
PROPARGITE JMPR 1977
IDENTITY
Chemical names
2-(4-tert-butylphenoxy)cyclohexyl prop-2-ynyl sulphite
2-(p-tert-butylphenoxy)cyclohexyl propargyl sulphite
Synonyms
Omite(R), DO 14, ENT 27226
Structural formula
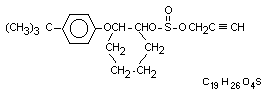 Other information on identity and properties
The technical grade material is a light to dark brown liquid of low
volatility. It is miscible with most organic solvents. Solubility in
water is about 0.5 mg/kg. The basic manufacturer states the
composition of the technical product to be:
85% active ingredient
7% "high molecular weight sulphites"
5% "unreacted starting materials"
1% propylene oxide stabilizer
(remainder unidentified)
Propargite is commercially available in 68.1% and 755% emulsion
concentrates, a 30% wettable powder, and a 4% dust. The manufacturer
states that the technical product shows no degradation after 12 months
when stabilized with propylene oxide. The odour of SO2 indicates
breakdown.
The product was introduced in the U.S.A. on an experimental basis in
1967 as a replacement for Aramite, another product of the same
manufacturer, which was withdrawn from use on food crops in the U.S.A.
The two compounds have similar chemical structures and properties.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Extensive degradation of propargite could be shown in in vitro as
well as in vivo experiments.
In vitro studies with liver homogenates showed that the major
primary metabolites appear to be propargyl alcohol, the glycol ether
and their conjugates, which are further degraded to secondary
metabolites (Sullivan, 1968).
In in vivo metabolism studies in rats that were orally treated with
a single dose of 200 mg/kg propargite, propargyl alcohol and glycol
ether were also found as primary metabolites in the excreta as well as
in tissue samples. Following analysis of the excreta and tissues only
about 5% of the administered dose was accounted for as unchanged
propargite or the primary metabolites, indicating that the primary
metabolites are further degraded (Sullivan, 1968).
The oral administration of a single close of 271 mg/kg benzene ring
labelled 140 propargite to rats was followed by fast excretion of the
radiocarbon. Within 72 hours postdosage, 47% of the administered dose
was eliminated via the urine. 32% via the faeces. The total carcass
contained approximately 90% of the administered radioactivity.
Identified metabolites excreted with the rat urine and faeces were
tert-butylpyrocatechol and glycol ether (Ryer and Sullivan, 1969a)
In cows treated with a single oral close of 4 mg/kg b.w. combined
non-radioactive and 14C labelled propargite a similar excretion
pattern was found. 54% of the administered dose was eliminated with
the urine, 17% with the faeces. Approximately 0.3% was found in the
milk. Identified metabolites were tert-butylpyrocatcohol, glycol
ether and tertbutylphenol (Ryer and Sullivan, 1969b).
In a feeding study, dairy cows were maintained on a diet containing
14C-labelled and unlabelled propargite at dosage levels of 3 and 20
ppm for 12 consecutive days. Peak urinary excretion observed at the
seventh test day reached values of 4 and 23 mg/kg respectively for the
3 and 20 ppm dietary level; peak faecal excretion was approximately 1
and 4 mg/kg. Maximum milk residue values reached on the fourth test
day were 0.02 and 0.06 mg/kg at the 3 and 20 ppm dose levels
respectively. Residues in selected tissues ranged from 0.02 to about
1.3 mg/kg (Kennedy et al., 1970).
Similar results were obtained after daily administration of a diet
containing 50 and 100 ppm propargite to lactating cows for a period of
27 days (Smith and Roger, 1975).
Pigs were maintained on a diet containing propargite at dosage levels
of 20 and 100 ppm for 30 days. Residues in kidney and liver were below
the detection limit of 0.1 mg/kg at both feeding levels and residues
in muscle and fat ranged from below 0.1 to about 2 mg/kg (Ladd et al.,
1974).
Residues studies in hens that were treated with 3, 10 and 30 ppm 14C
propargite in their diet for 30 days showed residue levels in eggs
from 0.13, 0.49 and 0-145 µg/kg respectively at the 3, 10 and 30 ppm
dose levels. Residues in muscle ranged from 2-12 µg/kg, liver 18-174
µg/kg kidney 2-25 µg/kg and fat 7-116 µg/kg depending on exposure
level (Jenkins et al., 1972).
After continuous exposure to a concentration of 0.025 mg/l 14C
propargite in water for 35 days tissue residues in edible portions of
fishes were about 2 mg/kg. After transfer to uncontaminated water 70%
of the radioactivity present in the edible tissues was eliminated
within 14 days (Sleight III, 1972),
The in vivo metabolism studies in several animal species confirmed
the rapid degradation of propargite already shown in in vitro
experiments. The metabolic reactions are hydrolysis of the parent
ester followed by other cleavage. The formed products are polar
alcoholic and phenolic compounds which can rapidly be excreted or
further metabolized and incorporated into natural tissue constituents
by biochemical reactions which are well documented in the literature.
See also the section "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on teratogenicity
Female rats were orally dosed with 0, 50 and 100 mg/kg from day 6
through 15 of gestation. The treatment had no effect on maternal body
weight, mortality or fertility. No reproductive adverse effects were
found with respect to implantation and resorption sites. Embryonic and
foetal development was not affected by the treatment
(Haley et al., 1972).
Special study on reproduction
Rat
See "Long term study".
Special study on carcinogenicity
Rat
See "Long term study".
Dog
See "Short term study".
Acute toxicity
Species Sex Route LD50 References
mg/kg
Rat M, F oral approx. 2200 Carson, 1963
Rabbit M, F dermal approx. 3200 Weir, 1967
Rat
Groups of 5 male and 5 female rats were treated for 90 days with
amounts of propargite in their diet to provide daily intakes of 10,
20, 40, 100 and 200 mg/kg b.w. corresponding to dietary levels in
adult animals of 200, 400, 800, 2000 and 4000 ppm respectively. The
control group existed of 15 male and 15 female rats. Treatment with
2000 and 4000 ppm caused a dose-related growth retardation and
reduction of food intake. Haematological examinations and clinical
chemistry tests revealed no abnormalities. At 2000 and 4000 ppm the
relative liver weights were increased, the relative kidney weights
were increased only in the 4000 ppm groups. Gross and microscopic
examinations of tissues disclosed no treatment-related pathological
alterations (Carson, 1964).
Dog
Groups of 3 male and 3 female dogs were maintained on a diet
containing propargite at dose levels of 0 and 2000 to 2500 ppm
(increase of dosage after 3 weeks) for 90 days. The treatment did not
affect appearance, behaviour, results of haematological. and clinical
chemistry tests and urinalyses. In most treated dogs reduced food
consumption and body weight loss and an increase of relative liver and
kidney weights were observed, No compound-related alterations were
found macroscopically. Histopathological examinations revealed an
increased amount of pigment in the liver reticuloendothelial cells and
increased haemosiderosis of the spleen (Holsing, 1968).
Groups of 8 dogs were fed a diet containing propargite at 100, 300 and
900 ppm for 24 months. The control group consisted of 12 animals. The
treatment had no effect on growth rate, results of clinical laboratory
studies, urinalyses and organ weights. Gross and microscopic
examination did not reveal abnormal pathological findings. No tumours
were found (Osert 1966).
Long term Study
Rat
Groups, each comprising 25 male and 25 female rats were fed dietary
levels of propargite to provide daily intakes of 5, 15 and 45 mg/kg
b.w. corresponding to adult levels of 100, 300 and 900 ppm for a
period of 24 months. The control group consisted of 37 animals of each
sex. After 26 test weeks without noticeable evidence of a
treatment-related effect, a supplementary study was started with a
control-and a test group comprising 25 male and 25 female rats
receiving 100 mg/kg b.w. corresponding to 2000 ppm for 78 weeks. Rats
that died during the study and those sacrificed after 104 and 78 weeks
were examined grossly and microscopically. The treatment had no effect
on appearance, behaviour, growth rate and mortality at the dietary
levels up to and including 900 ppm. At the 2000 ppm level at the end
of the study there was a reduction of food consumption and also a
reduction of the body weight gain of about 30%; mortality of the male
rats increased to 32% compared to 7% of the corresponding control.
Haematological examinations and clinical laboratory studies did not
reveal abnormal findings. Absolute and relative liver and kidney
weights of the animals treated with dietary levels of 100, 300 and 900
ppm which died or were sacrificed after 104 weeks showed inconsistent
variations without any dose-relationship. At 2000 ppm an increase of
the relative liver and kidney weights of about 30%. were observed.
Gross autopsy findings in 1 male (of 25) and 7 females (of 23) in the
2000 ppm group were enlarged and/or dark red lymph nodes which in some
cases were involved in abdominal masses. In the corresponding control
group 2 male rats of 29 animals showed this alteration. No
dose-dependent increase of the frequency of this lymph node alteration
was found at the 100, 300 and 900 ppm levels. A variety of benign and
malignant tumours was observed in the control and treated groups. No
dose-relationship of the frequency of tumours was found. Four sarcomas
were found in rats sacrificed after 78 weeks ingestion of 2000 ppm,
whereas in the corresponding control group no sarcomas were seen
(Oser, 1966).
Additionally a 3-generation reproductive was included in the 2-year
feeding experiment with 20 pairs of male and female rats from the
control and 100 ppm test groups. Dosage was raised to 300 ppm in the
F2 pups at the time of weaning. The treatment revealed no abnormal
findings (Oser, 1966).
COMMENTS
Propargite is readily absorbed from the gastrointestinal tract and is
excreted rapidly as metabolites which are formed mainly by hydrolysis
of the sulphite ester.
After feeding a diet containing 20 ppm of propargite the residues in
milk or in tissues were of the order of 0.1 mg/kg.
The compound is not teratogenic in the rat.
In a 3-generation reproductive study no adverse effects were revealed
at concentrations up to 300 ppm. This concentration is therefore
regarded as a no-effect level.
In a 2-year feeding study with up to 900 ppm in dogs which included
extensive laboratory and histological investigations, no adverse
effects were noted. A 2-year feeding study in rats up to 2000 ppm
showed appreciable but not dose-related variations in the relative
liver and kidney weights in all treated groups. However, no major
adverse effects were seen. Owing to a poor survival rate and the
termination of the study with the high dose animals after 78 weeks,
the finding that the tumour incidence was not elevated is of limited
value. This study was not considered to fulfil the criteria for an
acceptable lone-term study. Therefore a safety factor of 200 was used.
Propargite is structurally related to Aramite, a compound which is
suspected of being a carcinogen. Although there are no indications
that propargite acts similarly, the need for satisfactory
carcinogenicity studies was expressed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 300 mg/kg in the diet, equivalent to 15 mg/kg bw
Dog: 900 mg/kg in the diet, equivalent to 22 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Propargite is an acaracide said to be effective on a variety of mites
on food crops and ornamentals. Table 1 shows the use patterns
registered in the U.S.A. Information has been received that the
product is also used in Italy, Australia, New Zealand, South Africa,
Argentina, Mexico, and France. The government of the Netherlands has
informed the Meeting that the compound was used until 1976, at which
time its use was discontinued. A country statement was received from
New Zealand regarding uses and tolerances. The uses permitted in these
countries generally parallel those shown in Table 1. Significant
exceptions are included in the footnotes.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trails on twenty-six crops (Uniroyal, 1977). Most of the trials were
conducted in the U.S.A. A country statement was received from New
Zealand with data on peaches. Analyses in all U.S. trials, the
procedure described under "Methods of residue analysis" (PAM II,
1973). Analyses of untreated controls (crop blanks) were reported in
each experiment, along with the recovery of propargite from fortified
controls. Controls were generally reported as 0 and recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form when feasible.
The use patterns employed in Mexico and France on apples, peaches,
plums and citrus differed significantly from the other countries. In
the absence of residue data reflecting good agricultural practice in
those countries, it was not possible to predict residue levels. The
recommended DIRLs for apples, peaches, plums and citrus therefore are
based on the national use patterns which can be related to supervised
residue trails.
Alfalfa
There are no national tolerances or registrations for propargite use
on alfalfa. However, the submission to the Meeting contained some
residue data on alfalfa hay, fresh forage and trash. Single and
multiple applications were made at 1.4 to 4.7 kg a.i./ha. Residues on
fresh forage ranged 0.0 to 328 mg/kg at 28 and 0 day intervals from
treatment, respectively. Residues on hay and trash ranged from 2.6 to
100 mg/kg with intervals from treatment of 22 to 52 days. With a 28
day pre-harvest interval, MRLs of 50 and 75 mg/kg on fresh alfalfa and
alfalfa hay, respectively, would be adequate.
TABLE 1. Registered use patterns for propargite (USA)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Almond 30% WP 0.03-0.05%(4) 2 28(5) low volume
68.1% EC 2.5-3.4 kg/ha 1 post-harvest aerial
application
only
Apples 30% WP 0.04-0.05(1)(6) 3 7(2)(7)(12)
Apricots 30% WP 0.05%(4) 2 14(5) low volume
68.1% EC 1.7-2.5 kg/ha 2 post-harvest aerial
application
only
Cranberries 68.1% EC 1.6 kg/ha 1 14
Beans 75% EC 2.8 hg/ha(4) 2 7(fresh)(5)
28 (dry)
Corn 75% EC 1.9 kg/ha 1 30
(field)
Cotton 75% EC 0.85-1.9 kg/ha 3 before bolls open
Figs 30% WP 0.03-0.05%(3)(4)(11) 2 14
Grapes 30% WP 1.7-3.0 kg/ha 2 21
4% Dust 0.2 kg/ha 2 14
Grapefruit, 75% EC 0.025-0.05(4)(8) 2 7 (5)
oranges
Lemons 30% WP 0.05% (8) 2 7
Hops 68.1% EC 1.7 kg/ha(4) 2 14 (5)
30% WP 1.7 kg/ha 2 14
Mint 68.1% EC 1.7-2.5 kg/ha 2 14
Nectarines 30% WP 0.05% (4) 2 14 (5)
Peaches 30% WP 0.04-0.05%(4)(8) 2 14(5)(10)(13)
Peanuts 30% WP 1.0-1.7 kg/ha 2 14
Pears 30% WP 0.04-0.05%(8)
Plums 30% WP 0.04-0.05(4)(8) 2 14(5)(10)
Potatoes 75% EC 1.4-2.4 kg/ha 2 14
68.1% EC 1.7-2.5 kg/ha 2 14
TABLE 1. (Continued)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Sorghum 75% EC 1.5-1.9 kg/ha 1 30 silage
60 grain
Strawberries 30% WP 1.7-3.4 kg/ha(9) 3 3
4% Dust 1.8 kg/ha 3 3
Walnuts 68.1% EC 0.03-0.05% (4) 2 14 (5)
(1) France 0.12, Italy 0.06%
(2) France 21, Italy 15, New Zealand 14
(3) France 0.6-0.9 kg/ha
(4) Italy 0.06%
(5) Italy 21
(6) Mexico 0.08-0.12%
(7) Mexico 28
(8) Mexico 0.12%
(9) East U.S.A. 0.84 kg/ha
(10) Argentina 21
(11) Argentina 0.05%
(12) South Africa 14
(13) South Africa 21
Almonds
Field trials were conducted in California: 63 samples of nut meats
were anlayzed. Residues on the nut meats from two applications of 0.03
and 0.06% sprays were < 0.1 mg/kg at post-treatment intervals of 21
to 28 days.
Residues on almond hulls from treatment with 0.05% spray (30% WP)
followed by 3.4 kg a.i./ha (68.1% EC) ranged from 5.2 to 31 mg/kg with
pre-harvest intervals of 28-35 days. Under good agricultural practice,
residues are not likely to exceed 55 mg/kg.
Apples
Field trials were conducted in nine U.S.A. states, South Africa and
Australia, some 452 samples being analysed. The residue data are
summarized in Table 2. All controls were reported zero. Residues from
applications of up to 0.06% spray after 7 days were less than 3 mg/kg.
Residue data indicate that residues remain associated with the apple
pomace and are not destroyed in drying. Residues in the juice were
barely detectable (ca. 0.02 mg/kg) while residues in the wet and dried
pomace were 16-21 mg/kg and 56-80 mg/kg, respectively. Thus, residues
concentrate by factors of 15-22 in the manufacture of dried pomace
from treated apples (Uniroyal, 1972), An MRL of 80 mg/kg in dried
apple pomace is therefore appropriate.
TABLE 2. Range of residues in apples, mg/kg.
Application rate, Interval (days, last treatment to harvest)
% 0-1 6-7 14 21 28
0.03 0.1-3.1 0.6-1.8 0.2-1.0 0.2-0.7 0.2-0.9
0.06 0.3-6.3 0.5-1.6 0.3-2.4 0.5-1.1 0.2-0.6
Apricots, peaches and plums
Field trials were conducted in California, Connecticut, Georgia, North
and South Carolina, Maryland, Michigan, Pennsylvania, Washington, New
York and New Zealand. Some 385 test samples of fresh fruit and 65
controls were anlaysed from one and two applications at four dosage
rates, including the maximum and twice the maximum label rates (Table
1). The residue data are summarized in Table 3. Only one residue on
peaches was greater than 7 mg/kg at 14 days after treatment at the
maximum rate. Residues will not exceed 7 mg/kg from sprays up to
0.06%, provided that treatments are not made within 14 days of
harvest.
A study was conducted on plums (fresh prunes) using a standard
commercial drying process to determine residues on dried prunes. The
average residue value for the dried fruit was ca. 20% of the average
value for the fresh fruit. Thus no additional MRL is needed for the
dried fruit.
TABLE 3. Range of residues on apricots, peaches and plums, mg/kg
Application rate. Interval (days, last treatment to harvest)
% 0-1 6-7 13-14 21-23
0.03 0.4-12 0.2-7.0 0.4-10.0 0.3-5.0
0.05 0.7-6.7 2.6-14.0 0.6-5.6 0.0-1.7
0.06 1.3-17.0 1.3-8.7 0.2-5.5 0.3-9.3
0.10 1.6-27.0 0.3-8.4 0.8-11.0 0.8-3.5
Beans
Field trials were conducted in the states of Washington, California
and Idaho on snap beans, succulent lima beans and dry beans. 52
analyses were reported. Single and multiple applications were made at
the maximum and at twice the maximum label rate (Table 1), by aerial
and ground application. The residues are summarized in Table 4 for
fresh and dry beans, 7 and 28 days after the last treatment,
respectively. No residue data were submitted for bean vines; however
there is a restriction against the feeding or foraging of treated bean
vines. The only other feed item, cannery waste, would consist of cull
beans with some bean vines and pods present as a result of mechanical
harvesting. Residue levels would not be likely to exceed 20 mg/kg on
cannery waste.
TABLE 4. Range of residues in beans, mg/kg
Application rate, kg a.i./ha
2.8 5.6
Fresh (7 days after last treatment) <0.2-15 <0.2-12
Dry (29 days after last treatment <0.1-0.1 <0.1-0.24
Maize
Residue data for maize were made available from five U.S.A. sites, two
in California and one each in Colorado, Texas and Nebraska. Following
applications at the maximum and twice the maximum label rates (Table
1), silage stage maize sampled 28-35 days after application contained
maximum residues of 2.4 and 18 mg/kg, respectively; maize stover,
sampled after 47-105 days, contained maximum residues of 4 and 11
mg/kg, respectively. Interpolation of the 2X data shows that MRLs on
maize forage and fodder of 10 mg/kg are appropriate.
No detectable residues (< 0.1 mg/kg) were found on either the grain
or ears of maize sampled 60-105 days after the above applications. No
data on grain reflecting a 30 day pre-harvest interval were submitted.
However, since propargite does not translocate to any extent, residues
on grain are not expected to exceed 0.1 mg/kg.
Cotton
Five field studies from locations in California, Arkansas and
Mississippi were reported. The studies reflect treatment at the
maximum and twice the maximum label rate (Table 1). Analyses were
performed on 96 samples of cottonseed and processed products, hulls,
oil, meal and soapstock. All values on cottonseed were reported as
< 0.1 mg/kg. All samples of meal and soapstock contained
< 0.05 mg/kg as did all but on refined oil sample which contained a
level of 0.1 mg/kg after treatment at an excessive rate. Almost all of
the hull samples had low but detectable residues, 0.03-0.06 mg/kg.
Thus, an MRL of 0.1 mg/kg is appropriate for cottonseed. There is a
restriction in the U.S.A. against feeding treated foliage or cotton
trash to livestock.
Cranberries
Seven studies were reported from Massachusetts, with a total of 36
analyses. The studies reflect applications at 1.68 kg a.i./ha (4 by
ground and 3 by air) with one study at 3.36 kg a.i./ha. Several of the
studies show decline rates (at 7, 14 and 21 days). The residue
declines rapidly between 0 and 7 days and then shows little further
decline from 7 to 21 days. At the recommended rate (Table 1), residues
are not likely to exceed 10 mg/kg. Residues are summarized in Table 5.
TABLE 5. Range of residues in cranberries, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 21
1.68 2.2-11 <0.1-1.9 <0.1-7.1 0.1-1.0
3.36 3.3-14.9
Figs
The residue data for figs consists of four studies made in California,
including two decline studies. Various applications were made
including the maximum and twice the maximum label rate (Table 15).
Residues on fresh figs are summarized in Table 6. While the data are
limited, it is not likely that residues will exceed 3 mg/kg at 14 days
after treatment.
TABLE 6. Residues on figs, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0-1 7 13-14 21 28
1.27 3.3 1.9 0.8 0.45
2.02 1.6-2.8 1.0-1.4 0.2-1.4 0.3-0.6
4.04 2.5-5.3 2.2-3.2 0.7-4.2
Grapefruit, oranges and lemons
Field trials were conducted in Texas, Florida and California. Some 281
harvest samples were analyzed. One and two applications were made at
the recommended and twice the recommended rates (Table 1). The results
are summarized in Table 6. For applications up to 0.06% spray with a
7 day interval, 5 mg/kg would not be exceeded.
Washed oranges bearing residues of 1.9 mg/kg were processed to dried
pulp by a procedure simulating the commercial process. The residue in
the dried pulp was 15.5 mg/kg. The residue in the dried pulp is about
eight times that on the whole fruit, so 40 mg/kg is an appropriate
MRL.
TABLE 6. Range of residues in grapefruit, oranges and
lemons, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 28
0.03 0.3-0.5 0.2-0.8 0.2-0.3 0.9-1.0
0.05 1.7-3.6 0.2-4.8 1.1-2.1 0.6-3.1
0.06 1.0-1.4 0.9-2.5 0.0-0.9 0.6-1.2
0.10 1.0-5.1 0.8-7.2 1.2-3.2 1.2-2.9
Grapes
Residue trials were conducted in Washington and California, with
analyses of 254 samples of grapes. A wide range of experimental
conditions in the various field tests and the resulting variation in
residues preclude a tabular summary of the data. No residues exceeded
10 mg/kg from the maximum recommended rate with a 21 day interval
after treatment.
A residue concentration factor of 2.5 was observed in the commercial
production of raisins from fresh grapes. Thus a 25 mg/kg MRL for
raisins would be appropriate. The data showed a concentration of
residues on dried grape pomace to 4 times that found on fresh grapes,
therefore, 40 mg/kg would be appropriate for grape pomace.
Hops
Eight field trials were conducted in the State of Washington. Residues
on green hops resulting from three applications at the maximum and
twice the maximum label rate (Table 1) are summarized in Table 7.
Residues on green hops from good agricultural practices are not likely
to exceed 15 mg/kg. Residues on dried hops ranged from 11 to 30 mg/kg
14 days after treatment. A 30 mg/kg MRL would be appropriate for dried
hops since residues from two applications will probably be somewhat
lower than those noted above.
