
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
DICHLORVOS
IDENTITY
Synonym
DDVP
Chemical name
2,2-dichlorovinyl dimethyl phosphate
Formula
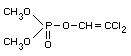 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. Dichlorvos is broken down rapidly in the
liver (Tracy, 1960; Tracy et al., 1960). Thus, the onset of poisoning
is rapid, and if recovery occurs, it is prompt (Klotzsche, 1956;
Durham et al., 1957; Gaines, 1960, Yamashita, 1962).
In blood from a human female with pseudocholinesterase deficiency,
0.185 µg of dichlorvos caused 50 per cent inhibition of the
erythrocyte esterase activity. The erythrocyte esterase activity in
normal blood was inhibited by only 15 per cent by 0.3 µg of dichlorvos
(Vigliani, 1966). Assays of whole blood from a cow given 1.0 mg/kg of
dichlorvos either orally or subcutaneously did not show any
cholinesterase inhibition; however, intravenous administration of the
same dose produced 29 per cent inhibition after 1-2 hours with rapid
recovery. In a goat given 1.52 mg/kg subcutaneously there was 44 per
cent inhibition in 1/4 hour; 46 per cent in 1/2 hour; 41 per cent in 1
hour and 35 per cent in 2 hours, followed by rapid recovery (Casida et
al., 1962).
The compound is not stored in the body nor excreted in the milk to any
appreciable extent in cows or rats, even when administered in doses
that produced severe poisoning (Tracy, 1960; Tracy et al. 1960).
With whole homogenates of liver, kidney, spleen and adrenals from rat
and rabbit, the principal labelled metabolite of dichlorvos-35P was
dimethylphosphate, 50-85 per cent, with the remaining radioactivity
appearing in demethyl-dichlorvos, monomethyl phosphate and inorganic
phosphate. In the plasma of both species, dimethyl phosphate accounted
for 98 to 100 per cent of the dichlorvos hydrolyzed. When dimethyl
32P-phosphate was incubated with rat plasma or liver homogenate, all
the radioactivity was recovered as unchanged dimethyl phosphate.
Dichlorvos was hydrolyzed by the soluble and mitochondrial fractions
of rat liver but not by the microsomes. Demethyl-dichlorvos was
hydrolyzed by the soluble fraction only. In rat liver homogenate, 2-3
times more dichloroacetaldehyde than inorganic phosphate was produced
from demethyl-dichlorvos. Inorganic phosphate was produced from
monomethyl phosphate in both plasma and liver homogenate. The reaction
was very slow. In soluble rat liver preparations, the
dichloroacetaldehyde released by the hydrolysis of dichlorvos was
reduced in the presence of DPNH to dichloroethanol. A very small
amount may have appeared as dichloroacetate. It is possible that a
pathway exists for conjugation of dichloroethanol with glucuronic acid
(Hodgson & Casida, 1962).
In rats given dichlorvos-32P, 10 mg/kg orally, rapid absorption,
distribution and hydrolysis took place. Female rats treated every 1/2
hour with 4 mg/kg of dichlorvos-32P for 2 hours and sacrificed 1/2
hour after the last dose showed mainly hydrolysis products in the
tissues. In mice given 4 doses of 10 mg/kg every 15 minutes, 95 per
cent of the dichlorvos administered was hydrolyzed in the liver,
kidney and small intestine. In male rats given
dimethyl-32P-phosphate, 500 mg/kg orally, necropsy 90 hours after
dosing indicated that almost the entire dose had been eliminated. The
urine contained only unmetabolized dimethyl-32P-phosphate, which
accounted for about 50 per cent of the radioactivity administered. The
tissues were almost devoid of radioactivity. In a rat given
demethyl-dichlorvos-32P, 500 mg/kg orally, about 14 per cent of the
dose was eliminated in the urine in 90 hours, and the tissue
distribution was similar to that of the rat which had received
dichlorvos. A very high proportion of the radioactivity was found in
bone, indicating rapid degradation to phosphoric acid. Metabolites in
the urine were 86 per cent, phosphoric acid and 14 per cent
demethyl-dichlorvos. From 67 to 100 per cent of the administered
radioactivity was recovered with 1 week in the combined urine and
faeces of cows, rats and a goat given various doses of
dichlorvos-32P. Excretion of radioactivity in the faeces accounted
for 11-15 per cent of the dose, except in cows treated orally, where
about 50 per cent of the radioactivity was excreted. The excreted
metabolites appeared to be demethyl-dichlorvos, dimethyl phosphate,
monomethyl phosphate and inorganic phosphate. Most of the
radioactivity in the milk of the cows and goat was due to hydrolysis
products. The level of organosoluble radioactivity was significantly
above background only within the first 2 hours. The highest excretion
level was found at 12 hours in the cows (Casida, McBride &
Niedermeier, 1962).
Both single and repeated large doses cause a stress-attributed
reduction in the eosinophil count of the peripheral blood of rats
(Klotzsche, 1956).
Experiments in rats showed that the liver is highly efficient in
detoxicating dichlorvos (Gaines, 1966) and that the compound is
absorbed from the gastrointestinal tract by the blood of the hepatic
portal system (Laws, 1966).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 124 Yamashita, 1962
Mouse i.p. 28* Casida et al., 1962
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56** Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80*** Durham et al., 1957
Chick, male Oral 14.8 Sherman & Ross, 1961
* Technical grade; purity not stated.
** Based on 99 per cent pure material.
*** Result of 2 tests on a technical preparation (90 per cent pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5-17.5 hours (Durham
et al., 1957).
Domestic animals. Horses tolerated a single dose of 50 mg/kg
dichlorvos in feed, but showed moderate acute poisoning when the
insecticide was given by stomach-tube at 25 mg/kg (Jackson et al.,
1960). An almost identical dose (27 mg/kg) given by stomach-tube
caused severe but non-fatal poisoning in a cow (Tracy et al., 1960).
Dichlorvos does not produce either immediate or delayed paralysis in
hens (Durham et al., 1957).
Man. Men withstood brief exposure (30-60 minutes) to concentrations
as high as 6.9 µg/l without depression of cholinesterase activity or
any other observed effect (Durham et al., 1959; Hayes, 1961). A single
exposure for 8 hours at a concentration ranging from 0.9 to 3.5 µg/l
produced slight inhibition of plasma cholinesterase activity in man
(Witter et al., 1961). Slightly higher concentrations or longer
periods of exposure, or both, produce measurable reduction of both the
red cell and plasma enzyme activity in men, monkeys and rats (Hayes,
1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood cholinesterase
activity but much larger doses are required to produce illness. Thus,
Durham et al. (1957) found that a dietary concentration of only 50 ppm
soon produced detectable lowering of plasma and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
diminution of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (Gaines, 1964).
When dichlorvos is given by stomach-tube it is not so well tolerated
as when the same dosage is absorbed from the diet gradually throughout
the day. Female rats tolerated doses of 10 and 20 mg/kg in the diet
but suffered severe, acute poisoning when given single doses of 30
mg/kg. Even at 30 mg/kg, the rats survived and the red cell
cholinesterase activity and growth of their litters were normal (Tracy
et al., 1960).
Reproduction studies in rats fed 0, 0.1, 1.0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers, and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965).
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13-0.37 mg/kg/day) produced no
detectable effect; levels equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33 per cent of control,
respectively, and led to some increase in the activity and
aggressiveness of the dogs (Blucher et al., 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in a
monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al., 1957).
Monkeys tolerated continuous exposure to concentrations ranging from
0.1-0.5 µg/l for 22 days without a definite change in cholinesterase
activity. They showed a definite depletion by the 50th day of exposure
(Durham et al., 1959), but the concentration of dichlorvos may have
exceeded 0.5 µg/l sometime between the 22nd and 50th day (Hayes,
1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24-1.48 µg/l, but they returned to normal in spite of
continuing exposure (Tracy et 1960).
Man. Men showed no change in cholinesterase activity when exposed
for 8-10 half-hour intervals, 4 nights per week, for 11 weeks to
concentrations from 0.07 to 0.66 µg/l (average 0.25 µg/l). They did
show a small but statistically significant depression of plasma enzyme
activity, but not of red cell enzyme activity, when the schedule of
dosing was maintained but the concentration increased to 0.40-0.55
µg/l (average 0.51 µg/l). The other parameters studied, including
complex reaction time, airway resistance and vision, remained normal,
(Rasussen et al., 1963). The tolerated inhaled dosage averaged 0.5
mg/man/day while the dosage that caused a slight fall of plasma
cholinesterase was 1.1 mg/man/day. The compound produced no decrease
in whole blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al., 1963; Gratz et
al., 1962).
When 15 subjects were exposed for 8 months to concentrations of 0.1
mg/M3 and higher of dichlorvos in the air, 5 showed a slight
reduction in plasma cholinesterase activity. When 36 subjects were
exposed to concentrations of less than 0.1 mg/M3, none showed any
reduction in plasma cholinesterase activity (Vigliani, 1966).
Long-term studies
No really long-term studies have been made of dichlorvos, nor do they
appear indicated because of the rapid action and excretion of the
compound.
Comments
Dichlorvos is rapidly metabolised in mammalian tissues to relatively
non-toxic metabolites which are rapidly excreted. From the data
available to date, the maximum level causing no significant
toxicological effect in man is 0.01 mg/kg/day by inhalation.
Continuous exposure of man to 0.1 mg/M3 caused some depression of
plasma cholinesterase activity without clinical signs. It would be
desirable to determine the maximum oral dose causing no inhibition of
cholinesterase activity in man. In short-term experiments in dogs, the
oral no-effect level was 0.37 mg/kg/day.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg/day.
Dog: 0.37 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatment
No information was available on the use of dichlorvos treatments
applied to food crops prior to harvest. Its potentialities in this
field seem limited by its lack of systemic properties and its short
persistence. It is used on beef and dairy cattle and on goats, sheep
and swine and in and around the buildings which house these animals.
(b) Post-harvest treatments
Strong & Spur (1961) showed that dichlorvos was active against a range
of insect pests of stored produce. Experimental work has since been
carried out on its application for the protection of cereals and
cereal products during storage and for the control of insects in
facilities where foods are stored, processed, handled or shipped.
