
PIRIMICARB JMPR 1978
Explanation
Pirimicarb was evaluated by the 1976 Meeting (FAO/WHO, 1977b).
A temporary ADI for man was established and temporary MRLs were
recommended for a wide range of fruits and vegetables.
Additional toxicological and residues data arising from the
1976 review are summarised in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special studies on plant metabolites
Rat
Groups of 40 rats (20 of each sex) were orally treated with
daily doses of 12.5 and 50 mg/kg of the plant metabolite carbamate
(R 34 885) for a period of 14 or 28 days. The control group
consisted of 10 animals of each sex. The treatment had no effect on
the clinical condition, behaviour and mean body weight gain. Either
showed the parameters of the haematological and bone marrow
examination and the values of the urinalyses any pathological
alternations. The results gave no evidence of hypochromic anaemia
as suggested in a previous study. The histopathological examination
revealed no abnormal findings.
In a similar experiment designed to study the inhibition of
the cholinesterase activity of the metabolite groups of 10 rats (5
males and 5 females) were treated with daily oral doses of 0, 3 and
12.5 mg/kg for 28 consecutive days. No significant inhibition of
the enzyme was found in erythrocytes and brain, whereas the plasma
cholinesterase activity showed a depression of 32% compared to
control in females after treatment with 28 doses of 12.5 mg/kg
(Parkinson, 1978a).
Groups of 40 rats (20 males and 20 females) were orally
treated with 25 and 100 mg/kg of the plant metabolite 5.6 -
dimethyl-2-methylamino-pyrimidin-4-yl dimethylcarbamate (R 34 836)
for 14 or 28 days. Additionally a control group consisting of 10
animals of each sex was included into the study. The test compound
had no adverse effect when administered at a dose level of 25
mg/kg; at 100 mg/kg however 5 male and 6 female animals died during
the study between two and seven doses with clinical signs of
cholinesterase inhibition. Some animals at 100 mg/kg lost weight.
The examination of blood and bone marrow revealed no abnormal
findings and gave no indication for hypochromic anaemia. The
results of urinalyses fell into normal limits with the only
exception of slightly higher urinary protein values in male animals
treated with 14 and 28 doses of 100 mg/kg respectively. No adverse
histopathological effects were found at 100 mg/kg.
To determine the degree of cholinesterase inhibition groups of
10 rats (5 male and 5 female animals) were orally dosed with 0,
1.5, 5, 25 and 100 mg/kg of the test compound for 28 days. The
cholinesterase activity was not affected in erythrocytes and brain,
the plasma cholinesterase activity however showed inhibition at 25
mg/kg after 28 days of about 30% in male and about 55% in female
animals; after 14 days of treatment with 100 mg/kg the depression
was 40%, after 28 days of treatment 63% in female animals only
(Parkinson, 1978b).
Short-term studies
Rat
In order to determine a no effect level as regards the growth
depression particularly in female rats as found in previous long
term studies, groups of 20 female rats were maintained on diets
containing 0, 100, 175, 250 and 750 ppm pirimicarb for a period of
8 weeks. The feeding did not adversely affect the general health
condition. At 750 ppm reduction of the body weight was observed,
accompanied by lower food consumption compared to the control
animals, especially in the first half of the study (Paul et al.,
1978).
In a paired feeding study female rats, fed with dietary levels
of 0, 250 and 750 ppm were paired with rats receiving restricted
diets at concentrations of 250 and 750 ppm respectively. A
treatment related growth depression was observed that was slight at
the 250 ppm dietary level and marked at 750 ppm after ad
libitum or restricted feeding. The results indicate that the
growth depression seems not to be due to reduced palatability of
the supplemented food (Richards et al., 1978).
Dog
To define the susceptibility to haemolytic anaemia in the
beagle strain (Jackson et al., 1977) another study was undertaken
employing the foxhound an experimental animal. Groups of dogs
received pirimicarb at dietary levels of 0 (2 animals) and 2 mg/kg
b.w. (2 animals) over a period of 16 weeks. A third group (6
animals) was maintained on a diet containing the test compound at
a level of 25/50 mg/kg The dose level of 50 mg/kg was subsequently
reduced back to 25 mg/kg as noon an marked haematological changes
appeared. The treatment was followed by a recovery period from week
17-23. One dog of the 25/50 mg/kg group was killed after a 2-week
treatment with 50 mg/kg pirimicarb, showing behavioural changes and
clinical deterioration as weight loss, inappetence and ataxia.
Loose faeces were seen in dogs from all treatment groups. At 25
mg/kg vomiting was observed sporadically. Over the first 4 weeks
the male animals showed reduced body weight gains. At 50 mg/kg
usually a few hours after feeding excessive salivation occurred;
some dogs showed toxic effects as laboured respiration, vomiting,
bloody faeces and flaccid muscles. The weight loss in some animals
was accompanied by a slight reduction in food intake. The treatment
at 50 mg/kg was associated with marked anaemia characterised by
reduction in haemoglobin, packed cell volume and erythrocyte count
and an increase in reticulocytes. The direct Coombs test failed to
produce positive results (Jackson and Royle, 1978a). The examination
of the bone marrow revealed a tendency to an increased number of
normoblasts and towards suppressed activity (hypoplasia). Anaemia,
reticulocytosis and bone marrow changes were reversible and normal
values were measured after the recovery period. Maximum inhibition
of plasma cholinesterase activity of about 50-79% and 80-90% was
measured at the 25 mg/kg and the 50 mg/kg dose level respectively.
No dose-related alternations as regards the biochemical parameters
GOT, GPT, SAP, Glucose and BUN and the common parameters of urinalyses
were found at any dose level. No abnormal macroscopic findings were
detected. Owing to the small number of control animals the
significance of various alterations in relative organ weight
particularly of the kidney and the spleen can't be determined (Fox,
1978).
Monkey
Groups of 4 rhesus monkeys (2 of each sex) were orally treated
with 2 and 25 mg/kg pirimicarb over a period of 91 days;
additionally 2 monkeys were used as controls. The treatment had no
effect on mortality, appearance, behaviour and body weight gain.
The parameters of the haematological examinations and the results
of urinalyses were within normal limits. There was no evidence of
haemolytic anaemia and all direct Coombs tests were negative
throughout the dosing period. The results of clinical chemistry
with respect to the parameters of Bilirubin, LAP, SGOT, SGPT, SAP,
total serum protein, plasma glucose, plasma urea showed no abnormal
alterations. A dose-related inhibition of the cholinesterase
activity in erythrocytes of 16 and 38% and in plasma of 8 and 74%
was found at the dose levels of 2 and 25 mg/kg 2 hours after
dosing. The examination of the bone marrow revealed no pathological
findings. The few incidental macroscopic findings are not
attributable to treatment and the organ weights were considered to
be within normal limits (Heywood et al., 1977).
In a similar study, groups of 4 rhesus monkeys of each sex
were orally dosed with 0, 2, 7 and 25 mg/kg pirmicarb for up to 17
weeks, followed by a recovery period of 8 weeks. The survival,
appearance and behaviour were not adversely affected by treatment
and the only clinical effect was loose faeces in animals of all
treatment groups. At 25 mg/kg a slight reduction In body weight
gain was observed in the female animals. The results of
haematological investigations gave no indication of a
treatment-related anaemia. The parameters of clinical chemistry and
urinalyses varied within normal limits. The inhibition of the
cholinesterase activity in erythrocytes was 19, 18 and 31% and in
plasma it was 23, 35 and 63% at the dose levels of 2, 7 and 25
mg/kg respectively. The cholinesterase activity measured after 2
weeks after cessation of treatment was normal. The findings of the
bone marrow examinations were normal as well as any macroscopic
post mortem findings. No group differences in organ weights were
discovered. In the direct Coombs test sporadically positive
reactions were found (Heywood et al., 1978a). Additional
serological studies on monkeys confirmed the weak positive
reactions in the Coombs test in some animals treated with 0, 2, 7
and 25 mg/kg pirimicarb (Jackson and Royle, 1978b). As could be
demonstrated in a test performed on 30 untreated monkeys isolated
positive reactions can occur spontaneously (Heywood et al., 1978b;
Jackson and Royle, 1978c).
COMMENTS
Previous studies in beagle dogs showed that the oral
administration of pirimicarb caused haemolytic anaemia at dose
levels of 10 mg/kg b.w. Concern was expressed by the 1976 Meeting
regarding the pathogenesis of the condition and the susceptibility
of other species than beagle dogs. Studies were performed recently
in the foxhound strain. The same haematologic changes were found
after oral treatment with 50 mg/kg. However, studies on rhesus
monkeys with administration of oral daily doses of 25 mg/kg for 17
weeks did not show my signs of haemolytic anaemia. The toxic
response of the dog therefore seems to be species specific.
