
FAO/PL:1967/M/11/1
WHO/Food Add./68.30
1967 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Rome, 4 - 11 December,
1967. (FAO/WHO, 1968)
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1968
CHLORDANE
This pesticide was evaluated by the 1965 Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues (FAO/WHO, 1965). At that time, the Joint Meeting
did not recommend an acceptable daily intake because of the concern
that the technical product might contain hexachlorocyclopentadiene
since no information was available on the composition of technical
chlordane and the nature and toxicity of terminal residues. Since that
time, a large amount of additional information has become available
and the above problems were reviewed by the IUPAC Commission on the
Chemical Nature of Terminal Pesticide Residues (IUPAC, 1967, 1968).
Therefore the previously published monograph has been greatly expanded
and is reproduced in its entirety below.
IDENTITY
Chemical names
1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetrahydro-4,7, methanoindane or
1,2,4,5,6,7,10,10-octachloro-4,7,8,9-tetrahydro-4,7-methyleneindane or
1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methanoindene.
Synonym
chlordan
Empirical formula
C10H6Cl8
Structural formula
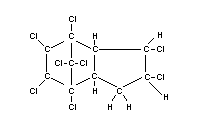 Other relevant chemical properties
Chlordane, as presently manufactured in the USA, is a uniform
technical product, the composition of which is controlled. Analytical
methods and reference standards have been made available periodically
by the manufacturer to regulatory agencies as a method of commercial
product control. Analytical methods for regulating the technical
formulation in commerce have become official methods after
collaborative studies by the Association of Official Analytical
Chemists (AOAC) in the USA and the European Commission for Methods of
Analysis of Pesticides (CIPAC). While hexachlorocyclo pentadiene is
used in the manufacture of the technical product, none of this
chemical remains unreacted and there is no evidence that it appears as
a constituent of the terminal residue in food.
There is some confusion in nomenclature. Gamma-chlordane is the
alpha-chlordane referred to by March (1952) (it is the component
present in the highest concentration in technical chlordane),
alpha-chlordane is the beta isomer described by March. Poonawalla and
Korte (1964), Ludwig (1966), and some other authors still use March's
designation. These two components together comprise approximately half
of technical chlordane.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Chlordane is absorbed from the gastrointestinal tract, the respiratory
tract, and through the skin (Ambrose et al., 1953). It is stored in
the adipose tissue of rats, sheep, goats, and cows and accumulates in
the milk.
Rats fed chlordane for six and a half months showed the following
levels in their abdominal fat at various feeding levels (in ppm):
intake 3, residue 5.2; intake 15, residue 32; intake 30, residue 76;
intake 60, residue 109 (Ingle, 1965).
Chlordane fed at 25 ppm in the diet for 8 weeks reached a maximum
level of 18 ppm in the fat of calves and 12 ppm in sheep. After
feeding was stopped, the residue was eliminated from calves in 20
weeks and from the sheep in 4 weeks (Claborn et al., 1953).
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlordane is
excreted in the urine of rabbits (Stohlman and Smith, 1950).
Cows grazing on pasture to which 0.5 pound of chlordane per acre had
been applied showed an average of 0.03 ppm of chlordane in their milk
(Westlake et al., 1963).
Both technical chlordane and one of its pure isomers (gamma-chlordane)
have been shown to have stimulating effects on rat liver microsomes
for the metabolism of certain drugs (Burns et al., 1965; Hart and
Fouts, 1963; Hart et al., 1963; Kuntzman, et al., 1964).
In vitro metabolism by hepatic microsomal enzymes from rats treated
8 days previously with single i.p. doses of 10 or 100 mg/kg of
chlordane in corn oil was increased for the 3 drugs reported
(hexobarbital, aminopyrine and chlorpromazine). The increases for
hexobarbital and aminopyrine were dose-related. In vivo hexobarbital
response, measured by sleeping time of mice, 1, 3 and 8 days after
single i.p. doses of 25 mg/kg body-weight technical chlordane or
gamma-chlordane, showed a decrease (shortened sleeping time), greatest
at day 3 but still marked on day 8 (Fouts, 1963).
The mechanism of action of chlordane is similar to that of
phenobarbital in stimulating hepatic drug metabolism in the rat. Both
agents increase liver weight, microsomal protein, microsomal NADPH
oxidase, and microsomal cytochrome CO-binding pigment. They both
stimulate drug metabolism in adrenalectomized or hypophysectomized
rats and promote the proliferation of smooth endoplasmic reticulum in
the liver cell, and ethionine blocks the enzyme stimulating effects of
both agents (Hart and Fouts, 1965).
Liver microsomal preparations from adult, female rats given 10 mg/kg
of chlordane intraperitoneally every other day for 14 days showed a
385 per cent increase in the biotransformation of 17ß-estradiol to
polar metabolites (Kuntzman et al., 1964). The intraperitoneal
injection of immature male rats with 50 mg/kg of chlordane daily for 4
days produced an increase of activity of the liver microsomal steroid
hydroxylases (Conney et al., 1967).
The toxicities of bishydroxycoumarin and phenylbutazone were reduced
in both rats and dogs pretreated by chlordane, and this effect was
accompanied by lower plasma levels of these drugs in the
chlordane-treated animals than in controls given the same doses (Welch
and Harrison, 1966). The toxicity of parathion was reduced in mice
pretreated 16 hours to 4 days previously with a single oral dose of
130 mg/kg of chlordane (Triolo and Coon, 1966).
Male rats weighing 60 to 80 gms were given 25 mg/kg of chlordane
intraperitoneally daily for 3 days, and were sacrificed 24 hours after
the last dose. The livers showed a proliferation of the
smooth-surfaced endoplasmic reticulum in the parenchymal cells,
associated with an increased activity of microsomal preparations from
such livers to metabolize hexobarbital, aminopyrine, zoxazolamine and
p-nitrobenzoic acid (Fouts and Rogers, 1965).
Pretreatment of rats for several days with chlordane increased the
LD50 of warfarin more than 10-fold, an effect associated with
decreased plasma levels of warfarin and an increased rate of
metabolism of the drug by liver microsomal enzymes (Ikeda et al.,
1966).
Technical chlordane and one of its pure isomers, gamma-chlordane,
administered to adult or weanling rats in single or 3 daily doses,
after 1 to 8 days increased the hepatic microsomal metabolism of
hexobarbital, aminopyrine, and chlorpromazine (Hart et al., 1963).
Treatment of newborn rabbits with 50 mg/kg of gamma-chlordane daily
for 3 days increased the activity of liver enzymes that metabolize
hexobarbital, aminopyrine and p-nitrobenzoic acid. Treatment of
lactating mothers with chlordane increased the drug metabolizing
enzymes in the nurslings (Fouts and Hart, 1965). Drug metabolism was
also stimulated in adult rabbits.
In dogs given 5 mg/kg of chlordane orally three times a week for 7
weeks the rate of metabolism of phenylbutazone was markedly increased.
This effect persisted as long as 21 weeks after termination of the
chlordane administration (Burns et al., 1965).
Chlordane has been demonstrated to stimulate the rate of drug
metabolism by the liver of the squirrel monkey (Cram et al., 1965).
For further information see the sections In Plants, and In
Animals.
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat Oral 200 - 590* Ambrose et al., 1953
Ingle, 1955
Stohlman et al., 1950
Oral 335 - 430 Gaines, 1960
150 - 225 Ingle, 1955
Mouse Oral 430 US Food and Drug Admin., 1947
Rabbit Oral 100 - 300* Stohlman et al., 1950
20 - 40 Ingle, 1955
Goat Oral 180 Welch, 1948
Sheep Oral 500 -1000 Welch, 1948
Chicken Oral 220 - 230 Turner and Eden, 1952
* The differences are explained by the use of different solvents,
and by the fact that the chlordane mentioned in the older
literature contained a considerable amount of the very toxic
hexachlorocyclopentadiene (Ingle, 1965; Lehman, 1952).
Man. A dose of 104 mg/kg proved fatal (Derbes et al., 1955). An
18-year old female showed convulsions but recovered after a dose of
approximately 30 mg/kg (amount retained after vomiting estimated to be
10 mg/kg). In two infants, 15 months and 3 years of age, 10 and 40
mg/kg respectively gave severe poisoning (Stormont and Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12 male
rats, all of them died within 10 days. At 500 ppm, 12/12 died within
70 days; at 300 ppm, 9/12 were alive after 100 days (Stohlman et al.,
1950).
Daily oral doses of 6.25 - 25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg, all
the animals died. Intracytoplasmic bodies in the liver-cells were
found at all levels and their number was in proportion to the dose
used (Ambrose et al., 1953).
Groups of 6 females and 6 males were fed 2.5 ppm or 25 ppm of a sample
of technical chlordane containing 60-75% chlordane and 25-40%
unrelated products for up to 9 months. Centrolobular cell hypertrophy,
peripheral migration of cytoplasmic granules and the presence of
cytoplasmic bodies were observed in 1 male at 2.5 ppm and in 5 males
at 12.5 ppm (Ortega et al., 1957).
Initial groups of 10 males and 20 females were used in a 3-generation
study at dietary levels of 0, 0.3, 3, 15, 30 and 60 ppm of technical
chlordane. Two litters in each filial generation were studied. Levels
up to and including 30 ppm had no effect on fertility, numbers of
young or litters, or weight, growth or mortality of the young animals
to weaning age. Autopsy of animals post-weaning showed no gross or
microscopic difference between the groups. At 60 ppm, there was a high
(10.6 per cent) mortality in the second F3 generation litters during
the latter part of the nursing period; these animals showed gross and
microscopic pathology comparable to that characteristic for chlordane
intoxication. However, survivors of this generation showed no tissue
changes at all. A third act of F3 litters at 60 ppm suffered 17 per
cent mortality during the nursing period, with symptomatology end
gross and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from the
60 ppm group and placed on chlordane-free diets for 30 days prior to
remating showed no difference in any respect from control litters. No
evidence of teratogenicity or tumorigenicity for chlordane was found
in this study (Ingle, 1967).
Dog. Chlordane was given in varying oral doses to dogs for 7 days,
convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but 700
mg/kg (highest dose) did not produce any effect (Batte and Turk,
1948).
Four groups of 2 to 4 dogs given chlordane orally in doses between 5
and 80 mg/kg body-weight daily all died within periods of 25 days to
93 weeks (Lehman, 1952).
Groups of 4-7 males and 4-7 females were fed 0, 0.3, 3, 15 and 30 ppm
of chlordane for 2 years. Abnormalities in the results of clinical
liver function tests were seen in the 15 and 30 ppm groups. In animals
selected for necropsy at the end of the first year, increased relative
liver weights and associated hepatocellular changes were found at 30
ppm; at the end of two years, dose-related increases in relative liver
weights were found at 15 and 30 ppm, with non-dose-related
hepatocellular changes. There was no difference between the severity
of the liver lesions of the 30 ppm animals and those of four animals
withdrawn from 30 ppm treatment for eight months prior to sacrifice.
Percutaneous liver biopsies on two animals of the 30 ppm group at 1, 3
and 6 months showed hepatocellular changes at 6 months but not at 1 or
3 months. No adverse effect was seen on behaviour, appearance,
survival, weight gain, blood picture or the results of periodic
physical examination, at all levels (Wazeter, 1967).
Sheep. Chlordane administered by stomach-tube to sheep in a dose of
0.5 g/kg body-weight produced toxic symptoms (incoordination, partial
blindness) in 5 to 6 days. A dose of 1 g/kg body-weight produced
severe respiratory and nervous symptoms at 16 hours and death after 48
hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each sex)
were given 2.5, 25 and 75 ppm of chlordane in the diet for 2 years.
The sample of chlordane used had an LD50 of 450 mg/kg (Lehman, 1951).
It was found that 25 and 75 ppm gave moderate to severe signs of
intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
Groups of 40 rats (20 males and 20 females) were fed concentrations of
5, 10, 30, 150 and 300 ppm of "technical chlordane" in the diet over a
2-year period.
Throughout the experiment tremors and convulsions appeared or could be
induced at 30 or more ppm. Following fasting, no neurological symptoms
appeared at 5 or 10 ppm. Growth rate was affected at 150 or 300 ppm.
Liver histological damage was observed in the form of hypertrophy of
centrolobular calls, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm, slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was carried
on between late 1953 and late 1955, "technical chlordane of recent
manufacture" was used. Groups of 40 rats were given chlordane at 2.5,
5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given no
chlordane. Changes concerning food consumption, growth and mortality
were seen only in the 300 ppm group. Liver cell changes were not
present in the animals given 2.5-25 ppm. At 50 ppm only "cytoplasmic
peripheralization" was present. At higher doses the changes were as
those previously described (Ingle, 1955).
In a study published in 1953, a sample of chlordane exhibiting an oral
LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each sex
were given 0, 10, 20, 40, 80, 160, 320, 640 and 1280 ppm of chlordane
in their diets for approximately 407 days. The animals at 640 and 1280
ppm died early. At lower dosages, survival was unaffected. Increased
liver-weight (in comparison with the control group) was observed over
320 ppm. In a sample of liver of a male at 320 ppm the average nuclear
volume was 377µ3 compared to 268µ3 in a control rat. Cytoplasmic
vacuoles containing fat and clusters of granules at the periphery of
the cytoplasm were often seen. In the males they were equivocal at 10
ppm, absent at 20 ppm and infrequent at 40 ppm. In the females these
lesions were common and were seen only at 80 ppm and over (Ambrose et
al., 1953).
Observations in man
Workers engaged in the manufacture and formulation of chlordane for
periods up to 15 years have exhibited no evidence of harmful effects
attributable to this insecticide (Princi and Spurbeck, 1951; Alvarez
and Hyman, 1953; Fishbein et al, 1964). In a survey of more than 1105
persons who had been engaged in pest control operations for 1 to 30
years (318 for 5 to 19 years), three cases of toxicity due to
chlordane were reported, the only symptoms specified being dizziness
and headache (Stein and Hayes, 1964).
Chlordane was not found in any of 282 human fat samples taken at
autopsy, though DDT and lindane were regularly detected and dieldrin
was frequently found (Hoffman et al., 1964).
Comments
Since chlordane stimulates drug metabolizing processes in the rat,
mouse, rabbit, dog and squirrel monkey, and since the microsomal
enzyme stimulating properties of chlordane are similar to those of
phenobarbital, which stimulates drug metabolism in man, it appears
extremely likely that chlordane also stimulates drug metabolism in
man. This effect in animals and the associated cellular changes in the
liver, considered characteristic of the chlorinated hydrocarbon
insecticides as a class, are thought by several workers not to
represent toxic effects but rather physiological adaptive processes.
Toxicological studies reported prior to 1953 are not contributory to
the evaluation of the currently manufactured product because of the
variability of composition before that time. The chlordane made for
commercial use since 1953, which has been used in the studies
reported, has a constancy of composition that makes the data adequate
for toxicological evaluation.
In none of the many toxicological studies done has there been any
evidence observed of tumorigenic or other irreversible pathologic
changes, even at the highest doses or feeding levels used.
On the basis of the results of two long-term feeding studies in the
rat, a no-effect level for the rat may be established at 20 ppm, or 1
mg/kg/day. A satisfactory 3-generation reproduction study in the rat
has been completed recently in which 30 ppm was without effect on
fertility, number or size of litters, or on the weight, growth or
mortality of the young to weaning age. Furthermore, there was no
evidence of teratogenicity. In the recently completed 2-year feeding
study in the dog, 3 ppm of chlordane was the highest level used that
had no effect in tests of liver function or on liver weight and
structure of the liver cell. Though the liver weight and cell
structure changes at 15 ppm may probably be classified as adaptive
rather than toxic in nature, the significance of the functional
changes at this level cannot be assessed in the present state of our
knowledge. Even though these changes consistently disappeared before
the end of the 2-year feeding period, and even though 15 and 30 ppm
produced no other adverse effects throughout the 2-year period, it
seems necessary at this time to take 3 ppm chlordane as the maximum
known no-effect level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat. 20 ppm in the diet, equivalent to 1 mg/kg/day
Dog. 3.0 ppm in the diet, equivalent to 0.075 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.001 mg/kg body weight
Further work required
See General Comments page 3 and 4.
Further work desirable
Toxicological data on alpha and gamma chlordane.
EVALUATION FOR TOLERANCES
USE PATTERN
Chlordane was the first cyclodiene insecticide and originally had a
broad scale and spectrum of use but was somewhat displaced by aldrin,
dieldrin, endrin and heptachlor because of their greater persistence
and effectiveness and lower cost. In recent years, to narrow the use
spectrum of aldrin, dieldrin, endrin and heptachlor, there is renewed
interest in the use of chlordane.
Several million pounds of chlordane have been used annually in North
America for more than 20 years of which between 30 and 40 per cent has
been used in agriculture. The USA and Canadian currently approved uses
represent the largest spectrum and potential scale of use in the world
and have been used an a guide to this evaluation.
Pre-harvest treatments
The most important use of chlordane in for soil treatment, with a
limited use for foliar application, the main crops being large root
crops, sugar beets, corn, small root vegetables, leafy and stalk
vegetables, cucurbits, leguminous vegetables, berry fruits, sugar
cane, pineapple and cereals. Some uses on forage crops are still
approved in the USA.
The literature suggests that in fifty or more countries, chlordane is
or can be used on sugar beets, potatoes, vegetables, tobacco, sugar
cane, ground nuts (peanuts), beans, bananas, coffee, deciduous fruits,
citrus, olives, cotton and tea bushes. However, information on the
scale of use and resulting residues is not generally available. In
other countries the scale of use is probably only a fraction of that
in the USA. Dosages vary between countries, with a range of 1 to 8
lbs/acre recommended for a variety of vegetable insect control
requirements.
Post-harvest treatments
No post-harvest uses are currently recommended.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The large amount of available information needs critical review
because of improvements in analytical methods since 1961 and recent
information on the chemical nature of terminal residues. Methods used
prior to 1963 were based on total chlorine or colorimetric
measurements and expressed results in terms of technical chlordane.
Recently developed methods identify individual components, the
summation of which frequently is approximately one half of that
reported by earlier non-specific methods. New data developed from 1965
to 1967 reports individual components of residues as alpha and
gamma-chlordane, sometimes with much smaller and insignificant
quantities of heptachlor and heptachlor epoxide. Small amounts of
gamma-chlordane will be found in some crops resulting from the use of
technical heptachlor in soil (Lichtenstein et al, 1967; Saha and
Stewart, 1967; Velsicol, 1967 a through d). Some authors have
recently added the various components of the residue and reported the
total as chlordane (e.g. Smith and Adams, 1965).
The available information concerning residues resulting from uses
constituting "good agricultural practice" (i.e. consistent with pest
control requirements, principally in the USA and Canada) are
summarized below.
Crop Residue (ppm) Reference
alfalfa 0.01 Ordas et al, 1956
" 0.16 Allen, 1963
barley, grain 0.0 - 0.06 Velsicol, 1967c
" , straw 0.98 - 2.65 " "
beans <0.01 Smith and Adams, 1965
beets 0.17 "
beet tops 0.01 - 0.1 "
bell peppers none recovered Muns et al, 1960
broccoli " " "
cabbage " " "
cantaloupe 0.08 "
" , meat and rind 0.01 - 0.1 Smith and Adams, 1965
carrots 1.5 Muns et al, 1960
" 0.01 - 0.13 Cook, 1960
" 0.16 (1) Lichtenstein et al, 1967
" , peel 0.0 - 0.7 Cook, 1960
collards none recovered Marth, 1962a
cucumbers 0.08 Muns et al, 1960
eggplant none recovered "
lettuce 0.04 "
peaches 0.8 Fahey et al, 1957
peanuts 1.74 ± 0.82 Morgan et al, 1967
pineapple 0.145, 0.43 (2) Perez-Escolar, 1959
potatoes <0.01 - 0.23 (3)
radishes 0.03 Lichtenstein et al, 1967
rutabagas 0.5 Muns et al, 1960
" 0.08 - 0.16 (4) Saha and Stewart, 1967
snap beans <0.01 Smith and Adams, 1965
strawberries <0.01 "
" none recovered Fahey, 1962
sweet potatoes 0.01 - 0.4 (5)
sugar beets 0.04 - 0.37 (6)
tomatoes 0.01 Muns et al, 1960
" <0.01 Smith and Adams, 1965
turnips 0.01 - 0.1 "
" 0.16 Muns et al, 1960
turnip tops 0.01 - 0.1 Smith and Adams, 1965
wine none (7) Painter et al, 1963
(1) soil contained 0.68 ppm gamma chlordane (from heptachlor application 5 years
previous).
(continued)
(2) resulting from one and two applications, respectively.
(3) overall range as reported by many authors, e.g. Lichtenstein, 1967; Smith
and Adams, 1965; Begg et al, 1960; USDA, 1964; USDA, 1966; Corley et al,
1965; Velsicol, 1967b.
(4) gamma chlordane from heptachlor
(5) overall range as reported by many authors, e.g. Muns et al, 1960; Smith and
Adams, 1965; Smith et al, 1964.
(6) overall range following different rates of application as reported by
many authors, e.g. Muns et al, 1960; Rusk and McDonough, 1966;
Velsicol, 1967d.
(7) 0.6 in must and lees.
Data are required from similar investigations using reliable
analytical procedures from other countries. Information pertaining to
successive crops grown in rotation over a period of years after
initial treatment would be of particular interest. Data are also
required on the residues which may occur in oil seeds as the result of
intentional use, or from growing these crops in rotations. This is of
particular importance where certain oil-containing crops are grown in
rotation with other crops, especially after maize.
In general these data suggest that the highest residues consistent
with good agricultural practice will result in the large root crops,
and under certain conditions in the leafy and stalk vegetables.
Considerable variation can be expected in residues found in the large
root crops, particularly potatoes, depending on soil type, variety of
potato and date of maturity. Recent information suggests that the
residues in leafy and stalk vegetables may in part be from direct
contamination from soil, rather than a true translocation into growing
parts other than the stem or stalk. The intermediate group in
magnitude of residues to be expected would include small root
vegetables, cucurbits and pineapple. Cereals, berry fruits, pod
vegetables and fruit vegetables of the tomato type are in the category
of lowest residues, usually well below 0.1 ppm. It is probable that
there is no true translocation of residues in the grain of most
cereals and that the residues found are due to mechanical
contamination by soil, dust, debris, etc., during harvesting
operations.
RESIDUES IN FOOD MOVING IN COMMERCE
Pesticide residues in commercial animal feeds are important sources of
residues in milk and other animal products. Data concerning chlordane
are available from one such survey in Tables 1 and 2 (Minyard and
Jackson, 1963).
TABLE I
The occurrence of chlordane in commercial feeds expressed in parts per billion
No. No. Range of chlordane Range of total
Feed samples positive residue (ppb) pesticide
residue (ppb)
16% protein Dairy 73 53 0-254 26 - 1153
18% protein Dairy 6 4 13- 39 55 - 713
20% protein Dairy 2 2 15- 34 139 - 274
32% protein Dairy 4 1 5 36 - 435
12% protein Fitting
and Freshening 2 1 12 49 - 60
14% protein Cattle 3 2 11- 12 47 - 183
20% protein Beef 1 1 45 343
16% protein Hog 3 0 - 34 - 62
16% protein Poultry 3 2 7- 11 80 - 291
TABLE II
Ranges and averages of insecticide residues found in 101 feeds expressed in parts per billion
Av. level of
No. Per cent Range of contamination of
Insecticide contaminated contaminated contamination contaminated foods
(ppb) (ppb)
lindane 101 100.0 5 - 79 16.0
aldrin 87 86.1 1 - 16 2.6
DDT 99 98.0 10 - 448 80.5
methoxychlor 46 45.5 10 - 580 74.0
endrin 59 58.4 4 - 27 10.6
chlordane 68 67.3 5 - 254 30.4
dieldrin 89 88.1 1 - 16 4.9
toxaphene 15 14.8 60 - 530 178.3
heptachlor 9 8.9 2 - 13 4.8
heptachlor epoxide 3 3.0 1 - 6 3.3
Soybeans follow maize in recommended crop rotations in the USA. Soil
used for maize production is treated with chlordane, aldrin or
heptachlor and may result in residues of chlordane in soybeans
subsequently grown in these soils. An interim report on a survey of
unintentional residues from soil and crop samples from 27 regional
special sampling locations in the USA was made available to the
meeting. Chlordane was detected in soils from 3 fields at an average
concentration of 0.26 ppm, soybeans from one of these fields
containing 0.03 ppm. Eight other soil samples contained an average
concentration of 0.50 ppm (range 0.09 to 1.11 ppm). No chlordane was
detected in soybean seed or plants from these latter soils (USDA,
1967a).
Information on sources of chlordane in total diet studies has been
available for five years. The earliest of these concerns 12,000
samples analyzed by paper chromatography and gas liquid chromatography
in the state of California (CSDA, 1962). No chlordane was found in
this survey.
Thirty-eight total diet studies in 1961-62, for six USA cities
resulted in only two samples containing chlordane at 0.01 and 0.03 ppm
(Fishbach, 1963).
Eighty-two foods collected from 18 markets in three different
geographical areas in the USA were prepared for consumption, resulting
in 216 composite samples, and then analysed (Duggan et al, 1966).
Chlordane was found in only one composite sample at 0.033 ppm in
"garden fruit" produce (i.e. raw peppers, fresh and canned tomatoes,
raw cucumbers, catsup, eggplant, raw and frozen summer squash).
Comprehensive unpublished information from the USA was made available
to the 1967 Joint Meeting (Duggan, 1967). This data is concerned with
both objective samples of food for the years 1964 -1966 inclusive and
total diet samples for the year ending April 1967.
TABLE III
Objective Samples - Chlordane
Year 1964 1965 1966 Total
Number of domestic samples 20,088 14,462 14,806 49,356
Per cent of samples positive 0.7 1.1 0.9 0.9
Number of import samples 1,224 1,217 1,399 3,840
Per cent of samples positive 0.2 0.9 0.1 0.4
Total Diet Samples - Chlordane
Leafy vegetables - 1 composite positive contained 0.02 ppm (29 negative)
Legume vegetables - 1 composite positive contained 0.005 ppm (29 negative).
In Table IV details of the incidence and levels of chlordane for 1966
are of considerable interest as to amount and sources of residues in
the diet of man in the USA.
Canadian information covers a 4.5 year period from 1963 to July 1967.
Of a total of 14,270 samples of food (12,186 domestic and 2,084
imports), eleven contained chlordane; one lot of imported green
peppers, >0.3 ppm; six lots of whole eggs, 1.8 to 11 ppm (all seven
in excess of tolerance). Three vegetable samples contained traces of
chlordane, and one lot of turnips contained residues in excess of 0.3
ppm (Swackhamer, 1967).