Trials have shown that 4 micrograms of dichlorvos vapour per litre of
air for six hours will control common stored product insects. Durham
et al. (1959) found that the tightness of the warehouse and the
temperature influences the amount required to obtain the desired
concentration in the air; but in fairly tight warehouses the necessary
concentration can be obtained by releasing dichlorvos at 50 mg/m3.
Dichlorvos was dispensed by Gillenwater & Harein (1964) from a
specially designed heat volatilizer containing impregnated resin
pellets. However, it could be applied as a pressurized aerosol
formulation although higher levels of residues might result from such
use, particularly in any foodstuffs situated close to the aerosol
dispensers.
Some tests have been conducted recently on dichlorvos impregnated
resin strips which show considerable promise for future extension of
its use in the control of insects in stored produce (Green et al.,
1966).
(c) Uses other than on food
For the control, primarily, of flies and, mosquitos dichlorvos is
being used both inside and outside of agricultural premises such as
barns, feed lots, milk rooms, poultry houses, stables, corrals,
holding pens and poultry pens and yards. It has been used for a number
of years for the control of Ephestia elutella (Hübner) and
Lasioderma serricorne (Fabricius) in tobacco warehouses and in
processing areas. The insecticide is applied into the air spaces after
working hours. Dichlorvos is also being used in the United States in
sprays (0.5 per cent for the control of insect pests such as ants,
bedbugs, cockroaches, flies, silverfish, spiders, ticks and wasps.
Tolerances
The use of dichlorvos in ways which might possibly leave residues in
food is quite recent. There appear to be no legal or other tolerances.
Residues resulting from supervised trials
There is only a limited amount of data available on residues in food
produced by dichlorvos. In a series of warehouse tests conducted in
the United States packaged noodles, raisins, beans, peanuts, flour and
sugar were exposed to 21 weekly treatments of dichlorvos applied as a
vapour at the rate of 50 mg/m3 per treatment. In each treatment, the
insecticide was vaporized slowly over a period of six hours. The
highest residue found after 21 applications in composite samples of
the various foods was 1.68 ppm in peanuts contained in burlap bags.
The residues in the other foods were well below 1 ppm (Unpublished
information received from US Department of Agriculture).
Following the work of Strong & Spur (1961) which showed that
dichlorvos was toxic to stored-product insects at very low
concentrations, preliminary tests were conducted in the United Kingdom
to assess its value for rapid disinfestation of grain where a long
residual life is unnecessary or undesirable. Feed barley was treated
with an emulsifiable formulation of dichlorvos as the grain was turned
from one bin into another. A deposit of 4 ppm appeared effective in
controlling the insects present but the insecticide disappeared very
quickly.
Resin strips measuring about 2-1/2" × 10" × 1/4" and containing about
18.6 per cent of dichlorvos by weight are under test to determine
their effectiveness in providing continuous control of flying and
crawling insects. The initial generation of dichlorvos by these strips
is approximately 40 mg/hr/strip and will produce a dichlorvos
concentration in the air of 1 mg/l at 30 per cent R.H. The emission
rate falls and after 30 days an almost constant rate of 3.0-4.0 mg of
dichlorvos is emitted producing a concentration of the insecticide in
the air of 0.075-0.1 µg/l of air. About 0.1 µg/l of dichlorvos is
produced for about two-thirds of the active life of the strips.
Residues in food moving in commerce
No information was available.
Fate of residues
Fragmentary data indicate that dichlorvos residues appearing in foods
from good agricultural practices are of a very low level and they
disappear quite rapidly when the treated foods are aired.
Green & Tyler (1966) found barley sprayed with dichlorvos at the rate
of 4 ppm while being turned from one bin to another had 1.9 ppm
(average) by analysis during treatment, 0.93 ppm after one week, 0.25
ppm after six weeks, 0.22 ppm after 10 weeks, and none could be
detected 15 weeks later.
A variety of foods were exposed in a room in which were hung
dichlorvos resin strips at the rate of 1 strip/1000 cu. ft for seven
weeks. The dichlorvos concentration in the air reached 0.33 µg/l after
24 hours and decreased to 0.03 µg/l after eight weeks. Residues of
dichlorvos in the various foods increased to maximum levels ranging
from 3.4 ppm for whole apples after 28 days' exposure down to 0.03 ppm
in sugar after 56 days. Whole apples, bacon and cheese had dichlorvos
residues greater than 1 ppm; banana (skin), currants (covered with
paper), dried milk (polyethylene bag) and surface samples of wheat,
cocoa beans and flour in hessian sacks had residues of 0.1-1 ppm; and
eggs, oranges, sugar, banana (edible part) had dichlorvos residues
below 0.1 ppm. On removal to an insecticide-free room, 20-70 per cent
of the residue was lost after four days, and 60-100 per cent after 10
days (Unpublished information).
Dichlorvos was added to rice at levels of 4.5 ppm and 19 ppm, and to
flour at 4.5 ppm and 14 ppm. Then the food was placed in sealed
containers and stored for 65 hours at room temperature. As in home
practice, the rice was washed with cold water and then cooked for
20-30 minutes until edible. There was 98 per cent less dichlorvos in
the rice after washing and cooking. The biscuits made from the treated
flour had 80 per cent and 60 per cent less dichlorvos, respectively
(Unpublished information).
Residues in meats were followed by exposure to dichlorvos labelled
with P32: the effects of cooking on such residues were investigated.
Dichlorvos penetrated only into the surface layers of the samples and
the residues in fat were generally lower than in the other samples.
Following the frying and boiling of steak containing dichlorvos
residues, the latter were completely destroyed and it was possible to
detect only products of hydrolysis (Miller & Aitken, 1965). Also after
the usual conservation of plums by thermalsterilization, dichlorvos
residues disappeared completely (Benes, personal communication).
Geisabühler & Haselbach (1963) concluded that the milling process does
not result in any considerable decrease of residues in wheat treated
with dichlorvos. During storage however the decomposition of the
residues proceeds much more quickly in milled wheat products than it
does in whole grains. No dichlorvos could be detected in samples of
white flour after one month of storage at room temperature.
Methods of residue analysis
The method which has been used for determining dichlorvos residues in
foods was developed by Shell Development Company. It is a
spectrophotometric method based on enzyme inhibition. It has a
sensitivity 0.10 ppm of dichlorvos.
Gas-liquid-chromatographic methods have been developed for
orgophosphorus compounds. It appears promising that this type of
analysis could be perfected for the determination of dichlorvos
residues.
RECOMMENDATIONS FOR TOLERANCES
No tolerances are recommended at this time because of insufficient
information on the specific uses of dichlorvos on foods and the
resulting residues. Recommendations for tolerances should be
considered at the next joint meeting.
Further work or information
Information is required on:
(a) range of commodities likely to be treated,
(b) occurrence of residue levels resulting from good pest control
practices,
(c) rate of disappearance through normal aging, processing, cooking,
etc.,
and
(d) chemical nature of terminal residues occurring in foods from good
pest control practices.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Blucher, W., Budd, E. R., Dewey, M. L., Eisenlord, G., Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Unpublished report
from the Hine Laboratories, San Francisco, California
Casida, J. E., McBride, L. & Niedermeier, R. P. (1962) J. Agr. Food
Chem., 10, 370
Durham, W. F., Gaines, T. B., McCauley, R. H., Sedlak, V. A., Mattson,
A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr. Hlth, 15,
340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes, W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 68
Gaines, T. B. (1964) Unpublished report
Gaines, T. B. (1966) Nature (Lond.) 209, 88
Gratz, N. G., Bracha, P. & Carmichael, A. G, (1962) WHO/Vector
Control/11
Hayes, W. J., jr (1961) Bull. Wld Hlth Org., 24, 629
Hodgson, E. & Casida, J. E. (1962) J. Agr. Food Chem., 10, 208
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960) J.
econ. Ent., 53, 602
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 2, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
Vigliani, E. C. (1966) Unpublished report to Shell Chemical Co.
Witherup, S., Caldwell, J. S. & Hull, L. (1965) Unpublished report
from the Kettering Laboratory, University of Cincinnati, Cincinnati,
Ohio
Witter, R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
REFERENCES PERTINENT TO AGRICULTURAL DATA
Durham, W. F., Hayes, W. J. & Mattson, A. M. (1959) Toxicological
Studies of DDVP in Tobacco Warehouses. Arch. industr. Hlth, 20:
202-10
Geissbühler, H. & Haselbach, C. (1963) On the behaviour of DDVP upon
storage and processing of insecticide treated cereals. (Report for
CIBA, Basle) Battelle Inst. Geneva, 30 October
Gillenwater, H. B. & Harein, P. K. (1964) A dispenser designed to
provide large quantities of insecticide vapor. J. Econ. Ent., 57
(5): 762-3
Green, A. A., Kane, J. & Gradidge, J. M. G. (1966) Experiments in the
Control of Ephestia elutella using Dichlorvos Vapour. J. Stored
Proc. Res., 2: 147-157
Green, A. A. & Tyler, P. S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus infesting
stored barley. J. Stored Prod. Res., 1: 273-295
Millar, K. R. & Aitken, W. M. (1965) Residues in meat following
exposure to P32 labelled dichlorvos vapour in an enclosed space.
N.Z. J. Agric. Res., 8 (2): 350-362
Strong, H. G. & Spur, D. E. (1961) Evaluation of Insecticides as
protectants against pests of stored grain and seeds. J. Econ. Ent.,
54 (2); 235-238
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. Dichlorvos is broken down rapidly in the
liver (Tracy, 1960; Tracy et al., 1960). Thus, the onset of poisoning
is rapid, and if recovery occurs, it is prompt (Klotzsche, 1956;
Durham et al., 1957; Gaines, 1960, Yamashita, 1962).
