
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
CHLORDANE
Explanation
This pesticide was first evaluated by the WHO Expert Committee on
Pesticide Residues at the 1963 and 1965 Joint FAO/WHO Meetings on
Pesticide Residues (FAO/WHO, 1964, 1965). A completely revised
monograph on chlordane was produced after the 1967 Joint Meeting
(FAO/WHO, 1968). Since that time additional data, particularly on the
metabolism of chlordane, have become available and these and pertinent
older data are summarized in this monograph addendum.
IDENTITY
The chemical name and structure of chlordane is given in the previous
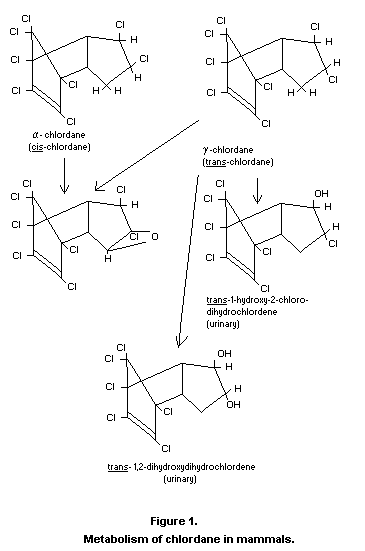
monograph (FAO/WHO, 1968). The configuration of the two isomers is
shown in Fig. 1.
Nomenclature of chlordane isomers
Three systems are now used in the literature to name the principal
isomers of chlordane. The oldest has been used since about 1947 by
Velsicol Chemical Corporation, in whose laboratories chlordane was
discovered. This nomenclature (hereinafter called System 1) was
employed in technical literature in documents considered by regulatory
agencies and in the identification of Reference Analytical Standards
which were freely distributed to researchers, regulatory laboratories
and quality control analysts. System 2 appeared in the technical
literature in the early 1950's: System 3 was first used in 1969.
The three systems are correlated in Table I, to which is appended some
references employing each. Unequivocal bases for experimental
distinction of the two isomers are chromatographic relative retention
times and accompanying infra-red spectra and NMR spectra (Velsicol,
1970).
TABLE I
Chlordane isomers
Correlation of nomenclature systems
System 1 System 2 System 3
Alpha-chlordane Beta-chlordane cis-chlordane
Gamma-chlordane Alpha-chlordane trans-chlordane
Examples of use of nomenclatures:
System
1. Velsicol Chemical Corp., technical documents for regulatory
agencies, publications and correspondence, Reference Analytical
Standards, since 1947; FAO/WHO, 1968; IUPAC, 1968, 1969.
2. March, 1952; Büchel et al., 1966; IUPAC 1967; Riemschneider,
1969.
3. Chau and Cochrane, 1969.
Designation of salient chromatographic peaks
In the analytical reports on constituents of residue, certain minor
components of chlordane are named by letters. The designations usually
correspond to those marked on a chromatogram presented to IUPAC
Commission on Terminal Pesticide Residues (Polen, 1966) or another set
used in the USFDA "Pesticide Analytical Manual, Vol. I" (1968). The
letter designations are compared in Table II, and the relevant
chromatograms of technical chlordane are given in Figure 2.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
The current knowledge concerning the metabolism of chlordane in
mammals is shown in Figure 1. Work in rats and rabbits leading to the
identification of the hydrophilic metabolites has already been
reviewed in the previous monograph (Poonawalla and Korte, 1964;
Ludwig, 1966; Korte, 1967a, 1967b). The metabolism of chlordane has
also been the subject of a general review of the metabolism of the
cyclodiene insecticides (Brooks, 1969).
Recently, another metabolite termed "oxychlordane" has been isolated
from the fat and milk of several animal species which had been fed
chlordane. Details of the levels found in the tissues are given in the
section entitled, "Fate of residues in animals". The structure (see
Figure 1) has been determined spectrographically and by chemical
synthesis by workers in two independent laboratories (Schwemmer et
al., 1970; Lawrence et al., 1970). "Oxychlordane" has not been
detected as a plant metabolite. Heptachlor and its metabolites may
occur as residues from the use of chlordane; although it is uncertain
if they are derived from the metabolism of chlordane or from the
presence of impurities of heptachlor in the chlordane used (Calo,
1970). (See also the monograph on heptachlor)
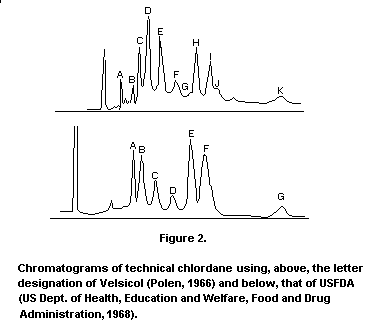 Chromatograms of technical chlordane using, above, the letter
designation of Velsicol (Polen, 1966) and below, that of USFDA (US
Dept. of Health, Education and Welfare, Food and Drug Administration,
1968).
TABLE II
Chlordane chromatographic peaks:
correlation of letter designations
Velsicol 1/ USFDA 2/
C A
D B
E C
F D
H 3/ E 3/
I 4/ F 4/
K G
1/ Polen, 1966
2/ US Dept. of Health, Education and Welfare, Food and Drug
Administration, 1968
3/ Peak for trans-chlordane
4/ Peak for cis-chlordane
No further experimental information is available to enable complete
elucidation of the significance of liver hypertrophy or the
stimulation of liver microsomal activity following administration of
chlordane. The significance of these changes was discussed in the
previous monograph on chlordane (FAO/WHO, 1968).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
In the 78-weeks feeding study in rats reviewed under "Long-term
studies", no tumours were evident when dietary levels up to 35 ppm of
the cis- or 75 ppm of the trans-isomer were fed (Ingle 1969).
There is no report that tumours have been looked for in any other
species fed chlordane.
Special studies on the metabolite "oxychlordane"
Rat
A group comprising two male and two female rats was fed a level of the
chlordane metabolite "oxychlordane" in their diet, sufficient to
maintain a daily intake of 2.0 mg/kg body-weight. The compound was fed
for 90 days, after which time the surviving animals were sacrificed
for autopsy. Body-weight increase, food consumption, mortality,
organ-weights and organ to body-weight ratios, behaviour, haematology
and blood chemistry and urologic studies were all considered to be
within the normal range for the strain of rat used. No gross
pathological abnormalities were evident, and no histopathological
lesions could be attributed to "oxychlordane". A parallel control
Group of rats fed a normal diet does not appear to have been examined
in this experiment (Plant et al., 1970). Feeding studies with
"oxychlordane" utilizing a larger number of animals are reported to be
in progress (Calo, 1970).
Special studies on the possible metabolite 1-hydroxychlordene
Rat
Groups of 25 males and 25 females were fed dietary levels of 0, 100,
250, 500, 1000, and 2000 ppm of 1-hydroxychlordene for up to 224 days.
After 110 days, three females from each feeding level were mated with
males at all levels. Mortality in all groups was low, and no real
differences existed. At 224 days, autopsy revealed no gross
abnormality. Histopathology of all visceral organs was negative,
except for the slight to moderate cytoplasmic margination of a few
liver cells at 1000 and 2000 ppm (Ingle, 1965).
Special studies on photodecomposition
Rat
No information is available on the mammalian toxicity of the
photoisomer of chlordane.
Acute toxicity
The acute toxicity of the two isomers of chlordane and some
metabolites is summarized in Table III.
Rats were fed for 28 days from weaning on either (a) a diet containing
3.5 percent protein as casein; (b) a diet containing a normal amount
of protein as casein or (c) a standard laboratory diet. A single oral
TABLE III
Acute toxicity of chlordane isomers and metabolites
LD50 mg/kg
Compound Animal body weight Reference
(oral)
cis-chlordane Rat (M) 392 Wazeter et al., 1968
trans-chlordane Rat (M) 327 Wazeter et al,, 1963
cis-trans-chlordane Rat (M) 371 Wazeter et al., 1968
(1:1 by weight)
oxychlordane Rat (M,F) 19.1 Kastri et al., 1969 a
chlordane Rat (M,F) >4600 Nastri et al., 1969 b
3-chlorochlordene Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxychlordene Rat (M,F) >4600 Mastri et al., 1969 b
chlordene epoxide Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxy 2,3-epoxy Rat (F) >4600 and Mastri et al., 1969 c
chlordene <10200
2 chlorochlordene Rat (F) >10200 Mastri et al., 1969 c
dose of chlordane was administered after the feeding period. The LD50
values for the three groups were 137, 267, or 311 mg/kg body-weight,
respectively. Clinical symptoms and pathology were the same in all
groups (Boyd and Taylor, 1969).
Long-term studies
Rat
Groups, each of 20 male and 20 female rats were fed dietary levels of
0, 5, 15, 25 or 35 ppm of cis-chlordane or 15, 25, 35 or 75 ppm of
trans-chlordane or 5, 15, 25, 35 or 50 ppm of a 1 : 1 mixture of
cis and trans-chlordane. In the group fed cis-chlordane, growth
retardation became apparent in the group fed 35 ppm after four months
in the males and after five months in the females; with
trans-chlordane, the 75 ppm group of males only displayed growth
retardation after eight months. With the mixture, growth retardation
was evident in both sexes fed 50 ppm, beginning early in the males and
later in the females. Growth retardation was not evident in any groups
fed lower doses of either isomer. Food consumption bore a relationship
to growth. Increased mortality for both sexes became significant in
the groups fed 35 ppm of cis-chlordane, 75 ppm of trans-chlordane
or 50 ppm of the cis-trans mixture.
Haematocrit was normal for all test groups. Autopsy revealed no gross
pathological lesion, in particular there was no evidence of tumours.
Histological examination of all organs showed no changes from feeding
chlordane at any level, except in the case of the liver. Compression
of sinusoids due to slight hepatic cell hypertrophy in the
centrolobular region and minimal bile duct proliferation were evident
at 35 ppm of cis-chlordane. The above were noted, but were minimal,
at 25 ppm of the same isomer. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region, minimal
perinuclear vacuolation and minimal cell hypertrophy with constriction
of sinusoids were noted at 75 ppm of the trans-chlordane. Slight
cytoplasmic homogeneity of hepatic cells in the centrolobular region
and occasional cytoplasmic margination were observed at 50 ppm of the
cis-trans mixture. The above alterations were minimal at 35 ppm of
the same mixture. No liver abnormalities were evident after feeding
lower levels of the chlordane isomers (Ingle, 1969)
COMMENT
The study on cis and trans-isomers of chlordane, which was
considered desirable at the 1967 Joint Meeting, has now been completed
and indicates that the no-effect levels of the two isomers and of the
technical product are all within the same range, The toxic metabolite,
oxychlordane, which becomes stored in the fat or excreted in the milk
of lactating animals fed high doses of chlordane, does not appear to
be a residue in plants. For this reason, the toxicity of oxychlordane
was not considered to be a matter of concern. No information is
available on the mammalian toxicity of the photoisomer of chlordane. A
satisfactory reproduction study in one species has been previously
reported and is considered adequate. However, in view of the
observations from other organochlorine compounds, an adequate
carcinogenicity study in another species is needed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 ppm of technical chlordane in the diet, equivalent to 1 mg/kg
body-weight/day
Dog: 3 ppm of technical chlordane in the diet, equivalent to 0.075
mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Several million pounds of chlordane have been used annually in a broad
spectrum of applications in the U.S.A. for nearly 25 years. Outside
the U.S.A. its use is somewhat less, but comparable.
Velsicol (1970) has presented the approximate distribution of use of
chlordane outside of the continental United States of America as
follows:
Area Percentage
Europe 55
North America (excludes U.S.A.) 15
South America 15
Asia 10
Africa 5
Total 100
In the U.S.A. about half and outside the U.S.A. about 10 percent of
chlordane usage is estimated to be non-agricultural (household pest
control, lawn protection, etc.)
Chlordane is used in protection of the following crops:
Europe: Potatoes, vegetables, small grains, sugar beets
and sugar cane.
Western hemisphere: Sugar cane, maize, small grains, potatoes and
vegetables.
Asia: Rice, cotton and jute.
Other uses of chlordane are in the protection of fruits, nuts and oil
seed crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A substantial amount of new information on the residues resulting from
supervised trials is made available to the Working Party. Present
application of electron capture gas chromatography on chlordane
residues has allowed fairly detailed evaluation of several
constituents of the residues. The available new information is
summarized in Table IV. The data are based on the records of Velsicol
Chemical Corporation (1970).
Evidence of residues in food in commerce or at consumption
In a series of papers (Duggan et al., 1966, 1967; Duggan and
Weatherwax, 1967; Duggan and Lipscomb, 1969) the pesticide residue
levels have been reported in 11 food and feed categories, as raw
agricultural products or ready-to-eat foods, domestic and imported,
and they have assessed the dietary intake during 1964 to 1968 in the
United States. The observations on chlordane levels are summarized in
Table V.
Chlordane was undetected in all but three categories of raw
agricultural products where levels were <0.005 ppm and at maximum
incidence of 3.3 percent in imported grain. Ready-to-eat foods in
these three categories contained less (in one category) or undetected
residues.
Chlordane residues found in vegetable oil seeds, oils and byproducts
are summarized in Table VI. The traces of chlordane were reported in
less than 1 percent of the sample of soybeans, but none appeared in
the finished oil. Chlordane residues were reported in 3.7 per cent of
the crude maize oil samples, but were not found in the finished oil. A
small number of samples of crude cottonseed oil showed occasional
residues of chlordane, but the concentration in finished oil was lower
by about 59 percent. No chlordane residues were observed in
oleomargarine or in the total diet composites consisting of salad
dressings, salad oils, mayonnaise, shortenings and peanut butter
(Duggan, 1968 a, 1968 b). These empirical observations corroborate the
evidence that commercial processing removes chlordane residues, if
present, from edible vegetable oils and oil products.
From 750 meal cake samples (Table VI), chlordane was detected in 0.7
percent of the soybean cakes and in 6.5 percent of the cottonseed
TABLE IV
Summary of maximum observed chlordane residues1,2
Treatment
maximum rate
Root vegetables lb/acre ppm
Parsnip 10 0.07
Potato 10 0.23
Rutabaga 10 0.5
Sugar beets (root) 10 0.11
(wet pulp) 10 0.15
(dried pulp) 10 0.3
(juice) 10 0.063
Sweet potato 10 0.04
Leafy and stalk vegetables
Asparagus 44 0.07
Broccoli 44 0.063
Brussel sprouts 44 o.36
Cabbage 44 0.09
Celery 124 0.063
Cauliflower 44 (foliar) 0.06
Mustard greens 10 0.07
Spinach 10 0.10
Swiss chard 10 0.07
Other vegetables
Snap beans 10 0.01
Grains
Barley (Grain) 104 (foliar and soil) 0.063
" (straw) 104 0.42
Oats (grain) 10 0.063
" (straw) 10 0.10
Rye (grain) 10 0:063
" (straw) 10 0 07
Wheat (grain) 4 (foliar) 0:063
" (straw) 4 ( " ) 0.38
" (grain) 10 0:063
" (straw) 10 0.10
Rice (grain) 10 (soil or seed dressing) 0.033
" (straw) 10 0.033
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Grains
Maize and "popcorn" (seed) 10 0.063
" " " (stalks and
leaves) 10 0.033
Sorghum (grain) 12 (soil or seed dressing) 0.063
" (stalks) 12 ( " ) 0.07
Cucurbits
Cantaloupe 34 (foliar) 0.1
Cucumber 34 ( " ) 0.063
Pumpkins (pulp) 44 ( " ) 0.063
" (rind) 44 ( " ) 0.10
" (pulp) 10 0.08
" (rind) 10 0.15
Squash 34 (foliar) 0.09
Watermelons 34 ( " ) 0.063
Fruits and nuts
Almonds 10 0.0063
Figs 10 0.0033 5
Filberts 10 0.0033
Olives 10 0.0063
Pecans 10 0.048
Pomegranate 10 0.0063 5
Walnuts 10 033 5
Bananas 10 0.033
Guavas 10 0 036
Mangoes 10 0:013 5
Passion fruit 10 0.035
Papayas 5 0.01
Oil seed crops
Cotton (seed) 1-2 (foliar, up 12 0.22
applications
(meal) 1-2 ( " ) 0.07
(crude oil) 1-2 ( " ) 0.11
(soap stock) 1-2 ( " ) 0.063
(stalks) 1-2 ( " ) 0.29
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Oil seed crops
Linseed (seed) 34 (foliar) 0.22
(meal) 34 ( " ) 0.09
(crude oil) 34 ( " ) 0.24
Soybeans (beans) 124 0.38
(crude oil) 124 0.42; 0.72
Rape (whole plants) 3 (foliar) 0.35
1 From soil treatment except where indicated; data not previously
published.
2 Sum of six constituents of technical chlordane.
3 Negative reading - value shown is sum of estimated lower limits of
sensitivity components.
4 Exceeds maximum rate permitted in U.S.A.
5 Shell or meat analyzed separately.
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large fruit N2 - N - N -
Small fruit N - N - N -
Grain & cereals
For human use 0.7 <0.005 3.3 <0.005 N -
For animal use N - N - - -
Vegetables
Leaf and stem N - N - N -
Vine and ear 2.0 <0.005 0.3 <0.005 2.7 <0.001
Root 1.9 <0.005 0.3 <0.005 N -
Potatoes - - - - N -
Beans N - N - N -
Eggs N - N - N -
Nuts N - N - N -
Processed meat
(canned frozen,
etc.)
Domestic N -
Imported N -
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Fluid milk
(fat basis) N -
Dairy products
(fat basis) N - N -
1 Extracted from USA-FDA surveillances programme report (Duggan, 1968b)
2 Not tabulated in reference, presumably because of insignificant
frequency of incidence
TABLE VI
Chlordane residues in vegetable oil seeds, oils and byproducts1
Crude oil Meal (cake) Edible oil
Product Number of Raw product
samples Incidence Average Incident Average Incident Average Incident Average
analysed percent ppm percent ppm percent ppm percent ppm
Soybeans 550 0.9 <0.001 - 2 - 2 0.7 <0.001 - 2 - 2
Groundnuts 177 - 2 - 2 - 2 - 2 - 2 - 2 - 2 - 2
Cottonseed 23 8.7 0.004 2.2 0.017 6.5 0.012 2.4 0.007
Maize 819 - 2 - 2 3.7 0.080 - 2 - 2
Total and
weighted 1569 0.4 <0.001 2.0 0.042 0.9 3 <0.001 3 0.04 <0.001
averages
1 Extracted from USA-FDA surveillance programne reports (Duggan, 1968a)
2 Not detected
3 Estimates based upon 750 samples for which cake data are given
cakes at average levels less than 0.001 and 0.012 ppm, respectively.
Of all the samples, 0.9 percent contained a detectable level (<0.001
ppm) of chlordane.
Dietary intake (milligrammes/day) generally appeared to be too low to
quantitate. Except for <0.001 mg/day (<1.7 percent of the ADI for a
60 kg man) from legumes during June 1966 to April 1967, no other
figures are listed for chlordane intake by Duggan and Lipscomb (1969).
The total diet studies in England and Wales (Abbott et al., 1969)
reveal no residues of chlordane.
FATE OF RESIDUES
In animals
An animal metabolite of chlordane, "oxychlordane", first reported in
1968 to the IUPAC Commission on Terminal Residues as an analytical
manifestation (IUPAC, 1969) has been isolated from pigs (Schwemmer et
al., 1970) and from milk and cheese (Lawrence et al., 1970). The
metabolite has been characterized with respect to analytical
parameters, propensity for occurrence in milk and storage in the fat
of dogs, cattle (beef and dairy) pigs and rats (Polen, 1970).
"Oxychlordane" levels in milk and some tissues of cows and goats,
following termination of ingestion of 10 ppm doses of chlordane
isomers for ten days, has been studied (Velsicol, 1970). Maximum
residues of "oxychlordane" found in milk were as follows:
Isomer fed Cow Goat
cis-chlordane 0.011 ppm 0.029 ppm
trans-chlordane 0.049 ppm 0.067 ppm
cis-+trans-chlordane 1:1 0.048 ppm 0.040 ppm
Highest milk level vs. feed level: goat 0.7 percent; cow 0.5 percent.
The residue levels in milk following termination of feeding of
chlordane declined to half in 10 to 12 days in cows and in 8 to 15
days in goats. Fifty days after chlordane withdrawal, the fat tissue
of cows contained up to 0.25 ppm and (2.5 percent of feeding level),
that of goats up to 0.4 ppm (4 percent of the feeding level) of
"oxychlordane". Some of this metabolite was occasionally found in
liver, but none in brain. The parent chlordane isomers could sometimes
be found in milk on the tenth day of feeding, but later none was
detected either in milk or in tissues (Singh et al., 1970 a, 1970 b).
At feeding levels of chlordane corresponding to proposed international
tolerances, oxychlordane is not detected in milk (sensitivity 0.005
ppm) but may be found in the fat of cattle at or below 0.03 ppm
(Polen, 1970).
When calves were fed for one month, one sixth of their ration, sugar
beet pulp derived from chlordane treated soil (Velsicol, 1970), the
maximum total residues found in the fat were, when fed with pulp from
the chlordane treatment year, up to 0.62 ppm and, when fed with pulp
from a treatment done a year prior to harvest, up to 0.06 ppm. Rates
of chlordane applications were 6-10 lb active ingredient per acre. The
total residue of the dry pulp varied from 0.05 to 0.30 ppm. The
residues found in liver were much less than those in fat, and none was
found in muscle (sensitivity 0.01 ppm).
