
FAO/PL:1967/M/11/1
WHO/Food Add./68.30
1967 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Rome, 4 - 11 December,
1967. (FAO/WHO, 1968)
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1968
DICHLORVOS
This pesticide was evaluated by the 1966 Joint Meeting of the FAO
Working Party and the WHO Expert Committee on Pesticide Residues
(FAO/WHO, 1967). Since the previous publication, the results of some
additional experimental work have been reported. This new work is
summarized and discussed in the following monograph addendum.
IDENTITY
Chemical names
2,2-dichlorovinyl dimethyl phosphate
0,0-dimethyl-2,2-dichlorovinyl phosphate
Synonym
DDVP
Names of Proprietary Products
Dichlorvos or formulations containing dichlorvos appear under trade
names of "Vapona", "Nuvan", "Nogos", "Cossman's Fly-Cake",
"Phoracide", "Herkol", "Alco Fly Fighter Insect Spray", "Lethalaire
Bantam 8", "Lethalaire F-83", "Real-Kill Fly and Mosquito Killer",
"Kill-Fly Resin Strip", "Misect", "Atgard V", "De-Pester Insect
Strip", "Vaponex", "Vaponicide", "Vaporette Bar", "Dedevap", "No-Pest
Strip".
Empirical formula
C4H7O4PCl2
Structural formula
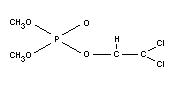 Relevant physical and chemical properties
The technical material usually contains 95 to 99 percent dichlorvos,
1 to 4 percent related compounds, and about 1 percent of unrelated
impurities. It is a clear, mobile liquid with a mild, rather pleasant
aromatic odor.
Boiling point: 84°C at 1 mm Hg
Specific gravity: 1.415 at 25°C
Vapor pressure: 0.01 mm Hg at 20°C
0.032 mm Hg at 32°C
0.30 mm Hg at 60°C
Solubility: About 3 percent in kerosene and
mineral oils and 1 percent in water
and glycerine. Miscible with
aromatic and chlorinated
hydrocarbon solvents.
Volatility: 145 mg/m3 at 20°C
350 mg/m3 at 30°C
800 mg/m3 at 40°C
Stability: Stable in organic solvents but
hydrolyzes in presence of water.
Alkalis accelerate and acids reduce
aqueous hydrolysis.
Composition of the Technical Product
Dichlorvos is available in oil solutions, emulsifiable concentrates,
aerosol formulations, and impregnated in polyvinyl chloride resin and
sold in the form of pellets, strips, blocks, and other forms. The
impregnated-resin stock usually contains about 20 percent of
dichlorvos by weight.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
In acute oral toxicity studies in male rats, the toxicity of
dichlorvos was markedly potentiated when administered in combination
with malathion. It was slightly potentiated when administered in
combination with trichlorofon, phosdrin and phosphamidon. No
potentiation was observed when it was administered in combination with
twenty-three other pesticides (Narcisse, 1967).
Short-term studies
Dog. For two years, groups of three males and three females were fed
diets to which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been
added. The individual portions were alloted weekly. Analyses of
composite samples representing the average diets over one week showed
respectively, 0.01, 0.09, 0.32, 3.2, 32 and 256 ppm of dichlorvos.
Erythrocyte and plasma cholinesterase activities were reduced at the
two highest levels; erythrocytic activity only was affected at the
nominal level of 10 ppm but recovered to essentially normal level at
the end of the feeding period. This recovery was also recorded at the
nominal 100 and 500 ppm levels. Brain cholinesterase, measured at the
end of the two year period, was not affected at any level.
Histological examination of major organs revealed dose-related
hepatocellular oedema, slight in degree and in one animal only at the
nominal 10 ppm level, and found to a more marked degree and in all
animals at the high level. Liver weights were slightly increased in
males at the nominal 100 ppm level and in both sexes at the highest
level. However, no difference from controls was seen in serum alkaline
phosphatase or transaminase activities, total serum proteins or A:G
ratios. No effect of dichlorvos was seen at any level on general
appearance, survival, rate of weight gain, food consumption,
peripheral blood picture or urine. (Jolley, Stemmer and Pfitzer,
1967).
Man. Dichlorvos was given orally to 4 separate 5-man groups at 1,
1.5, 2.0 and 2.5 mg doses. The 2.0 mg dose produced a depression of
plasma cholinesterase to 71 percent of control two days after a 28-day
feeding period. The plasma cholinesterase of the group given 2.5 mg
daily was depressed to 70 percent of control after feeding for 20
days. There was no significant effect on RBC cholinesterase at this
time. (Rider, et al., 1967).
Long-term studies
Rat. Initial groups of 40 males and 40 females were fed diets to
which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been added, for
two years. Five males and five females from each group were to be
selected for necropsy at 26, 52 and 78 weeks. Individual diets were
allotted weekly; analysis of composite samples representing average
diets over one week enabled estimates of average dietary
concentrations to be made; respectively, 0, 0.047, 0.467, 4.67, 46.7
and 234 ppm. Plasma and erythrocyte cholinesterase activities were
depressed at the high level and generally slightly depressed at the
nominal 100 ppm level. Brain cholinesterase, measured at 26, 52, 78
and 104 weeks, was depressed at the high level. Histological
examination of major organs disclosed fine vacuolization of the
hepatic cells of the high level animals, with some fatty change and
bile stasis. Fine vacuolization only was found in 80 percent of
females and 62 percent of males at the nominal 100 ppm level. However,
no effect was seen on serum total proteins or A:G ratios, and, in a
supplementary experiment, no effect on hexabarbital sleeping time was
found by prolonged feeding of dichlorvos up to 1000 ppm or by single
sub-lethal oral doses. No hepatocellular change was seen at the three
lower test levels. No effect of dichlorvos at any level was seen in
behaviour or mortality rate, rate of weight gain, food consumption,
terminal body and organ weights, peripheral blood picture, urine and
tumour incidence (Witherup, Stemmer and Pfitzer, 1967).
Comments
Recent information has disclosed that dichlorvos is rapidly converted
in plants, to dichloro-acetaldehyde and further to dichloroethanol. As
these metabolites are also known to occur in mammals after dichlorvos
administration, it was concluded that the assessment of the safe
levels of dichlorvos included that of the metabolites.
In two-year oral feeding studies on dogs and rats the decrease of the
cholinesterase activity of erythrocytes and plasma is proportional to
the applied dose of dichlorvos. Recovery to essentially normal level
at the end of the experiment is especially visible in dogs. Depression
of the brain cholinesterase activity could be ascertained in rats
only, but only at the highest nominal dietary level 500 ppm.
Cytoplasmatic vacuolisation in hepatic cello, while dose-related, may
be considered as a variation within physiological limits. In dogs the
increase in the weight of the liver at nominal levels of 100 ppm and
500 ppm was not associated with changes of liver function tests. No
stimulation or inhibition in the activity of microsomal enzymes were
proved in rats by means of indirect test using the hexabarbital
sleeping time.
For the establishment of the ADI the dose-related effect on the
cholinesterase activity resulting from the two year experiment on rats
and dogs can be taken into consideration. The oral level of 0.25
mg/kg/day was without toxicological effect in the rat. In dogs the
corresponding value was 0.08 mg/kg/day.
In man the dose of 0.033 mg/kg/day caused toxicologically
insignificant depression of plasma cholinesterase activity. The
erythrocyte cholinesterase was not affected.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.004 mg/kg body-weight.
EVALUATION FOR TOLERANCES
USE PATTERN
Pre-harvest treatments
Because of its very short persistence and lack of systemic action, the
use of dichlorvos on growing crops is limited and somewhat
specialized. Its most common pre-harvest use is for the control of
aphids, red spider mite (Tetranychus telarius), white fly
(Trialeurodes vaporariorum), leaf miners, and other pests of crops
growing in greenhouses; and for the control of mushroom flies in
mushroom houses. The crops and uses for which dichlorvos has been
registered or approved in various countries are as follows :
Cotton, date palms Iraq
Deciduous trees Austria, Bulgaria, Germany, Great
Britain, Hungary, Italy,
Netherlands, Switzerland
Greenhouses Austria, Bulgaria, Germany, Great
Britain, Hungary, Switzerland
Horticulture Austria, Great Britain
Mushroom houses Great Britain, Hungary,
Netherlands, United States
Ornamentals Germany, Great Britain, Switzerland
Vegetables Austria, Great Britain,
Netherlands, Switzerland
General use Austria, Ceylon, Chile, India,
Japan, Mexico, Pakistan, Venezuela
Dairy and meat United States
animals
Dichlorvos has been approved in the United Stated for direct
application to livestock, including lactating animals. It has also
been approved to control flies, fleas, and mites in animal barns,
piggeries, and chicken houses. In the United Kingdom it can be used in
pig and poultry houses for ectoparasite control.
Post-harvest treatments
To date, the use of dichlorvos on food as post-harvest treatments has
been largely experimental. However, the results have been so promising
that it may find extensive use for protecting raw and processed
agricultural products. Uses of dichlorvos investigated are as a direct
spray or dust treatment to grain; as aerosol or impregnated-resin
strips applied in the overhead space of storage bins for the control
of insects infesting grains; and as sprays, vapors, fogs, aerosols,
and impregnated-resin strips for controlling insects in facilities
where foods are stored, handled, processed, transported, and marketed.
Other uses
Dichlorvos has been used for a number of years in the U.S.A. and in
other countries as a spray or aerosol in tobacco warehouses for the
control of cigarette beetles (Lasioderma serricorne) and the tobacco
moth (Ephestia elutella) at the rates of 2 g/1,000 cu ft (71 mg/m3)
biweekly and 1 g/1,000 cu ft (35 mg/m3) weekly, respectively. It has
also been used in tobacco processing rooms after working hours at 0.5
to 1 g/1,000 cu ft (18 to 35 mg/m3) daily.
The principle use of dichlorvos, however, has been in the domestic and
public health field. In the U.S.A., formulations containing 0.5
percent of dichlorvos have been approved for the control of household
pests such as ante, bedbugs, cockroaches, flies, mosquitoes,
silverfish, spiders, ticks, and wasps in private and public buildings
and outdoors. Resin strips impregnated with dichlorvos have been
approved for use in the home kitchen in the United Kingdom and in
homes and certain commercial establishments in the United States.
Dichlorvos has been found effective for disinsection of aircraft
during flight. Vapor concentrations of 0.15 - 0.30 µg/litre of air
produced 100-percent mortality of flies and mosquitoes after 30-minute
exposures (Jensen, Flury, and Schoof, 1965). No effect was noticed on
the cholinesterase level of three individuals exposed 24 times to
30-minute aircraft treatments, during which time the dichlorvos vapor
concentration was 0.20 to 0.24 µg/litre of air (Schoof et al., 1961).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown under cover
Mushrooms
In the United States dichlorvos has been approved for mushroom houses
at the rate of 2 g/1,000 cu ft (71 mg/m3) and can be applied every 4
days provided no mushrooms are picked within 1 day after treatment. In
trials conducted by Snetsinger and Miner (1964) no dichlorvos residue
was found in mushrooms picked 24 hours after treatment at 4 g and 6 g
of dichlorvos per 1,000 cu ft (141 and 212 mg/m3) nor were cumulated
residues noted with consecutive applications. Ciba Laboratories Ltd.
(unpublished) reported residues of 0.85 ppm 24 hours after, 0.03 ppm
48 hours after, and 0.12 ppm 72 hours after treatment of the mushroom
house with dichlorvos at the rate of 4.8 g/1,000 cu ft (170 mg/m3).
Mushrooms exposed to application of 3 g/1,000 cu ft (106 mg/m3) had a
maximum of 0.3 ppm 2 hours after, 0.02 ppm 24 hours after, and 0.3 ppm
of dichlorvos 48 hours after treatment.
Lettuce
Tests conducted by Ciba Laboratories Ltd. (unpublished) with lettuce
grown in greenhouses produced the following residues after one
application :
Rate and method Dicohlorovos residues (ppm) at
of application intervals after harvest (hours)
1 2 24 48 72
4 g/1,000 cu ft
(141 mg/m3) by spray gun 78 53 4.9 - -
2 g/1,000 cu ft
(71 mg/m3) by microsol - - 2.1 0.4 0.2
generator
Rate and method Dicohlorovos residues (PPE) at
of application intervals after harvest (hours)
1 2 24 48 72
1 g/1,000 cu ft
(35 mg/m3) by microsol - - 1.3 0.4 0.3
generator
Shell Chemical Company (unpublished) reported lettuce growing in
greenhouses treated with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3)
had residues of 4.4 ppm 4 hours after treatment and no detectable
residue after 24 and 48 hours.
Tomatoes
Ciba Laboratories Ltd. (unpublished) reported tomatoes growing in a
greenhouse treated with dichlorvos at 1 g/1,000 cu ft (35 mg/m3)
(microsol generator) had residues of nil to 0.4 ppm. Shell Chemical
Company (unpublished) conducted test treatments with dichlorvos at 1.4
g/1,000 cu ft (49 mg/m3) on tomatoes growing in greenhouses. Residues
found at intervals after spraying were 0.13 ppm after 15 minutes, 0.10
ppm after 13 hours and nil after 23 hours.
Cucumbers
Shell Chemical Company Ltd. (unpublished) found no residues on
cucumbers sprayed with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3) 16,
24 and 48 hours after treatment. Ciba Laboratories Ltd. (unpublished)
found residues of 7.2 ppm 24 hours after, 0.2 ppm 48 hours after, and
0.02 ppm 72 hours after spraying with dichlorvos at the rate of 0.54
g/1,000 cu ft (19 mg/m3).
