
ENDOSULFAN
EXPLANATION
Endosulfan was evaluated for acceptable daily intake (ADI) by
the Joint Meeting of Pesticide Residues (JMPR) in 1963, 1965, 1967,
and 1968, and reviewed in 1971, 1974, 1975, 1982, and 1985 (Annex 1,
FAO/WHO 1964, 1965a, 1968a, 1969a, 1972a, 1975a, 1976a, 1983a, and
1986a). Toxicological monographs were prepared in 1964, 1965, 1967,
and 1968 (Annex 1, FAO/WHO 1964, 1965b, 1968b, and 1969b).
Essentially all of the toxicology data available to evaluate
endosulfan during these proceedings were presented at the meetings
held between 1963 and 1968. The 1967 JMPR was unable to set an ADI
for endosulfan, pending evaluation of the results of a reproduction
study in rats. The reproduction study was evaluated by the 1968
JMPR and a temporary ADI of 0 to 0.0075 milligrams/kilograms per
body weight (mg/kg bw) was determined, based partly on the results
of that study and long-term rat and dog feeding studies. The
chronic rat and dog feeding studies were characterized as providing
sufficient information on the long-term effects of endosulfan.
However, the rat reproduction and teratology studies were performed
by IBT and were not independently validated. In 1982, JMPR
recommended that the ADI be replaced by a temporary ADI at the same
level, pending receipt of data validation and/or replacement studies
for the non-validated data. The 1985 JMPR extended the temporary
ADI and concluded that a complete re-evaluation of endosulfan was
required with toxicological data obtained following up-to-date
protocols. Since that time additional toxicological data (including
replacement reproduction and teratology studies) have become
available and are reviewed in the monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspect
Absorption, distribution, and excretion
Rats
The pharmacokinetics of orally administered alpha and beta
endosulfan in rats and sheep was described in a recent review paper
(Stumpf & ABhauer, 1986). In addition, dermal absorption studies in
rats (Craine, 1986) and monkeys (Lachmann, 1987) were also
available.
Following the administration of a single oral dose (1.2 mg/kg)
of radiolabelled endosulfan to male rats, 40 to 60% of the substance
was absorbed over 48 hours. Approximately 54 to 82% was excreted
(15 to 22% in feces, 29 to 47% in bile, and 10 to 13% in urine).
Similar results were obtained following a single oral dose (2 mg/kg)
to female rats, i.e., 90% of the radiolabelled substance was
excreted in 120 hours, with an excretion T´ of 30 to 40 hours
(Stumpf & ABhauer, 1986).
During a 14-day study in the rat with doses of 5 or 25 mg/kg
administered in the daily diet, followed by a 14-day elimination
phase, 65% was excreted during the dosing period and an additional
8% during the ensuing 14-day elimination phase. Tissue distribution
studies conducted with the 5 mg/kg daily dietary dose indicated an
accumulation of endosulfan in rat kidney (value of 3 mg/kg). Levels
were lower in liver and body fat (< 1 mg/kg) and in muscle and
brain (< 0.07 mg/kg). After discontinuation of feeding the stored
radioactivity was rapidly eliminated with a T´ of 3 to 7 days
(Stumpf & ABhauer, 1986).
The dermal absorption of single doses (0.10, 0.76, or
10.13 mg/kg bw) of aqueous suspensions of 14C-endosulfan (94.6%
pure) was examined following exposure periods of 30 minutes to 24
hours to the suspension in 114 male Crl:CD(SD)BR rats. Over a
24-hour time period approximately 25% of each applied dose was
absorbed and 55% remained bound to skin; about 20% was removed by
washing. Of the 25% absorbed at 24 hours, about 10% was excreted in
feces and urine (Craine, 1986).
Monkeys
The dermal absorption of single doses (2.2 to 3.0 mg/kg) of
aqueous suspensions of 14C-endosulfan (94.6% pure) was studied
after a 10-hour exposure period to shaved skin in two Rhesus
monkeys. Absorption of endosulfan occurred as indicated by gradually
increasing levels of radioactivity in blood and plasma over the
first 24 to 36 hours of the study. Plateau levels occurred at 36
hours in blood (25 mg/mL) and plasma (35 mg/mL). At the end of the
experiment (96 hours), 4.3% and 3.7% of the administered radioactive
dose was excreted in feces and urine, respectively, 11% was detected
in treated skin, and 10.5% was found in the carcasses. The total
recovery at 96 hours averaged 51% for both monkeys. Tissue
distribution studies revealed low (< 0.05 ppm) levels of
radioactivity in kidney, fat, liver, and brain. The respective
levels in liver, fat, and kidney were 19X, 9X and 3X greater than
the levels found in blood (Lachmann, 1987).
Sheep
Following the administration of a single oral dose (0.3 mg/kg)
of radiolabelled endosulfan to sheep, 50% of the dose was excreted
in feces, 41% in urine, and 1% in milk, over a period of 22 days
(Stumpf & ABhauer, 1986).
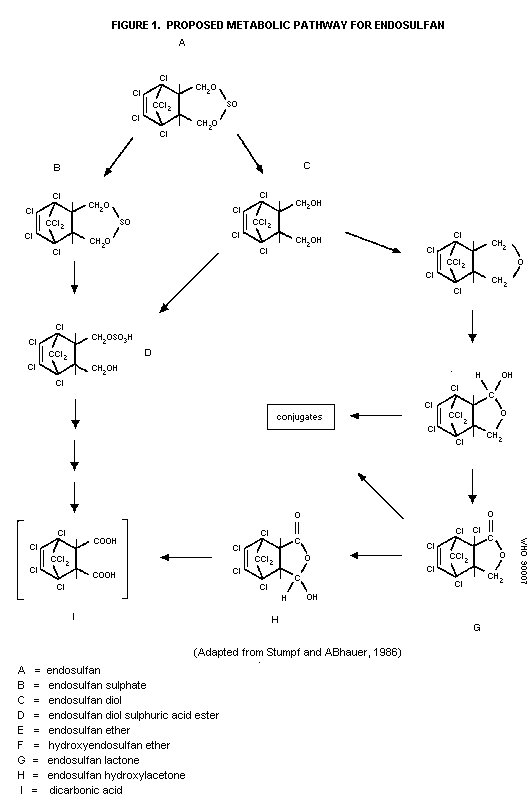
Biotransformation
Two biotransformation pathways have been described for
endosulfan in mammals (i.e., rats) (Stumpf & ABhauer, 1986). The
first pathways involves hydrolysis and oxidation to various
sulfur-free metabolites, namely endosulfan diol, endosulfan ether,
hydroxy-endosulfan ether, and endosulfan lactone (which is also
converted to endosulfan hydroxylactone in insects only), and polar
conjugates. The second pathway involves the formation of various
sulfur-containing metabolites, namely endosulfan sulfate, endosulfan
sulfuric acid ester, and endosulfan bicarbonic acid (postulated).
The proposed metabolic pathway is described in Figure 1.
Studies in the rat suggest that those components excreted
primarily in the feces after administration of a single oral dose
(1.2 mg/kg) of endosulfan are unchanged parent compound, endosulfan
sulfate, and all of the sulfur-free metabolites. The urine
contained mainly endosulfan diol and polar conjugates, in addition
to some unchanged parent compound (Stumpf & ABhauer, 1986).
Studies in monkeys following dermally administered
14C-endosulfan indicated that the unchanged parent compound was
present in small amounts of feces (0.1% of an applied dose) whereas
a metabolite referred to a "x" (presumed to be endosulfan
hydroxycarboxylic acid) was present to the extent of 0.4% of the
applied dose. In urine, endosulfan diol was the most prevalent
metabolite (1.5% of the dose), followed by "x" (0.6% of the dose)
plus negligible amounts of parent compound (Lachmann, 1987).
 Toxicological Studies
Acute toxicity
An acute nose-only inhalation toxicity test of endosulfan in
Wistar rats resulted in LC50 values of 0.0345 mg/L of air in males
and 0.9126 mg/L of air in females. Clinical signs of intoxication
were dyspnea, passivity, disequilibrium, trembling, tremors,
tono-clonic convulsions, and subdued reflex activity. Gross necropsy
changes in animals that died consisted of sporadic dark red pinhead
foci in the lungs (Hollander & Weigand, 1983).
Short-term studies
Mice
Groups of 10 male and 10 female Hoe:NMRKf(SPF 71) mice were fed
diets containing 0 or 18 ppm technical endosulfan for 42 days. The
18 ppm dose level was calculated to be equivalent to 3.7 mg/kg
bw/day in males and 4.6 mg/kg bw/day in females. The results
obtained indicated that deaths occurred in 2/10 treated mice (one
female on day 28 and another female on day 38), and absolute and
relative liver weights were significantly increased in females. In
addition, the body weight gain was slightly elevated throughout the
study, with the increase observed on day 36 being statistically
significant. There were no other changes in females or in males
with respect to food consumption, behaviour or general condition
(including ocular and dental examinations), or macroscopic
examinations of organs. The NOAEL was less than 4.6 mg/kg bw/day in
females (based on deaths and increased liver weights) and equal to
3.7 mg/kg bw/day in males (Donaubauer et al. 1985).
In a 13-week study, groups of 20 male and 20 female CD-1 mice
received dietary levels of 0, 2, 6, 18, or 54 ppm of technical
endosulfan. These concentrations were equivalent to doses of
approximately 0, 0.24-0.27, 0.74-0.80, 2.13-2.39, or 7.3-7.5 mg/kg
bw/day, respectively.
Adverse findings appeared to be confined primarily to the
highest dose level of endosulfan (i.e., 54 ppm). The observed
changes included deaths (12/20 males and 10/20 females at various
study intervals), convulsions and salivation (1/20 males and 1/20
females), reduced neutrophils (males on weeks 6 and 13), increased
total serum lipids (females on week 13), reduced spleen weight
(males), and slight vascular congestion in the lungs (11/20 males
and 6/20 females). All of those parameters were unchanged in all
other treatment groups. At dose levels of 6, 18, and 54 ppm, body
weight gain was depressed in male mice at study week 1 only, and
blood glucose levels were depressed in females at study week 6 only.
Neither of these changes persisted throughout the study and are thus
not considered to be of toxicological importance. Similarly, food
consumption was reduced only on study weeks 1 and 2 in mice of both
sexes at the 54 ppm dose level. From the above data, the NOAEL was
considered to be 18 ppm, equivalent to 2.13 to 2.39 mg/kg bw/day in
the diet, respectively, in male and female mice (Barnard et al.
1984).
Rats
Endosulfan was fed for 4 weeks in the daily diet to groups of
100 male Wistar rats at concentrations of 360 and 720 ppm. These
concentrations were said to be equivalent to doses of 34.0 and
67.8 mg/kg bw/day, respectively. An additional 20 male rats served
as control animals. At the end of 4 weeks treatment, one-half of
the animals in each group were killed (main study group) and the
other half were allowed to recover for 4 more weeks before being
sacrificed (recovery group). Rats were examined daily for behaviour
and general health conditions. Body weights and food consumption
were recorded weekly. At autopsy, all animals were examined
grossly, and the relative weights of three organs (liver, kidneys,
brain) were calculated. Histological exams were performed on the
left kidney, and a piece of liver from six animals/dose level; the
right liver, another piece of liver and the brain from two
animals/dose level were examined by electron microscopy. The
remaining parts of the above organs not used for histological study
were used to identify residues of endosulfan in tissue.
Two deaths occurred due to unknown causes (one rat at 360 ppm
on day 29 and one rat at 720 ppm on day 8). There were no
treatment-related effects in the appearance or general health of the
animals (including signs of neurological disturbances, corneal
opacity, oral mucosal lesions, or changes in dental growth), in body
weight gain, or in food consumption. The relative weights of the
liver were increased at both dose levels, and relative kidney and
brain weights were increased at the highest dose level. Upon gross
examination, the kidneys appeared darkened in colour at both dose
levels. Histopathological examination showed proliferation and
enlargement of lysosomes of the cells of the proximal convoluted
tubules, and granular pigmentation of the same cells, at both doses
of endosulfan. Lysosomal changes were not seen in brain or liver.
The above findings were not seen in animals in the 4-week recovery
group. The identification of residues of endosulfan in tissues was
unsuccessful. There was no NOAEL in this study due to the observed
renal and liver changes at 360 ppm, equivalent to 34.0 mg/kg bw/day
in the diet (Leist & Kramer, 1985).
In a follow-up report, the results of repeat tissue residue
analysis studies after dietary dosing with 360 and 720 ppm in Wistar
rats for 4 weeks, and with a recovery period of an additional 4
weeks, were made available. The findings indicated that
alpha-endosulfan, and to a considerably lesser extent
beta-endosulfan, was temporary stored in the kidneys in proportion
to the administered dose. Only endosulfan-sulfate and
endosulfan-lactone were detected in any appreciable quantities in
the kidneys. It was also noted that endosulfan and its metabolites
were minimally detectable in the blood. In the liver,
endosulfan-sulfate and endosulfan-lactone were the dominant
compounds, although their levels were many times lower than the
concentrations of alpha-endosulfan measured in the kidneys. It was
also observed that the storages were reversible; 30 days after the
end of the treatment phase alpha-endosulfan was detected only in
traces, and beta-endosulfan not at all (Leist & Mayer, 1987).
In a 13-week study, groups of 25 male and 25 female CD rats
were fed diets containing 0, 10, 30, 60, or 360 ppm technical
endosulfan. Daily dosages were equivalent to 0, 0.64, 1.92, 3.85,
and 23.41 mg/kg bw/day in males, and 0, 0.75, 2.26, 4.59, and
27.17 mg/kg bw in females. At the end of the 13-week treatment
period all animals were sacrificed except for 5 rats/sex dose group
that were withdrawn from treatment and followed for an additional
4-week recovery period. Ophthalmoscopic exams were performed on
control and high-dose rats before treatment and at week 13.
Neurological exams (locomotor reflexes) were performed on 10
rats/sex from the control and high-dose levels before treatment and
on weeks 2, 6, and 13; in addition, all animals were examined for
grip reflex and evidence of ataxia on week 13. Blood was sampled at
0, 6, and 12/13 weeks from 10 rats/sex/dose for assessment of
hematology and clinical chemistry parameters. A standard battery of
tests/examinations was performed and, in addition, blood and plasma
cholinesterase estimations were made at 5 and 12 weeks in 10
rats/sex/dose level. Urine was collected on weeks 4 and 13 for
standard urinalysis determinations. Similar studies were performed
in the rats that were allowed to complete the recovery period.
Three deaths occurred during the treatment period (1/20 female
controls, 1/20 females at 60 ppm due to anesthetic shock during
routine blood sampling, and 1/20 females at 320 ppm on day 19). No
deaths occurred in rats during the recovery period.
The only clinical sign of toxicity observed was hair loss in
females receiving 60 and 360 ppm endosulfan. Body weight gain was
reduced in male and female rats to a slight degree over the course
of the study. Food consumption was reduced in females given 360 ppm
during study weeks 1 and 2 only. All of these changes regressed by
the end of the recovery period. No adverse ophthalmological or
neurological findings were observed in treated rats.
A variety of hematological changes were observed during the
treatment period. Red blood cells were reduced at week 13 (and
after week 6) in male rats at doses of 30 to 360 ppm. MCV was
increased in female rats at weeks 13 and 6 at doses of 60 and
360 ppm. Finally, PCV was reduced at weeks 13 and 6 in male rats
given 360 ppm. In most cases, these effects were still evident in
rats, especially males, at recovery week 17. Changes involving
clinical chemistry parameters included increases in serum phosphorus
in females (weeks 12 and 6) at 30 and 360 ppm, reductions in serum
electrocytes (Na+ and K+) in males (weeks 12 and 6) at 60 and
360 ppm, and increased in total lipids and cholesterol in females
(weeks 12 and 6) at 360 ppm. In the case of urinalysis
determinations, a darkened urine was observed in male rats (week 13)
at 60 and 360 ppm, and in females (week 13) at 360 ppm. The urine
of the male rats at 60 and 360 ppm also contained elevated levels of
ketones and proteins. The clinical chemistry and urinalysis changes
regressed by the end of the recovery period.
Lower plasma and red blood cells cholinesterase activities were
observed in females (weeks 12 and 5) given 360 ppm endosulfan. In
contrast, increased brain cholinesterase activity was seen in
females treated with 60 and 360 ppm. These changes regressed during
the recovery period.
At necropsy, increases were noted in the relative weights of
the kidney (male rats at 60 and 360 ppm, and female rats at
360 ppm), liver (males and females at 360 ppm), brain (females at 60
and 360 ppm), and epididymes (males at 360 ppm). Increased kidney
weights were also seen in high-dose males at the end of the recovery
period. Macroscopic findings included enlarged kidneys and enlarged
liver. Histopathological findings also involved the kidneys and the
liver. Two main findings occurred in the kidneys. The first was
the observation of occasional cells of the proximal convoluted
tubules showing yellowish discoloration of the cytoplasma (incidence
in males). The second was another type of darker and more
particulate granular and/or clumped pigment in the straight portions
and occasionally in the proximal convoluted tubules. In addition,
male rats given 360 ppm endosulfan also showed yellowish coloured
protein aggregations in the proximal convoluted tubule and
intracytoplasmic eosinophilic droplets in the tubules. During the
recovery period the discoloration decreased whereas granular pigment
still persisted. In the liver, minimal centrilobular enlargement of
hepatocytes was observed. The liver change was not seen in the
recovery group rats.
The NOAEL is estimated to be 10 ppm in female rats, equivalent
to 0.75 mg/kg bw/day in the diet. The lowest effect level is 30 ppm
(i.e., 2.26 mg/kg bw/day) based upon the findings of increased serum
phosphorus levels and yellow discolored cells in the renal proximal
convoluted tubules of 1/20 females. Both of these changes were
produced by higher doses of endosulfan in female rats in a
dose-related fashion. The NOAEL for male rats is less than 10 ppm
(lowest dose tested), equivalent to 0.64 mg/kg bw/day in the diet.
This is based upon the finding of yellow discolored cells in the
renal proximal convoluted tubules in 14/20 males (Barnard et al.
1985).
In a dermal toxicity study, endosulfan was applied to the skin
of male and female Wistar rats (6/sex/dose) at doses of 0, 1, 3, 9,
and 27 mg/kg bw, and to males only at 81 mg/kg bw for 21
applications over 30 days. Toxic effects of the test compound
occurred at doses of 9 mg/kg bw or more and included deaths, liver
abnormalities (enlarged parenchymal cells with loss of cytoplasmic
basophilia, isolated cell necrosis, and frequent mitosis) in
occasional animals and significantly elevated absolute and relative
spleen weights in females. The deaths that were observed in female
rats were accompanied by tono-clonic convulsions. The NOAEL for
subchronic dermal toxicity is 3 mg/kg bw/day (Ebert et al. 1985a).
In a similar dermal toxicity study, endosulfan was applied to
the skin of Wistar rats at doses of 0, 12, 48, 96, and 192 mg/kg bw
in males and 0, 3, 6, 12, and 48 in females for 21 applications over
29 days. In this study, male rats exhibited increases in relative
kidney weights at doses of 12, 96 and 192 mg/kg bw (attributed by
the study authors to slightly lower body weights in males), deaths
in 36 percent of the animals at 192 mg/kg bw and a discrete
deposition of pigment in a few cells of the proximal convoluted
tubule in one high dose rat. In female rats, clinical signs of
toxicity were first observed at 12 mg/kg bw, and included
piloerection, salivation and lacrymation, blood from the eyes and
nose with increasing doses of the chemical, and deaths in 18% of the
animals due to the tono-clonic convulsions at 48 mg/kg bw. No other
treatment-related effects were noted (Ebert et al. 1985b).
In an inhalation study, groups of 15 male and 15 female SPF
Wistar rats were administered 0, 0.0005, 0.0010, and 0.0020 mg
endosulfan/L of air for 21 exposures (6 hours/day) for 29 days. Two
control groups (air and ethanol-PEG 400 vehicle) were used. Five
animals/test group were held for a 4-week recovery period after
receiving the test aerosol. The NOAEL is 0.001 mg/l of air in males
(based upon the decrement in weight gain of 12 to 16% seen in
recovery animals) (Hollander et al., 1984).
Dogs
Endosulfan technical was fed for one year in the daily diet to
groups of 6 male and 6 female beagle dogs at concentrations of 0, 3,
10, 30, and 30-60 ppm. The latter dietary level was increased in
stages from 30 to 45 ppm after 54 days of treatment, and then to
60 ppm after 106 days of treatment.
No spontaneous deaths were observed in endosulfan-treated
animals. However, 13 dogs were sacrificed in extremis. These
included one male in the 30 ppm dose group after 9 months due to a
poor overall general condition (resulting from refusal to eat and
severe purulent mediastinitis), and all high-dose males and females
after 4 to 5 months due to marked and progressive nervous symptoms.
These took the form of extreme sensitivity to noise and optic
stimuli, tonic contractions of muscles in the extremities, face and
cheeks, and weakened or abolished placing and righting reactions.
Similar nervous symptoms (i.e., violent contraction of the abdominal
muscles and convulsive movements of the cheeks) also occurred in
most of the surviving dogs in the 30 ppm dose group.
Food consumption was reduced in male and female dogs at 30 ppm
(on weeks 1 to 7) and at 3-60 ppm (from week 16 until the time of
sacrifice). Similarly, body weight gain was reduced in male dogs at
30 ppm (from week 43 onward) and in dogs of both sexes at 30-60 ppm
(from week 10 until the time of sacrifice). No adverse
ophthamological findings were observed in treated dogs, and tests to
examine hearing and dental status were normal. Standard
examinations for hematology, clinical chemistry, and urinalysis were
reported to be normal, as were determinations of cholinesterase
activity in serum, red cells, and brain. No treatment-related
alterations in bromsulphthalein retention of phenolsulphon-pthtalein
elimination were noted in special tests to assess hepatic and renal
function, respectively.
No dose-related changes in organ weight or in gross or
microscopic pathology were observed. The only histopatholopical
findings of interest were the occurrence of acute liver congestion
in 3/6 high-dose male dogs, and the presence of brown pigment in the
renal epithelium of 1/6 high-dose male dogs. The NOAEL in the study
was 10 ppm, equivalent to 0.65 mg/kg bw/day in the diet for males
and 0.57 mg/kg bw/day for females (Brunk, 1989).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice were fed diets containing endosulfan
technical (98.8% ppm) for 78 weeks. The time-weighted average (TWA)
dietary concentrations were 3.5 and 6.9 parts per million (ppm) for
males (50/dose group) and 2 and 3.9 ppm for females (50/dose group).
Twenty mice/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 0.52
and 1.0 mg/kg bw/day in males, and 0.3 and 0.58 mg/kg bw/day in
females.
High early mortality was observed among male mice. Eleven
high-dose males and two low-dose males died on week 19. Survival at
term was 15% (3/20) in control males, 38% (19/50) in low-dose males,
and 10% (5/50) in high-dose males. The deaths in the controls may
have been due to fighting. No common cause of death was found for
the high-dose males. Survival was high among all female mice (85%
of control, 94% of low dose, and 96% of high-dose females survived
to term). No effects of treatment on body weight or clinical signs
of toxicity were observed. No dose-dependent non-neoplastic lesions
were noted in female mice. However, the abbreviated lifespan of
male mice was considered to preclude any accurate analysis of
late-occurring tumours. The NOAEL in this study was less than
3.5 ppm (i.e., 0.25 mg/kg bw/day; lowest dose tested) in male mice
due to mortality, and 3.9 ppm (i.e., 0.58 mg/kg bw/day; highest dose
tested) in female mice (NCI, 1978).
A second chronic feeding study of endosulfan technical (97.9%
pure) was performed in Hoe:NMRKf(SPF 71) mice in which 60
animals/sex/dose level received dietary concentrations of 0, 2, 6,
and 18 pm for 24 months. An additional 10 mice/sex/dose were
sacrificed at interim periods of 12 and 18 months, respectively.