When cows were fed with rations containing potato and alfalfa grown in
chlordane treated soil (Velsicol, 1970), no detectable residues were
found. Residue of heptachlor, heptachlor epoxide, compounds C and E
(minor components of technical chlordane), cis-chlordane and
trans-chlordane were all less than 0.01 ppm.
Hens fed 0.08 ppm of chlordane in their ration produced eggs with no
detectable residues (Herrick et al., 1969).
In plants
Korte and Porter (1970) reported on the metabolism of
trans-chlordane in white cabbage and carrots. Tests were under
glasshouse conditions and are difficult to relate to practical
conditions.
White cabbage leaves, four weeks after foliar application of 14C
labelled trans-chlordane, contained less than 1 percent of the
radioactivity on the surface; 90 percent was in the leaves as three
conversion products, in addition to the parent compound. At ten weeks,
20 percent of the applied radioactivity remained, 80 percent of which
was from metabolites. Soil treatment of carrots with 14C-labelled
trans-chlordane resulted in 0.01 ppm in carrots (mainly metabolites)
and 0.06 ppm in leaves (two thirds of the parent compound) 12 weeks
after treatment.
Polen (1970 a, 1970 b) reports that all the evidence accumulated thus
indicates that "oxychlordane" is not normally present in the terminal
residues of chlordane in plants.
In soils
Miles at al. (1969) found in their study on metabolism of heptachlor
by soil micro-organisms that heptachlor epoxide, 1-hydroxychlordene,
1-hydroxy- 2,3-epoxychlordane and possibly 1-keto-2,3-epoxychlordane
are formed, but their importance under field conditions after
chlordane treatment has not been established.
Onsager et al. (1970) did not detect heptachlor epoxide in
chlordane-treated soil (or in sugar beets grown therein). The
biological half life of chlordane was estimated to be 14.3 months, in
good agreement with other published data.
Lichtenstein at al. (1970) reported that soils treated for five years
annually with technical heptachlor (25 lb heptachlor and about 7.5 lb
chlordane as an impurity - cumulative basis) contained 0.019 ppm of
chlordane isomers (in addition to heptachlor derivatives) ten years
after the first treatment.
Polen (1970 a, 1970 b) reports that "oxychlordane" has not been found
in the terminal residues of chlordane in soils.
Photochemical transformations
The possibility of conversion of constituents of residues from
technical chlordane by ultraviolet light in vitro has been
demonstrated by several workers (Fischler and Korte, 1969; Vollner et
al., 1969; Benson et al., 1969; Rosen et al., 1969; McGuire et al.,
1970). Present evidence indicates that the photochemical
transformation compounds do not contribute significantly, if at all,
to terminal chlordane residues under agricultural conditions.
Observations of the IUPAC Chlordane Working Party (IUPAC 1969) on gas
liquid chromatograms from foliar applications on beans and cabbage
revealed no new peaks during the ageing of the residues and hence gave
no indication of photolytic products. A more recent investigation
directed specifically toward evaluation of the effects, if any, of
sunlight on chlordane residues also found no significant indications
of the production of photolytic products under typical field
conditions (Polen, 1970).
Pure preparations of compounds which are possible constituents of
chlordane terminal residues (cis- and trans-chlordanes, chlordene,
heptachlor, heptachlor epoxide and nonachlor) wore exposed to sunlight
as films on glass, on wheat and on soil surfaces. On glass after 235
hours of exposure, only heptachlor epoxide and cis-chlordane were
converted, constituting respectively 54 and 1.6 percent of the
terminal residues. After 39 days of exposure, no photoreaction
products were detected on soil; on wheat (2 lb chlordane per acre,
foliar application) only photo-cis-chlordane was detected as 2
percent of the terminal residue.
In another set of observations, 30 randomly-selected crop and soil
samples from supervised field trials throughout the U.S.A. were
examined for presence of photo products in chlordane residues.
Application rates ranged from four to ten lb of technical chlordane
per acre, soil or foliar applications; sampling intervals were from 15
to 303 days after application. Samples were: grass; straw of rice and
rye; seeds of rice and cotton; potato culls; soil from chlordane
treatments on which was grown barley or rice; sugar beets; whole and
spent cossettes; spinach; cauliflower and broccoli. Generally, no
photo products were detected at a sensitivity level of 0.01 ppm. It
was concluded that typically photo products are not produced in
chlordane soil treatments and that, if present at all in terminal
residues from foliar treatment, the contribution to the terminal
residue is less than 2 percent.
In storage and processing
With a few exceptions, accumulated evidence indicates that normal food
processing tends to reduce or eliminate chlordane residue. The lower
incidence and levels of residues in ready-to-eat foods compared to raw
agricultural products is evident in US Total Diet Studies, and was
commented on above.
In root crops, consistent evidence shows that chlordane residues from
treatments with either technical chlordane or technical heptachlor, in
which chlordane is a minor component, are concentrated in the peel,
and that peeling and/or cooking reduces or eliminates the residue.
In potatoes, peeling removes virtually the entire residue from
chlordane treatments up to 10 lb/acre. Without peeling, baking removed
about 80 percent of the residue and boiling up to 30 percent. (Saha et
al., 1968).
The residue of trans-chlordane from technical heptachlor treatments
of rutabagas was concentrated in the peel, and about half of that
residue was removed by boiling. The pulp was free of chlordane residue
before and after cooking (Saha and Stewart, 1967).
Similar patterns are observed in processing carrots, turnips and
beets. No residues of chlordane are detected in boiled pulp of turnips
or beets. In carrots, a 98 percent reduction resulted from peeling
only and 63 percent from boiling alone (Saha, 1970).
In commercial canning, washing, peeling (potatoes, tomatoes, etc.) and
abrasive peeling (used, for example, in preparing carrots) have been
demonstrated to reduce surface-concentrated pesticide residues (Farrow
at al., 1969). No doubt the surface-bound residues of chlordane would
respond in a similar way.
Chlordane residues on cabbage or beans are not changed in level or
composition by simple ten minute cooking (Polen, 1968). The lack of
the loss of chlordane residues were due to the fact that chlordane is
chemically relatively stable, co-distillation did not occur and the
cooking water containing extractable residue was included in the
sample extracted for residue analysis.
Commercial processing of edible vegetable oil produces finished oil
practically free of residues of chlordane, as well as other
organochlorine pesticides (Gooding, 1966; IUPAC, 1970; Smith at al.,
1968).
Samples of milk containing chlordane manufactured into condensed,
dried whole and evaporated milk were reduced in chlordane content. The
following figures are the percentage reduction in chlordane content
(fat basis) of processed fractions: condensed milk 11 percent; spray
dried milk 25 percent; evaporated milk 45 percent and drum dried milk
55 percent (Liska and Stadelman, 1969).
Wheat flour and rice, each contaminated by exposure to chlordane
vapours, were processed to produce two cooked products: a baked cookie
and boiled rice. The baked wheat flour cookie contained an average of
50 percent less residue than the wheat and the cooked rice about 73
percent less than the raw product (Bevenue and Yeo, 1969).
METHODS OF RESIDUE ANALYSIS
Analytical methods 1/
Prior to about 1961, residues from application of technical chlordane
were determined by total organochlorine, colorimetric or bioassay
techniques. The information derived was limited by lack of specificity
and sensitivity. At the present time, procedures based on the use of
electron capture gas chromatography are recommended. Four specific
analytical methods for the multi-component residues of chlordane are
available (Velsicol, 1970).
The methodology is that adopted for organochlorine pesticides by the
IUPAC Commission on Pesticide Residue Analysis and incorporates
procedures which favour analysis of chlordane residues in several
substrates. Detection limits are 0.002-0.02, depending on the nature
of the sample. Analytical method AM 0506 (Velsicol, 1970) is used for
soil in order to determine residue as chlordane, which is defined as
the sum of residues (ppm) of unchanged compounds from technical
chlordane plus residues of their conversion products, if present in
minor proportions. Conversion products are estimated separately if
they are present in significant amounts. Analytical method AM 0507
(Velsicol, 1970) is applicable to plant tissues and crops such as,
fruits, vegetables, grains, etc. The residue is obtained as a sum of
residues (ppm) of unchanged compounds from technical chlordane plus
residues of their conversion products, photolytic and metabolic, if
present in minor proportions. Conversion products are estimated
separately if they are present in significant amounts. Analytical
method AM 0509 (Velsicol, 1970) is applicable to milk, milk products
(e.g. butter and cheese), animal tissues and various animal food
products. Residue of chlordane is the sum of residues (ppm) of
unchanged compounds from technical chlordane plus possible residues of
their metabolites, oxychlordane and heptachlor epoxide. Method AM 0508
(Velsicol, 1970) is a supplementary method to describe modifications
to existing analytical methods in order to identify and quantitate
1/ Copies of the analytical methods and reference analytical grade
samples of the compounds are available from Velsicol Chemical Corp.,
341 E. Ohio Street, Chicago, Illinois 60611, U.S.A.
minor components of technical chlordane or of possible conversion
products (metabolic, photolytic or others). This method applies the
procedures of the other methods referred; e.g. the following compounds
can be determined: photo-cis-chlordane, photo-heptachlor epoxide,
heptachlor epoxide, "oxychlordane", photo-heptachlor and
photo-chlordane.
Quantitation of residues in plant and animal products
Several procedures have been used under various circumstances to
quantitate the gas-chromatographic responses of chlordane residues. In
most instances, the values calculated are good approximations of total
residues, but fail to distinguish conversion products from unchanged
components of technical chlordane. The methods AM 0506 to 0509
inclusive allow for quantitation of conversion products if present.
A method used prior to about 1968 relied upon calibration of the
principal peak (trans-chlordane) in reference technical chlordane
and estimation of the total residue by assuming constancy of the
proportions of the components in the course of "weathering". This
technique overstated the residue of chlordane by about 20-70 percent
for foliar treatments and by roughly half that amount for residues
from soil treatment in which conversion compounds may be in somewhat
greater concentration (Velsicol, 1970). These factors should be given
consideration in evaluating residue data generated before about 1968.
The estimation of chlordane residues in plants and soils as the sum of
cis-and trans-isomers (FAO/WHO, 1968) gives values roughly equal
to a minimum of 70-80 percent of the total terminal residues of
chlordane. In animal products, the sum of "oxychlordane" plus
cis-and trans-chlordane comprises generally more than 90 percent
of the residues in the lipoid phase.
APPRAISAL
A comprehensive set of new data for evaluation of chlordane residues
was provided to answer the questions raised by the 1967 Joint Meeting,
to add new information to the Monograph, to review the temporary
tolerances recommended in 1967 and to make new recommendations.
Technical chlordane continues to be a standardized product produced by
a single manufacturer. One of the characteristics of the technical
chlordane for which the recommendations are made is its content of
heptachlor (10 ± 2 percent).
Chlordane is used, in addition to the U.S.A., especially in Europe and
to a smaller extent in other American countries, Asia and Africa. Soil
treatments represent the main usage of chlordane. Foliar applications,
with exception of cotton, are not considered to be recommended usages
any more. The use to forage crops has been discontinued, e.g. in the
U.S.A.
Thorough studies on the chlordane residues in foodstuffs and in meal
cakes are conducted in the U.S.A. Residues were found, but in three
categories of raw agricultural products levels were less than 0.005
ppm. Oil seed cakes from soybeans and cotton seeds contained in 0.7
and 6.5 percent of the samples had residues with average levels of
less than 0.001 and 0.012 ppm, respectively.
Dietary intakes of chlordane in the U.S.A., England and Wales have
proved to be negligible. In most cases, the residues in the diet
composites were less than the analytical detection limits. Due to
this, it has not been possible to make reliable estimates of daily
chlordane intake.
Food processing tends to reduce or eliminate chlordane residues;
especially those processes which include removal of the treated crop
surface, disposal of cooking water, vacuum distillation or other type
of dehydration processes are able to remove a substantial part of the
residues.
Residues resulting from growing crops in land treated in previous
years with chlordane has been studied. It was found that the residues
in sugar beets, grown in the same land in several subsequent years,
were proportional (about 10 percent) to the residues in the soil at
the time of planting. Thus the chlordane level left in the soil seems
to determine the residue level found in crops. Stability of chlordane
in soil is well studied.
An animal metabolite of chlordane, oxychlordane, has been found in fat
of animals ingesting chlordane, but at significantly lower levels than
the level of chlordane in feed. Information for recommending practical
residue limits for meat, milk, poultry and eggs is available.
So far there is no evidence that oxychlordane is a component of
terminal chlordane residues in plants or in soils.
Photochemical transformation products of chlordane, which are reported
to be formed in vitro, are most evidently not formed in soils. In
foliar treated plants, if at all present, they are estimated to
contribute to the terminal residue less than 2 percent.
Hydrophilic metabolites of chlordane are formed in plants; their
significance is under investigation.
Analytical methods, based on the methodology generally adopted for
organochlorine pesticides, have been developed to determine the
multi-component residues of chlordane as well as their metabolites
with detection limits of 0.02-0.002 ppm. Clarification of the
nomenclature of chlordane isomers and the letter designations of
chlordane chromatographic peaks have been made.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The recommendations made now are for the residues which result from
pre-plant soil applications at a maximum level of 10 lb /acre or from
seed dressing, except for cotton which may require multiple foliar
applications, before the bolls open, at 1-2 lb /acre. Good
agricultural practice for sugar beets would require that the soil
would not contain, from the treatments of the planting and previous
years, more than 2.5-5.0 lb /acre. In cool climates, the application
rates should be established to lower levels to compensate the greater
stability of the residues.
For regulatory purposes, the chlordane residues in plant products
should be determined as the sum of cis- and trans-chlordane and in
animal products as the sum of cis-chlordane, trans-chlordane and
"oxychlordane".
In view of the new data for evaluating chlordane residues, the
recommendations made by the 1967 Joint Meeting for temporary
tolerances and practical residue limits are replaced by new
recommendations for tolerances and practical residue limits.
TOLERANCES
ppm
Root vegetables (practically soil-free) 0.3
Potatoes, sweet potatoes, rutabagas, turnips,
parsnips, sugar beets and radishes
Leafy and stalk vegetables 0.2
Asparagus, broccoli, brussel sprouts, cabbage, celery,
cauliflower, mustard greens, spinach, Swiss chard
and lettuce
Other vegetables 0.02
Beans, peas, eggplants, tomatoes and collards
(= cole-worts)
Grains 0.05
Wheat, rye, oats, rice (polished), maize, sorghum
and popcorn
Cucurbits 0.1
Cantaloupes, cucumbers, pumpkins, squash and watermelons
Fruits and nuts 0.1
Almonds, bananas, figs, filberts, guavas, mangoes,
olives, passion fruit, papayas, pecans, pomegranates,
pineapple, strawberries and walnuts
TOLERANCES (cont'd)
ppm
Citrus, pome and stone fruits 0.02
Not specified above
Vegetable oils
Crude soybean and linseed oils 0.5
Crude cottonseed oil 0.1
Edible cottonseed and soybean oils 0.02
PRACTICAL RESIDUE LIMITS
Milk and milk products (fat basis) 0.05
Fat of meat 0.05
Fat of poultry 0.05
Eggs (shell free) 0.02
FURTHER WORK OR INFORMATION
REQUIRED (before June 1972)
Further information on chlordane residues in carrots resulting from
soil and seed treatments and from crop rotation on soils having been
treated with chlordane in previous years.
DESIRABLE
1. An adequate carcinogenicity study in a second species of animal.
2. Results of the investigation of the hydrophilic plant metabolites
of chlordane.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J. Sci.
Fd. Agr., 20: 245-249
Benson, W.R., Lombardo, R., Barron, R., Lustig, E., Mastbrook, D. and
Ross, R. (1969) Paper at American Chemical Society Meeting, New York,
N.Y., Sept. 1969
Bevenue, A., and Yeo, C.Y. (1969) Effects of the cooking process on
the removal of chlordane residues from wheat flour and rice. Bull.
Environm. Contamination Toxic., 4: 370-374
Boyd, E.M., and Taylor, F.I. (1969) The acute oral toxicity of
chlordane in albino rats fed for 28 days from weaning on a
protein-deficient diet. Industr. Med., 38 (12): 42-49
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Büchel, K.H., Ginsberg, A.E. and Fischer, R. (1966)
Cyclodin-Insektizid IV. Synthese und Struktur von Isomeren des
Chlordans. Chem. Ber., 99: 421-430
Calo, D. (1970) Chlordane. Section I. Related toxicology. Unpublished
summary prepared by Velsicol Chemical Corporation.
Chau, A.S. and Cochraney W.P. (1969) Cyclodiene chemistry. I
Derivative formation for the identification of heptachlor, heptachlor
epoxide, cis-chlordane, trans-chlordane and aldrin pesticide
residues by gas chromatography. J. ass. off. anal. Chem.,
52: 1092-1100
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide Monit.
J., 2: 140-152
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide Residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed. Pesticide Monit. J., 1,2: 2-12
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Duggan, R.E. (1968a) Residues in food and feed. Pesticide Monit. J.,
1,4: 2-7
Duggan, R.E. (Ed.) (1968b) Residues in food and feed. Pesticide Monit.
J., 2: 2-46
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pesticide
Monit. J., 2: 153-162
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23 (1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1: WHO/Food Add./27.65
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Farrow, R.P., Elkins, E.R., Rose, W.W., Lamb, F.C., Ralls, J.W. and
Mercer, W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews, 29:
73-87
Fischler, H.M., and Korte, F. (1969) Sensibilisierte und
unsensibilisierte Photoisomerisierung von Cyclodien-Insektizide.
Tetrahedron Rett. 32: 2793-2796
Gooding, C.M.B. (1966) Fate of chlorinated organic pesticide residues
in the production of edible vegetable oils. Chem. Ind., 1966: 344
Herrick, G.M., Fry, J.I., Fong, W.G., Golden, D.C. (1969) Insecticide
residues in eggs resulting from the dusting and short-term feeding of
low levels of chlorinated hydrocarbon insecticides to hens. J. Agr.
Fd. Chem., 17: 291-295
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rats for 224 days. Dept. of Zoology, Univ. of Illinois,
18 Oct. 1965, submitted by Velsicol Chemical Corporation
Ingle, L. (1969) Albino rats subjected to alpha and gamma chlordane
incorporated into the daily diet for 78 weeks. Unpublished report from
the University of Illinois
IUPAC. (1967) Commission on Terminal Residues. 1st Meeting. J. Assoc.
Offic. Anal. Chemists, 50: 1071-1088
IUPAC. (1968) Commission on Terminal Residues. 2nd Meeting. J. Assoc.
Offic. Anal. Chemists, 51: 372-378
IUPAC. (1969) Commission on Terminal Residues. Terminal chlordane
residues. 3rd. Meeting. J. Assoc. Offic. Anal. Chemists, 52: 300
IUPAC. (1970) Commission on Terminal Pesticide Residues. 4th Meeting.
Fate of organochlorine pesticides in vegetable oil processing. Comptes
Rendus-XXV Conference IUPAC p. 187-189. Also J. Assoc. Offic. Anal.
Chemists, 53: 987-1003, 1970
Korte, F. (1967a) Metabolism of 14C-labelled insecticides in
micro-organisms, insects and mammals. Botyukaguku, 32: 46-59
Korte, F. (1967b) Metabolism of chlorinated insecticides. Paper
submitted to IUPAC Commission on Terminal Pesticide Residues. Appendix
VI of the proceedings of the Commission, Vienna
Korte, F. and Porters P.E. (1970) Terminal residues of cyclodiene
insecticides. Meeting of IUPAC Commission on Terminal Residues
Erbach/Rheingau, 14-18 Sept. 1970
Lawrence, J.H., Barron, R.P., Chen, J-Y.T., Lombardo, P. and Benson,
W.R. (1970) Note of identification of a chlordane metabolite found in
milk and cheese. J. Ass. off. anal. Chem., 53: 261-262
Lichtenstein, E.P., Schultz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period, translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, B.J., Stadelman, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews 29: 61-72
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the detection of pesticide residues. Int. Atomic
Energy Agency Vienna p. 49-58
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Entomol., 45: 452-456
Martin, R.J. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III) Pesticide Monit. J., 1, 4: 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on synthetic (X) in male and female albino rats.
Unpublished report from Industrial Bio-Test Laboratories submitted to
Velsicol Chemical Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories submitted by Velsicol Chemical
Corporation. (IBT no.A7367), 23 June 1969
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories submitted by Velsicol
Chemical Corporation (IBT no.A7518) 21 August 1969
McGuire, R.R., Zabrik, M.J., Schuetz, R.D. and Flotard, R.D. (1970)
Photochemistry of bioactive compounds. J. Agr. Fd. Chem., 18:
319-321
Miles J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
Econ. Entomol., 62: 1334-1338
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugar beets. J. Econ.
