
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DICHLORVOS
Chemical name
O,O-dimethyl 2,2-dichlorovinyl phosphate
Synonyms
O,O-dimethyl,-2,2-dichlorovinyl phosphate, dimethyl
2,2-dichlorovinyl phosphate
Empirical formula
C4H7O4PCl2
Structural formula
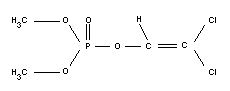 Relevant physical or chemical properties
The molecular weight of dichlorvos is 221 whereas those of the
other commonly used organo-phosphorus insecticides (e.g. parathion,
malathion) are in the order of 300. The low molecular weight is
associated with a relatively high vapour pressure as compared with
other organic phosphorus compounds but a low vapour pressure as
compared with compounds generally regarded as fumigants (see Table 1).
Dichlorvos penetrates closely packed material, such as grain,
very poorly, but it has exceptional ability to permeate air spaces at
a distance from the point of application in spite of considerable
ventilation.
Dichlorvos hydrolyses rapidly in the presence of moisture. The
initial breakdown products we considered to be dimethyl phosphoric
acid and dichloroacetaldehyde. The latter tends to be oxidized on
exposure to air to dichloroacetic acid. Formulations (and sometimes
areas treated with dichlorvos) will exhibit a sour or vinegar-like
odour due to the dichloroacetic acid if hydrolysis has occurred to any
extent. Dichlorvos itself is odourless at air concentrations
recommended or normally encountered.
Use
Dichlorvos is used to combat flying or other exposed insects in
buildings and other closed spaces, e.g. control of cigarette beetles
in tobacco warehouses and control of several species in greenhouses,
barns, poultry houses, mushroom houses, homes and restaurants and
other food handling establishments. It is now receiving final inflight
tests for the control of flies, mosquitos, and other disease vectors
in aircraft. It has shown promise in the control of malaria mosquitos
in houses. Its use for the control of pests of crops and of stored
products is being explored.
TABLE 1. MOLECULAR WEIGHT AND VAPOUR PRESSURE OF SELECTED COMPOUNDS
Compound Molecular weight Vapour pressure
(mm Hg)
Sarin 140 -
Dichlorvos 221 0.025 at 25°C
0.032 at 32.2°C
Parathion 291 0.0000378 at 20°C
Malathion 330 0.00004 at 30°C
Phosphine (PH3) 34 29 200 at 25°C
Cyanide (HCN) 27 760 at 26°C
Carbon tetrachloride 154 100 at 25°C
Tetrachlorethylene 166 18 at 25°C
Residues
To date, most of the residue data have been produced by employing
a non-specific cholinesterase inhibition (spectrophotometric) method
(Giang & Hall, 1951; Giang, Smith & Hall, 1956; USDA, 1965). The
sensitivity claimed for this method is 0.1 ppm in most of the products
examined to date, but in tobacco and high fat commodities sensitivity
is reduced to 0.5 ppm. A tentative method employing microcoulometric
gas chromotography sensitive to 0.05-0.1 ppm in some vegetables is
being evaluated (Kiigamagi & Terriere, 1964).
A negative test by the cholinesterase methods gives some degree
of assurance that residues are not present. Since one or more degraded
esters or metabolites of other organic phosphate insecticides are
commonly found if a positive cholinesterase test results, it is
necessary to at least employ the two methods described by Getz, 1962a
and 1962b, for initial identification of residues or degradation
products.
A specific insect bioassay, using Drosophila melanogaster has
been used to detect residues of dichlorvos (Sun & Johnson, 1963). This
method appears to be interesting due to its specificity.
To date no reports are available on effects of processing food
after treatment with dichlorvos (milling, baking, etc.).
Effect on treated crop
No reports are available to indicate whether or not dichlorvos
combines with food or alters its nutritive value.
BIOLOGICAL DATA
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. On the other hand, dichlorvos is broken
down rapidly in the liver (Tracy, 1960; Tracy et al., 1960). Thus, the
onset of poisoning is rapid and, if recovery occurs, it is prompt
(Klotzsche, 1956; Durham et al., 1957; Gaines, 1960; Yamashita, 1962).
The compound is not stored in the body. It is not excreted in the milk
of cows or rats even when administered in doses that produce severe
poisoning (Tracy, 1960; Tracy et al., 1960).
Both single and repeated large doses cause a reduction in the
eosinophil count of the peripheral blood of rats that was attributed
to stress (Klotzsche, 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56* Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80** Durham et al., 1957
Mouse Oral 124 Yamashita, 1962
(continued)
Animal Route LD50 mg/kg References
body-weight
Chick, male Oral 14.8 Sherman & Ross, 1961
* Based on 99% pure material.
** Result of 2 tests on a technical preparation (90% pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5 to 17.5 hours
(Durham et al., 1957).
Domestic animals. Horses tolerated a single dosage of
dichlorvos in feed at the rate of 50 mg/kg but showed moderate acute
poisoning when the insecticide was given by stomach-tube at the rate
of 25 mg/kg (Jackson et al. 1960). An almost identical dose (27 mg/kg)
given by stomach-tube caused severe but non-fatal poisoning in a cow
(Tracy et al. 1960).
Dichlorvos does not produce either immediate or delayed paralysis
in hens (Durham et al. 1957).