Two reports of no chlordane being found in British food (Robinson and
McGill, 1966; Egan et al, 1966) are difficult to evaluate because of
lack of information on current use of chlordane in Britain and the
countries from which some foods concerned were imported.
FATE OF RESIDUES
In soils
Chlordane residues in soil have been investigated since 1949. However,
prior to 1961 much of the information is of doubtful value. Sources of
information since then include : USDA, 1964; Landis, 1965; Edwards,
1965; Edwards, 1966; Rusk and McDonough, 1966; Chisholm et al, 1966;
Bess et al, 1966; USDA 1967b; Nash and Woolson, 1967; Saha and
Stewart, 1967; Velsicol, 1967a; Lichtenstein, 1967; and Majumder,
1967.
Table V summarizes comparative persistence of some organochlorine
insecticides based on the literature review of Edwards (1966) but
modified as concerns doses used for a particular pest as far as
chlordane is concerned.
Approximately 55 per cent of chlordane remains in soil after the first
year following application, compared with 26 per cent for aldrin, 75
per cent for dieldrin and 80 per cent for DDT, a situation which is
not consistent with the respective vapour pressures; hence other
factors must be operating.
Although most reports to date express residues in soil on the basis of
equivalents of technical chlordane, Saha and Stewart (1967) reported
11.9 per cent of the residue resulting from one use of heptachlor as
gamma-chlordane (one pound of heptachlor contains 35 per cent
gamma-chlordane).
TABLE IV
Incidence and levels of chlordane in objective samples of food of domestic origin*, USA, 1966 (Duggan, 1967)
Number of Samples
Raw Manufactured Processed Fish
Range agricultural dairy animal and Vegetable Processed
ppm products products feeds shellfish oils foods Total
T - 0.03 59 1 3 1 - - 64
0.04 - 0.10 17 - 4 2 - - 23
0.11 - 0.50 35 - 4 - 1 1 41
0.51 - 1.00 - - 1 - - - 1
1.01 - 1.50 - - 2 - - - 2
1.51 - 2.00 - - 1 - - - 1
Above 2.00 2 - - - - - 2
Total samples
containing 113 ** 1 15 3 1 1 134
chlordane
Total samples
examined 9,804 611 680 290 171 227 14,806 *
* Of an additional 1399 samples of imported foods, only 1 contained chlordane, and this in a raw
agricultural product in the range of - 0.03 ppm
** 55 residues were in root vegetables
30 residues were in vine and ear vegetables
TABLE V
Persistence of some organochlorine insecticides in soil
Insecticide Average dose active Time for 95% disappearance
ingredient, lbs/acre in years
Range Mean
aldrin 1 - 3 1 - 6 3
chlordane 1 - 2 3 - 5 4
DDT 1 - 2´ 4 - 30 10
dieldrin 1 - 3 5 - 25 8
heptachlor 1 - 3 3 - 5 3´
lindane 1 - 2´ 3 - 10 6´
telodrin ¨ - 1 2 - 7 4
In plants
Information on the chemical nature of the terminal residues from
weathering of external residues has recently become available
(Thurston, 1965; Klein and Link, 1967). A further review of new
unpublished information and work in progress is contained in the 1966
and 1967 Reports of the IUPAC Commission on Chemical Nature of
Terminal Pesticide Residues (IUPAC, 1967, 1968).
The principal terminal residues from weathering of chlordane on plants
have been identified as alpha- and gamma-chlordane by characteristic
"signature" peaks as determined by gas liquid chromatographic methods
and confirmed by infrared methods. The sequence of analyses taken
after agricultural applications of technical chlordane exhibit a
fairly constant pattern. Minute amounts of heptachlor or heptachlor
epoxide appear in some chromatograms but, for all practical purposes,
these can usually be ignored.
The loss of technical chlordane from plants by normal weathering is
extremely rapid. At the end of the seventh day only 7 ppm of a
starting load of 110 ppm remains on kale, and only 0.1 ppm, expressed
as technical chlordane equivalent, remains after 28 days (Klein and
Link, 1967).
Previous studies on weathering of residues have not taken account of
the possible formation of hydrophilic metabolites, an omission which
is not probably toxicologically important since the known hydrophilic
metabolites of at least gamma-chlordane are less toxic than
technical chlordane. However, such information in domestic animals
after ingestion of weathered residues would be important in
establishing the consumer hazard of residues in animal products.
Recent information on the production of hydrophilic metabolites of
gamma-chlordane in animals (Poonwalla and Korte, 1964; Ludwig, 1966)
suggests that similar investigations should be carried out in plants,
particularly from the stand-point of translocation and metabolism of
residues in soil. Work on that subject is now in progress by Korte and
will be reported to the IUPAC Commission on Terminal Residues in 1968.
Current indications are that substantial formation of hydrophilic
metabolites (of lower order of toxicity than alpha-, gamma-, and
technical chlordane) are formed in the stalk of plants (Korte, 1967
personal communication).
Gamma-chlordane residues can occur in plants as the result of use of
technical heptachlor in soil. An application of 6.6 kg/ha mixed into
soil results in 11.9 per cent of the soil residue being in the form of
gamma-chlordane. Rutabagas grown in this soil contained 0.008 ppm
gamma-chlordane, this being 13.3 per cent of the total residue
(heptachlor + heptachlor epoxide + gamma chlordane = 0.060 ppm), (Saha
and Stewart, 1967). Crops grown in soil containing 0.68 ppm
gamma-chlordane, resulting from heavy previous use of heptachlor,
contained harvest residues of gamma-chlordane of the following order :
radishes, 0.03 ppm; carrots, 0.16 ppm; potatoes, 0.03 ppm; pea greens,
not detected (Lichtenstein et al, 1967 in press).
In animals
An important recent development with a bearing on the evaluation of
the hazard of residues in food to humans is the evidence that in
mammals gamma-chlordane can be metabolized and detoxified to more
hydrophilic products which are largely excreted into the aqueous
phase. Rats receiving intravenous doses of gamma-chlordane 14C
excreted 22 per cent of the total dose within 60 hours in the form of
hydrophilic metabolites in feces. High percentages of hydrophilic
metabolites were detected in the entire alimentary tract and kidneys.
Unchanged gamma-chlordane was found only in subcutaneous fat
(Poonawalla and Korte, 1964). Weekly doses of gamma-chlordane 14C
administered by stomach tube for ten weeks to rabbits resulted in 47.2
per cent of the total administered radioactivity being excreted in
urine, and 22.7 per cent in feces, with only about 4 per cent in fatty
tissues. Unchanged gamma-chlordane could be detected only in
subcutaneous fat, while all other tissues contained hydrophilic
metabolites. In urine, only hydrophilic metabolites could be detected.
Two hydrophilic metabolites have been isolated, one identified as a
chloro-hydrine. The acute oral toxicity of this metabolite is lower
than that of chlordane. The LD50 value for mice is higher than 1800
mg/kg. The structural formula for this metabolite assigned is
1-hydroxy-2-chloro-dihydrochlordane. The second metabolite is more
hydrophilic than the first, with both chlorine atoms in position 1 and
2 of gamma-chlordane possibly replaced by hydroxylic groups (Ludwig,
1966). The first metabolite may also possibly be derived from
alpha-dihydro-heptachlor resulting from oxidative detoxication
metabolism of heptachlor. Brooks and Harrison, (1967) assign a
slightly different structure to this metabolite, designating it
possibly as an octochloro-compound.
These recent findings possibly explain why the analysis of more than
500 samples of human fat tissues in the USA did not detect chlordane
(Hoffman et al, 1964; Hoffman, 1963). In less than 50 per cent of
these samples, two peaks in GLC chromatograms were noted, representing
concentrations of 0.01 - 0.05 ppm chlorine. Assuming these peaks are
due to a pesticide, their region indicates they could be caused by
ronnel, heptachlor, heptachlor epoxide, endosulfan or chlordane, or
hydrophilic metabolites of chlordane only partially soluble in polar
solvents.
The weathered residues of plants consist largely of alpha- and
gamma-chlordane. If this also occurs in residues translocated from
soil to growing plants, their hazard to man, after ingestion and
detoxication to hydrophilic metabolites, would reasonably be much less
than that for technical chlordane.
Residues of chlordane in meat resulting from direct application to
animals has been reported (Claborn, 1956; Claborn et al, 1960).
Consequently, chlordane is not currently recommended for use in direct
treatment of animals. Residues in whole milk resulting from grazing
cows on chlordane-treated pastures as early as one day, one week, two
weeks and four weeks after treatment were dosage dependent. At 0.25
lb/acre, none was found; at 0.5 lb and 1.0 lb per acre, a maximum of
0.08 ppm at the end of 8 weeks of feeding was found together with
maximum residues of heptachlor epoxide of 0.04 ppm. (Westlake et al,
1963).
Alfalfa treated with chlordane at one and two pounds per acre resulted
in hay residues of 20.4 and 20.9 ppm. Cows receiving this alfalfa for
a period of 150 and 100 days produced milk that contained residues
ranging from none to 0.2 ppm maximum (Carter et al, 1956).
Both of these experiments demonstrate considerable variation in the
residues excreted in milk by individual animals and suggest that
average results from a large herd of dairy animals would produce
smaller residues in pooled milk.
New USA data have been developed recently from feeding alfalfa with
known residues to dairy cattle. A progress report reviewed by the
Joint Meeting suggests that feeding alfalfa hay treated at 1.0 and 2.0
lbs/acre to Holstein cows resulted in a daily intake of 10.6 mg and
15.9 mg/day. Milk samples analyzed during the 31-day feeding study
were all negative (at a sensitivity of 0.01 ppm), with the exception
of one animal on the first day of feeding (Velsicol, 1967e).
In storage and processing
Information on the behaviour of residues during storage and processing
is needed. Although in the past, terminal residues of chlordane have
been regarded as stable, one investigation to date suggests that at
least in the case of rutabagas, cooking in water results in total
disappearance of residues in pulp (sensitivity 2 ppb) and 40 per cent
disappearance from peel, most being lost in water and only about 13.5
per cent steam-distilled (Saha and Stewart, 1967). Similar data on
other foods would be most useful.
METHODS OF RESIDUE ANALYSIS
A number of multidetection systems are available for the detection and
determination of organochlorine compounds and most of these can be
used for residues of alpha- and gamma-chlordane. An example is the
AOAC (1966) system in which acetonitrile partition and florisil column
clean-up are used, the residues being identified by gas chromatography
coupled with thin-layer or paper chromatography. Both electron capture
and micro-coulometric detectors can be used: the application of these
to chlordane, residue analysis, with special reference to
interferences by toxaphene (should this also be present) has been
described by Klein and Link (1967). The multidetection systems, which
normally separate the two isomers, are sensitive to about 0.02 ppm of
each isomer. Alternative methods for the confirmation of the identity
of residues, e.g. using infra-red spectrophotometry, are also
available.
Since alpha- and gamma-chlordane have boon shown by Klein and Link
(1967) to weather to approximately the same extent in agricultural
application, it is convenient to sum the residues; whilst in these
circumstances it might theoretically be possible then to apply a
factor to express this sum in terms of original technical chlordane,
it is recommended that results be reported as the total of alpha- plus
gamma-chlordane.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Canada 0.3 on a wide range of
foods (approximately
50 individual crops
included)
(cont'd)
Country Tolerance, ppm Crop
European Economic
Community 0.2 in fruits and
vegetables proposed,
(combined total with a review date
of all cyclodiene of 1 January 1970.
residues)
Netherlands,
Belgium, 0.1
Luxembourg
USA 0.3 on a wide range of
foods (approximately
50 individual crops
included)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Temporary tolerances
The potential hazard of residues resulting from the use of chlordane
has been reduced by narrowing the spectrum of use. New information on
the chemical nature of terminal residues indicates that these are
largely alpha- and gamma-chlordane. The extensive available
information from objective samples and total diet studies
in the USA (where several million pounds a year have been used
in agriculture for twenty years) indicates insignificant loads of
residues in food as consumed.
In view of these data and the relatively narrow spectrum of currently
recommended use, the following temporary tolerances, to be reviewed
31 December, 1970, are recommended :
Large root crops 0.3 ppm
Potatoes, sweet potatoes, rutabagas, turnips (soil
treatment) with the restriction that these be used for
human consumption only. If culls are to be fed to
livestock, additional data are required on the number of
pounds per day which may be fed in rations of swine, beef
and dairy cattle, with resultant residue data in animal
products.
Leafy and stalk vegetables 0.3 ppm
including all cole crops, spinach, lettuce, celery
and asparagus (soil treatment).
Small root vegetables 0.2 ppm
including but not limited to onions, table beets,
radishes (excluding carrots) (soil treatments).
Cucurbits 0.2 ppm
Cucumbers, melons including canteloupe, pumpkin and
squash (soil treatments)
Pineapple (soil treatment) 0.2 ppm
Sugar beets (soil treatment) 0.1 ppm
provided data are developed on residues remaining in
sugar beet pulp and green tops and residues remaining
in animal products when amounts of these are included
in the ration are fed to swine, beef or dairy cattle.
Pod vegetables (in the pod) 0.1 ppm
peas, beans, etc. (soil treatment)
Berry fruits 0.1 ppm
bush, cane and strawberries (soil treatment)
Tomatoes and related garden fruits (soil treatment) 0.1 ppm
Sweet corn and popcorn (soil treatment) 0.1 ppm
Comments:
Whole oil seeds: data are incomplete and available analytical
results are inconsistent. Tolerances or practical residue limits will
be required if chlordane is used in soil or as a foliar treatment.
Citrus, pome and stone fruits: no tolerance required, since no
residues are likely to result in fruit from soil treatments.
The point of enforcement of these tolerances should be on the raw
agricultural products moving in commerce.
Practical residue limits
Cereals 0.1 ppm
The meeting realized that it may be necessary at some future date to
recommend practical residue limits for chlordane for animal products.
Data were not available in 1967 to indicate such need or the levels
required.
FURTHER WORK
Further work required before 30 June 1970
1. More evidence is needed on the extent to which chlordane is used
on particular crops in various countries, the residues which
occur in commerce and total diet studies in countries using this
pesticide or importing food.
2. Data are required on the fate of residues during the normal
course of storage and processing before consumption.
3. Information on residues resulting from growing crops in rotations
on land in which chlordane has been used previously.
4. Data are required on residues in oil seeds, in vegetable oil
after processing and in oil seed cake or meal which may become
animal feed.
Further work desirable
Many of the measurements of residues made prior to 1961 need to be
repeated with the aid of the more sensitive and specific analytical
techniques.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Alvarez, W.C. and Hyman, S. (1953) A.M.A. Arch. Indust. Hyg. Occup.
Med. 8, 480
Ambrose, A.M., Christensen, H.E., Robbins, D.J. and Rather, L.J.
(1953) A.M.A. Arch. Indust. Hyg. Occup. Med. 7, 197
Batte, E.G. and Turk, R.D. (1948) J. econ Ent., 41, 102-103.
Burns, J.J., Cucinell, S.A., Koster, R. and Conney, A.H. (1965) Ann.
N.Y. Acad. Sci. 123, 273.
Carter, R.J., Hubanks, P.E., Poos, F.W., Moore, L.A. and Ely, R.E.
(1953) J. Dairy Science, 36, 1172.
Claborn, H.V., Bowers, J.W., Wells, R.W., Radeleff, R.D. and
Nickerson, W.J. (1953). Agric. Chemicals, 8, 37
Conney, A.H., Welch, R.M., Kuntzman, R. and Burns, J.J. (1967) Clin.
Pharmacol. Therap. 8, 2
Cram, R.L. Juchau, M.R. and Fouts, J.R. (1965) J. Lab. Clin. Med.
66, 906
Derbes, J.V., Dent, H.J., Forrest, W.W. and Johnson, M.F. (1955)
J. Amer. med. Ass. 158, 1367
Fishbein, W.I., White, J.V. and Isaacs, H.J. (1964) Indust. Med. and
Surg. 33, 726
Fouts, J.R. (1963) Ann. N.Y. Acad. Sciences, 104, 875
Fouts, J.R. and Hart, L.G. (1965) Ann. N.Y. Acad. Sciences, 123, 245
Fouts, J.R. and Rogers, L.A. (1965) J. Pharmacol. Exper. Therap.
147, 112
Gaines, T.G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L.G. and Fouts, J.R. (1963) Proc. Soc. exp. Biol. (N.Y.), 114,
388
Hart, L.G. and Fouts, J.R. (1965) Biochem. Pharmacol. 14, 263
Hart, L.G., Shultice, R.W. and Fouts, J.R. (1963) Toxicol appl.
Pharmacol., 5, 371
Hoffman, W.S., Fishbein, W.I. and Andelman, M.B. (1964) Arch.
Environ. Health, 9, 387
Ikeda, M., Sezesny, B. and Barnes, M. (1966) Fed. Proc. 25, 417
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1955) Unpublished report submitted by Velsicol Corporation
Ingle, L. (1965) A Monograph on Chlordane, Ingle, Urbana, Illinois
Ingle, L. (1967) Unpublished report submitted by Velsicol Corporation
Kuntzman, R., Jacobson, M., Schneidman, K. and Conney, A.H. (1964)
J. Pharmacol. exper. Therap., 146, 280
Lehman, A.J. (1951) Assoc. Food and Drug Officials Bull., 15 122
Lehman, A.J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W.J. and Durham, W.F. (1957) A.M.A. Arch. Path.,
64 61, 4
Princi, F. and Spurbeck, G.H. (1951) A.M.A. Arch. Indust. Hyg.
Occup. Med., 3, 64
Stein, W.J. and Hayes, W.J., Jr. (1964) Indust. Med. and Surg., 33,
549
Stohlman, E.F. and Smith, M.I. (1950) Advances in Chem. Ser., 1, 228
Stohlman, E.F., Thorp, W.T.S. and Smith, M.I. (1950) Arch. industr.
Hyg., 1, 13-19
Stormont, R.T. and Conley, B.E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Triolo, A.J. and Coon, J.M. (1966) Ag. and Food Chem., 14, 549
Turner, H.F. and Eden, W.G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Wazeter, F.X. (1967) Unpublished report submitted by Velsicol
Corporation
Welch, H. (1948) J. econ. Entomol., 41, 36-39
Welch, R.M. and Harrison, Y. (1966) The Pharmacologist, 8, 217
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.
and Schutzmann, R.L. (1963) J. Ag. Food Chem., 11, 244
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Allen, W.R. (1963) Plant growth dilution and the decline of
insecticide residues from alfalfa in Manitoba. Proc. Ent. Soc.
Manitoba 19.
AOAC. (1966) Changes in methods of analysis. J. Assoc. Off. Anal.
Chem. 49 : 222-230.
Banham, F.L. (1961) The persistence of certain insecticides for
control of the tuber flea beetle, Epitrix tuberis (Gent.) in the
interior of British Columbia. Can. J. Plant Sci. 41 : 664.
Begg, J.A., Plummer, P.J.G., Konst, H. (1960) Insecticide residues in
potatoes after soil treatments for control of wireworms. Can. J. Plant
Sci. 40 : 680-669.
Bess, H.A., Ota, A.K., Kawanish, C. (1966) Persistence of soil
insecticides for control of subterranean termites. J. Econ. Ent. 59 :
911-915.
Brooks, G.T., Harrison, A. (1967) The toxicity of
alpha-dihydroheptachlor and related compounds to the housefly (M.
domestica L.) and their metabolism by housefly and pig liver
microsomes. Life Sciences 6 : 1439-1448.
Carter, R.H., Claborn, H.V., Woodard, G.T., Ely, R.E. (1956) Residues
in animal products. USDA Yearbook of Agriculture, 143-148.
Chisholm, R.D., Koblit, S.K.Y., Westlake, W.E. (1966) Estimation of
aldrin and chlordane residues in soils treated for termite control.
Pest Control 30 : 48-50-52-53.
Claborn, H.V. (1956) Insecticide residues in meat and milk. USDA ARS
33-25.
Claborn, H.V., Bushland, R.C., Mann, H.D., Ivey, M.C., Radeleff, R.D.
(1960) Meat and milk residues from livestock sprays. J. Agr. Food
Chem. 8 : 439.
Cook, W.C. (1960) Report of residue analysis. USDA, ARS., Ent. Res.
Div., Pesticide Chemicals Res. Br., Report No. PCY-60-21.
Corley, C., Miles, E.J., Shands, W. (1965) Chlorinated hydrocarbon
residues in potatoes. USDA, ARS., Ent. Res. Div., Pesticide Chemicals
Res. Br., Report No. PC-B-65-22.
California State Department of Agriculture. (1962) Chlordane residues
in commercial food channels. Personal Communication.
Duggan, R.E., Barry, H.C., Johnson, L.Y. (1966) Pesticide residues in
total diet samples. Science 151 : 101-104.
Duggan, R.E. (1967) Objective samples of food analysed by USFDA for
chlordane residues. 1964-1967. Personal Communication.
Edwards, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. Ecology and the Industrial Society. Vth
Symp. Brit. Ecol. Soc., Beackwell, Oxford. p.239.
Edwards, C.A. (1966) Insecticide residues in soils. Res. Rev. 13 :
83-132.
Egan, H., Holmes, D.C., Roburn, J., Tatton, J.O.G. (1966) Pesticide
residues in foodstuffs in Great Britain II. Persistent organochlorine
pesticide residues in selected foods. J. Sci. Food Agr. 17 : 563-569.
Fahey, J.E., Hamilton, D.W., Rusk, H.W. (1957) Effect of spray date on
residues of chlorinated hydrocarbon insecticides on peaches. J. Econ.
Entomol. 50 : 366.
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Ent. 55 : 179.
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65.
Fischback, H. (1963) Problems stemming from the refinement of
analytical methods. Publication No. 1082, Natl. Acad. of Sci. NRC
55-62.
Hoffman, W.S. (1963) Personal communication.
Hoffman, W.S., Fishbein, W.I., Andelman, M.B. (1964) The pesticide
content of human fat tissue. Arch. Env. Health 9 : 387-394.
IUPAC. (1967) 1966 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. 50 :
1081-1083.
IUPAC. (1968) 1967 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. (in
press).
Klein, A.K., Link, J.D. (1967) Field weathering of toxaphene and
chlordane. J. Assoc. Off. Analyt. Chem. 50 : 586-91.
Korte, F. (1967) Personal communication
Landis, B.J. (1965) Report of residue analysis; soil, DDT, aldrin and
chlordane. USDA, ARS, Ent. Res. Div., Pesticide Chemicals Br., Report
No. PCY-65-25.
Lichtenstein, E.P. (1967) Alpha-chlordane content of heptachlor
formulations. Personal communication.
Lichtenstein, E.P., Fuhnemann, Schultz. (1967) Use of carbon to reduce
the uptake of insecticidal soil residues by crop plants. Effects of
carbon on insecticide absorption and toxicity in soils. J. Agr. Food
Chem. (in press).
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the Detection of Pesticide Residues. International
Atomic Energy Agency, Vienna, pp. 49-58.
Majumder, S.K. (1967) A review of the problem of the toxicity of
pesticide chemicals in food in India. Ind. Food Packer 21 : 1-14.
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Ent. 45 : 452-456.
Marth, E.H. (1962) Chlorinated hydrocarbons deposited in biological
material. I. Plants and plant products. J. Milk Food Technol. 25 : 36.
Minyard, J.P., Jackson, E.R. (1963) Pesticide residues in commercial
animal feeds. J. Assoc. Off. Analyt. Chem. 46 : 843-859.
Morgan, L.W., Leuk, D.B., Beck, E.W., Wardham, D.W. (1967) Residues of
aldrin, chlordane, endrin and heptachlor in peanuts grown in treated
soil. J. Econ. Ent. 60 : 1289-1291.
Muns, R.P., Stone, M.W., Foley, F. (1960) Residues in vegetable crops
following soil applications of insecticides. J. Econ. Ent. 53 : 832.
Nash, R.G., Woolson, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science 157 : 924-927.
Ordas, E.P., Smith, V.C., Meyer, C.F. (1956) Spectrophotometric
determination of heptachlor and technical chlordane on food and forage
crops. J. Agr. Food Chem. 4 : 444.
Painter, Ruth R., Kilgore, W.W., Ough, C.S. (1963) Distribution of
pesticides in fermentation products obtained from artificially
fortified grape musts. J. Food Sci. 28 : 342.
Perez-Escolar, M.E. (1959) Control pineapple gummosis in Puerto Rico.
J. Agr. Univ. Puerto Rico 43 : 116.
Poonawalla, N.H., Korte, F. (1964) Metabolism of insecticides. VIII
Excretion, distribution and metabolism of alpha-chlordane-14C by
rats. Life Sci. 3 : 1497
Saha, J.G., Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide, and
gamma-chlordane residues in soil and rutabagas after soil and surface
treatments with heptachlor. Can. J. Plant Sci. 47 : 79-88.
Robinson, J., McGill, A.E.J. (1966) Organochlorine insecticide
residues in complete prepared meals in Great Britain in 1965. Nature
212 : 1037-1038.
Rusk, H.W., McDonough, L.M. (1966) Report of residue analysis; soils
and sugar beet roots. USDA, ARS, Ent. Res. Div., Pesticide Chemicals
Res. Br., Report Nos. PCY-67-6 and PCY-66-9.
Smith, F.F., Adams, H.R. (1965) Chlordane residues in crops. USDA,
ARS, Ent. Res. Div. Pesticide Chemicals Res. Br., Special Report
PC-B-65-26.
Smith, F.F., Reid, W.J., Cuthbert, F.P. (1964) Dieldrin and chlordane
residues in sweet potatoes. USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report PC-B-64-10.
Swackhamer, A.B. (1967) Personal communication
Thurston, A.D. (1965) Preliminary studies on weathering of chlordane
residues. J. Assoc. Off. Analyt. Chem. 48 : 952-4.
USDA. (1964) Chlorinated hydrocarbon pesticide residues in soil at
Battle Creek, Mich., 1963, USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report No. PL-V-64-10.
USDA. (1966) Report of residue analysis; potatoes, chlordane. ARS,
Ent. Res. Div., Pesticide Chemicals Res. Br., Report No. PCY-66-1.
USDA. (1967a) Monitoring chlorinated hydrocarbon insecticide residues
in soybeans, 1966. ARS, Plant Pest Control Division, interim report,
1967.
USDA. (1967b) Report of residue analysis; soil; DDT and chlordane.
USDA, ARS, Ent. Res. Div., Pesticide Chemicals Res. Br., Report No.
PCB-67-9.
Velsicol Corp. (1967a) Unpublished report on chlordane residues in
soil. Submitted to FAO.
