
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
PROPARGITE
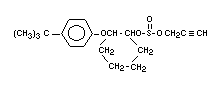 Explanation
Propargite was reviewed by the Joint Meeting of 1977 (FAO/WHO
1978)1 and a temporary ADI of 0-0.08 mg/kg b.w. was determined. The
JMPR requested that a carcinogenicity study be performed and submitted
for review prior to July 1981. The subject study was not made
available for review. The teratology study in rats was performed by
Industrial Bio-Test Laboratories (IBT) and a replacement study has
subsequently been provided and reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 20-30 pregnant Sprague-Dawley rats were administered
propargite by gavage at levels of 0, 6, 25 or 105 mg/kg/day from days
6 through 15 of gestation. Maize oil and aspirin served as the vehicle
(negative) and positive control groups, respectively. On day 20 of
gestation, all pregnant animals were sacrificed and the foetuses
delivered by caesarean section. Maternal observations included
cageside observations, body weights, numbers of corpora lutea,
implantation sites, resorption sites and live and dead foetuses. Sex
of foetuses and body weights of foetuses were also determined. Each
foetus was examined grossly for abnormalities, as well as for detailed
visceral and skeletal anomalies and/or variations.
1 See Annex 2 for WHO and FAO documentation.
There were no compound-related effects on maternal or foetal body
weights, pregnancy implantations, number of live/dead foetuses or
number of resorption sites, except in the positive control (aspirin,
250 mg/kg) groups where both maternal and foetal weights were
decreased, number of live foetuses per dam decreased and the number of
resorption sites were increased. The only noticeable effect of
propargite was an increase in maternal death at 105 mg/kg bw.
Visceral examination did not reveal any dose-related effects
other than small pups at 105 mg/kg bw. Again, in the positive controls
there were significant increases in spina bifida and
encephalomeningocele.
Skeletal variations consisted primarily of incomplete
ossification, missing sternebrae and retarded hyoid development, which
occurred with greater frequency at 25 and 105 mg/kg bw than in the
low-dose or vehicle control groups. Also noted at the high dose was
incomplete closure of the skull. These skeletal variations are common
in this species, are often considered an expression of maternal
toxicity and their biological significance is not clearly understood
at present. There were increased occurrences of these same variations
in the positive control group, along with fused/split ribs,
extranumery or rudimentary ribs and incomplete ossification of
extremities.
Propargite was not teratogenic in rats at any dose level
administered, although there was evidence of maternal toxicity at 25
and 105 mg/kg bw (Knickerbocker 1979).
COMMENTS
The ADI and supporting data were reviewed by the JMPR in 1977
(FAO/WHO 1978). No additional data has been received that would alter
those considerations.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 300 mg/kg in the diet, equivalent to 15 mg/kg bw.
Dog : 900 mg/kg in the diet, equivalent to 22 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.15 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Mutagenicity studies.
2. Observations in humans.
REFERENCES
Knickerbocker, M. Teratogenic evaluation of Omite technical in
1979 Sprague-Dawley rats. Food and Drug Research Laboratories,
Inc. Report submitted to the World Health Organization by
Uniroyal Chemical Co. (Unpublished)
PROPARGITE
Explanation
Propargite was reviewed by the Joint Meeting of 1977 (FAO/WHO
1978)1 and a temporary ADI of 0-0.08 mg/kg b.w. was determined. The
JMPR requested that a carcinogenicity study be performed and submitted
for review prior to July 1981. The subject study was not made
available for review. The teratology study in rats was performed by
Industrial Bio-Test Laboratories (IBT) and a replacement study has
subsequently been provided and reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 20-30 pregnant Sprague-Dawley rats were administered
propargite by gavage at levels of 0, 6, 25 or 105 mg/kg/day from days
6 through 15 of gestation. Maize oil and aspirin served as the vehicle
(negative) and positive control groups, respectively. On day 20 of
gestation, all pregnant animals were sacrificed and the foetuses
delivered by caesarean section. Maternal observations included
cageside observations, body weights, numbers of corpora lutea,
implantation sites, resorption sites and live and dead foetuses. Sex
of foetuses and body weights of foetuses were also determined. Each
foetus was examined grossly for abnormalities, as well as for detailed
visceral and skeletal anomalies and/or variations.
1 See Annex 2 for WHO and FAO documentation.
There were no compound-related effects on maternal or foetal body
weights, pregnancy implantations, number of live/dead foetuses or
number of resorption sites, except in the positive control (aspirin,
250 mg/kg) groups where both maternal and foetal weights were
decreased, number of live foetuses per dam decreased and the number of
resorption sites were increased. The only noticeable effect of
propargite was an increase in maternal death at 105 mg/kg bw.
Visceral examination did not reveal any dose-related effects
other than small pups at 105 mg/kg bw. Again, in the positive controls
there were significant increases in spina bifida and
encephalomeningocele.
Skeletal variations consisted primarily of incomplete
ossification, missing sternebrae and retarded hyoid development, which
occurred with greater frequency at 25 and 105 mg/kg bw than in the
low-dose or vehicle control groups. Also noted at the high dose was
incomplete closure of the skull. These skeletal variations are common
in this species, are often considered an expression of maternal
toxicity and their biological significance is not clearly understood
at present. There were increased occurrences of these same variations
in the positive control group, along with fused/split ribs,
extranumery or rudimentary ribs and incomplete ossification of
extremities.
Propargite was not teratogenic in rats at any dose level
administered, although there was evidence of maternal toxicity at 25
and 105 mg/kg bw (Knickerbocker 1979).
COMMENTS
The ADI and supporting data were reviewed by the JMPR in 1977
(FAO/WHO 1978). No additional data has been received that would alter
those considerations.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 300 mg/kg in the diet, equivalent to 15 mg/kg bw.
Dog : 900 mg/kg in the diet, equivalent to 22 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.15 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Mutagenicity studies.
2. Observations in humans.
REFERENCES
Knickerbocker, M. Teratogenic evaluation of Omite technical in
1979 Sprague-Dawley rats. Food and Drug Research Laboratories,
Inc. Report submitted to the World Health Organization by
Uniroyal Chemical Co. (Unpublished)
PROPARGITE
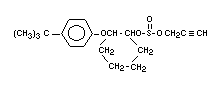 Explanation
Propargite was evaluated in 1977, 1978, 1979 and 1980 (FAO/WHO
1978b, 1979b, 1980b and 1981b)1 at which times several temporary
maximum residue levels were either estimated or revised. The Joint
Meeting has considered it desirable to have additional information
on the metabolic fate of propargite in plants, especially
characterization of substantial unidentified residues. Also desired
was data from supervised trials in countries other than the U.S. and
information on propargite residues on commodities in commerce.
Additional studies on the metabolism of propargite in plants and
residue data from countries other than the U.S. (including for tea and
resulting from discussions at the 14th Session of the Codex Committee
on Pesticides Residues) have been made available and are reviewed in
this monograph addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
No information on nationally-approved agricultural practices has
been provided to the Meeting for those commodities and for those
countries from which additional residue data are available. The
Meeting was informed that registration was discontinued in The
Netherlands in 1976.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Propargite residue data resulting from supervised trials in
several countries other than the U.S. on several commodities are
summarized in Table 1 (Uniroyal 1982). Since no nationally-approved
agricultural practice information for these countries or on these
commodities was available, previously submitted good agricultural
practice information from other countries was taken into consideration
to the extent possible in evaluating the data.
1 See Annex 2 for FAO and WHO documentation, JMPR 1982 389-398
Table 1. Propargite Residues from Supervised Trials
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Apples U.K. 1 2 0.75 57E
(0.07%)
(Ross) 1979 8 0.96 <0.03
16 0.80 0.04
23 0.56 <0.02
(Cox, O.P.) 7 0.68 <0.02
10 0.52 0.03
18 0.33 <0.02
(Lascton 1 0.86 57E 0 1.5-1.9 <0.02-0.3
Superb, Cox, (0.17%) (1.7 av.) 2 (0.1 av.) 2
Bramley, 1981 orchard 7 1.1-1.9
Egremont sprayer (1.5 av.) 2
Russet) 14 0.6-1.7
(1.1 av.) 2
21 0.51, 0.73
Poland
(Mcintosh, 1 0.75 30W 80 <0.05, <0.05, <0.05,
Jonathan, (0.075%) <0.05, <0.05
Lobo) high <0.05
volume
mist
blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
(Mcintosh, 1 0.9 30W 55 0.21, 0.09 <0.05, <0.05,
Starking, (0.06%) 0.39, 0.16 <0.05
Bancroft, high
Idamont) volume
mist
blower
(Mcintosh, 1 0.9 30W 55 <0.05,
Starking, (0.06%) <0.05
Boskop) <0.05
(Grenadier) U.K. 1 1.0 57E 7 1.1 0.04, 0.26,
1980 high 14 0.89 0.025
volume 21 1
mist
blower
(Cox) 2 1.0 57E 4 2.4 0.11, 0.02,
high 7 2 0.05
volume 14 1.5
mist blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Black currants 1 U.K. 1 0.86 57E 0 5.1 <0.03
(Baldwin) (0.043%) 7 2.5 <0.03
1981 Knapsack 14 1.2
21 3.5,
2.3
0 2 <0.02
7 0.94 <0.02
14 0.80
21 0.27
Hops, green U.K. 1 0.86 57E 0 12 <0.1
(Wye) 1981 (0.09%) 7 1.2 (3 samples)
mist blower 14 1.5
29 0.61 4
(WVG) 0 3.9 <0.1
7 3.1 (3 samples)
14 3.1
17 2.7 4
Plums, fresh U.K. 1 0.86 57E 0 0.29 <0.01
(Victoria) (0.09%) 0.35
orchard 7 0.53
sprayer 14 0.35
21 0.47
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
England
(Czar) 0 0.67 <0.01
7 1.5
1.1
14 0.58
(Victory) 0 0.33
7 0.28
14 0.16
21 0.13
Tea India 1 0.75 5 57E 7 0.45- 0.0 3
1976 0.66
(0.51 avg. of
6 samples)
1.5 5 57E 7 0.89
2.2
(1.3 avg. of
12 samples)
Tomatoes 1 South Africa 2 0.56 8 57E 1 7 1.2 0.014,
(0.07%) 2 7 0.85 <0.02,
1980 4 7 1.6 0.023
6 6 0.83
8 7 0.034
0.046
16 7 0.29
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
2 1.12 57E 1 7 3.6
(0.14%) 2 7 2.0
4 7 2.1
6 6 2.3
8 7 2.6
16 7 0.97
1 Residues corrected for analytical recoveries;
2 Four samples;
3 A 0.06 mg/kg limit of detection claimed;
4 Commercially dried;
5 Weather described as "wet";
6 6 days after first treatment;
7 Days after second treatment, the one day value is 7 days after first treatment;
8 Late frulting stage at treatment.
Except in the case of tea, in which Soxhlet extraction was used,
and black currants, where the analytical method was not identified,
the basic method (Devine and Sisken 1972) or modifications of it was
used for developing data in Table 1. Recoveries were generally 80% or
better.
Apples
Residues on several varieties of apples in the U.K. and Poland
resulting from up to two applications at rates and preharvest
intervals consistent with those on which the current 5 mg/kg limit is
based are within that limit. The 5 mg/kg limit is confirmed.
