. (When the incidence of pulmonary lesions was compared with a
large group of control mice examined in the laboratory over an 8-year
interval, the data did not show a significant increase in pulmonary
tumors as a result of pirimicarb in the diet. The controls used in
this study apparently had an extremely low incidence of lung lesion).
There was no evidence of excessive lymphoma or leukemia noted in the
study and detailed examination of the CNS did not indicate pirimicarb
to be a carcinogen in mice (Palmer and Samuels, 1974).
Groups of rats (48 males/group) were fed pirimicarb in the diet
at dose levels of 0,750 and 2,500 ppm for 2 years. Because of
respiratory infection, an antibiotic (oxytetracycline and sodium
sulphadimidine were administered in drinking water as needed.
Mortality was heavy after the 40th week predominantly as a result of
respiratory infection. Growth was reduced at 2,500 ppm but not at 750
ppm. An increased incidence of reticulum cell lymphoma was observed in
the study. It was concluded that this incidence was as a direct result
of the accompanying pulmonary infection. However, the infiltration
pattern of the tumor was mainly perivascular. A statistical treatment
of the tumor incidence did not confirm a relationship of tumor
incidence to the presence of pirimicarb in the diet.
In another study using a different strain, groups of rats (48
males/group) were fed diets for two years similar to those described
above. Again, an outbreak of respiratory disease within one year
limited the surviving animals at the conclusion of the study. However,
in contrast to the previous study, no reticulum cell lymphoma was
observed. The incidence of tumors was not different from controls. It
was concluded that pirimicarb did not produce an increased incidence
of tumors in male rats.
A third study was initiated from those animals discarded from the
three generation reproduction study. Groups of rats (24 of each
sex/group) previously exposed in utero and fed prior to weaning were
fed pirimicarb in the diet at dosage levels of 0 and 750 ppm for 2
years. Again, respiratory infection reduced survival of rats in this
study (rats were also treated with antibiotics to control the
infection). There was no difference relating to survival between the
control and treated groups. Growth of both sexes was reduced by
pirimicarb in the diet. Gross and microscopic analyses did not
indicate an increased incidence of tumors in the pirimicarb fed rats.
Based on lifetime exposure to pirimicarb from conception, there is no
carcinogenic effect noted in the rat (Samuels et al., 1975). However,
the undercurrent respiratory infection noted in all four long term
studies aimed at examining lung lesions precludes definitive
conclusions on this problem.
Special studies on teratology
Mouse
Groups of mice (25 mated females/group) were fed pirimicarb in
the diet at dosage levels of 0,40 and 500 ppm throughout pregnancy
(day 0 to day 18 of gestation). There were no parental deaths and no
abortions occurred. On sacrifice at day 18, fetuses were removed and
uteri examined for resorptions. Implantation was slightly reduced in
both pirimicarb groups. The mean litter weight and fetal weights were
reduced at the high dose level. There was no effect on the sex ratio
of fetuses.
No abnormalities were observed on gross examination or on
skeletal examination following clearing and alizarin straining
(Fletcher et al., 1971d).
Rat
Groups of rats (18-21 females/group) were fed pirimicarb in the
diet at dosages of 0, 250 and 750 ppm throughout pregnancy. Parental
growth was reduced at the high dose level. Total weight was also
reduced at the high dose level. No abnormalities were noted in the
treatment group attributable to pirimicarb in the diet (Fletcher et
al, 1971f).
Rabbit
Groups of pregnant rabbits (16 does/group) were administered
pirimicarb orally by gavage daily from day 1 to day 28 of gestation at
doses of 0,1.25, 2.50 and 5.0 mg/kg. Administration of 2.5 and 5 mg/kg
produced a maternally toxic response noted as a reduction of weight
gain. This was also reflected in reduced fetal weights which occurred
at the upper two dose levels. There were no teratogenic effects noted
on gross examination of tissue, or on skeletal examination of cleared,
stained fetuses. Pirimicarb has no teratogenic potential in rabbits. A
slight increase in the size of the brain of some litter mates was not
considered to be hydrocephalus and was not dose dependent (Hodge,
1974). On the basis of studies in three species, it appears that
pirimicarb has little teratological potential.
Special studies on mutagenesis
Mouse
Groups of mice (15 males/group) were administered pirimicarb
orally for 5 days by intubation at dosage levels of 0, 10 and 20
mg/kg. Two positive controls of 150 mg/kg or 100 mg/kg of ethyl
methanesulphonate (EMS) were administered either by a single ip
injection on the day before mating or by oral administration 5 days
prior to mating. Each male was mated to two virgin females at weekly
intervals for 8 weeks in a dominant lethal mutagenesis study. In an
evaluation of pregnancy, implantation and early and late death of
fetuses, pirimicarb treatment did not show differences from control
values. In contrast, EMS resulted in reduced pregnancy,
preimplantation loss and increased early death, judged to be a
significant mutagenic occurrence. There are no indications from this
study that pirimicarb would induce mutations in mice (McGregor, 1974).
Special studies on sensitization
Groups of guinea pigs (4 males/test group, 3 males as controls)
were treated on the ear for 3 days with a 10% (w/v) solution of either
technical or formulated pirimicarb. Four days later the animals were
challenged by applying doses of 0, 0.1, 1.0 and 10% solutions to the
shaved flank skin. Traces of erythema were noted but a primary
sensitization reaction was not observed (Parkinson, 1974c).
Special studies on inhalation toxicity
Pirimicarb has been recommended for use under enclosed glasshouse
conditions. Studies were performed on the acute inhalation toxicity
following exposure of rats to smoke generated in an enclosed chamber
at doses up to 300 mg/m3. Mortality was observed as were acute signs
of poisoning following a 6 hour exposure. Rats exposed to 75 mg/m3
for 6 hours showed clinical signs of poisoning while at a level of 15
mg/m3 no signs of poisoning over the 6 hour test exposure were
observed (Riley, 1972a). Rats exposed to 15 mg pirimicarb/m3, 6 hours
a day, 5 days/week for 3 weeks did not exhibit toxic signs of
poisoning. A slight plasma cholinesterase depression was observed
(Riley, 1972b). Acute inhalation studies on rats using a 50%
dispersible powder formulation showed that exposure for one hour at a
dust concentration of 20,000 mg/m3 caused no acute signs of
poisoning although plasma and RBC cholinesterase depression was noted
(Riley, 1972c). Rats exposed daily 5 days/week for 3 weeks (6
hours/day) to a "saturated vapor" of pirimicarb (air passed through
technical pirimicarb was not analyzed for the presence of pirimicarb)
did not display signs of poisoning or even of exposure (Clark, 1967b;
Riley, 1973).
Special studies on neurotoxicity
Four adult hens, administered a single oral dose of 25 mg
pirimicarb/kg by gavage, displayed cholinergic signs of poisoning but
no clinical indication of a delayed neurotoxic response (Clark,
1967b).
Four adult hens were administered pirimicarb by oral intubation
at a dose level of 25 mg/kg. They were observed for 21 days,
re-treated with the same dose and again observed for 21 days. No signs
of delayed neurological ataxia were observed (Fletcher, 1971a).
Special studies on potentiation
Groups of female rats (10 rats/group) were administered
pirimicarb alone and in combination with carbaryl or azinphos-methyl
orally by gavage. Slight changes in the projected LD50 values were
noted with azinphos-methyl when the materials were administered in
combination. No such changes were noted with carbaryl combinations.
There was no potentiation of the acute effects noted with simultaneous
oral administration of carbaryl while a slight potentiation of acute
toxicity was noted with azinphos-methyl (Parkinson, 1975d).
Special studies on plant metabolites
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-formylmethylamino pirimicarb
(R.34885, II) by gavage daily for 10 days at doses of 25 mg/kg/day.
Males developed a slight hypochromia, while a hypochromic anemia was
observed in females. No other toxic signs were observed. There was no
intubution of plasma, RBc or brain cholinesterase. Increased
hemopoietic activity was noted in the spleen (Fletcher et al., 1971a).
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-methylaminopirimicarb (R.34836,
III) by gavage daily for 10 days at doses of 100 mg/kg/day. Acute
signs of poisoning were noted immediately after each daily dose. These
signs abated within 2 hours. Plasma cholinesterase depression was
observed only in females. Hematological examination revealed
hypochromic anemia in females while males showed reticulocytosis and
slight hypochromia. No change in clotting time was seen. Increased
hemopoietic activity was seen in the spleen of males and females.
Changes in the thymus were also observed (Fletcher et al., 1971b).
Special studies on antidotes
Groups of female rats were administered pirimicarb by
subcutaneous injection (150 mg/kg - 2 X LD50). When signs of
poisoning were observed, atropine alone or in combination with P2S
was administered by S.C. injection. Atropine alone or in combination
with P2S was an effective antidote while P2S alone was not effective
in reducing the acute toxic effects (Clark, 1967b).
Eye and skin irritation
Technical pirimicarb and a 50% dispersible powder formulation
produced a slight occular irritation when administered to the
conjunctival sac of rabbits. One drop of a 5% solution or a suspension
of 25% (w/v) of technical pirimicarb in propylene glycol or 50% (w/v)
formulated product, instilled in the conjunctival sac, resulted in
slight pain, redness and transient slight corneal opacity. Pirimicarb
was classified as a mild irritant graded as 3-4 m a scale of 8 (Clark,
1967b; Fletcher, 1971b; Ferguson and Parkinson, 1973). Technical
pirimicarb applied to the intact or abraded skin of rabbits was
non-irritating when administered as a 25% suspension in propylene
glycol (Fletcher, 1971b; Parkinson, 1972). When a paste was made of a
50% dispersible powder formulation, pirimicarb was irritating
especially to abraded skin. No reaction was observed with intact,
undamaged skin (Parkinson, 1972). Technical pirimicarb (5% in
propyleneglycol) applied to the shaved back of rats repeatedly for 24
hours under a plastic film was not irritating to the skin (Fletcher,
1971b; Clerk 1967a).
Acute Toxicity
TABLE 3. Acute toxicity of pirimicarb
LD50
Species Sex Route (mg/kg) Reference
Rat F oral (fasted) 68 Clark, 1967a
(fed) 147-210 Clark, 1967a
F oral 101-147 Clark, 1967b;
Parkinson, 1975
F oral 165-221* Parkinson, 1972b
dermal >500 Clark, 1967b
TABLE 3. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
M&F ip 25-50 Clark, 1967b
F SC 75 Clark, 1967b
Mouse F oral 107 Clark, 1967b
Dog M&F oral 100-200 Clark, 1967b
Rabbit dermal >500 Clark, 1967b
Hen oral 25-50 Clark, 1967b
Rat inhalation approx. Riley, 1973
300 mg/m3
* pirimicarb stored at 37°C for up to 6 months showed no change in
acute toxicity value suggesting stability over this time interval.
Signs of poisoning: Within a few minutes of receiving a toxic oral
dose, signs of poisoning in a rat were observed including:
salivation, chromolacrymation, urination, defecation, miosis and
muscular fibrillations leading to fasciculations and death within
30 minutes. These are all typical signs of cholinergic
stimulation normally seen with parasympathomimetic agents.
Short term studies
Rabbit - dermal
Groups of rabbits (4 of each sex/group) were administered
pirimicarb dermally at a dose of 500 mg/kg for a 24 hour duration. The
skin was washed and the treatment repeated daily for 14 days. No
toxicity was observed and the animals appeared normal (Fletcher,
1971c).
Rat
Groups of rats (10 male and 10 female rats/group) were
administered pirimicarb daily by gavage at doses of 0 and 50 mg/kg for
10 days. A slight growth depression was noted but no mortality or
cumulative effects were seen. Hematology was normal and gross
examination of tissues and organs at the conclusion of the study
showed no adverse effects (Clark, 1967b).
TABLE 4. Acute toxicity of metabolites or impurities
LD50
Compound* Species Sex Route (mg/kg) Reference
pirimicarb Rat F oral 800-1600 Lefevre and
phenol (I) Parkinson, 1974
(R.31805)
N-methyl-N-formyl Rat F oral 50-100 Lefevre and
pirimicarb (II) Parkinson, 1974
(R.34885)
N-demethyl pirimicarb Rat F oral 200-400 Lefevre and
III) Parkinson, 1974
(R.34836)
N-di-demethyl Rat F oral 79 Parkinson, 1974a
pirimicarb (IV)
(R.35140)
N-demethyl phenol (V) Rat F oral 2000-2500 Lefevre and
(R.34865) Parkinson, 1974
N-di-demethyl phenol (VI) Rat F oral >2500 Parkinson, 1974a
(R.31680)
N,N-dimethyl Rat F oral 1455 Parkinson, 1975a
guanidine HCl
methyl guanidine SO4 Rat F oral 1105 Parkinson, 1975a
guanidine HCl Rat F oral 1105 Parkinson, 1975a
2-(dimethyl-amino)- Rat F oral 158 Parkinson, 1974b
6-methyl-4-pyrimidinyl
dimethylcarbamate
(R.42488)
2-(dimethyl-amino)-5, Rat F oral Zeta 1000 Parkinson, 1975b
6 dimethyl-4-pyrimidinyl
diethylcarbamate
(R.32444)
2-(dimethyl-amino)-5,6- Rat F oral Zeta 800 Parkinson, 1975c
dimethyl-4-pyrimidinyl
ethylmethylcarbmate
(R.33160)
* See figure 1 for structural definition of the numbered products.
Groups of rats (25 males and 25 females/group) were fed
pirimicarb in the diet for 90 days at dosage levels of 0, 250 and 750
ppm. A separate group of rats was administered pirimicarb by gavage,
daily at a dose of 25 mg/kg. There was no effect on growth, food
consumption or mortality in the dietary groups. Mortality was evident
in the rats dosed by gavage although the survivors showed normal
growth over the 90 day interval. Hematology parameters were
unaffected. A slight decline in plasma cholinesterase was observed in
the rats treated by gavage and at sporadic intervals in the 750 ppm
group. RBC and brain activity was normal. Gross and microscopic
analysis of tissues and organs examined at the conclusion of the study
was normal. Bone marrow cytology was not performed (Griffiths and
Conning, 1968).
A no-effect level of >750 ppm in the diet but lower than
25 mg/kg body weight administered by gavage was evidenced in this
study.
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
pirimicarb in the diet at dosage levels of 0, 4, 10 and 25 mg/kg for
90 days. Growth was not affected over the course of the study although
one of the male dogs at the highest level died during the course of
study. While the cause of death was not completely diagnosed, it was
probably related to anemia as the animal showed marked erythropoietic
hyperplasia and the presence of numerous nucleated red blood cells. A
macrocytic anemia was observed in two other dogs (one high and one
intermediate dose) at the conclusion of the study. Although hematology
parameters were not unusual an increased number of nucleated red blood
cells was observed in peripheral blood. Bone marrow examination showed
an increased nucleated cell count and reduced m/e ratio at all dose
levels. This abnormal count dropped slightly after a 28 day recovery
period, but was not down to normal. Serum folate, iron and vitamin
B12 levels were normal, suggesting that the hemolytic anemia was not
related to this type of deficiency.
Cholinesterase measurements showed depression of plasma enzymes
at the upper two dose levels. Brain and RBC cholinesterase activity as
measured by the delta pH method was unaffected by pirimicarb.
Gross and microscopic examinations of tissues and organs
indicated hemopoiesis in the spleen and lymph nodes of dogs showing
hematological signs of anemia. No effects on tissues and organs (other
than on bone marrow observations of all treated animals or on the
tissues and organs of the dog that died) were observed. A no-effect
level was not defined in this study. Delayed maturation of red blood
cells as evidenced by the presence of abnormal levels of nucleated red
blood cells was observed in all treated animals (Conning et al.,
1968).
Groups of dogs (4 beagle dogs of each sex/group) were fed diets
containing pirimicarb at dose levels of 0,0.4, 1.8 and 4.0 mg/kg body
weight for 90 days (the 4.0 mg/kg dose was maintained for 180 days).
There was no mortality and growth was unaffected by pirimicarb in the
diet. Clinical chemistry, hematology and urinalysis parameters were
normal. Bone marrow cytological examination again showed the presence
of nucleated red blood cell precursors at the highest dose tested. The
occurrence of these juvenile cells in marrow was not accompanied by
anemia as was evident In the previous study. Gross and microscopic
examination of other tissues and organs showed evidence of spleenic
hemopoiesis at the high dose level over the 6 month test. Other
tissues and organs were not affected. Plasma and brain cholinesterase
values were also normal (Conning et al., 1969).
In special studies to evaluate the hemolytic anemia observed in
dogs, four dogs (two male and two female) were fed pirimicarb in the
diet at levels of either 25 or 50 mg/kg for up to 110 weeks. Two of
the four dogs (1 male at 50 mg/kg and 1 female at 25 mg/kg) developed
anemia while two (1 female at 50 mg/kg and 1 male at 25 mg/kg) were
normal over the entire period. Peripheral blood showed changed in the
size and shape of red cells and the presence of some nucleated red
blood cells was observed. No abnormalities were seen in the white cell
series or platelets. Bone marrow samples showed a marked erythroid
hyperplasia. Attempts made to reduce the anemia with high therapeutic
doses of Vitamin B12, folic acid and folinic acid were negative. Red
blood cells from the anemic dogs were able to be agglutinated by
specific antigamma globulin serum while cells from normal dogs were
not. Free antibody was demonstrated in the sera of anemic dogs. The
antibody reacted with cells of the individual from which it was
removed and also with cells of normal-in-appearance (non-anemic)
pirimicarb-treated dogs. The antibody did not react with cells of
normal, untreated dogs. The presence of antibody and the demonstrated
effects of anemia were reversible (within 6 weeks) upon withdrawal of
the pirimicarb treatment. The pirimicarb treatment appears to have
been responsible for inducing changes in red blood cells of dogs
treated at higher levels. However, only some of the dogs responded
further in producing antibody that reacts with the altered cell
inducing anemia (Garner et al., 1972). In a further study to evaluate
a threshold of response of susceptible animals to pirimicarb, the two
susceptible dogs when allowed to recover from the induced-anemia were
fed levels of 1-2 mg/kg. One of the two dogs became anemic when
exposed for 14 weeks to 2 mg/kg. The other animal remained normal.
Further immunological studies using the susceptible dogs showed IgG
binding to cells of non-anemic dogs exposed to pirimicarb and
suggested that pirimicarb does not inhibit the IgG binding. It was
concluded that the antibody is not produced in direct relation to
pirimicarb alone, but possibly to a toxicant-red cell complex or to a
change induced by the toxicant (Jackson et al., 1974).
Groups of dogs (4 male and 4 female/group) were fed pirimicarb in
the diet for two years at dosage levels of 0, 0.4, 1.8 and 4.0 mg/kg
body weight. There were no effects on behaviour, growth or food
consumption over the course of the two years. Blood chemistry
including blood and brain cholinesterase and urinalysis parameters
were unaffected. Hematology was normal. The M/E ratio was decreased at
the high level of feeding at the conclusion of the study. There were
no unusual findings in bone marrow analysis although a slight increase
in a nucleated red blood cell content was evident at 4 mg/kg. Serum
iron and folio acid levels were unaffected by pirimicarb. Gross and
microscopic analysis of tissues and organs showed no effects
attributed to pirimicarb. Based on the data submitted a no-effect
level is 1.8 mg/kg body weight (Fletcher et al., 1971c.).
Long term study
Rat
Groups of rats (48 males and 48 females/group) were fed
pirimicarb in the diet at dosage levels of 0, 250, 500 and 750 ppm for
two years. There were no effects noted on survival. Growth was reduced
in females at all dose levels. There were no effects noted in males.
Total food intake of females was reduced. (Food conversion ratios
(ratio of food consumed to body weight gain) were reduced primarily in
the two higher dose groups).
No effects were noted on hematological parameters including
cholinesterase activity. Plasma cholinesterase was slightly reduced in
females. No effects were seen with RBC or brain cholinesterase. Gross
and microscopic examination of tissues and organs revealed only
reduced spleen weight of 750 ppm females. No other adverse effects
attributable to pirimicarb in the diet were noted. There was no
over-all increase in tumor incidence. An increase in the number of
localized astrocytomas in the high dosed males but not in the females
was not considered to be significant. Because of this slight effect
noted in males, a special study using males was initiated and is
summarized in the special study on carcinogenesis. A definitive
no-effect level wan not noted in this study, as dietary conversion
calculations do not support a firm conclusion of inappetance as a
basis for reduced growth. The marginal reduction of growth in females
suggests that a no-effect level is slightly below 250 ppm (Fletcher et
al., 1972).
OBSERVATIONS IN MAN
Cholinergic signs of poisoning accompanied by depression of
plasma (but not RBC) cholinesterase activity were observed in men
occupationally exposed to pirimicarb in the manufacture of formulated
products. In the course of investigating various procedures for safe
manufacturing conditions, it was observed that substantial vapor
toxicity was apparent as pirimicarb apparently volatalized at high
(65°C) temperatures and was inhaled by workers. Signs of exposure and
depression of plasma cholinesterase activity were recorded both of
which rapidly diminished with no apparent latent effects (Bagness et
al., 1975).
COMMENTS
Pirimicarb, an N,N-dimethylcarbamate ester, is rapidly absorbed
and metabolised in mammals by oxidative and/or hydrolytic pathways.
Only a few of the many potential metabolites have been isolated. Those
products containing an intact carbamate structure are as acutely toxic
as the parent molecule. Where cleavage of the ester groups occurs, the
acute toxicity is decreased markedly. With the exception of two
products observed on leaf surfaces (the N-formyl (methylamino) and
N-methylamino analogues of pirimicarb) and not in animal studies, all
other products identified are common to both metabolic pathways. It is
possible that these two are also produced as transitory products in
mammals. Pirimicarb is rapidly excreted from the body and neither
pirimicarb nor its metabolites accumulate in any tissue or organ. In
short term rat studies with the two carbamate plant metabolites,
administered at high doses, hypochromic anemia was observed. The
significance of this occurrence was not defined.
Pirimicarb is moderately toxic following acute administration to
various animal species. Enzyme kinetic studies on the in vivo and
in vitro inhibitory properties suggest that pirimicarb is a
rapidly reversible inhibitor of acetylcholinesterase as compared with
its effects on pseudo-cholinesterase. However, the stability of
inhibited (carbamoylated) plasma cholinesterase allowed an estimate to
be made of in vivo cholinesterase depression.
In several species no effects on reproduction (including
teratogenicity) were observed. A mutagenic response was negative as
evidenced by a mouse dominant lethal study. Pirimicarb did not induce
a delayed neurotoxic response, as generally noted with TOCP, in hens.
Short and long term studies in several animal species were performed.
In one long term study in the rat an increased number of astrocytomas
were noted in males, but this was not confirmed in two subsequent
studies. In a specific carcinogenic study in mice, a significant
increase in the incidence of pulmonary tumours was observed. However,
it was believed that the differences were due to an abnormally low
control value, thus negating the full evaluation of the study. None of
these studies was entirely adequate for evaluating the carcinogenic
potential, as chronic respiratory infection in each rat study limited
survival and a full interpretation of the data.
In short term studies with dogs, a dose dependent effect
manifested as hemolytic anemia changes was observed at high levels of
pirimicarb fed in the diet. Anemia did not occur in all dogs exposed
at high doses, and there was a suggestion of a sensitisation reaction.
In studies on the mechanism of action of pirimicarb with respect to
the hemolytic anemia, it was observed that an abnormal condition in
red blood cells was associated with the presence of pirimicarb. Also,
the presence of an increased number of a certain type of nucleated red
blood cells in bone marrow cytological examination, even in the
absence of anemia, suggested interference in a late stage of
development of erythrocytes induced by relatively high levels of
pirimicarb and complicated the full interpretation of the data. It was
suggested that hemolysis is caused by induction of a specific antibody
to the red blood cell following high level administration of
pirimicarb.
In a two year study with dogs of the same colony, no anemia was
observed although a dose dependent occurrence of nucleated red blood
cell precursors in the bone marrow was noted. At the low dose levels,
no effects on hematological or other parameters examined were
observed. Thus, in a susceptible species the effects noted over a
short term high level exposure were not cumulative and were not
manifested in a two year study. A similar hematological condition was
not reported in rodents exposed to pirimicarb in several long and
short term studies.
In a long term study with rats, growth depression was observed,
primarily at high levels. Marginal depression of growth was observed
in females (but not males) at the lowest dose level tested.
Calculation of food consumption was made with respect to growth. It
was concluded that growth depression may be related to inappentance
rather than being a specific toxicological effect.
In workers known to be occupationally exposed certain effects on
plasma cholinesterase activity were observed but no adverse long term
effects were noted.
The Meeting, in considering the toxicological properties of
pirimicarb, concluded with respect to the marginal growth depression
observed in rat species that a no-effect level would be slightly below
the lowest dose level tested.
The adverse effects on hematological parameters noted in the dog
were dose dependent and a no-effect level could be seen.
The carcinogenic potential of pirimicarb in rodents could not be
fully evaluated although the studies performed gave little concern to
the Meeting that pirimicarb was a potent carcinogen in the species
tested.
In consideration of both the long and short term studies in
several species a no-effect level was agreed upon from the data of the
2 year dog study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.8 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimicarb is a fast-acting, selective aphicide with contact,
translaminar and, more particularly, fumigant actions. It has proved
effective on all of the crops on which it has been tried against all
aphids including organophosphorus-resistant strains, except for
multiresistant strains of the damson hop aphid, Phorodon humuli.
Pirimicarb is harmless to useful parasites and predators, with
the exception of hoverflies. It is also of very low toxicity to bees.
This specificity makes pirimicarb a valuable chemical for use in
integrated control programmes.
It is active at rates as low as 0.125 kg ai/ha, depending on the
efficiency of crop spray cover and the species of aphid present.
Normal rates of commercial application are 0.25 kg ai/ha; but rates as
high as 0.50 kg ai/ha are recommended for some outlets. Applied as a
smoke, pirimicarb is normally effective at 15 mg/m3.
Pirimicarb possesses only limited persistence on crops.
Re-treatment may therefore be necessary after 10-14 days in those
situations where the potential for re-infestation remains high.
Pre-harvest withholding intervals range from zero for root crops, and
crops where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb was first synthesised in 1965. It was first registered
for use on peaches in Western Europe in 1969. It is now registered
world-wide for use on a wide range of crops, including fruit,
vegetables and cereals.
Use patterns in the Netherlands, South Africa, New Zealand and
Australia are summarized in Table 5.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pirimicarb has a half-life of one to three days on most crops.
Residue trials have been conducted on a wide range of crops in
numerous countries since 1967. The results of these studies are
reported by Manley (1972) and by Edwards and Dick (1976). They are
summarized in Tables 6-10. Total pyrimidine carbamate residues have
been studied, i.e. residues of pirimicarb and its two major
carbamate-containing metabolites on plants (the N-demethyl and
N-formyl (methylamino) analogues of pirimicarb, III and II, Figure I).
II is also commonly known as desmethyl-formamido-pirimicarb.
TABLE 5. Use pattern in various countries
Country Crop Formulation Rate Pre-harvest Interval
ai.
Netherlands Cucurbits (Eggplant, 10% smoke 10 g/700m3 3 days
cucumber, generator
gherkin,
melon)
Pepper 50% WP 25 g/100 l 3 days
Tomato
All other consumption 10% smoke 10 g/700 m3 1 March-31 Oct - 1 week
crops under glass generator 1 Nov-28 Feb - 2 weeks
All fruits & vegetables 50% WP 25 g/100 l 7 days
with the exception of
endive and lettuce under
glass and parsley and
celery
Sugarbeet, potatoes 50% WP 0.25 Kg/ha -
Cereals 50% WP 0.25 Kg/ha 4 weeks
South Africa Wheat WP 125 g/ha 7 days
Peaches WP 25 g/100 l 21 days
New Zealand Potatoes 250 g/220 l/ha Nil
Brassicas 250 g/200 l/ha 3 days
Australia Vegetables, fruits and hops 2 days
Leafy Vegetables (Tables 6 and 7)
The highest initial residues after spraying edible crops are
found in leafy vegetables. Lettuce contained total carbamate residues
of up to 20 mg/kg immediately after spraying at recommended use rates,
with the highest values occurring after indoor applications. However
decay rates are normally rapid (half-lives of 1-3 days outdoors) so
that after seven days in the outdoor situation or fourteen days
indoors total pyrimidine carbamate residues are normally less than
1 mg/kg.
Cucurbits and solanaceous fruits (Table 6)
A short pre-harvest withholding interval (e.g. 1-3 days) is
normally required in solanaceous fruits and cucurbits. Pirimicarb
residues in these crops can vary with the method of application and
with whether the crop is grown indoors or outside. Nevertheless after
recommended pre-harvest withholding intervals, total pyrimidine
carbamate residues are below 1 mg/kg and usually below 0.5 mg/kg. An
exception is chili peppers, grown outdoors, where residues are below 2
mg/kg after seven days.
Treatments with a smoke generator in the greenhouse give rise to
lower residues than corresponding spray treatments (Table 6).
Root crops and legumes (Table 8)
Pirimicarb does not move from the leaves into the storage organs
(roots and tubers) of root crops. Therefore root crops and tubers do
not normally contain significant pyrimidine carbamate residues after
spraying. Where residues are detected, these are due either to the
root being wholly or partly exposed to the spray (e.g. kohlrabi and
carrots) or to residues being transferred from the foliage during
sampling and/or sample preparation. Similarly, detectable residues are
not expected in peas and beans where the edible portion of the crop is
protected by pods. The low residues which sometimes occur,
<0.1 mg/kg, probably arise from inadvertent contamination during
sample preparation.
Runner and French beans exposed to the spray contained residues
up to 1.4 mg/kg immediately after spraying but decay was generally
rapid (half-life less than 1 day) so that total pyrimidine carbamate
residues after seven days were normally less than 0.5 (mg/kg).
Fruit (Table 9)
Residues in fruit are lower than in leafy vegetables. Levels are
generally below 2 mg/kg, even immediately after application. These
decay rapidly, with half-lives of 1-2 days. After seven days, total
pyrimidine carbamate residues are normally below 0.5 mg/kg and
TABLE 6. Residues of Pirimicarb And Its Two Major Carbamate-Containing Metabolites In Leafy
Vegetables, Cucurbits and Solanaceous Fruit From supervised Trials With Sprays Or
Smoke Generators
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Brussels sprouts Netherlands 0.14 1 3-4 0.11-0.13
U.K. 0.28 1 3 <0.14
0.28 3 3 0.28
U.S.A. 0.14 5-9 3 0.03-0.10
0.28 5-9 3 0.05-0.32
Broccoli U.S.A. 0.14 2-5 1 <0.05-0.71
0.28 2-5 1 <0.10-0.96
Cabbage (green) Australia 0.25* 1 7 0.21
Germany 0.30 1-2 7 0.57
New Zealand ( 0.28 1 7-8 0.11-0.30
U.K. (
U.S.A. 0.14 2-5 3 <0.05-0.08
0.28 2-5 3 <0.05-0.18
Cabbage (savoy) Germany 0.30 1 1-10 0.01
Japan 0.50* 4 7-8 0.02-0.78
Cauliflower Germany 0.30 1 7 0.03
(whole)
U.S.A. 0.14 1-7 3 <0.05
0.28 1-7 3 <0-05-0.275
Celery (outdoor) Netherlands 0.25 1 7 0.22-0.97
U.K. 0.28 1 7 0.18
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
(indoor) Netherlands 0.25 1 7 4.1
Endive Netherlands 0.28 1 7 <0.10
Lettuce (outdoor) Australia 0.28 1 0 7.5
2 1.4
5 0.23
9-16 ND**
0.56 1 0 19.6
2 2.4
5 0.33
9 0.38
13-16 ND
Germany 0.50 1 7 <0.01-0.05
U.K. 0.25* 1 7 <0.18-0.56
U.S.A. 0.14 3-7 1 <0.05-0.44
0.28 3-9 1 <0.05-0.97
(indoor) U.K. 0.25* 1 13-14 <0.18-0.47
Parsley (outdoor) Netherlands 0.25 1 15 0.15-0.65
(indoor) 0.25 1 15 4.7
Onions (salad) U.K. 0.16* 1 0 0.04-0.39
Spinach U.K. 0.50 1 7 <0.01-0.03
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Cucumbers Canada 0.28 1 3 0.04-0.13
U.K. 0.28 1 2 <0.10-0.34
Aubergines U.S.A. 0.28-0.42 7 3 <0.01
Gherkins Germany 0.3-0.5 1-3 2-3 0.01-0.05
U.K. 0.25* 1 3 0.45
Peppers (outdoor) Canada 0.14 3 3 0.05
(Bell) U.S.A. 0.14 3-6 1 0.05-0.34
0.28 4 1 0.05-0.43
(indoor) Canada 0.28 1 2 0.14
France (
Netherlands( 0.25* 1 3 <0.12-0.16
Peppers (outdoor) U.S.A. 0.14 2-10 7 0.05-0.83
(chili) 0.28 1-10 7 <0.05-1.26
Tomatoes Australia 0.25* 1 2 0.08
Canada 0.19+0.28 2 2 0.04
0.28 1 3 0.28
Germany 0.50 1-2 1 <0.01-0.04
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Japan 0.50* 2-4 1 0.10-0.61
U.K. 0.25* 2 2 <0.01-0.37
U.S.A. 0.28 7 3 <0.01
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Cucumbers Germany 30 3 1 0.29-0.32
Netherlands 20 1 0 0.32
15 1 1 0.03
U.K. 15 1 0 0.05-0.19
Endive Netherlands 12 1-2 13-14 0.02.-0.14
Lettuce Germany 14 3 14 0.46
Netherlands ( 15 1 14 0.02-0.85
U.K. (
Gherkins Germany 15-17 3 0 0.11
TABLE 6. (Cont'd.)
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Netherlands 14 1 1 0.02
Tomatoes Germany 14-16 3 0 0.01-0.08
Netherlands 13 1 1 0.01-0.02
U.K. 15 1 0 0.08-0.09
* Rate in g a.i./l
** Not detectable
consistently below 1 mg/kg. Pirimicarb does not penetrate the skin of
fruit and hence can if necessary be removed by peeling (Edwards and
Dick 1976; Manley 1969).
TABLE 7. Decline of pirimicarb residues on cabbages (New Zealand)
Application Residues in mg/kg, at intervals (days) after application
rate
No. kg a.i./ha 1 3 7 10 14 21
1 0.30 0.47 0.38 0.30 0.14 0.13 <0.06
1 0.50 0.49 0.39 0-30 0.24 0.20 <0.06
2 0.28 0.44 0.18 0.19
2 0.56 0.88 0.39 0.18
Cereals and seed crops (Tables 9 and 10)
Wheat grain analysed in husk contained total pyrimidine carbamate
residues of 1-3 mg/kg on day after application (Table 10). Half-lives
varied between one and seven days. No residues were detected in
dehusked grain 7 days after spraying.
Very low residues (<0.1 mg/kg) were detected in oil seed rape
seven days after application of pirimicarb.
FATE OF RESIDUES
In Plants
Pirimicarb is rapidly lost from plants on which it has a
half-life of 1-3 days. Volatilisation affords the primary means of
loss: the higher the ambient temperature, the greater the percentage
of pirimicarb lost by volatilisation (Manley, 1969; Bewick and Leahey,
1976; Davis and Hemingway, 1976; Hemingway and Davis, 1976).
Pirimicarb also undergoes photochemical and metabolic
degradation.
The major carbamate-containing degradation products are compounds
II and III. (Figure 1). Compound IV is only a minor
carbamate-containing degradation product. Other minor degradation
products include the water soluble hydroxypyrimidines I, V, and VI,
TABLE 8. Residues of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Root
Vegetables And Legumes From Supervised Spray Trials
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Beetroot U.K. 0.25* 1 0 0.02
Carrots
(submerged) U.K. 0.28 1 0 0.02-0.04
(tops of roots
exposed) U.K. 0.25* 1 7 0.11
Kohlrabi (roots) Germany 0.25 1 0 <0.01-0.23
0.25 2 0-10 <0.01
(leaves) Germany 0.25 1 7 0.09-0.11
0.25 2 1-10 <0.01
Parsnips U.K. 0.25* 1 0 <0.01
Potatoes Netherlands 0.25 1 0 <0.01
(aerial)
New Zealand ( 0.28 1 0 <0.01
U.K. (
U.S.A. 0.14 1-7 0 <0.01
0.28 1-7 0 <0.01
Radish Japan 0.25* 2-4 0 <0.01
Sugar beet Germany 0.30 1-2 0 <0.01-0.02
(roots)
(leaves) Germany 0.30 1-2 7-8 0.12-0.70
TABLE 8. (Cont'd.)
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Turnips U.K. 0.25* 1 0 0.04
Beans (broad) Germany 0.30 1 0 0.09
Beans (runner/ Germany 0.30 1 7 0.05
French)
U.K. 0.25* 1 7-8 <0.06.0.55
0.50 1 0 0.89-1.4
Peas Australia 0.28 4 0 0.06
Germany 0.30 1 0 0.10
Netherlands 0.25 1 0 0.03
U.K. 0.16* 1 0 <0.01
0.50 1 7 0.06-0.67
* indicates rate in terms of g ai per litre.