Entomol., 63: 1143-1146
Plank, J., Keplinger, M.L., Fancher, O.E. and Richter, W.R. (1970)
90-day subacute oral toxicity of synthetic "X" (oxychlordane) in
albino rats. Unpublished report from Industrial Bio-Test Laboratories
submitted to Velsicol Chemical Corporation
Polen, P.B. (1966) Chlordane: composition, analytical consideration
and terminal residues. IUPAC Commission on Terminal Pesticide
Residues, Geneva
Polen, P.B. (1968) Terminal residues in chlordane. Status Rept. 2.
Chlordane Working Party. IUPAC Commission on Terminal Pesticide
Residues
Polen, P.B. (1969) Report of chlordane working party to the third
meeting of the IUPAC Commission on Terminal Residues. J. Ass. Offic.
Anal. Chem., 52: 300
Polen, P.B., Hester, M. and Benziger, J. (1970a) Characterization of
oxychlordane, animal metabolite of chlordane. Bull. environm. Contam.
Toxicol., 5: (3)
Polen, P.B. (1970b) Terminal residues of chlordane. Meeting of IUPAC
Commission on Terminal Residues, Erbach/Rheingau, 14-18 Sept.
Poonawalla, N.H. and Korte, F. (1964) Metabolism of insecticides VIII.
Excretion, distribution and metabolism of -chlordane-14C by rats.
Life Sci., 3: 1497-1500
Riemschneider, R. (1969) The chemistry of the Insecticides of the
Diene Group. World Rev. Pest Control Part 2 4: 29-61
Rosen, J.D., Sutherland, D.J. and Khan, M.A.Q. (1969) Properties of
photo-isomers of heptachlor and isodrin. J. Agr. Fd. Chem., 17:
404-405
Saha, J.G. and Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide
and trans-chlordane residues in soil and rutabaga after soil and
surface treatments with heptachlor. Can. J. Plant Sci., 47: 79-88
Saha, J.G., Burrage, R.H., Nielson, H.A. and Simpson, E.C.R. (1968)
Chlordane: composition of commercial formulation residues in potatoes
grown in treated soil and their reduction by home processing.
Pesticide Progress Reports from Research Branch Establishments
(Canada), 6 (5): 117-121
Saha, J.G. (1970) Unpublished data
Schwemmer, B., Cochrane, W.P. and Polen, P.B. (1970) Oxychlordane,
animal metabolite of chlordane: isolation and synthesis. Science,
169: 1087
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970a) Alpha-gamma
chlordane milk residue study in cows. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970b) -1970- Alpha-gamma
chlordane milk residue study in goats. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Smith, K.J., Polen, P.B., de Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chemists Assoc. 45:
866-869
US Dept. of Health, Education and Welfare Food and Drug
Administration. (1968) Pesticide analytical manual Vol. I, Washington
D.C.
Velsicol. (ed. P.B. Polen) (1970) Chlordane: agricultural residues and
related toxicology prepared for the 1970 Joint FAO/WHO Meeting on
Pesticide Residues by Velsicol Chemical Corporation
Vollner, L., Klein, W. and Korte, F. (1969) Beiträge zur ökologischen
Chemie, Photoumlagerung der Komponenten des technischen Chlordans.
Tetrahedron Lett., 34: 2967-2970
Wazeter, F.X., Butler, R.H., Geil, R.G. and Rehkamper J.A. (1968)
Alpha chlordane, gamma chlordane, alpha-gamma chlordane. Comparative
acute oral toxicity (LD50) in male albino rats. Unpublished report
from International Research and Development Corporation for Velsicol
Chemical Corporation
Chromatograms of technical chlordane using, above, the letter
designation of Velsicol (Polen, 1966) and below, that of USFDA (US
Dept. of Health, Education and Welfare, Food and Drug Administration,
1968).
TABLE II
Chlordane chromatographic peaks:
correlation of letter designations
Velsicol 1/ USFDA 2/
C A
D B
E C
F D
H 3/ E 3/
I 4/ F 4/
K G
1/ Polen, 1966
2/ US Dept. of Health, Education and Welfare, Food and Drug
Administration, 1968
3/ Peak for trans-chlordane
4/ Peak for cis-chlordane
No further experimental information is available to enable complete
elucidation of the significance of liver hypertrophy or the
stimulation of liver microsomal activity following administration of
chlordane. The significance of these changes was discussed in the
previous monograph on chlordane (FAO/WHO, 1968).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
In the 78-weeks feeding study in rats reviewed under "Long-term
studies", no tumours were evident when dietary levels up to 35 ppm of
the cis- or 75 ppm of the trans-isomer were fed (Ingle 1969).
There is no report that tumours have been looked for in any other
species fed chlordane.
Special studies on the metabolite "oxychlordane"
Rat
A group comprising two male and two female rats was fed a level of the
chlordane metabolite "oxychlordane" in their diet, sufficient to
maintain a daily intake of 2.0 mg/kg body-weight. The compound was fed
for 90 days, after which time the surviving animals were sacrificed
for autopsy. Body-weight increase, food consumption, mortality,
organ-weights and organ to body-weight ratios, behaviour, haematology
and blood chemistry and urologic studies were all considered to be
within the normal range for the strain of rat used. No gross
pathological abnormalities were evident, and no histopathological
lesions could be attributed to "oxychlordane". A parallel control
Group of rats fed a normal diet does not appear to have been examined
in this experiment (Plant et al., 1970). Feeding studies with
"oxychlordane" utilizing a larger number of animals are reported to be
in progress (Calo, 1970).
Special studies on the possible metabolite 1-hydroxychlordene
Rat
Groups of 25 males and 25 females were fed dietary levels of 0, 100,
250, 500, 1000, and 2000 ppm of 1-hydroxychlordene for up to 224 days.
After 110 days, three females from each feeding level were mated with
males at all levels. Mortality in all groups was low, and no real
differences existed. At 224 days, autopsy revealed no gross
abnormality. Histopathology of all visceral organs was negative,
except for the slight to moderate cytoplasmic margination of a few
liver cells at 1000 and 2000 ppm (Ingle, 1965).
Special studies on photodecomposition
Rat
No information is available on the mammalian toxicity of the
photoisomer of chlordane.
Acute toxicity
The acute toxicity of the two isomers of chlordane and some
metabolites is summarized in Table III.
Rats were fed for 28 days from weaning on either (a) a diet containing
3.5 percent protein as casein; (b) a diet containing a normal amount
of protein as casein or (c) a standard laboratory diet. A single oral
TABLE III
Acute toxicity of chlordane isomers and metabolites
LD50 mg/kg
Compound Animal body weight Reference
(oral)
cis-chlordane Rat (M) 392 Wazeter et al., 1968
trans-chlordane Rat (M) 327 Wazeter et al,, 1963
cis-trans-chlordane Rat (M) 371 Wazeter et al., 1968
(1:1 by weight)
oxychlordane Rat (M,F) 19.1 Kastri et al., 1969 a
chlordane Rat (M,F) >4600 Nastri et al., 1969 b
3-chlorochlordene Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxychlordene Rat (M,F) >4600 Mastri et al., 1969 b
chlordene epoxide Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxy 2,3-epoxy Rat (F) >4600 and Mastri et al., 1969 c
chlordene <10200
2 chlorochlordene Rat (F) >10200 Mastri et al., 1969 c
dose of chlordane was administered after the feeding period. The LD50
values for the three groups were 137, 267, or 311 mg/kg body-weight,
respectively. Clinical symptoms and pathology were the same in all
groups (Boyd and Taylor, 1969).
Long-term studies
Rat
Groups, each of 20 male and 20 female rats were fed dietary levels of
0, 5, 15, 25 or 35 ppm of cis-chlordane or 15, 25, 35 or 75 ppm of
trans-chlordane or 5, 15, 25, 35 or 50 ppm of a 1 : 1 mixture of
cis and trans-chlordane. In the group fed cis-chlordane, growth
retardation became apparent in the group fed 35 ppm after four months
in the males and after five months in the females; with
trans-chlordane, the 75 ppm group of males only displayed growth
retardation after eight months. With the mixture, growth retardation
was evident in both sexes fed 50 ppm, beginning early in the males and
later in the females. Growth retardation was not evident in any groups
fed lower doses of either isomer. Food consumption bore a relationship
to growth. Increased mortality for both sexes became significant in
the groups fed 35 ppm of cis-chlordane, 75 ppm of trans-chlordane
or 50 ppm of the cis-trans mixture.
Haematocrit was normal for all test groups. Autopsy revealed no gross
pathological lesion, in particular there was no evidence of tumours.
Histological examination of all organs showed no changes from feeding
chlordane at any level, except in the case of the liver. Compression
of sinusoids due to slight hepatic cell hypertrophy in the
centrolobular region and minimal bile duct proliferation were evident
at 35 ppm of cis-chlordane. The above were noted, but were minimal,
at 25 ppm of the same isomer. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region, minimal
perinuclear vacuolation and minimal cell hypertrophy with constriction
of sinusoids were noted at 75 ppm of the trans-chlordane. Slight
cytoplasmic homogeneity of hepatic cells in the centrolobular region
and occasional cytoplasmic margination were observed at 50 ppm of the
cis-trans mixture. The above alterations were minimal at 35 ppm of
the same mixture. No liver abnormalities were evident after feeding
lower levels of the chlordane isomers (Ingle, 1969)
COMMENT
The study on cis and trans-isomers of chlordane, which was
considered desirable at the 1967 Joint Meeting, has now been completed
and indicates that the no-effect levels of the two isomers and of the
technical product are all within the same range, The toxic metabolite,
oxychlordane, which becomes stored in the fat or excreted in the milk
of lactating animals fed high doses of chlordane, does not appear to
be a residue in plants. For this reason, the toxicity of oxychlordane
was not considered to be a matter of concern. No information is
available on the mammalian toxicity of the photoisomer of chlordane. A
satisfactory reproduction study in one species has been previously
reported and is considered adequate. However, in view of the
observations from other organochlorine compounds, an adequate
carcinogenicity study in another species is needed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 ppm of technical chlordane in the diet, equivalent to 1 mg/kg
body-weight/day
Dog: 3 ppm of technical chlordane in the diet, equivalent to 0.075
mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Several million pounds of chlordane have been used annually in a broad
spectrum of applications in the U.S.A. for nearly 25 years. Outside
the U.S.A. its use is somewhat less, but comparable.
Velsicol (1970) has presented the approximate distribution of use of
chlordane outside of the continental United States of America as
follows:
Area Percentage
Europe 55
North America (excludes U.S.A.) 15
South America 15
Asia 10
Africa 5
Total 100
In the U.S.A. about half and outside the U.S.A. about 10 percent of
chlordane usage is estimated to be non-agricultural (household pest
control, lawn protection, etc.)
Chlordane is used in protection of the following crops:
Europe: Potatoes, vegetables, small grains, sugar beets
and sugar cane.
Western hemisphere: Sugar cane, maize, small grains, potatoes and
vegetables.
Asia: Rice, cotton and jute.
Other uses of chlordane are in the protection of fruits, nuts and oil
seed crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A substantial amount of new information on the residues resulting from
supervised trials is made available to the Working Party. Present
application of electron capture gas chromatography on chlordane
residues has allowed fairly detailed evaluation of several
constituents of the residues. The available new information is
summarized in Table IV. The data are based on the records of Velsicol
Chemical Corporation (1970).
Evidence of residues in food in commerce or at consumption
In a series of papers (Duggan et al., 1966, 1967; Duggan and
Weatherwax, 1967; Duggan and Lipscomb, 1969) the pesticide residue
levels have been reported in 11 food and feed categories, as raw
agricultural products or ready-to-eat foods, domestic and imported,
and they have assessed the dietary intake during 1964 to 1968 in the
United States. The observations on chlordane levels are summarized in
Table V.
Chlordane was undetected in all but three categories of raw
agricultural products where levels were <0.005 ppm and at maximum
incidence of 3.3 percent in imported grain. Ready-to-eat foods in
these three categories contained less (in one category) or undetected
residues.
Chlordane residues found in vegetable oil seeds, oils and byproducts
are summarized in Table VI. The traces of chlordane were reported in
less than 1 percent of the sample of soybeans, but none appeared in
the finished oil. Chlordane residues were reported in 3.7 per cent of
the crude maize oil samples, but were not found in the finished oil. A
small number of samples of crude cottonseed oil showed occasional
residues of chlordane, but the concentration in finished oil was lower
by about 59 percent. No chlordane residues were observed in
oleomargarine or in the total diet composites consisting of salad
dressings, salad oils, mayonnaise, shortenings and peanut butter
(Duggan, 1968 a, 1968 b). These empirical observations corroborate the
evidence that commercial processing removes chlordane residues, if
present, from edible vegetable oils and oil products.
From 750 meal cake samples (Table VI), chlordane was detected in 0.7
percent of the soybean cakes and in 6.5 percent of the cottonseed
TABLE IV
Summary of maximum observed chlordane residues1,2
Treatment
maximum rate
Root vegetables lb/acre ppm
Parsnip 10 0.07
Potato 10 0.23
Rutabaga 10 0.5
Sugar beets (root) 10 0.11
(wet pulp) 10 0.15
(dried pulp) 10 0.3
(juice) 10 0.063
Sweet potato 10 0.04
Leafy and stalk vegetables
Asparagus 44 0.07
Broccoli 44 0.063
Brussel sprouts 44 o.36
Cabbage 44 0.09
Celery 124 0.063
Cauliflower 44 (foliar) 0.06
Mustard greens 10 0.07
Spinach 10 0.10
Swiss chard 10 0.07
Other vegetables
Snap beans 10 0.01
Grains
Barley (Grain) 104 (foliar and soil) 0.063
" (straw) 104 0.42
Oats (grain) 10 0.063
" (straw) 10 0.10
Rye (grain) 10 0:063
" (straw) 10 0 07
Wheat (grain) 4 (foliar) 0:063
" (straw) 4 ( " ) 0.38
" (grain) 10 0:063
" (straw) 10 0.10
Rice (grain) 10 (soil or seed dressing) 0.033
" (straw) 10 0.033
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Grains
Maize and "popcorn" (seed) 10 0.063
" " " (stalks and
leaves) 10 0.033
Sorghum (grain) 12 (soil or seed dressing) 0.063
" (stalks) 12 ( " ) 0.07
Cucurbits
Cantaloupe 34 (foliar) 0.1
Cucumber 34 ( " ) 0.063
Pumpkins (pulp) 44 ( " ) 0.063
" (rind) 44 ( " ) 0.10
" (pulp) 10 0.08
" (rind) 10 0.15
Squash 34 (foliar) 0.09
Watermelons 34 ( " ) 0.063
Fruits and nuts
Almonds 10 0.0063
Figs 10 0.0033 5
Filberts 10 0.0033
Olives 10 0.0063
Pecans 10 0.048
Pomegranate 10 0.0063 5
Walnuts 10 033 5
Bananas 10 0.033
Guavas 10 0 036
Mangoes 10 0:013 5
Passion fruit 10 0.035
Papayas 5 0.01
Oil seed crops
Cotton (seed) 1-2 (foliar, up 12 0.22
applications
(meal) 1-2 ( " ) 0.07
(crude oil) 1-2 ( " ) 0.11
(soap stock) 1-2 ( " ) 0.063
(stalks) 1-2 ( " ) 0.29
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Oil seed crops
Linseed (seed) 34 (foliar) 0.22
(meal) 34 ( " ) 0.09
(crude oil) 34 ( " ) 0.24
Soybeans (beans) 124 0.38
(crude oil) 124 0.42; 0.72
Rape (whole plants) 3 (foliar) 0.35
1 From soil treatment except where indicated; data not previously
published.
2 Sum of six constituents of technical chlordane.
3 Negative reading - value shown is sum of estimated lower limits of
sensitivity components.
4 Exceeds maximum rate permitted in U.S.A.
5 Shell or meat analyzed separately.
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large fruit N2 - N - N -
Small fruit N - N - N -
Grain & cereals
For human use 0.7 <0.005 3.3 <0.005 N -
For animal use N - N - - -
Vegetables
Leaf and stem N - N - N -
Vine and ear 2.0 <0.005 0.3 <0.005 2.7 <0.001
Root 1.9 <0.005 0.3 <0.005 N -
Potatoes - - - - N -
Beans N - N - N -
Eggs N - N - N -
Nuts N - N - N -
Processed meat
(canned frozen,
etc.)
Domestic N -
Imported N -
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Fluid milk
(fat basis) N -
Dairy products
(fat basis) N - N -
1 Extracted from USA-FDA surveillances programme report (Duggan, 1968b)
2 Not tabulated in reference, presumably because of insignificant
frequency of incidence
TABLE VI
Chlordane residues in vegetable oil seeds, oils and byproducts1
Crude oil Meal (cake) Edible oil
Product Number of Raw product
samples Incidence Average Incident Average Incident Average Incident Average
analysed percent ppm percent ppm percent ppm percent ppm
Soybeans 550 0.9 <0.001 - 2 - 2 0.7 <0.001 - 2 - 2
Groundnuts 177 - 2 - 2 - 2 - 2 - 2 - 2 - 2 - 2
Cottonseed 23 8.7 0.004 2.2 0.017 6.5 0.012 2.4 0.007
Maize 819 - 2 - 2 3.7 0.080 - 2 - 2
Total and
weighted 1569 0.4 <0.001 2.0 0.042 0.9 3 <0.001 3 0.04 <0.001
averages
1 Extracted from USA-FDA surveillance programne reports (Duggan, 1968a)
2 Not detected
3 Estimates based upon 750 samples for which cake data are given
cakes at average levels less than 0.001 and 0.012 ppm, respectively.
Of all the samples, 0.9 percent contained a detectable level (<0.001
ppm) of chlordane.
Dietary intake (milligrammes/day) generally appeared to be too low to
quantitate. Except for <0.001 mg/day (<1.7 percent of the ADI for a
60 kg man) from legumes during June 1966 to April 1967, no other
figures are listed for chlordane intake by Duggan and Lipscomb (1969).
The total diet studies in England and Wales (Abbott et al., 1969)
reveal no residues of chlordane.
FATE OF RESIDUES
In animals
An animal metabolite of chlordane, "oxychlordane", first reported in
1968 to the IUPAC Commission on Terminal Residues as an analytical
manifestation (IUPAC, 1969) has been isolated from pigs (Schwemmer et
al., 1970) and from milk and cheese (Lawrence et al., 1970). The
metabolite has been characterized with respect to analytical
parameters, propensity for occurrence in milk and storage in the fat
of dogs, cattle (beef and dairy) pigs and rats (Polen, 1970).
"Oxychlordane" levels in milk and some tissues of cows and goats,
following termination of ingestion of 10 ppm doses of chlordane
isomers for ten days, has been studied (Velsicol, 1970). Maximum
residues of "oxychlordane" found in milk were as follows:
Isomer fed Cow Goat
cis-chlordane 0.011 ppm 0.029 ppm
trans-chlordane 0.049 ppm 0.067 ppm
cis-+trans-chlordane 1:1 0.048 ppm 0.040 ppm
Highest milk level vs. feed level: goat 0.7 percent; cow 0.5 percent.
The residue levels in milk following termination of feeding of
chlordane declined to half in 10 to 12 days in cows and in 8 to 15
days in goats. Fifty days after chlordane withdrawal, the fat tissue
of cows contained up to 0.25 ppm and (2.5 percent of feeding level),
that of goats up to 0.4 ppm (4 percent of the feeding level) of
"oxychlordane". Some of this metabolite was occasionally found in
liver, but none in brain. The parent chlordane isomers could sometimes
be found in milk on the tenth day of feeding, but later none was
detected either in milk or in tissues (Singh et al., 1970 a, 1970 b).
At feeding levels of chlordane corresponding to proposed international
tolerances, oxychlordane is not detected in milk (sensitivity 0.005
ppm) but may be found in the fat of cattle at or below 0.03 ppm
(Polen, 1970).
When calves were fed for one month, one sixth of their ration, sugar
beet pulp derived from chlordane treated soil (Velsicol, 1970), the
maximum total residues found in the fat were, when fed with pulp from
the chlordane treatment year, up to 0.62 ppm and, when fed with pulp
from a treatment done a year prior to harvest, up to 0.06 ppm. Rates
of chlordane applications were 6-10 lb active ingredient per acre. The
total residue of the dry pulp varied from 0.05 to 0.30 ppm. The
residues found in liver were much less than those in fat, and none was
found in muscle (sensitivity 0.01 ppm).
When cows were fed with rations containing potato and alfalfa grown in
chlordane treated soil (Velsicol, 1970), no detectable residues were
found. Residue of heptachlor, heptachlor epoxide, compounds C and E
(minor components of technical chlordane), cis-chlordane and
trans-chlordane were all less than 0.01 ppm.
Hens fed 0.08 ppm of chlordane in their ration produced eggs with no
detectable residues (Herrick et al., 1969).
In plants
Korte and Porter (1970) reported on the metabolism of
trans-chlordane in white cabbage and carrots. Tests were under
glasshouse conditions and are difficult to relate to practical
conditions.
White cabbage leaves, four weeks after foliar application of 14C
labelled trans-chlordane, contained less than 1 percent of the
radioactivity on the surface; 90 percent was in the leaves as three
conversion products, in addition to the parent compound. At ten weeks,
20 percent of the applied radioactivity remained, 80 percent of which
was from metabolites. Soil treatment of carrots with 14C-labelled
trans-chlordane resulted in 0.01 ppm in carrots (mainly metabolites)
and 0.06 ppm in leaves (two thirds of the parent compound) 12 weeks
after treatment.