The administered concentrations were calculated to be equivalent to
0, 0.28. 0.84, and 2.51 mg/kg bw/day in males and 0, 0.32, 0.097,
and 2.86 mg/kg bw/day in females.
Survival was significantly reduced in female mice with 18 ppm
endosulfan in the main portion of the study (total deaths in females
= 33/60 or 55% controls, 36/60 or 60% low dose, 38/60 or 63.3% mid
dose, and 43/60 or 71.7% high dose). Survival was not affected by
the test compound in male mice in the main study (total deaths in
males = 27/60 or 45% controls, 33/60 or 55% low dose, 37/60 or 61.7%
mid dose, and 35/60 or 58.3% high dose). There was no
compound-related effect on mortality in either female or male mice
at the 12- and 18-month interim sacrifice periods. Body weight
gain was slightly reduced (about 10%) in male mice throughout the
main study at 18 ppm endosulfan. Female weights were slightly
elevated in the 12- and 18-month satellite groups, body weight was
either slightly increased or remained unchanged from controls in
animals of all treatment groups. There were no treatment-related
effects on food consumption, behavioural or general health
conditions of the animals, or hematological or clinical chemistry
parameters.
Gross and microscopic examination were performed at 12, 18, and
24 months. Reductions in several organ weights occurred with the
18 ppm dose level of endosulfan; these included decreases in the
weights of the liver (males at 18 months only), and ovaries (females
at 12 and 18 months). No treatment-related pathological changes or
tumours were seen after gross or histopathological examination of
tissues at 12, 18, or 24 months. The NOAEL was 6 ppm endosulfan,
equivalent to 0.84 mg/kg bw/day in male mice and 0.97 mg/kg bw/day
in female mice (Donaubauer, 1988).
Rats
Groups of Osborne-Mendel rats were fed diets containing
endosulfan (technical grade; 98.8% pure) for 78 weeks. The TWA
dietary concentrations were 408 and 952 ppm for males (50/dose
group) and 223 and 445 ppm for females (50/dose group). Twenty
rats/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 20.4
and 47.6 mg/kg bw/day in males, and 11.1 and 22.1 mg/kg bw/day in
females.
Endosulfan produced early mortality (dose-related) in male
rats. By study week 54, 52% of the high-dose males died and by week
74, 88% were dead. The high-dose males were sacrificed on week 74.
For the low-dose males, only 10/50 (20%) survived to term, and these
were sacrificed on week 82. Survival was adequate in control males.
In the case of female rats, no compound-related deaths occurred (70%
of control, 60% of low dose, and 50% of high-dose females survived
to term). Body weight was also depressed in a dose-related fashion
in treated male rats from week 22 onward. At the time of sacrifice,
body weight was depressed about 25% in both low- and high-dose male
rats compared to the control male group. Body weight was not
markedly changed in female rats. Several clinical signs of toxicity
were also seen in treated rats of both sexes; these included hunched
appearance, reddened and squinted eyes, rough fur, and abdominal
urine stains.
Upon histopathological examination, toxic nephropathy was seen
in both male (0/20 controls, 47/50 low dose, 43/47 high dose) and
female (0/20 controls, 27/50 low dose, 29/50 high dose) rats. This
abnormality was characterized by degenerative changes in the
proximal convoluted tubules at the corticomedullary junction, with
cloudy swelling, fatty degeneration and necrosis of the tubular
epithelium. Some affected tubules contained hyaline casts and
enlarged dark-staining regenerative tubular epithelial cells, and
the kidneys often had inflammatory cell infiltration, fibrotic
changes, and focal mineralization. Parathyroid gland hyperplasia
was also seen in male rats (0/20 controls, 21/40 low dose, 18.47
high dose) and was attributed to the effects of chronic renal
failure. Medial calcification of the blood vessels, perhaps related
to the parathyroid hyperplasia, occurred frequently in treated male
rats. Finally, testicular atrophy occurred in male rats (3/19
control, 18/47 low dose, 24/47 high dose) and was characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules, multinucleated cells (fusion bodies), and
calcium deposition resulting in aspermatogenesis.
No conclusions concerning the carcinogenicity of endosulfan
could be drawn from the bioassay of male rats because of the high
early mortality and abbreviated lifespans of these animals.
Survival in female rats was considered to be sufficient for the
evaluation of late occurring tumours; endosulfan was not
carcinogenic in female rats. The results of this long-term bioassay
in rats indicate that the lowest dietary concentrations of
endosulfan tested in males (408 ppm TWA - equivalent to
20.4 mg/kg/day) and females (223 ppm TWA - equivalent to
11.1 mg/kg/day) were toxic and that a NOAEL could not be established
(NCI, 1978).
A second chronic feeding study of endosulfan technical (97.1%
pure) was performed in Charles River Crl:CD(SD)BR rats in which 50
animals/sex/dose level received dietary concentrations of 0, 3, 7.5,
15, and 75 ppm for 104 weeks. An additional 20 rats/sex/dose
received similar doses of endosulfan and were used to obtain
hematology, clinical chemistry, and urinalysis data at various
intervals throughout the study. Gross and microscopic examinations
were performed on all animals. The administered concentrations of
endosulfan were calculated to be equivalent to 0.1, 0.3, 0.6, and
2.9 mg/kg bw/day in males and 0.1, 0.4, 0.7, and 3.8 mg/kg bw/day in
females.
There were no compound-related effects on mortality, clinical
signs of toxicity, food and water consumption, or ophthalmoscopic
parameters. Adverse findings were mainly observed at the highest
dose of endosulfan. These included significant reductions in body
weight gain over the study in males (-17%) and females (-18%), a
reduced food utilization efficiency in females, hematological
changes at week 103 in males (increased platelets, and reductions in
mean corpuscular volume, total white cells, and lymphocytes), and
clinical chemistry changes at week 103 in males (increased plasma
globulin and urinary protein levels, and reduced GPT and serum
albumin levels). The reductions in total white cells and
lymphocytes, and the increased urinary protein levels, were said to
be within expected limits of variation. It should be noted that
body weight gain was also reduced in males given 15 ppm endosulfan
on study weeks 6 to 18 only (-9% reduction); this effect, however,
was not progressive after week 18 and was not considered to be
toxicologically significant.
Gross and microscopic examination of all animals in the study
indicated that the primary target organ for toxicity was the kidney.
This was noted from an increased number of enlarged kidneys seen
grossly for females at 75 ppm (10/70 controls, 18/70 at 3 ppm, 19/70
at 7.5 ppm, 17/70 at 15 ppm, and 26/70 at 75 ppm), and an elevated
incidence of marked progressive glomerulonephritis in males at
75 ppm, 24/70 at 15 ppm, and 30/70 at 75 ppm) and also in females at
75 ppm (1/70 controls, 6/70 at 3 ppm, 6/70 at 7.5 ppm, 5/70 at
15 ppm, and 8/70 at 75 ppm). In addition, blood vessel aneurysms
were elevated in male rats at 75 ppm (10/70 controls, 6/70 at 3 ppm,
14/70 at 7.5 ppm, 10/70 at 15 ppm, and 21/70 at 75 ppm). The
aneurysms were considered to be possibly related to the renal
lesions.
Other observations in the study at 75 ppm in male rats included
a greater number of enlarged lumbar lymph nodes (14/70 controls,
10/70 at 3 ppm, 8/70 at 7.5 ppm, 7/70 at 15 ppm, and 19/70 at
75 ppm), inflammatory cells in the liver (15/70 controls, 16/70 at
3 ppm, 15/70 at 7.5 ppm, 18/70 at 15 ppm, and 25/70 at 75 ppm), and
polyarteritis in the pancreas (0/70 controls, 2/70 at 3 ppm, 4/70 at
7.5 ppm, 1/70 at 15 ppm, and 8/70 at 75 ppm) and testes (16/70
controls, 15/70 at 3 ppm, 23/70 at 7.5 ppm, 18/70 at 15 ppm, and
27/70 at 75 ppm). Finally, the mean weights of the testes were
reduced at 15 ppm and 75 ppm of endosulfan, but the response was not
strictly dose-related and was within expected limits of variation.
Endosulfan was not carcinogenic in either male or female rats.
The NOAEL was said to be 15 ppm, equivalent to 0.6 mg/kg bw/day in
male rats and 0.7 mg/kg bw/day in female rats (Ruckman et al.
1988).
Reproduction studies
Rats
Male and female Charles River SD-CD rats were administered
endosulfan (97% purity) in the diet for two generations at
concentrations of 0, 3, 15, and 75 ppm (equal to approximately 0.2,
1.0, and 6.0 mg/kg bw/day). Females were mated to males on a
one-to-one basis with 32 males/32 females per dose in the F0
generation, and 28 males/28 females per dose for the F1 generation.
F0 generation rats were administered endosulfan from 6 weeks of age
through 18 weeks of age, at which time they were mated to produce
F1a litters. F1a young were sacrificed 21 days postpartum, and the
F0 generation remated to produce the F1b litters. After weaning of
the F1b pups, the F0 generation rats were sacrificed. The F1b rats
were continued on their respective diets for approximately 98 days
prior to mating to produce the F2a litters (which were sacrificed
after weaning) and the F2b litters (likewise sacrificed after
weaning). Endosulfan was administered continuously throughout the
experiment.
There were no compound-related effects on mortality, food/water
consumption, or body weight gain, although F1b females given 3 ppm
demonstrated slightly lower body weights than controls from week 4
through 36. Pregnancy rate, mating performance and gestation period
for both F0 and F1b generations were unaffected by treatment.
Both matings of the F0 generation demonstrated significant
decreases in litter weight at 75 ppm from day 4 through day 21 but
not in either F1b mating. Also, F0 generation litter weights were
significantly reduced on days 4 to 21 during the second mating at
15 ppm as well. Although the trend for cumulative litter loss (%)
was increased in the first mating at 75 ppm, it was not evident in
the second mating nor in either mating of the F1b generation. The
mean pup weight was unaffected throughout both F0 and F1b matings.
Sex ratio appeared to be unaffected throughout the study.
F0 and F1b generation parents presented evidence of increased
absolute organ weights for heart, liver, kidneys, and brain at
75 ppm. These were not demonstrated in the offspring from either
mating of these parents. There was no consistent dose-related
effect. Histopathology of F1b adults and F2b weanlings
demonstrated no compound-related effects. F0 and F1b generation
females and offspring were examined for skeletal and visceral
abnormalities and no consistent dose-related trends were evident,
except for isolated incidences of microphthalmia (2), anophthalmia
(2), kinked/missing tail (3), shortened/malformed digits or limbs
(5), retinal hemorrhage (1) and spina bifida (1).
There were no dose-related or compound-related effects on
reproductive performance. Decreases in litter weight at 15 and
75 ppm occurred in the F1a and F1b litters; however, this finding
was not considered to be adverse since the reductions in litter
weight were marginal and there were no significant corresponding
reductions in pup weight or litter size. The visceral and skeletal
examinations of fetuses for assessing teratogenic effects were
within normal variation for the strain/species studied. It is
considered that endosulfan, at dietary levels up to and including
75 ppm (approximately 6 mg/kg/day in the diet) caused no adverse
effects on reproduction in rats in this study (Edwards et al.
1984).
However, as noted below, renal changes occurred in F1b adult
rats at all of the doses of endosulfan that were tested. In an
addendum to the above study, a histological review of the kidneys of
adult rats from the F1b generation and of weanling rats from the F2b
generation was reported. The effort was initiated because yellowish
discoloration and pigment deposits were noted in renal proximal
convuluted tubules in rats in a 13-week oral toxicity study of
endosulfan followed by a 4-week withdrawal period (see Barnard
et al. 1985). The histological data obtained indicated the
presence of yellowish discolored cells in the proximal convoluted
tubules in F1b adult rats given dietary doses of 3, 15, and 75 ppm
endosulfan. The incidences in males were: 0/28 controls, 11/28 at
3 ppm, 13/28 at 15 ppm, and 28/28 at 75 ppm. The incidences in
females were: 0/28 controls, 0/28 at 3 ppm, 0/28 at 15 ppm, and 9/28
at 75 ppm. In addition, granular/clumped pigment was also noted at
a dietary dose of 75 ppm in F1b adult rats. The incidences at
75 ppm were 14/28 males and 1/28 females vs. an incidence of 0/28 in
males and females from the control, 3 ppm, and 15 ppm dose groups.
In the F2b generation weanling rats, no comparable renal changes
were observed following treatment with endosulfan (Offer, 1985).
These findings were not considered to be adverse since the yellow
pigment in kidneys was identified as endosulfan and its metabolites,
being stored and metabolized in lysosomes prior to excretion.
Special studies on teratogenicity
Rats
Groups of 25 pregnant CD Sprague-Dawley rats were administered
endosulfan (97.3% purity), via oral gavage, at dose levels of 0,
0.66, 2, and 6 mg/kg bw/day from days 6 to 19 of gestation.
(Day 0 = day sperm found). Ten additional animals were added to the
high dose (due to mortality) and five to the control groups (due to
loss of tissue during processing). On day 20 of gestation, all dams
were killed by asphyxiation with CO2 and fetuses delivered by
cesarean section.
Maternal toxicity was evident in the mid- and high-dose groups,
expressed by significantly reduced body weight and body weight gain
during gestation, and by various clinical signs of toxicity (e.g.,
rats rubbing their faces, alopecia, rough coats, lethargy,
flaccidity, and hyperactivity). Several animals died as the result
of improper gavage tube insertion. Fetal and embryolethality were
not apparent in any group and all pregnant animals had viable
litters. Mean fetal weight and crown-rump length were significantly
reduced in the high-dose group, but not in the lower dose groups. A
number of external, visceral, and skeletal abnormalities were
observed in the fetuses in the high-dose group. The most prominent
effect was a significant increase in the incidence of small fourth
and unossified fifth sternebrae in the high-dose fetuses. However,
there was no clear evidence of a teratogenic effect due to the
significant maternal toxicity expressed at the same level. The
NOAEL for maternal toxicity was 0.66 mg/kg bw/day and that for
fetotoxicity was 2 mg/kg bw/day by oral gavage (MacKenzie et al.
1980a).
A range-finding study with endosulfan in pregnant CD
Sprague-Dawley rats (4-6/group) was performed for the purpose of
setting doses for the above described rat teratology study.
Endosulfan was administered by gavage on days 6 to 19 of gestation
(Day 0 = day sperm found) at doses of 0 (corn oil vehicle), 1.25,
2.5, 5, 10, 20 and 40 mg/kg bw/day. Toxic signs in dams included
reductions in body weight gain at doses of 1.25 mg/kg bw/day and
greater, various clinically observed adverse effects at doses of
2.5 mg/kg bw/day and greater (e.g., salivation, piloerection, poor
muscle tone, lethargy, head-rubbing behaviour, hyperactivity,
spasticity, tremors and convulsions, and deaths (67 to 100%
mortality) at 10 mg/kg bw/day and greater. The changes were dose
related and most marked at higher doses of endosulfan. No effects
on pregnancy maintenance or fetal survival were observed (MacKenzie
et al. 1980b).
Rabbits
Groups of pregnant New Zealand White rabbits (20/group) were
administered endosulfan (97.3% purity) via oral gavage in corn oil
at dose levels of 0, 0.3, 0.7, and 1.8 mg/kg bw/day from days 6 to
28 of gestation (Day 0 = day of mating). Animals were observed
throughout the test period for toxic signs. Six additional pregnant
rabbits were added to the high-dose group due to mortality. On day
29 of gestation, the dams were asphyxiated with CO2, and the entire
reproductive tract was removed.
Maternal toxicity was evident at the high dose, expressed as
rapid breathing, hyperactivity, convulsions and mortality. Group
mean body weights were not affected by treatment. All pregnant dams
had viable fetuses, with the exception of one high-dose dam which
died on test and which had nine late resorptions. There were no
compound-related effects on the number of corpora lutea,
implantation efficiency, litter size, sex ratio, mean fetal length
or weight, or the number of live/dead or resorbed fetuses. There
were no gross external abnormalities and the visceral and skeletal
examinations revealed no dose-related increase in variations or
anomalies. The NOAEL for maternal toxicity was 0.7 mg/kg bw/day.
Endosulfan was not teratogenic in rabbits at doses up to and
including 1.8 mg/kg bw, although maternal toxicity was expressed at
that level (MacKenzie et al. 1981a).
A range-finding study with endosulfan in pregnant New Zealand
White rabbits (3 to 10/group) was conducted to set doses for the
above described rabbit teratology study. Endosulfan was
administered by gavage on days 6 to 28 of gestation (Day 0 = day of
mating) at doses of 0 (corn oil), 1, 2, 4, 8, and 12 mg/kg bw/day.
Standard observations and necropsy procedures were performed. Toxic
signs in dams included deaths at 2 mg/kg bw/day or more (20 to 100%
mortality) and various clinical adverse effects at 2 mg/kg bw/day or
more (e.g., hyperactivity, opisthotonos, clonic and tonic
convulsions, paralysis, tremors, and loss of mobility). Fetal
survival appeared to be adversely affected at 2 mg/kg bw or more as
indicated by an increase in the percent of dead fetuses and a
decrease in the percent of live fetuses in dams. No prominent
effects on weight gain or pregnancy rate occurred (MacKenzie et al.
1981b).
Special study on mutagenicity
Endosulfan was tested for mutagenic activity in a variety of
bacterial, yeast, and mammalian cell systems and was negative in
each test (Table 1).
TABLE 1. RESULTS OF MUTAGENICITY STUDIES ON ENDOSULFAN
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
Ames Testa S.typhmurium 6-5000 ug/plate Negative Shirasu et al. 1978
TA98, TA100, TA1535,
TA1537, TA1538
Yeast Forward Mutation Schizosaccharomyces pombe 62.5-500 ug/plate Negative Milone 1984a
Testa
Yeast Gene-Conversion/ S. cerevisiae 100-5000 ug/ml Negative Milone, 1984b
DNA Repair Testa (D4 strain)
Mouse Lymphoma Assaya Mouse L51786 TK+/- Cells 6.25-100 ug/ml Negative Cifone, 1984a
DNA Repair Assaya B. subtilis H17 and 20-2000 ug/disk Negative Shirasu et al. 1978
M45 (rec-)
Unscheduled DNA Synthesis Primary-Hepatocytes (Rat) 0.102-51 ug/ml Negative Cifone, 1984b
Unscheduled DNA Human Cell Line A 549 1-1000 ug/ml Negative Muller, 1988
Synthesisa
Micronucleus Assay NMRI Mice O.2-5 mg/kg, Negative Jung et al. 1983
(Bone Marrow) Orallyb
Micronucleus Assay Swiss Albino Male Mice 43.3 mg/kg Negative Usha Rani et al. 1980
(Bone Marrow) Orallyb
Chromosome Aberration Cultured Human Lymphocytes 0.05-100 ug/ml Negative Milone, 1986
Testa in vitro
TABLE 1 (CONTD).
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
In vivo Cytogenicity Male Albino Rats 11-55 mg/kg/day Negative Dikshith & Datta, 1978
Test (Bone Marrow Orally for 5 days
and Spermatogonia)
a With and without metbolic activation.
b Administered twice orally at an interval of 24 hours.
Special study on neurotoxicity
The acute delayed neurotoxicity of endosulfan was assessed in
adult domestic hens. The study consisted of three parts. First,
the LD50 determination was performed using oral (gavage) doses of
0, 40, 60, 90, 110, and 135 mg/kg bw endosulfan (5 hens/dose group).
The observed LD50 was 96 mg/kg bw. All deaths occurred within 2
hours of dosing. Toxic signs observed were subdued behaviour and
weight loss. Second, a protection assessment was performed using an
oral dose of 96 mg/kg bw of endosulfan alone, or in combination with
either phenobarbital (15 mg/kg bw i.m.), diazepam (2 mg/kg bw p.o.),
or atropine (10 mg/kg bw i.m.) plus 2-PAM (25 mg/kg bw i.m.). There
were 5 hens/group. The results did not provide evidence that any of
the protections reduced the number of mortalities occurring after
dosing with endosulfan. Third, a neurotoxicity assessment was
performed using groups of 10 hens. A vehicle control group was
dosed orally with corn oil, a positive control group with 500 mg/kg
bw TOCP, and four test groups were each dosed with the LD50
(96 mg/kg bw) dose of endosulfan. Dosing was followed by a 21-day
observation period, a redosing, and a final 21-day observation
period. Surviving hens were sacrificed at 42 days.
Histopathological examination of brain, spinal cord and peripheral
nerve tissues were performed on 9 of the 40 animals dosed with
endosulfan (these were the survivors to 42 days), 10/10 control
hens, and 10/10 TOCP-treated hens. The results indicated that
endosulfan did not produce any clinical signs of neurotoxicity or
neuropathological (i.e., histological) effects on the central or
peripheral nervous system. In contrast, hens treated with TOCP
displayed ataxia as well as significant axonal degeneration in the
spinal cord and in the peripheral (tibial) nerve (Roberts et al.
1983).
Special study on skin sensitization
Endosulfan had no sensitizing potential (delayed contact
hypersensitivity) in guinea pigs when tested by the Buehler method
(Jung & Weigand, 1983).
COMMENTS
In rats, the absorption of single oral doses of endosulfan
required 2 or more days; subsequent urinary and fecal excretion was
also slow, requiring 5 or more days. The biotransformation pathways
involve hydrolysis and oxidation to sulfur-free metabolites and
polar conjugates, and the formation of various sulfur-containing
moieties. Endosulfan preferentially accumulated in the kidney, with
lower levels in other tissues, the elimination half-time from
tissues being 3 to 7 days.
Endosulfan at dietary dose levels of 10 ppm and higher in
subchronic studies in rats produced yellow pigmentation of renal
cells. In contrast, renal cellular pigmentation was not observed in
subchronic studies in mice at similar dose levels (see reproduction
studies below).
In a one-year dietary study in dogs, doses of 30 to 60 ppm
resulted in early sacrifices due to clinical signs of neurotoxicity
(e.g., convulsive muscular activity). Reduced food consumption and
weight gain also occurred. In this study, the NOAEL was 10 ppm,
equal to 0.57 mg/kg bw/day.
Long-term carcinogenicity studies were performed in mice and
rats. Treatment-related increases in neoplasms were not observed.
The NOAELs (based on non-neoplastic effects) were 6 ppm (equal to
0.84 mg/kg bw/day) in mice and 15 ppm (equal to 0.6 mg/kg bw/day) in
rats.
A two-generation, 2 litters per generation reproduction study
was conducted in rats. Endosulfan was associated with a reduction
in weight of the F1a and Flb litters at dietary levels of 15 and
75 ppm, and the presence of yellowish pigment in cells in the renal
proximal convoluted tubules at dose levels of 3, 15 and 75 ppm in
F1b adult rats. The Meeting did not consider these findings to be
adverse since the reduction in litter weights were marginal and
occurred in the absence of corresponding reductions in pup weight or
litter size. The yellow pigment in kidneys was identified as
endosulfan and its metabolites, being stored and metabolized in
lysosomes prior to excretion.
In teratology studies performed in rats and rabbits, NOAELs of
approximately 0.7 and 1.8 mg/kg bw/day were identified for maternal
toxicity and fetotoxicity, respectively. Endosulfan was not
teratogenic at 6 mg/kg bw/day in rats and 1.8 mg/kg bw/day in
rabbits, the highest dose levels tested.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 6 ppm in the diet, equal to 0.84 mg/kg bw/day
Rat: 15 ppm in the diet, equal to 0.6 mg/kg bw/day
Dog: 10 ppm in the diet, equal to 0.57 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E., Gibson,
W.A., Rao, R., Offer, J.M., Gopinath, G., Almond, R.H. (1984)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice. Unpublished Report No. A29663 from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street,
A.E., Gibson, W.A., Gopinath, G., Majeed, S.K., Almond, R.J. (1985)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice followed by a 4-week withdrawal period. Unpublished Report
No. A30700 from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Brunk, R. (1989) Endosulfan-substance technical: Testing for
toxicity by repeated oral administration (1-year feeding study) to
beagle dogs. Unpublished Report No. 89-0188 from Pharma Research
Toxicology and Pathology, Hoechst AG. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Cifone, M.A. (1984a) Mutagenicity evaluation of HOE 002671-
substance technical in the mouse lymphoma forward mutation assay.
