
PIPERONYL BUTOXIDE
First draft prepared by
A. Moretto
Istituto di Medicina del Lavoro,
Universita degli Studi di Padova, Padua, Italy
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Studies with mixtures
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Piperonyl butoxide was previously evaluated toxicologically by
the JMPR in 1965, 1966, 1972, and 1992 (Annex I, references 3, 6, 18,
and 65). An ADI of 0-0.03 mg/kg bw was allocated in 1972 on the basis
of a one-year study in dogs. The 1992 JMPR confirmed the existing ADI
and recommended that piperonyl butoxide be reviewed again in 1995
after submission of studies on acute toxicity and teratogenicity in
rats, appropriate studies of genotoxicity, the results of an on-going
one-year study in dogs, an on-going study of carcinogenicity in mice,
and studies of carcinogenicity in rats and mice performed within the
US National Toxicology Program, and observations in humans.
Piperonyl butoxide was re-evaluated at the present Meeting within
the periodic review programme of the CCPR, and a monograph summarizing
all of the available data was prepared.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Male Swiss-Webster mice (18-20 g) were given piperonyl butoxide
labelled with 14C at either the alpha-methylene or the alpha-carbon
of the 2-(2-butoxyethoxy)ethoxymethyl side-chain by gavage at 1.7 mg
(5 µmol)/kg bw. Radiocarbon was determined in expired carbon dioxide
0.5, 1, 2, 4, and 6 h and every 6 h up to 48 h after treatment, and in
urine and faeces 12, 24, and 48 h after treatment. Two days after
administration of [methylene-14C]-piperonyl butoxide, 97.2% of the
radiolabel was recovered, with 75.5% in carbon dioxide, 6.1% in urine,
4% in faeces, and 6.8% in the carcass; after administration of
piperonyl butoxide labelled in the 2(2-butoxyethoxy)ethoxymethyl
side-chain, 75% of the radiolabel was recovered, with 65% in urine, 8%
in faeces, and < 1% in carbon dioxide. In the latter case, the
urinary metabolites were demethylated derivatives of the parent
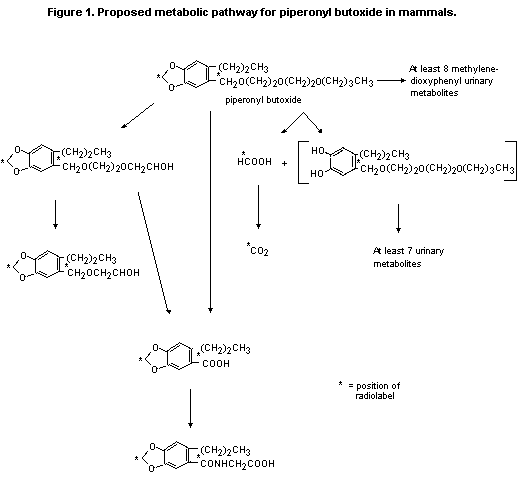
compound. Figure 1 shows the proposed metabolic pathway for piperonyl
butoxide in mammals (Kamienski & Casida, 1970).
In the same experiments, male Sprague-Dawley rats were given
3.4 mg (10 µmol)/kg bw labelled piperonyl butoxide by gavage. The
total label (in expired carbon dioxide and urine) recovered at 48 h
was 72-74%, and the pattern of excretion was similar to that observed
in the mouse (Kamienski & Casida, 1970).
Adult male Sprague-Dawley rats were given single intravenous
doses of piperonyl butoxide labelled with 14C in the methylenedioxy
or the alpha-methylene side-chain. Bile samples were collected from a
fistula, and 10 urine samples were taken at various intervals before
(one sample) and up to about 8 h after treatment; expired air was also
collected. More than 10 metabolites were detected, but not identified,
in urine and bile; the parent compound was identified only in fat
(9-18% of the total radiolabel) and lung (15-25%) after about 8 h.
After treatment with [14C-methylenedioxy]-piperonyl butoxide, about
40% of the label was recovered as carbon dioxide, < 1% in urine, and
3% in bile. After treatment with [14C-alpha-methylene]-piperonyl
butoxide, 25-47% of the label was found in the bile and about 5% in
urine; almost none was detected in expired air. The peak amount of
label was detected in bile < 30 min after injection with either
compound and in urine about 25 h later; significant amounts were
present in both urine and bile about 8 h later (Fishbein et al.,
1969).
A series of studies were performed on young male Charles River CD
rats with piperonyl butoxide labelled with 14C on the alpha-carbon
of the 2-(2-butoxyethoxy)ethoxymethyl side-chain. Four rats were given
a single dose of about 500 mg/kg bw (15.6 ± 0.4 µCi) by gavage. An
average of 0.18% of the administered radiolabel was expired as carbon
dioxide during the 24 h after treatment. Four rats were given a single
dose of about 500 mg/kg bw (14.4 ± 0.7 µCi) by gavage; blood samples
were then collected from the tail vein for 24 h and analysed for
radioactivity. Plasma radioactivity reached a peak 3-12 h after
treatment and dropped to about half the peak level within 24 h. Four
rats were given a single dose of about 500 mg/kg bw (14.1 ± 1.3 µCi)
by gavage; urine and faecal samples were collected and weighed 4, 8,
12, and 24 h after treatment and then every 24 h for seven days. Most
of the radiolabel was recovered in urine and faeces 12-2.4 h after
treatment; by 168 h after treatment, an average of about 38% of the
administered radioactivity was recovered in urine and 62% in faeces.
Twenty rats were given a single dose of about 500 mg/kg bw by
gavage; groups of five rats were killed 1, 6, 24, 48, and 168 h after
treatment, and several tissues were examined for radioactivity. At
each interval, the highest levels of radiolabel were found in the
gastrointestinal tract and its contents; high levels were also found
in lungs, liver, kidneys, fat, prostate, and seminal vescicles. At 1,
6, 24, 48. and 168 h, 62, 67, 37, 13, and 1%, respectively, of the
administered radiolabel was recovered. Five rats received piperonyl
butoxide at about 500 mg/kg bw per day for 13 days and then a single
dose of about 500 mg/kg bw of radiolabelled compound (9.6 ± 0.2 µCi)
by gavage. Most of the radiolabel was recovered in urine and faeces
12-48 h after treatment. By 168 h after treatment, an average of about
43% of the radiolabel was recovered in urine and 54% in faeces
(Selim, 1985).
(b) Effects on enzymes and other biochemical parameters
Many studies have been performed in vitro and in vivo to
elucidate the mechanism of action of piperonyl butoxide. A single
intraperitoneal injection of 450 mg/kg bw inhibited microsomal
mixed-function oxidases in the livers of male Swiss-Webster mice; the
duration and extent of inhibition depended on the substrate used
(Skrinjaric-Spoljar et al., (1971).
In male Swiss mice given a single intraperitoneal dose of
160 mg/kg bw of piperonyl butoxide, 50-60% inhibition of dimethyl-
aminopyrine and hexobarbital hydroxylases was found 1 h after
treatment (Jaffe et al., 1968).
 A dose-dependent, biphasic effect was observed on the ortho and
para hydroxylations of biphenyls by liver microsomes of mice treated
with 10-640 mg/kg bw of piperonyl butoxide intraperitoneally,
para-Hydroxylation was inhibited by 80% at the highest dose 30 min
after treatment but had recovered within 24 h; ortho-hydroxylation
was induced by up to 150% 1 h after treatment, and activity was still
elevated after 96 h in the groups at the highest dose (Jaffe et al.,
1969).
Piperonyl butoxide inhibits ethylmorphine N-demethylation by
liver microsomes in vitro. The inhibition is proportional to the
amount of cytochrome P450-piperonyl butoxide metabolite (unknown)
complex formed. Differences between liver microsomes from rats and
mice may account for the greater sensitivity of mice to inhibition of
drug metabolism by piperonyl butoxide: the Ki for inhibition of
ethylmorphine N-demethylase was found to be 19 µmol/litre in rats and
6 µmol/litre in mice (Franklin, 1972).
Mouse liver homogenates (20% w/v in 50 mmol/litre phosphate
buffer, pH 7.4) were fractioned into nuclear, mitochondrial,
microsomal, and soluble fractions, which were incubated with labelled
piperonyl butoxide. NAD, NADP, NADH, or NADPH was sometimes added as a
cofactor. Only the microsomal fraction had significant metabolic
activity in the presence of NADPH and, to a lesser extent, with NADH
(Kamienski & Casida, 1970).
Male CF-1 mice were given a single intraperitoneal dose of
piperonyl butoxide (purity, 87-89%) at 0.5-25 mg/kg bw 1 h before
intraperitoneal administration of 40 mg/kg bw pentobarbital or
100 mg/kg bw zoxazolamine. Pentobarbital-induced sleeping time was
significantly increased by 10 or 25 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 5,
10, or 25 mg/kg bw (Conney et al., 1972). Skinjaric-Spoljar et al.
(1971) found that a single intraperitoneal dose of 22 mg/kg bw
piperonyl butoxide to mice increased the hexobarbital (62.5 mg/kg bw
intraperitoneally) sleeping time from 28 to 100 min.
Male Sprague-Dawley rats were given single intraperitoneal doses
of piperonyl butoxide (purity, 87-89%) at 67-1000 mg/kg bw 1 h before
intraperitoneal administration of pentobarbital (25 mg/kg bw) or
zoxazolamine (70 mg/kg bw). Pentobarbital-induced sleeping time was
significantly increased by 1000 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 333
or 1000 mg/kg bw (Conney et al., 1972).
Male mice and rats were given single intraperitoneal doses of
piperonyl butoxide 1 h before injection of 200 mg/kg bw antipyrine.
The NOAELs for effects on antipyrine metabolism were 100 mg/kg bw in
rats and 0.5 mg/kg bw in mice (Conney et al., 1972).
Groups of six male weanling Sherman rats were fed technical-grade
piperonylbutoxide (purity, 80%) at dietary concentrations of 0, 1000,
5000, or 10 000 ppm for one, four, or eight weeks. The activities of
hexobarbital oxidase, aniline hydroxylase, para-nitroanisole
demethylase, nitroreductase, and glucuronyltransferase and the P450
content were increased two- to fourfold by 5000 or 10 000 ppm
piperonyl butoxide. Liver weights and microsomal protein content were
increased to a maximum of 50-70%. Electron microscopy showed
enlargement and extensive proliferation of the smooth endoplasmic
reticulum in liver parenchymal cells. The dose of 1000 ppm had minimal
effects on liver weight and on P450 and glucuronyl transferase
activity and no effect on proliferation of the smooth endoplasmic
reticulum. Maximal effects on P450 content and on the activity of
P450-related enzymes were observed after one week of treatment. The
maximal effect on glucuronyltransferase activity was observed between
four and eight weeks of treatment (Goldstein et al., 1973).
Piperonyl butoxide (purity unsepcified) dissolved in corn oil was
administered to 10 male Sprague-Dawley rats at 400 mg/kg bw
intraperitoneally; another group of animals received 100 mg/kg bw of
piperine intraperitoneally. Control animals received 2 ml/kg bw corn
oil. Groups of five animals were sacrificed 1 and 24 h after
treatment, and hepatic levels of cytochromes P450 and b5, proteins,
and the activities of NADPH cytochrome c reductase, benzphetamine
N-demethylase, aminopyrine N-demethylase, and aniline hydroxylase
were determined. The microsomal protein level was not affected by
treatment. Piperonyl butoxide decreased the P450 but not the b5 level
by about 30% after 1 h; however, after 24 h the contents were
increased by about 100 and 30%, respectively. NADPH cytochrome c
reductase activity was increased by 50% 24 h after treatment. The
activities of N-demethylases and aniline hydroxylase were decreased
by 20 and 50%, respectively, 1 h after administration of either
piperine or piperonyl butoxide. By 24 h, the activities were still low
in piperine-treated animals but had increased by 30-80% in those given
piperonyl butoxide (Dalvi & Dalvi, 1991).
Single intraperitoneal doses of 400 mg/kg bw piperonyl butoxide
were given to male CD-1 mice 1 h before a single intraperitoneal dose
of parathion-methyl, azinphos-methyl, parathion, or azinphos-ethyl, or
their oxygen analogues. The LD50 of the oxygen analogues was not
altered whereas that of the methyl homologues was reduced, with a
40-fold increase in the LD50 for parathion-methyl and a threefold
increase in that for azinphos-methyl; that of the ethyl homologues was
increased, with a 50% decrease in the LD50 for parathion and an 85%
decrease for azinphos ethyl. The plasma levels of all pesticides were
increased by three- to sevenfold in piperonyl butoxide-pretreated
animals 30 min after treatment (Levine & Murphy, 1977a). It has been
suggested that detoxification of parathion-methyl, but not that of
parathion- or azinphos-ethyl, continues through uninhibited
glutathione-dependent pathways (Mirer et al., 1977; Levine & Murphy,
1977b).
A single intraperitoneal dose of 600 mg/kg bw piperonyl butoxide
to three-month-old CD-1 mice reduced the hepatotoxicity (as assessed
by reduced glutathione content, plasma sorbitol dehydrogenase, and
histopathological liver necrosis) caused by an oral dose of 600 mg/kg
bw acetominophen given 2 h earlier or 1 h later. Since the hepatic
mixed-function oxidase system generates a toxic, electrophilic
metabolite of acetominophen, piperonyl butoxide probably acts by
inhibiting: that enzymatic system (Brady et al., 1988).
Pretreatment of groups of 10 male Syrian golden hamsters with
400 mg/kg bw piperonyl butoxide given subcutaneously 2 h before
subcutaneous injection of 17.8 mg/kg bw N-nitrosodiethylamine twice
weekly for 20 weeks inhibited the development of pulmonary
carcinogenesis by the nitrosamine. The numbers of animals with lung
tumors were 0 controls, 6 treated only with N-nitrosodiethylamine, 0
treated with piperonyl butoxide and nitrosamine, and 0 given only
piperonyl butoxide. Tracheal tumors were found in 0, 10, 5, and 0
animals in the four groups, respectively. The protective effect was
associated with reduced (by 50% or more) covalent binding of
N-[ethyl-1-14C]-nitroso-diethylamine to macromolecules in trachea,
lung, and liver (Schuller & McMahon, 1985).
Eight-week-old male CD-1 mice were fed diets containing piperonyl
butoxide at concentrations adjusted to provide doses of 0, 10, 30,
100, or 300 mg/kg bw per day or sodium phenobarbital at 0.05% (w/w)
for 42 days. Replicative DNA synthesis was studied by implanting
seven-day osmotic pumps to administer 5-bromo-2'-deoxyuridine (BrDU)
during days 0-7 and 35-42 of treatment; liver sections were
immunostained with an antibody to BrDU, and the percentage of
hepatocyte nuclei undergoing replicative DNA synthesis was calculated
by microscopic examination of at least 1000 nuclei. Xenobiotic
metabolism was assessed by measuring the liver P450 content and the
activities of 7-ethoxyresorufin O-deethylase, 7-pentoxyresorufin
O-depentylase, and ethylmorphine N-demethylase in liver
microsomes. Sodium phenobarbital was used as a positive control. The
relative liver weight was increased by about 15% after seven days of
treatment with 300 mg/kg bw per day of piperonyl butoxide and by about
20% with sodium phenobarbital; after 42 days, it was increased by
about 10% after treatment with 100 and by about 20% after treatment
with 300 mg/kg bw piperonyl butoxide. Midzonal liver-cell hypertrophy
was observed in mice given 300 mg/kg bw per day for seven or 42 days
or 100 mg/kg bw per day for 42 days. Sodium phenobarbital induced
centrilobular hypertrophy after either seven or 42 days of treatment.
Replicative DNA synthesis was induced to 350% by the high dose of
piperonyl butoxide and to 825% by sodium phenobarbital for seven days;
no significant increase was observed after 42 days at any dose. A
dose-related increase in microsomal protein and P450 content was
observed on day 42, and the increases were statistically significant
at doses > 100 mg/kg bw. Inconsistent increases were observed in
the activities of 7-ethoxyresorufin O-deethylase and 7-pento-
xyresorufin O-depentylase in piperonyl butoxide-treated mice, but a
dose-related increase from 20 to 50% in the activity of ethylmorphine
N-demethylase was seen on day 42. Treatment with sodium pheno-
barbital for 42 days resulted in increases in all of the measured
parameters. Piperonyl butoxide (and sodium phenobarbital) thus caused
a transient stimulation of liver-cell replication and a permanent
effect on liver weight, morphology, and enzyme induction at doses that
induced liver nodules in long-term studies (Phillips el al., 1995).
2. Toxicological studies
(a) Acute toxicity
Groups of five Sprague-Dawley CD rats of each sex were exposed by
inhalation for 4 h to a mean analytical concentration of 5.9 mg/litre
piperonyl butoxide (purity, 90.78%). The particle median aerodynamic
diameter was 2.6 µm; about 15% of the aerosol was < 1 µm in diameter
and about 95% < 10 µm. All animals survived the exposure and the
15-day observation period after treatment. Exposure caused excessive
lacrimation and salivation, nasal discharge and laboured breathing.
All animals recovered. No remarkable signs were seen post mortem
(Hoffman, 1991). Other data are reported in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were fed
diets containing 0, 1000, 3000, or 9000 ppm piperonyl butoxide (purity
unspecified) for 20 days. Animals were observed daily for mortality;
body weight was measured and clinical observations were made on days
1, 2, 3, 7, 14, and 20; the food consumption of five animals per cage
was measured on days 0-3, 3-7, 7-14, and 14-20. At the end of
treatment, blood clinical chemistry was assessed, liver, kidneys, and
spleen were weighed, and livers and kidneys were examined
histologically. No deaths occurred. The body weights of animals at the
high dose were about 15% lower than those of controls at the end of
the study, and those of females at the middle dose were about 10%
lower on day 2 and about 8% lower at termination (not statistically
significant). The lowered body weights were apparently associated with
decreased food intake. The weights of livers showed a dose-related
increase in both males (by 22% at the low dose, 63% at the middle
dose, and 79% at the high dose) and females (62% at the middle dose
and 78% at the high dose); at the high dose, the weights of the
kidneys were reduced by 29% in males and 18% in females, and the
weights of the spleen by 25% in males and 33%, in females. In animals
at the high dose, a 31% increase in serum cholesterol was seen in
males and a 67% increase in females, a 28% increase in serum
phospholipids was seen in males and a 38% increase in females, a 19%
Table 1. Acute toxicity of piperonyl butoxide in animals
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse NR Oral 4.0 NR Negherbon (1959)
Rat Male Oral 4.57 90.78 Gabriel (1991a)
Female 7.22
NR Oral 8.0-10.6 NR Sarles et al. (1949)
NR Oral 13.5 NR Lehman (1948)
NR Oral 11.5 NR Lehman (1951)
Male, female Inhalation (4 h) > 5900 90.78 Hoffman (1991)
NR Subcutaneous > 15.9 NR Sarles et al. (1949)
Rabbit Male, female Dermal > 2 90.78 Gabriel (1991b)
NR Oral 2.7-5.3 NR Sarles et al. (1949)
Cat NR Oral > 10.6 NR Sarles et al. (1949)
Dog NR Oral > 8.0 NR Sarles et al. (1949)
NR, Not reported
increase in total serum proteins occurred in males and an 18% increase
in females, and a 235% increase in gamma-glutamyl transpeptidase was
seen in females. Some of these levels were also increased in the
animals at the middle dose. Microscopic examination of the liver
showed hypertrophic hepatocytes, single-cell necrosis, and cell
infiltration in the centrilobular area in all mice at the high dose.
The kidneys were unremarkable. The NOAEL for effects on the liver was
1000 ppm, equivalent to 150 mg/kg bw per day (Fujitani et al.,
1993a).
Groups of 20 Crj:CD-1 male mice were fed diets containing 0,
1500, 3000, or 6000 ppm piperonyl butoxide (purity unspecified) for
seven weeks. Food intake was measured every week and behavioural tests
were performed at weeks 4 (exploratory behaviour), 6 (multiple water
T-maze), and 7 (exploratory behaviour) of treatment. A treatment-
related reduction in food intake was observed in animals at the middle
and high doses during the first week of treatment. No consistent,
significant effect was observed in the T-maze test or in the
exploratory behaviour test at week 4. At week 7, some parameters in
the exploratory behaviour test were altered (Tanaka, 1993).
Rats
Hypertrophy of periportal cells with slight fatty changes were
observed in rats fed a diet containing 5000 ppm piperonyl butoxide for
17 weeks (Lehman, 1952a,b).
Feeding of six weekly doses of up to 224 mg/kg bw of active
ingredient to rats caused no observable effects at autopsy three weeks
after the final dose, but few details were given (Sarles et al.,
1949).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were fed diets containing concentrations of
piperonyl butoxide adjusted to obtain doses of 0, 62.5, 125, 250, 500,
1000, or 2000 mg/kg bw per day for four weeks. All animals were
observed for mortality and morbidity twice daily, and body weight and
food consumption were measured weekly. Haematological and biochemical
tests were performed on day 24 or 25 of treatment. At termination,
survivors were necropsied, certain organs were weighed and microscopic
examinations were performed on several organs. Six animals died before
termination, but the deaths were considered to be due to anaesthesia
for bleeding. General signs of toxicity, such as prominent backbone,
thinness, poor fur condition, brown staining and piloerection, were
observed in animals at the highest dose. Body-weight gain was reduced
by about 20% in females at 500 mg/kg bw per day, by 27% in males and
37% in females at 1000 mg/kg bw per day, and by 66% in males and 92%
in females at 2000 mg/kg bw per day. These reductions were only
partially associated with reduced food intake. Haematology and
clinical biochemistry showed no significant alterations. Absolute and
relative liver weights were increased in animals given doses >
500 mg/kg bw per day; the relative liver weights were also increased
in males at 250 mg/kg bw per day. The relative weights of the
adrenals, kidneys, and brain were slightly increased in the group at
the highest dose and in some animals at 1000 mg/kg bw per day.
Eosinophilia and loss of vacuolation in hepatocytes were noted in all
treated animals, and the severity of these lesions was dose-related.
These may be adaptive changes. Necrosis of hepatocytes and cytoplasmic
inclusions were observed in animals at 1000 and 2000 mg/kg bw per day.
The NOAEL was 125 mg/kg bw per day on the basis of effects on the
liver (Modeweg-Hansen et al., 1984).
In a study preliminary to the study of carcinogenicity by the
same authors summarized below, groups of 10 Fischer 344/DuCrj rats of
each sex were fed diets containing 0, 2500, 5000, 10 000, 20 000, or
30 000 ppm piperonyl butoxide (purity, 89%) for 13 weeks. At
termination, survivors were necropsied, and limited microscopic
examination was performed. One male at the high dose died before
termination. The final body weights and body-weight gains were reduced
in a dose-related manner in all treated males; in females, a
significant effect was seen only at the two highest doses. Liver
weights were increased in all treated animals, by up to 50% in males
and 108% in females. Absolute kidney weights were increased in animals
at the high dose, and the relative weight was also increased in groups
at lower doses. Hepatocyte hypertrophy and focal necrosis were seen in
animals at higher doses (data not shown). No relevant macro- or
microscopic lesions were observed in the digestive tract, but the
caecum was not examined histologically. There was no NOAEL because of
effects on the liver at all doses (Maekawa et al., 1985).
Groups of 15 Charles River CD rats of each sex were exposed by
inhalation for 6 h per day, five days per week, for 13 weeks to
piperonyl butoxide (purity, 90.78%) at mean analytical concentrations
of 15, 74, 155, or 512 mg/m3. The highest concentration was the
maximum that could be generated at the appropriate particle size.
Fifteen controls were exposed to conditioned room air only. The
concentrations in chamber air were monitored gravimetrically four
times per day and by gas chromatography once a day. Particle size
distribution was measured once during each exposure. The test aerosol
had a mass median aerodynamic diameter of 1.7 µm with a geometric
standard deviation of 2.6. On average, 37% of the particles were <
10 µm in diameter and 29% < 1 µm. Abnormal signs were looked for once
during each exposure, and detailed physical examinations were
conducted on all animals once before treatment and weekly during
treatment. Ophthalmoscopic examinations were performed on all animals
before treatment and on the day before sacrifice. Body weight was
recorded before exposure, immediately before exposure on test day 1,
weekly thereafter, and just before sacrifice. At termination,
haematological and blood chemical parameters were evaluated in all
animals, selected organs were weighed, complete gross post-mortem
examinations were conducted, and selected tissues from all animals
were examined microscopically.
Body-weight gain and food consumption were not affected by
exposure, and all animals survived to the end of treatment.
Ophthalmoscopic examinations showed no indication of exposure-related
ocular effects. There was a dose-related increase in nasal discharge,
dried material on the facial area and extremities, and anogenital
staining in animals at 155 and 512 mg/m3. The serum levels of
aspartate and alanine aminotransferases and of glucose were slightly
decreased (10-28%), while blood urea nitrogen, total protein, and
albumin levels were slightly increased (4-10%) in animals of each sex
at the high dose. Not all of these differences were statistically
significant, and a dose-response relationship was not seen
consistently; however, a similar trend was seen in animals of each
sex. Statistically significant increases in the absolute and relative
weights of the livers and kidneys were seen in animals at the high
dose; the relative kidney weights were increased by 10-12%, and the
absolute and relative liver weights were both increased by 20-29%.
Relative liver weights were also significantly elevated (8-9%) in
males and females at 155 mg/m3. Vesiculation and vacuolation of the
hepatocellular cytoplasm was seen in almost all treated animals but
tended to be more pronounced in those at the highest level. No clear
dose-related increase in either incidence or severity was observed.
Squamous or squamoid metaplasia of the pseudostratified columnar
epithelium of the larynx was seen in several exposed animals and one
control female, the severity being greater in animals at the high
dose. Similar metaplastic changes were seen in the columnar epithelium
lining the ventral diverticulum in several animals at 512 mg/m3 and
in one female at 15 mg/m3. Hyperplasia and hyperkeratosis of the
squamous epithelium normally found in the larynx were seen in a small
number of animals at 512 mg/m3. Laryngeal mucosal inflammation was
seen in all treated animals and was slightly more severe in animals at
the highest dose. All of the changes were considered to be localized
responses indicative of irritation rather then systemic toxicity.
Other findings in tissues and organs examined macroscopically and
microscopically post mortem were unremarkable. No systemic toxicity
was seen at 155 mg/m3; effects on the liver and kidney were seen at
512 mg/m3 (Newton, 1992).
Groups of 10 male and 10 female Fischer 344/DuCrj rats were fed
diets containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide
(technical grade of unspecified purity) for 13 weeks. Animals were
observed daily for mortality; body weight and clinical signs were
monitored daily for the first five days and then twice weekly. Food
and water consumption was measured on days 4, 11, 18, and 39 for about
six rats in each group. At the end of treatment, haematological and
blood chemical parameters were determined, animals were necropsied,
and selected organs were weighed; the liver and kidneys were examined
histologically. There were no deaths. Clinical signs consisted of nose
bleeds from about day 2 to day 20 and dose-dependent abdominal
distension. In animals at the high dose, food consumption was reduced
by about 46% and water consumption by 28% on day 4 but not at other
times. The body weights of animals at the high dose were significantly
reduced, by 36% in males and 24% in females. Blood haemoglobin levels
were decreased in a dose-dependent manner, but the decrease was
statistically significant only in animals at the high dose (by 10-11%)
and females at the middle dose (by 7%). A reduced mean corpuscular
haemoglobin content was also observed in females at the high dose.
Serum albumin was increased by 16-23%, cholesterol by 88-93%,
gamma-glutamyl transpetidase activity to five to six times the control
values in males at the high dose, and urea nitrogen by 24% in males at
that dose. Serum protein was increased in all treated females and
serum phospholipid in females at the high dose; decreases were seen in
serum levels of triglyceride in all males and bilirubin and glucose in
males at the high dose. Absolute and relative liver weights were
increased in a dose-related manner, but were significantly increased
only in males at the middle dose (by 47%) and males and females at the
high dose (by 57-94%). Relative kidney weights were increased in a
dose-related manner to up to 1.42% of control values. Hypertrophic
hepatocytes containing a basophilic, fine granular substance were seen
in animals at the high dose. In the periportal area, marked
vacuolation of hepatocytes was observed, with occasional coagulative
necrosis and oval-cell proliferation. In male rats, atrophy of the
epithelium of the proximal convoluted tubules was observed in the
renal cortex. There was no NOAEL because of effects on the liver and
kidney (Fujitani et al., 1992).
In a subsequent study performed under the same experimental
conditions, haematological, clinical chemical, and morphological
observations after 1, 2, 4, or 12 weeks of treatment showed that all
of the alterations occurred in a time-dependent manner, and that some
of them (increased liver and kidney weights, morphological alterations
in the liver) were already present after one week (Fujitani et al.,
1993b).
Rabbits
Three weekly doses of up to 108 mg/kg bw active ingredient of a
5% emulsion of piperonyl butoxide showed no effects at autopsy three
weeks after the final dose, but few details were given (Sarles
et al., 1949).
Dogs
Groups of one to three dogs of each sex were given piperonyl
butoxide at 0, 3, 32, 106, or 320 mg/kg per day orally in capsules on
six days per week for one year. All dogs at the two highest doses lost
weight, but large variations in body-weight gain and the small number
of animals prevented meaningful comparisons between the controls and
animals at the lower doses. A dose-related increase in relative liver
weight (no statistical analysis reported) was observed, which was
associated with unspecified histopathological changes. Increased
kidney and adrenal weights were observed at 100 and 320 mg/kg bw per
day (Sarles & Vandergrift, 1952).
Groups of two beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for eight weeks at concentrations
corresponding to 500, 1000, 2000, or 3000 ppm active ingredient. The
diets were prepared weekly; the actual concentrations and the
homogeneity of the mixture were measured at three intervals and found
to be satisfactory. Animals were observed at least twice daily for
mortality and signs of toxicity; detailed observations were recorded
at least once weekly, and body weight and food consumption were
recorded weekly. Physical examinations and haematological and
biochemical tests were conducted on all animals before exposure and at
termination. All major tissues and organs were examined
microscopically post mortem. Selected organs were weighed. All
animals survived to study termination. Reduced body-weight gains were
seen in males and females receiving 1000 ppm (14 and 9% of the initial
body weight, respectively), 2000 ppm (6 and 7%), and 3000 ppm (7 and
4%) ppm in comparison with the control group (17% and 14% increases).
Decreased food consumption (by 19 and 23%) was observed animals at
3000 ppm. There were no treatment-related changes in haematological
parameters. Males and females at 2000 and 3000 ppm had alkaline
phosphatase activities that were about 1.5 times the control value and
increased absolute and relative liver and gall-bladder weights. No
macroscopic changes were observed that could be attributed to
treatment, and treatment-related microscopic changes were limited to
diffuse, mild hypertrophy of hepatocytes in all treated males and in
females at the two highest doses. There was no NOAEL because of
effects on the liver (Goldenthal, 1993a).
Groups of four beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for one year at concentrations
corresponding to 100, 600, and 2000 ppm active ingredient. The diets
were prepared weekly and stored at room temperature. The stability of
the compound was assessed twice: an initial test showed a loss of
about 10% over 10 days, possibly due to technical problems; a second
test showed 97-101% of the initial concentration on day 10. The actual
concentrations were measured 16 times and were found to be 87-110% of
the nominal concentrations. The homogeneity of the mixture was also
found to be satisfactory. The dogs were observed at least twice daily
for mortality and signs of toxicity; detailed observations were
recorded weekly. Body weight and food consumption were recorded weekly
for the first 14 weeks and every two weeks thereafter. Ophthalmoscopic
examinations were conducted before the beginning of treatment and at
termination. Physical examinations were conducted on all animals
before exposure and after 3, 6, 9, and 12 months of treatment.
Haematological and biochemical tests and urinalyses were conducted on
all animals after 6 and 12 months. All animals were examined
post mortem, and all major tissues and organs were examined
microscopically. Selected organs were weighed. All animals survived to
the end of the study, with no relevant clinical or ophthalmological
signs. The body weights of males and females receiving 2000 ppm
piperonyl butoxide were 2% lower to 3% higher than the initial
weights, whereas those of controls were 22-25% higher. Treatment-
related decreases in food consumption were observed in males at 600
(by 15%) and 2000 (by 20%) ppm. Small, not statistically significant,
not dose-related decreases in food consumption were observed in
treated females.
No treatment-related change in haematological parameters was
observed. The activity of alkaline phosphatase was increased to three
to five times the control values in animals at 2000 ppm after 6 and 12
months, and cholesterol levels were decreased (not statistically
significant) at these intervals in females. Males and females at this
dose had increased absolute (by 22 and 36%) and relative (by 52 and
86%) liver and gall-bladder weights, and small increases in thyroid
and parathyroid weights (average, 34%) were observed in females. These
changes were not associated with microscopic changes in the thyroid
and were considered to be of questionable biological significance. No
macroscopic pathological changes were observed that could be
attributed to treatment. Treatment-related histopathological changes
were limited to diffuse, mild hypertrophy of the hepatocytes in males
and females at 2000 ppm. The NOAEL was 100 ppm, equal to 16 mg/kg bw
per day, on the basis of effects on the liver, some clinical chemical
alterations, and reduced body-weight gain at 2000 ppm (Goldenthal,
1993b).
Monkeys
Two African green monkeys were given 32 or 106 mg/kg bw per day
piperonyl butoxide orally for six days per week for four weeks.
Minimal microscopic changes were observed in the livers (Sarles &
Vandergrift, 1952).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 (C57Bl/6 × C3H/Anf)F1 and (C57Bl/6 × AKR)F1 mice of
each sex received piperonyl butoxide (purity unspecified) at 100 mg/kg
bw or 'Butacide' at 464 mg/kg bw in 0..5% gelatin at seven days of age
by stomach tube and the same amount (not adjusted for increasing body
weight) daily up to four weeks of age; subsequently, the mice were fed
diets containing 300 ppm. The dose was reported to be the maximal
tolerated dose for infant and young mice. All animals were killed when
they were about 70 weeks of age, and their tumour incidences were
compared with those of 79-90 necropsied mice of each sex and strain,
which had either been untreated or had received gelatine only. No
significant difference in the incidence of tumours was found between
treated and control mice, although the authors concluded that
additional evaluation was required (Innes et al., 1969). This study
was not considered to be adequate by the Meeting.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for 30 weeks;
the doses were decreased to 500 or 2000 ppm for the following 82
weeks, due apparently to a greater than expected decrease in
body-weight gain. The control group consisted of 20 males and 20
females. Animals were observed twice daily for signs of toxicity; body
weight was recorded at least once per month. Necroscopic examination
was performed at termination on all moribund animals and on those
found dead, unless precluded by autolysis. Major tissues and organs
and all gross lesions were examined microscopically. At termination,
85% of control males and 75% of control females, 82% and 68% at the
low dose, and 84% and 70% at the high dose, respectively, were still
alive. Body weight was reduced in a dose-related manner, by up to
about 15% in females at the high dose at termination. No
treatment-related increase in tumour incidence was found, but
hepatocellular carcinomas occurred frequently, especially in males,
with incidences of 50% in controls, 34%, at the low dose, and 40% at
the high dose. No statistically significant differences were found in
the incidences of neoplastic and non-neoplastic lesions (US National
Cancer Institute, 1979)
Groups of 60 CD-1 mice of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) at concentrations adjusted to give
doses of 0 (two control groups), 30,100, or 300 mg/kg bw per day, for
78 weeks. The diets were prepared weekly and the concentrations
adjusted on the basis of body weights and food consumption. The diets
were analysed for the actual concentration, homogeneity, and stability
of piperonyl butoxide. The average actual concentrations at the low
and high doses were 99 and 95%. The homogeneity and stability (no
decrease in concentration after 14-21 days) of piperonyl butoxide in
the diet were considered to be satisfactory. Animals were observed
twice daily for signs of toxicity. Food consumption and body weight
were determined weekly for the first week and then every other week.
Haematological measurements were made for 10 rats of each sex in the
control and high-dose groups at week 52 and for all groups at
termination. All surviving animals were necropsied; major tissues and
organs from controls and animals at the high dose and the lungs,
liver, kidneys, and gross lesions from other groups were examined
microscopically. All slides from the livers underwent an independent
pathological review; the results reported here are the consensus or
majority opinions of all the pathologists involved.
No treatment-related clinical signs or palpable masses were
observed. The mortality rates were (males/females) 27/32, 32/27,
27/38, 22/33, and 40/18% in the first control group, animals at 30,
100, and 300 mg/kg bw per day, and the second control groups,
respectively. Body weights and body-weight gains of animals at the
high dose were slightly (occasionally statistically significantly)
decreased. No significant effects on food consumption were observed.
Haematological parameters were also unaffected by treatment. A
dose-related increase in absolute and relative liver weights was
observed in mice of each sex at the middle and high doses.
Hepatocellular hypertrophy was more frequent in males at the high
(72%) and middle doses (27%) than in controls (10 and 18%) and in
females at the high dose (15%; 0 and 7% in controls). Hepatocellular
hyperplasia (also called eosinophilic/clear-cell focus), characterized
by aggregates of hepatocytes with a swollen eosinophilic cytoplasm,
was present in 8% of males at the high dose, 2% of females at the
middle dose, and 7% at the high dose. The incidences of hepatocellular
hyperplasia were 0 and 3% in control males and 0% in both female
control groups. The incidence of hepatocellular adenomas was higher in
male mice at the middle (37%) and high (47%) doses than in either
control group (15 and 13%) and in females at the high dose (17%; 3% in
controls). The hepatocellular adenomas observed in many treated
animals were composed of large, polyhedral, densely-packed cells with
abundant granular eosinophilic cytoplasm. This appearance is different
from that of spontaneous adenomas found in CD-1 mice, where small to
medium, well-differentiated, basophilic cells, distributed in solid to
normal sinusoidal patterns, are found. Only the incidence of the
former type of adenoma was increased in piperonyl butoxide-treated
mice. A nonsignificantly increased incidence of hepatocellular
carcinomas was observed in males at the high dose (12%; 3% in
controls). No other treatment-related neoplastic or non-neoplastic
lesion was found. The NOAEL was 30 mg/kg bw per day on the basis of
effects on the liver (Hermanski & Wagner, 1993).
A 12-month study of carcinogenicity was conducted in male
Crj:CD-1 mice fed diets containing 0 ( n = 52), 6000 ( n = 53), or
12 000 ( n = 100) ppm piperonyl butoxide (purity, 94.3%; no
detectable safrole or isosafrole). Animals were observed daily for
mortality and morbidity; those that died or were sacrificed at
termination were necropsied, and gross lesions and the liver were
examined histologically. The mortality rates were 6% of controls, 2%
at the low dose, and 19% at the high dose. Terminal body weight was
found to have been reduced by 17% at the low dose and 29% at the high
dose. Hepatocellular hyperplasia was seen in 38% of animals at the low
dose and 8% at the high dose, hepatocellular adenomas in 13 and 22%,
hepatocellular carcinomas in 11 and 52%, and haemangioendothelial
sarcoma in 2 and 42%. It should be noted that the hepatocellular
adenomas and carcinomas may have obscured the true dose-response
relationship for hepatocellular hyperplasia. A condition termed
'post-necrotic peliosis', consisting of multifocal necrosis with blood
cysts, was also found in 17% of animals at the high dose. One control
animal showed hepatocellular hyperplasia and one had a hepatocellular
adenoma (Takahashi et al., 1994a).
Rats
Groups of 12 Wistar rats of each sex were fed diets containing 0,
100, 1000, 10 000, or 25 000 ppm piperonyl butoxide for two years, and
an additional group was fed a diet containing 1000 ppm piperonyl
butoxide plus 167 ppm pyrethrins. Animals were observed daily for
clinical signs of toxicity, and food consumption and body weight were
determined weekly. Autopsies were performed on all animals. Males and
females were paired for the duration of the study, except during
nursing (see section ( d)). Body-weight gain was reduced in animals
at 10 000 (by 10-20%) and 25 000 ppm (by about 90%), in association
with reduced food intake. All animals at the high dose were dead by
week 68; the mortality rates in the other groups were (males/females):
30/20%, 40/30%, 40/50%, and 60/60% for controls and animals at 100,
1000, and 10 000 ppm, respectively. The relative weights of livers and
kidneys were higher than those of controls in animals at 10 000 (by
40% for liver and kidney) and 25 000 ppm (by 275% for liver and 150%
for kidney). These effects were not associated with morphological
alterations. The incidence of tumours was not increased (Sarles &
Vandergrift, 1952).
Sixty Sprague-Dawley Crl:CDR (SD)BR rats of each sex were fed
diets containing piperonyl butoxide (purity, 89%) at concentrations
adjusted weekly (every two weeks after week 15) to achieve daily
intakes of 0 (two groups of controls), 30, 100, or 500 mg/kg bw per
day for 104-105 weeks. The stability and homogeneity of the diets and
the correspondence of actual to nominal concentrations were checked
before and throughout the study and found to be acceptable. The
animals were observed for mortality, clinical signs, food consumption,
body weight, and ophthalmoscopic, haematological, clinical
biochemical, urinary, and pathological parameters. Complete
histological examinations were made of animals in the two control
groups and at the high dose and of those animals at the low and
intermediate doses which died during the study. Histological
examination of animals at these doses killed at the end of the study
was limited to the liver, kidney, lung, thyroid, testis, epididymides,
ovary, and any observed abnormalities.
No clinical signs related to treatment were observed.
Sialodacryoadenitis was diagnosed in a large percentage of treated
animals in weeks 25-28 and weeks 63-67. Body weights were lower in
rats of each sex at 500 mg/kg bw per day than in controls throughout
the study. At week 104, the mean body weight was 20-30% lower than the
control value. A trivial reduction in food consumption was observed in
animals of each sex at the highest dose. Ophthalmoscopic examination
in week 99 revealed no changes attributable to treatment, and
haematological tests and urinalysis revealed no adverse effects.
Female rats receiving 500 mg/kg bw per day had higher cholesterol
levels than controls throughout the study and increased blood urea
nitrogen at 98 weeks. Other statistically significant differences in
biochemical parameters were of no biological relevance. At the end of
the study, the mortality rates were 82, 78, 87, 82, and 78% in males
and 55, 68, 63, 43, and 50% in females at 0 (two groups), 30, 100, and
500 mg/kg bw per day, respectively.
Increased liver weights were seen in animals of each sex at 100
and 500 mg/kg bw per day, corresponding to a higher incidence of
macroscopic enlargement associated with hyperplasia and hypertrophy of
centrilobular hepatocytes and enlarged eosinophilic cells, which
occasionally contained a brownish cytoplasmic pigment. Increased
kidney weights were observed in female rats at the two highest doses,
corresponding histologically to a higher incidence of chronic
interstitial glomerulonephritis. Other histological changes were
confined to the endocrine organs: Enlarged thyroid glands were
observed in animals of each sex at 500 mg/kg per day, corresponding
histologically to a higher incidence of generalized and focal
hyperplasia of follicles and increased pigment deposition in the
colloid. A slightly higher incidence of adrenal and ovarian
enlargement seen among females receiving 500 mg/kg per day was not
associated with histological changes. In males, there was a negative
trend in the presence of enlarged, focal, coarsely vacuolated cortical
cells in the adrenals. The combined incidence of bilateral and
unilateral testicular atrophy was comparable between groups, but there
were significant differences from controls in the incidences of
bilateral testicular atrophy at all doses, with 17, 33, 47, and 43% at
0, 30, 100, and 500 mg/kg bw per day, respectively. After an
additional, full review of the relevant data, this finding was
considered unlikely to be related to treatment because the atrophy was
not associated with degeneration of seminiferous tubules or
aspermatogenesis, and testicular weights were not decreased.
Morphometric analysis of the pituitary gland area showed reduced size
in males at the highest dose. A trend analysis of tumour incidence
with dose showed both increases (in the lymphoid system and thyroid)
and decreases (in mammary glands and pituitary). These differences
were not statistically significant in pairwise comparisons between
groups, and the incidences were within the range of those of
historical controls of this strain of rats. These observations provide
no evidence that the treatment had carcinogenic potential, but the
wide range of gross alterations, effects on organ weight, and
histological changes observed reflect the biological activity of the
test compound. The enlargement of the liver, the primary target organ,
is consistent with the activity of the compound as a hepatic enzyme
inducer. The wide differences in the incidences of morphological
changes and lesions in endocrine and hormone-sensitive organs between
controls and treated groups strongly support the interpretation that
they are the results of changes in hormonal levels brought about by
hepatic enzyme induction. The NOAEL was 30 mg/kg bw per day on the
basis of effects on the liver (Graham, 1987).