Polen (1970 a, 1970 b) reports that all the evidence accumulated thus
indicates that "oxychlordane" is not normally present in the terminal
residues of chlordane in plants.
In soils
Miles at al. (1969) found in their study on metabolism of heptachlor
by soil micro-organisms that heptachlor epoxide, 1-hydroxychlordene,
1-hydroxy- 2,3-epoxychlordane and possibly 1-keto-2,3-epoxychlordane
are formed, but their importance under field conditions after
chlordane treatment has not been established.
Onsager et al. (1970) did not detect heptachlor epoxide in
chlordane-treated soil (or in sugar beets grown therein). The
biological half life of chlordane was estimated to be 14.3 months, in
good agreement with other published data.
Lichtenstein at al. (1970) reported that soils treated for five years
annually with technical heptachlor (25 lb heptachlor and about 7.5 lb
chlordane as an impurity - cumulative basis) contained 0.019 ppm of
chlordane isomers (in addition to heptachlor derivatives) ten years
after the first treatment.
Polen (1970 a, 1970 b) reports that "oxychlordane" has not been found
in the terminal residues of chlordane in soils.
Photochemical transformations
The possibility of conversion of constituents of residues from
technical chlordane by ultraviolet light in vitro has been
demonstrated by several workers (Fischler and Korte, 1969; Vollner et
al., 1969; Benson et al., 1969; Rosen et al., 1969; McGuire et al.,
1970). Present evidence indicates that the photochemical
transformation compounds do not contribute significantly, if at all,
to terminal chlordane residues under agricultural conditions.
Observations of the IUPAC Chlordane Working Party (IUPAC 1969) on gas
liquid chromatograms from foliar applications on beans and cabbage
revealed no new peaks during the ageing of the residues and hence gave
no indication of photolytic products. A more recent investigation
directed specifically toward evaluation of the effects, if any, of
sunlight on chlordane residues also found no significant indications
of the production of photolytic products under typical field
conditions (Polen, 1970).
Pure preparations of compounds which are possible constituents of
chlordane terminal residues (cis- and trans-chlordanes, chlordene,
heptachlor, heptachlor epoxide and nonachlor) wore exposed to sunlight
as films on glass, on wheat and on soil surfaces. On glass after 235
hours of exposure, only heptachlor epoxide and cis-chlordane were
converted, constituting respectively 54 and 1.6 percent of the
terminal residues. After 39 days of exposure, no photoreaction
products were detected on soil; on wheat (2 lb chlordane per acre,
foliar application) only photo-cis-chlordane was detected as 2
percent of the terminal residue.
In another set of observations, 30 randomly-selected crop and soil
samples from supervised field trials throughout the U.S.A. were
examined for presence of photo products in chlordane residues.
Application rates ranged from four to ten lb of technical chlordane
per acre, soil or foliar applications; sampling intervals were from 15
to 303 days after application. Samples were: grass; straw of rice and
rye; seeds of rice and cotton; potato culls; soil from chlordane
treatments on which was grown barley or rice; sugar beets; whole and
spent cossettes; spinach; cauliflower and broccoli. Generally, no
photo products were detected at a sensitivity level of 0.01 ppm. It
was concluded that typically photo products are not produced in
chlordane soil treatments and that, if present at all in terminal
residues from foliar treatment, the contribution to the terminal
residue is less than 2 percent.
In storage and processing
With a few exceptions, accumulated evidence indicates that normal food
processing tends to reduce or eliminate chlordane residue. The lower
incidence and levels of residues in ready-to-eat foods compared to raw
agricultural products is evident in US Total Diet Studies, and was
commented on above.
In root crops, consistent evidence shows that chlordane residues from
treatments with either technical chlordane or technical heptachlor, in
which chlordane is a minor component, are concentrated in the peel,
and that peeling and/or cooking reduces or eliminates the residue.
In potatoes, peeling removes virtually the entire residue from
chlordane treatments up to 10 lb/acre. Without peeling, baking removed
about 80 percent of the residue and boiling up to 30 percent. (Saha et
al., 1968).
The residue of trans-chlordane from technical heptachlor treatments
of rutabagas was concentrated in the peel, and about half of that
residue was removed by boiling. The pulp was free of chlordane residue
before and after cooking (Saha and Stewart, 1967).
Similar patterns are observed in processing carrots, turnips and
beets. No residues of chlordane are detected in boiled pulp of turnips
or beets. In carrots, a 98 percent reduction resulted from peeling
only and 63 percent from boiling alone (Saha, 1970).
In commercial canning, washing, peeling (potatoes, tomatoes, etc.) and
abrasive peeling (used, for example, in preparing carrots) have been
demonstrated to reduce surface-concentrated pesticide residues (Farrow
at al., 1969). No doubt the surface-bound residues of chlordane would
respond in a similar way.
Chlordane residues on cabbage or beans are not changed in level or
composition by simple ten minute cooking (Polen, 1968). The lack of
the loss of chlordane residues were due to the fact that chlordane is
chemically relatively stable, co-distillation did not occur and the
cooking water containing extractable residue was included in the
sample extracted for residue analysis.
Commercial processing of edible vegetable oil produces finished oil
practically free of residues of chlordane, as well as other
organochlorine pesticides (Gooding, 1966; IUPAC, 1970; Smith at al.,
1968).
Samples of milk containing chlordane manufactured into condensed,
dried whole and evaporated milk were reduced in chlordane content. The
following figures are the percentage reduction in chlordane content
(fat basis) of processed fractions: condensed milk 11 percent; spray
dried milk 25 percent; evaporated milk 45 percent and drum dried milk
55 percent (Liska and Stadelman, 1969).
Wheat flour and rice, each contaminated by exposure to chlordane
vapours, were processed to produce two cooked products: a baked cookie
and boiled rice. The baked wheat flour cookie contained an average of
50 percent less residue than the wheat and the cooked rice about 73
percent less than the raw product (Bevenue and Yeo, 1969).
METHODS OF RESIDUE ANALYSIS
Analytical methods 1/
Prior to about 1961, residues from application of technical chlordane
were determined by total organochlorine, colorimetric or bioassay
techniques. The information derived was limited by lack of specificity
and sensitivity. At the present time, procedures based on the use of
electron capture gas chromatography are recommended. Four specific
analytical methods for the multi-component residues of chlordane are
available (Velsicol, 1970).
The methodology is that adopted for organochlorine pesticides by the
IUPAC Commission on Pesticide Residue Analysis and incorporates
procedures which favour analysis of chlordane residues in several
substrates. Detection limits are 0.002-0.02, depending on the nature
of the sample. Analytical method AM 0506 (Velsicol, 1970) is used for
soil in order to determine residue as chlordane, which is defined as
the sum of residues (ppm) of unchanged compounds from technical
chlordane plus residues of their conversion products, if present in
minor proportions. Conversion products are estimated separately if
they are present in significant amounts. Analytical method AM 0507
(Velsicol, 1970) is applicable to plant tissues and crops such as,
fruits, vegetables, grains, etc. The residue is obtained as a sum of
residues (ppm) of unchanged compounds from technical chlordane plus
residues of their conversion products, photolytic and metabolic, if
present in minor proportions. Conversion products are estimated
separately if they are present in significant amounts. Analytical
method AM 0509 (Velsicol, 1970) is applicable to milk, milk products
(e.g. butter and cheese), animal tissues and various animal food
products. Residue of chlordane is the sum of residues (ppm) of
unchanged compounds from technical chlordane plus possible residues of
their metabolites, oxychlordane and heptachlor epoxide. Method AM 0508
(Velsicol, 1970) is a supplementary method to describe modifications
to existing analytical methods in order to identify and quantitate
1/ Copies of the analytical methods and reference analytical grade
samples of the compounds are available from Velsicol Chemical Corp.,
341 E. Ohio Street, Chicago, Illinois 60611, U.S.A.
minor components of technical chlordane or of possible conversion
products (metabolic, photolytic or others). This method applies the
procedures of the other methods referred; e.g. the following compounds
can be determined: photo-cis-chlordane, photo-heptachlor epoxide,
heptachlor epoxide, "oxychlordane", photo-heptachlor and
photo-chlordane.
Quantitation of residues in plant and animal products
Several procedures have been used under various circumstances to
quantitate the gas-chromatographic responses of chlordane residues. In
most instances, the values calculated are good approximations of total
residues, but fail to distinguish conversion products from unchanged
components of technical chlordane. The methods AM 0506 to 0509
inclusive allow for quantitation of conversion products if present.
A method used prior to about 1968 relied upon calibration of the
principal peak (trans-chlordane) in reference technical chlordane
and estimation of the total residue by assuming constancy of the
proportions of the components in the course of "weathering". This
technique overstated the residue of chlordane by about 20-70 percent
for foliar treatments and by roughly half that amount for residues
from soil treatment in which conversion compounds may be in somewhat
greater concentration (Velsicol, 1970). These factors should be given
consideration in evaluating residue data generated before about 1968.
The estimation of chlordane residues in plants and soils as the sum of
cis-and trans-isomers (FAO/WHO, 1968) gives values roughly equal
to a minimum of 70-80 percent of the total terminal residues of
chlordane. In animal products, the sum of "oxychlordane" plus
cis-and trans-chlordane comprises generally more than 90 percent
of the residues in the lipoid phase.
APPRAISAL
A comprehensive set of new data for evaluation of chlordane residues
was provided to answer the questions raised by the 1967 Joint Meeting,
to add new information to the Monograph, to review the temporary
tolerances recommended in 1967 and to make new recommendations.
Technical chlordane continues to be a standardized product produced by
a single manufacturer. One of the characteristics of the technical
chlordane for which the recommendations are made is its content of
heptachlor (10 ± 2 percent).
Chlordane is used, in addition to the U.S.A., especially in Europe and
to a smaller extent in other American countries, Asia and Africa. Soil
treatments represent the main usage of chlordane. Foliar applications,
with exception of cotton, are not considered to be recommended usages
any more. The use to forage crops has been discontinued, e.g. in the
U.S.A.
Thorough studies on the chlordane residues in foodstuffs and in meal
cakes are conducted in the U.S.A. Residues were found, but in three
categories of raw agricultural products levels were less than 0.005
ppm. Oil seed cakes from soybeans and cotton seeds contained in 0.7
and 6.5 percent of the samples had residues with average levels of
less than 0.001 and 0.012 ppm, respectively.
Dietary intakes of chlordane in the U.S.A., England and Wales have
proved to be negligible. In most cases, the residues in the diet
composites were less than the analytical detection limits. Due to
this, it has not been possible to make reliable estimates of daily
chlordane intake.
Food processing tends to reduce or eliminate chlordane residues;
especially those processes which include removal of the treated crop
surface, disposal of cooking water, vacuum distillation or other type
of dehydration processes are able to remove a substantial part of the
residues.
Residues resulting from growing crops in land treated in previous
years with chlordane has been studied. It was found that the residues
in sugar beets, grown in the same land in several subsequent years,
were proportional (about 10 percent) to the residues in the soil at
the time of planting. Thus the chlordane level left in the soil seems
to determine the residue level found in crops. Stability of chlordane
in soil is well studied.
An animal metabolite of chlordane, oxychlordane, has been found in fat
of animals ingesting chlordane, but at significantly lower levels than
the level of chlordane in feed. Information for recommending practical
residue limits for meat, milk, poultry and eggs is available.
So far there is no evidence that oxychlordane is a component of
terminal chlordane residues in plants or in soils.
Photochemical transformation products of chlordane, which are reported
to be formed in vitro, are most evidently not formed in soils. In
foliar treated plants, if at all present, they are estimated to
contribute to the terminal residue less than 2 percent.
Hydrophilic metabolites of chlordane are formed in plants; their
significance is under investigation.
Analytical methods, based on the methodology generally adopted for
organochlorine pesticides, have been developed to determine the
multi-component residues of chlordane as well as their metabolites
with detection limits of 0.02-0.002 ppm. Clarification of the
nomenclature of chlordane isomers and the letter designations of
chlordane chromatographic peaks have been made.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The recommendations made now are for the residues which result from
pre-plant soil applications at a maximum level of 10 lb /acre or from
seed dressing, except for cotton which may require multiple foliar
applications, before the bolls open, at 1-2 lb /acre. Good
agricultural practice for sugar beets would require that the soil
would not contain, from the treatments of the planting and previous
years, more than 2.5-5.0 lb /acre. In cool climates, the application
rates should be established to lower levels to compensate the greater
stability of the residues.
For regulatory purposes, the chlordane residues in plant products
should be determined as the sum of cis- and trans-chlordane and in
animal products as the sum of cis-chlordane, trans-chlordane and
"oxychlordane".
In view of the new data for evaluating chlordane residues, the
recommendations made by the 1967 Joint Meeting for temporary
tolerances and practical residue limits are replaced by new
recommendations for tolerances and practical residue limits.
TOLERANCES
ppm
Root vegetables (practically soil-free) 0.3
Potatoes, sweet potatoes, rutabagas, turnips,
parsnips, sugar beets and radishes
Leafy and stalk vegetables 0.2
Asparagus, broccoli, brussel sprouts, cabbage, celery,
cauliflower, mustard greens, spinach, Swiss chard
and lettuce
Other vegetables 0.02
Beans, peas, eggplants, tomatoes and collards
(= cole-worts)
Grains 0.05
Wheat, rye, oats, rice (polished), maize, sorghum
and popcorn
Cucurbits 0.1
Cantaloupes, cucumbers, pumpkins, squash and watermelons
Fruits and nuts 0.1
Almonds, bananas, figs, filberts, guavas, mangoes,
olives, passion fruit, papayas, pecans, pomegranates,
pineapple, strawberries and walnuts
TOLERANCES (cont'd)
ppm
Citrus, pome and stone fruits 0.02
Not specified above
Vegetable oils
Crude soybean and linseed oils 0.5
Crude cottonseed oil 0.1
Edible cottonseed and soybean oils 0.02
PRACTICAL RESIDUE LIMITS
Milk and milk products (fat basis) 0.05
Fat of meat 0.05
Fat of poultry 0.05
Eggs (shell free) 0.02
FURTHER WORK OR INFORMATION
REQUIRED (before June 1972)
Further information on chlordane residues in carrots resulting from
soil and seed treatments and from crop rotation on soils having been
treated with chlordane in previous years.
DESIRABLE
1. An adequate carcinogenicity study in a second species of animal.
2. Results of the investigation of the hydrophilic plant metabolites
of chlordane.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J. Sci.
Fd. Agr., 20: 245-249
Benson, W.R., Lombardo, R., Barron, R., Lustig, E., Mastbrook, D. and
Ross, R. (1969) Paper at American Chemical Society Meeting, New York,
N.Y., Sept. 1969
Bevenue, A., and Yeo, C.Y. (1969) Effects of the cooking process on
the removal of chlordane residues from wheat flour and rice. Bull.
Environm. Contamination Toxic., 4: 370-374
Boyd, E.M., and Taylor, F.I. (1969) The acute oral toxicity of
chlordane in albino rats fed for 28 days from weaning on a
protein-deficient diet. Industr. Med., 38 (12): 42-49
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Büchel, K.H., Ginsberg, A.E. and Fischer, R. (1966)
Cyclodin-Insektizid IV. Synthese und Struktur von Isomeren des
Chlordans. Chem. Ber., 99: 421-430
Calo, D. (1970) Chlordane. Section I. Related toxicology. Unpublished
summary prepared by Velsicol Chemical Corporation.
Chau, A.S. and Cochraney W.P. (1969) Cyclodiene chemistry. I
Derivative formation for the identification of heptachlor, heptachlor
epoxide, cis-chlordane, trans-chlordane and aldrin pesticide
residues by gas chromatography. J. ass. off. anal. Chem.,
52: 1092-1100
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide Monit.
J., 2: 140-152
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide Residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed. Pesticide Monit. J., 1,2: 2-12
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Duggan, R.E. (1968a) Residues in food and feed. Pesticide Monit. J.,
1,4: 2-7
Duggan, R.E. (Ed.) (1968b) Residues in food and feed. Pesticide Monit.
J., 2: 2-46
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pesticide
Monit. J., 2: 153-162
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23 (1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1: WHO/Food Add./27.65
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Farrow, R.P., Elkins, E.R., Rose, W.W., Lamb, F.C., Ralls, J.W. and
Mercer, W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews, 29:
73-87
Fischler, H.M., and Korte, F. (1969) Sensibilisierte und
unsensibilisierte Photoisomerisierung von Cyclodien-Insektizide.
Tetrahedron Rett. 32: 2793-2796
Gooding, C.M.B. (1966) Fate of chlorinated organic pesticide residues
in the production of edible vegetable oils. Chem. Ind., 1966: 344
Herrick, G.M., Fry, J.I., Fong, W.G., Golden, D.C. (1969) Insecticide
residues in eggs resulting from the dusting and short-term feeding of
low levels of chlorinated hydrocarbon insecticides to hens. J. Agr.
Fd. Chem., 17: 291-295
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rats for 224 days. Dept. of Zoology, Univ. of Illinois,
18 Oct. 1965, submitted by Velsicol Chemical Corporation
Ingle, L. (1969) Albino rats subjected to alpha and gamma chlordane
incorporated into the daily diet for 78 weeks. Unpublished report from
the University of Illinois
IUPAC. (1967) Commission on Terminal Residues. 1st Meeting. J. Assoc.
Offic. Anal. Chemists, 50: 1071-1088
IUPAC. (1968) Commission on Terminal Residues. 2nd Meeting. J. Assoc.
Offic. Anal. Chemists, 51: 372-378
IUPAC. (1969) Commission on Terminal Residues. Terminal chlordane
residues. 3rd. Meeting. J. Assoc. Offic. Anal. Chemists, 52: 300
IUPAC. (1970) Commission on Terminal Pesticide Residues. 4th Meeting.
Fate of organochlorine pesticides in vegetable oil processing. Comptes
Rendus-XXV Conference IUPAC p. 187-189. Also J. Assoc. Offic. Anal.
Chemists, 53: 987-1003, 1970
Korte, F. (1967a) Metabolism of 14C-labelled insecticides in
micro-organisms, insects and mammals. Botyukaguku, 32: 46-59
Korte, F. (1967b) Metabolism of chlorinated insecticides. Paper
submitted to IUPAC Commission on Terminal Pesticide Residues. Appendix
VI of the proceedings of the Commission, Vienna
Korte, F. and Porters P.E. (1970) Terminal residues of cyclodiene
insecticides. Meeting of IUPAC Commission on Terminal Residues
Erbach/Rheingau, 14-18 Sept. 1970
Lawrence, J.H., Barron, R.P., Chen, J-Y.T., Lombardo, P. and Benson,
W.R. (1970) Note of identification of a chlordane metabolite found in
milk and cheese. J. Ass. off. anal. Chem., 53: 261-262
Lichtenstein, E.P., Schultz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period, translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, B.J., Stadelman, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews 29: 61-72
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the detection of pesticide residues. Int. Atomic
Energy Agency Vienna p. 49-58
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Entomol., 45: 452-456
Martin, R.J. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III) Pesticide Monit. J., 1, 4: 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on synthetic (X) in male and female albino rats.
Unpublished report from Industrial Bio-Test Laboratories submitted to
Velsicol Chemical Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories submitted by Velsicol Chemical
Corporation. (IBT no.A7367), 23 June 1969
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories submitted by Velsicol
Chemical Corporation (IBT no.A7518) 21 August 1969
McGuire, R.R., Zabrik, M.J., Schuetz, R.D. and Flotard, R.D. (1970)
Photochemistry of bioactive compounds. J. Agr. Fd. Chem., 18:
319-321
Miles J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
Econ. Entomol., 62: 1334-1338
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugar beets. J. Econ.
Entomol., 63: 1143-1146
Plank, J., Keplinger, M.L., Fancher, O.E. and Richter, W.R. (1970)
90-day subacute oral toxicity of synthetic "X" (oxychlordane) in
albino rats. Unpublished report from Industrial Bio-Test Laboratories
submitted to Velsicol Chemical Corporation
Polen, P.B. (1966) Chlordane: composition, analytical consideration
and terminal residues. IUPAC Commission on Terminal Pesticide
Residues, Geneva
Polen, P.B. (1968) Terminal residues in chlordane. Status Rept. 2.
Chlordane Working Party. IUPAC Commission on Terminal Pesticide
Residues
Polen, P.B. (1969) Report of chlordane working party to the third
meeting of the IUPAC Commission on Terminal Residues. J. Ass. Offic.
Anal. Chem., 52: 300
Polen, P.B., Hester, M. and Benziger, J. (1970a) Characterization of
oxychlordane, animal metabolite of chlordane. Bull. environm. Contam.
Toxicol., 5: (3)
Polen, P.B. (1970b) Terminal residues of chlordane. Meeting of IUPAC
Commission on Terminal Residues, Erbach/Rheingau, 14-18 Sept.
Poonawalla, N.H. and Korte, F. (1964) Metabolism of insecticides VIII.
Excretion, distribution and metabolism of -chlordane-14C by rats.
