
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
PIPERONYL BUTOXIDE
IDENTITY
Chemical name
5-[2-(2-butoxyethoxy)ethoxymethyl]-6-propyl-1,3-benzodioxole
or
3,4-methylenedioxy-6-propylbenzyl n-butyl diethyleneglycol ether
Formula
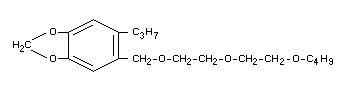 Note
The piperonyl butoxide used in commerce and in the work here reviewed
consists of a technical product containing not less than 80 per cent
of the above chemical together with related compounds which result
from the process of synthesis via the chloromethyl derivative of
dehydrosafrole and the sodium salt of the mono-n-butyl ether of
diethylene glycol.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract with
loss of appetite and prostration (Sarles et al., 1949). It may be that
these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949, Sarles &
Vandergrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandergrift, 1952).
In rats, large subcutaenous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandergrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects, piperonyl
butoxide synergizes pyrethrins by inhibiting lipase (esterase), but
his results were inconclusive.
In an experiment in which 87.6 per cent of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09 per cent was found
in the urine (Sarles & Vandergrift, 1952).
In vitro experiments using purified bovine erythrocyte acetyl
cholinesterase showed that malathion had decreased anti-cholinesterase
activity in the presence of piperonyl butoxide (Rai & Roan, 1956).
Piperonyl butoxide at dose levels of 0.1-1.0 ml per rat given by the
oral, intraperitoneal or intravenous routes retarded the elimination
of intravenously administered 3,4-benzpyrene. Detoxification and
biliary excretion of this carcinogen were also decreased. It was
suggested that the induced hepatic damage may have increased the
retention of the carcinogen (Falk et al., 1965).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 4030 U.S.F.D.A., 1946
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rat Oral 11500 Lehman, 1951
Rat s.c. >15900 Sarles et al., 1949
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of coumaphos and its phosphate by a factor of 4 to 6. There
is some evidence that piperonyl butoxide interferes with
detoxification of the organo-phosphorus insecticides (Robbins et al.,
1959). Apparently no additional toxicity was produced in rats when
one-sixth as much pyrethrin was added to their diet containing
piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandergrift, 1952).
Short-term studies
Mouse. Four groups of Swiss mice were given subcutaneous injections
of tricaprylin solutions containing one of the following compounds:
fluoromethane, tetrachlorodifluoroethane and trichlorotrifluoroethane
in concentrations of 10 per cent.; and piperonyl butoxide in a
concentration of 5 per cent. Two groups of mice were given the
following combinations by subcutaneous injection:
tetrachlorodifluoroethane (10 per cent) plus piperonyl butoxide (5 per
cent); and trichlorodifluoroethane (10 per cent) plus piperonyl
butoxide (5 per cent). The mice received the injections at the ages of
1, 7, 14 and 21 days. In those groups which received piperonyl
butoxide, either alone or in combination, the total dose of piperonyl
butoxide was about 5-10 g/kg body-weight. After 50-52 weeks, the
incidence of hepatomas in the groups which received the individual
compounds was 5 of 126 (about 4 per cent), and the total incidence in
the two groups which received piperonyl butoxide in combination with a
"Freon(R)" was 8 of 33 (about 24 per cent). No influence on the
incidence of malignant lymphomas was seen (Epstein et al., 1966).
Rat. In a 17-week study, a dietary level of 5000 ppm piperonyl
butoxide caused gross and tissue damage to liver (enlargement and
periportal hepatic cell hypertrophy with slight fatty change) and
kidney (renal tubular pigmentation of a "wear and tear" type) (Lehman,
1952 b and c).
Single weekly doses of between 530 and 4240 mg/kg body-weight
administered six times to rats, and of 1060-4240 mg/kg body-weight
administered three times to rabbits showed no effects at autopsy three
weeks after the final dose (Sarles et al., 1949).
A 31-day test in rats showed terminal anorexia. Early deaths were
largely due to damage of ganglionic cells of the brain stem. (Sarles &
Vandergrift, 1952).
Dog. Body-weight gain was reduced compared with controls in dogs
dosed with 32 mg/kg/day for 1 year; dogs dosed with 106 mg/kg/day or
higher lost weight. At 3 mg/kg/day there was a slight increase in
liver weight without gross or microscopic pathology. The kidneys and
adrenals were progressively enlarged at dosages of about 100 mg/kg/day
and above. Microscopic pathology was evident in the liver at dosage
rates of 32 mg/kg/day and over. Hepatoma and carcinoma were not seen
(Sarles & Vandergrift, 1952).
Monkey. At comparable dosage, symptomatology was somewhat less than
in dogs. Microscopical pathology of the liver in monkeys at 100
mg/kg/day was comparable to that in dogs receiving 30 mg/kg/day (a
dosage that produced no observed effect in the monkey). The apparent
difference in the sensitivity of the two species may be explained by
the shorter exposure of the monkey (1 month) compared with the dogs (1
year) (Sarles & Vandergrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide at
high as 1000 ppm caused no decrease in the growth rate of female rats;
concentrations as low as 100 ppm produced some reduction in the growth
rate of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
decreased food consumption (78 per cent of control). A concentration
of 25 000 ppm, reduced food consumption to 37 per cent of control and
stunted the animals. However, in subacute experiments, anorexia was
terminal and therefore not the simple effect of unpalatability of the
food. A concentration of 10 000 ppm caused a distinct increase in
mortality rate in both sexes evident in 2 years and a concentration of
25 000 killed about half the animals in half a year. Only
concentrations of 10 000 ppm or higher produced significant increase
in the relative weight of the liver and kidney. Liver changes were
found at levels of 10 000 ppm and more. Less marked changes occurred
in the kidney and adrenal. Benign or malignant tumours occurred in 30
per cent of the test animals but the authors claimed that their
occurrence was not related to piperonyl butoxide. Reproduction was
decreased by a dietary level of 10 000 ppm and stopped by a
concentration or 25 000 ppm (Sarles & Vandergrift, 1952).
Comments
So far as is known, the colorimetric tests used responded to piperonyl
butoxide only. Thus, the 12 per cent of the single dose administered
to the dog that was unaccounted for (see Biochemical aspects) may
have been the most important from a toxicological standpoint.
From the long-term studies in the rat a level of 100 ppm was without
toxicological effect when compared to the controls.
From the one-year study in the dog the dose of 3 mg/kg body-weight/day
was without toxicological effect.
For future toxicological studies, specifications of the test material
should be stated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per day.
Dog. 3 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.03 mg/kg body-weight
Further work required
Biochemical studies on the qualitative and quantitative aspects of
metabolism of the compound.
Studies on the effect of piperonyl butoxide on the liver of dogs. (For
details see Report of Scientific Group on Procedures for Investigating
Intentional and Unintentional Food Additives - July 1966).
The effects of this compound on reproduction in at leant one more
species.
Long-term feeding studies in another species, with careful observation
to detect any possible tumours. These studies should be done using
piperonyl butoxide alone and in combination with other agents, such as
pyrethrins and freons, with which it might be combined in practice.
Results of the above work should be made available not later than five
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
Piperonyl butoxide has little or no insecticidal activity. Its primary
use is as a synergist for pyrethrins. Piperonyl butoxide may act as a
true synergist, as an antioxidant, as an extender, or as a combination
of any two or all three. In those countries where tolerances have been
established for piperonyl butoxide and pyrethrins, the two materials
are usually formulated, in a ratio of about 10:1 (w/w), respectively.
(a) Pre-harvest treatments
Formulations containing piperonyl butoxide are employed for
controlling insects on growing plants just before harvest and on dairy
and meat animals. They have been used in many countries on growing
bush and vine fruits, deciduous fruits and nuts, forage crops, and on
dairy and meat animals. Malathion and other insecticides have replaced
synergized pyrethrins for some of the above uses and, therefore,
piperonyl butoxide is not used on food prior to harvesting as
extensively now as it was 10 or more years ago.
(b) Post-harvest treatments
Piperonyl butoxide, in combination with pyrethrins, has been used in a
spray or dust formulation on freshly picked fruits and vegetables
after harvest while in the field, in storage, or in processing plants
for the control of drosophila and other insects. It is also used
directly on dried fruits, treenuts, grains and oil seeds as a
protective treatment against insect infestation during storage.
Piperonyl butoxide is usually formulated in a 10:1 ratio with
pyrethrins for application as a spray or dust directly on the food
commodities as they are placed in containers or as they move on a
conveyor into storage. It is used with pyrethrins in water emulsion or
wettable powder formulations as surface sprays on stacked bagged
peanuts and other oil seeds, and on animal feeds. Piperonyl butoxide
is very commonly used in aerosols as space treatments in food
handling, processing and storage facilities.
As with pre-harvest treatments, piperonyl butoxide is not being used
as extensively as it was a number of years ago. Malathion has replaced
synergized pyrethrins in many of the above uses.
(c) Other uses
Piperonyl butoxide (50 mg/sq. ft) in combination with pyrethrins has
been found to be effective in protecting cereal products against
insect attack, when applied to the outside surface of the outer ply of
multiwall paper bags. This treatment is becoming widely used,
particularly in the United States, for cereal products destined for
storage or shipment overseas.
Perhaps the greatest use of piperonyl butoxide is in formulations to
treat farm buildings and food processing, handling, shipping, storage
and marketing facilities. The treatment consists of spraying the
floors, walls, working areas and machinery, applying it in an aerosol
form to the insect infested area, or both. Again, malathion has
replaced synergized pyrethrins for some of these uses.
