
FAO/PL:1967/M/11/1
WHO/Food Add./68.30
1967 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Rome, 4 - 11 December,
1967. (FAO/WHO, 1968)
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1968
ENDOSULFAN
IDENTITY
Chemical Names
6,7,8,9,10,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-
benzodioxathiepin 3-oxide.
alpha,ß-1,2,3,4,7,7-hexachlorbicyclo-(2,2,1)-hepten-2-bisoxymethylene-
5,6 sulfite
Synonym
Thiodan
Empirical Formula
C9H6Cl6O3S = 406.95
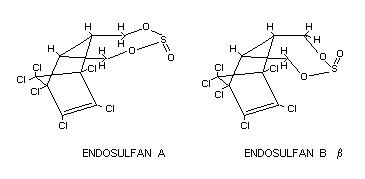
Structural Formulae
Technical endosulfan contains two stereoisomers, endosulfans A and B
in the proportion variously reported as from 4:1 to 7:3. The technical
material is a 90-95 per cent pure mixture of the two isomers.
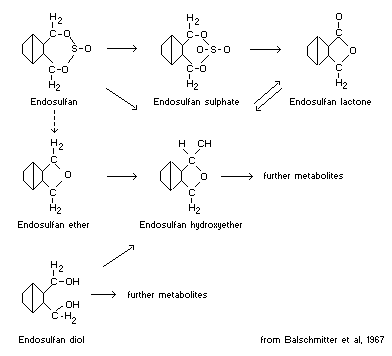
 Other relative chemical properties including metabolites
Balschmitter et al (1967) have considered all of the possible
metabolites of endosulfan which can hypothetically be formed on
hydrolysis, oxidation or reduction. They have investigated endosulfan
metabolism in the mouse and rat using thin layer and gas
chromatographic techniques and have identified five of the possible
metabolites, including endosulfan sulfate, diol, ether, hydroxyether
and lactone as illustrated in the following figure :
Other relative chemical properties including metabolites
Balschmitter et al (1967) have considered all of the possible
metabolites of endosulfan which can hypothetically be formed on
hydrolysis, oxidation or reduction. They have investigated endosulfan
metabolism in the mouse and rat using thin layer and gas
chromatographic techniques and have identified five of the possible
metabolites, including endosulfan sulfate, diol, ether, hydroxyether
and lactone as illustrated in the following figure :
 EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Endosulfan is rapidly absorbed from the intestinal tract and about 30
per cent of a lethal dose is eliminated during 24 hours in the rat
(Czech, 1958).
Leaves from apple trees sprayed with endosulfan contained an
ultraviolet irradiation product equal in toxicity to, and more
persistent than, the parent compound (Harrison, 1967).
Endosulfan given to mice in single doses or repeated daily for 49 days
was found in the tissues as the sulfate. When endosulfan, endosulfan
sulfate, the diol, or the ether were fed to mice, a metabolite
considered to be the diol appeared in the urine. Oral doses of the
isomers were partly excreted in the faeces unchanged, along with
endosulfan sulfate and the diol. No residue was found in blood or
brain, but traces of the sulfate appeared in kidney and muscle (Deema
et al., 1966).
When endosulfan was administered to rats, no unchanged endosulfan was
found in the urine. Two metabolites were found in rat urine 48 hours
after the animals were injected intraperitoneally with endosulfan;
these appeared to be conjugation products of the alcohol derivative of
endosulfan (Rahn, 1963).
Three female pigs weighing 31 to 39 kg were treated for 27, 54 and 81
days with technical endosulfan (97 per cent pure) in capsules with
their feed in an amount equivalent to 2 ppm in the diet. During the
test periods these animals received, respectively, total doses of 81.8
mg, 196.6 mg and 332.4 mg of endosulfan. At the end of each of those
treatments no endosulfan could be detected in any of 13 different
organs and tissues including liver and brain but excluding fat. In the
fat from five different areas only one of the isomers (endosulfan A)
was consistently found, at an average level of 0.06 ppm, with values
ranging from 0.01 to 0.1 ppm. No endosulfan B was detected in any of
the 15 fat analyses. Endosulfan sulfate was found in 3 of the 15
samples at levels of 0.04 to 0.05 ppm. The amount present after the
81-day trial was no higher than after 27 days. In a parallel test with
DDT administered to 3 pigs at a rate of 7 ppm in the diet, the level
in the fat was 8.3, 9.1 and 9.7 ppm, respectively, at the end of the
three test periods. In a further experiment, 5 pigs were given 2 ppm
endosulfan in their diet for 30 days. On the 11th day thereafter 0.002
ppm was found in one of five fat samples, and none was detectable
after 27 days (Maier-Bode, 1967a).
Milk cows fed 2 ppm endosulfan for 26 days excreted an average of 1
ppm unchanged in the faeces after the second day, or about 20 per cent
of the amount consumed. The milk contained 0.02 to 0.1 ppm endosulfan
sulfate and no unchanged material. The urine showed 0.1 to 1 ppm of
the alcohol derivative and was positive for an unidentified
metabolite. At the end of the test period no unchanged endosulfan was
detected in muscle, liver, kidney, brain or fat, though muscle
contained <0.01 ppm and fat 0.1 to 0.3 ppm of the sulfate (Gorbach,
1966).
When endosulfan was fed to 3 female pigs at the rate of 2 ppm for up
to 81 days, mean recoveries of endosulfan A and B and endosulfan
sulfate at 27, 54 and 81 days were 0.07, 0.09 and 0.04 ppm from body
fat but none was found in other tissues and organs examined. In
another experiment in which the pigs were fed endosulfan at 2 ppm for
30 days, the residue in the fat of those slaughtered on the last day
of feeding was 0.003 - 0.01 ppm, and the residues in the fat of those
slaughtered 11 days after the last administration was 0.002 ppm in 1
of the 5 samples and not detectable (<0.001 ppm) in the others
(Maier-Bode, 1967a).
Beef cattle that grazed for 31-36 days on Bermuda grass pasture
sprayed 7 days previously with endosulfan showed no endosulfan in
their body fat. No residue was found in the milk of cows fed silage
containing 0.41 to 2.35 ppm of endosulfan for 21 days. Two beef cattle
fed 5 and 2.5 mg/kg/day of endosulfan showed toxic effects in 2 and 13
days respectively. Treatment with 1.1 mg/kg/day produced no
intoxication, but resulted in residues in the fat (Beck et al., 1966).
Three geese were allowed to feed for 17 days on weeds in a strawberry
field sprayed twice, once before the trial and again after the first 7
days, with 50 per cent endosulfan wettable powder at 2-lb. per 100
gal. (amount per unit area not indicated). The geese showed no signs
of poisoning, and at the end of the 17-day feeding period no
endosulfan could be detected in the liver, kidney, fat or stomach
contents (Dustan, 1965).
Acute toxicity (technical grade)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 40-50 and 110* Hazelton Laboratories, 1957
Rat Intraperitoneal 8 Czech, 1958
* Dependent on the vehicle used
A dose of 15 mg/kg orally of purified endosulfan was estimated to be
an LD10 in mice (BALB/c strain). A dose of 20 mg/kg killed most of
the mice in 24 hours (Deema et al., 1966).
By oral administration in the rat the LD50 of endosulfan A is
reported to be 76 mg/kg, and of endosulfan B 240 mg/kg (Maier-Bode,
1967a).
Endosulfan is highly toxic to fish, lethal concentrations in the water
ranging from 0.001 to 0.0125 ppm for several species. In young birds
the LD50, in terms of ppm in the diet over a 10-day feeding period,
is reported as 270 ppm for the bobwhite, 620 ppm for the pheasant and
200 ppm for the mallard duck (Maier-Bode, 1967b).
Short-term studies
Rat. Rats tolerated daily 1.6 - 3.2 mg/kg body-weight orally for 12
weeks without any influence on growth-rate (Czech, 1958)
Dog. Endosulfan technical grade was administered daily in gelatin
capsules to 4 dogs for 3 days in a dose of 2.5 mg/kg body-weight.
Vomiting was observed in one dog and vomiting, tremors, convulsions,
rapid respiration, and mydriasis in 3 dogs. (Hazelton Laboratories,
1959).
Three groups of dogs each consisting of 2 males and 2 females were
given endosulfan orally in gelatin capsules 6 days a week for one year
in doses corresponding to 0.075, 0.25 and 0.75 mg/kg body-weight. No
signs of toxicity were observed. At autopsy gross and microscopic
examination of the tissues showed no difference between treated and
control animals (Hazelton Laboratories, 1959).
Long-term studies
Rat. Groups of 25 male and 25 female rats received 10, 30 and 100
ppm of endosulfan technical grade in the diet for 104 weeks. Survival
of the female rats in the 10-and 30-ppm groups was lower than that of
the female control group during the second year. In the 100-ppm female
group, survival was significantly lower after 26 weeks and
abnormalities were observed in weight gain and on haematological
examinations. At autopsy the relative weight of the testes in the
10-ppm male group was significantly lower than in the control group.
Consistent histopathological findings were apparent only in the
100-ppm male group. In these the kidneys were enlarged and there were
signs of renal tubular damage with interstitial nephritis. Hydropic
cells were seen in the liver. The tumour incidence was within normal
limits in all test groups (Hazelton Laboratories, 1959).
Comments
A number of studies on several species of animals have provided
evidence that endosulfan does not have cumulative properties. Failure
of endosulfan to accumulate in the body is also reflected in the
toxicity data on dogs, which showed marked toxic effects in 3 days
from 2.5 mg/kg/day but no effects from 0.75 mg/kg/day for one year.
Re-evaluation of the data for rats indicated that 30 ppm in the diet
can be considered a no effect level in this species.
TOXICOLOGICAL EVALUATION
Pending evaluation of the results on reproduction study in the rat,
the meeting was unable to set an ADI.
Further work required
Results of reproduction studies in the rat.
EVALUATION FOR TOLERANCES
USE PATTERN
Endosulfan use is approved in many countries for pest control in a
wide range of fruit and vegetables; it is also used on a number of
important non-food crops, e.g. cotton, tobacco. Various minimum
intervals between final application and harvest are in operation: for
European countries this varies from 15 to 42 days and averages about
30 days whilst in the United States intervals up to 30 days are used.
Endosulfan is not used in veterinary practice.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of over one thousand analyses, mainly using
microcoulometric gas chromatography, in field crop trials collected by
Hoechst are summarized in the following table. It is emphasized that
the dose-rates indicated have in many cases been chosen from a range
of dose-rates which have been studied and are in some cases higher
than the recommended dose rates. Whilst the information in the table
represents the summary of a much greater volume of data, the results
of individual trials have occasionally shown residues which are above
or below the general average levels indicated; however, the maximum
levels seldom exceed three times the general average levels indicated.
Only in a few cases have endosulfan sulphate residues been measured in
addition to endosulfan: for tomatoes about 0.1 ppm endosulfan sulphate
was found after 23 days and for alfalfa from 0.0 to 2.0 ppm
approximately although individual figures varied considerably.