TABLE 9. Residues Of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Fruit, Cereals
And Seed Crops From Supervised Spray Trials
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
Apples Canada 0.25 1 7 0.01-0.60
U.K. 0.16 2 7 0.09-0.37
Blackcurrants U.K. 0.16 1 7 0.01-0.08
Oranges Australia 0.25 1 7 <0.01 (whole fruit)
<0.02 (flesh)
0.32 (peel)
Peaches Australia 0.25 1 7 0.05
Italy ( 0.25 1-3 5-6 <0.10-0.27
Spain ( 0.50 1-3 5-6 0.20-0.36
Plums Canada 0.14* 1 7 0.36
0.28* 1 7 0.38
U.K. 0.25 1 7 0.06-0.11
Raspberries U.K. 0.16 1 7 0.02-0.09
Strawberries Australia 0.25 1 0 1.5
0.25 1 7 0.23
U.K. 0.25 1 7 0.07-0.09
Wheat (in husk) Australia 0.25* 1 7 0.33
Germany 0.25* 1-2 7 0.04-1.5
TABLE 9. (Cont'd.)
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
South Africa 0.25* 1-2 7 0.27-0.52
(de-husked) Netherlands 0.125* 1 7 <0.02
Oil seed rape Australia 0.28- 1-4 7 <0.10
Czechoslovakia 0.35*
* indicates rate in terms of kg a.i./ha
and guanidine and its 1-methyl and 1,1 dimethyl derivatives. This
pattern of degradation has been shown to take place in part or
completely on peach and sugar beet leaves (Manley, 1969), lettuce
(Hemingway and Davis, 1976) and cabbage (Davis and Hemingway, 1976).
The rate of degradation varies with crop type and weather conditions.
Thus in lettuce, seven days after application of pirimicarb 71% of the
radioactivity remaining was present as pirimicarb and the carbamates
II and III, whilst in cabbage the figure was 32%.
In lettuce plants sprayed with 2-14C-labelled pirimicarb four
weeks prior to harvest, 25% of the radioactivity associated with the
plants could not be extracted by refluxing with ethanol and was termed
`bound'. Pirimicarb, compounds III and V and the three guanidines
mentioned above were all shown to be minor constituents of the bound
residue. Approximately 40% of the bound residue was associated with
the cellulose and hemicellulose of the plant (Teal and Skidmore,
1976).
When pirimicarb was introduced systemically into broad bean
plants by stem injection, its half-life was less than one day. Again
products II and III were formed initially. However these and
pirimicarb were subsequently degraded, the carbamate groups being
quantitively eliminated as measured by the evolution of 14CO2 from
plants treated with 14C-carbonyl-labelled pirimicarb (Manley, 1969).
Spraying of potatoes with 2-14C-labelled pirimicarb at 0.42 kg
ai/ha resulted in very low residues of radioactivity in the tubers
(less than 0.05 mg/kg pirimicarb equivalents). More than 80% of this
low residue was present as unextractable materials or as a complex
mixture of water-soluble materials closely associated with plant
sugars and pigments (Bowker et al, 1975).
When pirimicarb sprayed at rates up to 5 kg ai per hectare, ie
20-40 times normal use rates, total pyrimidine carbamate residues in
"follow-up" crops at harvest extremely small - of the order of the
limit of determination of the analytical method or below (Edwards et
al, 1974 and 1975). Total radiolabelled residues in "follow-up" crops
at harvest as a result of applying 2-14C-labelled pirimicarb at 0.5
kg ai per hectare were also small - up to 0.14 mg/kg pirimicarb
equivalents. In lettuce, which contained the highest radioactive
residue, approximately 50% of the activity was due to a combination of
pirimicarb, compound III, the hydroxypyrimidines I, V and the three
guanidines (Hughes and Leahey, 1974; Leahey and Benwell, 1976).
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. Only traces of residues are transferred to
milk and tissues.
TABLE 10. Pirimicarb Residues on Wheat (South Africa) at Intervals
after Application
Residues, mg/kg
Application Days
rate No. of after Demethyl-
g a.i./ha Applications Application Pirimicarb pirimicarb
250 1 1 2.3 0.4
2 1.7 0.3
4 0.4 0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.7 0.4
2 0.6 0.3
4 0.2 0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.5
2 1.6 0.2
4 0.3 <0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.8 0.4
2 0.6 0.2
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.4
2 2.3 0.4
4 0.4 <0.2
7 0.3 <0.2
55 <0.1 <0.2
125 1 1 0.6 0.3
2 0.6 0.3
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
Results are the average of 3 determinations
Recovery pirimicarb 105% at 1 mg/kg
demethyl-pirimicarb 78% at 1 mg/kg
Results not corrected for recovery
Limit of detection pirimicarb 0.1 mg/kg
demethyl-pirimicarb 0.2 mg/kg
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum radioactive residue in the milk (0.25 mg/kg
pirimicarb equivalents) was detected one hour after dosing; 80-90% of
this residue was due to the hydroxypyrimidines I, V and VI (Figure 1).
Subsequent residues were 0.06 mg/kg pirimicarb equivalents or less.
The maximum residue in fat and meat tissues after twelve days was only
0.04 mg/kg pirimicarb equivalents (Hemingway et al 1976). See also
"Biotransformation".
In soil
Pirimicarb is extensively degraded in soil. Degradation occurs
most rapidly in less acid soils. In soils of pH6.5 and above,
pirimicarb has a half-life of up to 7 weeks under aerobic conditions.
The rate of degradation in more acid soils was considerably enhanced
by air drying. Although less important than soil pH, a higher organic
matter content also appears to enhance the rate of degradation of
pirimicarb (Hill et al 1975a; Hill and Stevens 1975).
The principal route of degradation in soil is by hydrolysis of
the carbamate moiety from the pyrimidine ring. N-dealkylation of the
2-dimethylamino group has also been observed. Cleavage of the
pyrimidine ring has been demonstrated by the loss of 14CO2 from
soils treated with 2-14C-labelled pirimicarb. Hydrolysis of the
carbamate moiety results from both biological and chemical soil
activity whereas pyrimidine ring cleavage is mediated only by
biological activity. Several fungi and actinomycete cultures are
capable of metabolising pirimicarb (Hill et al 1975a; Hill and Arnold,
1974; Manley et al 1972).
Compounds I, III and V (Figure 1) are all major soil metabolites.
Minor degradation products include the carbamates II and IV and the
hydroxy pyrimidine VI (Hill et al, 1975a). Compound I is the major
product of photochemical degradation of pirimicarb on the soil surface
(84% of the residue recovered after 20 days) (Leahey and Benwall,
1974).
After two years, more than 70% of the radioactivity resulting
from the application of 2-14C-labelled pirimicarb to acid soils was
not extracted by refluxing with acetone: water (5:1). Almost all of
this bound residue was associated with the fulvic acid fraction of the
soil. Up to 60% of the bound residue was due to pirimicarb plus the
hydroxypyrimidines I and V (Hill et al 1975b).
Pirimicarb and its soil degradation products are of low mobility
in soil; lower than atrazine which is recognized as being only
moderately mobile (Riley et al 1974). There is no significant
concentration of pirimicarb in "run-off" water or eroded soil (Gouman
and Hale, 1975).
In water
Pirimicarb undergoes rapid photochemical degradation in water:
over 50% of the applied pirimicarb is degraded in 24 hours at pH5-9.
There is no significant loss of pirimicarb by volatilisation from
water. Degradation products include the pyrimidine carbamates II-IV,
the hydroxypyrimidines I, V and VI, 1,1-dimethylguanidine and
1-methylguanidine. Only traces (<1%) of guanidine itself have been
detected (Bowker and Griggs, 1974; Lincoln and Hemingway 1974).
In washing and cooking
Residues of pirimicarb and its two major carbamate metabolites II
and III on lettuce were reduced by 38-49% by washing with cold water.
In addition, during cooking approximately 60% of the pirimicarb and
52-65% of the carbamate-containing metabolite residues were
transferred from Brussels sprouts and cabbage into the cooking water.
There was no detectable conversion to other products during the
washing or cooking processes (Edwards et al, 1976).
In storage
There was no loss of residue from deep-frozen samples fortified
with pirimicarb. Details are given in "Methods of residue analysis".
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Monitoring of pirimicarb residues in the Netherlands has shown
that residues in imported grapes and in all but one sample of
home-produced endive were within the national tolerance of 1 mg/kg.
METHODS OF RESIDUE ANALYSIS
A gas-chromatographic method using a nitrogen-selective detector
to detect residues of pirimicarb plus its two major
carbamate-containing degradation products (II and III, Figure 1) is
the preferred residue method (Zweig 1975).
Crop samples are macerated with choloroform in the presence of
anhydrous sodium sulphate. The extract is subjected to a solvent
partition clean-up during which
5,6-dimethyl-2-formylmethylamino-4-pyrimidinyl dimethylcarbamate (II)
is hydrolysed to 5,6-dimethyl-2-methylamino-4-pyrimidinyl
dimethylcarbamate (III). The dried extract in ethyl acetate is
evaporated to dryness and taken up into acetone, and an aliquot of
this solution is injected into a gas chromatograph fitted with a
nitrogen detector. The size of the peaks obtained at the retention
times of pirimicarb and of compound III are used to calculate residue
levels by comparison with peaks obtained by injection of standard
solutions of the two compounds (ICI Plant Protection Ltd., 1972).
Some crop extracts may contain co-extracted interfering
compounds. These can be removed by cleanup on a column of silica gel,
prior to gas-chromatographic determination (Edwards and Dick, 1976).
The limit of determination for pirimicarb and for compound III in
crops is normally 0.01 mg/kg. Under the conditions of chromatography
described in the method, retention times for pirimicarb and compound
III were found to be 0.7 and 1.5 minutes respectively (ICI Plant
Protection Ltd, 1972).
Methanol may be used as the extraction solvent instead of
chloroform. For crops containing a high percentage of water (i.e.
normal 'green' crops), chloroform and methanol are equally efficient
(Manley, 1969; Edwards and Dick, 1975). However for crops of very low
water content (e.g. dry leaf tea, cured tobacco and oil seed rape), it
is essential to use methanol to extract residues of pirimicarb and
compounds II and III completely (Edwards and Dick, 1976).
Hydrolysis of compound II to compound III in 0.1 M hydrochloric
acid during the solvent partition cleanup was shown to be complete
(Cox, 1973).
A colorometric method, involving hydrolysis to dimethylamine, is
also available for determining residues of pirimicarb metabolites II
and III in crops (Zweig, 1975). Crop samples are macerated with
chloroform and the extract washed successively with 0.1M sodium
hydroxide, water and a slightly acid phosphate buffer (ph 4.3). The
chloroform is evaporated under reduced pressure and the residue steam
distilled with 1 M sodium hydroxide, liberating dimethylamine which
is absorbed in 0.1 M hydrochloric acid and determined
absorptiometrically as the yellow-brown copper dimethydithiocarbamate
complex at 436nm. Where troublesome emulsions occur at the washing
stages, the sample is extracted with acetone. Emulsifying agents are
removed by solvent partition; pirimicarb and its carbamate-containing
metabolites are then extracted into chloroform which is treated as the
chloroform extract obtained above.
Residues of the dimethyl dithiocarbamate fungicides (thiram and
ziram) interfere with the determination of pirimicarb and the two
metabolites by this method. Where interfering residues are present, a
chloroform extract obtained as described in the preceding paragraph is
subjected to thin layer chromatography alongside a marker spot on
silica gel GF 254. The developed chromatogram is viewed under
ultra-violet light, the primicarb band is removed and steam distilled,
and the determination completed, as described above (ICI Plant
Protection Ltd. 1969). The limit of determination of the method is
normally 0.7 mg/kg pirimicarb or pirimicarb equivalents. Recoveries in
excess of 70% are normally obtained (Bullock, 1969). The determination
of residues of pirimicarb and compound II and III is not affected by
the presence of residues of the ethylene bisdithiocarbamate fungicides
(e.g. maneb and zineb), nor by the presence of residues of carbaryl,
as none of these compounds is hydrolysed to dimethylamine.
TABLE 11. Stability Of Pirimicarb Residues In Crops Stored At -14°C
Mean residues, mg/kg
CROP
Initially After After
3 6.5
months months
Apples 0.92 0.94 0.86
Potatoes 1.00 0.79 0.81
Lettuce 0.97 1.04 0.94
Cucumbers 0.91 1.05 0.81
Pirimicarb residues are stable at -14°C. Samples of apples,
potatoes, lettuces and cucumbers were spiked with pirimicarb at
1 mg/kg, stored at -14°C for up to six months and analysed for
pirimicarb by the gaschromatographic method described above (see Table
11). Statistical analysis of the individual residue figures and of the
determined recovery values showed that there were no significant
differences among the residue values due either to storage time or to
crop (Edwards and Atreya, 1974).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national tolerances have been established for residue of
pirimicarb plus its two major carbamate containing metabolites, II and
III (Table 12).
TABLE 12. National tolerances reported to the Meeting
Country Crop Tolerance mg/kg
Australia Vegetables, 1
fruit and hops 0.5
Hungary All crops 0.5
Japan Fruit and vegetables 0.3
TABLE 12. (Cont'd.)
Country Crop Tolerance mg/kg
New Zealand All crops 0.5
Switzerland Fruit and vegetables 1
USA Potatoes 0.1
Venezuela All crops 0.5
Netherlands Fruit and vegetables 1.0
Grain 0.05
Potatoes 0.05
APPRAISAL
Pirimicarb is a fast acting, selective aphicide which is now
registered in a number of countries for use on fruit, vegetables and
other crops. It has proved effective on all crops on which it has been
tried against all aphids including organophosphorus-resistant strains,
with the exception of multi-resistant strains of Phorodon humuli.
It is harmless to useful parasites and predators, with the exception
of hoverflies, and has a very low toxicity to bees.
In addition to its contact and translaminar actions pirimicarb is
effective as a fumigant. Technical pirimicarb is not less than 95%
pure and is normally 98% pure; the impurities contain up to 1.3% of
other pyrimidine carbamates. It is formulated as a 50% dispersible
powder and a 50% dispersible pellet or grain, as a ULV formulation and
in a 10% smoke generator. The usual rates of application are 0.25 kg
a.i./ha as a crop spray and 15 mg/m3 as a smoke.
Pirimicarb possesses only limited persistence on crops and the
pre-harvest withholding intervals range from zero for root crops, and
others where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb is rapidly lost from plants; it has a half-life of 1-3
days. Most of the loss is by volatilisation, the extent of which
increases with rise in the ambient temperature, but there is some
photochemical and metabolic degradation. There are two major
metabolites containing the carbamate moiety, the N-formyl(methylamino)
(II) and 2-methylamino (III) analogues of pirimicarb, which occur in
plants. Minor degradation products include a third carbamate, 2-amino
pirimicarb, the water-soluble 2-dimethylamino-, 2-methylamino- and
2-amino-hydroxypyrimidines and the corresponding guanidines. The
recommended residue methods for pirimicarb also estimate the two major
carbamate-containing metabolites.
Use of radio-labelled material shows that some part (25% in one
experiment) of the pesticide may become bound to plant materials (i.e.
not extractable with organic solvents) but, as far as can be judged,
the more toxic carbamate compounds are not major constituents of this.
Within the plant (stem injection of broad beans), metabolic products
II and III are formed initially but subsequently degraded. Transfer to
the tubers of sprayed potatoes is small. Follow-up crops grown on
heavily sprayed land contain only very small amounts (at the
analytical limit) of determinable residue. Washing heavily treated
lettuce with cold water reduces residues of pirimicarb by about 38%
and cooking Brussels sprouts and cabbage gives a 60% reduction.
Pirimicarb is concentrated in the skin of apples and the residues are
reduced below the level of detection on peeling.
The transfer of residues of pirimicarb from animal feed to the
milk and meat of cows appears to be very small.
In soil there is substantial hydrolysis of the carbamate moiety
from the pyrimidine ring and photochemical degradation on the soil
surface also very largely produces the hydroxypyrimidine V. Pirimicarb
and its degradation products are of low mobility in soil and there is
no significant concentration of pirimicarb in run-off water or eroded
soil.
Pirimicarb is rapidly degraded photochemically in water exposed
to bright sunlight (50% in 24 hours at pH 5 to 9) but decomposition is
slow in the dark.
Adequate methods of analysis are available for regulatory
purposes. A gas-chromatographic method using a nitrogen selective
detector to detect residues of pirimicarb plus its two major carbamate
degradation products II and III (II is converted to III during the
clean-up process) is the preferred method. The limit of determination
of pirimicarb and compound III in crops by this method is 0.01 mg/kg.
Total carbamate can also be determined by a colorimetric method for
which the limit of determination is 0.1 mg/kg and the pesticide
recovery better than 70%. Modifications of clean-up etc. are available
for both methods to deal with particular problems (Zweig, 1975).
Although little information is yet available on the residue of
pirimicarb in food moving in commerce, numerous results from
supervised residue trials on crops growing in a number of countries
enable recommendations to be made for certain groups of crops. Because
the maximum residue limits recommended for many leafy vegetables are
the same it would be reasonable to have a single recommendation for
this group.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the total residue of pirimicarb, its
N-formyl(methylamino) analogue (desmethylformamido-pirimicarb) and
desmethyl-pirimicarb expressed as pirimicarb.
Commodity Temporary Maximum Note:
Residue Limit
(mg/kg) Pirimicarb has
a half-life of
Chili peppers 2 1-3 days on
plants; leafy
Apples 1 commodities
Bell peppers 1 are harvested
Broccoli 1 7 days after
Brussels sprouts 1 application,
Cabbage 1 root crops are
Cauliflower 1 harvested 0-3
Celery 1 days after
Cucumber 1 application
Eggplant 1
Endive 1
Gherkins 1
Lettuce 1
Parsley 1
Tomatoes 1
Blackcurrants 0.5
Beans (with pod) 0.5
Kohlrabi 0.5
Onions 0.5
Peaches 0.5
Plums 0.5
Raspberries 0.5
Strawberries 0.5
Peas 0.2
Rapeseed 0.2
Beans (without pod) 0.1
Beetroot 0.05*
Citrus fruits 0.05*
Parsnips 0.05*
Potatoes 0.05*
Radishes 0.05*
Sugar beet 0.05*
Turnips 0.05*
Wheat 0.05*
FURTHER WORK OR INFORMATION
Required (by 30 June 1978)
1) Studies on other species, primarily primate, to define the range
of species' susceptibility to the hematologic changes noted in
the dog.
* At or about the limit of determination.
2) Definition of the susceptibility to hemolytic anemia in the
inbred beagle strain used in toxicological studies.
3) Studies to fully define the reduced growth noted in several
rodent dietary studies.
4) Immunological studies when appropriate in animals species other
than the clog to define further the significance (and possible
range of occurrence) of the hemolytic anemia.
5) Significance of hypochromic anemia associated with rats fed high
levels of plant metabolites.
Required (by 1980)
1. Carcinogenic study in an appropriate mammalian species using a
currently acceptable protocol.
Desirable
1. Survey of industrially exposed individuals by immunological (or
serological) techniques to define the potential occurrence of
antibodies following pirimicarb exposure.
2. More information on residues in grains other than wheat, e.g.,
barley, oats, rice, maize.
REFERENCES
Bagness, J.E., Hamer, C.M. and Willis, G.A., Pirimicarb
1975 Operational Experience During Formulation.
Unpublished report from ICI Plant Protection
Division submitted to the World Health
Organization by ICI Ltd.
Bewick, D.W. and Leahey, J.P. Pirimicarb : Identification
1976 Of Material Volatilised From The Surface Of A
Treated Lettuce Plant, ICI Plant Protection Ltd.
Report No. TXJ1289A (Unpublished).
Bowker, D.M. and Griggs, B.F. Pirimicarb : Fate In Water.
1974 ICI Plant Protection Ltd. Report No. AR2492A
(Unpublished).
Bowker,D.M., Griggs, B.F. and May, M.S. Pirimicarb Fate In
197e Potatoes. ICI Plant Protection Ltd. Report No.
AR2491 AR (Unpublished).
Bullock, D.J.W. A Colorimetric Method Of Analysis for Residues Of
1969 PP062 In Food Crops, ICI Plant Protection Ltd.
Report No. AR2091 AR (Unpublished).
Clark, D.G., The Influence of Feeding on the Oral Toxicity of
1967a PP 062 and PP159. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Clark, D.G., The Acute Toxicity of Technical PP062, Dispersible
1967b Powder (J.F.2130) and PP062 Granules (J.F. 2148).
Unpublished report from ICI Central Toxicology
Laboratory submitted to the World Health
Organization by ICI Ltd.
Cox, J.H. Determination of Pirimicarb and Metabolites In Potato
1973 Tubers, ICI America Inc. Report (Unpublished).
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1968 Toxicity of PP062 - Beagle Dogs (Part 1).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1969 Toxicity of PPO62 - Beagle Dogs (Part 2).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by IGI Ltd.
Daniel, J.W. and Bratt, H., Absorption and Excretion by Rats of
1968 the Carbamate Insecticide, PP062. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Davis, J.A. and Hemingway, R.J. Pirimicarb : Fate On Cabbage. IGI
1976 Plant Protection Division Report No. TMJ1348A
(Unpublished).
Edwards, M.J. and Atreya, N.C. Pirimicarb : Storage Stability
1974 of Residues In Deep Frozen Crop Samples. ICI Plant
Protection Ltd. Report No. TMJ1090A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb : Crop Extractability
1975 Study, ICI Plant Protection Ltd. Report No.
TMJ1166A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb Residue Summary Residues
1976 In Crops From Field Trials 1973-1975. ICI Plant
Protection Division Report No. TMJ1360B (Un
published).
Edwards, M.J. Dick, J.P. and Hayward, G.J. Pirimicarb Effect of
1976 Washing and Cooking upon Residues in Lettuce and
Brassicae Crops. ICI Plant Protection Division
Report No. TMJ1346A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1974 Carry Over Study On Lettuce And Radish. ICI Plant
Protection Ltd. Report No. AR2565A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1975 Carry Over Study On Carrots, ICI Plant Protection
Ltd. Report No. TMJ1155A (Unpublished).
Ferguson, D.R. and Parkinson, G.R., Pirimicarb (PP062)
1973 Eye Irritation of Technical Grade Pirimicarb and
50% Dispersible Powder Formulation (JF 2538,
`Pirimor') Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Neurotoxicity in Hens.
1971a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Irritancy Tests. Unpublished
1971b report from ICI Central Toxicology laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Pirimicarb (PP062) : Subacute Toxicity on Rabbit
1971c Skin. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K. and Sotheran, J., Pirimicarb (PP062): Three
1971 Generation Reproduction Study in Rats. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.
1971a Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylformamidopirimicarb.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.,
1971b Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylpirimicarb. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Litchfield, M.H. and Watson, M.,
1971c Pirimicarb (PP062): Two Year Feeding Study in
Beagle Dogs. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Hodge, M.C.E. and Kinch, D.A., Pirimicarb (PP062) :
1971d Teratological Studies in the Mouse. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. Forshaw, A.M. and Monks, I.H., Pirimicarb (PP062):
1971e Excretion and Metabolism in the Dog and Rat.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Kinch, D.A. and Hodge, M.C.E. Pirimicarb (PP062) :
1971f Teratological Studies in the Rat. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. and Bratt, H., Pirimicarb (PP062). Accumulation Study
1972 in Rats. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Clapp, M.J.L., Garner, R., Litchfield, M.H. and
1972 Wilson, J., `Pirimicarb', (PP062): Two Year
Feeding Study in the Rat. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Garner, R., Phillips, C.E., and Slack, P., Pirimicarb (PP062)
1972 Pirimicarb Anaemia (A Chemically-Induced
Autoimmune Haemolytic Anaemia). Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Gowman, M.A. and Hale, S.W. Pirimicarb : Loss From Soil In
1975 Run-Off Water and Eroded Soil. ICI Plant
Protection Ltd. Report No. TMJ1102A (Unpublished).
Griffiths, D. and Conning, D.M., Ninety Day Oral Toxicity
1968 of PP062 - Albino Rats. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Hemingway, R.J. and Davis, J.A. Pirimicarb : Fate on Lettuce.
1976 ICI Plant Protection Division Report No. TMJ1324A
(Unpublished).
Hemingway, R.J. Davis, J.A. and Cleverly, B.A. Pirimicarb:
1976 Ercretion and Metabolism of a Single Oral Dose
Administered to a Cow. Unpublished report No.
AR2663A from ICI Plant Protection Division,
submitted to the World Health Organization by ICI
Ltd.
Hill, I.R. and Arnold, D.J. Pirimicarb Metabolism by Micro-Organisms
1974 Isolated from Soil. ICI Plant Protection Ltd.
Report No. TMJ1119A (Unpublished).
Hill, I.R., Arnold, D.J., Minns, E.C. and Greaves, R. Pirimicarb :
1975a Laboratory Studies Of The Degradation Of Single
Applications Of The Pesticide In Soil In the
Absence Of Plants. ICI Plant Protection Ltd.
Report No. AR2555A (Unpublished).
Hill, I.R., Harvey, B.R. and Arnold, D.J. Pirimicarb Extraction And
1975b Fractionation Of 'Bound' Residues In Soil. ICI
Plant Protection Ltd. Report No. AR2579A
(Unpublished).
Hill, I.R. and Stevens, J.E. Pirimicarb : Losses of Radioactivity
From 14C-Pirimicarb Treated Soils, During Air
Drying. ICI Plant Protection Ltd. Report No.
TMJ1143A (Unpublished).
Hodge, M.C.E., Pirimicarb (PP062): Teratological Studies in
1974 the Rabbit. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Hughes, H.E. and Leahey, J.P. Pirimicarb : Uptake of Pirimicarb And
1974 Its Metabolites From Soil By Rotational Crops. ICI
Plant Protection Ltd. Report No. AR2553A
(Unpublished).
ICI Plant Protection Ltd. Residue Analytical Method No. 15 for
1972 The Determination Of Residues Of Pirimicarb And
Its Two Major Metabolites In Crops
(Gas-chromatographic Method) (Unpublished).
ICI Plant Protection Ltd. Provisional Analytical Method No. 306/A For
The Determination Of Pirimicarb Residues In Fruits
Vegetables And Grain (Unpublished).
Jackson, J.A., Chart, I.S., and Sanderson, J.H., A Pirimicarb -
1974 Induced Haemolytic Anemia in Dogs. Report of a
Special Study. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Leahey, J.P. and Benwell, M. Pirimicarb : Photodegradation On A
1974 Soil Surface. ICI Plant Protection Ltd. Report No.
AR25504 (Unpublished).
Leahey, J.P. and Benwell, M. Pirimicarb ; Uptake From Soil And
Metabolism In Lettuce And Carrots, ICI Plant
Protection Division Report No. TMJ1331A
(Unpublished).
Lefevre, V.K. and Parkinson, G.R., Pirimicarb (PPO62;
1974 2-dimethylamino-5, 6-Dimethylpyrimidin-4-yl
Dimethylcarbamate): Acute Oral Toxicities of Plant
and Mammalian Metabolites. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Lincoln, B.A. and Hemingway, R.J. Pirimicarb : Fate In Water. ICI
1974 Plant Protection Ltd. Report No. TMJ1116A
(Unpublished).
Litchfield, M.H., The Measurement of Blood Cholinesterase Activity
1968 in Animals Dosed with 4,5
Dimethyl-2-dimethylamino-6-pyrimidinyl Dimethyl
Carbamate (PP062): Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Manley, C.A. Pyrimidine Insecticides : The Fate of Pirimicarb
1969 On Surfaces And Within The Plant ICI Plant
Protection Ltd. Report No. AR2109A (Unpublished),
18-19 and Figure 14.
Manley, C. A. Residue Summary : Pirimicarb In Crops. ICI
1972 Plant Protection Ltd. Report No. TMJ585/2
(Unpublished).
Manley, C.A., Hill, I.R., Harper, P. Black L. and Brooks J.R.
1972 Pirimicarb : Fate In Soil. ICI Plant Protection
Ltd. Report No. TMJ788AR (Unpublished).
Main, A.R. Personal communication.
1976
McGregor, D.B., Dominant Lethal Study in Mice of ICI PP062.
1974 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Palmer, S. and Samuels, D.M., Pirimicarb: 80 Week Carcinogenic
1974 Study in Mice. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Irritation Studies of Technical
1972a and Formulated Material on Normal and Wounded
Rabbit Skin. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Acute Oral Toxicity Following
1972b Storage at 37°C for Three and Six Months.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, C.R., Pirimicarb Metabolites (R.31680 and R.35140):
1974a Acute Oral Toxicity. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.42488): Acute Oral
1974b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Skin Sensitization. Unpublished report
1974c from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Parkinson, G.R., Oral Toxicities of Three Guanidine Compounds.
1975a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.32444): Acute Oral
1975b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.33160) : Acute Oral
1975c Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Potentiation Studies With
1975d Azinphos-methyl and Carbaryl. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A. Pirimicarb (PP062): "Pirimor" Smoke Generators:
1972a Acute Inhalation Studies. Unpublished report from
ICI Central Toxicology Laboratories submitted to
the World Health Organization.
Riley, R.A., Pirimicarb (PP062): `Pirimor' Smoke Generators.
1972b Subacute Inhalation Studies. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A., Pirimicarb (PP062): Acute Inhalation Toxicity of
1972c `Pirimor' 50% Dispersible Powder. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Riley, R.A., Pirimicarb (PP062) Acute Inhalation Toxicity.
1973 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Riley, D., Coutts, J., Stevens, J.E. and Newby, S.E. Pirimicarb :
1974 Leaching in Soil ICI Plant Protection Ltd. Report
No. AR2556A (Unpublished).
Samuels, D.M., Hodge, M.G.E. and Palmer, S.M., Pirimicarb - Three
1975 Two Year Feeding Studies to Assess, Carcinogenic
Potential. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Teal, G. and Skidmore, M.W. Pirimicarb Investigation Of The Bound
1976 Residues On Lettuce. ICI Plant Protection Division
Report No. TMJ1323A (Unpublished).
Zweig, G. Analytical Methods For Pesticides, And Plant Growth
1975 Regulators. Academic Press, VII:399.
restricting conjugation. Small quantities of the hydroxypyrimidine
were recovered as conjugates but the major products were passed as
unconjugated metabolites in the urine of rats and dogs (Fletcher et
al., 1971e).
Two products were identified as degradation products in plants
which were not found in dog or rat urine, the 2-formyl (methylamino)
(R-34885,II) and the 2-methylamino (demethyl-pirimicarb, R34836, III)
derivatives containing the intact carbamate. While these were isolated
from plants and not animals, the probability exists that they are
formed in mammals as transitory products since the carbamate is labile
and subject to rapid cleavage. The identification of the 2-methylamino
(V) and the 2-amibo (VI) hydropyrimidine derivates of pirimicarb in
rat and dog urine suggest the same sequence of metabolism and
breakdown in animals as plants with quantitative differences in the
final products.
Effects on Enzymes and other biochemical parameters
Pirimicarb is an anticholinesterase agent and, as other
N-methyl-and N,N-dimethylcarbamates, elicits a parasympathomimetic
response in certain animals. Evaluation of reversible cholinesterase
inhibition by carbamate esters is difficult. Using an automated delta
pH method the following I50 values for some pirimicarb metabolites
were obtained (Table 1).
TABLE 1. Inhibition of cholinesterase by Pirimicarb metabolites
Metabolite I50
Molarity X 106
2-Methylamino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (III) 4.54
2-amino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (IV) 4.34
2-dimethylamino-5,6-dimethyl
-4-hydroxypyrimidine (I) 14.9 X 103
2-methylamino-5,6-dimethyl
-4-hydroxypyrimidine (V) 4.25 X 103
(Fletcher et al., 1971e)
Using a female cat, a time course study of cholinesterase
depression was run following acute poisoning by intravenous injection
of a dose of 20 mg/kg. Rapid in vivo depression of pretreatment
plasma cholinesterase activity was obtained which persisted for more
than 4´ hours, long after clinical signs of poisoning had stopped.
These results are summarized in Table 2.
TABLE 2. Symptoms and plasma cholinesterase depression in a cat
poisoned with pirimicarb
Time Signs of Poisoning Plasma Cholinesterase
(% Inhibition)
5 min. salivation, trembling 72
15 min. salivation, fibrillation 72
30 min. reduced fibrillation 73
1 hr. none 72
2 hr. 75
4´ hr. 73
(Clark, 1967b)
An attempt was made to resolve questions raised on the
reliability of the routine automated procedure used for the
determination of blood cholinesterase activity. The original method
used extended substrate incubation times and large dilutions. Both of
these factors are known to be responsible for enhancing spontaneous
reactivation of the carbamoylated enzyme. In an attempt to demonstrate
that the N,N-dimethylcarbamoylated cholinesterase is sufficiently
stable to be measured by techniques not normally employed, a shortened
incubation time (5.5 min. instead of 23 min) and reduced dilution were
used. Acetylcholine concentrations were not changed. With these
modifications a comparison of the method used routinely, a radiometric
(14C-acetylcholine) method and a modified automated delta Ph method
was made to evaluate cholinesterase depression in vivo in rats.
Rats were orally administered pirimicarb at 30 mg/kg and blood
removed after 1 hour. Dogs were administered pirimicarb at 30 mg/kg
dose and blood was sampled at one hour after treatment.
Cholinesterase depression was similar in all methods of assay
employed. In rats, male and female, plasma measured 50% inhibition by
all methods. In dogs, a comparison of the radiometric
14C-acetylcholine method with the automated delta Ph method showed no
inhibition of plasma cholinesterase at 4 mg/kg. In addition, plasma
cholinesterase from treated animals was found to be stable over a 24
hour interval at 4-5°C with no loss in inhibition spontaneously
regenerated activity).
From the foregoing study, it was concluded that a stable
carbamoylated enzyme analogous to a phosphorylated enzyme may be
formed with pirimicarb and the reduced activity can successfully be
measured by an automated delta Ph method. It was concluded that the
K3 step was slow and a stable intermediate was formed. The conclusion
of this study is that the method used in most studies to measure
cholinesterase activity did not underestimate the inhibition and give
misleading results (Litchfield, 1968).
In vitro studies in part confirmed these conclusions
especially with respect to pseudocholinesterase enzymes. The reaction
of pirimicarb and horse serum cholinesterase yielded a stable N,N-
dimethylcarbamoylated enzyme having a k3 of 5 X 10-4min-1.
However, with acetylcholinesterase (eel or RBC) the k3 is higher (3.7
X 10-2 min-1 and 0.9 X 10-2 min-1 respectively) and the spontaneous
reactivity is much greater. Thus, the use of plasma or serum
cholinesterase to assay pirimicarb inhibition would show adequate
values for the interaction of enzyme and inhibitor while the use of
RBC as other-acetylcholinesterase sources would be expected to give
misleading values especially where extended assay times or large
dilutions were a factor (Main, A.R. personal communication).
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of rats (12 male and 24 female rats/group) were fed
pirimicarb in the diet at 0,250 and 750 ppm and mated to begin a
3-generation, 2-litter per generation standard reproduction study. At
the conclusion of the study, tissues from 10 rats of each sex from the
F3b generation were examined microscopically. Four offspring were
observed to be malformed at birth (3 litter mates of the second
generation F2a - 250 ppm and one 750 ppm F2a fetus). Reduced body
weight of the parents (specifically females but often males as well)
was noted at both treatment levels. Growth of pups prior to weaning
was not affected by pirimicarb in the diet although a reduced growth
was seen thereafter in all three generations. The basic reproduction
indices normally measured (fertility, gestation, lactation and
viability) were not affected. Evaluation of parturition data suggests
no effects on the ability to maintain pregnancy. No adverse effects on
reproduction were observed with pirimicarb. Because malformations were
observed in only 4 fetuses of 2665 treated it was concluded that these
were not as a result of pirimicarb in the diet (Fletcher and Sotheran,
1971).
Special studies on carcinogenesis
Groups of mice (50 of each sex per group; SPF, Alderly Park
strain) were fed pirimicarb in the diet at levels of 0, 300 and 1500
ppm for 80 weeks. Mortality was reported to be a result of endemic
respiratory disease. The clinical condition of the mice at autopsy
confirmed a high incidence of respiratory disease. A slight body
weight reduction was noted only at the high dose level. No effects
were noted on food consumption or on gross and microscopic analysis of
most tissues and organs. The incidence of pulmonary tumors was
significantly higher in both treated groups than controls used in the
study. (When the incidence of pulmonary lesions was compared with a
large group of control mice examined in the laboratory over an 8-year
interval, the data did not show a significant increase in pulmonary
tumors as a result of pirimicarb in the diet. The controls used in
this study apparently had an extremely low incidence of lung lesion).
There was no evidence of excessive lymphoma or leukemia noted in the
study and detailed examination of the CNS did not indicate pirimicarb
to be a carcinogen in mice (Palmer and Samuels, 1974).
Groups of rats (48 males/group) were fed pirimicarb in the diet
at dose levels of 0,750 and 2,500 ppm for 2 years. Because of
respiratory infection, an antibiotic (oxytetracycline and sodium
sulphadimidine were administered in drinking water as needed.
Mortality was heavy after the 40th week predominantly as a result of
respiratory infection. Growth was reduced at 2,500 ppm but not at 750
ppm. An increased incidence of reticulum cell lymphoma was observed in
the study. It was concluded that this incidence was as a direct result
of the accompanying pulmonary infection. However, the infiltration
pattern of the tumor was mainly perivascular. A statistical treatment
of the tumor incidence did not confirm a relationship of tumor
incidence to the presence of pirimicarb in the diet.
In another study using a different strain, groups of rats (48
males/group) were fed diets for two years similar to those described
above. Again, an outbreak of respiratory disease within one year
limited the surviving animals at the conclusion of the study. However,
in contrast to the previous study, no reticulum cell lymphoma was
observed. The incidence of tumors was not different from controls. It
was concluded that pirimicarb did not produce an increased incidence
of tumors in male rats.
