
DICHLORVOS
First draft prepared by
A. Moretto
University of Padua, Padua, Italy
EXPLANATION
Dichlorvos was previously evaluated by the Joint Meeting in
1965, 1966, 1967, 1970 and 1977 (Annex I, references 3, 6, 8, 14,
28). An ADI of 0-0.004 mg/kg bw was allocated in 1966 and was
maintained at subsequent Meetings. The compound was re-evaluated by
the present Meeting on the basis of the CCPR periodic review
programme. Since 1977 many reviews on the toxicological aspects of
dichlorvos have been published. The most relevant are the IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans (IARC, 1979, 1991) and the IPCS Environmental Health
Criteria 79 on Dichlorvos (WHO, 1989). This monograph summarizes
new or not previously reviewed data as well as relevant data from
previous monographs and monograph addenda on dichlorvos.
BIOCHEMICAL ASPECTS
Many studies have been performed on the biochemical aspects of
dichlorvos and have been summarized by WHO (1989). Summaries of the
most relevant data are given here.
Absorption, distribution and elimination
Dichlorvos is readily absorbed via all routes of exposure. In
rats orally dosed with 32P-dichlorvos, 60-70% of administered
radioactivity was recovered in the urine and about 10% in the faeces
within 6 days after dosing. Radioactivity in bones slowly increased
with time because of the incorporation of 32P into the normal
phosphate pool. After the oral administration of
methyl-14C-dichlorvos to rats and mice, the excretion of
radioactivity was rapid. The major routes of elimination after 4
days were urine (approximately 60%) followed by expired air
(approximately 16%). Carcass contained about 5% of the administered
radioactivity 4 days after dosing. When vinyl-14C-dichlorvos was
used, less radioactivity was recovered in urine (10-30%) while
higher levels were recovered in liver, skin and carcass. In
carcass, levels of radioactivity were 14-34% after 1-4 days from
dosing mice, rats and Syrian hamsters. The higher levels found in
carcass indicate that the vinyl moiety enters the 2-carbon metabolic
pool. Similar results were obtained using the inhalation and
parenteral routes. After a single i.p. dose (10 mg/kg bw), the
dichlorvos concentration in brain peaked after 1 min. and than
disappeared with a half-life of less than 1 min (Nordgren et al.,
1978). Experiments with pregnant rabbits and pigs showed that
dichlorvos readily crosses the placenta (WHO, 1989).
Biotransformation
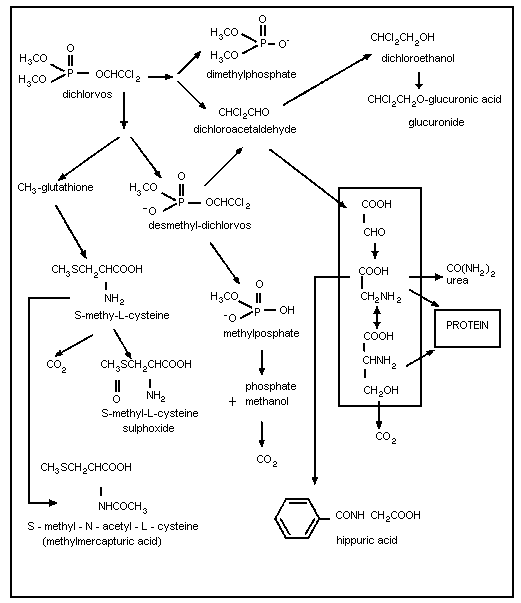
A scheme of the metabolites of dichlorvos in mammals is given
in Figure 1. Two main pathways are responsible for the rapid
degradation of dichlorvos. Desmethyl-dichlorvos is produced by a
glutathione-dependent enzymatic system while dimethylphosphate and
dichloroacetaldehyde are the hydrolysis products of an A-esterase
which is present mainly in plasma and liver (Reiner et al., 1975).
This appears to be the dominant pathway. The rate of hydrolysis of
dichlorvos by rat plasma has been determined to be 12 µmol/hour/ml
at 37 °C (Reiner et al., 1980). The metabolism of dichlorvos is
rapid and similar in the various species, including humans.
Differences between species are related to the rate of metabolite
formation rather than to the nature of the metabolites. The
metabolism of dichlorvos is so rapid that the half-life in blood
could not be determined in experimental animals, and it appears to
be shorter than 15 minutes. No evidence of the accumulation of
dichlorvos or potentially toxic metabolites has been found (WHO,
1989).
 Effects on enzymes and other biochemical parameters
In vitro studies showed that rodent plasma ChE is more
sensitive than erythrocyte and brain ChE to inhibition by
dichlorvos. Calculated I50s (concentrations which inhibit 50% of
enzyme activity) at 37 °C, pH 7.4-7.6, were 10-8 and 10-7 mol/l,
respectively, for an incubation time of 20 minutes (Asperen &
Dekhuijzen, 1958; Skrinjaric-Spoljar et al., 1973; Lotti &
Johnson, 1978). The rate of phosphorylation of rat brain ChE was
calculated to be 1.5 x 105 mol/l/min at 37 °C, pH 7.4-7.6
(Skrinjaric-Spoljar et al., 1973). Half-life of in vitro
reactivation (at 37 °C, pH 7.4-7.6) was found to be about 100
minutes; that of aging was found to be about 400 min. This means
that inhibited AChE almost completely reactivates within a few
hours, leaving a small fraction (20% or less) irreversibly inhibited
(i.e. aged) (Skrinjaric-Spoljar et al., 1973).
TOXICOLOGICAL STUDIES
Acute toxicity studies
The results of acute toxicity tests of dichlorvos administered
by various routes to different animal species are summarized in
Tables 1 and 2. Typical cholinergic signs are observed. WHO has
classified dichlorvos as highly hazardous (WHO, 1992).
Short-term toxicity studies
Mice
In a range-finding study, groups of 10 B6C3F1 mice/sex were
administered by corn oil gavage 0, 5, 10, 20, 40, 80, or 160 mg
dichlorvos/kg bw/day, 5 days per week for 13 weeks. Animals were
observed twice daily for clinical signs and body weight was recorded
weekly. Necropsy was performed on all animals. Oesophagus and
gastrointestinal tract of all animals dying after day 46 were
examined histologically. All vehicle controls and all animals in
the highest dose group with survivors were examined histologically.
All mice at 160 mg/kg bw/day and 5 male mice at 80 mg/kg bw/day died
before the end of the study. Final mean body weights in all treated
groups were comparable to controls. No gross or microscopic
pathologic effects were observed (Chan, 1989).
Rats
In a range-finding study, groups of 10 male and 10 female
F344/N rats were administered by corn oil gavage 0, 2, 4, 8, 16, 32,
or 64 mg dichlorvos/kg bw/day, 5 days per week for 13 weeks.
Animals were observed twice daily for clinical sign and body weights
were recorded weekly. Necropsy was performed on all animals.
Oesophagus and gastrointestinal tract of all animals dying after day
46 were examined histologically. All vehicle controls and all
animals in the highest dose group with survivors were examined
histologically. All rats at 32 and 64 mg/kg bw/day and 1 male and 4
females at 16 mg/kg bw/day died before the end of the study. Final
mean body weights of all dose groups were comparable to vehicle
controls. No gross or microscopic pathologic effects were observed
(Chan, 1989).
Table 1. Acute toxicity of dichlorvos (WHO, 1989)
Species Route LD50 (mg/kg bw)
Mouse oral 68-275
i.p. 28-41
i.v. 8-10
s.c. 13-33
Rat oral 30-110
dermal (24 h) 75-107
i.p. 18
s.c. 72
Guinea-pig s.c. 28
Hamster i.p. 30
Rabbit oral 13-23
dermal 205
Cat oral 28
Dog oral 100-316
Chicken oral 15
Swine oral 157
Table 2. Acute inhalation toxicity of dichlorvos (WHO, 1989)
Species Mode of exposure Time of exposure LC50
(hours) (µg/l)
Mouse whole body 4 13
head only 4 > 218
Rat whole body 4 15
whole body 1 140
head only 4 340
head only 1 455
head only 4 > 198
Dogs
Groups of 3 male and 3 female beagle dogs received 0, 2.3, 6.9,
12 or 23 mg dichlorvos/dog (93% in corn oil by gelatin capsule)
daily, for 90 days. The mean doses per group were equivalent to
0.3, 1, 1.5 or 3 mg/kg bw/day. No effects were observed on
mortality, growth, haematology, liver and kidney function, organ
weights, or at gross and histopathological examination. In the two
highest dose groups, the dogs showed excitement, increased activity
and aggression. Plasma and erythrocyte ChE activities, measured
initially and at intervals of approximately 2 weeks, were normal in
the lowest dose group (0.3 mg/kg bw/day) but inhibited in the other
dose groups (up to about 60% in the highest dose group). Inhibition
was lower from day 70 onwards. Brain ChE activity at termination
was decreased (to 33%of control activity) only in the highest dose
group. The NOAEL in this study was 1.5 mg/kg bw/day based on
reduction in brain ChE activity at termination. However, taking
into account the behavioural changes, a more conservative NOAEL can
be considered to be 1 mg/kg bw/day (Hine, 1962).
Pigs
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, or 16 mg
dichlorvos/kg bw/day (divided over 2 daily doses) for 30 days. No
effects were found on body-weight gain, haematology, or clinical
chemistry when compared with control animals fed a blank PVC
formulation. Plasma and erythrocyte ChE activities were
significantly inhibited in the 4 mg/kg bw/day group (37 and 34% of
controls, respectively) and in the 16 mg/kg bw/day group (33 and 21%
of controls, respectively). The NOAEL in this study was 1 mg/kg
bw/day based on inhibition of erythrocyte ChE activity (Stanton et
al., 1979).
Monkeys
Rhesus monkeys (4/sex) were continuously exposed to dichlorvos
vapour at an average actual concentration of 0.05 mg/m3 for 3
months. The control group consisted of 4 males and 1 female. No
adverse effects were observed in appearance, behaviour, or
haematological and clinical chemical determinations. Plasma ChE
activity was slightly reduced (up to 28% inhibition), while
erythrocyte ChE activity inhibition was 36%. No changes in nerve
maximum conduction velocities or muscular evoked action potentials
were induced by exposure to dichlorvos (Coulston & Griffin, 1977).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 100 male and 100 female C57BI/6/Bln mice (5-6 weeks
old) received by gavage 0.2 mg dichlorvos per mouse (97% in 0.2 ml
water), freshly prepared, either twice or 3 times per week for 50
weeks. Control groups received by gavage either 0.2 ml water, 3
times per week (about 50 males and 50 females), or no treatment (35
males and 35 females). Surviving animals were sacrificed after 110
weeks. This strain of mice is known for the spontaneous occurrence
of mixed lymphomas (reticulocell sarcoma type B).
From the age of 12 months onwards, some animals in all groups
developed interstitial pneumonia. The incidence of mixed lymphomas
was decreased in both test groups (26-60% in control groups, 23-30%
in treated groups). An increased incidence of focal hyperplasia
(transitional cell hyperplasia) of the urinary bladder was found in
both dichlorvos groups (0-8% in control groups, 5-10% in treated
groups). The authors concluded that no neoplastic lesions were
found which could be attributed to the treatment of the animals with
dichlorvos (Horn et al., 1987).
Dichlorvos was not co-carcinogenic in C57BI/6/Bln mice when
administered by gavage three times/week at 0.2 mg/animal to mice
subcutaneously injected with 50 µg N-nitrosodiethylamine per animal
weekly for 50 weeks followed by an observation period of up to 110
weeks (Horn et al., 1990).
Two groups of 50 male and 50 female B6C3F1 mice were fed
1000 or 2000 ppm dichlorvos (purity > 94%) in corn oil in the diet
for 2 weeks. Due to severe signs of intoxication, doses were
lowered to 300 and 600 ppm for the following 78 weeks. Samples of
the diets analyzed during the study showed that time-weighted
average concentrations were 318 and 635 ppm. Matched controls
consisted of 10 mice of each sex; the pooled controls from
simultaneous studies with other compounds consisted of 100 male and
80 female mice. All surviving mice were killed at 92-94 weeks.
Animals were observed twice daily for clinical signs.
Alopecia and rough hair coats were noted in many treated
animals, particularly in the male groups, beginning at week 20 and
persisting throughout the study. The average body weights of the
high-dose mice of both sexes were slightly decreased compared with
controls. The low-dose female group showed 74% survival at 90 weeks
compared to 84% and 90% in high-dose and control groups,
respectively. There was no significant increase in the incidence of
tumours attributable to dichlorvos in either sex.
Two squamous-cell carcinomas of the oesophagus (one in a
low-dose male and one in a high-dose female), 1 papilloma of the
oesophagus in a high-dose female and 3 cases of focal hyperplasia of
the oesophageal epithelium in low-dose males were recorded in the
treated mice. The significance of the findings in the treated mice
was considered uncertain because of insufficient information
concerning the spontaneous incidence of these lesions and lack of
statistical significance within the experiment. Dichlorvos (up to
635 ppm in the diet, equivalent to 95 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Studies in B6C3F1 mice given 0, 400 or 800 mg/litre
dichlorvos in drinking-water for two years and in CFE rats exposed
to 0, 0.05, 0.48, or 4.7 mg/m3 of dichlorvos 23 hours/day for 2
years were summarized in WHO (1989). There was no evidence of
carcinogenic effects in these studies (Blair et al., 1976;
Konihishi et al., 1981).
Groups of 50 B6C3F1 mice were given dichlorvos (99% purity)
by corn oil gavage at doses of 0, 10 or 20 (males) or 0, 20 or 40
(females) mg/kg bw/day daily, 5 days per week, for 103 weeks. The
dose volume of the corn oil was 10 ml/kg bw/day. Body weights were
recorded once weekly for the first 12 weeks and then monthly.
Animals were observed twice per day for clinical signs. Necropsy
and histological examinations were performed on all moribund animals
or at the end of the study.
Body-weight gain and survival did not significantly differ
between treated and control groups. No compound-related clinical
signs were observed. Blood cholinesterase activity was not
determined during the study. However, a separate study showed
inhibition of plasma ChE activity by 60-90% in all dose groups while
erythrocyte ChE activity was normal (see "Special studies on
Cholinesterase activity" for detailed description and comments).
The incidences of forestomach squamous cell papillomas were
1/50, 1/50 and 5/50 in control, low and high-dose males respectively
and 5/49, 6/49 and 18/50 in control, low and high-dose females,
respectively. The positive trend was statistically significant in
both sexes while by pairwise comparison only the incidence in high-
dose females was significantly higher than in controls. Two
forestomach squamous cell carcinomas were seen in high-dose females
and none in the other groups. No increase in the incidence of
forestomach hyperplasia was seen in the dosed mice compared with
vehicle controls (10-20%). In female mice the incidence of adenomas
and adenomas or carcinomas (combined) of the pituitary gland (12/45,
6/45 and 6/44 in control, low and high-dose groups, respectively)
and the incidence of lymphomas (16/50, 11/50 and 9/50 in control,
low and high-dose groups, respectively) showed a significant
negative trend. Based on the increased incidence of forestomach
papillomas, the NOAEL was 10 mg/kg bw/day (Chan, 1989; Chan et al.,
1991).
Rats
Groups of 40 male and 40 female weanling CD rats were fed diets
containing nominal concentrations of 0, 0.1, 1, 10, 100 or 500 ppm
dichlorvos (93% purity) for 2 years. Diets were prepared weekly.
Five males and 5 females from each group were killed after 6, 12 or
18 months. Analysis of diet samples showed a considerable loss of
dichlorvos associated with a gradual increase in
dichloroacetaldehyde (DCA) content (average concentrations ranging
from 0.01 to 28.6 ppm. The average actual concentrations of
dichlorvos in each diet were 0.05, 0.5, 4.7, 47 or 230 ppm. No
cholinergic signs were observed. No effects were seen on behaviour,
mortality rate, weight gain, food consumption, terminal body and
organ weights, haematology or urinalysis. Plasma and erythrocyte
ChE activities were measured 13 times during the study. In the 100
ppm group, plasma and erythrocyte ChE activities were reduced to
60-90% and to 50-90% of control activities, respectively; in the 500
ppm group, the activities were reduced to 20-70% and 20-60%,
respectively. Activities were higher toward the end of the study.
Brain ChE activity was decreased in the highest dose group by
45-47%, 24-43%, 24-38% and 5-15% after 6, 12, 18 and 24 months,
respectively. Histological examination of major organs (liver,
heart, lungs, kidneys, spleen, brain, gonads, pituitary, adrenals,
and thyroids) revealed hepatocellular fatty vacuolization in all 500
ppm rats, and in most females and several males at 100 ppm. No
effect was seen on serum total proteins or albumin: globulin ratio,
or on hexobarbital sleeping time. The tumour incidence was
comparable with that of the control group. The NOAEL, based on
brain ChE inhibition, was 100 ppm (actual concentration 47 ppm,
equivalent to 2.4 mg/kg bw/day) (Witherup et al., 1967).
Groups of 50 male and 50 female weanling CFE rats were exposed
(whole body) to nominal air concentrations of 0, 0.05, 0.5 or 5 mg
dichlorvos (97% purity)/m3 for 23 hours per day for two years. The
average actual dichlorvos concentrations were 0.05, 0.48, or 4.7
mg/m3. Body-weight gain was reduced in the two highest dose
groups. After two years of exposure, plasma and erythrocyte ChE
activities were reduced by 20-30% at 0.5 mg/m3 and by > 60% in the
highest dose group; brain ChE activity was reduced by 10%
(statistically significant) at 0.5 mg/m3 and by 80% in the highest
dose group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry
values, organ weights, or gross or microscopic examinations of major
organs. Ultrastructural examinations of bronchi and alveoli of rats
exposed to 0 or 5 mg/m3 showed no differences between the two
groups.
It was concluded that 2-year exposure to 0.05 mg/m3 dichlorvos
did not cause observable adverse effects to CFE rats (Blair et al.,
1976). It should be noted that in this study the rats were not only
exposed by inhalation but also via their food, drinking-water, and
by grooming. This resulted in additional oral ingestion of
dichlorvos (Stevenson & Blair, 1977).
Osborne-Mendel rats (50/sex) were fed 1000 ppm dichlorvos
(purity > 94%) in corn oil for 3 weeks. Due to severe cholinergic
signs, the dose was reduced to 300 ppm for the remaining 77 weeks.
Another group (50 males and 50 females) was fed 150 ppm dichlorvos
for 80 weeks. Samples of the diets analyzed during the study showed
that time-weighted average concentrations were 330 and 150 ppm,
respectively. Matched controls consisted of 10 rats of each sex;
the pooled controls from simultaneous studies of other compounds
consisted of 60 rats of each sex. Animals were observed twice daily
for clinical signs. All surviving rats were killed after 110-111
weeks.
The average body weights of the high-dose rats of both sexes
were slightly decreased compared with controls. There was no
significant increase in the incidence and type of tumours
attributable to dichlorvos in either sex. Dichlorvos (up to 330 ppm
in the diet, equivalent to about 30 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Groups of 70 and 100 rats per sex received 0.1 mg dichlorvos
(97%)/rat in 0.2 ml water by gavage twice or 3 times per week,
respectively, for 60 weeks. Thereafter, the animals were observed
for another 51 weeks. A control group of 60 animals of each sex
received 3 times per week 0.2 ml water. From the age of 10 months
onwards, the incidence of focal hyperplasia of urinary bladder and
of renal pelvis increased in males but decreased in females from
both test groups compared to controls. No neoplastic lesions were
found which could be attributed to treatment (Horn et al., 1988).
A study in Fischer 344 rats given drinking-water containing 0,
140 or 280 mg/litre dichlorvos for 108 weeks was summarized by WHO
(1989). There was no evidence of carcinogenicity (Enomoto et al.,
1981).
Groups of 50 male and 50 female F344/N rats were administered
dichlorvos by corn oil gavage at 0, 4 or 8 mg/kg bw/day (99%
purity), 5 days per week for 103 weeks. Body weights were recorded
once weekly for the first 12 weeks and then monthly. Animals were
observed twice daily for clinical signs. Necropsy and histologic
examinations were performed on all moribund animals or at the end of
the study.
Mild diarrhoea was observed in treated animals. ChE activities
were not determined during the study. However, a separate study
showed inhibition of plasma ChE activity by 50-80% in all dosed
groups but no erythrocyte ChE inhibition (see "Special studies on
cholinesterase activity" for detailed description and comments). No
significant differences in mean body weights or survival were
observed between any groups of either sex. Increased incidence of
cytoplasmic vacuolisation of liver was observed in dosed males and
of cortical cytoplasmic vacuolisation of adrenal glands in all dosed
males and in low-dose females. The incidence of pancreatic adenomas
(cross and horizontal tissue sections were analyzed), in control,
low and high-dose groups was 25/50, 30/50 and 33/50 in males and
2/50, 3/50 and 6/50 in females, respectively. The increased
incidence in treated males was statistically significant. The
incidence of mononuclear cell leukaemia (11/50, 20/50, 21/50 in
control, low and high-dose groups, respectively) was significantly
increased in the treated male rats compared with controls. The
incidence of mammary gland adenomas or fibroadenomas in female rats
was 11/50 in controls, 19/50 in the 4 mg/kg bw/day group and 17/50
in the 8 mg/kg bw/day group. The incidence in the low-dose group
was only slightly higher than the historical control values. Two
mammary gland carcinomas were observed in control and low-dose
groups. The authors concluded that there was evidence that
dichlorvos is carcinogenic to rats (Chan, 1989; Chan et al.,
1991). The Meeting observed that the incidence of pancreatic
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia, which is usually high and variable in
this strain of rats, was also of questionable biological
significance (Haseman et al., 1985).
Dichlorvos at 8 or 16 mg/kg bw/day was administered by gavage
to groups of 8-12 male F344 rats, either with or without leukaemia
transplant, for 5 days a week. Other groups of rats were either not
treated or given the leukaemia transplant only. At 70-days
post-transplant, the animals were killed. The rats dosed with
dichlorvos developed the disease earlier and the rate of tumour
progression was increased. Three out of 16 transplant recipients
dosed with 16 mg/kg bw/day died of leukaemia during the last week of
dosing. The severity of the mononuclear cell leukemia in the
transplant recipients, as measured by histopathological examination
of spleen and liver, was correlated with the changes in tumour
growth rates. However, no dose-response was found for spleen weight
and WBC count (Dieter et al., 1989).
Dogs
Groups of beagle dogs (3/sex) were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100 or 500 ppm dichlorvos (93%
purity) for 2 years. The average actual concentrations were 0,
0.09, 0.32, 3.2, 32 or 260 ppm dichlorvos; the average
dichloroacetaldehyde concentrations at the three highest dosages
were 0.6, 6.4 and 20 ppm. Since diet analysis was performed weekly
on a mixture of diet samples taken the previous 7 days, it is
likely, given the high volatility of dichlorvos, that at the end of
the week the actual concentration was much lower than 30% of the
nominal concentration.
No effects were seen on general appearance, survival, weight
gain, food consumption, haematology or urinalysis. Erythrocyte ChE
activity was reduced up to 50% of controls in the 10 ppm group with
recovery to control values at the end of the feeding period. At the
highest dose level, inhibition was more than 90% but complete
recovery was observed at 24 months. A similar pattern was observed
for plasma ChE activity. Brain ChE activity, measured at the end of
the study was similar to that of the controls in all treated groups.
Relative liver weights were slightly increased in males at 100 ppm
and in both sexes at 500 ppm. Histological examination of major
organs revealed slight dose-related cytoplasmic vacuolization and
enlargement of hepatocytes in animals at the two highest levels. No
differences were seen in serum alkaline phosphatase, transaminase
activities, total serum proteins or albumin:globulin ratios (Jolley
et al., 1967).
Reproduction studies
See also under special studies on testes.
Mice
Male and female Crl:CD3-1 mice were exposed to dichlorvos
concentrations of 0, 1.9, 3.0 or 4.6 mg/m3, generated from
dichlorvos-impregnated PVC strips in their cages. Exposure began
4 days prior to formation of breeding groups (3 females and 1 male)
and continued throughout pregnancy. Signs of intoxication were not
observed. Plasma ChE activity was significantly inhibited (by 90%,
93% and 95% in the 1.9, 3.0 and 4.6 mg/m3 groups, respectively)
when measured on day 4 after beginning of treatment. Gestation
length, number of litters, litter frequency and mean litter size
were comparable to controls. No grossly detectable congenital
anomalies were detected in any of the offspring (Casebolt et al.,
1990).
Rats
A 3-generation, 2-litter/generation reproduction study in rats
summarized by WHO (1989) was negative at doses up to 235 ppm in the
diet, equivalent to 12 mg/kg bw/day (Witherup et al., 1965).
Domestic animals
Several studies have been carried out with pregnant sows but no
adverse effects on piglets were observed with doses which inhibited
plasma and erythrocyte ChE activity in the sows (WHO, 1989).
Special studies on delayed neurotoxicity
Several studies have shown that a single dose of dichlorvos
does not produce delayed neurotoxicity in pre-medicated hens,
whether it is administered orally or subcutaneously (WHO, 1989).
However, Caroldi & Lotti (1981) reported mild signs of ataxia in
pre-medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg bw) and severe (> 80%) inhibition of NTE in peripheral
nerve, spinal cord and brain. Johnson (1978) did not observe ataxia
in pre-medicated hens given the same dose in the same way. However,
in this experiment spinal cord NTE inhibition was below the
threshold. When the dose was repeated 1-3 days after the first
dose, spinal cord NTE inhibition increased and the hens became
ataxic.
In a 90-day study, white leghorn hens were given dichlorvos
(99.9% purity) either dermally or orally. For oral administration,
a 10-20% solution of dichlorvos in corn oil in gelatin capsules was
used whereas, dermally, 1-20% emulsifiable concentrates in technical
grade xylene (containing 2% Triton X-100) were used. Dichlorvos at
doses greater than 1 mg/kg bw/day (dermal) or 6 mg/kg bw/day (oral)
led to cholinergic symptoms and death after 2-3 days. None of the
animals developed organophosphate-induced delayed neuropathy
(Francis et al., 1985).
In summary, it is possible to produce clinical neuropathy in
hens, but the doses required are far in excess of the LD50. This
is consistent with the low in vitro ratio of AChE I50/NTE I50 for
dichlorvos (Lotti & Johnson, 1978).
Special studies on embryotoxicity and teratogenicity
Several embryotoxicity/teratogenicity studies have been
performed with dichlorvos in mice, rats and rabbits. These studies
are summarized in WHO (1989). Dichlorvos given orally, by
inhalation or intraperitoneally, was not teratogenic at doses which
were toxic to the pregnant animals. However, in a study in rats
where a single i.p. dose of 15 mg/kg bw was given on day 11 of
gestation, 3/41 fetuses in the treated group had omphaloceles (0/50
in controls) (Kimbrough & Gaines, 1968). This finding has not been
confirmed in other studies.
Special studies on cholinesterase activity
This section summarizes studies in which the effect of
dichlorvos on ChE activity was the only parameter investigated.
Studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos in relation to the time
elapsed after dosing are summarized in Table 3. In general, the
maximum inhibition occurred within one hour followed by rapid
recovery.
Mice/Rats
Groups of 8-week old B6C3F1 mice and F344/N rats (10/sex)
were administered dichlorvos (99%) by corn oil gavage at daily doses
of 0, 5, 10, 20, or 40 mg/kg bw (mice) and 0, 2, 4, 8, or 16 mg/kg
bw (rats), five days per week for one week. Plasma and erythrocyte
ChE activities were measured on days 10 or 11, 25 or 26 and 32 or
33; blood was obtained about 3 hours after treatment.
Plasma ChE activity was significantly inhibited in all dose
groups of mice (50 and 90% inhibition in low and high-dose groups,
respectively) and rats (25 and 80% inhibition in low and high-dose
groups, respectively). At all time-points, erythrocyte ChE activity
in dosed and vehicle-control mice and rats was similar. However,
the timing for determination of the enzyme activities might have
underestimated the inhibition (Chan, 1989).
Rats
Reiner & Plestina (1979) compared in vivo reappearance of ChE
activity in rats after treatment with either dichlorvos (2.5 mg/kg
bw i.v.) or metrifonate (300 mg/kg bw i.v.). Half-lives of recovery
of brain and plasma ChE (acetylcholine was the substrate) were found
to be 2 and 2.5 hours, respectively, both in dichlorvos-treated and
in metrifonate-treated rats. This was consistent with in vitro
data (Skrinjaric-Spoljar et al., 1973).
In a study on the influence of temperature on ChE activity,
rats were injected i.p. with a single dose of 6.3 mg/kg dichlorvos
and kept at either 28 °C or 5 °C. The maximum inhibition of whole
blood ChE activity (40% and 50% in the two groups, respectively)
occurred after 0.5 hour. The animals kept at 5 °C showed slightly
less inhibition of whole blood ChE activity than those at room
temperature (Chattopadhyay et al., 1982).
Table 3. Time-related inhibition of ChE activity in animals after administration of a single dose of dichlorvos
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Mouse, male i.p. 10 15 min 70 Nordgren et al., (1978)
(n = 6-8) 60 min 50
2 hours 20
Mouse, male i.p. 15 2 hours 20 Cohen & Ehrich, 1976
Mouse, male i.p. 30 15 min 63 Cohen & Ehrich, 1976
(n = 4) 1 hour 50
5 hours 35
18 hours 10
Rat, male oral 1.6 2 hours 0 Pacheka et al., 1975
(n = 6) 10 2 hours 15
40 1 hour 60*
Rat, male oral 40 1 hour 70 Teichert et al., 1976
(n = 1)
Rat, male oral 40 5 min 45 Pacheka et al., 1975
(n = 6) 15 min 80
1 hour 85
2 hours 70
8 hours 35
24 hours 25
48 hours 6
Rat, male oral 50 15 min 68 80 97.91** Modak et al., 1975
(n = 6) 3 hours 73 37 68-70**
24 hours 39 24 31-38**
Table 3 (contd)
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Rat, male i.v. 2.5 30 min 60 85 Reiner & Plestina, 1979
(n = 5-12) 90 min 40 65
3 hours 7 44
12 hours 0 17
48 hours - 10
Dog, greyhounds oral 11 1 hour 90 93 Snow & Watson, 1973
1 male 2 hours 80 70
1 female 24 hours 15 35
72 hours 8 25
Dog, 2 male oral 22 fatal 90 95 66*** Snow & Watson, 1973
greyhounds,
1 female
crossbred
Dog, greyhounds oral 22 1 hour 88 83 Snow & Watson, 1973
and crossbred 3 hours 76 66
(n = 7-9) 6 hours 60 50
24 hours 30 30
48 hours 13 30
72 hours 5 35
Dog, beagle oral 50 2 hours 68 37 Ward & Glicksberg, 1971
sex not specified 24 hours 37 26
(n = 15) 5 days 8 22
14 days 5 20
21 days 0 8
Table 3 (contd)
* Daily dosing for 14 days caused 60% AChE inhibition when measured 24 hours after the last dose.
** Determinations were performed in striatum, hippocampus, medulla and cortex. No significant difference
was found between these brain areas.
*** Measured 20-155 min. after treatment.
When pregnant rats were given oral doses of 1.1 or 5.6 mg
dichlorvos/kg bw/day during days 14-21 of gestation, plasma ChE
activity of the high-dose mothers was inhibited (30-50%) but not
that of the young. Brain ChE activity did not show any significant
inhibition (Zalewska et al., 1977).
Rabbits
Dichlorvos, when infused into the ear vein of adult male
rabbits, produced dose- and time-related inhibition of whole blood
ChE activity during infusion. Spontaneous but incomplete recovery
to 60-80% of the normal activity occurred within 60-90 minutes after
infusion. Almost complete recovery was obtained by injecting oximes
up to 2.5 hours later (Shellenberger et al., 1965; Gough &
Shellenberger, 1977-1978; Shellenberger, 1980).
The progeny of rabbits, treated orally with 6 mg/kg bw/day for
the last 10 days of gestation, showed inhibition of brain ChE
activity at one day (30%) and 8 days of life (15%). Plasma ChE
activity was higher throughout days 1-16 of life (Maslinska &
Zalewska, 1978).
Guinea-pigs
Groups of 5 male and 5 female guinea-pigs were given daily
applications of 0, 25, 50 or 100 mg/kg bw/day dichlorvos (94%
purity) on the shorn skin for 8 days. All animals survived. A
dose-dependent inhibition of both plasma and erythrocyte ChE
activities occurred in all test groups. Recovery of plasma ChE
activities was complete within one week of the last exposure, and
that of erythrocyte ChE was complete within one week in the females
and 2 weeks in the males (Brown & Roberts, 1966).
Hens
Chickens dosed once orally with 1,3, or 6 mg/kg bw showed a
rapid inhibition of plasma ChE activity followed by recovery with an
half-life being estimated at three days (Rauws & van Logten, 1973).
White Leghorn pullets and hens were fed a diet containing 30
ppm dichlorvos for 35 days followed by a 21-day recovery period.
Plasma ChE activity was inhibited by 70% after four weeks of
treatment but returned to normal during the recovery period (Pym et
al., 1984).
Monkeys
Thirty-two Rhesus monkeys were given a pelleted PVC-resin
formulation containing 20% dichlorvos at dosages ranging from 1 to
16 mg dichlorvos/kg bw once daily or 1.6 and 4 mg dichlorvos/kg bw
twice daily for 10 to 21 consecutive days. None of the monkeys
showed overt signs of intoxication, although they ate less food and
had soft faeces. Plasma and erythrocyte ChE activities were reduced
by approximately 80% in all animals (irrespective of the dose) and
remained inhibited throughout the study. Plasma ChE activities
returned to normal values within approximately 3 weeks and the
erythrocyte ChE activities within 50 to 60 days following cessation
of exposure (Hass et al., 1972).
Special studies on genotoxicity
Methylating reactivity
In vitro studies
In a quantitative colour test in which methylation of
4-(p-nitro-benzyl) pyridine was measured to predict DNA alkylating
potential, dichlorvos gave a positive response, the reaction being
about 1/3 that of the known alkylating compound
methylmethanesulfonate (MMS) (Bedford & Robinson, 1972).
Alkylation by dichlorvos of calf thymus DNA, resulting in the
formation of N-7-methylguanine, was reported by Lofroth (1970).
Methylation by dichlorvos of isolated salmon sperm DNA and of
DNA from intact E.coli and from human HeLa cells broadly resembled
that by MMS (Lawley et al., 1974). In this study a very high
dichlorvos concentration (14 mmol/l) was used with isolated DNA
(12.5 mmol/l DNA-P). Dichlorvos concentrations with E. coli and
HeLa cells were 1-3 mmol/l. The rate of methylation of guanine-N-7
from salmon sperm DNA or from intact HeLa cell DNA by dichlorvos was
2-3 x 10-4 mol/l/min (calculated assuming guanine as 23-25% of DNA
with an average molecular weight of DNA base pair of 649, Lawley et
al., 1974). This is about 15 times lower than the rate of
methylation by MMS. This rate is to be compared with the rate of
reaction with AChE (phosphorylation) (1.5 x 105 mol/l/min at 37 °C)
(Skrinjaric-Spoljar et al., 1973). Therefore, the relative rate
of phosphorylation is about 9 orders of magnitude higher than that
of alkylation.
Labelled 7-methylguanine was present in both DNA and RNA
isolated from E. coli exposed to [Me-3H]-dichlorvos (1.1 mmol/l
for 4 hours). The methylating capability of dichlorvos was less, by
a factor of 10-100, than that of strongly genotoxic methylating
compounds (Wennerberg & Lofroth, 1974).
Incubation of bacteriophage R17 with 0-100 mmol
dichlorvos/litre for 90 hours did not result in methylation of the
phosphate groups of the RNA to any significant extent (Shooter,
1975).
In vivo studies
Mice were given i.p. injections of methyl-14C-dichlorvos (1.9
µmol/kg bw, approximately 420 µg/kg bw). The degree of alkylation
of guanine-N-7 in DNA isolated from soft tissues amounted to 8 x
10-13 mol methyl per gram of DNA (about 5 x 10-10 mol/mol guanine)
(Segerback, 1981; Segerback & Ehrenberg, 1981). From acute toxicity
data in mouse (see Table 1) it can be extrapolated that this dose
would cause 1 mol/mol phosphorylation of erythrocyte ChE.
DNA and RNA from the total soft tissues of male rats exposed to
atmospheres containing 0.064 mg/m3 (about 0.1% of the LC50) of
methyl-14C-dichlorvos for 12 hours did not show methylation of the
N-7 atom of guanine moieties. The exposure period constituted a
significant fraction of the half-life of the 7-methylguanine
moieties in DNA (Wooder et al., 1977; Wooder & Wright, 1981).
Excretion of labelled 7-methylguanine in the urine by NMRI mice
and rats injected i.p. with [Me-14C]-dichlorvos, or exposed by
inhalation for 2 hours (mice only) was reported by Wennerberg &
Lofroth (1974) and Lofroth & Wennerberg (1974). In rat urine,
labelled 3-methyladenine and 1-methyl-nicotinamide were also present
(Lofroth & Wennerberg, 1974). According to the authors, these
results demonstrate that dichlorvos spontaneously methylates guanine
and adenine moieties in nucleic acids. However, administration of
radiolabelled adenine and guanine to otherwise untreated rats gave
rise to the excretion of radiolabelled methylated purines in the
urine. Therefore, the detection of radiolabelled purines, per se,
in the urine of animals exposed to methyl-labelled methylating
agents does not constitute evidence for the spontaneous methylation
of the purine moieties of nucleosides or nucleic acids by
methylating agents (Wooder et al., 1978; Wooder & Wright, 1981).
Moreover, a natural biosynthetic pathway has been demonstrated
whereby the methyl carbon atoms of dichlorvos can be incorporated
into the heterocyclic rings and the methyl groups of urinary
7-methylguanine after entering the 1-C pools, in vivo (Wright et
al., 1979; Wooder & Wright, 1981).
Genotoxicity
In vitro studies
Several studies using bacteria and fungi as test organisms have
been carried out (Table 4). In most of the studies, only one, often
high, dichlorvos concentration was tested, sometimes resulting in
low survival of the test organism. The alkylating properties of
dichlorvos (see above) are most probably the cause of the mutagenic
action. This is suggested, for instance by data in E. coli
strains deficient at four repair loci (Bridges et al., 1973).
In vitro studies using mammalian cells are summarized in
Table 5. Dichlorvos caused cell transformation, mutations, DNA
strand breaks, sister chromatid exchanges and chromosomal
aberrations in cultured animal cells. In cultured human cells,
dichlorvos induced unscheduled DNA synthesis but did not induce
sister chromatid exchanges or chromosomal aberrations. Negative
results from chromosomal aberration tests in cultured human
lymphocytes were also cited by Fahrig (1974) and Wild (1975).
Dichlorvos (0.3 µg/ml) caused a synergised an increase in
sister chromatid exchanges in Chinese hamster ovary cells when
tested in combination with synthetic pyrethroids or propoxur which,
as such, were negative; however, in the combination phenothrin +
dichlorvos (0.3 µg/ml) the induction of SCE by phenothrin was
negative (Wang et al., 1988).
In vivo studies
In vivo studies are summarized in Table 6.
In Drosophila melanogaster, chromosomal aberrations but not
sex-linked recessive lethal mutations were induced. Negative
results were obtained in host-mediated, dominant lethal, sister
chromatid exchange and micronucleus assays (except on skin after
local application at cytotoxic doses). Dichlorvos did not induce
in vivo chromosomal aberrations in bone-marrow cells,
spermatocytes or spermatogonia, DNA strand breaks or unscheduled DNA
synthesis.
Special studies on liver microsomal enzymes
A decrease in liver microsomal cytochrome P-450 occurred in
rats after three daily i.p. injections with 6 mg dichlorvos/kg bw
(Purshottam & Kaveeshwar, 1982) but no effect on liver microsomal
UDP-Glucuronyl transferase was found in mice 4 hours after a single
i.p. injection of 25 mg/kg bw (Yoshida et al., 1976).
Pre-treatment of rats with three daily i.p. injections of
sodium phenobarbital did not significantly change mortality and
plasma ChE inhibition caused by an i.p. injection of dichlorvos (20
mg/kg bw) (Purshottam & Kaveeshwar, 1979).
Short- and long-term studies with mice, rats or dogs dosed
orally or intraperitoneally with dichlorvos did not show any effect
on microsomal drug metabolizing enzymes (Witherup et al., 1967;
Uchiyama et al., 1975; Farber et al., 1975).
Table 4. Mutagenicity tests on microorganisms in vitro
Test system Test object Concentration Purity Results 1 Reference
Mitotic A. nidulans strain p ? ? + Morpurgo et al., 1979
non-disjunction
and crossing over
Recombinant assay B. subtilis H17 Rc+ 2 mg/plate ? - Shirasu et al., 1976
M45 Rec- " + " "
Forward mutation A. nidulans strain 35 14 mg/disc ? + Bignami et al., 1977
E. coli B 5-25 mmol/l 95% +2 Wild, 1973
Gal RS ? ? + Fahrig, 1974
K12(5-MT) 0.3-3.2 mmol/l ? + Mohn, 1973
S. coelicolor A 3(2) his A1 5.6 mg/disc 99.9% + Carere et al., 1978a, 1978b
Reverse mutation E. coli B/r WP2 5 mg/plate > 97% +3 Moriya et al., 1978
S. typhimurium TA 1535 5 mg/plate > 97% +4 " "
E. coli WP2 hcr up to 5 mg/plate ? +3 Moriya et al., 1983
S. typhimurium TA 98 up to 5 mg/plate ? - " "
TA 100 " " ? + " "
TA 1535 " " ? ? " "
TA 1537 " " ? - " "
TA 1538 " " ? - " "
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation (cont'd) E. coli B/r Wp2( ); SR714 0.04-2.3 mmol/l 99% +3 Houk & DeMarini, 1987
CM 561 0.2% " +5 Bridges et al., 1973
CM 571; CM 611 " " -6 " "
WP2 " " +5 " "
WP2 uvr A " " +5 " "
WP2 micro drop of ? -7 Dean, 1972a
analytical grade,
technical grade
or 10% acqueous
solution/plate
K12HfrH 0.1% 97.5% + Voogd et al., 1972
C. freundii 425 0.05% or 0.1% " +8 " "
E. aerogenes 0.1% " + " "
K. pneumoniae 0.05% oe 0.1% " + " "
S. typhimurium 64-320 " " + " "
S. marcescens Hy/alpha 13 1.25-5 mg/disc ? + Dean, 1972a
Hy/alpha 21
E. coli WP2 WP2 5 µg/ml ? +9 Green et al., 1976
hcr+/hcr- 20-25 µl/disk 50% commercial +10 Nagy et al, 1975
WP2 5 mg/plate formulation " "
hcr+/hcr- ?
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation S. typhimurium TA 1535 5 mg/plate ? + Shirasu et al., 1976
(cont'd) TA 1536 " ? + " "
TA 1537 " ? - " "
TA 1538 " ? - " "
E. coli WP2 5 µl/plate11 ? + Hanna & Dyer, 1975
WP2 uvr A " ? + " "
WP 67 " ? + " "
CM 561 " ? - " "
CM 571 " ? - " "
CM 611 " ? - " "
S. typhimurium TA 1530
TA 1535 5 µl/plate ? + Hanna & Dyer, 1975
his C117 " ? + " "
his G46 " ? - " "
" ? - " "
P. aeroginusa PAO 38 0.08 mol/l ? + Dyer & Hanna, 1973
S. typhimurium his C117 0.03 mol/l ? + " "
S. typhimurium TA 98 ? ? -3 Braun et al., 1982
TA 100 ? ? +3 " "
TA 1535 ? ? -3 " "
TA 1536 ? ? -3 " "
TA 1537 ? ? -3 " "
TA 1538 ? ? -3 " "
TA 98 0.1-6.6 mg/plate12 99% -3 Chan, 1989:
Zeiger et al., 1988
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
TA 100 " " " +3 Carere et al., 1978a,b
TA 1535 2.8 mg/plate 99.9% -3 " "
TA 1536 " " " -3
TA 1537 " " "
S. typhimurium TA 1538 2.8 mg/plate 99.9% -3 Carere et al., 1978a,b
TA 1535 1.5 mg/ml 99.9% +
Schizosaccharmoyces pombe 1.5-14 mmol/plate13 > 99% +3 Gilot-Delhalle et al.,
ade 6 1983
Gene conversion S. cerevisiae D4 2-8 mg/ml > 97% +14,15 Dean et al., 1972
D4 6-40 mmol/l ? +2 Fahrig, 1973, 1974
632/4 ? ? - Guerzoni et al., 1976
+16 Griffin & Hill, 1978
DNA strand breaks E. coli K-12CR34Co1E1 1 mg/ml ?
Growth inhibition E. coli W3110 pol A+/pol A- 6.4 mmol/l ? + Rosenkranz, 1973
P. mirabilis PG 273; PG 713 ? ? +15 Braun et al., 1982
1 Without metabolic activation except where noted.
2 Positive controls (methyl methanesulfonate 1-4 mmol/l) yielded expected positive results.
3 Both with and without metabolic activation.
4 Only without metabolic activation.
5 Methyl methane sulfonate (0.04%) yielded positive responses.
6 Methyl methane sulfoante (0.04%) yielded negative responses.
7 Methyl methane sulfonate, N-methyl-N'-nitro-N-nitroso guanidine yielded positive response.
8 At 0.1%
9 Positive controls (methyl methanesulfonate 0.5-2.0 µg/ml) yielded expected positive results.
Table 4 (contd)
10 Positive controls (N-methyl-N'-nitro-nitroso guanidine, N-nitroso-N-methylurethane and acridium chloride)
yielded positive responses.
11 Toxic dose.
12 Toxic effect at 3.3 mg/plate.
13 The LD50 was 5.5 mmol/l.
14 Positive controls (ethyl methane sulfonate) yielded positive responses.
15 From 4 mg/ml.
16 Methyl methane sulfonate (2-10 mmol/l), N-methyl-N'-nitro-N-nitroso guanidine (3.4-6.8 mmol/l) yielded positive responses.
Table 5. Mutagenicity tests in mammalian cells in vitro
Test system Test object Concentration Purity Results Reference
Viral transformation Syrian hamster embryo 0.05-0.45 mmol/l ? +1 Hatch et al., 1986
cells/adenovirus SA7 cytotoxic
Gene mutation Chinese hamster V79 cells up to 1 mmol/l ? - Wild, 1975
(azaguanine resistance) 1.25-5 mmol/l ? +2 Aquilina et al., 1984
Mouse lymphoma L5178Y cells 6.25-50 nl/m3 ? +4,5 Chan, 1989
(trifluorothymidine resistance) 12.5-200 nl/m6 +5,7 Chan, 1989
DNA strand breaks Chinese hamster V79 cells 0.2% ? +8 Green et al., 1974
Sister chromatid exchange Primary rat tracheal epithelial cells 5-160 µg/ml9 93.9% +10,11 Lin et al., 1988
Chinese hamster ovary cells 0.03 and 0.1 mmol/l 98 + Nishio & Uyeki, 1981
0.1-0.5 mmol/l > 98% + Tezuka et al., 1980
0.3-1000 µg/ml ? +12 Wang et al., 1988
1-50 µg/ml ? +13 Chan, 1989
Human lymphocytes 2.5-10 µg/ml 99% - Nicholas et al., 1978
Human fetal lung fibroblasts 99% - Nicholas et al., 1978
Chromosomal aberrations Rat tracheal epithelial cells 5-160 µg/ml9 93.9% +11,14 Lin et al., 1988
Chinese hamster lung fibroblasts ? ? + Ishidate et al., 1981
Chinese hamster V79 cells 0.1-0.5 mmol/l > 98% +15 Tezuka et al., 1980
Chinese hamster ovary cells 16-160 µg/ml ? +16 Chan, 1989
Human lymphocytes 1-40 µg/ml > 99% -17 Dean, 1972b
Table 5 (contd)
Test system Test object Concentration Purity Results Reference
Unscheduled DNA synthesis Human lymphocytes 5-500 µg/ml 99.8% + Perocco & Fini, 1980
EUE human cells 6.5-650 mmol/l ? +18 Aquilina et al., 1984
DNA (sedimentation coefficient) Calf thymus DNA 0.1% ? + Rosenkranz &
Rozenkranz, 1972
DNA (thermolabile regions) Calf thymus DNA 45 mmol/l 99% - Olinski et al., 1980
DNA (resistance to micrococcal) Chinese hamster ovary cells 10 mmol/l ? + Nishio & Uyeki, 1980
1 Positive controls (benzo(a)pyrene 0.001-0.002 mmol/l) yielded expected positive responses.
2 Positive controls (ethyl methan sulfonate 20 mmol/l) yielded expected positive responses.
3 Growth inhibition from 12.5 nl/ml.
4 From 12.5 nl/ml.
5 Positive controls (methyl methanesulfonate 5 nl/ml) yielded expected positive responses.
6 Growth inhibition from 100 nl/ml.
7 From 100 nl/ml.
8 Negative at lower concentrations.
9 50% mortality was observed at 80 µg/ml.
10 From 10 µg/ml.
11 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 0.25-1 µg/ml, 50% mortality at 0.5 µg/ml)
yielded expected positive responses.
12 From 40 µg/ml.
13 From 10 µg/ml. When incubated with S9, positive response at 50 µg/ml.
14 From 80 µg/ml.
15 At 0.5 mmol/l.
16 At 160 µg/ml.
17 Cytotoxicity was observed at 5-40 µg/ml.
18 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine) yielded expected positive responses.