TABLE 7. Range of residues on green hops, mg/kg
Application rate, Interval (days last treatment to harvest)
kg a.i./ha 0 6-7 13-14 20
1.7 24-50 5-17 6-15 2-6
3.4 36-92 24-66 11-43 6-24
Mint
Field studies were conducted in Washington, Oregon and Idaho. A total
of 89 analyses were made on fresh hay, spent hay and mint oil,
including controls. In four studies, two applications were made at
rates of 2.5 and 5 kg a.i./ha resulting in maximum residues of 19 and
50 mg/kg respectively, 15-17 days after treatment. Three residue
decline studies were also submitted. Samples were taken 0, 1, 2 and 4
weeks after a single aerial application at 1 and 2 times the maximum
label rate (Table 1). From a decline curve based on these data,
residues resulting from the recommended use are ca. 50 mg/kg on fresh
mint hay.
Five samples of fresh mint hay bearing residues of 8.7-50 mg/kg were
fractionated by steam distillation and the resulting mint oil and
spent hay contained residues of 1.6-7.9 mg/kg and 12-97 mg/kg
respectively. Residues in mint oil are not likely to exceed those on
fresh mint hay and there is a restriction against feeding spent mint
hay to livestock.
Nectarines
Seven trials were conducted at locations in California and
Pennsylvania. One or two applications were made at one and two times
the maximum label rate (Table 1). The residue data are summarized in
Table 8. Residues were no higher than 3-4 mg/kg at the prescribed
pre-harvest interval. However, extrapolation of data from peaches,
apricots, and plums would indicate that a 7 mg/kg MRL would be
appropriate.
TABLE 8. Range of residues in nectarines, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 6-7 13-15 20-21
0.05 0.66-7.3 0.24-3.1 0.44-3.4 0.39-1.3
0.10 1-3-10.5 0.34-5.1 0.91-3.4 0.41-1.5
Peanuts
Residue studies were conducted in Georgia, Florida, North Carolina,
Oklahoma, Mississippi and Arkansas. Two applications were made in all
but four decline studies on peanut vines, where only one application
was made. Dosages ranged from 0.6 times to twice the maximum
recommended rate (Table 1). Six of the studies reflect a 14 day
preharvest interval, but in the other ten studies intervals were from
19 to 66 days.
Peanut kernels
All residue values were reported as < 0.1 mg/kg. Since no detectable
residues were found in peanuts, no MRLs are needed for peanut
by-products, meal, oil and soapstock.
Peanut hulls
Residues were < 0.1 mg/kg in five of seven studies. Residues of
0.14 mg/kg, 0.3 mg/kg and 1.2 mg/kg were found after applications at
twice, 6 times and 1.2 times the recommended rates respectively, in
the other two studies.
Peanut vines and hay
Residues were less than 10 mg/kg 14 days after application at the
maximum rate. The data indicate that residues decline rapidly from a
maximum of 38 mg/kg at 0 days to 3.6 mg/kg at 14 days.
Pears
Four field studies were conducted in California, Pennsylvania,
Michigan and Washington, one and two applications were made with 0.03
and 0.06% sprays. The residue data are summarized in Table 9. All
residues were less than 3 mg/kg, 14 days after treatment.
TABLE 9. Range of residues in pears, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 21
0.03 0.6-1.6 0.3-1.2 0-2.7 0.1-2.4
0.06 2.2-4.1 0.9-2.6 0.3-2.2 0.1-1.7
Potatoes
Seven residue studies were made available, four from Washington and
one each from Idaho, Vermont and Illinois. After two applications at
rates of 1.5 to 4.5 kg a.i./ha, all residues were less than the limit
of detection of the method (0.1 mg/kg) 14 days after treatment.
Sorghum
Field studies were conducted in Texas, Nebraska and Iowa on grain
sorghum. Applications were made at the maximum and twice the maximum
recommended rates (Table 1). Samples of grain and forage or fodder
were harvested at 28, 30, 44 and 50 days after application. Residues
in forage ranged from < 0.1 to 4.1 mg/kg. Residues in forage and
fodder are not likely to exceed 10 mg/kg 30 days after treatment.
Residue levels in grain ranged from < 0.1 to 2.4 mg/kg 60 days or
more after treatment. Thus an MRL of 5 mg/kg with a 60 day interval
after treatment is appropriate for sorghum grain.
Strawberries
Field trials were conducted in California, Louisiana and Florida.
Strawberries treated with two formulations in California, reflecting
three applications at the maximum recommended rate (Table 1) had
residues ranging from 0.19 to 4.0 mg/kg for 3 days after treatment.
Residues from applications of 0.84 and 1.7 kg/ha in Louisiana and
Florida are summarized in Table 10. Under good agricultural practice,
residues are not likely to exceed 7 mg/kg.
TABLE 10. Range of residues in stramberries, mg/kg
Application rate Interval (days, last treatment to harvest)
kg a.i./ha 0 3 7
1.7 0.9-2.0 0.3-3.8 0.2-0.7
3.4 1.4-4.1 0.7-9.2 0.4-1.1
Sugar beets
There are no national tolerances or registrations for propargite on
sugar beets; however, this use is pending in the U.S.A. The submission
to the Meeting therefore contained some residue data on sugar beet
tops, roots and by-products. Sugar beet root samples from Idaho,
Washington and California reflecting two treatments at 2.8 and 5.6 kg
a.i./ha and a 21 day interval from treatment# showed residues ranging
from 0.05 to 0.23 ind/kg and 0.13 to 0.28 mg/kg respectively.
In a residue decline study, on sugar beet tops, a single application
at 2.8 kg a.i./ha yielded residues of 40-114 mg/kg at 0 days.
20-80 mg/kg at 14-17 days and 2.4-34 mg/kg at 27-28 days. Adiitional
data for tops representing two ground treatments at 2.8 and 5.6
kg a.i./ha show residues ranging from 11-24 mg/kg and 19-59 mg/kg
respectively (21 day interval).
In order to obtain data on processing fractions, sugar beet roots
bearing 0.1 mg/kg residues were processed to wet and dry pulp,
molasses and sugar. Propargite residues in wet pulp, molasses and
sugar were less than or equal to 0.1 mg/kg. The data for dry pulp show
a maximum residue concentration factor of 5, with residues from
0.1-0.5 mg/kg.
Tea
There are no national tolerances or registrations for propargite in
tea, however, a tolerance for this use is pending in the U.S.A. The
submission to the Meeting contained some residue data on dried tea
leaves, both green and black. The data are summarized in Table 11.
TABLE 11. Range of residues in tea, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 28
0.31 <0.1-0.3 <0.1-0.2
0.75 48-178 1.1-4.2 <0.1-0.2 <0.1
Walnuts
Six field trials were conducted at locations in California. Both
30% WP and 75% EC formulations were applied at the maximum recommended
rate (Table 1). No residues were detected in any of the walnut meat
samples 45 to 48 days after treatment. Although the interval is
greater than that recommended (14 days), since propargite is
non-systemic, any residues found on nut meats would be expected to
arise as a result of contamination in de-hulling and de-shelling
processes. Under good agricultural practice, an MRL of 0.1 mg/kg (at
or about limit of determination) would be appropriate. There is a
restriction against grazing or feeding livestock on cover crops growm
in treated orchards, therefore no feed items are involved in this use.
Meat, milk, poultry and eggs
Propargite is used on a number of crops which yield by-products used
commercially for animal feeds. Primary sources would be dried citrus
pulp, sorghum grain, forage and fodder and maize forage and fodder.
Some additional contribution could occur from dried pomace, almond
hulls and dried grape pomace. Per this reason, some animal feeding
studies were submitted. These are also referred to in the section
"Biochemical aspects", where references are given.
In one study, cattle were fed at levels of 3 and 20 ppm in the total
dry diet for 12 days. Maximum residues in milk (whole milk basis) were
0.059-0.067 mg/kg. The latter is equivalent to 1.7 mg/kg on the fat
basis. Maximum residues in tissues were as follows: liver, 1.3 mg/kg,
kidney, 0.27 mg/kg, fat, 0.21 mg/kg and muscle, 0.18 mg/kg.
In a second study, cows received 0, 50 and 100 ppm in the diet for 27
days. Analysis of milk showed all samples at both feeding levels to be
< 0.08 mg/kg. Residues ranged up to 1.6 mg/kg on a fat basis.
Residues in muscle, kidney and liver from both feeding levels were
40.03 mg/kg (limit of determination). Residues in fat ranged from 0.32
to 0.42 mg/kg at the 10 and 100 ppm feeding levels, respectively.
Poultry were fed 3, 10 or 30 ppm in the diet for 30 days (3 chickens
in each group). Eggs were sampled daily and the animals were
sacrificed 24. hours after treatment. Maximum levels In eggs were
0.013 mg/kg at the 3 PPM level, 0.043 mg/kg at the 10 ppm feeding
level, and 0.106 mi/kg at the 30 ppm level. Liver tissue had the
highest residue levels ranging from 0.047 to 0.089 mg/kg at the 10
diet level and from 0.17 to 0.18 mg/kg at the 30 diet level.
Hogs were fed 20 and 100 ppm in the total diet. Two hogs at each level
and one control were maintained for 30 days. No residues
(< 0.1 mg/kg) were found in liver or kidney. No residues were found
in muscle at the 20 ppm level but up to 0.23 mg/kg at the 100 ppm
intake. In fat, residues up to 0.18 and 0.2 mg/kg were found at the 20
and 100 ppm intakes, respectively.
The studies show that residues may transfer to meat, milk, poultry and
eggs. A mixed ration for cattle could consist of 15% dried apple
pomace, 15% dried citrus pulp and 70% sorghum grain, forage and fodder
or maize forage and fodder. This diet could theoretically contain
propargite residues of ca. 25 mg/kg.
A maximum possible intake of 10 mg/kg could result from the hogging
down of peanut fields and other swine feed items such as maize grain
and cooked potatoes.
Poultry feed items, sorghum grain, grape pomace, maize grain and
cooked potatoes, could contribute ca. 8 mg/kg propargite to the diet.
Residues are unlikely to exceed 2 mg/kg in milk fat and 0.1 mg/kg in
the meat, meat by-product, and fat of cattle, goats, hogs, horses,
poultry and sheep.
FATE OF RESIDUES
In animals
The available data on metabolism by animals are more conclusive than
those on metabolism by animals are move conclusive than those on
metabolism by plants. See also "Biochemical aspects."
Propargite -14C feeding tests have been conducted on a number of
animals including the rat, cow, pig and chicken. Additional
information on degradation was available from in vitro experiments
with buffered liver homogenates.
A general metabolism scheme has been proposed for animals, part of
which has been verified experimentally, and part of which is based on
analogy with biochemical reactions reported in the literature.
Identification of some metabolites was by TLC comparison with prepared
standards. In other cases less positive identification was made by
partition co-effecients (p values) and solvent extraction scheme. In
cow liver the bulk of the 14C activity was identified only as polar
metabolites.
The experiments apparently were carried out over a number of years,
the studies in some animals being repeated with refinements in
analytical techniques. None of the work on propargite submitted to the
Meeting has been published. Certain general conclusions appear
justified. (a) Propargite is metabolized in animals to polar compounds
which are excreted, principally in the urine. (b) There is not a
pronounced tendency to storage or accumulation in the body, but the
compound is not completely metabolized because the parent compound was
the principal residue in the milk of cows, was tentatively identified
as a portion of the 14C activity in animal fat, and was found in
excreta. (c) The parent compound is detected in the liver only for
short periods after ingestion. d) The primary hydrolysis products, the
glycol ether and propargyl alcohol, are further degraded. (e) Some
polar metabolites are in conjugated forms.
A list of the metabolits and the sites in which they were reported is
given in Table 12. The sequence of the metabolic scheme is not
definitely known.
TABLE 12. Propargite metabolites
Compound Found in
Parent Cow: butter fat, body fat (1)
Rat: liver (1)
liver homogenate (1)
Glycol ether Cow: urine, faeces, milk (2)
Rat: faeces, liver (1)
liver homogenate (1)
Propargyl alcohol liver homogenate (1)
Butylphenol Cow: urine (2)
Rat: liver (1)
liver homogenate (1)
Butylcatechol Cow: urine (2)
Rat: urine, faeces (2)
Cyclohexandiol urine (2)
"Polar metabolites" Rat: liver (2)
Cow: liver (2)
(1) Identified by GLC or TLC
(2) Tentatively characterized by solvent
fractionation scheme, p values, literature analogies.
In plants
Propargite is generally considered to be "non-systemic" by mode of
miticidal action. According to information made available to the
Meeting aged residues are primarily surface residues of the parent
compound (Uniroyall 1977). Residue decline was attributed to
volatilization of the parent compound and hydrolysis to propargyl
alcohol and the "glycol ether",
2-(p-tert-butylphenoxycyclohexanol). The two hydrolysis products
have not been detected in samples by chemical analysis at a detection
level of 0.1 mg/kg. In experiments on bean plants to investigate
possible photolytic degradation, the same two compounds could not be
detected. It has been suggested that if the glycol ether and propargyl
alcohol are intermediates in the degradation scheme, their presence is
fleeting because of their volatility.
Other evidence cited in the manufacturer's submission on the behavior
of propargite in plants was: (a) comparison of surface and pulp
analysis of apples showed no absorption (of parent); (b) mites
confined to upper leaf surfaces were not affected by sprays on the
lower surfaces (c) there was no effect on mites of an adjacent leaf
when on leaf of a bean plant was treated; (d) volatility losses of the
parent compound under field conditions have been correlated with model
laboratory experiments (loss from aluminum foil).
The above experiments partially support the idea that propargite is
not translocated in plants and that the residue of concern is
propargite per se. However, much of the evidence is indirect, and it
would be desirable to have conventional metabolism studies on some
representative crops, using 35S and/or 14C, to confirm the fate of
propargite in plants.
In soil
Only a summary was made available (Uniroyall 1977). The half-life in a
variety of soils was said to range from 2 to 18 weeks. Leaching is
negligible. Laboratory and field studies have shown that propargite is
hydrolysed in soil. The major soil degradation product found was the
glycol ether.
Summary, metabolism
The available metabolism studies do not firmly identify the residue
components in plants or animals. However, there is sufficient
information to indicate a probable metabolic scheme. It is fairly
certain that the primary metabolites are the hydrolysis products
tert-butylphenoxycyclohexanol and propargyl alcohol. It is probable
that further breakdown to butylphenol and cyclohexanediol occurs. If
correlations are to be made between metabolic schemes and toxic
potential for propargite and aramite, it should be noted that both
compounds hydrolyze at the sulfite ester, aramite yielding
2-chloroethanol and propargite yielding propargyl alcohol. Whether any
further confirmatory studies are needed would depend on the toxic
potential of the postulated fragments.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Propargite is detected in the multi-residue screening procedure
employed by the Canadian Department of Health and Welfare, The
compound is eluted from Florisil in fraction 3 as described in the
method (15% acetone in hexane). Only 4 positive findings of propargite
were reported in the Canadian surveillance programs (as of 1977). The
highest residue was found in a nectarine sample at 0.57 mg/kg (Health
Protection Branch, 1977),
The U.S. Food and Drug Administration multi-residue method has not
been validated for propargite. The specific GLC/FPD analytical method
has not been applied in the U.S. surveillance programme. Therefore
propargite has not been reported in foods in channels of commerce in
the U.S.A. No reports from other governments have been received on
occurrence in commerce.
There were no data available on reduction of residues through washing,
cooking or processing. The principal exposure through ingestion would
be from fruits eaten raw and unpeeled.
METHODS OF RESIDUE ANALYSIS
The early residue data on propargite (mid 1960s) were obtained by gas
chromatography with flame ionization or microcouclometric (SO2)
detection. Some difficulties were experienced by government
laboratories in validation studies on the method at that time were
because the clean-up and extraction procedures were unsatisfactory.
Through a series of modifications, a basic procedure using gas
chromatography with flame photometric detection (FPB) has evolved
which is satisfactory for a wide variety of fruits, vegetables, and
animal tissues (Devine and Sisken, 1972).
In the basic method, residues are extracted with 1:1 hexane and
isopropanol, followed by an aqueous NaCl wash and Florisil column
chromatography. For oily or waxy samples an optional step is included
in which the residues are partitioned into nitromethane. Other
modifications are prescribed for certain crops. The method has been
validated in U.S. government laboratories on milk, apples and animal
tissues (PAM III, 1973). Depending on the substrate, the limit of
determination is about 0.05 to 0.2 mg/kg.
A similar GC-FPD method for propargite residue in citrus has been
reported by Westlake et al, (1971).
Propargite is not known to be recovered through the U.S. Food and Drug
Administration multiresidue procedure. Information received from
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Almonds 0.1
Almonds, hulls 55
Apples 3 3 3 3
Apples, pomace, dried 80
Apricots 7 3
Bananas 3
Beans, dry 0.2
Beans, succulent 20
Citrus, dried pulp 40
Corn, fodder 10
Corn, forage 10
Corn, grain 0.1
Cottonseed 0.1 3
Cranberries 10
Eggs 0.1
Figs 3
Grapefruit 5 5
Grapes 10 7
Grapes, pomace 40
Hops 15 3
Hops, dried 30 30
Lemons 5 5
Milk 0.08
NATIONAL TOLERANCES REPORTED TO THE MEETING (Continued)
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Milk, fat 2
Mint 50
Nectarines 4 3
Oranges 5 5
Passion fruit 3
Peaches 7 7 3
Peanuts 0.1
Peanuts, forage 10
Peanuts, hay 10
Peanuts, hulls 10 3
Pears 3 3 3
Plums (prunes) 7 3 3
Potatoes 0.1
Raisins 25
Sorghum, fodder 10
Sorghum, forage 10
Sorghum, grain 10
Strawberries 7 7 3
Walnuts 0.1
Meat, meat
by-products and fat
of cattle, goats, hogs,
horses, poultry and
sheep 0.1
Canadian authorities is that it is recovered through their
multiresidue procedure. It is eluted from Florisil in fraction 3 of
the Canadian procedure (15% acetone/hexane).
APPRAISAL
Propargite is an acaricide which has been used on a wide variety of
food crops and ornamentals since its introduction in 1967. Available
information indicates that the product is used in Australia, New
Zealand, Argentina, Mexico, France, South Africa, Italy and the U.S.A.
The primary manufacturer has submitted a report on supervised trials
on 26 crops, all of which were conducted in the U.S.A. Limited residue
data were received from New Zealand on peaches). U.S.A., Canada, New
Zealand and Australia have set national tolerances (MRLs) for
propargite.
Propargite is non-syrtemic by mode of action against mites, and data
indicate that residues which do occur are surface residues. Residues
on the harvested commodities are primarily propargite per se. No
significant metabolites or alteration products have been reported on
harvested crops. However, much of the evidence regarding the fate of
propargite on plants is indirect. It would be desirable to have 35S
or 14C radiotracer studies to confirm the degradation pathways in
plants.
Radioactively labelled propargite (14C) has been used in some animal
experiments, including studies on the rat, cow and chicken. Techniques
used to fractionate and identify the activity were not as definitive
as would be desired, but it is fairly certain that propargite is
hydrolysed in the body to tert-butylphenoxyoyclohexanol and
propargyl alcohol. It is probable that there is further degradation to
butylphenol and cyclohexanediol and excretion as conjugates in the
urine. However, some residues of unchanged parent compound do occur in
milk, body fat and briefly in liver. If any correlations are to be
made between the metabolism of propargite and the related compound
aramite, it may be significant to compare the toxic potential of the
hydrolysis product propargyl alcohol with that of the corresponding
hydrolysis product of eremite, ß-chloroethanol.
An analytical method suitable for regulatory purposes is available. It
is a gas chromatographic method (published) with flame photometric
detection that has been validated in government laboratories.
Propargite is also measured by the multi-residue method employed in
the Canadian surveillance programme. It has not been studies in the
U.S. Food and Drug Administration's multi-residue method.
RECOMMENDATIONS
Temporary maximum residue limits for propargite on the following
commodities are recommended.
Commodity Limit mg/kg Pre-harvest intervals on which
recommendations are based
(days)
Apple pomace 80
Alfalfa hay 75 28
Almond hulls 55 28
Alfalfa, fresh 50 28
Mint hay 50 14
Dried citrus pulp 40
Grape pomace 40
Dried hops 30
Raisins 25
Beans (in pod) 20 7
Corn fodder
and forage 10 30
Cranberries 10 14
Grapes 10 14-21
Peanut hay
and forage 10 14
Sorghum fodder
and forage 10 30 (if silaged)
Apricots, peaches,
plums, nectarines 7 14
Strawberries 7 3
Citrus 5 7
Sorghum grain 5 60
Apples, pears 3
Pigs 3 14
Milk (fat) 2
Beans, dry 0.2 28
Almonds 0.1* 28
Cottonseed 0.1* before bolls open
Eggs 0.1
Fat of poultry 0.1
Fat of meat 0.1
Maize (kernels) 0.1* 30
Milk, whole 0.1
Peanuts (kernels) 0.1* 14
Potatoes 0.1* 14
Walnuts 0.1* 14
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (Before July 1981)
1. A carcinogenic study
DESIRABLE
1. Mutagenicity studies
2. Observations in humans
3. Data from supervised trials in countries other than the USA
4. Information on the occurrence of residues on commodities in
commerce
5. Confirmation of the postulated degradation pathway in plants
using radio-labelled propargite.
REFERENCES
Carson S. (1963) Acute Oral LD50 in Rats. Unpublished report from
Food and Drug Research Lab., submitted by Uniroyal Chemcial, Inc.
Carson S. (1963) Subacute Feeding Studies with D-014 in Rats.
Unpublished report from Food and Drug Research Lab., submitted by
Uniroyal Chemical, Inc.
Haley, G., Kennedy, G.L. and Keplinger, M.L. (1972) Teratogenic Study
with Omite in Albino Rats. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Health Protection Branch (1977) Personal communication, Canada Bu.
Chem. Safety, Health Protection Branch to FAO Panel of Experts, 1977.
Holsing, G.C. (1968) 13-Week Dietary Feeding - Dogs. Unpublished
report from Hazleton Laboratories, Inc., submitted by Uniroyal
Chemical.
Jenkins, D.H., Kennedy, G. and Keplinger, M.L. (1972) Study with Omite
in White Leghorn Chickens. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Kennedy, G., Jenkins, D.H. and Keplinger, M.L. (1970) Metabolism of
14C-Omite in the Cow. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Ladd, R., Jenkins, D.H. and Keplinger, M.L. (1974) Tissue Residue
Study with Omite in Crossbred Swine. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Oser, B.L. (1966) Chronic (2-Year) Feeding Studies with D-014 in Rate
and Dogs. Unpublished report from Food and Drug Research Lab.9
submitted by Uniroyal Chemical.
PAM II Pesticide Analytical Manual, Vol. II, revised (1973); U.S.
Department of Health, Education and Welfare, Food and Drug
Administration.
Ryer, F.H. and Sullivany J.B. (1969) Rachiotracer Drug Metabolism
Study with Omite 14C. Unpublished report from Hazleton Laboratories,
Inc., submitted by Uniroyal Chemical.
Ryer, F.H. and Sullivany J.B. (1969b) The Fate of Omite 14C in the
Cow. Unpublished report from Hazleton Laboratoriesy Inc., submitted by
Uniroyal Chemical.
Sleight III, B.H. and Maceky K.J. (1972) 14C Omite Fish Accumulation.
Unpublished report from Bionomics, Inc., submitted by Uniroyal
Chemical.
Smith, K.S. and Roger, J.C. (1975) Omite Milk and Tissue Residue Study
in Dairy Cows: Unpublished report from Cannon Laboratories, submitted
by Uniroyal Chemical.
Sullivan, J.B. (1968) An Investigation of the In-Vitro and In-Vivo
Metabolism of Omite. Unpublished report from Hazleton Laboratories
Inc., submitted by Uniroyal Chemical.
Uniroyal Unpublished information (1977), Uniroyal Inc., to U.S.
Environmental Protection Agency.
Weir, RJ. (1967) Acute Dermal Application to Rabbits. Unpublished
report from Hazleton Laboratories Inc., submitted by Uniroyal
Chemical.