Unpublished Report No. A29801 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Cifone, M.A. (1984b) Evaluation of HOE 002671-substance technical
in the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished Report No. A29800 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-Endosulfan. Unpublished Report No. A-36685 from WIL Research
Laboratories, Inc., Ashland, Ohio. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Dikshith, T.S.S., Datta, K. (1978) Endosulfan: lack of cytogenetic
effects in male rats. Bull. Environ. Contam. Toxicol., 20:
826-833.
Donaubauer, H.H. (1988) Endosulfan-substance technical.
Carcinogenicity study in mice - 24 months feeding study.
Unpublished Report No. A38008 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Donaubauer, H.H., Leist, K.H., Kramer, M. (1985) Endosulfan-
substance technical: 42-day feeding study in mice. Unpublished
Report No. A38104 from Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Leist, K.H., Kramer, P. (1985a) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in Wistar rats. Unpublished Report
No. A30753 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Weigand, W., Kramer, P. (1985b) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in SPF Wistar rats. Unpublished Report
No. A30754 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Edwards, J.A., Reid, Y.J., Offer, J.M., Almond, R.H., Gibson, W.A.
(1984) Effects of Endosulfan-technical on reproductive function of
multiple generations in the rat. Unpublished Report No. A29428
(Huntingdon Report No. HST 204/83768) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W. (1983) Hoe 002671-active ingredient
technical. Testing for acute aerosol inhalation toxicity in male
and female SPF Wistar rats, 4 hours - LC50. Unpublished Report
No. A32087 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W., Kramer, P. (1984) Endosulfan-active
ingredient technical. Testing for subchronic inhalation toxicity -
21 exposures in 29 days in SPF Wistar rats. Unpublished Report
No. A29823 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Jung & Weigand, W. (1983) Hoe 002671-active ingredient technical.
Testing for sensitizing properties in female Perbright-White guinea
pigs. Unpublished Report No. A27248 from Pharma Forschung
Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Jung, Weigand, W., Kramer, P. (1983) Hoe 112671-active ingredient
technical. Micronucleus test in male and female NMRI mice following
oral administration. Unpublished Report No. A29689 from Pharma
Forschung Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Lachmann, G.(1987) Dermal absorption of 14C-Endosulfan in rhesus
monkeys. Unpublished Report No. A36685 from Battelle-Institut,
Frankfurt, FRG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Kramer, P. (1985) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A30776 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Mayer, D. (1987) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A37112 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Dickie, S.M., Jackson, T.A.,
Zelinger, D.J. (1981a) Teratology study with FMC 5462 in rabbits.
Unpublished Report No. A23192 (Raltech Study No. 80070) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1981b) Range-finding study with FMC 5462 in pregnant rabbits.
Unpublished Report No. A37682 (Raltech Study No. 79032) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1980a) Teratology study with FMC 5462 in rats. Unpublished
Report No. A21393 (Raltech Study No. 79041) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M. (1980b)
Range-finding study with FMC 5462 in pregnant rats. Unpublished
Report No. A37680 (Raltech Study No. 79031) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Milone, M.F. (1984a) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished Report No. A29312 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1984b) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Saccharomyces cerevisiae.
Unpublished Report No. A29313 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1986) Study of the capacity of the test article
Endosulfan, substance technical, to induce chromosome aberration in
human lymphocytes cultured in vitro. Unpublished Report No. A33127
from Instituto di Richerche Biomediche "Antoine Marxer", Italy.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Muller, W. (1988) Evaluation of Endosulfan-substance, technical in
the unscheduled DNA synthesis test in mammalian cells in vitro.
Unpublished Report No. A38455 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
National Cancer Institute (NCI) (1978) Bioassay of Endosulfan for
possible carcinogenicity. Technical Report No. 62 from US
Department of Health, Education and Welfare, NIH. Submitted to WHO
by Hoechst AG, Frankfurt, FRG.
Offer, J.M. (1985) Addendum to HST 204: effect of Endosulfan-
technical on the reproductive function of multiple generations in
the rat. Histopathological review of the kidneys in adult rats of
the F1B generation and in weanling rats of the F2B generation.
Unpublished Report No. A30757 (Huntingdon Addendum to Report HST
204/83768) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Cooke, L., Gibson,
W.A. (1983) Acute delayed neurotoxicity study with Endosulfan-
technical in the domestic hen. Unpublished Report No. A32153
(Huntingdon Report No. HST 225/83888) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed,
S.K., Anderson, A., Chanter, D.O. (1988) Endosulfan, active
technical ingredient. Combined chronic toxicity/carcinogenicity
study (104-week feeding in rats). Unpublished Report No. HST
289/881067) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Shirasu, Y., Moriya, M., Ohta, T. (1978) Microbial mutagenicity
testing on Endosulfan. Unpublished Report No. A21215 from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Stumpf, K., ABhauer, J. (1986) An up-to-date review of the
environmental safety of Endosulfan. Unpublished Report No. A35180
from the Analytical Laboratory, Hoechst AG. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Usha Rani, M.V., Reddy, O.S., Reddy, P.P. (1980) Mutagenicity
studies involving aldrin, dieldrin, endosulfan, dimethoate,
phosphamidon, carbaryl and ceresan. Bull. Environ. Contam.
Toxicol., 25: 277-282.
Toxicological Studies
Acute toxicity
An acute nose-only inhalation toxicity test of endosulfan in
Wistar rats resulted in LC50 values of 0.0345 mg/L of air in males
and 0.9126 mg/L of air in females. Clinical signs of intoxication
were dyspnea, passivity, disequilibrium, trembling, tremors,
tono-clonic convulsions, and subdued reflex activity. Gross necropsy
changes in animals that died consisted of sporadic dark red pinhead
foci in the lungs (Hollander & Weigand, 1983).
Short-term studies
Mice
Groups of 10 male and 10 female Hoe:NMRKf(SPF 71) mice were fed
diets containing 0 or 18 ppm technical endosulfan for 42 days. The
18 ppm dose level was calculated to be equivalent to 3.7 mg/kg
bw/day in males and 4.6 mg/kg bw/day in females. The results
obtained indicated that deaths occurred in 2/10 treated mice (one
female on day 28 and another female on day 38), and absolute and
relative liver weights were significantly increased in females. In
addition, the body weight gain was slightly elevated throughout the
study, with the increase observed on day 36 being statistically
significant. There were no other changes in females or in males
with respect to food consumption, behaviour or general condition
(including ocular and dental examinations), or macroscopic
examinations of organs. The NOAEL was less than 4.6 mg/kg bw/day in
females (based on deaths and increased liver weights) and equal to
3.7 mg/kg bw/day in males (Donaubauer et al. 1985).
In a 13-week study, groups of 20 male and 20 female CD-1 mice
received dietary levels of 0, 2, 6, 18, or 54 ppm of technical
endosulfan. These concentrations were equivalent to doses of
approximately 0, 0.24-0.27, 0.74-0.80, 2.13-2.39, or 7.3-7.5 mg/kg
bw/day, respectively.
Adverse findings appeared to be confined primarily to the
highest dose level of endosulfan (i.e., 54 ppm). The observed
changes included deaths (12/20 males and 10/20 females at various
study intervals), convulsions and salivation (1/20 males and 1/20
females), reduced neutrophils (males on weeks 6 and 13), increased
total serum lipids (females on week 13), reduced spleen weight
(males), and slight vascular congestion in the lungs (11/20 males
and 6/20 females). All of those parameters were unchanged in all
other treatment groups. At dose levels of 6, 18, and 54 ppm, body
weight gain was depressed in male mice at study week 1 only, and
blood glucose levels were depressed in females at study week 6 only.
Neither of these changes persisted throughout the study and are thus
not considered to be of toxicological importance. Similarly, food
consumption was reduced only on study weeks 1 and 2 in mice of both
sexes at the 54 ppm dose level. From the above data, the NOAEL was
considered to be 18 ppm, equivalent to 2.13 to 2.39 mg/kg bw/day in
the diet, respectively, in male and female mice (Barnard et al.
1984).
Rats
Endosulfan was fed for 4 weeks in the daily diet to groups of
100 male Wistar rats at concentrations of 360 and 720 ppm. These
concentrations were said to be equivalent to doses of 34.0 and
67.8 mg/kg bw/day, respectively. An additional 20 male rats served
as control animals. At the end of 4 weeks treatment, one-half of
the animals in each group were killed (main study group) and the
other half were allowed to recover for 4 more weeks before being
sacrificed (recovery group). Rats were examined daily for behaviour
and general health conditions. Body weights and food consumption
were recorded weekly. At autopsy, all animals were examined
grossly, and the relative weights of three organs (liver, kidneys,
brain) were calculated. Histological exams were performed on the
left kidney, and a piece of liver from six animals/dose level; the
right liver, another piece of liver and the brain from two
animals/dose level were examined by electron microscopy. The
remaining parts of the above organs not used for histological study
were used to identify residues of endosulfan in tissue.
Two deaths occurred due to unknown causes (one rat at 360 ppm
on day 29 and one rat at 720 ppm on day 8). There were no
treatment-related effects in the appearance or general health of the
animals (including signs of neurological disturbances, corneal
opacity, oral mucosal lesions, or changes in dental growth), in body
weight gain, or in food consumption. The relative weights of the
liver were increased at both dose levels, and relative kidney and
brain weights were increased at the highest dose level. Upon gross
examination, the kidneys appeared darkened in colour at both dose
levels. Histopathological examination showed proliferation and
enlargement of lysosomes of the cells of the proximal convoluted
tubules, and granular pigmentation of the same cells, at both doses
of endosulfan. Lysosomal changes were not seen in brain or liver.
The above findings were not seen in animals in the 4-week recovery
group. The identification of residues of endosulfan in tissues was
unsuccessful. There was no NOAEL in this study due to the observed
renal and liver changes at 360 ppm, equivalent to 34.0 mg/kg bw/day
in the diet (Leist & Kramer, 1985).
In a follow-up report, the results of repeat tissue residue
analysis studies after dietary dosing with 360 and 720 ppm in Wistar
rats for 4 weeks, and with a recovery period of an additional 4
weeks, were made available. The findings indicated that
alpha-endosulfan, and to a considerably lesser extent
beta-endosulfan, was temporary stored in the kidneys in proportion
to the administered dose. Only endosulfan-sulfate and
endosulfan-lactone were detected in any appreciable quantities in
the kidneys. It was also noted that endosulfan and its metabolites
were minimally detectable in the blood. In the liver,
endosulfan-sulfate and endosulfan-lactone were the dominant
compounds, although their levels were many times lower than the
concentrations of alpha-endosulfan measured in the kidneys. It was
also observed that the storages were reversible; 30 days after the
end of the treatment phase alpha-endosulfan was detected only in
traces, and beta-endosulfan not at all (Leist & Mayer, 1987).
In a 13-week study, groups of 25 male and 25 female CD rats
were fed diets containing 0, 10, 30, 60, or 360 ppm technical
endosulfan. Daily dosages were equivalent to 0, 0.64, 1.92, 3.85,
and 23.41 mg/kg bw/day in males, and 0, 0.75, 2.26, 4.59, and
27.17 mg/kg bw in females. At the end of the 13-week treatment
period all animals were sacrificed except for 5 rats/sex dose group
that were withdrawn from treatment and followed for an additional
4-week recovery period. Ophthalmoscopic exams were performed on
control and high-dose rats before treatment and at week 13.
Neurological exams (locomotor reflexes) were performed on 10
rats/sex from the control and high-dose levels before treatment and
on weeks 2, 6, and 13; in addition, all animals were examined for
grip reflex and evidence of ataxia on week 13. Blood was sampled at
0, 6, and 12/13 weeks from 10 rats/sex/dose for assessment of
hematology and clinical chemistry parameters. A standard battery of
tests/examinations was performed and, in addition, blood and plasma
cholinesterase estimations were made at 5 and 12 weeks in 10
rats/sex/dose level. Urine was collected on weeks 4 and 13 for
standard urinalysis determinations. Similar studies were performed
in the rats that were allowed to complete the recovery period.
Three deaths occurred during the treatment period (1/20 female
controls, 1/20 females at 60 ppm due to anesthetic shock during
routine blood sampling, and 1/20 females at 320 ppm on day 19). No
deaths occurred in rats during the recovery period.
The only clinical sign of toxicity observed was hair loss in
females receiving 60 and 360 ppm endosulfan. Body weight gain was
reduced in male and female rats to a slight degree over the course
of the study. Food consumption was reduced in females given 360 ppm
during study weeks 1 and 2 only. All of these changes regressed by
the end of the recovery period. No adverse ophthalmological or
neurological findings were observed in treated rats.
A variety of hematological changes were observed during the
treatment period. Red blood cells were reduced at week 13 (and
after week 6) in male rats at doses of 30 to 360 ppm. MCV was
increased in female rats at weeks 13 and 6 at doses of 60 and
360 ppm. Finally, PCV was reduced at weeks 13 and 6 in male rats
given 360 ppm. In most cases, these effects were still evident in
rats, especially males, at recovery week 17. Changes involving
clinical chemistry parameters included increases in serum phosphorus
in females (weeks 12 and 6) at 30 and 360 ppm, reductions in serum
electrocytes (Na+ and K+) in males (weeks 12 and 6) at 60 and
360 ppm, and increased in total lipids and cholesterol in females
(weeks 12 and 6) at 360 ppm. In the case of urinalysis
determinations, a darkened urine was observed in male rats (week 13)
at 60 and 360 ppm, and in females (week 13) at 360 ppm. The urine
of the male rats at 60 and 360 ppm also contained elevated levels of
ketones and proteins. The clinical chemistry and urinalysis changes
regressed by the end of the recovery period.
Lower plasma and red blood cells cholinesterase activities were
observed in females (weeks 12 and 5) given 360 ppm endosulfan. In
contrast, increased brain cholinesterase activity was seen in
females treated with 60 and 360 ppm. These changes regressed during
the recovery period.
At necropsy, increases were noted in the relative weights of
the kidney (male rats at 60 and 360 ppm, and female rats at
360 ppm), liver (males and females at 360 ppm), brain (females at 60
and 360 ppm), and epididymes (males at 360 ppm). Increased kidney
weights were also seen in high-dose males at the end of the recovery
period. Macroscopic findings included enlarged kidneys and enlarged
liver. Histopathological findings also involved the kidneys and the
liver. Two main findings occurred in the kidneys. The first was
the observation of occasional cells of the proximal convoluted
tubules showing yellowish discoloration of the cytoplasma (incidence
in males). The second was another type of darker and more
particulate granular and/or clumped pigment in the straight portions
and occasionally in the proximal convoluted tubules. In addition,
male rats given 360 ppm endosulfan also showed yellowish coloured
protein aggregations in the proximal convoluted tubule and
intracytoplasmic eosinophilic droplets in the tubules. During the
recovery period the discoloration decreased whereas granular pigment
still persisted. In the liver, minimal centrilobular enlargement of
hepatocytes was observed. The liver change was not seen in the
recovery group rats.
The NOAEL is estimated to be 10 ppm in female rats, equivalent
to 0.75 mg/kg bw/day in the diet. The lowest effect level is 30 ppm
(i.e., 2.26 mg/kg bw/day) based upon the findings of increased serum
phosphorus levels and yellow discolored cells in the renal proximal
convoluted tubules of 1/20 females. Both of these changes were
produced by higher doses of endosulfan in female rats in a
dose-related fashion. The NOAEL for male rats is less than 10 ppm
(lowest dose tested), equivalent to 0.64 mg/kg bw/day in the diet.
This is based upon the finding of yellow discolored cells in the
renal proximal convoluted tubules in 14/20 males (Barnard et al.
1985).
In a dermal toxicity study, endosulfan was applied to the skin
of male and female Wistar rats (6/sex/dose) at doses of 0, 1, 3, 9,
and 27 mg/kg bw, and to males only at 81 mg/kg bw for 21
applications over 30 days. Toxic effects of the test compound
occurred at doses of 9 mg/kg bw or more and included deaths, liver
abnormalities (enlarged parenchymal cells with loss of cytoplasmic
basophilia, isolated cell necrosis, and frequent mitosis) in
occasional animals and significantly elevated absolute and relative
spleen weights in females. The deaths that were observed in female
rats were accompanied by tono-clonic convulsions. The NOAEL for
subchronic dermal toxicity is 3 mg/kg bw/day (Ebert et al. 1985a).
In a similar dermal toxicity study, endosulfan was applied to
the skin of Wistar rats at doses of 0, 12, 48, 96, and 192 mg/kg bw
in males and 0, 3, 6, 12, and 48 in females for 21 applications over
29 days. In this study, male rats exhibited increases in relative
kidney weights at doses of 12, 96 and 192 mg/kg bw (attributed by
the study authors to slightly lower body weights in males), deaths
in 36 percent of the animals at 192 mg/kg bw and a discrete
deposition of pigment in a few cells of the proximal convoluted
tubule in one high dose rat. In female rats, clinical signs of
toxicity were first observed at 12 mg/kg bw, and included
piloerection, salivation and lacrymation, blood from the eyes and
nose with increasing doses of the chemical, and deaths in 18% of the
animals due to the tono-clonic convulsions at 48 mg/kg bw. No other
treatment-related effects were noted (Ebert et al. 1985b).
In an inhalation study, groups of 15 male and 15 female SPF
Wistar rats were administered 0, 0.0005, 0.0010, and 0.0020 mg
endosulfan/L of air for 21 exposures (6 hours/day) for 29 days. Two
control groups (air and ethanol-PEG 400 vehicle) were used. Five
animals/test group were held for a 4-week recovery period after
receiving the test aerosol. The NOAEL is 0.001 mg/l of air in males
(based upon the decrement in weight gain of 12 to 16% seen in
recovery animals) (Hollander et al., 1984).
Dogs
Endosulfan technical was fed for one year in the daily diet to
groups of 6 male and 6 female beagle dogs at concentrations of 0, 3,
10, 30, and 30-60 ppm. The latter dietary level was increased in
stages from 30 to 45 ppm after 54 days of treatment, and then to
60 ppm after 106 days of treatment.
No spontaneous deaths were observed in endosulfan-treated
animals. However, 13 dogs were sacrificed in extremis. These
included one male in the 30 ppm dose group after 9 months due to a
poor overall general condition (resulting from refusal to eat and
severe purulent mediastinitis), and all high-dose males and females
after 4 to 5 months due to marked and progressive nervous symptoms.
These took the form of extreme sensitivity to noise and optic
stimuli, tonic contractions of muscles in the extremities, face and
cheeks, and weakened or abolished placing and righting reactions.
Similar nervous symptoms (i.e., violent contraction of the abdominal
muscles and convulsive movements of the cheeks) also occurred in
most of the surviving dogs in the 30 ppm dose group.
Food consumption was reduced in male and female dogs at 30 ppm
(on weeks 1 to 7) and at 3-60 ppm (from week 16 until the time of
sacrifice). Similarly, body weight gain was reduced in male dogs at
30 ppm (from week 43 onward) and in dogs of both sexes at 30-60 ppm
(from week 10 until the time of sacrifice). No adverse
ophthamological findings were observed in treated dogs, and tests to
examine hearing and dental status were normal. Standard
examinations for hematology, clinical chemistry, and urinalysis were
reported to be normal, as were determinations of cholinesterase
activity in serum, red cells, and brain. No treatment-related
alterations in bromsulphthalein retention of phenolsulphon-pthtalein
elimination were noted in special tests to assess hepatic and renal
function, respectively.
No dose-related changes in organ weight or in gross or
microscopic pathology were observed. The only histopatholopical
findings of interest were the occurrence of acute liver congestion
in 3/6 high-dose male dogs, and the presence of brown pigment in the
renal epithelium of 1/6 high-dose male dogs. The NOAEL in the study
was 10 ppm, equivalent to 0.65 mg/kg bw/day in the diet for males
and 0.57 mg/kg bw/day for females (Brunk, 1989).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice were fed diets containing endosulfan
technical (98.8% ppm) for 78 weeks. The time-weighted average (TWA)
dietary concentrations were 3.5 and 6.9 parts per million (ppm) for
males (50/dose group) and 2 and 3.9 ppm for females (50/dose group).
Twenty mice/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 0.52
and 1.0 mg/kg bw/day in males, and 0.3 and 0.58 mg/kg bw/day in
females.
High early mortality was observed among male mice. Eleven
high-dose males and two low-dose males died on week 19. Survival at
term was 15% (3/20) in control males, 38% (19/50) in low-dose males,
and 10% (5/50) in high-dose males. The deaths in the controls may
have been due to fighting. No common cause of death was found for
the high-dose males. Survival was high among all female mice (85%
of control, 94% of low dose, and 96% of high-dose females survived
to term). No effects of treatment on body weight or clinical signs
of toxicity were observed. No dose-dependent non-neoplastic lesions
were noted in female mice. However, the abbreviated lifespan of
male mice was considered to preclude any accurate analysis of
late-occurring tumours. The NOAEL in this study was less than
3.5 ppm (i.e., 0.25 mg/kg bw/day; lowest dose tested) in male mice
due to mortality, and 3.9 ppm (i.e., 0.58 mg/kg bw/day; highest dose
tested) in female mice (NCI, 1978).
A second chronic feeding study of endosulfan technical (97.9%
pure) was performed in Hoe:NMRKf(SPF 71) mice in which 60
animals/sex/dose level received dietary concentrations of 0, 2, 6,
and 18 pm for 24 months. An additional 10 mice/sex/dose were
sacrificed at interim periods of 12 and 18 months, respectively.
The administered concentrations were calculated to be equivalent to
0, 0.28. 0.84, and 2.51 mg/kg bw/day in males and 0, 0.32, 0.097,
and 2.86 mg/kg bw/day in females.
Survival was significantly reduced in female mice with 18 ppm
endosulfan in the main portion of the study (total deaths in females
= 33/60 or 55% controls, 36/60 or 60% low dose, 38/60 or 63.3% mid
dose, and 43/60 or 71.7% high dose). Survival was not affected by
the test compound in male mice in the main study (total deaths in
males = 27/60 or 45% controls, 33/60 or 55% low dose, 37/60 or 61.7%
mid dose, and 35/60 or 58.3% high dose). There was no
compound-related effect on mortality in either female or male mice
at the 12- and 18-month interim sacrifice periods. Body weight
gain was slightly reduced (about 10%) in male mice throughout the
main study at 18 ppm endosulfan. Female weights were slightly
elevated in the 12- and 18-month satellite groups, body weight was
either slightly increased or remained unchanged from controls in
animals of all treatment groups. There were no treatment-related
effects on food consumption, behavioural or general health
conditions of the animals, or hematological or clinical chemistry
parameters.
Gross and microscopic examination were performed at 12, 18, and
24 months. Reductions in several organ weights occurred with the
18 ppm dose level of endosulfan; these included decreases in the
weights of the liver (males at 18 months only), and ovaries (females
at 12 and 18 months). No treatment-related pathological changes or
tumours were seen after gross or histopathological examination of
tissues at 12, 18, or 24 months. The NOAEL was 6 ppm endosulfan,
equivalent to 0.84 mg/kg bw/day in male mice and 0.97 mg/kg bw/day
in female mice (Donaubauer, 1988).
Rats
Groups of Osborne-Mendel rats were fed diets containing
endosulfan (technical grade; 98.8% pure) for 78 weeks. The TWA
dietary concentrations were 408 and 952 ppm for males (50/dose
group) and 223 and 445 ppm for females (50/dose group). Twenty
rats/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 20.4
and 47.6 mg/kg bw/day in males, and 11.1 and 22.1 mg/kg bw/day in
females.
Endosulfan produced early mortality (dose-related) in male
rats. By study week 54, 52% of the high-dose males died and by week
74, 88% were dead. The high-dose males were sacrificed on week 74.
For the low-dose males, only 10/50 (20%) survived to term, and these
were sacrificed on week 82. Survival was adequate in control males.