Life Sci., 3: 1497-1500
Riemschneider, R. (1969) The chemistry of the Insecticides of the
Diene Group. World Rev. Pest Control Part 2 4: 29-61
Rosen, J.D., Sutherland, D.J. and Khan, M.A.Q. (1969) Properties of
photo-isomers of heptachlor and isodrin. J. Agr. Fd. Chem., 17:
404-405
Saha, J.G. and Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide
and trans-chlordane residues in soil and rutabaga after soil and
surface treatments with heptachlor. Can. J. Plant Sci., 47: 79-88
Saha, J.G., Burrage, R.H., Nielson, H.A. and Simpson, E.C.R. (1968)
Chlordane: composition of commercial formulation residues in potatoes
grown in treated soil and their reduction by home processing.
Pesticide Progress Reports from Research Branch Establishments
(Canada), 6 (5): 117-121
Saha, J.G. (1970) Unpublished data
Schwemmer, B., Cochrane, W.P. and Polen, P.B. (1970) Oxychlordane,
animal metabolite of chlordane: isolation and synthesis. Science,
169: 1087
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970a) Alpha-gamma
chlordane milk residue study in cows. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970b) -1970- Alpha-gamma
chlordane milk residue study in goats. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Smith, K.J., Polen, P.B., de Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chemists Assoc. 45:
866-869
US Dept. of Health, Education and Welfare Food and Drug
Administration. (1968) Pesticide analytical manual Vol. I, Washington
D.C.
Velsicol. (ed. P.B. Polen) (1970) Chlordane: agricultural residues and
related toxicology prepared for the 1970 Joint FAO/WHO Meeting on
Pesticide Residues by Velsicol Chemical Corporation
Vollner, L., Klein, W. and Korte, F. (1969) Beiträge zur ökologischen
Chemie, Photoumlagerung der Komponenten des technischen Chlordans.
Tetrahedron Lett., 34: 2967-2970
Wazeter, F.X., Butler, R.H., Geil, R.G. and Rehkamper J.A. (1968)
Alpha chlordane, gamma chlordane, alpha-gamma chlordane. Comparative
acute oral toxicity (LD50) in male albino rats. Unpublished report
from International Research and Development Corporation for Velsicol
Chemical Corporation

 Chromatograms of technical chlordane using, above, the letter
designation of Velsicol (Polen, 1966) and below, that of USFDA (US
Dept. of Health, Education and Welfare, Food and Drug Administration,
1968).
TABLE II
Chlordane chromatographic peaks:
correlation of letter designations
Velsicol 1/ USFDA 2/
C A
D B
E C
F D
H 3/ E 3/
I 4/ F 4/
K G
1/ Polen, 1966
2/ US Dept. of Health, Education and Welfare, Food and Drug
Administration, 1968
3/ Peak for trans-chlordane
4/ Peak for cis-chlordane
No further experimental information is available to enable complete
elucidation of the significance of liver hypertrophy or the
stimulation of liver microsomal activity following administration of
chlordane. The significance of these changes was discussed in the
previous monograph on chlordane (FAO/WHO, 1968).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
In the 78-weeks feeding study in rats reviewed under "Long-term
studies", no tumours were evident when dietary levels up to 35 ppm of
the cis- or 75 ppm of the trans-isomer were fed (Ingle 1969).
There is no report that tumours have been looked for in any other
species fed chlordane.
Special studies on the metabolite "oxychlordane"
Rat
A group comprising two male and two female rats was fed a level of the
chlordane metabolite "oxychlordane" in their diet, sufficient to
maintain a daily intake of 2.0 mg/kg body-weight. The compound was fed
for 90 days, after which time the surviving animals were sacrificed
for autopsy. Body-weight increase, food consumption, mortality,
organ-weights and organ to body-weight ratios, behaviour, haematology
and blood chemistry and urologic studies were all considered to be
within the normal range for the strain of rat used. No gross
pathological abnormalities were evident, and no histopathological
lesions could be attributed to "oxychlordane". A parallel control
Group of rats fed a normal diet does not appear to have been examined
in this experiment (Plant et al., 1970). Feeding studies with
"oxychlordane" utilizing a larger number of animals are reported to be
in progress (Calo, 1970).
Special studies on the possible metabolite 1-hydroxychlordene
Rat
Groups of 25 males and 25 females were fed dietary levels of 0, 100,
250, 500, 1000, and 2000 ppm of 1-hydroxychlordene for up to 224 days.
After 110 days, three females from each feeding level were mated with
males at all levels. Mortality in all groups was low, and no real
differences existed. At 224 days, autopsy revealed no gross
abnormality. Histopathology of all visceral organs was negative,
except for the slight to moderate cytoplasmic margination of a few
liver cells at 1000 and 2000 ppm (Ingle, 1965).
Special studies on photodecomposition
Rat
No information is available on the mammalian toxicity of the
photoisomer of chlordane.
Acute toxicity
The acute toxicity of the two isomers of chlordane and some
metabolites is summarized in Table III.
Rats were fed for 28 days from weaning on either (a) a diet containing
3.5 percent protein as casein; (b) a diet containing a normal amount
of protein as casein or (c) a standard laboratory diet. A single oral
TABLE III
Acute toxicity of chlordane isomers and metabolites
LD50 mg/kg
Compound Animal body weight Reference
(oral)
cis-chlordane Rat (M) 392 Wazeter et al., 1968
trans-chlordane Rat (M) 327 Wazeter et al,, 1963
cis-trans-chlordane Rat (M) 371 Wazeter et al., 1968
(1:1 by weight)
oxychlordane Rat (M,F) 19.1 Kastri et al., 1969 a
chlordane Rat (M,F) >4600 Nastri et al., 1969 b
3-chlorochlordene Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxychlordene Rat (M,F) >4600 Mastri et al., 1969 b
chlordene epoxide Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxy 2,3-epoxy Rat (F) >4600 and Mastri et al., 1969 c
chlordene <10200
2 chlorochlordene Rat (F) >10200 Mastri et al., 1969 c
dose of chlordane was administered after the feeding period. The LD50
values for the three groups were 137, 267, or 311 mg/kg body-weight,
respectively. Clinical symptoms and pathology were the same in all
groups (Boyd and Taylor, 1969).
Long-term studies
Rat
Groups, each of 20 male and 20 female rats were fed dietary levels of
0, 5, 15, 25 or 35 ppm of cis-chlordane or 15, 25, 35 or 75 ppm of
trans-chlordane or 5, 15, 25, 35 or 50 ppm of a 1 : 1 mixture of
cis and trans-chlordane. In the group fed cis-chlordane, growth
retardation became apparent in the group fed 35 ppm after four months
in the males and after five months in the females; with
trans-chlordane, the 75 ppm group of males only displayed growth
retardation after eight months. With the mixture, growth retardation
was evident in both sexes fed 50 ppm, beginning early in the males and
later in the females. Growth retardation was not evident in any groups
fed lower doses of either isomer. Food consumption bore a relationship
to growth. Increased mortality for both sexes became significant in
the groups fed 35 ppm of cis-chlordane, 75 ppm of trans-chlordane
or 50 ppm of the cis-trans mixture.
Haematocrit was normal for all test groups. Autopsy revealed no gross
pathological lesion, in particular there was no evidence of tumours.
Histological examination of all organs showed no changes from feeding
chlordane at any level, except in the case of the liver. Compression
of sinusoids due to slight hepatic cell hypertrophy in the
centrolobular region and minimal bile duct proliferation were evident
at 35 ppm of cis-chlordane. The above were noted, but were minimal,
at 25 ppm of the same isomer. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region, minimal
perinuclear vacuolation and minimal cell hypertrophy with constriction
of sinusoids were noted at 75 ppm of the trans-chlordane. Slight
cytoplasmic homogeneity of hepatic cells in the centrolobular region
and occasional cytoplasmic margination were observed at 50 ppm of the
cis-trans mixture. The above alterations were minimal at 35 ppm of
the same mixture. No liver abnormalities were evident after feeding
lower levels of the chlordane isomers (Ingle, 1969)
COMMENT
The study on cis and trans-isomers of chlordane, which was
considered desirable at the 1967 Joint Meeting, has now been completed
and indicates that the no-effect levels of the two isomers and of the
technical product are all within the same range, The toxic metabolite,
oxychlordane, which becomes stored in the fat or excreted in the milk
of lactating animals fed high doses of chlordane, does not appear to
be a residue in plants. For this reason, the toxicity of oxychlordane
was not considered to be a matter of concern. No information is
available on the mammalian toxicity of the photoisomer of chlordane. A
satisfactory reproduction study in one species has been previously
reported and is considered adequate. However, in view of the
observations from other organochlorine compounds, an adequate
carcinogenicity study in another species is needed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 ppm of technical chlordane in the diet, equivalent to 1 mg/kg
body-weight/day
Dog: 3 ppm of technical chlordane in the diet, equivalent to 0.075
mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Several million pounds of chlordane have been used annually in a broad
spectrum of applications in the U.S.A. for nearly 25 years. Outside
the U.S.A. its use is somewhat less, but comparable.
Velsicol (1970) has presented the approximate distribution of use of
chlordane outside of the continental United States of America as
follows:
Area Percentage
Europe 55
North America (excludes U.S.A.) 15
South America 15
Asia 10
Africa 5
Total 100
In the U.S.A. about half and outside the U.S.A. about 10 percent of
chlordane usage is estimated to be non-agricultural (household pest
control, lawn protection, etc.)
Chlordane is used in protection of the following crops:
Europe: Potatoes, vegetables, small grains, sugar beets
and sugar cane.
Western hemisphere: Sugar cane, maize, small grains, potatoes and
vegetables.
Asia: Rice, cotton and jute.
Other uses of chlordane are in the protection of fruits, nuts and oil
seed crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A substantial amount of new information on the residues resulting from
supervised trials is made available to the Working Party. Present
application of electron capture gas chromatography on chlordane
residues has allowed fairly detailed evaluation of several
constituents of the residues. The available new information is
summarized in Table IV. The data are based on the records of Velsicol
Chemical Corporation (1970).
Evidence of residues in food in commerce or at consumption
In a series of papers (Duggan et al., 1966, 1967; Duggan and
Weatherwax, 1967; Duggan and Lipscomb, 1969) the pesticide residue
levels have been reported in 11 food and feed categories, as raw
agricultural products or ready-to-eat foods, domestic and imported,
and they have assessed the dietary intake during 1964 to 1968 in the
United States. The observations on chlordane levels are summarized in
Table V.
Chlordane was undetected in all but three categories of raw
agricultural products where levels were <0.005 ppm and at maximum
incidence of 3.3 percent in imported grain. Ready-to-eat foods in
these three categories contained less (in one category) or undetected
residues.
Chlordane residues found in vegetable oil seeds, oils and byproducts
are summarized in Table VI. The traces of chlordane were reported in
less than 1 percent of the sample of soybeans, but none appeared in
the finished oil. Chlordane residues were reported in 3.7 per cent of
the crude maize oil samples, but were not found in the finished oil. A
small number of samples of crude cottonseed oil showed occasional
residues of chlordane, but the concentration in finished oil was lower
by about 59 percent. No chlordane residues were observed in
oleomargarine or in the total diet composites consisting of salad
dressings, salad oils, mayonnaise, shortenings and peanut butter
(Duggan, 1968 a, 1968 b). These empirical observations corroborate the
evidence that commercial processing removes chlordane residues, if
present, from edible vegetable oils and oil products.
From 750 meal cake samples (Table VI), chlordane was detected in 0.7
percent of the soybean cakes and in 6.5 percent of the cottonseed
TABLE IV
Summary of maximum observed chlordane residues1,2
Treatment
maximum rate
Root vegetables lb/acre ppm
Parsnip 10 0.07
Potato 10 0.23
Rutabaga 10 0.5
Sugar beets (root) 10 0.11
(wet pulp) 10 0.15
(dried pulp) 10 0.3
(juice) 10 0.063
Sweet potato 10 0.04
Leafy and stalk vegetables
Asparagus 44 0.07
Broccoli 44 0.063
Brussel sprouts 44 o.36
Cabbage 44 0.09
Celery 124 0.063
Cauliflower 44 (foliar) 0.06
Mustard greens 10 0.07
Spinach 10 0.10
Swiss chard 10 0.07
Other vegetables
Snap beans 10 0.01
Grains
Barley (Grain) 104 (foliar and soil) 0.063
" (straw) 104 0.42
Oats (grain) 10 0.063
" (straw) 10 0.10
Rye (grain) 10 0:063
" (straw) 10 0 07
Wheat (grain) 4 (foliar) 0:063
" (straw) 4 ( " ) 0.38
" (grain) 10 0:063
" (straw) 10 0.10
Rice (grain) 10 (soil or seed dressing) 0.033
" (straw) 10 0.033
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Grains
Maize and "popcorn" (seed) 10 0.063
" " " (stalks and
leaves) 10 0.033
Sorghum (grain) 12 (soil or seed dressing) 0.063
" (stalks) 12 ( " ) 0.07
Cucurbits
Cantaloupe 34 (foliar) 0.1
Cucumber 34 ( " ) 0.063
Pumpkins (pulp) 44 ( " ) 0.063
" (rind) 44 ( " ) 0.10
" (pulp) 10 0.08
" (rind) 10 0.15
Squash 34 (foliar) 0.09
Watermelons 34 ( " ) 0.063
Fruits and nuts
Almonds 10 0.0063
Figs 10 0.0033 5
Filberts 10 0.0033
Olives 10 0.0063
Pecans 10 0.048
Pomegranate 10 0.0063 5
Walnuts 10 033 5
Bananas 10 0.033
Guavas 10 0 036
Mangoes 10 0:013 5
Passion fruit 10 0.035
Papayas 5 0.01
Oil seed crops
Cotton (seed) 1-2 (foliar, up 12 0.22
applications
(meal) 1-2 ( " ) 0.07
(crude oil) 1-2 ( " ) 0.11
(soap stock) 1-2 ( " ) 0.063
(stalks) 1-2 ( " ) 0.29
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Oil seed crops
Linseed (seed) 34 (foliar) 0.22
(meal) 34 ( " ) 0.09
(crude oil) 34 ( " ) 0.24
Soybeans (beans) 124 0.38
(crude oil) 124 0.42; 0.72
Rape (whole plants) 3 (foliar) 0.35
1 From soil treatment except where indicated; data not previously
published.
2 Sum of six constituents of technical chlordane.
3 Negative reading - value shown is sum of estimated lower limits of
sensitivity components.
4 Exceeds maximum rate permitted in U.S.A.
5 Shell or meat analyzed separately.
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large fruit N2 - N - N -
Small fruit N - N - N -
Grain & cereals
For human use 0.7 <0.005 3.3 <0.005 N -
For animal use N - N - - -
Vegetables
Leaf and stem N - N - N -
Vine and ear 2.0 <0.005 0.3 <0.005 2.7 <0.001
Root 1.9 <0.005 0.3 <0.005 N -
Potatoes - - - - N -
Beans N - N - N -
Eggs N - N - N -
Nuts N - N - N -
Processed meat
(canned frozen,
etc.)
Domestic N -
Imported N -
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Fluid milk
(fat basis) N -
Dairy products
(fat basis) N - N -
1 Extracted from USA-FDA surveillances programme report (Duggan, 1968b)
2 Not tabulated in reference, presumably because of insignificant
frequency of incidence
TABLE VI
Chlordane residues in vegetable oil seeds, oils and byproducts1
Crude oil Meal (cake) Edible oil
Product Number of Raw product
samples Incidence Average Incident Average Incident Average Incident Average
analysed percent ppm percent ppm percent ppm percent ppm
Soybeans 550 0.9 <0.001 - 2 - 2 0.7 <0.001 - 2 - 2
Groundnuts 177 - 2 - 2 - 2 - 2 - 2 - 2 - 2 - 2
Cottonseed 23 8.7 0.004 2.2 0.017 6.5 0.012 2.4 0.007
Maize 819 - 2 - 2 3.7 0.080 - 2 - 2
Total and
weighted 1569 0.4 <0.001 2.0 0.042 0.9 3 <0.001 3 0.04 <0.001
averages
1 Extracted from USA-FDA surveillance programne reports (Duggan, 1968a)
2 Not detected
3 Estimates based upon 750 samples for which cake data are given
cakes at average levels less than 0.001 and 0.012 ppm, respectively.
Of all the samples, 0.9 percent contained a detectable level (<0.001
ppm) of chlordane.
Dietary intake (milligrammes/day) generally appeared to be too low to
quantitate. Except for <0.001 mg/day (<1.7 percent of the ADI for a
60 kg man) from legumes during June 1966 to April 1967, no other
figures are listed for chlordane intake by Duggan and Lipscomb (1969).
The total diet studies in England and Wales (Abbott et al., 1969)
reveal no residues of chlordane.
FATE OF RESIDUES
In animals
An animal metabolite of chlordane, "oxychlordane", first reported in
1968 to the IUPAC Commission on Terminal Residues as an analytical
manifestation (IUPAC, 1969) has been isolated from pigs (Schwemmer et
al., 1970) and from milk and cheese (Lawrence et al., 1970). The
metabolite has been characterized with respect to analytical
parameters, propensity for occurrence in milk and storage in the fat
of dogs, cattle (beef and dairy) pigs and rats (Polen, 1970).
"Oxychlordane" levels in milk and some tissues of cows and goats,
following termination of ingestion of 10 ppm doses of chlordane
isomers for ten days, has been studied (Velsicol, 1970). Maximum
residues of "oxychlordane" found in milk were as follows:
Isomer fed Cow Goat
cis-chlordane 0.011 ppm 0.029 ppm
trans-chlordane 0.049 ppm 0.067 ppm
cis-+trans-chlordane 1:1 0.048 ppm 0.040 ppm
Highest milk level vs. feed level: goat 0.7 percent; cow 0.5 percent.
The residue levels in milk following termination of feeding of
chlordane declined to half in 10 to 12 days in cows and in 8 to 15
days in goats. Fifty days after chlordane withdrawal, the fat tissue
of cows contained up to 0.25 ppm and (2.5 percent of feeding level),
that of goats up to 0.4 ppm (4 percent of the feeding level) of
"oxychlordane". Some of this metabolite was occasionally found in
liver, but none in brain. The parent chlordane isomers could sometimes
be found in milk on the tenth day of feeding, but later none was
detected either in milk or in tissues (Singh et al., 1970 a, 1970 b).
At feeding levels of chlordane corresponding to proposed international
tolerances, oxychlordane is not detected in milk (sensitivity 0.005
ppm) but may be found in the fat of cattle at or below 0.03 ppm
(Polen, 1970).
When calves were fed for one month, one sixth of their ration, sugar
beet pulp derived from chlordane treated soil (Velsicol, 1970), the
maximum total residues found in the fat were, when fed with pulp from
the chlordane treatment year, up to 0.62 ppm and, when fed with pulp
from a treatment done a year prior to harvest, up to 0.06 ppm. Rates
of chlordane applications were 6-10 lb active ingredient per acre. The
total residue of the dry pulp varied from 0.05 to 0.30 ppm. The
residues found in liver were much less than those in fat, and none was
found in muscle (sensitivity 0.01 ppm).
When cows were fed with rations containing potato and alfalfa grown in
chlordane treated soil (Velsicol, 1970), no detectable residues were
found. Residue of heptachlor, heptachlor epoxide, compounds C and E
(minor components of technical chlordane), cis-chlordane and
trans-chlordane were all less than 0.01 ppm.
Hens fed 0.08 ppm of chlordane in their ration produced eggs with no
detectable residues (Herrick et al., 1969).
In plants
Korte and Porter (1970) reported on the metabolism of
trans-chlordane in white cabbage and carrots. Tests were under
glasshouse conditions and are difficult to relate to practical
conditions.
White cabbage leaves, four weeks after foliar application of 14C
labelled trans-chlordane, contained less than 1 percent of the
radioactivity on the surface; 90 percent was in the leaves as three
conversion products, in addition to the parent compound. At ten weeks,
20 percent of the applied radioactivity remained, 80 percent of which
was from metabolites. Soil treatment of carrots with 14C-labelled
trans-chlordane resulted in 0.01 ppm in carrots (mainly metabolites)
and 0.06 ppm in leaves (two thirds of the parent compound) 12 weeks
after treatment.
Polen (1970 a, 1970 b) reports that all the evidence accumulated thus
indicates that "oxychlordane" is not normally present in the terminal
residues of chlordane in plants.
In soils
Miles at al. (1969) found in their study on metabolism of heptachlor
by soil micro-organisms that heptachlor epoxide, 1-hydroxychlordene,
1-hydroxy- 2,3-epoxychlordane and possibly 1-keto-2,3-epoxychlordane
are formed, but their importance under field conditions after
chlordane treatment has not been established.
Onsager et al. (1970) did not detect heptachlor epoxide in
chlordane-treated soil (or in sugar beets grown therein). The
biological half life of chlordane was estimated to be 14.3 months, in
good agreement with other published data.
Lichtenstein at al. (1970) reported that soils treated for five years
annually with technical heptachlor (25 lb heptachlor and about 7.5 lb
chlordane as an impurity - cumulative basis) contained 0.019 ppm of
chlordane isomers (in addition to heptachlor derivatives) ten years
after the first treatment.
Polen (1970 a, 1970 b) reports that "oxychlordane" has not been found
in the terminal residues of chlordane in soils.
Photochemical transformations
The possibility of conversion of constituents of residues from
technical chlordane by ultraviolet light in vitro has been
demonstrated by several workers (Fischler and Korte, 1969; Vollner et
al., 1969; Benson et al., 1969; Rosen et al., 1969; McGuire et al.,
1970). Present evidence indicates that the photochemical
transformation compounds do not contribute significantly, if at all,
to terminal chlordane residues under agricultural conditions.
