
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
PIPERONYL BUTOXIDE
Chemical name
3,4-methylenedioxy-6-propylbenxyl n-butyl diethyleneglycol
ether
Synonyms
Alpha-[2-(2-n-butoxyethoxy)ethoxy]-4,5-methylendioxy-2-
propyltolune
or
6-(propylpiperonyl)-butyl carbityl ether
or
(3,4-methylenedioxy-6-propylbenzyl) (butyl diethylene glycol
ether) ether
Empirical formula
C19H30O5 (molecular weight 338)
Structural formula
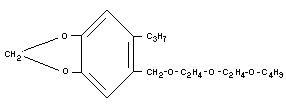 Relevant physical and chemical properties
Piperonyl butoxide is a derivative of piperic acid. Its
synergistic activity is believed to be due to the presence of the
methylenedioxy group in the molecular structure. It is synthesized
from safrole and the butyl ether of diethylene glycol.
Piperonyl butoxide is quite stable, resistant to hydrolysis,
oxidation and exposure to sunlight. Strong bases up to 1N
concentration and weak acids will not affect it, but strong acids will
destroy it. For a more complete description of the physical and
chemical characteristics see Negherbon (1959).
Uses
Alone, piperonyl butoxide is a compound of only mediocre
insecticidal power. It acts as an effective synergist to increase the
toxicity; "knockdown" and persistence of pyrethrins and allethrin. The
synergist action is so pronounced that the resulting kill of insects
is much greater than that which can be produced by pyrethrins alone
(Wachs, 1947; McAlister et al., 1947). It does not synergize or
potentiate DDT or nicotine. There are some suggestions that piperonyl
butoxide will potentiate the toxicity of organic phosphate
insecticides to insects (Robbins et al., 1959). Rotenone, ryanodine
and benzene hexachloride are activated by piperonyl butoxide but to a
lesser degree than pyrethrins (Negherbon, 1959).
Residues
Residues do result from use on foods, but the information on this
subject is incomplete.
In the analysis of residues, extraneous materials extracted from
natural products develop dark brown to black colours when heated with
concentrated phosphoric acid. These colours interfere with the residue
determination by colorimetric end procedure. Each food presents
different problems in removing interfering substances. Four methods
have been published by the Association of Official Agricultural
Chemists. In wheat, pinto beans, Alaska beans hulled rice, oats and
barley a sensitivity of 20 mmg of piperonyl butoxide of 0.5 ppm can be
obtained (AOAC, 1960; AOAC, 1963; Munday, 1963).
A method is reported for flour, grain and oil base materials, but
details are given only for oil base materials, containing 0.25-0.75
mg/ml (Jones et al., 1952). A colorimetric method for application to
fats, waxes and oils is suitable for 50-80 mmg working range (Williams
& Sweeney, 1956).
Effect on treated crop
No information available.
BIOLOGICAL DATA
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract
with loss of appetite and prostration (Sarles et al., 1949). It may be
that these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949; Sarles &
Vandegrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandegrift, 1952).
In rats, large subcutaneous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandegrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects,
piperonyl butoxide synergizes pyrethrins by inhibiting lipase
(esterase), but his results were inconclusive.
In an experiment in which 87.6% of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09% was found in the
urine (Sarles & Vandegrift, 1952). So far as is known, the
colorimetric tests used responded to piperonyl butoxide only. Thus,
the 12% of the dose that was unaccounted for may have been the most
important part from a toxicological standpoint.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 4030 Negherbon, 1959
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of courmaphos (a triphosphate) and its phosphate by a factor
of 4 to 6 (Robbins et al., 1959).
There is some evidence that the piperonyl butoxide interferes
with detoxification of the organo-phosphorus insecticides (Robbins et
al., 1959). However, apparently no additional toxicity was produced in
rats when one-sixth as much pyrethrins was added to their diet
containing piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandegrift, 1952).
Short-term studies
Monkey. At comparable dosage, symptomatology was somewhat less
than that in dogs. Microscopical pathology of the liver in monkeys on
a dosage of 100 mg/kg/day was comparable to that in dogs receiving 30
mg/kg/day (a dosage that produced no observed effect in the monkey).
The apparent difference in the susceptibility of the species may be
explained by the shorter exposure of the monkey (1 month) compared
with the dogs (1 year) (Sarles & Vandegrift, 1952).