Groups of 50 Fischer 344/Du Crj rats of each sex were fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide (purity, about
89%) for two years at doses selected on the basis of the results of
the 13-week study by the same authors, summarized above. Animals were
observed daily for clinical signs and mortality; food consumption was
measured monthly, but the frequency of body-weight measurement was not
stated. Treatment was stopped after 104 weeks, and surviving animals
were sacrificed after six more weeks on the basal diet. All animals,
including those sacrificed when moribund or found dead, were
necropsied and a complete histological examination was performed. The
mortality rates were (males/females) 16/14, 38/22, and 42/34% in
controls and animals at 5000 and 10 000 ppm; the increased mortality
was statistically significant for animals at the high dose. Just
before death, many animals showed signs of anaemia (not specified) and
had blood in their faeces. A dose-related reduction in body weight was
seen in animals of each sex (statistical analysis not reported), with
no concomitant decrease in food intake. No statistically significant
difference in the incidence of malignant or benign neoplasms was
found, except a decreased incidence of thyroid C-cell adenomas in
males at the high dose (9%; 44% in controls). Dose-related increases
were seen in the incidences of ulcers (0/0%, 35/2%, and 52/45% in male
and female controls and those at the low and high doses,
respectively), regenerative hyperplasia (0/0%, 13/0%, and 22/4%),
ossification in the ileocaecal mucosa (0/0%, 15/0%, and 20/0%), and
haemorrhage of the caecum and colon (0/0%, 10/0%, and 17/12%). The
main histological lesions were chronic ulcers with inflammatory-cell
infiltration and granulation, sometimes with ossification of the
surrounding mucosa. Most of deaths in treated animals before the end
of the study were attributed by the authors to lesions leading to
anaemia. Piperonyl butoxide was not found to be carcinogenic
(Maekawa et al., 1985).
Groups of 50 Fischer 344 rats of each sex were fed diets
containing 5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for
24 months; the control group consisted of 20 animals. The diets were
prepared freshly each week and stored at 7°C. Animals were observed
twice daily for mortality and underwent a complete clinical
examination monthly. The food consumption of 10 animals in each group
was measured during the first week of each month; body weights were
measured about twice monthly. Moribund animals were sacrificed and
necropsied and their organs were examined histologically. All
surviving animals were sacrificed and submitted to a complete
histopathological examination. All control females and about 80% of
treated females survived until termination, whereas no difference in
survival was found for males (70-75% survival in all groups). A
dose-related reduction in body weight was found for both females (16%
at the low dose and 24% at the high dose) and males (7% at the low and
16% at the high dose). No difference was found in the incidence of
malignant or benign neoplasms, except for that of lymphoreticular
malignant lymphoma which was significantly increased in treated
females (14% at the low and 30% at the high dose; 5% in controls) and
nonsignificantly decreased in males (30% at the low and 26% at the
high dose; 35% in controls). The author concluded that the results
were equivocal because of the variability in the historical control
data and the difficult diagnosis of these neoplasms (Cardy et al.,
1979; US National Cancer Institute, 1979).
Groups of 30-33 Fischer 344/DuCrj rats of each sex were fed diets
containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide (two
lots: purity, 94.5 and 94.3%; no detectable safrole or isosafrole) for
95 (males) or 96 (females) weeks. The study was ended before 104 weeks
because of the high mortality of males at 12 000 ppm (see below).
Animals were observed daily for clinical signs and mortality; body
weights were measured monthly. Food consumption was determined in a
preliminary experiment involving six rats of each sex per group
(duration not reported), which resulted in calculated compound intakes
of 537, 1061, and 2002 mg/kg per day by females and 547, 1052, and
1877 mg/kg per day by males at 6000, 12 000 and 24 000 ppm,
respectively. Rats that died were necropsied and observed for tumors
and non-neoplastic lesions, which were then examined microscopically.
At termination, surviving rats were necropsied; hepatic nodules (when
multiple nodules were present, three major ones were taken) and major
organs were weighed and examined histologically. Blood was collected
for haematological tests (including prothrombin and partial
thromboplastic times) and for standard clinical chemistry. The
mortality rates were 17, 23, 50, and 24% in males and 20, 10, 17, and
21% in females at 0, 6000, 12 000, and 24 000 ppm, respectively. Males
at 12 000 ppm began to die as early as week 40 and at a statistically
significantly different rate from other groups from week 45. Deaths
were most frequently associated with haemorrhage in the caecum. Body
weights were reduced in a dose-related manner in all treated animals
but statistically significantly only in animals at the middle and high
doses. Males and females at the high dose lost weight from week 60,
and at termination their body weights were about 50% of those of
controls. The weights of all organs except the liver were reduced in
animals at the high dose. The absolute liver weights were increased in
animals at 12 000 ppm (in males by 22% and in females by 51%) and in
females at 6000 ppm (by 29%). During the first month of treatment,
rough hair, lethargy, epistaxis, and a fall in food consumption (data
not reported) were observed in animals of each sex at 24 000 ppm.
Haematological tests showed a significant increase in hypochromic,
microcytic anaemia, the severity of which was dose-related, in all
treated animals. The most significant findings in plasma were
decreased cholinesterase activity and triglyceride contents in animals
at all doses and an increased urea nitrogen level in animals at the
high dose and in all treated females. Hepatic tumours were observed
from week 74 of treatment. Nodular lesions of the liver were observed
only in treated animals, and their incidence, numbers per rat, and
size were dose-related. Hepatocellular adenomas and carcinomas were
found in animals at the middle and high doses: the incidences of
adenomas were 27% in males and 13% in females at 12 000 ppm and 15% in
males and 30% in females at 24 000 ppm; the incidences of carcinoma
were 13% in males and 0% in females at 12 000 ppm and 73% in males and
46% in females at 24 000 ppm. The nodules in animals at the low dose
were classified as foci or hepatocellular focal hyperplasia. The only
other difference between groups was found for interstitial-cell
tumours of the testis, with incidences of 23/25 in controls, 19/23 at
6000 ppm, 13/15 at 12 000 ppm, and 15/25 at 24 000 ppm. Essential
(haemorrhagic) thrombocytaemia (defined as > 3 × 106 platelets per
microlitre, with a bimodal platelet distribution curve) was found in
0/24 male controls and in 6/23 males at 6000 ppm, 3/15 at 12 000 ppm,
and 9/24 at 24 000 ppm and in only 1/25 females at the high dose. Of
the non-neoplastic lesions, the following are significant: an
increased incidence of stomach haemorrhage (males: 9/33; 5/30 in
controls; females: 19/33; 2/30 in controls) at the high dose, an
increased incidence of haemorrhage and/or oedema of the caecum in all
treated males and in females at the middle dose, an increased
incidence of black kidneys in males at the middle and high doses and
all treated females, and an increased incidence of whitish spotting in
the lungs of males at the middle and high doses. Tubular dilatation,
distension of Bowman's space and interstitial fibrosis were found in
the kidneys of animals at the high dose (actual numbers not reported)
(Takahashi et al., 1994b).
(d) Reproductive toxicity
Mice
In a two-generation study, with one litter per generation, groups
of 10 male and 10 female Crj:CD-1 mice were fed diets containing 0,
1000, 2000, 4000, or 8000 ppm piperonyl butoxide (purity unspecified).
Animals of the F0 generation, five weeks old at the start of the
study, were mated for five days at nine weeks of age. Animals of the
F1 generation was removed from their dams at four weeks of age and
allocated randomly to continue treatment; the F2 generation was
produced similarly to the F1 generation. Individual food intake was
measured before mating and during mating, gestation, and lactation for
all generations; litter size and weight and sex ratio were measured at
birth, and pups were weighed 0, 4, 7, 14, and 21 days after birth.
Some neurobehavioural tests were performed during lactation of the
F2 generation and the results analysed on a litter basis. The tests
included surface righting and negative geotaxis on postnatal days 4
and 7, cliff avoidance on postnatal day 7, swimming behaviour on
postnatal days 4 and 14, and olfactory orientation on postnatal day
14. Food consumption was reduced by 4-44% in F0 animals at 8000 ppm
except during mating, by 47% in F1 animals at 8000 ppm during
lactation, and by 16-22% in F0 and F1 animals at 4000 ppm during
lactation. The mean litter size of F1 animals at 8000 ppm was not
significantly reduced (7.7; 10.6 in controls), but the mean F1
litter weight was reduced by 38% (statistically significant) at
8000 ppm and by 18% (not statistically significant) at 4000 ppm. F1
pups at 8000 ppm had a lower survival index at postnatal day 21 (63%;
91% in controls for males, statistically significant; 79%; 89% in
controls for females, not statistically significant). The weights of
all F1 pups were reduced, but there was no dose-response
relationship at 1000-4000 ppm. The results of neurobehavioural tests
for treated F1 animals were different from those of controls in some
instances, but no dose-related response was evident. The mean F2
litter size was significantly reduced at 4000 ppm (10.3; 13.1 in
controls) and 8000 ppm (7.0), and the mean F2 litter weights were
reduced in all treated groups by 13-57%. F2 pups at 8000 ppm had a
lower survival index than controls at postnatal day 21 (59% in males,
statistically significant; 79% in females, not statistically
significant), and F2 pup weights were reduced at 2000, 4000, and
8000 ppm. At 2000 and 4000 ppm, the effect was evident only from
postnatal day 4; at 1000 ppm, reductions in pup weights were observed
on postnatal day 4 in males and postnatal day 7 in animals of each
sex. There was no NOAEL because of effects on pup weights at all doses
(Tanaka et al., 1992).
Groups of 10 male and 10 female Crj:CD-1 mice were fed diets
containing 0,1500, 3000, or 6000 ppm piperonyl butoxide (purity
unspecified) for four weeks before mating (F0 generation), during
gestation, and until the F1 generation was eight weeks old.
Open-field activity was measured in the F0 generation after three
weeks of treatment. Litter size, pup weight, and some developmental
behavioural signs (surface righting, negative geotaxis, cliff
avoidance, swimming, and olfactory orientation) were measured during
lactation of the F1 generation; open-field activity was measured at
three and eight weeks of age and multiple water T-maze activity at six
weeks of age. A dose-related decrease in ambulation and rearing was
seen in the open-field test in males of the F0 generation, but only
the former was statistically significant at the high dose. Pup body
weight at birth was reduced in all groups; on postnatal day 21, the
body weight of animals at the middle dose was 7% lower than that of
controls, and that of animals at the high dose was 41% lower. The
survival indexes at postnatal day 21 were 79, 93, 80, and 52 in
controls and in mice at the low, middle, and high doses, respectively.
The results of the behavioural test during lactation were not
significant, except for reduced olfactory orientation in animals at
the middle and high doses. The results of the open-field test and the
multiple water T-maze test were not altered by treatment, except for a
few, not dose-related differences in some parameters (Tanaka, 1992).
Rats
In a two-litter, two-generation study of reproductive toxicity,
groups of 26 Sprague-Dawley CD rats of each sex, seven weeks of age,
received piperonyl butoxide in the diet at 0, 300, 1000, or 5000 ppm,
equal to 0, 20, 68, and 350 mg/kg bw per day for males (average food
intake calculated in weeks 1-28 of the study) and to 0, 29, 94, and
480 mg/kg bw per day for females (calculated in weeks 1-12 of the
study). The stability, homogeneity, and correspondence of the actual
concentrations in the diets to the nominal concentrations were checked
several times before and during the study and found to be acceptable.
Rats were maintained on their respective diets for 85 days before
mating, throughout the two mating periods, and until scheduled
sacrifice. The F1b generation litters were weaned on day 21
post partum, and groups of 26 rats of each sex were selected to form
the F1b adult generation. These animals were maintained on their
respective diets for 83 days before mating. Animals were observed for
signs of toxicity, and body weight and food consumption were recorded.
External and internal gross examinations were performed on adult rats
and on a selected number of weanlings; histopathological examination
was undertaken for the reproductive tracts of control animals, those
at the high dose, and those of rats at the low and middle doses that
failed to mate successfully in either mating period.
Clinical and pathological examinations showed no
treatment-related toxic effects in adult F0 or F1b animals. The
body weights of adult animals of each sex at 5000 ppm were lower than
those of controls from a few weeks after the beginning to the end of
the study. This effect occasionally corresponded to reduced food
intake. Mating performance, fertility indexes, gestation indexes,
length of gestation, and numbers of live and dead pups at birth were
not affected by treatment. The viability and lactation indexes,
clinical conditions, and pathological appearance of pups of all
generations were also unaffected. The body weights of male and female
pups of all generations at 5000 ppm were lower than those of control
pups. This effect was not detectable at birth but was seen as early as
day 4 post partum. The NOAEL for parental toxicity and pup development
was 1000 ppm, equal to 68 mg/kg bw per day (Robinson et al., 1986).
During the study of carcinogenicity reviewed above (Sarles &
Vandergrift, 1952), rats of each sex were pair-caged throughout the
experiment, except during nursing. No effect on reproductive
efficiency was seen in the first, second, or third generations (no
details given).
(e) Developmental toxicity
Mice
Groups of 20 pregnant Crj:CD-1 mice were given piperonyl butoxide
(purity, > 95%) in olive oil by gavage on day 9 of gestation at doses
of 0, 1065, 1385, or 1800 mg/kg bw. Dams were weighed on gestation
days 9 and 18 and then sacrificed, and the uteri were examined for the
presence and position of resorption sites, fetuses, and implantation
sites. Viable fetuses were weighed and examined for external
malformations and variations, and then fixed and stained for
visualization of the skeleton. No abnormal behaviour or mortality was
observed in dams. One dam at the middle dose and two at the high dose
aborted; the litters of one dam at the middle dose and three at the
high dose were resorbed. Maternal body-weight gain was comparable in
all groups. The total resorption rates were significantly greater at
the middle (26%) and high (32%) doses than in controls (6%), probably
due to total litter resorptions, since the number of viable fetuses
per dam was comparable in all groups. (The raw data were not available
to confirm the reviewer's hypothesis.) The average fetal body weight
was significantly reduced in males, by 3% at the middle dose and 7% at
the high dose, and in females, by 4% at the low dose, 5% at the middle
dose, and 6% at the high dose. Exencephaly, craniochisis, open
eyelids, omphalocele, kinky tail, and talipes varus were observed in
all groups. Oligodactyly in the forelimbs was found in none of the
controls but in one fetus at the low dose, four (2%) at the middle
dose, and 27 (6%) at the high dose. A single oral dose > 1065 mg/kg
bw per day of piperonyl butoxide to dams on day 9 of gestation was
thus embryo- and fetotoxic (Tanaka et al., 1994).
Rats
Groups of 20 pregnant COBS random-bred albino rats were given 0,
300, or 1000 mg/kg bw per day piperonyl butoxide (technical-grade;
purity unspecified) dissolved in corn oil by gavage on days 6-15 of
gestation. The doses were chosen after a pilot study in which six
animals per group were treated with 0, 100, 300, 1000, or 3000 mg/kg
bw per day; reduced body-weight gain was observed during gestation
days 6-15 at the highest dose. Pregnant animals were shipped from the
breeder to the test laboratory on day 1 of gestation and were observed
and weighed daily. On day 20 of gestation, they were sacrificed and
the usual parameters were determined. No signs of toxicity were
observed. Body-weight gain was reduced by 10% in animals at the low
dose and by 15% in those at the high dose, mainly between days 15 and
20 of gestation. Reproductive parameters were not significantly
affected by treatment. One female at 300 mg/kg bw resorbed 8 of 11
fetuses, and one at the high dose resorbed the entire litter. Fetuses
of treated dams had no internal, external, or skeletal malformations
that could be related to treatment. There was no NOAEL for maternal
toxicity because of reduced body-weight gain at both doses. The NOAEL
for embryo- and fetotoxicity was 1000 mg/kg bw per day on the basis of
the absence of any significant finding (Kennedy et al., 1977). This
report from Industrial Biotest Laboratories has not been validated by
a governmental agency and was therefore not taken into account by the
Meeting.
Groups of 17-20 pregnant Wistar rats were given piperonyl
butoxide (purity, 80%) at 0, 62.5, 125, 250, or 500 mg/kg bw dissolved
in corn oil by gavage on days 6-15 of gestation. They were sacrificed
on day 22 of gestation and necropsied, and their fetuses were removed
and examined for external, visceral, and skeletal changes. There were
no deaths or signs of toxicity in dams and no effect on the numbers of
corpora lutea, live fetuses, resorption sites plus dead fetuses,
anomalous fetuses, anomalous litters, or fetal body weight. The types
and incidences of anomalies in the treated groups were comparable to
those of the control group (data not shown). There was no evidence of
embryotoxicity, fetotoxicity, or teratogenicity, and no maternal
toxicity was elicited at the doses used (Khera et al., 1979).
Groups of 20 timed-pregnant Sprague-Dawley CD rats were given
undiluted piperonyl butoxide (purity, 90.78%) at 0, 200, 500, or
1000 mg/kg bw per day by gavage on days 6-15 of gestation. The doses
were chosen in a preliminary pilot study in which five animals per
group were treated with 0, 250, 500, 1000, 2000, or 4000 mg/kg bw per
day, and in which all animals at 4000 and four-fifths of those at
2000 mg/kg bw per day groups died or became moribund. Animals were
observed and weighed daily. On day 20 of gestation, they were
sacrificed and the usual parameters plus liver weight were determined.
No females aborted or delivered early, but wetness in the urogenital
area was observed in 13/24 dams at the highest dose, and stains of
urine, red urogenital discharge, and perinasal encrustation were seen
at lower incidences. Body-weight gain was reduced between days 6 and 9
of gestation in animals at the low dose (an effect considered to be
nonsignificant because of the low weight of non-pregnant animals) and
between days 6 and 15 in animals at the middle and high doses; these
reductions were associated with reduced food intake, resulting in a
significantly lower body weight (by about 5%) in animals at 500 and
1000 mg/kg bw per day on days 15 and 18 of gestation. Increased
absolute (by 8%) and relative (by 11%) weights were observed at the
high dose. Reproductive parameters were not significantly affected by
treatment. Fetuses of treated dams had no internal, external, or
skeletal malformations that could be related to treatment. The NOAEL
for maternal toxicity was 200 mg/kg bw per day on the basis of reduced
body-weight gain and food consumption, increased liver weight, and
signs of toxicity at higher doses. The NOAEL for embryo- and
fetotoxicity was 1000 mg/kg bw per day on the basis of the absence of
any significant finding (Chun & Neeper-Bradley, 1991).
Rabbits
In a range-finding study, five inseminated New Zealand white
rabbits were treated by gavage with piperonyl butoxide during
gestation days 7-19 at doses of 0, 50,100, 200, 300, or 400 mg/kg bw
per day in corn oil. All animals except one control survived until the
end of the study. Two animals each at 300 and 400 mg/kg bw per day and
one at 100 mg/kg bw per day group aborted all or part of their litters
between gestation days 22 and 26. Decreased defaecation was observed
at the highest dose, and animals at the two highest doses were thinner
than normal at the end of the treatment period. Significant reductions
in body-weight gain and some body-weight loss were observed in rabbits
at 300 and 400 mg/kg bw per day; a trivial reduction in body-weight
gain was observed in animals at 200 mg/kg bw per day. No consistent
effects were seen on uterine parameters. Doses of 50, 100, and
200 mg/kg bw per day were therefore chosen for the definitive study of
teratogenicity.
Groups of 16 inseminated New Zealand white rabbits were treated
by gavage with piperonyl butoxide (purity, 100%) during gestation days
7-19 at doses of 0, 50, 100, or 200 mg/kg bw per day in 0.5 ml/kg bw
corn oil. Caesarean sections were performed on gestation day 29, and
the fetuses were removed for teratological evaluation. All animals
survived until the end of the study. Decreased defaecation was
observed in animals at 100 and 200 mg/kg bw per day. Slight
body-weight loss was observed at the middle and high doses during the
treatment period, but there was substantial recovery of body weight
during the post-treatment period, so that the weight gain during the
overall gestation period was comparable to that of controls. The mean
post-implantation losses of treated does were slightly greater than
those of controls but were not dose-related. Malformations were
observed incidentally and were considered to be unrelated to
treatment. The numbers of fetuses with more full ribs than normal (45,
58, 59, and 60% at 0, 50, 100, and 200 mg/kg bw, respectively) or with
27 presacral vertebrae (20, 32, 27, and 40%, respectively) were
greater than those in controls, but the number of litters was not
different. There was no clear dose-effect relationship, and the
relevance of this finding is dubious. The NOAEL for maternal toxicity
was 50 mg/kg bw per day on the basis of decreased defaecation and
dose-related body-weight losses during the treatment period. The NOAEL
for teratogenicity was 200 mg/kg bw per day (Leng et al., 1986). The
1992 JMPR wrongly reported an NOAEL of 100 mg/kg bw per day.
(f) Genotoxicity
The results of studies of the genotoxicity of piperonyl butoxide
are reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Piperonyl butoxide was applied at 0.5 ml to intact, clipped skin
of six New Zealand white rabbits for 4 h under a semi-occlusive
dressing, and the treated areas were examined for erythema and oedema
up to 78 h after the contact period. An adjacent area of untreated
skin served as the control. Piperonyl butoxide was very mildly
irritating (Romanelli, 1991a). An older study reported similar results
(Sarles et al., 1949).
Piperonyl butoxide at 0.1 ml was instilled into the conjuntival
sac of one eye of six New Zealand white rabbits, and the treated eyes
were examined up 72 h after instillation. Conjunctival irritation was
observed, which fully recovered within 72 h. No corneal or iridal
lesions were observed (Romanelli, 1991b). An older study reported
similar results in rabbits, cats, and dogs (Sarles et al., 1949).
Table 2. Results of tests for the genotoxicity of piperonyl butoxide
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, TA100, 100-5000 µg/plate 90.78 Negativea Lawlor (1991)
TA1535, TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative White et al. (1977)
TA 100, TA1537
Reverse mutation E. coli WP2 1 mg/disc 80 Negative Ashwood-Smith
et al. (1972)
Gene mutation Mouse lymphoma L5178Y 6.3-100 µg/mlb NR Positive McGregor et al.
cells (1988)
Gene mutation Chinese hamster ovary, cells 10-100 µg/mlc NR Equivocal Tu et al. (1985)
25-500 µg/mld Negative
Unscheduled DNA Rat hepatocytes 1-100 µg/mle 90.78 Negative McKeon & Phil
synthesis (1991)
Unscheduled DNA Human liver slices 0.05-2.5 mmol/litre 90.78 Negative Lake (1995)
synthesis
Chromosomal Chinese hamster ovary cells 25-99.9 µg/ml 90.78 Negativea Murli (1991)
aberration 62.6-251 µg/mla
Table 2. (cont'd).
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Dominant lethal ICR/Ha Swiss mice 0, 200, or 1000 mg Commercial Equivocal Epstein et al. (1972)
mutation intraperitoneally or formulation
1000 mg/kg bw orally
× 5
NR, not reported
a With and without metabolic activation
b Lethal at 100 µg/ml
c Without metabolic activation; cytotoxic at > 30 µg/ml
d With metabolic activation; cytotoxic at > 300 µg/ml
e Cytotoxic at > 49.9 µg/ml
Skin sensitization was studied in 10 male Hartley guinea-pigs by
a modified Buehler method. A gauze patch containing 0.4 ml piperonyl
butoxide was applied for 6 h onto clipped skin, three times a week for
three weeks. After a two-week rest period, the animals were challenged
on another site with a single 6-h application. Chlorodinitrobenzene
(0.1% w/v in a 50% ethanol:0.9% saline solution) was used as the
positive control. No evidence of contact sensitization was observed
(Romanelli, 1991c). An older study also reported no response in
rabbits (Sarles et al., 1949)
(ii) Studies with mixtures
Neonatal ICR/Ha Swiss mice (n = 91) were given subcutaneous
injections of 5 mg piperonyl butoxide alone at the ages of one and
seven days and 10 mg at the ages of 14 and 21 days. Further groups
were given the same doses in combination with Freon-112 (n = 137) or
-113 (n = 94) at doses of 10 mg at one and seven days and 20 mg at 14
and 21 days. Control animals were treated with the solvent,
tricaprylin. All surviving animals were killed after 52 weeks. No
tumours were found in animals treated with piperonyl butoxide, but the
incidence of hepatomas in male mice given piperonyl butoxide plus
Freon was significantly increased when compared with that in groups
receiving solvent, piperonyl butoxide, or Freon alone (Epstein
et al., 1967).
Groups of 45 Sprague-Dawley CD rats of each sex were fed either
standard diets or diets containing 400 ppm pyrethrins plus 2000 ppm
piperonyl butoxide for 104 weeks. The diets were prepared daily from a
10-fold concentrated pre-mix prepared freshly once a week. The purity
of piperonyl butoxide was 90.5%, and that of pyrethrins was 53.1%
(batch used until week 55) or 52.4% (batch used from week 56 until
termination); the diets were prepared taking into account the purity
of the compounds. The actual concentrations and the stability of the
diet were not tested. Body weight and food consumption were determined
once a week. The average daily intakes were 79 and 101 mg/kg bw
piperonyl butoxide and 16 and 20 mg/kg bw pyrethrins for males and
females, respectively. Animals were observed for clinical signs, skin
lesions, and palpable masses. All animals found dead or moribund were
inspected, and macroscopic lesions were examined microscopically.
During week 101, urinalysis and haematological and clinical chemical
tests were performed in 10 animals per group. At termination, all
animals were observed grossly and selected organs were examined
microscopically.
No treatment-related clinical signs of toxicity were observed.
Mortality was 84 and 76% in control males and females and 80 and 58%
in treated males and females, respectively. The body weights of
treated females were about 20% lower than those of controls, mainly
during the first 78 weeks, while at termination treated animals were
about 10% lighter than controls; the difference was less evident
(about 5%) in males. Slightly reduced food intake was also observed
until week 27 of treatment. Haematological, clinical chemical, and
urinary parameters were not significantly altered in treated animals,
and macroscopic and microscopic examinations revealed no significant
treatment-related neoplastic or non-neoplastic effects (Hunter
et al., 1977).
3. Observations in humans
In nine men, antipyrine metabolism was not affected by a single
oral dose of 50 mg (0.71 mg/kg bw) piperonyl butoxide (Conney et al.,
1972).
Two male infants, whose mothers were sisters and who were born
within two weeks of each other, had coarctation of the aorta. Exposure
to insect repellents and insecticides, including piperonyl butoxide,
during week 8 of gestation was reported (Hall et al., 1975).
Percutaneous absorption of pyrethrin and piperonyl butoxide was
studied in six male volunteers. A formulation containing 0.3% pyrethin
and 3.0% piperonyl butoxide was spread on the ventral forearm for 30
min. This formulation was chosen because it corresponds to that of a
commercially available product used for the treatment of head lice.
The formulation contained either 14C-pyrethrin or 14C-piperonyl
butoxide. After application, urine was collected for up to seven days,
and percutaneous absorption was determined from total urinary
excretion of radioabel, assuming that 22.5% of pyrethin and 51.3% of
piperonyl butoxide are excreted in the urine after parenteral
injection, as was shown in monkeys. Absorption of pyrethrin was 1.9 ±
1.2% and that of piperonyl butoxide 2.1 ± 0.6% of the administered
dose. No radiolabel was found in blood taken 1 h after application.
The extrapolated absorption from human scalp was 7.5 ± 4.7% of
pyrethin and 8.3 ± 2.4% of piperonyl butoxide (Wester et al., 1994).
Comments
Piperonyl butoxide is metabolized by oxidation of the methylene
group of the methylenedioxyphenyl moiety to yield carbon dioxide. The
remainder of the molecule undergoes further degradation and is
excreted mainly in the urine. As it is an alternative substrate (and
therefore a competitive inhibitor) for the microsomal cytochrome P450
system, piperonyl butoxide inhibits the metabolism of several drugs
and pesticides. The mechanism of action of this inhibition has been
elucidated in several studies.
In male rats given single oral doses of about 500 mg/kg bw of
[methylene-alpha 14C]-piperonyl butoxide, the label reached a peak
in blood after 3-12 h and dropped by about 50% within 24 h. The
highest levels of radiolabel were found in the gastrointestinal tract
and its, contents, suggesting that enterohepatic circulation occurs.
High levels of radioactivity were also found in the lung, liver,
kidney, fat, prostate, and seminal vesicles. The excretion pattern was
unchanged after 14 repeated doses of piperonyl butoxide.
Piperonyl butoxide has negligible acute toxicity, and it has been
classified by WHO as unlikely to present an acute hazard in normal
use.
Both short-term and long-term studies show that the target organ
of the toxicity of piperonyl butoxide is the liver. Male animals were
slightly more sensitive than females. In a number of short-term
studies in rodents, hepatic toxicity was characterized by liver
enlargement with associated hypertrophic hepatocytes, focal necrosis,
and, at times, alteration of some clinical chemical parameters. The
NOAEL for liver toxicity was about 100 mg/kg bw per day.
In rats exposed to piperonyl butoxide by inhalation at 0, 15, 74,
155, or 512 mg/m3 for 6 h per day on five days a week for 13 weeks,
effects were seen on the liver at the highest dose. Irritation of the
upper airways was seen at all doses, with squamous metaplasia of the
larynx.
In a one-year study in dogs fed diets containing 0, 100, 600, or
2000 ppm piperonyl butoxide, reduced body-weight gain, increased liver
weight with hypertrophic hepatocytes, and alteration of some clinical
chemical parameters were observed at 2000 ppm. The NOAEL in this study
was 600 ppm, equal to 16 mg/kg bw per day.
Several studies have been conducted of carcinogenicity in mice
and rats, some of which were considered to be inadequate. In a
112-week study, mice were given diets containing 5000 or 10 000 ppm
piperonyl butoxide during weeks 1-30 and 500 or 2000 ppm during weeks
31-112. No increase in tumour incidence was observed. Mice were fed
diets that gave a daily intake of 0, 30, 100, or 300 mg/kg bw for 78
weeks. Eosinophilic foci and adenomas with eosinophilic cells were
observed more frequently in the livers of males at the middle dose and
males and females at the high dose. The NOAEL in this study was
30 mg/kg bw per day on the basis of effects on the liver.
In a 12-month study of carcinogenicity in the liver, male mice
were fed diets containing 0, 6000, or 12 000 ppm piperonyl butoxide.
Body weights were reduced in a dose-related manner, and increased
mortality was observed in animals at the high dose. Hepatocellular
adenomas and carcinomas were observed in treated groups. Piperonyl
butoxide was carcinogenic at doses that were toxic to the liver and
caused general toxicity.
In a two-year study in rats at dietary concentrations adjusted to
achieve doses of 0, 30, 100, or 500 mg/kg bw per day, increased liver
weights, with corresponding hyperplasia and hypertrophy of
hepatocytes, morphological changes, and lesions in the endocrine and
hormone-sensitive organs, were observed at 100 and 500 mg/kg bw per
day. These effects were considered to be secondary to the ability of
piperonyl butoxide to induce hepatic cytochrome P450 enzymes.
Piperonyl butoxide was not found to be carcinogenic in this study.
After reconsideration of the data on testes in this study, previously
evaluated by the 1992 JMPR, and taking into account the results of
other long-term studies, the Meeting concluded that the NOAEL was
30 mg/kg bw per day on the basis of effects on the liver.
Two studies of carcinogenicity were conducted in rats fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide. No significantly
increased incidence of neoplasia was found in treated rats. Effects on
body weight and mortality were observed at the highest dose. In one
study, ileocaecal lesions were observed in both groups. Another study
of carcinogenicity was conducted in rats fed diets containing 0, 6000,
12 000, or 24 000 ppm. Increased mortality was observed in females at
the middle dose. Absolute liver weights were increased in females at
the low dose and in animals of each sex at the high dose. Nodular
lesions of the liver were observed in treated animals, and their
incidence and severity were related to the dose. Hepatocellular
adenomas and carcinomas were observed in animals at the middle and
high doses. Gastric and caecal haemorrhages, renal lesions, anaemia,
and platelet alteration were observed in treated animals. Piperonyl
butoxide was carcinogenic at doses that caused general toxicity.
In a 104-week study in rats, piperonyl butoxide was given at
2000 ppm in the diet in combination with pyrethrins at 400 ppm. A
slight reduction in the body weights of treated females was the only
adverse effect observed.
A two-generation study of reproductive toxicity, with one litter
per generation, was conducted in mice fed diets containing 0, 1000,
2000, 4000, or 8000 ppm piperonyl butoxide. There was no NOAEL because
of reduced pup weight at all doses. Pup viability was reduced at the
highest dose. In a two-litter, two-generation study of reproductive
toxicity in rats at dietary levels of 0, 300, 1000, or 5000 ppm, the
NOAEL for parental toxicity and pup development was 1000 ppm (equal to
68 mg/kg bw per day) on the basis of lowered body weight at 5000 ppm.
Embryo- and fetotoxicity were observed when mice were given single
doses of piperonyl butoxide at 0, 1070, 1390, or 1800 mg/kg bw by
gavage on day 9 of gestation.
Piperonyl butoxide was not embryotoxic or teratogenic in rats or
rabbits. Maternal toxicity was found in rats at doses > 500 mg/kg
bw per day. The NOAEL was 200 mg/kg bw per day in a study of
developmental toxicity in rabbits given 0, 50, 100, or 200 mg/kg bw
per day by gavage. The incidence of common developmental variations,
such as more full ribs and more than 27 presacral vertebrae, was
increased in all treated groups. Since a clear dose-effect
relationship was lacking, the association of this finding with
treatment was considered dubious. The NOAEL for maternal toxicity was
50 mg/kg bw per day.
Piperonyl butoxide was a mild dermal and ocular irritant but not
a dermal sensitizer in rabbits.
Piperonyl butoxide was adequately tested for genotoxicity in a
range of assays in vivo and in vitro. The Meeting concluded that
it is not genotoxic.
A single dose of 0.71 mg/kg bw piperonyl butoxide did not alter
antipyrine metabolism in humans. A study in which a formulation
containing 3% piperonyl butoxide was spread onto the ventral forearm
of adult male volunteers indicated that about 8% of the applied dose
would be absorbed through the human scalp. These data did not
contribute directly to the establishment of the ADI.
An ADI of 0-0.2 mg/kg bw was established on the basis of the
NOAEL of 600 ppm (equal to 16 mg/kg bw per day) in the one-year study
in dogs, with a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 mg/kg bw per day (78-week dietary study of toxicity and
carcinogenicity)
Rat: 30 mg/kg bw per day (two-year dietary study of
carcinogenicity)
200 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
500 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
1000 ppm, equal to 68 mg/kg bw per day (study of
reproductive toxicity)
Rabbit: 50 mg/kg bw per day (study of developmental toxicity)
200 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 600 ppm, equal to 16 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Further observations in humans
2. Short-term studies with mixtures of piperonyl butoxide and
other active ingredients, in ratios relevant to human
exposure
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to piperonyl butoxide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, irritation, rabbit Mildly irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Not sensitizing
Inhalation, lethality, rat Lacrimation, salivation, nasal
discharge, and laboured breathing at
5.9 mg/litre for 4 h. No mortality
Oral, lethality, mouse, rat, cat, dog LD50 = 4-14 g/kg bw
Dermal, lethality, rabbit LD50 > 2 g/kg bw
Medium-term (1-26 weeks) Repeated oral, toxicity, mice, rats, NOAEL = 100 mg/kg bw per day; effects on
3-13 weeks liver
Repeated inhalation, toxicity, rats, 13 weeks Irritating to upper airways at 15 mg/m3 for
6 h per day, 5 days per week, with squamous
metaplasia of the larynx; effects on the liver at
512 mg/m3
Oral, developmental toxicity, rabbit NOAEL = 50 mg/kg bw per day for maternal
toxicity; no fetotoxicity or teratogenidty
Oral, reproductive toxicity, rat NOAEL = 68 mg/kg bw per day; maternal
and pup toxicity
Long-term (> one year) Repeated oral, toxicity, dog, one year NOAEL = 16 mg/kg bw per day
References
Ashwood-Smith, M.J., Trevino, J. & Ring, R. (1972) Mutagenicity of
dichlorvos. Nature, 240, 418-420. Brady, J.T., Montelius, D.A.,
Beierschmitt, W.P., Wyand, D.S., Khairalla, E.A. & Cohen, S.D.
(1988) Effect of piperonyl butoxide post-treatment on
acetaminophen hepatotoxicity. Biochem. Pharmacol.,
37, 2097-2099.
Cardy, R.H., Renne, R.A., Warner, J.W. & Cypher, R.L. (1979)
Carcinogenesis bioassay of technical-grade piperonyl butoxide in
F344 rats. J. Natl Cancer Inst., 62, 569-578.
Chun, J.S. & Neeper-Bradley, T.L. (1991) Developmental toxicity
evaluation of piperonyl butoxide administered by gavage to CD
(Sprague-Dawley) rats. Unpublished report No. 54-586 from Bushy
Run Research Center, Export, Pennsylvania, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Conney, A.H., Chang, R., Levin, W.M., Garbut, A., Munro-Faure, A.D.,
Peck, A.W. & Bye, A. (1972) Effects of piperonyl butoxide on drug
metabolism in rodents and man. Arch. Environ. Health,
24, 97-106.
Dalvi, R.R. & Dalvi P.S. (1991) Differences in the effects of piperine
and piperonyl butoxide on hepatic drug-metabolizing enzyme system
in rats. Drug Chem. Toxicol., 14, 219-229.
Epstein, S.S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel, N.
(1967) Synergistic toxicity and carcinogenicity of 'Freons' and
piperonyl butoxide. Nature, 214, 526-528.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 233, 288-325.
Fishbein, L., Falk, H.L., Fawker, J., Jordan, S. & Corbett, B. (1969)
The metabolism of piperonyl butoxide in the rat with 14C in the
methylenedioxy or alpha-methylene group. J. Chromatogr.,
41, 61-79.
Franklin, M.R. (1972) Inhibition of hepatic oxidative xenobiotic
metabolism by piperonyl butoxide. Biochem. Pharmacol.,
21, 3287-3299.
Fujitani, T., Ando, H., Fujitani, K., Ikeda, T., Kojima, A., Kubo, Y.,
Ogato, A., Oishi, S., Takahashi, O. & Yoneyama, M. (1992)
Sub-acute toxicity of piperonyl butoxide in F344 rats.
Toxicology, 72, 291-298.
Fujitani, T., Tanaka, T., Hashimoto, Y. & Yoneyama, M. (1993a)
Subacute toxicity of piperonyl butoxide in ICR mice.
Toxicology, 83, 93-100.
Fujitani, T., Tada, Y. & Yoneyama, M. (1993b) Hepatotoxicity of
piperonyl butoxide in male F344 rats. Toxicology, 84, 171-183.
Gabriel, D. (1991a) Acute oral toxicity, LD50-rats. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Gabriel, D. (1991b) Acute dermal toxicity, single level-rabbits.
Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldenthal, E.I. (1993a) Evaluation of piperonyl butoxide in an eight
week toxicity study in dogs. Unpublished report No. 542-004 from
International Research and Development Corp., Mattawan, Michigan,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Goldenthal, E.I. (1993b) Evaluation of piperonyl butoxide in a one
year chronic dietary toxicity study in dogs. Unpublished report
No. 542-005 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldstein, J.A., Hickman, P. & Kimbrough R.D. (1973) Effects of
purified and technical piperonyl butoxide on drug metabolizing
enzymes and ultrastructure of rat liver. Toxicol. Appl.
Pharmacol., 26, 444-458.
Graham, C (1987) 24-Month dietary toxicity and carcinogenicity study
of piperonyl butoxide in the albino rat. Unpublished report
No. 81690 from Bio-Research Ltd Laboratory, Seneville, Quebec,
Canada. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hall J.G., McLaughlin, J.F. & Stamm, S. (1975) Coarctation of the
aorta in male cousins with similar maternal environmental
exposure to insect repellent and insecticides. Pediatrics,
55, 425-427.
Hermanski, S.J. & Wagner, C.L. (1993) Chronic dietary oncogenicity
study with piperonyl butoxide in CD-1 mice. Unpublished report
No. 91N0134 from Bushy Run Research Center, Export, Pennsylvania,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hoffman, G.M. (1991) An acute inhalation toxicity study of piperonyl
butoxide in the rat. Unpublished report No. 91-8330 from
Bio/dynamics, East Millstone, New Jersey, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Hunter, B., Bridges, J.L. & Prentice, D.E. (1977) Long term feeding of
pyrethrins and piperonyl butoxide to rats. Unpublished report
from Huntington Research Centre, Huntington, Cambs, United
Kingdom. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Bates, R.R., Falk, H.L., Gart, J.J., Klein, M.,
Mitchell, I. & Peters, J. (1969) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Natl Cancer Inst., 42, 1101-1114.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1968)
In vivo inhibition of mouse liver microsomal hydroxylating
systems by methylenedioxyphenyl insecticidal synergists and
related compounds. Life Sci., 7, 1051-1062.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1969)
Bi-modal effect of piperonyl butoxide on the o- and
p-hydroxylations of biphenyl by mouse liver microsomes.
Biochem. Pharmacol., 18, 1045-1051.
Kamienski, F.X. & Casida, J.E. (1970) Importance of demethylenation in
the metabolism in vivo and in vitro of methylenedioxyphenyl
synergists and related compounds in mammals. Biochem.
Pharmacol., 19, 91-112.
Kennedy, G.L., Smith, S.H., Kinoshita, F.K., Keplinger, M.L. &
Calandra, J.C. (1977) Teratogenic evaluation of piperonyl
butoxide in the rat. Food Cosmet. Toxicol., 15, 337-339.
Khera, K.S., Whalen, C., Angers, G. & Trivett, G. (1979) Assessment of
the teratogenic potential of piperonyl butoxide, biphenyl, and
phosalone in the rat. Toxicol. Appl. Pharmacol., 47, 353-358.
Lake, B.G. (1995) An investigation of the effect of piperonyl butoxide
on unscheduled DNA synthesis in cultured human liver slices.
Unpublished report No. 1501/1 from BIBRA International,
Carshalton, Surrey, United Kingdom. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lawlor, T.E. (1991) Piperonyl butoxide in the Salmonella/mammalian-
microsome reverse mutation assay (Ames test) with a confirmatory
assay. Unpublished report No. 14413-0-401R from Hazleton
Washington, Kensington, Maryland, USA. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lehman, A.J. (1948) Q. Bull. Assoc. Food Drug Off., 12, 82.
Lehman, A.J. (1951) Q. Bull. Assoc. Food Drug Off., 15, 122.
Lehman, A.J. (1952a) Q. Bull. Assoc. Food Drug Off., 16, 43.
Lehman, A.J. (1952b) Q. Bull. Assoc. Food Drug Off., 16, 47.
Leng, J.M., Schwartz, C.A. & Schardein, J.L. (1986) Teratology study
in rabbits. Unpublished report No. 542-002 from International
Research and Development Corp., Mattawan, Michigan, USA.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Levine, B.S. & Murphy, S.D. (1977a) Esterase inhibition and
reactivation in relation to piperonyl butoxide-phosphorothionate
interactions. Toxicol. Appl. Pharmacol., 40, 379-391.
Levine, B.S. & Murphy, S.D. (1977b) Effect of piperonyl butoxide on
the metabolism of dimethyl and diethyl phosphorothionate
insecticides. Toxicol. Appl. Pharmacol., 40, 393-406.
Maekawa, A., Onodera, H., Furuta, K., Tanigawa, H., Ogiu, T. &
Hayashi, Y. (1985) Lack of evidence of carcinogenicity of
technical-grade piperonyl butoxide in F344 rats: Selective
induction of ileocaecal ulcers. Food Chem. Toxicol.,
7, 675-682.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded
chemicals. Environ. Mol. Mutag., 12, 85-154.
McKeon, M.E. & Phil, M. (1991) Piperonyl butoxide in the assay for
unscheduled DNA synthesis in rat liver primary cell cultures with
a confirmatory assay. Unpublished report No. 14413-O-447R from
Hazleton Washington, Kensington, Maryland, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Mirer, F.E., Levine, B.S. & Murphy, S.D. (1977) Parathion and methyl
parathion toxicity and metabolism in piperonyl butoxide and
diethyl maleate pretreated mice. Chem.-Biol. Interactions,
17, 99-112.
Modeweg-Hansen, L., Lalande, M., Bier, C., Losos, G. & Osborne, B.E.
