
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
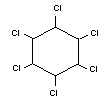
LINDANE
Chemical name
gamma-1,2,3,4,5,6-hexachlorocyclohexane
Synonym
gamma-BHC, gammexane, gamma-HCH
Empirical formula
C6H6Cl6
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Lindane is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, lindane is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of lindane
in growing animals is however, much lower than that of beta-BHC or of
DDT. In rats ingesting repeated small doses of lindane, the
concentration in the fat depots is in a state of equilibrium with the
concentration of the diet (Lehman, 1953). The data on lindane
metabolism are very incomplete. According to certain in vitro tests
it would seem that part is broken down in the liver. The unchanged
compound is eliminated in the faeces and urine and is found in the
milk (Ray et al., 1953; Van Asperen, 1957; Ware & Gilmore, 1959). Very
little information is available about its effects on basic enzyme
systems. Growing animals are particularly susceptible to its toxic
action.
Acute toxicity
From the viewpoint of acute toxicity, lindane is the most toxic
of all the isomers present in the technical hexachlorocyclohexanes. It
is a neurotropic poison causing excitation of the central nervous
system with convulsions followed - if the dose is sufficiently high
- by depressive phenomena leading to death from respiratory collapse.
Animal Route LD50 mg/kg References
body-weight
Rat Oral 125, 200 Dallemagne & Philippot,
1948
Lehman, 1948
Lehman, 1951
US Public Health
Service, 1956
177-230 Negherbon, 1959
Rat Intraperitoneal 35-85 Negherbon, 1959
Mouse Oral 86 Negherbon, 1959
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Negherbon, 1959
Rabbit Oral 60-200 Cameron, 1945
Negherbon, 1959
Dog Oral 40-200 Negherbon, 1959
(lethal)
Dog Intravenous 7.5 Negherbon, 1959
(lethal)
The fatal dose for man is estimated to be 150 mg/kg body-weight,
i.e., of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Short-term studies
Rat. When rats were fed a diet containing 400 ppm lindane for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given lindane by
stomach-tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous symptoms (trembling, spasms), fatty degeneration of the liver
and of the epithelium of the renal tubules, vacuolization of the
cerebral cells and a marked increase in mortality rate were observed.
With 10 mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950 and 1951; Kewitz, 1952; Kewitz & Reinert, 1952); when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of lindane, the general behaviour of the animals, their
body-weight, histological findings and the post-mortem examination
showed no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to successive administration
by the intramuscular route of daily doses of 40 mg lindane for 19
days, 80 mg for 9 days and 160 mg for three days. The animals died and
showed inter alia hepatic degeneration (Dallemagne & Philippot,
1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed
hepatic lesions (Woodward & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10%
solution of lindane in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of lindane brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied lindane during an
antimalaria campaign, about 46% became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal
applications of 0.5% lindane in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3% of lindane and were observed for 10 months. No abnormalities were
found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given
for 2 years diets containing 5 to 1600 ppm of the alpha- beta-, and
gamma-isomers of lindane. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of lindane, the lowest concentration causing significant
liver charges was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of
20 young rats (10 males and 10 females) were fed diets containing 25,
50 and 100 ppm of lindane throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration. 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets
containing 50 and 100 ppm of lindane; histological lesions were found
in the liver parenchyma (Ortega et al., 1957).
Twenty-five young rats were given lindane daily by stomach-tube
at a dosage of 10 µg/kg body-weight (corresponding to about 0.1 and
0.15 ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments on the experimental studies reported
In all these animal species, lindane has proved to be a
cumulative poison causing hepatic and renal lesions and disturbances
of the central nervous system. The dog seems particularly susceptible
to neurological effects.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty-five ppm of lindane approximately equivalent to 1.25 mg/kg
of bodyweight.
Estimate of the maximal acceptable daily intake for man
0-0.0125 mg/kg.
Further work desirable
Investigation on the chemical nature of the residue occurring in
the plant. Additional biochemical studies. Long-term feeding studies
in other species than the rat.
REFERENCES
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Dallemagne, M. J. & Philippot, E. (1948) Arch. Int. Pharm. Ther.,
76, 274
Doisy, E. A. J. & Bocklage, B. C. (1949) Proc. soc. exp. Biol.