19 Possible index of DNA alkylation.
20 Possibly indicating structural rearrangement of chromatin.
Table 6. Mutagenicity tests in vivo
Test system Test object Concentration Purity Results* Reference
Crossing over/ Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recombination ++++/dp b cn bw
Sex-linked Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recessive lethal Sobels & Todd, 1979
mutation
Oregan K 0.01-0.1 ppm in food ? -2 Kramers & Knapp, 1978
commercial formulation
Chromosomal Drosophila melanogaster 1 ppm in food ? + Gupta & Singh, 1974
aberration
Autosomal recessive Drosophila melanogaster 0.1-0.75 ppm in food for ? + Hanna & Dyer, 1975
levels 18 months
Host-mediated assay Salmonella typhimurium 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
G46 His- in mice (NMRI)
Salmonella typhimurium 8-10 mg/kg bw p.o. 97.5% -3 Voogd et al., 1972
(64-320) in mice (Swiss)
Serratia marcescens 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
(a 21 Leu-) in mice (NMRI)
Sacchoromyces cerevisiae 50 or 100 mg/kg bw p.o. > 97% -3 Dean et al., 1972
(D4) in mice (CF1)
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Dominant lethal Female mice (CF1) 0, 25 or 50 mg/kg bw p.o. > 97% -4 Dean & Blair, 1976
0, 2 or 8 mg/m3 inhalation, > 97% - Dean & Blair, 1976
from weaning to 11 weeks
of age
Male mice (ICR/Ha Swiss) 0, 5 or 10 mg/kg bw/day ? - Epstein et al., 1972
p.o. for 5 days
(8 weeks of mating)
Single i.p. injection of ? - Epstein et al., 1972
13 or 16.5 mg/kg bw
(8 weeks of mating)
Male mice (Q) 2 ppm in drinking water, 99% - Degraeve et al., 1984a
5 days/week for 7 week
10 mg/kg bw i.p. 99% -5 Moutschen-Dahmen et al., 1981
Male mice (CF1) 30 or 55 mg/m3 inhalation > 97% - Dean & Thorpe, 1972b
for 16 h
2.1 or 5.8 mg/m3 inhalation > 97% -6 Dean & Thorpe, 1972b
for 23 hours/day for 4 weeks
Sister chromatid Male mice (B6C3F1) 5-35 mg/kg bw i.p. 99% -7 Kligerman et al., 1985
exchange peripheral lymphocytes
Male mice (B6C3F1) 6-40 mg/kg bw i.p. ? -8 Chan, 1989
bone marrow cells
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Micronucleus test Mice (Swiss Webster) 0.0075-0.015 mg/kg bw/day ? -9 Paik & Lee, 1977
i.p. for 2 or 4 days
Micronucleus test Mice (HRA/Skh, hairless) skin painting with tech.grade +10 Tungul et al., 1991
(in vitro/in vivo) skin keratinocytes 0-228 µg
(in 100 µl acetone)
Chromosomal Chinese hamster, both 10-15 mg/kg bw p.o. > 97% -11 Dean & Thorpe 1972a
aberrations sexes bone-marrow cells
Male mice (Q) 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
bone-marrow cells 5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Degraeve et al., 1984b
Degraeve et al., 1984b
Male mice (B6C3F1) bone 6-40 mg/kg bw i.p. ? -12 Chan, 1989
marrow cells
Female Syrian golden 0, 3, 6, 15 or 30 mg/kg 50% +13 Dzwonkowska & Hübner, 1986
hamster commercial
formulation
bone-marrow cells bw i.p.
Mice (CF1), both sexes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h
5 mg/m3 inhalation > 97% > 97% -11 Dean & Thorpe, 1972a
23 hours/day for 21 days
Male Chinese hamsters 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h.
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Chromosomal Mice (Q) spermatocytes 2 ppm in drinking-water 99% - Moutschen-Dahmen et al., 1981;
aberrations 5 days/week for 7 weeks Degraeve et al., 1984a
(cont'd)
Chinese hamsters 15 mg/kg bw p.o. > 97% -11 Dean & Thorpe, 1972a
spermatocytes
Mice (Q) spermatocytes 10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984a
Mice (CF1) spermatocytes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
for 16h
5 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
23h/day for 21 days
Chinese hamster 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
spermatocytes for 16 h
Mice (CF1) spermatogonia 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984b
DNA strand breaks Rats (Wistar), both 10 mg/kg bw i.p. 99.8% -14 Wooder & Creedy, 1979
sexes rat liver cell DNA
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Unscheduled DNA Male rats (F344) 0, 2, 10 or 35 mg/kg ? - Mirsalis et al., 1989
synthesis hepatocytes bw p.o.
Mice (B6C3F1) (both sexes) 0, 10, 20, 40 or > 98% -15 Bedford, 1991
forestomachrats 100 mg/kg bw p.o.
1 Approximate LD50
2 Positive controls (2.5 mmol/l ethyl methanesulfonate) yielded expected positive responses.
3 Positive controls (400 mg/kg bw p.o. ethyl methanesulfonate) yielded expected positive responses.
4 Positive controls (100 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
5 Positive controls in 2nd and 5th week of mating.
6 Positive controls (2000 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
7 Positive controls (2-acetylaminofluorene, ethyl methane sulfonate and N-nitroso morpholine) yielded positive results.
All animals treated with 35 mg/kg bw i.p. died.
8 Positive controls (ethyl methane sulfonate 100 mg/kg bw p.o.) yielded expected positive responses.
9 Positive controls (cyclophosamide 30-240 mg/kg bw i.p.) yielded expected positive responses.
10 Recovery of plated cells in vitro after a single topical application of dichlorvos was about 50% of controls.
11 Positive controls (endoxan 100-200 mg/kg bw i.p.) yielded expected positive responses.
12 Positive controls (ethyl methane sulfonate 300-375 mg/kg bw p.o.) yielded expected positive responses.
13 LD50 = 30 mg/kg bw i.p. No dose related response. Positive controls (cyclophosphamide 40 s. mg/kg bw i.p.)
yielded expected positive responses.
14 Positive controls (methyl methane sulfonate 30-60 mg/kg bw i.p.) yielded expected positive responses.
15 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 200 mg/kg bw p.o.) yielded weakly positive results.
Dichlorvos treatment induced a hyperplastic response similar to that induced by the non-genotoxic carcinogen butylated
hydroxyamisole (300 mg/kg bw p.o.).
Special studies on metabolites
Acute and short-term toxicity studies
The intraperitoneal toxicity of metabolites of dichlorvos in
female mice is considerably less than that of dichlorvos. The LD50
(mg/kg bw) was 250 for dichloroacetic acid, 440 for
dichloroacetaldehyde and 890 for dichloroethanol. For other
metabolites it was > 1000 mg/kg bw. A short-term inhalation study
with dichloroacetaldehyde in rats did not show adverse effects at
concentrations up to 2 mg/m3 for 30 days (WHO, 1989).
Methylating reactivity
In a quantitative colour test in which methylation of
4-(p-nitrobenzyl) pyridine was measured to predict DNA alkylating
potential, desmethyl-dichlorvos, dimethyl phosphate,
dichloroethanol, dichloroacetaldehyde and dichloroacetic acid gave
no reaction (Bedford & Robinson, 1972).
Mutagenicity
In a rec-type repair test with Proteus mirabilis strains
PG713 and PG273, desmethyldichlorvos (10 or 40 µmol/plate) did not
induce base-pair substitutions or other DNA damage (Braun et al.,
1982).
Dichloroacetaldehyde (DCA) appeared to be mutagenic in the
Salmonella test using S. typhimurium TA 100 and TA 1535. The
mutagenicity decreased in the presence of a microsomal activation
system (Lofroth, 1978; Bignami et al., 1980). It was also
positive when tested in concentrations of 10-40 µg/plate in a
forward and a back mutation system in S. coelicolor and two
forward mutation systems in A. nidulans (Bignami et al., 1980).
DCA (0.01%) was not mutagenic to K. pneumoniae in a fluctuation
test (Voogd et al., 1972). DCA did not induce unscheduled DNA
synthesis in the human epithelial-like cell EUE as well as
ouabain-resistant mutations in cultured V-79 Chinese hamster cells
(Aquilina et al., 1984). In a dominant lethal assay with DCA
administered as a single i.p. injection of 176 mg/kg bw to two
strains of male mice (AB Jena-Halle and DBA), positive effects were
reported (Fischer et al., 1977). It should be noted, however,
that the dose used would never be reached after dichlorvos treatment
(i.p. LD50 = 28-41 mg/kg bw). Ramel (1981) reported negative
results in a dominant lethal study with another strain of mice.
No evidence for mutagenicity of dichloroethanol was obtained in
S. typhimurium TA 100 and TA 1535 (Lofroth, 1978; Bignami et al.,
1980). In S. coelicolor (test doses 60-80 µg/plate) and A.
nidulans (test doses 10-50 µg/plate), dichloroethanol was a weak
mutagen (Bignami et al., 1980). Dichloroethanol (1 M and 0.1%)
was mutagenic to K. pneumoniae in a fluctuation test (Voogd et
al., 1972).
Special studies on skin sensitization
In the guinea-pig maximization test by Magnusson & Kligman,
using intradermal and topical induction concentrations of 5% and 25%
in water respectively, a 0.5% challenge concentration caused
sensitization in 30-40% of the animals but a 0.05% challenge
concentration caused no sensitization (Matsushita et al., 1985).
Special studies on testes
Mice
Groups of 14 male NMRI/Han mice received either a single oral
dose of 40 mg/kg bw dichlorvos in olive oil or 18 daily oral doses
of 0 or 10 mg/kg bw dichlorvos in olive oil.
On days 9,18, 27, 36, 54 and 63, two animals from each group
were killed and their testes examined histologically. A significant
increase in the number of damaged seminiferous tubules
(desquamation, decreases in cell population, "holes") was observed
in both dichlorvos groups. The supporting Sertoli cells were also
damaged, which may have resulted in the above effects. In addition,
there was an increase in the number and hypertrophy of Leydig cells.
No explanation was given for these effects (Krause & Homola, 1972,
1974).
An increased incidence, just above background, of sperm
abnormalities, was observed in a screening study on hybrid mice
given five daily i.p. injections of 10 mg/kg bw dichlorvos
(approximately half the LD50). At lower doses, 1 mg/kg bw, the
number of sperm abnormalities was either similar to or lower than
those in the controls (Wyrobek & Bruce, 1975).
Rats
Groups of 16 male juvenile Wistar rats received either 20 mg/kg
bw dichlorvos in olive oil on days 4 and 5, 10 mg/kg bw dichlorvos
in olive oil daily from days 4 to 23, or 0.1 ml olive oil daily from
days 4 to 23. On days 6, 12, 18, 26, 34, and 50 of life, two rats
from each group were sacrificed. Histological examination of the
testes showed slight reduction in the number of spermatogenic cells
and Leydig cells. All changes were reversed by the 50th day of
life. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells (Krause et al.,
1976).
In a subsequent experiment measurement of testosterone
concentrations in the testes, and luteinizing hormone (LH) and
follicle stimulating hormone (FSH) concentrations in serum showed no
differences between treated animals and olive-oil treated controls
(Krause, 1977). However, in this study the use of a different
dosing regimen (10 mg/kg bw by gavage every other day for 2 weeks)
prevented a strict comparison with the earlier study by Krause et
al. (1976).
Fifty-five male Wistar rats (aged 5 months) were orally
administered dichlorvos at levels of 5 or 10 mg/kg bw every other
day for 8 weeks. Eleven rats were killed every 4 weeks. No change
was seen in body-weight gain or testes weight. The score values of
the seminal cellular system decreased after 4-8 weeks of treatment,
but were restored 8 weeks after the end of treatment (Fujita et
al., 1977).
Miscellaneous studies
Effects of inhaled ChE inhibitors, including dichlorvos
(dissolved in acetone), on bronchial tonus were studied in young
adult rats exposed head-only. Bronchoconstriction did not occur at
toxicologically significant doses. An increase in response to
acetylcholine provocation test was observed (Pauluhn et al.,
1987).
The effect of diet on the toxicity of dichlorvos was
investigated using young male rats kept for 30 days on the following
diets: high protein (HPD), low protein (LPD), high fat (HFD), and
standard (SD). Growth rates were normal except for a slightly
decreased body-weight gain in the HFD group. A single i.p.
injection of 50 mg/kg bw dichlorvos led to slightly higher mortality
in LPD rats and slightly lower mortality in HPD rats, compared with
SD rats (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or
HPD for 30 days. At the end of this period, a single i.p. dose of
dichlorvos (20 or 30 mg/kg bw) was administered. No difference in
plasma and erythrocyte ChE inhibition was found. In the case of the
HPD, the spontaneous recovery of plasma and erythrocyte ChE activity
was slightly reduced (Purshottam & Srivastava, 1984).
Observations in humans
In vitro studies
The sensitivity to inhibition by dichlorvos of erythrocyte and
brain ChE was higher than that of plasma ChE, the I50s being about
10-8 and 10-7 mol/l, respectively (Skrinjaric-Spoljar et al.,
1973; Carter & Maddux, 1974; Lotti & Johnson, 1978; Boyer, 1978;
Casale et al., 1989). The rate of phosphorylation of human
erythrocyte ChE was similar to that of rat brain ChE (1.2 x 105 and
1.5 x 105 mol/l/min at 37 °C, pH 7.4-7,6, respectively).
The rate of dichlorvos hydrolysis by human plasma at 37 °C was
found to be 7-11 µmol/hour/ml (Reiner et al., 1980; Traverso et
al., 1989).
Studies on volunteers
Six healthy male volunteers were given single oral doses of 7.5
mg/kg bw of metrifonate. Concentrations of metrifonate (trichlorfon)
and dichlorvos, which is a metrifonate rearrangement product, were
determined in whole blood at different times for up to 24 hours.
The half-life of metrifonate was about 2 hours. The concentrations
of dichlorvos closely followed those of metrifonate with a constant
ratio of 0.01-0.02. However, a half-life for dichlorvos of 2 hours
cannot be accepted because dichlorvos is continuously formed from
metrifonate (Abdi & Villen, 1991).
Groups of five young men received total daily doses of 1, 1.5,
2 or 2.5 mg dichlorvos in corn oil per person, divided in two
gelatin capsules administered at 08.00 h and at 15.00 h for 28 days.
A further group of ten men received 1.5 mg/day for 60 days. Control
groups of two men per dose level received gelatin capsules with corn
oil. Plasma and erythrocyte ChE activities were determined twice
weekly before, during and after dosing. Once each week a medical
interview, complete blood count, and urinalysis were done on each
subject. Before dosing and after the last dose SGOT, ALP, PT,
thymol turbidity, and total bilirubin were determined.
The 2.5 mg/day dose produced a decrease in plasma ChE activity
from the second week of treatment; dosing was discontinued after 20
days when plasma values showed a 30% decrease. The plasma ChE
activity returned to control values 15 days after dosing was ended.
The 2 mg/day dose produced also a reduction in plasma ChE activity
from the second week of treatment which reached a maximum inhibition
of 29% the second day after the last dose. The group receiving 1.5
mg/day for 28 days showed no change in plasma ChE activity while in
the group dosed for 60 days this activity was inhibited by 27%
compared to the control values. None of the groups showed an effect
on erythrocyte ChE activity, haematology, clinical chemistry or
urinalysis. The NOAEL in this study was the highest dose tested of
2.5 mg/day/man (approximately 0.03 mg/kg bw/day) based on the
absence of inhibition of erythrocyte ChE activity (Rider, 1967;
Rider et al., 1967, 1968).
Two groups of six men (21-45 years of age, 64-106 kg bw)
received 0.9 mg dichlorvos three times a day for 21 days, either in
a pre-meal capsule filled with cottonseed oil or in a gelatin salad
consumed during the course of the meal. Control subjects were dosed
with either cotton seed oil capsules or plain gelatin. No
cholinergic signs or symptoms were observed. Plasma ChE activity
was inhibited by 30-40%, the inhibition being higher when dichlorvos
in cotton seed oil was ingested before the meal. The half-life for
the regeneration of plasma ChE activity was 13.7 days. Erythrocyte
ChE activity was not reduced. The NOAEL in this study was 0.04
mg/kg bw/day, based on absence of erythrocyte cholinesterase
inhibition (Boyer et al., 1977).
A number of studies have been done with a slow-release PVC
formulation of dichlorvos intended for use as an anthelminticum.
Single oral doses of above 4 mg dichlorvos/kg bw resulted in
inhibition of erythrocyte ChE activity 24 hours after application,
the maximum being 46% at 32 mg/kg bw. Plasma ChE activity was
affected at single doses of 1 mg/kg bw (50% inhibition) and above.
However, no dichlorvos-related symptoms were observed. Repeated
oral dosing for 7 days produced clinical symptoms with doses of 8
mg/kg bw/day and above. Plasma ChE activity was inhibited by about
80% at all dose levels (1-16 mg/kg bw/day). Erythrocyte ChE
activity showed a dose-related decrease from 5-30% at 1 mg/kg bw/day
up to 50-80% inhibition at the highest dose. Blood count, urine,
liver function, PT and BUN were normal in all studies (Hine &
Slomka, 1968, 1970; Pena-Chavarria et al., 1969; Slomka & Hine,
1981).
In children (aged 7-18 years) orally treated with metrifonate
(7.5, 10.0 or 12.5 mg/kg bw), the half-lives of recovery of
inhibited erythrocyte AChE and plasma ChE were 15 and 6.7 days,
respectively (Reiner & Plestina, 1979). It is known that the
anticholinesterase activity of metrifonate is due to its
decomposition to dichlorvos (Reiner et al., 1975). Similar
half-lives for plasma ChE have been described by Boyer et al.,
(1977) after repeated oral dosing with dichlorvos and by Bisby &
Simpson (1975) after cutaneous exposure of a sprayman to dichlorvos.
This longer half-life contrasts with faster in vitro half-lives,
which are similar to those of the rodent (Skrinjaric-Spoljar et
al., 1973).
Several inhalation studies have been carried out and are
summarized by WHO (1989). These studies confirmed that plasma ChE
is more sensitive to inhibition by dichlorvos than RBC ChE. The
latter was found inhibited when the dichlorvos dose (concentration x
time) was higher than about 1500 mg/m3/min (WHO, 1989).
Thirteen men (31-53 years of age) were exposed to dichlorvos
strips (20% dichlorvos; 1 strip per 30 m3) for 3 months. No
biologically or statistically significant changes were observed in
their electromyography results nor in their whole blood ChE activity
(Ottevanger, 1975).
Poisoning incidents
Fournier et al. (1978) reported that dichlorvos was detected
in blood in three cases of human intoxication within 24 hours after
poisoning (exact timing not reported).
A woman who intentionally ingested an estimated 100 mg
dichlorvos/kg bw survived following intensive care for 14 days
(Watanabe et al., 1974). A suicide with a dichlorvos dose of
about 400 mg/kg bw succeeded in spite of treatment (Shinoda et al.,
1972). A female patient, aged 35 years, who had accidentally
ingested 60 g fluid Divipan (dichlorvos concentration not reported),
was comatose for a week and recovered slowly. Clinical and
electrophysiological examinations showed a pure motor form of
neuropathy, according to the authors (Vasilescu & Florescu, 1980).
Three cases of poisoning with dichlorvos taken orally in unspecified
but high quantities have been reported from India. The patients
first showed severe cholinergic signs for a few days. After
recovery, delayed neurotoxicity developed. Nerve conduction studies
showed a severe axonal degeneration (Wadia et al., 1985, 1987).
Thirteen cases of dichlorvos ingestion, either accidental or
deliberate, were reported from a hospital in Beijing, China. The
patients included 10 women and three men, aged 11-38 years. Twelve
of the 13 patients ingested 25-50 ml of dichlorvos (80% purity).
Ten cases were associated with acute pulmonary oedema. Two patients
died because OP poisoning was not diagnosed in time. The others
recovered completely 2-5 days after treatment (Li et al., 1989).
Occupational Exposure
A number of fatal and non-fatal dichlorvos poisoning cases have
been described and summarized by WHO (1989). Two workers who failed
to promptly wash off the concentrated formulation of dichlorvos,
which splashed on to their skin, died subsequently. However, in
those cases where the spilled solution was washed off immediately,
the victims showed symptoms of intoxication but recovered after
treatment. Occupational exposure of spraymen entails both dermal
and respiratory absorption of dichlorvos. When appropriate
protective equipment was not used, inhibition of plasma ChE and less
frequently of RBC ChE was found. Sometimes mild, short lasting,
cholinergic symptoms and signs have been reported (WHO, 1989).
Each of 13 pest-control operators carried out urban
pest-control work for one day in 4 houses using 230-330 g dichlorvos
as aerosol and 40-50 g dichlorvos as emulsion spray. At the end of
the day's work, an operator had an average dichlorvos residue of 0.8
mg/m2 on the back, 0.4 mg/m2 on the chest, and 11 mg/m2 on the
respirator filter. Urinary dimethylphosphate excretion in 3
applicators ranged from 0.32 to 1.39 micrograms on the day of
treatment, but approached the level of detection by the following
morning. Blood and urine analyses revealed no changes in various
clinical parameters, including serum cholinesterase levels (Das et
al., 1983).
COMMENTS
Dichlorvos is rapidly absorbed by all routes of exposure and
rapidly degraded. The metabolic pathways of dichlorvos are similar
in mammalian species, including humans. Metabolites are rapidly
excreted or incorporated into natural enzymatic pathways.
Dichlorvos has a marked acute oral toxicity with typical
cholinergic signs and has been classified by WHO as highly
hazardous.
Rat erythrocyte and brain cholinesterase inhibited by
dichlorvos spontaneously reactivates with a half-life of about two
hours both in vitro and in vivo.
Several carcinogenicity studies in mice and rats using routes
other than gavage were negative, even when doses causing signs of
toxicity were used. It should be noted that two squamous-cell
carcinomas of the oesophagus have been observed in treated mice in
one study.
In a carcinogenicity study in mice dichlorvos administered by
corn oil gavage (0, 10 or 20 mg/kg bw/day to males, and 0, 20 or 40
mg/kg bw/day to females), caused forestomach papillomas
(statistically-significant positive trend with increased incidence
in the high-dose female group). Elements of the mechanism by which
these papillomas might arise have not been established, but the
induction of hyperplasia in the forestomach was demonstrated.
Additionally, genotoxic effects might occur at high local
concentrations of dichlorvos (see below) as can be obtained in
gavage dosing but not in dietary exposure. Based on the increased
incidence of forestomach papillomas, the NOAEL was 10 mg/kg bw/day.
In a two-year feeding study in rats (0, 0.1, 1, 10, 100 or 500
ppm), no neoplastic lesions were attributed to treatment. The
NOAEL, based on brain cholinesterase inhibition, was 100 ppm (actual
concentration 47 ppm, equivalent to 2.4 mg/kg bw/day).
In a carcinogenicity study in Fischer 344 rats, dichlorvos
administered by corn oil gavage (0, 4 or 8 mg/kg bw/day) caused an
increased incidence of pancreatic adenomas (statistically
significant in males only), mononuclear cell leukemias
(statistically significant in males only, no dose response) and
mammary gland adenomas or fibroadenomas (females only, no dose
response, statistically-significant in the low-dose group only).
The Meeting observed that the incidence of pancreatic acinar
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia which is usually high and variable in
this strain of rats, was also of questionable biological
significance. The doses used significantly inhibited plasma, but
not erythrocyte, cholinesterase activity when measured three hours
after treatment. However, given the rapid recovery of erythrocyte
cholinesterase activity after inhibition by dichlorvos, the timing
might have underestimated the inhibition.
Dichlorvos has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. These data indicate that
dichlorvos is genotoxic in bacteria and cultured mammalian cells,
but that it is not clastogenic in vivo except under conditions
where an unusually high tissue dose can be attained. Dichloro-
acetaldehyde, a major metabolite of dichlorvos, is a weak bacterial
mutagen. Positive results have been reported in mice given a dose
of dichloroacetaldehyde far greater than that which could derive
from sublethal doses of dichlorvos. Dichlorvos has been shown to
methylate DNA in vitro at a rate that is 8-9 orders of magnitude
lower than the rate of phosphorylation. Therefore, DNA alkylation
is not likely to occur at doses of dichlorvos which are not
inhibitory to erythrocyte/brain cholinesterase.
A three-generation reproduction study in rats was negative at
doses up to 235 ppm in the diet, equivalent to 12 mg/kg bw/day. A
one-litter, one-generation study in mice in which dichlorvos was
administered by inhalation at doses which caused > 90% plasma
cholinesterase inhibition, but no signs of toxicity, was negative.
Dichlorvos caused reversible damage of seminiferous tubules, Leydig
and Sertoli cells at oral doses of 10 mg/kg bw daily for 18 days in
mice and at 5 mg/kg bw and above every other day for 8 weeks in
rats.
Dichlorvos appeared not to be teratogenic in mice, rats and
rabbits at doses which caused maternal toxicity.
Dichlorvos caused delayed polyneuropathy in hens at doses much
higher than the unprotected LD50. Cases of delayed polyneuropathy
also have been reported in humans after severe intoxications.
In humans, the rate of dichlorvos hydrolysis by plasma is
similar to that in rats. The rate of recovery of inhibited
erythrocyte and plasma cholinesterase activity in humans given
dichlorvos is much slower than in rats. Half-lives of recovery are
about 15 days in humans and about two hours in rats. A daily dose
of 1 mg/kg bw to male human volunteers for seven days caused 5-30%
inhibition of erythrocyte cholinesterase. The NOAEL in humans,
based on absence of erythrocyte cholinesterase inhibition in 12
volunteer males for 21 days was 0.04 mg/kg bw/day.
In 1986, the Joint Meeting discussed the significance of
carcinogenicity studies for organophosphorus pesticides and the
requirements for further studies (Section 3.1 of Annex 1, reference
47). At that time none of the organophosphorus pesticides had
caused a carcinogenic response in experimental animals. That Joint
Meeting recommended that, depending upon future evaluation on a case
by case basis, further consideration should be given to the need for
carcinogenicity studies for organophosphates.
In assessing the potential hazard to humans of residues of
dichlorvos, the following considerations were taken into account in
view of the weakly positive results in the gavage carcinogenicity
study in mice.
Organophosphorus esters used as insecticides react with
biological molecules by means of phosphorylation of serine
hydrolases and of alkylation of macromolecules. Phosphorylation of
acetylcholinesterase and alkylation of DNA are considered to account
for the acute cholinergic toxicity and initiation of the
carcinogenic process, respectively. These biochemical reactions
occur at different rates. When the rate of phosphorylation is
substantially higher than the rate of alkylation, in vivo
genotoxic effects are unlikely to occur because effective doses
cannot be achieved due to acute toxicity. Dichlorvos meets these
criteria, the rate of phosphorylation of acetylcholinesterase being
much faster (eight orders of magnitude) than that of alkylation of
several macromolecules, including DNA. Hence positive mutagenicity
tests were seen only in vitro and, as indicated in the 1986 Joint
Meeting report, carcinogenicity studies are unlikely to give more
information. The weak carcinogenic response of dichlorvos obtained
in mice in a corn oil gavage study should be interpreted as a local
effect of dichlorvos.
Information on comparative cholinergic toxicity might be of
critical relevance for the extrapolation of toxic effects (other
than acute effects) of organophosphates in experimental animals to
humans. The characteristics of the interactions of a given compound
with acetylcholinesterase (rates of phosphorylation, spontaneous
reactivation and ageing) from different species can be compared in
vitro. Also, the in vivo rate of reappearance of blood
acetylcholinesterase activity can be measured. In some cases,
metabolic degradation of organophosphates can be assessed
comparatively by measuring the level of serum A esterase, which
hydrolyses a given compound. All these data enable an improved
assessment of cholinergic toxicity of organophosphates in different
species. This knowledge may be of special significance in the case
of dimethyl phosphates since the rates of in vivo reactivation
vary substantially across species. Therefore, chronic dosing is
more critical for extrapolation from animal data to humans. In a
repeated dose regime, the longer the half-life of reactivation the
more rapid and/or more toxic will be the resulting effect (i.e. in a
chronic dosing regime, humans will survive much lower doses of
dichlorvos causing, when given alone, peak erythrocyte/brain
cholinesterase inhibition than those which can be reached in
rodents). Therefore, comparison between the in vivo rates of
recovery of enzyme activity will enable an assessment of the
repeated doses of compounds and the resulting cholinesterase
inhibition, which would represent the limiting factors for other
toxicities (including mutagenicity and carcinogenicity).
In the case of dichlorvos, the Meeting considered the
extrapolation of carcinogenicity data derived in rodents and its
applicability to human safety, and concluded that the compound would
not result in chronic human health hazards at doses below those
which result in acetylcholinesterase inhibition.
The Meeting maintained the ADI, which is based on studies in
humans with a NOAEL of 0.04 mg/kg bw/day, using a 10-fold safety
factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 mg/kg bw/day (two-year study)
Rat: 47 ppm in the diet, equivalent to 2.4 mg/kg bw/day
(two-year study)
Human: 0.04 mg/kg bw/day (21-day study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abdi, Y.A. & Villen, T. (1991) Pharmacokinetics of metrifonate and
its rearrangement product dichlorvos in whole blood. Pharmacol.
Toxicol. 68: 137-139.
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti,
E., Falcone, E. & Carere, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon and dichloroacetaldehyde. Pestic. Sci. 15:
439-442.
Asperen van, K., Dekhuijzen, H.M. (1958) A quantitative analysis of
the kinetics of cholinesterase inhibition in tissue homogenates of
mice and houseflies. Biochim. Biophys. Acta, 28: 307-337.
Bedford, C.T. & Robinson, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2: 307-337.
Benford, D.J. (1991) Investigation of the genotoxic and/or irritant
effects of dichlorvos on mouse forestomach. Unpublished report no.
RI90/0405 from Robens Institute of Health and Safety, Guildford,
Surrey, U.K.. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. & Morpurgo, G.
(1977) Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutat. Res. 46: 395-402.
Bignami, M., Conti, G., Conti, L., Crebelli, R., Misuraca, F.,
Ruglia, A.M., Randazzo, R., Sciandrello, G. & Carere, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor and Asperigillus nidulans.
Chem.-Biol. Interactions, 30: 9-23.
Bisby, J.A. & Simpson, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Austr. 2: 394-395.
Blair, D., Dix, K.M., Hunt, P.F., Thorpe, E., Stevenson, D.E. &
Walker, A.I.T. (1976) Dichlorvos - a 2-year inhalation
carcinogenesis study in rats. Arch. Toxicol. 35: 281-294.
Boyer, A.C. (1978) Factors which affect the in vitro inhibition of
human plasma cholinesterase. Toxicol. Appl. Pharmacol. 41: 475-
483.
Boyer, A.C., Brown, L.J., Slomka, M.B. & Hine, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. Appl. Pharmacol. 41:
389-394.
Braun, R., Schoneich, J., Weissflog, L. & Dedek, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair - direct alkylation versus metabolic activation and
breakdown. 1. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem.-Biol.
Interact. 39: 339-350.
Bridges, B.A., Mottershead, R.P., Green, M.H.L. & Gray, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulphonate for
Escherichia coli WP2 and some derivatives deficient in DNA repair.
Mutat. Res. 19: 295-303.
Brown, V.K.H. & Roberts, M (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to
guinea-pigs. Unpublished report no. IRR TL/40/66 from Shell Research
Ltd., Sittingbourne, UK. Submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Buselmayer, W., Rohrborn, G., Propping, P. (1972) Mutagenicity
investigations with pesticudes in the host-mediated assay and the
dominant lethal test in mice. Biol. Zbl. 91: 311-325 (in German).
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M., Raschetti,
R. (1978a) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res. 57: 277-286.
Carere, A., Ortali, V.A., Cardamone, G., Morpurgo, G. (1978b)
Mutagenicity of dichlorvos and other structurally related pesticides
in Salmonella and Streptomyces. Chem.-Biol. Interactions, 22:
297-308.
Caroldi, S., Lotti, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett. 9:
157-159.
Carter, M.K., Maddux B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. Appl. Pharmacol., 27: 456-463.
Casale, G.P., Bavari, S., Connolly, J.J. (1989) Inhibition of human
serum complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam. Appl. Toxicol. 12: 460-
468.
Casebolt, D.B., Leary, S.L., Undeutsch, L. (1990) Effects of
dichlorvos treatment on mouse reproduction. Lab. Anim. Care, 40:
65-67.
Chan, P.C. (1989) Toxicology and carcinogenesis studies of
dichlorvos (CAS No. 62-73-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, National Toxicology
Program, Technical Report Series NTP TR No.342.
Chan, P.C., Huff, J., Haseman, J.K., Alison, R. & Prejean, J.D.
(1991) Carcinogenesis studies of dichlorvos in Fischer rats and
B6C3F1 Mice. Jpn. J. Cancer Res. 82: 157-164.
Chattopadhyay, D.P., Dighe, S.K., Dube, D.K., & Purnanand (1982)
Changes in toxicity of DDVP, DFP, and parathion in rats under cold
environment. Bull. Environ. Contam. Toxicol. 29: 605-610.
Cohen, S.D., Ehrich, M. (1976) Cholinesterase and carboxyl esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. Appl. Pharmacol. 37: 39-48.
Coulston, F., Griffin, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP vapors.
Unpuplished report from Albany, New York, Institute of Comparative
and Human Toxicology and Holloman, Air Force Base, New Mexico,
Intern. Center of Environmental Safety. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Das, Y.T., Taskar, P.K., Brown, H.D., Chattopadhyay, S.K. (1983)
Exposure of professional pest control operator to dichlorvos (DDVP)
and residue on house structures. Toxicol. Letters, 17: 95-99.
Dean, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on micro-organisms. Arch. Toxikol. 30: 67-74.
Dean, B.J., (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol. 30: 75-85.
Dean, B.J. & Blair,D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres
containing dichlorvos. Mutat. Res. 40: 67-72.
Dean, B.J., Thorpe, E. (1972a) Cytogenetic studies with dichlorvos
in mice and Chinese hamsters. Arch. Toxikol. 30: 39-49.
Dean, B.J., Thorpe, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxicol. 30: 51-59.
Dean, B.J., Doak, S.M.A., Funnell, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium using
Saccharomyces cerevisiae. Arch. Toxicol. 30: 61-66.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984a) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol. 56: 66-67.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Letters, 21: 315-319.
Dieter, M.P., Jameson, C.W., French, J.E., Gangjee, S. (1989)
Development and validation of a cellular transplant model for
leukemia in Fischer rats: a short-term assay for potential
anti-leukemic chemicals. Leukemia Research, 13: 841-849.
Dyer, K.F., Hanna, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res. 21: 175-177.
Dzwonkowska, A., Hubner, H. (1986) Induction of chromosomal
aberrations in the Syriam hamster by insecticides tested in vivo.
Arch. Toxicol. 58: 152-156.
Enomoto, M.F., Nakadate, M., Ninomia, K., Hayakawa, Y., Ito, H.,
Igarashi, S., Uwanuma, Y., Nakasato, R., Hatanaka, J. (1981) Studies
on carcinogenicity of DDVP (2,2-dichlorovinyl dimethyl phosphate)
mixed in drinking-water in rats. Ministry of Health and Welfare,
Tokyo, Japan (Cooperative studies on carcinogenicity test on
mutagens) (Unpublished).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325.
Fahrig, R. (1973) (Genetic effects of organophosphorous
insecticides.) Die Naturwissenschaften, 60: 50-51 (in German).
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
IARC Scient. Publ. 10: 161-181.
Farber, T.M., Marks, E.M., Cerra, F.E. (1975) Dichlorvos and
microsomal enzyme activity. Fed. Proc. 34: 785.
Fischer, G.W., Schneider,P., Scheufler, H. (1977) (Mutagenicity of
dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester, possoble metabolites of the organophosphorus
pesticide trichlorphon.) Chem.-Biol. Interactions, 19: 205-213 (in
German).
Fournier, E., Sonnier, D., Dally, S., (1978) Detection and assay of
organophosphate pesticides in human blood by gas chromatography.
Clin. Toxicol. 12: 457-462.
Francis, B.M., Metcalf, R.L., Hansen, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. Environ. Sci. Health, B20: 73-95.
Fujita, K., Matsushima, S., Abe, E., Sasaki, K., Kurosawa, K. (1977)
(Examination of the effects of dichlorvos on the testis.) Nippon
Noson Igakkai Zasshi, 26: 328-329 (in Japanese) as quoted by WHO,
1989.
Gilot-Delhalle, J., Colizzi, A., Moutschen, J., Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade 6
locus of Schizosaccharomyces pombe. Mutat. Res: 117: 139-148.
Gough, B.J., Shellenberger, T.E. (1977-1978) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors
and reactivation with oximes. Drug and Chemical Toxicol. 1: 25-43.
Green, M.H.L., Medcalf, A.S.C., Arlett, C.F., Harcourt, S.A.,
Lehmann, A.R. (1974) DNA strand breakage caused by dichlorvos,
methyl methanesulphonate and iodoacetamide in Escherichia coli and
cultured Chinese hamster cells. Mutat. Res. 24: 365-378.
Green, M.H.L., Muriel, W.J., Bridges, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res. 38: 33-42.
Griffin III, D.E., Hill, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res. 52: 161-169.
Guerzoni, M.E., Del Cupolo, L., Ponti, I. (1976) (Mutagenic
activity of pesticides). Rev. Sci. Tecn. Alim. Nutr. Um. 6:
161-165 (in Italian).
Gupta, A.K., Singh, J. (1974) Dichlorvos (DDVP) induced breaks in
the salivary gland chromosomes of Drosophila melanogaster. Current
Science, 43: 661-662.
Hanna, P.J., Dyer, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res. 28: 405-420.
Haseman, J.K., Huff, J.E., Rao, G.N., Arnold, J.E., Boorman, G.A.,
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1
(B6C3F1) mice. J. Natl. Cancer Inst. 75: 975-984.
Hass, D.K., Collins, J.A., Kodama, J.K. (1972) Effects of orally
administered dichlorvos in Rhesus monkeys. J. Am. Vet. Med. 161:
714-719.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W.,
Schechtman, L.M. (1986) Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutag. 8:
515-531.
Hine, C.H. (1962) 90-day chronic toxicity studies of Vapona
insecticide for dogs. Unpublished report from San Francisco, The
Hine Laboratories, Report no. 1, September 1. Prepared for Shell
Chemical Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd,
Temana International Ltd.
Hine, C.H., Slomka, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
Hine, C.H., Slomka, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. Appl. Pharmacol. 17:
304-305.
Horn, K.H., Teichmann, B., Schramm, T. (1987) (Studies on
dichlorvos. I. Testing of dichlorvos for carcinogenic activity in
mice.) Archiv für Geschwulstforschung, 57: 353-360 (in German).
Horn, K.H., Teichmann, B., Schramm, T., Nischan, P. (1988) (Studies
on dichlorvos. II. Testing of dichlorvos for carcinogenic activity
in rats). Archiv für Geschwulstforschung, 58: 1-10 (in German).
Horn, K.H., Teichmann, B., Schramm, T. (1990) (Studies on
dichlorvos. III. Testing of dichlorvos for co-carcinogenic activity
in mice). Archiv für Geschwulstforschung, 60: 117-124 (in German).
Houk, V.S., DeMarini, D.M. (1987) Induction of prophage lambda by
chlorinated pesticides. Mutat. Res. 182: 193-201.
IARC (1979) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Some halogenated hydrocarbons, Volume 20:
97-127.
IARC (1991) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Occupational exposures in insecticide
application, and some pesticides. Volume 53: 267-307.
Ishidate, M., Sofuni, T., Yoshikawa, K. (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monograph on Cancer
Research, 27: 95-108.
Jayasuriya, V.U. de S., Ratnayake, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inform. Serv. 50: 184-186.
Johnson, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch. Toxicol.
41: 107-110.
Jolley, W.P., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of Vapona insecticide into their daily diet.
Unpublished report from Cincinnati, The Kettering Laboratory,
January 19. Prepared for Shell Chemical Company. Submitted to WHO by
Bayer AG, Ciba-Geigy Ltd, Temana International Ltd.
Kimbrough, R.D., Gaines, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. Environ.
Health, 16: 805-808.
Kligerman, A.D., Erexson, G.L., Wilmer, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res. 157: 181-187.
Konishi, Y., Denda, A., Kitaoka, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3F1 mice. Tokyo, Ministry of
Health and Welfare, Japan (unpublished).
Kramers, P.G.N., Knaap, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of Drosophila
melanogaster. Mutat. Res. 57: 103-105.
Krause, W. (1977) Influence of DDT, DDVP and malathion on FSH, LH
and testosterone serum levels and testosterone concentrations in
testis. Bull. Environ. Contam. Toxicol. 18: 231-242.
Krause, W., Homola, S. (1972) (Influence on spermatogenesis by DDVP
(dichlorvos).) Arch. Dermatol. Forschung, 244: 439-441 (in
German).
Krause, W., Homola, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat testis after the
application of dichlorvos (DDVP). Bull. Envirom. Contam. Toxicol.
11: 429-433 (study is performed in mice).
Krause, W., Hamm, K., Weissmuller, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. Environ. Contam. Toxicol. 15: 458-462.
Lawley, P.D., Shah, S.A., Orr, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (Dichlorvos, DDVP).
Chem.-Biol. Interactions, 8: 171-182.
Li, C., Miller, W.T., Jiang, J. (1989) Pulmonary edema due to
ingestion of organophosphate insecticide. Am. J. Roentgenol., 152:
265-266.
Lin, S.Y., Lee, T.C., Cheng, C.S., Wang, T.C. (1988) Cytotoxicity,
sister-chromatid exchange, chromosome aberration and transformation
induced by 2,2-dichlorovinyl-0,0-dimethyl phosphate. Mutat. Res.
206: 439-445.
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57: 393-394.
Lofroth, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
naturforsch. 29: 651.
Lofroth, G., Wennerberg, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch. 41:
215-221.
Lotti, M., Johnson, M.K. (1978) Neurotoxicity of organopho-sphorus
pesticides: Predictions can be based on in vitro studies with hen
and human enzymes. Arch. Toxicol. 41: 215-221.
Maslinska, D., Zalewska, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia Histochem. Cytochem. 16: 335-341.
Matsushita, T., Aoyama, K., Yoshimi, K., Fujita, Y., Ueda, A. (1985)
Allergic contact dermatitis from organophosphorus insecticides.
Ind. Health, 23: 145-153.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L., Loh, E.K., Hamilton,
C.M., Bakke, J.P., Spalding, J.W. (1989) Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: Testing of 24 compounds. Environ. Mol. Mutat.
14: 155-164.
Modak, A.T., Stavinoha, W.B., Weintraib, S.T. (1975) Dichlorvos and
the cholinergic system: effects on the cholinesterase and
acetylcholine and choline contents of rat tissues. Arch. Int.
Pharmacodyn. 217: 293-301.
Mohn, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides. Mutat.
Res. 20: 7-15.
Moriya, M., Kato, K., Shirasu, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res. 57: 259-263.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y., (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res. 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979) Analysis of mitotic nondisjunction with
Aspergillus nidulans. Environ. Health Aspects, 31: 81-95.
Moutschen-Dahmen, J., Moutschen-Dahmen, M., Degraeve, N. (1981)
Metrifonate and dichlorvos: cytogenetic investigations. Acta
Pharmacol. Toxicol. 49(V): 29-39.
Nagy, Zs., Mile, I., Antoni, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta Microbiol. Acad.
Sci. Hung. 22: 309-314.
NCI (National Cancer Institute) (1977) Bioassay of dichlorvos for
possible carcinogenicity. Bethesda, Maryland, USA.
Nicholas, A.H., Vienne, M., Berghe, H. van den (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Letters, 2:
271-275.
Nishio, A., Uyeki, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphate
insecticides and their oxygen analogs. J. Toxicol. Environ. Health,
8: 939-946.
Nishio, A., Uyeki, E.M. (1982) Nuclease-sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants.
Biochem. Biophys. Research Comm. 107: 485-491.
Nordgren, I., Bergstrom, M., Holmstedt, B., Sandoz, M. (1978)
Transformation and action of metrifonate. Arch Toxicol. 41: 31-41.
Olinski, R., Walter, Z., Wiaderkiewicz, R., Lukasova, E., Palecek,
E. (1980) Changes in DNA properties due to treatment with the
pesticides malathion and DDVP. Radiat. Environ. Biophys. 18:
65-72.
Ottevanger, C.F. (1975) (Neuromuscular function in people exposed to
dichlorvos resin strips. The importance of electromyography.)
Europ. J. Toxicol. 8: 192-195 (in French).
Pachecka, J., Sulinski, A., Ziolkowska, G. (1975) The activities of
some esterases of the rat brain after intoxication by
organophosphate insecticides, dichlorvos and trichlorphon.
Neuropat. Pol. 13: 455-462.
Paik, S.G., Lee, S.J. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool. 20:
19-28.
Pauluhn, J., Machemer, L., Kimmerle, G. (1987) Effects of inhaled
cholinesterase inhibitors on bronchial tonus and on plasma and
erythrocyte acetylcholine esterase activity in rats. Toxicology,
46: 177-190.
Pena-Chavarria, A., Swartzwelder, J.C., Villarejos, V.M., Kotcher,
E., Arguedas, J. (1969) Dichlorvos, an effective broad-spectrum
anthelmintic. Am. J. Trop. Med. Hyg. 18: 907-911.
Perocco, P., Fini, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
Purshottam, T., Kaveeshwar, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviation, Space,
and Environ. Med. 50: 581-584.
Purshottam, T., Srivastava, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
Environ. Health, 39: 425-430.
Pym, R.A., Singh, G., Gilbert, W.S., Armstrong, J.P., McCleary, B.V.
(1984) Effects of dichlorvos, maldison and pirimiphos-methyl on
food consumption, egg production, egg and tissue residues, and
plasma acetylcholinesterase inhibition in layer strain hens. Aust.
J. Exp. Agric. Anim. Husb. 24: 83-92.
Ramel, C. (1981) Does dichlorvos constitute a genotoxic hazard?
Progress in Mutat. Res. 2: 69-78.
Rauws, A.G., Logten, M.J. van (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in chicken.
Toxicology, 1: 29-41.
Reiner, E., Plestina, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by
0,0-dimethyl-2,2-dichlorovinyl phosphate. Toxicol. Appl. Pharmacol.
49: 451-454.
Reiner, E. Krauthacker, B., Simenon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
0,0-dimethyl-2,2,2-trichloro-1-hydroxyethylphosphonate
(trichlorphon). Biochem. Pharmacol. 24: 717-722.
Reiner, E., Simeon, V., Skrinjaric-Spoljar, M. (1980) Hydrolysis of
O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP) by esterases in
parasitic helminths, and in vertebrate plasma and erythrocytes.
Comp. Biochem. Physiol. 66: 149-152.
Rider, J.A. (1967) Determination of the minimal incipient toxicity
of dichlorvos in humans. Unpublished report from San Francisco,
Gastrointestinal Research Lab., October. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Rider, J.A., Moeller, H.C., Puletti, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies
with Guthion, and determination of incipient toxicity level of
dichlorvos in humans. Fed. Proc. 26: 427.
Rider, J.A., Moeller, H.C., Puletti, E.J., Swader, J. (1968) Studies
on the anticholinesterase effects of methyl parathion, Guthion,
dichlorvos, and Gardona in human subjects. Fed. Proc. 27: 597.
Rosenkranz, H.S. (1973) Preferential effect of dichlorvos (Vapona)
on bacteria deficient in DNA polymerase. Cancer Research, 23:
458-459.
Rosenkranz, H.S., Rosenkranz, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia, 28: 386-387.
Segerback, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP
(2,2-dichlorovinyl dimethylphosphate). Hereditas, 94: 73-76.
Segerback, D., Ehrenberg, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta Pharmacol. Toxicol. 49(V): 56-66.
Shellenberger, T.E. (1980) Organophosphate pesticides inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. Environ. Sci. Hlth. B15: 795-822.
Shellenberger, T.E., Newell, G.W., Okamoto, S.S., Sarros, A. (1965)
Response of rabbit whole-blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14:
943-952.
Shinoda, H., Ito, K., Matsunaga, T., Takada, T. & Nomura, T. (1972)
(Two suicides by acute pesticide intoxication). J. Jpn. Assoc.
Rural Med. 21: 242-249 (in Japanese).
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shooter, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating agents.
Chem. Biol. Interactions, 11: 575-588.
Skrinjaric-Spoljar, M., Simeon, V., Reiner, E. (1973) Spontaneous
reactivation and aging of dimethylphosphorylated
acetylcholinesterase and cholinesterase. Biochim. Biophys. Acta,
315: 636-369.
Slomka, M.B., Hine, C.H. (1981) Clinical pharmacology of dichlorvos.
Acta Parmacol. Toxicol. 49(V): 105-108.
Snow, D.H., Watson, A.D. (1973) The acute toxicity of dichlorvos in
the dog: 1. clinical observations and clinical pathology. Aust.
Vet. J. 49: 113-119.
Sobels, F.H., Todd, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res. 67: 89-92.
Stanton, H.C., Albert, J.R., Mersmann, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows.
Am. J. Vet. Research, 40: 315-320.
Stevenson, D.E., Blair, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Tox. 18: 215-217.
Teichert, K., Szymczyk, T., Consolo, S., Ladinsky, H. (1976) Effect
of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. Appl. Pharmacol. 35: 77-81.
Tezuka, H., Ando, N., Suzuki, R., Terahta, M., Moriya, M., Shira-su,
Y. (1980) Sister-chromatid exchanges and chromosomal aberrations in
cultured Chinese hamster cells treated with pesticides positive in
microbial reversion assays. Mutat. Res. 78: 177-191.
Traverso, R., Moretto, A., Lotti, M. (1989) Human serum
"A"-esterases. Hydrolysis of 0,0-dimethyl-2,2-dichlorovinyl
phosphate. Biochem. Pharmacol. 38: 671-676.
Tungul, A., Bonin, A.M., He, S., Baker, R.S. (1991) Micronuclei
induction by dichlorvos in the mouse skin. Mutagenesis, 6:
405-408.
Uchiyama, M., Yoshida, T., Homma, K., Hongo, T. (1975) Inhibition
of hepatic drug-metabolizing enzymes by thiophosphate insecticides
and its drug toxicological implications. Biochem. Pharmacol. 24:
1221-1225.
Vasilescu, C., Florescu, A. (1980) Clinical and electrophysiological
study of neuropathy after organophosphorous compounds poisoning.
Arch. Toxicol. 43: 305-315.
Voogd, C.E., Jacobs, J.J., Stel, J.J. van der (1972) On the
mutagenic action of dichlorvos. Mutat. Res. 16: 413-416.
Wadia, R.S., Shinde, S.N., Vaidya, S. (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurol. India, 33:
247-253.
Wadia, R.S., Chitra, S., Amin, R.B., Kiwalkar, R.S., Sardesai, H.V.
(1987) Electrophysiological studies in acute organophosphate
poisoning. J. Neurol. Neurosurg. Psychiatry, 50: 1142-1148.
Wang, T., Wu, C., Lin, J., Tarn, C., (1988) Dichlorvos potentiates
the insecticide-induced sister chromatid exchanges in Chinese
hamster ovary cells. Bull. Inst. Zool. Academia Sinica, 27:
111-117.