Dog. Dogs did not grow as fast as the controls when dosed at
the rate of 32 mg/kg/day for a year and lost weight when dosed at
rates of 106 mg/kg/day or higher. Even at 3 mg/kg/day, the dogs showed
some increase in liver weight and the increase was progressively
greater at higher dosage rates. The kidneys and adrenals were
progressively enlarged at dosages of about 100 mg/kg/day and above.
Microscopical pathology was evident in the liver at dosage rates of
about 30 mg/kg/day and over. Hepatoma and carcinoma did not occur in
the dog (Sarles & Vandegrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide
as high as 1000 ppm caused no decrease in the growth rate of female
rats; concentrations as low as 100 ppm produced some reduction in the
growth of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
subnormal food consumption (78% of control). A concentration of 25 000
ppm reduced food consumption to 37% of normal and stunted the animals.
However, in subacute experiments, anorexia was terminal and therefore
not the simple effect of unpalatability of the food. A concentration
of 10 000 ppm caused a distinct increase in mortality in both sexes
evident in 2 years and a concentration of 25 000 killed about half the
animals in half a year. Only concentrations of 10 000 ppm or higher
produced significant increase in the relative weight of the liver and
kidney. Some degree of liver pathology apparently occurred in all
groups of rats but was progressively more marked at dietary levels of
1000 ppm and more. Less marked changes occurred in the kidney and
adrenal. Benign or malignant tumours occurred in 30% of the test
animals but the authors claimed that their occurrence was not related
to piperonyl butoxide. Reproduction was decreased by a dietary level
of 10 000 ppm and stopped by a concentration of 25 000 ppm (Sarles &
Vandegrift, 1952).
Comments on the experimental work reported and evaluation
Sarles & Vandegrift (1952) made a distinction for the rat
between the ill-defined effects of 1000 ppm and the effects of 100
ppm, a level which they found to be "nontoxic". Furthermore, dogs
showed decreased growth and microscopical pathology of the liver at
about 30 mg/kg/day and some increase in liver weight at only 3
mg/kg/day.
The uses of piperonyl butoxide are such that only a small
portion of food would be expected to contain any. No report of actual
residues is available. Although there is no evidence that the
presently approved uses of piperonyl butoxide involve any danger there
is not enough information on the compound to allow the setting of an
acceptable daily intake figure for human beings.
Further work considered necessary
Determination of the nature and amount of the residues reaching
the consumer.
A level should be established that causes no significant effect
during long-term studies in at least 2 species. The question of
tumorigenicity should be re-explored, especially in rats. Biochemical
studies should be made on the qualitative and quantitative aspects of
metabolism of the compound.
REFERENCES
AOAC (1960) Official methods of Analysis of the Association of
Official Agricultural Chemists, ninth ed., Washington
AOAC (1963) J. Assoc. Offic. Agr. Chem., 46, 145
Chamberlain, R. H. (1950) Amer. J. Hyg., 52, 153
Jones, H. A. Ackermann, H. J. & Webster, M. E. (1952) J. Assoc.
Offic. Agr. Chem., 35, 771
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 12, 82
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 16, 3
McAlister, L. C. Jones, H. A. & Moore, D. H. (1947) J. econ. Ent.,
40, 906
Munday, W. H. (1963) J. Assoc. Offic. Agr. Chem., 46, 244
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ. Ent.,
52, 660
Sarles, M. P., Dove, W. E. & More, D. H. (1949) Amer. J. trop. Med.,
29, 151
Sarles, M. P. & Vandegrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
Wachs, H. (1947) Science, 105, 530
William H. L. & Sweeney, J. P. (1956) J. Assoc. Offic. Agr. Chem.,
39, 975
Relevant physical and chemical properties
Piperonyl butoxide is a derivative of piperic acid. Its
synergistic activity is believed to be due to the presence of the
methylenedioxy group in the molecular structure. It is synthesized
from safrole and the butyl ether of diethylene glycol.
Piperonyl butoxide is quite stable, resistant to hydrolysis,
oxidation and exposure to sunlight. Strong bases up to 1N
concentration and weak acids will not affect it, but strong acids will
destroy it. For a more complete description of the physical and
chemical characteristics see Negherbon (1959).