Crops grown in the open
Ciba Laboratories Ltd. (unpublished) carried out trials on the
application of dichlorvos to various vegetable crops in the field at
the rate of 1 g/100 sq ft (108 mg/m2) equivalent to about 1 g/1,000
cu ft (35 mg/m3) in greenhouses. The results were as follows :
Vegetable Dichlorvos residue (PP2)
at intervals after treatment
1 hour 72 hours 6 days
Dwarf beans 0.2 0.02 Nil
0.3 Nil Nil
Cauliflower 0.2 0.01 Nil
- 0.01 Nil
Spinach 0.03 0.02 Nil
0.03 0.03 Nil
Lettuce 15.4 0.03 Nil
21.7 0.02 Nil
Residues resulting from use on animals
Studies conducted by Casida, McBride and Niedermeier (1962) concluded
that dichlorvos sprayed, rubbed, or painted on milk cows appeared
unlikely to produce significant residues in milk. The organosoluble
insecticide equivalent in milk 12 and 24 hours after cows were fed
1 mg/kg and 2 mg/kg of dichlorvos was 0.46 ppb and 0.39 ppb, and 21.1
ppb and 7.3 ppb, respectively. Hens subjected to a dichlorvos spray of
1.5 g/1,000 cu ft (53 mg/m3) (twice recommended does) 5 times at
3-day intervals produced eggs with dichlorvos residues of 0.11 ppm or
less, and the dichlorvos residue in the edible tissue of the birds
sacrificed the day after the third treatment was loss than 0.1 ppm
(Ciba Laboratories Ltd., unpublished).
Residues resulting from use on cereals and cereal products
Trials conducted in the United Kingdom in 1964 by the Pest Infestation
Laboratory (unpublished) showed that feed barley treated with 4 ppm of
dichlorvos while being turned had 1.8 ppm of dichlorvos immediately
after treatment, 0.93 ppm after 1 week, 0.25 ppm after 6 weeks and
0.26 ppm after 10 weeks, and none could be detected after 15 weeks. In
another trial, Green and Tyler (1966) treated barley (13-15 percent
moisture) with 4 ppm dichlorvos. The dichlorvos residues on the grain
immediately after treatment and 1 and 2 weeks later were 0.53 to 0.63
ppm, 0.13 to 0.26 ppm, and 0.05 to 0.09 ppm, respectively. With drier
grain and improved treatment methods, they obtained higher and more
persistent residues with the same rate of application: 1.90 ppm during
treatment and 1.06 ppm 1 week after, 0.45 ppm 3 weeks after, 0.25 ppm
6 weeks after, and 0.26 ppm 10 weeks after treatment.
Strong and Sbur (1964) studied the persistency of dichlorvos as a
spray on wheat (16-percent moisture) at 10 ppm and found its toxicity
to the rice weevil (Sitophilus oryzea) was lost after 2 weeks at
high storage temperature; whereas when stored at 60°F (15.6°C), the
treated wheat still killed rice weevils for as long as 3 months'
storage.
Research conducted by the Stored-Product Insects R and D Laboratory,
U.S. Department of Agriculture, (unpublished) showed dichlorvos vapor
concentrations of 4 to 6 µg/1 for 6 hours [2 g/1,000 cu ft (71
mg/m3)] applied weekly will protect food in storage against insect
infestation. Dichlorvos residues in packaged noodles, raisins, rice,
beans, and sugar after 21 consecutive weekly applications were less
than 2 ppm.
Test conducted by Schulten and Kuyken (1966) on the effectiveness of
dichlorvos resin strips for the control of the cocoa moth (Ephestia
elutella) (Hübner) showed that 46 dichlorvos-impregnated strips (20
percent) in 2,200m3 of warehouse space produced air concentrations of
0.05 µg/1 in 27 days and 0.05 µg/1 in 54 days. The highest dichlorvos
residues in the stored cocoa beans were 0.02 - 0.03 ppm.
Residues in meat
Samples of mincemeat, bacon, steak, and fat were exposed in a hut at
daily intervals following a 30-minute treatment with vapors of pure
dichlorvos labeled with 32P. During the treatment, the dichlorvos
concentration was maintained at approximately 0.5 microgram/liter of
air and the prevailing temperature within the hut fell rapidly after
each application, and no insecticide was detectable in the air after 2
hours. Meat samples introduced into the hut immediately following
treatments still accumulated dichlorvos although they were of a very
low level. Those in fat were generally lower than those in the
corresponding steak, mincemeat, or bacon. The maximum dichlorvos
residues found after 12-hour exposures in the various types of meats
in the same order listed above were 0.08, 0.22, 0.19 and 0.19 ppm
(Millar and Aitken, 1965).
In 1964, the Shell Chemical Co. carried out an unpublished trial at a
meat factory in Britain. Dichlorvos was applied by a watering can at a
rate of 1.5 g/1,000 cu ft. The highest concentration of dichlorvos in
the atmosphere was found to be 0.7 microgram/liter one hour after
application. Nine hours after application, the atmospheric
concentration had dropped to 0.25 microgram/liter and to 0.08
microgram/liter by 24 hours. Residues of dichlorvos in samples of
cooked meat and offal exposed to the atmosphere over a 25-hour period
after application were less than 0.1 ppm. In processed meat, residues
reached 0.4 ppm within 1/2 hour of application and fell to less than
0.05 ppm after 25 hours.
Residues resulting from other uses
In trials conducted by Shell Chemical Company, U.S.A., (unpublished)
16 dichlorvos resin strips were used in a kitchen with 20,000 cu ft
(566 m3) and 4 resin strips in another kitchen with 2,352 cu ft (66.6
m3). Usual restaurant exhaust fans over the stoves ran
intermittently. Five days later, meals exposed to the insecticide
vapors for 24 hours contained dichlorvos residues of about 0.15 ppm.
Meals exposed in a similar manner 10 days after the strips were hung
had 0.05 ppm of dichlorvos. Other trials showed cheese from a cheese
factory using one 2- × 10-in (5- × 25.4-cm) strip per 1,000 cu ft
(28.3 m3) contained less than 0.01 ppm of the decomposition product
dichloroacetaldehyde. Fish exposed to the same concentration had no
more insecticide residue than unexposed fish.
FATE OF RESIDUES
In plants
It has been shown that dichlorvos residues in food disappear rather
rapidly. For example, the trials conducted by Ciba Laboratories Ltd.
(unpublished) showed that dichlorvos residue in mushrooms decreased
from 0.85 ppm 24 hours after treatment to 0.03 ppm 24 hours later.
Lettuce with 78 ppm of dichlorvos 1 hour after treatment had 0.02 ppm
72 hours later. Tests conducted by the Pest Infestation Laboratory in
the United Kingdom showed that the 1.8 ppm of dichlorvos on barley
immediately after treatment was down to 0.93 ppm in 1 week and to 0.25
ppm in 6 weeks. Ciba Ltd. (unpublished) found wheat grain with 60 ppm
of dichlorvos had 85 percent less dichlorvos after 1 month and 97.5
percent less after 4 months of storage. These tests also showed that
the higher the moisture content of the grain and of the storage
temperature, the faster the dichlorvos residue disappeared from the
grain.
In plant material the hydrolysis product dichloroacetaldehyde can be
present in rather significant quantities. In tests conducted by Shell
Chemical Company in the U.S. (unpublished), five vegetables were
sprayed with a solution of dichlorvos in acetone at rates calculated
to give 5 ppm residues on the commodities. Analyses were conducted of
the dichlorvos (DDVP) and of the dichloroacetaldehyde (DCA). The
results obtained were :
ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Cucumbers 6.1 0.1 3.9 0.2 2.6 0.1
(cont'd) ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Lettuce 3.6 0.06 0.9 0.06 Nil Nil
Mushrooms 1.2 0.06 Nil Nil Nil Nil
Spinach 6.5 0.3 2.0 0.2 1.0 0.1
Tomatoes 5.1 0.1 2.4 0.2 1.3 0.1
Studies conducted by Shell Development Company, U.S.A., (unpublished)
showed the acute oral toxicity to rats of dichlorvos when combined
with certain other insecticides could be more then additive
(potentiation). Of the 27 insecticides combined with dichlorvos, no
potentiation was found with 20, marginal potentiation with 3, and
positive potentiation with 4. The most marked potentiation was
obtained when dichlorvos was combined with malathion. The combined
LD50 acute oral toxicity to rats of the two pesticides was 135 mg/kg,
whereas the expected additive value was 1,298 mg/kg, or a 9.61-fold
increase.
In storage and processing
Processing and cooking also removed large percentages of dichlorvos
which may be present on food. For example, wheat with 23.8 ppm of
dichlorvos after treatment produced flour with 4.6 ppm of dichlorvos.
Three months later, the wheat had 2.8 ppm of dichlorvos and the white
flour milled from it had 1.7 ppm. The dichlorvos residue in this flour
after 14 days' storage fell below the limits of detection. Shell
Chemical Company, U.S.A. (unpublished) found that biscuits made from
flour containing 0.35 ppm and 1.8 ppm of dichlorvos had 80 and 60
percent less dichlorvos, respectively. Flour with 9.5 ppm of
dichlorvos heated for 30 minutes at 100°C, 150°C, and 200°C had 0.2
ppm, 0.03 ppm, and 0.02 ppm of dichlorvos, respectively. Rice with 5.3
ppm of dichlorvos had only 0.06 ppm after cooking. Miller and Aitken
(1965) found frying and cooking completely destroyed dichlorvos in the
meat, leaving only products of hydrolysis.
METHODS OF RESIDUE ANALYSIS
Shell Development Company analytical method MMS-30/64 entitled
"Determination of Vapona Insecticide in Crops and Animal Products,
Enzyme Inhibition-Spectrophotometric Method". Sensitivity is as low as
0.1 ppm of dichlorvos. This method is not specific for dichlorvos.
Shell Chemical Company analytical method PMS-G-900/60 entitled
"Determination of Dichloroacetaldehyde Residues in Crops and Animal
Tissues, GLC Electron Capture Method". Sensitivity is as low as 0.01
ppm.
Woodstock analytical method WAMS 32-1 entitled "Determination of
Vapona in Technical Grade Products, Formulations and Extracts from
Certain Crops and Similar Samples. - Gas-Liquid Chromatographic
Method."
Other gas-liquid chromatography methods for dichlorvos and its
breakdown products are being investigated.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Australia 2 fruit, vegetables and
grain recommended by the
Food Additive Committee
of National Health and
Medical Research
Canada None
Germany *
Holland 0.1 vegetables (including
mushrooms, roots, bulbs
and tubers) and fruits
Switzerland 0.1 vegetables
United States **
* The residue on edible crops may not exceed the lower limit of
detectability of the analytical methods.
** Petitions have been submitted to U.S. Food and Drug
Administration for approval of tolerances of 0.5 ppm of
dichlorvos in packaged processed foods resulting from space
treatments in warehouses and 0.25 ppm of dichlorvos in canned
tomatoes resulting from direct application to tomatoes for
Drosophila control.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Dichlorvos in/on food disappears very rapidly and to very low levels.
Processing and cooking further reduce dichlorvos residues in food.
In the hydrolysis of dichlorvos, dichloroacetaldehyde is formed and
this metabolite may be present in food in detectable amounts. The
temporary tolerances for dichlorvos residues, therefore, include
dichloroacetaldehyde when it is reported as being present.
Because an analytical method specific for dichlorvos that can be used
for regulatory purposes is not available at this time, the tolerances
being recommended for dichlorvos residues are temporary and are to be
reviewed by 31 December 1970.
The recommended temporary tolerances are :
Cereals 2.0
Cereal products and
fresh vegetables 0.3
Canned and frozen vegetables,
fresh fruit (other than citrus) 0.1
Considering the loss of dichlorvos that takes place by aging,
processing, and cooking and based on the ninth decile of consumption
derived from consumer intake studies, the amount of dichlorvos
reaching the consumer in the above foods will be less than 0.1 mg/day
of the 0.24 mg/day permitted by the ADI. The remainder will be
available for respiratory intake and for residues that may be present
in other foods.
FURTHER WORK
Further work required before 30 June 1970
A specific analytical method with a sensitivity of about 0.01 ppm
suitable for regulatory purposes.
Data on other uses of dichlorvos, the residues resulting from such
uses, and the affects of aging, processing, and cooking.
Actual dichlorvos residues present in food moving in commerce.
Total diet studies.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
FAO/WHO. (1967) FAO Mtg. Rept. PL:CP/15; WHO Food Add./67.32
Jolley, W.B., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International.
Narcisse, J.K. (1967) unpublished report submitted by Shell
International.
Rider, S.A., Moeller, H.C. and Puletti, E.J. (1967). Fed. Proc.,
26, 427
Witherup, S., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agric. Fd. Chem., 10 (5): 370 - 377.
Green, A.A., and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos, and fenitrothion for the control of Oryzaephilus
surinamensis (L) (Coleoptera, Silvanidae) infesting stored barley.
J. Stored Prod. Res., 1 (3): 273 - 285.
Jensen, Jens A., Flury, Vincent P. and Schoof, Herbert F. (1965)
Dichlorvos vapour disinsection of aircraft. Bull. Wld. Hlth. Org., 32:
175 - 180.