Significant dose-related cholinesterase inhibition in plasma
was observed in dogs and monkeys at dose levels of 25 mg/kg and
above. The additional studies in dog (foxhound) confirm the no
effect level established at the 1976 Meeting.
Additionally the results of supplementary rat studies were
submitted to consider the previously observed growth impairment in
female rats at 250 ppm in the diet. In one of the presented studies
a no effect level of 250 ppm was noted. In a corresponding paired
feeding study a slight reduction in body weight gain at 250 ppm
compared to the control was observed, suggesting 250 ppm to be the
borderline of biological effect. Growth impairment was not caused
by diminished food intake.
Special studies on two plant metabolites 5,6-dimethyl-2-methyl
formamido-pyrimidin4-yl dimethyl-carbamate (R 34 885) and
5,6-dimethyl-2-methylamino-pyrimidin-4-yl dimethylcarbamate (R 34
836) could not confirm the abnormal haematological findings of
hypochromic anaemia noted in previous studies which were reported
at the 1976 Meeting.
Data on carcinogenicity of pirimicarb were not submitted. New
data reduced some of the concerns of the previous Meeting and
allowed a higher value for the temporary ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Monkey: 2 mg/kg body weight day
Rat: 175 ppm in the diet equivalent to 9 mg/kg body
weight
Dog: 1.8 mg/kg body weight day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg b.w.
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues data in fruit and vegetables were reviewed at the
1976 Meeting. Additional data an alfalfa and cereals is reviewed
below.
Alfalfa (Medicago sativa)
At 0.07-0.14 kg ai per ha, pirimicarb controls the blue aphid,
Acyrthosiphon kondoi and the pea aphid, Acyrthosiphon
pisum, on alfalfa. The major use of alfalfa is as hay. Freshly
cut green standing alfalfa is also used as animal feed, cut either
on an "as needed" basis or for ensilation.
Seventeen alfalfa residue trials were conducted in seven
States of the USA during 1975 and 1976, including four aerial
trials. Samples for residue analysis normally consisted of 0.5-1 kg
of mature crop derived from 3-4 replicate plots. Samples were
usually transferred to cold storage at -18°C within three hours of
harvest, prior to analysis by gas chromatography (Edwards et al.,
1977).
Residues on green standing alfalfa decay rapidly; 50-75% of
the initial residue is lost in 1-3 days. One or more days after the
last application of 0.07-0.14 kg ai per ha, residues of pirimicarb
plus its two carbamate-containing plant metabolites, II and III
were less than 50 mg/kg experssed as pirimicarb equivalents on a
dry weight basis - see Table 1 and Figure 1 (Cox, 1977; Edwards et
al., 1977).
During the seven days after spraying, total
pyrimidine-carbamate residue levels in alfalfa hay, determined on
a fresh weight basic, did not decrease markedly because moisture
loss from the cut crop acted as a concentration step. However, when
calculated on a dry-weight basis, total carbamate residues were
seen to decrease; although the rate of decay was somewhat lower
than that found in standing green alfalfa, residue levels in hay
were still smaller than those on standing green alfalfa determined
on a dry-weight basis. Residues were consistently below 20 mg/kg in
hay, on a dry-weight basis, after last spraying at 0.07-0.14 kg ai
per ha (Table 2) (Cox, 1977; Edwards et al., 1977).
Cereals (Wheat, Triticum spp., Barley, Hordeum vulgare and
Oats, Avena sativa)
At 0.125-0.25 kg ai per ha pirimicarb controls the grain
aphid, Macrosiphum avenae, on cereals. Spraying is conducted at
an immature growth stage, normally several weeks before harvest.
Residue trials have been conducted in Australia, South Africa,
Canada, Denmark, Germany, the Netherlands and the UK. Approximately
1 kg samples were normally transferred to a deep freeze within a few
hours of harvest. Residues of pirimicarb and its two major
carbamate-contained plant metabolites were usually determined by
the gas-chromatographic method. As in the case of alfalfa residues
of pirimicarb on green standing cereals decay rapidly, 50% of the
initial residue being normally lost within 1-3 days. One day or
more after the application of 0.25 kg ai per ha, residues of
pirimicarb plus its two carbamate-containing plant metabolites, II
and III, were less than 5 mg/kg expressed as pirimicarb equivalents
- Table 3 and Figure 1 (Dick, 1978; Kennedy, 1978). There was no
obvious difference between residues on immature ears and stalks
(Dick, 1978). No residues were detected in grain and straw
harvested three weeks or more after spraying at 0.125-0.3 kg ai,
per ha (limit of determination; normally 0.01-0.04 mg/kg). See
Table 4 (Dick, 1978; Kennedy, 1978).
TABLE 1. Residues of pirimicarb in green standing alfalfa ("green chop"),
USA 1975-76
Application No. of Pre-harvest Total carbamate
rate applications Interval residues
(kg ai/ha) (days) (mg/kg)
Range Mean
0.07 1-3 0 1.4-26 13
1 1.3-14 6.2
2 0.92-9.7 3.9
3 0.92-6.4 3.7
7 0.48-3.5 2.1
0.14 1-4 0 6.6-79 27
1 3.1-45 12
2 1.9-46 8.7
3 1.0-27 6.5
7 0.57-11 3.0
0.28 1-4 0 12-38 29
1 6.8-22 14
2 5.5-14 9.5
3 2.6-11 7.4
7 1.0-5.8 2.6
* expressed as mg/kg pirimicarb equivalents and on a dry weight basis.
TABLE 2. Residues of pirimicarb in alfalfa hay, USA 1975-76
Application No. of Pre-harvest Total carbamate
rate applications interval residues
(kg ai/ha) days (mg/kg)
Range Mean
0.07 1-3 0-3 0.29-12 3.6
6-7 0.81-5.1 2.3
0.14 1-4 0-3 0.55-11 2.9
6-7 0.41-7.5 2.3
0.28 2-4 0-3 1.0-14 5.3
6-7 1.4-8.1 4.2
* expressed as mg/kg pirimicarb equivalents on a dry weight basis.
TABLE 3. Residues of pirimicarb in green standing wheat and barley, 1973-78
Country Application Pre-harvest Total carbamate
rate interval residues
(kg ai/ha) (days) (mg/kg)*
Range Mean
Wheat
Canada 0.14 1-2 0.89-3.9 2.4
5 0.76
7 < 0.04-0.98 0.51
South Africa 0.25 1 0.84-1.1 0.96
2 0.80-1.0 0.91
4 0.52-0.65 0.60
7 0.27-0.52 0.41
Germany 0.25 0 2.5-4.7 3.8
1 0.58-3.4 2.4
3 0.13-2.6 1.6
7 0.04-1.5 0.99
TABLE 3 (Continued)
Country Application Pre-harvest Total carbamate
rate interval residues
(kg ai/ha) (days) (mg/kg)*
Range Mean
Australia (0.025% ai 0 5.9
spray) 2 3.3
7 0.33
South Africa 0.5 1 2.0-2.6 2.3
2 1.5-2.0 1.7
4 1.3-1.3 1.3
7 0.62-1.0 0.79
Germany 0.5 0 2.2-2-4.5 3.1
1 0.47-1.7 1.2
3-4 0.19-1.0 0.48
7-8 0.05-0.21 0.21
Barley
Canada 0.14 1 2.6
5 0.51
7 0.42
* expressed as mg/kg pirimicarb equivalents on a fresh weight basis.
