
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
PIRIMICARB
Explanation
Pirimicarb was evaluated by the 1976, 1977 and 1979 Meetings.* A
temporary ADI for man was established and temporary maximum residue
limits (MRLs) were recommended on a wide range of crops. At an
evaluation of pirimicarb by the 1977 Meeting, it was recommended that
information on carcinogenicity in an appropriate mammalian species
using a currently acceptable protocol be available by 1980.
Information dealing with cytogenicity and foetal and maternal
brain acetylcholinesterase isoenzyme changes induced by pirimicarb and
additional data on residues are summarized in this monograph addendum.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Short-term studies
Rat
In order to determine a no-effect level as regards the growth
depression found in previous long-term studies, particularly in female
rats, groups of 20 female rats were maintained on diets containing 0,
100, 175, 250 and 750 ppm pirimicarb for a period of 8 weeks. The
feeding did not adversely affect the general health. At 750 ppm,
reduction of the body weight gain was observed, accompanied by lower
food consumption compared to the control animals, especially in the
first half of the study (Paul et al 1978).
In a paired feeding study, female rats fed with dietary levels of
0, 250 and 750 ppm were paired with rats receiving restricted diets at
concentrations of 250 and 750 ppm respectively. A treatment-related
growth depression was observed that was slight at the 250 ppm dietary
level and marked at 750 ppm after ad libitum or restricted feeding.
The results indicate that the growth depression seems not to be due to
reduced palatability of the supplemented food (Richards et al 1978).
Dog
To define the susceptibility to haemolytic anaemia in the beagle
strain (Jackson et al 1977) another study was undertaken employing
the foxhound as an experimental animal. Groups of dogs received
pirimicarb at dietary levels of 0 (2 animals) and 2 mg/kg bw
* See Annex II for FAO and WHO documentation.
(2 animals) over a period of 16 weeks. A third group (6 animals)
was maintained on a diet containing the test compound at a level of
25/50 mg/kg. The dose level of 50 mg/kg was subsequently reduced back
to 25 mg/kg as soon as marked haematological changes appeared. The
treatment was followed by a recovery period from week 17 to 23. One
dog of the 25/50 mg/kg group which showed behavioural changes and
clinical deterioration as weight loss, inappetence and ataxia, was
killed after a 2-week treatment with 50 mg/kg pirimicarb. Loose faeces
were seen in dogs from all treatment groups. At 25 mg/kg vomiting was
observed sporadically. Over the first 4 weeks the male animals showed
reduced body weight gains. At 50 mg/kg, usually a few hours after
feeding, excessive salivation occurred; some dogs showed toxic effects
as laboured respiration, vomiting, bloody faeces and flaccid muscles.
The weight loss in some animals was accompanied by a slight reduction
in food intake. The treatment at 50 mg/kg was associated with marked
anaemia characterized by reduction in haemoglobin, packed cell volume
and erythrocyte count and an increase in reticulocytes. The direct
Coombes test failed to produce positive results (Jackson and Royle
1978a). The examination of the bone marrow revealed a tendency to an
increased number of normoblasts and toward suppressed activity
(hypoplasia). Anaemia, reticulocytosis and bone marrow changes were
reversible and normal values were measured after the recovery period.
Maximum inhibition of plasma cholinesterase activity of about 50 to
79% and 80 to 90% was measured at the 25 mg/kg and the 50 mg/kg dose
level respectively. No dose-related alterations as regards the
biochemical parameters GOT, GPT, SAP, glucose and BUN and the common
parameters of urinalyses were found at any dose level. No abnormal
macroscopic findings were detected. Owing to the small number of
control animals the significance of various alterations in relative
organ weights, particularly of the kidney and the spleen, could not be
determined (Fox 1978).
Monkey
Groups of 4 rhesus monkeys (2 of each sex) were orally treated
with 2 and 25 mg/kg pirimicarb over a period of 91 days; additionally,
2 monkeys were used as controls. The treatment had no effect on
mortality, appearance, behaviour and body weight gain. The parameters
of the haematological examinations and the results of urinalyses were
within normal limits. There was no evidence of haemolytic anaemia and
all direct Coombs tests were negative throughout the dosing period.
The results of clinical chemistry with respect to the parameters of
bilirubin, LAP, SGOT, SGPT, SAP, total serum protein, plasma glucose,
and plasma urea showed no abnormal alterations. A dose-related
inhibition of the cholinesterase activity in the erythrocytes of 16%
and 38% and in plasma of 8% and 74% was found at the dose levels of
2 mg/kg and 25 mg/kg 2 h after dosing. The examination of the bone
marrow revealed no pathological findings. The few incidental
macroscopic findings are not attributable to treatment and the organ
weights were considered to be within normal limits (Heywood et al
1977).
In a similar study, groups of 4 rhesus monkeys of each sex were
orally dosed with 0, 2, 7 and 25 mg/kg pirimicarb for up to 17 weeks,
followed by a recovery period of 8 weeks. The survival, appearance and
behaviour were not adversely affected by treatment and the only
clinical effect was loose faeces in animals of all treatment groups.
At 25 mg/kg a slight reduction in body weight gain was observed in the
female animals. The results of haematological investigations gave no
indication of a treatment-related anaemia. The parameters of clinical
chemistry and urinalyses varied within normal limits. The inhibition
of the cholinesterase activity in erythrocytes was 19, 18 and 31% and
in plasma it was 23, 35 and 63% at the dose levels of 2, 7 and
25 mg/kg respectively. The cholinesterase activity measured after 2
weeks after cessation of treatment was normal. The findings of the
bone marrow examinations were normal as well as any macroscopic post
mortem findings. No group differences in organ weights were
discovered. In the direct Coombs test, sporadically positive reactions
were found (Heywood et al 1978a). Additional serological studies on
monkeys confirmed the weak positive reactions in the Coombs test in
some animals treated with 0, 2, 7 and 25 mg/kg pirimicarb (Jackson and
Royle: 1978b). As could be demonstrated in a test performed on 30
untreated monkeys isolated positive reactions can occur spontaneously
(Heywood et al 1978b; Jackson and Royle 1978c).
Special studies on plant metabolites
Rat
Groups of 40 rats (20 of each sex) were orally treated with daily
doses of 1.5 and 50 mg/kg of the plant metabolite carbamate (R 34885)
for a period of 14 or 28 days. The control group consisted of 10
animals of each sex. The treatment had no effect on the clinical
condition, behaviour and mean body weight gain. The results gave no
evidence of hypochromic anaemia as suggested in a previous study. The
histopathological examination revealed no abnormal findings.
In a similar experiment, designed to study the inhibition of the
cholinesterase activity of the metabolite, groups of 10 rats (5 males
and 5 females) were treated with daily oral doses of 0, 3 and
12.5 mg/kg for 28 consecutive days. No significant inhibition of the
enzyme was found in erythrocytes and brain, whereas the plasma
cholinesterase activity showed a depression of 32% compared to control
in females after treatment with 28 doses of 12.5 mg/ kg (Parkinson
1978a).
Groups of 40 rats (20 males and 20 females) were orally treated
with 25 and 100 mg/kg of the plant metabolite 5,6-dimethyl-2-
methylamino-pyrimidin-4-yl dimethylcarbamate (R34836) for 14 or 28
days. Additionally, a control group consisting of 10 animals of each
sex was included in the study. The test compound had no adverse effect
when administered at a dose level of 20 mg/kg; at 100 mg/kg, however,
5 male and 6 female animals died during the study between 2 and 7
doses with clinical signs of cholinesterase inhibition. Some animals
lost weight at 100 mg/kg. The examination of blood and bone marrow
revealed no abnormal findings and gave no indication for hypochromic
anaemia. The results of urinalyses fell into normal limits, with the
only exception of slightly higher urinary protein values in male
animals treated with 14 and 28 doses of 100 mg/kg respectively. No
adverse histopathological effects were found at 100 mg/kg.
To determine the degree of cholinesterase inhibition, groups of
10 rats (5 male and 5 female animals) were orally dosed with 0, 1.5,
5, 25 and 100 mg/kg of the test compound for 28 days. The
cholinesterase activity was not affected in erythrocytes and brain.
The plasma cholinesterase activity, however, showed inhibition at
25 mg/kg after 28 days of about 30% in male and about 55% in female
animals; after 14 days of treatment with 100 mg/kg the depression was
40%, after 28 days of treatment depression was 63% in female animals
only (Parkinson 1978b).
Special studies on mutagenicity
The mutagenic activity of a number of pesticides, e.g.,
pirimicarb, Polimartin and Cuprosan, was studied in human peripheral
blood lymphocyte cultures from two donors with different levels of
spontaneous chromosomal aberrations. Incubation of lymphocyte culture
with pirimicarb increased the yield of chromosomal aberrations
regardless of the level of spontaneous aberrations (Polimartin and
Cuprosan failed to induce chromosomal aberrations). The potential
mutagenicity of pirimicarb was also confirmed on the basis of its
cytogenic activity in mice bone marrow cells (Pilinskaya 1981).
Special studies on induction of foetal and maternal
acetylcholinesterase
isoenzymes
Pregnant rats (18th day) were orally given 20 mg/kg pirimicarb.
(Other insecticidal carbamates with anticholinesterase properties
which were analogously tested were: 50 mg/kg carbaryl, 0.1 mg/kg
aldicarb and 2.5 mg/kg carbaryl.) The acetylcholinesterase (ACLE)
isoenzymes from the brain of mothers and their foetuses were separated
by electrophoresis on polyacrylamide gel. The four carbamate derivates
caused a significant lowering of the percentage of the least mobile
isoenzyme (isoenzyme 1) in the mothers. Pirimicarb, aldicarb and
carbaryl also caused a significant decrease in the percentage of the
first foetal brain isoenzyme (Cambon et al 1980).
RESIDUES IN FOOD
Data reviewed by the 1976 and 1978 Meetings showed that
pirimicarb is rapidly lost from plants after spraying. Half of the
initial residue is lost, primarily by volatilization, in 1 to 3 days;
the higher the ambient temperature, the greater the percentage of
pirimicarb lost by volatilization. Pirimicarb also undergoes
photochemical and metabolic degradation. The major carbamate-
containing degradation products are compounds II and III (Figure 1).
Other degradation products include the hydroxypyrimidines V, VI and
VII which can be present either free or as conjugates. The
hydroxypyrimides are major metabolites of pirimicarb in the rat, dog
and cow. Guanidine and its 1-methyl and 1:1 - dimethyl derivatives are
also formed following the application of pirimicarb to plants.
A gas-chromatographic method using a nitrogen-selective detector
to determine residues of pirimicarb plus its two major carbamate-
containing plant metabolites Figure 1 (II and III) was reviewed as the
preferred crop residue method by the 1976 and 1978 Meetings. This
method was used during the residue determinations reviewed below, and
all values quotes are corrected for percentage recovery, unless stated
otherwise.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Cereal grains
At 0.125-0.25 kg a.i./ha pirimicarb controls the grain aphid,
Macrosiphum avenae, on cereals. Spraying is conducted at an immature
growth stage, normally several weeks before harvest.
As a result of the data available to it, the 1976 Meeting
proposed a temporary MRL in wheat grain of 0.05 mg/kg, at or about
the limit of determination. Data available in 1978 re-affirmed
the appropriateness of this proposal and facilitated similar
recommendations on barley and oats. The 1978 appraisal noted that no
data were available on residues in other grains such as maize or rice.
Further data now available on wheat and oats sprayed in Canada
with a 50% formulation re-affirm the appropriateness of the earlier
proposals (Table 1). The half-life for pirimicarb on both crops was
between 1 and 5 days (Bullock and Kennedy 1981).
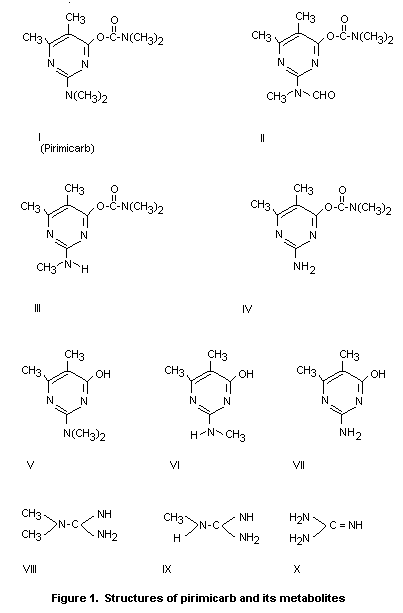 TABLE 1. Residues of pirimicarb in barley and wheat, Canada 1978
Application rate Crop part Total carbamate residues (mg/kg) after
Crop (kg a.i./ha) analysed (days)
1 5 7 14
Barley 0.07 Grain 0.47 0.15 0.11 0.04
0.14 Grain 0.82 0.30 0.14 0.07
0.07 Ears - - 0.04 <0.02
0.14 Ears - - 0.05 0.02
Wheat 0.07 Ears 0.10 0.02 <0.02 <0.02
0.14 Ears 0.93 0.41 0.05 <0.02
A trial was conducted in Japan in 1979, in which sweet corn was
sprayed at the unusually high rate of 2 kg a.i./ha, using a 48% WP
formulation and 2 000 to 4 000 l of water/ha. Residue samples taken 23
days later contained no detectable pyrimidine-carbamate residues
(limit of determination 0.02 mg/kg) (Bullock and Kennedy 1981). This
was an anticipated finding, as sweet corn on the cob is protected from
spray by the surrounding foliage.