(1984) A dietary dose range-finding study of piperonyl butoxide
in the albino rat. Unpublished report No. 81802B from
Bio-Research Laboratories, Edgewater, Maryland, USA. Submitted to
WHO by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Murli, H. (1991) Piperonyl butoxide: Measuring chromosomal aberrations
in Chinese hamster ovary (CHO) cells with multiple harvests under
conditions of metabolic activation. Unpublished report
No. 14413-0-437C from Hazleton Washington, Kensington, Maryland,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Negherbon, W.O. (1959) Handbook of Toxicology, Vol. 3, Philadelphia,
Saunders.
Newton, P.E. (1992) A subchronic (3-month) inhalation toxicity study
of piperonyl butoxide in the rat via whole-body exposures.
Unpublished report No. 91-8333 from Bio/dynamics, East Millstone,
New Jersey, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Phillips, J.C., Cunninghame, M.E., Price, R.J., Osimitz, T., Ganriel,
K.L., Preiss, F.J., Butler, W.H. & Lake, B.G. (1995) Effect of
piperonyl butoxide (PBO) on cell replication and xenobiotic
metabolism in mouse liver (Abstract). Toxicologist, 15, 230.
Robinson, K., Pinsonneault, L. & Procter, B.G. (1986) A two-generation
(two-litter) reproduction study of piperonyl butoxide
administered in the diet to the rat. Unpublished report No. 81689
from Bio-Research Laboratories Ltd, Montreal, Quebec, Canada.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991a) Primary skin irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991b) Primary eye irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991c) Guinea pig dermal sensitization-modified Buehler
method. Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Sarles, M.P. & Vandegrift, W.B. (1952) Chronic oral toxicity and
related studies on animals with the insecticide and pyrethrum
synergist, piperonyl butoxide. Am. J. Trop. Med. Hyg.,
1, 862-883.
Sarles, M.P., Dove, W.E. & Moore, D.H. (1949) Acute toxicity and
irritation tests on animals with the new insecticide, piperonyl
butoxide. Am J. Trop. Med., 29, 151-166.
Schuller, H.M. & McMahon, J.B. (1985) Inhibition of N-nitroso-
diethylamine-induced respiratory tract carcinogenesis by
piperonylbutoxide in hamsters. Cancer Res., 45, 2807-2812.
Selim, S. (1985) Single and repeated oral dose pharmacokinetic and
distribution studies of piperonyl butoxide. Unpublished report
No. WP509070/1 from Biological Test Center, Irvine, California,
USA. Submitted to WHO by Endura SA. Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Skrinijaric-Spoljar, M., Matthews, H.B., Engel, J.L. & Casida, J.E.
(1971) Response of hepatic microsomal mixed-function oxidases to
various types of insecticide chemical synergists administered to
mice. Biochem. Pharmacol., 20, 1607-1618.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994a) Piperonyl butoxide induces hepatocellular carcinoma in
male CD-1 mice. Arch. Toxicol., 68, 467-469.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994b) Chronic toxicity studies of piperonyl butoxide in F344
rats: Induction of hepatocellular carcinoma. Fundam. Appl.
Toxicol., 22, 293-303.
Tanaka, T. (1992) Effects of piperonyl butoxide on F1 generation mice.
Toxicol. Lett., 60, 83-90.
Tanaka, T. (1993) Behavioural effects of piperonyl butoxide in male
mice. Toxicol. Lett., 69, 155-161.
Tanaka, T., Takahashi, O. & Oishi, S. (1992) Reproductive and
neurobehavioural effects in three-generation toxicity study of
piperonyl butoxide administered to mice. Food Chem. Toxicol.,
30, 1015-1019.
Tanaka, T., Fujitani, T., Takahashi, O. & Oishi, S. (1994)
Developmental toxicity evaluation of piperonyl butoxide in CD-1
mice. Toxicol. Lett., 71, 123-129.
Tu, A.S., Coombes-Hallowell, W.E., Pallotta, S.L. & Catanzano, A.
(1985) Evaluation of piperonyl butoxide in the CHO/HGPRT mutation
assay with and without metabolic activation. Unpublished report
No. 53906 from Arthur D. Little, Inc., Cambridge, Massachusetts,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
US National Cancer Institute (1979) Bioassay of piperonyl butoxide for
possible carcinogenicity (DHEW Publ. No. 79-1375), Bethesda,
Maryland, USA, US Department of Health, Education, and Welfare.
Wester, R.C., Bucks, D.A.W. & Maibach, H.I. (1994) Human in vivo
percutaneous absorption of pyrethrin and piperonyl butoxide.
Food Chem. Toxicol., 32, 51-53.
White, T.J., Goodman, D., Shulgin, A.T., Castagnoli, N., Lee, R. &
Petrakis, N.L. (1977) Mutagenic activity of some centrally active
aromatic amines in Salmonella typhimurium. Mutat. Res.,
56, 199-202.
A dose-dependent, biphasic effect was observed on the ortho and
para hydroxylations of biphenyls by liver microsomes of mice treated
with 10-640 mg/kg bw of piperonyl butoxide intraperitoneally,
para-Hydroxylation was inhibited by 80% at the highest dose 30 min
after treatment but had recovered within 24 h; ortho-hydroxylation
was induced by up to 150% 1 h after treatment, and activity was still
elevated after 96 h in the groups at the highest dose (Jaffe et al.,
1969).
Piperonyl butoxide inhibits ethylmorphine N-demethylation by
liver microsomes in vitro. The inhibition is proportional to the
amount of cytochrome P450-piperonyl butoxide metabolite (unknown)
complex formed. Differences between liver microsomes from rats and
mice may account for the greater sensitivity of mice to inhibition of
drug metabolism by piperonyl butoxide: the Ki for inhibition of
ethylmorphine N-demethylase was found to be 19 µmol/litre in rats and
6 µmol/litre in mice (Franklin, 1972).
Mouse liver homogenates (20% w/v in 50 mmol/litre phosphate
buffer, pH 7.4) were fractioned into nuclear, mitochondrial,
microsomal, and soluble fractions, which were incubated with labelled
piperonyl butoxide. NAD, NADP, NADH, or NADPH was sometimes added as a
cofactor. Only the microsomal fraction had significant metabolic
activity in the presence of NADPH and, to a lesser extent, with NADH
(Kamienski & Casida, 1970).
Male CF-1 mice were given a single intraperitoneal dose of
piperonyl butoxide (purity, 87-89%) at 0.5-25 mg/kg bw 1 h before
intraperitoneal administration of 40 mg/kg bw pentobarbital or
100 mg/kg bw zoxazolamine. Pentobarbital-induced sleeping time was
significantly increased by 10 or 25 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 5,
10, or 25 mg/kg bw (Conney et al., 1972). Skinjaric-Spoljar et al.
(1971) found that a single intraperitoneal dose of 22 mg/kg bw
piperonyl butoxide to mice increased the hexobarbital (62.5 mg/kg bw
intraperitoneally) sleeping time from 28 to 100 min.
Male Sprague-Dawley rats were given single intraperitoneal doses
of piperonyl butoxide (purity, 87-89%) at 67-1000 mg/kg bw 1 h before
intraperitoneal administration of pentobarbital (25 mg/kg bw) or
zoxazolamine (70 mg/kg bw). Pentobarbital-induced sleeping time was
significantly increased by 1000 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 333
or 1000 mg/kg bw (Conney et al., 1972).
Male mice and rats were given single intraperitoneal doses of
piperonyl butoxide 1 h before injection of 200 mg/kg bw antipyrine.
The NOAELs for effects on antipyrine metabolism were 100 mg/kg bw in
rats and 0.5 mg/kg bw in mice (Conney et al., 1972).
Groups of six male weanling Sherman rats were fed technical-grade
piperonylbutoxide (purity, 80%) at dietary concentrations of 0, 1000,
5000, or 10 000 ppm for one, four, or eight weeks. The activities of
hexobarbital oxidase, aniline hydroxylase, para-nitroanisole
demethylase, nitroreductase, and glucuronyltransferase and the P450
content were increased two- to fourfold by 5000 or 10 000 ppm
piperonyl butoxide. Liver weights and microsomal protein content were
increased to a maximum of 50-70%. Electron microscopy showed
enlargement and extensive proliferation of the smooth endoplasmic
reticulum in liver parenchymal cells. The dose of 1000 ppm had minimal
effects on liver weight and on P450 and glucuronyl transferase
activity and no effect on proliferation of the smooth endoplasmic
reticulum. Maximal effects on P450 content and on the activity of
P450-related enzymes were observed after one week of treatment. The
maximal effect on glucuronyltransferase activity was observed between
four and eight weeks of treatment (Goldstein et al., 1973).
Piperonyl butoxide (purity unsepcified) dissolved in corn oil was
administered to 10 male Sprague-Dawley rats at 400 mg/kg bw
intraperitoneally; another group of animals received 100 mg/kg bw of
piperine intraperitoneally. Control animals received 2 ml/kg bw corn
oil. Groups of five animals were sacrificed 1 and 24 h after
treatment, and hepatic levels of cytochromes P450 and b5, proteins,
and the activities of NADPH cytochrome c reductase, benzphetamine
N-demethylase, aminopyrine N-demethylase, and aniline hydroxylase
were determined. The microsomal protein level was not affected by
treatment. Piperonyl butoxide decreased the P450 but not the b5 level
by about 30% after 1 h; however, after 24 h the contents were
increased by about 100 and 30%, respectively. NADPH cytochrome c
reductase activity was increased by 50% 24 h after treatment. The
activities of N-demethylases and aniline hydroxylase were decreased
by 20 and 50%, respectively, 1 h after administration of either
piperine or piperonyl butoxide. By 24 h, the activities were still low
in piperine-treated animals but had increased by 30-80% in those given
piperonyl butoxide (Dalvi & Dalvi, 1991).
Single intraperitoneal doses of 400 mg/kg bw piperonyl butoxide
were given to male CD-1 mice 1 h before a single intraperitoneal dose
of parathion-methyl, azinphos-methyl, parathion, or azinphos-ethyl, or
their oxygen analogues. The LD50 of the oxygen analogues was not
altered whereas that of the methyl homologues was reduced, with a
40-fold increase in the LD50 for parathion-methyl and a threefold
increase in that for azinphos-methyl; that of the ethyl homologues was
increased, with a 50% decrease in the LD50 for parathion and an 85%
decrease for azinphos ethyl. The plasma levels of all pesticides were
increased by three- to sevenfold in piperonyl butoxide-pretreated
animals 30 min after treatment (Levine & Murphy, 1977a). It has been
suggested that detoxification of parathion-methyl, but not that of
parathion- or azinphos-ethyl, continues through uninhibited
glutathione-dependent pathways (Mirer et al., 1977; Levine & Murphy,
1977b).
A single intraperitoneal dose of 600 mg/kg bw piperonyl butoxide
to three-month-old CD-1 mice reduced the hepatotoxicity (as assessed
by reduced glutathione content, plasma sorbitol dehydrogenase, and
histopathological liver necrosis) caused by an oral dose of 600 mg/kg
bw acetominophen given 2 h earlier or 1 h later. Since the hepatic
mixed-function oxidase system generates a toxic, electrophilic
metabolite of acetominophen, piperonyl butoxide probably acts by
inhibiting: that enzymatic system (Brady et al., 1988).
Pretreatment of groups of 10 male Syrian golden hamsters with
400 mg/kg bw piperonyl butoxide given subcutaneously 2 h before
subcutaneous injection of 17.8 mg/kg bw N-nitrosodiethylamine twice
weekly for 20 weeks inhibited the development of pulmonary
carcinogenesis by the nitrosamine. The numbers of animals with lung
tumors were 0 controls, 6 treated only with N-nitrosodiethylamine, 0
treated with piperonyl butoxide and nitrosamine, and 0 given only
piperonyl butoxide. Tracheal tumors were found in 0, 10, 5, and 0
animals in the four groups, respectively. The protective effect was
associated with reduced (by 50% or more) covalent binding of
N-[ethyl-1-14C]-nitroso-diethylamine to macromolecules in trachea,
lung, and liver (Schuller & McMahon, 1985).
Eight-week-old male CD-1 mice were fed diets containing piperonyl
butoxide at concentrations adjusted to provide doses of 0, 10, 30,
100, or 300 mg/kg bw per day or sodium phenobarbital at 0.05% (w/w)
for 42 days. Replicative DNA synthesis was studied by implanting
seven-day osmotic pumps to administer 5-bromo-2'-deoxyuridine (BrDU)
during days 0-7 and 35-42 of treatment; liver sections were
immunostained with an antibody to BrDU, and the percentage of
hepatocyte nuclei undergoing replicative DNA synthesis was calculated
by microscopic examination of at least 1000 nuclei. Xenobiotic
metabolism was assessed by measuring the liver P450 content and the
activities of 7-ethoxyresorufin O-deethylase, 7-pentoxyresorufin
O-depentylase, and ethylmorphine N-demethylase in liver
microsomes. Sodium phenobarbital was used as a positive control. The
relative liver weight was increased by about 15% after seven days of
treatment with 300 mg/kg bw per day of piperonyl butoxide and by about
20% with sodium phenobarbital; after 42 days, it was increased by
about 10% after treatment with 100 and by about 20% after treatment
with 300 mg/kg bw piperonyl butoxide. Midzonal liver-cell hypertrophy
was observed in mice given 300 mg/kg bw per day for seven or 42 days
or 100 mg/kg bw per day for 42 days. Sodium phenobarbital induced
centrilobular hypertrophy after either seven or 42 days of treatment.
Replicative DNA synthesis was induced to 350% by the high dose of
piperonyl butoxide and to 825% by sodium phenobarbital for seven days;
no significant increase was observed after 42 days at any dose. A
dose-related increase in microsomal protein and P450 content was
observed on day 42, and the increases were statistically significant
at doses > 100 mg/kg bw. Inconsistent increases were observed in
the activities of 7-ethoxyresorufin O-deethylase and 7-pento-
xyresorufin O-depentylase in piperonyl butoxide-treated mice, but a
dose-related increase from 20 to 50% in the activity of ethylmorphine
N-demethylase was seen on day 42. Treatment with sodium pheno-
barbital for 42 days resulted in increases in all of the measured
parameters. Piperonyl butoxide (and sodium phenobarbital) thus caused
a transient stimulation of liver-cell replication and a permanent
effect on liver weight, morphology, and enzyme induction at doses that
induced liver nodules in long-term studies (Phillips el al., 1995).
2. Toxicological studies
(a) Acute toxicity
Groups of five Sprague-Dawley CD rats of each sex were exposed by
inhalation for 4 h to a mean analytical concentration of 5.9 mg/litre
piperonyl butoxide (purity, 90.78%). The particle median aerodynamic
diameter was 2.6 µm; about 15% of the aerosol was < 1 µm in diameter
and about 95% < 10 µm. All animals survived the exposure and the
15-day observation period after treatment. Exposure caused excessive
lacrimation and salivation, nasal discharge and laboured breathing.
All animals recovered. No remarkable signs were seen post mortem
(Hoffman, 1991). Other data are reported in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were fed
diets containing 0, 1000, 3000, or 9000 ppm piperonyl butoxide (purity
unspecified) for 20 days. Animals were observed daily for mortality;
body weight was measured and clinical observations were made on days
1, 2, 3, 7, 14, and 20; the food consumption of five animals per cage
was measured on days 0-3, 3-7, 7-14, and 14-20. At the end of
treatment, blood clinical chemistry was assessed, liver, kidneys, and
spleen were weighed, and livers and kidneys were examined
histologically. No deaths occurred. The body weights of animals at the
high dose were about 15% lower than those of controls at the end of
the study, and those of females at the middle dose were about 10%
lower on day 2 and about 8% lower at termination (not statistically
significant). The lowered body weights were apparently associated with
decreased food intake. The weights of livers showed a dose-related
increase in both males (by 22% at the low dose, 63% at the middle
dose, and 79% at the high dose) and females (62% at the middle dose
and 78% at the high dose); at the high dose, the weights of the
kidneys were reduced by 29% in males and 18% in females, and the
weights of the spleen by 25% in males and 33%, in females. In animals
at the high dose, a 31% increase in serum cholesterol was seen in
males and a 67% increase in females, a 28% increase in serum
phospholipids was seen in males and a 38% increase in females, a 19%
Table 1. Acute toxicity of piperonyl butoxide in animals
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse NR Oral 4.0 NR Negherbon (1959)
Rat Male Oral 4.57 90.78 Gabriel (1991a)
Female 7.22
NR Oral 8.0-10.6 NR Sarles et al. (1949)
NR Oral 13.5 NR Lehman (1948)
NR Oral 11.5 NR Lehman (1951)
Male, female Inhalation (4 h) > 5900 90.78 Hoffman (1991)
NR Subcutaneous > 15.9 NR Sarles et al. (1949)
Rabbit Male, female Dermal > 2 90.78 Gabriel (1991b)
NR Oral 2.7-5.3 NR Sarles et al. (1949)
Cat NR Oral > 10.6 NR Sarles et al. (1949)
Dog NR Oral > 8.0 NR Sarles et al. (1949)
NR, Not reported
increase in total serum proteins occurred in males and an 18% increase
in females, and a 235% increase in gamma-glutamyl transpeptidase was
seen in females. Some of these levels were also increased in the
animals at the middle dose. Microscopic examination of the liver
showed hypertrophic hepatocytes, single-cell necrosis, and cell
infiltration in the centrilobular area in all mice at the high dose.
The kidneys were unremarkable. The NOAEL for effects on the liver was
1000 ppm, equivalent to 150 mg/kg bw per day (Fujitani et al.,
1993a).
Groups of 20 Crj:CD-1 male mice were fed diets containing 0,
1500, 3000, or 6000 ppm piperonyl butoxide (purity unspecified) for
seven weeks. Food intake was measured every week and behavioural tests
were performed at weeks 4 (exploratory behaviour), 6 (multiple water
T-maze), and 7 (exploratory behaviour) of treatment. A treatment-
related reduction in food intake was observed in animals at the middle
and high doses during the first week of treatment. No consistent,
significant effect was observed in the T-maze test or in the
exploratory behaviour test at week 4. At week 7, some parameters in
the exploratory behaviour test were altered (Tanaka, 1993).
Rats
Hypertrophy of periportal cells with slight fatty changes were
observed in rats fed a diet containing 5000 ppm piperonyl butoxide for
17 weeks (Lehman, 1952a,b).
Feeding of six weekly doses of up to 224 mg/kg bw of active
ingredient to rats caused no observable effects at autopsy three weeks
after the final dose, but few details were given (Sarles et al.,
1949).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were fed diets containing concentrations of
piperonyl butoxide adjusted to obtain doses of 0, 62.5, 125, 250, 500,
1000, or 2000 mg/kg bw per day for four weeks. All animals were
observed for mortality and morbidity twice daily, and body weight and
food consumption were measured weekly. Haematological and biochemical
tests were performed on day 24 or 25 of treatment. At termination,
survivors were necropsied, certain organs were weighed and microscopic
examinations were performed on several organs. Six animals died before
termination, but the deaths were considered to be due to anaesthesia
for bleeding. General signs of toxicity, such as prominent backbone,
thinness, poor fur condition, brown staining and piloerection, were
observed in animals at the highest dose. Body-weight gain was reduced
by about 20% in females at 500 mg/kg bw per day, by 27% in males and
37% in females at 1000 mg/kg bw per day, and by 66% in males and 92%
in females at 2000 mg/kg bw per day. These reductions were only
partially associated with reduced food intake. Haematology and
clinical biochemistry showed no significant alterations. Absolute and
relative liver weights were increased in animals given doses >
500 mg/kg bw per day; the relative liver weights were also increased
in males at 250 mg/kg bw per day. The relative weights of the
adrenals, kidneys, and brain were slightly increased in the group at
the highest dose and in some animals at 1000 mg/kg bw per day.
Eosinophilia and loss of vacuolation in hepatocytes were noted in all
treated animals, and the severity of these lesions was dose-related.
These may be adaptive changes. Necrosis of hepatocytes and cytoplasmic
inclusions were observed in animals at 1000 and 2000 mg/kg bw per day.
The NOAEL was 125 mg/kg bw per day on the basis of effects on the
liver (Modeweg-Hansen et al., 1984).
In a study preliminary to the study of carcinogenicity by the
same authors summarized below, groups of 10 Fischer 344/DuCrj rats of
each sex were fed diets containing 0, 2500, 5000, 10 000, 20 000, or
30 000 ppm piperonyl butoxide (purity, 89%) for 13 weeks. At
termination, survivors were necropsied, and limited microscopic
examination was performed. One male at the high dose died before
termination. The final body weights and body-weight gains were reduced
in a dose-related manner in all treated males; in females, a
significant effect was seen only at the two highest doses. Liver
weights were increased in all treated animals, by up to 50% in males
and 108% in females. Absolute kidney weights were increased in animals
at the high dose, and the relative weight was also increased in groups
at lower doses. Hepatocyte hypertrophy and focal necrosis were seen in
animals at higher doses (data not shown). No relevant macro- or
microscopic lesions were observed in the digestive tract, but the
caecum was not examined histologically. There was no NOAEL because of
effects on the liver at all doses (Maekawa et al., 1985).
Groups of 15 Charles River CD rats of each sex were exposed by
inhalation for 6 h per day, five days per week, for 13 weeks to
piperonyl butoxide (purity, 90.78%) at mean analytical concentrations
of 15, 74, 155, or 512 mg/m3. The highest concentration was the
maximum that could be generated at the appropriate particle size.
Fifteen controls were exposed to conditioned room air only. The
concentrations in chamber air were monitored gravimetrically four
times per day and by gas chromatography once a day. Particle size
distribution was measured once during each exposure. The test aerosol
had a mass median aerodynamic diameter of 1.7 µm with a geometric
standard deviation of 2.6. On average, 37% of the particles were <
10 µm in diameter and 29% < 1 µm. Abnormal signs were looked for once
during each exposure, and detailed physical examinations were
conducted on all animals once before treatment and weekly during
treatment. Ophthalmoscopic examinations were performed on all animals
before treatment and on the day before sacrifice. Body weight was
recorded before exposure, immediately before exposure on test day 1,
weekly thereafter, and just before sacrifice. At termination,
haematological and blood chemical parameters were evaluated in all
animals, selected organs were weighed, complete gross post-mortem
examinations were conducted, and selected tissues from all animals
were examined microscopically.
Body-weight gain and food consumption were not affected by
exposure, and all animals survived to the end of treatment.
Ophthalmoscopic examinations showed no indication of exposure-related
ocular effects. There was a dose-related increase in nasal discharge,
dried material on the facial area and extremities, and anogenital
staining in animals at 155 and 512 mg/m3. The serum levels of
aspartate and alanine aminotransferases and of glucose were slightly
decreased (10-28%), while blood urea nitrogen, total protein, and
albumin levels were slightly increased (4-10%) in animals of each sex
at the high dose. Not all of these differences were statistically
significant, and a dose-response relationship was not seen
consistently; however, a similar trend was seen in animals of each
sex. Statistically significant increases in the absolute and relative
weights of the livers and kidneys were seen in animals at the high
dose; the relative kidney weights were increased by 10-12%, and the
absolute and relative liver weights were both increased by 20-29%.
Relative liver weights were also significantly elevated (8-9%) in
males and females at 155 mg/m3. Vesiculation and vacuolation of the
hepatocellular cytoplasm was seen in almost all treated animals but
tended to be more pronounced in those at the highest level. No clear
dose-related increase in either incidence or severity was observed.
Squamous or squamoid metaplasia of the pseudostratified columnar
epithelium of the larynx was seen in several exposed animals and one
control female, the severity being greater in animals at the high
dose. Similar metaplastic changes were seen in the columnar epithelium
lining the ventral diverticulum in several animals at 512 mg/m3 and
in one female at 15 mg/m3. Hyperplasia and hyperkeratosis of the
squamous epithelium normally found in the larynx were seen in a small
number of animals at 512 mg/m3. Laryngeal mucosal inflammation was
seen in all treated animals and was slightly more severe in animals at
the highest dose. All of the changes were considered to be localized
responses indicative of irritation rather then systemic toxicity.
Other findings in tissues and organs examined macroscopically and
microscopically post mortem were unremarkable. No systemic toxicity
was seen at 155 mg/m3; effects on the liver and kidney were seen at
512 mg/m3 (Newton, 1992).
Groups of 10 male and 10 female Fischer 344/DuCrj rats were fed
diets containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide
(technical grade of unspecified purity) for 13 weeks. Animals were
observed daily for mortality; body weight and clinical signs were
monitored daily for the first five days and then twice weekly. Food
and water consumption was measured on days 4, 11, 18, and 39 for about
six rats in each group. At the end of treatment, haematological and
blood chemical parameters were determined, animals were necropsied,
and selected organs were weighed; the liver and kidneys were examined
histologically. There were no deaths. Clinical signs consisted of nose
bleeds from about day 2 to day 20 and dose-dependent abdominal
distension. In animals at the high dose, food consumption was reduced
by about 46% and water consumption by 28% on day 4 but not at other
times. The body weights of animals at the high dose were significantly
reduced, by 36% in males and 24% in females. Blood haemoglobin levels
were decreased in a dose-dependent manner, but the decrease was
statistically significant only in animals at the high dose (by 10-11%)
and females at the middle dose (by 7%). A reduced mean corpuscular
haemoglobin content was also observed in females at the high dose.
Serum albumin was increased by 16-23%, cholesterol by 88-93%,
gamma-glutamyl transpetidase activity to five to six times the control
values in males at the high dose, and urea nitrogen by 24% in males at
that dose. Serum protein was increased in all treated females and
serum phospholipid in females at the high dose; decreases were seen in
serum levels of triglyceride in all males and bilirubin and glucose in
males at the high dose. Absolute and relative liver weights were
increased in a dose-related manner, but were significantly increased
only in males at the middle dose (by 47%) and males and females at the
high dose (by 57-94%). Relative kidney weights were increased in a
dose-related manner to up to 1.42% of control values. Hypertrophic
hepatocytes containing a basophilic, fine granular substance were seen
in animals at the high dose. In the periportal area, marked
vacuolation of hepatocytes was observed, with occasional coagulative
necrosis and oval-cell proliferation. In male rats, atrophy of the
epithelium of the proximal convoluted tubules was observed in the
renal cortex. There was no NOAEL because of effects on the liver and
kidney (Fujitani et al., 1992).
In a subsequent study performed under the same experimental
conditions, haematological, clinical chemical, and morphological
observations after 1, 2, 4, or 12 weeks of treatment showed that all
of the alterations occurred in a time-dependent manner, and that some
of them (increased liver and kidney weights, morphological alterations
in the liver) were already present after one week (Fujitani et al.,
1993b).
Rabbits
Three weekly doses of up to 108 mg/kg bw active ingredient of a
5% emulsion of piperonyl butoxide showed no effects at autopsy three
weeks after the final dose, but few details were given (Sarles
et al., 1949).
Dogs
Groups of one to three dogs of each sex were given piperonyl
butoxide at 0, 3, 32, 106, or 320 mg/kg per day orally in capsules on
six days per week for one year. All dogs at the two highest doses lost
weight, but large variations in body-weight gain and the small number
of animals prevented meaningful comparisons between the controls and
animals at the lower doses. A dose-related increase in relative liver
weight (no statistical analysis reported) was observed, which was
associated with unspecified histopathological changes. Increased
kidney and adrenal weights were observed at 100 and 320 mg/kg bw per
day (Sarles & Vandergrift, 1952).
Groups of two beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for eight weeks at concentrations
corresponding to 500, 1000, 2000, or 3000 ppm active ingredient. The
diets were prepared weekly; the actual concentrations and the
homogeneity of the mixture were measured at three intervals and found
to be satisfactory. Animals were observed at least twice daily for
mortality and signs of toxicity; detailed observations were recorded
at least once weekly, and body weight and food consumption were
recorded weekly. Physical examinations and haematological and
biochemical tests were conducted on all animals before exposure and at
termination. All major tissues and organs were examined
microscopically post mortem. Selected organs were weighed. All
animals survived to study termination. Reduced body-weight gains were
seen in males and females receiving 1000 ppm (14 and 9% of the initial
body weight, respectively), 2000 ppm (6 and 7%), and 3000 ppm (7 and
4%) ppm in comparison with the control group (17% and 14% increases).
Decreased food consumption (by 19 and 23%) was observed animals at
3000 ppm. There were no treatment-related changes in haematological
parameters. Males and females at 2000 and 3000 ppm had alkaline
phosphatase activities that were about 1.5 times the control value and
increased absolute and relative liver and gall-bladder weights. No
macroscopic changes were observed that could be attributed to
treatment, and treatment-related microscopic changes were limited to
diffuse, mild hypertrophy of hepatocytes in all treated males and in
females at the two highest doses. There was no NOAEL because of
effects on the liver (Goldenthal, 1993a).
Groups of four beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for one year at concentrations
corresponding to 100, 600, and 2000 ppm active ingredient. The diets
were prepared weekly and stored at room temperature. The stability of
the compound was assessed twice: an initial test showed a loss of
about 10% over 10 days, possibly due to technical problems; a second
test showed 97-101% of the initial concentration on day 10. The actual
concentrations were measured 16 times and were found to be 87-110% of
the nominal concentrations. The homogeneity of the mixture was also
found to be satisfactory. The dogs were observed at least twice daily
for mortality and signs of toxicity; detailed observations were
recorded weekly. Body weight and food consumption were recorded weekly
for the first 14 weeks and every two weeks thereafter. Ophthalmoscopic
examinations were conducted before the beginning of treatment and at
termination. Physical examinations were conducted on all animals
before exposure and after 3, 6, 9, and 12 months of treatment.
Haematological and biochemical tests and urinalyses were conducted on
all animals after 6 and 12 months. All animals were examined
post mortem, and all major tissues and organs were examined
microscopically. Selected organs were weighed. All animals survived to
the end of the study, with no relevant clinical or ophthalmological
signs. The body weights of males and females receiving 2000 ppm
piperonyl butoxide were 2% lower to 3% higher than the initial
weights, whereas those of controls were 22-25% higher. Treatment-
related decreases in food consumption were observed in males at 600
(by 15%) and 2000 (by 20%) ppm. Small, not statistically significant,
not dose-related decreases in food consumption were observed in
treated females.
No treatment-related change in haematological parameters was
observed. The activity of alkaline phosphatase was increased to three
to five times the control values in animals at 2000 ppm after 6 and 12
months, and cholesterol levels were decreased (not statistically
significant) at these intervals in females. Males and females at this
dose had increased absolute (by 22 and 36%) and relative (by 52 and
86%) liver and gall-bladder weights, and small increases in thyroid
and parathyroid weights (average, 34%) were observed in females. These
changes were not associated with microscopic changes in the thyroid
and were considered to be of questionable biological significance. No
macroscopic pathological changes were observed that could be
attributed to treatment. Treatment-related histopathological changes
were limited to diffuse, mild hypertrophy of the hepatocytes in males
and females at 2000 ppm. The NOAEL was 100 ppm, equal to 16 mg/kg bw
per day, on the basis of effects on the liver, some clinical chemical
alterations, and reduced body-weight gain at 2000 ppm (Goldenthal,
1993b).
Monkeys
Two African green monkeys were given 32 or 106 mg/kg bw per day
piperonyl butoxide orally for six days per week for four weeks.
Minimal microscopic changes were observed in the livers (Sarles &
Vandergrift, 1952).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 (C57Bl/6 × C3H/Anf)F1 and (C57Bl/6 × AKR)F1 mice of
each sex received piperonyl butoxide (purity unspecified) at 100 mg/kg
bw or 'Butacide' at 464 mg/kg bw in 0..5% gelatin at seven days of age
by stomach tube and the same amount (not adjusted for increasing body
weight) daily up to four weeks of age; subsequently, the mice were fed
diets containing 300 ppm. The dose was reported to be the maximal
tolerated dose for infant and young mice. All animals were killed when
they were about 70 weeks of age, and their tumour incidences were
compared with those of 79-90 necropsied mice of each sex and strain,
which had either been untreated or had received gelatine only. No
significant difference in the incidence of tumours was found between
treated and control mice, although the authors concluded that
additional evaluation was required (Innes et al., 1969). This study
was not considered to be adequate by the Meeting.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for 30 weeks;
the doses were decreased to 500 or 2000 ppm for the following 82
weeks, due apparently to a greater than expected decrease in
body-weight gain. The control group consisted of 20 males and 20
females. Animals were observed twice daily for signs of toxicity; body
weight was recorded at least once per month. Necroscopic examination
was performed at termination on all moribund animals and on those
found dead, unless precluded by autolysis. Major tissues and organs
and all gross lesions were examined microscopically. At termination,
85% of control males and 75% of control females, 82% and 68% at the
low dose, and 84% and 70% at the high dose, respectively, were still
alive. Body weight was reduced in a dose-related manner, by up to
about 15% in females at the high dose at termination. No
treatment-related increase in tumour incidence was found, but
hepatocellular carcinomas occurred frequently, especially in males,
with incidences of 50% in controls, 34%, at the low dose, and 40% at
the high dose. No statistically significant differences were found in
the incidences of neoplastic and non-neoplastic lesions (US National
Cancer Institute, 1979)
Groups of 60 CD-1 mice of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) at concentrations adjusted to give
doses of 0 (two control groups), 30,100, or 300 mg/kg bw per day, for
78 weeks. The diets were prepared weekly and the concentrations
adjusted on the basis of body weights and food consumption. The diets
were analysed for the actual concentration, homogeneity, and stability
of piperonyl butoxide. The average actual concentrations at the low
and high doses were 99 and 95%. The homogeneity and stability (no
decrease in concentration after 14-21 days) of piperonyl butoxide in
the diet were considered to be satisfactory. Animals were observed
twice daily for signs of toxicity. Food consumption and body weight
were determined weekly for the first week and then every other week.
Haematological measurements were made for 10 rats of each sex in the
control and high-dose groups at week 52 and for all groups at
termination. All surviving animals were necropsied; major tissues and
organs from controls and animals at the high dose and the lungs,
liver, kidneys, and gross lesions from other groups were examined
microscopically. All slides from the livers underwent an independent
pathological review; the results reported here are the consensus or
majority opinions of all the pathologists involved.
No treatment-related clinical signs or palpable masses were
observed. The mortality rates were (males/females) 27/32, 32/27,
27/38, 22/33, and 40/18% in the first control group, animals at 30,
100, and 300 mg/kg bw per day, and the second control groups,
respectively. Body weights and body-weight gains of animals at the
high dose were slightly (occasionally statistically significantly)
decreased. No significant effects on food consumption were observed.
Haematological parameters were also unaffected by treatment. A
dose-related increase in absolute and relative liver weights was
observed in mice of each sex at the middle and high doses.
Hepatocellular hypertrophy was more frequent in males at the high
(72%) and middle doses (27%) than in controls (10 and 18%) and in
females at the high dose (15%; 0 and 7% in controls). Hepatocellular
hyperplasia (also called eosinophilic/clear-cell focus), characterized
by aggregates of hepatocytes with a swollen eosinophilic cytoplasm,
was present in 8% of males at the high dose, 2% of females at the
middle dose, and 7% at the high dose. The incidences of hepatocellular
hyperplasia were 0 and 3% in control males and 0% in both female
control groups. The incidence of hepatocellular adenomas was higher in
male mice at the middle (37%) and high (47%) doses than in either
control group (15 and 13%) and in females at the high dose (17%; 3% in
controls). The hepatocellular adenomas observed in many treated
animals were composed of large, polyhedral, densely-packed cells with
abundant granular eosinophilic cytoplasm. This appearance is different
from that of spontaneous adenomas found in CD-1 mice, where small to
medium, well-differentiated, basophilic cells, distributed in solid to
normal sinusoidal patterns, are found. Only the incidence of the
former type of adenoma was increased in piperonyl butoxide-treated
mice. A nonsignificantly increased incidence of hepatocellular
carcinomas was observed in males at the high dose (12%; 3% in
controls). No other treatment-related neoplastic or non-neoplastic
lesion was found. The NOAEL was 30 mg/kg bw per day on the basis of
effects on the liver (Hermanski & Wagner, 1993).
A 12-month study of carcinogenicity was conducted in male
Crj:CD-1 mice fed diets containing 0 ( n = 52), 6000 ( n = 53), or
12 000 ( n = 100) ppm piperonyl butoxide (purity, 94.3%; no
detectable safrole or isosafrole). Animals were observed daily for
mortality and morbidity; those that died or were sacrificed at
termination were necropsied, and gross lesions and the liver were
examined histologically. The mortality rates were 6% of controls, 2%
at the low dose, and 19% at the high dose. Terminal body weight was
found to have been reduced by 17% at the low dose and 29% at the high
dose. Hepatocellular hyperplasia was seen in 38% of animals at the low
dose and 8% at the high dose, hepatocellular adenomas in 13 and 22%,
hepatocellular carcinomas in 11 and 52%, and haemangioendothelial
sarcoma in 2 and 42%. It should be noted that the hepatocellular
adenomas and carcinomas may have obscured the true dose-response
relationship for hepatocellular hyperplasia. A condition termed
'post-necrotic peliosis', consisting of multifocal necrosis with blood
cysts, was also found in 17% of animals at the high dose. One control
animal showed hepatocellular hyperplasia and one had a hepatocellular
adenoma (Takahashi et al., 1994a).
Rats
Groups of 12 Wistar rats of each sex were fed diets containing 0,
100, 1000, 10 000, or 25 000 ppm piperonyl butoxide for two years, and
an additional group was fed a diet containing 1000 ppm piperonyl
butoxide plus 167 ppm pyrethrins. Animals were observed daily for
clinical signs of toxicity, and food consumption and body weight were
determined weekly. Autopsies were performed on all animals. Males and
females were paired for the duration of the study, except during
nursing (see section ( d)). Body-weight gain was reduced in animals
at 10 000 (by 10-20%) and 25 000 ppm (by about 90%), in association
with reduced food intake. All animals at the high dose were dead by
week 68; the mortality rates in the other groups were (males/females):
30/20%, 40/30%, 40/50%, and 60/60% for controls and animals at 100,
1000, and 10 000 ppm, respectively. The relative weights of livers and
kidneys were higher than those of controls in animals at 10 000 (by
40% for liver and kidney) and 25 000 ppm (by 275% for liver and 150%
for kidney). These effects were not associated with morphological
alterations. The incidence of tumours was not increased (Sarles &
Vandergrift, 1952).
Sixty Sprague-Dawley Crl:CDR (SD)BR rats of each sex were fed
diets containing piperonyl butoxide (purity, 89%) at concentrations
adjusted weekly (every two weeks after week 15) to achieve daily
intakes of 0 (two groups of controls), 30, 100, or 500 mg/kg bw per
day for 104-105 weeks. The stability and homogeneity of the diets and
the correspondence of actual to nominal concentrations were checked
before and throughout the study and found to be acceptable. The
animals were observed for mortality, clinical signs, food consumption,
body weight, and ophthalmoscopic, haematological, clinical
biochemical, urinary, and pathological parameters. Complete
histological examinations were made of animals in the two control
groups and at the high dose and of those animals at the low and
intermediate doses which died during the study. Histological
examination of animals at these doses killed at the end of the study
was limited to the liver, kidney, lung, thyroid, testis, epididymides,
ovary, and any observed abnormalities.
No clinical signs related to treatment were observed.
Sialodacryoadenitis was diagnosed in a large percentage of treated
animals in weeks 25-28 and weeks 63-67. Body weights were lower in
rats of each sex at 500 mg/kg bw per day than in controls throughout
the study. At week 104, the mean body weight was 20-30% lower than the
control value. A trivial reduction in food consumption was observed in
animals of each sex at the highest dose. Ophthalmoscopic examination
in week 99 revealed no changes attributable to treatment, and
haematological tests and urinalysis revealed no adverse effects.
Female rats receiving 500 mg/kg bw per day had higher cholesterol
levels than controls throughout the study and increased blood urea
nitrogen at 98 weeks. Other statistically significant differences in
biochemical parameters were of no biological relevance. At the end of
the study, the mortality rates were 82, 78, 87, 82, and 78% in males
and 55, 68, 63, 43, and 50% in females at 0 (two groups), 30, 100, and
500 mg/kg bw per day, respectively.
Increased liver weights were seen in animals of each sex at 100
and 500 mg/kg bw per day, corresponding to a higher incidence of
macroscopic enlargement associated with hyperplasia and hypertrophy of
centrilobular hepatocytes and enlarged eosinophilic cells, which
occasionally contained a brownish cytoplasmic pigment. Increased
kidney weights were observed in female rats at the two highest doses,
corresponding histologically to a higher incidence of chronic
interstitial glomerulonephritis. Other histological changes were
confined to the endocrine organs: Enlarged thyroid glands were
observed in animals of each sex at 500 mg/kg per day, corresponding
histologically to a higher incidence of generalized and focal
hyperplasia of follicles and increased pigment deposition in the
colloid. A slightly higher incidence of adrenal and ovarian
enlargement seen among females receiving 500 mg/kg per day was not
associated with histological changes. In males, there was a negative
trend in the presence of enlarged, focal, coarsely vacuolated cortical
cells in the adrenals. The combined incidence of bilateral and
unilateral testicular atrophy was comparable between groups, but there
were significant differences from controls in the incidences of
bilateral testicular atrophy at all doses, with 17, 33, 47, and 43% at
0, 30, 100, and 500 mg/kg bw per day, respectively. After an
additional, full review of the relevant data, this finding was
considered unlikely to be related to treatment because the atrophy was
not associated with degeneration of seminiferous tubules or
aspermatogenesis, and testicular weights were not decreased.
Morphometric analysis of the pituitary gland area showed reduced size
in males at the highest dose. A trend analysis of tumour incidence
with dose showed both increases (in the lymphoid system and thyroid)
and decreases (in mammary glands and pituitary). These differences
were not statistically significant in pairwise comparisons between
groups, and the incidences were within the range of those of
historical controls of this strain of rats. These observations provide
no evidence that the treatment had carcinogenic potential, but the
wide range of gross alterations, effects on organ weight, and
histological changes observed reflect the biological activity of the
test compound. The enlargement of the liver, the primary target organ,
is consistent with the activity of the compound as a hepatic enzyme
inducer. The wide differences in the incidences of morphological
changes and lesions in endocrine and hormone-sensitive organs between
controls and treated groups strongly support the interpretation that
they are the results of changes in hormonal levels brought about by
hepatic enzyme induction. The NOAEL was 30 mg/kg bw per day on the
basis of effects on the liver (Graham, 1987).
Groups of 50 Fischer 344/Du Crj rats of each sex were fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide (purity, about
89%) for two years at doses selected on the basis of the results of
the 13-week study by the same authors, summarized above. Animals were
observed daily for clinical signs and mortality; food consumption was
measured monthly, but the frequency of body-weight measurement was not
stated. Treatment was stopped after 104 weeks, and surviving animals
were sacrificed after six more weeks on the basal diet. All animals,
including those sacrificed when moribund or found dead, were
necropsied and a complete histological examination was performed. The
mortality rates were (males/females) 16/14, 38/22, and 42/34% in
controls and animals at 5000 and 10 000 ppm; the increased mortality
was statistically significant for animals at the high dose. Just
before death, many animals showed signs of anaemia (not specified) and
had blood in their faeces. A dose-related reduction in body weight was
seen in animals of each sex (statistical analysis not reported), with
no concomitant decrease in food intake. No statistically significant
difference in the incidence of malignant or benign neoplasms was
found, except a decreased incidence of thyroid C-cell adenomas in
males at the high dose (9%; 44% in controls). Dose-related increases
were seen in the incidences of ulcers (0/0%, 35/2%, and 52/45% in male
and female controls and those at the low and high doses,
respectively), regenerative hyperplasia (0/0%, 13/0%, and 22/4%),
ossification in the ileocaecal mucosa (0/0%, 15/0%, and 20/0%), and
haemorrhage of the caecum and colon (0/0%, 10/0%, and 17/12%). The
main histological lesions were chronic ulcers with inflammatory-cell
infiltration and granulation, sometimes with ossification of the
surrounding mucosa. Most of deaths in treated animals before the end
of the study were attributed by the authors to lesions leading to
anaemia. Piperonyl butoxide was not found to be carcinogenic
(Maekawa et al., 1985).