In blood from a human female with pseudocholinesterase deficiency,
0.185 µg of dichlorvos caused 50 per cent inhibition of the
erythrocyte esterase activity. The erythrocyte esterase activity in
normal blood was inhibited by only 15 per cent by 0.3 µg of dichlorvos
(Vigliani, 1966). Assays of whole blood from a cow given 1.0 mg/kg of
dichlorvos either orally or subcutaneously did not show any
cholinesterase inhibition; however, intravenous administration of the
same dose produced 29 per cent inhibition after 1-2 hours with rapid
recovery. In a goat given 1.52 mg/kg subcutaneously there was 44 per
cent inhibition in 1/4 hour; 46 per cent in 1/2 hour; 41 per cent in 1
hour and 35 per cent in 2 hours, followed by rapid recovery (Casida et
al., 1962).
The compound is not stored in the body nor excreted in the milk to any
appreciable extent in cows or rats, even when administered in doses
that produced severe poisoning (Tracy, 1960; Tracy et al. 1960).
With whole homogenates of liver, kidney, spleen and adrenals from rat
and rabbit, the principal labelled metabolite of dichlorvos-35P was
dimethylphosphate, 50-85 per cent, with the remaining radioactivity
appearing in demethyl-dichlorvos, monomethyl phosphate and inorganic
phosphate. In the plasma of both species, dimethyl phosphate accounted
for 98 to 100 per cent of the dichlorvos hydrolyzed. When dimethyl
32P-phosphate was incubated with rat plasma or liver homogenate, all
the radioactivity was recovered as unchanged dimethyl phosphate.
Dichlorvos was hydrolyzed by the soluble and mitochondrial fractions
of rat liver but not by the microsomes. Demethyl-dichlorvos was
hydrolyzed by the soluble fraction only. In rat liver homogenate, 2-3
times more dichloroacetaldehyde than inorganic phosphate was produced
from demethyl-dichlorvos. Inorganic phosphate was produced from
monomethyl phosphate in both plasma and liver homogenate. The reaction
was very slow. In soluble rat liver preparations, the
dichloroacetaldehyde released by the hydrolysis of dichlorvos was
reduced in the presence of DPNH to dichloroethanol. A very small
amount may have appeared as dichloroacetate. It is possible that a
pathway exists for conjugation of dichloroethanol with glucuronic acid
(Hodgson & Casida, 1962).
In rats given dichlorvos-32P, 10 mg/kg orally, rapid absorption,
distribution and hydrolysis took place. Female rats treated every 1/2
hour with 4 mg/kg of dichlorvos-32P for 2 hours and sacrificed 1/2
hour after the last dose showed mainly hydrolysis products in the
tissues. In mice given 4 doses of 10 mg/kg every 15 minutes, 95 per
cent of the dichlorvos administered was hydrolyzed in the liver,
kidney and small intestine. In male rats given
dimethyl-32P-phosphate, 500 mg/kg orally, necropsy 90 hours after
dosing indicated that almost the entire dose had been eliminated. The
urine contained only unmetabolized dimethyl-32P-phosphate, which
accounted for about 50 per cent of the radioactivity administered. The
tissues were almost devoid of radioactivity. In a rat given
demethyl-dichlorvos-32P, 500 mg/kg orally, about 14 per cent of the
dose was eliminated in the urine in 90 hours, and the tissue
distribution was similar to that of the rat which had received
dichlorvos. A very high proportion of the radioactivity was found in
bone, indicating rapid degradation to phosphoric acid. Metabolites in
the urine were 86 per cent, phosphoric acid and 14 per cent
demethyl-dichlorvos. From 67 to 100 per cent of the administered
radioactivity was recovered with 1 week in the combined urine and
faeces of cows, rats and a goat given various doses of
dichlorvos-32P. Excretion of radioactivity in the faeces accounted
for 11-15 per cent of the dose, except in cows treated orally, where
about 50 per cent of the radioactivity was excreted. The excreted
metabolites appeared to be demethyl-dichlorvos, dimethyl phosphate,
monomethyl phosphate and inorganic phosphate. Most of the
radioactivity in the milk of the cows and goat was due to hydrolysis
products. The level of organosoluble radioactivity was significantly
above background only within the first 2 hours. The highest excretion
level was found at 12 hours in the cows (Casida, McBride &
Niedermeier, 1962).
Both single and repeated large doses cause a stress-attributed
reduction in the eosinophil count of the peripheral blood of rats
(Klotzsche, 1956).
Experiments in rats showed that the liver is highly efficient in
detoxicating dichlorvos (Gaines, 1966) and that the compound is
absorbed from the gastrointestinal tract by the blood of the hepatic
portal system (Laws, 1966).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 124 Yamashita, 1962
Mouse i.p. 28* Casida et al., 1962
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56** Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80*** Durham et al., 1957
Chick, male Oral 14.8 Sherman & Ross, 1961
* Technical grade; purity not stated.
** Based on 99 per cent pure material.
*** Result of 2 tests on a technical preparation (90 per cent pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5-17.5 hours (Durham
et al., 1957).
Domestic animals. Horses tolerated a single dose of 50 mg/kg
dichlorvos in feed, but showed moderate acute poisoning when the
insecticide was given by stomach-tube at 25 mg/kg (Jackson et al.,
1960). An almost identical dose (27 mg/kg) given by stomach-tube
caused severe but non-fatal poisoning in a cow (Tracy et al., 1960).
Dichlorvos does not produce either immediate or delayed paralysis in
hens (Durham et al., 1957).
Man. Men withstood brief exposure (30-60 minutes) to concentrations
as high as 6.9 µg/l without depression of cholinesterase activity or
any other observed effect (Durham et al., 1959; Hayes, 1961). A single
exposure for 8 hours at a concentration ranging from 0.9 to 3.5 µg/l
produced slight inhibition of plasma cholinesterase activity in man
(Witter et al., 1961). Slightly higher concentrations or longer
periods of exposure, or both, produce measurable reduction of both the
red cell and plasma enzyme activity in men, monkeys and rats (Hayes,
1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood cholinesterase
activity but much larger doses are required to produce illness. Thus,
Durham et al. (1957) found that a dietary concentration of only 50 ppm
soon produced detectable lowering of plasma and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
diminution of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (Gaines, 1964).
When dichlorvos is given by stomach-tube it is not so well tolerated
as when the same dosage is absorbed from the diet gradually throughout
the day. Female rats tolerated doses of 10 and 20 mg/kg in the diet
but suffered severe, acute poisoning when given single doses of 30
mg/kg. Even at 30 mg/kg, the rats survived and the red cell
cholinesterase activity and growth of their litters were normal (Tracy
et al., 1960).
Reproduction studies in rats fed 0, 0.1, 1.0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers, and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965).
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13-0.37 mg/kg/day) produced no
detectable effect; levels equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33 per cent of control,
respectively, and led to some increase in the activity and
aggressiveness of the dogs (Blucher et al., 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in a
monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al., 1957).
Monkeys tolerated continuous exposure to concentrations ranging from
0.1-0.5 µg/l for 22 days without a definite change in cholinesterase
activity. They showed a definite depletion by the 50th day of exposure
(Durham et al., 1959), but the concentration of dichlorvos may have
exceeded 0.5 µg/l sometime between the 22nd and 50th day (Hayes,
1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24-1.48 µg/l, but they returned to normal in spite of
continuing exposure (Tracy et 1960).
Man. Men showed no change in cholinesterase activity when exposed
for 8-10 half-hour intervals, 4 nights per week, for 11 weeks to
concentrations from 0.07 to 0.66 µg/l (average 0.25 µg/l). They did
show a small but statistically significant depression of plasma enzyme
activity, but not of red cell enzyme activity, when the schedule of
dosing was maintained but the concentration increased to 0.40-0.55
µg/l (average 0.51 µg/l). The other parameters studied, including
complex reaction time, airway resistance and vision, remained normal,
(Rasussen et al., 1963). The tolerated inhaled dosage averaged 0.5
mg/man/day while the dosage that caused a slight fall of plasma
cholinesterase was 1.1 mg/man/day. The compound produced no decrease
in whole blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al., 1963; Gratz et
al., 1962).
When 15 subjects were exposed for 8 months to concentrations of 0.1
mg/M3 and higher of dichlorvos in the air, 5 showed a slight
reduction in plasma cholinesterase activity. When 36 subjects were
exposed to concentrations of less than 0.1 mg/M3, none showed any
reduction in plasma cholinesterase activity (Vigliani, 1966).
Long-term studies
No really long-term studies have been made of dichlorvos, nor do they
appear indicated because of the rapid action and excretion of the
compound.
Comments
Dichlorvos is rapidly metabolised in mammalian tissues to relatively
non-toxic metabolites which are rapidly excreted. From the data
available to date, the maximum level causing no significant
toxicological effect in man is 0.01 mg/kg/day by inhalation.
Continuous exposure of man to 0.1 mg/M3 caused some depression of
plasma cholinesterase activity without clinical signs. It would be
desirable to determine the maximum oral dose causing no inhibition of
cholinesterase activity in man. In short-term experiments in dogs, the
oral no-effect level was 0.37 mg/kg/day.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg/day.
Dog: 0.37 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatment
No information was available on the use of dichlorvos treatments
applied to food crops prior to harvest. Its potentialities in this
field seem limited by its lack of systemic properties and its short
persistence. It is used on beef and dairy cattle and on goats, sheep
and swine and in and around the buildings which house these animals.
(b) Post-harvest treatments
Strong & Spur (1961) showed that dichlorvos was active against a range
of insect pests of stored produce. Experimental work has since been
carried out on its application for the protection of cereals and
cereal products during storage and for the control of insects in
facilities where foods are stored, processed, handled or shipped.
Trials have shown that 4 micrograms of dichlorvos vapour per litre of
air for six hours will control common stored product insects. Durham
et al. (1959) found that the tightness of the warehouse and the
temperature influences the amount required to obtain the desired
concentration in the air; but in fairly tight warehouses the necessary
concentration can be obtained by releasing dichlorvos at 50 mg/m3.
Dichlorvos was dispensed by Gillenwater & Harein (1964) from a
specially designed heat volatilizer containing impregnated resin
pellets. However, it could be applied as a pressurized aerosol
formulation although higher levels of residues might result from such
use, particularly in any foodstuffs situated close to the aerosol
dispensers.