Millar, K.R. and Aitken, W.M. (1965) Residues in meat following
exposure to P-Labeled dichlorvos vapor in an enclosed space. N.Z. J1
Agric. Res., 8(2): 350 - 362.
Schoof, H.F., Jensen, J.A., Porter, J.E. and Maddock, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP vapour.
Bull. Wld. Hlth. Org., 24: 623 - 628.
Schulten, G.G.M. and Kuyken, W. (1966) Dichlorvos resin strips for
control of cocoa moth, Ephestia elutella. Int. Pest Control, 8(3):
18, 19, 21, 23.
Snetsinger, R. and Miner, D. (1964) Tests with dichlorvos vapours for
the control of mushroom flies. J. Econ. Ent., 57(1): 182 - 183.
Strong, R.G. and Sbur, D.E. (1964) Influence of grain moisture and
storage temperature on the effectiveness of five insecticides as grain
protectants. J. Econ. Ent., 57(1): 44 - 47.
Relevant physical and chemical properties
The technical material usually contains 95 to 99 percent dichlorvos,
1 to 4 percent related compounds, and about 1 percent of unrelated
impurities. It is a clear, mobile liquid with a mild, rather pleasant
aromatic odor.
Boiling point: 84°C at 1 mm Hg
Specific gravity: 1.415 at 25°C
Vapor pressure: 0.01 mm Hg at 20°C
0.032 mm Hg at 32°C
0.30 mm Hg at 60°C
Solubility: About 3 percent in kerosene and
mineral oils and 1 percent in water
and glycerine. Miscible with
aromatic and chlorinated
hydrocarbon solvents.
Volatility: 145 mg/m3 at 20°C
350 mg/m3 at 30°C
800 mg/m3 at 40°C
Stability: Stable in organic solvents but
hydrolyzes in presence of water.
Alkalis accelerate and acids reduce
aqueous hydrolysis.
Composition of the Technical Product
Dichlorvos is available in oil solutions, emulsifiable concentrates,
aerosol formulations, and impregnated in polyvinyl chloride resin and
sold in the form of pellets, strips, blocks, and other forms. The
impregnated-resin stock usually contains about 20 percent of
dichlorvos by weight.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
In acute oral toxicity studies in male rats, the toxicity of
dichlorvos was markedly potentiated when administered in combination
with malathion. It was slightly potentiated when administered in
combination with trichlorofon, phosdrin and phosphamidon. No
potentiation was observed when it was administered in combination with
twenty-three other pesticides (Narcisse, 1967).
Short-term studies
Dog. For two years, groups of three males and three females were fed
diets to which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been
added. The individual portions were alloted weekly. Analyses of
composite samples representing the average diets over one week showed
respectively, 0.01, 0.09, 0.32, 3.2, 32 and 256 ppm of dichlorvos.
Erythrocyte and plasma cholinesterase activities were reduced at the
two highest levels; erythrocytic activity only was affected at the
nominal level of 10 ppm but recovered to essentially normal level at
the end of the feeding period. This recovery was also recorded at the
nominal 100 and 500 ppm levels. Brain cholinesterase, measured at the
end of the two year period, was not affected at any level.
Histological examination of major organs revealed dose-related
hepatocellular oedema, slight in degree and in one animal only at the
nominal 10 ppm level, and found to a more marked degree and in all
animals at the high level. Liver weights were slightly increased in
males at the nominal 100 ppm level and in both sexes at the highest
level. However, no difference from controls was seen in serum alkaline
phosphatase or transaminase activities, total serum proteins or A:G
ratios. No effect of dichlorvos was seen at any level on general
appearance, survival, rate of weight gain, food consumption,
peripheral blood picture or urine. (Jolley, Stemmer and Pfitzer,
1967).
Man. Dichlorvos was given orally to 4 separate 5-man groups at 1,
1.5, 2.0 and 2.5 mg doses. The 2.0 mg dose produced a depression of
plasma cholinesterase to 71 percent of control two days after a 28-day
feeding period. The plasma cholinesterase of the group given 2.5 mg
daily was depressed to 70 percent of control after feeding for 20
days. There was no significant effect on RBC cholinesterase at this
time. (Rider, et al., 1967).
Long-term studies
Rat. Initial groups of 40 males and 40 females were fed diets to
which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been added, for
two years. Five males and five females from each group were to be
selected for necropsy at 26, 52 and 78 weeks. Individual diets were
allotted weekly; analysis of composite samples representing average
diets over one week enabled estimates of average dietary
concentrations to be made; respectively, 0, 0.047, 0.467, 4.67, 46.7
and 234 ppm. Plasma and erythrocyte cholinesterase activities were
depressed at the high level and generally slightly depressed at the
nominal 100 ppm level. Brain cholinesterase, measured at 26, 52, 78
and 104 weeks, was depressed at the high level. Histological
examination of major organs disclosed fine vacuolization of the
hepatic cells of the high level animals, with some fatty change and
bile stasis. Fine vacuolization only was found in 80 percent of
females and 62 percent of males at the nominal 100 ppm level. However,
no effect was seen on serum total proteins or A:G ratios, and, in a
supplementary experiment, no effect on hexabarbital sleeping time was
found by prolonged feeding of dichlorvos up to 1000 ppm or by single
sub-lethal oral doses. No hepatocellular change was seen at the three
lower test levels. No effect of dichlorvos at any level was seen in
behaviour or mortality rate, rate of weight gain, food consumption,
terminal body and organ weights, peripheral blood picture, urine and
tumour incidence (Witherup, Stemmer and Pfitzer, 1967).
Comments
Recent information has disclosed that dichlorvos is rapidly converted
in plants, to dichloro-acetaldehyde and further to dichloroethanol. As
these metabolites are also known to occur in mammals after dichlorvos
administration, it was concluded that the assessment of the safe
levels of dichlorvos included that of the metabolites.
In two-year oral feeding studies on dogs and rats the decrease of the
cholinesterase activity of erythrocytes and plasma is proportional to
the applied dose of dichlorvos. Recovery to essentially normal level
at the end of the experiment is especially visible in dogs. Depression
of the brain cholinesterase activity could be ascertained in rats
only, but only at the highest nominal dietary level 500 ppm.
Cytoplasmatic vacuolisation in hepatic cello, while dose-related, may
be considered as a variation within physiological limits. In dogs the
increase in the weight of the liver at nominal levels of 100 ppm and
500 ppm was not associated with changes of liver function tests. No
stimulation or inhibition in the activity of microsomal enzymes were
proved in rats by means of indirect test using the hexabarbital
sleeping time.
For the establishment of the ADI the dose-related effect on the
cholinesterase activity resulting from the two year experiment on rats
and dogs can be taken into consideration. The oral level of 0.25
mg/kg/day was without toxicological effect in the rat. In dogs the
corresponding value was 0.08 mg/kg/day.
In man the dose of 0.033 mg/kg/day caused toxicologically
insignificant depression of plasma cholinesterase activity. The
erythrocyte cholinesterase was not affected.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.004 mg/kg body-weight.
EVALUATION FOR TOLERANCES
USE PATTERN
Pre-harvest treatments
Because of its very short persistence and lack of systemic action, the
use of dichlorvos on growing crops is limited and somewhat
specialized. Its most common pre-harvest use is for the control of
aphids, red spider mite (Tetranychus telarius), white fly
(Trialeurodes vaporariorum), leaf miners, and other pests of crops
growing in greenhouses; and for the control of mushroom flies in
mushroom houses. The crops and uses for which dichlorvos has been
registered or approved in various countries are as follows :
Cotton, date palms Iraq
Deciduous trees Austria, Bulgaria, Germany, Great
Britain, Hungary, Italy,
Netherlands, Switzerland
Greenhouses Austria, Bulgaria, Germany, Great
Britain, Hungary, Switzerland
Horticulture Austria, Great Britain
Mushroom houses Great Britain, Hungary,
Netherlands, United States
Ornamentals Germany, Great Britain, Switzerland
Vegetables Austria, Great Britain,
Netherlands, Switzerland
General use Austria, Ceylon, Chile, India,
Japan, Mexico, Pakistan, Venezuela
Dairy and meat United States
animals
Dichlorvos has been approved in the United Stated for direct
application to livestock, including lactating animals. It has also
been approved to control flies, fleas, and mites in animal barns,
piggeries, and chicken houses. In the United Kingdom it can be used in
pig and poultry houses for ectoparasite control.
Post-harvest treatments
To date, the use of dichlorvos on food as post-harvest treatments has
been largely experimental. However, the results have been so promising
that it may find extensive use for protecting raw and processed
agricultural products. Uses of dichlorvos investigated are as a direct
spray or dust treatment to grain; as aerosol or impregnated-resin
strips applied in the overhead space of storage bins for the control
of insects infesting grains; and as sprays, vapors, fogs, aerosols,
and impregnated-resin strips for controlling insects in facilities
where foods are stored, handled, processed, transported, and marketed.
Other uses
Dichlorvos has been used for a number of years in the U.S.A. and in
other countries as a spray or aerosol in tobacco warehouses for the
control of cigarette beetles (Lasioderma serricorne) and the tobacco
moth (Ephestia elutella) at the rates of 2 g/1,000 cu ft (71 mg/m3)
biweekly and 1 g/1,000 cu ft (35 mg/m3) weekly, respectively. It has
also been used in tobacco processing rooms after working hours at 0.5
to 1 g/1,000 cu ft (18 to 35 mg/m3) daily.
The principle use of dichlorvos, however, has been in the domestic and
public health field. In the U.S.A., formulations containing 0.5
percent of dichlorvos have been approved for the control of household
pests such as ante, bedbugs, cockroaches, flies, mosquitoes,
silverfish, spiders, ticks, and wasps in private and public buildings
and outdoors. Resin strips impregnated with dichlorvos have been
approved for use in the home kitchen in the United Kingdom and in
homes and certain commercial establishments in the United States.
Dichlorvos has been found effective for disinsection of aircraft
during flight. Vapor concentrations of 0.15 - 0.30 µg/litre of air
produced 100-percent mortality of flies and mosquitoes after 30-minute
exposures (Jensen, Flury, and Schoof, 1965). No effect was noticed on
the cholinesterase level of three individuals exposed 24 times to
30-minute aircraft treatments, during which time the dichlorvos vapor
concentration was 0.20 to 0.24 µg/litre of air (Schoof et al., 1961).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown under cover
Mushrooms
In the United States dichlorvos has been approved for mushroom houses
at the rate of 2 g/1,000 cu ft (71 mg/m3) and can be applied every 4
days provided no mushrooms are picked within 1 day after treatment. In
trials conducted by Snetsinger and Miner (1964) no dichlorvos residue
was found in mushrooms picked 24 hours after treatment at 4 g and 6 g
of dichlorvos per 1,000 cu ft (141 and 212 mg/m3) nor were cumulated
residues noted with consecutive applications. Ciba Laboratories Ltd.
(unpublished) reported residues of 0.85 ppm 24 hours after, 0.03 ppm
48 hours after, and 0.12 ppm 72 hours after treatment of the mushroom
house with dichlorvos at the rate of 4.8 g/1,000 cu ft (170 mg/m3).
Mushrooms exposed to application of 3 g/1,000 cu ft (106 mg/m3) had a
maximum of 0.3 ppm 2 hours after, 0.02 ppm 24 hours after, and 0.3 ppm
of dichlorvos 48 hours after treatment.
Lettuce
Tests conducted by Ciba Laboratories Ltd. (unpublished) with lettuce
grown in greenhouses produced the following residues after one
application :
Rate and method Dicohlorovos residues (ppm) at
of application intervals after harvest (hours)
1 2 24 48 72
4 g/1,000 cu ft
(141 mg/m3) by spray gun 78 53 4.9 - -
2 g/1,000 cu ft
(71 mg/m3) by microsol - - 2.1 0.4 0.2
generator
Rate and method Dicohlorovos residues (PPE) at
of application intervals after harvest (hours)
1 2 24 48 72
1 g/1,000 cu ft
(35 mg/m3) by microsol - - 1.3 0.4 0.3
generator
Shell Chemical Company (unpublished) reported lettuce growing in
greenhouses treated with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3)
had residues of 4.4 ppm 4 hours after treatment and no detectable
residue after 24 and 48 hours.
Tomatoes
Ciba Laboratories Ltd. (unpublished) reported tomatoes growing in a
greenhouse treated with dichlorvos at 1 g/1,000 cu ft (35 mg/m3)
(microsol generator) had residues of nil to 0.4 ppm. Shell Chemical
Company (unpublished) conducted test treatments with dichlorvos at 1.4
g/1,000 cu ft (49 mg/m3) on tomatoes growing in greenhouses. Residues
found at intervals after spraying were 0.13 ppm after 15 minutes, 0.10
ppm after 13 hours and nil after 23 hours.
Cucumbers
Shell Chemical Company Ltd. (unpublished) found no residues on
cucumbers sprayed with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3) 16,
24 and 48 hours after treatment. Ciba Laboratories Ltd. (unpublished)
found residues of 7.2 ppm 24 hours after, 0.2 ppm 48 hours after, and
0.02 ppm 72 hours after spraying with dichlorvos at the rate of 0.54
g/1,000 cu ft (19 mg/m3).