Ward, F.P., Glicksberg, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Ass. 158:
457-461.
Watanabe, H., Shirai, O., Ebata, N. (1974/1976) (A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care.) Sapporo Igaku Zasshi, 43: 334-337 (in Japanese);
Pest. Abstr., 8: 94-95.
Weisburger, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D. (eds). Effects of chronic exposures
to pesticides on animal systems, pp. 165-176. New York, Raven Press.
Wennerberg, R., Lofroth, G. (1974) Formation of 7-methylguanine by
dichlorvos in bacteria and mice. Chem.-Biol. Interactions, 8:
339-348.
WHO (1989) Environmental Health Criteria 79: Dichlorvos.
International Programme on Chemical safety, Geneva, World Health
Organization.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wild, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of
dichlorvos and methyl methanesulphonate. Mutat. Res. 19: 33-41.
Wild, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res. 32: 133-150.
Witherup, S., Caldwell J.S., Hull, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their
offsprings by the introduction of Vapona insecticide into their
diets. Unpublished report by Kettering Laboratory.
Witherup, S., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction
of Vapona insecticide into their daily diets. Unpublished report
from Cincinnati, The Kettering Laboratory, February 14. Prepared for
Shell Chemical Company, submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Wooder, M.F., Creedy, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo. Unpublished
report. Sittingbourne, Shell Research Ltd., TLGR. 79.089.
Wooder, M.F., Wright, A.S. (1981) Alkylation of DNA by
organophosphorus pesticides. Acta Pharmacol. Toxicol. 49(V):
51-55.
Wooder, M.F., Wright, A.S., King, L.J. (1977) In vivo alkylation
studies with dichlorvos at practical use concentrations.
Chem.-Biol. Interact. 19: 25-46.
Wooder, M.F., Wright, A.S., Stevenson, D.E. (1978) Studies on the
in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol. 1: 505.
Wright, A.S., Hutson, D.H., Wooder, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol. 42: 1-18.
Wyrobek, A.J., Bruce, W.R. (1975) Chemical induction of sperm
abnormalities in mice. Proc. Nat. Acad. Sci. USA, 72: 4425-4429.
Yoshida, T., Nomura, M., Suzuki, Y. (1976) Inhibition of hepatic
UDP-glucuronyltransferase activity by organophosphate insecticides
and by carbon disulfide in mice. Bull. Environ. Contam. Toxicol.
15: 421-424.
Zalewska, Z., Rakowska, I., Matraszek, G., Sitkiewicz, D. (1977)
Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterase. Neuropat. Pol.
15: 255-262.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagenesis, 11(12): 1-157.
Effects on enzymes and other biochemical parameters
In vitro studies showed that rodent plasma ChE is more
sensitive than erythrocyte and brain ChE to inhibition by
dichlorvos. Calculated I50s (concentrations which inhibit 50% of
enzyme activity) at 37 °C, pH 7.4-7.6, were 10-8 and 10-7 mol/l,
respectively, for an incubation time of 20 minutes (Asperen &
Dekhuijzen, 1958; Skrinjaric-Spoljar et al., 1973; Lotti &
Johnson, 1978). The rate of phosphorylation of rat brain ChE was
calculated to be 1.5 x 105 mol/l/min at 37 °C, pH 7.4-7.6
(Skrinjaric-Spoljar et al., 1973). Half-life of in vitro
reactivation (at 37 °C, pH 7.4-7.6) was found to be about 100
minutes; that of aging was found to be about 400 min. This means
that inhibited AChE almost completely reactivates within a few
hours, leaving a small fraction (20% or less) irreversibly inhibited
(i.e. aged) (Skrinjaric-Spoljar et al., 1973).
TOXICOLOGICAL STUDIES
Acute toxicity studies
The results of acute toxicity tests of dichlorvos administered
by various routes to different animal species are summarized in
Tables 1 and 2. Typical cholinergic signs are observed. WHO has
classified dichlorvos as highly hazardous (WHO, 1992).
Short-term toxicity studies
Mice
In a range-finding study, groups of 10 B6C3F1 mice/sex were
administered by corn oil gavage 0, 5, 10, 20, 40, 80, or 160 mg
dichlorvos/kg bw/day, 5 days per week for 13 weeks. Animals were
observed twice daily for clinical signs and body weight was recorded
weekly. Necropsy was performed on all animals. Oesophagus and
gastrointestinal tract of all animals dying after day 46 were
examined histologically. All vehicle controls and all animals in
the highest dose group with survivors were examined histologically.
All mice at 160 mg/kg bw/day and 5 male mice at 80 mg/kg bw/day died
before the end of the study. Final mean body weights in all treated
groups were comparable to controls. No gross or microscopic
pathologic effects were observed (Chan, 1989).
Rats
In a range-finding study, groups of 10 male and 10 female
F344/N rats were administered by corn oil gavage 0, 2, 4, 8, 16, 32,
or 64 mg dichlorvos/kg bw/day, 5 days per week for 13 weeks.
Animals were observed twice daily for clinical sign and body weights
were recorded weekly. Necropsy was performed on all animals.
Oesophagus and gastrointestinal tract of all animals dying after day
46 were examined histologically. All vehicle controls and all
animals in the highest dose group with survivors were examined
histologically. All rats at 32 and 64 mg/kg bw/day and 1 male and 4
females at 16 mg/kg bw/day died before the end of the study. Final
mean body weights of all dose groups were comparable to vehicle
controls. No gross or microscopic pathologic effects were observed
(Chan, 1989).
Table 1. Acute toxicity of dichlorvos (WHO, 1989)
Species Route LD50 (mg/kg bw)
Mouse oral 68-275
i.p. 28-41
i.v. 8-10
s.c. 13-33
Rat oral 30-110
dermal (24 h) 75-107
i.p. 18
s.c. 72
Guinea-pig s.c. 28
Hamster i.p. 30
Rabbit oral 13-23
dermal 205
Cat oral 28
Dog oral 100-316
Chicken oral 15
Swine oral 157
Table 2. Acute inhalation toxicity of dichlorvos (WHO, 1989)
Species Mode of exposure Time of exposure LC50
(hours) (µg/l)
Mouse whole body 4 13
head only 4 > 218
Rat whole body 4 15
whole body 1 140
head only 4 340
head only 1 455
head only 4 > 198
Dogs
Groups of 3 male and 3 female beagle dogs received 0, 2.3, 6.9,
12 or 23 mg dichlorvos/dog (93% in corn oil by gelatin capsule)
daily, for 90 days. The mean doses per group were equivalent to
0.3, 1, 1.5 or 3 mg/kg bw/day. No effects were observed on
mortality, growth, haematology, liver and kidney function, organ
weights, or at gross and histopathological examination. In the two
highest dose groups, the dogs showed excitement, increased activity
and aggression. Plasma and erythrocyte ChE activities, measured
initially and at intervals of approximately 2 weeks, were normal in
the lowest dose group (0.3 mg/kg bw/day) but inhibited in the other
dose groups (up to about 60% in the highest dose group). Inhibition
was lower from day 70 onwards. Brain ChE activity at termination
was decreased (to 33%of control activity) only in the highest dose
group. The NOAEL in this study was 1.5 mg/kg bw/day based on
reduction in brain ChE activity at termination. However, taking
into account the behavioural changes, a more conservative NOAEL can
be considered to be 1 mg/kg bw/day (Hine, 1962).
Pigs
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, or 16 mg
dichlorvos/kg bw/day (divided over 2 daily doses) for 30 days. No
effects were found on body-weight gain, haematology, or clinical
chemistry when compared with control animals fed a blank PVC
formulation. Plasma and erythrocyte ChE activities were
significantly inhibited in the 4 mg/kg bw/day group (37 and 34% of
controls, respectively) and in the 16 mg/kg bw/day group (33 and 21%
of controls, respectively). The NOAEL in this study was 1 mg/kg
bw/day based on inhibition of erythrocyte ChE activity (Stanton et
al., 1979).
Monkeys
Rhesus monkeys (4/sex) were continuously exposed to dichlorvos
vapour at an average actual concentration of 0.05 mg/m3 for 3
months. The control group consisted of 4 males and 1 female. No
adverse effects were observed in appearance, behaviour, or
haematological and clinical chemical determinations. Plasma ChE
activity was slightly reduced (up to 28% inhibition), while
erythrocyte ChE activity inhibition was 36%. No changes in nerve
maximum conduction velocities or muscular evoked action potentials
were induced by exposure to dichlorvos (Coulston & Griffin, 1977).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 100 male and 100 female C57BI/6/Bln mice (5-6 weeks
old) received by gavage 0.2 mg dichlorvos per mouse (97% in 0.2 ml
water), freshly prepared, either twice or 3 times per week for 50
weeks. Control groups received by gavage either 0.2 ml water, 3
times per week (about 50 males and 50 females), or no treatment (35
males and 35 females). Surviving animals were sacrificed after 110
weeks. This strain of mice is known for the spontaneous occurrence
of mixed lymphomas (reticulocell sarcoma type B).
From the age of 12 months onwards, some animals in all groups
developed interstitial pneumonia. The incidence of mixed lymphomas
was decreased in both test groups (26-60% in control groups, 23-30%
in treated groups). An increased incidence of focal hyperplasia
(transitional cell hyperplasia) of the urinary bladder was found in
both dichlorvos groups (0-8% in control groups, 5-10% in treated
groups). The authors concluded that no neoplastic lesions were
found which could be attributed to the treatment of the animals with
dichlorvos (Horn et al., 1987).
Dichlorvos was not co-carcinogenic in C57BI/6/Bln mice when
administered by gavage three times/week at 0.2 mg/animal to mice
subcutaneously injected with 50 µg N-nitrosodiethylamine per animal
weekly for 50 weeks followed by an observation period of up to 110
weeks (Horn et al., 1990).
Two groups of 50 male and 50 female B6C3F1 mice were fed
1000 or 2000 ppm dichlorvos (purity > 94%) in corn oil in the diet
for 2 weeks. Due to severe signs of intoxication, doses were
lowered to 300 and 600 ppm for the following 78 weeks. Samples of
the diets analyzed during the study showed that time-weighted
average concentrations were 318 and 635 ppm. Matched controls
consisted of 10 mice of each sex; the pooled controls from
simultaneous studies with other compounds consisted of 100 male and
80 female mice. All surviving mice were killed at 92-94 weeks.
Animals were observed twice daily for clinical signs.
Alopecia and rough hair coats were noted in many treated
animals, particularly in the male groups, beginning at week 20 and
persisting throughout the study. The average body weights of the
high-dose mice of both sexes were slightly decreased compared with
controls. The low-dose female group showed 74% survival at 90 weeks
compared to 84% and 90% in high-dose and control groups,
respectively. There was no significant increase in the incidence of
tumours attributable to dichlorvos in either sex.
Two squamous-cell carcinomas of the oesophagus (one in a
low-dose male and one in a high-dose female), 1 papilloma of the
oesophagus in a high-dose female and 3 cases of focal hyperplasia of
the oesophageal epithelium in low-dose males were recorded in the
treated mice. The significance of the findings in the treated mice
was considered uncertain because of insufficient information
concerning the spontaneous incidence of these lesions and lack of
statistical significance within the experiment. Dichlorvos (up to
635 ppm in the diet, equivalent to 95 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Studies in B6C3F1 mice given 0, 400 or 800 mg/litre
dichlorvos in drinking-water for two years and in CFE rats exposed
to 0, 0.05, 0.48, or 4.7 mg/m3 of dichlorvos 23 hours/day for 2
years were summarized in WHO (1989). There was no evidence of
carcinogenic effects in these studies (Blair et al., 1976;
Konihishi et al., 1981).
Groups of 50 B6C3F1 mice were given dichlorvos (99% purity)
by corn oil gavage at doses of 0, 10 or 20 (males) or 0, 20 or 40
(females) mg/kg bw/day daily, 5 days per week, for 103 weeks. The
dose volume of the corn oil was 10 ml/kg bw/day. Body weights were
recorded once weekly for the first 12 weeks and then monthly.
Animals were observed twice per day for clinical signs. Necropsy
and histological examinations were performed on all moribund animals
or at the end of the study.
Body-weight gain and survival did not significantly differ
between treated and control groups. No compound-related clinical
signs were observed. Blood cholinesterase activity was not
determined during the study. However, a separate study showed
inhibition of plasma ChE activity by 60-90% in all dose groups while
erythrocyte ChE activity was normal (see "Special studies on
Cholinesterase activity" for detailed description and comments).
The incidences of forestomach squamous cell papillomas were
1/50, 1/50 and 5/50 in control, low and high-dose males respectively
and 5/49, 6/49 and 18/50 in control, low and high-dose females,
respectively. The positive trend was statistically significant in
both sexes while by pairwise comparison only the incidence in high-
dose females was significantly higher than in controls. Two
forestomach squamous cell carcinomas were seen in high-dose females
and none in the other groups. No increase in the incidence of
forestomach hyperplasia was seen in the dosed mice compared with
vehicle controls (10-20%). In female mice the incidence of adenomas
and adenomas or carcinomas (combined) of the pituitary gland (12/45,
6/45 and 6/44 in control, low and high-dose groups, respectively)
and the incidence of lymphomas (16/50, 11/50 and 9/50 in control,
low and high-dose groups, respectively) showed a significant
negative trend. Based on the increased incidence of forestomach
papillomas, the NOAEL was 10 mg/kg bw/day (Chan, 1989; Chan et al.,
1991).
Rats
Groups of 40 male and 40 female weanling CD rats were fed diets
containing nominal concentrations of 0, 0.1, 1, 10, 100 or 500 ppm
dichlorvos (93% purity) for 2 years. Diets were prepared weekly.
Five males and 5 females from each group were killed after 6, 12 or
18 months. Analysis of diet samples showed a considerable loss of
dichlorvos associated with a gradual increase in
dichloroacetaldehyde (DCA) content (average concentrations ranging
from 0.01 to 28.6 ppm. The average actual concentrations of
dichlorvos in each diet were 0.05, 0.5, 4.7, 47 or 230 ppm. No
cholinergic signs were observed. No effects were seen on behaviour,
mortality rate, weight gain, food consumption, terminal body and
organ weights, haematology or urinalysis. Plasma and erythrocyte
ChE activities were measured 13 times during the study. In the 100
ppm group, plasma and erythrocyte ChE activities were reduced to
60-90% and to 50-90% of control activities, respectively; in the 500
ppm group, the activities were reduced to 20-70% and 20-60%,
respectively. Activities were higher toward the end of the study.
Brain ChE activity was decreased in the highest dose group by
45-47%, 24-43%, 24-38% and 5-15% after 6, 12, 18 and 24 months,
respectively. Histological examination of major organs (liver,
heart, lungs, kidneys, spleen, brain, gonads, pituitary, adrenals,
and thyroids) revealed hepatocellular fatty vacuolization in all 500
ppm rats, and in most females and several males at 100 ppm. No
effect was seen on serum total proteins or albumin: globulin ratio,
or on hexobarbital sleeping time. The tumour incidence was
comparable with that of the control group. The NOAEL, based on
brain ChE inhibition, was 100 ppm (actual concentration 47 ppm,
equivalent to 2.4 mg/kg bw/day) (Witherup et al., 1967).
Groups of 50 male and 50 female weanling CFE rats were exposed
(whole body) to nominal air concentrations of 0, 0.05, 0.5 or 5 mg
dichlorvos (97% purity)/m3 for 23 hours per day for two years. The
average actual dichlorvos concentrations were 0.05, 0.48, or 4.7
mg/m3. Body-weight gain was reduced in the two highest dose
groups. After two years of exposure, plasma and erythrocyte ChE
activities were reduced by 20-30% at 0.5 mg/m3 and by > 60% in the
highest dose group; brain ChE activity was reduced by 10%
(statistically significant) at 0.5 mg/m3 and by 80% in the highest
dose group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry
values, organ weights, or gross or microscopic examinations of major
organs. Ultrastructural examinations of bronchi and alveoli of rats
exposed to 0 or 5 mg/m3 showed no differences between the two
groups.
It was concluded that 2-year exposure to 0.05 mg/m3 dichlorvos
did not cause observable adverse effects to CFE rats (Blair et al.,
1976). It should be noted that in this study the rats were not only
exposed by inhalation but also via their food, drinking-water, and
by grooming. This resulted in additional oral ingestion of
dichlorvos (Stevenson & Blair, 1977).
Osborne-Mendel rats (50/sex) were fed 1000 ppm dichlorvos
(purity > 94%) in corn oil for 3 weeks. Due to severe cholinergic
signs, the dose was reduced to 300 ppm for the remaining 77 weeks.
Another group (50 males and 50 females) was fed 150 ppm dichlorvos
for 80 weeks. Samples of the diets analyzed during the study showed
that time-weighted average concentrations were 330 and 150 ppm,
respectively. Matched controls consisted of 10 rats of each sex;
the pooled controls from simultaneous studies of other compounds
consisted of 60 rats of each sex. Animals were observed twice daily
for clinical signs. All surviving rats were killed after 110-111
weeks.
The average body weights of the high-dose rats of both sexes
were slightly decreased compared with controls. There was no
significant increase in the incidence and type of tumours
attributable to dichlorvos in either sex. Dichlorvos (up to 330 ppm
in the diet, equivalent to about 30 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Groups of 70 and 100 rats per sex received 0.1 mg dichlorvos
(97%)/rat in 0.2 ml water by gavage twice or 3 times per week,
respectively, for 60 weeks. Thereafter, the animals were observed
for another 51 weeks. A control group of 60 animals of each sex
received 3 times per week 0.2 ml water. From the age of 10 months
onwards, the incidence of focal hyperplasia of urinary bladder and
of renal pelvis increased in males but decreased in females from
both test groups compared to controls. No neoplastic lesions were
found which could be attributed to treatment (Horn et al., 1988).
A study in Fischer 344 rats given drinking-water containing 0,
140 or 280 mg/litre dichlorvos for 108 weeks was summarized by WHO
(1989). There was no evidence of carcinogenicity (Enomoto et al.,
1981).
Groups of 50 male and 50 female F344/N rats were administered
dichlorvos by corn oil gavage at 0, 4 or 8 mg/kg bw/day (99%
purity), 5 days per week for 103 weeks. Body weights were recorded
once weekly for the first 12 weeks and then monthly. Animals were
observed twice daily for clinical signs. Necropsy and histologic
examinations were performed on all moribund animals or at the end of
the study.
Mild diarrhoea was observed in treated animals. ChE activities
were not determined during the study. However, a separate study
showed inhibition of plasma ChE activity by 50-80% in all dosed
groups but no erythrocyte ChE inhibition (see "Special studies on
cholinesterase activity" for detailed description and comments). No
significant differences in mean body weights or survival were
observed between any groups of either sex. Increased incidence of
cytoplasmic vacuolisation of liver was observed in dosed males and
of cortical cytoplasmic vacuolisation of adrenal glands in all dosed
males and in low-dose females. The incidence of pancreatic adenomas
(cross and horizontal tissue sections were analyzed), in control,
low and high-dose groups was 25/50, 30/50 and 33/50 in males and
2/50, 3/50 and 6/50 in females, respectively. The increased
incidence in treated males was statistically significant. The
incidence of mononuclear cell leukaemia (11/50, 20/50, 21/50 in
control, low and high-dose groups, respectively) was significantly
increased in the treated male rats compared with controls. The
incidence of mammary gland adenomas or fibroadenomas in female rats
was 11/50 in controls, 19/50 in the 4 mg/kg bw/day group and 17/50
in the 8 mg/kg bw/day group. The incidence in the low-dose group
was only slightly higher than the historical control values. Two
mammary gland carcinomas were observed in control and low-dose
groups. The authors concluded that there was evidence that
dichlorvos is carcinogenic to rats (Chan, 1989; Chan et al.,
1991). The Meeting observed that the incidence of pancreatic
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia, which is usually high and variable in
this strain of rats, was also of questionable biological
significance (Haseman et al., 1985).
Dichlorvos at 8 or 16 mg/kg bw/day was administered by gavage
to groups of 8-12 male F344 rats, either with or without leukaemia
transplant, for 5 days a week. Other groups of rats were either not
treated or given the leukaemia transplant only. At 70-days
post-transplant, the animals were killed. The rats dosed with
dichlorvos developed the disease earlier and the rate of tumour
progression was increased. Three out of 16 transplant recipients
dosed with 16 mg/kg bw/day died of leukaemia during the last week of
dosing. The severity of the mononuclear cell leukemia in the
transplant recipients, as measured by histopathological examination
of spleen and liver, was correlated with the changes in tumour
growth rates. However, no dose-response was found for spleen weight
and WBC count (Dieter et al., 1989).
Dogs
Groups of beagle dogs (3/sex) were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100 or 500 ppm dichlorvos (93%
purity) for 2 years. The average actual concentrations were 0,
0.09, 0.32, 3.2, 32 or 260 ppm dichlorvos; the average
dichloroacetaldehyde concentrations at the three highest dosages
were 0.6, 6.4 and 20 ppm. Since diet analysis was performed weekly
on a mixture of diet samples taken the previous 7 days, it is
likely, given the high volatility of dichlorvos, that at the end of
the week the actual concentration was much lower than 30% of the
nominal concentration.
No effects were seen on general appearance, survival, weight
gain, food consumption, haematology or urinalysis. Erythrocyte ChE
activity was reduced up to 50% of controls in the 10 ppm group with
recovery to control values at the end of the feeding period. At the
highest dose level, inhibition was more than 90% but complete
recovery was observed at 24 months. A similar pattern was observed
for plasma ChE activity. Brain ChE activity, measured at the end of
the study was similar to that of the controls in all treated groups.
Relative liver weights were slightly increased in males at 100 ppm
and in both sexes at 500 ppm. Histological examination of major
organs revealed slight dose-related cytoplasmic vacuolization and
enlargement of hepatocytes in animals at the two highest levels. No
differences were seen in serum alkaline phosphatase, transaminase
activities, total serum proteins or albumin:globulin ratios (Jolley
et al., 1967).
Reproduction studies
See also under special studies on testes.
Mice
Male and female Crl:CD3-1 mice were exposed to dichlorvos
concentrations of 0, 1.9, 3.0 or 4.6 mg/m3, generated from
dichlorvos-impregnated PVC strips in their cages. Exposure began
4 days prior to formation of breeding groups (3 females and 1 male)
and continued throughout pregnancy. Signs of intoxication were not
observed. Plasma ChE activity was significantly inhibited (by 90%,
93% and 95% in the 1.9, 3.0 and 4.6 mg/m3 groups, respectively)
when measured on day 4 after beginning of treatment. Gestation
length, number of litters, litter frequency and mean litter size
were comparable to controls. No grossly detectable congenital
anomalies were detected in any of the offspring (Casebolt et al.,
1990).
Rats
A 3-generation, 2-litter/generation reproduction study in rats
summarized by WHO (1989) was negative at doses up to 235 ppm in the
diet, equivalent to 12 mg/kg bw/day (Witherup et al., 1965).
Domestic animals
Several studies have been carried out with pregnant sows but no
adverse effects on piglets were observed with doses which inhibited
plasma and erythrocyte ChE activity in the sows (WHO, 1989).
Special studies on delayed neurotoxicity
Several studies have shown that a single dose of dichlorvos
does not produce delayed neurotoxicity in pre-medicated hens,
whether it is administered orally or subcutaneously (WHO, 1989).
However, Caroldi & Lotti (1981) reported mild signs of ataxia in
pre-medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg bw) and severe (> 80%) inhibition of NTE in peripheral
nerve, spinal cord and brain. Johnson (1978) did not observe ataxia
in pre-medicated hens given the same dose in the same way. However,
in this experiment spinal cord NTE inhibition was below the
threshold. When the dose was repeated 1-3 days after the first
dose, spinal cord NTE inhibition increased and the hens became
ataxic.
In a 90-day study, white leghorn hens were given dichlorvos
(99.9% purity) either dermally or orally. For oral administration,
a 10-20% solution of dichlorvos in corn oil in gelatin capsules was
used whereas, dermally, 1-20% emulsifiable concentrates in technical
grade xylene (containing 2% Triton X-100) were used. Dichlorvos at
doses greater than 1 mg/kg bw/day (dermal) or 6 mg/kg bw/day (oral)
led to cholinergic symptoms and death after 2-3 days. None of the
animals developed organophosphate-induced delayed neuropathy
(Francis et al., 1985).
In summary, it is possible to produce clinical neuropathy in
hens, but the doses required are far in excess of the LD50. This
is consistent with the low in vitro ratio of AChE I50/NTE I50 for
dichlorvos (Lotti & Johnson, 1978).
Special studies on embryotoxicity and teratogenicity
Several embryotoxicity/teratogenicity studies have been
performed with dichlorvos in mice, rats and rabbits. These studies
are summarized in WHO (1989). Dichlorvos given orally, by
inhalation or intraperitoneally, was not teratogenic at doses which
were toxic to the pregnant animals. However, in a study in rats
where a single i.p. dose of 15 mg/kg bw was given on day 11 of
gestation, 3/41 fetuses in the treated group had omphaloceles (0/50
in controls) (Kimbrough & Gaines, 1968). This finding has not been
confirmed in other studies.
Special studies on cholinesterase activity
This section summarizes studies in which the effect of
dichlorvos on ChE activity was the only parameter investigated.
Studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos in relation to the time
elapsed after dosing are summarized in Table 3. In general, the
maximum inhibition occurred within one hour followed by rapid
recovery.
Mice/Rats
Groups of 8-week old B6C3F1 mice and F344/N rats (10/sex)
were administered dichlorvos (99%) by corn oil gavage at daily doses
of 0, 5, 10, 20, or 40 mg/kg bw (mice) and 0, 2, 4, 8, or 16 mg/kg
bw (rats), five days per week for one week. Plasma and erythrocyte
ChE activities were measured on days 10 or 11, 25 or 26 and 32 or
33; blood was obtained about 3 hours after treatment.
Plasma ChE activity was significantly inhibited in all dose
groups of mice (50 and 90% inhibition in low and high-dose groups,
respectively) and rats (25 and 80% inhibition in low and high-dose
groups, respectively). At all time-points, erythrocyte ChE activity
in dosed and vehicle-control mice and rats was similar. However,
the timing for determination of the enzyme activities might have
underestimated the inhibition (Chan, 1989).
Rats
Reiner & Plestina (1979) compared in vivo reappearance of ChE
activity in rats after treatment with either dichlorvos (2.5 mg/kg
bw i.v.) or metrifonate (300 mg/kg bw i.v.). Half-lives of recovery
of brain and plasma ChE (acetylcholine was the substrate) were found
to be 2 and 2.5 hours, respectively, both in dichlorvos-treated and
in metrifonate-treated rats. This was consistent with in vitro
data (Skrinjaric-Spoljar et al., 1973).
In a study on the influence of temperature on ChE activity,
rats were injected i.p. with a single dose of 6.3 mg/kg dichlorvos
and kept at either 28 °C or 5 °C. The maximum inhibition of whole
blood ChE activity (40% and 50% in the two groups, respectively)
occurred after 0.5 hour. The animals kept at 5 °C showed slightly
less inhibition of whole blood ChE activity than those at room
temperature (Chattopadhyay et al., 1982).
Table 3. Time-related inhibition of ChE activity in animals after administration of a single dose of dichlorvos
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Mouse, male i.p. 10 15 min 70 Nordgren et al., (1978)
(n = 6-8) 60 min 50
2 hours 20
Mouse, male i.p. 15 2 hours 20 Cohen & Ehrich, 1976
Mouse, male i.p. 30 15 min 63 Cohen & Ehrich, 1976
(n = 4) 1 hour 50
5 hours 35
18 hours 10
Rat, male oral 1.6 2 hours 0 Pacheka et al., 1975
(n = 6) 10 2 hours 15
40 1 hour 60*
Rat, male oral 40 1 hour 70 Teichert et al., 1976
(n = 1)
Rat, male oral 40 5 min 45 Pacheka et al., 1975
(n = 6) 15 min 80
1 hour 85
2 hours 70
8 hours 35
24 hours 25
48 hours 6
Rat, male oral 50 15 min 68 80 97.91** Modak et al., 1975
(n = 6) 3 hours 73 37 68-70**
24 hours 39 24 31-38**
Table 3 (contd)
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Rat, male i.v. 2.5 30 min 60 85 Reiner & Plestina, 1979
(n = 5-12) 90 min 40 65
3 hours 7 44
12 hours 0 17
48 hours - 10
Dog, greyhounds oral 11 1 hour 90 93 Snow & Watson, 1973
1 male 2 hours 80 70
1 female 24 hours 15 35
72 hours 8 25
Dog, 2 male oral 22 fatal 90 95 66*** Snow & Watson, 1973
greyhounds,
1 female
crossbred
Dog, greyhounds oral 22 1 hour 88 83 Snow & Watson, 1973
and crossbred 3 hours 76 66
(n = 7-9) 6 hours 60 50
24 hours 30 30
48 hours 13 30
72 hours 5 35
Dog, beagle oral 50 2 hours 68 37 Ward & Glicksberg, 1971
sex not specified 24 hours 37 26
(n = 15) 5 days 8 22
14 days 5 20
21 days 0 8
Table 3 (contd)
* Daily dosing for 14 days caused 60% AChE inhibition when measured 24 hours after the last dose.
** Determinations were performed in striatum, hippocampus, medulla and cortex. No significant difference
was found between these brain areas.
*** Measured 20-155 min. after treatment.
When pregnant rats were given oral doses of 1.1 or 5.6 mg
dichlorvos/kg bw/day during days 14-21 of gestation, plasma ChE
activity of the high-dose mothers was inhibited (30-50%) but not
that of the young. Brain ChE activity did not show any significant
inhibition (Zalewska et al., 1977).
Rabbits
Dichlorvos, when infused into the ear vein of adult male
rabbits, produced dose- and time-related inhibition of whole blood
ChE activity during infusion. Spontaneous but incomplete recovery
to 60-80% of the normal activity occurred within 60-90 minutes after
infusion. Almost complete recovery was obtained by injecting oximes
up to 2.5 hours later (Shellenberger et al., 1965; Gough &
Shellenberger, 1977-1978; Shellenberger, 1980).
The progeny of rabbits, treated orally with 6 mg/kg bw/day for
the last 10 days of gestation, showed inhibition of brain ChE
activity at one day (30%) and 8 days of life (15%). Plasma ChE
activity was higher throughout days 1-16 of life (Maslinska &
Zalewska, 1978).
Guinea-pigs
Groups of 5 male and 5 female guinea-pigs were given daily
applications of 0, 25, 50 or 100 mg/kg bw/day dichlorvos (94%
purity) on the shorn skin for 8 days. All animals survived. A
dose-dependent inhibition of both plasma and erythrocyte ChE
activities occurred in all test groups. Recovery of plasma ChE
activities was complete within one week of the last exposure, and
that of erythrocyte ChE was complete within one week in the females
and 2 weeks in the males (Brown & Roberts, 1966).
Hens
Chickens dosed once orally with 1,3, or 6 mg/kg bw showed a
rapid inhibition of plasma ChE activity followed by recovery with an
half-life being estimated at three days (Rauws & van Logten, 1973).
White Leghorn pullets and hens were fed a diet containing 30
ppm dichlorvos for 35 days followed by a 21-day recovery period.
Plasma ChE activity was inhibited by 70% after four weeks of
treatment but returned to normal during the recovery period (Pym et
al., 1984).
Monkeys
Thirty-two Rhesus monkeys were given a pelleted PVC-resin
formulation containing 20% dichlorvos at dosages ranging from 1 to
16 mg dichlorvos/kg bw once daily or 1.6 and 4 mg dichlorvos/kg bw
twice daily for 10 to 21 consecutive days. None of the monkeys
showed overt signs of intoxication, although they ate less food and
had soft faeces. Plasma and erythrocyte ChE activities were reduced
by approximately 80% in all animals (irrespective of the dose) and
remained inhibited throughout the study. Plasma ChE activities
returned to normal values within approximately 3 weeks and the
erythrocyte ChE activities within 50 to 60 days following cessation
of exposure (Hass et al., 1972).
Special studies on genotoxicity
Methylating reactivity
In vitro studies
In a quantitative colour test in which methylation of
4-(p-nitro-benzyl) pyridine was measured to predict DNA alkylating
potential, dichlorvos gave a positive response, the reaction being
about 1/3 that of the known alkylating compound
methylmethanesulfonate (MMS) (Bedford & Robinson, 1972).
Alkylation by dichlorvos of calf thymus DNA, resulting in the
formation of N-7-methylguanine, was reported by Lofroth (1970).
Methylation by dichlorvos of isolated salmon sperm DNA and of
DNA from intact E.coli and from human HeLa cells broadly resembled
that by MMS (Lawley et al., 1974). In this study a very high
dichlorvos concentration (14 mmol/l) was used with isolated DNA
(12.5 mmol/l DNA-P). Dichlorvos concentrations with E. coli and
HeLa cells were 1-3 mmol/l. The rate of methylation of guanine-N-7
from salmon sperm DNA or from intact HeLa cell DNA by dichlorvos was
2-3 x 10-4 mol/l/min (calculated assuming guanine as 23-25% of DNA
with an average molecular weight of DNA base pair of 649, Lawley et
al., 1974). This is about 15 times lower than the rate of
methylation by MMS. This rate is to be compared with the rate of
reaction with AChE (phosphorylation) (1.5 x 105 mol/l/min at 37 °C)
(Skrinjaric-Spoljar et al., 1973). Therefore, the relative rate
of phosphorylation is about 9 orders of magnitude higher than that
of alkylation.
Labelled 7-methylguanine was present in both DNA and RNA
isolated from E. coli exposed to [Me-3H]-dichlorvos (1.1 mmol/l
for 4 hours). The methylating capability of dichlorvos was less, by
a factor of 10-100, than that of strongly genotoxic methylating
compounds (Wennerberg & Lofroth, 1974).
Incubation of bacteriophage R17 with 0-100 mmol
dichlorvos/litre for 90 hours did not result in methylation of the
phosphate groups of the RNA to any significant extent (Shooter,
1975).
In vivo studies
Mice were given i.p. injections of methyl-14C-dichlorvos (1.9
µmol/kg bw, approximately 420 µg/kg bw). The degree of alkylation
of guanine-N-7 in DNA isolated from soft tissues amounted to 8 x
10-13 mol methyl per gram of DNA (about 5 x 10-10 mol/mol guanine)
(Segerback, 1981; Segerback & Ehrenberg, 1981). From acute toxicity
data in mouse (see Table 1) it can be extrapolated that this dose
would cause 1 mol/mol phosphorylation of erythrocyte ChE.
DNA and RNA from the total soft tissues of male rats exposed to
atmospheres containing 0.064 mg/m3 (about 0.1% of the LC50) of
methyl-14C-dichlorvos for 12 hours did not show methylation of the
N-7 atom of guanine moieties. The exposure period constituted a
significant fraction of the half-life of the 7-methylguanine
moieties in DNA (Wooder et al., 1977; Wooder & Wright, 1981).
Excretion of labelled 7-methylguanine in the urine by NMRI mice
and rats injected i.p. with [Me-14C]-dichlorvos, or exposed by
inhalation for 2 hours (mice only) was reported by Wennerberg &
Lofroth (1974) and Lofroth & Wennerberg (1974). In rat urine,
labelled 3-methyladenine and 1-methyl-nicotinamide were also present
(Lofroth & Wennerberg, 1974). According to the authors, these
results demonstrate that dichlorvos spontaneously methylates guanine
and adenine moieties in nucleic acids. However, administration of
radiolabelled adenine and guanine to otherwise untreated rats gave
rise to the excretion of radiolabelled methylated purines in the
urine. Therefore, the detection of radiolabelled purines, per se,
in the urine of animals exposed to methyl-labelled methylating
agents does not constitute evidence for the spontaneous methylation
of the purine moieties of nucleosides or nucleic acids by
methylating agents (Wooder et al., 1978; Wooder & Wright, 1981).
Moreover, a natural biosynthetic pathway has been demonstrated
whereby the methyl carbon atoms of dichlorvos can be incorporated
into the heterocyclic rings and the methyl groups of urinary
7-methylguanine after entering the 1-C pools, in vivo (Wright et
al., 1979; Wooder & Wright, 1981).
Genotoxicity
In vitro studies
Several studies using bacteria and fungi as test organisms have
been carried out (Table 4). In most of the studies, only one, often
high, dichlorvos concentration was tested, sometimes resulting in
low survival of the test organism. The alkylating properties of
dichlorvos (see above) are most probably the cause of the mutagenic
action. This is suggested, for instance by data in E. coli
strains deficient at four repair loci (Bridges et al., 1973).
In vitro studies using mammalian cells are summarized in
Table 5. Dichlorvos caused cell transformation, mutations, DNA
strand breaks, sister chromatid exchanges and chromosomal
aberrations in cultured animal cells. In cultured human cells,
dichlorvos induced unscheduled DNA synthesis but did not induce
sister chromatid exchanges or chromosomal aberrations. Negative
results from chromosomal aberration tests in cultured human
lymphocytes were also cited by Fahrig (1974) and Wild (1975).
Dichlorvos (0.3 µg/ml) caused a synergised an increase in
sister chromatid exchanges in Chinese hamster ovary cells when
tested in combination with synthetic pyrethroids or propoxur which,
as such, were negative; however, in the combination phenothrin +
dichlorvos (0.3 µg/ml) the induction of SCE by phenothrin was
negative (Wang et al., 1988).
In vivo studies
In vivo studies are summarized in Table 6.
In Drosophila melanogaster, chromosomal aberrations but not
sex-linked recessive lethal mutations were induced. Negative
results were obtained in host-mediated, dominant lethal, sister
chromatid exchange and micronucleus assays (except on skin after
local application at cytotoxic doses). Dichlorvos did not induce
in vivo chromosomal aberrations in bone-marrow cells,
spermatocytes or spermatogonia, DNA strand breaks or unscheduled DNA
synthesis.
Special studies on liver microsomal enzymes
A decrease in liver microsomal cytochrome P-450 occurred in
rats after three daily i.p. injections with 6 mg dichlorvos/kg bw
(Purshottam & Kaveeshwar, 1982) but no effect on liver microsomal
UDP-Glucuronyl transferase was found in mice 4 hours after a single
i.p. injection of 25 mg/kg bw (Yoshida et al., 1976).
Pre-treatment of rats with three daily i.p. injections of
sodium phenobarbital did not significantly change mortality and
plasma ChE inhibition caused by an i.p. injection of dichlorvos (20
mg/kg bw) (Purshottam & Kaveeshwar, 1979).
Short- and long-term studies with mice, rats or dogs dosed
orally or intraperitoneally with dichlorvos did not show any effect
on microsomal drug metabolizing enzymes (Witherup et al., 1967;
Uchiyama et al., 1975; Farber et al., 1975).
Table 4. Mutagenicity tests on microorganisms in vitro
Test system Test object Concentration Purity Results 1 Reference
Mitotic A. nidulans strain p ? ? + Morpurgo et al., 1979
non-disjunction
and crossing over
Recombinant assay B. subtilis H17 Rc+ 2 mg/plate ? - Shirasu et al., 1976
M45 Rec- " + " "
Forward mutation A. nidulans strain 35 14 mg/disc ? + Bignami et al., 1977
E. coli B 5-25 mmol/l 95% +2 Wild, 1973
Gal RS ? ? + Fahrig, 1974
K12(5-MT) 0.3-3.2 mmol/l ? + Mohn, 1973
S. coelicolor A 3(2) his A1 5.6 mg/disc 99.9% + Carere et al., 1978a, 1978b
Reverse mutation E. coli B/r WP2 5 mg/plate > 97% +3 Moriya et al., 1978
S. typhimurium TA 1535 5 mg/plate > 97% +4 " "
E. coli WP2 hcr up to 5 mg/plate ? +3 Moriya et al., 1983
S. typhimurium TA 98 up to 5 mg/plate ? - " "
TA 100 " " ? + " "
TA 1535 " " ? ? " "
TA 1537 " " ? - " "
TA 1538 " " ? - " "
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation (cont'd) E. coli B/r Wp2( ); SR714 0.04-2.3 mmol/l 99% +3 Houk & DeMarini, 1987
CM 561 0.2% " +5 Bridges et al., 1973
CM 571; CM 611 " " -6 " "
WP2 " " +5 " "
WP2 uvr A " " +5 " "
WP2 micro drop of ? -7 Dean, 1972a
analytical grade,
technical grade
or 10% acqueous
solution/plate
K12HfrH 0.1% 97.5% + Voogd et al., 1972
C. freundii 425 0.05% or 0.1% " +8 " "
E. aerogenes 0.1% " + " "
K. pneumoniae 0.05% oe 0.1% " + " "
S. typhimurium 64-320 " " + " "
S. marcescens Hy/alpha 13 1.25-5 mg/disc ? + Dean, 1972a
Hy/alpha 21
E. coli WP2 WP2 5 µg/ml ? +9 Green et al., 1976
hcr+/hcr- 20-25 µl/disk 50% commercial +10 Nagy et al, 1975
WP2 5 mg/plate formulation " "
hcr+/hcr- ?
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation S. typhimurium TA 1535 5 mg/plate ? + Shirasu et al., 1976
(cont'd) TA 1536 " ? + " "
TA 1537 " ? - " "
TA 1538 " ? - " "
E. coli WP2 5 µl/plate11 ? + Hanna & Dyer, 1975
WP2 uvr A " ? + " "
WP 67 " ? + " "
CM 561 " ? - " "
CM 571 " ? - " "
CM 611 " ? - " "
S. typhimurium TA 1530
TA 1535 5 µl/plate ? + Hanna & Dyer, 1975
his C117 " ? + " "
his G46 " ? - " "
" ? - " "
P. aeroginusa PAO 38 0.08 mol/l ? + Dyer & Hanna, 1973
S. typhimurium his C117 0.03 mol/l ? + " "
S. typhimurium TA 98 ? ? -3 Braun et al., 1982
TA 100 ? ? +3 " "
TA 1535 ? ? -3 " "
TA 1536 ? ? -3 " "
TA 1537 ? ? -3 " "
TA 1538 ? ? -3 " "
TA 98 0.1-6.6 mg/plate12 99% -3 Chan, 1989:
Zeiger et al., 1988
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
TA 100 " " " +3 Carere et al., 1978a,b
TA 1535 2.8 mg/plate 99.9% -3 " "
TA 1536 " " " -3
TA 1537 " " "
S. typhimurium TA 1538 2.8 mg/plate 99.9% -3 Carere et al., 1978a,b
TA 1535 1.5 mg/ml 99.9% +
Schizosaccharmoyces pombe 1.5-14 mmol/plate13 > 99% +3 Gilot-Delhalle et al.,
ade 6 1983
Gene conversion S. cerevisiae D4 2-8 mg/ml > 97% +14,15 Dean et al., 1972
D4 6-40 mmol/l ? +2 Fahrig, 1973, 1974
632/4 ? ? - Guerzoni et al., 1976
+16 Griffin & Hill, 1978
DNA strand breaks E. coli K-12CR34Co1E1 1 mg/ml ?
Growth inhibition E. coli W3110 pol A+/pol A- 6.4 mmol/l ? + Rosenkranz, 1973
P. mirabilis PG 273; PG 713 ? ? +15 Braun et al., 1982
1 Without metabolic activation except where noted.
2 Positive controls (methyl methanesulfonate 1-4 mmol/l) yielded expected positive results.
3 Both with and without metabolic activation.
4 Only without metabolic activation.
5 Methyl methane sulfonate (0.04%) yielded positive responses.
6 Methyl methane sulfoante (0.04%) yielded negative responses.
7 Methyl methane sulfonate, N-methyl-N'-nitro-N-nitroso guanidine yielded positive response.
8 At 0.1%
9 Positive controls (methyl methanesulfonate 0.5-2.0 µg/ml) yielded expected positive results.
Table 4 (contd)
10 Positive controls (N-methyl-N'-nitro-nitroso guanidine, N-nitroso-N-methylurethane and acridium chloride)
yielded positive responses.
11 Toxic dose.
12 Toxic effect at 3.3 mg/plate.
13 The LD50 was 5.5 mmol/l.
14 Positive controls (ethyl methane sulfonate) yielded positive responses.
15 From 4 mg/ml.
16 Methyl methane sulfonate (2-10 mmol/l), N-methyl-N'-nitro-N-nitroso guanidine (3.4-6.8 mmol/l) yielded positive responses.
Table 5. Mutagenicity tests in mammalian cells in vitro
Test system Test object Concentration Purity Results Reference
Viral transformation Syrian hamster embryo 0.05-0.45 mmol/l ? +1 Hatch et al., 1986
cells/adenovirus SA7 cytotoxic
Gene mutation Chinese hamster V79 cells up to 1 mmol/l ? - Wild, 1975
(azaguanine resistance) 1.25-5 mmol/l ? +2 Aquilina et al., 1984
Mouse lymphoma L5178Y cells 6.25-50 nl/m3 ? +4,5 Chan, 1989
(trifluorothymidine resistance) 12.5-200 nl/m6 +5,7 Chan, 1989
DNA strand breaks Chinese hamster V79 cells 0.2% ? +8 Green et al., 1974
Sister chromatid exchange Primary rat tracheal epithelial cells 5-160 µg/ml9 93.9% +10,11 Lin et al., 1988
Chinese hamster ovary cells 0.03 and 0.1 mmol/l 98 + Nishio & Uyeki, 1981
0.1-0.5 mmol/l > 98% + Tezuka et al., 1980
0.3-1000 µg/ml ? +12 Wang et al., 1988
1-50 µg/ml ? +13 Chan, 1989
Human lymphocytes 2.5-10 µg/ml 99% - Nicholas et al., 1978
Human fetal lung fibroblasts 99% - Nicholas et al., 1978
Chromosomal aberrations Rat tracheal epithelial cells 5-160 µg/ml9 93.9% +11,14 Lin et al., 1988
Chinese hamster lung fibroblasts ? ? + Ishidate et al., 1981
Chinese hamster V79 cells 0.1-0.5 mmol/l > 98% +15 Tezuka et al., 1980
Chinese hamster ovary cells 16-160 µg/ml ? +16 Chan, 1989
Human lymphocytes 1-40 µg/ml > 99% -17 Dean, 1972b
Table 5 (contd)
Test system Test object Concentration Purity Results Reference
Unscheduled DNA synthesis Human lymphocytes 5-500 µg/ml 99.8% + Perocco & Fini, 1980
EUE human cells 6.5-650 mmol/l ? +18 Aquilina et al., 1984
DNA (sedimentation coefficient) Calf thymus DNA 0.1% ? + Rosenkranz &
Rozenkranz, 1972
DNA (thermolabile regions) Calf thymus DNA 45 mmol/l 99% - Olinski et al., 1980
DNA (resistance to micrococcal) Chinese hamster ovary cells 10 mmol/l ? + Nishio & Uyeki, 1980
1 Positive controls (benzo(a)pyrene 0.001-0.002 mmol/l) yielded expected positive responses.
2 Positive controls (ethyl methan sulfonate 20 mmol/l) yielded expected positive responses.
3 Growth inhibition from 12.5 nl/ml.
4 From 12.5 nl/ml.
5 Positive controls (methyl methanesulfonate 5 nl/ml) yielded expected positive responses.
6 Growth inhibition from 100 nl/ml.
7 From 100 nl/ml.
8 Negative at lower concentrations.
9 50% mortality was observed at 80 µg/ml.
10 From 10 µg/ml.
11 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 0.25-1 µg/ml, 50% mortality at 0.5 µg/ml)
yielded expected positive responses.
12 From 40 µg/ml.
13 From 10 µg/ml. When incubated with S9, positive response at 50 µg/ml.
14 From 80 µg/ml.
15 At 0.5 mmol/l.
16 At 160 µg/ml.
17 Cytotoxicity was observed at 5-40 µg/ml.
18 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine) yielded expected positive responses.
19 Possible index of DNA alkylation.
20 Possibly indicating structural rearrangement of chromatin.
Table 6. Mutagenicity tests in vivo
Test system Test object Concentration Purity Results* Reference
Crossing over/ Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recombination ++++/dp b cn bw
Sex-linked Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recessive lethal Sobels & Todd, 1979
mutation
Oregan K 0.01-0.1 ppm in food ? -2 Kramers & Knapp, 1978
commercial formulation
Chromosomal Drosophila melanogaster 1 ppm in food ? + Gupta & Singh, 1974
aberration
Autosomal recessive Drosophila melanogaster 0.1-0.75 ppm in food for ? + Hanna & Dyer, 1975
levels 18 months
Host-mediated assay Salmonella typhimurium 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
G46 His- in mice (NMRI)
Salmonella typhimurium 8-10 mg/kg bw p.o. 97.5% -3 Voogd et al., 1972
(64-320) in mice (Swiss)
Serratia marcescens 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
(a 21 Leu-) in mice (NMRI)
Sacchoromyces cerevisiae 50 or 100 mg/kg bw p.o. > 97% -3 Dean et al., 1972
(D4) in mice (CF1)
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Dominant lethal Female mice (CF1) 0, 25 or 50 mg/kg bw p.o. > 97% -4 Dean & Blair, 1976
0, 2 or 8 mg/m3 inhalation, > 97% - Dean & Blair, 1976
from weaning to 11 weeks
of age
Male mice (ICR/Ha Swiss) 0, 5 or 10 mg/kg bw/day ? - Epstein et al., 1972
p.o. for 5 days
(8 weeks of mating)
Single i.p. injection of ? - Epstein et al., 1972
13 or 16.5 mg/kg bw
(8 weeks of mating)
Male mice (Q) 2 ppm in drinking water, 99% - Degraeve et al., 1984a
5 days/week for 7 week
10 mg/kg bw i.p. 99% -5 Moutschen-Dahmen et al., 1981
Male mice (CF1) 30 or 55 mg/m3 inhalation > 97% - Dean & Thorpe, 1972b
for 16 h
2.1 or 5.8 mg/m3 inhalation > 97% -6 Dean & Thorpe, 1972b
for 23 hours/day for 4 weeks
Sister chromatid Male mice (B6C3F1) 5-35 mg/kg bw i.p. 99% -7 Kligerman et al., 1985
exchange peripheral lymphocytes
Male mice (B6C3F1) 6-40 mg/kg bw i.p. ? -8 Chan, 1989
bone marrow cells
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Micronucleus test Mice (Swiss Webster) 0.0075-0.015 mg/kg bw/day ? -9 Paik & Lee, 1977
i.p. for 2 or 4 days
Micronucleus test Mice (HRA/Skh, hairless) skin painting with tech.grade +10 Tungul et al., 1991
(in vitro/in vivo) skin keratinocytes 0-228 µg
(in 100 µl acetone)
Chromosomal Chinese hamster, both 10-15 mg/kg bw p.o. > 97% -11 Dean & Thorpe 1972a
aberrations sexes bone-marrow cells
Male mice (Q) 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
bone-marrow cells 5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Degraeve et al., 1984b
Degraeve et al., 1984b
Male mice (B6C3F1) bone 6-40 mg/kg bw i.p. ? -12 Chan, 1989
marrow cells
Female Syrian golden 0, 3, 6, 15 or 30 mg/kg 50% +13 Dzwonkowska & Hübner, 1986
hamster commercial
formulation
bone-marrow cells bw i.p.