TABLE 4. Residues of Pirimicarb in cereal grains and straw, 1968-78
Application Pre-harvest Total carbamate
Country rate interval residues
(kg ai/ha) (days) (mg/kg)*
Range Mean
1. Wheat grain
Canada 0.125-0.14 14 <0.04
23 <0.04
92 <0.04
TABLE 4 (Continued)
Application Pre-harvest Total carbamate
Country rate interval residues
(kg ai/ha) (days) (mg/kg)*
Range Mean
Denmark 0.125 33 <0.01 <0.01
Germany 13-15 <0.01 <0.01
18-21 <0.01 <0.01
Holland 56 <0.01 <0.01
Denmark 0.28-0.3 33 <0.01 <0.01
Germany 6-9 <0.01-0.12 0.05
21-22 <0.01 <0.01
39-42 <0.01 <0.01
Holland 7 <0.01 <0.01
14 <0.01 <0.01
56 <0.01 <0.01
UK 70 <0.1** <0.1**
75-103 <0.01 <0.01
Barley grain
Canada 0.14 14 0.09
45 <0.04
Oat grain
Canada 0.14 52-71 <0.04 <0.04
2. Wheat straw
Germany 0.125 18-21 <0.01 <0.01
UK 0.28 75-103 <0.01 <0.01
Barley straw
Canada 0.14 14 0.26
* expressed as mg/kg pirimicarb equivalents on a fresh weight basis
** determined colorimetrically; limit of determination 0.1 mg/kg
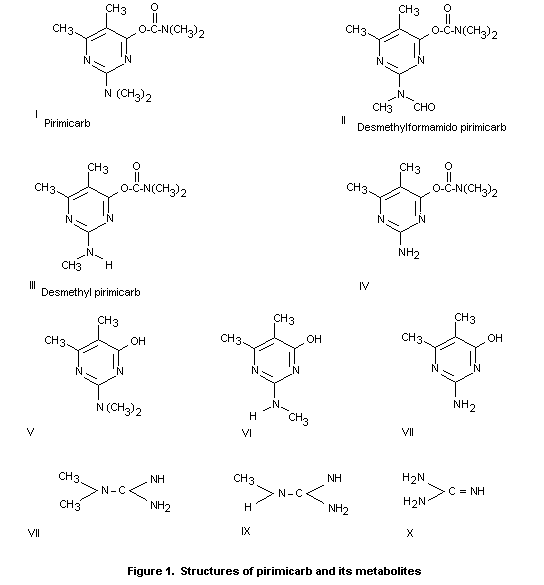 FATE OF RESIDUES
In plants
Data reviewed by the 1976 Meeting showed that pirimicarb is
rapidly lost from plants after spraying. Half of the initial residue
is lost in 1-3 days. Volatilisation affords the primary means of loss:
the higher the ambient temperature, the greater the percentage of
pirimicarb lost by volatilisation (FAO/WHO, 1977b). Pirimicarb also
undergoes photochemical and metabolic degradation. The major
carbamate-containing degradation products are compounds II And III
(Figure 1). Other degradation products include the hydroxypyrimidines
V, VI and VII, which can be present either free or as conjugates, and
guanidine and its 1-methyl and III methyl derivatives. A further
metabolite on cabbages has been characterized by its facile
degradation to the hydroxypyrimidine V and another metabolic component
was characterized as re-crystallising with 1,1-dimethylguanidine but
not susceptible to desorption from charcoal with a solution containing
a large quantity of 1,1-dimethylguanidine (Teal and Skidmore, 1978).
Some of the residue is not extracted by conventional techniques, 14%
of the radioactivity associated with cabbage leaves 3´ weeks after
applying 2 - 14C-labelled pirimicarb was not extracted by maceration
with methanol (Teal, 1978). In lettuce plants sprayed with 2-
14C-labelled pirimicarb four weeks prior to harvest, 25% of the
radioactivity associated with the plants could not be extracted with
ethanol. Pirimicarb compounds III and VI and the three guanidines
mentioned above were all shown to be minor constituents of this
unextracted residue. Approximately 40% of the unextracted
radioactivity was associated with the cellulose and hemicellulose of
the plant (FAO/WHO, 1977b).
The fate of pirimicarb on alfalfa (Davis and Hemingway, 1978a) is
similar to that reviewed previously on lettuce, cabbage, peach leaves
and sugar beet leaves.
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. The biotransformation of pirimicarb in cows
is similar to that in rat and dog. Only traces of residues are
transferred to milk and tissues (FAO/WHO, 1977b).
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum residue in the milk (0.25 mg/kg pirimicarb
equivalents) was detected one hour after dosing; 80-90% of the residue
was due to the hydroxypyrimidines V, VI and VII (Figure 1). Subsequent
residues were 0.06 mg/kg pirimicarb equivalents or less. The maximum
residue in fat and meat tissues after twelve days was only 0.04 mg/kg
pirimicarb equivalents (FAO/WHO, 1977b).
When 2-14C-labelled pirimicarb was administered to a lactating
goat for seven days at a rate equivalent to 37 ppm in the diet, 96% of
the radioactivity recovered in the excreta was in the urine.
Hydroxypyrimidines constituted the major residues - compound VI 35%,
compound VII 29% and compound V 2% of the radioactivity in the urine.
The total radioactive residue in the milk reached plateau levels of 0,
12 and 0.3 mg Pirimicarb equivalents /kg in the morning and afternoon
milkings respectively. Hydroxypyrimidines again constituted the major
components - VI and VII together accounting for more than 56% of the
radioactivity and V 5%. The goat was sacrificed four hours after
receiving the last dose. In the tissues the levels of radioactivity,
in pirimicarb equivalents, were muscle 0.45 mg/kg, fat, 0.18 mg/kg,
liver 1.8 mg/kg and kidney 2.3 mg/kg. Approximately 50-60% of the
extractable radioactivity in muscle, liver and kidney was due to the
hydroxypyrimidines V, VI and VII, the two latter predominating (Davis
and Hemingway, 1978b). The hydroxypyrimidines V-VII are also major
metabolites in the rat and dog and are of low mammalian toxicity
(FAO/WHO,1977b). In no instance were residues of pirimicarb or its
carbamate-containing metabolites detected in this study. The remainder
of the radioactivity present in tissues and milk was due to a mixture
of polar, water-soluble compounds (Davis and Hemingway, 1978b).
Groups of three barren Friesian cows were maintained for 28-29
days on diets containing approximately 20, 60 and 200 ppm pirimicarb,
applied as a spray to pelleted grass nuts. The majority of milk
samples obtained from the 20 ppm group did not contain detectable
carbamate residues (ie pirimicarb plus its two carbamate-containing
metabolites II and III; limit of determination 0.01 mg/kg pirimicarb
equivalents). Only trace residues were found in milk obtained from the
60 ppm group - up to approximately 0.02 mg/kg. Milk samples from the
200 ppm group contained mean residues of 0.07 mg/kg (range 0.02 - 0.13
mg/kg). Two animals in each group were sacrificed after 28-29 days and
the third after a further seven days on untreated diet. Total
carbamate residues in meat tissues were normally non-detectable (ie
less than 0.01 mg/kg pirimicarb equivalents) at all dose levels. Trace
residues were detected in a few muscle and fat samples but the total
carbamate residue was consistently below 0.05 mg/kg in all groups
(Edwards et al., 1978).
An oral dose of pirimicarb is rapidly excreted by hens. Only
traces of residues are transferred to eggs and tissues and the
hydroxypyrimidines V-VII constitute the major components of these
residues.
When a single oral dose of 2-14C-labelled pirimicarb was
administered at 8 mg/kg to a hen in peak egg production, 88% of the
administered radioactivity was recovered from the excreta during the
following three days (Hendley at al., 1978).
When 2-14C-labelled pirimicarb was administered to two hens in
full egg production, at a rate equivalent to 6 ppm in the diet for
fourteen days, total radioactive residues in eggs reached a plateau of
0.05-0.06 mg pirimicarb equivalents/kg in 5-8 days, when the residue
in the yolk was approximately twice that in the albumen. The
hydroxypyrimidines V-VII (Figure 1) constituted approximately 50% of
the total radioactivity in the yolks, with compounds VI and VII
predominating (each approximately 20% of the radioactivity in eggs).
The pyrimidine-carbamates I-IV together represented approximately 8%
of the total radioactivity in the yolks, the remainder of the
radioactivity was either not solubilised or was more polar than
compound VII. The hens were sacrificed three hours after receiving the
last dose. The tissues contained approximately 0.15 mg/kg (muscle),
0.35 mg/kg (liver) or 0.02 mg/kg (fat) pirimicarb equivalents. 48-63%
of the radioactivity in liver and muscle was due to the
hydroxypyrimidines V-VII, compounds VI and VII again predominating. No
more than 3.5% of the radioactivity in the tissues was due to the
pyrimidine-carbamates I-VI (Figure 1). About 25% of the radioactivity
in the tissues was due to compounds more polar than the
hydroxypyrimidine VII, including a conjugate or conjugates of VI
Hendley et al., 1978).
When a hen in full egg production was given nine daily doses of
2-14C-labelled pirimicarb at a rate equivalent to 60 ppm in the diet,
levels of total radioactivity in eggs and tissues were greater than
those in the equivalent hens dosed at the 6 ppm rate by the expected
factor of 10 (Hendley et al., 1978).
Groups of 40 hens in maximum egg production were maintained for
up to 28 day on diets containing 1.5, 4.6 and 14 ppm total pyrimidine
carbamates. Approximately 90% of the administered carbamate mixture
was pirimicarb itself and 10% the carbamate metabolites II and III. No
residues of either pirimicarb or compounds II and III were detected in
either yolks or albumen of the eggs or in muscle and skin at sacrifice
(limits of determination: 0.01 mg/kg for pirimicarb, 0.01-0.02 mg/kg
for compounds II and III determined jointly). Trace total carbamate
residues in liver did not exceed 0.05 mg/kg pirimicarb equivalents
(Edwards and Dick, 1978).