Pirimicarb is not registered for use on rice at this time and no
data on residues are available.
Citrus fruit
The 1976 Meeting proposed a temporary MRL in citrus fruits of
0.05 mg/kg, at or about the limit of determination of the analytical
method. Interpretation of much of the data available in 1976 was
rendered difficult by the fact that often the peel and flesh were
analysed separately. Whole fruits had been analysed on only a limited
number of occasions, although the available data justified the
proposed MRL. Supportive evidence was provided by the separate
analysis of peel and flesh of oranges treated in Australia and in
Spain. Residues were less than 0.02 mg/kg in edible flesh and did not
exceed 1 mg/kg in the peel. At the time, results for samples obtained
from Japan were deemed unrepresentative, particularly since there was
a concern over possible contamination of the edible flesh with
residues during peeling.
Aphids usually occur on citrus early in the season. This is
several months before the first fruits are mature. However, the
harvesting period often extends from the autumn through to the
following spring. As a result, fruit can remain on the tree at the
time of the first sprays against aphids during the following year, and
then a short pre-harvest withholding interval is appropriate.
Ferreira and Tainha (1981) in Portugal have now provided new
evidence of residues on whole oranges. In Portugal, pirimicarb is
approved as a 50% WP formulation for use in citrus at a spray
concentration of 37.5 g a.i./hl, applied to "run off". The pre-harvest
withholding interval is two weeks. Two spray applications were made,
fourteen days apart, to three separate replicate trees. Samples of 15
fruits were collected from each tree 1, 4, 7, 11, 21 28 and 35 days
after the second spray. Analyses by a slight modification of the
standard residue method yielded results as shown in Table 2.
TABLE 2. Residues of pirimicarb in organge, Portugal, 1980
Application Days between Total carbamate residues (mg/kg)
Rate last treatment
and harvest Tree No.11 Tree No.21 Tree No. 31 Mean
1 0.69 0.64 0.99 0.77
37.5 g a.i./h 4 0.27 0.27 0.52 0.35
applied to 7 0.27 0.30 0.35 0.31
"run off" , 11 0.37 0.16 0.30 0.28
twice, fourteen 14 0.21 0.14 0.22 0.19
days 21 0.14 0.20 0.29 0.21
apart. 28 0.20 0.22 0.28 0.23
35 0.18 0.20 0.24 0.21
1 mean of three analyses (uncorrected for recovery).
Residues in oranges are present mainly in the oil glands of the
peel, in which they tend to remain stable. Levels in the edible flesh
have been consistently below 1 mg/kg (Table 3) (Manley 1972; Edwards
and Dick 1976; Bullock and Kennedy 1981).
Apple and pear
The 1976 Meeting proposed a temporary MRL for apples at 1 mg/kg.
Additional residues data now available from trials in Canada in 1980
and Federal Republic of Germany in 1977 confirm the appropriateness of
this value (Table 4) (Bullock and Kennedy 1981).
TABLE 3. Residues of pirimicarb in orange, Australia and Japan, 1970-78.
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
Australia 50% WP 0.025 kg "High Whole 0 <0.01 Edwards and Dick
1973-4 a.i./hl volume" fruit 1 <0.01 1976
7 <0.01
14 <0.01
Skin 0 0.95
1 0.60
7 0.32
14 0.36
Flesh 0 <0.02
1 <0.02
7 <0.02
14 <0.02
Japan 50% WP 0.05 kg 5000 Skin 11 13.0 Manley
1970 a.i./hi 21 4.9 1972
(four 31 7.2
applications 42-43 2.1-3.0
over 9-10 49 0.72-2.4
weeks) 59-63 0.67-3.6
TABLE 3. (con't)
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
0.05 kg 5000 Skin 11 16.5
a.i./hl 31 5.1-11.6
(five 42-43 5.2
applications 49 2.4
over 9-10 59-63 1.6-4.7
weeks)
0.05 kg 5000 Flesh 11-63 <0.01-0.88
a.i./hi (mean 0.19;
(4-6 16 samples)
applications
over 9-11
weeks)
Japan 48% 0.05 kg 3300- Skin >123 days 0.03-0.34 Bullock and Kennedy
1977 Dispersible a.i./hl 5000 (four 1981
Grain (3 or 2 results)
applications Flesh <0.02
approx. (four
11 weeks results)
apart) Juice <0.02
(two
results)
TABLE 4. Residues of pirimicarb in apple, Canada 1980 and Federal Republic of Germany 1977
Country Rate of Days between Total Carbamate residues (mg/kg)
and application last application
Year Formulation (kg a.i./ha) and harvest Minimum Maximum Mean
Canada 50% WP 0.56 0 - - 0.58 (1)1
1980 3 - - 0.42 (1)
8 - - 0.19 (1)
14 - - 0.05 (1)
Germany 50% 0.5 0 0.42 0.69 0.49 (4)
1977 Dispersible 3 0.22 0.51 0.31 (4)
grain 7-8 0.17 0.51 0.27 (4)
10-11 0.16 0.45 0.21 (4)
14 0.17 0.49 0.24 (3)
20-21 0.09 0.49 0.13 (3)
1 Figures in parentheses are the numbers of results on which the means are based.
A trial was also carried out in the UK on pears, using a single
application of a 50% dispersible grain formulation at a rate of
0.21 kg a.i./ha. Samples for residue analysis were taken at 0, 2, 7
and 107 days. The initial total carbamate residue of 0.39 mg/kg
decayed slowly over the following seven days, to 0.27 mg/kg. No
residue was detected in the 107-day sample (limit of determination,
0.02 mg/kg) (Bullock and Kennedy 1981). Two trials were also carried
out on pears in Japan in 1973. Two or three applications of a 50%
dispersible powder formulation were made at 0.05 kg a.i./hl, at 2500 l
of spray per ha. Samples for residue analysis were taken 54 and 64
days after the last applications. As expected with such long
intervals, no residues were detected in any of the four samples
analysed (limit of determination, 0.02 mg/kg) (Edwards and Dick 1976).
Data reviewed at the 1976 Meeting indicated that pirimicarb
residue levels on apples tend to decline more slowly than on many
other crops, such as leafy vegetables. The data now available on pears
are consistent with the findings on apples, in terms both of the
levels of residues present and in the pattern of residue decline.
Pecan
At 0.0125-0.025 kg a.i./hl applied 'high volume', pirimicarb
controls the black margined aphid, Monellia costalis, yellow aphid,
Monellia sp. and black pecan aphid, Tinocallis caryaefoliae, on
pecans. Aphids usually occur on pecans early in the season, although a
second infestation can appear a few weeks before harvest. At that
time, the nut is still protected by the hull and as a result it is
unlikely that residues would be detected in the nut. That expectation
was confirmed in four trials conducted in the USA in 1975. Pirimicarb
was sprayed at 0.125 to 0.375 kg a.i./ha up to three times, using a
50% WP formulation. Nuts were harvested 17 to 77 days after the last
application. No pyrimidine-carbamate residues were detected in any of
the ten samples analysed (limit of determination, 0.05 mg/kg) (Ussary
1976).
Cotton
At 0.03 to 0.06 kg a.i./ha, pirimicarb controls the cotton aphid,
Aphis gossypii. Aphid attack on cotton occurs early in the growing
season and it is unlikely that a residue would be detected in the
seeds at harvest. This expectation was confirmed in six trials
conducted in the USA in 1976-78. Two to four applications of
pirimicarb were made using a 50% WP at 0.06 or 0.12 kg a.i./ha. The
cotton was harvested 40 to 122 days after the last application. No
pyrimidine-carbamate residues were detected in any of the ten samples
analysed (limit of determination, 0.05 mg/kg ) (Ussary 1979).
Leeks, spinach and watercress
Pirimicarb is particularly useful when a pre-harvest clean-up of
aphid infestations is required. Aphids controlled by the compound
include the peach-potato aphid, Myzus persicae, on leeks and
watercress, shallot aphid, Myzus ascalonicus, on leeks, and the
black bean aphid, Aphid fabae, on spinach.
Three trials were conducted on outdoor leeks in Germany in 1977.
A 50% dispersible grain formulation was applied on three occasions at
0.15 kg a.i./ha. Initial total carbamate residues on the leeks of 1.5
to 4.0 mg/kg declined rapidly with a half-life of less than one day
(Table 5) (Bullock and Kennedy 1981).
Work on three varieties of spinach was undertaken in Germany in
1974, using a single application of a 50% dispersible grain
formulation at 0.5 kg a.i./ha. In three further trials in Germany in
1977, a 50% dispersible grain formulation was applied on three
occasions at 0.15 kg a.i./ha. Initial total carbamate residues of
approximately 3 to 8 mg/kg declined rapidly with a half-life of less
than one day (Table 5) (Bullock and Kennedy 1981; Edwards and Dick
1976).
Three trials were conducted on watercress in the UK in 1972, in
which a 50% WP formulation was applied at 0.25 kg a.i./ha (recommended
rate) or 0.50 kg a.i./ha. Total carbamate residue levels on the
watercress again declined very quickly in the period immediately after
application (Table 5)(Bullock 1972).
The pattern of residues on these three crops are similar to those
reviewed on various leafy vegetables at the 1976 Meeting.
Glasshouse crops
Some additional data on residues of pirimicarb on glasshouse
crops have been received (Greve and van de Kamp 1974) and are recorded
in Table 6. They are in line with data previously received on these
crops.
Other crops
Trials involving the application of pirimicarb to broccoli,
Brussels sprouts, peas, potatoes, soybeans, sugarbeet and turnips in
various countries, gave rise to no residues above the limits of
determination (generally <0.02 mg/kg). Further trials data on
pirimicarb residues in some other fruits and vegetables are listed in
Table 7 (Bullock and Kennedy 1981).
TABLE 5. Residues of pirimicarb in leek, spinach and watercress1
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Leeks Germany 50% 0.15 0 1.5 4.0 2.6 (3)2
1977 dispersible × 3 1 0.3 0.65 0.48 (2)
grain 2 - - 0.15 (1)
4 0.06 0.12 0.09 (3)
7 0.02 0.05 0.04 (3)
10 <0.02 0.02 0.02 (3)
14 <0.02 0.03 0.02 (3)
Spinach Germany 50% 0.15 0 4.6 7.3 5.6 (3)
1977 dispersible × 3 1 0.72 2.0 1.3 (3)
grain 4 0.16 0.50 0.29 (3)
7 0.05 0.06 0.06 (3)
10 <0.02 0.02 0.02 (3)
Germany 50% 0.5 0 2.7 6.0 4.1 (3)
1974 dispersible (× 1) 1 1.5 2.1 1.7 (3)
grain 3 0.14 0.65 0.39 (3)
5 0.01 0.12 0.07 (3)
7 <0.01 0.03 0.02 (3)
10 <0.01 0.02 0.02 (3)
12 - - <0.01 (1)
TABLE 5. (con't)
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Watercress UK 50% WP 0.25 0 5.9 22 11 (3)
1972 (× 1) 1 - - 1.1 (1)
2 0.43 0.51 0.44 (3)
4 0.37 0.39 0.38 (3)
7 0.16 0.20 0.18 (2)
0.5 0 - - 13 (1)
(× 1) 2 - - 0.86 (1)
4 - - 0.95 (1)
7 - - 0.50 (1)
1 Bullock and Kennedy, 1981; Edwards and Dick, 1976;
2 Figures in parentheses are the numbers of results upon which the means are based.
TABLE 6. Residues of pirimicarb in glasshouse crops1
Crop Residues (mg/kg) at intervals (days) after treatment
0 3
Range Mean Range Mean
Eggplants 0.16-0.24 0.19 0.05 0.05
" 0.13-0.19 0.16 0.10-0.24 0.17
Gherkins 0.38-0.64 0.47 0.09-0.13 0.11
" <0.05 <0.05 <0.05 <0.05
Cucumber (whole) <0.05 <0.05 <0.05 <0.05
" (peel) 0.06-0.24 0.11 0.05-0.14 0.08
Tomato 0.12-0.21 0.14
" 0.09-0.13 0.12 <0.05 <0.05
Pepper (bell) 0.18-0.30 0.25 0.05-0.07 0.06
" " <0.05-0.11 <0.06 <0.05-0.14 <0.08
1 In each case, one treatment with 50% WP at 0.375 kg a.i./ha.
FATE OF RESIDUES
In processing
Two studies were conducted in the USA in 1977 to determine
residues of pirimicarb and its carbamate metabolites in processed
cotton fractions. Cotton plants were sprayed with a 50% WP formulation
of pirimicarb at 0.07 to 0.13 kg a.i./ha. Two to four applications
were made with an interval of 49 to 122 days between the last
application and harvest. The cotton was harvested, fractioned and
submitted to the laboratory for residue analysis. The results are
shown in Table 8. Although no pyrimidine-carbamate residues were
observed in cottonseed, slight residues of up to 0.05 mg/kg were
observed in crude oil. A trace was noted in cottonseed meal at the
0.13 kg a.i./ha rate (twice the maximum recommended). However, as no
residue was detected in the cottonseed, it is unlikely that residues
should be present in both the meal and oil. The trace residues in meal
are most likely due to lack of sample homogeneity or possibly to minor
contamination (Ussary 1979).