Groups of 50 Fischer 344 rats of each sex were fed diets
containing 5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for
24 months; the control group consisted of 20 animals. The diets were
prepared freshly each week and stored at 7°C. Animals were observed
twice daily for mortality and underwent a complete clinical
examination monthly. The food consumption of 10 animals in each group
was measured during the first week of each month; body weights were
measured about twice monthly. Moribund animals were sacrificed and
necropsied and their organs were examined histologically. All
surviving animals were sacrificed and submitted to a complete
histopathological examination. All control females and about 80% of
treated females survived until termination, whereas no difference in
survival was found for males (70-75% survival in all groups). A
dose-related reduction in body weight was found for both females (16%
at the low dose and 24% at the high dose) and males (7% at the low and
16% at the high dose). No difference was found in the incidence of
malignant or benign neoplasms, except for that of lymphoreticular
malignant lymphoma which was significantly increased in treated
females (14% at the low and 30% at the high dose; 5% in controls) and
nonsignificantly decreased in males (30% at the low and 26% at the
high dose; 35% in controls). The author concluded that the results
were equivocal because of the variability in the historical control
data and the difficult diagnosis of these neoplasms (Cardy et al.,
1979; US National Cancer Institute, 1979).
Groups of 30-33 Fischer 344/DuCrj rats of each sex were fed diets
containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide (two
lots: purity, 94.5 and 94.3%; no detectable safrole or isosafrole) for
95 (males) or 96 (females) weeks. The study was ended before 104 weeks
because of the high mortality of males at 12 000 ppm (see below).
Animals were observed daily for clinical signs and mortality; body
weights were measured monthly. Food consumption was determined in a
preliminary experiment involving six rats of each sex per group
(duration not reported), which resulted in calculated compound intakes
of 537, 1061, and 2002 mg/kg per day by females and 547, 1052, and
1877 mg/kg per day by males at 6000, 12 000 and 24 000 ppm,
respectively. Rats that died were necropsied and observed for tumors
and non-neoplastic lesions, which were then examined microscopically.
At termination, surviving rats were necropsied; hepatic nodules (when
multiple nodules were present, three major ones were taken) and major
organs were weighed and examined histologically. Blood was collected
for haematological tests (including prothrombin and partial
thromboplastic times) and for standard clinical chemistry. The
mortality rates were 17, 23, 50, and 24% in males and 20, 10, 17, and
21% in females at 0, 6000, 12 000, and 24 000 ppm, respectively. Males
at 12 000 ppm began to die as early as week 40 and at a statistically
significantly different rate from other groups from week 45. Deaths
were most frequently associated with haemorrhage in the caecum. Body
weights were reduced in a dose-related manner in all treated animals
but statistically significantly only in animals at the middle and high
doses. Males and females at the high dose lost weight from week 60,
and at termination their body weights were about 50% of those of
controls. The weights of all organs except the liver were reduced in
animals at the high dose. The absolute liver weights were increased in
animals at 12 000 ppm (in males by 22% and in females by 51%) and in
females at 6000 ppm (by 29%). During the first month of treatment,
rough hair, lethargy, epistaxis, and a fall in food consumption (data
not reported) were observed in animals of each sex at 24 000 ppm.
Haematological tests showed a significant increase in hypochromic,
microcytic anaemia, the severity of which was dose-related, in all
treated animals. The most significant findings in plasma were
decreased cholinesterase activity and triglyceride contents in animals
at all doses and an increased urea nitrogen level in animals at the
high dose and in all treated females. Hepatic tumours were observed
from week 74 of treatment. Nodular lesions of the liver were observed
only in treated animals, and their incidence, numbers per rat, and
size were dose-related. Hepatocellular adenomas and carcinomas were
found in animals at the middle and high doses: the incidences of
adenomas were 27% in males and 13% in females at 12 000 ppm and 15% in
males and 30% in females at 24 000 ppm; the incidences of carcinoma
were 13% in males and 0% in females at 12 000 ppm and 73% in males and
46% in females at 24 000 ppm. The nodules in animals at the low dose
were classified as foci or hepatocellular focal hyperplasia. The only
other difference between groups was found for interstitial-cell
tumours of the testis, with incidences of 23/25 in controls, 19/23 at
6000 ppm, 13/15 at 12 000 ppm, and 15/25 at 24 000 ppm. Essential
(haemorrhagic) thrombocytaemia (defined as > 3 × 106 platelets per
microlitre, with a bimodal platelet distribution curve) was found in
0/24 male controls and in 6/23 males at 6000 ppm, 3/15 at 12 000 ppm,
and 9/24 at 24 000 ppm and in only 1/25 females at the high dose. Of
the non-neoplastic lesions, the following are significant: an
increased incidence of stomach haemorrhage (males: 9/33; 5/30 in
controls; females: 19/33; 2/30 in controls) at the high dose, an
increased incidence of haemorrhage and/or oedema of the caecum in all
treated males and in females at the middle dose, an increased
incidence of black kidneys in males at the middle and high doses and
all treated females, and an increased incidence of whitish spotting in
the lungs of males at the middle and high doses. Tubular dilatation,
distension of Bowman's space and interstitial fibrosis were found in
the kidneys of animals at the high dose (actual numbers not reported)
(Takahashi et al., 1994b).
(d) Reproductive toxicity
Mice
In a two-generation study, with one litter per generation, groups
of 10 male and 10 female Crj:CD-1 mice were fed diets containing 0,
1000, 2000, 4000, or 8000 ppm piperonyl butoxide (purity unspecified).
Animals of the F0 generation, five weeks old at the start of the
study, were mated for five days at nine weeks of age. Animals of the
F1 generation was removed from their dams at four weeks of age and
allocated randomly to continue treatment; the F2 generation was
produced similarly to the F1 generation. Individual food intake was
measured before mating and during mating, gestation, and lactation for
all generations; litter size and weight and sex ratio were measured at
birth, and pups were weighed 0, 4, 7, 14, and 21 days after birth.
Some neurobehavioural tests were performed during lactation of the
F2 generation and the results analysed on a litter basis. The tests
included surface righting and negative geotaxis on postnatal days 4
and 7, cliff avoidance on postnatal day 7, swimming behaviour on
postnatal days 4 and 14, and olfactory orientation on postnatal day
14. Food consumption was reduced by 4-44% in F0 animals at 8000 ppm
except during mating, by 47% in F1 animals at 8000 ppm during
lactation, and by 16-22% in F0 and F1 animals at 4000 ppm during
lactation. The mean litter size of F1 animals at 8000 ppm was not
significantly reduced (7.7; 10.6 in controls), but the mean F1
litter weight was reduced by 38% (statistically significant) at
8000 ppm and by 18% (not statistically significant) at 4000 ppm. F1
pups at 8000 ppm had a lower survival index at postnatal day 21 (63%;
91% in controls for males, statistically significant; 79%; 89% in
controls for females, not statistically significant). The weights of
all F1 pups were reduced, but there was no dose-response
relationship at 1000-4000 ppm. The results of neurobehavioural tests
for treated F1 animals were different from those of controls in some
instances, but no dose-related response was evident. The mean F2
litter size was significantly reduced at 4000 ppm (10.3; 13.1 in
controls) and 8000 ppm (7.0), and the mean F2 litter weights were
reduced in all treated groups by 13-57%. F2 pups at 8000 ppm had a
lower survival index than controls at postnatal day 21 (59% in males,
statistically significant; 79% in females, not statistically
significant), and F2 pup weights were reduced at 2000, 4000, and
8000 ppm. At 2000 and 4000 ppm, the effect was evident only from
postnatal day 4; at 1000 ppm, reductions in pup weights were observed
on postnatal day 4 in males and postnatal day 7 in animals of each
sex. There was no NOAEL because of effects on pup weights at all doses
(Tanaka et al., 1992).
Groups of 10 male and 10 female Crj:CD-1 mice were fed diets
containing 0,1500, 3000, or 6000 ppm piperonyl butoxide (purity
unspecified) for four weeks before mating (F0 generation), during
gestation, and until the F1 generation was eight weeks old.
Open-field activity was measured in the F0 generation after three
weeks of treatment. Litter size, pup weight, and some developmental
behavioural signs (surface righting, negative geotaxis, cliff
avoidance, swimming, and olfactory orientation) were measured during
lactation of the F1 generation; open-field activity was measured at
three and eight weeks of age and multiple water T-maze activity at six
weeks of age. A dose-related decrease in ambulation and rearing was
seen in the open-field test in males of the F0 generation, but only
the former was statistically significant at the high dose. Pup body
weight at birth was reduced in all groups; on postnatal day 21, the
body weight of animals at the middle dose was 7% lower than that of
controls, and that of animals at the high dose was 41% lower. The
survival indexes at postnatal day 21 were 79, 93, 80, and 52 in
controls and in mice at the low, middle, and high doses, respectively.
The results of the behavioural test during lactation were not
significant, except for reduced olfactory orientation in animals at
the middle and high doses. The results of the open-field test and the
multiple water T-maze test were not altered by treatment, except for a
few, not dose-related differences in some parameters (Tanaka, 1992).
Rats
In a two-litter, two-generation study of reproductive toxicity,
groups of 26 Sprague-Dawley CD rats of each sex, seven weeks of age,
received piperonyl butoxide in the diet at 0, 300, 1000, or 5000 ppm,
equal to 0, 20, 68, and 350 mg/kg bw per day for males (average food
intake calculated in weeks 1-28 of the study) and to 0, 29, 94, and
480 mg/kg bw per day for females (calculated in weeks 1-12 of the
study). The stability, homogeneity, and correspondence of the actual
concentrations in the diets to the nominal concentrations were checked
several times before and during the study and found to be acceptable.
Rats were maintained on their respective diets for 85 days before
mating, throughout the two mating periods, and until scheduled
sacrifice. The F1b generation litters were weaned on day 21
post partum, and groups of 26 rats of each sex were selected to form
the F1b adult generation. These animals were maintained on their
respective diets for 83 days before mating. Animals were observed for
signs of toxicity, and body weight and food consumption were recorded.
External and internal gross examinations were performed on adult rats
and on a selected number of weanlings; histopathological examination
was undertaken for the reproductive tracts of control animals, those
at the high dose, and those of rats at the low and middle doses that
failed to mate successfully in either mating period.
Clinical and pathological examinations showed no
treatment-related toxic effects in adult F0 or F1b animals. The
body weights of adult animals of each sex at 5000 ppm were lower than
those of controls from a few weeks after the beginning to the end of
the study. This effect occasionally corresponded to reduced food
intake. Mating performance, fertility indexes, gestation indexes,
length of gestation, and numbers of live and dead pups at birth were
not affected by treatment. The viability and lactation indexes,
clinical conditions, and pathological appearance of pups of all
generations were also unaffected. The body weights of male and female
pups of all generations at 5000 ppm were lower than those of control
pups. This effect was not detectable at birth but was seen as early as
day 4 post partum. The NOAEL for parental toxicity and pup development
was 1000 ppm, equal to 68 mg/kg bw per day (Robinson et al., 1986).
During the study of carcinogenicity reviewed above (Sarles &
Vandergrift, 1952), rats of each sex were pair-caged throughout the
experiment, except during nursing. No effect on reproductive
efficiency was seen in the first, second, or third generations (no
details given).
(e) Developmental toxicity
Mice
Groups of 20 pregnant Crj:CD-1 mice were given piperonyl butoxide
(purity, > 95%) in olive oil by gavage on day 9 of gestation at doses
of 0, 1065, 1385, or 1800 mg/kg bw. Dams were weighed on gestation
days 9 and 18 and then sacrificed, and the uteri were examined for the
presence and position of resorption sites, fetuses, and implantation
sites. Viable fetuses were weighed and examined for external
malformations and variations, and then fixed and stained for
visualization of the skeleton. No abnormal behaviour or mortality was
observed in dams. One dam at the middle dose and two at the high dose
aborted; the litters of one dam at the middle dose and three at the
high dose were resorbed. Maternal body-weight gain was comparable in
all groups. The total resorption rates were significantly greater at
the middle (26%) and high (32%) doses than in controls (6%), probably
due to total litter resorptions, since the number of viable fetuses
per dam was comparable in all groups. (The raw data were not available
to confirm the reviewer's hypothesis.) The average fetal body weight
was significantly reduced in males, by 3% at the middle dose and 7% at
the high dose, and in females, by 4% at the low dose, 5% at the middle
dose, and 6% at the high dose. Exencephaly, craniochisis, open
eyelids, omphalocele, kinky tail, and talipes varus were observed in
all groups. Oligodactyly in the forelimbs was found in none of the
controls but in one fetus at the low dose, four (2%) at the middle
dose, and 27 (6%) at the high dose. A single oral dose > 1065 mg/kg
bw per day of piperonyl butoxide to dams on day 9 of gestation was
thus embryo- and fetotoxic (Tanaka et al., 1994).
Rats
Groups of 20 pregnant COBS random-bred albino rats were given 0,
300, or 1000 mg/kg bw per day piperonyl butoxide (technical-grade;
purity unspecified) dissolved in corn oil by gavage on days 6-15 of
gestation. The doses were chosen after a pilot study in which six
animals per group were treated with 0, 100, 300, 1000, or 3000 mg/kg
bw per day; reduced body-weight gain was observed during gestation
days 6-15 at the highest dose. Pregnant animals were shipped from the
breeder to the test laboratory on day 1 of gestation and were observed
and weighed daily. On day 20 of gestation, they were sacrificed and
the usual parameters were determined. No signs of toxicity were
observed. Body-weight gain was reduced by 10% in animals at the low
dose and by 15% in those at the high dose, mainly between days 15 and
20 of gestation. Reproductive parameters were not significantly
affected by treatment. One female at 300 mg/kg bw resorbed 8 of 11
fetuses, and one at the high dose resorbed the entire litter. Fetuses
of treated dams had no internal, external, or skeletal malformations
that could be related to treatment. There was no NOAEL for maternal
toxicity because of reduced body-weight gain at both doses. The NOAEL
for embryo- and fetotoxicity was 1000 mg/kg bw per day on the basis of
the absence of any significant finding (Kennedy et al., 1977). This
report from Industrial Biotest Laboratories has not been validated by
a governmental agency and was therefore not taken into account by the
Meeting.
Groups of 17-20 pregnant Wistar rats were given piperonyl
butoxide (purity, 80%) at 0, 62.5, 125, 250, or 500 mg/kg bw dissolved
in corn oil by gavage on days 6-15 of gestation. They were sacrificed
on day 22 of gestation and necropsied, and their fetuses were removed
and examined for external, visceral, and skeletal changes. There were
no deaths or signs of toxicity in dams and no effect on the numbers of
corpora lutea, live fetuses, resorption sites plus dead fetuses,
anomalous fetuses, anomalous litters, or fetal body weight. The types
and incidences of anomalies in the treated groups were comparable to
those of the control group (data not shown). There was no evidence of
embryotoxicity, fetotoxicity, or teratogenicity, and no maternal
toxicity was elicited at the doses used (Khera et al., 1979).
Groups of 20 timed-pregnant Sprague-Dawley CD rats were given
undiluted piperonyl butoxide (purity, 90.78%) at 0, 200, 500, or
1000 mg/kg bw per day by gavage on days 6-15 of gestation. The doses
were chosen in a preliminary pilot study in which five animals per
group were treated with 0, 250, 500, 1000, 2000, or 4000 mg/kg bw per
day, and in which all animals at 4000 and four-fifths of those at
2000 mg/kg bw per day groups died or became moribund. Animals were
observed and weighed daily. On day 20 of gestation, they were
sacrificed and the usual parameters plus liver weight were determined.
No females aborted or delivered early, but wetness in the urogenital
area was observed in 13/24 dams at the highest dose, and stains of
urine, red urogenital discharge, and perinasal encrustation were seen
at lower incidences. Body-weight gain was reduced between days 6 and 9
of gestation in animals at the low dose (an effect considered to be
nonsignificant because of the low weight of non-pregnant animals) and
between days 6 and 15 in animals at the middle and high doses; these
reductions were associated with reduced food intake, resulting in a
significantly lower body weight (by about 5%) in animals at 500 and
1000 mg/kg bw per day on days 15 and 18 of gestation. Increased
absolute (by 8%) and relative (by 11%) weights were observed at the
high dose. Reproductive parameters were not significantly affected by
treatment. Fetuses of treated dams had no internal, external, or
skeletal malformations that could be related to treatment. The NOAEL
for maternal toxicity was 200 mg/kg bw per day on the basis of reduced
body-weight gain and food consumption, increased liver weight, and
signs of toxicity at higher doses. The NOAEL for embryo- and
fetotoxicity was 1000 mg/kg bw per day on the basis of the absence of
any significant finding (Chun & Neeper-Bradley, 1991).
Rabbits
In a range-finding study, five inseminated New Zealand white
rabbits were treated by gavage with piperonyl butoxide during
gestation days 7-19 at doses of 0, 50,100, 200, 300, or 400 mg/kg bw
per day in corn oil. All animals except one control survived until the
end of the study. Two animals each at 300 and 400 mg/kg bw per day and
one at 100 mg/kg bw per day group aborted all or part of their litters
between gestation days 22 and 26. Decreased defaecation was observed
at the highest dose, and animals at the two highest doses were thinner
than normal at the end of the treatment period. Significant reductions
in body-weight gain and some body-weight loss were observed in rabbits
at 300 and 400 mg/kg bw per day; a trivial reduction in body-weight
gain was observed in animals at 200 mg/kg bw per day. No consistent
effects were seen on uterine parameters. Doses of 50, 100, and
200 mg/kg bw per day were therefore chosen for the definitive study of
teratogenicity.
Groups of 16 inseminated New Zealand white rabbits were treated
by gavage with piperonyl butoxide (purity, 100%) during gestation days
7-19 at doses of 0, 50, 100, or 200 mg/kg bw per day in 0.5 ml/kg bw
corn oil. Caesarean sections were performed on gestation day 29, and
the fetuses were removed for teratological evaluation. All animals
survived until the end of the study. Decreased defaecation was
observed in animals at 100 and 200 mg/kg bw per day. Slight
body-weight loss was observed at the middle and high doses during the
treatment period, but there was substantial recovery of body weight
during the post-treatment period, so that the weight gain during the
overall gestation period was comparable to that of controls. The mean
post-implantation losses of treated does were slightly greater than
those of controls but were not dose-related. Malformations were
observed incidentally and were considered to be unrelated to
treatment. The numbers of fetuses with more full ribs than normal (45,
58, 59, and 60% at 0, 50, 100, and 200 mg/kg bw, respectively) or with
27 presacral vertebrae (20, 32, 27, and 40%, respectively) were
greater than those in controls, but the number of litters was not
different. There was no clear dose-effect relationship, and the
relevance of this finding is dubious. The NOAEL for maternal toxicity
was 50 mg/kg bw per day on the basis of decreased defaecation and
dose-related body-weight losses during the treatment period. The NOAEL
for teratogenicity was 200 mg/kg bw per day (Leng et al., 1986). The
1992 JMPR wrongly reported an NOAEL of 100 mg/kg bw per day.
(f) Genotoxicity
The results of studies of the genotoxicity of piperonyl butoxide
are reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Piperonyl butoxide was applied at 0.5 ml to intact, clipped skin
of six New Zealand white rabbits for 4 h under a semi-occlusive
dressing, and the treated areas were examined for erythema and oedema
up to 78 h after the contact period. An adjacent area of untreated
skin served as the control. Piperonyl butoxide was very mildly
irritating (Romanelli, 1991a). An older study reported similar results
(Sarles et al., 1949).
Piperonyl butoxide at 0.1 ml was instilled into the conjuntival
sac of one eye of six New Zealand white rabbits, and the treated eyes
were examined up 72 h after instillation. Conjunctival irritation was
observed, which fully recovered within 72 h. No corneal or iridal
lesions were observed (Romanelli, 1991b). An older study reported
similar results in rabbits, cats, and dogs (Sarles et al., 1949).
Table 2. Results of tests for the genotoxicity of piperonyl butoxide
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, TA100, 100-5000 µg/plate 90.78 Negativea Lawlor (1991)
TA1535, TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative White et al. (1977)
TA 100, TA1537
Reverse mutation E. coli WP2 1 mg/disc 80 Negative Ashwood-Smith
et al. (1972)
Gene mutation Mouse lymphoma L5178Y 6.3-100 µg/mlb NR Positive McGregor et al.
cells (1988)
Gene mutation Chinese hamster ovary, cells 10-100 µg/mlc NR Equivocal Tu et al. (1985)
25-500 µg/mld Negative
Unscheduled DNA Rat hepatocytes 1-100 µg/mle 90.78 Negative McKeon & Phil
synthesis (1991)
Unscheduled DNA Human liver slices 0.05-2.5 mmol/litre 90.78 Negative Lake (1995)
synthesis
Chromosomal Chinese hamster ovary cells 25-99.9 µg/ml 90.78 Negativea Murli (1991)
aberration 62.6-251 µg/mla
Table 2. (cont'd).
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Dominant lethal ICR/Ha Swiss mice 0, 200, or 1000 mg Commercial Equivocal Epstein et al. (1972)
mutation intraperitoneally or formulation
1000 mg/kg bw orally
× 5
NR, not reported
a With and without metabolic activation
b Lethal at 100 µg/ml
c Without metabolic activation; cytotoxic at > 30 µg/ml
d With metabolic activation; cytotoxic at > 300 µg/ml
e Cytotoxic at > 49.9 µg/ml
Skin sensitization was studied in 10 male Hartley guinea-pigs by
a modified Buehler method. A gauze patch containing 0.4 ml piperonyl
butoxide was applied for 6 h onto clipped skin, three times a week for
three weeks. After a two-week rest period, the animals were challenged
on another site with a single 6-h application. Chlorodinitrobenzene
(0.1% w/v in a 50% ethanol:0.9% saline solution) was used as the
positive control. No evidence of contact sensitization was observed
(Romanelli, 1991c). An older study also reported no response in
rabbits (Sarles et al., 1949)
(ii) Studies with mixtures
Neonatal ICR/Ha Swiss mice (n = 91) were given subcutaneous
injections of 5 mg piperonyl butoxide alone at the ages of one and
seven days and 10 mg at the ages of 14 and 21 days. Further groups
were given the same doses in combination with Freon-112 (n = 137) or
-113 (n = 94) at doses of 10 mg at one and seven days and 20 mg at 14
and 21 days. Control animals were treated with the solvent,
tricaprylin. All surviving animals were killed after 52 weeks. No
tumours were found in animals treated with piperonyl butoxide, but the
incidence of hepatomas in male mice given piperonyl butoxide plus
Freon was significantly increased when compared with that in groups
receiving solvent, piperonyl butoxide, or Freon alone (Epstein
et al., 1967).
Groups of 45 Sprague-Dawley CD rats of each sex were fed either
standard diets or diets containing 400 ppm pyrethrins plus 2000 ppm
piperonyl butoxide for 104 weeks. The diets were prepared daily from a
10-fold concentrated pre-mix prepared freshly once a week. The purity
of piperonyl butoxide was 90.5%, and that of pyrethrins was 53.1%
(batch used until week 55) or 52.4% (batch used from week 56 until
termination); the diets were prepared taking into account the purity
of the compounds. The actual concentrations and the stability of the
diet were not tested. Body weight and food consumption were determined
once a week. The average daily intakes were 79 and 101 mg/kg bw
piperonyl butoxide and 16 and 20 mg/kg bw pyrethrins for males and
females, respectively. Animals were observed for clinical signs, skin
lesions, and palpable masses. All animals found dead or moribund were
inspected, and macroscopic lesions were examined microscopically.
During week 101, urinalysis and haematological and clinical chemical
tests were performed in 10 animals per group. At termination, all
animals were observed grossly and selected organs were examined
microscopically.
No treatment-related clinical signs of toxicity were observed.
Mortality was 84 and 76% in control males and females and 80 and 58%
in treated males and females, respectively. The body weights of
treated females were about 20% lower than those of controls, mainly
during the first 78 weeks, while at termination treated animals were
about 10% lighter than controls; the difference was less evident
(about 5%) in males. Slightly reduced food intake was also observed
until week 27 of treatment. Haematological, clinical chemical, and
urinary parameters were not significantly altered in treated animals,
and macroscopic and microscopic examinations revealed no significant
treatment-related neoplastic or non-neoplastic effects (Hunter
et al., 1977).
3. Observations in humans
In nine men, antipyrine metabolism was not affected by a single
oral dose of 50 mg (0.71 mg/kg bw) piperonyl butoxide (Conney et al.,
1972).
Two male infants, whose mothers were sisters and who were born
within two weeks of each other, had coarctation of the aorta. Exposure
to insect repellents and insecticides, including piperonyl butoxide,
during week 8 of gestation was reported (Hall et al., 1975).
Percutaneous absorption of pyrethrin and piperonyl butoxide was
studied in six male volunteers. A formulation containing 0.3% pyrethin
and 3.0% piperonyl butoxide was spread on the ventral forearm for 30
min. This formulation was chosen because it corresponds to that of a
commercially available product used for the treatment of head lice.
The formulation contained either 14C-pyrethrin or 14C-piperonyl
butoxide. After application, urine was collected for up to seven days,
and percutaneous absorption was determined from total urinary
excretion of radioabel, assuming that 22.5% of pyrethin and 51.3% of
piperonyl butoxide are excreted in the urine after parenteral
injection, as was shown in monkeys. Absorption of pyrethrin was 1.9 ±
1.2% and that of piperonyl butoxide 2.1 ± 0.6% of the administered
dose. No radiolabel was found in blood taken 1 h after application.
The extrapolated absorption from human scalp was 7.5 ± 4.7% of
pyrethin and 8.3 ± 2.4% of piperonyl butoxide (Wester et al., 1994).
Comments
Piperonyl butoxide is metabolized by oxidation of the methylene
group of the methylenedioxyphenyl moiety to yield carbon dioxide. The
remainder of the molecule undergoes further degradation and is
excreted mainly in the urine. As it is an alternative substrate (and
therefore a competitive inhibitor) for the microsomal cytochrome P450
system, piperonyl butoxide inhibits the metabolism of several drugs
and pesticides. The mechanism of action of this inhibition has been
elucidated in several studies.
In male rats given single oral doses of about 500 mg/kg bw of
[methylene-alpha 14C]-piperonyl butoxide, the label reached a peak
in blood after 3-12 h and dropped by about 50% within 24 h. The
highest levels of radiolabel were found in the gastrointestinal tract
and its, contents, suggesting that enterohepatic circulation occurs.
High levels of radioactivity were also found in the lung, liver,
kidney, fat, prostate, and seminal vesicles. The excretion pattern was
unchanged after 14 repeated doses of piperonyl butoxide.
Piperonyl butoxide has negligible acute toxicity, and it has been
classified by WHO as unlikely to present an acute hazard in normal
use.
Both short-term and long-term studies show that the target organ
of the toxicity of piperonyl butoxide is the liver. Male animals were
slightly more sensitive than females. In a number of short-term
studies in rodents, hepatic toxicity was characterized by liver
enlargement with associated hypertrophic hepatocytes, focal necrosis,
and, at times, alteration of some clinical chemical parameters. The
NOAEL for liver toxicity was about 100 mg/kg bw per day.
In rats exposed to piperonyl butoxide by inhalation at 0, 15, 74,
155, or 512 mg/m3 for 6 h per day on five days a week for 13 weeks,
effects were seen on the liver at the highest dose. Irritation of the
upper airways was seen at all doses, with squamous metaplasia of the
larynx.
In a one-year study in dogs fed diets containing 0, 100, 600, or
2000 ppm piperonyl butoxide, reduced body-weight gain, increased liver
weight with hypertrophic hepatocytes, and alteration of some clinical
chemical parameters were observed at 2000 ppm. The NOAEL in this study
was 600 ppm, equal to 16 mg/kg bw per day.
Several studies have been conducted of carcinogenicity in mice
and rats, some of which were considered to be inadequate. In a
112-week study, mice were given diets containing 5000 or 10 000 ppm
piperonyl butoxide during weeks 1-30 and 500 or 2000 ppm during weeks
31-112. No increase in tumour incidence was observed. Mice were fed
diets that gave a daily intake of 0, 30, 100, or 300 mg/kg bw for 78
weeks. Eosinophilic foci and adenomas with eosinophilic cells were
observed more frequently in the livers of males at the middle dose and
males and females at the high dose. The NOAEL in this study was
30 mg/kg bw per day on the basis of effects on the liver.
In a 12-month study of carcinogenicity in the liver, male mice
were fed diets containing 0, 6000, or 12 000 ppm piperonyl butoxide.
Body weights were reduced in a dose-related manner, and increased
mortality was observed in animals at the high dose. Hepatocellular
adenomas and carcinomas were observed in treated groups. Piperonyl
butoxide was carcinogenic at doses that were toxic to the liver and
caused general toxicity.
In a two-year study in rats at dietary concentrations adjusted to
achieve doses of 0, 30, 100, or 500 mg/kg bw per day, increased liver
weights, with corresponding hyperplasia and hypertrophy of
hepatocytes, morphological changes, and lesions in the endocrine and
hormone-sensitive organs, were observed at 100 and 500 mg/kg bw per
day. These effects were considered to be secondary to the ability of
piperonyl butoxide to induce hepatic cytochrome P450 enzymes.
Piperonyl butoxide was not found to be carcinogenic in this study.
After reconsideration of the data on testes in this study, previously
evaluated by the 1992 JMPR, and taking into account the results of
other long-term studies, the Meeting concluded that the NOAEL was
30 mg/kg bw per day on the basis of effects on the liver.
Two studies of carcinogenicity were conducted in rats fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide. No significantly
increased incidence of neoplasia was found in treated rats. Effects on
body weight and mortality were observed at the highest dose. In one
study, ileocaecal lesions were observed in both groups. Another study
of carcinogenicity was conducted in rats fed diets containing 0, 6000,
12 000, or 24 000 ppm. Increased mortality was observed in females at
the middle dose. Absolute liver weights were increased in females at
the low dose and in animals of each sex at the high dose. Nodular
lesions of the liver were observed in treated animals, and their
incidence and severity were related to the dose. Hepatocellular
adenomas and carcinomas were observed in animals at the middle and
high doses. Gastric and caecal haemorrhages, renal lesions, anaemia,
and platelet alteration were observed in treated animals. Piperonyl
butoxide was carcinogenic at doses that caused general toxicity.
In a 104-week study in rats, piperonyl butoxide was given at
2000 ppm in the diet in combination with pyrethrins at 400 ppm. A
slight reduction in the body weights of treated females was the only
adverse effect observed.
A two-generation study of reproductive toxicity, with one litter
per generation, was conducted in mice fed diets containing 0, 1000,
2000, 4000, or 8000 ppm piperonyl butoxide. There was no NOAEL because
of reduced pup weight at all doses. Pup viability was reduced at the
highest dose. In a two-litter, two-generation study of reproductive
toxicity in rats at dietary levels of 0, 300, 1000, or 5000 ppm, the
NOAEL for parental toxicity and pup development was 1000 ppm (equal to
68 mg/kg bw per day) on the basis of lowered body weight at 5000 ppm.
Embryo- and fetotoxicity were observed when mice were given single
doses of piperonyl butoxide at 0, 1070, 1390, or 1800 mg/kg bw by
gavage on day 9 of gestation.
Piperonyl butoxide was not embryotoxic or teratogenic in rats or
rabbits. Maternal toxicity was found in rats at doses > 500 mg/kg
bw per day. The NOAEL was 200 mg/kg bw per day in a study of
developmental toxicity in rabbits given 0, 50, 100, or 200 mg/kg bw
per day by gavage. The incidence of common developmental variations,
such as more full ribs and more than 27 presacral vertebrae, was
increased in all treated groups. Since a clear dose-effect
relationship was lacking, the association of this finding with
treatment was considered dubious. The NOAEL for maternal toxicity was
50 mg/kg bw per day.
Piperonyl butoxide was a mild dermal and ocular irritant but not
a dermal sensitizer in rabbits.
Piperonyl butoxide was adequately tested for genotoxicity in a
range of assays in vivo and in vitro. The Meeting concluded that
it is not genotoxic.
A single dose of 0.71 mg/kg bw piperonyl butoxide did not alter
antipyrine metabolism in humans. A study in which a formulation
containing 3% piperonyl butoxide was spread onto the ventral forearm
of adult male volunteers indicated that about 8% of the applied dose
would be absorbed through the human scalp. These data did not
contribute directly to the establishment of the ADI.
An ADI of 0-0.2 mg/kg bw was established on the basis of the
NOAEL of 600 ppm (equal to 16 mg/kg bw per day) in the one-year study
in dogs, with a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 mg/kg bw per day (78-week dietary study of toxicity and
carcinogenicity)
Rat: 30 mg/kg bw per day (two-year dietary study of
carcinogenicity)
200 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
500 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
1000 ppm, equal to 68 mg/kg bw per day (study of
reproductive toxicity)
Rabbit: 50 mg/kg bw per day (study of developmental toxicity)
200 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 600 ppm, equal to 16 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Further observations in humans
2. Short-term studies with mixtures of piperonyl butoxide and
other active ingredients, in ratios relevant to human
exposure
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to piperonyl butoxide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, irritation, rabbit Mildly irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Not sensitizing
Inhalation, lethality, rat Lacrimation, salivation, nasal
discharge, and laboured breathing at
5.9 mg/litre for 4 h. No mortality
Oral, lethality, mouse, rat, cat, dog LD50 = 4-14 g/kg bw
Dermal, lethality, rabbit LD50 > 2 g/kg bw
Medium-term (1-26 weeks) Repeated oral, toxicity, mice, rats, NOAEL = 100 mg/kg bw per day; effects on
3-13 weeks liver
Repeated inhalation, toxicity, rats, 13 weeks Irritating to upper airways at 15 mg/m3 for
6 h per day, 5 days per week, with squamous
metaplasia of the larynx; effects on the liver at
512 mg/m3
Oral, developmental toxicity, rabbit NOAEL = 50 mg/kg bw per day for maternal
toxicity; no fetotoxicity or teratogenidty
Oral, reproductive toxicity, rat NOAEL = 68 mg/kg bw per day; maternal
and pup toxicity
Long-term (> one year) Repeated oral, toxicity, dog, one year NOAEL = 16 mg/kg bw per day
References
Ashwood-Smith, M.J., Trevino, J. & Ring, R. (1972) Mutagenicity of
dichlorvos. Nature, 240, 418-420. Brady, J.T., Montelius, D.A.,
Beierschmitt, W.P., Wyand, D.S., Khairalla, E.A. & Cohen, S.D.
(1988) Effect of piperonyl butoxide post-treatment on
acetaminophen hepatotoxicity. Biochem. Pharmacol.,
37, 2097-2099.
Cardy, R.H., Renne, R.A., Warner, J.W. & Cypher, R.L. (1979)
Carcinogenesis bioassay of technical-grade piperonyl butoxide in
F344 rats. J. Natl Cancer Inst., 62, 569-578.
Chun, J.S. & Neeper-Bradley, T.L. (1991) Developmental toxicity
evaluation of piperonyl butoxide administered by gavage to CD
(Sprague-Dawley) rats. Unpublished report No. 54-586 from Bushy
Run Research Center, Export, Pennsylvania, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Conney, A.H., Chang, R., Levin, W.M., Garbut, A., Munro-Faure, A.D.,
Peck, A.W. & Bye, A. (1972) Effects of piperonyl butoxide on drug
metabolism in rodents and man. Arch. Environ. Health,
24, 97-106.
Dalvi, R.R. & Dalvi P.S. (1991) Differences in the effects of piperine
and piperonyl butoxide on hepatic drug-metabolizing enzyme system
in rats. Drug Chem. Toxicol., 14, 219-229.
Epstein, S.S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel, N.
(1967) Synergistic toxicity and carcinogenicity of 'Freons' and
piperonyl butoxide. Nature, 214, 526-528.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 233, 288-325.
Fishbein, L., Falk, H.L., Fawker, J., Jordan, S. & Corbett, B. (1969)
The metabolism of piperonyl butoxide in the rat with 14C in the
methylenedioxy or alpha-methylene group. J. Chromatogr.,
41, 61-79.
Franklin, M.R. (1972) Inhibition of hepatic oxidative xenobiotic
metabolism by piperonyl butoxide. Biochem. Pharmacol.,
21, 3287-3299.
Fujitani, T., Ando, H., Fujitani, K., Ikeda, T., Kojima, A., Kubo, Y.,
Ogato, A., Oishi, S., Takahashi, O. & Yoneyama, M. (1992)
Sub-acute toxicity of piperonyl butoxide in F344 rats.
Toxicology, 72, 291-298.
Fujitani, T., Tanaka, T., Hashimoto, Y. & Yoneyama, M. (1993a)
Subacute toxicity of piperonyl butoxide in ICR mice.
Toxicology, 83, 93-100.
Fujitani, T., Tada, Y. & Yoneyama, M. (1993b) Hepatotoxicity of
piperonyl butoxide in male F344 rats. Toxicology, 84, 171-183.
Gabriel, D. (1991a) Acute oral toxicity, LD50-rats. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Gabriel, D. (1991b) Acute dermal toxicity, single level-rabbits.
Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldenthal, E.I. (1993a) Evaluation of piperonyl butoxide in an eight
week toxicity study in dogs. Unpublished report No. 542-004 from
International Research and Development Corp., Mattawan, Michigan,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Goldenthal, E.I. (1993b) Evaluation of piperonyl butoxide in a one
year chronic dietary toxicity study in dogs. Unpublished report
No. 542-005 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldstein, J.A., Hickman, P. & Kimbrough R.D. (1973) Effects of
purified and technical piperonyl butoxide on drug metabolizing
enzymes and ultrastructure of rat liver. Toxicol. Appl.
Pharmacol., 26, 444-458.
Graham, C (1987) 24-Month dietary toxicity and carcinogenicity study
of piperonyl butoxide in the albino rat. Unpublished report
No. 81690 from Bio-Research Ltd Laboratory, Seneville, Quebec,
Canada. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hall J.G., McLaughlin, J.F. & Stamm, S. (1975) Coarctation of the
aorta in male cousins with similar maternal environmental
exposure to insect repellent and insecticides. Pediatrics,
55, 425-427.
Hermanski, S.J. & Wagner, C.L. (1993) Chronic dietary oncogenicity
study with piperonyl butoxide in CD-1 mice. Unpublished report
No. 91N0134 from Bushy Run Research Center, Export, Pennsylvania,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hoffman, G.M. (1991) An acute inhalation toxicity study of piperonyl
butoxide in the rat. Unpublished report No. 91-8330 from
Bio/dynamics, East Millstone, New Jersey, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Hunter, B., Bridges, J.L. & Prentice, D.E. (1977) Long term feeding of
pyrethrins and piperonyl butoxide to rats. Unpublished report
from Huntington Research Centre, Huntington, Cambs, United
Kingdom. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Bates, R.R., Falk, H.L., Gart, J.J., Klein, M.,
Mitchell, I. & Peters, J. (1969) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Natl Cancer Inst., 42, 1101-1114.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1968)
In vivo inhibition of mouse liver microsomal hydroxylating
systems by methylenedioxyphenyl insecticidal synergists and
related compounds. Life Sci., 7, 1051-1062.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1969)
Bi-modal effect of piperonyl butoxide on the o- and
p-hydroxylations of biphenyl by mouse liver microsomes.
Biochem. Pharmacol., 18, 1045-1051.
Kamienski, F.X. & Casida, J.E. (1970) Importance of demethylenation in
the metabolism in vivo and in vitro of methylenedioxyphenyl
synergists and related compounds in mammals. Biochem.
Pharmacol., 19, 91-112.
Kennedy, G.L., Smith, S.H., Kinoshita, F.K., Keplinger, M.L. &
Calandra, J.C. (1977) Teratogenic evaluation of piperonyl
butoxide in the rat. Food Cosmet. Toxicol., 15, 337-339.
Khera, K.S., Whalen, C., Angers, G. & Trivett, G. (1979) Assessment of
the teratogenic potential of piperonyl butoxide, biphenyl, and
phosalone in the rat. Toxicol. Appl. Pharmacol., 47, 353-358.
Lake, B.G. (1995) An investigation of the effect of piperonyl butoxide
on unscheduled DNA synthesis in cultured human liver slices.
Unpublished report No. 1501/1 from BIBRA International,
Carshalton, Surrey, United Kingdom. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lawlor, T.E. (1991) Piperonyl butoxide in the Salmonella/mammalian-
microsome reverse mutation assay (Ames test) with a confirmatory
assay. Unpublished report No. 14413-0-401R from Hazleton
Washington, Kensington, Maryland, USA. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lehman, A.J. (1948) Q. Bull. Assoc. Food Drug Off., 12, 82.
Lehman, A.J. (1951) Q. Bull. Assoc. Food Drug Off., 15, 122.
Lehman, A.J. (1952a) Q. Bull. Assoc. Food Drug Off., 16, 43.
Lehman, A.J. (1952b) Q. Bull. Assoc. Food Drug Off., 16, 47.
Leng, J.M., Schwartz, C.A. & Schardein, J.L. (1986) Teratology study
in rabbits. Unpublished report No. 542-002 from International
Research and Development Corp., Mattawan, Michigan, USA.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Levine, B.S. & Murphy, S.D. (1977a) Esterase inhibition and
reactivation in relation to piperonyl butoxide-phosphorothionate
interactions. Toxicol. Appl. Pharmacol., 40, 379-391.
Levine, B.S. & Murphy, S.D. (1977b) Effect of piperonyl butoxide on
the metabolism of dimethyl and diethyl phosphorothionate
insecticides. Toxicol. Appl. Pharmacol., 40, 393-406.
Maekawa, A., Onodera, H., Furuta, K., Tanigawa, H., Ogiu, T. &
Hayashi, Y. (1985) Lack of evidence of carcinogenicity of
technical-grade piperonyl butoxide in F344 rats: Selective
induction of ileocaecal ulcers. Food Chem. Toxicol.,
7, 675-682.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded
chemicals. Environ. Mol. Mutag., 12, 85-154.
McKeon, M.E. & Phil, M. (1991) Piperonyl butoxide in the assay for
unscheduled DNA synthesis in rat liver primary cell cultures with
a confirmatory assay. Unpublished report No. 14413-O-447R from
Hazleton Washington, Kensington, Maryland, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Mirer, F.E., Levine, B.S. & Murphy, S.D. (1977) Parathion and methyl
parathion toxicity and metabolism in piperonyl butoxide and
diethyl maleate pretreated mice. Chem.-Biol. Interactions,
17, 99-112.
Modeweg-Hansen, L., Lalande, M., Bier, C., Losos, G. & Osborne, B.E.
(1984) A dietary dose range-finding study of piperonyl butoxide
in the albino rat. Unpublished report No. 81802B from
Bio-Research Laboratories, Edgewater, Maryland, USA. Submitted to
WHO by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Murli, H. (1991) Piperonyl butoxide: Measuring chromosomal aberrations
in Chinese hamster ovary (CHO) cells with multiple harvests under
conditions of metabolic activation. Unpublished report
No. 14413-0-437C from Hazleton Washington, Kensington, Maryland,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Negherbon, W.O. (1959) Handbook of Toxicology, Vol. 3, Philadelphia,
Saunders.
Newton, P.E. (1992) A subchronic (3-month) inhalation toxicity study
of piperonyl butoxide in the rat via whole-body exposures.
Unpublished report No. 91-8333 from Bio/dynamics, East Millstone,
New Jersey, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Phillips, J.C., Cunninghame, M.E., Price, R.J., Osimitz, T., Ganriel,
K.L., Preiss, F.J., Butler, W.H. & Lake, B.G. (1995) Effect of
piperonyl butoxide (PBO) on cell replication and xenobiotic
metabolism in mouse liver (Abstract). Toxicologist, 15, 230.
Robinson, K., Pinsonneault, L. & Procter, B.G. (1986) A two-generation
(two-litter) reproduction study of piperonyl butoxide
administered in the diet to the rat. Unpublished report No. 81689
from Bio-Research Laboratories Ltd, Montreal, Quebec, Canada.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991a) Primary skin irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991b) Primary eye irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991c) Guinea pig dermal sensitization-modified Buehler
method. Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Sarles, M.P. & Vandegrift, W.B. (1952) Chronic oral toxicity and
related studies on animals with the insecticide and pyrethrum
synergist, piperonyl butoxide. Am. J. Trop. Med. Hyg.,
1, 862-883.
Sarles, M.P., Dove, W.E. & Moore, D.H. (1949) Acute toxicity and
irritation tests on animals with the new insecticide, piperonyl
butoxide. Am J. Trop. Med., 29, 151-166.