Summary of Field Residue Data for Endosulfan (Hoechst papers)
(all values are in parts per million)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Alfalfa 1 0.5 0.3 different methods
Corn 4 <0.5 Normal rate 1
Peas 1 0.1
Potatoes 11 <0.1
Soybeans 2 <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Watermelons 2 <0.1
Artichokes 1 <0.1
Broccoli 2 0.1 - 0.3 0.1
Brussels sprouts 3 1.3
Cabbage 1 1.0 <0.1
Celery 1 3 8 (sic)
Collards 5 0.5
Cucumber 4 <0.1
Dried lima beans 3 0 <0.1 60 days
Green lima beans 2´ <0.1
Kale 8 1.0 Normal rate 1
Lettuce 2 2.0 (0.1) Variable
Pepper 2 <0.1
Spinach 1 0.5
String beans 1 <0.1
Tomatoes 3 <0.1 Normal rate 1
Apples 3 0.5 <0.1
Cherries 2 1 0.3
Blackcurrant 4 0.2 0.1
Prunes 2´ (b) 0.2 0.1
Peaches 2 0.3 Variable data
Pears 1´ <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Strawberries 3 0.3
Sugarbeets 1 <0.1
* These are not necessarily the recommended dose rates
(b) lb per 100 gallons
This summary shows that, with the possible exception of celery,
lettuce, spinach and strawberries, harvest residue levels are probably
normally less than 0.1 ppm. Maier-Bode (1967) gives further field
trial residue data for most of these commodities and states that
endosulfan is less persistent on plant surfaces than residues of DDT,
aldrin or toxaphene and that residues of endosulfan A disappear more
quickly than those of endosulfan B. He also states that up to 0.3 ppm,
but usually less than 0.1 ppm, of endosulfan sulphate has been found
on leaves and fruits and that this compound is toxicologically
equivalent to technical endosulfan. Other metabolites, including
endosulfan diol and endosulfan ether, can also be detected
occasionally in plants but not in crops intended for human
consumption. Maier-Bode concludes that less than 0.5 ppm of residue is
found in the field 2 to 3 weeks after normal application to a large
number of fruits and vegetables and fodder plants; but that residues
in grass or clover are not apparently reduced by ensilage or on drying
for hay. As a general conclusion, when endosulfan is used to protect
food crops against insect infestation in accordance with good
agricultural practice, residues in the treated produce are unlikely to
exceed 0.5 ppm at harvest, except possibly for lettuce, celery and
brassicae which should not exceed 2.0 ppm. Not all samples of these
commodities will contain such amounts of residue, in fact only a small
proportion of each individual commodity is likely to be treated.
RESIDUES IN FOOD AT TIME OF CONSUMPTION
There is only very limited evidence suggesting that residues do not
normally occur in total diet studies in the United States.
FATE OF RESIDUES
General considerations
Both endosulfan isomers are slowly hydrolysed in acid, alkaline and
neutral environment to endosulfan alcohol and sulphur dioxide.
Although in some ways resembling the cyclodienes structurally, the
degree of persistence of endosulfan residues is not so great as for
aldrin or endrin residues but is similar to that of lindane. Sunlight
(or artificial ultra-violet irradiation) gives rise to some
transformation to the more persistent compound endosulfan sulphate
(6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,
3-benzodioxathiepin 3,3-dioxide) (United Kingdom, 1963; Forman, et al,
1965), the toxicity of which is similar to that of endosulfan. The
sulphate itself is further degraded to endosulfan alcohol. Harrison et
al (1967) have shown in field trials on black-currant that, unless
deliberately oversprayed, the total residue of endosulfan A,
endosulfan B and endosulfan sulphate does not normally exceed 0.5 ppm
at harvest.
In animals
Endosulfan does not accumulate in animals. Sheep fed 15 mg endosulfan
daily for 26 days showed 0.05 ppm of endosulfan sulphate in the milk,
this being the limit of detection by the analytical method used.
Similar experiments with cows fed a diet containing 5 ppm each of
endosulfan and endosulfan sulphate showed only endosulfan sulphate
residues in the milk up to 0.16 ppm (Hoechst, 1965). When sows are fed
a diet containing 2 ppm endosulfan for 81 days, residues (found only
in the fatty tissue) do not exceed 0.1 ppm (Maier-Bode, 1968)
Residues of endosulfan have not so far been detected in human fat in
Britain whereas residues of all of the other commonly used
organochlorine compounds have been reported (United Kingdom, 1963).
This may be due to the relatively small use of endosulfan in Britain,
but even in the United States, where it in used on a much larger
scale, its presence in human fat has not been reported.
In storage and processing
Little work has been done on the investigation of residue losses on
washing and other preparation for consumption
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis for endosulfan have been reviewed by
Maier-Bode (1968). A number of multidetection systems are available
for the detection and determination of organochlorine compounds and
most of these can be applied to residues of endosulfan A and
endosulfan B (Terranova and Ware, 1963; Zweig et al, 1960; Carroll,
1962; Burke and Mills, 1963; Byers, et al, 1965). These methods also
include the AOAC system (1966) though this, as normally carried out,
may not detect endosulfan B. The methods can usually also be used,
with some modifications to extraction and clean-up conditions where
necessary, for the detection and determination of residues of
endosulfan sulphate, as described by Harrison et al, 1967. A number of
thin layer chromatographic systems have been described and are useful
in this connection (Junichi and Tetsukichi, 1962; a and b; Abbott et
al, 1964; Walker and Berosa, 1963; Kovacs, 1966; Balschmitter and
Tölg, 1966, a and b). A number of general points in connection with
multidetection systems of residue analysis are set out in the
introductory note on page 4. The methods are normally sensitive to
about 0.05 ppm of each isomer or of endosulfan sulphate. Both electron
capture and microcoulometric detection methods may be used and
alternative methods for the confirmation of the identity of residues
such as infra-red spectrophotometry are available (Forman et al,
1965). Less sensitive and less specific colorimetric methods of
residue analysis, based on alkaline hydrolysis to give sulphur dioxide
which is then reacted with p-rosaniline and formaldehyde (Mohoney,
1962; Zweig, 1964) or with methanolic potassium hydroxide and pyridine
(Butler, et al, 1962; Maitlen et al, 1963) have also been described.
Biological methods based on pyridine toxicity to fish (Romer, 1960) or
Drosophila (Varis and Tittanen, 1963; Beye, 1962; Huesman, 1961) are
also available.
Endosulfan metabolites can also be detected and determined by many of
the multidetection systems described above provided a suitable
extraction method is used: Maier-Bode (1968) suggests benzene or
benzene-isopropanol as extraction solvents. The gas chromatography of
the sulphate, ether, hydroxyether and lactone using three different
columns has been described by Ballschmitter and Töld (1966a).
Whilst some of these techniques may require further study before they
can be applied generally to residue determinations in crops and total
diets, it is recommended that wherever possible results of such
analyses should record separately endosulfan A, endosulfan B and
endosulfan sulphate.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Benelux 0.5 fruit and vegetables
Canada 1.0 fruit
2.0 vegetables
Fed.Rep. Germany 0.5 fruit and vegetables
Switzerland 0.5 strawberries
United States 2.0 apples, apricots, artichokes, broccoli,
cabbage, cherries, cucumbers, egg-plants,
grapes, lettuce, melons, nectarines,
peaches, peas, peppers, plums, prunes,
pumpkins, squash, strawberries, tomatoes,
watercress.
FAO/WHO RECOMMENDATIONS FOR TOLERANCES
No acceptable daily intake level is available so no recommendation for
tolerances can be made at present.
FURTHER WORK
Further work required before tolerances can be recommended
1. Submission of data required for an estimation of acceptable daily
intake (see page 138).
2. Fuller details of the purity and composition or technical
endosulfan and the determination of residues of endosulfan A,
endosulfan B and endosulfan sulphate in treated produce and total
diets by appropriate methods of analysis.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Beck, E.W., Johnson, J.C., Woodham, D.W., Leuck, D.B., Dawsey, L.H.,
Robbins, J.E. and Bowman, M.C. (1966) J. econ. Entomol., 59, 1444.
Czech, M. (1958) Medizin u. Chemie., 6, 574
Deema, P., Thompson, E. and Ware, G.W. (1966) J. econ. Entomol., 59,
546
Dustan G.G. (1965) Pesticide Progress 3 : 131
Gorbach, S. (1966) Unpublished report.
Harrison, R.B., Holmes, D.C., Roburn, J. and Tatton, O'G (1967)
J. Sci. Food Agr. 18, 10
Hazelton Laboratories, (1957) Unpublished report
Hazelton Laboratories, (1959) Unpublished report
Maier-Bode, H. (1967a) Archiv. f. Pflanzenschutz., 3 : 201
Maier-Bode, H. (1967b) Residue Review, 21 (in press)
Rahn, H.W. (1963) Arch. internat. Pharmacodyn., 144: 126
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Abbott, D.C., Egan, H., Thomson, J. (1964) Thin-layer chromatography
of organochlorine pesticides, J. Chromatog. 16 : 481
AOAC. (1966) Changes in Methods of Analysis, J. Assoc. Offic. Analyt.
Chem., 49 : 222 - 230.
Ballschmitter, K., Tölg, G. (1966a) Metabolisms des Thiodans in
Insekten. Angew. Chem. 78: 775.
Ballschmitter, K., Tölg, G. (1966b) Fluorescenzindikatoran zum
Nachveis von Organchalogeniden in der Dünnschicht chromatographie.
Z. anal. Chem. 215 : 305
Ballschmitter, K., Schophan, I. and Tölg, G. (1967) The metabolization
of endosulfan in insects and mammals. Paper presented to the VI
International Plant Protection Congress, Vienna.
Beye, F. (1962) Biodetection of insecticides in Coleus blumei with
Drosophila melanogaster. Anzeiger f. Schadlingskunde, 35 : 9 - 12.
Burke, J. and Mills, P.A. (1963) Microcoulometric gas chromatographic
determination of thiodan and tedion in green vegetables. J. Assoc.
Offic. Agric. Chem., 46 : 177 - 182.
Butler, L.I., Maitland, J.C. and Fakey, J.E. (1962) Microdetermination
of thiodan residues. J. Agric. Food Chem. 10 : 479 - 481.
Byers, R.A., Woodham, D.W. and Bowman, M.C. (1965) Residues on coastal
Bermuda grass, trash and soil treated with granular endosulfan.
J. Econ. Entomol. 58 : 160 - 161.
Carroll, C.C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Reviews, 1 : 37 - 65.
Forman, S.E., Durbetaki, A.J., Cohen, M.V. and Olefson, R.A. (1965)
Conformational equilibria in cydic sulphites and sulphates:
configurations of the two isomeric thiodans. J. Org. Chem., 30 : 169 -
175.
Harrison, R.B., Ruzicka, J.H.A. and Tatton, J. O'G. (1967) Endosulfan
residues on blackcurrants. J. Sci. Food Agric. 18 : 262 - 264.
Hoechst. (1965) Farbwerke Hoechst AG. Data sheet for
endosulfan/thiodan : Frankfurt am-Main.
Huesman, H. (1961) Z. angew. Zool., 48 : 1 - 29.
Junichi, Y. and Tetsukichi, N. (1962a) Identification of organic
chlorinated pesticides by plate chromatography. Proc. Japan Acad. 38 :
129
Junichi, Y. and Tetsukichi, N. (1962b) Separation and identification
of organic chlorinated pesticides by plate chromatography. Kagaku
Keisatsu Kantyusho Hokoku, 15 : 321.
Kovacs, H.F. (1966) The rapid detection of chlorinated pesticide
residues by an improved thin-layer chromatographic technique.
J. Assoc. Offic. Agric. Chem. 49 : 365.
Maier-Bode, H. (1967) Meded. Landb. Hoogesch. Opzoek. Stn. Gent, in
press
Maier-Bode, H. (1968) Endosulfan. Residue Reviews, 21 : in press
Maitlen, J.C., Walker, K.C. and Westlake, W.E. (1963) An improved
colorimetric method for determining endosulfan residues in vegetables
and beef fat. J. Agric. Food Chem., 11 : 416 - 418.
