
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
DICHLOFLUANID
IDENTITY
Chemical name
N,N-dimethyl-N'-phenyl-(N'-fluorodichloromethylthio)-sulphamide
N'-dichlorofluoromethylthio-NN-dimethyl-N'-phenylsulphamide
Synonyms
Euparen (R), Bay 47 531
Structural formula
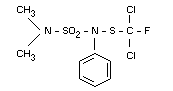 Other relevant chemical properties
The pure material is a white powder with slight characteristic odour,
m.p. 105.0-105.6°C; insoluble in water at 20°C, solubility in methanol
1.5 g/100 ml and in xylene 7.0 g/100 ml. The purity of the technical
material is at least 96 percent and the product contains
N',N'-dimethyl-N-phenyl sulphamide (DMSA) not more than 1 percent and
ionogenic chlorine max. 0.3 percent. The technical product is
formulated as 50 percent wettable powder and 7.5 percent dust. The
active ingredient decomposes in alkaline media and in the presence of
polysulphides. It is light-sensitive, but the discoloration induced
does not affect its biological activity.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
No information is available on the rate of absorption and on the
distribution of dichlofluanid in the animal body. Studies in rats
indicate that the absorption is small. Within 72 hours, 45-92 Percent
of the administered dose could be isolated from the fences. Most of
the dichlofluanid found was in an unchanged form; approximately 12
percent was isolated as N,N-dimethyl-N'-phenylsulphamide. After oral
administration of dichlofluanid the unchanged form of the compound van
not detectable in the serum or urine; however when either
dichlofluanid or its metabolite dimethylphenylsulphamide was
administered orally, four metabolites could be isolated from the
urine. The structures of those compounds have now been determined and
are given below:
Other relevant chemical properties
The pure material is a white powder with slight characteristic odour,
m.p. 105.0-105.6°C; insoluble in water at 20°C, solubility in methanol
1.5 g/100 ml and in xylene 7.0 g/100 ml. The purity of the technical
material is at least 96 percent and the product contains
N',N'-dimethyl-N-phenyl sulphamide (DMSA) not more than 1 percent and
ionogenic chlorine max. 0.3 percent. The technical product is
formulated as 50 percent wettable powder and 7.5 percent dust. The
active ingredient decomposes in alkaline media and in the presence of
polysulphides. It is light-sensitive, but the discoloration induced
does not affect its biological activity.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
No information is available on the rate of absorption and on the
distribution of dichlofluanid in the animal body. Studies in rats
indicate that the absorption is small. Within 72 hours, 45-92 Percent
of the administered dose could be isolated from the fences. Most of
the dichlofluanid found was in an unchanged form; approximately 12
percent was isolated as N,N-dimethyl-N'-phenylsulphamide. After oral
administration of dichlofluanid the unchanged form of the compound van
not detectable in the serum or urine; however when either
dichlofluanid or its metabolite dimethylphenylsulphamide was
administered orally, four metabolites could be isolated from the
urine. The structures of those compounds have now been determined and
are given below:
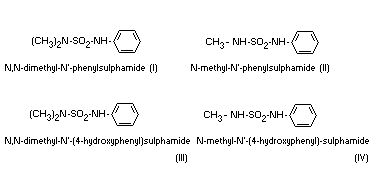 All the metabolites are excreted in the free-form and the metabolites
III and IV are also excreted as glucuronides. Metabolites I and II can
also be found in serum. The metabolites found in urine and serum after
oral administration of dichlofluanid are the same as those found after
oral administration of dimethylphenylsulphamide. From these studies it
is evident that dichlofluanid is absorbed only very slightly, if at
all, from the gastrointestines tract (Eben and Kimmerle, 1968, Bayer,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio-portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield N
N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 male and 20 female animals,
received dietary levels of 0, 150, 500, 1500 and 4500 ppm of
dichlofluanid for a period extending over three generations. Two
litters per generation wore followed and there were no deformities at
any dose-level. The groups fed 150, 500 and 1500 ppm displayed no
abnormal effects with respect to fertility, litter-size and percentage
survival to weaning. In the group given 4500 ppm there was no
difference from the control group in the F1b generation, but in the
F2b and succeeding generations to F3b, the body-weights of the
young animals were significantly lower both at birth and during
weaning. In the 4500 ppm group the lactation index was also slightly
reduced after one of the six matings (Löser, 1969).
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and 10,000
ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis, gross
and microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ-weights were comparable in the different
groups, except in the 10,000 ppm group where the female rate displayed
a decrease in the adrenal weight; and in both sexes an increase in the
liver-weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported. such might be
camouflaged by the histological changes due to infections (Lorke,
1965).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and Raymund, 1963
Mouse (M) i.p. 6.0 DuBois and Raymund, 1963
Chicken oral >1000 DuBois, 1963
Rat (M) i.p. 15 DuBois and Raymund, 1963
Rat (F) i.p. 15 Bayer, 1962
DuBois and Raymund, 1963
Rat (M) oral 500-1000 DuBois and Raymund, 1963
Rat (F) oral 525 Bayer, 1962
DuBois and Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and Raymund, 1963
Guinea-pig (M) oral 250 DuBois and Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly in a
decrease in activity which began several hours after administration of
the compound. With doses around the LD50, death occurred in one to
four days (DuBois and Raymund, 1963)
Short-term studies
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and 4500
ppm of dichlofluanid in their diets for four months. Behaviour, food
consumption, body-weight changes, mortality, function tests of the
liver and kidney urinalysis, gross pathology and final average
body-weights and average organ-weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body-weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300, 1000,
3000, and 10,000 ppm of dichlofluanid in their diets for four months.
Food consumption, growth, haematology, urinalysis, mortality, gross
and microscopic pathology were closely comparable in the 30 to 3000
ppm experimental groups and the control groups. The same was the case
with final average body and organ-weights except in the 3000 ppm group
where the male rats displayed reduction of heart-weights and the
female rate an increase in liver-weights. In the rats fed 10,000 ppm
there was a deterioration of general condition, decreased food intake,
smaller weight gains, and higher mortality than the control group. The
male rats showed a decrease in heart weights, while in both cases
there was a decrease in the weight of adrenal glands and an increase
in liver-weights. Histopathological changes were found in the liver
(vacuolization, inflation in size and shrunken nuclei of some cells),
kidneys (increased protein precipitation in the proximal tubuli) and
the spleen (reduction of the lymphatic tissue) (Bayer, 1964).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150, 500,
1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney-weights, while both sexes showed elevated
relative liver-weights, but normal enzyme function tests (Löser,
1968).
COMMENT
No information is available on the absorption or metabolism of
dichlofluanid in man or in animal species other than the rat. In
particular there is no information reported on the metabolism of the
dichlorofluoromethylthio-moiety. It is known that chemical hydrolysis
gives dichlorofluoromethanethiol and this observation is of some
concern when related to possible metabolic breakdown of dichlofluanid.
It cannot be assumed that the metabolism would ultimately result in
the formation of fluoride ions as the carbon-fluorine bond is known to
be highly resistant to cleavage, and thus there is a possibility that
other organofluorine compounds could result from the metabolism. This
possibility needs elucidation.
The 500 ppm level of dichlofluanid in the four-month study in dogs
seems to have no toxicological effects but no histological studies
were reported. In the four-month feeding study in the rat, the 1000
ppm dose level appeared to be without toxic effect. The long-term
study in rats was also not considered adequate because there was no
histological information provided. There are also no data from
observations in man nor from a one to two year study in a non-rodent
mammalian species.
For these reasons no acceptable daily intake could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop. Further
it is used or recommended for testing on tomato, cucumber, lettuce,
onion, cherry, plum, peach, currants, citrus fruits, and pecans.
It has good plant tolerance at the recommended concentrations,
although, according to location and variety, slight injuries may be
caused on stone fruits and on ornamentals (Anon., 1965). Some
interference in the act and development of fruit on some varieties of
strawberries has also been reported (Gourley, 1968). Dichlofluanid is
harmless to bees.
Safety intervals have been laid down in several European countries,
which differ according to the recommendations, crops and tolerances
where these are established.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Dichlofluanid is used for the treatment of ornamentals, especially for
the control of fungal diseases on roses. Dichlofluanid can be used for
control of powdery mildew without adversely affecting the quality of
tobacco. Dichlofluanid has a good side-effect against spider mites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following tables give the residues of dichlofluanid and its
metabolite DMSA found in the listed crops after application at the
recommended concentrations. The analyses were carried out by
Farbenfabriken Bayer AG, Germany, The State Institute of Agricultural
Chemistry, Finland, and various institutes (Anon., 1967, 1968, 1969).
The residues on grapes show large differences which may be dependent
upon several factors ouch as plot size, nature of grape development in
the different wine-growing districts, spraying technique and spraying
machine (Vogeler and Goeldner, 1967; Hurter at al., 1967). Also on
other crops the variation of residues greatly interferes with the
evaluation of data.
TABLE I
Residue data from field trials
Crop Number of Pre-harvest Residue at harvest (ppm)
treatments interval
(days) Dichlofluanid DMSA
Apples 1 - 12 10 - 14 0.2 - 3.2 not determined
Strawberries 1 - 4 11 - 14 n.d.- 3.6 0.25 - 3.1
Raspberries 3 - 4 7 5.9 - 13.8 0.65 - 6.4
13 - 14 2.3 - 10.5 0.45 - 4.0
Fresh currants 3 - 7 14 0.9 - 2.2 0.8 - 1.0
Grapes 2 - 6 40 - 50 0.7 - 10.9 0.25 - 4.4
Lettuce 5 - 6 14 n.d.- 0.3 0.8 - 2.5
Tomatoes 1 - 3 5 - 7 0.1 - 0.2 0.1 - 0.9
n.d. = not detected
FATE OF RESIDUES
General comments
Dichlofluanid is degraded to N', N'-dimethyl-N-phenyl sulphamide
(DMSA) under alkaline conditions in vitro, on the plant, and partly in
the gastrointestinal tract. (Also see entry under 'BIOCHEMICAL
ASPECTS' above). Amounts of residues on the plants are given Table I.
The metabolite DMSA is less toxic than dichlofluanid and is
fungicidally ineffective.
In animals
Dichlofluanid is either not resorbed at all from the gastrointestinal
tract or, if so, then only in a very slight extent. Dichlofluanid,
after being fed to rats, is degraded to DMSA.
In plants
Dimethylaminosulphanilide occurs as a metabolite of dichlofluanid on
all plants (Vogeler and Niessen, 1967).
The residue analyses show that the metabolite already forms on the day
dichlofluanid to applied. The proportion of metabolite in the total
residue varies according to the nature of the plant material. It does
not exceed the proportion of dichlofluanid, except in the case of
processed fruits and sometimes on strawberries. The "half-life" of
DMSA on apples is approximately 30 days; this value was determined in
an experiment in which formulated DMSA was sprayed (Vogeler, 1965).
The residue figures for dichlofluanid and its degradation product
combined are only slightly higher than the figures of dichlofluanid.
There is thus no evidence of buildup of the degradation product.
Dichlofluanid is relatively persistent, having a "half-life" of about
one week on strawberries. Consequently, the establishment of a
withholding period short enough to permit the use of dichlofluanid on
strawberries throughout the harvest period seems so far questionable
(Brewerton and Gibbs, 1968).