Velsicol Corp. (1967b) Unpublished report on residues in potatoes from
soil treatments with chlordane. Submitted to FAO.
Velsicol Corp. (1967c) Unpublished report on residues in barley grain
and straw from spraying growing barley at Fargo, N.D., 1966. Submitted
to FAO.
Velsicol Corp. (1967d) Unpublished report on chlordane residues in
sugar beets. Submitted to FAO.
Velsicol Corp. (1967e) Progress report on chlordane dairy feeding
study. Submitted to FAO.
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.,
Schutzmann, R.L. (1963) Chemical residues in the milk of cows grazed
on chlordane-treated pasture. J. Agr. Food Chem. 11 : 244.
Other relevant chemical properties
Chlordane, as presently manufactured in the USA, is a uniform
technical product, the composition of which is controlled. Analytical
methods and reference standards have been made available periodically
by the manufacturer to regulatory agencies as a method of commercial
product control. Analytical methods for regulating the technical
formulation in commerce have become official methods after
collaborative studies by the Association of Official Analytical
Chemists (AOAC) in the USA and the European Commission for Methods of
Analysis of Pesticides (CIPAC). While hexachlorocyclo pentadiene is
used in the manufacture of the technical product, none of this
chemical remains unreacted and there is no evidence that it appears as
a constituent of the terminal residue in food.
There is some confusion in nomenclature. Gamma-chlordane is the
alpha-chlordane referred to by March (1952) (it is the component
present in the highest concentration in technical chlordane),
alpha-chlordane is the beta isomer described by March. Poonawalla and
Korte (1964), Ludwig (1966), and some other authors still use March's
designation. These two components together comprise approximately half
of technical chlordane.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Chlordane is absorbed from the gastrointestinal tract, the respiratory
tract, and through the skin (Ambrose et al., 1953). It is stored in
the adipose tissue of rats, sheep, goats, and cows and accumulates in
the milk.
Rats fed chlordane for six and a half months showed the following
levels in their abdominal fat at various feeding levels (in ppm):
intake 3, residue 5.2; intake 15, residue 32; intake 30, residue 76;
intake 60, residue 109 (Ingle, 1965).
Chlordane fed at 25 ppm in the diet for 8 weeks reached a maximum
level of 18 ppm in the fat of calves and 12 ppm in sheep. After
feeding was stopped, the residue was eliminated from calves in 20
weeks and from the sheep in 4 weeks (Claborn et al., 1953).
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlordane is
excreted in the urine of rabbits (Stohlman and Smith, 1950).
Cows grazing on pasture to which 0.5 pound of chlordane per acre had
been applied showed an average of 0.03 ppm of chlordane in their milk
(Westlake et al., 1963).
Both technical chlordane and one of its pure isomers (gamma-chlordane)
have been shown to have stimulating effects on rat liver microsomes
for the metabolism of certain drugs (Burns et al., 1965; Hart and
Fouts, 1963; Hart et al., 1963; Kuntzman, et al., 1964).
In vitro metabolism by hepatic microsomal enzymes from rats treated
8 days previously with single i.p. doses of 10 or 100 mg/kg of
chlordane in corn oil was increased for the 3 drugs reported
(hexobarbital, aminopyrine and chlorpromazine). The increases for
hexobarbital and aminopyrine were dose-related. In vivo hexobarbital
response, measured by sleeping time of mice, 1, 3 and 8 days after
single i.p. doses of 25 mg/kg body-weight technical chlordane or
gamma-chlordane, showed a decrease (shortened sleeping time), greatest
at day 3 but still marked on day 8 (Fouts, 1963).
The mechanism of action of chlordane is similar to that of
phenobarbital in stimulating hepatic drug metabolism in the rat. Both
agents increase liver weight, microsomal protein, microsomal NADPH
oxidase, and microsomal cytochrome CO-binding pigment. They both
stimulate drug metabolism in adrenalectomized or hypophysectomized
rats and promote the proliferation of smooth endoplasmic reticulum in
the liver cell, and ethionine blocks the enzyme stimulating effects of
both agents (Hart and Fouts, 1965).
Liver microsomal preparations from adult, female rats given 10 mg/kg
of chlordane intraperitoneally every other day for 14 days showed a
385 per cent increase in the biotransformation of 17ß-estradiol to
polar metabolites (Kuntzman et al., 1964). The intraperitoneal
injection of immature male rats with 50 mg/kg of chlordane daily for 4
days produced an increase of activity of the liver microsomal steroid
hydroxylases (Conney et al., 1967).
The toxicities of bishydroxycoumarin and phenylbutazone were reduced
in both rats and dogs pretreated by chlordane, and this effect was
accompanied by lower plasma levels of these drugs in the
chlordane-treated animals than in controls given the same doses (Welch
and Harrison, 1966). The toxicity of parathion was reduced in mice
pretreated 16 hours to 4 days previously with a single oral dose of
130 mg/kg of chlordane (Triolo and Coon, 1966).
Male rats weighing 60 to 80 gms were given 25 mg/kg of chlordane
intraperitoneally daily for 3 days, and were sacrificed 24 hours after
the last dose. The livers showed a proliferation of the
smooth-surfaced endoplasmic reticulum in the parenchymal cells,
associated with an increased activity of microsomal preparations from
such livers to metabolize hexobarbital, aminopyrine, zoxazolamine and
p-nitrobenzoic acid (Fouts and Rogers, 1965).
Pretreatment of rats for several days with chlordane increased the
LD50 of warfarin more than 10-fold, an effect associated with
decreased plasma levels of warfarin and an increased rate of
metabolism of the drug by liver microsomal enzymes (Ikeda et al.,
1966).
Technical chlordane and one of its pure isomers, gamma-chlordane,
administered to adult or weanling rats in single or 3 daily doses,
after 1 to 8 days increased the hepatic microsomal metabolism of
hexobarbital, aminopyrine, and chlorpromazine (Hart et al., 1963).
Treatment of newborn rabbits with 50 mg/kg of gamma-chlordane daily
for 3 days increased the activity of liver enzymes that metabolize
hexobarbital, aminopyrine and p-nitrobenzoic acid. Treatment of
lactating mothers with chlordane increased the drug metabolizing
enzymes in the nurslings (Fouts and Hart, 1965). Drug metabolism was
also stimulated in adult rabbits.
In dogs given 5 mg/kg of chlordane orally three times a week for 7
weeks the rate of metabolism of phenylbutazone was markedly increased.
This effect persisted as long as 21 weeks after termination of the
chlordane administration (Burns et al., 1965).
Chlordane has been demonstrated to stimulate the rate of drug
metabolism by the liver of the squirrel monkey (Cram et al., 1965).
For further information see the sections In Plants, and In
Animals.
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat Oral 200 - 590* Ambrose et al., 1953
Ingle, 1955
Stohlman et al., 1950
Oral 335 - 430 Gaines, 1960
150 - 225 Ingle, 1955
Mouse Oral 430 US Food and Drug Admin., 1947
Rabbit Oral 100 - 300* Stohlman et al., 1950
20 - 40 Ingle, 1955
Goat Oral 180 Welch, 1948
Sheep Oral 500 -1000 Welch, 1948
Chicken Oral 220 - 230 Turner and Eden, 1952
* The differences are explained by the use of different solvents,
and by the fact that the chlordane mentioned in the older
literature contained a considerable amount of the very toxic
hexachlorocyclopentadiene (Ingle, 1965; Lehman, 1952).
Man. A dose of 104 mg/kg proved fatal (Derbes et al., 1955). An
18-year old female showed convulsions but recovered after a dose of
approximately 30 mg/kg (amount retained after vomiting estimated to be
10 mg/kg). In two infants, 15 months and 3 years of age, 10 and 40
mg/kg respectively gave severe poisoning (Stormont and Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12 male
rats, all of them died within 10 days. At 500 ppm, 12/12 died within
70 days; at 300 ppm, 9/12 were alive after 100 days (Stohlman et al.,
1950).
Daily oral doses of 6.25 - 25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg, all
the animals died. Intracytoplasmic bodies in the liver-cells were
found at all levels and their number was in proportion to the dose
used (Ambrose et al., 1953).
Groups of 6 females and 6 males were fed 2.5 ppm or 25 ppm of a sample
of technical chlordane containing 60-75% chlordane and 25-40%
unrelated products for up to 9 months. Centrolobular cell hypertrophy,
peripheral migration of cytoplasmic granules and the presence of
cytoplasmic bodies were observed in 1 male at 2.5 ppm and in 5 males
at 12.5 ppm (Ortega et al., 1957).
Initial groups of 10 males and 20 females were used in a 3-generation
study at dietary levels of 0, 0.3, 3, 15, 30 and 60 ppm of technical
chlordane. Two litters in each filial generation were studied. Levels
up to and including 30 ppm had no effect on fertility, numbers of
young or litters, or weight, growth or mortality of the young animals
to weaning age. Autopsy of animals post-weaning showed no gross or
microscopic difference between the groups. At 60 ppm, there was a high
(10.6 per cent) mortality in the second F3 generation litters during
the latter part of the nursing period; these animals showed gross and
microscopic pathology comparable to that characteristic for chlordane
intoxication. However, survivors of this generation showed no tissue
changes at all. A third act of F3 litters at 60 ppm suffered 17 per
cent mortality during the nursing period, with symptomatology end
gross and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from the
60 ppm group and placed on chlordane-free diets for 30 days prior to
remating showed no difference in any respect from control litters. No
evidence of teratogenicity or tumorigenicity for chlordane was found
in this study (Ingle, 1967).
Dog. Chlordane was given in varying oral doses to dogs for 7 days,
convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but 700
mg/kg (highest dose) did not produce any effect (Batte and Turk,
1948).
Four groups of 2 to 4 dogs given chlordane orally in doses between 5
and 80 mg/kg body-weight daily all died within periods of 25 days to
93 weeks (Lehman, 1952).
Groups of 4-7 males and 4-7 females were fed 0, 0.3, 3, 15 and 30 ppm
of chlordane for 2 years. Abnormalities in the results of clinical
liver function tests were seen in the 15 and 30 ppm groups. In animals
selected for necropsy at the end of the first year, increased relative
liver weights and associated hepatocellular changes were found at 30
ppm; at the end of two years, dose-related increases in relative liver
weights were found at 15 and 30 ppm, with non-dose-related
hepatocellular changes. There was no difference between the severity
of the liver lesions of the 30 ppm animals and those of four animals
withdrawn from 30 ppm treatment for eight months prior to sacrifice.
Percutaneous liver biopsies on two animals of the 30 ppm group at 1, 3
and 6 months showed hepatocellular changes at 6 months but not at 1 or
3 months. No adverse effect was seen on behaviour, appearance,
survival, weight gain, blood picture or the results of periodic
physical examination, at all levels (Wazeter, 1967).
Sheep. Chlordane administered by stomach-tube to sheep in a dose of
0.5 g/kg body-weight produced toxic symptoms (incoordination, partial
blindness) in 5 to 6 days. A dose of 1 g/kg body-weight produced
severe respiratory and nervous symptoms at 16 hours and death after 48
hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each sex)
were given 2.5, 25 and 75 ppm of chlordane in the diet for 2 years.
The sample of chlordane used had an LD50 of 450 mg/kg (Lehman, 1951).
It was found that 25 and 75 ppm gave moderate to severe signs of
intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
Groups of 40 rats (20 males and 20 females) were fed concentrations of
5, 10, 30, 150 and 300 ppm of "technical chlordane" in the diet over a
2-year period.
Throughout the experiment tremors and convulsions appeared or could be
induced at 30 or more ppm. Following fasting, no neurological symptoms
appeared at 5 or 10 ppm. Growth rate was affected at 150 or 300 ppm.
Liver histological damage was observed in the form of hypertrophy of
centrolobular calls, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm, slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was carried
on between late 1953 and late 1955, "technical chlordane of recent
manufacture" was used. Groups of 40 rats were given chlordane at 2.5,
5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given no
chlordane. Changes concerning food consumption, growth and mortality
were seen only in the 300 ppm group. Liver cell changes were not
present in the animals given 2.5-25 ppm. At 50 ppm only "cytoplasmic
peripheralization" was present. At higher doses the changes were as
those previously described (Ingle, 1955).
In a study published in 1953, a sample of chlordane exhibiting an oral
LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each sex
were given 0, 10, 20, 40, 80, 160, 320, 640 and 1280 ppm of chlordane
in their diets for approximately 407 days. The animals at 640 and 1280
ppm died early. At lower dosages, survival was unaffected. Increased
liver-weight (in comparison with the control group) was observed over
320 ppm. In a sample of liver of a male at 320 ppm the average nuclear
volume was 377µ3 compared to 268µ3 in a control rat. Cytoplasmic
vacuoles containing fat and clusters of granules at the periphery of
the cytoplasm were often seen. In the males they were equivocal at 10
ppm, absent at 20 ppm and infrequent at 40 ppm. In the females these
lesions were common and were seen only at 80 ppm and over (Ambrose et
al., 1953).
Observations in man
Workers engaged in the manufacture and formulation of chlordane for
periods up to 15 years have exhibited no evidence of harmful effects
attributable to this insecticide (Princi and Spurbeck, 1951; Alvarez
and Hyman, 1953; Fishbein et al, 1964). In a survey of more than 1105
persons who had been engaged in pest control operations for 1 to 30
years (318 for 5 to 19 years), three cases of toxicity due to
chlordane were reported, the only symptoms specified being dizziness
and headache (Stein and Hayes, 1964).
Chlordane was not found in any of 282 human fat samples taken at
autopsy, though DDT and lindane were regularly detected and dieldrin
was frequently found (Hoffman et al., 1964).
Comments
Since chlordane stimulates drug metabolizing processes in the rat,
mouse, rabbit, dog and squirrel monkey, and since the microsomal
enzyme stimulating properties of chlordane are similar to those of
phenobarbital, which stimulates drug metabolism in man, it appears
extremely likely that chlordane also stimulates drug metabolism in
man. This effect in animals and the associated cellular changes in the
liver, considered characteristic of the chlorinated hydrocarbon
insecticides as a class, are thought by several workers not to
represent toxic effects but rather physiological adaptive processes.
Toxicological studies reported prior to 1953 are not contributory to
the evaluation of the currently manufactured product because of the
variability of composition before that time. The chlordane made for
commercial use since 1953, which has been used in the studies
reported, has a constancy of composition that makes the data adequate
for toxicological evaluation.
In none of the many toxicological studies done has there been any
evidence observed of tumorigenic or other irreversible pathologic
changes, even at the highest doses or feeding levels used.
On the basis of the results of two long-term feeding studies in the
rat, a no-effect level for the rat may be established at 20 ppm, or 1
mg/kg/day. A satisfactory 3-generation reproduction study in the rat
has been completed recently in which 30 ppm was without effect on
fertility, number or size of litters, or on the weight, growth or
mortality of the young to weaning age. Furthermore, there was no
evidence of teratogenicity. In the recently completed 2-year feeding
study in the dog, 3 ppm of chlordane was the highest level used that
had no effect in tests of liver function or on liver weight and
structure of the liver cell. Though the liver weight and cell
structure changes at 15 ppm may probably be classified as adaptive
rather than toxic in nature, the significance of the functional
changes at this level cannot be assessed in the present state of our
knowledge. Even though these changes consistently disappeared before
the end of the 2-year feeding period, and even though 15 and 30 ppm
produced no other adverse effects throughout the 2-year period, it
seems necessary at this time to take 3 ppm chlordane as the maximum
known no-effect level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat. 20 ppm in the diet, equivalent to 1 mg/kg/day
Dog. 3.0 ppm in the diet, equivalent to 0.075 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.001 mg/kg body weight
Further work required
See General Comments page 3 and 4.
Further work desirable
Toxicological data on alpha and gamma chlordane.
EVALUATION FOR TOLERANCES
USE PATTERN
Chlordane was the first cyclodiene insecticide and originally had a
broad scale and spectrum of use but was somewhat displaced by aldrin,
dieldrin, endrin and heptachlor because of their greater persistence
and effectiveness and lower cost. In recent years, to narrow the use
spectrum of aldrin, dieldrin, endrin and heptachlor, there is renewed
interest in the use of chlordane.
Several million pounds of chlordane have been used annually in North
America for more than 20 years of which between 30 and 40 per cent has
been used in agriculture. The USA and Canadian currently approved uses
represent the largest spectrum and potential scale of use in the world
and have been used an a guide to this evaluation.
Pre-harvest treatments
The most important use of chlordane in for soil treatment, with a
limited use for foliar application, the main crops being large root
crops, sugar beets, corn, small root vegetables, leafy and stalk
vegetables, cucurbits, leguminous vegetables, berry fruits, sugar
cane, pineapple and cereals. Some uses on forage crops are still
approved in the USA.
The literature suggests that in fifty or more countries, chlordane is
or can be used on sugar beets, potatoes, vegetables, tobacco, sugar
cane, ground nuts (peanuts), beans, bananas, coffee, deciduous fruits,
citrus, olives, cotton and tea bushes. However, information on the
scale of use and resulting residues is not generally available. In
other countries the scale of use is probably only a fraction of that
in the USA. Dosages vary between countries, with a range of 1 to 8
lbs/acre recommended for a variety of vegetable insect control
requirements.
Post-harvest treatments
No post-harvest uses are currently recommended.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The large amount of available information needs critical review
because of improvements in analytical methods since 1961 and recent
information on the chemical nature of terminal residues. Methods used
prior to 1963 were based on total chlorine or colorimetric
measurements and expressed results in terms of technical chlordane.
Recently developed methods identify individual components, the
summation of which frequently is approximately one half of that
reported by earlier non-specific methods. New data developed from 1965
to 1967 reports individual components of residues as alpha and
gamma-chlordane, sometimes with much smaller and insignificant
quantities of heptachlor and heptachlor epoxide. Small amounts of
gamma-chlordane will be found in some crops resulting from the use of
technical heptachlor in soil (Lichtenstein et al, 1967; Saha and
Stewart, 1967; Velsicol, 1967 a through d). Some authors have
recently added the various components of the residue and reported the
total as chlordane (e.g. Smith and Adams, 1965).
The available information concerning residues resulting from uses
constituting "good agricultural practice" (i.e. consistent with pest
control requirements, principally in the USA and Canada) are
summarized below.
Crop Residue (ppm) Reference
alfalfa 0.01 Ordas et al, 1956
" 0.16 Allen, 1963
barley, grain 0.0 - 0.06 Velsicol, 1967c
" , straw 0.98 - 2.65 " "
beans <0.01 Smith and Adams, 1965
beets 0.17 "
beet tops 0.01 - 0.1 "
bell peppers none recovered Muns et al, 1960
broccoli " " "
cabbage " " "
cantaloupe 0.08 "
" , meat and rind 0.01 - 0.1 Smith and Adams, 1965
carrots 1.5 Muns et al, 1960
" 0.01 - 0.13 Cook, 1960
" 0.16 (1) Lichtenstein et al, 1967
" , peel 0.0 - 0.7 Cook, 1960
collards none recovered Marth, 1962a
cucumbers 0.08 Muns et al, 1960
eggplant none recovered "
lettuce 0.04 "
peaches 0.8 Fahey et al, 1957
peanuts 1.74 ± 0.82 Morgan et al, 1967
pineapple 0.145, 0.43 (2) Perez-Escolar, 1959
potatoes <0.01 - 0.23 (3)
radishes 0.03 Lichtenstein et al, 1967
rutabagas 0.5 Muns et al, 1960
" 0.08 - 0.16 (4) Saha and Stewart, 1967
snap beans <0.01 Smith and Adams, 1965
strawberries <0.01 "
" none recovered Fahey, 1962
sweet potatoes 0.01 - 0.4 (5)
sugar beets 0.04 - 0.37 (6)
tomatoes 0.01 Muns et al, 1960
" <0.01 Smith and Adams, 1965
turnips 0.01 - 0.1 "
" 0.16 Muns et al, 1960
turnip tops 0.01 - 0.1 Smith and Adams, 1965
wine none (7) Painter et al, 1963
(1) soil contained 0.68 ppm gamma chlordane (from heptachlor application 5 years
previous).
(continued)
(2) resulting from one and two applications, respectively.
(3) overall range as reported by many authors, e.g. Lichtenstein, 1967; Smith
and Adams, 1965; Begg et al, 1960; USDA, 1964; USDA, 1966; Corley et al,
1965; Velsicol, 1967b.
(4) gamma chlordane from heptachlor
(5) overall range as reported by many authors, e.g. Muns et al, 1960; Smith and
Adams, 1965; Smith et al, 1964.
(6) overall range following different rates of application as reported by
many authors, e.g. Muns et al, 1960; Rusk and McDonough, 1966;
Velsicol, 1967d.
(7) 0.6 in must and lees.
Data are required from similar investigations using reliable
analytical procedures from other countries. Information pertaining to
successive crops grown in rotation over a period of years after
initial treatment would be of particular interest. Data are also
required on the residues which may occur in oil seeds as the result of
intentional use, or from growing these crops in rotations. This is of
particular importance where certain oil-containing crops are grown in
rotation with other crops, especially after maize.
In general these data suggest that the highest residues consistent
with good agricultural practice will result in the large root crops,
and under certain conditions in the leafy and stalk vegetables.
Considerable variation can be expected in residues found in the large
root crops, particularly potatoes, depending on soil type, variety of
potato and date of maturity. Recent information suggests that the
residues in leafy and stalk vegetables may in part be from direct
contamination from soil, rather than a true translocation into growing
parts other than the stem or stalk. The intermediate group in
magnitude of residues to be expected would include small root
vegetables, cucurbits and pineapple. Cereals, berry fruits, pod
vegetables and fruit vegetables of the tomato type are in the category
of lowest residues, usually well below 0.1 ppm. It is probable that
there is no true translocation of residues in the grain of most
cereals and that the residues found are due to mechanical
contamination by soil, dust, debris, etc., during harvesting
operations.
RESIDUES IN FOOD MOVING IN COMMERCE
Pesticide residues in commercial animal feeds are important sources of
residues in milk and other animal products. Data concerning chlordane
are available from one such survey in Tables 1 and 2 (Minyard and
Jackson, 1963).
TABLE I
The occurrence of chlordane in commercial feeds expressed in parts per billion
No. No. Range of chlordane Range of total
Feed samples positive residue (ppb) pesticide
residue (ppb)
16% protein Dairy 73 53 0-254 26 - 1153
18% protein Dairy 6 4 13- 39 55 - 713
20% protein Dairy 2 2 15- 34 139 - 274
32% protein Dairy 4 1 5 36 - 435
12% protein Fitting
and Freshening 2 1 12 49 - 60
14% protein Cattle 3 2 11- 12 47 - 183
20% protein Beef 1 1 45 343
16% protein Hog 3 0 - 34 - 62
16% protein Poultry 3 2 7- 11 80 - 291
TABLE II
Ranges and averages of insecticide residues found in 101 feeds expressed in parts per billion
Av. level of
No. Per cent Range of contamination of
Insecticide contaminated contaminated contamination contaminated foods
(ppb) (ppb)
lindane 101 100.0 5 - 79 16.0
aldrin 87 86.1 1 - 16 2.6
DDT 99 98.0 10 - 448 80.5
methoxychlor 46 45.5 10 - 580 74.0
endrin 59 58.4 4 - 27 10.6
chlordane 68 67.3 5 - 254 30.4
dieldrin 89 88.1 1 - 16 4.9
toxaphene 15 14.8 60 - 530 178.3
heptachlor 9 8.9 2 - 13 4.8
heptachlor epoxide 3 3.0 1 - 6 3.3
Soybeans follow maize in recommended crop rotations in the USA. Soil
used for maize production is treated with chlordane, aldrin or
heptachlor and may result in residues of chlordane in soybeans
subsequently grown in these soils. An interim report on a survey of
unintentional residues from soil and crop samples from 27 regional
special sampling locations in the USA was made available to the
meeting. Chlordane was detected in soils from 3 fields at an average
concentration of 0.26 ppm, soybeans from one of these fields
containing 0.03 ppm. Eight other soil samples contained an average
concentration of 0.50 ppm (range 0.09 to 1.11 ppm). No chlordane was
detected in soybean seed or plants from these latter soils (USDA,
1967a).
Information on sources of chlordane in total diet studies has been
available for five years. The earliest of these concerns 12,000
samples analyzed by paper chromatography and gas liquid chromatography
in the state of California (CSDA, 1962). No chlordane was found in
this survey.
Thirty-eight total diet studies in 1961-62, for six USA cities
resulted in only two samples containing chlordane at 0.01 and 0.03 ppm
(Fishbach, 1963).
Eighty-two foods collected from 18 markets in three different
geographical areas in the USA were prepared for consumption, resulting
in 216 composite samples, and then analysed (Duggan et al, 1966).
Chlordane was found in only one composite sample at 0.033 ppm in
"garden fruit" produce (i.e. raw peppers, fresh and canned tomatoes,
raw cucumbers, catsup, eggplant, raw and frozen summer squash).
Comprehensive unpublished information from the USA was made available
to the 1967 Joint Meeting (Duggan, 1967). This data is concerned with
both objective samples of food for the years 1964 -1966 inclusive and
total diet samples for the year ending April 1967.
TABLE III
Objective Samples - Chlordane
Year 1964 1965 1966 Total
Number of domestic samples 20,088 14,462 14,806 49,356
Per cent of samples positive 0.7 1.1 0.9 0.9
Number of import samples 1,224 1,217 1,399 3,840
Per cent of samples positive 0.2 0.9 0.1 0.4
Total Diet Samples - Chlordane
Leafy vegetables - 1 composite positive contained 0.02 ppm (29 negative)
Legume vegetables - 1 composite positive contained 0.005 ppm (29 negative).
In Table IV details of the incidence and levels of chlordane for 1966
are of considerable interest as to amount and sources of residues in
the diet of man in the USA.
Canadian information covers a 4.5 year period from 1963 to July 1967.
Of a total of 14,270 samples of food (12,186 domestic and 2,084
imports), eleven contained chlordane; one lot of imported green
peppers, >0.3 ppm; six lots of whole eggs, 1.8 to 11 ppm (all seven
in excess of tolerance). Three vegetable samples contained traces of
chlordane, and one lot of turnips contained residues in excess of 0.3
ppm (Swackhamer, 1967).
Two reports of no chlordane being found in British food (Robinson and
McGill, 1966; Egan et al, 1966) are difficult to evaluate because of
lack of information on current use of chlordane in Britain and the
countries from which some foods concerned were imported.