Schuller, H.M. & McMahon, J.B. (1985) Inhibition of N-nitroso-
diethylamine-induced respiratory tract carcinogenesis by
piperonylbutoxide in hamsters. Cancer Res., 45, 2807-2812.
Selim, S. (1985) Single and repeated oral dose pharmacokinetic and
distribution studies of piperonyl butoxide. Unpublished report
No. WP509070/1 from Biological Test Center, Irvine, California,
USA. Submitted to WHO by Endura SA. Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Skrinijaric-Spoljar, M., Matthews, H.B., Engel, J.L. & Casida, J.E.
(1971) Response of hepatic microsomal mixed-function oxidases to
various types of insecticide chemical synergists administered to
mice. Biochem. Pharmacol., 20, 1607-1618.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994a) Piperonyl butoxide induces hepatocellular carcinoma in
male CD-1 mice. Arch. Toxicol., 68, 467-469.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994b) Chronic toxicity studies of piperonyl butoxide in F344
rats: Induction of hepatocellular carcinoma. Fundam. Appl.
Toxicol., 22, 293-303.
Tanaka, T. (1992) Effects of piperonyl butoxide on F1 generation mice.
Toxicol. Lett., 60, 83-90.
Tanaka, T. (1993) Behavioural effects of piperonyl butoxide in male
mice. Toxicol. Lett., 69, 155-161.
Tanaka, T., Takahashi, O. & Oishi, S. (1992) Reproductive and
neurobehavioural effects in three-generation toxicity study of
piperonyl butoxide administered to mice. Food Chem. Toxicol.,
30, 1015-1019.
Tanaka, T., Fujitani, T., Takahashi, O. & Oishi, S. (1994)
Developmental toxicity evaluation of piperonyl butoxide in CD-1
mice. Toxicol. Lett., 71, 123-129.
Tu, A.S., Coombes-Hallowell, W.E., Pallotta, S.L. & Catanzano, A.
(1985) Evaluation of piperonyl butoxide in the CHO/HGPRT mutation
assay with and without metabolic activation. Unpublished report
No. 53906 from Arthur D. Little, Inc., Cambridge, Massachusetts,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
US National Cancer Institute (1979) Bioassay of piperonyl butoxide for
possible carcinogenicity (DHEW Publ. No. 79-1375), Bethesda,
Maryland, USA, US Department of Health, Education, and Welfare.
Wester, R.C., Bucks, D.A.W. & Maibach, H.I. (1994) Human in vivo
percutaneous absorption of pyrethrin and piperonyl butoxide.
Food Chem. Toxicol., 32, 51-53.
White, T.J., Goodman, D., Shulgin, A.T., Castagnoli, N., Lee, R. &
Petrakis, N.L. (1977) Mutagenic activity of some centrally active
aromatic amines in Salmonella typhimurium. Mutat. Res.,
56, 199-202.
 A dose-dependent, biphasic effect was observed on the ortho and
para hydroxylations of biphenyls by liver microsomes of mice treated
with 10-640 mg/kg bw of piperonyl butoxide intraperitoneally,
para-Hydroxylation was inhibited by 80% at the highest dose 30 min
after treatment but had recovered within 24 h; ortho-hydroxylation
was induced by up to 150% 1 h after treatment, and activity was still
elevated after 96 h in the groups at the highest dose (Jaffe et al.,
1969).
Piperonyl butoxide inhibits ethylmorphine N-demethylation by
liver microsomes in vitro. The inhibition is proportional to the
amount of cytochrome P450-piperonyl butoxide metabolite (unknown)
complex formed. Differences between liver microsomes from rats and
mice may account for the greater sensitivity of mice to inhibition of
drug metabolism by piperonyl butoxide: the Ki for inhibition of
ethylmorphine N-demethylase was found to be 19 µmol/litre in rats and
6 µmol/litre in mice (Franklin, 1972).
Mouse liver homogenates (20% w/v in 50 mmol/litre phosphate
buffer, pH 7.4) were fractioned into nuclear, mitochondrial,
microsomal, and soluble fractions, which were incubated with labelled
piperonyl butoxide. NAD, NADP, NADH, or NADPH was sometimes added as a
cofactor. Only the microsomal fraction had significant metabolic
activity in the presence of NADPH and, to a lesser extent, with NADH
(Kamienski & Casida, 1970).
Male CF-1 mice were given a single intraperitoneal dose of
piperonyl butoxide (purity, 87-89%) at 0.5-25 mg/kg bw 1 h before
intraperitoneal administration of 40 mg/kg bw pentobarbital or
100 mg/kg bw zoxazolamine. Pentobarbital-induced sleeping time was
significantly increased by 10 or 25 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 5,
10, or 25 mg/kg bw (Conney et al., 1972). Skinjaric-Spoljar et al.
(1971) found that a single intraperitoneal dose of 22 mg/kg bw
piperonyl butoxide to mice increased the hexobarbital (62.5 mg/kg bw
intraperitoneally) sleeping time from 28 to 100 min.
Male Sprague-Dawley rats were given single intraperitoneal doses
of piperonyl butoxide (purity, 87-89%) at 67-1000 mg/kg bw 1 h before
intraperitoneal administration of pentobarbital (25 mg/kg bw) or
zoxazolamine (70 mg/kg bw). Pentobarbital-induced sleeping time was
significantly increased by 1000 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 333
or 1000 mg/kg bw (Conney et al., 1972).
Male mice and rats were given single intraperitoneal doses of
piperonyl butoxide 1 h before injection of 200 mg/kg bw antipyrine.
The NOAELs for effects on antipyrine metabolism were 100 mg/kg bw in
rats and 0.5 mg/kg bw in mice (Conney et al., 1972).
Groups of six male weanling Sherman rats were fed technical-grade
piperonylbutoxide (purity, 80%) at dietary concentrations of 0, 1000,
5000, or 10 000 ppm for one, four, or eight weeks. The activities of
hexobarbital oxidase, aniline hydroxylase, para-nitroanisole
demethylase, nitroreductase, and glucuronyltransferase and the P450
content were increased two- to fourfold by 5000 or 10 000 ppm
piperonyl butoxide. Liver weights and microsomal protein content were
increased to a maximum of 50-70%. Electron microscopy showed
enlargement and extensive proliferation of the smooth endoplasmic
reticulum in liver parenchymal cells. The dose of 1000 ppm had minimal
effects on liver weight and on P450 and glucuronyl transferase
activity and no effect on proliferation of the smooth endoplasmic
reticulum. Maximal effects on P450 content and on the activity of
P450-related enzymes were observed after one week of treatment. The
maximal effect on glucuronyltransferase activity was observed between
four and eight weeks of treatment (Goldstein et al., 1973).
Piperonyl butoxide (purity unsepcified) dissolved in corn oil was
administered to 10 male Sprague-Dawley rats at 400 mg/kg bw
intraperitoneally; another group of animals received 100 mg/kg bw of
piperine intraperitoneally. Control animals received 2 ml/kg bw corn
oil. Groups of five animals were sacrificed 1 and 24 h after
treatment, and hepatic levels of cytochromes P450 and b5, proteins,
and the activities of NADPH cytochrome c reductase, benzphetamine
N-demethylase, aminopyrine N-demethylase, and aniline hydroxylase
were determined. The microsomal protein level was not affected by
treatment. Piperonyl butoxide decreased the P450 but not the b5 level
by about 30% after 1 h; however, after 24 h the contents were
increased by about 100 and 30%, respectively. NADPH cytochrome c
reductase activity was increased by 50% 24 h after treatment. The
activities of N-demethylases and aniline hydroxylase were decreased
by 20 and 50%, respectively, 1 h after administration of either
piperine or piperonyl butoxide. By 24 h, the activities were still low
in piperine-treated animals but had increased by 30-80% in those given
piperonyl butoxide (Dalvi & Dalvi, 1991).
Single intraperitoneal doses of 400 mg/kg bw piperonyl butoxide
were given to male CD-1 mice 1 h before a single intraperitoneal dose
of parathion-methyl, azinphos-methyl, parathion, or azinphos-ethyl, or
their oxygen analogues. The LD50 of the oxygen analogues was not
altered whereas that of the methyl homologues was reduced, with a
40-fold increase in the LD50 for parathion-methyl and a threefold
increase in that for azinphos-methyl; that of the ethyl homologues was
increased, with a 50% decrease in the LD50 for parathion and an 85%
decrease for azinphos ethyl. The plasma levels of all pesticides were
increased by three- to sevenfold in piperonyl butoxide-pretreated
animals 30 min after treatment (Levine & Murphy, 1977a). It has been
suggested that detoxification of parathion-methyl, but not that of
parathion- or azinphos-ethyl, continues through uninhibited
glutathione-dependent pathways (Mirer et al., 1977; Levine & Murphy,
1977b).
A single intraperitoneal dose of 600 mg/kg bw piperonyl butoxide
to three-month-old CD-1 mice reduced the hepatotoxicity (as assessed
by reduced glutathione content, plasma sorbitol dehydrogenase, and
histopathological liver necrosis) caused by an oral dose of 600 mg/kg
bw acetominophen given 2 h earlier or 1 h later. Since the hepatic
mixed-function oxidase system generates a toxic, electrophilic
metabolite of acetominophen, piperonyl butoxide probably acts by
inhibiting: that enzymatic system (Brady et al., 1988).
Pretreatment of groups of 10 male Syrian golden hamsters with
400 mg/kg bw piperonyl butoxide given subcutaneously 2 h before
subcutaneous injection of 17.8 mg/kg bw N-nitrosodiethylamine twice
weekly for 20 weeks inhibited the development of pulmonary
carcinogenesis by the nitrosamine. The numbers of animals with lung
tumors were 0 controls, 6 treated only with N-nitrosodiethylamine, 0
treated with piperonyl butoxide and nitrosamine, and 0 given only
piperonyl butoxide. Tracheal tumors were found in 0, 10, 5, and 0
animals in the four groups, respectively. The protective effect was
associated with reduced (by 50% or more) covalent binding of
N-[ethyl-1-14C]-nitroso-diethylamine to macromolecules in trachea,
lung, and liver (Schuller & McMahon, 1985).
Eight-week-old male CD-1 mice were fed diets containing piperonyl
butoxide at concentrations adjusted to provide doses of 0, 10, 30,
100, or 300 mg/kg bw per day or sodium phenobarbital at 0.05% (w/w)
for 42 days. Replicative DNA synthesis was studied by implanting
seven-day osmotic pumps to administer 5-bromo-2'-deoxyuridine (BrDU)
during days 0-7 and 35-42 of treatment; liver sections were
immunostained with an antibody to BrDU, and the percentage of
hepatocyte nuclei undergoing replicative DNA synthesis was calculated
by microscopic examination of at least 1000 nuclei. Xenobiotic
metabolism was assessed by measuring the liver P450 content and the
activities of 7-ethoxyresorufin O-deethylase, 7-pentoxyresorufin
O-depentylase, and ethylmorphine N-demethylase in liver
microsomes. Sodium phenobarbital was used as a positive control. The
relative liver weight was increased by about 15% after seven days of
treatment with 300 mg/kg bw per day of piperonyl butoxide and by about
20% with sodium phenobarbital; after 42 days, it was increased by
about 10% after treatment with 100 and by about 20% after treatment
with 300 mg/kg bw piperonyl butoxide. Midzonal liver-cell hypertrophy
was observed in mice given 300 mg/kg bw per day for seven or 42 days
or 100 mg/kg bw per day for 42 days. Sodium phenobarbital induced
centrilobular hypertrophy after either seven or 42 days of treatment.
Replicative DNA synthesis was induced to 350% by the high dose of
piperonyl butoxide and to 825% by sodium phenobarbital for seven days;
no significant increase was observed after 42 days at any dose. A
dose-related increase in microsomal protein and P450 content was
observed on day 42, and the increases were statistically significant
at doses > 100 mg/kg bw. Inconsistent increases were observed in
the activities of 7-ethoxyresorufin O-deethylase and 7-pento-
xyresorufin O-depentylase in piperonyl butoxide-treated mice, but a
dose-related increase from 20 to 50% in the activity of ethylmorphine
N-demethylase was seen on day 42. Treatment with sodium pheno-
barbital for 42 days resulted in increases in all of the measured
parameters. Piperonyl butoxide (and sodium phenobarbital) thus caused
a transient stimulation of liver-cell replication and a permanent
effect on liver weight, morphology, and enzyme induction at doses that
induced liver nodules in long-term studies (Phillips el al., 1995).
2. Toxicological studies
(a) Acute toxicity
Groups of five Sprague-Dawley CD rats of each sex were exposed by
inhalation for 4 h to a mean analytical concentration of 5.9 mg/litre
piperonyl butoxide (purity, 90.78%). The particle median aerodynamic
diameter was 2.6 µm; about 15% of the aerosol was < 1 µm in diameter
and about 95% < 10 µm. All animals survived the exposure and the
15-day observation period after treatment. Exposure caused excessive
lacrimation and salivation, nasal discharge and laboured breathing.
All animals recovered. No remarkable signs were seen post mortem
(Hoffman, 1991). Other data are reported in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were fed
diets containing 0, 1000, 3000, or 9000 ppm piperonyl butoxide (purity
unspecified) for 20 days. Animals were observed daily for mortality;
body weight was measured and clinical observations were made on days
1, 2, 3, 7, 14, and 20; the food consumption of five animals per cage
was measured on days 0-3, 3-7, 7-14, and 14-20. At the end of
treatment, blood clinical chemistry was assessed, liver, kidneys, and
spleen were weighed, and livers and kidneys were examined
histologically. No deaths occurred. The body weights of animals at the
high dose were about 15% lower than those of controls at the end of
the study, and those of females at the middle dose were about 10%
lower on day 2 and about 8% lower at termination (not statistically
significant). The lowered body weights were apparently associated with
decreased food intake. The weights of livers showed a dose-related
increase in both males (by 22% at the low dose, 63% at the middle
dose, and 79% at the high dose) and females (62% at the middle dose
and 78% at the high dose); at the high dose, the weights of the
kidneys were reduced by 29% in males and 18% in females, and the
weights of the spleen by 25% in males and 33%, in females. In animals
at the high dose, a 31% increase in serum cholesterol was seen in
males and a 67% increase in females, a 28% increase in serum
phospholipids was seen in males and a 38% increase in females, a 19%
Table 1. Acute toxicity of piperonyl butoxide in animals
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse NR Oral 4.0 NR Negherbon (1959)
Rat Male Oral 4.57 90.78 Gabriel (1991a)
Female 7.22
NR Oral 8.0-10.6 NR Sarles et al. (1949)
NR Oral 13.5 NR Lehman (1948)
NR Oral 11.5 NR Lehman (1951)
Male, female Inhalation (4 h) > 5900 90.78 Hoffman (1991)
NR Subcutaneous > 15.9 NR Sarles et al. (1949)
Rabbit Male, female Dermal > 2 90.78 Gabriel (1991b)
NR Oral 2.7-5.3 NR Sarles et al. (1949)
Cat NR Oral > 10.6 NR Sarles et al. (1949)
Dog NR Oral > 8.0 NR Sarles et al. (1949)
NR, Not reported
increase in total serum proteins occurred in males and an 18% increase
in females, and a 235% increase in gamma-glutamyl transpeptidase was
seen in females. Some of these levels were also increased in the
animals at the middle dose. Microscopic examination of the liver
showed hypertrophic hepatocytes, single-cell necrosis, and cell
infiltration in the centrilobular area in all mice at the high dose.
The kidneys were unremarkable. The NOAEL for effects on the liver was
1000 ppm, equivalent to 150 mg/kg bw per day (Fujitani et al.,
1993a).
Groups of 20 Crj:CD-1 male mice were fed diets containing 0,
1500, 3000, or 6000 ppm piperonyl butoxide (purity unspecified) for
seven weeks. Food intake was measured every week and behavioural tests
were performed at weeks 4 (exploratory behaviour), 6 (multiple water
T-maze), and 7 (exploratory behaviour) of treatment. A treatment-
related reduction in food intake was observed in animals at the middle
and high doses during the first week of treatment. No consistent,
significant effect was observed in the T-maze test or in the
exploratory behaviour test at week 4. At week 7, some parameters in
the exploratory behaviour test were altered (Tanaka, 1993).
Rats
Hypertrophy of periportal cells with slight fatty changes were
observed in rats fed a diet containing 5000 ppm piperonyl butoxide for
17 weeks (Lehman, 1952a,b).
Feeding of six weekly doses of up to 224 mg/kg bw of active
ingredient to rats caused no observable effects at autopsy three weeks
after the final dose, but few details were given (Sarles et al.,
1949).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were fed diets containing concentrations of
piperonyl butoxide adjusted to obtain doses of 0, 62.5, 125, 250, 500,
1000, or 2000 mg/kg bw per day for four weeks. All animals were
observed for mortality and morbidity twice daily, and body weight and
food consumption were measured weekly. Haematological and biochemical
tests were performed on day 24 or 25 of treatment. At termination,
survivors were necropsied, certain organs were weighed and microscopic
examinations were performed on several organs. Six animals died before
termination, but the deaths were considered to be due to anaesthesia
for bleeding. General signs of toxicity, such as prominent backbone,
thinness, poor fur condition, brown staining and piloerection, were
observed in animals at the highest dose. Body-weight gain was reduced
by about 20% in females at 500 mg/kg bw per day, by 27% in males and
37% in females at 1000 mg/kg bw per day, and by 66% in males and 92%
in females at 2000 mg/kg bw per day. These reductions were only
partially associated with reduced food intake. Haematology and
clinical biochemistry showed no significant alterations. Absolute and
relative liver weights were increased in animals given doses >
500 mg/kg bw per day; the relative liver weights were also increased
in males at 250 mg/kg bw per day. The relative weights of the
adrenals, kidneys, and brain were slightly increased in the group at
the highest dose and in some animals at 1000 mg/kg bw per day.
Eosinophilia and loss of vacuolation in hepatocytes were noted in all
treated animals, and the severity of these lesions was dose-related.
These may be adaptive changes. Necrosis of hepatocytes and cytoplasmic
inclusions were observed in animals at 1000 and 2000 mg/kg bw per day.
The NOAEL was 125 mg/kg bw per day on the basis of effects on the
liver (Modeweg-Hansen et al., 1984).
In a study preliminary to the study of carcinogenicity by the
same authors summarized below, groups of 10 Fischer 344/DuCrj rats of
each sex were fed diets containing 0, 2500, 5000, 10 000, 20 000, or
30 000 ppm piperonyl butoxide (purity, 89%) for 13 weeks. At
termination, survivors were necropsied, and limited microscopic
examination was performed. One male at the high dose died before
termination. The final body weights and body-weight gains were reduced
in a dose-related manner in all treated males; in females, a
significant effect was seen only at the two highest doses. Liver
weights were increased in all treated animals, by up to 50% in males
and 108% in females. Absolute kidney weights were increased in animals
at the high dose, and the relative weight was also increased in groups
at lower doses. Hepatocyte hypertrophy and focal necrosis were seen in
animals at higher doses (data not shown). No relevant macro- or
microscopic lesions were observed in the digestive tract, but the
caecum was not examined histologically. There was no NOAEL because of
effects on the liver at all doses (Maekawa et al., 1985).
Groups of 15 Charles River CD rats of each sex were exposed by
inhalation for 6 h per day, five days per week, for 13 weeks to
piperonyl butoxide (purity, 90.78%) at mean analytical concentrations
of 15, 74, 155, or 512 mg/m3. The highest concentration was the
maximum that could be generated at the appropriate particle size.
Fifteen controls were exposed to conditioned room air only. The
concentrations in chamber air were monitored gravimetrically four
times per day and by gas chromatography once a day. Particle size
distribution was measured once during each exposure. The test aerosol
had a mass median aerodynamic diameter of 1.7 µm with a geometric
standard deviation of 2.6. On average, 37% of the particles were <
10 µm in diameter and 29% < 1 µm. Abnormal signs were looked for once
during each exposure, and detailed physical examinations were
conducted on all animals once before treatment and weekly during
treatment. Ophthalmoscopic examinations were performed on all animals
before treatment and on the day before sacrifice. Body weight was
recorded before exposure, immediately before exposure on test day 1,
weekly thereafter, and just before sacrifice. At termination,
haematological and blood chemical parameters were evaluated in all
animals, selected organs were weighed, complete gross post-mortem
examinations were conducted, and selected tissues from all animals
were examined microscopically.
Body-weight gain and food consumption were not affected by
exposure, and all animals survived to the end of treatment.
Ophthalmoscopic examinations showed no indication of exposure-related
ocular effects. There was a dose-related increase in nasal discharge,
dried material on the facial area and extremities, and anogenital
staining in animals at 155 and 512 mg/m3. The serum levels of
aspartate and alanine aminotransferases and of glucose were slightly
decreased (10-28%), while blood urea nitrogen, total protein, and
albumin levels were slightly increased (4-10%) in animals of each sex
at the high dose. Not all of these differences were statistically
significant, and a dose-response relationship was not seen
consistently; however, a similar trend was seen in animals of each
sex. Statistically significant increases in the absolute and relative
weights of the livers and kidneys were seen in animals at the high
dose; the relative kidney weights were increased by 10-12%, and the
absolute and relative liver weights were both increased by 20-29%.
Relative liver weights were also significantly elevated (8-9%) in
males and females at 155 mg/m3. Vesiculation and vacuolation of the
hepatocellular cytoplasm was seen in almost all treated animals but
tended to be more pronounced in those at the highest level. No clear
dose-related increase in either incidence or severity was observed.
Squamous or squamoid metaplasia of the pseudostratified columnar
epithelium of the larynx was seen in several exposed animals and one
control female, the severity being greater in animals at the high
dose. Similar metaplastic changes were seen in the columnar epithelium
lining the ventral diverticulum in several animals at 512 mg/m3 and
in one female at 15 mg/m3. Hyperplasia and hyperkeratosis of the
squamous epithelium normally found in the larynx were seen in a small
number of animals at 512 mg/m3. Laryngeal mucosal inflammation was
seen in all treated animals and was slightly more severe in animals at
the highest dose. All of the changes were considered to be localized
responses indicative of irritation rather then systemic toxicity.
Other findings in tissues and organs examined macroscopically and
microscopically post mortem were unremarkable. No systemic toxicity
was seen at 155 mg/m3; effects on the liver and kidney were seen at
512 mg/m3 (Newton, 1992).
Groups of 10 male and 10 female Fischer 344/DuCrj rats were fed
diets containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide
(technical grade of unspecified purity) for 13 weeks. Animals were
observed daily for mortality; body weight and clinical signs were
monitored daily for the first five days and then twice weekly. Food
and water consumption was measured on days 4, 11, 18, and 39 for about
six rats in each group. At the end of treatment, haematological and
blood chemical parameters were determined, animals were necropsied,
and selected organs were weighed; the liver and kidneys were examined
histologically. There were no deaths. Clinical signs consisted of nose
bleeds from about day 2 to day 20 and dose-dependent abdominal
distension. In animals at the high dose, food consumption was reduced
by about 46% and water consumption by 28% on day 4 but not at other
times. The body weights of animals at the high dose were significantly
reduced, by 36% in males and 24% in females. Blood haemoglobin levels
were decreased in a dose-dependent manner, but the decrease was
statistically significant only in animals at the high dose (by 10-11%)
and females at the middle dose (by 7%). A reduced mean corpuscular
haemoglobin content was also observed in females at the high dose.
Serum albumin was increased by 16-23%, cholesterol by 88-93%,
gamma-glutamyl transpetidase activity to five to six times the control
values in males at the high dose, and urea nitrogen by 24% in males at
that dose. Serum protein was increased in all treated females and
serum phospholipid in females at the high dose; decreases were seen in
serum levels of triglyceride in all males and bilirubin and glucose in
males at the high dose. Absolute and relative liver weights were
increased in a dose-related manner, but were significantly increased
only in males at the middle dose (by 47%) and males and females at the
high dose (by 57-94%). Relative kidney weights were increased in a
dose-related manner to up to 1.42% of control values. Hypertrophic
hepatocytes containing a basophilic, fine granular substance were seen
in animals at the high dose. In the periportal area, marked
vacuolation of hepatocytes was observed, with occasional coagulative
necrosis and oval-cell proliferation. In male rats, atrophy of the
epithelium of the proximal convoluted tubules was observed in the
renal cortex. There was no NOAEL because of effects on the liver and
kidney (Fujitani et al., 1992).
In a subsequent study performed under the same experimental
conditions, haematological, clinical chemical, and morphological
observations after 1, 2, 4, or 12 weeks of treatment showed that all
of the alterations occurred in a time-dependent manner, and that some
of them (increased liver and kidney weights, morphological alterations
in the liver) were already present after one week (Fujitani et al.,
1993b).
Rabbits
Three weekly doses of up to 108 mg/kg bw active ingredient of a
5% emulsion of piperonyl butoxide showed no effects at autopsy three
weeks after the final dose, but few details were given (Sarles
et al., 1949).
Dogs
Groups of one to three dogs of each sex were given piperonyl
butoxide at 0, 3, 32, 106, or 320 mg/kg per day orally in capsules on
six days per week for one year. All dogs at the two highest doses lost
weight, but large variations in body-weight gain and the small number
of animals prevented meaningful comparisons between the controls and
animals at the lower doses. A dose-related increase in relative liver
weight (no statistical analysis reported) was observed, which was
associated with unspecified histopathological changes. Increased
kidney and adrenal weights were observed at 100 and 320 mg/kg bw per
day (Sarles & Vandergrift, 1952).
Groups of two beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for eight weeks at concentrations
corresponding to 500, 1000, 2000, or 3000 ppm active ingredient. The
diets were prepared weekly; the actual concentrations and the
homogeneity of the mixture were measured at three intervals and found
to be satisfactory. Animals were observed at least twice daily for
mortality and signs of toxicity; detailed observations were recorded
at least once weekly, and body weight and food consumption were
recorded weekly. Physical examinations and haematological and
biochemical tests were conducted on all animals before exposure and at
termination. All major tissues and organs were examined
microscopically post mortem. Selected organs were weighed. All
animals survived to study termination. Reduced body-weight gains were
seen in males and females receiving 1000 ppm (14 and 9% of the initial
body weight, respectively), 2000 ppm (6 and 7%), and 3000 ppm (7 and
4%) ppm in comparison with the control group (17% and 14% increases).
Decreased food consumption (by 19 and 23%) was observed animals at
3000 ppm. There were no treatment-related changes in haematological
parameters. Males and females at 2000 and 3000 ppm had alkaline
phosphatase activities that were about 1.5 times the control value and
increased absolute and relative liver and gall-bladder weights. No
macroscopic changes were observed that could be attributed to
treatment, and treatment-related microscopic changes were limited to
diffuse, mild hypertrophy of hepatocytes in all treated males and in
females at the two highest doses. There was no NOAEL because of
effects on the liver (Goldenthal, 1993a).
Groups of four beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for one year at concentrations
corresponding to 100, 600, and 2000 ppm active ingredient. The diets
were prepared weekly and stored at room temperature. The stability of
the compound was assessed twice: an initial test showed a loss of
about 10% over 10 days, possibly due to technical problems; a second
test showed 97-101% of the initial concentration on day 10. The actual
concentrations were measured 16 times and were found to be 87-110% of
the nominal concentrations. The homogeneity of the mixture was also
found to be satisfactory. The dogs were observed at least twice daily
for mortality and signs of toxicity; detailed observations were
recorded weekly. Body weight and food consumption were recorded weekly
for the first 14 weeks and every two weeks thereafter. Ophthalmoscopic
examinations were conducted before the beginning of treatment and at
termination. Physical examinations were conducted on all animals
before exposure and after 3, 6, 9, and 12 months of treatment.
Haematological and biochemical tests and urinalyses were conducted on
all animals after 6 and 12 months. All animals were examined
post mortem, and all major tissues and organs were examined
microscopically. Selected organs were weighed. All animals survived to
the end of the study, with no relevant clinical or ophthalmological
signs. The body weights of males and females receiving 2000 ppm
piperonyl butoxide were 2% lower to 3% higher than the initial
weights, whereas those of controls were 22-25% higher. Treatment-
related decreases in food consumption were observed in males at 600
(by 15%) and 2000 (by 20%) ppm. Small, not statistically significant,
not dose-related decreases in food consumption were observed in
treated females.
No treatment-related change in haematological parameters was
observed. The activity of alkaline phosphatase was increased to three
to five times the control values in animals at 2000 ppm after 6 and 12
months, and cholesterol levels were decreased (not statistically
significant) at these intervals in females. Males and females at this
dose had increased absolute (by 22 and 36%) and relative (by 52 and
86%) liver and gall-bladder weights, and small increases in thyroid
and parathyroid weights (average, 34%) were observed in females. These
changes were not associated with microscopic changes in the thyroid
and were considered to be of questionable biological significance. No
macroscopic pathological changes were observed that could be
attributed to treatment. Treatment-related histopathological changes
were limited to diffuse, mild hypertrophy of the hepatocytes in males
and females at 2000 ppm. The NOAEL was 100 ppm, equal to 16 mg/kg bw
per day, on the basis of effects on the liver, some clinical chemical
alterations, and reduced body-weight gain at 2000 ppm (Goldenthal,
1993b).
Monkeys
Two African green monkeys were given 32 or 106 mg/kg bw per day
piperonyl butoxide orally for six days per week for four weeks.
Minimal microscopic changes were observed in the livers (Sarles &
Vandergrift, 1952).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 (C57Bl/6 × C3H/Anf)F1 and (C57Bl/6 × AKR)F1 mice of
each sex received piperonyl butoxide (purity unspecified) at 100 mg/kg
bw or 'Butacide' at 464 mg/kg bw in 0..5% gelatin at seven days of age
by stomach tube and the same amount (not adjusted for increasing body
weight) daily up to four weeks of age; subsequently, the mice were fed
diets containing 300 ppm. The dose was reported to be the maximal
tolerated dose for infant and young mice. All animals were killed when
they were about 70 weeks of age, and their tumour incidences were
compared with those of 79-90 necropsied mice of each sex and strain,
which had either been untreated or had received gelatine only. No
significant difference in the incidence of tumours was found between
treated and control mice, although the authors concluded that
additional evaluation was required (Innes et al., 1969). This study
was not considered to be adequate by the Meeting.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for 30 weeks;
the doses were decreased to 500 or 2000 ppm for the following 82
weeks, due apparently to a greater than expected decrease in
body-weight gain. The control group consisted of 20 males and 20
females. Animals were observed twice daily for signs of toxicity; body
weight was recorded at least once per month. Necroscopic examination
was performed at termination on all moribund animals and on those
found dead, unless precluded by autolysis. Major tissues and organs
and all gross lesions were examined microscopically. At termination,
85% of control males and 75% of control females, 82% and 68% at the
low dose, and 84% and 70% at the high dose, respectively, were still
alive. Body weight was reduced in a dose-related manner, by up to
about 15% in females at the high dose at termination. No
treatment-related increase in tumour incidence was found, but
hepatocellular carcinomas occurred frequently, especially in males,
with incidences of 50% in controls, 34%, at the low dose, and 40% at
the high dose. No statistically significant differences were found in
the incidences of neoplastic and non-neoplastic lesions (US National
Cancer Institute, 1979)
Groups of 60 CD-1 mice of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) at concentrations adjusted to give
doses of 0 (two control groups), 30,100, or 300 mg/kg bw per day, for
78 weeks. The diets were prepared weekly and the concentrations
adjusted on the basis of body weights and food consumption. The diets
were analysed for the actual concentration, homogeneity, and stability
of piperonyl butoxide. The average actual concentrations at the low
and high doses were 99 and 95%. The homogeneity and stability (no
decrease in concentration after 14-21 days) of piperonyl butoxide in
the diet were considered to be satisfactory. Animals were observed
twice daily for signs of toxicity. Food consumption and body weight
were determined weekly for the first week and then every other week.
Haematological measurements were made for 10 rats of each sex in the
control and high-dose groups at week 52 and for all groups at
termination. All surviving animals were necropsied; major tissues and
organs from controls and animals at the high dose and the lungs,
liver, kidneys, and gross lesions from other groups were examined
microscopically. All slides from the livers underwent an independent
pathological review; the results reported here are the consensus or
majority opinions of all the pathologists involved.
No treatment-related clinical signs or palpable masses were
observed. The mortality rates were (males/females) 27/32, 32/27,
27/38, 22/33, and 40/18% in the first control group, animals at 30,
100, and 300 mg/kg bw per day, and the second control groups,
respectively. Body weights and body-weight gains of animals at the
high dose were slightly (occasionally statistically significantly)
decreased. No significant effects on food consumption were observed.
Haematological parameters were also unaffected by treatment. A
dose-related increase in absolute and relative liver weights was
observed in mice of each sex at the middle and high doses.
Hepatocellular hypertrophy was more frequent in males at the high
(72%) and middle doses (27%) than in controls (10 and 18%) and in
females at the high dose (15%; 0 and 7% in controls). Hepatocellular
hyperplasia (also called eosinophilic/clear-cell focus), characterized
by aggregates of hepatocytes with a swollen eosinophilic cytoplasm,
was present in 8% of males at the high dose, 2% of females at the
middle dose, and 7% at the high dose. The incidences of hepatocellular
hyperplasia were 0 and 3% in control males and 0% in both female
control groups. The incidence of hepatocellular adenomas was higher in
male mice at the middle (37%) and high (47%) doses than in either
control group (15 and 13%) and in females at the high dose (17%; 3% in
controls). The hepatocellular adenomas observed in many treated
animals were composed of large, polyhedral, densely-packed cells with
abundant granular eosinophilic cytoplasm. This appearance is different
from that of spontaneous adenomas found in CD-1 mice, where small to
medium, well-differentiated, basophilic cells, distributed in solid to
normal sinusoidal patterns, are found. Only the incidence of the
former type of adenoma was increased in piperonyl butoxide-treated
mice. A nonsignificantly increased incidence of hepatocellular
carcinomas was observed in males at the high dose (12%; 3% in
controls). No other treatment-related neoplastic or non-neoplastic
lesion was found. The NOAEL was 30 mg/kg bw per day on the basis of
effects on the liver (Hermanski & Wagner, 1993).
A 12-month study of carcinogenicity was conducted in male
Crj:CD-1 mice fed diets containing 0 ( n = 52), 6000 ( n = 53), or
12 000 ( n = 100) ppm piperonyl butoxide (purity, 94.3%; no
detectable safrole or isosafrole). Animals were observed daily for
mortality and morbidity; those that died or were sacrificed at
termination were necropsied, and gross lesions and the liver were
examined histologically. The mortality rates were 6% of controls, 2%
at the low dose, and 19% at the high dose. Terminal body weight was
found to have been reduced by 17% at the low dose and 29% at the high
dose. Hepatocellular hyperplasia was seen in 38% of animals at the low
dose and 8% at the high dose, hepatocellular adenomas in 13 and 22%,
hepatocellular carcinomas in 11 and 52%, and haemangioendothelial
sarcoma in 2 and 42%. It should be noted that the hepatocellular
adenomas and carcinomas may have obscured the true dose-response
relationship for hepatocellular hyperplasia. A condition termed
'post-necrotic peliosis', consisting of multifocal necrosis with blood
cysts, was also found in 17% of animals at the high dose. One control
animal showed hepatocellular hyperplasia and one had a hepatocellular
adenoma (Takahashi et al., 1994a).
Rats
Groups of 12 Wistar rats of each sex were fed diets containing 0,
100, 1000, 10 000, or 25 000 ppm piperonyl butoxide for two years, and
an additional group was fed a diet containing 1000 ppm piperonyl
butoxide plus 167 ppm pyrethrins. Animals were observed daily for
clinical signs of toxicity, and food consumption and body weight were
determined weekly. Autopsies were performed on all animals. Males and
females were paired for the duration of the study, except during
nursing (see section ( d)). Body-weight gain was reduced in animals
at 10 000 (by 10-20%) and 25 000 ppm (by about 90%), in association
with reduced food intake. All animals at the high dose were dead by
week 68; the mortality rates in the other groups were (males/females):
30/20%, 40/30%, 40/50%, and 60/60% for controls and animals at 100,
1000, and 10 000 ppm, respectively. The relative weights of livers and
kidneys were higher than those of controls in animals at 10 000 (by
40% for liver and kidney) and 25 000 ppm (by 275% for liver and 150%
for kidney). These effects were not associated with morphological
alterations. The incidence of tumours was not increased (Sarles &
Vandergrift, 1952).
Sixty Sprague-Dawley Crl:CDR (SD)BR rats of each sex were fed
diets containing piperonyl butoxide (purity, 89%) at concentrations
adjusted weekly (every two weeks after week 15) to achieve daily
intakes of 0 (two groups of controls), 30, 100, or 500 mg/kg bw per
day for 104-105 weeks. The stability and homogeneity of the diets and
the correspondence of actual to nominal concentrations were checked
before and throughout the study and found to be acceptable. The
animals were observed for mortality, clinical signs, food consumption,
body weight, and ophthalmoscopic, haematological, clinical
biochemical, urinary, and pathological parameters. Complete
histological examinations were made of animals in the two control
groups and at the high dose and of those animals at the low and
intermediate doses which died during the study. Histological
examination of animals at these doses killed at the end of the study
was limited to the liver, kidney, lung, thyroid, testis, epididymides,
ovary, and any observed abnormalities.
No clinical signs related to treatment were observed.
Sialodacryoadenitis was diagnosed in a large percentage of treated
animals in weeks 25-28 and weeks 63-67. Body weights were lower in
rats of each sex at 500 mg/kg bw per day than in controls throughout
the study. At week 104, the mean body weight was 20-30% lower than the
control value. A trivial reduction in food consumption was observed in
animals of each sex at the highest dose. Ophthalmoscopic examination
in week 99 revealed no changes attributable to treatment, and
haematological tests and urinalysis revealed no adverse effects.
Female rats receiving 500 mg/kg bw per day had higher cholesterol
levels than controls throughout the study and increased blood urea
nitrogen at 98 weeks. Other statistically significant differences in
biochemical parameters were of no biological relevance. At the end of
the study, the mortality rates were 82, 78, 87, 82, and 78% in males
and 55, 68, 63, 43, and 50% in females at 0 (two groups), 30, 100, and
500 mg/kg bw per day, respectively.
Increased liver weights were seen in animals of each sex at 100
and 500 mg/kg bw per day, corresponding to a higher incidence of
macroscopic enlargement associated with hyperplasia and hypertrophy of
centrilobular hepatocytes and enlarged eosinophilic cells, which
occasionally contained a brownish cytoplasmic pigment. Increased
kidney weights were observed in female rats at the two highest doses,
corresponding histologically to a higher incidence of chronic
interstitial glomerulonephritis. Other histological changes were
confined to the endocrine organs: Enlarged thyroid glands were
observed in animals of each sex at 500 mg/kg per day, corresponding
histologically to a higher incidence of generalized and focal
hyperplasia of follicles and increased pigment deposition in the
colloid. A slightly higher incidence of adrenal and ovarian
enlargement seen among females receiving 500 mg/kg per day was not
associated with histological changes. In males, there was a negative
trend in the presence of enlarged, focal, coarsely vacuolated cortical
cells in the adrenals. The combined incidence of bilateral and
unilateral testicular atrophy was comparable between groups, but there
were significant differences from controls in the incidences of
bilateral testicular atrophy at all doses, with 17, 33, 47, and 43% at
0, 30, 100, and 500 mg/kg bw per day, respectively. After an
additional, full review of the relevant data, this finding was
considered unlikely to be related to treatment because the atrophy was
not associated with degeneration of seminiferous tubules or
aspermatogenesis, and testicular weights were not decreased.
Morphometric analysis of the pituitary gland area showed reduced size
in males at the highest dose. A trend analysis of tumour incidence
with dose showed both increases (in the lymphoid system and thyroid)
and decreases (in mammary glands and pituitary). These differences
were not statistically significant in pairwise comparisons between
groups, and the incidences were within the range of those of
historical controls of this strain of rats. These observations provide
no evidence that the treatment had carcinogenic potential, but the
wide range of gross alterations, effects on organ weight, and
histological changes observed reflect the biological activity of the
test compound. The enlargement of the liver, the primary target organ,
is consistent with the activity of the compound as a hepatic enzyme
inducer. The wide differences in the incidences of morphological
changes and lesions in endocrine and hormone-sensitive organs between
controls and treated groups strongly support the interpretation that
they are the results of changes in hormonal levels brought about by
hepatic enzyme induction. The NOAEL was 30 mg/kg bw per day on the
basis of effects on the liver (Graham, 1987).
Groups of 50 Fischer 344/Du Crj rats of each sex were fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide (purity, about
89%) for two years at doses selected on the basis of the results of
the 13-week study by the same authors, summarized above. Animals were
observed daily for clinical signs and mortality; food consumption was
measured monthly, but the frequency of body-weight measurement was not
stated. Treatment was stopped after 104 weeks, and surviving animals
were sacrificed after six more weeks on the basal diet. All animals,
including those sacrificed when moribund or found dead, were
necropsied and a complete histological examination was performed. The
mortality rates were (males/females) 16/14, 38/22, and 42/34% in
controls and animals at 5000 and 10 000 ppm; the increased mortality
was statistically significant for animals at the high dose. Just
before death, many animals showed signs of anaemia (not specified) and
had blood in their faeces. A dose-related reduction in body weight was
seen in animals of each sex (statistical analysis not reported), with
no concomitant decrease in food intake. No statistically significant
difference in the incidence of malignant or benign neoplasms was
found, except a decreased incidence of thyroid C-cell adenomas in
males at the high dose (9%; 44% in controls). Dose-related increases
were seen in the incidences of ulcers (0/0%, 35/2%, and 52/45% in male
and female controls and those at the low and high doses,
respectively), regenerative hyperplasia (0/0%, 13/0%, and 22/4%),
ossification in the ileocaecal mucosa (0/0%, 15/0%, and 20/0%), and
haemorrhage of the caecum and colon (0/0%, 10/0%, and 17/12%). The
main histological lesions were chronic ulcers with inflammatory-cell
infiltration and granulation, sometimes with ossification of the
surrounding mucosa. Most of deaths in treated animals before the end
of the study were attributed by the authors to lesions leading to
anaemia. Piperonyl butoxide was not found to be carcinogenic
(Maekawa et al., 1985).
Groups of 50 Fischer 344 rats of each sex were fed diets
containing 5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for
24 months; the control group consisted of 20 animals. The diets were
prepared freshly each week and stored at 7°C. Animals were observed
twice daily for mortality and underwent a complete clinical
examination monthly. The food consumption of 10 animals in each group
was measured during the first week of each month; body weights were
measured about twice monthly. Moribund animals were sacrificed and
necropsied and their organs were examined histologically. All
surviving animals were sacrificed and submitted to a complete
histopathological examination. All control females and about 80% of
treated females survived until termination, whereas no difference in
survival was found for males (70-75% survival in all groups). A
dose-related reduction in body weight was found for both females (16%
at the low dose and 24% at the high dose) and males (7% at the low and
16% at the high dose). No difference was found in the incidence of
malignant or benign neoplasms, except for that of lymphoreticular
malignant lymphoma which was significantly increased in treated
females (14% at the low and 30% at the high dose; 5% in controls) and
nonsignificantly decreased in males (30% at the low and 26% at the
high dose; 35% in controls). The author concluded that the results
were equivocal because of the variability in the historical control
data and the difficult diagnosis of these neoplasms (Cardy et al.,
1979; US National Cancer Institute, 1979).
Groups of 30-33 Fischer 344/DuCrj rats of each sex were fed diets
containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide (two
lots: purity, 94.5 and 94.3%; no detectable safrole or isosafrole) for
95 (males) or 96 (females) weeks. The study was ended before 104 weeks
because of the high mortality of males at 12 000 ppm (see below).
Animals were observed daily for clinical signs and mortality; body
weights were measured monthly. Food consumption was determined in a
preliminary experiment involving six rats of each sex per group
(duration not reported), which resulted in calculated compound intakes
of 537, 1061, and 2002 mg/kg per day by females and 547, 1052, and
1877 mg/kg per day by males at 6000, 12 000 and 24 000 ppm,
respectively. Rats that died were necropsied and observed for tumors
and non-neoplastic lesions, which were then examined microscopically.