Westlake, W.E., Gunther, P.A. and Jeppson, L.R. J. Agr. Fd. Chem.,
19:894.
Other information on identity and properties
The technical grade material is a light to dark brown liquid of low
volatility. It is miscible with most organic solvents. Solubility in
water is about 0.5 mg/kg. The basic manufacturer states the
composition of the technical product to be:
85% active ingredient
7% "high molecular weight sulphites"
5% "unreacted starting materials"
1% propylene oxide stabilizer
(remainder unidentified)
Propargite is commercially available in 68.1% and 755% emulsion
concentrates, a 30% wettable powder, and a 4% dust. The manufacturer
states that the technical product shows no degradation after 12 months
when stabilized with propylene oxide. The odour of SO2 indicates
breakdown.
The product was introduced in the U.S.A. on an experimental basis in
1967 as a replacement for Aramite, another product of the same
manufacturer, which was withdrawn from use on food crops in the U.S.A.
The two compounds have similar chemical structures and properties.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Extensive degradation of propargite could be shown in in vitro as
well as in vivo experiments.
In vitro studies with liver homogenates showed that the major
primary metabolites appear to be propargyl alcohol, the glycol ether
and their conjugates, which are further degraded to secondary
metabolites (Sullivan, 1968).
In in vivo metabolism studies in rats that were orally treated with
a single dose of 200 mg/kg propargite, propargyl alcohol and glycol
ether were also found as primary metabolites in the excreta as well as
in tissue samples. Following analysis of the excreta and tissues only
about 5% of the administered dose was accounted for as unchanged
propargite or the primary metabolites, indicating that the primary
metabolites are further degraded (Sullivan, 1968).
The oral administration of a single close of 271 mg/kg benzene ring
labelled 140 propargite to rats was followed by fast excretion of the
radiocarbon. Within 72 hours postdosage, 47% of the administered dose
was eliminated via the urine. 32% via the faeces. The total carcass
contained approximately 90% of the administered radioactivity.
Identified metabolites excreted with the rat urine and faeces were
tert-butylpyrocatechol and glycol ether (Ryer and Sullivan, 1969a)
In cows treated with a single oral close of 4 mg/kg b.w. combined
non-radioactive and 14C labelled propargite a similar excretion
pattern was found. 54% of the administered dose was eliminated with
the urine, 17% with the faeces. Approximately 0.3% was found in the
milk. Identified metabolites were tert-butylpyrocatcohol, glycol
ether and tertbutylphenol (Ryer and Sullivan, 1969b).
In a feeding study, dairy cows were maintained on a diet containing
14C-labelled and unlabelled propargite at dosage levels of 3 and 20
ppm for 12 consecutive days. Peak urinary excretion observed at the
seventh test day reached values of 4 and 23 mg/kg respectively for the
3 and 20 ppm dietary level; peak faecal excretion was approximately 1
and 4 mg/kg. Maximum milk residue values reached on the fourth test
day were 0.02 and 0.06 mg/kg at the 3 and 20 ppm dose levels
respectively. Residues in selected tissues ranged from 0.02 to about
1.3 mg/kg (Kennedy et al., 1970).
Similar results were obtained after daily administration of a diet
containing 50 and 100 ppm propargite to lactating cows for a period of
27 days (Smith and Roger, 1975).
Pigs were maintained on a diet containing propargite at dosage levels
of 20 and 100 ppm for 30 days. Residues in kidney and liver were below
the detection limit of 0.1 mg/kg at both feeding levels and residues
in muscle and fat ranged from below 0.1 to about 2 mg/kg (Ladd et al.,
1974).
Residues studies in hens that were treated with 3, 10 and 30 ppm 14C
propargite in their diet for 30 days showed residue levels in eggs
from 0.13, 0.49 and 0-145 µg/kg respectively at the 3, 10 and 30 ppm
dose levels. Residues in muscle ranged from 2-12 µg/kg, liver 18-174
µg/kg kidney 2-25 µg/kg and fat 7-116 µg/kg depending on exposure
level (Jenkins et al., 1972).
After continuous exposure to a concentration of 0.025 mg/l 14C
propargite in water for 35 days tissue residues in edible portions of
fishes were about 2 mg/kg. After transfer to uncontaminated water 70%
of the radioactivity present in the edible tissues was eliminated
within 14 days (Sleight III, 1972),
The in vivo metabolism studies in several animal species confirmed
the rapid degradation of propargite already shown in in vitro
experiments. The metabolic reactions are hydrolysis of the parent
ester followed by other cleavage. The formed products are polar
alcoholic and phenolic compounds which can rapidly be excreted or
further metabolized and incorporated into natural tissue constituents
by biochemical reactions which are well documented in the literature.
See also the section "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on teratogenicity
Female rats were orally dosed with 0, 50 and 100 mg/kg from day 6
through 15 of gestation. The treatment had no effect on maternal body
weight, mortality or fertility. No reproductive adverse effects were
found with respect to implantation and resorption sites. Embryonic and
foetal development was not affected by the treatment
(Haley et al., 1972).
Special study on reproduction
Rat
See "Long term study".
Special study on carcinogenicity
Rat
See "Long term study".
Dog
See "Short term study".
Acute toxicity
Species Sex Route LD50 References
mg/kg
Rat M, F oral approx. 2200 Carson, 1963
Rabbit M, F dermal approx. 3200 Weir, 1967
Rat
Groups of 5 male and 5 female rats were treated for 90 days with
amounts of propargite in their diet to provide daily intakes of 10,
20, 40, 100 and 200 mg/kg b.w. corresponding to dietary levels in
adult animals of 200, 400, 800, 2000 and 4000 ppm respectively. The
control group existed of 15 male and 15 female rats. Treatment with
2000 and 4000 ppm caused a dose-related growth retardation and
reduction of food intake. Haematological examinations and clinical
chemistry tests revealed no abnormalities. At 2000 and 4000 ppm the
relative liver weights were increased, the relative kidney weights
were increased only in the 4000 ppm groups. Gross and microscopic
examinations of tissues disclosed no treatment-related pathological
alterations (Carson, 1964).
Dog
Groups of 3 male and 3 female dogs were maintained on a diet
containing propargite at dose levels of 0 and 2000 to 2500 ppm
(increase of dosage after 3 weeks) for 90 days. The treatment did not
affect appearance, behaviour, results of haematological. and clinical
chemistry tests and urinalyses. In most treated dogs reduced food
consumption and body weight loss and an increase of relative liver and
kidney weights were observed, No compound-related alterations were
found macroscopically. Histopathological examinations revealed an
increased amount of pigment in the liver reticuloendothelial cells and
increased haemosiderosis of the spleen (Holsing, 1968).
Groups of 8 dogs were fed a diet containing propargite at 100, 300 and
900 ppm for 24 months. The control group consisted of 12 animals. The
treatment had no effect on growth rate, results of clinical laboratory
studies, urinalyses and organ weights. Gross and microscopic
examination did not reveal abnormal pathological findings. No tumours
were found (Osert 1966).
Long term Study
Rat
Groups, each comprising 25 male and 25 female rats were fed dietary
levels of propargite to provide daily intakes of 5, 15 and 45 mg/kg
b.w. corresponding to adult levels of 100, 300 and 900 ppm for a
period of 24 months. The control group consisted of 37 animals of each
sex. After 26 test weeks without noticeable evidence of a
treatment-related effect, a supplementary study was started with a
control-and a test group comprising 25 male and 25 female rats
receiving 100 mg/kg b.w. corresponding to 2000 ppm for 78 weeks. Rats
that died during the study and those sacrificed after 104 and 78 weeks
were examined grossly and microscopically. The treatment had no effect
on appearance, behaviour, growth rate and mortality at the dietary
levels up to and including 900 ppm. At the 2000 ppm level at the end
of the study there was a reduction of food consumption and also a
reduction of the body weight gain of about 30%; mortality of the male
rats increased to 32% compared to 7% of the corresponding control.
Haematological examinations and clinical laboratory studies did not
reveal abnormal findings. Absolute and relative liver and kidney
weights of the animals treated with dietary levels of 100, 300 and 900
ppm which died or were sacrificed after 104 weeks showed inconsistent
variations without any dose-relationship. At 2000 ppm an increase of
the relative liver and kidney weights of about 30%. were observed.
Gross autopsy findings in 1 male (of 25) and 7 females (of 23) in the
2000 ppm group were enlarged and/or dark red lymph nodes which in some
cases were involved in abdominal masses. In the corresponding control
group 2 male rats of 29 animals showed this alteration. No
dose-dependent increase of the frequency of this lymph node alteration
was found at the 100, 300 and 900 ppm levels. A variety of benign and
malignant tumours was observed in the control and treated groups. No
dose-relationship of the frequency of tumours was found. Four sarcomas
were found in rats sacrificed after 78 weeks ingestion of 2000 ppm,
whereas in the corresponding control group no sarcomas were seen
(Oser, 1966).
Additionally a 3-generation reproductive was included in the 2-year
feeding experiment with 20 pairs of male and female rats from the
control and 100 ppm test groups. Dosage was raised to 300 ppm in the
F2 pups at the time of weaning. The treatment revealed no abnormal
findings (Oser, 1966).
COMMENTS
Propargite is readily absorbed from the gastrointestinal tract and is
excreted rapidly as metabolites which are formed mainly by hydrolysis
of the sulphite ester.
After feeding a diet containing 20 ppm of propargite the residues in
milk or in tissues were of the order of 0.1 mg/kg.
The compound is not teratogenic in the rat.
In a 3-generation reproductive study no adverse effects were revealed
at concentrations up to 300 ppm. This concentration is therefore
regarded as a no-effect level.
In a 2-year feeding study with up to 900 ppm in dogs which included
extensive laboratory and histological investigations, no adverse
effects were noted. A 2-year feeding study in rats up to 2000 ppm
showed appreciable but not dose-related variations in the relative
liver and kidney weights in all treated groups. However, no major
adverse effects were seen. Owing to a poor survival rate and the
termination of the study with the high dose animals after 78 weeks,
the finding that the tumour incidence was not elevated is of limited
value. This study was not considered to fulfil the criteria for an
acceptable lone-term study. Therefore a safety factor of 200 was used.
Propargite is structurally related to Aramite, a compound which is
suspected of being a carcinogen. Although there are no indications
that propargite acts similarly, the need for satisfactory
carcinogenicity studies was expressed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 300 mg/kg in the diet, equivalent to 15 mg/kg bw
Dog: 900 mg/kg in the diet, equivalent to 22 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Propargite is an acaracide said to be effective on a variety of mites
on food crops and ornamentals. Table 1 shows the use patterns
registered in the U.S.A. Information has been received that the
product is also used in Italy, Australia, New Zealand, South Africa,
Argentina, Mexico, and France. The government of the Netherlands has
informed the Meeting that the compound was used until 1976, at which
time its use was discontinued. A country statement was received from
New Zealand regarding uses and tolerances. The uses permitted in these
countries generally parallel those shown in Table 1. Significant
exceptions are included in the footnotes.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trails on twenty-six crops (Uniroyal, 1977). Most of the trials were
conducted in the U.S.A. A country statement was received from New
Zealand with data on peaches. Analyses in all U.S. trials, the
procedure described under "Methods of residue analysis" (PAM II,
1973). Analyses of untreated controls (crop blanks) were reported in
each experiment, along with the recovery of propargite from fortified
controls. Controls were generally reported as 0 and recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form when feasible.
The use patterns employed in Mexico and France on apples, peaches,
plums and citrus differed significantly from the other countries. In
the absence of residue data reflecting good agricultural practice in
those countries, it was not possible to predict residue levels. The
recommended DIRLs for apples, peaches, plums and citrus therefore are
based on the national use patterns which can be related to supervised
residue trails.
Alfalfa
There are no national tolerances or registrations for propargite use
on alfalfa. However, the submission to the Meeting contained some
residue data on alfalfa hay, fresh forage and trash. Single and
multiple applications were made at 1.4 to 4.7 kg a.i./ha. Residues on
fresh forage ranged 0.0 to 328 mg/kg at 28 and 0 day intervals from
treatment, respectively. Residues on hay and trash ranged from 2.6 to
100 mg/kg with intervals from treatment of 22 to 52 days. With a 28
day pre-harvest interval, MRLs of 50 and 75 mg/kg on fresh alfalfa and
alfalfa hay, respectively, would be adequate.
TABLE 1. Registered use patterns for propargite (USA)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Almond 30% WP 0.03-0.05%(4) 2 28(5) low volume
68.1% EC 2.5-3.4 kg/ha 1 post-harvest aerial
application
only
Apples 30% WP 0.04-0.05(1)(6) 3 7(2)(7)(12)
Apricots 30% WP 0.05%(4) 2 14(5) low volume
68.1% EC 1.7-2.5 kg/ha 2 post-harvest aerial
application
only
Cranberries 68.1% EC 1.6 kg/ha 1 14
Beans 75% EC 2.8 hg/ha(4) 2 7(fresh)(5)
28 (dry)
Corn 75% EC 1.9 kg/ha 1 30
(field)
Cotton 75% EC 0.85-1.9 kg/ha 3 before bolls open
Figs 30% WP 0.03-0.05%(3)(4)(11) 2 14
Grapes 30% WP 1.7-3.0 kg/ha 2 21
4% Dust 0.2 kg/ha 2 14
Grapefruit, 75% EC 0.025-0.05(4)(8) 2 7 (5)
oranges
Lemons 30% WP 0.05% (8) 2 7
Hops 68.1% EC 1.7 kg/ha(4) 2 14 (5)
30% WP 1.7 kg/ha 2 14
Mint 68.1% EC 1.7-2.5 kg/ha 2 14
Nectarines 30% WP 0.05% (4) 2 14 (5)
Peaches 30% WP 0.04-0.05%(4)(8) 2 14(5)(10)(13)
Peanuts 30% WP 1.0-1.7 kg/ha 2 14
Pears 30% WP 0.04-0.05%(8)
Plums 30% WP 0.04-0.05(4)(8) 2 14(5)(10)
Potatoes 75% EC 1.4-2.4 kg/ha 2 14
68.1% EC 1.7-2.5 kg/ha 2 14
TABLE 1. (Continued)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Sorghum 75% EC 1.5-1.9 kg/ha 1 30 silage
60 grain
Strawberries 30% WP 1.7-3.4 kg/ha(9) 3 3
4% Dust 1.8 kg/ha 3 3
Walnuts 68.1% EC 0.03-0.05% (4) 2 14 (5)
(1) France 0.12, Italy 0.06%
(2) France 21, Italy 15, New Zealand 14
(3) France 0.6-0.9 kg/ha
(4) Italy 0.06%
(5) Italy 21
(6) Mexico 0.08-0.12%
(7) Mexico 28
(8) Mexico 0.12%
(9) East U.S.A. 0.84 kg/ha
(10) Argentina 21
(11) Argentina 0.05%
(12) South Africa 14
(13) South Africa 21
Almonds
Field trials were conducted in California: 63 samples of nut meats
were anlayzed. Residues on the nut meats from two applications of 0.03
and 0.06% sprays were < 0.1 mg/kg at post-treatment intervals of 21
to 28 days.
Residues on almond hulls from treatment with 0.05% spray (30% WP)
followed by 3.4 kg a.i./ha (68.1% EC) ranged from 5.2 to 31 mg/kg with
pre-harvest intervals of 28-35 days. Under good agricultural practice,
residues are not likely to exceed 55 mg/kg.
Apples
Field trials were conducted in nine U.S.A. states, South Africa and
Australia, some 452 samples being analysed. The residue data are
summarized in Table 2. All controls were reported zero. Residues from
applications of up to 0.06% spray after 7 days were less than 3 mg/kg.
Residue data indicate that residues remain associated with the apple
pomace and are not destroyed in drying. Residues in the juice were
barely detectable (ca. 0.02 mg/kg) while residues in the wet and dried
pomace were 16-21 mg/kg and 56-80 mg/kg, respectively. Thus, residues
concentrate by factors of 15-22 in the manufacture of dried pomace
from treated apples (Uniroyal, 1972), An MRL of 80 mg/kg in dried
apple pomace is therefore appropriate.
TABLE 2. Range of residues in apples, mg/kg.
Application rate, Interval (days, last treatment to harvest)
% 0-1 6-7 14 21 28
0.03 0.1-3.1 0.6-1.8 0.2-1.0 0.2-0.7 0.2-0.9
0.06 0.3-6.3 0.5-1.6 0.3-2.4 0.5-1.1 0.2-0.6
Apricots, peaches and plums
Field trials were conducted in California, Connecticut, Georgia, North
and South Carolina, Maryland, Michigan, Pennsylvania, Washington, New
York and New Zealand. Some 385 test samples of fresh fruit and 65
controls were anlaysed from one and two applications at four dosage
rates, including the maximum and twice the maximum label rates (Table
1). The residue data are summarized in Table 3. Only one residue on
peaches was greater than 7 mg/kg at 14 days after treatment at the
maximum rate. Residues will not exceed 7 mg/kg from sprays up to
0.06%, provided that treatments are not made within 14 days of
harvest.
A study was conducted on plums (fresh prunes) using a standard
commercial drying process to determine residues on dried prunes. The
average residue value for the dried fruit was ca. 20% of the average
value for the fresh fruit. Thus no additional MRL is needed for the
dried fruit.
TABLE 3. Range of residues on apricots, peaches and plums, mg/kg
Application rate. Interval (days, last treatment to harvest)
% 0-1 6-7 13-14 21-23
0.03 0.4-12 0.2-7.0 0.4-10.0 0.3-5.0
0.05 0.7-6.7 2.6-14.0 0.6-5.6 0.0-1.7
0.06 1.3-17.0 1.3-8.7 0.2-5.5 0.3-9.3
0.10 1.6-27.0 0.3-8.4 0.8-11.0 0.8-3.5
Beans
Field trials were conducted in the states of Washington, California
and Idaho on snap beans, succulent lima beans and dry beans. 52
analyses were reported. Single and multiple applications were made at
the maximum and at twice the maximum label rate (Table 1), by aerial
and ground application. The residues are summarized in Table 4 for
fresh and dry beans, 7 and 28 days after the last treatment,
respectively. No residue data were submitted for bean vines; however
there is a restriction against the feeding or foraging of treated bean
vines. The only other feed item, cannery waste, would consist of cull
beans with some bean vines and pods present as a result of mechanical
harvesting. Residue levels would not be likely to exceed 20 mg/kg on
cannery waste.
TABLE 4. Range of residues in beans, mg/kg
Application rate, kg a.i./ha
2.8 5.6
Fresh (7 days after last treatment) <0.2-15 <0.2-12
Dry (29 days after last treatment <0.1-0.1 <0.1-0.24
Maize
Residue data for maize were made available from five U.S.A. sites, two
in California and one each in Colorado, Texas and Nebraska. Following
applications at the maximum and twice the maximum label rates (Table
1), silage stage maize sampled 28-35 days after application contained
maximum residues of 2.4 and 18 mg/kg, respectively; maize stover,
sampled after 47-105 days, contained maximum residues of 4 and 11
mg/kg, respectively. Interpolation of the 2X data shows that MRLs on
maize forage and fodder of 10 mg/kg are appropriate.
No detectable residues (< 0.1 mg/kg) were found on either the grain
or ears of maize sampled 60-105 days after the above applications. No
data on grain reflecting a 30 day pre-harvest interval were submitted.
However, since propargite does not translocate to any extent, residues
on grain are not expected to exceed 0.1 mg/kg.
Cotton
Five field studies from locations in California, Arkansas and
Mississippi were reported. The studies reflect treatment at the
maximum and twice the maximum label rate (Table 1). Analyses were
performed on 96 samples of cottonseed and processed products, hulls,
oil, meal and soapstock. All values on cottonseed were reported as
< 0.1 mg/kg. All samples of meal and soapstock contained
< 0.05 mg/kg as did all but on refined oil sample which contained a
level of 0.1 mg/kg after treatment at an excessive rate. Almost all of
the hull samples had low but detectable residues, 0.03-0.06 mg/kg.
Thus, an MRL of 0.1 mg/kg is appropriate for cottonseed. There is a
restriction in the U.S.A. against feeding treated foliage or cotton
trash to livestock.
Cranberries
Seven studies were reported from Massachusetts, with a total of 36
analyses. The studies reflect applications at 1.68 kg a.i./ha (4 by
ground and 3 by air) with one study at 3.36 kg a.i./ha. Several of the
studies show decline rates (at 7, 14 and 21 days). The residue
declines rapidly between 0 and 7 days and then shows little further
decline from 7 to 21 days. At the recommended rate (Table 1), residues
are not likely to exceed 10 mg/kg. Residues are summarized in Table 5.
TABLE 5. Range of residues in cranberries, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 21
1.68 2.2-11 <0.1-1.9 <0.1-7.1 0.1-1.0
3.36 3.3-14.9
Figs
The residue data for figs consists of four studies made in California,
including two decline studies. Various applications were made
including the maximum and twice the maximum label rate (Table 15).
Residues on fresh figs are summarized in Table 6. While the data are
limited, it is not likely that residues will exceed 3 mg/kg at 14 days
after treatment.
TABLE 6. Residues on figs, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0-1 7 13-14 21 28
1.27 3.3 1.9 0.8 0.45
2.02 1.6-2.8 1.0-1.4 0.2-1.4 0.3-0.6
4.04 2.5-5.3 2.2-3.2 0.7-4.2
Grapefruit, oranges and lemons
Field trials were conducted in Texas, Florida and California. Some 281
harvest samples were analyzed. One and two applications were made at
the recommended and twice the recommended rates (Table 1). The results
are summarized in Table 6. For applications up to 0.06% spray with a
7 day interval, 5 mg/kg would not be exceeded.
Washed oranges bearing residues of 1.9 mg/kg were processed to dried
pulp by a procedure simulating the commercial process. The residue in
the dried pulp was 15.5 mg/kg. The residue in the dried pulp is about
eight times that on the whole fruit, so 40 mg/kg is an appropriate
MRL.
TABLE 6. Range of residues in grapefruit, oranges and
lemons, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 28
0.03 0.3-0.5 0.2-0.8 0.2-0.3 0.9-1.0
0.05 1.7-3.6 0.2-4.8 1.1-2.1 0.6-3.1
0.06 1.0-1.4 0.9-2.5 0.0-0.9 0.6-1.2
0.10 1.0-5.1 0.8-7.2 1.2-3.2 1.2-2.9
Grapes
Residue trials were conducted in Washington and California, with
analyses of 254 samples of grapes. A wide range of experimental
conditions in the various field tests and the resulting variation in
residues preclude a tabular summary of the data. No residues exceeded
10 mg/kg from the maximum recommended rate with a 21 day interval
after treatment.
A residue concentration factor of 2.5 was observed in the commercial
production of raisins from fresh grapes. Thus a 25 mg/kg MRL for
raisins would be appropriate. The data showed a concentration of
residues on dried grape pomace to 4 times that found on fresh grapes,
therefore, 40 mg/kg would be appropriate for grape pomace.
Hops
Eight field trials were conducted in the State of Washington. Residues
on green hops resulting from three applications at the maximum and
twice the maximum label rate (Table 1) are summarized in Table 7.
Residues on green hops from good agricultural practices are not likely
to exceed 15 mg/kg. Residues on dried hops ranged from 11 to 30 mg/kg
14 days after treatment. A 30 mg/kg MRL would be appropriate for dried
hops since residues from two applications will probably be somewhat
lower than those noted above.
TABLE 7. Range of residues on green hops, mg/kg
Application rate, Interval (days last treatment to harvest)
kg a.i./ha 0 6-7 13-14 20
1.7 24-50 5-17 6-15 2-6
3.4 36-92 24-66 11-43 6-24
Mint
Field studies were conducted in Washington, Oregon and Idaho. A total
of 89 analyses were made on fresh hay, spent hay and mint oil,
including controls. In four studies, two applications were made at
rates of 2.5 and 5 kg a.i./ha resulting in maximum residues of 19 and
50 mg/kg respectively, 15-17 days after treatment. Three residue
decline studies were also submitted. Samples were taken 0, 1, 2 and 4
weeks after a single aerial application at 1 and 2 times the maximum
label rate (Table 1). From a decline curve based on these data,
residues resulting from the recommended use are ca. 50 mg/kg on fresh
mint hay.