Uses
Alone, piperonyl butoxide is a compound of only mediocre
insecticidal power. It acts as an effective synergist to increase the
toxicity; "knockdown" and persistence of pyrethrins and allethrin. The
synergist action is so pronounced that the resulting kill of insects
is much greater than that which can be produced by pyrethrins alone
(Wachs, 1947; McAlister et al., 1947). It does not synergize or
potentiate DDT or nicotine. There are some suggestions that piperonyl
butoxide will potentiate the toxicity of organic phosphate
insecticides to insects (Robbins et al., 1959). Rotenone, ryanodine
and benzene hexachloride are activated by piperonyl butoxide but to a
lesser degree than pyrethrins (Negherbon, 1959).
Residues
Residues do result from use on foods, but the information on this
subject is incomplete.
In the analysis of residues, extraneous materials extracted from
natural products develop dark brown to black colours when heated with
concentrated phosphoric acid. These colours interfere with the residue
determination by colorimetric end procedure. Each food presents
different problems in removing interfering substances. Four methods
have been published by the Association of Official Agricultural
Chemists. In wheat, pinto beans, Alaska beans hulled rice, oats and
barley a sensitivity of 20 mmg of piperonyl butoxide of 0.5 ppm can be
obtained (AOAC, 1960; AOAC, 1963; Munday, 1963).
A method is reported for flour, grain and oil base materials, but
details are given only for oil base materials, containing 0.25-0.75
mg/ml (Jones et al., 1952). A colorimetric method for application to
fats, waxes and oils is suitable for 50-80 mmg working range (Williams
& Sweeney, 1956).
Effect on treated crop
No information available.
BIOLOGICAL DATA
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract
with loss of appetite and prostration (Sarles et al., 1949). It may be
that these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949; Sarles &
Vandegrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandegrift, 1952).
In rats, large subcutaneous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandegrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects,
piperonyl butoxide synergizes pyrethrins by inhibiting lipase
(esterase), but his results were inconclusive.
In an experiment in which 87.6% of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09% was found in the
urine (Sarles & Vandegrift, 1952). So far as is known, the
colorimetric tests used responded to piperonyl butoxide only. Thus,
the 12% of the dose that was unaccounted for may have been the most
important part from a toxicological standpoint.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 4030 Negherbon, 1959
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of courmaphos (a triphosphate) and its phosphate by a factor
of 4 to 6 (Robbins et al., 1959).
There is some evidence that the piperonyl butoxide interferes
with detoxification of the organo-phosphorus insecticides (Robbins et
al., 1959). However, apparently no additional toxicity was produced in
rats when one-sixth as much pyrethrins was added to their diet
containing piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandegrift, 1952).
Short-term studies
Monkey. At comparable dosage, symptomatology was somewhat less
than that in dogs. Microscopical pathology of the liver in monkeys on
a dosage of 100 mg/kg/day was comparable to that in dogs receiving 30
mg/kg/day (a dosage that produced no observed effect in the monkey).
The apparent difference in the susceptibility of the species may be
explained by the shorter exposure of the monkey (1 month) compared
with the dogs (1 year) (Sarles & Vandegrift, 1952).
Dog. Dogs did not grow as fast as the controls when dosed at
the rate of 32 mg/kg/day for a year and lost weight when dosed at
rates of 106 mg/kg/day or higher. Even at 3 mg/kg/day, the dogs showed
some increase in liver weight and the increase was progressively
greater at higher dosage rates. The kidneys and adrenals were
progressively enlarged at dosages of about 100 mg/kg/day and above.
Microscopical pathology was evident in the liver at dosage rates of
about 30 mg/kg/day and over. Hepatoma and carcinoma did not occur in
the dog (Sarles & Vandegrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide
as high as 1000 ppm caused no decrease in the growth rate of female
rats; concentrations as low as 100 ppm produced some reduction in the
growth of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
subnormal food consumption (78% of control). A concentration of 25 000
ppm reduced food consumption to 37% of normal and stunted the animals.