Black Currants
Residues resulting from one application at 0.86 kg/ha in England
range from a high level of 5.1 mg/kg on day of application to
0.27 mg/kg at 21 days after last application. Although maximum
residues are less than the 10 mg/kg limits for other small fruits, a
limit could not be estimated in the absence of nationally approved
agricultural information for currants. In addition to good
agricultural practice information from U.K., additional residue data
and good agricultural information from countries other than U.K. would
be desirable for estimating maximum residue levels for black currants.
The manufacturer has informed the Meeting that additional studies are
in progress.
Hops
No information was available on nationally approved agricultural
practices in the country from which additional residue data were
available. With a single application at half the maximum rate, the two
applications and 14-day preharvest interval used as a basis for the
current 30 mg/kg limit, residues are well below that limit on both
green and dried hops. However, there is a possibility that field
incurred residues in dried hops, both in the new studies and those
previously reviewed, were not quantitatively extracted by the standard
analytical procedure (see discussion under "Tea"),
Plums
Although no information on nationally approved agricultural
practice was available from the country in which the residue trials
were conducted, residues from single applications of the EC
formulation at application rates similar to those for other
formulations used as a basis for the current 7 mg/kg limit on plums
are well below that limit, even on day of application. The current
limit is based on a 14-day interval from last application to harvest.
Tea
The current 5 mg/kg limit is based on application of a 57E
formulation at a rate of 0.75 kg a.i./ha and a 7-day preharvest
interval. Maximum residues in several samples based on this good
agricultural practice (confirmed to this Meeting) were approximately
4 mg/kg (FAO/WHO 1978b, 1979b). Based on data submitted to this
Meeting, analytical methodology used in developing previously reviewed
residue data does not efficiently extract residues from tea, dry or
manufactured. The comparative extraction efficiencies of a Soxhlet
extraction (see Methods of Analysis) and the standard procedure
(Devine and Sisken 1972) are summarized in Table 2.
Table 2. Comparative Extraction of Propargite from Tea (mg/kg)
Sample Extraction Technique
Standard Procedure Soxhlet Factor
1 0.2 1.0 5
2 0.3 0.9 3
3 (sun dried) 0.6 1.5 2.5
4 (sun dried) 0.57 1.2 2.1
5 (sun dried) 0.52 1.2 2.3
6 (manufactured) 1.0 2.0 2
7 (manufactured) 1.3 2.0 1.5
8 (manufactured) 1.0 2.2 2.2
Average 2.6
Additional residue data (Table 1) in which Soxhlet extraction was
utilized suggest that the current 5 mg/kg limit is adequate, even
after using twice the recommended application rate. These data are
consistent with much of the data previously reviewed. However, because
residues from several samples previously reviewed (which were analysed
by the standard procedure) were approximately 4 mg/kg, a 2-2.5 times
increase in extraction efficiency would result in residues of about
10 mg/kg.
Other information provided to the Meeting shows that even with
the standard procedure rehydration of sun-dried tea by soaking
overnight with "2 times amount of water" can double the extraction
efficiency.
Tomatoes
If the formulation and uses employed during the field trials
indicated in Table 1 represent that country's nationally approved
agricultural practices, the current 2 mg/kg limit for tomatoes is too
low. A 5 mg/kg limit may be needed.
FATE OF RESIDUES
In Plants
It is known that propargite is the largest component of the
residue in plants and that the residue is primarily a surface one.
There is evidence that it is not translocated to an appreciable extent
and that losses are largely through volatilization. It has been
hypothesized that propargyl alcohol and the propargite glycol ether
(tertbutylphenoxycyclohexanol) are the primary metabolites, with
further degradation to other moieties, but there has been little
experimental evidence to confirm this. There has been some evidence
for trace amounts of the glycol ether. Unidentified residues in plants
have varied from 6-50% of total residues, with some not entirely
conclusive evidence in peaches that no one component accounts for
appreciably more than 10% of the total extractable residue. The Joint
Meeting considered additional studies on the metabolic fate of
propargite in plants desirable, especially for unidentified residues
and the possibility of residues of the glycol ether. Additional
studies have been provided in response to this request for further
information.
The metabolic fate of propargite in green beans, green bean
callus tissue and maize callus tissue was investigated (Lengen 1982).
Potted greenhouse grown green beans were sprayed or painted with
14C-labelled propargite in a formulation with a specific activity of
0.5 ÁCi/ÁM. The spray application was at a rate equivalent to 4.2 kg
propargite/ha and beans were harvested 7 days later.
Bean callus tissue was initiated from small pieces of immature
pods, grown for two weeks, injected with the labelled formulation and
harvested seven days after treatment. Similar experiments were
conducted in maize callus (roots).
Samples were extracted with methanol, some aliquots of which were
partitioned further with chloroform. After concentration under a flow
of nitrogen, using two solvent systems and X-ray film for location
of bands, extracted residues were characterized by thin-layer
chromatography. Mass spectrometry was used to confirm the identity
of propargite. Unextracted residues were determined by combustion.
Total residues in sprayed beans were 22 mg/kg at seven days. The
distribution of propargite, the glycol ether and unidentified
residues in bean and corn tissues are summarized in Table 3. The
chromatographic behaviour, partitioning characteristics and
distribution of residues extracted from beans are summarized in
Table 4.
Table 3 Distribution 1 of Residues in Propargite-Treated Beans 2
and Bean and Maize Callus Tissue
Sprayed Bean Bean Callus Maize Callus
Propargite 80.2 76.0 96.0
Propargite 1.0 2.4 <1
glycol ether
Polar 3 9.9 12.2 1.1
Unextracted 4.1 2.8 <1
1 Percent of total residue;
2 Total residues equivalent to 22 mg/kg;
3 Resolved into a minimum of six components.
Table 3 confirmed earlier studies, which show that propargite is
the principal residue and that low residues of the glycol ether may be
present. The level of unidentified residues in beans at 14% is within
the range found in previous studies on plants, but less than the
maximum. Propargite is shown to be less extensively metabolized in
maize callus than in beans.
Table 4 confirms that most of the residue is propargite which is
organosoluble, and supports earlier findings suggesting the presence
of glycol ether. If present, the ether comprises approximately 1% or
less of the total in sprayed beans. Most of the aqueous partition
fraction is polar material, although comparable levels of polar
materials are also found in the chloroform fraction. Table 4 also
shows that the 10% polar materials of Table 3 are composed of at least
six unidentified components. No one of the six bands comprises more
than 8.4% (band one) of the total (aqueous and organosoluble)
extracted residue. Further investigation of band one with a second
solvent system shows it to consist of eight unidentified and
unquantified components in bean pods and six in maize callus. Three of
the six in corn callus co-chromatograph with three found in the beans.
Table 4. Distrlhution of 14C-Labelled Components on TLC of 14C-Omite Treated Blue Lake Bean Tissue 1
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
1 0.0-0.03 3.2 (3.0) 3.2 (3.1) 5.4 (4.9)
2 0.03-0.11 0.7 (0.6) 0.9 (0.9) 1.0 (0.9)
3 0.11-0.19 3 0.9 (0.8) 1.0 (1.0) 2.5 (2.3)
4 0.19-0.27 0.9 (0.8) 0.9 (0.9) 1.2 (1.1)
5 0.27-0.37 5.1 (4.7) 4.6 (4.4) 6.0 (5.5)
6(Omite) 0.37-0.36 87.3 (81.0) 83.9 (80.7) 81.9 (74.9)
7 0.46-1.0 1.9 (1.8) 5.4 (5.2) 1.8 (1.6)
Table 4. (con't)
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
Percent of metabolites in the aqueous fraction 2
1 0.0-0.03 75.8 (5.4) 72.1 (2.7) 87.2 (7.4)
2,3,4,5 0.03-0.21 8.0 (0.6) 8.3 (0.3) 5.1 (0.4)
6 (Omite) 0.21-0.29 9.6 (0.7) 13.0 (0.5) 3.6 (0.3)
7 0.29-1.0 6.6 (0.5) 6.6 (0.3) 4.1 (0.3)
1 TLC = double or single development in cyclohexane-ethyl acetate (9:1); interval is 7 days.
2 The Rfs and percentages are an average of two plates.
3 Co-chromatography with glycol ether.
METHODS OF RESIDUE ANALYSIS
The analytical method for Soxhlet extraction of propargite from
tea was provided to the Meeting and was used to develop the data in
Table 1. Twenty-five grams of tea in a Soxhlet thimble underwent
extraction in a unit containing 300 ml hexane for 16 hours. A 10 g
aliquot was eluted from a 15 cm florosil (activated at 130░C) column
with 2% acetone in hexane after an initial elution with 100 ml of
benzene, which was discarded. After concentration to 2 ml, GLC
analysis was conducted, using a sulphur specific flame photometric
detector. The limit of detection was said to be approximately
0.05 mg/kg, although of two untreated samples analysed when the
standard procedure was compared to Soxhlet extraction, residues were
listed as 0.0 and 1.96 mg/kg. Residues in two untreated samples during
the field trials were listed as 0.0 mg/kg.
The analytical method was validated at 0.5 to 2 mg/kg
fortification levels with recoveries >81%. It would appear that
analytical methods of choice for tea should include a Soxhlet
extraction step. The standard analytical method, while not preferred
for tea, can be improved for tea by a rehydration step if it is used.
APPRAISAL
The Meeting was provided with additional studies characterizing
propargite residues in plants and results from some additional residue
studies in countries other than the U.S. Both items of information had
been considered desirable by the Joint Meeting.
No information was available on nationally approved uses in the
countries from which residue data were available. Based on good
agricultural practices, the current limit for apples, hops and plums
could be confirmed. However, new information on the efficiency of
extraction of propargite residues from dried commodities leaves some
doubt as to the adequacy of the current limit for hops. Additional
residue data from field incurred residues in dried hops resulting from
good agricultural practices expected to give maximum residues is
desirable, using an analytical method employing a Soxhlet extraction
step. An alternative, but less desirable, analytical method could be
the standard procedure modified width a rehydration step.
The available data and lack of information on nationally approved
agricultural practices does not permit estimation of a limit on black
currants at this time.
New information on the extraction efficiency of the standard
analytical procedure for propargite in dried tea supports an increase
in that limit and suggests that analyses of all dried commodities for
propargite should use an analytical method employing a Soxhlet type
extraction step. The standard analytical method of Levine and Sisken
modified with a rehydration step appears to be an alternative but less
desirable method for dried commodities.
In the case of tomatoes, additional data suggest that the current
2 mg/kg limit may be too low. However, in the absence of information
on nationally approved agricultural practices in countries from which
data have been provided, there is no basis for revising the current
limit.
Recently conducted studies on the metabolic fate of propargite in
beans confirm that propargite is the major single residue and support
earlier studies suggesting the presence of low levels of propargite
glycol ether (1% as compared to 7% in an earlier study on dried bean
pods). Qualitatively, unidentified moieties are shown to consist
largely of polar materials, which is similar to findings in studies
previously reviewed by the Joint Meeting. Quantitatively, and under
different experimental conditions (including some substantially longer
preharvest intervals), some of the earlier studies have shown
unidentified residues to constitute over half of the total residue.
In those earlier metabolism studies in dry bean pods (FAO/WHO
1981b) residues were extracted with methanol/acetone, whereas in field
trials on dry beans (FAO/WHO 1978b) residues were low but the standard
extraction procedure utilizes hexane/isopropanol, which may not have
extracted all of the polar materials. In the studies submitted to this
Meeting, unidentified residues constituted approximately 20% of the
total and extraction was with methanol.