Rainfalls may cause considerable falls in the residues of
dichlofluanid.
In soil
Data on dichlofluanid residues in soil are not available.
In storage and processing
Nothing is known about the degradation of dichlofluanid on fruit in
storage. There are few figures available on effects of washing and
peeling of fruit on the residue levels (Table II). No final
conclusions can be made although the results indicate that variable
amounts of residues could be washed off and a substantial part of
residue was removed by peeling.
There was a decrease of the amount of residues in the canning process
of strawberries (Kavanagh at al., 1968). Following applications at the
recommended dosage, residues on deep-frozen strawberries amounted up
to 1.5 ppm; on the other hand, canned fruits and juice originating
from the same sample contained a maximum of about 0.1 ppm. Following
application at twice the recommended dosage, canned strawberries
contained up to 0.88 ppm. The reduction of residues in the canning
process is presumably canned by washing with water. According to
Kavanagh et al., DMSA was not detected in any sample of frozen or
canned fruit.
It has been reported that the dichlofluanid treatment of strawberries
would tend to increase the sweetness and acidity of the berries (Kirby
and Arthey, 1966).
Strawberry jam made from treated fruits contained dichlofluanid
residues of less than 0.1 ppm and DMSA residues of between 0.15 and
0.4 ppm. The content of residues in the treated strawberries amounted
to 0.4 to 1.15 ppm dichlofluanid and 0.8 to 0.85 ppm DMSA (Maier-Bode,
1966).
To investigate the residues in wine field-treated grapes as well as
marc, must, and wine yielded from them were analysed (see also Vogeler
and Goeldner, 1967) (Table III). The analyses showed that these wines
contained no residue of dichlofluanid and only small residues of DMSA.
A further 20 analyses of different wines made from treated grapes
showed no residues of dichlofluanid and the amounts of DMSA residues
found ranged from "not detectable" to 2.95 ppm. In these analyses the
amount of the residue on the grapes was unknown. In the course of wine
processing, the residues are removed by the pressing process and
pre-clarification (desliming) of the must. In marc very high residues
are found.
TABLE II
Effect of washing and peeling on residue levels
Pre-harvest Residue at Residue after Residue after
interval harvest washing peeling
Crop (days) (ppm) (ppm) (ppm)
Apple 14 2.0 1.3 0.4
14 3.3 4.0 1.5
Strawberry 14 0.07 0.03 -
19 0.5 0.15 -
19 0.6 0.20 -
TABLE III
Dichlofluanid and DMSA residues in grapes and must, marc, and wine made
from treated grapes
Residue
Pre-harvest (ppm)
Number of interval
Crop treatments (days) Dichlofluanid DMSA
Grapes 2 - 9 21 - 95 0.4 - 5.4 0.25 - 1.35
Must, not
clarified n.d. - 2.0 0.3 - 0.95
Must,
clarified n.d. - 0.05 0.25 - 0.4
Marc 0.4 - 53.0 8.9 - 47.0
Smashed 1.2 - 5.0 not determined
Peels 0.6 - 11.1 not determined
Wine n.d. - 3.9 n.d. - 2.95
n.d. = not detected
METHODS OF RESIDUE ANALYSIS
The most important method for determining residues of dichlofluanid is
gas chromatography using an electron capture detector. This method may
be used for analysing apples, strawberries, raspberries, currants,
lettuce, tomatoes, wins and grapes (Vogeler and Niessen, 1967).
Methods based on the same principle have been described for analysis
of strawberries (Eades and Gardiner, 1967) and grapes (Hurter et al.,
1966).
Dichlofluanid may also be determined by polarography (Nangniot at al.,
1967) and by colorimetry (Vogeler and Niessen, 1967). The colorimetric
method is based on saponification of the parent compound to the
metabolite DMSA and determination of aniline by diazotization and
coupling with N-(1-naphtyl)-ethylene-diamine. By this method, the sum
of parent compound and metabolite is obtained so that the metabolite
is determined alone in a second analytical procedure in which alkaline
saponification of the parent compound is omitted.
The parent compound and the metabolite DMSA can be determined in one
analytical procedure by gas chromatography and colorimetry after
column-chromatographic separation (Vogeler and Niessen, 1967). The
sensitivity of the colorimetric method is approximately 0.1 ppm and
that of the gas chromatographic method is 0.1 ppm or less depending
upon the response of the detector.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Saf. Int./
Country Crop Tol./ppm Days
Austria General 14
General 15.0 proposed
Belgium General 14
Fruits and vegetables 5.0
except potatoes
Denmark General 14
Finland General 14
France Grapes, strawberries 7
"Kupfer-Euparen (6341)" Grapes 7
Germany
(Fed.Rep.) Pome fruit 7
Cane and bush fruit 7*
Strawberries 2.0 + 2.0 DMSA 14
Tomatoes 1.0 + 1.0 DMSA 3*
Apples and pears 0.5 + 0.5 DMSA
Italy WP: General 20
Grapes 40
Strawberries 20
6012 (40% + 10% Cu) General 15
"Ramato blu (15% + 30% Cu)" General 7
Netherlands General 5.0
Strawberries, raspberries,
blackberries 7
Currants 21
Strawberries grown under glass 14
Norway General 7
Poland Fruits, vegetables, field crops 14
Grapes 42
(cont'd)
Saf. Int./
Country Crop Tol./ppm Days
Sweden General 7
Switzerland General 21
Grapes 1.0
Strawberries 7.0
United Kingdom Strawberries, raspberries 14
Blackberries, blackcurrants,
gooseberries, loganberries,
onions, cauliflower (grown under
glass), lettuce (grown under
glass), lettuce (field grown) 21
Yugoslavia Strawberries 2.0 + 2.0 DMSA 14
Grapes 30
* subject to official approval
APPRAISAL
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop, and
mildew of roses. Further, it is used or recommended for testing on
tomato, cucumber, lettuce, onion, cherry, plump peach, currants,
citrus fruits and pecans. Concentration of the sprays is recommended
to 0.075-0.12 percent active ingredient. Dichlofluanid has a good
side-effect against spider mites. Although there is a good plant
tolerance to dichlofluanid, slight injuries may be caused, according
to location and variety, on some stone fruits. It is harmless to bees.
Dichlofluanid is used in many European countries and the safety
intervals from the last treatment to the harvest vary from three to
forty days. The national tolerances applied vary from 1 to 7 ppm for
dichlofluanid alone or for dichlofluanid plus its metabolite DMSA. It
is formulated as 50 percent wettable powder and 7.5 percent dust.
The residue data available to the meeting were obtained from
supervised field trials in Germany, England and Finland. Initial
residues of dichlofluanid had usually degraded by one half within a
week; on grapes more persistent residues were found. By washing,
peeling and processing, residues are partly removed.
Dichlofluanid is degraded to N',N'-dimethyl-N-phenyl sulphamide (DMSA)
under alkaline conditions in vitro, on all plants, and in the
gastrointestinal tract. In addition to DMSA, in urine three other
metabolites of dichlofluanid are detected and identified. Residue data
on DMSA are available and its "half-life" on apples is determined (30
days). In processed strawberries both dichlofluanid and DMSA are
found. Wines produced from treated grapes are found to contain both
dichlofluanid and DMSA. The occurrence of DMSA seems to be, however,
more relevant than that of the parent compound. In plants or plant
products DMSA is the only degradation product of dichlofluanid so far
identified.
The documentation on dichlofluanid includes methods of residue
analysis based on GLC, polarography and colorimetry. Both the parent
compound and DMSA can be determined. A sensitivity of 0.1 ppm in plant
material can be reached.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
As no acceptable daily intake was established, no tolerances were
recommended.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Further information on the absorption and metabolism of the
compound particularly with regard to the fate of the
fluorine-containing portion of the molecule.
2. A long-term feeding study in the rat including a histological
examination of all major organs.
3. A 1-2 year feeding study in a non-rodent mammalian species.
4. Information on the composition of the technical dichlofluanid,
including the impurities.
5. More detailed information on the nature and magnitude of terminal
residues in plants including data on the fluorine-containing moiety
of the molecule.
6. Information about possible degradation mechanism of the molecule by
the action of sulfhydryl compounds in vitro and in vivo.
7. Data on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues from different
countries, especially on those crops which have shown inconsistency
of residue data. Data on degradation products of dichlofluanid, if
important in magnitude or toxicologically, should be included.
8. Data on residue levels in raw agricultural products moving in
commerce.
9. Qualitative and quantitative data on fate of residues in washing,
blanching and storing and thermal processing of treated crops.
10. Data concerning the possible occurrence of the parent compound in
wines produced from treated grapes.
DESIRABLE
1. Metabolism in animal species, other than the rat.
2. Metabolic studies and other observations in man.
3. Information on the fate of the compound in soil.
4. Evaluation of the analytical methods by collaborative studies for
regulatory purposes.
REFERENCES
Anon. (1965) (R)Euparen (Bay 47531). Pflanzenschutz. Farbenfabriken
Bayer AG. Technical Information Sheet
Anon. (1967) Investigations on pesticide residues. Publications of the
State Institute of Agricultural Chemistry. Tikkurila, Finland, No.1.
Anon. (1968) Ibid. No.2
Anon. (1969) Ibid. No.4
Bayer (1962) Product Kü 13-032-C. Unpub. Rept. prepared and submitted
by the Institute of Toxicology, Farbenfabriken Bayer AG.
Bayer (1964) Report of 4-months feeding study on rats with active
ingredient 47531. Unpub. Rept. prepared and submitted by the Institute
of Toxicology, Farbenfabriken Bayer AG.
Bayer (1969) Dichlofluanid. Unpub. Summary Rept. prepared and
submitted by Farbenfabriken Bayer AG.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
Residues on Strawberries. New Zealand J. Agr. Res. 11:784-88
DuBois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Unpub. Rept. submitted by Farbenfabriken Bayer
AG.
DuBois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Unpub. Rept. submitted by
Farbenfabriken Bayer A.G.
Eades, J.F. and Gardiner, K.D. (1967) Estimation of Dichlofluanid
Residues in Strawberries. Chemistry and Industry 32:1539-60
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Unpub. Rept. prepared and submitted by the Institute of
Toxicology, Farbenfabriken Bayer AG.
Gourley, C.O. (1968) Fungicidal control of Botrytis cinerea on four
strawberry varieties. Can. J. Plant Sci. 48:267-72
Hurter, J., Mayer, K. and Zürrer, A. (1966) Gärhemmung durch
Fungizidrückstände Schweizerische Z. Obst- und Weinbau 102:592-7
Hurter, J., Lauber, H.P., Mayer, K., Schüepp, H. and Bolay, A. (1967)
Rückstandsmenge auf Weintrauben und Gärnerlauf nach Behandlung mit
Dichlofluanid und Folpet. Schweizerische Z. Obst-und Weinbau 103.201-9
Kavanagh, T., Gardiner, K.D., O'Callaghan, F.F. and Eades, J.F.K.
(1968) Fungicidal Control of Botrytis of Strawberries and Laboratory
Determination of Residues and Flavour. Meded. Rijksfac.
Landbouwwetensch. 33:959-68
Kirby, A.H.M. and Arthey, V.D. (1966) The influence of grey mold
fungicides on the flavour of canned strawberries. Meded. Rijksfac.