FATE OF RESIDUES
In soils
Chlordane residues in soil have been investigated since 1949. However,
prior to 1961 much of the information is of doubtful value. Sources of
information since then include : USDA, 1964; Landis, 1965; Edwards,
1965; Edwards, 1966; Rusk and McDonough, 1966; Chisholm et al, 1966;
Bess et al, 1966; USDA 1967b; Nash and Woolson, 1967; Saha and
Stewart, 1967; Velsicol, 1967a; Lichtenstein, 1967; and Majumder,
1967.
Table V summarizes comparative persistence of some organochlorine
insecticides based on the literature review of Edwards (1966) but
modified as concerns doses used for a particular pest as far as
chlordane is concerned.
Approximately 55 per cent of chlordane remains in soil after the first
year following application, compared with 26 per cent for aldrin, 75
per cent for dieldrin and 80 per cent for DDT, a situation which is
not consistent with the respective vapour pressures; hence other
factors must be operating.
Although most reports to date express residues in soil on the basis of
equivalents of technical chlordane, Saha and Stewart (1967) reported
11.9 per cent of the residue resulting from one use of heptachlor as
gamma-chlordane (one pound of heptachlor contains 35 per cent
gamma-chlordane).
TABLE IV
Incidence and levels of chlordane in objective samples of food of domestic origin*, USA, 1966 (Duggan, 1967)
Number of Samples
Raw Manufactured Processed Fish
Range agricultural dairy animal and Vegetable Processed
ppm products products feeds shellfish oils foods Total
T - 0.03 59 1 3 1 - - 64
0.04 - 0.10 17 - 4 2 - - 23
0.11 - 0.50 35 - 4 - 1 1 41
0.51 - 1.00 - - 1 - - - 1
1.01 - 1.50 - - 2 - - - 2
1.51 - 2.00 - - 1 - - - 1
Above 2.00 2 - - - - - 2
Total samples
containing 113 ** 1 15 3 1 1 134
chlordane
Total samples
examined 9,804 611 680 290 171 227 14,806 *
* Of an additional 1399 samples of imported foods, only 1 contained chlordane, and this in a raw
agricultural product in the range of - 0.03 ppm
** 55 residues were in root vegetables
30 residues were in vine and ear vegetables
TABLE V
Persistence of some organochlorine insecticides in soil
Insecticide Average dose active Time for 95% disappearance
ingredient, lbs/acre in years
Range Mean
aldrin 1 - 3 1 - 6 3
chlordane 1 - 2 3 - 5 4
DDT 1 - 2´ 4 - 30 10
dieldrin 1 - 3 5 - 25 8
heptachlor 1 - 3 3 - 5 3´
lindane 1 - 2´ 3 - 10 6´
telodrin ¨ - 1 2 - 7 4
In plants
Information on the chemical nature of the terminal residues from
weathering of external residues has recently become available
(Thurston, 1965; Klein and Link, 1967). A further review of new
unpublished information and work in progress is contained in the 1966
and 1967 Reports of the IUPAC Commission on Chemical Nature of
Terminal Pesticide Residues (IUPAC, 1967, 1968).
The principal terminal residues from weathering of chlordane on plants
have been identified as alpha- and gamma-chlordane by characteristic
"signature" peaks as determined by gas liquid chromatographic methods
and confirmed by infrared methods. The sequence of analyses taken
after agricultural applications of technical chlordane exhibit a
fairly constant pattern. Minute amounts of heptachlor or heptachlor
epoxide appear in some chromatograms but, for all practical purposes,
these can usually be ignored.
The loss of technical chlordane from plants by normal weathering is
extremely rapid. At the end of the seventh day only 7 ppm of a
starting load of 110 ppm remains on kale, and only 0.1 ppm, expressed
as technical chlordane equivalent, remains after 28 days (Klein and
Link, 1967).
Previous studies on weathering of residues have not taken account of
the possible formation of hydrophilic metabolites, an omission which
is not probably toxicologically important since the known hydrophilic
metabolites of at least gamma-chlordane are less toxic than
technical chlordane. However, such information in domestic animals
after ingestion of weathered residues would be important in
establishing the consumer hazard of residues in animal products.
Recent information on the production of hydrophilic metabolites of
gamma-chlordane in animals (Poonwalla and Korte, 1964; Ludwig, 1966)
suggests that similar investigations should be carried out in plants,
particularly from the stand-point of translocation and metabolism of
residues in soil. Work on that subject is now in progress by Korte and
will be reported to the IUPAC Commission on Terminal Residues in 1968.
Current indications are that substantial formation of hydrophilic
metabolites (of lower order of toxicity than alpha-, gamma-, and
technical chlordane) are formed in the stalk of plants (Korte, 1967
personal communication).
Gamma-chlordane residues can occur in plants as the result of use of
technical heptachlor in soil. An application of 6.6 kg/ha mixed into
soil results in 11.9 per cent of the soil residue being in the form of
gamma-chlordane. Rutabagas grown in this soil contained 0.008 ppm
gamma-chlordane, this being 13.3 per cent of the total residue
(heptachlor + heptachlor epoxide + gamma chlordane = 0.060 ppm), (Saha
and Stewart, 1967). Crops grown in soil containing 0.68 ppm
gamma-chlordane, resulting from heavy previous use of heptachlor,
contained harvest residues of gamma-chlordane of the following order :
radishes, 0.03 ppm; carrots, 0.16 ppm; potatoes, 0.03 ppm; pea greens,
not detected (Lichtenstein et al, 1967 in press).
In animals
An important recent development with a bearing on the evaluation of
the hazard of residues in food to humans is the evidence that in
mammals gamma-chlordane can be metabolized and detoxified to more
hydrophilic products which are largely excreted into the aqueous
phase. Rats receiving intravenous doses of gamma-chlordane 14C
excreted 22 per cent of the total dose within 60 hours in the form of
hydrophilic metabolites in feces. High percentages of hydrophilic
metabolites were detected in the entire alimentary tract and kidneys.
Unchanged gamma-chlordane was found only in subcutaneous fat
(Poonawalla and Korte, 1964). Weekly doses of gamma-chlordane 14C
administered by stomach tube for ten weeks to rabbits resulted in 47.2
per cent of the total administered radioactivity being excreted in
urine, and 22.7 per cent in feces, with only about 4 per cent in fatty
tissues. Unchanged gamma-chlordane could be detected only in
subcutaneous fat, while all other tissues contained hydrophilic
metabolites. In urine, only hydrophilic metabolites could be detected.
Two hydrophilic metabolites have been isolated, one identified as a
chloro-hydrine. The acute oral toxicity of this metabolite is lower
than that of chlordane. The LD50 value for mice is higher than 1800
mg/kg. The structural formula for this metabolite assigned is
1-hydroxy-2-chloro-dihydrochlordane. The second metabolite is more
hydrophilic than the first, with both chlorine atoms in position 1 and
2 of gamma-chlordane possibly replaced by hydroxylic groups (Ludwig,
1966). The first metabolite may also possibly be derived from
alpha-dihydro-heptachlor resulting from oxidative detoxication
metabolism of heptachlor. Brooks and Harrison, (1967) assign a
slightly different structure to this metabolite, designating it
possibly as an octochloro-compound.
These recent findings possibly explain why the analysis of more than
500 samples of human fat tissues in the USA did not detect chlordane
(Hoffman et al, 1964; Hoffman, 1963). In less than 50 per cent of
these samples, two peaks in GLC chromatograms were noted, representing
concentrations of 0.01 - 0.05 ppm chlorine. Assuming these peaks are
due to a pesticide, their region indicates they could be caused by
ronnel, heptachlor, heptachlor epoxide, endosulfan or chlordane, or
hydrophilic metabolites of chlordane only partially soluble in polar
solvents.
The weathered residues of plants consist largely of alpha- and
gamma-chlordane. If this also occurs in residues translocated from
soil to growing plants, their hazard to man, after ingestion and
detoxication to hydrophilic metabolites, would reasonably be much less
than that for technical chlordane.
Residues of chlordane in meat resulting from direct application to
animals has been reported (Claborn, 1956; Claborn et al, 1960).
Consequently, chlordane is not currently recommended for use in direct
treatment of animals. Residues in whole milk resulting from grazing
cows on chlordane-treated pastures as early as one day, one week, two
weeks and four weeks after treatment were dosage dependent. At 0.25
lb/acre, none was found; at 0.5 lb and 1.0 lb per acre, a maximum of
0.08 ppm at the end of 8 weeks of feeding was found together with
maximum residues of heptachlor epoxide of 0.04 ppm. (Westlake et al,
1963).
Alfalfa treated with chlordane at one and two pounds per acre resulted
in hay residues of 20.4 and 20.9 ppm. Cows receiving this alfalfa for
a period of 150 and 100 days produced milk that contained residues
ranging from none to 0.2 ppm maximum (Carter et al, 1956).
Both of these experiments demonstrate considerable variation in the
residues excreted in milk by individual animals and suggest that
average results from a large herd of dairy animals would produce
smaller residues in pooled milk.
New USA data have been developed recently from feeding alfalfa with
known residues to dairy cattle. A progress report reviewed by the
Joint Meeting suggests that feeding alfalfa hay treated at 1.0 and 2.0
lbs/acre to Holstein cows resulted in a daily intake of 10.6 mg and
15.9 mg/day. Milk samples analyzed during the 31-day feeding study
were all negative (at a sensitivity of 0.01 ppm), with the exception
of one animal on the first day of feeding (Velsicol, 1967e).
In storage and processing
Information on the behaviour of residues during storage and processing
is needed. Although in the past, terminal residues of chlordane have
been regarded as stable, one investigation to date suggests that at
least in the case of rutabagas, cooking in water results in total
disappearance of residues in pulp (sensitivity 2 ppb) and 40 per cent
disappearance from peel, most being lost in water and only about 13.5
per cent steam-distilled (Saha and Stewart, 1967). Similar data on
other foods would be most useful.
METHODS OF RESIDUE ANALYSIS
A number of multidetection systems are available for the detection and
determination of organochlorine compounds and most of these can be
used for residues of alpha- and gamma-chlordane. An example is the
AOAC (1966) system in which acetonitrile partition and florisil column
clean-up are used, the residues being identified by gas chromatography
coupled with thin-layer or paper chromatography. Both electron capture
and micro-coulometric detectors can be used: the application of these
to chlordane, residue analysis, with special reference to
interferences by toxaphene (should this also be present) has been
described by Klein and Link (1967). The multidetection systems, which
normally separate the two isomers, are sensitive to about 0.02 ppm of
each isomer. Alternative methods for the confirmation of the identity
of residues, e.g. using infra-red spectrophotometry, are also
available.
Since alpha- and gamma-chlordane have boon shown by Klein and Link
(1967) to weather to approximately the same extent in agricultural
application, it is convenient to sum the residues; whilst in these
circumstances it might theoretically be possible then to apply a
factor to express this sum in terms of original technical chlordane,
it is recommended that results be reported as the total of alpha- plus
gamma-chlordane.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Canada 0.3 on a wide range of
foods (approximately
50 individual crops
included)
(cont'd)
Country Tolerance, ppm Crop
European Economic
Community 0.2 in fruits and
vegetables proposed,
(combined total with a review date
of all cyclodiene of 1 January 1970.
residues)
Netherlands,
Belgium, 0.1
Luxembourg
USA 0.3 on a wide range of
foods (approximately
50 individual crops
included)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Temporary tolerances
The potential hazard of residues resulting from the use of chlordane
has been reduced by narrowing the spectrum of use. New information on
the chemical nature of terminal residues indicates that these are
largely alpha- and gamma-chlordane. The extensive available
information from objective samples and total diet studies
in the USA (where several million pounds a year have been used
in agriculture for twenty years) indicates insignificant loads of
residues in food as consumed.
In view of these data and the relatively narrow spectrum of currently
recommended use, the following temporary tolerances, to be reviewed
31 December, 1970, are recommended :
Large root crops 0.3 ppm
Potatoes, sweet potatoes, rutabagas, turnips (soil
treatment) with the restriction that these be used for
human consumption only. If culls are to be fed to
livestock, additional data are required on the number of
pounds per day which may be fed in rations of swine, beef
and dairy cattle, with resultant residue data in animal
products.
Leafy and stalk vegetables 0.3 ppm
including all cole crops, spinach, lettuce, celery
and asparagus (soil treatment).
Small root vegetables 0.2 ppm
including but not limited to onions, table beets,
radishes (excluding carrots) (soil treatments).
Cucurbits 0.2 ppm
Cucumbers, melons including canteloupe, pumpkin and
squash (soil treatments)
Pineapple (soil treatment) 0.2 ppm
Sugar beets (soil treatment) 0.1 ppm
provided data are developed on residues remaining in
sugar beet pulp and green tops and residues remaining
in animal products when amounts of these are included
in the ration are fed to swine, beef or dairy cattle.
Pod vegetables (in the pod) 0.1 ppm
peas, beans, etc. (soil treatment)
Berry fruits 0.1 ppm
bush, cane and strawberries (soil treatment)
Tomatoes and related garden fruits (soil treatment) 0.1 ppm
Sweet corn and popcorn (soil treatment) 0.1 ppm
Comments:
Whole oil seeds: data are incomplete and available analytical
results are inconsistent. Tolerances or practical residue limits will
be required if chlordane is used in soil or as a foliar treatment.
Citrus, pome and stone fruits: no tolerance required, since no
residues are likely to result in fruit from soil treatments.
The point of enforcement of these tolerances should be on the raw
agricultural products moving in commerce.
Practical residue limits
Cereals 0.1 ppm
The meeting realized that it may be necessary at some future date to
recommend practical residue limits for chlordane for animal products.
Data were not available in 1967 to indicate such need or the levels
required.
FURTHER WORK
Further work required before 30 June 1970
1. More evidence is needed on the extent to which chlordane is used
on particular crops in various countries, the residues which
occur in commerce and total diet studies in countries using this
pesticide or importing food.
2. Data are required on the fate of residues during the normal
course of storage and processing before consumption.
3. Information on residues resulting from growing crops in rotations
on land in which chlordane has been used previously.
4. Data are required on residues in oil seeds, in vegetable oil
after processing and in oil seed cake or meal which may become
animal feed.
Further work desirable
Many of the measurements of residues made prior to 1961 need to be
repeated with the aid of the more sensitive and specific analytical
techniques.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Alvarez, W.C. and Hyman, S. (1953) A.M.A. Arch. Indust. Hyg. Occup.
Med. 8, 480
Ambrose, A.M., Christensen, H.E., Robbins, D.J. and Rather, L.J.
(1953) A.M.A. Arch. Indust. Hyg. Occup. Med. 7, 197
Batte, E.G. and Turk, R.D. (1948) J. econ Ent., 41, 102-103.
Burns, J.J., Cucinell, S.A., Koster, R. and Conney, A.H. (1965) Ann.
N.Y. Acad. Sci. 123, 273.
Carter, R.J., Hubanks, P.E., Poos, F.W., Moore, L.A. and Ely, R.E.
(1953) J. Dairy Science, 36, 1172.
Claborn, H.V., Bowers, J.W., Wells, R.W., Radeleff, R.D. and
Nickerson, W.J. (1953). Agric. Chemicals, 8, 37
Conney, A.H., Welch, R.M., Kuntzman, R. and Burns, J.J. (1967) Clin.
Pharmacol. Therap. 8, 2
Cram, R.L. Juchau, M.R. and Fouts, J.R. (1965) J. Lab. Clin. Med.
66, 906
Derbes, J.V., Dent, H.J., Forrest, W.W. and Johnson, M.F. (1955)
J. Amer. med. Ass. 158, 1367
Fishbein, W.I., White, J.V. and Isaacs, H.J. (1964) Indust. Med. and
Surg. 33, 726
Fouts, J.R. (1963) Ann. N.Y. Acad. Sciences, 104, 875
Fouts, J.R. and Hart, L.G. (1965) Ann. N.Y. Acad. Sciences, 123, 245
Fouts, J.R. and Rogers, L.A. (1965) J. Pharmacol. Exper. Therap.
147, 112
Gaines, T.G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L.G. and Fouts, J.R. (1963) Proc. Soc. exp. Biol. (N.Y.), 114,
388
Hart, L.G. and Fouts, J.R. (1965) Biochem. Pharmacol. 14, 263
Hart, L.G., Shultice, R.W. and Fouts, J.R. (1963) Toxicol appl.
Pharmacol., 5, 371
Hoffman, W.S., Fishbein, W.I. and Andelman, M.B. (1964) Arch.
Environ. Health, 9, 387
Ikeda, M., Sezesny, B. and Barnes, M. (1966) Fed. Proc. 25, 417
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1955) Unpublished report submitted by Velsicol Corporation
Ingle, L. (1965) A Monograph on Chlordane, Ingle, Urbana, Illinois
Ingle, L. (1967) Unpublished report submitted by Velsicol Corporation
Kuntzman, R., Jacobson, M., Schneidman, K. and Conney, A.H. (1964)
J. Pharmacol. exper. Therap., 146, 280
Lehman, A.J. (1951) Assoc. Food and Drug Officials Bull., 15 122
Lehman, A.J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W.J. and Durham, W.F. (1957) A.M.A. Arch. Path.,
64 61, 4
Princi, F. and Spurbeck, G.H. (1951) A.M.A. Arch. Indust. Hyg.
Occup. Med., 3, 64
Stein, W.J. and Hayes, W.J., Jr. (1964) Indust. Med. and Surg., 33,
549
Stohlman, E.F. and Smith, M.I. (1950) Advances in Chem. Ser., 1, 228
Stohlman, E.F., Thorp, W.T.S. and Smith, M.I. (1950) Arch. industr.
Hyg., 1, 13-19
Stormont, R.T. and Conley, B.E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Triolo, A.J. and Coon, J.M. (1966) Ag. and Food Chem., 14, 549
Turner, H.F. and Eden, W.G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Wazeter, F.X. (1967) Unpublished report submitted by Velsicol
Corporation
Welch, H. (1948) J. econ. Entomol., 41, 36-39
Welch, R.M. and Harrison, Y. (1966) The Pharmacologist, 8, 217
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.
and Schutzmann, R.L. (1963) J. Ag. Food Chem., 11, 244
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Allen, W.R. (1963) Plant growth dilution and the decline of
insecticide residues from alfalfa in Manitoba. Proc. Ent. Soc.
Manitoba 19.
AOAC. (1966) Changes in methods of analysis. J. Assoc. Off. Anal.
Chem. 49 : 222-230.
Banham, F.L. (1961) The persistence of certain insecticides for
control of the tuber flea beetle, Epitrix tuberis (Gent.) in the
interior of British Columbia. Can. J. Plant Sci. 41 : 664.
Begg, J.A., Plummer, P.J.G., Konst, H. (1960) Insecticide residues in
potatoes after soil treatments for control of wireworms. Can. J. Plant
Sci. 40 : 680-669.
Bess, H.A., Ota, A.K., Kawanish, C. (1966) Persistence of soil
insecticides for control of subterranean termites. J. Econ. Ent. 59 :
911-915.
Brooks, G.T., Harrison, A. (1967) The toxicity of
alpha-dihydroheptachlor and related compounds to the housefly (M.
domestica L.) and their metabolism by housefly and pig liver
microsomes. Life Sciences 6 : 1439-1448.
Carter, R.H., Claborn, H.V., Woodard, G.T., Ely, R.E. (1956) Residues
in animal products. USDA Yearbook of Agriculture, 143-148.
Chisholm, R.D., Koblit, S.K.Y., Westlake, W.E. (1966) Estimation of
aldrin and chlordane residues in soils treated for termite control.
Pest Control 30 : 48-50-52-53.
Claborn, H.V. (1956) Insecticide residues in meat and milk. USDA ARS
33-25.
Claborn, H.V., Bushland, R.C., Mann, H.D., Ivey, M.C., Radeleff, R.D.
(1960) Meat and milk residues from livestock sprays. J. Agr. Food
Chem. 8 : 439.
Cook, W.C. (1960) Report of residue analysis. USDA, ARS., Ent. Res.
Div., Pesticide Chemicals Res. Br., Report No. PCY-60-21.
Corley, C., Miles, E.J., Shands, W. (1965) Chlorinated hydrocarbon
residues in potatoes. USDA, ARS., Ent. Res. Div., Pesticide Chemicals
Res. Br., Report No. PC-B-65-22.
California State Department of Agriculture. (1962) Chlordane residues
in commercial food channels. Personal Communication.
Duggan, R.E., Barry, H.C., Johnson, L.Y. (1966) Pesticide residues in
total diet samples. Science 151 : 101-104.
Duggan, R.E. (1967) Objective samples of food analysed by USFDA for
chlordane residues. 1964-1967. Personal Communication.
Edwards, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. Ecology and the Industrial Society. Vth
Symp. Brit. Ecol. Soc., Beackwell, Oxford. p.239.
Edwards, C.A. (1966) Insecticide residues in soils. Res. Rev. 13 :
83-132.
Egan, H., Holmes, D.C., Roburn, J., Tatton, J.O.G. (1966) Pesticide
residues in foodstuffs in Great Britain II. Persistent organochlorine
pesticide residues in selected foods. J. Sci. Food Agr. 17 : 563-569.
Fahey, J.E., Hamilton, D.W., Rusk, H.W. (1957) Effect of spray date on
residues of chlorinated hydrocarbon insecticides on peaches. J. Econ.
Entomol. 50 : 366.
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Ent. 55 : 179.
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65.
Fischback, H. (1963) Problems stemming from the refinement of
analytical methods. Publication No. 1082, Natl. Acad. of Sci. NRC
55-62.
Hoffman, W.S. (1963) Personal communication.
Hoffman, W.S., Fishbein, W.I., Andelman, M.B. (1964) The pesticide
content of human fat tissue. Arch. Env. Health 9 : 387-394.
IUPAC. (1967) 1966 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. 50 :
1081-1083.
IUPAC. (1968) 1967 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. (in
press).
Klein, A.K., Link, J.D. (1967) Field weathering of toxaphene and
chlordane. J. Assoc. Off. Analyt. Chem. 50 : 586-91.
Korte, F. (1967) Personal communication
Landis, B.J. (1965) Report of residue analysis; soil, DDT, aldrin and
chlordane. USDA, ARS, Ent. Res. Div., Pesticide Chemicals Br., Report
No. PCY-65-25.
Lichtenstein, E.P. (1967) Alpha-chlordane content of heptachlor
formulations. Personal communication.
Lichtenstein, E.P., Fuhnemann, Schultz. (1967) Use of carbon to reduce
the uptake of insecticidal soil residues by crop plants. Effects of
carbon on insecticide absorption and toxicity in soils. J. Agr. Food
Chem. (in press).
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the Detection of Pesticide Residues. International
Atomic Energy Agency, Vienna, pp. 49-58.
Majumder, S.K. (1967) A review of the problem of the toxicity of
pesticide chemicals in food in India. Ind. Food Packer 21 : 1-14.
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Ent. 45 : 452-456.
Marth, E.H. (1962) Chlorinated hydrocarbons deposited in biological
material. I. Plants and plant products. J. Milk Food Technol. 25 : 36.
Minyard, J.P., Jackson, E.R. (1963) Pesticide residues in commercial
animal feeds. J. Assoc. Off. Analyt. Chem. 46 : 843-859.
Morgan, L.W., Leuk, D.B., Beck, E.W., Wardham, D.W. (1967) Residues of
aldrin, chlordane, endrin and heptachlor in peanuts grown in treated
soil. J. Econ. Ent. 60 : 1289-1291.
Muns, R.P., Stone, M.W., Foley, F. (1960) Residues in vegetable crops
following soil applications of insecticides. J. Econ. Ent. 53 : 832.
Nash, R.G., Woolson, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science 157 : 924-927.
Ordas, E.P., Smith, V.C., Meyer, C.F. (1956) Spectrophotometric
determination of heptachlor and technical chlordane on food and forage
crops. J. Agr. Food Chem. 4 : 444.
Painter, Ruth R., Kilgore, W.W., Ough, C.S. (1963) Distribution of
pesticides in fermentation products obtained from artificially
fortified grape musts. J. Food Sci. 28 : 342.
Perez-Escolar, M.E. (1959) Control pineapple gummosis in Puerto Rico.
J. Agr. Univ. Puerto Rico 43 : 116.
Poonawalla, N.H., Korte, F. (1964) Metabolism of insecticides. VIII
Excretion, distribution and metabolism of alpha-chlordane-14C by
rats. Life Sci. 3 : 1497
Saha, J.G., Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide, and
gamma-chlordane residues in soil and rutabagas after soil and surface
treatments with heptachlor. Can. J. Plant Sci. 47 : 79-88.
Robinson, J., McGill, A.E.J. (1966) Organochlorine insecticide
residues in complete prepared meals in Great Britain in 1965. Nature
212 : 1037-1038.
Rusk, H.W., McDonough, L.M. (1966) Report of residue analysis; soils
and sugar beet roots. USDA, ARS, Ent. Res. Div., Pesticide Chemicals
Res. Br., Report Nos. PCY-67-6 and PCY-66-9.
Smith, F.F., Adams, H.R. (1965) Chlordane residues in crops. USDA,
ARS, Ent. Res. Div. Pesticide Chemicals Res. Br., Special Report
PC-B-65-26.
Smith, F.F., Reid, W.J., Cuthbert, F.P. (1964) Dieldrin and chlordane
residues in sweet potatoes. USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report PC-B-64-10.
Swackhamer, A.B. (1967) Personal communication
Thurston, A.D. (1965) Preliminary studies on weathering of chlordane
residues. J. Assoc. Off. Analyt. Chem. 48 : 952-4.
USDA. (1964) Chlorinated hydrocarbon pesticide residues in soil at
Battle Creek, Mich., 1963, USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report No. PL-V-64-10.
USDA. (1966) Report of residue analysis; potatoes, chlordane. ARS,
Ent. Res. Div., Pesticide Chemicals Res. Br., Report No. PCY-66-1.
USDA. (1967a) Monitoring chlorinated hydrocarbon insecticide residues
in soybeans, 1966. ARS, Plant Pest Control Division, interim report,
1967.
USDA. (1967b) Report of residue analysis; soil; DDT and chlordane.
USDA, ARS, Ent. Res. Div., Pesticide Chemicals Res. Br., Report No.
PCB-67-9.
Velsicol Corp. (1967a) Unpublished report on chlordane residues in
soil. Submitted to FAO.
Velsicol Corp. (1967b) Unpublished report on residues in potatoes from
soil treatments with chlordane. Submitted to FAO.
Velsicol Corp. (1967c) Unpublished report on residues in barley grain
and straw from spraying growing barley at Fargo, N.D., 1966. Submitted
to FAO.
Velsicol Corp. (1967d) Unpublished report on chlordane residues in
sugar beets. Submitted to FAO.
