
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
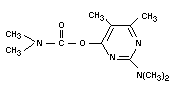
PIRIMICARB
 Explanation
Pirimicarb was evaluated by the Joint Meeting in 1976 and 1978
(FAO/WHO 1977 and 1979)1. The temporary ADI established in 1976 was
re-assessed in 1978 and 0-0.01 mg/kg bw was recommended. The Joint
Meeting concluded that, although it was not unduly concerned about the
carcinogenic potential of pirimicarb, it wished to see a further
carcinogenicity study undertaken in an appropriate mammalian species
using a current accepted protocol. A mouse oncogenicity study was
performed and is reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Groups of Alderley Park Swiss derived mice (60/sex/group) were
fed diets containing pirimicarb at levels of 0 (2 groups), 200, 400
and 1 600 ppm for up to 96 weeks. Animals were examined throughout the
study for abnormal clinical or behavioural conditions, body weights
were determined and food consumption was measured at regular
intervals. Complete histopathology, including bone marrow smears, were
performed on all animals. There was no evidence of bacterial, viral or
parasitic infections in any of the animals examined during the study.
Body weights of the 1 600 ppm male and female rats were
significantly decreased (p <0.01) throughout the study. Food
consumption and food utilization were variably decreased throughout
the test, as well as increased food wastage, primarily in the high
dose group animals. Mortality rates for males were comparable among
all treatment and control groups, whereas, mortality was significantly
increased for high dose females. There was a significant increase in
the finding of lymphosarcoma among the high dose females during weeks
27-52, but the incidence of lymphosarcoma overall was comparable among
all treatment and control groups.
1 See Annex 2 for WHO and FAO documentation.
The incidence of pulmonary tumours (pulmonary adenomas) in the
high dose males and females is not biologically significant when
compared with historical control data and the considerations from the
first mouse study (Palmer and Samuels 1974).
Hepatocellular nodules, classed as Type A or Type B, were
significantly increased in the high dose group when compared to
controls. No consistent trend in either Type A or B nodule was
determined, and therefore, total incidence of liver nodules, per se,
was evaluated. The incidence of these tumours was not significantly
increased among groups through week 78 of the study, as determined by
intercurrent deaths, but became significantly different from week 79
to termination. There was no clear dose response, the mortality rate
was increased for low and high dose males from weeks 76 to
termination, and there was no previous identification of liver
involvement or target organ toxicity to the liver. These support the
conslusion that pirimicarb was not oncogenic in mice under the
conditions of this study (Litchfield et al 1980).
Special Studies on Mutagenicity
Rat - cytogenetic study
Six-week-old, Wistar-derived outbreed male rats (180-220 g) were
administered pirimicarb dissolved in maize oil (10, 50 and 100 mg/kg)
by oral gavage. There were five experimental groups with eight
animals/group. The negative and positive (EMS) control groups
consisted of a total of 12 animals at each dosing regime. Six or 24
hours after a single dose and 6 hours after the five consecutive daily
doses of the test compound, animals were given a single IP treatment
of 3 mg/kg colchicine and sacrificed two hours later by cervical
dislocation. Chromosome slides were prepared and 50 cells from each
animal were examined and scored for chromatid or chromosome gaps,
chromatid breaks, fragments and other complex abnormalities.
Pirimicarb did not induce chromosomal alterations in bone marrow
cells of Wistar-derived outbred male rats when administered via oral
gavage (Anderson et al 1980).
Salmonella/microsome reverse mutations
Pirimicarb, dissolved in DMSO at five concentrations (4, 20, 100,
500 and 2 500 µg/plate), was evaluated in two separate studies for
mutagenic activity by the plate-incorporation method of the Ames
Salmonella reverse mutation test, in the presence and absence of
mammalian metabolic activation from rat liver enzymes. Five histidine-
requiring strains of Salmonella typhimurium for detecting base-pair
substitution, (TA 100, TA 1535) and frameshift mutation (TA 98, TA
1537 and TA 1538) were used in the histidine-reversion assay. The
in vitro mammalian metabolic activation system consisted of liver
homogenate fraction "S-9" from Aroclor 1254 treated Sprague-Dawley
albino rats and the co-factor solution described by Ames.