While the new studies do give further confirmation of earlier
findings, no new information is available on the actual identity of
metabolites/degradation products that would allow confirmation of the
postulated metabolic pathway of propargite in plants. The additional
study indicating that no one component of the metabolites constitutes
more than 10% of the total residue in beans does give some
reassurance. If this same statement could be made under those
conditions resulting in unidentified residues at over 50% of total
residue, even greater assurance would be given. Under these
circumstances, the actual identity of the major unidentified residues
is still desirable.
RECOMMENDATIONS
In the case of tea, the Meeting concluded that the existing
temporary limit should be revised as indicated. For other commodities
data either confirmed existing limits or were insufficient to support
revision of existing limits of estimation of new levels.
Commodity Estimated Maximum Residue Level (mg/kg)
Tea 10 (5)
FURTHER WORK OR INFORMATION
Desirable
1. Information on nationally approved good agricultural practices,
especially from those countries in which field trials have been
conducted and for which residue data have been provided to the
1982 Joint Meeting, or which may be provided to a future Joint
Meeting.
2. Residue data from field incurred residues in dried hops resulting
from good agricultural practices expected to result in maximum
residues and analysed by a validated analytical method utilizing
a Soxhlet type extraction step.
3. Actual identification of the major propargite plant metabolite
degradation products.
4. Information on the occurrence of residues in commodities in
commerce.
REFERENCES
Devine, J.M. and Sisken, H.R. Use of flame photometric detector
1972 for determining residues of Omite [2-(p tert-
butylphenoxy)cyclohexy) propargyl sulfite] Journal of
Agricultural Food Chem. 20: 59-61.
Lengen, M. Propargite metabolism in beans and corn, Project No. 8107,
1982 June 1982. Studies conducted by Galye H. Davidonis and Ralph
O. Mumma, Pennsylvania State University 9 June 1982.
(Unpublished)
Uniroyal. Residue data submitted to the 1982 Joint Meeting by Uniroyal
1982 Chemical, Division of Uniroyal, Inc,, Agricultural Chemicals
Research and Development, 74 Amity Road, Bethany, CT 06525.
Determination of Omite residues in apples, J. 2774,
DM79/2381D, J2774, DM79/237D, 20 May 1980; 217/4; M81/19B,
C, D and E, M81/20B, C, D and E; M81/21B, C, D and E;
M81/22B, C, D and E, 28 October, 1981; J3225, M80/250,
M80/251, 28 November, 1980. Residues of propargite in apples
by Dr. Boleslaw Salmonowicz, March 1982. Determination of
Omite residues in Currants, 217/1; M81/25, 217/1, M81/26,
28 October 1981; Determination of Omite residues in Hops,
21713; M81/23, 21713, M81/24, July 1982; Determination of
Omite residues in plums, 217/2, 217/2, M81/27, 217/2,
M81/28, 28 October 1981.
Omite residues in tomatoes, J2911, 8 May 1980.
Data submitted to the 1982 JMPR and initially developed and
submitted 19 January 1977 to the U.S. Environmental
Protection Agency in support of U.S. pesticide tolerance
petition FAP6H5100. (Unpublished)
Explanation
Propargite was evaluated in 1977, 1978, 1979 and 1980 (FAO/WHO
1978b, 1979b, 1980b and 1981b)1 at which times several temporary
maximum residue levels were either estimated or revised. The Joint
Meeting has considered it desirable to have additional information
on the metabolic fate of propargite in plants, especially
characterization of substantial unidentified residues. Also desired
was data from supervised trials in countries other than the U.S. and
information on propargite residues on commodities in commerce.
Additional studies on the metabolism of propargite in plants and
residue data from countries other than the U.S. (including for tea and
resulting from discussions at the 14th Session of the Codex Committee
on Pesticides Residues) have been made available and are reviewed in
this monograph addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
No information on nationally-approved agricultural practices has
been provided to the Meeting for those commodities and for those
countries from which additional residue data are available. The
Meeting was informed that registration was discontinued in The
Netherlands in 1976.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Propargite residue data resulting from supervised trials in
several countries other than the U.S. on several commodities are
summarized in Table 1 (Uniroyal 1982). Since no nationally-approved
agricultural practice information for these countries or on these
commodities was available, previously submitted good agricultural
practice information from other countries was taken into consideration
to the extent possible in evaluating the data.
1 See Annex 2 for FAO and WHO documentation, JMPR 1982 389-398
Table 1. Propargite Residues from Supervised Trials
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Apples U.K. 1 2 0.75 57E
(0.07%)
(Ross) 1979 8 0.96 <0.03
16 0.80 0.04
23 0.56 <0.02
(Cox, O.P.) 7 0.68 <0.02
10 0.52 0.03
18 0.33 <0.02
(Lascton 1 0.86 57E 0 1.5-1.9 <0.02-0.3
Superb, Cox, (0.17%) (1.7 av.) 2 (0.1 av.) 2
Bramley, 1981 orchard 7 1.1-1.9
Egremont sprayer (1.5 av.) 2
Russet) 14 0.6-1.7
(1.1 av.) 2
21 0.51, 0.73
Poland
(Mcintosh, 1 0.75 30W 80 <0.05, <0.05, <0.05,
Jonathan, (0.075%) <0.05, <0.05
Lobo) high <0.05
volume
mist
blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
(Mcintosh, 1 0.9 30W 55 0.21, 0.09 <0.05, <0.05,
Starking, (0.06%) 0.39, 0.16 <0.05
Bancroft, high
Idamont) volume
mist
blower
(Mcintosh, 1 0.9 30W 55 <0.05,
Starking, (0.06%) <0.05
Boskop) <0.05
(Grenadier) U.K. 1 1.0 57E 7 1.1 0.04, 0.26,
1980 high 14 0.89 0.025
volume 21 1
mist
blower
(Cox) 2 1.0 57E 4 2.4 0.11, 0.02,
high 7 2 0.05
volume 14 1.5
mist blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Black currants 1 U.K. 1 0.86 57E 0 5.1 <0.03
(Baldwin) (0.043%) 7 2.5 <0.03
1981 Knapsack 14 1.2
21 3.5,
2.3
0 2 <0.02
7 0.94 <0.02
14 0.80
21 0.27
Hops, green U.K. 1 0.86 57E 0 12 <0.1
(Wye) 1981 (0.09%) 7 1.2 (3 samples)
mist blower 14 1.5
29 0.61 4
(WVG) 0 3.9 <0.1
7 3.1 (3 samples)
14 3.1
17 2.7 4
Plums, fresh U.K. 1 0.86 57E 0 0.29 <0.01
(Victoria) (0.09%) 0.35
orchard 7 0.53
sprayer 14 0.35
21 0.47
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
England
(Czar) 0 0.67 <0.01
7 1.5
1.1
14 0.58
(Victory) 0 0.33
7 0.28
14 0.16
21 0.13
Tea India 1 0.75 5 57E 7 0.45- 0.0 3
1976 0.66
(0.51 avg. of
6 samples)
1.5 5 57E 7 0.89
2.2
(1.3 avg. of
12 samples)
Tomatoes 1 South Africa 2 0.56 8 57E 1 7 1.2 0.014,
(0.07%) 2 7 0.85 <0.02,
1980 4 7 1.6 0.023
6 6 0.83
8 7 0.034
0.046
16 7 0.29
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
2 1.12 57E 1 7 3.6
(0.14%) 2 7 2.0
4 7 2.1
6 6 2.3
8 7 2.6
16 7 0.97
1 Residues corrected for analytical recoveries;
2 Four samples;
3 A 0.06 mg/kg limit of detection claimed;
4 Commercially dried;
5 Weather described as "wet";
6 6 days after first treatment;
7 Days after second treatment, the one day value is 7 days after first treatment;
8 Late frulting stage at treatment.
Except in the case of tea, in which Soxhlet extraction was used,
and black currants, where the analytical method was not identified,
the basic method (Devine and Sisken 1972) or modifications of it was
used for developing data in Table 1. Recoveries were generally 80% or
better.
Apples
Residues on several varieties of apples in the U.K. and Poland
resulting from up to two applications at rates and preharvest
intervals consistent with those on which the current 5 mg/kg limit is
based are within that limit. The 5 mg/kg limit is confirmed.
Black Currants
Residues resulting from one application at 0.86 kg/ha in England
range from a high level of 5.1 mg/kg on day of application to
0.27 mg/kg at 21 days after last application. Although maximum
residues are less than the 10 mg/kg limits for other small fruits, a
limit could not be estimated in the absence of nationally approved
agricultural information for currants. In addition to good
agricultural practice information from U.K., additional residue data
and good agricultural information from countries other than U.K. would
be desirable for estimating maximum residue levels for black currants.
The manufacturer has informed the Meeting that additional studies are
in progress.
Hops
No information was available on nationally approved agricultural
practices in the country from which additional residue data were
available. With a single application at half the maximum rate, the two
applications and 14-day preharvest interval used as a basis for the
current 30 mg/kg limit, residues are well below that limit on both
green and dried hops. However, there is a possibility that field
incurred residues in dried hops, both in the new studies and those
previously reviewed, were not quantitatively extracted by the standard
analytical procedure (see discussion under "Tea"),
Plums
Although no information on nationally approved agricultural
practice was available from the country in which the residue trials
were conducted, residues from single applications of the EC
formulation at application rates similar to those for other
formulations used as a basis for the current 7 mg/kg limit on plums
are well below that limit, even on day of application. The current
limit is based on a 14-day interval from last application to harvest.
Tea
The current 5 mg/kg limit is based on application of a 57E
formulation at a rate of 0.75 kg a.i./ha and a 7-day preharvest
interval. Maximum residues in several samples based on this good
agricultural practice (confirmed to this Meeting) were approximately
4 mg/kg (FAO/WHO 1978b, 1979b). Based on data submitted to this
Meeting, analytical methodology used in developing previously reviewed
residue data does not efficiently extract residues from tea, dry or
manufactured. The comparative extraction efficiencies of a Soxhlet
extraction (see Methods of Analysis) and the standard procedure
(Devine and Sisken 1972) are summarized in Table 2.
Table 2. Comparative Extraction of Propargite from Tea (mg/kg)
Sample Extraction Technique
Standard Procedure Soxhlet Factor
1 0.2 1.0 5
2 0.3 0.9 3
3 (sun dried) 0.6 1.5 2.5
4 (sun dried) 0.57 1.2 2.1
5 (sun dried) 0.52 1.2 2.3
6 (manufactured) 1.0 2.0 2
7 (manufactured) 1.3 2.0 1.5
8 (manufactured) 1.0 2.2 2.2
Average 2.6
Additional residue data (Table 1) in which Soxhlet extraction was
utilized suggest that the current 5 mg/kg limit is adequate, even
after using twice the recommended application rate. These data are
consistent with much of the data previously reviewed. However, because
residues from several samples previously reviewed (which were analysed
by the standard procedure) were approximately 4 mg/kg, a 2-2.5 times
increase in extraction efficiency would result in residues of about
10 mg/kg.
Other information provided to the Meeting shows that even with
the standard procedure rehydration of sun-dried tea by soaking
overnight with "2 times amount of water" can double the extraction
efficiency.
Tomatoes
If the formulation and uses employed during the field trials
indicated in Table 1 represent that country's nationally approved
agricultural practices, the current 2 mg/kg limit for tomatoes is too
low. A 5 mg/kg limit may be needed.
FATE OF RESIDUES
In Plants
It is known that propargite is the largest component of the
residue in plants and that the residue is primarily a surface one.