Mohoney, C.H. (1962) Flavour and quality changes in fruits and
vegetables in the United States caused by application of pesticide
chemicals. Residue Reviews, 1 : 11 - 23.
Romer, D. (1960) Determination of insecticide residues and assay of
lasting coatings. Anzeiger f. Schadlingskunde, 33 : 138 - 140.
Terranova, A.C. and Ware, G.W. (1963) Studies of endosulfan in bean
plants by paper and gas chromatography. J. Econ. Entomol., 56 : 596
- 599.
United Kingdom. (1963) Report of the Government Chemist, H.M.
Stationery Office, London. p.78
Varis, A.L. and Tittanen, K. (1963) Montalous Koetoiminta, 17 : 252
- 255.
Walker, K.C. and Berosa, M. (1963) Thin-layer chromatography for
insecticide analysis. J. Assoc. Offic. Agric. Chem. 46 : 250.
Zweig, G. (editor). (1964) Pesticides, Plant Growth Regulators and
Food Additives, Vol. II, p. 511 London, Academic Press.
Zweig, G., Archer, T.E. and Robenstein, D. (1960) Residue analysis of
endosulfan by combination of gas chromatography and infrared
spectrophotometry. J. Agric. Food Chem. 8 : 403 - 405.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Endosulfan is rapidly absorbed from the intestinal tract and about 30
per cent of a lethal dose is eliminated during 24 hours in the rat
(Czech, 1958).
Leaves from apple trees sprayed with endosulfan contained an
ultraviolet irradiation product equal in toxicity to, and more
persistent than, the parent compound (Harrison, 1967).
Endosulfan given to mice in single doses or repeated daily for 49 days
was found in the tissues as the sulfate. When endosulfan, endosulfan
sulfate, the diol, or the ether were fed to mice, a metabolite
considered to be the diol appeared in the urine. Oral doses of the
isomers were partly excreted in the faeces unchanged, along with
endosulfan sulfate and the diol. No residue was found in blood or
brain, but traces of the sulfate appeared in kidney and muscle (Deema
et al., 1966).
When endosulfan was administered to rats, no unchanged endosulfan was
found in the urine. Two metabolites were found in rat urine 48 hours
after the animals were injected intraperitoneally with endosulfan;
these appeared to be conjugation products of the alcohol derivative of
endosulfan (Rahn, 1963).
Three female pigs weighing 31 to 39 kg were treated for 27, 54 and 81
days with technical endosulfan (97 per cent pure) in capsules with
their feed in an amount equivalent to 2 ppm in the diet. During the
test periods these animals received, respectively, total doses of 81.8
mg, 196.6 mg and 332.4 mg of endosulfan. At the end of each of those
treatments no endosulfan could be detected in any of 13 different
organs and tissues including liver and brain but excluding fat. In the
fat from five different areas only one of the isomers (endosulfan A)
was consistently found, at an average level of 0.06 ppm, with values
ranging from 0.01 to 0.1 ppm. No endosulfan B was detected in any of
the 15 fat analyses. Endosulfan sulfate was found in 3 of the 15
samples at levels of 0.04 to 0.05 ppm. The amount present after the
81-day trial was no higher than after 27 days. In a parallel test with
DDT administered to 3 pigs at a rate of 7 ppm in the diet, the level
in the fat was 8.3, 9.1 and 9.7 ppm, respectively, at the end of the
three test periods. In a further experiment, 5 pigs were given 2 ppm
endosulfan in their diet for 30 days. On the 11th day thereafter 0.002
ppm was found in one of five fat samples, and none was detectable
after 27 days (Maier-Bode, 1967a).
Milk cows fed 2 ppm endosulfan for 26 days excreted an average of 1
ppm unchanged in the faeces after the second day, or about 20 per cent
of the amount consumed. The milk contained 0.02 to 0.1 ppm endosulfan
sulfate and no unchanged material. The urine showed 0.1 to 1 ppm of
the alcohol derivative and was positive for an unidentified
metabolite. At the end of the test period no unchanged endosulfan was
detected in muscle, liver, kidney, brain or fat, though muscle
contained <0.01 ppm and fat 0.1 to 0.3 ppm of the sulfate (Gorbach,
1966).
When endosulfan was fed to 3 female pigs at the rate of 2 ppm for up
to 81 days, mean recoveries of endosulfan A and B and endosulfan
sulfate at 27, 54 and 81 days were 0.07, 0.09 and 0.04 ppm from body
fat but none was found in other tissues and organs examined. In
another experiment in which the pigs were fed endosulfan at 2 ppm for
30 days, the residue in the fat of those slaughtered on the last day
of feeding was 0.003 - 0.01 ppm, and the residues in the fat of those
slaughtered 11 days after the last administration was 0.002 ppm in 1
of the 5 samples and not detectable (<0.001 ppm) in the others
(Maier-Bode, 1967a).
Beef cattle that grazed for 31-36 days on Bermuda grass pasture
sprayed 7 days previously with endosulfan showed no endosulfan in
their body fat. No residue was found in the milk of cows fed silage
containing 0.41 to 2.35 ppm of endosulfan for 21 days. Two beef cattle
fed 5 and 2.5 mg/kg/day of endosulfan showed toxic effects in 2 and 13
days respectively. Treatment with 1.1 mg/kg/day produced no
intoxication, but resulted in residues in the fat (Beck et al., 1966).
Three geese were allowed to feed for 17 days on weeds in a strawberry
field sprayed twice, once before the trial and again after the first 7
days, with 50 per cent endosulfan wettable powder at 2-lb. per 100
gal. (amount per unit area not indicated). The geese showed no signs
of poisoning, and at the end of the 17-day feeding period no
endosulfan could be detected in the liver, kidney, fat or stomach
contents (Dustan, 1965).
Acute toxicity (technical grade)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 40-50 and 110* Hazelton Laboratories, 1957
Rat Intraperitoneal 8 Czech, 1958
* Dependent on the vehicle used
A dose of 15 mg/kg orally of purified endosulfan was estimated to be
an LD10 in mice (BALB/c strain). A dose of 20 mg/kg killed most of
the mice in 24 hours (Deema et al., 1966).
By oral administration in the rat the LD50 of endosulfan A is
reported to be 76 mg/kg, and of endosulfan B 240 mg/kg (Maier-Bode,
1967a).
Endosulfan is highly toxic to fish, lethal concentrations in the water
ranging from 0.001 to 0.0125 ppm for several species. In young birds
the LD50, in terms of ppm in the diet over a 10-day feeding period,
is reported as 270 ppm for the bobwhite, 620 ppm for the pheasant and
200 ppm for the mallard duck (Maier-Bode, 1967b).
Short-term studies
Rat. Rats tolerated daily 1.6 - 3.2 mg/kg body-weight orally for 12
weeks without any influence on growth-rate (Czech, 1958)
Dog. Endosulfan technical grade was administered daily in gelatin
capsules to 4 dogs for 3 days in a dose of 2.5 mg/kg body-weight.
Vomiting was observed in one dog and vomiting, tremors, convulsions,
rapid respiration, and mydriasis in 3 dogs. (Hazelton Laboratories,
1959).
Three groups of dogs each consisting of 2 males and 2 females were
given endosulfan orally in gelatin capsules 6 days a week for one year
in doses corresponding to 0.075, 0.25 and 0.75 mg/kg body-weight. No
signs of toxicity were observed. At autopsy gross and microscopic
examination of the tissues showed no difference between treated and
control animals (Hazelton Laboratories, 1959).
Long-term studies
Rat. Groups of 25 male and 25 female rats received 10, 30 and 100
ppm of endosulfan technical grade in the diet for 104 weeks. Survival
of the female rats in the 10-and 30-ppm groups was lower than that of
the female control group during the second year. In the 100-ppm female
group, survival was significantly lower after 26 weeks and
abnormalities were observed in weight gain and on haematological
examinations. At autopsy the relative weight of the testes in the
10-ppm male group was significantly lower than in the control group.
Consistent histopathological findings were apparent only in the
100-ppm male group. In these the kidneys were enlarged and there were
signs of renal tubular damage with interstitial nephritis. Hydropic
cells were seen in the liver. The tumour incidence was within normal
limits in all test groups (Hazelton Laboratories, 1959).
Comments
A number of studies on several species of animals have provided
evidence that endosulfan does not have cumulative properties. Failure
of endosulfan to accumulate in the body is also reflected in the
toxicity data on dogs, which showed marked toxic effects in 3 days
from 2.5 mg/kg/day but no effects from 0.75 mg/kg/day for one year.
Re-evaluation of the data for rats indicated that 30 ppm in the diet
can be considered a no effect level in this species.
TOXICOLOGICAL EVALUATION
Pending evaluation of the results on reproduction study in the rat,
the meeting was unable to set an ADI.
Further work required
Results of reproduction studies in the rat.
EVALUATION FOR TOLERANCES
USE PATTERN
Endosulfan use is approved in many countries for pest control in a
wide range of fruit and vegetables; it is also used on a number of
important non-food crops, e.g. cotton, tobacco. Various minimum
intervals between final application and harvest are in operation: for
European countries this varies from 15 to 42 days and averages about
30 days whilst in the United States intervals up to 30 days are used.
Endosulfan is not used in veterinary practice.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of over one thousand analyses, mainly using
microcoulometric gas chromatography, in field crop trials collected by
Hoechst are summarized in the following table. It is emphasized that
the dose-rates indicated have in many cases been chosen from a range
of dose-rates which have been studied and are in some cases higher
than the recommended dose rates. Whilst the information in the table
represents the summary of a much greater volume of data, the results
of individual trials have occasionally shown residues which are above
or below the general average levels indicated; however, the maximum
levels seldom exceed three times the general average levels indicated.
Only in a few cases have endosulfan sulphate residues been measured in
addition to endosulfan: for tomatoes about 0.1 ppm endosulfan sulphate
was found after 23 days and for alfalfa from 0.0 to 2.0 ppm
approximately although individual figures varied considerably.
Summary of Field Residue Data for Endosulfan (Hoechst papers)
(all values are in parts per million)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Alfalfa 1 0.5 0.3 different methods
Corn 4 <0.5 Normal rate 1
Peas 1 0.1
Potatoes 11 <0.1
Soybeans 2 <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Watermelons 2 <0.1
Artichokes 1 <0.1
Broccoli 2 0.1 - 0.3 0.1
Brussels sprouts 3 1.3
Cabbage 1 1.0 <0.1
Celery 1 3 8 (sic)
Collards 5 0.5
Cucumber 4 <0.1
Dried lima beans 3 0 <0.1 60 days
Green lima beans 2´ <0.1
Kale 8 1.0 Normal rate 1
Lettuce 2 2.0 (0.1) Variable
Pepper 2 <0.1
Spinach 1 0.5
String beans 1 <0.1
Tomatoes 3 <0.1 Normal rate 1
Apples 3 0.5 <0.1
Cherries 2 1 0.3
Blackcurrant 4 0.2 0.1
Prunes 2´ (b) 0.2 0.1
Peaches 2 0.3 Variable data
Pears 1´ <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Strawberries 3 0.3
Sugarbeets 1 <0.1
* These are not necessarily the recommended dose rates
(b) lb per 100 gallons
This summary shows that, with the possible exception of celery,
lettuce, spinach and strawberries, harvest residue levels are probably
normally less than 0.1 ppm. Maier-Bode (1967) gives further field
trial residue data for most of these commodities and states that
endosulfan is less persistent on plant surfaces than residues of DDT,
aldrin or toxaphene and that residues of endosulfan A disappear more
quickly than those of endosulfan B. He also states that up to 0.3 ppm,
but usually less than 0.1 ppm, of endosulfan sulphate has been found
on leaves and fruits and that this compound is toxicologically
equivalent to technical endosulfan. Other metabolites, including
endosulfan diol and endosulfan ether, can also be detected
occasionally in plants but not in crops intended for human
consumption. Maier-Bode concludes that less than 0.5 ppm of residue is
found in the field 2 to 3 weeks after normal application to a large
number of fruits and vegetables and fodder plants; but that residues
in grass or clover are not apparently reduced by ensilage or on drying
for hay. As a general conclusion, when endosulfan is used to protect
food crops against insect infestation in accordance with good
agricultural practice, residues in the treated produce are unlikely to
exceed 0.5 ppm at harvest, except possibly for lettuce, celery and
brassicae which should not exceed 2.0 ppm. Not all samples of these
commodities will contain such amounts of residue, in fact only a small
proportion of each individual commodity is likely to be treated.