Piperonyl butoxide is present in most of the pyrethrins formulations
used for household insect control but the ratio of the two compounds
in such formulations varies considerably.
Tolerances (established or considered)
Country Product Parts per million
Brazil cereals 10
Canada grain sorghum 8
barley, buckwheat, corn 20
pop-corn, rice, rye, wheat
Czechoslovakia grain -
Germany grain 15
Italy cereals 20
Netherlands cereal 10
Tolerances (cont'd)
Country Product Parts per million
USA bush and vine fruits exempt
cottonseed
(post-harvest) 8
deciduous fruits and
nuts exempt
flax 8
forage crops exempt
fruits and nuts 8
(post-harvest)
grain (post-harvest) 20
mushrooms exempt
peanuts
(post-harvest 8
vegetables exempt
vegetables
(post-harvest) 8
Residues resulting from supervised trials
No data were available on the fate of piperonyl butoxide residues on
growing crops, and on fruits and vegetables which had received
post-harvest treatments. The bulk of the information available on the
fate of piperonyl butoxide residues has been obtained from
post-harvest application to cereals, cereal products, dried fruits and
dried citrus pulp animal feed. The evaluation of the use of the
insecticide mixture for protecting stored wheat is described by
Walkden and Nelson (1959): an unpublished document from the US
Department of Agriculture also was reviewed by the meeting and
provided the following information:
In order to protect grain from insect attack, piperonyl butoxide, in
combination with pyrethrins, is applied to various grains at rates
equivalent to 14.2 ppm on wheat, 15.2 ppm on shelled corn, 26.7 ppm on
oats, 17.8 ppm on barley, 15.2 ppm on rye and 19.0 ppm on rough rice.
Some 30 per cent or more of the insecticide is normally lost during
application; furthermore, the deterioration is rather rapid during the
first few months after storage.
Two space treatments applied respectively at rates of two and four
ounces of 0.5 per cent piperonyl butoxide in combination with 0.4 per
cent pyrethrins per 1000 cubic feet produced a maximum of piperonyl
butoxide residue of 1.5 ppm on the top 1-1/2 inches of exposed flour.
When dried fruit (apricots, peaches, pears) were exposed to 10
treatments, at two and three day intervals over a period of one month,
with a formulation containing 0.5 per cent of pyrethrins, 1.0 per cent
of piperonyl butoxide, 1.67 per cent of MGK 264(R) and 96.83 per
cent of light petroleum distillate applied at the rate of one gallon
per 50 000 cubic feet of space, the maximum residues obtained were 1.9
ppm of pyrethrins, 7.7 ppm of piperonyl butoxide and 7.2 ppm of MGK
264(R).
Bagged dried citrus animal feed was exposed to weekly treatments over
a three month period to aerosol formulations containing 0.2 per cent
of pyrethrins and two per cent of piperonyl butoxide applied at the
rate of 2.5 pints/1000 cu. ft of total warehouse space. The maximum
piperonyl butoxide residue obtained in the feed during the entire
period was 5.5 ppm. A similar result was obtained with a wettable
powder formulation (Laudani et al. 1959).
Multiwall paper bags with special insect-tight closures and pyrethrins
with piperonyl butoxide (540 mg/m2) applied to the outer surface
have provided effective protection to cereal products against outside
insect infestation during long-term storage period, and the maximum
piperonyl butoxide residues from composite samples were:
Rice - 5.5 ppm, non-fat dried milk 2.5 ppm; dry beans - 0.5 ppm,
flour - 6.0 ppm.
A similar test involving cornmeal stored for six months in bags
treated with pyrethrins and piperonyl butoxide (540 mg/m2) showed a
maximum of piperonyl butoxide in the cornmeal to be 10.7 in 50 lb.
bags and 3.3 ppm in 100 lb. bags.
Residues in food moving in commerce
In 99 samples taken from cargoes shipped from all over the world to
Rotterdam and Amsterdam, piperonyl butoxide was found in 11 samples.
The residues ranged from 0.1 to 1.0 ppm. The limit of the sensitivity
of the method used was 0.5 ppm.
Residues at time of consumption
At the time of preparation of this report no data were available on
the fate of piperonyl butoxide residues on or in fresh fruit,
dried-fruit, tree nuts, fresh and dried vegetables. Information is
available on the fate of piperonyl butoxide on cereals but this is not
as complete as it should be.
In controlled studies the deposit on wheat, from an application dosage
of 13.2 ppm, was shown to be 5.6 ppm in two months and 3.6 ppm in four
months after treatment. On another occasion a theoretical 17.4 ppm was
down to 3 ppm after two months of storage. Since the treatment is used
only on grain going into storage for three months, or longer, it can
be assumed that there would be at least a 50 percent loss of residue.
Other studies have also shown that when treated wheat is milled most
of the remaining residue goes into the screenings and scourings. A
very small percentage of the piperonyl butoxide ends up in the flour
traction. These same studies showed milled fractions of treated corn
had either none or a very small percentage of the piperonyl butoxide
originally present. Cooking reduced the residue from 4.7 to 0.6 ppm.
Methods of residue analysis
The method of Jones, Ackerman and Webster (1952) as modified by
Williams and Sweeney (1956), in which the colour produced on treatment
with tannic acid in phosphoric-acetic acid is measured, is suitable
for the determination of residues of piperonyl butoxide. The clean-up
techniques as appropriate to various foodstuffs are indicated. The
method is in general sensitive to 0.1 ppm piperonyl butoxide. Beroza
(1963) has described a thin-layer chromatographic method which should
be capable of development into a satisfactory technique for many
foodstuffs.
RECOMMENDATIONS FOR TOLERANCES
The primary use of piperonyl butoxide has been as a synergist for
pyrethrins which have been used rather extensively in the past to
protect a variety of foods, raw and processed, against insect
infestation. Malathion and certain other insecticides have proved to
be successful for similar purposes during recent years. Therefore
piperonyl butoxide is probably used less than previously for purposes
that may lead to residues in foods.
Information is lacking on the identity, persistence and effects of the
breakdown products of piperonyl butoxide and the other related
compounds present in piperonyl butoxide concentrates.
Although some information is available on the residues and their fate
on or in cereal and cereal products, there are insufficient data on
residues obtained from good agricultural practices on fresh and dried
fruits, tree nuts, fresh and dried vegetables and peanuts and other
oil seeds. Similarly there are very few data on the actual occurrence
of residues in food in commerce. The above information should be
obtained and considered before tolerances are established. Therefore,
tolerances for piperonyl butoxide at this time should be temporary
and reviewed in three years.
The proposed temporary tolerances are:
Cereal and cereal products 20 ppm
Fresh fruits for canning only 8 ppm
Dried fruits 8 ppm
Tree nuts 8 ppm
Dried vegetables 8 ppm
Peanuts and oil seeds 8 ppm
At these levels, which are considered to be those which might possibly
result from adequately supervised use on the crops in question the
intake would not reach the acceptable daily figure, using the ninth
decile food consumption figures for the United States of America and
disregarding other safeguarding as outlined in Part I of the report on
this meeting.
The need for treatment, with insecticides containing piperonyl
butoxide, of fresh fruits for canning is questionable. If such a need
exists, the countries interested should provide information on the
extent of its use and on the resulting residues.
Further work or information
(a) Further information is desirable on the levels of piperonyl
butoxide obtained on the various foods and the effect of handling,
cleaning, processing and storing.
(b) Research should be conducted to determine the identity,
persistency and toxicological effects of the breakdown products of
piperonyl butoxide and the related compounds present with piperonyl
butoxide.
(c) The analytical methods for piperonyl butoxide should be reviewed.
A faster, more sensitive method is desirable.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Chamberlain, R. W. (1950) Amer. J. Hyg., 52, 153
Epstein, S. S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel,
N. (1966) Unpublished report
Falk, H. L., Thompson, S. J. & Kotin, P. (1965) Arch. Environ.
Health, 10, 847
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952 a) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 3
Lehman, A. J. (1952 b) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1952 c) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Rai, L. & Roan, C. C. (1956) J. econ. Entomol., 49, 591
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ.
Entomol., 52, 660
Sarles, M. P., Dove, W. E. & Moore, D. H. (1949) Amer. J. trop.
Med., 29, 151
Sarles, M. P. & Vandergrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
U.S. Food and Drug Admin., (1946) Unpublished data
REFERENCES PERTINENT TO AGRICULTURAL DATA
Beroza, M. (1963) Identification of 3,4-Methylenedioxyphenyl
Synergists by Thin-layer Chromatography. Agric. Food Chem., 11 (1):
51-53
Jones, H. A., Kerby, G. F. & E. J. Incho. (1952) Insect proofing of
paper. Chemical Specialities Manufacturers Association 38th Mid-Year
Meeting, Boston, Mass., USA
Jones, H. A., Akerman, H. J. & M. E. Webster. (1952) The colorimetric
determination of piperonyl butoxide. J. Assoc. Offic. Agric. Chem.,
35: 771-780
Laudani, H., Gillenwater, H. B., Kantack, B. H. & M. F. Phillips.
(1959) The protection of citrus pulp against insect infestation with
surface applications of pyrethrum - piperonyl butoxide wettable
powder. J. Econ. Ent., 52 (2): 224-7
Walkden, H. H. & H. D. Nelson. (1959) Evaluation of synergized
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Agriculture, Marketing Research Report No.
322
Williams, H. L. & Sweeney, J. P. (1956) Isolation of piperonyl
butoxide from oils, fats and waxes. J. Assoc. Offic. Agric. Food
Chem., 11: 51-54
Note
The piperonyl butoxide used in commerce and in the work here reviewed
consists of a technical product containing not less than 80 per cent
of the above chemical together with related compounds which result
from the process of synthesis via the chloromethyl derivative of
dehydrosafrole and the sodium salt of the mono-n-butyl ether of
diethylene glycol.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract with
loss of appetite and prostration (Sarles et al., 1949). It may be that
these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949, Sarles &
Vandergrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandergrift, 1952).
