
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
PROPARGITE
First draft prepared by
E. Bosshard
Federal Office of Agriculture, Section Crop Protection Products, Bern,
Switzerland
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: Cell proliferation
Observations in humans
Comments
Evaluation
References
Explanation
Propargite is an acaricide which has been used on a wide variety
of food crops since its introduction in 1967. The compound was
assessed toxicologically by the 1977, 1980, and 1982 Joint Meetings
(Annex 1, references 28, 34, and 38). The 1977 Meeting established
a temporary ADI of 0-0.08 mg/kg bw on the basis of a NOAEL of 300 ppm
(equivalent to 15 mg/kg bw per day) in a three-generation study of
reproductive toxicity. Because a long-term toxicity study in rats
reported in 1966 was considered by the Meeting to be inadequate, a
safety factor of 200 was used. The results of a long-term study of
carcinogenicity in mice were made available to the Meeting in 1980; no
carcinogenic effects were observed. In a study of teratogenicity in
rats, delayed maturation was observed, and the 1980 Meeting concluded
that this effect should be clarified. The 1982 Meeting re-evaluated
the results of this study and concluded that propargite was not
teratogenic in rats. That Meeting established an ADI of 0-0.15 mg/kg
bw on the basis of a NOAEL of 15 mg/kg bw per day in the earlier
multigeneration study of reproductive toxicity and a safety factor of
100. Propargite was re-evaluated by the present Meeting in the context
of the periodic review programme of the Codex Committee on Pesticide
Residues.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Mice
Groups of 10 CD-1 mice of each sex were given a single dose of
[14C-phenyl]propargite by gavage at 150 mg/kg bw. Urine and faeces
were collected before dosing and at 24-h intervals after dosing until
termination at 168 h. The animals were observed for clinical signs of
toxicity twice a day. No abnormal behaviour or overt signs of toxicity
were observed. Most of the administered radiolabel was eliminated
within the first 24 h of dosing in animals of each sex. Urinary
excretion over the 168-h collection period accounted for 59% of the
administered dose in males and 47% in females, and faecal elimination
accounted for 42% in males and 53% in females. The results indicate
that the route of elimination is sex-dependent, as urinary excretion
was lower and faecal elimination correspondingly higher in females. A
large percentage of the dose was either not absorbed or was eliminated
in the faeces by biliary excretion (Trela, 1991).
Rats
Groups of four male Sprague-Dawley rats received dermal
applications of technical-grade [14C]propargite in 20%
2-propanol/water at doses of 0.05, 0.5, or 5.0 mg/kg bw on skin that
had been clipped 24 h before dosing. The material was left on the skin
for 0 (high dose only), 2, 4, 8, or 24 h, and, except for the 0-h
application, the sites were covered with a nonocclusive patch and a
protective device. At the end of exposure, the application site of the
animals exposed for 0, 2, and 4 h was washed with soap and water,
while those exposed for 8 and 24 h were maintained for a further 21
days. Urine and faeces were collected throughout treatment and at 24-h
intervals from animals maintained after removal of the test material.
Blood samples were collected from each animal at termination. The
absorbed dose was considered to be the sum of the radiolabel found in
the carcass, blood, skin, urine, faeces, and cage washes. At the low
dose, 22% had been absorbed after 2 h, 33% after 4 h, 18% after 8 h
plus 21 days, and 20% after 24 h plus 21 days, indicating similar
values for all lengths of exposure. Most of the material was thus
absorbed within the first 4 h of treatment. Animals exposed to the
intermediate dose absorbed 21% in 2 h, 7% in 4 h, 14% in 8 h, and 13%
in 24 h. At the high dose, absorption accounted for 32% of the dose
after 2 or 4 h. Urinary excretion accounted for 10% of the low dose
and faecal excretion for 7% and 9% after exposure for 8 and 24 h,
respectively. At the intermediate dose, urinary excretion represented
8% after 8 h and 7% after 24 h and faecal excretion represented 6 and
5%, respectively. At the high dose, urinary and faecal excretion
accounted for 3% after 8 and 24 h (Andre et al., 1990a).
Groups of four male Sprague-Dawley rats were given dermal
applications of a 14C-radiolabelled formulation containing 85%
technical-grade propargite, diluents, and wetting and dispersing
agents at doses of 0.05, 0.5, or 5.0 mg/kg bw on their backs and were
treated as in the study described above. At the low dose, absorption
amounted to 5% of the dose at 2 h, 15% at 4 h, 13% at 8 h, and 17% at
24 h. The corresponding values were 9%, 14%, 5%, and 7% at the
intermediate dose and 2%, 8%, 9%, 7%, and 9% at the high dose. These
results again show that most of the material was absorbed during the
first 4 h after application (Mizens et al., 1990).
In a third study with a similar design, groups of four rats
received dermal applications of a 14C-radiolabelled formulation
containing 85% technical-grade propargite, surfactants, and solvents
at doses of 0.05, 0.5, or 5.0 mg/kg bw. The only difference from the
preceding studies was that the animals exposed for 8 or 24 h were
maintained for only 5 days after removal of the test material.
Absorption accounted for 9-11% of the administered low dose, 7-11% of
the intermediate dose, and 2-5% of the high dose. At the low dose,
urinary excretion accounted for 4% of the dose after 8 or 24 h of
exposure and faecal excretion for 2-3%. At the intermediate dose,
urinary excretion accounted for 5% and faecal excretion for 3% after
24 h. At the high dose, urinary excretion accounted for 1% after 8 h
and 3% after 24 h and the faecal excretion for 1% and 2%,
respectively. Most of the material was absorbed during the first 2 h
of exposure (Andre et al., 1990b).
In a study of identical design but with another formulation of
propargite, about 3% of the material had been absorbed after exposure
for 2, 4, or 8 h to the low and intermediate doses, while 9% of the
low dose was absorbed within 24 h. The absorption rates after the
different lengths of exposure varied between 4% and 9%, with a mean
rate of 6% of the administered dose. At the low dose, urinary and
faecal excretion represented 1% after 8 h and 3% in urine and 5% in
faeces after 24 h. At the intermediate dose, urinary and faecal
excretion accounted for 1-2% for the different lengths of exposure. At
the high dose, urinary excretion accounted for 2% after 8 h and 4%
after 24 h and faecal excretion for 1% at 8 or 24 h (Andre et al.,
1990c).
Groups of four male and four female Sprague-Dawley rats were
given single oral doses of [14C-phenyl]propargite at doses of 75 or
262 mg/kg bw for females and 83 or 232 mg/kg bw for males, and excreta
were collected over 96 h. Males excreted an average of 43% and 46% of
the high and low doses in urine, respectively, and in females, the
corresponding values were 36% and 42%. Faecal elimination accounted
for 46% and 42% of the high and low doses in males and 59% and 50% in
females, respectively. The amounts of radiolabel remaining in the
tissues 96 h after dosing were highest in the gastrointestinal tract,
liver, and kidney, accounting for about 1% of the high administered
dose. High-performance liquid chromatography (HPLC) of the urinary
samples indicated that propargite is extensively metabolized in rats.
The results also indicate a sex difference in the urinary metabolite
profile, although the metabolites were not identified (Knipe, 1986,
1987).
The results of a study of the pharmacokinetics of
[14C]propargite after administration of a single oral dose were
compared with those of a study in which rats were fed unlabelled
propargite for 13 weeks before receiving a single oral dose of
[14C]propargite. The comparisons comprised the concentrations of
radiolabel in urine, faeces, and tissue samples and the HPLC profiles
of the urine samples. In the pharmacokinetics study, groups of eight
CD rats of each sex received a single oral dose of 0, 25, 60, or 200
mg/kg bw [14C-phenyl]propargite, and urine and faeces were collected
up to 96 h after dosing. Two animals of each sex were killed at 6, 24,
48, and 96 h, and all controls were killed at 96 h. In the study of
toxicity, described in detail in section 2 (b), groups of rats were
maintained on a diet containing propargite at concentrations of 0,
100, 1000, or 2000 ppm for at least 13 weeks, and then two rats of
each sex per group were dosed by gavage with [14C]propargite and
housed in individual metabolism cages for 96 h. Blood samples were
collected 1, 2, 4, 8, 24, 48, 72, and 96 h after dosing, and excreta
were collected at 0-6, 6-12, 12-24, 24-48, 48-72, and 72-96 h. At the
end of 96 h, each rat was anaesthetized and lungs, liver, kidneys,
spleen, stomach, intestines, fat, and muscle were collected.
Radiolabel was measured in the excreta, and the HPLC profiles of the
radiolabelled material in urine excreted over 0-24 h after
administration of [14C]propargite were determined for each rat.
In the pharmacokinetics study, peak urinary excretion occurred at
24 h at all doses. The mean total urinary excretion over the 96-h
collection period accounted for 40% of administered radiolabel at 25
mg/kg bw, 37% at 60 mg/kg bw, and 23% at 200 mg/kg bw; the
corresponding values for faecal excretion were 56%, 74%, and 73%,
respectively. The time of peak faecal excretion was dose-dependent:
the higher the dose, the later the peak excretion. The radiolabel
measured in tissues accounted for about 1.6% of the administered dose
at 25 mg/kg bw, 2.2% at 60 mg/kg bw, and 3.7% at 200 mg/kg bw. The
highest concentrations were found in intestine, fat, liver, and
muscle. In the toxicity study, the elimination of radiolabel followed
a similar pattern, with peak urinary excretion between 12 and 24 h and
peak faecal elimination between 24 and 48 h. The radiolabel excreted
in urine acounted for 28% of the administered dose at 100 ppm, 31% at
1000 ppm, and 28% at 2000 ppm; the corresponding values in faeces were
35%, 31%, and 29%, respectively. The total tissue residues constituted
0.6-1.5% of the dose, resulting in low total recoveries of only 68% at
100 ppm, 79% at 1000 ppm, and 67% at 2000 ppm. The highest
concentrations were found in intestine, liver, fat, and muscle. The
results of this comparative study indicate a similar pattern of
elimination in male and female rats given single doses or prolonged
pretreatment. The profile of urinary metabolites in female rats in
both studies indicated the presence of a further metabolite (Gay,
1987; Banijamali & Tortora, 1988a,b).
Male and female Sprague-Dawley (CD/BR) rats were treated with
[14C-phenyl]propargite or unlabelled propargite in various regimens.
A planned group treated by intravenous injection was not included
since propargite was found to be insufficiently soluble in
physiological saline or water. The test material was thus administered
by gavage in corn oil. Six rats of each sex received a single dose of
[14C]propargite at 25 mg/kg bw; 18 rats of each sex received
unlabelled material at a dose of 25 mg/kg bw per day for 14 days, and
then six rats of each sex received a single dose of 25 mg/kg bw
[14C]propargite, three of each sex received no further treatment,
and the other preconditioned animals were discarded; and six rats of
each sex received a single dose of 200 mg/kg bw [14C]propargite.
Urine and faeces were collected from all animals before dosing with
radiolabelled propargite and 6, 24, 36, and 48 h after dosing and
thereafter at 24-h intervals until termination at 96 h. The animals
were than killed, blood samples were taken, and necropsy was performed
for collection of selected tissues.
Observation for clinical signs revealed soft faeces or diarrhoea
in several animals about 12 h after dosing, which was attributed to
the corn oil vehicle. About 24 h after dosing, several rats at the
high dose had hunched posture, rough coats, and decreased activity.
Peak urinary excretion were observed between 6 and 24 h in animals at
the low dose and preconditioned animals and between 6 and 36 h in
those at the high dose. Total urinary excretion over 96 h accounted
for 61% of the administered dose in males and 50% in females at the
low dose, 53% in males and 40% in females that had been
preconditioned, and 30% in males and 34% in females at the high dose.
These results indicate a sex-dependent excretion pattern at the low
dose but not at the high dose. Preconditioning slightly decreased the
extent of elimination and the urinary excretion rate. The
concentrations of radiolabel in urine samples from preconditioned
animals that were not treated with [14C]propargite were below the
detection limit. Peak excretion in the faeces was found between 6 and
24 h with all three treatments. Total faecal elimination over 96 h
accounted for 51% of the administered dose in males and 61% in females
at the low dose, 75% in males and 70% in females at the high dose, and
63% in males and 72% in females that had been preconditioned. Thus,
elimination in faeces was slightly greater after preconditioning, and
a corresponding decrease in urinary excretion was found. The slightly
increased faecal elimination and slightly decreased urinary excretion
at the high dose indicate that urinary excretion may have become
saturated. The total radiolabel in the tissues accounted for
approximately 1-1.5% of the administered dose in both male and female
treated animals; the highest concentrations were found in liver,
corresponding to about 0.2% of the administered dose in all groups
(Johnson, 1990).
Groups of two rats of each sex were given
[14C-phenyl]propargite by gavage at a dose of 51.7 mg/kg bw, and
expired air was collected for 24 h and urine and faeces at 24-h
intervals for 168 h. Only trace amounts, accounting for up to 0.04% of
the administered dose, were found in expired air. In male rats, 66% of
the administered dose was found in urine and 28% in faeces, while in
female rats 49% was in urine and 37% in faeces. The results indicate
that the radiolabel was located on a portion of the molecule that did
not undergo metabolism to carbon dioxide or other volatile components
that could be expected in expired air (Andre et al., 1989).
Mice and rats
Groups of five male and five female Sprague-Dawley CD/Br rats and
five male and five female CD-1 mice were treated with
[14C-2,3-propargyl]propargite by gavage at a single dose of 200
mg/kg bw for rats and 150 mg/kg bw for mice. Serial samples of urine,
faeces, and expired air were collected until 120 h after dosing, when
the animals were killed and selected tissues were analysed for
radiolabel. Peak urinary excretion was observed between 6 and 36 h
after dosing in rats and 0-24 h after dosing in mice. The total
urinary excretion accounted for 36% of the administered dose in male
and 38% in female rats, and 40% in male and 33% in female mice. Peak
faecal excretion occurred between 6 and 36 h after dosing in rats and
0-6 h after dosing in mice. The total faecal elimination accounted for
38% of the administered dose in male and 35% in female rats, and 38%
in male and 55% in female mice. The radiolabel in expired air
accounted for 7-12% of the administered dose, with no significant
difference between sexes or species. The total radiolabel in tissues
accounted for 1-2% of the administered dose, the highest concentration
being found in liver in both species (Mahon, 1993).
In another comparative study in Sprague-Dawley CD/Br rats and
CD-1 mice, plasma pharmacokinetics and biliary excretion were
evaluated. The study was reported in several parts and an overview
provided by Gay (1994).
In the pharmacokinetics study [14C-phenyl]propargite was
administered by gavage in corn oil to groups of 17 male and 17 female
rats and 25 male and 25 female mice at a single dose of 150 mg/kg bw.
Groups of 19 rats and 28 mice of each sex were also given intravenous
injections of 20 mg/kg bw. Blood samples were taken before treatment
and 0.5, 1, 2, 4, 8, 12, 24, 36, and 48 h after oral administration
and 2, 5, 10, 15, and 30 min and 1, 1.5, 4, 12, 24, and 48 h after
intravenous administration. Since fasting is believed to reduce the
effects of factors that might interfere with absorption of chemicals,
the animals were fasted before oral dosing -- rats for about 12 h and
mice for about 4 h. Food was also withheld after dosing, for 4-5 h for
rats and for 1-2 h for mice. All animals were observed for clinical
signs of toxicity at least once a day during the study. Seven mice
were found dead after the initial blood collection. Since rats are
more sensitive than mice to repeated dosing but no rats were found
dead in this study, it is presumed that the deaths of the mice were
due to stress during blood collection rather than to the toxicity of
propargite. No other abnormal clinical changes were observed in rats
or mice. Pharmacokinetics was determined from the profiles of plasma
concentrations over time. After oral administration, the profiles for
both sexes and both species fit a one-compartment model with
first-order absorption and elimination. Absorption was dependent on
species but not sex, as the compound was absorbed up to seven times
more rapidly in mice than in rats, with peak absorption rates of 9-11
µg/ml in rats and 12-14 µg/ml in mice. The elimination half-times were
8-9 h in mice and 10-11 h in rats. Although the rate of absorption was
faster in mice, the absolute bioavailability, 74-80%, showed no clear
difference between species or sexes. After intravenous administration,
the plasma concentration-time profiles for both sexes and species were
biphasic and fit an open two-compartment model with first-order
elimination. The peak absorption rates were 34 µg/ml in mice and 40-47
µg/ml in rats, with half-times of 2-5.5 h in mice and 4 h in rats. The
clearance rates in rats were about two times lower than in mice and
were independent of sex (Sabourin et al., 1994).
The second part of the study involved an investigation of the
pharmacokinetics of biliary elimination after a single oral dose of
150 mg/kg bw [14C-phenyl]propargite to groups of five rats and five
mice of each sex. All animals were fasted before oral dosing. Bile,
blood, urine, and faeces were collected over 48 h, and individual
urinary and faecal sampling was performed 12, 24, and 48 h after
administration. The main route of elimination of radiolabel in rats
and mice was the faeces, which accounted for 64% of the administered
dose in rats and 45% in mice; urinary excretion accounted for 11% and
4% in rats and mice, respectively. In both species, up to 0.02% of the
administered dose was found in blood. The total eliminated in bile of
rats and mice was similar, accounting for 15% in both species, but the
time course of elimination was different. The concentration in bile
showed a plateau between 12 and 36 h, and the mean elimination
half-time was 21 h in rats and 9 h in mice. Moreover, mice showed a
higher mean integrated area under the curve of concentration-time and
a higher maximum plasma concentration. Thus, small species-dependent
differences were found in pharmacokinetics, but there were no
significant differences between the sexes (Andre & Laveglia, 1994).
In the third part of the study, plasma and bile samples from the
first two parts of the study were analysed for metabolites by HPLC.
Plasma and bile samples collected at 4, 24, and 48 h were pooled to
provide sufficient material for analysis. The results of this study
are presented below (Banijamali et al., 1994).
(b) Biotransformation
(i) Propargite
Rats
Six male rats were given [14C-phenyl]propargite as a single
oral dose of 1.5 g/kg bw, and urine and faeces were collected at
intervals over 72 h. The total urinary excretion accounted for 12% of
the administered dose. Propargite was rapidly degraded to more polar
products, and metabolism of the cyclohexyl ring was strongly favoured.
Five urinary metabolites but no parent compound were excreted in the
urine. The metabolites isolated from the urine were
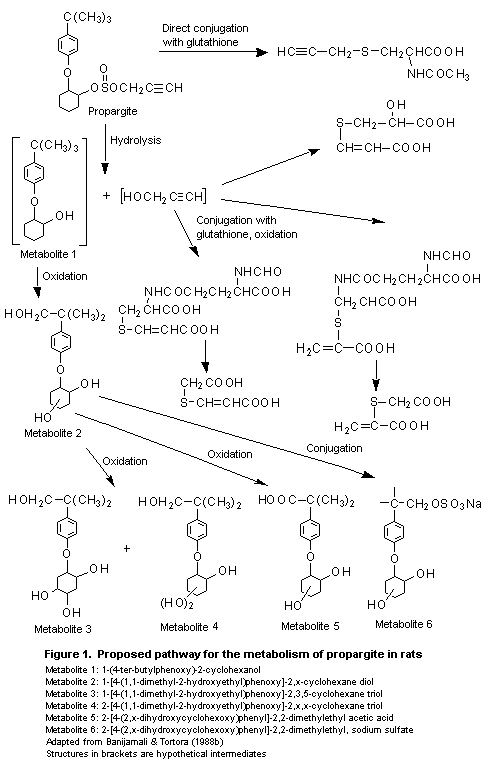
1-(4- ter-butylphenoxy)-2-cyclohexanol (1, see Figure 1),
1-[4-(1,1-dimethyl-2-hydroxyethyl)-phenoxy]-2,x-cyclohexane diol (2);
1-[4-(1,1-dimethyl-2-hydroxyethyl)phenoxy]-2,3,5-cyclohexane triol
(3); 2-[4-(1,1-dimethyl-2-hydroxyethyl) phenoxy]-2,x,x-cyclohexane
triol (4); 2-[4-(2,x-dihyroxycyclohexoxy)-phenyl]-2,2-dimethylethyl
acetic acid (5), and
2-[4-(2,x-dihyroxycyclohexoxy)phenyl]-2,2-dimethylethyl, sodium
sulfate (6) (Banijamali & Tortora, 1988b).
Investigations during a 13-week study in rats revealed the
presence of a sixth urinary metabolite which was identified as
1-[4-(2,4,5-trihydroxycyclohexoxy)phenyl]-2,2-dimethyl acetic acid
(Banijamali, 1989a).
In the study of Johnson (1990) described above, in which a single
oral dose of 25 mg/kg bw with and without preconditioning or 200 mg/kg
bw were given to groups of male and female rats, metabolites were
identified in faecal samples collected between 6 and 24 h after
dosing. Most of the radiolabel in faeces was associated with unchanged
parent compound. A small amount of the hydrolysis product
1-[4-(1,1-dimethylethyl)phenoxy]-2-cyclohexanol (TBPC) was present in
all extracts, and three polar components were identified as the acetic
acid derivative (carboxy-TBPC), the cyclohexane triol derivative
(HOMe-TBPC-triol), and the cyclohexane diol derivative
(carboxy-TBPC-diol). All of these metabolites were also found in urine
(Banijamali & Nag, 1990).
Mice and rats
Differences in the tumorigenic response of mice and rats in
long-term studies of the toxicity of propargite led to a series of
comparative studies of pharmacokinetics and metabolism. Plasma and
bile samples from the studies described above (Andre & Laveglia, 1994;
Sabourin et al., 1994) were thus used for metabolite profiling. The
three times selected for pooling of bile samples were 4, 24, and 48 h
after administration, the 4-h period representing the peak or early
plateau of the biliary concentration-time curves, the 24-h period
representing the plateau or elimination phase, and 48 h being the last
collection. Plasma samples collected at 0.5, 2, 8, and 24 h for rats
and at 1, 4, 12, and 24 h for mice were pooled for each species and
sex. Metabolism was found to be rapid and extensive, and no parent
compound was found in the bile of either species; in contrast, a small
amount of propargite was found in plasma, constituting < 4%, except
in plasma from male mice, where it represented about 10% of the
radiolabelled residue. HPLC analysis of bile from male and female rats
and mice indicated the presence of six metabolites which are formed as
a result of hydrolysis of the propynyl sulfite side-chain of
propargite, subsequent oxidation of the tert-butyl moiety, and
hydroxylation of the cyclohexyl moiety. The latter reaction yields
various stereoisomers. Four major metabolites were observed in the
pooled plasma samples, with qualitatively similar metabolite profiles
in the two species. The main metabolites in bile and plasma from
 female rats and male mice were
1-[4-(1,1-dimethyl-2-hydroxyethyl)phenoxy]-2-cyclohexanol (HOMe-TBPC)
and the corresponding diol derivative carboxy-TBPC-diol). No
consistent qualitative or quantitative species differences were found
in the metabolite profiles of propargite in bile and plasma
(Banijamali et al., 1994).
In another comparative study, the metabolites found in the faeces
of male and female CD-1 mice treated with [14C-phenyl]propargite
were characterized and compared with those identified previously in
the faeces of male and female rats (Banijamali & Nag, 1990; Johnson,
1990). The mice were given a single oral dose of 150 mg/kg bw of the
compound by gavage, whereas the rats received a single oral dose of
200 mg/kg bw (Johnson, 1990). Male mice excreted 42% of the
administered dose in the faeces and female mice about 53%, while in
rats faecal excretion accounted for 75% of the administered dose in
males and 70% in females. Peak faecal excretion of radiolabel was
observed between 0 and 24 h in both species. The profile of faecal
metabolites of mice and rats was qualitatively similar. The radiolabel
was associated with unchanged parent compound, the hydrolysis product
propargite glycol ether, and the polar metabolites hxdroxylated
tert-butyl and hydroxylated cyclohexyl propargite glycol ether. Rat
faeces contained a substantially higher percentage of unabsorbed
propargite than mouse faeces. The finding that faeces of female mice
contained more propargite (49% of total faecal residue) than faeces of
male mice (25%) suggests that faecal elimination is sex-dependent.
Moreover, male mouse faeces contained a higher percentage of polar
metabolites (56% of total faecal residue) than those of female mice
(36%). Mouse faeces contained both a greater number and a greater
percentage of polar metabolites than rat faeces, indicating more
extensive metabolism of propargite in mice than rats (Banijamali &
Nag, 1991).
Goats
One dairy goat was given [14C-phenyl]propargite at a dose of
675 mg/kg bw per day on 3 consecutive day, during which time urine,
faeces, and milk were collected. The animal was killed 8 h after the
last dose, and liver, kidney, muscle, fat, and bile samples were
collected. The highest concentration of radiolabel was found in bile,
followed by liver, kidney, fat, and muscle. The bile contained 0.29%
of the administered dose, urine 16%, faeces 14%, and milk 0.1%.
Overall, 34% of the administered dose was recovered; the remaining
radiolabel was presumed to have remained in the gastrointestinal tract
(Byrd, 1988). HPLC analysis revealed metabolism of the cyclohexyl and
tert-butyl group resulting in a number of polar metabolic products,
including carboxy-TBPC-diol, carboxy-TBPC,
1-[4-(1,dimethylethyl)-phenoxy]-2,x-cyclohexanediol, and TBPC in milk
and tissues. Small quantities of unchanged propargite were found in
milk, fat, and liver (Banijamali, 1989b).
After oral administration of [14C-phenyl]propargite in capsules
at doses of 65 or 325 mg/kg bw per day for 3 days to two lactating
goats, metabolites were identified in milk and edible tissues. The
liver contained 14 metabolites, kidney 12, muscle 9, milk 7, and fat
6. Seven of the 14 metabolites isolated from liver were glucuronide or
sulfate conjugates. The metabolism of propargite in goats thus appears
to involve hydrolysis of the propynyl sulfite side-chain followed by
aliphatic and/or alicyclic hydroxylation of the tert-butyl methyl
and cyclohexyl groups to form TBPC-diol and HOMe-TBPC, respectively.
These metabolites undergo further oxidation to yield HOMe-TBPC-diol,
carboxy-TBPC, carboxy-TBPC-diol, and carboxy-TBPC-triol. Some of these
metabolites subsequently undergo conjugation to form glucuronides and
sulfates. The results of these studies indicate similar metabolism in
rats and goats (Banijamali & Lau, 1996).
(ii) Propargyl alcohol and propargyl propargite
In order to learn more about the fate of the propynyl sulfite
side-chain of the propargite molecule, the metabolism of propargyl
alcohol, which may be released from propargite, was studied.
[1,2,3-13C-, 2,3-14C]Propargyl alcohol was administered to groups
of eight male Sprague-Dawley rats by gavage at a dose of 40 mg/kg bw,
and samples of urine, faeces, and expired air (four animals) were
collected 24, 48, 72, and 96 h after treatment. The rats were killed
at 96 h. Radiolabel associated with the parent compound represented
56% of the administered dose in urine, 12% in faeces, and 7% in
expired air. These results are consistent with those of another study
of the degradation and expiratory elimination of this compound (Mahon,
1993). Only 4-6% of the administered dose was recovered in the
carcass. Most of the radiolabel was excreted within the first 24 h of
administration in urine and expired air. The peak elimination in
faeces also occurred within 24 h of dosing and continued for 48 h. The
proposed metabolic pathway involves oxidation of propargyl alcohol to
2-propynoic acid and further detoxification by glutathione conjugation
to yield the following final products:
3,3-bis[(2-(acetylamino)-2-carboxyethyl)thio]-1-propanol,
3-(carboxymethylthio)-2-propenoic acid;
2(methylthio)-3-(methylsulfinyl)-2-propenoic acid;
3-{[2-(acetylamino)-2-carboxyethyl]thio}-3-[(2-amino-2-carboxyethyl)
thio]1-propanol; and
3-{[2-(acetylamino)-2-carboxyethyl]-sulfinyl}-3-{[2-(acetylamino)-2-
carboxyethyl]thio}-1-propanol. These metabolites have not been
reported previously and represent the first examples of multiple
glutathione additions to the carbon-carbon triple bond (Banijamali,
1998).
[1,2,3-13C-, 2,3-14C-propargyl]Propargite was administered
orally to male SpragueDawley rats at a dose of 150 mg/kg bw, and urine
and faeces were collected at 24-h intervals until study termination
96 h after dosing. The total radiolabel excreted in urine accounted
for 25% of the administered dose, and that in faeces for 48%. The six
major metabolites identified in rat urine were
3-(carboxymethylthio)-2-propenoic acid,
2-(carboxymethylthio)-2-propenoic acid,
2-(acetyl-amino)-3-(2-propynylthio)propanoic acid,
3-[(2-carboxy-2-hydroxyethyl)thio]-2-propenoic acid,
3- (N-formylglutamylcysteinyl)-2-propenoic acid, and
2- (N-formylglutamylcysteinyl)-2-propenoic acid. These metabolites
were the result of conjugation with glutathione followed by enzymatic
degradation. Seven metabolites were identified tentatively in faeces
(Banijamali, 1999).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity conducted since the
earlier evaluation (Table 1) are consistent with the previous data.
The clinical observations made most frequently after oral
administration included urogenital staining and abnormal defaecation,
hypoactivity, and swollen, red paws. Most of the treated animals lost
weight during a few days after dosing. Those that died had dark-red
areas, thickened mucosa, and red foci in the stomach. All of the
deaths occurred during the second week, with no sex difference
(Kiplinger, 1993a).
After inhalation, the commonest observations were laboured
breathing and various secretory responses. About one-third of the
treated animals lost weight during the first week after exposure, and
treatment-related reddening of the lungs was seen in some animals
found dead or killed at term. There was no sex difference in
lethality. All deaths occurred 1-17 days after exposure (Hoffman,
1992).
After dermal exposure, none of the animals died and all gained
weight during the 24-day observation period. All rabbits showed severe
erythema and oedema, and eschar formation, fissuring, desquamation,
and a white-yellow exudate on the application site appeared during the
second week of the study and persisted until day 14. Thickened skin
and desquamation within the application site were seen on all rabbits
at necropsy (Kiplinger, 1993b).
Male and female New Zealand white rabbits received dermal
applications of 0.5 ml of undiluted technical-grade propargite
(purity, 90.3%) and were observed at 0.5, 24, 48, and 72 h, daily
through day 14, and on day 21, when they were killed. Irritation was
seen during the first few days after application, consisting of
moderate erythema and slight-to-moderate oedema, but on days 5-9
severe erythema and oedema, fissuring, eschar formation, and
desquamation were observed. The severe irritation had decreased to
slight oedema and erythema by day 21, indicating reversibility of the
reaction (Kiplinger, 1993c).
Table 1. Acute toxicity of technical-grade propargite (purity, 90.3%)
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or mg/L)
Rat Crl:CD BR M Gastric intubation 2600 Kiplinger (1993a)
F 2900
Both 2800
Rat Crl:CD BR M Inhalation (4 h) 0.95 Hoffman (1992)
F 0.95
Both 0.89
Rabbit New Zealand white M Dermal (24 h) > 4000 Kiplinger (1993b)
F > 4000
Both > 4000
Male and female New Zealand white rabbits received 0.1 ml of
undiluted technical-grade propargite (purity, 90.3%) into the
conjunctival sac and were observed at 1, 24, 48, and 72 h and on days
4, 7, 10, 14, 17, and 21 after application. Reactions were observed in
the conjunctiva, cornea, and iris. The effects in cornea and iris had
subsided by day 10 or earlier, and the corneal effects had cleared by
day 21 (Kiplinger, 1993d).
In a modified Buehler test, male and female Hartley albino
guinea-pigs received three topical applications of 0.1%
technical-grade propargite (purity, 90.3%) in ethanol for induction, a
challenge dose of 0.2% propargite in acetone) two weeks later, and a
second challenge (0.1 and 0.2% in acetone) after a 1-week
interruption. The applications were left in place for 6 h. A positive
control (dinitrochlorobenzene) and an untreated control group were
included. No evidence of sensitization was seen (Kiplinger, 1993e).
(b) Short-term studies of toxicity
Rats
Propargite was administered in the diet to groups of five rats of
each sex at concentrations of 0 (15 animals), 200, 400, 800, 2000, or
4000 ppm for 90 days. Reduced food consumption and body-weight gain
were observed at the two higher concentrations. Haematological and
clinical chemical parameters (glucose and urea nitrogen) were not
affected by treatment. The relative weights of the liver, kidney,
adrenals, and gonads were increased at the two higher concentrations.
No treatment-related macroscopic or microscopic alterations were
observed (Carson, 1964).
Groups of 10 male and 10 female Crl:CD (SD) BR rats were
maintained on diets containing technical-grade propargite (purity,
87.2%) at concentrations of 0, 100, 1000, or 2000 ppm (equivalent to
0, 5, 50, and 100 mg/kg bw per day) for at least 13 weeks. At the end
of the study, all surviving rats were anaesthetized, weighed, bled,
killed, and necropsied. All tissues from control rats and those at the
highest dose, the lungs, liver, and kidneys from rats at 100 or
1000 ppm, and all macroscopic lesions from all rats were examined
microscopically. No deaths occurred during the study. All animals at
2000 ppm had rough coats throughout the study, and many were thin with
a hunched posture, alopecia, and rhinorrhoea. The body weights of rats
at 1000 and 2000 ppm were significantly lower than those of controls
throughout the study, by 30% at 1000 ppm and 69% in males and 52% in
females at 2000 ppm. The body-weight gains of males were significantly
reduced during week 10 at 100 ppm, during most of the study at
1000 ppm, and throughout the study at 2000 ppm. The significantly
reduced body-weight gain at 100 ppm during week 10 did not result in a
significant reduction in the cumulative body-weight gain at study
termination or in any other adverse effect and was considered not to
be toxicologically relevant. The body-weight gains of females were
significantly lower during weeks 1 and 5 at 1000 ppm and during weeks
1-4 at 2000 ppm. The reduction in the body-weight gain of males at the
end of the study in comparison with controls was 4% at 100 ppm, 30% at
1000 ppm, and 69% at 2000 ppm, and the corresponding reductions in
females were 6%, 31%, and 52%, respectively. The reductions at 1000
and 2000 ppm were significant. The reduced body-weight gain was
associated with a dose-dependent reduction in food consumption at the
two higher doses.
Various haematological and clinical chemical parameters were
altered at the two higher doses, including significantly increased
erthrocyte count and haemoglobin values in males at 2000 ppm and
significantly decreased mean corpuscular volume and mean corpuscular
haemoglobin in males at 1000 and 2000 ppm and females at 2000 ppm. A
non-dose-related but significant increase in platelet count in females
at 1000 ppm and an increased erythrocyte count in males at 100 ppm
were considered to be of no toxicological significance. The blood
glucose concentration was significantly lower in males and females at
1000 and 2000 ppm, and urea nitrogen was significantly higher and
creatinine significantly lower in animals of each sex at 2000 ppm.
Reduced total protein, albumin, and globulin values were found in
males at 2000 ppm and in females at 1000 ppm and 2000 ppm. An
increased albumin:globulin ratio was found in males and females at
2000 ppm. The concentrations of calcium, inorganic phosphorus,
potassium, and chloride showed significant alterations at 2000 ppm.
Most of the changes observed were considered to be associated with the
decreased food consumption and body weight. The absolute weights of
the kidneys and liver were significantly reduced in animals of each
sex at 2000 ppm, and the absolute weight of the testis was reduced in
males at this dose. The relative weights of the kidney, liver, and
testis were increased in a dose-related manner at 1000 and 2000 ppm.
Macroscopic and microscopic examinations did not reveal alterations
attributable to treatment. The NOAEL was 100 ppm, equivalent to 5
mg/kg bw per day, on the basis of effects on body weight and changes
in clinical chemical parameters at higher doses (Kehoe, 1988).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of technical-grade propargite (purity, 85%) at
doses of 0, 0.1, 1.0, 10, or 100 mg/kg bw per day, 5 days per week for
3 weeks. No deaths occurred during the study, and no clinical signs
were observed at any dose. Food consumption was slightly reduced at
all doses, and the mean body weightswere slightly reduced in treated
males and slightly increased in treated females, with no clear
dose-response relationship, indicating no consistent treatment-related
effect. Biochemical parameters were not affected by treatment, whereas
haematological investigations revealed a significant increase in the
number of segmented neutrophils at the end of the study in males at
100 mg/kg bw. Signs of dermal irritation were seen at the application
site in all treated groups, with a dose-related increase in incidence
and severity. The signs consisted of erythema, oedema, eschar
formation, exfoliation, atonia, desquamation, fissuring, blanching,
and coriaceousness. The dermal effects occurred earlier with
increasing dose. The observed changes were graded as mild-to-severe in
animals at 10 and 100 mg/kg bw per day, mild-to-moderate at 1 mg/kg bw
per day, and mild at 0.1 mg/kg bw per day. No changes in organ weights
were observed, and no additional macroscopic changes were seen.
Dose-related microscopic changes were confined to the application
site, which were in the incidence and severity of acanthosis and
hyperkeratosis. The incidence of dermal inflammation was similar in
all treated groups, and necrosis was observed at doses > 1 mg/kg bw
per day. The NOEL for systemic toxicity was 100 mg/kg bw per day, the
highest dose tested, if the increased number of segmented neutrophils
in males at this dose is considered to be a borderline effect. No NOEL
could be identified for local irritation (Goldenthal, 1989).
Dogs
Groups of three beagle dogs of each sex were given diets
containing propargite (purity not specified) at concentrations of 0,
2000 ppm (weeks 1-3), and 2500 ppm (weeks 4-13), equal to 55 mg/kg bw
per day for males and 67 mg/kg bw per day for females (mean values of
2000 and 2500 ppm). Appearance, behaviour, and signs of toxicity were
recorded daily and body weights and food consumption weekly. Clinical
chemistry was evaluated once initially and 1 and 3 months after the
start of the study. Gross necropsy was performed on all dogs killed
at13 weeks, and the weights of the thyroid, heart, liver, spleen,
kidneys, adrenals, and testis were recorded; all organs were examined
microscopically. Most of the treated animals had a reduced appetite,
particularly during the first half of the study, and weight loss was
seen. Two males showed reduction in various haematological parameters,
including haematocrit and erythrocyte count after 3 months of
treatment, and all treated males had slightly increased aspartate
aminotransferase activity. Changes in organ weights showed no clear
dose-related pattern, and the increased relative liver weight was
probably a consequence of the reduced body weights. No macroscopic
changes were observed. Microscopic findings considered to be related
to treatment were increased pigmentation in the reticuloendothelial
cells in the liver and increased haemosiderin deposits in the spleen,
which may also be related to the decreased food consumption (Holsing &
Kundzins, 1968).
Groups of six beagle dogs of each sex recieved diets containing
technical-grade propargite (purity, 88.6%) at concentrations of 0,
160, 1250, or 2500 ppm, equivalent to 4, 30, and 48 mg/kg bw, for 1
year. The high concentration was reduced to 1875 ppm at week 9 after
observation of excessive body-weight loss. Treatment did not induce
ocular abnormalities or any treatment-related changes in clinical
chemical or urinary parameters. Two animals at the high dose died with
marked body-weight loss, and the remaining animals at this dose were
thin throughout most of the study and appeared to be dehydrated during
the last few months. Pronounced body-weight loss occurred during the
first 8 weeks at the high dose, by 2.6 kg in males and 1.9 kg in
females, and at the intermediate dose, by 0.4 kg in males and 0.5 kg
in females. Once the dose had been reduced to 1875 ppm, the
body-weight loss was less pronounced; however, the weight gain of
animals at 1250 ppm was markedly lower than that of controls. The food
consumption of animals at the high dose was reduced throughout the
study, perhaps indicating unpalatability, although it tended to
increase from week 9. Effects on haematological parameters included
reduced haemoglobin, haematocrit, and erythrocyte values in males and
females at the high dose and reduced haematocrit in males at the
intermediate dose at 3 and 6 months and at termination. In females,
these changes were less pronounced and were observed only after
3 months of treatment. Platelet counts were elevated in females at
1250 and 1875 ppm at all intervals and in males at the high dose after
6 and 12 months. Decreased absolute weights and increased relative
weights of many organs were observed in animals at the high dose and
occasionally in those at the intermediate dose. These changes are
considered to be due to the reduced body weight at this dose level.
The macroscopic changes consisted of an increased incidence of
red-tan-white foci in the lungs of males at the high dose and a higher
incidence of involution of the thymus at doses > 1250 ppm. Changes
in the bone marrow consisted of a greater incidence and severity of
erythroid-myeloid depletion and atrophy in animals at the high dose.
Histologically, the changes in the lung consisted of congestion and
inflammatory changes and fibrous and alveolar/bronchiolar epithelial
hyperplasia. In the stomach, dilated mucosal glands and vacuoles in
parietal cells were seen, but these changes showed no clear
dose-response relationship and their relation to treatment is
questionable. The NOAEL was 160 ppm, equivalent to 4 mg/kg bw per day,
on the basis of body-weight loss, reduced food consumption, and
histopathological alterations in the thymus and bone marrow at higher
concentrations (Atkinson, 1991).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a 30-day range-finding study, groups of 10 mice of each sex
received diets containing propargite at concentrations of 0, 600, 900,
1350, 2000, or 3000 ppm. Gross observations, body weight, food
consumption, gross necroscopy, and organ weights were recorded.
Body-weight loss and changes in organ weights were the predominant
effects. Minimal effects were considered to have occurred at 1000 ppm
(Gallo & Bailey, 1976), which was selected as the highest dose for the
study described below.
Groups of 15 CD-1 mice of each sex received diets containing
propargite (purity, 88.5% during the first 12 months and 84.3% during
the remaining 6 months) in corn oil at concentrations of 0, 500, or
1000 ppm, equivalent to 0, 75, and 150 mg/kg bw per day, for 52 weeks;
and groups of 60 mice of each sex received diets containing the
compound at concentrations of 0, 50, 160, 500, or 1000 ppm for
78 weeks, equivalent to 0, 7.5, 24, 75, and 150 mg/kg bw per day. The
animals were observed daily for changes in general appearance,
behaviour, appetite, toxic effects, and deaths. Body weights were
determined weekly for the first 28 weeks and every two weeks
thereafter. Food consumption was recorded weekly. Before the start of
treatment and at 52 and 78 weeks, leukocyte and erythrocyte counts
were determined in 10 animals of each sex per group, and before
termination differential leukocyte counts were made in 10 animals of
each sex per group. Gross necropsy was carried out on all animals
found dead or killed when moribund and on all animals killed at the
scheduled time. Essentially all organs, including the head, tongue,
ear, and nose, were saved, and the fresh weights of the liver, spleen,
kidneys, heart, adrenals, thyroid, and gonads were recorded.
Furthermore, sections of the spinal cord, an additional lobe of the
liver, the gall-bladder, an additional section of the uterus to
include the cervix, the nasal cavity, and the middle ear were taken
for histopathological examination.
Males at doses > 160 ppm had a lower mortality rate than
controls at 52 weeks, the survival rates being > 85%; after 78
weeks, the survival rates were 38% at 50 ppm and 60% at 500 ppm. The
variations seen in body-weight gain were not dose-related. Males
treated for 78 weeks showed a non-dose-related increase in body-weight
gain when compared with controls, whereas the females showed a
non-dose-related decrease. A similar, inconsistent pattern was found
in animals treated for 52 weeks, and these variations are considered
to be unrelated to treatment and of no toxicological significance.
Treatment did not affect food consumption or haematological
parameters, and no unexpected gross alterations were seen. An increase
in the relative weight of the kidney males treated for 52 weeks can
probably to be attributed to the reduced body-weight gain of these
animals. Females showed increased absolute and relative weights of the
adrenals at both 500 and 1000 ppm and a non-dose-related increase in
the absolute and relative weights of the thyroid at 500 ppm. The
relationship of the effects on the thyroid to treatment is
questionable. Animals treated for 78 weeks had reduced absolute and
relative weights of the kidney and uterus at concentrations > 160
ppm, and a non-dose-related increase in uterine weight was observed at
500 and 1000 ppm in females treated for 78 weeks and at 160, 500, and
1000 ppm in females treated for 52 weeks. Statistical significance was
attained only in females at 1000 ppm killed at 78 weeks.
Histopathological examination showed no treatment-related alterations
in tissues or organs, and no correlation was found between the
occurrence or type of neoplasms and treatment. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of changes in the
weights of the kidney and uterus at higher doses (Cox & Re, 1979;
Becci, 1980).