In the case of female rats, no compound-related deaths occurred (70%
of control, 60% of low dose, and 50% of high-dose females survived
to term). Body weight was also depressed in a dose-related fashion
in treated male rats from week 22 onward. At the time of sacrifice,
body weight was depressed about 25% in both low- and high-dose male
rats compared to the control male group. Body weight was not
markedly changed in female rats. Several clinical signs of toxicity
were also seen in treated rats of both sexes; these included hunched
appearance, reddened and squinted eyes, rough fur, and abdominal
urine stains.
Upon histopathological examination, toxic nephropathy was seen
in both male (0/20 controls, 47/50 low dose, 43/47 high dose) and
female (0/20 controls, 27/50 low dose, 29/50 high dose) rats. This
abnormality was characterized by degenerative changes in the
proximal convoluted tubules at the corticomedullary junction, with
cloudy swelling, fatty degeneration and necrosis of the tubular
epithelium. Some affected tubules contained hyaline casts and
enlarged dark-staining regenerative tubular epithelial cells, and
the kidneys often had inflammatory cell infiltration, fibrotic
changes, and focal mineralization. Parathyroid gland hyperplasia
was also seen in male rats (0/20 controls, 21/40 low dose, 18.47
high dose) and was attributed to the effects of chronic renal
failure. Medial calcification of the blood vessels, perhaps related
to the parathyroid hyperplasia, occurred frequently in treated male
rats. Finally, testicular atrophy occurred in male rats (3/19
control, 18/47 low dose, 24/47 high dose) and was characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules, multinucleated cells (fusion bodies), and
calcium deposition resulting in aspermatogenesis.
No conclusions concerning the carcinogenicity of endosulfan
could be drawn from the bioassay of male rats because of the high
early mortality and abbreviated lifespans of these animals.
Survival in female rats was considered to be sufficient for the
evaluation of late occurring tumours; endosulfan was not
carcinogenic in female rats. The results of this long-term bioassay
in rats indicate that the lowest dietary concentrations of
endosulfan tested in males (408 ppm TWA - equivalent to
20.4 mg/kg/day) and females (223 ppm TWA - equivalent to
11.1 mg/kg/day) were toxic and that a NOAEL could not be established
(NCI, 1978).
A second chronic feeding study of endosulfan technical (97.1%
pure) was performed in Charles River Crl:CD(SD)BR rats in which 50
animals/sex/dose level received dietary concentrations of 0, 3, 7.5,
15, and 75 ppm for 104 weeks. An additional 20 rats/sex/dose
received similar doses of endosulfan and were used to obtain
hematology, clinical chemistry, and urinalysis data at various
intervals throughout the study. Gross and microscopic examinations
were performed on all animals. The administered concentrations of
endosulfan were calculated to be equivalent to 0.1, 0.3, 0.6, and
2.9 mg/kg bw/day in males and 0.1, 0.4, 0.7, and 3.8 mg/kg bw/day in
females.
There were no compound-related effects on mortality, clinical
signs of toxicity, food and water consumption, or ophthalmoscopic
parameters. Adverse findings were mainly observed at the highest
dose of endosulfan. These included significant reductions in body
weight gain over the study in males (-17%) and females (-18%), a
reduced food utilization efficiency in females, hematological
changes at week 103 in males (increased platelets, and reductions in
mean corpuscular volume, total white cells, and lymphocytes), and
clinical chemistry changes at week 103 in males (increased plasma
globulin and urinary protein levels, and reduced GPT and serum
albumin levels). The reductions in total white cells and
lymphocytes, and the increased urinary protein levels, were said to
be within expected limits of variation. It should be noted that
body weight gain was also reduced in males given 15 ppm endosulfan
on study weeks 6 to 18 only (-9% reduction); this effect, however,
was not progressive after week 18 and was not considered to be
toxicologically significant.
Gross and microscopic examination of all animals in the study
indicated that the primary target organ for toxicity was the kidney.
This was noted from an increased number of enlarged kidneys seen
grossly for females at 75 ppm (10/70 controls, 18/70 at 3 ppm, 19/70
at 7.5 ppm, 17/70 at 15 ppm, and 26/70 at 75 ppm), and an elevated
incidence of marked progressive glomerulonephritis in males at
75 ppm, 24/70 at 15 ppm, and 30/70 at 75 ppm) and also in females at
75 ppm (1/70 controls, 6/70 at 3 ppm, 6/70 at 7.5 ppm, 5/70 at
15 ppm, and 8/70 at 75 ppm). In addition, blood vessel aneurysms
were elevated in male rats at 75 ppm (10/70 controls, 6/70 at 3 ppm,
14/70 at 7.5 ppm, 10/70 at 15 ppm, and 21/70 at 75 ppm). The
aneurysms were considered to be possibly related to the renal
lesions.
Other observations in the study at 75 ppm in male rats included
a greater number of enlarged lumbar lymph nodes (14/70 controls,
10/70 at 3 ppm, 8/70 at 7.5 ppm, 7/70 at 15 ppm, and 19/70 at
75 ppm), inflammatory cells in the liver (15/70 controls, 16/70 at
3 ppm, 15/70 at 7.5 ppm, 18/70 at 15 ppm, and 25/70 at 75 ppm), and
polyarteritis in the pancreas (0/70 controls, 2/70 at 3 ppm, 4/70 at
7.5 ppm, 1/70 at 15 ppm, and 8/70 at 75 ppm) and testes (16/70
controls, 15/70 at 3 ppm, 23/70 at 7.5 ppm, 18/70 at 15 ppm, and
27/70 at 75 ppm). Finally, the mean weights of the testes were
reduced at 15 ppm and 75 ppm of endosulfan, but the response was not
strictly dose-related and was within expected limits of variation.
Endosulfan was not carcinogenic in either male or female rats.
The NOAEL was said to be 15 ppm, equivalent to 0.6 mg/kg bw/day in
male rats and 0.7 mg/kg bw/day in female rats (Ruckman et al.
1988).
Reproduction studies
Rats
Male and female Charles River SD-CD rats were administered
endosulfan (97% purity) in the diet for two generations at
concentrations of 0, 3, 15, and 75 ppm (equal to approximately 0.2,
1.0, and 6.0 mg/kg bw/day). Females were mated to males on a
one-to-one basis with 32 males/32 females per dose in the F0
generation, and 28 males/28 females per dose for the F1 generation.
F0 generation rats were administered endosulfan from 6 weeks of age
through 18 weeks of age, at which time they were mated to produce
F1a litters. F1a young were sacrificed 21 days postpartum, and the
F0 generation remated to produce the F1b litters. After weaning of
the F1b pups, the F0 generation rats were sacrificed. The F1b rats
were continued on their respective diets for approximately 98 days
prior to mating to produce the F2a litters (which were sacrificed
after weaning) and the F2b litters (likewise sacrificed after
weaning). Endosulfan was administered continuously throughout the
experiment.
There were no compound-related effects on mortality, food/water
consumption, or body weight gain, although F1b females given 3 ppm
demonstrated slightly lower body weights than controls from week 4
through 36. Pregnancy rate, mating performance and gestation period
for both F0 and F1b generations were unaffected by treatment.
Both matings of the F0 generation demonstrated significant
decreases in litter weight at 75 ppm from day 4 through day 21 but
not in either F1b mating. Also, F0 generation litter weights were
significantly reduced on days 4 to 21 during the second mating at
15 ppm as well. Although the trend for cumulative litter loss (%)
was increased in the first mating at 75 ppm, it was not evident in
the second mating nor in either mating of the F1b generation. The
mean pup weight was unaffected throughout both F0 and F1b matings.
Sex ratio appeared to be unaffected throughout the study.
F0 and F1b generation parents presented evidence of increased
absolute organ weights for heart, liver, kidneys, and brain at
75 ppm. These were not demonstrated in the offspring from either
mating of these parents. There was no consistent dose-related
effect. Histopathology of F1b adults and F2b weanlings
demonstrated no compound-related effects. F0 and F1b generation
females and offspring were examined for skeletal and visceral
abnormalities and no consistent dose-related trends were evident,
except for isolated incidences of microphthalmia (2), anophthalmia
(2), kinked/missing tail (3), shortened/malformed digits or limbs
(5), retinal hemorrhage (1) and spina bifida (1).
There were no dose-related or compound-related effects on
reproductive performance. Decreases in litter weight at 15 and
75 ppm occurred in the F1a and F1b litters; however, this finding
was not considered to be adverse since the reductions in litter
weight were marginal and there were no significant corresponding
reductions in pup weight or litter size. The visceral and skeletal
examinations of fetuses for assessing teratogenic effects were
within normal variation for the strain/species studied. It is
considered that endosulfan, at dietary levels up to and including
75 ppm (approximately 6 mg/kg/day in the diet) caused no adverse
effects on reproduction in rats in this study (Edwards et al.
1984).
However, as noted below, renal changes occurred in F1b adult
rats at all of the doses of endosulfan that were tested. In an
addendum to the above study, a histological review of the kidneys of
adult rats from the F1b generation and of weanling rats from the F2b
generation was reported. The effort was initiated because yellowish
discoloration and pigment deposits were noted in renal proximal
convuluted tubules in rats in a 13-week oral toxicity study of
endosulfan followed by a 4-week withdrawal period (see Barnard
et al. 1985). The histological data obtained indicated the
presence of yellowish discolored cells in the proximal convoluted
tubules in F1b adult rats given dietary doses of 3, 15, and 75 ppm
endosulfan. The incidences in males were: 0/28 controls, 11/28 at
3 ppm, 13/28 at 15 ppm, and 28/28 at 75 ppm. The incidences in
females were: 0/28 controls, 0/28 at 3 ppm, 0/28 at 15 ppm, and 9/28
at 75 ppm. In addition, granular/clumped pigment was also noted at
a dietary dose of 75 ppm in F1b adult rats. The incidences at
75 ppm were 14/28 males and 1/28 females vs. an incidence of 0/28 in
males and females from the control, 3 ppm, and 15 ppm dose groups.
In the F2b generation weanling rats, no comparable renal changes
were observed following treatment with endosulfan (Offer, 1985).
These findings were not considered to be adverse since the yellow
pigment in kidneys was identified as endosulfan and its metabolites,
being stored and metabolized in lysosomes prior to excretion.
Special studies on teratogenicity
Rats
Groups of 25 pregnant CD Sprague-Dawley rats were administered
endosulfan (97.3% purity), via oral gavage, at dose levels of 0,
0.66, 2, and 6 mg/kg bw/day from days 6 to 19 of gestation.
(Day 0 = day sperm found). Ten additional animals were added to the
high dose (due to mortality) and five to the control groups (due to
loss of tissue during processing). On day 20 of gestation, all dams
were killed by asphyxiation with CO2 and fetuses delivered by
cesarean section.
Maternal toxicity was evident in the mid- and high-dose groups,
expressed by significantly reduced body weight and body weight gain
during gestation, and by various clinical signs of toxicity (e.g.,
rats rubbing their faces, alopecia, rough coats, lethargy,
flaccidity, and hyperactivity). Several animals died as the result
of improper gavage tube insertion. Fetal and embryolethality were
not apparent in any group and all pregnant animals had viable
litters. Mean fetal weight and crown-rump length were significantly
reduced in the high-dose group, but not in the lower dose groups. A
number of external, visceral, and skeletal abnormalities were
observed in the fetuses in the high-dose group. The most prominent
effect was a significant increase in the incidence of small fourth
and unossified fifth sternebrae in the high-dose fetuses. However,
there was no clear evidence of a teratogenic effect due to the
significant maternal toxicity expressed at the same level. The
NOAEL for maternal toxicity was 0.66 mg/kg bw/day and that for
fetotoxicity was 2 mg/kg bw/day by oral gavage (MacKenzie et al.
1980a).
A range-finding study with endosulfan in pregnant CD
Sprague-Dawley rats (4-6/group) was performed for the purpose of
setting doses for the above described rat teratology study.
Endosulfan was administered by gavage on days 6 to 19 of gestation
(Day 0 = day sperm found) at doses of 0 (corn oil vehicle), 1.25,
2.5, 5, 10, 20 and 40 mg/kg bw/day. Toxic signs in dams included
reductions in body weight gain at doses of 1.25 mg/kg bw/day and
greater, various clinically observed adverse effects at doses of
2.5 mg/kg bw/day and greater (e.g., salivation, piloerection, poor
muscle tone, lethargy, head-rubbing behaviour, hyperactivity,
spasticity, tremors and convulsions, and deaths (67 to 100%
mortality) at 10 mg/kg bw/day and greater. The changes were dose
related and most marked at higher doses of endosulfan. No effects
on pregnancy maintenance or fetal survival were observed (MacKenzie
et al. 1980b).
Rabbits
Groups of pregnant New Zealand White rabbits (20/group) were
administered endosulfan (97.3% purity) via oral gavage in corn oil
at dose levels of 0, 0.3, 0.7, and 1.8 mg/kg bw/day from days 6 to
28 of gestation (Day 0 = day of mating). Animals were observed
throughout the test period for toxic signs. Six additional pregnant
rabbits were added to the high-dose group due to mortality. On day
29 of gestation, the dams were asphyxiated with CO2, and the entire
reproductive tract was removed.
Maternal toxicity was evident at the high dose, expressed as
rapid breathing, hyperactivity, convulsions and mortality. Group
mean body weights were not affected by treatment. All pregnant dams
had viable fetuses, with the exception of one high-dose dam which
died on test and which had nine late resorptions. There were no
compound-related effects on the number of corpora lutea,
implantation efficiency, litter size, sex ratio, mean fetal length
or weight, or the number of live/dead or resorbed fetuses. There
were no gross external abnormalities and the visceral and skeletal
examinations revealed no dose-related increase in variations or
anomalies. The NOAEL for maternal toxicity was 0.7 mg/kg bw/day.
Endosulfan was not teratogenic in rabbits at doses up to and
including 1.8 mg/kg bw, although maternal toxicity was expressed at
that level (MacKenzie et al. 1981a).
A range-finding study with endosulfan in pregnant New Zealand
White rabbits (3 to 10/group) was conducted to set doses for the
above described rabbit teratology study. Endosulfan was
administered by gavage on days 6 to 28 of gestation (Day 0 = day of
mating) at doses of 0 (corn oil), 1, 2, 4, 8, and 12 mg/kg bw/day.
Standard observations and necropsy procedures were performed. Toxic
signs in dams included deaths at 2 mg/kg bw/day or more (20 to 100%
mortality) and various clinical adverse effects at 2 mg/kg bw/day or
more (e.g., hyperactivity, opisthotonos, clonic and tonic
convulsions, paralysis, tremors, and loss of mobility). Fetal
survival appeared to be adversely affected at 2 mg/kg bw or more as
indicated by an increase in the percent of dead fetuses and a
decrease in the percent of live fetuses in dams. No prominent
effects on weight gain or pregnancy rate occurred (MacKenzie et al.
1981b).
Special study on mutagenicity
Endosulfan was tested for mutagenic activity in a variety of
bacterial, yeast, and mammalian cell systems and was negative in
each test (Table 1).
TABLE 1. RESULTS OF MUTAGENICITY STUDIES ON ENDOSULFAN
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
Ames Testa S.typhmurium 6-5000 ug/plate Negative Shirasu et al. 1978
TA98, TA100, TA1535,
TA1537, TA1538
Yeast Forward Mutation Schizosaccharomyces pombe 62.5-500 ug/plate Negative Milone 1984a
Testa
Yeast Gene-Conversion/ S. cerevisiae 100-5000 ug/ml Negative Milone, 1984b
DNA Repair Testa (D4 strain)
Mouse Lymphoma Assaya Mouse L51786 TK+/- Cells 6.25-100 ug/ml Negative Cifone, 1984a
DNA Repair Assaya B. subtilis H17 and 20-2000 ug/disk Negative Shirasu et al. 1978
M45 (rec-)
Unscheduled DNA Synthesis Primary-Hepatocytes (Rat) 0.102-51 ug/ml Negative Cifone, 1984b
Unscheduled DNA Human Cell Line A 549 1-1000 ug/ml Negative Muller, 1988
Synthesisa
Micronucleus Assay NMRI Mice O.2-5 mg/kg, Negative Jung et al. 1983
(Bone Marrow) Orallyb
Micronucleus Assay Swiss Albino Male Mice 43.3 mg/kg Negative Usha Rani et al. 1980
(Bone Marrow) Orallyb
Chromosome Aberration Cultured Human Lymphocytes 0.05-100 ug/ml Negative Milone, 1986
Testa in vitro
TABLE 1 (CONTD).
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
In vivo Cytogenicity Male Albino Rats 11-55 mg/kg/day Negative Dikshith & Datta, 1978
Test (Bone Marrow Orally for 5 days
and Spermatogonia)
a With and without metbolic activation.
b Administered twice orally at an interval of 24 hours.
Special study on neurotoxicity
The acute delayed neurotoxicity of endosulfan was assessed in
adult domestic hens. The study consisted of three parts. First,
the LD50 determination was performed using oral (gavage) doses of
0, 40, 60, 90, 110, and 135 mg/kg bw endosulfan (5 hens/dose group).
The observed LD50 was 96 mg/kg bw. All deaths occurred within 2
hours of dosing. Toxic signs observed were subdued behaviour and
weight loss. Second, a protection assessment was performed using an
oral dose of 96 mg/kg bw of endosulfan alone, or in combination with
either phenobarbital (15 mg/kg bw i.m.), diazepam (2 mg/kg bw p.o.),
or atropine (10 mg/kg bw i.m.) plus 2-PAM (25 mg/kg bw i.m.). There
were 5 hens/group. The results did not provide evidence that any of
the protections reduced the number of mortalities occurring after
dosing with endosulfan. Third, a neurotoxicity assessment was
performed using groups of 10 hens. A vehicle control group was
dosed orally with corn oil, a positive control group with 500 mg/kg
bw TOCP, and four test groups were each dosed with the LD50
(96 mg/kg bw) dose of endosulfan. Dosing was followed by a 21-day
observation period, a redosing, and a final 21-day observation
period. Surviving hens were sacrificed at 42 days.
Histopathological examination of brain, spinal cord and peripheral
nerve tissues were performed on 9 of the 40 animals dosed with
endosulfan (these were the survivors to 42 days), 10/10 control
hens, and 10/10 TOCP-treated hens. The results indicated that
endosulfan did not produce any clinical signs of neurotoxicity or
neuropathological (i.e., histological) effects on the central or
peripheral nervous system. In contrast, hens treated with TOCP
displayed ataxia as well as significant axonal degeneration in the
spinal cord and in the peripheral (tibial) nerve (Roberts et al.
1983).
Special study on skin sensitization
Endosulfan had no sensitizing potential (delayed contact
hypersensitivity) in guinea pigs when tested by the Buehler method
(Jung & Weigand, 1983).
COMMENTS
In rats, the absorption of single oral doses of endosulfan
required 2 or more days; subsequent urinary and fecal excretion was
also slow, requiring 5 or more days. The biotransformation pathways
involve hydrolysis and oxidation to sulfur-free metabolites and
polar conjugates, and the formation of various sulfur-containing
moieties. Endosulfan preferentially accumulated in the kidney, with
lower levels in other tissues, the elimination half-time from
tissues being 3 to 7 days.
Endosulfan at dietary dose levels of 10 ppm and higher in
subchronic studies in rats produced yellow pigmentation of renal
cells. In contrast, renal cellular pigmentation was not observed in
subchronic studies in mice at similar dose levels (see reproduction
studies below).
In a one-year dietary study in dogs, doses of 30 to 60 ppm
resulted in early sacrifices due to clinical signs of neurotoxicity
(e.g., convulsive muscular activity). Reduced food consumption and
weight gain also occurred. In this study, the NOAEL was 10 ppm,
equal to 0.57 mg/kg bw/day.
Long-term carcinogenicity studies were performed in mice and
rats. Treatment-related increases in neoplasms were not observed.
The NOAELs (based on non-neoplastic effects) were 6 ppm (equal to
0.84 mg/kg bw/day) in mice and 15 ppm (equal to 0.6 mg/kg bw/day) in
rats.
A two-generation, 2 litters per generation reproduction study
was conducted in rats. Endosulfan was associated with a reduction
in weight of the F1a and Flb litters at dietary levels of 15 and
75 ppm, and the presence of yellowish pigment in cells in the renal
proximal convoluted tubules at dose levels of 3, 15 and 75 ppm in
F1b adult rats. The Meeting did not consider these findings to be
adverse since the reduction in litter weights were marginal and
occurred in the absence of corresponding reductions in pup weight or
litter size. The yellow pigment in kidneys was identified as
endosulfan and its metabolites, being stored and metabolized in
lysosomes prior to excretion.
In teratology studies performed in rats and rabbits, NOAELs of
approximately 0.7 and 1.8 mg/kg bw/day were identified for maternal
toxicity and fetotoxicity, respectively. Endosulfan was not
teratogenic at 6 mg/kg bw/day in rats and 1.8 mg/kg bw/day in
rabbits, the highest dose levels tested.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 6 ppm in the diet, equal to 0.84 mg/kg bw/day
Rat: 15 ppm in the diet, equal to 0.6 mg/kg bw/day
Dog: 10 ppm in the diet, equal to 0.57 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E., Gibson,
W.A., Rao, R., Offer, J.M., Gopinath, G., Almond, R.H. (1984)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice. Unpublished Report No. A29663 from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street,
A.E., Gibson, W.A., Gopinath, G., Majeed, S.K., Almond, R.J. (1985)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice followed by a 4-week withdrawal period. Unpublished Report
No. A30700 from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Brunk, R. (1989) Endosulfan-substance technical: Testing for
toxicity by repeated oral administration (1-year feeding study) to
beagle dogs. Unpublished Report No. 89-0188 from Pharma Research
Toxicology and Pathology, Hoechst AG. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Cifone, M.A. (1984a) Mutagenicity evaluation of HOE 002671-
substance technical in the mouse lymphoma forward mutation assay.
Unpublished Report No. A29801 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Cifone, M.A. (1984b) Evaluation of HOE 002671-substance technical
in the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished Report No. A29800 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-Endosulfan. Unpublished Report No. A-36685 from WIL Research
Laboratories, Inc., Ashland, Ohio. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Dikshith, T.S.S., Datta, K. (1978) Endosulfan: lack of cytogenetic
effects in male rats. Bull. Environ. Contam. Toxicol., 20:
826-833.
Donaubauer, H.H. (1988) Endosulfan-substance technical.
Carcinogenicity study in mice - 24 months feeding study.
Unpublished Report No. A38008 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Donaubauer, H.H., Leist, K.H., Kramer, M. (1985) Endosulfan-
substance technical: 42-day feeding study in mice. Unpublished
Report No. A38104 from Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Leist, K.H., Kramer, P. (1985a) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in Wistar rats. Unpublished Report
No. A30753 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Weigand, W., Kramer, P. (1985b) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in SPF Wistar rats. Unpublished Report
No. A30754 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Edwards, J.A., Reid, Y.J., Offer, J.M., Almond, R.H., Gibson, W.A.
(1984) Effects of Endosulfan-technical on reproductive function of
multiple generations in the rat. Unpublished Report No. A29428
(Huntingdon Report No. HST 204/83768) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W. (1983) Hoe 002671-active ingredient
technical. Testing for acute aerosol inhalation toxicity in male
and female SPF Wistar rats, 4 hours - LC50. Unpublished Report
No. A32087 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W., Kramer, P. (1984) Endosulfan-active
ingredient technical. Testing for subchronic inhalation toxicity -
21 exposures in 29 days in SPF Wistar rats. Unpublished Report
No. A29823 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Jung & Weigand, W. (1983) Hoe 002671-active ingredient technical.
Testing for sensitizing properties in female Perbright-White guinea
pigs. Unpublished Report No. A27248 from Pharma Forschung
Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Jung, Weigand, W., Kramer, P. (1983) Hoe 112671-active ingredient
technical. Micronucleus test in male and female NMRI mice following
oral administration. Unpublished Report No. A29689 from Pharma
Forschung Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Lachmann, G.(1987) Dermal absorption of 14C-Endosulfan in rhesus
monkeys. Unpublished Report No. A36685 from Battelle-Institut,
Frankfurt, FRG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Kramer, P. (1985) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A30776 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Mayer, D. (1987) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A37112 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Dickie, S.M., Jackson, T.A.,
Zelinger, D.J. (1981a) Teratology study with FMC 5462 in rabbits.