Five samples of fresh mint hay bearing residues of 8.7-50 mg/kg were
fractionated by steam distillation and the resulting mint oil and
spent hay contained residues of 1.6-7.9 mg/kg and 12-97 mg/kg
respectively. Residues in mint oil are not likely to exceed those on
fresh mint hay and there is a restriction against feeding spent mint
hay to livestock.
Nectarines
Seven trials were conducted at locations in California and
Pennsylvania. One or two applications were made at one and two times
the maximum label rate (Table 1). The residue data are summarized in
Table 8. Residues were no higher than 3-4 mg/kg at the prescribed
pre-harvest interval. However, extrapolation of data from peaches,
apricots, and plums would indicate that a 7 mg/kg MRL would be
appropriate.
TABLE 8. Range of residues in nectarines, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 6-7 13-15 20-21
0.05 0.66-7.3 0.24-3.1 0.44-3.4 0.39-1.3
0.10 1-3-10.5 0.34-5.1 0.91-3.4 0.41-1.5
Peanuts
Residue studies were conducted in Georgia, Florida, North Carolina,
Oklahoma, Mississippi and Arkansas. Two applications were made in all
but four decline studies on peanut vines, where only one application
was made. Dosages ranged from 0.6 times to twice the maximum
recommended rate (Table 1). Six of the studies reflect a 14 day
preharvest interval, but in the other ten studies intervals were from
19 to 66 days.
Peanut kernels
All residue values were reported as < 0.1 mg/kg. Since no detectable
residues were found in peanuts, no MRLs are needed for peanut
by-products, meal, oil and soapstock.
Peanut hulls
Residues were < 0.1 mg/kg in five of seven studies. Residues of
0.14 mg/kg, 0.3 mg/kg and 1.2 mg/kg were found after applications at
twice, 6 times and 1.2 times the recommended rates respectively, in
the other two studies.
Peanut vines and hay
Residues were less than 10 mg/kg 14 days after application at the
maximum rate. The data indicate that residues decline rapidly from a
maximum of 38 mg/kg at 0 days to 3.6 mg/kg at 14 days.
Pears
Four field studies were conducted in California, Pennsylvania,
Michigan and Washington, one and two applications were made with 0.03
and 0.06% sprays. The residue data are summarized in Table 9. All
residues were less than 3 mg/kg, 14 days after treatment.
TABLE 9. Range of residues in pears, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 21
0.03 0.6-1.6 0.3-1.2 0-2.7 0.1-2.4
0.06 2.2-4.1 0.9-2.6 0.3-2.2 0.1-1.7
Potatoes
Seven residue studies were made available, four from Washington and
one each from Idaho, Vermont and Illinois. After two applications at
rates of 1.5 to 4.5 kg a.i./ha, all residues were less than the limit
of detection of the method (0.1 mg/kg) 14 days after treatment.
Sorghum
Field studies were conducted in Texas, Nebraska and Iowa on grain
sorghum. Applications were made at the maximum and twice the maximum
recommended rates (Table 1). Samples of grain and forage or fodder
were harvested at 28, 30, 44 and 50 days after application. Residues
in forage ranged from < 0.1 to 4.1 mg/kg. Residues in forage and
fodder are not likely to exceed 10 mg/kg 30 days after treatment.
Residue levels in grain ranged from < 0.1 to 2.4 mg/kg 60 days or
more after treatment. Thus an MRL of 5 mg/kg with a 60 day interval
after treatment is appropriate for sorghum grain.
Strawberries
Field trials were conducted in California, Louisiana and Florida.
Strawberries treated with two formulations in California, reflecting
three applications at the maximum recommended rate (Table 1) had
residues ranging from 0.19 to 4.0 mg/kg for 3 days after treatment.
Residues from applications of 0.84 and 1.7 kg/ha in Louisiana and
Florida are summarized in Table 10. Under good agricultural practice,
residues are not likely to exceed 7 mg/kg.
TABLE 10. Range of residues in stramberries, mg/kg
Application rate Interval (days, last treatment to harvest)
kg a.i./ha 0 3 7
1.7 0.9-2.0 0.3-3.8 0.2-0.7
3.4 1.4-4.1 0.7-9.2 0.4-1.1
Sugar beets
There are no national tolerances or registrations for propargite on
sugar beets; however, this use is pending in the U.S.A. The submission
to the Meeting therefore contained some residue data on sugar beet
tops, roots and by-products. Sugar beet root samples from Idaho,
Washington and California reflecting two treatments at 2.8 and 5.6 kg
a.i./ha and a 21 day interval from treatment# showed residues ranging
from 0.05 to 0.23 ind/kg and 0.13 to 0.28 mg/kg respectively.
In a residue decline study, on sugar beet tops, a single application
at 2.8 kg a.i./ha yielded residues of 40-114 mg/kg at 0 days.
20-80 mg/kg at 14-17 days and 2.4-34 mg/kg at 27-28 days. Adiitional
data for tops representing two ground treatments at 2.8 and 5.6
kg a.i./ha show residues ranging from 11-24 mg/kg and 19-59 mg/kg
respectively (21 day interval).
In order to obtain data on processing fractions, sugar beet roots
bearing 0.1 mg/kg residues were processed to wet and dry pulp,
molasses and sugar. Propargite residues in wet pulp, molasses and
sugar were less than or equal to 0.1 mg/kg. The data for dry pulp show
a maximum residue concentration factor of 5, with residues from
0.1-0.5 mg/kg.
Tea
There are no national tolerances or registrations for propargite in
tea, however, a tolerance for this use is pending in the U.S.A. The
submission to the Meeting contained some residue data on dried tea
leaves, both green and black. The data are summarized in Table 11.
TABLE 11. Range of residues in tea, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 28
0.31 <0.1-0.3 <0.1-0.2
0.75 48-178 1.1-4.2 <0.1-0.2 <0.1
Walnuts
Six field trials were conducted at locations in California. Both
30% WP and 75% EC formulations were applied at the maximum recommended
rate (Table 1). No residues were detected in any of the walnut meat
samples 45 to 48 days after treatment. Although the interval is
greater than that recommended (14 days), since propargite is
non-systemic, any residues found on nut meats would be expected to
arise as a result of contamination in de-hulling and de-shelling
processes. Under good agricultural practice, an MRL of 0.1 mg/kg (at
or about limit of determination) would be appropriate. There is a
restriction against grazing or feeding livestock on cover crops growm
in treated orchards, therefore no feed items are involved in this use.
Meat, milk, poultry and eggs
Propargite is used on a number of crops which yield by-products used
commercially for animal feeds. Primary sources would be dried citrus
pulp, sorghum grain, forage and fodder and maize forage and fodder.
Some additional contribution could occur from dried pomace, almond
hulls and dried grape pomace. Per this reason, some animal feeding
studies were submitted. These are also referred to in the section
"Biochemical aspects", where references are given.
In one study, cattle were fed at levels of 3 and 20 ppm in the total
dry diet for 12 days. Maximum residues in milk (whole milk basis) were
0.059-0.067 mg/kg. The latter is equivalent to 1.7 mg/kg on the fat
basis. Maximum residues in tissues were as follows: liver, 1.3 mg/kg,
kidney, 0.27 mg/kg, fat, 0.21 mg/kg and muscle, 0.18 mg/kg.
In a second study, cows received 0, 50 and 100 ppm in the diet for 27
days. Analysis of milk showed all samples at both feeding levels to be
< 0.08 mg/kg. Residues ranged up to 1.6 mg/kg on a fat basis.
Residues in muscle, kidney and liver from both feeding levels were
40.03 mg/kg (limit of determination). Residues in fat ranged from 0.32
to 0.42 mg/kg at the 10 and 100 ppm feeding levels, respectively.
Poultry were fed 3, 10 or 30 ppm in the diet for 30 days (3 chickens
in each group). Eggs were sampled daily and the animals were
sacrificed 24. hours after treatment. Maximum levels In eggs were
0.013 mg/kg at the 3 PPM level, 0.043 mg/kg at the 10 ppm feeding
level, and 0.106 mi/kg at the 30 ppm level. Liver tissue had the
highest residue levels ranging from 0.047 to 0.089 mg/kg at the 10
diet level and from 0.17 to 0.18 mg/kg at the 30 diet level.
Hogs were fed 20 and 100 ppm in the total diet. Two hogs at each level
and one control were maintained for 30 days. No residues
(< 0.1 mg/kg) were found in liver or kidney. No residues were found
in muscle at the 20 ppm level but up to 0.23 mg/kg at the 100 ppm
intake. In fat, residues up to 0.18 and 0.2 mg/kg were found at the 20
and 100 ppm intakes, respectively.
The studies show that residues may transfer to meat, milk, poultry and
eggs. A mixed ration for cattle could consist of 15% dried apple
pomace, 15% dried citrus pulp and 70% sorghum grain, forage and fodder
or maize forage and fodder. This diet could theoretically contain
propargite residues of ca. 25 mg/kg.
A maximum possible intake of 10 mg/kg could result from the hogging
down of peanut fields and other swine feed items such as maize grain
and cooked potatoes.
Poultry feed items, sorghum grain, grape pomace, maize grain and
cooked potatoes, could contribute ca. 8 mg/kg propargite to the diet.
Residues are unlikely to exceed 2 mg/kg in milk fat and 0.1 mg/kg in
the meat, meat by-product, and fat of cattle, goats, hogs, horses,
poultry and sheep.
FATE OF RESIDUES
In animals
The available data on metabolism by animals are more conclusive than
those on metabolism by animals are move conclusive than those on
metabolism by plants. See also "Biochemical aspects."
Propargite -14C feeding tests have been conducted on a number of
animals including the rat, cow, pig and chicken. Additional
information on degradation was available from in vitro experiments
with buffered liver homogenates.
A general metabolism scheme has been proposed for animals, part of
which has been verified experimentally, and part of which is based on
analogy with biochemical reactions reported in the literature.
Identification of some metabolites was by TLC comparison with prepared
standards. In other cases less positive identification was made by
partition co-effecients (p values) and solvent extraction scheme. In
cow liver the bulk of the 14C activity was identified only as polar
metabolites.
The experiments apparently were carried out over a number of years,
the studies in some animals being repeated with refinements in
analytical techniques. None of the work on propargite submitted to the
Meeting has been published. Certain general conclusions appear
justified. (a) Propargite is metabolized in animals to polar compounds
which are excreted, principally in the urine. (b) There is not a
pronounced tendency to storage or accumulation in the body, but the
compound is not completely metabolized because the parent compound was
the principal residue in the milk of cows, was tentatively identified
as a portion of the 14C activity in animal fat, and was found in
excreta. (c) The parent compound is detected in the liver only for
short periods after ingestion. d) The primary hydrolysis products, the
glycol ether and propargyl alcohol, are further degraded. (e) Some
polar metabolites are in conjugated forms.
A list of the metabolits and the sites in which they were reported is
given in Table 12. The sequence of the metabolic scheme is not
definitely known.
TABLE 12. Propargite metabolites
Compound Found in
Parent Cow: butter fat, body fat (1)
Rat: liver (1)
liver homogenate (1)
Glycol ether Cow: urine, faeces, milk (2)
Rat: faeces, liver (1)
liver homogenate (1)
Propargyl alcohol liver homogenate (1)
Butylphenol Cow: urine (2)
Rat: liver (1)
liver homogenate (1)
Butylcatechol Cow: urine (2)
Rat: urine, faeces (2)
Cyclohexandiol urine (2)
"Polar metabolites" Rat: liver (2)
Cow: liver (2)
(1) Identified by GLC or TLC
(2) Tentatively characterized by solvent
fractionation scheme, p values, literature analogies.
In plants
Propargite is generally considered to be "non-systemic" by mode of
miticidal action. According to information made available to the
Meeting aged residues are primarily surface residues of the parent
compound (Uniroyall 1977). Residue decline was attributed to
volatilization of the parent compound and hydrolysis to propargyl
alcohol and the "glycol ether",
2-(p-tert-butylphenoxycyclohexanol). The two hydrolysis products
have not been detected in samples by chemical analysis at a detection
level of 0.1 mg/kg. In experiments on bean plants to investigate
possible photolytic degradation, the same two compounds could not be
detected. It has been suggested that if the glycol ether and propargyl
alcohol are intermediates in the degradation scheme, their presence is
fleeting because of their volatility.
Other evidence cited in the manufacturer's submission on the behavior
of propargite in plants was: (a) comparison of surface and pulp
analysis of apples showed no absorption (of parent); (b) mites
confined to upper leaf surfaces were not affected by sprays on the
lower surfaces (c) there was no effect on mites of an adjacent leaf
when on leaf of a bean plant was treated; (d) volatility losses of the
parent compound under field conditions have been correlated with model
laboratory experiments (loss from aluminum foil).
The above experiments partially support the idea that propargite is
not translocated in plants and that the residue of concern is
propargite per se. However, much of the evidence is indirect, and it
would be desirable to have conventional metabolism studies on some
representative crops, using 35S and/or 14C, to confirm the fate of
propargite in plants.
In soil
Only a summary was made available (Uniroyall 1977). The half-life in a
variety of soils was said to range from 2 to 18 weeks. Leaching is
negligible. Laboratory and field studies have shown that propargite is
hydrolysed in soil. The major soil degradation product found was the
glycol ether.
Summary, metabolism
The available metabolism studies do not firmly identify the residue
components in plants or animals. However, there is sufficient
information to indicate a probable metabolic scheme. It is fairly
certain that the primary metabolites are the hydrolysis products
tert-butylphenoxycyclohexanol and propargyl alcohol. It is probable
that further breakdown to butylphenol and cyclohexanediol occurs. If
correlations are to be made between metabolic schemes and toxic
potential for propargite and aramite, it should be noted that both
compounds hydrolyze at the sulfite ester, aramite yielding
2-chloroethanol and propargite yielding propargyl alcohol. Whether any
further confirmatory studies are needed would depend on the toxic
potential of the postulated fragments.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Propargite is detected in the multi-residue screening procedure
employed by the Canadian Department of Health and Welfare, The
compound is eluted from Florisil in fraction 3 as described in the
method (15% acetone in hexane). Only 4 positive findings of propargite
were reported in the Canadian surveillance programs (as of 1977). The
highest residue was found in a nectarine sample at 0.57 mg/kg (Health
Protection Branch, 1977),
The U.S. Food and Drug Administration multi-residue method has not
been validated for propargite. The specific GLC/FPD analytical method
has not been applied in the U.S. surveillance programme. Therefore
propargite has not been reported in foods in channels of commerce in
the U.S.A. No reports from other governments have been received on
occurrence in commerce.
There were no data available on reduction of residues through washing,
cooking or processing. The principal exposure through ingestion would
be from fruits eaten raw and unpeeled.
METHODS OF RESIDUE ANALYSIS
The early residue data on propargite (mid 1960s) were obtained by gas
chromatography with flame ionization or microcouclometric (SO2)
detection. Some difficulties were experienced by government
laboratories in validation studies on the method at that time were
because the clean-up and extraction procedures were unsatisfactory.
Through a series of modifications, a basic procedure using gas
chromatography with flame photometric detection (FPB) has evolved
which is satisfactory for a wide variety of fruits, vegetables, and
animal tissues (Devine and Sisken, 1972).
In the basic method, residues are extracted with 1:1 hexane and
isopropanol, followed by an aqueous NaCl wash and Florisil column
chromatography. For oily or waxy samples an optional step is included
in which the residues are partitioned into nitromethane. Other
modifications are prescribed for certain crops. The method has been
validated in U.S. government laboratories on milk, apples and animal
tissues (PAM III, 1973). Depending on the substrate, the limit of
determination is about 0.05 to 0.2 mg/kg.
A similar GC-FPD method for propargite residue in citrus has been
reported by Westlake et al, (1971).
Propargite is not known to be recovered through the U.S. Food and Drug
Administration multiresidue procedure. Information received from
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Almonds 0.1
Almonds, hulls 55
Apples 3 3 3 3
Apples, pomace, dried 80
Apricots 7 3
Bananas 3
Beans, dry 0.2
Beans, succulent 20
Citrus, dried pulp 40
Corn, fodder 10
Corn, forage 10
Corn, grain 0.1
Cottonseed 0.1 3
Cranberries 10
Eggs 0.1
Figs 3
Grapefruit 5 5
Grapes 10 7
Grapes, pomace 40
Hops 15 3
Hops, dried 30 30
Lemons 5 5
Milk 0.08
NATIONAL TOLERANCES REPORTED TO THE MEETING (Continued)
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Milk, fat 2
Mint 50
Nectarines 4 3
Oranges 5 5
Passion fruit 3
Peaches 7 7 3
Peanuts 0.1
Peanuts, forage 10
Peanuts, hay 10
Peanuts, hulls 10 3
Pears 3 3 3
Plums (prunes) 7 3 3
Potatoes 0.1
Raisins 25
Sorghum, fodder 10
Sorghum, forage 10
Sorghum, grain 10
Strawberries 7 7 3
Walnuts 0.1
Meat, meat
by-products and fat
of cattle, goats, hogs,
horses, poultry and
sheep 0.1
Canadian authorities is that it is recovered through their
multiresidue procedure. It is eluted from Florisil in fraction 3 of
the Canadian procedure (15% acetone/hexane).
APPRAISAL
Propargite is an acaricide which has been used on a wide variety of
food crops and ornamentals since its introduction in 1967. Available
information indicates that the product is used in Australia, New
Zealand, Argentina, Mexico, France, South Africa, Italy and the U.S.A.
The primary manufacturer has submitted a report on supervised trials
on 26 crops, all of which were conducted in the U.S.A. Limited residue
data were received from New Zealand on peaches). U.S.A., Canada, New
Zealand and Australia have set national tolerances (MRLs) for
propargite.
Propargite is non-syrtemic by mode of action against mites, and data
indicate that residues which do occur are surface residues. Residues
on the harvested commodities are primarily propargite per se. No
significant metabolites or alteration products have been reported on
harvested crops. However, much of the evidence regarding the fate of
propargite on plants is indirect. It would be desirable to have 35S
or 14C radiotracer studies to confirm the degradation pathways in
plants.
Radioactively labelled propargite (14C) has been used in some animal
experiments, including studies on the rat, cow and chicken. Techniques
used to fractionate and identify the activity were not as definitive
as would be desired, but it is fairly certain that propargite is
hydrolysed in the body to tert-butylphenoxyoyclohexanol and
propargyl alcohol. It is probable that there is further degradation to
butylphenol and cyclohexanediol and excretion as conjugates in the
urine. However, some residues of unchanged parent compound do occur in
milk, body fat and briefly in liver. If any correlations are to be
made between the metabolism of propargite and the related compound
aramite, it may be significant to compare the toxic potential of the
hydrolysis product propargyl alcohol with that of the corresponding
hydrolysis product of eremite, ß-chloroethanol.
An analytical method suitable for regulatory purposes is available. It
is a gas chromatographic method (published) with flame photometric
detection that has been validated in government laboratories.
Propargite is also measured by the multi-residue method employed in
the Canadian surveillance programme. It has not been studies in the
U.S. Food and Drug Administration's multi-residue method.
RECOMMENDATIONS
Temporary maximum residue limits for propargite on the following
commodities are recommended.
Commodity Limit mg/kg Pre-harvest intervals on which
recommendations are based
(days)
Apple pomace 80
Alfalfa hay 75 28
Almond hulls 55 28
Alfalfa, fresh 50 28
Mint hay 50 14
Dried citrus pulp 40
Grape pomace 40
Dried hops 30
Raisins 25
Beans (in pod) 20 7
Corn fodder
and forage 10 30
Cranberries 10 14
Grapes 10 14-21
Peanut hay
and forage 10 14
Sorghum fodder
and forage 10 30 (if silaged)
Apricots, peaches,
plums, nectarines 7 14
Strawberries 7 3
Citrus 5 7
Sorghum grain 5 60
Apples, pears 3
Pigs 3 14
Milk (fat) 2
Beans, dry 0.2 28
Almonds 0.1* 28
Cottonseed 0.1* before bolls open
Eggs 0.1
Fat of poultry 0.1
Fat of meat 0.1
Maize (kernels) 0.1* 30
Milk, whole 0.1
Peanuts (kernels) 0.1* 14
Potatoes 0.1* 14
Walnuts 0.1* 14
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (Before July 1981)
1. A carcinogenic study
DESIRABLE
1. Mutagenicity studies
2. Observations in humans
3. Data from supervised trials in countries other than the USA
4. Information on the occurrence of residues on commodities in
commerce
5. Confirmation of the postulated degradation pathway in plants
using radio-labelled propargite.
REFERENCES
Carson S. (1963) Acute Oral LD50 in Rats. Unpublished report from
Food and Drug Research Lab., submitted by Uniroyal Chemcial, Inc.
Carson S. (1963) Subacute Feeding Studies with D-014 in Rats.
Unpublished report from Food and Drug Research Lab., submitted by
Uniroyal Chemical, Inc.
Haley, G., Kennedy, G.L. and Keplinger, M.L. (1972) Teratogenic Study
with Omite in Albino Rats. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Health Protection Branch (1977) Personal communication, Canada Bu.
Chem. Safety, Health Protection Branch to FAO Panel of Experts, 1977.
Holsing, G.C. (1968) 13-Week Dietary Feeding - Dogs. Unpublished
report from Hazleton Laboratories, Inc., submitted by Uniroyal
Chemical.
Jenkins, D.H., Kennedy, G. and Keplinger, M.L. (1972) Study with Omite
in White Leghorn Chickens. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Kennedy, G., Jenkins, D.H. and Keplinger, M.L. (1970) Metabolism of
14C-Omite in the Cow. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Ladd, R., Jenkins, D.H. and Keplinger, M.L. (1974) Tissue Residue
Study with Omite in Crossbred Swine. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Oser, B.L. (1966) Chronic (2-Year) Feeding Studies with D-014 in Rate
and Dogs. Unpublished report from Food and Drug Research Lab.9
submitted by Uniroyal Chemical.
PAM II Pesticide Analytical Manual, Vol. II, revised (1973); U.S.
Department of Health, Education and Welfare, Food and Drug
Administration.
Ryer, F.H. and Sullivany J.B. (1969) Rachiotracer Drug Metabolism
Study with Omite 14C. Unpublished report from Hazleton Laboratories,
Inc., submitted by Uniroyal Chemical.
Ryer, F.H. and Sullivany J.B. (1969b) The Fate of Omite 14C in the
Cow. Unpublished report from Hazleton Laboratoriesy Inc., submitted by
Uniroyal Chemical.
Sleight III, B.H. and Maceky K.J. (1972) 14C Omite Fish Accumulation.
Unpublished report from Bionomics, Inc., submitted by Uniroyal
Chemical.
Smith, K.S. and Roger, J.C. (1975) Omite Milk and Tissue Residue Study
in Dairy Cows: Unpublished report from Cannon Laboratories, submitted
by Uniroyal Chemical.
Sullivan, J.B. (1968) An Investigation of the In-Vitro and In-Vivo
Metabolism of Omite. Unpublished report from Hazleton Laboratories
Inc., submitted by Uniroyal Chemical.
Uniroyal Unpublished information (1977), Uniroyal Inc., to U.S.
Environmental Protection Agency.
Weir, RJ. (1967) Acute Dermal Application to Rabbits. Unpublished
report from Hazleton Laboratories Inc., submitted by Uniroyal
Chemical.
Westlake, W.E., Gunther, P.A. and Jeppson, L.R. J. Agr. Fd. Chem.,
19:894.