Crops grown in the open
Ciba Laboratories Ltd. (unpublished) carried out trials on the
application of dichlorvos to various vegetable crops in the field at
the rate of 1 g/100 sq ft (108 mg/m2) equivalent to about 1 g/1,000
cu ft (35 mg/m3) in greenhouses. The results were as follows :
Vegetable Dichlorvos residue (PP2)
at intervals after treatment
1 hour 72 hours 6 days
Dwarf beans 0.2 0.02 Nil
0.3 Nil Nil
Cauliflower 0.2 0.01 Nil
- 0.01 Nil
Spinach 0.03 0.02 Nil
0.03 0.03 Nil
Lettuce 15.4 0.03 Nil
21.7 0.02 Nil
Residues resulting from use on animals
Studies conducted by Casida, McBride and Niedermeier (1962) concluded
that dichlorvos sprayed, rubbed, or painted on milk cows appeared
unlikely to produce significant residues in milk. The organosoluble
insecticide equivalent in milk 12 and 24 hours after cows were fed
1 mg/kg and 2 mg/kg of dichlorvos was 0.46 ppb and 0.39 ppb, and 21.1
ppb and 7.3 ppb, respectively. Hens subjected to a dichlorvos spray of
1.5 g/1,000 cu ft (53 mg/m3) (twice recommended does) 5 times at
3-day intervals produced eggs with dichlorvos residues of 0.11 ppm or
less, and the dichlorvos residue in the edible tissue of the birds
sacrificed the day after the third treatment was loss than 0.1 ppm
(Ciba Laboratories Ltd., unpublished).
Residues resulting from use on cereals and cereal products
Trials conducted in the United Kingdom in 1964 by the Pest Infestation
Laboratory (unpublished) showed that feed barley treated with 4 ppm of
dichlorvos while being turned had 1.8 ppm of dichlorvos immediately
after treatment, 0.93 ppm after 1 week, 0.25 ppm after 6 weeks and
0.26 ppm after 10 weeks, and none could be detected after 15 weeks. In
another trial, Green and Tyler (1966) treated barley (13-15 percent
moisture) with 4 ppm dichlorvos. The dichlorvos residues on the grain
immediately after treatment and 1 and 2 weeks later were 0.53 to 0.63
ppm, 0.13 to 0.26 ppm, and 0.05 to 0.09 ppm, respectively. With drier
grain and improved treatment methods, they obtained higher and more
persistent residues with the same rate of application: 1.90 ppm during
treatment and 1.06 ppm 1 week after, 0.45 ppm 3 weeks after, 0.25 ppm
6 weeks after, and 0.26 ppm 10 weeks after treatment.
Strong and Sbur (1964) studied the persistency of dichlorvos as a
spray on wheat (16-percent moisture) at 10 ppm and found its toxicity
to the rice weevil (Sitophilus oryzea) was lost after 2 weeks at
high storage temperature; whereas when stored at 60°F (15.6°C), the
treated wheat still killed rice weevils for as long as 3 months'
storage.
Research conducted by the Stored-Product Insects R and D Laboratory,
U.S. Department of Agriculture, (unpublished) showed dichlorvos vapor
concentrations of 4 to 6 µg/1 for 6 hours [2 g/1,000 cu ft (71
mg/m3)] applied weekly will protect food in storage against insect
infestation. Dichlorvos residues in packaged noodles, raisins, rice,
beans, and sugar after 21 consecutive weekly applications were less
than 2 ppm.
Test conducted by Schulten and Kuyken (1966) on the effectiveness of
dichlorvos resin strips for the control of the cocoa moth (Ephestia
elutella) (Hübner) showed that 46 dichlorvos-impregnated strips (20
percent) in 2,200m3 of warehouse space produced air concentrations of
0.05 µg/1 in 27 days and 0.05 µg/1 in 54 days. The highest dichlorvos
residues in the stored cocoa beans were 0.02 - 0.03 ppm.
Residues in meat
Samples of mincemeat, bacon, steak, and fat were exposed in a hut at
daily intervals following a 30-minute treatment with vapors of pure
dichlorvos labeled with 32P. During the treatment, the dichlorvos
concentration was maintained at approximately 0.5 microgram/liter of
air and the prevailing temperature within the hut fell rapidly after
each application, and no insecticide was detectable in the air after 2
hours. Meat samples introduced into the hut immediately following
treatments still accumulated dichlorvos although they were of a very
low level. Those in fat were generally lower than those in the
corresponding steak, mincemeat, or bacon. The maximum dichlorvos
residues found after 12-hour exposures in the various types of meats
in the same order listed above were 0.08, 0.22, 0.19 and 0.19 ppm
(Millar and Aitken, 1965).
In 1964, the Shell Chemical Co. carried out an unpublished trial at a
meat factory in Britain. Dichlorvos was applied by a watering can at a
rate of 1.5 g/1,000 cu ft. The highest concentration of dichlorvos in
the atmosphere was found to be 0.7 microgram/liter one hour after
application. Nine hours after application, the atmospheric
concentration had dropped to 0.25 microgram/liter and to 0.08
microgram/liter by 24 hours. Residues of dichlorvos in samples of
cooked meat and offal exposed to the atmosphere over a 25-hour period
after application were less than 0.1 ppm. In processed meat, residues
reached 0.4 ppm within 1/2 hour of application and fell to less than
0.05 ppm after 25 hours.
Residues resulting from other uses
In trials conducted by Shell Chemical Company, U.S.A., (unpublished)
16 dichlorvos resin strips were used in a kitchen with 20,000 cu ft
(566 m3) and 4 resin strips in another kitchen with 2,352 cu ft (66.6
m3). Usual restaurant exhaust fans over the stoves ran
intermittently. Five days later, meals exposed to the insecticide
vapors for 24 hours contained dichlorvos residues of about 0.15 ppm.
Meals exposed in a similar manner 10 days after the strips were hung
had 0.05 ppm of dichlorvos. Other trials showed cheese from a cheese
factory using one 2- × 10-in (5- × 25.4-cm) strip per 1,000 cu ft
(28.3 m3) contained less than 0.01 ppm of the decomposition product
dichloroacetaldehyde. Fish exposed to the same concentration had no
more insecticide residue than unexposed fish.
FATE OF RESIDUES
In plants
It has been shown that dichlorvos residues in food disappear rather
rapidly. For example, the trials conducted by Ciba Laboratories Ltd.
(unpublished) showed that dichlorvos residue in mushrooms decreased
from 0.85 ppm 24 hours after treatment to 0.03 ppm 24 hours later.
Lettuce with 78 ppm of dichlorvos 1 hour after treatment had 0.02 ppm
72 hours later. Tests conducted by the Pest Infestation Laboratory in
the United Kingdom showed that the 1.8 ppm of dichlorvos on barley
immediately after treatment was down to 0.93 ppm in 1 week and to 0.25
ppm in 6 weeks. Ciba Ltd. (unpublished) found wheat grain with 60 ppm
of dichlorvos had 85 percent less dichlorvos after 1 month and 97.5
percent less after 4 months of storage. These tests also showed that
the higher the moisture content of the grain and of the storage
temperature, the faster the dichlorvos residue disappeared from the
grain.
In plant material the hydrolysis product dichloroacetaldehyde can be
present in rather significant quantities. In tests conducted by Shell
Chemical Company in the U.S. (unpublished), five vegetables were
sprayed with a solution of dichlorvos in acetone at rates calculated
to give 5 ppm residues on the commodities. Analyses were conducted of
the dichlorvos (DDVP) and of the dichloroacetaldehyde (DCA). The
results obtained were :
ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Cucumbers 6.1 0.1 3.9 0.2 2.6 0.1
(cont'd) ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Lettuce 3.6 0.06 0.9 0.06 Nil Nil
Mushrooms 1.2 0.06 Nil Nil Nil Nil
Spinach 6.5 0.3 2.0 0.2 1.0 0.1
Tomatoes 5.1 0.1 2.4 0.2 1.3 0.1
Studies conducted by Shell Development Company, U.S.A., (unpublished)
showed the acute oral toxicity to rats of dichlorvos when combined
with certain other insecticides could be more then additive
(potentiation). Of the 27 insecticides combined with dichlorvos, no
potentiation was found with 20, marginal potentiation with 3, and
positive potentiation with 4. The most marked potentiation was
obtained when dichlorvos was combined with malathion. The combined
LD50 acute oral toxicity to rats of the two pesticides was 135 mg/kg,
whereas the expected additive value was 1,298 mg/kg, or a 9.61-fold
increase.
In storage and processing
Processing and cooking also removed large percentages of dichlorvos
which may be present on food. For example, wheat with 23.8 ppm of
dichlorvos after treatment produced flour with 4.6 ppm of dichlorvos.
Three months later, the wheat had 2.8 ppm of dichlorvos and the white
flour milled from it had 1.7 ppm. The dichlorvos residue in this flour
after 14 days' storage fell below the limits of detection. Shell
Chemical Company, U.S.A. (unpublished) found that biscuits made from
flour containing 0.35 ppm and 1.8 ppm of dichlorvos had 80 and 60
percent less dichlorvos, respectively. Flour with 9.5 ppm of
dichlorvos heated for 30 minutes at 100°C, 150°C, and 200°C had 0.2
ppm, 0.03 ppm, and 0.02 ppm of dichlorvos, respectively. Rice with 5.3
ppm of dichlorvos had only 0.06 ppm after cooking. Miller and Aitken
(1965) found frying and cooking completely destroyed dichlorvos in the
meat, leaving only products of hydrolysis.
METHODS OF RESIDUE ANALYSIS
Shell Development Company analytical method MMS-30/64 entitled
"Determination of Vapona Insecticide in Crops and Animal Products,
Enzyme Inhibition-Spectrophotometric Method". Sensitivity is as low as
0.1 ppm of dichlorvos. This method is not specific for dichlorvos.
Shell Chemical Company analytical method PMS-G-900/60 entitled
"Determination of Dichloroacetaldehyde Residues in Crops and Animal
Tissues, GLC Electron Capture Method". Sensitivity is as low as 0.01
ppm.
Woodstock analytical method WAMS 32-1 entitled "Determination of
Vapona in Technical Grade Products, Formulations and Extracts from
Certain Crops and Similar Samples. - Gas-Liquid Chromatographic
Method."
Other gas-liquid chromatography methods for dichlorvos and its
breakdown products are being investigated.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Australia 2 fruit, vegetables and
grain recommended by the
Food Additive Committee
of National Health and
Medical Research
Canada None
Germany *
Holland 0.1 vegetables (including
mushrooms, roots, bulbs
and tubers) and fruits
Switzerland 0.1 vegetables
United States **
* The residue on edible crops may not exceed the lower limit of
detectability of the analytical methods.
** Petitions have been submitted to U.S. Food and Drug
Administration for approval of tolerances of 0.5 ppm of
dichlorvos in packaged processed foods resulting from space
treatments in warehouses and 0.25 ppm of dichlorvos in canned
tomatoes resulting from direct application to tomatoes for
Drosophila control.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Dichlorvos in/on food disappears very rapidly and to very low levels.
Processing and cooking further reduce dichlorvos residues in food.
In the hydrolysis of dichlorvos, dichloroacetaldehyde is formed and
this metabolite may be present in food in detectable amounts. The
temporary tolerances for dichlorvos residues, therefore, include
dichloroacetaldehyde when it is reported as being present.
Because an analytical method specific for dichlorvos that can be used
for regulatory purposes is not available at this time, the tolerances
being recommended for dichlorvos residues are temporary and are to be
reviewed by 31 December 1970.
The recommended temporary tolerances are :
Cereals 2.0
Cereal products and
fresh vegetables 0.3
Canned and frozen vegetables,
fresh fruit (other than citrus) 0.1
Considering the loss of dichlorvos that takes place by aging,
processing, and cooking and based on the ninth decile of consumption
derived from consumer intake studies, the amount of dichlorvos
reaching the consumer in the above foods will be less than 0.1 mg/day
of the 0.24 mg/day permitted by the ADI. The remainder will be
available for respiratory intake and for residues that may be present
in other foods.
FURTHER WORK
Further work required before 30 June 1970
A specific analytical method with a sensitivity of about 0.01 ppm
suitable for regulatory purposes.
Data on other uses of dichlorvos, the residues resulting from such
uses, and the affects of aging, processing, and cooking.
Actual dichlorvos residues present in food moving in commerce.
Total diet studies.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
FAO/WHO. (1967) FAO Mtg. Rept. PL:CP/15; WHO Food Add./67.32
Jolley, W.B., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International.
Narcisse, J.K. (1967) unpublished report submitted by Shell
International.
Rider, S.A., Moeller, H.C. and Puletti, E.J. (1967). Fed. Proc.,
26, 427
Witherup, S., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agric. Fd. Chem., 10 (5): 370 - 377.
Green, A.A., and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos, and fenitrothion for the control of Oryzaephilus
surinamensis (L) (Coleoptera, Silvanidae) infesting stored barley.
J. Stored Prod. Res., 1 (3): 273 - 285.
Jensen, Jens A., Flury, Vincent P. and Schoof, Herbert F. (1965)
Dichlorvos vapour disinsection of aircraft. Bull. Wld. Hlth. Org., 32:
175 - 180.
Millar, K.R. and Aitken, W.M. (1965) Residues in meat following
exposure to P-Labeled dichlorvos vapor in an enclosed space. N.Z. J1
Agric. Res., 8(2): 350 - 362.
Schoof, H.F., Jensen, J.A., Porter, J.E. and Maddock, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP vapour.
Bull. Wld. Hlth. Org., 24: 623 - 628.