Rats
Propargite was administered to groups of 25 male and 25 female
FDRL rats in the diet at concentrations of 0 (37 animals), 100, 300,
or 900 ppm, equivalent to 0, 5, 15, and 45 mg/kg bw per day for 2
years. After the study had been in progress for 26 weeks, an
additional treatment group was included at a concentration of 2000 ppm
(equivalent to 100 mg/kg bw per day) as well as an additional control
group, because the lower doses had no effect; these groups were
treated for 78 weeks. The appearance, behaviour, and survival of the
animals was recorded daily, and body weight and food consumption were
recorded weekly. The efficiency of food use was calculated for the
first 3 months. Haematological and clinical chemical parameters were
evaluated in all animals at 12, 26, 52, 78, and 104 weeks. All rats
that died or were killed when moribund or at the end of the study were
examined macroscopically. Sections of the main organs from half of the
animals at 900 and 2000 ppm and their corresponding controls and of
the liver, kidneys, bone marrow, and thymus of the remaining rats were
examined histologically.
Treatment did not affect the appearance or behaviour of the
animals. The survival rate of males at 2000 ppm was lower than that in
the other treated groups and the control group. During the first 12
weeks of treatment, no difference in body weight or food intake was
observed between treated and the control groups, but later, the
body-weight gain of males at 900 ppm and of all treated females was
lower than that of controls, with no dose-response relationship.
Whereas the changes observed at 900 ppm were slight, a marked
reduction in body-weight gain was seen at 2000 ppm, and food intake
was also reduced at this dose. Haemoglobin, haematocrit, and leukocyte
values showed no treatment-related change at concentrations up to 2000
ppm. The absolute weights of the livers of rats at 900 ppm and of
females at 300 ppm were increased, and the relative weights were also
increased, attaining statistical significance in males at 900 ppm and
in females at concentrations > 300 ppm. In animals at 2000 ppm, the
absolute weight of the liver was decreased and the relative weight
increased, the changes being statistically significant. The relative
weight of the kidney was increased in males at 900 ppm after 104 weeks
of treatment, whereas in animals treated at 2000 ppm for 78 weeks, the
absolute weight of the kidneys was decreased and the relative weight
was increased in animals of each sex. Gross observation at autopsy
showed no treatment-related changes. The total incidences of sarcomas
and carcinomas were 6/85 in controls, 6/44 at 100 ppm, 8/42 at 300
ppm, 9/39 at 900 ppm, and 4/26 at 2000 ppm. The NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day, on the basis of changes in organ
weights (Oser, 1966). The low survival rate of animals at the highest
dose limited the relevance of the findings, and this study was
considered by the 1977 Meeting to be inadequate for evaluating the
carcinogenicity of propargite.
On the basis of the results of a 90-day study which indicated a
maximum tolerated dose of 1000 ppm (Kehoe, 1988), 800 ppm was selected
as the highest dose in a 2-year study in Crl:CDBR rats. Groups of 60
rats of each sex were given diets containing propargite (purity,
87.2%) at concentrations of 0, 50, 80, 400, or 800 ppm, equal to 2, 4,
19, and 39 mg/kg bw per day for males and 3, 5, 24, and 49 mg/kg bw
per day for females. Owing to low survival rates in males at the high
dose (30% after 103 weeks), this group was killed at 103 weeks. An
interim sacrifice was made at 53 weeks. The test diets were found to
be of adequate homogeneity and stability.
The mortality rate of males at 400 and 800 ppm was increased
towards the end of the study, with a significant positive trend,
although the group differences were not large enough to reach
statistical significance. The body weights of males at 800 ppm were
significantly reduced at the end of the study. Females showed reduced
body weight at various times during the study but no significant
overall reduction. Body-weight gain was reduced in a dose-related
manner, by 5% at 80 ppm, 12% at 400 ppm, and 18% at 800 ppm in males
at the end of the study, with statistical significance at 400 and 800
ppm. In females, body-weight gain was reduced by 15% at the high dose,
but with no statistical significance. At interim sacrifice, the
body-weight gain was reduced by 6% at 400 ppm and 20% at 800 ppm in
males and by 4% at 400 ppm and 30% at 800 ppm in females. Food
consumption was reduced at concentrations > 400 ppm in animals of
each sex. No compound-related differences in clinical signs were seen
between control and treated groups, and no treatment-related
ophthalmoscopic changes were found. The changes in haematological
parameters consisted of an increased reticulocyte count, mostly due to
decreases in three animals, and a reduced erythrocyte count in males
at 800 ppm at termination, indicating the presence of hypoxia and/or
accelerated erythropoiesis. The effects on clinical chemistry
consisted of decreased total serum protein values in males at 400 and
800 ppm and in females at 800 ppm at 26 weeks and a corresponding
decrease in total serum calcium. After 26 weeks, decreased aspartate-
and alanine aminotransferase activities were seen, with no clear
dose-response relationship. A dose-related decrease in aspartate
aminotransferase activity was observed in females at 400 and 800 ppm
after 52 weeks of treatment, probably as a result of the impaired
nutritional status of animals at these doses.
Necropsy of animals that died during the first 64 weeks of the
study showed no gross, compound-related changes in tissues. From week
65, several males at the high dose were found to have abdominal
masses, mostly in the small intestine and principally in the jejunum.
At the end of the study, males at 400 and 800 ppm and females at 800
ppm showed high frequencies of this treatment-related effect, the
incidences of masses in the jejunum being 0% in controls, 0% at
50 ppm, 0% at 80 ppm, 17% at 400 ppm, and 25% at 800 ppm in males and
0% in controls, 2% at 50 ppm, 0% at 80 ppm, 2% at 400 ppm, and 15% at
800 ppm in females. No other gross tissue alterations attributable to
treatment were found. No compound- or dose-related changes were found
in the absolute weights of the organs of treated animals, but
alterations were found in the relative weights of various organs: At
week 53, the relative weights of the livers of treated females showed
a dose-related increase over that of controls, which attained
statistical significance only in animals at 50, 400, and 800 ppm.
These changes in relative liver weights were not accompanied by
biochemical or histopathological alterations, suggesting that they
were due to the reductions in body-weight gain in females at 400 and
800 ppm and that the significant increase at 50 ppm (not supported by
a similar change at 80 ppm) is an incidental finding. Increased
relative liver weights were also observed in males at the high dose at
interim sacrifice, without reaching statistical significance, again
probably reflecting the reduced body-weight gain of these animals. At
termination, slightly increased relative liver weights were observed
in animals of each sex, but with no statistical significance. At the
interim sacrifice, the relative weights of the kidneys were also found
to be increased in animals at the high dose and in females at 80 ppm.
Histological examination revealed no treatment-related
non-neoplastic alterations and no compound-related neoplastic findings
through week 52, but during the second year of the study high
frequencies of undifferentiated sarcomas of the jejunum in males at
400 and 800 ppm and in females at 800 ppm were recorded (Table 2).
Single cases were also found in other dose groups. A clear
dose-related increase in the incidence of sarcoma was found in males
at 400 and 800 ppm and in females at 800 ppm. The incidences in all
treated female rats were also increased when compared with controls,
but with no dose-response relationship. The incidence of
undifferentiated sarcomas in duodenum, subcutaneous tissue, jejunum,
and the thoracic cavity in rats of this strain in previous studies was
reported to be 0.2% in males and 0% in females, but no
undifferentiated sarcomas were found in the jejunum. Electron
microscopic examination of abdominal tumour masses from three males at
400 ppm and one at 800 ppm revealed one malignant schwannoma, one
undifferentiated sarcoma, and one fibrosarcoma, suggesting that
propargite induced proliferation of mesenchymal tumours in various
stages of differentiation. The tumour incidences correlated with the
observations of abdominal masses. The NOAEL for systemic toxicity and
carcinogenicity was 80 ppm, equal to 4 mg/kg bw per day, on the basis
of effects on body weight, food consumption, and clinical chemical
parameters and an increased incidence of jejunal sarcomas at higher
doses (Trutter, 1991).
Dogs
Groups of six beagle dogs of each sex were maintained on a diet
containing propargite at concentrations of 0, 100, 300, or 900 ppm for
1 h per day, 6 days a week for 2 years. The dogs were observed daily
for appearance, behaviour, signs of toxicity, and neurological
reflexes. Body weight and food intake were recorded weekly for the
first 12 weeks and every 2 weeks thereafter. Haematological, clinical
chemical and urinary parameters were determined at 26, 52, 78, and
106 weeks. One animal of each sex per group was killed after 1 year
and examined grossly. A survivors were killed after 2 years and
examined. Treatment did not adversely affect the appearance,
behaviour, body weight, haematological or clinical chemical or
microscopic appearance. No indication of carcinogenicity was found
(Oser, 1966).
(d) Genotoxicity
The results of tests for the genotoxicity of propargite are
summarized in Table 3.
Table 2. Incidences of undifferentiated sarcoma of the jejunum in rats fed propargite in the diet for 2 years
Sex Dose Tumour incidence
(ppm)
Unscheduled deaths Interim sacrifice Terminal sacrifice Total
No. of No. % No. of No. % No. of No. % No. of No. %
rats rats rats rats
Male 0 26 0 0 9 0 0 24 0 0 59 0 0
50 16 0 0 0 31 0 0 47 0 0
80 23 0 0 0 23 0 0 46 0 0
400 32 9 28 0 17 2 12 49 11 22
800 35 20 57 10 0 0 15 4 27 60 24 40
Female 0 27 0 0 10 0 0 20 0 0 57 0 0
50 29 0 0 0 20 1 5 49 1 2
80 20 1 5 0 29 0 0 49 1 2
400 28 0 0 0 20 1 5 48 1 2
800 25 8 32 10 0 0 21 4 19 56 12 21
Table 3. Results of tests for the genotoxicity of propargite
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.001-5 µl/plate NR Negative ± S9 Brusick & Weir
TA98, TA100, in DMSO (1977)
TA1535, TA1537,
TA1538;
S. cerevisiae D4
Reverse mutation S. typhimurium 10-5000 µg/plate 90.9 Negative ± S9 Shirasu et al. (1979)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr
Reverse mutation S. typhimurium 10-300 µl/plate 90a Positive ± S9 Lawlor (1991)
TA98, TA100, at > 10 µl/ml
TA1535, TA1537, in TA100
TA1538
Gene mutation B. subtilis H17, M45 1-100% v/v in DMSO 90.9 Negative Shirasu et al. (1979)
Gene mutation Chinese hamster 1-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 10-75 µg/ml +S9 (1993)
locus in DMSO
Gene mutation Chinese hamster 0.2-4.2 µg/ml 90 Negative Bigger & Clarke
ovary cells, Hprt -S9; 10-50 µg/ml (1993)
locus +S9 in DMSO
Gene mutation Chinese hamster 0.5-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 5-5 µg/ml +S9 (1993)
locus in acetone
Table 3. (continued)
End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Chinese hamster 25-200 µg/ml NR Negative ± S9 Kirkland (1985)
aberrations ovary cells
DNA repair Rat hepatocytes 0.0167-0.5 µg/ml 97 Negative Barfknecht (1987)
in acetone
In vivo
Micronucleus ICR mouse 37.5, 75, 150 mg/kg 89.6 Negative Putman & Young
formation bw i.p in corn oil (1994)
NR, not reported; i.p., intraperitoneally
a Technical-grade containing epoxidized soya bean oil as stabilizer
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of rats were maintained on diets containing propargite at
concentrations of 0 or 100 ppm. When the rats were about 100 days of
age and sexually mature, 20 pairs of males and females were followed
through two reproductive cycles. The first litters were discarded. At
weaning of the second litter, 10 rats of each sex in each group were
selected for the F1 generation. A similar procedure was chosen for
selection of the F2 generation. The dose of the F2 pups was
increased to 300 ppm, and the F3 pups received 300 ppm throughout
treatment. The pups were raised to maturity and the cycle repeated in
this and the succeeding generation. Treatment did not have adverse
effects on the dams, the reproduction indices, or their pups. The NOEL
was 100 ppm, the only dose tested, equivalent to 5 mg/kg bw per day
(Oser, 1966).
Groups of 25 immature Crl:CDBR albino rats of each sex were fed
diets that contained technical-grade propargite (purity, 87.2%) at
concentrations of 0, 80, 400, or 800 ppm, equivalent to 0, 4, 20, and
40 mg/kg bw per day, for 10 weeks before mating and throughout mating,
gestation, lactation, and weaning of the F1a pups. After weaning,
these pups were killed and discarded, and the F0 animals were mated
again to produce F1b litters. After weaning, the pups were assigned
at random to four groups of 25 animals of each sex, and the F0
adults were killed and necropsied. The selected F1b animals were fed
the diets for at least 10 weeks before mating and throughout
gestation, lactation, and weaning of F2a pups. After these pups were
weaned, they were killed and discarded, and the F1b animals were
mated again to produce F2b litters. After these pups had been
weaned, they were killed and discarded, and the F1b adults were
killed and necropsied. The body weights of males were recorded on the
first day of treatment, weekly thereafter, and on the day of necropsy,
whereas females were weighed on the first day of treatment, weekly
before mating, on days 0, 7, 14, and 20 of gestation and lactation,
and on the day of necropsy. Food consumption was recorded weekly only
before mating of the F0 and F1b generations and not during the
gestation and lactation periods. All F0 and F1b adults were
examined macroscopically, and the reproductive organs of those at 0
and 800 ppm were examined microscopically.
No treatment-related clinical signs were noted in these animals,
and their survival was not adversely affected. The treatment resulted
in dose-related reductions in body weight and body-weight gain of both
sexes of both generations at 400 and 800 ppm during various phases of
the study. Body weight was reduced by 5-10% in males at 400 ppm and by
18-28% in both generations at 800 ppm before and after mating.
Body-weight gain showed a corresponding pattern, with reductions of up
to 20% in males at 400 ppm and up to 60% at 800 ppm. Similar
reductions were found in females: at 400 ppm, the maximum reduction
during premating, gestation, and lactation was 10%, and at 800 ppm the
reduction was up to 22%. The body-weight gains of females were reduced
by up to 30% at 400 ppm and up to 90% at 800 ppm before mating. Marked
differences in the body-weight gain of females were seen in the
premating, gestation, and lactation periods. In all generations,
lactating dams treated with 400 and 800 ppm had greater body-weight
gain than controls, perhaps because some controls lost weight during
the second half of the lactation period. Males of the F0 generation
at 400 ppm showed a dose-related reduction in mean food consumption
during weeks 0-1, 4-5, and 8-9 before mating, and the food consumption
of males at 800 ppm was consistently lower before mating. The mean
food consumption of F0 females at 400 ppm was lower only during week
6-7, but that of females at 800 ppm was reduced throughout the
premating period. The mean food consumption of males and females of
the F1b generation showed a dose-related reduction at 400 and 800
ppm. Mating and fertility parameters and gestation indexes were not
affected by treatment, and no treatment-related differences in litter
size or sex ratio were seen. Pup weight per litter was reduced in all
generations at 400 ppm on lactation days 7, 14, and 21 and for litters
at 800 ppm on lactation days 0, 4, 7, 14 ,and 21. The maximum
reduction in the weight of pups at 800 ppm group on lactation day 21
was 44%. There were no treatment-related macroscopic or microscopic
changes. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per day, on
the basis of effects on the body weights of dams and pups at higher
doses (Kehoe, 1990).
In a study designed to clarify the reduced pup weights during
lactation at concentrations of 400 and 800 ppm, a cross-fostering
study was initiated in which groups of Crl: CD VAF/Plus rats were
given diets containing technical-grade propargite (purity, 89.9%) at
concentrations of 0 (100 animals of each sex), 400 ppm (30 of each
sex), or 800 ppm (60 of each sex). The F0 parents were given the
diets for 70 days before mating, and F0 males were killed one week
after mating. The F0 females that had delivered were killed on
lactation day 21, and those that did not deliver within 25 days after
mating were also necropsied. On lactation day 21, the F1 offspring
were necropsied, with special attention to the reproductive organs. On
lactation day 0, the litters were cross-fostered to dams in other
groups and those of the control group to other dams within the group.
Selected dams in the control group and at 800 ppm were allowed to keep
their own litters. The new groups were thus: 15 untreated dams with
their own untreated litters (controls); 20 untreated dams
cross-fostering untreated litters; 20 untreated dams cross-fostering
litters treated at 400 ppm; 20 untreated dams cross-fostering litters
treated at 800 ppm; 20 dams treated at 400 ppm cross-fostering
untreated litters; 20 dams at 800 ppm cross-fostering untreated
litters; and 20 dams at 800 ppm with their own litters treated at 800
ppm. All animals were observed for deaths and signs of toxicity twice
daily. Body weights were recorded weekly for males and for females
until evidence of copulation was seen and on days 0, 7, 14, and 20 of
gestation and days 0, 7, 14, and 21 of lactation. The food consumption
of adult rats was measured weekly except during mating in weeks 11-13
and, for females, during gestation and lactation. The litters were
reduced randomly to four pups of each sex on lactation day 4 to
provide homogeneous groups for evaluation of nursing, survival, and
pup growth. The culled pups were examined externally. The litters were
caged with their dams for 3 weeks after birth. Throughout lactation,
the dams and their pups were observed daily for survival and
behavioural abnormalities in nesting and nursing. Pups were weighed on
days 0, 4, 7, 14, and 21 of lactation, the day of termination.
Treatment had no effect on the appearance or behaviour of the
F0 animals. A treatment-related reduction in body weight relative to
the controls was seen in males and females at 800 ppm during
premating, by 16% in males and 9% in females at the end of premating.
During gestation, the mean maternal body weight and body-weight gain
were reduced at the 800 ppm; during lactation, the mean body weights
of dams at 800 ppm cross-fostering untreated litters and and of dams
at 800 ppm with their own litters remained lower than those of
controls. At the beginning of lactation, reduced body weights were
observed in all cross-fostering groups relative to the controls, with
reductions of 5% in untreated dams cross-fostering untreated litters,
4% in untreated dams cross-fostering litters treated at 400 ppm, 2% in
untreated dams cross-fostering litters at 800 ppm, 3% in dams treated
at 400 ppm cross-fostering untreated litters, 7% in dams at 800 ppm
cross-fostering untreated litters, and 12% in dams at 800 ppm with
their own litters treated at 800 ppm. At the end of the lactation
period, the reductions were 3%, 6%, 2%, 0%, 7%, and 10% in these
groups, respectively. Body-weight gain during lactation showed marked
variation among the different groups which was unrelated to dose, the
changes over the 21-day period corresponding to 31%, -17%, 10%, 52%,
14%, and 28% in the six groups, respectively. Food consumption was
also reduced, by up to 20% at 800 ppm in males and females before
mating. During gestation, the food consumption of dams at 800 ppm was
reduced to about 11%. During lactation, the food consumption in the
different groups was 103%, 101%, 106%, 98%, 83%, and 80% relative to
the controls, respectively.
No treatment-related differences in litter size, viability on day
0, or the survival index of the F1 offspring was observed. From
birth (lactation day 0), the mean body weights of male and female pups
born to dams treated at 800 ppm were statistically significantly
reduced when compared with the control group, whereas the weights of
female pups born to dams given 400 ppm were significantly increased,
an effect that was considered biologically irrelevant. On lactation
day 0, the body weights of untreated pups nursed by their untreated
dams and of pups at 400 ppm fostered by untreated dams were unchanged.
The weights of pups at 800 ppm fostered by untreated dams were
nonsignificantly reduced on days 0 and 4; those of untreated pups
fostered by dams at 400 ppm were nonsignificantly reduced on days 0
and 4 but significantly reduced on days 14 and 21 of lactation; the
weights of untreated pups fostered by dams at 800 ppm and those of
pups treated at 800 ppm and fostered by dams at the same dose were
nonsignificantly reduced on day 0 but significantly reduced on days 7,
14, and 21. There were no treatment-related differences in the
incidences of malformations, developmental variations, or macroscopic
and microscopic appearance in the F1 offspring. The results of this
study indicate that the effects on pup weight are the result of
toxicity in the dams (York, 1992).
(ii) Developmental toxicity
Rats
Groups of a minimum of 20 females Sprague-Dawley rats were given
technical-grade propargite as a suspension in corn oil by gavage at
doses of 0, 6, 25, or 105 mg/kg bw per day on days 6-15 of gestation.
At least 30 females were used as vehicle controls and for a positive
control group receiving an aqueous suspension of aspirin at a dose of
250 mg/kg bw per day. The original doses were set at 0, 25, 105, and
450 mg/kg bw per day, but dams at 450 mg/kg bw per day died after only
3 or 4 days of treatment and therefore this group was terminated. The
females at 105 mg/kg bw per day showed signs of toxicity, including
bloody nasal and vaginal discharges and urinary incontinence; this
dose was continued as the high dose, 25 mg/kg bw per day as the
intermediate dose, and an additional dose of 6 mg/kg bw per day as the
low dose. No clinical signs were observed at 6 or 25 mg/kg bw per day.
The body weight and body-weight gain of dams at 105 mg/kg bw per day
were lower than those of the other groups, but the difference did not
attain statistical significance. The body weight and body-weight gain
of the positive controls were significantly reduced at the end of the
treatment period. The reproductive performance of the treated dams, as
measured by pregnancy rate and the numbers of implantations,
resorptions, and live and dead fetuses, was similar at all doses. When
the fetuses of treated dams were evaluated for skeletal anomalies,
those at 25 and 105 mg/kg bw per day showed statistically significant
increases in the incidence of missing sternebrae, with 3% of fetuses
and 24% of litters at 25 mg/kg bw per day and 16% of fetuses and 41%
of litters at 105 mg/kg bw per day affected. The control incidence was
1% of fetuses and 6% of litters. The incidences of incomplete
ossification of vertebrae were also increased, with 28%/88%
(fetuses/litters) at 6 mg/kg bw per day, 23%/71% at 25 mg/kg bw per
day, and 30%/86% at 105 mg/kg bw per day; the control incidence was
13%/58%. Statistically significant increases were also observed in the
incidence of incomplete closure of the skull at 105 mg/kg bw per day
and in the incidence of reduced or missing hyoid, with 2%/10% at 25
mg/kg bw per day, and 5%/14% at 105 mg/kg bw per day, and 0.4%/3% in
the control group and 0.5%/4% at 6 mg/kg bw per day. The incidence of
haemorrhagic abdomen was increased at 25 and 105 mg/kg bw per day,
with no clear dose-response relationship. Most of these parameters
were also adversely affected in the dams in the positive control
group. In these animals, the incidence of resorption sites was
increased and the number of live fetuses and fetal weight were
significantly reduced, and the body weights of the dams were reduced.
The fetuses of the positive controls showed various skeletal and
soft-tissue abnormalities at significantly higher incidences than in
the untreated controls. The NOAEL for maternal toxicity was 25 mg/kg
bw per day, but no NOAEL could be identified for developmental
toxicity (Knickerbocker & Re, 1979).
Groups of 45 female Crl:CD rats were given technical-grade
propargite (purity, 85%) by gavage at doses of 0, 6, 12, 18, 25, or
105 mg/kg bw per day on days 6-15 of gestation. On day 20, the pups of
20 gravid females were removed surgically and examined. The remaining
animals were allowed to deliver, and they and their pups were observed
until day 21 of lactation, when they were necropsied. There were no
treatment-related effects on behaviour or survival. Animals at the
highest dose had anogenital and body surface staining, and the body
weights were significantly reduced by 5% when compared with controls.
The adjusted body weight (body weight on day 20 of gestation minus the
weight of uterus and contents) of animals at this dose showed a
similar reduction (7%) on days 6-9 of gestation. The body-weight gain
during days 6-15 of gestation was similar at the four lower doses but
was reduced at the highest dose. The body weights during lactation did
not differ with dose, and those of animals at the highest dose were
similar to those of controls. No sign of developmental toxicity was
seen, as measured by pre- and postimplantation loss, the numbers of
implantations and corpora lutea, pup survival index, or fetal body
weight. Slight reductions in the numbers of live offspring at day 0 of
lactation were observed, with no clear dose-response relationship; the
greatest reduction, 96.4%, was found at the highest dose. The indexes
of pup survival and mortality calculated on a fetal basis showed no
treatment-related reduction, but pup mortality analysed on a litter
basis showed a statistically significant increase in the number of
litters with deaths per total litters on day 7 at the highest dose. No
treatment-related difference in the incidence of malformations or of
developmental variations was seen. The NOAEL was 25 mg/kg bw per day
on the basis of effects on the body weight of dams and slight effects
on postnatal mortality at the higher dose (Schardein, 1990).
Rabbits
Groups of 17 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by intubation at
doses of 0, 2, 6, 10, or 18 mg/kg bw per day on days 6-18 of
gestation. Maternal toxicity, manifested by higher mortality rates at
6, 10, and 18 mg/kg bw per day, attained statistical significance at
18 mg/kg bw per day. Adipsia and anorexia were seen in all groups but
at a markedly higher frequency at the three higher doses than in
controls. Maternal body weight and body-weight gain showed
dose-related reductions at doses > 6 mg/kg bw per day, resulting in
reduced overall weight gain up to day 29 at 6 and 10 mg/kg bw per day
and weight loss at 18 mg/kg bw per day. The finding of brown areas in
the gastric mucosa in most animals at the highest dose at necropsy was
considered to be related to treatment. Pregnancy rates were not
adversely affected, but gravid uterine weights showed a dose-related
reduction at all doses. As the litter size also showed a dose-related
reduction, the reduction in uterine weight might reflect the smaller
litter sizes. Fewer implantations were found in all treated groups,
with the fewest at 18 mg/kg bw per day; the mean number of resorptions
was slightly increased at 10 and 18 mg/kg bw per day, and the mean
incidence of resorptions calculated on a per litter basis was markedly
increased at these doses. Treated groups had fewer live fetuses per
litter, and the fetal viability index calculated on a per litter basis
was decreased at 10 and 18 mg/kg bw per day. The mean fetal body
weights were reduced at the highest dose.
Increased incidences of visceral and skeletal malformations were
seen at doses > 6 mg/kg bw per day. One fetus at 10 mg/kg bw per
day had an enlarged, domed head (hydrocephaly), resulting in an
incidence of 1.6%, and two fetuses from the same litter at 18 mg/kg bw
per day also had hydrocephaly, resulting in an incidence of 9%; the
incidence in controls was 0% and that in historical controls was
0.11%. One fetus at the highest dose had an incomplete diaphragm
(incidence, 5%; 0% in controls, 0.04% in historical controls) and
other visceral alterations such as small lungs and kidneys. The
treatment-related skeletal variants included delayed ossification of
the skull and bone alignment of sternebrae. Skull closure that was
only 75% complete was seen more frequently at doses > 6 mg/kg bw
per day, resulting in incidences of 12%, 13%, and 10%, respectively,
with an incidence of 4% in the concurrent control group and in animals
at 2 mg/kg bw per day. The absence of a doserelated increase at the
highest dose might be a consequence of the small number of fetuses
available for examination (21 compared with 62-115 in the other
groups). The historical incidence for 75% skull closure was 5.7%.
Malaligned or fused sternebrae were found at incidences of 0% in
controls and 2%, 3%, 8%, and 0% at the four doses, respectively, with
a historical control incidence of 1.8%. The NOAEL for maternal and
fetal toxicity was 2 mg/kg bw per day (Serota et al., 1983).
Groups of 25 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by gavage at
doses of 0, 2, 4, 6, 8, or 10 mg/kg bw per day on days 7-19 of
gestation. The fetuses were removed surgically on day 29 and examined
for teratological changes. Dams at 8 and 10 mg/kg bw per day showed
signs of toxicity during the treatment period, including reduced
body-weight gain, and body-weight loss was observed during the second
half of the treatment period at 8 mg/kg bw per day and throughout
treatment at 10 mg/kg bw per day; however, the body weights at the end
of gestation were not significantly reduced. Three females at 4 mg/kg
bw per day, one at 8 mg/kg bw per day, and four at 10 mg/kg bw per day
aborted between days 18 and 25 of gestation. The toxicological
significance of the abortions at 4 and 8 mg/kg bw per day is
questionable because of the lack of a dose-response relationship, but
the abortions at 10 mg/kg bw per day are considered to be related to
treatment as they were accompanied by signs of systemic toxicity in
the dams. Fetal viability, fetal body weights, the mean numbers of
pre- and postimplantation losses, and the total numbers of
implantations and corpora lutea were comparable in all groups,
including controls. Higher incidences (on fetal and litter bases) of
fused sternebrae were found at 8 and 10 mg/kg bw per day, with
incidences of 0/0 % (fetuses/litters), 2/7%, 0.8/6%, 0/0%, 2/11%, and
8/38% at the 0, 2, 4, 6, 8, and 10 mg/kg bw per day, respectively.
This malformation is reported to occur spontaneously at an incidence
of up to about 5%. The NOAEL for maternal and developmental toxicity
was 6 mg/kg bw per day (Schardein, 1989).
(f) Special studies: Cell proliferation
In the 2-year study in CD rats of Trutter (1991), described
above, treatment with propargite resulted in a statistically
significant increase in the incidence of undifferentiated sarcomas in
the jejunum in animals of each sex given dietary concentrations
> 400 ppm. In order to investigate the underlying mechanism and to
establish a NOAEL for the relevant parameter, comparative studies were
conducted in CD rats and CD-1 mice over 1 or 4 weeks. Male rats were
given technical-grade propargite in the diet at 0 or 800 ppm or 0 or
80 ppm, female rats were given diets containing 0, 40, or 800 ppm, and
male mice received diets containing 0 or 1000 ppm. The groups
consisted of 12 controls and 22 treated animals. Body weight, food
consumption, and clinical observations were recorded weekly during the
study. Osmotic pumps containing 5-bromo-2'-deoxyuridine (BrdU), which
is incorporated into the DNA of replicating cells and used to detect
proliferating cells by immunohistochemistry, were placed
subcutaneously in half of the animals on day 1 or 20 of the study, and
the animals were killed and necropsied 1 week after implantation. All
animals were processed for immunohistochemistry and histopathology
(haematoxylin and eosin staining). Sections of the jejunum were
collected to determine cell proliferation in three layers of smooth
muscle. Cells that incorporated BrdU were identified by the presence
of chromagen over their nuclei, and cell proliferation was expressed
as unit length labelling index (number of labelled cells per square
millimetre). Positive staining in the epithelium of the duodenum was
considered to indicate systemic delivery of BrdU.
The body weights of rats receiving 800 ppm were reduced,
especially in males, and food consumption was lower. The body weights
and food consumption of the mice were not affected. After 1 week,
increased total smooth muscle cell proliferation was observed at 800
ppm in male and female rats, and the response was statistically and
biologically significant, the latter being defined as a twofold or
greater increase in cell proliferation in treated animals when
compared with controls. No cell proliferation was induced at lower
doses in rats, and no cell proliferation occurred in male mice at 1000
ppm. No biologically significant increase in cell proliferation was
found after 4 weeks of treatment, although male rats at 800 ppm showed
a statistically significant increase. The NOEL for cell proliferation
was 40 ppm, equal to 2 mg/kg bw per day (Eldridge, 1994).
A similar study was conducted to investigate a possible strain
specificity of the cell proliferative response. Groups of 11 Wistar
(WKY) rats of each sex received technical-grade propargite (purity,
88.6%) in the diet at a concentration of 900 ppm for 1 week, while six
animals of each sex received the diet alone. On the first day of the
study, osmotic pumps containing BrdU were placed subcutaneously, and
the rats were killed and necropsied 1 week later. All animals were
processed for immunohistochemistry and histopathology as described
above. Body weight, food consumption, and clinical observations were
recorded at the end of treatment. Body-weight gain and food
consumption were decreased in rats of each sex throughout the study.
No biologically significant increase in cell proliferation was
observed, and a statistically significant increase was found only in
the outer layer of the smooth muscle of the jejunum in female rats.
This isolated finding was not considered to be biologically
significant, and the combined results for all three layers did not
attain statistical significance. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in any animal. These
results are consistent with the lack of tumorigenic response in male
and female Wistar rats treated with propargite at 900 ppm for 2 years
(Eldridge, 1995; Oser, 1966).
Groups of 10 Charles River CD rats of each sex received
technical-grade propargite in the diet at a concentration of 0 or 400
ppm for 1 week. On the first day, all rats received osmotic pumps
containing BrdU. The animals were observed for clinical signs, body
weight, and food consumption. On day 7, the animals were killed, and
the jejunum and the duodenum were examined macroscopically and
microscopically. No clinical signs and no effect on body-weight gain
or food consumption were seen. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in the jejunum.
Statistically and biologically significant increases in cell
proliferation were found in jejunal smooth muscle in males and females
(Goldenthal, 1999).
Groups of CD rats were given diets containing propargite (purity,
93%) at doses up to 800 ppm, according to the following design, with
evaluations of cell proliferation and histopathological changes after
4, 8, 12, 16, and 20 months. Groups of 90 males were given diets
containing concentrations of 0 or 800 ppm, equal to 0 and 42 mg/kg bw
per day; 6 weeks later, groups of five males were given concentrations
of 0, 80, or 400 ppm, equal to 0, 4, and 21 mg/kg bw per day, and
groups of 55 females were given concentrations of 0, 40, 400, or 800
ppm, equal to 0, 6, 28, and 55 mg/kg bw per day. Each rat was observed
twice daily for death and signs of toxicity. Clinical examinations
were conducted once weekly, and body weights and food consumption were
recorded weekly for the first 16 weeks and every 4 weeks thereafter.
Ten males at 0 and 800 ppm were killed after 4, 8, 12, 16, and 20
months, and the first 10 rats in the remaining groups were killed
after 4, 12, and 20 months. One week before scheduled necropsy,
osmotic pumps containing BrdU were implanted subcutaneously into the
backs of the animals. Standard immunohistochemical methods were used
to stain tissues for BrdU, and staining was evaluated by nuclear
labelling. Three smooth muscle layers were evaluated. Jejunal tissues
stained with haematoxylin and eosin, serial to those stained for BrdU,
were examined for histopathological changes. Ten animals in each group
were killed 6 or 7 days after implantation of the pumps. Sections of
jejunum, ileum, duodenum, and stomach were collected at the 4,- 12-,
and 20-month interim sacrifices, while only sections of jejunum and
duodenum were collected at the 8- and 16-month sacrifices. The
remaining tissues from each animal and the carcass were discarded
without further necropsy, and no further microscopic evaluation was
conducted.
The survival of control and treated animals was similar, and no
treatment-related clinical signs were observed. Body weights were
decreased by 17% in males and 13% in females at 800 ppm, and a
statistically significant decrease in body weight was observed in
males at 400 ppm, which did not result in a significant overall
reduction in body weight at the end of the study; no effect on body
weights was observed in females at this dose. The average food
consumption was reduced in animals at 800 ppm and sporadically in
those at 400 ppm. No treatment-related macroscopic changes were found
in animals killed at 4, 8, 12, or 16 months, but treatment-related
jejunal masses or nodules were observed at 20 months in males at 400
and 800 ppm and in females at 800 ppm. No statistically significant
increase in cell proliferation was detected in the smooth muscle of
the jejunum of rats treated for up to 20 months, but at that time an
at least twofold increase in cell proliferation was seen in male rats
given 800 ppm. At 4, 8, and 12 months, less cell proliferation was
seen in males at 800 ppm than in controls. These findings are
consistent with the reduced total number of cells at the same times.
At 16 months, the difference in the total number of cells between
controls and males at 800 ppm began to decrease, resulting in similar
cell proliferation in the two groups, and by 20 months both cell
proliferation and the total number of cells were greater in the rats
at the high dose than in controls. This effect appears to be a
compensatory response to apparent suppression of cell turnover in the
smooth muscle cells of the jejunum in these animals, which was first
apparent at 16 months. By 20 months, the smooth muscle cells had
overcompensated for the inhibition in cell turnover, resulting in
increased cell proliferation and total cell number. Although the
increase in cell proliferation was not statistically significant, a
twofold or greater increase in the unit length labelling index is
considered to reflect a cell proliferative response. Histopathological
evaluation revealed no hyperplasia, cytotoxicity, or inflammation in
the jejunum. The NOAEL for systemic toxicity was 80 ppm, equal to 4
mg/kg bw per day, on the basis of slight effects on body-weight gain
and jejunal masses at 400 ppm. The NOAEL for cell proliferation was
400 ppm, equal to 21 mg/kg bw per day (Goldenthal, 1998).
The results of these studies suggest that sustained cell
proliferation is not an etiological factor in tumour formation at the
jejunum. Furthermore, no lesions indicative of toxicity were
identified as precursors of tumour formation. The results suggest a
mitogenic mode of action rather than a cytotoxic action, and this
conclusion is supported by the species- and strain-dependent
differences in the carcinogenicity of propargite and the corresponding
species- and strain-dependent differences in jejunal cell
proliferative response.
3. Observations in humans
Propargite is an irritant in humans and may also be a sensitizer,
as several outbreaks of dermatitis were observed in field workers
exposed to this compound (Lee et al., 1981). An outbreak of poisoning
and dermatitis was seen among workers exposed to a formulation of
propargite in Japan in 1970: after use of the miticide on orange
trees, 40 out of 47 workers developed dermatitis and 43 showed signs
of noncutaneous effects, including irritation of the respiratory
tract. Dermatitis was also observed in mixers, loaders, and field
workers in California, USA. Outbreaks of dermatitis in subsequent
years have been reviewed (O'Malley, 1997). The environmental half-time
of propargite of 5-11 days may allow prolonged residual action which
might be responsible for the outbreaks of dermatitis in field workers
(Abrams et al., 1991).
Comments
The results of various studies of the pharmacokinetics of single
oral doses of propargite in CD rats showed that gastrointestinal
absorption was inversely related to the administered dose. After
administration of a single oral dose of [14C-phenyl]ring-labelled
propargite to rats, 20-50% of the administered dose was excreted in
the urine and 40-75% in faeces; about 1.5% of the administered dose
was found in tissues, with slightly higher urinary excretion and
correspondingly less faecal elimination in males. Roughly similar
results were found in CD mice treated in the same way.
In rats, dermal absorption of propargite occurred mainly within
the first 4 h after application. The absorption amounted to up to 33%
of the administered dose of technical material and 3-17% of the
administered doses of various formulations.
The metabolism of propargite has been elucidated in a series of
studies in rats and mice. The compound was rapidly degraded to polar
metabolites, metabolism of the cyclohexyl ring predominating. Most of
the radiolabel in rat faeces was associated with unchanged parent
compound and with a few metabolites, which were also found in urine.
In comparative studies in rats and mice, no parent compound was found
in the bile of either species, whereas the plasma contained small
amounts of unchanged propargite; six metabolites were found in rat and
mouse bile. The metabolites are formed as a result of hydrolysis of
the propynyl sulfite side-chain, subsequent oxidation and conjugation
of the tert-butyl moiety, and hydroxylation of the cyclohexyl
moiety. No consistent quantitative or qualitative species differences
in bile and plasma metabolite profiles were found.
In further studies of the metabolism of propargite with a
radiolabel located in the propynyl sulfite side-chain and with
radiolabelled propargyl alcohol, an additional metabolic pathway was
identified which involves metabolism of the side-chain by glutathione
conjugation.
Propargite is of low acute toxicity, with an oral LD50 in rats
of 2800 mg/kg bw, but it irritates the skin and eyes. Propargite did
not show dermal sensitizing potential in the Buehler test in
guinea-pigs. WHO (1999) has classified propargite as 'slightly
hazardous'.
In short-term studies in mice, rats, and dogs, the signs of
systemic toxicity included effects on body weight and on
haematological and clinical chemical parameters. In a study in which
CD rats were fed propargite for three months, the NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day. In dogs, dietary administration of
propargite for 1 year resulted in a NOAEL of 160 ppm, equivalent to 4
mg/kg bw per day, on the basis of effects on body weight and on
various haematological parameters and histopathological changes in the
thymus and bone marrow. In a 21-day study in rabbits treated dermally,
the NOAEL for systemic toxicity was < 100 mg/kg bw per day, whereas
no NOAEL for local irritation was found.
In long-term studies, the most significant toxicological finding
was the occurrence of jejunal sarcomas in CD (Crl:CDBR) rats, whereas
no carcinogenic effect was observed in CD-1 mice or Wistar (FDRL)
rats. In a 78-week study in CD-1 mice carried out in 1979, the NOAEL
was 50 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
changes in organ weights. In the study in Wistar (FDRL) rats carried
out in 1966, the NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day,
also on the basis of changes in organ weights.
In a two-year study reported in 1991 in which CD (Crl:CDBR) rats
were given diets containing propargite at concentrations of 0, 50, 80,
400, or 800 ppm (equal to 0, 2, 4, 19, and 39 mg/kg bw per day in
males and 0, 3, 5, 24, and 49 mg/kg bw per day in females), males at
400 and 800 ppm showed a dose-related increase in the incidence of
undifferentiated sarcomas in the jejunum, a very rare tumour. Females
also showed a clear tumorigenic response but with a different
dose-response relationship, since a high incidence (21%) of jejunal
tumours in the smooth muscle was observed at the highest concentration
of 800 ppm but low incidences (one animal in each group) at 50, 80,
and 400 ppm. Given the rarity of sarcomas arising at this site (0% in
concurrent and historical female controls, 0% in concurrent male
controls, and 0.2% in historical male controls), a NOAEL for
tumorigenicity could not be identified in this study. In a 20-month
study reported in 1998 in which CD rats were given dietary
concentrations of 0, 80, 400, or 800 ppm (equal to 0, 4, 21, and 42
mg/kg bw per day) for males and 0, 40, 400, or 800 ppm (equal to 0, 6,
28, and 55 mg/kg bw per day) for females, the appearance of jejunal
masses at 400 and 800 ppm in males and at 400 ppm in females suggested
that the tumorigenic response is a reproducible effect. Reduced cell
proliferation in jejunal smooth muscle layers and decreased jejunal
cell division were found at various times during the study. After 20
months, cell division and cell proliferation were found to be
increased only in males at 800 ppm, with no corresponding response in
females or in males at lower doses. No hyperplasia, cytotoxicity, or
inflammation was found in the jejunal epithelium.
Several short-term (1 or 4 weeks) studies of cell proliferation
were conducted in male and female CD rats at concentrations of up to
800 ppm, in Wistar rats (WKY strain) at 900 ppm, and in male CD-1 mice
at 1000 ppm. The NOAEL for cell proliferation in the CD rat was 40 ppm
(equal to 2 mg/kg bw per day) in females and 80 ppm (equal to 4 mg/kg
bw per day) in males, both being the lowest doses tested. A
proliferative response was observed at 800 ppm in both male and female
rats after 1 week of treatment, whereas the response was observed only
in males after 4 weeks of treatment at this concentration; no cell
proliferation was observed at 40 and 80 ppm. No cell proliferation was
found in Wistar rats (WKY) treated with 900 ppm or in CD-1 mice at
1000 ppm in the same study design.
The results of the long-term studies in CD rats and the
short-term studies of cell proliferation indicate that the cell
proliferation induced by propargite in the jejunum is characterized by
an initial transient proliferative response, lasting for at least 4
weeks in males and for only about 1 week in females. This profile of
cell proliferation suggests that the underlying mechanism for tumour
formation in the jejunum of CD rats may be related to the mitogenic
activity of the compound.
The observed dose-response relationship for tumour formation,
with a greater increase in tumour incidence in males than in females,
correlates with the duration and degree of jejunal cell proliferation.
The prolonged duration of cell proliferation in male rats was
associated with a more pronounced tumorigenic response than in
females. Furthermore, propargite did not induce cell proliferation in
species and strains in which no carcinogenic activity was observed.
The available database does not further illuminate the causal
relationship between cell proliferation and tumorigenicity, and no
explanation was offered of the species, strain, and sex specificity or
of the association between the presence of an early, transient cell
proliferation response and the occurrence of jejunal tumours in CD
rats and the consistent absence of these findings in Wistar rats and
CD-1 mice. Nevertheless, the association between proliferation and
tumorigenic activity was recognized by the Meeting. The Meeting also
noted that the NOAEL for cell proliferation of 40 ppm is very close to
the lowest tumorigenic concentration in female rats of 50 ppm. The
available database did not, however, clarify the dose-response
relationship found at low tumorigenic doses in females and offered no
further scientific evidence to support use of cell proliferation as a
marker of potential carcinogenicity.
An adequate range of studies for genotoxicity was conducted, and
the results were consistently negative. Therefore the Meeting
concluded that propargite is not genotoxic. The finding of
consistently negative results in numerous tests for genotoxicity
provides further support for the conclusion that propargite causes
tumours by a non-genotoxic mechanism.
In a three-generation study of reproductive toxicity, reduced
body-weight gain was seen at concentrations of 400 ppm (equivalent to
20 mg/kg bw per day) and above in parental animals and in pups during
lactation. The results of a subsequent cross-fostering study showed
that the growth retardation of the pups was reversible and was due to
maternal toxicity. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per
day, on the basis of reduced body-weight gain in parental animals and
pups.