Unpublished Report No. A23192 (Raltech Study No. 80070) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1981b) Range-finding study with FMC 5462 in pregnant rabbits.
Unpublished Report No. A37682 (Raltech Study No. 79032) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1980a) Teratology study with FMC 5462 in rats. Unpublished
Report No. A21393 (Raltech Study No. 79041) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M. (1980b)
Range-finding study with FMC 5462 in pregnant rats. Unpublished
Report No. A37680 (Raltech Study No. 79031) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Milone, M.F. (1984a) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished Report No. A29312 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1984b) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Saccharomyces cerevisiae.
Unpublished Report No. A29313 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1986) Study of the capacity of the test article
Endosulfan, substance technical, to induce chromosome aberration in
human lymphocytes cultured in vitro. Unpublished Report No. A33127
from Instituto di Richerche Biomediche "Antoine Marxer", Italy.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Muller, W. (1988) Evaluation of Endosulfan-substance, technical in
the unscheduled DNA synthesis test in mammalian cells in vitro.
Unpublished Report No. A38455 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
National Cancer Institute (NCI) (1978) Bioassay of Endosulfan for
possible carcinogenicity. Technical Report No. 62 from US
Department of Health, Education and Welfare, NIH. Submitted to WHO
by Hoechst AG, Frankfurt, FRG.
Offer, J.M. (1985) Addendum to HST 204: effect of Endosulfan-
technical on the reproductive function of multiple generations in
the rat. Histopathological review of the kidneys in adult rats of
the F1B generation and in weanling rats of the F2B generation.
Unpublished Report No. A30757 (Huntingdon Addendum to Report HST
204/83768) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Cooke, L., Gibson,
W.A. (1983) Acute delayed neurotoxicity study with Endosulfan-
technical in the domestic hen. Unpublished Report No. A32153
(Huntingdon Report No. HST 225/83888) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed,
S.K., Anderson, A., Chanter, D.O. (1988) Endosulfan, active
technical ingredient. Combined chronic toxicity/carcinogenicity
study (104-week feeding in rats). Unpublished Report No. HST
289/881067) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Shirasu, Y., Moriya, M., Ohta, T. (1978) Microbial mutagenicity
testing on Endosulfan. Unpublished Report No. A21215 from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Stumpf, K., ABhauer, J. (1986) An up-to-date review of the
environmental safety of Endosulfan. Unpublished Report No. A35180
from the Analytical Laboratory, Hoechst AG. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Usha Rani, M.V., Reddy, O.S., Reddy, P.P. (1980) Mutagenicity
studies involving aldrin, dieldrin, endosulfan, dimethoate,
phosphamidon, carbaryl and ceresan. Bull. Environ. Contam.
Toxicol., 25: 277-282.
 Toxicological Studies
Acute toxicity
An acute nose-only inhalation toxicity test of endosulfan in
Wistar rats resulted in LC50 values of 0.0345 mg/L of air in males
and 0.9126 mg/L of air in females. Clinical signs of intoxication
were dyspnea, passivity, disequilibrium, trembling, tremors,
tono-clonic convulsions, and subdued reflex activity. Gross necropsy
changes in animals that died consisted of sporadic dark red pinhead
foci in the lungs (Hollander & Weigand, 1983).
Short-term studies
Mice
Groups of 10 male and 10 female Hoe:NMRKf(SPF 71) mice were fed
diets containing 0 or 18 ppm technical endosulfan for 42 days. The
18 ppm dose level was calculated to be equivalent to 3.7 mg/kg
bw/day in males and 4.6 mg/kg bw/day in females. The results
obtained indicated that deaths occurred in 2/10 treated mice (one
female on day 28 and another female on day 38), and absolute and
relative liver weights were significantly increased in females. In
addition, the body weight gain was slightly elevated throughout the
study, with the increase observed on day 36 being statistically
significant. There were no other changes in females or in males
with respect to food consumption, behaviour or general condition
(including ocular and dental examinations), or macroscopic
examinations of organs. The NOAEL was less than 4.6 mg/kg bw/day in
females (based on deaths and increased liver weights) and equal to
3.7 mg/kg bw/day in males (Donaubauer et al. 1985).
In a 13-week study, groups of 20 male and 20 female CD-1 mice
received dietary levels of 0, 2, 6, 18, or 54 ppm of technical
endosulfan. These concentrations were equivalent to doses of
approximately 0, 0.24-0.27, 0.74-0.80, 2.13-2.39, or 7.3-7.5 mg/kg
bw/day, respectively.
Adverse findings appeared to be confined primarily to the
highest dose level of endosulfan (i.e., 54 ppm). The observed
changes included deaths (12/20 males and 10/20 females at various
study intervals), convulsions and salivation (1/20 males and 1/20
females), reduced neutrophils (males on weeks 6 and 13), increased
total serum lipids (females on week 13), reduced spleen weight
(males), and slight vascular congestion in the lungs (11/20 males
and 6/20 females). All of those parameters were unchanged in all
other treatment groups. At dose levels of 6, 18, and 54 ppm, body
weight gain was depressed in male mice at study week 1 only, and
blood glucose levels were depressed in females at study week 6 only.
Neither of these changes persisted throughout the study and are thus
not considered to be of toxicological importance. Similarly, food
consumption was reduced only on study weeks 1 and 2 in mice of both
sexes at the 54 ppm dose level. From the above data, the NOAEL was
considered to be 18 ppm, equivalent to 2.13 to 2.39 mg/kg bw/day in
the diet, respectively, in male and female mice (Barnard et al.
1984).
Rats
Endosulfan was fed for 4 weeks in the daily diet to groups of
100 male Wistar rats at concentrations of 360 and 720 ppm. These
concentrations were said to be equivalent to doses of 34.0 and
67.8 mg/kg bw/day, respectively. An additional 20 male rats served
as control animals. At the end of 4 weeks treatment, one-half of
the animals in each group were killed (main study group) and the
other half were allowed to recover for 4 more weeks before being
sacrificed (recovery group). Rats were examined daily for behaviour
and general health conditions. Body weights and food consumption
were recorded weekly. At autopsy, all animals were examined
grossly, and the relative weights of three organs (liver, kidneys,
brain) were calculated. Histological exams were performed on the
left kidney, and a piece of liver from six animals/dose level; the
right liver, another piece of liver and the brain from two
animals/dose level were examined by electron microscopy. The
remaining parts of the above organs not used for histological study
were used to identify residues of endosulfan in tissue.
Two deaths occurred due to unknown causes (one rat at 360 ppm
on day 29 and one rat at 720 ppm on day 8). There were no
treatment-related effects in the appearance or general health of the
animals (including signs of neurological disturbances, corneal
opacity, oral mucosal lesions, or changes in dental growth), in body
weight gain, or in food consumption. The relative weights of the
liver were increased at both dose levels, and relative kidney and
brain weights were increased at the highest dose level. Upon gross
examination, the kidneys appeared darkened in colour at both dose
levels. Histopathological examination showed proliferation and
enlargement of lysosomes of the cells of the proximal convoluted
tubules, and granular pigmentation of the same cells, at both doses
of endosulfan. Lysosomal changes were not seen in brain or liver.
The above findings were not seen in animals in the 4-week recovery
group. The identification of residues of endosulfan in tissues was
unsuccessful. There was no NOAEL in this study due to the observed
renal and liver changes at 360 ppm, equivalent to 34.0 mg/kg bw/day
in the diet (Leist & Kramer, 1985).
In a follow-up report, the results of repeat tissue residue
analysis studies after dietary dosing with 360 and 720 ppm in Wistar
rats for 4 weeks, and with a recovery period of an additional 4
weeks, were made available. The findings indicated that
alpha-endosulfan, and to a considerably lesser extent
beta-endosulfan, was temporary stored in the kidneys in proportion
to the administered dose. Only endosulfan-sulfate and
endosulfan-lactone were detected in any appreciable quantities in
the kidneys. It was also noted that endosulfan and its metabolites
were minimally detectable in the blood. In the liver,
endosulfan-sulfate and endosulfan-lactone were the dominant
compounds, although their levels were many times lower than the
concentrations of alpha-endosulfan measured in the kidneys. It was
also observed that the storages were reversible; 30 days after the
end of the treatment phase alpha-endosulfan was detected only in
traces, and beta-endosulfan not at all (Leist & Mayer, 1987).
In a 13-week study, groups of 25 male and 25 female CD rats
were fed diets containing 0, 10, 30, 60, or 360 ppm technical
endosulfan. Daily dosages were equivalent to 0, 0.64, 1.92, 3.85,
and 23.41 mg/kg bw/day in males, and 0, 0.75, 2.26, 4.59, and
27.17 mg/kg bw in females. At the end of the 13-week treatment
period all animals were sacrificed except for 5 rats/sex dose group
that were withdrawn from treatment and followed for an additional
4-week recovery period. Ophthalmoscopic exams were performed on
control and high-dose rats before treatment and at week 13.
Neurological exams (locomotor reflexes) were performed on 10
rats/sex from the control and high-dose levels before treatment and
on weeks 2, 6, and 13; in addition, all animals were examined for
grip reflex and evidence of ataxia on week 13. Blood was sampled at
0, 6, and 12/13 weeks from 10 rats/sex/dose for assessment of
hematology and clinical chemistry parameters. A standard battery of
tests/examinations was performed and, in addition, blood and plasma
cholinesterase estimations were made at 5 and 12 weeks in 10
rats/sex/dose level. Urine was collected on weeks 4 and 13 for
standard urinalysis determinations. Similar studies were performed
in the rats that were allowed to complete the recovery period.
Three deaths occurred during the treatment period (1/20 female
controls, 1/20 females at 60 ppm due to anesthetic shock during
routine blood sampling, and 1/20 females at 320 ppm on day 19). No
deaths occurred in rats during the recovery period.
The only clinical sign of toxicity observed was hair loss in
females receiving 60 and 360 ppm endosulfan. Body weight gain was
reduced in male and female rats to a slight degree over the course
of the study. Food consumption was reduced in females given 360 ppm
during study weeks 1 and 2 only. All of these changes regressed by
the end of the recovery period. No adverse ophthalmological or
neurological findings were observed in treated rats.
A variety of hematological changes were observed during the
treatment period. Red blood cells were reduced at week 13 (and
after week 6) in male rats at doses of 30 to 360 ppm. MCV was
increased in female rats at weeks 13 and 6 at doses of 60 and
360 ppm. Finally, PCV was reduced at weeks 13 and 6 in male rats
given 360 ppm. In most cases, these effects were still evident in
rats, especially males, at recovery week 17. Changes involving
clinical chemistry parameters included increases in serum phosphorus
in females (weeks 12 and 6) at 30 and 360 ppm, reductions in serum
electrocytes (Na+ and K+) in males (weeks 12 and 6) at 60 and
360 ppm, and increased in total lipids and cholesterol in females
(weeks 12 and 6) at 360 ppm. In the case of urinalysis
determinations, a darkened urine was observed in male rats (week 13)
at 60 and 360 ppm, and in females (week 13) at 360 ppm. The urine
of the male rats at 60 and 360 ppm also contained elevated levels of
ketones and proteins. The clinical chemistry and urinalysis changes
regressed by the end of the recovery period.
Lower plasma and red blood cells cholinesterase activities were
observed in females (weeks 12 and 5) given 360 ppm endosulfan. In
contrast, increased brain cholinesterase activity was seen in
females treated with 60 and 360 ppm. These changes regressed during
the recovery period.
At necropsy, increases were noted in the relative weights of
the kidney (male rats at 60 and 360 ppm, and female rats at
360 ppm), liver (males and females at 360 ppm), brain (females at 60
and 360 ppm), and epididymes (males at 360 ppm). Increased kidney
weights were also seen in high-dose males at the end of the recovery
period. Macroscopic findings included enlarged kidneys and enlarged
liver. Histopathological findings also involved the kidneys and the
liver. Two main findings occurred in the kidneys. The first was
the observation of occasional cells of the proximal convoluted
tubules showing yellowish discoloration of the cytoplasma (incidence
in males). The second was another type of darker and more
particulate granular and/or clumped pigment in the straight portions
and occasionally in the proximal convoluted tubules. In addition,
male rats given 360 ppm endosulfan also showed yellowish coloured
protein aggregations in the proximal convoluted tubule and
intracytoplasmic eosinophilic droplets in the tubules. During the
recovery period the discoloration decreased whereas granular pigment
still persisted. In the liver, minimal centrilobular enlargement of
hepatocytes was observed. The liver change was not seen in the
recovery group rats.
The NOAEL is estimated to be 10 ppm in female rats, equivalent
to 0.75 mg/kg bw/day in the diet. The lowest effect level is 30 ppm
(i.e., 2.26 mg/kg bw/day) based upon the findings of increased serum
phosphorus levels and yellow discolored cells in the renal proximal
convoluted tubules of 1/20 females. Both of these changes were
produced by higher doses of endosulfan in female rats in a
dose-related fashion. The NOAEL for male rats is less than 10 ppm
(lowest dose tested), equivalent to 0.64 mg/kg bw/day in the diet.
This is based upon the finding of yellow discolored cells in the
renal proximal convoluted tubules in 14/20 males (Barnard et al.
1985).
In a dermal toxicity study, endosulfan was applied to the skin
of male and female Wistar rats (6/sex/dose) at doses of 0, 1, 3, 9,
and 27 mg/kg bw, and to males only at 81 mg/kg bw for 21
applications over 30 days. Toxic effects of the test compound
occurred at doses of 9 mg/kg bw or more and included deaths, liver
abnormalities (enlarged parenchymal cells with loss of cytoplasmic
basophilia, isolated cell necrosis, and frequent mitosis) in
occasional animals and significantly elevated absolute and relative
spleen weights in females. The deaths that were observed in female
rats were accompanied by tono-clonic convulsions. The NOAEL for
subchronic dermal toxicity is 3 mg/kg bw/day (Ebert et al. 1985a).
In a similar dermal toxicity study, endosulfan was applied to
the skin of Wistar rats at doses of 0, 12, 48, 96, and 192 mg/kg bw
in males and 0, 3, 6, 12, and 48 in females for 21 applications over
29 days. In this study, male rats exhibited increases in relative
kidney weights at doses of 12, 96 and 192 mg/kg bw (attributed by
the study authors to slightly lower body weights in males), deaths
in 36 percent of the animals at 192 mg/kg bw and a discrete
deposition of pigment in a few cells of the proximal convoluted
tubule in one high dose rat. In female rats, clinical signs of
toxicity were first observed at 12 mg/kg bw, and included
piloerection, salivation and lacrymation, blood from the eyes and
nose with increasing doses of the chemical, and deaths in 18% of the
animals due to the tono-clonic convulsions at 48 mg/kg bw. No other
treatment-related effects were noted (Ebert et al. 1985b).
In an inhalation study, groups of 15 male and 15 female SPF
Wistar rats were administered 0, 0.0005, 0.0010, and 0.0020 mg
endosulfan/L of air for 21 exposures (6 hours/day) for 29 days. Two
control groups (air and ethanol-PEG 400 vehicle) were used. Five
animals/test group were held for a 4-week recovery period after
receiving the test aerosol. The NOAEL is 0.001 mg/l of air in males
(based upon the decrement in weight gain of 12 to 16% seen in
recovery animals) (Hollander et al., 1984).
Dogs
Endosulfan technical was fed for one year in the daily diet to
groups of 6 male and 6 female beagle dogs at concentrations of 0, 3,
10, 30, and 30-60 ppm. The latter dietary level was increased in
stages from 30 to 45 ppm after 54 days of treatment, and then to
60 ppm after 106 days of treatment.
No spontaneous deaths were observed in endosulfan-treated
animals. However, 13 dogs were sacrificed in extremis. These
included one male in the 30 ppm dose group after 9 months due to a
poor overall general condition (resulting from refusal to eat and
severe purulent mediastinitis), and all high-dose males and females
after 4 to 5 months due to marked and progressive nervous symptoms.
These took the form of extreme sensitivity to noise and optic
stimuli, tonic contractions of muscles in the extremities, face and
cheeks, and weakened or abolished placing and righting reactions.
Similar nervous symptoms (i.e., violent contraction of the abdominal
muscles and convulsive movements of the cheeks) also occurred in
most of the surviving dogs in the 30 ppm dose group.
Food consumption was reduced in male and female dogs at 30 ppm
(on weeks 1 to 7) and at 3-60 ppm (from week 16 until the time of
sacrifice). Similarly, body weight gain was reduced in male dogs at
30 ppm (from week 43 onward) and in dogs of both sexes at 30-60 ppm
(from week 10 until the time of sacrifice). No adverse
ophthamological findings were observed in treated dogs, and tests to
examine hearing and dental status were normal. Standard
examinations for hematology, clinical chemistry, and urinalysis were
reported to be normal, as were determinations of cholinesterase
activity in serum, red cells, and brain. No treatment-related
alterations in bromsulphthalein retention of phenolsulphon-pthtalein
elimination were noted in special tests to assess hepatic and renal
function, respectively.
No dose-related changes in organ weight or in gross or
microscopic pathology were observed. The only histopatholopical
findings of interest were the occurrence of acute liver congestion
in 3/6 high-dose male dogs, and the presence of brown pigment in the
renal epithelium of 1/6 high-dose male dogs. The NOAEL in the study
was 10 ppm, equivalent to 0.65 mg/kg bw/day in the diet for males
and 0.57 mg/kg bw/day for females (Brunk, 1989).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice were fed diets containing endosulfan
technical (98.8% ppm) for 78 weeks. The time-weighted average (TWA)
dietary concentrations were 3.5 and 6.9 parts per million (ppm) for
males (50/dose group) and 2 and 3.9 ppm for females (50/dose group).
Twenty mice/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 0.52
and 1.0 mg/kg bw/day in males, and 0.3 and 0.58 mg/kg bw/day in
females.
High early mortality was observed among male mice. Eleven
high-dose males and two low-dose males died on week 19. Survival at
term was 15% (3/20) in control males, 38% (19/50) in low-dose males,
and 10% (5/50) in high-dose males. The deaths in the controls may
have been due to fighting. No common cause of death was found for
the high-dose males. Survival was high among all female mice (85%
of control, 94% of low dose, and 96% of high-dose females survived
to term). No effects of treatment on body weight or clinical signs
of toxicity were observed. No dose-dependent non-neoplastic lesions
were noted in female mice. However, the abbreviated lifespan of
male mice was considered to preclude any accurate analysis of
late-occurring tumours. The NOAEL in this study was less than
3.5 ppm (i.e., 0.25 mg/kg bw/day; lowest dose tested) in male mice
due to mortality, and 3.9 ppm (i.e., 0.58 mg/kg bw/day; highest dose
tested) in female mice (NCI, 1978).
A second chronic feeding study of endosulfan technical (97.9%
pure) was performed in Hoe:NMRKf(SPF 71) mice in which 60
animals/sex/dose level received dietary concentrations of 0, 2, 6,
and 18 pm for 24 months. An additional 10 mice/sex/dose were
sacrificed at interim periods of 12 and 18 months, respectively.
The administered concentrations were calculated to be equivalent to
0, 0.28. 0.84, and 2.51 mg/kg bw/day in males and 0, 0.32, 0.097,
and 2.86 mg/kg bw/day in females.
Survival was significantly reduced in female mice with 18 ppm
endosulfan in the main portion of the study (total deaths in females
= 33/60 or 55% controls, 36/60 or 60% low dose, 38/60 or 63.3% mid
dose, and 43/60 or 71.7% high dose). Survival was not affected by
the test compound in male mice in the main study (total deaths in
males = 27/60 or 45% controls, 33/60 or 55% low dose, 37/60 or 61.7%
mid dose, and 35/60 or 58.3% high dose). There was no
compound-related effect on mortality in either female or male mice
at the 12- and 18-month interim sacrifice periods. Body weight
gain was slightly reduced (about 10%) in male mice throughout the
main study at 18 ppm endosulfan. Female weights were slightly
elevated in the 12- and 18-month satellite groups, body weight was
either slightly increased or remained unchanged from controls in
animals of all treatment groups. There were no treatment-related
effects on food consumption, behavioural or general health
conditions of the animals, or hematological or clinical chemistry
parameters.
Gross and microscopic examination were performed at 12, 18, and
24 months. Reductions in several organ weights occurred with the
18 ppm dose level of endosulfan; these included decreases in the
weights of the liver (males at 18 months only), and ovaries (females
at 12 and 18 months). No treatment-related pathological changes or
tumours were seen after gross or histopathological examination of
tissues at 12, 18, or 24 months. The NOAEL was 6 ppm endosulfan,
equivalent to 0.84 mg/kg bw/day in male mice and 0.97 mg/kg bw/day
in female mice (Donaubauer, 1988).
Rats
Groups of Osborne-Mendel rats were fed diets containing
endosulfan (technical grade; 98.8% pure) for 78 weeks. The TWA
dietary concentrations were 408 and 952 ppm for males (50/dose
group) and 223 and 445 ppm for females (50/dose group). Twenty
rats/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 20.4
and 47.6 mg/kg bw/day in males, and 11.1 and 22.1 mg/kg bw/day in
females.
Endosulfan produced early mortality (dose-related) in male
rats. By study week 54, 52% of the high-dose males died and by week
74, 88% were dead. The high-dose males were sacrificed on week 74.
For the low-dose males, only 10/50 (20%) survived to term, and these
were sacrificed on week 82. Survival was adequate in control males.
In the case of female rats, no compound-related deaths occurred (70%
of control, 60% of low dose, and 50% of high-dose females survived
to term). Body weight was also depressed in a dose-related fashion
in treated male rats from week 22 onward. At the time of sacrifice,
body weight was depressed about 25% in both low- and high-dose male
rats compared to the control male group. Body weight was not
markedly changed in female rats. Several clinical signs of toxicity
were also seen in treated rats of both sexes; these included hunched
appearance, reddened and squinted eyes, rough fur, and abdominal
urine stains.
Upon histopathological examination, toxic nephropathy was seen
in both male (0/20 controls, 47/50 low dose, 43/47 high dose) and
female (0/20 controls, 27/50 low dose, 29/50 high dose) rats. This
abnormality was characterized by degenerative changes in the
proximal convoluted tubules at the corticomedullary junction, with
cloudy swelling, fatty degeneration and necrosis of the tubular
epithelium. Some affected tubules contained hyaline casts and
enlarged dark-staining regenerative tubular epithelial cells, and
the kidneys often had inflammatory cell infiltration, fibrotic
changes, and focal mineralization. Parathyroid gland hyperplasia
was also seen in male rats (0/20 controls, 21/40 low dose, 18.47
high dose) and was attributed to the effects of chronic renal
failure. Medial calcification of the blood vessels, perhaps related
to the parathyroid hyperplasia, occurred frequently in treated male
rats. Finally, testicular atrophy occurred in male rats (3/19
control, 18/47 low dose, 24/47 high dose) and was characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules, multinucleated cells (fusion bodies), and
calcium deposition resulting in aspermatogenesis.
No conclusions concerning the carcinogenicity of endosulfan
could be drawn from the bioassay of male rats because of the high
early mortality and abbreviated lifespans of these animals.
Survival in female rats was considered to be sufficient for the
evaluation of late occurring tumours; endosulfan was not
carcinogenic in female rats. The results of this long-term bioassay
in rats indicate that the lowest dietary concentrations of
endosulfan tested in males (408 ppm TWA - equivalent to
20.4 mg/kg/day) and females (223 ppm TWA - equivalent to
11.1 mg/kg/day) were toxic and that a NOAEL could not be established
(NCI, 1978).