There is evidence that it is not translocated to an appreciable extent
and that losses are largely through volatilization. It has been
hypothesized that propargyl alcohol and the propargite glycol ether
(tertbutylphenoxycyclohexanol) are the primary metabolites, with
further degradation to other moieties, but there has been little
experimental evidence to confirm this. There has been some evidence
for trace amounts of the glycol ether. Unidentified residues in plants
have varied from 6-50% of total residues, with some not entirely
conclusive evidence in peaches that no one component accounts for
appreciably more than 10% of the total extractable residue. The Joint
Meeting considered additional studies on the metabolic fate of
propargite in plants desirable, especially for unidentified residues
and the possibility of residues of the glycol ether. Additional
studies have been provided in response to this request for further
information.
The metabolic fate of propargite in green beans, green bean
callus tissue and maize callus tissue was investigated (Lengen 1982).
Potted greenhouse grown green beans were sprayed or painted with
14C-labelled propargite in a formulation with a specific activity of
0.5 ÁCi/ÁM. The spray application was at a rate equivalent to 4.2 kg
propargite/ha and beans were harvested 7 days later.
Bean callus tissue was initiated from small pieces of immature
pods, grown for two weeks, injected with the labelled formulation and
harvested seven days after treatment. Similar experiments were
conducted in maize callus (roots).
Samples were extracted with methanol, some aliquots of which were
partitioned further with chloroform. After concentration under a flow
of nitrogen, using two solvent systems and X-ray film for location
of bands, extracted residues were characterized by thin-layer
chromatography. Mass spectrometry was used to confirm the identity
of propargite. Unextracted residues were determined by combustion.
Total residues in sprayed beans were 22 mg/kg at seven days. The
distribution of propargite, the glycol ether and unidentified
residues in bean and corn tissues are summarized in Table 3. The
chromatographic behaviour, partitioning characteristics and
distribution of residues extracted from beans are summarized in
Table 4.
Table 3 Distribution 1 of Residues in Propargite-Treated Beans 2
and Bean and Maize Callus Tissue
Sprayed Bean Bean Callus Maize Callus
Propargite 80.2 76.0 96.0
Propargite 1.0 2.4 <1
glycol ether
Polar 3 9.9 12.2 1.1
Unextracted 4.1 2.8 <1
1 Percent of total residue;
2 Total residues equivalent to 22 mg/kg;
3 Resolved into a minimum of six components.
Table 3 confirmed earlier studies, which show that propargite is
the principal residue and that low residues of the glycol ether may be
present. The level of unidentified residues in beans at 14% is within
the range found in previous studies on plants, but less than the
maximum. Propargite is shown to be less extensively metabolized in
maize callus than in beans.
Table 4 confirms that most of the residue is propargite which is
organosoluble, and supports earlier findings suggesting the presence
of glycol ether. If present, the ether comprises approximately 1% or
less of the total in sprayed beans. Most of the aqueous partition
fraction is polar material, although comparable levels of polar
materials are also found in the chloroform fraction. Table 4 also
shows that the 10% polar materials of Table 3 are composed of at least
six unidentified components. No one of the six bands comprises more
than 8.4% (band one) of the total (aqueous and organosoluble)
extracted residue. Further investigation of band one with a second
solvent system shows it to consist of eight unidentified and
unquantified components in bean pods and six in maize callus. Three of
the six in corn callus co-chromatograph with three found in the beans.
Table 4. Distrlhution of 14C-Labelled Components on TLC of 14C-Omite Treated Blue Lake Bean Tissue 1
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
1 0.0-0.03 3.2 (3.0) 3.2 (3.1) 5.4 (4.9)
2 0.03-0.11 0.7 (0.6) 0.9 (0.9) 1.0 (0.9)
3 0.11-0.19 3 0.9 (0.8) 1.0 (1.0) 2.5 (2.3)
4 0.19-0.27 0.9 (0.8) 0.9 (0.9) 1.2 (1.1)
5 0.27-0.37 5.1 (4.7) 4.6 (4.4) 6.0 (5.5)
6(Omite) 0.37-0.36 87.3 (81.0) 83.9 (80.7) 81.9 (74.9)
7 0.46-1.0 1.9 (1.8) 5.4 (5.2) 1.8 (1.6)
Table 4. (con't)
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
Percent of metabolites in the aqueous fraction 2
1 0.0-0.03 75.8 (5.4) 72.1 (2.7) 87.2 (7.4)
2,3,4,5 0.03-0.21 8.0 (0.6) 8.3 (0.3) 5.1 (0.4)
6 (Omite) 0.21-0.29 9.6 (0.7) 13.0 (0.5) 3.6 (0.3)
7 0.29-1.0 6.6 (0.5) 6.6 (0.3) 4.1 (0.3)
1 TLC = double or single development in cyclohexane-ethyl acetate (9:1); interval is 7 days.
2 The Rfs and percentages are an average of two plates.
3 Co-chromatography with glycol ether.
METHODS OF RESIDUE ANALYSIS
The analytical method for Soxhlet extraction of propargite from
tea was provided to the Meeting and was used to develop the data in
Table 1. Twenty-five grams of tea in a Soxhlet thimble underwent
extraction in a unit containing 300 ml hexane for 16 hours. A 10 g
aliquot was eluted from a 15 cm florosil (activated at 130░C) column
with 2% acetone in hexane after an initial elution with 100 ml of
benzene, which was discarded. After concentration to 2 ml, GLC
analysis was conducted, using a sulphur specific flame photometric
detector. The limit of detection was said to be approximately
0.05 mg/kg, although of two untreated samples analysed when the
standard procedure was compared to Soxhlet extraction, residues were
listed as 0.0 and 1.96 mg/kg. Residues in two untreated samples during
the field trials were listed as 0.0 mg/kg.
The analytical method was validated at 0.5 to 2 mg/kg
fortification levels with recoveries >81%. It would appear that
analytical methods of choice for tea should include a Soxhlet
extraction step. The standard analytical method, while not preferred
for tea, can be improved for tea by a rehydration step if it is used.
APPRAISAL
The Meeting was provided with additional studies characterizing
propargite residues in plants and results from some additional residue
studies in countries other than the U.S. Both items of information had
been considered desirable by the Joint Meeting.
No information was available on nationally approved uses in the
countries from which residue data were available. Based on good
agricultural practices, the current limit for apples, hops and plums
could be confirmed. However, new information on the efficiency of
extraction of propargite residues from dried commodities leaves some
doubt as to the adequacy of the current limit for hops. Additional
residue data from field incurred residues in dried hops resulting from
good agricultural practices expected to give maximum residues is
desirable, using an analytical method employing a Soxhlet extraction
step. An alternative, but less desirable, analytical method could be
the standard procedure modified width a rehydration step.
The available data and lack of information on nationally approved
agricultural practices does not permit estimation of a limit on black
currants at this time.
New information on the extraction efficiency of the standard
analytical procedure for propargite in dried tea supports an increase
in that limit and suggests that analyses of all dried commodities for
propargite should use an analytical method employing a Soxhlet type
extraction step. The standard analytical method of Levine and Sisken
modified with a rehydration step appears to be an alternative but less
desirable method for dried commodities.
In the case of tomatoes, additional data suggest that the current
2 mg/kg limit may be too low. However, in the absence of information
on nationally approved agricultural practices in countries from which
data have been provided, there is no basis for revising the current
limit.
Recently conducted studies on the metabolic fate of propargite in
beans confirm that propargite is the major single residue and support
earlier studies suggesting the presence of low levels of propargite
glycol ether (1% as compared to 7% in an earlier study on dried bean
pods). Qualitatively, unidentified moieties are shown to consist
largely of polar materials, which is similar to findings in studies
previously reviewed by the Joint Meeting. Quantitatively, and under
different experimental conditions (including some substantially longer
preharvest intervals), some of the earlier studies have shown
unidentified residues to constitute over half of the total residue.
In those earlier metabolism studies in dry bean pods (FAO/WHO
1981b) residues were extracted with methanol/acetone, whereas in field
trials on dry beans (FAO/WHO 1978b) residues were low but the standard
extraction procedure utilizes hexane/isopropanol, which may not have
extracted all of the polar materials. In the studies submitted to this
Meeting, unidentified residues constituted approximately 20% of the
total and extraction was with methanol.
While the new studies do give further confirmation of earlier
findings, no new information is available on the actual identity of
metabolites/degradation products that would allow confirmation of the
postulated metabolic pathway of propargite in plants. The additional
study indicating that no one component of the metabolites constitutes
more than 10% of the total residue in beans does give some
reassurance. If this same statement could be made under those
conditions resulting in unidentified residues at over 50% of total
residue, even greater assurance would be given. Under these
circumstances, the actual identity of the major unidentified residues
is still desirable.
RECOMMENDATIONS
In the case of tea, the Meeting concluded that the existing
temporary limit should be revised as indicated. For other commodities
data either confirmed existing limits or were insufficient to support
revision of existing limits of estimation of new levels.
Commodity Estimated Maximum Residue Level (mg/kg)
Tea 10 (5)
FURTHER WORK OR INFORMATION
Desirable
1. Information on nationally approved good agricultural practices,
especially from those countries in which field trials have been
conducted and for which residue data have been provided to the
1982 Joint Meeting, or which may be provided to a future Joint
Meeting.
2. Residue data from field incurred residues in dried hops resulting
from good agricultural practices expected to result in maximum
residues and analysed by a validated analytical method utilizing
a Soxhlet type extraction step.
3. Actual identification of the major propargite plant metabolite
degradation products.
4. Information on the occurrence of residues in commodities in
commerce.
REFERENCES
Devine, J.M. and Sisken, H.R. Use of flame photometric detector
1972 for determining residues of Omite [2-(p tert-
butylphenoxy)cyclohexy) propargyl sulfite] Journal of
Agricultural Food Chem. 20: 59-61.
Lengen, M. Propargite metabolism in beans and corn, Project No. 8107,
1982 June 1982. Studies conducted by Galye H. Davidonis and Ralph
O. Mumma, Pennsylvania State University 9 June 1982.
(Unpublished)
Uniroyal. Residue data submitted to the 1982 Joint Meeting by Uniroyal
1982 Chemical, Division of Uniroyal, Inc,, Agricultural Chemicals
Research and Development, 74 Amity Road, Bethany, CT 06525.
Determination of Omite residues in apples, J. 2774,
DM79/2381D, J2774, DM79/237D, 20 May 1980; 217/4; M81/19B,
C, D and E, M81/20B, C, D and E; M81/21B, C, D and E;
M81/22B, C, D and E, 28 October, 1981; J3225, M80/250,
M80/251, 28 November, 1980. Residues of propargite in apples
by Dr. Boleslaw Salmonowicz, March 1982. Determination of
Omite residues in Currants, 217/1; M81/25, 217/1, M81/26,
28 October 1981; Determination of Omite residues in Hops,
21713; M81/23, 21713, M81/24, July 1982; Determination of
Omite residues in plums, 217/2, 217/2, M81/27, 217/2,
M81/28, 28 October 1981.
Omite residues in tomatoes, J2911, 8 May 1980.