Observations of the IUPAC Chlordane Working Party (IUPAC 1969) on gas
liquid chromatograms from foliar applications on beans and cabbage
revealed no new peaks during the ageing of the residues and hence gave
no indication of photolytic products. A more recent investigation
directed specifically toward evaluation of the effects, if any, of
sunlight on chlordane residues also found no significant indications
of the production of photolytic products under typical field
conditions (Polen, 1970).
Pure preparations of compounds which are possible constituents of
chlordane terminal residues (cis- and trans-chlordanes, chlordene,
heptachlor, heptachlor epoxide and nonachlor) wore exposed to sunlight
as films on glass, on wheat and on soil surfaces. On glass after 235
hours of exposure, only heptachlor epoxide and cis-chlordane were
converted, constituting respectively 54 and 1.6 percent of the
terminal residues. After 39 days of exposure, no photoreaction
products were detected on soil; on wheat (2 lb chlordane per acre,
foliar application) only photo-cis-chlordane was detected as 2
percent of the terminal residue.
In another set of observations, 30 randomly-selected crop and soil
samples from supervised field trials throughout the U.S.A. were
examined for presence of photo products in chlordane residues.
Application rates ranged from four to ten lb of technical chlordane
per acre, soil or foliar applications; sampling intervals were from 15
to 303 days after application. Samples were: grass; straw of rice and
rye; seeds of rice and cotton; potato culls; soil from chlordane
treatments on which was grown barley or rice; sugar beets; whole and
spent cossettes; spinach; cauliflower and broccoli. Generally, no
photo products were detected at a sensitivity level of 0.01 ppm. It
was concluded that typically photo products are not produced in
chlordane soil treatments and that, if present at all in terminal
residues from foliar treatment, the contribution to the terminal
residue is less than 2 percent.
In storage and processing
With a few exceptions, accumulated evidence indicates that normal food
processing tends to reduce or eliminate chlordane residue. The lower
incidence and levels of residues in ready-to-eat foods compared to raw
agricultural products is evident in US Total Diet Studies, and was
commented on above.
In root crops, consistent evidence shows that chlordane residues from
treatments with either technical chlordane or technical heptachlor, in
which chlordane is a minor component, are concentrated in the peel,
and that peeling and/or cooking reduces or eliminates the residue.
In potatoes, peeling removes virtually the entire residue from
chlordane treatments up to 10 lb/acre. Without peeling, baking removed
about 80 percent of the residue and boiling up to 30 percent. (Saha et
al., 1968).
The residue of trans-chlordane from technical heptachlor treatments
of rutabagas was concentrated in the peel, and about half of that
residue was removed by boiling. The pulp was free of chlordane residue
before and after cooking (Saha and Stewart, 1967).
Similar patterns are observed in processing carrots, turnips and
beets. No residues of chlordane are detected in boiled pulp of turnips
or beets. In carrots, a 98 percent reduction resulted from peeling
only and 63 percent from boiling alone (Saha, 1970).
In commercial canning, washing, peeling (potatoes, tomatoes, etc.) and
abrasive peeling (used, for example, in preparing carrots) have been
demonstrated to reduce surface-concentrated pesticide residues (Farrow
at al., 1969). No doubt the surface-bound residues of chlordane would
respond in a similar way.
Chlordane residues on cabbage or beans are not changed in level or
composition by simple ten minute cooking (Polen, 1968). The lack of
the loss of chlordane residues were due to the fact that chlordane is
chemically relatively stable, co-distillation did not occur and the
cooking water containing extractable residue was included in the
sample extracted for residue analysis.
Commercial processing of edible vegetable oil produces finished oil
practically free of residues of chlordane, as well as other
organochlorine pesticides (Gooding, 1966; IUPAC, 1970; Smith at al.,
1968).
Samples of milk containing chlordane manufactured into condensed,
dried whole and evaporated milk were reduced in chlordane content. The
following figures are the percentage reduction in chlordane content
(fat basis) of processed fractions: condensed milk 11 percent; spray
dried milk 25 percent; evaporated milk 45 percent and drum dried milk
55 percent (Liska and Stadelman, 1969).
Wheat flour and rice, each contaminated by exposure to chlordane
vapours, were processed to produce two cooked products: a baked cookie
and boiled rice. The baked wheat flour cookie contained an average of
50 percent less residue than the wheat and the cooked rice about 73
percent less than the raw product (Bevenue and Yeo, 1969).
METHODS OF RESIDUE ANALYSIS
Analytical methods 1/
Prior to about 1961, residues from application of technical chlordane
were determined by total organochlorine, colorimetric or bioassay
techniques. The information derived was limited by lack of specificity
and sensitivity. At the present time, procedures based on the use of
electron capture gas chromatography are recommended. Four specific
analytical methods for the multi-component residues of chlordane are
available (Velsicol, 1970).
The methodology is that adopted for organochlorine pesticides by the
IUPAC Commission on Pesticide Residue Analysis and incorporates
procedures which favour analysis of chlordane residues in several
substrates. Detection limits are 0.002-0.02, depending on the nature
of the sample. Analytical method AM 0506 (Velsicol, 1970) is used for
soil in order to determine residue as chlordane, which is defined as
the sum of residues (ppm) of unchanged compounds from technical
chlordane plus residues of their conversion products, if present in
minor proportions. Conversion products are estimated separately if
they are present in significant amounts. Analytical method AM 0507
(Velsicol, 1970) is applicable to plant tissues and crops such as,
fruits, vegetables, grains, etc. The residue is obtained as a sum of
residues (ppm) of unchanged compounds from technical chlordane plus
residues of their conversion products, photolytic and metabolic, if
present in minor proportions. Conversion products are estimated
separately if they are present in significant amounts. Analytical
method AM 0509 (Velsicol, 1970) is applicable to milk, milk products
(e.g. butter and cheese), animal tissues and various animal food
products. Residue of chlordane is the sum of residues (ppm) of
unchanged compounds from technical chlordane plus possible residues of
their metabolites, oxychlordane and heptachlor epoxide. Method AM 0508
(Velsicol, 1970) is a supplementary method to describe modifications
to existing analytical methods in order to identify and quantitate
1/ Copies of the analytical methods and reference analytical grade
samples of the compounds are available from Velsicol Chemical Corp.,
341 E. Ohio Street, Chicago, Illinois 60611, U.S.A.
minor components of technical chlordane or of possible conversion
products (metabolic, photolytic or others). This method applies the
procedures of the other methods referred; e.g. the following compounds
can be determined: photo-cis-chlordane, photo-heptachlor epoxide,
heptachlor epoxide, "oxychlordane", photo-heptachlor and
photo-chlordane.
Quantitation of residues in plant and animal products
Several procedures have been used under various circumstances to
quantitate the gas-chromatographic responses of chlordane residues. In
most instances, the values calculated are good approximations of total
residues, but fail to distinguish conversion products from unchanged
components of technical chlordane. The methods AM 0506 to 0509
inclusive allow for quantitation of conversion products if present.
A method used prior to about 1968 relied upon calibration of the
principal peak (trans-chlordane) in reference technical chlordane
and estimation of the total residue by assuming constancy of the
proportions of the components in the course of "weathering". This
technique overstated the residue of chlordane by about 20-70 percent
for foliar treatments and by roughly half that amount for residues
from soil treatment in which conversion compounds may be in somewhat
greater concentration (Velsicol, 1970). These factors should be given
consideration in evaluating residue data generated before about 1968.
The estimation of chlordane residues in plants and soils as the sum of
cis-and trans-isomers (FAO/WHO, 1968) gives values roughly equal
to a minimum of 70-80 percent of the total terminal residues of
chlordane. In animal products, the sum of "oxychlordane" plus
cis-and trans-chlordane comprises generally more than 90 percent
of the residues in the lipoid phase.
APPRAISAL
A comprehensive set of new data for evaluation of chlordane residues
was provided to answer the questions raised by the 1967 Joint Meeting,
to add new information to the Monograph, to review the temporary
tolerances recommended in 1967 and to make new recommendations.
Technical chlordane continues to be a standardized product produced by
a single manufacturer. One of the characteristics of the technical
chlordane for which the recommendations are made is its content of
heptachlor (10 ± 2 percent).
Chlordane is used, in addition to the U.S.A., especially in Europe and
to a smaller extent in other American countries, Asia and Africa. Soil
treatments represent the main usage of chlordane. Foliar applications,
with exception of cotton, are not considered to be recommended usages
any more. The use to forage crops has been discontinued, e.g. in the
U.S.A.
Thorough studies on the chlordane residues in foodstuffs and in meal
cakes are conducted in the U.S.A. Residues were found, but in three
categories of raw agricultural products levels were less than 0.005
ppm. Oil seed cakes from soybeans and cotton seeds contained in 0.7
and 6.5 percent of the samples had residues with average levels of
less than 0.001 and 0.012 ppm, respectively.
Dietary intakes of chlordane in the U.S.A., England and Wales have
proved to be negligible. In most cases, the residues in the diet
composites were less than the analytical detection limits. Due to
this, it has not been possible to make reliable estimates of daily
chlordane intake.
Food processing tends to reduce or eliminate chlordane residues;
especially those processes which include removal of the treated crop
surface, disposal of cooking water, vacuum distillation or other type
of dehydration processes are able to remove a substantial part of the
residues.
Residues resulting from growing crops in land treated in previous
years with chlordane has been studied. It was found that the residues
in sugar beets, grown in the same land in several subsequent years,
were proportional (about 10 percent) to the residues in the soil at
the time of planting. Thus the chlordane level left in the soil seems
to determine the residue level found in crops. Stability of chlordane
in soil is well studied.
An animal metabolite of chlordane, oxychlordane, has been found in fat
of animals ingesting chlordane, but at significantly lower levels than
the level of chlordane in feed. Information for recommending practical
residue limits for meat, milk, poultry and eggs is available.
So far there is no evidence that oxychlordane is a component of
terminal chlordane residues in plants or in soils.
Photochemical transformation products of chlordane, which are reported
to be formed in vitro, are most evidently not formed in soils. In
foliar treated plants, if at all present, they are estimated to
contribute to the terminal residue less than 2 percent.
Hydrophilic metabolites of chlordane are formed in plants; their
significance is under investigation.
Analytical methods, based on the methodology generally adopted for
organochlorine pesticides, have been developed to determine the
multi-component residues of chlordane as well as their metabolites
with detection limits of 0.02-0.002 ppm. Clarification of the
nomenclature of chlordane isomers and the letter designations of
chlordane chromatographic peaks have been made.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The recommendations made now are for the residues which result from
pre-plant soil applications at a maximum level of 10 lb /acre or from
seed dressing, except for cotton which may require multiple foliar
applications, before the bolls open, at 1-2 lb /acre. Good
agricultural practice for sugar beets would require that the soil
would not contain, from the treatments of the planting and previous
years, more than 2.5-5.0 lb /acre. In cool climates, the application
rates should be established to lower levels to compensate the greater
stability of the residues.
For regulatory purposes, the chlordane residues in plant products
should be determined as the sum of cis- and trans-chlordane and in
animal products as the sum of cis-chlordane, trans-chlordane and
"oxychlordane".
In view of the new data for evaluating chlordane residues, the
recommendations made by the 1967 Joint Meeting for temporary
tolerances and practical residue limits are replaced by new
recommendations for tolerances and practical residue limits.
TOLERANCES
ppm
Root vegetables (practically soil-free) 0.3
Potatoes, sweet potatoes, rutabagas, turnips,
parsnips, sugar beets and radishes
Leafy and stalk vegetables 0.2
Asparagus, broccoli, brussel sprouts, cabbage, celery,
cauliflower, mustard greens, spinach, Swiss chard
and lettuce
Other vegetables 0.02
Beans, peas, eggplants, tomatoes and collards
(= cole-worts)
Grains 0.05
Wheat, rye, oats, rice (polished), maize, sorghum
and popcorn
Cucurbits 0.1
Cantaloupes, cucumbers, pumpkins, squash and watermelons
Fruits and nuts 0.1
Almonds, bananas, figs, filberts, guavas, mangoes,
olives, passion fruit, papayas, pecans, pomegranates,
pineapple, strawberries and walnuts
TOLERANCES (cont'd)
ppm
Citrus, pome and stone fruits 0.02
Not specified above
Vegetable oils
Crude soybean and linseed oils 0.5
Crude cottonseed oil 0.1
Edible cottonseed and soybean oils 0.02
PRACTICAL RESIDUE LIMITS
Milk and milk products (fat basis) 0.05
Fat of meat 0.05
Fat of poultry 0.05
Eggs (shell free) 0.02
FURTHER WORK OR INFORMATION
REQUIRED (before June 1972)
Further information on chlordane residues in carrots resulting from
soil and seed treatments and from crop rotation on soils having been
treated with chlordane in previous years.
DESIRABLE
1. An adequate carcinogenicity study in a second species of animal.
2. Results of the investigation of the hydrophilic plant metabolites
of chlordane.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J. Sci.
Fd. Agr., 20: 245-249
Benson, W.R., Lombardo, R., Barron, R., Lustig, E., Mastbrook, D. and
Ross, R. (1969) Paper at American Chemical Society Meeting, New York,
N.Y., Sept. 1969
Bevenue, A., and Yeo, C.Y. (1969) Effects of the cooking process on
the removal of chlordane residues from wheat flour and rice. Bull.
Environm. Contamination Toxic., 4: 370-374
Boyd, E.M., and Taylor, F.I. (1969) The acute oral toxicity of
chlordane in albino rats fed for 28 days from weaning on a
protein-deficient diet. Industr. Med., 38 (12): 42-49
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Büchel, K.H., Ginsberg, A.E. and Fischer, R. (1966)
Cyclodin-Insektizid IV. Synthese und Struktur von Isomeren des
Chlordans. Chem. Ber., 99: 421-430
Calo, D. (1970) Chlordane. Section I. Related toxicology. Unpublished
summary prepared by Velsicol Chemical Corporation.
Chau, A.S. and Cochraney W.P. (1969) Cyclodiene chemistry. I
Derivative formation for the identification of heptachlor, heptachlor
epoxide, cis-chlordane, trans-chlordane and aldrin pesticide
residues by gas chromatography. J. ass. off. anal. Chem.,
52: 1092-1100
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide Monit.
J., 2: 140-152
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide Residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed. Pesticide Monit. J., 1,2: 2-12
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Duggan, R.E. (1968a) Residues in food and feed. Pesticide Monit. J.,
1,4: 2-7
Duggan, R.E. (Ed.) (1968b) Residues in food and feed. Pesticide Monit.
J., 2: 2-46
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pesticide
Monit. J., 2: 153-162
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23 (1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1: WHO/Food Add./27.65
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Farrow, R.P., Elkins, E.R., Rose, W.W., Lamb, F.C., Ralls, J.W. and
Mercer, W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews, 29:
73-87
Fischler, H.M., and Korte, F. (1969) Sensibilisierte und
unsensibilisierte Photoisomerisierung von Cyclodien-Insektizide.
Tetrahedron Rett. 32: 2793-2796
Gooding, C.M.B. (1966) Fate of chlorinated organic pesticide residues
in the production of edible vegetable oils. Chem. Ind., 1966: 344
Herrick, G.M., Fry, J.I., Fong, W.G., Golden, D.C. (1969) Insecticide
residues in eggs resulting from the dusting and short-term feeding of
low levels of chlorinated hydrocarbon insecticides to hens. J. Agr.
Fd. Chem., 17: 291-295
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rats for 224 days. Dept. of Zoology, Univ. of Illinois,
18 Oct. 1965, submitted by Velsicol Chemical Corporation
Ingle, L. (1969) Albino rats subjected to alpha and gamma chlordane
incorporated into the daily diet for 78 weeks. Unpublished report from
the University of Illinois
IUPAC. (1967) Commission on Terminal Residues. 1st Meeting. J. Assoc.
Offic. Anal. Chemists, 50: 1071-1088
IUPAC. (1968) Commission on Terminal Residues. 2nd Meeting. J. Assoc.
Offic. Anal. Chemists, 51: 372-378
IUPAC. (1969) Commission on Terminal Residues. Terminal chlordane
residues. 3rd. Meeting. J. Assoc. Offic. Anal. Chemists, 52: 300
IUPAC. (1970) Commission on Terminal Pesticide Residues. 4th Meeting.
Fate of organochlorine pesticides in vegetable oil processing. Comptes
Rendus-XXV Conference IUPAC p. 187-189. Also J. Assoc. Offic. Anal.
Chemists, 53: 987-1003, 1970
Korte, F. (1967a) Metabolism of 14C-labelled insecticides in
micro-organisms, insects and mammals. Botyukaguku, 32: 46-59
Korte, F. (1967b) Metabolism of chlorinated insecticides. Paper
submitted to IUPAC Commission on Terminal Pesticide Residues. Appendix
VI of the proceedings of the Commission, Vienna
Korte, F. and Porters P.E. (1970) Terminal residues of cyclodiene
insecticides. Meeting of IUPAC Commission on Terminal Residues
Erbach/Rheingau, 14-18 Sept. 1970
Lawrence, J.H., Barron, R.P., Chen, J-Y.T., Lombardo, P. and Benson,
W.R. (1970) Note of identification of a chlordane metabolite found in
milk and cheese. J. Ass. off. anal. Chem., 53: 261-262
Lichtenstein, E.P., Schultz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period, translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, B.J., Stadelman, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews 29: 61-72
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the detection of pesticide residues. Int. Atomic
Energy Agency Vienna p. 49-58
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Entomol., 45: 452-456
Martin, R.J. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III) Pesticide Monit. J., 1, 4: 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on synthetic (X) in male and female albino rats.
Unpublished report from Industrial Bio-Test Laboratories submitted to
Velsicol Chemical Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories submitted by Velsicol Chemical
Corporation. (IBT no.A7367), 23 June 1969
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories submitted by Velsicol
Chemical Corporation (IBT no.A7518) 21 August 1969
McGuire, R.R., Zabrik, M.J., Schuetz, R.D. and Flotard, R.D. (1970)
Photochemistry of bioactive compounds. J. Agr. Fd. Chem., 18:
319-321
Miles J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
Econ. Entomol., 62: 1334-1338
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugar beets. J. Econ.
Entomol., 63: 1143-1146
Plank, J., Keplinger, M.L., Fancher, O.E. and Richter, W.R. (1970)
90-day subacute oral toxicity of synthetic "X" (oxychlordane) in
albino rats. Unpublished report from Industrial Bio-Test Laboratories
submitted to Velsicol Chemical Corporation
Polen, P.B. (1966) Chlordane: composition, analytical consideration
and terminal residues. IUPAC Commission on Terminal Pesticide
Residues, Geneva
Polen, P.B. (1968) Terminal residues in chlordane. Status Rept. 2.
Chlordane Working Party. IUPAC Commission on Terminal Pesticide
Residues
Polen, P.B. (1969) Report of chlordane working party to the third
meeting of the IUPAC Commission on Terminal Residues. J. Ass. Offic.
Anal. Chem., 52: 300
Polen, P.B., Hester, M. and Benziger, J. (1970a) Characterization of
oxychlordane, animal metabolite of chlordane. Bull. environm. Contam.
Toxicol., 5: (3)
Polen, P.B. (1970b) Terminal residues of chlordane. Meeting of IUPAC
Commission on Terminal Residues, Erbach/Rheingau, 14-18 Sept.
Poonawalla, N.H. and Korte, F. (1964) Metabolism of insecticides VIII.
Excretion, distribution and metabolism of -chlordane-14C by rats.
Life Sci., 3: 1497-1500
Riemschneider, R. (1969) The chemistry of the Insecticides of the
Diene Group. World Rev. Pest Control Part 2 4: 29-61
Rosen, J.D., Sutherland, D.J. and Khan, M.A.Q. (1969) Properties of
photo-isomers of heptachlor and isodrin. J. Agr. Fd. Chem., 17:
404-405
Saha, J.G. and Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide
and trans-chlordane residues in soil and rutabaga after soil and
surface treatments with heptachlor. Can. J. Plant Sci., 47: 79-88
Saha, J.G., Burrage, R.H., Nielson, H.A. and Simpson, E.C.R. (1968)
Chlordane: composition of commercial formulation residues in potatoes
grown in treated soil and their reduction by home processing.
Pesticide Progress Reports from Research Branch Establishments
(Canada), 6 (5): 117-121
Saha, J.G. (1970) Unpublished data
Schwemmer, B., Cochrane, W.P. and Polen, P.B. (1970) Oxychlordane,
animal metabolite of chlordane: isolation and synthesis. Science,
169: 1087
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970a) Alpha-gamma
chlordane milk residue study in cows. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970b) -1970- Alpha-gamma
chlordane milk residue study in goats. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Smith, K.J., Polen, P.B., de Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chemists Assoc. 45:
866-869
US Dept. of Health, Education and Welfare Food and Drug
Administration. (1968) Pesticide analytical manual Vol. I, Washington
D.C.
Velsicol. (ed. P.B. Polen) (1970) Chlordane: agricultural residues and
related toxicology prepared for the 1970 Joint FAO/WHO Meeting on
Pesticide Residues by Velsicol Chemical Corporation
Vollner, L., Klein, W. and Korte, F. (1969) Beiträge zur ökologischen
Chemie, Photoumlagerung der Komponenten des technischen Chlordans.