RESIDUES IN FOOD AT TIME OF CONSUMPTION
There is only very limited evidence suggesting that residues do not
normally occur in total diet studies in the United States.
FATE OF RESIDUES
General considerations
Both endosulfan isomers are slowly hydrolysed in acid, alkaline and
neutral environment to endosulfan alcohol and sulphur dioxide.
Although in some ways resembling the cyclodienes structurally, the
degree of persistence of endosulfan residues is not so great as for
aldrin or endrin residues but is similar to that of lindane. Sunlight
(or artificial ultra-violet irradiation) gives rise to some
transformation to the more persistent compound endosulfan sulphate
(6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,
3-benzodioxathiepin 3,3-dioxide) (United Kingdom, 1963; Forman, et al,
1965), the toxicity of which is similar to that of endosulfan. The
sulphate itself is further degraded to endosulfan alcohol. Harrison et
al (1967) have shown in field trials on black-currant that, unless
deliberately oversprayed, the total residue of endosulfan A,
endosulfan B and endosulfan sulphate does not normally exceed 0.5 ppm
at harvest.
In animals
Endosulfan does not accumulate in animals. Sheep fed 15 mg endosulfan
daily for 26 days showed 0.05 ppm of endosulfan sulphate in the milk,
this being the limit of detection by the analytical method used.
Similar experiments with cows fed a diet containing 5 ppm each of
endosulfan and endosulfan sulphate showed only endosulfan sulphate
residues in the milk up to 0.16 ppm (Hoechst, 1965). When sows are fed
a diet containing 2 ppm endosulfan for 81 days, residues (found only
in the fatty tissue) do not exceed 0.1 ppm (Maier-Bode, 1968)
Residues of endosulfan have not so far been detected in human fat in
Britain whereas residues of all of the other commonly used
organochlorine compounds have been reported (United Kingdom, 1963).
This may be due to the relatively small use of endosulfan in Britain,
but even in the United States, where it in used on a much larger
scale, its presence in human fat has not been reported.
In storage and processing
Little work has been done on the investigation of residue losses on
washing and other preparation for consumption
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis for endosulfan have been reviewed by
Maier-Bode (1968). A number of multidetection systems are available
for the detection and determination of organochlorine compounds and
most of these can be applied to residues of endosulfan A and
endosulfan B (Terranova and Ware, 1963; Zweig et al, 1960; Carroll,
1962; Burke and Mills, 1963; Byers, et al, 1965). These methods also
include the AOAC system (1966) though this, as normally carried out,
may not detect endosulfan B. The methods can usually also be used,
with some modifications to extraction and clean-up conditions where
necessary, for the detection and determination of residues of
endosulfan sulphate, as described by Harrison et al, 1967. A number of
thin layer chromatographic systems have been described and are useful
in this connection (Junichi and Tetsukichi, 1962; a and b; Abbott et
al, 1964; Walker and Berosa, 1963; Kovacs, 1966; Balschmitter and
Tölg, 1966, a and b). A number of general points in connection with
multidetection systems of residue analysis are set out in the
introductory note on page 4. The methods are normally sensitive to
about 0.05 ppm of each isomer or of endosulfan sulphate. Both electron
capture and microcoulometric detection methods may be used and
alternative methods for the confirmation of the identity of residues
such as infra-red spectrophotometry are available (Forman et al,
1965). Less sensitive and less specific colorimetric methods of
residue analysis, based on alkaline hydrolysis to give sulphur dioxide
which is then reacted with p-rosaniline and formaldehyde (Mohoney,
1962; Zweig, 1964) or with methanolic potassium hydroxide and pyridine
(Butler, et al, 1962; Maitlen et al, 1963) have also been described.
Biological methods based on pyridine toxicity to fish (Romer, 1960) or
Drosophila (Varis and Tittanen, 1963; Beye, 1962; Huesman, 1961) are
also available.
Endosulfan metabolites can also be detected and determined by many of
the multidetection systems described above provided a suitable
extraction method is used: Maier-Bode (1968) suggests benzene or
benzene-isopropanol as extraction solvents. The gas chromatography of
the sulphate, ether, hydroxyether and lactone using three different
columns has been described by Ballschmitter and Töld (1966a).
Whilst some of these techniques may require further study before they
can be applied generally to residue determinations in crops and total
diets, it is recommended that wherever possible results of such
analyses should record separately endosulfan A, endosulfan B and
endosulfan sulphate.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Benelux 0.5 fruit and vegetables
Canada 1.0 fruit
2.0 vegetables
Fed.Rep. Germany 0.5 fruit and vegetables
Switzerland 0.5 strawberries
United States 2.0 apples, apricots, artichokes, broccoli,
cabbage, cherries, cucumbers, egg-plants,
grapes, lettuce, melons, nectarines,
peaches, peas, peppers, plums, prunes,
pumpkins, squash, strawberries, tomatoes,
watercress.
FAO/WHO RECOMMENDATIONS FOR TOLERANCES
No acceptable daily intake level is available so no recommendation for
tolerances can be made at present.
FURTHER WORK
Further work required before tolerances can be recommended
1. Submission of data required for an estimation of acceptable daily
intake (see page 138).
2. Fuller details of the purity and composition or technical
endosulfan and the determination of residues of endosulfan A,
endosulfan B and endosulfan sulphate in treated produce and total
diets by appropriate methods of analysis.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Beck, E.W., Johnson, J.C., Woodham, D.W., Leuck, D.B., Dawsey, L.H.,
Robbins, J.E. and Bowman, M.C. (1966) J. econ. Entomol., 59, 1444.
Czech, M. (1958) Medizin u. Chemie., 6, 574
Deema, P., Thompson, E. and Ware, G.W. (1966) J. econ. Entomol., 59,
546
Dustan G.G. (1965) Pesticide Progress 3 : 131
Gorbach, S. (1966) Unpublished report.
Harrison, R.B., Holmes, D.C., Roburn, J. and Tatton, O'G (1967)
J. Sci. Food Agr. 18, 10
Hazelton Laboratories, (1957) Unpublished report
Hazelton Laboratories, (1959) Unpublished report
Maier-Bode, H. (1967a) Archiv. f. Pflanzenschutz., 3 : 201
Maier-Bode, H. (1967b) Residue Review, 21 (in press)
Rahn, H.W. (1963) Arch. internat. Pharmacodyn., 144: 126
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Abbott, D.C., Egan, H., Thomson, J. (1964) Thin-layer chromatography
of organochlorine pesticides, J. Chromatog. 16 : 481
AOAC. (1966) Changes in Methods of Analysis, J. Assoc. Offic. Analyt.
Chem., 49 : 222 - 230.
Ballschmitter, K., Tölg, G. (1966a) Metabolisms des Thiodans in
Insekten. Angew. Chem. 78: 775.
Ballschmitter, K., Tölg, G. (1966b) Fluorescenzindikatoran zum
Nachveis von Organchalogeniden in der Dünnschicht chromatographie.
Z. anal. Chem. 215 : 305
Ballschmitter, K., Schophan, I. and Tölg, G. (1967) The metabolization
of endosulfan in insects and mammals. Paper presented to the VI
International Plant Protection Congress, Vienna.
Beye, F. (1962) Biodetection of insecticides in Coleus blumei with
Drosophila melanogaster. Anzeiger f. Schadlingskunde, 35 : 9 - 12.
Burke, J. and Mills, P.A. (1963) Microcoulometric gas chromatographic
determination of thiodan and tedion in green vegetables. J. Assoc.
Offic. Agric. Chem., 46 : 177 - 182.
Butler, L.I., Maitland, J.C. and Fakey, J.E. (1962) Microdetermination
of thiodan residues. J. Agric. Food Chem. 10 : 479 - 481.
Byers, R.A., Woodham, D.W. and Bowman, M.C. (1965) Residues on coastal
Bermuda grass, trash and soil treated with granular endosulfan.
J. Econ. Entomol. 58 : 160 - 161.
Carroll, C.C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Reviews, 1 : 37 - 65.
Forman, S.E., Durbetaki, A.J., Cohen, M.V. and Olefson, R.A. (1965)
Conformational equilibria in cydic sulphites and sulphates:
configurations of the two isomeric thiodans. J. Org. Chem., 30 : 169 -
175.
Harrison, R.B., Ruzicka, J.H.A. and Tatton, J. O'G. (1967) Endosulfan
residues on blackcurrants. J. Sci. Food Agric. 18 : 262 - 264.
Hoechst. (1965) Farbwerke Hoechst AG. Data sheet for
endosulfan/thiodan : Frankfurt am-Main.
Huesman, H. (1961) Z. angew. Zool., 48 : 1 - 29.
Junichi, Y. and Tetsukichi, N. (1962a) Identification of organic
chlorinated pesticides by plate chromatography. Proc. Japan Acad. 38 :
129
Junichi, Y. and Tetsukichi, N. (1962b) Separation and identification
of organic chlorinated pesticides by plate chromatography. Kagaku
Keisatsu Kantyusho Hokoku, 15 : 321.
Kovacs, H.F. (1966) The rapid detection of chlorinated pesticide
residues by an improved thin-layer chromatographic technique.
J. Assoc. Offic. Agric. Chem. 49 : 365.
Maier-Bode, H. (1967) Meded. Landb. Hoogesch. Opzoek. Stn. Gent, in
press
Maier-Bode, H. (1968) Endosulfan. Residue Reviews, 21 : in press
Maitlen, J.C., Walker, K.C. and Westlake, W.E. (1963) An improved
colorimetric method for determining endosulfan residues in vegetables
and beef fat. J. Agric. Food Chem., 11 : 416 - 418.
Mohoney, C.H. (1962) Flavour and quality changes in fruits and
vegetables in the United States caused by application of pesticide
chemicals. Residue Reviews, 1 : 11 - 23.
Romer, D. (1960) Determination of insecticide residues and assay of
lasting coatings. Anzeiger f. Schadlingskunde, 33 : 138 - 140.
Terranova, A.C. and Ware, G.W. (1963) Studies of endosulfan in bean
plants by paper and gas chromatography. J. Econ. Entomol., 56 : 596
- 599.
United Kingdom. (1963) Report of the Government Chemist, H.M.
Stationery Office, London. p.78
Varis, A.L. and Tittanen, K. (1963) Montalous Koetoiminta, 17 : 252
- 255.