Velsicol Corp. (1967e) Progress report on chlordane dairy feeding
study. Submitted to FAO.
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.,
Schutzmann, R.L. (1963) Chemical residues in the milk of cows grazed
on chlordane-treated pasture. J. Agr. Food Chem. 11 : 244.
 Other relevant chemical properties
Chlordane, as presently manufactured in the USA, is a uniform
technical product, the composition of which is controlled. Analytical
methods and reference standards have been made available periodically
by the manufacturer to regulatory agencies as a method of commercial
product control. Analytical methods for regulating the technical
formulation in commerce have become official methods after
collaborative studies by the Association of Official Analytical
Chemists (AOAC) in the USA and the European Commission for Methods of
Analysis of Pesticides (CIPAC). While hexachlorocyclo pentadiene is
used in the manufacture of the technical product, none of this
chemical remains unreacted and there is no evidence that it appears as
a constituent of the terminal residue in food.
There is some confusion in nomenclature. Gamma-chlordane is the
alpha-chlordane referred to by March (1952) (it is the component
present in the highest concentration in technical chlordane),
alpha-chlordane is the beta isomer described by March. Poonawalla and
Korte (1964), Ludwig (1966), and some other authors still use March's
designation. These two components together comprise approximately half
of technical chlordane.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Chlordane is absorbed from the gastrointestinal tract, the respiratory
tract, and through the skin (Ambrose et al., 1953). It is stored in
the adipose tissue of rats, sheep, goats, and cows and accumulates in
the milk.
Rats fed chlordane for six and a half months showed the following
levels in their abdominal fat at various feeding levels (in ppm):
intake 3, residue 5.2; intake 15, residue 32; intake 30, residue 76;
intake 60, residue 109 (Ingle, 1965).
Chlordane fed at 25 ppm in the diet for 8 weeks reached a maximum
level of 18 ppm in the fat of calves and 12 ppm in sheep. After
feeding was stopped, the residue was eliminated from calves in 20
weeks and from the sheep in 4 weeks (Claborn et al., 1953).
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlordane is
excreted in the urine of rabbits (Stohlman and Smith, 1950).
Cows grazing on pasture to which 0.5 pound of chlordane per acre had
been applied showed an average of 0.03 ppm of chlordane in their milk
(Westlake et al., 1963).
Both technical chlordane and one of its pure isomers (gamma-chlordane)
have been shown to have stimulating effects on rat liver microsomes
for the metabolism of certain drugs (Burns et al., 1965; Hart and
Fouts, 1963; Hart et al., 1963; Kuntzman, et al., 1964).
In vitro metabolism by hepatic microsomal enzymes from rats treated
8 days previously with single i.p. doses of 10 or 100 mg/kg of
chlordane in corn oil was increased for the 3 drugs reported
(hexobarbital, aminopyrine and chlorpromazine). The increases for
hexobarbital and aminopyrine were dose-related. In vivo hexobarbital
response, measured by sleeping time of mice, 1, 3 and 8 days after
single i.p. doses of 25 mg/kg body-weight technical chlordane or
gamma-chlordane, showed a decrease (shortened sleeping time), greatest
at day 3 but still marked on day 8 (Fouts, 1963).
The mechanism of action of chlordane is similar to that of
phenobarbital in stimulating hepatic drug metabolism in the rat. Both
agents increase liver weight, microsomal protein, microsomal NADPH
oxidase, and microsomal cytochrome CO-binding pigment. They both
stimulate drug metabolism in adrenalectomized or hypophysectomized
rats and promote the proliferation of smooth endoplasmic reticulum in
the liver cell, and ethionine blocks the enzyme stimulating effects of
both agents (Hart and Fouts, 1965).
Liver microsomal preparations from adult, female rats given 10 mg/kg
of chlordane intraperitoneally every other day for 14 days showed a
385 per cent increase in the biotransformation of 17ß-estradiol to
polar metabolites (Kuntzman et al., 1964). The intraperitoneal
injection of immature male rats with 50 mg/kg of chlordane daily for 4
days produced an increase of activity of the liver microsomal steroid
hydroxylases (Conney et al., 1967).
The toxicities of bishydroxycoumarin and phenylbutazone were reduced
in both rats and dogs pretreated by chlordane, and this effect was
accompanied by lower plasma levels of these drugs in the
chlordane-treated animals than in controls given the same doses (Welch
and Harrison, 1966). The toxicity of parathion was reduced in mice
pretreated 16 hours to 4 days previously with a single oral dose of
130 mg/kg of chlordane (Triolo and Coon, 1966).
Male rats weighing 60 to 80 gms were given 25 mg/kg of chlordane
intraperitoneally daily for 3 days, and were sacrificed 24 hours after
the last dose. The livers showed a proliferation of the
smooth-surfaced endoplasmic reticulum in the parenchymal cells,
associated with an increased activity of microsomal preparations from
such livers to metabolize hexobarbital, aminopyrine, zoxazolamine and
p-nitrobenzoic acid (Fouts and Rogers, 1965).
Pretreatment of rats for several days with chlordane increased the
LD50 of warfarin more than 10-fold, an effect associated with
decreased plasma levels of warfarin and an increased rate of
metabolism of the drug by liver microsomal enzymes (Ikeda et al.,
1966).
Technical chlordane and one of its pure isomers, gamma-chlordane,
administered to adult or weanling rats in single or 3 daily doses,
after 1 to 8 days increased the hepatic microsomal metabolism of
hexobarbital, aminopyrine, and chlorpromazine (Hart et al., 1963).
Treatment of newborn rabbits with 50 mg/kg of gamma-chlordane daily
for 3 days increased the activity of liver enzymes that metabolize
hexobarbital, aminopyrine and p-nitrobenzoic acid. Treatment of
lactating mothers with chlordane increased the drug metabolizing
enzymes in the nurslings (Fouts and Hart, 1965). Drug metabolism was
also stimulated in adult rabbits.
In dogs given 5 mg/kg of chlordane orally three times a week for 7
weeks the rate of metabolism of phenylbutazone was markedly increased.
This effect persisted as long as 21 weeks after termination of the
chlordane administration (Burns et al., 1965).
Chlordane has been demonstrated to stimulate the rate of drug
metabolism by the liver of the squirrel monkey (Cram et al., 1965).
For further information see the sections In Plants, and In
Animals.
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat Oral 200 - 590* Ambrose et al., 1953
Ingle, 1955
Stohlman et al., 1950
Oral 335 - 430 Gaines, 1960
150 - 225 Ingle, 1955
Mouse Oral 430 US Food and Drug Admin., 1947
Rabbit Oral 100 - 300* Stohlman et al., 1950
20 - 40 Ingle, 1955
Goat Oral 180 Welch, 1948
Sheep Oral 500 -1000 Welch, 1948
Chicken Oral 220 - 230 Turner and Eden, 1952
* The differences are explained by the use of different solvents,
and by the fact that the chlordane mentioned in the older
literature contained a considerable amount of the very toxic
hexachlorocyclopentadiene (Ingle, 1965; Lehman, 1952).
Man. A dose of 104 mg/kg proved fatal (Derbes et al., 1955). An
18-year old female showed convulsions but recovered after a dose of
approximately 30 mg/kg (amount retained after vomiting estimated to be
10 mg/kg). In two infants, 15 months and 3 years of age, 10 and 40
mg/kg respectively gave severe poisoning (Stormont and Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12 male
rats, all of them died within 10 days. At 500 ppm, 12/12 died within
70 days; at 300 ppm, 9/12 were alive after 100 days (Stohlman et al.,
1950).
Daily oral doses of 6.25 - 25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg, all
the animals died. Intracytoplasmic bodies in the liver-cells were
found at all levels and their number was in proportion to the dose
used (Ambrose et al., 1953).
Groups of 6 females and 6 males were fed 2.5 ppm or 25 ppm of a sample
of technical chlordane containing 60-75% chlordane and 25-40%
unrelated products for up to 9 months. Centrolobular cell hypertrophy,
peripheral migration of cytoplasmic granules and the presence of
cytoplasmic bodies were observed in 1 male at 2.5 ppm and in 5 males
at 12.5 ppm (Ortega et al., 1957).
Initial groups of 10 males and 20 females were used in a 3-generation
study at dietary levels of 0, 0.3, 3, 15, 30 and 60 ppm of technical
chlordane. Two litters in each filial generation were studied. Levels
up to and including 30 ppm had no effect on fertility, numbers of
young or litters, or weight, growth or mortality of the young animals
to weaning age. Autopsy of animals post-weaning showed no gross or
microscopic difference between the groups. At 60 ppm, there was a high
(10.6 per cent) mortality in the second F3 generation litters during
the latter part of the nursing period; these animals showed gross and
microscopic pathology comparable to that characteristic for chlordane
intoxication. However, survivors of this generation showed no tissue
changes at all. A third act of F3 litters at 60 ppm suffered 17 per
cent mortality during the nursing period, with symptomatology end
gross and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from the
60 ppm group and placed on chlordane-free diets for 30 days prior to
remating showed no difference in any respect from control litters. No
evidence of teratogenicity or tumorigenicity for chlordane was found
in this study (Ingle, 1967).
Dog. Chlordane was given in varying oral doses to dogs for 7 days,
convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but 700
mg/kg (highest dose) did not produce any effect (Batte and Turk,
1948).
Four groups of 2 to 4 dogs given chlordane orally in doses between 5
and 80 mg/kg body-weight daily all died within periods of 25 days to
93 weeks (Lehman, 1952).
Groups of 4-7 males and 4-7 females were fed 0, 0.3, 3, 15 and 30 ppm
of chlordane for 2 years. Abnormalities in the results of clinical
liver function tests were seen in the 15 and 30 ppm groups. In animals
selected for necropsy at the end of the first year, increased relative
liver weights and associated hepatocellular changes were found at 30
ppm; at the end of two years, dose-related increases in relative liver
weights were found at 15 and 30 ppm, with non-dose-related
hepatocellular changes. There was no difference between the severity
of the liver lesions of the 30 ppm animals and those of four animals
withdrawn from 30 ppm treatment for eight months prior to sacrifice.
Percutaneous liver biopsies on two animals of the 30 ppm group at 1, 3
and 6 months showed hepatocellular changes at 6 months but not at 1 or
3 months. No adverse effect was seen on behaviour, appearance,
survival, weight gain, blood picture or the results of periodic
physical examination, at all levels (Wazeter, 1967).
Sheep. Chlordane administered by stomach-tube to sheep in a dose of
0.5 g/kg body-weight produced toxic symptoms (incoordination, partial
blindness) in 5 to 6 days. A dose of 1 g/kg body-weight produced
severe respiratory and nervous symptoms at 16 hours and death after 48
hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each sex)
were given 2.5, 25 and 75 ppm of chlordane in the diet for 2 years.
The sample of chlordane used had an LD50 of 450 mg/kg (Lehman, 1951).
It was found that 25 and 75 ppm gave moderate to severe signs of
intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
Groups of 40 rats (20 males and 20 females) were fed concentrations of
5, 10, 30, 150 and 300 ppm of "technical chlordane" in the diet over a
2-year period.
Throughout the experiment tremors and convulsions appeared or could be
induced at 30 or more ppm. Following fasting, no neurological symptoms
appeared at 5 or 10 ppm. Growth rate was affected at 150 or 300 ppm.
Liver histological damage was observed in the form of hypertrophy of
centrolobular calls, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm, slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was carried
on between late 1953 and late 1955, "technical chlordane of recent
manufacture" was used. Groups of 40 rats were given chlordane at 2.5,
5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given no
chlordane. Changes concerning food consumption, growth and mortality
were seen only in the 300 ppm group. Liver cell changes were not
present in the animals given 2.5-25 ppm. At 50 ppm only "cytoplasmic
peripheralization" was present. At higher doses the changes were as
those previously described (Ingle, 1955).
In a study published in 1953, a sample of chlordane exhibiting an oral
LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each sex
were given 0, 10, 20, 40, 80, 160, 320, 640 and 1280 ppm of chlordane
in their diets for approximately 407 days. The animals at 640 and 1280
ppm died early. At lower dosages, survival was unaffected. Increased
liver-weight (in comparison with the control group) was observed over
320 ppm. In a sample of liver of a male at 320 ppm the average nuclear
volume was 377µ3 compared to 268µ3 in a control rat. Cytoplasmic
vacuoles containing fat and clusters of granules at the periphery of
the cytoplasm were often seen. In the males they were equivocal at 10
ppm, absent at 20 ppm and infrequent at 40 ppm. In the females these
lesions were common and were seen only at 80 ppm and over (Ambrose et
al., 1953).
Observations in man
Workers engaged in the manufacture and formulation of chlordane for
periods up to 15 years have exhibited no evidence of harmful effects
attributable to this insecticide (Princi and Spurbeck, 1951; Alvarez
and Hyman, 1953; Fishbein et al, 1964). In a survey of more than 1105
persons who had been engaged in pest control operations for 1 to 30
years (318 for 5 to 19 years), three cases of toxicity due to
chlordane were reported, the only symptoms specified being dizziness
and headache (Stein and Hayes, 1964).
Chlordane was not found in any of 282 human fat samples taken at
autopsy, though DDT and lindane were regularly detected and dieldrin
was frequently found (Hoffman et al., 1964).
Comments
Since chlordane stimulates drug metabolizing processes in the rat,
mouse, rabbit, dog and squirrel monkey, and since the microsomal
enzyme stimulating properties of chlordane are similar to those of
phenobarbital, which stimulates drug metabolism in man, it appears
extremely likely that chlordane also stimulates drug metabolism in
man. This effect in animals and the associated cellular changes in the
liver, considered characteristic of the chlorinated hydrocarbon
insecticides as a class, are thought by several workers not to
represent toxic effects but rather physiological adaptive processes.
Toxicological studies reported prior to 1953 are not contributory to
the evaluation of the currently manufactured product because of the
variability of composition before that time. The chlordane made for
commercial use since 1953, which has been used in the studies
reported, has a constancy of composition that makes the data adequate
for toxicological evaluation.
In none of the many toxicological studies done has there been any
evidence observed of tumorigenic or other irreversible pathologic
changes, even at the highest doses or feeding levels used.
On the basis of the results of two long-term feeding studies in the
rat, a no-effect level for the rat may be established at 20 ppm, or 1
mg/kg/day. A satisfactory 3-generation reproduction study in the rat
has been completed recently in which 30 ppm was without effect on
fertility, number or size of litters, or on the weight, growth or
mortality of the young to weaning age. Furthermore, there was no
evidence of teratogenicity. In the recently completed 2-year feeding
study in the dog, 3 ppm of chlordane was the highest level used that
had no effect in tests of liver function or on liver weight and
structure of the liver cell. Though the liver weight and cell
structure changes at 15 ppm may probably be classified as adaptive
rather than toxic in nature, the significance of the functional
changes at this level cannot be assessed in the present state of our
knowledge. Even though these changes consistently disappeared before
the end of the 2-year feeding period, and even though 15 and 30 ppm
produced no other adverse effects throughout the 2-year period, it
seems necessary at this time to take 3 ppm chlordane as the maximum
known no-effect level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat. 20 ppm in the diet, equivalent to 1 mg/kg/day
Dog. 3.0 ppm in the diet, equivalent to 0.075 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.001 mg/kg body weight
Further work required
See General Comments page 3 and 4.
Further work desirable
Toxicological data on alpha and gamma chlordane.
EVALUATION FOR TOLERANCES
USE PATTERN
Chlordane was the first cyclodiene insecticide and originally had a
broad scale and spectrum of use but was somewhat displaced by aldrin,
dieldrin, endrin and heptachlor because of their greater persistence
and effectiveness and lower cost. In recent years, to narrow the use
spectrum of aldrin, dieldrin, endrin and heptachlor, there is renewed
interest in the use of chlordane.
Several million pounds of chlordane have been used annually in North
America for more than 20 years of which between 30 and 40 per cent has
been used in agriculture. The USA and Canadian currently approved uses
represent the largest spectrum and potential scale of use in the world
and have been used an a guide to this evaluation.
Pre-harvest treatments
The most important use of chlordane in for soil treatment, with a
limited use for foliar application, the main crops being large root
crops, sugar beets, corn, small root vegetables, leafy and stalk
vegetables, cucurbits, leguminous vegetables, berry fruits, sugar
cane, pineapple and cereals. Some uses on forage crops are still
approved in the USA.
The literature suggests that in fifty or more countries, chlordane is
or can be used on sugar beets, potatoes, vegetables, tobacco, sugar
cane, ground nuts (peanuts), beans, bananas, coffee, deciduous fruits,
citrus, olives, cotton and tea bushes. However, information on the
scale of use and resulting residues is not generally available. In
other countries the scale of use is probably only a fraction of that
in the USA. Dosages vary between countries, with a range of 1 to 8
lbs/acre recommended for a variety of vegetable insect control
requirements.
Post-harvest treatments
No post-harvest uses are currently recommended.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The large amount of available information needs critical review
because of improvements in analytical methods since 1961 and recent
information on the chemical nature of terminal residues. Methods used
prior to 1963 were based on total chlorine or colorimetric
measurements and expressed results in terms of technical chlordane.
Recently developed methods identify individual components, the
summation of which frequently is approximately one half of that
reported by earlier non-specific methods. New data developed from 1965
to 1967 reports individual components of residues as alpha and
gamma-chlordane, sometimes with much smaller and insignificant
quantities of heptachlor and heptachlor epoxide. Small amounts of
gamma-chlordane will be found in some crops resulting from the use of
technical heptachlor in soil (Lichtenstein et al, 1967; Saha and
Stewart, 1967; Velsicol, 1967 a through d). Some authors have
recently added the various components of the residue and reported the
total as chlordane (e.g. Smith and Adams, 1965).
The available information concerning residues resulting from uses
constituting "good agricultural practice" (i.e. consistent with pest
control requirements, principally in the USA and Canada) are
summarized below.
Crop Residue (ppm) Reference
alfalfa 0.01 Ordas et al, 1956
" 0.16 Allen, 1963
barley, grain 0.0 - 0.06 Velsicol, 1967c
" , straw 0.98 - 2.65 " "
beans <0.01 Smith and Adams, 1965
beets 0.17 "
beet tops 0.01 - 0.1 "
bell peppers none recovered Muns et al, 1960
broccoli " " "
cabbage " " "
cantaloupe 0.08 "
" , meat and rind 0.01 - 0.1 Smith and Adams, 1965
carrots 1.5 Muns et al, 1960
" 0.01 - 0.13 Cook, 1960
" 0.16 (1) Lichtenstein et al, 1967
" , peel 0.0 - 0.7 Cook, 1960
collards none recovered Marth, 1962a
cucumbers 0.08 Muns et al, 1960
eggplant none recovered "
lettuce 0.04 "
peaches 0.8 Fahey et al, 1957
peanuts 1.74 ± 0.82 Morgan et al, 1967
pineapple 0.145, 0.43 (2) Perez-Escolar, 1959
potatoes <0.01 - 0.23 (3)
radishes 0.03 Lichtenstein et al, 1967
rutabagas 0.5 Muns et al, 1960
" 0.08 - 0.16 (4) Saha and Stewart, 1967
snap beans <0.01 Smith and Adams, 1965
strawberries <0.01 "
" none recovered Fahey, 1962
sweet potatoes 0.01 - 0.4 (5)
sugar beets 0.04 - 0.37 (6)
tomatoes 0.01 Muns et al, 1960
" <0.01 Smith and Adams, 1965
turnips 0.01 - 0.1 "
" 0.16 Muns et al, 1960
turnip tops 0.01 - 0.1 Smith and Adams, 1965
wine none (7) Painter et al, 1963
(1) soil contained 0.68 ppm gamma chlordane (from heptachlor application 5 years
previous).
(continued)
(2) resulting from one and two applications, respectively.
(3) overall range as reported by many authors, e.g. Lichtenstein, 1967; Smith
and Adams, 1965; Begg et al, 1960; USDA, 1964; USDA, 1966; Corley et al,
1965; Velsicol, 1967b.
(4) gamma chlordane from heptachlor
(5) overall range as reported by many authors, e.g. Muns et al, 1960; Smith and
Adams, 1965; Smith et al, 1964.
(6) overall range following different rates of application as reported by
many authors, e.g. Muns et al, 1960; Rusk and McDonough, 1966;
Velsicol, 1967d.
(7) 0.6 in must and lees.
Data are required from similar investigations using reliable
analytical procedures from other countries. Information pertaining to
successive crops grown in rotation over a period of years after
initial treatment would be of particular interest. Data are also
required on the residues which may occur in oil seeds as the result of
intentional use, or from growing these crops in rotations. This is of
particular importance where certain oil-containing crops are grown in
rotation with other crops, especially after maize.
In general these data suggest that the highest residues consistent
with good agricultural practice will result in the large root crops,
and under certain conditions in the leafy and stalk vegetables.
Considerable variation can be expected in residues found in the large
root crops, particularly potatoes, depending on soil type, variety of
potato and date of maturity. Recent information suggests that the
residues in leafy and stalk vegetables may in part be from direct
contamination from soil, rather than a true translocation into growing
parts other than the stem or stalk. The intermediate group in
magnitude of residues to be expected would include small root
vegetables, cucurbits and pineapple. Cereals, berry fruits, pod
vegetables and fruit vegetables of the tomato type are in the category
of lowest residues, usually well below 0.1 ppm. It is probable that
there is no true translocation of residues in the grain of most
cereals and that the residues found are due to mechanical
contamination by soil, dust, debris, etc., during harvesting
operations.
RESIDUES IN FOOD MOVING IN COMMERCE
Pesticide residues in commercial animal feeds are important sources of
residues in milk and other animal products. Data concerning chlordane
are available from one such survey in Tables 1 and 2 (Minyard and
Jackson, 1963).
TABLE I
The occurrence of chlordane in commercial feeds expressed in parts per billion
No. No. Range of chlordane Range of total
Feed samples positive residue (ppb) pesticide
residue (ppb)
16% protein Dairy 73 53 0-254 26 - 1153
18% protein Dairy 6 4 13- 39 55 - 713
20% protein Dairy 2 2 15- 34 139 - 274
32% protein Dairy 4 1 5 36 - 435
12% protein Fitting
and Freshening 2 1 12 49 - 60
14% protein Cattle 3 2 11- 12 47 - 183
20% protein Beef 1 1 45 343
16% protein Hog 3 0 - 34 - 62
16% protein Poultry 3 2 7- 11 80 - 291
TABLE II
Ranges and averages of insecticide residues found in 101 feeds expressed in parts per billion
Av. level of
No. Per cent Range of contamination of
Insecticide contaminated contaminated contamination contaminated foods
(ppb) (ppb)
lindane 101 100.0 5 - 79 16.0
aldrin 87 86.1 1 - 16 2.6
DDT 99 98.0 10 - 448 80.5
methoxychlor 46 45.5 10 - 580 74.0
endrin 59 58.4 4 - 27 10.6
chlordane 68 67.3 5 - 254 30.4
dieldrin 89 88.1 1 - 16 4.9
toxaphene 15 14.8 60 - 530 178.3
heptachlor 9 8.9 2 - 13 4.8
heptachlor epoxide 3 3.0 1 - 6 3.3
Soybeans follow maize in recommended crop rotations in the USA. Soil
used for maize production is treated with chlordane, aldrin or
heptachlor and may result in residues of chlordane in soybeans
subsequently grown in these soils. An interim report on a survey of
unintentional residues from soil and crop samples from 27 regional
special sampling locations in the USA was made available to the
meeting. Chlordane was detected in soils from 3 fields at an average
concentration of 0.26 ppm, soybeans from one of these fields
containing 0.03 ppm. Eight other soil samples contained an average
concentration of 0.50 ppm (range 0.09 to 1.11 ppm). No chlordane was
detected in soybean seed or plants from these latter soils (USDA,
1967a).
Information on sources of chlordane in total diet studies has been
available for five years. The earliest of these concerns 12,000
samples analyzed by paper chromatography and gas liquid chromatography
in the state of California (CSDA, 1962). No chlordane was found in
this survey.
Thirty-eight total diet studies in 1961-62, for six USA cities
resulted in only two samples containing chlordane at 0.01 and 0.03 ppm
(Fishbach, 1963).
Eighty-two foods collected from 18 markets in three different
geographical areas in the USA were prepared for consumption, resulting
in 216 composite samples, and then analysed (Duggan et al, 1966).
Chlordane was found in only one composite sample at 0.033 ppm in
"garden fruit" produce (i.e. raw peppers, fresh and canned tomatoes,
raw cucumbers, catsup, eggplant, raw and frozen summer squash).
Comprehensive unpublished information from the USA was made available
to the 1967 Joint Meeting (Duggan, 1967). This data is concerned with
both objective samples of food for the years 1964 -1966 inclusive and
total diet samples for the year ending April 1967.
TABLE III
Objective Samples - Chlordane
Year 1964 1965 1966 Total
Number of domestic samples 20,088 14,462 14,806 49,356
Per cent of samples positive 0.7 1.1 0.9 0.9
Number of import samples 1,224 1,217 1,399 3,840
Per cent of samples positive 0.2 0.9 0.1 0.4
Total Diet Samples - Chlordane
Leafy vegetables - 1 composite positive contained 0.02 ppm (29 negative)
Legume vegetables - 1 composite positive contained 0.005 ppm (29 negative).
In Table IV details of the incidence and levels of chlordane for 1966
are of considerable interest as to amount and sources of residues in
the diet of man in the USA.
Canadian information covers a 4.5 year period from 1963 to July 1967.
Of a total of 14,270 samples of food (12,186 domestic and 2,084
imports), eleven contained chlordane; one lot of imported green
peppers, >0.3 ppm; six lots of whole eggs, 1.8 to 11 ppm (all seven
in excess of tolerance). Three vegetable samples contained traces of
chlordane, and one lot of turnips contained residues in excess of 0.3
ppm (Swackhamer, 1967).
Two reports of no chlordane being found in British food (Robinson and
McGill, 1966; Egan et al, 1966) are difficult to evaluate because of
lack of information on current use of chlordane in Britain and the
countries from which some foods concerned were imported.
FATE OF RESIDUES
In soils
Chlordane residues in soil have been investigated since 1949. However,
prior to 1961 much of the information is of doubtful value. Sources of
information since then include : USDA, 1964; Landis, 1965; Edwards,
1965; Edwards, 1966; Rusk and McDonough, 1966; Chisholm et al, 1966;
Bess et al, 1966; USDA 1967b; Nash and Woolson, 1967; Saha and
Stewart, 1967; Velsicol, 1967a; Lichtenstein, 1967; and Majumder,
1967.