At termination, surviving rats were necropsied; hepatic nodules (when
multiple nodules were present, three major ones were taken) and major
organs were weighed and examined histologically. Blood was collected
for haematological tests (including prothrombin and partial
thromboplastic times) and for standard clinical chemistry. The
mortality rates were 17, 23, 50, and 24% in males and 20, 10, 17, and
21% in females at 0, 6000, 12 000, and 24 000 ppm, respectively. Males
at 12 000 ppm began to die as early as week 40 and at a statistically
significantly different rate from other groups from week 45. Deaths
were most frequently associated with haemorrhage in the caecum. Body
weights were reduced in a dose-related manner in all treated animals
but statistically significantly only in animals at the middle and high
doses. Males and females at the high dose lost weight from week 60,
and at termination their body weights were about 50% of those of
controls. The weights of all organs except the liver were reduced in
animals at the high dose. The absolute liver weights were increased in
animals at 12 000 ppm (in males by 22% and in females by 51%) and in
females at 6000 ppm (by 29%). During the first month of treatment,
rough hair, lethargy, epistaxis, and a fall in food consumption (data
not reported) were observed in animals of each sex at 24 000 ppm.
Haematological tests showed a significant increase in hypochromic,
microcytic anaemia, the severity of which was dose-related, in all
treated animals. The most significant findings in plasma were
decreased cholinesterase activity and triglyceride contents in animals
at all doses and an increased urea nitrogen level in animals at the
high dose and in all treated females. Hepatic tumours were observed
from week 74 of treatment. Nodular lesions of the liver were observed
only in treated animals, and their incidence, numbers per rat, and
size were dose-related. Hepatocellular adenomas and carcinomas were
found in animals at the middle and high doses: the incidences of
adenomas were 27% in males and 13% in females at 12 000 ppm and 15% in
males and 30% in females at 24 000 ppm; the incidences of carcinoma
were 13% in males and 0% in females at 12 000 ppm and 73% in males and
46% in females at 24 000 ppm. The nodules in animals at the low dose
were classified as foci or hepatocellular focal hyperplasia. The only
other difference between groups was found for interstitial-cell
tumours of the testis, with incidences of 23/25 in controls, 19/23 at
6000 ppm, 13/15 at 12 000 ppm, and 15/25 at 24 000 ppm. Essential
(haemorrhagic) thrombocytaemia (defined as > 3 × 106 platelets per
microlitre, with a bimodal platelet distribution curve) was found in
0/24 male controls and in 6/23 males at 6000 ppm, 3/15 at 12 000 ppm,
and 9/24 at 24 000 ppm and in only 1/25 females at the high dose. Of
the non-neoplastic lesions, the following are significant: an
increased incidence of stomach haemorrhage (males: 9/33; 5/30 in
controls; females: 19/33; 2/30 in controls) at the high dose, an
increased incidence of haemorrhage and/or oedema of the caecum in all
treated males and in females at the middle dose, an increased
incidence of black kidneys in males at the middle and high doses and
all treated females, and an increased incidence of whitish spotting in
the lungs of males at the middle and high doses. Tubular dilatation,
distension of Bowman's space and interstitial fibrosis were found in
the kidneys of animals at the high dose (actual numbers not reported)
(Takahashi et al., 1994b).
(d) Reproductive toxicity
Mice
In a two-generation study, with one litter per generation, groups
of 10 male and 10 female Crj:CD-1 mice were fed diets containing 0,
1000, 2000, 4000, or 8000 ppm piperonyl butoxide (purity unspecified).
Animals of the F0 generation, five weeks old at the start of the
study, were mated for five days at nine weeks of age. Animals of the
F1 generation was removed from their dams at four weeks of age and
allocated randomly to continue treatment; the F2 generation was
produced similarly to the F1 generation. Individual food intake was
measured before mating and during mating, gestation, and lactation for
all generations; litter size and weight and sex ratio were measured at
birth, and pups were weighed 0, 4, 7, 14, and 21 days after birth.
Some neurobehavioural tests were performed during lactation of the
F2 generation and the results analysed on a litter basis. The tests
included surface righting and negative geotaxis on postnatal days 4
and 7, cliff avoidance on postnatal day 7, swimming behaviour on
postnatal days 4 and 14, and olfactory orientation on postnatal day
14. Food consumption was reduced by 4-44% in F0 animals at 8000 ppm
except during mating, by 47% in F1 animals at 8000 ppm during
lactation, and by 16-22% in F0 and F1 animals at 4000 ppm during
lactation. The mean litter size of F1 animals at 8000 ppm was not
significantly reduced (7.7; 10.6 in controls), but the mean F1
litter weight was reduced by 38% (statistically significant) at
8000 ppm and by 18% (not statistically significant) at 4000 ppm. F1
pups at 8000 ppm had a lower survival index at postnatal day 21 (63%;
91% in controls for males, statistically significant; 79%; 89% in
controls for females, not statistically significant). The weights of
all F1 pups were reduced, but there was no dose-response
relationship at 1000-4000 ppm. The results of neurobehavioural tests
for treated F1 animals were different from those of controls in some
instances, but no dose-related response was evident. The mean F2
litter size was significantly reduced at 4000 ppm (10.3; 13.1 in
controls) and 8000 ppm (7.0), and the mean F2 litter weights were
reduced in all treated groups by 13-57%. F2 pups at 8000 ppm had a
lower survival index than controls at postnatal day 21 (59% in males,
statistically significant; 79% in females, not statistically
significant), and F2 pup weights were reduced at 2000, 4000, and
8000 ppm. At 2000 and 4000 ppm, the effect was evident only from
postnatal day 4; at 1000 ppm, reductions in pup weights were observed
on postnatal day 4 in males and postnatal day 7 in animals of each
sex. There was no NOAEL because of effects on pup weights at all doses
(Tanaka et al., 1992).
Groups of 10 male and 10 female Crj:CD-1 mice were fed diets
containing 0,1500, 3000, or 6000 ppm piperonyl butoxide (purity
unspecified) for four weeks before mating (F0 generation), during
gestation, and until the F1 generation was eight weeks old.
Open-field activity was measured in the F0 generation after three
weeks of treatment. Litter size, pup weight, and some developmental
behavioural signs (surface righting, negative geotaxis, cliff
avoidance, swimming, and olfactory orientation) were measured during
lactation of the F1 generation; open-field activity was measured at
three and eight weeks of age and multiple water T-maze activity at six
weeks of age. A dose-related decrease in ambulation and rearing was
seen in the open-field test in males of the F0 generation, but only
the former was statistically significant at the high dose. Pup body
weight at birth was reduced in all groups; on postnatal day 21, the
body weight of animals at the middle dose was 7% lower than that of
controls, and that of animals at the high dose was 41% lower. The
survival indexes at postnatal day 21 were 79, 93, 80, and 52 in
controls and in mice at the low, middle, and high doses, respectively.
The results of the behavioural test during lactation were not
significant, except for reduced olfactory orientation in animals at
the middle and high doses. The results of the open-field test and the
multiple water T-maze test were not altered by treatment, except for a
few, not dose-related differences in some parameters (Tanaka, 1992).
Rats
In a two-litter, two-generation study of reproductive toxicity,
groups of 26 Sprague-Dawley CD rats of each sex, seven weeks of age,
received piperonyl butoxide in the diet at 0, 300, 1000, or 5000 ppm,
equal to 0, 20, 68, and 350 mg/kg bw per day for males (average food
intake calculated in weeks 1-28 of the study) and to 0, 29, 94, and
480 mg/kg bw per day for females (calculated in weeks 1-12 of the
study). The stability, homogeneity, and correspondence of the actual
concentrations in the diets to the nominal concentrations were checked
several times before and during the study and found to be acceptable.
Rats were maintained on their respective diets for 85 days before
mating, throughout the two mating periods, and until scheduled
sacrifice. The F1b generation litters were weaned on day 21
post partum, and groups of 26 rats of each sex were selected to form
the F1b adult generation. These animals were maintained on their
respective diets for 83 days before mating. Animals were observed for
signs of toxicity, and body weight and food consumption were recorded.
External and internal gross examinations were performed on adult rats
and on a selected number of weanlings; histopathological examination
was undertaken for the reproductive tracts of control animals, those
at the high dose, and those of rats at the low and middle doses that
failed to mate successfully in either mating period.
Clinical and pathological examinations showed no
treatment-related toxic effects in adult F0 or F1b animals. The
body weights of adult animals of each sex at 5000 ppm were lower than
those of controls from a few weeks after the beginning to the end of
the study. This effect occasionally corresponded to reduced food
intake. Mating performance, fertility indexes, gestation indexes,
length of gestation, and numbers of live and dead pups at birth were
not affected by treatment. The viability and lactation indexes,
clinical conditions, and pathological appearance of pups of all
generations were also unaffected. The body weights of male and female
pups of all generations at 5000 ppm were lower than those of control
pups. This effect was not detectable at birth but was seen as early as
day 4 post partum. The NOAEL for parental toxicity and pup development
was 1000 ppm, equal to 68 mg/kg bw per day (Robinson et al., 1986).
During the study of carcinogenicity reviewed above (Sarles &
Vandergrift, 1952), rats of each sex were pair-caged throughout the
experiment, except during nursing. No effect on reproductive
efficiency was seen in the first, second, or third generations (no
details given).
(e) Developmental toxicity
Mice
Groups of 20 pregnant Crj:CD-1 mice were given piperonyl butoxide
(purity, > 95%) in olive oil by gavage on day 9 of gestation at doses
of 0, 1065, 1385, or 1800 mg/kg bw. Dams were weighed on gestation
days 9 and 18 and then sacrificed, and the uteri were examined for the
presence and position of resorption sites, fetuses, and implantation
sites. Viable fetuses were weighed and examined for external
malformations and variations, and then fixed and stained for
visualization of the skeleton. No abnormal behaviour or mortality was
observed in dams. One dam at the middle dose and two at the high dose
aborted; the litters of one dam at the middle dose and three at the
high dose were resorbed. Maternal body-weight gain was comparable in
all groups. The total resorption rates were significantly greater at
the middle (26%) and high (32%) doses than in controls (6%), probably
due to total litter resorptions, since the number of viable fetuses
per dam was comparable in all groups. (The raw data were not available
to confirm the reviewer's hypothesis.) The average fetal body weight
was significantly reduced in males, by 3% at the middle dose and 7% at
the high dose, and in females, by 4% at the low dose, 5% at the middle
dose, and 6% at the high dose. Exencephaly, craniochisis, open
eyelids, omphalocele, kinky tail, and talipes varus were observed in
all groups. Oligodactyly in the forelimbs was found in none of the
controls but in one fetus at the low dose, four (2%) at the middle
dose, and 27 (6%) at the high dose. A single oral dose > 1065 mg/kg
bw per day of piperonyl butoxide to dams on day 9 of gestation was
thus embryo- and fetotoxic (Tanaka et al., 1994).
Rats
Groups of 20 pregnant COBS random-bred albino rats were given 0,
300, or 1000 mg/kg bw per day piperonyl butoxide (technical-grade;
purity unspecified) dissolved in corn oil by gavage on days 6-15 of
gestation. The doses were chosen after a pilot study in which six
animals per group were treated with 0, 100, 300, 1000, or 3000 mg/kg
bw per day; reduced body-weight gain was observed during gestation
days 6-15 at the highest dose. Pregnant animals were shipped from the
breeder to the test laboratory on day 1 of gestation and were observed
and weighed daily. On day 20 of gestation, they were sacrificed and
the usual parameters were determined. No signs of toxicity were
observed. Body-weight gain was reduced by 10% in animals at the low
dose and by 15% in those at the high dose, mainly between days 15 and
20 of gestation. Reproductive parameters were not significantly
affected by treatment. One female at 300 mg/kg bw resorbed 8 of 11
fetuses, and one at the high dose resorbed the entire litter. Fetuses
of treated dams had no internal, external, or skeletal malformations
that could be related to treatment. There was no NOAEL for maternal
toxicity because of reduced body-weight gain at both doses. The NOAEL
for embryo- and fetotoxicity was 1000 mg/kg bw per day on the basis of
the absence of any significant finding (Kennedy et al., 1977). This
report from Industrial Biotest Laboratories has not been validated by
a governmental agency and was therefore not taken into account by the
Meeting.
Groups of 17-20 pregnant Wistar rats were given piperonyl
butoxide (purity, 80%) at 0, 62.5, 125, 250, or 500 mg/kg bw dissolved
in corn oil by gavage on days 6-15 of gestation. They were sacrificed
on day 22 of gestation and necropsied, and their fetuses were removed
and examined for external, visceral, and skeletal changes. There were
no deaths or signs of toxicity in dams and no effect on the numbers of
corpora lutea, live fetuses, resorption sites plus dead fetuses,
anomalous fetuses, anomalous litters, or fetal body weight. The types
and incidences of anomalies in the treated groups were comparable to
those of the control group (data not shown). There was no evidence of
embryotoxicity, fetotoxicity, or teratogenicity, and no maternal
toxicity was elicited at the doses used (Khera et al., 1979).
Groups of 20 timed-pregnant Sprague-Dawley CD rats were given
undiluted piperonyl butoxide (purity, 90.78%) at 0, 200, 500, or
1000 mg/kg bw per day by gavage on days 6-15 of gestation. The doses
were chosen in a preliminary pilot study in which five animals per
group were treated with 0, 250, 500, 1000, 2000, or 4000 mg/kg bw per
day, and in which all animals at 4000 and four-fifths of those at
2000 mg/kg bw per day groups died or became moribund. Animals were
observed and weighed daily. On day 20 of gestation, they were
sacrificed and the usual parameters plus liver weight were determined.
No females aborted or delivered early, but wetness in the urogenital
area was observed in 13/24 dams at the highest dose, and stains of
urine, red urogenital discharge, and perinasal encrustation were seen
at lower incidences. Body-weight gain was reduced between days 6 and 9
of gestation in animals at the low dose (an effect considered to be
nonsignificant because of the low weight of non-pregnant animals) and
between days 6 and 15 in animals at the middle and high doses; these
reductions were associated with reduced food intake, resulting in a
significantly lower body weight (by about 5%) in animals at 500 and
1000 mg/kg bw per day on days 15 and 18 of gestation. Increased
absolute (by 8%) and relative (by 11%) weights were observed at the
high dose. Reproductive parameters were not significantly affected by
treatment. Fetuses of treated dams had no internal, external, or
skeletal malformations that could be related to treatment. The NOAEL
for maternal toxicity was 200 mg/kg bw per day on the basis of reduced
body-weight gain and food consumption, increased liver weight, and
signs of toxicity at higher doses. The NOAEL for embryo- and
fetotoxicity was 1000 mg/kg bw per day on the basis of the absence of
any significant finding (Chun & Neeper-Bradley, 1991).
Rabbits
In a range-finding study, five inseminated New Zealand white
rabbits were treated by gavage with piperonyl butoxide during
gestation days 7-19 at doses of 0, 50,100, 200, 300, or 400 mg/kg bw
per day in corn oil. All animals except one control survived until the
end of the study. Two animals each at 300 and 400 mg/kg bw per day and
one at 100 mg/kg bw per day group aborted all or part of their litters
between gestation days 22 and 26. Decreased defaecation was observed
at the highest dose, and animals at the two highest doses were thinner
than normal at the end of the treatment period. Significant reductions
in body-weight gain and some body-weight loss were observed in rabbits
at 300 and 400 mg/kg bw per day; a trivial reduction in body-weight
gain was observed in animals at 200 mg/kg bw per day. No consistent
effects were seen on uterine parameters. Doses of 50, 100, and
200 mg/kg bw per day were therefore chosen for the definitive study of
teratogenicity.
Groups of 16 inseminated New Zealand white rabbits were treated
by gavage with piperonyl butoxide (purity, 100%) during gestation days
7-19 at doses of 0, 50, 100, or 200 mg/kg bw per day in 0.5 ml/kg bw
corn oil. Caesarean sections were performed on gestation day 29, and
the fetuses were removed for teratological evaluation. All animals
survived until the end of the study. Decreased defaecation was
observed in animals at 100 and 200 mg/kg bw per day. Slight
body-weight loss was observed at the middle and high doses during the
treatment period, but there was substantial recovery of body weight
during the post-treatment period, so that the weight gain during the
overall gestation period was comparable to that of controls. The mean
post-implantation losses of treated does were slightly greater than
those of controls but were not dose-related. Malformations were
observed incidentally and were considered to be unrelated to
treatment. The numbers of fetuses with more full ribs than normal (45,
58, 59, and 60% at 0, 50, 100, and 200 mg/kg bw, respectively) or with
27 presacral vertebrae (20, 32, 27, and 40%, respectively) were
greater than those in controls, but the number of litters was not
different. There was no clear dose-effect relationship, and the
relevance of this finding is dubious. The NOAEL for maternal toxicity
was 50 mg/kg bw per day on the basis of decreased defaecation and
dose-related body-weight losses during the treatment period. The NOAEL
for teratogenicity was 200 mg/kg bw per day (Leng et al., 1986). The
1992 JMPR wrongly reported an NOAEL of 100 mg/kg bw per day.
(f) Genotoxicity
The results of studies of the genotoxicity of piperonyl butoxide
are reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Piperonyl butoxide was applied at 0.5 ml to intact, clipped skin
of six New Zealand white rabbits for 4 h under a semi-occlusive
dressing, and the treated areas were examined for erythema and oedema
up to 78 h after the contact period. An adjacent area of untreated
skin served as the control. Piperonyl butoxide was very mildly
irritating (Romanelli, 1991a). An older study reported similar results
(Sarles et al., 1949).
Piperonyl butoxide at 0.1 ml was instilled into the conjuntival
sac of one eye of six New Zealand white rabbits, and the treated eyes
were examined up 72 h after instillation. Conjunctival irritation was
observed, which fully recovered within 72 h. No corneal or iridal
lesions were observed (Romanelli, 1991b). An older study reported
similar results in rabbits, cats, and dogs (Sarles et al., 1949).
Table 2. Results of tests for the genotoxicity of piperonyl butoxide
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, TA100, 100-5000 µg/plate 90.78 Negativea Lawlor (1991)
TA1535, TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative White et al. (1977)
TA 100, TA1537
Reverse mutation E. coli WP2 1 mg/disc 80 Negative Ashwood-Smith
et al. (1972)
Gene mutation Mouse lymphoma L5178Y 6.3-100 µg/mlb NR Positive McGregor et al.
cells (1988)
Gene mutation Chinese hamster ovary, cells 10-100 µg/mlc NR Equivocal Tu et al. (1985)
25-500 µg/mld Negative
Unscheduled DNA Rat hepatocytes 1-100 µg/mle 90.78 Negative McKeon & Phil
synthesis (1991)
Unscheduled DNA Human liver slices 0.05-2.5 mmol/litre 90.78 Negative Lake (1995)
synthesis
Chromosomal Chinese hamster ovary cells 25-99.9 µg/ml 90.78 Negativea Murli (1991)
aberration 62.6-251 µg/mla
Table 2. (cont'd).
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Dominant lethal ICR/Ha Swiss mice 0, 200, or 1000 mg Commercial Equivocal Epstein et al. (1972)
mutation intraperitoneally or formulation
1000 mg/kg bw orally
× 5
NR, not reported
a With and without metabolic activation
b Lethal at 100 µg/ml
c Without metabolic activation; cytotoxic at > 30 µg/ml
d With metabolic activation; cytotoxic at > 300 µg/ml
e Cytotoxic at > 49.9 µg/ml
Skin sensitization was studied in 10 male Hartley guinea-pigs by
a modified Buehler method. A gauze patch containing 0.4 ml piperonyl
butoxide was applied for 6 h onto clipped skin, three times a week for
three weeks. After a two-week rest period, the animals were challenged
on another site with a single 6-h application. Chlorodinitrobenzene
(0.1% w/v in a 50% ethanol:0.9% saline solution) was used as the
positive control. No evidence of contact sensitization was observed
(Romanelli, 1991c). An older study also reported no response in
rabbits (Sarles et al., 1949)
(ii) Studies with mixtures
Neonatal ICR/Ha Swiss mice (n = 91) were given subcutaneous
injections of 5 mg piperonyl butoxide alone at the ages of one and
seven days and 10 mg at the ages of 14 and 21 days. Further groups
were given the same doses in combination with Freon-112 (n = 137) or
-113 (n = 94) at doses of 10 mg at one and seven days and 20 mg at 14
and 21 days. Control animals were treated with the solvent,
tricaprylin. All surviving animals were killed after 52 weeks. No
tumours were found in animals treated with piperonyl butoxide, but the
incidence of hepatomas in male mice given piperonyl butoxide plus
Freon was significantly increased when compared with that in groups
receiving solvent, piperonyl butoxide, or Freon alone (Epstein
et al., 1967).
Groups of 45 Sprague-Dawley CD rats of each sex were fed either
standard diets or diets containing 400 ppm pyrethrins plus 2000 ppm
piperonyl butoxide for 104 weeks. The diets were prepared daily from a
10-fold concentrated pre-mix prepared freshly once a week. The purity
of piperonyl butoxide was 90.5%, and that of pyrethrins was 53.1%
(batch used until week 55) or 52.4% (batch used from week 56 until
termination); the diets were prepared taking into account the purity
of the compounds. The actual concentrations and the stability of the
diet were not tested. Body weight and food consumption were determined
once a week. The average daily intakes were 79 and 101 mg/kg bw
piperonyl butoxide and 16 and 20 mg/kg bw pyrethrins for males and
females, respectively. Animals were observed for clinical signs, skin
lesions, and palpable masses. All animals found dead or moribund were
inspected, and macroscopic lesions were examined microscopically.
During week 101, urinalysis and haematological and clinical chemical
tests were performed in 10 animals per group. At termination, all
animals were observed grossly and selected organs were examined
microscopically.
No treatment-related clinical signs of toxicity were observed.
Mortality was 84 and 76% in control males and females and 80 and 58%
in treated males and females, respectively. The body weights of
treated females were about 20% lower than those of controls, mainly
during the first 78 weeks, while at termination treated animals were
about 10% lighter than controls; the difference was less evident
(about 5%) in males. Slightly reduced food intake was also observed
until week 27 of treatment. Haematological, clinical chemical, and
urinary parameters were not significantly altered in treated animals,
and macroscopic and microscopic examinations revealed no significant
treatment-related neoplastic or non-neoplastic effects (Hunter
et al., 1977).
3. Observations in humans
In nine men, antipyrine metabolism was not affected by a single
oral dose of 50 mg (0.71 mg/kg bw) piperonyl butoxide (Conney et al.,
1972).
Two male infants, whose mothers were sisters and who were born
within two weeks of each other, had coarctation of the aorta. Exposure
to insect repellents and insecticides, including piperonyl butoxide,
during week 8 of gestation was reported (Hall et al., 1975).
Percutaneous absorption of pyrethrin and piperonyl butoxide was
studied in six male volunteers. A formulation containing 0.3% pyrethin
and 3.0% piperonyl butoxide was spread on the ventral forearm for 30
min. This formulation was chosen because it corresponds to that of a
commercially available product used for the treatment of head lice.
The formulation contained either 14C-pyrethrin or 14C-piperonyl
butoxide. After application, urine was collected for up to seven days,
and percutaneous absorption was determined from total urinary
excretion of radioabel, assuming that 22.5% of pyrethin and 51.3% of
piperonyl butoxide are excreted in the urine after parenteral
injection, as was shown in monkeys. Absorption of pyrethrin was 1.9 ±
1.2% and that of piperonyl butoxide 2.1 ± 0.6% of the administered
dose. No radiolabel was found in blood taken 1 h after application.
The extrapolated absorption from human scalp was 7.5 ± 4.7% of
pyrethin and 8.3 ± 2.4% of piperonyl butoxide (Wester et al., 1994).
Comments
Piperonyl butoxide is metabolized by oxidation of the methylene
group of the methylenedioxyphenyl moiety to yield carbon dioxide. The
remainder of the molecule undergoes further degradation and is
excreted mainly in the urine. As it is an alternative substrate (and
therefore a competitive inhibitor) for the microsomal cytochrome P450
system, piperonyl butoxide inhibits the metabolism of several drugs
and pesticides. The mechanism of action of this inhibition has been
elucidated in several studies.
In male rats given single oral doses of about 500 mg/kg bw of
[methylene-alpha 14C]-piperonyl butoxide, the label reached a peak
in blood after 3-12 h and dropped by about 50% within 24 h. The
highest levels of radiolabel were found in the gastrointestinal tract
and its, contents, suggesting that enterohepatic circulation occurs.
High levels of radioactivity were also found in the lung, liver,
kidney, fat, prostate, and seminal vesicles. The excretion pattern was
unchanged after 14 repeated doses of piperonyl butoxide.
Piperonyl butoxide has negligible acute toxicity, and it has been
classified by WHO as unlikely to present an acute hazard in normal
use.
Both short-term and long-term studies show that the target organ
of the toxicity of piperonyl butoxide is the liver. Male animals were
slightly more sensitive than females. In a number of short-term
studies in rodents, hepatic toxicity was characterized by liver
enlargement with associated hypertrophic hepatocytes, focal necrosis,
and, at times, alteration of some clinical chemical parameters. The
NOAEL for liver toxicity was about 100 mg/kg bw per day.
In rats exposed to piperonyl butoxide by inhalation at 0, 15, 74,
155, or 512 mg/m3 for 6 h per day on five days a week for 13 weeks,
effects were seen on the liver at the highest dose. Irritation of the
upper airways was seen at all doses, with squamous metaplasia of the
larynx.
In a one-year study in dogs fed diets containing 0, 100, 600, or
2000 ppm piperonyl butoxide, reduced body-weight gain, increased liver
weight with hypertrophic hepatocytes, and alteration of some clinical
chemical parameters were observed at 2000 ppm. The NOAEL in this study
was 600 ppm, equal to 16 mg/kg bw per day.
Several studies have been conducted of carcinogenicity in mice
and rats, some of which were considered to be inadequate. In a
112-week study, mice were given diets containing 5000 or 10 000 ppm
piperonyl butoxide during weeks 1-30 and 500 or 2000 ppm during weeks
31-112. No increase in tumour incidence was observed. Mice were fed
diets that gave a daily intake of 0, 30, 100, or 300 mg/kg bw for 78
weeks. Eosinophilic foci and adenomas with eosinophilic cells were
observed more frequently in the livers of males at the middle dose and
males and females at the high dose. The NOAEL in this study was
30 mg/kg bw per day on the basis of effects on the liver.
In a 12-month study of carcinogenicity in the liver, male mice
were fed diets containing 0, 6000, or 12 000 ppm piperonyl butoxide.
Body weights were reduced in a dose-related manner, and increased
mortality was observed in animals at the high dose. Hepatocellular
adenomas and carcinomas were observed in treated groups. Piperonyl
butoxide was carcinogenic at doses that were toxic to the liver and
caused general toxicity.
In a two-year study in rats at dietary concentrations adjusted to
achieve doses of 0, 30, 100, or 500 mg/kg bw per day, increased liver
weights, with corresponding hyperplasia and hypertrophy of
hepatocytes, morphological changes, and lesions in the endocrine and
hormone-sensitive organs, were observed at 100 and 500 mg/kg bw per
day. These effects were considered to be secondary to the ability of
piperonyl butoxide to induce hepatic cytochrome P450 enzymes.
Piperonyl butoxide was not found to be carcinogenic in this study.
After reconsideration of the data on testes in this study, previously
evaluated by the 1992 JMPR, and taking into account the results of
other long-term studies, the Meeting concluded that the NOAEL was
30 mg/kg bw per day on the basis of effects on the liver.
Two studies of carcinogenicity were conducted in rats fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide. No significantly
increased incidence of neoplasia was found in treated rats. Effects on
body weight and mortality were observed at the highest dose. In one
study, ileocaecal lesions were observed in both groups. Another study
of carcinogenicity was conducted in rats fed diets containing 0, 6000,
12 000, or 24 000 ppm. Increased mortality was observed in females at
the middle dose. Absolute liver weights were increased in females at
the low dose and in animals of each sex at the high dose. Nodular
lesions of the liver were observed in treated animals, and their
incidence and severity were related to the dose. Hepatocellular
adenomas and carcinomas were observed in animals at the middle and
high doses. Gastric and caecal haemorrhages, renal lesions, anaemia,
and platelet alteration were observed in treated animals. Piperonyl
butoxide was carcinogenic at doses that caused general toxicity.
In a 104-week study in rats, piperonyl butoxide was given at
2000 ppm in the diet in combination with pyrethrins at 400 ppm. A
slight reduction in the body weights of treated females was the only
adverse effect observed.
A two-generation study of reproductive toxicity, with one litter
per generation, was conducted in mice fed diets containing 0, 1000,
2000, 4000, or 8000 ppm piperonyl butoxide. There was no NOAEL because
of reduced pup weight at all doses. Pup viability was reduced at the
highest dose. In a two-litter, two-generation study of reproductive
toxicity in rats at dietary levels of 0, 300, 1000, or 5000 ppm, the
NOAEL for parental toxicity and pup development was 1000 ppm (equal to
68 mg/kg bw per day) on the basis of lowered body weight at 5000 ppm.
Embryo- and fetotoxicity were observed when mice were given single
doses of piperonyl butoxide at 0, 1070, 1390, or 1800 mg/kg bw by
gavage on day 9 of gestation.
Piperonyl butoxide was not embryotoxic or teratogenic in rats or
rabbits. Maternal toxicity was found in rats at doses > 500 mg/kg
bw per day. The NOAEL was 200 mg/kg bw per day in a study of
developmental toxicity in rabbits given 0, 50, 100, or 200 mg/kg bw
per day by gavage. The incidence of common developmental variations,
such as more full ribs and more than 27 presacral vertebrae, was
increased in all treated groups. Since a clear dose-effect
relationship was lacking, the association of this finding with
treatment was considered dubious. The NOAEL for maternal toxicity was
50 mg/kg bw per day.
Piperonyl butoxide was a mild dermal and ocular irritant but not
a dermal sensitizer in rabbits.
Piperonyl butoxide was adequately tested for genotoxicity in a
range of assays in vivo and in vitro. The Meeting concluded that
it is not genotoxic.
A single dose of 0.71 mg/kg bw piperonyl butoxide did not alter
antipyrine metabolism in humans. A study in which a formulation
containing 3% piperonyl butoxide was spread onto the ventral forearm
of adult male volunteers indicated that about 8% of the applied dose
would be absorbed through the human scalp. These data did not
contribute directly to the establishment of the ADI.
An ADI of 0-0.2 mg/kg bw was established on the basis of the
NOAEL of 600 ppm (equal to 16 mg/kg bw per day) in the one-year study
in dogs, with a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 mg/kg bw per day (78-week dietary study of toxicity and
carcinogenicity)
Rat: 30 mg/kg bw per day (two-year dietary study of
carcinogenicity)
200 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
500 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
1000 ppm, equal to 68 mg/kg bw per day (study of
reproductive toxicity)
Rabbit: 50 mg/kg bw per day (study of developmental toxicity)
200 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 600 ppm, equal to 16 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Further observations in humans
2. Short-term studies with mixtures of piperonyl butoxide and
other active ingredients, in ratios relevant to human
exposure
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to piperonyl butoxide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, irritation, rabbit Mildly irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Not sensitizing
Inhalation, lethality, rat Lacrimation, salivation, nasal
discharge, and laboured breathing at
5.9 mg/litre for 4 h. No mortality
Oral, lethality, mouse, rat, cat, dog LD50 = 4-14 g/kg bw
Dermal, lethality, rabbit LD50 > 2 g/kg bw
Medium-term (1-26 weeks) Repeated oral, toxicity, mice, rats, NOAEL = 100 mg/kg bw per day; effects on
3-13 weeks liver
Repeated inhalation, toxicity, rats, 13 weeks Irritating to upper airways at 15 mg/m3 for
6 h per day, 5 days per week, with squamous
metaplasia of the larynx; effects on the liver at
512 mg/m3
Oral, developmental toxicity, rabbit NOAEL = 50 mg/kg bw per day for maternal
toxicity; no fetotoxicity or teratogenidty
Oral, reproductive toxicity, rat NOAEL = 68 mg/kg bw per day; maternal
and pup toxicity
Long-term (> one year) Repeated oral, toxicity, dog, one year NOAEL = 16 mg/kg bw per day
References
Ashwood-Smith, M.J., Trevino, J. & Ring, R. (1972) Mutagenicity of
dichlorvos. Nature, 240, 418-420. Brady, J.T., Montelius, D.A.,
Beierschmitt, W.P., Wyand, D.S., Khairalla, E.A. & Cohen, S.D.
(1988) Effect of piperonyl butoxide post-treatment on
acetaminophen hepatotoxicity. Biochem. Pharmacol.,
37, 2097-2099.
Cardy, R.H., Renne, R.A., Warner, J.W. & Cypher, R.L. (1979)
Carcinogenesis bioassay of technical-grade piperonyl butoxide in
F344 rats. J. Natl Cancer Inst., 62, 569-578.
Chun, J.S. & Neeper-Bradley, T.L. (1991) Developmental toxicity
evaluation of piperonyl butoxide administered by gavage to CD
(Sprague-Dawley) rats. Unpublished report No. 54-586 from Bushy
Run Research Center, Export, Pennsylvania, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Conney, A.H., Chang, R., Levin, W.M., Garbut, A., Munro-Faure, A.D.,
Peck, A.W. & Bye, A. (1972) Effects of piperonyl butoxide on drug
metabolism in rodents and man. Arch. Environ. Health,
24, 97-106.
Dalvi, R.R. & Dalvi P.S. (1991) Differences in the effects of piperine
and piperonyl butoxide on hepatic drug-metabolizing enzyme system
in rats. Drug Chem. Toxicol., 14, 219-229.
Epstein, S.S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel, N.
(1967) Synergistic toxicity and carcinogenicity of 'Freons' and
piperonyl butoxide. Nature, 214, 526-528.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 233, 288-325.
Fishbein, L., Falk, H.L., Fawker, J., Jordan, S. & Corbett, B. (1969)
The metabolism of piperonyl butoxide in the rat with 14C in the
methylenedioxy or alpha-methylene group. J. Chromatogr.,
41, 61-79.
Franklin, M.R. (1972) Inhibition of hepatic oxidative xenobiotic
metabolism by piperonyl butoxide. Biochem. Pharmacol.,
21, 3287-3299.
Fujitani, T., Ando, H., Fujitani, K., Ikeda, T., Kojima, A., Kubo, Y.,
Ogato, A., Oishi, S., Takahashi, O. & Yoneyama, M. (1992)
Sub-acute toxicity of piperonyl butoxide in F344 rats.
Toxicology, 72, 291-298.
Fujitani, T., Tanaka, T., Hashimoto, Y. & Yoneyama, M. (1993a)
Subacute toxicity of piperonyl butoxide in ICR mice.
Toxicology, 83, 93-100.
Fujitani, T., Tada, Y. & Yoneyama, M. (1993b) Hepatotoxicity of
piperonyl butoxide in male F344 rats. Toxicology, 84, 171-183.
Gabriel, D. (1991a) Acute oral toxicity, LD50-rats. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Gabriel, D. (1991b) Acute dermal toxicity, single level-rabbits.
Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldenthal, E.I. (1993a) Evaluation of piperonyl butoxide in an eight
week toxicity study in dogs. Unpublished report No. 542-004 from
International Research and Development Corp., Mattawan, Michigan,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Goldenthal, E.I. (1993b) Evaluation of piperonyl butoxide in a one
year chronic dietary toxicity study in dogs. Unpublished report
No. 542-005 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldstein, J.A., Hickman, P. & Kimbrough R.D. (1973) Effects of
purified and technical piperonyl butoxide on drug metabolizing
enzymes and ultrastructure of rat liver. Toxicol. Appl.
Pharmacol., 26, 444-458.
Graham, C (1987) 24-Month dietary toxicity and carcinogenicity study
of piperonyl butoxide in the albino rat. Unpublished report
No. 81690 from Bio-Research Ltd Laboratory, Seneville, Quebec,
Canada. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hall J.G., McLaughlin, J.F. & Stamm, S. (1975) Coarctation of the
aorta in male cousins with similar maternal environmental
exposure to insect repellent and insecticides. Pediatrics,
55, 425-427.
Hermanski, S.J. & Wagner, C.L. (1993) Chronic dietary oncogenicity
study with piperonyl butoxide in CD-1 mice. Unpublished report
No. 91N0134 from Bushy Run Research Center, Export, Pennsylvania,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hoffman, G.M. (1991) An acute inhalation toxicity study of piperonyl
butoxide in the rat. Unpublished report No. 91-8330 from
Bio/dynamics, East Millstone, New Jersey, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Hunter, B., Bridges, J.L. & Prentice, D.E. (1977) Long term feeding of
pyrethrins and piperonyl butoxide to rats. Unpublished report
from Huntington Research Centre, Huntington, Cambs, United
Kingdom. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Bates, R.R., Falk, H.L., Gart, J.J., Klein, M.,
Mitchell, I. & Peters, J. (1969) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Natl Cancer Inst., 42, 1101-1114.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1968)
In vivo inhibition of mouse liver microsomal hydroxylating
systems by methylenedioxyphenyl insecticidal synergists and
related compounds. Life Sci., 7, 1051-1062.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1969)
Bi-modal effect of piperonyl butoxide on the o- and
p-hydroxylations of biphenyl by mouse liver microsomes.
Biochem. Pharmacol., 18, 1045-1051.
Kamienski, F.X. & Casida, J.E. (1970) Importance of demethylenation in
the metabolism in vivo and in vitro of methylenedioxyphenyl
synergists and related compounds in mammals. Biochem.
Pharmacol., 19, 91-112.
Kennedy, G.L., Smith, S.H., Kinoshita, F.K., Keplinger, M.L. &
Calandra, J.C. (1977) Teratogenic evaluation of piperonyl
butoxide in the rat. Food Cosmet. Toxicol., 15, 337-339.
Khera, K.S., Whalen, C., Angers, G. & Trivett, G. (1979) Assessment of
the teratogenic potential of piperonyl butoxide, biphenyl, and
phosalone in the rat. Toxicol. Appl. Pharmacol., 47, 353-358.
Lake, B.G. (1995) An investigation of the effect of piperonyl butoxide
on unscheduled DNA synthesis in cultured human liver slices.
Unpublished report No. 1501/1 from BIBRA International,
Carshalton, Surrey, United Kingdom. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lawlor, T.E. (1991) Piperonyl butoxide in the Salmonella/mammalian-
microsome reverse mutation assay (Ames test) with a confirmatory
assay. Unpublished report No. 14413-0-401R from Hazleton
Washington, Kensington, Maryland, USA. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lehman, A.J. (1948) Q. Bull. Assoc. Food Drug Off., 12, 82.
Lehman, A.J. (1951) Q. Bull. Assoc. Food Drug Off., 15, 122.
Lehman, A.J. (1952a) Q. Bull. Assoc. Food Drug Off., 16, 43.
Lehman, A.J. (1952b) Q. Bull. Assoc. Food Drug Off., 16, 47.
Leng, J.M., Schwartz, C.A. & Schardein, J.L. (1986) Teratology study
in rabbits. Unpublished report No. 542-002 from International
Research and Development Corp., Mattawan, Michigan, USA.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Levine, B.S. & Murphy, S.D. (1977a) Esterase inhibition and
reactivation in relation to piperonyl butoxide-phosphorothionate
interactions. Toxicol. Appl. Pharmacol., 40, 379-391.
Levine, B.S. & Murphy, S.D. (1977b) Effect of piperonyl butoxide on
the metabolism of dimethyl and diethyl phosphorothionate
insecticides. Toxicol. Appl. Pharmacol., 40, 393-406.
Maekawa, A., Onodera, H., Furuta, K., Tanigawa, H., Ogiu, T. &
Hayashi, Y. (1985) Lack of evidence of carcinogenicity of
technical-grade piperonyl butoxide in F344 rats: Selective
induction of ileocaecal ulcers. Food Chem. Toxicol.,
7, 675-682.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded
chemicals. Environ. Mol. Mutag., 12, 85-154.
McKeon, M.E. & Phil, M. (1991) Piperonyl butoxide in the assay for
unscheduled DNA synthesis in rat liver primary cell cultures with
a confirmatory assay. Unpublished report No. 14413-O-447R from
Hazleton Washington, Kensington, Maryland, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Mirer, F.E., Levine, B.S. & Murphy, S.D. (1977) Parathion and methyl
parathion toxicity and metabolism in piperonyl butoxide and
diethyl maleate pretreated mice. Chem.-Biol. Interactions,
17, 99-112.
Modeweg-Hansen, L., Lalande, M., Bier, C., Losos, G. & Osborne, B.E.
(1984) A dietary dose range-finding study of piperonyl butoxide
in the albino rat. Unpublished report No. 81802B from
Bio-Research Laboratories, Edgewater, Maryland, USA. Submitted to
WHO by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Murli, H. (1991) Piperonyl butoxide: Measuring chromosomal aberrations
in Chinese hamster ovary (CHO) cells with multiple harvests under
conditions of metabolic activation. Unpublished report
No. 14413-0-437C from Hazleton Washington, Kensington, Maryland,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Negherbon, W.O. (1959) Handbook of Toxicology, Vol. 3, Philadelphia,
Saunders.
Newton, P.E. (1992) A subchronic (3-month) inhalation toxicity study
of piperonyl butoxide in the rat via whole-body exposures.
Unpublished report No. 91-8333 from Bio/dynamics, East Millstone,
New Jersey, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Phillips, J.C., Cunninghame, M.E., Price, R.J., Osimitz, T., Ganriel,
K.L., Preiss, F.J., Butler, W.H. & Lake, B.G. (1995) Effect of
piperonyl butoxide (PBO) on cell replication and xenobiotic
metabolism in mouse liver (Abstract). Toxicologist, 15, 230.
Robinson, K., Pinsonneault, L. & Procter, B.G. (1986) A two-generation
(two-litter) reproduction study of piperonyl butoxide
administered in the diet to the rat. Unpublished report No. 81689
from Bio-Research Laboratories Ltd, Montreal, Quebec, Canada.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991a) Primary skin irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991b) Primary eye irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991c) Guinea pig dermal sensitization-modified Buehler
method. Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Sarles, M.P. & Vandegrift, W.B. (1952) Chronic oral toxicity and
related studies on animals with the insecticide and pyrethrum
synergist, piperonyl butoxide. Am. J. Trop. Med. Hyg.,
1, 862-883.
Sarles, M.P., Dove, W.E. & Moore, D.H. (1949) Acute toxicity and
irritation tests on animals with the new insecticide, piperonyl
butoxide. Am J. Trop. Med., 29, 151-166.
Schuller, H.M. & McMahon, J.B. (1985) Inhibition of N-nitroso-
diethylamine-induced respiratory tract carcinogenesis by
piperonylbutoxide in hamsters. Cancer Res., 45, 2807-2812.
Selim, S. (1985) Single and repeated oral dose pharmacokinetic and
distribution studies of piperonyl butoxide. Unpublished report
No. WP509070/1 from Biological Test Center, Irvine, California,
USA. Submitted to WHO by Endura SA. Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Skrinijaric-Spoljar, M., Matthews, H.B., Engel, J.L. & Casida, J.E.
(1971) Response of hepatic microsomal mixed-function oxidases to
various types of insecticide chemical synergists administered to
mice. Biochem. Pharmacol., 20, 1607-1618.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994a) Piperonyl butoxide induces hepatocellular carcinoma in
male CD-1 mice. Arch. Toxicol., 68, 467-469.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994b) Chronic toxicity studies of piperonyl butoxide in F344
rats: Induction of hepatocellular carcinoma. Fundam. Appl.
Toxicol., 22, 293-303.
Tanaka, T. (1992) Effects of piperonyl butoxide on F1 generation mice.
Toxicol. Lett., 60, 83-90.
Tanaka, T. (1993) Behavioural effects of piperonyl butoxide in male
mice. Toxicol. Lett., 69, 155-161.
Tanaka, T., Takahashi, O. & Oishi, S. (1992) Reproductive and
neurobehavioural effects in three-generation toxicity study of
piperonyl butoxide administered to mice. Food Chem. Toxicol.,
30, 1015-1019.
Tanaka, T., Fujitani, T., Takahashi, O. & Oishi, S. (1994)
Developmental toxicity evaluation of piperonyl butoxide in CD-1
mice. Toxicol. Lett., 71, 123-129.
Tu, A.S., Coombes-Hallowell, W.E., Pallotta, S.L. & Catanzano, A.
(1985) Evaluation of piperonyl butoxide in the CHO/HGPRT mutation
assay with and without metabolic activation. Unpublished report
No. 53906 from Arthur D. Little, Inc., Cambridge, Massachusetts,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
US National Cancer Institute (1979) Bioassay of piperonyl butoxide for
possible carcinogenicity (DHEW Publ. No. 79-1375), Bethesda,
Maryland, USA, US Department of Health, Education, and Welfare.