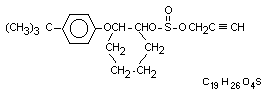 Other information on identity and properties
The technical grade material is a light to dark brown liquid of low
volatility. It is miscible with most organic solvents. Solubility in
water is about 0.5 mg/kg. The basic manufacturer states the
composition of the technical product to be:
85% active ingredient
7% "high molecular weight sulphites"
5% "unreacted starting materials"
1% propylene oxide stabilizer
(remainder unidentified)
Propargite is commercially available in 68.1% and 755% emulsion
concentrates, a 30% wettable powder, and a 4% dust. The manufacturer
states that the technical product shows no degradation after 12 months
when stabilized with propylene oxide. The odour of SO2 indicates
breakdown.
The product was introduced in the U.S.A. on an experimental basis in
1967 as a replacement for Aramite, another product of the same
manufacturer, which was withdrawn from use on food crops in the U.S.A.
The two compounds have similar chemical structures and properties.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Extensive degradation of propargite could be shown in in vitro as
well as in vivo experiments.
In vitro studies with liver homogenates showed that the major
primary metabolites appear to be propargyl alcohol, the glycol ether
and their conjugates, which are further degraded to secondary
metabolites (Sullivan, 1968).
In in vivo metabolism studies in rats that were orally treated with
a single dose of 200 mg/kg propargite, propargyl alcohol and glycol
ether were also found as primary metabolites in the excreta as well as
in tissue samples. Following analysis of the excreta and tissues only
about 5% of the administered dose was accounted for as unchanged
propargite or the primary metabolites, indicating that the primary
metabolites are further degraded (Sullivan, 1968).
The oral administration of a single close of 271 mg/kg benzene ring
labelled 140 propargite to rats was followed by fast excretion of the
radiocarbon. Within 72 hours postdosage, 47% of the administered dose
was eliminated via the urine. 32% via the faeces. The total carcass
contained approximately 90% of the administered radioactivity.
Identified metabolites excreted with the rat urine and faeces were
tert-butylpyrocatechol and glycol ether (Ryer and Sullivan, 1969a)
In cows treated with a single oral close of 4 mg/kg b.w. combined
non-radioactive and 14C labelled propargite a similar excretion
pattern was found. 54% of the administered dose was eliminated with
the urine, 17% with the faeces. Approximately 0.3% was found in the
milk. Identified metabolites were tert-butylpyrocatcohol, glycol
ether and tertbutylphenol (Ryer and Sullivan, 1969b).
In a feeding study, dairy cows were maintained on a diet containing
14C-labelled and unlabelled propargite at dosage levels of 3 and 20
ppm for 12 consecutive days. Peak urinary excretion observed at the
seventh test day reached values of 4 and 23 mg/kg respectively for the
3 and 20 ppm dietary level; peak faecal excretion was approximately 1
and 4 mg/kg. Maximum milk residue values reached on the fourth test
day were 0.02 and 0.06 mg/kg at the 3 and 20 ppm dose levels
respectively. Residues in selected tissues ranged from 0.02 to about
1.3 mg/kg (Kennedy et al., 1970).
Similar results were obtained after daily administration of a diet
containing 50 and 100 ppm propargite to lactating cows for a period of
27 days (Smith and Roger, 1975).
Pigs were maintained on a diet containing propargite at dosage levels
of 20 and 100 ppm for 30 days. Residues in kidney and liver were below
the detection limit of 0.1 mg/kg at both feeding levels and residues
in muscle and fat ranged from below 0.1 to about 2 mg/kg (Ladd et al.,
1974).
Residues studies in hens that were treated with 3, 10 and 30 ppm 14C
propargite in their diet for 30 days showed residue levels in eggs
from 0.13, 0.49 and 0-145 µg/kg respectively at the 3, 10 and 30 ppm
dose levels. Residues in muscle ranged from 2-12 µg/kg, liver 18-174
µg/kg kidney 2-25 µg/kg and fat 7-116 µg/kg depending on exposure
level (Jenkins et al., 1972).
After continuous exposure to a concentration of 0.025 mg/l 14C
propargite in water for 35 days tissue residues in edible portions of
fishes were about 2 mg/kg. After transfer to uncontaminated water 70%
of the radioactivity present in the edible tissues was eliminated
within 14 days (Sleight III, 1972),
The in vivo metabolism studies in several animal species confirmed
the rapid degradation of propargite already shown in in vitro
experiments. The metabolic reactions are hydrolysis of the parent
ester followed by other cleavage. The formed products are polar
alcoholic and phenolic compounds which can rapidly be excreted or
further metabolized and incorporated into natural tissue constituents
by biochemical reactions which are well documented in the literature.
See also the section "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on teratogenicity
Female rats were orally dosed with 0, 50 and 100 mg/kg from day 6
through 15 of gestation. The treatment had no effect on maternal body
weight, mortality or fertility. No reproductive adverse effects were
found with respect to implantation and resorption sites. Embryonic and
foetal development was not affected by the treatment
(Haley et al., 1972).
Special study on reproduction
Rat
See "Long term study".
Special study on carcinogenicity
Rat
See "Long term study".
Dog
See "Short term study".
Acute toxicity
Species Sex Route LD50 References
mg/kg
Rat M, F oral approx. 2200 Carson, 1963
Rabbit M, F dermal approx. 3200 Weir, 1967
Rat
Groups of 5 male and 5 female rats were treated for 90 days with
amounts of propargite in their diet to provide daily intakes of 10,
20, 40, 100 and 200 mg/kg b.w. corresponding to dietary levels in
adult animals of 200, 400, 800, 2000 and 4000 ppm respectively. The
control group existed of 15 male and 15 female rats. Treatment with
2000 and 4000 ppm caused a dose-related growth retardation and
reduction of food intake. Haematological examinations and clinical
chemistry tests revealed no abnormalities. At 2000 and 4000 ppm the
relative liver weights were increased, the relative kidney weights
were increased only in the 4000 ppm groups. Gross and microscopic
examinations of tissues disclosed no treatment-related pathological
alterations (Carson, 1964).
Dog
Groups of 3 male and 3 female dogs were maintained on a diet
containing propargite at dose levels of 0 and 2000 to 2500 ppm
(increase of dosage after 3 weeks) for 90 days. The treatment did not
affect appearance, behaviour, results of haematological. and clinical
chemistry tests and urinalyses. In most treated dogs reduced food
consumption and body weight loss and an increase of relative liver and
kidney weights were observed, No compound-related alterations were
found macroscopically. Histopathological examinations revealed an
increased amount of pigment in the liver reticuloendothelial cells and
increased haemosiderosis of the spleen (Holsing, 1968).
Groups of 8 dogs were fed a diet containing propargite at 100, 300 and
900 ppm for 24 months. The control group consisted of 12 animals. The
treatment had no effect on growth rate, results of clinical laboratory
studies, urinalyses and organ weights. Gross and microscopic
examination did not reveal abnormal pathological findings. No tumours
were found (Osert 1966).
Long term Study
Rat
Groups, each comprising 25 male and 25 female rats were fed dietary
levels of propargite to provide daily intakes of 5, 15 and 45 mg/kg
b.w. corresponding to adult levels of 100, 300 and 900 ppm for a
period of 24 months. The control group consisted of 37 animals of each
sex. After 26 test weeks without noticeable evidence of a
treatment-related effect, a supplementary study was started with a
control-and a test group comprising 25 male and 25 female rats
receiving 100 mg/kg b.w. corresponding to 2000 ppm for 78 weeks. Rats
that died during the study and those sacrificed after 104 and 78 weeks
were examined grossly and microscopically. The treatment had no effect
on appearance, behaviour, growth rate and mortality at the dietary
levels up to and including 900 ppm. At the 2000 ppm level at the end
of the study there was a reduction of food consumption and also a
reduction of the body weight gain of about 30%; mortality of the male
rats increased to 32% compared to 7% of the corresponding control.
Haematological examinations and clinical laboratory studies did not
reveal abnormal findings. Absolute and relative liver and kidney
weights of the animals treated with dietary levels of 100, 300 and 900
ppm which died or were sacrificed after 104 weeks showed inconsistent
variations without any dose-relationship. At 2000 ppm an increase of
the relative liver and kidney weights of about 30%. were observed.
Gross autopsy findings in 1 male (of 25) and 7 females (of 23) in the
2000 ppm group were enlarged and/or dark red lymph nodes which in some
cases were involved in abdominal masses. In the corresponding control
group 2 male rats of 29 animals showed this alteration. No
dose-dependent increase of the frequency of this lymph node alteration
was found at the 100, 300 and 900 ppm levels. A variety of benign and
malignant tumours was observed in the control and treated groups. No
dose-relationship of the frequency of tumours was found. Four sarcomas
were found in rats sacrificed after 78 weeks ingestion of 2000 ppm,
whereas in the corresponding control group no sarcomas were seen
(Oser, 1966).
Additionally a 3-generation reproductive was included in the 2-year
feeding experiment with 20 pairs of male and female rats from the
control and 100 ppm test groups. Dosage was raised to 300 ppm in the
F2 pups at the time of weaning. The treatment revealed no abnormal
findings (Oser, 1966).
COMMENTS
Propargite is readily absorbed from the gastrointestinal tract and is
excreted rapidly as metabolites which are formed mainly by hydrolysis
of the sulphite ester.
After feeding a diet containing 20 ppm of propargite the residues in
milk or in tissues were of the order of 0.1 mg/kg.
The compound is not teratogenic in the rat.
In a 3-generation reproductive study no adverse effects were revealed
at concentrations up to 300 ppm. This concentration is therefore
regarded as a no-effect level.
In a 2-year feeding study with up to 900 ppm in dogs which included
extensive laboratory and histological investigations, no adverse
effects were noted. A 2-year feeding study in rats up to 2000 ppm
showed appreciable but not dose-related variations in the relative
liver and kidney weights in all treated groups. However, no major
adverse effects were seen. Owing to a poor survival rate and the
termination of the study with the high dose animals after 78 weeks,
the finding that the tumour incidence was not elevated is of limited
value. This study was not considered to fulfil the criteria for an
acceptable lone-term study. Therefore a safety factor of 200 was used.
Propargite is structurally related to Aramite, a compound which is
suspected of being a carcinogen. Although there are no indications
that propargite acts similarly, the need for satisfactory
carcinogenicity studies was expressed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 300 mg/kg in the diet, equivalent to 15 mg/kg bw
Dog: 900 mg/kg in the diet, equivalent to 22 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Propargite is an acaracide said to be effective on a variety of mites
on food crops and ornamentals. Table 1 shows the use patterns
registered in the U.S.A. Information has been received that the
product is also used in Italy, Australia, New Zealand, South Africa,
Argentina, Mexico, and France. The government of the Netherlands has
informed the Meeting that the compound was used until 1976, at which
time its use was discontinued. A country statement was received from
New Zealand regarding uses and tolerances. The uses permitted in these
countries generally parallel those shown in Table 1. Significant
exceptions are included in the footnotes.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trails on twenty-six crops (Uniroyal, 1977). Most of the trials were
conducted in the U.S.A. A country statement was received from New
Zealand with data on peaches. Analyses in all U.S. trials, the
procedure described under "Methods of residue analysis" (PAM II,
1973). Analyses of untreated controls (crop blanks) were reported in
each experiment, along with the recovery of propargite from fortified
controls. Controls were generally reported as 0 and recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form when feasible.
The use patterns employed in Mexico and France on apples, peaches,
plums and citrus differed significantly from the other countries. In
the absence of residue data reflecting good agricultural practice in
those countries, it was not possible to predict residue levels. The
recommended DIRLs for apples, peaches, plums and citrus therefore are
based on the national use patterns which can be related to supervised
residue trails.
Alfalfa
There are no national tolerances or registrations for propargite use
on alfalfa. However, the submission to the Meeting contained some
residue data on alfalfa hay, fresh forage and trash. Single and
multiple applications were made at 1.4 to 4.7 kg a.i./ha. Residues on
fresh forage ranged 0.0 to 328 mg/kg at 28 and 0 day intervals from
treatment, respectively. Residues on hay and trash ranged from 2.6 to
100 mg/kg with intervals from treatment of 22 to 52 days. With a 28
day pre-harvest interval, MRLs of 50 and 75 mg/kg on fresh alfalfa and
alfalfa hay, respectively, would be adequate.
TABLE 1. Registered use patterns for propargite (USA)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Almond 30% WP 0.03-0.05%(4) 2 28(5) low volume
68.1% EC 2.5-3.4 kg/ha 1 post-harvest aerial
application
only
Apples 30% WP 0.04-0.05(1)(6) 3 7(2)(7)(12)
Apricots 30% WP 0.05%(4) 2 14(5) low volume
68.1% EC 1.7-2.5 kg/ha 2 post-harvest aerial
application
only
Cranberries 68.1% EC 1.6 kg/ha 1 14
Beans 75% EC 2.8 hg/ha(4) 2 7(fresh)(5)
28 (dry)
Corn 75% EC 1.9 kg/ha 1 30
(field)
Cotton 75% EC 0.85-1.9 kg/ha 3 before bolls open
Figs 30% WP 0.03-0.05%(3)(4)(11) 2 14
Grapes 30% WP 1.7-3.0 kg/ha 2 21
4% Dust 0.2 kg/ha 2 14
Grapefruit, 75% EC 0.025-0.05(4)(8) 2 7 (5)
oranges
Lemons 30% WP 0.05% (8) 2 7
Hops 68.1% EC 1.7 kg/ha(4) 2 14 (5)
30% WP 1.7 kg/ha 2 14
Mint 68.1% EC 1.7-2.5 kg/ha 2 14
Nectarines 30% WP 0.05% (4) 2 14 (5)
Peaches 30% WP 0.04-0.05%(4)(8) 2 14(5)(10)(13)
Peanuts 30% WP 1.0-1.7 kg/ha 2 14
Pears 30% WP 0.04-0.05%(8)
Plums 30% WP 0.04-0.05(4)(8) 2 14(5)(10)
Potatoes 75% EC 1.4-2.4 kg/ha 2 14
68.1% EC 1.7-2.5 kg/ha 2 14
TABLE 1. (Continued)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Sorghum 75% EC 1.5-1.9 kg/ha 1 30 silage
60 grain
Strawberries 30% WP 1.7-3.4 kg/ha(9) 3 3
4% Dust 1.8 kg/ha 3 3
Walnuts 68.1% EC 0.03-0.05% (4) 2 14 (5)
(1) France 0.12, Italy 0.06%
(2) France 21, Italy 15, New Zealand 14
(3) France 0.6-0.9 kg/ha
(4) Italy 0.06%
(5) Italy 21
(6) Mexico 0.08-0.12%
(7) Mexico 28
(8) Mexico 0.12%
(9) East U.S.A. 0.84 kg/ha
(10) Argentina 21
(11) Argentina 0.05%
(12) South Africa 14
(13) South Africa 21
Almonds
Field trials were conducted in California: 63 samples of nut meats
were anlayzed. Residues on the nut meats from two applications of 0.03
and 0.06% sprays were < 0.1 mg/kg at post-treatment intervals of 21
to 28 days.
Residues on almond hulls from treatment with 0.05% spray (30% WP)
followed by 3.4 kg a.i./ha (68.1% EC) ranged from 5.2 to 31 mg/kg with
pre-harvest intervals of 28-35 days. Under good agricultural practice,
residues are not likely to exceed 55 mg/kg.
Apples
Field trials were conducted in nine U.S.A. states, South Africa and
Australia, some 452 samples being analysed. The residue data are
summarized in Table 2. All controls were reported zero. Residues from
applications of up to 0.06% spray after 7 days were less than 3 mg/kg.
Residue data indicate that residues remain associated with the apple
pomace and are not destroyed in drying. Residues in the juice were
barely detectable (ca. 0.02 mg/kg) while residues in the wet and dried
pomace were 16-21 mg/kg and 56-80 mg/kg, respectively. Thus, residues
concentrate by factors of 15-22 in the manufacture of dried pomace
from treated apples (Uniroyal, 1972), An MRL of 80 mg/kg in dried
apple pomace is therefore appropriate.
TABLE 2. Range of residues in apples, mg/kg.
Application rate, Interval (days, last treatment to harvest)
% 0-1 6-7 14 21 28
0.03 0.1-3.1 0.6-1.8 0.2-1.0 0.2-0.7 0.2-0.9
0.06 0.3-6.3 0.5-1.6 0.3-2.4 0.5-1.1 0.2-0.6
Apricots, peaches and plums
Field trials were conducted in California, Connecticut, Georgia, North
and South Carolina, Maryland, Michigan, Pennsylvania, Washington, New
York and New Zealand. Some 385 test samples of fresh fruit and 65
controls were anlaysed from one and two applications at four dosage
rates, including the maximum and twice the maximum label rates (Table
1). The residue data are summarized in Table 3. Only one residue on
peaches was greater than 7 mg/kg at 14 days after treatment at the
maximum rate. Residues will not exceed 7 mg/kg from sprays up to
0.06%, provided that treatments are not made within 14 days of
harvest.
A study was conducted on plums (fresh prunes) using a standard
commercial drying process to determine residues on dried prunes. The
average residue value for the dried fruit was ca. 20% of the average
value for the fresh fruit. Thus no additional MRL is needed for the
dried fruit.
TABLE 3. Range of residues on apricots, peaches and plums, mg/kg
Application rate. Interval (days, last treatment to harvest)
% 0-1 6-7 13-14 21-23
0.03 0.4-12 0.2-7.0 0.4-10.0 0.3-5.0
0.05 0.7-6.7 2.6-14.0 0.6-5.6 0.0-1.7
0.06 1.3-17.0 1.3-8.7 0.2-5.5 0.3-9.3
0.10 1.6-27.0 0.3-8.4 0.8-11.0 0.8-3.5
Beans
Field trials were conducted in the states of Washington, California
and Idaho on snap beans, succulent lima beans and dry beans. 52
analyses were reported. Single and multiple applications were made at
the maximum and at twice the maximum label rate (Table 1), by aerial
and ground application. The residues are summarized in Table 4 for
fresh and dry beans, 7 and 28 days after the last treatment,
respectively. No residue data were submitted for bean vines; however
there is a restriction against the feeding or foraging of treated bean
vines. The only other feed item, cannery waste, would consist of cull
beans with some bean vines and pods present as a result of mechanical
harvesting. Residue levels would not be likely to exceed 20 mg/kg on
cannery waste.
TABLE 4. Range of residues in beans, mg/kg
Application rate, kg a.i./ha
2.8 5.6
Fresh (7 days after last treatment) <0.2-15 <0.2-12
Dry (29 days after last treatment <0.1-0.1 <0.1-0.24
Maize
Residue data for maize were made available from five U.S.A. sites, two
in California and one each in Colorado, Texas and Nebraska. Following
applications at the maximum and twice the maximum label rates (Table
1), silage stage maize sampled 28-35 days after application contained
maximum residues of 2.4 and 18 mg/kg, respectively; maize stover,
sampled after 47-105 days, contained maximum residues of 4 and 11
mg/kg, respectively. Interpolation of the 2X data shows that MRLs on
maize forage and fodder of 10 mg/kg are appropriate.
No detectable residues (< 0.1 mg/kg) were found on either the grain
or ears of maize sampled 60-105 days after the above applications. No
data on grain reflecting a 30 day pre-harvest interval were submitted.
However, since propargite does not translocate to any extent, residues
on grain are not expected to exceed 0.1 mg/kg.
Cotton
Five field studies from locations in California, Arkansas and
Mississippi were reported. The studies reflect treatment at the
maximum and twice the maximum label rate (Table 1). Analyses were
performed on 96 samples of cottonseed and processed products, hulls,
oil, meal and soapstock. All values on cottonseed were reported as
< 0.1 mg/kg. All samples of meal and soapstock contained
< 0.05 mg/kg as did all but on refined oil sample which contained a
level of 0.1 mg/kg after treatment at an excessive rate. Almost all of
the hull samples had low but detectable residues, 0.03-0.06 mg/kg.
Thus, an MRL of 0.1 mg/kg is appropriate for cottonseed. There is a
restriction in the U.S.A. against feeding treated foliage or cotton
trash to livestock.
Cranberries
Seven studies were reported from Massachusetts, with a total of 36
analyses. The studies reflect applications at 1.68 kg a.i./ha (4 by
ground and 3 by air) with one study at 3.36 kg a.i./ha. Several of the
studies show decline rates (at 7, 14 and 21 days). The residue
declines rapidly between 0 and 7 days and then shows little further
decline from 7 to 21 days. At the recommended rate (Table 1), residues
are not likely to exceed 10 mg/kg. Residues are summarized in Table 5.
TABLE 5. Range of residues in cranberries, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 21
1.68 2.2-11 <0.1-1.9 <0.1-7.1 0.1-1.0
3.36 3.3-14.9
Figs
The residue data for figs consists of four studies made in California,
including two decline studies. Various applications were made
including the maximum and twice the maximum label rate (Table 15).
Residues on fresh figs are summarized in Table 6. While the data are
limited, it is not likely that residues will exceed 3 mg/kg at 14 days
after treatment.
TABLE 6. Residues on figs, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0-1 7 13-14 21 28
1.27 3.3 1.9 0.8 0.45
2.02 1.6-2.8 1.0-1.4 0.2-1.4 0.3-0.6
4.04 2.5-5.3 2.2-3.2 0.7-4.2
Grapefruit, oranges and lemons
Field trials were conducted in Texas, Florida and California. Some 281
harvest samples were analyzed. One and two applications were made at
the recommended and twice the recommended rates (Table 1). The results
are summarized in Table 6. For applications up to 0.06% spray with a
7 day interval, 5 mg/kg would not be exceeded.
Washed oranges bearing residues of 1.9 mg/kg were processed to dried
pulp by a procedure simulating the commercial process. The residue in
the dried pulp was 15.5 mg/kg. The residue in the dried pulp is about
eight times that on the whole fruit, so 40 mg/kg is an appropriate
MRL.
TABLE 6. Range of residues in grapefruit, oranges and
lemons, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 28
0.03 0.3-0.5 0.2-0.8 0.2-0.3 0.9-1.0
0.05 1.7-3.6 0.2-4.8 1.1-2.1 0.6-3.1
0.06 1.0-1.4 0.9-2.5 0.0-0.9 0.6-1.2
0.10 1.0-5.1 0.8-7.2 1.2-3.2 1.2-2.9
Grapes
Residue trials were conducted in Washington and California, with
analyses of 254 samples of grapes. A wide range of experimental
conditions in the various field tests and the resulting variation in
residues preclude a tabular summary of the data. No residues exceeded
10 mg/kg from the maximum recommended rate with a 21 day interval
after treatment.
A residue concentration factor of 2.5 was observed in the commercial
production of raisins from fresh grapes. Thus a 25 mg/kg MRL for
raisins would be appropriate. The data showed a concentration of
residues on dried grape pomace to 4 times that found on fresh grapes,
therefore, 40 mg/kg would be appropriate for grape pomace.
Hops
Eight field trials were conducted in the State of Washington. Residues
on green hops resulting from three applications at the maximum and
twice the maximum label rate (Table 1) are summarized in Table 7.
Residues on green hops from good agricultural practices are not likely
to exceed 15 mg/kg. Residues on dried hops ranged from 11 to 30 mg/kg
14 days after treatment. A 30 mg/kg MRL would be appropriate for dried
hops since residues from two applications will probably be somewhat
lower than those noted above.
TABLE 7. Range of residues on green hops, mg/kg
Application rate, Interval (days last treatment to harvest)
kg a.i./ha 0 6-7 13-14 20
1.7 24-50 5-17 6-15 2-6
3.4 36-92 24-66 11-43 6-24
Mint
Field studies were conducted in Washington, Oregon and Idaho. A total
of 89 analyses were made on fresh hay, spent hay and mint oil,
including controls. In four studies, two applications were made at
rates of 2.5 and 5 kg a.i./ha resulting in maximum residues of 19 and
50 mg/kg respectively, 15-17 days after treatment. Three residue
decline studies were also submitted. Samples were taken 0, 1, 2 and 4
weeks after a single aerial application at 1 and 2 times the maximum
label rate (Table 1). From a decline curve based on these data,
residues resulting from the recommended use are ca. 50 mg/kg on fresh
mint hay.