Two studies of developmental toxicity were conducted in rats and
two in rabbits. In a study in Sprague-Dawley rats reported in 1979,
fetal effects such as increased incidences of incomplete vertebral
ossification and missing sternebrae and hyoids were observed in
treated animals. Not all of the findings were dose-related and they
were not reproduced in a study reported in 1990. The NOAEL was 25
mg/kg bw per day on the basis of reduced body-weight gain in dams and
a slight increase in the postnatal mortality rate at the highest dose
in the latter study. In the two studies in rabbits, dated 1983 and
1989, fetal effects indicative of developmental retardation were
observed at maternally toxic doses of 6 mg/kg bw per day and above,
and an increased incidence of hydrocephaly was seen at higher doses in
the first study. Most of the fetal effects, including hydrocephaly,
were not confirmed in the second study, in which effects were observed
only at doses of 8 mg/kg bw per day and higher. The overall NOAEL in
rabbits was 4 mg/kg bw per day. No evidence for teratogenicity was
found in these studies.
The Meeting allocated an ADI of 0-0.01 mg/kg bw on the basis of
the LOAEL of 50 ppm (equal to 3 mg/kg bw per day) for tumorigenicity
in female CD rats, with a safety factor of 300. This safety factor was
chosen to account for the lack of a NOAEL in this study and the nature
of the end-point (tumorigenesis) and to encompass the NOAEL in the
same rat strain for increased cell proliferation in the jejunum, the
site of tumour formation.
The Meeting concluded that it was unnecessary to determine an
acute reference dose because of the low acute toxicity of propargite.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (effects on organ
weights in a 78-week study of toxicity and carcinogenicity)
Rat: < 50 ppm, equal to 3 mg/kg bw per day (lowest concentration
tested; tumorigenicity in a 2-year study)
80 ppm, equivalent to 4 mg/kg bw per day (maternal and fetal
toxicity in a three-generation study)
25 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Rabbit: 4 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Dog: 160 ppm, equivalent to 4 mg/kg bw per day (toxicity in a
1-year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Further studies on the mechanism of tumorigenic activity
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to propargite
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid and incomplete (50% in rats and mice)
Dermal absorption 30%, rats
Distribution Highest concentrations in intestine, liver (rats and mice)
Potential for accumulation None
Rate and extent of excretion Rapid excretion in rats and mice (urinary, approximately 50%;
faecal, 50%)
Metabolism in animals Rapid degradation to numerous polar metabolites, no parent
compound in bile or urine; hydrolysis of propynyl sulfite
side-chain and subsequent oxidation of tert-butyl moiety and
hydroxylation of cyclohexyl moiety, conjugation
Toxicologically significant compounds Parent compound; animal and plant metabolites (animals, plants,
and environment) similar
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 4000 mg/kg bw
Rat, LC50, inhalation 0.89 mg/L (4 h)
Dermal irritation Irritating to rabbit skin
Ocular irritation Irritating to rabbit eye
Dermal sensitization Not sensitizing in guinea-pigs (Buehler test)
Short-term toxicity
Target/critical effect Haematological system
Lowest relevant oral NOAEL Dog: 1 year, 160 ppm (4 mg/kg bw per day)
Lowest relevant dermal NOAEL Rabbit: 21 days, < 100 mg/kg bw per day (systemic toxicity)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Intestine (jejunal tumours in CD rats), haematological system
Lowest relevant NOAEL Rat (CD): 50 ppm (females; 3 mg/kg bw per day), 2-year study
Carcinogenicity Carcinogenic in CD rats but not in FDRL rats or
CD-1 mice
Reproductive toxicity
Reproductive target/critical effect Reduced pup weight at maternally toxic doses
Lowest relevant reproductive NOAEL Rat: 80 ppm (4 mg/kg bw per day), three-generation study
Developmental target/critical effect Rat: fetotoxicity at maternally toxic doses
Rabbit: fetotoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rabbit: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Other toxicological studies Transient cell proliferation in jejunal smooth-muscle cells
in CD rats
Medical data Dermatitis in field workers
Summary Value Study Safety factor
ADI 0-0.01mg/kg bw CD rats, 2-year study 300
Acute RfD Unnecessary
References
Abrams, K., Hogan, D.J. & Maibach, H.I. (1991) Pesticide-related
dermatoses in agricultural workers. Occupational Medicine:
State of the Art Reviews, Vol. 6, pp. 463-491.
Andre, J.C. & Laveglia, J. (1994) Pharmacokinetics of 14C-Omite: A
comparative study in rats and mice, Part II, elimination of
14C-Omite equivalents in bile following oral administration.
Unpublished report No. 93-0284 from Ricerca, Inc., Department of
Toxicology and Animal Metabolism, Painesville, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1989) Study to
determine if rats expire radiolabel in air after oral
administration of 14C-Omite. Unpublished report No.
3374-89-0211-AM-001 from Ricerca, Inc., Department of Toxicology
and Animal Metabolism, Painesville, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990a) Study of the
dermal absorption of technical 14C-Omite by male Sprague-Dawley
rats. Unpublished report No. 3452-89-0308-AM-001 from Ricerca,
Inc., Department of Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990b) Study of the
dermal absorption of 14C-Omite-6E by male Sprague-Dawley rats.
Unpublished report No. 3451-89-0307-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990c) Study of the
dermal absorption of 14C-Comite by male Sprague-Dawley rats.
Unpublished report No. 3450-89-0306-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Atkinson, J.E. (1991) A chronic (1 year) oral toxicity study in the
dog with Omite via the diet. Unpublished report No. 88-3377 from
Bio/dynamics, Inc., New Jersey, USA. Submitted by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1989a) Identification of 14C-Omite urinary
metabolite in rats. Part 2: Identification of the sixth
metabolite of Omite in female rat urine. Unpublished report No.
8706 from Uniroyal Chemical Co., Inc., Middlebury, Connecticut,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Banijamali, A.R. (1989b) Identification of 14C-Omite metabolites in
a lactating goat. Unpublished report No. 8869 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1998) Identification of metabolites of [1,2,3-13C]
propargyl alcohol in rat urine by 13C NMR and mass
spectrometry. Unpublished report No. 96038 Part I from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. (1999) Identification of metabolites of
[1,2,3-13C,2,3,-14C-propargyl]propargite in male
Sprague-Dawley rats. Unpublished report No. 98156 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Lau, R.C.M. (1996) 14C-Propargite nature of the
residue in lactating goat. Unpublished report No. 95157 (Uniroyal
Project)/ No. 95123g Southwest Bio-Labs from Uniroyal Chemical
Co. Inc., Middlebury, Connecticut, USA and South West Bio-Labs,
Inc., Las Cruces, New Mexico, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Nag, J.K. (1990) The identification of Omite
metabolites in rats; Amendment No. 4: Fecal metabolism of Omite.
Unpublished report No. 8706 from Uniroyal Chemical Co. Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co. Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. &Nag, J.K. (1991) Identification of [phenyl-U-14C]
Omite fecal metabolites in mice and comparison to rat's.
Unpublished report No. 9107 from Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Tortora, N.J. (1988a) Identification of 14C-Omite
urinary metabolite in rats. Unpublished report No. 8706 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co. Inc., Middlebury, USA.
Banijamali, A.R. & Tortora, N.J. (1988b) Comparison of subchronic vs
single oral dose in the disposition and metabolism of Omite in
rats. Unpublished report No. 87122 from Biotek, Inc., Woburn,
Massachusetts (Project No. 8719), Hazleton Laboratories America,
Inc., Madison, WIisconsin (Project No. 8721) and Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R., Kratky, E. & Gay, M.H. (1994) Pharmacokinetics of
14C-Omite: a comparative study in rats and mice - Part III -
Analysis. Unpublished report No. 9395 from Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Barfknecht, T.R. (1987) Rat hepatocyte primary culture/DNA repair
test. Unpublished report No. PH 311-UN-001-87 from Pharmakon
Research International, Inc., Pennsylvania, USA. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Becci, P.J. (1980) Supplementary pathology report for chronic
oncogenic evaluation of Omite in CD-1 mice. Unpublished report
No. 5036 from Food and Drug Research Laboratories, Inc., New
York, USA. Submitted to WHO by Uniroyal Chemical, Inc., Bethany,
Connecticut, USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (DMSO). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (acetone). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Brusick, D.J. & Weir, R.J. (1977) Mutagenicity evaluation of DO14.
Unpublished report No. 2683 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by Uniroyal Chemical,
Bethany, Connecticut, USA.
Butterworth, B.E. (1991) Chemically induced cell proliferation as a
predictive assay for potential carcinogenicity. In: Butterworth,
B.E., Slaga, T.J., Farland, W. & McClain, M., eds, Chemically
Induced Cell Proliferation: Implications for Risk Assessment, pp.
457-467.
Byrd, J.W. (1988) 14C-Omite goat metabolism study. Unpublished
report No. 8734g from Southwest Bio-Labs, Inc., Las Cruces, NM,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Carson, S. (1964) Subacute feeding studies with D-014 in rats.
Unpublished report No. 85603 from Food and Drug Research
Laboratories, Inc., New York, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Cox, G.E. & Re, T.A. (1979) Chronic oncogenic evaluation of Omite in
CD-1 mice following 78-weeks of dietary treatment. Unpublished
report No. 5036 from Food and Drug Research Laboratories, Inc.,
New York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Eldridge, S. (1994) Effect of dietary Omite technical on cell
proliferation in jejunum of rats and mice. Unpublished report No.
6030-10 (ManTech), 93-45 and 93-131 (PAI) from Pathology
Associates, Inc., Durham, North Carolina, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Eldridge, S. (1995) Effect of dietary Omite technical on cell
proliferation in jejunum of Wistar rats. Unpublished report No.
6030-017 (ManTech), 94-91 (PAI) from Pathology Associates
International, Durham, North Carolina, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gallo, M.A. & Bailey, D.E. (1976) Range finding study with Omite in
albino mice. Unpublished report No. 5028 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to WHO by
Uniroyal Chemical, Inc., Bethany, Connecticut, USA.
Gay, M.H. (1987) Rat metabolism of 14C-Omite. Final report.
Unpublished report No. 87002 from Biotek, Inc., Woburn,
Massachusetts, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Gay, M.H. (1994) Pharmacokinetics of 14C-Omite: a comparative study
in rats and mice (overview). Unpublished report No. 9395 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc.
Goldenthal, E.I. (1989) 21-day repeat dose dermal toxicity study in
rabbits. Unpublished report No. IRDC 399-098 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1998) Twenty month dietary toxicity study in rats
with Omite. Unpublished report No. 399-187 from MPI Research,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Goldenthal, E.I. (1999) Effect of one week dietary administration of
Omite technical (400 ppm) on jejunal cell proliferation in
Sprague-Dawley rats. Unpublished report No. 399-206 from MPI
Research, Inc., Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
propargite in the rat. Unpublished report No. 91-8372 from
Bio/dynamics, Inc., New Jersey, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Holsing, G.C. & Kundzins, L. (1968) 13-Week dietary feeding study-dogs
with D-014. Unpublished report No.798-140 from Hazleton
Laboratories, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Incorporated, Bethany, Connecticut, USA.
Johnson, J.D. (1990) Metabolism of 14C-Omite in rats: Dosing, sample
collection and total 14C-residue determinations. Unpublished
report Uniroyal No. 8998, Battelle No. NO967-2700 from Battelle
Columbus Division, Columbus, Ohio, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Kehoe, D.F. (1988) Subchronic toxicity and kinetic study with Omite
technical in rats. Unpublished report No. HLA 6111-107 from
Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kehoe, D.F. (1990) Two-generation reproduction study with Omite
technical in rats (two litters per generation). Unpublished
report No. HLA 6111-108 from Hazleton Laboratories America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Uniroyal Chemical
Co., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993a) Acute oral toxicity study in albino rats with
Omite Technical. Unpublished report No. WIL-155012 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993b) Acute dermal toxicity study in albino rabbits
with Omite technical. Unpublished report No. WIL-155013 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993c) Primary dermal irritation study in albino
rabbits with Omite technical. Unpublished report No. WIL-155014
from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kiplinger, G.R. (1993d) Primary eye irritation study in albino rabbits
with Omite technical. Unpublished report No. WIL-155015 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993e) Skin sensitization study in albino guinea pigs
with Omite technical. Unpublished report No. WIL-155016 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co. Inc., Bethany, Connecticut, USA.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of D-014 by its effects on cultured chinese hamster
ovary (CHO) cells using an in vitro cytogenetics assay.
Unpublished report No. URC1/CHO/AR/KF14/CH3 from Microtest
Research Lim, York, United Kingdom. Submitted to WHO by Uniroyal
Chemical Co., Bethany, Connecticut, USA.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of Omite
technical in Sprague-Dawley rats. Unpublished report No. 5992 (b)
from Food and Drug Research Laboratories, Inc., Maspeth, New
York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Knipe, J.O. (1986) The disposition and metabolism of 14C-Omite in
male and female rats. Unpublished report No. 8589 from Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Knipe, J.O. (1987) Progress report. The metabolism of 14C-Omite in
rats. Unpublished report No. 8706 from Uniroyal Chemical Co.,
Inc., Naugatuck, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Lawlor, T.E. (1991) Mutagenicity test on Omite ESO in the
Salmonella/reverse mutation assay (Ames test) preincubation
method. Unpublished report No. 12514-0-420 from Hazleton
Washington, Inc., Kensington, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Lee, S.L., Chin, Y.W. & Kim, J.S. (1981) A study on hypersensitivity
of Korean farmers to various agrochemicals. Determination of
concentration for patch-test of fruit-tree agrochemicals and
hypersensitivity of orange orchard farmers in Che-ju Do, Korea.
Seoul Med. J., 22, 137-142.
Mahon, C.R. (1993) Comparative metabolism of 14C-propargyl-Omite in
rats and mice. Unpublished report No. 91188 (Uniroyal Study
No.)/No. SC 910217 (Battelle Study No.) from Battelle Laboratory,
Columbus, Ohio, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA.
Mizens, M., Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990)
Study of the dermal absorption of 14C-Omite-30W by male
Sprague-Dawley rats. Unpublished report No. 3449-89-0305-AM-001
from Ricerca, Inc., Dept. Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
O'Malley, M.A. (1997) Skin reactions to pesticides. Occupational
Medicine: State of the Art Reviews, Vol. 12, pp. 327-345.
Oser, B.L. (1966) Chronic (2-year) feeding studies with D-014 in rats
and dogs. Unpublished report No. 86000/86014 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to
Naugatuck Chemical, Connecticut, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Putman, D.L. & Young, R.R. (1994) Micronucleus cytogenetic assay in
mice. Unpublished report No. G94AP36.122 from Microbiological
Associates, Inc., Rockville, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Sabourin, P.J., Trigg, N.J., Mahon, C.R. & Johnson, J.D. (1994)
Pharmacokinetics of 14C-Omite: A comparative study in rats and
mice - Part I - 14C-Omite equivalents in blood following oral
and intravenous administration. Unpublished report No. SC930180
from Battelle Laboratory, Columbus, Ohio, USA. Submitted to WHO
by Uniroyal Co., Inc., Middlebury, Connecticut, USA.
Schardein, J.L. (1989) Developmental toxicity study in New Zealand
white rabbits. Unpublished report No. 399-097 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
Schardein, J.L. (1990) Developmental toxicity study in rats.
Unpublished report No. 399-096 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Serota, D.G., Wolfe, G.W., Durloo, R.S. & Fezio, W.L. (1983)
Teratology study in rabbits Omite technical, revised final
report. Unpublished report No. 798-195 from Hazleton Laboratories
America, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Chemical, Bethany, Connecticut, USA.
Shirasu, Y., Moriya, M. & Watanabe, K. (1979) Microbial mutagenicity
testing on BPPS (Propargite). Unpublished report from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Uniroyal Chemical, Bethany, Connecticut, USA.
Trela, B.A. (1991) Metabolism of 14C-Omite in mice: Dosing, sample
collection, and 14C-residue determinations. Unpublished report
No. SC910015 from Battelle Columbus Division, Columbus, Ohio.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Trutter, J.A. (1991) Combined chronic toxicity and oncogenicity study
in rats with Omite technical. Unpublished report No. HLA/HWA
798-220 from Hazleton Laboratories America, Inc., Virginia, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety. York, R.G.
(1992) A cross-fostering reproduction study with Omite technical
in rats. Unpublished report No. 399-113 from International
Research and Development Corporation, Matttawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
female rats and male mice were
1-[4-(1,1-dimethyl-2-hydroxyethyl)phenoxy]-2-cyclohexanol (HOMe-TBPC)
and the corresponding diol derivative carboxy-TBPC-diol). No
consistent qualitative or quantitative species differences were found
in the metabolite profiles of propargite in bile and plasma
(Banijamali et al., 1994).
In another comparative study, the metabolites found in the faeces
of male and female CD-1 mice treated with [14C-phenyl]propargite
were characterized and compared with those identified previously in
the faeces of male and female rats (Banijamali & Nag, 1990; Johnson,
1990). The mice were given a single oral dose of 150 mg/kg bw of the
compound by gavage, whereas the rats received a single oral dose of
200 mg/kg bw (Johnson, 1990). Male mice excreted 42% of the
administered dose in the faeces and female mice about 53%, while in
rats faecal excretion accounted for 75% of the administered dose in
males and 70% in females. Peak faecal excretion of radiolabel was
observed between 0 and 24 h in both species. The profile of faecal
metabolites of mice and rats was qualitatively similar. The radiolabel
was associated with unchanged parent compound, the hydrolysis product
propargite glycol ether, and the polar metabolites hxdroxylated
tert-butyl and hydroxylated cyclohexyl propargite glycol ether. Rat
faeces contained a substantially higher percentage of unabsorbed
propargite than mouse faeces. The finding that faeces of female mice
contained more propargite (49% of total faecal residue) than faeces of
male mice (25%) suggests that faecal elimination is sex-dependent.
Moreover, male mouse faeces contained a higher percentage of polar
metabolites (56% of total faecal residue) than those of female mice
(36%). Mouse faeces contained both a greater number and a greater
percentage of polar metabolites than rat faeces, indicating more
extensive metabolism of propargite in mice than rats (Banijamali &
Nag, 1991).
Goats
One dairy goat was given [14C-phenyl]propargite at a dose of
675 mg/kg bw per day on 3 consecutive day, during which time urine,
faeces, and milk were collected. The animal was killed 8 h after the
last dose, and liver, kidney, muscle, fat, and bile samples were
collected. The highest concentration of radiolabel was found in bile,
followed by liver, kidney, fat, and muscle. The bile contained 0.29%
of the administered dose, urine 16%, faeces 14%, and milk 0.1%.
Overall, 34% of the administered dose was recovered; the remaining
radiolabel was presumed to have remained in the gastrointestinal tract
(Byrd, 1988). HPLC analysis revealed metabolism of the cyclohexyl and
tert-butyl group resulting in a number of polar metabolic products,
including carboxy-TBPC-diol, carboxy-TBPC,
1-[4-(1,dimethylethyl)-phenoxy]-2,x-cyclohexanediol, and TBPC in milk
and tissues. Small quantities of unchanged propargite were found in
milk, fat, and liver (Banijamali, 1989b).
After oral administration of [14C-phenyl]propargite in capsules
at doses of 65 or 325 mg/kg bw per day for 3 days to two lactating
goats, metabolites were identified in milk and edible tissues. The
liver contained 14 metabolites, kidney 12, muscle 9, milk 7, and fat
6. Seven of the 14 metabolites isolated from liver were glucuronide or
sulfate conjugates. The metabolism of propargite in goats thus appears
to involve hydrolysis of the propynyl sulfite side-chain followed by
aliphatic and/or alicyclic hydroxylation of the tert-butyl methyl
and cyclohexyl groups to form TBPC-diol and HOMe-TBPC, respectively.
These metabolites undergo further oxidation to yield HOMe-TBPC-diol,
carboxy-TBPC, carboxy-TBPC-diol, and carboxy-TBPC-triol. Some of these
metabolites subsequently undergo conjugation to form glucuronides and
sulfates. The results of these studies indicate similar metabolism in
rats and goats (Banijamali & Lau, 1996).
(ii) Propargyl alcohol and propargyl propargite
In order to learn more about the fate of the propynyl sulfite
side-chain of the propargite molecule, the metabolism of propargyl
alcohol, which may be released from propargite, was studied.
[1,2,3-13C-, 2,3-14C]Propargyl alcohol was administered to groups
of eight male Sprague-Dawley rats by gavage at a dose of 40 mg/kg bw,
and samples of urine, faeces, and expired air (four animals) were
collected 24, 48, 72, and 96 h after treatment. The rats were killed
at 96 h. Radiolabel associated with the parent compound represented
56% of the administered dose in urine, 12% in faeces, and 7% in
expired air. These results are consistent with those of another study
of the degradation and expiratory elimination of this compound (Mahon,
1993). Only 4-6% of the administered dose was recovered in the
carcass. Most of the radiolabel was excreted within the first 24 h of
administration in urine and expired air. The peak elimination in
faeces also occurred within 24 h of dosing and continued for 48 h. The
proposed metabolic pathway involves oxidation of propargyl alcohol to
2-propynoic acid and further detoxification by glutathione conjugation
to yield the following final products:
3,3-bis[(2-(acetylamino)-2-carboxyethyl)thio]-1-propanol,
3-(carboxymethylthio)-2-propenoic acid;
2(methylthio)-3-(methylsulfinyl)-2-propenoic acid;
3-{[2-(acetylamino)-2-carboxyethyl]thio}-3-[(2-amino-2-carboxyethyl)
thio]1-propanol; and
3-{[2-(acetylamino)-2-carboxyethyl]-sulfinyl}-3-{[2-(acetylamino)-2-
carboxyethyl]thio}-1-propanol. These metabolites have not been
reported previously and represent the first examples of multiple
glutathione additions to the carbon-carbon triple bond (Banijamali,
1998).
[1,2,3-13C-, 2,3-14C-propargyl]Propargite was administered
orally to male SpragueDawley rats at a dose of 150 mg/kg bw, and urine
and faeces were collected at 24-h intervals until study termination
96 h after dosing. The total radiolabel excreted in urine accounted
for 25% of the administered dose, and that in faeces for 48%. The six
major metabolites identified in rat urine were
3-(carboxymethylthio)-2-propenoic acid,
2-(carboxymethylthio)-2-propenoic acid,
2-(acetyl-amino)-3-(2-propynylthio)propanoic acid,
3-[(2-carboxy-2-hydroxyethyl)thio]-2-propenoic acid,
3- (N-formylglutamylcysteinyl)-2-propenoic acid, and
2- (N-formylglutamylcysteinyl)-2-propenoic acid. These metabolites
were the result of conjugation with glutathione followed by enzymatic
degradation. Seven metabolites were identified tentatively in faeces
(Banijamali, 1999).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity conducted since the
earlier evaluation (Table 1) are consistent with the previous data.
The clinical observations made most frequently after oral
administration included urogenital staining and abnormal defaecation,
hypoactivity, and swollen, red paws. Most of the treated animals lost
weight during a few days after dosing. Those that died had dark-red
areas, thickened mucosa, and red foci in the stomach. All of the
deaths occurred during the second week, with no sex difference
(Kiplinger, 1993a).
After inhalation, the commonest observations were laboured
breathing and various secretory responses. About one-third of the
treated animals lost weight during the first week after exposure, and
treatment-related reddening of the lungs was seen in some animals
found dead or killed at term. There was no sex difference in
lethality. All deaths occurred 1-17 days after exposure (Hoffman,
1992).
After dermal exposure, none of the animals died and all gained
weight during the 24-day observation period. All rabbits showed severe
erythema and oedema, and eschar formation, fissuring, desquamation,
and a white-yellow exudate on the application site appeared during the
second week of the study and persisted until day 14. Thickened skin
and desquamation within the application site were seen on all rabbits
at necropsy (Kiplinger, 1993b).
Male and female New Zealand white rabbits received dermal
applications of 0.5 ml of undiluted technical-grade propargite
(purity, 90.3%) and were observed at 0.5, 24, 48, and 72 h, daily
through day 14, and on day 21, when they were killed. Irritation was
seen during the first few days after application, consisting of
moderate erythema and slight-to-moderate oedema, but on days 5-9
severe erythema and oedema, fissuring, eschar formation, and
desquamation were observed. The severe irritation had decreased to
slight oedema and erythema by day 21, indicating reversibility of the
reaction (Kiplinger, 1993c).
Table 1. Acute toxicity of technical-grade propargite (purity, 90.3%)
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or mg/L)
Rat Crl:CD BR M Gastric intubation 2600 Kiplinger (1993a)
F 2900
Both 2800
Rat Crl:CD BR M Inhalation (4 h) 0.95 Hoffman (1992)
F 0.95
Both 0.89
Rabbit New Zealand white M Dermal (24 h) > 4000 Kiplinger (1993b)
F > 4000
Both > 4000
Male and female New Zealand white rabbits received 0.1 ml of
undiluted technical-grade propargite (purity, 90.3%) into the
conjunctival sac and were observed at 1, 24, 48, and 72 h and on days
4, 7, 10, 14, 17, and 21 after application. Reactions were observed in
the conjunctiva, cornea, and iris. The effects in cornea and iris had
subsided by day 10 or earlier, and the corneal effects had cleared by
day 21 (Kiplinger, 1993d).
In a modified Buehler test, male and female Hartley albino
guinea-pigs received three topical applications of 0.1%
technical-grade propargite (purity, 90.3%) in ethanol for induction, a
challenge dose of 0.2% propargite in acetone) two weeks later, and a
second challenge (0.1 and 0.2% in acetone) after a 1-week
interruption. The applications were left in place for 6 h. A positive
control (dinitrochlorobenzene) and an untreated control group were
included. No evidence of sensitization was seen (Kiplinger, 1993e).
(b) Short-term studies of toxicity
Rats
Propargite was administered in the diet to groups of five rats of
each sex at concentrations of 0 (15 animals), 200, 400, 800, 2000, or
4000 ppm for 90 days. Reduced food consumption and body-weight gain
were observed at the two higher concentrations. Haematological and
clinical chemical parameters (glucose and urea nitrogen) were not
affected by treatment. The relative weights of the liver, kidney,
adrenals, and gonads were increased at the two higher concentrations.
No treatment-related macroscopic or microscopic alterations were
observed (Carson, 1964).
Groups of 10 male and 10 female Crl:CD (SD) BR rats were
maintained on diets containing technical-grade propargite (purity,
87.2%) at concentrations of 0, 100, 1000, or 2000 ppm (equivalent to
0, 5, 50, and 100 mg/kg bw per day) for at least 13 weeks. At the end
of the study, all surviving rats were anaesthetized, weighed, bled,
killed, and necropsied. All tissues from control rats and those at the
highest dose, the lungs, liver, and kidneys from rats at 100 or
1000 ppm, and all macroscopic lesions from all rats were examined
microscopically. No deaths occurred during the study. All animals at
2000 ppm had rough coats throughout the study, and many were thin with
a hunched posture, alopecia, and rhinorrhoea. The body weights of rats
at 1000 and 2000 ppm were significantly lower than those of controls
throughout the study, by 30% at 1000 ppm and 69% in males and 52% in
females at 2000 ppm. The body-weight gains of males were significantly
reduced during week 10 at 100 ppm, during most of the study at
1000 ppm, and throughout the study at 2000 ppm. The significantly
reduced body-weight gain at 100 ppm during week 10 did not result in a
significant reduction in the cumulative body-weight gain at study
termination or in any other adverse effect and was considered not to
be toxicologically relevant. The body-weight gains of females were
significantly lower during weeks 1 and 5 at 1000 ppm and during weeks
1-4 at 2000 ppm. The reduction in the body-weight gain of males at the
end of the study in comparison with controls was 4% at 100 ppm, 30% at
1000 ppm, and 69% at 2000 ppm, and the corresponding reductions in
females were 6%, 31%, and 52%, respectively. The reductions at 1000
and 2000 ppm were significant. The reduced body-weight gain was
associated with a dose-dependent reduction in food consumption at the
two higher doses.
Various haematological and clinical chemical parameters were
altered at the two higher doses, including significantly increased
erthrocyte count and haemoglobin values in males at 2000 ppm and
significantly decreased mean corpuscular volume and mean corpuscular
haemoglobin in males at 1000 and 2000 ppm and females at 2000 ppm. A
non-dose-related but significant increase in platelet count in females
at 1000 ppm and an increased erythrocyte count in males at 100 ppm
were considered to be of no toxicological significance. The blood
glucose concentration was significantly lower in males and females at
1000 and 2000 ppm, and urea nitrogen was significantly higher and
creatinine significantly lower in animals of each sex at 2000 ppm.
Reduced total protein, albumin, and globulin values were found in
males at 2000 ppm and in females at 1000 ppm and 2000 ppm. An
increased albumin:globulin ratio was found in males and females at
2000 ppm. The concentrations of calcium, inorganic phosphorus,
potassium, and chloride showed significant alterations at 2000 ppm.
Most of the changes observed were considered to be associated with the
decreased food consumption and body weight. The absolute weights of
the kidneys and liver were significantly reduced in animals of each
sex at 2000 ppm, and the absolute weight of the testis was reduced in
males at this dose. The relative weights of the kidney, liver, and
testis were increased in a dose-related manner at 1000 and 2000 ppm.
Macroscopic and microscopic examinations did not reveal alterations
attributable to treatment. The NOAEL was 100 ppm, equivalent to 5
mg/kg bw per day, on the basis of effects on body weight and changes
in clinical chemical parameters at higher doses (Kehoe, 1988).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of technical-grade propargite (purity, 85%) at
doses of 0, 0.1, 1.0, 10, or 100 mg/kg bw per day, 5 days per week for
3 weeks. No deaths occurred during the study, and no clinical signs
were observed at any dose. Food consumption was slightly reduced at
all doses, and the mean body weightswere slightly reduced in treated
males and slightly increased in treated females, with no clear
dose-response relationship, indicating no consistent treatment-related
effect. Biochemical parameters were not affected by treatment, whereas
haematological investigations revealed a significant increase in the
number of segmented neutrophils at the end of the study in males at
100 mg/kg bw. Signs of dermal irritation were seen at the application
site in all treated groups, with a dose-related increase in incidence
and severity. The signs consisted of erythema, oedema, eschar
formation, exfoliation, atonia, desquamation, fissuring, blanching,
and coriaceousness. The dermal effects occurred earlier with
increasing dose. The observed changes were graded as mild-to-severe in
animals at 10 and 100 mg/kg bw per day, mild-to-moderate at 1 mg/kg bw
per day, and mild at 0.1 mg/kg bw per day. No changes in organ weights
were observed, and no additional macroscopic changes were seen.
Dose-related microscopic changes were confined to the application
site, which were in the incidence and severity of acanthosis and
hyperkeratosis. The incidence of dermal inflammation was similar in
all treated groups, and necrosis was observed at doses > 1 mg/kg bw
per day. The NOEL for systemic toxicity was 100 mg/kg bw per day, the
highest dose tested, if the increased number of segmented neutrophils
in males at this dose is considered to be a borderline effect. No NOEL
could be identified for local irritation (Goldenthal, 1989).
Dogs
Groups of three beagle dogs of each sex were given diets
containing propargite (purity not specified) at concentrations of 0,
2000 ppm (weeks 1-3), and 2500 ppm (weeks 4-13), equal to 55 mg/kg bw
per day for males and 67 mg/kg bw per day for females (mean values of
2000 and 2500 ppm). Appearance, behaviour, and signs of toxicity were
recorded daily and body weights and food consumption weekly. Clinical
chemistry was evaluated once initially and 1 and 3 months after the
start of the study. Gross necropsy was performed on all dogs killed
at13 weeks, and the weights of the thyroid, heart, liver, spleen,
kidneys, adrenals, and testis were recorded; all organs were examined
microscopically. Most of the treated animals had a reduced appetite,
particularly during the first half of the study, and weight loss was
seen. Two males showed reduction in various haematological parameters,
including haematocrit and erythrocyte count after 3 months of
treatment, and all treated males had slightly increased aspartate
aminotransferase activity. Changes in organ weights showed no clear
dose-related pattern, and the increased relative liver weight was
probably a consequence of the reduced body weights. No macroscopic
changes were observed. Microscopic findings considered to be related
to treatment were increased pigmentation in the reticuloendothelial
cells in the liver and increased haemosiderin deposits in the spleen,
which may also be related to the decreased food consumption (Holsing &
Kundzins, 1968).
Groups of six beagle dogs of each sex recieved diets containing
technical-grade propargite (purity, 88.6%) at concentrations of 0,
160, 1250, or 2500 ppm, equivalent to 4, 30, and 48 mg/kg bw, for 1
year. The high concentration was reduced to 1875 ppm at week 9 after
observation of excessive body-weight loss. Treatment did not induce
ocular abnormalities or any treatment-related changes in clinical
chemical or urinary parameters. Two animals at the high dose died with
marked body-weight loss, and the remaining animals at this dose were
thin throughout most of the study and appeared to be dehydrated during
the last few months. Pronounced body-weight loss occurred during the
first 8 weeks at the high dose, by 2.6 kg in males and 1.9 kg in
females, and at the intermediate dose, by 0.4 kg in males and 0.5 kg
in females. Once the dose had been reduced to 1875 ppm, the
body-weight loss was less pronounced; however, the weight gain of
animals at 1250 ppm was markedly lower than that of controls. The food
consumption of animals at the high dose was reduced throughout the
study, perhaps indicating unpalatability, although it tended to
increase from week 9. Effects on haematological parameters included
reduced haemoglobin, haematocrit, and erythrocyte values in males and
females at the high dose and reduced haematocrit in males at the
intermediate dose at 3 and 6 months and at termination. In females,
these changes were less pronounced and were observed only after
3 months of treatment. Platelet counts were elevated in females at
1250 and 1875 ppm at all intervals and in males at the high dose after
6 and 12 months. Decreased absolute weights and increased relative
weights of many organs were observed in animals at the high dose and
occasionally in those at the intermediate dose. These changes are
considered to be due to the reduced body weight at this dose level.
The macroscopic changes consisted of an increased incidence of
red-tan-white foci in the lungs of males at the high dose and a higher
incidence of involution of the thymus at doses > 1250 ppm. Changes
in the bone marrow consisted of a greater incidence and severity of
erythroid-myeloid depletion and atrophy in animals at the high dose.
Histologically, the changes in the lung consisted of congestion and
inflammatory changes and fibrous and alveolar/bronchiolar epithelial
hyperplasia. In the stomach, dilated mucosal glands and vacuoles in
parietal cells were seen, but these changes showed no clear
dose-response relationship and their relation to treatment is
questionable. The NOAEL was 160 ppm, equivalent to 4 mg/kg bw per day,
on the basis of body-weight loss, reduced food consumption, and
histopathological alterations in the thymus and bone marrow at higher
concentrations (Atkinson, 1991).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a 30-day range-finding study, groups of 10 mice of each sex
received diets containing propargite at concentrations of 0, 600, 900,
1350, 2000, or 3000 ppm. Gross observations, body weight, food
consumption, gross necroscopy, and organ weights were recorded.
Body-weight loss and changes in organ weights were the predominant
effects. Minimal effects were considered to have occurred at 1000 ppm
(Gallo & Bailey, 1976), which was selected as the highest dose for the
study described below.
Groups of 15 CD-1 mice of each sex received diets containing
propargite (purity, 88.5% during the first 12 months and 84.3% during
the remaining 6 months) in corn oil at concentrations of 0, 500, or
1000 ppm, equivalent to 0, 75, and 150 mg/kg bw per day, for 52 weeks;
and groups of 60 mice of each sex received diets containing the
compound at concentrations of 0, 50, 160, 500, or 1000 ppm for
78 weeks, equivalent to 0, 7.5, 24, 75, and 150 mg/kg bw per day. The
animals were observed daily for changes in general appearance,
behaviour, appetite, toxic effects, and deaths. Body weights were
determined weekly for the first 28 weeks and every two weeks
thereafter. Food consumption was recorded weekly. Before the start of
treatment and at 52 and 78 weeks, leukocyte and erythrocyte counts
were determined in 10 animals of each sex per group, and before
termination differential leukocyte counts were made in 10 animals of
each sex per group. Gross necropsy was carried out on all animals
found dead or killed when moribund and on all animals killed at the
scheduled time. Essentially all organs, including the head, tongue,
ear, and nose, were saved, and the fresh weights of the liver, spleen,
kidneys, heart, adrenals, thyroid, and gonads were recorded.
Furthermore, sections of the spinal cord, an additional lobe of the
liver, the gall-bladder, an additional section of the uterus to
include the cervix, the nasal cavity, and the middle ear were taken
for histopathological examination.
Males at doses > 160 ppm had a lower mortality rate than
controls at 52 weeks, the survival rates being > 85%; after 78
weeks, the survival rates were 38% at 50 ppm and 60% at 500 ppm. The
variations seen in body-weight gain were not dose-related. Males
treated for 78 weeks showed a non-dose-related increase in body-weight
gain when compared with controls, whereas the females showed a
non-dose-related decrease. A similar, inconsistent pattern was found
in animals treated for 52 weeks, and these variations are considered
to be unrelated to treatment and of no toxicological significance.
Treatment did not affect food consumption or haematological
parameters, and no unexpected gross alterations were seen. An increase
in the relative weight of the kidney males treated for 52 weeks can
probably to be attributed to the reduced body-weight gain of these
animals. Females showed increased absolute and relative weights of the
adrenals at both 500 and 1000 ppm and a non-dose-related increase in
the absolute and relative weights of the thyroid at 500 ppm. The
relationship of the effects on the thyroid to treatment is
questionable. Animals treated for 78 weeks had reduced absolute and
relative weights of the kidney and uterus at concentrations > 160
ppm, and a non-dose-related increase in uterine weight was observed at
500 and 1000 ppm in females treated for 78 weeks and at 160, 500, and
1000 ppm in females treated for 52 weeks. Statistical significance was
attained only in females at 1000 ppm killed at 78 weeks.
Histopathological examination showed no treatment-related alterations
in tissues or organs, and no correlation was found between the
occurrence or type of neoplasms and treatment. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of changes in the
weights of the kidney and uterus at higher doses (Cox & Re, 1979;
Becci, 1980).
Rats
Propargite was administered to groups of 25 male and 25 female
FDRL rats in the diet at concentrations of 0 (37 animals), 100, 300,
or 900 ppm, equivalent to 0, 5, 15, and 45 mg/kg bw per day for 2
years. After the study had been in progress for 26 weeks, an
additional treatment group was included at a concentration of 2000 ppm
(equivalent to 100 mg/kg bw per day) as well as an additional control
group, because the lower doses had no effect; these groups were
treated for 78 weeks. The appearance, behaviour, and survival of the
animals was recorded daily, and body weight and food consumption were
recorded weekly. The efficiency of food use was calculated for the
first 3 months. Haematological and clinical chemical parameters were
evaluated in all animals at 12, 26, 52, 78, and 104 weeks. All rats
that died or were killed when moribund or at the end of the study were
examined macroscopically. Sections of the main organs from half of the
animals at 900 and 2000 ppm and their corresponding controls and of
the liver, kidneys, bone marrow, and thymus of the remaining rats were
examined histologically.
Treatment did not affect the appearance or behaviour of the
animals. The survival rate of males at 2000 ppm was lower than that in
the other treated groups and the control group. During the first 12
weeks of treatment, no difference in body weight or food intake was
observed between treated and the control groups, but later, the
body-weight gain of males at 900 ppm and of all treated females was
lower than that of controls, with no dose-response relationship.
Whereas the changes observed at 900 ppm were slight, a marked
reduction in body-weight gain was seen at 2000 ppm, and food intake
was also reduced at this dose. Haemoglobin, haematocrit, and leukocyte
values showed no treatment-related change at concentrations up to 2000
ppm. The absolute weights of the livers of rats at 900 ppm and of
females at 300 ppm were increased, and the relative weights were also
increased, attaining statistical significance in males at 900 ppm and
in females at concentrations > 300 ppm. In animals at 2000 ppm, the
absolute weight of the liver was decreased and the relative weight
increased, the changes being statistically significant. The relative
weight of the kidney was increased in males at 900 ppm after 104 weeks
of treatment, whereas in animals treated at 2000 ppm for 78 weeks, the
absolute weight of the kidneys was decreased and the relative weight
was increased in animals of each sex. Gross observation at autopsy
showed no treatment-related changes. The total incidences of sarcomas
and carcinomas were 6/85 in controls, 6/44 at 100 ppm, 8/42 at 300
ppm, 9/39 at 900 ppm, and 4/26 at 2000 ppm. The NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day, on the basis of changes in organ
weights (Oser, 1966). The low survival rate of animals at the highest
dose limited the relevance of the findings, and this study was
considered by the 1977 Meeting to be inadequate for evaluating the
carcinogenicity of propargite.
On the basis of the results of a 90-day study which indicated a
maximum tolerated dose of 1000 ppm (Kehoe, 1988), 800 ppm was selected
as the highest dose in a 2-year study in Crl:CDBR rats. Groups of 60
rats of each sex were given diets containing propargite (purity,
87.2%) at concentrations of 0, 50, 80, 400, or 800 ppm, equal to 2, 4,
19, and 39 mg/kg bw per day for males and 3, 5, 24, and 49 mg/kg bw
per day for females. Owing to low survival rates in males at the high
dose (30% after 103 weeks), this group was killed at 103 weeks. An
interim sacrifice was made at 53 weeks. The test diets were found to
be of adequate homogeneity and stability.
The mortality rate of males at 400 and 800 ppm was increased
towards the end of the study, with a significant positive trend,
although the group differences were not large enough to reach
statistical significance. The body weights of males at 800 ppm were
significantly reduced at the end of the study. Females showed reduced
body weight at various times during the study but no significant
overall reduction. Body-weight gain was reduced in a dose-related
manner, by 5% at 80 ppm, 12% at 400 ppm, and 18% at 800 ppm in males
at the end of the study, with statistical significance at 400 and 800
ppm. In females, body-weight gain was reduced by 15% at the high dose,
but with no statistical significance. At interim sacrifice, the
body-weight gain was reduced by 6% at 400 ppm and 20% at 800 ppm in
males and by 4% at 400 ppm and 30% at 800 ppm in females. Food
consumption was reduced at concentrations > 400 ppm in animals of
each sex. No compound-related differences in clinical signs were seen
between control and treated groups, and no treatment-related
ophthalmoscopic changes were found. The changes in haematological
parameters consisted of an increased reticulocyte count, mostly due to
decreases in three animals, and a reduced erythrocyte count in males
at 800 ppm at termination, indicating the presence of hypoxia and/or
accelerated erythropoiesis. The effects on clinical chemistry
consisted of decreased total serum protein values in males at 400 and
800 ppm and in females at 800 ppm at 26 weeks and a corresponding
decrease in total serum calcium. After 26 weeks, decreased aspartate-
and alanine aminotransferase activities were seen, with no clear
dose-response relationship. A dose-related decrease in aspartate
aminotransferase activity was observed in females at 400 and 800 ppm
after 52 weeks of treatment, probably as a result of the impaired
nutritional status of animals at these doses.
Necropsy of animals that died during the first 64 weeks of the
study showed no gross, compound-related changes in tissues. From week
65, several males at the high dose were found to have abdominal
masses, mostly in the small intestine and principally in the jejunum.
At the end of the study, males at 400 and 800 ppm and females at 800
ppm showed high frequencies of this treatment-related effect, the
incidences of masses in the jejunum being 0% in controls, 0% at
50 ppm, 0% at 80 ppm, 17% at 400 ppm, and 25% at 800 ppm in males and
0% in controls, 2% at 50 ppm, 0% at 80 ppm, 2% at 400 ppm, and 15% at
800 ppm in females. No other gross tissue alterations attributable to
treatment were found. No compound- or dose-related changes were found
in the absolute weights of the organs of treated animals, but
alterations were found in the relative weights of various organs: At
week 53, the relative weights of the livers of treated females showed
a dose-related increase over that of controls, which attained
statistical significance only in animals at 50, 400, and 800 ppm.
These changes in relative liver weights were not accompanied by
biochemical or histopathological alterations, suggesting that they
were due to the reductions in body-weight gain in females at 400 and
800 ppm and that the significant increase at 50 ppm (not supported by
a similar change at 80 ppm) is an incidental finding. Increased
relative liver weights were also observed in males at the high dose at
interim sacrifice, without reaching statistical significance, again
probably reflecting the reduced body-weight gain of these animals. At
termination, slightly increased relative liver weights were observed
in animals of each sex, but with no statistical significance. At the
interim sacrifice, the relative weights of the kidneys were also found
to be increased in animals at the high dose and in females at 80 ppm.