Mice (CF1), both sexes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h
5 mg/m3 inhalation > 97% > 97% -11 Dean & Thorpe, 1972a
23 hours/day for 21 days
Male Chinese hamsters 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h.
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Chromosomal Mice (Q) spermatocytes 2 ppm in drinking-water 99% - Moutschen-Dahmen et al., 1981;
aberrations 5 days/week for 7 weeks Degraeve et al., 1984a
(cont'd)
Chinese hamsters 15 mg/kg bw p.o. > 97% -11 Dean & Thorpe, 1972a
spermatocytes
Mice (Q) spermatocytes 10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984a
Mice (CF1) spermatocytes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
for 16h
5 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
23h/day for 21 days
Chinese hamster 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
spermatocytes for 16 h
Mice (CF1) spermatogonia 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984b
DNA strand breaks Rats (Wistar), both 10 mg/kg bw i.p. 99.8% -14 Wooder & Creedy, 1979
sexes rat liver cell DNA
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Unscheduled DNA Male rats (F344) 0, 2, 10 or 35 mg/kg ? - Mirsalis et al., 1989
synthesis hepatocytes bw p.o.
Mice (B6C3F1) (both sexes) 0, 10, 20, 40 or > 98% -15 Bedford, 1991
forestomachrats 100 mg/kg bw p.o.
1 Approximate LD50
2 Positive controls (2.5 mmol/l ethyl methanesulfonate) yielded expected positive responses.
3 Positive controls (400 mg/kg bw p.o. ethyl methanesulfonate) yielded expected positive responses.
4 Positive controls (100 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
5 Positive controls in 2nd and 5th week of mating.
6 Positive controls (2000 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
7 Positive controls (2-acetylaminofluorene, ethyl methane sulfonate and N-nitroso morpholine) yielded positive results.
All animals treated with 35 mg/kg bw i.p. died.
8 Positive controls (ethyl methane sulfonate 100 mg/kg bw p.o.) yielded expected positive responses.
9 Positive controls (cyclophosamide 30-240 mg/kg bw i.p.) yielded expected positive responses.
10 Recovery of plated cells in vitro after a single topical application of dichlorvos was about 50% of controls.
11 Positive controls (endoxan 100-200 mg/kg bw i.p.) yielded expected positive responses.
12 Positive controls (ethyl methane sulfonate 300-375 mg/kg bw p.o.) yielded expected positive responses.
13 LD50 = 30 mg/kg bw i.p. No dose related response. Positive controls (cyclophosphamide 40 s. mg/kg bw i.p.)
yielded expected positive responses.
14 Positive controls (methyl methane sulfonate 30-60 mg/kg bw i.p.) yielded expected positive responses.
15 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 200 mg/kg bw p.o.) yielded weakly positive results.
Dichlorvos treatment induced a hyperplastic response similar to that induced by the non-genotoxic carcinogen butylated
hydroxyamisole (300 mg/kg bw p.o.).
Special studies on metabolites
Acute and short-term toxicity studies
The intraperitoneal toxicity of metabolites of dichlorvos in
female mice is considerably less than that of dichlorvos. The LD50
(mg/kg bw) was 250 for dichloroacetic acid, 440 for
dichloroacetaldehyde and 890 for dichloroethanol. For other
metabolites it was > 1000 mg/kg bw. A short-term inhalation study
with dichloroacetaldehyde in rats did not show adverse effects at
concentrations up to 2 mg/m3 for 30 days (WHO, 1989).
Methylating reactivity
In a quantitative colour test in which methylation of
4-(p-nitrobenzyl) pyridine was measured to predict DNA alkylating
potential, desmethyl-dichlorvos, dimethyl phosphate,
dichloroethanol, dichloroacetaldehyde and dichloroacetic acid gave
no reaction (Bedford & Robinson, 1972).
Mutagenicity
In a rec-type repair test with Proteus mirabilis strains
PG713 and PG273, desmethyldichlorvos (10 or 40 µmol/plate) did not
induce base-pair substitutions or other DNA damage (Braun et al.,
1982).
Dichloroacetaldehyde (DCA) appeared to be mutagenic in the
Salmonella test using S. typhimurium TA 100 and TA 1535. The
mutagenicity decreased in the presence of a microsomal activation
system (Lofroth, 1978; Bignami et al., 1980). It was also
positive when tested in concentrations of 10-40 µg/plate in a
forward and a back mutation system in S. coelicolor and two
forward mutation systems in A. nidulans (Bignami et al., 1980).
DCA (0.01%) was not mutagenic to K. pneumoniae in a fluctuation
test (Voogd et al., 1972). DCA did not induce unscheduled DNA
synthesis in the human epithelial-like cell EUE as well as
ouabain-resistant mutations in cultured V-79 Chinese hamster cells
(Aquilina et al., 1984). In a dominant lethal assay with DCA
administered as a single i.p. injection of 176 mg/kg bw to two
strains of male mice (AB Jena-Halle and DBA), positive effects were
reported (Fischer et al., 1977). It should be noted, however,
that the dose used would never be reached after dichlorvos treatment
(i.p. LD50 = 28-41 mg/kg bw). Ramel (1981) reported negative
results in a dominant lethal study with another strain of mice.
No evidence for mutagenicity of dichloroethanol was obtained in
S. typhimurium TA 100 and TA 1535 (Lofroth, 1978; Bignami et al.,
1980). In S. coelicolor (test doses 60-80 µg/plate) and A.
nidulans (test doses 10-50 µg/plate), dichloroethanol was a weak
mutagen (Bignami et al., 1980). Dichloroethanol (1 M and 0.1%)
was mutagenic to K. pneumoniae in a fluctuation test (Voogd et
al., 1972).
Special studies on skin sensitization
In the guinea-pig maximization test by Magnusson & Kligman,
using intradermal and topical induction concentrations of 5% and 25%
in water respectively, a 0.5% challenge concentration caused
sensitization in 30-40% of the animals but a 0.05% challenge
concentration caused no sensitization (Matsushita et al., 1985).
Special studies on testes
Mice
Groups of 14 male NMRI/Han mice received either a single oral
dose of 40 mg/kg bw dichlorvos in olive oil or 18 daily oral doses
of 0 or 10 mg/kg bw dichlorvos in olive oil.
On days 9,18, 27, 36, 54 and 63, two animals from each group
were killed and their testes examined histologically. A significant
increase in the number of damaged seminiferous tubules
(desquamation, decreases in cell population, "holes") was observed
in both dichlorvos groups. The supporting Sertoli cells were also
damaged, which may have resulted in the above effects. In addition,
there was an increase in the number and hypertrophy of Leydig cells.
No explanation was given for these effects (Krause & Homola, 1972,
1974).
An increased incidence, just above background, of sperm
abnormalities, was observed in a screening study on hybrid mice
given five daily i.p. injections of 10 mg/kg bw dichlorvos
(approximately half the LD50). At lower doses, 1 mg/kg bw, the
number of sperm abnormalities was either similar to or lower than
those in the controls (Wyrobek & Bruce, 1975).
Rats
Groups of 16 male juvenile Wistar rats received either 20 mg/kg
bw dichlorvos in olive oil on days 4 and 5, 10 mg/kg bw dichlorvos
in olive oil daily from days 4 to 23, or 0.1 ml olive oil daily from
days 4 to 23. On days 6, 12, 18, 26, 34, and 50 of life, two rats
from each group were sacrificed. Histological examination of the
testes showed slight reduction in the number of spermatogenic cells
and Leydig cells. All changes were reversed by the 50th day of
life. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells (Krause et al.,
1976).
In a subsequent experiment measurement of testosterone
concentrations in the testes, and luteinizing hormone (LH) and
follicle stimulating hormone (FSH) concentrations in serum showed no
differences between treated animals and olive-oil treated controls
(Krause, 1977). However, in this study the use of a different
dosing regimen (10 mg/kg bw by gavage every other day for 2 weeks)
prevented a strict comparison with the earlier study by Krause et
al. (1976).
Fifty-five male Wistar rats (aged 5 months) were orally
administered dichlorvos at levels of 5 or 10 mg/kg bw every other
day for 8 weeks. Eleven rats were killed every 4 weeks. No change
was seen in body-weight gain or testes weight. The score values of
the seminal cellular system decreased after 4-8 weeks of treatment,
but were restored 8 weeks after the end of treatment (Fujita et
al., 1977).
Miscellaneous studies
Effects of inhaled ChE inhibitors, including dichlorvos
(dissolved in acetone), on bronchial tonus were studied in young
adult rats exposed head-only. Bronchoconstriction did not occur at
toxicologically significant doses. An increase in response to
acetylcholine provocation test was observed (Pauluhn et al.,
1987).
The effect of diet on the toxicity of dichlorvos was
investigated using young male rats kept for 30 days on the following
diets: high protein (HPD), low protein (LPD), high fat (HFD), and
standard (SD). Growth rates were normal except for a slightly
decreased body-weight gain in the HFD group. A single i.p.
injection of 50 mg/kg bw dichlorvos led to slightly higher mortality
in LPD rats and slightly lower mortality in HPD rats, compared with
SD rats (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or
HPD for 30 days. At the end of this period, a single i.p. dose of
dichlorvos (20 or 30 mg/kg bw) was administered. No difference in
plasma and erythrocyte ChE inhibition was found. In the case of the
HPD, the spontaneous recovery of plasma and erythrocyte ChE activity
was slightly reduced (Purshottam & Srivastava, 1984).
Observations in humans
In vitro studies
The sensitivity to inhibition by dichlorvos of erythrocyte and
brain ChE was higher than that of plasma ChE, the I50s being about
10-8 and 10-7 mol/l, respectively (Skrinjaric-Spoljar et al.,
1973; Carter & Maddux, 1974; Lotti & Johnson, 1978; Boyer, 1978;
Casale et al., 1989). The rate of phosphorylation of human
erythrocyte ChE was similar to that of rat brain ChE (1.2 x 105 and
1.5 x 105 mol/l/min at 37 °C, pH 7.4-7,6, respectively).
The rate of dichlorvos hydrolysis by human plasma at 37 °C was
found to be 7-11 µmol/hour/ml (Reiner et al., 1980; Traverso et
al., 1989).
Studies on volunteers
Six healthy male volunteers were given single oral doses of 7.5
mg/kg bw of metrifonate. Concentrations of metrifonate (trichlorfon)
and dichlorvos, which is a metrifonate rearrangement product, were
determined in whole blood at different times for up to 24 hours.
The half-life of metrifonate was about 2 hours. The concentrations
of dichlorvos closely followed those of metrifonate with a constant
ratio of 0.01-0.02. However, a half-life for dichlorvos of 2 hours
cannot be accepted because dichlorvos is continuously formed from
metrifonate (Abdi & Villen, 1991).
Groups of five young men received total daily doses of 1, 1.5,
2 or 2.5 mg dichlorvos in corn oil per person, divided in two
gelatin capsules administered at 08.00 h and at 15.00 h for 28 days.
A further group of ten men received 1.5 mg/day for 60 days. Control
groups of two men per dose level received gelatin capsules with corn
oil. Plasma and erythrocyte ChE activities were determined twice
weekly before, during and after dosing. Once each week a medical
interview, complete blood count, and urinalysis were done on each
subject. Before dosing and after the last dose SGOT, ALP, PT,
thymol turbidity, and total bilirubin were determined.
The 2.5 mg/day dose produced a decrease in plasma ChE activity
from the second week of treatment; dosing was discontinued after 20
days when plasma values showed a 30% decrease. The plasma ChE
activity returned to control values 15 days after dosing was ended.
The 2 mg/day dose produced also a reduction in plasma ChE activity
from the second week of treatment which reached a maximum inhibition
of 29% the second day after the last dose. The group receiving 1.5
mg/day for 28 days showed no change in plasma ChE activity while in
the group dosed for 60 days this activity was inhibited by 27%
compared to the control values. None of the groups showed an effect
on erythrocyte ChE activity, haematology, clinical chemistry or
urinalysis. The NOAEL in this study was the highest dose tested of
2.5 mg/day/man (approximately 0.03 mg/kg bw/day) based on the
absence of inhibition of erythrocyte ChE activity (Rider, 1967;
Rider et al., 1967, 1968).
Two groups of six men (21-45 years of age, 64-106 kg bw)
received 0.9 mg dichlorvos three times a day for 21 days, either in
a pre-meal capsule filled with cottonseed oil or in a gelatin salad
consumed during the course of the meal. Control subjects were dosed
with either cotton seed oil capsules or plain gelatin. No
cholinergic signs or symptoms were observed. Plasma ChE activity
was inhibited by 30-40%, the inhibition being higher when dichlorvos
in cotton seed oil was ingested before the meal. The half-life for
the regeneration of plasma ChE activity was 13.7 days. Erythrocyte
ChE activity was not reduced. The NOAEL in this study was 0.04
mg/kg bw/day, based on absence of erythrocyte cholinesterase
inhibition (Boyer et al., 1977).
A number of studies have been done with a slow-release PVC
formulation of dichlorvos intended for use as an anthelminticum.
Single oral doses of above 4 mg dichlorvos/kg bw resulted in
inhibition of erythrocyte ChE activity 24 hours after application,
the maximum being 46% at 32 mg/kg bw. Plasma ChE activity was
affected at single doses of 1 mg/kg bw (50% inhibition) and above.
However, no dichlorvos-related symptoms were observed. Repeated
oral dosing for 7 days produced clinical symptoms with doses of 8
mg/kg bw/day and above. Plasma ChE activity was inhibited by about
80% at all dose levels (1-16 mg/kg bw/day). Erythrocyte ChE
activity showed a dose-related decrease from 5-30% at 1 mg/kg bw/day
up to 50-80% inhibition at the highest dose. Blood count, urine,
liver function, PT and BUN were normal in all studies (Hine &
Slomka, 1968, 1970; Pena-Chavarria et al., 1969; Slomka & Hine,
1981).
In children (aged 7-18 years) orally treated with metrifonate
(7.5, 10.0 or 12.5 mg/kg bw), the half-lives of recovery of
inhibited erythrocyte AChE and plasma ChE were 15 and 6.7 days,
respectively (Reiner & Plestina, 1979). It is known that the
anticholinesterase activity of metrifonate is due to its
decomposition to dichlorvos (Reiner et al., 1975). Similar
half-lives for plasma ChE have been described by Boyer et al.,
(1977) after repeated oral dosing with dichlorvos and by Bisby &
Simpson (1975) after cutaneous exposure of a sprayman to dichlorvos.
This longer half-life contrasts with faster in vitro half-lives,
which are similar to those of the rodent (Skrinjaric-Spoljar et
al., 1973).
Several inhalation studies have been carried out and are
summarized by WHO (1989). These studies confirmed that plasma ChE
is more sensitive to inhibition by dichlorvos than RBC ChE. The
latter was found inhibited when the dichlorvos dose (concentration x
time) was higher than about 1500 mg/m3/min (WHO, 1989).
Thirteen men (31-53 years of age) were exposed to dichlorvos
strips (20% dichlorvos; 1 strip per 30 m3) for 3 months. No
biologically or statistically significant changes were observed in
their electromyography results nor in their whole blood ChE activity
(Ottevanger, 1975).
Poisoning incidents
Fournier et al. (1978) reported that dichlorvos was detected
in blood in three cases of human intoxication within 24 hours after
poisoning (exact timing not reported).
A woman who intentionally ingested an estimated 100 mg
dichlorvos/kg bw survived following intensive care for 14 days
(Watanabe et al., 1974). A suicide with a dichlorvos dose of
about 400 mg/kg bw succeeded in spite of treatment (Shinoda et al.,
1972). A female patient, aged 35 years, who had accidentally
ingested 60 g fluid Divipan (dichlorvos concentration not reported),
was comatose for a week and recovered slowly. Clinical and
electrophysiological examinations showed a pure motor form of
neuropathy, according to the authors (Vasilescu & Florescu, 1980).
Three cases of poisoning with dichlorvos taken orally in unspecified
but high quantities have been reported from India. The patients
first showed severe cholinergic signs for a few days. After
recovery, delayed neurotoxicity developed. Nerve conduction studies
showed a severe axonal degeneration (Wadia et al., 1985, 1987).
Thirteen cases of dichlorvos ingestion, either accidental or
deliberate, were reported from a hospital in Beijing, China. The
patients included 10 women and three men, aged 11-38 years. Twelve
of the 13 patients ingested 25-50 ml of dichlorvos (80% purity).
Ten cases were associated with acute pulmonary oedema. Two patients
died because OP poisoning was not diagnosed in time. The others
recovered completely 2-5 days after treatment (Li et al., 1989).
Occupational Exposure
A number of fatal and non-fatal dichlorvos poisoning cases have
been described and summarized by WHO (1989). Two workers who failed
to promptly wash off the concentrated formulation of dichlorvos,
which splashed on to their skin, died subsequently. However, in
those cases where the spilled solution was washed off immediately,
the victims showed symptoms of intoxication but recovered after
treatment. Occupational exposure of spraymen entails both dermal
and respiratory absorption of dichlorvos. When appropriate
protective equipment was not used, inhibition of plasma ChE and less
frequently of RBC ChE was found. Sometimes mild, short lasting,
cholinergic symptoms and signs have been reported (WHO, 1989).
Each of 13 pest-control operators carried out urban
pest-control work for one day in 4 houses using 230-330 g dichlorvos
as aerosol and 40-50 g dichlorvos as emulsion spray. At the end of
the day's work, an operator had an average dichlorvos residue of 0.8
mg/m2 on the back, 0.4 mg/m2 on the chest, and 11 mg/m2 on the
respirator filter. Urinary dimethylphosphate excretion in 3
applicators ranged from 0.32 to 1.39 micrograms on the day of
treatment, but approached the level of detection by the following
morning. Blood and urine analyses revealed no changes in various
clinical parameters, including serum cholinesterase levels (Das et
al., 1983).
COMMENTS
Dichlorvos is rapidly absorbed by all routes of exposure and
rapidly degraded. The metabolic pathways of dichlorvos are similar
in mammalian species, including humans. Metabolites are rapidly
excreted or incorporated into natural enzymatic pathways.
Dichlorvos has a marked acute oral toxicity with typical
cholinergic signs and has been classified by WHO as highly
hazardous.
Rat erythrocyte and brain cholinesterase inhibited by
dichlorvos spontaneously reactivates with a half-life of about two
hours both in vitro and in vivo.
Several carcinogenicity studies in mice and rats using routes
other than gavage were negative, even when doses causing signs of
toxicity were used. It should be noted that two squamous-cell
carcinomas of the oesophagus have been observed in treated mice in
one study.
In a carcinogenicity study in mice dichlorvos administered by
corn oil gavage (0, 10 or 20 mg/kg bw/day to males, and 0, 20 or 40
mg/kg bw/day to females), caused forestomach papillomas
(statistically-significant positive trend with increased incidence
in the high-dose female group). Elements of the mechanism by which
these papillomas might arise have not been established, but the
induction of hyperplasia in the forestomach was demonstrated.
Additionally, genotoxic effects might occur at high local
concentrations of dichlorvos (see below) as can be obtained in
gavage dosing but not in dietary exposure. Based on the increased
incidence of forestomach papillomas, the NOAEL was 10 mg/kg bw/day.
In a two-year feeding study in rats (0, 0.1, 1, 10, 100 or 500
ppm), no neoplastic lesions were attributed to treatment. The
NOAEL, based on brain cholinesterase inhibition, was 100 ppm (actual
concentration 47 ppm, equivalent to 2.4 mg/kg bw/day).
In a carcinogenicity study in Fischer 344 rats, dichlorvos
administered by corn oil gavage (0, 4 or 8 mg/kg bw/day) caused an
increased incidence of pancreatic adenomas (statistically
significant in males only), mononuclear cell leukemias
(statistically significant in males only, no dose response) and
mammary gland adenomas or fibroadenomas (females only, no dose
response, statistically-significant in the low-dose group only).
The Meeting observed that the incidence of pancreatic acinar
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia which is usually high and variable in
this strain of rats, was also of questionable biological
significance. The doses used significantly inhibited plasma, but
not erythrocyte, cholinesterase activity when measured three hours
after treatment. However, given the rapid recovery of erythrocyte
cholinesterase activity after inhibition by dichlorvos, the timing
might have underestimated the inhibition.
Dichlorvos has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. These data indicate that
dichlorvos is genotoxic in bacteria and cultured mammalian cells,
but that it is not clastogenic in vivo except under conditions
where an unusually high tissue dose can be attained. Dichloro-
acetaldehyde, a major metabolite of dichlorvos, is a weak bacterial
mutagen. Positive results have been reported in mice given a dose
of dichloroacetaldehyde far greater than that which could derive
from sublethal doses of dichlorvos. Dichlorvos has been shown to
methylate DNA in vitro at a rate that is 8-9 orders of magnitude
lower than the rate of phosphorylation. Therefore, DNA alkylation
is not likely to occur at doses of dichlorvos which are not
inhibitory to erythrocyte/brain cholinesterase.
A three-generation reproduction study in rats was negative at
doses up to 235 ppm in the diet, equivalent to 12 mg/kg bw/day. A
one-litter, one-generation study in mice in which dichlorvos was
administered by inhalation at doses which caused > 90% plasma
cholinesterase inhibition, but no signs of toxicity, was negative.
Dichlorvos caused reversible damage of seminiferous tubules, Leydig
and Sertoli cells at oral doses of 10 mg/kg bw daily for 18 days in
mice and at 5 mg/kg bw and above every other day for 8 weeks in
rats.
Dichlorvos appeared not to be teratogenic in mice, rats and
rabbits at doses which caused maternal toxicity.
Dichlorvos caused delayed polyneuropathy in hens at doses much
higher than the unprotected LD50. Cases of delayed polyneuropathy
also have been reported in humans after severe intoxications.
In humans, the rate of dichlorvos hydrolysis by plasma is
similar to that in rats. The rate of recovery of inhibited
erythrocyte and plasma cholinesterase activity in humans given
dichlorvos is much slower than in rats. Half-lives of recovery are
about 15 days in humans and about two hours in rats. A daily dose
of 1 mg/kg bw to male human volunteers for seven days caused 5-30%
inhibition of erythrocyte cholinesterase. The NOAEL in humans,
based on absence of erythrocyte cholinesterase inhibition in 12
volunteer males for 21 days was 0.04 mg/kg bw/day.
In 1986, the Joint Meeting discussed the significance of
carcinogenicity studies for organophosphorus pesticides and the
requirements for further studies (Section 3.1 of Annex 1, reference
47). At that time none of the organophosphorus pesticides had
caused a carcinogenic response in experimental animals. That Joint
Meeting recommended that, depending upon future evaluation on a case
by case basis, further consideration should be given to the need for
carcinogenicity studies for organophosphates.
In assessing the potential hazard to humans of residues of
dichlorvos, the following considerations were taken into account in
view of the weakly positive results in the gavage carcinogenicity
study in mice.
Organophosphorus esters used as insecticides react with
biological molecules by means of phosphorylation of serine
hydrolases and of alkylation of macromolecules. Phosphorylation of
acetylcholinesterase and alkylation of DNA are considered to account
for the acute cholinergic toxicity and initiation of the
carcinogenic process, respectively. These biochemical reactions
occur at different rates. When the rate of phosphorylation is
substantially higher than the rate of alkylation, in vivo
genotoxic effects are unlikely to occur because effective doses
cannot be achieved due to acute toxicity. Dichlorvos meets these
criteria, the rate of phosphorylation of acetylcholinesterase being
much faster (eight orders of magnitude) than that of alkylation of
several macromolecules, including DNA. Hence positive mutagenicity
tests were seen only in vitro and, as indicated in the 1986 Joint
Meeting report, carcinogenicity studies are unlikely to give more
information. The weak carcinogenic response of dichlorvos obtained
in mice in a corn oil gavage study should be interpreted as a local
effect of dichlorvos.
Information on comparative cholinergic toxicity might be of
critical relevance for the extrapolation of toxic effects (other
than acute effects) of organophosphates in experimental animals to
humans. The characteristics of the interactions of a given compound
with acetylcholinesterase (rates of phosphorylation, spontaneous
reactivation and ageing) from different species can be compared in
vitro. Also, the in vivo rate of reappearance of blood
acetylcholinesterase activity can be measured. In some cases,
metabolic degradation of organophosphates can be assessed
comparatively by measuring the level of serum A esterase, which
hydrolyses a given compound. All these data enable an improved
assessment of cholinergic toxicity of organophosphates in different
species. This knowledge may be of special significance in the case
of dimethyl phosphates since the rates of in vivo reactivation
vary substantially across species. Therefore, chronic dosing is
more critical for extrapolation from animal data to humans. In a
repeated dose regime, the longer the half-life of reactivation the
more rapid and/or more toxic will be the resulting effect (i.e. in a
chronic dosing regime, humans will survive much lower doses of
dichlorvos causing, when given alone, peak erythrocyte/brain
cholinesterase inhibition than those which can be reached in
rodents). Therefore, comparison between the in vivo rates of
recovery of enzyme activity will enable an assessment of the
repeated doses of compounds and the resulting cholinesterase
inhibition, which would represent the limiting factors for other
toxicities (including mutagenicity and carcinogenicity).
In the case of dichlorvos, the Meeting considered the
extrapolation of carcinogenicity data derived in rodents and its
applicability to human safety, and concluded that the compound would
not result in chronic human health hazards at doses below those
which result in acetylcholinesterase inhibition.
The Meeting maintained the ADI, which is based on studies in
humans with a NOAEL of 0.04 mg/kg bw/day, using a 10-fold safety
factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 mg/kg bw/day (two-year study)
Rat: 47 ppm in the diet, equivalent to 2.4 mg/kg bw/day
(two-year study)
Human: 0.04 mg/kg bw/day (21-day study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abdi, Y.A. & Villen, T. (1991) Pharmacokinetics of metrifonate and
its rearrangement product dichlorvos in whole blood. Pharmacol.
Toxicol. 68: 137-139.
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti,
E., Falcone, E. & Carere, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon and dichloroacetaldehyde. Pestic. Sci. 15:
439-442.
Asperen van, K., Dekhuijzen, H.M. (1958) A quantitative analysis of
the kinetics of cholinesterase inhibition in tissue homogenates of
mice and houseflies. Biochim. Biophys. Acta, 28: 307-337.
Bedford, C.T. & Robinson, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2: 307-337.
Benford, D.J. (1991) Investigation of the genotoxic and/or irritant
effects of dichlorvos on mouse forestomach. Unpublished report no.
RI90/0405 from Robens Institute of Health and Safety, Guildford,
Surrey, U.K.. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. & Morpurgo, G.
(1977) Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutat. Res. 46: 395-402.
Bignami, M., Conti, G., Conti, L., Crebelli, R., Misuraca, F.,
Ruglia, A.M., Randazzo, R., Sciandrello, G. & Carere, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor and Asperigillus nidulans.
Chem.-Biol. Interactions, 30: 9-23.
Bisby, J.A. & Simpson, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Austr. 2: 394-395.
Blair, D., Dix, K.M., Hunt, P.F., Thorpe, E., Stevenson, D.E. &
Walker, A.I.T. (1976) Dichlorvos - a 2-year inhalation
carcinogenesis study in rats. Arch. Toxicol. 35: 281-294.
Boyer, A.C. (1978) Factors which affect the in vitro inhibition of
human plasma cholinesterase. Toxicol. Appl. Pharmacol. 41: 475-
483.
Boyer, A.C., Brown, L.J., Slomka, M.B. & Hine, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. Appl. Pharmacol. 41:
389-394.
Braun, R., Schoneich, J., Weissflog, L. & Dedek, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair - direct alkylation versus metabolic activation and
breakdown. 1. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem.-Biol.
Interact. 39: 339-350.
Bridges, B.A., Mottershead, R.P., Green, M.H.L. & Gray, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulphonate for
Escherichia coli WP2 and some derivatives deficient in DNA repair.
Mutat. Res. 19: 295-303.
Brown, V.K.H. & Roberts, M (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to
guinea-pigs. Unpublished report no. IRR TL/40/66 from Shell Research
Ltd., Sittingbourne, UK. Submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Buselmayer, W., Rohrborn, G., Propping, P. (1972) Mutagenicity
investigations with pesticudes in the host-mediated assay and the
dominant lethal test in mice. Biol. Zbl. 91: 311-325 (in German).
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M., Raschetti,
R. (1978a) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res. 57: 277-286.
Carere, A., Ortali, V.A., Cardamone, G., Morpurgo, G. (1978b)
Mutagenicity of dichlorvos and other structurally related pesticides
in Salmonella and Streptomyces. Chem.-Biol. Interactions, 22:
297-308.
Caroldi, S., Lotti, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett. 9:
157-159.
Carter, M.K., Maddux B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. Appl. Pharmacol., 27: 456-463.
Casale, G.P., Bavari, S., Connolly, J.J. (1989) Inhibition of human
serum complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam. Appl. Toxicol. 12: 460-
468.
Casebolt, D.B., Leary, S.L., Undeutsch, L. (1990) Effects of
dichlorvos treatment on mouse reproduction. Lab. Anim. Care, 40:
65-67.
Chan, P.C. (1989) Toxicology and carcinogenesis studies of
dichlorvos (CAS No. 62-73-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, National Toxicology
Program, Technical Report Series NTP TR No.342.
Chan, P.C., Huff, J., Haseman, J.K., Alison, R. & Prejean, J.D.
(1991) Carcinogenesis studies of dichlorvos in Fischer rats and
B6C3F1 Mice. Jpn. J. Cancer Res. 82: 157-164.
Chattopadhyay, D.P., Dighe, S.K., Dube, D.K., & Purnanand (1982)
Changes in toxicity of DDVP, DFP, and parathion in rats under cold
environment. Bull. Environ. Contam. Toxicol. 29: 605-610.
Cohen, S.D., Ehrich, M. (1976) Cholinesterase and carboxyl esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. Appl. Pharmacol. 37: 39-48.
Coulston, F., Griffin, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP vapors.
Unpuplished report from Albany, New York, Institute of Comparative
and Human Toxicology and Holloman, Air Force Base, New Mexico,
Intern. Center of Environmental Safety. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Das, Y.T., Taskar, P.K., Brown, H.D., Chattopadhyay, S.K. (1983)
Exposure of professional pest control operator to dichlorvos (DDVP)
and residue on house structures. Toxicol. Letters, 17: 95-99.
Dean, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on micro-organisms. Arch. Toxikol. 30: 67-74.
Dean, B.J., (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol. 30: 75-85.
Dean, B.J. & Blair,D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres
containing dichlorvos. Mutat. Res. 40: 67-72.
Dean, B.J., Thorpe, E. (1972a) Cytogenetic studies with dichlorvos
in mice and Chinese hamsters. Arch. Toxikol. 30: 39-49.
Dean, B.J., Thorpe, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxicol. 30: 51-59.
Dean, B.J., Doak, S.M.A., Funnell, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium using
Saccharomyces cerevisiae. Arch. Toxicol. 30: 61-66.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984a) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol. 56: 66-67.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Letters, 21: 315-319.
Dieter, M.P., Jameson, C.W., French, J.E., Gangjee, S. (1989)
Development and validation of a cellular transplant model for
leukemia in Fischer rats: a short-term assay for potential
anti-leukemic chemicals. Leukemia Research, 13: 841-849.
Dyer, K.F., Hanna, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res. 21: 175-177.
Dzwonkowska, A., Hubner, H. (1986) Induction of chromosomal
aberrations in the Syriam hamster by insecticides tested in vivo.
Arch. Toxicol. 58: 152-156.
Enomoto, M.F., Nakadate, M., Ninomia, K., Hayakawa, Y., Ito, H.,
Igarashi, S., Uwanuma, Y., Nakasato, R., Hatanaka, J. (1981) Studies
on carcinogenicity of DDVP (2,2-dichlorovinyl dimethyl phosphate)
mixed in drinking-water in rats. Ministry of Health and Welfare,
Tokyo, Japan (Cooperative studies on carcinogenicity test on
mutagens) (Unpublished).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325.
Fahrig, R. (1973) (Genetic effects of organophosphorous
insecticides.) Die Naturwissenschaften, 60: 50-51 (in German).
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
IARC Scient. Publ. 10: 161-181.
Farber, T.M., Marks, E.M., Cerra, F.E. (1975) Dichlorvos and
microsomal enzyme activity. Fed. Proc. 34: 785.
Fischer, G.W., Schneider,P., Scheufler, H. (1977) (Mutagenicity of
dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester, possoble metabolites of the organophosphorus
pesticide trichlorphon.) Chem.-Biol. Interactions, 19: 205-213 (in
German).
Fournier, E., Sonnier, D., Dally, S., (1978) Detection and assay of
organophosphate pesticides in human blood by gas chromatography.
Clin. Toxicol. 12: 457-462.
Francis, B.M., Metcalf, R.L., Hansen, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. Environ. Sci. Health, B20: 73-95.
Fujita, K., Matsushima, S., Abe, E., Sasaki, K., Kurosawa, K. (1977)
(Examination of the effects of dichlorvos on the testis.) Nippon
Noson Igakkai Zasshi, 26: 328-329 (in Japanese) as quoted by WHO,
1989.
Gilot-Delhalle, J., Colizzi, A., Moutschen, J., Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade 6
locus of Schizosaccharomyces pombe. Mutat. Res: 117: 139-148.
Gough, B.J., Shellenberger, T.E. (1977-1978) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors
and reactivation with oximes. Drug and Chemical Toxicol. 1: 25-43.
Green, M.H.L., Medcalf, A.S.C., Arlett, C.F., Harcourt, S.A.,
Lehmann, A.R. (1974) DNA strand breakage caused by dichlorvos,
methyl methanesulphonate and iodoacetamide in Escherichia coli and
cultured Chinese hamster cells. Mutat. Res. 24: 365-378.
Green, M.H.L., Muriel, W.J., Bridges, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res. 38: 33-42.
Griffin III, D.E., Hill, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res. 52: 161-169.
Guerzoni, M.E., Del Cupolo, L., Ponti, I. (1976) (Mutagenic
activity of pesticides). Rev. Sci. Tecn. Alim. Nutr. Um. 6:
161-165 (in Italian).
Gupta, A.K., Singh, J. (1974) Dichlorvos (DDVP) induced breaks in
the salivary gland chromosomes of Drosophila melanogaster. Current
Science, 43: 661-662.
Hanna, P.J., Dyer, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res. 28: 405-420.
Haseman, J.K., Huff, J.E., Rao, G.N., Arnold, J.E., Boorman, G.A.,
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1
(B6C3F1) mice. J. Natl. Cancer Inst. 75: 975-984.
Hass, D.K., Collins, J.A., Kodama, J.K. (1972) Effects of orally
administered dichlorvos in Rhesus monkeys. J. Am. Vet. Med. 161:
714-719.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W.,
Schechtman, L.M. (1986) Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutag. 8:
515-531.
Hine, C.H. (1962) 90-day chronic toxicity studies of Vapona
insecticide for dogs. Unpublished report from San Francisco, The
Hine Laboratories, Report no. 1, September 1. Prepared for Shell
Chemical Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd,
Temana International Ltd.
Hine, C.H., Slomka, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
Hine, C.H., Slomka, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. Appl. Pharmacol. 17:
304-305.
Horn, K.H., Teichmann, B., Schramm, T. (1987) (Studies on
dichlorvos. I. Testing of dichlorvos for carcinogenic activity in
mice.) Archiv für Geschwulstforschung, 57: 353-360 (in German).
Horn, K.H., Teichmann, B., Schramm, T., Nischan, P. (1988) (Studies
on dichlorvos. II. Testing of dichlorvos for carcinogenic activity
in rats). Archiv für Geschwulstforschung, 58: 1-10 (in German).
Horn, K.H., Teichmann, B., Schramm, T. (1990) (Studies on
dichlorvos. III. Testing of dichlorvos for co-carcinogenic activity
in mice). Archiv für Geschwulstforschung, 60: 117-124 (in German).
Houk, V.S., DeMarini, D.M. (1987) Induction of prophage lambda by
chlorinated pesticides. Mutat. Res. 182: 193-201.
IARC (1979) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Some halogenated hydrocarbons, Volume 20:
97-127.
IARC (1991) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Occupational exposures in insecticide
application, and some pesticides. Volume 53: 267-307.
Ishidate, M., Sofuni, T., Yoshikawa, K. (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monograph on Cancer
Research, 27: 95-108.
Jayasuriya, V.U. de S., Ratnayake, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inform. Serv. 50: 184-186.
Johnson, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch. Toxicol.
41: 107-110.
Jolley, W.P., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of Vapona insecticide into their daily diet.
Unpublished report from Cincinnati, The Kettering Laboratory,
January 19. Prepared for Shell Chemical Company. Submitted to WHO by
Bayer AG, Ciba-Geigy Ltd, Temana International Ltd.
Kimbrough, R.D., Gaines, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. Environ.
Health, 16: 805-808.
Kligerman, A.D., Erexson, G.L., Wilmer, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res. 157: 181-187.
Konishi, Y., Denda, A., Kitaoka, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3F1 mice. Tokyo, Ministry of
Health and Welfare, Japan (unpublished).
Kramers, P.G.N., Knaap, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of Drosophila
melanogaster. Mutat. Res. 57: 103-105.
Krause, W. (1977) Influence of DDT, DDVP and malathion on FSH, LH
and testosterone serum levels and testosterone concentrations in
testis. Bull. Environ. Contam. Toxicol. 18: 231-242.
Krause, W., Homola, S. (1972) (Influence on spermatogenesis by DDVP
(dichlorvos).) Arch. Dermatol. Forschung, 244: 439-441 (in
German).
Krause, W., Homola, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat testis after the
application of dichlorvos (DDVP). Bull. Envirom. Contam. Toxicol.
11: 429-433 (study is performed in mice).
Krause, W., Hamm, K., Weissmuller, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. Environ. Contam. Toxicol. 15: 458-462.
Lawley, P.D., Shah, S.A., Orr, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (Dichlorvos, DDVP).
Chem.-Biol. Interactions, 8: 171-182.
Li, C., Miller, W.T., Jiang, J. (1989) Pulmonary edema due to
ingestion of organophosphate insecticide. Am. J. Roentgenol., 152:
265-266.
Lin, S.Y., Lee, T.C., Cheng, C.S., Wang, T.C. (1988) Cytotoxicity,
sister-chromatid exchange, chromosome aberration and transformation
induced by 2,2-dichlorovinyl-0,0-dimethyl phosphate. Mutat. Res.
206: 439-445.
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57: 393-394.
Lofroth, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
naturforsch. 29: 651.
Lofroth, G., Wennerberg, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch. 41:
215-221.
Lotti, M., Johnson, M.K. (1978) Neurotoxicity of organopho-sphorus
pesticides: Predictions can be based on in vitro studies with hen
and human enzymes. Arch. Toxicol. 41: 215-221.
Maslinska, D., Zalewska, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia Histochem. Cytochem. 16: 335-341.
Matsushita, T., Aoyama, K., Yoshimi, K., Fujita, Y., Ueda, A. (1985)
Allergic contact dermatitis from organophosphorus insecticides.
Ind. Health, 23: 145-153.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L., Loh, E.K., Hamilton,
C.M., Bakke, J.P., Spalding, J.W. (1989) Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: Testing of 24 compounds. Environ. Mol. Mutat.
14: 155-164.
Modak, A.T., Stavinoha, W.B., Weintraib, S.T. (1975) Dichlorvos and
the cholinergic system: effects on the cholinesterase and
acetylcholine and choline contents of rat tissues. Arch. Int.
Pharmacodyn. 217: 293-301.
Mohn, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides. Mutat.
Res. 20: 7-15.
Moriya, M., Kato, K., Shirasu, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res. 57: 259-263.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y., (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res. 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979) Analysis of mitotic nondisjunction with
Aspergillus nidulans. Environ. Health Aspects, 31: 81-95.
Moutschen-Dahmen, J., Moutschen-Dahmen, M., Degraeve, N. (1981)
Metrifonate and dichlorvos: cytogenetic investigations. Acta
Pharmacol. Toxicol. 49(V): 29-39.
Nagy, Zs., Mile, I., Antoni, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta Microbiol. Acad.
Sci. Hung. 22: 309-314.
NCI (National Cancer Institute) (1977) Bioassay of dichlorvos for
possible carcinogenicity. Bethesda, Maryland, USA.
Nicholas, A.H., Vienne, M., Berghe, H. van den (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Letters, 2:
271-275.
Nishio, A., Uyeki, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphate
insecticides and their oxygen analogs. J. Toxicol. Environ. Health,
8: 939-946.
Nishio, A., Uyeki, E.M. (1982) Nuclease-sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants.
Biochem. Biophys. Research Comm. 107: 485-491.
Nordgren, I., Bergstrom, M., Holmstedt, B., Sandoz, M. (1978)
Transformation and action of metrifonate. Arch Toxicol. 41: 31-41.
Olinski, R., Walter, Z., Wiaderkiewicz, R., Lukasova, E., Palecek,
E. (1980) Changes in DNA properties due to treatment with the
pesticides malathion and DDVP. Radiat. Environ. Biophys. 18:
65-72.
Ottevanger, C.F. (1975) (Neuromuscular function in people exposed to
dichlorvos resin strips. The importance of electromyography.)
Europ. J. Toxicol. 8: 192-195 (in French).
Pachecka, J., Sulinski, A., Ziolkowska, G. (1975) The activities of
some esterases of the rat brain after intoxication by
organophosphate insecticides, dichlorvos and trichlorphon.
Neuropat. Pol. 13: 455-462.
Paik, S.G., Lee, S.J. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool. 20:
19-28.
Pauluhn, J., Machemer, L., Kimmerle, G. (1987) Effects of inhaled
cholinesterase inhibitors on bronchial tonus and on plasma and
erythrocyte acetylcholine esterase activity in rats. Toxicology,
46: 177-190.
Pena-Chavarria, A., Swartzwelder, J.C., Villarejos, V.M., Kotcher,
E., Arguedas, J. (1969) Dichlorvos, an effective broad-spectrum
anthelmintic. Am. J. Trop. Med. Hyg. 18: 907-911.
Perocco, P., Fini, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
Purshottam, T., Kaveeshwar, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviation, Space,
and Environ. Med. 50: 581-584.
Purshottam, T., Srivastava, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
Environ. Health, 39: 425-430.
Pym, R.A., Singh, G., Gilbert, W.S., Armstrong, J.P., McCleary, B.V.
(1984) Effects of dichlorvos, maldison and pirimiphos-methyl on
food consumption, egg production, egg and tissue residues, and
plasma acetylcholinesterase inhibition in layer strain hens. Aust.
J. Exp. Agric. Anim. Husb. 24: 83-92.
Ramel, C. (1981) Does dichlorvos constitute a genotoxic hazard?
Progress in Mutat. Res. 2: 69-78.
Rauws, A.G., Logten, M.J. van (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in chicken.
Toxicology, 1: 29-41.
Reiner, E., Plestina, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by
0,0-dimethyl-2,2-dichlorovinyl phosphate. Toxicol. Appl. Pharmacol.
49: 451-454.
Reiner, E. Krauthacker, B., Simenon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
0,0-dimethyl-2,2,2-trichloro-1-hydroxyethylphosphonate
(trichlorphon). Biochem. Pharmacol. 24: 717-722.
Reiner, E., Simeon, V., Skrinjaric-Spoljar, M. (1980) Hydrolysis of
O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP) by esterases in
parasitic helminths, and in vertebrate plasma and erythrocytes.
Comp. Biochem. Physiol. 66: 149-152.
Rider, J.A. (1967) Determination of the minimal incipient toxicity
of dichlorvos in humans. Unpublished report from San Francisco,
Gastrointestinal Research Lab., October. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Rider, J.A., Moeller, H.C., Puletti, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies
with Guthion, and determination of incipient toxicity level of
dichlorvos in humans. Fed. Proc. 26: 427.
Rider, J.A., Moeller, H.C., Puletti, E.J., Swader, J. (1968) Studies
on the anticholinesterase effects of methyl parathion, Guthion,
dichlorvos, and Gardona in human subjects. Fed. Proc. 27: 597.
Rosenkranz, H.S. (1973) Preferential effect of dichlorvos (Vapona)
on bacteria deficient in DNA polymerase. Cancer Research, 23:
458-459.
Rosenkranz, H.S., Rosenkranz, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia, 28: 386-387.
Segerback, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP
(2,2-dichlorovinyl dimethylphosphate). Hereditas, 94: 73-76.
Segerback, D., Ehrenberg, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta Pharmacol. Toxicol. 49(V): 56-66.
Shellenberger, T.E. (1980) Organophosphate pesticides inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. Environ. Sci. Hlth. B15: 795-822.
Shellenberger, T.E., Newell, G.W., Okamoto, S.S., Sarros, A. (1965)
Response of rabbit whole-blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14:
943-952.
Shinoda, H., Ito, K., Matsunaga, T., Takada, T. & Nomura, T. (1972)
(Two suicides by acute pesticide intoxication). J. Jpn. Assoc.
Rural Med. 21: 242-249 (in Japanese).
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shooter, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating agents.
Chem. Biol. Interactions, 11: 575-588.
Skrinjaric-Spoljar, M., Simeon, V., Reiner, E. (1973) Spontaneous
reactivation and aging of dimethylphosphorylated
acetylcholinesterase and cholinesterase. Biochim. Biophys. Acta,
315: 636-369.
Slomka, M.B., Hine, C.H. (1981) Clinical pharmacology of dichlorvos.
Acta Parmacol. Toxicol. 49(V): 105-108.
Snow, D.H., Watson, A.D. (1973) The acute toxicity of dichlorvos in
the dog: 1. clinical observations and clinical pathology. Aust.
Vet. J. 49: 113-119.
Sobels, F.H., Todd, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res. 67: 89-92.
Stanton, H.C., Albert, J.R., Mersmann, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows.
Am. J. Vet. Research, 40: 315-320.
Stevenson, D.E., Blair, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Tox. 18: 215-217.
Teichert, K., Szymczyk, T., Consolo, S., Ladinsky, H. (1976) Effect
of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. Appl. Pharmacol. 35: 77-81.
Tezuka, H., Ando, N., Suzuki, R., Terahta, M., Moriya, M., Shira-su,
Y. (1980) Sister-chromatid exchanges and chromosomal aberrations in
cultured Chinese hamster cells treated with pesticides positive in
microbial reversion assays. Mutat. Res. 78: 177-191.
Traverso, R., Moretto, A., Lotti, M. (1989) Human serum
"A"-esterases. Hydrolysis of 0,0-dimethyl-2,2-dichlorovinyl
phosphate. Biochem. Pharmacol. 38: 671-676.
Tungul, A., Bonin, A.M., He, S., Baker, R.S. (1991) Micronuclei
induction by dichlorvos in the mouse skin. Mutagenesis, 6:
405-408.
Uchiyama, M., Yoshida, T., Homma, K., Hongo, T. (1975) Inhibition
of hepatic drug-metabolizing enzymes by thiophosphate insecticides
and its drug toxicological implications. Biochem. Pharmacol. 24:
1221-1225.
Vasilescu, C., Florescu, A. (1980) Clinical and electrophysiological
study of neuropathy after organophosphorous compounds poisoning.
Arch. Toxicol. 43: 305-315.
Voogd, C.E., Jacobs, J.J., Stel, J.J. van der (1972) On the
mutagenic action of dichlorvos. Mutat. Res. 16: 413-416.
Wadia, R.S., Shinde, S.N., Vaidya, S. (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurol. India, 33:
247-253.
Wadia, R.S., Chitra, S., Amin, R.B., Kiwalkar, R.S., Sardesai, H.V.
(1987) Electrophysiological studies in acute organophosphate
poisoning. J. Neurol. Neurosurg. Psychiatry, 50: 1142-1148.
Wang, T., Wu, C., Lin, J., Tarn, C., (1988) Dichlorvos potentiates
the insecticide-induced sister chromatid exchanges in Chinese
hamster ovary cells. Bull. Inst. Zool. Academia Sinica, 27:
111-117.
Ward, F.P., Glicksberg, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Ass. 158:
457-461.
Watanabe, H., Shirai, O., Ebata, N. (1974/1976) (A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care.) Sapporo Igaku Zasshi, 43: 334-337 (in Japanese);
Pest. Abstr., 8: 94-95.
Weisburger, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D. (eds). Effects of chronic exposures
to pesticides on animal systems, pp. 165-176. New York, Raven Press.
Wennerberg, R., Lofroth, G. (1974) Formation of 7-methylguanine by
dichlorvos in bacteria and mice. Chem.-Biol. Interactions, 8:
339-348.
WHO (1989) Environmental Health Criteria 79: Dichlorvos.
International Programme on Chemical safety, Geneva, World Health
Organization.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wild, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of
dichlorvos and methyl methanesulphonate. Mutat. Res. 19: 33-41.
Wild, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res. 32: 133-150.
Witherup, S., Caldwell J.S., Hull, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their
offsprings by the introduction of Vapona insecticide into their
diets. Unpublished report by Kettering Laboratory.
Witherup, S., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction
of Vapona insecticide into their daily diets. Unpublished report
from Cincinnati, The Kettering Laboratory, February 14. Prepared for
Shell Chemical Company, submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Wooder, M.F., Creedy, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo. Unpublished
report. Sittingbourne, Shell Research Ltd., TLGR. 79.089.
Wooder, M.F., Wright, A.S. (1981) Alkylation of DNA by
organophosphorus pesticides. Acta Pharmacol. Toxicol. 49(V):
51-55.
Wooder, M.F., Wright, A.S., King, L.J. (1977) In vivo alkylation
studies with dichlorvos at practical use concentrations.