Landbouwwetensch. 31:1011-20
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Rept. prepared and
submitted by the Institute of Toxicology Farbenfabriken Bayer AG.
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Unpub. Rept. from the
Institute of Toxicology, Farbenfabriken Bayer AG.
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Unpub. Rept. from the Institute of Toxicology, Farbenfabriken Bayer
AG.
Löser, E. (1969) Bayer 47531, Generationaversuche an Ratten. Unpub.
Rept. prepared and submitted by the Institute of Toxicology,
Farbenfabriken Bayer AG.
Maier-Bode, E. (1966) Pharmakologisches Institut, Bonn. Unpub. Ref.
Farbenfabriken Bayer AG.
Nangniot, P., Vervier, R. and Martens, P.H. (1967) Le dosage de la
N,N-diméthyl-N-phényl -(N'-flourdichlorméthylthio) -sulfamide (Euparen)
on fruits. Bull. Rech. Agr. Gembloux 2:284-93
Nangniot, P., Vervier, R. and Martens, P.E. (1967) The use of
polarography to determine small amounts of fungicides on insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulfamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Vogeler, K. (1965) Farbenfabriken Bayer AG., Biologisches Institut,
Leverkusen. Unpub.
Vogeler, K. and Goeldner, H. (1967) Untersuchungen über Rückstände
nach Anwendung von Euparen an Weintrauben. Schweizerische Z. Obst- und
Weinbau 103:494-504
Volgeler, K. and Niessen, H. (1967) Kolorimetrische und
gas-chromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz - Nachrichten "Bayer" 20:534-49
All the metabolites are excreted in the free-form and the metabolites
III and IV are also excreted as glucuronides. Metabolites I and II can
also be found in serum. The metabolites found in urine and serum after
oral administration of dichlofluanid are the same as those found after
oral administration of dimethylphenylsulphamide. From these studies it
is evident that dichlofluanid is absorbed only very slightly, if at
all, from the gastrointestines tract (Eben and Kimmerle, 1968, Bayer,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio-portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield N
N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 male and 20 female animals,
received dietary levels of 0, 150, 500, 1500 and 4500 ppm of
dichlofluanid for a period extending over three generations. Two
litters per generation wore followed and there were no deformities at
any dose-level. The groups fed 150, 500 and 1500 ppm displayed no
abnormal effects with respect to fertility, litter-size and percentage
survival to weaning. In the group given 4500 ppm there was no
difference from the control group in the F1b generation, but in the
F2b and succeeding generations to F3b, the body-weights of the
young animals were significantly lower both at birth and during
weaning. In the 4500 ppm group the lactation index was also slightly
reduced after one of the six matings (Löser, 1969).
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and 10,000
ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis, gross
and microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ-weights were comparable in the different
groups, except in the 10,000 ppm group where the female rate displayed
a decrease in the adrenal weight; and in both sexes an increase in the
liver-weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported. such might be
camouflaged by the histological changes due to infections (Lorke,
1965).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and Raymund, 1963
Mouse (M) i.p. 6.0 DuBois and Raymund, 1963
Chicken oral >1000 DuBois, 1963
Rat (M) i.p. 15 DuBois and Raymund, 1963
Rat (F) i.p. 15 Bayer, 1962
DuBois and Raymund, 1963
Rat (M) oral 500-1000 DuBois and Raymund, 1963
Rat (F) oral 525 Bayer, 1962
DuBois and Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and Raymund, 1963
Guinea-pig (M) oral 250 DuBois and Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly in a
decrease in activity which began several hours after administration of
the compound. With doses around the LD50, death occurred in one to
four days (DuBois and Raymund, 1963)
Short-term studies
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and 4500
ppm of dichlofluanid in their diets for four months. Behaviour, food
consumption, body-weight changes, mortality, function tests of the
liver and kidney urinalysis, gross pathology and final average
body-weights and average organ-weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body-weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300, 1000,
3000, and 10,000 ppm of dichlofluanid in their diets for four months.
Food consumption, growth, haematology, urinalysis, mortality, gross
and microscopic pathology were closely comparable in the 30 to 3000
ppm experimental groups and the control groups. The same was the case
with final average body and organ-weights except in the 3000 ppm group
where the male rats displayed reduction of heart-weights and the
female rate an increase in liver-weights. In the rats fed 10,000 ppm
there was a deterioration of general condition, decreased food intake,
smaller weight gains, and higher mortality than the control group. The
male rats showed a decrease in heart weights, while in both cases
there was a decrease in the weight of adrenal glands and an increase
in liver-weights. Histopathological changes were found in the liver
(vacuolization, inflation in size and shrunken nuclei of some cells),
kidneys (increased protein precipitation in the proximal tubuli) and
the spleen (reduction of the lymphatic tissue) (Bayer, 1964).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150, 500,
1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney-weights, while both sexes showed elevated
relative liver-weights, but normal enzyme function tests (Löser,
1968).
COMMENT
No information is available on the absorption or metabolism of
dichlofluanid in man or in animal species other than the rat. In
particular there is no information reported on the metabolism of the
dichlorofluoromethylthio-moiety. It is known that chemical hydrolysis
gives dichlorofluoromethanethiol and this observation is of some
concern when related to possible metabolic breakdown of dichlofluanid.
It cannot be assumed that the metabolism would ultimately result in
the formation of fluoride ions as the carbon-fluorine bond is known to
be highly resistant to cleavage, and thus there is a possibility that
other organofluorine compounds could result from the metabolism. This
possibility needs elucidation.
The 500 ppm level of dichlofluanid in the four-month study in dogs
seems to have no toxicological effects but no histological studies
were reported. In the four-month feeding study in the rat, the 1000
ppm dose level appeared to be without toxic effect. The long-term
study in rats was also not considered adequate because there was no
histological information provided. There are also no data from
observations in man nor from a one to two year study in a non-rodent
mammalian species.
For these reasons no acceptable daily intake could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop. Further
it is used or recommended for testing on tomato, cucumber, lettuce,
onion, cherry, plum, peach, currants, citrus fruits, and pecans.
It has good plant tolerance at the recommended concentrations,
although, according to location and variety, slight injuries may be
caused on stone fruits and on ornamentals (Anon., 1965). Some
interference in the act and development of fruit on some varieties of
strawberries has also been reported (Gourley, 1968). Dichlofluanid is
harmless to bees.
Safety intervals have been laid down in several European countries,
which differ according to the recommendations, crops and tolerances
where these are established.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Dichlofluanid is used for the treatment of ornamentals, especially for
the control of fungal diseases on roses. Dichlofluanid can be used for
control of powdery mildew without adversely affecting the quality of
tobacco. Dichlofluanid has a good side-effect against spider mites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following tables give the residues of dichlofluanid and its
metabolite DMSA found in the listed crops after application at the
recommended concentrations. The analyses were carried out by
Farbenfabriken Bayer AG, Germany, The State Institute of Agricultural
Chemistry, Finland, and various institutes (Anon., 1967, 1968, 1969).
The residues on grapes show large differences which may be dependent
upon several factors ouch as plot size, nature of grape development in
the different wine-growing districts, spraying technique and spraying
machine (Vogeler and Goeldner, 1967; Hurter at al., 1967). Also on
other crops the variation of residues greatly interferes with the
evaluation of data.
TABLE I
Residue data from field trials
Crop Number of Pre-harvest Residue at harvest (ppm)
treatments interval
(days) Dichlofluanid DMSA
Apples 1 - 12 10 - 14 0.2 - 3.2 not determined
Strawberries 1 - 4 11 - 14 n.d.- 3.6 0.25 - 3.1
Raspberries 3 - 4 7 5.9 - 13.8 0.65 - 6.4
13 - 14 2.3 - 10.5 0.45 - 4.0
Fresh currants 3 - 7 14 0.9 - 2.2 0.8 - 1.0
Grapes 2 - 6 40 - 50 0.7 - 10.9 0.25 - 4.4
Lettuce 5 - 6 14 n.d.- 0.3 0.8 - 2.5
Tomatoes 1 - 3 5 - 7 0.1 - 0.2 0.1 - 0.9
n.d. = not detected
FATE OF RESIDUES
General comments
Dichlofluanid is degraded to N', N'-dimethyl-N-phenyl sulphamide
(DMSA) under alkaline conditions in vitro, on the plant, and partly in
the gastrointestinal tract. (Also see entry under 'BIOCHEMICAL
ASPECTS' above). Amounts of residues on the plants are given Table I.
The metabolite DMSA is less toxic than dichlofluanid and is
fungicidally ineffective.
In animals
Dichlofluanid is either not resorbed at all from the gastrointestinal
tract or, if so, then only in a very slight extent. Dichlofluanid,
after being fed to rats, is degraded to DMSA.
In plants
Dimethylaminosulphanilide occurs as a metabolite of dichlofluanid on
all plants (Vogeler and Niessen, 1967).
The residue analyses show that the metabolite already forms on the day
dichlofluanid to applied. The proportion of metabolite in the total
residue varies according to the nature of the plant material. It does
not exceed the proportion of dichlofluanid, except in the case of
processed fruits and sometimes on strawberries. The "half-life" of
DMSA on apples is approximately 30 days; this value was determined in
an experiment in which formulated DMSA was sprayed (Vogeler, 1965).
The residue figures for dichlofluanid and its degradation product
combined are only slightly higher than the figures of dichlofluanid.
There is thus no evidence of buildup of the degradation product.
Dichlofluanid is relatively persistent, having a "half-life" of about
one week on strawberries. Consequently, the establishment of a
withholding period short enough to permit the use of dichlofluanid on
strawberries throughout the harvest period seems so far questionable
(Brewerton and Gibbs, 1968).
Rainfalls may cause considerable falls in the residues of
dichlofluanid.
In soil
Data on dichlofluanid residues in soil are not available.
In storage and processing
Nothing is known about the degradation of dichlofluanid on fruit in
storage. There are few figures available on effects of washing and
peeling of fruit on the residue levels (Table II). No final
conclusions can be made although the results indicate that variable
amounts of residues could be washed off and a substantial part of
residue was removed by peeling.
There was a decrease of the amount of residues in the canning process
of strawberries (Kavanagh at al., 1968). Following applications at the
recommended dosage, residues on deep-frozen strawberries amounted up
to 1.5 ppm; on the other hand, canned fruits and juice originating
from the same sample contained a maximum of about 0.1 ppm. Following
application at twice the recommended dosage, canned strawberries
contained up to 0.88 ppm. The reduction of residues in the canning
process is presumably canned by washing with water. According to
Kavanagh et al., DMSA was not detected in any sample of frozen or
canned fruit.
It has been reported that the dichlofluanid treatment of strawberries
would tend to increase the sweetness and acidity of the berries (Kirby
and Arthey, 1966).
Strawberry jam made from treated fruits contained dichlofluanid
residues of less than 0.1 ppm and DMSA residues of between 0.15 and
0.4 ppm. The content of residues in the treated strawberries amounted
to 0.4 to 1.15 ppm dichlofluanid and 0.8 to 0.85 ppm DMSA (Maier-Bode,
1966).