In rats, large subcutaenous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandergrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects, piperonyl
butoxide synergizes pyrethrins by inhibiting lipase (esterase), but
his results were inconclusive.
In an experiment in which 87.6 per cent of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09 per cent was found
in the urine (Sarles & Vandergrift, 1952).
In vitro experiments using purified bovine erythrocyte acetyl
cholinesterase showed that malathion had decreased anti-cholinesterase
activity in the presence of piperonyl butoxide (Rai & Roan, 1956).
Piperonyl butoxide at dose levels of 0.1-1.0 ml per rat given by the
oral, intraperitoneal or intravenous routes retarded the elimination
of intravenously administered 3,4-benzpyrene. Detoxification and
biliary excretion of this carcinogen were also decreased. It was
suggested that the induced hepatic damage may have increased the
retention of the carcinogen (Falk et al., 1965).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 4030 U.S.F.D.A., 1946
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rat Oral 11500 Lehman, 1951
Rat s.c. >15900 Sarles et al., 1949
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of coumaphos and its phosphate by a factor of 4 to 6. There
is some evidence that piperonyl butoxide interferes with
detoxification of the organo-phosphorus insecticides (Robbins et al.,
1959). Apparently no additional toxicity was produced in rats when
one-sixth as much pyrethrin was added to their diet containing
piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandergrift, 1952).
Short-term studies
Mouse. Four groups of Swiss mice were given subcutaneous injections
of tricaprylin solutions containing one of the following compounds:
fluoromethane, tetrachlorodifluoroethane and trichlorotrifluoroethane
in concentrations of 10 per cent.; and piperonyl butoxide in a
concentration of 5 per cent. Two groups of mice were given the
following combinations by subcutaneous injection:
tetrachlorodifluoroethane (10 per cent) plus piperonyl butoxide (5 per
cent); and trichlorodifluoroethane (10 per cent) plus piperonyl
butoxide (5 per cent). The mice received the injections at the ages of
1, 7, 14 and 21 days. In those groups which received piperonyl
butoxide, either alone or in combination, the total dose of piperonyl
butoxide was about 5-10 g/kg body-weight. After 50-52 weeks, the
incidence of hepatomas in the groups which received the individual
compounds was 5 of 126 (about 4 per cent), and the total incidence in
the two groups which received piperonyl butoxide in combination with a
"Freon(R)" was 8 of 33 (about 24 per cent). No influence on the
incidence of malignant lymphomas was seen (Epstein et al., 1966).
Rat. In a 17-week study, a dietary level of 5000 ppm piperonyl
butoxide caused gross and tissue damage to liver (enlargement and
periportal hepatic cell hypertrophy with slight fatty change) and
kidney (renal tubular pigmentation of a "wear and tear" type) (Lehman,
1952 b and c).
Single weekly doses of between 530 and 4240 mg/kg body-weight
administered six times to rats, and of 1060-4240 mg/kg body-weight
administered three times to rabbits showed no effects at autopsy three
weeks after the final dose (Sarles et al., 1949).
A 31-day test in rats showed terminal anorexia. Early deaths were
largely due to damage of ganglionic cells of the brain stem. (Sarles &
Vandergrift, 1952).
Dog. Body-weight gain was reduced compared with controls in dogs
dosed with 32 mg/kg/day for 1 year; dogs dosed with 106 mg/kg/day or
higher lost weight. At 3 mg/kg/day there was a slight increase in
liver weight without gross or microscopic pathology. The kidneys and
adrenals were progressively enlarged at dosages of about 100 mg/kg/day
and above. Microscopic pathology was evident in the liver at dosage
rates of 32 mg/kg/day and over. Hepatoma and carcinoma were not seen
(Sarles & Vandergrift, 1952).
Monkey. At comparable dosage, symptomatology was somewhat less than
in dogs. Microscopical pathology of the liver in monkeys at 100
mg/kg/day was comparable to that in dogs receiving 30 mg/kg/day (a
dosage that produced no observed effect in the monkey). The apparent
difference in the sensitivity of the two species may be explained by
the shorter exposure of the monkey (1 month) compared with the dogs (1
year) (Sarles & Vandergrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide at
high as 1000 ppm caused no decrease in the growth rate of female rats;
concentrations as low as 100 ppm produced some reduction in the growth
rate of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
decreased food consumption (78 per cent of control). A concentration
of 25 000 ppm, reduced food consumption to 37 per cent of control and
stunted the animals. However, in subacute experiments, anorexia was
terminal and therefore not the simple effect of unpalatability of the
food. A concentration of 10 000 ppm caused a distinct increase in
mortality rate in both sexes evident in 2 years and a concentration of
25 000 killed about half the animals in half a year. Only
concentrations of 10 000 ppm or higher produced significant increase
in the relative weight of the liver and kidney. Liver changes were
found at levels of 10 000 ppm and more. Less marked changes occurred
in the kidney and adrenal. Benign or malignant tumours occurred in 30
per cent of the test animals but the authors claimed that their
occurrence was not related to piperonyl butoxide. Reproduction was
decreased by a dietary level of 10 000 ppm and stopped by a
concentration or 25 000 ppm (Sarles & Vandergrift, 1952).
Comments
So far as is known, the colorimetric tests used responded to piperonyl
butoxide only. Thus, the 12 per cent of the single dose administered
to the dog that was unaccounted for (see Biochemical aspects) may
have been the most important from a toxicological standpoint.
From the long-term studies in the rat a level of 100 ppm was without
toxicological effect when compared to the controls.
From the one-year study in the dog the dose of 3 mg/kg body-weight/day
was without toxicological effect.
For future toxicological studies, specifications of the test material
should be stated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per day.
Dog. 3 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.03 mg/kg body-weight
Further work required
Biochemical studies on the qualitative and quantitative aspects of
metabolism of the compound.
Studies on the effect of piperonyl butoxide on the liver of dogs. (For
details see Report of Scientific Group on Procedures for Investigating
Intentional and Unintentional Food Additives - July 1966).
The effects of this compound on reproduction in at leant one more
species.
Long-term feeding studies in another species, with careful observation
to detect any possible tumours. These studies should be done using
piperonyl butoxide alone and in combination with other agents, such as
pyrethrins and freons, with which it might be combined in practice.
Results of the above work should be made available not later than five
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
Piperonyl butoxide has little or no insecticidal activity. Its primary
use is as a synergist for pyrethrins. Piperonyl butoxide may act as a
true synergist, as an antioxidant, as an extender, or as a combination
of any two or all three. In those countries where tolerances have been
established for piperonyl butoxide and pyrethrins, the two materials
are usually formulated, in a ratio of about 10:1 (w/w), respectively.
(a) Pre-harvest treatments
Formulations containing piperonyl butoxide are employed for
controlling insects on growing plants just before harvest and on dairy
and meat animals. They have been used in many countries on growing
bush and vine fruits, deciduous fruits and nuts, forage crops, and on
dairy and meat animals. Malathion and other insecticides have replaced
synergized pyrethrins for some of the above uses and, therefore,
piperonyl butoxide is not used on food prior to harvesting as
extensively now as it was 10 or more years ago.
(b) Post-harvest treatments
Piperonyl butoxide, in combination with pyrethrins, has been used in a
spray or dust formulation on freshly picked fruits and vegetables
after harvest while in the field, in storage, or in processing plants
for the control of drosophila and other insects. It is also used
directly on dried fruits, treenuts, grains and oil seeds as a
protective treatment against insect infestation during storage.
Piperonyl butoxide is usually formulated in a 10:1 ratio with
pyrethrins for application as a spray or dust directly on the food
commodities as they are placed in containers or as they move on a
conveyor into storage. It is used with pyrethrins in water emulsion or
wettable powder formulations as surface sprays on stacked bagged
peanuts and other oil seeds, and on animal feeds. Piperonyl butoxide
is very commonly used in aerosols as space treatments in food
handling, processing and storage facilities.
As with pre-harvest treatments, piperonyl butoxide is not being used
as extensively as it was a number of years ago. Malathion has replaced
synergized pyrethrins in many of the above uses.
(c) Other uses
Piperonyl butoxide (50 mg/sq. ft) in combination with pyrethrins has
been found to be effective in protecting cereal products against
insect attack, when applied to the outside surface of the outer ply of
multiwall paper bags. This treatment is becoming widely used,
particularly in the United States, for cereal products destined for
storage or shipment overseas.
Perhaps the greatest use of piperonyl butoxide is in formulations to
treat farm buildings and food processing, handling, shipping, storage
and marketing facilities. The treatment consists of spraying the
floors, walls, working areas and machinery, applying it in an aerosol
form to the insect infested area, or both. Again, malathion has
replaced synergized pyrethrins for some of these uses.
Piperonyl butoxide is present in most of the pyrethrins formulations
used for household insect control but the ratio of the two compounds
in such formulations varies considerably.