(N.Y.), 71, 490
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Phamacol.
exp. Ther., 100, 59
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel-Forsch., 1, 356
Kewitz, H. (1952) Arch. exp. Path. Phamakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. Path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. Path. Pharmakol., 227, 183
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) Arch. Path., 64,
614
Ray, E. E. et al., (1953) J. Amer. vet. med. Ass., 123, 448
Truhaut, R. (1954) Communication au symposium intern. de la prevention
du cancer, Sao Paulo
United States Public Health Service (1956) Clinical memoranda on
economic poisons
Van Asperen, K. (1957) C.R. 4è Congrès Intern. de lutte contre les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. Econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wassermann, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu,
G. & Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 387
BIOLOGICAL DATA
Biochemical aspects
Lindane is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, lindane is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of lindane
in growing animals is however, much lower than that of beta-BHC or of
DDT. In rats ingesting repeated small doses of lindane, the
concentration in the fat depots is in a state of equilibrium with the
concentration of the diet (Lehman, 1953). The data on lindane
metabolism are very incomplete. According to certain in vitro tests
it would seem that part is broken down in the liver. The unchanged
compound is eliminated in the faeces and urine and is found in the
milk (Ray et al., 1953; Van Asperen, 1957; Ware & Gilmore, 1959). Very
little information is available about its effects on basic enzyme
systems. Growing animals are particularly susceptible to its toxic
action.
Acute toxicity
From the viewpoint of acute toxicity, lindane is the most toxic
of all the isomers present in the technical hexachlorocyclohexanes. It
is a neurotropic poison causing excitation of the central nervous
system with convulsions followed - if the dose is sufficiently high
- by depressive phenomena leading to death from respiratory collapse.
Animal Route LD50 mg/kg References
body-weight
Rat Oral 125, 200 Dallemagne & Philippot,
1948
Lehman, 1948
Lehman, 1951
US Public Health
Service, 1956
177-230 Negherbon, 1959
Rat Intraperitoneal 35-85 Negherbon, 1959
Mouse Oral 86 Negherbon, 1959
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Negherbon, 1959
Rabbit Oral 60-200 Cameron, 1945
Negherbon, 1959
Dog Oral 40-200 Negherbon, 1959
(lethal)
Dog Intravenous 7.5 Negherbon, 1959
(lethal)
The fatal dose for man is estimated to be 150 mg/kg body-weight,
i.e., of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Short-term studies
Rat. When rats were fed a diet containing 400 ppm lindane for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given lindane by
stomach-tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous symptoms (trembling, spasms), fatty degeneration of the liver
and of the epithelium of the renal tubules, vacuolization of the
cerebral cells and a marked increase in mortality rate were observed.
With 10 mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950 and 1951; Kewitz, 1952; Kewitz & Reinert, 1952); when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of lindane, the general behaviour of the animals, their
body-weight, histological findings and the post-mortem examination
showed no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to successive administration
by the intramuscular route of daily doses of 40 mg lindane for 19
days, 80 mg for 9 days and 160 mg for three days. The animals died and
showed inter alia hepatic degeneration (Dallemagne & Philippot,
1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed
hepatic lesions (Woodward & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10%
solution of lindane in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of lindane brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied lindane during an
antimalaria campaign, about 46% became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal
applications of 0.5% lindane in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3% of lindane and were observed for 10 months. No abnormalities were
found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given
for 2 years diets containing 5 to 1600 ppm of the alpha- beta-, and
gamma-isomers of lindane. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of lindane, the lowest concentration causing significant
liver charges was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of
20 young rats (10 males and 10 females) were fed diets containing 25,
50 and 100 ppm of lindane throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration. 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets
containing 50 and 100 ppm of lindane; histological lesions were found
in the liver parenchyma (Ortega et al., 1957).
Twenty-five young rats were given lindane daily by stomach-tube
at a dosage of 10 µg/kg body-weight (corresponding to about 0.1 and
0.15 ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments on the experimental studies reported
In all these animal species, lindane has proved to be a
cumulative poison causing hepatic and renal lesions and disturbances
of the central nervous system. The dog seems particularly susceptible
to neurological effects.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty-five ppm of lindane approximately equivalent to 1.25 mg/kg
of bodyweight.
Estimate of the maximal acceptable daily intake for man
0-0.0125 mg/kg.
Further work desirable
Investigation on the chemical nature of the residue occurring in
the plant. Additional biochemical studies. Long-term feeding studies
in other species than the rat.
REFERENCES
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Dallemagne, M. J. & Philippot, E. (1948) Arch. Int. Pharm. Ther.,
76, 274
Doisy, E. A. J. & Bocklage, B. C. (1949) Proc. soc. exp. Biol.