To investigate the residues in wine field-treated grapes as well as
marc, must, and wine yielded from them were analysed (see also Vogeler
and Goeldner, 1967) (Table III). The analyses showed that these wines
contained no residue of dichlofluanid and only small residues of DMSA.
A further 20 analyses of different wines made from treated grapes
showed no residues of dichlofluanid and the amounts of DMSA residues
found ranged from "not detectable" to 2.95 ppm. In these analyses the
amount of the residue on the grapes was unknown. In the course of wine
processing, the residues are removed by the pressing process and
pre-clarification (desliming) of the must. In marc very high residues
are found.
TABLE II
Effect of washing and peeling on residue levels
Pre-harvest Residue at Residue after Residue after
interval harvest washing peeling
Crop (days) (ppm) (ppm) (ppm)
Apple 14 2.0 1.3 0.4
14 3.3 4.0 1.5
Strawberry 14 0.07 0.03 -
19 0.5 0.15 -
19 0.6 0.20 -
TABLE III
Dichlofluanid and DMSA residues in grapes and must, marc, and wine made
from treated grapes
Residue
Pre-harvest (ppm)
Number of interval
Crop treatments (days) Dichlofluanid DMSA
Grapes 2 - 9 21 - 95 0.4 - 5.4 0.25 - 1.35
Must, not
clarified n.d. - 2.0 0.3 - 0.95
Must,
clarified n.d. - 0.05 0.25 - 0.4
Marc 0.4 - 53.0 8.9 - 47.0
Smashed 1.2 - 5.0 not determined
Peels 0.6 - 11.1 not determined
Wine n.d. - 3.9 n.d. - 2.95
n.d. = not detected
METHODS OF RESIDUE ANALYSIS
The most important method for determining residues of dichlofluanid is
gas chromatography using an electron capture detector. This method may
be used for analysing apples, strawberries, raspberries, currants,
lettuce, tomatoes, wins and grapes (Vogeler and Niessen, 1967).
Methods based on the same principle have been described for analysis
of strawberries (Eades and Gardiner, 1967) and grapes (Hurter et al.,
1966).
Dichlofluanid may also be determined by polarography (Nangniot at al.,
1967) and by colorimetry (Vogeler and Niessen, 1967). The colorimetric
method is based on saponification of the parent compound to the
metabolite DMSA and determination of aniline by diazotization and
coupling with N-(1-naphtyl)-ethylene-diamine. By this method, the sum
of parent compound and metabolite is obtained so that the metabolite
is determined alone in a second analytical procedure in which alkaline
saponification of the parent compound is omitted.
The parent compound and the metabolite DMSA can be determined in one
analytical procedure by gas chromatography and colorimetry after
column-chromatographic separation (Vogeler and Niessen, 1967). The
sensitivity of the colorimetric method is approximately 0.1 ppm and
that of the gas chromatographic method is 0.1 ppm or less depending
upon the response of the detector.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Saf. Int./
Country Crop Tol./ppm Days
Austria General 14
General 15.0 proposed
Belgium General 14
Fruits and vegetables 5.0
except potatoes
Denmark General 14
Finland General 14
France Grapes, strawberries 7
"Kupfer-Euparen (6341)" Grapes 7
Germany
(Fed.Rep.) Pome fruit 7
Cane and bush fruit 7*
Strawberries 2.0 + 2.0 DMSA 14
Tomatoes 1.0 + 1.0 DMSA 3*
Apples and pears 0.5 + 0.5 DMSA
Italy WP: General 20
Grapes 40
Strawberries 20
6012 (40% + 10% Cu) General 15
"Ramato blu (15% + 30% Cu)" General 7
Netherlands General 5.0
Strawberries, raspberries,
blackberries 7
Currants 21
Strawberries grown under glass 14
Norway General 7
Poland Fruits, vegetables, field crops 14
Grapes 42
(cont'd)
Saf. Int./
Country Crop Tol./ppm Days
Sweden General 7
Switzerland General 21
Grapes 1.0
Strawberries 7.0
United Kingdom Strawberries, raspberries 14
Blackberries, blackcurrants,
gooseberries, loganberries,
onions, cauliflower (grown under
glass), lettuce (grown under
glass), lettuce (field grown) 21
Yugoslavia Strawberries 2.0 + 2.0 DMSA 14
Grapes 30
* subject to official approval
APPRAISAL
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop, and
mildew of roses. Further, it is used or recommended for testing on
tomato, cucumber, lettuce, onion, cherry, plump peach, currants,
citrus fruits and pecans. Concentration of the sprays is recommended
to 0.075-0.12 percent active ingredient. Dichlofluanid has a good
side-effect against spider mites. Although there is a good plant
tolerance to dichlofluanid, slight injuries may be caused, according
to location and variety, on some stone fruits. It is harmless to bees.
Dichlofluanid is used in many European countries and the safety
intervals from the last treatment to the harvest vary from three to
forty days. The national tolerances applied vary from 1 to 7 ppm for
dichlofluanid alone or for dichlofluanid plus its metabolite DMSA. It
is formulated as 50 percent wettable powder and 7.5 percent dust.
The residue data available to the meeting were obtained from
supervised field trials in Germany, England and Finland. Initial
residues of dichlofluanid had usually degraded by one half within a
week; on grapes more persistent residues were found. By washing,
peeling and processing, residues are partly removed.
Dichlofluanid is degraded to N',N'-dimethyl-N-phenyl sulphamide (DMSA)
under alkaline conditions in vitro, on all plants, and in the
gastrointestinal tract. In addition to DMSA, in urine three other
metabolites of dichlofluanid are detected and identified. Residue data
on DMSA are available and its "half-life" on apples is determined (30
days). In processed strawberries both dichlofluanid and DMSA are
found. Wines produced from treated grapes are found to contain both
dichlofluanid and DMSA. The occurrence of DMSA seems to be, however,
more relevant than that of the parent compound. In plants or plant
products DMSA is the only degradation product of dichlofluanid so far
identified.
The documentation on dichlofluanid includes methods of residue
analysis based on GLC, polarography and colorimetry. Both the parent
compound and DMSA can be determined. A sensitivity of 0.1 ppm in plant
material can be reached.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
As no acceptable daily intake was established, no tolerances were
recommended.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Further information on the absorption and metabolism of the
compound particularly with regard to the fate of the
fluorine-containing portion of the molecule.
2. A long-term feeding study in the rat including a histological
examination of all major organs.
3. A 1-2 year feeding study in a non-rodent mammalian species.
4. Information on the composition of the technical dichlofluanid,
including the impurities.
5. More detailed information on the nature and magnitude of terminal
residues in plants including data on the fluorine-containing moiety
of the molecule.
6. Information about possible degradation mechanism of the molecule by
the action of sulfhydryl compounds in vitro and in vivo.
7. Data on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues from different
countries, especially on those crops which have shown inconsistency
of residue data. Data on degradation products of dichlofluanid, if
important in magnitude or toxicologically, should be included.
8. Data on residue levels in raw agricultural products moving in
commerce.
9. Qualitative and quantitative data on fate of residues in washing,
blanching and storing and thermal processing of treated crops.
10. Data concerning the possible occurrence of the parent compound in
wines produced from treated grapes.
DESIRABLE
1. Metabolism in animal species, other than the rat.
2. Metabolic studies and other observations in man.
3. Information on the fate of the compound in soil.
4. Evaluation of the analytical methods by collaborative studies for
regulatory purposes.
REFERENCES
Anon. (1965) (R)Euparen (Bay 47531). Pflanzenschutz. Farbenfabriken
Bayer AG. Technical Information Sheet
Anon. (1967) Investigations on pesticide residues. Publications of the
State Institute of Agricultural Chemistry. Tikkurila, Finland, No.1.
Anon. (1968) Ibid. No.2
Anon. (1969) Ibid. No.4
Bayer (1962) Product Kü 13-032-C. Unpub. Rept. prepared and submitted
by the Institute of Toxicology, Farbenfabriken Bayer AG.
Bayer (1964) Report of 4-months feeding study on rats with active
ingredient 47531. Unpub. Rept. prepared and submitted by the Institute
of Toxicology, Farbenfabriken Bayer AG.
Bayer (1969) Dichlofluanid. Unpub. Summary Rept. prepared and
submitted by Farbenfabriken Bayer AG.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
Residues on Strawberries. New Zealand J. Agr. Res. 11:784-88
DuBois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Unpub. Rept. submitted by Farbenfabriken Bayer
AG.
DuBois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Unpub. Rept. submitted by
Farbenfabriken Bayer A.G.
Eades, J.F. and Gardiner, K.D. (1967) Estimation of Dichlofluanid
Residues in Strawberries. Chemistry and Industry 32:1539-60
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Unpub. Rept. prepared and submitted by the Institute of
Toxicology, Farbenfabriken Bayer AG.
Gourley, C.O. (1968) Fungicidal control of Botrytis cinerea on four
strawberry varieties. Can. J. Plant Sci. 48:267-72
Hurter, J., Mayer, K. and Zürrer, A. (1966) Gärhemmung durch
Fungizidrückstände Schweizerische Z. Obst- und Weinbau 102:592-7
Hurter, J., Lauber, H.P., Mayer, K., Schüepp, H. and Bolay, A. (1967)
Rückstandsmenge auf Weintrauben und Gärnerlauf nach Behandlung mit
Dichlofluanid und Folpet. Schweizerische Z. Obst-und Weinbau 103.201-9
Kavanagh, T., Gardiner, K.D., O'Callaghan, F.F. and Eades, J.F.K.
(1968) Fungicidal Control of Botrytis of Strawberries and Laboratory
Determination of Residues and Flavour. Meded. Rijksfac.
Landbouwwetensch. 33:959-68
Kirby, A.H.M. and Arthey, V.D. (1966) The influence of grey mold
fungicides on the flavour of canned strawberries. Meded. Rijksfac.
Landbouwwetensch. 31:1011-20
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Rept. prepared and
submitted by the Institute of Toxicology Farbenfabriken Bayer AG.
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Unpub. Rept. from the
Institute of Toxicology, Farbenfabriken Bayer AG.
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Unpub. Rept. from the Institute of Toxicology, Farbenfabriken Bayer
AG.
Löser, E. (1969) Bayer 47531, Generationaversuche an Ratten. Unpub.
Rept. prepared and submitted by the Institute of Toxicology,
Farbenfabriken Bayer AG.
Maier-Bode, E. (1966) Pharmakologisches Institut, Bonn. Unpub. Ref.
Farbenfabriken Bayer AG.
Nangniot, P., Vervier, R. and Martens, P.H. (1967) Le dosage de la
N,N-diméthyl-N-phényl -(N'-flourdichlorméthylthio) -sulfamide (Euparen)
on fruits. Bull. Rech. Agr. Gembloux 2:284-93
Nangniot, P., Vervier, R. and Martens, P.E. (1967) The use of
polarography to determine small amounts of fungicides on insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulfamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Vogeler, K. (1965) Farbenfabriken Bayer AG., Biologisches Institut,
Leverkusen. Unpub.