METHODS OF RESIDUE ANALYSIS
A gas chromatographic method using a nitrogen-selective detector
to determine residues of pirimicarb plus its two major carbamate
containing plant metabolites (II and III, Figure 1) was reviewed by
the 1976 meeting as the preferred crop residue method (FAO/WHO,
1977b). With modified extraction procedures, this method is suitable
for the determination of these residues in products of animal origin.
Milk and eggs are extracted with a mixture of acetonitrile and
chloroform. Tissues are extracted with methanol in the presence of
anhydrous sodium sulphate. Clean-up by solvent partition and
determination by gas chromatography are as reviewed at the 1976
Meeting (ICI Ltd., 1978).
Acetonitrile/chloroform has been shown to be an efficient solvent
for extracting aged residues of pirimicarb and of the two metabolites
from milk. Greater than 80% extraction efficiency was obtained. The
low residue levels in tissues precluded a meaningful extractability
study but by analogy with studies on the extractability of these
compounds from a wide range of crop substrates, methanol is considered
to be the most acceptable extraction solvent for tissues (Edwards et
al., 1978;). Mean percentage recovery values for samples fortified in
the range 0.005/0.01-0.01/0.25 mg/kg were 76-92% for milk, 76-98% for
egg fractions, 80-89% for cow tissues and 62-96% for hen tissues
(Edwards et al., 1978; Edwards and Dick, 1978).
APPRAISAL
Further data on residues of pirimicarb on alfalfa, wheat, barley
and oats were reviewed, together with information concerning likely
levels in milk, eggs and meat arising from treated animal feeds.
Available methods of analysis have been improved and extended to cover
products of animal origin. The data provided are sufficient to
recommend additional temporary maximum residue limits for residues of
pirimicarb in alfalfa, barley, oats, milk, meat and eggs. No data were
available on residues in other grains each as maize or rice.
RECOMMENDATIONS
The following temporary maximum residue limits refer to the sum
of pirimicarb, its N-formyl(methylamino) analogue (desmethylformanido
pirimicarb) and desmethyl pirimicarb.
Commodity Temporary MRL (mg/kg)
Alfalfa (green) 50 (dry weight basis)
Alfalfa hay 20 (dry weight basis)
Barley 0.05*
Oats 0.05*
Eggs 0.05*
Meat 0.05*
Milk 0.05*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1980)
1. Carcinogenicity study in an appropriate mammalian species using a
currently acceptable protocol.
REFERENCES
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Unpublished
(1978) report from Hazelton Laboratories Europe Ltd.,
No. 1371-72/2, submitted by ICI Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
(1977) Street A.E. Pirimicarb preliminary oral toxicity
study in rhesus monkeys (repeated dosages for 13
weeks). Unpublished report from Huntingdon
Research Centre, No. 164/77825, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
1978a Street, A.E. Pirimicarb oral toxicity study in
rhesus monkeys (repeated dosage for 17 weeks,
followed by a recovery period of 8 weeks).
Unpublished report (final report) from Huntingdon
Research Centre, No. 198/78444, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H. and Brown, G.
(1978b) Pirimicarb comparative data from naive rhesus
monkeys (white blood cell counts and Direct Coombs
Tests). Unpublished report from Huntingdon
Research Centre, No. ICI 219/78445, submitted by
ICI Ltd.
Jackson, J.A., Chart, I.S., Sanderson, J. H. and Garner, R.
(1977) Pirimicarb induced immune hemolytic anaemia in
dogs. Scand. J. Haematol. 19, 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study
(1978a) in foxhounds. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/420, submitted by ICI Ltd.
Jackson, J.A. and Royle, G. Pirimicarb: oral toxicity study in
(1978b) rhesus monkeys. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory No.
CTL/P/421, submitted by ICI Ltd.
Jackson, J.A. and Royle. G. Pirimicarb: comparative data from
1978c) naive rhesus monkeys (white blood cell counts and
Direct Coombs Tests). "Addendum: results of
additional haematological and serological
investigations". Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/419,
submitted by ICI.
Kennedy, S.H. Residues data on pirimicarb in cereals: Canada.
(1978) ICI Plant Protection Division data (unpublished).
Parkinson, G.R. Pirimicarb metabolite R 34 885: subacute oral
(1978a) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/402,
submitted by ICI.
Parkinson, G.R. Pirimicarb metabolite R 34 836: subacute oral
(1978b) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/401,
submitted by ICI.
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M.
(1978) Pirimicarb: growth study to determine a no effect
level in the female rat. Unpublished report from
ICI Central Toxicology Laboratory No. CTL/P/408
submitted by ICI.
Richards, D., Banham, P.B. and Weight. T.M. Pirimicarb paired
(1978) feeding study in a female rat. Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/407, submitted by ICI.
Teal, G. and Skidmore, M.W. Pirimicarb: metabolism on cabbage plants
(1978) in the field, ICI Plant Protection Division Report
No. RJ00033A (unpublished).
FATE OF RESIDUES
In plants
Data reviewed by the 1976 Meeting showed that pirimicarb is
rapidly lost from plants after spraying. Half of the initial residue
is lost in 1-3 days. Volatilisation affords the primary means of loss:
the higher the ambient temperature, the greater the percentage of
pirimicarb lost by volatilisation (FAO/WHO, 1977b). Pirimicarb also
undergoes photochemical and metabolic degradation. The major
carbamate-containing degradation products are compounds II And III
(Figure 1). Other degradation products include the hydroxypyrimidines
V, VI and VII, which can be present either free or as conjugates, and
guanidine and its 1-methyl and III methyl derivatives. A further
metabolite on cabbages has been characterized by its facile
degradation to the hydroxypyrimidine V and another metabolic component
was characterized as re-crystallising with 1,1-dimethylguanidine but
not susceptible to desorption from charcoal with a solution containing
a large quantity of 1,1-dimethylguanidine (Teal and Skidmore, 1978).
Some of the residue is not extracted by conventional techniques, 14%
of the radioactivity associated with cabbage leaves 3´ weeks after
applying 2 - 14C-labelled pirimicarb was not extracted by maceration
with methanol (Teal, 1978). In lettuce plants sprayed with 2-
14C-labelled pirimicarb four weeks prior to harvest, 25% of the
radioactivity associated with the plants could not be extracted with
ethanol. Pirimicarb compounds III and VI and the three guanidines
mentioned above were all shown to be minor constituents of this
unextracted residue. Approximately 40% of the unextracted
radioactivity was associated with the cellulose and hemicellulose of
the plant (FAO/WHO, 1977b).
The fate of pirimicarb on alfalfa (Davis and Hemingway, 1978a) is
similar to that reviewed previously on lettuce, cabbage, peach leaves
and sugar beet leaves.
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. The biotransformation of pirimicarb in cows
is similar to that in rat and dog. Only traces of residues are
transferred to milk and tissues (FAO/WHO, 1977b).
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum residue in the milk (0.25 mg/kg pirimicarb
equivalents) was detected one hour after dosing; 80-90% of the residue
was due to the hydroxypyrimidines V, VI and VII (Figure 1). Subsequent
residues were 0.06 mg/kg pirimicarb equivalents or less. The maximum
residue in fat and meat tissues after twelve days was only 0.04 mg/kg
pirimicarb equivalents (FAO/WHO, 1977b).
When 2-14C-labelled pirimicarb was administered to a lactating
goat for seven days at a rate equivalent to 37 ppm in the diet, 96% of
the radioactivity recovered in the excreta was in the urine.
Hydroxypyrimidines constituted the major residues - compound VI 35%,
compound VII 29% and compound V 2% of the radioactivity in the urine.
The total radioactive residue in the milk reached plateau levels of 0,
12 and 0.3 mg Pirimicarb equivalents /kg in the morning and afternoon
milkings respectively. Hydroxypyrimidines again constituted the major
components - VI and VII together accounting for more than 56% of the
radioactivity and V 5%. The goat was sacrificed four hours after
receiving the last dose. In the tissues the levels of radioactivity,
in pirimicarb equivalents, were muscle 0.45 mg/kg, fat, 0.18 mg/kg,
liver 1.8 mg/kg and kidney 2.3 mg/kg. Approximately 50-60% of the
extractable radioactivity in muscle, liver and kidney was due to the
hydroxypyrimidines V, VI and VII, the two latter predominating (Davis
and Hemingway, 1978b). The hydroxypyrimidines V-VII are also major
metabolites in the rat and dog and are of low mammalian toxicity
(FAO/WHO,1977b). In no instance were residues of pirimicarb or its
carbamate-containing metabolites detected in this study. The remainder
of the radioactivity present in tissues and milk was due to a mixture
of polar, water-soluble compounds (Davis and Hemingway, 1978b).