TABLE 7. Pirimicarb residues in some fruits and vegetables
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Cherry Fed.Rep.Germany 1977 0 1.5 - 1.9 1.4
4 0.03- 0.89 0.41
7 0.02- 0.52 0.26
14 <0.02- 0.33 0.15
UK 1976 62 <0.02 <0.02
Peach Japan 1976 27-61 0.13- 0.40 0.28
Canada 1980 60-107 <0.02 <0.02
Plum Canada 1976 21-30 0.14- 0.17 0.15
Pepper Denmark 1976 0 - 0.13
3 - 0.10
7 - 0.04
14 - 0.02
Tomato Canada 1976 1 - 0.10
3 - 0.03
7 - <0.02
Denmark 1977 0 - 0.46
3 - 0.16
7 - 0.09
14 - 0.02
Cucumber Denmark 1977 0 0.13, 0.47 -
3 0.08, 0.30 -
7 0.07, 0.38 -
14 0.02, 0.05 -
21 0.02, 0.07 -
TABLE 7. (con't)
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Parsley Netherlands 1976 0 - 23
1 - 15
3 - 8.6
7 - 6.3
14 - 4.6
TABLE 8. Pirimicarb residues on cottonseed fractions, USA 1977
Residue (mg/kg)1
Type of No of Days after last
Sample applications application Control 0.07 kg ai/A 0.13 kg ai/A4
to harvest P2 DMP3 Total P DMP Total P DMP Total
Cottonseed 4 49 ND ND ND ND ND ND ND ND ND
Hulls ND ND ND ND ND ND
Meal ND TR 0.01 ND 0.01
Crude oil ND ND ND 0.02 0.01 0.03
Cottonseed 2 122 ND ND ND
Hulls ND ND ND
Meal ND ND ND
Crude oil 0.04 0.01 0.05
1 ND=none detected (<0.01 ppm of the individual compounds);
2 P = pirimicarb;
3 DMP = desmethyl pirimicarb;
4 Twice maximum use rate.
METHODS OF ANALYSIS
The preferred method for determining residues of pirimicarb and
its carbamate-containing metabolites in crops and in products of
animal origin is by gas-liquid chromatography with selective nitrogen
detection. The method was reviewed at the 1976 and 1978 Meetings.
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs listed in Table 9 have been established for
residues of pirimicarb plus its two carbamate-containing metabolites,
II and III (Figure 1).
TABLE 9. National maximum residue limits reported to the Meeting
Country Crop MRL (mg/kg)
Australia Vegetables, fruit and hops 0.5
Belgium Potato 0.5
Other crops 0.3
Brazil Cabbage, cauliflower 0.5
Aubergine, cucumber, pepper, tomato 0.2
Potato 0.05
Fed. Rep. of Lettuce, cherry 1.0 ) from
Germany Other leaf, sprout and fruit vegetables 0.5 ) 1.1.82
Cereals, potato, sugarbeet 0.1 )
France Cereals 0.05 (proposed)
Hungary Fruit 0.5
Vegetables 1
Other crops 0.5
Japan Fruits and vegetables 0.3
Netherlands Fruits, vegetables, herbs and spices 1.0
Grain, potato 0.05
New Zealand Beans, lettuce, pea, brassicae, tomato 1
Potato, fodder crops 0.5
TABLE 9. (con't)
Country Crop MRL (mg/kg)
South Africa Cruciferae, oats, sorghum, wheat, peach 0.3
Peanut, pecan, potato 0.05
Switzerland Fruits and vegetables 1
USA Potato 0.1
Venezuela All Crops 0.5
EVALUATION
COMMENTS AND APPRAISAL
The Meeting was informed that the data requested by the 1978
Meeting would not be available until 1982. The Meeting therefore
agreed to extend the existing temporary ADI until 1982.
Additional data on residues of pirimicarb in a range of crops
have been received and reviewed. The pattern of residue data is
generally similar to that reviewed at previous meetings, although the
new data on oranges show the need for an upward revision of the
present limit to cover residues arising from use close to harvest in
some countries. On outdoor vegetables and field crops the residues
decline very quickly after spraying; on fruits, however, decline is
slower. Data on pears, together with old and new data on apples,
allowed a recommendation for limits on pome fruits. Additional and
altered limits are proposed.
RECOMMENDATIONS OF RESIDUE LIMITS
The following new or altered temporary limits refer to the sum of
pirimicarb, its N-formyl (methylamino) analogue (desmethylformamido
pirimicarb) and desmethyl pirimicarb.
Pre-harvest interval on
Temporary MRL which the recommendations
Commodity (mg/kg) are based (days)
Pome fruits 1 7
Spinach 1 3-4
Watercress 1 2-4
Leeks 0.5 1-2
Oranges 0.5 7-14
Citrus fruits
(other than oranges) 0.051 -
Cottonseed 0.051 -
Pecan 0.051 -
Sweet corn 0.051 -
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Data on residues on oranges due to applications made close to
harvest in countries other than Portugal.
2. Data on residues on other citrus fruits from countries where
applications close to harvest are recommended or permitted.
REFERENCES
Bullock, D.J.W. Pirimicarb residues data on watercress: UK 1972. ICI
1972 Plant Protection Division. (Unpublished)
Bullock, D.J.W. and Kennedy, S.H. Pirimicarb : residues in crops from
1981 field trials during 1976-80. ICI Plant Protection Division
Report no. TMJ 1883A. (Unpublished)
Cambon, C., Declume, C. and Derace, R. Foetal and maternal rat brain
1980 acetylcholinesterase: isoenzymes changes following
insecticidal carbamate derivates poisoning. Archives of
Toxicology 45: 257-262.
Edwards, M.J. and Dick, J.P. Pirimicarb residue summary: Residues in
1976 crops from field trials during 1973-1975. ICI Plant
Protection Division Report No. TMJ 1360B. (Unpublished)
Ferreira, J.R. and Tainha, A. Residues of pirimicarb in field-treated
1981 oranges. Ministry of Agriculture and Fisheries, Toxicology
and Analytical services Directorate, Portugal. Report PPA
(TR)-12/81. (Unpublished)
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Report from
1978 Hazelton Labs. Europe Ltd., no. 1371-72/2, submitted by ICI
Ltd. to WHO. (Unpublished)
Greve, P.A. and van de Kamp, C.G. Residuen van bestrijdingsmiddeln in
1974 de groenteteelt onder glas. Report 96/74 Tox - ROB. Report
from the Netherlands. (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and Street,
1977 A.E. Pirimicarb preliminary oral toxicity study in rhesus
monkeys (repeated dosages for 13 weeks). Report from
Huntingdon Research Centre, no. 164/77825, submitted by ICI
Ltd. to WHO (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H,, Brown, G. and Street,
1978a A.E. Pirimicarb oral toxicity study in rhesus monkeys
(repeated dosages for 17 weeks, followed by a recovery
period of 8 weeks). Report (final report) from Huntingdon
Research Centre no. 198/78444, submitted by ICI Ltd. to WHO.
(Unpublished)
1978b Pirimicarb comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs Tests). Report
from Huntingdon Research Centre, No. 219/78445, submitted by
ICI Ltd. to WHO. (Unpublished)
Jackson, J.A., Chart, I.S., Sanderson, J.H. and Garner, R. Pirimicarb
1977 induced immune hemolytic anaemia in dogs, Scandinavian
Journal of Haematology, 19: 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study in
1978a foxhounds. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/420, submitted by ICI Ltd.
(Unpublished)
Jackson, J.A.; and Royle, G. Pirimicarb: oral toxicity study in rhesus
1978b monkeys. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/421, submitted by ICI Ltd.
(Unpublished)
1978c Pirimicarb: comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs tests). Addendum:
results of additional serological investigations. Report
from ICI Central Toxicology Laboratory, no. CTL/P/419
submitted by ICI Ltd. (Unpublished)
Manley, C.A. residue summary: Pirimicarb in crops. ICI Plant
1972 Protection Division Report no. TMJ 585/2. (Unpublished)
Parkinson, G.R. Pirimicarb metabolite R 34885: subacute oral toxicity
1978a to rats. Report from ICI Central Toxicology Laboratory, No.
CTL/P/402, submitted by ICI to WHO. (Unpublished)
1978b Pirimicarb metabolite R34836: subacute oral toxicity to
rats. Report from ICI Central Toxicology Laboratory, no.
CTL/P/401, submitted by ICI to WHO. (Unpublished)
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M. Pirimicarb:
1978 growth study to determine a no-effect level in the female
rat. Report from ICI Central Toxicology Laboratory no.
CTL/P/408, submitted by ICI to WHO. (Unpublished)
Pilinskaya, M.A. Cytogenic effect of a number of pesticides in a
1981 culture of human peripheral blood lymphocytes with different
levels of spontaneous aberrations. Tsitologiya i Genetika,
15(2) : 82-84
Richards, D., Banhan, P.B. and Weight, T M. Pirimicarb paired feeding
study in a female rat. Report from ICI Central Toxicology
Laboratory, no. CTL/P/407. Submitted by ICI to WHO.
(Unpublished)
Ussary, J.P. Pirimicarb residues in pecan, 1975. ICI Americas Inc.
1976 Reports. (Unpublished)
1979 Pirimicarb residues on cottonseed. ICI Americas Inc, Report
no. TMU 0442/B. (Unpublished)
TABLE 1. Residues of pirimicarb in barley and wheat, Canada 1978
Application rate Crop part Total carbamate residues (mg/kg) after
Crop (kg a.i./ha) analysed (days)
1 5 7 14
Barley 0.07 Grain 0.47 0.15 0.11 0.04
0.14 Grain 0.82 0.30 0.14 0.07
0.07 Ears - - 0.04 <0.02
0.14 Ears - - 0.05 0.02
Wheat 0.07 Ears 0.10 0.02 <0.02 <0.02
0.14 Ears 0.93 0.41 0.05 <0.02
A trial was conducted in Japan in 1979, in which sweet corn was
sprayed at the unusually high rate of 2 kg a.i./ha, using a 48% WP
formulation and 2 000 to 4 000 l of water/ha. Residue samples taken 23
days later contained no detectable pyrimidine-carbamate residues
(limit of determination 0.02 mg/kg) (Bullock and Kennedy 1981). This
was an anticipated finding, as sweet corn on the cob is protected from
spray by the surrounding foliage.
Pirimicarb is not registered for use on rice at this time and no
data on residues are available.
Citrus fruit
The 1976 Meeting proposed a temporary MRL in citrus fruits of
0.05 mg/kg, at or about the limit of determination of the analytical
method. Interpretation of much of the data available in 1976 was
rendered difficult by the fact that often the peel and flesh were
analysed separately. Whole fruits had been analysed on only a limited
number of occasions, although the available data justified the
proposed MRL. Supportive evidence was provided by the separate
analysis of peel and flesh of oranges treated in Australia and in
Spain. Residues were less than 0.02 mg/kg in edible flesh and did not
exceed 1 mg/kg in the peel. At the time, results for samples obtained
from Japan were deemed unrepresentative, particularly since there was
a concern over possible contamination of the edible flesh with
residues during peeling.
Aphids usually occur on citrus early in the season. This is
several months before the first fruits are mature. However, the
harvesting period often extends from the autumn through to the
following spring. As a result, fruit can remain on the tree at the
time of the first sprays against aphids during the following year, and
then a short pre-harvest withholding interval is appropriate.
Ferreira and Tainha (1981) in Portugal have now provided new
evidence of residues on whole oranges. In Portugal, pirimicarb is
approved as a 50% WP formulation for use in citrus at a spray
concentration of 37.5 g a.i./hl, applied to "run off". The pre-harvest
withholding interval is two weeks. Two spray applications were made,
fourteen days apart, to three separate replicate trees. Samples of 15
fruits were collected from each tree 1, 4, 7, 11, 21 28 and 35 days
after the second spray. Analyses by a slight modification of the
standard residue method yielded results as shown in Table 2.
TABLE 2. Residues of pirimicarb in organge, Portugal, 1980
Application Days between Total carbamate residues (mg/kg)
Rate last treatment
and harvest Tree No.11 Tree No.21 Tree No. 31 Mean
1 0.69 0.64 0.99 0.77
37.5 g a.i./h 4 0.27 0.27 0.52 0.35
applied to 7 0.27 0.30 0.35 0.31
"run off" , 11 0.37 0.16 0.30 0.28
twice, fourteen 14 0.21 0.14 0.22 0.19
days 21 0.14 0.20 0.29 0.21
apart. 28 0.20 0.22 0.28 0.23
35 0.18 0.20 0.24 0.21
1 mean of three analyses (uncorrected for recovery).
Residues in oranges are present mainly in the oil glands of the
peel, in which they tend to remain stable. Levels in the edible flesh
have been consistently below 1 mg/kg (Table 3) (Manley 1972; Edwards
and Dick 1976; Bullock and Kennedy 1981).
Apple and pear
The 1976 Meeting proposed a temporary MRL for apples at 1 mg/kg.
Additional residues data now available from trials in Canada in 1980
and Federal Republic of Germany in 1977 confirm the appropriateness of
this value (Table 4) (Bullock and Kennedy 1981).
TABLE 3. Residues of pirimicarb in orange, Australia and Japan, 1970-78.