Vogeler, K. and Goeldner, H. (1967) Untersuchungen über Rückstände
nach Anwendung von Euparen an Weintrauben. Schweizerische Z. Obst- und
Weinbau 103:494-504
Volgeler, K. and Niessen, H. (1967) Kolorimetrische und
gas-chromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz - Nachrichten "Bayer" 20:534-49
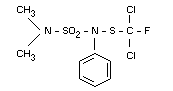 Other relevant chemical properties
The pure material is a white powder with slight characteristic odour,
m.p. 105.0-105.6°C; insoluble in water at 20°C, solubility in methanol
1.5 g/100 ml and in xylene 7.0 g/100 ml. The purity of the technical
material is at least 96 percent and the product contains
N',N'-dimethyl-N-phenyl sulphamide (DMSA) not more than 1 percent and
ionogenic chlorine max. 0.3 percent. The technical product is
formulated as 50 percent wettable powder and 7.5 percent dust. The
active ingredient decomposes in alkaline media and in the presence of
polysulphides. It is light-sensitive, but the discoloration induced
does not affect its biological activity.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
No information is available on the rate of absorption and on the
distribution of dichlofluanid in the animal body. Studies in rats
indicate that the absorption is small. Within 72 hours, 45-92 Percent
of the administered dose could be isolated from the fences. Most of
the dichlofluanid found was in an unchanged form; approximately 12
percent was isolated as N,N-dimethyl-N'-phenylsulphamide. After oral
administration of dichlofluanid the unchanged form of the compound van
not detectable in the serum or urine; however when either
dichlofluanid or its metabolite dimethylphenylsulphamide was
administered orally, four metabolites could be isolated from the
urine. The structures of those compounds have now been determined and
are given below:
Other relevant chemical properties
The pure material is a white powder with slight characteristic odour,
m.p. 105.0-105.6°C; insoluble in water at 20°C, solubility in methanol
1.5 g/100 ml and in xylene 7.0 g/100 ml. The purity of the technical
material is at least 96 percent and the product contains
N',N'-dimethyl-N-phenyl sulphamide (DMSA) not more than 1 percent and
ionogenic chlorine max. 0.3 percent. The technical product is
formulated as 50 percent wettable powder and 7.5 percent dust. The
active ingredient decomposes in alkaline media and in the presence of
polysulphides. It is light-sensitive, but the discoloration induced
does not affect its biological activity.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
No information is available on the rate of absorption and on the
distribution of dichlofluanid in the animal body. Studies in rats
indicate that the absorption is small. Within 72 hours, 45-92 Percent
of the administered dose could be isolated from the fences. Most of
the dichlofluanid found was in an unchanged form; approximately 12
percent was isolated as N,N-dimethyl-N'-phenylsulphamide. After oral
administration of dichlofluanid the unchanged form of the compound van
not detectable in the serum or urine; however when either
dichlofluanid or its metabolite dimethylphenylsulphamide was
administered orally, four metabolites could be isolated from the
urine. The structures of those compounds have now been determined and
are given below:
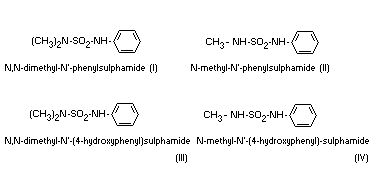 All the metabolites are excreted in the free-form and the metabolites
III and IV are also excreted as glucuronides. Metabolites I and II can
also be found in serum. The metabolites found in urine and serum after
oral administration of dichlofluanid are the same as those found after
oral administration of dimethylphenylsulphamide. From these studies it
is evident that dichlofluanid is absorbed only very slightly, if at
all, from the gastrointestines tract (Eben and Kimmerle, 1968, Bayer,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio-portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield N
N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 male and 20 female animals,
received dietary levels of 0, 150, 500, 1500 and 4500 ppm of
dichlofluanid for a period extending over three generations. Two
litters per generation wore followed and there were no deformities at
any dose-level. The groups fed 150, 500 and 1500 ppm displayed no
abnormal effects with respect to fertility, litter-size and percentage
survival to weaning. In the group given 4500 ppm there was no
difference from the control group in the F1b generation, but in the
F2b and succeeding generations to F3b, the body-weights of the
young animals were significantly lower both at birth and during
weaning. In the 4500 ppm group the lactation index was also slightly
reduced after one of the six matings (Löser, 1969).
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and 10,000
ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis, gross
and microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ-weights were comparable in the different
groups, except in the 10,000 ppm group where the female rate displayed
a decrease in the adrenal weight; and in both sexes an increase in the
liver-weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported. such might be
camouflaged by the histological changes due to infections (Lorke,
1965).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and Raymund, 1963
Mouse (M) i.p. 6.0 DuBois and Raymund, 1963
Chicken oral >1000 DuBois, 1963
Rat (M) i.p. 15 DuBois and Raymund, 1963
Rat (F) i.p. 15 Bayer, 1962
DuBois and Raymund, 1963
Rat (M) oral 500-1000 DuBois and Raymund, 1963
Rat (F) oral 525 Bayer, 1962
DuBois and Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and Raymund, 1963
Guinea-pig (M) oral 250 DuBois and Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly in a
decrease in activity which began several hours after administration of
the compound. With doses around the LD50, death occurred in one to
four days (DuBois and Raymund, 1963)
Short-term studies
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and 4500
ppm of dichlofluanid in their diets for four months. Behaviour, food
consumption, body-weight changes, mortality, function tests of the
liver and kidney urinalysis, gross pathology and final average
body-weights and average organ-weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body-weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300, 1000,
3000, and 10,000 ppm of dichlofluanid in their diets for four months.
Food consumption, growth, haematology, urinalysis, mortality, gross
and microscopic pathology were closely comparable in the 30 to 3000
ppm experimental groups and the control groups. The same was the case
with final average body and organ-weights except in the 3000 ppm group
where the male rats displayed reduction of heart-weights and the
female rate an increase in liver-weights. In the rats fed 10,000 ppm
there was a deterioration of general condition, decreased food intake,
smaller weight gains, and higher mortality than the control group. The
male rats showed a decrease in heart weights, while in both cases
there was a decrease in the weight of adrenal glands and an increase
in liver-weights. Histopathological changes were found in the liver
(vacuolization, inflation in size and shrunken nuclei of some cells),
kidneys (increased protein precipitation in the proximal tubuli) and
the spleen (reduction of the lymphatic tissue) (Bayer, 1964).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150, 500,
1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney-weights, while both sexes showed elevated
relative liver-weights, but normal enzyme function tests (Löser,
1968).
COMMENT
No information is available on the absorption or metabolism of
dichlofluanid in man or in animal species other than the rat. In
particular there is no information reported on the metabolism of the
dichlorofluoromethylthio-moiety. It is known that chemical hydrolysis
gives dichlorofluoromethanethiol and this observation is of some
concern when related to possible metabolic breakdown of dichlofluanid.
It cannot be assumed that the metabolism would ultimately result in
the formation of fluoride ions as the carbon-fluorine bond is known to
be highly resistant to cleavage, and thus there is a possibility that
other organofluorine compounds could result from the metabolism. This
possibility needs elucidation.
The 500 ppm level of dichlofluanid in the four-month study in dogs
seems to have no toxicological effects but no histological studies
were reported. In the four-month feeding study in the rat, the 1000
ppm dose level appeared to be without toxic effect. The long-term
study in rats was also not considered adequate because there was no
histological information provided. There are also no data from
observations in man nor from a one to two year study in a non-rodent
mammalian species.
For these reasons no acceptable daily intake could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop. Further
it is used or recommended for testing on tomato, cucumber, lettuce,
onion, cherry, plum, peach, currants, citrus fruits, and pecans.
It has good plant tolerance at the recommended concentrations,
although, according to location and variety, slight injuries may be
caused on stone fruits and on ornamentals (Anon., 1965). Some
interference in the act and development of fruit on some varieties of
strawberries has also been reported (Gourley, 1968). Dichlofluanid is
harmless to bees.
Safety intervals have been laid down in several European countries,
which differ according to the recommendations, crops and tolerances
where these are established.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Dichlofluanid is used for the treatment of ornamentals, especially for
the control of fungal diseases on roses. Dichlofluanid can be used for
control of powdery mildew without adversely affecting the quality of
tobacco. Dichlofluanid has a good side-effect against spider mites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following tables give the residues of dichlofluanid and its
metabolite DMSA found in the listed crops after application at the
recommended concentrations. The analyses were carried out by
Farbenfabriken Bayer AG, Germany, The State Institute of Agricultural
Chemistry, Finland, and various institutes (Anon., 1967, 1968, 1969).
The residues on grapes show large differences which may be dependent
upon several factors ouch as plot size, nature of grape development in
the different wine-growing districts, spraying technique and spraying
machine (Vogeler and Goeldner, 1967; Hurter at al., 1967). Also on
other crops the variation of residues greatly interferes with the
evaluation of data.
TABLE I
Residue data from field trials
Crop Number of Pre-harvest Residue at harvest (ppm)
treatments interval
(days) Dichlofluanid DMSA
Apples 1 - 12 10 - 14 0.2 - 3.2 not determined
Strawberries 1 - 4 11 - 14 n.d.- 3.6 0.25 - 3.1
Raspberries 3 - 4 7 5.9 - 13.8 0.65 - 6.4
13 - 14 2.3 - 10.5 0.45 - 4.0
Fresh currants 3 - 7 14 0.9 - 2.2 0.8 - 1.0
Grapes 2 - 6 40 - 50 0.7 - 10.9 0.25 - 4.4
Lettuce 5 - 6 14 n.d.- 0.3 0.8 - 2.5
Tomatoes 1 - 3 5 - 7 0.1 - 0.2 0.1 - 0.9
n.d. = not detected
FATE OF RESIDUES
General comments
Dichlofluanid is degraded to N', N'-dimethyl-N-phenyl sulphamide
(DMSA) under alkaline conditions in vitro, on the plant, and partly in
the gastrointestinal tract. (Also see entry under 'BIOCHEMICAL
ASPECTS' above). Amounts of residues on the plants are given Table I.
The metabolite DMSA is less toxic than dichlofluanid and is
fungicidally ineffective.
In animals
Dichlofluanid is either not resorbed at all from the gastrointestinal
tract or, if so, then only in a very slight extent. Dichlofluanid,
after being fed to rats, is degraded to DMSA.
In plants
Dimethylaminosulphanilide occurs as a metabolite of dichlofluanid on
all plants (Vogeler and Niessen, 1967).
The residue analyses show that the metabolite already forms on the day
dichlofluanid to applied. The proportion of metabolite in the total
residue varies according to the nature of the plant material. It does
not exceed the proportion of dichlofluanid, except in the case of
processed fruits and sometimes on strawberries. The "half-life" of
DMSA on apples is approximately 30 days; this value was determined in
an experiment in which formulated DMSA was sprayed (Vogeler, 1965).
The residue figures for dichlofluanid and its degradation product
combined are only slightly higher than the figures of dichlofluanid.
There is thus no evidence of buildup of the degradation product.
Dichlofluanid is relatively persistent, having a "half-life" of about
one week on strawberries. Consequently, the establishment of a
withholding period short enough to permit the use of dichlofluanid on
strawberries throughout the harvest period seems so far questionable
(Brewerton and Gibbs, 1968).
Rainfalls may cause considerable falls in the residues of
dichlofluanid.
In soil
Data on dichlofluanid residues in soil are not available.