Groups of three barren Friesian cows were maintained for 28-29
days on diets containing approximately 20, 60 and 200 ppm pirimicarb,
applied as a spray to pelleted grass nuts. The majority of milk
samples obtained from the 20 ppm group did not contain detectable
carbamate residues (ie pirimicarb plus its two carbamate-containing
metabolites II and III; limit of determination 0.01 mg/kg pirimicarb
equivalents). Only trace residues were found in milk obtained from the
60 ppm group - up to approximately 0.02 mg/kg. Milk samples from the
200 ppm group contained mean residues of 0.07 mg/kg (range 0.02 - 0.13
mg/kg). Two animals in each group were sacrificed after 28-29 days and
the third after a further seven days on untreated diet. Total
carbamate residues in meat tissues were normally non-detectable (ie
less than 0.01 mg/kg pirimicarb equivalents) at all dose levels. Trace
residues were detected in a few muscle and fat samples but the total
carbamate residue was consistently below 0.05 mg/kg in all groups
(Edwards et al., 1978).
An oral dose of pirimicarb is rapidly excreted by hens. Only
traces of residues are transferred to eggs and tissues and the
hydroxypyrimidines V-VII constitute the major components of these
residues.
When a single oral dose of 2-14C-labelled pirimicarb was
administered at 8 mg/kg to a hen in peak egg production, 88% of the
administered radioactivity was recovered from the excreta during the
following three days (Hendley at al., 1978).
When 2-14C-labelled pirimicarb was administered to two hens in
full egg production, at a rate equivalent to 6 ppm in the diet for
fourteen days, total radioactive residues in eggs reached a plateau of
0.05-0.06 mg pirimicarb equivalents/kg in 5-8 days, when the residue
in the yolk was approximately twice that in the albumen. The
hydroxypyrimidines V-VII (Figure 1) constituted approximately 50% of
the total radioactivity in the yolks, with compounds VI and VII
predominating (each approximately 20% of the radioactivity in eggs).
The pyrimidine-carbamates I-IV together represented approximately 8%
of the total radioactivity in the yolks, the remainder of the
radioactivity was either not solubilised or was more polar than
compound VII. The hens were sacrificed three hours after receiving the
last dose. The tissues contained approximately 0.15 mg/kg (muscle),
0.35 mg/kg (liver) or 0.02 mg/kg (fat) pirimicarb equivalents. 48-63%
of the radioactivity in liver and muscle was due to the
hydroxypyrimidines V-VII, compounds VI and VII again predominating. No
more than 3.5% of the radioactivity in the tissues was due to the
pyrimidine-carbamates I-VI (Figure 1). About 25% of the radioactivity
in the tissues was due to compounds more polar than the
hydroxypyrimidine VII, including a conjugate or conjugates of VI
Hendley et al., 1978).
When a hen in full egg production was given nine daily doses of
2-14C-labelled pirimicarb at a rate equivalent to 60 ppm in the diet,
levels of total radioactivity in eggs and tissues were greater than
those in the equivalent hens dosed at the 6 ppm rate by the expected
factor of 10 (Hendley et al., 1978).
Groups of 40 hens in maximum egg production were maintained for
up to 28 day on diets containing 1.5, 4.6 and 14 ppm total pyrimidine
carbamates. Approximately 90% of the administered carbamate mixture
was pirimicarb itself and 10% the carbamate metabolites II and III. No
residues of either pirimicarb or compounds II and III were detected in
either yolks or albumen of the eggs or in muscle and skin at sacrifice
(limits of determination: 0.01 mg/kg for pirimicarb, 0.01-0.02 mg/kg
for compounds II and III determined jointly). Trace total carbamate
residues in liver did not exceed 0.05 mg/kg pirimicarb equivalents
(Edwards and Dick, 1978).
METHODS OF RESIDUE ANALYSIS
A gas chromatographic method using a nitrogen-selective detector
to determine residues of pirimicarb plus its two major carbamate
containing plant metabolites (II and III, Figure 1) was reviewed by
the 1976 meeting as the preferred crop residue method (FAO/WHO,
1977b). With modified extraction procedures, this method is suitable
for the determination of these residues in products of animal origin.
Milk and eggs are extracted with a mixture of acetonitrile and
chloroform. Tissues are extracted with methanol in the presence of
anhydrous sodium sulphate. Clean-up by solvent partition and
determination by gas chromatography are as reviewed at the 1976
Meeting (ICI Ltd., 1978).
Acetonitrile/chloroform has been shown to be an efficient solvent
for extracting aged residues of pirimicarb and of the two metabolites
from milk. Greater than 80% extraction efficiency was obtained. The
low residue levels in tissues precluded a meaningful extractability
study but by analogy with studies on the extractability of these
compounds from a wide range of crop substrates, methanol is considered
to be the most acceptable extraction solvent for tissues (Edwards et
al., 1978;). Mean percentage recovery values for samples fortified in
the range 0.005/0.01-0.01/0.25 mg/kg were 76-92% for milk, 76-98% for
egg fractions, 80-89% for cow tissues and 62-96% for hen tissues
(Edwards et al., 1978; Edwards and Dick, 1978).
APPRAISAL
Further data on residues of pirimicarb on alfalfa, wheat, barley
and oats were reviewed, together with information concerning likely
levels in milk, eggs and meat arising from treated animal feeds.
Available methods of analysis have been improved and extended to cover
products of animal origin. The data provided are sufficient to
recommend additional temporary maximum residue limits for residues of
pirimicarb in alfalfa, barley, oats, milk, meat and eggs. No data were
available on residues in other grains each as maize or rice.
RECOMMENDATIONS
The following temporary maximum residue limits refer to the sum
of pirimicarb, its N-formyl(methylamino) analogue (desmethylformanido
pirimicarb) and desmethyl pirimicarb.
Commodity Temporary MRL (mg/kg)
Alfalfa (green) 50 (dry weight basis)
Alfalfa hay 20 (dry weight basis)
Barley 0.05*
Oats 0.05*
Eggs 0.05*
Meat 0.05*
Milk 0.05*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1980)
1. Carcinogenicity study in an appropriate mammalian species using a
currently acceptable protocol.
REFERENCES
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Unpublished
(1978) report from Hazelton Laboratories Europe Ltd.,
No. 1371-72/2, submitted by ICI Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
(1977) Street A.E. Pirimicarb preliminary oral toxicity
study in rhesus monkeys (repeated dosages for 13
weeks). Unpublished report from Huntingdon
Research Centre, No. 164/77825, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
1978a Street, A.E. Pirimicarb oral toxicity study in
rhesus monkeys (repeated dosage for 17 weeks,
followed by a recovery period of 8 weeks).
Unpublished report (final report) from Huntingdon
Research Centre, No. 198/78444, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H. and Brown, G.
(1978b) Pirimicarb comparative data from naive rhesus
monkeys (white blood cell counts and Direct Coombs
Tests). Unpublished report from Huntingdon
Research Centre, No. ICI 219/78445, submitted by
ICI Ltd.
Jackson, J.A., Chart, I.S., Sanderson, J. H. and Garner, R.
(1977) Pirimicarb induced immune hemolytic anaemia in
dogs. Scand. J. Haematol. 19, 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study
(1978a) in foxhounds. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/420, submitted by ICI Ltd.
Jackson, J.A. and Royle, G. Pirimicarb: oral toxicity study in
(1978b) rhesus monkeys. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory No.
CTL/P/421, submitted by ICI Ltd.
Jackson, J.A. and Royle. G. Pirimicarb: comparative data from
1978c) naive rhesus monkeys (white blood cell counts and
Direct Coombs Tests). "Addendum: results of
additional haematological and serological
investigations". Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/419,
submitted by ICI.
Kennedy, S.H. Residues data on pirimicarb in cereals: Canada.
(1978) ICI Plant Protection Division data (unpublished).
Parkinson, G.R. Pirimicarb metabolite R 34 885: subacute oral
(1978a) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/402,
submitted by ICI.
Parkinson, G.R. Pirimicarb metabolite R 34 836: subacute oral
(1978b) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/401,
submitted by ICI.
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M.
(1978) Pirimicarb: growth study to determine a no effect
level in the female rat. Unpublished report from
ICI Central Toxicology Laboratory No. CTL/P/408
submitted by ICI.