Some tests have been conducted recently on dichlorvos impregnated
resin strips which show considerable promise for future extension of
its use in the control of insects in stored produce (Green et al.,
1966).
(c) Uses other than on food
For the control, primarily, of flies and, mosquitos dichlorvos is
being used both inside and outside of agricultural premises such as
barns, feed lots, milk rooms, poultry houses, stables, corrals,
holding pens and poultry pens and yards. It has been used for a number
of years for the control of Ephestia elutella (Hübner) and
Lasioderma serricorne (Fabricius) in tobacco warehouses and in
processing areas. The insecticide is applied into the air spaces after
working hours. Dichlorvos is also being used in the United States in
sprays (0.5 per cent for the control of insect pests such as ants,
bedbugs, cockroaches, flies, silverfish, spiders, ticks and wasps.
Tolerances
The use of dichlorvos in ways which might possibly leave residues in
food is quite recent. There appear to be no legal or other tolerances.
Residues resulting from supervised trials
There is only a limited amount of data available on residues in food
produced by dichlorvos. In a series of warehouse tests conducted in
the United States packaged noodles, raisins, beans, peanuts, flour and
sugar were exposed to 21 weekly treatments of dichlorvos applied as a
vapour at the rate of 50 mg/m3 per treatment. In each treatment, the
insecticide was vaporized slowly over a period of six hours. The
highest residue found after 21 applications in composite samples of
the various foods was 1.68 ppm in peanuts contained in burlap bags.
The residues in the other foods were well below 1 ppm (Unpublished
information received from US Department of Agriculture).
Following the work of Strong & Spur (1961) which showed that
dichlorvos was toxic to stored-product insects at very low
concentrations, preliminary tests were conducted in the United Kingdom
to assess its value for rapid disinfestation of grain where a long
residual life is unnecessary or undesirable. Feed barley was treated
with an emulsifiable formulation of dichlorvos as the grain was turned
from one bin into another. A deposit of 4 ppm appeared effective in
controlling the insects present but the insecticide disappeared very
quickly.
Resin strips measuring about 2-1/2" × 10" × 1/4" and containing about
18.6 per cent of dichlorvos by weight are under test to determine
their effectiveness in providing continuous control of flying and
crawling insects. The initial generation of dichlorvos by these strips
is approximately 40 mg/hr/strip and will produce a dichlorvos
concentration in the air of 1 mg/l at 30 per cent R.H. The emission
rate falls and after 30 days an almost constant rate of 3.0-4.0 mg of
dichlorvos is emitted producing a concentration of the insecticide in
the air of 0.075-0.1 µg/l of air. About 0.1 µg/l of dichlorvos is
produced for about two-thirds of the active life of the strips.
Residues in food moving in commerce
No information was available.
Fate of residues
Fragmentary data indicate that dichlorvos residues appearing in foods
from good agricultural practices are of a very low level and they
disappear quite rapidly when the treated foods are aired.
Green & Tyler (1966) found barley sprayed with dichlorvos at the rate
of 4 ppm while being turned from one bin to another had 1.9 ppm
(average) by analysis during treatment, 0.93 ppm after one week, 0.25
ppm after six weeks, 0.22 ppm after 10 weeks, and none could be
detected 15 weeks later.
A variety of foods were exposed in a room in which were hung
dichlorvos resin strips at the rate of 1 strip/1000 cu. ft for seven
weeks. The dichlorvos concentration in the air reached 0.33 µg/l after
24 hours and decreased to 0.03 µg/l after eight weeks. Residues of
dichlorvos in the various foods increased to maximum levels ranging
from 3.4 ppm for whole apples after 28 days' exposure down to 0.03 ppm
in sugar after 56 days. Whole apples, bacon and cheese had dichlorvos
residues greater than 1 ppm; banana (skin), currants (covered with
paper), dried milk (polyethylene bag) and surface samples of wheat,
cocoa beans and flour in hessian sacks had residues of 0.1-1 ppm; and
eggs, oranges, sugar, banana (edible part) had dichlorvos residues
below 0.1 ppm. On removal to an insecticide-free room, 20-70 per cent
of the residue was lost after four days, and 60-100 per cent after 10
days (Unpublished information).
Dichlorvos was added to rice at levels of 4.5 ppm and 19 ppm, and to
flour at 4.5 ppm and 14 ppm. Then the food was placed in sealed
containers and stored for 65 hours at room temperature. As in home
practice, the rice was washed with cold water and then cooked for
20-30 minutes until edible. There was 98 per cent less dichlorvos in
the rice after washing and cooking. The biscuits made from the treated
flour had 80 per cent and 60 per cent less dichlorvos, respectively
(Unpublished information).
Residues in meats were followed by exposure to dichlorvos labelled
with P32: the effects of cooking on such residues were investigated.
Dichlorvos penetrated only into the surface layers of the samples and
the residues in fat were generally lower than in the other samples.
Following the frying and boiling of steak containing dichlorvos
residues, the latter were completely destroyed and it was possible to
detect only products of hydrolysis (Miller & Aitken, 1965). Also after
the usual conservation of plums by thermalsterilization, dichlorvos
residues disappeared completely (Benes, personal communication).
Geisabühler & Haselbach (1963) concluded that the milling process does
not result in any considerable decrease of residues in wheat treated
with dichlorvos. During storage however the decomposition of the
residues proceeds much more quickly in milled wheat products than it
does in whole grains. No dichlorvos could be detected in samples of
white flour after one month of storage at room temperature.
Methods of residue analysis
The method which has been used for determining dichlorvos residues in
foods was developed by Shell Development Company. It is a
spectrophotometric method based on enzyme inhibition. It has a
sensitivity 0.10 ppm of dichlorvos.
Gas-liquid-chromatographic methods have been developed for
orgophosphorus compounds. It appears promising that this type of
analysis could be perfected for the determination of dichlorvos
residues.
RECOMMENDATIONS FOR TOLERANCES
No tolerances are recommended at this time because of insufficient
information on the specific uses of dichlorvos on foods and the
resulting residues. Recommendations for tolerances should be
considered at the next joint meeting.
Further work or information
Information is required on:
(a) range of commodities likely to be treated,
(b) occurrence of residue levels resulting from good pest control
practices,
(c) rate of disappearance through normal aging, processing, cooking,
etc.,
and
(d) chemical nature of terminal residues occurring in foods from good
pest control practices.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Blucher, W., Budd, E. R., Dewey, M. L., Eisenlord, G., Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Unpublished report
from the Hine Laboratories, San Francisco, California
Casida, J. E., McBride, L. & Niedermeier, R. P. (1962) J. Agr. Food
Chem., 10, 370
Durham, W. F., Gaines, T. B., McCauley, R. H., Sedlak, V. A., Mattson,
A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr. Hlth, 15,
340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes, W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 68
Gaines, T. B. (1964) Unpublished report
Gaines, T. B. (1966) Nature (Lond.) 209, 88
Gratz, N. G., Bracha, P. & Carmichael, A. G, (1962) WHO/Vector
Control/11
Hayes, W. J., jr (1961) Bull. Wld Hlth Org., 24, 629
Hodgson, E. & Casida, J. E. (1962) J. Agr. Food Chem., 10, 208
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960) J.
econ. Ent., 53, 602
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 2, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
Vigliani, E. C. (1966) Unpublished report to Shell Chemical Co.
Witherup, S., Caldwell, J. S. & Hull, L. (1965) Unpublished report
from the Kettering Laboratory, University of Cincinnati, Cincinnati,
Ohio
Witter, R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
REFERENCES PERTINENT TO AGRICULTURAL DATA
Durham, W. F., Hayes, W. J. & Mattson, A. M. (1959) Toxicological
Studies of DDVP in Tobacco Warehouses. Arch. industr. Hlth, 20:
202-10
Geissbühler, H. & Haselbach, C. (1963) On the behaviour of DDVP upon
storage and processing of insecticide treated cereals. (Report for
CIBA, Basle) Battelle Inst. Geneva, 30 October
Gillenwater, H. B. & Harein, P. K. (1964) A dispenser designed to
provide large quantities of insecticide vapor. J. Econ. Ent., 57
(5): 762-3
Green, A. A., Kane, J. & Gradidge, J. M. G. (1966) Experiments in the
Control of Ephestia elutella using Dichlorvos Vapour. J. Stored
Proc. Res., 2: 147-157
Green, A. A. & Tyler, P. S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus infesting
stored barley. J. Stored Prod. Res., 1: 273-295
Millar, K. R. & Aitken, W. M. (1965) Residues in meat following
exposure to P32 labelled dichlorvos vapour in an enclosed space.
N.Z. J. Agric. Res., 8 (2): 350-362
Strong, H. G. & Spur, D. E. (1961) Evaluation of Insecticides as
protectants against pests of stored grain and seeds. J. Econ. Ent.,
54 (2); 235-238
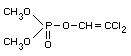 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. Dichlorvos is broken down rapidly in the
liver (Tracy, 1960; Tracy et al., 1960). Thus, the onset of poisoning
is rapid, and if recovery occurs, it is prompt (Klotzsche, 1956;
Durham et al., 1957; Gaines, 1960, Yamashita, 1962).
In blood from a human female with pseudocholinesterase deficiency,
0.185 µg of dichlorvos caused 50 per cent inhibition of the
erythrocyte esterase activity. The erythrocyte esterase activity in
normal blood was inhibited by only 15 per cent by 0.3 µg of dichlorvos
(Vigliani, 1966). Assays of whole blood from a cow given 1.0 mg/kg of
dichlorvos either orally or subcutaneously did not show any
cholinesterase inhibition; however, intravenous administration of the
same dose produced 29 per cent inhibition after 1-2 hours with rapid
recovery. In a goat given 1.52 mg/kg subcutaneously there was 44 per
cent inhibition in 1/4 hour; 46 per cent in 1/2 hour; 41 per cent in 1
hour and 35 per cent in 2 hours, followed by rapid recovery (Casida et
al., 1962).
The compound is not stored in the body nor excreted in the milk to any
appreciable extent in cows or rats, even when administered in doses
that produced severe poisoning (Tracy, 1960; Tracy et al. 1960).