Schulten, G.G.M. and Kuyken, W. (1966) Dichlorvos resin strips for
control of cocoa moth, Ephestia elutella. Int. Pest Control, 8(3):
18, 19, 21, 23.
Snetsinger, R. and Miner, D. (1964) Tests with dichlorvos vapours for
the control of mushroom flies. J. Econ. Ent., 57(1): 182 - 183.
Strong, R.G. and Sbur, D.E. (1964) Influence of grain moisture and
storage temperature on the effectiveness of five insecticides as grain
protectants. J. Econ. Ent., 57(1): 44 - 47.
 Relevant physical and chemical properties
The technical material usually contains 95 to 99 percent dichlorvos,
1 to 4 percent related compounds, and about 1 percent of unrelated
impurities. It is a clear, mobile liquid with a mild, rather pleasant
aromatic odor.
Boiling point: 84°C at 1 mm Hg
Specific gravity: 1.415 at 25°C
Vapor pressure: 0.01 mm Hg at 20°C
0.032 mm Hg at 32°C
0.30 mm Hg at 60°C
Solubility: About 3 percent in kerosene and
mineral oils and 1 percent in water
and glycerine. Miscible with
aromatic and chlorinated
hydrocarbon solvents.
Volatility: 145 mg/m3 at 20°C
350 mg/m3 at 30°C
800 mg/m3 at 40°C
Stability: Stable in organic solvents but
hydrolyzes in presence of water.
Alkalis accelerate and acids reduce
aqueous hydrolysis.
Composition of the Technical Product
Dichlorvos is available in oil solutions, emulsifiable concentrates,
aerosol formulations, and impregnated in polyvinyl chloride resin and
sold in the form of pellets, strips, blocks, and other forms. The
impregnated-resin stock usually contains about 20 percent of
dichlorvos by weight.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
In acute oral toxicity studies in male rats, the toxicity of
dichlorvos was markedly potentiated when administered in combination
with malathion. It was slightly potentiated when administered in
combination with trichlorofon, phosdrin and phosphamidon. No
potentiation was observed when it was administered in combination with
twenty-three other pesticides (Narcisse, 1967).
Short-term studies
Dog. For two years, groups of three males and three females were fed
diets to which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been
added. The individual portions were alloted weekly. Analyses of
composite samples representing the average diets over one week showed
respectively, 0.01, 0.09, 0.32, 3.2, 32 and 256 ppm of dichlorvos.
Erythrocyte and plasma cholinesterase activities were reduced at the
two highest levels; erythrocytic activity only was affected at the
nominal level of 10 ppm but recovered to essentially normal level at
the end of the feeding period. This recovery was also recorded at the
nominal 100 and 500 ppm levels. Brain cholinesterase, measured at the
end of the two year period, was not affected at any level.
Histological examination of major organs revealed dose-related
hepatocellular oedema, slight in degree and in one animal only at the
nominal 10 ppm level, and found to a more marked degree and in all
animals at the high level. Liver weights were slightly increased in
males at the nominal 100 ppm level and in both sexes at the highest
level. However, no difference from controls was seen in serum alkaline
phosphatase or transaminase activities, total serum proteins or A:G
ratios. No effect of dichlorvos was seen at any level on general
appearance, survival, rate of weight gain, food consumption,
peripheral blood picture or urine. (Jolley, Stemmer and Pfitzer,
1967).
Man. Dichlorvos was given orally to 4 separate 5-man groups at 1,
1.5, 2.0 and 2.5 mg doses. The 2.0 mg dose produced a depression of
plasma cholinesterase to 71 percent of control two days after a 28-day
feeding period. The plasma cholinesterase of the group given 2.5 mg
daily was depressed to 70 percent of control after feeding for 20
days. There was no significant effect on RBC cholinesterase at this
time. (Rider, et al., 1967).
Long-term studies
Rat. Initial groups of 40 males and 40 females were fed diets to
which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been added, for
two years. Five males and five females from each group were to be
selected for necropsy at 26, 52 and 78 weeks. Individual diets were
allotted weekly; analysis of composite samples representing average
diets over one week enabled estimates of average dietary
concentrations to be made; respectively, 0, 0.047, 0.467, 4.67, 46.7
and 234 ppm. Plasma and erythrocyte cholinesterase activities were
depressed at the high level and generally slightly depressed at the
nominal 100 ppm level. Brain cholinesterase, measured at 26, 52, 78
and 104 weeks, was depressed at the high level. Histological
examination of major organs disclosed fine vacuolization of the
hepatic cells of the high level animals, with some fatty change and
bile stasis. Fine vacuolization only was found in 80 percent of
females and 62 percent of males at the nominal 100 ppm level. However,
no effect was seen on serum total proteins or A:G ratios, and, in a
supplementary experiment, no effect on hexabarbital sleeping time was
found by prolonged feeding of dichlorvos up to 1000 ppm or by single
sub-lethal oral doses. No hepatocellular change was seen at the three
lower test levels. No effect of dichlorvos at any level was seen in
behaviour or mortality rate, rate of weight gain, food consumption,
terminal body and organ weights, peripheral blood picture, urine and
tumour incidence (Witherup, Stemmer and Pfitzer, 1967).
Comments
Recent information has disclosed that dichlorvos is rapidly converted
in plants, to dichloro-acetaldehyde and further to dichloroethanol. As
these metabolites are also known to occur in mammals after dichlorvos
administration, it was concluded that the assessment of the safe
levels of dichlorvos included that of the metabolites.
In two-year oral feeding studies on dogs and rats the decrease of the
cholinesterase activity of erythrocytes and plasma is proportional to
the applied dose of dichlorvos. Recovery to essentially normal level
at the end of the experiment is especially visible in dogs. Depression
of the brain cholinesterase activity could be ascertained in rats
only, but only at the highest nominal dietary level 500 ppm.
Cytoplasmatic vacuolisation in hepatic cello, while dose-related, may
be considered as a variation within physiological limits. In dogs the
increase in the weight of the liver at nominal levels of 100 ppm and
500 ppm was not associated with changes of liver function tests. No
stimulation or inhibition in the activity of microsomal enzymes were
proved in rats by means of indirect test using the hexabarbital
sleeping time.
For the establishment of the ADI the dose-related effect on the
cholinesterase activity resulting from the two year experiment on rats
and dogs can be taken into consideration. The oral level of 0.25
mg/kg/day was without toxicological effect in the rat. In dogs the
corresponding value was 0.08 mg/kg/day.
In man the dose of 0.033 mg/kg/day caused toxicologically
insignificant depression of plasma cholinesterase activity. The
erythrocyte cholinesterase was not affected.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.004 mg/kg body-weight.
EVALUATION FOR TOLERANCES
USE PATTERN
Pre-harvest treatments
Because of its very short persistence and lack of systemic action, the
use of dichlorvos on growing crops is limited and somewhat
specialized. Its most common pre-harvest use is for the control of
aphids, red spider mite (Tetranychus telarius), white fly
(Trialeurodes vaporariorum), leaf miners, and other pests of crops
growing in greenhouses; and for the control of mushroom flies in
mushroom houses. The crops and uses for which dichlorvos has been
registered or approved in various countries are as follows :
Cotton, date palms Iraq
Deciduous trees Austria, Bulgaria, Germany, Great
Britain, Hungary, Italy,
Netherlands, Switzerland
Greenhouses Austria, Bulgaria, Germany, Great
Britain, Hungary, Switzerland
Horticulture Austria, Great Britain
Mushroom houses Great Britain, Hungary,
Netherlands, United States
Ornamentals Germany, Great Britain, Switzerland
Vegetables Austria, Great Britain,
Netherlands, Switzerland
General use Austria, Ceylon, Chile, India,
Japan, Mexico, Pakistan, Venezuela
Dairy and meat United States
animals
Dichlorvos has been approved in the United Stated for direct
application to livestock, including lactating animals. It has also
been approved to control flies, fleas, and mites in animal barns,
piggeries, and chicken houses. In the United Kingdom it can be used in
pig and poultry houses for ectoparasite control.
Post-harvest treatments
To date, the use of dichlorvos on food as post-harvest treatments has
been largely experimental. However, the results have been so promising
that it may find extensive use for protecting raw and processed
agricultural products. Uses of dichlorvos investigated are as a direct
spray or dust treatment to grain; as aerosol or impregnated-resin
strips applied in the overhead space of storage bins for the control
of insects infesting grains; and as sprays, vapors, fogs, aerosols,
and impregnated-resin strips for controlling insects in facilities
where foods are stored, handled, processed, transported, and marketed.
Other uses
Dichlorvos has been used for a number of years in the U.S.A. and in
other countries as a spray or aerosol in tobacco warehouses for the
control of cigarette beetles (Lasioderma serricorne) and the tobacco
moth (Ephestia elutella) at the rates of 2 g/1,000 cu ft (71 mg/m3)
biweekly and 1 g/1,000 cu ft (35 mg/m3) weekly, respectively. It has
also been used in tobacco processing rooms after working hours at 0.5
to 1 g/1,000 cu ft (18 to 35 mg/m3) daily.
The principle use of dichlorvos, however, has been in the domestic and
public health field. In the U.S.A., formulations containing 0.5
percent of dichlorvos have been approved for the control of household
pests such as ante, bedbugs, cockroaches, flies, mosquitoes,
silverfish, spiders, ticks, and wasps in private and public buildings
and outdoors. Resin strips impregnated with dichlorvos have been
approved for use in the home kitchen in the United Kingdom and in
homes and certain commercial establishments in the United States.
Dichlorvos has been found effective for disinsection of aircraft
during flight. Vapor concentrations of 0.15 - 0.30 µg/litre of air
produced 100-percent mortality of flies and mosquitoes after 30-minute
exposures (Jensen, Flury, and Schoof, 1965). No effect was noticed on
the cholinesterase level of three individuals exposed 24 times to
30-minute aircraft treatments, during which time the dichlorvos vapor
concentration was 0.20 to 0.24 µg/litre of air (Schoof et al., 1961).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown under cover
Mushrooms
In the United States dichlorvos has been approved for mushroom houses
at the rate of 2 g/1,000 cu ft (71 mg/m3) and can be applied every 4
days provided no mushrooms are picked within 1 day after treatment. In
trials conducted by Snetsinger and Miner (1964) no dichlorvos residue
was found in mushrooms picked 24 hours after treatment at 4 g and 6 g
of dichlorvos per 1,000 cu ft (141 and 212 mg/m3) nor were cumulated
residues noted with consecutive applications. Ciba Laboratories Ltd.
(unpublished) reported residues of 0.85 ppm 24 hours after, 0.03 ppm
48 hours after, and 0.12 ppm 72 hours after treatment of the mushroom
house with dichlorvos at the rate of 4.8 g/1,000 cu ft (170 mg/m3).
Mushrooms exposed to application of 3 g/1,000 cu ft (106 mg/m3) had a
maximum of 0.3 ppm 2 hours after, 0.02 ppm 24 hours after, and 0.3 ppm
of dichlorvos 48 hours after treatment.
Lettuce
Tests conducted by Ciba Laboratories Ltd. (unpublished) with lettuce
grown in greenhouses produced the following residues after one
application :
Rate and method Dicohlorovos residues (ppm) at
of application intervals after harvest (hours)
1 2 24 48 72
4 g/1,000 cu ft
(141 mg/m3) by spray gun 78 53 4.9 - -
2 g/1,000 cu ft
(71 mg/m3) by microsol - - 2.1 0.4 0.2
generator
Rate and method Dicohlorovos residues (PPE) at
of application intervals after harvest (hours)
1 2 24 48 72
1 g/1,000 cu ft
(35 mg/m3) by microsol - - 1.3 0.4 0.3
generator
Shell Chemical Company (unpublished) reported lettuce growing in
greenhouses treated with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3)
had residues of 4.4 ppm 4 hours after treatment and no detectable
residue after 24 and 48 hours.
Tomatoes
Ciba Laboratories Ltd. (unpublished) reported tomatoes growing in a
greenhouse treated with dichlorvos at 1 g/1,000 cu ft (35 mg/m3)
(microsol generator) had residues of nil to 0.4 ppm. Shell Chemical
Company (unpublished) conducted test treatments with dichlorvos at 1.4
g/1,000 cu ft (49 mg/m3) on tomatoes growing in greenhouses. Residues
found at intervals after spraying were 0.13 ppm after 15 minutes, 0.10
ppm after 13 hours and nil after 23 hours.
Cucumbers
Shell Chemical Company Ltd. (unpublished) found no residues on
cucumbers sprayed with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3) 16,
24 and 48 hours after treatment. Ciba Laboratories Ltd. (unpublished)
found residues of 7.2 ppm 24 hours after, 0.2 ppm 48 hours after, and
0.02 ppm 72 hours after spraying with dichlorvos at the rate of 0.54
g/1,000 cu ft (19 mg/m3).