Chem.-Biol. Interact. 19: 25-46.
Wooder, M.F., Wright, A.S., Stevenson, D.E. (1978) Studies on the
in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol. 1: 505.
Wright, A.S., Hutson, D.H., Wooder, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol. 42: 1-18.
Wyrobek, A.J., Bruce, W.R. (1975) Chemical induction of sperm
abnormalities in mice. Proc. Nat. Acad. Sci. USA, 72: 4425-4429.
Yoshida, T., Nomura, M., Suzuki, Y. (1976) Inhibition of hepatic
UDP-glucuronyltransferase activity by organophosphate insecticides
and by carbon disulfide in mice. Bull. Environ. Contam. Toxicol.
15: 421-424.
Zalewska, Z., Rakowska, I., Matraszek, G., Sitkiewicz, D. (1977)
Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterase. Neuropat. Pol.
15: 255-262.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagenesis, 11(12): 1-157.
 Effects on enzymes and other biochemical parameters
In vitro studies showed that rodent plasma ChE is more
sensitive than erythrocyte and brain ChE to inhibition by
dichlorvos. Calculated I50s (concentrations which inhibit 50% of
enzyme activity) at 37 °C, pH 7.4-7.6, were 10-8 and 10-7 mol/l,
respectively, for an incubation time of 20 minutes (Asperen &
Dekhuijzen, 1958; Skrinjaric-Spoljar et al., 1973; Lotti &
Johnson, 1978). The rate of phosphorylation of rat brain ChE was
calculated to be 1.5 x 105 mol/l/min at 37 °C, pH 7.4-7.6
(Skrinjaric-Spoljar et al., 1973). Half-life of in vitro
reactivation (at 37 °C, pH 7.4-7.6) was found to be about 100
minutes; that of aging was found to be about 400 min. This means
that inhibited AChE almost completely reactivates within a few
hours, leaving a small fraction (20% or less) irreversibly inhibited
(i.e. aged) (Skrinjaric-Spoljar et al., 1973).
TOXICOLOGICAL STUDIES
Acute toxicity studies
The results of acute toxicity tests of dichlorvos administered
by various routes to different animal species are summarized in
Tables 1 and 2. Typical cholinergic signs are observed. WHO has
classified dichlorvos as highly hazardous (WHO, 1992).
Short-term toxicity studies
Mice
In a range-finding study, groups of 10 B6C3F1 mice/sex were
administered by corn oil gavage 0, 5, 10, 20, 40, 80, or 160 mg
dichlorvos/kg bw/day, 5 days per week for 13 weeks. Animals were
observed twice daily for clinical signs and body weight was recorded
weekly. Necropsy was performed on all animals. Oesophagus and
gastrointestinal tract of all animals dying after day 46 were
examined histologically. All vehicle controls and all animals in
the highest dose group with survivors were examined histologically.
All mice at 160 mg/kg bw/day and 5 male mice at 80 mg/kg bw/day died
before the end of the study. Final mean body weights in all treated
groups were comparable to controls. No gross or microscopic
pathologic effects were observed (Chan, 1989).
Rats
In a range-finding study, groups of 10 male and 10 female
F344/N rats were administered by corn oil gavage 0, 2, 4, 8, 16, 32,
or 64 mg dichlorvos/kg bw/day, 5 days per week for 13 weeks.
Animals were observed twice daily for clinical sign and body weights
were recorded weekly. Necropsy was performed on all animals.
Oesophagus and gastrointestinal tract of all animals dying after day
46 were examined histologically. All vehicle controls and all
animals in the highest dose group with survivors were examined
histologically. All rats at 32 and 64 mg/kg bw/day and 1 male and 4
females at 16 mg/kg bw/day died before the end of the study. Final
mean body weights of all dose groups were comparable to vehicle
controls. No gross or microscopic pathologic effects were observed
(Chan, 1989).
Table 1. Acute toxicity of dichlorvos (WHO, 1989)
Species Route LD50 (mg/kg bw)
Mouse oral 68-275
i.p. 28-41
i.v. 8-10
s.c. 13-33
Rat oral 30-110
dermal (24 h) 75-107
i.p. 18
s.c. 72
Guinea-pig s.c. 28
Hamster i.p. 30
Rabbit oral 13-23
dermal 205
Cat oral 28
Dog oral 100-316
Chicken oral 15
Swine oral 157
Table 2. Acute inhalation toxicity of dichlorvos (WHO, 1989)
Species Mode of exposure Time of exposure LC50
(hours) (µg/l)
Mouse whole body 4 13
head only 4 > 218
Rat whole body 4 15
whole body 1 140
head only 4 340
head only 1 455
head only 4 > 198
Dogs
Groups of 3 male and 3 female beagle dogs received 0, 2.3, 6.9,
12 or 23 mg dichlorvos/dog (93% in corn oil by gelatin capsule)
daily, for 90 days. The mean doses per group were equivalent to
0.3, 1, 1.5 or 3 mg/kg bw/day. No effects were observed on
mortality, growth, haematology, liver and kidney function, organ
weights, or at gross and histopathological examination. In the two
highest dose groups, the dogs showed excitement, increased activity
and aggression. Plasma and erythrocyte ChE activities, measured
initially and at intervals of approximately 2 weeks, were normal in
the lowest dose group (0.3 mg/kg bw/day) but inhibited in the other
dose groups (up to about 60% in the highest dose group). Inhibition
was lower from day 70 onwards. Brain ChE activity at termination
was decreased (to 33%of control activity) only in the highest dose
group. The NOAEL in this study was 1.5 mg/kg bw/day based on
reduction in brain ChE activity at termination. However, taking
into account the behavioural changes, a more conservative NOAEL can
be considered to be 1 mg/kg bw/day (Hine, 1962).
Pigs
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, or 16 mg
dichlorvos/kg bw/day (divided over 2 daily doses) for 30 days. No
effects were found on body-weight gain, haematology, or clinical
chemistry when compared with control animals fed a blank PVC
formulation. Plasma and erythrocyte ChE activities were
significantly inhibited in the 4 mg/kg bw/day group (37 and 34% of
controls, respectively) and in the 16 mg/kg bw/day group (33 and 21%
of controls, respectively). The NOAEL in this study was 1 mg/kg
bw/day based on inhibition of erythrocyte ChE activity (Stanton et
al., 1979).
Monkeys
Rhesus monkeys (4/sex) were continuously exposed to dichlorvos
vapour at an average actual concentration of 0.05 mg/m3 for 3
months. The control group consisted of 4 males and 1 female. No
adverse effects were observed in appearance, behaviour, or
haematological and clinical chemical determinations. Plasma ChE
activity was slightly reduced (up to 28% inhibition), while
erythrocyte ChE activity inhibition was 36%. No changes in nerve
maximum conduction velocities or muscular evoked action potentials
were induced by exposure to dichlorvos (Coulston & Griffin, 1977).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 100 male and 100 female C57BI/6/Bln mice (5-6 weeks
old) received by gavage 0.2 mg dichlorvos per mouse (97% in 0.2 ml
water), freshly prepared, either twice or 3 times per week for 50
weeks. Control groups received by gavage either 0.2 ml water, 3
times per week (about 50 males and 50 females), or no treatment (35
males and 35 females). Surviving animals were sacrificed after 110
weeks. This strain of mice is known for the spontaneous occurrence
of mixed lymphomas (reticulocell sarcoma type B).
From the age of 12 months onwards, some animals in all groups
developed interstitial pneumonia. The incidence of mixed lymphomas
was decreased in both test groups (26-60% in control groups, 23-30%
in treated groups). An increased incidence of focal hyperplasia
(transitional cell hyperplasia) of the urinary bladder was found in
both dichlorvos groups (0-8% in control groups, 5-10% in treated
groups). The authors concluded that no neoplastic lesions were
found which could be attributed to the treatment of the animals with
dichlorvos (Horn et al., 1987).
Dichlorvos was not co-carcinogenic in C57BI/6/Bln mice when
administered by gavage three times/week at 0.2 mg/animal to mice
subcutaneously injected with 50 µg N-nitrosodiethylamine per animal
weekly for 50 weeks followed by an observation period of up to 110
weeks (Horn et al., 1990).
Two groups of 50 male and 50 female B6C3F1 mice were fed
1000 or 2000 ppm dichlorvos (purity > 94%) in corn oil in the diet
for 2 weeks. Due to severe signs of intoxication, doses were
lowered to 300 and 600 ppm for the following 78 weeks. Samples of
the diets analyzed during the study showed that time-weighted
average concentrations were 318 and 635 ppm. Matched controls
consisted of 10 mice of each sex; the pooled controls from
simultaneous studies with other compounds consisted of 100 male and
80 female mice. All surviving mice were killed at 92-94 weeks.
Animals were observed twice daily for clinical signs.
Alopecia and rough hair coats were noted in many treated
animals, particularly in the male groups, beginning at week 20 and
persisting throughout the study. The average body weights of the
high-dose mice of both sexes were slightly decreased compared with
controls. The low-dose female group showed 74% survival at 90 weeks
compared to 84% and 90% in high-dose and control groups,
respectively. There was no significant increase in the incidence of
tumours attributable to dichlorvos in either sex.
Two squamous-cell carcinomas of the oesophagus (one in a
low-dose male and one in a high-dose female), 1 papilloma of the
oesophagus in a high-dose female and 3 cases of focal hyperplasia of
the oesophageal epithelium in low-dose males were recorded in the
treated mice. The significance of the findings in the treated mice
was considered uncertain because of insufficient information
concerning the spontaneous incidence of these lesions and lack of
statistical significance within the experiment. Dichlorvos (up to
635 ppm in the diet, equivalent to 95 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Studies in B6C3F1 mice given 0, 400 or 800 mg/litre
dichlorvos in drinking-water for two years and in CFE rats exposed
to 0, 0.05, 0.48, or 4.7 mg/m3 of dichlorvos 23 hours/day for 2
years were summarized in WHO (1989). There was no evidence of
carcinogenic effects in these studies (Blair et al., 1976;
Konihishi et al., 1981).
Groups of 50 B6C3F1 mice were given dichlorvos (99% purity)
by corn oil gavage at doses of 0, 10 or 20 (males) or 0, 20 or 40
(females) mg/kg bw/day daily, 5 days per week, for 103 weeks. The
dose volume of the corn oil was 10 ml/kg bw/day. Body weights were
recorded once weekly for the first 12 weeks and then monthly.
Animals were observed twice per day for clinical signs. Necropsy
and histological examinations were performed on all moribund animals
or at the end of the study.
Body-weight gain and survival did not significantly differ
between treated and control groups. No compound-related clinical
signs were observed. Blood cholinesterase activity was not
determined during the study. However, a separate study showed
inhibition of plasma ChE activity by 60-90% in all dose groups while
erythrocyte ChE activity was normal (see "Special studies on
Cholinesterase activity" for detailed description and comments).
The incidences of forestomach squamous cell papillomas were
1/50, 1/50 and 5/50 in control, low and high-dose males respectively
and 5/49, 6/49 and 18/50 in control, low and high-dose females,
respectively. The positive trend was statistically significant in
both sexes while by pairwise comparison only the incidence in high-
dose females was significantly higher than in controls. Two
forestomach squamous cell carcinomas were seen in high-dose females
and none in the other groups. No increase in the incidence of
forestomach hyperplasia was seen in the dosed mice compared with
vehicle controls (10-20%). In female mice the incidence of adenomas
and adenomas or carcinomas (combined) of the pituitary gland (12/45,
6/45 and 6/44 in control, low and high-dose groups, respectively)
and the incidence of lymphomas (16/50, 11/50 and 9/50 in control,
low and high-dose groups, respectively) showed a significant
negative trend. Based on the increased incidence of forestomach
papillomas, the NOAEL was 10 mg/kg bw/day (Chan, 1989; Chan et al.,
1991).
Rats
Groups of 40 male and 40 female weanling CD rats were fed diets
containing nominal concentrations of 0, 0.1, 1, 10, 100 or 500 ppm
dichlorvos (93% purity) for 2 years. Diets were prepared weekly.
Five males and 5 females from each group were killed after 6, 12 or
18 months. Analysis of diet samples showed a considerable loss of
dichlorvos associated with a gradual increase in
dichloroacetaldehyde (DCA) content (average concentrations ranging
from 0.01 to 28.6 ppm. The average actual concentrations of
dichlorvos in each diet were 0.05, 0.5, 4.7, 47 or 230 ppm. No
cholinergic signs were observed. No effects were seen on behaviour,
mortality rate, weight gain, food consumption, terminal body and
organ weights, haematology or urinalysis. Plasma and erythrocyte
ChE activities were measured 13 times during the study. In the 100
ppm group, plasma and erythrocyte ChE activities were reduced to
60-90% and to 50-90% of control activities, respectively; in the 500
ppm group, the activities were reduced to 20-70% and 20-60%,
respectively. Activities were higher toward the end of the study.
Brain ChE activity was decreased in the highest dose group by
45-47%, 24-43%, 24-38% and 5-15% after 6, 12, 18 and 24 months,
respectively. Histological examination of major organs (liver,
heart, lungs, kidneys, spleen, brain, gonads, pituitary, adrenals,
and thyroids) revealed hepatocellular fatty vacuolization in all 500
ppm rats, and in most females and several males at 100 ppm. No
effect was seen on serum total proteins or albumin: globulin ratio,
or on hexobarbital sleeping time. The tumour incidence was
comparable with that of the control group. The NOAEL, based on
brain ChE inhibition, was 100 ppm (actual concentration 47 ppm,
equivalent to 2.4 mg/kg bw/day) (Witherup et al., 1967).
Groups of 50 male and 50 female weanling CFE rats were exposed
(whole body) to nominal air concentrations of 0, 0.05, 0.5 or 5 mg
dichlorvos (97% purity)/m3 for 23 hours per day for two years. The
average actual dichlorvos concentrations were 0.05, 0.48, or 4.7
mg/m3. Body-weight gain was reduced in the two highest dose
groups. After two years of exposure, plasma and erythrocyte ChE
activities were reduced by 20-30% at 0.5 mg/m3 and by > 60% in the
highest dose group; brain ChE activity was reduced by 10%
(statistically significant) at 0.5 mg/m3 and by 80% in the highest
dose group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry
values, organ weights, or gross or microscopic examinations of major
organs. Ultrastructural examinations of bronchi and alveoli of rats
exposed to 0 or 5 mg/m3 showed no differences between the two
groups.
It was concluded that 2-year exposure to 0.05 mg/m3 dichlorvos
did not cause observable adverse effects to CFE rats (Blair et al.,
1976). It should be noted that in this study the rats were not only
exposed by inhalation but also via their food, drinking-water, and
by grooming. This resulted in additional oral ingestion of
dichlorvos (Stevenson & Blair, 1977).
Osborne-Mendel rats (50/sex) were fed 1000 ppm dichlorvos
(purity > 94%) in corn oil for 3 weeks. Due to severe cholinergic
signs, the dose was reduced to 300 ppm for the remaining 77 weeks.
Another group (50 males and 50 females) was fed 150 ppm dichlorvos
for 80 weeks. Samples of the diets analyzed during the study showed
that time-weighted average concentrations were 330 and 150 ppm,
respectively. Matched controls consisted of 10 rats of each sex;
the pooled controls from simultaneous studies of other compounds
consisted of 60 rats of each sex. Animals were observed twice daily
for clinical signs. All surviving rats were killed after 110-111
weeks.
The average body weights of the high-dose rats of both sexes
were slightly decreased compared with controls. There was no
significant increase in the incidence and type of tumours
attributable to dichlorvos in either sex. Dichlorvos (up to 330 ppm
in the diet, equivalent to about 30 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Groups of 70 and 100 rats per sex received 0.1 mg dichlorvos
(97%)/rat in 0.2 ml water by gavage twice or 3 times per week,
respectively, for 60 weeks. Thereafter, the animals were observed
for another 51 weeks. A control group of 60 animals of each sex
received 3 times per week 0.2 ml water. From the age of 10 months
onwards, the incidence of focal hyperplasia of urinary bladder and
of renal pelvis increased in males but decreased in females from
both test groups compared to controls. No neoplastic lesions were
found which could be attributed to treatment (Horn et al., 1988).
A study in Fischer 344 rats given drinking-water containing 0,
140 or 280 mg/litre dichlorvos for 108 weeks was summarized by WHO
(1989). There was no evidence of carcinogenicity (Enomoto et al.,
1981).
Groups of 50 male and 50 female F344/N rats were administered
dichlorvos by corn oil gavage at 0, 4 or 8 mg/kg bw/day (99%
purity), 5 days per week for 103 weeks. Body weights were recorded
once weekly for the first 12 weeks and then monthly. Animals were
observed twice daily for clinical signs. Necropsy and histologic
examinations were performed on all moribund animals or at the end of
the study.
Mild diarrhoea was observed in treated animals. ChE activities
were not determined during the study. However, a separate study
showed inhibition of plasma ChE activity by 50-80% in all dosed
groups but no erythrocyte ChE inhibition (see "Special studies on
cholinesterase activity" for detailed description and comments). No
significant differences in mean body weights or survival were
observed between any groups of either sex. Increased incidence of
cytoplasmic vacuolisation of liver was observed in dosed males and
of cortical cytoplasmic vacuolisation of adrenal glands in all dosed
males and in low-dose females. The incidence of pancreatic adenomas
(cross and horizontal tissue sections were analyzed), in control,
low and high-dose groups was 25/50, 30/50 and 33/50 in males and
2/50, 3/50 and 6/50 in females, respectively. The increased
incidence in treated males was statistically significant. The
incidence of mononuclear cell leukaemia (11/50, 20/50, 21/50 in
control, low and high-dose groups, respectively) was significantly
increased in the treated male rats compared with controls. The
incidence of mammary gland adenomas or fibroadenomas in female rats
was 11/50 in controls, 19/50 in the 4 mg/kg bw/day group and 17/50
in the 8 mg/kg bw/day group. The incidence in the low-dose group
was only slightly higher than the historical control values. Two
mammary gland carcinomas were observed in control and low-dose
groups. The authors concluded that there was evidence that
dichlorvos is carcinogenic to rats (Chan, 1989; Chan et al.,
1991). The Meeting observed that the incidence of pancreatic
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia, which is usually high and variable in
this strain of rats, was also of questionable biological
significance (Haseman et al., 1985).
Dichlorvos at 8 or 16 mg/kg bw/day was administered by gavage
to groups of 8-12 male F344 rats, either with or without leukaemia
transplant, for 5 days a week. Other groups of rats were either not
treated or given the leukaemia transplant only. At 70-days
post-transplant, the animals were killed. The rats dosed with
dichlorvos developed the disease earlier and the rate of tumour
progression was increased. Three out of 16 transplant recipients
dosed with 16 mg/kg bw/day died of leukaemia during the last week of
dosing. The severity of the mononuclear cell leukemia in the
transplant recipients, as measured by histopathological examination
of spleen and liver, was correlated with the changes in tumour
growth rates. However, no dose-response was found for spleen weight
and WBC count (Dieter et al., 1989).
Dogs
Groups of beagle dogs (3/sex) were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100 or 500 ppm dichlorvos (93%
purity) for 2 years. The average actual concentrations were 0,
0.09, 0.32, 3.2, 32 or 260 ppm dichlorvos; the average
dichloroacetaldehyde concentrations at the three highest dosages
were 0.6, 6.4 and 20 ppm. Since diet analysis was performed weekly
on a mixture of diet samples taken the previous 7 days, it is
likely, given the high volatility of dichlorvos, that at the end of
the week the actual concentration was much lower than 30% of the
nominal concentration.
No effects were seen on general appearance, survival, weight
gain, food consumption, haematology or urinalysis. Erythrocyte ChE
activity was reduced up to 50% of controls in the 10 ppm group with
recovery to control values at the end of the feeding period. At the
highest dose level, inhibition was more than 90% but complete
recovery was observed at 24 months. A similar pattern was observed
for plasma ChE activity. Brain ChE activity, measured at the end of
the study was similar to that of the controls in all treated groups.
Relative liver weights were slightly increased in males at 100 ppm
and in both sexes at 500 ppm. Histological examination of major
organs revealed slight dose-related cytoplasmic vacuolization and
enlargement of hepatocytes in animals at the two highest levels. No
differences were seen in serum alkaline phosphatase, transaminase
activities, total serum proteins or albumin:globulin ratios (Jolley
et al., 1967).
Reproduction studies
See also under special studies on testes.
Mice
Male and female Crl:CD3-1 mice were exposed to dichlorvos
concentrations of 0, 1.9, 3.0 or 4.6 mg/m3, generated from
dichlorvos-impregnated PVC strips in their cages. Exposure began
4 days prior to formation of breeding groups (3 females and 1 male)
and continued throughout pregnancy. Signs of intoxication were not
observed. Plasma ChE activity was significantly inhibited (by 90%,
93% and 95% in the 1.9, 3.0 and 4.6 mg/m3 groups, respectively)
when measured on day 4 after beginning of treatment. Gestation
length, number of litters, litter frequency and mean litter size
were comparable to controls. No grossly detectable congenital
anomalies were detected in any of the offspring (Casebolt et al.,
1990).
Rats
A 3-generation, 2-litter/generation reproduction study in rats
summarized by WHO (1989) was negative at doses up to 235 ppm in the
diet, equivalent to 12 mg/kg bw/day (Witherup et al., 1965).
Domestic animals
Several studies have been carried out with pregnant sows but no
adverse effects on piglets were observed with doses which inhibited
plasma and erythrocyte ChE activity in the sows (WHO, 1989).
Special studies on delayed neurotoxicity
Several studies have shown that a single dose of dichlorvos
does not produce delayed neurotoxicity in pre-medicated hens,
whether it is administered orally or subcutaneously (WHO, 1989).
However, Caroldi & Lotti (1981) reported mild signs of ataxia in
pre-medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg bw) and severe (> 80%) inhibition of NTE in peripheral
nerve, spinal cord and brain. Johnson (1978) did not observe ataxia
in pre-medicated hens given the same dose in the same way. However,
in this experiment spinal cord NTE inhibition was below the
threshold. When the dose was repeated 1-3 days after the first
dose, spinal cord NTE inhibition increased and the hens became
ataxic.
In a 90-day study, white leghorn hens were given dichlorvos
(99.9% purity) either dermally or orally. For oral administration,
a 10-20% solution of dichlorvos in corn oil in gelatin capsules was
used whereas, dermally, 1-20% emulsifiable concentrates in technical
grade xylene (containing 2% Triton X-100) were used. Dichlorvos at
doses greater than 1 mg/kg bw/day (dermal) or 6 mg/kg bw/day (oral)
led to cholinergic symptoms and death after 2-3 days. None of the
animals developed organophosphate-induced delayed neuropathy
(Francis et al., 1985).
In summary, it is possible to produce clinical neuropathy in
hens, but the doses required are far in excess of the LD50. This
is consistent with the low in vitro ratio of AChE I50/NTE I50 for
dichlorvos (Lotti & Johnson, 1978).
Special studies on embryotoxicity and teratogenicity
Several embryotoxicity/teratogenicity studies have been
performed with dichlorvos in mice, rats and rabbits. These studies
are summarized in WHO (1989). Dichlorvos given orally, by
inhalation or intraperitoneally, was not teratogenic at doses which
were toxic to the pregnant animals. However, in a study in rats
where a single i.p. dose of 15 mg/kg bw was given on day 11 of
gestation, 3/41 fetuses in the treated group had omphaloceles (0/50
in controls) (Kimbrough & Gaines, 1968). This finding has not been
confirmed in other studies.
Special studies on cholinesterase activity
This section summarizes studies in which the effect of
dichlorvos on ChE activity was the only parameter investigated.
Studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos in relation to the time
elapsed after dosing are summarized in Table 3. In general, the
maximum inhibition occurred within one hour followed by rapid
recovery.
Mice/Rats
Groups of 8-week old B6C3F1 mice and F344/N rats (10/sex)
were administered dichlorvos (99%) by corn oil gavage at daily doses
of 0, 5, 10, 20, or 40 mg/kg bw (mice) and 0, 2, 4, 8, or 16 mg/kg
bw (rats), five days per week for one week. Plasma and erythrocyte
ChE activities were measured on days 10 or 11, 25 or 26 and 32 or
33; blood was obtained about 3 hours after treatment.
Plasma ChE activity was significantly inhibited in all dose
groups of mice (50 and 90% inhibition in low and high-dose groups,
respectively) and rats (25 and 80% inhibition in low and high-dose
groups, respectively). At all time-points, erythrocyte ChE activity
in dosed and vehicle-control mice and rats was similar. However,
the timing for determination of the enzyme activities might have
underestimated the inhibition (Chan, 1989).
Rats
Reiner & Plestina (1979) compared in vivo reappearance of ChE
activity in rats after treatment with either dichlorvos (2.5 mg/kg
bw i.v.) or metrifonate (300 mg/kg bw i.v.). Half-lives of recovery
of brain and plasma ChE (acetylcholine was the substrate) were found
to be 2 and 2.5 hours, respectively, both in dichlorvos-treated and
in metrifonate-treated rats. This was consistent with in vitro
data (Skrinjaric-Spoljar et al., 1973).
In a study on the influence of temperature on ChE activity,
rats were injected i.p. with a single dose of 6.3 mg/kg dichlorvos
and kept at either 28 °C or 5 °C. The maximum inhibition of whole
blood ChE activity (40% and 50% in the two groups, respectively)
occurred after 0.5 hour. The animals kept at 5 °C showed slightly
less inhibition of whole blood ChE activity than those at room
temperature (Chattopadhyay et al., 1982).
Table 3. Time-related inhibition of ChE activity in animals after administration of a single dose of dichlorvos
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Mouse, male i.p. 10 15 min 70 Nordgren et al., (1978)
(n = 6-8) 60 min 50
2 hours 20
Mouse, male i.p. 15 2 hours 20 Cohen & Ehrich, 1976
Mouse, male i.p. 30 15 min 63 Cohen & Ehrich, 1976
(n = 4) 1 hour 50
5 hours 35
18 hours 10
Rat, male oral 1.6 2 hours 0 Pacheka et al., 1975
(n = 6) 10 2 hours 15
40 1 hour 60*
Rat, male oral 40 1 hour 70 Teichert et al., 1976
(n = 1)
Rat, male oral 40 5 min 45 Pacheka et al., 1975
(n = 6) 15 min 80
1 hour 85
2 hours 70
8 hours 35
24 hours 25
48 hours 6
Rat, male oral 50 15 min 68 80 97.91** Modak et al., 1975
(n = 6) 3 hours 73 37 68-70**
24 hours 39 24 31-38**
Table 3 (contd)
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Rat, male i.v. 2.5 30 min 60 85 Reiner & Plestina, 1979
(n = 5-12) 90 min 40 65
3 hours 7 44
12 hours 0 17
48 hours - 10
Dog, greyhounds oral 11 1 hour 90 93 Snow & Watson, 1973
1 male 2 hours 80 70
1 female 24 hours 15 35
72 hours 8 25
Dog, 2 male oral 22 fatal 90 95 66*** Snow & Watson, 1973
greyhounds,
1 female
crossbred
Dog, greyhounds oral 22 1 hour 88 83 Snow & Watson, 1973
and crossbred 3 hours 76 66
(n = 7-9) 6 hours 60 50
24 hours 30 30
48 hours 13 30
72 hours 5 35
Dog, beagle oral 50 2 hours 68 37 Ward & Glicksberg, 1971
sex not specified 24 hours 37 26
(n = 15) 5 days 8 22
14 days 5 20
21 days 0 8
Table 3 (contd)
* Daily dosing for 14 days caused 60% AChE inhibition when measured 24 hours after the last dose.
** Determinations were performed in striatum, hippocampus, medulla and cortex. No significant difference
was found between these brain areas.
*** Measured 20-155 min. after treatment.
When pregnant rats were given oral doses of 1.1 or 5.6 mg
dichlorvos/kg bw/day during days 14-21 of gestation, plasma ChE
activity of the high-dose mothers was inhibited (30-50%) but not
that of the young. Brain ChE activity did not show any significant
inhibition (Zalewska et al., 1977).
Rabbits
Dichlorvos, when infused into the ear vein of adult male
rabbits, produced dose- and time-related inhibition of whole blood
ChE activity during infusion. Spontaneous but incomplete recovery
to 60-80% of the normal activity occurred within 60-90 minutes after
infusion. Almost complete recovery was obtained by injecting oximes
up to 2.5 hours later (Shellenberger et al., 1965; Gough &
Shellenberger, 1977-1978; Shellenberger, 1980).
The progeny of rabbits, treated orally with 6 mg/kg bw/day for
the last 10 days of gestation, showed inhibition of brain ChE
activity at one day (30%) and 8 days of life (15%). Plasma ChE
activity was higher throughout days 1-16 of life (Maslinska &
Zalewska, 1978).
Guinea-pigs
Groups of 5 male and 5 female guinea-pigs were given daily
applications of 0, 25, 50 or 100 mg/kg bw/day dichlorvos (94%
purity) on the shorn skin for 8 days. All animals survived. A
dose-dependent inhibition of both plasma and erythrocyte ChE
activities occurred in all test groups. Recovery of plasma ChE
activities was complete within one week of the last exposure, and
that of erythrocyte ChE was complete within one week in the females
and 2 weeks in the males (Brown & Roberts, 1966).
Hens
Chickens dosed once orally with 1,3, or 6 mg/kg bw showed a
rapid inhibition of plasma ChE activity followed by recovery with an
half-life being estimated at three days (Rauws & van Logten, 1973).
White Leghorn pullets and hens were fed a diet containing 30
ppm dichlorvos for 35 days followed by a 21-day recovery period.
Plasma ChE activity was inhibited by 70% after four weeks of
treatment but returned to normal during the recovery period (Pym et
al., 1984).
Monkeys
Thirty-two Rhesus monkeys were given a pelleted PVC-resin
formulation containing 20% dichlorvos at dosages ranging from 1 to
16 mg dichlorvos/kg bw once daily or 1.6 and 4 mg dichlorvos/kg bw
twice daily for 10 to 21 consecutive days. None of the monkeys
showed overt signs of intoxication, although they ate less food and
had soft faeces. Plasma and erythrocyte ChE activities were reduced
by approximately 80% in all animals (irrespective of the dose) and
remained inhibited throughout the study. Plasma ChE activities
returned to normal values within approximately 3 weeks and the
erythrocyte ChE activities within 50 to 60 days following cessation
of exposure (Hass et al., 1972).
Special studies on genotoxicity
Methylating reactivity
In vitro studies
In a quantitative colour test in which methylation of
4-(p-nitro-benzyl) pyridine was measured to predict DNA alkylating
potential, dichlorvos gave a positive response, the reaction being
about 1/3 that of the known alkylating compound
methylmethanesulfonate (MMS) (Bedford & Robinson, 1972).
Alkylation by dichlorvos of calf thymus DNA, resulting in the
formation of N-7-methylguanine, was reported by Lofroth (1970).
Methylation by dichlorvos of isolated salmon sperm DNA and of
DNA from intact E.coli and from human HeLa cells broadly resembled
that by MMS (Lawley et al., 1974). In this study a very high
dichlorvos concentration (14 mmol/l) was used with isolated DNA
(12.5 mmol/l DNA-P). Dichlorvos concentrations with E. coli and
HeLa cells were 1-3 mmol/l. The rate of methylation of guanine-N-7
from salmon sperm DNA or from intact HeLa cell DNA by dichlorvos was
2-3 x 10-4 mol/l/min (calculated assuming guanine as 23-25% of DNA
with an average molecular weight of DNA base pair of 649, Lawley et
al., 1974). This is about 15 times lower than the rate of
methylation by MMS. This rate is to be compared with the rate of
reaction with AChE (phosphorylation) (1.5 x 105 mol/l/min at 37 °C)
(Skrinjaric-Spoljar et al., 1973). Therefore, the relative rate
of phosphorylation is about 9 orders of magnitude higher than that
of alkylation.
Labelled 7-methylguanine was present in both DNA and RNA
isolated from E. coli exposed to [Me-3H]-dichlorvos (1.1 mmol/l
for 4 hours). The methylating capability of dichlorvos was less, by
a factor of 10-100, than that of strongly genotoxic methylating
compounds (Wennerberg & Lofroth, 1974).
Incubation of bacteriophage R17 with 0-100 mmol
dichlorvos/litre for 90 hours did not result in methylation of the
phosphate groups of the RNA to any significant extent (Shooter,
1975).
In vivo studies
Mice were given i.p. injections of methyl-14C-dichlorvos (1.9
µmol/kg bw, approximately 420 µg/kg bw). The degree of alkylation
of guanine-N-7 in DNA isolated from soft tissues amounted to 8 x
10-13 mol methyl per gram of DNA (about 5 x 10-10 mol/mol guanine)
(Segerback, 1981; Segerback & Ehrenberg, 1981). From acute toxicity
data in mouse (see Table 1) it can be extrapolated that this dose
would cause 1 mol/mol phosphorylation of erythrocyte ChE.
DNA and RNA from the total soft tissues of male rats exposed to
atmospheres containing 0.064 mg/m3 (about 0.1% of the LC50) of
methyl-14C-dichlorvos for 12 hours did not show methylation of the
N-7 atom of guanine moieties. The exposure period constituted a
significant fraction of the half-life of the 7-methylguanine
moieties in DNA (Wooder et al., 1977; Wooder & Wright, 1981).
Excretion of labelled 7-methylguanine in the urine by NMRI mice
and rats injected i.p. with [Me-14C]-dichlorvos, or exposed by
inhalation for 2 hours (mice only) was reported by Wennerberg &
Lofroth (1974) and Lofroth & Wennerberg (1974). In rat urine,
labelled 3-methyladenine and 1-methyl-nicotinamide were also present
(Lofroth & Wennerberg, 1974). According to the authors, these
results demonstrate that dichlorvos spontaneously methylates guanine
and adenine moieties in nucleic acids. However, administration of
radiolabelled adenine and guanine to otherwise untreated rats gave
rise to the excretion of radiolabelled methylated purines in the
urine. Therefore, the detection of radiolabelled purines, per se,
in the urine of animals exposed to methyl-labelled methylating
agents does not constitute evidence for the spontaneous methylation
of the purine moieties of nucleosides or nucleic acids by
methylating agents (Wooder et al., 1978; Wooder & Wright, 1981).
Moreover, a natural biosynthetic pathway has been demonstrated
whereby the methyl carbon atoms of dichlorvos can be incorporated
into the heterocyclic rings and the methyl groups of urinary
7-methylguanine after entering the 1-C pools, in vivo (Wright et
al., 1979; Wooder & Wright, 1981).
Genotoxicity
In vitro studies
Several studies using bacteria and fungi as test organisms have
been carried out (Table 4). In most of the studies, only one, often
high, dichlorvos concentration was tested, sometimes resulting in
low survival of the test organism. The alkylating properties of
dichlorvos (see above) are most probably the cause of the mutagenic
action. This is suggested, for instance by data in E. coli
strains deficient at four repair loci (Bridges et al., 1973).
In vitro studies using mammalian cells are summarized in
Table 5. Dichlorvos caused cell transformation, mutations, DNA
strand breaks, sister chromatid exchanges and chromosomal
aberrations in cultured animal cells. In cultured human cells,
dichlorvos induced unscheduled DNA synthesis but did not induce
sister chromatid exchanges or chromosomal aberrations. Negative
results from chromosomal aberration tests in cultured human
lymphocytes were also cited by Fahrig (1974) and Wild (1975).
Dichlorvos (0.3 µg/ml) caused a synergised an increase in
sister chromatid exchanges in Chinese hamster ovary cells when
tested in combination with synthetic pyrethroids or propoxur which,
as such, were negative; however, in the combination phenothrin +
dichlorvos (0.3 µg/ml) the induction of SCE by phenothrin was
negative (Wang et al., 1988).
In vivo studies
In vivo studies are summarized in Table 6.
In Drosophila melanogaster, chromosomal aberrations but not
sex-linked recessive lethal mutations were induced. Negative
results were obtained in host-mediated, dominant lethal, sister
chromatid exchange and micronucleus assays (except on skin after
local application at cytotoxic doses). Dichlorvos did not induce
in vivo chromosomal aberrations in bone-marrow cells,
spermatocytes or spermatogonia, DNA strand breaks or unscheduled DNA
synthesis.
Special studies on liver microsomal enzymes
A decrease in liver microsomal cytochrome P-450 occurred in
rats after three daily i.p. injections with 6 mg dichlorvos/kg bw
(Purshottam & Kaveeshwar, 1982) but no effect on liver microsomal
UDP-Glucuronyl transferase was found in mice 4 hours after a single
i.p. injection of 25 mg/kg bw (Yoshida et al., 1976).
Pre-treatment of rats with three daily i.p. injections of
sodium phenobarbital did not significantly change mortality and
plasma ChE inhibition caused by an i.p. injection of dichlorvos (20
mg/kg bw) (Purshottam & Kaveeshwar, 1979).
Short- and long-term studies with mice, rats or dogs dosed
orally or intraperitoneally with dichlorvos did not show any effect
on microsomal drug metabolizing enzymes (Witherup et al., 1967;
Uchiyama et al., 1975; Farber et al., 1975).
Table 4. Mutagenicity tests on microorganisms in vitro
Test system Test object Concentration Purity Results 1 Reference
Mitotic A. nidulans strain p ? ? + Morpurgo et al., 1979
non-disjunction
and crossing over
Recombinant assay B. subtilis H17 Rc+ 2 mg/plate ? - Shirasu et al., 1976
M45 Rec- " + " "
Forward mutation A. nidulans strain 35 14 mg/disc ? + Bignami et al., 1977
E. coli B 5-25 mmol/l 95% +2 Wild, 1973
Gal RS ? ? + Fahrig, 1974
K12(5-MT) 0.3-3.2 mmol/l ? + Mohn, 1973
S. coelicolor A 3(2) his A1 5.6 mg/disc 99.9% + Carere et al., 1978a, 1978b
Reverse mutation E. coli B/r WP2 5 mg/plate > 97% +3 Moriya et al., 1978
S. typhimurium TA 1535 5 mg/plate > 97% +4 " "
E. coli WP2 hcr up to 5 mg/plate ? +3 Moriya et al., 1983
S. typhimurium TA 98 up to 5 mg/plate ? - " "
TA 100 " " ? + " "
TA 1535 " " ? ? " "
TA 1537 " " ? - " "
TA 1538 " " ? - " "
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation (cont'd) E. coli B/r Wp2( ); SR714 0.04-2.3 mmol/l 99% +3 Houk & DeMarini, 1987
CM 561 0.2% " +5 Bridges et al., 1973
CM 571; CM 611 " " -6 " "
WP2 " " +5 " "
WP2 uvr A " " +5 " "
WP2 micro drop of ? -7 Dean, 1972a
analytical grade,
technical grade
or 10% acqueous
solution/plate
K12HfrH 0.1% 97.5% + Voogd et al., 1972
C. freundii 425 0.05% or 0.1% " +8 " "
E. aerogenes 0.1% " + " "
K. pneumoniae 0.05% oe 0.1% " + " "
S. typhimurium 64-320 " " + " "
S. marcescens Hy/alpha 13 1.25-5 mg/disc ? + Dean, 1972a
Hy/alpha 21
E. coli WP2 WP2 5 µg/ml ? +9 Green et al., 1976
hcr+/hcr- 20-25 µl/disk 50% commercial +10 Nagy et al, 1975
WP2 5 mg/plate formulation " "
hcr+/hcr- ?
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation S. typhimurium TA 1535 5 mg/plate ? + Shirasu et al., 1976
(cont'd) TA 1536 " ? + " "
TA 1537 " ? - " "
TA 1538 " ? - " "
E. coli WP2 5 µl/plate11 ? + Hanna & Dyer, 1975
WP2 uvr A " ? + " "
WP 67 " ? + " "
CM 561 " ? - " "
CM 571 " ? - " "
CM 611 " ? - " "
S. typhimurium TA 1530
TA 1535 5 µl/plate ? + Hanna & Dyer, 1975
his C117 " ? + " "
his G46 " ? - " "
" ? - " "
P. aeroginusa PAO 38 0.08 mol/l ? + Dyer & Hanna, 1973
S. typhimurium his C117 0.03 mol/l ? + " "
S. typhimurium TA 98 ? ? -3 Braun et al., 1982
TA 100 ? ? +3 " "
TA 1535 ? ? -3 " "
TA 1536 ? ? -3 " "
TA 1537 ? ? -3 " "
TA 1538 ? ? -3 " "
TA 98 0.1-6.6 mg/plate12 99% -3 Chan, 1989:
Zeiger et al., 1988
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
TA 100 " " " +3 Carere et al., 1978a,b
TA 1535 2.8 mg/plate 99.9% -3 " "
TA 1536 " " " -3
TA 1537 " " "
S. typhimurium TA 1538 2.8 mg/plate 99.9% -3 Carere et al., 1978a,b
TA 1535 1.5 mg/ml 99.9% +
Schizosaccharmoyces pombe 1.5-14 mmol/plate13 > 99% +3 Gilot-Delhalle et al.,
ade 6 1983
Gene conversion S. cerevisiae D4 2-8 mg/ml > 97% +14,15 Dean et al., 1972
D4 6-40 mmol/l ? +2 Fahrig, 1973, 1974
632/4 ? ? - Guerzoni et al., 1976
+16 Griffin & Hill, 1978
DNA strand breaks E. coli K-12CR34Co1E1 1 mg/ml ?
Growth inhibition E. coli W3110 pol A+/pol A- 6.4 mmol/l ? + Rosenkranz, 1973
P. mirabilis PG 273; PG 713 ? ? +15 Braun et al., 1982
1 Without metabolic activation except where noted.
2 Positive controls (methyl methanesulfonate 1-4 mmol/l) yielded expected positive results.
3 Both with and without metabolic activation.
4 Only without metabolic activation.
5 Methyl methane sulfonate (0.04%) yielded positive responses.
6 Methyl methane sulfoante (0.04%) yielded negative responses.
7 Methyl methane sulfonate, N-methyl-N'-nitro-N-nitroso guanidine yielded positive response.
8 At 0.1%
9 Positive controls (methyl methanesulfonate 0.5-2.0 µg/ml) yielded expected positive results.
Table 4 (contd)
10 Positive controls (N-methyl-N'-nitro-nitroso guanidine, N-nitroso-N-methylurethane and acridium chloride)
yielded positive responses.
11 Toxic dose.
12 Toxic effect at 3.3 mg/plate.
13 The LD50 was 5.5 mmol/l.
14 Positive controls (ethyl methane sulfonate) yielded positive responses.
15 From 4 mg/ml.
16 Methyl methane sulfonate (2-10 mmol/l), N-methyl-N'-nitro-N-nitroso guanidine (3.4-6.8 mmol/l) yielded positive responses.
Table 5. Mutagenicity tests in mammalian cells in vitro
Test system Test object Concentration Purity Results Reference
Viral transformation Syrian hamster embryo 0.05-0.45 mmol/l ? +1 Hatch et al., 1986
cells/adenovirus SA7 cytotoxic
Gene mutation Chinese hamster V79 cells up to 1 mmol/l ? - Wild, 1975
(azaguanine resistance) 1.25-5 mmol/l ? +2 Aquilina et al., 1984
Mouse lymphoma L5178Y cells 6.25-50 nl/m3 ? +4,5 Chan, 1989
(trifluorothymidine resistance) 12.5-200 nl/m6 +5,7 Chan, 1989
DNA strand breaks Chinese hamster V79 cells 0.2% ? +8 Green et al., 1974
Sister chromatid exchange Primary rat tracheal epithelial cells 5-160 µg/ml9 93.9% +10,11 Lin et al., 1988
Chinese hamster ovary cells 0.03 and 0.1 mmol/l 98 + Nishio & Uyeki, 1981
0.1-0.5 mmol/l > 98% + Tezuka et al., 1980
0.3-1000 µg/ml ? +12 Wang et al., 1988
1-50 µg/ml ? +13 Chan, 1989
Human lymphocytes 2.5-10 µg/ml 99% - Nicholas et al., 1978
Human fetal lung fibroblasts 99% - Nicholas et al., 1978
Chromosomal aberrations Rat tracheal epithelial cells 5-160 µg/ml9 93.9% +11,14 Lin et al., 1988
Chinese hamster lung fibroblasts ? ? + Ishidate et al., 1981
Chinese hamster V79 cells 0.1-0.5 mmol/l > 98% +15 Tezuka et al., 1980
Chinese hamster ovary cells 16-160 µg/ml ? +16 Chan, 1989
Human lymphocytes 1-40 µg/ml > 99% -17 Dean, 1972b
Table 5 (contd)
Test system Test object Concentration Purity Results Reference
Unscheduled DNA synthesis Human lymphocytes 5-500 µg/ml 99.8% + Perocco & Fini, 1980
EUE human cells 6.5-650 mmol/l ? +18 Aquilina et al., 1984
DNA (sedimentation coefficient) Calf thymus DNA 0.1% ? + Rosenkranz &
Rozenkranz, 1972
DNA (thermolabile regions) Calf thymus DNA 45 mmol/l 99% - Olinski et al., 1980
DNA (resistance to micrococcal) Chinese hamster ovary cells 10 mmol/l ? + Nishio & Uyeki, 1980
1 Positive controls (benzo(a)pyrene 0.001-0.002 mmol/l) yielded expected positive responses.
2 Positive controls (ethyl methan sulfonate 20 mmol/l) yielded expected positive responses.
3 Growth inhibition from 12.5 nl/ml.
4 From 12.5 nl/ml.
5 Positive controls (methyl methanesulfonate 5 nl/ml) yielded expected positive responses.
6 Growth inhibition from 100 nl/ml.
7 From 100 nl/ml.
8 Negative at lower concentrations.
9 50% mortality was observed at 80 µg/ml.
10 From 10 µg/ml.
11 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 0.25-1 µg/ml, 50% mortality at 0.5 µg/ml)
yielded expected positive responses.
12 From 40 µg/ml.
13 From 10 µg/ml. When incubated with S9, positive response at 50 µg/ml.
14 From 80 µg/ml.
15 At 0.5 mmol/l.
16 At 160 µg/ml.
17 Cytotoxicity was observed at 5-40 µg/ml.
18 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine) yielded expected positive responses.
19 Possible index of DNA alkylation.
20 Possibly indicating structural rearrangement of chromatin.
Table 6. Mutagenicity tests in vivo
Test system Test object Concentration Purity Results* Reference
Crossing over/ Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recombination ++++/dp b cn bw
Sex-linked Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recessive lethal Sobels & Todd, 1979
mutation
Oregan K 0.01-0.1 ppm in food ? -2 Kramers & Knapp, 1978
commercial formulation
Chromosomal Drosophila melanogaster 1 ppm in food ? + Gupta & Singh, 1974
aberration
Autosomal recessive Drosophila melanogaster 0.1-0.75 ppm in food for ? + Hanna & Dyer, 1975
levels 18 months
Host-mediated assay Salmonella typhimurium 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
G46 His- in mice (NMRI)
Salmonella typhimurium 8-10 mg/kg bw p.o. 97.5% -3 Voogd et al., 1972
(64-320) in mice (Swiss)
Serratia marcescens 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
(a 21 Leu-) in mice (NMRI)
Sacchoromyces cerevisiae 50 or 100 mg/kg bw p.o. > 97% -3 Dean et al., 1972
(D4) in mice (CF1)
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Dominant lethal Female mice (CF1) 0, 25 or 50 mg/kg bw p.o. > 97% -4 Dean & Blair, 1976
0, 2 or 8 mg/m3 inhalation, > 97% - Dean & Blair, 1976
from weaning to 11 weeks
of age
Male mice (ICR/Ha Swiss) 0, 5 or 10 mg/kg bw/day ? - Epstein et al., 1972
p.o. for 5 days
(8 weeks of mating)
Single i.p. injection of ? - Epstein et al., 1972
13 or 16.5 mg/kg bw
(8 weeks of mating)
Male mice (Q) 2 ppm in drinking water, 99% - Degraeve et al., 1984a
5 days/week for 7 week
10 mg/kg bw i.p. 99% -5 Moutschen-Dahmen et al., 1981
Male mice (CF1) 30 or 55 mg/m3 inhalation > 97% - Dean & Thorpe, 1972b
for 16 h
2.1 or 5.8 mg/m3 inhalation > 97% -6 Dean & Thorpe, 1972b
for 23 hours/day for 4 weeks
Sister chromatid Male mice (B6C3F1) 5-35 mg/kg bw i.p. 99% -7 Kligerman et al., 1985
exchange peripheral lymphocytes
Male mice (B6C3F1) 6-40 mg/kg bw i.p. ? -8 Chan, 1989
bone marrow cells
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Micronucleus test Mice (Swiss Webster) 0.0075-0.015 mg/kg bw/day ? -9 Paik & Lee, 1977
i.p. for 2 or 4 days
Micronucleus test Mice (HRA/Skh, hairless) skin painting with tech.grade +10 Tungul et al., 1991
(in vitro/in vivo) skin keratinocytes 0-228 µg
(in 100 µl acetone)
Chromosomal Chinese hamster, both 10-15 mg/kg bw p.o. > 97% -11 Dean & Thorpe 1972a
aberrations sexes bone-marrow cells
Male mice (Q) 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
bone-marrow cells 5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Degraeve et al., 1984b
Degraeve et al., 1984b
Male mice (B6C3F1) bone 6-40 mg/kg bw i.p. ? -12 Chan, 1989
marrow cells
Female Syrian golden 0, 3, 6, 15 or 30 mg/kg 50% +13 Dzwonkowska & Hübner, 1986
hamster commercial
formulation
bone-marrow cells bw i.p.
Mice (CF1), both sexes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h
5 mg/m3 inhalation > 97% > 97% -11 Dean & Thorpe, 1972a
23 hours/day for 21 days
Male Chinese hamsters 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h.
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Chromosomal Mice (Q) spermatocytes 2 ppm in drinking-water 99% - Moutschen-Dahmen et al., 1981;
aberrations 5 days/week for 7 weeks Degraeve et al., 1984a
(cont'd)
Chinese hamsters 15 mg/kg bw p.o. > 97% -11 Dean & Thorpe, 1972a
spermatocytes
Mice (Q) spermatocytes 10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984a
Mice (CF1) spermatocytes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
for 16h
5 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
23h/day for 21 days
Chinese hamster 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
spermatocytes for 16 h
Mice (CF1) spermatogonia 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984b
DNA strand breaks Rats (Wistar), both 10 mg/kg bw i.p. 99.8% -14 Wooder & Creedy, 1979
sexes rat liver cell DNA
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Unscheduled DNA Male rats (F344) 0, 2, 10 or 35 mg/kg ? - Mirsalis et al., 1989
synthesis hepatocytes bw p.o.