With whole homogenates of liver, kidney, spleen and adrenals from rat
and rabbit, the principal labelled metabolite of dichlorvos-35P was
dimethylphosphate, 50-85 per cent, with the remaining radioactivity
appearing in demethyl-dichlorvos, monomethyl phosphate and inorganic
phosphate. In the plasma of both species, dimethyl phosphate accounted
for 98 to 100 per cent of the dichlorvos hydrolyzed. When dimethyl
32P-phosphate was incubated with rat plasma or liver homogenate, all
the radioactivity was recovered as unchanged dimethyl phosphate.
Dichlorvos was hydrolyzed by the soluble and mitochondrial fractions
of rat liver but not by the microsomes. Demethyl-dichlorvos was
hydrolyzed by the soluble fraction only. In rat liver homogenate, 2-3
times more dichloroacetaldehyde than inorganic phosphate was produced
from demethyl-dichlorvos. Inorganic phosphate was produced from
monomethyl phosphate in both plasma and liver homogenate. The reaction
was very slow. In soluble rat liver preparations, the
dichloroacetaldehyde released by the hydrolysis of dichlorvos was
reduced in the presence of DPNH to dichloroethanol. A very small
amount may have appeared as dichloroacetate. It is possible that a
pathway exists for conjugation of dichloroethanol with glucuronic acid
(Hodgson & Casida, 1962).
In rats given dichlorvos-32P, 10 mg/kg orally, rapid absorption,
distribution and hydrolysis took place. Female rats treated every 1/2
hour with 4 mg/kg of dichlorvos-32P for 2 hours and sacrificed 1/2
hour after the last dose showed mainly hydrolysis products in the
tissues. In mice given 4 doses of 10 mg/kg every 15 minutes, 95 per
cent of the dichlorvos administered was hydrolyzed in the liver,
kidney and small intestine. In male rats given
dimethyl-32P-phosphate, 500 mg/kg orally, necropsy 90 hours after
dosing indicated that almost the entire dose had been eliminated. The
urine contained only unmetabolized dimethyl-32P-phosphate, which
accounted for about 50 per cent of the radioactivity administered. The
tissues were almost devoid of radioactivity. In a rat given
demethyl-dichlorvos-32P, 500 mg/kg orally, about 14 per cent of the
dose was eliminated in the urine in 90 hours, and the tissue
distribution was similar to that of the rat which had received
dichlorvos. A very high proportion of the radioactivity was found in
bone, indicating rapid degradation to phosphoric acid. Metabolites in
the urine were 86 per cent, phosphoric acid and 14 per cent
demethyl-dichlorvos. From 67 to 100 per cent of the administered
radioactivity was recovered with 1 week in the combined urine and
faeces of cows, rats and a goat given various doses of
dichlorvos-32P. Excretion of radioactivity in the faeces accounted
for 11-15 per cent of the dose, except in cows treated orally, where
about 50 per cent of the radioactivity was excreted. The excreted
metabolites appeared to be demethyl-dichlorvos, dimethyl phosphate,
monomethyl phosphate and inorganic phosphate. Most of the
radioactivity in the milk of the cows and goat was due to hydrolysis
products. The level of organosoluble radioactivity was significantly
above background only within the first 2 hours. The highest excretion
level was found at 12 hours in the cows (Casida, McBride &
Niedermeier, 1962).
Both single and repeated large doses cause a stress-attributed
reduction in the eosinophil count of the peripheral blood of rats
(Klotzsche, 1956).
Experiments in rats showed that the liver is highly efficient in
detoxicating dichlorvos (Gaines, 1966) and that the compound is
absorbed from the gastrointestinal tract by the blood of the hepatic
portal system (Laws, 1966).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 124 Yamashita, 1962
Mouse i.p. 28* Casida et al., 1962
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56** Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80*** Durham et al., 1957
Chick, male Oral 14.8 Sherman & Ross, 1961
* Technical grade; purity not stated.
** Based on 99 per cent pure material.
*** Result of 2 tests on a technical preparation (90 per cent pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5-17.5 hours (Durham
et al., 1957).
Domestic animals. Horses tolerated a single dose of 50 mg/kg
dichlorvos in feed, but showed moderate acute poisoning when the
insecticide was given by stomach-tube at 25 mg/kg (Jackson et al.,
1960). An almost identical dose (27 mg/kg) given by stomach-tube
caused severe but non-fatal poisoning in a cow (Tracy et al., 1960).
Dichlorvos does not produce either immediate or delayed paralysis in
hens (Durham et al., 1957).
Man. Men withstood brief exposure (30-60 minutes) to concentrations
as high as 6.9 µg/l without depression of cholinesterase activity or
any other observed effect (Durham et al., 1959; Hayes, 1961). A single
exposure for 8 hours at a concentration ranging from 0.9 to 3.5 µg/l
produced slight inhibition of plasma cholinesterase activity in man
(Witter et al., 1961). Slightly higher concentrations or longer
periods of exposure, or both, produce measurable reduction of both the
red cell and plasma enzyme activity in men, monkeys and rats (Hayes,
1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood cholinesterase
activity but much larger doses are required to produce illness. Thus,
Durham et al. (1957) found that a dietary concentration of only 50 ppm
soon produced detectable lowering of plasma and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
diminution of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (Gaines, 1964).
When dichlorvos is given by stomach-tube it is not so well tolerated
as when the same dosage is absorbed from the diet gradually throughout
the day. Female rats tolerated doses of 10 and 20 mg/kg in the diet
but suffered severe, acute poisoning when given single doses of 30
mg/kg. Even at 30 mg/kg, the rats survived and the red cell
cholinesterase activity and growth of their litters were normal (Tracy
et al., 1960).
Reproduction studies in rats fed 0, 0.1, 1.0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers, and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965).
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13-0.37 mg/kg/day) produced no
detectable effect; levels equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33 per cent of control,
respectively, and led to some increase in the activity and
aggressiveness of the dogs (Blucher et al., 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in a
monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al., 1957).
Monkeys tolerated continuous exposure to concentrations ranging from
0.1-0.5 µg/l for 22 days without a definite change in cholinesterase
activity. They showed a definite depletion by the 50th day of exposure
(Durham et al., 1959), but the concentration of dichlorvos may have
exceeded 0.5 µg/l sometime between the 22nd and 50th day (Hayes,
1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24-1.48 µg/l, but they returned to normal in spite of
continuing exposure (Tracy et 1960).
Man. Men showed no change in cholinesterase activity when exposed
for 8-10 half-hour intervals, 4 nights per week, for 11 weeks to
concentrations from 0.07 to 0.66 µg/l (average 0.25 µg/l). They did
show a small but statistically significant depression of plasma enzyme
activity, but not of red cell enzyme activity, when the schedule of
dosing was maintained but the concentration increased to 0.40-0.55
µg/l (average 0.51 µg/l). The other parameters studied, including
complex reaction time, airway resistance and vision, remained normal,
(Rasussen et al., 1963). The tolerated inhaled dosage averaged 0.5
mg/man/day while the dosage that caused a slight fall of plasma
cholinesterase was 1.1 mg/man/day. The compound produced no decrease
in whole blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al., 1963; Gratz et
al., 1962).
When 15 subjects were exposed for 8 months to concentrations of 0.1
mg/M3 and higher of dichlorvos in the air, 5 showed a slight
reduction in plasma cholinesterase activity. When 36 subjects were
exposed to concentrations of less than 0.1 mg/M3, none showed any
reduction in plasma cholinesterase activity (Vigliani, 1966).
Long-term studies
No really long-term studies have been made of dichlorvos, nor do they
appear indicated because of the rapid action and excretion of the
compound.
Comments
Dichlorvos is rapidly metabolised in mammalian tissues to relatively
non-toxic metabolites which are rapidly excreted. From the data
available to date, the maximum level causing no significant
toxicological effect in man is 0.01 mg/kg/day by inhalation.
Continuous exposure of man to 0.1 mg/M3 caused some depression of
plasma cholinesterase activity without clinical signs. It would be
desirable to determine the maximum oral dose causing no inhibition of
cholinesterase activity in man. In short-term experiments in dogs, the
oral no-effect level was 0.37 mg/kg/day.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg/day.
Dog: 0.37 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatment
No information was available on the use of dichlorvos treatments
applied to food crops prior to harvest. Its potentialities in this
field seem limited by its lack of systemic properties and its short
persistence. It is used on beef and dairy cattle and on goats, sheep
and swine and in and around the buildings which house these animals.
(b) Post-harvest treatments
Strong & Spur (1961) showed that dichlorvos was active against a range
of insect pests of stored produce. Experimental work has since been
carried out on its application for the protection of cereals and
cereal products during storage and for the control of insects in
facilities where foods are stored, processed, handled or shipped.
Trials have shown that 4 micrograms of dichlorvos vapour per litre of
air for six hours will control common stored product insects. Durham
et al. (1959) found that the tightness of the warehouse and the
temperature influences the amount required to obtain the desired
concentration in the air; but in fairly tight warehouses the necessary
concentration can be obtained by releasing dichlorvos at 50 mg/m3.
Dichlorvos was dispensed by Gillenwater & Harein (1964) from a
specially designed heat volatilizer containing impregnated resin
pellets. However, it could be applied as a pressurized aerosol
formulation although higher levels of residues might result from such
use, particularly in any foodstuffs situated close to the aerosol
dispensers.
Some tests have been conducted recently on dichlorvos impregnated
resin strips which show considerable promise for future extension of
its use in the control of insects in stored produce (Green et al.,
1966).
(c) Uses other than on food
For the control, primarily, of flies and, mosquitos dichlorvos is
being used both inside and outside of agricultural premises such as
barns, feed lots, milk rooms, poultry houses, stables, corrals,
holding pens and poultry pens and yards. It has been used for a number
of years for the control of Ephestia elutella (Hübner) and
Lasioderma serricorne (Fabricius) in tobacco warehouses and in
processing areas. The insecticide is applied into the air spaces after
working hours. Dichlorvos is also being used in the United States in
sprays (0.5 per cent for the control of insect pests such as ants,
bedbugs, cockroaches, flies, silverfish, spiders, ticks and wasps.