Crops grown in the open
Ciba Laboratories Ltd. (unpublished) carried out trials on the
application of dichlorvos to various vegetable crops in the field at
the rate of 1 g/100 sq ft (108 mg/m2) equivalent to about 1 g/1,000
cu ft (35 mg/m3) in greenhouses. The results were as follows :
Vegetable Dichlorvos residue (PP2)
at intervals after treatment
1 hour 72 hours 6 days
Dwarf beans 0.2 0.02 Nil
0.3 Nil Nil
Cauliflower 0.2 0.01 Nil
- 0.01 Nil
Spinach 0.03 0.02 Nil
0.03 0.03 Nil
Lettuce 15.4 0.03 Nil
21.7 0.02 Nil
Residues resulting from use on animals
Studies conducted by Casida, McBride and Niedermeier (1962) concluded
that dichlorvos sprayed, rubbed, or painted on milk cows appeared
unlikely to produce significant residues in milk. The organosoluble
insecticide equivalent in milk 12 and 24 hours after cows were fed
1 mg/kg and 2 mg/kg of dichlorvos was 0.46 ppb and 0.39 ppb, and 21.1
ppb and 7.3 ppb, respectively. Hens subjected to a dichlorvos spray of
1.5 g/1,000 cu ft (53 mg/m3) (twice recommended does) 5 times at
3-day intervals produced eggs with dichlorvos residues of 0.11 ppm or
less, and the dichlorvos residue in the edible tissue of the birds
sacrificed the day after the third treatment was loss than 0.1 ppm
(Ciba Laboratories Ltd., unpublished).
Residues resulting from use on cereals and cereal products
Trials conducted in the United Kingdom in 1964 by the Pest Infestation
Laboratory (unpublished) showed that feed barley treated with 4 ppm of
dichlorvos while being turned had 1.8 ppm of dichlorvos immediately
after treatment, 0.93 ppm after 1 week, 0.25 ppm after 6 weeks and
0.26 ppm after 10 weeks, and none could be detected after 15 weeks. In
another trial, Green and Tyler (1966) treated barley (13-15 percent
moisture) with 4 ppm dichlorvos. The dichlorvos residues on the grain
immediately after treatment and 1 and 2 weeks later were 0.53 to 0.63
ppm, 0.13 to 0.26 ppm, and 0.05 to 0.09 ppm, respectively. With drier
grain and improved treatment methods, they obtained higher and more
persistent residues with the same rate of application: 1.90 ppm during
treatment and 1.06 ppm 1 week after, 0.45 ppm 3 weeks after, 0.25 ppm
6 weeks after, and 0.26 ppm 10 weeks after treatment.
Strong and Sbur (1964) studied the persistency of dichlorvos as a
spray on wheat (16-percent moisture) at 10 ppm and found its toxicity
to the rice weevil (Sitophilus oryzea) was lost after 2 weeks at
high storage temperature; whereas when stored at 60°F (15.6°C), the
treated wheat still killed rice weevils for as long as 3 months'
storage.
Research conducted by the Stored-Product Insects R and D Laboratory,
U.S. Department of Agriculture, (unpublished) showed dichlorvos vapor
concentrations of 4 to 6 µg/1 for 6 hours [2 g/1,000 cu ft (71
mg/m3)] applied weekly will protect food in storage against insect
infestation. Dichlorvos residues in packaged noodles, raisins, rice,
beans, and sugar after 21 consecutive weekly applications were less
than 2 ppm.
Test conducted by Schulten and Kuyken (1966) on the effectiveness of
dichlorvos resin strips for the control of the cocoa moth (Ephestia
elutella) (Hübner) showed that 46 dichlorvos-impregnated strips (20
percent) in 2,200m3 of warehouse space produced air concentrations of
0.05 µg/1 in 27 days and 0.05 µg/1 in 54 days. The highest dichlorvos
residues in the stored cocoa beans were 0.02 - 0.03 ppm.
Residues in meat
Samples of mincemeat, bacon, steak, and fat were exposed in a hut at
daily intervals following a 30-minute treatment with vapors of pure
dichlorvos labeled with 32P. During the treatment, the dichlorvos
concentration was maintained at approximately 0.5 microgram/liter of
air and the prevailing temperature within the hut fell rapidly after
each application, and no insecticide was detectable in the air after 2
hours. Meat samples introduced into the hut immediately following
treatments still accumulated dichlorvos although they were of a very
low level. Those in fat were generally lower than those in the
corresponding steak, mincemeat, or bacon. The maximum dichlorvos
residues found after 12-hour exposures in the various types of meats
in the same order listed above were 0.08, 0.22, 0.19 and 0.19 ppm
(Millar and Aitken, 1965).
In 1964, the Shell Chemical Co. carried out an unpublished trial at a
meat factory in Britain. Dichlorvos was applied by a watering can at a
rate of 1.5 g/1,000 cu ft. The highest concentration of dichlorvos in
the atmosphere was found to be 0.7 microgram/liter one hour after
application. Nine hours after application, the atmospheric
concentration had dropped to 0.25 microgram/liter and to 0.08
microgram/liter by 24 hours. Residues of dichlorvos in samples of
cooked meat and offal exposed to the atmosphere over a 25-hour period
after application were less than 0.1 ppm. In processed meat, residues
reached 0.4 ppm within 1/2 hour of application and fell to less than
0.05 ppm after 25 hours.
Residues resulting from other uses
In trials conducted by Shell Chemical Company, U.S.A., (unpublished)
16 dichlorvos resin strips were used in a kitchen with 20,000 cu ft
(566 m3) and 4 resin strips in another kitchen with 2,352 cu ft (66.6
m3). Usual restaurant exhaust fans over the stoves ran
intermittently. Five days later, meals exposed to the insecticide
vapors for 24 hours contained dichlorvos residues of about 0.15 ppm.
Meals exposed in a similar manner 10 days after the strips were hung
had 0.05 ppm of dichlorvos. Other trials showed cheese from a cheese
factory using one 2- × 10-in (5- × 25.4-cm) strip per 1,000 cu ft
(28.3 m3) contained less than 0.01 ppm of the decomposition product
dichloroacetaldehyde. Fish exposed to the same concentration had no
more insecticide residue than unexposed fish.
FATE OF RESIDUES
In plants
It has been shown that dichlorvos residues in food disappear rather
rapidly. For example, the trials conducted by Ciba Laboratories Ltd.
(unpublished) showed that dichlorvos residue in mushrooms decreased
from 0.85 ppm 24 hours after treatment to 0.03 ppm 24 hours later.
Lettuce with 78 ppm of dichlorvos 1 hour after treatment had 0.02 ppm
72 hours later. Tests conducted by the Pest Infestation Laboratory in
the United Kingdom showed that the 1.8 ppm of dichlorvos on barley
immediately after treatment was down to 0.93 ppm in 1 week and to 0.25
ppm in 6 weeks. Ciba Ltd. (unpublished) found wheat grain with 60 ppm
of dichlorvos had 85 percent less dichlorvos after 1 month and 97.5
percent less after 4 months of storage. These tests also showed that
the higher the moisture content of the grain and of the storage
temperature, the faster the dichlorvos residue disappeared from the
grain.
In plant material the hydrolysis product dichloroacetaldehyde can be
present in rather significant quantities. In tests conducted by Shell
Chemical Company in the U.S. (unpublished), five vegetables were
sprayed with a solution of dichlorvos in acetone at rates calculated
to give 5 ppm residues on the commodities. Analyses were conducted of
the dichlorvos (DDVP) and of the dichloroacetaldehyde (DCA). The
results obtained were :
ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Cucumbers 6.1 0.1 3.9 0.2 2.6 0.1
(cont'd) ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Lettuce 3.6 0.06 0.9 0.06 Nil Nil
Mushrooms 1.2 0.06 Nil Nil Nil Nil
Spinach 6.5 0.3 2.0 0.2 1.0 0.1
Tomatoes 5.1 0.1 2.4 0.2 1.3 0.1
Studies conducted by Shell Development Company, U.S.A., (unpublished)
showed the acute oral toxicity to rats of dichlorvos when combined
with certain other insecticides could be more then additive
(potentiation). Of the 27 insecticides combined with dichlorvos, no
potentiation was found with 20, marginal potentiation with 3, and
positive potentiation with 4. The most marked potentiation was
obtained when dichlorvos was combined with malathion. The combined
LD50 acute oral toxicity to rats of the two pesticides was 135 mg/kg,
whereas the expected additive value was 1,298 mg/kg, or a 9.61-fold
increase.
In storage and processing
Processing and cooking also removed large percentages of dichlorvos
which may be present on food. For example, wheat with 23.8 ppm of
dichlorvos after treatment produced flour with 4.6 ppm of dichlorvos.
Three months later, the wheat had 2.8 ppm of dichlorvos and the white
flour milled from it had 1.7 ppm. The dichlorvos residue in this flour
after 14 days' storage fell below the limits of detection. Shell
Chemical Company, U.S.A. (unpublished) found that biscuits made from
flour containing 0.35 ppm and 1.8 ppm of dichlorvos had 80 and 60
percent less dichlorvos, respectively. Flour with 9.5 ppm of
dichlorvos heated for 30 minutes at 100°C, 150°C, and 200°C had 0.2
ppm, 0.03 ppm, and 0.02 ppm of dichlorvos, respectively. Rice with 5.3
ppm of dichlorvos had only 0.06 ppm after cooking. Miller and Aitken
(1965) found frying and cooking completely destroyed dichlorvos in the
meat, leaving only products of hydrolysis.
METHODS OF RESIDUE ANALYSIS
Shell Development Company analytical method MMS-30/64 entitled
"Determination of Vapona Insecticide in Crops and Animal Products,
Enzyme Inhibition-Spectrophotometric Method". Sensitivity is as low as
0.1 ppm of dichlorvos. This method is not specific for dichlorvos.
Shell Chemical Company analytical method PMS-G-900/60 entitled
"Determination of Dichloroacetaldehyde Residues in Crops and Animal
Tissues, GLC Electron Capture Method". Sensitivity is as low as 0.01
ppm.
Woodstock analytical method WAMS 32-1 entitled "Determination of
Vapona in Technical Grade Products, Formulations and Extracts from
Certain Crops and Similar Samples. - Gas-Liquid Chromatographic
Method."
Other gas-liquid chromatography methods for dichlorvos and its
breakdown products are being investigated.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Australia 2 fruit, vegetables and
grain recommended by the
Food Additive Committee
of National Health and
Medical Research
Canada None
Germany *
Holland 0.1 vegetables (including
mushrooms, roots, bulbs
and tubers) and fruits
Switzerland 0.1 vegetables
United States **
* The residue on edible crops may not exceed the lower limit of
detectability of the analytical methods.
** Petitions have been submitted to U.S. Food and Drug
Administration for approval of tolerances of 0.5 ppm of
dichlorvos in packaged processed foods resulting from space
treatments in warehouses and 0.25 ppm of dichlorvos in canned
tomatoes resulting from direct application to tomatoes for
Drosophila control.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Dichlorvos in/on food disappears very rapidly and to very low levels.
Processing and cooking further reduce dichlorvos residues in food.
In the hydrolysis of dichlorvos, dichloroacetaldehyde is formed and
this metabolite may be present in food in detectable amounts. The
temporary tolerances for dichlorvos residues, therefore, include
dichloroacetaldehyde when it is reported as being present.
Because an analytical method specific for dichlorvos that can be used
for regulatory purposes is not available at this time, the tolerances
being recommended for dichlorvos residues are temporary and are to be
reviewed by 31 December 1970.
The recommended temporary tolerances are :
Cereals 2.0
Cereal products and
fresh vegetables 0.3
Canned and frozen vegetables,
fresh fruit (other than citrus) 0.1
Considering the loss of dichlorvos that takes place by aging,
processing, and cooking and based on the ninth decile of consumption
derived from consumer intake studies, the amount of dichlorvos
reaching the consumer in the above foods will be less than 0.1 mg/day
of the 0.24 mg/day permitted by the ADI. The remainder will be
available for respiratory intake and for residues that may be present
in other foods.
FURTHER WORK
Further work required before 30 June 1970
A specific analytical method with a sensitivity of about 0.01 ppm
suitable for regulatory purposes.
Data on other uses of dichlorvos, the residues resulting from such
uses, and the affects of aging, processing, and cooking.
Actual dichlorvos residues present in food moving in commerce.
Total diet studies.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
FAO/WHO. (1967) FAO Mtg. Rept. PL:CP/15; WHO Food Add./67.32
Jolley, W.B., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International.
Narcisse, J.K. (1967) unpublished report submitted by Shell
International.
Rider, S.A., Moeller, H.C. and Puletti, E.J. (1967). Fed. Proc.,
26, 427
Witherup, S., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agric. Fd. Chem., 10 (5): 370 - 377.
Green, A.A., and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos, and fenitrothion for the control of Oryzaephilus
surinamensis (L) (Coleoptera, Silvanidae) infesting stored barley.
J. Stored Prod. Res., 1 (3): 273 - 285.
Jensen, Jens A., Flury, Vincent P. and Schoof, Herbert F. (1965)
Dichlorvos vapour disinsection of aircraft. Bull. Wld. Hlth. Org., 32:
175 - 180.
Millar, K.R. and Aitken, W.M. (1965) Residues in meat following
exposure to P-Labeled dichlorvos vapor in an enclosed space. N.Z. J1
Agric. Res., 8(2): 350 - 362.
Schoof, H.F., Jensen, J.A., Porter, J.E. and Maddock, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP vapour.
Bull. Wld. Hlth. Org., 24: 623 - 628.
Schulten, G.G.M. and Kuyken, W. (1966) Dichlorvos resin strips for
control of cocoa moth, Ephestia elutella. Int. Pest Control, 8(3):
18, 19, 21, 23.
Snetsinger, R. and Miner, D. (1964) Tests with dichlorvos vapours for
the control of mushroom flies. J. Econ. Ent., 57(1): 182 - 183.
Strong, R.G. and Sbur, D.E. (1964) Influence of grain moisture and
storage temperature on the effectiveness of five insecticides as grain
protectants. J. Econ. Ent., 57(1): 44 - 47.