Walker, K.C. and Berosa, M. (1963) Thin-layer chromatography for
insecticide analysis. J. Assoc. Offic. Agric. Chem. 46 : 250.
Zweig, G. (editor). (1964) Pesticides, Plant Growth Regulators and
Food Additives, Vol. II, p. 511 London, Academic Press.
Zweig, G., Archer, T.E. and Robenstein, D. (1960) Residue analysis of
endosulfan by combination of gas chromatography and infrared
spectrophotometry. J. Agric. Food Chem. 8 : 403 - 405.
 Other relative chemical properties including metabolites
Balschmitter et al (1967) have considered all of the possible
metabolites of endosulfan which can hypothetically be formed on
hydrolysis, oxidation or reduction. They have investigated endosulfan
metabolism in the mouse and rat using thin layer and gas
chromatographic techniques and have identified five of the possible
metabolites, including endosulfan sulfate, diol, ether, hydroxyether
and lactone as illustrated in the following figure :
Other relative chemical properties including metabolites
Balschmitter et al (1967) have considered all of the possible
metabolites of endosulfan which can hypothetically be formed on
hydrolysis, oxidation or reduction. They have investigated endosulfan
metabolism in the mouse and rat using thin layer and gas
chromatographic techniques and have identified five of the possible
metabolites, including endosulfan sulfate, diol, ether, hydroxyether
and lactone as illustrated in the following figure :
 EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Endosulfan is rapidly absorbed from the intestinal tract and about 30
per cent of a lethal dose is eliminated during 24 hours in the rat
(Czech, 1958).
Leaves from apple trees sprayed with endosulfan contained an
ultraviolet irradiation product equal in toxicity to, and more
persistent than, the parent compound (Harrison, 1967).
Endosulfan given to mice in single doses or repeated daily for 49 days
was found in the tissues as the sulfate. When endosulfan, endosulfan
sulfate, the diol, or the ether were fed to mice, a metabolite
considered to be the diol appeared in the urine. Oral doses of the
isomers were partly excreted in the faeces unchanged, along with
endosulfan sulfate and the diol. No residue was found in blood or
brain, but traces of the sulfate appeared in kidney and muscle (Deema
et al., 1966).
When endosulfan was administered to rats, no unchanged endosulfan was
found in the urine. Two metabolites were found in rat urine 48 hours
after the animals were injected intraperitoneally with endosulfan;
these appeared to be conjugation products of the alcohol derivative of
endosulfan (Rahn, 1963).
Three female pigs weighing 31 to 39 kg were treated for 27, 54 and 81
days with technical endosulfan (97 per cent pure) in capsules with
their feed in an amount equivalent to 2 ppm in the diet. During the
test periods these animals received, respectively, total doses of 81.8
mg, 196.6 mg and 332.4 mg of endosulfan. At the end of each of those
treatments no endosulfan could be detected in any of 13 different
organs and tissues including liver and brain but excluding fat. In the
fat from five different areas only one of the isomers (endosulfan A)
was consistently found, at an average level of 0.06 ppm, with values
ranging from 0.01 to 0.1 ppm. No endosulfan B was detected in any of
the 15 fat analyses. Endosulfan sulfate was found in 3 of the 15
samples at levels of 0.04 to 0.05 ppm. The amount present after the
81-day trial was no higher than after 27 days. In a parallel test with
DDT administered to 3 pigs at a rate of 7 ppm in the diet, the level
in the fat was 8.3, 9.1 and 9.7 ppm, respectively, at the end of the
three test periods. In a further experiment, 5 pigs were given 2 ppm
endosulfan in their diet for 30 days. On the 11th day thereafter 0.002
ppm was found in one of five fat samples, and none was detectable
after 27 days (Maier-Bode, 1967a).
Milk cows fed 2 ppm endosulfan for 26 days excreted an average of 1
ppm unchanged in the faeces after the second day, or about 20 per cent
of the amount consumed. The milk contained 0.02 to 0.1 ppm endosulfan
sulfate and no unchanged material. The urine showed 0.1 to 1 ppm of
the alcohol derivative and was positive for an unidentified
metabolite. At the end of the test period no unchanged endosulfan was
detected in muscle, liver, kidney, brain or fat, though muscle
contained <0.01 ppm and fat 0.1 to 0.3 ppm of the sulfate (Gorbach,
1966).
When endosulfan was fed to 3 female pigs at the rate of 2 ppm for up
to 81 days, mean recoveries of endosulfan A and B and endosulfan
sulfate at 27, 54 and 81 days were 0.07, 0.09 and 0.04 ppm from body
fat but none was found in other tissues and organs examined. In
another experiment in which the pigs were fed endosulfan at 2 ppm for
30 days, the residue in the fat of those slaughtered on the last day
of feeding was 0.003 - 0.01 ppm, and the residues in the fat of those
slaughtered 11 days after the last administration was 0.002 ppm in 1
of the 5 samples and not detectable (<0.001 ppm) in the others
(Maier-Bode, 1967a).
Beef cattle that grazed for 31-36 days on Bermuda grass pasture
sprayed 7 days previously with endosulfan showed no endosulfan in
their body fat. No residue was found in the milk of cows fed silage
containing 0.41 to 2.35 ppm of endosulfan for 21 days. Two beef cattle
fed 5 and 2.5 mg/kg/day of endosulfan showed toxic effects in 2 and 13
days respectively. Treatment with 1.1 mg/kg/day produced no
intoxication, but resulted in residues in the fat (Beck et al., 1966).
Three geese were allowed to feed for 17 days on weeds in a strawberry
field sprayed twice, once before the trial and again after the first 7
days, with 50 per cent endosulfan wettable powder at 2-lb. per 100
gal. (amount per unit area not indicated). The geese showed no signs
of poisoning, and at the end of the 17-day feeding period no
endosulfan could be detected in the liver, kidney, fat or stomach
contents (Dustan, 1965).
Acute toxicity (technical grade)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 40-50 and 110* Hazelton Laboratories, 1957
Rat Intraperitoneal 8 Czech, 1958
* Dependent on the vehicle used
A dose of 15 mg/kg orally of purified endosulfan was estimated to be
an LD10 in mice (BALB/c strain). A dose of 20 mg/kg killed most of
the mice in 24 hours (Deema et al., 1966).
By oral administration in the rat the LD50 of endosulfan A is
reported to be 76 mg/kg, and of endosulfan B 240 mg/kg (Maier-Bode,
1967a).
Endosulfan is highly toxic to fish, lethal concentrations in the water
ranging from 0.001 to 0.0125 ppm for several species. In young birds
the LD50, in terms of ppm in the diet over a 10-day feeding period,
is reported as 270 ppm for the bobwhite, 620 ppm for the pheasant and
200 ppm for the mallard duck (Maier-Bode, 1967b).
Short-term studies
Rat. Rats tolerated daily 1.6 - 3.2 mg/kg body-weight orally for 12
weeks without any influence on growth-rate (Czech, 1958)
Dog. Endosulfan technical grade was administered daily in gelatin
capsules to 4 dogs for 3 days in a dose of 2.5 mg/kg body-weight.
Vomiting was observed in one dog and vomiting, tremors, convulsions,
rapid respiration, and mydriasis in 3 dogs. (Hazelton Laboratories,
1959).
Three groups of dogs each consisting of 2 males and 2 females were
given endosulfan orally in gelatin capsules 6 days a week for one year
in doses corresponding to 0.075, 0.25 and 0.75 mg/kg body-weight. No
signs of toxicity were observed. At autopsy gross and microscopic
examination of the tissues showed no difference between treated and
control animals (Hazelton Laboratories, 1959).
Long-term studies
Rat. Groups of 25 male and 25 female rats received 10, 30 and 100
ppm of endosulfan technical grade in the diet for 104 weeks. Survival
of the female rats in the 10-and 30-ppm groups was lower than that of
the female control group during the second year. In the 100-ppm female
group, survival was significantly lower after 26 weeks and
abnormalities were observed in weight gain and on haematological
examinations. At autopsy the relative weight of the testes in the
10-ppm male group was significantly lower than in the control group.
Consistent histopathological findings were apparent only in the
100-ppm male group. In these the kidneys were enlarged and there were
signs of renal tubular damage with interstitial nephritis. Hydropic
cells were seen in the liver. The tumour incidence was within normal
limits in all test groups (Hazelton Laboratories, 1959).
Comments
A number of studies on several species of animals have provided
evidence that endosulfan does not have cumulative properties. Failure
of endosulfan to accumulate in the body is also reflected in the
toxicity data on dogs, which showed marked toxic effects in 3 days
from 2.5 mg/kg/day but no effects from 0.75 mg/kg/day for one year.
Re-evaluation of the data for rats indicated that 30 ppm in the diet
can be considered a no effect level in this species.
TOXICOLOGICAL EVALUATION
Pending evaluation of the results on reproduction study in the rat,
the meeting was unable to set an ADI.
Further work required
Results of reproduction studies in the rat.
EVALUATION FOR TOLERANCES
USE PATTERN
Endosulfan use is approved in many countries for pest control in a
wide range of fruit and vegetables; it is also used on a number of
important non-food crops, e.g. cotton, tobacco. Various minimum
intervals between final application and harvest are in operation: for
European countries this varies from 15 to 42 days and averages about
30 days whilst in the United States intervals up to 30 days are used.
Endosulfan is not used in veterinary practice.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of over one thousand analyses, mainly using
microcoulometric gas chromatography, in field crop trials collected by
Hoechst are summarized in the following table. It is emphasized that
the dose-rates indicated have in many cases been chosen from a range
of dose-rates which have been studied and are in some cases higher
than the recommended dose rates. Whilst the information in the table
represents the summary of a much greater volume of data, the results
of individual trials have occasionally shown residues which are above
or below the general average levels indicated; however, the maximum
levels seldom exceed three times the general average levels indicated.
Only in a few cases have endosulfan sulphate residues been measured in
addition to endosulfan: for tomatoes about 0.1 ppm endosulfan sulphate
was found after 23 days and for alfalfa from 0.0 to 2.0 ppm
approximately although individual figures varied considerably.
Summary of Field Residue Data for Endosulfan (Hoechst papers)
(all values are in parts per million)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Alfalfa 1 0.5 0.3 different methods
Corn 4 <0.5 Normal rate 1
Peas 1 0.1
Potatoes 11 <0.1
Soybeans 2 <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Watermelons 2 <0.1
Artichokes 1 <0.1
Broccoli 2 0.1 - 0.3 0.1
Brussels sprouts 3 1.3
Cabbage 1 1.0 <0.1
Celery 1 3 8 (sic)
Collards 5 0.5
Cucumber 4 <0.1
Dried lima beans 3 0 <0.1 60 days
Green lima beans 2´ <0.1
Kale 8 1.0 Normal rate 1
Lettuce 2 2.0 (0.1) Variable
Pepper 2 <0.1
Spinach 1 0.5
String beans 1 <0.1
Tomatoes 3 <0.1 Normal rate 1
Apples 3 0.5 <0.1
Cherries 2 1 0.3
Blackcurrant 4 0.2 0.1
Prunes 2´ (b) 0.2 0.1
Peaches 2 0.3 Variable data
Pears 1´ <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Strawberries 3 0.3
Sugarbeets 1 <0.1
* These are not necessarily the recommended dose rates
(b) lb per 100 gallons
This summary shows that, with the possible exception of celery,
lettuce, spinach and strawberries, harvest residue levels are probably
normally less than 0.1 ppm. Maier-Bode (1967) gives further field
trial residue data for most of these commodities and states that
endosulfan is less persistent on plant surfaces than residues of DDT,
aldrin or toxaphene and that residues of endosulfan A disappear more
quickly than those of endosulfan B. He also states that up to 0.3 ppm,
but usually less than 0.1 ppm, of endosulfan sulphate has been found
on leaves and fruits and that this compound is toxicologically
equivalent to technical endosulfan. Other metabolites, including
endosulfan diol and endosulfan ether, can also be detected
occasionally in plants but not in crops intended for human
consumption. Maier-Bode concludes that less than 0.5 ppm of residue is
found in the field 2 to 3 weeks after normal application to a large
number of fruits and vegetables and fodder plants; but that residues
in grass or clover are not apparently reduced by ensilage or on drying
for hay. As a general conclusion, when endosulfan is used to protect
food crops against insect infestation in accordance with good
agricultural practice, residues in the treated produce are unlikely to
exceed 0.5 ppm at harvest, except possibly for lettuce, celery and
brassicae which should not exceed 2.0 ppm. Not all samples of these
commodities will contain such amounts of residue, in fact only a small
proportion of each individual commodity is likely to be treated.