Richards, D., Banham, P.B. and Weight. T.M. Pirimicarb paired
(1978) feeding study in a female rat. Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/407, submitted by ICI.
Teal, G. and Skidmore, M.W. Pirimicarb: metabolism on cabbage plants
(1978) in the field, ICI Plant Protection Division Report
No. RJ00033A (unpublished).
 FATE OF RESIDUES
In plants
Data reviewed by the 1976 Meeting showed that pirimicarb is
rapidly lost from plants after spraying. Half of the initial residue
is lost in 1-3 days. Volatilisation affords the primary means of loss:
the higher the ambient temperature, the greater the percentage of
pirimicarb lost by volatilisation (FAO/WHO, 1977b). Pirimicarb also
undergoes photochemical and metabolic degradation. The major
carbamate-containing degradation products are compounds II And III
(Figure 1). Other degradation products include the hydroxypyrimidines
V, VI and VII, which can be present either free or as conjugates, and
guanidine and its 1-methyl and III methyl derivatives. A further
metabolite on cabbages has been characterized by its facile
degradation to the hydroxypyrimidine V and another metabolic component
was characterized as re-crystallising with 1,1-dimethylguanidine but
not susceptible to desorption from charcoal with a solution containing
a large quantity of 1,1-dimethylguanidine (Teal and Skidmore, 1978).
Some of the residue is not extracted by conventional techniques, 14%
of the radioactivity associated with cabbage leaves 3´ weeks after
applying 2 - 14C-labelled pirimicarb was not extracted by maceration
with methanol (Teal, 1978). In lettuce plants sprayed with 2-
14C-labelled pirimicarb four weeks prior to harvest, 25% of the
radioactivity associated with the plants could not be extracted with
ethanol. Pirimicarb compounds III and VI and the three guanidines
mentioned above were all shown to be minor constituents of this
unextracted residue. Approximately 40% of the unextracted
radioactivity was associated with the cellulose and hemicellulose of
the plant (FAO/WHO, 1977b).
The fate of pirimicarb on alfalfa (Davis and Hemingway, 1978a) is
similar to that reviewed previously on lettuce, cabbage, peach leaves
and sugar beet leaves.
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. The biotransformation of pirimicarb in cows
is similar to that in rat and dog. Only traces of residues are
transferred to milk and tissues (FAO/WHO, 1977b).
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum residue in the milk (0.25 mg/kg pirimicarb
equivalents) was detected one hour after dosing; 80-90% of the residue
was due to the hydroxypyrimidines V, VI and VII (Figure 1). Subsequent
residues were 0.06 mg/kg pirimicarb equivalents or less. The maximum
residue in fat and meat tissues after twelve days was only 0.04 mg/kg
pirimicarb equivalents (FAO/WHO, 1977b).
When 2-14C-labelled pirimicarb was administered to a lactating
goat for seven days at a rate equivalent to 37 ppm in the diet, 96% of
the radioactivity recovered in the excreta was in the urine.
Hydroxypyrimidines constituted the major residues - compound VI 35%,
compound VII 29% and compound V 2% of the radioactivity in the urine.
The total radioactive residue in the milk reached plateau levels of 0,
12 and 0.3 mg Pirimicarb equivalents /kg in the morning and afternoon
milkings respectively. Hydroxypyrimidines again constituted the major
components - VI and VII together accounting for more than 56% of the
radioactivity and V 5%. The goat was sacrificed four hours after
receiving the last dose. In the tissues the levels of radioactivity,
in pirimicarb equivalents, were muscle 0.45 mg/kg, fat, 0.18 mg/kg,
liver 1.8 mg/kg and kidney 2.3 mg/kg. Approximately 50-60% of the
extractable radioactivity in muscle, liver and kidney was due to the
hydroxypyrimidines V, VI and VII, the two latter predominating (Davis
and Hemingway, 1978b). The hydroxypyrimidines V-VII are also major
metabolites in the rat and dog and are of low mammalian toxicity
(FAO/WHO,1977b). In no instance were residues of pirimicarb or its
carbamate-containing metabolites detected in this study. The remainder
of the radioactivity present in tissues and milk was due to a mixture
of polar, water-soluble compounds (Davis and Hemingway, 1978b).
Groups of three barren Friesian cows were maintained for 28-29
days on diets containing approximately 20, 60 and 200 ppm pirimicarb,
applied as a spray to pelleted grass nuts. The majority of milk
samples obtained from the 20 ppm group did not contain detectable
carbamate residues (ie pirimicarb plus its two carbamate-containing
metabolites II and III; limit of determination 0.01 mg/kg pirimicarb
equivalents). Only trace residues were found in milk obtained from the
60 ppm group - up to approximately 0.02 mg/kg. Milk samples from the
200 ppm group contained mean residues of 0.07 mg/kg (range 0.02 - 0.13
mg/kg). Two animals in each group were sacrificed after 28-29 days and
the third after a further seven days on untreated diet. Total
carbamate residues in meat tissues were normally non-detectable (ie
less than 0.01 mg/kg pirimicarb equivalents) at all dose levels. Trace
residues were detected in a few muscle and fat samples but the total
carbamate residue was consistently below 0.05 mg/kg in all groups
(Edwards et al., 1978).
An oral dose of pirimicarb is rapidly excreted by hens. Only
traces of residues are transferred to eggs and tissues and the
hydroxypyrimidines V-VII constitute the major components of these
residues.
When a single oral dose of 2-14C-labelled pirimicarb was
administered at 8 mg/kg to a hen in peak egg production, 88% of the
administered radioactivity was recovered from the excreta during the
following three days (Hendley at al., 1978).
When 2-14C-labelled pirimicarb was administered to two hens in
full egg production, at a rate equivalent to 6 ppm in the diet for
fourteen days, total radioactive residues in eggs reached a plateau of
0.05-0.06 mg pirimicarb equivalents/kg in 5-8 days, when the residue
in the yolk was approximately twice that in the albumen. The
hydroxypyrimidines V-VII (Figure 1) constituted approximately 50% of
the total radioactivity in the yolks, with compounds VI and VII
predominating (each approximately 20% of the radioactivity in eggs).
The pyrimidine-carbamates I-IV together represented approximately 8%
of the total radioactivity in the yolks, the remainder of the
radioactivity was either not solubilised or was more polar than
compound VII. The hens were sacrificed three hours after receiving the
last dose. The tissues contained approximately 0.15 mg/kg (muscle),
0.35 mg/kg (liver) or 0.02 mg/kg (fat) pirimicarb equivalents. 48-63%
of the radioactivity in liver and muscle was due to the
hydroxypyrimidines V-VII, compounds VI and VII again predominating. No
more than 3.5% of the radioactivity in the tissues was due to the
pyrimidine-carbamates I-VI (Figure 1). About 25% of the radioactivity
in the tissues was due to compounds more polar than the
hydroxypyrimidine VII, including a conjugate or conjugates of VI
Hendley et al., 1978).
When a hen in full egg production was given nine daily doses of
2-14C-labelled pirimicarb at a rate equivalent to 60 ppm in the diet,
levels of total radioactivity in eggs and tissues were greater than
those in the equivalent hens dosed at the 6 ppm rate by the expected
factor of 10 (Hendley et al., 1978).
Groups of 40 hens in maximum egg production were maintained for
up to 28 day on diets containing 1.5, 4.6 and 14 ppm total pyrimidine
carbamates. Approximately 90% of the administered carbamate mixture
was pirimicarb itself and 10% the carbamate metabolites II and III. No
residues of either pirimicarb or compounds II and III were detected in
either yolks or albumen of the eggs or in muscle and skin at sacrifice
(limits of determination: 0.01 mg/kg for pirimicarb, 0.01-0.02 mg/kg
for compounds II and III determined jointly). Trace total carbamate
residues in liver did not exceed 0.05 mg/kg pirimicarb equivalents
(Edwards and Dick, 1978).
METHODS OF RESIDUE ANALYSIS
A gas chromatographic method using a nitrogen-selective detector
to determine residues of pirimicarb plus its two major carbamate
containing plant metabolites (II and III, Figure 1) was reviewed by
the 1976 meeting as the preferred crop residue method (FAO/WHO,
1977b). With modified extraction procedures, this method is suitable
for the determination of these residues in products of animal origin.
Milk and eggs are extracted with a mixture of acetonitrile and
chloroform. Tissues are extracted with methanol in the presence of
anhydrous sodium sulphate. Clean-up by solvent partition and
determination by gas chromatography are as reviewed at the 1976
Meeting (ICI Ltd., 1978).