A third study was initiated from those animals discarded from the
three generation reproduction study. Groups of rats (24 of each
sex/group) previously exposed in utero and fed prior to weaning were
fed pirimicarb in the diet at dosage levels of 0 and 750 ppm for 2
years. Again, respiratory infection reduced survival of rats in this
study (rats were also treated with antibiotics to control the
infection). There was no difference relating to survival between the
control and treated groups. Growth of both sexes was reduced by
pirimicarb in the diet. Gross and microscopic analyses did not
indicate an increased incidence of tumors in the pirimicarb fed rats.
Based on lifetime exposure to pirimicarb from conception, there is no
carcinogenic effect noted in the rat (Samuels et al., 1975). However,
the undercurrent respiratory infection noted in all four long term
studies aimed at examining lung lesions precludes definitive
conclusions on this problem.
Special studies on teratology
Mouse
Groups of mice (25 mated females/group) were fed pirimicarb in
the diet at dosage levels of 0,40 and 500 ppm throughout pregnancy
(day 0 to day 18 of gestation). There were no parental deaths and no
abortions occurred. On sacrifice at day 18, fetuses were removed and
uteri examined for resorptions. Implantation was slightly reduced in
both pirimicarb groups. The mean litter weight and fetal weights were
reduced at the high dose level. There was no effect on the sex ratio
of fetuses.
No abnormalities were observed on gross examination or on
skeletal examination following clearing and alizarin straining
(Fletcher et al., 1971d).
Rat
Groups of rats (18-21 females/group) were fed pirimicarb in the
diet at dosages of 0, 250 and 750 ppm throughout pregnancy. Parental
growth was reduced at the high dose level. Total weight was also
reduced at the high dose level. No abnormalities were noted in the
treatment group attributable to pirimicarb in the diet (Fletcher et
al, 1971f).
Rabbit
Groups of pregnant rabbits (16 does/group) were administered
pirimicarb orally by gavage daily from day 1 to day 28 of gestation at
doses of 0,1.25, 2.50 and 5.0 mg/kg. Administration of 2.5 and 5 mg/kg
produced a maternally toxic response noted as a reduction of weight
gain. This was also reflected in reduced fetal weights which occurred
at the upper two dose levels. There were no teratogenic effects noted
on gross examination of tissue, or on skeletal examination of cleared,
stained fetuses. Pirimicarb has no teratogenic potential in rabbits. A
slight increase in the size of the brain of some litter mates was not
considered to be hydrocephalus and was not dose dependent (Hodge,
1974). On the basis of studies in three species, it appears that
pirimicarb has little teratological potential.
Special studies on mutagenesis
Mouse
Groups of mice (15 males/group) were administered pirimicarb
orally for 5 days by intubation at dosage levels of 0, 10 and 20
mg/kg. Two positive controls of 150 mg/kg or 100 mg/kg of ethyl
methanesulphonate (EMS) were administered either by a single ip
injection on the day before mating or by oral administration 5 days
prior to mating. Each male was mated to two virgin females at weekly
intervals for 8 weeks in a dominant lethal mutagenesis study. In an
evaluation of pregnancy, implantation and early and late death of
fetuses, pirimicarb treatment did not show differences from control
values. In contrast, EMS resulted in reduced pregnancy,
preimplantation loss and increased early death, judged to be a
significant mutagenic occurrence. There are no indications from this
study that pirimicarb would induce mutations in mice (McGregor, 1974).
Special studies on sensitization
Groups of guinea pigs (4 males/test group, 3 males as controls)
were treated on the ear for 3 days with a 10% (w/v) solution of either
technical or formulated pirimicarb. Four days later the animals were
challenged by applying doses of 0, 0.1, 1.0 and 10% solutions to the
shaved flank skin. Traces of erythema were noted but a primary
sensitization reaction was not observed (Parkinson, 1974c).
Special studies on inhalation toxicity
Pirimicarb has been recommended for use under enclosed glasshouse
conditions. Studies were performed on the acute inhalation toxicity
following exposure of rats to smoke generated in an enclosed chamber
at doses up to 300 mg/m3. Mortality was observed as were acute signs
of poisoning following a 6 hour exposure. Rats exposed to 75 mg/m3
for 6 hours showed clinical signs of poisoning while at a level of 15
mg/m3 no signs of poisoning over the 6 hour test exposure were
observed (Riley, 1972a). Rats exposed to 15 mg pirimicarb/m3, 6 hours
a day, 5 days/week for 3 weeks did not exhibit toxic signs of
poisoning. A slight plasma cholinesterase depression was observed
(Riley, 1972b). Acute inhalation studies on rats using a 50%
dispersible powder formulation showed that exposure for one hour at a
dust concentration of 20,000 mg/m3 caused no acute signs of
poisoning although plasma and RBC cholinesterase depression was noted
(Riley, 1972c). Rats exposed daily 5 days/week for 3 weeks (6
hours/day) to a "saturated vapor" of pirimicarb (air passed through
technical pirimicarb was not analyzed for the presence of pirimicarb)
did not display signs of poisoning or even of exposure (Clark, 1967b;
Riley, 1973).
Special studies on neurotoxicity
Four adult hens, administered a single oral dose of 25 mg
pirimicarb/kg by gavage, displayed cholinergic signs of poisoning but
no clinical indication of a delayed neurotoxic response (Clark,
1967b).
Four adult hens were administered pirimicarb by oral intubation
at a dose level of 25 mg/kg. They were observed for 21 days,
re-treated with the same dose and again observed for 21 days. No signs
of delayed neurological ataxia were observed (Fletcher, 1971a).
Special studies on potentiation
Groups of female rats (10 rats/group) were administered
pirimicarb alone and in combination with carbaryl or azinphos-methyl
orally by gavage. Slight changes in the projected LD50 values were
noted with azinphos-methyl when the materials were administered in
combination. No such changes were noted with carbaryl combinations.
There was no potentiation of the acute effects noted with simultaneous
oral administration of carbaryl while a slight potentiation of acute
toxicity was noted with azinphos-methyl (Parkinson, 1975d).
Special studies on plant metabolites
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-formylmethylamino pirimicarb
(R.34885, II) by gavage daily for 10 days at doses of 25 mg/kg/day.
Males developed a slight hypochromia, while a hypochromic anemia was
observed in females. No other toxic signs were observed. There was no
intubution of plasma, RBc or brain cholinesterase. Increased
hemopoietic activity was noted in the spleen (Fletcher et al., 1971a).
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-methylaminopirimicarb (R.34836,
III) by gavage daily for 10 days at doses of 100 mg/kg/day. Acute
signs of poisoning were noted immediately after each daily dose. These
signs abated within 2 hours. Plasma cholinesterase depression was
observed only in females. Hematological examination revealed
hypochromic anemia in females while males showed reticulocytosis and
slight hypochromia. No change in clotting time was seen. Increased
hemopoietic activity was seen in the spleen of males and females.
Changes in the thymus were also observed (Fletcher et al., 1971b).
Special studies on antidotes
Groups of female rats were administered pirimicarb by
subcutaneous injection (150 mg/kg - 2 X LD50). When signs of
poisoning were observed, atropine alone or in combination with P2S
was administered by S.C. injection. Atropine alone or in combination
with P2S was an effective antidote while P2S alone was not effective
in reducing the acute toxic effects (Clark, 1967b).
Eye and skin irritation
Technical pirimicarb and a 50% dispersible powder formulation
produced a slight occular irritation when administered to the
conjunctival sac of rabbits. One drop of a 5% solution or a suspension
of 25% (w/v) of technical pirimicarb in propylene glycol or 50% (w/v)
formulated product, instilled in the conjunctival sac, resulted in
slight pain, redness and transient slight corneal opacity. Pirimicarb
was classified as a mild irritant graded as 3-4 m a scale of 8 (Clark,
1967b; Fletcher, 1971b; Ferguson and Parkinson, 1973). Technical
pirimicarb applied to the intact or abraded skin of rabbits was
non-irritating when administered as a 25% suspension in propylene
glycol (Fletcher, 1971b; Parkinson, 1972). When a paste was made of a
50% dispersible powder formulation, pirimicarb was irritating
especially to abraded skin. No reaction was observed with intact,
undamaged skin (Parkinson, 1972). Technical pirimicarb (5% in
propyleneglycol) applied to the shaved back of rats repeatedly for 24
hours under a plastic film was not irritating to the skin (Fletcher,
1971b; Clerk 1967a).
Acute Toxicity
TABLE 3. Acute toxicity of pirimicarb
LD50
Species Sex Route (mg/kg) Reference
Rat F oral (fasted) 68 Clark, 1967a
(fed) 147-210 Clark, 1967a
F oral 101-147 Clark, 1967b;
Parkinson, 1975
F oral 165-221* Parkinson, 1972b
dermal >500 Clark, 1967b
TABLE 3. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
M&F ip 25-50 Clark, 1967b
F SC 75 Clark, 1967b
Mouse F oral 107 Clark, 1967b
Dog M&F oral 100-200 Clark, 1967b
Rabbit dermal >500 Clark, 1967b
Hen oral 25-50 Clark, 1967b
Rat inhalation approx. Riley, 1973
300 mg/m3
* pirimicarb stored at 37°C for up to 6 months showed no change in
acute toxicity value suggesting stability over this time interval.
Signs of poisoning: Within a few minutes of receiving a toxic oral
dose, signs of poisoning in a rat were observed including:
salivation, chromolacrymation, urination, defecation, miosis and
muscular fibrillations leading to fasciculations and death within
30 minutes. These are all typical signs of cholinergic
stimulation normally seen with parasympathomimetic agents.
Short term studies
Rabbit - dermal
Groups of rabbits (4 of each sex/group) were administered
pirimicarb dermally at a dose of 500 mg/kg for a 24 hour duration. The
skin was washed and the treatment repeated daily for 14 days. No
toxicity was observed and the animals appeared normal (Fletcher,
1971c).
Rat
Groups of rats (10 male and 10 female rats/group) were
administered pirimicarb daily by gavage at doses of 0 and 50 mg/kg for
10 days. A slight growth depression was noted but no mortality or
cumulative effects were seen. Hematology was normal and gross
examination of tissues and organs at the conclusion of the study
showed no adverse effects (Clark, 1967b).
TABLE 4. Acute toxicity of metabolites or impurities
LD50
Compound* Species Sex Route (mg/kg) Reference
pirimicarb Rat F oral 800-1600 Lefevre and
phenol (I) Parkinson, 1974
(R.31805)
N-methyl-N-formyl Rat F oral 50-100 Lefevre and
pirimicarb (II) Parkinson, 1974
(R.34885)
N-demethyl pirimicarb Rat F oral 200-400 Lefevre and
III) Parkinson, 1974
(R.34836)
N-di-demethyl Rat F oral 79 Parkinson, 1974a
pirimicarb (IV)
(R.35140)
N-demethyl phenol (V) Rat F oral 2000-2500 Lefevre and
(R.34865) Parkinson, 1974
N-di-demethyl phenol (VI) Rat F oral >2500 Parkinson, 1974a
(R.31680)
N,N-dimethyl Rat F oral 1455 Parkinson, 1975a
guanidine HCl
methyl guanidine SO4 Rat F oral 1105 Parkinson, 1975a
guanidine HCl Rat F oral 1105 Parkinson, 1975a
2-(dimethyl-amino)- Rat F oral 158 Parkinson, 1974b
6-methyl-4-pyrimidinyl
dimethylcarbamate
(R.42488)
2-(dimethyl-amino)-5, Rat F oral Zeta 1000 Parkinson, 1975b
6 dimethyl-4-pyrimidinyl
diethylcarbamate
(R.32444)
2-(dimethyl-amino)-5,6- Rat F oral Zeta 800 Parkinson, 1975c
dimethyl-4-pyrimidinyl
ethylmethylcarbmate
(R.33160)
* See figure 1 for structural definition of the numbered products.
Groups of rats (25 males and 25 females/group) were fed
pirimicarb in the diet for 90 days at dosage levels of 0, 250 and 750
ppm. A separate group of rats was administered pirimicarb by gavage,
daily at a dose of 25 mg/kg. There was no effect on growth, food
consumption or mortality in the dietary groups. Mortality was evident
in the rats dosed by gavage although the survivors showed normal
growth over the 90 day interval. Hematology parameters were
unaffected. A slight decline in plasma cholinesterase was observed in
the rats treated by gavage and at sporadic intervals in the 750 ppm
group. RBC and brain activity was normal. Gross and microscopic
analysis of tissues and organs examined at the conclusion of the study
was normal. Bone marrow cytology was not performed (Griffiths and
Conning, 1968).
A no-effect level of >750 ppm in the diet but lower than
25 mg/kg body weight administered by gavage was evidenced in this
study.
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
pirimicarb in the diet at dosage levels of 0, 4, 10 and 25 mg/kg for
90 days. Growth was not affected over the course of the study although
one of the male dogs at the highest level died during the course of
study. While the cause of death was not completely diagnosed, it was
probably related to anemia as the animal showed marked erythropoietic
hyperplasia and the presence of numerous nucleated red blood cells. A
macrocytic anemia was observed in two other dogs (one high and one
intermediate dose) at the conclusion of the study. Although hematology
parameters were not unusual an increased number of nucleated red blood
cells was observed in peripheral blood. Bone marrow examination showed
an increased nucleated cell count and reduced m/e ratio at all dose
levels. This abnormal count dropped slightly after a 28 day recovery
period, but was not down to normal. Serum folate, iron and vitamin
B12 levels were normal, suggesting that the hemolytic anemia was not
related to this type of deficiency.
Cholinesterase measurements showed depression of plasma enzymes
at the upper two dose levels. Brain and RBC cholinesterase activity as
measured by the delta pH method was unaffected by pirimicarb.
Gross and microscopic examinations of tissues and organs
indicated hemopoiesis in the spleen and lymph nodes of dogs showing
hematological signs of anemia. No effects on tissues and organs (other
than on bone marrow observations of all treated animals or on the
tissues and organs of the dog that died) were observed. A no-effect
level was not defined in this study. Delayed maturation of red blood
cells as evidenced by the presence of abnormal levels of nucleated red
blood cells was observed in all treated animals (Conning et al.,
1968).
Groups of dogs (4 beagle dogs of each sex/group) were fed diets
containing pirimicarb at dose levels of 0,0.4, 1.8 and 4.0 mg/kg body
weight for 90 days (the 4.0 mg/kg dose was maintained for 180 days).
There was no mortality and growth was unaffected by pirimicarb in the
diet. Clinical chemistry, hematology and urinalysis parameters were
normal. Bone marrow cytological examination again showed the presence
of nucleated red blood cell precursors at the highest dose tested. The
occurrence of these juvenile cells in marrow was not accompanied by
anemia as was evident In the previous study. Gross and microscopic
examination of other tissues and organs showed evidence of spleenic
hemopoiesis at the high dose level over the 6 month test. Other
tissues and organs were not affected. Plasma and brain cholinesterase
values were also normal (Conning et al., 1969).
In special studies to evaluate the hemolytic anemia observed in
dogs, four dogs (two male and two female) were fed pirimicarb in the
diet at levels of either 25 or 50 mg/kg for up to 110 weeks. Two of
the four dogs (1 male at 50 mg/kg and 1 female at 25 mg/kg) developed
anemia while two (1 female at 50 mg/kg and 1 male at 25 mg/kg) were
normal over the entire period. Peripheral blood showed changed in the
size and shape of red cells and the presence of some nucleated red
blood cells was observed. No abnormalities were seen in the white cell
series or platelets. Bone marrow samples showed a marked erythroid
hyperplasia. Attempts made to reduce the anemia with high therapeutic
doses of Vitamin B12, folic acid and folinic acid were negative. Red
blood cells from the anemic dogs were able to be agglutinated by
specific antigamma globulin serum while cells from normal dogs were
not. Free antibody was demonstrated in the sera of anemic dogs. The
antibody reacted with cells of the individual from which it was
removed and also with cells of normal-in-appearance (non-anemic)
pirimicarb-treated dogs. The antibody did not react with cells of
normal, untreated dogs. The presence of antibody and the demonstrated
effects of anemia were reversible (within 6 weeks) upon withdrawal of
the pirimicarb treatment. The pirimicarb treatment appears to have
been responsible for inducing changes in red blood cells of dogs
treated at higher levels. However, only some of the dogs responded
further in producing antibody that reacts with the altered cell
inducing anemia (Garner et al., 1972). In a further study to evaluate
a threshold of response of susceptible animals to pirimicarb, the two
susceptible dogs when allowed to recover from the induced-anemia were
fed levels of 1-2 mg/kg. One of the two dogs became anemic when
exposed for 14 weeks to 2 mg/kg. The other animal remained normal.
Further immunological studies using the susceptible dogs showed IgG
binding to cells of non-anemic dogs exposed to pirimicarb and
suggested that pirimicarb does not inhibit the IgG binding. It was
concluded that the antibody is not produced in direct relation to
pirimicarb alone, but possibly to a toxicant-red cell complex or to a
change induced by the toxicant (Jackson et al., 1974).
Groups of dogs (4 male and 4 female/group) were fed pirimicarb in
the diet for two years at dosage levels of 0, 0.4, 1.8 and 4.0 mg/kg
body weight. There were no effects on behaviour, growth or food
consumption over the course of the two years. Blood chemistry
including blood and brain cholinesterase and urinalysis parameters
were unaffected. Hematology was normal. The M/E ratio was decreased at
the high level of feeding at the conclusion of the study. There were
no unusual findings in bone marrow analysis although a slight increase
in a nucleated red blood cell content was evident at 4 mg/kg. Serum
iron and folio acid levels were unaffected by pirimicarb. Gross and
microscopic analysis of tissues and organs showed no effects
attributed to pirimicarb. Based on the data submitted a no-effect
level is 1.8 mg/kg body weight (Fletcher et al., 1971c.).
Long term study
Rat
Groups of rats (48 males and 48 females/group) were fed
pirimicarb in the diet at dosage levels of 0, 250, 500 and 750 ppm for
two years. There were no effects noted on survival. Growth was reduced
in females at all dose levels. There were no effects noted in males.
Total food intake of females was reduced. (Food conversion ratios
(ratio of food consumed to body weight gain) were reduced primarily in
the two higher dose groups).
No effects were noted on hematological parameters including
cholinesterase activity. Plasma cholinesterase was slightly reduced in
females. No effects were seen with RBC or brain cholinesterase. Gross
and microscopic examination of tissues and organs revealed only
reduced spleen weight of 750 ppm females. No other adverse effects
attributable to pirimicarb in the diet were noted. There was no
over-all increase in tumor incidence. An increase in the number of
localized astrocytomas in the high dosed males but not in the females
was not considered to be significant. Because of this slight effect
noted in males, a special study using males was initiated and is
summarized in the special study on carcinogenesis. A definitive
no-effect level wan not noted in this study, as dietary conversion
calculations do not support a firm conclusion of inappetance as a
basis for reduced growth. The marginal reduction of growth in females
suggests that a no-effect level is slightly below 250 ppm (Fletcher et
al., 1972).
OBSERVATIONS IN MAN
Cholinergic signs of poisoning accompanied by depression of
plasma (but not RBC) cholinesterase activity were observed in men
occupationally exposed to pirimicarb in the manufacture of formulated
products. In the course of investigating various procedures for safe
manufacturing conditions, it was observed that substantial vapor
toxicity was apparent as pirimicarb apparently volatalized at high
(65°C) temperatures and was inhaled by workers. Signs of exposure and
depression of plasma cholinesterase activity were recorded both of
which rapidly diminished with no apparent latent effects (Bagness et
al., 1975).
COMMENTS
Pirimicarb, an N,N-dimethylcarbamate ester, is rapidly absorbed
and metabolised in mammals by oxidative and/or hydrolytic pathways.
Only a few of the many potential metabolites have been isolated. Those
products containing an intact carbamate structure are as acutely toxic
as the parent molecule. Where cleavage of the ester groups occurs, the
acute toxicity is decreased markedly. With the exception of two
products observed on leaf surfaces (the N-formyl (methylamino) and
N-methylamino analogues of pirimicarb) and not in animal studies, all
other products identified are common to both metabolic pathways. It is
possible that these two are also produced as transitory products in
mammals. Pirimicarb is rapidly excreted from the body and neither
pirimicarb nor its metabolites accumulate in any tissue or organ. In
short term rat studies with the two carbamate plant metabolites,
administered at high doses, hypochromic anemia was observed. The
significance of this occurrence was not defined.
Pirimicarb is moderately toxic following acute administration to
various animal species. Enzyme kinetic studies on the in vivo and
in vitro inhibitory properties suggest that pirimicarb is a
rapidly reversible inhibitor of acetylcholinesterase as compared with
its effects on pseudo-cholinesterase. However, the stability of
inhibited (carbamoylated) plasma cholinesterase allowed an estimate to
be made of in vivo cholinesterase depression.
In several species no effects on reproduction (including
teratogenicity) were observed. A mutagenic response was negative as
evidenced by a mouse dominant lethal study. Pirimicarb did not induce
a delayed neurotoxic response, as generally noted with TOCP, in hens.
Short and long term studies in several animal species were performed.
In one long term study in the rat an increased number of astrocytomas
were noted in males, but this was not confirmed in two subsequent
studies. In a specific carcinogenic study in mice, a significant
increase in the incidence of pulmonary tumours was observed. However,
it was believed that the differences were due to an abnormally low
control value, thus negating the full evaluation of the study. None of
these studies was entirely adequate for evaluating the carcinogenic
potential, as chronic respiratory infection in each rat study limited
survival and a full interpretation of the data.
In short term studies with dogs, a dose dependent effect
manifested as hemolytic anemia changes was observed at high levels of
pirimicarb fed in the diet. Anemia did not occur in all dogs exposed
at high doses, and there was a suggestion of a sensitisation reaction.
In studies on the mechanism of action of pirimicarb with respect to
the hemolytic anemia, it was observed that an abnormal condition in
red blood cells was associated with the presence of pirimicarb. Also,
the presence of an increased number of a certain type of nucleated red
blood cells in bone marrow cytological examination, even in the
absence of anemia, suggested interference in a late stage of
development of erythrocytes induced by relatively high levels of
pirimicarb and complicated the full interpretation of the data. It was
suggested that hemolysis is caused by induction of a specific antibody
to the red blood cell following high level administration of
pirimicarb.
In a two year study with dogs of the same colony, no anemia was
observed although a dose dependent occurrence of nucleated red blood
cell precursors in the bone marrow was noted. At the low dose levels,
no effects on hematological or other parameters examined were
observed. Thus, in a susceptible species the effects noted over a
short term high level exposure were not cumulative and were not
manifested in a two year study. A similar hematological condition was
not reported in rodents exposed to pirimicarb in several long and
short term studies.
In a long term study with rats, growth depression was observed,
primarily at high levels. Marginal depression of growth was observed
in females (but not males) at the lowest dose level tested.
Calculation of food consumption was made with respect to growth. It
was concluded that growth depression may be related to inappentance
rather than being a specific toxicological effect.
In workers known to be occupationally exposed certain effects on
plasma cholinesterase activity were observed but no adverse long term
effects were noted.
The Meeting, in considering the toxicological properties of
pirimicarb, concluded with respect to the marginal growth depression
observed in rat species that a no-effect level would be slightly below
the lowest dose level tested.
The adverse effects on hematological parameters noted in the dog
were dose dependent and a no-effect level could be seen.
The carcinogenic potential of pirimicarb in rodents could not be
fully evaluated although the studies performed gave little concern to
the Meeting that pirimicarb was a potent carcinogen in the species
tested.
In consideration of both the long and short term studies in
several species a no-effect level was agreed upon from the data of the
2 year dog study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.8 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimicarb is a fast-acting, selective aphicide with contact,
translaminar and, more particularly, fumigant actions. It has proved
effective on all of the crops on which it has been tried against all
aphids including organophosphorus-resistant strains, except for
multiresistant strains of the damson hop aphid, Phorodon humuli.
Pirimicarb is harmless to useful parasites and predators, with
the exception of hoverflies. It is also of very low toxicity to bees.
This specificity makes pirimicarb a valuable chemical for use in
integrated control programmes.
It is active at rates as low as 0.125 kg ai/ha, depending on the
efficiency of crop spray cover and the species of aphid present.
Normal rates of commercial application are 0.25 kg ai/ha; but rates as
high as 0.50 kg ai/ha are recommended for some outlets. Applied as a
smoke, pirimicarb is normally effective at 15 mg/m3.
Pirimicarb possesses only limited persistence on crops.
Re-treatment may therefore be necessary after 10-14 days in those
situations where the potential for re-infestation remains high.
Pre-harvest withholding intervals range from zero for root crops, and
crops where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb was first synthesised in 1965. It was first registered
for use on peaches in Western Europe in 1969. It is now registered
world-wide for use on a wide range of crops, including fruit,
vegetables and cereals.
Use patterns in the Netherlands, South Africa, New Zealand and
Australia are summarized in Table 5.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pirimicarb has a half-life of one to three days on most crops.
Residue trials have been conducted on a wide range of crops in
numerous countries since 1967. The results of these studies are
reported by Manley (1972) and by Edwards and Dick (1976). They are
summarized in Tables 6-10. Total pyrimidine carbamate residues have
been studied, i.e. residues of pirimicarb and its two major
carbamate-containing metabolites on plants (the N-demethyl and
N-formyl (methylamino) analogues of pirimicarb, III and II, Figure I).
II is also commonly known as desmethyl-formamido-pirimicarb.
TABLE 5. Use pattern in various countries
Country Crop Formulation Rate Pre-harvest Interval
ai.
Netherlands Cucurbits (Eggplant, 10% smoke 10 g/700m3 3 days
cucumber, generator
gherkin,
melon)
Pepper 50% WP 25 g/100 l 3 days
Tomato
All other consumption 10% smoke 10 g/700 m3 1 March-31 Oct - 1 week
crops under glass generator 1 Nov-28 Feb - 2 weeks
All fruits & vegetables 50% WP 25 g/100 l 7 days
with the exception of
endive and lettuce under
glass and parsley and
celery
Sugarbeet, potatoes 50% WP 0.25 Kg/ha -
Cereals 50% WP 0.25 Kg/ha 4 weeks
South Africa Wheat WP 125 g/ha 7 days
Peaches WP 25 g/100 l 21 days
New Zealand Potatoes 250 g/220 l/ha Nil
Brassicas 250 g/200 l/ha 3 days
Australia Vegetables, fruits and hops 2 days
Leafy Vegetables (Tables 6 and 7)
The highest initial residues after spraying edible crops are
found in leafy vegetables. Lettuce contained total carbamate residues
of up to 20 mg/kg immediately after spraying at recommended use rates,
with the highest values occurring after indoor applications. However
decay rates are normally rapid (half-lives of 1-3 days outdoors) so
that after seven days in the outdoor situation or fourteen days
indoors total pyrimidine carbamate residues are normally less than
1 mg/kg.
Cucurbits and solanaceous fruits (Table 6)
A short pre-harvest withholding interval (e.g. 1-3 days) is
normally required in solanaceous fruits and cucurbits. Pirimicarb
residues in these crops can vary with the method of application and
with whether the crop is grown indoors or outside. Nevertheless after
recommended pre-harvest withholding intervals, total pyrimidine
carbamate residues are below 1 mg/kg and usually below 0.5 mg/kg. An
exception is chili peppers, grown outdoors, where residues are below 2
mg/kg after seven days.
Treatments with a smoke generator in the greenhouse give rise to
lower residues than corresponding spray treatments (Table 6).
Root crops and legumes (Table 8)
Pirimicarb does not move from the leaves into the storage organs
(roots and tubers) of root crops. Therefore root crops and tubers do
not normally contain significant pyrimidine carbamate residues after
spraying. Where residues are detected, these are due either to the
root being wholly or partly exposed to the spray (e.g. kohlrabi and
carrots) or to residues being transferred from the foliage during
sampling and/or sample preparation. Similarly, detectable residues are
not expected in peas and beans where the edible portion of the crop is
protected by pods. The low residues which sometimes occur,
<0.1 mg/kg, probably arise from inadvertent contamination during
sample preparation.
Runner and French beans exposed to the spray contained residues
up to 1.4 mg/kg immediately after spraying but decay was generally
rapid (half-life less than 1 day) so that total pyrimidine carbamate
residues after seven days were normally less than 0.5 (mg/kg).
Fruit (Table 9)
Residues in fruit are lower than in leafy vegetables. Levels are
generally below 2 mg/kg, even immediately after application. These
decay rapidly, with half-lives of 1-2 days. After seven days, total
pyrimidine carbamate residues are normally below 0.5 mg/kg and
TABLE 6. Residues of Pirimicarb And Its Two Major Carbamate-Containing Metabolites In Leafy
Vegetables, Cucurbits and Solanaceous Fruit From supervised Trials With Sprays Or
Smoke Generators
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Brussels sprouts Netherlands 0.14 1 3-4 0.11-0.13
U.K. 0.28 1 3 <0.14
0.28 3 3 0.28
U.S.A. 0.14 5-9 3 0.03-0.10
0.28 5-9 3 0.05-0.32
Broccoli U.S.A. 0.14 2-5 1 <0.05-0.71
0.28 2-5 1 <0.10-0.96
Cabbage (green) Australia 0.25* 1 7 0.21
Germany 0.30 1-2 7 0.57
New Zealand ( 0.28 1 7-8 0.11-0.30
U.K. (
U.S.A. 0.14 2-5 3 <0.05-0.08
0.28 2-5 3 <0.05-0.18
Cabbage (savoy) Germany 0.30 1 1-10 0.01
Japan 0.50* 4 7-8 0.02-0.78
Cauliflower Germany 0.30 1 7 0.03
(whole)
U.S.A. 0.14 1-7 3 <0.05
0.28 1-7 3 <0-05-0.275
Celery (outdoor) Netherlands 0.25 1 7 0.22-0.97
U.K. 0.28 1 7 0.18
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
(indoor) Netherlands 0.25 1 7 4.1
Endive Netherlands 0.28 1 7 <0.10
Lettuce (outdoor) Australia 0.28 1 0 7.5
2 1.4
5 0.23
9-16 ND**
0.56 1 0 19.6
2 2.4
5 0.33
9 0.38
13-16 ND
Germany 0.50 1 7 <0.01-0.05
U.K. 0.25* 1 7 <0.18-0.56
U.S.A. 0.14 3-7 1 <0.05-0.44
0.28 3-9 1 <0.05-0.97
(indoor) U.K. 0.25* 1 13-14 <0.18-0.47
Parsley (outdoor) Netherlands 0.25 1 15 0.15-0.65
(indoor) 0.25 1 15 4.7
Onions (salad) U.K. 0.16* 1 0 0.04-0.39
Spinach U.K. 0.50 1 7 <0.01-0.03
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Cucumbers Canada 0.28 1 3 0.04-0.13
U.K. 0.28 1 2 <0.10-0.34
Aubergines U.S.A. 0.28-0.42 7 3 <0.01
Gherkins Germany 0.3-0.5 1-3 2-3 0.01-0.05
U.K. 0.25* 1 3 0.45
Peppers (outdoor) Canada 0.14 3 3 0.05
(Bell) U.S.A. 0.14 3-6 1 0.05-0.34
0.28 4 1 0.05-0.43
(indoor) Canada 0.28 1 2 0.14
France (
Netherlands( 0.25* 1 3 <0.12-0.16
Peppers (outdoor) U.S.A. 0.14 2-10 7 0.05-0.83
(chili) 0.28 1-10 7 <0.05-1.26
Tomatoes Australia 0.25* 1 2 0.08
Canada 0.19+0.28 2 2 0.04
0.28 1 3 0.28
Germany 0.50 1-2 1 <0.01-0.04
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Japan 0.50* 2-4 1 0.10-0.61
U.K. 0.25* 2 2 <0.01-0.37
U.S.A. 0.28 7 3 <0.01
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Cucumbers Germany 30 3 1 0.29-0.32
Netherlands 20 1 0 0.32
15 1 1 0.03
U.K. 15 1 0 0.05-0.19
Endive Netherlands 12 1-2 13-14 0.02.-0.14
Lettuce Germany 14 3 14 0.46
Netherlands ( 15 1 14 0.02-0.85
U.K. (
Gherkins Germany 15-17 3 0 0.11
TABLE 6. (Cont'd.)
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Netherlands 14 1 1 0.02
Tomatoes Germany 14-16 3 0 0.01-0.08
Netherlands 13 1 1 0.01-0.02
U.K. 15 1 0 0.08-0.09
* Rate in g a.i./l
** Not detectable
consistently below 1 mg/kg. Pirimicarb does not penetrate the skin of
fruit and hence can if necessary be removed by peeling (Edwards and
Dick 1976; Manley 1969).
TABLE 7. Decline of pirimicarb residues on cabbages (New Zealand)
Application Residues in mg/kg, at intervals (days) after application
rate
No. kg a.i./ha 1 3 7 10 14 21
1 0.30 0.47 0.38 0.30 0.14 0.13 <0.06
1 0.50 0.49 0.39 0-30 0.24 0.20 <0.06
2 0.28 0.44 0.18 0.19
2 0.56 0.88 0.39 0.18
Cereals and seed crops (Tables 9 and 10)
Wheat grain analysed in husk contained total pyrimidine carbamate
residues of 1-3 mg/kg on day after application (Table 10). Half-lives
varied between one and seven days. No residues were detected in
dehusked grain 7 days after spraying.
Very low residues (<0.1 mg/kg) were detected in oil seed rape
seven days after application of pirimicarb.
FATE OF RESIDUES
In Plants
Pirimicarb is rapidly lost from plants on which it has a
half-life of 1-3 days. Volatilisation affords the primary means of
loss: the higher the ambient temperature, the greater the percentage
of pirimicarb lost by volatilisation (Manley, 1969; Bewick and Leahey,
1976; Davis and Hemingway, 1976; Hemingway and Davis, 1976).
Pirimicarb also undergoes photochemical and metabolic
degradation.
The major carbamate-containing degradation products are compounds
II and III. (Figure 1). Compound IV is only a minor
carbamate-containing degradation product. Other minor degradation
products include the water soluble hydroxypyrimidines I, V, and VI,
TABLE 8. Residues of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Root
Vegetables And Legumes From Supervised Spray Trials
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Beetroot U.K. 0.25* 1 0 0.02
Carrots
(submerged) U.K. 0.28 1 0 0.02-0.04
(tops of roots
exposed) U.K. 0.25* 1 7 0.11
Kohlrabi (roots) Germany 0.25 1 0 <0.01-0.23
0.25 2 0-10 <0.01
(leaves) Germany 0.25 1 7 0.09-0.11
0.25 2 1-10 <0.01
Parsnips U.K. 0.25* 1 0 <0.01
Potatoes Netherlands 0.25 1 0 <0.01
(aerial)
New Zealand ( 0.28 1 0 <0.01
U.K. (
U.S.A. 0.14 1-7 0 <0.01
0.28 1-7 0 <0.01
Radish Japan 0.25* 2-4 0 <0.01
Sugar beet Germany 0.30 1-2 0 <0.01-0.02
(roots)
(leaves) Germany 0.30 1-2 7-8 0.12-0.70
TABLE 8. (Cont'd.)
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Turnips U.K. 0.25* 1 0 0.04
Beans (broad) Germany 0.30 1 0 0.09
Beans (runner/ Germany 0.30 1 7 0.05
French)
U.K. 0.25* 1 7-8 <0.06.0.55
0.50 1 0 0.89-1.4
Peas Australia 0.28 4 0 0.06
Germany 0.30 1 0 0.10
Netherlands 0.25 1 0 0.03
U.K. 0.16* 1 0 <0.01
0.50 1 7 0.06-0.67
* indicates rate in terms of g ai per litre.
TABLE 9. Residues Of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Fruit, Cereals
And Seed Crops From Supervised Spray Trials
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
Apples Canada 0.25 1 7 0.01-0.60
U.K. 0.16 2 7 0.09-0.37
Blackcurrants U.K. 0.16 1 7 0.01-0.08
Oranges Australia 0.25 1 7 <0.01 (whole fruit)
<0.02 (flesh)
0.32 (peel)
Peaches Australia 0.25 1 7 0.05
Italy ( 0.25 1-3 5-6 <0.10-0.27
Spain ( 0.50 1-3 5-6 0.20-0.36
Plums Canada 0.14* 1 7 0.36
0.28* 1 7 0.38
U.K. 0.25 1 7 0.06-0.11
Raspberries U.K. 0.16 1 7 0.02-0.09
Strawberries Australia 0.25 1 0 1.5
0.25 1 7 0.23
U.K. 0.25 1 7 0.07-0.09
Wheat (in husk) Australia 0.25* 1 7 0.33
Germany 0.25* 1-2 7 0.04-1.5
TABLE 9. (Cont'd.)
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
South Africa 0.25* 1-2 7 0.27-0.52
(de-husked) Netherlands 0.125* 1 7 <0.02
Oil seed rape Australia 0.28- 1-4 7 <0.10
Czechoslovakia 0.35*
* indicates rate in terms of kg a.i./ha
and guanidine and its 1-methyl and 1,1 dimethyl derivatives. This
pattern of degradation has been shown to take place in part or
completely on peach and sugar beet leaves (Manley, 1969), lettuce
(Hemingway and Davis, 1976) and cabbage (Davis and Hemingway, 1976).
The rate of degradation varies with crop type and weather conditions.
Thus in lettuce, seven days after application of pirimicarb 71% of the
radioactivity remaining was present as pirimicarb and the carbamates
II and III, whilst in cabbage the figure was 32%.