Tolerances
The use of dichlorvos in ways which might possibly leave residues in
food is quite recent. There appear to be no legal or other tolerances.
Residues resulting from supervised trials
There is only a limited amount of data available on residues in food
produced by dichlorvos. In a series of warehouse tests conducted in
the United States packaged noodles, raisins, beans, peanuts, flour and
sugar were exposed to 21 weekly treatments of dichlorvos applied as a
vapour at the rate of 50 mg/m3 per treatment. In each treatment, the
insecticide was vaporized slowly over a period of six hours. The
highest residue found after 21 applications in composite samples of
the various foods was 1.68 ppm in peanuts contained in burlap bags.
The residues in the other foods were well below 1 ppm (Unpublished
information received from US Department of Agriculture).
Following the work of Strong & Spur (1961) which showed that
dichlorvos was toxic to stored-product insects at very low
concentrations, preliminary tests were conducted in the United Kingdom
to assess its value for rapid disinfestation of grain where a long
residual life is unnecessary or undesirable. Feed barley was treated
with an emulsifiable formulation of dichlorvos as the grain was turned
from one bin into another. A deposit of 4 ppm appeared effective in
controlling the insects present but the insecticide disappeared very
quickly.
Resin strips measuring about 2-1/2" × 10" × 1/4" and containing about
18.6 per cent of dichlorvos by weight are under test to determine
their effectiveness in providing continuous control of flying and
crawling insects. The initial generation of dichlorvos by these strips
is approximately 40 mg/hr/strip and will produce a dichlorvos
concentration in the air of 1 mg/l at 30 per cent R.H. The emission
rate falls and after 30 days an almost constant rate of 3.0-4.0 mg of
dichlorvos is emitted producing a concentration of the insecticide in
the air of 0.075-0.1 µg/l of air. About 0.1 µg/l of dichlorvos is
produced for about two-thirds of the active life of the strips.
Residues in food moving in commerce
No information was available.
Fate of residues
Fragmentary data indicate that dichlorvos residues appearing in foods
from good agricultural practices are of a very low level and they
disappear quite rapidly when the treated foods are aired.
Green & Tyler (1966) found barley sprayed with dichlorvos at the rate
of 4 ppm while being turned from one bin to another had 1.9 ppm
(average) by analysis during treatment, 0.93 ppm after one week, 0.25
ppm after six weeks, 0.22 ppm after 10 weeks, and none could be
detected 15 weeks later.
A variety of foods were exposed in a room in which were hung
dichlorvos resin strips at the rate of 1 strip/1000 cu. ft for seven
weeks. The dichlorvos concentration in the air reached 0.33 µg/l after
24 hours and decreased to 0.03 µg/l after eight weeks. Residues of
dichlorvos in the various foods increased to maximum levels ranging
from 3.4 ppm for whole apples after 28 days' exposure down to 0.03 ppm
in sugar after 56 days. Whole apples, bacon and cheese had dichlorvos
residues greater than 1 ppm; banana (skin), currants (covered with
paper), dried milk (polyethylene bag) and surface samples of wheat,
cocoa beans and flour in hessian sacks had residues of 0.1-1 ppm; and
eggs, oranges, sugar, banana (edible part) had dichlorvos residues
below 0.1 ppm. On removal to an insecticide-free room, 20-70 per cent
of the residue was lost after four days, and 60-100 per cent after 10
days (Unpublished information).
Dichlorvos was added to rice at levels of 4.5 ppm and 19 ppm, and to
flour at 4.5 ppm and 14 ppm. Then the food was placed in sealed
containers and stored for 65 hours at room temperature. As in home
practice, the rice was washed with cold water and then cooked for
20-30 minutes until edible. There was 98 per cent less dichlorvos in
the rice after washing and cooking. The biscuits made from the treated
flour had 80 per cent and 60 per cent less dichlorvos, respectively
(Unpublished information).
Residues in meats were followed by exposure to dichlorvos labelled
with P32: the effects of cooking on such residues were investigated.
Dichlorvos penetrated only into the surface layers of the samples and
the residues in fat were generally lower than in the other samples.
Following the frying and boiling of steak containing dichlorvos
residues, the latter were completely destroyed and it was possible to
detect only products of hydrolysis (Miller & Aitken, 1965). Also after
the usual conservation of plums by thermalsterilization, dichlorvos
residues disappeared completely (Benes, personal communication).
Geisabühler & Haselbach (1963) concluded that the milling process does
not result in any considerable decrease of residues in wheat treated
with dichlorvos. During storage however the decomposition of the
residues proceeds much more quickly in milled wheat products than it
does in whole grains. No dichlorvos could be detected in samples of
white flour after one month of storage at room temperature.
Methods of residue analysis
The method which has been used for determining dichlorvos residues in
foods was developed by Shell Development Company. It is a
spectrophotometric method based on enzyme inhibition. It has a
sensitivity 0.10 ppm of dichlorvos.
Gas-liquid-chromatographic methods have been developed for
orgophosphorus compounds. It appears promising that this type of
analysis could be perfected for the determination of dichlorvos
residues.
RECOMMENDATIONS FOR TOLERANCES
No tolerances are recommended at this time because of insufficient
information on the specific uses of dichlorvos on foods and the
resulting residues. Recommendations for tolerances should be
considered at the next joint meeting.
Further work or information
Information is required on:
(a) range of commodities likely to be treated,
(b) occurrence of residue levels resulting from good pest control
practices,
(c) rate of disappearance through normal aging, processing, cooking,
etc.,
and
(d) chemical nature of terminal residues occurring in foods from good
pest control practices.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Blucher, W., Budd, E. R., Dewey, M. L., Eisenlord, G., Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Unpublished report
from the Hine Laboratories, San Francisco, California
Casida, J. E., McBride, L. & Niedermeier, R. P. (1962) J. Agr. Food
Chem., 10, 370
Durham, W. F., Gaines, T. B., McCauley, R. H., Sedlak, V. A., Mattson,
A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr. Hlth, 15,
340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes, W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 68
Gaines, T. B. (1964) Unpublished report
Gaines, T. B. (1966) Nature (Lond.) 209, 88
Gratz, N. G., Bracha, P. & Carmichael, A. G, (1962) WHO/Vector
Control/11
Hayes, W. J., jr (1961) Bull. Wld Hlth Org., 24, 629
Hodgson, E. & Casida, J. E. (1962) J. Agr. Food Chem., 10, 208
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960) J.
econ. Ent., 53, 602
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 2, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
Vigliani, E. C. (1966) Unpublished report to Shell Chemical Co.
Witherup, S., Caldwell, J. S. & Hull, L. (1965) Unpublished report
from the Kettering Laboratory, University of Cincinnati, Cincinnati,
Ohio
Witter, R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
REFERENCES PERTINENT TO AGRICULTURAL DATA
Durham, W. F., Hayes, W. J. & Mattson, A. M. (1959) Toxicological
Studies of DDVP in Tobacco Warehouses. Arch. industr. Hlth, 20:
202-10
Geissbühler, H. & Haselbach, C. (1963) On the behaviour of DDVP upon
storage and processing of insecticide treated cereals. (Report for
CIBA, Basle) Battelle Inst. Geneva, 30 October
Gillenwater, H. B. & Harein, P. K. (1964) A dispenser designed to
provide large quantities of insecticide vapor. J. Econ. Ent., 57
(5): 762-3
Green, A. A., Kane, J. & Gradidge, J. M. G. (1966) Experiments in the
Control of Ephestia elutella using Dichlorvos Vapour. J. Stored
Proc. Res., 2: 147-157
Green, A. A. & Tyler, P. S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus infesting
stored barley. J. Stored Prod. Res., 1: 273-295
Millar, K. R. & Aitken, W. M. (1965) Residues in meat following
exposure to P32 labelled dichlorvos vapour in an enclosed space.
N.Z. J. Agric. Res., 8 (2): 350-362
Strong, H. G. & Spur, D. E. (1961) Evaluation of Insecticides as
protectants against pests of stored grain and seeds. J. Econ. Ent.,
54 (2); 235-238
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Dichlorvos inhibits cholinesterase activity and thus causes
parasympathomimetic effects. It requires no metabolic conversion, but
inhibits the enzyme directly. Dichlorvos is broken down rapidly in the
liver (Tracy, 1960; Tracy et al., 1960). Thus, the onset of poisoning
is rapid, and if recovery occurs, it is prompt (Klotzsche, 1956;
Durham et al., 1957; Gaines, 1960, Yamashita, 1962).
In blood from a human female with pseudocholinesterase deficiency,
0.185 µg of dichlorvos caused 50 per cent inhibition of the
erythrocyte esterase activity. The erythrocyte esterase activity in
normal blood was inhibited by only 15 per cent by 0.3 µg of dichlorvos
(Vigliani, 1966). Assays of whole blood from a cow given 1.0 mg/kg of
dichlorvos either orally or subcutaneously did not show any
cholinesterase inhibition; however, intravenous administration of the
same dose produced 29 per cent inhibition after 1-2 hours with rapid
recovery. In a goat given 1.52 mg/kg subcutaneously there was 44 per
cent inhibition in 1/4 hour; 46 per cent in 1/2 hour; 41 per cent in 1
hour and 35 per cent in 2 hours, followed by rapid recovery (Casida et
al., 1962).
The compound is not stored in the body nor excreted in the milk to any
appreciable extent in cows or rats, even when administered in doses
that produced severe poisoning (Tracy, 1960; Tracy et al. 1960).
With whole homogenates of liver, kidney, spleen and adrenals from rat
and rabbit, the principal labelled metabolite of dichlorvos-35P was
dimethylphosphate, 50-85 per cent, with the remaining radioactivity
appearing in demethyl-dichlorvos, monomethyl phosphate and inorganic
phosphate. In the plasma of both species, dimethyl phosphate accounted
for 98 to 100 per cent of the dichlorvos hydrolyzed. When dimethyl
32P-phosphate was incubated with rat plasma or liver homogenate, all
the radioactivity was recovered as unchanged dimethyl phosphate.