Data submitted to the 1982 JMPR and initially developed and
submitted 19 January 1977 to the U.S. Environmental
Protection Agency in support of U.S. pesticide tolerance
petition FAP6H5100. (Unpublished)
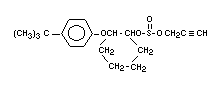 Explanation
Propargite was reviewed by the Joint Meeting of 1977 (FAO/WHO
1978)1 and a temporary ADI of 0-0.08 mg/kg b.w. was determined. The
JMPR requested that a carcinogenicity study be performed and submitted
for review prior to July 1981. The subject study was not made
available for review. The teratology study in rats was performed by
Industrial Bio-Test Laboratories (IBT) and a replacement study has
subsequently been provided and reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 20-30 pregnant Sprague-Dawley rats were administered
propargite by gavage at levels of 0, 6, 25 or 105 mg/kg/day from days
6 through 15 of gestation. Maize oil and aspirin served as the vehicle
(negative) and positive control groups, respectively. On day 20 of
gestation, all pregnant animals were sacrificed and the foetuses
delivered by caesarean section. Maternal observations included
cageside observations, body weights, numbers of corpora lutea,
implantation sites, resorption sites and live and dead foetuses. Sex
of foetuses and body weights of foetuses were also determined. Each
foetus was examined grossly for abnormalities, as well as for detailed
visceral and skeletal anomalies and/or variations.
1 See Annex 2 for WHO and FAO documentation.
There were no compound-related effects on maternal or foetal body
weights, pregnancy implantations, number of live/dead foetuses or
number of resorption sites, except in the positive control (aspirin,
250 mg/kg) groups where both maternal and foetal weights were
decreased, number of live foetuses per dam decreased and the number of
resorption sites were increased. The only noticeable effect of
propargite was an increase in maternal death at 105 mg/kg bw.
Visceral examination did not reveal any dose-related effects
other than small pups at 105 mg/kg bw. Again, in the positive controls
there were significant increases in spina bifida and
encephalomeningocele.
Skeletal variations consisted primarily of incomplete
ossification, missing sternebrae and retarded hyoid development, which
occurred with greater frequency at 25 and 105 mg/kg bw than in the
low-dose or vehicle control groups. Also noted at the high dose was
incomplete closure of the skull. These skeletal variations are common
in this species, are often considered an expression of maternal
toxicity and their biological significance is not clearly understood
at present. There were increased occurrences of these same variations
in the positive control group, along with fused/split ribs,
extranumery or rudimentary ribs and incomplete ossification of
extremities.
Propargite was not teratogenic in rats at any dose level
administered, although there was evidence of maternal toxicity at 25
and 105 mg/kg bw (Knickerbocker 1979).
COMMENTS
The ADI and supporting data were reviewed by the JMPR in 1977
(FAO/WHO 1978). No additional data has been received that would alter
those considerations.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 300 mg/kg in the diet, equivalent to 15 mg/kg bw.
Dog : 900 mg/kg in the diet, equivalent to 22 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.15 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Mutagenicity studies.
2. Observations in humans.
REFERENCES
Knickerbocker, M. Teratogenic evaluation of Omite technical in
1979 Sprague-Dawley rats. Food and Drug Research Laboratories,
Inc. Report submitted to the World Health Organization by
Uniroyal Chemical Co. (Unpublished)
PROPARGITE
Explanation
Propargite was reviewed by the Joint Meeting of 1977 (FAO/WHO
1978)1 and a temporary ADI of 0-0.08 mg/kg b.w. was determined. The
JMPR requested that a carcinogenicity study be performed and submitted
for review prior to July 1981. The subject study was not made
available for review. The teratology study in rats was performed by
Industrial Bio-Test Laboratories (IBT) and a replacement study has
subsequently been provided and reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 20-30 pregnant Sprague-Dawley rats were administered
propargite by gavage at levels of 0, 6, 25 or 105 mg/kg/day from days
6 through 15 of gestation. Maize oil and aspirin served as the vehicle
(negative) and positive control groups, respectively. On day 20 of
gestation, all pregnant animals were sacrificed and the foetuses
delivered by caesarean section. Maternal observations included
cageside observations, body weights, numbers of corpora lutea,
implantation sites, resorption sites and live and dead foetuses. Sex
of foetuses and body weights of foetuses were also determined. Each
foetus was examined grossly for abnormalities, as well as for detailed
visceral and skeletal anomalies and/or variations.
1 See Annex 2 for WHO and FAO documentation.
There were no compound-related effects on maternal or foetal body
weights, pregnancy implantations, number of live/dead foetuses or
number of resorption sites, except in the positive control (aspirin,
250 mg/kg) groups where both maternal and foetal weights were
decreased, number of live foetuses per dam decreased and the number of
resorption sites were increased. The only noticeable effect of
propargite was an increase in maternal death at 105 mg/kg bw.
Visceral examination did not reveal any dose-related effects
other than small pups at 105 mg/kg bw. Again, in the positive controls
there were significant increases in spina bifida and
encephalomeningocele.
Skeletal variations consisted primarily of incomplete
ossification, missing sternebrae and retarded hyoid development, which
occurred with greater frequency at 25 and 105 mg/kg bw than in the
low-dose or vehicle control groups. Also noted at the high dose was
incomplete closure of the skull. These skeletal variations are common
in this species, are often considered an expression of maternal
toxicity and their biological significance is not clearly understood
at present. There were increased occurrences of these same variations
in the positive control group, along with fused/split ribs,
extranumery or rudimentary ribs and incomplete ossification of
extremities.
Propargite was not teratogenic in rats at any dose level
administered, although there was evidence of maternal toxicity at 25
and 105 mg/kg bw (Knickerbocker 1979).
COMMENTS
The ADI and supporting data were reviewed by the JMPR in 1977
(FAO/WHO 1978). No additional data has been received that would alter
those considerations.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 300 mg/kg in the diet, equivalent to 15 mg/kg bw.
Dog : 900 mg/kg in the diet, equivalent to 22 mg/kg bw.
Estimate of Acceptable Daily Intake for Man
0 - 0.15 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
1. Mutagenicity studies.
2. Observations in humans.
REFERENCES
Knickerbocker, M. Teratogenic evaluation of Omite technical in
1979 Sprague-Dawley rats. Food and Drug Research Laboratories,
Inc. Report submitted to the World Health Organization by
Uniroyal Chemical Co. (Unpublished)
PROPARGITE
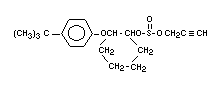 Explanation
Propargite was evaluated in 1977, 1978, 1979 and 1980 (FAO/WHO
1978b, 1979b, 1980b and 1981b)1 at which times several temporary
maximum residue levels were either estimated or revised. The Joint
Meeting has considered it desirable to have additional information
on the metabolic fate of propargite in plants, especially
characterization of substantial unidentified residues. Also desired
was data from supervised trials in countries other than the U.S. and
information on propargite residues on commodities in commerce.
Additional studies on the metabolism of propargite in plants and
residue data from countries other than the U.S. (including for tea and
resulting from discussions at the 14th Session of the Codex Committee
on Pesticides Residues) have been made available and are reviewed in
this monograph addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
No information on nationally-approved agricultural practices has
been provided to the Meeting for those commodities and for those
countries from which additional residue data are available. The
Meeting was informed that registration was discontinued in The
Netherlands in 1976.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Propargite residue data resulting from supervised trials in
several countries other than the U.S. on several commodities are
summarized in Table 1 (Uniroyal 1982). Since no nationally-approved
agricultural practice information for these countries or on these
commodities was available, previously submitted good agricultural
practice information from other countries was taken into consideration
to the extent possible in evaluating the data.
1 See Annex 2 for FAO and WHO documentation, JMPR 1982 389-398
Table 1. Propargite Residues from Supervised Trials
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Apples U.K. 1 2 0.75 57E
(0.07%)
(Ross) 1979 8 0.96 <0.03
16 0.80 0.04
23 0.56 <0.02
(Cox, O.P.) 7 0.68 <0.02
10 0.52 0.03
18 0.33 <0.02
(Lascton 1 0.86 57E 0 1.5-1.9 <0.02-0.3
Superb, Cox, (0.17%) (1.7 av.) 2 (0.1 av.) 2
Bramley, 1981 orchard 7 1.1-1.9
Egremont sprayer (1.5 av.) 2
Russet) 14 0.6-1.7
(1.1 av.) 2
21 0.51, 0.73
Poland
(Mcintosh, 1 0.75 30W 80 <0.05, <0.05, <0.05,
Jonathan, (0.075%) <0.05, <0.05
Lobo) high <0.05
volume
mist
blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
(Mcintosh, 1 0.9 30W 55 0.21, 0.09 <0.05, <0.05,
Starking, (0.06%) 0.39, 0.16 <0.05
Bancroft, high
Idamont) volume
mist
blower
(Mcintosh, 1 0.9 30W 55 <0.05,
Starking, (0.06%) <0.05
Boskop) <0.05
(Grenadier) U.K. 1 1.0 57E 7 1.1 0.04, 0.26,
1980 high 14 0.89 0.025
volume 21 1
mist
blower
(Cox) 2 1.0 57E 4 2.4 0.11, 0.02,
high 7 2 0.05
volume 14 1.5
mist blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Black currants 1 U.K. 1 0.86 57E 0 5.1 <0.03
(Baldwin) (0.043%) 7 2.5 <0.03
1981 Knapsack 14 1.2
21 3.5,
2.3
0 2 <0.02
7 0.94 <0.02
14 0.80
21 0.27
Hops, green U.K. 1 0.86 57E 0 12 <0.1
(Wye) 1981 (0.09%) 7 1.2 (3 samples)
mist blower 14 1.5
29 0.61 4
(WVG) 0 3.9 <0.1
7 3.1 (3 samples)
14 3.1
17 2.7 4
Plums, fresh U.K. 1 0.86 57E 0 0.29 <0.01
(Victoria) (0.09%) 0.35
orchard 7 0.53
sprayer 14 0.35
21 0.47
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
England
(Czar) 0 0.67 <0.01
7 1.5
1.1
14 0.58
(Victory) 0 0.33
7 0.28
14 0.16
21 0.13
Tea India 1 0.75 5 57E 7 0.45- 0.0 3
1976 0.66
(0.51 avg. of
6 samples)
1.5 5 57E 7 0.89
2.2
(1.3 avg. of
12 samples)
Tomatoes 1 South Africa 2 0.56 8 57E 1 7 1.2 0.014,
(0.07%) 2 7 0.85 <0.02,
1980 4 7 1.6 0.023
6 6 0.83
8 7 0.034
0.046
16 7 0.29
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
2 1.12 57E 1 7 3.6
(0.14%) 2 7 2.0
4 7 2.1
6 6 2.3
8 7 2.6
16 7 0.97
1 Residues corrected for analytical recoveries;
2 Four samples;
3 A 0.06 mg/kg limit of detection claimed;
4 Commercially dried;
5 Weather described as "wet";
6 6 days after first treatment;
7 Days after second treatment, the one day value is 7 days after first treatment;
8 Late frulting stage at treatment.
Except in the case of tea, in which Soxhlet extraction was used,
and black currants, where the analytical method was not identified,
the basic method (Devine and Sisken 1972) or modifications of it was
used for developing data in Table 1. Recoveries were generally 80% or
better.
Apples
Residues on several varieties of apples in the U.K. and Poland
resulting from up to two applications at rates and preharvest
intervals consistent with those on which the current 5 mg/kg limit is
based are within that limit. The 5 mg/kg limit is confirmed.
Black Currants
Residues resulting from one application at 0.86 kg/ha in England
range from a high level of 5.1 mg/kg on day of application to
0.27 mg/kg at 21 days after last application. Although maximum
residues are less than the 10 mg/kg limits for other small fruits, a
limit could not be estimated in the absence of nationally approved
agricultural information for currants. In addition to good
agricultural practice information from U.K., additional residue data
and good agricultural information from countries other than U.K. would
be desirable for estimating maximum residue levels for black currants.