(N.Y.), 71, 490
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Phamacol.
exp. Ther., 100, 59
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel-Forsch., 1, 356
Kewitz, H. (1952) Arch. exp. Path. Phamakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. Path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. Path. Pharmakol., 227, 183
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) Arch. Path., 64,
614
Ray, E. E. et al., (1953) J. Amer. vet. med. Ass., 123, 448
Truhaut, R. (1954) Communication au symposium intern. de la prevention
du cancer, Sao Paulo
United States Public Health Service (1956) Clinical memoranda on
economic poisons
Van Asperen, K. (1957) C.R. 4è Congrès Intern. de lutte contre les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. Econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wassermann, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu,
G. & Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 387
 BIOLOGICAL DATA
Biochemical aspects
Lindane is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, lindane is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of lindane
in growing animals is however, much lower than that of beta-BHC or of
DDT. In rats ingesting repeated small doses of lindane, the
concentration in the fat depots is in a state of equilibrium with the
concentration of the diet (Lehman, 1953). The data on lindane
metabolism are very incomplete. According to certain in vitro tests
it would seem that part is broken down in the liver. The unchanged
compound is eliminated in the faeces and urine and is found in the
milk (Ray et al., 1953; Van Asperen, 1957; Ware & Gilmore, 1959). Very
little information is available about its effects on basic enzyme
systems. Growing animals are particularly susceptible to its toxic
action.
Acute toxicity
From the viewpoint of acute toxicity, lindane is the most toxic
of all the isomers present in the technical hexachlorocyclohexanes. It
is a neurotropic poison causing excitation of the central nervous
system with convulsions followed - if the dose is sufficiently high
- by depressive phenomena leading to death from respiratory collapse.
Animal Route LD50 mg/kg References
body-weight
Rat Oral 125, 200 Dallemagne & Philippot,
1948
Lehman, 1948
Lehman, 1951
US Public Health
Service, 1956
177-230 Negherbon, 1959
Rat Intraperitoneal 35-85 Negherbon, 1959
Mouse Oral 86 Negherbon, 1959
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Negherbon, 1959
Rabbit Oral 60-200 Cameron, 1945
Negherbon, 1959
Dog Oral 40-200 Negherbon, 1959
(lethal)
Dog Intravenous 7.5 Negherbon, 1959
(lethal)
The fatal dose for man is estimated to be 150 mg/kg body-weight,
i.e., of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Short-term studies
Rat. When rats were fed a diet containing 400 ppm lindane for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given lindane by
stomach-tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous symptoms (trembling, spasms), fatty degeneration of the liver
and of the epithelium of the renal tubules, vacuolization of the
cerebral cells and a marked increase in mortality rate were observed.
With 10 mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950 and 1951; Kewitz, 1952; Kewitz & Reinert, 1952); when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of lindane, the general behaviour of the animals, their
body-weight, histological findings and the post-mortem examination
showed no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to successive administration
by the intramuscular route of daily doses of 40 mg lindane for 19
days, 80 mg for 9 days and 160 mg for three days. The animals died and
showed inter alia hepatic degeneration (Dallemagne & Philippot,
1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed
hepatic lesions (Woodward & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10%
solution of lindane in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of lindane brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied lindane during an
antimalaria campaign, about 46% became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal
applications of 0.5% lindane in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3% of lindane and were observed for 10 months. No abnormalities were
found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given
for 2 years diets containing 5 to 1600 ppm of the alpha- beta-, and
gamma-isomers of lindane. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of lindane, the lowest concentration causing significant
liver charges was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of
20 young rats (10 males and 10 females) were fed diets containing 25,
50 and 100 ppm of lindane throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration. 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets
containing 50 and 100 ppm of lindane; histological lesions were found
in the liver parenchyma (Ortega et al., 1957).
Twenty-five young rats were given lindane daily by stomach-tube
at a dosage of 10 µg/kg body-weight (corresponding to about 0.1 and
0.15 ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments on the experimental studies reported
In all these animal species, lindane has proved to be a
cumulative poison causing hepatic and renal lesions and disturbances
of the central nervous system. The dog seems particularly susceptible
to neurological effects.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty-five ppm of lindane approximately equivalent to 1.25 mg/kg
of bodyweight.
Estimate of the maximal acceptable daily intake for man
0-0.0125 mg/kg.
Further work desirable
Investigation on the chemical nature of the residue occurring in
the plant. Additional biochemical studies. Long-term feeding studies
in other species than the rat.
REFERENCES
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Dallemagne, M. J. & Philippot, E. (1948) Arch. Int. Pharm. Ther.,
76, 274
Doisy, E. A. J. & Bocklage, B. C. (1949) Proc. soc. exp. Biol.