Histological examination revealed no treatment-related
non-neoplastic alterations and no compound-related neoplastic findings
through week 52, but during the second year of the study high
frequencies of undifferentiated sarcomas of the jejunum in males at
400 and 800 ppm and in females at 800 ppm were recorded (Table 2).
Single cases were also found in other dose groups. A clear
dose-related increase in the incidence of sarcoma was found in males
at 400 and 800 ppm and in females at 800 ppm. The incidences in all
treated female rats were also increased when compared with controls,
but with no dose-response relationship. The incidence of
undifferentiated sarcomas in duodenum, subcutaneous tissue, jejunum,
and the thoracic cavity in rats of this strain in previous studies was
reported to be 0.2% in males and 0% in females, but no
undifferentiated sarcomas were found in the jejunum. Electron
microscopic examination of abdominal tumour masses from three males at
400 ppm and one at 800 ppm revealed one malignant schwannoma, one
undifferentiated sarcoma, and one fibrosarcoma, suggesting that
propargite induced proliferation of mesenchymal tumours in various
stages of differentiation. The tumour incidences correlated with the
observations of abdominal masses. The NOAEL for systemic toxicity and
carcinogenicity was 80 ppm, equal to 4 mg/kg bw per day, on the basis
of effects on body weight, food consumption, and clinical chemical
parameters and an increased incidence of jejunal sarcomas at higher
doses (Trutter, 1991).
Dogs
Groups of six beagle dogs of each sex were maintained on a diet
containing propargite at concentrations of 0, 100, 300, or 900 ppm for
1 h per day, 6 days a week for 2 years. The dogs were observed daily
for appearance, behaviour, signs of toxicity, and neurological
reflexes. Body weight and food intake were recorded weekly for the
first 12 weeks and every 2 weeks thereafter. Haematological, clinical
chemical and urinary parameters were determined at 26, 52, 78, and
106 weeks. One animal of each sex per group was killed after 1 year
and examined grossly. A survivors were killed after 2 years and
examined. Treatment did not adversely affect the appearance,
behaviour, body weight, haematological or clinical chemical or
microscopic appearance. No indication of carcinogenicity was found
(Oser, 1966).
(d) Genotoxicity
The results of tests for the genotoxicity of propargite are
summarized in Table 3.
Table 2. Incidences of undifferentiated sarcoma of the jejunum in rats fed propargite in the diet for 2 years
Sex Dose Tumour incidence
(ppm)
Unscheduled deaths Interim sacrifice Terminal sacrifice Total
No. of No. % No. of No. % No. of No. % No. of No. %
rats rats rats rats
Male 0 26 0 0 9 0 0 24 0 0 59 0 0
50 16 0 0 0 31 0 0 47 0 0
80 23 0 0 0 23 0 0 46 0 0
400 32 9 28 0 17 2 12 49 11 22
800 35 20 57 10 0 0 15 4 27 60 24 40
Female 0 27 0 0 10 0 0 20 0 0 57 0 0
50 29 0 0 0 20 1 5 49 1 2
80 20 1 5 0 29 0 0 49 1 2
400 28 0 0 0 20 1 5 48 1 2
800 25 8 32 10 0 0 21 4 19 56 12 21
Table 3. Results of tests for the genotoxicity of propargite
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.001-5 µl/plate NR Negative ± S9 Brusick & Weir
TA98, TA100, in DMSO (1977)
TA1535, TA1537,
TA1538;
S. cerevisiae D4
Reverse mutation S. typhimurium 10-5000 µg/plate 90.9 Negative ± S9 Shirasu et al. (1979)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr
Reverse mutation S. typhimurium 10-300 µl/plate 90a Positive ± S9 Lawlor (1991)
TA98, TA100, at > 10 µl/ml
TA1535, TA1537, in TA100
TA1538
Gene mutation B. subtilis H17, M45 1-100% v/v in DMSO 90.9 Negative Shirasu et al. (1979)
Gene mutation Chinese hamster 1-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 10-75 µg/ml +S9 (1993)
locus in DMSO
Gene mutation Chinese hamster 0.2-4.2 µg/ml 90 Negative Bigger & Clarke
ovary cells, Hprt -S9; 10-50 µg/ml (1993)
locus +S9 in DMSO
Gene mutation Chinese hamster 0.5-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 5-5 µg/ml +S9 (1993)
locus in acetone
Table 3. (continued)
End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Chinese hamster 25-200 µg/ml NR Negative ± S9 Kirkland (1985)
aberrations ovary cells
DNA repair Rat hepatocytes 0.0167-0.5 µg/ml 97 Negative Barfknecht (1987)
in acetone
In vivo
Micronucleus ICR mouse 37.5, 75, 150 mg/kg 89.6 Negative Putman & Young
formation bw i.p in corn oil (1994)
NR, not reported; i.p., intraperitoneally
a Technical-grade containing epoxidized soya bean oil as stabilizer
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of rats were maintained on diets containing propargite at
concentrations of 0 or 100 ppm. When the rats were about 100 days of
age and sexually mature, 20 pairs of males and females were followed
through two reproductive cycles. The first litters were discarded. At
weaning of the second litter, 10 rats of each sex in each group were
selected for the F1 generation. A similar procedure was chosen for
selection of the F2 generation. The dose of the F2 pups was
increased to 300 ppm, and the F3 pups received 300 ppm throughout
treatment. The pups were raised to maturity and the cycle repeated in
this and the succeeding generation. Treatment did not have adverse
effects on the dams, the reproduction indices, or their pups. The NOEL
was 100 ppm, the only dose tested, equivalent to 5 mg/kg bw per day
(Oser, 1966).
Groups of 25 immature Crl:CDBR albino rats of each sex were fed
diets that contained technical-grade propargite (purity, 87.2%) at
concentrations of 0, 80, 400, or 800 ppm, equivalent to 0, 4, 20, and
40 mg/kg bw per day, for 10 weeks before mating and throughout mating,
gestation, lactation, and weaning of the F1a pups. After weaning,
these pups were killed and discarded, and the F0 animals were mated
again to produce F1b litters. After weaning, the pups were assigned
at random to four groups of 25 animals of each sex, and the F0
adults were killed and necropsied. The selected F1b animals were fed
the diets for at least 10 weeks before mating and throughout
gestation, lactation, and weaning of F2a pups. After these pups were
weaned, they were killed and discarded, and the F1b animals were
mated again to produce F2b litters. After these pups had been
weaned, they were killed and discarded, and the F1b adults were
killed and necropsied. The body weights of males were recorded on the
first day of treatment, weekly thereafter, and on the day of necropsy,
whereas females were weighed on the first day of treatment, weekly
before mating, on days 0, 7, 14, and 20 of gestation and lactation,
and on the day of necropsy. Food consumption was recorded weekly only
before mating of the F0 and F1b generations and not during the
gestation and lactation periods. All F0 and F1b adults were
examined macroscopically, and the reproductive organs of those at 0
and 800 ppm were examined microscopically.
No treatment-related clinical signs were noted in these animals,
and their survival was not adversely affected. The treatment resulted
in dose-related reductions in body weight and body-weight gain of both
sexes of both generations at 400 and 800 ppm during various phases of
the study. Body weight was reduced by 5-10% in males at 400 ppm and by
18-28% in both generations at 800 ppm before and after mating.
Body-weight gain showed a corresponding pattern, with reductions of up
to 20% in males at 400 ppm and up to 60% at 800 ppm. Similar
reductions were found in females: at 400 ppm, the maximum reduction
during premating, gestation, and lactation was 10%, and at 800 ppm the
reduction was up to 22%. The body-weight gains of females were reduced
by up to 30% at 400 ppm and up to 90% at 800 ppm before mating. Marked
differences in the body-weight gain of females were seen in the
premating, gestation, and lactation periods. In all generations,
lactating dams treated with 400 and 800 ppm had greater body-weight
gain than controls, perhaps because some controls lost weight during
the second half of the lactation period. Males of the F0 generation
at 400 ppm showed a dose-related reduction in mean food consumption
during weeks 0-1, 4-5, and 8-9 before mating, and the food consumption
of males at 800 ppm was consistently lower before mating. The mean
food consumption of F0 females at 400 ppm was lower only during week
6-7, but that of females at 800 ppm was reduced throughout the
premating period. The mean food consumption of males and females of
the F1b generation showed a dose-related reduction at 400 and 800
ppm. Mating and fertility parameters and gestation indexes were not
affected by treatment, and no treatment-related differences in litter
size or sex ratio were seen. Pup weight per litter was reduced in all
generations at 400 ppm on lactation days 7, 14, and 21 and for litters
at 800 ppm on lactation days 0, 4, 7, 14 ,and 21. The maximum
reduction in the weight of pups at 800 ppm group on lactation day 21
was 44%. There were no treatment-related macroscopic or microscopic
changes. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per day, on
the basis of effects on the body weights of dams and pups at higher
doses (Kehoe, 1990).
In a study designed to clarify the reduced pup weights during
lactation at concentrations of 400 and 800 ppm, a cross-fostering
study was initiated in which groups of Crl: CD VAF/Plus rats were
given diets containing technical-grade propargite (purity, 89.9%) at
concentrations of 0 (100 animals of each sex), 400 ppm (30 of each
sex), or 800 ppm (60 of each sex). The F0 parents were given the
diets for 70 days before mating, and F0 males were killed one week
after mating. The F0 females that had delivered were killed on
lactation day 21, and those that did not deliver within 25 days after
mating were also necropsied. On lactation day 21, the F1 offspring
were necropsied, with special attention to the reproductive organs. On
lactation day 0, the litters were cross-fostered to dams in other
groups and those of the control group to other dams within the group.
Selected dams in the control group and at 800 ppm were allowed to keep
their own litters. The new groups were thus: 15 untreated dams with
their own untreated litters (controls); 20 untreated dams
cross-fostering untreated litters; 20 untreated dams cross-fostering
litters treated at 400 ppm; 20 untreated dams cross-fostering litters
treated at 800 ppm; 20 dams treated at 400 ppm cross-fostering
untreated litters; 20 dams at 800 ppm cross-fostering untreated
litters; and 20 dams at 800 ppm with their own litters treated at 800
ppm. All animals were observed for deaths and signs of toxicity twice
daily. Body weights were recorded weekly for males and for females
until evidence of copulation was seen and on days 0, 7, 14, and 20 of
gestation and days 0, 7, 14, and 21 of lactation. The food consumption
of adult rats was measured weekly except during mating in weeks 11-13
and, for females, during gestation and lactation. The litters were
reduced randomly to four pups of each sex on lactation day 4 to
provide homogeneous groups for evaluation of nursing, survival, and
pup growth. The culled pups were examined externally. The litters were
caged with their dams for 3 weeks after birth. Throughout lactation,
the dams and their pups were observed daily for survival and
behavioural abnormalities in nesting and nursing. Pups were weighed on
days 0, 4, 7, 14, and 21 of lactation, the day of termination.
Treatment had no effect on the appearance or behaviour of the
F0 animals. A treatment-related reduction in body weight relative to
the controls was seen in males and females at 800 ppm during
premating, by 16% in males and 9% in females at the end of premating.
During gestation, the mean maternal body weight and body-weight gain
were reduced at the 800 ppm; during lactation, the mean body weights
of dams at 800 ppm cross-fostering untreated litters and and of dams
at 800 ppm with their own litters remained lower than those of
controls. At the beginning of lactation, reduced body weights were
observed in all cross-fostering groups relative to the controls, with
reductions of 5% in untreated dams cross-fostering untreated litters,
4% in untreated dams cross-fostering litters treated at 400 ppm, 2% in
untreated dams cross-fostering litters at 800 ppm, 3% in dams treated
at 400 ppm cross-fostering untreated litters, 7% in dams at 800 ppm
cross-fostering untreated litters, and 12% in dams at 800 ppm with
their own litters treated at 800 ppm. At the end of the lactation
period, the reductions were 3%, 6%, 2%, 0%, 7%, and 10% in these
groups, respectively. Body-weight gain during lactation showed marked
variation among the different groups which was unrelated to dose, the
changes over the 21-day period corresponding to 31%, -17%, 10%, 52%,
14%, and 28% in the six groups, respectively. Food consumption was
also reduced, by up to 20% at 800 ppm in males and females before
mating. During gestation, the food consumption of dams at 800 ppm was
reduced to about 11%. During lactation, the food consumption in the
different groups was 103%, 101%, 106%, 98%, 83%, and 80% relative to
the controls, respectively.
No treatment-related differences in litter size, viability on day
0, or the survival index of the F1 offspring was observed. From
birth (lactation day 0), the mean body weights of male and female pups
born to dams treated at 800 ppm were statistically significantly
reduced when compared with the control group, whereas the weights of
female pups born to dams given 400 ppm were significantly increased,
an effect that was considered biologically irrelevant. On lactation
day 0, the body weights of untreated pups nursed by their untreated
dams and of pups at 400 ppm fostered by untreated dams were unchanged.
The weights of pups at 800 ppm fostered by untreated dams were
nonsignificantly reduced on days 0 and 4; those of untreated pups
fostered by dams at 400 ppm were nonsignificantly reduced on days 0
and 4 but significantly reduced on days 14 and 21 of lactation; the
weights of untreated pups fostered by dams at 800 ppm and those of
pups treated at 800 ppm and fostered by dams at the same dose were
nonsignificantly reduced on day 0 but significantly reduced on days 7,
14, and 21. There were no treatment-related differences in the
incidences of malformations, developmental variations, or macroscopic
and microscopic appearance in the F1 offspring. The results of this
study indicate that the effects on pup weight are the result of
toxicity in the dams (York, 1992).
(ii) Developmental toxicity
Rats
Groups of a minimum of 20 females Sprague-Dawley rats were given
technical-grade propargite as a suspension in corn oil by gavage at
doses of 0, 6, 25, or 105 mg/kg bw per day on days 6-15 of gestation.
At least 30 females were used as vehicle controls and for a positive
control group receiving an aqueous suspension of aspirin at a dose of
250 mg/kg bw per day. The original doses were set at 0, 25, 105, and
450 mg/kg bw per day, but dams at 450 mg/kg bw per day died after only
3 or 4 days of treatment and therefore this group was terminated. The
females at 105 mg/kg bw per day showed signs of toxicity, including
bloody nasal and vaginal discharges and urinary incontinence; this
dose was continued as the high dose, 25 mg/kg bw per day as the
intermediate dose, and an additional dose of 6 mg/kg bw per day as the
low dose. No clinical signs were observed at 6 or 25 mg/kg bw per day.
The body weight and body-weight gain of dams at 105 mg/kg bw per day
were lower than those of the other groups, but the difference did not
attain statistical significance. The body weight and body-weight gain
of the positive controls were significantly reduced at the end of the
treatment period. The reproductive performance of the treated dams, as
measured by pregnancy rate and the numbers of implantations,
resorptions, and live and dead fetuses, was similar at all doses. When
the fetuses of treated dams were evaluated for skeletal anomalies,
those at 25 and 105 mg/kg bw per day showed statistically significant
increases in the incidence of missing sternebrae, with 3% of fetuses
and 24% of litters at 25 mg/kg bw per day and 16% of fetuses and 41%
of litters at 105 mg/kg bw per day affected. The control incidence was
1% of fetuses and 6% of litters. The incidences of incomplete
ossification of vertebrae were also increased, with 28%/88%
(fetuses/litters) at 6 mg/kg bw per day, 23%/71% at 25 mg/kg bw per
day, and 30%/86% at 105 mg/kg bw per day; the control incidence was
13%/58%. Statistically significant increases were also observed in the
incidence of incomplete closure of the skull at 105 mg/kg bw per day
and in the incidence of reduced or missing hyoid, with 2%/10% at 25
mg/kg bw per day, and 5%/14% at 105 mg/kg bw per day, and 0.4%/3% in
the control group and 0.5%/4% at 6 mg/kg bw per day. The incidence of
haemorrhagic abdomen was increased at 25 and 105 mg/kg bw per day,
with no clear dose-response relationship. Most of these parameters
were also adversely affected in the dams in the positive control
group. In these animals, the incidence of resorption sites was
increased and the number of live fetuses and fetal weight were
significantly reduced, and the body weights of the dams were reduced.
The fetuses of the positive controls showed various skeletal and
soft-tissue abnormalities at significantly higher incidences than in
the untreated controls. The NOAEL for maternal toxicity was 25 mg/kg
bw per day, but no NOAEL could be identified for developmental
toxicity (Knickerbocker & Re, 1979).
Groups of 45 female Crl:CD rats were given technical-grade
propargite (purity, 85%) by gavage at doses of 0, 6, 12, 18, 25, or
105 mg/kg bw per day on days 6-15 of gestation. On day 20, the pups of
20 gravid females were removed surgically and examined. The remaining
animals were allowed to deliver, and they and their pups were observed
until day 21 of lactation, when they were necropsied. There were no
treatment-related effects on behaviour or survival. Animals at the
highest dose had anogenital and body surface staining, and the body
weights were significantly reduced by 5% when compared with controls.
The adjusted body weight (body weight on day 20 of gestation minus the
weight of uterus and contents) of animals at this dose showed a
similar reduction (7%) on days 6-9 of gestation. The body-weight gain
during days 6-15 of gestation was similar at the four lower doses but
was reduced at the highest dose. The body weights during lactation did
not differ with dose, and those of animals at the highest dose were
similar to those of controls. No sign of developmental toxicity was
seen, as measured by pre- and postimplantation loss, the numbers of
implantations and corpora lutea, pup survival index, or fetal body
weight. Slight reductions in the numbers of live offspring at day 0 of
lactation were observed, with no clear dose-response relationship; the
greatest reduction, 96.4%, was found at the highest dose. The indexes
of pup survival and mortality calculated on a fetal basis showed no
treatment-related reduction, but pup mortality analysed on a litter
basis showed a statistically significant increase in the number of
litters with deaths per total litters on day 7 at the highest dose. No
treatment-related difference in the incidence of malformations or of
developmental variations was seen. The NOAEL was 25 mg/kg bw per day
on the basis of effects on the body weight of dams and slight effects
on postnatal mortality at the higher dose (Schardein, 1990).
Rabbits
Groups of 17 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by intubation at
doses of 0, 2, 6, 10, or 18 mg/kg bw per day on days 6-18 of
gestation. Maternal toxicity, manifested by higher mortality rates at
6, 10, and 18 mg/kg bw per day, attained statistical significance at
18 mg/kg bw per day. Adipsia and anorexia were seen in all groups but
at a markedly higher frequency at the three higher doses than in
controls. Maternal body weight and body-weight gain showed
dose-related reductions at doses > 6 mg/kg bw per day, resulting in
reduced overall weight gain up to day 29 at 6 and 10 mg/kg bw per day
and weight loss at 18 mg/kg bw per day. The finding of brown areas in
the gastric mucosa in most animals at the highest dose at necropsy was
considered to be related to treatment. Pregnancy rates were not
adversely affected, but gravid uterine weights showed a dose-related
reduction at all doses. As the litter size also showed a dose-related
reduction, the reduction in uterine weight might reflect the smaller
litter sizes. Fewer implantations were found in all treated groups,
with the fewest at 18 mg/kg bw per day; the mean number of resorptions
was slightly increased at 10 and 18 mg/kg bw per day, and the mean
incidence of resorptions calculated on a per litter basis was markedly
increased at these doses. Treated groups had fewer live fetuses per
litter, and the fetal viability index calculated on a per litter basis
was decreased at 10 and 18 mg/kg bw per day. The mean fetal body
weights were reduced at the highest dose.
Increased incidences of visceral and skeletal malformations were
seen at doses > 6 mg/kg bw per day. One fetus at 10 mg/kg bw per
day had an enlarged, domed head (hydrocephaly), resulting in an
incidence of 1.6%, and two fetuses from the same litter at 18 mg/kg bw
per day also had hydrocephaly, resulting in an incidence of 9%; the
incidence in controls was 0% and that in historical controls was
0.11%. One fetus at the highest dose had an incomplete diaphragm
(incidence, 5%; 0% in controls, 0.04% in historical controls) and
other visceral alterations such as small lungs and kidneys. The
treatment-related skeletal variants included delayed ossification of
the skull and bone alignment of sternebrae. Skull closure that was
only 75% complete was seen more frequently at doses > 6 mg/kg bw
per day, resulting in incidences of 12%, 13%, and 10%, respectively,
with an incidence of 4% in the concurrent control group and in animals
at 2 mg/kg bw per day. The absence of a doserelated increase at the
highest dose might be a consequence of the small number of fetuses
available for examination (21 compared with 62-115 in the other
groups). The historical incidence for 75% skull closure was 5.7%.
Malaligned or fused sternebrae were found at incidences of 0% in
controls and 2%, 3%, 8%, and 0% at the four doses, respectively, with
a historical control incidence of 1.8%. The NOAEL for maternal and
fetal toxicity was 2 mg/kg bw per day (Serota et al., 1983).
Groups of 25 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by gavage at
doses of 0, 2, 4, 6, 8, or 10 mg/kg bw per day on days 7-19 of
gestation. The fetuses were removed surgically on day 29 and examined
for teratological changes. Dams at 8 and 10 mg/kg bw per day showed
signs of toxicity during the treatment period, including reduced
body-weight gain, and body-weight loss was observed during the second
half of the treatment period at 8 mg/kg bw per day and throughout
treatment at 10 mg/kg bw per day; however, the body weights at the end
of gestation were not significantly reduced. Three females at 4 mg/kg
bw per day, one at 8 mg/kg bw per day, and four at 10 mg/kg bw per day
aborted between days 18 and 25 of gestation. The toxicological
significance of the abortions at 4 and 8 mg/kg bw per day is
questionable because of the lack of a dose-response relationship, but
the abortions at 10 mg/kg bw per day are considered to be related to
treatment as they were accompanied by signs of systemic toxicity in
the dams. Fetal viability, fetal body weights, the mean numbers of
pre- and postimplantation losses, and the total numbers of
implantations and corpora lutea were comparable in all groups,
including controls. Higher incidences (on fetal and litter bases) of
fused sternebrae were found at 8 and 10 mg/kg bw per day, with
incidences of 0/0 % (fetuses/litters), 2/7%, 0.8/6%, 0/0%, 2/11%, and
8/38% at the 0, 2, 4, 6, 8, and 10 mg/kg bw per day, respectively.
This malformation is reported to occur spontaneously at an incidence
of up to about 5%. The NOAEL for maternal and developmental toxicity
was 6 mg/kg bw per day (Schardein, 1989).
(f) Special studies: Cell proliferation
In the 2-year study in CD rats of Trutter (1991), described
above, treatment with propargite resulted in a statistically
significant increase in the incidence of undifferentiated sarcomas in
the jejunum in animals of each sex given dietary concentrations
> 400 ppm. In order to investigate the underlying mechanism and to
establish a NOAEL for the relevant parameter, comparative studies were
conducted in CD rats and CD-1 mice over 1 or 4 weeks. Male rats were
given technical-grade propargite in the diet at 0 or 800 ppm or 0 or
80 ppm, female rats were given diets containing 0, 40, or 800 ppm, and
male mice received diets containing 0 or 1000 ppm. The groups
consisted of 12 controls and 22 treated animals. Body weight, food
consumption, and clinical observations were recorded weekly during the
study. Osmotic pumps containing 5-bromo-2'-deoxyuridine (BrdU), which
is incorporated into the DNA of replicating cells and used to detect
proliferating cells by immunohistochemistry, were placed
subcutaneously in half of the animals on day 1 or 20 of the study, and
the animals were killed and necropsied 1 week after implantation. All
animals were processed for immunohistochemistry and histopathology
(haematoxylin and eosin staining). Sections of the jejunum were
collected to determine cell proliferation in three layers of smooth
muscle. Cells that incorporated BrdU were identified by the presence
of chromagen over their nuclei, and cell proliferation was expressed
as unit length labelling index (number of labelled cells per square
millimetre). Positive staining in the epithelium of the duodenum was
considered to indicate systemic delivery of BrdU.
The body weights of rats receiving 800 ppm were reduced,
especially in males, and food consumption was lower. The body weights
and food consumption of the mice were not affected. After 1 week,
increased total smooth muscle cell proliferation was observed at 800
ppm in male and female rats, and the response was statistically and
biologically significant, the latter being defined as a twofold or
greater increase in cell proliferation in treated animals when
compared with controls. No cell proliferation was induced at lower
doses in rats, and no cell proliferation occurred in male mice at 1000
ppm. No biologically significant increase in cell proliferation was
found after 4 weeks of treatment, although male rats at 800 ppm showed
a statistically significant increase. The NOEL for cell proliferation
was 40 ppm, equal to 2 mg/kg bw per day (Eldridge, 1994).
A similar study was conducted to investigate a possible strain
specificity of the cell proliferative response. Groups of 11 Wistar
(WKY) rats of each sex received technical-grade propargite (purity,
88.6%) in the diet at a concentration of 900 ppm for 1 week, while six
animals of each sex received the diet alone. On the first day of the
study, osmotic pumps containing BrdU were placed subcutaneously, and
the rats were killed and necropsied 1 week later. All animals were
processed for immunohistochemistry and histopathology as described
above. Body weight, food consumption, and clinical observations were
recorded at the end of treatment. Body-weight gain and food
consumption were decreased in rats of each sex throughout the study.
No biologically significant increase in cell proliferation was
observed, and a statistically significant increase was found only in
the outer layer of the smooth muscle of the jejunum in female rats.
This isolated finding was not considered to be biologically
significant, and the combined results for all three layers did not
attain statistical significance. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in any animal. These
results are consistent with the lack of tumorigenic response in male
and female Wistar rats treated with propargite at 900 ppm for 2 years
(Eldridge, 1995; Oser, 1966).
Groups of 10 Charles River CD rats of each sex received
technical-grade propargite in the diet at a concentration of 0 or 400
ppm for 1 week. On the first day, all rats received osmotic pumps
containing BrdU. The animals were observed for clinical signs, body
weight, and food consumption. On day 7, the animals were killed, and
the jejunum and the duodenum were examined macroscopically and
microscopically. No clinical signs and no effect on body-weight gain
or food consumption were seen. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in the jejunum.
Statistically and biologically significant increases in cell
proliferation were found in jejunal smooth muscle in males and females
(Goldenthal, 1999).
Groups of CD rats were given diets containing propargite (purity,
93%) at doses up to 800 ppm, according to the following design, with
evaluations of cell proliferation and histopathological changes after
4, 8, 12, 16, and 20 months. Groups of 90 males were given diets
containing concentrations of 0 or 800 ppm, equal to 0 and 42 mg/kg bw
per day; 6 weeks later, groups of five males were given concentrations
of 0, 80, or 400 ppm, equal to 0, 4, and 21 mg/kg bw per day, and
groups of 55 females were given concentrations of 0, 40, 400, or 800
ppm, equal to 0, 6, 28, and 55 mg/kg bw per day. Each rat was observed
twice daily for death and signs of toxicity. Clinical examinations
were conducted once weekly, and body weights and food consumption were
recorded weekly for the first 16 weeks and every 4 weeks thereafter.
Ten males at 0 and 800 ppm were killed after 4, 8, 12, 16, and 20
months, and the first 10 rats in the remaining groups were killed
after 4, 12, and 20 months. One week before scheduled necropsy,
osmotic pumps containing BrdU were implanted subcutaneously into the
backs of the animals. Standard immunohistochemical methods were used
to stain tissues for BrdU, and staining was evaluated by nuclear
labelling. Three smooth muscle layers were evaluated. Jejunal tissues
stained with haematoxylin and eosin, serial to those stained for BrdU,
were examined for histopathological changes. Ten animals in each group
were killed 6 or 7 days after implantation of the pumps. Sections of
jejunum, ileum, duodenum, and stomach were collected at the 4,- 12-,
and 20-month interim sacrifices, while only sections of jejunum and
duodenum were collected at the 8- and 16-month sacrifices. The
remaining tissues from each animal and the carcass were discarded
without further necropsy, and no further microscopic evaluation was
conducted.
The survival of control and treated animals was similar, and no
treatment-related clinical signs were observed. Body weights were
decreased by 17% in males and 13% in females at 800 ppm, and a
statistically significant decrease in body weight was observed in
males at 400 ppm, which did not result in a significant overall
reduction in body weight at the end of the study; no effect on body
weights was observed in females at this dose. The average food
consumption was reduced in animals at 800 ppm and sporadically in
those at 400 ppm. No treatment-related macroscopic changes were found
in animals killed at 4, 8, 12, or 16 months, but treatment-related
jejunal masses or nodules were observed at 20 months in males at 400
and 800 ppm and in females at 800 ppm. No statistically significant
increase in cell proliferation was detected in the smooth muscle of
the jejunum of rats treated for up to 20 months, but at that time an
at least twofold increase in cell proliferation was seen in male rats
given 800 ppm. At 4, 8, and 12 months, less cell proliferation was
seen in males at 800 ppm than in controls. These findings are
consistent with the reduced total number of cells at the same times.
At 16 months, the difference in the total number of cells between
controls and males at 800 ppm began to decrease, resulting in similar
cell proliferation in the two groups, and by 20 months both cell
proliferation and the total number of cells were greater in the rats
at the high dose than in controls. This effect appears to be a
compensatory response to apparent suppression of cell turnover in the
smooth muscle cells of the jejunum in these animals, which was first
apparent at 16 months. By 20 months, the smooth muscle cells had
overcompensated for the inhibition in cell turnover, resulting in
increased cell proliferation and total cell number. Although the
increase in cell proliferation was not statistically significant, a
twofold or greater increase in the unit length labelling index is
considered to reflect a cell proliferative response. Histopathological
evaluation revealed no hyperplasia, cytotoxicity, or inflammation in
the jejunum. The NOAEL for systemic toxicity was 80 ppm, equal to 4
mg/kg bw per day, on the basis of slight effects on body-weight gain
and jejunal masses at 400 ppm. The NOAEL for cell proliferation was
400 ppm, equal to 21 mg/kg bw per day (Goldenthal, 1998).
The results of these studies suggest that sustained cell
proliferation is not an etiological factor in tumour formation at the
jejunum. Furthermore, no lesions indicative of toxicity were
identified as precursors of tumour formation. The results suggest a
mitogenic mode of action rather than a cytotoxic action, and this
conclusion is supported by the species- and strain-dependent
differences in the carcinogenicity of propargite and the corresponding
species- and strain-dependent differences in jejunal cell
proliferative response.
3. Observations in humans
Propargite is an irritant in humans and may also be a sensitizer,
as several outbreaks of dermatitis were observed in field workers
exposed to this compound (Lee et al., 1981). An outbreak of poisoning
and dermatitis was seen among workers exposed to a formulation of
propargite in Japan in 1970: after use of the miticide on orange
trees, 40 out of 47 workers developed dermatitis and 43 showed signs
of noncutaneous effects, including irritation of the respiratory
tract. Dermatitis was also observed in mixers, loaders, and field
workers in California, USA. Outbreaks of dermatitis in subsequent
years have been reviewed (O'Malley, 1997). The environmental half-time
of propargite of 5-11 days may allow prolonged residual action which
might be responsible for the outbreaks of dermatitis in field workers
(Abrams et al., 1991).
Comments
The results of various studies of the pharmacokinetics of single
oral doses of propargite in CD rats showed that gastrointestinal
absorption was inversely related to the administered dose. After
administration of a single oral dose of [14C-phenyl]ring-labelled
propargite to rats, 20-50% of the administered dose was excreted in
the urine and 40-75% in faeces; about 1.5% of the administered dose
was found in tissues, with slightly higher urinary excretion and
correspondingly less faecal elimination in males. Roughly similar
results were found in CD mice treated in the same way.
In rats, dermal absorption of propargite occurred mainly within
the first 4 h after application. The absorption amounted to up to 33%
of the administered dose of technical material and 3-17% of the
administered doses of various formulations.
The metabolism of propargite has been elucidated in a series of
studies in rats and mice. The compound was rapidly degraded to polar
metabolites, metabolism of the cyclohexyl ring predominating. Most of
the radiolabel in rat faeces was associated with unchanged parent
compound and with a few metabolites, which were also found in urine.
In comparative studies in rats and mice, no parent compound was found
in the bile of either species, whereas the plasma contained small
amounts of unchanged propargite; six metabolites were found in rat and
mouse bile. The metabolites are formed as a result of hydrolysis of
the propynyl sulfite side-chain, subsequent oxidation and conjugation
of the tert-butyl moiety, and hydroxylation of the cyclohexyl
moiety. No consistent quantitative or qualitative species differences
in bile and plasma metabolite profiles were found.
In further studies of the metabolism of propargite with a
radiolabel located in the propynyl sulfite side-chain and with
radiolabelled propargyl alcohol, an additional metabolic pathway was
identified which involves metabolism of the side-chain by glutathione
conjugation.
Propargite is of low acute toxicity, with an oral LD50 in rats
of 2800 mg/kg bw, but it irritates the skin and eyes. Propargite did
not show dermal sensitizing potential in the Buehler test in
guinea-pigs. WHO (1999) has classified propargite as 'slightly
hazardous'.
In short-term studies in mice, rats, and dogs, the signs of
systemic toxicity included effects on body weight and on
haematological and clinical chemical parameters. In a study in which
CD rats were fed propargite for three months, the NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day. In dogs, dietary administration of
propargite for 1 year resulted in a NOAEL of 160 ppm, equivalent to 4
mg/kg bw per day, on the basis of effects on body weight and on
various haematological parameters and histopathological changes in the
thymus and bone marrow. In a 21-day study in rabbits treated dermally,
the NOAEL for systemic toxicity was < 100 mg/kg bw per day, whereas
no NOAEL for local irritation was found.
In long-term studies, the most significant toxicological finding
was the occurrence of jejunal sarcomas in CD (Crl:CDBR) rats, whereas
no carcinogenic effect was observed in CD-1 mice or Wistar (FDRL)
rats. In a 78-week study in CD-1 mice carried out in 1979, the NOAEL
was 50 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
changes in organ weights. In the study in Wistar (FDRL) rats carried
out in 1966, the NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day,
also on the basis of changes in organ weights.
In a two-year study reported in 1991 in which CD (Crl:CDBR) rats
were given diets containing propargite at concentrations of 0, 50, 80,
400, or 800 ppm (equal to 0, 2, 4, 19, and 39 mg/kg bw per day in
males and 0, 3, 5, 24, and 49 mg/kg bw per day in females), males at
400 and 800 ppm showed a dose-related increase in the incidence of
undifferentiated sarcomas in the jejunum, a very rare tumour. Females
also showed a clear tumorigenic response but with a different
dose-response relationship, since a high incidence (21%) of jejunal
tumours in the smooth muscle was observed at the highest concentration
of 800 ppm but low incidences (one animal in each group) at 50, 80,
and 400 ppm. Given the rarity of sarcomas arising at this site (0% in
concurrent and historical female controls, 0% in concurrent male
controls, and 0.2% in historical male controls), a NOAEL for
tumorigenicity could not be identified in this study. In a 20-month
study reported in 1998 in which CD rats were given dietary
concentrations of 0, 80, 400, or 800 ppm (equal to 0, 4, 21, and 42
mg/kg bw per day) for males and 0, 40, 400, or 800 ppm (equal to 0, 6,
28, and 55 mg/kg bw per day) for females, the appearance of jejunal
masses at 400 and 800 ppm in males and at 400 ppm in females suggested
that the tumorigenic response is a reproducible effect. Reduced cell
proliferation in jejunal smooth muscle layers and decreased jejunal
cell division were found at various times during the study. After 20
months, cell division and cell proliferation were found to be
increased only in males at 800 ppm, with no corresponding response in
females or in males at lower doses. No hyperplasia, cytotoxicity, or
inflammation was found in the jejunal epithelium.
Several short-term (1 or 4 weeks) studies of cell proliferation
were conducted in male and female CD rats at concentrations of up to
800 ppm, in Wistar rats (WKY strain) at 900 ppm, and in male CD-1 mice
at 1000 ppm. The NOAEL for cell proliferation in the CD rat was 40 ppm
(equal to 2 mg/kg bw per day) in females and 80 ppm (equal to 4 mg/kg
bw per day) in males, both being the lowest doses tested. A
proliferative response was observed at 800 ppm in both male and female
rats after 1 week of treatment, whereas the response was observed only
in males after 4 weeks of treatment at this concentration; no cell
proliferation was observed at 40 and 80 ppm. No cell proliferation was
found in Wistar rats (WKY) treated with 900 ppm or in CD-1 mice at
1000 ppm in the same study design.
The results of the long-term studies in CD rats and the
short-term studies of cell proliferation indicate that the cell
proliferation induced by propargite in the jejunum is characterized by
an initial transient proliferative response, lasting for at least 4
weeks in males and for only about 1 week in females. This profile of
cell proliferation suggests that the underlying mechanism for tumour
formation in the jejunum of CD rats may be related to the mitogenic
activity of the compound.
The observed dose-response relationship for tumour formation,
with a greater increase in tumour incidence in males than in females,
correlates with the duration and degree of jejunal cell proliferation.
The prolonged duration of cell proliferation in male rats was
associated with a more pronounced tumorigenic response than in
females. Furthermore, propargite did not induce cell proliferation in
species and strains in which no carcinogenic activity was observed.
The available database does not further illuminate the causal
relationship between cell proliferation and tumorigenicity, and no
explanation was offered of the species, strain, and sex specificity or
of the association between the presence of an early, transient cell
proliferation response and the occurrence of jejunal tumours in CD
rats and the consistent absence of these findings in Wistar rats and
CD-1 mice. Nevertheless, the association between proliferation and
tumorigenic activity was recognized by the Meeting. The Meeting also
noted that the NOAEL for cell proliferation of 40 ppm is very close to
the lowest tumorigenic concentration in female rats of 50 ppm. The
available database did not, however, clarify the dose-response
relationship found at low tumorigenic doses in females and offered no
further scientific evidence to support use of cell proliferation as a
marker of potential carcinogenicity.
An adequate range of studies for genotoxicity was conducted, and
the results were consistently negative. Therefore the Meeting
concluded that propargite is not genotoxic. The finding of
consistently negative results in numerous tests for genotoxicity
provides further support for the conclusion that propargite causes
tumours by a non-genotoxic mechanism.
In a three-generation study of reproductive toxicity, reduced
body-weight gain was seen at concentrations of 400 ppm (equivalent to
20 mg/kg bw per day) and above in parental animals and in pups during
lactation. The results of a subsequent cross-fostering study showed
that the growth retardation of the pups was reversible and was due to
maternal toxicity. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per
day, on the basis of reduced body-weight gain in parental animals and
pups.
Two studies of developmental toxicity were conducted in rats and
two in rabbits. In a study in Sprague-Dawley rats reported in 1979,
fetal effects such as increased incidences of incomplete vertebral
ossification and missing sternebrae and hyoids were observed in
treated animals. Not all of the findings were dose-related and they
were not reproduced in a study reported in 1990. The NOAEL was 25
mg/kg bw per day on the basis of reduced body-weight gain in dams and
a slight increase in the postnatal mortality rate at the highest dose
in the latter study. In the two studies in rabbits, dated 1983 and
1989, fetal effects indicative of developmental retardation were
observed at maternally toxic doses of 6 mg/kg bw per day and above,
and an increased incidence of hydrocephaly was seen at higher doses in
the first study. Most of the fetal effects, including hydrocephaly,
were not confirmed in the second study, in which effects were observed
only at doses of 8 mg/kg bw per day and higher. The overall NOAEL in
rabbits was 4 mg/kg bw per day. No evidence for teratogenicity was
found in these studies.
The Meeting allocated an ADI of 0-0.01 mg/kg bw on the basis of
the LOAEL of 50 ppm (equal to 3 mg/kg bw per day) for tumorigenicity
in female CD rats, with a safety factor of 300. This safety factor was
chosen to account for the lack of a NOAEL in this study and the nature
of the end-point (tumorigenesis) and to encompass the NOAEL in the
same rat strain for increased cell proliferation in the jejunum, the
site of tumour formation.
The Meeting concluded that it was unnecessary to determine an
acute reference dose because of the low acute toxicity of propargite.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (effects on organ
weights in a 78-week study of toxicity and carcinogenicity)
Rat: < 50 ppm, equal to 3 mg/kg bw per day (lowest concentration
tested; tumorigenicity in a 2-year study)
80 ppm, equivalent to 4 mg/kg bw per day (maternal and fetal
toxicity in a three-generation study)
25 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Rabbit: 4 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Dog: 160 ppm, equivalent to 4 mg/kg bw per day (toxicity in a
1-year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Further studies on the mechanism of tumorigenic activity
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to propargite
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid and incomplete (50% in rats and mice)
Dermal absorption 30%, rats
Distribution Highest concentrations in intestine, liver (rats and mice)
Potential for accumulation None
Rate and extent of excretion Rapid excretion in rats and mice (urinary, approximately 50%;
faecal, 50%)
Metabolism in animals Rapid degradation to numerous polar metabolites, no parent
compound in bile or urine; hydrolysis of propynyl sulfite
side-chain and subsequent oxidation of tert-butyl moiety and
hydroxylation of cyclohexyl moiety, conjugation
Toxicologically significant compounds Parent compound; animal and plant metabolites (animals, plants,
and environment) similar
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 4000 mg/kg bw
Rat, LC50, inhalation 0.89 mg/L (4 h)
Dermal irritation Irritating to rabbit skin
Ocular irritation Irritating to rabbit eye
Dermal sensitization Not sensitizing in guinea-pigs (Buehler test)
Short-term toxicity
Target/critical effect Haematological system
Lowest relevant oral NOAEL Dog: 1 year, 160 ppm (4 mg/kg bw per day)
Lowest relevant dermal NOAEL Rabbit: 21 days, < 100 mg/kg bw per day (systemic toxicity)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Intestine (jejunal tumours in CD rats), haematological system
Lowest relevant NOAEL Rat (CD): 50 ppm (females; 3 mg/kg bw per day), 2-year study
Carcinogenicity Carcinogenic in CD rats but not in FDRL rats or
CD-1 mice
Reproductive toxicity
Reproductive target/critical effect Reduced pup weight at maternally toxic doses
Lowest relevant reproductive NOAEL Rat: 80 ppm (4 mg/kg bw per day), three-generation study
Developmental target/critical effect Rat: fetotoxicity at maternally toxic doses
Rabbit: fetotoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rabbit: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Other toxicological studies Transient cell proliferation in jejunal smooth-muscle cells
in CD rats
Medical data Dermatitis in field workers
Summary Value Study Safety factor
ADI 0-0.01mg/kg bw CD rats, 2-year study 300
Acute RfD Unnecessary
References
Abrams, K., Hogan, D.J. & Maibach, H.I. (1991) Pesticide-related
dermatoses in agricultural workers. Occupational Medicine:
State of the Art Reviews, Vol. 6, pp. 463-491.
Andre, J.C. & Laveglia, J. (1994) Pharmacokinetics of 14C-Omite: A
comparative study in rats and mice, Part II, elimination of
14C-Omite equivalents in bile following oral administration.
Unpublished report No. 93-0284 from Ricerca, Inc., Department of
Toxicology and Animal Metabolism, Painesville, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1989) Study to
determine if rats expire radiolabel in air after oral
administration of 14C-Omite. Unpublished report No.
3374-89-0211-AM-001 from Ricerca, Inc., Department of Toxicology
and Animal Metabolism, Painesville, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990a) Study of the
dermal absorption of technical 14C-Omite by male Sprague-Dawley
rats. Unpublished report No. 3452-89-0308-AM-001 from Ricerca,
Inc., Department of Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990b) Study of the
dermal absorption of 14C-Omite-6E by male Sprague-Dawley rats.
Unpublished report No. 3451-89-0307-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990c) Study of the
dermal absorption of 14C-Comite by male Sprague-Dawley rats.
Unpublished report No. 3450-89-0306-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Atkinson, J.E. (1991) A chronic (1 year) oral toxicity study in the
dog with Omite via the diet. Unpublished report No. 88-3377 from
Bio/dynamics, Inc., New Jersey, USA. Submitted by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1989a) Identification of 14C-Omite urinary
metabolite in rats. Part 2: Identification of the sixth
metabolite of Omite in female rat urine. Unpublished report No.
8706 from Uniroyal Chemical Co., Inc., Middlebury, Connecticut,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Banijamali, A.R. (1989b) Identification of 14C-Omite metabolites in
a lactating goat. Unpublished report No. 8869 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1998) Identification of metabolites of [1,2,3-13C]
propargyl alcohol in rat urine by 13C NMR and mass
spectrometry. Unpublished report No. 96038 Part I from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. (1999) Identification of metabolites of
[1,2,3-13C,2,3,-14C-propargyl]propargite in male
Sprague-Dawley rats. Unpublished report No. 98156 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Lau, R.C.M. (1996) 14C-Propargite nature of the
residue in lactating goat. Unpublished report No. 95157 (Uniroyal
Project)/ No. 95123g Southwest Bio-Labs from Uniroyal Chemical
Co. Inc., Middlebury, Connecticut, USA and South West Bio-Labs,
Inc., Las Cruces, New Mexico, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Nag, J.K. (1990) The identification of Omite
metabolites in rats; Amendment No. 4: Fecal metabolism of Omite.