Acetonitrile/chloroform has been shown to be an efficient solvent
for extracting aged residues of pirimicarb and of the two metabolites
from milk. Greater than 80% extraction efficiency was obtained. The
low residue levels in tissues precluded a meaningful extractability
study but by analogy with studies on the extractability of these
compounds from a wide range of crop substrates, methanol is considered
to be the most acceptable extraction solvent for tissues (Edwards et
al., 1978;). Mean percentage recovery values for samples fortified in
the range 0.005/0.01-0.01/0.25 mg/kg were 76-92% for milk, 76-98% for
egg fractions, 80-89% for cow tissues and 62-96% for hen tissues
(Edwards et al., 1978; Edwards and Dick, 1978).
APPRAISAL
Further data on residues of pirimicarb on alfalfa, wheat, barley
and oats were reviewed, together with information concerning likely
levels in milk, eggs and meat arising from treated animal feeds.
Available methods of analysis have been improved and extended to cover
products of animal origin. The data provided are sufficient to
recommend additional temporary maximum residue limits for residues of
pirimicarb in alfalfa, barley, oats, milk, meat and eggs. No data were
available on residues in other grains each as maize or rice.
RECOMMENDATIONS
The following temporary maximum residue limits refer to the sum
of pirimicarb, its N-formyl(methylamino) analogue (desmethylformanido
pirimicarb) and desmethyl pirimicarb.
Commodity Temporary MRL (mg/kg)
Alfalfa (green) 50 (dry weight basis)
Alfalfa hay 20 (dry weight basis)
Barley 0.05*
Oats 0.05*
Eggs 0.05*
Meat 0.05*
Milk 0.05*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1980)
1. Carcinogenicity study in an appropriate mammalian species using a
currently acceptable protocol.
REFERENCES
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Unpublished
(1978) report from Hazelton Laboratories Europe Ltd.,
No. 1371-72/2, submitted by ICI Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
(1977) Street A.E. Pirimicarb preliminary oral toxicity
study in rhesus monkeys (repeated dosages for 13
weeks). Unpublished report from Huntingdon
Research Centre, No. 164/77825, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
1978a Street, A.E. Pirimicarb oral toxicity study in
rhesus monkeys (repeated dosage for 17 weeks,
followed by a recovery period of 8 weeks).
Unpublished report (final report) from Huntingdon
Research Centre, No. 198/78444, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H. and Brown, G.
(1978b) Pirimicarb comparative data from naive rhesus
monkeys (white blood cell counts and Direct Coombs
Tests). Unpublished report from Huntingdon
Research Centre, No. ICI 219/78445, submitted by
ICI Ltd.
Jackson, J.A., Chart, I.S., Sanderson, J. H. and Garner, R.
(1977) Pirimicarb induced immune hemolytic anaemia in
dogs. Scand. J. Haematol. 19, 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study
(1978a) in foxhounds. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/420, submitted by ICI Ltd.
Jackson, J.A. and Royle, G. Pirimicarb: oral toxicity study in
(1978b) rhesus monkeys. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory No.
CTL/P/421, submitted by ICI Ltd.
Jackson, J.A. and Royle. G. Pirimicarb: comparative data from
1978c) naive rhesus monkeys (white blood cell counts and
Direct Coombs Tests). "Addendum: results of
additional haematological and serological
investigations". Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/419,
submitted by ICI.
Kennedy, S.H. Residues data on pirimicarb in cereals: Canada.
(1978) ICI Plant Protection Division data (unpublished).
Parkinson, G.R. Pirimicarb metabolite R 34 885: subacute oral
(1978a) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/402,
submitted by ICI.
Parkinson, G.R. Pirimicarb metabolite R 34 836: subacute oral
(1978b) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/401,
submitted by ICI.
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M.
(1978) Pirimicarb: growth study to determine a no effect
level in the female rat. Unpublished report from
ICI Central Toxicology Laboratory No. CTL/P/408
submitted by ICI.
Richards, D., Banham, P.B. and Weight. T.M. Pirimicarb paired
(1978) feeding study in a female rat. Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/407, submitted by ICI.
Teal, G. and Skidmore, M.W. Pirimicarb: metabolism on cabbage plants
(1978) in the field, ICI Plant Protection Division Report
No. RJ00033A (unpublished).
FATE OF RESIDUES
In plants
Data reviewed by the 1976 Meeting showed that pirimicarb is
rapidly lost from plants after spraying. Half of the initial residue
is lost in 1-3 days. Volatilisation affords the primary means of loss:
the higher the ambient temperature, the greater the percentage of
pirimicarb lost by volatilisation (FAO/WHO, 1977b). Pirimicarb also
undergoes photochemical and metabolic degradation. The major
carbamate-containing degradation products are compounds II And III
(Figure 1). Other degradation products include the hydroxypyrimidines
V, VI and VII, which can be present either free or as conjugates, and
guanidine and its 1-methyl and III methyl derivatives. A further
metabolite on cabbages has been characterized by its facile
degradation to the hydroxypyrimidine V and another metabolic component
was characterized as re-crystallising with 1,1-dimethylguanidine but
not susceptible to desorption from charcoal with a solution containing
a large quantity of 1,1-dimethylguanidine (Teal and Skidmore, 1978).
Some of the residue is not extracted by conventional techniques, 14%
of the radioactivity associated with cabbage leaves 3´ weeks after
applying 2 - 14C-labelled pirimicarb was not extracted by maceration
with methanol (Teal, 1978). In lettuce plants sprayed with 2-
14C-labelled pirimicarb four weeks prior to harvest, 25% of the
radioactivity associated with the plants could not be extracted with
ethanol. Pirimicarb compounds III and VI and the three guanidines
mentioned above were all shown to be minor constituents of this
unextracted residue. Approximately 40% of the unextracted
radioactivity was associated with the cellulose and hemicellulose of
the plant (FAO/WHO, 1977b).
The fate of pirimicarb on alfalfa (Davis and Hemingway, 1978a) is
similar to that reviewed previously on lettuce, cabbage, peach leaves
and sugar beet leaves.
In animals
An oral dose of pirimicarb is rapidly excreted by cows,
principally in the urine. The biotransformation of pirimicarb in cows
is similar to that in rat and dog. Only traces of residues are
transferred to milk and tissues (FAO/WHO, 1977b).
When a single oral dose of 2-14C-labelled pirimicarb was
administered to a cow at 1 mg/kg (equivalent to approximately 33 ppm
in the diet), the radioactivity was quantitatively recovered during
the following twelve days, principally in the urine (95.6%) but also
in the faeces (4.3%). Only 0.29% of the radioactivity was recovered in
the milk. The maximum residue in the milk (0.25 mg/kg pirimicarb
equivalents) was detected one hour after dosing; 80-90% of the residue
was due to the hydroxypyrimidines V, VI and VII (Figure 1). Subsequent
residues were 0.06 mg/kg pirimicarb equivalents or less. The maximum
residue in fat and meat tissues after twelve days was only 0.04 mg/kg
pirimicarb equivalents (FAO/WHO, 1977b).
When 2-14C-labelled pirimicarb was administered to a lactating
goat for seven days at a rate equivalent to 37 ppm in the diet, 96% of
the radioactivity recovered in the excreta was in the urine.
Hydroxypyrimidines constituted the major residues - compound VI 35%,
compound VII 29% and compound V 2% of the radioactivity in the urine.
The total radioactive residue in the milk reached plateau levels of 0,
12 and 0.3 mg Pirimicarb equivalents /kg in the morning and afternoon
milkings respectively. Hydroxypyrimidines again constituted the major
components - VI and VII together accounting for more than 56% of the
radioactivity and V 5%. The goat was sacrificed four hours after
receiving the last dose. In the tissues the levels of radioactivity,
in pirimicarb equivalents, were muscle 0.45 mg/kg, fat, 0.18 mg/kg,
liver 1.8 mg/kg and kidney 2.3 mg/kg. Approximately 50-60% of the
extractable radioactivity in muscle, liver and kidney was due to the
hydroxypyrimidines V, VI and VII, the two latter predominating (Davis
and Hemingway, 1978b). The hydroxypyrimidines V-VII are also major
metabolites in the rat and dog and are of low mammalian toxicity
(FAO/WHO,1977b). In no instance were residues of pirimicarb or its
carbamate-containing metabolites detected in this study. The remainder
of the radioactivity present in tissues and milk was due to a mixture
of polar, water-soluble compounds (Davis and Hemingway, 1978b).