Five samples of fresh mint hay bearing residues of 8.7-50 mg/kg were
fractionated by steam distillation and the resulting mint oil and
spent hay contained residues of 1.6-7.9 mg/kg and 12-97 mg/kg
respectively. Residues in mint oil are not likely to exceed those on
fresh mint hay and there is a restriction against feeding spent mint
hay to livestock.
Nectarines
Seven trials were conducted at locations in California and
Pennsylvania. One or two applications were made at one and two times
the maximum label rate (Table 1). The residue data are summarized in
Table 8. Residues were no higher than 3-4 mg/kg at the prescribed
pre-harvest interval. However, extrapolation of data from peaches,
apricots, and plums would indicate that a 7 mg/kg MRL would be
appropriate.
TABLE 8. Range of residues in nectarines, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 6-7 13-15 20-21
0.05 0.66-7.3 0.24-3.1 0.44-3.4 0.39-1.3
0.10 1-3-10.5 0.34-5.1 0.91-3.4 0.41-1.5
Peanuts
Residue studies were conducted in Georgia, Florida, North Carolina,
Oklahoma, Mississippi and Arkansas. Two applications were made in all
but four decline studies on peanut vines, where only one application
was made. Dosages ranged from 0.6 times to twice the maximum
recommended rate (Table 1). Six of the studies reflect a 14 day
preharvest interval, but in the other ten studies intervals were from
19 to 66 days.
Peanut kernels
All residue values were reported as < 0.1 mg/kg. Since no detectable
residues were found in peanuts, no MRLs are needed for peanut
by-products, meal, oil and soapstock.
Peanut hulls
Residues were < 0.1 mg/kg in five of seven studies. Residues of
0.14 mg/kg, 0.3 mg/kg and 1.2 mg/kg were found after applications at
twice, 6 times and 1.2 times the recommended rates respectively, in
the other two studies.
Peanut vines and hay
Residues were less than 10 mg/kg 14 days after application at the
maximum rate. The data indicate that residues decline rapidly from a
maximum of 38 mg/kg at 0 days to 3.6 mg/kg at 14 days.
Pears
Four field studies were conducted in California, Pennsylvania,
Michigan and Washington, one and two applications were made with 0.03
and 0.06% sprays. The residue data are summarized in Table 9. All
residues were less than 3 mg/kg, 14 days after treatment.
TABLE 9. Range of residues in pears, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 21
0.03 0.6-1.6 0.3-1.2 0-2.7 0.1-2.4
0.06 2.2-4.1 0.9-2.6 0.3-2.2 0.1-1.7
Potatoes
Seven residue studies were made available, four from Washington and
one each from Idaho, Vermont and Illinois. After two applications at
rates of 1.5 to 4.5 kg a.i./ha, all residues were less than the limit
of detection of the method (0.1 mg/kg) 14 days after treatment.
Sorghum
Field studies were conducted in Texas, Nebraska and Iowa on grain
sorghum. Applications were made at the maximum and twice the maximum
recommended rates (Table 1). Samples of grain and forage or fodder
were harvested at 28, 30, 44 and 50 days after application. Residues
in forage ranged from < 0.1 to 4.1 mg/kg. Residues in forage and
fodder are not likely to exceed 10 mg/kg 30 days after treatment.
Residue levels in grain ranged from < 0.1 to 2.4 mg/kg 60 days or
more after treatment. Thus an MRL of 5 mg/kg with a 60 day interval
after treatment is appropriate for sorghum grain.
Strawberries
Field trials were conducted in California, Louisiana and Florida.
Strawberries treated with two formulations in California, reflecting
three applications at the maximum recommended rate (Table 1) had
residues ranging from 0.19 to 4.0 mg/kg for 3 days after treatment.
Residues from applications of 0.84 and 1.7 kg/ha in Louisiana and
Florida are summarized in Table 10. Under good agricultural practice,
residues are not likely to exceed 7 mg/kg.
TABLE 10. Range of residues in stramberries, mg/kg
Application rate Interval (days, last treatment to harvest)
kg a.i./ha 0 3 7
1.7 0.9-2.0 0.3-3.8 0.2-0.7
3.4 1.4-4.1 0.7-9.2 0.4-1.1
Sugar beets
There are no national tolerances or registrations for propargite on
sugar beets; however, this use is pending in the U.S.A. The submission
to the Meeting therefore contained some residue data on sugar beet
tops, roots and by-products. Sugar beet root samples from Idaho,
Washington and California reflecting two treatments at 2.8 and 5.6 kg
a.i./ha and a 21 day interval from treatment# showed residues ranging
from 0.05 to 0.23 ind/kg and 0.13 to 0.28 mg/kg respectively.
In a residue decline study, on sugar beet tops, a single application
at 2.8 kg a.i./ha yielded residues of 40-114 mg/kg at 0 days.
20-80 mg/kg at 14-17 days and 2.4-34 mg/kg at 27-28 days. Adiitional
data for tops representing two ground treatments at 2.8 and 5.6
kg a.i./ha show residues ranging from 11-24 mg/kg and 19-59 mg/kg
respectively (21 day interval).
In order to obtain data on processing fractions, sugar beet roots
bearing 0.1 mg/kg residues were processed to wet and dry pulp,
molasses and sugar. Propargite residues in wet pulp, molasses and
sugar were less than or equal to 0.1 mg/kg. The data for dry pulp show
a maximum residue concentration factor of 5, with residues from
0.1-0.5 mg/kg.
Tea
There are no national tolerances or registrations for propargite in
tea, however, a tolerance for this use is pending in the U.S.A. The
submission to the Meeting contained some residue data on dried tea
leaves, both green and black. The data are summarized in Table 11.
TABLE 11. Range of residues in tea, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 28
0.31 <0.1-0.3 <0.1-0.2
0.75 48-178 1.1-4.2 <0.1-0.2 <0.1
Walnuts
Six field trials were conducted at locations in California. Both
30% WP and 75% EC formulations were applied at the maximum recommended
rate (Table 1). No residues were detected in any of the walnut meat
samples 45 to 48 days after treatment. Although the interval is
greater than that recommended (14 days), since propargite is
non-systemic, any residues found on nut meats would be expected to
arise as a result of contamination in de-hulling and de-shelling
processes. Under good agricultural practice, an MRL of 0.1 mg/kg (at
or about limit of determination) would be appropriate. There is a
restriction against grazing or feeding livestock on cover crops growm
in treated orchards, therefore no feed items are involved in this use.
Meat, milk, poultry and eggs
Propargite is used on a number of crops which yield by-products used
commercially for animal feeds. Primary sources would be dried citrus
pulp, sorghum grain, forage and fodder and maize forage and fodder.
Some additional contribution could occur from dried pomace, almond
hulls and dried grape pomace. Per this reason, some animal feeding
studies were submitted. These are also referred to in the section
"Biochemical aspects", where references are given.
In one study, cattle were fed at levels of 3 and 20 ppm in the total
dry diet for 12 days. Maximum residues in milk (whole milk basis) were
0.059-0.067 mg/kg. The latter is equivalent to 1.7 mg/kg on the fat
basis. Maximum residues in tissues were as follows: liver, 1.3 mg/kg,
kidney, 0.27 mg/kg, fat, 0.21 mg/kg and muscle, 0.18 mg/kg.
In a second study, cows received 0, 50 and 100 ppm in the diet for 27
days. Analysis of milk showed all samples at both feeding levels to be
< 0.08 mg/kg. Residues ranged up to 1.6 mg/kg on a fat basis.
Residues in muscle, kidney and liver from both feeding levels were
40.03 mg/kg (limit of determination). Residues in fat ranged from 0.32
to 0.42 mg/kg at the 10 and 100 ppm feeding levels, respectively.
Poultry were fed 3, 10 or 30 ppm in the diet for 30 days (3 chickens
in each group). Eggs were sampled daily and the animals were
sacrificed 24. hours after treatment. Maximum levels In eggs were
0.013 mg/kg at the 3 PPM level, 0.043 mg/kg at the 10 ppm feeding
level, and 0.106 mi/kg at the 30 ppm level. Liver tissue had the
highest residue levels ranging from 0.047 to 0.089 mg/kg at the 10
diet level and from 0.17 to 0.18 mg/kg at the 30 diet level.
Hogs were fed 20 and 100 ppm in the total diet. Two hogs at each level
and one control were maintained for 30 days. No residues
(< 0.1 mg/kg) were found in liver or kidney. No residues were found
in muscle at the 20 ppm level but up to 0.23 mg/kg at the 100 ppm
intake. In fat, residues up to 0.18 and 0.2 mg/kg were found at the 20
and 100 ppm intakes, respectively.
The studies show that residues may transfer to meat, milk, poultry and
eggs. A mixed ration for cattle could consist of 15% dried apple
pomace, 15% dried citrus pulp and 70% sorghum grain, forage and fodder
or maize forage and fodder. This diet could theoretically contain
propargite residues of ca. 25 mg/kg.
A maximum possible intake of 10 mg/kg could result from the hogging
down of peanut fields and other swine feed items such as maize grain
and cooked potatoes.
Poultry feed items, sorghum grain, grape pomace, maize grain and
cooked potatoes, could contribute ca. 8 mg/kg propargite to the diet.
Residues are unlikely to exceed 2 mg/kg in milk fat and 0.1 mg/kg in
the meat, meat by-product, and fat of cattle, goats, hogs, horses,
poultry and sheep.
FATE OF RESIDUES
In animals
The available data on metabolism by animals are more conclusive than
those on metabolism by animals are move conclusive than those on
metabolism by plants. See also "Biochemical aspects."
Propargite -14C feeding tests have been conducted on a number of
animals including the rat, cow, pig and chicken. Additional
information on degradation was available from in vitro experiments
with buffered liver homogenates.
A general metabolism scheme has been proposed for animals, part of
which has been verified experimentally, and part of which is based on
analogy with biochemical reactions reported in the literature.
Identification of some metabolites was by TLC comparison with prepared
standards. In other cases less positive identification was made by
partition co-effecients (p values) and solvent extraction scheme. In
cow liver the bulk of the 14C activity was identified only as polar
metabolites.
The experiments apparently were carried out over a number of years,
the studies in some animals being repeated with refinements in
analytical techniques. None of the work on propargite submitted to the
Meeting has been published. Certain general conclusions appear
justified. (a) Propargite is metabolized in animals to polar compounds
which are excreted, principally in the urine. (b) There is not a
pronounced tendency to storage or accumulation in the body, but the
compound is not completely metabolized because the parent compound was
the principal residue in the milk of cows, was tentatively identified
as a portion of the 14C activity in animal fat, and was found in
excreta. (c) The parent compound is detected in the liver only for
short periods after ingestion. d) The primary hydrolysis products, the
glycol ether and propargyl alcohol, are further degraded. (e) Some
polar metabolites are in conjugated forms.
A list of the metabolits and the sites in which they were reported is
given in Table 12. The sequence of the metabolic scheme is not
definitely known.
TABLE 12. Propargite metabolites
Compound Found in
Parent Cow: butter fat, body fat (1)
Rat: liver (1)
liver homogenate (1)
Glycol ether Cow: urine, faeces, milk (2)
Rat: faeces, liver (1)
liver homogenate (1)
Propargyl alcohol liver homogenate (1)
Butylphenol Cow: urine (2)
Rat: liver (1)
liver homogenate (1)
Butylcatechol Cow: urine (2)
Rat: urine, faeces (2)
Cyclohexandiol urine (2)
"Polar metabolites" Rat: liver (2)
Cow: liver (2)
(1) Identified by GLC or TLC
(2) Tentatively characterized by solvent
fractionation scheme, p values, literature analogies.
In plants
Propargite is generally considered to be "non-systemic" by mode of
miticidal action. According to information made available to the
Meeting aged residues are primarily surface residues of the parent
compound (Uniroyall 1977). Residue decline was attributed to
volatilization of the parent compound and hydrolysis to propargyl
alcohol and the "glycol ether",
2-(p-tert-butylphenoxycyclohexanol). The two hydrolysis products
have not been detected in samples by chemical analysis at a detection
level of 0.1 mg/kg. In experiments on bean plants to investigate
possible photolytic degradation, the same two compounds could not be
detected. It has been suggested that if the glycol ether and propargyl
alcohol are intermediates in the degradation scheme, their presence is
fleeting because of their volatility.
Other evidence cited in the manufacturer's submission on the behavior
of propargite in plants was: (a) comparison of surface and pulp
analysis of apples showed no absorption (of parent); (b) mites
confined to upper leaf surfaces were not affected by sprays on the
lower surfaces (c) there was no effect on mites of an adjacent leaf
when on leaf of a bean plant was treated; (d) volatility losses of the
parent compound under field conditions have been correlated with model
laboratory experiments (loss from aluminum foil).
The above experiments partially support the idea that propargite is
not translocated in plants and that the residue of concern is
propargite per se. However, much of the evidence is indirect, and it
would be desirable to have conventional metabolism studies on some
representative crops, using 35S and/or 14C, to confirm the fate of
propargite in plants.
In soil
Only a summary was made available (Uniroyall 1977). The half-life in a
variety of soils was said to range from 2 to 18 weeks. Leaching is
negligible. Laboratory and field studies have shown that propargite is
hydrolysed in soil. The major soil degradation product found was the
glycol ether.
Summary, metabolism
The available metabolism studies do not firmly identify the residue
components in plants or animals. However, there is sufficient
information to indicate a probable metabolic scheme. It is fairly
certain that the primary metabolites are the hydrolysis products
tert-butylphenoxycyclohexanol and propargyl alcohol. It is probable
that further breakdown to butylphenol and cyclohexanediol occurs. If
correlations are to be made between metabolic schemes and toxic
potential for propargite and aramite, it should be noted that both
compounds hydrolyze at the sulfite ester, aramite yielding
2-chloroethanol and propargite yielding propargyl alcohol. Whether any
further confirmatory studies are needed would depend on the toxic
potential of the postulated fragments.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Propargite is detected in the multi-residue screening procedure
employed by the Canadian Department of Health and Welfare, The
compound is eluted from Florisil in fraction 3 as described in the
method (15% acetone in hexane). Only 4 positive findings of propargite
were reported in the Canadian surveillance programs (as of 1977). The
highest residue was found in a nectarine sample at 0.57 mg/kg (Health
Protection Branch, 1977),
The U.S. Food and Drug Administration multi-residue method has not
been validated for propargite. The specific GLC/FPD analytical method
has not been applied in the U.S. surveillance programme. Therefore
propargite has not been reported in foods in channels of commerce in
the U.S.A. No reports from other governments have been received on
occurrence in commerce.
There were no data available on reduction of residues through washing,
cooking or processing. The principal exposure through ingestion would
be from fruits eaten raw and unpeeled.
METHODS OF RESIDUE ANALYSIS
The early residue data on propargite (mid 1960s) were obtained by gas
chromatography with flame ionization or microcouclometric (SO2)
detection. Some difficulties were experienced by government
laboratories in validation studies on the method at that time were
because the clean-up and extraction procedures were unsatisfactory.
Through a series of modifications, a basic procedure using gas
chromatography with flame photometric detection (FPB) has evolved
which is satisfactory for a wide variety of fruits, vegetables, and
animal tissues (Devine and Sisken, 1972).
In the basic method, residues are extracted with 1:1 hexane and
isopropanol, followed by an aqueous NaCl wash and Florisil column
chromatography. For oily or waxy samples an optional step is included
in which the residues are partitioned into nitromethane. Other
modifications are prescribed for certain crops. The method has been
validated in U.S. government laboratories on milk, apples and animal
tissues (PAM III, 1973). Depending on the substrate, the limit of
determination is about 0.05 to 0.2 mg/kg.
A similar GC-FPD method for propargite residue in citrus has been
reported by Westlake et al, (1971).
Propargite is not known to be recovered through the U.S. Food and Drug
Administration multiresidue procedure. Information received from
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Almonds 0.1
Almonds, hulls 55
Apples 3 3 3 3
Apples, pomace, dried 80
Apricots 7 3
Bananas 3
Beans, dry 0.2
Beans, succulent 20
Citrus, dried pulp 40
Corn, fodder 10
Corn, forage 10
Corn, grain 0.1
Cottonseed 0.1 3
Cranberries 10
Eggs 0.1
Figs 3
Grapefruit 5 5
Grapes 10 7
Grapes, pomace 40
Hops 15 3
Hops, dried 30 30
Lemons 5 5
Milk 0.08
NATIONAL TOLERANCES REPORTED TO THE MEETING (Continued)
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Milk, fat 2
Mint 50
Nectarines 4 3
Oranges 5 5
Passion fruit 3
Peaches 7 7 3
Peanuts 0.1
Peanuts, forage 10
Peanuts, hay 10
Peanuts, hulls 10 3
Pears 3 3 3
Plums (prunes) 7 3 3
Potatoes 0.1
Raisins 25
Sorghum, fodder 10
Sorghum, forage 10
Sorghum, grain 10
Strawberries 7 7 3
Walnuts 0.1
Meat, meat
by-products and fat
of cattle, goats, hogs,
horses, poultry and
sheep 0.1
Canadian authorities is that it is recovered through their
multiresidue procedure. It is eluted from Florisil in fraction 3 of
the Canadian procedure (15% acetone/hexane).
APPRAISAL
Propargite is an acaricide which has been used on a wide variety of
food crops and ornamentals since its introduction in 1967. Available
information indicates that the product is used in Australia, New
Zealand, Argentina, Mexico, France, South Africa, Italy and the U.S.A.
The primary manufacturer has submitted a report on supervised trials
on 26 crops, all of which were conducted in the U.S.A. Limited residue
data were received from New Zealand on peaches). U.S.A., Canada, New
Zealand and Australia have set national tolerances (MRLs) for
propargite.
Propargite is non-syrtemic by mode of action against mites, and data
indicate that residues which do occur are surface residues. Residues
on the harvested commodities are primarily propargite per se. No
significant metabolites or alteration products have been reported on
harvested crops. However, much of the evidence regarding the fate of
propargite on plants is indirect. It would be desirable to have 35S
or 14C radiotracer studies to confirm the degradation pathways in
plants.
Radioactively labelled propargite (14C) has been used in some animal
experiments, including studies on the rat, cow and chicken. Techniques
used to fractionate and identify the activity were not as definitive
as would be desired, but it is fairly certain that propargite is
hydrolysed in the body to tert-butylphenoxyoyclohexanol and
propargyl alcohol. It is probable that there is further degradation to
butylphenol and cyclohexanediol and excretion as conjugates in the
urine. However, some residues of unchanged parent compound do occur in
milk, body fat and briefly in liver. If any correlations are to be
made between the metabolism of propargite and the related compound
aramite, it may be significant to compare the toxic potential of the
hydrolysis product propargyl alcohol with that of the corresponding
hydrolysis product of eremite, ß-chloroethanol.
An analytical method suitable for regulatory purposes is available. It
is a gas chromatographic method (published) with flame photometric
detection that has been validated in government laboratories.
Propargite is also measured by the multi-residue method employed in
the Canadian surveillance programme. It has not been studies in the
U.S. Food and Drug Administration's multi-residue method.
RECOMMENDATIONS
Temporary maximum residue limits for propargite on the following
commodities are recommended.
Commodity Limit mg/kg Pre-harvest intervals on which
recommendations are based
(days)
Apple pomace 80
Alfalfa hay 75 28
Almond hulls 55 28
Alfalfa, fresh 50 28
Mint hay 50 14
Dried citrus pulp 40
Grape pomace 40
Dried hops 30
Raisins 25
Beans (in pod) 20 7
Corn fodder
and forage 10 30
Cranberries 10 14
Grapes 10 14-21
Peanut hay
and forage 10 14
Sorghum fodder
and forage 10 30 (if silaged)
Apricots, peaches,
plums, nectarines 7 14
Strawberries 7 3
Citrus 5 7
Sorghum grain 5 60
Apples, pears 3
Pigs 3 14
Milk (fat) 2
Beans, dry 0.2 28
Almonds 0.1* 28
Cottonseed 0.1* before bolls open
Eggs 0.1
Fat of poultry 0.1
Fat of meat 0.1
Maize (kernels) 0.1* 30
Milk, whole 0.1
Peanuts (kernels) 0.1* 14
Potatoes 0.1* 14
Walnuts 0.1* 14
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (Before July 1981)
1. A carcinogenic study
DESIRABLE
1. Mutagenicity studies
2. Observations in humans
3. Data from supervised trials in countries other than the USA
4. Information on the occurrence of residues on commodities in
commerce
5. Confirmation of the postulated degradation pathway in plants
using radio-labelled propargite.
REFERENCES
Carson S. (1963) Acute Oral LD50 in Rats. Unpublished report from
Food and Drug Research Lab., submitted by Uniroyal Chemcial, Inc.
Carson S. (1963) Subacute Feeding Studies with D-014 in Rats.
Unpublished report from Food and Drug Research Lab., submitted by
Uniroyal Chemical, Inc.
Haley, G., Kennedy, G.L. and Keplinger, M.L. (1972) Teratogenic Study
with Omite in Albino Rats. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Health Protection Branch (1977) Personal communication, Canada Bu.
Chem. Safety, Health Protection Branch to FAO Panel of Experts, 1977.
Holsing, G.C. (1968) 13-Week Dietary Feeding - Dogs. Unpublished
report from Hazleton Laboratories, Inc., submitted by Uniroyal
Chemical.
Jenkins, D.H., Kennedy, G. and Keplinger, M.L. (1972) Study with Omite
in White Leghorn Chickens. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Kennedy, G., Jenkins, D.H. and Keplinger, M.L. (1970) Metabolism of
14C-Omite in the Cow. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Ladd, R., Jenkins, D.H. and Keplinger, M.L. (1974) Tissue Residue
Study with Omite in Crossbred Swine. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Oser, B.L. (1966) Chronic (2-Year) Feeding Studies with D-014 in Rate
and Dogs. Unpublished report from Food and Drug Research Lab.9
submitted by Uniroyal Chemical.
PAM II Pesticide Analytical Manual, Vol. II, revised (1973); U.S.
Department of Health, Education and Welfare, Food and Drug
Administration.
Ryer, F.H. and Sullivany J.B. (1969) Rachiotracer Drug Metabolism
Study with Omite 14C. Unpublished report from Hazleton Laboratories,
Inc., submitted by Uniroyal Chemical.
Ryer, F.H. and Sullivany J.B. (1969b) The Fate of Omite 14C in the
Cow. Unpublished report from Hazleton Laboratoriesy Inc., submitted by
Uniroyal Chemical.
Sleight III, B.H. and Maceky K.J. (1972) 14C Omite Fish Accumulation.
Unpublished report from Bionomics, Inc., submitted by Uniroyal
Chemical.
Smith, K.S. and Roger, J.C. (1975) Omite Milk and Tissue Residue Study
in Dairy Cows: Unpublished report from Cannon Laboratories, submitted
by Uniroyal Chemical.
Sullivan, J.B. (1968) An Investigation of the In-Vitro and In-Vivo
Metabolism of Omite. Unpublished report from Hazleton Laboratories
Inc., submitted by Uniroyal Chemical.
Uniroyal Unpublished information (1977), Uniroyal Inc., to U.S.
Environmental Protection Agency.
Weir, RJ. (1967) Acute Dermal Application to Rabbits. Unpublished
report from Hazleton Laboratories Inc., submitted by Uniroyal
Chemical.
Westlake, W.E., Gunther, P.A. and Jeppson, L.R. J. Agr. Fd. Chem.,
19:894.
Other information on identity and properties
The technical grade material is a light to dark brown liquid of low
volatility. It is miscible with most organic solvents. Solubility in
water is about 0.5 mg/kg. The basic manufacturer states the
composition of the technical product to be:
85% active ingredient
7% "high molecular weight sulphites"
5% "unreacted starting materials"
1% propylene oxide stabilizer
(remainder unidentified)
Propargite is commercially available in 68.1% and 755% emulsion
concentrates, a 30% wettable powder, and a 4% dust. The manufacturer
states that the technical product shows no degradation after 12 months
when stabilized with propylene oxide. The odour of SO2 indicates
breakdown.