A second chronic feeding study of endosulfan technical (97.1%
pure) was performed in Charles River Crl:CD(SD)BR rats in which 50
animals/sex/dose level received dietary concentrations of 0, 3, 7.5,
15, and 75 ppm for 104 weeks. An additional 20 rats/sex/dose
received similar doses of endosulfan and were used to obtain
hematology, clinical chemistry, and urinalysis data at various
intervals throughout the study. Gross and microscopic examinations
were performed on all animals. The administered concentrations of
endosulfan were calculated to be equivalent to 0.1, 0.3, 0.6, and
2.9 mg/kg bw/day in males and 0.1, 0.4, 0.7, and 3.8 mg/kg bw/day in
females.
There were no compound-related effects on mortality, clinical
signs of toxicity, food and water consumption, or ophthalmoscopic
parameters. Adverse findings were mainly observed at the highest
dose of endosulfan. These included significant reductions in body
weight gain over the study in males (-17%) and females (-18%), a
reduced food utilization efficiency in females, hematological
changes at week 103 in males (increased platelets, and reductions in
mean corpuscular volume, total white cells, and lymphocytes), and
clinical chemistry changes at week 103 in males (increased plasma
globulin and urinary protein levels, and reduced GPT and serum
albumin levels). The reductions in total white cells and
lymphocytes, and the increased urinary protein levels, were said to
be within expected limits of variation. It should be noted that
body weight gain was also reduced in males given 15 ppm endosulfan
on study weeks 6 to 18 only (-9% reduction); this effect, however,
was not progressive after week 18 and was not considered to be
toxicologically significant.
Gross and microscopic examination of all animals in the study
indicated that the primary target organ for toxicity was the kidney.
This was noted from an increased number of enlarged kidneys seen
grossly for females at 75 ppm (10/70 controls, 18/70 at 3 ppm, 19/70
at 7.5 ppm, 17/70 at 15 ppm, and 26/70 at 75 ppm), and an elevated
incidence of marked progressive glomerulonephritis in males at
75 ppm, 24/70 at 15 ppm, and 30/70 at 75 ppm) and also in females at
75 ppm (1/70 controls, 6/70 at 3 ppm, 6/70 at 7.5 ppm, 5/70 at
15 ppm, and 8/70 at 75 ppm). In addition, blood vessel aneurysms
were elevated in male rats at 75 ppm (10/70 controls, 6/70 at 3 ppm,
14/70 at 7.5 ppm, 10/70 at 15 ppm, and 21/70 at 75 ppm). The
aneurysms were considered to be possibly related to the renal
lesions.
Other observations in the study at 75 ppm in male rats included
a greater number of enlarged lumbar lymph nodes (14/70 controls,
10/70 at 3 ppm, 8/70 at 7.5 ppm, 7/70 at 15 ppm, and 19/70 at
75 ppm), inflammatory cells in the liver (15/70 controls, 16/70 at
3 ppm, 15/70 at 7.5 ppm, 18/70 at 15 ppm, and 25/70 at 75 ppm), and
polyarteritis in the pancreas (0/70 controls, 2/70 at 3 ppm, 4/70 at
7.5 ppm, 1/70 at 15 ppm, and 8/70 at 75 ppm) and testes (16/70
controls, 15/70 at 3 ppm, 23/70 at 7.5 ppm, 18/70 at 15 ppm, and
27/70 at 75 ppm). Finally, the mean weights of the testes were
reduced at 15 ppm and 75 ppm of endosulfan, but the response was not
strictly dose-related and was within expected limits of variation.
Endosulfan was not carcinogenic in either male or female rats.
The NOAEL was said to be 15 ppm, equivalent to 0.6 mg/kg bw/day in
male rats and 0.7 mg/kg bw/day in female rats (Ruckman et al.
1988).
Reproduction studies
Rats
Male and female Charles River SD-CD rats were administered
endosulfan (97% purity) in the diet for two generations at
concentrations of 0, 3, 15, and 75 ppm (equal to approximately 0.2,
1.0, and 6.0 mg/kg bw/day). Females were mated to males on a
one-to-one basis with 32 males/32 females per dose in the F0
generation, and 28 males/28 females per dose for the F1 generation.
F0 generation rats were administered endosulfan from 6 weeks of age
through 18 weeks of age, at which time they were mated to produce
F1a litters. F1a young were sacrificed 21 days postpartum, and the
F0 generation remated to produce the F1b litters. After weaning of
the F1b pups, the F0 generation rats were sacrificed. The F1b rats
were continued on their respective diets for approximately 98 days
prior to mating to produce the F2a litters (which were sacrificed
after weaning) and the F2b litters (likewise sacrificed after
weaning). Endosulfan was administered continuously throughout the
experiment.
There were no compound-related effects on mortality, food/water
consumption, or body weight gain, although F1b females given 3 ppm
demonstrated slightly lower body weights than controls from week 4
through 36. Pregnancy rate, mating performance and gestation period
for both F0 and F1b generations were unaffected by treatment.
Both matings of the F0 generation demonstrated significant
decreases in litter weight at 75 ppm from day 4 through day 21 but
not in either F1b mating. Also, F0 generation litter weights were
significantly reduced on days 4 to 21 during the second mating at
15 ppm as well. Although the trend for cumulative litter loss (%)
was increased in the first mating at 75 ppm, it was not evident in
the second mating nor in either mating of the F1b generation. The
mean pup weight was unaffected throughout both F0 and F1b matings.
Sex ratio appeared to be unaffected throughout the study.
F0 and F1b generation parents presented evidence of increased
absolute organ weights for heart, liver, kidneys, and brain at
75 ppm. These were not demonstrated in the offspring from either
mating of these parents. There was no consistent dose-related
effect. Histopathology of F1b adults and F2b weanlings
demonstrated no compound-related effects. F0 and F1b generation
females and offspring were examined for skeletal and visceral
abnormalities and no consistent dose-related trends were evident,
except for isolated incidences of microphthalmia (2), anophthalmia
(2), kinked/missing tail (3), shortened/malformed digits or limbs
(5), retinal hemorrhage (1) and spina bifida (1).
There were no dose-related or compound-related effects on
reproductive performance. Decreases in litter weight at 15 and
75 ppm occurred in the F1a and F1b litters; however, this finding
was not considered to be adverse since the reductions in litter
weight were marginal and there were no significant corresponding
reductions in pup weight or litter size. The visceral and skeletal
examinations of fetuses for assessing teratogenic effects were
within normal variation for the strain/species studied. It is
considered that endosulfan, at dietary levels up to and including
75 ppm (approximately 6 mg/kg/day in the diet) caused no adverse
effects on reproduction in rats in this study (Edwards et al.
1984).
However, as noted below, renal changes occurred in F1b adult
rats at all of the doses of endosulfan that were tested. In an
addendum to the above study, a histological review of the kidneys of
adult rats from the F1b generation and of weanling rats from the F2b
generation was reported. The effort was initiated because yellowish
discoloration and pigment deposits were noted in renal proximal
convuluted tubules in rats in a 13-week oral toxicity study of
endosulfan followed by a 4-week withdrawal period (see Barnard
et al. 1985). The histological data obtained indicated the
presence of yellowish discolored cells in the proximal convoluted
tubules in F1b adult rats given dietary doses of 3, 15, and 75 ppm
endosulfan. The incidences in males were: 0/28 controls, 11/28 at
3 ppm, 13/28 at 15 ppm, and 28/28 at 75 ppm. The incidences in
females were: 0/28 controls, 0/28 at 3 ppm, 0/28 at 15 ppm, and 9/28
at 75 ppm. In addition, granular/clumped pigment was also noted at
a dietary dose of 75 ppm in F1b adult rats. The incidences at
75 ppm were 14/28 males and 1/28 females vs. an incidence of 0/28 in
males and females from the control, 3 ppm, and 15 ppm dose groups.
In the F2b generation weanling rats, no comparable renal changes
were observed following treatment with endosulfan (Offer, 1985).
These findings were not considered to be adverse since the yellow
pigment in kidneys was identified as endosulfan and its metabolites,
being stored and metabolized in lysosomes prior to excretion.
Special studies on teratogenicity
Rats
Groups of 25 pregnant CD Sprague-Dawley rats were administered
endosulfan (97.3% purity), via oral gavage, at dose levels of 0,
0.66, 2, and 6 mg/kg bw/day from days 6 to 19 of gestation.
(Day 0 = day sperm found). Ten additional animals were added to the
high dose (due to mortality) and five to the control groups (due to
loss of tissue during processing). On day 20 of gestation, all dams
were killed by asphyxiation with CO2 and fetuses delivered by
cesarean section.
Maternal toxicity was evident in the mid- and high-dose groups,
expressed by significantly reduced body weight and body weight gain
during gestation, and by various clinical signs of toxicity (e.g.,
rats rubbing their faces, alopecia, rough coats, lethargy,
flaccidity, and hyperactivity). Several animals died as the result
of improper gavage tube insertion. Fetal and embryolethality were
not apparent in any group and all pregnant animals had viable
litters. Mean fetal weight and crown-rump length were significantly
reduced in the high-dose group, but not in the lower dose groups. A
number of external, visceral, and skeletal abnormalities were
observed in the fetuses in the high-dose group. The most prominent
effect was a significant increase in the incidence of small fourth
and unossified fifth sternebrae in the high-dose fetuses. However,
there was no clear evidence of a teratogenic effect due to the
significant maternal toxicity expressed at the same level. The
NOAEL for maternal toxicity was 0.66 mg/kg bw/day and that for
fetotoxicity was 2 mg/kg bw/day by oral gavage (MacKenzie et al.
1980a).
A range-finding study with endosulfan in pregnant CD
Sprague-Dawley rats (4-6/group) was performed for the purpose of
setting doses for the above described rat teratology study.
Endosulfan was administered by gavage on days 6 to 19 of gestation
(Day 0 = day sperm found) at doses of 0 (corn oil vehicle), 1.25,
2.5, 5, 10, 20 and 40 mg/kg bw/day. Toxic signs in dams included
reductions in body weight gain at doses of 1.25 mg/kg bw/day and
greater, various clinically observed adverse effects at doses of
2.5 mg/kg bw/day and greater (e.g., salivation, piloerection, poor
muscle tone, lethargy, head-rubbing behaviour, hyperactivity,
spasticity, tremors and convulsions, and deaths (67 to 100%
mortality) at 10 mg/kg bw/day and greater. The changes were dose
related and most marked at higher doses of endosulfan. No effects
on pregnancy maintenance or fetal survival were observed (MacKenzie
et al. 1980b).
Rabbits
Groups of pregnant New Zealand White rabbits (20/group) were
administered endosulfan (97.3% purity) via oral gavage in corn oil
at dose levels of 0, 0.3, 0.7, and 1.8 mg/kg bw/day from days 6 to
28 of gestation (Day 0 = day of mating). Animals were observed
throughout the test period for toxic signs. Six additional pregnant
rabbits were added to the high-dose group due to mortality. On day
29 of gestation, the dams were asphyxiated with CO2, and the entire
reproductive tract was removed.
Maternal toxicity was evident at the high dose, expressed as
rapid breathing, hyperactivity, convulsions and mortality. Group
mean body weights were not affected by treatment. All pregnant dams
had viable fetuses, with the exception of one high-dose dam which
died on test and which had nine late resorptions. There were no
compound-related effects on the number of corpora lutea,
implantation efficiency, litter size, sex ratio, mean fetal length
or weight, or the number of live/dead or resorbed fetuses. There
were no gross external abnormalities and the visceral and skeletal
examinations revealed no dose-related increase in variations or
anomalies. The NOAEL for maternal toxicity was 0.7 mg/kg bw/day.
Endosulfan was not teratogenic in rabbits at doses up to and
including 1.8 mg/kg bw, although maternal toxicity was expressed at
that level (MacKenzie et al. 1981a).
A range-finding study with endosulfan in pregnant New Zealand
White rabbits (3 to 10/group) was conducted to set doses for the
above described rabbit teratology study. Endosulfan was
administered by gavage on days 6 to 28 of gestation (Day 0 = day of
mating) at doses of 0 (corn oil), 1, 2, 4, 8, and 12 mg/kg bw/day.
Standard observations and necropsy procedures were performed. Toxic
signs in dams included deaths at 2 mg/kg bw/day or more (20 to 100%
mortality) and various clinical adverse effects at 2 mg/kg bw/day or
more (e.g., hyperactivity, opisthotonos, clonic and tonic
convulsions, paralysis, tremors, and loss of mobility). Fetal
survival appeared to be adversely affected at 2 mg/kg bw or more as
indicated by an increase in the percent of dead fetuses and a
decrease in the percent of live fetuses in dams. No prominent
effects on weight gain or pregnancy rate occurred (MacKenzie et al.
1981b).
Special study on mutagenicity
Endosulfan was tested for mutagenic activity in a variety of
bacterial, yeast, and mammalian cell systems and was negative in
each test (Table 1).
TABLE 1. RESULTS OF MUTAGENICITY STUDIES ON ENDOSULFAN
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
Ames Testa S.typhmurium 6-5000 ug/plate Negative Shirasu et al. 1978
TA98, TA100, TA1535,
TA1537, TA1538
Yeast Forward Mutation Schizosaccharomyces pombe 62.5-500 ug/plate Negative Milone 1984a
Testa
Yeast Gene-Conversion/ S. cerevisiae 100-5000 ug/ml Negative Milone, 1984b
DNA Repair Testa (D4 strain)
Mouse Lymphoma Assaya Mouse L51786 TK+/- Cells 6.25-100 ug/ml Negative Cifone, 1984a
DNA Repair Assaya B. subtilis H17 and 20-2000 ug/disk Negative Shirasu et al. 1978
M45 (rec-)
Unscheduled DNA Synthesis Primary-Hepatocytes (Rat) 0.102-51 ug/ml Negative Cifone, 1984b
Unscheduled DNA Human Cell Line A 549 1-1000 ug/ml Negative Muller, 1988
Synthesisa
Micronucleus Assay NMRI Mice O.2-5 mg/kg, Negative Jung et al. 1983
(Bone Marrow) Orallyb
Micronucleus Assay Swiss Albino Male Mice 43.3 mg/kg Negative Usha Rani et al. 1980
(Bone Marrow) Orallyb
Chromosome Aberration Cultured Human Lymphocytes 0.05-100 ug/ml Negative Milone, 1986
Testa in vitro
TABLE 1 (CONTD).
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
In vivo Cytogenicity Male Albino Rats 11-55 mg/kg/day Negative Dikshith & Datta, 1978
Test (Bone Marrow Orally for 5 days
and Spermatogonia)
a With and without metbolic activation.
b Administered twice orally at an interval of 24 hours.
Special study on neurotoxicity
The acute delayed neurotoxicity of endosulfan was assessed in
adult domestic hens. The study consisted of three parts. First,
the LD50 determination was performed using oral (gavage) doses of
0, 40, 60, 90, 110, and 135 mg/kg bw endosulfan (5 hens/dose group).
The observed LD50 was 96 mg/kg bw. All deaths occurred within 2
hours of dosing. Toxic signs observed were subdued behaviour and
weight loss. Second, a protection assessment was performed using an
oral dose of 96 mg/kg bw of endosulfan alone, or in combination with
either phenobarbital (15 mg/kg bw i.m.), diazepam (2 mg/kg bw p.o.),
or atropine (10 mg/kg bw i.m.) plus 2-PAM (25 mg/kg bw i.m.). There
were 5 hens/group. The results did not provide evidence that any of
the protections reduced the number of mortalities occurring after
dosing with endosulfan. Third, a neurotoxicity assessment was
performed using groups of 10 hens. A vehicle control group was
dosed orally with corn oil, a positive control group with 500 mg/kg
bw TOCP, and four test groups were each dosed with the LD50
(96 mg/kg bw) dose of endosulfan. Dosing was followed by a 21-day
observation period, a redosing, and a final 21-day observation
period. Surviving hens were sacrificed at 42 days.
Histopathological examination of brain, spinal cord and peripheral
nerve tissues were performed on 9 of the 40 animals dosed with
endosulfan (these were the survivors to 42 days), 10/10 control
hens, and 10/10 TOCP-treated hens. The results indicated that
endosulfan did not produce any clinical signs of neurotoxicity or
neuropathological (i.e., histological) effects on the central or
peripheral nervous system. In contrast, hens treated with TOCP
displayed ataxia as well as significant axonal degeneration in the
spinal cord and in the peripheral (tibial) nerve (Roberts et al.
1983).
Special study on skin sensitization
Endosulfan had no sensitizing potential (delayed contact
hypersensitivity) in guinea pigs when tested by the Buehler method
(Jung & Weigand, 1983).
COMMENTS
In rats, the absorption of single oral doses of endosulfan
required 2 or more days; subsequent urinary and fecal excretion was
also slow, requiring 5 or more days. The biotransformation pathways
involve hydrolysis and oxidation to sulfur-free metabolites and
polar conjugates, and the formation of various sulfur-containing
moieties. Endosulfan preferentially accumulated in the kidney, with
lower levels in other tissues, the elimination half-time from
tissues being 3 to 7 days.
Endosulfan at dietary dose levels of 10 ppm and higher in
subchronic studies in rats produced yellow pigmentation of renal
cells. In contrast, renal cellular pigmentation was not observed in
subchronic studies in mice at similar dose levels (see reproduction
studies below).
In a one-year dietary study in dogs, doses of 30 to 60 ppm
resulted in early sacrifices due to clinical signs of neurotoxicity
(e.g., convulsive muscular activity). Reduced food consumption and
weight gain also occurred. In this study, the NOAEL was 10 ppm,
equal to 0.57 mg/kg bw/day.
Long-term carcinogenicity studies were performed in mice and
rats. Treatment-related increases in neoplasms were not observed.
The NOAELs (based on non-neoplastic effects) were 6 ppm (equal to
0.84 mg/kg bw/day) in mice and 15 ppm (equal to 0.6 mg/kg bw/day) in
rats.
A two-generation, 2 litters per generation reproduction study
was conducted in rats. Endosulfan was associated with a reduction
in weight of the F1a and Flb litters at dietary levels of 15 and
75 ppm, and the presence of yellowish pigment in cells in the renal
proximal convoluted tubules at dose levels of 3, 15 and 75 ppm in
F1b adult rats. The Meeting did not consider these findings to be
adverse since the reduction in litter weights were marginal and
occurred in the absence of corresponding reductions in pup weight or
litter size. The yellow pigment in kidneys was identified as
endosulfan and its metabolites, being stored and metabolized in
lysosomes prior to excretion.
In teratology studies performed in rats and rabbits, NOAELs of
approximately 0.7 and 1.8 mg/kg bw/day were identified for maternal
toxicity and fetotoxicity, respectively. Endosulfan was not
teratogenic at 6 mg/kg bw/day in rats and 1.8 mg/kg bw/day in
rabbits, the highest dose levels tested.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 6 ppm in the diet, equal to 0.84 mg/kg bw/day
Rat: 15 ppm in the diet, equal to 0.6 mg/kg bw/day
Dog: 10 ppm in the diet, equal to 0.57 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E., Gibson,
W.A., Rao, R., Offer, J.M., Gopinath, G., Almond, R.H. (1984)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice. Unpublished Report No. A29663 from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street,
A.E., Gibson, W.A., Gopinath, G., Majeed, S.K., Almond, R.J. (1985)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice followed by a 4-week withdrawal period. Unpublished Report
No. A30700 from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Brunk, R. (1989) Endosulfan-substance technical: Testing for
toxicity by repeated oral administration (1-year feeding study) to
beagle dogs. Unpublished Report No. 89-0188 from Pharma Research
Toxicology and Pathology, Hoechst AG. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Cifone, M.A. (1984a) Mutagenicity evaluation of HOE 002671-
substance technical in the mouse lymphoma forward mutation assay.
Unpublished Report No. A29801 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Cifone, M.A. (1984b) Evaluation of HOE 002671-substance technical
in the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished Report No. A29800 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-Endosulfan. Unpublished Report No. A-36685 from WIL Research
Laboratories, Inc., Ashland, Ohio. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Dikshith, T.S.S., Datta, K. (1978) Endosulfan: lack of cytogenetic
effects in male rats. Bull. Environ. Contam. Toxicol., 20:
826-833.
Donaubauer, H.H. (1988) Endosulfan-substance technical.
Carcinogenicity study in mice - 24 months feeding study.
Unpublished Report No. A38008 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Donaubauer, H.H., Leist, K.H., Kramer, M. (1985) Endosulfan-
substance technical: 42-day feeding study in mice. Unpublished
Report No. A38104 from Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Leist, K.H., Kramer, P. (1985a) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in Wistar rats. Unpublished Report
No. A30753 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Weigand, W., Kramer, P. (1985b) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in SPF Wistar rats. Unpublished Report
No. A30754 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Edwards, J.A., Reid, Y.J., Offer, J.M., Almond, R.H., Gibson, W.A.
(1984) Effects of Endosulfan-technical on reproductive function of
multiple generations in the rat. Unpublished Report No. A29428
(Huntingdon Report No. HST 204/83768) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W. (1983) Hoe 002671-active ingredient
technical. Testing for acute aerosol inhalation toxicity in male
and female SPF Wistar rats, 4 hours - LC50. Unpublished Report
No. A32087 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W., Kramer, P. (1984) Endosulfan-active
ingredient technical. Testing for subchronic inhalation toxicity -
21 exposures in 29 days in SPF Wistar rats. Unpublished Report
No. A29823 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Jung & Weigand, W. (1983) Hoe 002671-active ingredient technical.
Testing for sensitizing properties in female Perbright-White guinea
pigs. Unpublished Report No. A27248 from Pharma Forschung
Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Jung, Weigand, W., Kramer, P. (1983) Hoe 112671-active ingredient
technical. Micronucleus test in male and female NMRI mice following
oral administration. Unpublished Report No. A29689 from Pharma
Forschung Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Lachmann, G.(1987) Dermal absorption of 14C-Endosulfan in rhesus
monkeys. Unpublished Report No. A36685 from Battelle-Institut,
Frankfurt, FRG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Kramer, P. (1985) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A30776 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Mayer, D. (1987) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A37112 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Dickie, S.M., Jackson, T.A.,
Zelinger, D.J. (1981a) Teratology study with FMC 5462 in rabbits.
Unpublished Report No. A23192 (Raltech Study No. 80070) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1981b) Range-finding study with FMC 5462 in pregnant rabbits.
Unpublished Report No. A37682 (Raltech Study No. 79032) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1980a) Teratology study with FMC 5462 in rats. Unpublished
Report No. A21393 (Raltech Study No. 79041) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M. (1980b)
Range-finding study with FMC 5462 in pregnant rats. Unpublished
Report No. A37680 (Raltech Study No. 79031) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Milone, M.F. (1984a) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished Report No. A29312 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1984b) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Saccharomyces cerevisiae.
Unpublished Report No. A29313 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1986) Study of the capacity of the test article
Endosulfan, substance technical, to induce chromosome aberration in
human lymphocytes cultured in vitro. Unpublished Report No. A33127
from Instituto di Richerche Biomediche "Antoine Marxer", Italy.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Muller, W. (1988) Evaluation of Endosulfan-substance, technical in
the unscheduled DNA synthesis test in mammalian cells in vitro.
Unpublished Report No. A38455 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
National Cancer Institute (NCI) (1978) Bioassay of Endosulfan for
possible carcinogenicity. Technical Report No. 62 from US
Department of Health, Education and Welfare, NIH. Submitted to WHO
by Hoechst AG, Frankfurt, FRG.
Offer, J.M. (1985) Addendum to HST 204: effect of Endosulfan-
technical on the reproductive function of multiple generations in
the rat. Histopathological review of the kidneys in adult rats of
the F1B generation and in weanling rats of the F2B generation.