(N.Y.), 71, 490
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Phamacol.
exp. Ther., 100, 59
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel-Forsch., 1, 356
Kewitz, H. (1952) Arch. exp. Path. Phamakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. Path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. Path. Pharmakol., 227, 183
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) Arch. Path., 64,
614
Ray, E. E. et al., (1953) J. Amer. vet. med. Ass., 123, 448
Truhaut, R. (1954) Communication au symposium intern. de la prevention
du cancer, Sao Paulo
United States Public Health Service (1956) Clinical memoranda on
economic poisons
Van Asperen, K. (1957) C.R. 4è Congrès Intern. de lutte contre les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. Econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wassermann, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu,
G. & Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 387
BIOLOGICAL DATA
Biochemical aspects
Lindane is absorbed from the digestive tract, particularly in the
presence of lipids. Once absorbed, lindane is distributed to various
tissues or organs, accumulating above all in the fat depots (Van
Asperen, 1957), liver and kidneys. The rate of accumulation of lindane
in growing animals is however, much lower than that of beta-BHC or of
DDT. In rats ingesting repeated small doses of lindane, the
concentration in the fat depots is in a state of equilibrium with the
concentration of the diet (Lehman, 1953). The data on lindane
metabolism are very incomplete. According to certain in vitro tests
it would seem that part is broken down in the liver. The unchanged
compound is eliminated in the faeces and urine and is found in the
milk (Ray et al., 1953; Van Asperen, 1957; Ware & Gilmore, 1959). Very
little information is available about its effects on basic enzyme
systems. Growing animals are particularly susceptible to its toxic
action.
Acute toxicity
From the viewpoint of acute toxicity, lindane is the most toxic
of all the isomers present in the technical hexachlorocyclohexanes. It
is a neurotropic poison causing excitation of the central nervous
system with convulsions followed - if the dose is sufficiently high
- by depressive phenomena leading to death from respiratory collapse.
Animal Route LD50 mg/kg References
body-weight
Rat Oral 125, 200 Dallemagne & Philippot,
1948
Lehman, 1948
Lehman, 1951
US Public Health
Service, 1956
177-230 Negherbon, 1959
Rat Intraperitoneal 35-85 Negherbon, 1959
Mouse Oral 86 Negherbon, 1959
Guinea-pig Oral 100-127 Lehman, 1948
Lehman, 1951
Negherbon, 1959
Rabbit Oral 60-200 Cameron, 1945
Negherbon, 1959
Dog Oral 40-200 Negherbon, 1959
(lethal)
Dog Intravenous 7.5 Negherbon, 1959
(lethal)
The fatal dose for man is estimated to be 150 mg/kg body-weight,
i.e., of the order of 10 g for an adult weighing 70 kg. Children are
particularly susceptible.
Short-term studies
Rat. When rats were fed a diet containing 400 ppm lindane for 4
weeks, hepatic lesions appeared (Doisy & Bocklage, 1949).
Forty-eight rats (24 male and 24 female) were given lindane by
stomach-tube in a daily dose of 32 mg/kg body-weight for 6 months.
Nervous symptoms (trembling, spasms), fatty degeneration of the liver
and of the epithelium of the renal tubules, vacuolization of the
cerebral cells and a marked increase in mortality rate were observed.
With 10 mg/kg body-weight for 17 months no abnormalities were revealed
(Klimmer, 1955). These findings of functional disturbances in the
central nervous system were in accordance with previous results
(Herken, 1950 and 1951; Kewitz, 1952; Kewitz & Reinert, 1952); when 60
rats were fed for 12 months with a diet containing 2, 3, 4, 5 and 10
ppm of lindane, the general behaviour of the animals, their
body-weight, histological findings and the post-mortem examination
showed no abnormalities (Melis, 1955).
Rabbit. Two rabbits were subjected to successive administration
by the intramuscular route of daily doses of 40 mg lindane for 19
days, 80 mg for 9 days and 160 mg for three days. The animals died and
showed inter alia hepatic degeneration (Dallemagne & Philippot,
1948).
Dog. Dogs fed 10 mg/kg body-weight for 18 to 49 days showed
hepatic lesions (Woodward & Hagan, 1947).
Five dogs were given daily intramuscular injections of a 10%
solution of lindane in oil ranging from 20 to 390 mg/kg body-weight.
This repeated administration of lindane brought about convulsions,
paralysis and anorexia (Dallemagne & Philippot, 1948).
Man. Of 148 spray operators who applied lindane during an
antimalaria campaign, about 46% became affected. The clinical
examination showed digestive and respiratory disorders, cutaneous
symptoms, and neurological effects (asthma, polyneuritis). There is a
lack of precise information on the chemical composition of the
hexachlorocyclohexane employed, on the conditions of application and
on the amount absorbed (Wassermann et al., 1960; Wassermann et al.,
1961).