Tolerances (established or considered)
Country Product Parts per million
Brazil cereals 10
Canada grain sorghum 8
barley, buckwheat, corn 20
pop-corn, rice, rye, wheat
Czechoslovakia grain -
Germany grain 15
Italy cereals 20
Netherlands cereal 10
Tolerances (cont'd)
Country Product Parts per million
USA bush and vine fruits exempt
cottonseed
(post-harvest) 8
deciduous fruits and
nuts exempt
flax 8
forage crops exempt
fruits and nuts 8
(post-harvest)
grain (post-harvest) 20
mushrooms exempt
peanuts
(post-harvest 8
vegetables exempt
vegetables
(post-harvest) 8
Residues resulting from supervised trials
No data were available on the fate of piperonyl butoxide residues on
growing crops, and on fruits and vegetables which had received
post-harvest treatments. The bulk of the information available on the
fate of piperonyl butoxide residues has been obtained from
post-harvest application to cereals, cereal products, dried fruits and
dried citrus pulp animal feed. The evaluation of the use of the
insecticide mixture for protecting stored wheat is described by
Walkden and Nelson (1959): an unpublished document from the US
Department of Agriculture also was reviewed by the meeting and
provided the following information:
In order to protect grain from insect attack, piperonyl butoxide, in
combination with pyrethrins, is applied to various grains at rates
equivalent to 14.2 ppm on wheat, 15.2 ppm on shelled corn, 26.7 ppm on
oats, 17.8 ppm on barley, 15.2 ppm on rye and 19.0 ppm on rough rice.
Some 30 per cent or more of the insecticide is normally lost during
application; furthermore, the deterioration is rather rapid during the
first few months after storage.
Two space treatments applied respectively at rates of two and four
ounces of 0.5 per cent piperonyl butoxide in combination with 0.4 per
cent pyrethrins per 1000 cubic feet produced a maximum of piperonyl
butoxide residue of 1.5 ppm on the top 1-1/2 inches of exposed flour.
When dried fruit (apricots, peaches, pears) were exposed to 10
treatments, at two and three day intervals over a period of one month,
with a formulation containing 0.5 per cent of pyrethrins, 1.0 per cent
of piperonyl butoxide, 1.67 per cent of MGK 264(R) and 96.83 per
cent of light petroleum distillate applied at the rate of one gallon
per 50 000 cubic feet of space, the maximum residues obtained were 1.9
ppm of pyrethrins, 7.7 ppm of piperonyl butoxide and 7.2 ppm of MGK
264(R).
Bagged dried citrus animal feed was exposed to weekly treatments over
a three month period to aerosol formulations containing 0.2 per cent
of pyrethrins and two per cent of piperonyl butoxide applied at the
rate of 2.5 pints/1000 cu. ft of total warehouse space. The maximum
piperonyl butoxide residue obtained in the feed during the entire
period was 5.5 ppm. A similar result was obtained with a wettable
powder formulation (Laudani et al. 1959).
Multiwall paper bags with special insect-tight closures and pyrethrins
with piperonyl butoxide (540 mg/m2) applied to the outer surface
have provided effective protection to cereal products against outside
insect infestation during long-term storage period, and the maximum
piperonyl butoxide residues from composite samples were:
Rice - 5.5 ppm, non-fat dried milk 2.5 ppm; dry beans - 0.5 ppm,
flour - 6.0 ppm.
A similar test involving cornmeal stored for six months in bags
treated with pyrethrins and piperonyl butoxide (540 mg/m2) showed a
maximum of piperonyl butoxide in the cornmeal to be 10.7 in 50 lb.
bags and 3.3 ppm in 100 lb. bags.
Residues in food moving in commerce
In 99 samples taken from cargoes shipped from all over the world to
Rotterdam and Amsterdam, piperonyl butoxide was found in 11 samples.
The residues ranged from 0.1 to 1.0 ppm. The limit of the sensitivity
of the method used was 0.5 ppm.
Residues at time of consumption
At the time of preparation of this report no data were available on
the fate of piperonyl butoxide residues on or in fresh fruit,
dried-fruit, tree nuts, fresh and dried vegetables. Information is
available on the fate of piperonyl butoxide on cereals but this is not
as complete as it should be.
In controlled studies the deposit on wheat, from an application dosage
of 13.2 ppm, was shown to be 5.6 ppm in two months and 3.6 ppm in four
months after treatment. On another occasion a theoretical 17.4 ppm was
down to 3 ppm after two months of storage. Since the treatment is used
only on grain going into storage for three months, or longer, it can
be assumed that there would be at least a 50 percent loss of residue.
Other studies have also shown that when treated wheat is milled most
of the remaining residue goes into the screenings and scourings. A
very small percentage of the piperonyl butoxide ends up in the flour
traction. These same studies showed milled fractions of treated corn
had either none or a very small percentage of the piperonyl butoxide
originally present. Cooking reduced the residue from 4.7 to 0.6 ppm.
Methods of residue analysis
The method of Jones, Ackerman and Webster (1952) as modified by
Williams and Sweeney (1956), in which the colour produced on treatment
with tannic acid in phosphoric-acetic acid is measured, is suitable
for the determination of residues of piperonyl butoxide. The clean-up
techniques as appropriate to various foodstuffs are indicated. The
method is in general sensitive to 0.1 ppm piperonyl butoxide. Beroza
(1963) has described a thin-layer chromatographic method which should
be capable of development into a satisfactory technique for many
foodstuffs.
RECOMMENDATIONS FOR TOLERANCES
The primary use of piperonyl butoxide has been as a synergist for
pyrethrins which have been used rather extensively in the past to
protect a variety of foods, raw and processed, against insect
infestation. Malathion and certain other insecticides have proved to
be successful for similar purposes during recent years. Therefore
piperonyl butoxide is probably used less than previously for purposes
that may lead to residues in foods.
Information is lacking on the identity, persistence and effects of the
breakdown products of piperonyl butoxide and the other related
compounds present in piperonyl butoxide concentrates.
Although some information is available on the residues and their fate
on or in cereal and cereal products, there are insufficient data on
residues obtained from good agricultural practices on fresh and dried
fruits, tree nuts, fresh and dried vegetables and peanuts and other
oil seeds. Similarly there are very few data on the actual occurrence
of residues in food in commerce. The above information should be
obtained and considered before tolerances are established. Therefore,
tolerances for piperonyl butoxide at this time should be temporary
and reviewed in three years.
The proposed temporary tolerances are:
Cereal and cereal products 20 ppm
Fresh fruits for canning only 8 ppm
Dried fruits 8 ppm
Tree nuts 8 ppm
Dried vegetables 8 ppm
Peanuts and oil seeds 8 ppm
At these levels, which are considered to be those which might possibly
result from adequately supervised use on the crops in question the
intake would not reach the acceptable daily figure, using the ninth
decile food consumption figures for the United States of America and
disregarding other safeguarding as outlined in Part I of the report on
this meeting.
The need for treatment, with insecticides containing piperonyl
butoxide, of fresh fruits for canning is questionable. If such a need
exists, the countries interested should provide information on the
extent of its use and on the resulting residues.
Further work or information
(a) Further information is desirable on the levels of piperonyl
butoxide obtained on the various foods and the effect of handling,
cleaning, processing and storing.
(b) Research should be conducted to determine the identity,
persistency and toxicological effects of the breakdown products of
piperonyl butoxide and the related compounds present with piperonyl
butoxide.
(c) The analytical methods for piperonyl butoxide should be reviewed.
A faster, more sensitive method is desirable.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Chamberlain, R. W. (1950) Amer. J. Hyg., 52, 153
Epstein, S. S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel,
N. (1966) Unpublished report
Falk, H. L., Thompson, S. J. & Kotin, P. (1965) Arch. Environ.
Health, 10, 847
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952 a) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 3
Lehman, A. J. (1952 b) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1952 c) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Rai, L. & Roan, C. C. (1956) J. econ. Entomol., 49, 591
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ.
Entomol., 52, 660
Sarles, M. P., Dove, W. E. & Moore, D. H. (1949) Amer. J. trop.
Med., 29, 151
Sarles, M. P. & Vandergrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
U.S. Food and Drug Admin., (1946) Unpublished data
REFERENCES PERTINENT TO AGRICULTURAL DATA
Beroza, M. (1963) Identification of 3,4-Methylenedioxyphenyl
Synergists by Thin-layer Chromatography. Agric. Food Chem., 11 (1):
51-53
Jones, H. A., Kerby, G. F. & E. J. Incho. (1952) Insect proofing of
paper. Chemical Specialities Manufacturers Association 38th Mid-Year
Meeting, Boston, Mass., USA
Jones, H. A., Akerman, H. J. & M. E. Webster. (1952) The colorimetric
determination of piperonyl butoxide. J. Assoc. Offic. Agric. Chem.,
35: 771-780
Laudani, H., Gillenwater, H. B., Kantack, B. H. & M. F. Phillips.
(1959) The protection of citrus pulp against insect infestation with
surface applications of pyrethrum - piperonyl butoxide wettable
powder. J. Econ. Ent., 52 (2): 224-7
Walkden, H. H. & H. D. Nelson. (1959) Evaluation of synergized
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Agriculture, Marketing Research Report No.
322
Williams, H. L. & Sweeney, J. P. (1956) Isolation of piperonyl
butoxide from oils, fats and waxes. J. Assoc. Offic. Agric. Food
Chem., 11: 51-54
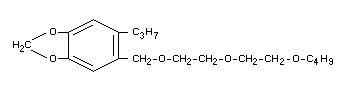 Note
The piperonyl butoxide used in commerce and in the work here reviewed
consists of a technical product containing not less than 80 per cent
of the above chemical together with related compounds which result
from the process of synthesis via the chloromethyl derivative of
dehydrosafrole and the sodium salt of the mono-n-butyl ether of
diethylene glycol.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract with
loss of appetite and prostration (Sarles et al., 1949). It may be that
these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949, Sarles &
Vandergrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandergrift, 1952).
In rats, large subcutaenous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandergrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects, piperonyl
butoxide synergizes pyrethrins by inhibiting lipase (esterase), but
his results were inconclusive.