In lettuce plants sprayed with 2-14C-labelled pirimicarb four
weeks prior to harvest, 25% of the radioactivity associated with the
plants could not be extracted by refluxing with ethanol and was termed
`bound'. Pirimicarb, compounds III and V and the three guanidines
mentioned above were all shown to be minor constituents of the bound
residue. Approximately 40% of the bound residue was associated with
the cellulose and hemicellulose of the plant (Teal and Skidmore,
1976).
When pirimicarb was introduced systemically into broad bean
plants by stem injection, its half-life was less than one day. Again
products II and III were formed initially. However these and
pirimicarb were subsequently degraded, the carbamate groups being
quantitively eliminated as measured by the evolution of 14CO2 from
plants treated with 14C-carbonyl-labelled pirimicarb (Manley, 1969).
Spraying of potatoes with 2-14C-labelled pirimicarb at 0.42 kg
ai/ha resulted in very low residues of radioactivity in the tubers
(less than 0.05 mg/kg pirimicarb equivalents). More than 80% of this
low residue was present as unextractable materials or as a complex
mixture of water-soluble materials closely associated with plant
sugars and pigments (Bowker et al, 1975).
When pirimicarb sprayed at rates up to 5 kg ai per hectare, ie
20-40 times normal use rates, total pyrimidine carbamate residues in
"follow-up" crops at harvest extremely small - of the order of the
limit of determination of the analytical method or below (Edwards et
al, 1974 and 1975). Total radiolabelled residues in "follow-up" crops
at harvest as a result of applying 2-14C-labelled pirimicarb at 0.5
kg ai per hectare were also small - up to 0.14 mg/kg pirimicarb
equivalents. In lettuce, which contained the highest radioactive
residue, approximately 50% of the activity was due to a combination of
pirimicarb, compound III, the hydroxypyrimidines I, V and the three
guanidines (Hughes and Leahey, 1974; Leahey and Benwell, 1976).
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. Only traces of residues are transferred to
milk and tissues.
TABLE 10. Pirimicarb Residues on Wheat (South Africa) at Intervals
after Application
Residues, mg/kg
Application Days
rate No. of after Demethyl-
g a.i./ha Applications Application Pirimicarb pirimicarb
250 1 1 2.3 0.4
2 1.7 0.3
4 0.4 0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.7 0.4
2 0.6 0.3
4 0.2 0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.5
2 1.6 0.2
4 0.3 <0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.8 0.4
2 0.6 0.2
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.4
2 2.3 0.4
4 0.4 <0.2
7 0.3 <0.2
55 <0.1 <0.2
125 1 1 0.6 0.3
2 0.6 0.3
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
Results are the average of 3 determinations
Recovery pirimicarb 105% at 1 mg/kg
demethyl-pirimicarb 78% at 1 mg/kg
Results not corrected for recovery
Limit of detection pirimicarb 0.1 mg/kg
demethyl-pirimicarb 0.2 mg/kg
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum radioactive residue in the milk (0.25 mg/kg
pirimicarb equivalents) was detected one hour after dosing; 80-90% of
this residue was due to the hydroxypyrimidines I, V and VI (Figure 1).
Subsequent residues were 0.06 mg/kg pirimicarb equivalents or less.
The maximum residue in fat and meat tissues after twelve days was only
0.04 mg/kg pirimicarb equivalents (Hemingway et al 1976). See also
"Biotransformation".
In soil
Pirimicarb is extensively degraded in soil. Degradation occurs
most rapidly in less acid soils. In soils of pH6.5 and above,
pirimicarb has a half-life of up to 7 weeks under aerobic conditions.
The rate of degradation in more acid soils was considerably enhanced
by air drying. Although less important than soil pH, a higher organic
matter content also appears to enhance the rate of degradation of
pirimicarb (Hill et al 1975a; Hill and Stevens 1975).
The principal route of degradation in soil is by hydrolysis of
the carbamate moiety from the pyrimidine ring. N-dealkylation of the
2-dimethylamino group has also been observed. Cleavage of the
pyrimidine ring has been demonstrated by the loss of 14CO2 from
soils treated with 2-14C-labelled pirimicarb. Hydrolysis of the
carbamate moiety results from both biological and chemical soil
activity whereas pyrimidine ring cleavage is mediated only by
biological activity. Several fungi and actinomycete cultures are
capable of metabolising pirimicarb (Hill et al 1975a; Hill and Arnold,
1974; Manley et al 1972).
Compounds I, III and V (Figure 1) are all major soil metabolites.
Minor degradation products include the carbamates II and IV and the
hydroxy pyrimidine VI (Hill et al, 1975a). Compound I is the major
product of photochemical degradation of pirimicarb on the soil surface
(84% of the residue recovered after 20 days) (Leahey and Benwall,
1974).
After two years, more than 70% of the radioactivity resulting
from the application of 2-14C-labelled pirimicarb to acid soils was
not extracted by refluxing with acetone: water (5:1). Almost all of
this bound residue was associated with the fulvic acid fraction of the
soil. Up to 60% of the bound residue was due to pirimicarb plus the
hydroxypyrimidines I and V (Hill et al 1975b).
Pirimicarb and its soil degradation products are of low mobility
in soil; lower than atrazine which is recognized as being only
moderately mobile (Riley et al 1974). There is no significant
concentration of pirimicarb in "run-off" water or eroded soil (Gouman
and Hale, 1975).
In water
Pirimicarb undergoes rapid photochemical degradation in water:
over 50% of the applied pirimicarb is degraded in 24 hours at pH5-9.
There is no significant loss of pirimicarb by volatilisation from
water. Degradation products include the pyrimidine carbamates II-IV,
the hydroxypyrimidines I, V and VI, 1,1-dimethylguanidine and
1-methylguanidine. Only traces (<1%) of guanidine itself have been
detected (Bowker and Griggs, 1974; Lincoln and Hemingway 1974).
In washing and cooking
Residues of pirimicarb and its two major carbamate metabolites II
and III on lettuce were reduced by 38-49% by washing with cold water.
In addition, during cooking approximately 60% of the pirimicarb and
52-65% of the carbamate-containing metabolite residues were
transferred from Brussels sprouts and cabbage into the cooking water.
There was no detectable conversion to other products during the
washing or cooking processes (Edwards et al, 1976).
In storage
There was no loss of residue from deep-frozen samples fortified
with pirimicarb. Details are given in "Methods of residue analysis".
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Monitoring of pirimicarb residues in the Netherlands has shown
that residues in imported grapes and in all but one sample of
home-produced endive were within the national tolerance of 1 mg/kg.
METHODS OF RESIDUE ANALYSIS
A gas-chromatographic method using a nitrogen-selective detector
to detect residues of pirimicarb plus its two major
carbamate-containing degradation products (II and III, Figure 1) is
the preferred residue method (Zweig 1975).
Crop samples are macerated with choloroform in the presence of
anhydrous sodium sulphate. The extract is subjected to a solvent
partition clean-up during which
5,6-dimethyl-2-formylmethylamino-4-pyrimidinyl dimethylcarbamate (II)
is hydrolysed to 5,6-dimethyl-2-methylamino-4-pyrimidinyl
dimethylcarbamate (III). The dried extract in ethyl acetate is
evaporated to dryness and taken up into acetone, and an aliquot of
this solution is injected into a gas chromatograph fitted with a
nitrogen detector. The size of the peaks obtained at the retention
times of pirimicarb and of compound III are used to calculate residue
levels by comparison with peaks obtained by injection of standard
solutions of the two compounds (ICI Plant Protection Ltd., 1972).
Some crop extracts may contain co-extracted interfering
compounds. These can be removed by cleanup on a column of silica gel,
prior to gas-chromatographic determination (Edwards and Dick, 1976).
The limit of determination for pirimicarb and for compound III in
crops is normally 0.01 mg/kg. Under the conditions of chromatography
described in the method, retention times for pirimicarb and compound
III were found to be 0.7 and 1.5 minutes respectively (ICI Plant
Protection Ltd, 1972).
Methanol may be used as the extraction solvent instead of
chloroform. For crops containing a high percentage of water (i.e.
normal 'green' crops), chloroform and methanol are equally efficient
(Manley, 1969; Edwards and Dick, 1975). However for crops of very low
water content (e.g. dry leaf tea, cured tobacco and oil seed rape), it
is essential to use methanol to extract residues of pirimicarb and
compounds II and III completely (Edwards and Dick, 1976).
Hydrolysis of compound II to compound III in 0.1 M hydrochloric
acid during the solvent partition cleanup was shown to be complete
(Cox, 1973).
A colorometric method, involving hydrolysis to dimethylamine, is
also available for determining residues of pirimicarb metabolites II
and III in crops (Zweig, 1975). Crop samples are macerated with
chloroform and the extract washed successively with 0.1M sodium
hydroxide, water and a slightly acid phosphate buffer (ph 4.3). The
chloroform is evaporated under reduced pressure and the residue steam
distilled with 1 M sodium hydroxide, liberating dimethylamine which
is absorbed in 0.1 M hydrochloric acid and determined
absorptiometrically as the yellow-brown copper dimethydithiocarbamate
complex at 436nm. Where troublesome emulsions occur at the washing
stages, the sample is extracted with acetone. Emulsifying agents are
removed by solvent partition; pirimicarb and its carbamate-containing
metabolites are then extracted into chloroform which is treated as the
chloroform extract obtained above.
Residues of the dimethyl dithiocarbamate fungicides (thiram and
ziram) interfere with the determination of pirimicarb and the two
metabolites by this method. Where interfering residues are present, a
chloroform extract obtained as described in the preceding paragraph is
subjected to thin layer chromatography alongside a marker spot on
silica gel GF 254. The developed chromatogram is viewed under
ultra-violet light, the primicarb band is removed and steam distilled,
and the determination completed, as described above (ICI Plant
Protection Ltd. 1969). The limit of determination of the method is
normally 0.7 mg/kg pirimicarb or pirimicarb equivalents. Recoveries in
excess of 70% are normally obtained (Bullock, 1969). The determination
of residues of pirimicarb and compound II and III is not affected by
the presence of residues of the ethylene bisdithiocarbamate fungicides
(e.g. maneb and zineb), nor by the presence of residues of carbaryl,
as none of these compounds is hydrolysed to dimethylamine.
TABLE 11. Stability Of Pirimicarb Residues In Crops Stored At -14°C
Mean residues, mg/kg
CROP
Initially After After
3 6.5
months months
Apples 0.92 0.94 0.86
Potatoes 1.00 0.79 0.81
Lettuce 0.97 1.04 0.94
Cucumbers 0.91 1.05 0.81
Pirimicarb residues are stable at -14°C. Samples of apples,
potatoes, lettuces and cucumbers were spiked with pirimicarb at
1 mg/kg, stored at -14°C for up to six months and analysed for
pirimicarb by the gaschromatographic method described above (see Table
11). Statistical analysis of the individual residue figures and of the
determined recovery values showed that there were no significant
differences among the residue values due either to storage time or to
crop (Edwards and Atreya, 1974).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national tolerances have been established for residue of
pirimicarb plus its two major carbamate containing metabolites, II and
III (Table 12).
TABLE 12. National tolerances reported to the Meeting
Country Crop Tolerance mg/kg
Australia Vegetables, 1
fruit and hops 0.5
Hungary All crops 0.5
Japan Fruit and vegetables 0.3
TABLE 12. (Cont'd.)
Country Crop Tolerance mg/kg
New Zealand All crops 0.5
Switzerland Fruit and vegetables 1
USA Potatoes 0.1
Venezuela All crops 0.5
Netherlands Fruit and vegetables 1.0
Grain 0.05
Potatoes 0.05
APPRAISAL
Pirimicarb is a fast acting, selective aphicide which is now
registered in a number of countries for use on fruit, vegetables and
other crops. It has proved effective on all crops on which it has been
tried against all aphids including organophosphorus-resistant strains,
with the exception of multi-resistant strains of Phorodon humuli.
It is harmless to useful parasites and predators, with the exception
of hoverflies, and has a very low toxicity to bees.
In addition to its contact and translaminar actions pirimicarb is
effective as a fumigant. Technical pirimicarb is not less than 95%
pure and is normally 98% pure; the impurities contain up to 1.3% of
other pyrimidine carbamates. It is formulated as a 50% dispersible
powder and a 50% dispersible pellet or grain, as a ULV formulation and
in a 10% smoke generator. The usual rates of application are 0.25 kg
a.i./ha as a crop spray and 15 mg/m3 as a smoke.
Pirimicarb possesses only limited persistence on crops and the
pre-harvest withholding intervals range from zero for root crops, and
others where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb is rapidly lost from plants; it has a half-life of 1-3
days. Most of the loss is by volatilisation, the extent of which
increases with rise in the ambient temperature, but there is some
photochemical and metabolic degradation. There are two major
metabolites containing the carbamate moiety, the N-formyl(methylamino)
(II) and 2-methylamino (III) analogues of pirimicarb, which occur in
plants. Minor degradation products include a third carbamate, 2-amino
pirimicarb, the water-soluble 2-dimethylamino-, 2-methylamino- and
2-amino-hydroxypyrimidines and the corresponding guanidines. The
recommended residue methods for pirimicarb also estimate the two major
carbamate-containing metabolites.
Use of radio-labelled material shows that some part (25% in one
experiment) of the pesticide may become bound to plant materials (i.e.
not extractable with organic solvents) but, as far as can be judged,
the more toxic carbamate compounds are not major constituents of this.
Within the plant (stem injection of broad beans), metabolic products
II and III are formed initially but subsequently degraded. Transfer to
the tubers of sprayed potatoes is small. Follow-up crops grown on
heavily sprayed land contain only very small amounts (at the
analytical limit) of determinable residue. Washing heavily treated
lettuce with cold water reduces residues of pirimicarb by about 38%
and cooking Brussels sprouts and cabbage gives a 60% reduction.
Pirimicarb is concentrated in the skin of apples and the residues are
reduced below the level of detection on peeling.
The transfer of residues of pirimicarb from animal feed to the
milk and meat of cows appears to be very small.
In soil there is substantial hydrolysis of the carbamate moiety
from the pyrimidine ring and photochemical degradation on the soil
surface also very largely produces the hydroxypyrimidine V. Pirimicarb
and its degradation products are of low mobility in soil and there is
no significant concentration of pirimicarb in run-off water or eroded
soil.
Pirimicarb is rapidly degraded photochemically in water exposed
to bright sunlight (50% in 24 hours at pH 5 to 9) but decomposition is
slow in the dark.
Adequate methods of analysis are available for regulatory
purposes. A gas-chromatographic method using a nitrogen selective
detector to detect residues of pirimicarb plus its two major carbamate
degradation products II and III (II is converted to III during the
clean-up process) is the preferred method. The limit of determination
of pirimicarb and compound III in crops by this method is 0.01 mg/kg.
Total carbamate can also be determined by a colorimetric method for
which the limit of determination is 0.1 mg/kg and the pesticide
recovery better than 70%. Modifications of clean-up etc. are available
for both methods to deal with particular problems (Zweig, 1975).
Although little information is yet available on the residue of
pirimicarb in food moving in commerce, numerous results from
supervised residue trials on crops growing in a number of countries
enable recommendations to be made for certain groups of crops. Because
the maximum residue limits recommended for many leafy vegetables are
the same it would be reasonable to have a single recommendation for
this group.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the total residue of pirimicarb, its
N-formyl(methylamino) analogue (desmethylformamido-pirimicarb) and
desmethyl-pirimicarb expressed as pirimicarb.
Commodity Temporary Maximum Note:
Residue Limit
(mg/kg) Pirimicarb has
a half-life of
Chili peppers 2 1-3 days on
plants; leafy
Apples 1 commodities
Bell peppers 1 are harvested
Broccoli 1 7 days after
Brussels sprouts 1 application,
Cabbage 1 root crops are
Cauliflower 1 harvested 0-3
Celery 1 days after
Cucumber 1 application
Eggplant 1
Endive 1
Gherkins 1
Lettuce 1
Parsley 1
Tomatoes 1
Blackcurrants 0.5
Beans (with pod) 0.5
Kohlrabi 0.5
Onions 0.5
Peaches 0.5
Plums 0.5
Raspberries 0.5
Strawberries 0.5
Peas 0.2
Rapeseed 0.2
Beans (without pod) 0.1
Beetroot 0.05*
Citrus fruits 0.05*
Parsnips 0.05*
Potatoes 0.05*
Radishes 0.05*
Sugar beet 0.05*
Turnips 0.05*
Wheat 0.05*
FURTHER WORK OR INFORMATION
Required (by 30 June 1978)
1) Studies on other species, primarily primate, to define the range
of species' susceptibility to the hematologic changes noted in
the dog.
* At or about the limit of determination.
2) Definition of the susceptibility to hemolytic anemia in the
inbred beagle strain used in toxicological studies.
3) Studies to fully define the reduced growth noted in several
rodent dietary studies.
4) Immunological studies when appropriate in animals species other
than the clog to define further the significance (and possible
range of occurrence) of the hemolytic anemia.
5) Significance of hypochromic anemia associated with rats fed high
levels of plant metabolites.
Required (by 1980)
1. Carcinogenic study in an appropriate mammalian species using a
currently acceptable protocol.
Desirable
1. Survey of industrially exposed individuals by immunological (or
serological) techniques to define the potential occurrence of
antibodies following pirimicarb exposure.
2. More information on residues in grains other than wheat, e.g.,
barley, oats, rice, maize.
REFERENCES
Bagness, J.E., Hamer, C.M. and Willis, G.A., Pirimicarb
1975 Operational Experience During Formulation.
Unpublished report from ICI Plant Protection
Division submitted to the World Health
Organization by ICI Ltd.
Bewick, D.W. and Leahey, J.P. Pirimicarb : Identification
1976 Of Material Volatilised From The Surface Of A
Treated Lettuce Plant, ICI Plant Protection Ltd.
Report No. TXJ1289A (Unpublished).
Bowker, D.M. and Griggs, B.F. Pirimicarb : Fate In Water.
1974 ICI Plant Protection Ltd. Report No. AR2492A
(Unpublished).
Bowker,D.M., Griggs, B.F. and May, M.S. Pirimicarb Fate In
197e Potatoes. ICI Plant Protection Ltd. Report No.
AR2491 AR (Unpublished).
Bullock, D.J.W. A Colorimetric Method Of Analysis for Residues Of
1969 PP062 In Food Crops, ICI Plant Protection Ltd.
Report No. AR2091 AR (Unpublished).
Clark, D.G., The Influence of Feeding on the Oral Toxicity of
1967a PP 062 and PP159. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Clark, D.G., The Acute Toxicity of Technical PP062, Dispersible
1967b Powder (J.F.2130) and PP062 Granules (J.F. 2148).
Unpublished report from ICI Central Toxicology
Laboratory submitted to the World Health
Organization by ICI Ltd.
Cox, J.H. Determination of Pirimicarb and Metabolites In Potato
1973 Tubers, ICI America Inc. Report (Unpublished).
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1968 Toxicity of PP062 - Beagle Dogs (Part 1).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1969 Toxicity of PPO62 - Beagle Dogs (Part 2).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by IGI Ltd.
Daniel, J.W. and Bratt, H., Absorption and Excretion by Rats of
1968 the Carbamate Insecticide, PP062. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Davis, J.A. and Hemingway, R.J. Pirimicarb : Fate On Cabbage. IGI
1976 Plant Protection Division Report No. TMJ1348A
(Unpublished).
Edwards, M.J. and Atreya, N.C. Pirimicarb : Storage Stability
1974 of Residues In Deep Frozen Crop Samples. ICI Plant
Protection Ltd. Report No. TMJ1090A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb : Crop Extractability
1975 Study, ICI Plant Protection Ltd. Report No.
TMJ1166A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb Residue Summary Residues
1976 In Crops From Field Trials 1973-1975. ICI Plant
Protection Division Report No. TMJ1360B (Un
published).
Edwards, M.J. Dick, J.P. and Hayward, G.J. Pirimicarb Effect of
1976 Washing and Cooking upon Residues in Lettuce and
Brassicae Crops. ICI Plant Protection Division
Report No. TMJ1346A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1974 Carry Over Study On Lettuce And Radish. ICI Plant
Protection Ltd. Report No. AR2565A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1975 Carry Over Study On Carrots, ICI Plant Protection
Ltd. Report No. TMJ1155A (Unpublished).
Ferguson, D.R. and Parkinson, G.R., Pirimicarb (PP062)
1973 Eye Irritation of Technical Grade Pirimicarb and
50% Dispersible Powder Formulation (JF 2538,
`Pirimor') Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Neurotoxicity in Hens.
1971a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Irritancy Tests. Unpublished
1971b report from ICI Central Toxicology laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Pirimicarb (PP062) : Subacute Toxicity on Rabbit
1971c Skin. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K. and Sotheran, J., Pirimicarb (PP062): Three
1971 Generation Reproduction Study in Rats. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.
1971a Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylformamidopirimicarb.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.,
1971b Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylpirimicarb. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Litchfield, M.H. and Watson, M.,
1971c Pirimicarb (PP062): Two Year Feeding Study in
Beagle Dogs. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Hodge, M.C.E. and Kinch, D.A., Pirimicarb (PP062) :
1971d Teratological Studies in the Mouse. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. Forshaw, A.M. and Monks, I.H., Pirimicarb (PP062):
1971e Excretion and Metabolism in the Dog and Rat.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Kinch, D.A. and Hodge, M.C.E. Pirimicarb (PP062) :
1971f Teratological Studies in the Rat. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. and Bratt, H., Pirimicarb (PP062). Accumulation Study
1972 in Rats. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Clapp, M.J.L., Garner, R., Litchfield, M.H. and
1972 Wilson, J., `Pirimicarb', (PP062): Two Year
Feeding Study in the Rat. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Garner, R., Phillips, C.E., and Slack, P., Pirimicarb (PP062)
1972 Pirimicarb Anaemia (A Chemically-Induced
Autoimmune Haemolytic Anaemia). Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Gowman, M.A. and Hale, S.W. Pirimicarb : Loss From Soil In
1975 Run-Off Water and Eroded Soil. ICI Plant
Protection Ltd. Report No. TMJ1102A (Unpublished).
Griffiths, D. and Conning, D.M., Ninety Day Oral Toxicity
1968 of PP062 - Albino Rats. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Hemingway, R.J. and Davis, J.A. Pirimicarb : Fate on Lettuce.
1976 ICI Plant Protection Division Report No. TMJ1324A
(Unpublished).
Hemingway, R.J. Davis, J.A. and Cleverly, B.A. Pirimicarb:
1976 Ercretion and Metabolism of a Single Oral Dose
Administered to a Cow. Unpublished report No.
AR2663A from ICI Plant Protection Division,
submitted to the World Health Organization by ICI
Ltd.
Hill, I.R. and Arnold, D.J. Pirimicarb Metabolism by Micro-Organisms
1974 Isolated from Soil. ICI Plant Protection Ltd.
Report No. TMJ1119A (Unpublished).
Hill, I.R., Arnold, D.J., Minns, E.C. and Greaves, R. Pirimicarb :
1975a Laboratory Studies Of The Degradation Of Single
Applications Of The Pesticide In Soil In the
Absence Of Plants. ICI Plant Protection Ltd.
Report No. AR2555A (Unpublished).
Hill, I.R., Harvey, B.R. and Arnold, D.J. Pirimicarb Extraction And
1975b Fractionation Of 'Bound' Residues In Soil. ICI
Plant Protection Ltd. Report No. AR2579A
(Unpublished).
Hill, I.R. and Stevens, J.E. Pirimicarb : Losses of Radioactivity
From 14C-Pirimicarb Treated Soils, During Air
Drying. ICI Plant Protection Ltd. Report No.
TMJ1143A (Unpublished).
Hodge, M.C.E., Pirimicarb (PP062): Teratological Studies in
1974 the Rabbit. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Hughes, H.E. and Leahey, J.P. Pirimicarb : Uptake of Pirimicarb And
1974 Its Metabolites From Soil By Rotational Crops. ICI
Plant Protection Ltd. Report No. AR2553A
(Unpublished).
ICI Plant Protection Ltd. Residue Analytical Method No. 15 for
1972 The Determination Of Residues Of Pirimicarb And
Its Two Major Metabolites In Crops
(Gas-chromatographic Method) (Unpublished).
ICI Plant Protection Ltd. Provisional Analytical Method No. 306/A For
The Determination Of Pirimicarb Residues In Fruits
Vegetables And Grain (Unpublished).
Jackson, J.A., Chart, I.S., and Sanderson, J.H., A Pirimicarb -
1974 Induced Haemolytic Anemia in Dogs. Report of a
Special Study. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Leahey, J.P. and Benwell, M. Pirimicarb : Photodegradation On A
1974 Soil Surface. ICI Plant Protection Ltd. Report No.
AR25504 (Unpublished).
Leahey, J.P. and Benwell, M. Pirimicarb ; Uptake From Soil And
Metabolism In Lettuce And Carrots, ICI Plant
Protection Division Report No. TMJ1331A
(Unpublished).
Lefevre, V.K. and Parkinson, G.R., Pirimicarb (PPO62;
1974 2-dimethylamino-5, 6-Dimethylpyrimidin-4-yl
Dimethylcarbamate): Acute Oral Toxicities of Plant
and Mammalian Metabolites. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Lincoln, B.A. and Hemingway, R.J. Pirimicarb : Fate In Water. ICI
1974 Plant Protection Ltd. Report No. TMJ1116A
(Unpublished).
Litchfield, M.H., The Measurement of Blood Cholinesterase Activity
1968 in Animals Dosed with 4,5
Dimethyl-2-dimethylamino-6-pyrimidinyl Dimethyl
Carbamate (PP062): Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Manley, C.A. Pyrimidine Insecticides : The Fate of Pirimicarb
1969 On Surfaces And Within The Plant ICI Plant
Protection Ltd. Report No. AR2109A (Unpublished),
18-19 and Figure 14.
Manley, C. A. Residue Summary : Pirimicarb In Crops. ICI
1972 Plant Protection Ltd. Report No. TMJ585/2
(Unpublished).
Manley, C.A., Hill, I.R., Harper, P. Black L. and Brooks J.R.
1972 Pirimicarb : Fate In Soil. ICI Plant Protection
Ltd. Report No. TMJ788AR (Unpublished).
Main, A.R. Personal communication.
1976
McGregor, D.B., Dominant Lethal Study in Mice of ICI PP062.
1974 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Palmer, S. and Samuels, D.M., Pirimicarb: 80 Week Carcinogenic
1974 Study in Mice. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Irritation Studies of Technical
1972a and Formulated Material on Normal and Wounded
Rabbit Skin. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Acute Oral Toxicity Following
1972b Storage at 37°C for Three and Six Months.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, C.R., Pirimicarb Metabolites (R.31680 and R.35140):
1974a Acute Oral Toxicity. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.42488): Acute Oral
1974b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Skin Sensitization. Unpublished report
1974c from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Parkinson, G.R., Oral Toxicities of Three Guanidine Compounds.
1975a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.32444): Acute Oral
1975b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.33160) : Acute Oral
1975c Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Potentiation Studies With
1975d Azinphos-methyl and Carbaryl. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A. Pirimicarb (PP062): "Pirimor" Smoke Generators:
1972a Acute Inhalation Studies. Unpublished report from
ICI Central Toxicology Laboratories submitted to
the World Health Organization.
Riley, R.A., Pirimicarb (PP062): `Pirimor' Smoke Generators.
1972b Subacute Inhalation Studies. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A., Pirimicarb (PP062): Acute Inhalation Toxicity of
1972c `Pirimor' 50% Dispersible Powder. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Riley, R.A., Pirimicarb (PP062) Acute Inhalation Toxicity.
1973 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Riley, D., Coutts, J., Stevens, J.E. and Newby, S.E. Pirimicarb :
1974 Leaching in Soil ICI Plant Protection Ltd. Report
No. AR2556A (Unpublished).
Samuels, D.M., Hodge, M.G.E. and Palmer, S.M., Pirimicarb - Three
1975 Two Year Feeding Studies to Assess, Carcinogenic
Potential. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Teal, G. and Skidmore, M.W. Pirimicarb Investigation Of The Bound
1976 Residues On Lettuce. ICI Plant Protection Division
Report No. TMJ1323A (Unpublished).
Zweig, G. Analytical Methods For Pesticides, And Plant Growth
1975 Regulators. Academic Press, VII:399.
 Other information on identity and properties
Primicarb is a colourless, crystalline solid. It melts at
90.5°C.
Vapour pressure (torr):
1.58 x 10-5 at 25°C
3.0 x 10-5 at 30°C
4.4. x 10-5 at 35°C
1.8 x 10-5 at 65°C
Solubility (g per 100 ml at 25°C).
Water 0.27
Methanol 23
Ethanol 25
Acetone 40
Xylene 29
Chloroform 32
Pirimicarb is stable at temperatures up to 37°C for at least
two years.
Pirimicarb forms stable salts with both organic and
inorganic acids. It is decomposed by prolonged boiling with acids
or alkalis to 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine
and dimethylamine. The hydroxypyrimidine is stable in boiling
acid and alkaline solutions and forms stable salts with both
acids and bases.
Purity
Not less than 95% pure, usually 98%. Impurities contain a
maximum of 1.3% of other pyrimidine carbamates. Formulated
products include a 50% ai. dispersible grain, 25% and 50%
dispersible powders, a 10% ai smoke generator, a 5% ai ultra-low
volume formulation and a 5% ai emulsifiable concentrate.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption Distribution and Excretion
Pirimicarb is rapidly absorbed, distributed, metabolized and
excreted in mammals. In studies with dogs and rats administered
subacute doses of 14C-labelled pirimicarb (ring or carbonyl
position), excretion was rapid. In dogs, within one day 74-86% of
the administered dose of ring labelled pirimicarb was eliminated,
primarily in the urine. Over the following 3 days, minor
quantities were released. In total, from 86 to 94% of the
administered ring labelled dose was recovered (79-88% in urine,
6-7% in feces). With carbonyl (C=0) labelled pirimicarb, only
15-26% of the dose administered to dogs was recovered,
predominantly in urine. The unrecovered product (14CO2) was
believed to be eliminated rapidly in expired air as suggested by
studies where rats were administered 14C-carbonyl-pirimicarb by
Other information on identity and properties
Primicarb is a colourless, crystalline solid. It melts at
90.5°C.
Vapour pressure (torr):
1.58 x 10-5 at 25°C
3.0 x 10-5 at 30°C
4.4. x 10-5 at 35°C
1.8 x 10-5 at 65°C
Solubility (g per 100 ml at 25°C).
Water 0.27
Methanol 23
Ethanol 25
Acetone 40
Xylene 29
Chloroform 32
Pirimicarb is stable at temperatures up to 37°C for at least
two years.
Pirimicarb forms stable salts with both organic and
inorganic acids. It is decomposed by prolonged boiling with acids
or alkalis to 2-dimethylamino-5,6-dimethyl-4-hydroxypyrimidine
and dimethylamine. The hydroxypyrimidine is stable in boiling
acid and alkaline solutions and forms stable salts with both
acids and bases.
Purity
Not less than 95% pure, usually 98%. Impurities contain a
maximum of 1.3% of other pyrimidine carbamates. Formulated
products include a 50% ai. dispersible grain, 25% and 50%
dispersible powders, a 10% ai smoke generator, a 5% ai ultra-low
volume formulation and a 5% ai emulsifiable concentrate.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption Distribution and Excretion
Pirimicarb is rapidly absorbed, distributed, metabolized and
excreted in mammals. In studies with dogs and rats administered
subacute doses of 14C-labelled pirimicarb (ring or carbonyl
position), excretion was rapid. In dogs, within one day 74-86% of
the administered dose of ring labelled pirimicarb was eliminated,
primarily in the urine. Over the following 3 days, minor
quantities were released. In total, from 86 to 94% of the
administered ring labelled dose was recovered (79-88% in urine,
6-7% in feces). With carbonyl (C=0) labelled pirimicarb, only
15-26% of the dose administered to dogs was recovered,
predominantly in urine. The unrecovered product (14CO2) was
believed to be eliminated rapidly in expired air as suggested by
studies where rats were administered 14C-carbonyl-pirimicarb by
 oral gavage or intraperitoneal injection. Over 50% of the
administered dose was expired as 14CO2 within 5 hours following
administration by either route (Fletcher et al., 1971e).
Further studies on the absorption, tissue distribution and
excretion in rats following oral or ip injection again suggested
the same pattern of rapid absorption, degradation of the
carbamate ester to CO2 and minor (15%) elimination of
radioactivity in the urine (Daniel and Bratt, 1968). There was
essentially no storage in the body of animals (rats) sacrificed 8
days after treatment. Measurement of abdominal fat from rats
orally administered pirimicarb (50 mg/IC9) and sacrificed 24
hours after treatment (either a single application or up to 4
daily treatments) showed no accumulation of pirimicarb in adipose
tissue (Fletcher and Bratt, 1972).
Pirimicarb administered to a lactating cow at a dose of 1
mg/kg was rapidly excreted in the urine (96%) and feces (4%). A
trace of material (<0.3% of the recovered dose) was observed in
milk with a maximum occurring within one hour of treatment
(Hemingway et al., 1976). Pirimicarb is not expected to
accumulate in the body following repeated exposures (Fletcher and
Bratt, 1972).
Biotransformation
Qualitative identification of the urinary metabolites from
rats and dogs administered pirimicarb by oral gavage or
intraperitoneal injection showed the same series of products. The
major metabolites were identified as the hydroxy pyrimidine
(pyrimidinol) derived from pirimicarb with modifications of the
alkyl substituents of the aromatic moiety. A proposed metabolic
scheme for the degradation of pirimicarb is seen in Figure 1
(only a few of the many compounds proposed have been isolated).
As expected oxidative and hydrolytic mechanisms act on various
components of the molecule, namely the carbonyl ester, the
N(CH3)2 of both the ring and carbamate moiety and the CH3
groups of the 5 and 6 positions of the pyrimidine nucleus.
Quantitative determination of isolated metabolites from the urine
of rats, dogs and a lactating cow show essentially the same
pattern of metabolism. The following four metabolites were
recovered from urine:
Metabolite % of dose administered
Rat Dog Cow
2-dimethylamino-5,6-dimethyl 16.3 6.4 10
-4-hydrozypyrimidine (I)
Metabolite % of dose administered
Rat Dog Cow
2-methylamino-5,6-dimethyl 40.9 20.7 41
-4-hydroxypyrimidine (V)
2-amino-5,6-dimethyl 12.9 16.5 21
-4-hydroxypyrimidine (VI)
2-dimethylamino-6-hydroxymethyl 5.7 1.8 -
-5-methyl-4-hydroxyprimidine
(VII)
The major metabolites in milk of a lactating cow were also I, V and VI
above in a similar ratio. Conjugation of hydroxypyrimidines was not
evident in these studies. There were few aglycones recovered following
treatment of urinary products with glucuronidase and chromatographic
characterization of organic soluble products. It was assumed that the
metabolites predominated in the tautomeric pyrimidone configuration,
oral gavage or intraperitoneal injection. Over 50% of the
administered dose was expired as 14CO2 within 5 hours following
administration by either route (Fletcher et al., 1971e).
Further studies on the absorption, tissue distribution and
excretion in rats following oral or ip injection again suggested
the same pattern of rapid absorption, degradation of the
carbamate ester to CO2 and minor (15%) elimination of
radioactivity in the urine (Daniel and Bratt, 1968). There was
essentially no storage in the body of animals (rats) sacrificed 8
days after treatment. Measurement of abdominal fat from rats
orally administered pirimicarb (50 mg/IC9) and sacrificed 24
hours after treatment (either a single application or up to 4
daily treatments) showed no accumulation of pirimicarb in adipose
tissue (Fletcher and Bratt, 1972).
Pirimicarb administered to a lactating cow at a dose of 1
mg/kg was rapidly excreted in the urine (96%) and feces (4%). A
trace of material (<0.3% of the recovered dose) was observed in
milk with a maximum occurring within one hour of treatment
(Hemingway et al., 1976). Pirimicarb is not expected to
accumulate in the body following repeated exposures (Fletcher and
Bratt, 1972).
Biotransformation
Qualitative identification of the urinary metabolites from
rats and dogs administered pirimicarb by oral gavage or
intraperitoneal injection showed the same series of products. The
major metabolites were identified as the hydroxy pyrimidine
(pyrimidinol) derived from pirimicarb with modifications of the
alkyl substituents of the aromatic moiety. A proposed metabolic
scheme for the degradation of pirimicarb is seen in Figure 1
(only a few of the many compounds proposed have been isolated).
As expected oxidative and hydrolytic mechanisms act on various
components of the molecule, namely the carbonyl ester, the
N(CH3)2 of both the ring and carbamate moiety and the CH3
groups of the 5 and 6 positions of the pyrimidine nucleus.
Quantitative determination of isolated metabolites from the urine
of rats, dogs and a lactating cow show essentially the same
pattern of metabolism. The following four metabolites were
recovered from urine:
Metabolite % of dose administered
Rat Dog Cow
2-dimethylamino-5,6-dimethyl 16.3 6.4 10
-4-hydrozypyrimidine (I)
Metabolite % of dose administered
Rat Dog Cow
2-methylamino-5,6-dimethyl 40.9 20.7 41
-4-hydroxypyrimidine (V)
2-amino-5,6-dimethyl 12.9 16.5 21
-4-hydroxypyrimidine (VI)
2-dimethylamino-6-hydroxymethyl 5.7 1.8 -
-5-methyl-4-hydroxyprimidine
(VII)
The major metabolites in milk of a lactating cow were also I, V and VI
above in a similar ratio. Conjugation of hydroxypyrimidines was not
evident in these studies. There were few aglycones recovered following
treatment of urinary products with glucuronidase and chromatographic
characterization of organic soluble products. It was assumed that the
metabolites predominated in the tautomeric pyrimidone configuration,
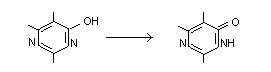 restricting conjugation. Small quantities of the hydroxypyrimidine
were recovered as conjugates but the major products were passed as
unconjugated metabolites in the urine of rats and dogs (Fletcher et
al., 1971e).