Pirimicarb did not demonstrate a dose-related increase in the
number of histidine-independent colonies, failed to induce any
biologically significant change in the reversion frequency to
histidine independence with or without the addition of metabolic
activation preparation in the study, and thus, was not mutagenic at
the dose levels tested (4 through 2 500 µg/plate) (Trueman 1980;
Longstaff 1978).
COMMENTS
Pirimicarb was last evaluated by the JMPR in 1978, when the
Meeting expressed concern for the previously conducted rat and mouse
oncogenicity studies. An additional long-term evaluation was requested
and has been submitted and reviewed. It was concluded that pirimicarb
was not carcinogenic in the rodent species tested, under the
conditions of the study.
Pirimicarb was also evaluated for mutagenic activity in a battery
of in vivo and in vitro studies and all results were negative. In
the absence of positive findings in the mutagenicity and
carcinogenicity studies, the concerns previously expressed have been
resolved and an ADI has been established.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Monkey : 2 mg/kg bw/day,
Rat : 175 ppm in the diet, equivalent to 9 mg/kg bw
Dog : 1.8 mg/kg bw/day.
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
REFERENCES
Anderson, D., Richardson, C.R., Howard, C.A., Bradbrook, C., Salt,
1980 M.J. and Cook S.K. Pirimicarb: a cytogenetic study in the
rat. Report by ICI Central Toxicology Laboratory No.
CTL/P/525, submitted by ICI to WHO. (Unpublished)
Litchfield, M.H., Sotherson, M.F. and Jackson, D.G. Pirimicarb:
1980 Lifetime feeding study in the mouse. CTL Study No. PM 0002,
submitted by ICI to WHO.
Longstaff, E. Pirimicarb; short-term predictive tests for
1978 carcinogenicity results from the Salmonella/microsome
reverse mutation test. Report by ICI Central Toxicology
Laboratory, No. CTL/P/428, submitted by ICI to WHO.
(Unpublished)
Palmer, S.M. and Samuels, D.M. Pirimicarb: 80-week carcinogenic study
1974 in mice. CTL Study No. HO/CTL/P121/B, submitted by ICI to
WHO.
Trueman, R.W. An examination of pirimicarb for potential mutagenicity
1980 using the Salmonella/microsome reverse mutation assay. CTL
Report No. Y00032/001/002, submitted by ICI to WHO.
Explanation
Pirimicarb was evaluated by the Joint Meeting in 1976 and 1978
(FAO/WHO 1977 and 1979)1. The temporary ADI established in 1976 was
re-assessed in 1978 and 0-0.01 mg/kg bw was recommended. The Joint
Meeting concluded that, although it was not unduly concerned about the
carcinogenic potential of pirimicarb, it wished to see a further
carcinogenicity study undertaken in an appropriate mammalian species
using a current accepted protocol. A mouse oncogenicity study was
performed and is reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Groups of Alderley Park Swiss derived mice (60/sex/group) were
fed diets containing pirimicarb at levels of 0 (2 groups), 200, 400
and 1 600 ppm for up to 96 weeks. Animals were examined throughout the
study for abnormal clinical or behavioural conditions, body weights
were determined and food consumption was measured at regular
intervals. Complete histopathology, including bone marrow smears, were
performed on all animals. There was no evidence of bacterial, viral or
parasitic infections in any of the animals examined during the study.
Body weights of the 1 600 ppm male and female rats were
significantly decreased (p <0.01) throughout the study. Food
consumption and food utilization were variably decreased throughout
the test, as well as increased food wastage, primarily in the high
dose group animals. Mortality rates for males were comparable among
all treatment and control groups, whereas, mortality was significantly
increased for high dose females. There was a significant increase in
the finding of lymphosarcoma among the high dose females during weeks
27-52, but the incidence of lymphosarcoma overall was comparable among
all treatment and control groups.
1 See Annex 2 for WHO and FAO documentation.
The incidence of pulmonary tumours (pulmonary adenomas) in the
high dose males and females is not biologically significant when
compared with historical control data and the considerations from the
first mouse study (Palmer and Samuels 1974).