In an experiment in which 87.6 per cent of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09 per cent was found
in the urine (Sarles & Vandergrift, 1952).
In vitro experiments using purified bovine erythrocyte acetyl
cholinesterase showed that malathion had decreased anti-cholinesterase
activity in the presence of piperonyl butoxide (Rai & Roan, 1956).
Piperonyl butoxide at dose levels of 0.1-1.0 ml per rat given by the
oral, intraperitoneal or intravenous routes retarded the elimination
of intravenously administered 3,4-benzpyrene. Detoxification and
biliary excretion of this carcinogen were also decreased. It was
suggested that the induced hepatic damage may have increased the
retention of the carcinogen (Falk et al., 1965).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 4030 U.S.F.D.A., 1946
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rat Oral 11500 Lehman, 1951
Rat s.c. >15900 Sarles et al., 1949
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of coumaphos and its phosphate by a factor of 4 to 6. There
is some evidence that piperonyl butoxide interferes with
detoxification of the organo-phosphorus insecticides (Robbins et al.,
1959). Apparently no additional toxicity was produced in rats when
one-sixth as much pyrethrin was added to their diet containing
piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandergrift, 1952).
Short-term studies
Mouse. Four groups of Swiss mice were given subcutaneous injections
of tricaprylin solutions containing one of the following compounds:
fluoromethane, tetrachlorodifluoroethane and trichlorotrifluoroethane
in concentrations of 10 per cent.; and piperonyl butoxide in a
concentration of 5 per cent. Two groups of mice were given the
following combinations by subcutaneous injection:
tetrachlorodifluoroethane (10 per cent) plus piperonyl butoxide (5 per
cent); and trichlorodifluoroethane (10 per cent) plus piperonyl
butoxide (5 per cent). The mice received the injections at the ages of
1, 7, 14 and 21 days. In those groups which received piperonyl
butoxide, either alone or in combination, the total dose of piperonyl
butoxide was about 5-10 g/kg body-weight. After 50-52 weeks, the
incidence of hepatomas in the groups which received the individual
compounds was 5 of 126 (about 4 per cent), and the total incidence in
the two groups which received piperonyl butoxide in combination with a
"Freon(R)" was 8 of 33 (about 24 per cent). No influence on the
incidence of malignant lymphomas was seen (Epstein et al., 1966).
Rat. In a 17-week study, a dietary level of 5000 ppm piperonyl
butoxide caused gross and tissue damage to liver (enlargement and
periportal hepatic cell hypertrophy with slight fatty change) and
kidney (renal tubular pigmentation of a "wear and tear" type) (Lehman,
1952 b and c).
Single weekly doses of between 530 and 4240 mg/kg body-weight
administered six times to rats, and of 1060-4240 mg/kg body-weight
administered three times to rabbits showed no effects at autopsy three
weeks after the final dose (Sarles et al., 1949).
A 31-day test in rats showed terminal anorexia. Early deaths were
largely due to damage of ganglionic cells of the brain stem. (Sarles &
Vandergrift, 1952).
Dog. Body-weight gain was reduced compared with controls in dogs
dosed with 32 mg/kg/day for 1 year; dogs dosed with 106 mg/kg/day or
higher lost weight. At 3 mg/kg/day there was a slight increase in
liver weight without gross or microscopic pathology. The kidneys and
adrenals were progressively enlarged at dosages of about 100 mg/kg/day
and above. Microscopic pathology was evident in the liver at dosage
rates of 32 mg/kg/day and over. Hepatoma and carcinoma were not seen
(Sarles & Vandergrift, 1952).
Monkey. At comparable dosage, symptomatology was somewhat less than
in dogs. Microscopical pathology of the liver in monkeys at 100
mg/kg/day was comparable to that in dogs receiving 30 mg/kg/day (a
dosage that produced no observed effect in the monkey). The apparent
difference in the sensitivity of the two species may be explained by
the shorter exposure of the monkey (1 month) compared with the dogs (1
year) (Sarles & Vandergrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide at
high as 1000 ppm caused no decrease in the growth rate of female rats;
concentrations as low as 100 ppm produced some reduction in the growth
rate of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
decreased food consumption (78 per cent of control). A concentration
of 25 000 ppm, reduced food consumption to 37 per cent of control and
stunted the animals. However, in subacute experiments, anorexia was
terminal and therefore not the simple effect of unpalatability of the
food. A concentration of 10 000 ppm caused a distinct increase in
mortality rate in both sexes evident in 2 years and a concentration of
25 000 killed about half the animals in half a year. Only
concentrations of 10 000 ppm or higher produced significant increase
in the relative weight of the liver and kidney. Liver changes were
found at levels of 10 000 ppm and more. Less marked changes occurred
in the kidney and adrenal. Benign or malignant tumours occurred in 30
per cent of the test animals but the authors claimed that their
occurrence was not related to piperonyl butoxide. Reproduction was
decreased by a dietary level of 10 000 ppm and stopped by a
concentration or 25 000 ppm (Sarles & Vandergrift, 1952).
Comments
So far as is known, the colorimetric tests used responded to piperonyl
butoxide only. Thus, the 12 per cent of the single dose administered
to the dog that was unaccounted for (see Biochemical aspects) may
have been the most important from a toxicological standpoint.
From the long-term studies in the rat a level of 100 ppm was without
toxicological effect when compared to the controls.
From the one-year study in the dog the dose of 3 mg/kg body-weight/day
was without toxicological effect.
For future toxicological studies, specifications of the test material
should be stated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per day.
Dog. 3 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.03 mg/kg body-weight
Further work required
Biochemical studies on the qualitative and quantitative aspects of
metabolism of the compound.
Studies on the effect of piperonyl butoxide on the liver of dogs. (For
details see Report of Scientific Group on Procedures for Investigating
Intentional and Unintentional Food Additives - July 1966).
The effects of this compound on reproduction in at leant one more
species.
Long-term feeding studies in another species, with careful observation
to detect any possible tumours. These studies should be done using
piperonyl butoxide alone and in combination with other agents, such as
pyrethrins and freons, with which it might be combined in practice.
Results of the above work should be made available not later than five
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
Piperonyl butoxide has little or no insecticidal activity. Its primary
use is as a synergist for pyrethrins. Piperonyl butoxide may act as a
true synergist, as an antioxidant, as an extender, or as a combination
of any two or all three. In those countries where tolerances have been
established for piperonyl butoxide and pyrethrins, the two materials
are usually formulated, in a ratio of about 10:1 (w/w), respectively.
(a) Pre-harvest treatments
Formulations containing piperonyl butoxide are employed for
controlling insects on growing plants just before harvest and on dairy
and meat animals. They have been used in many countries on growing
bush and vine fruits, deciduous fruits and nuts, forage crops, and on
dairy and meat animals. Malathion and other insecticides have replaced
synergized pyrethrins for some of the above uses and, therefore,
piperonyl butoxide is not used on food prior to harvesting as
extensively now as it was 10 or more years ago.
(b) Post-harvest treatments
Piperonyl butoxide, in combination with pyrethrins, has been used in a
spray or dust formulation on freshly picked fruits and vegetables
after harvest while in the field, in storage, or in processing plants
for the control of drosophila and other insects. It is also used
directly on dried fruits, treenuts, grains and oil seeds as a
protective treatment against insect infestation during storage.
Piperonyl butoxide is usually formulated in a 10:1 ratio with
pyrethrins for application as a spray or dust directly on the food
commodities as they are placed in containers or as they move on a
conveyor into storage. It is used with pyrethrins in water emulsion or
wettable powder formulations as surface sprays on stacked bagged
peanuts and other oil seeds, and on animal feeds. Piperonyl butoxide
is very commonly used in aerosols as space treatments in food
handling, processing and storage facilities.
As with pre-harvest treatments, piperonyl butoxide is not being used
as extensively as it was a number of years ago. Malathion has replaced
synergized pyrethrins in many of the above uses.
(c) Other uses
Piperonyl butoxide (50 mg/sq. ft) in combination with pyrethrins has
been found to be effective in protecting cereal products against
insect attack, when applied to the outside surface of the outer ply of
multiwall paper bags. This treatment is becoming widely used,
particularly in the United States, for cereal products destined for
storage or shipment overseas.
Perhaps the greatest use of piperonyl butoxide is in formulations to
treat farm buildings and food processing, handling, shipping, storage
and marketing facilities. The treatment consists of spraying the
floors, walls, working areas and machinery, applying it in an aerosol
form to the insect infested area, or both. Again, malathion has
replaced synergized pyrethrins for some of these uses.
Piperonyl butoxide is present in most of the pyrethrins formulations
used for household insect control but the ratio of the two compounds
in such formulations varies considerably.
Tolerances (established or considered)
Country Product Parts per million
Brazil cereals 10
Canada grain sorghum 8
barley, buckwheat, corn 20
pop-corn, rice, rye, wheat
Czechoslovakia grain -
Germany grain 15
Italy cereals 20
Netherlands cereal 10
Tolerances (cont'd)
Country Product Parts per million
USA bush and vine fruits exempt
cottonseed
(post-harvest) 8
deciduous fruits and
nuts exempt
flax 8
forage crops exempt
fruits and nuts 8
(post-harvest)
grain (post-harvest) 20
mushrooms exempt
peanuts
(post-harvest 8
vegetables exempt
vegetables
(post-harvest) 8
Residues resulting from supervised trials
No data were available on the fate of piperonyl butoxide residues on
growing crops, and on fruits and vegetables which had received
post-harvest treatments. The bulk of the information available on the
fate of piperonyl butoxide residues has been obtained from
post-harvest application to cereals, cereal products, dried fruits and
dried citrus pulp animal feed. The evaluation of the use of the
insecticide mixture for protecting stored wheat is described by
Walkden and Nelson (1959): an unpublished document from the US
Department of Agriculture also was reviewed by the meeting and
provided the following information:
In order to protect grain from insect attack, piperonyl butoxide, in
combination with pyrethrins, is applied to various grains at rates
equivalent to 14.2 ppm on wheat, 15.2 ppm on shelled corn, 26.7 ppm on
oats, 17.8 ppm on barley, 15.2 ppm on rye and 19.0 ppm on rough rice.