Unpublished Report No. A30757 (Huntingdon Addendum to Report HST
204/83768) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Cooke, L., Gibson,
W.A. (1983) Acute delayed neurotoxicity study with Endosulfan-
technical in the domestic hen. Unpublished Report No. A32153
(Huntingdon Report No. HST 225/83888) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed,
S.K., Anderson, A., Chanter, D.O. (1988) Endosulfan, active
technical ingredient. Combined chronic toxicity/carcinogenicity
study (104-week feeding in rats). Unpublished Report No. HST
289/881067) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Shirasu, Y., Moriya, M., Ohta, T. (1978) Microbial mutagenicity
testing on Endosulfan. Unpublished Report No. A21215 from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Stumpf, K., ABhauer, J. (1986) An up-to-date review of the
environmental safety of Endosulfan. Unpublished Report No. A35180
from the Analytical Laboratory, Hoechst AG. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Usha Rani, M.V., Reddy, O.S., Reddy, P.P. (1980) Mutagenicity
studies involving aldrin, dieldrin, endosulfan, dimethoate,
phosphamidon, carbaryl and ceresan. Bull. Environ. Contam.
Toxicol., 25: 277-282.
Toxicological Studies
Acute toxicity
An acute nose-only inhalation toxicity test of endosulfan in
Wistar rats resulted in LC50 values of 0.0345 mg/L of air in males
and 0.9126 mg/L of air in females. Clinical signs of intoxication
were dyspnea, passivity, disequilibrium, trembling, tremors,
tono-clonic convulsions, and subdued reflex activity. Gross necropsy
changes in animals that died consisted of sporadic dark red pinhead
foci in the lungs (Hollander & Weigand, 1983).
Short-term studies
Mice
Groups of 10 male and 10 female Hoe:NMRKf(SPF 71) mice were fed
diets containing 0 or 18 ppm technical endosulfan for 42 days. The
18 ppm dose level was calculated to be equivalent to 3.7 mg/kg
bw/day in males and 4.6 mg/kg bw/day in females. The results
obtained indicated that deaths occurred in 2/10 treated mice (one
female on day 28 and another female on day 38), and absolute and
relative liver weights were significantly increased in females. In
addition, the body weight gain was slightly elevated throughout the
study, with the increase observed on day 36 being statistically
significant. There were no other changes in females or in males
with respect to food consumption, behaviour or general condition
(including ocular and dental examinations), or macroscopic
examinations of organs. The NOAEL was less than 4.6 mg/kg bw/day in
females (based on deaths and increased liver weights) and equal to
3.7 mg/kg bw/day in males (Donaubauer et al. 1985).
In a 13-week study, groups of 20 male and 20 female CD-1 mice
received dietary levels of 0, 2, 6, 18, or 54 ppm of technical
endosulfan. These concentrations were equivalent to doses of
approximately 0, 0.24-0.27, 0.74-0.80, 2.13-2.39, or 7.3-7.5 mg/kg
bw/day, respectively.
Adverse findings appeared to be confined primarily to the
highest dose level of endosulfan (i.e., 54 ppm). The observed
changes included deaths (12/20 males and 10/20 females at various
study intervals), convulsions and salivation (1/20 males and 1/20
females), reduced neutrophils (males on weeks 6 and 13), increased
total serum lipids (females on week 13), reduced spleen weight
(males), and slight vascular congestion in the lungs (11/20 males
and 6/20 females). All of those parameters were unchanged in all
other treatment groups. At dose levels of 6, 18, and 54 ppm, body
weight gain was depressed in male mice at study week 1 only, and
blood glucose levels were depressed in females at study week 6 only.
Neither of these changes persisted throughout the study and are thus
not considered to be of toxicological importance. Similarly, food
consumption was reduced only on study weeks 1 and 2 in mice of both
sexes at the 54 ppm dose level. From the above data, the NOAEL was
considered to be 18 ppm, equivalent to 2.13 to 2.39 mg/kg bw/day in
the diet, respectively, in male and female mice (Barnard et al.
1984).
Rats
Endosulfan was fed for 4 weeks in the daily diet to groups of
100 male Wistar rats at concentrations of 360 and 720 ppm. These
concentrations were said to be equivalent to doses of 34.0 and
67.8 mg/kg bw/day, respectively. An additional 20 male rats served
as control animals. At the end of 4 weeks treatment, one-half of
the animals in each group were killed (main study group) and the
other half were allowed to recover for 4 more weeks before being
sacrificed (recovery group). Rats were examined daily for behaviour
and general health conditions. Body weights and food consumption
were recorded weekly. At autopsy, all animals were examined
grossly, and the relative weights of three organs (liver, kidneys,
brain) were calculated. Histological exams were performed on the
left kidney, and a piece of liver from six animals/dose level; the
right liver, another piece of liver and the brain from two
animals/dose level were examined by electron microscopy. The
remaining parts of the above organs not used for histological study
were used to identify residues of endosulfan in tissue.
Two deaths occurred due to unknown causes (one rat at 360 ppm
on day 29 and one rat at 720 ppm on day 8). There were no
treatment-related effects in the appearance or general health of the
animals (including signs of neurological disturbances, corneal
opacity, oral mucosal lesions, or changes in dental growth), in body
weight gain, or in food consumption. The relative weights of the
liver were increased at both dose levels, and relative kidney and
brain weights were increased at the highest dose level. Upon gross
examination, the kidneys appeared darkened in colour at both dose
levels. Histopathological examination showed proliferation and
enlargement of lysosomes of the cells of the proximal convoluted
tubules, and granular pigmentation of the same cells, at both doses
of endosulfan. Lysosomal changes were not seen in brain or liver.
The above findings were not seen in animals in the 4-week recovery
group. The identification of residues of endosulfan in tissues was
unsuccessful. There was no NOAEL in this study due to the observed
renal and liver changes at 360 ppm, equivalent to 34.0 mg/kg bw/day
in the diet (Leist & Kramer, 1985).
In a follow-up report, the results of repeat tissue residue
analysis studies after dietary dosing with 360 and 720 ppm in Wistar
rats for 4 weeks, and with a recovery period of an additional 4
weeks, were made available. The findings indicated that
alpha-endosulfan, and to a considerably lesser extent
beta-endosulfan, was temporary stored in the kidneys in proportion
to the administered dose. Only endosulfan-sulfate and
endosulfan-lactone were detected in any appreciable quantities in
the kidneys. It was also noted that endosulfan and its metabolites
were minimally detectable in the blood. In the liver,
endosulfan-sulfate and endosulfan-lactone were the dominant
compounds, although their levels were many times lower than the
concentrations of alpha-endosulfan measured in the kidneys. It was
also observed that the storages were reversible; 30 days after the
end of the treatment phase alpha-endosulfan was detected only in
traces, and beta-endosulfan not at all (Leist & Mayer, 1987).
In a 13-week study, groups of 25 male and 25 female CD rats
were fed diets containing 0, 10, 30, 60, or 360 ppm technical
endosulfan. Daily dosages were equivalent to 0, 0.64, 1.92, 3.85,
and 23.41 mg/kg bw/day in males, and 0, 0.75, 2.26, 4.59, and
27.17 mg/kg bw in females. At the end of the 13-week treatment
period all animals were sacrificed except for 5 rats/sex dose group
that were withdrawn from treatment and followed for an additional
4-week recovery period. Ophthalmoscopic exams were performed on
control and high-dose rats before treatment and at week 13.
Neurological exams (locomotor reflexes) were performed on 10
rats/sex from the control and high-dose levels before treatment and
on weeks 2, 6, and 13; in addition, all animals were examined for
grip reflex and evidence of ataxia on week 13. Blood was sampled at
0, 6, and 12/13 weeks from 10 rats/sex/dose for assessment of
hematology and clinical chemistry parameters. A standard battery of
tests/examinations was performed and, in addition, blood and plasma
cholinesterase estimations were made at 5 and 12 weeks in 10
rats/sex/dose level. Urine was collected on weeks 4 and 13 for
standard urinalysis determinations. Similar studies were performed
in the rats that were allowed to complete the recovery period.
Three deaths occurred during the treatment period (1/20 female
controls, 1/20 females at 60 ppm due to anesthetic shock during
routine blood sampling, and 1/20 females at 320 ppm on day 19). No
deaths occurred in rats during the recovery period.
The only clinical sign of toxicity observed was hair loss in
females receiving 60 and 360 ppm endosulfan. Body weight gain was
reduced in male and female rats to a slight degree over the course
of the study. Food consumption was reduced in females given 360 ppm
during study weeks 1 and 2 only. All of these changes regressed by
the end of the recovery period. No adverse ophthalmological or
neurological findings were observed in treated rats.
A variety of hematological changes were observed during the
treatment period. Red blood cells were reduced at week 13 (and
after week 6) in male rats at doses of 30 to 360 ppm. MCV was
increased in female rats at weeks 13 and 6 at doses of 60 and
360 ppm. Finally, PCV was reduced at weeks 13 and 6 in male rats
given 360 ppm. In most cases, these effects were still evident in
rats, especially males, at recovery week 17. Changes involving
clinical chemistry parameters included increases in serum phosphorus
in females (weeks 12 and 6) at 30 and 360 ppm, reductions in serum
electrocytes (Na+ and K+) in males (weeks 12 and 6) at 60 and
360 ppm, and increased in total lipids and cholesterol in females
(weeks 12 and 6) at 360 ppm. In the case of urinalysis
determinations, a darkened urine was observed in male rats (week 13)
at 60 and 360 ppm, and in females (week 13) at 360 ppm. The urine
of the male rats at 60 and 360 ppm also contained elevated levels of
ketones and proteins. The clinical chemistry and urinalysis changes
regressed by the end of the recovery period.
Lower plasma and red blood cells cholinesterase activities were
observed in females (weeks 12 and 5) given 360 ppm endosulfan. In
contrast, increased brain cholinesterase activity was seen in
females treated with 60 and 360 ppm. These changes regressed during
the recovery period.
At necropsy, increases were noted in the relative weights of
the kidney (male rats at 60 and 360 ppm, and female rats at
360 ppm), liver (males and females at 360 ppm), brain (females at 60
and 360 ppm), and epididymes (males at 360 ppm). Increased kidney
weights were also seen in high-dose males at the end of the recovery
period. Macroscopic findings included enlarged kidneys and enlarged
liver. Histopathological findings also involved the kidneys and the
liver. Two main findings occurred in the kidneys. The first was
the observation of occasional cells of the proximal convoluted
tubules showing yellowish discoloration of the cytoplasma (incidence
in males). The second was another type of darker and more
particulate granular and/or clumped pigment in the straight portions
and occasionally in the proximal convoluted tubules. In addition,
male rats given 360 ppm endosulfan also showed yellowish coloured
protein aggregations in the proximal convoluted tubule and
intracytoplasmic eosinophilic droplets in the tubules. During the
recovery period the discoloration decreased whereas granular pigment
still persisted. In the liver, minimal centrilobular enlargement of
hepatocytes was observed. The liver change was not seen in the
recovery group rats.
The NOAEL is estimated to be 10 ppm in female rats, equivalent
to 0.75 mg/kg bw/day in the diet. The lowest effect level is 30 ppm
(i.e., 2.26 mg/kg bw/day) based upon the findings of increased serum
phosphorus levels and yellow discolored cells in the renal proximal
convoluted tubules of 1/20 females. Both of these changes were
produced by higher doses of endosulfan in female rats in a
dose-related fashion. The NOAEL for male rats is less than 10 ppm
(lowest dose tested), equivalent to 0.64 mg/kg bw/day in the diet.
This is based upon the finding of yellow discolored cells in the
renal proximal convoluted tubules in 14/20 males (Barnard et al.
1985).
In a dermal toxicity study, endosulfan was applied to the skin
of male and female Wistar rats (6/sex/dose) at doses of 0, 1, 3, 9,
and 27 mg/kg bw, and to males only at 81 mg/kg bw for 21
applications over 30 days. Toxic effects of the test compound
occurred at doses of 9 mg/kg bw or more and included deaths, liver
abnormalities (enlarged parenchymal cells with loss of cytoplasmic
basophilia, isolated cell necrosis, and frequent mitosis) in
occasional animals and significantly elevated absolute and relative
spleen weights in females. The deaths that were observed in female
rats were accompanied by tono-clonic convulsions. The NOAEL for
subchronic dermal toxicity is 3 mg/kg bw/day (Ebert et al. 1985a).
In a similar dermal toxicity study, endosulfan was applied to
the skin of Wistar rats at doses of 0, 12, 48, 96, and 192 mg/kg bw
in males and 0, 3, 6, 12, and 48 in females for 21 applications over
29 days. In this study, male rats exhibited increases in relative
kidney weights at doses of 12, 96 and 192 mg/kg bw (attributed by
the study authors to slightly lower body weights in males), deaths
in 36 percent of the animals at 192 mg/kg bw and a discrete
deposition of pigment in a few cells of the proximal convoluted
tubule in one high dose rat. In female rats, clinical signs of
toxicity were first observed at 12 mg/kg bw, and included
piloerection, salivation and lacrymation, blood from the eyes and
nose with increasing doses of the chemical, and deaths in 18% of the
animals due to the tono-clonic convulsions at 48 mg/kg bw. No other
treatment-related effects were noted (Ebert et al. 1985b).
In an inhalation study, groups of 15 male and 15 female SPF
Wistar rats were administered 0, 0.0005, 0.0010, and 0.0020 mg
endosulfan/L of air for 21 exposures (6 hours/day) for 29 days. Two
control groups (air and ethanol-PEG 400 vehicle) were used. Five
animals/test group were held for a 4-week recovery period after
receiving the test aerosol. The NOAEL is 0.001 mg/l of air in males
(based upon the decrement in weight gain of 12 to 16% seen in
recovery animals) (Hollander et al., 1984).
Dogs
Endosulfan technical was fed for one year in the daily diet to
groups of 6 male and 6 female beagle dogs at concentrations of 0, 3,
10, 30, and 30-60 ppm. The latter dietary level was increased in
stages from 30 to 45 ppm after 54 days of treatment, and then to
60 ppm after 106 days of treatment.
No spontaneous deaths were observed in endosulfan-treated
animals. However, 13 dogs were sacrificed in extremis. These
included one male in the 30 ppm dose group after 9 months due to a
poor overall general condition (resulting from refusal to eat and
severe purulent mediastinitis), and all high-dose males and females
after 4 to 5 months due to marked and progressive nervous symptoms.
These took the form of extreme sensitivity to noise and optic
stimuli, tonic contractions of muscles in the extremities, face and
cheeks, and weakened or abolished placing and righting reactions.
Similar nervous symptoms (i.e., violent contraction of the abdominal
muscles and convulsive movements of the cheeks) also occurred in
most of the surviving dogs in the 30 ppm dose group.
Food consumption was reduced in male and female dogs at 30 ppm
(on weeks 1 to 7) and at 3-60 ppm (from week 16 until the time of
sacrifice). Similarly, body weight gain was reduced in male dogs at
30 ppm (from week 43 onward) and in dogs of both sexes at 30-60 ppm
(from week 10 until the time of sacrifice). No adverse
ophthamological findings were observed in treated dogs, and tests to
examine hearing and dental status were normal. Standard
examinations for hematology, clinical chemistry, and urinalysis were
reported to be normal, as were determinations of cholinesterase
activity in serum, red cells, and brain. No treatment-related
alterations in bromsulphthalein retention of phenolsulphon-pthtalein
elimination were noted in special tests to assess hepatic and renal
function, respectively.
No dose-related changes in organ weight or in gross or
microscopic pathology were observed. The only histopatholopical
findings of interest were the occurrence of acute liver congestion
in 3/6 high-dose male dogs, and the presence of brown pigment in the
renal epithelium of 1/6 high-dose male dogs. The NOAEL in the study
was 10 ppm, equivalent to 0.65 mg/kg bw/day in the diet for males
and 0.57 mg/kg bw/day for females (Brunk, 1989).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice were fed diets containing endosulfan
technical (98.8% ppm) for 78 weeks. The time-weighted average (TWA)
dietary concentrations were 3.5 and 6.9 parts per million (ppm) for
males (50/dose group) and 2 and 3.9 ppm for females (50/dose group).
Twenty mice/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 0.52
and 1.0 mg/kg bw/day in males, and 0.3 and 0.58 mg/kg bw/day in
females.
High early mortality was observed among male mice. Eleven
high-dose males and two low-dose males died on week 19. Survival at
term was 15% (3/20) in control males, 38% (19/50) in low-dose males,
and 10% (5/50) in high-dose males. The deaths in the controls may
have been due to fighting. No common cause of death was found for
the high-dose males. Survival was high among all female mice (85%
of control, 94% of low dose, and 96% of high-dose females survived
to term). No effects of treatment on body weight or clinical signs
of toxicity were observed. No dose-dependent non-neoplastic lesions
were noted in female mice. However, the abbreviated lifespan of
male mice was considered to preclude any accurate analysis of
late-occurring tumours. The NOAEL in this study was less than
3.5 ppm (i.e., 0.25 mg/kg bw/day; lowest dose tested) in male mice
due to mortality, and 3.9 ppm (i.e., 0.58 mg/kg bw/day; highest dose
tested) in female mice (NCI, 1978).
A second chronic feeding study of endosulfan technical (97.9%
pure) was performed in Hoe:NMRKf(SPF 71) mice in which 60
animals/sex/dose level received dietary concentrations of 0, 2, 6,
and 18 pm for 24 months. An additional 10 mice/sex/dose were
sacrificed at interim periods of 12 and 18 months, respectively.
The administered concentrations were calculated to be equivalent to
0, 0.28. 0.84, and 2.51 mg/kg bw/day in males and 0, 0.32, 0.097,
and 2.86 mg/kg bw/day in females.
Survival was significantly reduced in female mice with 18 ppm
endosulfan in the main portion of the study (total deaths in females
= 33/60 or 55% controls, 36/60 or 60% low dose, 38/60 or 63.3% mid
dose, and 43/60 or 71.7% high dose). Survival was not affected by
the test compound in male mice in the main study (total deaths in
males = 27/60 or 45% controls, 33/60 or 55% low dose, 37/60 or 61.7%
mid dose, and 35/60 or 58.3% high dose). There was no
compound-related effect on mortality in either female or male mice
at the 12- and 18-month interim sacrifice periods. Body weight
gain was slightly reduced (about 10%) in male mice throughout the
main study at 18 ppm endosulfan. Female weights were slightly
elevated in the 12- and 18-month satellite groups, body weight was
either slightly increased or remained unchanged from controls in
animals of all treatment groups. There were no treatment-related
effects on food consumption, behavioural or general health
conditions of the animals, or hematological or clinical chemistry
parameters.
Gross and microscopic examination were performed at 12, 18, and
24 months. Reductions in several organ weights occurred with the
18 ppm dose level of endosulfan; these included decreases in the
weights of the liver (males at 18 months only), and ovaries (females
at 12 and 18 months). No treatment-related pathological changes or
tumours were seen after gross or histopathological examination of
tissues at 12, 18, or 24 months. The NOAEL was 6 ppm endosulfan,
equivalent to 0.84 mg/kg bw/day in male mice and 0.97 mg/kg bw/day
in female mice (Donaubauer, 1988).
Rats
Groups of Osborne-Mendel rats were fed diets containing
endosulfan (technical grade; 98.8% pure) for 78 weeks. The TWA
dietary concentrations were 408 and 952 ppm for males (50/dose
group) and 223 and 445 ppm for females (50/dose group). Twenty
rats/sex were used as concurrent controls. The TWA dietary
concentrations of endosulfan were equivalent to approximately 20.4
and 47.6 mg/kg bw/day in males, and 11.1 and 22.1 mg/kg bw/day in
females.
Endosulfan produced early mortality (dose-related) in male
rats. By study week 54, 52% of the high-dose males died and by week
74, 88% were dead. The high-dose males were sacrificed on week 74.
For the low-dose males, only 10/50 (20%) survived to term, and these
were sacrificed on week 82. Survival was adequate in control males.
In the case of female rats, no compound-related deaths occurred (70%
of control, 60% of low dose, and 50% of high-dose females survived
to term). Body weight was also depressed in a dose-related fashion
in treated male rats from week 22 onward. At the time of sacrifice,
body weight was depressed about 25% in both low- and high-dose male
rats compared to the control male group. Body weight was not
markedly changed in female rats. Several clinical signs of toxicity
were also seen in treated rats of both sexes; these included hunched
appearance, reddened and squinted eyes, rough fur, and abdominal
urine stains.
Upon histopathological examination, toxic nephropathy was seen
in both male (0/20 controls, 47/50 low dose, 43/47 high dose) and
female (0/20 controls, 27/50 low dose, 29/50 high dose) rats. This
abnormality was characterized by degenerative changes in the
proximal convoluted tubules at the corticomedullary junction, with
cloudy swelling, fatty degeneration and necrosis of the tubular
epithelium. Some affected tubules contained hyaline casts and
enlarged dark-staining regenerative tubular epithelial cells, and
the kidneys often had inflammatory cell infiltration, fibrotic
changes, and focal mineralization. Parathyroid gland hyperplasia
was also seen in male rats (0/20 controls, 21/40 low dose, 18.47
high dose) and was attributed to the effects of chronic renal
failure. Medial calcification of the blood vessels, perhaps related
to the parathyroid hyperplasia, occurred frequently in treated male
rats. Finally, testicular atrophy occurred in male rats (3/19
control, 18/47 low dose, 24/47 high dose) and was characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules, multinucleated cells (fusion bodies), and
calcium deposition resulting in aspermatogenesis.
No conclusions concerning the carcinogenicity of endosulfan
could be drawn from the bioassay of male rats because of the high
early mortality and abbreviated lifespans of these animals.
Survival in female rats was considered to be sufficient for the
evaluation of late occurring tumours; endosulfan was not
carcinogenic in female rats. The results of this long-term bioassay
in rats indicate that the lowest dietary concentrations of
endosulfan tested in males (408 ppm TWA - equivalent to
20.4 mg/kg/day) and females (223 ppm TWA - equivalent to
11.1 mg/kg/day) were toxic and that a NOAEL could not be established
(NCI, 1978).
A second chronic feeding study of endosulfan technical (97.1%
pure) was performed in Charles River Crl:CD(SD)BR rats in which 50
animals/sex/dose level received dietary concentrations of 0, 3, 7.5,
15, and 75 ppm for 104 weeks. An additional 20 rats/sex/dose
received similar doses of endosulfan and were used to obtain
hematology, clinical chemistry, and urinalysis data at various
intervals throughout the study. Gross and microscopic examinations
were performed on all animals. The administered concentrations of
endosulfan were calculated to be equivalent to 0.1, 0.3, 0.6, and
2.9 mg/kg bw/day in males and 0.1, 0.4, 0.7, and 3.8 mg/kg bw/day in
females.
There were no compound-related effects on mortality, clinical
signs of toxicity, food and water consumption, or ophthalmoscopic
parameters. Adverse findings were mainly observed at the highest
dose of endosulfan. These included significant reductions in body
weight gain over the study in males (-17%) and females (-18%), a
reduced food utilization efficiency in females, hematological
changes at week 103 in males (increased platelets, and reductions in
mean corpuscular volume, total white cells, and lymphocytes), and
clinical chemistry changes at week 103 in males (increased plasma
globulin and urinary protein levels, and reduced GPT and serum
albumin levels). The reductions in total white cells and
lymphocytes, and the increased urinary protein levels, were said to
be within expected limits of variation. It should be noted that
body weight gain was also reduced in males given 15 ppm endosulfan
on study weeks 6 to 18 only (-9% reduction); this effect, however,
was not progressive after week 18 and was not considered to be
toxicologically significant.
Gross and microscopic examination of all animals in the study
indicated that the primary target organ for toxicity was the kidney.
This was noted from an increased number of enlarged kidneys seen
grossly for females at 75 ppm (10/70 controls, 18/70 at 3 ppm, 19/70
at 7.5 ppm, 17/70 at 15 ppm, and 26/70 at 75 ppm), and an elevated
incidence of marked progressive glomerulonephritis in males at
75 ppm, 24/70 at 15 ppm, and 30/70 at 75 ppm) and also in females at
75 ppm (1/70 controls, 6/70 at 3 ppm, 6/70 at 7.5 ppm, 5/70 at
15 ppm, and 8/70 at 75 ppm). In addition, blood vessel aneurysms
were elevated in male rats at 75 ppm (10/70 controls, 6/70 at 3 ppm,
14/70 at 7.5 ppm, 10/70 at 15 ppm, and 21/70 at 75 ppm). The
aneurysms were considered to be possibly related to the renal
lesions.