Hepatocellular nodules, classed as Type A or Type B, were
significantly increased in the high dose group when compared to
controls. No consistent trend in either Type A or B nodule was
determined, and therefore, total incidence of liver nodules, per se,
was evaluated. The incidence of these tumours was not significantly
increased among groups through week 78 of the study, as determined by
intercurrent deaths, but became significantly different from week 79
to termination. There was no clear dose response, the mortality rate
was increased for low and high dose males from weeks 76 to
termination, and there was no previous identification of liver
involvement or target organ toxicity to the liver. These support the
conslusion that pirimicarb was not oncogenic in mice under the
conditions of this study (Litchfield et al 1980).
Special Studies on Mutagenicity
Rat - cytogenetic study
Six-week-old, Wistar-derived outbreed male rats (180-220 g) were
administered pirimicarb dissolved in maize oil (10, 50 and 100 mg/kg)
by oral gavage. There were five experimental groups with eight
animals/group. The negative and positive (EMS) control groups
consisted of a total of 12 animals at each dosing regime. Six or 24
hours after a single dose and 6 hours after the five consecutive daily
doses of the test compound, animals were given a single IP treatment
of 3 mg/kg colchicine and sacrificed two hours later by cervical
dislocation. Chromosome slides were prepared and 50 cells from each
animal were examined and scored for chromatid or chromosome gaps,
chromatid breaks, fragments and other complex abnormalities.
Pirimicarb did not induce chromosomal alterations in bone marrow
cells of Wistar-derived outbred male rats when administered via oral
gavage (Anderson et al 1980).
Salmonella/microsome reverse mutations
Pirimicarb, dissolved in DMSO at five concentrations (4, 20, 100,
500 and 2 500 µg/plate), was evaluated in two separate studies for
mutagenic activity by the plate-incorporation method of the Ames
Salmonella reverse mutation test, in the presence and absence of
mammalian metabolic activation from rat liver enzymes. Five histidine-
requiring strains of Salmonella typhimurium for detecting base-pair
substitution, (TA 100, TA 1535) and frameshift mutation (TA 98, TA
1537 and TA 1538) were used in the histidine-reversion assay. The
in vitro mammalian metabolic activation system consisted of liver
homogenate fraction "S-9" from Aroclor 1254 treated Sprague-Dawley
albino rats and the co-factor solution described by Ames.
Pirimicarb did not demonstrate a dose-related increase in the
number of histidine-independent colonies, failed to induce any
biologically significant change in the reversion frequency to
histidine independence with or without the addition of metabolic
activation preparation in the study, and thus, was not mutagenic at
the dose levels tested (4 through 2 500 µg/plate) (Trueman 1980;
Longstaff 1978).
COMMENTS
Pirimicarb was last evaluated by the JMPR in 1978, when the
Meeting expressed concern for the previously conducted rat and mouse
oncogenicity studies. An additional long-term evaluation was requested
and has been submitted and reviewed. It was concluded that pirimicarb
was not carcinogenic in the rodent species tested, under the
conditions of the study.
Pirimicarb was also evaluated for mutagenic activity in a battery
of in vivo and in vitro studies and all results were negative. In
the absence of positive findings in the mutagenicity and
carcinogenicity studies, the concerns previously expressed have been
resolved and an ADI has been established.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Monkey : 2 mg/kg bw/day,
Rat : 175 ppm in the diet, equivalent to 9 mg/kg bw
Dog : 1.8 mg/kg bw/day.
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
REFERENCES
Anderson, D., Richardson, C.R., Howard, C.A., Bradbrook, C., Salt,
1980 M.J. and Cook S.K. Pirimicarb: a cytogenetic study in the
rat. Report by ICI Central Toxicology Laboratory No.
CTL/P/525, submitted by ICI to WHO. (Unpublished)
Litchfield, M.H., Sotherson, M.F. and Jackson, D.G. Pirimicarb:
1980 Lifetime feeding study in the mouse. CTL Study No. PM 0002,
submitted by ICI to WHO.
Longstaff, E. Pirimicarb; short-term predictive tests for
1978 carcinogenicity results from the Salmonella/microsome
reverse mutation test. Report by ICI Central Toxicology
Laboratory, No. CTL/P/428, submitted by ICI to WHO.
(Unpublished)
Palmer, S.M. and Samuels, D.M. Pirimicarb: 80-week carcinogenic study
1974 in mice. CTL Study No. HO/CTL/P121/B, submitted by ICI to
WHO.
Trueman, R.W. An examination of pirimicarb for potential mutagenicity
1980 using the Salmonella/microsome reverse mutation assay. CTL
Report No. Y00032/001/002, submitted by ICI to WHO.