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
Australia 50% WP 0.025 kg "High Whole 0 <0.01 Edwards and Dick
1973-4 a.i./hl volume" fruit 1 <0.01 1976
7 <0.01
14 <0.01
Skin 0 0.95
1 0.60
7 0.32
14 0.36
Flesh 0 <0.02
1 <0.02
7 <0.02
14 <0.02
Japan 50% WP 0.05 kg 5000 Skin 11 13.0 Manley
1970 a.i./hi 21 4.9 1972
(four 31 7.2
applications 42-43 2.1-3.0
over 9-10 49 0.72-2.4
weeks) 59-63 0.67-3.6
TABLE 3. (con't)
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
0.05 kg 5000 Skin 11 16.5
a.i./hl 31 5.1-11.6
(five 42-43 5.2
applications 49 2.4
over 9-10 59-63 1.6-4.7
weeks)
0.05 kg 5000 Flesh 11-63 <0.01-0.88
a.i./hi (mean 0.19;
(4-6 16 samples)
applications
over 9-11
weeks)
Japan 48% 0.05 kg 3300- Skin >123 days 0.03-0.34 Bullock and Kennedy
1977 Dispersible a.i./hl 5000 (four 1981
Grain (3 or 2 results)
applications Flesh <0.02
approx. (four
11 weeks results)
apart) Juice <0.02
(two
results)
TABLE 4. Residues of pirimicarb in apple, Canada 1980 and Federal Republic of Germany 1977
Country Rate of Days between Total Carbamate residues (mg/kg)
and application last application
Year Formulation (kg a.i./ha) and harvest Minimum Maximum Mean
Canada 50% WP 0.56 0 - - 0.58 (1)1
1980 3 - - 0.42 (1)
8 - - 0.19 (1)
14 - - 0.05 (1)
Germany 50% 0.5 0 0.42 0.69 0.49 (4)
1977 Dispersible 3 0.22 0.51 0.31 (4)
grain 7-8 0.17 0.51 0.27 (4)
10-11 0.16 0.45 0.21 (4)
14 0.17 0.49 0.24 (3)
20-21 0.09 0.49 0.13 (3)
1 Figures in parentheses are the numbers of results on which the means are based.
A trial was also carried out in the UK on pears, using a single
application of a 50% dispersible grain formulation at a rate of
0.21 kg a.i./ha. Samples for residue analysis were taken at 0, 2, 7
and 107 days. The initial total carbamate residue of 0.39 mg/kg
decayed slowly over the following seven days, to 0.27 mg/kg. No
residue was detected in the 107-day sample (limit of determination,
0.02 mg/kg) (Bullock and Kennedy 1981). Two trials were also carried
out on pears in Japan in 1973. Two or three applications of a 50%
dispersible powder formulation were made at 0.05 kg a.i./hl, at 2500 l
of spray per ha. Samples for residue analysis were taken 54 and 64
days after the last applications. As expected with such long
intervals, no residues were detected in any of the four samples
analysed (limit of determination, 0.02 mg/kg) (Edwards and Dick 1976).
Data reviewed at the 1976 Meeting indicated that pirimicarb
residue levels on apples tend to decline more slowly than on many
other crops, such as leafy vegetables. The data now available on pears
are consistent with the findings on apples, in terms both of the
levels of residues present and in the pattern of residue decline.
Pecan
At 0.0125-0.025 kg a.i./hl applied 'high volume', pirimicarb
controls the black margined aphid, Monellia costalis, yellow aphid,
Monellia sp. and black pecan aphid, Tinocallis caryaefoliae, on
pecans. Aphids usually occur on pecans early in the season, although a
second infestation can appear a few weeks before harvest. At that
time, the nut is still protected by the hull and as a result it is
unlikely that residues would be detected in the nut. That expectation
was confirmed in four trials conducted in the USA in 1975. Pirimicarb
was sprayed at 0.125 to 0.375 kg a.i./ha up to three times, using a
50% WP formulation. Nuts were harvested 17 to 77 days after the last
application. No pyrimidine-carbamate residues were detected in any of
the ten samples analysed (limit of determination, 0.05 mg/kg) (Ussary
1976).
Cotton
At 0.03 to 0.06 kg a.i./ha, pirimicarb controls the cotton aphid,
Aphis gossypii. Aphid attack on cotton occurs early in the growing
season and it is unlikely that a residue would be detected in the
seeds at harvest. This expectation was confirmed in six trials
conducted in the USA in 1976-78. Two to four applications of
pirimicarb were made using a 50% WP at 0.06 or 0.12 kg a.i./ha. The
cotton was harvested 40 to 122 days after the last application. No
pyrimidine-carbamate residues were detected in any of the ten samples
analysed (limit of determination, 0.05 mg/kg ) (Ussary 1979).
Leeks, spinach and watercress
Pirimicarb is particularly useful when a pre-harvest clean-up of
aphid infestations is required. Aphids controlled by the compound
include the peach-potato aphid, Myzus persicae, on leeks and
watercress, shallot aphid, Myzus ascalonicus, on leeks, and the
black bean aphid, Aphid fabae, on spinach.
Three trials were conducted on outdoor leeks in Germany in 1977.
A 50% dispersible grain formulation was applied on three occasions at
0.15 kg a.i./ha. Initial total carbamate residues on the leeks of 1.5
to 4.0 mg/kg declined rapidly with a half-life of less than one day
(Table 5) (Bullock and Kennedy 1981).
Work on three varieties of spinach was undertaken in Germany in
1974, using a single application of a 50% dispersible grain
formulation at 0.5 kg a.i./ha. In three further trials in Germany in
1977, a 50% dispersible grain formulation was applied on three
occasions at 0.15 kg a.i./ha. Initial total carbamate residues of
approximately 3 to 8 mg/kg declined rapidly with a half-life of less
than one day (Table 5) (Bullock and Kennedy 1981; Edwards and Dick
1976).
Three trials were conducted on watercress in the UK in 1972, in
which a 50% WP formulation was applied at 0.25 kg a.i./ha (recommended
rate) or 0.50 kg a.i./ha. Total carbamate residue levels on the
watercress again declined very quickly in the period immediately after
application (Table 5)(Bullock 1972).
The pattern of residues on these three crops are similar to those
reviewed on various leafy vegetables at the 1976 Meeting.
Glasshouse crops
Some additional data on residues of pirimicarb on glasshouse
crops have been received (Greve and van de Kamp 1974) and are recorded
in Table 6. They are in line with data previously received on these
crops.
Other crops
Trials involving the application of pirimicarb to broccoli,
Brussels sprouts, peas, potatoes, soybeans, sugarbeet and turnips in
various countries, gave rise to no residues above the limits of
determination (generally <0.02 mg/kg). Further trials data on
pirimicarb residues in some other fruits and vegetables are listed in
Table 7 (Bullock and Kennedy 1981).
TABLE 5. Residues of pirimicarb in leek, spinach and watercress1
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Leeks Germany 50% 0.15 0 1.5 4.0 2.6 (3)2
1977 dispersible × 3 1 0.3 0.65 0.48 (2)
grain 2 - - 0.15 (1)
4 0.06 0.12 0.09 (3)
7 0.02 0.05 0.04 (3)
10 <0.02 0.02 0.02 (3)
14 <0.02 0.03 0.02 (3)
Spinach Germany 50% 0.15 0 4.6 7.3 5.6 (3)
1977 dispersible × 3 1 0.72 2.0 1.3 (3)
grain 4 0.16 0.50 0.29 (3)
7 0.05 0.06 0.06 (3)
10 <0.02 0.02 0.02 (3)
Germany 50% 0.5 0 2.7 6.0 4.1 (3)
1974 dispersible (× 1) 1 1.5 2.1 1.7 (3)
grain 3 0.14 0.65 0.39 (3)
5 0.01 0.12 0.07 (3)
7 <0.01 0.03 0.02 (3)
10 <0.01 0.02 0.02 (3)
12 - - <0.01 (1)
TABLE 5. (con't)
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Watercress UK 50% WP 0.25 0 5.9 22 11 (3)
1972 (× 1) 1 - - 1.1 (1)
2 0.43 0.51 0.44 (3)
4 0.37 0.39 0.38 (3)
7 0.16 0.20 0.18 (2)
0.5 0 - - 13 (1)
(× 1) 2 - - 0.86 (1)
4 - - 0.95 (1)
7 - - 0.50 (1)
1 Bullock and Kennedy, 1981; Edwards and Dick, 1976;
2 Figures in parentheses are the numbers of results upon which the means are based.
TABLE 6. Residues of pirimicarb in glasshouse crops1
Crop Residues (mg/kg) at intervals (days) after treatment
0 3
Range Mean Range Mean
Eggplants 0.16-0.24 0.19 0.05 0.05
" 0.13-0.19 0.16 0.10-0.24 0.17
Gherkins 0.38-0.64 0.47 0.09-0.13 0.11
" <0.05 <0.05 <0.05 <0.05
Cucumber (whole) <0.05 <0.05 <0.05 <0.05
" (peel) 0.06-0.24 0.11 0.05-0.14 0.08
Tomato 0.12-0.21 0.14
" 0.09-0.13 0.12 <0.05 <0.05
Pepper (bell) 0.18-0.30 0.25 0.05-0.07 0.06
" " <0.05-0.11 <0.06 <0.05-0.14 <0.08
1 In each case, one treatment with 50% WP at 0.375 kg a.i./ha.
FATE OF RESIDUES
In processing
Two studies were conducted in the USA in 1977 to determine
residues of pirimicarb and its carbamate metabolites in processed
cotton fractions. Cotton plants were sprayed with a 50% WP formulation
of pirimicarb at 0.07 to 0.13 kg a.i./ha. Two to four applications
were made with an interval of 49 to 122 days between the last
application and harvest. The cotton was harvested, fractioned and
submitted to the laboratory for residue analysis. The results are
shown in Table 8. Although no pyrimidine-carbamate residues were
observed in cottonseed, slight residues of up to 0.05 mg/kg were
observed in crude oil. A trace was noted in cottonseed meal at the
0.13 kg a.i./ha rate (twice the maximum recommended). However, as no
residue was detected in the cottonseed, it is unlikely that residues
should be present in both the meal and oil. The trace residues in meal
are most likely due to lack of sample homogeneity or possibly to minor
contamination (Ussary 1979).
TABLE 7. Pirimicarb residues in some fruits and vegetables
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Cherry Fed.Rep.Germany 1977 0 1.5 - 1.9 1.4
4 0.03- 0.89 0.41
7 0.02- 0.52 0.26
14 <0.02- 0.33 0.15
UK 1976 62 <0.02 <0.02
Peach Japan 1976 27-61 0.13- 0.40 0.28
Canada 1980 60-107 <0.02 <0.02
Plum Canada 1976 21-30 0.14- 0.17 0.15
Pepper Denmark 1976 0 - 0.13
3 - 0.10
7 - 0.04
14 - 0.02
Tomato Canada 1976 1 - 0.10
3 - 0.03
7 - <0.02
Denmark 1977 0 - 0.46
3 - 0.16
7 - 0.09
14 - 0.02
Cucumber Denmark 1977 0 0.13, 0.47 -
3 0.08, 0.30 -
7 0.07, 0.38 -
14 0.02, 0.05 -
21 0.02, 0.07 -
TABLE 7. (con't)
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Parsley Netherlands 1976 0 - 23
1 - 15
3 - 8.6
7 - 6.3
14 - 4.6
TABLE 8. Pirimicarb residues on cottonseed fractions, USA 1977
Residue (mg/kg)1
Type of No of Days after last
Sample applications application Control 0.07 kg ai/A 0.13 kg ai/A4
to harvest P2 DMP3 Total P DMP Total P DMP Total
Cottonseed 4 49 ND ND ND ND ND ND ND ND ND
Hulls ND ND ND ND ND ND
Meal ND TR 0.01 ND 0.01
Crude oil ND ND ND 0.02 0.01 0.03
Cottonseed 2 122 ND ND ND
Hulls ND ND ND
Meal ND ND ND
Crude oil 0.04 0.01 0.05
1 ND=none detected (<0.01 ppm of the individual compounds);
2 P = pirimicarb;
3 DMP = desmethyl pirimicarb;
4 Twice maximum use rate.
METHODS OF ANALYSIS
The preferred method for determining residues of pirimicarb and
its carbamate-containing metabolites in crops and in products of
animal origin is by gas-liquid chromatography with selective nitrogen
detection. The method was reviewed at the 1976 and 1978 Meetings.
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs listed in Table 9 have been established for
residues of pirimicarb plus its two carbamate-containing metabolites,
II and III (Figure 1).
TABLE 9. National maximum residue limits reported to the Meeting
Country Crop MRL (mg/kg)
Australia Vegetables, fruit and hops 0.5
Belgium Potato 0.5
Other crops 0.3
Brazil Cabbage, cauliflower 0.5
Aubergine, cucumber, pepper, tomato 0.2
Potato 0.05
Fed. Rep. of Lettuce, cherry 1.0 ) from
Germany Other leaf, sprout and fruit vegetables 0.5 ) 1.1.82
Cereals, potato, sugarbeet 0.1 )
France Cereals 0.05 (proposed)
Hungary Fruit 0.5
Vegetables 1
Other crops 0.5
Japan Fruits and vegetables 0.3
Netherlands Fruits, vegetables, herbs and spices 1.0
Grain, potato 0.05
New Zealand Beans, lettuce, pea, brassicae, tomato 1
Potato, fodder crops 0.5
TABLE 9. (con't)
Country Crop MRL (mg/kg)
South Africa Cruciferae, oats, sorghum, wheat, peach 0.3
Peanut, pecan, potato 0.05
Switzerland Fruits and vegetables 1
USA Potato 0.1
Venezuela All Crops 0.5
EVALUATION
COMMENTS AND APPRAISAL
The Meeting was informed that the data requested by the 1978
Meeting would not be available until 1982. The Meeting therefore
agreed to extend the existing temporary ADI until 1982.