In storage and processing
Nothing is known about the degradation of dichlofluanid on fruit in
storage. There are few figures available on effects of washing and
peeling of fruit on the residue levels (Table II). No final
conclusions can be made although the results indicate that variable
amounts of residues could be washed off and a substantial part of
residue was removed by peeling.
There was a decrease of the amount of residues in the canning process
of strawberries (Kavanagh at al., 1968). Following applications at the
recommended dosage, residues on deep-frozen strawberries amounted up
to 1.5 ppm; on the other hand, canned fruits and juice originating
from the same sample contained a maximum of about 0.1 ppm. Following
application at twice the recommended dosage, canned strawberries
contained up to 0.88 ppm. The reduction of residues in the canning
process is presumably canned by washing with water. According to
Kavanagh et al., DMSA was not detected in any sample of frozen or
canned fruit.
It has been reported that the dichlofluanid treatment of strawberries
would tend to increase the sweetness and acidity of the berries (Kirby
and Arthey, 1966).
Strawberry jam made from treated fruits contained dichlofluanid
residues of less than 0.1 ppm and DMSA residues of between 0.15 and
0.4 ppm. The content of residues in the treated strawberries amounted
to 0.4 to 1.15 ppm dichlofluanid and 0.8 to 0.85 ppm DMSA (Maier-Bode,
1966).
To investigate the residues in wine field-treated grapes as well as
marc, must, and wine yielded from them were analysed (see also Vogeler
and Goeldner, 1967) (Table III). The analyses showed that these wines
contained no residue of dichlofluanid and only small residues of DMSA.
A further 20 analyses of different wines made from treated grapes
showed no residues of dichlofluanid and the amounts of DMSA residues
found ranged from "not detectable" to 2.95 ppm. In these analyses the
amount of the residue on the grapes was unknown. In the course of wine
processing, the residues are removed by the pressing process and
pre-clarification (desliming) of the must. In marc very high residues
are found.
TABLE II
Effect of washing and peeling on residue levels
Pre-harvest Residue at Residue after Residue after
interval harvest washing peeling
Crop (days) (ppm) (ppm) (ppm)
Apple 14 2.0 1.3 0.4
14 3.3 4.0 1.5
Strawberry 14 0.07 0.03 -
19 0.5 0.15 -
19 0.6 0.20 -
TABLE III
Dichlofluanid and DMSA residues in grapes and must, marc, and wine made
from treated grapes
Residue
Pre-harvest (ppm)
Number of interval
Crop treatments (days) Dichlofluanid DMSA
Grapes 2 - 9 21 - 95 0.4 - 5.4 0.25 - 1.35
Must, not
clarified n.d. - 2.0 0.3 - 0.95
Must,
clarified n.d. - 0.05 0.25 - 0.4
Marc 0.4 - 53.0 8.9 - 47.0
Smashed 1.2 - 5.0 not determined
Peels 0.6 - 11.1 not determined
Wine n.d. - 3.9 n.d. - 2.95
n.d. = not detected
METHODS OF RESIDUE ANALYSIS
The most important method for determining residues of dichlofluanid is
gas chromatography using an electron capture detector. This method may
be used for analysing apples, strawberries, raspberries, currants,
lettuce, tomatoes, wins and grapes (Vogeler and Niessen, 1967).
Methods based on the same principle have been described for analysis
of strawberries (Eades and Gardiner, 1967) and grapes (Hurter et al.,
1966).
Dichlofluanid may also be determined by polarography (Nangniot at al.,
1967) and by colorimetry (Vogeler and Niessen, 1967). The colorimetric
method is based on saponification of the parent compound to the
metabolite DMSA and determination of aniline by diazotization and
coupling with N-(1-naphtyl)-ethylene-diamine. By this method, the sum
of parent compound and metabolite is obtained so that the metabolite
is determined alone in a second analytical procedure in which alkaline
saponification of the parent compound is omitted.
The parent compound and the metabolite DMSA can be determined in one
analytical procedure by gas chromatography and colorimetry after
column-chromatographic separation (Vogeler and Niessen, 1967). The
sensitivity of the colorimetric method is approximately 0.1 ppm and
that of the gas chromatographic method is 0.1 ppm or less depending
upon the response of the detector.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Saf. Int./
Country Crop Tol./ppm Days
Austria General 14
General 15.0 proposed
Belgium General 14
Fruits and vegetables 5.0
except potatoes
Denmark General 14
Finland General 14
France Grapes, strawberries 7
"Kupfer-Euparen (6341)" Grapes 7
Germany
(Fed.Rep.) Pome fruit 7
Cane and bush fruit 7*
Strawberries 2.0 + 2.0 DMSA 14
Tomatoes 1.0 + 1.0 DMSA 3*
Apples and pears 0.5 + 0.5 DMSA
Italy WP: General 20
Grapes 40
Strawberries 20
6012 (40% + 10% Cu) General 15
"Ramato blu (15% + 30% Cu)" General 7
Netherlands General 5.0
Strawberries, raspberries,
blackberries 7
Currants 21
Strawberries grown under glass 14
Norway General 7
Poland Fruits, vegetables, field crops 14
Grapes 42
(cont'd)
Saf. Int./
Country Crop Tol./ppm Days
Sweden General 7
Switzerland General 21
Grapes 1.0
Strawberries 7.0
United Kingdom Strawberries, raspberries 14
Blackberries, blackcurrants,
gooseberries, loganberries,
onions, cauliflower (grown under
glass), lettuce (grown under
glass), lettuce (field grown) 21
Yugoslavia Strawberries 2.0 + 2.0 DMSA 14
Grapes 30
* subject to official approval
APPRAISAL
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop, and
mildew of roses. Further, it is used or recommended for testing on
tomato, cucumber, lettuce, onion, cherry, plump peach, currants,
citrus fruits and pecans. Concentration of the sprays is recommended
to 0.075-0.12 percent active ingredient. Dichlofluanid has a good
side-effect against spider mites. Although there is a good plant
tolerance to dichlofluanid, slight injuries may be caused, according
to location and variety, on some stone fruits. It is harmless to bees.
Dichlofluanid is used in many European countries and the safety
intervals from the last treatment to the harvest vary from three to
forty days. The national tolerances applied vary from 1 to 7 ppm for
dichlofluanid alone or for dichlofluanid plus its metabolite DMSA. It
is formulated as 50 percent wettable powder and 7.5 percent dust.
The residue data available to the meeting were obtained from
supervised field trials in Germany, England and Finland. Initial
residues of dichlofluanid had usually degraded by one half within a
week; on grapes more persistent residues were found. By washing,
peeling and processing, residues are partly removed.
Dichlofluanid is degraded to N',N'-dimethyl-N-phenyl sulphamide (DMSA)
under alkaline conditions in vitro, on all plants, and in the
gastrointestinal tract. In addition to DMSA, in urine three other
metabolites of dichlofluanid are detected and identified. Residue data
on DMSA are available and its "half-life" on apples is determined (30
days). In processed strawberries both dichlofluanid and DMSA are
found. Wines produced from treated grapes are found to contain both
dichlofluanid and DMSA. The occurrence of DMSA seems to be, however,
more relevant than that of the parent compound. In plants or plant
products DMSA is the only degradation product of dichlofluanid so far
identified.
The documentation on dichlofluanid includes methods of residue
analysis based on GLC, polarography and colorimetry. Both the parent
compound and DMSA can be determined. A sensitivity of 0.1 ppm in plant
material can be reached.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
As no acceptable daily intake was established, no tolerances were
recommended.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Further information on the absorption and metabolism of the
compound particularly with regard to the fate of the
fluorine-containing portion of the molecule.
2. A long-term feeding study in the rat including a histological
examination of all major organs.
3. A 1-2 year feeding study in a non-rodent mammalian species.
4. Information on the composition of the technical dichlofluanid,
including the impurities.
5. More detailed information on the nature and magnitude of terminal
residues in plants including data on the fluorine-containing moiety
of the molecule.
6. Information about possible degradation mechanism of the molecule by
the action of sulfhydryl compounds in vitro and in vivo.
7. Data on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues from different
countries, especially on those crops which have shown inconsistency
of residue data. Data on degradation products of dichlofluanid, if
important in magnitude or toxicologically, should be included.
8. Data on residue levels in raw agricultural products moving in
commerce.
9. Qualitative and quantitative data on fate of residues in washing,
blanching and storing and thermal processing of treated crops.
10. Data concerning the possible occurrence of the parent compound in
wines produced from treated grapes.
DESIRABLE
1. Metabolism in animal species, other than the rat.
2. Metabolic studies and other observations in man.
3. Information on the fate of the compound in soil.
4. Evaluation of the analytical methods by collaborative studies for
regulatory purposes.
REFERENCES
Anon. (1965) (R)Euparen (Bay 47531). Pflanzenschutz. Farbenfabriken
Bayer AG. Technical Information Sheet
Anon. (1967) Investigations on pesticide residues. Publications of the
State Institute of Agricultural Chemistry. Tikkurila, Finland, No.1.
Anon. (1968) Ibid. No.2
Anon. (1969) Ibid. No.4
Bayer (1962) Product Kü 13-032-C. Unpub. Rept. prepared and submitted
by the Institute of Toxicology, Farbenfabriken Bayer AG.
Bayer (1964) Report of 4-months feeding study on rats with active
ingredient 47531. Unpub. Rept. prepared and submitted by the Institute
of Toxicology, Farbenfabriken Bayer AG.
Bayer (1969) Dichlofluanid. Unpub. Summary Rept. prepared and
submitted by Farbenfabriken Bayer AG.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
Residues on Strawberries. New Zealand J. Agr. Res. 11:784-88
DuBois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Unpub. Rept. submitted by Farbenfabriken Bayer
AG.
DuBois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Unpub. Rept. submitted by
Farbenfabriken Bayer A.G.
Eades, J.F. and Gardiner, K.D. (1967) Estimation of Dichlofluanid
Residues in Strawberries. Chemistry and Industry 32:1539-60
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Unpub. Rept. prepared and submitted by the Institute of
Toxicology, Farbenfabriken Bayer AG.
Gourley, C.O. (1968) Fungicidal control of Botrytis cinerea on four
strawberry varieties. Can. J. Plant Sci. 48:267-72
Hurter, J., Mayer, K. and Zürrer, A. (1966) Gärhemmung durch
Fungizidrückstände Schweizerische Z. Obst- und Weinbau 102:592-7
Hurter, J., Lauber, H.P., Mayer, K., Schüepp, H. and Bolay, A. (1967)
Rückstandsmenge auf Weintrauben und Gärnerlauf nach Behandlung mit
Dichlofluanid und Folpet. Schweizerische Z. Obst-und Weinbau 103.201-9
Kavanagh, T., Gardiner, K.D., O'Callaghan, F.F. and Eades, J.F.K.
(1968) Fungicidal Control of Botrytis of Strawberries and Laboratory
Determination of Residues and Flavour. Meded. Rijksfac.
Landbouwwetensch. 33:959-68
Kirby, A.H.M. and Arthey, V.D. (1966) The influence of grey mold
fungicides on the flavour of canned strawberries. Meded. Rijksfac.
Landbouwwetensch. 31:1011-20
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Rept. prepared and
submitted by the Institute of Toxicology Farbenfabriken Bayer AG.