Man. Men withstood brief exposure (30-60 minutes) to
concentrations as high as 6.9 µg/l without depression of
cholinesterase activity or any other observed effect (Durham et al.
1959; Hayes, 1961). A single exposure for 8 hours at a concentration
ranging from 0.9 to 3.5 µg/l produced slight inhibition of plasma
cholinesterase activity in man (Witter et al. 1961). Slightly higher
concentrations or longer periods of exposure, or both, produce
measurable reduction of both the red cell and plasma enzyme activity
in men, monkeys and rats (Hayes, 1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood
cholinesterase activity but much larger doses are required to produce
illness. Thus, Durham et al. (1957) found that a dietary concentration
of only 50 ppm soon produced detectable lowering of plasm and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
dimunition of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (T. B. Gaines, unpublished results).
When dichlorvos is given by stomach-tube it is not so well
tolerated as when the same dosage is absorbed from the diet gradually
throughout the day. Thus, Tracy et al. (1960) found that female rats
tolerated doses of 10 and 20 mg/kg but suffered severe, acute
poisoning when given doses of 30 mg/kg. Even at 30 mg/kg, the rats
survived and the red cell cholinesterase activity and growth of their
litters were normal.
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13 to 0.37 mg/kg/day) produced no
detectable effect; rates equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33% of normal, respectively,
and to some increase in the activity and aggressiveness of the dogs
(Blucher et al. 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in
a monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al. 1957).
Monkeys tolerated continuous exposure to concentrations ranging
from 0.1-0.5 µg/l for 22 days without a definite change in
cholinesterase activity. They showed a definite depletion by the 50th
day of exposure (Durham et al. 1959), but the concentration of the
insecticide may have exceeded 0.5 µg/l sometime between the 22nd and
50th day (Hayes, 1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24 to 1.48 µg/l, but they returned to normal in spite
of continuing exposure (Tracy et al. 1960).
Man. Men showed no change in cholinesterase activity when
exposed for 8-10 half-hour intervals, 4 nights per week, for 11 weeks
to concentrations ranging from 0.07 to 0.66 µg/l (average 0.25 µg/l).
They did show a small but statistically significant depression of
plasm enzyme activity, but not of red cell enzyme activity, when the
schedule of dosing was maintained but the concentration increased to
0.40.0.55 µg/l (average 0.51 µg/l). The other parameters studied,
including complex reaction time, airway resistance and vision,
remained normal (Rasmussen et al. 1963). The tolerated inhaled dosage
averaged 0.5 mg/man/day while the dosage that caused a slight fall of
plasma cholinesterase, was 1.1 mg/man/day. The compound produced no
decrease in blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al. 1963; Gratz et
al. 1962).
Experiments on rats show that the liver is highly efficient in
detoxicating dichlorvos (Gaines, T. B., in preparation) and that the
compound is absorbed from the gastro-intestinal tract by the blood of
the hepatic portal system (Laws, E. R., in preparation). Thus, a given
dose of dichlorvos absorbed after ingestion is less toxic than the
same dose absorbed in an identical time following inhalation.
Long-term studies
No really long-term studies have been made of dichlorvos, nor do
they appear indicated because of the rapid action and excretion of the
compound.
Comments on experimental work reported and evaluation
Dichlorvos has a greater effect when inhaled than when given by
mouth. So far the only maximum level causing no significant
toxicological effect for man has been determined by inhalation
experiments and is 0.01 mg/kg/day. In dogs, the maximum oral no-effect
level was 0.37 mg/kg/day. Until more data on oral toxicity to man are
forthcoming, an acceptable daily intake cannot be established.
Further work required
Determination of oral toxicity for man.
REFERENCES
Blucher, W., Budd, E. R. Dewey, M. L., Eisenlord, G. Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Report from the
Hine Laboratories, San Francisco, California
Durham, W. F., Gaines, T. B., McCauley, R. H., jr, Sedlak, V. A.,
Mattson, A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr.
Hlth, 15, 340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes. W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gaines, T. B., A technique for studying the net effect of metabolism
of compounds in the liver on their toxicity (In preparation)
Gaines, T. B., Effect of dietary DDVP on reproduction in rats and on
survival of their offspring. Unpublished results
Getz, M. E. (1962a) J. Ass. Offic. Agr. Chem., 45, 397
Getz, M. E. (1962b) J. Ass. Offic. Agr. Chem., 45, 393
Giang, P. A. & Hall, S. A. (1951) Anal. Chem., 23, 1830
Giang, P. A., Smith, F. F. & Hall, S. A. (1956) J. Agr. Food Chem.,
4, 621
Gratz, N. G., Bracha, P. & Carmichael, A. G. (1962) WHO/Vector
Control/11, 49 pp.
Hayes, W. J., jr (1961) Bull, Wld Hlth Org., 24, 629
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960)
J. econ. Ent., 53, 602
Kiigamagi, V. & Terriere, L. C. (1964) Shell Agr. Chem. Div., New York
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Laws, E. R., jr, The absorption of DDVP (In preparation)
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 3, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Sun, Y. P. & Johnson, E. R. (1963) J. Ass. Offic. Agr. Chem., 46,
524
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
USDA. 1965 Method Dr. 5e-62 dated 1 March 1962, rev. Nov. 20 1965.