Mice (B6C3F1) (both sexes) 0, 10, 20, 40 or > 98% -15 Bedford, 1991
forestomachrats 100 mg/kg bw p.o.
1 Approximate LD50
2 Positive controls (2.5 mmol/l ethyl methanesulfonate) yielded expected positive responses.
3 Positive controls (400 mg/kg bw p.o. ethyl methanesulfonate) yielded expected positive responses.
4 Positive controls (100 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
5 Positive controls in 2nd and 5th week of mating.
6 Positive controls (2000 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
7 Positive controls (2-acetylaminofluorene, ethyl methane sulfonate and N-nitroso morpholine) yielded positive results.
All animals treated with 35 mg/kg bw i.p. died.
8 Positive controls (ethyl methane sulfonate 100 mg/kg bw p.o.) yielded expected positive responses.
9 Positive controls (cyclophosamide 30-240 mg/kg bw i.p.) yielded expected positive responses.
10 Recovery of plated cells in vitro after a single topical application of dichlorvos was about 50% of controls.
11 Positive controls (endoxan 100-200 mg/kg bw i.p.) yielded expected positive responses.
12 Positive controls (ethyl methane sulfonate 300-375 mg/kg bw p.o.) yielded expected positive responses.
13 LD50 = 30 mg/kg bw i.p. No dose related response. Positive controls (cyclophosphamide 40 s. mg/kg bw i.p.)
yielded expected positive responses.
14 Positive controls (methyl methane sulfonate 30-60 mg/kg bw i.p.) yielded expected positive responses.
15 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 200 mg/kg bw p.o.) yielded weakly positive results.
Dichlorvos treatment induced a hyperplastic response similar to that induced by the non-genotoxic carcinogen butylated
hydroxyamisole (300 mg/kg bw p.o.).
Special studies on metabolites
Acute and short-term toxicity studies
The intraperitoneal toxicity of metabolites of dichlorvos in
female mice is considerably less than that of dichlorvos. The LD50
(mg/kg bw) was 250 for dichloroacetic acid, 440 for
dichloroacetaldehyde and 890 for dichloroethanol. For other
metabolites it was > 1000 mg/kg bw. A short-term inhalation study
with dichloroacetaldehyde in rats did not show adverse effects at
concentrations up to 2 mg/m3 for 30 days (WHO, 1989).
Methylating reactivity
In a quantitative colour test in which methylation of
4-(p-nitrobenzyl) pyridine was measured to predict DNA alkylating
potential, desmethyl-dichlorvos, dimethyl phosphate,
dichloroethanol, dichloroacetaldehyde and dichloroacetic acid gave
no reaction (Bedford & Robinson, 1972).
Mutagenicity
In a rec-type repair test with Proteus mirabilis strains
PG713 and PG273, desmethyldichlorvos (10 or 40 µmol/plate) did not
induce base-pair substitutions or other DNA damage (Braun et al.,
1982).
Dichloroacetaldehyde (DCA) appeared to be mutagenic in the
Salmonella test using S. typhimurium TA 100 and TA 1535. The
mutagenicity decreased in the presence of a microsomal activation
system (Lofroth, 1978; Bignami et al., 1980). It was also
positive when tested in concentrations of 10-40 µg/plate in a
forward and a back mutation system in S. coelicolor and two
forward mutation systems in A. nidulans (Bignami et al., 1980).
DCA (0.01%) was not mutagenic to K. pneumoniae in a fluctuation
test (Voogd et al., 1972). DCA did not induce unscheduled DNA
synthesis in the human epithelial-like cell EUE as well as
ouabain-resistant mutations in cultured V-79 Chinese hamster cells
(Aquilina et al., 1984). In a dominant lethal assay with DCA
administered as a single i.p. injection of 176 mg/kg bw to two
strains of male mice (AB Jena-Halle and DBA), positive effects were
reported (Fischer et al., 1977). It should be noted, however,
that the dose used would never be reached after dichlorvos treatment
(i.p. LD50 = 28-41 mg/kg bw). Ramel (1981) reported negative
results in a dominant lethal study with another strain of mice.
No evidence for mutagenicity of dichloroethanol was obtained in
S. typhimurium TA 100 and TA 1535 (Lofroth, 1978; Bignami et al.,
1980). In S. coelicolor (test doses 60-80 µg/plate) and A.
nidulans (test doses 10-50 µg/plate), dichloroethanol was a weak
mutagen (Bignami et al., 1980). Dichloroethanol (1 M and 0.1%)
was mutagenic to K. pneumoniae in a fluctuation test (Voogd et
al., 1972).
Special studies on skin sensitization
In the guinea-pig maximization test by Magnusson & Kligman,
using intradermal and topical induction concentrations of 5% and 25%
in water respectively, a 0.5% challenge concentration caused
sensitization in 30-40% of the animals but a 0.05% challenge
concentration caused no sensitization (Matsushita et al., 1985).
Special studies on testes
Mice
Groups of 14 male NMRI/Han mice received either a single oral
dose of 40 mg/kg bw dichlorvos in olive oil or 18 daily oral doses
of 0 or 10 mg/kg bw dichlorvos in olive oil.
On days 9,18, 27, 36, 54 and 63, two animals from each group
were killed and their testes examined histologically. A significant
increase in the number of damaged seminiferous tubules
(desquamation, decreases in cell population, "holes") was observed
in both dichlorvos groups. The supporting Sertoli cells were also
damaged, which may have resulted in the above effects. In addition,
there was an increase in the number and hypertrophy of Leydig cells.
No explanation was given for these effects (Krause & Homola, 1972,
1974).
An increased incidence, just above background, of sperm
abnormalities, was observed in a screening study on hybrid mice
given five daily i.p. injections of 10 mg/kg bw dichlorvos
(approximately half the LD50). At lower doses, 1 mg/kg bw, the
number of sperm abnormalities was either similar to or lower than
those in the controls (Wyrobek & Bruce, 1975).
Rats
Groups of 16 male juvenile Wistar rats received either 20 mg/kg
bw dichlorvos in olive oil on days 4 and 5, 10 mg/kg bw dichlorvos
in olive oil daily from days 4 to 23, or 0.1 ml olive oil daily from
days 4 to 23. On days 6, 12, 18, 26, 34, and 50 of life, two rats
from each group were sacrificed. Histological examination of the
testes showed slight reduction in the number of spermatogenic cells
and Leydig cells. All changes were reversed by the 50th day of
life. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells (Krause et al.,
1976).
In a subsequent experiment measurement of testosterone
concentrations in the testes, and luteinizing hormone (LH) and
follicle stimulating hormone (FSH) concentrations in serum showed no
differences between treated animals and olive-oil treated controls
(Krause, 1977). However, in this study the use of a different
dosing regimen (10 mg/kg bw by gavage every other day for 2 weeks)
prevented a strict comparison with the earlier study by Krause et
al. (1976).
Fifty-five male Wistar rats (aged 5 months) were orally
administered dichlorvos at levels of 5 or 10 mg/kg bw every other
day for 8 weeks. Eleven rats were killed every 4 weeks. No change
was seen in body-weight gain or testes weight. The score values of
the seminal cellular system decreased after 4-8 weeks of treatment,
but were restored 8 weeks after the end of treatment (Fujita et
al., 1977).
Miscellaneous studies
Effects of inhaled ChE inhibitors, including dichlorvos
(dissolved in acetone), on bronchial tonus were studied in young
adult rats exposed head-only. Bronchoconstriction did not occur at
toxicologically significant doses. An increase in response to
acetylcholine provocation test was observed (Pauluhn et al.,
1987).
The effect of diet on the toxicity of dichlorvos was
investigated using young male rats kept for 30 days on the following
diets: high protein (HPD), low protein (LPD), high fat (HFD), and
standard (SD). Growth rates were normal except for a slightly
decreased body-weight gain in the HFD group. A single i.p.
injection of 50 mg/kg bw dichlorvos led to slightly higher mortality
in LPD rats and slightly lower mortality in HPD rats, compared with
SD rats (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or
HPD for 30 days. At the end of this period, a single i.p. dose of
dichlorvos (20 or 30 mg/kg bw) was administered. No difference in
plasma and erythrocyte ChE inhibition was found. In the case of the
HPD, the spontaneous recovery of plasma and erythrocyte ChE activity
was slightly reduced (Purshottam & Srivastava, 1984).
Observations in humans
In vitro studies
The sensitivity to inhibition by dichlorvos of erythrocyte and
brain ChE was higher than that of plasma ChE, the I50s being about
10-8 and 10-7 mol/l, respectively (Skrinjaric-Spoljar et al.,
1973; Carter & Maddux, 1974; Lotti & Johnson, 1978; Boyer, 1978;
Casale et al., 1989). The rate of phosphorylation of human
erythrocyte ChE was similar to that of rat brain ChE (1.2 x 105 and
1.5 x 105 mol/l/min at 37 °C, pH 7.4-7,6, respectively).
The rate of dichlorvos hydrolysis by human plasma at 37 °C was
found to be 7-11 µmol/hour/ml (Reiner et al., 1980; Traverso et
al., 1989).
Studies on volunteers
Six healthy male volunteers were given single oral doses of 7.5
mg/kg bw of metrifonate. Concentrations of metrifonate (trichlorfon)
and dichlorvos, which is a metrifonate rearrangement product, were
determined in whole blood at different times for up to 24 hours.
The half-life of metrifonate was about 2 hours. The concentrations
of dichlorvos closely followed those of metrifonate with a constant
ratio of 0.01-0.02. However, a half-life for dichlorvos of 2 hours
cannot be accepted because dichlorvos is continuously formed from
metrifonate (Abdi & Villen, 1991).
Groups of five young men received total daily doses of 1, 1.5,
2 or 2.5 mg dichlorvos in corn oil per person, divided in two
gelatin capsules administered at 08.00 h and at 15.00 h for 28 days.
A further group of ten men received 1.5 mg/day for 60 days. Control
groups of two men per dose level received gelatin capsules with corn
oil. Plasma and erythrocyte ChE activities were determined twice
weekly before, during and after dosing. Once each week a medical
interview, complete blood count, and urinalysis were done on each
subject. Before dosing and after the last dose SGOT, ALP, PT,
thymol turbidity, and total bilirubin were determined.
The 2.5 mg/day dose produced a decrease in plasma ChE activity
from the second week of treatment; dosing was discontinued after 20
days when plasma values showed a 30% decrease. The plasma ChE
activity returned to control values 15 days after dosing was ended.
The 2 mg/day dose produced also a reduction in plasma ChE activity
from the second week of treatment which reached a maximum inhibition
of 29% the second day after the last dose. The group receiving 1.5
mg/day for 28 days showed no change in plasma ChE activity while in
the group dosed for 60 days this activity was inhibited by 27%
compared to the control values. None of the groups showed an effect
on erythrocyte ChE activity, haematology, clinical chemistry or
urinalysis. The NOAEL in this study was the highest dose tested of
2.5 mg/day/man (approximately 0.03 mg/kg bw/day) based on the
absence of inhibition of erythrocyte ChE activity (Rider, 1967;
Rider et al., 1967, 1968).
Two groups of six men (21-45 years of age, 64-106 kg bw)
received 0.9 mg dichlorvos three times a day for 21 days, either in
a pre-meal capsule filled with cottonseed oil or in a gelatin salad
consumed during the course of the meal. Control subjects were dosed
with either cotton seed oil capsules or plain gelatin. No
cholinergic signs or symptoms were observed. Plasma ChE activity
was inhibited by 30-40%, the inhibition being higher when dichlorvos
in cotton seed oil was ingested before the meal. The half-life for
the regeneration of plasma ChE activity was 13.7 days. Erythrocyte
ChE activity was not reduced. The NOAEL in this study was 0.04
mg/kg bw/day, based on absence of erythrocyte cholinesterase
inhibition (Boyer et al., 1977).
A number of studies have been done with a slow-release PVC
formulation of dichlorvos intended for use as an anthelminticum.
Single oral doses of above 4 mg dichlorvos/kg bw resulted in
inhibition of erythrocyte ChE activity 24 hours after application,
the maximum being 46% at 32 mg/kg bw. Plasma ChE activity was
affected at single doses of 1 mg/kg bw (50% inhibition) and above.
However, no dichlorvos-related symptoms were observed. Repeated
oral dosing for 7 days produced clinical symptoms with doses of 8
mg/kg bw/day and above. Plasma ChE activity was inhibited by about
80% at all dose levels (1-16 mg/kg bw/day). Erythrocyte ChE
activity showed a dose-related decrease from 5-30% at 1 mg/kg bw/day
up to 50-80% inhibition at the highest dose. Blood count, urine,
liver function, PT and BUN were normal in all studies (Hine &
Slomka, 1968, 1970; Pena-Chavarria et al., 1969; Slomka & Hine,
1981).
In children (aged 7-18 years) orally treated with metrifonate
(7.5, 10.0 or 12.5 mg/kg bw), the half-lives of recovery of
inhibited erythrocyte AChE and plasma ChE were 15 and 6.7 days,
respectively (Reiner & Plestina, 1979). It is known that the
anticholinesterase activity of metrifonate is due to its
decomposition to dichlorvos (Reiner et al., 1975). Similar
half-lives for plasma ChE have been described by Boyer et al.,
(1977) after repeated oral dosing with dichlorvos and by Bisby &
Simpson (1975) after cutaneous exposure of a sprayman to dichlorvos.
This longer half-life contrasts with faster in vitro half-lives,
which are similar to those of the rodent (Skrinjaric-Spoljar et
al., 1973).
Several inhalation studies have been carried out and are
summarized by WHO (1989). These studies confirmed that plasma ChE
is more sensitive to inhibition by dichlorvos than RBC ChE. The
latter was found inhibited when the dichlorvos dose (concentration x
time) was higher than about 1500 mg/m3/min (WHO, 1989).
Thirteen men (31-53 years of age) were exposed to dichlorvos
strips (20% dichlorvos; 1 strip per 30 m3) for 3 months. No
biologically or statistically significant changes were observed in
their electromyography results nor in their whole blood ChE activity
(Ottevanger, 1975).
Poisoning incidents
Fournier et al. (1978) reported that dichlorvos was detected
in blood in three cases of human intoxication within 24 hours after
poisoning (exact timing not reported).
A woman who intentionally ingested an estimated 100 mg
dichlorvos/kg bw survived following intensive care for 14 days
(Watanabe et al., 1974). A suicide with a dichlorvos dose of
about 400 mg/kg bw succeeded in spite of treatment (Shinoda et al.,
1972). A female patient, aged 35 years, who had accidentally
ingested 60 g fluid Divipan (dichlorvos concentration not reported),
was comatose for a week and recovered slowly. Clinical and
electrophysiological examinations showed a pure motor form of
neuropathy, according to the authors (Vasilescu & Florescu, 1980).
Three cases of poisoning with dichlorvos taken orally in unspecified
but high quantities have been reported from India. The patients
first showed severe cholinergic signs for a few days. After
recovery, delayed neurotoxicity developed. Nerve conduction studies
showed a severe axonal degeneration (Wadia et al., 1985, 1987).
Thirteen cases of dichlorvos ingestion, either accidental or
deliberate, were reported from a hospital in Beijing, China. The
patients included 10 women and three men, aged 11-38 years. Twelve
of the 13 patients ingested 25-50 ml of dichlorvos (80% purity).
Ten cases were associated with acute pulmonary oedema. Two patients
died because OP poisoning was not diagnosed in time. The others
recovered completely 2-5 days after treatment (Li et al., 1989).
Occupational Exposure
A number of fatal and non-fatal dichlorvos poisoning cases have
been described and summarized by WHO (1989). Two workers who failed
to promptly wash off the concentrated formulation of dichlorvos,
which splashed on to their skin, died subsequently. However, in
those cases where the spilled solution was washed off immediately,
the victims showed symptoms of intoxication but recovered after
treatment. Occupational exposure of spraymen entails both dermal
and respiratory absorption of dichlorvos. When appropriate
protective equipment was not used, inhibition of plasma ChE and less
frequently of RBC ChE was found. Sometimes mild, short lasting,
cholinergic symptoms and signs have been reported (WHO, 1989).
Each of 13 pest-control operators carried out urban
pest-control work for one day in 4 houses using 230-330 g dichlorvos
as aerosol and 40-50 g dichlorvos as emulsion spray. At the end of
the day's work, an operator had an average dichlorvos residue of 0.8
mg/m2 on the back, 0.4 mg/m2 on the chest, and 11 mg/m2 on the
respirator filter. Urinary dimethylphosphate excretion in 3
applicators ranged from 0.32 to 1.39 micrograms on the day of
treatment, but approached the level of detection by the following
morning. Blood and urine analyses revealed no changes in various
clinical parameters, including serum cholinesterase levels (Das et
al., 1983).
COMMENTS
Dichlorvos is rapidly absorbed by all routes of exposure and
rapidly degraded. The metabolic pathways of dichlorvos are similar
in mammalian species, including humans. Metabolites are rapidly
excreted or incorporated into natural enzymatic pathways.
Dichlorvos has a marked acute oral toxicity with typical
cholinergic signs and has been classified by WHO as highly
hazardous.
Rat erythrocyte and brain cholinesterase inhibited by
dichlorvos spontaneously reactivates with a half-life of about two
hours both in vitro and in vivo.
Several carcinogenicity studies in mice and rats using routes
other than gavage were negative, even when doses causing signs of
toxicity were used. It should be noted that two squamous-cell
carcinomas of the oesophagus have been observed in treated mice in
one study.
In a carcinogenicity study in mice dichlorvos administered by
corn oil gavage (0, 10 or 20 mg/kg bw/day to males, and 0, 20 or 40
mg/kg bw/day to females), caused forestomach papillomas
(statistically-significant positive trend with increased incidence
in the high-dose female group). Elements of the mechanism by which
these papillomas might arise have not been established, but the
induction of hyperplasia in the forestomach was demonstrated.
Additionally, genotoxic effects might occur at high local
concentrations of dichlorvos (see below) as can be obtained in
gavage dosing but not in dietary exposure. Based on the increased
incidence of forestomach papillomas, the NOAEL was 10 mg/kg bw/day.
In a two-year feeding study in rats (0, 0.1, 1, 10, 100 or 500
ppm), no neoplastic lesions were attributed to treatment. The
NOAEL, based on brain cholinesterase inhibition, was 100 ppm (actual
concentration 47 ppm, equivalent to 2.4 mg/kg bw/day).
In a carcinogenicity study in Fischer 344 rats, dichlorvos
administered by corn oil gavage (0, 4 or 8 mg/kg bw/day) caused an
increased incidence of pancreatic adenomas (statistically
significant in males only), mononuclear cell leukemias
(statistically significant in males only, no dose response) and
mammary gland adenomas or fibroadenomas (females only, no dose
response, statistically-significant in the low-dose group only).
The Meeting observed that the incidence of pancreatic acinar
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia which is usually high and variable in
this strain of rats, was also of questionable biological
significance. The doses used significantly inhibited plasma, but
not erythrocyte, cholinesterase activity when measured three hours
after treatment. However, given the rapid recovery of erythrocyte
cholinesterase activity after inhibition by dichlorvos, the timing
might have underestimated the inhibition.
Dichlorvos has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. These data indicate that
dichlorvos is genotoxic in bacteria and cultured mammalian cells,
but that it is not clastogenic in vivo except under conditions
where an unusually high tissue dose can be attained. Dichloro-
acetaldehyde, a major metabolite of dichlorvos, is a weak bacterial
mutagen. Positive results have been reported in mice given a dose
of dichloroacetaldehyde far greater than that which could derive
from sublethal doses of dichlorvos. Dichlorvos has been shown to
methylate DNA in vitro at a rate that is 8-9 orders of magnitude
lower than the rate of phosphorylation. Therefore, DNA alkylation
is not likely to occur at doses of dichlorvos which are not
inhibitory to erythrocyte/brain cholinesterase.
A three-generation reproduction study in rats was negative at
doses up to 235 ppm in the diet, equivalent to 12 mg/kg bw/day. A
one-litter, one-generation study in mice in which dichlorvos was
administered by inhalation at doses which caused > 90% plasma
cholinesterase inhibition, but no signs of toxicity, was negative.
Dichlorvos caused reversible damage of seminiferous tubules, Leydig
and Sertoli cells at oral doses of 10 mg/kg bw daily for 18 days in
mice and at 5 mg/kg bw and above every other day for 8 weeks in
rats.
Dichlorvos appeared not to be teratogenic in mice, rats and
rabbits at doses which caused maternal toxicity.
Dichlorvos caused delayed polyneuropathy in hens at doses much
higher than the unprotected LD50. Cases of delayed polyneuropathy
also have been reported in humans after severe intoxications.
In humans, the rate of dichlorvos hydrolysis by plasma is
similar to that in rats. The rate of recovery of inhibited
erythrocyte and plasma cholinesterase activity in humans given
dichlorvos is much slower than in rats. Half-lives of recovery are
about 15 days in humans and about two hours in rats. A daily dose
of 1 mg/kg bw to male human volunteers for seven days caused 5-30%
inhibition of erythrocyte cholinesterase. The NOAEL in humans,
based on absence of erythrocyte cholinesterase inhibition in 12
volunteer males for 21 days was 0.04 mg/kg bw/day.
In 1986, the Joint Meeting discussed the significance of
carcinogenicity studies for organophosphorus pesticides and the
requirements for further studies (Section 3.1 of Annex 1, reference
47). At that time none of the organophosphorus pesticides had
caused a carcinogenic response in experimental animals. That Joint
Meeting recommended that, depending upon future evaluation on a case
by case basis, further consideration should be given to the need for
carcinogenicity studies for organophosphates.
In assessing the potential hazard to humans of residues of
dichlorvos, the following considerations were taken into account in
view of the weakly positive results in the gavage carcinogenicity
study in mice.
Organophosphorus esters used as insecticides react with
biological molecules by means of phosphorylation of serine
hydrolases and of alkylation of macromolecules. Phosphorylation of
acetylcholinesterase and alkylation of DNA are considered to account
for the acute cholinergic toxicity and initiation of the
carcinogenic process, respectively. These biochemical reactions
occur at different rates. When the rate of phosphorylation is
substantially higher than the rate of alkylation, in vivo
genotoxic effects are unlikely to occur because effective doses
cannot be achieved due to acute toxicity. Dichlorvos meets these
criteria, the rate of phosphorylation of acetylcholinesterase being
much faster (eight orders of magnitude) than that of alkylation of
several macromolecules, including DNA. Hence positive mutagenicity
tests were seen only in vitro and, as indicated in the 1986 Joint
Meeting report, carcinogenicity studies are unlikely to give more
information. The weak carcinogenic response of dichlorvos obtained
in mice in a corn oil gavage study should be interpreted as a local
effect of dichlorvos.
Information on comparative cholinergic toxicity might be of
critical relevance for the extrapolation of toxic effects (other
than acute effects) of organophosphates in experimental animals to
humans. The characteristics of the interactions of a given compound
with acetylcholinesterase (rates of phosphorylation, spontaneous
reactivation and ageing) from different species can be compared in
vitro. Also, the in vivo rate of reappearance of blood
acetylcholinesterase activity can be measured. In some cases,
metabolic degradation of organophosphates can be assessed
comparatively by measuring the level of serum A esterase, which
hydrolyses a given compound. All these data enable an improved
assessment of cholinergic toxicity of organophosphates in different
species. This knowledge may be of special significance in the case
of dimethyl phosphates since the rates of in vivo reactivation
vary substantially across species. Therefore, chronic dosing is
more critical for extrapolation from animal data to humans. In a
repeated dose regime, the longer the half-life of reactivation the
more rapid and/or more toxic will be the resulting effect (i.e. in a
chronic dosing regime, humans will survive much lower doses of
dichlorvos causing, when given alone, peak erythrocyte/brain
cholinesterase inhibition than those which can be reached in
rodents). Therefore, comparison between the in vivo rates of
recovery of enzyme activity will enable an assessment of the
repeated doses of compounds and the resulting cholinesterase
inhibition, which would represent the limiting factors for other
toxicities (including mutagenicity and carcinogenicity).
In the case of dichlorvos, the Meeting considered the
extrapolation of carcinogenicity data derived in rodents and its
applicability to human safety, and concluded that the compound would
not result in chronic human health hazards at doses below those
which result in acetylcholinesterase inhibition.
The Meeting maintained the ADI, which is based on studies in
humans with a NOAEL of 0.04 mg/kg bw/day, using a 10-fold safety
factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 mg/kg bw/day (two-year study)
Rat: 47 ppm in the diet, equivalent to 2.4 mg/kg bw/day
(two-year study)
Human: 0.04 mg/kg bw/day (21-day study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abdi, Y.A. & Villen, T. (1991) Pharmacokinetics of metrifonate and
its rearrangement product dichlorvos in whole blood. Pharmacol.
Toxicol. 68: 137-139.
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti,
E., Falcone, E. & Carere, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon and dichloroacetaldehyde. Pestic. Sci. 15:
439-442.
Asperen van, K., Dekhuijzen, H.M. (1958) A quantitative analysis of
the kinetics of cholinesterase inhibition in tissue homogenates of
mice and houseflies. Biochim. Biophys. Acta, 28: 307-337.
Bedford, C.T. & Robinson, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2: 307-337.
Benford, D.J. (1991) Investigation of the genotoxic and/or irritant
effects of dichlorvos on mouse forestomach. Unpublished report no.
RI90/0405 from Robens Institute of Health and Safety, Guildford,
Surrey, U.K.. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. & Morpurgo, G.
(1977) Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutat. Res. 46: 395-402.
Bignami, M., Conti, G., Conti, L., Crebelli, R., Misuraca, F.,
Ruglia, A.M., Randazzo, R., Sciandrello, G. & Carere, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor and Asperigillus nidulans.
Chem.-Biol. Interactions, 30: 9-23.
Bisby, J.A. & Simpson, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Austr. 2: 394-395.
Blair, D., Dix, K.M., Hunt, P.F., Thorpe, E., Stevenson, D.E. &
Walker, A.I.T. (1976) Dichlorvos - a 2-year inhalation
carcinogenesis study in rats. Arch. Toxicol. 35: 281-294.
Boyer, A.C. (1978) Factors which affect the in vitro inhibition of
human plasma cholinesterase. Toxicol. Appl. Pharmacol. 41: 475-
483.
Boyer, A.C., Brown, L.J., Slomka, M.B. & Hine, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. Appl. Pharmacol. 41:
389-394.
Braun, R., Schoneich, J., Weissflog, L. & Dedek, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair - direct alkylation versus metabolic activation and
breakdown. 1. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem.-Biol.
Interact. 39: 339-350.
Bridges, B.A., Mottershead, R.P., Green, M.H.L. & Gray, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulphonate for
Escherichia coli WP2 and some derivatives deficient in DNA repair.
Mutat. Res. 19: 295-303.
Brown, V.K.H. & Roberts, M (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to
guinea-pigs. Unpublished report no. IRR TL/40/66 from Shell Research
Ltd., Sittingbourne, UK. Submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Buselmayer, W., Rohrborn, G., Propping, P. (1972) Mutagenicity
investigations with pesticudes in the host-mediated assay and the
dominant lethal test in mice. Biol. Zbl. 91: 311-325 (in German).
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M., Raschetti,
R. (1978a) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res. 57: 277-286.
Carere, A., Ortali, V.A., Cardamone, G., Morpurgo, G. (1978b)
Mutagenicity of dichlorvos and other structurally related pesticides
in Salmonella and Streptomyces. Chem.-Biol. Interactions, 22:
297-308.
Caroldi, S., Lotti, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett. 9:
157-159.
Carter, M.K., Maddux B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. Appl. Pharmacol., 27: 456-463.
Casale, G.P., Bavari, S., Connolly, J.J. (1989) Inhibition of human
serum complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam. Appl. Toxicol. 12: 460-
468.
Casebolt, D.B., Leary, S.L., Undeutsch, L. (1990) Effects of
dichlorvos treatment on mouse reproduction. Lab. Anim. Care, 40:
65-67.
Chan, P.C. (1989) Toxicology and carcinogenesis studies of
dichlorvos (CAS No. 62-73-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, National Toxicology
Program, Technical Report Series NTP TR No.342.
Chan, P.C., Huff, J., Haseman, J.K., Alison, R. & Prejean, J.D.
(1991) Carcinogenesis studies of dichlorvos in Fischer rats and
B6C3F1 Mice. Jpn. J. Cancer Res. 82: 157-164.
Chattopadhyay, D.P., Dighe, S.K., Dube, D.K., & Purnanand (1982)
Changes in toxicity of DDVP, DFP, and parathion in rats under cold
environment. Bull. Environ. Contam. Toxicol. 29: 605-610.
Cohen, S.D., Ehrich, M. (1976) Cholinesterase and carboxyl esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. Appl. Pharmacol. 37: 39-48.
Coulston, F., Griffin, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP vapors.
Unpuplished report from Albany, New York, Institute of Comparative
and Human Toxicology and Holloman, Air Force Base, New Mexico,
Intern. Center of Environmental Safety. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Das, Y.T., Taskar, P.K., Brown, H.D., Chattopadhyay, S.K. (1983)
Exposure of professional pest control operator to dichlorvos (DDVP)
and residue on house structures. Toxicol. Letters, 17: 95-99.
Dean, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on micro-organisms. Arch. Toxikol. 30: 67-74.
Dean, B.J., (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol. 30: 75-85.
Dean, B.J. & Blair,D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres
containing dichlorvos. Mutat. Res. 40: 67-72.
Dean, B.J., Thorpe, E. (1972a) Cytogenetic studies with dichlorvos
in mice and Chinese hamsters. Arch. Toxikol. 30: 39-49.
Dean, B.J., Thorpe, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxicol. 30: 51-59.
Dean, B.J., Doak, S.M.A., Funnell, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium using
Saccharomyces cerevisiae. Arch. Toxicol. 30: 61-66.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984a) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol. 56: 66-67.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Letters, 21: 315-319.
Dieter, M.P., Jameson, C.W., French, J.E., Gangjee, S. (1989)
Development and validation of a cellular transplant model for
leukemia in Fischer rats: a short-term assay for potential
anti-leukemic chemicals. Leukemia Research, 13: 841-849.
Dyer, K.F., Hanna, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res. 21: 175-177.
Dzwonkowska, A., Hubner, H. (1986) Induction of chromosomal
aberrations in the Syriam hamster by insecticides tested in vivo.
Arch. Toxicol. 58: 152-156.
Enomoto, M.F., Nakadate, M., Ninomia, K., Hayakawa, Y., Ito, H.,
Igarashi, S., Uwanuma, Y., Nakasato, R., Hatanaka, J. (1981) Studies
on carcinogenicity of DDVP (2,2-dichlorovinyl dimethyl phosphate)
mixed in drinking-water in rats. Ministry of Health and Welfare,
Tokyo, Japan (Cooperative studies on carcinogenicity test on
mutagens) (Unpublished).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325.
Fahrig, R. (1973) (Genetic effects of organophosphorous
insecticides.) Die Naturwissenschaften, 60: 50-51 (in German).
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
IARC Scient. Publ. 10: 161-181.
Farber, T.M., Marks, E.M., Cerra, F.E. (1975) Dichlorvos and
microsomal enzyme activity. Fed. Proc. 34: 785.
Fischer, G.W., Schneider,P., Scheufler, H. (1977) (Mutagenicity of
dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester, possoble metabolites of the organophosphorus
pesticide trichlorphon.) Chem.-Biol. Interactions, 19: 205-213 (in
German).
Fournier, E., Sonnier, D., Dally, S., (1978) Detection and assay of
organophosphate pesticides in human blood by gas chromatography.
Clin. Toxicol. 12: 457-462.
Francis, B.M., Metcalf, R.L., Hansen, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. Environ. Sci. Health, B20: 73-95.
Fujita, K., Matsushima, S., Abe, E., Sasaki, K., Kurosawa, K. (1977)
(Examination of the effects of dichlorvos on the testis.) Nippon
Noson Igakkai Zasshi, 26: 328-329 (in Japanese) as quoted by WHO,
1989.
Gilot-Delhalle, J., Colizzi, A., Moutschen, J., Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade 6
locus of Schizosaccharomyces pombe. Mutat. Res: 117: 139-148.
Gough, B.J., Shellenberger, T.E. (1977-1978) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors
and reactivation with oximes. Drug and Chemical Toxicol. 1: 25-43.
Green, M.H.L., Medcalf, A.S.C., Arlett, C.F., Harcourt, S.A.,
Lehmann, A.R. (1974) DNA strand breakage caused by dichlorvos,
methyl methanesulphonate and iodoacetamide in Escherichia coli and
cultured Chinese hamster cells. Mutat. Res. 24: 365-378.
Green, M.H.L., Muriel, W.J., Bridges, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res. 38: 33-42.
Griffin III, D.E., Hill, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res. 52: 161-169.
Guerzoni, M.E., Del Cupolo, L., Ponti, I. (1976) (Mutagenic
activity of pesticides). Rev. Sci. Tecn. Alim. Nutr. Um. 6:
161-165 (in Italian).
Gupta, A.K., Singh, J. (1974) Dichlorvos (DDVP) induced breaks in
the salivary gland chromosomes of Drosophila melanogaster. Current
Science, 43: 661-662.
Hanna, P.J., Dyer, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res. 28: 405-420.
Haseman, J.K., Huff, J.E., Rao, G.N., Arnold, J.E., Boorman, G.A.,
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1
(B6C3F1) mice. J. Natl. Cancer Inst. 75: 975-984.
Hass, D.K., Collins, J.A., Kodama, J.K. (1972) Effects of orally
administered dichlorvos in Rhesus monkeys. J. Am. Vet. Med. 161:
714-719.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W.,
Schechtman, L.M. (1986) Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutag. 8:
515-531.
Hine, C.H. (1962) 90-day chronic toxicity studies of Vapona
insecticide for dogs. Unpublished report from San Francisco, The
Hine Laboratories, Report no. 1, September 1. Prepared for Shell
Chemical Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd,
Temana International Ltd.
Hine, C.H., Slomka, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
Hine, C.H., Slomka, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. Appl. Pharmacol. 17:
304-305.
Horn, K.H., Teichmann, B., Schramm, T. (1987) (Studies on
dichlorvos. I. Testing of dichlorvos for carcinogenic activity in
mice.) Archiv für Geschwulstforschung, 57: 353-360 (in German).
Horn, K.H., Teichmann, B., Schramm, T., Nischan, P. (1988) (Studies
on dichlorvos. II. Testing of dichlorvos for carcinogenic activity
in rats). Archiv für Geschwulstforschung, 58: 1-10 (in German).
Horn, K.H., Teichmann, B., Schramm, T. (1990) (Studies on
dichlorvos. III. Testing of dichlorvos for co-carcinogenic activity
in mice). Archiv für Geschwulstforschung, 60: 117-124 (in German).
Houk, V.S., DeMarini, D.M. (1987) Induction of prophage lambda by
chlorinated pesticides. Mutat. Res. 182: 193-201.
IARC (1979) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Some halogenated hydrocarbons, Volume 20:
97-127.
IARC (1991) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Occupational exposures in insecticide
application, and some pesticides. Volume 53: 267-307.
Ishidate, M., Sofuni, T., Yoshikawa, K. (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monograph on Cancer
Research, 27: 95-108.
Jayasuriya, V.U. de S., Ratnayake, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inform. Serv. 50: 184-186.
Johnson, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch. Toxicol.
41: 107-110.
Jolley, W.P., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of Vapona insecticide into their daily diet.
Unpublished report from Cincinnati, The Kettering Laboratory,
January 19. Prepared for Shell Chemical Company. Submitted to WHO by
Bayer AG, Ciba-Geigy Ltd, Temana International Ltd.
Kimbrough, R.D., Gaines, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. Environ.
Health, 16: 805-808.
Kligerman, A.D., Erexson, G.L., Wilmer, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res. 157: 181-187.
Konishi, Y., Denda, A., Kitaoka, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3F1 mice. Tokyo, Ministry of
Health and Welfare, Japan (unpublished).
Kramers, P.G.N., Knaap, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of Drosophila
melanogaster. Mutat. Res. 57: 103-105.
Krause, W. (1977) Influence of DDT, DDVP and malathion on FSH, LH
and testosterone serum levels and testosterone concentrations in
testis. Bull. Environ. Contam. Toxicol. 18: 231-242.
Krause, W., Homola, S. (1972) (Influence on spermatogenesis by DDVP
(dichlorvos).) Arch. Dermatol. Forschung, 244: 439-441 (in
German).
Krause, W., Homola, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat testis after the
application of dichlorvos (DDVP). Bull. Envirom. Contam. Toxicol.
11: 429-433 (study is performed in mice).
Krause, W., Hamm, K., Weissmuller, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. Environ. Contam. Toxicol. 15: 458-462.
Lawley, P.D., Shah, S.A., Orr, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (Dichlorvos, DDVP).
Chem.-Biol. Interactions, 8: 171-182.
Li, C., Miller, W.T., Jiang, J. (1989) Pulmonary edema due to
ingestion of organophosphate insecticide. Am. J. Roentgenol., 152:
265-266.
Lin, S.Y., Lee, T.C., Cheng, C.S., Wang, T.C. (1988) Cytotoxicity,
sister-chromatid exchange, chromosome aberration and transformation
induced by 2,2-dichlorovinyl-0,0-dimethyl phosphate. Mutat. Res.
206: 439-445.
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57: 393-394.
Lofroth, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
naturforsch. 29: 651.
Lofroth, G., Wennerberg, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch. 41:
215-221.
Lotti, M., Johnson, M.K. (1978) Neurotoxicity of organopho-sphorus
pesticides: Predictions can be based on in vitro studies with hen
and human enzymes. Arch. Toxicol. 41: 215-221.
Maslinska, D., Zalewska, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia Histochem. Cytochem. 16: 335-341.
Matsushita, T., Aoyama, K., Yoshimi, K., Fujita, Y., Ueda, A. (1985)
Allergic contact dermatitis from organophosphorus insecticides.
Ind. Health, 23: 145-153.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L., Loh, E.K., Hamilton,
C.M., Bakke, J.P., Spalding, J.W. (1989) Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: Testing of 24 compounds. Environ. Mol. Mutat.
14: 155-164.
Modak, A.T., Stavinoha, W.B., Weintraib, S.T. (1975) Dichlorvos and
the cholinergic system: effects on the cholinesterase and
acetylcholine and choline contents of rat tissues. Arch. Int.
Pharmacodyn. 217: 293-301.
Mohn, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides. Mutat.
Res. 20: 7-15.
Moriya, M., Kato, K., Shirasu, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res. 57: 259-263.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y., (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res. 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979) Analysis of mitotic nondisjunction with
Aspergillus nidulans. Environ. Health Aspects, 31: 81-95.
Moutschen-Dahmen, J., Moutschen-Dahmen, M., Degraeve, N. (1981)
Metrifonate and dichlorvos: cytogenetic investigations. Acta
Pharmacol. Toxicol. 49(V): 29-39.
Nagy, Zs., Mile, I., Antoni, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta Microbiol. Acad.
Sci. Hung. 22: 309-314.
NCI (National Cancer Institute) (1977) Bioassay of dichlorvos for
possible carcinogenicity. Bethesda, Maryland, USA.
Nicholas, A.H., Vienne, M., Berghe, H. van den (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Letters, 2:
271-275.
Nishio, A., Uyeki, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphate
insecticides and their oxygen analogs. J. Toxicol. Environ. Health,
8: 939-946.
Nishio, A., Uyeki, E.M. (1982) Nuclease-sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants.
Biochem. Biophys. Research Comm. 107: 485-491.
Nordgren, I., Bergstrom, M., Holmstedt, B., Sandoz, M. (1978)
Transformation and action of metrifonate. Arch Toxicol. 41: 31-41.
Olinski, R., Walter, Z., Wiaderkiewicz, R., Lukasova, E., Palecek,
E. (1980) Changes in DNA properties due to treatment with the
pesticides malathion and DDVP. Radiat. Environ. Biophys. 18:
65-72.
Ottevanger, C.F. (1975) (Neuromuscular function in people exposed to
dichlorvos resin strips. The importance of electromyography.)
Europ. J. Toxicol. 8: 192-195 (in French).
Pachecka, J., Sulinski, A., Ziolkowska, G. (1975) The activities of
some esterases of the rat brain after intoxication by
organophosphate insecticides, dichlorvos and trichlorphon.
Neuropat. Pol. 13: 455-462.
Paik, S.G., Lee, S.J. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool. 20:
19-28.
Pauluhn, J., Machemer, L., Kimmerle, G. (1987) Effects of inhaled
cholinesterase inhibitors on bronchial tonus and on plasma and
erythrocyte acetylcholine esterase activity in rats. Toxicology,
46: 177-190.
Pena-Chavarria, A., Swartzwelder, J.C., Villarejos, V.M., Kotcher,
E., Arguedas, J. (1969) Dichlorvos, an effective broad-spectrum
anthelmintic. Am. J. Trop. Med. Hyg. 18: 907-911.
Perocco, P., Fini, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
Purshottam, T., Kaveeshwar, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviation, Space,
and Environ. Med. 50: 581-584.
Purshottam, T., Srivastava, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
Environ. Health, 39: 425-430.
Pym, R.A., Singh, G., Gilbert, W.S., Armstrong, J.P., McCleary, B.V.
(1984) Effects of dichlorvos, maldison and pirimiphos-methyl on
food consumption, egg production, egg and tissue residues, and
plasma acetylcholinesterase inhibition in layer strain hens. Aust.
J. Exp. Agric. Anim. Husb. 24: 83-92.
Ramel, C. (1981) Does dichlorvos constitute a genotoxic hazard?
Progress in Mutat. Res. 2: 69-78.
Rauws, A.G., Logten, M.J. van (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in chicken.
Toxicology, 1: 29-41.
Reiner, E., Plestina, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by
0,0-dimethyl-2,2-dichlorovinyl phosphate. Toxicol. Appl. Pharmacol.
49: 451-454.
Reiner, E. Krauthacker, B., Simenon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
0,0-dimethyl-2,2,2-trichloro-1-hydroxyethylphosphonate
(trichlorphon). Biochem. Pharmacol. 24: 717-722.
Reiner, E., Simeon, V., Skrinjaric-Spoljar, M. (1980) Hydrolysis of
O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP) by esterases in
parasitic helminths, and in vertebrate plasma and erythrocytes.
Comp. Biochem. Physiol. 66: 149-152.
Rider, J.A. (1967) Determination of the minimal incipient toxicity
of dichlorvos in humans. Unpublished report from San Francisco,
Gastrointestinal Research Lab., October. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Rider, J.A., Moeller, H.C., Puletti, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies
with Guthion, and determination of incipient toxicity level of
dichlorvos in humans. Fed. Proc. 26: 427.
Rider, J.A., Moeller, H.C., Puletti, E.J., Swader, J. (1968) Studies
on the anticholinesterase effects of methyl parathion, Guthion,
dichlorvos, and Gardona in human subjects. Fed. Proc. 27: 597.
Rosenkranz, H.S. (1973) Preferential effect of dichlorvos (Vapona)
on bacteria deficient in DNA polymerase. Cancer Research, 23:
458-459.
Rosenkranz, H.S., Rosenkranz, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia, 28: 386-387.
Segerback, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP
(2,2-dichlorovinyl dimethylphosphate). Hereditas, 94: 73-76.
Segerback, D., Ehrenberg, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta Pharmacol. Toxicol. 49(V): 56-66.
Shellenberger, T.E. (1980) Organophosphate pesticides inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. Environ. Sci. Hlth. B15: 795-822.
Shellenberger, T.E., Newell, G.W., Okamoto, S.S., Sarros, A. (1965)
Response of rabbit whole-blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14:
943-952.
Shinoda, H., Ito, K., Matsunaga, T., Takada, T. & Nomura, T. (1972)
(Two suicides by acute pesticide intoxication). J. Jpn. Assoc.
Rural Med. 21: 242-249 (in Japanese).
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shooter, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating agents.
Chem. Biol. Interactions, 11: 575-588.
Skrinjaric-Spoljar, M., Simeon, V., Reiner, E. (1973) Spontaneous
reactivation and aging of dimethylphosphorylated
acetylcholinesterase and cholinesterase. Biochim. Biophys. Acta,
315: 636-369.
Slomka, M.B., Hine, C.H. (1981) Clinical pharmacology of dichlorvos.
Acta Parmacol. Toxicol. 49(V): 105-108.
Snow, D.H., Watson, A.D. (1973) The acute toxicity of dichlorvos in
the dog: 1. clinical observations and clinical pathology. Aust.
Vet. J. 49: 113-119.
Sobels, F.H., Todd, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res. 67: 89-92.
Stanton, H.C., Albert, J.R., Mersmann, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows.
Am. J. Vet. Research, 40: 315-320.
Stevenson, D.E., Blair, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Tox. 18: 215-217.
Teichert, K., Szymczyk, T., Consolo, S., Ladinsky, H. (1976) Effect
of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. Appl. Pharmacol. 35: 77-81.
Tezuka, H., Ando, N., Suzuki, R., Terahta, M., Moriya, M., Shira-su,
Y. (1980) Sister-chromatid exchanges and chromosomal aberrations in
cultured Chinese hamster cells treated with pesticides positive in
microbial reversion assays. Mutat. Res. 78: 177-191.
Traverso, R., Moretto, A., Lotti, M. (1989) Human serum
"A"-esterases. Hydrolysis of 0,0-dimethyl-2,2-dichlorovinyl
phosphate. Biochem. Pharmacol. 38: 671-676.
Tungul, A., Bonin, A.M., He, S., Baker, R.S. (1991) Micronuclei
induction by dichlorvos in the mouse skin. Mutagenesis, 6:
405-408.
Uchiyama, M., Yoshida, T., Homma, K., Hongo, T. (1975) Inhibition
of hepatic drug-metabolizing enzymes by thiophosphate insecticides
and its drug toxicological implications. Biochem. Pharmacol. 24:
1221-1225.
Vasilescu, C., Florescu, A. (1980) Clinical and electrophysiological
study of neuropathy after organophosphorous compounds poisoning.
Arch. Toxicol. 43: 305-315.
Voogd, C.E., Jacobs, J.J., Stel, J.J. van der (1972) On the
mutagenic action of dichlorvos. Mutat. Res. 16: 413-416.
Wadia, R.S., Shinde, S.N., Vaidya, S. (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurol. India, 33:
247-253.
Wadia, R.S., Chitra, S., Amin, R.B., Kiwalkar, R.S., Sardesai, H.V.
(1987) Electrophysiological studies in acute organophosphate
poisoning. J. Neurol. Neurosurg. Psychiatry, 50: 1142-1148.
Wang, T., Wu, C., Lin, J., Tarn, C., (1988) Dichlorvos potentiates
the insecticide-induced sister chromatid exchanges in Chinese
hamster ovary cells. Bull. Inst. Zool. Academia Sinica, 27:
111-117.
Ward, F.P., Glicksberg, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Ass. 158:
457-461.
Watanabe, H., Shirai, O., Ebata, N. (1974/1976) (A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care.) Sapporo Igaku Zasshi, 43: 334-337 (in Japanese);
Pest. Abstr., 8: 94-95.
Weisburger, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D. (eds). Effects of chronic exposures
to pesticides on animal systems, pp. 165-176. New York, Raven Press.
Wennerberg, R., Lofroth, G. (1974) Formation of 7-methylguanine by
dichlorvos in bacteria and mice. Chem.-Biol. Interactions, 8:
339-348.
WHO (1989) Environmental Health Criteria 79: Dichlorvos.
International Programme on Chemical safety, Geneva, World Health
Organization.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wild, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of
dichlorvos and methyl methanesulphonate. Mutat. Res. 19: 33-41.
Wild, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res. 32: 133-150.
Witherup, S., Caldwell J.S., Hull, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their
offsprings by the introduction of Vapona insecticide into their
diets. Unpublished report by Kettering Laboratory.
Witherup, S., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction
of Vapona insecticide into their daily diets. Unpublished report
from Cincinnati, The Kettering Laboratory, February 14. Prepared for
Shell Chemical Company, submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Wooder, M.F., Creedy, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo. Unpublished
report. Sittingbourne, Shell Research Ltd., TLGR. 79.089.
Wooder, M.F., Wright, A.S. (1981) Alkylation of DNA by
organophosphorus pesticides. Acta Pharmacol. Toxicol. 49(V):
51-55.
Wooder, M.F., Wright, A.S., King, L.J. (1977) In vivo alkylation
studies with dichlorvos at practical use concentrations.
Chem.-Biol. Interact. 19: 25-46.
Wooder, M.F., Wright, A.S., Stevenson, D.E. (1978) Studies on the
in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol. 1: 505.
Wright, A.S., Hutson, D.H., Wooder, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol. 42: 1-18.
Wyrobek, A.J., Bruce, W.R. (1975) Chemical induction of sperm
abnormalities in mice. Proc. Nat. Acad. Sci. USA, 72: 4425-4429.
Yoshida, T., Nomura, M., Suzuki, Y. (1976) Inhibition of hepatic
UDP-glucuronyltransferase activity by organophosphate insecticides
and by carbon disulfide in mice. Bull. Environ. Contam. Toxicol.
15: 421-424.
Zalewska, Z., Rakowska, I., Matraszek, G., Sitkiewicz, D. (1977)
Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterase. Neuropat. Pol.
15: 255-262.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagenesis, 11(12): 1-157.
Effects on enzymes and other biochemical parameters
In vitro studies showed that rodent plasma ChE is more
sensitive than erythrocyte and brain ChE to inhibition by
dichlorvos. Calculated I50s (concentrations which inhibit 50% of
enzyme activity) at 37 °C, pH 7.4-7.6, were 10-8 and 10-7 mol/l,
respectively, for an incubation time of 20 minutes (Asperen &
Dekhuijzen, 1958; Skrinjaric-Spoljar et al., 1973; Lotti &
Johnson, 1978). The rate of phosphorylation of rat brain ChE was
calculated to be 1.5 x 105 mol/l/min at 37 °C, pH 7.4-7.6
(Skrinjaric-Spoljar et al., 1973). Half-life of in vitro
reactivation (at 37 °C, pH 7.4-7.6) was found to be about 100
minutes; that of aging was found to be about 400 min. This means
that inhibited AChE almost completely reactivates within a few
hours, leaving a small fraction (20% or less) irreversibly inhibited
(i.e. aged) (Skrinjaric-Spoljar et al., 1973).