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Unpub. Rept. from the
Institute of Toxicology, Farbenfabriken Bayer AG.
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Unpub. Rept. from the Institute of Toxicology, Farbenfabriken Bayer
AG.
Löser, E. (1969) Bayer 47531, Generationaversuche an Ratten. Unpub.
Rept. prepared and submitted by the Institute of Toxicology,
Farbenfabriken Bayer AG.
Maier-Bode, E. (1966) Pharmakologisches Institut, Bonn. Unpub. Ref.
Farbenfabriken Bayer AG.
Nangniot, P., Vervier, R. and Martens, P.H. (1967) Le dosage de la
N,N-diméthyl-N-phényl -(N'-flourdichlorméthylthio) -sulfamide (Euparen)
on fruits. Bull. Rech. Agr. Gembloux 2:284-93
Nangniot, P., Vervier, R. and Martens, P.E. (1967) The use of
polarography to determine small amounts of fungicides on insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulfamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Vogeler, K. (1965) Farbenfabriken Bayer AG., Biologisches Institut,
Leverkusen. Unpub.
Vogeler, K. and Goeldner, H. (1967) Untersuchungen über Rückstände
nach Anwendung von Euparen an Weintrauben. Schweizerische Z. Obst- und
Weinbau 103:494-504
Volgeler, K. and Niessen, H. (1967) Kolorimetrische und
gas-chromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz - Nachrichten "Bayer" 20:534-49
All the metabolites are excreted in the free-form and the metabolites
III and IV are also excreted as glucuronides. Metabolites I and II can
also be found in serum. The metabolites found in urine and serum after
oral administration of dichlofluanid are the same as those found after
oral administration of dimethylphenylsulphamide. From these studies it
is evident that dichlofluanid is absorbed only very slightly, if at
all, from the gastrointestines tract (Eben and Kimmerle, 1968, Bayer,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio-portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield N
N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 male and 20 female animals,
received dietary levels of 0, 150, 500, 1500 and 4500 ppm of
dichlofluanid for a period extending over three generations. Two
litters per generation wore followed and there were no deformities at
any dose-level. The groups fed 150, 500 and 1500 ppm displayed no
abnormal effects with respect to fertility, litter-size and percentage
survival to weaning. In the group given 4500 ppm there was no
difference from the control group in the F1b generation, but in the
F2b and succeeding generations to F3b, the body-weights of the
young animals were significantly lower both at birth and during
weaning. In the 4500 ppm group the lactation index was also slightly
reduced after one of the six matings (Löser, 1969).
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and 10,000
ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis, gross
and microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ-weights were comparable in the different
groups, except in the 10,000 ppm group where the female rate displayed
a decrease in the adrenal weight; and in both sexes an increase in the
liver-weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported. such might be
camouflaged by the histological changes due to infections (Lorke,
1965).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and Raymund, 1963
Mouse (M) i.p. 6.0 DuBois and Raymund, 1963
Chicken oral >1000 DuBois, 1963
Rat (M) i.p. 15 DuBois and Raymund, 1963
Rat (F) i.p. 15 Bayer, 1962
DuBois and Raymund, 1963
Rat (M) oral 500-1000 DuBois and Raymund, 1963
Rat (F) oral 525 Bayer, 1962
DuBois and Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and Raymund, 1963
Guinea-pig (M) oral 250 DuBois and Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly in a
decrease in activity which began several hours after administration of
the compound. With doses around the LD50, death occurred in one to
four days (DuBois and Raymund, 1963)
Short-term studies
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and 4500
ppm of dichlofluanid in their diets for four months. Behaviour, food
consumption, body-weight changes, mortality, function tests of the
liver and kidney urinalysis, gross pathology and final average
body-weights and average organ-weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body-weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300, 1000,
3000, and 10,000 ppm of dichlofluanid in their diets for four months.
Food consumption, growth, haematology, urinalysis, mortality, gross
and microscopic pathology were closely comparable in the 30 to 3000
ppm experimental groups and the control groups. The same was the case
with final average body and organ-weights except in the 3000 ppm group
where the male rats displayed reduction of heart-weights and the
female rate an increase in liver-weights. In the rats fed 10,000 ppm
there was a deterioration of general condition, decreased food intake,
smaller weight gains, and higher mortality than the control group. The
male rats showed a decrease in heart weights, while in both cases
there was a decrease in the weight of adrenal glands and an increase
in liver-weights. Histopathological changes were found in the liver
(vacuolization, inflation in size and shrunken nuclei of some cells),
kidneys (increased protein precipitation in the proximal tubuli) and
the spleen (reduction of the lymphatic tissue) (Bayer, 1964).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150, 500,
1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney-weights, while both sexes showed elevated
relative liver-weights, but normal enzyme function tests (Löser,
1968).
COMMENT
No information is available on the absorption or metabolism of
dichlofluanid in man or in animal species other than the rat. In
particular there is no information reported on the metabolism of the
dichlorofluoromethylthio-moiety. It is known that chemical hydrolysis
gives dichlorofluoromethanethiol and this observation is of some
concern when related to possible metabolic breakdown of dichlofluanid.
It cannot be assumed that the metabolism would ultimately result in
the formation of fluoride ions as the carbon-fluorine bond is known to
be highly resistant to cleavage, and thus there is a possibility that
other organofluorine compounds could result from the metabolism. This
possibility needs elucidation.
The 500 ppm level of dichlofluanid in the four-month study in dogs
seems to have no toxicological effects but no histological studies
were reported. In the four-month feeding study in the rat, the 1000
ppm dose level appeared to be without toxic effect. The long-term
study in rats was also not considered adequate because there was no
histological information provided. There are also no data from
observations in man nor from a one to two year study in a non-rodent
mammalian species.
For these reasons no acceptable daily intake could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop. Further
it is used or recommended for testing on tomato, cucumber, lettuce,
onion, cherry, plum, peach, currants, citrus fruits, and pecans.
It has good plant tolerance at the recommended concentrations,
although, according to location and variety, slight injuries may be
caused on stone fruits and on ornamentals (Anon., 1965). Some
interference in the act and development of fruit on some varieties of
strawberries has also been reported (Gourley, 1968). Dichlofluanid is
harmless to bees.
Safety intervals have been laid down in several European countries,
which differ according to the recommendations, crops and tolerances
where these are established.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Dichlofluanid is used for the treatment of ornamentals, especially for
the control of fungal diseases on roses. Dichlofluanid can be used for
control of powdery mildew without adversely affecting the quality of
tobacco. Dichlofluanid has a good side-effect against spider mites.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The following tables give the residues of dichlofluanid and its
metabolite DMSA found in the listed crops after application at the
recommended concentrations. The analyses were carried out by
Farbenfabriken Bayer AG, Germany, The State Institute of Agricultural
Chemistry, Finland, and various institutes (Anon., 1967, 1968, 1969).
The residues on grapes show large differences which may be dependent
upon several factors ouch as plot size, nature of grape development in
the different wine-growing districts, spraying technique and spraying
machine (Vogeler and Goeldner, 1967; Hurter at al., 1967). Also on
other crops the variation of residues greatly interferes with the
evaluation of data.
TABLE I
Residue data from field trials
Crop Number of Pre-harvest Residue at harvest (ppm)
treatments interval
(days) Dichlofluanid DMSA
Apples 1 - 12 10 - 14 0.2 - 3.2 not determined
Strawberries 1 - 4 11 - 14 n.d.- 3.6 0.25 - 3.1
Raspberries 3 - 4 7 5.9 - 13.8 0.65 - 6.4
13 - 14 2.3 - 10.5 0.45 - 4.0
Fresh currants 3 - 7 14 0.9 - 2.2 0.8 - 1.0
Grapes 2 - 6 40 - 50 0.7 - 10.9 0.25 - 4.4
Lettuce 5 - 6 14 n.d.- 0.3 0.8 - 2.5
Tomatoes 1 - 3 5 - 7 0.1 - 0.2 0.1 - 0.9
n.d. = not detected
FATE OF RESIDUES
General comments
Dichlofluanid is degraded to N', N'-dimethyl-N-phenyl sulphamide
(DMSA) under alkaline conditions in vitro, on the plant, and partly in
the gastrointestinal tract. (Also see entry under 'BIOCHEMICAL
ASPECTS' above). Amounts of residues on the plants are given Table I.
The metabolite DMSA is less toxic than dichlofluanid and is
fungicidally ineffective.
In animals
Dichlofluanid is either not resorbed at all from the gastrointestinal
tract or, if so, then only in a very slight extent. Dichlofluanid,
after being fed to rats, is degraded to DMSA.
In plants
Dimethylaminosulphanilide occurs as a metabolite of dichlofluanid on
all plants (Vogeler and Niessen, 1967).
The residue analyses show that the metabolite already forms on the day
dichlofluanid to applied. The proportion of metabolite in the total
residue varies according to the nature of the plant material. It does
not exceed the proportion of dichlofluanid, except in the case of
processed fruits and sometimes on strawberries. The "half-life" of
DMSA on apples is approximately 30 days; this value was determined in
an experiment in which formulated DMSA was sprayed (Vogeler, 1965).
The residue figures for dichlofluanid and its degradation product
combined are only slightly higher than the figures of dichlofluanid.
There is thus no evidence of buildup of the degradation product.
Dichlofluanid is relatively persistent, having a "half-life" of about
one week on strawberries. Consequently, the establishment of a
withholding period short enough to permit the use of dichlofluanid on
strawberries throughout the harvest period seems so far questionable
(Brewerton and Gibbs, 1968).
Rainfalls may cause considerable falls in the residues of
dichlofluanid.
In soil
Data on dichlofluanid residues in soil are not available.
In storage and processing
Nothing is known about the degradation of dichlofluanid on fruit in
storage. There are few figures available on effects of washing and
peeling of fruit on the residue levels (Table II). No final
conclusions can be made although the results indicate that variable
amounts of residues could be washed off and a substantial part of
residue was removed by peeling.
There was a decrease of the amount of residues in the canning process
of strawberries (Kavanagh at al., 1968). Following applications at the
recommended dosage, residues on deep-frozen strawberries amounted up
to 1.5 ppm; on the other hand, canned fruits and juice originating
from the same sample contained a maximum of about 0.1 ppm. Following
application at twice the recommended dosage, canned strawberries
contained up to 0.88 ppm. The reduction of residues in the canning
process is presumably canned by washing with water. According to
Kavanagh et al., DMSA was not detected in any sample of frozen or
canned fruit.
It has been reported that the dichlofluanid treatment of strawberries
would tend to increase the sweetness and acidity of the berries (Kirby
and Arthey, 1966).
Strawberry jam made from treated fruits contained dichlofluanid
residues of less than 0.1 ppm and DMSA residues of between 0.15 and
0.4 ppm. The content of residues in the treated strawberries amounted
to 0.4 to 1.15 ppm dichlofluanid and 0.8 to 0.85 ppm DMSA (Maier-Bode,
1966).
To investigate the residues in wine field-treated grapes as well as
marc, must, and wine yielded from them were analysed (see also Vogeler
and Goeldner, 1967) (Table III). The analyses showed that these wines
contained no residue of dichlofluanid and only small residues of DMSA.