Some 30 per cent or more of the insecticide is normally lost during
application; furthermore, the deterioration is rather rapid during the
first few months after storage.
Two space treatments applied respectively at rates of two and four
ounces of 0.5 per cent piperonyl butoxide in combination with 0.4 per
cent pyrethrins per 1000 cubic feet produced a maximum of piperonyl
butoxide residue of 1.5 ppm on the top 1-1/2 inches of exposed flour.
When dried fruit (apricots, peaches, pears) were exposed to 10
treatments, at two and three day intervals over a period of one month,
with a formulation containing 0.5 per cent of pyrethrins, 1.0 per cent
of piperonyl butoxide, 1.67 per cent of MGK 264(R) and 96.83 per
cent of light petroleum distillate applied at the rate of one gallon
per 50 000 cubic feet of space, the maximum residues obtained were 1.9
ppm of pyrethrins, 7.7 ppm of piperonyl butoxide and 7.2 ppm of MGK
264(R).
Bagged dried citrus animal feed was exposed to weekly treatments over
a three month period to aerosol formulations containing 0.2 per cent
of pyrethrins and two per cent of piperonyl butoxide applied at the
rate of 2.5 pints/1000 cu. ft of total warehouse space. The maximum
piperonyl butoxide residue obtained in the feed during the entire
period was 5.5 ppm. A similar result was obtained with a wettable
powder formulation (Laudani et al. 1959).
Multiwall paper bags with special insect-tight closures and pyrethrins
with piperonyl butoxide (540 mg/m2) applied to the outer surface
have provided effective protection to cereal products against outside
insect infestation during long-term storage period, and the maximum
piperonyl butoxide residues from composite samples were:
Rice - 5.5 ppm, non-fat dried milk 2.5 ppm; dry beans - 0.5 ppm,
flour - 6.0 ppm.
A similar test involving cornmeal stored for six months in bags
treated with pyrethrins and piperonyl butoxide (540 mg/m2) showed a
maximum of piperonyl butoxide in the cornmeal to be 10.7 in 50 lb.
bags and 3.3 ppm in 100 lb. bags.
Residues in food moving in commerce
In 99 samples taken from cargoes shipped from all over the world to
Rotterdam and Amsterdam, piperonyl butoxide was found in 11 samples.
The residues ranged from 0.1 to 1.0 ppm. The limit of the sensitivity
of the method used was 0.5 ppm.
Residues at time of consumption
At the time of preparation of this report no data were available on
the fate of piperonyl butoxide residues on or in fresh fruit,
dried-fruit, tree nuts, fresh and dried vegetables. Information is
available on the fate of piperonyl butoxide on cereals but this is not
as complete as it should be.
In controlled studies the deposit on wheat, from an application dosage
of 13.2 ppm, was shown to be 5.6 ppm in two months and 3.6 ppm in four
months after treatment. On another occasion a theoretical 17.4 ppm was
down to 3 ppm after two months of storage. Since the treatment is used
only on grain going into storage for three months, or longer, it can
be assumed that there would be at least a 50 percent loss of residue.
Other studies have also shown that when treated wheat is milled most
of the remaining residue goes into the screenings and scourings. A
very small percentage of the piperonyl butoxide ends up in the flour
traction. These same studies showed milled fractions of treated corn
had either none or a very small percentage of the piperonyl butoxide
originally present. Cooking reduced the residue from 4.7 to 0.6 ppm.
Methods of residue analysis
The method of Jones, Ackerman and Webster (1952) as modified by
Williams and Sweeney (1956), in which the colour produced on treatment
with tannic acid in phosphoric-acetic acid is measured, is suitable
for the determination of residues of piperonyl butoxide. The clean-up
techniques as appropriate to various foodstuffs are indicated. The
method is in general sensitive to 0.1 ppm piperonyl butoxide. Beroza
(1963) has described a thin-layer chromatographic method which should
be capable of development into a satisfactory technique for many
foodstuffs.
RECOMMENDATIONS FOR TOLERANCES
The primary use of piperonyl butoxide has been as a synergist for
pyrethrins which have been used rather extensively in the past to
protect a variety of foods, raw and processed, against insect
infestation. Malathion and certain other insecticides have proved to
be successful for similar purposes during recent years. Therefore
piperonyl butoxide is probably used less than previously for purposes
that may lead to residues in foods.
Information is lacking on the identity, persistence and effects of the
breakdown products of piperonyl butoxide and the other related
compounds present in piperonyl butoxide concentrates.
Although some information is available on the residues and their fate
on or in cereal and cereal products, there are insufficient data on
residues obtained from good agricultural practices on fresh and dried
fruits, tree nuts, fresh and dried vegetables and peanuts and other
oil seeds. Similarly there are very few data on the actual occurrence
of residues in food in commerce. The above information should be
obtained and considered before tolerances are established. Therefore,
tolerances for piperonyl butoxide at this time should be temporary
and reviewed in three years.
The proposed temporary tolerances are:
Cereal and cereal products 20 ppm
Fresh fruits for canning only 8 ppm
Dried fruits 8 ppm
Tree nuts 8 ppm
Dried vegetables 8 ppm
Peanuts and oil seeds 8 ppm
At these levels, which are considered to be those which might possibly
result from adequately supervised use on the crops in question the
intake would not reach the acceptable daily figure, using the ninth
decile food consumption figures for the United States of America and
disregarding other safeguarding as outlined in Part I of the report on
this meeting.
The need for treatment, with insecticides containing piperonyl
butoxide, of fresh fruits for canning is questionable. If such a need
exists, the countries interested should provide information on the
extent of its use and on the resulting residues.
Further work or information
(a) Further information is desirable on the levels of piperonyl
butoxide obtained on the various foods and the effect of handling,
cleaning, processing and storing.
(b) Research should be conducted to determine the identity,
persistency and toxicological effects of the breakdown products of
piperonyl butoxide and the related compounds present with piperonyl
butoxide.
(c) The analytical methods for piperonyl butoxide should be reviewed.
A faster, more sensitive method is desirable.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Chamberlain, R. W. (1950) Amer. J. Hyg., 52, 153
Epstein, S. S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel,
N. (1966) Unpublished report
Falk, H. L., Thompson, S. J. & Kotin, P. (1965) Arch. Environ.
Health, 10, 847
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952 a) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 3
Lehman, A. J. (1952 b) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1952 c) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Rai, L. & Roan, C. C. (1956) J. econ. Entomol., 49, 591
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ.
Entomol., 52, 660
Sarles, M. P., Dove, W. E. & Moore, D. H. (1949) Amer. J. trop.
Med., 29, 151
Sarles, M. P. & Vandergrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
U.S. Food and Drug Admin., (1946) Unpublished data
REFERENCES PERTINENT TO AGRICULTURAL DATA
Beroza, M. (1963) Identification of 3,4-Methylenedioxyphenyl
Synergists by Thin-layer Chromatography. Agric. Food Chem., 11 (1):
51-53
Jones, H. A., Kerby, G. F. & E. J. Incho. (1952) Insect proofing of
paper. Chemical Specialities Manufacturers Association 38th Mid-Year
Meeting, Boston, Mass., USA
Jones, H. A., Akerman, H. J. & M. E. Webster. (1952) The colorimetric
determination of piperonyl butoxide. J. Assoc. Offic. Agric. Chem.,
35: 771-780
Laudani, H., Gillenwater, H. B., Kantack, B. H. & M. F. Phillips.
(1959) The protection of citrus pulp against insect infestation with
surface applications of pyrethrum - piperonyl butoxide wettable
powder. J. Econ. Ent., 52 (2): 224-7
Walkden, H. H. & H. D. Nelson. (1959) Evaluation of synergized
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Agriculture, Marketing Research Report No.
322
Williams, H. L. & Sweeney, J. P. (1956) Isolation of piperonyl
butoxide from oils, fats and waxes. J. Assoc. Offic. Agric. Food
Chem., 11: 51-54
Note
The piperonyl butoxide used in commerce and in the work here reviewed
consists of a technical product containing not less than 80 per cent
of the above chemical together with related compounds which result
from the process of synthesis via the chloromethyl derivative of
dehydrosafrole and the sodium salt of the mono-n-butyl ether of
diethylene glycol.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
High oral doses produce haemorrhage into the intestinal tract with
loss of appetite and prostration (Sarles et al., 1949). It may be that
these are the effects of local irritation and that the
hyperexcitability and convulsions produced by large dermal doses
(Lehman, 1952) are more indicative of the action of the absorbed drug.
The compound produces liver injury (Sarles et al., 1949, Sarles &
Vandergrift, 1952), and at least in dogs, and in rats at high dosage
levels, liver injury was recognized as the cause of death (Sarles &
Vandergrift, 1952).
In rats, large subcutaenous doses produce an increased bleeding
tendency and "rusty" (bloody) urine (Sarles et al., 1949). Massive
bleeding was found in some animals at autopsy (Sarles & Vandergrift,
1952).
Chamberlain (1950) explored the hypothesis that, in insects, piperonyl
butoxide synergizes pyrethrins by inhibiting lipase (esterase), but
his results were inconclusive.