Additional data on residues of pirimicarb in a range of crops
have been received and reviewed. The pattern of residue data is
generally similar to that reviewed at previous meetings, although the
new data on oranges show the need for an upward revision of the
present limit to cover residues arising from use close to harvest in
some countries. On outdoor vegetables and field crops the residues
decline very quickly after spraying; on fruits, however, decline is
slower. Data on pears, together with old and new data on apples,
allowed a recommendation for limits on pome fruits. Additional and
altered limits are proposed.
RECOMMENDATIONS OF RESIDUE LIMITS
The following new or altered temporary limits refer to the sum of
pirimicarb, its N-formyl (methylamino) analogue (desmethylformamido
pirimicarb) and desmethyl pirimicarb.
Pre-harvest interval on
Temporary MRL which the recommendations
Commodity (mg/kg) are based (days)
Pome fruits 1 7
Spinach 1 3-4
Watercress 1 2-4
Leeks 0.5 1-2
Oranges 0.5 7-14
Citrus fruits
(other than oranges) 0.051 -
Cottonseed 0.051 -
Pecan 0.051 -
Sweet corn 0.051 -
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Data on residues on oranges due to applications made close to
harvest in countries other than Portugal.
2. Data on residues on other citrus fruits from countries where
applications close to harvest are recommended or permitted.
REFERENCES
Bullock, D.J.W. Pirimicarb residues data on watercress: UK 1972. ICI
1972 Plant Protection Division. (Unpublished)
Bullock, D.J.W. and Kennedy, S.H. Pirimicarb : residues in crops from
1981 field trials during 1976-80. ICI Plant Protection Division
Report no. TMJ 1883A. (Unpublished)
Cambon, C., Declume, C. and Derace, R. Foetal and maternal rat brain
1980 acetylcholinesterase: isoenzymes changes following
insecticidal carbamate derivates poisoning. Archives of
Toxicology 45: 257-262.
Edwards, M.J. and Dick, J.P. Pirimicarb residue summary: Residues in
1976 crops from field trials during 1973-1975. ICI Plant
Protection Division Report No. TMJ 1360B. (Unpublished)
Ferreira, J.R. and Tainha, A. Residues of pirimicarb in field-treated
1981 oranges. Ministry of Agriculture and Fisheries, Toxicology
and Analytical services Directorate, Portugal. Report PPA
(TR)-12/81. (Unpublished)
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Report from
1978 Hazelton Labs. Europe Ltd., no. 1371-72/2, submitted by ICI
Ltd. to WHO. (Unpublished)
Greve, P.A. and van de Kamp, C.G. Residuen van bestrijdingsmiddeln in
1974 de groenteteelt onder glas. Report 96/74 Tox - ROB. Report
from the Netherlands. (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and Street,
1977 A.E. Pirimicarb preliminary oral toxicity study in rhesus
monkeys (repeated dosages for 13 weeks). Report from
Huntingdon Research Centre, no. 164/77825, submitted by ICI
Ltd. to WHO (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H,, Brown, G. and Street,
1978a A.E. Pirimicarb oral toxicity study in rhesus monkeys
(repeated dosages for 17 weeks, followed by a recovery
period of 8 weeks). Report (final report) from Huntingdon
Research Centre no. 198/78444, submitted by ICI Ltd. to WHO.
(Unpublished)
1978b Pirimicarb comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs Tests). Report
from Huntingdon Research Centre, No. 219/78445, submitted by
ICI Ltd. to WHO. (Unpublished)
Jackson, J.A., Chart, I.S., Sanderson, J.H. and Garner, R. Pirimicarb
1977 induced immune hemolytic anaemia in dogs, Scandinavian
Journal of Haematology, 19: 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study in
1978a foxhounds. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/420, submitted by ICI Ltd.
(Unpublished)
Jackson, J.A.; and Royle, G. Pirimicarb: oral toxicity study in rhesus
1978b monkeys. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/421, submitted by ICI Ltd.
(Unpublished)
1978c Pirimicarb: comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs tests). Addendum:
results of additional serological investigations. Report
from ICI Central Toxicology Laboratory, no. CTL/P/419
submitted by ICI Ltd. (Unpublished)
Manley, C.A. residue summary: Pirimicarb in crops. ICI Plant
1972 Protection Division Report no. TMJ 585/2. (Unpublished)
Parkinson, G.R. Pirimicarb metabolite R 34885: subacute oral toxicity
1978a to rats. Report from ICI Central Toxicology Laboratory, No.
CTL/P/402, submitted by ICI to WHO. (Unpublished)
1978b Pirimicarb metabolite R34836: subacute oral toxicity to
rats. Report from ICI Central Toxicology Laboratory, no.
CTL/P/401, submitted by ICI to WHO. (Unpublished)
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M. Pirimicarb:
1978 growth study to determine a no-effect level in the female
rat. Report from ICI Central Toxicology Laboratory no.
CTL/P/408, submitted by ICI to WHO. (Unpublished)
Pilinskaya, M.A. Cytogenic effect of a number of pesticides in a
1981 culture of human peripheral blood lymphocytes with different
levels of spontaneous aberrations. Tsitologiya i Genetika,
15(2) : 82-84
Richards, D., Banhan, P.B. and Weight, T M. Pirimicarb paired feeding
study in a female rat. Report from ICI Central Toxicology
Laboratory, no. CTL/P/407. Submitted by ICI to WHO.
(Unpublished)
Ussary, J.P. Pirimicarb residues in pecan, 1975. ICI Americas Inc.
1976 Reports. (Unpublished)
1979 Pirimicarb residues on cottonseed. ICI Americas Inc, Report
no. TMU 0442/B. (Unpublished)
 TABLE 1. Residues of pirimicarb in barley and wheat, Canada 1978
Application rate Crop part Total carbamate residues (mg/kg) after
Crop (kg a.i./ha) analysed (days)
1 5 7 14
Barley 0.07 Grain 0.47 0.15 0.11 0.04
0.14 Grain 0.82 0.30 0.14 0.07
0.07 Ears - - 0.04 <0.02
0.14 Ears - - 0.05 0.02
Wheat 0.07 Ears 0.10 0.02 <0.02 <0.02
0.14 Ears 0.93 0.41 0.05 <0.02
A trial was conducted in Japan in 1979, in which sweet corn was
sprayed at the unusually high rate of 2 kg a.i./ha, using a 48% WP
formulation and 2 000 to 4 000 l of water/ha. Residue samples taken 23
days later contained no detectable pyrimidine-carbamate residues
(limit of determination 0.02 mg/kg) (Bullock and Kennedy 1981). This
was an anticipated finding, as sweet corn on the cob is protected from
spray by the surrounding foliage.
Pirimicarb is not registered for use on rice at this time and no
data on residues are available.
Citrus fruit
The 1976 Meeting proposed a temporary MRL in citrus fruits of
0.05 mg/kg, at or about the limit of determination of the analytical
method. Interpretation of much of the data available in 1976 was
rendered difficult by the fact that often the peel and flesh were
analysed separately. Whole fruits had been analysed on only a limited
number of occasions, although the available data justified the
proposed MRL. Supportive evidence was provided by the separate
analysis of peel and flesh of oranges treated in Australia and in
Spain. Residues were less than 0.02 mg/kg in edible flesh and did not
exceed 1 mg/kg in the peel. At the time, results for samples obtained
from Japan were deemed unrepresentative, particularly since there was
a concern over possible contamination of the edible flesh with
residues during peeling.
Aphids usually occur on citrus early in the season. This is
several months before the first fruits are mature. However, the
harvesting period often extends from the autumn through to the
following spring. As a result, fruit can remain on the tree at the
time of the first sprays against aphids during the following year, and
then a short pre-harvest withholding interval is appropriate.
Ferreira and Tainha (1981) in Portugal have now provided new
evidence of residues on whole oranges. In Portugal, pirimicarb is
approved as a 50% WP formulation for use in citrus at a spray
concentration of 37.5 g a.i./hl, applied to "run off". The pre-harvest
withholding interval is two weeks. Two spray applications were made,
fourteen days apart, to three separate replicate trees. Samples of 15
fruits were collected from each tree 1, 4, 7, 11, 21 28 and 35 days
after the second spray. Analyses by a slight modification of the
standard residue method yielded results as shown in Table 2.
TABLE 2. Residues of pirimicarb in organge, Portugal, 1980
Application Days between Total carbamate residues (mg/kg)
Rate last treatment
and harvest Tree No.11 Tree No.21 Tree No. 31 Mean
1 0.69 0.64 0.99 0.77
37.5 g a.i./h 4 0.27 0.27 0.52 0.35
applied to 7 0.27 0.30 0.35 0.31
"run off" , 11 0.37 0.16 0.30 0.28
twice, fourteen 14 0.21 0.14 0.22 0.19
days 21 0.14 0.20 0.29 0.21
apart. 28 0.20 0.22 0.28 0.23
35 0.18 0.20 0.24 0.21
1 mean of three analyses (uncorrected for recovery).
Residues in oranges are present mainly in the oil glands of the
peel, in which they tend to remain stable. Levels in the edible flesh
have been consistently below 1 mg/kg (Table 3) (Manley 1972; Edwards
and Dick 1976; Bullock and Kennedy 1981).
Apple and pear
The 1976 Meeting proposed a temporary MRL for apples at 1 mg/kg.
Additional residues data now available from trials in Canada in 1980
and Federal Republic of Germany in 1977 confirm the appropriateness of
this value (Table 4) (Bullock and Kennedy 1981).
TABLE 3. Residues of pirimicarb in orange, Australia and Japan, 1970-78.
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
Australia 50% WP 0.025 kg "High Whole 0 <0.01 Edwards and Dick
1973-4 a.i./hl volume" fruit 1 <0.01 1976
7 <0.01
14 <0.01
Skin 0 0.95
1 0.60
7 0.32
14 0.36
Flesh 0 <0.02
1 <0.02
7 <0.02
14 <0.02
Japan 50% WP 0.05 kg 5000 Skin 11 13.0 Manley
1970 a.i./hi 21 4.9 1972
(four 31 7.2
applications 42-43 2.1-3.0
over 9-10 49 0.72-2.4
weeks) 59-63 0.67-3.6
TABLE 3. (con't)
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
0.05 kg 5000 Skin 11 16.5
a.i./hl 31 5.1-11.6
(five 42-43 5.2
applications 49 2.4
over 9-10 59-63 1.6-4.7
weeks)
0.05 kg 5000 Flesh 11-63 <0.01-0.88
a.i./hi (mean 0.19;
(4-6 16 samples)
applications
over 9-11
weeks)
Japan 48% 0.05 kg 3300- Skin >123 days 0.03-0.34 Bullock and Kennedy
1977 Dispersible a.i./hl 5000 (four 1981
Grain (3 or 2 results)
applications Flesh <0.02
approx. (four
11 weeks results)
apart) Juice <0.02
(two
results)
TABLE 4. Residues of pirimicarb in apple, Canada 1980 and Federal Republic of Germany 1977
Country Rate of Days between Total Carbamate residues (mg/kg)
and application last application
Year Formulation (kg a.i./ha) and harvest Minimum Maximum Mean
Canada 50% WP 0.56 0 - - 0.58 (1)1
1980 3 - - 0.42 (1)
8 - - 0.19 (1)
14 - - 0.05 (1)
Germany 50% 0.5 0 0.42 0.69 0.49 (4)
1977 Dispersible 3 0.22 0.51 0.31 (4)
grain 7-8 0.17 0.51 0.27 (4)
10-11 0.16 0.45 0.21 (4)
14 0.17 0.49 0.24 (3)
20-21 0.09 0.49 0.13 (3)
1 Figures in parentheses are the numbers of results on which the means are based.
A trial was also carried out in the UK on pears, using a single
application of a 50% dispersible grain formulation at a rate of
0.21 kg a.i./ha. Samples for residue analysis were taken at 0, 2, 7
and 107 days. The initial total carbamate residue of 0.39 mg/kg
decayed slowly over the following seven days, to 0.27 mg/kg. No
residue was detected in the 107-day sample (limit of determination,
0.02 mg/kg) (Bullock and Kennedy 1981). Two trials were also carried
out on pears in Japan in 1973. Two or three applications of a 50%
dispersible powder formulation were made at 0.05 kg a.i./hl, at 2500 l
of spray per ha. Samples for residue analysis were taken 54 and 64
days after the last applications. As expected with such long
intervals, no residues were detected in any of the four samples
analysed (limit of determination, 0.02 mg/kg) (Edwards and Dick 1976).
Data reviewed at the 1976 Meeting indicated that pirimicarb
residue levels on apples tend to decline more slowly than on many
other crops, such as leafy vegetables. The data now available on pears
are consistent with the findings on apples, in terms both of the
levels of residues present and in the pattern of residue decline.
Pecan
At 0.0125-0.025 kg a.i./hl applied 'high volume', pirimicarb
controls the black margined aphid, Monellia costalis, yellow aphid,
Monellia sp. and black pecan aphid, Tinocallis caryaefoliae, on
pecans. Aphids usually occur on pecans early in the season, although a
second infestation can appear a few weeks before harvest. At that
time, the nut is still protected by the hull and as a result it is
unlikely that residues would be detected in the nut. That expectation
was confirmed in four trials conducted in the USA in 1975. Pirimicarb
was sprayed at 0.125 to 0.375 kg a.i./ha up to three times, using a
50% WP formulation. Nuts were harvested 17 to 77 days after the last
application. No pyrimidine-carbamate residues were detected in any of
the ten samples analysed (limit of determination, 0.05 mg/kg) (Ussary
1976).