The manufacturer has informed the Meeting that additional studies are
in progress.
Hops
No information was available on nationally approved agricultural
practices in the country from which additional residue data were
available. With a single application at half the maximum rate, the two
applications and 14-day preharvest interval used as a basis for the
current 30 mg/kg limit, residues are well below that limit on both
green and dried hops. However, there is a possibility that field
incurred residues in dried hops, both in the new studies and those
previously reviewed, were not quantitatively extracted by the standard
analytical procedure (see discussion under "Tea"),
Plums
Although no information on nationally approved agricultural
practice was available from the country in which the residue trials
were conducted, residues from single applications of the EC
formulation at application rates similar to those for other
formulations used as a basis for the current 7 mg/kg limit on plums
are well below that limit, even on day of application. The current
limit is based on a 14-day interval from last application to harvest.
Tea
The current 5 mg/kg limit is based on application of a 57E
formulation at a rate of 0.75 kg a.i./ha and a 7-day preharvest
interval. Maximum residues in several samples based on this good
agricultural practice (confirmed to this Meeting) were approximately
4 mg/kg (FAO/WHO 1978b, 1979b). Based on data submitted to this
Meeting, analytical methodology used in developing previously reviewed
residue data does not efficiently extract residues from tea, dry or
manufactured. The comparative extraction efficiencies of a Soxhlet
extraction (see Methods of Analysis) and the standard procedure
(Devine and Sisken 1972) are summarized in Table 2.
Table 2. Comparative Extraction of Propargite from Tea (mg/kg)
Sample Extraction Technique
Standard Procedure Soxhlet Factor
1 0.2 1.0 5
2 0.3 0.9 3
3 (sun dried) 0.6 1.5 2.5
4 (sun dried) 0.57 1.2 2.1
5 (sun dried) 0.52 1.2 2.3
6 (manufactured) 1.0 2.0 2
7 (manufactured) 1.3 2.0 1.5
8 (manufactured) 1.0 2.2 2.2
Average 2.6
Additional residue data (Table 1) in which Soxhlet extraction was
utilized suggest that the current 5 mg/kg limit is adequate, even
after using twice the recommended application rate. These data are
consistent with much of the data previously reviewed. However, because
residues from several samples previously reviewed (which were analysed
by the standard procedure) were approximately 4 mg/kg, a 2-2.5 times
increase in extraction efficiency would result in residues of about
10 mg/kg.
Other information provided to the Meeting shows that even with
the standard procedure rehydration of sun-dried tea by soaking
overnight with "2 times amount of water" can double the extraction
efficiency.
Tomatoes
If the formulation and uses employed during the field trials
indicated in Table 1 represent that country's nationally approved
agricultural practices, the current 2 mg/kg limit for tomatoes is too
low. A 5 mg/kg limit may be needed.
FATE OF RESIDUES
In Plants
It is known that propargite is the largest component of the
residue in plants and that the residue is primarily a surface one.
There is evidence that it is not translocated to an appreciable extent
and that losses are largely through volatilization. It has been
hypothesized that propargyl alcohol and the propargite glycol ether
(tertbutylphenoxycyclohexanol) are the primary metabolites, with
further degradation to other moieties, but there has been little
experimental evidence to confirm this. There has been some evidence
for trace amounts of the glycol ether. Unidentified residues in plants
have varied from 6-50% of total residues, with some not entirely
conclusive evidence in peaches that no one component accounts for
appreciably more than 10% of the total extractable residue. The Joint
Meeting considered additional studies on the metabolic fate of
propargite in plants desirable, especially for unidentified residues
and the possibility of residues of the glycol ether. Additional
studies have been provided in response to this request for further
information.
The metabolic fate of propargite in green beans, green bean
callus tissue and maize callus tissue was investigated (Lengen 1982).
Potted greenhouse grown green beans were sprayed or painted with
14C-labelled propargite in a formulation with a specific activity of
0.5 ÁCi/ÁM. The spray application was at a rate equivalent to 4.2 kg
propargite/ha and beans were harvested 7 days later.
Bean callus tissue was initiated from small pieces of immature
pods, grown for two weeks, injected with the labelled formulation and
harvested seven days after treatment. Similar experiments were
conducted in maize callus (roots).
Samples were extracted with methanol, some aliquots of which were
partitioned further with chloroform. After concentration under a flow
of nitrogen, using two solvent systems and X-ray film for location
of bands, extracted residues were characterized by thin-layer
chromatography. Mass spectrometry was used to confirm the identity
of propargite. Unextracted residues were determined by combustion.
Total residues in sprayed beans were 22 mg/kg at seven days. The
distribution of propargite, the glycol ether and unidentified
residues in bean and corn tissues are summarized in Table 3. The
chromatographic behaviour, partitioning characteristics and
distribution of residues extracted from beans are summarized in
Table 4.
Table 3 Distribution 1 of Residues in Propargite-Treated Beans 2
and Bean and Maize Callus Tissue
Sprayed Bean Bean Callus Maize Callus
Propargite 80.2 76.0 96.0
Propargite 1.0 2.4 <1
glycol ether
Polar 3 9.9 12.2 1.1
Unextracted 4.1 2.8 <1
1 Percent of total residue;
2 Total residues equivalent to 22 mg/kg;
3 Resolved into a minimum of six components.
Table 3 confirmed earlier studies, which show that propargite is
the principal residue and that low residues of the glycol ether may be
present. The level of unidentified residues in beans at 14% is within
the range found in previous studies on plants, but less than the
maximum. Propargite is shown to be less extensively metabolized in
maize callus than in beans.
Table 4 confirms that most of the residue is propargite which is
organosoluble, and supports earlier findings suggesting the presence
of glycol ether. If present, the ether comprises approximately 1% or
less of the total in sprayed beans. Most of the aqueous partition
fraction is polar material, although comparable levels of polar
materials are also found in the chloroform fraction. Table 4 also
shows that the 10% polar materials of Table 3 are composed of at least
six unidentified components. No one of the six bands comprises more
than 8.4% (band one) of the total (aqueous and organosoluble)
extracted residue. Further investigation of band one with a second
solvent system shows it to consist of eight unidentified and
unquantified components in bean pods and six in maize callus. Three of
the six in corn callus co-chromatograph with three found in the beans.
Table 4. Distrlhution of 14C-Labelled Components on TLC of 14C-Omite Treated Blue Lake Bean Tissue 1
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
1 0.0-0.03 3.2 (3.0) 3.2 (3.1) 5.4 (4.9)
2 0.03-0.11 0.7 (0.6) 0.9 (0.9) 1.0 (0.9)
3 0.11-0.19 3 0.9 (0.8) 1.0 (1.0) 2.5 (2.3)
4 0.19-0.27 0.9 (0.8) 0.9 (0.9) 1.2 (1.1)
5 0.27-0.37 5.1 (4.7) 4.6 (4.4) 6.0 (5.5)
6(Omite) 0.37-0.36 87.3 (81.0) 83.9 (80.7) 81.9 (74.9)
7 0.46-1.0 1.9 (1.8) 5.4 (5.2) 1.8 (1.6)
Table 4. (con't)
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
Percent of metabolites in the aqueous fraction 2
1 0.0-0.03 75.8 (5.4) 72.1 (2.7) 87.2 (7.4)
2,3,4,5 0.03-0.21 8.0 (0.6) 8.3 (0.3) 5.1 (0.4)
6 (Omite) 0.21-0.29 9.6 (0.7) 13.0 (0.5) 3.6 (0.3)
7 0.29-1.0 6.6 (0.5) 6.6 (0.3) 4.1 (0.3)
1 TLC = double or single development in cyclohexane-ethyl acetate (9:1); interval is 7 days.
2 The Rfs and percentages are an average of two plates.
3 Co-chromatography with glycol ether.
METHODS OF RESIDUE ANALYSIS
The analytical method for Soxhlet extraction of propargite from
tea was provided to the Meeting and was used to develop the data in
Table 1. Twenty-five grams of tea in a Soxhlet thimble underwent
extraction in a unit containing 300 ml hexane for 16 hours. A 10 g
aliquot was eluted from a 15 cm florosil (activated at 130░C) column
with 2% acetone in hexane after an initial elution with 100 ml of
benzene, which was discarded. After concentration to 2 ml, GLC
analysis was conducted, using a sulphur specific flame photometric
detector. The limit of detection was said to be approximately
0.05 mg/kg, although of two untreated samples analysed when the
standard procedure was compared to Soxhlet extraction, residues were
listed as 0.0 and 1.96 mg/kg. Residues in two untreated samples during
the field trials were listed as 0.0 mg/kg.
The analytical method was validated at 0.5 to 2 mg/kg
fortification levels with recoveries >81%. It would appear that
analytical methods of choice for tea should include a Soxhlet
extraction step. The standard analytical method, while not preferred
for tea, can be improved for tea by a rehydration step if it is used.
APPRAISAL
The Meeting was provided with additional studies characterizing
propargite residues in plants and results from some additional residue
studies in countries other than the U.S. Both items of information had
been considered desirable by the Joint Meeting.
No information was available on nationally approved uses in the
countries from which residue data were available. Based on good
agricultural practices, the current limit for apples, hops and plums
could be confirmed. However, new information on the efficiency of
extraction of propargite residues from dried commodities leaves some
doubt as to the adequacy of the current limit for hops. Additional
residue data from field incurred residues in dried hops resulting from
good agricultural practices expected to give maximum residues is
desirable, using an analytical method employing a Soxhlet extraction
step. An alternative, but less desirable, analytical method could be
the standard procedure modified width a rehydration step.
The available data and lack of information on nationally approved
agricultural practices does not permit estimation of a limit on black
currants at this time.
New information on the extraction efficiency of the standard
analytical procedure for propargite in dried tea supports an increase
in that limit and suggests that analyses of all dried commodities for
propargite should use an analytical method employing a Soxhlet type
extraction step. The standard analytical method of Levine and Sisken
modified with a rehydration step appears to be an alternative but less
desirable method for dried commodities.
In the case of tomatoes, additional data suggest that the current
2 mg/kg limit may be too low. However, in the absence of information
on nationally approved agricultural practices in countries from which
data have been provided, there is no basis for revising the current
limit.
Recently conducted studies on the metabolic fate of propargite in
beans confirm that propargite is the major single residue and support
earlier studies suggesting the presence of low levels of propargite
glycol ether (1% as compared to 7% in an earlier study on dried bean
pods). Qualitatively, unidentified moieties are shown to consist
largely of polar materials, which is similar to findings in studies
previously reviewed by the Joint Meeting. Quantitatively, and under
different experimental conditions (including some substantially longer
preharvest intervals), some of the earlier studies have shown
unidentified residues to constitute over half of the total residue.
In those earlier metabolism studies in dry bean pods (FAO/WHO
1981b) residues were extracted with methanol/acetone, whereas in field
trials on dry beans (FAO/WHO 1978b) residues were low but the standard
extraction procedure utilizes hexane/isopropanol, which may not have
extracted all of the polar materials. In the studies submitted to this
Meeting, unidentified residues constituted approximately 20% of the
total and extraction was with methanol.