Long-term studies
Mouse. Groups of 30 mice each were subjected to dermal
applications of 0.5% lindane in acetone. The applications were given
twice weekly over a period of 15 months. Another group of animals
received subcutaneous implantation of paraffin wax pellets containing
3% of lindane and were observed for 10 months. No abnormalities were
found (Orr, 1948).
Rat. Groups of 20 rats (10 males and 10 females) were given
for 2 years diets containing 5 to 1600 ppm of the alpha- beta-, and
gamma-isomers of lindane. These experiments clearly showed that the
gamma-isomer was the least toxic and the beta-isomer the most toxic.
The organs injured were the liver and to a lesser extent the kidneys.
In the case of lindane, the lowest concentration causing significant
liver charges was 100 ppm. No significant effect was noted at
concentrations of 50 ppm or lower (Fitzhugh et al., 1950).
With the aim of detecting any carcinogenic action, 3 groups of
20 young rats (10 males and 10 females) were fed diets containing 25,
50 and 100 ppm of lindane throughout their life-span. At the 2 highest
concentrations, slight hypertrophy of the liver was observed, while
with 100 ppm there was a slight tendency to fatty degeneration of this
organ. At the lowest concentration. 25 ppm, there was no toxic effect,
in particular, no histological lesions were detected in the liver or
kidney. None of the concentrations used gave any significant increase
in the percentage of tumours as compared with the control group
(Truhaut, 1954).
Groups of 12 rats (6 males and 6 females) were fed diets
containing 50 and 100 ppm of lindane; histological lesions were found
in the liver parenchyma (Ortega et al., 1957).
Twenty-five young rats were given lindane daily by stomach-tube
at a dosage of 10 µg/kg body-weight (corresponding to about 0.1 and
0.15 ppm) for 17 months. No abnormalities were found (Klimmer, 1955).
Comments on the experimental studies reported
In all these animal species, lindane has proved to be a
cumulative poison causing hepatic and renal lesions and disturbances
of the central nervous system. The dog seems particularly susceptible
to neurological effects.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty-five ppm of lindane approximately equivalent to 1.25 mg/kg
of bodyweight.
Estimate of the maximal acceptable daily intake for man
0-0.0125 mg/kg.
Further work desirable
Investigation on the chemical nature of the residue occurring in
the plant. Additional biochemical studies. Long-term feeding studies
in other species than the rat.
REFERENCES
Cameron, G. R. (1945) Brit. med. Bull., 3, 780
Dallemagne, M. J. & Philippot, E. (1948) Arch. Int. Pharm. Ther.,
76, 274
Doisy, E. A. J. & Bocklage, B. C. (1949) Proc. soc. exp. Biol.
(N.Y.), 71, 490
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1950) J. Phamacol.
exp. Ther., 100, 59
Herken, H. (1950) Arztl. Wschr., 5, 193
Herken, H. (1951) Arzneimittel-Forsch., 1, 356
Kewitz, H. (1952) Arch. exp. Path. Phamakol., 216, 161
Kewitz, H. & Reinert, H. (1952) Arch. exp. Path. Pharmakol., 215,
93
Klimmer, O. R. (1955) Arch. exp. Path. Pharmakol., 227, 183
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1953) Quart. Bull. Assoc. Food and Drug Officials
U.S., 17, 3-12
Melis, R. (1955) Nuovi Ann. Ig., 6 (2), 90
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Orr, J. W. (1948) Nature, 162, 189
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) Arch. Path., 64,
614
Ray, E. E. et al., (1953) J. Amer. vet. med. Ass., 123, 448
Truhaut, R. (1954) Communication au symposium intern. de la prevention
du cancer, Sao Paulo
United States Public Health Service (1956) Clinical memoranda on
economic poisons
Van Asperen, K. (1957) C.R. 4è Congrès Intern. de lutte contre les
ennemis des plantes, Hambourg, 2, 1619
Ware, G. W. & Gilmore, L. O. (1959) J. Econ. Ent., 52, 349
Wasserman, M., Sandulescu, G., Iliescu, S. & Mandric, G. (1960)
Arch. Mal. prof., 21, 195
Wassermann, M., Pendefunda, G., Merling, M., Mihail, G., Sandulescu,
G. & Vancea, G. (1961) Arch. Mal. prof., 22, 308
Woodard, G. & Hagan, E. C. (1947) Fed. Proc., 6, 387