RESIDUES IN FOOD AT TIME OF CONSUMPTION
There is only very limited evidence suggesting that residues do not
normally occur in total diet studies in the United States.
FATE OF RESIDUES
General considerations
Both endosulfan isomers are slowly hydrolysed in acid, alkaline and
neutral environment to endosulfan alcohol and sulphur dioxide.
Although in some ways resembling the cyclodienes structurally, the
degree of persistence of endosulfan residues is not so great as for
aldrin or endrin residues but is similar to that of lindane. Sunlight
(or artificial ultra-violet irradiation) gives rise to some
transformation to the more persistent compound endosulfan sulphate
(6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,
3-benzodioxathiepin 3,3-dioxide) (United Kingdom, 1963; Forman, et al,
1965), the toxicity of which is similar to that of endosulfan. The
sulphate itself is further degraded to endosulfan alcohol. Harrison et
al (1967) have shown in field trials on black-currant that, unless
deliberately oversprayed, the total residue of endosulfan A,
endosulfan B and endosulfan sulphate does not normally exceed 0.5 ppm
at harvest.
In animals
Endosulfan does not accumulate in animals. Sheep fed 15 mg endosulfan
daily for 26 days showed 0.05 ppm of endosulfan sulphate in the milk,
this being the limit of detection by the analytical method used.
Similar experiments with cows fed a diet containing 5 ppm each of
endosulfan and endosulfan sulphate showed only endosulfan sulphate
residues in the milk up to 0.16 ppm (Hoechst, 1965). When sows are fed
a diet containing 2 ppm endosulfan for 81 days, residues (found only
in the fatty tissue) do not exceed 0.1 ppm (Maier-Bode, 1968)
Residues of endosulfan have not so far been detected in human fat in
Britain whereas residues of all of the other commonly used
organochlorine compounds have been reported (United Kingdom, 1963).
This may be due to the relatively small use of endosulfan in Britain,
but even in the United States, where it in used on a much larger
scale, its presence in human fat has not been reported.
In storage and processing
Little work has been done on the investigation of residue losses on
washing and other preparation for consumption
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis for endosulfan have been reviewed by
Maier-Bode (1968). A number of multidetection systems are available
for the detection and determination of organochlorine compounds and
most of these can be applied to residues of endosulfan A and
endosulfan B (Terranova and Ware, 1963; Zweig et al, 1960; Carroll,
1962; Burke and Mills, 1963; Byers, et al, 1965). These methods also
include the AOAC system (1966) though this, as normally carried out,
may not detect endosulfan B. The methods can usually also be used,
with some modifications to extraction and clean-up conditions where
necessary, for the detection and determination of residues of
endosulfan sulphate, as described by Harrison et al, 1967. A number of
thin layer chromatographic systems have been described and are useful
in this connection (Junichi and Tetsukichi, 1962; a and b; Abbott et
al, 1964; Walker and Berosa, 1963; Kovacs, 1966; Balschmitter and
Tölg, 1966, a and b). A number of general points in connection with
multidetection systems of residue analysis are set out in the
introductory note on page 4. The methods are normally sensitive to
about 0.05 ppm of each isomer or of endosulfan sulphate. Both electron
capture and microcoulometric detection methods may be used and
alternative methods for the confirmation of the identity of residues
such as infra-red spectrophotometry are available (Forman et al,
1965). Less sensitive and less specific colorimetric methods of
residue analysis, based on alkaline hydrolysis to give sulphur dioxide
which is then reacted with p-rosaniline and formaldehyde (Mohoney,
1962; Zweig, 1964) or with methanolic potassium hydroxide and pyridine
(Butler, et al, 1962; Maitlen et al, 1963) have also been described.
Biological methods based on pyridine toxicity to fish (Romer, 1960) or
Drosophila (Varis and Tittanen, 1963; Beye, 1962; Huesman, 1961) are
also available.
Endosulfan metabolites can also be detected and determined by many of
the multidetection systems described above provided a suitable
extraction method is used: Maier-Bode (1968) suggests benzene or
benzene-isopropanol as extraction solvents. The gas chromatography of
the sulphate, ether, hydroxyether and lactone using three different
columns has been described by Ballschmitter and Töld (1966a).
Whilst some of these techniques may require further study before they
can be applied generally to residue determinations in crops and total
diets, it is recommended that wherever possible results of such
analyses should record separately endosulfan A, endosulfan B and
endosulfan sulphate.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Benelux 0.5 fruit and vegetables
Canada 1.0 fruit
2.0 vegetables
Fed.Rep. Germany 0.5 fruit and vegetables
Switzerland 0.5 strawberries
United States 2.0 apples, apricots, artichokes, broccoli,
cabbage, cherries, cucumbers, egg-plants,
grapes, lettuce, melons, nectarines,
peaches, peas, peppers, plums, prunes,
pumpkins, squash, strawberries, tomatoes,
watercress.
FAO/WHO RECOMMENDATIONS FOR TOLERANCES
No acceptable daily intake level is available so no recommendation for
tolerances can be made at present.
FURTHER WORK
Further work required before tolerances can be recommended
1. Submission of data required for an estimation of acceptable daily
intake (see page 138).
2. Fuller details of the purity and composition or technical
endosulfan and the determination of residues of endosulfan A,
endosulfan B and endosulfan sulphate in treated produce and total
diets by appropriate methods of analysis.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Beck, E.W., Johnson, J.C., Woodham, D.W., Leuck, D.B., Dawsey, L.H.,
Robbins, J.E. and Bowman, M.C. (1966) J. econ. Entomol., 59, 1444.
Czech, M. (1958) Medizin u. Chemie., 6, 574
Deema, P., Thompson, E. and Ware, G.W. (1966) J. econ. Entomol., 59,
546
Dustan G.G. (1965) Pesticide Progress 3 : 131
Gorbach, S. (1966) Unpublished report.
Harrison, R.B., Holmes, D.C., Roburn, J. and Tatton, O'G (1967)
J. Sci. Food Agr. 18, 10
Hazelton Laboratories, (1957) Unpublished report
Hazelton Laboratories, (1959) Unpublished report
Maier-Bode, H. (1967a) Archiv. f. Pflanzenschutz., 3 : 201
Maier-Bode, H. (1967b) Residue Review, 21 (in press)
Rahn, H.W. (1963) Arch. internat. Pharmacodyn., 144: 126
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Abbott, D.C., Egan, H., Thomson, J. (1964) Thin-layer chromatography
of organochlorine pesticides, J. Chromatog. 16 : 481
AOAC. (1966) Changes in Methods of Analysis, J. Assoc. Offic. Analyt.
Chem., 49 : 222 - 230.
Ballschmitter, K., Tölg, G. (1966a) Metabolisms des Thiodans in
Insekten. Angew. Chem. 78: 775.
Ballschmitter, K., Tölg, G. (1966b) Fluorescenzindikatoran zum
Nachveis von Organchalogeniden in der Dünnschicht chromatographie.
Z. anal. Chem. 215 : 305
Ballschmitter, K., Schophan, I. and Tölg, G. (1967) The metabolization
of endosulfan in insects and mammals. Paper presented to the VI
International Plant Protection Congress, Vienna.
Beye, F. (1962) Biodetection of insecticides in Coleus blumei with
Drosophila melanogaster. Anzeiger f. Schadlingskunde, 35 : 9 - 12.
Burke, J. and Mills, P.A. (1963) Microcoulometric gas chromatographic
determination of thiodan and tedion in green vegetables. J. Assoc.
Offic. Agric. Chem., 46 : 177 - 182.
Butler, L.I., Maitland, J.C. and Fakey, J.E. (1962) Microdetermination
of thiodan residues. J. Agric. Food Chem. 10 : 479 - 481.
Byers, R.A., Woodham, D.W. and Bowman, M.C. (1965) Residues on coastal
Bermuda grass, trash and soil treated with granular endosulfan.
J. Econ. Entomol. 58 : 160 - 161.
Carroll, C.C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Reviews, 1 : 37 - 65.
Forman, S.E., Durbetaki, A.J., Cohen, M.V. and Olefson, R.A. (1965)
Conformational equilibria in cydic sulphites and sulphates:
configurations of the two isomeric thiodans. J. Org. Chem., 30 : 169 -
175.
Harrison, R.B., Ruzicka, J.H.A. and Tatton, J. O'G. (1967) Endosulfan
residues on blackcurrants. J. Sci. Food Agric. 18 : 262 - 264.
Hoechst. (1965) Farbwerke Hoechst AG. Data sheet for
endosulfan/thiodan : Frankfurt am-Main.
Huesman, H. (1961) Z. angew. Zool., 48 : 1 - 29.
Junichi, Y. and Tetsukichi, N. (1962a) Identification of organic
chlorinated pesticides by plate chromatography. Proc. Japan Acad. 38 :
129
Junichi, Y. and Tetsukichi, N. (1962b) Separation and identification
of organic chlorinated pesticides by plate chromatography. Kagaku
Keisatsu Kantyusho Hokoku, 15 : 321.
Kovacs, H.F. (1966) The rapid detection of chlorinated pesticide
residues by an improved thin-layer chromatographic technique.
J. Assoc. Offic. Agric. Chem. 49 : 365.
Maier-Bode, H. (1967) Meded. Landb. Hoogesch. Opzoek. Stn. Gent, in
press
Maier-Bode, H. (1968) Endosulfan. Residue Reviews, 21 : in press
Maitlen, J.C., Walker, K.C. and Westlake, W.E. (1963) An improved
colorimetric method for determining endosulfan residues in vegetables
and beef fat. J. Agric. Food Chem., 11 : 416 - 418.
Mohoney, C.H. (1962) Flavour and quality changes in fruits and
vegetables in the United States caused by application of pesticide
chemicals. Residue Reviews, 1 : 11 - 23.
Romer, D. (1960) Determination of insecticide residues and assay of
lasting coatings. Anzeiger f. Schadlingskunde, 33 : 138 - 140.
Terranova, A.C. and Ware, G.W. (1963) Studies of endosulfan in bean
plants by paper and gas chromatography. J. Econ. Entomol., 56 : 596
- 599.
United Kingdom. (1963) Report of the Government Chemist, H.M.
Stationery Office, London. p.78
Varis, A.L. and Tittanen, K. (1963) Montalous Koetoiminta, 17 : 252
- 255.