While the new studies do give further confirmation of earlier
findings, no new information is available on the actual identity of
metabolites/degradation products that would allow confirmation of the
postulated metabolic pathway of propargite in plants. The additional
study indicating that no one component of the metabolites constitutes
more than 10% of the total residue in beans does give some
reassurance. If this same statement could be made under those
conditions resulting in unidentified residues at over 50% of total
residue, even greater assurance would be given. Under these
circumstances, the actual identity of the major unidentified residues
is still desirable.
RECOMMENDATIONS
In the case of tea, the Meeting concluded that the existing
temporary limit should be revised as indicated. For other commodities
data either confirmed existing limits or were insufficient to support
revision of existing limits of estimation of new levels.
Commodity Estimated Maximum Residue Level (mg/kg)
Tea 10 (5)
FURTHER WORK OR INFORMATION
Desirable
1. Information on nationally approved good agricultural practices,
especially from those countries in which field trials have been
conducted and for which residue data have been provided to the
1982 Joint Meeting, or which may be provided to a future Joint
Meeting.
2. Residue data from field incurred residues in dried hops resulting
from good agricultural practices expected to result in maximum
residues and analysed by a validated analytical method utilizing
a Soxhlet type extraction step.
3. Actual identification of the major propargite plant metabolite
degradation products.
4. Information on the occurrence of residues in commodities in
commerce.
REFERENCES
Devine, J.M. and Sisken, H.R. Use of flame photometric detector
1972 for determining residues of Omite [2-(p tert-
butylphenoxy)cyclohexy) propargyl sulfite] Journal of
Agricultural Food Chem. 20: 59-61.
Lengen, M. Propargite metabolism in beans and corn, Project No. 8107,
1982 June 1982. Studies conducted by Galye H. Davidonis and Ralph
O. Mumma, Pennsylvania State University 9 June 1982.
(Unpublished)
Uniroyal. Residue data submitted to the 1982 Joint Meeting by Uniroyal
1982 Chemical, Division of Uniroyal, Inc,, Agricultural Chemicals
Research and Development, 74 Amity Road, Bethany, CT 06525.
Determination of Omite residues in apples, J. 2774,
DM79/2381D, J2774, DM79/237D, 20 May 1980; 217/4; M81/19B,
C, D and E, M81/20B, C, D and E; M81/21B, C, D and E;
M81/22B, C, D and E, 28 October, 1981; J3225, M80/250,
M80/251, 28 November, 1980. Residues of propargite in apples
by Dr. Boleslaw Salmonowicz, March 1982. Determination of
Omite residues in Currants, 217/1; M81/25, 217/1, M81/26,
28 October 1981; Determination of Omite residues in Hops,
21713; M81/23, 21713, M81/24, July 1982; Determination of
Omite residues in plums, 217/2, 217/2, M81/27, 217/2,
M81/28, 28 October 1981.
Omite residues in tomatoes, J2911, 8 May 1980.
Data submitted to the 1982 JMPR and initially developed and
submitted 19 January 1977 to the U.S. Environmental
Protection Agency in support of U.S. pesticide tolerance
petition FAP6H5100. (Unpublished)
Explanation
Propargite was evaluated in 1977, 1978, 1979 and 1980 (FAO/WHO
1978b, 1979b, 1980b and 1981b)1 at which times several temporary
maximum residue levels were either estimated or revised. The Joint
Meeting has considered it desirable to have additional information
on the metabolic fate of propargite in plants, especially
characterization of substantial unidentified residues. Also desired
was data from supervised trials in countries other than the U.S. and
information on propargite residues on commodities in commerce.
Additional studies on the metabolism of propargite in plants and
residue data from countries other than the U.S. (including for tea and
resulting from discussions at the 14th Session of the Codex Committee
on Pesticides Residues) have been made available and are reviewed in
this monograph addendum.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
No information on nationally-approved agricultural practices has
been provided to the Meeting for those commodities and for those
countries from which additional residue data are available. The
Meeting was informed that registration was discontinued in The
Netherlands in 1976.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Propargite residue data resulting from supervised trials in
several countries other than the U.S. on several commodities are
summarized in Table 1 (Uniroyal 1982). Since no nationally-approved
agricultural practice information for these countries or on these
commodities was available, previously submitted good agricultural
practice information from other countries was taken into consideration
to the extent possible in evaluating the data.
1 See Annex 2 for FAO and WHO documentation, JMPR 1982 389-398
Table 1. Propargite Residues from Supervised Trials
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Apples U.K. 1 2 0.75 57E
(0.07%)
(Ross) 1979 8 0.96 <0.03
16 0.80 0.04
23 0.56 <0.02
(Cox, O.P.) 7 0.68 <0.02
10 0.52 0.03
18 0.33 <0.02
(Lascton 1 0.86 57E 0 1.5-1.9 <0.02-0.3
Superb, Cox, (0.17%) (1.7 av.) 2 (0.1 av.) 2
Bramley, 1981 orchard 7 1.1-1.9
Egremont sprayer (1.5 av.) 2
Russet) 14 0.6-1.7
(1.1 av.) 2
21 0.51, 0.73
Poland
(Mcintosh, 1 0.75 30W 80 <0.05, <0.05, <0.05,
Jonathan, (0.075%) <0.05, <0.05
Lobo) high <0.05
volume
mist
blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
(Mcintosh, 1 0.9 30W 55 0.21, 0.09 <0.05, <0.05,
Starking, (0.06%) 0.39, 0.16 <0.05
Bancroft, high
Idamont) volume
mist
blower
(Mcintosh, 1 0.9 30W 55 <0.05,
Starking, (0.06%) <0.05
Boskop) <0.05
(Grenadier) U.K. 1 1.0 57E 7 1.1 0.04, 0.26,
1980 high 14 0.89 0.025
volume 21 1
mist
blower
(Cox) 2 1.0 57E 4 2.4 0.11, 0.02,
high 7 2 0.05
volume 14 1.5
mist blower
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
Black currants 1 U.K. 1 0.86 57E 0 5.1 <0.03
(Baldwin) (0.043%) 7 2.5 <0.03
1981 Knapsack 14 1.2
21 3.5,
2.3
0 2 <0.02
7 0.94 <0.02
14 0.80
21 0.27
Hops, green U.K. 1 0.86 57E 0 12 <0.1
(Wye) 1981 (0.09%) 7 1.2 (3 samples)
mist blower 14 1.5
29 0.61 4
(WVG) 0 3.9 <0.1
7 3.1 (3 samples)
14 3.1
17 2.7 4
Plums, fresh U.K. 1 0.86 57E 0 0.29 <0.01
(Victoria) (0.09%) 0.35
orchard 7 0.53
sprayer 14 0.35
21 0.47
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
England
(Czar) 0 0.67 <0.01
7 1.5
1.1
14 0.58
(Victory) 0 0.33
7 0.28
14 0.16
21 0.13
Tea India 1 0.75 5 57E 7 0.45- 0.0 3
1976 0.66
(0.51 avg. of
6 samples)
1.5 5 57E 7 0.89
2.2
(1.3 avg. of
12 samples)
Tomatoes 1 South Africa 2 0.56 8 57E 1 7 1.2 0.014,
(0.07%) 2 7 0.85 <0.02,
1980 4 7 1.6 0.023
6 6 0.83
8 7 0.034
0.046
16 7 0.29
Table 1. (con't)
Application Residues (mg/kg)
Crop Country/
(Variety) Year No. Rate Formulation Interval
(kg a.i./ha) (day) Untreated
2 1.12 57E 1 7 3.6
(0.14%) 2 7 2.0
4 7 2.1
6 6 2.3
8 7 2.6
16 7 0.97
1 Residues corrected for analytical recoveries;
2 Four samples;
3 A 0.06 mg/kg limit of detection claimed;
4 Commercially dried;
5 Weather described as "wet";
6 6 days after first treatment;
7 Days after second treatment, the one day value is 7 days after first treatment;
8 Late frulting stage at treatment.
Except in the case of tea, in which Soxhlet extraction was used,
and black currants, where the analytical method was not identified,
the basic method (Devine and Sisken 1972) or modifications of it was
used for developing data in Table 1. Recoveries were generally 80% or
better.
Apples
Residues on several varieties of apples in the U.K. and Poland
resulting from up to two applications at rates and preharvest
intervals consistent with those on which the current 5 mg/kg limit is
based are within that limit. The 5 mg/kg limit is confirmed.
Black Currants
Residues resulting from one application at 0.86 kg/ha in England
range from a high level of 5.1 mg/kg on day of application to
0.27 mg/kg at 21 days after last application. Although maximum
residues are less than the 10 mg/kg limits for other small fruits, a
limit could not be estimated in the absence of nationally approved
agricultural information for currants. In addition to good
agricultural practice information from U.K., additional residue data
and good agricultural information from countries other than U.K. would
be desirable for estimating maximum residue levels for black currants.
The manufacturer has informed the Meeting that additional studies are
in progress.
Hops
No information was available on nationally approved agricultural
practices in the country from which additional residue data were
available. With a single application at half the maximum rate, the two
applications and 14-day preharvest interval used as a basis for the
current 30 mg/kg limit, residues are well below that limit on both
green and dried hops. However, there is a possibility that field
incurred residues in dried hops, both in the new studies and those
previously reviewed, were not quantitatively extracted by the standard
analytical procedure (see discussion under "Tea"),
Plums
Although no information on nationally approved agricultural
practice was available from the country in which the residue trials
were conducted, residues from single applications of the EC
formulation at application rates similar to those for other
formulations used as a basis for the current 7 mg/kg limit on plums
are well below that limit, even on day of application. The current
limit is based on a 14-day interval from last application to harvest.
Tea
The current 5 mg/kg limit is based on application of a 57E
formulation at a rate of 0.75 kg a.i./ha and a 7-day preharvest
interval. Maximum residues in several samples based on this good
agricultural practice (confirmed to this Meeting) were approximately
4 mg/kg (FAO/WHO 1978b, 1979b). Based on data submitted to this
Meeting, analytical methodology used in developing previously reviewed
residue data does not efficiently extract residues from tea, dry or
manufactured. The comparative extraction efficiencies of a Soxhlet
extraction (see Methods of Analysis) and the standard procedure
(Devine and Sisken 1972) are summarized in Table 2.
Table 2. Comparative Extraction of Propargite from Tea (mg/kg)
Sample Extraction Technique
Standard Procedure Soxhlet Factor
1 0.2 1.0 5
2 0.3 0.9 3
3 (sun dried) 0.6 1.5 2.5
4 (sun dried) 0.57 1.2 2.1
5 (sun dried) 0.52 1.2 2.3
6 (manufactured) 1.0 2.0 2
7 (manufactured) 1.3 2.0 1.5
8 (manufactured) 1.0 2.2 2.2
Average 2.6
Additional residue data (Table 1) in which Soxhlet extraction was
utilized suggest that the current 5 mg/kg limit is adequate, even
after using twice the recommended application rate. These data are
consistent with much of the data previously reviewed. However, because
residues from several samples previously reviewed (which were analysed
by the standard procedure) were approximately 4 mg/kg, a 2-2.5 times
increase in extraction efficiency would result in residues of about
10 mg/kg.
Other information provided to the Meeting shows that even with
the standard procedure rehydration of sun-dried tea by soaking
overnight with "2 times amount of water" can double the extraction
efficiency.
Tomatoes
If the formulation and uses employed during the field trials
indicated in Table 1 represent that country's nationally approved
agricultural practices, the current 2 mg/kg limit for tomatoes is too
low. A 5 mg/kg limit may be needed.
FATE OF RESIDUES
In Plants
It is known that propargite is the largest component of the
residue in plants and that the residue is primarily a surface one.