Unpublished report No. 8706 from Uniroyal Chemical Co. Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co. Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. &Nag, J.K. (1991) Identification of [phenyl-U-14C]
Omite fecal metabolites in mice and comparison to rat's.
Unpublished report No. 9107 from Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Tortora, N.J. (1988a) Identification of 14C-Omite
urinary metabolite in rats. Unpublished report No. 8706 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co. Inc., Middlebury, USA.
Banijamali, A.R. & Tortora, N.J. (1988b) Comparison of subchronic vs
single oral dose in the disposition and metabolism of Omite in
rats. Unpublished report No. 87122 from Biotek, Inc., Woburn,
Massachusetts (Project No. 8719), Hazleton Laboratories America,
Inc., Madison, WIisconsin (Project No. 8721) and Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R., Kratky, E. & Gay, M.H. (1994) Pharmacokinetics of
14C-Omite: a comparative study in rats and mice - Part III -
Analysis. Unpublished report No. 9395 from Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Barfknecht, T.R. (1987) Rat hepatocyte primary culture/DNA repair
test. Unpublished report No. PH 311-UN-001-87 from Pharmakon
Research International, Inc., Pennsylvania, USA. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Becci, P.J. (1980) Supplementary pathology report for chronic
oncogenic evaluation of Omite in CD-1 mice. Unpublished report
No. 5036 from Food and Drug Research Laboratories, Inc., New
York, USA. Submitted to WHO by Uniroyal Chemical, Inc., Bethany,
Connecticut, USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (DMSO). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (acetone). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Brusick, D.J. & Weir, R.J. (1977) Mutagenicity evaluation of DO14.
Unpublished report No. 2683 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by Uniroyal Chemical,
Bethany, Connecticut, USA.
Butterworth, B.E. (1991) Chemically induced cell proliferation as a
predictive assay for potential carcinogenicity. In: Butterworth,
B.E., Slaga, T.J., Farland, W. & McClain, M., eds, Chemically
Induced Cell Proliferation: Implications for Risk Assessment, pp.
457-467.
Byrd, J.W. (1988) 14C-Omite goat metabolism study. Unpublished
report No. 8734g from Southwest Bio-Labs, Inc., Las Cruces, NM,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Carson, S. (1964) Subacute feeding studies with D-014 in rats.
Unpublished report No. 85603 from Food and Drug Research
Laboratories, Inc., New York, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Cox, G.E. & Re, T.A. (1979) Chronic oncogenic evaluation of Omite in
CD-1 mice following 78-weeks of dietary treatment. Unpublished
report No. 5036 from Food and Drug Research Laboratories, Inc.,
New York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Eldridge, S. (1994) Effect of dietary Omite technical on cell
proliferation in jejunum of rats and mice. Unpublished report No.
6030-10 (ManTech), 93-45 and 93-131 (PAI) from Pathology
Associates, Inc., Durham, North Carolina, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Eldridge, S. (1995) Effect of dietary Omite technical on cell
proliferation in jejunum of Wistar rats. Unpublished report No.
6030-017 (ManTech), 94-91 (PAI) from Pathology Associates
International, Durham, North Carolina, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gallo, M.A. & Bailey, D.E. (1976) Range finding study with Omite in
albino mice. Unpublished report No. 5028 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to WHO by
Uniroyal Chemical, Inc., Bethany, Connecticut, USA.
Gay, M.H. (1987) Rat metabolism of 14C-Omite. Final report.
Unpublished report No. 87002 from Biotek, Inc., Woburn,
Massachusetts, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Gay, M.H. (1994) Pharmacokinetics of 14C-Omite: a comparative study
in rats and mice (overview). Unpublished report No. 9395 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc.
Goldenthal, E.I. (1989) 21-day repeat dose dermal toxicity study in
rabbits. Unpublished report No. IRDC 399-098 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1998) Twenty month dietary toxicity study in rats
with Omite. Unpublished report No. 399-187 from MPI Research,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Goldenthal, E.I. (1999) Effect of one week dietary administration of
Omite technical (400 ppm) on jejunal cell proliferation in
Sprague-Dawley rats. Unpublished report No. 399-206 from MPI
Research, Inc., Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
propargite in the rat. Unpublished report No. 91-8372 from
Bio/dynamics, Inc., New Jersey, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Holsing, G.C. & Kundzins, L. (1968) 13-Week dietary feeding study-dogs
with D-014. Unpublished report No.798-140 from Hazleton
Laboratories, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Incorporated, Bethany, Connecticut, USA.
Johnson, J.D. (1990) Metabolism of 14C-Omite in rats: Dosing, sample
collection and total 14C-residue determinations. Unpublished
report Uniroyal No. 8998, Battelle No. NO967-2700 from Battelle
Columbus Division, Columbus, Ohio, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Kehoe, D.F. (1988) Subchronic toxicity and kinetic study with Omite
technical in rats. Unpublished report No. HLA 6111-107 from
Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kehoe, D.F. (1990) Two-generation reproduction study with Omite
technical in rats (two litters per generation). Unpublished
report No. HLA 6111-108 from Hazleton Laboratories America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Uniroyal Chemical
Co., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993a) Acute oral toxicity study in albino rats with
Omite Technical. Unpublished report No. WIL-155012 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993b) Acute dermal toxicity study in albino rabbits
with Omite technical. Unpublished report No. WIL-155013 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993c) Primary dermal irritation study in albino
rabbits with Omite technical. Unpublished report No. WIL-155014
from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kiplinger, G.R. (1993d) Primary eye irritation study in albino rabbits
with Omite technical. Unpublished report No. WIL-155015 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993e) Skin sensitization study in albino guinea pigs
with Omite technical. Unpublished report No. WIL-155016 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co. Inc., Bethany, Connecticut, USA.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of D-014 by its effects on cultured chinese hamster
ovary (CHO) cells using an in vitro cytogenetics assay.
Unpublished report No. URC1/CHO/AR/KF14/CH3 from Microtest
Research Lim, York, United Kingdom. Submitted to WHO by Uniroyal
Chemical Co., Bethany, Connecticut, USA.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of Omite
technical in Sprague-Dawley rats. Unpublished report No. 5992 (b)
from Food and Drug Research Laboratories, Inc., Maspeth, New
York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Knipe, J.O. (1986) The disposition and metabolism of 14C-Omite in
male and female rats. Unpublished report No. 8589 from Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Knipe, J.O. (1987) Progress report. The metabolism of 14C-Omite in
rats. Unpublished report No. 8706 from Uniroyal Chemical Co.,
Inc., Naugatuck, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Lawlor, T.E. (1991) Mutagenicity test on Omite ESO in the
Salmonella/reverse mutation assay (Ames test) preincubation
method. Unpublished report No. 12514-0-420 from Hazleton
Washington, Inc., Kensington, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Lee, S.L., Chin, Y.W. & Kim, J.S. (1981) A study on hypersensitivity
of Korean farmers to various agrochemicals. Determination of
concentration for patch-test of fruit-tree agrochemicals and
hypersensitivity of orange orchard farmers in Che-ju Do, Korea.
Seoul Med. J., 22, 137-142.
Mahon, C.R. (1993) Comparative metabolism of 14C-propargyl-Omite in
rats and mice. Unpublished report No. 91188 (Uniroyal Study
No.)/No. SC 910217 (Battelle Study No.) from Battelle Laboratory,
Columbus, Ohio, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA.
Mizens, M., Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990)
Study of the dermal absorption of 14C-Omite-30W by male
Sprague-Dawley rats. Unpublished report No. 3449-89-0305-AM-001
from Ricerca, Inc., Dept. Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
O'Malley, M.A. (1997) Skin reactions to pesticides. Occupational
Medicine: State of the Art Reviews, Vol. 12, pp. 327-345.
Oser, B.L. (1966) Chronic (2-year) feeding studies with D-014 in rats
and dogs. Unpublished report No. 86000/86014 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to
Naugatuck Chemical, Connecticut, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Putman, D.L. & Young, R.R. (1994) Micronucleus cytogenetic assay in
mice. Unpublished report No. G94AP36.122 from Microbiological
Associates, Inc., Rockville, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Sabourin, P.J., Trigg, N.J., Mahon, C.R. & Johnson, J.D. (1994)
Pharmacokinetics of 14C-Omite: A comparative study in rats and
mice - Part I - 14C-Omite equivalents in blood following oral
and intravenous administration. Unpublished report No. SC930180
from Battelle Laboratory, Columbus, Ohio, USA. Submitted to WHO
by Uniroyal Co., Inc., Middlebury, Connecticut, USA.
Schardein, J.L. (1989) Developmental toxicity study in New Zealand
white rabbits. Unpublished report No. 399-097 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
Schardein, J.L. (1990) Developmental toxicity study in rats.
Unpublished report No. 399-096 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Serota, D.G., Wolfe, G.W., Durloo, R.S. & Fezio, W.L. (1983)
Teratology study in rabbits Omite technical, revised final
report. Unpublished report No. 798-195 from Hazleton Laboratories
America, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Chemical, Bethany, Connecticut, USA.
Shirasu, Y., Moriya, M. & Watanabe, K. (1979) Microbial mutagenicity
testing on BPPS (Propargite). Unpublished report from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Uniroyal Chemical, Bethany, Connecticut, USA.
Trela, B.A. (1991) Metabolism of 14C-Omite in mice: Dosing, sample
collection, and 14C-residue determinations. Unpublished report
No. SC910015 from Battelle Columbus Division, Columbus, Ohio.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Trutter, J.A. (1991) Combined chronic toxicity and oncogenicity study
in rats with Omite technical. Unpublished report No. HLA/HWA
798-220 from Hazleton Laboratories America, Inc., Virginia, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety. York, R.G.
(1992) A cross-fostering reproduction study with Omite technical
in rats. Unpublished report No. 399-113 from International
Research and Development Corporation, Matttawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
 female rats and male mice were
1-[4-(1,1-dimethyl-2-hydroxyethyl)phenoxy]-2-cyclohexanol (HOMe-TBPC)
and the corresponding diol derivative carboxy-TBPC-diol). No
consistent qualitative or quantitative species differences were found
in the metabolite profiles of propargite in bile and plasma
(Banijamali et al., 1994).
In another comparative study, the metabolites found in the faeces
of male and female CD-1 mice treated with [14C-phenyl]propargite
were characterized and compared with those identified previously in
the faeces of male and female rats (Banijamali & Nag, 1990; Johnson,
1990). The mice were given a single oral dose of 150 mg/kg bw of the
compound by gavage, whereas the rats received a single oral dose of
200 mg/kg bw (Johnson, 1990). Male mice excreted 42% of the
administered dose in the faeces and female mice about 53%, while in
rats faecal excretion accounted for 75% of the administered dose in
males and 70% in females. Peak faecal excretion of radiolabel was
observed between 0 and 24 h in both species. The profile of faecal
metabolites of mice and rats was qualitatively similar. The radiolabel
was associated with unchanged parent compound, the hydrolysis product
propargite glycol ether, and the polar metabolites hxdroxylated
tert-butyl and hydroxylated cyclohexyl propargite glycol ether. Rat
faeces contained a substantially higher percentage of unabsorbed
propargite than mouse faeces. The finding that faeces of female mice
contained more propargite (49% of total faecal residue) than faeces of
male mice (25%) suggests that faecal elimination is sex-dependent.
Moreover, male mouse faeces contained a higher percentage of polar
metabolites (56% of total faecal residue) than those of female mice
(36%). Mouse faeces contained both a greater number and a greater
percentage of polar metabolites than rat faeces, indicating more
extensive metabolism of propargite in mice than rats (Banijamali &
Nag, 1991).
Goats
One dairy goat was given [14C-phenyl]propargite at a dose of
675 mg/kg bw per day on 3 consecutive day, during which time urine,
faeces, and milk were collected. The animal was killed 8 h after the
last dose, and liver, kidney, muscle, fat, and bile samples were
collected. The highest concentration of radiolabel was found in bile,
followed by liver, kidney, fat, and muscle. The bile contained 0.29%
of the administered dose, urine 16%, faeces 14%, and milk 0.1%.
Overall, 34% of the administered dose was recovered; the remaining
radiolabel was presumed to have remained in the gastrointestinal tract
(Byrd, 1988). HPLC analysis revealed metabolism of the cyclohexyl and
tert-butyl group resulting in a number of polar metabolic products,
including carboxy-TBPC-diol, carboxy-TBPC,
1-[4-(1,dimethylethyl)-phenoxy]-2,x-cyclohexanediol, and TBPC in milk
and tissues. Small quantities of unchanged propargite were found in
milk, fat, and liver (Banijamali, 1989b).
After oral administration of [14C-phenyl]propargite in capsules
at doses of 65 or 325 mg/kg bw per day for 3 days to two lactating
goats, metabolites were identified in milk and edible tissues. The
liver contained 14 metabolites, kidney 12, muscle 9, milk 7, and fat
6. Seven of the 14 metabolites isolated from liver were glucuronide or
sulfate conjugates. The metabolism of propargite in goats thus appears
to involve hydrolysis of the propynyl sulfite side-chain followed by
aliphatic and/or alicyclic hydroxylation of the tert-butyl methyl
and cyclohexyl groups to form TBPC-diol and HOMe-TBPC, respectively.
These metabolites undergo further oxidation to yield HOMe-TBPC-diol,
carboxy-TBPC, carboxy-TBPC-diol, and carboxy-TBPC-triol. Some of these
metabolites subsequently undergo conjugation to form glucuronides and
sulfates. The results of these studies indicate similar metabolism in
rats and goats (Banijamali & Lau, 1996).
(ii) Propargyl alcohol and propargyl propargite
In order to learn more about the fate of the propynyl sulfite
side-chain of the propargite molecule, the metabolism of propargyl
alcohol, which may be released from propargite, was studied.
[1,2,3-13C-, 2,3-14C]Propargyl alcohol was administered to groups
of eight male Sprague-Dawley rats by gavage at a dose of 40 mg/kg bw,
and samples of urine, faeces, and expired air (four animals) were
collected 24, 48, 72, and 96 h after treatment. The rats were killed
at 96 h. Radiolabel associated with the parent compound represented
56% of the administered dose in urine, 12% in faeces, and 7% in
expired air. These results are consistent with those of another study
of the degradation and expiratory elimination of this compound (Mahon,
1993). Only 4-6% of the administered dose was recovered in the
carcass. Most of the radiolabel was excreted within the first 24 h of
administration in urine and expired air. The peak elimination in
faeces also occurred within 24 h of dosing and continued for 48 h. The
proposed metabolic pathway involves oxidation of propargyl alcohol to
2-propynoic acid and further detoxification by glutathione conjugation
to yield the following final products:
3,3-bis[(2-(acetylamino)-2-carboxyethyl)thio]-1-propanol,
3-(carboxymethylthio)-2-propenoic acid;
2(methylthio)-3-(methylsulfinyl)-2-propenoic acid;
3-{[2-(acetylamino)-2-carboxyethyl]thio}-3-[(2-amino-2-carboxyethyl)
thio]1-propanol; and
3-{[2-(acetylamino)-2-carboxyethyl]-sulfinyl}-3-{[2-(acetylamino)-2-
carboxyethyl]thio}-1-propanol. These metabolites have not been
reported previously and represent the first examples of multiple
glutathione additions to the carbon-carbon triple bond (Banijamali,
1998).
[1,2,3-13C-, 2,3-14C-propargyl]Propargite was administered
orally to male SpragueDawley rats at a dose of 150 mg/kg bw, and urine
and faeces were collected at 24-h intervals until study termination
96 h after dosing. The total radiolabel excreted in urine accounted
for 25% of the administered dose, and that in faeces for 48%. The six
major metabolites identified in rat urine were
3-(carboxymethylthio)-2-propenoic acid,
2-(carboxymethylthio)-2-propenoic acid,
2-(acetyl-amino)-3-(2-propynylthio)propanoic acid,
3-[(2-carboxy-2-hydroxyethyl)thio]-2-propenoic acid,
3- (N-formylglutamylcysteinyl)-2-propenoic acid, and
2- (N-formylglutamylcysteinyl)-2-propenoic acid. These metabolites
were the result of conjugation with glutathione followed by enzymatic
degradation. Seven metabolites were identified tentatively in faeces
(Banijamali, 1999).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity conducted since the
earlier evaluation (Table 1) are consistent with the previous data.
The clinical observations made most frequently after oral
administration included urogenital staining and abnormal defaecation,
hypoactivity, and swollen, red paws. Most of the treated animals lost
weight during a few days after dosing. Those that died had dark-red
areas, thickened mucosa, and red foci in the stomach. All of the
deaths occurred during the second week, with no sex difference
(Kiplinger, 1993a).
After inhalation, the commonest observations were laboured
breathing and various secretory responses. About one-third of the
treated animals lost weight during the first week after exposure, and
treatment-related reddening of the lungs was seen in some animals
found dead or killed at term. There was no sex difference in
lethality. All deaths occurred 1-17 days after exposure (Hoffman,
1992).
After dermal exposure, none of the animals died and all gained
weight during the 24-day observation period. All rabbits showed severe
erythema and oedema, and eschar formation, fissuring, desquamation,
and a white-yellow exudate on the application site appeared during the
second week of the study and persisted until day 14. Thickened skin
and desquamation within the application site were seen on all rabbits
at necropsy (Kiplinger, 1993b).
Male and female New Zealand white rabbits received dermal
applications of 0.5 ml of undiluted technical-grade propargite
(purity, 90.3%) and were observed at 0.5, 24, 48, and 72 h, daily
through day 14, and on day 21, when they were killed. Irritation was
seen during the first few days after application, consisting of
moderate erythema and slight-to-moderate oedema, but on days 5-9
severe erythema and oedema, fissuring, eschar formation, and
desquamation were observed. The severe irritation had decreased to
slight oedema and erythema by day 21, indicating reversibility of the
reaction (Kiplinger, 1993c).
Table 1. Acute toxicity of technical-grade propargite (purity, 90.3%)
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or mg/L)
Rat Crl:CD BR M Gastric intubation 2600 Kiplinger (1993a)
F 2900
Both 2800
Rat Crl:CD BR M Inhalation (4 h) 0.95 Hoffman (1992)
F 0.95
Both 0.89
Rabbit New Zealand white M Dermal (24 h) > 4000 Kiplinger (1993b)
F > 4000
Both > 4000
Male and female New Zealand white rabbits received 0.1 ml of
undiluted technical-grade propargite (purity, 90.3%) into the
conjunctival sac and were observed at 1, 24, 48, and 72 h and on days
4, 7, 10, 14, 17, and 21 after application. Reactions were observed in
the conjunctiva, cornea, and iris. The effects in cornea and iris had
subsided by day 10 or earlier, and the corneal effects had cleared by
day 21 (Kiplinger, 1993d).
In a modified Buehler test, male and female Hartley albino
guinea-pigs received three topical applications of 0.1%
technical-grade propargite (purity, 90.3%) in ethanol for induction, a
challenge dose of 0.2% propargite in acetone) two weeks later, and a
second challenge (0.1 and 0.2% in acetone) after a 1-week
interruption. The applications were left in place for 6 h. A positive
control (dinitrochlorobenzene) and an untreated control group were
included. No evidence of sensitization was seen (Kiplinger, 1993e).
(b) Short-term studies of toxicity
Rats
Propargite was administered in the diet to groups of five rats of
each sex at concentrations of 0 (15 animals), 200, 400, 800, 2000, or
4000 ppm for 90 days. Reduced food consumption and body-weight gain
were observed at the two higher concentrations. Haematological and
clinical chemical parameters (glucose and urea nitrogen) were not
affected by treatment. The relative weights of the liver, kidney,
adrenals, and gonads were increased at the two higher concentrations.
No treatment-related macroscopic or microscopic alterations were
observed (Carson, 1964).
Groups of 10 male and 10 female Crl:CD (SD) BR rats were
maintained on diets containing technical-grade propargite (purity,
87.2%) at concentrations of 0, 100, 1000, or 2000 ppm (equivalent to
0, 5, 50, and 100 mg/kg bw per day) for at least 13 weeks. At the end
of the study, all surviving rats were anaesthetized, weighed, bled,
killed, and necropsied. All tissues from control rats and those at the
highest dose, the lungs, liver, and kidneys from rats at 100 or
1000 ppm, and all macroscopic lesions from all rats were examined
microscopically. No deaths occurred during the study. All animals at
2000 ppm had rough coats throughout the study, and many were thin with
a hunched posture, alopecia, and rhinorrhoea. The body weights of rats
at 1000 and 2000 ppm were significantly lower than those of controls
throughout the study, by 30% at 1000 ppm and 69% in males and 52% in
females at 2000 ppm. The body-weight gains of males were significantly
reduced during week 10 at 100 ppm, during most of the study at
1000 ppm, and throughout the study at 2000 ppm. The significantly
reduced body-weight gain at 100 ppm during week 10 did not result in a
significant reduction in the cumulative body-weight gain at study
termination or in any other adverse effect and was considered not to
be toxicologically relevant. The body-weight gains of females were
significantly lower during weeks 1 and 5 at 1000 ppm and during weeks
1-4 at 2000 ppm. The reduction in the body-weight gain of males at the
end of the study in comparison with controls was 4% at 100 ppm, 30% at
1000 ppm, and 69% at 2000 ppm, and the corresponding reductions in
females were 6%, 31%, and 52%, respectively. The reductions at 1000
and 2000 ppm were significant. The reduced body-weight gain was
associated with a dose-dependent reduction in food consumption at the
two higher doses.
Various haematological and clinical chemical parameters were
altered at the two higher doses, including significantly increased
erthrocyte count and haemoglobin values in males at 2000 ppm and
significantly decreased mean corpuscular volume and mean corpuscular
haemoglobin in males at 1000 and 2000 ppm and females at 2000 ppm. A
non-dose-related but significant increase in platelet count in females
at 1000 ppm and an increased erythrocyte count in males at 100 ppm
were considered to be of no toxicological significance. The blood
glucose concentration was significantly lower in males and females at
1000 and 2000 ppm, and urea nitrogen was significantly higher and
creatinine significantly lower in animals of each sex at 2000 ppm.
Reduced total protein, albumin, and globulin values were found in
males at 2000 ppm and in females at 1000 ppm and 2000 ppm. An
increased albumin:globulin ratio was found in males and females at
2000 ppm. The concentrations of calcium, inorganic phosphorus,
potassium, and chloride showed significant alterations at 2000 ppm.
Most of the changes observed were considered to be associated with the
decreased food consumption and body weight. The absolute weights of
the kidneys and liver were significantly reduced in animals of each
sex at 2000 ppm, and the absolute weight of the testis was reduced in
males at this dose. The relative weights of the kidney, liver, and
testis were increased in a dose-related manner at 1000 and 2000 ppm.
Macroscopic and microscopic examinations did not reveal alterations
attributable to treatment. The NOAEL was 100 ppm, equivalent to 5
mg/kg bw per day, on the basis of effects on body weight and changes
in clinical chemical parameters at higher doses (Kehoe, 1988).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of technical-grade propargite (purity, 85%) at
doses of 0, 0.1, 1.0, 10, or 100 mg/kg bw per day, 5 days per week for
3 weeks. No deaths occurred during the study, and no clinical signs
were observed at any dose. Food consumption was slightly reduced at
all doses, and the mean body weightswere slightly reduced in treated
males and slightly increased in treated females, with no clear
dose-response relationship, indicating no consistent treatment-related
effect. Biochemical parameters were not affected by treatment, whereas
haematological investigations revealed a significant increase in the
number of segmented neutrophils at the end of the study in males at
100 mg/kg bw. Signs of dermal irritation were seen at the application
site in all treated groups, with a dose-related increase in incidence
and severity. The signs consisted of erythema, oedema, eschar
formation, exfoliation, atonia, desquamation, fissuring, blanching,
and coriaceousness. The dermal effects occurred earlier with
increasing dose. The observed changes were graded as mild-to-severe in
animals at 10 and 100 mg/kg bw per day, mild-to-moderate at 1 mg/kg bw
per day, and mild at 0.1 mg/kg bw per day. No changes in organ weights
were observed, and no additional macroscopic changes were seen.
Dose-related microscopic changes were confined to the application
site, which were in the incidence and severity of acanthosis and
hyperkeratosis. The incidence of dermal inflammation was similar in
all treated groups, and necrosis was observed at doses > 1 mg/kg bw
per day. The NOEL for systemic toxicity was 100 mg/kg bw per day, the
highest dose tested, if the increased number of segmented neutrophils
in males at this dose is considered to be a borderline effect. No NOEL
could be identified for local irritation (Goldenthal, 1989).
Dogs
Groups of three beagle dogs of each sex were given diets
containing propargite (purity not specified) at concentrations of 0,
2000 ppm (weeks 1-3), and 2500 ppm (weeks 4-13), equal to 55 mg/kg bw
per day for males and 67 mg/kg bw per day for females (mean values of
2000 and 2500 ppm). Appearance, behaviour, and signs of toxicity were
recorded daily and body weights and food consumption weekly. Clinical
chemistry was evaluated once initially and 1 and 3 months after the
start of the study. Gross necropsy was performed on all dogs killed
at13 weeks, and the weights of the thyroid, heart, liver, spleen,
kidneys, adrenals, and testis were recorded; all organs were examined
microscopically. Most of the treated animals had a reduced appetite,
particularly during the first half of the study, and weight loss was
seen. Two males showed reduction in various haematological parameters,
including haematocrit and erythrocyte count after 3 months of
treatment, and all treated males had slightly increased aspartate
aminotransferase activity. Changes in organ weights showed no clear
dose-related pattern, and the increased relative liver weight was
probably a consequence of the reduced body weights. No macroscopic
changes were observed. Microscopic findings considered to be related
to treatment were increased pigmentation in the reticuloendothelial
cells in the liver and increased haemosiderin deposits in the spleen,
which may also be related to the decreased food consumption (Holsing &
Kundzins, 1968).
Groups of six beagle dogs of each sex recieved diets containing
technical-grade propargite (purity, 88.6%) at concentrations of 0,
160, 1250, or 2500 ppm, equivalent to 4, 30, and 48 mg/kg bw, for 1
year. The high concentration was reduced to 1875 ppm at week 9 after
observation of excessive body-weight loss. Treatment did not induce
ocular abnormalities or any treatment-related changes in clinical
chemical or urinary parameters. Two animals at the high dose died with
marked body-weight loss, and the remaining animals at this dose were
thin throughout most of the study and appeared to be dehydrated during
the last few months. Pronounced body-weight loss occurred during the
first 8 weeks at the high dose, by 2.6 kg in males and 1.9 kg in
females, and at the intermediate dose, by 0.4 kg in males and 0.5 kg
in females. Once the dose had been reduced to 1875 ppm, the
body-weight loss was less pronounced; however, the weight gain of
animals at 1250 ppm was markedly lower than that of controls. The food
consumption of animals at the high dose was reduced throughout the
study, perhaps indicating unpalatability, although it tended to
increase from week 9. Effects on haematological parameters included
reduced haemoglobin, haematocrit, and erythrocyte values in males and
females at the high dose and reduced haematocrit in males at the
intermediate dose at 3 and 6 months and at termination. In females,
these changes were less pronounced and were observed only after
3 months of treatment. Platelet counts were elevated in females at
1250 and 1875 ppm at all intervals and in males at the high dose after
6 and 12 months. Decreased absolute weights and increased relative
weights of many organs were observed in animals at the high dose and
occasionally in those at the intermediate dose. These changes are
considered to be due to the reduced body weight at this dose level.
The macroscopic changes consisted of an increased incidence of
red-tan-white foci in the lungs of males at the high dose and a higher
incidence of involution of the thymus at doses > 1250 ppm. Changes
in the bone marrow consisted of a greater incidence and severity of
erythroid-myeloid depletion and atrophy in animals at the high dose.
Histologically, the changes in the lung consisted of congestion and
inflammatory changes and fibrous and alveolar/bronchiolar epithelial
hyperplasia. In the stomach, dilated mucosal glands and vacuoles in
parietal cells were seen, but these changes showed no clear
dose-response relationship and their relation to treatment is
questionable. The NOAEL was 160 ppm, equivalent to 4 mg/kg bw per day,
on the basis of body-weight loss, reduced food consumption, and
histopathological alterations in the thymus and bone marrow at higher
concentrations (Atkinson, 1991).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a 30-day range-finding study, groups of 10 mice of each sex
received diets containing propargite at concentrations of 0, 600, 900,
1350, 2000, or 3000 ppm. Gross observations, body weight, food
consumption, gross necroscopy, and organ weights were recorded.
Body-weight loss and changes in organ weights were the predominant
effects. Minimal effects were considered to have occurred at 1000 ppm
(Gallo & Bailey, 1976), which was selected as the highest dose for the
study described below.
Groups of 15 CD-1 mice of each sex received diets containing
propargite (purity, 88.5% during the first 12 months and 84.3% during
the remaining 6 months) in corn oil at concentrations of 0, 500, or
1000 ppm, equivalent to 0, 75, and 150 mg/kg bw per day, for 52 weeks;
and groups of 60 mice of each sex received diets containing the
compound at concentrations of 0, 50, 160, 500, or 1000 ppm for
78 weeks, equivalent to 0, 7.5, 24, 75, and 150 mg/kg bw per day. The
animals were observed daily for changes in general appearance,
behaviour, appetite, toxic effects, and deaths. Body weights were
determined weekly for the first 28 weeks and every two weeks
thereafter. Food consumption was recorded weekly. Before the start of
treatment and at 52 and 78 weeks, leukocyte and erythrocyte counts
were determined in 10 animals of each sex per group, and before
termination differential leukocyte counts were made in 10 animals of
each sex per group. Gross necropsy was carried out on all animals
found dead or killed when moribund and on all animals killed at the
scheduled time. Essentially all organs, including the head, tongue,
ear, and nose, were saved, and the fresh weights of the liver, spleen,
kidneys, heart, adrenals, thyroid, and gonads were recorded.
Furthermore, sections of the spinal cord, an additional lobe of the
liver, the gall-bladder, an additional section of the uterus to
include the cervix, the nasal cavity, and the middle ear were taken
for histopathological examination.
Males at doses > 160 ppm had a lower mortality rate than
controls at 52 weeks, the survival rates being > 85%; after 78
weeks, the survival rates were 38% at 50 ppm and 60% at 500 ppm. The
variations seen in body-weight gain were not dose-related. Males
treated for 78 weeks showed a non-dose-related increase in body-weight
gain when compared with controls, whereas the females showed a
non-dose-related decrease. A similar, inconsistent pattern was found
in animals treated for 52 weeks, and these variations are considered
to be unrelated to treatment and of no toxicological significance.
Treatment did not affect food consumption or haematological
parameters, and no unexpected gross alterations were seen. An increase
in the relative weight of the kidney males treated for 52 weeks can
probably to be attributed to the reduced body-weight gain of these
animals. Females showed increased absolute and relative weights of the
adrenals at both 500 and 1000 ppm and a non-dose-related increase in
the absolute and relative weights of the thyroid at 500 ppm. The
relationship of the effects on the thyroid to treatment is
questionable. Animals treated for 78 weeks had reduced absolute and
relative weights of the kidney and uterus at concentrations > 160
ppm, and a non-dose-related increase in uterine weight was observed at
500 and 1000 ppm in females treated for 78 weeks and at 160, 500, and
1000 ppm in females treated for 52 weeks. Statistical significance was
attained only in females at 1000 ppm killed at 78 weeks.
Histopathological examination showed no treatment-related alterations
in tissues or organs, and no correlation was found between the
occurrence or type of neoplasms and treatment. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of changes in the
weights of the kidney and uterus at higher doses (Cox & Re, 1979;
Becci, 1980).
Rats
Propargite was administered to groups of 25 male and 25 female
FDRL rats in the diet at concentrations of 0 (37 animals), 100, 300,
or 900 ppm, equivalent to 0, 5, 15, and 45 mg/kg bw per day for 2
years. After the study had been in progress for 26 weeks, an
additional treatment group was included at a concentration of 2000 ppm
(equivalent to 100 mg/kg bw per day) as well as an additional control
group, because the lower doses had no effect; these groups were
treated for 78 weeks. The appearance, behaviour, and survival of the
animals was recorded daily, and body weight and food consumption were
recorded weekly. The efficiency of food use was calculated for the
first 3 months. Haematological and clinical chemical parameters were
evaluated in all animals at 12, 26, 52, 78, and 104 weeks. All rats
that died or were killed when moribund or at the end of the study were
examined macroscopically. Sections of the main organs from half of the
animals at 900 and 2000 ppm and their corresponding controls and of
the liver, kidneys, bone marrow, and thymus of the remaining rats were
examined histologically.
Treatment did not affect the appearance or behaviour of the
animals. The survival rate of males at 2000 ppm was lower than that in
the other treated groups and the control group. During the first 12
weeks of treatment, no difference in body weight or food intake was
observed between treated and the control groups, but later, the
body-weight gain of males at 900 ppm and of all treated females was
lower than that of controls, with no dose-response relationship.
Whereas the changes observed at 900 ppm were slight, a marked
reduction in body-weight gain was seen at 2000 ppm, and food intake
was also reduced at this dose. Haemoglobin, haematocrit, and leukocyte
values showed no treatment-related change at concentrations up to 2000
ppm. The absolute weights of the livers of rats at 900 ppm and of
females at 300 ppm were increased, and the relative weights were also
increased, attaining statistical significance in males at 900 ppm and
in females at concentrations > 300 ppm. In animals at 2000 ppm, the
absolute weight of the liver was decreased and the relative weight
increased, the changes being statistically significant. The relative
weight of the kidney was increased in males at 900 ppm after 104 weeks
of treatment, whereas in animals treated at 2000 ppm for 78 weeks, the
absolute weight of the kidneys was decreased and the relative weight
was increased in animals of each sex. Gross observation at autopsy
showed no treatment-related changes. The total incidences of sarcomas
and carcinomas were 6/85 in controls, 6/44 at 100 ppm, 8/42 at 300
ppm, 9/39 at 900 ppm, and 4/26 at 2000 ppm. The NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day, on the basis of changes in organ
weights (Oser, 1966). The low survival rate of animals at the highest
dose limited the relevance of the findings, and this study was
considered by the 1977 Meeting to be inadequate for evaluating the
carcinogenicity of propargite.
On the basis of the results of a 90-day study which indicated a
maximum tolerated dose of 1000 ppm (Kehoe, 1988), 800 ppm was selected
as the highest dose in a 2-year study in Crl:CDBR rats. Groups of 60
rats of each sex were given diets containing propargite (purity,
87.2%) at concentrations of 0, 50, 80, 400, or 800 ppm, equal to 2, 4,
19, and 39 mg/kg bw per day for males and 3, 5, 24, and 49 mg/kg bw
per day for females. Owing to low survival rates in males at the high
dose (30% after 103 weeks), this group was killed at 103 weeks. An
interim sacrifice was made at 53 weeks. The test diets were found to
be of adequate homogeneity and stability.
The mortality rate of males at 400 and 800 ppm was increased
towards the end of the study, with a significant positive trend,
although the group differences were not large enough to reach
statistical significance. The body weights of males at 800 ppm were
significantly reduced at the end of the study. Females showed reduced
body weight at various times during the study but no significant
overall reduction. Body-weight gain was reduced in a dose-related
manner, by 5% at 80 ppm, 12% at 400 ppm, and 18% at 800 ppm in males
at the end of the study, with statistical significance at 400 and 800
ppm. In females, body-weight gain was reduced by 15% at the high dose,
but with no statistical significance. At interim sacrifice, the
body-weight gain was reduced by 6% at 400 ppm and 20% at 800 ppm in
males and by 4% at 400 ppm and 30% at 800 ppm in females. Food
consumption was reduced at concentrations > 400 ppm in animals of
each sex. No compound-related differences in clinical signs were seen
between control and treated groups, and no treatment-related
ophthalmoscopic changes were found. The changes in haematological
parameters consisted of an increased reticulocyte count, mostly due to
decreases in three animals, and a reduced erythrocyte count in males
at 800 ppm at termination, indicating the presence of hypoxia and/or
accelerated erythropoiesis. The effects on clinical chemistry
consisted of decreased total serum protein values in males at 400 and
800 ppm and in females at 800 ppm at 26 weeks and a corresponding
decrease in total serum calcium. After 26 weeks, decreased aspartate-
and alanine aminotransferase activities were seen, with no clear
dose-response relationship. A dose-related decrease in aspartate
aminotransferase activity was observed in females at 400 and 800 ppm
after 52 weeks of treatment, probably as a result of the impaired
nutritional status of animals at these doses.
Necropsy of animals that died during the first 64 weeks of the
study showed no gross, compound-related changes in tissues. From week
65, several males at the high dose were found to have abdominal
masses, mostly in the small intestine and principally in the jejunum.
At the end of the study, males at 400 and 800 ppm and females at 800
ppm showed high frequencies of this treatment-related effect, the
incidences of masses in the jejunum being 0% in controls, 0% at
50 ppm, 0% at 80 ppm, 17% at 400 ppm, and 25% at 800 ppm in males and
0% in controls, 2% at 50 ppm, 0% at 80 ppm, 2% at 400 ppm, and 15% at
800 ppm in females. No other gross tissue alterations attributable to
treatment were found. No compound- or dose-related changes were found
in the absolute weights of the organs of treated animals, but
alterations were found in the relative weights of various organs: At
week 53, the relative weights of the livers of treated females showed
a dose-related increase over that of controls, which attained
statistical significance only in animals at 50, 400, and 800 ppm.
These changes in relative liver weights were not accompanied by
biochemical or histopathological alterations, suggesting that they
were due to the reductions in body-weight gain in females at 400 and
800 ppm and that the significant increase at 50 ppm (not supported by
a similar change at 80 ppm) is an incidental finding. Increased
relative liver weights were also observed in males at the high dose at
interim sacrifice, without reaching statistical significance, again
probably reflecting the reduced body-weight gain of these animals. At
termination, slightly increased relative liver weights were observed
in animals of each sex, but with no statistical significance. At the
interim sacrifice, the relative weights of the kidneys were also found
to be increased in animals at the high dose and in females at 80 ppm.
Histological examination revealed no treatment-related
non-neoplastic alterations and no compound-related neoplastic findings
through week 52, but during the second year of the study high
frequencies of undifferentiated sarcomas of the jejunum in males at
400 and 800 ppm and in females at 800 ppm were recorded (Table 2).
Single cases were also found in other dose groups. A clear
dose-related increase in the incidence of sarcoma was found in males
at 400 and 800 ppm and in females at 800 ppm. The incidences in all
treated female rats were also increased when compared with controls,
but with no dose-response relationship. The incidence of
undifferentiated sarcomas in duodenum, subcutaneous tissue, jejunum,
and the thoracic cavity in rats of this strain in previous studies was
reported to be 0.2% in males and 0% in females, but no
undifferentiated sarcomas were found in the jejunum. Electron
microscopic examination of abdominal tumour masses from three males at
400 ppm and one at 800 ppm revealed one malignant schwannoma, one
undifferentiated sarcoma, and one fibrosarcoma, suggesting that
propargite induced proliferation of mesenchymal tumours in various
stages of differentiation. The tumour incidences correlated with the
observations of abdominal masses. The NOAEL for systemic toxicity and
carcinogenicity was 80 ppm, equal to 4 mg/kg bw per day, on the basis
of effects on body weight, food consumption, and clinical chemical
parameters and an increased incidence of jejunal sarcomas at higher
doses (Trutter, 1991).
Dogs
Groups of six beagle dogs of each sex were maintained on a diet
containing propargite at concentrations of 0, 100, 300, or 900 ppm for
1 h per day, 6 days a week for 2 years. The dogs were observed daily
for appearance, behaviour, signs of toxicity, and neurological
reflexes. Body weight and food intake were recorded weekly for the
first 12 weeks and every 2 weeks thereafter. Haematological, clinical
chemical and urinary parameters were determined at 26, 52, 78, and
106 weeks. One animal of each sex per group was killed after 1 year
and examined grossly. A survivors were killed after 2 years and
examined. Treatment did not adversely affect the appearance,
behaviour, body weight, haematological or clinical chemical or
microscopic appearance. No indication of carcinogenicity was found
(Oser, 1966).
(d) Genotoxicity
The results of tests for the genotoxicity of propargite are
summarized in Table 3.
Table 2. Incidences of undifferentiated sarcoma of the jejunum in rats fed propargite in the diet for 2 years
Sex Dose Tumour incidence
(ppm)
Unscheduled deaths Interim sacrifice Terminal sacrifice Total
No. of No. % No. of No. % No. of No. % No. of No. %
rats rats rats rats
Male 0 26 0 0 9 0 0 24 0 0 59 0 0
50 16 0 0 0 31 0 0 47 0 0
80 23 0 0 0 23 0 0 46 0 0
400 32 9 28 0 17 2 12 49 11 22
800 35 20 57 10 0 0 15 4 27 60 24 40
Female 0 27 0 0 10 0 0 20 0 0 57 0 0
50 29 0 0 0 20 1 5 49 1 2
80 20 1 5 0 29 0 0 49 1 2
400 28 0 0 0 20 1 5 48 1 2
800 25 8 32 10 0 0 21 4 19 56 12 21
Table 3. Results of tests for the genotoxicity of propargite
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.001-5 µl/plate NR Negative ± S9 Brusick & Weir
TA98, TA100, in DMSO (1977)
TA1535, TA1537,
TA1538;
S. cerevisiae D4
Reverse mutation S. typhimurium 10-5000 µg/plate 90.9 Negative ± S9 Shirasu et al. (1979)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr
Reverse mutation S. typhimurium 10-300 µl/plate 90a Positive ± S9 Lawlor (1991)
TA98, TA100, at > 10 µl/ml
TA1535, TA1537, in TA100
TA1538
Gene mutation B. subtilis H17, M45 1-100% v/v in DMSO 90.9 Negative Shirasu et al. (1979)
Gene mutation Chinese hamster 1-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 10-75 µg/ml +S9 (1993)
locus in DMSO
Gene mutation Chinese hamster 0.2-4.2 µg/ml 90 Negative Bigger & Clarke
ovary cells, Hprt -S9; 10-50 µg/ml (1993)
locus +S9 in DMSO
Gene mutation Chinese hamster 0.5-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 5-5 µg/ml +S9 (1993)
locus in acetone
Table 3. (continued)
End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Chinese hamster 25-200 µg/ml NR Negative ± S9 Kirkland (1985)
aberrations ovary cells
DNA repair Rat hepatocytes 0.0167-0.5 µg/ml 97 Negative Barfknecht (1987)
in acetone
In vivo
Micronucleus ICR mouse 37.5, 75, 150 mg/kg 89.6 Negative Putman & Young
formation bw i.p in corn oil (1994)
NR, not reported; i.p., intraperitoneally
a Technical-grade containing epoxidized soya bean oil as stabilizer
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of rats were maintained on diets containing propargite at
concentrations of 0 or 100 ppm. When the rats were about 100 days of
age and sexually mature, 20 pairs of males and females were followed
through two reproductive cycles. The first litters were discarded. At
weaning of the second litter, 10 rats of each sex in each group were
selected for the F1 generation. A similar procedure was chosen for
selection of the F2 generation. The dose of the F2 pups was
increased to 300 ppm, and the F3 pups received 300 ppm throughout
treatment. The pups were raised to maturity and the cycle repeated in
this and the succeeding generation. Treatment did not have adverse
effects on the dams, the reproduction indices, or their pups. The NOEL
was 100 ppm, the only dose tested, equivalent to 5 mg/kg bw per day
(Oser, 1966).
Groups of 25 immature Crl:CDBR albino rats of each sex were fed
diets that contained technical-grade propargite (purity, 87.2%) at
concentrations of 0, 80, 400, or 800 ppm, equivalent to 0, 4, 20, and
40 mg/kg bw per day, for 10 weeks before mating and throughout mating,
gestation, lactation, and weaning of the F1a pups. After weaning,
these pups were killed and discarded, and the F0 animals were mated
again to produce F1b litters. After weaning, the pups were assigned
at random to four groups of 25 animals of each sex, and the F0
adults were killed and necropsied. The selected F1b animals were fed
the diets for at least 10 weeks before mating and throughout
gestation, lactation, and weaning of F2a pups. After these pups were
weaned, they were killed and discarded, and the F1b animals were
mated again to produce F2b litters. After these pups had been
weaned, they were killed and discarded, and the F1b adults were
killed and necropsied. The body weights of males were recorded on the
first day of treatment, weekly thereafter, and on the day of necropsy,
whereas females were weighed on the first day of treatment, weekly
before mating, on days 0, 7, 14, and 20 of gestation and lactation,
and on the day of necropsy. Food consumption was recorded weekly only
before mating of the F0 and F1b generations and not during the
gestation and lactation periods. All F0 and F1b adults were
examined macroscopically, and the reproductive organs of those at 0
and 800 ppm were examined microscopically.