Walker, K.C. and Berosa, M. (1963) Thin-layer chromatography for
insecticide analysis. J. Assoc. Offic. Agric. Chem. 46 : 250.
Zweig, G. (editor). (1964) Pesticides, Plant Growth Regulators and
Food Additives, Vol. II, p. 511 London, Academic Press.
Zweig, G., Archer, T.E. and Robenstein, D. (1960) Residue analysis of
endosulfan by combination of gas chromatography and infrared
spectrophotometry. J. Agric. Food Chem. 8 : 403 - 405.
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Endosulfan is rapidly absorbed from the intestinal tract and about 30
per cent of a lethal dose is eliminated during 24 hours in the rat
(Czech, 1958).
Leaves from apple trees sprayed with endosulfan contained an
ultraviolet irradiation product equal in toxicity to, and more
persistent than, the parent compound (Harrison, 1967).
Endosulfan given to mice in single doses or repeated daily for 49 days
was found in the tissues as the sulfate. When endosulfan, endosulfan
sulfate, the diol, or the ether were fed to mice, a metabolite
considered to be the diol appeared in the urine. Oral doses of the
isomers were partly excreted in the faeces unchanged, along with
endosulfan sulfate and the diol. No residue was found in blood or
brain, but traces of the sulfate appeared in kidney and muscle (Deema
et al., 1966).
When endosulfan was administered to rats, no unchanged endosulfan was
found in the urine. Two metabolites were found in rat urine 48 hours
after the animals were injected intraperitoneally with endosulfan;
these appeared to be conjugation products of the alcohol derivative of
endosulfan (Rahn, 1963).
Three female pigs weighing 31 to 39 kg were treated for 27, 54 and 81
days with technical endosulfan (97 per cent pure) in capsules with
their feed in an amount equivalent to 2 ppm in the diet. During the
test periods these animals received, respectively, total doses of 81.8
mg, 196.6 mg and 332.4 mg of endosulfan. At the end of each of those
treatments no endosulfan could be detected in any of 13 different
organs and tissues including liver and brain but excluding fat. In the
fat from five different areas only one of the isomers (endosulfan A)
was consistently found, at an average level of 0.06 ppm, with values
ranging from 0.01 to 0.1 ppm. No endosulfan B was detected in any of
the 15 fat analyses. Endosulfan sulfate was found in 3 of the 15
samples at levels of 0.04 to 0.05 ppm. The amount present after the
81-day trial was no higher than after 27 days. In a parallel test with
DDT administered to 3 pigs at a rate of 7 ppm in the diet, the level
in the fat was 8.3, 9.1 and 9.7 ppm, respectively, at the end of the
three test periods. In a further experiment, 5 pigs were given 2 ppm
endosulfan in their diet for 30 days. On the 11th day thereafter 0.002
ppm was found in one of five fat samples, and none was detectable
after 27 days (Maier-Bode, 1967a).
Milk cows fed 2 ppm endosulfan for 26 days excreted an average of 1
ppm unchanged in the faeces after the second day, or about 20 per cent
of the amount consumed. The milk contained 0.02 to 0.1 ppm endosulfan
sulfate and no unchanged material. The urine showed 0.1 to 1 ppm of
the alcohol derivative and was positive for an unidentified
metabolite. At the end of the test period no unchanged endosulfan was
detected in muscle, liver, kidney, brain or fat, though muscle
contained <0.01 ppm and fat 0.1 to 0.3 ppm of the sulfate (Gorbach,
1966).
When endosulfan was fed to 3 female pigs at the rate of 2 ppm for up
to 81 days, mean recoveries of endosulfan A and B and endosulfan
sulfate at 27, 54 and 81 days were 0.07, 0.09 and 0.04 ppm from body
fat but none was found in other tissues and organs examined. In
another experiment in which the pigs were fed endosulfan at 2 ppm for
30 days, the residue in the fat of those slaughtered on the last day
of feeding was 0.003 - 0.01 ppm, and the residues in the fat of those
slaughtered 11 days after the last administration was 0.002 ppm in 1
of the 5 samples and not detectable (<0.001 ppm) in the others
(Maier-Bode, 1967a).
Beef cattle that grazed for 31-36 days on Bermuda grass pasture
sprayed 7 days previously with endosulfan showed no endosulfan in
their body fat. No residue was found in the milk of cows fed silage
containing 0.41 to 2.35 ppm of endosulfan for 21 days. Two beef cattle
fed 5 and 2.5 mg/kg/day of endosulfan showed toxic effects in 2 and 13
days respectively. Treatment with 1.1 mg/kg/day produced no
intoxication, but resulted in residues in the fat (Beck et al., 1966).
Three geese were allowed to feed for 17 days on weeds in a strawberry
field sprayed twice, once before the trial and again after the first 7
days, with 50 per cent endosulfan wettable powder at 2-lb. per 100
gal. (amount per unit area not indicated). The geese showed no signs
of poisoning, and at the end of the 17-day feeding period no
endosulfan could be detected in the liver, kidney, fat or stomach
contents (Dustan, 1965).
Acute toxicity (technical grade)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 40-50 and 110* Hazelton Laboratories, 1957
Rat Intraperitoneal 8 Czech, 1958
* Dependent on the vehicle used
A dose of 15 mg/kg orally of purified endosulfan was estimated to be
an LD10 in mice (BALB/c strain). A dose of 20 mg/kg killed most of
the mice in 24 hours (Deema et al., 1966).
By oral administration in the rat the LD50 of endosulfan A is
reported to be 76 mg/kg, and of endosulfan B 240 mg/kg (Maier-Bode,
1967a).
Endosulfan is highly toxic to fish, lethal concentrations in the water
ranging from 0.001 to 0.0125 ppm for several species. In young birds
the LD50, in terms of ppm in the diet over a 10-day feeding period,
is reported as 270 ppm for the bobwhite, 620 ppm for the pheasant and
200 ppm for the mallard duck (Maier-Bode, 1967b).
Short-term studies
Rat. Rats tolerated daily 1.6 - 3.2 mg/kg body-weight orally for 12
weeks without any influence on growth-rate (Czech, 1958)
Dog. Endosulfan technical grade was administered daily in gelatin
capsules to 4 dogs for 3 days in a dose of 2.5 mg/kg body-weight.
Vomiting was observed in one dog and vomiting, tremors, convulsions,
rapid respiration, and mydriasis in 3 dogs. (Hazelton Laboratories,
1959).
Three groups of dogs each consisting of 2 males and 2 females were
given endosulfan orally in gelatin capsules 6 days a week for one year
in doses corresponding to 0.075, 0.25 and 0.75 mg/kg body-weight. No
signs of toxicity were observed. At autopsy gross and microscopic
examination of the tissues showed no difference between treated and
control animals (Hazelton Laboratories, 1959).
Long-term studies
Rat. Groups of 25 male and 25 female rats received 10, 30 and 100
ppm of endosulfan technical grade in the diet for 104 weeks. Survival
of the female rats in the 10-and 30-ppm groups was lower than that of
the female control group during the second year. In the 100-ppm female
group, survival was significantly lower after 26 weeks and
abnormalities were observed in weight gain and on haematological
examinations. At autopsy the relative weight of the testes in the
10-ppm male group was significantly lower than in the control group.
Consistent histopathological findings were apparent only in the
100-ppm male group. In these the kidneys were enlarged and there were
signs of renal tubular damage with interstitial nephritis. Hydropic
cells were seen in the liver. The tumour incidence was within normal
limits in all test groups (Hazelton Laboratories, 1959).
Comments
A number of studies on several species of animals have provided
evidence that endosulfan does not have cumulative properties. Failure
of endosulfan to accumulate in the body is also reflected in the
toxicity data on dogs, which showed marked toxic effects in 3 days
from 2.5 mg/kg/day but no effects from 0.75 mg/kg/day for one year.
Re-evaluation of the data for rats indicated that 30 ppm in the diet
can be considered a no effect level in this species.
TOXICOLOGICAL EVALUATION
Pending evaluation of the results on reproduction study in the rat,
the meeting was unable to set an ADI.
Further work required
Results of reproduction studies in the rat.
EVALUATION FOR TOLERANCES
USE PATTERN
Endosulfan use is approved in many countries for pest control in a
wide range of fruit and vegetables; it is also used on a number of
important non-food crops, e.g. cotton, tobacco. Various minimum
intervals between final application and harvest are in operation: for
European countries this varies from 15 to 42 days and averages about
30 days whilst in the United States intervals up to 30 days are used.
Endosulfan is not used in veterinary practice.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results of over one thousand analyses, mainly using
microcoulometric gas chromatography, in field crop trials collected by
Hoechst are summarized in the following table. It is emphasized that
the dose-rates indicated have in many cases been chosen from a range
of dose-rates which have been studied and are in some cases higher
than the recommended dose rates. Whilst the information in the table
represents the summary of a much greater volume of data, the results
of individual trials have occasionally shown residues which are above
or below the general average levels indicated; however, the maximum
levels seldom exceed three times the general average levels indicated.
Only in a few cases have endosulfan sulphate residues been measured in
addition to endosulfan: for tomatoes about 0.1 ppm endosulfan sulphate
was found after 23 days and for alfalfa from 0.0 to 2.0 ppm
approximately although individual figures varied considerably.
Summary of Field Residue Data for Endosulfan (Hoechst papers)
(all values are in parts per million)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Alfalfa 1 0.5 0.3 different methods
Corn 4 <0.5 Normal rate 1
Peas 1 0.1
Potatoes 11 <0.1
Soybeans 2 <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Watermelons 2 <0.1
Artichokes 1 <0.1
Broccoli 2 0.1 - 0.3 0.1
Brussels sprouts 3 1.3
Cabbage 1 1.0 <0.1
Celery 1 3 8 (sic)
Collards 5 0.5
Cucumber 4 <0.1
Dried lima beans 3 0 <0.1 60 days
Green lima beans 2´ <0.1
Kale 8 1.0 Normal rate 1
Lettuce 2 2.0 (0.1) Variable
Pepper 2 <0.1
Spinach 1 0.5
String beans 1 <0.1
Tomatoes 3 <0.1 Normal rate 1
Apples 3 0.5 <0.1
Cherries 2 1 0.3
Blackcurrant 4 0.2 0.1
Prunes 2´ (b) 0.2 0.1
Peaches 2 0.3 Variable data
Pears 1´ <0.1
Summary of Field Residue Data (cont'd)
* Total Dose General Average
a.i. lb/acre Residue at: Note
15 days 30 days
Strawberries 3 0.3
Sugarbeets 1 <0.1
* These are not necessarily the recommended dose rates
(b) lb per 100 gallons
This summary shows that, with the possible exception of celery,
lettuce, spinach and strawberries, harvest residue levels are probably
normally less than 0.1 ppm. Maier-Bode (1967) gives further field
trial residue data for most of these commodities and states that
endosulfan is less persistent on plant surfaces than residues of DDT,
aldrin or toxaphene and that residues of endosulfan A disappear more
quickly than those of endosulfan B. He also states that up to 0.3 ppm,
but usually less than 0.1 ppm, of endosulfan sulphate has been found
on leaves and fruits and that this compound is toxicologically
equivalent to technical endosulfan. Other metabolites, including
endosulfan diol and endosulfan ether, can also be detected
occasionally in plants but not in crops intended for human
consumption. Maier-Bode concludes that less than 0.5 ppm of residue is
found in the field 2 to 3 weeks after normal application to a large
number of fruits and vegetables and fodder plants; but that residues
in grass or clover are not apparently reduced by ensilage or on drying
for hay. As a general conclusion, when endosulfan is used to protect
food crops against insect infestation in accordance with good
agricultural practice, residues in the treated produce are unlikely to
exceed 0.5 ppm at harvest, except possibly for lettuce, celery and
brassicae which should not exceed 2.0 ppm. Not all samples of these
commodities will contain such amounts of residue, in fact only a small
proportion of each individual commodity is likely to be treated.