Wester, R.C., Bucks, D.A.W. & Maibach, H.I. (1994) Human in vivo
percutaneous absorption of pyrethrin and piperonyl butoxide.
Food Chem. Toxicol., 32, 51-53.
White, T.J., Goodman, D., Shulgin, A.T., Castagnoli, N., Lee, R. &
Petrakis, N.L. (1977) Mutagenic activity of some centrally active
aromatic amines in Salmonella typhimurium. Mutat. Res.,
56, 199-202.
A dose-dependent, biphasic effect was observed on the ortho and
para hydroxylations of biphenyls by liver microsomes of mice treated
with 10-640 mg/kg bw of piperonyl butoxide intraperitoneally,
para-Hydroxylation was inhibited by 80% at the highest dose 30 min
after treatment but had recovered within 24 h; ortho-hydroxylation
was induced by up to 150% 1 h after treatment, and activity was still
elevated after 96 h in the groups at the highest dose (Jaffe et al.,
1969).
Piperonyl butoxide inhibits ethylmorphine N-demethylation by
liver microsomes in vitro. The inhibition is proportional to the
amount of cytochrome P450-piperonyl butoxide metabolite (unknown)
complex formed. Differences between liver microsomes from rats and
mice may account for the greater sensitivity of mice to inhibition of
drug metabolism by piperonyl butoxide: the Ki for inhibition of
ethylmorphine N-demethylase was found to be 19 µmol/litre in rats and
6 µmol/litre in mice (Franklin, 1972).
Mouse liver homogenates (20% w/v in 50 mmol/litre phosphate
buffer, pH 7.4) were fractioned into nuclear, mitochondrial,
microsomal, and soluble fractions, which were incubated with labelled
piperonyl butoxide. NAD, NADP, NADH, or NADPH was sometimes added as a
cofactor. Only the microsomal fraction had significant metabolic
activity in the presence of NADPH and, to a lesser extent, with NADH
(Kamienski & Casida, 1970).
Male CF-1 mice were given a single intraperitoneal dose of
piperonyl butoxide (purity, 87-89%) at 0.5-25 mg/kg bw 1 h before
intraperitoneal administration of 40 mg/kg bw pentobarbital or
100 mg/kg bw zoxazolamine. Pentobarbital-induced sleeping time was
significantly increased by 10 or 25 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 5,
10, or 25 mg/kg bw (Conney et al., 1972). Skinjaric-Spoljar et al.
(1971) found that a single intraperitoneal dose of 22 mg/kg bw
piperonyl butoxide to mice increased the hexobarbital (62.5 mg/kg bw
intraperitoneally) sleeping time from 28 to 100 min.
Male Sprague-Dawley rats were given single intraperitoneal doses
of piperonyl butoxide (purity, 87-89%) at 67-1000 mg/kg bw 1 h before
intraperitoneal administration of pentobarbital (25 mg/kg bw) or
zoxazolamine (70 mg/kg bw). Pentobarbital-induced sleeping time was
significantly increased by 1000 mg/kg bw piperonyl butoxide, and
zoxazolamine-induced paralysis time was significantly increased by 333
or 1000 mg/kg bw (Conney et al., 1972).
Male mice and rats were given single intraperitoneal doses of
piperonyl butoxide 1 h before injection of 200 mg/kg bw antipyrine.
The NOAELs for effects on antipyrine metabolism were 100 mg/kg bw in
rats and 0.5 mg/kg bw in mice (Conney et al., 1972).
Groups of six male weanling Sherman rats were fed technical-grade
piperonylbutoxide (purity, 80%) at dietary concentrations of 0, 1000,
5000, or 10 000 ppm for one, four, or eight weeks. The activities of
hexobarbital oxidase, aniline hydroxylase, para-nitroanisole
demethylase, nitroreductase, and glucuronyltransferase and the P450
content were increased two- to fourfold by 5000 or 10 000 ppm
piperonyl butoxide. Liver weights and microsomal protein content were
increased to a maximum of 50-70%. Electron microscopy showed
enlargement and extensive proliferation of the smooth endoplasmic
reticulum in liver parenchymal cells. The dose of 1000 ppm had minimal
effects on liver weight and on P450 and glucuronyl transferase
activity and no effect on proliferation of the smooth endoplasmic
reticulum. Maximal effects on P450 content and on the activity of
P450-related enzymes were observed after one week of treatment. The
maximal effect on glucuronyltransferase activity was observed between
four and eight weeks of treatment (Goldstein et al., 1973).
Piperonyl butoxide (purity unsepcified) dissolved in corn oil was
administered to 10 male Sprague-Dawley rats at 400 mg/kg bw
intraperitoneally; another group of animals received 100 mg/kg bw of
piperine intraperitoneally. Control animals received 2 ml/kg bw corn
oil. Groups of five animals were sacrificed 1 and 24 h after
treatment, and hepatic levels of cytochromes P450 and b5, proteins,
and the activities of NADPH cytochrome c reductase, benzphetamine
N-demethylase, aminopyrine N-demethylase, and aniline hydroxylase
were determined. The microsomal protein level was not affected by
treatment. Piperonyl butoxide decreased the P450 but not the b5 level
by about 30% after 1 h; however, after 24 h the contents were
increased by about 100 and 30%, respectively. NADPH cytochrome c
reductase activity was increased by 50% 24 h after treatment. The
activities of N-demethylases and aniline hydroxylase were decreased
by 20 and 50%, respectively, 1 h after administration of either
piperine or piperonyl butoxide. By 24 h, the activities were still low
in piperine-treated animals but had increased by 30-80% in those given
piperonyl butoxide (Dalvi & Dalvi, 1991).
Single intraperitoneal doses of 400 mg/kg bw piperonyl butoxide
were given to male CD-1 mice 1 h before a single intraperitoneal dose
of parathion-methyl, azinphos-methyl, parathion, or azinphos-ethyl, or
their oxygen analogues. The LD50 of the oxygen analogues was not
altered whereas that of the methyl homologues was reduced, with a
40-fold increase in the LD50 for parathion-methyl and a threefold
increase in that for azinphos-methyl; that of the ethyl homologues was
increased, with a 50% decrease in the LD50 for parathion and an 85%
decrease for azinphos ethyl. The plasma levels of all pesticides were
increased by three- to sevenfold in piperonyl butoxide-pretreated
animals 30 min after treatment (Levine & Murphy, 1977a). It has been
suggested that detoxification of parathion-methyl, but not that of
parathion- or azinphos-ethyl, continues through uninhibited
glutathione-dependent pathways (Mirer et al., 1977; Levine & Murphy,
1977b).
A single intraperitoneal dose of 600 mg/kg bw piperonyl butoxide
to three-month-old CD-1 mice reduced the hepatotoxicity (as assessed
by reduced glutathione content, plasma sorbitol dehydrogenase, and
histopathological liver necrosis) caused by an oral dose of 600 mg/kg
bw acetominophen given 2 h earlier or 1 h later. Since the hepatic
mixed-function oxidase system generates a toxic, electrophilic
metabolite of acetominophen, piperonyl butoxide probably acts by
inhibiting: that enzymatic system (Brady et al., 1988).
Pretreatment of groups of 10 male Syrian golden hamsters with
400 mg/kg bw piperonyl butoxide given subcutaneously 2 h before
subcutaneous injection of 17.8 mg/kg bw N-nitrosodiethylamine twice
weekly for 20 weeks inhibited the development of pulmonary
carcinogenesis by the nitrosamine. The numbers of animals with lung
tumors were 0 controls, 6 treated only with N-nitrosodiethylamine, 0
treated with piperonyl butoxide and nitrosamine, and 0 given only
piperonyl butoxide. Tracheal tumors were found in 0, 10, 5, and 0
animals in the four groups, respectively. The protective effect was
associated with reduced (by 50% or more) covalent binding of
N-[ethyl-1-14C]-nitroso-diethylamine to macromolecules in trachea,
lung, and liver (Schuller & McMahon, 1985).
Eight-week-old male CD-1 mice were fed diets containing piperonyl
butoxide at concentrations adjusted to provide doses of 0, 10, 30,
100, or 300 mg/kg bw per day or sodium phenobarbital at 0.05% (w/w)
for 42 days. Replicative DNA synthesis was studied by implanting
seven-day osmotic pumps to administer 5-bromo-2'-deoxyuridine (BrDU)
during days 0-7 and 35-42 of treatment; liver sections were
immunostained with an antibody to BrDU, and the percentage of
hepatocyte nuclei undergoing replicative DNA synthesis was calculated
by microscopic examination of at least 1000 nuclei. Xenobiotic
metabolism was assessed by measuring the liver P450 content and the
activities of 7-ethoxyresorufin O-deethylase, 7-pentoxyresorufin
O-depentylase, and ethylmorphine N-demethylase in liver
microsomes. Sodium phenobarbital was used as a positive control. The
relative liver weight was increased by about 15% after seven days of
treatment with 300 mg/kg bw per day of piperonyl butoxide and by about
20% with sodium phenobarbital; after 42 days, it was increased by
about 10% after treatment with 100 and by about 20% after treatment
with 300 mg/kg bw piperonyl butoxide. Midzonal liver-cell hypertrophy
was observed in mice given 300 mg/kg bw per day for seven or 42 days
or 100 mg/kg bw per day for 42 days. Sodium phenobarbital induced
centrilobular hypertrophy after either seven or 42 days of treatment.
Replicative DNA synthesis was induced to 350% by the high dose of
piperonyl butoxide and to 825% by sodium phenobarbital for seven days;
no significant increase was observed after 42 days at any dose. A
dose-related increase in microsomal protein and P450 content was
observed on day 42, and the increases were statistically significant
at doses > 100 mg/kg bw. Inconsistent increases were observed in
the activities of 7-ethoxyresorufin O-deethylase and 7-pento-
xyresorufin O-depentylase in piperonyl butoxide-treated mice, but a
dose-related increase from 20 to 50% in the activity of ethylmorphine
N-demethylase was seen on day 42. Treatment with sodium pheno-
barbital for 42 days resulted in increases in all of the measured
parameters. Piperonyl butoxide (and sodium phenobarbital) thus caused
a transient stimulation of liver-cell replication and a permanent
effect on liver weight, morphology, and enzyme induction at doses that
induced liver nodules in long-term studies (Phillips el al., 1995).
2. Toxicological studies
(a) Acute toxicity
Groups of five Sprague-Dawley CD rats of each sex were exposed by
inhalation for 4 h to a mean analytical concentration of 5.9 mg/litre
piperonyl butoxide (purity, 90.78%). The particle median aerodynamic
diameter was 2.6 µm; about 15% of the aerosol was < 1 µm in diameter
and about 95% < 10 µm. All animals survived the exposure and the
15-day observation period after treatment. Exposure caused excessive
lacrimation and salivation, nasal discharge and laboured breathing.
All animals recovered. No remarkable signs were seen post mortem
(Hoffman, 1991). Other data are reported in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were fed
diets containing 0, 1000, 3000, or 9000 ppm piperonyl butoxide (purity
unspecified) for 20 days. Animals were observed daily for mortality;
body weight was measured and clinical observations were made on days
1, 2, 3, 7, 14, and 20; the food consumption of five animals per cage
was measured on days 0-3, 3-7, 7-14, and 14-20. At the end of
treatment, blood clinical chemistry was assessed, liver, kidneys, and
spleen were weighed, and livers and kidneys were examined
histologically. No deaths occurred. The body weights of animals at the
high dose were about 15% lower than those of controls at the end of
the study, and those of females at the middle dose were about 10%
lower on day 2 and about 8% lower at termination (not statistically
significant). The lowered body weights were apparently associated with
decreased food intake. The weights of livers showed a dose-related
increase in both males (by 22% at the low dose, 63% at the middle
dose, and 79% at the high dose) and females (62% at the middle dose
and 78% at the high dose); at the high dose, the weights of the
kidneys were reduced by 29% in males and 18% in females, and the
weights of the spleen by 25% in males and 33%, in females. In animals
at the high dose, a 31% increase in serum cholesterol was seen in
males and a 67% increase in females, a 28% increase in serum
phospholipids was seen in males and a 38% increase in females, a 19%
Table 1. Acute toxicity of piperonyl butoxide in animals
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse NR Oral 4.0 NR Negherbon (1959)
Rat Male Oral 4.57 90.78 Gabriel (1991a)
Female 7.22
NR Oral 8.0-10.6 NR Sarles et al. (1949)
NR Oral 13.5 NR Lehman (1948)
NR Oral 11.5 NR Lehman (1951)
Male, female Inhalation (4 h) > 5900 90.78 Hoffman (1991)
NR Subcutaneous > 15.9 NR Sarles et al. (1949)
Rabbit Male, female Dermal > 2 90.78 Gabriel (1991b)
NR Oral 2.7-5.3 NR Sarles et al. (1949)
Cat NR Oral > 10.6 NR Sarles et al. (1949)
Dog NR Oral > 8.0 NR Sarles et al. (1949)
NR, Not reported
increase in total serum proteins occurred in males and an 18% increase
in females, and a 235% increase in gamma-glutamyl transpeptidase was
seen in females. Some of these levels were also increased in the
animals at the middle dose. Microscopic examination of the liver
showed hypertrophic hepatocytes, single-cell necrosis, and cell
infiltration in the centrilobular area in all mice at the high dose.
The kidneys were unremarkable. The NOAEL for effects on the liver was
1000 ppm, equivalent to 150 mg/kg bw per day (Fujitani et al.,
1993a).
Groups of 20 Crj:CD-1 male mice were fed diets containing 0,
1500, 3000, or 6000 ppm piperonyl butoxide (purity unspecified) for
seven weeks. Food intake was measured every week and behavioural tests
were performed at weeks 4 (exploratory behaviour), 6 (multiple water
T-maze), and 7 (exploratory behaviour) of treatment. A treatment-
related reduction in food intake was observed in animals at the middle
and high doses during the first week of treatment. No consistent,
significant effect was observed in the T-maze test or in the
exploratory behaviour test at week 4. At week 7, some parameters in
the exploratory behaviour test were altered (Tanaka, 1993).
Rats
Hypertrophy of periportal cells with slight fatty changes were
observed in rats fed a diet containing 5000 ppm piperonyl butoxide for
17 weeks (Lehman, 1952a,b).
Feeding of six weekly doses of up to 224 mg/kg bw of active
ingredient to rats caused no observable effects at autopsy three weeks
after the final dose, but few details were given (Sarles et al.,
1949).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were fed diets containing concentrations of
piperonyl butoxide adjusted to obtain doses of 0, 62.5, 125, 250, 500,
1000, or 2000 mg/kg bw per day for four weeks. All animals were
observed for mortality and morbidity twice daily, and body weight and
food consumption were measured weekly. Haematological and biochemical
tests were performed on day 24 or 25 of treatment. At termination,
survivors were necropsied, certain organs were weighed and microscopic
examinations were performed on several organs. Six animals died before
termination, but the deaths were considered to be due to anaesthesia
for bleeding. General signs of toxicity, such as prominent backbone,
thinness, poor fur condition, brown staining and piloerection, were
observed in animals at the highest dose. Body-weight gain was reduced
by about 20% in females at 500 mg/kg bw per day, by 27% in males and
37% in females at 1000 mg/kg bw per day, and by 66% in males and 92%
in females at 2000 mg/kg bw per day. These reductions were only
partially associated with reduced food intake. Haematology and
clinical biochemistry showed no significant alterations. Absolute and
relative liver weights were increased in animals given doses >
500 mg/kg bw per day; the relative liver weights were also increased
in males at 250 mg/kg bw per day. The relative weights of the
adrenals, kidneys, and brain were slightly increased in the group at
the highest dose and in some animals at 1000 mg/kg bw per day.
Eosinophilia and loss of vacuolation in hepatocytes were noted in all
treated animals, and the severity of these lesions was dose-related.
These may be adaptive changes. Necrosis of hepatocytes and cytoplasmic
inclusions were observed in animals at 1000 and 2000 mg/kg bw per day.
The NOAEL was 125 mg/kg bw per day on the basis of effects on the
liver (Modeweg-Hansen et al., 1984).
In a study preliminary to the study of carcinogenicity by the
same authors summarized below, groups of 10 Fischer 344/DuCrj rats of
each sex were fed diets containing 0, 2500, 5000, 10 000, 20 000, or
30 000 ppm piperonyl butoxide (purity, 89%) for 13 weeks. At
termination, survivors were necropsied, and limited microscopic
examination was performed. One male at the high dose died before
termination. The final body weights and body-weight gains were reduced
in a dose-related manner in all treated males; in females, a
significant effect was seen only at the two highest doses. Liver
weights were increased in all treated animals, by up to 50% in males
and 108% in females. Absolute kidney weights were increased in animals
at the high dose, and the relative weight was also increased in groups
at lower doses. Hepatocyte hypertrophy and focal necrosis were seen in
animals at higher doses (data not shown). No relevant macro- or
microscopic lesions were observed in the digestive tract, but the
caecum was not examined histologically. There was no NOAEL because of
effects on the liver at all doses (Maekawa et al., 1985).
Groups of 15 Charles River CD rats of each sex were exposed by
inhalation for 6 h per day, five days per week, for 13 weeks to
piperonyl butoxide (purity, 90.78%) at mean analytical concentrations
of 15, 74, 155, or 512 mg/m3. The highest concentration was the
maximum that could be generated at the appropriate particle size.
Fifteen controls were exposed to conditioned room air only. The
concentrations in chamber air were monitored gravimetrically four
times per day and by gas chromatography once a day. Particle size
distribution was measured once during each exposure. The test aerosol
had a mass median aerodynamic diameter of 1.7 µm with a geometric
standard deviation of 2.6. On average, 37% of the particles were <
10 µm in diameter and 29% < 1 µm. Abnormal signs were looked for once
during each exposure, and detailed physical examinations were
conducted on all animals once before treatment and weekly during
treatment. Ophthalmoscopic examinations were performed on all animals
before treatment and on the day before sacrifice. Body weight was
recorded before exposure, immediately before exposure on test day 1,
weekly thereafter, and just before sacrifice. At termination,
haematological and blood chemical parameters were evaluated in all
animals, selected organs were weighed, complete gross post-mortem
examinations were conducted, and selected tissues from all animals
were examined microscopically.
Body-weight gain and food consumption were not affected by
exposure, and all animals survived to the end of treatment.
Ophthalmoscopic examinations showed no indication of exposure-related
ocular effects. There was a dose-related increase in nasal discharge,
dried material on the facial area and extremities, and anogenital
staining in animals at 155 and 512 mg/m3. The serum levels of
aspartate and alanine aminotransferases and of glucose were slightly
decreased (10-28%), while blood urea nitrogen, total protein, and
albumin levels were slightly increased (4-10%) in animals of each sex
at the high dose. Not all of these differences were statistically
significant, and a dose-response relationship was not seen
consistently; however, a similar trend was seen in animals of each
sex. Statistically significant increases in the absolute and relative
weights of the livers and kidneys were seen in animals at the high
dose; the relative kidney weights were increased by 10-12%, and the
absolute and relative liver weights were both increased by 20-29%.
Relative liver weights were also significantly elevated (8-9%) in
males and females at 155 mg/m3. Vesiculation and vacuolation of the
hepatocellular cytoplasm was seen in almost all treated animals but
tended to be more pronounced in those at the highest level. No clear
dose-related increase in either incidence or severity was observed.
Squamous or squamoid metaplasia of the pseudostratified columnar
epithelium of the larynx was seen in several exposed animals and one
control female, the severity being greater in animals at the high
dose. Similar metaplastic changes were seen in the columnar epithelium
lining the ventral diverticulum in several animals at 512 mg/m3 and
in one female at 15 mg/m3. Hyperplasia and hyperkeratosis of the
squamous epithelium normally found in the larynx were seen in a small
number of animals at 512 mg/m3. Laryngeal mucosal inflammation was
seen in all treated animals and was slightly more severe in animals at
the highest dose. All of the changes were considered to be localized
responses indicative of irritation rather then systemic toxicity.
Other findings in tissues and organs examined macroscopically and
microscopically post mortem were unremarkable. No systemic toxicity
was seen at 155 mg/m3; effects on the liver and kidney were seen at
512 mg/m3 (Newton, 1992).
Groups of 10 male and 10 female Fischer 344/DuCrj rats were fed
diets containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide
(technical grade of unspecified purity) for 13 weeks. Animals were
observed daily for mortality; body weight and clinical signs were
monitored daily for the first five days and then twice weekly. Food
and water consumption was measured on days 4, 11, 18, and 39 for about
six rats in each group. At the end of treatment, haematological and
blood chemical parameters were determined, animals were necropsied,
and selected organs were weighed; the liver and kidneys were examined
histologically. There were no deaths. Clinical signs consisted of nose
bleeds from about day 2 to day 20 and dose-dependent abdominal
distension. In animals at the high dose, food consumption was reduced
by about 46% and water consumption by 28% on day 4 but not at other
times. The body weights of animals at the high dose were significantly
reduced, by 36% in males and 24% in females. Blood haemoglobin levels
were decreased in a dose-dependent manner, but the decrease was
statistically significant only in animals at the high dose (by 10-11%)
and females at the middle dose (by 7%). A reduced mean corpuscular
haemoglobin content was also observed in females at the high dose.
Serum albumin was increased by 16-23%, cholesterol by 88-93%,
gamma-glutamyl transpetidase activity to five to six times the control
values in males at the high dose, and urea nitrogen by 24% in males at
that dose. Serum protein was increased in all treated females and
serum phospholipid in females at the high dose; decreases were seen in
serum levels of triglyceride in all males and bilirubin and glucose in
males at the high dose. Absolute and relative liver weights were
increased in a dose-related manner, but were significantly increased
only in males at the middle dose (by 47%) and males and females at the
high dose (by 57-94%). Relative kidney weights were increased in a
dose-related manner to up to 1.42% of control values. Hypertrophic
hepatocytes containing a basophilic, fine granular substance were seen
in animals at the high dose. In the periportal area, marked
vacuolation of hepatocytes was observed, with occasional coagulative
necrosis and oval-cell proliferation. In male rats, atrophy of the
epithelium of the proximal convoluted tubules was observed in the
renal cortex. There was no NOAEL because of effects on the liver and
kidney (Fujitani et al., 1992).
In a subsequent study performed under the same experimental
conditions, haematological, clinical chemical, and morphological
observations after 1, 2, 4, or 12 weeks of treatment showed that all
of the alterations occurred in a time-dependent manner, and that some
of them (increased liver and kidney weights, morphological alterations
in the liver) were already present after one week (Fujitani et al.,
1993b).
Rabbits
Three weekly doses of up to 108 mg/kg bw active ingredient of a
5% emulsion of piperonyl butoxide showed no effects at autopsy three
weeks after the final dose, but few details were given (Sarles
et al., 1949).
Dogs
Groups of one to three dogs of each sex were given piperonyl
butoxide at 0, 3, 32, 106, or 320 mg/kg per day orally in capsules on
six days per week for one year. All dogs at the two highest doses lost
weight, but large variations in body-weight gain and the small number
of animals prevented meaningful comparisons between the controls and
animals at the lower doses. A dose-related increase in relative liver
weight (no statistical analysis reported) was observed, which was
associated with unspecified histopathological changes. Increased
kidney and adrenal weights were observed at 100 and 320 mg/kg bw per
day (Sarles & Vandergrift, 1952).
Groups of two beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for eight weeks at concentrations
corresponding to 500, 1000, 2000, or 3000 ppm active ingredient. The
diets were prepared weekly; the actual concentrations and the
homogeneity of the mixture were measured at three intervals and found
to be satisfactory. Animals were observed at least twice daily for
mortality and signs of toxicity; detailed observations were recorded
at least once weekly, and body weight and food consumption were
recorded weekly. Physical examinations and haematological and
biochemical tests were conducted on all animals before exposure and at
termination. All major tissues and organs were examined
microscopically post mortem. Selected organs were weighed. All
animals survived to study termination. Reduced body-weight gains were
seen in males and females receiving 1000 ppm (14 and 9% of the initial
body weight, respectively), 2000 ppm (6 and 7%), and 3000 ppm (7 and
4%) ppm in comparison with the control group (17% and 14% increases).
Decreased food consumption (by 19 and 23%) was observed animals at
3000 ppm. There were no treatment-related changes in haematological
parameters. Males and females at 2000 and 3000 ppm had alkaline
phosphatase activities that were about 1.5 times the control value and
increased absolute and relative liver and gall-bladder weights. No
macroscopic changes were observed that could be attributed to
treatment, and treatment-related microscopic changes were limited to
diffuse, mild hypertrophy of hepatocytes in all treated males and in
females at the two highest doses. There was no NOAEL because of
effects on the liver (Goldenthal, 1993a).
Groups of four beagle dogs of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) for one year at concentrations
corresponding to 100, 600, and 2000 ppm active ingredient. The diets
were prepared weekly and stored at room temperature. The stability of
the compound was assessed twice: an initial test showed a loss of
about 10% over 10 days, possibly due to technical problems; a second
test showed 97-101% of the initial concentration on day 10. The actual
concentrations were measured 16 times and were found to be 87-110% of
the nominal concentrations. The homogeneity of the mixture was also
found to be satisfactory. The dogs were observed at least twice daily
for mortality and signs of toxicity; detailed observations were
recorded weekly. Body weight and food consumption were recorded weekly
for the first 14 weeks and every two weeks thereafter. Ophthalmoscopic
examinations were conducted before the beginning of treatment and at
termination. Physical examinations were conducted on all animals
before exposure and after 3, 6, 9, and 12 months of treatment.
Haematological and biochemical tests and urinalyses were conducted on
all animals after 6 and 12 months. All animals were examined
post mortem, and all major tissues and organs were examined
microscopically. Selected organs were weighed. All animals survived to
the end of the study, with no relevant clinical or ophthalmological
signs. The body weights of males and females receiving 2000 ppm
piperonyl butoxide were 2% lower to 3% higher than the initial
weights, whereas those of controls were 22-25% higher. Treatment-
related decreases in food consumption were observed in males at 600
(by 15%) and 2000 (by 20%) ppm. Small, not statistically significant,
not dose-related decreases in food consumption were observed in
treated females.
No treatment-related change in haematological parameters was
observed. The activity of alkaline phosphatase was increased to three
to five times the control values in animals at 2000 ppm after 6 and 12
months, and cholesterol levels were decreased (not statistically
significant) at these intervals in females. Males and females at this
dose had increased absolute (by 22 and 36%) and relative (by 52 and
86%) liver and gall-bladder weights, and small increases in thyroid
and parathyroid weights (average, 34%) were observed in females. These
changes were not associated with microscopic changes in the thyroid
and were considered to be of questionable biological significance. No
macroscopic pathological changes were observed that could be
attributed to treatment. Treatment-related histopathological changes
were limited to diffuse, mild hypertrophy of the hepatocytes in males
and females at 2000 ppm. The NOAEL was 100 ppm, equal to 16 mg/kg bw
per day, on the basis of effects on the liver, some clinical chemical
alterations, and reduced body-weight gain at 2000 ppm (Goldenthal,
1993b).
Monkeys
Two African green monkeys were given 32 or 106 mg/kg bw per day
piperonyl butoxide orally for six days per week for four weeks.
Minimal microscopic changes were observed in the livers (Sarles &
Vandergrift, 1952).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 (C57Bl/6 × C3H/Anf)F1 and (C57Bl/6 × AKR)F1 mice of
each sex received piperonyl butoxide (purity unspecified) at 100 mg/kg
bw or 'Butacide' at 464 mg/kg bw in 0..5% gelatin at seven days of age
by stomach tube and the same amount (not adjusted for increasing body
weight) daily up to four weeks of age; subsequently, the mice were fed
diets containing 300 ppm. The dose was reported to be the maximal
tolerated dose for infant and young mice. All animals were killed when
they were about 70 weeks of age, and their tumour incidences were
compared with those of 79-90 necropsied mice of each sex and strain,
which had either been untreated or had received gelatine only. No
significant difference in the incidence of tumours was found between
treated and control mice, although the authors concluded that
additional evaluation was required (Innes et al., 1969). This study
was not considered to be adequate by the Meeting.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for 30 weeks;
the doses were decreased to 500 or 2000 ppm for the following 82
weeks, due apparently to a greater than expected decrease in
body-weight gain. The control group consisted of 20 males and 20
females. Animals were observed twice daily for signs of toxicity; body
weight was recorded at least once per month. Necroscopic examination
was performed at termination on all moribund animals and on those
found dead, unless precluded by autolysis. Major tissues and organs
and all gross lesions were examined microscopically. At termination,
85% of control males and 75% of control females, 82% and 68% at the
low dose, and 84% and 70% at the high dose, respectively, were still
alive. Body weight was reduced in a dose-related manner, by up to
about 15% in females at the high dose at termination. No
treatment-related increase in tumour incidence was found, but
hepatocellular carcinomas occurred frequently, especially in males,
with incidences of 50% in controls, 34%, at the low dose, and 40% at
the high dose. No statistically significant differences were found in
the incidences of neoplastic and non-neoplastic lesions (US National
Cancer Institute, 1979)
Groups of 60 CD-1 mice of each sex were fed diets containing
piperonyl butoxide (purity, 90.78%) at concentrations adjusted to give
doses of 0 (two control groups), 30,100, or 300 mg/kg bw per day, for
78 weeks. The diets were prepared weekly and the concentrations
adjusted on the basis of body weights and food consumption. The diets
were analysed for the actual concentration, homogeneity, and stability
of piperonyl butoxide. The average actual concentrations at the low
and high doses were 99 and 95%. The homogeneity and stability (no
decrease in concentration after 14-21 days) of piperonyl butoxide in
the diet were considered to be satisfactory. Animals were observed
twice daily for signs of toxicity. Food consumption and body weight
were determined weekly for the first week and then every other week.
Haematological measurements were made for 10 rats of each sex in the
control and high-dose groups at week 52 and for all groups at
termination. All surviving animals were necropsied; major tissues and
organs from controls and animals at the high dose and the lungs,
liver, kidneys, and gross lesions from other groups were examined
microscopically. All slides from the livers underwent an independent
pathological review; the results reported here are the consensus or
majority opinions of all the pathologists involved.
No treatment-related clinical signs or palpable masses were
observed. The mortality rates were (males/females) 27/32, 32/27,
27/38, 22/33, and 40/18% in the first control group, animals at 30,
100, and 300 mg/kg bw per day, and the second control groups,
respectively. Body weights and body-weight gains of animals at the
high dose were slightly (occasionally statistically significantly)
decreased. No significant effects on food consumption were observed.
Haematological parameters were also unaffected by treatment. A
dose-related increase in absolute and relative liver weights was
observed in mice of each sex at the middle and high doses.
Hepatocellular hypertrophy was more frequent in males at the high
(72%) and middle doses (27%) than in controls (10 and 18%) and in
females at the high dose (15%; 0 and 7% in controls). Hepatocellular
hyperplasia (also called eosinophilic/clear-cell focus), characterized
by aggregates of hepatocytes with a swollen eosinophilic cytoplasm,
was present in 8% of males at the high dose, 2% of females at the
middle dose, and 7% at the high dose. The incidences of hepatocellular
hyperplasia were 0 and 3% in control males and 0% in both female
control groups. The incidence of hepatocellular adenomas was higher in
male mice at the middle (37%) and high (47%) doses than in either
control group (15 and 13%) and in females at the high dose (17%; 3% in
controls). The hepatocellular adenomas observed in many treated
animals were composed of large, polyhedral, densely-packed cells with
abundant granular eosinophilic cytoplasm. This appearance is different
from that of spontaneous adenomas found in CD-1 mice, where small to
medium, well-differentiated, basophilic cells, distributed in solid to
normal sinusoidal patterns, are found. Only the incidence of the
former type of adenoma was increased in piperonyl butoxide-treated
mice. A nonsignificantly increased incidence of hepatocellular
carcinomas was observed in males at the high dose (12%; 3% in
controls). No other treatment-related neoplastic or non-neoplastic
lesion was found. The NOAEL was 30 mg/kg bw per day on the basis of
effects on the liver (Hermanski & Wagner, 1993).
A 12-month study of carcinogenicity was conducted in male
Crj:CD-1 mice fed diets containing 0 ( n = 52), 6000 ( n = 53), or
12 000 ( n = 100) ppm piperonyl butoxide (purity, 94.3%; no
detectable safrole or isosafrole). Animals were observed daily for
mortality and morbidity; those that died or were sacrificed at
termination were necropsied, and gross lesions and the liver were
examined histologically. The mortality rates were 6% of controls, 2%
at the low dose, and 19% at the high dose. Terminal body weight was
found to have been reduced by 17% at the low dose and 29% at the high
dose. Hepatocellular hyperplasia was seen in 38% of animals at the low
dose and 8% at the high dose, hepatocellular adenomas in 13 and 22%,
hepatocellular carcinomas in 11 and 52%, and haemangioendothelial
sarcoma in 2 and 42%. It should be noted that the hepatocellular
adenomas and carcinomas may have obscured the true dose-response
relationship for hepatocellular hyperplasia. A condition termed
'post-necrotic peliosis', consisting of multifocal necrosis with blood
cysts, was also found in 17% of animals at the high dose. One control
animal showed hepatocellular hyperplasia and one had a hepatocellular
adenoma (Takahashi et al., 1994a).
Rats
Groups of 12 Wistar rats of each sex were fed diets containing 0,
100, 1000, 10 000, or 25 000 ppm piperonyl butoxide for two years, and
an additional group was fed a diet containing 1000 ppm piperonyl
butoxide plus 167 ppm pyrethrins. Animals were observed daily for
clinical signs of toxicity, and food consumption and body weight were
determined weekly. Autopsies were performed on all animals. Males and
females were paired for the duration of the study, except during
nursing (see section ( d)). Body-weight gain was reduced in animals
at 10 000 (by 10-20%) and 25 000 ppm (by about 90%), in association
with reduced food intake. All animals at the high dose were dead by
week 68; the mortality rates in the other groups were (males/females):
30/20%, 40/30%, 40/50%, and 60/60% for controls and animals at 100,
1000, and 10 000 ppm, respectively. The relative weights of livers and
kidneys were higher than those of controls in animals at 10 000 (by
40% for liver and kidney) and 25 000 ppm (by 275% for liver and 150%
for kidney). These effects were not associated with morphological
alterations. The incidence of tumours was not increased (Sarles &
Vandergrift, 1952).
Sixty Sprague-Dawley Crl:CDR (SD)BR rats of each sex were fed
diets containing piperonyl butoxide (purity, 89%) at concentrations
adjusted weekly (every two weeks after week 15) to achieve daily
intakes of 0 (two groups of controls), 30, 100, or 500 mg/kg bw per
day for 104-105 weeks. The stability and homogeneity of the diets and
the correspondence of actual to nominal concentrations were checked
before and throughout the study and found to be acceptable. The
animals were observed for mortality, clinical signs, food consumption,
body weight, and ophthalmoscopic, haematological, clinical
biochemical, urinary, and pathological parameters. Complete
histological examinations were made of animals in the two control
groups and at the high dose and of those animals at the low and
intermediate doses which died during the study. Histological
examination of animals at these doses killed at the end of the study
was limited to the liver, kidney, lung, thyroid, testis, epididymides,
ovary, and any observed abnormalities.
No clinical signs related to treatment were observed.
Sialodacryoadenitis was diagnosed in a large percentage of treated
animals in weeks 25-28 and weeks 63-67. Body weights were lower in
rats of each sex at 500 mg/kg bw per day than in controls throughout
the study. At week 104, the mean body weight was 20-30% lower than the
control value. A trivial reduction in food consumption was observed in
animals of each sex at the highest dose. Ophthalmoscopic examination
in week 99 revealed no changes attributable to treatment, and
haematological tests and urinalysis revealed no adverse effects.
Female rats receiving 500 mg/kg bw per day had higher cholesterol
levels than controls throughout the study and increased blood urea
nitrogen at 98 weeks. Other statistically significant differences in
biochemical parameters were of no biological relevance. At the end of
the study, the mortality rates were 82, 78, 87, 82, and 78% in males
and 55, 68, 63, 43, and 50% in females at 0 (two groups), 30, 100, and
500 mg/kg bw per day, respectively.
Increased liver weights were seen in animals of each sex at 100
and 500 mg/kg bw per day, corresponding to a higher incidence of
macroscopic enlargement associated with hyperplasia and hypertrophy of
centrilobular hepatocytes and enlarged eosinophilic cells, which
occasionally contained a brownish cytoplasmic pigment. Increased
kidney weights were observed in female rats at the two highest doses,
corresponding histologically to a higher incidence of chronic
interstitial glomerulonephritis. Other histological changes were
confined to the endocrine organs: Enlarged thyroid glands were
observed in animals of each sex at 500 mg/kg per day, corresponding
histologically to a higher incidence of generalized and focal
hyperplasia of follicles and increased pigment deposition in the
colloid. A slightly higher incidence of adrenal and ovarian
enlargement seen among females receiving 500 mg/kg per day was not
associated with histological changes. In males, there was a negative
trend in the presence of enlarged, focal, coarsely vacuolated cortical
cells in the adrenals. The combined incidence of bilateral and
unilateral testicular atrophy was comparable between groups, but there
were significant differences from controls in the incidences of
bilateral testicular atrophy at all doses, with 17, 33, 47, and 43% at
0, 30, 100, and 500 mg/kg bw per day, respectively. After an
additional, full review of the relevant data, this finding was
considered unlikely to be related to treatment because the atrophy was
not associated with degeneration of seminiferous tubules or
aspermatogenesis, and testicular weights were not decreased.
Morphometric analysis of the pituitary gland area showed reduced size
in males at the highest dose. A trend analysis of tumour incidence
with dose showed both increases (in the lymphoid system and thyroid)
and decreases (in mammary glands and pituitary). These differences
were not statistically significant in pairwise comparisons between
groups, and the incidences were within the range of those of
historical controls of this strain of rats. These observations provide
no evidence that the treatment had carcinogenic potential, but the
wide range of gross alterations, effects on organ weight, and
histological changes observed reflect the biological activity of the
test compound. The enlargement of the liver, the primary target organ,
is consistent with the activity of the compound as a hepatic enzyme
inducer. The wide differences in the incidences of morphological
changes and lesions in endocrine and hormone-sensitive organs between
controls and treated groups strongly support the interpretation that
they are the results of changes in hormonal levels brought about by
hepatic enzyme induction. The NOAEL was 30 mg/kg bw per day on the
basis of effects on the liver (Graham, 1987).
Groups of 50 Fischer 344/Du Crj rats of each sex were fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide (purity, about
89%) for two years at doses selected on the basis of the results of
the 13-week study by the same authors, summarized above. Animals were
observed daily for clinical signs and mortality; food consumption was
measured monthly, but the frequency of body-weight measurement was not
stated. Treatment was stopped after 104 weeks, and surviving animals
were sacrificed after six more weeks on the basal diet. All animals,
including those sacrificed when moribund or found dead, were
necropsied and a complete histological examination was performed. The
mortality rates were (males/females) 16/14, 38/22, and 42/34% in
controls and animals at 5000 and 10 000 ppm; the increased mortality
was statistically significant for animals at the high dose. Just
before death, many animals showed signs of anaemia (not specified) and
had blood in their faeces. A dose-related reduction in body weight was
seen in animals of each sex (statistical analysis not reported), with
no concomitant decrease in food intake. No statistically significant
difference in the incidence of malignant or benign neoplasms was
found, except a decreased incidence of thyroid C-cell adenomas in
males at the high dose (9%; 44% in controls). Dose-related increases
were seen in the incidences of ulcers (0/0%, 35/2%, and 52/45% in male
and female controls and those at the low and high doses,
respectively), regenerative hyperplasia (0/0%, 13/0%, and 22/4%),
ossification in the ileocaecal mucosa (0/0%, 15/0%, and 20/0%), and
haemorrhage of the caecum and colon (0/0%, 10/0%, and 17/12%). The
main histological lesions were chronic ulcers with inflammatory-cell
infiltration and granulation, sometimes with ossification of the
surrounding mucosa. Most of deaths in treated animals before the end
of the study were attributed by the authors to lesions leading to
anaemia. Piperonyl butoxide was not found to be carcinogenic
(Maekawa et al., 1985).
Groups of 50 Fischer 344 rats of each sex were fed diets
containing 5000 or 10 000 ppm piperonyl butoxide (purity, 88.4%) for
24 months; the control group consisted of 20 animals. The diets were
prepared freshly each week and stored at 7°C. Animals were observed
twice daily for mortality and underwent a complete clinical
examination monthly. The food consumption of 10 animals in each group
was measured during the first week of each month; body weights were
measured about twice monthly. Moribund animals were sacrificed and
necropsied and their organs were examined histologically. All
surviving animals were sacrificed and submitted to a complete
histopathological examination. All control females and about 80% of
treated females survived until termination, whereas no difference in
survival was found for males (70-75% survival in all groups). A
dose-related reduction in body weight was found for both females (16%
at the low dose and 24% at the high dose) and males (7% at the low and
16% at the high dose). No difference was found in the incidence of
malignant or benign neoplasms, except for that of lymphoreticular
malignant lymphoma which was significantly increased in treated
females (14% at the low and 30% at the high dose; 5% in controls) and
nonsignificantly decreased in males (30% at the low and 26% at the
high dose; 35% in controls). The author concluded that the results
were equivocal because of the variability in the historical control
data and the difficult diagnosis of these neoplasms (Cardy et al.,
1979; US National Cancer Institute, 1979).
Groups of 30-33 Fischer 344/DuCrj rats of each sex were fed diets
containing 0, 6000, 12 000, or 24 000 ppm piperonyl butoxide (two
lots: purity, 94.5 and 94.3%; no detectable safrole or isosafrole) for
95 (males) or 96 (females) weeks. The study was ended before 104 weeks
because of the high mortality of males at 12 000 ppm (see below).
Animals were observed daily for clinical signs and mortality; body
weights were measured monthly. Food consumption was determined in a
preliminary experiment involving six rats of each sex per group
(duration not reported), which resulted in calculated compound intakes
of 537, 1061, and 2002 mg/kg per day by females and 547, 1052, and
1877 mg/kg per day by males at 6000, 12 000 and 24 000 ppm,
respectively. Rats that died were necropsied and observed for tumors
and non-neoplastic lesions, which were then examined microscopically.
At termination, surviving rats were necropsied; hepatic nodules (when
multiple nodules were present, three major ones were taken) and major
organs were weighed and examined histologically. Blood was collected
for haematological tests (including prothrombin and partial
thromboplastic times) and for standard clinical chemistry. The
mortality rates were 17, 23, 50, and 24% in males and 20, 10, 17, and
21% in females at 0, 6000, 12 000, and 24 000 ppm, respectively. Males
at 12 000 ppm began to die as early as week 40 and at a statistically
significantly different rate from other groups from week 45. Deaths
were most frequently associated with haemorrhage in the caecum. Body
weights were reduced in a dose-related manner in all treated animals
but statistically significantly only in animals at the middle and high
doses. Males and females at the high dose lost weight from week 60,
and at termination their body weights were about 50% of those of
controls. The weights of all organs except the liver were reduced in
animals at the high dose. The absolute liver weights were increased in
animals at 12 000 ppm (in males by 22% and in females by 51%) and in
females at 6000 ppm (by 29%). During the first month of treatment,
rough hair, lethargy, epistaxis, and a fall in food consumption (data
not reported) were observed in animals of each sex at 24 000 ppm.