 Explanation
Pirimicarb was evaluated by the Joint Meeting in 1976 and 1978
(FAO/WHO 1977 and 1979)1. The temporary ADI established in 1976 was
re-assessed in 1978 and 0-0.01 mg/kg bw was recommended. The Joint
Meeting concluded that, although it was not unduly concerned about the
carcinogenic potential of pirimicarb, it wished to see a further
carcinogenicity study undertaken in an appropriate mammalian species
using a current accepted protocol. A mouse oncogenicity study was
performed and is reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Groups of Alderley Park Swiss derived mice (60/sex/group) were
fed diets containing pirimicarb at levels of 0 (2 groups), 200, 400
and 1 600 ppm for up to 96 weeks. Animals were examined throughout the
study for abnormal clinical or behavioural conditions, body weights
were determined and food consumption was measured at regular
intervals. Complete histopathology, including bone marrow smears, were
performed on all animals. There was no evidence of bacterial, viral or
parasitic infections in any of the animals examined during the study.
Body weights of the 1 600 ppm male and female rats were
significantly decreased (p <0.01) throughout the study. Food
consumption and food utilization were variably decreased throughout
the test, as well as increased food wastage, primarily in the high
dose group animals. Mortality rates for males were comparable among
all treatment and control groups, whereas, mortality was significantly
increased for high dose females. There was a significant increase in
the finding of lymphosarcoma among the high dose females during weeks
27-52, but the incidence of lymphosarcoma overall was comparable among
all treatment and control groups.
1 See Annex 2 for WHO and FAO documentation.
The incidence of pulmonary tumours (pulmonary adenomas) in the
high dose males and females is not biologically significant when
compared with historical control data and the considerations from the
first mouse study (Palmer and Samuels 1974).
Hepatocellular nodules, classed as Type A or Type B, were
significantly increased in the high dose group when compared to
controls. No consistent trend in either Type A or B nodule was
determined, and therefore, total incidence of liver nodules, per se,
was evaluated. The incidence of these tumours was not significantly
increased among groups through week 78 of the study, as determined by
intercurrent deaths, but became significantly different from week 79
to termination. There was no clear dose response, the mortality rate
was increased for low and high dose males from weeks 76 to
termination, and there was no previous identification of liver
involvement or target organ toxicity to the liver. These support the
conslusion that pirimicarb was not oncogenic in mice under the
conditions of this study (Litchfield et al 1980).
Special Studies on Mutagenicity
Rat - cytogenetic study
Six-week-old, Wistar-derived outbreed male rats (180-220 g) were
administered pirimicarb dissolved in maize oil (10, 50 and 100 mg/kg)
by oral gavage. There were five experimental groups with eight
animals/group. The negative and positive (EMS) control groups
consisted of a total of 12 animals at each dosing regime. Six or 24
hours after a single dose and 6 hours after the five consecutive daily
doses of the test compound, animals were given a single IP treatment
of 3 mg/kg colchicine and sacrificed two hours later by cervical
dislocation. Chromosome slides were prepared and 50 cells from each
animal were examined and scored for chromatid or chromosome gaps,
chromatid breaks, fragments and other complex abnormalities.
Pirimicarb did not induce chromosomal alterations in bone marrow
cells of Wistar-derived outbred male rats when administered via oral
gavage (Anderson et al 1980).
Salmonella/microsome reverse mutations
Pirimicarb, dissolved in DMSO at five concentrations (4, 20, 100,
500 and 2 500 µg/plate), was evaluated in two separate studies for
mutagenic activity by the plate-incorporation method of the Ames
Salmonella reverse mutation test, in the presence and absence of
mammalian metabolic activation from rat liver enzymes. Five histidine-
requiring strains of Salmonella typhimurium for detecting base-pair
substitution, (TA 100, TA 1535) and frameshift mutation (TA 98, TA
1537 and TA 1538) were used in the histidine-reversion assay. The
in vitro mammalian metabolic activation system consisted of liver
homogenate fraction "S-9" from Aroclor 1254 treated Sprague-Dawley
albino rats and the co-factor solution described by Ames.