Stored Prod. Insect Lab., Agr. Res. Serv., USDA, Savannah, Ga
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
Witherup, S., & Schlecht, H. (1962) Report from the Kettering
Laboratory, University of Cincinnati, Cincinnati, Ohio, 14 pp.
Witter. R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Relevant physical or chemical properties
The molecular weight of dichlorvos is 221 whereas those of the
other commonly used organo-phosphorus insecticides (e.g. parathion,
malathion) are in the order of 300. The low molecular weight is
associated with a relatively high vapour pressure as compared with
other organic phosphorus compounds but a low vapour pressure as
compared with compounds generally regarded as fumigants (see Table 1).
Dichlorvos penetrates closely packed material, such as grain,
very poorly, but it has exceptional ability to permeate air spaces at
a distance from the point of application in spite of considerable
ventilation.
Dichlorvos hydrolyses rapidly in the presence of moisture. The
initial breakdown products we considered to be dimethyl phosphoric
acid and dichloroacetaldehyde. The latter tends to be oxidized on
exposure to air to dichloroacetic acid. Formulations (and sometimes
areas treated with dichlorvos) will exhibit a sour or vinegar-like
odour due to the dichloroacetic acid if hydrolysis has occurred to any
extent. Dichlorvos itself is odourless at air concentrations
recommended or normally encountered.
Use
Dichlorvos is used to combat flying or other exposed insects in
buildings and other closed spaces, e.g. control of cigarette beetles
in tobacco warehouses and control of several species in greenhouses,
barns, poultry houses, mushroom houses, homes and restaurants and
other food handling establishments. It is now receiving final inflight
tests for the control of flies, mosquitos, and other disease vectors
in aircraft. It has shown promise in the control of malaria mosquitos
in houses. Its use for the control of pests of crops and of stored
products is being explored.
TABLE 1. MOLECULAR WEIGHT AND VAPOUR PRESSURE OF SELECTED COMPOUNDS
Compound Molecular weight Vapour pressure
(mm Hg)
Sarin 140 -
Dichlorvos 221 0.025 at 25°C
0.032 at 32.2°C
Parathion 291 0.0000378 at 20°C
Malathion 330 0.00004 at 30°C
Phosphine (PH3) 34 29 200 at 25°C
Cyanide (HCN) 27 760 at 26°C
Carbon tetrachloride 154 100 at 25°C
Tetrachlorethylene 166 18 at 25°C
Residues
To date, most of the residue data have been produced by employing
a non-specific cholinesterase inhibition (spectrophotometric) method
(Giang & Hall, 1951; Giang, Smith & Hall, 1956; USDA, 1965). The
sensitivity claimed for this method is 0.1 ppm in most of the products
examined to date, but in tobacco and high fat commodities sensitivity
is reduced to 0.5 ppm. A tentative method employing microcoulometric
gas chromotography sensitive to 0.05-0.1 ppm in some vegetables is
being evaluated (Kiigamagi & Terriere, 1964).
A negative test by the cholinesterase methods gives some degree
of assurance that residues are not present. Since one or more degraded
esters or metabolites of other organic phosphate insecticides are
commonly found if a positive cholinesterase test results, it is
necessary to at least employ the two methods described by Getz, 1962a
and 1962b, for initial identification of residues or degradation
products.
A specific insect bioassay, using Drosophila melanogaster has
been used to detect residues of dichlorvos (Sun & Johnson, 1963). This
method appears to be interesting due to its specificity.
To date no reports are available on effects of processing food
after treatment with dichlorvos (milling, baking, etc.).
Effect on treated crop
No reports are available to indicate whether or not dichlorvos
combines with food or alters its nutritive value.
BIOLOGICAL DATA
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. On the other hand, dichlorvos is broken
down rapidly in the liver (Tracy, 1960; Tracy et al., 1960). Thus, the
onset of poisoning is rapid and, if recovery occurs, it is prompt
(Klotzsche, 1956; Durham et al., 1957; Gaines, 1960; Yamashita, 1962).
The compound is not stored in the body. It is not excreted in the milk
of cows or rats even when administered in doses that produce severe
poisoning (Tracy, 1960; Tracy et al., 1960).
Both single and repeated large doses cause a reduction in the
eosinophil count of the peripheral blood of rats that was attributed
to stress (Klotzsche, 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56* Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80** Durham et al., 1957
Mouse Oral 124 Yamashita, 1962
(continued)
Animal Route LD50 mg/kg References
body-weight
Chick, male Oral 14.8 Sherman & Ross, 1961
* Based on 99% pure material.
** Result of 2 tests on a technical preparation (90% pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5 to 17.5 hours
(Durham et al., 1957).
Domestic animals. Horses tolerated a single dosage of
dichlorvos in feed at the rate of 50 mg/kg but showed moderate acute
poisoning when the insecticide was given by stomach-tube at the rate
of 25 mg/kg (Jackson et al. 1960). An almost identical dose (27 mg/kg)
given by stomach-tube caused severe but non-fatal poisoning in a cow
(Tracy et al. 1960).
Dichlorvos does not produce either immediate or delayed paralysis
in hens (Durham et al. 1957).
Man. Men withstood brief exposure (30-60 minutes) to
concentrations as high as 6.9 µg/l without depression of
cholinesterase activity or any other observed effect (Durham et al.