Other observations in the study at 75 ppm in male rats included
a greater number of enlarged lumbar lymph nodes (14/70 controls,
10/70 at 3 ppm, 8/70 at 7.5 ppm, 7/70 at 15 ppm, and 19/70 at
75 ppm), inflammatory cells in the liver (15/70 controls, 16/70 at
3 ppm, 15/70 at 7.5 ppm, 18/70 at 15 ppm, and 25/70 at 75 ppm), and
polyarteritis in the pancreas (0/70 controls, 2/70 at 3 ppm, 4/70 at
7.5 ppm, 1/70 at 15 ppm, and 8/70 at 75 ppm) and testes (16/70
controls, 15/70 at 3 ppm, 23/70 at 7.5 ppm, 18/70 at 15 ppm, and
27/70 at 75 ppm). Finally, the mean weights of the testes were
reduced at 15 ppm and 75 ppm of endosulfan, but the response was not
strictly dose-related and was within expected limits of variation.
Endosulfan was not carcinogenic in either male or female rats.
The NOAEL was said to be 15 ppm, equivalent to 0.6 mg/kg bw/day in
male rats and 0.7 mg/kg bw/day in female rats (Ruckman et al.
1988).
Reproduction studies
Rats
Male and female Charles River SD-CD rats were administered
endosulfan (97% purity) in the diet for two generations at
concentrations of 0, 3, 15, and 75 ppm (equal to approximately 0.2,
1.0, and 6.0 mg/kg bw/day). Females were mated to males on a
one-to-one basis with 32 males/32 females per dose in the F0
generation, and 28 males/28 females per dose for the F1 generation.
F0 generation rats were administered endosulfan from 6 weeks of age
through 18 weeks of age, at which time they were mated to produce
F1a litters. F1a young were sacrificed 21 days postpartum, and the
F0 generation remated to produce the F1b litters. After weaning of
the F1b pups, the F0 generation rats were sacrificed. The F1b rats
were continued on their respective diets for approximately 98 days
prior to mating to produce the F2a litters (which were sacrificed
after weaning) and the F2b litters (likewise sacrificed after
weaning). Endosulfan was administered continuously throughout the
experiment.
There were no compound-related effects on mortality, food/water
consumption, or body weight gain, although F1b females given 3 ppm
demonstrated slightly lower body weights than controls from week 4
through 36. Pregnancy rate, mating performance and gestation period
for both F0 and F1b generations were unaffected by treatment.
Both matings of the F0 generation demonstrated significant
decreases in litter weight at 75 ppm from day 4 through day 21 but
not in either F1b mating. Also, F0 generation litter weights were
significantly reduced on days 4 to 21 during the second mating at
15 ppm as well. Although the trend for cumulative litter loss (%)
was increased in the first mating at 75 ppm, it was not evident in
the second mating nor in either mating of the F1b generation. The
mean pup weight was unaffected throughout both F0 and F1b matings.
Sex ratio appeared to be unaffected throughout the study.
F0 and F1b generation parents presented evidence of increased
absolute organ weights for heart, liver, kidneys, and brain at
75 ppm. These were not demonstrated in the offspring from either
mating of these parents. There was no consistent dose-related
effect. Histopathology of F1b adults and F2b weanlings
demonstrated no compound-related effects. F0 and F1b generation
females and offspring were examined for skeletal and visceral
abnormalities and no consistent dose-related trends were evident,
except for isolated incidences of microphthalmia (2), anophthalmia
(2), kinked/missing tail (3), shortened/malformed digits or limbs
(5), retinal hemorrhage (1) and spina bifida (1).
There were no dose-related or compound-related effects on
reproductive performance. Decreases in litter weight at 15 and
75 ppm occurred in the F1a and F1b litters; however, this finding
was not considered to be adverse since the reductions in litter
weight were marginal and there were no significant corresponding
reductions in pup weight or litter size. The visceral and skeletal
examinations of fetuses for assessing teratogenic effects were
within normal variation for the strain/species studied. It is
considered that endosulfan, at dietary levels up to and including
75 ppm (approximately 6 mg/kg/day in the diet) caused no adverse
effects on reproduction in rats in this study (Edwards et al.
1984).
However, as noted below, renal changes occurred in F1b adult
rats at all of the doses of endosulfan that were tested. In an
addendum to the above study, a histological review of the kidneys of
adult rats from the F1b generation and of weanling rats from the F2b
generation was reported. The effort was initiated because yellowish
discoloration and pigment deposits were noted in renal proximal
convuluted tubules in rats in a 13-week oral toxicity study of
endosulfan followed by a 4-week withdrawal period (see Barnard
et al. 1985). The histological data obtained indicated the
presence of yellowish discolored cells in the proximal convoluted
tubules in F1b adult rats given dietary doses of 3, 15, and 75 ppm
endosulfan. The incidences in males were: 0/28 controls, 11/28 at
3 ppm, 13/28 at 15 ppm, and 28/28 at 75 ppm. The incidences in
females were: 0/28 controls, 0/28 at 3 ppm, 0/28 at 15 ppm, and 9/28
at 75 ppm. In addition, granular/clumped pigment was also noted at
a dietary dose of 75 ppm in F1b adult rats. The incidences at
75 ppm were 14/28 males and 1/28 females vs. an incidence of 0/28 in
males and females from the control, 3 ppm, and 15 ppm dose groups.
In the F2b generation weanling rats, no comparable renal changes
were observed following treatment with endosulfan (Offer, 1985).
These findings were not considered to be adverse since the yellow
pigment in kidneys was identified as endosulfan and its metabolites,
being stored and metabolized in lysosomes prior to excretion.
Special studies on teratogenicity
Rats
Groups of 25 pregnant CD Sprague-Dawley rats were administered
endosulfan (97.3% purity), via oral gavage, at dose levels of 0,
0.66, 2, and 6 mg/kg bw/day from days 6 to 19 of gestation.
(Day 0 = day sperm found). Ten additional animals were added to the
high dose (due to mortality) and five to the control groups (due to
loss of tissue during processing). On day 20 of gestation, all dams
were killed by asphyxiation with CO2 and fetuses delivered by
cesarean section.
Maternal toxicity was evident in the mid- and high-dose groups,
expressed by significantly reduced body weight and body weight gain
during gestation, and by various clinical signs of toxicity (e.g.,
rats rubbing their faces, alopecia, rough coats, lethargy,
flaccidity, and hyperactivity). Several animals died as the result
of improper gavage tube insertion. Fetal and embryolethality were
not apparent in any group and all pregnant animals had viable
litters. Mean fetal weight and crown-rump length were significantly
reduced in the high-dose group, but not in the lower dose groups. A
number of external, visceral, and skeletal abnormalities were
observed in the fetuses in the high-dose group. The most prominent
effect was a significant increase in the incidence of small fourth
and unossified fifth sternebrae in the high-dose fetuses. However,
there was no clear evidence of a teratogenic effect due to the
significant maternal toxicity expressed at the same level. The
NOAEL for maternal toxicity was 0.66 mg/kg bw/day and that for
fetotoxicity was 2 mg/kg bw/day by oral gavage (MacKenzie et al.
1980a).
A range-finding study with endosulfan in pregnant CD
Sprague-Dawley rats (4-6/group) was performed for the purpose of
setting doses for the above described rat teratology study.
Endosulfan was administered by gavage on days 6 to 19 of gestation
(Day 0 = day sperm found) at doses of 0 (corn oil vehicle), 1.25,
2.5, 5, 10, 20 and 40 mg/kg bw/day. Toxic signs in dams included
reductions in body weight gain at doses of 1.25 mg/kg bw/day and
greater, various clinically observed adverse effects at doses of
2.5 mg/kg bw/day and greater (e.g., salivation, piloerection, poor
muscle tone, lethargy, head-rubbing behaviour, hyperactivity,
spasticity, tremors and convulsions, and deaths (67 to 100%
mortality) at 10 mg/kg bw/day and greater. The changes were dose
related and most marked at higher doses of endosulfan. No effects
on pregnancy maintenance or fetal survival were observed (MacKenzie
et al. 1980b).
Rabbits
Groups of pregnant New Zealand White rabbits (20/group) were
administered endosulfan (97.3% purity) via oral gavage in corn oil
at dose levels of 0, 0.3, 0.7, and 1.8 mg/kg bw/day from days 6 to
28 of gestation (Day 0 = day of mating). Animals were observed
throughout the test period for toxic signs. Six additional pregnant
rabbits were added to the high-dose group due to mortality. On day
29 of gestation, the dams were asphyxiated with CO2, and the entire
reproductive tract was removed.
Maternal toxicity was evident at the high dose, expressed as
rapid breathing, hyperactivity, convulsions and mortality. Group
mean body weights were not affected by treatment. All pregnant dams
had viable fetuses, with the exception of one high-dose dam which
died on test and which had nine late resorptions. There were no
compound-related effects on the number of corpora lutea,
implantation efficiency, litter size, sex ratio, mean fetal length
or weight, or the number of live/dead or resorbed fetuses. There
were no gross external abnormalities and the visceral and skeletal
examinations revealed no dose-related increase in variations or
anomalies. The NOAEL for maternal toxicity was 0.7 mg/kg bw/day.
Endosulfan was not teratogenic in rabbits at doses up to and
including 1.8 mg/kg bw, although maternal toxicity was expressed at
that level (MacKenzie et al. 1981a).
A range-finding study with endosulfan in pregnant New Zealand
White rabbits (3 to 10/group) was conducted to set doses for the
above described rabbit teratology study. Endosulfan was
administered by gavage on days 6 to 28 of gestation (Day 0 = day of
mating) at doses of 0 (corn oil), 1, 2, 4, 8, and 12 mg/kg bw/day.
Standard observations and necropsy procedures were performed. Toxic
signs in dams included deaths at 2 mg/kg bw/day or more (20 to 100%
mortality) and various clinical adverse effects at 2 mg/kg bw/day or
more (e.g., hyperactivity, opisthotonos, clonic and tonic
convulsions, paralysis, tremors, and loss of mobility). Fetal
survival appeared to be adversely affected at 2 mg/kg bw or more as
indicated by an increase in the percent of dead fetuses and a
decrease in the percent of live fetuses in dams. No prominent
effects on weight gain or pregnancy rate occurred (MacKenzie et al.
1981b).
Special study on mutagenicity
Endosulfan was tested for mutagenic activity in a variety of
bacterial, yeast, and mammalian cell systems and was negative in
each test (Table 1).
TABLE 1. RESULTS OF MUTAGENICITY STUDIES ON ENDOSULFAN
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
Ames Testa S.typhmurium 6-5000 ug/plate Negative Shirasu et al. 1978
TA98, TA100, TA1535,
TA1537, TA1538
Yeast Forward Mutation Schizosaccharomyces pombe 62.5-500 ug/plate Negative Milone 1984a
Testa
Yeast Gene-Conversion/ S. cerevisiae 100-5000 ug/ml Negative Milone, 1984b
DNA Repair Testa (D4 strain)
Mouse Lymphoma Assaya Mouse L51786 TK+/- Cells 6.25-100 ug/ml Negative Cifone, 1984a
DNA Repair Assaya B. subtilis H17 and 20-2000 ug/disk Negative Shirasu et al. 1978
M45 (rec-)
Unscheduled DNA Synthesis Primary-Hepatocytes (Rat) 0.102-51 ug/ml Negative Cifone, 1984b
Unscheduled DNA Human Cell Line A 549 1-1000 ug/ml Negative Muller, 1988
Synthesisa
Micronucleus Assay NMRI Mice O.2-5 mg/kg, Negative Jung et al. 1983
(Bone Marrow) Orallyb
Micronucleus Assay Swiss Albino Male Mice 43.3 mg/kg Negative Usha Rani et al. 1980
(Bone Marrow) Orallyb
Chromosome Aberration Cultured Human Lymphocytes 0.05-100 ug/ml Negative Milone, 1986
Testa in vitro
TABLE 1 (CONTD).
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ENDOSULFAN RESULTS REFERENCE
In vivo Cytogenicity Male Albino Rats 11-55 mg/kg/day Negative Dikshith & Datta, 1978
Test (Bone Marrow Orally for 5 days
and Spermatogonia)
a With and without metbolic activation.
b Administered twice orally at an interval of 24 hours.
Special study on neurotoxicity
The acute delayed neurotoxicity of endosulfan was assessed in
adult domestic hens. The study consisted of three parts. First,
the LD50 determination was performed using oral (gavage) doses of
0, 40, 60, 90, 110, and 135 mg/kg bw endosulfan (5 hens/dose group).
The observed LD50 was 96 mg/kg bw. All deaths occurred within 2
hours of dosing. Toxic signs observed were subdued behaviour and
weight loss. Second, a protection assessment was performed using an
oral dose of 96 mg/kg bw of endosulfan alone, or in combination with
either phenobarbital (15 mg/kg bw i.m.), diazepam (2 mg/kg bw p.o.),
or atropine (10 mg/kg bw i.m.) plus 2-PAM (25 mg/kg bw i.m.). There
were 5 hens/group. The results did not provide evidence that any of
the protections reduced the number of mortalities occurring after
dosing with endosulfan. Third, a neurotoxicity assessment was
performed using groups of 10 hens. A vehicle control group was
dosed orally with corn oil, a positive control group with 500 mg/kg
bw TOCP, and four test groups were each dosed with the LD50
(96 mg/kg bw) dose of endosulfan. Dosing was followed by a 21-day
observation period, a redosing, and a final 21-day observation
period. Surviving hens were sacrificed at 42 days.
Histopathological examination of brain, spinal cord and peripheral
nerve tissues were performed on 9 of the 40 animals dosed with
endosulfan (these were the survivors to 42 days), 10/10 control
hens, and 10/10 TOCP-treated hens. The results indicated that
endosulfan did not produce any clinical signs of neurotoxicity or
neuropathological (i.e., histological) effects on the central or
peripheral nervous system. In contrast, hens treated with TOCP
displayed ataxia as well as significant axonal degeneration in the
spinal cord and in the peripheral (tibial) nerve (Roberts et al.
1983).
Special study on skin sensitization
Endosulfan had no sensitizing potential (delayed contact
hypersensitivity) in guinea pigs when tested by the Buehler method
(Jung & Weigand, 1983).
COMMENTS
In rats, the absorption of single oral doses of endosulfan
required 2 or more days; subsequent urinary and fecal excretion was
also slow, requiring 5 or more days. The biotransformation pathways
involve hydrolysis and oxidation to sulfur-free metabolites and
polar conjugates, and the formation of various sulfur-containing
moieties. Endosulfan preferentially accumulated in the kidney, with
lower levels in other tissues, the elimination half-time from
tissues being 3 to 7 days.
Endosulfan at dietary dose levels of 10 ppm and higher in
subchronic studies in rats produced yellow pigmentation of renal
cells. In contrast, renal cellular pigmentation was not observed in
subchronic studies in mice at similar dose levels (see reproduction
studies below).
In a one-year dietary study in dogs, doses of 30 to 60 ppm
resulted in early sacrifices due to clinical signs of neurotoxicity
(e.g., convulsive muscular activity). Reduced food consumption and
weight gain also occurred. In this study, the NOAEL was 10 ppm,
equal to 0.57 mg/kg bw/day.
Long-term carcinogenicity studies were performed in mice and
rats. Treatment-related increases in neoplasms were not observed.
The NOAELs (based on non-neoplastic effects) were 6 ppm (equal to
0.84 mg/kg bw/day) in mice and 15 ppm (equal to 0.6 mg/kg bw/day) in
rats.
A two-generation, 2 litters per generation reproduction study
was conducted in rats. Endosulfan was associated with a reduction
in weight of the F1a and Flb litters at dietary levels of 15 and
75 ppm, and the presence of yellowish pigment in cells in the renal
proximal convoluted tubules at dose levels of 3, 15 and 75 ppm in
F1b adult rats. The Meeting did not consider these findings to be
adverse since the reduction in litter weights were marginal and
occurred in the absence of corresponding reductions in pup weight or
litter size. The yellow pigment in kidneys was identified as
endosulfan and its metabolites, being stored and metabolized in
lysosomes prior to excretion.
In teratology studies performed in rats and rabbits, NOAELs of
approximately 0.7 and 1.8 mg/kg bw/day were identified for maternal
toxicity and fetotoxicity, respectively. Endosulfan was not
teratogenic at 6 mg/kg bw/day in rats and 1.8 mg/kg bw/day in
rabbits, the highest dose levels tested.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 6 ppm in the diet, equal to 0.84 mg/kg bw/day
Rat: 15 ppm in the diet, equal to 0.6 mg/kg bw/day
Dog: 10 ppm in the diet, equal to 0.57 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E., Gibson,
W.A., Rao, R., Offer, J.M., Gopinath, G., Almond, R.H. (1984)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice. Unpublished Report No. A29663 from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street,
A.E., Gibson, W.A., Gopinath, G., Majeed, S.K., Almond, R.J. (1985)
Endosulfan-active ingredient technical: 13-week toxicity study in
mice followed by a 4-week withdrawal period. Unpublished Report
No. A30700 from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Brunk, R. (1989) Endosulfan-substance technical: Testing for
toxicity by repeated oral administration (1-year feeding study) to
beagle dogs. Unpublished Report No. 89-0188 from Pharma Research
Toxicology and Pathology, Hoechst AG. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
Cifone, M.A. (1984a) Mutagenicity evaluation of HOE 002671-
substance technical in the mouse lymphoma forward mutation assay.
Unpublished Report No. A29801 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Cifone, M.A. (1984b) Evaluation of HOE 002671-substance technical
in the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished Report No. A29800 from Litton Bionetics, Inc.,
Kensington, Maryland. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-Endosulfan. Unpublished Report No. A-36685 from WIL Research
Laboratories, Inc., Ashland, Ohio. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Dikshith, T.S.S., Datta, K. (1978) Endosulfan: lack of cytogenetic
effects in male rats. Bull. Environ. Contam. Toxicol., 20:
826-833.
Donaubauer, H.H. (1988) Endosulfan-substance technical.
Carcinogenicity study in mice - 24 months feeding study.
Unpublished Report No. A38008 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Donaubauer, H.H., Leist, K.H., Kramer, M. (1985) Endosulfan-
substance technical: 42-day feeding study in mice. Unpublished
Report No. A38104 from Pharma Research Toxicology and Pathology,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Leist, K.H., Kramer, P. (1985a) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in Wistar rats. Unpublished Report
No. A30753 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Ebert, L., Weigand, W., Kramer, P. (1985b) Endosulfan-active
ingredient technical. Testing for sub-chronic dermal toxicity (21
applications over 30 days) in SPF Wistar rats. Unpublished Report
No. A30754 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Edwards, J.A., Reid, Y.J., Offer, J.M., Almond, R.H., Gibson, W.A.
(1984) Effects of Endosulfan-technical on reproductive function of
multiple generations in the rat. Unpublished Report No. A29428
(Huntingdon Report No. HST 204/83768) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W. (1983) Hoe 002671-active ingredient
technical. Testing for acute aerosol inhalation toxicity in male
and female SPF Wistar rats, 4 hours - LC50. Unpublished Report
No. A32087 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Hollander, H., Weigand, W., Kramer, P. (1984) Endosulfan-active
ingredient technical. Testing for subchronic inhalation toxicity -
21 exposures in 29 days in SPF Wistar rats. Unpublished Report
No. A29823 from Pharma Forschung Toxikologie, Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt, FRG.
Jung & Weigand, W. (1983) Hoe 002671-active ingredient technical.
Testing for sensitizing properties in female Perbright-White guinea
pigs. Unpublished Report No. A27248 from Pharma Forschung
Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Jung, Weigand, W., Kramer, P. (1983) Hoe 112671-active ingredient
technical. Micronucleus test in male and female NMRI mice following
oral administration. Unpublished Report No. A29689 from Pharma
Forschung Toxikologie, Hoechst AG. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Lachmann, G.(1987) Dermal absorption of 14C-Endosulfan in rhesus
monkeys. Unpublished Report No. A36685 from Battelle-Institut,
Frankfurt, FRG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Kramer, P. (1985) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A30776 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Leist, K.H., Mayer, D. (1987) Endosulfan-active ingredient
technical. 30-day feeding study in adult male Wistar rats.
Unpublished Report No. A37112 from Pharma Forschung Toxikologie,
Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Dickie, S.M., Jackson, T.A.,
Zelinger, D.J. (1981a) Teratology study with FMC 5462 in rabbits.
Unpublished Report No. A23192 (Raltech Study No. 80070) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1981b) Range-finding study with FMC 5462 in pregnant rabbits.
Unpublished Report No. A37682 (Raltech Study No. 79032) from
Raltech Scientific Services, Wisconsin. Submitted to WHO by Hoechst
AG, Frankfurt, FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M., Zelinger,
D.J. (1980a) Teratology study with FMC 5462 in rats. Unpublished
Report No. A21393 (Raltech Study No. 79041) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
MacKenzie, K.M., Felton, S.M., Jackson, T.A., Balk, M. (1980b)
Range-finding study with FMC 5462 in pregnant rats. Unpublished
Report No. A37680 (Raltech Study No. 79031) from Raltech Scientific
Services, Wisconsin. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
Milone, M.F. (1984a) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished Report No. A29312 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1984b) Study of the mutagenic activity in vitro of
the compound endosulfan-technical with Saccharomyces cerevisiae.
Unpublished Report No. A29313 from Instituto di Richerche
Biomediche "Antoine Marxer", Italy. Submitted to WHO by Hoechst AG,
Frankfurt, FRG.
Milone, M.F. (1986) Study of the capacity of the test article
Endosulfan, substance technical, to induce chromosome aberration in
human lymphocytes cultured in vitro. Unpublished Report No. A33127
from Instituto di Richerche Biomediche "Antoine Marxer", Italy.
Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Muller, W. (1988) Evaluation of Endosulfan-substance, technical in
the unscheduled DNA synthesis test in mammalian cells in vitro.
Unpublished Report No. A38455 from Pharma Research Toxicology and
Pathology, Hoechst AG. Submitted to WHO by Hoechst AG, Frankfurt,
FRG.
National Cancer Institute (NCI) (1978) Bioassay of Endosulfan for
possible carcinogenicity. Technical Report No. 62 from US
Department of Health, Education and Welfare, NIH. Submitted to WHO
by Hoechst AG, Frankfurt, FRG.
Offer, J.M. (1985) Addendum to HST 204: effect of Endosulfan-
technical on the reproductive function of multiple generations in
the rat. Histopathological review of the kidneys in adult rats of
the F1B generation and in weanling rats of the F2B generation.
Unpublished Report No. A30757 (Huntingdon Addendum to Report HST
204/83768) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Cooke, L., Gibson,
W.A. (1983) Acute delayed neurotoxicity study with Endosulfan-
technical in the domestic hen. Unpublished Report No. A32153
(Huntingdon Report No. HST 225/83888) from Huntingdon Research
Centre, England. Submitted to WHO by Hoechst AG, Frankfurt, FRG.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed,
S.K., Anderson, A., Chanter, D.O. (1988) Endosulfan, active
technical ingredient. Combined chronic toxicity/carcinogenicity
study (104-week feeding in rats). Unpublished Report No. HST
289/881067) from Huntingdon Research Centre, England. Submitted to
WHO by Hoechst AG, Frankfurt, FRG.
Shirasu, Y., Moriya, M., Ohta, T. (1978) Microbial mutagenicity
testing on Endosulfan. Unpublished Report No. A21215 from Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Stumpf, K., ABhauer, J. (1986) An up-to-date review of the
environmental safety of Endosulfan. Unpublished Report No. A35180
from the Analytical Laboratory, Hoechst AG. Submitted to WHO by
Hoechst AG, Frankfurt, FRG.
Usha Rani, M.V., Reddy, O.S., Reddy, P.P. (1980) Mutagenicity
studies involving aldrin, dieldrin, endosulfan, dimethoate,
phosphamidon, carbaryl and ceresan. Bull. Environ. Contam.
Toxicol., 25: 277-282.