TOXICOLOGICAL STUDIES
Acute toxicity studies
The results of acute toxicity tests of dichlorvos administered
by various routes to different animal species are summarized in
Tables 1 and 2. Typical cholinergic signs are observed. WHO has
classified dichlorvos as highly hazardous (WHO, 1992).
Short-term toxicity studies
Mice
In a range-finding study, groups of 10 B6C3F1 mice/sex were
administered by corn oil gavage 0, 5, 10, 20, 40, 80, or 160 mg
dichlorvos/kg bw/day, 5 days per week for 13 weeks. Animals were
observed twice daily for clinical signs and body weight was recorded
weekly. Necropsy was performed on all animals. Oesophagus and
gastrointestinal tract of all animals dying after day 46 were
examined histologically. All vehicle controls and all animals in
the highest dose group with survivors were examined histologically.
All mice at 160 mg/kg bw/day and 5 male mice at 80 mg/kg bw/day died
before the end of the study. Final mean body weights in all treated
groups were comparable to controls. No gross or microscopic
pathologic effects were observed (Chan, 1989).
Rats
In a range-finding study, groups of 10 male and 10 female
F344/N rats were administered by corn oil gavage 0, 2, 4, 8, 16, 32,
or 64 mg dichlorvos/kg bw/day, 5 days per week for 13 weeks.
Animals were observed twice daily for clinical sign and body weights
were recorded weekly. Necropsy was performed on all animals.
Oesophagus and gastrointestinal tract of all animals dying after day
46 were examined histologically. All vehicle controls and all
animals in the highest dose group with survivors were examined
histologically. All rats at 32 and 64 mg/kg bw/day and 1 male and 4
females at 16 mg/kg bw/day died before the end of the study. Final
mean body weights of all dose groups were comparable to vehicle
controls. No gross or microscopic pathologic effects were observed
(Chan, 1989).
Table 1. Acute toxicity of dichlorvos (WHO, 1989)
Species Route LD50 (mg/kg bw)
Mouse oral 68-275
i.p. 28-41
i.v. 8-10
s.c. 13-33
Rat oral 30-110
dermal (24 h) 75-107
i.p. 18
s.c. 72
Guinea-pig s.c. 28
Hamster i.p. 30
Rabbit oral 13-23
dermal 205
Cat oral 28
Dog oral 100-316
Chicken oral 15
Swine oral 157
Table 2. Acute inhalation toxicity of dichlorvos (WHO, 1989)
Species Mode of exposure Time of exposure LC50
(hours) (µg/l)
Mouse whole body 4 13
head only 4 > 218
Rat whole body 4 15
whole body 1 140
head only 4 340
head only 1 455
head only 4 > 198
Dogs
Groups of 3 male and 3 female beagle dogs received 0, 2.3, 6.9,
12 or 23 mg dichlorvos/dog (93% in corn oil by gelatin capsule)
daily, for 90 days. The mean doses per group were equivalent to
0.3, 1, 1.5 or 3 mg/kg bw/day. No effects were observed on
mortality, growth, haematology, liver and kidney function, organ
weights, or at gross and histopathological examination. In the two
highest dose groups, the dogs showed excitement, increased activity
and aggression. Plasma and erythrocyte ChE activities, measured
initially and at intervals of approximately 2 weeks, were normal in
the lowest dose group (0.3 mg/kg bw/day) but inhibited in the other
dose groups (up to about 60% in the highest dose group). Inhibition
was lower from day 70 onwards. Brain ChE activity at termination
was decreased (to 33%of control activity) only in the highest dose
group. The NOAEL in this study was 1.5 mg/kg bw/day based on
reduction in brain ChE activity at termination. However, taking
into account the behavioural changes, a more conservative NOAEL can
be considered to be 1 mg/kg bw/day (Hine, 1962).
Pigs
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, or 16 mg
dichlorvos/kg bw/day (divided over 2 daily doses) for 30 days. No
effects were found on body-weight gain, haematology, or clinical
chemistry when compared with control animals fed a blank PVC
formulation. Plasma and erythrocyte ChE activities were
significantly inhibited in the 4 mg/kg bw/day group (37 and 34% of
controls, respectively) and in the 16 mg/kg bw/day group (33 and 21%
of controls, respectively). The NOAEL in this study was 1 mg/kg
bw/day based on inhibition of erythrocyte ChE activity (Stanton et
al., 1979).
Monkeys
Rhesus monkeys (4/sex) were continuously exposed to dichlorvos
vapour at an average actual concentration of 0.05 mg/m3 for 3
months. The control group consisted of 4 males and 1 female. No
adverse effects were observed in appearance, behaviour, or
haematological and clinical chemical determinations. Plasma ChE
activity was slightly reduced (up to 28% inhibition), while
erythrocyte ChE activity inhibition was 36%. No changes in nerve
maximum conduction velocities or muscular evoked action potentials
were induced by exposure to dichlorvos (Coulston & Griffin, 1977).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 100 male and 100 female C57BI/6/Bln mice (5-6 weeks
old) received by gavage 0.2 mg dichlorvos per mouse (97% in 0.2 ml
water), freshly prepared, either twice or 3 times per week for 50
weeks. Control groups received by gavage either 0.2 ml water, 3
times per week (about 50 males and 50 females), or no treatment (35
males and 35 females). Surviving animals were sacrificed after 110
weeks. This strain of mice is known for the spontaneous occurrence
of mixed lymphomas (reticulocell sarcoma type B).
From the age of 12 months onwards, some animals in all groups
developed interstitial pneumonia. The incidence of mixed lymphomas
was decreased in both test groups (26-60% in control groups, 23-30%
in treated groups). An increased incidence of focal hyperplasia
(transitional cell hyperplasia) of the urinary bladder was found in
both dichlorvos groups (0-8% in control groups, 5-10% in treated
groups). The authors concluded that no neoplastic lesions were
found which could be attributed to the treatment of the animals with
dichlorvos (Horn et al., 1987).
Dichlorvos was not co-carcinogenic in C57BI/6/Bln mice when
administered by gavage three times/week at 0.2 mg/animal to mice
subcutaneously injected with 50 µg N-nitrosodiethylamine per animal
weekly for 50 weeks followed by an observation period of up to 110
weeks (Horn et al., 1990).
Two groups of 50 male and 50 female B6C3F1 mice were fed
1000 or 2000 ppm dichlorvos (purity > 94%) in corn oil in the diet
for 2 weeks. Due to severe signs of intoxication, doses were
lowered to 300 and 600 ppm for the following 78 weeks. Samples of
the diets analyzed during the study showed that time-weighted
average concentrations were 318 and 635 ppm. Matched controls
consisted of 10 mice of each sex; the pooled controls from
simultaneous studies with other compounds consisted of 100 male and
80 female mice. All surviving mice were killed at 92-94 weeks.
Animals were observed twice daily for clinical signs.
Alopecia and rough hair coats were noted in many treated
animals, particularly in the male groups, beginning at week 20 and
persisting throughout the study. The average body weights of the
high-dose mice of both sexes were slightly decreased compared with
controls. The low-dose female group showed 74% survival at 90 weeks
compared to 84% and 90% in high-dose and control groups,
respectively. There was no significant increase in the incidence of
tumours attributable to dichlorvos in either sex.
Two squamous-cell carcinomas of the oesophagus (one in a
low-dose male and one in a high-dose female), 1 papilloma of the
oesophagus in a high-dose female and 3 cases of focal hyperplasia of
the oesophageal epithelium in low-dose males were recorded in the
treated mice. The significance of the findings in the treated mice
was considered uncertain because of insufficient information
concerning the spontaneous incidence of these lesions and lack of
statistical significance within the experiment. Dichlorvos (up to
635 ppm in the diet, equivalent to 95 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Studies in B6C3F1 mice given 0, 400 or 800 mg/litre
dichlorvos in drinking-water for two years and in CFE rats exposed
to 0, 0.05, 0.48, or 4.7 mg/m3 of dichlorvos 23 hours/day for 2
years were summarized in WHO (1989). There was no evidence of
carcinogenic effects in these studies (Blair et al., 1976;
Konihishi et al., 1981).
Groups of 50 B6C3F1 mice were given dichlorvos (99% purity)
by corn oil gavage at doses of 0, 10 or 20 (males) or 0, 20 or 40
(females) mg/kg bw/day daily, 5 days per week, for 103 weeks. The
dose volume of the corn oil was 10 ml/kg bw/day. Body weights were
recorded once weekly for the first 12 weeks and then monthly.
Animals were observed twice per day for clinical signs. Necropsy
and histological examinations were performed on all moribund animals
or at the end of the study.
Body-weight gain and survival did not significantly differ
between treated and control groups. No compound-related clinical
signs were observed. Blood cholinesterase activity was not
determined during the study. However, a separate study showed
inhibition of plasma ChE activity by 60-90% in all dose groups while
erythrocyte ChE activity was normal (see "Special studies on
Cholinesterase activity" for detailed description and comments).
The incidences of forestomach squamous cell papillomas were
1/50, 1/50 and 5/50 in control, low and high-dose males respectively
and 5/49, 6/49 and 18/50 in control, low and high-dose females,
respectively. The positive trend was statistically significant in
both sexes while by pairwise comparison only the incidence in high-
dose females was significantly higher than in controls. Two
forestomach squamous cell carcinomas were seen in high-dose females
and none in the other groups. No increase in the incidence of
forestomach hyperplasia was seen in the dosed mice compared with
vehicle controls (10-20%). In female mice the incidence of adenomas
and adenomas or carcinomas (combined) of the pituitary gland (12/45,
6/45 and 6/44 in control, low and high-dose groups, respectively)
and the incidence of lymphomas (16/50, 11/50 and 9/50 in control,
low and high-dose groups, respectively) showed a significant
negative trend. Based on the increased incidence of forestomach
papillomas, the NOAEL was 10 mg/kg bw/day (Chan, 1989; Chan et al.,
1991).
Rats
Groups of 40 male and 40 female weanling CD rats were fed diets
containing nominal concentrations of 0, 0.1, 1, 10, 100 or 500 ppm
dichlorvos (93% purity) for 2 years. Diets were prepared weekly.
Five males and 5 females from each group were killed after 6, 12 or
18 months. Analysis of diet samples showed a considerable loss of
dichlorvos associated with a gradual increase in
dichloroacetaldehyde (DCA) content (average concentrations ranging
from 0.01 to 28.6 ppm. The average actual concentrations of
dichlorvos in each diet were 0.05, 0.5, 4.7, 47 or 230 ppm. No
cholinergic signs were observed. No effects were seen on behaviour,
mortality rate, weight gain, food consumption, terminal body and
organ weights, haematology or urinalysis. Plasma and erythrocyte
ChE activities were measured 13 times during the study. In the 100
ppm group, plasma and erythrocyte ChE activities were reduced to
60-90% and to 50-90% of control activities, respectively; in the 500
ppm group, the activities were reduced to 20-70% and 20-60%,
respectively. Activities were higher toward the end of the study.
Brain ChE activity was decreased in the highest dose group by
45-47%, 24-43%, 24-38% and 5-15% after 6, 12, 18 and 24 months,
respectively. Histological examination of major organs (liver,
heart, lungs, kidneys, spleen, brain, gonads, pituitary, adrenals,
and thyroids) revealed hepatocellular fatty vacuolization in all 500
ppm rats, and in most females and several males at 100 ppm. No
effect was seen on serum total proteins or albumin: globulin ratio,
or on hexobarbital sleeping time. The tumour incidence was
comparable with that of the control group. The NOAEL, based on
brain ChE inhibition, was 100 ppm (actual concentration 47 ppm,
equivalent to 2.4 mg/kg bw/day) (Witherup et al., 1967).
Groups of 50 male and 50 female weanling CFE rats were exposed
(whole body) to nominal air concentrations of 0, 0.05, 0.5 or 5 mg
dichlorvos (97% purity)/m3 for 23 hours per day for two years. The
average actual dichlorvos concentrations were 0.05, 0.48, or 4.7
mg/m3. Body-weight gain was reduced in the two highest dose
groups. After two years of exposure, plasma and erythrocyte ChE
activities were reduced by 20-30% at 0.5 mg/m3 and by > 60% in the
highest dose group; brain ChE activity was reduced by 10%
(statistically significant) at 0.5 mg/m3 and by 80% in the highest
dose group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry
values, organ weights, or gross or microscopic examinations of major
organs. Ultrastructural examinations of bronchi and alveoli of rats
exposed to 0 or 5 mg/m3 showed no differences between the two
groups.
It was concluded that 2-year exposure to 0.05 mg/m3 dichlorvos
did not cause observable adverse effects to CFE rats (Blair et al.,
1976). It should be noted that in this study the rats were not only
exposed by inhalation but also via their food, drinking-water, and
by grooming. This resulted in additional oral ingestion of
dichlorvos (Stevenson & Blair, 1977).
Osborne-Mendel rats (50/sex) were fed 1000 ppm dichlorvos
(purity > 94%) in corn oil for 3 weeks. Due to severe cholinergic
signs, the dose was reduced to 300 ppm for the remaining 77 weeks.
Another group (50 males and 50 females) was fed 150 ppm dichlorvos
for 80 weeks. Samples of the diets analyzed during the study showed
that time-weighted average concentrations were 330 and 150 ppm,
respectively. Matched controls consisted of 10 rats of each sex;
the pooled controls from simultaneous studies of other compounds
consisted of 60 rats of each sex. Animals were observed twice daily
for clinical signs. All surviving rats were killed after 110-111
weeks.
The average body weights of the high-dose rats of both sexes
were slightly decreased compared with controls. There was no
significant increase in the incidence and type of tumours
attributable to dichlorvos in either sex. Dichlorvos (up to 330 ppm
in the diet, equivalent to about 30 mg/kg bw/day) was not
demonstrated to be carcinogenic in this study (NCI, 1977;
Weisburger, 1982).
Groups of 70 and 100 rats per sex received 0.1 mg dichlorvos
(97%)/rat in 0.2 ml water by gavage twice or 3 times per week,
respectively, for 60 weeks. Thereafter, the animals were observed
for another 51 weeks. A control group of 60 animals of each sex
received 3 times per week 0.2 ml water. From the age of 10 months
onwards, the incidence of focal hyperplasia of urinary bladder and
of renal pelvis increased in males but decreased in females from
both test groups compared to controls. No neoplastic lesions were
found which could be attributed to treatment (Horn et al., 1988).
A study in Fischer 344 rats given drinking-water containing 0,
140 or 280 mg/litre dichlorvos for 108 weeks was summarized by WHO
(1989). There was no evidence of carcinogenicity (Enomoto et al.,
1981).
Groups of 50 male and 50 female F344/N rats were administered
dichlorvos by corn oil gavage at 0, 4 or 8 mg/kg bw/day (99%
purity), 5 days per week for 103 weeks. Body weights were recorded
once weekly for the first 12 weeks and then monthly. Animals were
observed twice daily for clinical signs. Necropsy and histologic
examinations were performed on all moribund animals or at the end of
the study.
Mild diarrhoea was observed in treated animals. ChE activities
were not determined during the study. However, a separate study
showed inhibition of plasma ChE activity by 50-80% in all dosed
groups but no erythrocyte ChE inhibition (see "Special studies on
cholinesterase activity" for detailed description and comments). No
significant differences in mean body weights or survival were
observed between any groups of either sex. Increased incidence of
cytoplasmic vacuolisation of liver was observed in dosed males and
of cortical cytoplasmic vacuolisation of adrenal glands in all dosed
males and in low-dose females. The incidence of pancreatic adenomas
(cross and horizontal tissue sections were analyzed), in control,
low and high-dose groups was 25/50, 30/50 and 33/50 in males and
2/50, 3/50 and 6/50 in females, respectively. The increased
incidence in treated males was statistically significant. The
incidence of mononuclear cell leukaemia (11/50, 20/50, 21/50 in
control, low and high-dose groups, respectively) was significantly
increased in the treated male rats compared with controls. The
incidence of mammary gland adenomas or fibroadenomas in female rats
was 11/50 in controls, 19/50 in the 4 mg/kg bw/day group and 17/50
in the 8 mg/kg bw/day group. The incidence in the low-dose group
was only slightly higher than the historical control values. Two
mammary gland carcinomas were observed in control and low-dose
groups. The authors concluded that there was evidence that
dichlorvos is carcinogenic to rats (Chan, 1989; Chan et al.,
1991). The Meeting observed that the incidence of pancreatic
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia, which is usually high and variable in
this strain of rats, was also of questionable biological
significance (Haseman et al., 1985).
Dichlorvos at 8 or 16 mg/kg bw/day was administered by gavage
to groups of 8-12 male F344 rats, either with or without leukaemia
transplant, for 5 days a week. Other groups of rats were either not
treated or given the leukaemia transplant only. At 70-days
post-transplant, the animals were killed. The rats dosed with
dichlorvos developed the disease earlier and the rate of tumour
progression was increased. Three out of 16 transplant recipients
dosed with 16 mg/kg bw/day died of leukaemia during the last week of
dosing. The severity of the mononuclear cell leukemia in the
transplant recipients, as measured by histopathological examination
of spleen and liver, was correlated with the changes in tumour
growth rates. However, no dose-response was found for spleen weight
and WBC count (Dieter et al., 1989).
Dogs
Groups of beagle dogs (3/sex) were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100 or 500 ppm dichlorvos (93%
purity) for 2 years. The average actual concentrations were 0,
0.09, 0.32, 3.2, 32 or 260 ppm dichlorvos; the average
dichloroacetaldehyde concentrations at the three highest dosages
were 0.6, 6.4 and 20 ppm. Since diet analysis was performed weekly
on a mixture of diet samples taken the previous 7 days, it is
likely, given the high volatility of dichlorvos, that at the end of
the week the actual concentration was much lower than 30% of the
nominal concentration.
No effects were seen on general appearance, survival, weight
gain, food consumption, haematology or urinalysis. Erythrocyte ChE
activity was reduced up to 50% of controls in the 10 ppm group with
recovery to control values at the end of the feeding period. At the
highest dose level, inhibition was more than 90% but complete
recovery was observed at 24 months. A similar pattern was observed
for plasma ChE activity. Brain ChE activity, measured at the end of
the study was similar to that of the controls in all treated groups.
Relative liver weights were slightly increased in males at 100 ppm
and in both sexes at 500 ppm. Histological examination of major
organs revealed slight dose-related cytoplasmic vacuolization and
enlargement of hepatocytes in animals at the two highest levels. No
differences were seen in serum alkaline phosphatase, transaminase
activities, total serum proteins or albumin:globulin ratios (Jolley
et al., 1967).
Reproduction studies
See also under special studies on testes.
Mice
Male and female Crl:CD3-1 mice were exposed to dichlorvos
concentrations of 0, 1.9, 3.0 or 4.6 mg/m3, generated from
dichlorvos-impregnated PVC strips in their cages. Exposure began
4 days prior to formation of breeding groups (3 females and 1 male)
and continued throughout pregnancy. Signs of intoxication were not
observed. Plasma ChE activity was significantly inhibited (by 90%,
93% and 95% in the 1.9, 3.0 and 4.6 mg/m3 groups, respectively)
when measured on day 4 after beginning of treatment. Gestation
length, number of litters, litter frequency and mean litter size
were comparable to controls. No grossly detectable congenital
anomalies were detected in any of the offspring (Casebolt et al.,
1990).
Rats
A 3-generation, 2-litter/generation reproduction study in rats
summarized by WHO (1989) was negative at doses up to 235 ppm in the
diet, equivalent to 12 mg/kg bw/day (Witherup et al., 1965).
Domestic animals
Several studies have been carried out with pregnant sows but no
adverse effects on piglets were observed with doses which inhibited
plasma and erythrocyte ChE activity in the sows (WHO, 1989).
Special studies on delayed neurotoxicity
Several studies have shown that a single dose of dichlorvos
does not produce delayed neurotoxicity in pre-medicated hens,
whether it is administered orally or subcutaneously (WHO, 1989).
However, Caroldi & Lotti (1981) reported mild signs of ataxia in
pre-medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg bw) and severe (> 80%) inhibition of NTE in peripheral
nerve, spinal cord and brain. Johnson (1978) did not observe ataxia
in pre-medicated hens given the same dose in the same way. However,
in this experiment spinal cord NTE inhibition was below the
threshold. When the dose was repeated 1-3 days after the first
dose, spinal cord NTE inhibition increased and the hens became
ataxic.
In a 90-day study, white leghorn hens were given dichlorvos
(99.9% purity) either dermally or orally. For oral administration,
a 10-20% solution of dichlorvos in corn oil in gelatin capsules was
used whereas, dermally, 1-20% emulsifiable concentrates in technical
grade xylene (containing 2% Triton X-100) were used. Dichlorvos at
doses greater than 1 mg/kg bw/day (dermal) or 6 mg/kg bw/day (oral)
led to cholinergic symptoms and death after 2-3 days. None of the
animals developed organophosphate-induced delayed neuropathy
(Francis et al., 1985).
In summary, it is possible to produce clinical neuropathy in
hens, but the doses required are far in excess of the LD50. This
is consistent with the low in vitro ratio of AChE I50/NTE I50 for
dichlorvos (Lotti & Johnson, 1978).
Special studies on embryotoxicity and teratogenicity
Several embryotoxicity/teratogenicity studies have been
performed with dichlorvos in mice, rats and rabbits. These studies
are summarized in WHO (1989). Dichlorvos given orally, by
inhalation or intraperitoneally, was not teratogenic at doses which
were toxic to the pregnant animals. However, in a study in rats
where a single i.p. dose of 15 mg/kg bw was given on day 11 of
gestation, 3/41 fetuses in the treated group had omphaloceles (0/50
in controls) (Kimbrough & Gaines, 1968). This finding has not been
confirmed in other studies.
Special studies on cholinesterase activity
This section summarizes studies in which the effect of
dichlorvos on ChE activity was the only parameter investigated.
Studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos in relation to the time
elapsed after dosing are summarized in Table 3. In general, the
maximum inhibition occurred within one hour followed by rapid
recovery.
Mice/Rats
Groups of 8-week old B6C3F1 mice and F344/N rats (10/sex)
were administered dichlorvos (99%) by corn oil gavage at daily doses
of 0, 5, 10, 20, or 40 mg/kg bw (mice) and 0, 2, 4, 8, or 16 mg/kg
bw (rats), five days per week for one week. Plasma and erythrocyte
ChE activities were measured on days 10 or 11, 25 or 26 and 32 or
33; blood was obtained about 3 hours after treatment.
Plasma ChE activity was significantly inhibited in all dose
groups of mice (50 and 90% inhibition in low and high-dose groups,
respectively) and rats (25 and 80% inhibition in low and high-dose
groups, respectively). At all time-points, erythrocyte ChE activity
in dosed and vehicle-control mice and rats was similar. However,
the timing for determination of the enzyme activities might have
underestimated the inhibition (Chan, 1989).
Rats
Reiner & Plestina (1979) compared in vivo reappearance of ChE
activity in rats after treatment with either dichlorvos (2.5 mg/kg
bw i.v.) or metrifonate (300 mg/kg bw i.v.). Half-lives of recovery
of brain and plasma ChE (acetylcholine was the substrate) were found
to be 2 and 2.5 hours, respectively, both in dichlorvos-treated and
in metrifonate-treated rats. This was consistent with in vitro
data (Skrinjaric-Spoljar et al., 1973).
In a study on the influence of temperature on ChE activity,
rats were injected i.p. with a single dose of 6.3 mg/kg dichlorvos
and kept at either 28 °C or 5 °C. The maximum inhibition of whole
blood ChE activity (40% and 50% in the two groups, respectively)
occurred after 0.5 hour. The animals kept at 5 °C showed slightly
less inhibition of whole blood ChE activity than those at room
temperature (Chattopadhyay et al., 1982).
Table 3. Time-related inhibition of ChE activity in animals after administration of a single dose of dichlorvos
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Mouse, male i.p. 10 15 min 70 Nordgren et al., (1978)
(n = 6-8) 60 min 50
2 hours 20
Mouse, male i.p. 15 2 hours 20 Cohen & Ehrich, 1976
Mouse, male i.p. 30 15 min 63 Cohen & Ehrich, 1976
(n = 4) 1 hour 50
5 hours 35
18 hours 10
Rat, male oral 1.6 2 hours 0 Pacheka et al., 1975
(n = 6) 10 2 hours 15
40 1 hour 60*
Rat, male oral 40 1 hour 70 Teichert et al., 1976
(n = 1)
Rat, male oral 40 5 min 45 Pacheka et al., 1975
(n = 6) 15 min 80
1 hour 85
2 hours 70
8 hours 35
24 hours 25
48 hours 6
Rat, male oral 50 15 min 68 80 97.91** Modak et al., 1975
(n = 6) 3 hours 73 37 68-70**
24 hours 39 24 31-38**
Table 3 (contd)
Species Route Dose Time Mean % inhibition Reference
(mg/kg bw)
plasma erythr. brain
ChE AChE AChE
Rat, male i.v. 2.5 30 min 60 85 Reiner & Plestina, 1979
(n = 5-12) 90 min 40 65
3 hours 7 44
12 hours 0 17
48 hours - 10
Dog, greyhounds oral 11 1 hour 90 93 Snow & Watson, 1973
1 male 2 hours 80 70
1 female 24 hours 15 35
72 hours 8 25
Dog, 2 male oral 22 fatal 90 95 66*** Snow & Watson, 1973
greyhounds,
1 female
crossbred
Dog, greyhounds oral 22 1 hour 88 83 Snow & Watson, 1973
and crossbred 3 hours 76 66
(n = 7-9) 6 hours 60 50
24 hours 30 30
48 hours 13 30
72 hours 5 35
Dog, beagle oral 50 2 hours 68 37 Ward & Glicksberg, 1971
sex not specified 24 hours 37 26
(n = 15) 5 days 8 22
14 days 5 20
21 days 0 8
Table 3 (contd)
* Daily dosing for 14 days caused 60% AChE inhibition when measured 24 hours after the last dose.
** Determinations were performed in striatum, hippocampus, medulla and cortex. No significant difference
was found between these brain areas.
*** Measured 20-155 min. after treatment.
When pregnant rats were given oral doses of 1.1 or 5.6 mg
dichlorvos/kg bw/day during days 14-21 of gestation, plasma ChE
activity of the high-dose mothers was inhibited (30-50%) but not
that of the young. Brain ChE activity did not show any significant
inhibition (Zalewska et al., 1977).
Rabbits
Dichlorvos, when infused into the ear vein of adult male
rabbits, produced dose- and time-related inhibition of whole blood
ChE activity during infusion. Spontaneous but incomplete recovery
to 60-80% of the normal activity occurred within 60-90 minutes after
infusion. Almost complete recovery was obtained by injecting oximes
up to 2.5 hours later (Shellenberger et al., 1965; Gough &
Shellenberger, 1977-1978; Shellenberger, 1980).
The progeny of rabbits, treated orally with 6 mg/kg bw/day for
the last 10 days of gestation, showed inhibition of brain ChE
activity at one day (30%) and 8 days of life (15%). Plasma ChE
activity was higher throughout days 1-16 of life (Maslinska &
Zalewska, 1978).
Guinea-pigs
Groups of 5 male and 5 female guinea-pigs were given daily
applications of 0, 25, 50 or 100 mg/kg bw/day dichlorvos (94%
purity) on the shorn skin for 8 days. All animals survived. A
dose-dependent inhibition of both plasma and erythrocyte ChE
activities occurred in all test groups. Recovery of plasma ChE
activities was complete within one week of the last exposure, and
that of erythrocyte ChE was complete within one week in the females
and 2 weeks in the males (Brown & Roberts, 1966).
Hens
Chickens dosed once orally with 1,3, or 6 mg/kg bw showed a
rapid inhibition of plasma ChE activity followed by recovery with an
half-life being estimated at three days (Rauws & van Logten, 1973).
White Leghorn pullets and hens were fed a diet containing 30
ppm dichlorvos for 35 days followed by a 21-day recovery period.
Plasma ChE activity was inhibited by 70% after four weeks of
treatment but returned to normal during the recovery period (Pym et
al., 1984).
Monkeys
Thirty-two Rhesus monkeys were given a pelleted PVC-resin
formulation containing 20% dichlorvos at dosages ranging from 1 to
16 mg dichlorvos/kg bw once daily or 1.6 and 4 mg dichlorvos/kg bw
twice daily for 10 to 21 consecutive days. None of the monkeys
showed overt signs of intoxication, although they ate less food and
had soft faeces. Plasma and erythrocyte ChE activities were reduced
by approximately 80% in all animals (irrespective of the dose) and
remained inhibited throughout the study. Plasma ChE activities
returned to normal values within approximately 3 weeks and the
erythrocyte ChE activities within 50 to 60 days following cessation
of exposure (Hass et al., 1972).
Special studies on genotoxicity
Methylating reactivity
In vitro studies
In a quantitative colour test in which methylation of
4-(p-nitro-benzyl) pyridine was measured to predict DNA alkylating
potential, dichlorvos gave a positive response, the reaction being
about 1/3 that of the known alkylating compound
methylmethanesulfonate (MMS) (Bedford & Robinson, 1972).
Alkylation by dichlorvos of calf thymus DNA, resulting in the
formation of N-7-methylguanine, was reported by Lofroth (1970).
Methylation by dichlorvos of isolated salmon sperm DNA and of
DNA from intact E.coli and from human HeLa cells broadly resembled
that by MMS (Lawley et al., 1974). In this study a very high
dichlorvos concentration (14 mmol/l) was used with isolated DNA
(12.5 mmol/l DNA-P). Dichlorvos concentrations with E. coli and
HeLa cells were 1-3 mmol/l. The rate of methylation of guanine-N-7
from salmon sperm DNA or from intact HeLa cell DNA by dichlorvos was
2-3 x 10-4 mol/l/min (calculated assuming guanine as 23-25% of DNA
with an average molecular weight of DNA base pair of 649, Lawley et
al., 1974). This is about 15 times lower than the rate of
methylation by MMS. This rate is to be compared with the rate of
reaction with AChE (phosphorylation) (1.5 x 105 mol/l/min at 37 °C)
(Skrinjaric-Spoljar et al., 1973). Therefore, the relative rate
of phosphorylation is about 9 orders of magnitude higher than that
of alkylation.
Labelled 7-methylguanine was present in both DNA and RNA
isolated from E. coli exposed to [Me-3H]-dichlorvos (1.1 mmol/l
for 4 hours). The methylating capability of dichlorvos was less, by
a factor of 10-100, than that of strongly genotoxic methylating
compounds (Wennerberg & Lofroth, 1974).
Incubation of bacteriophage R17 with 0-100 mmol
dichlorvos/litre for 90 hours did not result in methylation of the
phosphate groups of the RNA to any significant extent (Shooter,
1975).
In vivo studies
Mice were given i.p. injections of methyl-14C-dichlorvos (1.9
µmol/kg bw, approximately 420 µg/kg bw). The degree of alkylation
of guanine-N-7 in DNA isolated from soft tissues amounted to 8 x
10-13 mol methyl per gram of DNA (about 5 x 10-10 mol/mol guanine)
(Segerback, 1981; Segerback & Ehrenberg, 1981). From acute toxicity
data in mouse (see Table 1) it can be extrapolated that this dose
would cause 1 mol/mol phosphorylation of erythrocyte ChE.
DNA and RNA from the total soft tissues of male rats exposed to
atmospheres containing 0.064 mg/m3 (about 0.1% of the LC50) of
methyl-14C-dichlorvos for 12 hours did not show methylation of the
N-7 atom of guanine moieties. The exposure period constituted a
significant fraction of the half-life of the 7-methylguanine
moieties in DNA (Wooder et al., 1977; Wooder & Wright, 1981).
Excretion of labelled 7-methylguanine in the urine by NMRI mice
and rats injected i.p. with [Me-14C]-dichlorvos, or exposed by
inhalation for 2 hours (mice only) was reported by Wennerberg &
Lofroth (1974) and Lofroth & Wennerberg (1974). In rat urine,
labelled 3-methyladenine and 1-methyl-nicotinamide were also present
(Lofroth & Wennerberg, 1974). According to the authors, these
results demonstrate that dichlorvos spontaneously methylates guanine
and adenine moieties in nucleic acids. However, administration of
radiolabelled adenine and guanine to otherwise untreated rats gave
rise to the excretion of radiolabelled methylated purines in the
urine. Therefore, the detection of radiolabelled purines, per se,
in the urine of animals exposed to methyl-labelled methylating
agents does not constitute evidence for the spontaneous methylation
of the purine moieties of nucleosides or nucleic acids by
methylating agents (Wooder et al., 1978; Wooder & Wright, 1981).
Moreover, a natural biosynthetic pathway has been demonstrated
whereby the methyl carbon atoms of dichlorvos can be incorporated
into the heterocyclic rings and the methyl groups of urinary
7-methylguanine after entering the 1-C pools, in vivo (Wright et
al., 1979; Wooder & Wright, 1981).
Genotoxicity
In vitro studies
Several studies using bacteria and fungi as test organisms have
been carried out (Table 4). In most of the studies, only one, often
high, dichlorvos concentration was tested, sometimes resulting in
low survival of the test organism. The alkylating properties of
dichlorvos (see above) are most probably the cause of the mutagenic
action. This is suggested, for instance by data in E. coli
strains deficient at four repair loci (Bridges et al., 1973).
In vitro studies using mammalian cells are summarized in
Table 5. Dichlorvos caused cell transformation, mutations, DNA
strand breaks, sister chromatid exchanges and chromosomal
aberrations in cultured animal cells. In cultured human cells,
dichlorvos induced unscheduled DNA synthesis but did not induce
sister chromatid exchanges or chromosomal aberrations. Negative
results from chromosomal aberration tests in cultured human
lymphocytes were also cited by Fahrig (1974) and Wild (1975).
Dichlorvos (0.3 µg/ml) caused a synergised an increase in
sister chromatid exchanges in Chinese hamster ovary cells when
tested in combination with synthetic pyrethroids or propoxur which,
as such, were negative; however, in the combination phenothrin +
dichlorvos (0.3 µg/ml) the induction of SCE by phenothrin was
negative (Wang et al., 1988).
In vivo studies
In vivo studies are summarized in Table 6.
In Drosophila melanogaster, chromosomal aberrations but not
sex-linked recessive lethal mutations were induced. Negative
results were obtained in host-mediated, dominant lethal, sister
chromatid exchange and micronucleus assays (except on skin after
local application at cytotoxic doses). Dichlorvos did not induce
in vivo chromosomal aberrations in bone-marrow cells,
spermatocytes or spermatogonia, DNA strand breaks or unscheduled DNA
synthesis.
Special studies on liver microsomal enzymes
A decrease in liver microsomal cytochrome P-450 occurred in
rats after three daily i.p. injections with 6 mg dichlorvos/kg bw
(Purshottam & Kaveeshwar, 1982) but no effect on liver microsomal
UDP-Glucuronyl transferase was found in mice 4 hours after a single
i.p. injection of 25 mg/kg bw (Yoshida et al., 1976).
Pre-treatment of rats with three daily i.p. injections of
sodium phenobarbital did not significantly change mortality and
plasma ChE inhibition caused by an i.p. injection of dichlorvos (20
mg/kg bw) (Purshottam & Kaveeshwar, 1979).
Short- and long-term studies with mice, rats or dogs dosed
orally or intraperitoneally with dichlorvos did not show any effect
on microsomal drug metabolizing enzymes (Witherup et al., 1967;
Uchiyama et al., 1975; Farber et al., 1975).
Table 4. Mutagenicity tests on microorganisms in vitro
Test system Test object Concentration Purity Results 1 Reference
Mitotic A. nidulans strain p ? ? + Morpurgo et al., 1979
non-disjunction
and crossing over
Recombinant assay B. subtilis H17 Rc+ 2 mg/plate ? - Shirasu et al., 1976
M45 Rec- " + " "
Forward mutation A. nidulans strain 35 14 mg/disc ? + Bignami et al., 1977
E. coli B 5-25 mmol/l 95% +2 Wild, 1973
Gal RS ? ? + Fahrig, 1974
K12(5-MT) 0.3-3.2 mmol/l ? + Mohn, 1973
S. coelicolor A 3(2) his A1 5.6 mg/disc 99.9% + Carere et al., 1978a, 1978b
Reverse mutation E. coli B/r WP2 5 mg/plate > 97% +3 Moriya et al., 1978
S. typhimurium TA 1535 5 mg/plate > 97% +4 " "
E. coli WP2 hcr up to 5 mg/plate ? +3 Moriya et al., 1983
S. typhimurium TA 98 up to 5 mg/plate ? - " "
TA 100 " " ? + " "
TA 1535 " " ? ? " "
TA 1537 " " ? - " "
TA 1538 " " ? - " "
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation (cont'd) E. coli B/r Wp2( ); SR714 0.04-2.3 mmol/l 99% +3 Houk & DeMarini, 1987
CM 561 0.2% " +5 Bridges et al., 1973
CM 571; CM 611 " " -6 " "
WP2 " " +5 " "
WP2 uvr A " " +5 " "
WP2 micro drop of ? -7 Dean, 1972a
analytical grade,
technical grade
or 10% acqueous
solution/plate
K12HfrH 0.1% 97.5% + Voogd et al., 1972
C. freundii 425 0.05% or 0.1% " +8 " "
E. aerogenes 0.1% " + " "
K. pneumoniae 0.05% oe 0.1% " + " "
S. typhimurium 64-320 " " + " "
S. marcescens Hy/alpha 13 1.25-5 mg/disc ? + Dean, 1972a
Hy/alpha 21
E. coli WP2 WP2 5 µg/ml ? +9 Green et al., 1976
hcr+/hcr- 20-25 µl/disk 50% commercial +10 Nagy et al, 1975
WP2 5 mg/plate formulation " "
hcr+/hcr- ?
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
Reverse mutation S. typhimurium TA 1535 5 mg/plate ? + Shirasu et al., 1976
(cont'd) TA 1536 " ? + " "
TA 1537 " ? - " "
TA 1538 " ? - " "
E. coli WP2 5 µl/plate11 ? + Hanna & Dyer, 1975
WP2 uvr A " ? + " "
WP 67 " ? + " "
CM 561 " ? - " "
CM 571 " ? - " "
CM 611 " ? - " "
S. typhimurium TA 1530
TA 1535 5 µl/plate ? + Hanna & Dyer, 1975
his C117 " ? + " "
his G46 " ? - " "
" ? - " "
P. aeroginusa PAO 38 0.08 mol/l ? + Dyer & Hanna, 1973
S. typhimurium his C117 0.03 mol/l ? + " "
S. typhimurium TA 98 ? ? -3 Braun et al., 1982
TA 100 ? ? +3 " "
TA 1535 ? ? -3 " "
TA 1536 ? ? -3 " "
TA 1537 ? ? -3 " "
TA 1538 ? ? -3 " "
TA 98 0.1-6.6 mg/plate12 99% -3 Chan, 1989:
Zeiger et al., 1988
Table 4 (contd)
Test system Test object Concentration Purity Results 1 Reference
TA 100 " " " +3 Carere et al., 1978a,b
TA 1535 2.8 mg/plate 99.9% -3 " "
TA 1536 " " " -3
TA 1537 " " "
S. typhimurium TA 1538 2.8 mg/plate 99.9% -3 Carere et al., 1978a,b
TA 1535 1.5 mg/ml 99.9% +
Schizosaccharmoyces pombe 1.5-14 mmol/plate13 > 99% +3 Gilot-Delhalle et al.,
ade 6 1983
Gene conversion S. cerevisiae D4 2-8 mg/ml > 97% +14,15 Dean et al., 1972
D4 6-40 mmol/l ? +2 Fahrig, 1973, 1974
632/4 ? ? - Guerzoni et al., 1976
+16 Griffin & Hill, 1978
DNA strand breaks E. coli K-12CR34Co1E1 1 mg/ml ?
Growth inhibition E. coli W3110 pol A+/pol A- 6.4 mmol/l ? + Rosenkranz, 1973
P. mirabilis PG 273; PG 713 ? ? +15 Braun et al., 1982
1 Without metabolic activation except where noted.
2 Positive controls (methyl methanesulfonate 1-4 mmol/l) yielded expected positive results.
3 Both with and without metabolic activation.
4 Only without metabolic activation.
5 Methyl methane sulfonate (0.04%) yielded positive responses.
6 Methyl methane sulfoante (0.04%) yielded negative responses.
7 Methyl methane sulfonate, N-methyl-N'-nitro-N-nitroso guanidine yielded positive response.
8 At 0.1%
9 Positive controls (methyl methanesulfonate 0.5-2.0 µg/ml) yielded expected positive results.
Table 4 (contd)
10 Positive controls (N-methyl-N'-nitro-nitroso guanidine, N-nitroso-N-methylurethane and acridium chloride)
yielded positive responses.
11 Toxic dose.
12 Toxic effect at 3.3 mg/plate.
13 The LD50 was 5.5 mmol/l.
14 Positive controls (ethyl methane sulfonate) yielded positive responses.
15 From 4 mg/ml.
16 Methyl methane sulfonate (2-10 mmol/l), N-methyl-N'-nitro-N-nitroso guanidine (3.4-6.8 mmol/l) yielded positive responses.
Table 5. Mutagenicity tests in mammalian cells in vitro
Test system Test object Concentration Purity Results Reference
Viral transformation Syrian hamster embryo 0.05-0.45 mmol/l ? +1 Hatch et al., 1986
cells/adenovirus SA7 cytotoxic
Gene mutation Chinese hamster V79 cells up to 1 mmol/l ? - Wild, 1975
(azaguanine resistance) 1.25-5 mmol/l ? +2 Aquilina et al., 1984
Mouse lymphoma L5178Y cells 6.25-50 nl/m3 ? +4,5 Chan, 1989
(trifluorothymidine resistance) 12.5-200 nl/m6 +5,7 Chan, 1989
DNA strand breaks Chinese hamster V79 cells 0.2% ? +8 Green et al., 1974
Sister chromatid exchange Primary rat tracheal epithelial cells 5-160 µg/ml9 93.9% +10,11 Lin et al., 1988
Chinese hamster ovary cells 0.03 and 0.1 mmol/l 98 + Nishio & Uyeki, 1981
0.1-0.5 mmol/l > 98% + Tezuka et al., 1980
0.3-1000 µg/ml ? +12 Wang et al., 1988
1-50 µg/ml ? +13 Chan, 1989
Human lymphocytes 2.5-10 µg/ml 99% - Nicholas et al., 1978
Human fetal lung fibroblasts 99% - Nicholas et al., 1978
Chromosomal aberrations Rat tracheal epithelial cells 5-160 µg/ml9 93.9% +11,14 Lin et al., 1988
Chinese hamster lung fibroblasts ? ? + Ishidate et al., 1981
Chinese hamster V79 cells 0.1-0.5 mmol/l > 98% +15 Tezuka et al., 1980
Chinese hamster ovary cells 16-160 µg/ml ? +16 Chan, 1989
Human lymphocytes 1-40 µg/ml > 99% -17 Dean, 1972b
Table 5 (contd)
Test system Test object Concentration Purity Results Reference
Unscheduled DNA synthesis Human lymphocytes 5-500 µg/ml 99.8% + Perocco & Fini, 1980
EUE human cells 6.5-650 mmol/l ? +18 Aquilina et al., 1984
DNA (sedimentation coefficient) Calf thymus DNA 0.1% ? + Rosenkranz &
Rozenkranz, 1972
DNA (thermolabile regions) Calf thymus DNA 45 mmol/l 99% - Olinski et al., 1980
DNA (resistance to micrococcal) Chinese hamster ovary cells 10 mmol/l ? + Nishio & Uyeki, 1980
1 Positive controls (benzo(a)pyrene 0.001-0.002 mmol/l) yielded expected positive responses.
2 Positive controls (ethyl methan sulfonate 20 mmol/l) yielded expected positive responses.
3 Growth inhibition from 12.5 nl/ml.
4 From 12.5 nl/ml.
5 Positive controls (methyl methanesulfonate 5 nl/ml) yielded expected positive responses.
6 Growth inhibition from 100 nl/ml.
7 From 100 nl/ml.
8 Negative at lower concentrations.
9 50% mortality was observed at 80 µg/ml.
10 From 10 µg/ml.
11 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 0.25-1 µg/ml, 50% mortality at 0.5 µg/ml)
yielded expected positive responses.
12 From 40 µg/ml.
13 From 10 µg/ml. When incubated with S9, positive response at 50 µg/ml.
14 From 80 µg/ml.
15 At 0.5 mmol/l.
16 At 160 µg/ml.
17 Cytotoxicity was observed at 5-40 µg/ml.
18 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine) yielded expected positive responses.
19 Possible index of DNA alkylation.
20 Possibly indicating structural rearrangement of chromatin.
Table 6. Mutagenicity tests in vivo
Test system Test object Concentration Purity Results* Reference
Crossing over/ Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recombination ++++/dp b cn bw
Sex-linked Drosophila melanogaster 0.035%1 ? - Jayasuriya & Ratnayaka, 1973
recessive lethal Sobels & Todd, 1979
mutation
Oregan K 0.01-0.1 ppm in food ? -2 Kramers & Knapp, 1978
commercial formulation
Chromosomal Drosophila melanogaster 1 ppm in food ? + Gupta & Singh, 1974
aberration
Autosomal recessive Drosophila melanogaster 0.1-0.75 ppm in food for ? + Hanna & Dyer, 1975
levels 18 months
Host-mediated assay Salmonella typhimurium 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
G46 His- in mice (NMRI)
Salmonella typhimurium 8-10 mg/kg bw p.o. 97.5% -3 Voogd et al., 1972
(64-320) in mice (Swiss)
Serratia marcescens 25 mg/kg bw s.c. ? - Buselmayer et al., 1972
(a 21 Leu-) in mice (NMRI)
Sacchoromyces cerevisiae 50 or 100 mg/kg bw p.o. > 97% -3 Dean et al., 1972
(D4) in mice (CF1)
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Dominant lethal Female mice (CF1) 0, 25 or 50 mg/kg bw p.o. > 97% -4 Dean & Blair, 1976
0, 2 or 8 mg/m3 inhalation, > 97% - Dean & Blair, 1976
from weaning to 11 weeks
of age
Male mice (ICR/Ha Swiss) 0, 5 or 10 mg/kg bw/day ? - Epstein et al., 1972
p.o. for 5 days
(8 weeks of mating)
Single i.p. injection of ? - Epstein et al., 1972
13 or 16.5 mg/kg bw
(8 weeks of mating)
Male mice (Q) 2 ppm in drinking water, 99% - Degraeve et al., 1984a
5 days/week for 7 week
10 mg/kg bw i.p. 99% -5 Moutschen-Dahmen et al., 1981
Male mice (CF1) 30 or 55 mg/m3 inhalation > 97% - Dean & Thorpe, 1972b
for 16 h
2.1 or 5.8 mg/m3 inhalation > 97% -6 Dean & Thorpe, 1972b
for 23 hours/day for 4 weeks
Sister chromatid Male mice (B6C3F1) 5-35 mg/kg bw i.p. 99% -7 Kligerman et al., 1985
exchange peripheral lymphocytes
Male mice (B6C3F1) 6-40 mg/kg bw i.p. ? -8 Chan, 1989
bone marrow cells
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Micronucleus test Mice (Swiss Webster) 0.0075-0.015 mg/kg bw/day ? -9 Paik & Lee, 1977
i.p. for 2 or 4 days
Micronucleus test Mice (HRA/Skh, hairless) skin painting with tech.grade +10 Tungul et al., 1991
(in vitro/in vivo) skin keratinocytes 0-228 µg
(in 100 µl acetone)
Chromosomal Chinese hamster, both 10-15 mg/kg bw p.o. > 97% -11 Dean & Thorpe 1972a
aberrations sexes bone-marrow cells
Male mice (Q) 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
bone-marrow cells 5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Degraeve et al., 1984b
Degraeve et al., 1984b
Male mice (B6C3F1) bone 6-40 mg/kg bw i.p. ? -12 Chan, 1989
marrow cells
Female Syrian golden 0, 3, 6, 15 or 30 mg/kg 50% +13 Dzwonkowska & Hübner, 1986
hamster commercial
formulation
bone-marrow cells bw i.p.
Mice (CF1), both sexes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h
5 mg/m3 inhalation > 97% > 97% -11 Dean & Thorpe, 1972a
23 hours/day for 21 days
Male Chinese hamsters 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
bone-marrow cells for 16h.
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Chromosomal Mice (Q) spermatocytes 2 ppm in drinking-water 99% - Moutschen-Dahmen et al., 1981;
aberrations 5 days/week for 7 weeks Degraeve et al., 1984a
(cont'd)
Chinese hamsters 15 mg/kg bw p.o. > 97% -11 Dean & Thorpe, 1972a
spermatocytes
Mice (Q) spermatocytes 10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984a
Mice (CF1) spermatocytes 64-72 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
for 16h
5 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
23h/day for 21 days
Chinese hamster 28-36 mg/m3 inhalation > 97% -11 Dean & Thorpe, 1972a
spermatocytes for 16 h
Mice (CF1) spermatogonia 2 ppm in drinking-water, 99% - Moutschen-Dahmen et al., 1981;
5 days/week for 7 weeks Degraeve et al., 1984b
10 mg/kg bw i.p. 99% - Moutschen-Dahmen et al., 1981;
Degraeve et al., 1984b
DNA strand breaks Rats (Wistar), both 10 mg/kg bw i.p. 99.8% -14 Wooder & Creedy, 1979
sexes rat liver cell DNA
Table 6 (contd)
Test system Test object Concentration Purity Results* Reference
Unscheduled DNA Male rats (F344) 0, 2, 10 or 35 mg/kg ? - Mirsalis et al., 1989
synthesis hepatocytes bw p.o.
Mice (B6C3F1) (both sexes) 0, 10, 20, 40 or > 98% -15 Bedford, 1991
forestomachrats 100 mg/kg bw p.o.
1 Approximate LD50
2 Positive controls (2.5 mmol/l ethyl methanesulfonate) yielded expected positive responses.
3 Positive controls (400 mg/kg bw p.o. ethyl methanesulfonate) yielded expected positive responses.
4 Positive controls (100 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
5 Positive controls in 2nd and 5th week of mating.
6 Positive controls (2000 mg/kg bw p.o. methyl methanesulfonate) yielded expected positive responses.
7 Positive controls (2-acetylaminofluorene, ethyl methane sulfonate and N-nitroso morpholine) yielded positive results.
All animals treated with 35 mg/kg bw i.p. died.