1959; Hayes, 1961). A single exposure for 8 hours at a concentration
ranging from 0.9 to 3.5 µg/l produced slight inhibition of plasma
cholinesterase activity in man (Witter et al. 1961). Slightly higher
concentrations or longer periods of exposure, or both, produce
measurable reduction of both the red cell and plasma enzyme activity
in men, monkeys and rats (Hayes, 1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood
cholinesterase activity but much larger doses are required to produce
illness. Thus, Durham et al. (1957) found that a dietary concentration
of only 50 ppm soon produced detectable lowering of plasm and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
dimunition of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (T. B. Gaines, unpublished results).
When dichlorvos is given by stomach-tube it is not so well
tolerated as when the same dosage is absorbed from the diet gradually
throughout the day. Thus, Tracy et al. (1960) found that female rats
tolerated doses of 10 and 20 mg/kg but suffered severe, acute
poisoning when given doses of 30 mg/kg. Even at 30 mg/kg, the rats
survived and the red cell cholinesterase activity and growth of their
litters were normal.
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13 to 0.37 mg/kg/day) produced no
detectable effect; rates equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33% of normal, respectively,
and to some increase in the activity and aggressiveness of the dogs
(Blucher et al. 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in
a monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al. 1957).
Monkeys tolerated continuous exposure to concentrations ranging
from 0.1-0.5 µg/l for 22 days without a definite change in
cholinesterase activity. They showed a definite depletion by the 50th
day of exposure (Durham et al. 1959), but the concentration of the
insecticide may have exceeded 0.5 µg/l sometime between the 22nd and
50th day (Hayes, 1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24 to 1.48 µg/l, but they returned to normal in spite
of continuing exposure (Tracy et al. 1960).
Man. Men showed no change in cholinesterase activity when
exposed for 8-10 half-hour intervals, 4 nights per week, for 11 weeks
to concentrations ranging from 0.07 to 0.66 µg/l (average 0.25 µg/l).
They did show a small but statistically significant depression of
plasm enzyme activity, but not of red cell enzyme activity, when the
schedule of dosing was maintained but the concentration increased to
0.40.0.55 µg/l (average 0.51 µg/l). The other parameters studied,
including complex reaction time, airway resistance and vision,
remained normal (Rasmussen et al. 1963). The tolerated inhaled dosage
averaged 0.5 mg/man/day while the dosage that caused a slight fall of
plasma cholinesterase, was 1.1 mg/man/day. The compound produced no
decrease in blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al. 1963; Gratz et
al. 1962).
Experiments on rats show that the liver is highly efficient in
detoxicating dichlorvos (Gaines, T. B., in preparation) and that the
compound is absorbed from the gastro-intestinal tract by the blood of
the hepatic portal system (Laws, E. R., in preparation). Thus, a given
dose of dichlorvos absorbed after ingestion is less toxic than the
same dose absorbed in an identical time following inhalation.
Long-term studies
No really long-term studies have been made of dichlorvos, nor do
they appear indicated because of the rapid action and excretion of the
compound.
Comments on experimental work reported and evaluation
Dichlorvos has a greater effect when inhaled than when given by
mouth. So far the only maximum level causing no significant
toxicological effect for man has been determined by inhalation
experiments and is 0.01 mg/kg/day. In dogs, the maximum oral no-effect
level was 0.37 mg/kg/day. Until more data on oral toxicity to man are
forthcoming, an acceptable daily intake cannot be established.
Further work required
Determination of oral toxicity for man.
REFERENCES
Blucher, W., Budd, E. R. Dewey, M. L., Eisenlord, G. Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Report from the
Hine Laboratories, San Francisco, California
Durham, W. F., Gaines, T. B., McCauley, R. H., jr, Sedlak, V. A.,
Mattson, A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr.
Hlth, 15, 340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes. W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gaines, T. B., A technique for studying the net effect of metabolism
of compounds in the liver on their toxicity (In preparation)
Gaines, T. B., Effect of dietary DDVP on reproduction in rats and on
survival of their offspring. Unpublished results
Getz, M. E. (1962a) J. Ass. Offic. Agr. Chem., 45, 397
Getz, M. E. (1962b) J. Ass. Offic. Agr. Chem., 45, 393
Giang, P. A. & Hall, S. A. (1951) Anal. Chem., 23, 1830
Giang, P. A., Smith, F. F. & Hall, S. A. (1956) J. Agr. Food Chem.,
4, 621
Gratz, N. G., Bracha, P. & Carmichael, A. G. (1962) WHO/Vector
Control/11, 49 pp.
Hayes, W. J., jr (1961) Bull, Wld Hlth Org., 24, 629
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960)
J. econ. Ent., 53, 602
Kiigamagi, V. & Terriere, L. C. (1964) Shell Agr. Chem. Div., New York
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Laws, E. R., jr, The absorption of DDVP (In preparation)
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 3, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Sun, Y. P. & Johnson, E. R. (1963) J. Ass. Offic. Agr. Chem., 46,
524
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
USDA. 1965 Method Dr. 5e-62 dated 1 March 1962, rev. Nov. 20 1965.