RESIDUES IN FOOD AT TIME OF CONSUMPTION
There is only very limited evidence suggesting that residues do not
normally occur in total diet studies in the United States.
FATE OF RESIDUES
General considerations
Both endosulfan isomers are slowly hydrolysed in acid, alkaline and
neutral environment to endosulfan alcohol and sulphur dioxide.
Although in some ways resembling the cyclodienes structurally, the
degree of persistence of endosulfan residues is not so great as for
aldrin or endrin residues but is similar to that of lindane. Sunlight
(or artificial ultra-violet irradiation) gives rise to some
transformation to the more persistent compound endosulfan sulphate
(6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,
3-benzodioxathiepin 3,3-dioxide) (United Kingdom, 1963; Forman, et al,
1965), the toxicity of which is similar to that of endosulfan. The
sulphate itself is further degraded to endosulfan alcohol. Harrison et
al (1967) have shown in field trials on black-currant that, unless
deliberately oversprayed, the total residue of endosulfan A,
endosulfan B and endosulfan sulphate does not normally exceed 0.5 ppm
at harvest.
In animals
Endosulfan does not accumulate in animals. Sheep fed 15 mg endosulfan
daily for 26 days showed 0.05 ppm of endosulfan sulphate in the milk,
this being the limit of detection by the analytical method used.
Similar experiments with cows fed a diet containing 5 ppm each of
endosulfan and endosulfan sulphate showed only endosulfan sulphate
residues in the milk up to 0.16 ppm (Hoechst, 1965). When sows are fed
a diet containing 2 ppm endosulfan for 81 days, residues (found only
in the fatty tissue) do not exceed 0.1 ppm (Maier-Bode, 1968)
Residues of endosulfan have not so far been detected in human fat in
Britain whereas residues of all of the other commonly used
organochlorine compounds have been reported (United Kingdom, 1963).
This may be due to the relatively small use of endosulfan in Britain,
but even in the United States, where it in used on a much larger
scale, its presence in human fat has not been reported.
In storage and processing
Little work has been done on the investigation of residue losses on
washing and other preparation for consumption
METHODS OF RESIDUE ANALYSIS
Methods of residue analysis for endosulfan have been reviewed by
Maier-Bode (1968). A number of multidetection systems are available
for the detection and determination of organochlorine compounds and
most of these can be applied to residues of endosulfan A and
endosulfan B (Terranova and Ware, 1963; Zweig et al, 1960; Carroll,
1962; Burke and Mills, 1963; Byers, et al, 1965). These methods also
include the AOAC system (1966) though this, as normally carried out,
may not detect endosulfan B. The methods can usually also be used,
with some modifications to extraction and clean-up conditions where
necessary, for the detection and determination of residues of
endosulfan sulphate, as described by Harrison et al, 1967. A number of
thin layer chromatographic systems have been described and are useful
in this connection (Junichi and Tetsukichi, 1962; a and b; Abbott et
al, 1964; Walker and Berosa, 1963; Kovacs, 1966; Balschmitter and
Tölg, 1966, a and b). A number of general points in connection with
multidetection systems of residue analysis are set out in the
introductory note on page 4. The methods are normally sensitive to
about 0.05 ppm of each isomer or of endosulfan sulphate. Both electron
capture and microcoulometric detection methods may be used and
alternative methods for the confirmation of the identity of residues
such as infra-red spectrophotometry are available (Forman et al,
1965). Less sensitive and less specific colorimetric methods of
residue analysis, based on alkaline hydrolysis to give sulphur dioxide
which is then reacted with p-rosaniline and formaldehyde (Mohoney,
1962; Zweig, 1964) or with methanolic potassium hydroxide and pyridine
(Butler, et al, 1962; Maitlen et al, 1963) have also been described.
Biological methods based on pyridine toxicity to fish (Romer, 1960) or
Drosophila (Varis and Tittanen, 1963; Beye, 1962; Huesman, 1961) are
also available.
Endosulfan metabolites can also be detected and determined by many of
the multidetection systems described above provided a suitable
extraction method is used: Maier-Bode (1968) suggests benzene or
benzene-isopropanol as extraction solvents. The gas chromatography of
the sulphate, ether, hydroxyether and lactone using three different
columns has been described by Ballschmitter and Töld (1966a).
Whilst some of these techniques may require further study before they
can be applied generally to residue determinations in crops and total
diets, it is recommended that wherever possible results of such
analyses should record separately endosulfan A, endosulfan B and
endosulfan sulphate.
NATIONAL TOLERANCES
Country Tolerance, ppm Crop
Benelux 0.5 fruit and vegetables
Canada 1.0 fruit
2.0 vegetables
Fed.Rep. Germany 0.5 fruit and vegetables
Switzerland 0.5 strawberries
United States 2.0 apples, apricots, artichokes, broccoli,
cabbage, cherries, cucumbers, egg-plants,
grapes, lettuce, melons, nectarines,
peaches, peas, peppers, plums, prunes,
pumpkins, squash, strawberries, tomatoes,
watercress.
FAO/WHO RECOMMENDATIONS FOR TOLERANCES
No acceptable daily intake level is available so no recommendation for
tolerances can be made at present.
FURTHER WORK
Further work required before tolerances can be recommended
1. Submission of data required for an estimation of acceptable daily
intake (see page 138).
2. Fuller details of the purity and composition or technical
endosulfan and the determination of residues of endosulfan A,
endosulfan B and endosulfan sulphate in treated produce and total
diets by appropriate methods of analysis.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Beck, E.W., Johnson, J.C., Woodham, D.W., Leuck, D.B., Dawsey, L.H.,
Robbins, J.E. and Bowman, M.C. (1966) J. econ. Entomol., 59, 1444.
Czech, M. (1958) Medizin u. Chemie., 6, 574
Deema, P., Thompson, E. and Ware, G.W. (1966) J. econ. Entomol., 59,
546
Dustan G.G. (1965) Pesticide Progress 3 : 131
Gorbach, S. (1966) Unpublished report.
Harrison, R.B., Holmes, D.C., Roburn, J. and Tatton, O'G (1967)
J. Sci. Food Agr. 18, 10
Hazelton Laboratories, (1957) Unpublished report
Hazelton Laboratories, (1959) Unpublished report
Maier-Bode, H. (1967a) Archiv. f. Pflanzenschutz., 3 : 201
Maier-Bode, H. (1967b) Residue Review, 21 (in press)
Rahn, H.W. (1963) Arch. internat. Pharmacodyn., 144: 126
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
Abbott, D.C., Egan, H., Thomson, J. (1964) Thin-layer chromatography
of organochlorine pesticides, J. Chromatog. 16 : 481
AOAC. (1966) Changes in Methods of Analysis, J. Assoc. Offic. Analyt.
Chem., 49 : 222 - 230.
Ballschmitter, K., Tölg, G. (1966a) Metabolisms des Thiodans in
Insekten. Angew. Chem. 78: 775.
Ballschmitter, K., Tölg, G. (1966b) Fluorescenzindikatoran zum
Nachveis von Organchalogeniden in der Dünnschicht chromatographie.
Z. anal. Chem. 215 : 305
Ballschmitter, K., Schophan, I. and Tölg, G. (1967) The metabolization
of endosulfan in insects and mammals. Paper presented to the VI
International Plant Protection Congress, Vienna.
Beye, F. (1962) Biodetection of insecticides in Coleus blumei with
Drosophila melanogaster. Anzeiger f. Schadlingskunde, 35 : 9 - 12.
Burke, J. and Mills, P.A. (1963) Microcoulometric gas chromatographic
determination of thiodan and tedion in green vegetables. J. Assoc.
Offic. Agric. Chem., 46 : 177 - 182.
Butler, L.I., Maitland, J.C. and Fakey, J.E. (1962) Microdetermination
of thiodan residues. J. Agric. Food Chem. 10 : 479 - 481.
Byers, R.A., Woodham, D.W. and Bowman, M.C. (1965) Residues on coastal
Bermuda grass, trash and soil treated with granular endosulfan.
J. Econ. Entomol. 58 : 160 - 161.
Carroll, C.C. (1962) Pesticide residue analysis by microcoulometric
gas chromatography. Residue Reviews, 1 : 37 - 65.
Forman, S.E., Durbetaki, A.J., Cohen, M.V. and Olefson, R.A. (1965)
Conformational equilibria in cydic sulphites and sulphates:
configurations of the two isomeric thiodans. J. Org. Chem., 30 : 169 -
175.
Harrison, R.B., Ruzicka, J.H.A. and Tatton, J. O'G. (1967) Endosulfan
residues on blackcurrants. J. Sci. Food Agric. 18 : 262 - 264.
Hoechst. (1965) Farbwerke Hoechst AG. Data sheet for
endosulfan/thiodan : Frankfurt am-Main.
Huesman, H. (1961) Z. angew. Zool., 48 : 1 - 29.
Junichi, Y. and Tetsukichi, N. (1962a) Identification of organic
chlorinated pesticides by plate chromatography. Proc. Japan Acad. 38 :
129
Junichi, Y. and Tetsukichi, N. (1962b) Separation and identification
of organic chlorinated pesticides by plate chromatography. Kagaku
Keisatsu Kantyusho Hokoku, 15 : 321.
Kovacs, H.F. (1966) The rapid detection of chlorinated pesticide
residues by an improved thin-layer chromatographic technique.
J. Assoc. Offic. Agric. Chem. 49 : 365.
Maier-Bode, H. (1967) Meded. Landb. Hoogesch. Opzoek. Stn. Gent, in
press
Maier-Bode, H. (1968) Endosulfan. Residue Reviews, 21 : in press
Maitlen, J.C., Walker, K.C. and Westlake, W.E. (1963) An improved
colorimetric method for determining endosulfan residues in vegetables
and beef fat. J. Agric. Food Chem., 11 : 416 - 418.
Mohoney, C.H. (1962) Flavour and quality changes in fruits and
vegetables in the United States caused by application of pesticide
chemicals. Residue Reviews, 1 : 11 - 23.
Romer, D. (1960) Determination of insecticide residues and assay of
lasting coatings. Anzeiger f. Schadlingskunde, 33 : 138 - 140.
Terranova, A.C. and Ware, G.W. (1963) Studies of endosulfan in bean
plants by paper and gas chromatography. J. Econ. Entomol., 56 : 596
- 599.
United Kingdom. (1963) Report of the Government Chemist, H.M.
Stationery Office, London. p.78
Varis, A.L. and Tittanen, K. (1963) Montalous Koetoiminta, 17 : 252
- 255.
Walker, K.C. and Berosa, M. (1963) Thin-layer chromatography for
insecticide analysis. J. Assoc. Offic. Agric. Chem. 46 : 250.
Zweig, G. (editor). (1964) Pesticides, Plant Growth Regulators and
Food Additives, Vol. II, p. 511 London, Academic Press.
Zweig, G., Archer, T.E. and Robenstein, D. (1960) Residue analysis of
endosulfan by combination of gas chromatography and infrared
spectrophotometry. J. Agric. Food Chem. 8 : 403 - 405.