In an experiment in which 87.6 per cent of a large dose given to a dog
was recovered (chiefly from the faeces), only 0.09 per cent was found
in the urine (Sarles & Vandergrift, 1952).
In vitro experiments using purified bovine erythrocyte acetyl
cholinesterase showed that malathion had decreased anti-cholinesterase
activity in the presence of piperonyl butoxide (Rai & Roan, 1956).
Piperonyl butoxide at dose levels of 0.1-1.0 ml per rat given by the
oral, intraperitoneal or intravenous routes retarded the elimination
of intravenously administered 3,4-benzpyrene. Detoxification and
biliary excretion of this carcinogen were also decreased. It was
suggested that the induced hepatic damage may have increased the
retention of the carcinogen (Falk et al., 1965).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 4030 U.S.F.D.A., 1946
Rat Oral 7960-10600 Sarles et al., 1949
Rat Oral 13500 Lehman, 1948
Rat Oral 11500 Lehman, 1951
Rat s.c. >15900 Sarles et al., 1949
Rabbit Oral 2650-5300 Sarles et al., 1949
Cat Oral >10600 Sarles et al., 1949
Dog Oral >7950 Sarles et al., 1949
Simultaneous administration of piperonyl butoxide potentiates the
toxicity of coumaphos and its phosphate by a factor of 4 to 6. There
is some evidence that piperonyl butoxide interferes with
detoxification of the organo-phosphorus insecticides (Robbins et al.,
1959). Apparently no additional toxicity was produced in rats when
one-sixth as much pyrethrin was added to their diet containing
piperonyl butoxide at a concentration of 1000 ppm (Sarles &
Vandergrift, 1952).
Short-term studies
Mouse. Four groups of Swiss mice were given subcutaneous injections
of tricaprylin solutions containing one of the following compounds:
fluoromethane, tetrachlorodifluoroethane and trichlorotrifluoroethane
in concentrations of 10 per cent.; and piperonyl butoxide in a
concentration of 5 per cent. Two groups of mice were given the
following combinations by subcutaneous injection:
tetrachlorodifluoroethane (10 per cent) plus piperonyl butoxide (5 per
cent); and trichlorodifluoroethane (10 per cent) plus piperonyl
butoxide (5 per cent). The mice received the injections at the ages of
1, 7, 14 and 21 days. In those groups which received piperonyl
butoxide, either alone or in combination, the total dose of piperonyl
butoxide was about 5-10 g/kg body-weight. After 50-52 weeks, the
incidence of hepatomas in the groups which received the individual
compounds was 5 of 126 (about 4 per cent), and the total incidence in
the two groups which received piperonyl butoxide in combination with a
"Freon(R)" was 8 of 33 (about 24 per cent). No influence on the
incidence of malignant lymphomas was seen (Epstein et al., 1966).
Rat. In a 17-week study, a dietary level of 5000 ppm piperonyl
butoxide caused gross and tissue damage to liver (enlargement and
periportal hepatic cell hypertrophy with slight fatty change) and
kidney (renal tubular pigmentation of a "wear and tear" type) (Lehman,
1952 b and c).
Single weekly doses of between 530 and 4240 mg/kg body-weight
administered six times to rats, and of 1060-4240 mg/kg body-weight
administered three times to rabbits showed no effects at autopsy three
weeks after the final dose (Sarles et al., 1949).
A 31-day test in rats showed terminal anorexia. Early deaths were
largely due to damage of ganglionic cells of the brain stem. (Sarles &
Vandergrift, 1952).
Dog. Body-weight gain was reduced compared with controls in dogs
dosed with 32 mg/kg/day for 1 year; dogs dosed with 106 mg/kg/day or
higher lost weight. At 3 mg/kg/day there was a slight increase in
liver weight without gross or microscopic pathology. The kidneys and
adrenals were progressively enlarged at dosages of about 100 mg/kg/day
and above. Microscopic pathology was evident in the liver at dosage
rates of 32 mg/kg/day and over. Hepatoma and carcinoma were not seen
(Sarles & Vandergrift, 1952).
Monkey. At comparable dosage, symptomatology was somewhat less than
in dogs. Microscopical pathology of the liver in monkeys at 100
mg/kg/day was comparable to that in dogs receiving 30 mg/kg/day (a
dosage that produced no observed effect in the monkey). The apparent
difference in the sensitivity of the two species may be explained by
the shorter exposure of the monkey (1 month) compared with the dogs (1
year) (Sarles & Vandergrift, 1952).
Long-term studies
Rat. In two-year studies, concentrations of piperonyl butoxide at
high as 1000 ppm caused no decrease in the growth rate of female rats;
concentrations as low as 100 ppm produced some reduction in the growth
rate of males, but the difference was not considered significant. A
concentration of 10 000 ppm caused a significant reduction in the
growth rate of both sexes that was accounted for, at least in part, by
decreased food consumption (78 per cent of control). A concentration
of 25 000 ppm, reduced food consumption to 37 per cent of control and
stunted the animals. However, in subacute experiments, anorexia was
terminal and therefore not the simple effect of unpalatability of the
food. A concentration of 10 000 ppm caused a distinct increase in
mortality rate in both sexes evident in 2 years and a concentration of
25 000 killed about half the animals in half a year. Only
concentrations of 10 000 ppm or higher produced significant increase
in the relative weight of the liver and kidney. Liver changes were
found at levels of 10 000 ppm and more. Less marked changes occurred
in the kidney and adrenal. Benign or malignant tumours occurred in 30
per cent of the test animals but the authors claimed that their
occurrence was not related to piperonyl butoxide. Reproduction was
decreased by a dietary level of 10 000 ppm and stopped by a
concentration or 25 000 ppm (Sarles & Vandergrift, 1952).
Comments
So far as is known, the colorimetric tests used responded to piperonyl
butoxide only. Thus, the 12 per cent of the single dose administered
to the dog that was unaccounted for (see Biochemical aspects) may
have been the most important from a toxicological standpoint.
From the long-term studies in the rat a level of 100 ppm was without
toxicological effect when compared to the controls.
From the one-year study in the dog the dose of 3 mg/kg body-weight/day
was without toxicological effect.
For future toxicological studies, specifications of the test material
should be stated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg body-weight per day.
Dog. 3 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.03 mg/kg body-weight
Further work required
Biochemical studies on the qualitative and quantitative aspects of
metabolism of the compound.
Studies on the effect of piperonyl butoxide on the liver of dogs. (For
details see Report of Scientific Group on Procedures for Investigating
Intentional and Unintentional Food Additives - July 1966).
The effects of this compound on reproduction in at leant one more
species.
Long-term feeding studies in another species, with careful observation
to detect any possible tumours. These studies should be done using
piperonyl butoxide alone and in combination with other agents, such as
pyrethrins and freons, with which it might be combined in practice.
Results of the above work should be made available not later than five
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use Pattern
Piperonyl butoxide has little or no insecticidal activity. Its primary
use is as a synergist for pyrethrins. Piperonyl butoxide may act as a
true synergist, as an antioxidant, as an extender, or as a combination
of any two or all three. In those countries where tolerances have been
established for piperonyl butoxide and pyrethrins, the two materials
are usually formulated, in a ratio of about 10:1 (w/w), respectively.
(a) Pre-harvest treatments
Formulations containing piperonyl butoxide are employed for
controlling insects on growing plants just before harvest and on dairy
and meat animals. They have been used in many countries on growing
bush and vine fruits, deciduous fruits and nuts, forage crops, and on
dairy and meat animals. Malathion and other insecticides have replaced
synergized pyrethrins for some of the above uses and, therefore,
piperonyl butoxide is not used on food prior to harvesting as
extensively now as it was 10 or more years ago.
(b) Post-harvest treatments
Piperonyl butoxide, in combination with pyrethrins, has been used in a
spray or dust formulation on freshly picked fruits and vegetables
after harvest while in the field, in storage, or in processing plants
for the control of drosophila and other insects. It is also used
directly on dried fruits, treenuts, grains and oil seeds as a
protective treatment against insect infestation during storage.
Piperonyl butoxide is usually formulated in a 10:1 ratio with
pyrethrins for application as a spray or dust directly on the food
commodities as they are placed in containers or as they move on a
conveyor into storage. It is used with pyrethrins in water emulsion or
wettable powder formulations as surface sprays on stacked bagged
peanuts and other oil seeds, and on animal feeds. Piperonyl butoxide
is very commonly used in aerosols as space treatments in food
handling, processing and storage facilities.
As with pre-harvest treatments, piperonyl butoxide is not being used
as extensively as it was a number of years ago. Malathion has replaced
synergized pyrethrins in many of the above uses.
(c) Other uses
Piperonyl butoxide (50 mg/sq. ft) in combination with pyrethrins has
been found to be effective in protecting cereal products against
insect attack, when applied to the outside surface of the outer ply of
multiwall paper bags. This treatment is becoming widely used,
particularly in the United States, for cereal products destined for
storage or shipment overseas.
Perhaps the greatest use of piperonyl butoxide is in formulations to
treat farm buildings and food processing, handling, shipping, storage
and marketing facilities. The treatment consists of spraying the
floors, walls, working areas and machinery, applying it in an aerosol
form to the insect infested area, or both. Again, malathion has
replaced synergized pyrethrins for some of these uses.
Piperonyl butoxide is present in most of the pyrethrins formulations
used for household insect control but the ratio of the two compounds
in such formulations varies considerably.