The product was introduced in the U.S.A. on an experimental basis in
1967 as a replacement for Aramite, another product of the same
manufacturer, which was withdrawn from use on food crops in the U.S.A.
The two compounds have similar chemical structures and properties.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, excretion and biotransformation
Extensive degradation of propargite could be shown in in vitro as
well as in vivo experiments.
In vitro studies with liver homogenates showed that the major
primary metabolites appear to be propargyl alcohol, the glycol ether
and their conjugates, which are further degraded to secondary
metabolites (Sullivan, 1968).
In in vivo metabolism studies in rats that were orally treated with
a single dose of 200 mg/kg propargite, propargyl alcohol and glycol
ether were also found as primary metabolites in the excreta as well as
in tissue samples. Following analysis of the excreta and tissues only
about 5% of the administered dose was accounted for as unchanged
propargite or the primary metabolites, indicating that the primary
metabolites are further degraded (Sullivan, 1968).
The oral administration of a single close of 271 mg/kg benzene ring
labelled 140 propargite to rats was followed by fast excretion of the
radiocarbon. Within 72 hours postdosage, 47% of the administered dose
was eliminated via the urine. 32% via the faeces. The total carcass
contained approximately 90% of the administered radioactivity.
Identified metabolites excreted with the rat urine and faeces were
tert-butylpyrocatechol and glycol ether (Ryer and Sullivan, 1969a)
In cows treated with a single oral close of 4 mg/kg b.w. combined
non-radioactive and 14C labelled propargite a similar excretion
pattern was found. 54% of the administered dose was eliminated with
the urine, 17% with the faeces. Approximately 0.3% was found in the
milk. Identified metabolites were tert-butylpyrocatcohol, glycol
ether and tertbutylphenol (Ryer and Sullivan, 1969b).
In a feeding study, dairy cows were maintained on a diet containing
14C-labelled and unlabelled propargite at dosage levels of 3 and 20
ppm for 12 consecutive days. Peak urinary excretion observed at the
seventh test day reached values of 4 and 23 mg/kg respectively for the
3 and 20 ppm dietary level; peak faecal excretion was approximately 1
and 4 mg/kg. Maximum milk residue values reached on the fourth test
day were 0.02 and 0.06 mg/kg at the 3 and 20 ppm dose levels
respectively. Residues in selected tissues ranged from 0.02 to about
1.3 mg/kg (Kennedy et al., 1970).
Similar results were obtained after daily administration of a diet
containing 50 and 100 ppm propargite to lactating cows for a period of
27 days (Smith and Roger, 1975).
Pigs were maintained on a diet containing propargite at dosage levels
of 20 and 100 ppm for 30 days. Residues in kidney and liver were below
the detection limit of 0.1 mg/kg at both feeding levels and residues
in muscle and fat ranged from below 0.1 to about 2 mg/kg (Ladd et al.,
1974).
Residues studies in hens that were treated with 3, 10 and 30 ppm 14C
propargite in their diet for 30 days showed residue levels in eggs
from 0.13, 0.49 and 0-145 µg/kg respectively at the 3, 10 and 30 ppm
dose levels. Residues in muscle ranged from 2-12 µg/kg, liver 18-174
µg/kg kidney 2-25 µg/kg and fat 7-116 µg/kg depending on exposure
level (Jenkins et al., 1972).
After continuous exposure to a concentration of 0.025 mg/l 14C
propargite in water for 35 days tissue residues in edible portions of
fishes were about 2 mg/kg. After transfer to uncontaminated water 70%
of the radioactivity present in the edible tissues was eliminated
within 14 days (Sleight III, 1972),
The in vivo metabolism studies in several animal species confirmed
the rapid degradation of propargite already shown in in vitro
experiments. The metabolic reactions are hydrolysis of the parent
ester followed by other cleavage. The formed products are polar
alcoholic and phenolic compounds which can rapidly be excreted or
further metabolized and incorporated into natural tissue constituents
by biochemical reactions which are well documented in the literature.
See also the section "Fate of residues", "In animals".
TOXICOLOGICAL STUDIES
Special study on teratogenicity
Female rats were orally dosed with 0, 50 and 100 mg/kg from day 6
through 15 of gestation. The treatment had no effect on maternal body
weight, mortality or fertility. No reproductive adverse effects were
found with respect to implantation and resorption sites. Embryonic and
foetal development was not affected by the treatment
(Haley et al., 1972).
Special study on reproduction
Rat
See "Long term study".
Special study on carcinogenicity
Rat
See "Long term study".
Dog
See "Short term study".
Acute toxicity
Species Sex Route LD50 References
mg/kg
Rat M, F oral approx. 2200 Carson, 1963
Rabbit M, F dermal approx. 3200 Weir, 1967
Rat
Groups of 5 male and 5 female rats were treated for 90 days with
amounts of propargite in their diet to provide daily intakes of 10,
20, 40, 100 and 200 mg/kg b.w. corresponding to dietary levels in
adult animals of 200, 400, 800, 2000 and 4000 ppm respectively. The
control group existed of 15 male and 15 female rats. Treatment with
2000 and 4000 ppm caused a dose-related growth retardation and
reduction of food intake. Haematological examinations and clinical
chemistry tests revealed no abnormalities. At 2000 and 4000 ppm the
relative liver weights were increased, the relative kidney weights
were increased only in the 4000 ppm groups. Gross and microscopic
examinations of tissues disclosed no treatment-related pathological
alterations (Carson, 1964).
Dog
Groups of 3 male and 3 female dogs were maintained on a diet
containing propargite at dose levels of 0 and 2000 to 2500 ppm
(increase of dosage after 3 weeks) for 90 days. The treatment did not
affect appearance, behaviour, results of haematological. and clinical
chemistry tests and urinalyses. In most treated dogs reduced food
consumption and body weight loss and an increase of relative liver and
kidney weights were observed, No compound-related alterations were
found macroscopically. Histopathological examinations revealed an
increased amount of pigment in the liver reticuloendothelial cells and
increased haemosiderosis of the spleen (Holsing, 1968).
Groups of 8 dogs were fed a diet containing propargite at 100, 300 and
900 ppm for 24 months. The control group consisted of 12 animals. The
treatment had no effect on growth rate, results of clinical laboratory
studies, urinalyses and organ weights. Gross and microscopic
examination did not reveal abnormal pathological findings. No tumours
were found (Osert 1966).
Long term Study
Rat
Groups, each comprising 25 male and 25 female rats were fed dietary
levels of propargite to provide daily intakes of 5, 15 and 45 mg/kg
b.w. corresponding to adult levels of 100, 300 and 900 ppm for a
period of 24 months. The control group consisted of 37 animals of each
sex. After 26 test weeks without noticeable evidence of a
treatment-related effect, a supplementary study was started with a
control-and a test group comprising 25 male and 25 female rats
receiving 100 mg/kg b.w. corresponding to 2000 ppm for 78 weeks. Rats
that died during the study and those sacrificed after 104 and 78 weeks
were examined grossly and microscopically. The treatment had no effect
on appearance, behaviour, growth rate and mortality at the dietary
levels up to and including 900 ppm. At the 2000 ppm level at the end
of the study there was a reduction of food consumption and also a
reduction of the body weight gain of about 30%; mortality of the male
rats increased to 32% compared to 7% of the corresponding control.
Haematological examinations and clinical laboratory studies did not
reveal abnormal findings. Absolute and relative liver and kidney
weights of the animals treated with dietary levels of 100, 300 and 900
ppm which died or were sacrificed after 104 weeks showed inconsistent
variations without any dose-relationship. At 2000 ppm an increase of
the relative liver and kidney weights of about 30%. were observed.
Gross autopsy findings in 1 male (of 25) and 7 females (of 23) in the
2000 ppm group were enlarged and/or dark red lymph nodes which in some
cases were involved in abdominal masses. In the corresponding control
group 2 male rats of 29 animals showed this alteration. No
dose-dependent increase of the frequency of this lymph node alteration
was found at the 100, 300 and 900 ppm levels. A variety of benign and
malignant tumours was observed in the control and treated groups. No
dose-relationship of the frequency of tumours was found. Four sarcomas
were found in rats sacrificed after 78 weeks ingestion of 2000 ppm,
whereas in the corresponding control group no sarcomas were seen
(Oser, 1966).
Additionally a 3-generation reproductive was included in the 2-year
feeding experiment with 20 pairs of male and female rats from the
control and 100 ppm test groups. Dosage was raised to 300 ppm in the
F2 pups at the time of weaning. The treatment revealed no abnormal
findings (Oser, 1966).
COMMENTS
Propargite is readily absorbed from the gastrointestinal tract and is
excreted rapidly as metabolites which are formed mainly by hydrolysis
of the sulphite ester.
After feeding a diet containing 20 ppm of propargite the residues in
milk or in tissues were of the order of 0.1 mg/kg.
The compound is not teratogenic in the rat.
In a 3-generation reproductive study no adverse effects were revealed
at concentrations up to 300 ppm. This concentration is therefore
regarded as a no-effect level.
In a 2-year feeding study with up to 900 ppm in dogs which included
extensive laboratory and histological investigations, no adverse
effects were noted. A 2-year feeding study in rats up to 2000 ppm
showed appreciable but not dose-related variations in the relative
liver and kidney weights in all treated groups. However, no major
adverse effects were seen. Owing to a poor survival rate and the
termination of the study with the high dose animals after 78 weeks,
the finding that the tumour incidence was not elevated is of limited
value. This study was not considered to fulfil the criteria for an
acceptable lone-term study. Therefore a safety factor of 200 was used.
Propargite is structurally related to Aramite, a compound which is
suspected of being a carcinogen. Although there are no indications
that propargite acts similarly, the need for satisfactory
carcinogenicity studies was expressed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 300 mg/kg in the diet, equivalent to 15 mg/kg bw
Dog: 900 mg/kg in the diet, equivalent to 22 mg/kg bw
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Propargite is an acaracide said to be effective on a variety of mites
on food crops and ornamentals. Table 1 shows the use patterns
registered in the U.S.A. Information has been received that the
product is also used in Italy, Australia, New Zealand, South Africa,
Argentina, Mexico, and France. The government of the Netherlands has
informed the Meeting that the compound was used until 1976, at which
time its use was discontinued. A country statement was received from
New Zealand regarding uses and tolerances. The uses permitted in these
countries generally parallel those shown in Table 1. Significant
exceptions are included in the footnotes.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A submission to the Joint Meeting contained data from supervised
trails on twenty-six crops (Uniroyal, 1977). Most of the trials were
conducted in the U.S.A. A country statement was received from New
Zealand with data on peaches. Analyses in all U.S. trials, the
procedure described under "Methods of residue analysis" (PAM II,
1973). Analyses of untreated controls (crop blanks) were reported in
each experiment, along with the recovery of propargite from fortified
controls. Controls were generally reported as 0 and recoveries were
adequate. A brief discussion of the residue finding for each crop
follows. Data are summarized in tabular form when feasible.
The use patterns employed in Mexico and France on apples, peaches,
plums and citrus differed significantly from the other countries. In
the absence of residue data reflecting good agricultural practice in
those countries, it was not possible to predict residue levels. The
recommended DIRLs for apples, peaches, plums and citrus therefore are
based on the national use patterns which can be related to supervised
residue trails.
Alfalfa
There are no national tolerances or registrations for propargite use
on alfalfa. However, the submission to the Meeting contained some
residue data on alfalfa hay, fresh forage and trash. Single and
multiple applications were made at 1.4 to 4.7 kg a.i./ha. Residues on
fresh forage ranged 0.0 to 328 mg/kg at 28 and 0 day intervals from
treatment, respectively. Residues on hay and trash ranged from 2.6 to
100 mg/kg with intervals from treatment of 22 to 52 days. With a 28
day pre-harvest interval, MRLs of 50 and 75 mg/kg on fresh alfalfa and
alfalfa hay, respectively, would be adequate.
TABLE 1. Registered use patterns for propargite (USA)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Almond 30% WP 0.03-0.05%(4) 2 28(5) low volume
68.1% EC 2.5-3.4 kg/ha 1 post-harvest aerial
application
only
Apples 30% WP 0.04-0.05(1)(6) 3 7(2)(7)(12)
Apricots 30% WP 0.05%(4) 2 14(5) low volume
68.1% EC 1.7-2.5 kg/ha 2 post-harvest aerial
application
only
Cranberries 68.1% EC 1.6 kg/ha 1 14
Beans 75% EC 2.8 hg/ha(4) 2 7(fresh)(5)
28 (dry)
Corn 75% EC 1.9 kg/ha 1 30
(field)
Cotton 75% EC 0.85-1.9 kg/ha 3 before bolls open
Figs 30% WP 0.03-0.05%(3)(4)(11) 2 14
Grapes 30% WP 1.7-3.0 kg/ha 2 21
4% Dust 0.2 kg/ha 2 14
Grapefruit, 75% EC 0.025-0.05(4)(8) 2 7 (5)
oranges
Lemons 30% WP 0.05% (8) 2 7
Hops 68.1% EC 1.7 kg/ha(4) 2 14 (5)
30% WP 1.7 kg/ha 2 14
Mint 68.1% EC 1.7-2.5 kg/ha 2 14
Nectarines 30% WP 0.05% (4) 2 14 (5)
Peaches 30% WP 0.04-0.05%(4)(8) 2 14(5)(10)(13)
Peanuts 30% WP 1.0-1.7 kg/ha 2 14
Pears 30% WP 0.04-0.05%(8)
Plums 30% WP 0.04-0.05(4)(8) 2 14(5)(10)
Potatoes 75% EC 1.4-2.4 kg/ha 2 14
68.1% EC 1.7-2.5 kg/ha 2 14
TABLE 1. (Continued)
Application No. of Pre-harvest
Crop Formulation rate, a.i. treatments Interval (days) Notes
Sorghum 75% EC 1.5-1.9 kg/ha 1 30 silage
60 grain
Strawberries 30% WP 1.7-3.4 kg/ha(9) 3 3
4% Dust 1.8 kg/ha 3 3
Walnuts 68.1% EC 0.03-0.05% (4) 2 14 (5)
(1) France 0.12, Italy 0.06%
(2) France 21, Italy 15, New Zealand 14
(3) France 0.6-0.9 kg/ha
(4) Italy 0.06%
(5) Italy 21
(6) Mexico 0.08-0.12%
(7) Mexico 28
(8) Mexico 0.12%
(9) East U.S.A. 0.84 kg/ha
(10) Argentina 21
(11) Argentina 0.05%
(12) South Africa 14
(13) South Africa 21
Almonds
Field trials were conducted in California: 63 samples of nut meats
were anlayzed. Residues on the nut meats from two applications of 0.03
and 0.06% sprays were < 0.1 mg/kg at post-treatment intervals of 21
to 28 days.
Residues on almond hulls from treatment with 0.05% spray (30% WP)
followed by 3.4 kg a.i./ha (68.1% EC) ranged from 5.2 to 31 mg/kg with
pre-harvest intervals of 28-35 days. Under good agricultural practice,
residues are not likely to exceed 55 mg/kg.
Apples
Field trials were conducted in nine U.S.A. states, South Africa and
Australia, some 452 samples being analysed. The residue data are
summarized in Table 2. All controls were reported zero. Residues from
applications of up to 0.06% spray after 7 days were less than 3 mg/kg.
Residue data indicate that residues remain associated with the apple
pomace and are not destroyed in drying. Residues in the juice were
barely detectable (ca. 0.02 mg/kg) while residues in the wet and dried
pomace were 16-21 mg/kg and 56-80 mg/kg, respectively. Thus, residues
concentrate by factors of 15-22 in the manufacture of dried pomace
from treated apples (Uniroyal, 1972), An MRL of 80 mg/kg in dried
apple pomace is therefore appropriate.
TABLE 2. Range of residues in apples, mg/kg.
Application rate, Interval (days, last treatment to harvest)
% 0-1 6-7 14 21 28
0.03 0.1-3.1 0.6-1.8 0.2-1.0 0.2-0.7 0.2-0.9
0.06 0.3-6.3 0.5-1.6 0.3-2.4 0.5-1.1 0.2-0.6
Apricots, peaches and plums
Field trials were conducted in California, Connecticut, Georgia, North
and South Carolina, Maryland, Michigan, Pennsylvania, Washington, New
York and New Zealand. Some 385 test samples of fresh fruit and 65
controls were anlaysed from one and two applications at four dosage
rates, including the maximum and twice the maximum label rates (Table
1). The residue data are summarized in Table 3. Only one residue on
peaches was greater than 7 mg/kg at 14 days after treatment at the
maximum rate. Residues will not exceed 7 mg/kg from sprays up to
0.06%, provided that treatments are not made within 14 days of
harvest.
A study was conducted on plums (fresh prunes) using a standard
commercial drying process to determine residues on dried prunes. The
average residue value for the dried fruit was ca. 20% of the average
value for the fresh fruit. Thus no additional MRL is needed for the
dried fruit.
TABLE 3. Range of residues on apricots, peaches and plums, mg/kg
Application rate. Interval (days, last treatment to harvest)
% 0-1 6-7 13-14 21-23
0.03 0.4-12 0.2-7.0 0.4-10.0 0.3-5.0
0.05 0.7-6.7 2.6-14.0 0.6-5.6 0.0-1.7
0.06 1.3-17.0 1.3-8.7 0.2-5.5 0.3-9.3
0.10 1.6-27.0 0.3-8.4 0.8-11.0 0.8-3.5
Beans
Field trials were conducted in the states of Washington, California
and Idaho on snap beans, succulent lima beans and dry beans. 52
analyses were reported. Single and multiple applications were made at
the maximum and at twice the maximum label rate (Table 1), by aerial
and ground application. The residues are summarized in Table 4 for
fresh and dry beans, 7 and 28 days after the last treatment,
respectively. No residue data were submitted for bean vines; however
there is a restriction against the feeding or foraging of treated bean
vines. The only other feed item, cannery waste, would consist of cull
beans with some bean vines and pods present as a result of mechanical
harvesting. Residue levels would not be likely to exceed 20 mg/kg on
cannery waste.
TABLE 4. Range of residues in beans, mg/kg
Application rate, kg a.i./ha
2.8 5.6
Fresh (7 days after last treatment) <0.2-15 <0.2-12
Dry (29 days after last treatment <0.1-0.1 <0.1-0.24
Maize
Residue data for maize were made available from five U.S.A. sites, two
in California and one each in Colorado, Texas and Nebraska. Following
applications at the maximum and twice the maximum label rates (Table
1), silage stage maize sampled 28-35 days after application contained
maximum residues of 2.4 and 18 mg/kg, respectively; maize stover,
sampled after 47-105 days, contained maximum residues of 4 and 11
mg/kg, respectively. Interpolation of the 2X data shows that MRLs on
maize forage and fodder of 10 mg/kg are appropriate.
No detectable residues (< 0.1 mg/kg) were found on either the grain
or ears of maize sampled 60-105 days after the above applications. No
data on grain reflecting a 30 day pre-harvest interval were submitted.
However, since propargite does not translocate to any extent, residues
on grain are not expected to exceed 0.1 mg/kg.
Cotton
Five field studies from locations in California, Arkansas and
Mississippi were reported. The studies reflect treatment at the
maximum and twice the maximum label rate (Table 1). Analyses were
performed on 96 samples of cottonseed and processed products, hulls,
oil, meal and soapstock. All values on cottonseed were reported as
< 0.1 mg/kg. All samples of meal and soapstock contained
< 0.05 mg/kg as did all but on refined oil sample which contained a
level of 0.1 mg/kg after treatment at an excessive rate. Almost all of
the hull samples had low but detectable residues, 0.03-0.06 mg/kg.
Thus, an MRL of 0.1 mg/kg is appropriate for cottonseed. There is a
restriction in the U.S.A. against feeding treated foliage or cotton
trash to livestock.
Cranberries
Seven studies were reported from Massachusetts, with a total of 36
analyses. The studies reflect applications at 1.68 kg a.i./ha (4 by
ground and 3 by air) with one study at 3.36 kg a.i./ha. Several of the
studies show decline rates (at 7, 14 and 21 days). The residue
declines rapidly between 0 and 7 days and then shows little further
decline from 7 to 21 days. At the recommended rate (Table 1), residues
are not likely to exceed 10 mg/kg. Residues are summarized in Table 5.
TABLE 5. Range of residues in cranberries, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 21
1.68 2.2-11 <0.1-1.9 <0.1-7.1 0.1-1.0
3.36 3.3-14.9
Figs
The residue data for figs consists of four studies made in California,
including two decline studies. Various applications were made
including the maximum and twice the maximum label rate (Table 15).
Residues on fresh figs are summarized in Table 6. While the data are
limited, it is not likely that residues will exceed 3 mg/kg at 14 days
after treatment.
TABLE 6. Residues on figs, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0-1 7 13-14 21 28
1.27 3.3 1.9 0.8 0.45
2.02 1.6-2.8 1.0-1.4 0.2-1.4 0.3-0.6
4.04 2.5-5.3 2.2-3.2 0.7-4.2
Grapefruit, oranges and lemons
Field trials were conducted in Texas, Florida and California. Some 281
harvest samples were analyzed. One and two applications were made at
the recommended and twice the recommended rates (Table 1). The results
are summarized in Table 6. For applications up to 0.06% spray with a
7 day interval, 5 mg/kg would not be exceeded.
Washed oranges bearing residues of 1.9 mg/kg were processed to dried
pulp by a procedure simulating the commercial process. The residue in
the dried pulp was 15.5 mg/kg. The residue in the dried pulp is about
eight times that on the whole fruit, so 40 mg/kg is an appropriate
MRL.
TABLE 6. Range of residues in grapefruit, oranges and
lemons, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 28
0.03 0.3-0.5 0.2-0.8 0.2-0.3 0.9-1.0
0.05 1.7-3.6 0.2-4.8 1.1-2.1 0.6-3.1
0.06 1.0-1.4 0.9-2.5 0.0-0.9 0.6-1.2
0.10 1.0-5.1 0.8-7.2 1.2-3.2 1.2-2.9
Grapes
Residue trials were conducted in Washington and California, with
analyses of 254 samples of grapes. A wide range of experimental
conditions in the various field tests and the resulting variation in
residues preclude a tabular summary of the data. No residues exceeded
10 mg/kg from the maximum recommended rate with a 21 day interval
after treatment.
A residue concentration factor of 2.5 was observed in the commercial
production of raisins from fresh grapes. Thus a 25 mg/kg MRL for
raisins would be appropriate. The data showed a concentration of
residues on dried grape pomace to 4 times that found on fresh grapes,
therefore, 40 mg/kg would be appropriate for grape pomace.
Hops
Eight field trials were conducted in the State of Washington. Residues
on green hops resulting from three applications at the maximum and
twice the maximum label rate (Table 1) are summarized in Table 7.
Residues on green hops from good agricultural practices are not likely
to exceed 15 mg/kg. Residues on dried hops ranged from 11 to 30 mg/kg
14 days after treatment. A 30 mg/kg MRL would be appropriate for dried
hops since residues from two applications will probably be somewhat
lower than those noted above.
TABLE 7. Range of residues on green hops, mg/kg
Application rate, Interval (days last treatment to harvest)
kg a.i./ha 0 6-7 13-14 20
1.7 24-50 5-17 6-15 2-6
3.4 36-92 24-66 11-43 6-24
Mint
Field studies were conducted in Washington, Oregon and Idaho. A total
of 89 analyses were made on fresh hay, spent hay and mint oil,
including controls. In four studies, two applications were made at
rates of 2.5 and 5 kg a.i./ha resulting in maximum residues of 19 and
50 mg/kg respectively, 15-17 days after treatment. Three residue
decline studies were also submitted. Samples were taken 0, 1, 2 and 4
weeks after a single aerial application at 1 and 2 times the maximum
label rate (Table 1). From a decline curve based on these data,
residues resulting from the recommended use are ca. 50 mg/kg on fresh
mint hay.