Dichlorvos was hydrolyzed by the soluble and mitochondrial fractions
of rat liver but not by the microsomes. Demethyl-dichlorvos was
hydrolyzed by the soluble fraction only. In rat liver homogenate, 2-3
times more dichloroacetaldehyde than inorganic phosphate was produced
from demethyl-dichlorvos. Inorganic phosphate was produced from
monomethyl phosphate in both plasma and liver homogenate. The reaction
was very slow. In soluble rat liver preparations, the
dichloroacetaldehyde released by the hydrolysis of dichlorvos was
reduced in the presence of DPNH to dichloroethanol. A very small
amount may have appeared as dichloroacetate. It is possible that a
pathway exists for conjugation of dichloroethanol with glucuronic acid
(Hodgson & Casida, 1962).
In rats given dichlorvos-32P, 10 mg/kg orally, rapid absorption,
distribution and hydrolysis took place. Female rats treated every 1/2
hour with 4 mg/kg of dichlorvos-32P for 2 hours and sacrificed 1/2
hour after the last dose showed mainly hydrolysis products in the
tissues. In mice given 4 doses of 10 mg/kg every 15 minutes, 95 per
cent of the dichlorvos administered was hydrolyzed in the liver,
kidney and small intestine. In male rats given
dimethyl-32P-phosphate, 500 mg/kg orally, necropsy 90 hours after
dosing indicated that almost the entire dose had been eliminated. The
urine contained only unmetabolized dimethyl-32P-phosphate, which
accounted for about 50 per cent of the radioactivity administered. The
tissues were almost devoid of radioactivity. In a rat given
demethyl-dichlorvos-32P, 500 mg/kg orally, about 14 per cent of the
dose was eliminated in the urine in 90 hours, and the tissue
distribution was similar to that of the rat which had received
dichlorvos. A very high proportion of the radioactivity was found in
bone, indicating rapid degradation to phosphoric acid. Metabolites in
the urine were 86 per cent, phosphoric acid and 14 per cent
demethyl-dichlorvos. From 67 to 100 per cent of the administered
radioactivity was recovered with 1 week in the combined urine and
faeces of cows, rats and a goat given various doses of
dichlorvos-32P. Excretion of radioactivity in the faeces accounted
for 11-15 per cent of the dose, except in cows treated orally, where
about 50 per cent of the radioactivity was excreted. The excreted
metabolites appeared to be demethyl-dichlorvos, dimethyl phosphate,
monomethyl phosphate and inorganic phosphate. Most of the
radioactivity in the milk of the cows and goat was due to hydrolysis
products. The level of organosoluble radioactivity was significantly
above background only within the first 2 hours. The highest excretion
level was found at 12 hours in the cows (Casida, McBride &
Niedermeier, 1962).
Both single and repeated large doses cause a stress-attributed
reduction in the eosinophil count of the peripheral blood of rats
(Klotzsche, 1956).
Experiments in rats showed that the liver is highly efficient in
detoxicating dichlorvos (Gaines, 1966) and that the compound is
absorbed from the gastrointestinal tract by the blood of the hepatic
portal system (Laws, 1966).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 124 Yamashita, 1962
Mouse i.p. 28* Casida et al., 1962
Rat Oral 73 Klotzsche, 1956
Rat, male Oral 80 Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 56** Durham et al., 1957
Gaines, 1960
Mattson et al., 1955
Rat, female Oral 80*** Durham et al., 1957
Chick, male Oral 14.8 Sherman & Ross, 1961
* Technical grade; purity not stated.
** Based on 99 per cent pure material.
*** Result of 2 tests on a technical preparation (90 per cent pure).
Rat. Under laboratory conditions it was possible to produce
concentrations ranging from 31 to 118 µg/l in an exposure chamber.
Under the severest conditions of respiratory exposure, rats showed
signs of poisoning within 2 hours and died in 4.5-17.5 hours (Durham
et al., 1957).
Domestic animals. Horses tolerated a single dose of 50 mg/kg
dichlorvos in feed, but showed moderate acute poisoning when the
insecticide was given by stomach-tube at 25 mg/kg (Jackson et al.,
1960). An almost identical dose (27 mg/kg) given by stomach-tube
caused severe but non-fatal poisoning in a cow (Tracy et al., 1960).
Dichlorvos does not produce either immediate or delayed paralysis in
hens (Durham et al., 1957).
Man. Men withstood brief exposure (30-60 minutes) to concentrations
as high as 6.9 µg/l without depression of cholinesterase activity or
any other observed effect (Durham et al., 1959; Hayes, 1961). A single
exposure for 8 hours at a concentration ranging from 0.9 to 3.5 µg/l
produced slight inhibition of plasma cholinesterase activity in man
(Witter et al., 1961). Slightly higher concentrations or longer
periods of exposure, or both, produce measurable reduction of both the
red cell and plasma enzyme activity in men, monkeys and rats (Hayes,
1961).
Short-term studies
Rat. Relatively small repeated doses lower the blood cholinesterase
activity but much larger doses are required to produce illness. Thus,
Durham et al. (1957) found that a dietary concentration of only 50 ppm
soon produced detectable lowering of plasma and red cell
cholinesterase activity in female rats, but a dietary level of 1000
ppm (about 50 mg/kg/day) was tolerated for 90 days without any
diminution of growth or sign of intoxication. Male and female rats
reproduced as well as controls when maintained on a dietary level of
100 ppm (Gaines, 1964).
When dichlorvos is given by stomach-tube it is not so well tolerated
as when the same dosage is absorbed from the diet gradually throughout
the day. Female rats tolerated doses of 10 and 20 mg/kg in the diet
but suffered severe, acute poisoning when given single doses of 30
mg/kg. Even at 30 mg/kg, the rats survived and the red cell
cholinesterase activity and growth of their litters were normal (Tracy
et al., 1960).
Reproduction studies in rats fed 0, 0.1, 1.0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers, and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965).
Dog. Dichlorvos given to dogs by capsule at rates equivalent to
dietary levels of 5 and 15 ppm (0.13-0.37 mg/kg/day) produced no
detectable effect; levels equivalent to 25 ppm and 50 ppm depressed
brain cholinesterase activity to 88 and 33 per cent of control,
respectively, and led to some increase in the activity and
aggressiveness of the dogs (Blucher et al., 1962).
Monkey. A dermal dose of 50 mg/kg produced cholinergic signs in a
monkey 20 minutes after administration, and it died after 8 daily
doses at this rate. Higher dosage rates produced even more rapid onset
of illness, even though a single dose of 100 mg/kg was not fatal
(Durham et al., 1957).
Monkeys tolerated continuous exposure to concentrations ranging from
0.1-0.5 µg/l for 22 days without a definite change in cholinesterase
activity. They showed a definite depletion by the 50th day of exposure
(Durham et al., 1959), but the concentration of dichlorvos may have
exceeded 0.5 µg/l sometime between the 22nd and 50th day (Hayes,
1961).
Horse. Horses showed mild depression of red cell but not plasma
cholinesterase activity when exposed continuously to concentrations
ranging from 0.24-1.48 µg/l, but they returned to normal in spite of
continuing exposure (Tracy et 1960).
Man. Men showed no change in cholinesterase activity when exposed
for 8-10 half-hour intervals, 4 nights per week, for 11 weeks to
concentrations from 0.07 to 0.66 µg/l (average 0.25 µg/l). They did
show a small but statistically significant depression of plasma enzyme
activity, but not of red cell enzyme activity, when the schedule of
dosing was maintained but the concentration increased to 0.40-0.55
µg/l (average 0.51 µg/l). The other parameters studied, including
complex reaction time, airway resistance and vision, remained normal,
(Rasussen et al., 1963). The tolerated inhaled dosage averaged 0.5
mg/man/day while the dosage that caused a slight fall of plasma
cholinesterase was 1.1 mg/man/day. The compound produced no decrease
in whole blood cholinesterase activity and no other indication of
injury when used for malaria control (Funckes et al., 1963; Gratz et
al., 1962).
When 15 subjects were exposed for 8 months to concentrations of 0.1
mg/M3 and higher of dichlorvos in the air, 5 showed a slight
reduction in plasma cholinesterase activity. When 36 subjects were
exposed to concentrations of less than 0.1 mg/M3, none showed any
reduction in plasma cholinesterase activity (Vigliani, 1966).
Long-term studies
No really long-term studies have been made of dichlorvos, nor do they
appear indicated because of the rapid action and excretion of the
compound.
Comments
Dichlorvos is rapidly metabolised in mammalian tissues to relatively
non-toxic metabolites which are rapidly excreted. From the data
available to date, the maximum level causing no significant
toxicological effect in man is 0.01 mg/kg/day by inhalation.
Continuous exposure of man to 0.1 mg/M3 caused some depression of
plasma cholinesterase activity without clinical signs. It would be
desirable to determine the maximum oral dose causing no inhibition of
cholinesterase activity in man. In short-term experiments in dogs, the
oral no-effect level was 0.37 mg/kg/day.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg/day.
Dog: 0.37 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatment
No information was available on the use of dichlorvos treatments
applied to food crops prior to harvest. Its potentialities in this
field seem limited by its lack of systemic properties and its short
persistence. It is used on beef and dairy cattle and on goats, sheep
and swine and in and around the buildings which house these animals.
(b) Post-harvest treatments
Strong & Spur (1961) showed that dichlorvos was active against a range
of insect pests of stored produce. Experimental work has since been
carried out on its application for the protection of cereals and
cereal products during storage and for the control of insects in
facilities where foods are stored, processed, handled or shipped.
Trials have shown that 4 micrograms of dichlorvos vapour per litre of
air for six hours will control common stored product insects. Durham
et al. (1959) found that the tightness of the warehouse and the
temperature influences the amount required to obtain the desired
concentration in the air; but in fairly tight warehouses the necessary
concentration can be obtained by releasing dichlorvos at 50 mg/m3.
Dichlorvos was dispensed by Gillenwater & Harein (1964) from a
specially designed heat volatilizer containing impregnated resin
pellets. However, it could be applied as a pressurized aerosol
formulation although higher levels of residues might result from such
use, particularly in any foodstuffs situated close to the aerosol
dispensers.