A further 20 analyses of different wines made from treated grapes
showed no residues of dichlofluanid and the amounts of DMSA residues
found ranged from "not detectable" to 2.95 ppm. In these analyses the
amount of the residue on the grapes was unknown. In the course of wine
processing, the residues are removed by the pressing process and
pre-clarification (desliming) of the must. In marc very high residues
are found.
TABLE II
Effect of washing and peeling on residue levels
Pre-harvest Residue at Residue after Residue after
interval harvest washing peeling
Crop (days) (ppm) (ppm) (ppm)
Apple 14 2.0 1.3 0.4
14 3.3 4.0 1.5
Strawberry 14 0.07 0.03 -
19 0.5 0.15 -
19 0.6 0.20 -
TABLE III
Dichlofluanid and DMSA residues in grapes and must, marc, and wine made
from treated grapes
Residue
Pre-harvest (ppm)
Number of interval
Crop treatments (days) Dichlofluanid DMSA
Grapes 2 - 9 21 - 95 0.4 - 5.4 0.25 - 1.35
Must, not
clarified n.d. - 2.0 0.3 - 0.95
Must,
clarified n.d. - 0.05 0.25 - 0.4
Marc 0.4 - 53.0 8.9 - 47.0
Smashed 1.2 - 5.0 not determined
Peels 0.6 - 11.1 not determined
Wine n.d. - 3.9 n.d. - 2.95
n.d. = not detected
METHODS OF RESIDUE ANALYSIS
The most important method for determining residues of dichlofluanid is
gas chromatography using an electron capture detector. This method may
be used for analysing apples, strawberries, raspberries, currants,
lettuce, tomatoes, wins and grapes (Vogeler and Niessen, 1967).
Methods based on the same principle have been described for analysis
of strawberries (Eades and Gardiner, 1967) and grapes (Hurter et al.,
1966).
Dichlofluanid may also be determined by polarography (Nangniot at al.,
1967) and by colorimetry (Vogeler and Niessen, 1967). The colorimetric
method is based on saponification of the parent compound to the
metabolite DMSA and determination of aniline by diazotization and
coupling with N-(1-naphtyl)-ethylene-diamine. By this method, the sum
of parent compound and metabolite is obtained so that the metabolite
is determined alone in a second analytical procedure in which alkaline
saponification of the parent compound is omitted.
The parent compound and the metabolite DMSA can be determined in one
analytical procedure by gas chromatography and colorimetry after
column-chromatographic separation (Vogeler and Niessen, 1967). The
sensitivity of the colorimetric method is approximately 0.1 ppm and
that of the gas chromatographic method is 0.1 ppm or less depending
upon the response of the detector.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Saf. Int./
Country Crop Tol./ppm Days
Austria General 14
General 15.0 proposed
Belgium General 14
Fruits and vegetables 5.0
except potatoes
Denmark General 14
Finland General 14
France Grapes, strawberries 7
"Kupfer-Euparen (6341)" Grapes 7
Germany
(Fed.Rep.) Pome fruit 7
Cane and bush fruit 7*
Strawberries 2.0 + 2.0 DMSA 14
Tomatoes 1.0 + 1.0 DMSA 3*
Apples and pears 0.5 + 0.5 DMSA
Italy WP: General 20
Grapes 40
Strawberries 20
6012 (40% + 10% Cu) General 15
"Ramato blu (15% + 30% Cu)" General 7
Netherlands General 5.0
Strawberries, raspberries,
blackberries 7
Currants 21
Strawberries grown under glass 14
Norway General 7
Poland Fruits, vegetables, field crops 14
Grapes 42
(cont'd)
Saf. Int./
Country Crop Tol./ppm Days
Sweden General 7
Switzerland General 21
Grapes 1.0
Strawberries 7.0
United Kingdom Strawberries, raspberries 14
Blackberries, blackcurrants,
gooseberries, loganberries,
onions, cauliflower (grown under
glass), lettuce (grown under
glass), lettuce (field grown) 21
Yugoslavia Strawberries 2.0 + 2.0 DMSA 14
Grapes 30
* subject to official approval
APPRAISAL
Dichlofluanid is a fungicide with a broad spectrum of activity. It is
chiefly used for controlling scab of apple and pear, Botrytis of
strawberry and grape vine, Peronospora of grape vine and hop, and
mildew of roses. Further, it is used or recommended for testing on
tomato, cucumber, lettuce, onion, cherry, plump peach, currants,
citrus fruits and pecans. Concentration of the sprays is recommended
to 0.075-0.12 percent active ingredient. Dichlofluanid has a good
side-effect against spider mites. Although there is a good plant
tolerance to dichlofluanid, slight injuries may be caused, according
to location and variety, on some stone fruits. It is harmless to bees.
Dichlofluanid is used in many European countries and the safety
intervals from the last treatment to the harvest vary from three to
forty days. The national tolerances applied vary from 1 to 7 ppm for
dichlofluanid alone or for dichlofluanid plus its metabolite DMSA. It
is formulated as 50 percent wettable powder and 7.5 percent dust.
The residue data available to the meeting were obtained from
supervised field trials in Germany, England and Finland. Initial
residues of dichlofluanid had usually degraded by one half within a
week; on grapes more persistent residues were found. By washing,
peeling and processing, residues are partly removed.
Dichlofluanid is degraded to N',N'-dimethyl-N-phenyl sulphamide (DMSA)
under alkaline conditions in vitro, on all plants, and in the
gastrointestinal tract. In addition to DMSA, in urine three other
metabolites of dichlofluanid are detected and identified. Residue data
on DMSA are available and its "half-life" on apples is determined (30
days). In processed strawberries both dichlofluanid and DMSA are
found. Wines produced from treated grapes are found to contain both
dichlofluanid and DMSA. The occurrence of DMSA seems to be, however,
more relevant than that of the parent compound. In plants or plant
products DMSA is the only degradation product of dichlofluanid so far
identified.
The documentation on dichlofluanid includes methods of residue
analysis based on GLC, polarography and colorimetry. Both the parent
compound and DMSA can be determined. A sensitivity of 0.1 ppm in plant
material can be reached.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
As no acceptable daily intake was established, no tolerances were
recommended.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Further information on the absorption and metabolism of the
compound particularly with regard to the fate of the
fluorine-containing portion of the molecule.
2. A long-term feeding study in the rat including a histological
examination of all major organs.
3. A 1-2 year feeding study in a non-rodent mammalian species.
4. Information on the composition of the technical dichlofluanid,
including the impurities.
5. More detailed information on the nature and magnitude of terminal
residues in plants including data on the fluorine-containing moiety
of the molecule.
6. Information about possible degradation mechanism of the molecule by
the action of sulfhydryl compounds in vitro and in vivo.
7. Data on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues from different
countries, especially on those crops which have shown inconsistency
of residue data. Data on degradation products of dichlofluanid, if
important in magnitude or toxicologically, should be included.
8. Data on residue levels in raw agricultural products moving in
commerce.
9. Qualitative and quantitative data on fate of residues in washing,
blanching and storing and thermal processing of treated crops.
10. Data concerning the possible occurrence of the parent compound in
wines produced from treated grapes.
DESIRABLE
1. Metabolism in animal species, other than the rat.
2. Metabolic studies and other observations in man.
3. Information on the fate of the compound in soil.
4. Evaluation of the analytical methods by collaborative studies for
regulatory purposes.
REFERENCES
Anon. (1965) (R)Euparen (Bay 47531). Pflanzenschutz. Farbenfabriken
Bayer AG. Technical Information Sheet
Anon. (1967) Investigations on pesticide residues. Publications of the
State Institute of Agricultural Chemistry. Tikkurila, Finland, No.1.
Anon. (1968) Ibid. No.2
Anon. (1969) Ibid. No.4
Bayer (1962) Product Kü 13-032-C. Unpub. Rept. prepared and submitted
by the Institute of Toxicology, Farbenfabriken Bayer AG.
Bayer (1964) Report of 4-months feeding study on rats with active
ingredient 47531. Unpub. Rept. prepared and submitted by the Institute
of Toxicology, Farbenfabriken Bayer AG.
Bayer (1969) Dichlofluanid. Unpub. Summary Rept. prepared and
submitted by Farbenfabriken Bayer AG.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
Residues on Strawberries. New Zealand J. Agr. Res. 11:784-88
DuBois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Unpub. Rept. submitted by Farbenfabriken Bayer
AG.
DuBois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Unpub. Rept. submitted by
Farbenfabriken Bayer A.G.
Eades, J.F. and Gardiner, K.D. (1967) Estimation of Dichlofluanid
Residues in Strawberries. Chemistry and Industry 32:1539-60
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Unpub. Rept. prepared and submitted by the Institute of
Toxicology, Farbenfabriken Bayer AG.
Gourley, C.O. (1968) Fungicidal control of Botrytis cinerea on four
strawberry varieties. Can. J. Plant Sci. 48:267-72
Hurter, J., Mayer, K. and Zürrer, A. (1966) Gärhemmung durch
Fungizidrückstände Schweizerische Z. Obst- und Weinbau 102:592-7
Hurter, J., Lauber, H.P., Mayer, K., Schüepp, H. and Bolay, A. (1967)
Rückstandsmenge auf Weintrauben und Gärnerlauf nach Behandlung mit
Dichlofluanid und Folpet. Schweizerische Z. Obst-und Weinbau 103.201-9
Kavanagh, T., Gardiner, K.D., O'Callaghan, F.F. and Eades, J.F.K.
(1968) Fungicidal Control of Botrytis of Strawberries and Laboratory
Determination of Residues and Flavour. Meded. Rijksfac.
Landbouwwetensch. 33:959-68
Kirby, A.H.M. and Arthey, V.D. (1966) The influence of grey mold
fungicides on the flavour of canned strawberries. Meded. Rijksfac.
Landbouwwetensch. 31:1011-20
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Rept. prepared and
submitted by the Institute of Toxicology Farbenfabriken Bayer AG.
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Unpub. Rept. from the
Institute of Toxicology, Farbenfabriken Bayer AG.
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Unpub. Rept. from the Institute of Toxicology, Farbenfabriken Bayer
AG.
Löser, E. (1969) Bayer 47531, Generationaversuche an Ratten. Unpub.
Rept. prepared and submitted by the Institute of Toxicology,
Farbenfabriken Bayer AG.
Maier-Bode, E. (1966) Pharmakologisches Institut, Bonn. Unpub. Ref.
Farbenfabriken Bayer AG.
Nangniot, P., Vervier, R. and Martens, P.H. (1967) Le dosage de la
N,N-diméthyl-N-phényl -(N'-flourdichlorméthylthio) -sulfamide (Euparen)
on fruits. Bull. Rech. Agr. Gembloux 2:284-93
Nangniot, P., Vervier, R. and Martens, P.E. (1967) The use of
polarography to determine small amounts of fungicides on insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulfamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Vogeler, K. (1965) Farbenfabriken Bayer AG., Biologisches Institut,
Leverkusen. Unpub.
Vogeler, K. and Goeldner, H. (1967) Untersuchungen über Rückstände
nach Anwendung von Euparen an Weintrauben. Schweizerische Z. Obst- und
Weinbau 103:494-504
Volgeler, K. and Niessen, H. (1967) Kolorimetrische und
gas-chromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz - Nachrichten "Bayer" 20:534-49