Two products were identified as degradation products in plants
which were not found in dog or rat urine, the 2-formyl (methylamino)
(R-34885,II) and the 2-methylamino (demethyl-pirimicarb, R34836, III)
derivatives containing the intact carbamate. While these were isolated
from plants and not animals, the probability exists that they are
formed in mammals as transitory products since the carbamate is labile
and subject to rapid cleavage. The identification of the 2-methylamino
(V) and the 2-amibo (VI) hydropyrimidine derivates of pirimicarb in
rat and dog urine suggest the same sequence of metabolism and
breakdown in animals as plants with quantitative differences in the
final products.
Effects on Enzymes and other biochemical parameters
Pirimicarb is an anticholinesterase agent and, as other
N-methyl-and N,N-dimethylcarbamates, elicits a parasympathomimetic
response in certain animals. Evaluation of reversible cholinesterase
inhibition by carbamate esters is difficult. Using an automated delta
pH method the following I50 values for some pirimicarb metabolites
were obtained (Table 1).
TABLE 1. Inhibition of cholinesterase by Pirimicarb metabolites
Metabolite I50
Molarity X 106
2-Methylamino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (III) 4.54
2-amino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (IV) 4.34
2-dimethylamino-5,6-dimethyl
-4-hydroxypyrimidine (I) 14.9 X 103
2-methylamino-5,6-dimethyl
-4-hydroxypyrimidine (V) 4.25 X 103
(Fletcher et al., 1971e)
Using a female cat, a time course study of cholinesterase
depression was run following acute poisoning by intravenous injection
of a dose of 20 mg/kg. Rapid in vivo depression of pretreatment
plasma cholinesterase activity was obtained which persisted for more
than 4´ hours, long after clinical signs of poisoning had stopped.
These results are summarized in Table 2.
TABLE 2. Symptoms and plasma cholinesterase depression in a cat
poisoned with pirimicarb
Time Signs of Poisoning Plasma Cholinesterase
(% Inhibition)
5 min. salivation, trembling 72
15 min. salivation, fibrillation 72
30 min. reduced fibrillation 73
1 hr. none 72
2 hr. 75
4´ hr. 73
(Clark, 1967b)
An attempt was made to resolve questions raised on the
reliability of the routine automated procedure used for the
determination of blood cholinesterase activity. The original method
used extended substrate incubation times and large dilutions. Both of
these factors are known to be responsible for enhancing spontaneous
reactivation of the carbamoylated enzyme. In an attempt to demonstrate
that the N,N-dimethylcarbamoylated cholinesterase is sufficiently
stable to be measured by techniques not normally employed, a shortened
incubation time (5.5 min. instead of 23 min) and reduced dilution were
used. Acetylcholine concentrations were not changed. With these
modifications a comparison of the method used routinely, a radiometric
(14C-acetylcholine) method and a modified automated delta Ph method
was made to evaluate cholinesterase depression in vivo in rats.
Rats were orally administered pirimicarb at 30 mg/kg and blood
removed after 1 hour. Dogs were administered pirimicarb at 30 mg/kg
dose and blood was sampled at one hour after treatment.
Cholinesterase depression was similar in all methods of assay
employed. In rats, male and female, plasma measured 50% inhibition by
all methods. In dogs, a comparison of the radiometric
14C-acetylcholine method with the automated delta Ph method showed no
inhibition of plasma cholinesterase at 4 mg/kg. In addition, plasma
cholinesterase from treated animals was found to be stable over a 24
hour interval at 4-5°C with no loss in inhibition spontaneously
regenerated activity).
From the foregoing study, it was concluded that a stable
carbamoylated enzyme analogous to a phosphorylated enzyme may be
formed with pirimicarb and the reduced activity can successfully be
measured by an automated delta Ph method. It was concluded that the
K3 step was slow and a stable intermediate was formed. The conclusion
of this study is that the method used in most studies to measure
cholinesterase activity did not underestimate the inhibition and give
misleading results (Litchfield, 1968).
In vitro studies in part confirmed these conclusions
especially with respect to pseudocholinesterase enzymes. The reaction
of pirimicarb and horse serum cholinesterase yielded a stable N,N-
dimethylcarbamoylated enzyme having a k3 of 5 X 10-4min-1.
However, with acetylcholinesterase (eel or RBC) the k3 is higher (3.7
X 10-2 min-1 and 0.9 X 10-2 min-1 respectively) and the spontaneous
reactivity is much greater. Thus, the use of plasma or serum
cholinesterase to assay pirimicarb inhibition would show adequate
values for the interaction of enzyme and inhibitor while the use of
RBC as other-acetylcholinesterase sources would be expected to give
misleading values especially where extended assay times or large
dilutions were a factor (Main, A.R. personal communication).
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of rats (12 male and 24 female rats/group) were fed
pirimicarb in the diet at 0,250 and 750 ppm and mated to begin a
3-generation, 2-litter per generation standard reproduction study. At
the conclusion of the study, tissues from 10 rats of each sex from the
F3b generation were examined microscopically. Four offspring were
observed to be malformed at birth (3 litter mates of the second
generation F2a - 250 ppm and one 750 ppm F2a fetus). Reduced body
weight of the parents (specifically females but often males as well)
was noted at both treatment levels. Growth of pups prior to weaning
was not affected by pirimicarb in the diet although a reduced growth
was seen thereafter in all three generations. The basic reproduction
indices normally measured (fertility, gestation, lactation and
viability) were not affected. Evaluation of parturition data suggests
no effects on the ability to maintain pregnancy. No adverse effects on
reproduction were observed with pirimicarb. Because malformations were
observed in only 4 fetuses of 2665 treated it was concluded that these
were not as a result of pirimicarb in the diet (Fletcher and Sotheran,
1971).
Special studies on carcinogenesis
Groups of mice (50 of each sex per group; SPF, Alderly Park
strain) were fed pirimicarb in the diet at levels of 0, 300 and 1500
ppm for 80 weeks. Mortality was reported to be a result of endemic
respiratory disease. The clinical condition of the mice at autopsy
confirmed a high incidence of respiratory disease. A slight body
weight reduction was noted only at the high dose level. No effects
were noted on food consumption or on gross and microscopic analysis of
most tissues and organs. The incidence of pulmonary tumors was
significantly higher in both treated groups than controls used in the
study. (When the incidence of pulmonary lesions was compared with a
large group of control mice examined in the laboratory over an 8-year
interval, the data did not show a significant increase in pulmonary
tumors as a result of pirimicarb in the diet. The controls used in
this study apparently had an extremely low incidence of lung lesion).
There was no evidence of excessive lymphoma or leukemia noted in the
study and detailed examination of the CNS did not indicate pirimicarb
to be a carcinogen in mice (Palmer and Samuels, 1974).
Groups of rats (48 males/group) were fed pirimicarb in the diet
at dose levels of 0,750 and 2,500 ppm for 2 years. Because of
respiratory infection, an antibiotic (oxytetracycline and sodium
sulphadimidine were administered in drinking water as needed.
Mortality was heavy after the 40th week predominantly as a result of
respiratory infection. Growth was reduced at 2,500 ppm but not at 750
ppm. An increased incidence of reticulum cell lymphoma was observed in
the study. It was concluded that this incidence was as a direct result
of the accompanying pulmonary infection. However, the infiltration
pattern of the tumor was mainly perivascular. A statistical treatment
of the tumor incidence did not confirm a relationship of tumor
incidence to the presence of pirimicarb in the diet.
In another study using a different strain, groups of rats (48
males/group) were fed diets for two years similar to those described
above. Again, an outbreak of respiratory disease within one year
limited the surviving animals at the conclusion of the study. However,
in contrast to the previous study, no reticulum cell lymphoma was
observed. The incidence of tumors was not different from controls. It
was concluded that pirimicarb did not produce an increased incidence
of tumors in male rats.
A third study was initiated from those animals discarded from the
three generation reproduction study. Groups of rats (24 of each
sex/group) previously exposed in utero and fed prior to weaning were
fed pirimicarb in the diet at dosage levels of 0 and 750 ppm for 2
years. Again, respiratory infection reduced survival of rats in this
study (rats were also treated with antibiotics to control the
infection). There was no difference relating to survival between the
control and treated groups. Growth of both sexes was reduced by
pirimicarb in the diet. Gross and microscopic analyses did not
indicate an increased incidence of tumors in the pirimicarb fed rats.
Based on lifetime exposure to pirimicarb from conception, there is no
carcinogenic effect noted in the rat (Samuels et al., 1975). However,
the undercurrent respiratory infection noted in all four long term
studies aimed at examining lung lesions precludes definitive
conclusions on this problem.
Special studies on teratology
Mouse
Groups of mice (25 mated females/group) were fed pirimicarb in
the diet at dosage levels of 0,40 and 500 ppm throughout pregnancy
(day 0 to day 18 of gestation). There were no parental deaths and no
abortions occurred. On sacrifice at day 18, fetuses were removed and
uteri examined for resorptions. Implantation was slightly reduced in
both pirimicarb groups. The mean litter weight and fetal weights were
reduced at the high dose level. There was no effect on the sex ratio
of fetuses.
No abnormalities were observed on gross examination or on
skeletal examination following clearing and alizarin straining
(Fletcher et al., 1971d).
Rat
Groups of rats (18-21 females/group) were fed pirimicarb in the
diet at dosages of 0, 250 and 750 ppm throughout pregnancy. Parental
growth was reduced at the high dose level. Total weight was also
reduced at the high dose level. No abnormalities were noted in the
treatment group attributable to pirimicarb in the diet (Fletcher et
al, 1971f).
Rabbit
Groups of pregnant rabbits (16 does/group) were administered
pirimicarb orally by gavage daily from day 1 to day 28 of gestation at
doses of 0,1.25, 2.50 and 5.0 mg/kg. Administration of 2.5 and 5 mg/kg
produced a maternally toxic response noted as a reduction of weight
gain. This was also reflected in reduced fetal weights which occurred
at the upper two dose levels. There were no teratogenic effects noted
on gross examination of tissue, or on skeletal examination of cleared,
stained fetuses. Pirimicarb has no teratogenic potential in rabbits. A
slight increase in the size of the brain of some litter mates was not
considered to be hydrocephalus and was not dose dependent (Hodge,
1974). On the basis of studies in three species, it appears that
pirimicarb has little teratological potential.
Special studies on mutagenesis
Mouse
Groups of mice (15 males/group) were administered pirimicarb
orally for 5 days by intubation at dosage levels of 0, 10 and 20
mg/kg. Two positive controls of 150 mg/kg or 100 mg/kg of ethyl
methanesulphonate (EMS) were administered either by a single ip
injection on the day before mating or by oral administration 5 days
prior to mating. Each male was mated to two virgin females at weekly
intervals for 8 weeks in a dominant lethal mutagenesis study. In an
evaluation of pregnancy, implantation and early and late death of
fetuses, pirimicarb treatment did not show differences from control
values. In contrast, EMS resulted in reduced pregnancy,
preimplantation loss and increased early death, judged to be a
significant mutagenic occurrence. There are no indications from this
study that pirimicarb would induce mutations in mice (McGregor, 1974).
Special studies on sensitization
Groups of guinea pigs (4 males/test group, 3 males as controls)
were treated on the ear for 3 days with a 10% (w/v) solution of either
technical or formulated pirimicarb. Four days later the animals were
challenged by applying doses of 0, 0.1, 1.0 and 10% solutions to the
shaved flank skin. Traces of erythema were noted but a primary
sensitization reaction was not observed (Parkinson, 1974c).
Special studies on inhalation toxicity
Pirimicarb has been recommended for use under enclosed glasshouse
conditions. Studies were performed on the acute inhalation toxicity
following exposure of rats to smoke generated in an enclosed chamber
at doses up to 300 mg/m3. Mortality was observed as were acute signs
of poisoning following a 6 hour exposure. Rats exposed to 75 mg/m3
for 6 hours showed clinical signs of poisoning while at a level of 15
mg/m3 no signs of poisoning over the 6 hour test exposure were
observed (Riley, 1972a). Rats exposed to 15 mg pirimicarb/m3, 6 hours
a day, 5 days/week for 3 weeks did not exhibit toxic signs of
poisoning. A slight plasma cholinesterase depression was observed
(Riley, 1972b). Acute inhalation studies on rats using a 50%
dispersible powder formulation showed that exposure for one hour at a
dust concentration of 20,000 mg/m3 caused no acute signs of
poisoning although plasma and RBC cholinesterase depression was noted
(Riley, 1972c). Rats exposed daily 5 days/week for 3 weeks (6
hours/day) to a "saturated vapor" of pirimicarb (air passed through
technical pirimicarb was not analyzed for the presence of pirimicarb)
did not display signs of poisoning or even of exposure (Clark, 1967b;
Riley, 1973).
Special studies on neurotoxicity
Four adult hens, administered a single oral dose of 25 mg
pirimicarb/kg by gavage, displayed cholinergic signs of poisoning but
no clinical indication of a delayed neurotoxic response (Clark,
1967b).
Four adult hens were administered pirimicarb by oral intubation
at a dose level of 25 mg/kg. They were observed for 21 days,
re-treated with the same dose and again observed for 21 days. No signs
of delayed neurological ataxia were observed (Fletcher, 1971a).
Special studies on potentiation
Groups of female rats (10 rats/group) were administered
pirimicarb alone and in combination with carbaryl or azinphos-methyl
orally by gavage. Slight changes in the projected LD50 values were
noted with azinphos-methyl when the materials were administered in
combination. No such changes were noted with carbaryl combinations.
There was no potentiation of the acute effects noted with simultaneous
oral administration of carbaryl while a slight potentiation of acute
toxicity was noted with azinphos-methyl (Parkinson, 1975d).
Special studies on plant metabolites
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-formylmethylamino pirimicarb
(R.34885, II) by gavage daily for 10 days at doses of 25 mg/kg/day.
Males developed a slight hypochromia, while a hypochromic anemia was
observed in females. No other toxic signs were observed. There was no
intubution of plasma, RBc or brain cholinesterase. Increased
hemopoietic activity was noted in the spleen (Fletcher et al., 1971a).
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-methylaminopirimicarb (R.34836,
III) by gavage daily for 10 days at doses of 100 mg/kg/day. Acute
signs of poisoning were noted immediately after each daily dose. These
signs abated within 2 hours. Plasma cholinesterase depression was
observed only in females. Hematological examination revealed
hypochromic anemia in females while males showed reticulocytosis and
slight hypochromia. No change in clotting time was seen. Increased
hemopoietic activity was seen in the spleen of males and females.
Changes in the thymus were also observed (Fletcher et al., 1971b).
Special studies on antidotes
Groups of female rats were administered pirimicarb by
subcutaneous injection (150 mg/kg - 2 X LD50). When signs of
poisoning were observed, atropine alone or in combination with P2S
was administered by S.C. injection. Atropine alone or in combination
with P2S was an effective antidote while P2S alone was not effective
in reducing the acute toxic effects (Clark, 1967b).
Eye and skin irritation
Technical pirimicarb and a 50% dispersible powder formulation
produced a slight occular irritation when administered to the
conjunctival sac of rabbits. One drop of a 5% solution or a suspension
of 25% (w/v) of technical pirimicarb in propylene glycol or 50% (w/v)
formulated product, instilled in the conjunctival sac, resulted in
slight pain, redness and transient slight corneal opacity. Pirimicarb
was classified as a mild irritant graded as 3-4 m a scale of 8 (Clark,
1967b; Fletcher, 1971b; Ferguson and Parkinson, 1973). Technical
pirimicarb applied to the intact or abraded skin of rabbits was
non-irritating when administered as a 25% suspension in propylene
glycol (Fletcher, 1971b; Parkinson, 1972). When a paste was made of a
50% dispersible powder formulation, pirimicarb was irritating
especially to abraded skin. No reaction was observed with intact,
undamaged skin (Parkinson, 1972). Technical pirimicarb (5% in
propyleneglycol) applied to the shaved back of rats repeatedly for 24
hours under a plastic film was not irritating to the skin (Fletcher,
1971b; Clerk 1967a).
Acute Toxicity
TABLE 3. Acute toxicity of pirimicarb
LD50
Species Sex Route (mg/kg) Reference
Rat F oral (fasted) 68 Clark, 1967a
(fed) 147-210 Clark, 1967a
F oral 101-147 Clark, 1967b;
Parkinson, 1975
F oral 165-221* Parkinson, 1972b
dermal >500 Clark, 1967b
TABLE 3. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
M&F ip 25-50 Clark, 1967b
F SC 75 Clark, 1967b
Mouse F oral 107 Clark, 1967b
Dog M&F oral 100-200 Clark, 1967b
Rabbit dermal >500 Clark, 1967b
Hen oral 25-50 Clark, 1967b
Rat inhalation approx. Riley, 1973
300 mg/m3
* pirimicarb stored at 37°C for up to 6 months showed no change in
acute toxicity value suggesting stability over this time interval.
Signs of poisoning: Within a few minutes of receiving a toxic oral
dose, signs of poisoning in a rat were observed including:
salivation, chromolacrymation, urination, defecation, miosis and
muscular fibrillations leading to fasciculations and death within
30 minutes. These are all typical signs of cholinergic
stimulation normally seen with parasympathomimetic agents.
Short term studies
Rabbit - dermal
Groups of rabbits (4 of each sex/group) were administered
pirimicarb dermally at a dose of 500 mg/kg for a 24 hour duration. The
skin was washed and the treatment repeated daily for 14 days. No
toxicity was observed and the animals appeared normal (Fletcher,
1971c).
Rat
Groups of rats (10 male and 10 female rats/group) were
administered pirimicarb daily by gavage at doses of 0 and 50 mg/kg for
10 days. A slight growth depression was noted but no mortality or
cumulative effects were seen. Hematology was normal and gross
examination of tissues and organs at the conclusion of the study
showed no adverse effects (Clark, 1967b).
TABLE 4. Acute toxicity of metabolites or impurities
LD50
Compound* Species Sex Route (mg/kg) Reference
pirimicarb Rat F oral 800-1600 Lefevre and
phenol (I) Parkinson, 1974
(R.31805)
N-methyl-N-formyl Rat F oral 50-100 Lefevre and
pirimicarb (II) Parkinson, 1974
(R.34885)
N-demethyl pirimicarb Rat F oral 200-400 Lefevre and
III) Parkinson, 1974
(R.34836)
N-di-demethyl Rat F oral 79 Parkinson, 1974a
pirimicarb (IV)
(R.35140)
N-demethyl phenol (V) Rat F oral 2000-2500 Lefevre and
(R.34865) Parkinson, 1974
N-di-demethyl phenol (VI) Rat F oral >2500 Parkinson, 1974a
(R.31680)
N,N-dimethyl Rat F oral 1455 Parkinson, 1975a
guanidine HCl
methyl guanidine SO4 Rat F oral 1105 Parkinson, 1975a
guanidine HCl Rat F oral 1105 Parkinson, 1975a
2-(dimethyl-amino)- Rat F oral 158 Parkinson, 1974b
6-methyl-4-pyrimidinyl
dimethylcarbamate
(R.42488)
2-(dimethyl-amino)-5, Rat F oral Zeta 1000 Parkinson, 1975b
6 dimethyl-4-pyrimidinyl
diethylcarbamate
(R.32444)
2-(dimethyl-amino)-5,6- Rat F oral Zeta 800 Parkinson, 1975c
dimethyl-4-pyrimidinyl
ethylmethylcarbmate
(R.33160)
* See figure 1 for structural definition of the numbered products.
Groups of rats (25 males and 25 females/group) were fed
pirimicarb in the diet for 90 days at dosage levels of 0, 250 and 750
ppm. A separate group of rats was administered pirimicarb by gavage,
daily at a dose of 25 mg/kg. There was no effect on growth, food
consumption or mortality in the dietary groups. Mortality was evident
in the rats dosed by gavage although the survivors showed normal
growth over the 90 day interval. Hematology parameters were
unaffected. A slight decline in plasma cholinesterase was observed in
the rats treated by gavage and at sporadic intervals in the 750 ppm
group. RBC and brain activity was normal. Gross and microscopic
analysis of tissues and organs examined at the conclusion of the study
was normal. Bone marrow cytology was not performed (Griffiths and
Conning, 1968).
A no-effect level of >750 ppm in the diet but lower than
25 mg/kg body weight administered by gavage was evidenced in this
study.
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
pirimicarb in the diet at dosage levels of 0, 4, 10 and 25 mg/kg for
90 days. Growth was not affected over the course of the study although
one of the male dogs at the highest level died during the course of
study. While the cause of death was not completely diagnosed, it was
probably related to anemia as the animal showed marked erythropoietic
hyperplasia and the presence of numerous nucleated red blood cells. A
macrocytic anemia was observed in two other dogs (one high and one
intermediate dose) at the conclusion of the study. Although hematology
parameters were not unusual an increased number of nucleated red blood
cells was observed in peripheral blood. Bone marrow examination showed
an increased nucleated cell count and reduced m/e ratio at all dose
levels. This abnormal count dropped slightly after a 28 day recovery
period, but was not down to normal. Serum folate, iron and vitamin
B12 levels were normal, suggesting that the hemolytic anemia was not
related to this type of deficiency.
Cholinesterase measurements showed depression of plasma enzymes
at the upper two dose levels. Brain and RBC cholinesterase activity as
measured by the delta pH method was unaffected by pirimicarb.
Gross and microscopic examinations of tissues and organs
indicated hemopoiesis in the spleen and lymph nodes of dogs showing
hematological signs of anemia. No effects on tissues and organs (other
than on bone marrow observations of all treated animals or on the
tissues and organs of the dog that died) were observed. A no-effect
level was not defined in this study. Delayed maturation of red blood
cells as evidenced by the presence of abnormal levels of nucleated red
blood cells was observed in all treated animals (Conning et al.,
1968).
Groups of dogs (4 beagle dogs of each sex/group) were fed diets
containing pirimicarb at dose levels of 0,0.4, 1.8 and 4.0 mg/kg body
weight for 90 days (the 4.0 mg/kg dose was maintained for 180 days).
There was no mortality and growth was unaffected by pirimicarb in the
diet. Clinical chemistry, hematology and urinalysis parameters were
normal. Bone marrow cytological examination again showed the presence
of nucleated red blood cell precursors at the highest dose tested. The
occurrence of these juvenile cells in marrow was not accompanied by
anemia as was evident In the previous study. Gross and microscopic
examination of other tissues and organs showed evidence of spleenic
hemopoiesis at the high dose level over the 6 month test. Other
tissues and organs were not affected. Plasma and brain cholinesterase
values were also normal (Conning et al., 1969).
In special studies to evaluate the hemolytic anemia observed in
dogs, four dogs (two male and two female) were fed pirimicarb in the
diet at levels of either 25 or 50 mg/kg for up to 110 weeks. Two of
the four dogs (1 male at 50 mg/kg and 1 female at 25 mg/kg) developed
anemia while two (1 female at 50 mg/kg and 1 male at 25 mg/kg) were
normal over the entire period. Peripheral blood showed changed in the
size and shape of red cells and the presence of some nucleated red
blood cells was observed. No abnormalities were seen in the white cell
series or platelets. Bone marrow samples showed a marked erythroid
hyperplasia. Attempts made to reduce the anemia with high therapeutic
doses of Vitamin B12, folic acid and folinic acid were negative. Red
blood cells from the anemic dogs were able to be agglutinated by
specific antigamma globulin serum while cells from normal dogs were
not. Free antibody was demonstrated in the sera of anemic dogs. The
antibody reacted with cells of the individual from which it was
removed and also with cells of normal-in-appearance (non-anemic)
pirimicarb-treated dogs. The antibody did not react with cells of
normal, untreated dogs. The presence of antibody and the demonstrated
effects of anemia were reversible (within 6 weeks) upon withdrawal of
the pirimicarb treatment. The pirimicarb treatment appears to have
been responsible for inducing changes in red blood cells of dogs
treated at higher levels. However, only some of the dogs responded
further in producing antibody that reacts with the altered cell
inducing anemia (Garner et al., 1972). In a further study to evaluate
a threshold of response of susceptible animals to pirimicarb, the two
susceptible dogs when allowed to recover from the induced-anemia were
fed levels of 1-2 mg/kg. One of the two dogs became anemic when
exposed for 14 weeks to 2 mg/kg. The other animal remained normal.
Further immunological studies using the susceptible dogs showed IgG
binding to cells of non-anemic dogs exposed to pirimicarb and
suggested that pirimicarb does not inhibit the IgG binding. It was
concluded that the antibody is not produced in direct relation to
pirimicarb alone, but possibly to a toxicant-red cell complex or to a
change induced by the toxicant (Jackson et al., 1974).
Groups of dogs (4 male and 4 female/group) were fed pirimicarb in
the diet for two years at dosage levels of 0, 0.4, 1.8 and 4.0 mg/kg
body weight. There were no effects on behaviour, growth or food
consumption over the course of the two years. Blood chemistry
including blood and brain cholinesterase and urinalysis parameters
were unaffected. Hematology was normal. The M/E ratio was decreased at
the high level of feeding at the conclusion of the study. There were
no unusual findings in bone marrow analysis although a slight increase
in a nucleated red blood cell content was evident at 4 mg/kg. Serum
iron and folio acid levels were unaffected by pirimicarb. Gross and
microscopic analysis of tissues and organs showed no effects
attributed to pirimicarb. Based on the data submitted a no-effect
level is 1.8 mg/kg body weight (Fletcher et al., 1971c.).
Long term study
Rat
Groups of rats (48 males and 48 females/group) were fed
pirimicarb in the diet at dosage levels of 0, 250, 500 and 750 ppm for
two years. There were no effects noted on survival. Growth was reduced
in females at all dose levels. There were no effects noted in males.
Total food intake of females was reduced. (Food conversion ratios
(ratio of food consumed to body weight gain) were reduced primarily in
the two higher dose groups).
No effects were noted on hematological parameters including
cholinesterase activity. Plasma cholinesterase was slightly reduced in
females. No effects were seen with RBC or brain cholinesterase. Gross
and microscopic examination of tissues and organs revealed only
reduced spleen weight of 750 ppm females. No other adverse effects
attributable to pirimicarb in the diet were noted. There was no
over-all increase in tumor incidence. An increase in the number of
localized astrocytomas in the high dosed males but not in the females
was not considered to be significant. Because of this slight effect
noted in males, a special study using males was initiated and is
summarized in the special study on carcinogenesis. A definitive
no-effect level wan not noted in this study, as dietary conversion
calculations do not support a firm conclusion of inappetance as a
basis for reduced growth. The marginal reduction of growth in females
suggests that a no-effect level is slightly below 250 ppm (Fletcher et
al., 1972).
OBSERVATIONS IN MAN
Cholinergic signs of poisoning accompanied by depression of
plasma (but not RBC) cholinesterase activity were observed in men
occupationally exposed to pirimicarb in the manufacture of formulated
products. In the course of investigating various procedures for safe
manufacturing conditions, it was observed that substantial vapor
toxicity was apparent as pirimicarb apparently volatalized at high
(65°C) temperatures and was inhaled by workers. Signs of exposure and
depression of plasma cholinesterase activity were recorded both of
which rapidly diminished with no apparent latent effects (Bagness et
al., 1975).
COMMENTS
Pirimicarb, an N,N-dimethylcarbamate ester, is rapidly absorbed
and metabolised in mammals by oxidative and/or hydrolytic pathways.
Only a few of the many potential metabolites have been isolated. Those
products containing an intact carbamate structure are as acutely toxic
as the parent molecule. Where cleavage of the ester groups occurs, the
acute toxicity is decreased markedly. With the exception of two
products observed on leaf surfaces (the N-formyl (methylamino) and
N-methylamino analogues of pirimicarb) and not in animal studies, all
other products identified are common to both metabolic pathways. It is
possible that these two are also produced as transitory products in
mammals. Pirimicarb is rapidly excreted from the body and neither
pirimicarb nor its metabolites accumulate in any tissue or organ. In
short term rat studies with the two carbamate plant metabolites,
administered at high doses, hypochromic anemia was observed. The
significance of this occurrence was not defined.
Pirimicarb is moderately toxic following acute administration to
various animal species. Enzyme kinetic studies on the in vivo and
in vitro inhibitory properties suggest that pirimicarb is a
rapidly reversible inhibitor of acetylcholinesterase as compared with
its effects on pseudo-cholinesterase. However, the stability of
inhibited (carbamoylated) plasma cholinesterase allowed an estimate to
be made of in vivo cholinesterase depression.
In several species no effects on reproduction (including
teratogenicity) were observed. A mutagenic response was negative as
evidenced by a mouse dominant lethal study. Pirimicarb did not induce
a delayed neurotoxic response, as generally noted with TOCP, in hens.
Short and long term studies in several animal species were performed.
In one long term study in the rat an increased number of astrocytomas
were noted in males, but this was not confirmed in two subsequent
studies. In a specific carcinogenic study in mice, a significant
increase in the incidence of pulmonary tumours was observed. However,
it was believed that the differences were due to an abnormally low
control value, thus negating the full evaluation of the study. None of
these studies was entirely adequate for evaluating the carcinogenic
potential, as chronic respiratory infection in each rat study limited
survival and a full interpretation of the data.
In short term studies with dogs, a dose dependent effect
manifested as hemolytic anemia changes was observed at high levels of
pirimicarb fed in the diet. Anemia did not occur in all dogs exposed
at high doses, and there was a suggestion of a sensitisation reaction.
In studies on the mechanism of action of pirimicarb with respect to
the hemolytic anemia, it was observed that an abnormal condition in
red blood cells was associated with the presence of pirimicarb. Also,
the presence of an increased number of a certain type of nucleated red
blood cells in bone marrow cytological examination, even in the
absence of anemia, suggested interference in a late stage of
development of erythrocytes induced by relatively high levels of
pirimicarb and complicated the full interpretation of the data. It was
suggested that hemolysis is caused by induction of a specific antibody
to the red blood cell following high level administration of
pirimicarb.
In a two year study with dogs of the same colony, no anemia was
observed although a dose dependent occurrence of nucleated red blood
cell precursors in the bone marrow was noted. At the low dose levels,
no effects on hematological or other parameters examined were
observed. Thus, in a susceptible species the effects noted over a
short term high level exposure were not cumulative and were not
manifested in a two year study. A similar hematological condition was
not reported in rodents exposed to pirimicarb in several long and
short term studies.
In a long term study with rats, growth depression was observed,
primarily at high levels. Marginal depression of growth was observed
in females (but not males) at the lowest dose level tested.
Calculation of food consumption was made with respect to growth. It
was concluded that growth depression may be related to inappentance
rather than being a specific toxicological effect.
In workers known to be occupationally exposed certain effects on
plasma cholinesterase activity were observed but no adverse long term
effects were noted.
The Meeting, in considering the toxicological properties of
pirimicarb, concluded with respect to the marginal growth depression
observed in rat species that a no-effect level would be slightly below
the lowest dose level tested.
The adverse effects on hematological parameters noted in the dog
were dose dependent and a no-effect level could be seen.
The carcinogenic potential of pirimicarb in rodents could not be
fully evaluated although the studies performed gave little concern to
the Meeting that pirimicarb was a potent carcinogen in the species
tested.
In consideration of both the long and short term studies in
several species a no-effect level was agreed upon from the data of the
2 year dog study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.8 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimicarb is a fast-acting, selective aphicide with contact,
translaminar and, more particularly, fumigant actions. It has proved
effective on all of the crops on which it has been tried against all
aphids including organophosphorus-resistant strains, except for
multiresistant strains of the damson hop aphid, Phorodon humuli.
Pirimicarb is harmless to useful parasites and predators, with
the exception of hoverflies. It is also of very low toxicity to bees.
This specificity makes pirimicarb a valuable chemical for use in
integrated control programmes.
It is active at rates as low as 0.125 kg ai/ha, depending on the
efficiency of crop spray cover and the species of aphid present.
Normal rates of commercial application are 0.25 kg ai/ha; but rates as
high as 0.50 kg ai/ha are recommended for some outlets. Applied as a
smoke, pirimicarb is normally effective at 15 mg/m3.
Pirimicarb possesses only limited persistence on crops.
Re-treatment may therefore be necessary after 10-14 days in those
situations where the potential for re-infestation remains high.
Pre-harvest withholding intervals range from zero for root crops, and
crops where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb was first synthesised in 1965. It was first registered
for use on peaches in Western Europe in 1969. It is now registered
world-wide for use on a wide range of crops, including fruit,
vegetables and cereals.
Use patterns in the Netherlands, South Africa, New Zealand and
Australia are summarized in Table 5.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pirimicarb has a half-life of one to three days on most crops.
Residue trials have been conducted on a wide range of crops in
numerous countries since 1967. The results of these studies are
reported by Manley (1972) and by Edwards and Dick (1976). They are
summarized in Tables 6-10. Total pyrimidine carbamate residues have
been studied, i.e. residues of pirimicarb and its two major
carbamate-containing metabolites on plants (the N-demethyl and
N-formyl (methylamino) analogues of pirimicarb, III and II, Figure I).
II is also commonly known as desmethyl-formamido-pirimicarb.
TABLE 5. Use pattern in various countries
Country Crop Formulation Rate Pre-harvest Interval
ai.
Netherlands Cucurbits (Eggplant, 10% smoke 10 g/700m3 3 days
cucumber, generator
gherkin,
melon)
Pepper 50% WP 25 g/100 l 3 days
Tomato
All other consumption 10% smoke 10 g/700 m3 1 March-31 Oct - 1 week
crops under glass generator 1 Nov-28 Feb - 2 weeks
All fruits & vegetables 50% WP 25 g/100 l 7 days
with the exception of
endive and lettuce under
glass and parsley and
celery
Sugarbeet, potatoes 50% WP 0.25 Kg/ha -
Cereals 50% WP 0.25 Kg/ha 4 weeks
South Africa Wheat WP 125 g/ha 7 days
Peaches WP 25 g/100 l 21 days
New Zealand Potatoes 250 g/220 l/ha Nil
Brassicas 250 g/200 l/ha 3 days
Australia Vegetables, fruits and hops 2 days
Leafy Vegetables (Tables 6 and 7)
The highest initial residues after spraying edible crops are
found in leafy vegetables. Lettuce contained total carbamate residues
of up to 20 mg/kg immediately after spraying at recommended use rates,
with the highest values occurring after indoor applications. However
decay rates are normally rapid (half-lives of 1-3 days outdoors) so
that after seven days in the outdoor situation or fourteen days
indoors total pyrimidine carbamate residues are normally less than
1 mg/kg.
Cucurbits and solanaceous fruits (Table 6)
A short pre-harvest withholding interval (e.g. 1-3 days) is
normally required in solanaceous fruits and cucurbits. Pirimicarb
residues in these crops can vary with the method of application and
with whether the crop is grown indoors or outside. Nevertheless after
recommended pre-harvest withholding intervals, total pyrimidine
carbamate residues are below 1 mg/kg and usually below 0.5 mg/kg. An
exception is chili peppers, grown outdoors, where residues are below 2
mg/kg after seven days.
Treatments with a smoke generator in the greenhouse give rise to
lower residues than corresponding spray treatments (Table 6).
Root crops and legumes (Table 8)
Pirimicarb does not move from the leaves into the storage organs
(roots and tubers) of root crops. Therefore root crops and tubers do
not normally contain significant pyrimidine carbamate residues after
spraying. Where residues are detected, these are due either to the
root being wholly or partly exposed to the spray (e.g. kohlrabi and
carrots) or to residues being transferred from the foliage during
sampling and/or sample preparation. Similarly, detectable residues are
not expected in peas and beans where the edible portion of the crop is
protected by pods. The low residues which sometimes occur,
<0.1 mg/kg, probably arise from inadvertent contamination during
sample preparation.
Runner and French beans exposed to the spray contained residues
up to 1.4 mg/kg immediately after spraying but decay was generally
rapid (half-life less than 1 day) so that total pyrimidine carbamate
residues after seven days were normally less than 0.5 (mg/kg).