Relevant physical and chemical properties
The technical material usually contains 95 to 99 percent dichlorvos,
1 to 4 percent related compounds, and about 1 percent of unrelated
impurities. It is a clear, mobile liquid with a mild, rather pleasant
aromatic odor.
Boiling point: 84°C at 1 mm Hg
Specific gravity: 1.415 at 25°C
Vapor pressure: 0.01 mm Hg at 20°C
0.032 mm Hg at 32°C
0.30 mm Hg at 60°C
Solubility: About 3 percent in kerosene and
mineral oils and 1 percent in water
and glycerine. Miscible with
aromatic and chlorinated
hydrocarbon solvents.
Volatility: 145 mg/m3 at 20°C
350 mg/m3 at 30°C
800 mg/m3 at 40°C
Stability: Stable in organic solvents but
hydrolyzes in presence of water.
Alkalis accelerate and acids reduce
aqueous hydrolysis.
Composition of the Technical Product
Dichlorvos is available in oil solutions, emulsifiable concentrates,
aerosol formulations, and impregnated in polyvinyl chloride resin and
sold in the form of pellets, strips, blocks, and other forms. The
impregnated-resin stock usually contains about 20 percent of
dichlorvos by weight.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
In acute oral toxicity studies in male rats, the toxicity of
dichlorvos was markedly potentiated when administered in combination
with malathion. It was slightly potentiated when administered in
combination with trichlorofon, phosdrin and phosphamidon. No
potentiation was observed when it was administered in combination with
twenty-three other pesticides (Narcisse, 1967).
Short-term studies
Dog. For two years, groups of three males and three females were fed
diets to which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been
added. The individual portions were alloted weekly. Analyses of
composite samples representing the average diets over one week showed
respectively, 0.01, 0.09, 0.32, 3.2, 32 and 256 ppm of dichlorvos.
Erythrocyte and plasma cholinesterase activities were reduced at the
two highest levels; erythrocytic activity only was affected at the
nominal level of 10 ppm but recovered to essentially normal level at
the end of the feeding period. This recovery was also recorded at the
nominal 100 and 500 ppm levels. Brain cholinesterase, measured at the
end of the two year period, was not affected at any level.
Histological examination of major organs revealed dose-related
hepatocellular oedema, slight in degree and in one animal only at the
nominal 10 ppm level, and found to a more marked degree and in all
animals at the high level. Liver weights were slightly increased in
males at the nominal 100 ppm level and in both sexes at the highest
level. However, no difference from controls was seen in serum alkaline
phosphatase or transaminase activities, total serum proteins or A:G
ratios. No effect of dichlorvos was seen at any level on general
appearance, survival, rate of weight gain, food consumption,
peripheral blood picture or urine. (Jolley, Stemmer and Pfitzer,
1967).
Man. Dichlorvos was given orally to 4 separate 5-man groups at 1,
1.5, 2.0 and 2.5 mg doses. The 2.0 mg dose produced a depression of
plasma cholinesterase to 71 percent of control two days after a 28-day
feeding period. The plasma cholinesterase of the group given 2.5 mg
daily was depressed to 70 percent of control after feeding for 20
days. There was no significant effect on RBC cholinesterase at this
time. (Rider, et al., 1967).
Long-term studies
Rat. Initial groups of 40 males and 40 females were fed diets to
which 0, 0.1, 1, 10, 100 and 500 ppm of dichlorvos had been added, for
two years. Five males and five females from each group were to be
selected for necropsy at 26, 52 and 78 weeks. Individual diets were
allotted weekly; analysis of composite samples representing average
diets over one week enabled estimates of average dietary
concentrations to be made; respectively, 0, 0.047, 0.467, 4.67, 46.7
and 234 ppm. Plasma and erythrocyte cholinesterase activities were
depressed at the high level and generally slightly depressed at the
nominal 100 ppm level. Brain cholinesterase, measured at 26, 52, 78
and 104 weeks, was depressed at the high level. Histological
examination of major organs disclosed fine vacuolization of the
hepatic cells of the high level animals, with some fatty change and
bile stasis. Fine vacuolization only was found in 80 percent of
females and 62 percent of males at the nominal 100 ppm level. However,
no effect was seen on serum total proteins or A:G ratios, and, in a
supplementary experiment, no effect on hexabarbital sleeping time was
found by prolonged feeding of dichlorvos up to 1000 ppm or by single
sub-lethal oral doses. No hepatocellular change was seen at the three
lower test levels. No effect of dichlorvos at any level was seen in
behaviour or mortality rate, rate of weight gain, food consumption,
terminal body and organ weights, peripheral blood picture, urine and
tumour incidence (Witherup, Stemmer and Pfitzer, 1967).
Comments
Recent information has disclosed that dichlorvos is rapidly converted
in plants, to dichloro-acetaldehyde and further to dichloroethanol. As
these metabolites are also known to occur in mammals after dichlorvos
administration, it was concluded that the assessment of the safe
levels of dichlorvos included that of the metabolites.
In two-year oral feeding studies on dogs and rats the decrease of the
cholinesterase activity of erythrocytes and plasma is proportional to
the applied dose of dichlorvos. Recovery to essentially normal level
at the end of the experiment is especially visible in dogs. Depression
of the brain cholinesterase activity could be ascertained in rats
only, but only at the highest nominal dietary level 500 ppm.
Cytoplasmatic vacuolisation in hepatic cello, while dose-related, may
be considered as a variation within physiological limits. In dogs the
increase in the weight of the liver at nominal levels of 100 ppm and
500 ppm was not associated with changes of liver function tests. No
stimulation or inhibition in the activity of microsomal enzymes were
proved in rats by means of indirect test using the hexabarbital
sleeping time.
For the establishment of the ADI the dose-related effect on the
cholinesterase activity resulting from the two year experiment on rats
and dogs can be taken into consideration. The oral level of 0.25
mg/kg/day was without toxicological effect in the rat. In dogs the
corresponding value was 0.08 mg/kg/day.
In man the dose of 0.033 mg/kg/day caused toxicologically
insignificant depression of plasma cholinesterase activity. The
erythrocyte cholinesterase was not affected.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.004 mg/kg body-weight.
EVALUATION FOR TOLERANCES
USE PATTERN
Pre-harvest treatments
Because of its very short persistence and lack of systemic action, the
use of dichlorvos on growing crops is limited and somewhat
specialized. Its most common pre-harvest use is for the control of
aphids, red spider mite (Tetranychus telarius), white fly
(Trialeurodes vaporariorum), leaf miners, and other pests of crops
growing in greenhouses; and for the control of mushroom flies in
mushroom houses. The crops and uses for which dichlorvos has been
registered or approved in various countries are as follows :
Cotton, date palms Iraq
Deciduous trees Austria, Bulgaria, Germany, Great
Britain, Hungary, Italy,
Netherlands, Switzerland
Greenhouses Austria, Bulgaria, Germany, Great
Britain, Hungary, Switzerland
Horticulture Austria, Great Britain
Mushroom houses Great Britain, Hungary,
Netherlands, United States
Ornamentals Germany, Great Britain, Switzerland
Vegetables Austria, Great Britain,
Netherlands, Switzerland
General use Austria, Ceylon, Chile, India,
Japan, Mexico, Pakistan, Venezuela
Dairy and meat United States
animals
Dichlorvos has been approved in the United Stated for direct
application to livestock, including lactating animals. It has also
been approved to control flies, fleas, and mites in animal barns,
piggeries, and chicken houses. In the United Kingdom it can be used in
pig and poultry houses for ectoparasite control.
Post-harvest treatments
To date, the use of dichlorvos on food as post-harvest treatments has
been largely experimental. However, the results have been so promising
that it may find extensive use for protecting raw and processed
agricultural products. Uses of dichlorvos investigated are as a direct
spray or dust treatment to grain; as aerosol or impregnated-resin
strips applied in the overhead space of storage bins for the control
of insects infesting grains; and as sprays, vapors, fogs, aerosols,
and impregnated-resin strips for controlling insects in facilities
where foods are stored, handled, processed, transported, and marketed.
Other uses
Dichlorvos has been used for a number of years in the U.S.A. and in
other countries as a spray or aerosol in tobacco warehouses for the
control of cigarette beetles (Lasioderma serricorne) and the tobacco
moth (Ephestia elutella) at the rates of 2 g/1,000 cu ft (71 mg/m3)
biweekly and 1 g/1,000 cu ft (35 mg/m3) weekly, respectively. It has
also been used in tobacco processing rooms after working hours at 0.5
to 1 g/1,000 cu ft (18 to 35 mg/m3) daily.
The principle use of dichlorvos, however, has been in the domestic and
public health field. In the U.S.A., formulations containing 0.5
percent of dichlorvos have been approved for the control of household
pests such as ante, bedbugs, cockroaches, flies, mosquitoes,
silverfish, spiders, ticks, and wasps in private and public buildings
and outdoors. Resin strips impregnated with dichlorvos have been
approved for use in the home kitchen in the United Kingdom and in
homes and certain commercial establishments in the United States.
Dichlorvos has been found effective for disinsection of aircraft
during flight. Vapor concentrations of 0.15 - 0.30 µg/litre of air
produced 100-percent mortality of flies and mosquitoes after 30-minute
exposures (Jensen, Flury, and Schoof, 1965). No effect was noticed on
the cholinesterase level of three individuals exposed 24 times to
30-minute aircraft treatments, during which time the dichlorvos vapor
concentration was 0.20 to 0.24 µg/litre of air (Schoof et al., 1961).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown under cover
Mushrooms
In the United States dichlorvos has been approved for mushroom houses
at the rate of 2 g/1,000 cu ft (71 mg/m3) and can be applied every 4
days provided no mushrooms are picked within 1 day after treatment. In
trials conducted by Snetsinger and Miner (1964) no dichlorvos residue
was found in mushrooms picked 24 hours after treatment at 4 g and 6 g
of dichlorvos per 1,000 cu ft (141 and 212 mg/m3) nor were cumulated
residues noted with consecutive applications. Ciba Laboratories Ltd.
(unpublished) reported residues of 0.85 ppm 24 hours after, 0.03 ppm
48 hours after, and 0.12 ppm 72 hours after treatment of the mushroom
house with dichlorvos at the rate of 4.8 g/1,000 cu ft (170 mg/m3).
Mushrooms exposed to application of 3 g/1,000 cu ft (106 mg/m3) had a
maximum of 0.3 ppm 2 hours after, 0.02 ppm 24 hours after, and 0.3 ppm
of dichlorvos 48 hours after treatment.
Lettuce
Tests conducted by Ciba Laboratories Ltd. (unpublished) with lettuce
grown in greenhouses produced the following residues after one
application :
Rate and method Dicohlorovos residues (ppm) at
of application intervals after harvest (hours)
1 2 24 48 72
4 g/1,000 cu ft
(141 mg/m3) by spray gun 78 53 4.9 - -
2 g/1,000 cu ft
(71 mg/m3) by microsol - - 2.1 0.4 0.2
generator
Rate and method Dicohlorovos residues (PPE) at
of application intervals after harvest (hours)
1 2 24 48 72
1 g/1,000 cu ft
(35 mg/m3) by microsol - - 1.3 0.4 0.3
generator
Shell Chemical Company (unpublished) reported lettuce growing in
greenhouses treated with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3)
had residues of 4.4 ppm 4 hours after treatment and no detectable
residue after 24 and 48 hours.
Tomatoes
Ciba Laboratories Ltd. (unpublished) reported tomatoes growing in a
greenhouse treated with dichlorvos at 1 g/1,000 cu ft (35 mg/m3)
(microsol generator) had residues of nil to 0.4 ppm. Shell Chemical
Company (unpublished) conducted test treatments with dichlorvos at 1.4
g/1,000 cu ft (49 mg/m3) on tomatoes growing in greenhouses. Residues
found at intervals after spraying were 0.13 ppm after 15 minutes, 0.10
ppm after 13 hours and nil after 23 hours.
Cucumbers
Shell Chemical Company Ltd. (unpublished) found no residues on
cucumbers sprayed with dichlorvos at 1.4 g/1,000 cu ft (49 mg/m3) 16,
24 and 48 hours after treatment. Ciba Laboratories Ltd. (unpublished)
found residues of 7.2 ppm 24 hours after, 0.2 ppm 48 hours after, and
0.02 ppm 72 hours after spraying with dichlorvos at the rate of 0.54
g/1,000 cu ft (19 mg/m3).
Crops grown in the open
Ciba Laboratories Ltd. (unpublished) carried out trials on the
application of dichlorvos to various vegetable crops in the field at
the rate of 1 g/100 sq ft (108 mg/m2) equivalent to about 1 g/1,000
cu ft (35 mg/m3) in greenhouses. The results were as follows :
Vegetable Dichlorvos residue (PP2)
at intervals after treatment
1 hour 72 hours 6 days
Dwarf beans 0.2 0.02 Nil
0.3 Nil Nil
Cauliflower 0.2 0.01 Nil
- 0.01 Nil
Spinach 0.03 0.02 Nil
0.03 0.03 Nil
Lettuce 15.4 0.03 Nil
21.7 0.02 Nil
Residues resulting from use on animals
Studies conducted by Casida, McBride and Niedermeier (1962) concluded
that dichlorvos sprayed, rubbed, or painted on milk cows appeared
unlikely to produce significant residues in milk. The organosoluble
insecticide equivalent in milk 12 and 24 hours after cows were fed
1 mg/kg and 2 mg/kg of dichlorvos was 0.46 ppb and 0.39 ppb, and 21.1
ppb and 7.3 ppb, respectively. Hens subjected to a dichlorvos spray of
1.5 g/1,000 cu ft (53 mg/m3) (twice recommended does) 5 times at
3-day intervals produced eggs with dichlorvos residues of 0.11 ppm or
less, and the dichlorvos residue in the edible tissue of the birds
sacrificed the day after the third treatment was loss than 0.1 ppm
(Ciba Laboratories Ltd., unpublished).