Tetrahedron Lett., 34: 2967-2970
Wazeter, F.X., Butler, R.H., Geil, R.G. and Rehkamper J.A. (1968)
Alpha chlordane, gamma chlordane, alpha-gamma chlordane. Comparative
acute oral toxicity (LD50) in male albino rats. Unpublished report
from International Research and Development Corporation for Velsicol
Chemical Corporation
Chromatograms of technical chlordane using, above, the letter
designation of Velsicol (Polen, 1966) and below, that of USFDA (US
Dept. of Health, Education and Welfare, Food and Drug Administration,
1968).
TABLE II
Chlordane chromatographic peaks:
correlation of letter designations
Velsicol 1/ USFDA 2/
C A
D B
E C
F D
H 3/ E 3/
I 4/ F 4/
K G
1/ Polen, 1966
2/ US Dept. of Health, Education and Welfare, Food and Drug
Administration, 1968
3/ Peak for trans-chlordane
4/ Peak for cis-chlordane
No further experimental information is available to enable complete
elucidation of the significance of liver hypertrophy or the
stimulation of liver microsomal activity following administration of
chlordane. The significance of these changes was discussed in the
previous monograph on chlordane (FAO/WHO, 1968).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Rat
In the 78-weeks feeding study in rats reviewed under "Long-term
studies", no tumours were evident when dietary levels up to 35 ppm of
the cis- or 75 ppm of the trans-isomer were fed (Ingle 1969).
There is no report that tumours have been looked for in any other
species fed chlordane.
Special studies on the metabolite "oxychlordane"
Rat
A group comprising two male and two female rats was fed a level of the
chlordane metabolite "oxychlordane" in their diet, sufficient to
maintain a daily intake of 2.0 mg/kg body-weight. The compound was fed
for 90 days, after which time the surviving animals were sacrificed
for autopsy. Body-weight increase, food consumption, mortality,
organ-weights and organ to body-weight ratios, behaviour, haematology
and blood chemistry and urologic studies were all considered to be
within the normal range for the strain of rat used. No gross
pathological abnormalities were evident, and no histopathological
lesions could be attributed to "oxychlordane". A parallel control
Group of rats fed a normal diet does not appear to have been examined
in this experiment (Plant et al., 1970). Feeding studies with
"oxychlordane" utilizing a larger number of animals are reported to be
in progress (Calo, 1970).
Special studies on the possible metabolite 1-hydroxychlordene
Rat
Groups of 25 males and 25 females were fed dietary levels of 0, 100,
250, 500, 1000, and 2000 ppm of 1-hydroxychlordene for up to 224 days.
After 110 days, three females from each feeding level were mated with
males at all levels. Mortality in all groups was low, and no real
differences existed. At 224 days, autopsy revealed no gross
abnormality. Histopathology of all visceral organs was negative,
except for the slight to moderate cytoplasmic margination of a few
liver cells at 1000 and 2000 ppm (Ingle, 1965).
Special studies on photodecomposition
Rat
No information is available on the mammalian toxicity of the
photoisomer of chlordane.
Acute toxicity
The acute toxicity of the two isomers of chlordane and some
metabolites is summarized in Table III.
Rats were fed for 28 days from weaning on either (a) a diet containing
3.5 percent protein as casein; (b) a diet containing a normal amount
of protein as casein or (c) a standard laboratory diet. A single oral
TABLE III
Acute toxicity of chlordane isomers and metabolites
LD50 mg/kg
Compound Animal body weight Reference
(oral)
cis-chlordane Rat (M) 392 Wazeter et al., 1968
trans-chlordane Rat (M) 327 Wazeter et al,, 1963
cis-trans-chlordane Rat (M) 371 Wazeter et al., 1968
(1:1 by weight)
oxychlordane Rat (M,F) 19.1 Kastri et al., 1969 a
chlordane Rat (M,F) >4600 Nastri et al., 1969 b
3-chlorochlordene Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxychlordene Rat (M,F) >4600 Mastri et al., 1969 b
chlordene epoxide Rat (M,F) >4600 Mastri et al., 1969 b
1-hydroxy 2,3-epoxy Rat (F) >4600 and Mastri et al., 1969 c
chlordene <10200
2 chlorochlordene Rat (F) >10200 Mastri et al., 1969 c
dose of chlordane was administered after the feeding period. The LD50
values for the three groups were 137, 267, or 311 mg/kg body-weight,
respectively. Clinical symptoms and pathology were the same in all
groups (Boyd and Taylor, 1969).
Long-term studies
Rat
Groups, each of 20 male and 20 female rats were fed dietary levels of
0, 5, 15, 25 or 35 ppm of cis-chlordane or 15, 25, 35 or 75 ppm of
trans-chlordane or 5, 15, 25, 35 or 50 ppm of a 1 : 1 mixture of
cis and trans-chlordane. In the group fed cis-chlordane, growth
retardation became apparent in the group fed 35 ppm after four months
in the males and after five months in the females; with
trans-chlordane, the 75 ppm group of males only displayed growth
retardation after eight months. With the mixture, growth retardation
was evident in both sexes fed 50 ppm, beginning early in the males and
later in the females. Growth retardation was not evident in any groups
fed lower doses of either isomer. Food consumption bore a relationship
to growth. Increased mortality for both sexes became significant in
the groups fed 35 ppm of cis-chlordane, 75 ppm of trans-chlordane
or 50 ppm of the cis-trans mixture.
Haematocrit was normal for all test groups. Autopsy revealed no gross
pathological lesion, in particular there was no evidence of tumours.
Histological examination of all organs showed no changes from feeding
chlordane at any level, except in the case of the liver. Compression
of sinusoids due to slight hepatic cell hypertrophy in the
centrolobular region and minimal bile duct proliferation were evident
at 35 ppm of cis-chlordane. The above were noted, but were minimal,
at 25 ppm of the same isomer. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region, minimal
perinuclear vacuolation and minimal cell hypertrophy with constriction
of sinusoids were noted at 75 ppm of the trans-chlordane. Slight
cytoplasmic homogeneity of hepatic cells in the centrolobular region
and occasional cytoplasmic margination were observed at 50 ppm of the
cis-trans mixture. The above alterations were minimal at 35 ppm of
the same mixture. No liver abnormalities were evident after feeding
lower levels of the chlordane isomers (Ingle, 1969)
COMMENT
The study on cis and trans-isomers of chlordane, which was
considered desirable at the 1967 Joint Meeting, has now been completed
and indicates that the no-effect levels of the two isomers and of the
technical product are all within the same range, The toxic metabolite,
oxychlordane, which becomes stored in the fat or excreted in the milk
of lactating animals fed high doses of chlordane, does not appear to
be a residue in plants. For this reason, the toxicity of oxychlordane
was not considered to be a matter of concern. No information is
available on the mammalian toxicity of the photoisomer of chlordane. A
satisfactory reproduction study in one species has been previously
reported and is considered adequate. However, in view of the
observations from other organochlorine compounds, an adequate
carcinogenicity study in another species is needed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 20 ppm of technical chlordane in the diet, equivalent to 1 mg/kg
body-weight/day
Dog: 3 ppm of technical chlordane in the diet, equivalent to 0.075
mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Several million pounds of chlordane have been used annually in a broad
spectrum of applications in the U.S.A. for nearly 25 years. Outside
the U.S.A. its use is somewhat less, but comparable.
Velsicol (1970) has presented the approximate distribution of use of
chlordane outside of the continental United States of America as
follows:
Area Percentage
Europe 55
North America (excludes U.S.A.) 15
South America 15
Asia 10
Africa 5
Total 100
In the U.S.A. about half and outside the U.S.A. about 10 percent of
chlordane usage is estimated to be non-agricultural (household pest
control, lawn protection, etc.)
Chlordane is used in protection of the following crops:
Europe: Potatoes, vegetables, small grains, sugar beets
and sugar cane.
Western hemisphere: Sugar cane, maize, small grains, potatoes and
vegetables.
Asia: Rice, cotton and jute.
Other uses of chlordane are in the protection of fruits, nuts and oil
seed crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A substantial amount of new information on the residues resulting from
supervised trials is made available to the Working Party. Present
application of electron capture gas chromatography on chlordane
residues has allowed fairly detailed evaluation of several
constituents of the residues. The available new information is
summarized in Table IV. The data are based on the records of Velsicol
Chemical Corporation (1970).
Evidence of residues in food in commerce or at consumption
In a series of papers (Duggan et al., 1966, 1967; Duggan and
Weatherwax, 1967; Duggan and Lipscomb, 1969) the pesticide residue
levels have been reported in 11 food and feed categories, as raw
agricultural products or ready-to-eat foods, domestic and imported,
and they have assessed the dietary intake during 1964 to 1968 in the
United States. The observations on chlordane levels are summarized in
Table V.
Chlordane was undetected in all but three categories of raw
agricultural products where levels were <0.005 ppm and at maximum
incidence of 3.3 percent in imported grain. Ready-to-eat foods in
these three categories contained less (in one category) or undetected
residues.
Chlordane residues found in vegetable oil seeds, oils and byproducts
are summarized in Table VI. The traces of chlordane were reported in
less than 1 percent of the sample of soybeans, but none appeared in
the finished oil. Chlordane residues were reported in 3.7 per cent of
the crude maize oil samples, but were not found in the finished oil. A
small number of samples of crude cottonseed oil showed occasional
residues of chlordane, but the concentration in finished oil was lower
by about 59 percent. No chlordane residues were observed in
oleomargarine or in the total diet composites consisting of salad
dressings, salad oils, mayonnaise, shortenings and peanut butter
(Duggan, 1968 a, 1968 b). These empirical observations corroborate the
evidence that commercial processing removes chlordane residues, if
present, from edible vegetable oils and oil products.
From 750 meal cake samples (Table VI), chlordane was detected in 0.7
percent of the soybean cakes and in 6.5 percent of the cottonseed
TABLE IV
Summary of maximum observed chlordane residues1,2
Treatment
maximum rate
Root vegetables lb/acre ppm
Parsnip 10 0.07
Potato 10 0.23
Rutabaga 10 0.5
Sugar beets (root) 10 0.11
(wet pulp) 10 0.15
(dried pulp) 10 0.3
(juice) 10 0.063
Sweet potato 10 0.04
Leafy and stalk vegetables
Asparagus 44 0.07
Broccoli 44 0.063
Brussel sprouts 44 o.36
Cabbage 44 0.09
Celery 124 0.063
Cauliflower 44 (foliar) 0.06
Mustard greens 10 0.07
Spinach 10 0.10
Swiss chard 10 0.07
Other vegetables
Snap beans 10 0.01
Grains
Barley (Grain) 104 (foliar and soil) 0.063
" (straw) 104 0.42
Oats (grain) 10 0.063
" (straw) 10 0.10
Rye (grain) 10 0:063
" (straw) 10 0 07
Wheat (grain) 4 (foliar) 0:063
" (straw) 4 ( " ) 0.38
" (grain) 10 0:063
" (straw) 10 0.10
Rice (grain) 10 (soil or seed dressing) 0.033
" (straw) 10 0.033
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Grains
Maize and "popcorn" (seed) 10 0.063
" " " (stalks and
leaves) 10 0.033
Sorghum (grain) 12 (soil or seed dressing) 0.063
" (stalks) 12 ( " ) 0.07
Cucurbits
Cantaloupe 34 (foliar) 0.1
Cucumber 34 ( " ) 0.063
Pumpkins (pulp) 44 ( " ) 0.063
" (rind) 44 ( " ) 0.10
" (pulp) 10 0.08
" (rind) 10 0.15
Squash 34 (foliar) 0.09
Watermelons 34 ( " ) 0.063
Fruits and nuts
Almonds 10 0.0063
Figs 10 0.0033 5
Filberts 10 0.0033
Olives 10 0.0063
Pecans 10 0.048
Pomegranate 10 0.0063 5
Walnuts 10 033 5
Bananas 10 0.033
Guavas 10 0 036
Mangoes 10 0:013 5
Passion fruit 10 0.035
Papayas 5 0.01
Oil seed crops
Cotton (seed) 1-2 (foliar, up 12 0.22
applications
(meal) 1-2 ( " ) 0.07
(crude oil) 1-2 ( " ) 0.11
(soap stock) 1-2 ( " ) 0.063
(stalks) 1-2 ( " ) 0.29
TABLE IV (cont'd)
Treatment
maximum rate
lb/acre ppm
Oil seed crops
Linseed (seed) 34 (foliar) 0.22
(meal) 34 ( " ) 0.09
(crude oil) 34 ( " ) 0.24
Soybeans (beans) 124 0.38
(crude oil) 124 0.42; 0.72
Rape (whole plants) 3 (foliar) 0.35
1 From soil treatment except where indicated; data not previously
published.
2 Sum of six constituents of technical chlordane.
3 Negative reading - value shown is sum of estimated lower limits of
sensitivity components.
4 Exceeds maximum rate permitted in U.S.A.
5 Shell or meat analyzed separately.
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large fruit N2 - N - N -
Small fruit N - N - N -
Grain & cereals
For human use 0.7 <0.005 3.3 <0.005 N -
For animal use N - N - - -
Vegetables
Leaf and stem N - N - N -
Vine and ear 2.0 <0.005 0.3 <0.005 2.7 <0.001
Root 1.9 <0.005 0.3 <0.005 N -
Potatoes - - - - N -
Beans N - N - N -
Eggs N - N - N -
Nuts N - N - N -
Processed meat
(canned frozen,
etc.)
Domestic N -
Imported N -
TABLE V
Chlordane residues in food and feed.1
Raw agricultural product
Food category Domestic Import Ready-to-eat food
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Fluid milk
(fat basis) N -
Dairy products
(fat basis) N - N -
1 Extracted from USA-FDA surveillances programme report (Duggan, 1968b)
2 Not tabulated in reference, presumably because of insignificant
frequency of incidence
TABLE VI
Chlordane residues in vegetable oil seeds, oils and byproducts1
Crude oil Meal (cake) Edible oil
Product Number of Raw product
samples Incidence Average Incident Average Incident Average Incident Average
analysed percent ppm percent ppm percent ppm percent ppm
Soybeans 550 0.9 <0.001 - 2 - 2 0.7 <0.001 - 2 - 2
Groundnuts 177 - 2 - 2 - 2 - 2 - 2 - 2 - 2 - 2
Cottonseed 23 8.7 0.004 2.2 0.017 6.5 0.012 2.4 0.007
Maize 819 - 2 - 2 3.7 0.080 - 2 - 2
Total and
weighted 1569 0.4 <0.001 2.0 0.042 0.9 3 <0.001 3 0.04 <0.001
averages
1 Extracted from USA-FDA surveillance programne reports (Duggan, 1968a)
2 Not detected
3 Estimates based upon 750 samples for which cake data are given
cakes at average levels less than 0.001 and 0.012 ppm, respectively.
Of all the samples, 0.9 percent contained a detectable level (<0.001
ppm) of chlordane.
Dietary intake (milligrammes/day) generally appeared to be too low to
quantitate. Except for <0.001 mg/day (<1.7 percent of the ADI for a
60 kg man) from legumes during June 1966 to April 1967, no other
figures are listed for chlordane intake by Duggan and Lipscomb (1969).
The total diet studies in England and Wales (Abbott et al., 1969)
reveal no residues of chlordane.
FATE OF RESIDUES
In animals
An animal metabolite of chlordane, "oxychlordane", first reported in
1968 to the IUPAC Commission on Terminal Residues as an analytical
manifestation (IUPAC, 1969) has been isolated from pigs (Schwemmer et
al., 1970) and from milk and cheese (Lawrence et al., 1970). The
metabolite has been characterized with respect to analytical
parameters, propensity for occurrence in milk and storage in the fat
of dogs, cattle (beef and dairy) pigs and rats (Polen, 1970).
"Oxychlordane" levels in milk and some tissues of cows and goats,
following termination of ingestion of 10 ppm doses of chlordane
isomers for ten days, has been studied (Velsicol, 1970). Maximum
residues of "oxychlordane" found in milk were as follows:
Isomer fed Cow Goat
cis-chlordane 0.011 ppm 0.029 ppm
trans-chlordane 0.049 ppm 0.067 ppm
cis-+trans-chlordane 1:1 0.048 ppm 0.040 ppm
Highest milk level vs. feed level: goat 0.7 percent; cow 0.5 percent.
The residue levels in milk following termination of feeding of
chlordane declined to half in 10 to 12 days in cows and in 8 to 15
days in goats. Fifty days after chlordane withdrawal, the fat tissue
of cows contained up to 0.25 ppm and (2.5 percent of feeding level),
that of goats up to 0.4 ppm (4 percent of the feeding level) of
"oxychlordane". Some of this metabolite was occasionally found in
liver, but none in brain. The parent chlordane isomers could sometimes
be found in milk on the tenth day of feeding, but later none was
detected either in milk or in tissues (Singh et al., 1970 a, 1970 b).
At feeding levels of chlordane corresponding to proposed international
tolerances, oxychlordane is not detected in milk (sensitivity 0.005
ppm) but may be found in the fat of cattle at or below 0.03 ppm
(Polen, 1970).
When calves were fed for one month, one sixth of their ration, sugar
beet pulp derived from chlordane treated soil (Velsicol, 1970), the
maximum total residues found in the fat were, when fed with pulp from
the chlordane treatment year, up to 0.62 ppm and, when fed with pulp
from a treatment done a year prior to harvest, up to 0.06 ppm. Rates
of chlordane applications were 6-10 lb active ingredient per acre. The
total residue of the dry pulp varied from 0.05 to 0.30 ppm. The
residues found in liver were much less than those in fat, and none was
found in muscle (sensitivity 0.01 ppm).
When cows were fed with rations containing potato and alfalfa grown in
chlordane treated soil (Velsicol, 1970), no detectable residues were
found. Residue of heptachlor, heptachlor epoxide, compounds C and E
(minor components of technical chlordane), cis-chlordane and
trans-chlordane were all less than 0.01 ppm.
Hens fed 0.08 ppm of chlordane in their ration produced eggs with no
detectable residues (Herrick et al., 1969).
In plants
Korte and Porter (1970) reported on the metabolism of
trans-chlordane in white cabbage and carrots. Tests were under
glasshouse conditions and are difficult to relate to practical
conditions.
White cabbage leaves, four weeks after foliar application of 14C
labelled trans-chlordane, contained less than 1 percent of the
radioactivity on the surface; 90 percent was in the leaves as three
conversion products, in addition to the parent compound. At ten weeks,
20 percent of the applied radioactivity remained, 80 percent of which
was from metabolites. Soil treatment of carrots with 14C-labelled
trans-chlordane resulted in 0.01 ppm in carrots (mainly metabolites)
and 0.06 ppm in leaves (two thirds of the parent compound) 12 weeks
after treatment.
Polen (1970 a, 1970 b) reports that all the evidence accumulated thus
indicates that "oxychlordane" is not normally present in the terminal
residues of chlordane in plants.
In soils
Miles at al. (1969) found in their study on metabolism of heptachlor
by soil micro-organisms that heptachlor epoxide, 1-hydroxychlordene,
1-hydroxy- 2,3-epoxychlordane and possibly 1-keto-2,3-epoxychlordane
are formed, but their importance under field conditions after
chlordane treatment has not been established.
Onsager et al. (1970) did not detect heptachlor epoxide in
chlordane-treated soil (or in sugar beets grown therein). The
biological half life of chlordane was estimated to be 14.3 months, in
good agreement with other published data.
Lichtenstein at al. (1970) reported that soils treated for five years
annually with technical heptachlor (25 lb heptachlor and about 7.5 lb
chlordane as an impurity - cumulative basis) contained 0.019 ppm of
chlordane isomers (in addition to heptachlor derivatives) ten years
after the first treatment.
Polen (1970 a, 1970 b) reports that "oxychlordane" has not been found
in the terminal residues of chlordane in soils.
Photochemical transformations
The possibility of conversion of constituents of residues from
technical chlordane by ultraviolet light in vitro has been
demonstrated by several workers (Fischler and Korte, 1969; Vollner et
al., 1969; Benson et al., 1969; Rosen et al., 1969; McGuire et al.,
1970). Present evidence indicates that the photochemical
transformation compounds do not contribute significantly, if at all,
to terminal chlordane residues under agricultural conditions.
Observations of the IUPAC Chlordane Working Party (IUPAC 1969) on gas
liquid chromatograms from foliar applications on beans and cabbage
revealed no new peaks during the ageing of the residues and hence gave
no indication of photolytic products. A more recent investigation
directed specifically toward evaluation of the effects, if any, of
sunlight on chlordane residues also found no significant indications
of the production of photolytic products under typical field
conditions (Polen, 1970).
Pure preparations of compounds which are possible constituents of
chlordane terminal residues (cis- and trans-chlordanes, chlordene,
heptachlor, heptachlor epoxide and nonachlor) wore exposed to sunlight
as films on glass, on wheat and on soil surfaces. On glass after 235
hours of exposure, only heptachlor epoxide and cis-chlordane were
converted, constituting respectively 54 and 1.6 percent of the
terminal residues. After 39 days of exposure, no photoreaction
products were detected on soil; on wheat (2 lb chlordane per acre,
foliar application) only photo-cis-chlordane was detected as 2
percent of the terminal residue.