Stored Prod. Insect Lab., Agr. Res. Serv., USDA, Savannah, Ga
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
Witherup, S., & Schlecht, H. (1962) Report from the Kettering
Laboratory, University of Cincinnati, Cincinnati, Ohio, 14 pp.
Witter. R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
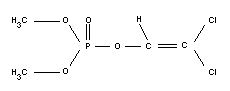 Relevant physical or chemical properties
The molecular weight of dichlorvos is 221 whereas those of the
other commonly used organo-phosphorus insecticides (e.g. parathion,
malathion) are in the order of 300. The low molecular weight is
associated with a relatively high vapour pressure as compared with
other organic phosphorus compounds but a low vapour pressure as
compared with compounds generally regarded as fumigants (see Table 1).
Dichlorvos penetrates closely packed material, such as grain,
very poorly, but it has exceptional ability to permeate air spaces at
a distance from the point of application in spite of considerable
ventilation.
Dichlorvos hydrolyses rapidly in the presence of moisture. The
initial breakdown products we considered to be dimethyl phosphoric
acid and dichloroacetaldehyde. The latter tends to be oxidized on
exposure to air to dichloroacetic acid. Formulations (and sometimes
areas treated with dichlorvos) will exhibit a sour or vinegar-like
odour due to the dichloroacetic acid if hydrolysis has occurred to any
extent. Dichlorvos itself is odourless at air concentrations
recommended or normally encountered.
Use
Dichlorvos is used to combat flying or other exposed insects in
buildings and other closed spaces, e.g. control of cigarette beetles
in tobacco warehouses and control of several species in greenhouses,
barns, poultry houses, mushroom houses, homes and restaurants and
other food handling establishments. It is now receiving final inflight
tests for the control of flies, mosquitos, and other disease vectors
in aircraft. It has shown promise in the control of malaria mosquitos
in houses. Its use for the control of pests of crops and of stored
products is being explored.
TABLE 1. MOLECULAR WEIGHT AND VAPOUR PRESSURE OF SELECTED COMPOUNDS
Compound Molecular weight Vapour pressure
(mm Hg)
Sarin 140 -
Dichlorvos 221 0.025 at 25°C
0.032 at 32.2°C
Parathion 291 0.0000378 at 20°C
Malathion 330 0.00004 at 30°C
Phosphine (PH3) 34 29 200 at 25°C
Cyanide (HCN) 27 760 at 26°C
Carbon tetrachloride 154 100 at 25°C
Tetrachlorethylene 166 18 at 25°C
Residues
To date, most of the residue data have been produced by employing
a non-specific cholinesterase inhibition (spectrophotometric) method
(Giang & Hall, 1951; Giang, Smith & Hall, 1956; USDA, 1965). The
sensitivity claimed for this method is 0.1 ppm in most of the products
examined to date, but in tobacco and high fat commodities sensitivity
is reduced to 0.5 ppm. A tentative method employing microcoulometric
gas chromotography sensitive to 0.05-0.1 ppm in some vegetables is
being evaluated (Kiigamagi & Terriere, 1964).
A negative test by the cholinesterase methods gives some degree
of assurance that residues are not present. Since one or more degraded
esters or metabolites of other organic phosphate insecticides are
commonly found if a positive cholinesterase test results, it is
necessary to at least employ the two methods described by Getz, 1962a
and 1962b, for initial identification of residues or degradation
products.
A specific insect bioassay, using Drosophila melanogaster has
been used to detect residues of dichlorvos (Sun & Johnson, 1963). This
method appears to be interesting due to its specificity.
To date no reports are available on effects of processing food
after treatment with dichlorvos (milling, baking, etc.).
Effect on treated crop
No reports are available to indicate whether or not dichlorvos
combines with food or alters its nutritive value.
BIOLOGICAL DATA
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. On the other hand, dichlorvos is broken
down rapidly in the liver (Tracy, 1960; Tracy et al., 1960). Thus, the
onset of poisoning is rapid and, if recovery occurs, it is prompt
(Klotzsche, 1956; Durham et al., 1957; Gaines, 1960; Yamashita, 1962).
The compound is not stored in the body. It is not excreted in the milk
of cows or rats even when administered in doses that produce severe
poisoning (Tracy, 1960; Tracy et al., 1960).
Both single and repeated large doses cause a reduction in the
eosinophil count of the peripheral blood of rats that was attributed
to stress (Klotzsche, 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56* Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80** Durham et al., 1957
Mouse Oral 124 Yamashita, 1962
(continued)
Animal Route LD50 mg/kg References
body-weight
Chick, male Oral 14.8 Sherman & Ross, 1961
* Based on 99% pure material.
** Result of 2 tests on a technical preparation (90% pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5 to 17.5 hours
(Durham et al., 1957).
Domestic animals. Horses tolerated a single dosage of
dichlorvos in feed at the rate of 50 mg/kg but showed moderate acute
poisoning when the insecticide was given by stomach-tube at the rate
of 25 mg/kg (Jackson et al. 1960). An almost identical dose (27 mg/kg)
given by stomach-tube caused severe but non-fatal poisoning in a cow
(Tracy et al. 1960).
Dichlorvos does not produce either immediate or delayed paralysis
in hens (Durham et al. 1957).
Man. Men withstood brief exposure (30-60 minutes) to
concentrations as high as 6.9 µg/l without depression of
cholinesterase activity or any other observed effect (Durham et al.