However, in subacute experiments, anorexia was terminal and therefore
not the simple effect of unpalatability of the food. A concentration
of 10 000 ppm caused a distinct increase in mortality in both sexes
evident in 2 years and a concentration of 25 000 killed about half the
animals in half a year. Only concentrations of 10 000 ppm or higher
produced significant increase in the relative weight of the liver and
kidney. Some degree of liver pathology apparently occurred in all
groups of rats but was progressively more marked at dietary levels of
1000 ppm and more. Less marked changes occurred in the kidney and
adrenal. Benign or malignant tumours occurred in 30% of the test
animals but the authors claimed that their occurrence was not related
to piperonyl butoxide. Reproduction was decreased by a dietary level
of 10 000 ppm and stopped by a concentration of 25 000 ppm (Sarles &
Vandegrift, 1952).
Comments on the experimental work reported and evaluation
Sarles & Vandegrift (1952) made a distinction for the rat
between the ill-defined effects of 1000 ppm and the effects of 100
ppm, a level which they found to be "nontoxic". Furthermore, dogs
showed decreased growth and microscopical pathology of the liver at
about 30 mg/kg/day and some increase in liver weight at only 3
mg/kg/day.
The uses of piperonyl butoxide are such that only a small
portion of food would be expected to contain any. No report of actual
residues is available. Although there is no evidence that the
presently approved uses of piperonyl butoxide involve any danger there
is not enough information on the compound to allow the setting of an
acceptable daily intake figure for human beings.
Further work considered necessary
Determination of the nature and amount of the residues reaching
the consumer.
A level should be established that causes no significant effect
during long-term studies in at least 2 species. The question of
tumorigenicity should be re-explored, especially in rats. Biochemical
studies should be made on the qualitative and quantitative aspects of
metabolism of the compound.
REFERENCES
AOAC (1960) Official methods of Analysis of the Association of
Official Agricultural Chemists, ninth ed., Washington
AOAC (1963) J. Assoc. Offic. Agr. Chem., 46, 145
Chamberlain, R. H. (1950) Amer. J. Hyg., 52, 153
Jones, H. A. Ackermann, H. J. & Webster, M. E. (1952) J. Assoc.
Offic. Agr. Chem., 35, 771
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 12, 82
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 16, 3
McAlister, L. C. Jones, H. A. & Moore, D. H. (1947) J. econ. Ent.,
40, 906
Munday, W. H. (1963) J. Assoc. Offic. Agr. Chem., 46, 244
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ. Ent.,
52, 660
Sarles, M. P., Dove, W. E. & More, D. H. (1949) Amer. J. trop. Med.,
29, 151
Sarles, M. P. & Vandegrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
Wachs, H. (1947) Science, 105, 530
William H. L. & Sweeney, J. P. (1956) J. Assoc. Offic. Agr. Chem.,
39, 975
 Relevant physical and chemical properties
Piperonyl butoxide is a derivative of piperic acid. Its
synergistic activity is believed to be due to the presence of the
methylenedioxy group in the molecular structure. It is synthesized
from safrole and the butyl ether of diethylene glycol.
Piperonyl butoxide is quite stable, resistant to hydrolysis,
oxidation and exposure to sunlight. Strong bases up to 1N
concentration and weak acids will not affect it, but strong acids will
destroy it. For a more complete description of the physical and
chemical characteristics see Negherbon (1959).
Uses
Alone, piperonyl butoxide is a compound of only mediocre
insecticidal power. It acts as an effective synergist to increase the
toxicity; "knockdown" and persistence of pyrethrins and allethrin. The
synergist action is so pronounced that the resulting kill of insects
is much greater than that which can be produced by pyrethrins alone
(Wachs, 1947; McAlister et al., 1947). It does not synergize or
potentiate DDT or nicotine. There are some suggestions that piperonyl
butoxide will potentiate the toxicity of organic phosphate
insecticides to insects (Robbins et al., 1959). Rotenone, ryanodine
and benzene hexachloride are activated by piperonyl butoxide but to a
lesser degree than pyrethrins (Negherbon, 1959).
Residues
Residues do result from use on foods, but the information on this
subject is incomplete.
In the analysis of residues, extraneous materials extracted from
natural products develop dark brown to black colours when heated with
concentrated phosphoric acid. These colours interfere with the residue
determination by colorimetric end procedure. Each food presents
different problems in removing interfering substances. Four methods
have been published by the Association of Official Agricultural
Chemists. In wheat, pinto beans, Alaska beans hulled rice, oats and
barley a sensitivity of 20 mmg of piperonyl butoxide of 0.5 ppm can be
obtained (AOAC, 1960; AOAC, 1963; Munday, 1963).