No treatment-related clinical signs were noted in these animals,
and their survival was not adversely affected. The treatment resulted
in dose-related reductions in body weight and body-weight gain of both
sexes of both generations at 400 and 800 ppm during various phases of
the study. Body weight was reduced by 5-10% in males at 400 ppm and by
18-28% in both generations at 800 ppm before and after mating.
Body-weight gain showed a corresponding pattern, with reductions of up
to 20% in males at 400 ppm and up to 60% at 800 ppm. Similar
reductions were found in females: at 400 ppm, the maximum reduction
during premating, gestation, and lactation was 10%, and at 800 ppm the
reduction was up to 22%. The body-weight gains of females were reduced
by up to 30% at 400 ppm and up to 90% at 800 ppm before mating. Marked
differences in the body-weight gain of females were seen in the
premating, gestation, and lactation periods. In all generations,
lactating dams treated with 400 and 800 ppm had greater body-weight
gain than controls, perhaps because some controls lost weight during
the second half of the lactation period. Males of the F0 generation
at 400 ppm showed a dose-related reduction in mean food consumption
during weeks 0-1, 4-5, and 8-9 before mating, and the food consumption
of males at 800 ppm was consistently lower before mating. The mean
food consumption of F0 females at 400 ppm was lower only during week
6-7, but that of females at 800 ppm was reduced throughout the
premating period. The mean food consumption of males and females of
the F1b generation showed a dose-related reduction at 400 and 800
ppm. Mating and fertility parameters and gestation indexes were not
affected by treatment, and no treatment-related differences in litter
size or sex ratio were seen. Pup weight per litter was reduced in all
generations at 400 ppm on lactation days 7, 14, and 21 and for litters
at 800 ppm on lactation days 0, 4, 7, 14 ,and 21. The maximum
reduction in the weight of pups at 800 ppm group on lactation day 21
was 44%. There were no treatment-related macroscopic or microscopic
changes. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per day, on
the basis of effects on the body weights of dams and pups at higher
doses (Kehoe, 1990).
In a study designed to clarify the reduced pup weights during
lactation at concentrations of 400 and 800 ppm, a cross-fostering
study was initiated in which groups of Crl: CD VAF/Plus rats were
given diets containing technical-grade propargite (purity, 89.9%) at
concentrations of 0 (100 animals of each sex), 400 ppm (30 of each
sex), or 800 ppm (60 of each sex). The F0 parents were given the
diets for 70 days before mating, and F0 males were killed one week
after mating. The F0 females that had delivered were killed on
lactation day 21, and those that did not deliver within 25 days after
mating were also necropsied. On lactation day 21, the F1 offspring
were necropsied, with special attention to the reproductive organs. On
lactation day 0, the litters were cross-fostered to dams in other
groups and those of the control group to other dams within the group.
Selected dams in the control group and at 800 ppm were allowed to keep
their own litters. The new groups were thus: 15 untreated dams with
their own untreated litters (controls); 20 untreated dams
cross-fostering untreated litters; 20 untreated dams cross-fostering
litters treated at 400 ppm; 20 untreated dams cross-fostering litters
treated at 800 ppm; 20 dams treated at 400 ppm cross-fostering
untreated litters; 20 dams at 800 ppm cross-fostering untreated
litters; and 20 dams at 800 ppm with their own litters treated at 800
ppm. All animals were observed for deaths and signs of toxicity twice
daily. Body weights were recorded weekly for males and for females
until evidence of copulation was seen and on days 0, 7, 14, and 20 of
gestation and days 0, 7, 14, and 21 of lactation. The food consumption
of adult rats was measured weekly except during mating in weeks 11-13
and, for females, during gestation and lactation. The litters were
reduced randomly to four pups of each sex on lactation day 4 to
provide homogeneous groups for evaluation of nursing, survival, and
pup growth. The culled pups were examined externally. The litters were
caged with their dams for 3 weeks after birth. Throughout lactation,
the dams and their pups were observed daily for survival and
behavioural abnormalities in nesting and nursing. Pups were weighed on
days 0, 4, 7, 14, and 21 of lactation, the day of termination.
Treatment had no effect on the appearance or behaviour of the
F0 animals. A treatment-related reduction in body weight relative to
the controls was seen in males and females at 800 ppm during
premating, by 16% in males and 9% in females at the end of premating.
During gestation, the mean maternal body weight and body-weight gain
were reduced at the 800 ppm; during lactation, the mean body weights
of dams at 800 ppm cross-fostering untreated litters and and of dams
at 800 ppm with their own litters remained lower than those of
controls. At the beginning of lactation, reduced body weights were
observed in all cross-fostering groups relative to the controls, with
reductions of 5% in untreated dams cross-fostering untreated litters,
4% in untreated dams cross-fostering litters treated at 400 ppm, 2% in
untreated dams cross-fostering litters at 800 ppm, 3% in dams treated
at 400 ppm cross-fostering untreated litters, 7% in dams at 800 ppm
cross-fostering untreated litters, and 12% in dams at 800 ppm with
their own litters treated at 800 ppm. At the end of the lactation
period, the reductions were 3%, 6%, 2%, 0%, 7%, and 10% in these
groups, respectively. Body-weight gain during lactation showed marked
variation among the different groups which was unrelated to dose, the
changes over the 21-day period corresponding to 31%, -17%, 10%, 52%,
14%, and 28% in the six groups, respectively. Food consumption was
also reduced, by up to 20% at 800 ppm in males and females before
mating. During gestation, the food consumption of dams at 800 ppm was
reduced to about 11%. During lactation, the food consumption in the
different groups was 103%, 101%, 106%, 98%, 83%, and 80% relative to
the controls, respectively.
No treatment-related differences in litter size, viability on day
0, or the survival index of the F1 offspring was observed. From
birth (lactation day 0), the mean body weights of male and female pups
born to dams treated at 800 ppm were statistically significantly
reduced when compared with the control group, whereas the weights of
female pups born to dams given 400 ppm were significantly increased,
an effect that was considered biologically irrelevant. On lactation
day 0, the body weights of untreated pups nursed by their untreated
dams and of pups at 400 ppm fostered by untreated dams were unchanged.
The weights of pups at 800 ppm fostered by untreated dams were
nonsignificantly reduced on days 0 and 4; those of untreated pups
fostered by dams at 400 ppm were nonsignificantly reduced on days 0
and 4 but significantly reduced on days 14 and 21 of lactation; the
weights of untreated pups fostered by dams at 800 ppm and those of
pups treated at 800 ppm and fostered by dams at the same dose were
nonsignificantly reduced on day 0 but significantly reduced on days 7,
14, and 21. There were no treatment-related differences in the
incidences of malformations, developmental variations, or macroscopic
and microscopic appearance in the F1 offspring. The results of this
study indicate that the effects on pup weight are the result of
toxicity in the dams (York, 1992).
(ii) Developmental toxicity
Rats
Groups of a minimum of 20 females Sprague-Dawley rats were given
technical-grade propargite as a suspension in corn oil by gavage at
doses of 0, 6, 25, or 105 mg/kg bw per day on days 6-15 of gestation.
At least 30 females were used as vehicle controls and for a positive
control group receiving an aqueous suspension of aspirin at a dose of
250 mg/kg bw per day. The original doses were set at 0, 25, 105, and
450 mg/kg bw per day, but dams at 450 mg/kg bw per day died after only
3 or 4 days of treatment and therefore this group was terminated. The
females at 105 mg/kg bw per day showed signs of toxicity, including
bloody nasal and vaginal discharges and urinary incontinence; this
dose was continued as the high dose, 25 mg/kg bw per day as the
intermediate dose, and an additional dose of 6 mg/kg bw per day as the
low dose. No clinical signs were observed at 6 or 25 mg/kg bw per day.
The body weight and body-weight gain of dams at 105 mg/kg bw per day
were lower than those of the other groups, but the difference did not
attain statistical significance. The body weight and body-weight gain
of the positive controls were significantly reduced at the end of the
treatment period. The reproductive performance of the treated dams, as
measured by pregnancy rate and the numbers of implantations,
resorptions, and live and dead fetuses, was similar at all doses. When
the fetuses of treated dams were evaluated for skeletal anomalies,
those at 25 and 105 mg/kg bw per day showed statistically significant
increases in the incidence of missing sternebrae, with 3% of fetuses
and 24% of litters at 25 mg/kg bw per day and 16% of fetuses and 41%
of litters at 105 mg/kg bw per day affected. The control incidence was
1% of fetuses and 6% of litters. The incidences of incomplete
ossification of vertebrae were also increased, with 28%/88%
(fetuses/litters) at 6 mg/kg bw per day, 23%/71% at 25 mg/kg bw per
day, and 30%/86% at 105 mg/kg bw per day; the control incidence was
13%/58%. Statistically significant increases were also observed in the
incidence of incomplete closure of the skull at 105 mg/kg bw per day
and in the incidence of reduced or missing hyoid, with 2%/10% at 25
mg/kg bw per day, and 5%/14% at 105 mg/kg bw per day, and 0.4%/3% in
the control group and 0.5%/4% at 6 mg/kg bw per day. The incidence of
haemorrhagic abdomen was increased at 25 and 105 mg/kg bw per day,
with no clear dose-response relationship. Most of these parameters
were also adversely affected in the dams in the positive control
group. In these animals, the incidence of resorption sites was
increased and the number of live fetuses and fetal weight were
significantly reduced, and the body weights of the dams were reduced.
The fetuses of the positive controls showed various skeletal and
soft-tissue abnormalities at significantly higher incidences than in
the untreated controls. The NOAEL for maternal toxicity was 25 mg/kg
bw per day, but no NOAEL could be identified for developmental
toxicity (Knickerbocker & Re, 1979).
Groups of 45 female Crl:CD rats were given technical-grade
propargite (purity, 85%) by gavage at doses of 0, 6, 12, 18, 25, or
105 mg/kg bw per day on days 6-15 of gestation. On day 20, the pups of
20 gravid females were removed surgically and examined. The remaining
animals were allowed to deliver, and they and their pups were observed
until day 21 of lactation, when they were necropsied. There were no
treatment-related effects on behaviour or survival. Animals at the
highest dose had anogenital and body surface staining, and the body
weights were significantly reduced by 5% when compared with controls.
The adjusted body weight (body weight on day 20 of gestation minus the
weight of uterus and contents) of animals at this dose showed a
similar reduction (7%) on days 6-9 of gestation. The body-weight gain
during days 6-15 of gestation was similar at the four lower doses but
was reduced at the highest dose. The body weights during lactation did
not differ with dose, and those of animals at the highest dose were
similar to those of controls. No sign of developmental toxicity was
seen, as measured by pre- and postimplantation loss, the numbers of
implantations and corpora lutea, pup survival index, or fetal body
weight. Slight reductions in the numbers of live offspring at day 0 of
lactation were observed, with no clear dose-response relationship; the
greatest reduction, 96.4%, was found at the highest dose. The indexes
of pup survival and mortality calculated on a fetal basis showed no
treatment-related reduction, but pup mortality analysed on a litter
basis showed a statistically significant increase in the number of
litters with deaths per total litters on day 7 at the highest dose. No
treatment-related difference in the incidence of malformations or of
developmental variations was seen. The NOAEL was 25 mg/kg bw per day
on the basis of effects on the body weight of dams and slight effects
on postnatal mortality at the higher dose (Schardein, 1990).
Rabbits
Groups of 17 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by intubation at
doses of 0, 2, 6, 10, or 18 mg/kg bw per day on days 6-18 of
gestation. Maternal toxicity, manifested by higher mortality rates at
6, 10, and 18 mg/kg bw per day, attained statistical significance at
18 mg/kg bw per day. Adipsia and anorexia were seen in all groups but
at a markedly higher frequency at the three higher doses than in
controls. Maternal body weight and body-weight gain showed
dose-related reductions at doses > 6 mg/kg bw per day, resulting in
reduced overall weight gain up to day 29 at 6 and 10 mg/kg bw per day
and weight loss at 18 mg/kg bw per day. The finding of brown areas in
the gastric mucosa in most animals at the highest dose at necropsy was
considered to be related to treatment. Pregnancy rates were not
adversely affected, but gravid uterine weights showed a dose-related
reduction at all doses. As the litter size also showed a dose-related
reduction, the reduction in uterine weight might reflect the smaller
litter sizes. Fewer implantations were found in all treated groups,
with the fewest at 18 mg/kg bw per day; the mean number of resorptions
was slightly increased at 10 and 18 mg/kg bw per day, and the mean
incidence of resorptions calculated on a per litter basis was markedly
increased at these doses. Treated groups had fewer live fetuses per
litter, and the fetal viability index calculated on a per litter basis
was decreased at 10 and 18 mg/kg bw per day. The mean fetal body
weights were reduced at the highest dose.
Increased incidences of visceral and skeletal malformations were
seen at doses > 6 mg/kg bw per day. One fetus at 10 mg/kg bw per
day had an enlarged, domed head (hydrocephaly), resulting in an
incidence of 1.6%, and two fetuses from the same litter at 18 mg/kg bw
per day also had hydrocephaly, resulting in an incidence of 9%; the
incidence in controls was 0% and that in historical controls was
0.11%. One fetus at the highest dose had an incomplete diaphragm
(incidence, 5%; 0% in controls, 0.04% in historical controls) and
other visceral alterations such as small lungs and kidneys. The
treatment-related skeletal variants included delayed ossification of
the skull and bone alignment of sternebrae. Skull closure that was
only 75% complete was seen more frequently at doses > 6 mg/kg bw
per day, resulting in incidences of 12%, 13%, and 10%, respectively,
with an incidence of 4% in the concurrent control group and in animals
at 2 mg/kg bw per day. The absence of a doserelated increase at the
highest dose might be a consequence of the small number of fetuses
available for examination (21 compared with 62-115 in the other
groups). The historical incidence for 75% skull closure was 5.7%.
Malaligned or fused sternebrae were found at incidences of 0% in
controls and 2%, 3%, 8%, and 0% at the four doses, respectively, with
a historical control incidence of 1.8%. The NOAEL for maternal and
fetal toxicity was 2 mg/kg bw per day (Serota et al., 1983).
Groups of 25 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by gavage at
doses of 0, 2, 4, 6, 8, or 10 mg/kg bw per day on days 7-19 of
gestation. The fetuses were removed surgically on day 29 and examined
for teratological changes. Dams at 8 and 10 mg/kg bw per day showed
signs of toxicity during the treatment period, including reduced
body-weight gain, and body-weight loss was observed during the second
half of the treatment period at 8 mg/kg bw per day and throughout
treatment at 10 mg/kg bw per day; however, the body weights at the end
of gestation were not significantly reduced. Three females at 4 mg/kg
bw per day, one at 8 mg/kg bw per day, and four at 10 mg/kg bw per day
aborted between days 18 and 25 of gestation. The toxicological
significance of the abortions at 4 and 8 mg/kg bw per day is
questionable because of the lack of a dose-response relationship, but
the abortions at 10 mg/kg bw per day are considered to be related to
treatment as they were accompanied by signs of systemic toxicity in
the dams. Fetal viability, fetal body weights, the mean numbers of
pre- and postimplantation losses, and the total numbers of
implantations and corpora lutea were comparable in all groups,
including controls. Higher incidences (on fetal and litter bases) of
fused sternebrae were found at 8 and 10 mg/kg bw per day, with
incidences of 0/0 % (fetuses/litters), 2/7%, 0.8/6%, 0/0%, 2/11%, and
8/38% at the 0, 2, 4, 6, 8, and 10 mg/kg bw per day, respectively.
This malformation is reported to occur spontaneously at an incidence
of up to about 5%. The NOAEL for maternal and developmental toxicity
was 6 mg/kg bw per day (Schardein, 1989).
(f) Special studies: Cell proliferation
In the 2-year study in CD rats of Trutter (1991), described
above, treatment with propargite resulted in a statistically
significant increase in the incidence of undifferentiated sarcomas in
the jejunum in animals of each sex given dietary concentrations
> 400 ppm. In order to investigate the underlying mechanism and to
establish a NOAEL for the relevant parameter, comparative studies were
conducted in CD rats and CD-1 mice over 1 or 4 weeks. Male rats were
given technical-grade propargite in the diet at 0 or 800 ppm or 0 or
80 ppm, female rats were given diets containing 0, 40, or 800 ppm, and
male mice received diets containing 0 or 1000 ppm. The groups
consisted of 12 controls and 22 treated animals. Body weight, food
consumption, and clinical observations were recorded weekly during the
study. Osmotic pumps containing 5-bromo-2'-deoxyuridine (BrdU), which
is incorporated into the DNA of replicating cells and used to detect
proliferating cells by immunohistochemistry, were placed
subcutaneously in half of the animals on day 1 or 20 of the study, and
the animals were killed and necropsied 1 week after implantation. All
animals were processed for immunohistochemistry and histopathology
(haematoxylin and eosin staining). Sections of the jejunum were
collected to determine cell proliferation in three layers of smooth
muscle. Cells that incorporated BrdU were identified by the presence
of chromagen over their nuclei, and cell proliferation was expressed
as unit length labelling index (number of labelled cells per square
millimetre). Positive staining in the epithelium of the duodenum was
considered to indicate systemic delivery of BrdU.
The body weights of rats receiving 800 ppm were reduced,
especially in males, and food consumption was lower. The body weights
and food consumption of the mice were not affected. After 1 week,
increased total smooth muscle cell proliferation was observed at 800
ppm in male and female rats, and the response was statistically and
biologically significant, the latter being defined as a twofold or
greater increase in cell proliferation in treated animals when
compared with controls. No cell proliferation was induced at lower
doses in rats, and no cell proliferation occurred in male mice at 1000
ppm. No biologically significant increase in cell proliferation was
found after 4 weeks of treatment, although male rats at 800 ppm showed
a statistically significant increase. The NOEL for cell proliferation
was 40 ppm, equal to 2 mg/kg bw per day (Eldridge, 1994).
A similar study was conducted to investigate a possible strain
specificity of the cell proliferative response. Groups of 11 Wistar
(WKY) rats of each sex received technical-grade propargite (purity,
88.6%) in the diet at a concentration of 900 ppm for 1 week, while six
animals of each sex received the diet alone. On the first day of the
study, osmotic pumps containing BrdU were placed subcutaneously, and
the rats were killed and necropsied 1 week later. All animals were
processed for immunohistochemistry and histopathology as described
above. Body weight, food consumption, and clinical observations were
recorded at the end of treatment. Body-weight gain and food
consumption were decreased in rats of each sex throughout the study.
No biologically significant increase in cell proliferation was
observed, and a statistically significant increase was found only in
the outer layer of the smooth muscle of the jejunum in female rats.
This isolated finding was not considered to be biologically
significant, and the combined results for all three layers did not
attain statistical significance. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in any animal. These
results are consistent with the lack of tumorigenic response in male
and female Wistar rats treated with propargite at 900 ppm for 2 years
(Eldridge, 1995; Oser, 1966).
Groups of 10 Charles River CD rats of each sex received
technical-grade propargite in the diet at a concentration of 0 or 400
ppm for 1 week. On the first day, all rats received osmotic pumps
containing BrdU. The animals were observed for clinical signs, body
weight, and food consumption. On day 7, the animals were killed, and
the jejunum and the duodenum were examined macroscopically and
microscopically. No clinical signs and no effect on body-weight gain
or food consumption were seen. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in the jejunum.
Statistically and biologically significant increases in cell
proliferation were found in jejunal smooth muscle in males and females
(Goldenthal, 1999).
Groups of CD rats were given diets containing propargite (purity,
93%) at doses up to 800 ppm, according to the following design, with
evaluations of cell proliferation and histopathological changes after
4, 8, 12, 16, and 20 months. Groups of 90 males were given diets
containing concentrations of 0 or 800 ppm, equal to 0 and 42 mg/kg bw
per day; 6 weeks later, groups of five males were given concentrations
of 0, 80, or 400 ppm, equal to 0, 4, and 21 mg/kg bw per day, and
groups of 55 females were given concentrations of 0, 40, 400, or 800
ppm, equal to 0, 6, 28, and 55 mg/kg bw per day. Each rat was observed
twice daily for death and signs of toxicity. Clinical examinations
were conducted once weekly, and body weights and food consumption were
recorded weekly for the first 16 weeks and every 4 weeks thereafter.
Ten males at 0 and 800 ppm were killed after 4, 8, 12, 16, and 20
months, and the first 10 rats in the remaining groups were killed
after 4, 12, and 20 months. One week before scheduled necropsy,
osmotic pumps containing BrdU were implanted subcutaneously into the
backs of the animals. Standard immunohistochemical methods were used
to stain tissues for BrdU, and staining was evaluated by nuclear
labelling. Three smooth muscle layers were evaluated. Jejunal tissues
stained with haematoxylin and eosin, serial to those stained for BrdU,
were examined for histopathological changes. Ten animals in each group
were killed 6 or 7 days after implantation of the pumps. Sections of
jejunum, ileum, duodenum, and stomach were collected at the 4,- 12-,
and 20-month interim sacrifices, while only sections of jejunum and
duodenum were collected at the 8- and 16-month sacrifices. The
remaining tissues from each animal and the carcass were discarded
without further necropsy, and no further microscopic evaluation was
conducted.
The survival of control and treated animals was similar, and no
treatment-related clinical signs were observed. Body weights were
decreased by 17% in males and 13% in females at 800 ppm, and a
statistically significant decrease in body weight was observed in
males at 400 ppm, which did not result in a significant overall
reduction in body weight at the end of the study; no effect on body
weights was observed in females at this dose. The average food
consumption was reduced in animals at 800 ppm and sporadically in
those at 400 ppm. No treatment-related macroscopic changes were found
in animals killed at 4, 8, 12, or 16 months, but treatment-related
jejunal masses or nodules were observed at 20 months in males at 400
and 800 ppm and in females at 800 ppm. No statistically significant
increase in cell proliferation was detected in the smooth muscle of
the jejunum of rats treated for up to 20 months, but at that time an
at least twofold increase in cell proliferation was seen in male rats
given 800 ppm. At 4, 8, and 12 months, less cell proliferation was
seen in males at 800 ppm than in controls. These findings are
consistent with the reduced total number of cells at the same times.
At 16 months, the difference in the total number of cells between
controls and males at 800 ppm began to decrease, resulting in similar
cell proliferation in the two groups, and by 20 months both cell
proliferation and the total number of cells were greater in the rats
at the high dose than in controls. This effect appears to be a
compensatory response to apparent suppression of cell turnover in the
smooth muscle cells of the jejunum in these animals, which was first
apparent at 16 months. By 20 months, the smooth muscle cells had
overcompensated for the inhibition in cell turnover, resulting in
increased cell proliferation and total cell number. Although the
increase in cell proliferation was not statistically significant, a
twofold or greater increase in the unit length labelling index is
considered to reflect a cell proliferative response. Histopathological
evaluation revealed no hyperplasia, cytotoxicity, or inflammation in
the jejunum. The NOAEL for systemic toxicity was 80 ppm, equal to 4
mg/kg bw per day, on the basis of slight effects on body-weight gain
and jejunal masses at 400 ppm. The NOAEL for cell proliferation was
400 ppm, equal to 21 mg/kg bw per day (Goldenthal, 1998).
The results of these studies suggest that sustained cell
proliferation is not an etiological factor in tumour formation at the
jejunum. Furthermore, no lesions indicative of toxicity were
identified as precursors of tumour formation. The results suggest a
mitogenic mode of action rather than a cytotoxic action, and this
conclusion is supported by the species- and strain-dependent
differences in the carcinogenicity of propargite and the corresponding
species- and strain-dependent differences in jejunal cell
proliferative response.
3. Observations in humans
Propargite is an irritant in humans and may also be a sensitizer,
as several outbreaks of dermatitis were observed in field workers
exposed to this compound (Lee et al., 1981). An outbreak of poisoning
and dermatitis was seen among workers exposed to a formulation of
propargite in Japan in 1970: after use of the miticide on orange
trees, 40 out of 47 workers developed dermatitis and 43 showed signs
of noncutaneous effects, including irritation of the respiratory
tract. Dermatitis was also observed in mixers, loaders, and field
workers in California, USA. Outbreaks of dermatitis in subsequent
years have been reviewed (O'Malley, 1997). The environmental half-time
of propargite of 5-11 days may allow prolonged residual action which
might be responsible for the outbreaks of dermatitis in field workers
(Abrams et al., 1991).
Comments
The results of various studies of the pharmacokinetics of single
oral doses of propargite in CD rats showed that gastrointestinal
absorption was inversely related to the administered dose. After
administration of a single oral dose of [14C-phenyl]ring-labelled
propargite to rats, 20-50% of the administered dose was excreted in
the urine and 40-75% in faeces; about 1.5% of the administered dose
was found in tissues, with slightly higher urinary excretion and
correspondingly less faecal elimination in males. Roughly similar
results were found in CD mice treated in the same way.
In rats, dermal absorption of propargite occurred mainly within
the first 4 h after application. The absorption amounted to up to 33%
of the administered dose of technical material and 3-17% of the
administered doses of various formulations.
The metabolism of propargite has been elucidated in a series of
studies in rats and mice. The compound was rapidly degraded to polar
metabolites, metabolism of the cyclohexyl ring predominating. Most of
the radiolabel in rat faeces was associated with unchanged parent
compound and with a few metabolites, which were also found in urine.
In comparative studies in rats and mice, no parent compound was found
in the bile of either species, whereas the plasma contained small
amounts of unchanged propargite; six metabolites were found in rat and
mouse bile. The metabolites are formed as a result of hydrolysis of
the propynyl sulfite side-chain, subsequent oxidation and conjugation
of the tert-butyl moiety, and hydroxylation of the cyclohexyl
moiety. No consistent quantitative or qualitative species differences
in bile and plasma metabolite profiles were found.
In further studies of the metabolism of propargite with a
radiolabel located in the propynyl sulfite side-chain and with
radiolabelled propargyl alcohol, an additional metabolic pathway was
identified which involves metabolism of the side-chain by glutathione
conjugation.
Propargite is of low acute toxicity, with an oral LD50 in rats
of 2800 mg/kg bw, but it irritates the skin and eyes. Propargite did
not show dermal sensitizing potential in the Buehler test in
guinea-pigs. WHO (1999) has classified propargite as 'slightly
hazardous'.
In short-term studies in mice, rats, and dogs, the signs of
systemic toxicity included effects on body weight and on
haematological and clinical chemical parameters. In a study in which
CD rats were fed propargite for three months, the NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day. In dogs, dietary administration of
propargite for 1 year resulted in a NOAEL of 160 ppm, equivalent to 4
mg/kg bw per day, on the basis of effects on body weight and on
various haematological parameters and histopathological changes in the
thymus and bone marrow. In a 21-day study in rabbits treated dermally,
the NOAEL for systemic toxicity was < 100 mg/kg bw per day, whereas
no NOAEL for local irritation was found.
In long-term studies, the most significant toxicological finding
was the occurrence of jejunal sarcomas in CD (Crl:CDBR) rats, whereas
no carcinogenic effect was observed in CD-1 mice or Wistar (FDRL)
rats. In a 78-week study in CD-1 mice carried out in 1979, the NOAEL
was 50 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
changes in organ weights. In the study in Wistar (FDRL) rats carried
out in 1966, the NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day,
also on the basis of changes in organ weights.
In a two-year study reported in 1991 in which CD (Crl:CDBR) rats
were given diets containing propargite at concentrations of 0, 50, 80,
400, or 800 ppm (equal to 0, 2, 4, 19, and 39 mg/kg bw per day in
males and 0, 3, 5, 24, and 49 mg/kg bw per day in females), males at
400 and 800 ppm showed a dose-related increase in the incidence of
undifferentiated sarcomas in the jejunum, a very rare tumour. Females
also showed a clear tumorigenic response but with a different
dose-response relationship, since a high incidence (21%) of jejunal
tumours in the smooth muscle was observed at the highest concentration
of 800 ppm but low incidences (one animal in each group) at 50, 80,
and 400 ppm. Given the rarity of sarcomas arising at this site (0% in
concurrent and historical female controls, 0% in concurrent male
controls, and 0.2% in historical male controls), a NOAEL for
tumorigenicity could not be identified in this study. In a 20-month
study reported in 1998 in which CD rats were given dietary
concentrations of 0, 80, 400, or 800 ppm (equal to 0, 4, 21, and 42
mg/kg bw per day) for males and 0, 40, 400, or 800 ppm (equal to 0, 6,
28, and 55 mg/kg bw per day) for females, the appearance of jejunal
masses at 400 and 800 ppm in males and at 400 ppm in females suggested
that the tumorigenic response is a reproducible effect. Reduced cell
proliferation in jejunal smooth muscle layers and decreased jejunal
cell division were found at various times during the study. After 20
months, cell division and cell proliferation were found to be
increased only in males at 800 ppm, with no corresponding response in
females or in males at lower doses. No hyperplasia, cytotoxicity, or
inflammation was found in the jejunal epithelium.
Several short-term (1 or 4 weeks) studies of cell proliferation
were conducted in male and female CD rats at concentrations of up to
800 ppm, in Wistar rats (WKY strain) at 900 ppm, and in male CD-1 mice
at 1000 ppm. The NOAEL for cell proliferation in the CD rat was 40 ppm
(equal to 2 mg/kg bw per day) in females and 80 ppm (equal to 4 mg/kg
bw per day) in males, both being the lowest doses tested. A
proliferative response was observed at 800 ppm in both male and female
rats after 1 week of treatment, whereas the response was observed only
in males after 4 weeks of treatment at this concentration; no cell
proliferation was observed at 40 and 80 ppm. No cell proliferation was
found in Wistar rats (WKY) treated with 900 ppm or in CD-1 mice at
1000 ppm in the same study design.
The results of the long-term studies in CD rats and the
short-term studies of cell proliferation indicate that the cell
proliferation induced by propargite in the jejunum is characterized by
an initial transient proliferative response, lasting for at least 4
weeks in males and for only about 1 week in females. This profile of
cell proliferation suggests that the underlying mechanism for tumour
formation in the jejunum of CD rats may be related to the mitogenic
activity of the compound.
The observed dose-response relationship for tumour formation,
with a greater increase in tumour incidence in males than in females,
correlates with the duration and degree of jejunal cell proliferation.
The prolonged duration of cell proliferation in male rats was
associated with a more pronounced tumorigenic response than in
females. Furthermore, propargite did not induce cell proliferation in
species and strains in which no carcinogenic activity was observed.
The available database does not further illuminate the causal
relationship between cell proliferation and tumorigenicity, and no
explanation was offered of the species, strain, and sex specificity or
of the association between the presence of an early, transient cell
proliferation response and the occurrence of jejunal tumours in CD
rats and the consistent absence of these findings in Wistar rats and
CD-1 mice. Nevertheless, the association between proliferation and
tumorigenic activity was recognized by the Meeting. The Meeting also
noted that the NOAEL for cell proliferation of 40 ppm is very close to
the lowest tumorigenic concentration in female rats of 50 ppm. The
available database did not, however, clarify the dose-response
relationship found at low tumorigenic doses in females and offered no
further scientific evidence to support use of cell proliferation as a
marker of potential carcinogenicity.
An adequate range of studies for genotoxicity was conducted, and
the results were consistently negative. Therefore the Meeting
concluded that propargite is not genotoxic. The finding of
consistently negative results in numerous tests for genotoxicity
provides further support for the conclusion that propargite causes
tumours by a non-genotoxic mechanism.
In a three-generation study of reproductive toxicity, reduced
body-weight gain was seen at concentrations of 400 ppm (equivalent to
20 mg/kg bw per day) and above in parental animals and in pups during
lactation. The results of a subsequent cross-fostering study showed
that the growth retardation of the pups was reversible and was due to
maternal toxicity. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per
day, on the basis of reduced body-weight gain in parental animals and
pups.
Two studies of developmental toxicity were conducted in rats and
two in rabbits. In a study in Sprague-Dawley rats reported in 1979,
fetal effects such as increased incidences of incomplete vertebral
ossification and missing sternebrae and hyoids were observed in
treated animals. Not all of the findings were dose-related and they
were not reproduced in a study reported in 1990. The NOAEL was 25
mg/kg bw per day on the basis of reduced body-weight gain in dams and
a slight increase in the postnatal mortality rate at the highest dose
in the latter study. In the two studies in rabbits, dated 1983 and
1989, fetal effects indicative of developmental retardation were
observed at maternally toxic doses of 6 mg/kg bw per day and above,
and an increased incidence of hydrocephaly was seen at higher doses in
the first study. Most of the fetal effects, including hydrocephaly,
were not confirmed in the second study, in which effects were observed
only at doses of 8 mg/kg bw per day and higher. The overall NOAEL in
rabbits was 4 mg/kg bw per day. No evidence for teratogenicity was
found in these studies.
The Meeting allocated an ADI of 0-0.01 mg/kg bw on the basis of
the LOAEL of 50 ppm (equal to 3 mg/kg bw per day) for tumorigenicity
in female CD rats, with a safety factor of 300. This safety factor was
chosen to account for the lack of a NOAEL in this study and the nature
of the end-point (tumorigenesis) and to encompass the NOAEL in the
same rat strain for increased cell proliferation in the jejunum, the
site of tumour formation.
The Meeting concluded that it was unnecessary to determine an
acute reference dose because of the low acute toxicity of propargite.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (effects on organ
weights in a 78-week study of toxicity and carcinogenicity)
Rat: < 50 ppm, equal to 3 mg/kg bw per day (lowest concentration
tested; tumorigenicity in a 2-year study)
80 ppm, equivalent to 4 mg/kg bw per day (maternal and fetal
toxicity in a three-generation study)
25 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Rabbit: 4 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Dog: 160 ppm, equivalent to 4 mg/kg bw per day (toxicity in a
1-year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Further studies on the mechanism of tumorigenic activity
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to propargite
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid and incomplete (50% in rats and mice)
Dermal absorption 30%, rats
Distribution Highest concentrations in intestine, liver (rats and mice)
Potential for accumulation None
Rate and extent of excretion Rapid excretion in rats and mice (urinary, approximately 50%;
faecal, 50%)
Metabolism in animals Rapid degradation to numerous polar metabolites, no parent
compound in bile or urine; hydrolysis of propynyl sulfite
side-chain and subsequent oxidation of tert-butyl moiety and
hydroxylation of cyclohexyl moiety, conjugation
Toxicologically significant compounds Parent compound; animal and plant metabolites (animals, plants,
and environment) similar
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 4000 mg/kg bw
Rat, LC50, inhalation 0.89 mg/L (4 h)
Dermal irritation Irritating to rabbit skin
Ocular irritation Irritating to rabbit eye
Dermal sensitization Not sensitizing in guinea-pigs (Buehler test)
Short-term toxicity
Target/critical effect Haematological system
Lowest relevant oral NOAEL Dog: 1 year, 160 ppm (4 mg/kg bw per day)
Lowest relevant dermal NOAEL Rabbit: 21 days, < 100 mg/kg bw per day (systemic toxicity)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Intestine (jejunal tumours in CD rats), haematological system
Lowest relevant NOAEL Rat (CD): 50 ppm (females; 3 mg/kg bw per day), 2-year study
Carcinogenicity Carcinogenic in CD rats but not in FDRL rats or
CD-1 mice
Reproductive toxicity
Reproductive target/critical effect Reduced pup weight at maternally toxic doses
Lowest relevant reproductive NOAEL Rat: 80 ppm (4 mg/kg bw per day), three-generation study
Developmental target/critical effect Rat: fetotoxicity at maternally toxic doses
Rabbit: fetotoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rabbit: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Other toxicological studies Transient cell proliferation in jejunal smooth-muscle cells
in CD rats
Medical data Dermatitis in field workers
Summary Value Study Safety factor
ADI 0-0.01mg/kg bw CD rats, 2-year study 300
Acute RfD Unnecessary
References
Abrams, K., Hogan, D.J. & Maibach, H.I. (1991) Pesticide-related
dermatoses in agricultural workers. Occupational Medicine:
State of the Art Reviews, Vol. 6, pp. 463-491.
Andre, J.C. & Laveglia, J. (1994) Pharmacokinetics of 14C-Omite: A
comparative study in rats and mice, Part II, elimination of
14C-Omite equivalents in bile following oral administration.
Unpublished report No. 93-0284 from Ricerca, Inc., Department of
Toxicology and Animal Metabolism, Painesville, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1989) Study to
determine if rats expire radiolabel in air after oral
administration of 14C-Omite. Unpublished report No.
3374-89-0211-AM-001 from Ricerca, Inc., Department of Toxicology
and Animal Metabolism, Painesville, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990a) Study of the
dermal absorption of technical 14C-Omite by male Sprague-Dawley
rats. Unpublished report No. 3452-89-0308-AM-001 from Ricerca,
Inc., Department of Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990b) Study of the
dermal absorption of 14C-Omite-6E by male Sprague-Dawley rats.
Unpublished report No. 3451-89-0307-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990c) Study of the
dermal absorption of 14C-Comite by male Sprague-Dawley rats.
Unpublished report No. 3450-89-0306-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Atkinson, J.E. (1991) A chronic (1 year) oral toxicity study in the
dog with Omite via the diet. Unpublished report No. 88-3377 from
Bio/dynamics, Inc., New Jersey, USA. Submitted by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1989a) Identification of 14C-Omite urinary
metabolite in rats. Part 2: Identification of the sixth
metabolite of Omite in female rat urine. Unpublished report No.
8706 from Uniroyal Chemical Co., Inc., Middlebury, Connecticut,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Banijamali, A.R. (1989b) Identification of 14C-Omite metabolites in
a lactating goat. Unpublished report No. 8869 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1998) Identification of metabolites of [1,2,3-13C]
propargyl alcohol in rat urine by 13C NMR and mass
spectrometry. Unpublished report No. 96038 Part I from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. (1999) Identification of metabolites of
[1,2,3-13C,2,3,-14C-propargyl]propargite in male
Sprague-Dawley rats. Unpublished report No. 98156 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Lau, R.C.M. (1996) 14C-Propargite nature of the
residue in lactating goat. Unpublished report No. 95157 (Uniroyal
Project)/ No. 95123g Southwest Bio-Labs from Uniroyal Chemical
Co. Inc., Middlebury, Connecticut, USA and South West Bio-Labs,
Inc., Las Cruces, New Mexico, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Nag, J.K. (1990) The identification of Omite
metabolites in rats; Amendment No. 4: Fecal metabolism of Omite.
Unpublished report No. 8706 from Uniroyal Chemical Co. Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co. Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. &Nag, J.K. (1991) Identification of [phenyl-U-14C]
Omite fecal metabolites in mice and comparison to rat's.
Unpublished report No. 9107 from Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Tortora, N.J. (1988a) Identification of 14C-Omite
urinary metabolite in rats. Unpublished report No. 8706 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co. Inc., Middlebury, USA.
Banijamali, A.R. & Tortora, N.J. (1988b) Comparison of subchronic vs
single oral dose in the disposition and metabolism of Omite in
rats. Unpublished report No. 87122 from Biotek, Inc., Woburn,
Massachusetts (Project No. 8719), Hazleton Laboratories America,
Inc., Madison, WIisconsin (Project No. 8721) and Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R., Kratky, E. & Gay, M.H. (1994) Pharmacokinetics of
14C-Omite: a comparative study in rats and mice - Part III -
Analysis. Unpublished report No. 9395 from Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Barfknecht, T.R. (1987) Rat hepatocyte primary culture/DNA repair
test. Unpublished report No. PH 311-UN-001-87 from Pharmakon
Research International, Inc., Pennsylvania, USA. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Becci, P.J. (1980) Supplementary pathology report for chronic
oncogenic evaluation of Omite in CD-1 mice. Unpublished report
No. 5036 from Food and Drug Research Laboratories, Inc., New
York, USA. Submitted to WHO by Uniroyal Chemical, Inc., Bethany,
Connecticut, USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (DMSO). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (acetone). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Brusick, D.J. & Weir, R.J. (1977) Mutagenicity evaluation of DO14.
Unpublished report No. 2683 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by Uniroyal Chemical,
Bethany, Connecticut, USA.
Butterworth, B.E. (1991) Chemically induced cell proliferation as a
predictive assay for potential carcinogenicity. In: Butterworth,
B.E., Slaga, T.J., Farland, W. & McClain, M., eds, Chemically
Induced Cell Proliferation: Implications for Risk Assessment, pp.
457-467.
Byrd, J.W. (1988) 14C-Omite goat metabolism study. Unpublished
report No. 8734g from Southwest Bio-Labs, Inc., Las Cruces, NM,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Carson, S. (1964) Subacute feeding studies with D-014 in rats.
Unpublished report No. 85603 from Food and Drug Research
Laboratories, Inc., New York, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Cox, G.E. & Re, T.A. (1979) Chronic oncogenic evaluation of Omite in
CD-1 mice following 78-weeks of dietary treatment. Unpublished
report No. 5036 from Food and Drug Research Laboratories, Inc.,
New York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Eldridge, S. (1994) Effect of dietary Omite technical on cell
proliferation in jejunum of rats and mice. Unpublished report No.
6030-10 (ManTech), 93-45 and 93-131 (PAI) from Pathology
Associates, Inc., Durham, North Carolina, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Eldridge, S. (1995) Effect of dietary Omite technical on cell
proliferation in jejunum of Wistar rats. Unpublished report No.
6030-017 (ManTech), 94-91 (PAI) from Pathology Associates
International, Durham, North Carolina, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gallo, M.A. & Bailey, D.E. (1976) Range finding study with Omite in
albino mice. Unpublished report No. 5028 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to WHO by
Uniroyal Chemical, Inc., Bethany, Connecticut, USA.
Gay, M.H. (1987) Rat metabolism of 14C-Omite. Final report.
Unpublished report No. 87002 from Biotek, Inc., Woburn,
Massachusetts, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Gay, M.H. (1994) Pharmacokinetics of 14C-Omite: a comparative study
in rats and mice (overview). Unpublished report No. 9395 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc.
Goldenthal, E.I. (1989) 21-day repeat dose dermal toxicity study in
rabbits. Unpublished report No. IRDC 399-098 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1998) Twenty month dietary toxicity study in rats
with Omite. Unpublished report No. 399-187 from MPI Research,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Goldenthal, E.I. (1999) Effect of one week dietary administration of
Omite technical (400 ppm) on jejunal cell proliferation in
Sprague-Dawley rats. Unpublished report No. 399-206 from MPI
Research, Inc., Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
propargite in the rat. Unpublished report No. 91-8372 from
Bio/dynamics, Inc., New Jersey, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Holsing, G.C. & Kundzins, L. (1968) 13-Week dietary feeding study-dogs
with D-014. Unpublished report No.798-140 from Hazleton
Laboratories, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Incorporated, Bethany, Connecticut, USA.
Johnson, J.D. (1990) Metabolism of 14C-Omite in rats: Dosing, sample
collection and total 14C-residue determinations. Unpublished
report Uniroyal No. 8998, Battelle No. NO967-2700 from Battelle
Columbus Division, Columbus, Ohio, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Kehoe, D.F. (1988) Subchronic toxicity and kinetic study with Omite
technical in rats. Unpublished report No. HLA 6111-107 from
Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kehoe, D.F. (1990) Two-generation reproduction study with Omite
technical in rats (two litters per generation). Unpublished
report No. HLA 6111-108 from Hazleton Laboratories America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Uniroyal Chemical
Co., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993a) Acute oral toxicity study in albino rats with
Omite Technical. Unpublished report No. WIL-155012 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993b) Acute dermal toxicity study in albino rabbits
with Omite technical. Unpublished report No. WIL-155013 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993c) Primary dermal irritation study in albino
rabbits with Omite technical. Unpublished report No. WIL-155014
from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kiplinger, G.R. (1993d) Primary eye irritation study in albino rabbits
with Omite technical. Unpublished report No. WIL-155015 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993e) Skin sensitization study in albino guinea pigs
with Omite technical. Unpublished report No. WIL-155016 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co. Inc., Bethany, Connecticut, USA.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of D-014 by its effects on cultured chinese hamster
ovary (CHO) cells using an in vitro cytogenetics assay.