Cotton
At 0.03 to 0.06 kg a.i./ha, pirimicarb controls the cotton aphid,
Aphis gossypii. Aphid attack on cotton occurs early in the growing
season and it is unlikely that a residue would be detected in the
seeds at harvest. This expectation was confirmed in six trials
conducted in the USA in 1976-78. Two to four applications of
pirimicarb were made using a 50% WP at 0.06 or 0.12 kg a.i./ha. The
cotton was harvested 40 to 122 days after the last application. No
pyrimidine-carbamate residues were detected in any of the ten samples
analysed (limit of determination, 0.05 mg/kg ) (Ussary 1979).
Leeks, spinach and watercress
Pirimicarb is particularly useful when a pre-harvest clean-up of
aphid infestations is required. Aphids controlled by the compound
include the peach-potato aphid, Myzus persicae, on leeks and
watercress, shallot aphid, Myzus ascalonicus, on leeks, and the
black bean aphid, Aphid fabae, on spinach.
Three trials were conducted on outdoor leeks in Germany in 1977.
A 50% dispersible grain formulation was applied on three occasions at
0.15 kg a.i./ha. Initial total carbamate residues on the leeks of 1.5
to 4.0 mg/kg declined rapidly with a half-life of less than one day
(Table 5) (Bullock and Kennedy 1981).
Work on three varieties of spinach was undertaken in Germany in
1974, using a single application of a 50% dispersible grain
formulation at 0.5 kg a.i./ha. In three further trials in Germany in
1977, a 50% dispersible grain formulation was applied on three
occasions at 0.15 kg a.i./ha. Initial total carbamate residues of
approximately 3 to 8 mg/kg declined rapidly with a half-life of less
than one day (Table 5) (Bullock and Kennedy 1981; Edwards and Dick
1976).
Three trials were conducted on watercress in the UK in 1972, in
which a 50% WP formulation was applied at 0.25 kg a.i./ha (recommended
rate) or 0.50 kg a.i./ha. Total carbamate residue levels on the
watercress again declined very quickly in the period immediately after
application (Table 5)(Bullock 1972).
The pattern of residues on these three crops are similar to those
reviewed on various leafy vegetables at the 1976 Meeting.
Glasshouse crops
Some additional data on residues of pirimicarb on glasshouse
crops have been received (Greve and van de Kamp 1974) and are recorded
in Table 6. They are in line with data previously received on these
crops.
Other crops
Trials involving the application of pirimicarb to broccoli,
Brussels sprouts, peas, potatoes, soybeans, sugarbeet and turnips in
various countries, gave rise to no residues above the limits of
determination (generally <0.02 mg/kg). Further trials data on
pirimicarb residues in some other fruits and vegetables are listed in
Table 7 (Bullock and Kennedy 1981).
TABLE 5. Residues of pirimicarb in leek, spinach and watercress1
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Leeks Germany 50% 0.15 0 1.5 4.0 2.6 (3)2
1977 dispersible × 3 1 0.3 0.65 0.48 (2)
grain 2 - - 0.15 (1)
4 0.06 0.12 0.09 (3)
7 0.02 0.05 0.04 (3)
10 <0.02 0.02 0.02 (3)
14 <0.02 0.03 0.02 (3)
Spinach Germany 50% 0.15 0 4.6 7.3 5.6 (3)
1977 dispersible × 3 1 0.72 2.0 1.3 (3)
grain 4 0.16 0.50 0.29 (3)
7 0.05 0.06 0.06 (3)
10 <0.02 0.02 0.02 (3)
Germany 50% 0.5 0 2.7 6.0 4.1 (3)
1974 dispersible (× 1) 1 1.5 2.1 1.7 (3)
grain 3 0.14 0.65 0.39 (3)
5 0.01 0.12 0.07 (3)
7 <0.01 0.03 0.02 (3)
10 <0.01 0.02 0.02 (3)
12 - - <0.01 (1)
TABLE 5. (con't)
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Watercress UK 50% WP 0.25 0 5.9 22 11 (3)
1972 (× 1) 1 - - 1.1 (1)
2 0.43 0.51 0.44 (3)
4 0.37 0.39 0.38 (3)
7 0.16 0.20 0.18 (2)
0.5 0 - - 13 (1)
(× 1) 2 - - 0.86 (1)
4 - - 0.95 (1)
7 - - 0.50 (1)
1 Bullock and Kennedy, 1981; Edwards and Dick, 1976;
2 Figures in parentheses are the numbers of results upon which the means are based.
TABLE 6. Residues of pirimicarb in glasshouse crops1
Crop Residues (mg/kg) at intervals (days) after treatment
0 3
Range Mean Range Mean
Eggplants 0.16-0.24 0.19 0.05 0.05
" 0.13-0.19 0.16 0.10-0.24 0.17
Gherkins 0.38-0.64 0.47 0.09-0.13 0.11
" <0.05 <0.05 <0.05 <0.05
Cucumber (whole) <0.05 <0.05 <0.05 <0.05
" (peel) 0.06-0.24 0.11 0.05-0.14 0.08
Tomato 0.12-0.21 0.14
" 0.09-0.13 0.12 <0.05 <0.05
Pepper (bell) 0.18-0.30 0.25 0.05-0.07 0.06
" " <0.05-0.11 <0.06 <0.05-0.14 <0.08
1 In each case, one treatment with 50% WP at 0.375 kg a.i./ha.
FATE OF RESIDUES
In processing
Two studies were conducted in the USA in 1977 to determine
residues of pirimicarb and its carbamate metabolites in processed
cotton fractions. Cotton plants were sprayed with a 50% WP formulation
of pirimicarb at 0.07 to 0.13 kg a.i./ha. Two to four applications
were made with an interval of 49 to 122 days between the last
application and harvest. The cotton was harvested, fractioned and
submitted to the laboratory for residue analysis. The results are
shown in Table 8. Although no pyrimidine-carbamate residues were
observed in cottonseed, slight residues of up to 0.05 mg/kg were
observed in crude oil. A trace was noted in cottonseed meal at the
0.13 kg a.i./ha rate (twice the maximum recommended). However, as no
residue was detected in the cottonseed, it is unlikely that residues
should be present in both the meal and oil. The trace residues in meal
are most likely due to lack of sample homogeneity or possibly to minor
contamination (Ussary 1979).
TABLE 7. Pirimicarb residues in some fruits and vegetables
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Cherry Fed.Rep.Germany 1977 0 1.5 - 1.9 1.4
4 0.03- 0.89 0.41
7 0.02- 0.52 0.26
14 <0.02- 0.33 0.15
UK 1976 62 <0.02 <0.02
Peach Japan 1976 27-61 0.13- 0.40 0.28
Canada 1980 60-107 <0.02 <0.02
Plum Canada 1976 21-30 0.14- 0.17 0.15
Pepper Denmark 1976 0 - 0.13
3 - 0.10
7 - 0.04
14 - 0.02
Tomato Canada 1976 1 - 0.10
3 - 0.03
7 - <0.02
Denmark 1977 0 - 0.46
3 - 0.16
7 - 0.09
14 - 0.02
Cucumber Denmark 1977 0 0.13, 0.47 -
3 0.08, 0.30 -
7 0.07, 0.38 -
14 0.02, 0.05 -
21 0.02, 0.07 -
TABLE 7. (con't)
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Parsley Netherlands 1976 0 - 23
1 - 15
3 - 8.6
7 - 6.3
14 - 4.6
TABLE 8. Pirimicarb residues on cottonseed fractions, USA 1977
Residue (mg/kg)1
Type of No of Days after last
Sample applications application Control 0.07 kg ai/A 0.13 kg ai/A4
to harvest P2 DMP3 Total P DMP Total P DMP Total
Cottonseed 4 49 ND ND ND ND ND ND ND ND ND
Hulls ND ND ND ND ND ND
Meal ND TR 0.01 ND 0.01
Crude oil ND ND ND 0.02 0.01 0.03
Cottonseed 2 122 ND ND ND
Hulls ND ND ND
Meal ND ND ND
Crude oil 0.04 0.01 0.05
1 ND=none detected (<0.01 ppm of the individual compounds);
2 P = pirimicarb;
3 DMP = desmethyl pirimicarb;
4 Twice maximum use rate.
METHODS OF ANALYSIS
The preferred method for determining residues of pirimicarb and
its carbamate-containing metabolites in crops and in products of
animal origin is by gas-liquid chromatography with selective nitrogen
detection. The method was reviewed at the 1976 and 1978 Meetings.
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs listed in Table 9 have been established for
residues of pirimicarb plus its two carbamate-containing metabolites,
II and III (Figure 1).
TABLE 9. National maximum residue limits reported to the Meeting
Country Crop MRL (mg/kg)
Australia Vegetables, fruit and hops 0.5
Belgium Potato 0.5
Other crops 0.3
Brazil Cabbage, cauliflower 0.5
Aubergine, cucumber, pepper, tomato 0.2
Potato 0.05
Fed. Rep. of Lettuce, cherry 1.0 ) from
Germany Other leaf, sprout and fruit vegetables 0.5 ) 1.1.82
Cereals, potato, sugarbeet 0.1 )
France Cereals 0.05 (proposed)
Hungary Fruit 0.5
Vegetables 1
Other crops 0.5
Japan Fruits and vegetables 0.3
Netherlands Fruits, vegetables, herbs and spices 1.0
Grain, potato 0.05
New Zealand Beans, lettuce, pea, brassicae, tomato 1
Potato, fodder crops 0.5
TABLE 9. (con't)
Country Crop MRL (mg/kg)
South Africa Cruciferae, oats, sorghum, wheat, peach 0.3
Peanut, pecan, potato 0.05
Switzerland Fruits and vegetables 1
USA Potato 0.1
Venezuela All Crops 0.5
EVALUATION
COMMENTS AND APPRAISAL
The Meeting was informed that the data requested by the 1978
Meeting would not be available until 1982. The Meeting therefore
agreed to extend the existing temporary ADI until 1982.
Additional data on residues of pirimicarb in a range of crops
have been received and reviewed. The pattern of residue data is
generally similar to that reviewed at previous meetings, although the
new data on oranges show the need for an upward revision of the
present limit to cover residues arising from use close to harvest in
some countries. On outdoor vegetables and field crops the residues
decline very quickly after spraying; on fruits, however, decline is
slower. Data on pears, together with old and new data on apples,
allowed a recommendation for limits on pome fruits. Additional and
altered limits are proposed.
RECOMMENDATIONS OF RESIDUE LIMITS
The following new or altered temporary limits refer to the sum of
pirimicarb, its N-formyl (methylamino) analogue (desmethylformamido
pirimicarb) and desmethyl pirimicarb.
Pre-harvest interval on
Temporary MRL which the recommendations
Commodity (mg/kg) are based (days)
Pome fruits 1 7
Spinach 1 3-4
Watercress 1 2-4
Leeks 0.5 1-2
Oranges 0.5 7-14
Citrus fruits
(other than oranges) 0.051 -
Cottonseed 0.051 -
Pecan 0.051 -
Sweet corn 0.051 -
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Data on residues on oranges due to applications made close to
harvest in countries other than Portugal.
2. Data on residues on other citrus fruits from countries where
applications close to harvest are recommended or permitted.
REFERENCES
Bullock, D.J.W. Pirimicarb residues data on watercress: UK 1972. ICI
1972 Plant Protection Division. (Unpublished)
Bullock, D.J.W. and Kennedy, S.H. Pirimicarb : residues in crops from
1981 field trials during 1976-80. ICI Plant Protection Division
Report no. TMJ 1883A. (Unpublished)
Cambon, C., Declume, C. and Derace, R. Foetal and maternal rat brain
1980 acetylcholinesterase: isoenzymes changes following
insecticidal carbamate derivates poisoning. Archives of
Toxicology 45: 257-262.
Edwards, M.J. and Dick, J.P. Pirimicarb residue summary: Residues in
1976 crops from field trials during 1973-1975. ICI Plant
Protection Division Report No. TMJ 1360B. (Unpublished)
Ferreira, J.R. and Tainha, A. Residues of pirimicarb in field-treated
1981 oranges. Ministry of Agriculture and Fisheries, Toxicology
and Analytical services Directorate, Portugal. Report PPA
(TR)-12/81. (Unpublished)
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Report from
1978 Hazelton Labs. Europe Ltd., no. 1371-72/2, submitted by ICI
Ltd. to WHO. (Unpublished)
Greve, P.A. and van de Kamp, C.G. Residuen van bestrijdingsmiddeln in
1974 de groenteteelt onder glas. Report 96/74 Tox - ROB. Report
from the Netherlands. (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and Street,
1977 A.E. Pirimicarb preliminary oral toxicity study in rhesus
monkeys (repeated dosages for 13 weeks). Report from
Huntingdon Research Centre, no. 164/77825, submitted by ICI
Ltd. to WHO (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H,, Brown, G. and Street,
1978a A.E. Pirimicarb oral toxicity study in rhesus monkeys
(repeated dosages for 17 weeks, followed by a recovery
period of 8 weeks). Report (final report) from Huntingdon
Research Centre no. 198/78444, submitted by ICI Ltd. to WHO.