A method is reported for flour, grain and oil base materials, but
details are given only for oil base materials, containing 0.25-0.75
mg/ml (Jones et al., 1952). A colorimetric method for application to
fats, waxes and oils is suitable for 50-80 mmg working range (Williams
& Sweeney, 1956).
Effect on treated crop
No information available.
BIOLOGICAL DATA
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract
with loss of appetite and prostration (Sarles et al., 1949). It may be
that these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949; Sarles &
Vandegrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandegrift, 1952).
In rats, large subcutaneous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandegrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects,
piperonyl butoxide synergizes pyrethrins by inhibiting lipase
(esterase), but his results were inconclusive.
In an experiment in which 87.6% of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09% was found in the
urine (Sarles & Vandegrift, 1952). So far as is known, the
colorimetric tests used responded to piperonyl butoxide only. Thus,
the 12% of the dose that was unaccounted for may have been the most
important part from a toxicological standpoint.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 4030 Negherbon, 1959
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of courmaphos (a triphosphate) and its phosphate by a factor
of 4 to 6 (Robbins et al., 1959).
There is some evidence that the piperonyl butoxide interferes
with detoxification of the organo-phosphorus insecticides (Robbins et
al., 1959). However, apparently no additional toxicity was produced in
rats when one-sixth as much pyrethrins was added to their diet
containing piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandegrift, 1952).
Short-term studies
Monkey. At comparable dosage, symptomatology was somewhat less
than that in dogs. Microscopical pathology of the liver in monkeys on
a dosage of 100 mg/kg/day was comparable to that in dogs receiving 30
mg/kg/day (a dosage that produced no observed effect in the monkey).
The apparent difference in the susceptibility of the species may be
explained by the shorter exposure of the monkey (1 month) compared
with the dogs (1 year) (Sarles & Vandegrift, 1952).
Dog. Dogs did not grow as fast as the controls when dosed at
the rate of 32 mg/kg/day for a year and lost weight when dosed at
rates of 106 mg/kg/day or higher. Even at 3 mg/kg/day, the dogs showed
some increase in liver weight and the increase was progressively
greater at higher dosage rates. The kidneys and adrenals were
progressively enlarged at dosages of about 100 mg/kg/day and above.
Microscopical pathology was evident in the liver at dosage rates of
about 30 mg/kg/day and over. Hepatoma and carcinoma did not occur in
the dog (Sarles & Vandegrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide
as high as 1000 ppm caused no decrease in the growth rate of female
rats; concentrations as low as 100 ppm produced some reduction in the
growth of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
subnormal food consumption (78% of control). A concentration of 25 000
ppm reduced food consumption to 37% of normal and stunted the animals.
However, in subacute experiments, anorexia was terminal and therefore
not the simple effect of unpalatability of the food. A concentration
of 10 000 ppm caused a distinct increase in mortality in both sexes
evident in 2 years and a concentration of 25 000 killed about half the
animals in half a year. Only concentrations of 10 000 ppm or higher
produced significant increase in the relative weight of the liver and
kidney. Some degree of liver pathology apparently occurred in all
groups of rats but was progressively more marked at dietary levels of
1000 ppm and more. Less marked changes occurred in the kidney and
adrenal. Benign or malignant tumours occurred in 30% of the test
animals but the authors claimed that their occurrence was not related
to piperonyl butoxide. Reproduction was decreased by a dietary level
of 10 000 ppm and stopped by a concentration of 25 000 ppm (Sarles &
Vandegrift, 1952).
Comments on the experimental work reported and evaluation
Sarles & Vandegrift (1952) made a distinction for the rat
between the ill-defined effects of 1000 ppm and the effects of 100
ppm, a level which they found to be "nontoxic". Furthermore, dogs
showed decreased growth and microscopical pathology of the liver at
about 30 mg/kg/day and some increase in liver weight at only 3
mg/kg/day.
The uses of piperonyl butoxide are such that only a small
portion of food would be expected to contain any. No report of actual
residues is available. Although there is no evidence that the
presently approved uses of piperonyl butoxide involve any danger there
is not enough information on the compound to allow the setting of an
acceptable daily intake figure for human beings.
Further work considered necessary
Determination of the nature and amount of the residues reaching
the consumer.