Haematological tests showed a significant increase in hypochromic,
microcytic anaemia, the severity of which was dose-related, in all
treated animals. The most significant findings in plasma were
decreased cholinesterase activity and triglyceride contents in animals
at all doses and an increased urea nitrogen level in animals at the
high dose and in all treated females. Hepatic tumours were observed
from week 74 of treatment. Nodular lesions of the liver were observed
only in treated animals, and their incidence, numbers per rat, and
size were dose-related. Hepatocellular adenomas and carcinomas were
found in animals at the middle and high doses: the incidences of
adenomas were 27% in males and 13% in females at 12 000 ppm and 15% in
males and 30% in females at 24 000 ppm; the incidences of carcinoma
were 13% in males and 0% in females at 12 000 ppm and 73% in males and
46% in females at 24 000 ppm. The nodules in animals at the low dose
were classified as foci or hepatocellular focal hyperplasia. The only
other difference between groups was found for interstitial-cell
tumours of the testis, with incidences of 23/25 in controls, 19/23 at
6000 ppm, 13/15 at 12 000 ppm, and 15/25 at 24 000 ppm. Essential
(haemorrhagic) thrombocytaemia (defined as > 3 × 106 platelets per
microlitre, with a bimodal platelet distribution curve) was found in
0/24 male controls and in 6/23 males at 6000 ppm, 3/15 at 12 000 ppm,
and 9/24 at 24 000 ppm and in only 1/25 females at the high dose. Of
the non-neoplastic lesions, the following are significant: an
increased incidence of stomach haemorrhage (males: 9/33; 5/30 in
controls; females: 19/33; 2/30 in controls) at the high dose, an
increased incidence of haemorrhage and/or oedema of the caecum in all
treated males and in females at the middle dose, an increased
incidence of black kidneys in males at the middle and high doses and
all treated females, and an increased incidence of whitish spotting in
the lungs of males at the middle and high doses. Tubular dilatation,
distension of Bowman's space and interstitial fibrosis were found in
the kidneys of animals at the high dose (actual numbers not reported)
(Takahashi et al., 1994b).
(d) Reproductive toxicity
Mice
In a two-generation study, with one litter per generation, groups
of 10 male and 10 female Crj:CD-1 mice were fed diets containing 0,
1000, 2000, 4000, or 8000 ppm piperonyl butoxide (purity unspecified).
Animals of the F0 generation, five weeks old at the start of the
study, were mated for five days at nine weeks of age. Animals of the
F1 generation was removed from their dams at four weeks of age and
allocated randomly to continue treatment; the F2 generation was
produced similarly to the F1 generation. Individual food intake was
measured before mating and during mating, gestation, and lactation for
all generations; litter size and weight and sex ratio were measured at
birth, and pups were weighed 0, 4, 7, 14, and 21 days after birth.
Some neurobehavioural tests were performed during lactation of the
F2 generation and the results analysed on a litter basis. The tests
included surface righting and negative geotaxis on postnatal days 4
and 7, cliff avoidance on postnatal day 7, swimming behaviour on
postnatal days 4 and 14, and olfactory orientation on postnatal day
14. Food consumption was reduced by 4-44% in F0 animals at 8000 ppm
except during mating, by 47% in F1 animals at 8000 ppm during
lactation, and by 16-22% in F0 and F1 animals at 4000 ppm during
lactation. The mean litter size of F1 animals at 8000 ppm was not
significantly reduced (7.7; 10.6 in controls), but the mean F1
litter weight was reduced by 38% (statistically significant) at
8000 ppm and by 18% (not statistically significant) at 4000 ppm. F1
pups at 8000 ppm had a lower survival index at postnatal day 21 (63%;
91% in controls for males, statistically significant; 79%; 89% in
controls for females, not statistically significant). The weights of
all F1 pups were reduced, but there was no dose-response
relationship at 1000-4000 ppm. The results of neurobehavioural tests
for treated F1 animals were different from those of controls in some
instances, but no dose-related response was evident. The mean F2
litter size was significantly reduced at 4000 ppm (10.3; 13.1 in
controls) and 8000 ppm (7.0), and the mean F2 litter weights were
reduced in all treated groups by 13-57%. F2 pups at 8000 ppm had a
lower survival index than controls at postnatal day 21 (59% in males,
statistically significant; 79% in females, not statistically
significant), and F2 pup weights were reduced at 2000, 4000, and
8000 ppm. At 2000 and 4000 ppm, the effect was evident only from
postnatal day 4; at 1000 ppm, reductions in pup weights were observed
on postnatal day 4 in males and postnatal day 7 in animals of each
sex. There was no NOAEL because of effects on pup weights at all doses
(Tanaka et al., 1992).
Groups of 10 male and 10 female Crj:CD-1 mice were fed diets
containing 0,1500, 3000, or 6000 ppm piperonyl butoxide (purity
unspecified) for four weeks before mating (F0 generation), during
gestation, and until the F1 generation was eight weeks old.
Open-field activity was measured in the F0 generation after three
weeks of treatment. Litter size, pup weight, and some developmental
behavioural signs (surface righting, negative geotaxis, cliff
avoidance, swimming, and olfactory orientation) were measured during
lactation of the F1 generation; open-field activity was measured at
three and eight weeks of age and multiple water T-maze activity at six
weeks of age. A dose-related decrease in ambulation and rearing was
seen in the open-field test in males of the F0 generation, but only
the former was statistically significant at the high dose. Pup body
weight at birth was reduced in all groups; on postnatal day 21, the
body weight of animals at the middle dose was 7% lower than that of
controls, and that of animals at the high dose was 41% lower. The
survival indexes at postnatal day 21 were 79, 93, 80, and 52 in
controls and in mice at the low, middle, and high doses, respectively.
The results of the behavioural test during lactation were not
significant, except for reduced olfactory orientation in animals at
the middle and high doses. The results of the open-field test and the
multiple water T-maze test were not altered by treatment, except for a
few, not dose-related differences in some parameters (Tanaka, 1992).
Rats
In a two-litter, two-generation study of reproductive toxicity,
groups of 26 Sprague-Dawley CD rats of each sex, seven weeks of age,
received piperonyl butoxide in the diet at 0, 300, 1000, or 5000 ppm,
equal to 0, 20, 68, and 350 mg/kg bw per day for males (average food
intake calculated in weeks 1-28 of the study) and to 0, 29, 94, and
480 mg/kg bw per day for females (calculated in weeks 1-12 of the
study). The stability, homogeneity, and correspondence of the actual
concentrations in the diets to the nominal concentrations were checked
several times before and during the study and found to be acceptable.
Rats were maintained on their respective diets for 85 days before
mating, throughout the two mating periods, and until scheduled
sacrifice. The F1b generation litters were weaned on day 21
post partum, and groups of 26 rats of each sex were selected to form
the F1b adult generation. These animals were maintained on their
respective diets for 83 days before mating. Animals were observed for
signs of toxicity, and body weight and food consumption were recorded.
External and internal gross examinations were performed on adult rats
and on a selected number of weanlings; histopathological examination
was undertaken for the reproductive tracts of control animals, those
at the high dose, and those of rats at the low and middle doses that
failed to mate successfully in either mating period.
Clinical and pathological examinations showed no
treatment-related toxic effects in adult F0 or F1b animals. The
body weights of adult animals of each sex at 5000 ppm were lower than
those of controls from a few weeks after the beginning to the end of
the study. This effect occasionally corresponded to reduced food
intake. Mating performance, fertility indexes, gestation indexes,
length of gestation, and numbers of live and dead pups at birth were
not affected by treatment. The viability and lactation indexes,
clinical conditions, and pathological appearance of pups of all
generations were also unaffected. The body weights of male and female
pups of all generations at 5000 ppm were lower than those of control
pups. This effect was not detectable at birth but was seen as early as
day 4 post partum. The NOAEL for parental toxicity and pup development
was 1000 ppm, equal to 68 mg/kg bw per day (Robinson et al., 1986).
During the study of carcinogenicity reviewed above (Sarles &
Vandergrift, 1952), rats of each sex were pair-caged throughout the
experiment, except during nursing. No effect on reproductive
efficiency was seen in the first, second, or third generations (no
details given).
(e) Developmental toxicity
Mice
Groups of 20 pregnant Crj:CD-1 mice were given piperonyl butoxide
(purity, > 95%) in olive oil by gavage on day 9 of gestation at doses
of 0, 1065, 1385, or 1800 mg/kg bw. Dams were weighed on gestation
days 9 and 18 and then sacrificed, and the uteri were examined for the
presence and position of resorption sites, fetuses, and implantation
sites. Viable fetuses were weighed and examined for external
malformations and variations, and then fixed and stained for
visualization of the skeleton. No abnormal behaviour or mortality was
observed in dams. One dam at the middle dose and two at the high dose
aborted; the litters of one dam at the middle dose and three at the
high dose were resorbed. Maternal body-weight gain was comparable in
all groups. The total resorption rates were significantly greater at
the middle (26%) and high (32%) doses than in controls (6%), probably
due to total litter resorptions, since the number of viable fetuses
per dam was comparable in all groups. (The raw data were not available
to confirm the reviewer's hypothesis.) The average fetal body weight
was significantly reduced in males, by 3% at the middle dose and 7% at
the high dose, and in females, by 4% at the low dose, 5% at the middle
dose, and 6% at the high dose. Exencephaly, craniochisis, open
eyelids, omphalocele, kinky tail, and talipes varus were observed in
all groups. Oligodactyly in the forelimbs was found in none of the
controls but in one fetus at the low dose, four (2%) at the middle
dose, and 27 (6%) at the high dose. A single oral dose > 1065 mg/kg
bw per day of piperonyl butoxide to dams on day 9 of gestation was
thus embryo- and fetotoxic (Tanaka et al., 1994).
Rats
Groups of 20 pregnant COBS random-bred albino rats were given 0,
300, or 1000 mg/kg bw per day piperonyl butoxide (technical-grade;
purity unspecified) dissolved in corn oil by gavage on days 6-15 of
gestation. The doses were chosen after a pilot study in which six
animals per group were treated with 0, 100, 300, 1000, or 3000 mg/kg
bw per day; reduced body-weight gain was observed during gestation
days 6-15 at the highest dose. Pregnant animals were shipped from the
breeder to the test laboratory on day 1 of gestation and were observed
and weighed daily. On day 20 of gestation, they were sacrificed and
the usual parameters were determined. No signs of toxicity were
observed. Body-weight gain was reduced by 10% in animals at the low
dose and by 15% in those at the high dose, mainly between days 15 and
20 of gestation. Reproductive parameters were not significantly
affected by treatment. One female at 300 mg/kg bw resorbed 8 of 11
fetuses, and one at the high dose resorbed the entire litter. Fetuses
of treated dams had no internal, external, or skeletal malformations
that could be related to treatment. There was no NOAEL for maternal
toxicity because of reduced body-weight gain at both doses. The NOAEL
for embryo- and fetotoxicity was 1000 mg/kg bw per day on the basis of
the absence of any significant finding (Kennedy et al., 1977). This
report from Industrial Biotest Laboratories has not been validated by
a governmental agency and was therefore not taken into account by the
Meeting.
Groups of 17-20 pregnant Wistar rats were given piperonyl
butoxide (purity, 80%) at 0, 62.5, 125, 250, or 500 mg/kg bw dissolved
in corn oil by gavage on days 6-15 of gestation. They were sacrificed
on day 22 of gestation and necropsied, and their fetuses were removed
and examined for external, visceral, and skeletal changes. There were
no deaths or signs of toxicity in dams and no effect on the numbers of
corpora lutea, live fetuses, resorption sites plus dead fetuses,
anomalous fetuses, anomalous litters, or fetal body weight. The types
and incidences of anomalies in the treated groups were comparable to
those of the control group (data not shown). There was no evidence of
embryotoxicity, fetotoxicity, or teratogenicity, and no maternal
toxicity was elicited at the doses used (Khera et al., 1979).
Groups of 20 timed-pregnant Sprague-Dawley CD rats were given
undiluted piperonyl butoxide (purity, 90.78%) at 0, 200, 500, or
1000 mg/kg bw per day by gavage on days 6-15 of gestation. The doses
were chosen in a preliminary pilot study in which five animals per
group were treated with 0, 250, 500, 1000, 2000, or 4000 mg/kg bw per
day, and in which all animals at 4000 and four-fifths of those at
2000 mg/kg bw per day groups died or became moribund. Animals were
observed and weighed daily. On day 20 of gestation, they were
sacrificed and the usual parameters plus liver weight were determined.
No females aborted or delivered early, but wetness in the urogenital
area was observed in 13/24 dams at the highest dose, and stains of
urine, red urogenital discharge, and perinasal encrustation were seen
at lower incidences. Body-weight gain was reduced between days 6 and 9
of gestation in animals at the low dose (an effect considered to be
nonsignificant because of the low weight of non-pregnant animals) and
between days 6 and 15 in animals at the middle and high doses; these
reductions were associated with reduced food intake, resulting in a
significantly lower body weight (by about 5%) in animals at 500 and
1000 mg/kg bw per day on days 15 and 18 of gestation. Increased
absolute (by 8%) and relative (by 11%) weights were observed at the
high dose. Reproductive parameters were not significantly affected by
treatment. Fetuses of treated dams had no internal, external, or
skeletal malformations that could be related to treatment. The NOAEL
for maternal toxicity was 200 mg/kg bw per day on the basis of reduced
body-weight gain and food consumption, increased liver weight, and
signs of toxicity at higher doses. The NOAEL for embryo- and
fetotoxicity was 1000 mg/kg bw per day on the basis of the absence of
any significant finding (Chun & Neeper-Bradley, 1991).
Rabbits
In a range-finding study, five inseminated New Zealand white
rabbits were treated by gavage with piperonyl butoxide during
gestation days 7-19 at doses of 0, 50,100, 200, 300, or 400 mg/kg bw
per day in corn oil. All animals except one control survived until the
end of the study. Two animals each at 300 and 400 mg/kg bw per day and
one at 100 mg/kg bw per day group aborted all or part of their litters
between gestation days 22 and 26. Decreased defaecation was observed
at the highest dose, and animals at the two highest doses were thinner
than normal at the end of the treatment period. Significant reductions
in body-weight gain and some body-weight loss were observed in rabbits
at 300 and 400 mg/kg bw per day; a trivial reduction in body-weight
gain was observed in animals at 200 mg/kg bw per day. No consistent
effects were seen on uterine parameters. Doses of 50, 100, and
200 mg/kg bw per day were therefore chosen for the definitive study of
teratogenicity.
Groups of 16 inseminated New Zealand white rabbits were treated
by gavage with piperonyl butoxide (purity, 100%) during gestation days
7-19 at doses of 0, 50, 100, or 200 mg/kg bw per day in 0.5 ml/kg bw
corn oil. Caesarean sections were performed on gestation day 29, and
the fetuses were removed for teratological evaluation. All animals
survived until the end of the study. Decreased defaecation was
observed in animals at 100 and 200 mg/kg bw per day. Slight
body-weight loss was observed at the middle and high doses during the
treatment period, but there was substantial recovery of body weight
during the post-treatment period, so that the weight gain during the
overall gestation period was comparable to that of controls. The mean
post-implantation losses of treated does were slightly greater than
those of controls but were not dose-related. Malformations were
observed incidentally and were considered to be unrelated to
treatment. The numbers of fetuses with more full ribs than normal (45,
58, 59, and 60% at 0, 50, 100, and 200 mg/kg bw, respectively) or with
27 presacral vertebrae (20, 32, 27, and 40%, respectively) were
greater than those in controls, but the number of litters was not
different. There was no clear dose-effect relationship, and the
relevance of this finding is dubious. The NOAEL for maternal toxicity
was 50 mg/kg bw per day on the basis of decreased defaecation and
dose-related body-weight losses during the treatment period. The NOAEL
for teratogenicity was 200 mg/kg bw per day (Leng et al., 1986). The
1992 JMPR wrongly reported an NOAEL of 100 mg/kg bw per day.
(f) Genotoxicity
The results of studies of the genotoxicity of piperonyl butoxide
are reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Piperonyl butoxide was applied at 0.5 ml to intact, clipped skin
of six New Zealand white rabbits for 4 h under a semi-occlusive
dressing, and the treated areas were examined for erythema and oedema
up to 78 h after the contact period. An adjacent area of untreated
skin served as the control. Piperonyl butoxide was very mildly
irritating (Romanelli, 1991a). An older study reported similar results
(Sarles et al., 1949).
Piperonyl butoxide at 0.1 ml was instilled into the conjuntival
sac of one eye of six New Zealand white rabbits, and the treated eyes
were examined up 72 h after instillation. Conjunctival irritation was
observed, which fully recovered within 72 h. No corneal or iridal
lesions were observed (Romanelli, 1991b). An older study reported
similar results in rabbits, cats, and dogs (Sarles et al., 1949).
Table 2. Results of tests for the genotoxicity of piperonyl butoxide
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, TA100, 100-5000 µg/plate 90.78 Negativea Lawlor (1991)
TA1535, TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative White et al. (1977)
TA 100, TA1537
Reverse mutation E. coli WP2 1 mg/disc 80 Negative Ashwood-Smith
et al. (1972)
Gene mutation Mouse lymphoma L5178Y 6.3-100 µg/mlb NR Positive McGregor et al.
cells (1988)
Gene mutation Chinese hamster ovary, cells 10-100 µg/mlc NR Equivocal Tu et al. (1985)
25-500 µg/mld Negative
Unscheduled DNA Rat hepatocytes 1-100 µg/mle 90.78 Negative McKeon & Phil
synthesis (1991)
Unscheduled DNA Human liver slices 0.05-2.5 mmol/litre 90.78 Negative Lake (1995)
synthesis
Chromosomal Chinese hamster ovary cells 25-99.9 µg/ml 90.78 Negativea Murli (1991)
aberration 62.6-251 µg/mla
Table 2. (cont'd).
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo
Dominant lethal ICR/Ha Swiss mice 0, 200, or 1000 mg Commercial Equivocal Epstein et al. (1972)
mutation intraperitoneally or formulation
1000 mg/kg bw orally
× 5
NR, not reported
a With and without metabolic activation
b Lethal at 100 µg/ml
c Without metabolic activation; cytotoxic at > 30 µg/ml
d With metabolic activation; cytotoxic at > 300 µg/ml
e Cytotoxic at > 49.9 µg/ml
Skin sensitization was studied in 10 male Hartley guinea-pigs by
a modified Buehler method. A gauze patch containing 0.4 ml piperonyl
butoxide was applied for 6 h onto clipped skin, three times a week for
three weeks. After a two-week rest period, the animals were challenged
on another site with a single 6-h application. Chlorodinitrobenzene
(0.1% w/v in a 50% ethanol:0.9% saline solution) was used as the
positive control. No evidence of contact sensitization was observed
(Romanelli, 1991c). An older study also reported no response in
rabbits (Sarles et al., 1949)
(ii) Studies with mixtures
Neonatal ICR/Ha Swiss mice (n = 91) were given subcutaneous
injections of 5 mg piperonyl butoxide alone at the ages of one and
seven days and 10 mg at the ages of 14 and 21 days. Further groups
were given the same doses in combination with Freon-112 (n = 137) or
-113 (n = 94) at doses of 10 mg at one and seven days and 20 mg at 14
and 21 days. Control animals were treated with the solvent,
tricaprylin. All surviving animals were killed after 52 weeks. No
tumours were found in animals treated with piperonyl butoxide, but the
incidence of hepatomas in male mice given piperonyl butoxide plus
Freon was significantly increased when compared with that in groups
receiving solvent, piperonyl butoxide, or Freon alone (Epstein
et al., 1967).
Groups of 45 Sprague-Dawley CD rats of each sex were fed either
standard diets or diets containing 400 ppm pyrethrins plus 2000 ppm
piperonyl butoxide for 104 weeks. The diets were prepared daily from a
10-fold concentrated pre-mix prepared freshly once a week. The purity
of piperonyl butoxide was 90.5%, and that of pyrethrins was 53.1%
(batch used until week 55) or 52.4% (batch used from week 56 until
termination); the diets were prepared taking into account the purity
of the compounds. The actual concentrations and the stability of the
diet were not tested. Body weight and food consumption were determined
once a week. The average daily intakes were 79 and 101 mg/kg bw
piperonyl butoxide and 16 and 20 mg/kg bw pyrethrins for males and
females, respectively. Animals were observed for clinical signs, skin
lesions, and palpable masses. All animals found dead or moribund were
inspected, and macroscopic lesions were examined microscopically.
During week 101, urinalysis and haematological and clinical chemical
tests were performed in 10 animals per group. At termination, all
animals were observed grossly and selected organs were examined
microscopically.
No treatment-related clinical signs of toxicity were observed.
Mortality was 84 and 76% in control males and females and 80 and 58%
in treated males and females, respectively. The body weights of
treated females were about 20% lower than those of controls, mainly
during the first 78 weeks, while at termination treated animals were
about 10% lighter than controls; the difference was less evident
(about 5%) in males. Slightly reduced food intake was also observed
until week 27 of treatment. Haematological, clinical chemical, and
urinary parameters were not significantly altered in treated animals,
and macroscopic and microscopic examinations revealed no significant
treatment-related neoplastic or non-neoplastic effects (Hunter
et al., 1977).
3. Observations in humans
In nine men, antipyrine metabolism was not affected by a single
oral dose of 50 mg (0.71 mg/kg bw) piperonyl butoxide (Conney et al.,
1972).
Two male infants, whose mothers were sisters and who were born
within two weeks of each other, had coarctation of the aorta. Exposure
to insect repellents and insecticides, including piperonyl butoxide,
during week 8 of gestation was reported (Hall et al., 1975).
Percutaneous absorption of pyrethrin and piperonyl butoxide was
studied in six male volunteers. A formulation containing 0.3% pyrethin
and 3.0% piperonyl butoxide was spread on the ventral forearm for 30
min. This formulation was chosen because it corresponds to that of a
commercially available product used for the treatment of head lice.
The formulation contained either 14C-pyrethrin or 14C-piperonyl
butoxide. After application, urine was collected for up to seven days,
and percutaneous absorption was determined from total urinary
excretion of radioabel, assuming that 22.5% of pyrethin and 51.3% of
piperonyl butoxide are excreted in the urine after parenteral
injection, as was shown in monkeys. Absorption of pyrethrin was 1.9 ±
1.2% and that of piperonyl butoxide 2.1 ± 0.6% of the administered
dose. No radiolabel was found in blood taken 1 h after application.
The extrapolated absorption from human scalp was 7.5 ± 4.7% of
pyrethin and 8.3 ± 2.4% of piperonyl butoxide (Wester et al., 1994).
Comments
Piperonyl butoxide is metabolized by oxidation of the methylene
group of the methylenedioxyphenyl moiety to yield carbon dioxide. The
remainder of the molecule undergoes further degradation and is
excreted mainly in the urine. As it is an alternative substrate (and
therefore a competitive inhibitor) for the microsomal cytochrome P450
system, piperonyl butoxide inhibits the metabolism of several drugs
and pesticides. The mechanism of action of this inhibition has been
elucidated in several studies.
In male rats given single oral doses of about 500 mg/kg bw of
[methylene-alpha 14C]-piperonyl butoxide, the label reached a peak
in blood after 3-12 h and dropped by about 50% within 24 h. The
highest levels of radiolabel were found in the gastrointestinal tract
and its, contents, suggesting that enterohepatic circulation occurs.
High levels of radioactivity were also found in the lung, liver,
kidney, fat, prostate, and seminal vesicles. The excretion pattern was
unchanged after 14 repeated doses of piperonyl butoxide.
Piperonyl butoxide has negligible acute toxicity, and it has been
classified by WHO as unlikely to present an acute hazard in normal
use.
Both short-term and long-term studies show that the target organ
of the toxicity of piperonyl butoxide is the liver. Male animals were
slightly more sensitive than females. In a number of short-term
studies in rodents, hepatic toxicity was characterized by liver
enlargement with associated hypertrophic hepatocytes, focal necrosis,
and, at times, alteration of some clinical chemical parameters. The
NOAEL for liver toxicity was about 100 mg/kg bw per day.
In rats exposed to piperonyl butoxide by inhalation at 0, 15, 74,
155, or 512 mg/m3 for 6 h per day on five days a week for 13 weeks,
effects were seen on the liver at the highest dose. Irritation of the
upper airways was seen at all doses, with squamous metaplasia of the
larynx.
In a one-year study in dogs fed diets containing 0, 100, 600, or
2000 ppm piperonyl butoxide, reduced body-weight gain, increased liver
weight with hypertrophic hepatocytes, and alteration of some clinical
chemical parameters were observed at 2000 ppm. The NOAEL in this study
was 600 ppm, equal to 16 mg/kg bw per day.
Several studies have been conducted of carcinogenicity in mice
and rats, some of which were considered to be inadequate. In a
112-week study, mice were given diets containing 5000 or 10 000 ppm
piperonyl butoxide during weeks 1-30 and 500 or 2000 ppm during weeks
31-112. No increase in tumour incidence was observed. Mice were fed
diets that gave a daily intake of 0, 30, 100, or 300 mg/kg bw for 78
weeks. Eosinophilic foci and adenomas with eosinophilic cells were
observed more frequently in the livers of males at the middle dose and
males and females at the high dose. The NOAEL in this study was
30 mg/kg bw per day on the basis of effects on the liver.
In a 12-month study of carcinogenicity in the liver, male mice
were fed diets containing 0, 6000, or 12 000 ppm piperonyl butoxide.
Body weights were reduced in a dose-related manner, and increased
mortality was observed in animals at the high dose. Hepatocellular
adenomas and carcinomas were observed in treated groups. Piperonyl
butoxide was carcinogenic at doses that were toxic to the liver and
caused general toxicity.
In a two-year study in rats at dietary concentrations adjusted to
achieve doses of 0, 30, 100, or 500 mg/kg bw per day, increased liver
weights, with corresponding hyperplasia and hypertrophy of
hepatocytes, morphological changes, and lesions in the endocrine and
hormone-sensitive organs, were observed at 100 and 500 mg/kg bw per
day. These effects were considered to be secondary to the ability of
piperonyl butoxide to induce hepatic cytochrome P450 enzymes.
Piperonyl butoxide was not found to be carcinogenic in this study.
After reconsideration of the data on testes in this study, previously
evaluated by the 1992 JMPR, and taking into account the results of
other long-term studies, the Meeting concluded that the NOAEL was
30 mg/kg bw per day on the basis of effects on the liver.
Two studies of carcinogenicity were conducted in rats fed diets
containing 0, 5000, or 10 000 ppm piperonyl butoxide. No significantly
increased incidence of neoplasia was found in treated rats. Effects on
body weight and mortality were observed at the highest dose. In one
study, ileocaecal lesions were observed in both groups. Another study
of carcinogenicity was conducted in rats fed diets containing 0, 6000,
12 000, or 24 000 ppm. Increased mortality was observed in females at
the middle dose. Absolute liver weights were increased in females at
the low dose and in animals of each sex at the high dose. Nodular
lesions of the liver were observed in treated animals, and their
incidence and severity were related to the dose. Hepatocellular
adenomas and carcinomas were observed in animals at the middle and
high doses. Gastric and caecal haemorrhages, renal lesions, anaemia,
and platelet alteration were observed in treated animals. Piperonyl
butoxide was carcinogenic at doses that caused general toxicity.
In a 104-week study in rats, piperonyl butoxide was given at
2000 ppm in the diet in combination with pyrethrins at 400 ppm. A
slight reduction in the body weights of treated females was the only
adverse effect observed.
A two-generation study of reproductive toxicity, with one litter
per generation, was conducted in mice fed diets containing 0, 1000,
2000, 4000, or 8000 ppm piperonyl butoxide. There was no NOAEL because
of reduced pup weight at all doses. Pup viability was reduced at the
highest dose. In a two-litter, two-generation study of reproductive
toxicity in rats at dietary levels of 0, 300, 1000, or 5000 ppm, the
NOAEL for parental toxicity and pup development was 1000 ppm (equal to
68 mg/kg bw per day) on the basis of lowered body weight at 5000 ppm.
Embryo- and fetotoxicity were observed when mice were given single
doses of piperonyl butoxide at 0, 1070, 1390, or 1800 mg/kg bw by
gavage on day 9 of gestation.
Piperonyl butoxide was not embryotoxic or teratogenic in rats or
rabbits. Maternal toxicity was found in rats at doses > 500 mg/kg
bw per day. The NOAEL was 200 mg/kg bw per day in a study of
developmental toxicity in rabbits given 0, 50, 100, or 200 mg/kg bw
per day by gavage. The incidence of common developmental variations,
such as more full ribs and more than 27 presacral vertebrae, was
increased in all treated groups. Since a clear dose-effect
relationship was lacking, the association of this finding with
treatment was considered dubious. The NOAEL for maternal toxicity was
50 mg/kg bw per day.
Piperonyl butoxide was a mild dermal and ocular irritant but not
a dermal sensitizer in rabbits.
Piperonyl butoxide was adequately tested for genotoxicity in a
range of assays in vivo and in vitro. The Meeting concluded that
it is not genotoxic.
A single dose of 0.71 mg/kg bw piperonyl butoxide did not alter
antipyrine metabolism in humans. A study in which a formulation
containing 3% piperonyl butoxide was spread onto the ventral forearm
of adult male volunteers indicated that about 8% of the applied dose
would be absorbed through the human scalp. These data did not
contribute directly to the establishment of the ADI.
An ADI of 0-0.2 mg/kg bw was established on the basis of the
NOAEL of 600 ppm (equal to 16 mg/kg bw per day) in the one-year study
in dogs, with a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 mg/kg bw per day (78-week dietary study of toxicity and
carcinogenicity)
Rat: 30 mg/kg bw per day (two-year dietary study of
carcinogenicity)
200 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
500 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
1000 ppm, equal to 68 mg/kg bw per day (study of
reproductive toxicity)
Rabbit: 50 mg/kg bw per day (study of developmental toxicity)
200 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 600 ppm, equal to 16 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Further observations in humans
2. Short-term studies with mixtures of piperonyl butoxide and
other active ingredients, in ratios relevant to human
exposure
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to piperonyl butoxide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, irritation, rabbit Mildly irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Not sensitizing
Inhalation, lethality, rat Lacrimation, salivation, nasal
discharge, and laboured breathing at
5.9 mg/litre for 4 h. No mortality
Oral, lethality, mouse, rat, cat, dog LD50 = 4-14 g/kg bw
Dermal, lethality, rabbit LD50 > 2 g/kg bw
Medium-term (1-26 weeks) Repeated oral, toxicity, mice, rats, NOAEL = 100 mg/kg bw per day; effects on
3-13 weeks liver
Repeated inhalation, toxicity, rats, 13 weeks Irritating to upper airways at 15 mg/m3 for
6 h per day, 5 days per week, with squamous
metaplasia of the larynx; effects on the liver at
512 mg/m3
Oral, developmental toxicity, rabbit NOAEL = 50 mg/kg bw per day for maternal
toxicity; no fetotoxicity or teratogenidty
Oral, reproductive toxicity, rat NOAEL = 68 mg/kg bw per day; maternal
and pup toxicity
Long-term (> one year) Repeated oral, toxicity, dog, one year NOAEL = 16 mg/kg bw per day
References
Ashwood-Smith, M.J., Trevino, J. & Ring, R. (1972) Mutagenicity of
dichlorvos. Nature, 240, 418-420. Brady, J.T., Montelius, D.A.,
Beierschmitt, W.P., Wyand, D.S., Khairalla, E.A. & Cohen, S.D.
(1988) Effect of piperonyl butoxide post-treatment on
acetaminophen hepatotoxicity. Biochem. Pharmacol.,
37, 2097-2099.
Cardy, R.H., Renne, R.A., Warner, J.W. & Cypher, R.L. (1979)
Carcinogenesis bioassay of technical-grade piperonyl butoxide in
F344 rats. J. Natl Cancer Inst., 62, 569-578.
Chun, J.S. & Neeper-Bradley, T.L. (1991) Developmental toxicity
evaluation of piperonyl butoxide administered by gavage to CD
(Sprague-Dawley) rats. Unpublished report No. 54-586 from Bushy
Run Research Center, Export, Pennsylvania, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Conney, A.H., Chang, R., Levin, W.M., Garbut, A., Munro-Faure, A.D.,
Peck, A.W. & Bye, A. (1972) Effects of piperonyl butoxide on drug
metabolism in rodents and man. Arch. Environ. Health,
24, 97-106.
Dalvi, R.R. & Dalvi P.S. (1991) Differences in the effects of piperine
and piperonyl butoxide on hepatic drug-metabolizing enzyme system
in rats. Drug Chem. Toxicol., 14, 219-229.
Epstein, S.S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel, N.
(1967) Synergistic toxicity and carcinogenicity of 'Freons' and
piperonyl butoxide. Nature, 214, 526-528.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W. & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in
the mouse. Toxicol. Appl. Pharmacol., 233, 288-325.
Fishbein, L., Falk, H.L., Fawker, J., Jordan, S. & Corbett, B. (1969)
The metabolism of piperonyl butoxide in the rat with 14C in the
methylenedioxy or alpha-methylene group. J. Chromatogr.,
41, 61-79.
Franklin, M.R. (1972) Inhibition of hepatic oxidative xenobiotic
metabolism by piperonyl butoxide. Biochem. Pharmacol.,
21, 3287-3299.
Fujitani, T., Ando, H., Fujitani, K., Ikeda, T., Kojima, A., Kubo, Y.,
Ogato, A., Oishi, S., Takahashi, O. & Yoneyama, M. (1992)
Sub-acute toxicity of piperonyl butoxide in F344 rats.
Toxicology, 72, 291-298.
Fujitani, T., Tanaka, T., Hashimoto, Y. & Yoneyama, M. (1993a)
Subacute toxicity of piperonyl butoxide in ICR mice.
Toxicology, 83, 93-100.
Fujitani, T., Tada, Y. & Yoneyama, M. (1993b) Hepatotoxicity of
piperonyl butoxide in male F344 rats. Toxicology, 84, 171-183.
Gabriel, D. (1991a) Acute oral toxicity, LD50-rats. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Gabriel, D. (1991b) Acute dermal toxicity, single level-rabbits.
Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldenthal, E.I. (1993a) Evaluation of piperonyl butoxide in an eight
week toxicity study in dogs. Unpublished report No. 542-004 from
International Research and Development Corp., Mattawan, Michigan,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Goldenthal, E.I. (1993b) Evaluation of piperonyl butoxide in a one
year chronic dietary toxicity study in dogs. Unpublished report
No. 542-005 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Goldstein, J.A., Hickman, P. & Kimbrough R.D. (1973) Effects of
purified and technical piperonyl butoxide on drug metabolizing
enzymes and ultrastructure of rat liver. Toxicol. Appl.
Pharmacol., 26, 444-458.
Graham, C (1987) 24-Month dietary toxicity and carcinogenicity study
of piperonyl butoxide in the albino rat. Unpublished report
No. 81690 from Bio-Research Ltd Laboratory, Seneville, Quebec,
Canada. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hall J.G., McLaughlin, J.F. & Stamm, S. (1975) Coarctation of the
aorta in male cousins with similar maternal environmental
exposure to insect repellent and insecticides. Pediatrics,
55, 425-427.
Hermanski, S.J. & Wagner, C.L. (1993) Chronic dietary oncogenicity
study with piperonyl butoxide in CD-1 mice. Unpublished report
No. 91N0134 from Bushy Run Research Center, Export, Pennsylvania,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Hoffman, G.M. (1991) An acute inhalation toxicity study of piperonyl
butoxide in the rat. Unpublished report No. 91-8330 from
Bio/dynamics, East Millstone, New Jersey, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Hunter, B., Bridges, J.L. & Prentice, D.E. (1977) Long term feeding of
pyrethrins and piperonyl butoxide to rats. Unpublished report
from Huntington Research Centre, Huntington, Cambs, United
Kingdom. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Bates, R.R., Falk, H.L., Gart, J.J., Klein, M.,
Mitchell, I. & Peters, J. (1969) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: a preliminary
note. J. Natl Cancer Inst., 42, 1101-1114.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1968)
In vivo inhibition of mouse liver microsomal hydroxylating
systems by methylenedioxyphenyl insecticidal synergists and
related compounds. Life Sci., 7, 1051-1062.
Jaffe, H., Fuji, K., Guerin, H., Sengupta, M. & Epstein, S.S. (1969)
Bi-modal effect of piperonyl butoxide on the o- and
p-hydroxylations of biphenyl by mouse liver microsomes.
Biochem. Pharmacol., 18, 1045-1051.
Kamienski, F.X. & Casida, J.E. (1970) Importance of demethylenation in
the metabolism in vivo and in vitro of methylenedioxyphenyl
synergists and related compounds in mammals. Biochem.
Pharmacol., 19, 91-112.
Kennedy, G.L., Smith, S.H., Kinoshita, F.K., Keplinger, M.L. &
Calandra, J.C. (1977) Teratogenic evaluation of piperonyl
butoxide in the rat. Food Cosmet. Toxicol., 15, 337-339.
Khera, K.S., Whalen, C., Angers, G. & Trivett, G. (1979) Assessment of
the teratogenic potential of piperonyl butoxide, biphenyl, and
phosalone in the rat. Toxicol. Appl. Pharmacol., 47, 353-358.
Lake, B.G. (1995) An investigation of the effect of piperonyl butoxide
on unscheduled DNA synthesis in cultured human liver slices.
Unpublished report No. 1501/1 from BIBRA International,
Carshalton, Surrey, United Kingdom. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lawlor, T.E. (1991) Piperonyl butoxide in the Salmonella/mammalian-
microsome reverse mutation assay (Ames test) with a confirmatory
assay. Unpublished report No. 14413-0-401R from Hazleton
Washington, Kensington, Maryland, USA. Submitted to WHO by Endura
SA, Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Lehman, A.J. (1948) Q. Bull. Assoc. Food Drug Off., 12, 82.
Lehman, A.J. (1951) Q. Bull. Assoc. Food Drug Off., 15, 122.
Lehman, A.J. (1952a) Q. Bull. Assoc. Food Drug Off., 16, 43.
Lehman, A.J. (1952b) Q. Bull. Assoc. Food Drug Off., 16, 47.
Leng, J.M., Schwartz, C.A. & Schardein, J.L. (1986) Teratology study
in rabbits. Unpublished report No. 542-002 from International
Research and Development Corp., Mattawan, Michigan, USA.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Levine, B.S. & Murphy, S.D. (1977a) Esterase inhibition and
reactivation in relation to piperonyl butoxide-phosphorothionate
interactions. Toxicol. Appl. Pharmacol., 40, 379-391.
Levine, B.S. & Murphy, S.D. (1977b) Effect of piperonyl butoxide on
the metabolism of dimethyl and diethyl phosphorothionate
insecticides. Toxicol. Appl. Pharmacol., 40, 393-406.
Maekawa, A., Onodera, H., Furuta, K., Tanigawa, H., Ogiu, T. &
Hayashi, Y. (1985) Lack of evidence of carcinogenicity of
technical-grade piperonyl butoxide in F344 rats: Selective
induction of ileocaecal ulcers. Food Chem. Toxicol.,
7, 675-682.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Responses of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay. III. 72 coded
chemicals. Environ. Mol. Mutag., 12, 85-154.
McKeon, M.E. & Phil, M. (1991) Piperonyl butoxide in the assay for
unscheduled DNA synthesis in rat liver primary cell cultures with
a confirmatory assay. Unpublished report No. 14413-O-447R from
Hazleton Washington, Kensington, Maryland, USA. Submitted to WHO
by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Mirer, F.E., Levine, B.S. & Murphy, S.D. (1977) Parathion and methyl
parathion toxicity and metabolism in piperonyl butoxide and
diethyl maleate pretreated mice. Chem.-Biol. Interactions,
17, 99-112.
Modeweg-Hansen, L., Lalande, M., Bier, C., Losos, G. & Osborne, B.E.
(1984) A dietary dose range-finding study of piperonyl butoxide
in the albino rat. Unpublished report No. 81802B from
Bio-Research Laboratories, Edgewater, Maryland, USA. Submitted to
WHO by Endura SA, Bologna, Italy, for the Piperonyl Butoxide Task
Force, Washington DC, USA.
Murli, H. (1991) Piperonyl butoxide: Measuring chromosomal aberrations
in Chinese hamster ovary (CHO) cells with multiple harvests under
conditions of metabolic activation. Unpublished report
No. 14413-0-437C from Hazleton Washington, Kensington, Maryland,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Negherbon, W.O. (1959) Handbook of Toxicology, Vol. 3, Philadelphia,
Saunders.
Newton, P.E. (1992) A subchronic (3-month) inhalation toxicity study
of piperonyl butoxide in the rat via whole-body exposures.
Unpublished report No. 91-8333 from Bio/dynamics, East Millstone,
New Jersey, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Phillips, J.C., Cunninghame, M.E., Price, R.J., Osimitz, T., Ganriel,
K.L., Preiss, F.J., Butler, W.H. & Lake, B.G. (1995) Effect of
piperonyl butoxide (PBO) on cell replication and xenobiotic
metabolism in mouse liver (Abstract). Toxicologist, 15, 230.
Robinson, K., Pinsonneault, L. & Procter, B.G. (1986) A two-generation
(two-litter) reproduction study of piperonyl butoxide
administered in the diet to the rat. Unpublished report No. 81689
from Bio-Research Laboratories Ltd, Montreal, Quebec, Canada.
Submitted to WHO by Endura SA, Bologna, Italy, for the Piperonyl
Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991a) Primary skin irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991b) Primary eye irritation-rabbits. Unpublished
report No. 91-7317A from Biosearch Inc., Philadelphia,
Pennsylvania, USA. Submitted to WHO by Endura SA, Bologna, Italy,
for the Piperonyl Butoxide Task Force, Washington DC, USA.
Romanelli, P. (1991c) Guinea pig dermal sensitization-modified Buehler
method. Unpublished report No. 91-7317A from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Endura SA,
Bologna, Italy, for the Piperonyl Butoxide Task Force,
Washington DC, USA.
Sarles, M.P. & Vandegrift, W.B. (1952) Chronic oral toxicity and
related studies on animals with the insecticide and pyrethrum
synergist, piperonyl butoxide. Am. J. Trop. Med. Hyg.,
1, 862-883.
Sarles, M.P., Dove, W.E. & Moore, D.H. (1949) Acute toxicity and
irritation tests on animals with the new insecticide, piperonyl
butoxide. Am J. Trop. Med., 29, 151-166.
Schuller, H.M. & McMahon, J.B. (1985) Inhibition of N-nitroso-
diethylamine-induced respiratory tract carcinogenesis by
piperonylbutoxide in hamsters. Cancer Res., 45, 2807-2812.
Selim, S. (1985) Single and repeated oral dose pharmacokinetic and
distribution studies of piperonyl butoxide. Unpublished report
No. WP509070/1 from Biological Test Center, Irvine, California,
USA. Submitted to WHO by Endura SA. Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
Skrinijaric-Spoljar, M., Matthews, H.B., Engel, J.L. & Casida, J.E.
(1971) Response of hepatic microsomal mixed-function oxidases to
various types of insecticide chemical synergists administered to
mice. Biochem. Pharmacol., 20, 1607-1618.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994a) Piperonyl butoxide induces hepatocellular carcinoma in
male CD-1 mice. Arch. Toxicol., 68, 467-469.
Takahashi, O., Oishi, T., Fujitani, T., Tanaka, T. & Yoneyama, M.
(1994b) Chronic toxicity studies of piperonyl butoxide in F344
rats: Induction of hepatocellular carcinoma. Fundam. Appl.
Toxicol., 22, 293-303.
Tanaka, T. (1992) Effects of piperonyl butoxide on F1 generation mice.
Toxicol. Lett., 60, 83-90.
Tanaka, T. (1993) Behavioural effects of piperonyl butoxide in male
mice. Toxicol. Lett., 69, 155-161.
Tanaka, T., Takahashi, O. & Oishi, S. (1992) Reproductive and
neurobehavioural effects in three-generation toxicity study of
piperonyl butoxide administered to mice. Food Chem. Toxicol.,
30, 1015-1019.
Tanaka, T., Fujitani, T., Takahashi, O. & Oishi, S. (1994)
Developmental toxicity evaluation of piperonyl butoxide in CD-1
mice. Toxicol. Lett., 71, 123-129.
Tu, A.S., Coombes-Hallowell, W.E., Pallotta, S.L. & Catanzano, A.
(1985) Evaluation of piperonyl butoxide in the CHO/HGPRT mutation
assay with and without metabolic activation. Unpublished report
No. 53906 from Arthur D. Little, Inc., Cambridge, Massachusetts,
USA. Submitted to WHO by Endura SA, Bologna, Italy, for the
Piperonyl Butoxide Task Force, Washington DC, USA.
US National Cancer Institute (1979) Bioassay of piperonyl butoxide for
possible carcinogenicity (DHEW Publ. No. 79-1375), Bethesda,
Maryland, USA, US Department of Health, Education, and Welfare.
Wester, R.C., Bucks, D.A.W. & Maibach, H.I. (1994) Human in vivo
percutaneous absorption of pyrethrin and piperonyl butoxide.
Food Chem. Toxicol., 32, 51-53.
White, T.J., Goodman, D., Shulgin, A.T., Castagnoli, N., Lee, R. &
Petrakis, N.L. (1977) Mutagenic activity of some centrally active
aromatic amines in Salmonella typhimurium. Mutat. Res.,
56, 199-202.