1959; Hayes, 1961). A single exposure for 8 hours at a concentration
ranging from 0.9 to 3.5 µg/l produced slight inhibition of plasma
cholinesterase activity in man (Witter et al. 1961). Slightly higher
concentrations or longer periods of exposure, or both, produce
measurable reduction of both the red cell and plasma enzyme activity
in men, monkeys and rats (Hayes, 1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood
cholinesterase activity but much larger doses are required to produce
illness. Thus, Durham et al. (1957) found that a dietary concentration
of only 50 ppm soon produced detectable lowering of plasm and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
dimunition of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (T. B. Gaines, unpublished results).
When dichlorvos is given by stomach-tube it is not so well
tolerated as when the same dosage is absorbed from the diet gradually
throughout the day. Thus, Tracy et al. (1960) found that female rats
tolerated doses of 10 and 20 mg/kg but suffered severe, acute
poisoning when given doses of 30 mg/kg. Even at 30 mg/kg, the rats
survived and the red cell cholinesterase activity and growth of their
litters were normal.
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13 to 0.37 mg/kg/day) produced no
detectable effect; rates equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33% of normal, respectively,
and to some increase in the activity and aggressiveness of the dogs
(Blucher et al. 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in
a monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al. 1957).
Monkeys tolerated continuous exposure to concentrations ranging
from 0.1-0.5 µg/l for 22 days without a definite change in
cholinesterase activity. They showed a definite depletion by the 50th
day of exposure (Durham et al. 1959), but the concentration of the
insecticide may have exceeded 0.5 µg/l sometime between the 22nd and
50th day (Hayes, 1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24 to 1.48 µg/l, but they returned to normal in spite
of continuing exposure (Tracy et al. 1960).
Man. Men showed no change in cholinesterase activity when
exposed for 8-10 half-hour intervals, 4 nights per week, for 11 weeks
to concentrations ranging from 0.07 to 0.66 µg/l (average 0.25 µg/l).
They did show a small but statistically significant depression of
plasm enzyme activity, but not of red cell enzyme activity, when the
schedule of dosing was maintained but the concentration increased to
0.40.0.55 µg/l (average 0.51 µg/l). The other parameters studied,
including complex reaction time, airway resistance and vision,
remained normal (Rasmussen et al. 1963). The tolerated inhaled dosage
averaged 0.5 mg/man/day while the dosage that caused a slight fall of
plasma cholinesterase, was 1.1 mg/man/day. The compound produced no
decrease in blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al. 1963; Gratz et
al. 1962).
Experiments on rats show that the liver is highly efficient in
detoxicating dichlorvos (Gaines, T. B., in preparation) and that the
compound is absorbed from the gastro-intestinal tract by the blood of
the hepatic portal system (Laws, E. R., in preparation). Thus, a given
dose of dichlorvos absorbed after ingestion is less toxic than the
same dose absorbed in an identical time following inhalation.
Long-term studies
No really long-term studies have been made of dichlorvos, nor do
they appear indicated because of the rapid action and excretion of the
compound.
Comments on experimental work reported and evaluation
Dichlorvos has a greater effect when inhaled than when given by
mouth. So far the only maximum level causing no significant
toxicological effect for man has been determined by inhalation
experiments and is 0.01 mg/kg/day. In dogs, the maximum oral no-effect
level was 0.37 mg/kg/day. Until more data on oral toxicity to man are
forthcoming, an acceptable daily intake cannot be established.
Further work required
Determination of oral toxicity for man.
REFERENCES
Blucher, W., Budd, E. R. Dewey, M. L., Eisenlord, G. Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Report from the
Hine Laboratories, San Francisco, California
Durham, W. F., Gaines, T. B., McCauley, R. H., jr, Sedlak, V. A.,
Mattson, A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr.
Hlth, 15, 340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes. W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gaines, T. B., A technique for studying the net effect of metabolism
of compounds in the liver on their toxicity (In preparation)
Gaines, T. B., Effect of dietary DDVP on reproduction in rats and on
survival of their offspring. Unpublished results
Getz, M. E. (1962a) J. Ass. Offic. Agr. Chem., 45, 397
Getz, M. E. (1962b) J. Ass. Offic. Agr. Chem., 45, 393
Giang, P. A. & Hall, S. A. (1951) Anal. Chem., 23, 1830
Giang, P. A., Smith, F. F. & Hall, S. A. (1956) J. Agr. Food Chem.,
4, 621
Gratz, N. G., Bracha, P. & Carmichael, A. G. (1962) WHO/Vector
Control/11, 49 pp.
Hayes, W. J., jr (1961) Bull, Wld Hlth Org., 24, 629
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960)
J. econ. Ent., 53, 602
Kiigamagi, V. & Terriere, L. C. (1964) Shell Agr. Chem. Div., New York
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Laws, E. R., jr, The absorption of DDVP (In preparation)
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 3, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Sun, Y. P. & Johnson, E. R. (1963) J. Ass. Offic. Agr. Chem., 46,
524
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
USDA. 1965 Method Dr. 5e-62 dated 1 March 1962, rev. Nov. 20 1965.
Stored Prod. Insect Lab., Agr. Res. Serv., USDA, Savannah, Ga
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
Witherup, S., & Schlecht, H. (1962) Report from the Kettering
Laboratory, University of Cincinnati, Cincinnati, Ohio, 14 pp.