Fruit (Table 9)
Residues in fruit are lower than in leafy vegetables. Levels are
generally below 2 mg/kg, even immediately after application. These
decay rapidly, with half-lives of 1-2 days. After seven days, total
pyrimidine carbamate residues are normally below 0.5 mg/kg and
TABLE 6. Residues of Pirimicarb And Its Two Major Carbamate-Containing Metabolites In Leafy
Vegetables, Cucurbits and Solanaceous Fruit From supervised Trials With Sprays Or
Smoke Generators
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Brussels sprouts Netherlands 0.14 1 3-4 0.11-0.13
U.K. 0.28 1 3 <0.14
0.28 3 3 0.28
U.S.A. 0.14 5-9 3 0.03-0.10
0.28 5-9 3 0.05-0.32
Broccoli U.S.A. 0.14 2-5 1 <0.05-0.71
0.28 2-5 1 <0.10-0.96
Cabbage (green) Australia 0.25* 1 7 0.21
Germany 0.30 1-2 7 0.57
New Zealand ( 0.28 1 7-8 0.11-0.30
U.K. (
U.S.A. 0.14 2-5 3 <0.05-0.08
0.28 2-5 3 <0.05-0.18
Cabbage (savoy) Germany 0.30 1 1-10 0.01
Japan 0.50* 4 7-8 0.02-0.78
Cauliflower Germany 0.30 1 7 0.03
(whole)
U.S.A. 0.14 1-7 3 <0.05
0.28 1-7 3 <0-05-0.275
Celery (outdoor) Netherlands 0.25 1 7 0.22-0.97
U.K. 0.28 1 7 0.18
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
(indoor) Netherlands 0.25 1 7 4.1
Endive Netherlands 0.28 1 7 <0.10
Lettuce (outdoor) Australia 0.28 1 0 7.5
2 1.4
5 0.23
9-16 ND**
0.56 1 0 19.6
2 2.4
5 0.33
9 0.38
13-16 ND
Germany 0.50 1 7 <0.01-0.05
U.K. 0.25* 1 7 <0.18-0.56
U.S.A. 0.14 3-7 1 <0.05-0.44
0.28 3-9 1 <0.05-0.97
(indoor) U.K. 0.25* 1 13-14 <0.18-0.47
Parsley (outdoor) Netherlands 0.25 1 15 0.15-0.65
(indoor) 0.25 1 15 4.7
Onions (salad) U.K. 0.16* 1 0 0.04-0.39
Spinach U.K. 0.50 1 7 <0.01-0.03
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Cucumbers Canada 0.28 1 3 0.04-0.13
U.K. 0.28 1 2 <0.10-0.34
Aubergines U.S.A. 0.28-0.42 7 3 <0.01
Gherkins Germany 0.3-0.5 1-3 2-3 0.01-0.05
U.K. 0.25* 1 3 0.45
Peppers (outdoor) Canada 0.14 3 3 0.05
(Bell) U.S.A. 0.14 3-6 1 0.05-0.34
0.28 4 1 0.05-0.43
(indoor) Canada 0.28 1 2 0.14
France (
Netherlands( 0.25* 1 3 <0.12-0.16
Peppers (outdoor) U.S.A. 0.14 2-10 7 0.05-0.83
(chili) 0.28 1-10 7 <0.05-1.26
Tomatoes Australia 0.25* 1 2 0.08
Canada 0.19+0.28 2 2 0.04
0.28 1 3 0.28
Germany 0.50 1-2 1 <0.01-0.04
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Japan 0.50* 2-4 1 0.10-0.61
U.K. 0.25* 2 2 <0.01-0.37
U.S.A. 0.28 7 3 <0.01
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Cucumbers Germany 30 3 1 0.29-0.32
Netherlands 20 1 0 0.32
15 1 1 0.03
U.K. 15 1 0 0.05-0.19
Endive Netherlands 12 1-2 13-14 0.02.-0.14
Lettuce Germany 14 3 14 0.46
Netherlands ( 15 1 14 0.02-0.85
U.K. (
Gherkins Germany 15-17 3 0 0.11
TABLE 6. (Cont'd.)
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Netherlands 14 1 1 0.02
Tomatoes Germany 14-16 3 0 0.01-0.08
Netherlands 13 1 1 0.01-0.02
U.K. 15 1 0 0.08-0.09
* Rate in g a.i./l
** Not detectable
consistently below 1 mg/kg. Pirimicarb does not penetrate the skin of
fruit and hence can if necessary be removed by peeling (Edwards and
Dick 1976; Manley 1969).
TABLE 7. Decline of pirimicarb residues on cabbages (New Zealand)
Application Residues in mg/kg, at intervals (days) after application
rate
No. kg a.i./ha 1 3 7 10 14 21
1 0.30 0.47 0.38 0.30 0.14 0.13 <0.06
1 0.50 0.49 0.39 0-30 0.24 0.20 <0.06
2 0.28 0.44 0.18 0.19
2 0.56 0.88 0.39 0.18
Cereals and seed crops (Tables 9 and 10)
Wheat grain analysed in husk contained total pyrimidine carbamate
residues of 1-3 mg/kg on day after application (Table 10). Half-lives
varied between one and seven days. No residues were detected in
dehusked grain 7 days after spraying.
Very low residues (<0.1 mg/kg) were detected in oil seed rape
seven days after application of pirimicarb.
FATE OF RESIDUES
In Plants
Pirimicarb is rapidly lost from plants on which it has a
half-life of 1-3 days. Volatilisation affords the primary means of
loss: the higher the ambient temperature, the greater the percentage
of pirimicarb lost by volatilisation (Manley, 1969; Bewick and Leahey,
1976; Davis and Hemingway, 1976; Hemingway and Davis, 1976).
Pirimicarb also undergoes photochemical and metabolic
degradation.
The major carbamate-containing degradation products are compounds
II and III. (Figure 1). Compound IV is only a minor
carbamate-containing degradation product. Other minor degradation
products include the water soluble hydroxypyrimidines I, V, and VI,
TABLE 8. Residues of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Root
Vegetables And Legumes From Supervised Spray Trials
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Beetroot U.K. 0.25* 1 0 0.02
Carrots
(submerged) U.K. 0.28 1 0 0.02-0.04
(tops of roots
exposed) U.K. 0.25* 1 7 0.11
Kohlrabi (roots) Germany 0.25 1 0 <0.01-0.23
0.25 2 0-10 <0.01
(leaves) Germany 0.25 1 7 0.09-0.11
0.25 2 1-10 <0.01
Parsnips U.K. 0.25* 1 0 <0.01
Potatoes Netherlands 0.25 1 0 <0.01
(aerial)
New Zealand ( 0.28 1 0 <0.01
U.K. (
U.S.A. 0.14 1-7 0 <0.01
0.28 1-7 0 <0.01
Radish Japan 0.25* 2-4 0 <0.01
Sugar beet Germany 0.30 1-2 0 <0.01-0.02
(roots)
(leaves) Germany 0.30 1-2 7-8 0.12-0.70
TABLE 8. (Cont'd.)
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Turnips U.K. 0.25* 1 0 0.04
Beans (broad) Germany 0.30 1 0 0.09
Beans (runner/ Germany 0.30 1 7 0.05
French)
U.K. 0.25* 1 7-8 <0.06.0.55
0.50 1 0 0.89-1.4
Peas Australia 0.28 4 0 0.06
Germany 0.30 1 0 0.10
Netherlands 0.25 1 0 0.03
U.K. 0.16* 1 0 <0.01
0.50 1 7 0.06-0.67
* indicates rate in terms of g ai per litre.
TABLE 9. Residues Of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Fruit, Cereals
And Seed Crops From Supervised Spray Trials
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
Apples Canada 0.25 1 7 0.01-0.60
U.K. 0.16 2 7 0.09-0.37
Blackcurrants U.K. 0.16 1 7 0.01-0.08
Oranges Australia 0.25 1 7 <0.01 (whole fruit)
<0.02 (flesh)
0.32 (peel)
Peaches Australia 0.25 1 7 0.05
Italy ( 0.25 1-3 5-6 <0.10-0.27
Spain ( 0.50 1-3 5-6 0.20-0.36
Plums Canada 0.14* 1 7 0.36
0.28* 1 7 0.38
U.K. 0.25 1 7 0.06-0.11
Raspberries U.K. 0.16 1 7 0.02-0.09
Strawberries Australia 0.25 1 0 1.5
0.25 1 7 0.23
U.K. 0.25 1 7 0.07-0.09
Wheat (in husk) Australia 0.25* 1 7 0.33
Germany 0.25* 1-2 7 0.04-1.5
TABLE 9. (Cont'd.)
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
South Africa 0.25* 1-2 7 0.27-0.52
(de-husked) Netherlands 0.125* 1 7 <0.02
Oil seed rape Australia 0.28- 1-4 7 <0.10
Czechoslovakia 0.35*
* indicates rate in terms of kg a.i./ha
and guanidine and its 1-methyl and 1,1 dimethyl derivatives. This
pattern of degradation has been shown to take place in part or
completely on peach and sugar beet leaves (Manley, 1969), lettuce
(Hemingway and Davis, 1976) and cabbage (Davis and Hemingway, 1976).
The rate of degradation varies with crop type and weather conditions.
Thus in lettuce, seven days after application of pirimicarb 71% of the
radioactivity remaining was present as pirimicarb and the carbamates
II and III, whilst in cabbage the figure was 32%.
In lettuce plants sprayed with 2-14C-labelled pirimicarb four
weeks prior to harvest, 25% of the radioactivity associated with the
plants could not be extracted by refluxing with ethanol and was termed
`bound'. Pirimicarb, compounds III and V and the three guanidines
mentioned above were all shown to be minor constituents of the bound
residue. Approximately 40% of the bound residue was associated with
the cellulose and hemicellulose of the plant (Teal and Skidmore,
1976).
When pirimicarb was introduced systemically into broad bean
plants by stem injection, its half-life was less than one day. Again
products II and III were formed initially. However these and
pirimicarb were subsequently degraded, the carbamate groups being
quantitively eliminated as measured by the evolution of 14CO2 from
plants treated with 14C-carbonyl-labelled pirimicarb (Manley, 1969).
Spraying of potatoes with 2-14C-labelled pirimicarb at 0.42 kg
ai/ha resulted in very low residues of radioactivity in the tubers
(less than 0.05 mg/kg pirimicarb equivalents). More than 80% of this
low residue was present as unextractable materials or as a complex
mixture of water-soluble materials closely associated with plant
sugars and pigments (Bowker et al, 1975).
When pirimicarb sprayed at rates up to 5 kg ai per hectare, ie
20-40 times normal use rates, total pyrimidine carbamate residues in
"follow-up" crops at harvest extremely small - of the order of the
limit of determination of the analytical method or below (Edwards et
al, 1974 and 1975). Total radiolabelled residues in "follow-up" crops
at harvest as a result of applying 2-14C-labelled pirimicarb at 0.5
kg ai per hectare were also small - up to 0.14 mg/kg pirimicarb
equivalents. In lettuce, which contained the highest radioactive
residue, approximately 50% of the activity was due to a combination of
pirimicarb, compound III, the hydroxypyrimidines I, V and the three
guanidines (Hughes and Leahey, 1974; Leahey and Benwell, 1976).
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. Only traces of residues are transferred to
milk and tissues.
TABLE 10. Pirimicarb Residues on Wheat (South Africa) at Intervals
after Application
Residues, mg/kg
Application Days
rate No. of after Demethyl-
g a.i./ha Applications Application Pirimicarb pirimicarb
250 1 1 2.3 0.4
2 1.7 0.3
4 0.4 0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.7 0.4
2 0.6 0.3
4 0.2 0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.5
2 1.6 0.2
4 0.3 <0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.8 0.4
2 0.6 0.2
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.4
2 2.3 0.4
4 0.4 <0.2
7 0.3 <0.2
55 <0.1 <0.2
125 1 1 0.6 0.3
2 0.6 0.3
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
Results are the average of 3 determinations
Recovery pirimicarb 105% at 1 mg/kg
demethyl-pirimicarb 78% at 1 mg/kg
Results not corrected for recovery
Limit of detection pirimicarb 0.1 mg/kg
demethyl-pirimicarb 0.2 mg/kg
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum radioactive residue in the milk (0.25 mg/kg
pirimicarb equivalents) was detected one hour after dosing; 80-90% of
this residue was due to the hydroxypyrimidines I, V and VI (Figure 1).
Subsequent residues were 0.06 mg/kg pirimicarb equivalents or less.
The maximum residue in fat and meat tissues after twelve days was only
0.04 mg/kg pirimicarb equivalents (Hemingway et al 1976). See also
"Biotransformation".
In soil
Pirimicarb is extensively degraded in soil. Degradation occurs
most rapidly in less acid soils. In soils of pH6.5 and above,
pirimicarb has a half-life of up to 7 weeks under aerobic conditions.
The rate of degradation in more acid soils was considerably enhanced
by air drying. Although less important than soil pH, a higher organic
matter content also appears to enhance the rate of degradation of
pirimicarb (Hill et al 1975a; Hill and Stevens 1975).
The principal route of degradation in soil is by hydrolysis of
the carbamate moiety from the pyrimidine ring. N-dealkylation of the
2-dimethylamino group has also been observed. Cleavage of the
pyrimidine ring has been demonstrated by the loss of 14CO2 from
soils treated with 2-14C-labelled pirimicarb. Hydrolysis of the
carbamate moiety results from both biological and chemical soil
activity whereas pyrimidine ring cleavage is mediated only by
biological activity. Several fungi and actinomycete cultures are
capable of metabolising pirimicarb (Hill et al 1975a; Hill and Arnold,
1974; Manley et al 1972).
Compounds I, III and V (Figure 1) are all major soil metabolites.
Minor degradation products include the carbamates II and IV and the
hydroxy pyrimidine VI (Hill et al, 1975a). Compound I is the major
product of photochemical degradation of pirimicarb on the soil surface
(84% of the residue recovered after 20 days) (Leahey and Benwall,
1974).
After two years, more than 70% of the radioactivity resulting
from the application of 2-14C-labelled pirimicarb to acid soils was
not extracted by refluxing with acetone: water (5:1). Almost all of
this bound residue was associated with the fulvic acid fraction of the
soil. Up to 60% of the bound residue was due to pirimicarb plus the
hydroxypyrimidines I and V (Hill et al 1975b).
Pirimicarb and its soil degradation products are of low mobility
in soil; lower than atrazine which is recognized as being only
moderately mobile (Riley et al 1974). There is no significant
concentration of pirimicarb in "run-off" water or eroded soil (Gouman
and Hale, 1975).
In water
Pirimicarb undergoes rapid photochemical degradation in water:
over 50% of the applied pirimicarb is degraded in 24 hours at pH5-9.
There is no significant loss of pirimicarb by volatilisation from
water. Degradation products include the pyrimidine carbamates II-IV,
the hydroxypyrimidines I, V and VI, 1,1-dimethylguanidine and
1-methylguanidine. Only traces (<1%) of guanidine itself have been
detected (Bowker and Griggs, 1974; Lincoln and Hemingway 1974).
In washing and cooking
Residues of pirimicarb and its two major carbamate metabolites II
and III on lettuce were reduced by 38-49% by washing with cold water.
In addition, during cooking approximately 60% of the pirimicarb and
52-65% of the carbamate-containing metabolite residues were
transferred from Brussels sprouts and cabbage into the cooking water.
There was no detectable conversion to other products during the
washing or cooking processes (Edwards et al, 1976).
In storage
There was no loss of residue from deep-frozen samples fortified
with pirimicarb. Details are given in "Methods of residue analysis".
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Monitoring of pirimicarb residues in the Netherlands has shown
that residues in imported grapes and in all but one sample of
home-produced endive were within the national tolerance of 1 mg/kg.
METHODS OF RESIDUE ANALYSIS
A gas-chromatographic method using a nitrogen-selective detector
to detect residues of pirimicarb plus its two major
carbamate-containing degradation products (II and III, Figure 1) is
the preferred residue method (Zweig 1975).
Crop samples are macerated with choloroform in the presence of
anhydrous sodium sulphate. The extract is subjected to a solvent
partition clean-up during which
5,6-dimethyl-2-formylmethylamino-4-pyrimidinyl dimethylcarbamate (II)
is hydrolysed to 5,6-dimethyl-2-methylamino-4-pyrimidinyl
dimethylcarbamate (III). The dried extract in ethyl acetate is
evaporated to dryness and taken up into acetone, and an aliquot of
this solution is injected into a gas chromatograph fitted with a
nitrogen detector. The size of the peaks obtained at the retention
times of pirimicarb and of compound III are used to calculate residue
levels by comparison with peaks obtained by injection of standard
solutions of the two compounds (ICI Plant Protection Ltd., 1972).
Some crop extracts may contain co-extracted interfering
compounds. These can be removed by cleanup on a column of silica gel,
prior to gas-chromatographic determination (Edwards and Dick, 1976).
The limit of determination for pirimicarb and for compound III in
crops is normally 0.01 mg/kg. Under the conditions of chromatography
described in the method, retention times for pirimicarb and compound
III were found to be 0.7 and 1.5 minutes respectively (ICI Plant
Protection Ltd, 1972).
Methanol may be used as the extraction solvent instead of
chloroform. For crops containing a high percentage of water (i.e.
normal 'green' crops), chloroform and methanol are equally efficient
(Manley, 1969; Edwards and Dick, 1975). However for crops of very low
water content (e.g. dry leaf tea, cured tobacco and oil seed rape), it
is essential to use methanol to extract residues of pirimicarb and
compounds II and III completely (Edwards and Dick, 1976).
Hydrolysis of compound II to compound III in 0.1 M hydrochloric
acid during the solvent partition cleanup was shown to be complete
(Cox, 1973).
A colorometric method, involving hydrolysis to dimethylamine, is
also available for determining residues of pirimicarb metabolites II
and III in crops (Zweig, 1975). Crop samples are macerated with
chloroform and the extract washed successively with 0.1M sodium
hydroxide, water and a slightly acid phosphate buffer (ph 4.3). The
chloroform is evaporated under reduced pressure and the residue steam
distilled with 1 M sodium hydroxide, liberating dimethylamine which
is absorbed in 0.1 M hydrochloric acid and determined
absorptiometrically as the yellow-brown copper dimethydithiocarbamate
complex at 436nm. Where troublesome emulsions occur at the washing
stages, the sample is extracted with acetone. Emulsifying agents are
removed by solvent partition; pirimicarb and its carbamate-containing
metabolites are then extracted into chloroform which is treated as the
chloroform extract obtained above.
Residues of the dimethyl dithiocarbamate fungicides (thiram and
ziram) interfere with the determination of pirimicarb and the two
metabolites by this method. Where interfering residues are present, a
chloroform extract obtained as described in the preceding paragraph is
subjected to thin layer chromatography alongside a marker spot on
silica gel GF 254. The developed chromatogram is viewed under
ultra-violet light, the primicarb band is removed and steam distilled,
and the determination completed, as described above (ICI Plant
Protection Ltd. 1969). The limit of determination of the method is
normally 0.7 mg/kg pirimicarb or pirimicarb equivalents. Recoveries in
excess of 70% are normally obtained (Bullock, 1969). The determination
of residues of pirimicarb and compound II and III is not affected by
the presence of residues of the ethylene bisdithiocarbamate fungicides
(e.g. maneb and zineb), nor by the presence of residues of carbaryl,
as none of these compounds is hydrolysed to dimethylamine.
TABLE 11. Stability Of Pirimicarb Residues In Crops Stored At -14°C
Mean residues, mg/kg
CROP
Initially After After
3 6.5
months months
Apples 0.92 0.94 0.86
Potatoes 1.00 0.79 0.81
Lettuce 0.97 1.04 0.94
Cucumbers 0.91 1.05 0.81
Pirimicarb residues are stable at -14°C. Samples of apples,
potatoes, lettuces and cucumbers were spiked with pirimicarb at
1 mg/kg, stored at -14°C for up to six months and analysed for
pirimicarb by the gaschromatographic method described above (see Table
11). Statistical analysis of the individual residue figures and of the
determined recovery values showed that there were no significant
differences among the residue values due either to storage time or to
crop (Edwards and Atreya, 1974).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national tolerances have been established for residue of
pirimicarb plus its two major carbamate containing metabolites, II and
III (Table 12).
TABLE 12. National tolerances reported to the Meeting
Country Crop Tolerance mg/kg
Australia Vegetables, 1
fruit and hops 0.5
Hungary All crops 0.5
Japan Fruit and vegetables 0.3
TABLE 12. (Cont'd.)
Country Crop Tolerance mg/kg
New Zealand All crops 0.5
Switzerland Fruit and vegetables 1
USA Potatoes 0.1
Venezuela All crops 0.5
Netherlands Fruit and vegetables 1.0
Grain 0.05
Potatoes 0.05
APPRAISAL
Pirimicarb is a fast acting, selective aphicide which is now
registered in a number of countries for use on fruit, vegetables and
other crops. It has proved effective on all crops on which it has been
tried against all aphids including organophosphorus-resistant strains,
with the exception of multi-resistant strains of Phorodon humuli.
It is harmless to useful parasites and predators, with the exception
of hoverflies, and has a very low toxicity to bees.
In addition to its contact and translaminar actions pirimicarb is
effective as a fumigant. Technical pirimicarb is not less than 95%
pure and is normally 98% pure; the impurities contain up to 1.3% of
other pyrimidine carbamates. It is formulated as a 50% dispersible
powder and a 50% dispersible pellet or grain, as a ULV formulation and
in a 10% smoke generator. The usual rates of application are 0.25 kg
a.i./ha as a crop spray and 15 mg/m3 as a smoke.
Pirimicarb possesses only limited persistence on crops and the
pre-harvest withholding intervals range from zero for root crops, and
others where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb is rapidly lost from plants; it has a half-life of 1-3
days. Most of the loss is by volatilisation, the extent of which
increases with rise in the ambient temperature, but there is some
photochemical and metabolic degradation. There are two major
metabolites containing the carbamate moiety, the N-formyl(methylamino)
(II) and 2-methylamino (III) analogues of pirimicarb, which occur in
plants. Minor degradation products include a third carbamate, 2-amino
pirimicarb, the water-soluble 2-dimethylamino-, 2-methylamino- and
2-amino-hydroxypyrimidines and the corresponding guanidines. The
recommended residue methods for pirimicarb also estimate the two major
carbamate-containing metabolites.
Use of radio-labelled material shows that some part (25% in one
experiment) of the pesticide may become bound to plant materials (i.e.
not extractable with organic solvents) but, as far as can be judged,
the more toxic carbamate compounds are not major constituents of this.
Within the plant (stem injection of broad beans), metabolic products
II and III are formed initially but subsequently degraded. Transfer to
the tubers of sprayed potatoes is small. Follow-up crops grown on
heavily sprayed land contain only very small amounts (at the
analytical limit) of determinable residue. Washing heavily treated
lettuce with cold water reduces residues of pirimicarb by about 38%
and cooking Brussels sprouts and cabbage gives a 60% reduction.
Pirimicarb is concentrated in the skin of apples and the residues are
reduced below the level of detection on peeling.
The transfer of residues of pirimicarb from animal feed to the
milk and meat of cows appears to be very small.
In soil there is substantial hydrolysis of the carbamate moiety
from the pyrimidine ring and photochemical degradation on the soil
surface also very largely produces the hydroxypyrimidine V. Pirimicarb
and its degradation products are of low mobility in soil and there is
no significant concentration of pirimicarb in run-off water or eroded
soil.
Pirimicarb is rapidly degraded photochemically in water exposed
to bright sunlight (50% in 24 hours at pH 5 to 9) but decomposition is
slow in the dark.
Adequate methods of analysis are available for regulatory
purposes. A gas-chromatographic method using a nitrogen selective
detector to detect residues of pirimicarb plus its two major carbamate
degradation products II and III (II is converted to III during the
clean-up process) is the preferred method. The limit of determination
of pirimicarb and compound III in crops by this method is 0.01 mg/kg.
Total carbamate can also be determined by a colorimetric method for
which the limit of determination is 0.1 mg/kg and the pesticide
recovery better than 70%. Modifications of clean-up etc. are available
for both methods to deal with particular problems (Zweig, 1975).
Although little information is yet available on the residue of
pirimicarb in food moving in commerce, numerous results from
supervised residue trials on crops growing in a number of countries
enable recommendations to be made for certain groups of crops. Because
the maximum residue limits recommended for many leafy vegetables are
the same it would be reasonable to have a single recommendation for
this group.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the total residue of pirimicarb, its
N-formyl(methylamino) analogue (desmethylformamido-pirimicarb) and
desmethyl-pirimicarb expressed as pirimicarb.
Commodity Temporary Maximum Note:
Residue Limit
(mg/kg) Pirimicarb has
a half-life of
Chili peppers 2 1-3 days on
plants; leafy
Apples 1 commodities
Bell peppers 1 are harvested
Broccoli 1 7 days after
Brussels sprouts 1 application,
Cabbage 1 root crops are
Cauliflower 1 harvested 0-3
Celery 1 days after
Cucumber 1 application
Eggplant 1
Endive 1
Gherkins 1
Lettuce 1
Parsley 1
Tomatoes 1
Blackcurrants 0.5
Beans (with pod) 0.5
Kohlrabi 0.5
Onions 0.5
Peaches 0.5
Plums 0.5
Raspberries 0.5
Strawberries 0.5
Peas 0.2
Rapeseed 0.2
Beans (without pod) 0.1
Beetroot 0.05*
Citrus fruits 0.05*
Parsnips 0.05*
Potatoes 0.05*
Radishes 0.05*
Sugar beet 0.05*
Turnips 0.05*
Wheat 0.05*
FURTHER WORK OR INFORMATION
Required (by 30 June 1978)
1) Studies on other species, primarily primate, to define the range
of species' susceptibility to the hematologic changes noted in
the dog.
* At or about the limit of determination.
2) Definition of the susceptibility to hemolytic anemia in the
inbred beagle strain used in toxicological studies.
3) Studies to fully define the reduced growth noted in several
rodent dietary studies.
4) Immunological studies when appropriate in animals species other
than the clog to define further the significance (and possible
range of occurrence) of the hemolytic anemia.
5) Significance of hypochromic anemia associated with rats fed high
levels of plant metabolites.
Required (by 1980)
1. Carcinogenic study in an appropriate mammalian species using a
currently acceptable protocol.
Desirable
1. Survey of industrially exposed individuals by immunological (or
serological) techniques to define the potential occurrence of
antibodies following pirimicarb exposure.
2. More information on residues in grains other than wheat, e.g.,
barley, oats, rice, maize.
REFERENCES
Bagness, J.E., Hamer, C.M. and Willis, G.A., Pirimicarb
1975 Operational Experience During Formulation.
Unpublished report from ICI Plant Protection
Division submitted to the World Health
Organization by ICI Ltd.
Bewick, D.W. and Leahey, J.P. Pirimicarb : Identification
1976 Of Material Volatilised From The Surface Of A
Treated Lettuce Plant, ICI Plant Protection Ltd.
Report No. TXJ1289A (Unpublished).
Bowker, D.M. and Griggs, B.F. Pirimicarb : Fate In Water.
1974 ICI Plant Protection Ltd. Report No. AR2492A
(Unpublished).
Bowker,D.M., Griggs, B.F. and May, M.S. Pirimicarb Fate In
197e Potatoes. ICI Plant Protection Ltd. Report No.
AR2491 AR (Unpublished).
Bullock, D.J.W. A Colorimetric Method Of Analysis for Residues Of
1969 PP062 In Food Crops, ICI Plant Protection Ltd.
Report No. AR2091 AR (Unpublished).
Clark, D.G., The Influence of Feeding on the Oral Toxicity of
1967a PP 062 and PP159. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Clark, D.G., The Acute Toxicity of Technical PP062, Dispersible
1967b Powder (J.F.2130) and PP062 Granules (J.F. 2148).
Unpublished report from ICI Central Toxicology
Laboratory submitted to the World Health
Organization by ICI Ltd.
Cox, J.H. Determination of Pirimicarb and Metabolites In Potato
1973 Tubers, ICI America Inc. Report (Unpublished).
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1968 Toxicity of PP062 - Beagle Dogs (Part 1).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1969 Toxicity of PPO62 - Beagle Dogs (Part 2).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by IGI Ltd.
Daniel, J.W. and Bratt, H., Absorption and Excretion by Rats of
1968 the Carbamate Insecticide, PP062. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Davis, J.A. and Hemingway, R.J. Pirimicarb : Fate On Cabbage. IGI
1976 Plant Protection Division Report No. TMJ1348A
(Unpublished).
Edwards, M.J. and Atreya, N.C. Pirimicarb : Storage Stability
1974 of Residues In Deep Frozen Crop Samples. ICI Plant
Protection Ltd. Report No. TMJ1090A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb : Crop Extractability
1975 Study, ICI Plant Protection Ltd. Report No.
TMJ1166A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb Residue Summary Residues
1976 In Crops From Field Trials 1973-1975. ICI Plant
Protection Division Report No. TMJ1360B (Un
published).
Edwards, M.J. Dick, J.P. and Hayward, G.J. Pirimicarb Effect of
1976 Washing and Cooking upon Residues in Lettuce and
Brassicae Crops. ICI Plant Protection Division
Report No. TMJ1346A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1974 Carry Over Study On Lettuce And Radish. ICI Plant
Protection Ltd. Report No. AR2565A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1975 Carry Over Study On Carrots, ICI Plant Protection
Ltd. Report No. TMJ1155A (Unpublished).
Ferguson, D.R. and Parkinson, G.R., Pirimicarb (PP062)
1973 Eye Irritation of Technical Grade Pirimicarb and
50% Dispersible Powder Formulation (JF 2538,
`Pirimor') Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Neurotoxicity in Hens.
1971a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Irritancy Tests. Unpublished
1971b report from ICI Central Toxicology laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Pirimicarb (PP062) : Subacute Toxicity on Rabbit
1971c Skin. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K. and Sotheran, J., Pirimicarb (PP062): Three
1971 Generation Reproduction Study in Rats. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.
1971a Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylformamidopirimicarb.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.,
1971b Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylpirimicarb. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Litchfield, M.H. and Watson, M.,
1971c Pirimicarb (PP062): Two Year Feeding Study in
Beagle Dogs. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Hodge, M.C.E. and Kinch, D.A., Pirimicarb (PP062) :
1971d Teratological Studies in the Mouse. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. Forshaw, A.M. and Monks, I.H., Pirimicarb (PP062):
1971e Excretion and Metabolism in the Dog and Rat.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Kinch, D.A. and Hodge, M.C.E. Pirimicarb (PP062) :
1971f Teratological Studies in the Rat. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. and Bratt, H., Pirimicarb (PP062). Accumulation Study
1972 in Rats. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Clapp, M.J.L., Garner, R., Litchfield, M.H. and
1972 Wilson, J., `Pirimicarb', (PP062): Two Year
Feeding Study in the Rat. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Garner, R., Phillips, C.E., and Slack, P., Pirimicarb (PP062)
1972 Pirimicarb Anaemia (A Chemically-Induced
Autoimmune Haemolytic Anaemia). Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Gowman, M.A. and Hale, S.W. Pirimicarb : Loss From Soil In
1975 Run-Off Water and Eroded Soil. ICI Plant
Protection Ltd. Report No. TMJ1102A (Unpublished).
Griffiths, D. and Conning, D.M., Ninety Day Oral Toxicity
1968 of PP062 - Albino Rats. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Hemingway, R.J. and Davis, J.A. Pirimicarb : Fate on Lettuce.
1976 ICI Plant Protection Division Report No. TMJ1324A
(Unpublished).
Hemingway, R.J. Davis, J.A. and Cleverly, B.A. Pirimicarb:
1976 Ercretion and Metabolism of a Single Oral Dose
Administered to a Cow. Unpublished report No.
AR2663A from ICI Plant Protection Division,
submitted to the World Health Organization by ICI
Ltd.
Hill, I.R. and Arnold, D.J. Pirimicarb Metabolism by Micro-Organisms
1974 Isolated from Soil. ICI Plant Protection Ltd.
Report No. TMJ1119A (Unpublished).
Hill, I.R., Arnold, D.J., Minns, E.C. and Greaves, R. Pirimicarb :
1975a Laboratory Studies Of The Degradation Of Single
Applications Of The Pesticide In Soil In the
Absence Of Plants. ICI Plant Protection Ltd.
Report No. AR2555A (Unpublished).
Hill, I.R., Harvey, B.R. and Arnold, D.J. Pirimicarb Extraction And
1975b Fractionation Of 'Bound' Residues In Soil. ICI
Plant Protection Ltd. Report No. AR2579A
(Unpublished).
Hill, I.R. and Stevens, J.E. Pirimicarb : Losses of Radioactivity
From 14C-Pirimicarb Treated Soils, During Air
Drying. ICI Plant Protection Ltd. Report No.
TMJ1143A (Unpublished).
Hodge, M.C.E., Pirimicarb (PP062): Teratological Studies in
1974 the Rabbit. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Hughes, H.E. and Leahey, J.P. Pirimicarb : Uptake of Pirimicarb And
1974 Its Metabolites From Soil By Rotational Crops. ICI
Plant Protection Ltd. Report No. AR2553A
(Unpublished).
ICI Plant Protection Ltd. Residue Analytical Method No. 15 for
1972 The Determination Of Residues Of Pirimicarb And
Its Two Major Metabolites In Crops
(Gas-chromatographic Method) (Unpublished).
ICI Plant Protection Ltd. Provisional Analytical Method No. 306/A For
The Determination Of Pirimicarb Residues In Fruits
Vegetables And Grain (Unpublished).
Jackson, J.A., Chart, I.S., and Sanderson, J.H., A Pirimicarb -
1974 Induced Haemolytic Anemia in Dogs. Report of a
Special Study. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Leahey, J.P. and Benwell, M. Pirimicarb : Photodegradation On A
1974 Soil Surface. ICI Plant Protection Ltd. Report No.
AR25504 (Unpublished).
Leahey, J.P. and Benwell, M. Pirimicarb ; Uptake From Soil And
Metabolism In Lettuce And Carrots, ICI Plant
Protection Division Report No. TMJ1331A
(Unpublished).
Lefevre, V.K. and Parkinson, G.R., Pirimicarb (PPO62;
1974 2-dimethylamino-5, 6-Dimethylpyrimidin-4-yl
Dimethylcarbamate): Acute Oral Toxicities of Plant
and Mammalian Metabolites. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Lincoln, B.A. and Hemingway, R.J. Pirimicarb : Fate In Water. ICI
1974 Plant Protection Ltd. Report No. TMJ1116A
(Unpublished).
Litchfield, M.H., The Measurement of Blood Cholinesterase Activity
1968 in Animals Dosed with 4,5
Dimethyl-2-dimethylamino-6-pyrimidinyl Dimethyl
Carbamate (PP062): Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Manley, C.A. Pyrimidine Insecticides : The Fate of Pirimicarb
1969 On Surfaces And Within The Plant ICI Plant
Protection Ltd. Report No. AR2109A (Unpublished),
18-19 and Figure 14.
Manley, C. A. Residue Summary : Pirimicarb In Crops. ICI
1972 Plant Protection Ltd. Report No. TMJ585/2
(Unpublished).
Manley, C.A., Hill, I.R., Harper, P. Black L. and Brooks J.R.
1972 Pirimicarb : Fate In Soil. ICI Plant Protection
Ltd. Report No. TMJ788AR (Unpublished).
Main, A.R. Personal communication.
1976
McGregor, D.B., Dominant Lethal Study in Mice of ICI PP062.
1974 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Palmer, S. and Samuels, D.M., Pirimicarb: 80 Week Carcinogenic
1974 Study in Mice. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Irritation Studies of Technical
1972a and Formulated Material on Normal and Wounded
Rabbit Skin. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Acute Oral Toxicity Following
1972b Storage at 37°C for Three and Six Months.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, C.R., Pirimicarb Metabolites (R.31680 and R.35140):
1974a Acute Oral Toxicity. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.42488): Acute Oral
1974b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Skin Sensitization. Unpublished report
1974c from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Parkinson, G.R., Oral Toxicities of Three Guanidine Compounds.
1975a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.32444): Acute Oral
1975b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.33160) : Acute Oral
1975c Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Potentiation Studies With
1975d Azinphos-methyl and Carbaryl. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A. Pirimicarb (PP062): "Pirimor" Smoke Generators:
1972a Acute Inhalation Studies. Unpublished report from
ICI Central Toxicology Laboratories submitted to
the World Health Organization.
Riley, R.A., Pirimicarb (PP062): `Pirimor' Smoke Generators.
1972b Subacute Inhalation Studies. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A., Pirimicarb (PP062): Acute Inhalation Toxicity of
1972c `Pirimor' 50% Dispersible Powder. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Riley, R.A., Pirimicarb (PP062) Acute Inhalation Toxicity.
1973 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Riley, D., Coutts, J., Stevens, J.E. and Newby, S.E. Pirimicarb :
1974 Leaching in Soil ICI Plant Protection Ltd. Report
No. AR2556A (Unpublished).
Samuels, D.M., Hodge, M.G.E. and Palmer, S.M., Pirimicarb - Three
1975 Two Year Feeding Studies to Assess, Carcinogenic
Potential. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Teal, G. and Skidmore, M.W. Pirimicarb Investigation Of The Bound
1976 Residues On Lettuce. ICI Plant Protection Division
Report No. TMJ1323A (Unpublished).
Zweig, G. Analytical Methods For Pesticides, And Plant Growth
1975 Regulators. Academic Press, VII:399.
restricting conjugation. Small quantities of the hydroxypyrimidine
were recovered as conjugates but the major products were passed as
unconjugated metabolites in the urine of rats and dogs (Fletcher et
al., 1971e).
Two products were identified as degradation products in plants
which were not found in dog or rat urine, the 2-formyl (methylamino)
(R-34885,II) and the 2-methylamino (demethyl-pirimicarb, R34836, III)
derivatives containing the intact carbamate. While these were isolated
from plants and not animals, the probability exists that they are
formed in mammals as transitory products since the carbamate is labile
and subject to rapid cleavage. The identification of the 2-methylamino
(V) and the 2-amibo (VI) hydropyrimidine derivates of pirimicarb in
rat and dog urine suggest the same sequence of metabolism and
breakdown in animals as plants with quantitative differences in the
final products.
Effects on Enzymes and other biochemical parameters
Pirimicarb is an anticholinesterase agent and, as other
N-methyl-and N,N-dimethylcarbamates, elicits a parasympathomimetic
response in certain animals. Evaluation of reversible cholinesterase
inhibition by carbamate esters is difficult. Using an automated delta
pH method the following I50 values for some pirimicarb metabolites
were obtained (Table 1).