Table V summarizes comparative persistence of some organochlorine
insecticides based on the literature review of Edwards (1966) but
modified as concerns doses used for a particular pest as far as
chlordane is concerned.
Approximately 55 per cent of chlordane remains in soil after the first
year following application, compared with 26 per cent for aldrin, 75
per cent for dieldrin and 80 per cent for DDT, a situation which is
not consistent with the respective vapour pressures; hence other
factors must be operating.
Although most reports to date express residues in soil on the basis of
equivalents of technical chlordane, Saha and Stewart (1967) reported
11.9 per cent of the residue resulting from one use of heptachlor as
gamma-chlordane (one pound of heptachlor contains 35 per cent
gamma-chlordane).
TABLE IV
Incidence and levels of chlordane in objective samples of food of domestic origin*, USA, 1966 (Duggan, 1967)
Number of Samples
Raw Manufactured Processed Fish
Range agricultural dairy animal and Vegetable Processed
ppm products products feeds shellfish oils foods Total
T - 0.03 59 1 3 1 - - 64
0.04 - 0.10 17 - 4 2 - - 23
0.11 - 0.50 35 - 4 - 1 1 41
0.51 - 1.00 - - 1 - - - 1
1.01 - 1.50 - - 2 - - - 2
1.51 - 2.00 - - 1 - - - 1
Above 2.00 2 - - - - - 2
Total samples
containing 113 ** 1 15 3 1 1 134
chlordane
Total samples
examined 9,804 611 680 290 171 227 14,806 *
* Of an additional 1399 samples of imported foods, only 1 contained chlordane, and this in a raw
agricultural product in the range of - 0.03 ppm
** 55 residues were in root vegetables
30 residues were in vine and ear vegetables
TABLE V
Persistence of some organochlorine insecticides in soil
Insecticide Average dose active Time for 95% disappearance
ingredient, lbs/acre in years
Range Mean
aldrin 1 - 3 1 - 6 3
chlordane 1 - 2 3 - 5 4
DDT 1 - 2´ 4 - 30 10
dieldrin 1 - 3 5 - 25 8
heptachlor 1 - 3 3 - 5 3´
lindane 1 - 2´ 3 - 10 6´
telodrin ¨ - 1 2 - 7 4
In plants
Information on the chemical nature of the terminal residues from
weathering of external residues has recently become available
(Thurston, 1965; Klein and Link, 1967). A further review of new
unpublished information and work in progress is contained in the 1966
and 1967 Reports of the IUPAC Commission on Chemical Nature of
Terminal Pesticide Residues (IUPAC, 1967, 1968).
The principal terminal residues from weathering of chlordane on plants
have been identified as alpha- and gamma-chlordane by characteristic
"signature" peaks as determined by gas liquid chromatographic methods
and confirmed by infrared methods. The sequence of analyses taken
after agricultural applications of technical chlordane exhibit a
fairly constant pattern. Minute amounts of heptachlor or heptachlor
epoxide appear in some chromatograms but, for all practical purposes,
these can usually be ignored.
The loss of technical chlordane from plants by normal weathering is
extremely rapid. At the end of the seventh day only 7 ppm of a
starting load of 110 ppm remains on kale, and only 0.1 ppm, expressed
as technical chlordane equivalent, remains after 28 days (Klein and
Link, 1967).
Previous studies on weathering of residues have not taken account of
the possible formation of hydrophilic metabolites, an omission which
is not probably toxicologically important since the known hydrophilic
metabolites of at least gamma-chlordane are less toxic than
technical chlordane. However, such information in domestic animals
after ingestion of weathered residues would be important in
establishing the consumer hazard of residues in animal products.
Recent information on the production of hydrophilic metabolites of
gamma-chlordane in animals (Poonwalla and Korte, 1964; Ludwig, 1966)
suggests that similar investigations should be carried out in plants,
particularly from the stand-point of translocation and metabolism of
residues in soil. Work on that subject is now in progress by Korte and
will be reported to the IUPAC Commission on Terminal Residues in 1968.
Current indications are that substantial formation of hydrophilic
metabolites (of lower order of toxicity than alpha-, gamma-, and
technical chlordane) are formed in the stalk of plants (Korte, 1967
personal communication).
Gamma-chlordane residues can occur in plants as the result of use of
technical heptachlor in soil. An application of 6.6 kg/ha mixed into
soil results in 11.9 per cent of the soil residue being in the form of
gamma-chlordane. Rutabagas grown in this soil contained 0.008 ppm
gamma-chlordane, this being 13.3 per cent of the total residue
(heptachlor + heptachlor epoxide + gamma chlordane = 0.060 ppm), (Saha
and Stewart, 1967). Crops grown in soil containing 0.68 ppm
gamma-chlordane, resulting from heavy previous use of heptachlor,
contained harvest residues of gamma-chlordane of the following order :
radishes, 0.03 ppm; carrots, 0.16 ppm; potatoes, 0.03 ppm; pea greens,
not detected (Lichtenstein et al, 1967 in press).
In animals
An important recent development with a bearing on the evaluation of
the hazard of residues in food to humans is the evidence that in
mammals gamma-chlordane can be metabolized and detoxified to more
hydrophilic products which are largely excreted into the aqueous
phase. Rats receiving intravenous doses of gamma-chlordane 14C
excreted 22 per cent of the total dose within 60 hours in the form of
hydrophilic metabolites in feces. High percentages of hydrophilic
metabolites were detected in the entire alimentary tract and kidneys.
Unchanged gamma-chlordane was found only in subcutaneous fat
(Poonawalla and Korte, 1964). Weekly doses of gamma-chlordane 14C
administered by stomach tube for ten weeks to rabbits resulted in 47.2
per cent of the total administered radioactivity being excreted in
urine, and 22.7 per cent in feces, with only about 4 per cent in fatty
tissues. Unchanged gamma-chlordane could be detected only in
subcutaneous fat, while all other tissues contained hydrophilic
metabolites. In urine, only hydrophilic metabolites could be detected.
Two hydrophilic metabolites have been isolated, one identified as a
chloro-hydrine. The acute oral toxicity of this metabolite is lower
than that of chlordane. The LD50 value for mice is higher than 1800
mg/kg. The structural formula for this metabolite assigned is
1-hydroxy-2-chloro-dihydrochlordane. The second metabolite is more
hydrophilic than the first, with both chlorine atoms in position 1 and
2 of gamma-chlordane possibly replaced by hydroxylic groups (Ludwig,
1966). The first metabolite may also possibly be derived from
alpha-dihydro-heptachlor resulting from oxidative detoxication
metabolism of heptachlor. Brooks and Harrison, (1967) assign a
slightly different structure to this metabolite, designating it
possibly as an octochloro-compound.
These recent findings possibly explain why the analysis of more than
500 samples of human fat tissues in the USA did not detect chlordane
(Hoffman et al, 1964; Hoffman, 1963). In less than 50 per cent of
these samples, two peaks in GLC chromatograms were noted, representing
concentrations of 0.01 - 0.05 ppm chlorine. Assuming these peaks are
due to a pesticide, their region indicates they could be caused by
ronnel, heptachlor, heptachlor epoxide, endosulfan or chlordane, or
hydrophilic metabolites of chlordane only partially soluble in polar
solvents.
The weathered residues of plants consist largely of alpha- and
gamma-chlordane. If this also occurs in residues translocated from
soil to growing plants, their hazard to man, after ingestion and
detoxication to hydrophilic metabolites, would reasonably be much less
than that for technical chlordane.
Residues of chlordane in meat resulting from direct application to
animals has been reported (Claborn, 1956; Claborn et al, 1960).
Consequently, chlordane is not currently recommended for use in direct
treatment of animals. Residues in whole milk resulting from grazing
cows on chlordane-treated pastures as early as one day, one week, two
weeks and four weeks after treatment were dosage dependent. At 0.25
lb/acre, none was found; at 0.5 lb and 1.0 lb per acre, a maximum of
0.08 ppm at the end of 8 weeks of feeding was found together with
maximum residues of heptachlor epoxide of 0.04 ppm. (Westlake et al,
1963).
Alfalfa treated with chlordane at one and two pounds per acre resulted
in hay residues of 20.4 and 20.9 ppm. Cows receiving this alfalfa for
a period of 150 and 100 days produced milk that contained residues
ranging from none to 0.2 ppm maximum (Carter et al, 1956).
Both of these experiments demonstrate considerable variation in the
residues excreted in milk by individual animals and suggest that
average results from a large herd of dairy animals would produce
smaller residues in pooled milk.
New USA data have been developed recently from feeding alfalfa with
known residues to dairy cattle. A progress report reviewed by the
Joint Meeting suggests that feeding alfalfa hay treated at 1.0 and 2.0
lbs/acre to Holstein cows resulted in a daily intake of 10.6 mg and
15.9 mg/day. Milk samples analyzed during the 31-day feeding study
were all negative (at a sensitivity of 0.01 ppm), with the exception
of one animal on the first day of feeding (Velsicol, 1967e).
In storage and processing
Information on the behaviour of residues during storage and processing
is needed. Although in the past, terminal residues of chlordane have
been regarded as stable, one investigation to date suggests that at
least in the case of rutabagas, cooking in water results in total
disappearance of residues in pulp (sensitivity 2 ppb) and 40 per cent
disappearance from peel, most being lost in water and only about 13.5
per cent steam-distilled (Saha and Stewart, 1967). Similar data on
other foods would be most useful.
METHODS OF RESIDUE ANALYSIS
A number of multidetection systems are available for the detection and
determination of organochlorine compounds and most of these can be
used for residues of alpha- and gamma-chlordane. An example is the
AOAC (1966) system in which acetonitrile partition and florisil column
clean-up are used, the residues being identified by gas chromatography
coupled with thin-layer or paper chromatography. Both electron capture
and micro-coulometric detectors can be used: the application of these
to chlordane, residue analysis, with special reference to
interferences by toxaphene (should this also be present) has been
described by Klein and Link (1967). The multidetection systems, which
normally separate the two isomers, are sensitive to about 0.02 ppm of
each isomer. Alternative methods for the confirmation of the identity
of residues, e.g. using infra-red spectrophotometry, are also
available.
Since alpha- and gamma-chlordane have boon shown by Klein and Link
(1967) to weather to approximately the same extent in agricultural
application, it is convenient to sum the residues; whilst in these
circumstances it might theoretically be possible then to apply a
factor to express this sum in terms of original technical chlordane,
it is recommended that results be reported as the total of alpha- plus
gamma-chlordane.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Canada 0.3 on a wide range of
foods (approximately
50 individual crops
included)
(cont'd)
Country Tolerance, ppm Crop
European Economic
Community 0.2 in fruits and
vegetables proposed,
(combined total with a review date
of all cyclodiene of 1 January 1970.
residues)
Netherlands,
Belgium, 0.1
Luxembourg
USA 0.3 on a wide range of
foods (approximately
50 individual crops
included)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Temporary tolerances
The potential hazard of residues resulting from the use of chlordane
has been reduced by narrowing the spectrum of use. New information on
the chemical nature of terminal residues indicates that these are
largely alpha- and gamma-chlordane. The extensive available
information from objective samples and total diet studies
in the USA (where several million pounds a year have been used
in agriculture for twenty years) indicates insignificant loads of
residues in food as consumed.
In view of these data and the relatively narrow spectrum of currently
recommended use, the following temporary tolerances, to be reviewed
31 December, 1970, are recommended :
Large root crops 0.3 ppm
Potatoes, sweet potatoes, rutabagas, turnips (soil
treatment) with the restriction that these be used for
human consumption only. If culls are to be fed to
livestock, additional data are required on the number of
pounds per day which may be fed in rations of swine, beef
and dairy cattle, with resultant residue data in animal
products.
Leafy and stalk vegetables 0.3 ppm
including all cole crops, spinach, lettuce, celery
and asparagus (soil treatment).
Small root vegetables 0.2 ppm
including but not limited to onions, table beets,
radishes (excluding carrots) (soil treatments).
Cucurbits 0.2 ppm
Cucumbers, melons including canteloupe, pumpkin and
squash (soil treatments)
Pineapple (soil treatment) 0.2 ppm
Sugar beets (soil treatment) 0.1 ppm
provided data are developed on residues remaining in
sugar beet pulp and green tops and residues remaining
in animal products when amounts of these are included
in the ration are fed to swine, beef or dairy cattle.
Pod vegetables (in the pod) 0.1 ppm
peas, beans, etc. (soil treatment)
Berry fruits 0.1 ppm
bush, cane and strawberries (soil treatment)
Tomatoes and related garden fruits (soil treatment) 0.1 ppm
Sweet corn and popcorn (soil treatment) 0.1 ppm
Comments:
Whole oil seeds: data are incomplete and available analytical
results are inconsistent. Tolerances or practical residue limits will
be required if chlordane is used in soil or as a foliar treatment.
Citrus, pome and stone fruits: no tolerance required, since no
residues are likely to result in fruit from soil treatments.
The point of enforcement of these tolerances should be on the raw
agricultural products moving in commerce.
Practical residue limits
Cereals 0.1 ppm
The meeting realized that it may be necessary at some future date to
recommend practical residue limits for chlordane for animal products.
Data were not available in 1967 to indicate such need or the levels
required.
FURTHER WORK
Further work required before 30 June 1970
1. More evidence is needed on the extent to which chlordane is used
on particular crops in various countries, the residues which
occur in commerce and total diet studies in countries using this
pesticide or importing food.
2. Data are required on the fate of residues during the normal
course of storage and processing before consumption.
3. Information on residues resulting from growing crops in rotations
on land in which chlordane has been used previously.
4. Data are required on residues in oil seeds, in vegetable oil
after processing and in oil seed cake or meal which may become
animal feed.
Further work desirable
Many of the measurements of residues made prior to 1961 need to be
repeated with the aid of the more sensitive and specific analytical
techniques.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Alvarez, W.C. and Hyman, S. (1953) A.M.A. Arch. Indust. Hyg. Occup.
Med. 8, 480
Ambrose, A.M., Christensen, H.E., Robbins, D.J. and Rather, L.J.
(1953) A.M.A. Arch. Indust. Hyg. Occup. Med. 7, 197
Batte, E.G. and Turk, R.D. (1948) J. econ Ent., 41, 102-103.
Burns, J.J., Cucinell, S.A., Koster, R. and Conney, A.H. (1965) Ann.
N.Y. Acad. Sci. 123, 273.
Carter, R.J., Hubanks, P.E., Poos, F.W., Moore, L.A. and Ely, R.E.
(1953) J. Dairy Science, 36, 1172.
Claborn, H.V., Bowers, J.W., Wells, R.W., Radeleff, R.D. and
Nickerson, W.J. (1953). Agric. Chemicals, 8, 37
Conney, A.H., Welch, R.M., Kuntzman, R. and Burns, J.J. (1967) Clin.
Pharmacol. Therap. 8, 2
Cram, R.L. Juchau, M.R. and Fouts, J.R. (1965) J. Lab. Clin. Med.
66, 906
Derbes, J.V., Dent, H.J., Forrest, W.W. and Johnson, M.F. (1955)
J. Amer. med. Ass. 158, 1367
Fishbein, W.I., White, J.V. and Isaacs, H.J. (1964) Indust. Med. and
Surg. 33, 726
Fouts, J.R. (1963) Ann. N.Y. Acad. Sciences, 104, 875
Fouts, J.R. and Hart, L.G. (1965) Ann. N.Y. Acad. Sciences, 123, 245
Fouts, J.R. and Rogers, L.A. (1965) J. Pharmacol. Exper. Therap.
147, 112
Gaines, T.G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L.G. and Fouts, J.R. (1963) Proc. Soc. exp. Biol. (N.Y.), 114,
388
Hart, L.G. and Fouts, J.R. (1965) Biochem. Pharmacol. 14, 263
Hart, L.G., Shultice, R.W. and Fouts, J.R. (1963) Toxicol appl.
Pharmacol., 5, 371
Hoffman, W.S., Fishbein, W.I. and Andelman, M.B. (1964) Arch.
Environ. Health, 9, 387
Ikeda, M., Sezesny, B. and Barnes, M. (1966) Fed. Proc. 25, 417
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1955) Unpublished report submitted by Velsicol Corporation
Ingle, L. (1965) A Monograph on Chlordane, Ingle, Urbana, Illinois
Ingle, L. (1967) Unpublished report submitted by Velsicol Corporation
Kuntzman, R., Jacobson, M., Schneidman, K. and Conney, A.H. (1964)
J. Pharmacol. exper. Therap., 146, 280
Lehman, A.J. (1951) Assoc. Food and Drug Officials Bull., 15 122
Lehman, A.J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W.J. and Durham, W.F. (1957) A.M.A. Arch. Path.,
64 61, 4
Princi, F. and Spurbeck, G.H. (1951) A.M.A. Arch. Indust. Hyg.
Occup. Med., 3, 64
Stein, W.J. and Hayes, W.J., Jr. (1964) Indust. Med. and Surg., 33,
549
Stohlman, E.F. and Smith, M.I. (1950) Advances in Chem. Ser., 1, 228
Stohlman, E.F., Thorp, W.T.S. and Smith, M.I. (1950) Arch. industr.
Hyg., 1, 13-19
Stormont, R.T. and Conley, B.E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Triolo, A.J. and Coon, J.M. (1966) Ag. and Food Chem., 14, 549
Turner, H.F. and Eden, W.G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Wazeter, F.X. (1967) Unpublished report submitted by Velsicol
Corporation
Welch, H. (1948) J. econ. Entomol., 41, 36-39
Welch, R.M. and Harrison, Y. (1966) The Pharmacologist, 8, 217
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.
and Schutzmann, R.L. (1963) J. Ag. Food Chem., 11, 244
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Allen, W.R. (1963) Plant growth dilution and the decline of
insecticide residues from alfalfa in Manitoba. Proc. Ent. Soc.
Manitoba 19.
AOAC. (1966) Changes in methods of analysis. J. Assoc. Off. Anal.
Chem. 49 : 222-230.
Banham, F.L. (1961) The persistence of certain insecticides for
control of the tuber flea beetle, Epitrix tuberis (Gent.) in the
interior of British Columbia. Can. J. Plant Sci. 41 : 664.
Begg, J.A., Plummer, P.J.G., Konst, H. (1960) Insecticide residues in
potatoes after soil treatments for control of wireworms. Can. J. Plant
Sci. 40 : 680-669.
Bess, H.A., Ota, A.K., Kawanish, C. (1966) Persistence of soil
insecticides for control of subterranean termites. J. Econ. Ent. 59 :
911-915.
Brooks, G.T., Harrison, A. (1967) The toxicity of
alpha-dihydroheptachlor and related compounds to the housefly (M.
domestica L.) and their metabolism by housefly and pig liver
microsomes. Life Sciences 6 : 1439-1448.
Carter, R.H., Claborn, H.V., Woodard, G.T., Ely, R.E. (1956) Residues
in animal products. USDA Yearbook of Agriculture, 143-148.
Chisholm, R.D., Koblit, S.K.Y., Westlake, W.E. (1966) Estimation of
aldrin and chlordane residues in soils treated for termite control.
Pest Control 30 : 48-50-52-53.
Claborn, H.V. (1956) Insecticide residues in meat and milk. USDA ARS
33-25.
Claborn, H.V., Bushland, R.C., Mann, H.D., Ivey, M.C., Radeleff, R.D.
(1960) Meat and milk residues from livestock sprays. J. Agr. Food
Chem. 8 : 439.
Cook, W.C. (1960) Report of residue analysis. USDA, ARS., Ent. Res.
Div., Pesticide Chemicals Res. Br., Report No. PCY-60-21.
Corley, C., Miles, E.J., Shands, W. (1965) Chlorinated hydrocarbon
residues in potatoes. USDA, ARS., Ent. Res. Div., Pesticide Chemicals
Res. Br., Report No. PC-B-65-22.
California State Department of Agriculture. (1962) Chlordane residues
in commercial food channels. Personal Communication.
Duggan, R.E., Barry, H.C., Johnson, L.Y. (1966) Pesticide residues in
total diet samples. Science 151 : 101-104.
Duggan, R.E. (1967) Objective samples of food analysed by USFDA for
chlordane residues. 1964-1967. Personal Communication.
Edwards, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. Ecology and the Industrial Society. Vth
Symp. Brit. Ecol. Soc., Beackwell, Oxford. p.239.
Edwards, C.A. (1966) Insecticide residues in soils. Res. Rev. 13 :
83-132.
Egan, H., Holmes, D.C., Roburn, J., Tatton, J.O.G. (1966) Pesticide
residues in foodstuffs in Great Britain II. Persistent organochlorine
pesticide residues in selected foods. J. Sci. Food Agr. 17 : 563-569.
Fahey, J.E., Hamilton, D.W., Rusk, H.W. (1957) Effect of spray date on
residues of chlorinated hydrocarbon insecticides on peaches. J. Econ.
Entomol. 50 : 366.
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Ent. 55 : 179.
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65.
Fischback, H. (1963) Problems stemming from the refinement of
analytical methods. Publication No. 1082, Natl. Acad. of Sci. NRC
55-62.
Hoffman, W.S. (1963) Personal communication.
Hoffman, W.S., Fishbein, W.I., Andelman, M.B. (1964) The pesticide
content of human fat tissue. Arch. Env. Health 9 : 387-394.
IUPAC. (1967) 1966 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. 50 :
1081-1083.
IUPAC. (1968) 1967 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. (in
press).
Klein, A.K., Link, J.D. (1967) Field weathering of toxaphene and
chlordane. J. Assoc. Off. Analyt. Chem. 50 : 586-91.
Korte, F. (1967) Personal communication
Landis, B.J. (1965) Report of residue analysis; soil, DDT, aldrin and
chlordane. USDA, ARS, Ent. Res. Div., Pesticide Chemicals Br., Report
No. PCY-65-25.
Lichtenstein, E.P. (1967) Alpha-chlordane content of heptachlor
formulations. Personal communication.
Lichtenstein, E.P., Fuhnemann, Schultz. (1967) Use of carbon to reduce
the uptake of insecticidal soil residues by crop plants. Effects of
carbon on insecticide absorption and toxicity in soils. J. Agr. Food
Chem. (in press).
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the Detection of Pesticide Residues. International
Atomic Energy Agency, Vienna, pp. 49-58.
Majumder, S.K. (1967) A review of the problem of the toxicity of
pesticide chemicals in food in India. Ind. Food Packer 21 : 1-14.
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Ent. 45 : 452-456.
Marth, E.H. (1962) Chlorinated hydrocarbons deposited in biological
material. I. Plants and plant products. J. Milk Food Technol. 25 : 36.
Minyard, J.P., Jackson, E.R. (1963) Pesticide residues in commercial
animal feeds. J. Assoc. Off. Analyt. Chem. 46 : 843-859.
Morgan, L.W., Leuk, D.B., Beck, E.W., Wardham, D.W. (1967) Residues of
aldrin, chlordane, endrin and heptachlor in peanuts grown in treated
soil. J. Econ. Ent. 60 : 1289-1291.
Muns, R.P., Stone, M.W., Foley, F. (1960) Residues in vegetable crops
following soil applications of insecticides. J. Econ. Ent. 53 : 832.
Nash, R.G., Woolson, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science 157 : 924-927.
Ordas, E.P., Smith, V.C., Meyer, C.F. (1956) Spectrophotometric
determination of heptachlor and technical chlordane on food and forage
crops. J. Agr. Food Chem. 4 : 444.
Painter, Ruth R., Kilgore, W.W., Ough, C.S. (1963) Distribution of
pesticides in fermentation products obtained from artificially
fortified grape musts. J. Food Sci. 28 : 342.
Perez-Escolar, M.E. (1959) Control pineapple gummosis in Puerto Rico.
J. Agr. Univ. Puerto Rico 43 : 116.
Poonawalla, N.H., Korte, F. (1964) Metabolism of insecticides. VIII
Excretion, distribution and metabolism of alpha-chlordane-14C by
rats. Life Sci. 3 : 1497
Saha, J.G., Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide, and
gamma-chlordane residues in soil and rutabagas after soil and surface
treatments with heptachlor. Can. J. Plant Sci. 47 : 79-88.
Robinson, J., McGill, A.E.J. (1966) Organochlorine insecticide
residues in complete prepared meals in Great Britain in 1965. Nature
212 : 1037-1038.
Rusk, H.W., McDonough, L.M. (1966) Report of residue analysis; soils
and sugar beet roots. USDA, ARS, Ent. Res. Div., Pesticide Chemicals
Res. Br., Report Nos. PCY-67-6 and PCY-66-9.
Smith, F.F., Adams, H.R. (1965) Chlordane residues in crops. USDA,
ARS, Ent. Res. Div. Pesticide Chemicals Res. Br., Special Report
PC-B-65-26.
Smith, F.F., Reid, W.J., Cuthbert, F.P. (1964) Dieldrin and chlordane
residues in sweet potatoes. USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report PC-B-64-10.
Swackhamer, A.B. (1967) Personal communication
Thurston, A.D. (1965) Preliminary studies on weathering of chlordane
residues. J. Assoc. Off. Analyt. Chem. 48 : 952-4.
USDA. (1964) Chlorinated hydrocarbon pesticide residues in soil at
Battle Creek, Mich., 1963, USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report No. PL-V-64-10.
USDA. (1966) Report of residue analysis; potatoes, chlordane. ARS,
Ent. Res. Div., Pesticide Chemicals Res. Br., Report No. PCY-66-1.
USDA. (1967a) Monitoring chlorinated hydrocarbon insecticide residues
in soybeans, 1966. ARS, Plant Pest Control Division, interim report,
1967.
USDA. (1967b) Report of residue analysis; soil; DDT and chlordane.
USDA, ARS, Ent. Res. Div., Pesticide Chemicals Res. Br., Report No.
PCB-67-9.
Velsicol Corp. (1967a) Unpublished report on chlordane residues in
soil. Submitted to FAO.
Velsicol Corp. (1967b) Unpublished report on residues in potatoes from
soil treatments with chlordane. Submitted to FAO.
Velsicol Corp. (1967c) Unpublished report on residues in barley grain
and straw from spraying growing barley at Fargo, N.D., 1966. Submitted
to FAO.
Velsicol Corp. (1967d) Unpublished report on chlordane residues in
sugar beets. Submitted to FAO.
Velsicol Corp. (1967e) Progress report on chlordane dairy feeding
study. Submitted to FAO.
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.,
Schutzmann, R.L. (1963) Chemical residues in the milk of cows grazed
on chlordane-treated pasture. J. Agr. Food Chem. 11 : 244.