Witter. R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Relevant physical or chemical properties
The molecular weight of dichlorvos is 221 whereas those of the
other commonly used organo-phosphorus insecticides (e.g. parathion,
malathion) are in the order of 300. The low molecular weight is
associated with a relatively high vapour pressure as compared with
other organic phosphorus compounds but a low vapour pressure as
compared with compounds generally regarded as fumigants (see Table 1).
Dichlorvos penetrates closely packed material, such as grain,
very poorly, but it has exceptional ability to permeate air spaces at
a distance from the point of application in spite of considerable
ventilation.
Dichlorvos hydrolyses rapidly in the presence of moisture. The
initial breakdown products we considered to be dimethyl phosphoric
acid and dichloroacetaldehyde. The latter tends to be oxidized on
exposure to air to dichloroacetic acid. Formulations (and sometimes
areas treated with dichlorvos) will exhibit a sour or vinegar-like
odour due to the dichloroacetic acid if hydrolysis has occurred to any
extent. Dichlorvos itself is odourless at air concentrations
recommended or normally encountered.
Use
Dichlorvos is used to combat flying or other exposed insects in
buildings and other closed spaces, e.g. control of cigarette beetles
in tobacco warehouses and control of several species in greenhouses,
barns, poultry houses, mushroom houses, homes and restaurants and
other food handling establishments. It is now receiving final inflight
tests for the control of flies, mosquitos, and other disease vectors
in aircraft. It has shown promise in the control of malaria mosquitos
in houses. Its use for the control of pests of crops and of stored
products is being explored.
TABLE 1. MOLECULAR WEIGHT AND VAPOUR PRESSURE OF SELECTED COMPOUNDS
Compound Molecular weight Vapour pressure
(mm Hg)
Sarin 140 -
Dichlorvos 221 0.025 at 25°C
0.032 at 32.2°C
Parathion 291 0.0000378 at 20°C
Malathion 330 0.00004 at 30°C
Phosphine (PH3) 34 29 200 at 25°C
Cyanide (HCN) 27 760 at 26°C
Carbon tetrachloride 154 100 at 25°C
Tetrachlorethylene 166 18 at 25°C
Residues
To date, most of the residue data have been produced by employing
a non-specific cholinesterase inhibition (spectrophotometric) method
(Giang & Hall, 1951; Giang, Smith & Hall, 1956; USDA, 1965). The
sensitivity claimed for this method is 0.1 ppm in most of the products
examined to date, but in tobacco and high fat commodities sensitivity
is reduced to 0.5 ppm. A tentative method employing microcoulometric
gas chromotography sensitive to 0.05-0.1 ppm in some vegetables is
being evaluated (Kiigamagi & Terriere, 1964).
A negative test by the cholinesterase methods gives some degree
of assurance that residues are not present. Since one or more degraded
esters or metabolites of other organic phosphate insecticides are
commonly found if a positive cholinesterase test results, it is
necessary to at least employ the two methods described by Getz, 1962a
and 1962b, for initial identification of residues or degradation
products.
A specific insect bioassay, using Drosophila melanogaster has
been used to detect residues of dichlorvos (Sun & Johnson, 1963). This
method appears to be interesting due to its specificity.
To date no reports are available on effects of processing food
after treatment with dichlorvos (milling, baking, etc.).
Effect on treated crop
No reports are available to indicate whether or not dichlorvos
combines with food or alters its nutritive value.
BIOLOGICAL DATA
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. On the other hand, dichlorvos is broken
down rapidly in the liver (Tracy, 1960; Tracy et al., 1960). Thus, the
onset of poisoning is rapid and, if recovery occurs, it is prompt
(Klotzsche, 1956; Durham et al., 1957; Gaines, 1960; Yamashita, 1962).
The compound is not stored in the body. It is not excreted in the milk
of cows or rats even when administered in doses that produce severe
poisoning (Tracy, 1960; Tracy et al., 1960).
Both single and repeated large doses cause a reduction in the
eosinophil count of the peripheral blood of rats that was attributed
to stress (Klotzsche, 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56* Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80** Durham et al., 1957
Mouse Oral 124 Yamashita, 1962
(continued)
Animal Route LD50 mg/kg References
body-weight
Chick, male Oral 14.8 Sherman & Ross, 1961
* Based on 99% pure material.
** Result of 2 tests on a technical preparation (90% pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5 to 17.5 hours
(Durham et al., 1957).
Domestic animals. Horses tolerated a single dosage of
dichlorvos in feed at the rate of 50 mg/kg but showed moderate acute
poisoning when the insecticide was given by stomach-tube at the rate
of 25 mg/kg (Jackson et al. 1960). An almost identical dose (27 mg/kg)
given by stomach-tube caused severe but non-fatal poisoning in a cow
(Tracy et al. 1960).
Dichlorvos does not produce either immediate or delayed paralysis
in hens (Durham et al. 1957).
Man. Men withstood brief exposure (30-60 minutes) to
concentrations as high as 6.9 µg/l without depression of
cholinesterase activity or any other observed effect (Durham et al.