Groups of three barren Friesian cows were maintained for 28-29
days on diets containing approximately 20, 60 and 200 ppm pirimicarb,
applied as a spray to pelleted grass nuts. The majority of milk
samples obtained from the 20 ppm group did not contain detectable
carbamate residues (ie pirimicarb plus its two carbamate-containing
metabolites II and III; limit of determination 0.01 mg/kg pirimicarb
equivalents). Only trace residues were found in milk obtained from the
60 ppm group - up to approximately 0.02 mg/kg. Milk samples from the
200 ppm group contained mean residues of 0.07 mg/kg (range 0.02 - 0.13
mg/kg). Two animals in each group were sacrificed after 28-29 days and
the third after a further seven days on untreated diet. Total
carbamate residues in meat tissues were normally non-detectable (ie
less than 0.01 mg/kg pirimicarb equivalents) at all dose levels. Trace
residues were detected in a few muscle and fat samples but the total
carbamate residue was consistently below 0.05 mg/kg in all groups
(Edwards et al., 1978).
An oral dose of pirimicarb is rapidly excreted by hens. Only
traces of residues are transferred to eggs and tissues and the
hydroxypyrimidines V-VII constitute the major components of these
residues.
When a single oral dose of 2-14C-labelled pirimicarb was
administered at 8 mg/kg to a hen in peak egg production, 88% of the
administered radioactivity was recovered from the excreta during the
following three days (Hendley at al., 1978).
When 2-14C-labelled pirimicarb was administered to two hens in
full egg production, at a rate equivalent to 6 ppm in the diet for
fourteen days, total radioactive residues in eggs reached a plateau of
0.05-0.06 mg pirimicarb equivalents/kg in 5-8 days, when the residue
in the yolk was approximately twice that in the albumen. The
hydroxypyrimidines V-VII (Figure 1) constituted approximately 50% of
the total radioactivity in the yolks, with compounds VI and VII
predominating (each approximately 20% of the radioactivity in eggs).
The pyrimidine-carbamates I-IV together represented approximately 8%
of the total radioactivity in the yolks, the remainder of the
radioactivity was either not solubilised or was more polar than
compound VII. The hens were sacrificed three hours after receiving the
last dose. The tissues contained approximately 0.15 mg/kg (muscle),
0.35 mg/kg (liver) or 0.02 mg/kg (fat) pirimicarb equivalents. 48-63%
of the radioactivity in liver and muscle was due to the
hydroxypyrimidines V-VII, compounds VI and VII again predominating. No
more than 3.5% of the radioactivity in the tissues was due to the
pyrimidine-carbamates I-VI (Figure 1). About 25% of the radioactivity
in the tissues was due to compounds more polar than the
hydroxypyrimidine VII, including a conjugate or conjugates of VI
Hendley et al., 1978).
When a hen in full egg production was given nine daily doses of
2-14C-labelled pirimicarb at a rate equivalent to 60 ppm in the diet,
levels of total radioactivity in eggs and tissues were greater than
those in the equivalent hens dosed at the 6 ppm rate by the expected
factor of 10 (Hendley et al., 1978).
Groups of 40 hens in maximum egg production were maintained for
up to 28 day on diets containing 1.5, 4.6 and 14 ppm total pyrimidine
carbamates. Approximately 90% of the administered carbamate mixture
was pirimicarb itself and 10% the carbamate metabolites II and III. No
residues of either pirimicarb or compounds II and III were detected in
either yolks or albumen of the eggs or in muscle and skin at sacrifice
(limits of determination: 0.01 mg/kg for pirimicarb, 0.01-0.02 mg/kg
for compounds II and III determined jointly). Trace total carbamate
residues in liver did not exceed 0.05 mg/kg pirimicarb equivalents
(Edwards and Dick, 1978).
METHODS OF RESIDUE ANALYSIS
A gas chromatographic method using a nitrogen-selective detector
to determine residues of pirimicarb plus its two major carbamate
containing plant metabolites (II and III, Figure 1) was reviewed by
the 1976 meeting as the preferred crop residue method (FAO/WHO,
1977b). With modified extraction procedures, this method is suitable
for the determination of these residues in products of animal origin.
Milk and eggs are extracted with a mixture of acetonitrile and
chloroform. Tissues are extracted with methanol in the presence of
anhydrous sodium sulphate. Clean-up by solvent partition and
determination by gas chromatography are as reviewed at the 1976
Meeting (ICI Ltd., 1978).
Acetonitrile/chloroform has been shown to be an efficient solvent
for extracting aged residues of pirimicarb and of the two metabolites
from milk. Greater than 80% extraction efficiency was obtained. The
low residue levels in tissues precluded a meaningful extractability
study but by analogy with studies on the extractability of these
compounds from a wide range of crop substrates, methanol is considered
to be the most acceptable extraction solvent for tissues (Edwards et
al., 1978;). Mean percentage recovery values for samples fortified in
the range 0.005/0.01-0.01/0.25 mg/kg were 76-92% for milk, 76-98% for
egg fractions, 80-89% for cow tissues and 62-96% for hen tissues
(Edwards et al., 1978; Edwards and Dick, 1978).
APPRAISAL
Further data on residues of pirimicarb on alfalfa, wheat, barley
and oats were reviewed, together with information concerning likely
levels in milk, eggs and meat arising from treated animal feeds.
Available methods of analysis have been improved and extended to cover
products of animal origin. The data provided are sufficient to
recommend additional temporary maximum residue limits for residues of
pirimicarb in alfalfa, barley, oats, milk, meat and eggs. No data were
available on residues in other grains each as maize or rice.
RECOMMENDATIONS
The following temporary maximum residue limits refer to the sum
of pirimicarb, its N-formyl(methylamino) analogue (desmethylformanido
pirimicarb) and desmethyl pirimicarb.
Commodity Temporary MRL (mg/kg)
Alfalfa (green) 50 (dry weight basis)
Alfalfa hay 20 (dry weight basis)
Barley 0.05*
Oats 0.05*
Eggs 0.05*
Meat 0.05*
Milk 0.05*
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1980)
1. Carcinogenicity study in an appropriate mammalian species using a
currently acceptable protocol.
REFERENCES
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Unpublished
(1978) report from Hazelton Laboratories Europe Ltd.,
No. 1371-72/2, submitted by ICI Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
(1977) Street A.E. Pirimicarb preliminary oral toxicity
study in rhesus monkeys (repeated dosages for 13
weeks). Unpublished report from Huntingdon
Research Centre, No. 164/77825, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and
1978a Street, A.E. Pirimicarb oral toxicity study in
rhesus monkeys (repeated dosage for 17 weeks,
followed by a recovery period of 8 weeks).
Unpublished report (final report) from Huntingdon
Research Centre, No. 198/78444, submitted by ICI
Ltd.
Heywood, R., Sortwell, R.J., Pulsford, A.H. and Brown, G.
(1978b) Pirimicarb comparative data from naive rhesus
monkeys (white blood cell counts and Direct Coombs
Tests). Unpublished report from Huntingdon
Research Centre, No. ICI 219/78445, submitted by
ICI Ltd.
Jackson, J.A., Chart, I.S., Sanderson, J. H. and Garner, R.
(1977) Pirimicarb induced immune hemolytic anaemia in
dogs. Scand. J. Haematol. 19, 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study
(1978a) in foxhounds. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/420, submitted by ICI Ltd.
Jackson, J.A. and Royle, G. Pirimicarb: oral toxicity study in
(1978b) rhesus monkeys. "Addendum: results of additional
aerological investigations". Unpublished report
from ICI Central Toxicology Laboratory No.
CTL/P/421, submitted by ICI Ltd.
Jackson, J.A. and Royle. G. Pirimicarb: comparative data from
1978c) naive rhesus monkeys (white blood cell counts and
Direct Coombs Tests). "Addendum: results of
additional haematological and serological
investigations". Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/419,
submitted by ICI.
Kennedy, S.H. Residues data on pirimicarb in cereals: Canada.
(1978) ICI Plant Protection Division data (unpublished).
Parkinson, G.R. Pirimicarb metabolite R 34 885: subacute oral
(1978a) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/402,
submitted by ICI.
Parkinson, G.R. Pirimicarb metabolite R 34 836: subacute oral
(1978b) toxicity to rats. Unpublished report from ICI
Central Toxicology Laboratory, No. CTL/P/401,
submitted by ICI.
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M.
(1978) Pirimicarb: growth study to determine a no effect
level in the female rat. Unpublished report from
ICI Central Toxicology Laboratory No. CTL/P/408
submitted by ICI.
Richards, D., Banham, P.B. and Weight. T.M. Pirimicarb paired
(1978) feeding study in a female rat. Unpublished report
from ICI Central Toxicology Laboratory, No.
CTL/P/407, submitted by ICI.
Teal, G. and Skidmore, M.W. Pirimicarb: metabolism on cabbage plants
(1978) in the field, ICI Plant Protection Division Report
No. RJ00033A (unpublished).