Pirimicarb did not demonstrate a dose-related increase in the
number of histidine-independent colonies, failed to induce any
biologically significant change in the reversion frequency to
histidine independence with or without the addition of metabolic
activation preparation in the study, and thus, was not mutagenic at
the dose levels tested (4 through 2 500 µg/plate) (Trueman 1980;
Longstaff 1978).
COMMENTS
Pirimicarb was last evaluated by the JMPR in 1978, when the
Meeting expressed concern for the previously conducted rat and mouse
oncogenicity studies. An additional long-term evaluation was requested
and has been submitted and reviewed. It was concluded that pirimicarb
was not carcinogenic in the rodent species tested, under the
conditions of the study.
Pirimicarb was also evaluated for mutagenic activity in a battery
of in vivo and in vitro studies and all results were negative. In
the absence of positive findings in the mutagenicity and
carcinogenicity studies, the concerns previously expressed have been
resolved and an ADI has been established.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Monkey : 2 mg/kg bw/day,
Rat : 175 ppm in the diet, equivalent to 9 mg/kg bw
Dog : 1.8 mg/kg bw/day.
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
REFERENCES
Anderson, D., Richardson, C.R., Howard, C.A., Bradbrook, C., Salt,
1980 M.J. and Cook S.K. Pirimicarb: a cytogenetic study in the
rat. Report by ICI Central Toxicology Laboratory No.
CTL/P/525, submitted by ICI to WHO. (Unpublished)
Litchfield, M.H., Sotherson, M.F. and Jackson, D.G. Pirimicarb:
1980 Lifetime feeding study in the mouse. CTL Study No. PM 0002,
submitted by ICI to WHO.
Longstaff, E. Pirimicarb; short-term predictive tests for
1978 carcinogenicity results from the Salmonella/microsome
reverse mutation test. Report by ICI Central Toxicology
Laboratory, No. CTL/P/428, submitted by ICI to WHO.
(Unpublished)
Palmer, S.M. and Samuels, D.M. Pirimicarb: 80-week carcinogenic study
1974 in mice. CTL Study No. HO/CTL/P121/B, submitted by ICI to
WHO.
Trueman, R.W. An examination of pirimicarb for potential mutagenicity
1980 using the Salmonella/microsome reverse mutation assay. CTL
Report No. Y00032/001/002, submitted by ICI to WHO.
Explanation
Pirimicarb was evaluated by the Joint Meeting in 1976 and 1978
(FAO/WHO 1977 and 1979)1. The temporary ADI established in 1976 was
re-assessed in 1978 and 0-0.01 mg/kg bw was recommended. The Joint
Meeting concluded that, although it was not unduly concerned about the
carcinogenic potential of pirimicarb, it wished to see a further
carcinogenicity study undertaken in an appropriate mammalian species
using a current accepted protocol. A mouse oncogenicity study was
performed and is reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
Groups of Alderley Park Swiss derived mice (60/sex/group) were
fed diets containing pirimicarb at levels of 0 (2 groups), 200, 400
and 1 600 ppm for up to 96 weeks. Animals were examined throughout the
study for abnormal clinical or behavioural conditions, body weights
were determined and food consumption was measured at regular
intervals. Complete histopathology, including bone marrow smears, were
performed on all animals. There was no evidence of bacterial, viral or
parasitic infections in any of the animals examined during the study.
Body weights of the 1 600 ppm male and female rats were
significantly decreased (p <0.01) throughout the study. Food
consumption and food utilization were variably decreased throughout
the test, as well as increased food wastage, primarily in the high
dose group animals. Mortality rates for males were comparable among
all treatment and control groups, whereas, mortality was significantly
increased for high dose females. There was a significant increase in
the finding of lymphosarcoma among the high dose females during weeks
27-52, but the incidence of lymphosarcoma overall was comparable among
all treatment and control groups.
1 See Annex 2 for WHO and FAO documentation.
The incidence of pulmonary tumours (pulmonary adenomas) in the
high dose males and females is not biologically significant when
compared with historical control data and the considerations from the
first mouse study (Palmer and Samuels 1974).
Hepatocellular nodules, classed as Type A or Type B, were
significantly increased in the high dose group when compared to
controls. No consistent trend in either Type A or B nodule was
determined, and therefore, total incidence of liver nodules, per se,
was evaluated. The incidence of these tumours was not significantly
increased among groups through week 78 of the study, as determined by
intercurrent deaths, but became significantly different from week 79
to termination. There was no clear dose response, the mortality rate
was increased for low and high dose males from weeks 76 to
termination, and there was no previous identification of liver
involvement or target organ toxicity to the liver. These support the
conslusion that pirimicarb was not oncogenic in mice under the
conditions of this study (Litchfield et al 1980).