Other relevant chemical properties
Chlordane, as presently manufactured in the USA, is a uniform
technical product, the composition of which is controlled. Analytical
methods and reference standards have been made available periodically
by the manufacturer to regulatory agencies as a method of commercial
product control. Analytical methods for regulating the technical
formulation in commerce have become official methods after
collaborative studies by the Association of Official Analytical
Chemists (AOAC) in the USA and the European Commission for Methods of
Analysis of Pesticides (CIPAC). While hexachlorocyclo pentadiene is
used in the manufacture of the technical product, none of this
chemical remains unreacted and there is no evidence that it appears as
a constituent of the terminal residue in food.
There is some confusion in nomenclature. Gamma-chlordane is the
alpha-chlordane referred to by March (1952) (it is the component
present in the highest concentration in technical chlordane),
alpha-chlordane is the beta isomer described by March. Poonawalla and
Korte (1964), Ludwig (1966), and some other authors still use March's
designation. These two components together comprise approximately half
of technical chlordane.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Chlordane is absorbed from the gastrointestinal tract, the respiratory
tract, and through the skin (Ambrose et al., 1953). It is stored in
the adipose tissue of rats, sheep, goats, and cows and accumulates in
the milk.
Rats fed chlordane for six and a half months showed the following
levels in their abdominal fat at various feeding levels (in ppm):
intake 3, residue 5.2; intake 15, residue 32; intake 30, residue 76;
intake 60, residue 109 (Ingle, 1965).
Chlordane fed at 25 ppm in the diet for 8 weeks reached a maximum
level of 18 ppm in the fat of calves and 12 ppm in sheep. After
feeding was stopped, the residue was eliminated from calves in 20
weeks and from the sheep in 4 weeks (Claborn et al., 1953).
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlordane is
excreted in the urine of rabbits (Stohlman and Smith, 1950).
Cows grazing on pasture to which 0.5 pound of chlordane per acre had
been applied showed an average of 0.03 ppm of chlordane in their milk
(Westlake et al., 1963).
Both technical chlordane and one of its pure isomers (gamma-chlordane)
have been shown to have stimulating effects on rat liver microsomes
for the metabolism of certain drugs (Burns et al., 1965; Hart and
Fouts, 1963; Hart et al., 1963; Kuntzman, et al., 1964).
In vitro metabolism by hepatic microsomal enzymes from rats treated
8 days previously with single i.p. doses of 10 or 100 mg/kg of
chlordane in corn oil was increased for the 3 drugs reported
(hexobarbital, aminopyrine and chlorpromazine). The increases for
hexobarbital and aminopyrine were dose-related. In vivo hexobarbital
response, measured by sleeping time of mice, 1, 3 and 8 days after
single i.p. doses of 25 mg/kg body-weight technical chlordane or
gamma-chlordane, showed a decrease (shortened sleeping time), greatest
at day 3 but still marked on day 8 (Fouts, 1963).
The mechanism of action of chlordane is similar to that of
phenobarbital in stimulating hepatic drug metabolism in the rat. Both
agents increase liver weight, microsomal protein, microsomal NADPH
oxidase, and microsomal cytochrome CO-binding pigment. They both
stimulate drug metabolism in adrenalectomized or hypophysectomized
rats and promote the proliferation of smooth endoplasmic reticulum in
the liver cell, and ethionine blocks the enzyme stimulating effects of
both agents (Hart and Fouts, 1965).
Liver microsomal preparations from adult, female rats given 10 mg/kg
of chlordane intraperitoneally every other day for 14 days showed a
385 per cent increase in the biotransformation of 17ß-estradiol to
polar metabolites (Kuntzman et al., 1964). The intraperitoneal
injection of immature male rats with 50 mg/kg of chlordane daily for 4
days produced an increase of activity of the liver microsomal steroid
hydroxylases (Conney et al., 1967).
The toxicities of bishydroxycoumarin and phenylbutazone were reduced
in both rats and dogs pretreated by chlordane, and this effect was
accompanied by lower plasma levels of these drugs in the
chlordane-treated animals than in controls given the same doses (Welch
and Harrison, 1966). The toxicity of parathion was reduced in mice
pretreated 16 hours to 4 days previously with a single oral dose of
130 mg/kg of chlordane (Triolo and Coon, 1966).
Male rats weighing 60 to 80 gms were given 25 mg/kg of chlordane
intraperitoneally daily for 3 days, and were sacrificed 24 hours after
the last dose. The livers showed a proliferation of the
smooth-surfaced endoplasmic reticulum in the parenchymal cells,
associated with an increased activity of microsomal preparations from
such livers to metabolize hexobarbital, aminopyrine, zoxazolamine and
p-nitrobenzoic acid (Fouts and Rogers, 1965).
Pretreatment of rats for several days with chlordane increased the
LD50 of warfarin more than 10-fold, an effect associated with
decreased plasma levels of warfarin and an increased rate of
metabolism of the drug by liver microsomal enzymes (Ikeda et al.,
1966).
Technical chlordane and one of its pure isomers, gamma-chlordane,
administered to adult or weanling rats in single or 3 daily doses,
after 1 to 8 days increased the hepatic microsomal metabolism of
hexobarbital, aminopyrine, and chlorpromazine (Hart et al., 1963).
Treatment of newborn rabbits with 50 mg/kg of gamma-chlordane daily
for 3 days increased the activity of liver enzymes that metabolize
hexobarbital, aminopyrine and p-nitrobenzoic acid. Treatment of
lactating mothers with chlordane increased the drug metabolizing
enzymes in the nurslings (Fouts and Hart, 1965). Drug metabolism was
also stimulated in adult rabbits.
In dogs given 5 mg/kg of chlordane orally three times a week for 7
weeks the rate of metabolism of phenylbutazone was markedly increased.
This effect persisted as long as 21 weeks after termination of the
chlordane administration (Burns et al., 1965).
Chlordane has been demonstrated to stimulate the rate of drug
metabolism by the liver of the squirrel monkey (Cram et al., 1965).
For further information see the sections In Plants, and In
Animals.
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat Oral 200 - 590* Ambrose et al., 1953
Ingle, 1955
Stohlman et al., 1950
Oral 335 - 430 Gaines, 1960
150 - 225 Ingle, 1955
Mouse Oral 430 US Food and Drug Admin., 1947
Rabbit Oral 100 - 300* Stohlman et al., 1950
20 - 40 Ingle, 1955
Goat Oral 180 Welch, 1948
Sheep Oral 500 -1000 Welch, 1948
Chicken Oral 220 - 230 Turner and Eden, 1952
* The differences are explained by the use of different solvents,
and by the fact that the chlordane mentioned in the older
literature contained a considerable amount of the very toxic
hexachlorocyclopentadiene (Ingle, 1965; Lehman, 1952).
Man. A dose of 104 mg/kg proved fatal (Derbes et al., 1955). An
18-year old female showed convulsions but recovered after a dose of
approximately 30 mg/kg (amount retained after vomiting estimated to be
10 mg/kg). In two infants, 15 months and 3 years of age, 10 and 40
mg/kg respectively gave severe poisoning (Stormont and Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12 male
rats, all of them died within 10 days. At 500 ppm, 12/12 died within
70 days; at 300 ppm, 9/12 were alive after 100 days (Stohlman et al.,
1950).
Daily oral doses of 6.25 - 25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg, all
the animals died. Intracytoplasmic bodies in the liver-cells were
found at all levels and their number was in proportion to the dose
used (Ambrose et al., 1953).
Groups of 6 females and 6 males were fed 2.5 ppm or 25 ppm of a sample
of technical chlordane containing 60-75% chlordane and 25-40%
unrelated products for up to 9 months. Centrolobular cell hypertrophy,
peripheral migration of cytoplasmic granules and the presence of
cytoplasmic bodies were observed in 1 male at 2.5 ppm and in 5 males
at 12.5 ppm (Ortega et al., 1957).
Initial groups of 10 males and 20 females were used in a 3-generation
study at dietary levels of 0, 0.3, 3, 15, 30 and 60 ppm of technical
chlordane. Two litters in each filial generation were studied. Levels
up to and including 30 ppm had no effect on fertility, numbers of
young or litters, or weight, growth or mortality of the young animals
to weaning age. Autopsy of animals post-weaning showed no gross or
microscopic difference between the groups. At 60 ppm, there was a high
(10.6 per cent) mortality in the second F3 generation litters during
the latter part of the nursing period; these animals showed gross and
microscopic pathology comparable to that characteristic for chlordane
intoxication. However, survivors of this generation showed no tissue
changes at all. A third act of F3 litters at 60 ppm suffered 17 per
cent mortality during the nursing period, with symptomatology end
gross and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from the
60 ppm group and placed on chlordane-free diets for 30 days prior to
remating showed no difference in any respect from control litters. No
evidence of teratogenicity or tumorigenicity for chlordane was found
in this study (Ingle, 1967).
Dog. Chlordane was given in varying oral doses to dogs for 7 days,
convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but 700
mg/kg (highest dose) did not produce any effect (Batte and Turk,
1948).
Four groups of 2 to 4 dogs given chlordane orally in doses between 5
and 80 mg/kg body-weight daily all died within periods of 25 days to
93 weeks (Lehman, 1952).
Groups of 4-7 males and 4-7 females were fed 0, 0.3, 3, 15 and 30 ppm
of chlordane for 2 years. Abnormalities in the results of clinical
liver function tests were seen in the 15 and 30 ppm groups. In animals
selected for necropsy at the end of the first year, increased relative
liver weights and associated hepatocellular changes were found at 30
ppm; at the end of two years, dose-related increases in relative liver
weights were found at 15 and 30 ppm, with non-dose-related
hepatocellular changes. There was no difference between the severity
of the liver lesions of the 30 ppm animals and those of four animals
withdrawn from 30 ppm treatment for eight months prior to sacrifice.
Percutaneous liver biopsies on two animals of the 30 ppm group at 1, 3
and 6 months showed hepatocellular changes at 6 months but not at 1 or
3 months. No adverse effect was seen on behaviour, appearance,
survival, weight gain, blood picture or the results of periodic
physical examination, at all levels (Wazeter, 1967).
Sheep. Chlordane administered by stomach-tube to sheep in a dose of
0.5 g/kg body-weight produced toxic symptoms (incoordination, partial
blindness) in 5 to 6 days. A dose of 1 g/kg body-weight produced
severe respiratory and nervous symptoms at 16 hours and death after 48
hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each sex)
were given 2.5, 25 and 75 ppm of chlordane in the diet for 2 years.
The sample of chlordane used had an LD50 of 450 mg/kg (Lehman, 1951).
It was found that 25 and 75 ppm gave moderate to severe signs of
intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
Groups of 40 rats (20 males and 20 females) were fed concentrations of
5, 10, 30, 150 and 300 ppm of "technical chlordane" in the diet over a
2-year period.
Throughout the experiment tremors and convulsions appeared or could be
induced at 30 or more ppm. Following fasting, no neurological symptoms
appeared at 5 or 10 ppm. Growth rate was affected at 150 or 300 ppm.
Liver histological damage was observed in the form of hypertrophy of
centrolobular calls, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm, slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was carried
on between late 1953 and late 1955, "technical chlordane of recent
manufacture" was used. Groups of 40 rats were given chlordane at 2.5,
5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given no
chlordane. Changes concerning food consumption, growth and mortality
were seen only in the 300 ppm group. Liver cell changes were not
present in the animals given 2.5-25 ppm. At 50 ppm only "cytoplasmic
peripheralization" was present. At higher doses the changes were as
those previously described (Ingle, 1955).
In a study published in 1953, a sample of chlordane exhibiting an oral
LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each sex
were given 0, 10, 20, 40, 80, 160, 320, 640 and 1280 ppm of chlordane
in their diets for approximately 407 days. The animals at 640 and 1280
ppm died early. At lower dosages, survival was unaffected. Increased
liver-weight (in comparison with the control group) was observed over
320 ppm. In a sample of liver of a male at 320 ppm the average nuclear
volume was 377µ3 compared to 268µ3 in a control rat. Cytoplasmic
vacuoles containing fat and clusters of granules at the periphery of
the cytoplasm were often seen. In the males they were equivocal at 10
ppm, absent at 20 ppm and infrequent at 40 ppm. In the females these
lesions were common and were seen only at 80 ppm and over (Ambrose et
al., 1953).
Observations in man
Workers engaged in the manufacture and formulation of chlordane for
periods up to 15 years have exhibited no evidence of harmful effects
attributable to this insecticide (Princi and Spurbeck, 1951; Alvarez
and Hyman, 1953; Fishbein et al, 1964). In a survey of more than 1105
persons who had been engaged in pest control operations for 1 to 30
years (318 for 5 to 19 years), three cases of toxicity due to
chlordane were reported, the only symptoms specified being dizziness
and headache (Stein and Hayes, 1964).
Chlordane was not found in any of 282 human fat samples taken at
autopsy, though DDT and lindane were regularly detected and dieldrin
was frequently found (Hoffman et al., 1964).
Comments
Since chlordane stimulates drug metabolizing processes in the rat,
mouse, rabbit, dog and squirrel monkey, and since the microsomal
enzyme stimulating properties of chlordane are similar to those of
phenobarbital, which stimulates drug metabolism in man, it appears
extremely likely that chlordane also stimulates drug metabolism in
man. This effect in animals and the associated cellular changes in the
liver, considered characteristic of the chlorinated hydrocarbon
insecticides as a class, are thought by several workers not to
represent toxic effects but rather physiological adaptive processes.
Toxicological studies reported prior to 1953 are not contributory to
the evaluation of the currently manufactured product because of the
variability of composition before that time. The chlordane made for
commercial use since 1953, which has been used in the studies
reported, has a constancy of composition that makes the data adequate
for toxicological evaluation.
In none of the many toxicological studies done has there been any
evidence observed of tumorigenic or other irreversible pathologic
changes, even at the highest doses or feeding levels used.
On the basis of the results of two long-term feeding studies in the
rat, a no-effect level for the rat may be established at 20 ppm, or 1
mg/kg/day. A satisfactory 3-generation reproduction study in the rat
has been completed recently in which 30 ppm was without effect on
fertility, number or size of litters, or on the weight, growth or
mortality of the young to weaning age. Furthermore, there was no
evidence of teratogenicity. In the recently completed 2-year feeding
study in the dog, 3 ppm of chlordane was the highest level used that
had no effect in tests of liver function or on liver weight and
structure of the liver cell. Though the liver weight and cell
structure changes at 15 ppm may probably be classified as adaptive
rather than toxic in nature, the significance of the functional
changes at this level cannot be assessed in the present state of our
knowledge. Even though these changes consistently disappeared before
the end of the 2-year feeding period, and even though 15 and 30 ppm
produced no other adverse effects throughout the 2-year period, it
seems necessary at this time to take 3 ppm chlordane as the maximum
known no-effect level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat. 20 ppm in the diet, equivalent to 1 mg/kg/day
Dog. 3.0 ppm in the diet, equivalent to 0.075 mg/kg/day
Estimate of acceptable daily intake for man
0 - 0.001 mg/kg body weight
Further work required
See General Comments page 3 and 4.
Further work desirable
Toxicological data on alpha and gamma chlordane.
EVALUATION FOR TOLERANCES
USE PATTERN
Chlordane was the first cyclodiene insecticide and originally had a
broad scale and spectrum of use but was somewhat displaced by aldrin,
dieldrin, endrin and heptachlor because of their greater persistence
and effectiveness and lower cost. In recent years, to narrow the use
spectrum of aldrin, dieldrin, endrin and heptachlor, there is renewed
interest in the use of chlordane.
Several million pounds of chlordane have been used annually in North
America for more than 20 years of which between 30 and 40 per cent has
been used in agriculture. The USA and Canadian currently approved uses
represent the largest spectrum and potential scale of use in the world
and have been used an a guide to this evaluation.
Pre-harvest treatments
The most important use of chlordane in for soil treatment, with a
limited use for foliar application, the main crops being large root
crops, sugar beets, corn, small root vegetables, leafy and stalk
vegetables, cucurbits, leguminous vegetables, berry fruits, sugar
cane, pineapple and cereals. Some uses on forage crops are still
approved in the USA.
The literature suggests that in fifty or more countries, chlordane is
or can be used on sugar beets, potatoes, vegetables, tobacco, sugar
cane, ground nuts (peanuts), beans, bananas, coffee, deciduous fruits,
citrus, olives, cotton and tea bushes. However, information on the
scale of use and resulting residues is not generally available. In
other countries the scale of use is probably only a fraction of that
in the USA. Dosages vary between countries, with a range of 1 to 8
lbs/acre recommended for a variety of vegetable insect control
requirements.
Post-harvest treatments
No post-harvest uses are currently recommended.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The large amount of available information needs critical review
because of improvements in analytical methods since 1961 and recent
information on the chemical nature of terminal residues. Methods used
prior to 1963 were based on total chlorine or colorimetric
measurements and expressed results in terms of technical chlordane.
Recently developed methods identify individual components, the
summation of which frequently is approximately one half of that
reported by earlier non-specific methods. New data developed from 1965
to 1967 reports individual components of residues as alpha and
gamma-chlordane, sometimes with much smaller and insignificant
quantities of heptachlor and heptachlor epoxide. Small amounts of
gamma-chlordane will be found in some crops resulting from the use of
technical heptachlor in soil (Lichtenstein et al, 1967; Saha and
Stewart, 1967; Velsicol, 1967 a through d). Some authors have
recently added the various components of the residue and reported the
total as chlordane (e.g. Smith and Adams, 1965).
The available information concerning residues resulting from uses
constituting "good agricultural practice" (i.e. consistent with pest
control requirements, principally in the USA and Canada) are
summarized below.
Crop Residue (ppm) Reference
alfalfa 0.01 Ordas et al, 1956
" 0.16 Allen, 1963
barley, grain 0.0 - 0.06 Velsicol, 1967c
" , straw 0.98 - 2.65 " "
beans <0.01 Smith and Adams, 1965
beets 0.17 "
beet tops 0.01 - 0.1 "
bell peppers none recovered Muns et al, 1960
broccoli " " "
cabbage " " "
cantaloupe 0.08 "
" , meat and rind 0.01 - 0.1 Smith and Adams, 1965
carrots 1.5 Muns et al, 1960
" 0.01 - 0.13 Cook, 1960
" 0.16 (1) Lichtenstein et al, 1967
" , peel 0.0 - 0.7 Cook, 1960
collards none recovered Marth, 1962a
cucumbers 0.08 Muns et al, 1960
eggplant none recovered "
lettuce 0.04 "
peaches 0.8 Fahey et al, 1957
peanuts 1.74 ± 0.82 Morgan et al, 1967
pineapple 0.145, 0.43 (2) Perez-Escolar, 1959
potatoes <0.01 - 0.23 (3)
radishes 0.03 Lichtenstein et al, 1967
rutabagas 0.5 Muns et al, 1960
" 0.08 - 0.16 (4) Saha and Stewart, 1967
snap beans <0.01 Smith and Adams, 1965
strawberries <0.01 "
" none recovered Fahey, 1962
sweet potatoes 0.01 - 0.4 (5)
sugar beets 0.04 - 0.37 (6)
tomatoes 0.01 Muns et al, 1960
" <0.01 Smith and Adams, 1965
turnips 0.01 - 0.1 "
" 0.16 Muns et al, 1960
turnip tops 0.01 - 0.1 Smith and Adams, 1965
wine none (7) Painter et al, 1963
(1) soil contained 0.68 ppm gamma chlordane (from heptachlor application 5 years
previous).
(continued)
(2) resulting from one and two applications, respectively.
(3) overall range as reported by many authors, e.g. Lichtenstein, 1967; Smith
and Adams, 1965; Begg et al, 1960; USDA, 1964; USDA, 1966; Corley et al,
1965; Velsicol, 1967b.
(4) gamma chlordane from heptachlor
(5) overall range as reported by many authors, e.g. Muns et al, 1960; Smith and
Adams, 1965; Smith et al, 1964.
(6) overall range following different rates of application as reported by
many authors, e.g. Muns et al, 1960; Rusk and McDonough, 1966;
Velsicol, 1967d.
(7) 0.6 in must and lees.
Data are required from similar investigations using reliable
analytical procedures from other countries. Information pertaining to
successive crops grown in rotation over a period of years after
initial treatment would be of particular interest. Data are also
required on the residues which may occur in oil seeds as the result of
intentional use, or from growing these crops in rotations. This is of
particular importance where certain oil-containing crops are grown in
rotation with other crops, especially after maize.
In general these data suggest that the highest residues consistent
with good agricultural practice will result in the large root crops,
and under certain conditions in the leafy and stalk vegetables.
Considerable variation can be expected in residues found in the large
root crops, particularly potatoes, depending on soil type, variety of
potato and date of maturity. Recent information suggests that the
residues in leafy and stalk vegetables may in part be from direct
contamination from soil, rather than a true translocation into growing
parts other than the stem or stalk. The intermediate group in
magnitude of residues to be expected would include small root
vegetables, cucurbits and pineapple. Cereals, berry fruits, pod
vegetables and fruit vegetables of the tomato type are in the category
of lowest residues, usually well below 0.1 ppm. It is probable that
there is no true translocation of residues in the grain of most
cereals and that the residues found are due to mechanical
contamination by soil, dust, debris, etc., during harvesting
operations.
RESIDUES IN FOOD MOVING IN COMMERCE
Pesticide residues in commercial animal feeds are important sources of
residues in milk and other animal products. Data concerning chlordane
are available from one such survey in Tables 1 and 2 (Minyard and
Jackson, 1963).
TABLE I
The occurrence of chlordane in commercial feeds expressed in parts per billion
No. No. Range of chlordane Range of total
Feed samples positive residue (ppb) pesticide
residue (ppb)
16% protein Dairy 73 53 0-254 26 - 1153
18% protein Dairy 6 4 13- 39 55 - 713
20% protein Dairy 2 2 15- 34 139 - 274
32% protein Dairy 4 1 5 36 - 435
12% protein Fitting
and Freshening 2 1 12 49 - 60
14% protein Cattle 3 2 11- 12 47 - 183
20% protein Beef 1 1 45 343
16% protein Hog 3 0 - 34 - 62
16% protein Poultry 3 2 7- 11 80 - 291
TABLE II
Ranges and averages of insecticide residues found in 101 feeds expressed in parts per billion
Av. level of
No. Per cent Range of contamination of
Insecticide contaminated contaminated contamination contaminated foods
(ppb) (ppb)
lindane 101 100.0 5 - 79 16.0
aldrin 87 86.1 1 - 16 2.6
DDT 99 98.0 10 - 448 80.5
methoxychlor 46 45.5 10 - 580 74.0
endrin 59 58.4 4 - 27 10.6
chlordane 68 67.3 5 - 254 30.4
dieldrin 89 88.1 1 - 16 4.9
toxaphene 15 14.8 60 - 530 178.3
heptachlor 9 8.9 2 - 13 4.8
heptachlor epoxide 3 3.0 1 - 6 3.3
Soybeans follow maize in recommended crop rotations in the USA. Soil
used for maize production is treated with chlordane, aldrin or
heptachlor and may result in residues of chlordane in soybeans
subsequently grown in these soils. An interim report on a survey of
unintentional residues from soil and crop samples from 27 regional
special sampling locations in the USA was made available to the
meeting. Chlordane was detected in soils from 3 fields at an average
concentration of 0.26 ppm, soybeans from one of these fields
containing 0.03 ppm. Eight other soil samples contained an average
concentration of 0.50 ppm (range 0.09 to 1.11 ppm). No chlordane was
detected in soybean seed or plants from these latter soils (USDA,
1967a).
Information on sources of chlordane in total diet studies has been
available for five years. The earliest of these concerns 12,000
samples analyzed by paper chromatography and gas liquid chromatography
in the state of California (CSDA, 1962). No chlordane was found in
this survey.
Thirty-eight total diet studies in 1961-62, for six USA cities
resulted in only two samples containing chlordane at 0.01 and 0.03 ppm
(Fishbach, 1963).
Eighty-two foods collected from 18 markets in three different
geographical areas in the USA were prepared for consumption, resulting
in 216 composite samples, and then analysed (Duggan et al, 1966).
Chlordane was found in only one composite sample at 0.033 ppm in
"garden fruit" produce (i.e. raw peppers, fresh and canned tomatoes,
raw cucumbers, catsup, eggplant, raw and frozen summer squash).
Comprehensive unpublished information from the USA was made available
to the 1967 Joint Meeting (Duggan, 1967). This data is concerned with
both objective samples of food for the years 1964 -1966 inclusive and
total diet samples for the year ending April 1967.
TABLE III
Objective Samples - Chlordane
Year 1964 1965 1966 Total
Number of domestic samples 20,088 14,462 14,806 49,356
Per cent of samples positive 0.7 1.1 0.9 0.9
Number of import samples 1,224 1,217 1,399 3,840
Per cent of samples positive 0.2 0.9 0.1 0.4
Total Diet Samples - Chlordane
Leafy vegetables - 1 composite positive contained 0.02 ppm (29 negative)
Legume vegetables - 1 composite positive contained 0.005 ppm (29 negative).
In Table IV details of the incidence and levels of chlordane for 1966
are of considerable interest as to amount and sources of residues in
the diet of man in the USA.
Canadian information covers a 4.5 year period from 1963 to July 1967.
Of a total of 14,270 samples of food (12,186 domestic and 2,084
imports), eleven contained chlordane; one lot of imported green
peppers, >0.3 ppm; six lots of whole eggs, 1.8 to 11 ppm (all seven
in excess of tolerance). Three vegetable samples contained traces of
chlordane, and one lot of turnips contained residues in excess of 0.3
ppm (Swackhamer, 1967).
Two reports of no chlordane being found in British food (Robinson and
McGill, 1966; Egan et al, 1966) are difficult to evaluate because of
lack of information on current use of chlordane in Britain and the
countries from which some foods concerned were imported.
FATE OF RESIDUES
In soils
Chlordane residues in soil have been investigated since 1949. However,
prior to 1961 much of the information is of doubtful value. Sources of
information since then include : USDA, 1964; Landis, 1965; Edwards,
1965; Edwards, 1966; Rusk and McDonough, 1966; Chisholm et al, 1966;
Bess et al, 1966; USDA 1967b; Nash and Woolson, 1967; Saha and
Stewart, 1967; Velsicol, 1967a; Lichtenstein, 1967; and Majumder,
1967.
Table V summarizes comparative persistence of some organochlorine
insecticides based on the literature review of Edwards (1966) but
modified as concerns doses used for a particular pest as far as
chlordane is concerned.
Approximately 55 per cent of chlordane remains in soil after the first
year following application, compared with 26 per cent for aldrin, 75
per cent for dieldrin and 80 per cent for DDT, a situation which is
not consistent with the respective vapour pressures; hence other
factors must be operating.