Unpublished report No. URC1/CHO/AR/KF14/CH3 from Microtest
Research Lim, York, United Kingdom. Submitted to WHO by Uniroyal
Chemical Co., Bethany, Connecticut, USA.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of Omite
technical in Sprague-Dawley rats. Unpublished report No. 5992 (b)
from Food and Drug Research Laboratories, Inc., Maspeth, New
York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Knipe, J.O. (1986) The disposition and metabolism of 14C-Omite in
male and female rats. Unpublished report No. 8589 from Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Knipe, J.O. (1987) Progress report. The metabolism of 14C-Omite in
rats. Unpublished report No. 8706 from Uniroyal Chemical Co.,
Inc., Naugatuck, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Lawlor, T.E. (1991) Mutagenicity test on Omite ESO in the
Salmonella/reverse mutation assay (Ames test) preincubation
method. Unpublished report No. 12514-0-420 from Hazleton
Washington, Inc., Kensington, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Lee, S.L., Chin, Y.W. & Kim, J.S. (1981) A study on hypersensitivity
of Korean farmers to various agrochemicals. Determination of
concentration for patch-test of fruit-tree agrochemicals and
hypersensitivity of orange orchard farmers in Che-ju Do, Korea.
Seoul Med. J., 22, 137-142.
Mahon, C.R. (1993) Comparative metabolism of 14C-propargyl-Omite in
rats and mice. Unpublished report No. 91188 (Uniroyal Study
No.)/No. SC 910217 (Battelle Study No.) from Battelle Laboratory,
Columbus, Ohio, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA.
Mizens, M., Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990)
Study of the dermal absorption of 14C-Omite-30W by male
Sprague-Dawley rats. Unpublished report No. 3449-89-0305-AM-001
from Ricerca, Inc., Dept. Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
O'Malley, M.A. (1997) Skin reactions to pesticides. Occupational
Medicine: State of the Art Reviews, Vol. 12, pp. 327-345.
Oser, B.L. (1966) Chronic (2-year) feeding studies with D-014 in rats
and dogs. Unpublished report No. 86000/86014 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to
Naugatuck Chemical, Connecticut, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Putman, D.L. & Young, R.R. (1994) Micronucleus cytogenetic assay in
mice. Unpublished report No. G94AP36.122 from Microbiological
Associates, Inc., Rockville, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Sabourin, P.J., Trigg, N.J., Mahon, C.R. & Johnson, J.D. (1994)
Pharmacokinetics of 14C-Omite: A comparative study in rats and
mice - Part I - 14C-Omite equivalents in blood following oral
and intravenous administration. Unpublished report No. SC930180
from Battelle Laboratory, Columbus, Ohio, USA. Submitted to WHO
by Uniroyal Co., Inc., Middlebury, Connecticut, USA.
Schardein, J.L. (1989) Developmental toxicity study in New Zealand
white rabbits. Unpublished report No. 399-097 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
Schardein, J.L. (1990) Developmental toxicity study in rats.
Unpublished report No. 399-096 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Serota, D.G., Wolfe, G.W., Durloo, R.S. & Fezio, W.L. (1983)
Teratology study in rabbits Omite technical, revised final
report. Unpublished report No. 798-195 from Hazleton Laboratories
America, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Chemical, Bethany, Connecticut, USA.
Shirasu, Y., Moriya, M. & Watanabe, K. (1979) Microbial mutagenicity
testing on BPPS (Propargite). Unpublished report from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Uniroyal Chemical, Bethany, Connecticut, USA.
Trela, B.A. (1991) Metabolism of 14C-Omite in mice: Dosing, sample
collection, and 14C-residue determinations. Unpublished report
No. SC910015 from Battelle Columbus Division, Columbus, Ohio.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Trutter, J.A. (1991) Combined chronic toxicity and oncogenicity study
in rats with Omite technical. Unpublished report No. HLA/HWA
798-220 from Hazleton Laboratories America, Inc., Virginia, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety. York, R.G.
(1992) A cross-fostering reproduction study with Omite technical
in rats. Unpublished report No. 399-113 from International
Research and Development Corporation, Matttawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
female rats and male mice were
1-[4-(1,1-dimethyl-2-hydroxyethyl)phenoxy]-2-cyclohexanol (HOMe-TBPC)
and the corresponding diol derivative carboxy-TBPC-diol). No
consistent qualitative or quantitative species differences were found
in the metabolite profiles of propargite in bile and plasma
(Banijamali et al., 1994).
In another comparative study, the metabolites found in the faeces
of male and female CD-1 mice treated with [14C-phenyl]propargite
were characterized and compared with those identified previously in
the faeces of male and female rats (Banijamali & Nag, 1990; Johnson,
1990). The mice were given a single oral dose of 150 mg/kg bw of the
compound by gavage, whereas the rats received a single oral dose of
200 mg/kg bw (Johnson, 1990). Male mice excreted 42% of the
administered dose in the faeces and female mice about 53%, while in
rats faecal excretion accounted for 75% of the administered dose in
males and 70% in females. Peak faecal excretion of radiolabel was
observed between 0 and 24 h in both species. The profile of faecal
metabolites of mice and rats was qualitatively similar. The radiolabel
was associated with unchanged parent compound, the hydrolysis product
propargite glycol ether, and the polar metabolites hxdroxylated
tert-butyl and hydroxylated cyclohexyl propargite glycol ether. Rat
faeces contained a substantially higher percentage of unabsorbed
propargite than mouse faeces. The finding that faeces of female mice
contained more propargite (49% of total faecal residue) than faeces of
male mice (25%) suggests that faecal elimination is sex-dependent.
Moreover, male mouse faeces contained a higher percentage of polar
metabolites (56% of total faecal residue) than those of female mice
(36%). Mouse faeces contained both a greater number and a greater
percentage of polar metabolites than rat faeces, indicating more
extensive metabolism of propargite in mice than rats (Banijamali &
Nag, 1991).
Goats
One dairy goat was given [14C-phenyl]propargite at a dose of
675 mg/kg bw per day on 3 consecutive day, during which time urine,
faeces, and milk were collected. The animal was killed 8 h after the
last dose, and liver, kidney, muscle, fat, and bile samples were
collected. The highest concentration of radiolabel was found in bile,
followed by liver, kidney, fat, and muscle. The bile contained 0.29%
of the administered dose, urine 16%, faeces 14%, and milk 0.1%.
Overall, 34% of the administered dose was recovered; the remaining
radiolabel was presumed to have remained in the gastrointestinal tract
(Byrd, 1988). HPLC analysis revealed metabolism of the cyclohexyl and
tert-butyl group resulting in a number of polar metabolic products,
including carboxy-TBPC-diol, carboxy-TBPC,
1-[4-(1,dimethylethyl)-phenoxy]-2,x-cyclohexanediol, and TBPC in milk
and tissues. Small quantities of unchanged propargite were found in
milk, fat, and liver (Banijamali, 1989b).
After oral administration of [14C-phenyl]propargite in capsules
at doses of 65 or 325 mg/kg bw per day for 3 days to two lactating
goats, metabolites were identified in milk and edible tissues. The
liver contained 14 metabolites, kidney 12, muscle 9, milk 7, and fat
6. Seven of the 14 metabolites isolated from liver were glucuronide or
sulfate conjugates. The metabolism of propargite in goats thus appears
to involve hydrolysis of the propynyl sulfite side-chain followed by
aliphatic and/or alicyclic hydroxylation of the tert-butyl methyl
and cyclohexyl groups to form TBPC-diol and HOMe-TBPC, respectively.
These metabolites undergo further oxidation to yield HOMe-TBPC-diol,
carboxy-TBPC, carboxy-TBPC-diol, and carboxy-TBPC-triol. Some of these
metabolites subsequently undergo conjugation to form glucuronides and
sulfates. The results of these studies indicate similar metabolism in
rats and goats (Banijamali & Lau, 1996).
(ii) Propargyl alcohol and propargyl propargite
In order to learn more about the fate of the propynyl sulfite
side-chain of the propargite molecule, the metabolism of propargyl
alcohol, which may be released from propargite, was studied.
[1,2,3-13C-, 2,3-14C]Propargyl alcohol was administered to groups
of eight male Sprague-Dawley rats by gavage at a dose of 40 mg/kg bw,
and samples of urine, faeces, and expired air (four animals) were
collected 24, 48, 72, and 96 h after treatment. The rats were killed
at 96 h. Radiolabel associated with the parent compound represented
56% of the administered dose in urine, 12% in faeces, and 7% in
expired air. These results are consistent with those of another study
of the degradation and expiratory elimination of this compound (Mahon,
1993). Only 4-6% of the administered dose was recovered in the
carcass. Most of the radiolabel was excreted within the first 24 h of
administration in urine and expired air. The peak elimination in
faeces also occurred within 24 h of dosing and continued for 48 h. The
proposed metabolic pathway involves oxidation of propargyl alcohol to
2-propynoic acid and further detoxification by glutathione conjugation
to yield the following final products:
3,3-bis[(2-(acetylamino)-2-carboxyethyl)thio]-1-propanol,
3-(carboxymethylthio)-2-propenoic acid;
2(methylthio)-3-(methylsulfinyl)-2-propenoic acid;
3-{[2-(acetylamino)-2-carboxyethyl]thio}-3-[(2-amino-2-carboxyethyl)
thio]1-propanol; and
3-{[2-(acetylamino)-2-carboxyethyl]-sulfinyl}-3-{[2-(acetylamino)-2-
carboxyethyl]thio}-1-propanol. These metabolites have not been
reported previously and represent the first examples of multiple
glutathione additions to the carbon-carbon triple bond (Banijamali,
1998).
[1,2,3-13C-, 2,3-14C-propargyl]Propargite was administered
orally to male SpragueDawley rats at a dose of 150 mg/kg bw, and urine
and faeces were collected at 24-h intervals until study termination
96 h after dosing. The total radiolabel excreted in urine accounted
for 25% of the administered dose, and that in faeces for 48%. The six
major metabolites identified in rat urine were
3-(carboxymethylthio)-2-propenoic acid,
2-(carboxymethylthio)-2-propenoic acid,
2-(acetyl-amino)-3-(2-propynylthio)propanoic acid,
3-[(2-carboxy-2-hydroxyethyl)thio]-2-propenoic acid,
3- (N-formylglutamylcysteinyl)-2-propenoic acid, and
2- (N-formylglutamylcysteinyl)-2-propenoic acid. These metabolites
were the result of conjugation with glutathione followed by enzymatic
degradation. Seven metabolites were identified tentatively in faeces
(Banijamali, 1999).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity conducted since the
earlier evaluation (Table 1) are consistent with the previous data.
The clinical observations made most frequently after oral
administration included urogenital staining and abnormal defaecation,
hypoactivity, and swollen, red paws. Most of the treated animals lost
weight during a few days after dosing. Those that died had dark-red
areas, thickened mucosa, and red foci in the stomach. All of the
deaths occurred during the second week, with no sex difference
(Kiplinger, 1993a).
After inhalation, the commonest observations were laboured
breathing and various secretory responses. About one-third of the
treated animals lost weight during the first week after exposure, and
treatment-related reddening of the lungs was seen in some animals
found dead or killed at term. There was no sex difference in
lethality. All deaths occurred 1-17 days after exposure (Hoffman,
1992).
After dermal exposure, none of the animals died and all gained
weight during the 24-day observation period. All rabbits showed severe
erythema and oedema, and eschar formation, fissuring, desquamation,
and a white-yellow exudate on the application site appeared during the
second week of the study and persisted until day 14. Thickened skin
and desquamation within the application site were seen on all rabbits
at necropsy (Kiplinger, 1993b).
Male and female New Zealand white rabbits received dermal
applications of 0.5 ml of undiluted technical-grade propargite
(purity, 90.3%) and were observed at 0.5, 24, 48, and 72 h, daily
through day 14, and on day 21, when they were killed. Irritation was
seen during the first few days after application, consisting of
moderate erythema and slight-to-moderate oedema, but on days 5-9
severe erythema and oedema, fissuring, eschar formation, and
desquamation were observed. The severe irritation had decreased to
slight oedema and erythema by day 21, indicating reversibility of the
reaction (Kiplinger, 1993c).
Table 1. Acute toxicity of technical-grade propargite (purity, 90.3%)
Species Strain Sex Route LD50 or LC50 Reference
(mg/kg bw or mg/L)
Rat Crl:CD BR M Gastric intubation 2600 Kiplinger (1993a)
F 2900
Both 2800
Rat Crl:CD BR M Inhalation (4 h) 0.95 Hoffman (1992)
F 0.95
Both 0.89
Rabbit New Zealand white M Dermal (24 h) > 4000 Kiplinger (1993b)
F > 4000
Both > 4000
Male and female New Zealand white rabbits received 0.1 ml of
undiluted technical-grade propargite (purity, 90.3%) into the
conjunctival sac and were observed at 1, 24, 48, and 72 h and on days
4, 7, 10, 14, 17, and 21 after application. Reactions were observed in
the conjunctiva, cornea, and iris. The effects in cornea and iris had
subsided by day 10 or earlier, and the corneal effects had cleared by
day 21 (Kiplinger, 1993d).
In a modified Buehler test, male and female Hartley albino
guinea-pigs received three topical applications of 0.1%
technical-grade propargite (purity, 90.3%) in ethanol for induction, a
challenge dose of 0.2% propargite in acetone) two weeks later, and a
second challenge (0.1 and 0.2% in acetone) after a 1-week
interruption. The applications were left in place for 6 h. A positive
control (dinitrochlorobenzene) and an untreated control group were
included. No evidence of sensitization was seen (Kiplinger, 1993e).
(b) Short-term studies of toxicity
Rats
Propargite was administered in the diet to groups of five rats of
each sex at concentrations of 0 (15 animals), 200, 400, 800, 2000, or
4000 ppm for 90 days. Reduced food consumption and body-weight gain
were observed at the two higher concentrations. Haematological and
clinical chemical parameters (glucose and urea nitrogen) were not
affected by treatment. The relative weights of the liver, kidney,
adrenals, and gonads were increased at the two higher concentrations.
No treatment-related macroscopic or microscopic alterations were
observed (Carson, 1964).
Groups of 10 male and 10 female Crl:CD (SD) BR rats were
maintained on diets containing technical-grade propargite (purity,
87.2%) at concentrations of 0, 100, 1000, or 2000 ppm (equivalent to
0, 5, 50, and 100 mg/kg bw per day) for at least 13 weeks. At the end
of the study, all surviving rats were anaesthetized, weighed, bled,
killed, and necropsied. All tissues from control rats and those at the
highest dose, the lungs, liver, and kidneys from rats at 100 or
1000 ppm, and all macroscopic lesions from all rats were examined
microscopically. No deaths occurred during the study. All animals at
2000 ppm had rough coats throughout the study, and many were thin with
a hunched posture, alopecia, and rhinorrhoea. The body weights of rats
at 1000 and 2000 ppm were significantly lower than those of controls
throughout the study, by 30% at 1000 ppm and 69% in males and 52% in
females at 2000 ppm. The body-weight gains of males were significantly
reduced during week 10 at 100 ppm, during most of the study at
1000 ppm, and throughout the study at 2000 ppm. The significantly
reduced body-weight gain at 100 ppm during week 10 did not result in a
significant reduction in the cumulative body-weight gain at study
termination or in any other adverse effect and was considered not to
be toxicologically relevant. The body-weight gains of females were
significantly lower during weeks 1 and 5 at 1000 ppm and during weeks
1-4 at 2000 ppm. The reduction in the body-weight gain of males at the
end of the study in comparison with controls was 4% at 100 ppm, 30% at
1000 ppm, and 69% at 2000 ppm, and the corresponding reductions in
females were 6%, 31%, and 52%, respectively. The reductions at 1000
and 2000 ppm were significant. The reduced body-weight gain was
associated with a dose-dependent reduction in food consumption at the
two higher doses.
Various haematological and clinical chemical parameters were
altered at the two higher doses, including significantly increased
erthrocyte count and haemoglobin values in males at 2000 ppm and
significantly decreased mean corpuscular volume and mean corpuscular
haemoglobin in males at 1000 and 2000 ppm and females at 2000 ppm. A
non-dose-related but significant increase in platelet count in females
at 1000 ppm and an increased erythrocyte count in males at 100 ppm
were considered to be of no toxicological significance. The blood
glucose concentration was significantly lower in males and females at
1000 and 2000 ppm, and urea nitrogen was significantly higher and
creatinine significantly lower in animals of each sex at 2000 ppm.
Reduced total protein, albumin, and globulin values were found in
males at 2000 ppm and in females at 1000 ppm and 2000 ppm. An
increased albumin:globulin ratio was found in males and females at
2000 ppm. The concentrations of calcium, inorganic phosphorus,
potassium, and chloride showed significant alterations at 2000 ppm.
Most of the changes observed were considered to be associated with the
decreased food consumption and body weight. The absolute weights of
the kidneys and liver were significantly reduced in animals of each
sex at 2000 ppm, and the absolute weight of the testis was reduced in
males at this dose. The relative weights of the kidney, liver, and
testis were increased in a dose-related manner at 1000 and 2000 ppm.
Macroscopic and microscopic examinations did not reveal alterations
attributable to treatment. The NOAEL was 100 ppm, equivalent to 5
mg/kg bw per day, on the basis of effects on body weight and changes
in clinical chemical parameters at higher doses (Kehoe, 1988).
Rabbits
Groups of five New Zealand white rabbits of each sex received
dermal applications of technical-grade propargite (purity, 85%) at
doses of 0, 0.1, 1.0, 10, or 100 mg/kg bw per day, 5 days per week for
3 weeks. No deaths occurred during the study, and no clinical signs
were observed at any dose. Food consumption was slightly reduced at
all doses, and the mean body weightswere slightly reduced in treated
males and slightly increased in treated females, with no clear
dose-response relationship, indicating no consistent treatment-related
effect. Biochemical parameters were not affected by treatment, whereas
haematological investigations revealed a significant increase in the
number of segmented neutrophils at the end of the study in males at
100 mg/kg bw. Signs of dermal irritation were seen at the application
site in all treated groups, with a dose-related increase in incidence
and severity. The signs consisted of erythema, oedema, eschar
formation, exfoliation, atonia, desquamation, fissuring, blanching,
and coriaceousness. The dermal effects occurred earlier with
increasing dose. The observed changes were graded as mild-to-severe in
animals at 10 and 100 mg/kg bw per day, mild-to-moderate at 1 mg/kg bw
per day, and mild at 0.1 mg/kg bw per day. No changes in organ weights
were observed, and no additional macroscopic changes were seen.
Dose-related microscopic changes were confined to the application
site, which were in the incidence and severity of acanthosis and
hyperkeratosis. The incidence of dermal inflammation was similar in
all treated groups, and necrosis was observed at doses > 1 mg/kg bw
per day. The NOEL for systemic toxicity was 100 mg/kg bw per day, the
highest dose tested, if the increased number of segmented neutrophils
in males at this dose is considered to be a borderline effect. No NOEL
could be identified for local irritation (Goldenthal, 1989).
Dogs
Groups of three beagle dogs of each sex were given diets
containing propargite (purity not specified) at concentrations of 0,
2000 ppm (weeks 1-3), and 2500 ppm (weeks 4-13), equal to 55 mg/kg bw
per day for males and 67 mg/kg bw per day for females (mean values of
2000 and 2500 ppm). Appearance, behaviour, and signs of toxicity were
recorded daily and body weights and food consumption weekly. Clinical
chemistry was evaluated once initially and 1 and 3 months after the
start of the study. Gross necropsy was performed on all dogs killed
at13 weeks, and the weights of the thyroid, heart, liver, spleen,
kidneys, adrenals, and testis were recorded; all organs were examined
microscopically. Most of the treated animals had a reduced appetite,
particularly during the first half of the study, and weight loss was
seen. Two males showed reduction in various haematological parameters,
including haematocrit and erythrocyte count after 3 months of
treatment, and all treated males had slightly increased aspartate
aminotransferase activity. Changes in organ weights showed no clear
dose-related pattern, and the increased relative liver weight was
probably a consequence of the reduced body weights. No macroscopic
changes were observed. Microscopic findings considered to be related
to treatment were increased pigmentation in the reticuloendothelial
cells in the liver and increased haemosiderin deposits in the spleen,
which may also be related to the decreased food consumption (Holsing &
Kundzins, 1968).
Groups of six beagle dogs of each sex recieved diets containing
technical-grade propargite (purity, 88.6%) at concentrations of 0,
160, 1250, or 2500 ppm, equivalent to 4, 30, and 48 mg/kg bw, for 1
year. The high concentration was reduced to 1875 ppm at week 9 after
observation of excessive body-weight loss. Treatment did not induce
ocular abnormalities or any treatment-related changes in clinical
chemical or urinary parameters. Two animals at the high dose died with
marked body-weight loss, and the remaining animals at this dose were
thin throughout most of the study and appeared to be dehydrated during
the last few months. Pronounced body-weight loss occurred during the
first 8 weeks at the high dose, by 2.6 kg in males and 1.9 kg in
females, and at the intermediate dose, by 0.4 kg in males and 0.5 kg
in females. Once the dose had been reduced to 1875 ppm, the
body-weight loss was less pronounced; however, the weight gain of
animals at 1250 ppm was markedly lower than that of controls. The food
consumption of animals at the high dose was reduced throughout the
study, perhaps indicating unpalatability, although it tended to
increase from week 9. Effects on haematological parameters included
reduced haemoglobin, haematocrit, and erythrocyte values in males and
females at the high dose and reduced haematocrit in males at the
intermediate dose at 3 and 6 months and at termination. In females,
these changes were less pronounced and were observed only after
3 months of treatment. Platelet counts were elevated in females at
1250 and 1875 ppm at all intervals and in males at the high dose after
6 and 12 months. Decreased absolute weights and increased relative
weights of many organs were observed in animals at the high dose and
occasionally in those at the intermediate dose. These changes are
considered to be due to the reduced body weight at this dose level.
The macroscopic changes consisted of an increased incidence of
red-tan-white foci in the lungs of males at the high dose and a higher
incidence of involution of the thymus at doses > 1250 ppm. Changes
in the bone marrow consisted of a greater incidence and severity of
erythroid-myeloid depletion and atrophy in animals at the high dose.
Histologically, the changes in the lung consisted of congestion and
inflammatory changes and fibrous and alveolar/bronchiolar epithelial
hyperplasia. In the stomach, dilated mucosal glands and vacuoles in
parietal cells were seen, but these changes showed no clear
dose-response relationship and their relation to treatment is
questionable. The NOAEL was 160 ppm, equivalent to 4 mg/kg bw per day,
on the basis of body-weight loss, reduced food consumption, and
histopathological alterations in the thymus and bone marrow at higher
concentrations (Atkinson, 1991).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a 30-day range-finding study, groups of 10 mice of each sex
received diets containing propargite at concentrations of 0, 600, 900,
1350, 2000, or 3000 ppm. Gross observations, body weight, food
consumption, gross necroscopy, and organ weights were recorded.
Body-weight loss and changes in organ weights were the predominant
effects. Minimal effects were considered to have occurred at 1000 ppm
(Gallo & Bailey, 1976), which was selected as the highest dose for the
study described below.
Groups of 15 CD-1 mice of each sex received diets containing
propargite (purity, 88.5% during the first 12 months and 84.3% during
the remaining 6 months) in corn oil at concentrations of 0, 500, or
1000 ppm, equivalent to 0, 75, and 150 mg/kg bw per day, for 52 weeks;
and groups of 60 mice of each sex received diets containing the
compound at concentrations of 0, 50, 160, 500, or 1000 ppm for
78 weeks, equivalent to 0, 7.5, 24, 75, and 150 mg/kg bw per day. The
animals were observed daily for changes in general appearance,
behaviour, appetite, toxic effects, and deaths. Body weights were
determined weekly for the first 28 weeks and every two weeks
thereafter. Food consumption was recorded weekly. Before the start of
treatment and at 52 and 78 weeks, leukocyte and erythrocyte counts
were determined in 10 animals of each sex per group, and before
termination differential leukocyte counts were made in 10 animals of
each sex per group. Gross necropsy was carried out on all animals
found dead or killed when moribund and on all animals killed at the
scheduled time. Essentially all organs, including the head, tongue,
ear, and nose, were saved, and the fresh weights of the liver, spleen,
kidneys, heart, adrenals, thyroid, and gonads were recorded.
Furthermore, sections of the spinal cord, an additional lobe of the
liver, the gall-bladder, an additional section of the uterus to
include the cervix, the nasal cavity, and the middle ear were taken
for histopathological examination.
Males at doses > 160 ppm had a lower mortality rate than
controls at 52 weeks, the survival rates being > 85%; after 78
weeks, the survival rates were 38% at 50 ppm and 60% at 500 ppm. The
variations seen in body-weight gain were not dose-related. Males
treated for 78 weeks showed a non-dose-related increase in body-weight
gain when compared with controls, whereas the females showed a
non-dose-related decrease. A similar, inconsistent pattern was found
in animals treated for 52 weeks, and these variations are considered
to be unrelated to treatment and of no toxicological significance.
Treatment did not affect food consumption or haematological
parameters, and no unexpected gross alterations were seen. An increase
in the relative weight of the kidney males treated for 52 weeks can
probably to be attributed to the reduced body-weight gain of these
animals. Females showed increased absolute and relative weights of the
adrenals at both 500 and 1000 ppm and a non-dose-related increase in
the absolute and relative weights of the thyroid at 500 ppm. The
relationship of the effects on the thyroid to treatment is
questionable. Animals treated for 78 weeks had reduced absolute and
relative weights of the kidney and uterus at concentrations > 160
ppm, and a non-dose-related increase in uterine weight was observed at
500 and 1000 ppm in females treated for 78 weeks and at 160, 500, and
1000 ppm in females treated for 52 weeks. Statistical significance was
attained only in females at 1000 ppm killed at 78 weeks.
Histopathological examination showed no treatment-related alterations
in tissues or organs, and no correlation was found between the
occurrence or type of neoplasms and treatment. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of changes in the
weights of the kidney and uterus at higher doses (Cox & Re, 1979;
Becci, 1980).
Rats
Propargite was administered to groups of 25 male and 25 female
FDRL rats in the diet at concentrations of 0 (37 animals), 100, 300,
or 900 ppm, equivalent to 0, 5, 15, and 45 mg/kg bw per day for 2
years. After the study had been in progress for 26 weeks, an
additional treatment group was included at a concentration of 2000 ppm
(equivalent to 100 mg/kg bw per day) as well as an additional control
group, because the lower doses had no effect; these groups were
treated for 78 weeks. The appearance, behaviour, and survival of the
animals was recorded daily, and body weight and food consumption were
recorded weekly. The efficiency of food use was calculated for the
first 3 months. Haematological and clinical chemical parameters were
evaluated in all animals at 12, 26, 52, 78, and 104 weeks. All rats
that died or were killed when moribund or at the end of the study were
examined macroscopically. Sections of the main organs from half of the
animals at 900 and 2000 ppm and their corresponding controls and of
the liver, kidneys, bone marrow, and thymus of the remaining rats were
examined histologically.
Treatment did not affect the appearance or behaviour of the
animals. The survival rate of males at 2000 ppm was lower than that in
the other treated groups and the control group. During the first 12
weeks of treatment, no difference in body weight or food intake was
observed between treated and the control groups, but later, the
body-weight gain of males at 900 ppm and of all treated females was
lower than that of controls, with no dose-response relationship.
Whereas the changes observed at 900 ppm were slight, a marked
reduction in body-weight gain was seen at 2000 ppm, and food intake
was also reduced at this dose. Haemoglobin, haematocrit, and leukocyte
values showed no treatment-related change at concentrations up to 2000
ppm. The absolute weights of the livers of rats at 900 ppm and of
females at 300 ppm were increased, and the relative weights were also
increased, attaining statistical significance in males at 900 ppm and
in females at concentrations > 300 ppm. In animals at 2000 ppm, the
absolute weight of the liver was decreased and the relative weight
increased, the changes being statistically significant. The relative
weight of the kidney was increased in males at 900 ppm after 104 weeks
of treatment, whereas in animals treated at 2000 ppm for 78 weeks, the
absolute weight of the kidneys was decreased and the relative weight
was increased in animals of each sex. Gross observation at autopsy
showed no treatment-related changes. The total incidences of sarcomas
and carcinomas were 6/85 in controls, 6/44 at 100 ppm, 8/42 at 300
ppm, 9/39 at 900 ppm, and 4/26 at 2000 ppm. The NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day, on the basis of changes in organ
weights (Oser, 1966). The low survival rate of animals at the highest
dose limited the relevance of the findings, and this study was
considered by the 1977 Meeting to be inadequate for evaluating the
carcinogenicity of propargite.
On the basis of the results of a 90-day study which indicated a
maximum tolerated dose of 1000 ppm (Kehoe, 1988), 800 ppm was selected
as the highest dose in a 2-year study in Crl:CDBR rats. Groups of 60
rats of each sex were given diets containing propargite (purity,
87.2%) at concentrations of 0, 50, 80, 400, or 800 ppm, equal to 2, 4,
19, and 39 mg/kg bw per day for males and 3, 5, 24, and 49 mg/kg bw
per day for females. Owing to low survival rates in males at the high
dose (30% after 103 weeks), this group was killed at 103 weeks. An
interim sacrifice was made at 53 weeks. The test diets were found to
be of adequate homogeneity and stability.
The mortality rate of males at 400 and 800 ppm was increased
towards the end of the study, with a significant positive trend,
although the group differences were not large enough to reach
statistical significance. The body weights of males at 800 ppm were
significantly reduced at the end of the study. Females showed reduced
body weight at various times during the study but no significant
overall reduction. Body-weight gain was reduced in a dose-related
manner, by 5% at 80 ppm, 12% at 400 ppm, and 18% at 800 ppm in males
at the end of the study, with statistical significance at 400 and 800
ppm. In females, body-weight gain was reduced by 15% at the high dose,
but with no statistical significance. At interim sacrifice, the
body-weight gain was reduced by 6% at 400 ppm and 20% at 800 ppm in
males and by 4% at 400 ppm and 30% at 800 ppm in females. Food
consumption was reduced at concentrations > 400 ppm in animals of
each sex. No compound-related differences in clinical signs were seen
between control and treated groups, and no treatment-related
ophthalmoscopic changes were found. The changes in haematological
parameters consisted of an increased reticulocyte count, mostly due to
decreases in three animals, and a reduced erythrocyte count in males
at 800 ppm at termination, indicating the presence of hypoxia and/or
accelerated erythropoiesis. The effects on clinical chemistry
consisted of decreased total serum protein values in males at 400 and
800 ppm and in females at 800 ppm at 26 weeks and a corresponding
decrease in total serum calcium. After 26 weeks, decreased aspartate-
and alanine aminotransferase activities were seen, with no clear
dose-response relationship. A dose-related decrease in aspartate
aminotransferase activity was observed in females at 400 and 800 ppm
after 52 weeks of treatment, probably as a result of the impaired
nutritional status of animals at these doses.
Necropsy of animals that died during the first 64 weeks of the
study showed no gross, compound-related changes in tissues. From week
65, several males at the high dose were found to have abdominal
masses, mostly in the small intestine and principally in the jejunum.
At the end of the study, males at 400 and 800 ppm and females at 800
ppm showed high frequencies of this treatment-related effect, the
incidences of masses in the jejunum being 0% in controls, 0% at
50 ppm, 0% at 80 ppm, 17% at 400 ppm, and 25% at 800 ppm in males and
0% in controls, 2% at 50 ppm, 0% at 80 ppm, 2% at 400 ppm, and 15% at
800 ppm in females. No other gross tissue alterations attributable to
treatment were found. No compound- or dose-related changes were found
in the absolute weights of the organs of treated animals, but
alterations were found in the relative weights of various organs: At
week 53, the relative weights of the livers of treated females showed
a dose-related increase over that of controls, which attained
statistical significance only in animals at 50, 400, and 800 ppm.
These changes in relative liver weights were not accompanied by
biochemical or histopathological alterations, suggesting that they
were due to the reductions in body-weight gain in females at 400 and
800 ppm and that the significant increase at 50 ppm (not supported by
a similar change at 80 ppm) is an incidental finding. Increased
relative liver weights were also observed in males at the high dose at
interim sacrifice, without reaching statistical significance, again
probably reflecting the reduced body-weight gain of these animals. At
termination, slightly increased relative liver weights were observed
in animals of each sex, but with no statistical significance. At the
interim sacrifice, the relative weights of the kidneys were also found
to be increased in animals at the high dose and in females at 80 ppm.
Histological examination revealed no treatment-related
non-neoplastic alterations and no compound-related neoplastic findings
through week 52, but during the second year of the study high
frequencies of undifferentiated sarcomas of the jejunum in males at
400 and 800 ppm and in females at 800 ppm were recorded (Table 2).
Single cases were also found in other dose groups. A clear
dose-related increase in the incidence of sarcoma was found in males
at 400 and 800 ppm and in females at 800 ppm. The incidences in all
treated female rats were also increased when compared with controls,
but with no dose-response relationship. The incidence of
undifferentiated sarcomas in duodenum, subcutaneous tissue, jejunum,
and the thoracic cavity in rats of this strain in previous studies was
reported to be 0.2% in males and 0% in females, but no
undifferentiated sarcomas were found in the jejunum. Electron
microscopic examination of abdominal tumour masses from three males at
400 ppm and one at 800 ppm revealed one malignant schwannoma, one
undifferentiated sarcoma, and one fibrosarcoma, suggesting that
propargite induced proliferation of mesenchymal tumours in various
stages of differentiation. The tumour incidences correlated with the
observations of abdominal masses. The NOAEL for systemic toxicity and
carcinogenicity was 80 ppm, equal to 4 mg/kg bw per day, on the basis
of effects on body weight, food consumption, and clinical chemical
parameters and an increased incidence of jejunal sarcomas at higher
doses (Trutter, 1991).
Dogs
Groups of six beagle dogs of each sex were maintained on a diet
containing propargite at concentrations of 0, 100, 300, or 900 ppm for
1 h per day, 6 days a week for 2 years. The dogs were observed daily
for appearance, behaviour, signs of toxicity, and neurological
reflexes. Body weight and food intake were recorded weekly for the
first 12 weeks and every 2 weeks thereafter. Haematological, clinical
chemical and urinary parameters were determined at 26, 52, 78, and
106 weeks. One animal of each sex per group was killed after 1 year
and examined grossly. A survivors were killed after 2 years and
examined. Treatment did not adversely affect the appearance,
behaviour, body weight, haematological or clinical chemical or
microscopic appearance. No indication of carcinogenicity was found
(Oser, 1966).
(d) Genotoxicity
The results of tests for the genotoxicity of propargite are
summarized in Table 3.
Table 2. Incidences of undifferentiated sarcoma of the jejunum in rats fed propargite in the diet for 2 years
Sex Dose Tumour incidence
(ppm)
Unscheduled deaths Interim sacrifice Terminal sacrifice Total
No. of No. % No. of No. % No. of No. % No. of No. %
rats rats rats rats
Male 0 26 0 0 9 0 0 24 0 0 59 0 0
50 16 0 0 0 31 0 0 47 0 0
80 23 0 0 0 23 0 0 46 0 0
400 32 9 28 0 17 2 12 49 11 22
800 35 20 57 10 0 0 15 4 27 60 24 40
Female 0 27 0 0 10 0 0 20 0 0 57 0 0
50 29 0 0 0 20 1 5 49 1 2
80 20 1 5 0 29 0 0 49 1 2
400 28 0 0 0 20 1 5 48 1 2
800 25 8 32 10 0 0 21 4 19 56 12 21
Table 3. Results of tests for the genotoxicity of propargite
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0.001-5 µl/plate NR Negative ± S9 Brusick & Weir
TA98, TA100, in DMSO (1977)
TA1535, TA1537,
TA1538;
S. cerevisiae D4
Reverse mutation S. typhimurium 10-5000 µg/plate 90.9 Negative ± S9 Shirasu et al. (1979)
TA98, TA100,
TA1535, TA1537,
TA1538; E. coli
WP2 hcr
Reverse mutation S. typhimurium 10-300 µl/plate 90a Positive ± S9 Lawlor (1991)
TA98, TA100, at > 10 µl/ml
TA1535, TA1537, in TA100
TA1538
Gene mutation B. subtilis H17, M45 1-100% v/v in DMSO 90.9 Negative Shirasu et al. (1979)
Gene mutation Chinese hamster 1-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 10-75 µg/ml +S9 (1993)
locus in DMSO
Gene mutation Chinese hamster 0.2-4.2 µg/ml 90 Negative Bigger & Clarke
ovary cells, Hprt -S9; 10-50 µg/ml (1993)
locus +S9 in DMSO
Gene mutation Chinese hamster 0.5-5 µg/ml -S9 90 Negative Bigger & Clarke
ovary cells, Hprt 5-5 µg/ml +S9 (1993)
locus in acetone
Table 3. (continued)
End-point Test object Concentration Purity Results Reference
(%)
Chromosomal Chinese hamster 25-200 µg/ml NR Negative ± S9 Kirkland (1985)
aberrations ovary cells
DNA repair Rat hepatocytes 0.0167-0.5 µg/ml 97 Negative Barfknecht (1987)
in acetone
In vivo
Micronucleus ICR mouse 37.5, 75, 150 mg/kg 89.6 Negative Putman & Young
formation bw i.p in corn oil (1994)
NR, not reported; i.p., intraperitoneally
a Technical-grade containing epoxidized soya bean oil as stabilizer
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of rats were maintained on diets containing propargite at
concentrations of 0 or 100 ppm. When the rats were about 100 days of
age and sexually mature, 20 pairs of males and females were followed
through two reproductive cycles. The first litters were discarded. At
weaning of the second litter, 10 rats of each sex in each group were
selected for the F1 generation. A similar procedure was chosen for
selection of the F2 generation. The dose of the F2 pups was
increased to 300 ppm, and the F3 pups received 300 ppm throughout
treatment. The pups were raised to maturity and the cycle repeated in
this and the succeeding generation. Treatment did not have adverse
effects on the dams, the reproduction indices, or their pups. The NOEL
was 100 ppm, the only dose tested, equivalent to 5 mg/kg bw per day
(Oser, 1966).
Groups of 25 immature Crl:CDBR albino rats of each sex were fed
diets that contained technical-grade propargite (purity, 87.2%) at
concentrations of 0, 80, 400, or 800 ppm, equivalent to 0, 4, 20, and
40 mg/kg bw per day, for 10 weeks before mating and throughout mating,
gestation, lactation, and weaning of the F1a pups. After weaning,
these pups were killed and discarded, and the F0 animals were mated
again to produce F1b litters. After weaning, the pups were assigned
at random to four groups of 25 animals of each sex, and the F0
adults were killed and necropsied. The selected F1b animals were fed
the diets for at least 10 weeks before mating and throughout
gestation, lactation, and weaning of F2a pups. After these pups were
weaned, they were killed and discarded, and the F1b animals were
mated again to produce F2b litters. After these pups had been
weaned, they were killed and discarded, and the F1b adults were
killed and necropsied. The body weights of males were recorded on the
first day of treatment, weekly thereafter, and on the day of necropsy,
whereas females were weighed on the first day of treatment, weekly
before mating, on days 0, 7, 14, and 20 of gestation and lactation,
and on the day of necropsy. Food consumption was recorded weekly only
before mating of the F0 and F1b generations and not during the
gestation and lactation periods. All F0 and F1b adults were
examined macroscopically, and the reproductive organs of those at 0
and 800 ppm were examined microscopically.
No treatment-related clinical signs were noted in these animals,
and their survival was not adversely affected. The treatment resulted
in dose-related reductions in body weight and body-weight gain of both
sexes of both generations at 400 and 800 ppm during various phases of
the study. Body weight was reduced by 5-10% in males at 400 ppm and by
18-28% in both generations at 800 ppm before and after mating.
Body-weight gain showed a corresponding pattern, with reductions of up
to 20% in males at 400 ppm and up to 60% at 800 ppm. Similar
reductions were found in females: at 400 ppm, the maximum reduction
during premating, gestation, and lactation was 10%, and at 800 ppm the
reduction was up to 22%. The body-weight gains of females were reduced
by up to 30% at 400 ppm and up to 90% at 800 ppm before mating. Marked
differences in the body-weight gain of females were seen in the
premating, gestation, and lactation periods. In all generations,
lactating dams treated with 400 and 800 ppm had greater body-weight
gain than controls, perhaps because some controls lost weight during
the second half of the lactation period. Males of the F0 generation
at 400 ppm showed a dose-related reduction in mean food consumption
during weeks 0-1, 4-5, and 8-9 before mating, and the food consumption
of males at 800 ppm was consistently lower before mating. The mean
food consumption of F0 females at 400 ppm was lower only during week
6-7, but that of females at 800 ppm was reduced throughout the
premating period. The mean food consumption of males and females of
the F1b generation showed a dose-related reduction at 400 and 800
ppm. Mating and fertility parameters and gestation indexes were not
affected by treatment, and no treatment-related differences in litter
size or sex ratio were seen. Pup weight per litter was reduced in all
generations at 400 ppm on lactation days 7, 14, and 21 and for litters
at 800 ppm on lactation days 0, 4, 7, 14 ,and 21. The maximum
reduction in the weight of pups at 800 ppm group on lactation day 21
was 44%. There were no treatment-related macroscopic or microscopic
changes. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per day, on
the basis of effects on the body weights of dams and pups at higher
doses (Kehoe, 1990).
In a study designed to clarify the reduced pup weights during
lactation at concentrations of 400 and 800 ppm, a cross-fostering
study was initiated in which groups of Crl: CD VAF/Plus rats were
given diets containing technical-grade propargite (purity, 89.9%) at
concentrations of 0 (100 animals of each sex), 400 ppm (30 of each
sex), or 800 ppm (60 of each sex). The F0 parents were given the
diets for 70 days before mating, and F0 males were killed one week
after mating. The F0 females that had delivered were killed on
lactation day 21, and those that did not deliver within 25 days after
mating were also necropsied. On lactation day 21, the F1 offspring
were necropsied, with special attention to the reproductive organs. On
lactation day 0, the litters were cross-fostered to dams in other
groups and those of the control group to other dams within the group.
Selected dams in the control group and at 800 ppm were allowed to keep
their own litters. The new groups were thus: 15 untreated dams with
their own untreated litters (controls); 20 untreated dams
cross-fostering untreated litters; 20 untreated dams cross-fostering
litters treated at 400 ppm; 20 untreated dams cross-fostering litters
treated at 800 ppm; 20 dams treated at 400 ppm cross-fostering
untreated litters; 20 dams at 800 ppm cross-fostering untreated
litters; and 20 dams at 800 ppm with their own litters treated at 800
ppm. All animals were observed for deaths and signs of toxicity twice
daily. Body weights were recorded weekly for males and for females
until evidence of copulation was seen and on days 0, 7, 14, and 20 of
gestation and days 0, 7, 14, and 21 of lactation. The food consumption
of adult rats was measured weekly except during mating in weeks 11-13
and, for females, during gestation and lactation. The litters were
reduced randomly to four pups of each sex on lactation day 4 to
provide homogeneous groups for evaluation of nursing, survival, and
pup growth. The culled pups were examined externally. The litters were
caged with their dams for 3 weeks after birth. Throughout lactation,
the dams and their pups were observed daily for survival and
behavioural abnormalities in nesting and nursing. Pups were weighed on
days 0, 4, 7, 14, and 21 of lactation, the day of termination.
Treatment had no effect on the appearance or behaviour of the
F0 animals. A treatment-related reduction in body weight relative to
the controls was seen in males and females at 800 ppm during
premating, by 16% in males and 9% in females at the end of premating.
During gestation, the mean maternal body weight and body-weight gain
were reduced at the 800 ppm; during lactation, the mean body weights
of dams at 800 ppm cross-fostering untreated litters and and of dams
at 800 ppm with their own litters remained lower than those of
controls. At the beginning of lactation, reduced body weights were
observed in all cross-fostering groups relative to the controls, with
reductions of 5% in untreated dams cross-fostering untreated litters,
4% in untreated dams cross-fostering litters treated at 400 ppm, 2% in
untreated dams cross-fostering litters at 800 ppm, 3% in dams treated
at 400 ppm cross-fostering untreated litters, 7% in dams at 800 ppm
cross-fostering untreated litters, and 12% in dams at 800 ppm with
their own litters treated at 800 ppm. At the end of the lactation
period, the reductions were 3%, 6%, 2%, 0%, 7%, and 10% in these
groups, respectively. Body-weight gain during lactation showed marked
variation among the different groups which was unrelated to dose, the
changes over the 21-day period corresponding to 31%, -17%, 10%, 52%,
14%, and 28% in the six groups, respectively. Food consumption was
also reduced, by up to 20% at 800 ppm in males and females before
mating. During gestation, the food consumption of dams at 800 ppm was
reduced to about 11%. During lactation, the food consumption in the
different groups was 103%, 101%, 106%, 98%, 83%, and 80% relative to
the controls, respectively.