There is evidence that it is not translocated to an appreciable extent
and that losses are largely through volatilization. It has been
hypothesized that propargyl alcohol and the propargite glycol ether
(tertbutylphenoxycyclohexanol) are the primary metabolites, with
further degradation to other moieties, but there has been little
experimental evidence to confirm this. There has been some evidence
for trace amounts of the glycol ether. Unidentified residues in plants
have varied from 6-50% of total residues, with some not entirely
conclusive evidence in peaches that no one component accounts for
appreciably more than 10% of the total extractable residue. The Joint
Meeting considered additional studies on the metabolic fate of
propargite in plants desirable, especially for unidentified residues
and the possibility of residues of the glycol ether. Additional
studies have been provided in response to this request for further
information.
The metabolic fate of propargite in green beans, green bean
callus tissue and maize callus tissue was investigated (Lengen 1982).
Potted greenhouse grown green beans were sprayed or painted with
14C-labelled propargite in a formulation with a specific activity of
0.5 ÁCi/ÁM. The spray application was at a rate equivalent to 4.2 kg
propargite/ha and beans were harvested 7 days later.
Bean callus tissue was initiated from small pieces of immature
pods, grown for two weeks, injected with the labelled formulation and
harvested seven days after treatment. Similar experiments were
conducted in maize callus (roots).
Samples were extracted with methanol, some aliquots of which were
partitioned further with chloroform. After concentration under a flow
of nitrogen, using two solvent systems and X-ray film for location
of bands, extracted residues were characterized by thin-layer
chromatography. Mass spectrometry was used to confirm the identity
of propargite. Unextracted residues were determined by combustion.
Total residues in sprayed beans were 22 mg/kg at seven days. The
distribution of propargite, the glycol ether and unidentified
residues in bean and corn tissues are summarized in Table 3. The
chromatographic behaviour, partitioning characteristics and
distribution of residues extracted from beans are summarized in
Table 4.
Table 3 Distribution 1 of Residues in Propargite-Treated Beans 2
and Bean and Maize Callus Tissue
Sprayed Bean Bean Callus Maize Callus
Propargite 80.2 76.0 96.0
Propargite 1.0 2.4 <1
glycol ether
Polar 3 9.9 12.2 1.1
Unextracted 4.1 2.8 <1
1 Percent of total residue;
2 Total residues equivalent to 22 mg/kg;
3 Resolved into a minimum of six components.
Table 3 confirmed earlier studies, which show that propargite is
the principal residue and that low residues of the glycol ether may be
present. The level of unidentified residues in beans at 14% is within
the range found in previous studies on plants, but less than the
maximum. Propargite is shown to be less extensively metabolized in
maize callus than in beans.
Table 4 confirms that most of the residue is propargite which is
organosoluble, and supports earlier findings suggesting the presence
of glycol ether. If present, the ether comprises approximately 1% or
less of the total in sprayed beans. Most of the aqueous partition
fraction is polar material, although comparable levels of polar
materials are also found in the chloroform fraction. Table 4 also
shows that the 10% polar materials of Table 3 are composed of at least
six unidentified components. No one of the six bands comprises more
than 8.4% (band one) of the total (aqueous and organosoluble)
extracted residue. Further investigation of band one with a second
solvent system shows it to consist of eight unidentified and
unquantified components in bean pods and six in maize callus. Three of
the six in corn callus co-chromatograph with three found in the beans.
Table 4. Distrlhution of 14C-Labelled Components on TLC of 14C-Omite Treated Blue Lake Bean Tissue 1
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
1 0.0-0.03 3.2 (3.0) 3.2 (3.1) 5.4 (4.9)
2 0.03-0.11 0.7 (0.6) 0.9 (0.9) 1.0 (0.9)
3 0.11-0.19 3 0.9 (0.8) 1.0 (1.0) 2.5 (2.3)
4 0.19-0.27 0.9 (0.8) 0.9 (0.9) 1.2 (1.1)
5 0.27-0.37 5.1 (4.7) 4.6 (4.4) 6.0 (5.5)
6(Omite) 0.37-0.36 87.3 (81.0) 83.9 (80.7) 81.9 (74.9)
7 0.46-1.0 1.9 (1.8) 5.4 (5.2) 1.8 (1.6)
Table 4. (con't)
Percent of metabolites in the chloroform fraction 2
Sprayed pods Painted pods Callus
Band % in % of % in % of % in % of
No. Rf Fraction Total Fraction Total Fraction Total
Percent of metabolites in the aqueous fraction 2
1 0.0-0.03 75.8 (5.4) 72.1 (2.7) 87.2 (7.4)
2,3,4,5 0.03-0.21 8.0 (0.6) 8.3 (0.3) 5.1 (0.4)
6 (Omite) 0.21-0.29 9.6 (0.7) 13.0 (0.5) 3.6 (0.3)
7 0.29-1.0 6.6 (0.5) 6.6 (0.3) 4.1 (0.3)
1 TLC = double or single development in cyclohexane-ethyl acetate (9:1); interval is 7 days.
2 The Rfs and percentages are an average of two plates.
3 Co-chromatography with glycol ether.
METHODS OF RESIDUE ANALYSIS
The analytical method for Soxhlet extraction of propargite from
tea was provided to the Meeting and was used to develop the data in
Table 1. Twenty-five grams of tea in a Soxhlet thimble underwent
extraction in a unit containing 300 ml hexane for 16 hours. A 10 g
aliquot was eluted from a 15 cm florosil (activated at 130░C) column
with 2% acetone in hexane after an initial elution with 100 ml of
benzene, which was discarded. After concentration to 2 ml, GLC
analysis was conducted, using a sulphur specific flame photometric
detector. The limit of detection was said to be approximately
0.05 mg/kg, although of two untreated samples analysed when the
standard procedure was compared to Soxhlet extraction, residues were
listed as 0.0 and 1.96 mg/kg. Residues in two untreated samples during
the field trials were listed as 0.0 mg/kg.
The analytical method was validated at 0.5 to 2 mg/kg
fortification levels with recoveries >81%. It would appear that
analytical methods of choice for tea should include a Soxhlet
extraction step. The standard analytical method, while not preferred
for tea, can be improved for tea by a rehydration step if it is used.
APPRAISAL
The Meeting was provided with additional studies characterizing
propargite residues in plants and results from some additional residue
studies in countries other than the U.S. Both items of information had
been considered desirable by the Joint Meeting.
No information was available on nationally approved uses in the
countries from which residue data were available. Based on good
agricultural practices, the current limit for apples, hops and plums
could be confirmed. However, new information on the efficiency of
extraction of propargite residues from dried commodities leaves some
doubt as to the adequacy of the current limit for hops. Additional
residue data from field incurred residues in dried hops resulting from
good agricultural practices expected to give maximum residues is
desirable, using an analytical method employing a Soxhlet extraction
step. An alternative, but less desirable, analytical method could be
the standard procedure modified width a rehydration step.
The available data and lack of information on nationally approved
agricultural practices does not permit estimation of a limit on black
currants at this time.
New information on the extraction efficiency of the standard
analytical procedure for propargite in dried tea supports an increase
in that limit and suggests that analyses of all dried commodities for
propargite should use an analytical method employing a Soxhlet type
extraction step. The standard analytical method of Levine and Sisken
modified with a rehydration step appears to be an alternative but less
desirable method for dried commodities.
In the case of tomatoes, additional data suggest that the current
2 mg/kg limit may be too low. However, in the absence of information
on nationally approved agricultural practices in countries from which
data have been provided, there is no basis for revising the current
limit.
Recently conducted studies on the metabolic fate of propargite in
beans confirm that propargite is the major single residue and support
earlier studies suggesting the presence of low levels of propargite
glycol ether (1% as compared to 7% in an earlier study on dried bean
pods). Qualitatively, unidentified moieties are shown to consist
largely of polar materials, which is similar to findings in studies
previously reviewed by the Joint Meeting. Quantitatively, and under
different experimental conditions (including some substantially longer
preharvest intervals), some of the earlier studies have shown
unidentified residues to constitute over half of the total residue.
In those earlier metabolism studies in dry bean pods (FAO/WHO
1981b) residues were extracted with methanol/acetone, whereas in field
trials on dry beans (FAO/WHO 1978b) residues were low but the standard
extraction procedure utilizes hexane/isopropanol, which may not have
extracted all of the polar materials. In the studies submitted to this
Meeting, unidentified residues constituted approximately 20% of the
total and extraction was with methanol.
While the new studies do give further confirmation of earlier
findings, no new information is available on the actual identity of
metabolites/degradation products that would allow confirmation of the
postulated metabolic pathway of propargite in plants. The additional
study indicating that no one component of the metabolites constitutes
more than 10% of the total residue in beans does give some
reassurance. If this same statement could be made under those
conditions resulting in unidentified residues at over 50% of total
residue, even greater assurance would be given. Under these
circumstances, the actual identity of the major unidentified residues
is still desirable.
RECOMMENDATIONS
In the case of tea, the Meeting concluded that the existing
temporary limit should be revised as indicated. For other commodities
data either confirmed existing limits or were insufficient to support
revision of existing limits of estimation of new levels.
Commodity Estimated Maximum Residue Level (mg/kg)
Tea 10 (5)
FURTHER WORK OR INFORMATION
Desirable
1. Information on nationally approved good agricultural practices,
especially from those countries in which field trials have been
conducted and for which residue data have been provided to the
1982 Joint Meeting, or which may be provided to a future Joint
Meeting.
2. Residue data from field incurred residues in dried hops resulting
from good agricultural practices expected to result in maximum
residues and analysed by a validated analytical method utilizing
a Soxhlet type extraction step.
3. Actual identification of the major propargite plant metabolite
degradation products.
4. Information on the occurrence of residues in commodities in
commerce.
REFERENCES
Devine, J.M. and Sisken, H.R. Use of flame photometric detector
1972 for determining residues of Omite [2-(p tert-
butylphenoxy)cyclohexy) propargyl sulfite] Journal of
Agricultural Food Chem. 20: 59-61.
Lengen, M. Propargite metabolism in beans and corn, Project No. 8107,
1982 June 1982. Studies conducted by Galye H. Davidonis and Ralph
O. Mumma, Pennsylvania State University 9 June 1982.
(Unpublished)
Uniroyal. Residue data submitted to the 1982 Joint Meeting by Uniroyal
1982 Chemical, Division of Uniroyal, Inc,, Agricultural Chemicals
Research and Development, 74 Amity Road, Bethany, CT 06525.
Determination of Omite residues in apples, J. 2774,
DM79/2381D, J2774, DM79/237D, 20 May 1980; 217/4; M81/19B,
C, D and E, M81/20B, C, D and E; M81/21B, C, D and E;
M81/22B, C, D and E, 28 October, 1981; J3225, M80/250,
M80/251, 28 November, 1980. Residues of propargite in apples
by Dr. Boleslaw Salmonowicz, March 1982. Determination of
Omite residues in Currants, 217/1; M81/25, 217/1, M81/26,
28 October 1981; Determination of Omite residues in Hops,
21713; M81/23, 21713, M81/24, July 1982; Determination of
Omite residues in plums, 217/2, 217/2, M81/27, 217/2,
M81/28, 28 October 1981.
Omite residues in tomatoes, J2911, 8 May 1980.
Data submitted to the 1982 JMPR and initially developed and
submitted 19 January 1977 to the U.S. Environmental
Protection Agency in support of U.S. pesticide tolerance
petition FAP6H5100. (Unpublished)