TABLE 1. Inhibition of cholinesterase by Pirimicarb metabolites
Metabolite I50
Molarity X 106
2-Methylamino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (III) 4.54
2-amino-5,6-dimethyl-4-
pyrimidinyl dimethylcarbamate (IV) 4.34
2-dimethylamino-5,6-dimethyl
-4-hydroxypyrimidine (I) 14.9 X 103
2-methylamino-5,6-dimethyl
-4-hydroxypyrimidine (V) 4.25 X 103
(Fletcher et al., 1971e)
Using a female cat, a time course study of cholinesterase
depression was run following acute poisoning by intravenous injection
of a dose of 20 mg/kg. Rapid in vivo depression of pretreatment
plasma cholinesterase activity was obtained which persisted for more
than 4´ hours, long after clinical signs of poisoning had stopped.
These results are summarized in Table 2.
TABLE 2. Symptoms and plasma cholinesterase depression in a cat
poisoned with pirimicarb
Time Signs of Poisoning Plasma Cholinesterase
(% Inhibition)
5 min. salivation, trembling 72
15 min. salivation, fibrillation 72
30 min. reduced fibrillation 73
1 hr. none 72
2 hr. 75
4´ hr. 73
(Clark, 1967b)
An attempt was made to resolve questions raised on the
reliability of the routine automated procedure used for the
determination of blood cholinesterase activity. The original method
used extended substrate incubation times and large dilutions. Both of
these factors are known to be responsible for enhancing spontaneous
reactivation of the carbamoylated enzyme. In an attempt to demonstrate
that the N,N-dimethylcarbamoylated cholinesterase is sufficiently
stable to be measured by techniques not normally employed, a shortened
incubation time (5.5 min. instead of 23 min) and reduced dilution were
used. Acetylcholine concentrations were not changed. With these
modifications a comparison of the method used routinely, a radiometric
(14C-acetylcholine) method and a modified automated delta Ph method
was made to evaluate cholinesterase depression in vivo in rats.
Rats were orally administered pirimicarb at 30 mg/kg and blood
removed after 1 hour. Dogs were administered pirimicarb at 30 mg/kg
dose and blood was sampled at one hour after treatment.
Cholinesterase depression was similar in all methods of assay
employed. In rats, male and female, plasma measured 50% inhibition by
all methods. In dogs, a comparison of the radiometric
14C-acetylcholine method with the automated delta Ph method showed no
inhibition of plasma cholinesterase at 4 mg/kg. In addition, plasma
cholinesterase from treated animals was found to be stable over a 24
hour interval at 4-5°C with no loss in inhibition spontaneously
regenerated activity).
From the foregoing study, it was concluded that a stable
carbamoylated enzyme analogous to a phosphorylated enzyme may be
formed with pirimicarb and the reduced activity can successfully be
measured by an automated delta Ph method. It was concluded that the
K3 step was slow and a stable intermediate was formed. The conclusion
of this study is that the method used in most studies to measure
cholinesterase activity did not underestimate the inhibition and give
misleading results (Litchfield, 1968).
In vitro studies in part confirmed these conclusions
especially with respect to pseudocholinesterase enzymes. The reaction
of pirimicarb and horse serum cholinesterase yielded a stable N,N-
dimethylcarbamoylated enzyme having a k3 of 5 X 10-4min-1.
However, with acetylcholinesterase (eel or RBC) the k3 is higher (3.7
X 10-2 min-1 and 0.9 X 10-2 min-1 respectively) and the spontaneous
reactivity is much greater. Thus, the use of plasma or serum
cholinesterase to assay pirimicarb inhibition would show adequate
values for the interaction of enzyme and inhibitor while the use of
RBC as other-acetylcholinesterase sources would be expected to give
misleading values especially where extended assay times or large
dilutions were a factor (Main, A.R. personal communication).
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of rats (12 male and 24 female rats/group) were fed
pirimicarb in the diet at 0,250 and 750 ppm and mated to begin a
3-generation, 2-litter per generation standard reproduction study. At
the conclusion of the study, tissues from 10 rats of each sex from the
F3b generation were examined microscopically. Four offspring were
observed to be malformed at birth (3 litter mates of the second
generation F2a - 250 ppm and one 750 ppm F2a fetus). Reduced body
weight of the parents (specifically females but often males as well)
was noted at both treatment levels. Growth of pups prior to weaning
was not affected by pirimicarb in the diet although a reduced growth
was seen thereafter in all three generations. The basic reproduction
indices normally measured (fertility, gestation, lactation and
viability) were not affected. Evaluation of parturition data suggests
no effects on the ability to maintain pregnancy. No adverse effects on
reproduction were observed with pirimicarb. Because malformations were
observed in only 4 fetuses of 2665 treated it was concluded that these
were not as a result of pirimicarb in the diet (Fletcher and Sotheran,
1971).
Special studies on carcinogenesis
Groups of mice (50 of each sex per group; SPF, Alderly Park
strain) were fed pirimicarb in the diet at levels of 0, 300 and 1500
ppm for 80 weeks. Mortality was reported to be a result of endemic
respiratory disease. The clinical condition of the mice at autopsy
confirmed a high incidence of respiratory disease. A slight body
weight reduction was noted only at the high dose level. No effects
were noted on food consumption or on gross and microscopic analysis of
most tissues and organs. The incidence of pulmonary tumors was
significantly higher in both treated groups than controls used in the
study. (When the incidence of pulmonary lesions was compared with a
large group of control mice examined in the laboratory over an 8-year
interval, the data did not show a significant increase in pulmonary
tumors as a result of pirimicarb in the diet. The controls used in
this study apparently had an extremely low incidence of lung lesion).
There was no evidence of excessive lymphoma or leukemia noted in the
study and detailed examination of the CNS did not indicate pirimicarb
to be a carcinogen in mice (Palmer and Samuels, 1974).
Groups of rats (48 males/group) were fed pirimicarb in the diet
at dose levels of 0,750 and 2,500 ppm for 2 years. Because of
respiratory infection, an antibiotic (oxytetracycline and sodium
sulphadimidine were administered in drinking water as needed.
Mortality was heavy after the 40th week predominantly as a result of
respiratory infection. Growth was reduced at 2,500 ppm but not at 750
ppm. An increased incidence of reticulum cell lymphoma was observed in
the study. It was concluded that this incidence was as a direct result
of the accompanying pulmonary infection. However, the infiltration
pattern of the tumor was mainly perivascular. A statistical treatment
of the tumor incidence did not confirm a relationship of tumor
incidence to the presence of pirimicarb in the diet.
In another study using a different strain, groups of rats (48
males/group) were fed diets for two years similar to those described
above. Again, an outbreak of respiratory disease within one year
limited the surviving animals at the conclusion of the study. However,
in contrast to the previous study, no reticulum cell lymphoma was
observed. The incidence of tumors was not different from controls. It
was concluded that pirimicarb did not produce an increased incidence
of tumors in male rats.
A third study was initiated from those animals discarded from the
three generation reproduction study. Groups of rats (24 of each
sex/group) previously exposed in utero and fed prior to weaning were
fed pirimicarb in the diet at dosage levels of 0 and 750 ppm for 2
years. Again, respiratory infection reduced survival of rats in this
study (rats were also treated with antibiotics to control the
infection). There was no difference relating to survival between the
control and treated groups. Growth of both sexes was reduced by
pirimicarb in the diet. Gross and microscopic analyses did not
indicate an increased incidence of tumors in the pirimicarb fed rats.
Based on lifetime exposure to pirimicarb from conception, there is no
carcinogenic effect noted in the rat (Samuels et al., 1975). However,
the undercurrent respiratory infection noted in all four long term
studies aimed at examining lung lesions precludes definitive
conclusions on this problem.
Special studies on teratology
Mouse
Groups of mice (25 mated females/group) were fed pirimicarb in
the diet at dosage levels of 0,40 and 500 ppm throughout pregnancy
(day 0 to day 18 of gestation). There were no parental deaths and no
abortions occurred. On sacrifice at day 18, fetuses were removed and
uteri examined for resorptions. Implantation was slightly reduced in
both pirimicarb groups. The mean litter weight and fetal weights were
reduced at the high dose level. There was no effect on the sex ratio
of fetuses.
No abnormalities were observed on gross examination or on
skeletal examination following clearing and alizarin straining
(Fletcher et al., 1971d).
Rat
Groups of rats (18-21 females/group) were fed pirimicarb in the
diet at dosages of 0, 250 and 750 ppm throughout pregnancy. Parental
growth was reduced at the high dose level. Total weight was also
reduced at the high dose level. No abnormalities were noted in the
treatment group attributable to pirimicarb in the diet (Fletcher et
al, 1971f).
Rabbit
Groups of pregnant rabbits (16 does/group) were administered
pirimicarb orally by gavage daily from day 1 to day 28 of gestation at
doses of 0,1.25, 2.50 and 5.0 mg/kg. Administration of 2.5 and 5 mg/kg
produced a maternally toxic response noted as a reduction of weight
gain. This was also reflected in reduced fetal weights which occurred
at the upper two dose levels. There were no teratogenic effects noted
on gross examination of tissue, or on skeletal examination of cleared,
stained fetuses. Pirimicarb has no teratogenic potential in rabbits. A
slight increase in the size of the brain of some litter mates was not
considered to be hydrocephalus and was not dose dependent (Hodge,
1974). On the basis of studies in three species, it appears that
pirimicarb has little teratological potential.
Special studies on mutagenesis
Mouse
Groups of mice (15 males/group) were administered pirimicarb
orally for 5 days by intubation at dosage levels of 0, 10 and 20
mg/kg. Two positive controls of 150 mg/kg or 100 mg/kg of ethyl
methanesulphonate (EMS) were administered either by a single ip
injection on the day before mating or by oral administration 5 days
prior to mating. Each male was mated to two virgin females at weekly
intervals for 8 weeks in a dominant lethal mutagenesis study. In an
evaluation of pregnancy, implantation and early and late death of
fetuses, pirimicarb treatment did not show differences from control
values. In contrast, EMS resulted in reduced pregnancy,
preimplantation loss and increased early death, judged to be a
significant mutagenic occurrence. There are no indications from this
study that pirimicarb would induce mutations in mice (McGregor, 1974).
Special studies on sensitization
Groups of guinea pigs (4 males/test group, 3 males as controls)
were treated on the ear for 3 days with a 10% (w/v) solution of either
technical or formulated pirimicarb. Four days later the animals were
challenged by applying doses of 0, 0.1, 1.0 and 10% solutions to the
shaved flank skin. Traces of erythema were noted but a primary
sensitization reaction was not observed (Parkinson, 1974c).
Special studies on inhalation toxicity
Pirimicarb has been recommended for use under enclosed glasshouse
conditions. Studies were performed on the acute inhalation toxicity
following exposure of rats to smoke generated in an enclosed chamber
at doses up to 300 mg/m3. Mortality was observed as were acute signs
of poisoning following a 6 hour exposure. Rats exposed to 75 mg/m3
for 6 hours showed clinical signs of poisoning while at a level of 15
mg/m3 no signs of poisoning over the 6 hour test exposure were
observed (Riley, 1972a). Rats exposed to 15 mg pirimicarb/m3, 6 hours
a day, 5 days/week for 3 weeks did not exhibit toxic signs of
poisoning. A slight plasma cholinesterase depression was observed
(Riley, 1972b). Acute inhalation studies on rats using a 50%
dispersible powder formulation showed that exposure for one hour at a
dust concentration of 20,000 mg/m3 caused no acute signs of
poisoning although plasma and RBC cholinesterase depression was noted
(Riley, 1972c). Rats exposed daily 5 days/week for 3 weeks (6
hours/day) to a "saturated vapor" of pirimicarb (air passed through
technical pirimicarb was not analyzed for the presence of pirimicarb)
did not display signs of poisoning or even of exposure (Clark, 1967b;
Riley, 1973).
Special studies on neurotoxicity
Four adult hens, administered a single oral dose of 25 mg
pirimicarb/kg by gavage, displayed cholinergic signs of poisoning but
no clinical indication of a delayed neurotoxic response (Clark,
1967b).
Four adult hens were administered pirimicarb by oral intubation
at a dose level of 25 mg/kg. They were observed for 21 days,
re-treated with the same dose and again observed for 21 days. No signs
of delayed neurological ataxia were observed (Fletcher, 1971a).
Special studies on potentiation
Groups of female rats (10 rats/group) were administered
pirimicarb alone and in combination with carbaryl or azinphos-methyl
orally by gavage. Slight changes in the projected LD50 values were
noted with azinphos-methyl when the materials were administered in
combination. No such changes were noted with carbaryl combinations.
There was no potentiation of the acute effects noted with simultaneous
oral administration of carbaryl while a slight potentiation of acute
toxicity was noted with azinphos-methyl (Parkinson, 1975d).
Special studies on plant metabolites
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-formylmethylamino pirimicarb
(R.34885, II) by gavage daily for 10 days at doses of 25 mg/kg/day.
Males developed a slight hypochromia, while a hypochromic anemia was
observed in females. No other toxic signs were observed. There was no
intubution of plasma, RBc or brain cholinesterase. Increased
hemopoietic activity was noted in the spleen (Fletcher et al., 1971a).
Groups of rats (10 male and 10 female rats/group) were
administered the plant metabolite 2-methylaminopirimicarb (R.34836,
III) by gavage daily for 10 days at doses of 100 mg/kg/day. Acute
signs of poisoning were noted immediately after each daily dose. These
signs abated within 2 hours. Plasma cholinesterase depression was
observed only in females. Hematological examination revealed
hypochromic anemia in females while males showed reticulocytosis and
slight hypochromia. No change in clotting time was seen. Increased
hemopoietic activity was seen in the spleen of males and females.
Changes in the thymus were also observed (Fletcher et al., 1971b).
Special studies on antidotes
Groups of female rats were administered pirimicarb by
subcutaneous injection (150 mg/kg - 2 X LD50). When signs of
poisoning were observed, atropine alone or in combination with P2S
was administered by S.C. injection. Atropine alone or in combination
with P2S was an effective antidote while P2S alone was not effective
in reducing the acute toxic effects (Clark, 1967b).
Eye and skin irritation
Technical pirimicarb and a 50% dispersible powder formulation
produced a slight occular irritation when administered to the
conjunctival sac of rabbits. One drop of a 5% solution or a suspension
of 25% (w/v) of technical pirimicarb in propylene glycol or 50% (w/v)
formulated product, instilled in the conjunctival sac, resulted in
slight pain, redness and transient slight corneal opacity. Pirimicarb
was classified as a mild irritant graded as 3-4 m a scale of 8 (Clark,
1967b; Fletcher, 1971b; Ferguson and Parkinson, 1973). Technical
pirimicarb applied to the intact or abraded skin of rabbits was
non-irritating when administered as a 25% suspension in propylene
glycol (Fletcher, 1971b; Parkinson, 1972). When a paste was made of a
50% dispersible powder formulation, pirimicarb was irritating
especially to abraded skin. No reaction was observed with intact,
undamaged skin (Parkinson, 1972). Technical pirimicarb (5% in
propyleneglycol) applied to the shaved back of rats repeatedly for 24
hours under a plastic film was not irritating to the skin (Fletcher,
1971b; Clerk 1967a).
Acute Toxicity
TABLE 3. Acute toxicity of pirimicarb
LD50
Species Sex Route (mg/kg) Reference
Rat F oral (fasted) 68 Clark, 1967a
(fed) 147-210 Clark, 1967a
F oral 101-147 Clark, 1967b;
Parkinson, 1975
F oral 165-221* Parkinson, 1972b
dermal >500 Clark, 1967b
TABLE 3. (Cont'd.)
LD50
Species Sex Route (mg/kg) Reference
M&F ip 25-50 Clark, 1967b
F SC 75 Clark, 1967b
Mouse F oral 107 Clark, 1967b
Dog M&F oral 100-200 Clark, 1967b
Rabbit dermal >500 Clark, 1967b
Hen oral 25-50 Clark, 1967b
Rat inhalation approx. Riley, 1973
300 mg/m3
* pirimicarb stored at 37°C for up to 6 months showed no change in
acute toxicity value suggesting stability over this time interval.
Signs of poisoning: Within a few minutes of receiving a toxic oral
dose, signs of poisoning in a rat were observed including:
salivation, chromolacrymation, urination, defecation, miosis and
muscular fibrillations leading to fasciculations and death within
30 minutes. These are all typical signs of cholinergic
stimulation normally seen with parasympathomimetic agents.
Short term studies
Rabbit - dermal
Groups of rabbits (4 of each sex/group) were administered
pirimicarb dermally at a dose of 500 mg/kg for a 24 hour duration. The
skin was washed and the treatment repeated daily for 14 days. No
toxicity was observed and the animals appeared normal (Fletcher,
1971c).
Rat
Groups of rats (10 male and 10 female rats/group) were
administered pirimicarb daily by gavage at doses of 0 and 50 mg/kg for
10 days. A slight growth depression was noted but no mortality or
cumulative effects were seen. Hematology was normal and gross
examination of tissues and organs at the conclusion of the study
showed no adverse effects (Clark, 1967b).
TABLE 4. Acute toxicity of metabolites or impurities
LD50
Compound* Species Sex Route (mg/kg) Reference
pirimicarb Rat F oral 800-1600 Lefevre and
phenol (I) Parkinson, 1974
(R.31805)
N-methyl-N-formyl Rat F oral 50-100 Lefevre and
pirimicarb (II) Parkinson, 1974
(R.34885)
N-demethyl pirimicarb Rat F oral 200-400 Lefevre and
III) Parkinson, 1974
(R.34836)
N-di-demethyl Rat F oral 79 Parkinson, 1974a
pirimicarb (IV)
(R.35140)
N-demethyl phenol (V) Rat F oral 2000-2500 Lefevre and
(R.34865) Parkinson, 1974
N-di-demethyl phenol (VI) Rat F oral >2500 Parkinson, 1974a
(R.31680)
N,N-dimethyl Rat F oral 1455 Parkinson, 1975a
guanidine HCl
methyl guanidine SO4 Rat F oral 1105 Parkinson, 1975a
guanidine HCl Rat F oral 1105 Parkinson, 1975a
2-(dimethyl-amino)- Rat F oral 158 Parkinson, 1974b
6-methyl-4-pyrimidinyl
dimethylcarbamate
(R.42488)
2-(dimethyl-amino)-5, Rat F oral Zeta 1000 Parkinson, 1975b
6 dimethyl-4-pyrimidinyl
diethylcarbamate
(R.32444)
2-(dimethyl-amino)-5,6- Rat F oral Zeta 800 Parkinson, 1975c
dimethyl-4-pyrimidinyl
ethylmethylcarbmate
(R.33160)
* See figure 1 for structural definition of the numbered products.
Groups of rats (25 males and 25 females/group) were fed
pirimicarb in the diet for 90 days at dosage levels of 0, 250 and 750
ppm. A separate group of rats was administered pirimicarb by gavage,
daily at a dose of 25 mg/kg. There was no effect on growth, food
consumption or mortality in the dietary groups. Mortality was evident
in the rats dosed by gavage although the survivors showed normal
growth over the 90 day interval. Hematology parameters were
unaffected. A slight decline in plasma cholinesterase was observed in
the rats treated by gavage and at sporadic intervals in the 750 ppm
group. RBC and brain activity was normal. Gross and microscopic
analysis of tissues and organs examined at the conclusion of the study
was normal. Bone marrow cytology was not performed (Griffiths and
Conning, 1968).
A no-effect level of >750 ppm in the diet but lower than
25 mg/kg body weight administered by gavage was evidenced in this
study.
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
pirimicarb in the diet at dosage levels of 0, 4, 10 and 25 mg/kg for
90 days. Growth was not affected over the course of the study although
one of the male dogs at the highest level died during the course of
study. While the cause of death was not completely diagnosed, it was
probably related to anemia as the animal showed marked erythropoietic
hyperplasia and the presence of numerous nucleated red blood cells. A
macrocytic anemia was observed in two other dogs (one high and one
intermediate dose) at the conclusion of the study. Although hematology
parameters were not unusual an increased number of nucleated red blood
cells was observed in peripheral blood. Bone marrow examination showed
an increased nucleated cell count and reduced m/e ratio at all dose
levels. This abnormal count dropped slightly after a 28 day recovery
period, but was not down to normal. Serum folate, iron and vitamin
B12 levels were normal, suggesting that the hemolytic anemia was not
related to this type of deficiency.
Cholinesterase measurements showed depression of plasma enzymes
at the upper two dose levels. Brain and RBC cholinesterase activity as
measured by the delta pH method was unaffected by pirimicarb.
Gross and microscopic examinations of tissues and organs
indicated hemopoiesis in the spleen and lymph nodes of dogs showing
hematological signs of anemia. No effects on tissues and organs (other
than on bone marrow observations of all treated animals or on the
tissues and organs of the dog that died) were observed. A no-effect
level was not defined in this study. Delayed maturation of red blood
cells as evidenced by the presence of abnormal levels of nucleated red
blood cells was observed in all treated animals (Conning et al.,
1968).
Groups of dogs (4 beagle dogs of each sex/group) were fed diets
containing pirimicarb at dose levels of 0,0.4, 1.8 and 4.0 mg/kg body
weight for 90 days (the 4.0 mg/kg dose was maintained for 180 days).
There was no mortality and growth was unaffected by pirimicarb in the
diet. Clinical chemistry, hematology and urinalysis parameters were
normal. Bone marrow cytological examination again showed the presence
of nucleated red blood cell precursors at the highest dose tested. The
occurrence of these juvenile cells in marrow was not accompanied by
anemia as was evident In the previous study. Gross and microscopic
examination of other tissues and organs showed evidence of spleenic
hemopoiesis at the high dose level over the 6 month test. Other
tissues and organs were not affected. Plasma and brain cholinesterase
values were also normal (Conning et al., 1969).
In special studies to evaluate the hemolytic anemia observed in
dogs, four dogs (two male and two female) were fed pirimicarb in the
diet at levels of either 25 or 50 mg/kg for up to 110 weeks. Two of
the four dogs (1 male at 50 mg/kg and 1 female at 25 mg/kg) developed
anemia while two (1 female at 50 mg/kg and 1 male at 25 mg/kg) were
normal over the entire period. Peripheral blood showed changed in the
size and shape of red cells and the presence of some nucleated red
blood cells was observed. No abnormalities were seen in the white cell
series or platelets. Bone marrow samples showed a marked erythroid
hyperplasia. Attempts made to reduce the anemia with high therapeutic
doses of Vitamin B12, folic acid and folinic acid were negative. Red
blood cells from the anemic dogs were able to be agglutinated by
specific antigamma globulin serum while cells from normal dogs were
not. Free antibody was demonstrated in the sera of anemic dogs. The
antibody reacted with cells of the individual from which it was
removed and also with cells of normal-in-appearance (non-anemic)
pirimicarb-treated dogs. The antibody did not react with cells of
normal, untreated dogs. The presence of antibody and the demonstrated
effects of anemia were reversible (within 6 weeks) upon withdrawal of
the pirimicarb treatment. The pirimicarb treatment appears to have
been responsible for inducing changes in red blood cells of dogs
treated at higher levels. However, only some of the dogs responded
further in producing antibody that reacts with the altered cell
inducing anemia (Garner et al., 1972). In a further study to evaluate
a threshold of response of susceptible animals to pirimicarb, the two
susceptible dogs when allowed to recover from the induced-anemia were
fed levels of 1-2 mg/kg. One of the two dogs became anemic when
exposed for 14 weeks to 2 mg/kg. The other animal remained normal.
Further immunological studies using the susceptible dogs showed IgG
binding to cells of non-anemic dogs exposed to pirimicarb and
suggested that pirimicarb does not inhibit the IgG binding. It was
concluded that the antibody is not produced in direct relation to
pirimicarb alone, but possibly to a toxicant-red cell complex or to a
change induced by the toxicant (Jackson et al., 1974).
Groups of dogs (4 male and 4 female/group) were fed pirimicarb in
the diet for two years at dosage levels of 0, 0.4, 1.8 and 4.0 mg/kg
body weight. There were no effects on behaviour, growth or food
consumption over the course of the two years. Blood chemistry
including blood and brain cholinesterase and urinalysis parameters
were unaffected. Hematology was normal. The M/E ratio was decreased at
the high level of feeding at the conclusion of the study. There were
no unusual findings in bone marrow analysis although a slight increase
in a nucleated red blood cell content was evident at 4 mg/kg. Serum
iron and folio acid levels were unaffected by pirimicarb. Gross and
microscopic analysis of tissues and organs showed no effects
attributed to pirimicarb. Based on the data submitted a no-effect
level is 1.8 mg/kg body weight (Fletcher et al., 1971c.).
Long term study
Rat
Groups of rats (48 males and 48 females/group) were fed
pirimicarb in the diet at dosage levels of 0, 250, 500 and 750 ppm for
two years. There were no effects noted on survival. Growth was reduced
in females at all dose levels. There were no effects noted in males.
Total food intake of females was reduced. (Food conversion ratios
(ratio of food consumed to body weight gain) were reduced primarily in
the two higher dose groups).
No effects were noted on hematological parameters including
cholinesterase activity. Plasma cholinesterase was slightly reduced in
females. No effects were seen with RBC or brain cholinesterase. Gross
and microscopic examination of tissues and organs revealed only
reduced spleen weight of 750 ppm females. No other adverse effects
attributable to pirimicarb in the diet were noted. There was no
over-all increase in tumor incidence. An increase in the number of
localized astrocytomas in the high dosed males but not in the females
was not considered to be significant. Because of this slight effect
noted in males, a special study using males was initiated and is
summarized in the special study on carcinogenesis. A definitive
no-effect level wan not noted in this study, as dietary conversion
calculations do not support a firm conclusion of inappetance as a
basis for reduced growth. The marginal reduction of growth in females
suggests that a no-effect level is slightly below 250 ppm (Fletcher et
al., 1972).
OBSERVATIONS IN MAN
Cholinergic signs of poisoning accompanied by depression of
plasma (but not RBC) cholinesterase activity were observed in men
occupationally exposed to pirimicarb in the manufacture of formulated
products. In the course of investigating various procedures for safe
manufacturing conditions, it was observed that substantial vapor
toxicity was apparent as pirimicarb apparently volatalized at high
(65°C) temperatures and was inhaled by workers. Signs of exposure and
depression of plasma cholinesterase activity were recorded both of
which rapidly diminished with no apparent latent effects (Bagness et
al., 1975).
COMMENTS
Pirimicarb, an N,N-dimethylcarbamate ester, is rapidly absorbed
and metabolised in mammals by oxidative and/or hydrolytic pathways.
Only a few of the many potential metabolites have been isolated. Those
products containing an intact carbamate structure are as acutely toxic
as the parent molecule. Where cleavage of the ester groups occurs, the
acute toxicity is decreased markedly. With the exception of two
products observed on leaf surfaces (the N-formyl (methylamino) and
N-methylamino analogues of pirimicarb) and not in animal studies, all
other products identified are common to both metabolic pathways. It is
possible that these two are also produced as transitory products in
mammals. Pirimicarb is rapidly excreted from the body and neither
pirimicarb nor its metabolites accumulate in any tissue or organ. In
short term rat studies with the two carbamate plant metabolites,
administered at high doses, hypochromic anemia was observed. The
significance of this occurrence was not defined.
Pirimicarb is moderately toxic following acute administration to
various animal species. Enzyme kinetic studies on the in vivo and
in vitro inhibitory properties suggest that pirimicarb is a
rapidly reversible inhibitor of acetylcholinesterase as compared with
its effects on pseudo-cholinesterase. However, the stability of
inhibited (carbamoylated) plasma cholinesterase allowed an estimate to
be made of in vivo cholinesterase depression.
In several species no effects on reproduction (including
teratogenicity) were observed. A mutagenic response was negative as
evidenced by a mouse dominant lethal study. Pirimicarb did not induce
a delayed neurotoxic response, as generally noted with TOCP, in hens.
Short and long term studies in several animal species were performed.
In one long term study in the rat an increased number of astrocytomas
were noted in males, but this was not confirmed in two subsequent
studies. In a specific carcinogenic study in mice, a significant
increase in the incidence of pulmonary tumours was observed. However,
it was believed that the differences were due to an abnormally low
control value, thus negating the full evaluation of the study. None of
these studies was entirely adequate for evaluating the carcinogenic
potential, as chronic respiratory infection in each rat study limited
survival and a full interpretation of the data.
In short term studies with dogs, a dose dependent effect
manifested as hemolytic anemia changes was observed at high levels of
pirimicarb fed in the diet. Anemia did not occur in all dogs exposed
at high doses, and there was a suggestion of a sensitisation reaction.
In studies on the mechanism of action of pirimicarb with respect to
the hemolytic anemia, it was observed that an abnormal condition in
red blood cells was associated with the presence of pirimicarb. Also,
the presence of an increased number of a certain type of nucleated red
blood cells in bone marrow cytological examination, even in the
absence of anemia, suggested interference in a late stage of
development of erythrocytes induced by relatively high levels of
pirimicarb and complicated the full interpretation of the data. It was
suggested that hemolysis is caused by induction of a specific antibody
to the red blood cell following high level administration of
pirimicarb.
In a two year study with dogs of the same colony, no anemia was
observed although a dose dependent occurrence of nucleated red blood
cell precursors in the bone marrow was noted. At the low dose levels,
no effects on hematological or other parameters examined were
observed. Thus, in a susceptible species the effects noted over a
short term high level exposure were not cumulative and were not
manifested in a two year study. A similar hematological condition was
not reported in rodents exposed to pirimicarb in several long and
short term studies.
In a long term study with rats, growth depression was observed,
primarily at high levels. Marginal depression of growth was observed
in females (but not males) at the lowest dose level tested.
Calculation of food consumption was made with respect to growth. It
was concluded that growth depression may be related to inappentance
rather than being a specific toxicological effect.
In workers known to be occupationally exposed certain effects on
plasma cholinesterase activity were observed but no adverse long term
effects were noted.
The Meeting, in considering the toxicological properties of
pirimicarb, concluded with respect to the marginal growth depression
observed in rat species that a no-effect level would be slightly below
the lowest dose level tested.
The adverse effects on hematological parameters noted in the dog
were dose dependent and a no-effect level could be seen.
The carcinogenic potential of pirimicarb in rodents could not be
fully evaluated although the studies performed gave little concern to
the Meeting that pirimicarb was a potent carcinogen in the species
tested.
In consideration of both the long and short term studies in
several species a no-effect level was agreed upon from the data of the
2 year dog study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.8 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pirimicarb is a fast-acting, selective aphicide with contact,
translaminar and, more particularly, fumigant actions. It has proved
effective on all of the crops on which it has been tried against all
aphids including organophosphorus-resistant strains, except for
multiresistant strains of the damson hop aphid, Phorodon humuli.
Pirimicarb is harmless to useful parasites and predators, with
the exception of hoverflies. It is also of very low toxicity to bees.
This specificity makes pirimicarb a valuable chemical for use in
integrated control programmes.
It is active at rates as low as 0.125 kg ai/ha, depending on the
efficiency of crop spray cover and the species of aphid present.
Normal rates of commercial application are 0.25 kg ai/ha; but rates as
high as 0.50 kg ai/ha are recommended for some outlets. Applied as a
smoke, pirimicarb is normally effective at 15 mg/m3.
Pirimicarb possesses only limited persistence on crops.
Re-treatment may therefore be necessary after 10-14 days in those
situations where the potential for re-infestation remains high.
Pre-harvest withholding intervals range from zero for root crops, and
crops where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb was first synthesised in 1965. It was first registered
for use on peaches in Western Europe in 1969. It is now registered
world-wide for use on a wide range of crops, including fruit,
vegetables and cereals.
Use patterns in the Netherlands, South Africa, New Zealand and
Australia are summarized in Table 5.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pirimicarb has a half-life of one to three days on most crops.
Residue trials have been conducted on a wide range of crops in
numerous countries since 1967. The results of these studies are
reported by Manley (1972) and by Edwards and Dick (1976). They are
summarized in Tables 6-10. Total pyrimidine carbamate residues have
been studied, i.e. residues of pirimicarb and its two major
carbamate-containing metabolites on plants (the N-demethyl and
N-formyl (methylamino) analogues of pirimicarb, III and II, Figure I).
II is also commonly known as desmethyl-formamido-pirimicarb.
TABLE 5. Use pattern in various countries
Country Crop Formulation Rate Pre-harvest Interval
ai.
Netherlands Cucurbits (Eggplant, 10% smoke 10 g/700m3 3 days
cucumber, generator
gherkin,
melon)
Pepper 50% WP 25 g/100 l 3 days
Tomato
All other consumption 10% smoke 10 g/700 m3 1 March-31 Oct - 1 week
crops under glass generator 1 Nov-28 Feb - 2 weeks
All fruits & vegetables 50% WP 25 g/100 l 7 days
with the exception of
endive and lettuce under
glass and parsley and
celery
Sugarbeet, potatoes 50% WP 0.25 Kg/ha -
Cereals 50% WP 0.25 Kg/ha 4 weeks
South Africa Wheat WP 125 g/ha 7 days
Peaches WP 25 g/100 l 21 days
New Zealand Potatoes 250 g/220 l/ha Nil
Brassicas 250 g/200 l/ha 3 days
Australia Vegetables, fruits and hops 2 days
Leafy Vegetables (Tables 6 and 7)
The highest initial residues after spraying edible crops are
found in leafy vegetables. Lettuce contained total carbamate residues
of up to 20 mg/kg immediately after spraying at recommended use rates,
with the highest values occurring after indoor applications. However
decay rates are normally rapid (half-lives of 1-3 days outdoors) so
that after seven days in the outdoor situation or fourteen days
indoors total pyrimidine carbamate residues are normally less than
1 mg/kg.
Cucurbits and solanaceous fruits (Table 6)
A short pre-harvest withholding interval (e.g. 1-3 days) is
normally required in solanaceous fruits and cucurbits. Pirimicarb
residues in these crops can vary with the method of application and
with whether the crop is grown indoors or outside. Nevertheless after
recommended pre-harvest withholding intervals, total pyrimidine
carbamate residues are below 1 mg/kg and usually below 0.5 mg/kg. An
exception is chili peppers, grown outdoors, where residues are below 2
mg/kg after seven days.
Treatments with a smoke generator in the greenhouse give rise to
lower residues than corresponding spray treatments (Table 6).
Root crops and legumes (Table 8)
Pirimicarb does not move from the leaves into the storage organs
(roots and tubers) of root crops. Therefore root crops and tubers do
not normally contain significant pyrimidine carbamate residues after
spraying. Where residues are detected, these are due either to the
root being wholly or partly exposed to the spray (e.g. kohlrabi and
carrots) or to residues being transferred from the foliage during
sampling and/or sample preparation. Similarly, detectable residues are
not expected in peas and beans where the edible portion of the crop is
protected by pods. The low residues which sometimes occur,
<0.1 mg/kg, probably arise from inadvertent contamination during
sample preparation.
Runner and French beans exposed to the spray contained residues
up to 1.4 mg/kg immediately after spraying but decay was generally
rapid (half-life less than 1 day) so that total pyrimidine carbamate
residues after seven days were normally less than 0.5 (mg/kg).
Fruit (Table 9)
Residues in fruit are lower than in leafy vegetables. Levels are
generally below 2 mg/kg, even immediately after application. These
decay rapidly, with half-lives of 1-2 days. After seven days, total
pyrimidine carbamate residues are normally below 0.5 mg/kg and
TABLE 6. Residues of Pirimicarb And Its Two Major Carbamate-Containing Metabolites In Leafy
Vegetables, Cucurbits and Solanaceous Fruit From supervised Trials With Sprays Or
Smoke Generators
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Brussels sprouts Netherlands 0.14 1 3-4 0.11-0.13
U.K. 0.28 1 3 <0.14
0.28 3 3 0.28
U.S.A. 0.14 5-9 3 0.03-0.10
0.28 5-9 3 0.05-0.32
Broccoli U.S.A. 0.14 2-5 1 <0.05-0.71
0.28 2-5 1 <0.10-0.96
Cabbage (green) Australia 0.25* 1 7 0.21
Germany 0.30 1-2 7 0.57
New Zealand ( 0.28 1 7-8 0.11-0.30
U.K. (
U.S.A. 0.14 2-5 3 <0.05-0.08
0.28 2-5 3 <0.05-0.18
Cabbage (savoy) Germany 0.30 1 1-10 0.01
Japan 0.50* 4 7-8 0.02-0.78
Cauliflower Germany 0.30 1 7 0.03
(whole)
U.S.A. 0.14 1-7 3 <0.05
0.28 1-7 3 <0-05-0.275
Celery (outdoor) Netherlands 0.25 1 7 0.22-0.97
U.K. 0.28 1 7 0.18
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
(indoor) Netherlands 0.25 1 7 4.1
Endive Netherlands 0.28 1 7 <0.10
Lettuce (outdoor) Australia 0.28 1 0 7.5
2 1.4
5 0.23
9-16 ND**
0.56 1 0 19.6
2 2.4
5 0.33
9 0.38
13-16 ND
Germany 0.50 1 7 <0.01-0.05
U.K. 0.25* 1 7 <0.18-0.56
U.S.A. 0.14 3-7 1 <0.05-0.44
0.28 3-9 1 <0.05-0.97
(indoor) U.K. 0.25* 1 13-14 <0.18-0.47
Parsley (outdoor) Netherlands 0.25 1 15 0.15-0.65
(indoor) 0.25 1 15 4.7
Onions (salad) U.K. 0.16* 1 0 0.04-0.39
Spinach U.K. 0.50 1 7 <0.01-0.03
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Cucumbers Canada 0.28 1 3 0.04-0.13
U.K. 0.28 1 2 <0.10-0.34
Aubergines U.S.A. 0.28-0.42 7 3 <0.01
Gherkins Germany 0.3-0.5 1-3 2-3 0.01-0.05
U.K. 0.25* 1 3 0.45
Peppers (outdoor) Canada 0.14 3 3 0.05
(Bell) U.S.A. 0.14 3-6 1 0.05-0.34
0.28 4 1 0.05-0.43
(indoor) Canada 0.28 1 2 0.14
France (
Netherlands( 0.25* 1 3 <0.12-0.16
Peppers (outdoor) U.S.A. 0.14 2-10 7 0.05-0.83
(chili) 0.28 1-10 7 <0.05-1.26
Tomatoes Australia 0.25* 1 2 0.08
Canada 0.19+0.28 2 2 0.04
0.28 1 3 0.28
Germany 0.50 1-2 1 <0.01-0.04
TABLE 6. (Cont'd.)