No treatment-related differences in litter size, viability on day
0, or the survival index of the F1 offspring was observed. From
birth (lactation day 0), the mean body weights of male and female pups
born to dams treated at 800 ppm were statistically significantly
reduced when compared with the control group, whereas the weights of
female pups born to dams given 400 ppm were significantly increased,
an effect that was considered biologically irrelevant. On lactation
day 0, the body weights of untreated pups nursed by their untreated
dams and of pups at 400 ppm fostered by untreated dams were unchanged.
The weights of pups at 800 ppm fostered by untreated dams were
nonsignificantly reduced on days 0 and 4; those of untreated pups
fostered by dams at 400 ppm were nonsignificantly reduced on days 0
and 4 but significantly reduced on days 14 and 21 of lactation; the
weights of untreated pups fostered by dams at 800 ppm and those of
pups treated at 800 ppm and fostered by dams at the same dose were
nonsignificantly reduced on day 0 but significantly reduced on days 7,
14, and 21. There were no treatment-related differences in the
incidences of malformations, developmental variations, or macroscopic
and microscopic appearance in the F1 offspring. The results of this
study indicate that the effects on pup weight are the result of
toxicity in the dams (York, 1992).
(ii) Developmental toxicity
Rats
Groups of a minimum of 20 females Sprague-Dawley rats were given
technical-grade propargite as a suspension in corn oil by gavage at
doses of 0, 6, 25, or 105 mg/kg bw per day on days 6-15 of gestation.
At least 30 females were used as vehicle controls and for a positive
control group receiving an aqueous suspension of aspirin at a dose of
250 mg/kg bw per day. The original doses were set at 0, 25, 105, and
450 mg/kg bw per day, but dams at 450 mg/kg bw per day died after only
3 or 4 days of treatment and therefore this group was terminated. The
females at 105 mg/kg bw per day showed signs of toxicity, including
bloody nasal and vaginal discharges and urinary incontinence; this
dose was continued as the high dose, 25 mg/kg bw per day as the
intermediate dose, and an additional dose of 6 mg/kg bw per day as the
low dose. No clinical signs were observed at 6 or 25 mg/kg bw per day.
The body weight and body-weight gain of dams at 105 mg/kg bw per day
were lower than those of the other groups, but the difference did not
attain statistical significance. The body weight and body-weight gain
of the positive controls were significantly reduced at the end of the
treatment period. The reproductive performance of the treated dams, as
measured by pregnancy rate and the numbers of implantations,
resorptions, and live and dead fetuses, was similar at all doses. When
the fetuses of treated dams were evaluated for skeletal anomalies,
those at 25 and 105 mg/kg bw per day showed statistically significant
increases in the incidence of missing sternebrae, with 3% of fetuses
and 24% of litters at 25 mg/kg bw per day and 16% of fetuses and 41%
of litters at 105 mg/kg bw per day affected. The control incidence was
1% of fetuses and 6% of litters. The incidences of incomplete
ossification of vertebrae were also increased, with 28%/88%
(fetuses/litters) at 6 mg/kg bw per day, 23%/71% at 25 mg/kg bw per
day, and 30%/86% at 105 mg/kg bw per day; the control incidence was
13%/58%. Statistically significant increases were also observed in the
incidence of incomplete closure of the skull at 105 mg/kg bw per day
and in the incidence of reduced or missing hyoid, with 2%/10% at 25
mg/kg bw per day, and 5%/14% at 105 mg/kg bw per day, and 0.4%/3% in
the control group and 0.5%/4% at 6 mg/kg bw per day. The incidence of
haemorrhagic abdomen was increased at 25 and 105 mg/kg bw per day,
with no clear dose-response relationship. Most of these parameters
were also adversely affected in the dams in the positive control
group. In these animals, the incidence of resorption sites was
increased and the number of live fetuses and fetal weight were
significantly reduced, and the body weights of the dams were reduced.
The fetuses of the positive controls showed various skeletal and
soft-tissue abnormalities at significantly higher incidences than in
the untreated controls. The NOAEL for maternal toxicity was 25 mg/kg
bw per day, but no NOAEL could be identified for developmental
toxicity (Knickerbocker & Re, 1979).
Groups of 45 female Crl:CD rats were given technical-grade
propargite (purity, 85%) by gavage at doses of 0, 6, 12, 18, 25, or
105 mg/kg bw per day on days 6-15 of gestation. On day 20, the pups of
20 gravid females were removed surgically and examined. The remaining
animals were allowed to deliver, and they and their pups were observed
until day 21 of lactation, when they were necropsied. There were no
treatment-related effects on behaviour or survival. Animals at the
highest dose had anogenital and body surface staining, and the body
weights were significantly reduced by 5% when compared with controls.
The adjusted body weight (body weight on day 20 of gestation minus the
weight of uterus and contents) of animals at this dose showed a
similar reduction (7%) on days 6-9 of gestation. The body-weight gain
during days 6-15 of gestation was similar at the four lower doses but
was reduced at the highest dose. The body weights during lactation did
not differ with dose, and those of animals at the highest dose were
similar to those of controls. No sign of developmental toxicity was
seen, as measured by pre- and postimplantation loss, the numbers of
implantations and corpora lutea, pup survival index, or fetal body
weight. Slight reductions in the numbers of live offspring at day 0 of
lactation were observed, with no clear dose-response relationship; the
greatest reduction, 96.4%, was found at the highest dose. The indexes
of pup survival and mortality calculated on a fetal basis showed no
treatment-related reduction, but pup mortality analysed on a litter
basis showed a statistically significant increase in the number of
litters with deaths per total litters on day 7 at the highest dose. No
treatment-related difference in the incidence of malformations or of
developmental variations was seen. The NOAEL was 25 mg/kg bw per day
on the basis of effects on the body weight of dams and slight effects
on postnatal mortality at the higher dose (Schardein, 1990).
Rabbits
Groups of 17 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by intubation at
doses of 0, 2, 6, 10, or 18 mg/kg bw per day on days 6-18 of
gestation. Maternal toxicity, manifested by higher mortality rates at
6, 10, and 18 mg/kg bw per day, attained statistical significance at
18 mg/kg bw per day. Adipsia and anorexia were seen in all groups but
at a markedly higher frequency at the three higher doses than in
controls. Maternal body weight and body-weight gain showed
dose-related reductions at doses > 6 mg/kg bw per day, resulting in
reduced overall weight gain up to day 29 at 6 and 10 mg/kg bw per day
and weight loss at 18 mg/kg bw per day. The finding of brown areas in
the gastric mucosa in most animals at the highest dose at necropsy was
considered to be related to treatment. Pregnancy rates were not
adversely affected, but gravid uterine weights showed a dose-related
reduction at all doses. As the litter size also showed a dose-related
reduction, the reduction in uterine weight might reflect the smaller
litter sizes. Fewer implantations were found in all treated groups,
with the fewest at 18 mg/kg bw per day; the mean number of resorptions
was slightly increased at 10 and 18 mg/kg bw per day, and the mean
incidence of resorptions calculated on a per litter basis was markedly
increased at these doses. Treated groups had fewer live fetuses per
litter, and the fetal viability index calculated on a per litter basis
was decreased at 10 and 18 mg/kg bw per day. The mean fetal body
weights were reduced at the highest dose.
Increased incidences of visceral and skeletal malformations were
seen at doses > 6 mg/kg bw per day. One fetus at 10 mg/kg bw per
day had an enlarged, domed head (hydrocephaly), resulting in an
incidence of 1.6%, and two fetuses from the same litter at 18 mg/kg bw
per day also had hydrocephaly, resulting in an incidence of 9%; the
incidence in controls was 0% and that in historical controls was
0.11%. One fetus at the highest dose had an incomplete diaphragm
(incidence, 5%; 0% in controls, 0.04% in historical controls) and
other visceral alterations such as small lungs and kidneys. The
treatment-related skeletal variants included delayed ossification of
the skull and bone alignment of sternebrae. Skull closure that was
only 75% complete was seen more frequently at doses > 6 mg/kg bw
per day, resulting in incidences of 12%, 13%, and 10%, respectively,
with an incidence of 4% in the concurrent control group and in animals
at 2 mg/kg bw per day. The absence of a doserelated increase at the
highest dose might be a consequence of the small number of fetuses
available for examination (21 compared with 62-115 in the other
groups). The historical incidence for 75% skull closure was 5.7%.
Malaligned or fused sternebrae were found at incidences of 0% in
controls and 2%, 3%, 8%, and 0% at the four doses, respectively, with
a historical control incidence of 1.8%. The NOAEL for maternal and
fetal toxicity was 2 mg/kg bw per day (Serota et al., 1983).
Groups of 25 female New Zealand white rabbits were given
technical-grade propargite (purity, 85%) in corn oil by gavage at
doses of 0, 2, 4, 6, 8, or 10 mg/kg bw per day on days 7-19 of
gestation. The fetuses were removed surgically on day 29 and examined
for teratological changes. Dams at 8 and 10 mg/kg bw per day showed
signs of toxicity during the treatment period, including reduced
body-weight gain, and body-weight loss was observed during the second
half of the treatment period at 8 mg/kg bw per day and throughout
treatment at 10 mg/kg bw per day; however, the body weights at the end
of gestation were not significantly reduced. Three females at 4 mg/kg
bw per day, one at 8 mg/kg bw per day, and four at 10 mg/kg bw per day
aborted between days 18 and 25 of gestation. The toxicological
significance of the abortions at 4 and 8 mg/kg bw per day is
questionable because of the lack of a dose-response relationship, but
the abortions at 10 mg/kg bw per day are considered to be related to
treatment as they were accompanied by signs of systemic toxicity in
the dams. Fetal viability, fetal body weights, the mean numbers of
pre- and postimplantation losses, and the total numbers of
implantations and corpora lutea were comparable in all groups,
including controls. Higher incidences (on fetal and litter bases) of
fused sternebrae were found at 8 and 10 mg/kg bw per day, with
incidences of 0/0 % (fetuses/litters), 2/7%, 0.8/6%, 0/0%, 2/11%, and
8/38% at the 0, 2, 4, 6, 8, and 10 mg/kg bw per day, respectively.
This malformation is reported to occur spontaneously at an incidence
of up to about 5%. The NOAEL for maternal and developmental toxicity
was 6 mg/kg bw per day (Schardein, 1989).
(f) Special studies: Cell proliferation
In the 2-year study in CD rats of Trutter (1991), described
above, treatment with propargite resulted in a statistically
significant increase in the incidence of undifferentiated sarcomas in
the jejunum in animals of each sex given dietary concentrations
> 400 ppm. In order to investigate the underlying mechanism and to
establish a NOAEL for the relevant parameter, comparative studies were
conducted in CD rats and CD-1 mice over 1 or 4 weeks. Male rats were
given technical-grade propargite in the diet at 0 or 800 ppm or 0 or
80 ppm, female rats were given diets containing 0, 40, or 800 ppm, and
male mice received diets containing 0 or 1000 ppm. The groups
consisted of 12 controls and 22 treated animals. Body weight, food
consumption, and clinical observations were recorded weekly during the
study. Osmotic pumps containing 5-bromo-2'-deoxyuridine (BrdU), which
is incorporated into the DNA of replicating cells and used to detect
proliferating cells by immunohistochemistry, were placed
subcutaneously in half of the animals on day 1 or 20 of the study, and
the animals were killed and necropsied 1 week after implantation. All
animals were processed for immunohistochemistry and histopathology
(haematoxylin and eosin staining). Sections of the jejunum were
collected to determine cell proliferation in three layers of smooth
muscle. Cells that incorporated BrdU were identified by the presence
of chromagen over their nuclei, and cell proliferation was expressed
as unit length labelling index (number of labelled cells per square
millimetre). Positive staining in the epithelium of the duodenum was
considered to indicate systemic delivery of BrdU.
The body weights of rats receiving 800 ppm were reduced,
especially in males, and food consumption was lower. The body weights
and food consumption of the mice were not affected. After 1 week,
increased total smooth muscle cell proliferation was observed at 800
ppm in male and female rats, and the response was statistically and
biologically significant, the latter being defined as a twofold or
greater increase in cell proliferation in treated animals when
compared with controls. No cell proliferation was induced at lower
doses in rats, and no cell proliferation occurred in male mice at 1000
ppm. No biologically significant increase in cell proliferation was
found after 4 weeks of treatment, although male rats at 800 ppm showed
a statistically significant increase. The NOEL for cell proliferation
was 40 ppm, equal to 2 mg/kg bw per day (Eldridge, 1994).
A similar study was conducted to investigate a possible strain
specificity of the cell proliferative response. Groups of 11 Wistar
(WKY) rats of each sex received technical-grade propargite (purity,
88.6%) in the diet at a concentration of 900 ppm for 1 week, while six
animals of each sex received the diet alone. On the first day of the
study, osmotic pumps containing BrdU were placed subcutaneously, and
the rats were killed and necropsied 1 week later. All animals were
processed for immunohistochemistry and histopathology as described
above. Body weight, food consumption, and clinical observations were
recorded at the end of treatment. Body-weight gain and food
consumption were decreased in rats of each sex throughout the study.
No biologically significant increase in cell proliferation was
observed, and a statistically significant increase was found only in
the outer layer of the smooth muscle of the jejunum in female rats.
This isolated finding was not considered to be biologically
significant, and the combined results for all three layers did not
attain statistical significance. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in any animal. These
results are consistent with the lack of tumorigenic response in male
and female Wistar rats treated with propargite at 900 ppm for 2 years
(Eldridge, 1995; Oser, 1966).
Groups of 10 Charles River CD rats of each sex received
technical-grade propargite in the diet at a concentration of 0 or 400
ppm for 1 week. On the first day, all rats received osmotic pumps
containing BrdU. The animals were observed for clinical signs, body
weight, and food consumption. On day 7, the animals were killed, and
the jejunum and the duodenum were examined macroscopically and
microscopically. No clinical signs and no effect on body-weight gain
or food consumption were seen. Histopathological evaluation revealed
no hyperplasia, cytotoxicity, or inflammation in the jejunum.
Statistically and biologically significant increases in cell
proliferation were found in jejunal smooth muscle in males and females
(Goldenthal, 1999).
Groups of CD rats were given diets containing propargite (purity,
93%) at doses up to 800 ppm, according to the following design, with
evaluations of cell proliferation and histopathological changes after
4, 8, 12, 16, and 20 months. Groups of 90 males were given diets
containing concentrations of 0 or 800 ppm, equal to 0 and 42 mg/kg bw
per day; 6 weeks later, groups of five males were given concentrations
of 0, 80, or 400 ppm, equal to 0, 4, and 21 mg/kg bw per day, and
groups of 55 females were given concentrations of 0, 40, 400, or 800
ppm, equal to 0, 6, 28, and 55 mg/kg bw per day. Each rat was observed
twice daily for death and signs of toxicity. Clinical examinations
were conducted once weekly, and body weights and food consumption were
recorded weekly for the first 16 weeks and every 4 weeks thereafter.
Ten males at 0 and 800 ppm were killed after 4, 8, 12, 16, and 20
months, and the first 10 rats in the remaining groups were killed
after 4, 12, and 20 months. One week before scheduled necropsy,
osmotic pumps containing BrdU were implanted subcutaneously into the
backs of the animals. Standard immunohistochemical methods were used
to stain tissues for BrdU, and staining was evaluated by nuclear
labelling. Three smooth muscle layers were evaluated. Jejunal tissues
stained with haematoxylin and eosin, serial to those stained for BrdU,
were examined for histopathological changes. Ten animals in each group
were killed 6 or 7 days after implantation of the pumps. Sections of
jejunum, ileum, duodenum, and stomach were collected at the 4,- 12-,
and 20-month interim sacrifices, while only sections of jejunum and
duodenum were collected at the 8- and 16-month sacrifices. The
remaining tissues from each animal and the carcass were discarded
without further necropsy, and no further microscopic evaluation was
conducted.
The survival of control and treated animals was similar, and no
treatment-related clinical signs were observed. Body weights were
decreased by 17% in males and 13% in females at 800 ppm, and a
statistically significant decrease in body weight was observed in
males at 400 ppm, which did not result in a significant overall
reduction in body weight at the end of the study; no effect on body
weights was observed in females at this dose. The average food
consumption was reduced in animals at 800 ppm and sporadically in
those at 400 ppm. No treatment-related macroscopic changes were found
in animals killed at 4, 8, 12, or 16 months, but treatment-related
jejunal masses or nodules were observed at 20 months in males at 400
and 800 ppm and in females at 800 ppm. No statistically significant
increase in cell proliferation was detected in the smooth muscle of
the jejunum of rats treated for up to 20 months, but at that time an
at least twofold increase in cell proliferation was seen in male rats
given 800 ppm. At 4, 8, and 12 months, less cell proliferation was
seen in males at 800 ppm than in controls. These findings are
consistent with the reduced total number of cells at the same times.
At 16 months, the difference in the total number of cells between
controls and males at 800 ppm began to decrease, resulting in similar
cell proliferation in the two groups, and by 20 months both cell
proliferation and the total number of cells were greater in the rats
at the high dose than in controls. This effect appears to be a
compensatory response to apparent suppression of cell turnover in the
smooth muscle cells of the jejunum in these animals, which was first
apparent at 16 months. By 20 months, the smooth muscle cells had
overcompensated for the inhibition in cell turnover, resulting in
increased cell proliferation and total cell number. Although the
increase in cell proliferation was not statistically significant, a
twofold or greater increase in the unit length labelling index is
considered to reflect a cell proliferative response. Histopathological
evaluation revealed no hyperplasia, cytotoxicity, or inflammation in
the jejunum. The NOAEL for systemic toxicity was 80 ppm, equal to 4
mg/kg bw per day, on the basis of slight effects on body-weight gain
and jejunal masses at 400 ppm. The NOAEL for cell proliferation was
400 ppm, equal to 21 mg/kg bw per day (Goldenthal, 1998).
The results of these studies suggest that sustained cell
proliferation is not an etiological factor in tumour formation at the
jejunum. Furthermore, no lesions indicative of toxicity were
identified as precursors of tumour formation. The results suggest a
mitogenic mode of action rather than a cytotoxic action, and this
conclusion is supported by the species- and strain-dependent
differences in the carcinogenicity of propargite and the corresponding
species- and strain-dependent differences in jejunal cell
proliferative response.
3. Observations in humans
Propargite is an irritant in humans and may also be a sensitizer,
as several outbreaks of dermatitis were observed in field workers
exposed to this compound (Lee et al., 1981). An outbreak of poisoning
and dermatitis was seen among workers exposed to a formulation of
propargite in Japan in 1970: after use of the miticide on orange
trees, 40 out of 47 workers developed dermatitis and 43 showed signs
of noncutaneous effects, including irritation of the respiratory
tract. Dermatitis was also observed in mixers, loaders, and field
workers in California, USA. Outbreaks of dermatitis in subsequent
years have been reviewed (O'Malley, 1997). The environmental half-time
of propargite of 5-11 days may allow prolonged residual action which
might be responsible for the outbreaks of dermatitis in field workers
(Abrams et al., 1991).
Comments
The results of various studies of the pharmacokinetics of single
oral doses of propargite in CD rats showed that gastrointestinal
absorption was inversely related to the administered dose. After
administration of a single oral dose of [14C-phenyl]ring-labelled
propargite to rats, 20-50% of the administered dose was excreted in
the urine and 40-75% in faeces; about 1.5% of the administered dose
was found in tissues, with slightly higher urinary excretion and
correspondingly less faecal elimination in males. Roughly similar
results were found in CD mice treated in the same way.
In rats, dermal absorption of propargite occurred mainly within
the first 4 h after application. The absorption amounted to up to 33%
of the administered dose of technical material and 3-17% of the
administered doses of various formulations.
The metabolism of propargite has been elucidated in a series of
studies in rats and mice. The compound was rapidly degraded to polar
metabolites, metabolism of the cyclohexyl ring predominating. Most of
the radiolabel in rat faeces was associated with unchanged parent
compound and with a few metabolites, which were also found in urine.
In comparative studies in rats and mice, no parent compound was found
in the bile of either species, whereas the plasma contained small
amounts of unchanged propargite; six metabolites were found in rat and
mouse bile. The metabolites are formed as a result of hydrolysis of
the propynyl sulfite side-chain, subsequent oxidation and conjugation
of the tert-butyl moiety, and hydroxylation of the cyclohexyl
moiety. No consistent quantitative or qualitative species differences
in bile and plasma metabolite profiles were found.
In further studies of the metabolism of propargite with a
radiolabel located in the propynyl sulfite side-chain and with
radiolabelled propargyl alcohol, an additional metabolic pathway was
identified which involves metabolism of the side-chain by glutathione
conjugation.
Propargite is of low acute toxicity, with an oral LD50 in rats
of 2800 mg/kg bw, but it irritates the skin and eyes. Propargite did
not show dermal sensitizing potential in the Buehler test in
guinea-pigs. WHO (1999) has classified propargite as 'slightly
hazardous'.
In short-term studies in mice, rats, and dogs, the signs of
systemic toxicity included effects on body weight and on
haematological and clinical chemical parameters. In a study in which
CD rats were fed propargite for three months, the NOAEL was 100 ppm,
equivalent to 5 mg/kg bw per day. In dogs, dietary administration of
propargite for 1 year resulted in a NOAEL of 160 ppm, equivalent to 4
mg/kg bw per day, on the basis of effects on body weight and on
various haematological parameters and histopathological changes in the
thymus and bone marrow. In a 21-day study in rabbits treated dermally,
the NOAEL for systemic toxicity was < 100 mg/kg bw per day, whereas
no NOAEL for local irritation was found.
In long-term studies, the most significant toxicological finding
was the occurrence of jejunal sarcomas in CD (Crl:CDBR) rats, whereas
no carcinogenic effect was observed in CD-1 mice or Wistar (FDRL)
rats. In a 78-week study in CD-1 mice carried out in 1979, the NOAEL
was 50 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
changes in organ weights. In the study in Wistar (FDRL) rats carried
out in 1966, the NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day,
also on the basis of changes in organ weights.
In a two-year study reported in 1991 in which CD (Crl:CDBR) rats
were given diets containing propargite at concentrations of 0, 50, 80,
400, or 800 ppm (equal to 0, 2, 4, 19, and 39 mg/kg bw per day in
males and 0, 3, 5, 24, and 49 mg/kg bw per day in females), males at
400 and 800 ppm showed a dose-related increase in the incidence of
undifferentiated sarcomas in the jejunum, a very rare tumour. Females
also showed a clear tumorigenic response but with a different
dose-response relationship, since a high incidence (21%) of jejunal
tumours in the smooth muscle was observed at the highest concentration
of 800 ppm but low incidences (one animal in each group) at 50, 80,
and 400 ppm. Given the rarity of sarcomas arising at this site (0% in
concurrent and historical female controls, 0% in concurrent male
controls, and 0.2% in historical male controls), a NOAEL for
tumorigenicity could not be identified in this study. In a 20-month
study reported in 1998 in which CD rats were given dietary
concentrations of 0, 80, 400, or 800 ppm (equal to 0, 4, 21, and 42
mg/kg bw per day) for males and 0, 40, 400, or 800 ppm (equal to 0, 6,
28, and 55 mg/kg bw per day) for females, the appearance of jejunal
masses at 400 and 800 ppm in males and at 400 ppm in females suggested
that the tumorigenic response is a reproducible effect. Reduced cell
proliferation in jejunal smooth muscle layers and decreased jejunal
cell division were found at various times during the study. After 20
months, cell division and cell proliferation were found to be
increased only in males at 800 ppm, with no corresponding response in
females or in males at lower doses. No hyperplasia, cytotoxicity, or
inflammation was found in the jejunal epithelium.
Several short-term (1 or 4 weeks) studies of cell proliferation
were conducted in male and female CD rats at concentrations of up to
800 ppm, in Wistar rats (WKY strain) at 900 ppm, and in male CD-1 mice
at 1000 ppm. The NOAEL for cell proliferation in the CD rat was 40 ppm
(equal to 2 mg/kg bw per day) in females and 80 ppm (equal to 4 mg/kg
bw per day) in males, both being the lowest doses tested. A
proliferative response was observed at 800 ppm in both male and female
rats after 1 week of treatment, whereas the response was observed only
in males after 4 weeks of treatment at this concentration; no cell
proliferation was observed at 40 and 80 ppm. No cell proliferation was
found in Wistar rats (WKY) treated with 900 ppm or in CD-1 mice at
1000 ppm in the same study design.
The results of the long-term studies in CD rats and the
short-term studies of cell proliferation indicate that the cell
proliferation induced by propargite in the jejunum is characterized by
an initial transient proliferative response, lasting for at least 4
weeks in males and for only about 1 week in females. This profile of
cell proliferation suggests that the underlying mechanism for tumour
formation in the jejunum of CD rats may be related to the mitogenic
activity of the compound.
The observed dose-response relationship for tumour formation,
with a greater increase in tumour incidence in males than in females,
correlates with the duration and degree of jejunal cell proliferation.
The prolonged duration of cell proliferation in male rats was
associated with a more pronounced tumorigenic response than in
females. Furthermore, propargite did not induce cell proliferation in
species and strains in which no carcinogenic activity was observed.
The available database does not further illuminate the causal
relationship between cell proliferation and tumorigenicity, and no
explanation was offered of the species, strain, and sex specificity or
of the association between the presence of an early, transient cell
proliferation response and the occurrence of jejunal tumours in CD
rats and the consistent absence of these findings in Wistar rats and
CD-1 mice. Nevertheless, the association between proliferation and
tumorigenic activity was recognized by the Meeting. The Meeting also
noted that the NOAEL for cell proliferation of 40 ppm is very close to
the lowest tumorigenic concentration in female rats of 50 ppm. The
available database did not, however, clarify the dose-response
relationship found at low tumorigenic doses in females and offered no
further scientific evidence to support use of cell proliferation as a
marker of potential carcinogenicity.
An adequate range of studies for genotoxicity was conducted, and
the results were consistently negative. Therefore the Meeting
concluded that propargite is not genotoxic. The finding of
consistently negative results in numerous tests for genotoxicity
provides further support for the conclusion that propargite causes
tumours by a non-genotoxic mechanism.
In a three-generation study of reproductive toxicity, reduced
body-weight gain was seen at concentrations of 400 ppm (equivalent to
20 mg/kg bw per day) and above in parental animals and in pups during
lactation. The results of a subsequent cross-fostering study showed
that the growth retardation of the pups was reversible and was due to
maternal toxicity. The NOAEL was 80 ppm, equivalent to 4 mg/kg bw per
day, on the basis of reduced body-weight gain in parental animals and
pups.
Two studies of developmental toxicity were conducted in rats and
two in rabbits. In a study in Sprague-Dawley rats reported in 1979,
fetal effects such as increased incidences of incomplete vertebral
ossification and missing sternebrae and hyoids were observed in
treated animals. Not all of the findings were dose-related and they
were not reproduced in a study reported in 1990. The NOAEL was 25
mg/kg bw per day on the basis of reduced body-weight gain in dams and
a slight increase in the postnatal mortality rate at the highest dose
in the latter study. In the two studies in rabbits, dated 1983 and
1989, fetal effects indicative of developmental retardation were
observed at maternally toxic doses of 6 mg/kg bw per day and above,
and an increased incidence of hydrocephaly was seen at higher doses in
the first study. Most of the fetal effects, including hydrocephaly,
were not confirmed in the second study, in which effects were observed
only at doses of 8 mg/kg bw per day and higher. The overall NOAEL in
rabbits was 4 mg/kg bw per day. No evidence for teratogenicity was
found in these studies.
The Meeting allocated an ADI of 0-0.01 mg/kg bw on the basis of
the LOAEL of 50 ppm (equal to 3 mg/kg bw per day) for tumorigenicity
in female CD rats, with a safety factor of 300. This safety factor was
chosen to account for the lack of a NOAEL in this study and the nature
of the end-point (tumorigenesis) and to encompass the NOAEL in the
same rat strain for increased cell proliferation in the jejunum, the
site of tumour formation.
The Meeting concluded that it was unnecessary to determine an
acute reference dose because of the low acute toxicity of propargite.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (effects on organ
weights in a 78-week study of toxicity and carcinogenicity)
Rat: < 50 ppm, equal to 3 mg/kg bw per day (lowest concentration
tested; tumorigenicity in a 2-year study)
80 ppm, equivalent to 4 mg/kg bw per day (maternal and fetal
toxicity in a three-generation study)
25 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Rabbit: 4 mg/kg bw per day (maternal and fetal toxicity in studies
of developmental toxicity)
Dog: 160 ppm, equivalent to 4 mg/kg bw per day (toxicity in a
1-year study)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Further studies on the mechanism of tumorigenic activity
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to propargite
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid and incomplete (50% in rats and mice)
Dermal absorption 30%, rats
Distribution Highest concentrations in intestine, liver (rats and mice)
Potential for accumulation None
Rate and extent of excretion Rapid excretion in rats and mice (urinary, approximately 50%;
faecal, 50%)
Metabolism in animals Rapid degradation to numerous polar metabolites, no parent
compound in bile or urine; hydrolysis of propynyl sulfite
side-chain and subsequent oxidation of tert-butyl moiety and
hydroxylation of cyclohexyl moiety, conjugation
Toxicologically significant compounds Parent compound; animal and plant metabolites (animals, plants,
and environment) similar
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 4000 mg/kg bw
Rat, LC50, inhalation 0.89 mg/L (4 h)
Dermal irritation Irritating to rabbit skin
Ocular irritation Irritating to rabbit eye
Dermal sensitization Not sensitizing in guinea-pigs (Buehler test)
Short-term toxicity
Target/critical effect Haematological system
Lowest relevant oral NOAEL Dog: 1 year, 160 ppm (4 mg/kg bw per day)
Lowest relevant dermal NOAEL Rabbit: 21 days, < 100 mg/kg bw per day (systemic toxicity)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Intestine (jejunal tumours in CD rats), haematological system
Lowest relevant NOAEL Rat (CD): 50 ppm (females; 3 mg/kg bw per day), 2-year study
Carcinogenicity Carcinogenic in CD rats but not in FDRL rats or
CD-1 mice
Reproductive toxicity
Reproductive target/critical effect Reduced pup weight at maternally toxic doses
Lowest relevant reproductive NOAEL Rat: 80 ppm (4 mg/kg bw per day), three-generation study
Developmental target/critical effect Rat: fetotoxicity at maternally toxic doses
Rabbit: fetotoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rabbit: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Other toxicological studies Transient cell proliferation in jejunal smooth-muscle cells
in CD rats
Medical data Dermatitis in field workers
Summary Value Study Safety factor
ADI 0-0.01mg/kg bw CD rats, 2-year study 300
Acute RfD Unnecessary
References
Abrams, K., Hogan, D.J. & Maibach, H.I. (1991) Pesticide-related
dermatoses in agricultural workers. Occupational Medicine:
State of the Art Reviews, Vol. 6, pp. 463-491.
Andre, J.C. & Laveglia, J. (1994) Pharmacokinetics of 14C-Omite: A
comparative study in rats and mice, Part II, elimination of
14C-Omite equivalents in bile following oral administration.
Unpublished report No. 93-0284 from Ricerca, Inc., Department of
Toxicology and Animal Metabolism, Painesville, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1989) Study to
determine if rats expire radiolabel in air after oral
administration of 14C-Omite. Unpublished report No.
3374-89-0211-AM-001 from Ricerca, Inc., Department of Toxicology
and Animal Metabolism, Painesville, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990a) Study of the
dermal absorption of technical 14C-Omite by male Sprague-Dawley
rats. Unpublished report No. 3452-89-0308-AM-001 from Ricerca,
Inc., Department of Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990b) Study of the
dermal absorption of 14C-Omite-6E by male Sprague-Dawley rats.
Unpublished report No. 3451-89-0307-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990c) Study of the
dermal absorption of 14C-Comite by male Sprague-Dawley rats.
Unpublished report No. 3450-89-0306-AM-001 from Ricerca, Inc.,
Department of Toxicology and Animal Metabolism, Painesville,
Ohio, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA.
Atkinson, J.E. (1991) A chronic (1 year) oral toxicity study in the
dog with Omite via the diet. Unpublished report No. 88-3377 from
Bio/dynamics, Inc., New Jersey, USA. Submitted by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1989a) Identification of 14C-Omite urinary
metabolite in rats. Part 2: Identification of the sixth
metabolite of Omite in female rat urine. Unpublished report No.
8706 from Uniroyal Chemical Co., Inc., Middlebury, Connecticut,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Banijamali, A.R. (1989b) Identification of 14C-Omite metabolites in
a lactating goat. Unpublished report No. 8869 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R. (1998) Identification of metabolites of [1,2,3-13C]
propargyl alcohol in rat urine by 13C NMR and mass
spectrometry. Unpublished report No. 96038 Part I from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. (1999) Identification of metabolites of
[1,2,3-13C,2,3,-14C-propargyl]propargite in male
Sprague-Dawley rats. Unpublished report No. 98156 from Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Lau, R.C.M. (1996) 14C-Propargite nature of the
residue in lactating goat. Unpublished report No. 95157 (Uniroyal
Project)/ No. 95123g Southwest Bio-Labs from Uniroyal Chemical
Co. Inc., Middlebury, Connecticut, USA and South West Bio-Labs,
Inc., Las Cruces, New Mexico, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Nag, J.K. (1990) The identification of Omite
metabolites in rats; Amendment No. 4: Fecal metabolism of Omite.
Unpublished report No. 8706 from Uniroyal Chemical Co. Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co. Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. &Nag, J.K. (1991) Identification of [phenyl-U-14C]
Omite fecal metabolites in mice and comparison to rat's.
Unpublished report No. 9107 from Uniroyal Chemical Co., Inc.,
Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Banijamali, A.R. & Tortora, N.J. (1988a) Identification of 14C-Omite
urinary metabolite in rats. Unpublished report No. 8706 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co. Inc., Middlebury, USA.
Banijamali, A.R. & Tortora, N.J. (1988b) Comparison of subchronic vs
single oral dose in the disposition and metabolism of Omite in
rats. Unpublished report No. 87122 from Biotek, Inc., Woburn,
Massachusetts (Project No. 8719), Hazleton Laboratories America,
Inc., Madison, WIisconsin (Project No. 8721) and Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Banijamali, A.R., Kratky, E. & Gay, M.H. (1994) Pharmacokinetics of
14C-Omite: a comparative study in rats and mice - Part III -
Analysis. Unpublished report No. 9395 from Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Middlebury, Connecticut, USA.
Barfknecht, T.R. (1987) Rat hepatocyte primary culture/DNA repair
test. Unpublished report No. PH 311-UN-001-87 from Pharmakon
Research International, Inc., Pennsylvania, USA. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Becci, P.J. (1980) Supplementary pathology report for chronic
oncogenic evaluation of Omite in CD-1 mice. Unpublished report
No. 5036 from Food and Drug Research Laboratories, Inc., New
York, USA. Submitted to WHO by Uniroyal Chemical, Inc., Bethany,
Connecticut, USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (DMSO). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Bigger, C.A.H. & Clarke, J.J. (1993) CHO/HGPRT mutation assay with
confirmation (acetone). Unpublished report No. TC864.332001 from
Microbiological Associates, Inc., Rockville, Maryland, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany,
Connecticut,USA.
Brusick, D.J. & Weir, R.J. (1977) Mutagenicity evaluation of DO14.
Unpublished report No. 2683 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by Uniroyal Chemical,
Bethany, Connecticut, USA.
Butterworth, B.E. (1991) Chemically induced cell proliferation as a
predictive assay for potential carcinogenicity. In: Butterworth,
B.E., Slaga, T.J., Farland, W. & McClain, M., eds, Chemically
Induced Cell Proliferation: Implications for Risk Assessment, pp.
457-467.
Byrd, J.W. (1988) 14C-Omite goat metabolism study. Unpublished
report No. 8734g from Southwest Bio-Labs, Inc., Las Cruces, NM,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Carson, S. (1964) Subacute feeding studies with D-014 in rats.
Unpublished report No. 85603 from Food and Drug Research
Laboratories, Inc., New York, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Cox, G.E. & Re, T.A. (1979) Chronic oncogenic evaluation of Omite in
CD-1 mice following 78-weeks of dietary treatment. Unpublished
report No. 5036 from Food and Drug Research Laboratories, Inc.,
New York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Eldridge, S. (1994) Effect of dietary Omite technical on cell
proliferation in jejunum of rats and mice. Unpublished report No.
6030-10 (ManTech), 93-45 and 93-131 (PAI) from Pathology
Associates, Inc., Durham, North Carolina, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Eldridge, S. (1995) Effect of dietary Omite technical on cell
proliferation in jejunum of Wistar rats. Unpublished report No.
6030-017 (ManTech), 94-91 (PAI) from Pathology Associates
International, Durham, North Carolina, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gallo, M.A. & Bailey, D.E. (1976) Range finding study with Omite in
albino mice. Unpublished report No. 5028 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to WHO by
Uniroyal Chemical, Inc., Bethany, Connecticut, USA.
Gay, M.H. (1987) Rat metabolism of 14C-Omite. Final report.
Unpublished report No. 87002 from Biotek, Inc., Woburn,
Massachusetts, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Gay, M.H. (1994) Pharmacokinetics of 14C-Omite: a comparative study
in rats and mice (overview). Unpublished report No. 9395 from
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc.
Goldenthal, E.I. (1989) 21-day repeat dose dermal toxicity study in
rabbits. Unpublished report No. IRDC 399-098 from International
Research and Development Corporation, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1998) Twenty month dietary toxicity study in rats
with Omite. Unpublished report No. 399-187 from MPI Research,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Goldenthal, E.I. (1999) Effect of one week dietary administration of
Omite technical (400 ppm) on jejunal cell proliferation in
Sprague-Dawley rats. Unpublished report No. 399-206 from MPI
Research, Inc., Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
propargite in the rat. Unpublished report No. 91-8372 from
Bio/dynamics, Inc., New Jersey, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Holsing, G.C. & Kundzins, L. (1968) 13-Week dietary feeding study-dogs
with D-014. Unpublished report No.798-140 from Hazleton
Laboratories, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Incorporated, Bethany, Connecticut, USA.
Johnson, J.D. (1990) Metabolism of 14C-Omite in rats: Dosing, sample
collection and total 14C-residue determinations. Unpublished
report Uniroyal No. 8998, Battelle No. NO967-2700 from Battelle
Columbus Division, Columbus, Ohio, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Middlebury, Connecticut, USA.
Kehoe, D.F. (1988) Subchronic toxicity and kinetic study with Omite
technical in rats. Unpublished report No. HLA 6111-107 from
Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kehoe, D.F. (1990) Two-generation reproduction study with Omite
technical in rats (two litters per generation). Unpublished
report No. HLA 6111-108 from Hazleton Laboratories America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Uniroyal Chemical
Co., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993a) Acute oral toxicity study in albino rats with
Omite Technical. Unpublished report No. WIL-155012 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993b) Acute dermal toxicity study in albino rabbits
with Omite technical. Unpublished report No. WIL-155013 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993c) Primary dermal irritation study in albino
rabbits with Omite technical. Unpublished report No. WIL-155014
from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Kiplinger, G.R. (1993d) Primary eye irritation study in albino rabbits
with Omite technical. Unpublished report No. WIL-155015 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kiplinger, G.R. (1993e) Skin sensitization study in albino guinea pigs
with Omite technical. Unpublished report No. WIL-155016 from WIL
Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO
by Uniroyal Chemical Co. Inc., Bethany, Connecticut, USA.
Kirkland, D.J. (1985) Study to evaluate the chromosome damaging
potential of D-014 by its effects on cultured chinese hamster
ovary (CHO) cells using an in vitro cytogenetics assay.
Unpublished report No. URC1/CHO/AR/KF14/CH3 from Microtest
Research Lim, York, United Kingdom. Submitted to WHO by Uniroyal
Chemical Co., Bethany, Connecticut, USA.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of Omite
technical in Sprague-Dawley rats. Unpublished report No. 5992 (b)
from Food and Drug Research Laboratories, Inc., Maspeth, New
York, USA. Submitted to WHO by Uniroyal Chemical, Bethany,
Connecticut, USA.
Knipe, J.O. (1986) The disposition and metabolism of 14C-Omite in
male and female rats. Unpublished report No. 8589 from Uniroyal
Chemical Co., Inc., Naugatuck, Connecticut, USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Knipe, J.O. (1987) Progress report. The metabolism of 14C-Omite in
rats. Unpublished report No. 8706 from Uniroyal Chemical Co.,
Inc., Naugatuck, Connecticut, USA. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Lawlor, T.E. (1991) Mutagenicity test on Omite ESO in the
Salmonella/reverse mutation assay (Ames test) preincubation
method. Unpublished report No. 12514-0-420 from Hazleton
Washington, Inc., Kensington, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Lee, S.L., Chin, Y.W. & Kim, J.S. (1981) A study on hypersensitivity
of Korean farmers to various agrochemicals. Determination of
concentration for patch-test of fruit-tree agrochemicals and
hypersensitivity of orange orchard farmers in Che-ju Do, Korea.
Seoul Med. J., 22, 137-142.
Mahon, C.R. (1993) Comparative metabolism of 14C-propargyl-Omite in
rats and mice. Unpublished report No. 91188 (Uniroyal Study
No.)/No. SC 910217 (Battelle Study No.) from Battelle Laboratory,
Columbus, Ohio, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Middlebury, Connecticut, USA.
Mizens, M., Andre, J.C., Marciniszyn, J.P. & Killeen, J.C. (1990)
Study of the dermal absorption of 14C-Omite-30W by male
Sprague-Dawley rats. Unpublished report No. 3449-89-0305-AM-001
from Ricerca, Inc., Dept. Toxicology and Animal Metabolism,
Painesville, Ohio, USA. Submitted to WHO by Uniroyal Chemical
Co., Inc., Middlebury, Connecticut, USA.
O'Malley, M.A. (1997) Skin reactions to pesticides. Occupational
Medicine: State of the Art Reviews, Vol. 12, pp. 327-345.
Oser, B.L. (1966) Chronic (2-year) feeding studies with D-014 in rats
and dogs. Unpublished report No. 86000/86014 from Food and Drug
Research Laboratories, Inc., New York, USA. Submitted to
Naugatuck Chemical, Connecticut, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Putman, D.L. & Young, R.R. (1994) Micronucleus cytogenetic assay in
mice. Unpublished report No. G94AP36.122 from Microbiological
Associates, Inc., Rockville, Maryland, USA. Submitted to WHO by
Uniroyal Chemical Co., Bethany, Connecticut, USA.
Sabourin, P.J., Trigg, N.J., Mahon, C.R. & Johnson, J.D. (1994)
Pharmacokinetics of 14C-Omite: A comparative study in rats and
mice - Part I - 14C-Omite equivalents in blood following oral
and intravenous administration. Unpublished report No. SC930180
from Battelle Laboratory, Columbus, Ohio, USA. Submitted to WHO
by Uniroyal Co., Inc., Middlebury, Connecticut, USA.
Schardein, J.L. (1989) Developmental toxicity study in New Zealand
white rabbits. Unpublished report No. 399-097 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
Schardein, J.L. (1990) Developmental toxicity study in rats.
Unpublished report No. 399-096 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Serota, D.G., Wolfe, G.W., Durloo, R.S. & Fezio, W.L. (1983)
Teratology study in rabbits Omite technical, revised final
report. Unpublished report No. 798-195 from Hazleton Laboratories
America, Inc., Virginia, USA. Submitted to WHO by Uniroyal
Chemical, Bethany, Connecticut, USA.
Shirasu, Y., Moriya, M. & Watanabe, K. (1979) Microbial mutagenicity
testing on BPPS (Propargite). Unpublished report from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Uniroyal Chemical, Bethany, Connecticut, USA.
Trela, B.A. (1991) Metabolism of 14C-Omite in mice: Dosing, sample
collection, and 14C-residue determinations. Unpublished report
No. SC910015 from Battelle Columbus Division, Columbus, Ohio.
Submitted to WHO by Uniroyal Chemical Co., Inc., Middlebury,
Connecticut, USA.
Trutter, J.A. (1991) Combined chronic toxicity and oncogenicity study
in rats with Omite technical. Unpublished report No. HLA/HWA
798-220 from Hazleton Laboratories America, Inc., Virginia, USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety. York, R.G.
(1992) A cross-fostering reproduction study with Omite technical
in rats. Unpublished report No. 399-113 from International
Research and Development Corporation, Matttawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Co., Bethany, Connecticut,
USA.