Special Studies on Mutagenicity
Rat - cytogenetic study
Six-week-old, Wistar-derived outbreed male rats (180-220 g) were
administered pirimicarb dissolved in maize oil (10, 50 and 100 mg/kg)
by oral gavage. There were five experimental groups with eight
animals/group. The negative and positive (EMS) control groups
consisted of a total of 12 animals at each dosing regime. Six or 24
hours after a single dose and 6 hours after the five consecutive daily
doses of the test compound, animals were given a single IP treatment
of 3 mg/kg colchicine and sacrificed two hours later by cervical
dislocation. Chromosome slides were prepared and 50 cells from each
animal were examined and scored for chromatid or chromosome gaps,
chromatid breaks, fragments and other complex abnormalities.
Pirimicarb did not induce chromosomal alterations in bone marrow
cells of Wistar-derived outbred male rats when administered via oral
gavage (Anderson et al 1980).
Salmonella/microsome reverse mutations
Pirimicarb, dissolved in DMSO at five concentrations (4, 20, 100,
500 and 2 500 µg/plate), was evaluated in two separate studies for
mutagenic activity by the plate-incorporation method of the Ames
Salmonella reverse mutation test, in the presence and absence of
mammalian metabolic activation from rat liver enzymes. Five histidine-
requiring strains of Salmonella typhimurium for detecting base-pair
substitution, (TA 100, TA 1535) and frameshift mutation (TA 98, TA
1537 and TA 1538) were used in the histidine-reversion assay. The
in vitro mammalian metabolic activation system consisted of liver
homogenate fraction "S-9" from Aroclor 1254 treated Sprague-Dawley
albino rats and the co-factor solution described by Ames.
Pirimicarb did not demonstrate a dose-related increase in the
number of histidine-independent colonies, failed to induce any
biologically significant change in the reversion frequency to
histidine independence with or without the addition of metabolic
activation preparation in the study, and thus, was not mutagenic at
the dose levels tested (4 through 2 500 µg/plate) (Trueman 1980;
Longstaff 1978).
COMMENTS
Pirimicarb was last evaluated by the JMPR in 1978, when the
Meeting expressed concern for the previously conducted rat and mouse
oncogenicity studies. An additional long-term evaluation was requested
and has been submitted and reviewed. It was concluded that pirimicarb
was not carcinogenic in the rodent species tested, under the
conditions of the study.
Pirimicarb was also evaluated for mutagenic activity in a battery
of in vivo and in vitro studies and all results were negative. In
the absence of positive findings in the mutagenicity and
carcinogenicity studies, the concerns previously expressed have been
resolved and an ADI has been established.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Monkey : 2 mg/kg bw/day,
Rat : 175 ppm in the diet, equivalent to 9 mg/kg bw
Dog : 1.8 mg/kg bw/day.
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
REFERENCES
Anderson, D., Richardson, C.R., Howard, C.A., Bradbrook, C., Salt,
1980 M.J. and Cook S.K. Pirimicarb: a cytogenetic study in the
rat. Report by ICI Central Toxicology Laboratory No.
CTL/P/525, submitted by ICI to WHO. (Unpublished)
Litchfield, M.H., Sotherson, M.F. and Jackson, D.G. Pirimicarb:
1980 Lifetime feeding study in the mouse. CTL Study No. PM 0002,
submitted by ICI to WHO.
Longstaff, E. Pirimicarb; short-term predictive tests for
1978 carcinogenicity results from the Salmonella/microsome
reverse mutation test. Report by ICI Central Toxicology
Laboratory, No. CTL/P/428, submitted by ICI to WHO.
(Unpublished)
Palmer, S.M. and Samuels, D.M. Pirimicarb: 80-week carcinogenic study
1974 in mice. CTL Study No. HO/CTL/P121/B, submitted by ICI to
WHO.
Trueman, R.W. An examination of pirimicarb for potential mutagenicity
1980 using the Salmonella/microsome reverse mutation assay. CTL
Report No. Y00032/001/002, submitted by ICI to WHO.