(Unpublished)
1978b Pirimicarb comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs Tests). Report
from Huntingdon Research Centre, No. 219/78445, submitted by
ICI Ltd. to WHO. (Unpublished)
Jackson, J.A., Chart, I.S., Sanderson, J.H. and Garner, R. Pirimicarb
1977 induced immune hemolytic anaemia in dogs, Scandinavian
Journal of Haematology, 19: 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study in
1978a foxhounds. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/420, submitted by ICI Ltd.
(Unpublished)
Jackson, J.A.; and Royle, G. Pirimicarb: oral toxicity study in rhesus
1978b monkeys. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/421, submitted by ICI Ltd.
(Unpublished)
1978c Pirimicarb: comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs tests). Addendum:
results of additional serological investigations. Report
from ICI Central Toxicology Laboratory, no. CTL/P/419
submitted by ICI Ltd. (Unpublished)
Manley, C.A. residue summary: Pirimicarb in crops. ICI Plant
1972 Protection Division Report no. TMJ 585/2. (Unpublished)
Parkinson, G.R. Pirimicarb metabolite R 34885: subacute oral toxicity
1978a to rats. Report from ICI Central Toxicology Laboratory, No.
CTL/P/402, submitted by ICI to WHO. (Unpublished)
1978b Pirimicarb metabolite R34836: subacute oral toxicity to
rats. Report from ICI Central Toxicology Laboratory, no.
CTL/P/401, submitted by ICI to WHO. (Unpublished)
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M. Pirimicarb:
1978 growth study to determine a no-effect level in the female
rat. Report from ICI Central Toxicology Laboratory no.
CTL/P/408, submitted by ICI to WHO. (Unpublished)
Pilinskaya, M.A. Cytogenic effect of a number of pesticides in a
1981 culture of human peripheral blood lymphocytes with different
levels of spontaneous aberrations. Tsitologiya i Genetika,
15(2) : 82-84
Richards, D., Banhan, P.B. and Weight, T M. Pirimicarb paired feeding
study in a female rat. Report from ICI Central Toxicology
Laboratory, no. CTL/P/407. Submitted by ICI to WHO.
(Unpublished)
Ussary, J.P. Pirimicarb residues in pecan, 1975. ICI Americas Inc.
1976 Reports. (Unpublished)
1979 Pirimicarb residues on cottonseed. ICI Americas Inc, Report
no. TMU 0442/B. (Unpublished)
TABLE 1. Residues of pirimicarb in barley and wheat, Canada 1978
Application rate Crop part Total carbamate residues (mg/kg) after
Crop (kg a.i./ha) analysed (days)
1 5 7 14
Barley 0.07 Grain 0.47 0.15 0.11 0.04
0.14 Grain 0.82 0.30 0.14 0.07
0.07 Ears - - 0.04 <0.02
0.14 Ears - - 0.05 0.02
Wheat 0.07 Ears 0.10 0.02 <0.02 <0.02
0.14 Ears 0.93 0.41 0.05 <0.02
A trial was conducted in Japan in 1979, in which sweet corn was
sprayed at the unusually high rate of 2 kg a.i./ha, using a 48% WP
formulation and 2 000 to 4 000 l of water/ha. Residue samples taken 23
days later contained no detectable pyrimidine-carbamate residues
(limit of determination 0.02 mg/kg) (Bullock and Kennedy 1981). This
was an anticipated finding, as sweet corn on the cob is protected from
spray by the surrounding foliage.
Pirimicarb is not registered for use on rice at this time and no
data on residues are available.
Citrus fruit
The 1976 Meeting proposed a temporary MRL in citrus fruits of
0.05 mg/kg, at or about the limit of determination of the analytical
method. Interpretation of much of the data available in 1976 was
rendered difficult by the fact that often the peel and flesh were
analysed separately. Whole fruits had been analysed on only a limited
number of occasions, although the available data justified the
proposed MRL. Supportive evidence was provided by the separate
analysis of peel and flesh of oranges treated in Australia and in
Spain. Residues were less than 0.02 mg/kg in edible flesh and did not
exceed 1 mg/kg in the peel. At the time, results for samples obtained
from Japan were deemed unrepresentative, particularly since there was
a concern over possible contamination of the edible flesh with
residues during peeling.
Aphids usually occur on citrus early in the season. This is
several months before the first fruits are mature. However, the
harvesting period often extends from the autumn through to the
following spring. As a result, fruit can remain on the tree at the
time of the first sprays against aphids during the following year, and
then a short pre-harvest withholding interval is appropriate.
Ferreira and Tainha (1981) in Portugal have now provided new
evidence of residues on whole oranges. In Portugal, pirimicarb is
approved as a 50% WP formulation for use in citrus at a spray
concentration of 37.5 g a.i./hl, applied to "run off". The pre-harvest
withholding interval is two weeks. Two spray applications were made,
fourteen days apart, to three separate replicate trees. Samples of 15
fruits were collected from each tree 1, 4, 7, 11, 21 28 and 35 days
after the second spray. Analyses by a slight modification of the
standard residue method yielded results as shown in Table 2.
TABLE 2. Residues of pirimicarb in organge, Portugal, 1980
Application Days between Total carbamate residues (mg/kg)
Rate last treatment
and harvest Tree No.11 Tree No.21 Tree No. 31 Mean
1 0.69 0.64 0.99 0.77
37.5 g a.i./h 4 0.27 0.27 0.52 0.35
applied to 7 0.27 0.30 0.35 0.31
"run off" , 11 0.37 0.16 0.30 0.28
twice, fourteen 14 0.21 0.14 0.22 0.19
days 21 0.14 0.20 0.29 0.21
apart. 28 0.20 0.22 0.28 0.23
35 0.18 0.20 0.24 0.21
1 mean of three analyses (uncorrected for recovery).
Residues in oranges are present mainly in the oil glands of the
peel, in which they tend to remain stable. Levels in the edible flesh
have been consistently below 1 mg/kg (Table 3) (Manley 1972; Edwards
and Dick 1976; Bullock and Kennedy 1981).
Apple and pear
The 1976 Meeting proposed a temporary MRL for apples at 1 mg/kg.
Additional residues data now available from trials in Canada in 1980
and Federal Republic of Germany in 1977 confirm the appropriateness of
this value (Table 4) (Bullock and Kennedy 1981).
TABLE 3. Residues of pirimicarb in orange, Australia and Japan, 1970-78.
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
Australia 50% WP 0.025 kg "High Whole 0 <0.01 Edwards and Dick
1973-4 a.i./hl volume" fruit 1 <0.01 1976
7 <0.01
14 <0.01
Skin 0 0.95
1 0.60
7 0.32
14 0.36
Flesh 0 <0.02
1 <0.02
7 <0.02
14 <0.02
Japan 50% WP 0.05 kg 5000 Skin 11 13.0 Manley
1970 a.i./hi 21 4.9 1972
(four 31 7.2
applications 42-43 2.1-3.0
over 9-10 49 0.72-2.4
weeks) 59-63 0.67-3.6
TABLE 3. (con't)
Days
Country Rate Volume Crop between Total Reference
and Fomulation of of spray part last carbamate
Year application (l/ha) analysed application residues
and (mg/kg)
harvest
0.05 kg 5000 Skin 11 16.5
a.i./hl 31 5.1-11.6
(five 42-43 5.2
applications 49 2.4
over 9-10 59-63 1.6-4.7
weeks)
0.05 kg 5000 Flesh 11-63 <0.01-0.88
a.i./hi (mean 0.19;
(4-6 16 samples)
applications
over 9-11
weeks)
Japan 48% 0.05 kg 3300- Skin >123 days 0.03-0.34 Bullock and Kennedy
1977 Dispersible a.i./hl 5000 (four 1981
Grain (3 or 2 results)
applications Flesh <0.02
approx. (four
11 weeks results)
apart) Juice <0.02
(two
results)
TABLE 4. Residues of pirimicarb in apple, Canada 1980 and Federal Republic of Germany 1977
Country Rate of Days between Total Carbamate residues (mg/kg)
and application last application
Year Formulation (kg a.i./ha) and harvest Minimum Maximum Mean
Canada 50% WP 0.56 0 - - 0.58 (1)1
1980 3 - - 0.42 (1)
8 - - 0.19 (1)
14 - - 0.05 (1)
Germany 50% 0.5 0 0.42 0.69 0.49 (4)
1977 Dispersible 3 0.22 0.51 0.31 (4)
grain 7-8 0.17 0.51 0.27 (4)
10-11 0.16 0.45 0.21 (4)
14 0.17 0.49 0.24 (3)
20-21 0.09 0.49 0.13 (3)
1 Figures in parentheses are the numbers of results on which the means are based.
A trial was also carried out in the UK on pears, using a single
application of a 50% dispersible grain formulation at a rate of
0.21 kg a.i./ha. Samples for residue analysis were taken at 0, 2, 7
and 107 days. The initial total carbamate residue of 0.39 mg/kg
decayed slowly over the following seven days, to 0.27 mg/kg. No
residue was detected in the 107-day sample (limit of determination,
0.02 mg/kg) (Bullock and Kennedy 1981). Two trials were also carried
out on pears in Japan in 1973. Two or three applications of a 50%
dispersible powder formulation were made at 0.05 kg a.i./hl, at 2500 l
of spray per ha. Samples for residue analysis were taken 54 and 64
days after the last applications. As expected with such long
intervals, no residues were detected in any of the four samples
analysed (limit of determination, 0.02 mg/kg) (Edwards and Dick 1976).
Data reviewed at the 1976 Meeting indicated that pirimicarb
residue levels on apples tend to decline more slowly than on many
other crops, such as leafy vegetables. The data now available on pears
are consistent with the findings on apples, in terms both of the
levels of residues present and in the pattern of residue decline.
Pecan
At 0.0125-0.025 kg a.i./hl applied 'high volume', pirimicarb
controls the black margined aphid, Monellia costalis, yellow aphid,
Monellia sp. and black pecan aphid, Tinocallis caryaefoliae, on
pecans. Aphids usually occur on pecans early in the season, although a
second infestation can appear a few weeks before harvest. At that
time, the nut is still protected by the hull and as a result it is
unlikely that residues would be detected in the nut. That expectation
was confirmed in four trials conducted in the USA in 1975. Pirimicarb
was sprayed at 0.125 to 0.375 kg a.i./ha up to three times, using a
50% WP formulation. Nuts were harvested 17 to 77 days after the last
application. No pyrimidine-carbamate residues were detected in any of
the ten samples analysed (limit of determination, 0.05 mg/kg) (Ussary
1976).
Cotton
At 0.03 to 0.06 kg a.i./ha, pirimicarb controls the cotton aphid,
Aphis gossypii. Aphid attack on cotton occurs early in the growing
season and it is unlikely that a residue would be detected in the
seeds at harvest. This expectation was confirmed in six trials
conducted in the USA in 1976-78. Two to four applications of
pirimicarb were made using a 50% WP at 0.06 or 0.12 kg a.i./ha. The
cotton was harvested 40 to 122 days after the last application. No
pyrimidine-carbamate residues were detected in any of the ten samples
analysed (limit of determination, 0.05 mg/kg ) (Ussary 1979).
Leeks, spinach and watercress
Pirimicarb is particularly useful when a pre-harvest clean-up of
aphid infestations is required. Aphids controlled by the compound
include the peach-potato aphid, Myzus persicae, on leeks and
watercress, shallot aphid, Myzus ascalonicus, on leeks, and the
black bean aphid, Aphid fabae, on spinach.
Three trials were conducted on outdoor leeks in Germany in 1977.
A 50% dispersible grain formulation was applied on three occasions at
0.15 kg a.i./ha. Initial total carbamate residues on the leeks of 1.5
to 4.0 mg/kg declined rapidly with a half-life of less than one day
(Table 5) (Bullock and Kennedy 1981).
Work on three varieties of spinach was undertaken in Germany in
1974, using a single application of a 50% dispersible grain
formulation at 0.5 kg a.i./ha. In three further trials in Germany in
1977, a 50% dispersible grain formulation was applied on three
occasions at 0.15 kg a.i./ha. Initial total carbamate residues of
approximately 3 to 8 mg/kg declined rapidly with a half-life of less
than one day (Table 5) (Bullock and Kennedy 1981; Edwards and Dick
1976).
Three trials were conducted on watercress in the UK in 1972, in
which a 50% WP formulation was applied at 0.25 kg a.i./ha (recommended
rate) or 0.50 kg a.i./ha. Total carbamate residue levels on the
watercress again declined very quickly in the period immediately after
application (Table 5)(Bullock 1972).
The pattern of residues on these three crops are similar to those
reviewed on various leafy vegetables at the 1976 Meeting.
Glasshouse crops
Some additional data on residues of pirimicarb on glasshouse
crops have been received (Greve and van de Kamp 1974) and are recorded
in Table 6. They are in line with data previously received on these
crops.
Other crops
Trials involving the application of pirimicarb to broccoli,
Brussels sprouts, peas, potatoes, soybeans, sugarbeet and turnips in
various countries, gave rise to no residues above the limits of
determination (generally <0.02 mg/kg). Further trials data on
pirimicarb residues in some other fruits and vegetables are listed in
Table 7 (Bullock and Kennedy 1981).