A level should be established that causes no significant effect
during long-term studies in at least 2 species. The question of
tumorigenicity should be re-explored, especially in rats. Biochemical
studies should be made on the qualitative and quantitative aspects of
metabolism of the compound.
REFERENCES
AOAC (1960) Official methods of Analysis of the Association of
Official Agricultural Chemists, ninth ed., Washington
AOAC (1963) J. Assoc. Offic. Agr. Chem., 46, 145
Chamberlain, R. H. (1950) Amer. J. Hyg., 52, 153
Jones, H. A. Ackermann, H. J. & Webster, M. E. (1952) J. Assoc.
Offic. Agr. Chem., 35, 771
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 12, 82
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 16, 3
McAlister, L. C. Jones, H. A. & Moore, D. H. (1947) J. econ. Ent.,
40, 906
Munday, W. H. (1963) J. Assoc. Offic. Agr. Chem., 46, 244
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ. Ent.,
52, 660
Sarles, M. P., Dove, W. E. & More, D. H. (1949) Amer. J. trop. Med.,
29, 151
Sarles, M. P. & Vandegrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
Wachs, H. (1947) Science, 105, 530
William H. L. & Sweeney, J. P. (1956) J. Assoc. Offic. Agr. Chem.,
39, 975
Relevant physical and chemical properties
Piperonyl butoxide is a derivative of piperic acid. Its
synergistic activity is believed to be due to the presence of the
methylenedioxy group in the molecular structure. It is synthesized
from safrole and the butyl ether of diethylene glycol.
Piperonyl butoxide is quite stable, resistant to hydrolysis,
oxidation and exposure to sunlight. Strong bases up to 1N
concentration and weak acids will not affect it, but strong acids will
destroy it. For a more complete description of the physical and
chemical characteristics see Negherbon (1959).
Uses
Alone, piperonyl butoxide is a compound of only mediocre
insecticidal power. It acts as an effective synergist to increase the
toxicity; "knockdown" and persistence of pyrethrins and allethrin. The
synergist action is so pronounced that the resulting kill of insects
is much greater than that which can be produced by pyrethrins alone
(Wachs, 1947; McAlister et al., 1947). It does not synergize or
potentiate DDT or nicotine. There are some suggestions that piperonyl
butoxide will potentiate the toxicity of organic phosphate
insecticides to insects (Robbins et al., 1959). Rotenone, ryanodine
and benzene hexachloride are activated by piperonyl butoxide but to a
lesser degree than pyrethrins (Negherbon, 1959).
Residues
Residues do result from use on foods, but the information on this
subject is incomplete.
In the analysis of residues, extraneous materials extracted from
natural products develop dark brown to black colours when heated with
concentrated phosphoric acid. These colours interfere with the residue
determination by colorimetric end procedure. Each food presents
different problems in removing interfering substances. Four methods
have been published by the Association of Official Agricultural
Chemists. In wheat, pinto beans, Alaska beans hulled rice, oats and
barley a sensitivity of 20 mmg of piperonyl butoxide of 0.5 ppm can be
obtained (AOAC, 1960; AOAC, 1963; Munday, 1963).
A method is reported for flour, grain and oil base materials, but
details are given only for oil base materials, containing 0.25-0.75
mg/ml (Jones et al., 1952). A colorimetric method for application to
fats, waxes and oils is suitable for 50-80 mmg working range (Williams
& Sweeney, 1956).
Effect on treated crop
No information available.
BIOLOGICAL DATA
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract
with loss of appetite and prostration (Sarles et al., 1949). It may be
that these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949; Sarles &
Vandegrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandegrift, 1952).
In rats, large subcutaneous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandegrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects,
piperonyl butoxide synergizes pyrethrins by inhibiting lipase
(esterase), but his results were inconclusive.
In an experiment in which 87.6% of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09% was found in the
urine (Sarles & Vandegrift, 1952). So far as is known, the
colorimetric tests used responded to piperonyl butoxide only. Thus,
the 12% of the dose that was unaccounted for may have been the most
important part from a toxicological standpoint.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 4030 Negherbon, 1959
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of courmaphos (a triphosphate) and its phosphate by a factor
of 4 to 6 (Robbins et al., 1959).
There is some evidence that the piperonyl butoxide interferes
with detoxification of the organo-phosphorus insecticides (Robbins et
al., 1959). However, apparently no additional toxicity was produced in
rats when one-sixth as much pyrethrins was added to their diet
containing piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandegrift, 1952).