Although most reports to date express residues in soil on the basis of
equivalents of technical chlordane, Saha and Stewart (1967) reported
11.9 per cent of the residue resulting from one use of heptachlor as
gamma-chlordane (one pound of heptachlor contains 35 per cent
gamma-chlordane).
TABLE IV
Incidence and levels of chlordane in objective samples of food of domestic origin*, USA, 1966 (Duggan, 1967)
Number of Samples
Raw Manufactured Processed Fish
Range agricultural dairy animal and Vegetable Processed
ppm products products feeds shellfish oils foods Total
T - 0.03 59 1 3 1 - - 64
0.04 - 0.10 17 - 4 2 - - 23
0.11 - 0.50 35 - 4 - 1 1 41
0.51 - 1.00 - - 1 - - - 1
1.01 - 1.50 - - 2 - - - 2
1.51 - 2.00 - - 1 - - - 1
Above 2.00 2 - - - - - 2
Total samples
containing 113 ** 1 15 3 1 1 134
chlordane
Total samples
examined 9,804 611 680 290 171 227 14,806 *
* Of an additional 1399 samples of imported foods, only 1 contained chlordane, and this in a raw
agricultural product in the range of - 0.03 ppm
** 55 residues were in root vegetables
30 residues were in vine and ear vegetables
TABLE V
Persistence of some organochlorine insecticides in soil
Insecticide Average dose active Time for 95% disappearance
ingredient, lbs/acre in years
Range Mean
aldrin 1 - 3 1 - 6 3
chlordane 1 - 2 3 - 5 4
DDT 1 - 2´ 4 - 30 10
dieldrin 1 - 3 5 - 25 8
heptachlor 1 - 3 3 - 5 3´
lindane 1 - 2´ 3 - 10 6´
telodrin ¨ - 1 2 - 7 4
In plants
Information on the chemical nature of the terminal residues from
weathering of external residues has recently become available
(Thurston, 1965; Klein and Link, 1967). A further review of new
unpublished information and work in progress is contained in the 1966
and 1967 Reports of the IUPAC Commission on Chemical Nature of
Terminal Pesticide Residues (IUPAC, 1967, 1968).
The principal terminal residues from weathering of chlordane on plants
have been identified as alpha- and gamma-chlordane by characteristic
"signature" peaks as determined by gas liquid chromatographic methods
and confirmed by infrared methods. The sequence of analyses taken
after agricultural applications of technical chlordane exhibit a
fairly constant pattern. Minute amounts of heptachlor or heptachlor
epoxide appear in some chromatograms but, for all practical purposes,
these can usually be ignored.
The loss of technical chlordane from plants by normal weathering is
extremely rapid. At the end of the seventh day only 7 ppm of a
starting load of 110 ppm remains on kale, and only 0.1 ppm, expressed
as technical chlordane equivalent, remains after 28 days (Klein and
Link, 1967).
Previous studies on weathering of residues have not taken account of
the possible formation of hydrophilic metabolites, an omission which
is not probably toxicologically important since the known hydrophilic
metabolites of at least gamma-chlordane are less toxic than
technical chlordane. However, such information in domestic animals
after ingestion of weathered residues would be important in
establishing the consumer hazard of residues in animal products.
Recent information on the production of hydrophilic metabolites of
gamma-chlordane in animals (Poonwalla and Korte, 1964; Ludwig, 1966)
suggests that similar investigations should be carried out in plants,
particularly from the stand-point of translocation and metabolism of
residues in soil. Work on that subject is now in progress by Korte and
will be reported to the IUPAC Commission on Terminal Residues in 1968.
Current indications are that substantial formation of hydrophilic
metabolites (of lower order of toxicity than alpha-, gamma-, and
technical chlordane) are formed in the stalk of plants (Korte, 1967
personal communication).
Gamma-chlordane residues can occur in plants as the result of use of
technical heptachlor in soil. An application of 6.6 kg/ha mixed into
soil results in 11.9 per cent of the soil residue being in the form of
gamma-chlordane. Rutabagas grown in this soil contained 0.008 ppm
gamma-chlordane, this being 13.3 per cent of the total residue
(heptachlor + heptachlor epoxide + gamma chlordane = 0.060 ppm), (Saha
and Stewart, 1967). Crops grown in soil containing 0.68 ppm
gamma-chlordane, resulting from heavy previous use of heptachlor,
contained harvest residues of gamma-chlordane of the following order :
radishes, 0.03 ppm; carrots, 0.16 ppm; potatoes, 0.03 ppm; pea greens,
not detected (Lichtenstein et al, 1967 in press).
In animals
An important recent development with a bearing on the evaluation of
the hazard of residues in food to humans is the evidence that in
mammals gamma-chlordane can be metabolized and detoxified to more
hydrophilic products which are largely excreted into the aqueous
phase. Rats receiving intravenous doses of gamma-chlordane 14C
excreted 22 per cent of the total dose within 60 hours in the form of
hydrophilic metabolites in feces. High percentages of hydrophilic
metabolites were detected in the entire alimentary tract and kidneys.
Unchanged gamma-chlordane was found only in subcutaneous fat
(Poonawalla and Korte, 1964). Weekly doses of gamma-chlordane 14C
administered by stomach tube for ten weeks to rabbits resulted in 47.2
per cent of the total administered radioactivity being excreted in
urine, and 22.7 per cent in feces, with only about 4 per cent in fatty
tissues. Unchanged gamma-chlordane could be detected only in
subcutaneous fat, while all other tissues contained hydrophilic
metabolites. In urine, only hydrophilic metabolites could be detected.
Two hydrophilic metabolites have been isolated, one identified as a
chloro-hydrine. The acute oral toxicity of this metabolite is lower
than that of chlordane. The LD50 value for mice is higher than 1800
mg/kg. The structural formula for this metabolite assigned is
1-hydroxy-2-chloro-dihydrochlordane. The second metabolite is more
hydrophilic than the first, with both chlorine atoms in position 1 and
2 of gamma-chlordane possibly replaced by hydroxylic groups (Ludwig,
1966). The first metabolite may also possibly be derived from
alpha-dihydro-heptachlor resulting from oxidative detoxication
metabolism of heptachlor. Brooks and Harrison, (1967) assign a
slightly different structure to this metabolite, designating it
possibly as an octochloro-compound.
These recent findings possibly explain why the analysis of more than
500 samples of human fat tissues in the USA did not detect chlordane
(Hoffman et al, 1964; Hoffman, 1963). In less than 50 per cent of
these samples, two peaks in GLC chromatograms were noted, representing
concentrations of 0.01 - 0.05 ppm chlorine. Assuming these peaks are
due to a pesticide, their region indicates they could be caused by
ronnel, heptachlor, heptachlor epoxide, endosulfan or chlordane, or
hydrophilic metabolites of chlordane only partially soluble in polar
solvents.
The weathered residues of plants consist largely of alpha- and
gamma-chlordane. If this also occurs in residues translocated from
soil to growing plants, their hazard to man, after ingestion and
detoxication to hydrophilic metabolites, would reasonably be much less
than that for technical chlordane.
Residues of chlordane in meat resulting from direct application to
animals has been reported (Claborn, 1956; Claborn et al, 1960).
Consequently, chlordane is not currently recommended for use in direct
treatment of animals. Residues in whole milk resulting from grazing
cows on chlordane-treated pastures as early as one day, one week, two
weeks and four weeks after treatment were dosage dependent. At 0.25
lb/acre, none was found; at 0.5 lb and 1.0 lb per acre, a maximum of
0.08 ppm at the end of 8 weeks of feeding was found together with
maximum residues of heptachlor epoxide of 0.04 ppm. (Westlake et al,
1963).
Alfalfa treated with chlordane at one and two pounds per acre resulted
in hay residues of 20.4 and 20.9 ppm. Cows receiving this alfalfa for
a period of 150 and 100 days produced milk that contained residues
ranging from none to 0.2 ppm maximum (Carter et al, 1956).
Both of these experiments demonstrate considerable variation in the
residues excreted in milk by individual animals and suggest that
average results from a large herd of dairy animals would produce
smaller residues in pooled milk.
New USA data have been developed recently from feeding alfalfa with
known residues to dairy cattle. A progress report reviewed by the
Joint Meeting suggests that feeding alfalfa hay treated at 1.0 and 2.0
lbs/acre to Holstein cows resulted in a daily intake of 10.6 mg and
15.9 mg/day. Milk samples analyzed during the 31-day feeding study
were all negative (at a sensitivity of 0.01 ppm), with the exception
of one animal on the first day of feeding (Velsicol, 1967e).
In storage and processing
Information on the behaviour of residues during storage and processing
is needed. Although in the past, terminal residues of chlordane have
been regarded as stable, one investigation to date suggests that at
least in the case of rutabagas, cooking in water results in total
disappearance of residues in pulp (sensitivity 2 ppb) and 40 per cent
disappearance from peel, most being lost in water and only about 13.5
per cent steam-distilled (Saha and Stewart, 1967). Similar data on
other foods would be most useful.
METHODS OF RESIDUE ANALYSIS
A number of multidetection systems are available for the detection and
determination of organochlorine compounds and most of these can be
used for residues of alpha- and gamma-chlordane. An example is the
AOAC (1966) system in which acetonitrile partition and florisil column
clean-up are used, the residues being identified by gas chromatography
coupled with thin-layer or paper chromatography. Both electron capture
and micro-coulometric detectors can be used: the application of these
to chlordane, residue analysis, with special reference to
interferences by toxaphene (should this also be present) has been
described by Klein and Link (1967). The multidetection systems, which
normally separate the two isomers, are sensitive to about 0.02 ppm of
each isomer. Alternative methods for the confirmation of the identity
of residues, e.g. using infra-red spectrophotometry, are also
available.
Since alpha- and gamma-chlordane have boon shown by Klein and Link
(1967) to weather to approximately the same extent in agricultural
application, it is convenient to sum the residues; whilst in these
circumstances it might theoretically be possible then to apply a
factor to express this sum in terms of original technical chlordane,
it is recommended that results be reported as the total of alpha- plus
gamma-chlordane.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Canada 0.3 on a wide range of
foods (approximately
50 individual crops
included)
(cont'd)
Country Tolerance, ppm Crop
European Economic
Community 0.2 in fruits and
vegetables proposed,
(combined total with a review date
of all cyclodiene of 1 January 1970.
residues)
Netherlands,
Belgium, 0.1
Luxembourg
USA 0.3 on a wide range of
foods (approximately
50 individual crops
included)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Temporary tolerances
The potential hazard of residues resulting from the use of chlordane
has been reduced by narrowing the spectrum of use. New information on
the chemical nature of terminal residues indicates that these are
largely alpha- and gamma-chlordane. The extensive available
information from objective samples and total diet studies
in the USA (where several million pounds a year have been used
in agriculture for twenty years) indicates insignificant loads of
residues in food as consumed.
In view of these data and the relatively narrow spectrum of currently
recommended use, the following temporary tolerances, to be reviewed
31 December, 1970, are recommended :
Large root crops 0.3 ppm
Potatoes, sweet potatoes, rutabagas, turnips (soil
treatment) with the restriction that these be used for
human consumption only. If culls are to be fed to
livestock, additional data are required on the number of
pounds per day which may be fed in rations of swine, beef
and dairy cattle, with resultant residue data in animal
products.
Leafy and stalk vegetables 0.3 ppm
including all cole crops, spinach, lettuce, celery
and asparagus (soil treatment).
Small root vegetables 0.2 ppm
including but not limited to onions, table beets,
radishes (excluding carrots) (soil treatments).
Cucurbits 0.2 ppm
Cucumbers, melons including canteloupe, pumpkin and
squash (soil treatments)
Pineapple (soil treatment) 0.2 ppm
Sugar beets (soil treatment) 0.1 ppm
provided data are developed on residues remaining in
sugar beet pulp and green tops and residues remaining
in animal products when amounts of these are included
in the ration are fed to swine, beef or dairy cattle.
Pod vegetables (in the pod) 0.1 ppm
peas, beans, etc. (soil treatment)
Berry fruits 0.1 ppm
bush, cane and strawberries (soil treatment)
Tomatoes and related garden fruits (soil treatment) 0.1 ppm
Sweet corn and popcorn (soil treatment) 0.1 ppm
Comments:
Whole oil seeds: data are incomplete and available analytical
results are inconsistent. Tolerances or practical residue limits will
be required if chlordane is used in soil or as a foliar treatment.
Citrus, pome and stone fruits: no tolerance required, since no
residues are likely to result in fruit from soil treatments.
The point of enforcement of these tolerances should be on the raw
agricultural products moving in commerce.
Practical residue limits
Cereals 0.1 ppm
The meeting realized that it may be necessary at some future date to
recommend practical residue limits for chlordane for animal products.
Data were not available in 1967 to indicate such need or the levels
required.
FURTHER WORK
Further work required before 30 June 1970
1. More evidence is needed on the extent to which chlordane is used
on particular crops in various countries, the residues which
occur in commerce and total diet studies in countries using this
pesticide or importing food.
2. Data are required on the fate of residues during the normal
course of storage and processing before consumption.
3. Information on residues resulting from growing crops in rotations
on land in which chlordane has been used previously.
4. Data are required on residues in oil seeds, in vegetable oil
after processing and in oil seed cake or meal which may become
animal feed.
Further work desirable
Many of the measurements of residues made prior to 1961 need to be
repeated with the aid of the more sensitive and specific analytical
techniques.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Alvarez, W.C. and Hyman, S. (1953) A.M.A. Arch. Indust. Hyg. Occup.
Med. 8, 480
Ambrose, A.M., Christensen, H.E., Robbins, D.J. and Rather, L.J.
(1953) A.M.A. Arch. Indust. Hyg. Occup. Med. 7, 197
Batte, E.G. and Turk, R.D. (1948) J. econ Ent., 41, 102-103.
Burns, J.J., Cucinell, S.A., Koster, R. and Conney, A.H. (1965) Ann.
N.Y. Acad. Sci. 123, 273.
Carter, R.J., Hubanks, P.E., Poos, F.W., Moore, L.A. and Ely, R.E.
(1953) J. Dairy Science, 36, 1172.
Claborn, H.V., Bowers, J.W., Wells, R.W., Radeleff, R.D. and
Nickerson, W.J. (1953). Agric. Chemicals, 8, 37
Conney, A.H., Welch, R.M., Kuntzman, R. and Burns, J.J. (1967) Clin.
Pharmacol. Therap. 8, 2
Cram, R.L. Juchau, M.R. and Fouts, J.R. (1965) J. Lab. Clin. Med.
66, 906
Derbes, J.V., Dent, H.J., Forrest, W.W. and Johnson, M.F. (1955)
J. Amer. med. Ass. 158, 1367
Fishbein, W.I., White, J.V. and Isaacs, H.J. (1964) Indust. Med. and
Surg. 33, 726
Fouts, J.R. (1963) Ann. N.Y. Acad. Sciences, 104, 875
Fouts, J.R. and Hart, L.G. (1965) Ann. N.Y. Acad. Sciences, 123, 245
Fouts, J.R. and Rogers, L.A. (1965) J. Pharmacol. Exper. Therap.
147, 112
Gaines, T.G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L.G. and Fouts, J.R. (1963) Proc. Soc. exp. Biol. (N.Y.), 114,
388
Hart, L.G. and Fouts, J.R. (1965) Biochem. Pharmacol. 14, 263
Hart, L.G., Shultice, R.W. and Fouts, J.R. (1963) Toxicol appl.
Pharmacol., 5, 371
Hoffman, W.S., Fishbein, W.I. and Andelman, M.B. (1964) Arch.
Environ. Health, 9, 387
Ikeda, M., Sezesny, B. and Barnes, M. (1966) Fed. Proc. 25, 417
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1955) Unpublished report submitted by Velsicol Corporation
Ingle, L. (1965) A Monograph on Chlordane, Ingle, Urbana, Illinois
Ingle, L. (1967) Unpublished report submitted by Velsicol Corporation
Kuntzman, R., Jacobson, M., Schneidman, K. and Conney, A.H. (1964)
J. Pharmacol. exper. Therap., 146, 280
Lehman, A.J. (1951) Assoc. Food and Drug Officials Bull., 15 122
Lehman, A.J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W.J. and Durham, W.F. (1957) A.M.A. Arch. Path.,
64 61, 4
Princi, F. and Spurbeck, G.H. (1951) A.M.A. Arch. Indust. Hyg.
Occup. Med., 3, 64
Stein, W.J. and Hayes, W.J., Jr. (1964) Indust. Med. and Surg., 33,
549
Stohlman, E.F. and Smith, M.I. (1950) Advances in Chem. Ser., 1, 228
Stohlman, E.F., Thorp, W.T.S. and Smith, M.I. (1950) Arch. industr.
Hyg., 1, 13-19
Stormont, R.T. and Conley, B.E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Triolo, A.J. and Coon, J.M. (1966) Ag. and Food Chem., 14, 549
Turner, H.F. and Eden, W.G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Wazeter, F.X. (1967) Unpublished report submitted by Velsicol
Corporation
Welch, H. (1948) J. econ. Entomol., 41, 36-39
Welch, R.M. and Harrison, Y. (1966) The Pharmacologist, 8, 217
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.
and Schutzmann, R.L. (1963) J. Ag. Food Chem., 11, 244
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Allen, W.R. (1963) Plant growth dilution and the decline of
insecticide residues from alfalfa in Manitoba. Proc. Ent. Soc.
Manitoba 19.
AOAC. (1966) Changes in methods of analysis. J. Assoc. Off. Anal.
Chem. 49 : 222-230.
Banham, F.L. (1961) The persistence of certain insecticides for
control of the tuber flea beetle, Epitrix tuberis (Gent.) in the
interior of British Columbia. Can. J. Plant Sci. 41 : 664.
Begg, J.A., Plummer, P.J.G., Konst, H. (1960) Insecticide residues in
potatoes after soil treatments for control of wireworms. Can. J. Plant
Sci. 40 : 680-669.
Bess, H.A., Ota, A.K., Kawanish, C. (1966) Persistence of soil
insecticides for control of subterranean termites. J. Econ. Ent. 59 :
911-915.
Brooks, G.T., Harrison, A. (1967) The toxicity of
alpha-dihydroheptachlor and related compounds to the housefly (M.
domestica L.) and their metabolism by housefly and pig liver
microsomes. Life Sciences 6 : 1439-1448.
Carter, R.H., Claborn, H.V., Woodard, G.T., Ely, R.E. (1956) Residues
in animal products. USDA Yearbook of Agriculture, 143-148.
Chisholm, R.D., Koblit, S.K.Y., Westlake, W.E. (1966) Estimation of
aldrin and chlordane residues in soils treated for termite control.
Pest Control 30 : 48-50-52-53.
Claborn, H.V. (1956) Insecticide residues in meat and milk. USDA ARS
33-25.
Claborn, H.V., Bushland, R.C., Mann, H.D., Ivey, M.C., Radeleff, R.D.
(1960) Meat and milk residues from livestock sprays. J. Agr. Food
Chem. 8 : 439.
Cook, W.C. (1960) Report of residue analysis. USDA, ARS., Ent. Res.
Div., Pesticide Chemicals Res. Br., Report No. PCY-60-21.
Corley, C., Miles, E.J., Shands, W. (1965) Chlorinated hydrocarbon
residues in potatoes. USDA, ARS., Ent. Res. Div., Pesticide Chemicals
Res. Br., Report No. PC-B-65-22.
California State Department of Agriculture. (1962) Chlordane residues
in commercial food channels. Personal Communication.
Duggan, R.E., Barry, H.C., Johnson, L.Y. (1966) Pesticide residues in
total diet samples. Science 151 : 101-104.
Duggan, R.E. (1967) Objective samples of food analysed by USFDA for
chlordane residues. 1964-1967. Personal Communication.
Edwards, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. Ecology and the Industrial Society. Vth
Symp. Brit. Ecol. Soc., Beackwell, Oxford. p.239.
Edwards, C.A. (1966) Insecticide residues in soils. Res. Rev. 13 :
83-132.
Egan, H., Holmes, D.C., Roburn, J., Tatton, J.O.G. (1966) Pesticide
residues in foodstuffs in Great Britain II. Persistent organochlorine
pesticide residues in selected foods. J. Sci. Food Agr. 17 : 563-569.
Fahey, J.E., Hamilton, D.W., Rusk, H.W. (1957) Effect of spray date on
residues of chlorinated hydrocarbon insecticides on peaches. J. Econ.
Entomol. 50 : 366.
Fahey, J.E., Rodriguez, J.G., Rusk, H.W., Chaplin, C.E. (1962)
Chemical evaluation of pesticide residues on strawberries. J. Econ.
Ent. 55 : 179.
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65.
Fischback, H. (1963) Problems stemming from the refinement of
analytical methods. Publication No. 1082, Natl. Acad. of Sci. NRC
55-62.
Hoffman, W.S. (1963) Personal communication.
Hoffman, W.S., Fishbein, W.I., Andelman, M.B. (1964) The pesticide
content of human fat tissue. Arch. Env. Health 9 : 387-394.
IUPAC. (1967) 1966 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. 50 :
1081-1083.
IUPAC. (1968) 1967 Report of the IUPAC Commission on Chemical Nature
of Terminal Pesticide Residues. J. Assoc. Off. Analyt. Chem. (in
press).
Klein, A.K., Link, J.D. (1967) Field weathering of toxaphene and
chlordane. J. Assoc. Off. Analyt. Chem. 50 : 586-91.
Korte, F. (1967) Personal communication
Landis, B.J. (1965) Report of residue analysis; soil, DDT, aldrin and
chlordane. USDA, ARS, Ent. Res. Div., Pesticide Chemicals Br., Report
No. PCY-65-25.
Lichtenstein, E.P. (1967) Alpha-chlordane content of heptachlor
formulations. Personal communication.
Lichtenstein, E.P., Fuhnemann, Schultz. (1967) Use of carbon to reduce
the uptake of insecticidal soil residues by crop plants. Effects of
carbon on insecticide absorption and toxicity in soils. J. Agr. Food
Chem. (in press).
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the Detection of Pesticide Residues. International
Atomic Energy Agency, Vienna, pp. 49-58.
Majumder, S.K. (1967) A review of the problem of the toxicity of
pesticide chemicals in food in India. Ind. Food Packer 21 : 1-14.
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Ent. 45 : 452-456.
Marth, E.H. (1962) Chlorinated hydrocarbons deposited in biological
material. I. Plants and plant products. J. Milk Food Technol. 25 : 36.
Minyard, J.P., Jackson, E.R. (1963) Pesticide residues in commercial
animal feeds. J. Assoc. Off. Analyt. Chem. 46 : 843-859.
Morgan, L.W., Leuk, D.B., Beck, E.W., Wardham, D.W. (1967) Residues of
aldrin, chlordane, endrin and heptachlor in peanuts grown in treated
soil. J. Econ. Ent. 60 : 1289-1291.
Muns, R.P., Stone, M.W., Foley, F. (1960) Residues in vegetable crops
following soil applications of insecticides. J. Econ. Ent. 53 : 832.
Nash, R.G., Woolson, E.A. (1967) Persistence of chlorinated
hydrocarbon insecticides in soils. Science 157 : 924-927.
Ordas, E.P., Smith, V.C., Meyer, C.F. (1956) Spectrophotometric
determination of heptachlor and technical chlordane on food and forage
crops. J. Agr. Food Chem. 4 : 444.
Painter, Ruth R., Kilgore, W.W., Ough, C.S. (1963) Distribution of
pesticides in fermentation products obtained from artificially
fortified grape musts. J. Food Sci. 28 : 342.
Perez-Escolar, M.E. (1959) Control pineapple gummosis in Puerto Rico.
J. Agr. Univ. Puerto Rico 43 : 116.
Poonawalla, N.H., Korte, F. (1964) Metabolism of insecticides. VIII
Excretion, distribution and metabolism of alpha-chlordane-14C by
rats. Life Sci. 3 : 1497
Saha, J.G., Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide, and
gamma-chlordane residues in soil and rutabagas after soil and surface
treatments with heptachlor. Can. J. Plant Sci. 47 : 79-88.
Robinson, J., McGill, A.E.J. (1966) Organochlorine insecticide
residues in complete prepared meals in Great Britain in 1965. Nature
212 : 1037-1038.
Rusk, H.W., McDonough, L.M. (1966) Report of residue analysis; soils
and sugar beet roots. USDA, ARS, Ent. Res. Div., Pesticide Chemicals
Res. Br., Report Nos. PCY-67-6 and PCY-66-9.
Smith, F.F., Adams, H.R. (1965) Chlordane residues in crops. USDA,
ARS, Ent. Res. Div. Pesticide Chemicals Res. Br., Special Report
PC-B-65-26.
Smith, F.F., Reid, W.J., Cuthbert, F.P. (1964) Dieldrin and chlordane
residues in sweet potatoes. USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report PC-B-64-10.
Swackhamer, A.B. (1967) Personal communication
Thurston, A.D. (1965) Preliminary studies on weathering of chlordane
residues. J. Assoc. Off. Analyt. Chem. 48 : 952-4.
USDA. (1964) Chlorinated hydrocarbon pesticide residues in soil at
Battle Creek, Mich., 1963, USDA, ARS, Ent. Res. Div., Pesticide
Chemicals Res. Br., Special Report No. PL-V-64-10.
USDA. (1966) Report of residue analysis; potatoes, chlordane. ARS,
Ent. Res. Div., Pesticide Chemicals Res. Br., Report No. PCY-66-1.
USDA. (1967a) Monitoring chlorinated hydrocarbon insecticide residues
in soybeans, 1966. ARS, Plant Pest Control Division, interim report,
1967.
USDA. (1967b) Report of residue analysis; soil; DDT and chlordane.
USDA, ARS, Ent. Res. Div., Pesticide Chemicals Res. Br., Report No.
PCB-67-9.
Velsicol Corp. (1967a) Unpublished report on chlordane residues in
soil. Submitted to FAO.
Velsicol Corp. (1967b) Unpublished report on residues in potatoes from
soil treatments with chlordane. Submitted to FAO.
Velsicol Corp. (1967c) Unpublished report on residues in barley grain
and straw from spraying growing barley at Fargo, N.D., 1966. Submitted
to FAO.
Velsicol Corp. (1967d) Unpublished report on chlordane residues in
sugar beets. Submitted to FAO.
Velsicol Corp. (1967e) Progress report on chlordane dairy feeding
study. Submitted to FAO.
Westlake, W.E., Corley, C., Murphy, R.T., Barthel, W.F., Bryant, H.,
Schutzmann, R.L. (1963) Chemical residues in the milk of cows grazed
on chlordane-treated pasture. J. Agr. Food Chem. 11 : 244.