Residues resulting from use on cereals and cereal products
Trials conducted in the United Kingdom in 1964 by the Pest Infestation
Laboratory (unpublished) showed that feed barley treated with 4 ppm of
dichlorvos while being turned had 1.8 ppm of dichlorvos immediately
after treatment, 0.93 ppm after 1 week, 0.25 ppm after 6 weeks and
0.26 ppm after 10 weeks, and none could be detected after 15 weeks. In
another trial, Green and Tyler (1966) treated barley (13-15 percent
moisture) with 4 ppm dichlorvos. The dichlorvos residues on the grain
immediately after treatment and 1 and 2 weeks later were 0.53 to 0.63
ppm, 0.13 to 0.26 ppm, and 0.05 to 0.09 ppm, respectively. With drier
grain and improved treatment methods, they obtained higher and more
persistent residues with the same rate of application: 1.90 ppm during
treatment and 1.06 ppm 1 week after, 0.45 ppm 3 weeks after, 0.25 ppm
6 weeks after, and 0.26 ppm 10 weeks after treatment.
Strong and Sbur (1964) studied the persistency of dichlorvos as a
spray on wheat (16-percent moisture) at 10 ppm and found its toxicity
to the rice weevil (Sitophilus oryzea) was lost after 2 weeks at
high storage temperature; whereas when stored at 60°F (15.6°C), the
treated wheat still killed rice weevils for as long as 3 months'
storage.
Research conducted by the Stored-Product Insects R and D Laboratory,
U.S. Department of Agriculture, (unpublished) showed dichlorvos vapor
concentrations of 4 to 6 µg/1 for 6 hours [2 g/1,000 cu ft (71
mg/m3)] applied weekly will protect food in storage against insect
infestation. Dichlorvos residues in packaged noodles, raisins, rice,
beans, and sugar after 21 consecutive weekly applications were less
than 2 ppm.
Test conducted by Schulten and Kuyken (1966) on the effectiveness of
dichlorvos resin strips for the control of the cocoa moth (Ephestia
elutella) (Hübner) showed that 46 dichlorvos-impregnated strips (20
percent) in 2,200m3 of warehouse space produced air concentrations of
0.05 µg/1 in 27 days and 0.05 µg/1 in 54 days. The highest dichlorvos
residues in the stored cocoa beans were 0.02 - 0.03 ppm.
Residues in meat
Samples of mincemeat, bacon, steak, and fat were exposed in a hut at
daily intervals following a 30-minute treatment with vapors of pure
dichlorvos labeled with 32P. During the treatment, the dichlorvos
concentration was maintained at approximately 0.5 microgram/liter of
air and the prevailing temperature within the hut fell rapidly after
each application, and no insecticide was detectable in the air after 2
hours. Meat samples introduced into the hut immediately following
treatments still accumulated dichlorvos although they were of a very
low level. Those in fat were generally lower than those in the
corresponding steak, mincemeat, or bacon. The maximum dichlorvos
residues found after 12-hour exposures in the various types of meats
in the same order listed above were 0.08, 0.22, 0.19 and 0.19 ppm
(Millar and Aitken, 1965).
In 1964, the Shell Chemical Co. carried out an unpublished trial at a
meat factory in Britain. Dichlorvos was applied by a watering can at a
rate of 1.5 g/1,000 cu ft. The highest concentration of dichlorvos in
the atmosphere was found to be 0.7 microgram/liter one hour after
application. Nine hours after application, the atmospheric
concentration had dropped to 0.25 microgram/liter and to 0.08
microgram/liter by 24 hours. Residues of dichlorvos in samples of
cooked meat and offal exposed to the atmosphere over a 25-hour period
after application were less than 0.1 ppm. In processed meat, residues
reached 0.4 ppm within 1/2 hour of application and fell to less than
0.05 ppm after 25 hours.
Residues resulting from other uses
In trials conducted by Shell Chemical Company, U.S.A., (unpublished)
16 dichlorvos resin strips were used in a kitchen with 20,000 cu ft
(566 m3) and 4 resin strips in another kitchen with 2,352 cu ft (66.6
m3). Usual restaurant exhaust fans over the stoves ran
intermittently. Five days later, meals exposed to the insecticide
vapors for 24 hours contained dichlorvos residues of about 0.15 ppm.
Meals exposed in a similar manner 10 days after the strips were hung
had 0.05 ppm of dichlorvos. Other trials showed cheese from a cheese
factory using one 2- × 10-in (5- × 25.4-cm) strip per 1,000 cu ft
(28.3 m3) contained less than 0.01 ppm of the decomposition product
dichloroacetaldehyde. Fish exposed to the same concentration had no
more insecticide residue than unexposed fish.
FATE OF RESIDUES
In plants
It has been shown that dichlorvos residues in food disappear rather
rapidly. For example, the trials conducted by Ciba Laboratories Ltd.
(unpublished) showed that dichlorvos residue in mushrooms decreased
from 0.85 ppm 24 hours after treatment to 0.03 ppm 24 hours later.
Lettuce with 78 ppm of dichlorvos 1 hour after treatment had 0.02 ppm
72 hours later. Tests conducted by the Pest Infestation Laboratory in
the United Kingdom showed that the 1.8 ppm of dichlorvos on barley
immediately after treatment was down to 0.93 ppm in 1 week and to 0.25
ppm in 6 weeks. Ciba Ltd. (unpublished) found wheat grain with 60 ppm
of dichlorvos had 85 percent less dichlorvos after 1 month and 97.5
percent less after 4 months of storage. These tests also showed that
the higher the moisture content of the grain and of the storage
temperature, the faster the dichlorvos residue disappeared from the
grain.
In plant material the hydrolysis product dichloroacetaldehyde can be
present in rather significant quantities. In tests conducted by Shell
Chemical Company in the U.S. (unpublished), five vegetables were
sprayed with a solution of dichlorvos in acetone at rates calculated
to give 5 ppm residues on the commodities. Analyses were conducted of
the dichlorvos (DDVP) and of the dichloroacetaldehyde (DCA). The
results obtained were :
ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Cucumbers 6.1 0.1 3.9 0.2 2.6 0.1
(cont'd) ppm after aging
Crops 1 hour 3 days 7 days
DDVP DCA DDVP DCA DDVP DCA
Lettuce 3.6 0.06 0.9 0.06 Nil Nil
Mushrooms 1.2 0.06 Nil Nil Nil Nil
Spinach 6.5 0.3 2.0 0.2 1.0 0.1
Tomatoes 5.1 0.1 2.4 0.2 1.3 0.1
Studies conducted by Shell Development Company, U.S.A., (unpublished)
showed the acute oral toxicity to rats of dichlorvos when combined
with certain other insecticides could be more then additive
(potentiation). Of the 27 insecticides combined with dichlorvos, no
potentiation was found with 20, marginal potentiation with 3, and
positive potentiation with 4. The most marked potentiation was
obtained when dichlorvos was combined with malathion. The combined
LD50 acute oral toxicity to rats of the two pesticides was 135 mg/kg,
whereas the expected additive value was 1,298 mg/kg, or a 9.61-fold
increase.
In storage and processing
Processing and cooking also removed large percentages of dichlorvos
which may be present on food. For example, wheat with 23.8 ppm of
dichlorvos after treatment produced flour with 4.6 ppm of dichlorvos.
Three months later, the wheat had 2.8 ppm of dichlorvos and the white
flour milled from it had 1.7 ppm. The dichlorvos residue in this flour
after 14 days' storage fell below the limits of detection. Shell
Chemical Company, U.S.A. (unpublished) found that biscuits made from
flour containing 0.35 ppm and 1.8 ppm of dichlorvos had 80 and 60
percent less dichlorvos, respectively. Flour with 9.5 ppm of
dichlorvos heated for 30 minutes at 100°C, 150°C, and 200°C had 0.2
ppm, 0.03 ppm, and 0.02 ppm of dichlorvos, respectively. Rice with 5.3
ppm of dichlorvos had only 0.06 ppm after cooking. Miller and Aitken
(1965) found frying and cooking completely destroyed dichlorvos in the
meat, leaving only products of hydrolysis.
METHODS OF RESIDUE ANALYSIS
Shell Development Company analytical method MMS-30/64 entitled
"Determination of Vapona Insecticide in Crops and Animal Products,
Enzyme Inhibition-Spectrophotometric Method". Sensitivity is as low as
0.1 ppm of dichlorvos. This method is not specific for dichlorvos.
Shell Chemical Company analytical method PMS-G-900/60 entitled
"Determination of Dichloroacetaldehyde Residues in Crops and Animal
Tissues, GLC Electron Capture Method". Sensitivity is as low as 0.01
ppm.
Woodstock analytical method WAMS 32-1 entitled "Determination of
Vapona in Technical Grade Products, Formulations and Extracts from
Certain Crops and Similar Samples. - Gas-Liquid Chromatographic
Method."
Other gas-liquid chromatography methods for dichlorvos and its
breakdown products are being investigated.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Australia 2 fruit, vegetables and
grain recommended by the
Food Additive Committee
of National Health and
Medical Research
Canada None
Germany *
Holland 0.1 vegetables (including
mushrooms, roots, bulbs
and tubers) and fruits
Switzerland 0.1 vegetables
United States **
* The residue on edible crops may not exceed the lower limit of
detectability of the analytical methods.
** Petitions have been submitted to U.S. Food and Drug
Administration for approval of tolerances of 0.5 ppm of
dichlorvos in packaged processed foods resulting from space
treatments in warehouses and 0.25 ppm of dichlorvos in canned
tomatoes resulting from direct application to tomatoes for
Drosophila control.
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances
Dichlorvos in/on food disappears very rapidly and to very low levels.
Processing and cooking further reduce dichlorvos residues in food.
In the hydrolysis of dichlorvos, dichloroacetaldehyde is formed and
this metabolite may be present in food in detectable amounts. The
temporary tolerances for dichlorvos residues, therefore, include
dichloroacetaldehyde when it is reported as being present.
Because an analytical method specific for dichlorvos that can be used
for regulatory purposes is not available at this time, the tolerances
being recommended for dichlorvos residues are temporary and are to be
reviewed by 31 December 1970.
The recommended temporary tolerances are :
Cereals 2.0
Cereal products and
fresh vegetables 0.3
Canned and frozen vegetables,
fresh fruit (other than citrus) 0.1
Considering the loss of dichlorvos that takes place by aging,
processing, and cooking and based on the ninth decile of consumption
derived from consumer intake studies, the amount of dichlorvos
reaching the consumer in the above foods will be less than 0.1 mg/day
of the 0.24 mg/day permitted by the ADI. The remainder will be
available for respiratory intake and for residues that may be present
in other foods.
FURTHER WORK
Further work required before 30 June 1970
A specific analytical method with a sensitivity of about 0.01 ppm
suitable for regulatory purposes.
Data on other uses of dichlorvos, the residues resulting from such
uses, and the affects of aging, processing, and cooking.
Actual dichlorvos residues present in food moving in commerce.
Total diet studies.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
FAO/WHO. (1967) FAO Mtg. Rept. PL:CP/15; WHO Food Add./67.32
Jolley, W.B., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International.
Narcisse, J.K. (1967) unpublished report submitted by Shell
International.
Rider, S.A., Moeller, H.C. and Puletti, E.J. (1967). Fed. Proc.,
26, 427
Witherup, S., Stemmer, K.L. and Pfitzer, E.A. (1967) unpublished
report submitted by Shell International
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agric. Fd. Chem., 10 (5): 370 - 377.
Green, A.A., and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos, and fenitrothion for the control of Oryzaephilus
surinamensis (L) (Coleoptera, Silvanidae) infesting stored barley.
J. Stored Prod. Res., 1 (3): 273 - 285.
Jensen, Jens A., Flury, Vincent P. and Schoof, Herbert F. (1965)
Dichlorvos vapour disinsection of aircraft. Bull. Wld. Hlth. Org., 32:
175 - 180.
Millar, K.R. and Aitken, W.M. (1965) Residues in meat following
exposure to P-Labeled dichlorvos vapor in an enclosed space. N.Z. J1
Agric. Res., 8(2): 350 - 362.
Schoof, H.F., Jensen, J.A., Porter, J.E. and Maddock, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP vapour.
Bull. Wld. Hlth. Org., 24: 623 - 628.
Schulten, G.G.M. and Kuyken, W. (1966) Dichlorvos resin strips for
control of cocoa moth, Ephestia elutella. Int. Pest Control, 8(3):
18, 19, 21, 23.
Snetsinger, R. and Miner, D. (1964) Tests with dichlorvos vapours for
the control of mushroom flies. J. Econ. Ent., 57(1): 182 - 183.
Strong, R.G. and Sbur, D.E. (1964) Influence of grain moisture and
storage temperature on the effectiveness of five insecticides as grain
protectants. J. Econ. Ent., 57(1): 44 - 47.