Short-term studies
Monkey. At comparable dosage, symptomatology was somewhat less
than that in dogs. Microscopical pathology of the liver in monkeys on
a dosage of 100 mg/kg/day was comparable to that in dogs receiving 30
mg/kg/day (a dosage that produced no observed effect in the monkey).
The apparent difference in the susceptibility of the species may be
explained by the shorter exposure of the monkey (1 month) compared
with the dogs (1 year) (Sarles & Vandegrift, 1952).
Dog. Dogs did not grow as fast as the controls when dosed at
the rate of 32 mg/kg/day for a year and lost weight when dosed at
rates of 106 mg/kg/day or higher. Even at 3 mg/kg/day, the dogs showed
some increase in liver weight and the increase was progressively
greater at higher dosage rates. The kidneys and adrenals were
progressively enlarged at dosages of about 100 mg/kg/day and above.
Microscopical pathology was evident in the liver at dosage rates of
about 30 mg/kg/day and over. Hepatoma and carcinoma did not occur in
the dog (Sarles & Vandegrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide
as high as 1000 ppm caused no decrease in the growth rate of female
rats; concentrations as low as 100 ppm produced some reduction in the
growth of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
subnormal food consumption (78% of control). A concentration of 25 000
ppm reduced food consumption to 37% of normal and stunted the animals.
However, in subacute experiments, anorexia was terminal and therefore
not the simple effect of unpalatability of the food. A concentration
of 10 000 ppm caused a distinct increase in mortality in both sexes
evident in 2 years and a concentration of 25 000 killed about half the
animals in half a year. Only concentrations of 10 000 ppm or higher
produced significant increase in the relative weight of the liver and
kidney. Some degree of liver pathology apparently occurred in all
groups of rats but was progressively more marked at dietary levels of
1000 ppm and more. Less marked changes occurred in the kidney and
adrenal. Benign or malignant tumours occurred in 30% of the test
animals but the authors claimed that their occurrence was not related
to piperonyl butoxide. Reproduction was decreased by a dietary level
of 10 000 ppm and stopped by a concentration of 25 000 ppm (Sarles &
Vandegrift, 1952).
Comments on the experimental work reported and evaluation
Sarles & Vandegrift (1952) made a distinction for the rat
between the ill-defined effects of 1000 ppm and the effects of 100
ppm, a level which they found to be "nontoxic". Furthermore, dogs
showed decreased growth and microscopical pathology of the liver at
about 30 mg/kg/day and some increase in liver weight at only 3
mg/kg/day.
The uses of piperonyl butoxide are such that only a small
portion of food would be expected to contain any. No report of actual
residues is available. Although there is no evidence that the
presently approved uses of piperonyl butoxide involve any danger there
is not enough information on the compound to allow the setting of an
acceptable daily intake figure for human beings.
Further work considered necessary
Determination of the nature and amount of the residues reaching
the consumer.
A level should be established that causes no significant effect
during long-term studies in at least 2 species. The question of
tumorigenicity should be re-explored, especially in rats. Biochemical
studies should be made on the qualitative and quantitative aspects of
metabolism of the compound.
REFERENCES
AOAC (1960) Official methods of Analysis of the Association of
Official Agricultural Chemists, ninth ed., Washington
AOAC (1963) J. Assoc. Offic. Agr. Chem., 46, 145
Chamberlain, R. H. (1950) Amer. J. Hyg., 52, 153
Jones, H. A. Ackermann, H. J. & Webster, M. E. (1952) J. Assoc.
Offic. Agr. Chem., 35, 771
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 12, 82
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials of
U.S., 16, 3
McAlister, L. C. Jones, H. A. & Moore, D. H. (1947) J. econ. Ent.,
40, 906
Munday, W. H. (1963) J. Assoc. Offic. Agr. Chem., 46, 244
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ. Ent.,
52, 660
Sarles, M. P., Dove, W. E. & More, D. H. (1949) Amer. J. trop. Med.,
29, 151
Sarles, M. P. & Vandegrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
Wachs, H. (1947) Science, 105, 530
William H. L. & Sweeney, J. P. (1956) J. Assoc. Offic. Agr. Chem.,
39, 975