SPRAY USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Japan 0.50* 2-4 1 0.10-0.61
U.K. 0.25* 2 2 <0.01-0.37
U.S.A. 0.28 7 3 <0.01
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Cucumbers Germany 30 3 1 0.29-0.32
Netherlands 20 1 0 0.32
15 1 1 0.03
U.K. 15 1 0 0.05-0.19
Endive Netherlands 12 1-2 13-14 0.02.-0.14
Lettuce Germany 14 3 14 0.46
Netherlands ( 15 1 14 0.02-0.85
U.K. (
Gherkins Germany 15-17 3 0 0.11
TABLE 6. (Cont'd.)
SMOKE GENERATOR USES
Crop Country Application No. of Pre-harvest Total carbamate
Rate Applications Interval Residues Range
mg ai/m3 (Days) (mg/kg)
Netherlands 14 1 1 0.02
Tomatoes Germany 14-16 3 0 0.01-0.08
Netherlands 13 1 1 0.01-0.02
U.K. 15 1 0 0.08-0.09
* Rate in g a.i./l
** Not detectable
consistently below 1 mg/kg. Pirimicarb does not penetrate the skin of
fruit and hence can if necessary be removed by peeling (Edwards and
Dick 1976; Manley 1969).
TABLE 7. Decline of pirimicarb residues on cabbages (New Zealand)
Application Residues in mg/kg, at intervals (days) after application
rate
No. kg a.i./ha 1 3 7 10 14 21
1 0.30 0.47 0.38 0.30 0.14 0.13 <0.06
1 0.50 0.49 0.39 0-30 0.24 0.20 <0.06
2 0.28 0.44 0.18 0.19
2 0.56 0.88 0.39 0.18
Cereals and seed crops (Tables 9 and 10)
Wheat grain analysed in husk contained total pyrimidine carbamate
residues of 1-3 mg/kg on day after application (Table 10). Half-lives
varied between one and seven days. No residues were detected in
dehusked grain 7 days after spraying.
Very low residues (<0.1 mg/kg) were detected in oil seed rape
seven days after application of pirimicarb.
FATE OF RESIDUES
In Plants
Pirimicarb is rapidly lost from plants on which it has a
half-life of 1-3 days. Volatilisation affords the primary means of
loss: the higher the ambient temperature, the greater the percentage
of pirimicarb lost by volatilisation (Manley, 1969; Bewick and Leahey,
1976; Davis and Hemingway, 1976; Hemingway and Davis, 1976).
Pirimicarb also undergoes photochemical and metabolic
degradation.
The major carbamate-containing degradation products are compounds
II and III. (Figure 1). Compound IV is only a minor
carbamate-containing degradation product. Other minor degradation
products include the water soluble hydroxypyrimidines I, V, and VI,
TABLE 8. Residues of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Root
Vegetables And Legumes From Supervised Spray Trials
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Beetroot U.K. 0.25* 1 0 0.02
Carrots
(submerged) U.K. 0.28 1 0 0.02-0.04
(tops of roots
exposed) U.K. 0.25* 1 7 0.11
Kohlrabi (roots) Germany 0.25 1 0 <0.01-0.23
0.25 2 0-10 <0.01
(leaves) Germany 0.25 1 7 0.09-0.11
0.25 2 1-10 <0.01
Parsnips U.K. 0.25* 1 0 <0.01
Potatoes Netherlands 0.25 1 0 <0.01
(aerial)
New Zealand ( 0.28 1 0 <0.01
U.K. (
U.S.A. 0.14 1-7 0 <0.01
0.28 1-7 0 <0.01
Radish Japan 0.25* 2-4 0 <0.01
Sugar beet Germany 0.30 1-2 0 <0.01-0.02
(roots)
(leaves) Germany 0.30 1-2 7-8 0.12-0.70
TABLE 8. (Cont'd.)
Vegetable Country Application No. of Pre-harvest Total Carbamate
Rate Applications Interval Residues Range
kg ai/ha (Days) (mg/kg)
Turnips U.K. 0.25* 1 0 0.04
Beans (broad) Germany 0.30 1 0 0.09
Beans (runner/ Germany 0.30 1 7 0.05
French)
U.K. 0.25* 1 7-8 <0.06.0.55
0.50 1 0 0.89-1.4
Peas Australia 0.28 4 0 0.06
Germany 0.30 1 0 0.10
Netherlands 0.25 1 0 0.03
U.K. 0.16* 1 0 <0.01
0.50 1 7 0.06-0.67
* indicates rate in terms of g ai per litre.
TABLE 9. Residues Of Pirimicarb And Its Two Major Carbamate Containing Metabolites In Fruit, Cereals
And Seed Crops From Supervised Spray Trials
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
Apples Canada 0.25 1 7 0.01-0.60
U.K. 0.16 2 7 0.09-0.37
Blackcurrants U.K. 0.16 1 7 0.01-0.08
Oranges Australia 0.25 1 7 <0.01 (whole fruit)
<0.02 (flesh)
0.32 (peel)
Peaches Australia 0.25 1 7 0.05
Italy ( 0.25 1-3 5-6 <0.10-0.27
Spain ( 0.50 1-3 5-6 0.20-0.36
Plums Canada 0.14* 1 7 0.36
0.28* 1 7 0.38
U.K. 0.25 1 7 0.06-0.11
Raspberries U.K. 0.16 1 7 0.02-0.09
Strawberries Australia 0.25 1 0 1.5
0.25 1 7 0.23
U.K. 0.25 1 7 0.07-0.09
Wheat (in husk) Australia 0.25* 1 7 0.33
Germany 0.25* 1-2 7 0.04-1.5
TABLE 9. (Cont'd.)
Crop Country Application No of Pre-harvest Total carbamate
Rate Applications Interval Residue Range
g ai/l (Days) (mg/kg)
South Africa 0.25* 1-2 7 0.27-0.52
(de-husked) Netherlands 0.125* 1 7 <0.02
Oil seed rape Australia 0.28- 1-4 7 <0.10
Czechoslovakia 0.35*
* indicates rate in terms of kg a.i./ha
and guanidine and its 1-methyl and 1,1 dimethyl derivatives. This
pattern of degradation has been shown to take place in part or
completely on peach and sugar beet leaves (Manley, 1969), lettuce
(Hemingway and Davis, 1976) and cabbage (Davis and Hemingway, 1976).
The rate of degradation varies with crop type and weather conditions.
Thus in lettuce, seven days after application of pirimicarb 71% of the
radioactivity remaining was present as pirimicarb and the carbamates
II and III, whilst in cabbage the figure was 32%.
In lettuce plants sprayed with 2-14C-labelled pirimicarb four
weeks prior to harvest, 25% of the radioactivity associated with the
plants could not be extracted by refluxing with ethanol and was termed
`bound'. Pirimicarb, compounds III and V and the three guanidines
mentioned above were all shown to be minor constituents of the bound
residue. Approximately 40% of the bound residue was associated with
the cellulose and hemicellulose of the plant (Teal and Skidmore,
1976).
When pirimicarb was introduced systemically into broad bean
plants by stem injection, its half-life was less than one day. Again
products II and III were formed initially. However these and
pirimicarb were subsequently degraded, the carbamate groups being
quantitively eliminated as measured by the evolution of 14CO2 from
plants treated with 14C-carbonyl-labelled pirimicarb (Manley, 1969).
Spraying of potatoes with 2-14C-labelled pirimicarb at 0.42 kg
ai/ha resulted in very low residues of radioactivity in the tubers
(less than 0.05 mg/kg pirimicarb equivalents). More than 80% of this
low residue was present as unextractable materials or as a complex
mixture of water-soluble materials closely associated with plant
sugars and pigments (Bowker et al, 1975).
When pirimicarb sprayed at rates up to 5 kg ai per hectare, ie
20-40 times normal use rates, total pyrimidine carbamate residues in
"follow-up" crops at harvest extremely small - of the order of the
limit of determination of the analytical method or below (Edwards et
al, 1974 and 1975). Total radiolabelled residues in "follow-up" crops
at harvest as a result of applying 2-14C-labelled pirimicarb at 0.5
kg ai per hectare were also small - up to 0.14 mg/kg pirimicarb
equivalents. In lettuce, which contained the highest radioactive
residue, approximately 50% of the activity was due to a combination of
pirimicarb, compound III, the hydroxypyrimidines I, V and the three
guanidines (Hughes and Leahey, 1974; Leahey and Benwell, 1976).
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. Only traces of residues are transferred to
milk and tissues.
TABLE 10. Pirimicarb Residues on Wheat (South Africa) at Intervals
after Application
Residues, mg/kg
Application Days
rate No. of after Demethyl-
g a.i./ha Applications Application Pirimicarb pirimicarb
250 1 1 2.3 0.4
2 1.7 0.3
4 0.4 0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.7 0.4
2 0.6 0.3
4 0.2 0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.5
2 1.6 0.2
4 0.3 <0.2
7 0.2 <0.2
55 <0.1 <0.2
125 1 1 0.8 0.4
2 0.6 0.2
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
250 1 1 2.5 0.4
2 2.3 0.4
4 0.4 <0.2
7 0.3 <0.2
55 <0.1 <0.2
125 1 1 0.6 0.3
2 0.6 0.3
4 0.1 <0.2
7 <0.1 <0.2
55 <0.1 <0.2
Results are the average of 3 determinations
Recovery pirimicarb 105% at 1 mg/kg
demethyl-pirimicarb 78% at 1 mg/kg
Results not corrected for recovery
Limit of detection pirimicarb 0.1 mg/kg
demethyl-pirimicarb 0.2 mg/kg
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum radioactive residue in the milk (0.25 mg/kg
pirimicarb equivalents) was detected one hour after dosing; 80-90% of
this residue was due to the hydroxypyrimidines I, V and VI (Figure 1).
Subsequent residues were 0.06 mg/kg pirimicarb equivalents or less.
The maximum residue in fat and meat tissues after twelve days was only
0.04 mg/kg pirimicarb equivalents (Hemingway et al 1976). See also
"Biotransformation".
In soil
Pirimicarb is extensively degraded in soil. Degradation occurs
most rapidly in less acid soils. In soils of pH6.5 and above,
pirimicarb has a half-life of up to 7 weeks under aerobic conditions.
The rate of degradation in more acid soils was considerably enhanced
by air drying. Although less important than soil pH, a higher organic
matter content also appears to enhance the rate of degradation of
pirimicarb (Hill et al 1975a; Hill and Stevens 1975).
The principal route of degradation in soil is by hydrolysis of
the carbamate moiety from the pyrimidine ring. N-dealkylation of the
2-dimethylamino group has also been observed. Cleavage of the
pyrimidine ring has been demonstrated by the loss of 14CO2 from
soils treated with 2-14C-labelled pirimicarb. Hydrolysis of the
carbamate moiety results from both biological and chemical soil
activity whereas pyrimidine ring cleavage is mediated only by
biological activity. Several fungi and actinomycete cultures are
capable of metabolising pirimicarb (Hill et al 1975a; Hill and Arnold,
1974; Manley et al 1972).
Compounds I, III and V (Figure 1) are all major soil metabolites.
Minor degradation products include the carbamates II and IV and the
hydroxy pyrimidine VI (Hill et al, 1975a). Compound I is the major
product of photochemical degradation of pirimicarb on the soil surface
(84% of the residue recovered after 20 days) (Leahey and Benwall,
1974).
After two years, more than 70% of the radioactivity resulting
from the application of 2-14C-labelled pirimicarb to acid soils was
not extracted by refluxing with acetone: water (5:1). Almost all of
this bound residue was associated with the fulvic acid fraction of the
soil. Up to 60% of the bound residue was due to pirimicarb plus the
hydroxypyrimidines I and V (Hill et al 1975b).
Pirimicarb and its soil degradation products are of low mobility
in soil; lower than atrazine which is recognized as being only
moderately mobile (Riley et al 1974). There is no significant
concentration of pirimicarb in "run-off" water or eroded soil (Gouman
and Hale, 1975).
In water
Pirimicarb undergoes rapid photochemical degradation in water:
over 50% of the applied pirimicarb is degraded in 24 hours at pH5-9.
There is no significant loss of pirimicarb by volatilisation from
water. Degradation products include the pyrimidine carbamates II-IV,
the hydroxypyrimidines I, V and VI, 1,1-dimethylguanidine and
1-methylguanidine. Only traces (<1%) of guanidine itself have been
detected (Bowker and Griggs, 1974; Lincoln and Hemingway 1974).
In washing and cooking
Residues of pirimicarb and its two major carbamate metabolites II
and III on lettuce were reduced by 38-49% by washing with cold water.
In addition, during cooking approximately 60% of the pirimicarb and
52-65% of the carbamate-containing metabolite residues were
transferred from Brussels sprouts and cabbage into the cooking water.
There was no detectable conversion to other products during the
washing or cooking processes (Edwards et al, 1976).
In storage
There was no loss of residue from deep-frozen samples fortified
with pirimicarb. Details are given in "Methods of residue analysis".
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Monitoring of pirimicarb residues in the Netherlands has shown
that residues in imported grapes and in all but one sample of
home-produced endive were within the national tolerance of 1 mg/kg.
METHODS OF RESIDUE ANALYSIS
A gas-chromatographic method using a nitrogen-selective detector
to detect residues of pirimicarb plus its two major
carbamate-containing degradation products (II and III, Figure 1) is
the preferred residue method (Zweig 1975).
Crop samples are macerated with choloroform in the presence of
anhydrous sodium sulphate. The extract is subjected to a solvent
partition clean-up during which
5,6-dimethyl-2-formylmethylamino-4-pyrimidinyl dimethylcarbamate (II)
is hydrolysed to 5,6-dimethyl-2-methylamino-4-pyrimidinyl
dimethylcarbamate (III). The dried extract in ethyl acetate is
evaporated to dryness and taken up into acetone, and an aliquot of
this solution is injected into a gas chromatograph fitted with a
nitrogen detector. The size of the peaks obtained at the retention
times of pirimicarb and of compound III are used to calculate residue
levels by comparison with peaks obtained by injection of standard
solutions of the two compounds (ICI Plant Protection Ltd., 1972).
Some crop extracts may contain co-extracted interfering
compounds. These can be removed by cleanup on a column of silica gel,
prior to gas-chromatographic determination (Edwards and Dick, 1976).
The limit of determination for pirimicarb and for compound III in
crops is normally 0.01 mg/kg. Under the conditions of chromatography
described in the method, retention times for pirimicarb and compound
III were found to be 0.7 and 1.5 minutes respectively (ICI Plant
Protection Ltd, 1972).
Methanol may be used as the extraction solvent instead of
chloroform. For crops containing a high percentage of water (i.e.
normal 'green' crops), chloroform and methanol are equally efficient
(Manley, 1969; Edwards and Dick, 1975). However for crops of very low
water content (e.g. dry leaf tea, cured tobacco and oil seed rape), it
is essential to use methanol to extract residues of pirimicarb and
compounds II and III completely (Edwards and Dick, 1976).
Hydrolysis of compound II to compound III in 0.1 M hydrochloric
acid during the solvent partition cleanup was shown to be complete
(Cox, 1973).
A colorometric method, involving hydrolysis to dimethylamine, is
also available for determining residues of pirimicarb metabolites II
and III in crops (Zweig, 1975). Crop samples are macerated with
chloroform and the extract washed successively with 0.1M sodium
hydroxide, water and a slightly acid phosphate buffer (ph 4.3). The
chloroform is evaporated under reduced pressure and the residue steam
distilled with 1 M sodium hydroxide, liberating dimethylamine which
is absorbed in 0.1 M hydrochloric acid and determined
absorptiometrically as the yellow-brown copper dimethydithiocarbamate
complex at 436nm. Where troublesome emulsions occur at the washing
stages, the sample is extracted with acetone. Emulsifying agents are
removed by solvent partition; pirimicarb and its carbamate-containing
metabolites are then extracted into chloroform which is treated as the
chloroform extract obtained above.
Residues of the dimethyl dithiocarbamate fungicides (thiram and
ziram) interfere with the determination of pirimicarb and the two
metabolites by this method. Where interfering residues are present, a
chloroform extract obtained as described in the preceding paragraph is
subjected to thin layer chromatography alongside a marker spot on
silica gel GF 254. The developed chromatogram is viewed under
ultra-violet light, the primicarb band is removed and steam distilled,
and the determination completed, as described above (ICI Plant
Protection Ltd. 1969). The limit of determination of the method is
normally 0.7 mg/kg pirimicarb or pirimicarb equivalents. Recoveries in
excess of 70% are normally obtained (Bullock, 1969). The determination
of residues of pirimicarb and compound II and III is not affected by
the presence of residues of the ethylene bisdithiocarbamate fungicides
(e.g. maneb and zineb), nor by the presence of residues of carbaryl,
as none of these compounds is hydrolysed to dimethylamine.
TABLE 11. Stability Of Pirimicarb Residues In Crops Stored At -14°C
Mean residues, mg/kg
CROP
Initially After After
3 6.5
months months
Apples 0.92 0.94 0.86
Potatoes 1.00 0.79 0.81
Lettuce 0.97 1.04 0.94
Cucumbers 0.91 1.05 0.81
Pirimicarb residues are stable at -14°C. Samples of apples,
potatoes, lettuces and cucumbers were spiked with pirimicarb at
1 mg/kg, stored at -14°C for up to six months and analysed for
pirimicarb by the gaschromatographic method described above (see Table
11). Statistical analysis of the individual residue figures and of the
determined recovery values showed that there were no significant
differences among the residue values due either to storage time or to
crop (Edwards and Atreya, 1974).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following national tolerances have been established for residue of
pirimicarb plus its two major carbamate containing metabolites, II and
III (Table 12).
TABLE 12. National tolerances reported to the Meeting
Country Crop Tolerance mg/kg
Australia Vegetables, 1
fruit and hops 0.5
Hungary All crops 0.5
Japan Fruit and vegetables 0.3
TABLE 12. (Cont'd.)
Country Crop Tolerance mg/kg
New Zealand All crops 0.5
Switzerland Fruit and vegetables 1
USA Potatoes 0.1
Venezuela All crops 0.5
Netherlands Fruit and vegetables 1.0
Grain 0.05
Potatoes 0.05
APPRAISAL
Pirimicarb is a fast acting, selective aphicide which is now
registered in a number of countries for use on fruit, vegetables and
other crops. It has proved effective on all crops on which it has been
tried against all aphids including organophosphorus-resistant strains,
with the exception of multi-resistant strains of Phorodon humuli.
It is harmless to useful parasites and predators, with the exception
of hoverflies, and has a very low toxicity to bees.
In addition to its contact and translaminar actions pirimicarb is
effective as a fumigant. Technical pirimicarb is not less than 95%
pure and is normally 98% pure; the impurities contain up to 1.3% of
other pyrimidine carbamates. It is formulated as a 50% dispersible
powder and a 50% dispersible pellet or grain, as a ULV formulation and
in a 10% smoke generator. The usual rates of application are 0.25 kg
a.i./ha as a crop spray and 15 mg/m3 as a smoke.
Pirimicarb possesses only limited persistence on crops and the
pre-harvest withholding intervals range from zero for root crops, and
others where the edible portion is protected from the spray, to seven
days for leafy vegetables.
Pirimicarb is rapidly lost from plants; it has a half-life of 1-3
days. Most of the loss is by volatilisation, the extent of which
increases with rise in the ambient temperature, but there is some
photochemical and metabolic degradation. There are two major
metabolites containing the carbamate moiety, the N-formyl(methylamino)
(II) and 2-methylamino (III) analogues of pirimicarb, which occur in
plants. Minor degradation products include a third carbamate, 2-amino
pirimicarb, the water-soluble 2-dimethylamino-, 2-methylamino- and
2-amino-hydroxypyrimidines and the corresponding guanidines. The
recommended residue methods for pirimicarb also estimate the two major
carbamate-containing metabolites.
Use of radio-labelled material shows that some part (25% in one
experiment) of the pesticide may become bound to plant materials (i.e.
not extractable with organic solvents) but, as far as can be judged,
the more toxic carbamate compounds are not major constituents of this.
Within the plant (stem injection of broad beans), metabolic products
II and III are formed initially but subsequently degraded. Transfer to
the tubers of sprayed potatoes is small. Follow-up crops grown on
heavily sprayed land contain only very small amounts (at the
analytical limit) of determinable residue. Washing heavily treated
lettuce with cold water reduces residues of pirimicarb by about 38%
and cooking Brussels sprouts and cabbage gives a 60% reduction.
Pirimicarb is concentrated in the skin of apples and the residues are
reduced below the level of detection on peeling.
The transfer of residues of pirimicarb from animal feed to the
milk and meat of cows appears to be very small.
In soil there is substantial hydrolysis of the carbamate moiety
from the pyrimidine ring and photochemical degradation on the soil
surface also very largely produces the hydroxypyrimidine V. Pirimicarb
and its degradation products are of low mobility in soil and there is
no significant concentration of pirimicarb in run-off water or eroded
soil.
Pirimicarb is rapidly degraded photochemically in water exposed
to bright sunlight (50% in 24 hours at pH 5 to 9) but decomposition is
slow in the dark.
Adequate methods of analysis are available for regulatory
purposes. A gas-chromatographic method using a nitrogen selective
detector to detect residues of pirimicarb plus its two major carbamate
degradation products II and III (II is converted to III during the
clean-up process) is the preferred method. The limit of determination
of pirimicarb and compound III in crops by this method is 0.01 mg/kg.
Total carbamate can also be determined by a colorimetric method for
which the limit of determination is 0.1 mg/kg and the pesticide
recovery better than 70%. Modifications of clean-up etc. are available
for both methods to deal with particular problems (Zweig, 1975).
Although little information is yet available on the residue of
pirimicarb in food moving in commerce, numerous results from
supervised residue trials on crops growing in a number of countries
enable recommendations to be made for certain groups of crops. Because
the maximum residue limits recommended for many leafy vegetables are
the same it would be reasonable to have a single recommendation for
this group.
RECOMMENDATIONS
The following temporary maximum residue limits are recommended.
They refer to the total residue of pirimicarb, its
N-formyl(methylamino) analogue (desmethylformamido-pirimicarb) and
desmethyl-pirimicarb expressed as pirimicarb.
Commodity Temporary Maximum Note:
Residue Limit
(mg/kg) Pirimicarb has
a half-life of
Chili peppers 2 1-3 days on
plants; leafy
Apples 1 commodities
Bell peppers 1 are harvested
Broccoli 1 7 days after
Brussels sprouts 1 application,
Cabbage 1 root crops are
Cauliflower 1 harvested 0-3
Celery 1 days after
Cucumber 1 application
Eggplant 1
Endive 1
Gherkins 1
Lettuce 1
Parsley 1
Tomatoes 1
Blackcurrants 0.5
Beans (with pod) 0.5
Kohlrabi 0.5
Onions 0.5
Peaches 0.5
Plums 0.5
Raspberries 0.5
Strawberries 0.5
Peas 0.2
Rapeseed 0.2
Beans (without pod) 0.1
Beetroot 0.05*
Citrus fruits 0.05*
Parsnips 0.05*
Potatoes 0.05*
Radishes 0.05*
Sugar beet 0.05*
Turnips 0.05*
Wheat 0.05*
FURTHER WORK OR INFORMATION
Required (by 30 June 1978)
1) Studies on other species, primarily primate, to define the range
of species' susceptibility to the hematologic changes noted in
the dog.
* At or about the limit of determination.
2) Definition of the susceptibility to hemolytic anemia in the
inbred beagle strain used in toxicological studies.
3) Studies to fully define the reduced growth noted in several
rodent dietary studies.
4) Immunological studies when appropriate in animals species other
than the clog to define further the significance (and possible
range of occurrence) of the hemolytic anemia.
5) Significance of hypochromic anemia associated with rats fed high
levels of plant metabolites.
Required (by 1980)
1. Carcinogenic study in an appropriate mammalian species using a
currently acceptable protocol.
Desirable
1. Survey of industrially exposed individuals by immunological (or
serological) techniques to define the potential occurrence of
antibodies following pirimicarb exposure.
2. More information on residues in grains other than wheat, e.g.,
barley, oats, rice, maize.
REFERENCES
Bagness, J.E., Hamer, C.M. and Willis, G.A., Pirimicarb
1975 Operational Experience During Formulation.
Unpublished report from ICI Plant Protection
Division submitted to the World Health
Organization by ICI Ltd.
Bewick, D.W. and Leahey, J.P. Pirimicarb : Identification
1976 Of Material Volatilised From The Surface Of A
Treated Lettuce Plant, ICI Plant Protection Ltd.
Report No. TXJ1289A (Unpublished).
Bowker, D.M. and Griggs, B.F. Pirimicarb : Fate In Water.
1974 ICI Plant Protection Ltd. Report No. AR2492A
(Unpublished).
Bowker,D.M., Griggs, B.F. and May, M.S. Pirimicarb Fate In
197e Potatoes. ICI Plant Protection Ltd. Report No.
AR2491 AR (Unpublished).
Bullock, D.J.W. A Colorimetric Method Of Analysis for Residues Of
1969 PP062 In Food Crops, ICI Plant Protection Ltd.
Report No. AR2091 AR (Unpublished).
Clark, D.G., The Influence of Feeding on the Oral Toxicity of
1967a PP 062 and PP159. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Clark, D.G., The Acute Toxicity of Technical PP062, Dispersible
1967b Powder (J.F.2130) and PP062 Granules (J.F. 2148).
Unpublished report from ICI Central Toxicology
Laboratory submitted to the World Health
Organization by ICI Ltd.
Cox, J.H. Determination of Pirimicarb and Metabolites In Potato
1973 Tubers, ICI America Inc. Report (Unpublished).
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1968 Toxicity of PP062 - Beagle Dogs (Part 1).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Conning, D.M., Garner, R. and Griffiths, D., Ninety Day Oral
1969 Toxicity of PPO62 - Beagle Dogs (Part 2).
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by IGI Ltd.
Daniel, J.W. and Bratt, H., Absorption and Excretion by Rats of
1968 the Carbamate Insecticide, PP062. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Davis, J.A. and Hemingway, R.J. Pirimicarb : Fate On Cabbage. IGI
1976 Plant Protection Division Report No. TMJ1348A
(Unpublished).
Edwards, M.J. and Atreya, N.C. Pirimicarb : Storage Stability
1974 of Residues In Deep Frozen Crop Samples. ICI Plant
Protection Ltd. Report No. TMJ1090A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb : Crop Extractability
1975 Study, ICI Plant Protection Ltd. Report No.
TMJ1166A (Unpublished).
Edwards, M.J. and Dick, J.P. Pirimicarb Residue Summary Residues
1976 In Crops From Field Trials 1973-1975. ICI Plant
Protection Division Report No. TMJ1360B (Un
published).
Edwards, M.J. Dick, J.P. and Hayward, G.J. Pirimicarb Effect of
1976 Washing and Cooking upon Residues in Lettuce and
Brassicae Crops. ICI Plant Protection Division
Report No. TMJ1346A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1974 Carry Over Study On Lettuce And Radish. ICI Plant
Protection Ltd. Report No. AR2565A (Unpublished).
Edwards, M.J., Dick, J.P. and White D. Pirimicarb : Soil Residue
1975 Carry Over Study On Carrots, ICI Plant Protection
Ltd. Report No. TMJ1155A (Unpublished).
Ferguson, D.R. and Parkinson, G.R., Pirimicarb (PP062)
1973 Eye Irritation of Technical Grade Pirimicarb and
50% Dispersible Powder Formulation (JF 2538,
`Pirimor') Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Neurotoxicity in Hens.
1971a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Pirimicarb (PP062) : Irritancy Tests. Unpublished
1971b report from ICI Central Toxicology laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Pirimicarb (PP062) : Subacute Toxicity on Rabbit
1971c Skin. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K. and Sotheran, J., Pirimicarb (PP062): Three
1971 Generation Reproduction Study in Rats. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.
1971a Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylformamidopirimicarb.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Garner, R., Parkinson, G.R. and Pratt, I.S.,
1971b Pirimicarb (PP062) Subacute Oral Toxicity of the
Plant Metabolite, Demethylpirimicarb. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K., Garner, R., Litchfield, M.H. and Watson, M.,
1971c Pirimicarb (PP062): Two Year Feeding Study in
Beagle Dogs. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Hodge, M.C.E. and Kinch, D.A., Pirimicarb (PP062) :
1971d Teratological Studies in the Mouse. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. Forshaw, A.M. and Monks, I.H., Pirimicarb (PP062):
1971e Excretion and Metabolism in the Dog and Rat.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Fletcher, K., Kinch, D.A. and Hodge, M.C.E. Pirimicarb (PP062) :
1971f Teratological Studies in the Rat. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Fletcher, K. and Bratt, H., Pirimicarb (PP062). Accumulation Study
1972 in Rats. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Fletcher, K., Clapp, M.J.L., Garner, R., Litchfield, M.H. and
1972 Wilson, J., `Pirimicarb', (PP062): Two Year
Feeding Study in the Rat. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Garner, R., Phillips, C.E., and Slack, P., Pirimicarb (PP062)
1972 Pirimicarb Anaemia (A Chemically-Induced
Autoimmune Haemolytic Anaemia). Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Gowman, M.A. and Hale, S.W. Pirimicarb : Loss From Soil In
1975 Run-Off Water and Eroded Soil. ICI Plant
Protection Ltd. Report No. TMJ1102A (Unpublished).
Griffiths, D. and Conning, D.M., Ninety Day Oral Toxicity
1968 of PP062 - Albino Rats. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Hemingway, R.J. and Davis, J.A. Pirimicarb : Fate on Lettuce.
1976 ICI Plant Protection Division Report No. TMJ1324A
(Unpublished).
Hemingway, R.J. Davis, J.A. and Cleverly, B.A. Pirimicarb:
1976 Ercretion and Metabolism of a Single Oral Dose
Administered to a Cow. Unpublished report No.
AR2663A from ICI Plant Protection Division,
submitted to the World Health Organization by ICI
Ltd.
Hill, I.R. and Arnold, D.J. Pirimicarb Metabolism by Micro-Organisms
1974 Isolated from Soil. ICI Plant Protection Ltd.
Report No. TMJ1119A (Unpublished).
Hill, I.R., Arnold, D.J., Minns, E.C. and Greaves, R. Pirimicarb :
1975a Laboratory Studies Of The Degradation Of Single
Applications Of The Pesticide In Soil In the
Absence Of Plants. ICI Plant Protection Ltd.
Report No. AR2555A (Unpublished).
Hill, I.R., Harvey, B.R. and Arnold, D.J. Pirimicarb Extraction And
1975b Fractionation Of 'Bound' Residues In Soil. ICI
Plant Protection Ltd. Report No. AR2579A
(Unpublished).
Hill, I.R. and Stevens, J.E. Pirimicarb : Losses of Radioactivity
From 14C-Pirimicarb Treated Soils, During Air
Drying. ICI Plant Protection Ltd. Report No.
TMJ1143A (Unpublished).
Hodge, M.C.E., Pirimicarb (PP062): Teratological Studies in
1974 the Rabbit. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Hughes, H.E. and Leahey, J.P. Pirimicarb : Uptake of Pirimicarb And
1974 Its Metabolites From Soil By Rotational Crops. ICI
Plant Protection Ltd. Report No. AR2553A
(Unpublished).
ICI Plant Protection Ltd. Residue Analytical Method No. 15 for
1972 The Determination Of Residues Of Pirimicarb And
Its Two Major Metabolites In Crops
(Gas-chromatographic Method) (Unpublished).
ICI Plant Protection Ltd. Provisional Analytical Method No. 306/A For
The Determination Of Pirimicarb Residues In Fruits
Vegetables And Grain (Unpublished).
Jackson, J.A., Chart, I.S., and Sanderson, J.H., A Pirimicarb -
1974 Induced Haemolytic Anemia in Dogs. Report of a
Special Study. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Leahey, J.P. and Benwell, M. Pirimicarb : Photodegradation On A
1974 Soil Surface. ICI Plant Protection Ltd. Report No.
AR25504 (Unpublished).
Leahey, J.P. and Benwell, M. Pirimicarb ; Uptake From Soil And
Metabolism In Lettuce And Carrots, ICI Plant
Protection Division Report No. TMJ1331A
(Unpublished).
Lefevre, V.K. and Parkinson, G.R., Pirimicarb (PPO62;
1974 2-dimethylamino-5, 6-Dimethylpyrimidin-4-yl
Dimethylcarbamate): Acute Oral Toxicities of Plant
and Mammalian Metabolites. Unpublished report from
ICI Central Toxicology Laboratory, submitted to
the World Health Organization by ICI Ltd.
Lincoln, B.A. and Hemingway, R.J. Pirimicarb : Fate In Water. ICI
1974 Plant Protection Ltd. Report No. TMJ1116A
(Unpublished).
Litchfield, M.H., The Measurement of Blood Cholinesterase Activity
1968 in Animals Dosed with 4,5
Dimethyl-2-dimethylamino-6-pyrimidinyl Dimethyl
Carbamate (PP062): Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Manley, C.A. Pyrimidine Insecticides : The Fate of Pirimicarb
1969 On Surfaces And Within The Plant ICI Plant
Protection Ltd. Report No. AR2109A (Unpublished),
18-19 and Figure 14.
Manley, C. A. Residue Summary : Pirimicarb In Crops. ICI
1972 Plant Protection Ltd. Report No. TMJ585/2
(Unpublished).
Manley, C.A., Hill, I.R., Harper, P. Black L. and Brooks J.R.
1972 Pirimicarb : Fate In Soil. ICI Plant Protection
Ltd. Report No. TMJ788AR (Unpublished).
Main, A.R. Personal communication.
1976
McGregor, D.B., Dominant Lethal Study in Mice of ICI PP062.
1974 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Palmer, S. and Samuels, D.M., Pirimicarb: 80 Week Carcinogenic
1974 Study in Mice. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Irritation Studies of Technical
1972a and Formulated Material on Normal and Wounded
Rabbit Skin. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb (PP062): Acute Oral Toxicity Following
1972b Storage at 37°C for Three and Six Months.
Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, C.R., Pirimicarb Metabolites (R.31680 and R.35140):
1974a Acute Oral Toxicity. Unpublished report from ICI
Central Toxicology Laboratory, submitted to the
World Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.42488): Acute Oral
1974b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Skin Sensitization. Unpublished report
1974c from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Parkinson, G.R., Oral Toxicities of Three Guanidine Compounds.
1975a Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.32444): Acute Oral
1975b Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb Impurity (R.33160) : Acute Oral
1975c Toxicity. Unpublished report from ICI Central
Toxicology Laboratory, submitted to the World
Health Organization by ICI Ltd.
Parkinson, G.R., Pirimicarb: Potentiation Studies With
1975d Azinphos-methyl and Carbaryl. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A. Pirimicarb (PP062): "Pirimor" Smoke Generators:
1972a Acute Inhalation Studies. Unpublished report from
ICI Central Toxicology Laboratories submitted to
the World Health Organization.
Riley, R.A., Pirimicarb (PP062): `Pirimor' Smoke Generators.
1972b Subacute Inhalation Studies. Unpublished report
from ICI Central Toxicology Laboratory, submitted
to the World Health Organization by ICI Ltd.
Riley, R.A., Pirimicarb (PP062): Acute Inhalation Toxicity of
1972c `Pirimor' 50% Dispersible Powder. Unpublished
report from ICI Central Toxicology Laboratory,
submitted to the World Health Organization by ICI
Ltd.
Riley, R.A., Pirimicarb (PP062) Acute Inhalation Toxicity.
1973 Unpublished report from ICI Central Toxicology
Laboratory, submitted to the World Health
Organization by ICI Ltd.
Riley, D., Coutts, J., Stevens, J.E. and Newby, S.E. Pirimicarb :
1974 Leaching in Soil ICI Plant Protection Ltd. Report
No. AR2556A (Unpublished).
Samuels, D.M., Hodge, M.G.E. and Palmer, S.M., Pirimicarb - Three
1975 Two Year Feeding Studies to Assess, Carcinogenic
Potential. Unpublished report from ICI Central
Toxicology laboratory, submitted to the World
Health Organization by ICI Ltd.
Teal, G. and Skidmore, M.W. Pirimicarb Investigation Of The Bound
1976 Residues On Lettuce. ICI Plant Protection Division
Report No. TMJ1323A (Unpublished).
Zweig, G. Analytical Methods For Pesticides, And Plant Growth
1975 Regulators. Academic Press, VII:399.