Tolerances (established or considered)
Country Product Parts per million
Brazil cereals 10
Canada grain sorghum 8
barley, buckwheat, corn 20
pop-corn, rice, rye, wheat
Czechoslovakia grain -
Germany grain 15
Italy cereals 20
Netherlands cereal 10
Tolerances (cont'd)
Country Product Parts per million
USA bush and vine fruits exempt
cottonseed
(post-harvest) 8
deciduous fruits and
nuts exempt
flax 8
forage crops exempt
fruits and nuts 8
(post-harvest)
grain (post-harvest) 20
mushrooms exempt
peanuts
(post-harvest 8
vegetables exempt
vegetables
(post-harvest) 8
Residues resulting from supervised trials
No data were available on the fate of piperonyl butoxide residues on
growing crops, and on fruits and vegetables which had received
post-harvest treatments. The bulk of the information available on the
fate of piperonyl butoxide residues has been obtained from
post-harvest application to cereals, cereal products, dried fruits and
dried citrus pulp animal feed. The evaluation of the use of the
insecticide mixture for protecting stored wheat is described by
Walkden and Nelson (1959): an unpublished document from the US
Department of Agriculture also was reviewed by the meeting and
provided the following information:
In order to protect grain from insect attack, piperonyl butoxide, in
combination with pyrethrins, is applied to various grains at rates
equivalent to 14.2 ppm on wheat, 15.2 ppm on shelled corn, 26.7 ppm on
oats, 17.8 ppm on barley, 15.2 ppm on rye and 19.0 ppm on rough rice.
Some 30 per cent or more of the insecticide is normally lost during
application; furthermore, the deterioration is rather rapid during the
first few months after storage.
Two space treatments applied respectively at rates of two and four
ounces of 0.5 per cent piperonyl butoxide in combination with 0.4 per
cent pyrethrins per 1000 cubic feet produced a maximum of piperonyl
butoxide residue of 1.5 ppm on the top 1-1/2 inches of exposed flour.
When dried fruit (apricots, peaches, pears) were exposed to 10
treatments, at two and three day intervals over a period of one month,
with a formulation containing 0.5 per cent of pyrethrins, 1.0 per cent
of piperonyl butoxide, 1.67 per cent of MGK 264(R) and 96.83 per
cent of light petroleum distillate applied at the rate of one gallon
per 50 000 cubic feet of space, the maximum residues obtained were 1.9
ppm of pyrethrins, 7.7 ppm of piperonyl butoxide and 7.2 ppm of MGK
264(R).
Bagged dried citrus animal feed was exposed to weekly treatments over
a three month period to aerosol formulations containing 0.2 per cent
of pyrethrins and two per cent of piperonyl butoxide applied at the
rate of 2.5 pints/1000 cu. ft of total warehouse space. The maximum
piperonyl butoxide residue obtained in the feed during the entire
period was 5.5 ppm. A similar result was obtained with a wettable
powder formulation (Laudani et al. 1959).
Multiwall paper bags with special insect-tight closures and pyrethrins
with piperonyl butoxide (540 mg/m2) applied to the outer surface
have provided effective protection to cereal products against outside
insect infestation during long-term storage period, and the maximum
piperonyl butoxide residues from composite samples were:
Rice - 5.5 ppm, non-fat dried milk 2.5 ppm; dry beans - 0.5 ppm,
flour - 6.0 ppm.
A similar test involving cornmeal stored for six months in bags
treated with pyrethrins and piperonyl butoxide (540 mg/m2) showed a
maximum of piperonyl butoxide in the cornmeal to be 10.7 in 50 lb.
bags and 3.3 ppm in 100 lb. bags.
Residues in food moving in commerce
In 99 samples taken from cargoes shipped from all over the world to
Rotterdam and Amsterdam, piperonyl butoxide was found in 11 samples.
The residues ranged from 0.1 to 1.0 ppm. The limit of the sensitivity
of the method used was 0.5 ppm.
Residues at time of consumption
At the time of preparation of this report no data were available on
the fate of piperonyl butoxide residues on or in fresh fruit,
dried-fruit, tree nuts, fresh and dried vegetables. Information is
available on the fate of piperonyl butoxide on cereals but this is not
as complete as it should be.
In controlled studies the deposit on wheat, from an application dosage
of 13.2 ppm, was shown to be 5.6 ppm in two months and 3.6 ppm in four
months after treatment. On another occasion a theoretical 17.4 ppm was
down to 3 ppm after two months of storage. Since the treatment is used
only on grain going into storage for three months, or longer, it can
be assumed that there would be at least a 50 percent loss of residue.
Other studies have also shown that when treated wheat is milled most
of the remaining residue goes into the screenings and scourings. A
very small percentage of the piperonyl butoxide ends up in the flour
traction. These same studies showed milled fractions of treated corn
had either none or a very small percentage of the piperonyl butoxide
originally present. Cooking reduced the residue from 4.7 to 0.6 ppm.
Methods of residue analysis
The method of Jones, Ackerman and Webster (1952) as modified by
Williams and Sweeney (1956), in which the colour produced on treatment
with tannic acid in phosphoric-acetic acid is measured, is suitable
for the determination of residues of piperonyl butoxide. The clean-up
techniques as appropriate to various foodstuffs are indicated. The
method is in general sensitive to 0.1 ppm piperonyl butoxide. Beroza
(1963) has described a thin-layer chromatographic method which should
be capable of development into a satisfactory technique for many
foodstuffs.
RECOMMENDATIONS FOR TOLERANCES
The primary use of piperonyl butoxide has been as a synergist for
pyrethrins which have been used rather extensively in the past to
protect a variety of foods, raw and processed, against insect
infestation. Malathion and certain other insecticides have proved to
be successful for similar purposes during recent years. Therefore
piperonyl butoxide is probably used less than previously for purposes
that may lead to residues in foods.
Information is lacking on the identity, persistence and effects of the
breakdown products of piperonyl butoxide and the other related
compounds present in piperonyl butoxide concentrates.
Although some information is available on the residues and their fate
on or in cereal and cereal products, there are insufficient data on
residues obtained from good agricultural practices on fresh and dried
fruits, tree nuts, fresh and dried vegetables and peanuts and other
oil seeds. Similarly there are very few data on the actual occurrence
of residues in food in commerce. The above information should be
obtained and considered before tolerances are established. Therefore,
tolerances for piperonyl butoxide at this time should be temporary
and reviewed in three years.
The proposed temporary tolerances are:
Cereal and cereal products 20 ppm
Fresh fruits for canning only 8 ppm
Dried fruits 8 ppm
Tree nuts 8 ppm
Dried vegetables 8 ppm
Peanuts and oil seeds 8 ppm
At these levels, which are considered to be those which might possibly
result from adequately supervised use on the crops in question the
intake would not reach the acceptable daily figure, using the ninth
decile food consumption figures for the United States of America and
disregarding other safeguarding as outlined in Part I of the report on
this meeting.
The need for treatment, with insecticides containing piperonyl
butoxide, of fresh fruits for canning is questionable. If such a need
exists, the countries interested should provide information on the
extent of its use and on the resulting residues.
Further work or information
(a) Further information is desirable on the levels of piperonyl
butoxide obtained on the various foods and the effect of handling,
cleaning, processing and storing.
(b) Research should be conducted to determine the identity,
persistency and toxicological effects of the breakdown products of
piperonyl butoxide and the related compounds present with piperonyl
butoxide.
(c) The analytical methods for piperonyl butoxide should be reviewed.
A faster, more sensitive method is desirable.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Chamberlain, R. W. (1950) Amer. J. Hyg., 52, 153
Epstein, S. S., Joshi, S., Andrea, J., Clapp, P., Falk, H. & Mantel,
N. (1966) Unpublished report
Falk, H. L., Thompson, S. J. & Kotin, P. (1965) Arch. Environ.
Health, 10, 847
Lehman, A. J. (1948) Quart. Bull. Assoc. Food and Drug Officials
U.S., 12, 82
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1952 a) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 3
Lehman, A. J. (1952 b) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1952 c) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Rai, L. & Roan, C. C. (1956) J. econ. Entomol., 49, 591
Robbins, W. E., Hopkins, T. L. & Darrow, D. I. (1959) J. econ.
Entomol., 52, 660
Sarles, M. P., Dove, W. E. & Moore, D. H. (1949) Amer. J. trop.
Med., 29, 151
Sarles, M. P. & Vandergrift, W. B. (1952) Amer. J. trop. Med. Hyg.,
1, 862
U.S. Food and Drug Admin., (1946) Unpublished data
REFERENCES PERTINENT TO AGRICULTURAL DATA
Beroza, M. (1963) Identification of 3,4-Methylenedioxyphenyl
Synergists by Thin-layer Chromatography. Agric. Food Chem., 11 (1):
51-53
Jones, H. A., Kerby, G. F. & E. J. Incho. (1952) Insect proofing of
paper. Chemical Specialities Manufacturers Association 38th Mid-Year
Meeting, Boston, Mass., USA
Jones, H. A., Akerman, H. J. & M. E. Webster. (1952) The colorimetric
determination of piperonyl butoxide. J. Assoc. Offic. Agric. Chem.,
35: 771-780
Laudani, H., Gillenwater, H. B., Kantack, B. H. & M. F. Phillips.
(1959) The protection of citrus pulp against insect infestation with
surface applications of pyrethrum - piperonyl butoxide wettable
powder. J. Econ. Ent., 52 (2): 224-7
Walkden, H. H. & H. D. Nelson. (1959) Evaluation of synergized
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Agriculture, Marketing Research Report No.
322
Williams, H. L. & Sweeney, J. P. (1956) Isolation of piperonyl
butoxide from oils, fats and waxes. J. Assoc. Offic. Agric. Food
Chem., 11: 51-54