In another set of observations, 30 randomly-selected crop and soil
samples from supervised field trials throughout the U.S.A. were
examined for presence of photo products in chlordane residues.
Application rates ranged from four to ten lb of technical chlordane
per acre, soil or foliar applications; sampling intervals were from 15
to 303 days after application. Samples were: grass; straw of rice and
rye; seeds of rice and cotton; potato culls; soil from chlordane
treatments on which was grown barley or rice; sugar beets; whole and
spent cossettes; spinach; cauliflower and broccoli. Generally, no
photo products were detected at a sensitivity level of 0.01 ppm. It
was concluded that typically photo products are not produced in
chlordane soil treatments and that, if present at all in terminal
residues from foliar treatment, the contribution to the terminal
residue is less than 2 percent.
In storage and processing
With a few exceptions, accumulated evidence indicates that normal food
processing tends to reduce or eliminate chlordane residue. The lower
incidence and levels of residues in ready-to-eat foods compared to raw
agricultural products is evident in US Total Diet Studies, and was
commented on above.
In root crops, consistent evidence shows that chlordane residues from
treatments with either technical chlordane or technical heptachlor, in
which chlordane is a minor component, are concentrated in the peel,
and that peeling and/or cooking reduces or eliminates the residue.
In potatoes, peeling removes virtually the entire residue from
chlordane treatments up to 10 lb/acre. Without peeling, baking removed
about 80 percent of the residue and boiling up to 30 percent. (Saha et
al., 1968).
The residue of trans-chlordane from technical heptachlor treatments
of rutabagas was concentrated in the peel, and about half of that
residue was removed by boiling. The pulp was free of chlordane residue
before and after cooking (Saha and Stewart, 1967).
Similar patterns are observed in processing carrots, turnips and
beets. No residues of chlordane are detected in boiled pulp of turnips
or beets. In carrots, a 98 percent reduction resulted from peeling
only and 63 percent from boiling alone (Saha, 1970).
In commercial canning, washing, peeling (potatoes, tomatoes, etc.) and
abrasive peeling (used, for example, in preparing carrots) have been
demonstrated to reduce surface-concentrated pesticide residues (Farrow
at al., 1969). No doubt the surface-bound residues of chlordane would
respond in a similar way.
Chlordane residues on cabbage or beans are not changed in level or
composition by simple ten minute cooking (Polen, 1968). The lack of
the loss of chlordane residues were due to the fact that chlordane is
chemically relatively stable, co-distillation did not occur and the
cooking water containing extractable residue was included in the
sample extracted for residue analysis.
Commercial processing of edible vegetable oil produces finished oil
practically free of residues of chlordane, as well as other
organochlorine pesticides (Gooding, 1966; IUPAC, 1970; Smith at al.,
1968).
Samples of milk containing chlordane manufactured into condensed,
dried whole and evaporated milk were reduced in chlordane content. The
following figures are the percentage reduction in chlordane content
(fat basis) of processed fractions: condensed milk 11 percent; spray
dried milk 25 percent; evaporated milk 45 percent and drum dried milk
55 percent (Liska and Stadelman, 1969).
Wheat flour and rice, each contaminated by exposure to chlordane
vapours, were processed to produce two cooked products: a baked cookie
and boiled rice. The baked wheat flour cookie contained an average of
50 percent less residue than the wheat and the cooked rice about 73
percent less than the raw product (Bevenue and Yeo, 1969).
METHODS OF RESIDUE ANALYSIS
Analytical methods 1/
Prior to about 1961, residues from application of technical chlordane
were determined by total organochlorine, colorimetric or bioassay
techniques. The information derived was limited by lack of specificity
and sensitivity. At the present time, procedures based on the use of
electron capture gas chromatography are recommended. Four specific
analytical methods for the multi-component residues of chlordane are
available (Velsicol, 1970).
The methodology is that adopted for organochlorine pesticides by the
IUPAC Commission on Pesticide Residue Analysis and incorporates
procedures which favour analysis of chlordane residues in several
substrates. Detection limits are 0.002-0.02, depending on the nature
of the sample. Analytical method AM 0506 (Velsicol, 1970) is used for
soil in order to determine residue as chlordane, which is defined as
the sum of residues (ppm) of unchanged compounds from technical
chlordane plus residues of their conversion products, if present in
minor proportions. Conversion products are estimated separately if
they are present in significant amounts. Analytical method AM 0507
(Velsicol, 1970) is applicable to plant tissues and crops such as,
fruits, vegetables, grains, etc. The residue is obtained as a sum of
residues (ppm) of unchanged compounds from technical chlordane plus
residues of their conversion products, photolytic and metabolic, if
present in minor proportions. Conversion products are estimated
separately if they are present in significant amounts. Analytical
method AM 0509 (Velsicol, 1970) is applicable to milk, milk products
(e.g. butter and cheese), animal tissues and various animal food
products. Residue of chlordane is the sum of residues (ppm) of
unchanged compounds from technical chlordane plus possible residues of
their metabolites, oxychlordane and heptachlor epoxide. Method AM 0508
(Velsicol, 1970) is a supplementary method to describe modifications
to existing analytical methods in order to identify and quantitate
1/ Copies of the analytical methods and reference analytical grade
samples of the compounds are available from Velsicol Chemical Corp.,
341 E. Ohio Street, Chicago, Illinois 60611, U.S.A.
minor components of technical chlordane or of possible conversion
products (metabolic, photolytic or others). This method applies the
procedures of the other methods referred; e.g. the following compounds
can be determined: photo-cis-chlordane, photo-heptachlor epoxide,
heptachlor epoxide, "oxychlordane", photo-heptachlor and
photo-chlordane.
Quantitation of residues in plant and animal products
Several procedures have been used under various circumstances to
quantitate the gas-chromatographic responses of chlordane residues. In
most instances, the values calculated are good approximations of total
residues, but fail to distinguish conversion products from unchanged
components of technical chlordane. The methods AM 0506 to 0509
inclusive allow for quantitation of conversion products if present.
A method used prior to about 1968 relied upon calibration of the
principal peak (trans-chlordane) in reference technical chlordane
and estimation of the total residue by assuming constancy of the
proportions of the components in the course of "weathering". This
technique overstated the residue of chlordane by about 20-70 percent
for foliar treatments and by roughly half that amount for residues
from soil treatment in which conversion compounds may be in somewhat
greater concentration (Velsicol, 1970). These factors should be given
consideration in evaluating residue data generated before about 1968.
The estimation of chlordane residues in plants and soils as the sum of
cis-and trans-isomers (FAO/WHO, 1968) gives values roughly equal
to a minimum of 70-80 percent of the total terminal residues of
chlordane. In animal products, the sum of "oxychlordane" plus
cis-and trans-chlordane comprises generally more than 90 percent
of the residues in the lipoid phase.
APPRAISAL
A comprehensive set of new data for evaluation of chlordane residues
was provided to answer the questions raised by the 1967 Joint Meeting,
to add new information to the Monograph, to review the temporary
tolerances recommended in 1967 and to make new recommendations.
Technical chlordane continues to be a standardized product produced by
a single manufacturer. One of the characteristics of the technical
chlordane for which the recommendations are made is its content of
heptachlor (10 ± 2 percent).
Chlordane is used, in addition to the U.S.A., especially in Europe and
to a smaller extent in other American countries, Asia and Africa. Soil
treatments represent the main usage of chlordane. Foliar applications,
with exception of cotton, are not considered to be recommended usages
any more. The use to forage crops has been discontinued, e.g. in the
U.S.A.
Thorough studies on the chlordane residues in foodstuffs and in meal
cakes are conducted in the U.S.A. Residues were found, but in three
categories of raw agricultural products levels were less than 0.005
ppm. Oil seed cakes from soybeans and cotton seeds contained in 0.7
and 6.5 percent of the samples had residues with average levels of
less than 0.001 and 0.012 ppm, respectively.
Dietary intakes of chlordane in the U.S.A., England and Wales have
proved to be negligible. In most cases, the residues in the diet
composites were less than the analytical detection limits. Due to
this, it has not been possible to make reliable estimates of daily
chlordane intake.
Food processing tends to reduce or eliminate chlordane residues;
especially those processes which include removal of the treated crop
surface, disposal of cooking water, vacuum distillation or other type
of dehydration processes are able to remove a substantial part of the
residues.
Residues resulting from growing crops in land treated in previous
years with chlordane has been studied. It was found that the residues
in sugar beets, grown in the same land in several subsequent years,
were proportional (about 10 percent) to the residues in the soil at
the time of planting. Thus the chlordane level left in the soil seems
to determine the residue level found in crops. Stability of chlordane
in soil is well studied.
An animal metabolite of chlordane, oxychlordane, has been found in fat
of animals ingesting chlordane, but at significantly lower levels than
the level of chlordane in feed. Information for recommending practical
residue limits for meat, milk, poultry and eggs is available.
So far there is no evidence that oxychlordane is a component of
terminal chlordane residues in plants or in soils.
Photochemical transformation products of chlordane, which are reported
to be formed in vitro, are most evidently not formed in soils. In
foliar treated plants, if at all present, they are estimated to
contribute to the terminal residue less than 2 percent.
Hydrophilic metabolites of chlordane are formed in plants; their
significance is under investigation.
Analytical methods, based on the methodology generally adopted for
organochlorine pesticides, have been developed to determine the
multi-component residues of chlordane as well as their metabolites
with detection limits of 0.02-0.002 ppm. Clarification of the
nomenclature of chlordane isomers and the letter designations of
chlordane chromatographic peaks have been made.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The recommendations made now are for the residues which result from
pre-plant soil applications at a maximum level of 10 lb /acre or from
seed dressing, except for cotton which may require multiple foliar
applications, before the bolls open, at 1-2 lb /acre. Good
agricultural practice for sugar beets would require that the soil
would not contain, from the treatments of the planting and previous
years, more than 2.5-5.0 lb /acre. In cool climates, the application
rates should be established to lower levels to compensate the greater
stability of the residues.
For regulatory purposes, the chlordane residues in plant products
should be determined as the sum of cis- and trans-chlordane and in
animal products as the sum of cis-chlordane, trans-chlordane and
"oxychlordane".
In view of the new data for evaluating chlordane residues, the
recommendations made by the 1967 Joint Meeting for temporary
tolerances and practical residue limits are replaced by new
recommendations for tolerances and practical residue limits.
TOLERANCES
ppm
Root vegetables (practically soil-free) 0.3
Potatoes, sweet potatoes, rutabagas, turnips,
parsnips, sugar beets and radishes
Leafy and stalk vegetables 0.2
Asparagus, broccoli, brussel sprouts, cabbage, celery,
cauliflower, mustard greens, spinach, Swiss chard
and lettuce
Other vegetables 0.02
Beans, peas, eggplants, tomatoes and collards
(= cole-worts)
Grains 0.05
Wheat, rye, oats, rice (polished), maize, sorghum
and popcorn
Cucurbits 0.1
Cantaloupes, cucumbers, pumpkins, squash and watermelons
Fruits and nuts 0.1
Almonds, bananas, figs, filberts, guavas, mangoes,
olives, passion fruit, papayas, pecans, pomegranates,
pineapple, strawberries and walnuts
TOLERANCES (cont'd)
ppm
Citrus, pome and stone fruits 0.02
Not specified above
Vegetable oils
Crude soybean and linseed oils 0.5
Crude cottonseed oil 0.1
Edible cottonseed and soybean oils 0.02
PRACTICAL RESIDUE LIMITS
Milk and milk products (fat basis) 0.05
Fat of meat 0.05
Fat of poultry 0.05
Eggs (shell free) 0.02
FURTHER WORK OR INFORMATION
REQUIRED (before June 1972)
Further information on chlordane residues in carrots resulting from
soil and seed treatments and from crop rotation on soils having been
treated with chlordane in previous years.
DESIRABLE
1. An adequate carcinogenicity study in a second species of animal.
2. Results of the investigation of the hydrophilic plant metabolites
of chlordane.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J. Sci.
Fd. Agr., 20: 245-249
Benson, W.R., Lombardo, R., Barron, R., Lustig, E., Mastbrook, D. and
Ross, R. (1969) Paper at American Chemical Society Meeting, New York,
N.Y., Sept. 1969
Bevenue, A., and Yeo, C.Y. (1969) Effects of the cooking process on
the removal of chlordane residues from wheat flour and rice. Bull.
Environm. Contamination Toxic., 4: 370-374
Boyd, E.M., and Taylor, F.I. (1969) The acute oral toxicity of
chlordane in albino rats fed for 28 days from weaning on a
protein-deficient diet. Industr. Med., 38 (12): 42-49
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Büchel, K.H., Ginsberg, A.E. and Fischer, R. (1966)
Cyclodin-Insektizid IV. Synthese und Struktur von Isomeren des
Chlordans. Chem. Ber., 99: 421-430
Calo, D. (1970) Chlordane. Section I. Related toxicology. Unpublished
summary prepared by Velsicol Chemical Corporation.
Chau, A.S. and Cochraney W.P. (1969) Cyclodiene chemistry. I
Derivative formation for the identification of heptachlor, heptachlor
epoxide, cis-chlordane, trans-chlordane and aldrin pesticide
residues by gas chromatography. J. ass. off. anal. Chem.,
52: 1092-1100
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide Monit.
J., 2: 140-152
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide Residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed. Pesticide Monit. J., 1,2: 2-12
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Duggan, R.E. (1968a) Residues in food and feed. Pesticide Monit. J.,
1,4: 2-7
Duggan, R.E. (Ed.) (1968b) Residues in food and feed. Pesticide Monit.
J., 2: 2-46
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pesticide
Monit. J., 2: 153-162
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23 (1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1: WHO/Food Add./27.65
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Farrow, R.P., Elkins, E.R., Rose, W.W., Lamb, F.C., Ralls, J.W. and
Mercer, W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews, 29:
73-87
Fischler, H.M., and Korte, F. (1969) Sensibilisierte und
unsensibilisierte Photoisomerisierung von Cyclodien-Insektizide.
Tetrahedron Rett. 32: 2793-2796
Gooding, C.M.B. (1966) Fate of chlorinated organic pesticide residues
in the production of edible vegetable oils. Chem. Ind., 1966: 344
Herrick, G.M., Fry, J.I., Fong, W.G., Golden, D.C. (1969) Insecticide
residues in eggs resulting from the dusting and short-term feeding of
low levels of chlorinated hydrocarbon insecticides to hens. J. Agr.
Fd. Chem., 17: 291-295
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rats for 224 days. Dept. of Zoology, Univ. of Illinois,
18 Oct. 1965, submitted by Velsicol Chemical Corporation
Ingle, L. (1969) Albino rats subjected to alpha and gamma chlordane
incorporated into the daily diet for 78 weeks. Unpublished report from
the University of Illinois
IUPAC. (1967) Commission on Terminal Residues. 1st Meeting. J. Assoc.
Offic. Anal. Chemists, 50: 1071-1088
IUPAC. (1968) Commission on Terminal Residues. 2nd Meeting. J. Assoc.
Offic. Anal. Chemists, 51: 372-378
IUPAC. (1969) Commission on Terminal Residues. Terminal chlordane
residues. 3rd. Meeting. J. Assoc. Offic. Anal. Chemists, 52: 300
IUPAC. (1970) Commission on Terminal Pesticide Residues. 4th Meeting.
Fate of organochlorine pesticides in vegetable oil processing. Comptes
Rendus-XXV Conference IUPAC p. 187-189. Also J. Assoc. Offic. Anal.
Chemists, 53: 987-1003, 1970
Korte, F. (1967a) Metabolism of 14C-labelled insecticides in
micro-organisms, insects and mammals. Botyukaguku, 32: 46-59
Korte, F. (1967b) Metabolism of chlorinated insecticides. Paper
submitted to IUPAC Commission on Terminal Pesticide Residues. Appendix
VI of the proceedings of the Commission, Vienna
Korte, F. and Porters P.E. (1970) Terminal residues of cyclodiene
insecticides. Meeting of IUPAC Commission on Terminal Residues
Erbach/Rheingau, 14-18 Sept. 1970
Lawrence, J.H., Barron, R.P., Chen, J-Y.T., Lombardo, P. and Benson,
W.R. (1970) Note of identification of a chlordane metabolite found in
milk and cheese. J. Ass. off. anal. Chem., 53: 261-262
Lichtenstein, E.P., Schultz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period, translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, B.J., Stadelman, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews 29: 61-72
Ludwig, G. (1966) Isolation and identification of metabolites of some
chlorinated insecticides and their detection by analytical methods.
Radioisotopes in the detection of pesticide residues. Int. Atomic
Energy Agency Vienna p. 49-58
March, R.B. (1952) The resolution and chemical and biological
characterization of some constituents of technical chlordane. J. Econ.
Entomol., 45: 452-456
Martin, R.J. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III) Pesticide Monit. J., 1, 4: 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on synthetic (X) in male and female albino rats.
Unpublished report from Industrial Bio-Test Laboratories submitted to
Velsicol Chemical Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories submitted by Velsicol Chemical
Corporation. (IBT no.A7367), 23 June 1969
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969c) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories submitted by Velsicol
Chemical Corporation (IBT no.A7518) 21 August 1969
McGuire, R.R., Zabrik, M.J., Schuetz, R.D. and Flotard, R.D. (1970)
Photochemistry of bioactive compounds. J. Agr. Fd. Chem., 18:
319-321
Miles J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
Econ. Entomol., 62: 1334-1338
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugar beets. J. Econ.
Entomol., 63: 1143-1146
Plank, J., Keplinger, M.L., Fancher, O.E. and Richter, W.R. (1970)
90-day subacute oral toxicity of synthetic "X" (oxychlordane) in
albino rats. Unpublished report from Industrial Bio-Test Laboratories
submitted to Velsicol Chemical Corporation
Polen, P.B. (1966) Chlordane: composition, analytical consideration
and terminal residues. IUPAC Commission on Terminal Pesticide
Residues, Geneva
Polen, P.B. (1968) Terminal residues in chlordane. Status Rept. 2.
Chlordane Working Party. IUPAC Commission on Terminal Pesticide
Residues
Polen, P.B. (1969) Report of chlordane working party to the third
meeting of the IUPAC Commission on Terminal Residues. J. Ass. Offic.
Anal. Chem., 52: 300
Polen, P.B., Hester, M. and Benziger, J. (1970a) Characterization of
oxychlordane, animal metabolite of chlordane. Bull. environm. Contam.
Toxicol., 5: (3)
Polen, P.B. (1970b) Terminal residues of chlordane. Meeting of IUPAC
Commission on Terminal Residues, Erbach/Rheingau, 14-18 Sept.
Poonawalla, N.H. and Korte, F. (1964) Metabolism of insecticides VIII.
Excretion, distribution and metabolism of -chlordane-14C by rats.
Life Sci., 3: 1497-1500
Riemschneider, R. (1969) The chemistry of the Insecticides of the
Diene Group. World Rev. Pest Control Part 2 4: 29-61
Rosen, J.D., Sutherland, D.J. and Khan, M.A.Q. (1969) Properties of
photo-isomers of heptachlor and isodrin. J. Agr. Fd. Chem., 17:
404-405
Saha, J.G. and Stewart, W.W.A. (1967) Heptachlor, heptachlor epoxide
and trans-chlordane residues in soil and rutabaga after soil and
surface treatments with heptachlor. Can. J. Plant Sci., 47: 79-88
Saha, J.G., Burrage, R.H., Nielson, H.A. and Simpson, E.C.R. (1968)
Chlordane: composition of commercial formulation residues in potatoes
grown in treated soil and their reduction by home processing.
Pesticide Progress Reports from Research Branch Establishments
(Canada), 6 (5): 117-121
Saha, J.G. (1970) Unpublished data
Schwemmer, B., Cochrane, W.P. and Polen, P.B. (1970) Oxychlordane,
animal metabolite of chlordane: isolation and synthesis. Science,
169: 1087
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970a) Alpha-gamma
chlordane milk residue study in cows. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Singh, V.K., Perkins, C.T. and Koch, D.A. (1970b) -1970- Alpha-gamma
chlordane milk residue study in goats. Unpublished report from the
Bio-Toxicological Research Associates, 31 March 1970, submitted by
Velsicol Chemical Corporation
Smith, K.J., Polen, P.B., de Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chemists Assoc. 45:
866-869
US Dept. of Health, Education and Welfare Food and Drug
Administration. (1968) Pesticide analytical manual Vol. I, Washington
D.C.
Velsicol. (ed. P.B. Polen) (1970) Chlordane: agricultural residues and
related toxicology prepared for the 1970 Joint FAO/WHO Meeting on
Pesticide Residues by Velsicol Chemical Corporation
Vollner, L., Klein, W. and Korte, F. (1969) Beiträge zur ökologischen
Chemie, Photoumlagerung der Komponenten des technischen Chlordans.
Tetrahedron Lett., 34: 2967-2970
Wazeter, F.X., Butler, R.H., Geil, R.G. and Rehkamper J.A. (1968)
Alpha chlordane, gamma chlordane, alpha-gamma chlordane. Comparative
acute oral toxicity (LD50) in male albino rats. Unpublished report
from International Research and Development Corporation for Velsicol
Chemical Corporation