Five samples of fresh mint hay bearing residues of 8.7-50 mg/kg were
fractionated by steam distillation and the resulting mint oil and
spent hay contained residues of 1.6-7.9 mg/kg and 12-97 mg/kg
respectively. Residues in mint oil are not likely to exceed those on
fresh mint hay and there is a restriction against feeding spent mint
hay to livestock.
Nectarines
Seven trials were conducted at locations in California and
Pennsylvania. One or two applications were made at one and two times
the maximum label rate (Table 1). The residue data are summarized in
Table 8. Residues were no higher than 3-4 mg/kg at the prescribed
pre-harvest interval. However, extrapolation of data from peaches,
apricots, and plums would indicate that a 7 mg/kg MRL would be
appropriate.
TABLE 8. Range of residues in nectarines, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 6-7 13-15 20-21
0.05 0.66-7.3 0.24-3.1 0.44-3.4 0.39-1.3
0.10 1-3-10.5 0.34-5.1 0.91-3.4 0.41-1.5
Peanuts
Residue studies were conducted in Georgia, Florida, North Carolina,
Oklahoma, Mississippi and Arkansas. Two applications were made in all
but four decline studies on peanut vines, where only one application
was made. Dosages ranged from 0.6 times to twice the maximum
recommended rate (Table 1). Six of the studies reflect a 14 day
preharvest interval, but in the other ten studies intervals were from
19 to 66 days.
Peanut kernels
All residue values were reported as < 0.1 mg/kg. Since no detectable
residues were found in peanuts, no MRLs are needed for peanut
by-products, meal, oil and soapstock.
Peanut hulls
Residues were < 0.1 mg/kg in five of seven studies. Residues of
0.14 mg/kg, 0.3 mg/kg and 1.2 mg/kg were found after applications at
twice, 6 times and 1.2 times the recommended rates respectively, in
the other two studies.
Peanut vines and hay
Residues were less than 10 mg/kg 14 days after application at the
maximum rate. The data indicate that residues decline rapidly from a
maximum of 38 mg/kg at 0 days to 3.6 mg/kg at 14 days.
Pears
Four field studies were conducted in California, Pennsylvania,
Michigan and Washington, one and two applications were made with 0.03
and 0.06% sprays. The residue data are summarized in Table 9. All
residues were less than 3 mg/kg, 14 days after treatment.
TABLE 9. Range of residues in pears, mg/kg
Application rate, Interval (days, last treatment to harvest)
% 0 7 14 21
0.03 0.6-1.6 0.3-1.2 0-2.7 0.1-2.4
0.06 2.2-4.1 0.9-2.6 0.3-2.2 0.1-1.7
Potatoes
Seven residue studies were made available, four from Washington and
one each from Idaho, Vermont and Illinois. After two applications at
rates of 1.5 to 4.5 kg a.i./ha, all residues were less than the limit
of detection of the method (0.1 mg/kg) 14 days after treatment.
Sorghum
Field studies were conducted in Texas, Nebraska and Iowa on grain
sorghum. Applications were made at the maximum and twice the maximum
recommended rates (Table 1). Samples of grain and forage or fodder
were harvested at 28, 30, 44 and 50 days after application. Residues
in forage ranged from < 0.1 to 4.1 mg/kg. Residues in forage and
fodder are not likely to exceed 10 mg/kg 30 days after treatment.
Residue levels in grain ranged from < 0.1 to 2.4 mg/kg 60 days or
more after treatment. Thus an MRL of 5 mg/kg with a 60 day interval
after treatment is appropriate for sorghum grain.
Strawberries
Field trials were conducted in California, Louisiana and Florida.
Strawberries treated with two formulations in California, reflecting
three applications at the maximum recommended rate (Table 1) had
residues ranging from 0.19 to 4.0 mg/kg for 3 days after treatment.
Residues from applications of 0.84 and 1.7 kg/ha in Louisiana and
Florida are summarized in Table 10. Under good agricultural practice,
residues are not likely to exceed 7 mg/kg.
TABLE 10. Range of residues in stramberries, mg/kg
Application rate Interval (days, last treatment to harvest)
kg a.i./ha 0 3 7
1.7 0.9-2.0 0.3-3.8 0.2-0.7
3.4 1.4-4.1 0.7-9.2 0.4-1.1
Sugar beets
There are no national tolerances or registrations for propargite on
sugar beets; however, this use is pending in the U.S.A. The submission
to the Meeting therefore contained some residue data on sugar beet
tops, roots and by-products. Sugar beet root samples from Idaho,
Washington and California reflecting two treatments at 2.8 and 5.6 kg
a.i./ha and a 21 day interval from treatment# showed residues ranging
from 0.05 to 0.23 ind/kg and 0.13 to 0.28 mg/kg respectively.
In a residue decline study, on sugar beet tops, a single application
at 2.8 kg a.i./ha yielded residues of 40-114 mg/kg at 0 days.
20-80 mg/kg at 14-17 days and 2.4-34 mg/kg at 27-28 days. Adiitional
data for tops representing two ground treatments at 2.8 and 5.6
kg a.i./ha show residues ranging from 11-24 mg/kg and 19-59 mg/kg
respectively (21 day interval).
In order to obtain data on processing fractions, sugar beet roots
bearing 0.1 mg/kg residues were processed to wet and dry pulp,
molasses and sugar. Propargite residues in wet pulp, molasses and
sugar were less than or equal to 0.1 mg/kg. The data for dry pulp show
a maximum residue concentration factor of 5, with residues from
0.1-0.5 mg/kg.
Tea
There are no national tolerances or registrations for propargite in
tea, however, a tolerance for this use is pending in the U.S.A. The
submission to the Meeting contained some residue data on dried tea
leaves, both green and black. The data are summarized in Table 11.
TABLE 11. Range of residues in tea, mg/kg
Application rate, Interval (days, last treatment to harvest)
kg a.i./ha 0 7 14 28
0.31 <0.1-0.3 <0.1-0.2
0.75 48-178 1.1-4.2 <0.1-0.2 <0.1
Walnuts
Six field trials were conducted at locations in California. Both
30% WP and 75% EC formulations were applied at the maximum recommended
rate (Table 1). No residues were detected in any of the walnut meat
samples 45 to 48 days after treatment. Although the interval is
greater than that recommended (14 days), since propargite is
non-systemic, any residues found on nut meats would be expected to
arise as a result of contamination in de-hulling and de-shelling
processes. Under good agricultural practice, an MRL of 0.1 mg/kg (at
or about limit of determination) would be appropriate. There is a
restriction against grazing or feeding livestock on cover crops growm
in treated orchards, therefore no feed items are involved in this use.
Meat, milk, poultry and eggs
Propargite is used on a number of crops which yield by-products used
commercially for animal feeds. Primary sources would be dried citrus
pulp, sorghum grain, forage and fodder and maize forage and fodder.
Some additional contribution could occur from dried pomace, almond
hulls and dried grape pomace. Per this reason, some animal feeding
studies were submitted. These are also referred to in the section
"Biochemical aspects", where references are given.
In one study, cattle were fed at levels of 3 and 20 ppm in the total
dry diet for 12 days. Maximum residues in milk (whole milk basis) were
0.059-0.067 mg/kg. The latter is equivalent to 1.7 mg/kg on the fat
basis. Maximum residues in tissues were as follows: liver, 1.3 mg/kg,
kidney, 0.27 mg/kg, fat, 0.21 mg/kg and muscle, 0.18 mg/kg.
In a second study, cows received 0, 50 and 100 ppm in the diet for 27
days. Analysis of milk showed all samples at both feeding levels to be
< 0.08 mg/kg. Residues ranged up to 1.6 mg/kg on a fat basis.
Residues in muscle, kidney and liver from both feeding levels were
40.03 mg/kg (limit of determination). Residues in fat ranged from 0.32
to 0.42 mg/kg at the 10 and 100 ppm feeding levels, respectively.
Poultry were fed 3, 10 or 30 ppm in the diet for 30 days (3 chickens
in each group). Eggs were sampled daily and the animals were
sacrificed 24. hours after treatment. Maximum levels In eggs were
0.013 mg/kg at the 3 PPM level, 0.043 mg/kg at the 10 ppm feeding
level, and 0.106 mi/kg at the 30 ppm level. Liver tissue had the
highest residue levels ranging from 0.047 to 0.089 mg/kg at the 10
diet level and from 0.17 to 0.18 mg/kg at the 30 diet level.
Hogs were fed 20 and 100 ppm in the total diet. Two hogs at each level
and one control were maintained for 30 days. No residues
(< 0.1 mg/kg) were found in liver or kidney. No residues were found
in muscle at the 20 ppm level but up to 0.23 mg/kg at the 100 ppm
intake. In fat, residues up to 0.18 and 0.2 mg/kg were found at the 20
and 100 ppm intakes, respectively.
The studies show that residues may transfer to meat, milk, poultry and
eggs. A mixed ration for cattle could consist of 15% dried apple
pomace, 15% dried citrus pulp and 70% sorghum grain, forage and fodder
or maize forage and fodder. This diet could theoretically contain
propargite residues of ca. 25 mg/kg.
A maximum possible intake of 10 mg/kg could result from the hogging
down of peanut fields and other swine feed items such as maize grain
and cooked potatoes.
Poultry feed items, sorghum grain, grape pomace, maize grain and
cooked potatoes, could contribute ca. 8 mg/kg propargite to the diet.
Residues are unlikely to exceed 2 mg/kg in milk fat and 0.1 mg/kg in
the meat, meat by-product, and fat of cattle, goats, hogs, horses,
poultry and sheep.
FATE OF RESIDUES
In animals
The available data on metabolism by animals are more conclusive than
those on metabolism by animals are move conclusive than those on
metabolism by plants. See also "Biochemical aspects."
Propargite -14C feeding tests have been conducted on a number of
animals including the rat, cow, pig and chicken. Additional
information on degradation was available from in vitro experiments
with buffered liver homogenates.
A general metabolism scheme has been proposed for animals, part of
which has been verified experimentally, and part of which is based on
analogy with biochemical reactions reported in the literature.
Identification of some metabolites was by TLC comparison with prepared
standards. In other cases less positive identification was made by
partition co-effecients (p values) and solvent extraction scheme. In
cow liver the bulk of the 14C activity was identified only as polar
metabolites.
The experiments apparently were carried out over a number of years,
the studies in some animals being repeated with refinements in
analytical techniques. None of the work on propargite submitted to the
Meeting has been published. Certain general conclusions appear
justified. (a) Propargite is metabolized in animals to polar compounds
which are excreted, principally in the urine. (b) There is not a
pronounced tendency to storage or accumulation in the body, but the
compound is not completely metabolized because the parent compound was
the principal residue in the milk of cows, was tentatively identified
as a portion of the 14C activity in animal fat, and was found in
excreta. (c) The parent compound is detected in the liver only for
short periods after ingestion. d) The primary hydrolysis products, the
glycol ether and propargyl alcohol, are further degraded. (e) Some
polar metabolites are in conjugated forms.
A list of the metabolits and the sites in which they were reported is
given in Table 12. The sequence of the metabolic scheme is not
definitely known.
TABLE 12. Propargite metabolites
Compound Found in
Parent Cow: butter fat, body fat (1)
Rat: liver (1)
liver homogenate (1)
Glycol ether Cow: urine, faeces, milk (2)
Rat: faeces, liver (1)
liver homogenate (1)
Propargyl alcohol liver homogenate (1)
Butylphenol Cow: urine (2)
Rat: liver (1)
liver homogenate (1)
Butylcatechol Cow: urine (2)
Rat: urine, faeces (2)
Cyclohexandiol urine (2)
"Polar metabolites" Rat: liver (2)
Cow: liver (2)
(1) Identified by GLC or TLC
(2) Tentatively characterized by solvent
fractionation scheme, p values, literature analogies.
In plants
Propargite is generally considered to be "non-systemic" by mode of
miticidal action. According to information made available to the
Meeting aged residues are primarily surface residues of the parent
compound (Uniroyall 1977). Residue decline was attributed to
volatilization of the parent compound and hydrolysis to propargyl
alcohol and the "glycol ether",
2-(p-tert-butylphenoxycyclohexanol). The two hydrolysis products
have not been detected in samples by chemical analysis at a detection
level of 0.1 mg/kg. In experiments on bean plants to investigate
possible photolytic degradation, the same two compounds could not be
detected. It has been suggested that if the glycol ether and propargyl
alcohol are intermediates in the degradation scheme, their presence is
fleeting because of their volatility.
Other evidence cited in the manufacturer's submission on the behavior
of propargite in plants was: (a) comparison of surface and pulp
analysis of apples showed no absorption (of parent); (b) mites
confined to upper leaf surfaces were not affected by sprays on the
lower surfaces (c) there was no effect on mites of an adjacent leaf
when on leaf of a bean plant was treated; (d) volatility losses of the
parent compound under field conditions have been correlated with model
laboratory experiments (loss from aluminum foil).
The above experiments partially support the idea that propargite is
not translocated in plants and that the residue of concern is
propargite per se. However, much of the evidence is indirect, and it
would be desirable to have conventional metabolism studies on some
representative crops, using 35S and/or 14C, to confirm the fate of
propargite in plants.
In soil
Only a summary was made available (Uniroyall 1977). The half-life in a
variety of soils was said to range from 2 to 18 weeks. Leaching is
negligible. Laboratory and field studies have shown that propargite is
hydrolysed in soil. The major soil degradation product found was the
glycol ether.
Summary, metabolism
The available metabolism studies do not firmly identify the residue
components in plants or animals. However, there is sufficient
information to indicate a probable metabolic scheme. It is fairly
certain that the primary metabolites are the hydrolysis products
tert-butylphenoxycyclohexanol and propargyl alcohol. It is probable
that further breakdown to butylphenol and cyclohexanediol occurs. If
correlations are to be made between metabolic schemes and toxic
potential for propargite and aramite, it should be noted that both
compounds hydrolyze at the sulfite ester, aramite yielding
2-chloroethanol and propargite yielding propargyl alcohol. Whether any
further confirmatory studies are needed would depend on the toxic
potential of the postulated fragments.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Propargite is detected in the multi-residue screening procedure
employed by the Canadian Department of Health and Welfare, The
compound is eluted from Florisil in fraction 3 as described in the
method (15% acetone in hexane). Only 4 positive findings of propargite
were reported in the Canadian surveillance programs (as of 1977). The
highest residue was found in a nectarine sample at 0.57 mg/kg (Health
Protection Branch, 1977),
The U.S. Food and Drug Administration multi-residue method has not
been validated for propargite. The specific GLC/FPD analytical method
has not been applied in the U.S. surveillance programme. Therefore
propargite has not been reported in foods in channels of commerce in
the U.S.A. No reports from other governments have been received on
occurrence in commerce.
There were no data available on reduction of residues through washing,
cooking or processing. The principal exposure through ingestion would
be from fruits eaten raw and unpeeled.
METHODS OF RESIDUE ANALYSIS
The early residue data on propargite (mid 1960s) were obtained by gas
chromatography with flame ionization or microcouclometric (SO2)
detection. Some difficulties were experienced by government
laboratories in validation studies on the method at that time were
because the clean-up and extraction procedures were unsatisfactory.
Through a series of modifications, a basic procedure using gas
chromatography with flame photometric detection (FPB) has evolved
which is satisfactory for a wide variety of fruits, vegetables, and
animal tissues (Devine and Sisken, 1972).
In the basic method, residues are extracted with 1:1 hexane and
isopropanol, followed by an aqueous NaCl wash and Florisil column
chromatography. For oily or waxy samples an optional step is included
in which the residues are partitioned into nitromethane. Other
modifications are prescribed for certain crops. The method has been
validated in U.S. government laboratories on milk, apples and animal
tissues (PAM III, 1973). Depending on the substrate, the limit of
determination is about 0.05 to 0.2 mg/kg.
A similar GC-FPD method for propargite residue in citrus has been
reported by Westlake et al, (1971).
Propargite is not known to be recovered through the U.S. Food and Drug
Administration multiresidue procedure. Information received from
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Almonds 0.1
Almonds, hulls 55
Apples 3 3 3 3
Apples, pomace, dried 80
Apricots 7 3
Bananas 3
Beans, dry 0.2
Beans, succulent 20
Citrus, dried pulp 40
Corn, fodder 10
Corn, forage 10
Corn, grain 0.1
Cottonseed 0.1 3
Cranberries 10
Eggs 0.1
Figs 3
Grapefruit 5 5
Grapes 10 7
Grapes, pomace 40
Hops 15 3
Hops, dried 30 30
Lemons 5 5
Milk 0.08
NATIONAL TOLERANCES REPORTED TO THE MEETING (Continued)
Tolerance, mg/kg
Commodity USA Canada New Zealand Australia
Milk, fat 2
Mint 50
Nectarines 4 3
Oranges 5 5
Passion fruit 3
Peaches 7 7 3
Peanuts 0.1
Peanuts, forage 10
Peanuts, hay 10
Peanuts, hulls 10 3
Pears 3 3 3
Plums (prunes) 7 3 3
Potatoes 0.1
Raisins 25
Sorghum, fodder 10
Sorghum, forage 10
Sorghum, grain 10
Strawberries 7 7 3
Walnuts 0.1
Meat, meat
by-products and fat
of cattle, goats, hogs,
horses, poultry and
sheep 0.1
Canadian authorities is that it is recovered through their
multiresidue procedure. It is eluted from Florisil in fraction 3 of
the Canadian procedure (15% acetone/hexane).
APPRAISAL
Propargite is an acaricide which has been used on a wide variety of
food crops and ornamentals since its introduction in 1967. Available
information indicates that the product is used in Australia, New
Zealand, Argentina, Mexico, France, South Africa, Italy and the U.S.A.
The primary manufacturer has submitted a report on supervised trials
on 26 crops, all of which were conducted in the U.S.A. Limited residue
data were received from New Zealand on peaches). U.S.A., Canada, New
Zealand and Australia have set national tolerances (MRLs) for
propargite.
Propargite is non-syrtemic by mode of action against mites, and data
indicate that residues which do occur are surface residues. Residues
on the harvested commodities are primarily propargite per se. No
significant metabolites or alteration products have been reported on
harvested crops. However, much of the evidence regarding the fate of
propargite on plants is indirect. It would be desirable to have 35S
or 14C radiotracer studies to confirm the degradation pathways in
plants.
Radioactively labelled propargite (14C) has been used in some animal
experiments, including studies on the rat, cow and chicken. Techniques
used to fractionate and identify the activity were not as definitive
as would be desired, but it is fairly certain that propargite is
hydrolysed in the body to tert-butylphenoxyoyclohexanol and
propargyl alcohol. It is probable that there is further degradation to
butylphenol and cyclohexanediol and excretion as conjugates in the
urine. However, some residues of unchanged parent compound do occur in
milk, body fat and briefly in liver. If any correlations are to be
made between the metabolism of propargite and the related compound
aramite, it may be significant to compare the toxic potential of the
hydrolysis product propargyl alcohol with that of the corresponding
hydrolysis product of eremite, ß-chloroethanol.
An analytical method suitable for regulatory purposes is available. It
is a gas chromatographic method (published) with flame photometric
detection that has been validated in government laboratories.
Propargite is also measured by the multi-residue method employed in
the Canadian surveillance programme. It has not been studies in the
U.S. Food and Drug Administration's multi-residue method.
RECOMMENDATIONS
Temporary maximum residue limits for propargite on the following
commodities are recommended.
Commodity Limit mg/kg Pre-harvest intervals on which
recommendations are based
(days)
Apple pomace 80
Alfalfa hay 75 28
Almond hulls 55 28
Alfalfa, fresh 50 28
Mint hay 50 14
Dried citrus pulp 40
Grape pomace 40
Dried hops 30
Raisins 25
Beans (in pod) 20 7
Corn fodder
and forage 10 30
Cranberries 10 14
Grapes 10 14-21
Peanut hay
and forage 10 14
Sorghum fodder
and forage 10 30 (if silaged)
Apricots, peaches,
plums, nectarines 7 14
Strawberries 7 3
Citrus 5 7
Sorghum grain 5 60
Apples, pears 3
Pigs 3 14
Milk (fat) 2
Beans, dry 0.2 28
Almonds 0.1* 28
Cottonseed 0.1* before bolls open
Eggs 0.1
Fat of poultry 0.1
Fat of meat 0.1
Maize (kernels) 0.1* 30
Milk, whole 0.1
Peanuts (kernels) 0.1* 14
Potatoes 0.1* 14
Walnuts 0.1* 14
* At or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (Before July 1981)
1. A carcinogenic study
DESIRABLE
1. Mutagenicity studies
2. Observations in humans
3. Data from supervised trials in countries other than the USA
4. Information on the occurrence of residues on commodities in
commerce
5. Confirmation of the postulated degradation pathway in plants
using radio-labelled propargite.
REFERENCES
Carson S. (1963) Acute Oral LD50 in Rats. Unpublished report from
Food and Drug Research Lab., submitted by Uniroyal Chemcial, Inc.
Carson S. (1963) Subacute Feeding Studies with D-014 in Rats.
Unpublished report from Food and Drug Research Lab., submitted by
Uniroyal Chemical, Inc.
Haley, G., Kennedy, G.L. and Keplinger, M.L. (1972) Teratogenic Study
with Omite in Albino Rats. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Health Protection Branch (1977) Personal communication, Canada Bu.
Chem. Safety, Health Protection Branch to FAO Panel of Experts, 1977.
Holsing, G.C. (1968) 13-Week Dietary Feeding - Dogs. Unpublished
report from Hazleton Laboratories, Inc., submitted by Uniroyal
Chemical.
Jenkins, D.H., Kennedy, G. and Keplinger, M.L. (1972) Study with Omite
in White Leghorn Chickens. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Kennedy, G., Jenkins, D.H. and Keplinger, M.L. (1970) Metabolism of
14C-Omite in the Cow. Unpublished report from Industrial Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Ladd, R., Jenkins, D.H. and Keplinger, M.L. (1974) Tissue Residue
Study with Omite in Crossbred Swine. Unpublished report from Bio-Test
Laboratories, submitted by Uniroyal Chemical.
Oser, B.L. (1966) Chronic (2-Year) Feeding Studies with D-014 in Rate
and Dogs. Unpublished report from Food and Drug Research Lab.9
submitted by Uniroyal Chemical.
PAM II Pesticide Analytical Manual, Vol. II, revised (1973); U.S.
Department of Health, Education and Welfare, Food and Drug
Administration.
Ryer, F.H. and Sullivany J.B. (1969) Rachiotracer Drug Metabolism
Study with Omite 14C. Unpublished report from Hazleton Laboratories,
Inc., submitted by Uniroyal Chemical.
Ryer, F.H. and Sullivany J.B. (1969b) The Fate of Omite 14C in the
Cow. Unpublished report from Hazleton Laboratoriesy Inc., submitted by
Uniroyal Chemical.
Sleight III, B.H. and Maceky K.J. (1972) 14C Omite Fish Accumulation.
Unpublished report from Bionomics, Inc., submitted by Uniroyal
Chemical.
Smith, K.S. and Roger, J.C. (1975) Omite Milk and Tissue Residue Study
in Dairy Cows: Unpublished report from Cannon Laboratories, submitted
by Uniroyal Chemical.
Sullivan, J.B. (1968) An Investigation of the In-Vitro and In-Vivo
Metabolism of Omite. Unpublished report from Hazleton Laboratories
Inc., submitted by Uniroyal Chemical.
Uniroyal Unpublished information (1977), Uniroyal Inc., to U.S.
Environmental Protection Agency.
Weir, RJ. (1967) Acute Dermal Application to Rabbits. Unpublished
report from Hazleton Laboratories Inc., submitted by Uniroyal
Chemical.
Westlake, W.E., Gunther, P.A. and Jeppson, L.R. J. Agr. Fd. Chem.,
19:894.