1959; Hayes, 1961). A single exposure for 8 hours at a concentration
ranging from 0.9 to 3.5 µg/l produced slight inhibition of plasma
cholinesterase activity in man (Witter et al. 1961). Slightly higher
concentrations or longer periods of exposure, or both, produce
measurable reduction of both the red cell and plasma enzyme activity
in men, monkeys and rats (Hayes, 1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood
cholinesterase activity but much larger doses are required to produce
illness. Thus, Durham et al. (1957) found that a dietary concentration
of only 50 ppm soon produced detectable lowering of plasm and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
dimunition of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (T. B. Gaines, unpublished results).
When dichlorvos is given by stomach-tube it is not so well
tolerated as when the same dosage is absorbed from the diet gradually
throughout the day. Thus, Tracy et al. (1960) found that female rats
tolerated doses of 10 and 20 mg/kg but suffered severe, acute
poisoning when given doses of 30 mg/kg. Even at 30 mg/kg, the rats
survived and the red cell cholinesterase activity and growth of their
litters were normal.
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13 to 0.37 mg/kg/day) produced no
detectable effect; rates equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33% of normal, respectively,
and to some increase in the activity and aggressiveness of the dogs
(Blucher et al. 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in
a monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al. 1957).
Monkeys tolerated continuous exposure to concentrations ranging
from 0.1-0.5 µg/l for 22 days without a definite change in
cholinesterase activity. They showed a definite depletion by the 50th
day of exposure (Durham et al. 1959), but the concentration of the
insecticide may have exceeded 0.5 µg/l sometime between the 22nd and
50th day (Hayes, 1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24 to 1.48 µg/l, but they returned to normal in spite
of continuing exposure (Tracy et al. 1960).
Man. Men showed no change in cholinesterase activity when
exposed for 8-10 half-hour intervals, 4 nights per week, for 11 weeks
to concentrations ranging from 0.07 to 0.66 µg/l (average 0.25 µg/l).
They did show a small but statistically significant depression of
plasm enzyme activity, but not of red cell enzyme activity, when the
schedule of dosing was maintained but the concentration increased to
0.40.0.55 µg/l (average 0.51 µg/l). The other parameters studied,
including complex reaction time, airway resistance and vision,
remained normal (Rasmussen et al. 1963). The tolerated inhaled dosage
averaged 0.5 mg/man/day while the dosage that caused a slight fall of
plasma cholinesterase, was 1.1 mg/man/day. The compound produced no
decrease in blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al. 1963; Gratz et
al. 1962).
Experiments on rats show that the liver is highly efficient in
detoxicating dichlorvos (Gaines, T. B., in preparation) and that the
compound is absorbed from the gastro-intestinal tract by the blood of
the hepatic portal system (Laws, E. R., in preparation). Thus, a given
dose of dichlorvos absorbed after ingestion is less toxic than the
same dose absorbed in an identical time following inhalation.
Long-term studies
No really long-term studies have been made of dichlorvos, nor do
they appear indicated because of the rapid action and excretion of the
compound.
Comments on experimental work reported and evaluation
Dichlorvos has a greater effect when inhaled than when given by
mouth. So far the only maximum level causing no significant
toxicological effect for man has been determined by inhalation
experiments and is 0.01 mg/kg/day. In dogs, the maximum oral no-effect
level was 0.37 mg/kg/day. Until more data on oral toxicity to man are
forthcoming, an acceptable daily intake cannot be established.
Further work required
Determination of oral toxicity for man.
REFERENCES
Blucher, W., Budd, E. R. Dewey, M. L., Eisenlord, G. Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Report from the
Hine Laboratories, San Francisco, California
Durham, W. F., Gaines, T. B., McCauley, R. H., jr, Sedlak, V. A.,
Mattson, A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr.
Hlth, 15, 340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes. W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Gaines, T. B., A technique for studying the net effect of metabolism
of compounds in the liver on their toxicity (In preparation)
Gaines, T. B., Effect of dietary DDVP on reproduction in rats and on
survival of their offspring. Unpublished results
Getz, M. E. (1962a) J. Ass. Offic. Agr. Chem., 45, 397
Getz, M. E. (1962b) J. Ass. Offic. Agr. Chem., 45, 393
Giang, P. A. & Hall, S. A. (1951) Anal. Chem., 23, 1830
Giang, P. A., Smith, F. F. & Hall, S. A. (1956) J. Agr. Food Chem.,
4, 621
Gratz, N. G., Bracha, P. & Carmichael, A. G. (1962) WHO/Vector
Control/11, 49 pp.
Hayes, W. J., jr (1961) Bull, Wld Hlth Org., 24, 629
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960)
J. econ. Ent., 53, 602
Kiigamagi, V. & Terriere, L. C. (1964) Shell Agr. Chem. Div., New York
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Laws, E. R., jr, The absorption of DDVP (In preparation)
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 3, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Sun, Y. P. & Johnson, E. R. (1963) J. Ass. Offic. Agr. Chem., 46,
524
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
USDA. 1965 Method Dr. 5e-62 dated 1 March 1962, rev. Nov. 20 1965.
Stored Prod. Insect Lab., Agr. Res. Serv., USDA, Savannah, Ga
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
Witherup, S., & Schlecht, H. (1962) Report from the Kettering
Laboratory, University of Cincinnati, Cincinnati, Ohio, 14 pp.
Witter. R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