Some tests have been conducted recently on dichlorvos impregnated
resin strips which show considerable promise for future extension of
its use in the control of insects in stored produce (Green et al.,
1966).
(c) Uses other than on food
For the control, primarily, of flies and, mosquitos dichlorvos is
being used both inside and outside of agricultural premises such as
barns, feed lots, milk rooms, poultry houses, stables, corrals,
holding pens and poultry pens and yards. It has been used for a number
of years for the control of Ephestia elutella (Hübner) and
Lasioderma serricorne (Fabricius) in tobacco warehouses and in
processing areas. The insecticide is applied into the air spaces after
working hours. Dichlorvos is also being used in the United States in
sprays (0.5 per cent for the control of insect pests such as ants,
bedbugs, cockroaches, flies, silverfish, spiders, ticks and wasps.
Tolerances
The use of dichlorvos in ways which might possibly leave residues in
food is quite recent. There appear to be no legal or other tolerances.
Residues resulting from supervised trials
There is only a limited amount of data available on residues in food
produced by dichlorvos. In a series of warehouse tests conducted in
the United States packaged noodles, raisins, beans, peanuts, flour and
sugar were exposed to 21 weekly treatments of dichlorvos applied as a
vapour at the rate of 50 mg/m3 per treatment. In each treatment, the
insecticide was vaporized slowly over a period of six hours. The
highest residue found after 21 applications in composite samples of
the various foods was 1.68 ppm in peanuts contained in burlap bags.
The residues in the other foods were well below 1 ppm (Unpublished
information received from US Department of Agriculture).
Following the work of Strong & Spur (1961) which showed that
dichlorvos was toxic to stored-product insects at very low
concentrations, preliminary tests were conducted in the United Kingdom
to assess its value for rapid disinfestation of grain where a long
residual life is unnecessary or undesirable. Feed barley was treated
with an emulsifiable formulation of dichlorvos as the grain was turned
from one bin into another. A deposit of 4 ppm appeared effective in
controlling the insects present but the insecticide disappeared very
quickly.
Resin strips measuring about 2-1/2" × 10" × 1/4" and containing about
18.6 per cent of dichlorvos by weight are under test to determine
their effectiveness in providing continuous control of flying and
crawling insects. The initial generation of dichlorvos by these strips
is approximately 40 mg/hr/strip and will produce a dichlorvos
concentration in the air of 1 mg/l at 30 per cent R.H. The emission
rate falls and after 30 days an almost constant rate of 3.0-4.0 mg of
dichlorvos is emitted producing a concentration of the insecticide in
the air of 0.075-0.1 µg/l of air. About 0.1 µg/l of dichlorvos is
produced for about two-thirds of the active life of the strips.
Residues in food moving in commerce
No information was available.
Fate of residues
Fragmentary data indicate that dichlorvos residues appearing in foods
from good agricultural practices are of a very low level and they
disappear quite rapidly when the treated foods are aired.
Green & Tyler (1966) found barley sprayed with dichlorvos at the rate
of 4 ppm while being turned from one bin to another had 1.9 ppm
(average) by analysis during treatment, 0.93 ppm after one week, 0.25
ppm after six weeks, 0.22 ppm after 10 weeks, and none could be
detected 15 weeks later.
A variety of foods were exposed in a room in which were hung
dichlorvos resin strips at the rate of 1 strip/1000 cu. ft for seven
weeks. The dichlorvos concentration in the air reached 0.33 µg/l after
24 hours and decreased to 0.03 µg/l after eight weeks. Residues of
dichlorvos in the various foods increased to maximum levels ranging
from 3.4 ppm for whole apples after 28 days' exposure down to 0.03 ppm
in sugar after 56 days. Whole apples, bacon and cheese had dichlorvos
residues greater than 1 ppm; banana (skin), currants (covered with
paper), dried milk (polyethylene bag) and surface samples of wheat,
cocoa beans and flour in hessian sacks had residues of 0.1-1 ppm; and
eggs, oranges, sugar, banana (edible part) had dichlorvos residues
below 0.1 ppm. On removal to an insecticide-free room, 20-70 per cent
of the residue was lost after four days, and 60-100 per cent after 10
days (Unpublished information).
Dichlorvos was added to rice at levels of 4.5 ppm and 19 ppm, and to
flour at 4.5 ppm and 14 ppm. Then the food was placed in sealed
containers and stored for 65 hours at room temperature. As in home
practice, the rice was washed with cold water and then cooked for
20-30 minutes until edible. There was 98 per cent less dichlorvos in
the rice after washing and cooking. The biscuits made from the treated
flour had 80 per cent and 60 per cent less dichlorvos, respectively
(Unpublished information).
Residues in meats were followed by exposure to dichlorvos labelled
with P32: the effects of cooking on such residues were investigated.
Dichlorvos penetrated only into the surface layers of the samples and
the residues in fat were generally lower than in the other samples.
Following the frying and boiling of steak containing dichlorvos
residues, the latter were completely destroyed and it was possible to
detect only products of hydrolysis (Miller & Aitken, 1965). Also after
the usual conservation of plums by thermalsterilization, dichlorvos
residues disappeared completely (Benes, personal communication).
Geisabühler & Haselbach (1963) concluded that the milling process does
not result in any considerable decrease of residues in wheat treated
with dichlorvos. During storage however the decomposition of the
residues proceeds much more quickly in milled wheat products than it
does in whole grains. No dichlorvos could be detected in samples of
white flour after one month of storage at room temperature.
Methods of residue analysis
The method which has been used for determining dichlorvos residues in
foods was developed by Shell Development Company. It is a
spectrophotometric method based on enzyme inhibition. It has a
sensitivity 0.10 ppm of dichlorvos.
Gas-liquid-chromatographic methods have been developed for
orgophosphorus compounds. It appears promising that this type of
analysis could be perfected for the determination of dichlorvos
residues.
RECOMMENDATIONS FOR TOLERANCES
No tolerances are recommended at this time because of insufficient
information on the specific uses of dichlorvos on foods and the
resulting residues. Recommendations for tolerances should be
considered at the next joint meeting.
Further work or information
Information is required on:
(a) range of commodities likely to be treated,
(b) occurrence of residue levels resulting from good pest control
practices,
(c) rate of disappearance through normal aging, processing, cooking,
etc.,
and
(d) chemical nature of terminal residues occurring in foods from good
pest control practices.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Blucher, W., Budd, E. R., Dewey, M. L., Eisenlord, G., Hine, C. H.,
Loquvam, G. L., Powers, M. T. & Riggs, C. W. (1962) Unpublished report
from the Hine Laboratories, San Francisco, California
Casida, J. E., McBride, L. & Niedermeier, R. P. (1962) J. Agr. Food
Chem., 10, 370
Durham, W. F., Gaines, T. B., McCauley, R. H., Sedlak, V. A., Mattson,
A. M. & Hayes, W. J., jr (1957) A. M. A. Arch. industr. Hlth, 15,
340
Durham, W. F., Hayes, W. J., jr & Mattson, A. M. (1959) A. M. A.
Arch. industr. Hlth., 20, 202
Funckes, A. J., Miller, S. & Hayes, W. J., jr (1963) Bull. Wld Hlth
Org., 29, 243
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 68
Gaines, T. B. (1964) Unpublished report
Gaines, T. B. (1966) Nature (Lond.) 209, 88
Gratz, N. G., Bracha, P. & Carmichael, A. G, (1962) WHO/Vector
Control/11
Hayes, W. J., jr (1961) Bull. Wld Hlth Org., 24, 629
Hodgson, E. & Casida, J. E. (1962) J. Agr. Food Chem., 10, 208
Jackson, J. B., Drummond, R. O., Buck, W. B. & Hunt, L. M. (1960) J.
econ. Ent., 53, 602
Klotzsche, C. (1956) Z. Angew. Zool., 1, 87
Mattson, A. M., Spillane, J. T. & Pearce, G. W. (1955) J. Agr. Food
Chem., 2, 319
Rasmussen, W. A., Jensen, J. A., Stein, W. J. & Hayes, W. J., jr
(1963) Aerospace Med., 34, 594
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Tracy, R. L. (1960) Soap Chem. Spec., 36, 74
Tracy, R. L., Woodcock, J. G. & Chodroff, S. (1960) J. econ. Ent.,
53, 593
Vigliani, E. C. (1966) Unpublished report to Shell Chemical Co.
Witherup, S., Caldwell, J. S. & Hull, L. (1965) Unpublished report
from the Kettering Laboratory, University of Cincinnati, Cincinnati,
Ohio
Witter, R. F., Gaines, T. B., Short, J. G., Sedlak, V. A. & Maddock,
D. R. (1961) Bull. Wld Hlth Org., 24, 635
Yamashita, K. (1962) Industr. Med. Surg., 31, 170
REFERENCES PERTINENT TO AGRICULTURAL DATA
Durham, W. F., Hayes, W. J. & Mattson, A. M. (1959) Toxicological
Studies of DDVP in Tobacco Warehouses. Arch. industr. Hlth, 20:
202-10
Geissbühler, H. & Haselbach, C. (1963) On the behaviour of DDVP upon
storage and processing of insecticide treated cereals. (Report for
CIBA, Basle) Battelle Inst. Geneva, 30 October
Gillenwater, H. B. & Harein, P. K. (1964) A dispenser designed to
provide large quantities of insecticide vapor. J. Econ. Ent., 57
(5): 762-3
Green, A. A., Kane, J. & Gradidge, J. M. G. (1966) Experiments in the
Control of Ephestia elutella using Dichlorvos Vapour. J. Stored
Proc. Res., 2: 147-157
Green, A. A. & Tyler, P. S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus infesting
stored barley. J. Stored Prod. Res., 1: 273-295
Millar, K. R. & Aitken, W. M. (1965) Residues in meat following
exposure to P32 labelled dichlorvos vapour in an enclosed space.
N.Z. J. Agric. Res., 8 (2): 350-362
Strong, H. G. & Spur, D. E. (1961) Evaluation of Insecticides as
protectants against pests of stored grain and seeds. J. Econ. Ent.,
54 (2); 235-238