8 Positive controls (ethyl methane sulfonate 100 mg/kg bw p.o.) yielded expected positive responses.
9 Positive controls (cyclophosamide 30-240 mg/kg bw i.p.) yielded expected positive responses.
10 Recovery of plated cells in vitro after a single topical application of dichlorvos was about 50% of controls.
11 Positive controls (endoxan 100-200 mg/kg bw i.p.) yielded expected positive responses.
12 Positive controls (ethyl methane sulfonate 300-375 mg/kg bw p.o.) yielded expected positive responses.
13 LD50 = 30 mg/kg bw i.p. No dose related response. Positive controls (cyclophosphamide 40 s. mg/kg bw i.p.)
yielded expected positive responses.
14 Positive controls (methyl methane sulfonate 30-60 mg/kg bw i.p.) yielded expected positive responses.
15 Positive controls (N-methyl-N'-nitro-N-nitroso guanidine 200 mg/kg bw p.o.) yielded weakly positive results.
Dichlorvos treatment induced a hyperplastic response similar to that induced by the non-genotoxic carcinogen butylated
hydroxyamisole (300 mg/kg bw p.o.).
Special studies on metabolites
Acute and short-term toxicity studies
The intraperitoneal toxicity of metabolites of dichlorvos in
female mice is considerably less than that of dichlorvos. The LD50
(mg/kg bw) was 250 for dichloroacetic acid, 440 for
dichloroacetaldehyde and 890 for dichloroethanol. For other
metabolites it was > 1000 mg/kg bw. A short-term inhalation study
with dichloroacetaldehyde in rats did not show adverse effects at
concentrations up to 2 mg/m3 for 30 days (WHO, 1989).
Methylating reactivity
In a quantitative colour test in which methylation of
4-(p-nitrobenzyl) pyridine was measured to predict DNA alkylating
potential, desmethyl-dichlorvos, dimethyl phosphate,
dichloroethanol, dichloroacetaldehyde and dichloroacetic acid gave
no reaction (Bedford & Robinson, 1972).
Mutagenicity
In a rec-type repair test with Proteus mirabilis strains
PG713 and PG273, desmethyldichlorvos (10 or 40 µmol/plate) did not
induce base-pair substitutions or other DNA damage (Braun et al.,
1982).
Dichloroacetaldehyde (DCA) appeared to be mutagenic in the
Salmonella test using S. typhimurium TA 100 and TA 1535. The
mutagenicity decreased in the presence of a microsomal activation
system (Lofroth, 1978; Bignami et al., 1980). It was also
positive when tested in concentrations of 10-40 µg/plate in a
forward and a back mutation system in S. coelicolor and two
forward mutation systems in A. nidulans (Bignami et al., 1980).
DCA (0.01%) was not mutagenic to K. pneumoniae in a fluctuation
test (Voogd et al., 1972). DCA did not induce unscheduled DNA
synthesis in the human epithelial-like cell EUE as well as
ouabain-resistant mutations in cultured V-79 Chinese hamster cells
(Aquilina et al., 1984). In a dominant lethal assay with DCA
administered as a single i.p. injection of 176 mg/kg bw to two
strains of male mice (AB Jena-Halle and DBA), positive effects were
reported (Fischer et al., 1977). It should be noted, however,
that the dose used would never be reached after dichlorvos treatment
(i.p. LD50 = 28-41 mg/kg bw). Ramel (1981) reported negative
results in a dominant lethal study with another strain of mice.
No evidence for mutagenicity of dichloroethanol was obtained in
S. typhimurium TA 100 and TA 1535 (Lofroth, 1978; Bignami et al.,
1980). In S. coelicolor (test doses 60-80 µg/plate) and A.
nidulans (test doses 10-50 µg/plate), dichloroethanol was a weak
mutagen (Bignami et al., 1980). Dichloroethanol (1 M and 0.1%)
was mutagenic to K. pneumoniae in a fluctuation test (Voogd et
al., 1972).
Special studies on skin sensitization
In the guinea-pig maximization test by Magnusson & Kligman,
using intradermal and topical induction concentrations of 5% and 25%
in water respectively, a 0.5% challenge concentration caused
sensitization in 30-40% of the animals but a 0.05% challenge
concentration caused no sensitization (Matsushita et al., 1985).
Special studies on testes
Mice
Groups of 14 male NMRI/Han mice received either a single oral
dose of 40 mg/kg bw dichlorvos in olive oil or 18 daily oral doses
of 0 or 10 mg/kg bw dichlorvos in olive oil.
On days 9,18, 27, 36, 54 and 63, two animals from each group
were killed and their testes examined histologically. A significant
increase in the number of damaged seminiferous tubules
(desquamation, decreases in cell population, "holes") was observed
in both dichlorvos groups. The supporting Sertoli cells were also
damaged, which may have resulted in the above effects. In addition,
there was an increase in the number and hypertrophy of Leydig cells.
No explanation was given for these effects (Krause & Homola, 1972,
1974).
An increased incidence, just above background, of sperm
abnormalities, was observed in a screening study on hybrid mice
given five daily i.p. injections of 10 mg/kg bw dichlorvos
(approximately half the LD50). At lower doses, 1 mg/kg bw, the
number of sperm abnormalities was either similar to or lower than
those in the controls (Wyrobek & Bruce, 1975).
Rats
Groups of 16 male juvenile Wistar rats received either 20 mg/kg
bw dichlorvos in olive oil on days 4 and 5, 10 mg/kg bw dichlorvos
in olive oil daily from days 4 to 23, or 0.1 ml olive oil daily from
days 4 to 23. On days 6, 12, 18, 26, 34, and 50 of life, two rats
from each group were sacrificed. Histological examination of the
testes showed slight reduction in the number of spermatogenic cells
and Leydig cells. All changes were reversed by the 50th day of
life. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells (Krause et al.,
1976).
In a subsequent experiment measurement of testosterone
concentrations in the testes, and luteinizing hormone (LH) and
follicle stimulating hormone (FSH) concentrations in serum showed no
differences between treated animals and olive-oil treated controls
(Krause, 1977). However, in this study the use of a different
dosing regimen (10 mg/kg bw by gavage every other day for 2 weeks)
prevented a strict comparison with the earlier study by Krause et
al. (1976).
Fifty-five male Wistar rats (aged 5 months) were orally
administered dichlorvos at levels of 5 or 10 mg/kg bw every other
day for 8 weeks. Eleven rats were killed every 4 weeks. No change
was seen in body-weight gain or testes weight. The score values of
the seminal cellular system decreased after 4-8 weeks of treatment,
but were restored 8 weeks after the end of treatment (Fujita et
al., 1977).
Miscellaneous studies
Effects of inhaled ChE inhibitors, including dichlorvos
(dissolved in acetone), on bronchial tonus were studied in young
adult rats exposed head-only. Bronchoconstriction did not occur at
toxicologically significant doses. An increase in response to
acetylcholine provocation test was observed (Pauluhn et al.,
1987).
The effect of diet on the toxicity of dichlorvos was
investigated using young male rats kept for 30 days on the following
diets: high protein (HPD), low protein (LPD), high fat (HFD), and
standard (SD). Growth rates were normal except for a slightly
decreased body-weight gain in the HFD group. A single i.p.
injection of 50 mg/kg bw dichlorvos led to slightly higher mortality
in LPD rats and slightly lower mortality in HPD rats, compared with
SD rats (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or
HPD for 30 days. At the end of this period, a single i.p. dose of
dichlorvos (20 or 30 mg/kg bw) was administered. No difference in
plasma and erythrocyte ChE inhibition was found. In the case of the
HPD, the spontaneous recovery of plasma and erythrocyte ChE activity
was slightly reduced (Purshottam & Srivastava, 1984).
Observations in humans
In vitro studies
The sensitivity to inhibition by dichlorvos of erythrocyte and
brain ChE was higher than that of plasma ChE, the I50s being about
10-8 and 10-7 mol/l, respectively (Skrinjaric-Spoljar et al.,
1973; Carter & Maddux, 1974; Lotti & Johnson, 1978; Boyer, 1978;
Casale et al., 1989). The rate of phosphorylation of human
erythrocyte ChE was similar to that of rat brain ChE (1.2 x 105 and
1.5 x 105 mol/l/min at 37 °C, pH 7.4-7,6, respectively).
The rate of dichlorvos hydrolysis by human plasma at 37 °C was
found to be 7-11 µmol/hour/ml (Reiner et al., 1980; Traverso et
al., 1989).
Studies on volunteers
Six healthy male volunteers were given single oral doses of 7.5
mg/kg bw of metrifonate. Concentrations of metrifonate (trichlorfon)
and dichlorvos, which is a metrifonate rearrangement product, were
determined in whole blood at different times for up to 24 hours.
The half-life of metrifonate was about 2 hours. The concentrations
of dichlorvos closely followed those of metrifonate with a constant
ratio of 0.01-0.02. However, a half-life for dichlorvos of 2 hours
cannot be accepted because dichlorvos is continuously formed from
metrifonate (Abdi & Villen, 1991).
Groups of five young men received total daily doses of 1, 1.5,
2 or 2.5 mg dichlorvos in corn oil per person, divided in two
gelatin capsules administered at 08.00 h and at 15.00 h for 28 days.
A further group of ten men received 1.5 mg/day for 60 days. Control
groups of two men per dose level received gelatin capsules with corn
oil. Plasma and erythrocyte ChE activities were determined twice
weekly before, during and after dosing. Once each week a medical
interview, complete blood count, and urinalysis were done on each
subject. Before dosing and after the last dose SGOT, ALP, PT,
thymol turbidity, and total bilirubin were determined.
The 2.5 mg/day dose produced a decrease in plasma ChE activity
from the second week of treatment; dosing was discontinued after 20
days when plasma values showed a 30% decrease. The plasma ChE
activity returned to control values 15 days after dosing was ended.
The 2 mg/day dose produced also a reduction in plasma ChE activity
from the second week of treatment which reached a maximum inhibition
of 29% the second day after the last dose. The group receiving 1.5
mg/day for 28 days showed no change in plasma ChE activity while in
the group dosed for 60 days this activity was inhibited by 27%
compared to the control values. None of the groups showed an effect
on erythrocyte ChE activity, haematology, clinical chemistry or
urinalysis. The NOAEL in this study was the highest dose tested of
2.5 mg/day/man (approximately 0.03 mg/kg bw/day) based on the
absence of inhibition of erythrocyte ChE activity (Rider, 1967;
Rider et al., 1967, 1968).
Two groups of six men (21-45 years of age, 64-106 kg bw)
received 0.9 mg dichlorvos three times a day for 21 days, either in
a pre-meal capsule filled with cottonseed oil or in a gelatin salad
consumed during the course of the meal. Control subjects were dosed
with either cotton seed oil capsules or plain gelatin. No
cholinergic signs or symptoms were observed. Plasma ChE activity
was inhibited by 30-40%, the inhibition being higher when dichlorvos
in cotton seed oil was ingested before the meal. The half-life for
the regeneration of plasma ChE activity was 13.7 days. Erythrocyte
ChE activity was not reduced. The NOAEL in this study was 0.04
mg/kg bw/day, based on absence of erythrocyte cholinesterase
inhibition (Boyer et al., 1977).
A number of studies have been done with a slow-release PVC
formulation of dichlorvos intended for use as an anthelminticum.
Single oral doses of above 4 mg dichlorvos/kg bw resulted in
inhibition of erythrocyte ChE activity 24 hours after application,
the maximum being 46% at 32 mg/kg bw. Plasma ChE activity was
affected at single doses of 1 mg/kg bw (50% inhibition) and above.
However, no dichlorvos-related symptoms were observed. Repeated
oral dosing for 7 days produced clinical symptoms with doses of 8
mg/kg bw/day and above. Plasma ChE activity was inhibited by about
80% at all dose levels (1-16 mg/kg bw/day). Erythrocyte ChE
activity showed a dose-related decrease from 5-30% at 1 mg/kg bw/day
up to 50-80% inhibition at the highest dose. Blood count, urine,
liver function, PT and BUN were normal in all studies (Hine &
Slomka, 1968, 1970; Pena-Chavarria et al., 1969; Slomka & Hine,
1981).
In children (aged 7-18 years) orally treated with metrifonate
(7.5, 10.0 or 12.5 mg/kg bw), the half-lives of recovery of
inhibited erythrocyte AChE and plasma ChE were 15 and 6.7 days,
respectively (Reiner & Plestina, 1979). It is known that the
anticholinesterase activity of metrifonate is due to its
decomposition to dichlorvos (Reiner et al., 1975). Similar
half-lives for plasma ChE have been described by Boyer et al.,
(1977) after repeated oral dosing with dichlorvos and by Bisby &
Simpson (1975) after cutaneous exposure of a sprayman to dichlorvos.
This longer half-life contrasts with faster in vitro half-lives,
which are similar to those of the rodent (Skrinjaric-Spoljar et
al., 1973).
Several inhalation studies have been carried out and are
summarized by WHO (1989). These studies confirmed that plasma ChE
is more sensitive to inhibition by dichlorvos than RBC ChE. The
latter was found inhibited when the dichlorvos dose (concentration x
time) was higher than about 1500 mg/m3/min (WHO, 1989).
Thirteen men (31-53 years of age) were exposed to dichlorvos
strips (20% dichlorvos; 1 strip per 30 m3) for 3 months. No
biologically or statistically significant changes were observed in
their electromyography results nor in their whole blood ChE activity
(Ottevanger, 1975).
Poisoning incidents
Fournier et al. (1978) reported that dichlorvos was detected
in blood in three cases of human intoxication within 24 hours after
poisoning (exact timing not reported).
A woman who intentionally ingested an estimated 100 mg
dichlorvos/kg bw survived following intensive care for 14 days
(Watanabe et al., 1974). A suicide with a dichlorvos dose of
about 400 mg/kg bw succeeded in spite of treatment (Shinoda et al.,
1972). A female patient, aged 35 years, who had accidentally
ingested 60 g fluid Divipan (dichlorvos concentration not reported),
was comatose for a week and recovered slowly. Clinical and
electrophysiological examinations showed a pure motor form of
neuropathy, according to the authors (Vasilescu & Florescu, 1980).
Three cases of poisoning with dichlorvos taken orally in unspecified
but high quantities have been reported from India. The patients
first showed severe cholinergic signs for a few days. After
recovery, delayed neurotoxicity developed. Nerve conduction studies
showed a severe axonal degeneration (Wadia et al., 1985, 1987).
Thirteen cases of dichlorvos ingestion, either accidental or
deliberate, were reported from a hospital in Beijing, China. The
patients included 10 women and three men, aged 11-38 years. Twelve
of the 13 patients ingested 25-50 ml of dichlorvos (80% purity).
Ten cases were associated with acute pulmonary oedema. Two patients
died because OP poisoning was not diagnosed in time. The others
recovered completely 2-5 days after treatment (Li et al., 1989).
Occupational Exposure
A number of fatal and non-fatal dichlorvos poisoning cases have
been described and summarized by WHO (1989). Two workers who failed
to promptly wash off the concentrated formulation of dichlorvos,
which splashed on to their skin, died subsequently. However, in
those cases where the spilled solution was washed off immediately,
the victims showed symptoms of intoxication but recovered after
treatment. Occupational exposure of spraymen entails both dermal
and respiratory absorption of dichlorvos. When appropriate
protective equipment was not used, inhibition of plasma ChE and less
frequently of RBC ChE was found. Sometimes mild, short lasting,
cholinergic symptoms and signs have been reported (WHO, 1989).
Each of 13 pest-control operators carried out urban
pest-control work for one day in 4 houses using 230-330 g dichlorvos
as aerosol and 40-50 g dichlorvos as emulsion spray. At the end of
the day's work, an operator had an average dichlorvos residue of 0.8
mg/m2 on the back, 0.4 mg/m2 on the chest, and 11 mg/m2 on the
respirator filter. Urinary dimethylphosphate excretion in 3
applicators ranged from 0.32 to 1.39 micrograms on the day of
treatment, but approached the level of detection by the following
morning. Blood and urine analyses revealed no changes in various
clinical parameters, including serum cholinesterase levels (Das et
al., 1983).
COMMENTS
Dichlorvos is rapidly absorbed by all routes of exposure and
rapidly degraded. The metabolic pathways of dichlorvos are similar
in mammalian species, including humans. Metabolites are rapidly
excreted or incorporated into natural enzymatic pathways.
Dichlorvos has a marked acute oral toxicity with typical
cholinergic signs and has been classified by WHO as highly
hazardous.
Rat erythrocyte and brain cholinesterase inhibited by
dichlorvos spontaneously reactivates with a half-life of about two
hours both in vitro and in vivo.
Several carcinogenicity studies in mice and rats using routes
other than gavage were negative, even when doses causing signs of
toxicity were used. It should be noted that two squamous-cell
carcinomas of the oesophagus have been observed in treated mice in
one study.
In a carcinogenicity study in mice dichlorvos administered by
corn oil gavage (0, 10 or 20 mg/kg bw/day to males, and 0, 20 or 40
mg/kg bw/day to females), caused forestomach papillomas
(statistically-significant positive trend with increased incidence
in the high-dose female group). Elements of the mechanism by which
these papillomas might arise have not been established, but the
induction of hyperplasia in the forestomach was demonstrated.
Additionally, genotoxic effects might occur at high local
concentrations of dichlorvos (see below) as can be obtained in
gavage dosing but not in dietary exposure. Based on the increased
incidence of forestomach papillomas, the NOAEL was 10 mg/kg bw/day.
In a two-year feeding study in rats (0, 0.1, 1, 10, 100 or 500
ppm), no neoplastic lesions were attributed to treatment. The
NOAEL, based on brain cholinesterase inhibition, was 100 ppm (actual
concentration 47 ppm, equivalent to 2.4 mg/kg bw/day).
In a carcinogenicity study in Fischer 344 rats, dichlorvos
administered by corn oil gavage (0, 4 or 8 mg/kg bw/day) caused an
increased incidence of pancreatic adenomas (statistically
significant in males only), mononuclear cell leukemias
(statistically significant in males only, no dose response) and
mammary gland adenomas or fibroadenomas (females only, no dose
response, statistically-significant in the low-dose group only).
The Meeting observed that the incidence of pancreatic acinar
adenomas in male control rats was unusually high and therefore the
higher incidence found in treated animals was considered of
questionable biological significance. The increased incidence of
mononuclear cell leukaemia which is usually high and variable in
this strain of rats, was also of questionable biological
significance. The doses used significantly inhibited plasma, but
not erythrocyte, cholinesterase activity when measured three hours
after treatment. However, given the rapid recovery of erythrocyte
cholinesterase activity after inhibition by dichlorvos, the timing
might have underestimated the inhibition.
Dichlorvos has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. These data indicate that
dichlorvos is genotoxic in bacteria and cultured mammalian cells,
but that it is not clastogenic in vivo except under conditions
where an unusually high tissue dose can be attained. Dichloro-
acetaldehyde, a major metabolite of dichlorvos, is a weak bacterial
mutagen. Positive results have been reported in mice given a dose
of dichloroacetaldehyde far greater than that which could derive
from sublethal doses of dichlorvos. Dichlorvos has been shown to
methylate DNA in vitro at a rate that is 8-9 orders of magnitude
lower than the rate of phosphorylation. Therefore, DNA alkylation
is not likely to occur at doses of dichlorvos which are not
inhibitory to erythrocyte/brain cholinesterase.
A three-generation reproduction study in rats was negative at
doses up to 235 ppm in the diet, equivalent to 12 mg/kg bw/day. A
one-litter, one-generation study in mice in which dichlorvos was
administered by inhalation at doses which caused > 90% plasma
cholinesterase inhibition, but no signs of toxicity, was negative.
Dichlorvos caused reversible damage of seminiferous tubules, Leydig
and Sertoli cells at oral doses of 10 mg/kg bw daily for 18 days in
mice and at 5 mg/kg bw and above every other day for 8 weeks in
rats.
Dichlorvos appeared not to be teratogenic in mice, rats and
rabbits at doses which caused maternal toxicity.
Dichlorvos caused delayed polyneuropathy in hens at doses much
higher than the unprotected LD50. Cases of delayed polyneuropathy
also have been reported in humans after severe intoxications.
In humans, the rate of dichlorvos hydrolysis by plasma is
similar to that in rats. The rate of recovery of inhibited
erythrocyte and plasma cholinesterase activity in humans given
dichlorvos is much slower than in rats. Half-lives of recovery are
about 15 days in humans and about two hours in rats. A daily dose
of 1 mg/kg bw to male human volunteers for seven days caused 5-30%
inhibition of erythrocyte cholinesterase. The NOAEL in humans,
based on absence of erythrocyte cholinesterase inhibition in 12
volunteer males for 21 days was 0.04 mg/kg bw/day.
In 1986, the Joint Meeting discussed the significance of
carcinogenicity studies for organophosphorus pesticides and the
requirements for further studies (Section 3.1 of Annex 1, reference
47). At that time none of the organophosphorus pesticides had
caused a carcinogenic response in experimental animals. That Joint
Meeting recommended that, depending upon future evaluation on a case
by case basis, further consideration should be given to the need for
carcinogenicity studies for organophosphates.
In assessing the potential hazard to humans of residues of
dichlorvos, the following considerations were taken into account in
view of the weakly positive results in the gavage carcinogenicity
study in mice.
Organophosphorus esters used as insecticides react with
biological molecules by means of phosphorylation of serine
hydrolases and of alkylation of macromolecules. Phosphorylation of
acetylcholinesterase and alkylation of DNA are considered to account
for the acute cholinergic toxicity and initiation of the
carcinogenic process, respectively. These biochemical reactions
occur at different rates. When the rate of phosphorylation is
substantially higher than the rate of alkylation, in vivo
genotoxic effects are unlikely to occur because effective doses
cannot be achieved due to acute toxicity. Dichlorvos meets these
criteria, the rate of phosphorylation of acetylcholinesterase being
much faster (eight orders of magnitude) than that of alkylation of
several macromolecules, including DNA. Hence positive mutagenicity
tests were seen only in vitro and, as indicated in the 1986 Joint
Meeting report, carcinogenicity studies are unlikely to give more
information. The weak carcinogenic response of dichlorvos obtained
in mice in a corn oil gavage study should be interpreted as a local
effect of dichlorvos.
Information on comparative cholinergic toxicity might be of
critical relevance for the extrapolation of toxic effects (other
than acute effects) of organophosphates in experimental animals to
humans. The characteristics of the interactions of a given compound
with acetylcholinesterase (rates of phosphorylation, spontaneous
reactivation and ageing) from different species can be compared in
vitro. Also, the in vivo rate of reappearance of blood
acetylcholinesterase activity can be measured. In some cases,
metabolic degradation of organophosphates can be assessed
comparatively by measuring the level of serum A esterase, which
hydrolyses a given compound. All these data enable an improved
assessment of cholinergic toxicity of organophosphates in different
species. This knowledge may be of special significance in the case
of dimethyl phosphates since the rates of in vivo reactivation
vary substantially across species. Therefore, chronic dosing is
more critical for extrapolation from animal data to humans. In a
repeated dose regime, the longer the half-life of reactivation the
more rapid and/or more toxic will be the resulting effect (i.e. in a
chronic dosing regime, humans will survive much lower doses of
dichlorvos causing, when given alone, peak erythrocyte/brain
cholinesterase inhibition than those which can be reached in
rodents). Therefore, comparison between the in vivo rates of
recovery of enzyme activity will enable an assessment of the
repeated doses of compounds and the resulting cholinesterase
inhibition, which would represent the limiting factors for other
toxicities (including mutagenicity and carcinogenicity).
In the case of dichlorvos, the Meeting considered the
extrapolation of carcinogenicity data derived in rodents and its
applicability to human safety, and concluded that the compound would
not result in chronic human health hazards at doses below those
which result in acetylcholinesterase inhibition.
The Meeting maintained the ADI, which is based on studies in
humans with a NOAEL of 0.04 mg/kg bw/day, using a 10-fold safety
factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 mg/kg bw/day (two-year study)
Rat: 47 ppm in the diet, equivalent to 2.4 mg/kg bw/day
(two-year study)
Human: 0.04 mg/kg bw/day (21-day study)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abdi, Y.A. & Villen, T. (1991) Pharmacokinetics of metrifonate and
its rearrangement product dichlorvos in whole blood. Pharmacol.
Toxicol. 68: 137-139.
Aquilina, G., Benigni, R., Bignami, M., Calcagnile, A., Dogliotti,
E., Falcone, E. & Carere, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon and dichloroacetaldehyde. Pestic. Sci. 15:
439-442.
Asperen van, K., Dekhuijzen, H.M. (1958) A quantitative analysis of
the kinetics of cholinesterase inhibition in tissue homogenates of
mice and houseflies. Biochim. Biophys. Acta, 28: 307-337.
Bedford, C.T. & Robinson, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2: 307-337.
Benford, D.J. (1991) Investigation of the genotoxic and/or irritant
effects of dichlorvos on mouse forestomach. Unpublished report no.
RI90/0405 from Robens Institute of Health and Safety, Guildford,
Surrey, U.K.. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Bignami, M., Aulicino, F., Velcich, A., Carere, A. & Morpurgo, G.
(1977) Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutat. Res. 46: 395-402.
Bignami, M., Conti, G., Conti, L., Crebelli, R., Misuraca, F.,
Ruglia, A.M., Randazzo, R., Sciandrello, G. & Carere, A. (1980)
Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella
typhimurium, Streptomyces coelicolor and Asperigillus nidulans.
Chem.-Biol. Interactions, 30: 9-23.
Bisby, J.A. & Simpson, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Austr. 2: 394-395.
Blair, D., Dix, K.M., Hunt, P.F., Thorpe, E., Stevenson, D.E. &
Walker, A.I.T. (1976) Dichlorvos - a 2-year inhalation
carcinogenesis study in rats. Arch. Toxicol. 35: 281-294.
Boyer, A.C. (1978) Factors which affect the in vitro inhibition of
human plasma cholinesterase. Toxicol. Appl. Pharmacol. 41: 475-
483.
Boyer, A.C., Brown, L.J., Slomka, M.B. & Hine, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. Appl. Pharmacol. 41:
389-394.
Braun, R., Schoneich, J., Weissflog, L. & Dedek, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair - direct alkylation versus metabolic activation and
breakdown. 1. Butonate, vinylbutonate, trichlorfon, dichlorvos,
demethyl dichlorvos and demethyl vinylbutonate. Chem.-Biol.
Interact. 39: 339-350.
Bridges, B.A., Mottershead, R.P., Green, M.H.L. & Gray, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulphonate for
Escherichia coli WP2 and some derivatives deficient in DNA repair.
Mutat. Res. 19: 295-303.
Brown, V.K.H. & Roberts, M (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to
guinea-pigs. Unpublished report no. IRR TL/40/66 from Shell Research
Ltd., Sittingbourne, UK. Submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Buselmayer, W., Rohrborn, G., Propping, P. (1972) Mutagenicity
investigations with pesticudes in the host-mediated assay and the
dominant lethal test in mice. Biol. Zbl. 91: 311-325 (in German).
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M., Raschetti,
R. (1978a) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res. 57: 277-286.
Carere, A., Ortali, V.A., Cardamone, G., Morpurgo, G. (1978b)
Mutagenicity of dichlorvos and other structurally related pesticides
in Salmonella and Streptomyces. Chem.-Biol. Interactions, 22:
297-308.
Caroldi, S., Lotti, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett. 9:
157-159.
Carter, M.K., Maddux B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. Appl. Pharmacol., 27: 456-463.
Casale, G.P., Bavari, S., Connolly, J.J. (1989) Inhibition of human
serum complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam. Appl. Toxicol. 12: 460-
468.
Casebolt, D.B., Leary, S.L., Undeutsch, L. (1990) Effects of
dichlorvos treatment on mouse reproduction. Lab. Anim. Care, 40:
65-67.
Chan, P.C. (1989) Toxicology and carcinogenesis studies of
dichlorvos (CAS No. 62-73-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, National Toxicology
Program, Technical Report Series NTP TR No.342.
Chan, P.C., Huff, J., Haseman, J.K., Alison, R. & Prejean, J.D.
(1991) Carcinogenesis studies of dichlorvos in Fischer rats and
B6C3F1 Mice. Jpn. J. Cancer Res. 82: 157-164.
Chattopadhyay, D.P., Dighe, S.K., Dube, D.K., & Purnanand (1982)
Changes in toxicity of DDVP, DFP, and parathion in rats under cold
environment. Bull. Environ. Contam. Toxicol. 29: 605-610.
Cohen, S.D., Ehrich, M. (1976) Cholinesterase and carboxyl esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. Appl. Pharmacol. 37: 39-48.
Coulston, F., Griffin, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP vapors.
Unpuplished report from Albany, New York, Institute of Comparative
and Human Toxicology and Holloman, Air Force Base, New Mexico,
Intern. Center of Environmental Safety. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Das, Y.T., Taskar, P.K., Brown, H.D., Chattopadhyay, S.K. (1983)
Exposure of professional pest control operator to dichlorvos (DDVP)
and residue on house structures. Toxicol. Letters, 17: 95-99.
Dean, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on micro-organisms. Arch. Toxikol. 30: 67-74.
Dean, B.J., (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol. 30: 75-85.
Dean, B.J. & Blair,D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres
containing dichlorvos. Mutat. Res. 40: 67-72.
Dean, B.J., Thorpe, E. (1972a) Cytogenetic studies with dichlorvos
in mice and Chinese hamsters. Arch. Toxikol. 30: 39-49.
Dean, B.J., Thorpe, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxicol. 30: 51-59.
Dean, B.J., Doak, S.M.A., Funnell, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium using
Saccharomyces cerevisiae. Arch. Toxicol. 30: 61-66.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984a) Cytogenetic and
genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol. 56: 66-67.
Degraeve, N., Chollet, M.C., Moutschen, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Letters, 21: 315-319.
Dieter, M.P., Jameson, C.W., French, J.E., Gangjee, S. (1989)
Development and validation of a cellular transplant model for
leukemia in Fischer rats: a short-term assay for potential
anti-leukemic chemicals. Leukemia Research, 13: 841-849.
Dyer, K.F., Hanna, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res. 21: 175-177.
Dzwonkowska, A., Hubner, H. (1986) Induction of chromosomal
aberrations in the Syriam hamster by insecticides tested in vivo.
Arch. Toxicol. 58: 152-156.
Enomoto, M.F., Nakadate, M., Ninomia, K., Hayakawa, Y., Ito, H.,
Igarashi, S., Uwanuma, Y., Nakasato, R., Hatanaka, J. (1981) Studies
on carcinogenicity of DDVP (2,2-dichlorovinyl dimethyl phosphate)
mixed in drinking-water in rats. Ministry of Health and Welfare,
Tokyo, Japan (Cooperative studies on carcinogenicity test on
mutagens) (Unpublished).
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325.
Fahrig, R. (1973) (Genetic effects of organophosphorous
insecticides.) Die Naturwissenschaften, 60: 50-51 (in German).
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
IARC Scient. Publ. 10: 161-181.
Farber, T.M., Marks, E.M., Cerra, F.E. (1975) Dichlorvos and
microsomal enzyme activity. Fed. Proc. 34: 785.
Fischer, G.W., Schneider,P., Scheufler, H. (1977) (Mutagenicity of
dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester, possoble metabolites of the organophosphorus
pesticide trichlorphon.) Chem.-Biol. Interactions, 19: 205-213 (in
German).
Fournier, E., Sonnier, D., Dally, S., (1978) Detection and assay of
organophosphate pesticides in human blood by gas chromatography.
Clin. Toxicol. 12: 457-462.
Francis, B.M., Metcalf, R.L., Hansen, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. Environ. Sci. Health, B20: 73-95.
Fujita, K., Matsushima, S., Abe, E., Sasaki, K., Kurosawa, K. (1977)
(Examination of the effects of dichlorvos on the testis.) Nippon
Noson Igakkai Zasshi, 26: 328-329 (in Japanese) as quoted by WHO,
1989.
Gilot-Delhalle, J., Colizzi, A., Moutschen, J., Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade 6
locus of Schizosaccharomyces pombe. Mutat. Res: 117: 139-148.
Gough, B.J., Shellenberger, T.E. (1977-1978) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors
and reactivation with oximes. Drug and Chemical Toxicol. 1: 25-43.
Green, M.H.L., Medcalf, A.S.C., Arlett, C.F., Harcourt, S.A.,
Lehmann, A.R. (1974) DNA strand breakage caused by dichlorvos,
methyl methanesulphonate and iodoacetamide in Escherichia coli and
cultured Chinese hamster cells. Mutat. Res. 24: 365-378.
Green, M.H.L., Muriel, W.J., Bridges, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res. 38: 33-42.
Griffin III, D.E., Hill, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res. 52: 161-169.
Guerzoni, M.E., Del Cupolo, L., Ponti, I. (1976) (Mutagenic
activity of pesticides). Rev. Sci. Tecn. Alim. Nutr. Um. 6:
161-165 (in Italian).
Gupta, A.K., Singh, J. (1974) Dichlorvos (DDVP) induced breaks in
the salivary gland chromosomes of Drosophila melanogaster. Current
Science, 43: 661-662.
Hanna, P.J., Dyer, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res. 28: 405-420.
Haseman, J.K., Huff, J.E., Rao, G.N., Arnold, J.E., Boorman, G.A.,
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1
(B6C3F1) mice. J. Natl. Cancer Inst. 75: 975-984.
Hass, D.K., Collins, J.A., Kodama, J.K. (1972) Effects of orally
administered dichlorvos in Rhesus monkeys. J. Am. Vet. Med. 161:
714-719.
Hatch, G.G., Anderson, T.M., Lubet, R.A., Kouri, R.E., Putman, D.L.,
Cameron, J.W., Nims, R.W., Most, B., Spalding, J.W., Tennant, R.W.,
Schechtman, L.M. (1986) Chemical enhancement of SA7 virus
transformation of hamster embryo cells: Evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutag. 8:
515-531.
Hine, C.H. (1962) 90-day chronic toxicity studies of Vapona
insecticide for dogs. Unpublished report from San Francisco, The
Hine Laboratories, Report no. 1, September 1. Prepared for Shell
Chemical Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd,
Temana International Ltd.
Hine, C.H., Slomka, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
Hine, C.H., Slomka, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. Appl. Pharmacol. 17:
304-305.
Horn, K.H., Teichmann, B., Schramm, T. (1987) (Studies on
dichlorvos. I. Testing of dichlorvos for carcinogenic activity in
mice.) Archiv für Geschwulstforschung, 57: 353-360 (in German).
Horn, K.H., Teichmann, B., Schramm, T., Nischan, P. (1988) (Studies
on dichlorvos. II. Testing of dichlorvos for carcinogenic activity
in rats). Archiv für Geschwulstforschung, 58: 1-10 (in German).
Horn, K.H., Teichmann, B., Schramm, T. (1990) (Studies on
dichlorvos. III. Testing of dichlorvos for co-carcinogenic activity
in mice). Archiv für Geschwulstforschung, 60: 117-124 (in German).
Houk, V.S., DeMarini, D.M. (1987) Induction of prophage lambda by
chlorinated pesticides. Mutat. Res. 182: 193-201.
IARC (1979) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Some halogenated hydrocarbons, Volume 20:
97-127.
IARC (1991) Monographs on the evaluation of the carcinogenic risk
of chemicals to humans. Occupational exposures in insecticide
application, and some pesticides. Volume 53: 267-307.
Ishidate, M., Sofuni, T., Yoshikawa, K. (1981) Chromosomal
aberration tests in vitro as a primary screening tool for
environmental mutagens and/or carcinogens. GANN Monograph on Cancer
Research, 27: 95-108.
Jayasuriya, V.U. de S., Ratnayake, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inform. Serv. 50: 184-186.
Johnson, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch. Toxicol.
41: 107-110.
Jolley, W.P., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of Vapona insecticide into their daily diet.
Unpublished report from Cincinnati, The Kettering Laboratory,
January 19. Prepared for Shell Chemical Company. Submitted to WHO by
Bayer AG, Ciba-Geigy Ltd, Temana International Ltd.
Kimbrough, R.D., Gaines, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. Environ.
Health, 16: 805-808.
Kligerman, A.D., Erexson, G.L., Wilmer, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res. 157: 181-187.
Konishi, Y., Denda, A., Kitaoka, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3F1 mice. Tokyo, Ministry of
Health and Welfare, Japan (unpublished).
Kramers, P.G.N., Knaap, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of Drosophila
melanogaster. Mutat. Res. 57: 103-105.
Krause, W. (1977) Influence of DDT, DDVP and malathion on FSH, LH
and testosterone serum levels and testosterone concentrations in
testis. Bull. Environ. Contam. Toxicol. 18: 231-242.
Krause, W., Homola, S. (1972) (Influence on spermatogenesis by DDVP
(dichlorvos).) Arch. Dermatol. Forschung, 244: 439-441 (in
German).
Krause, W., Homola, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat testis after the
application of dichlorvos (DDVP). Bull. Envirom. Contam. Toxicol.
11: 429-433 (study is performed in mice).
Krause, W., Hamm, K., Weissmuller, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. Environ. Contam. Toxicol. 15: 458-462.
Lawley, P.D., Shah, S.A., Orr, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (Dichlorvos, DDVP).
Chem.-Biol. Interactions, 8: 171-182.
Li, C., Miller, W.T., Jiang, J. (1989) Pulmonary edema due to
ingestion of organophosphate insecticide. Am. J. Roentgenol., 152:
265-266.
Lin, S.Y., Lee, T.C., Cheng, C.S., Wang, T.C. (1988) Cytotoxicity,
sister-chromatid exchange, chromosome aberration and transformation
induced by 2,2-dichlorovinyl-0,0-dimethyl phosphate. Mutat. Res.
206: 439-445.
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57: 393-394.
Lofroth, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
naturforsch. 29: 651.
Lofroth, G., Wennerberg, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch. 41:
215-221.
Lotti, M., Johnson, M.K. (1978) Neurotoxicity of organopho-sphorus
pesticides: Predictions can be based on in vitro studies with hen
and human enzymes. Arch. Toxicol. 41: 215-221.
Maslinska, D., Zalewska, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia Histochem. Cytochem. 16: 335-341.
Matsushita, T., Aoyama, K., Yoshimi, K., Fujita, Y., Ueda, A. (1985)
Allergic contact dermatitis from organophosphorus insecticides.
Ind. Health, 23: 145-153.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L., Loh, E.K., Hamilton,
C.M., Bakke, J.P., Spalding, J.W. (1989) Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: Testing of 24 compounds. Environ. Mol. Mutat.
14: 155-164.
Modak, A.T., Stavinoha, W.B., Weintraib, S.T. (1975) Dichlorvos and
the cholinergic system: effects on the cholinesterase and
acetylcholine and choline contents of rat tissues. Arch. Int.
Pharmacodyn. 217: 293-301.
Mohn, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides. Mutat.
Res. 20: 7-15.
Moriya, M., Kato, K., Shirasu, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res. 57: 259-263.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y., (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res. 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979) Analysis of mitotic nondisjunction with
Aspergillus nidulans. Environ. Health Aspects, 31: 81-95.
Moutschen-Dahmen, J., Moutschen-Dahmen, M., Degraeve, N. (1981)
Metrifonate and dichlorvos: cytogenetic investigations. Acta
Pharmacol. Toxicol. 49(V): 29-39.
Nagy, Zs., Mile, I., Antoni, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta Microbiol. Acad.
Sci. Hung. 22: 309-314.
NCI (National Cancer Institute) (1977) Bioassay of dichlorvos for
possible carcinogenicity. Bethesda, Maryland, USA.
Nicholas, A.H., Vienne, M., Berghe, H. van den (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Letters, 2:
271-275.
Nishio, A., Uyeki, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphate
insecticides and their oxygen analogs. J. Toxicol. Environ. Health,
8: 939-946.
Nishio, A., Uyeki, E.M. (1982) Nuclease-sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants.
Biochem. Biophys. Research Comm. 107: 485-491.
Nordgren, I., Bergstrom, M., Holmstedt, B., Sandoz, M. (1978)
Transformation and action of metrifonate. Arch Toxicol. 41: 31-41.
Olinski, R., Walter, Z., Wiaderkiewicz, R., Lukasova, E., Palecek,
E. (1980) Changes in DNA properties due to treatment with the
pesticides malathion and DDVP. Radiat. Environ. Biophys. 18:
65-72.
Ottevanger, C.F. (1975) (Neuromuscular function in people exposed to
dichlorvos resin strips. The importance of electromyography.)
Europ. J. Toxicol. 8: 192-195 (in French).
Pachecka, J., Sulinski, A., Ziolkowska, G. (1975) The activities of
some esterases of the rat brain after intoxication by
organophosphate insecticides, dichlorvos and trichlorphon.
Neuropat. Pol. 13: 455-462.
Paik, S.G., Lee, S.J. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool. 20:
19-28.
Pauluhn, J., Machemer, L., Kimmerle, G. (1987) Effects of inhaled
cholinesterase inhibitors on bronchial tonus and on plasma and
erythrocyte acetylcholine esterase activity in rats. Toxicology,
46: 177-190.
Pena-Chavarria, A., Swartzwelder, J.C., Villarejos, V.M., Kotcher,
E., Arguedas, J. (1969) Dichlorvos, an effective broad-spectrum
anthelmintic. Am. J. Trop. Med. Hyg. 18: 907-911.
Perocco, P., Fini, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
Purshottam, T., Kaveeshwar, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviation, Space,
and Environ. Med. 50: 581-584.
Purshottam, T., Srivastava, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
Environ. Health, 39: 425-430.
Pym, R.A., Singh, G., Gilbert, W.S., Armstrong, J.P., McCleary, B.V.
(1984) Effects of dichlorvos, maldison and pirimiphos-methyl on
food consumption, egg production, egg and tissue residues, and
plasma acetylcholinesterase inhibition in layer strain hens. Aust.
J. Exp. Agric. Anim. Husb. 24: 83-92.
Ramel, C. (1981) Does dichlorvos constitute a genotoxic hazard?
Progress in Mutat. Res. 2: 69-78.
Rauws, A.G., Logten, M.J. van (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in chicken.
Toxicology, 1: 29-41.
Reiner, E., Plestina, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by
0,0-dimethyl-2,2-dichlorovinyl phosphate. Toxicol. Appl. Pharmacol.
49: 451-454.
Reiner, E. Krauthacker, B., Simenon, V. & Skrinjaric-Spoljar, M.
(1975) Mechanism of inhibition in vitro of mammalian
acetylcholinesterase and cholinesterase in solutions of
0,0-dimethyl-2,2,2-trichloro-1-hydroxyethylphosphonate
(trichlorphon). Biochem. Pharmacol. 24: 717-722.
Reiner, E., Simeon, V., Skrinjaric-Spoljar, M. (1980) Hydrolysis of
O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP) by esterases in
parasitic helminths, and in vertebrate plasma and erythrocytes.
Comp. Biochem. Physiol. 66: 149-152.
Rider, J.A. (1967) Determination of the minimal incipient toxicity
of dichlorvos in humans. Unpublished report from San Francisco,
Gastrointestinal Research Lab., October. Prepared for Shell Chemical
Company. Submitted to WHO by Bayer AG, Ciba-Geigy Ltd, Temana
International Ltd.
Rider, J.A., Moeller, H.C., Puletti, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies
with Guthion, and determination of incipient toxicity level of
dichlorvos in humans. Fed. Proc. 26: 427.
Rider, J.A., Moeller, H.C., Puletti, E.J., Swader, J. (1968) Studies
on the anticholinesterase effects of methyl parathion, Guthion,
dichlorvos, and Gardona in human subjects. Fed. Proc. 27: 597.
Rosenkranz, H.S. (1973) Preferential effect of dichlorvos (Vapona)
on bacteria deficient in DNA polymerase. Cancer Research, 23:
458-459.
Rosenkranz, H.S., Rosenkranz, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia, 28: 386-387.
Segerback, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP
(2,2-dichlorovinyl dimethylphosphate). Hereditas, 94: 73-76.
Segerback, D., Ehrenberg, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta Pharmacol. Toxicol. 49(V): 56-66.
Shellenberger, T.E. (1980) Organophosphate pesticides inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. Environ. Sci. Hlth. B15: 795-822.
Shellenberger, T.E., Newell, G.W., Okamoto, S.S., Sarros, A. (1965)
Response of rabbit whole-blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14:
943-952.
Shinoda, H., Ito, K., Matsunaga, T., Takada, T. & Nomura, T. (1972)
(Two suicides by acute pesticide intoxication). J. Jpn. Assoc.
Rural Med. 21: 242-249 (in Japanese).
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A., Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shooter, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating agents.
Chem. Biol. Interactions, 11: 575-588.
Skrinjaric-Spoljar, M., Simeon, V., Reiner, E. (1973) Spontaneous
reactivation and aging of dimethylphosphorylated
acetylcholinesterase and cholinesterase. Biochim. Biophys. Acta,
315: 636-369.
Slomka, M.B., Hine, C.H. (1981) Clinical pharmacology of dichlorvos.
Acta Parmacol. Toxicol. 49(V): 105-108.
Snow, D.H., Watson, A.D. (1973) The acute toxicity of dichlorvos in
the dog: 1. clinical observations and clinical pathology. Aust.
Vet. J. 49: 113-119.
Sobels, F.H., Todd, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res. 67: 89-92.
Stanton, H.C., Albert, J.R., Mersmann, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows.
Am. J. Vet. Research, 40: 315-320.
Stevenson, D.E., Blair, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Tox. 18: 215-217.
Teichert, K., Szymczyk, T., Consolo, S., Ladinsky, H. (1976) Effect
of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. Appl. Pharmacol. 35: 77-81.
Tezuka, H., Ando, N., Suzuki, R., Terahta, M., Moriya, M., Shira-su,
Y. (1980) Sister-chromatid exchanges and chromosomal aberrations in
cultured Chinese hamster cells treated with pesticides positive in
microbial reversion assays. Mutat. Res. 78: 177-191.
Traverso, R., Moretto, A., Lotti, M. (1989) Human serum
"A"-esterases. Hydrolysis of 0,0-dimethyl-2,2-dichlorovinyl
phosphate. Biochem. Pharmacol. 38: 671-676.
Tungul, A., Bonin, A.M., He, S., Baker, R.S. (1991) Micronuclei
induction by dichlorvos in the mouse skin. Mutagenesis, 6:
405-408.
Uchiyama, M., Yoshida, T., Homma, K., Hongo, T. (1975) Inhibition
of hepatic drug-metabolizing enzymes by thiophosphate insecticides
and its drug toxicological implications. Biochem. Pharmacol. 24:
1221-1225.
Vasilescu, C., Florescu, A. (1980) Clinical and electrophysiological
study of neuropathy after organophosphorous compounds poisoning.
Arch. Toxicol. 43: 305-315.
Voogd, C.E., Jacobs, J.J., Stel, J.J. van der (1972) On the
mutagenic action of dichlorvos. Mutat. Res. 16: 413-416.
Wadia, R.S., Shinde, S.N., Vaidya, S. (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurol. India, 33:
247-253.
Wadia, R.S., Chitra, S., Amin, R.B., Kiwalkar, R.S., Sardesai, H.V.
(1987) Electrophysiological studies in acute organophosphate
poisoning. J. Neurol. Neurosurg. Psychiatry, 50: 1142-1148.
Wang, T., Wu, C., Lin, J., Tarn, C., (1988) Dichlorvos potentiates
the insecticide-induced sister chromatid exchanges in Chinese
hamster ovary cells. Bull. Inst. Zool. Academia Sinica, 27:
111-117.
Ward, F.P., Glicksberg, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Ass. 158:
457-461.
Watanabe, H., Shirai, O., Ebata, N. (1974/1976) (A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care.) Sapporo Igaku Zasshi, 43: 334-337 (in Japanese);
Pest. Abstr., 8: 94-95.
Weisburger, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D. (eds). Effects of chronic exposures
to pesticides on animal systems, pp. 165-176. New York, Raven Press.
Wennerberg, R., Lofroth, G. (1974) Formation of 7-methylguanine by
dichlorvos in bacteria and mice. Chem.-Biol. Interactions, 8:
339-348.
WHO (1989) Environmental Health Criteria 79: Dichlorvos.
International Programme on Chemical safety, Geneva, World Health
Organization.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wild, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of
dichlorvos and methyl methanesulphonate. Mutat. Res. 19: 33-41.
Wild, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res. 32: 133-150.
Witherup, S., Caldwell J.S., Hull, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their
offsprings by the introduction of Vapona insecticide into their
diets. Unpublished report by Kettering Laboratory.
Witherup, S., Stemmer, K.L., Pfitzer, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction
of Vapona insecticide into their daily diets. Unpublished report
from Cincinnati, The Kettering Laboratory, February 14. Prepared for
Shell Chemical Company, submitted to WHO by Bayer AG, Ciba-Geigy
Ltd, Temana International Ltd.
Wooder, M.F., Creedy, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo. Unpublished
report. Sittingbourne, Shell Research Ltd., TLGR. 79.089.
Wooder, M.F., Wright, A.S. (1981) Alkylation of DNA by
organophosphorus pesticides. Acta Pharmacol. Toxicol. 49(V):
51-55.
Wooder, M.F., Wright, A.S., King, L.J. (1977) In vivo alkylation
studies with dichlorvos at practical use concentrations.
Chem.-Biol. Interact. 19: 25-46.
Wooder, M.F., Wright, A.S., Stevenson, D.E. (1978) Studies on the
in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol. 1: 505.
Wright, A.S., Hutson, D.H., Wooder, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol. 42: 1-18.
Wyrobek, A.J., Bruce, W.R. (1975) Chemical induction of sperm
abnormalities in mice. Proc. Nat. Acad. Sci. USA, 72: 4425-4429.
Yoshida, T., Nomura, M., Suzuki, Y. (1976) Inhibition of hepatic
UDP-glucuronyltransferase activity by organophosphate insecticides
and by carbon disulfide in mice. Bull. Environ. Contam. Toxicol.
15: 421-424.
Zalewska, Z., Rakowska, I., Matraszek, G., Sitkiewicz, D. (1977)
Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterase. Neuropat. Pol.
15: 255-262.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagenesis, 11(12): 1-157.