TABLE 5. Residues of pirimicarb in leek, spinach and watercress1
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Leeks Germany 50% 0.15 0 1.5 4.0 2.6 (3)2
1977 dispersible × 3 1 0.3 0.65 0.48 (2)
grain 2 - - 0.15 (1)
4 0.06 0.12 0.09 (3)
7 0.02 0.05 0.04 (3)
10 <0.02 0.02 0.02 (3)
14 <0.02 0.03 0.02 (3)
Spinach Germany 50% 0.15 0 4.6 7.3 5.6 (3)
1977 dispersible × 3 1 0.72 2.0 1.3 (3)
grain 4 0.16 0.50 0.29 (3)
7 0.05 0.06 0.06 (3)
10 <0.02 0.02 0.02 (3)
Germany 50% 0.5 0 2.7 6.0 4.1 (3)
1974 dispersible (× 1) 1 1.5 2.1 1.7 (3)
grain 3 0.14 0.65 0.39 (3)
5 0.01 0.12 0.07 (3)
7 <0.01 0.03 0.02 (3)
10 <0.01 0.02 0.02 (3)
12 - - <0.01 (1)
TABLE 5. (con't)
Country Rate of Days Between Total Carbamate Residues
Crop and Formulation application last application (mg/kg)
Year (kg a.i./ha) and harvest
Minimum Maximum Mean
Watercress UK 50% WP 0.25 0 5.9 22 11 (3)
1972 (× 1) 1 - - 1.1 (1)
2 0.43 0.51 0.44 (3)
4 0.37 0.39 0.38 (3)
7 0.16 0.20 0.18 (2)
0.5 0 - - 13 (1)
(× 1) 2 - - 0.86 (1)
4 - - 0.95 (1)
7 - - 0.50 (1)
1 Bullock and Kennedy, 1981; Edwards and Dick, 1976;
2 Figures in parentheses are the numbers of results upon which the means are based.
TABLE 6. Residues of pirimicarb in glasshouse crops1
Crop Residues (mg/kg) at intervals (days) after treatment
0 3
Range Mean Range Mean
Eggplants 0.16-0.24 0.19 0.05 0.05
" 0.13-0.19 0.16 0.10-0.24 0.17
Gherkins 0.38-0.64 0.47 0.09-0.13 0.11
" <0.05 <0.05 <0.05 <0.05
Cucumber (whole) <0.05 <0.05 <0.05 <0.05
" (peel) 0.06-0.24 0.11 0.05-0.14 0.08
Tomato 0.12-0.21 0.14
" 0.09-0.13 0.12 <0.05 <0.05
Pepper (bell) 0.18-0.30 0.25 0.05-0.07 0.06
" " <0.05-0.11 <0.06 <0.05-0.14 <0.08
1 In each case, one treatment with 50% WP at 0.375 kg a.i./ha.
FATE OF RESIDUES
In processing
Two studies were conducted in the USA in 1977 to determine
residues of pirimicarb and its carbamate metabolites in processed
cotton fractions. Cotton plants were sprayed with a 50% WP formulation
of pirimicarb at 0.07 to 0.13 kg a.i./ha. Two to four applications
were made with an interval of 49 to 122 days between the last
application and harvest. The cotton was harvested, fractioned and
submitted to the laboratory for residue analysis. The results are
shown in Table 8. Although no pyrimidine-carbamate residues were
observed in cottonseed, slight residues of up to 0.05 mg/kg were
observed in crude oil. A trace was noted in cottonseed meal at the
0.13 kg a.i./ha rate (twice the maximum recommended). However, as no
residue was detected in the cottonseed, it is unlikely that residues
should be present in both the meal and oil. The trace residues in meal
are most likely due to lack of sample homogeneity or possibly to minor
contamination (Ussary 1979).
TABLE 7. Pirimicarb residues in some fruits and vegetables
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Cherry Fed.Rep.Germany 1977 0 1.5 - 1.9 1.4
4 0.03- 0.89 0.41
7 0.02- 0.52 0.26
14 <0.02- 0.33 0.15
UK 1976 62 <0.02 <0.02
Peach Japan 1976 27-61 0.13- 0.40 0.28
Canada 1980 60-107 <0.02 <0.02
Plum Canada 1976 21-30 0.14- 0.17 0.15
Pepper Denmark 1976 0 - 0.13
3 - 0.10
7 - 0.04
14 - 0.02
Tomato Canada 1976 1 - 0.10
3 - 0.03
7 - <0.02
Denmark 1977 0 - 0.46
3 - 0.16
7 - 0.09
14 - 0.02
Cucumber Denmark 1977 0 0.13, 0.47 -
3 0.08, 0.30 -
7 0.07, 0.38 -
14 0.02, 0.05 -
21 0.02, 0.07 -
TABLE 7. (con't)
Pre-harvest Pirimicarb residues (mg/kg)
Crop Country Year Interval (days) Range Mean
Parsley Netherlands 1976 0 - 23
1 - 15
3 - 8.6
7 - 6.3
14 - 4.6
TABLE 8. Pirimicarb residues on cottonseed fractions, USA 1977
Residue (mg/kg)1
Type of No of Days after last
Sample applications application Control 0.07 kg ai/A 0.13 kg ai/A4
to harvest P2 DMP3 Total P DMP Total P DMP Total
Cottonseed 4 49 ND ND ND ND ND ND ND ND ND
Hulls ND ND ND ND ND ND
Meal ND TR 0.01 ND 0.01
Crude oil ND ND ND 0.02 0.01 0.03
Cottonseed 2 122 ND ND ND
Hulls ND ND ND
Meal ND ND ND
Crude oil 0.04 0.01 0.05
1 ND=none detected (<0.01 ppm of the individual compounds);
2 P = pirimicarb;
3 DMP = desmethyl pirimicarb;
4 Twice maximum use rate.
METHODS OF ANALYSIS
The preferred method for determining residues of pirimicarb and
its carbamate-containing metabolites in crops and in products of
animal origin is by gas-liquid chromatography with selective nitrogen
detection. The method was reviewed at the 1976 and 1978 Meetings.
NATIONAL MAXIMUM RESIDUE LIMITS
The national MRLs listed in Table 9 have been established for
residues of pirimicarb plus its two carbamate-containing metabolites,
II and III (Figure 1).
TABLE 9. National maximum residue limits reported to the Meeting
Country Crop MRL (mg/kg)
Australia Vegetables, fruit and hops 0.5
Belgium Potato 0.5
Other crops 0.3
Brazil Cabbage, cauliflower 0.5
Aubergine, cucumber, pepper, tomato 0.2
Potato 0.05
Fed. Rep. of Lettuce, cherry 1.0 ) from
Germany Other leaf, sprout and fruit vegetables 0.5 ) 1.1.82
Cereals, potato, sugarbeet 0.1 )
France Cereals 0.05 (proposed)
Hungary Fruit 0.5
Vegetables 1
Other crops 0.5
Japan Fruits and vegetables 0.3
Netherlands Fruits, vegetables, herbs and spices 1.0
Grain, potato 0.05
New Zealand Beans, lettuce, pea, brassicae, tomato 1
Potato, fodder crops 0.5
TABLE 9. (con't)
Country Crop MRL (mg/kg)
South Africa Cruciferae, oats, sorghum, wheat, peach 0.3
Peanut, pecan, potato 0.05
Switzerland Fruits and vegetables 1
USA Potato 0.1
Venezuela All Crops 0.5
EVALUATION
COMMENTS AND APPRAISAL
The Meeting was informed that the data requested by the 1978
Meeting would not be available until 1982. The Meeting therefore
agreed to extend the existing temporary ADI until 1982.
Additional data on residues of pirimicarb in a range of crops
have been received and reviewed. The pattern of residue data is
generally similar to that reviewed at previous meetings, although the
new data on oranges show the need for an upward revision of the
present limit to cover residues arising from use close to harvest in
some countries. On outdoor vegetables and field crops the residues
decline very quickly after spraying; on fruits, however, decline is
slower. Data on pears, together with old and new data on apples,
allowed a recommendation for limits on pome fruits. Additional and
altered limits are proposed.
RECOMMENDATIONS OF RESIDUE LIMITS
The following new or altered temporary limits refer to the sum of
pirimicarb, its N-formyl (methylamino) analogue (desmethylformamido
pirimicarb) and desmethyl pirimicarb.
Pre-harvest interval on
Temporary MRL which the recommendations
Commodity (mg/kg) are based (days)
Pome fruits 1 7
Spinach 1 3-4
Watercress 1 2-4
Leeks 0.5 1-2
Oranges 0.5 7-14
Citrus fruits
(other than oranges) 0.051 -
Cottonseed 0.051 -
Pecan 0.051 -
Sweet corn 0.051 -
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Desirable
1. Data on residues on oranges due to applications made close to
harvest in countries other than Portugal.
2. Data on residues on other citrus fruits from countries where
applications close to harvest are recommended or permitted.
REFERENCES
Bullock, D.J.W. Pirimicarb residues data on watercress: UK 1972. ICI
1972 Plant Protection Division. (Unpublished)
Bullock, D.J.W. and Kennedy, S.H. Pirimicarb : residues in crops from
1981 field trials during 1976-80. ICI Plant Protection Division
Report no. TMJ 1883A. (Unpublished)
Cambon, C., Declume, C. and Derace, R. Foetal and maternal rat brain
1980 acetylcholinesterase: isoenzymes changes following
insecticidal carbamate derivates poisoning. Archives of
Toxicology 45: 257-262.
Edwards, M.J. and Dick, J.P. Pirimicarb residue summary: Residues in
1976 crops from field trials during 1973-1975. ICI Plant
Protection Division Report No. TMJ 1360B. (Unpublished)
Ferreira, J.R. and Tainha, A. Residues of pirimicarb in field-treated
1981 oranges. Ministry of Agriculture and Fisheries, Toxicology
and Analytical services Directorate, Portugal. Report PPA
(TR)-12/81. (Unpublished)
Fox, T. Pirimicarb dietary toxicity study in foxhounds. Report from
1978 Hazelton Labs. Europe Ltd., no. 1371-72/2, submitted by ICI
Ltd. to WHO. (Unpublished)
Greve, P.A. and van de Kamp, C.G. Residuen van bestrijdingsmiddeln in
1974 de groenteteelt onder glas. Report 96/74 Tox - ROB. Report
from the Netherlands. (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H., Brown, G. and Street,
1977 A.E. Pirimicarb preliminary oral toxicity study in rhesus
monkeys (repeated dosages for 13 weeks). Report from
Huntingdon Research Centre, no. 164/77825, submitted by ICI
Ltd. to WHO (Unpublished)
Heywood, R., Sortwell, R.J., Pulsford, A.H,, Brown, G. and Street,
1978a A.E. Pirimicarb oral toxicity study in rhesus monkeys
(repeated dosages for 17 weeks, followed by a recovery
period of 8 weeks). Report (final report) from Huntingdon
Research Centre no. 198/78444, submitted by ICI Ltd. to WHO.
(Unpublished)
1978b Pirimicarb comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs Tests). Report
from Huntingdon Research Centre, No. 219/78445, submitted by
ICI Ltd. to WHO. (Unpublished)
Jackson, J.A., Chart, I.S., Sanderson, J.H. and Garner, R. Pirimicarb
1977 induced immune hemolytic anaemia in dogs, Scandinavian
Journal of Haematology, 19: 360-366.
Jackson, J.A. and Royle, G. Pirimicarb: dietary toxicity study in
1978a foxhounds. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/420, submitted by ICI Ltd.
(Unpublished)
Jackson, J.A.; and Royle, G. Pirimicarb: oral toxicity study in rhesus
1978b monkeys. Addendum: results of additional serological
investigations. Report from ICI Central Toxicology
Laboratory, no. CTL/P/421, submitted by ICI Ltd.
(Unpublished)
1978c Pirimicarb: comparative data from native rhesus monkeys
(white blood cell counts and direct Coombs tests). Addendum:
results of additional serological investigations. Report
from ICI Central Toxicology Laboratory, no. CTL/P/419
submitted by ICI Ltd. (Unpublished)
Manley, C.A. residue summary: Pirimicarb in crops. ICI Plant
1972 Protection Division Report no. TMJ 585/2. (Unpublished)
Parkinson, G.R. Pirimicarb metabolite R 34885: subacute oral toxicity
1978a to rats. Report from ICI Central Toxicology Laboratory, No.
CTL/P/402, submitted by ICI to WHO. (Unpublished)
1978b Pirimicarb metabolite R34836: subacute oral toxicity to
rats. Report from ICI Central Toxicology Laboratory, no.
CTL/P/401, submitted by ICI to WHO. (Unpublished)
Paul, J.D., Richards, D., Banham, P.B. and Weight, T.M. Pirimicarb:
1978 growth study to determine a no-effect level in the female
rat. Report from ICI Central Toxicology Laboratory no.
CTL/P/408, submitted by ICI to WHO. (Unpublished)
Pilinskaya, M.A. Cytogenic effect of a number of pesticides in a
1981 culture of human peripheral blood lymphocytes with different
levels of spontaneous aberrations. Tsitologiya i Genetika,
15(2) : 82-84
Richards, D., Banhan, P.B. and Weight, T M. Pirimicarb paired feeding
study in a female rat. Report from ICI Central Toxicology
Laboratory, no. CTL/P/407. Submitted by ICI to WHO.
(Unpublished)
Ussary, J.P. Pirimicarb residues in pecan, 1975. ICI Americas Inc.
1976 Reports. (Unpublished)
1979 Pirimicarb residues on cottonseed. ICI Americas Inc, Report
no. TMU 0442/B. (Unpublished)
