
DICHLOFLUANID JMPR 1974
Explanation
In 1969 the Joint Meeting evaluated dichlofluanid in the light of
the information then available. No ADI could be established and no
tolerances were recommended.
The Meeting listed 10 items on which further work or information
was required before tolerances could be recommended (FAO/WHO, 1970).
New information has been received on toxicology, composition of
the technical product, rates and frequencies of application,
pre-harvest intervals, residues and the influence of residues on the
fermentation of must. This information is summarized in the following
monograph addendum.
IDENTITY
Synonyms
Elvaron(R)
Other relevant chemical and physical properties
The purity of the technical material is at least 96%, and the
maximum levels of the impurities are as shown in Table 1.
In practice, the average content of dichlofluanid in the
technical product is approximately 98%, so that the by-products
normally amount to only about half of the amounts given in Table 1.
TABLE 1. Maximum levels of impurities in dichlofluanid
Maximum
Impurity content, %
N,N-dimethyl-N-phenylsulphamide (DMSA) 1.5
N,N-dimethylbenzylamine (C6H5-CH2-N(CH3)2) 1.0
"difluoro-dichlofluanid" (R-CF2Cl) 0.3
chlorides (expressed as HCl) 0.3
up to 6 unidentified trace impurities, total 1.0
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
No information is available on the rate of absorption or on the
distribution of dichlofluanid in the animal body. Studies in rats
indicate that the absorption is small. Within 72 hours, 45-92 percent
of the administered dose could be isolated from the faeces. Most of
the dichlofluanid found was in an unchanged form; approximately 12
percent was isolated as N,N-dimethyl-N'-phenylsulphamide. After oral
administration of dichlofluanid the unchanged form of the compound was
not detectable in the serum or urine; however when either
dichlofluanid or its metabolite dimethylphenylsulphamide was
administered orally, four metabolites could be isolated from the
urine. The structures of these compounds have now been determined and
are given below:
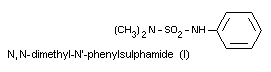 N,N-dimethyl-N'-phenylsulphamide (I)
N,N-dimethyl-N'-phenylsulphamide (I)
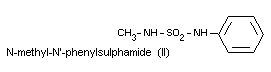 N-methyl-N'-phenylsulphamide (II)
N-methyl-N'-phenylsulphamide (II)
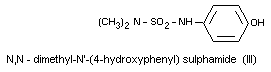 N,N-dimethyl-N'-(4-hydroxyphenyl) sulphamide (III)
N,N-dimethyl-N'-(4-hydroxyphenyl) sulphamide (III)
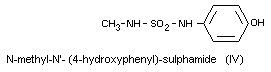 N-methyl-N'-(4-hydroxyphenyl)-sulphamide (IV)
All the metabolites are excreted in the free form and the
metabolites III and IV are also excreted as glucuronides. Metabolites
I and II can also be found in serum. The metabolites found in urine
and serum after oral administration of dichlofluanid are the same as
those found after oral administration of dimethylphenylsulphamide.
From these studies it is evident that dichlofluanid is absorbed only
very slightly from the G.I. tract (Eben and Kimmerle, 1968; Anon.,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield
N,N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
TOXICOLOGICAL STUDIES
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and
10 000 ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis gross and
microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ weights were comparable in the different
groups, except in the 10 000 ppm group where the female rats displayed
a decrease in the adrenal weight, and in both sexes an increase in the
liver weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported, such changes might
be camouflaged by the histological changes due to infections (Lorke,
1965).
Special studies on mutagenicity
Mouse
A dominant-lethal-test was carried out by administering 500 mg
dichlofluanid/kg bodyweight to twenty male mice. Untreated female mice
mated with three males did not show any difference in the early and
late resorptions, the number of corpora lutea or living and dead
foetuses when compared to those mated with control males. This study
at one relatively high dose level, did not reveal any dominant lethal
mutagenic effect of dichlofluanid (Machemer, 1974b).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 males and 20 females received
dietary levels of 0, 150, 500, 1500 and 4500 ppm of dichlofluanid
(90.2% pure; 8% inert material; 0.3% DMSA) for a period extending over
three generations. Two litters per generation were studied. No
information is available on the selection of animals from one
generation to the next. None of the dosed groups indicated any
abnormal effects on fertility throughout all three generations. The
groups fed 150, 500 and 1500 ppm displayed no abnormal effects
compared to controls with respect to group averages of birth weight,
litter-size and percentage of survival to weaning. Data for still
births and resorptions are not given in the report of this study. Only
in the group given 4500 ppm, the body weights of the young animals
were lower both at birth and at weaning in all generations except the
F1a at birth. The lactation index was also slightly reduced in one of
the six matings at this level. Although the experiment was not
designed to study teratological effects it may be noted that no
malformations were seen at any dose level (Löser, 1969a).
An additional study of the histology of organs of some animals of
the F3b generation showed no dose related effects of dichlofluanid
(Vince and Spicer, 1971).
Groups of 20-23 pregnant female rats were administered
dichlofluanid by stomach tube, on days 6 to 15 of gestation, at doses
of 30, 100, and 300 mg/kg/day. Symptoms of toxicity were observed in
all test groups and body-weight gains were decreased compared to the
controls both during the ten days treatment and during the total
gestation period. The effect was similar in all test groups and not
dose-dependent. The number of implantations, resorptions and foetuses
were within normal limits in all groups, whereas the average weight of
the placenta and the foetuses were slightly decreased in the groups
administered 300 mg/kg, possibly owing to the general toxic effect on
the maternal animals. In this study comprising only doses with
maternal toxicity no indication of teratogenic potential of
dichlofluanid was found (Machemer, 1974a).
Acute toxicity
TABLE 2. Acute toxicity of dichlofluanid
LD50mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and
Raymund, 1963
Mouse (M) i.p. 6.0 Dubois and
Raymund, 1963
Chicken oral >1000 Dubois, 1963
Rat (M) i.p. 15 Dubois and
Raymund, 1963
Rat (F) i.p. 15 Anon., 1962
DuBois and
Raymund, 1963
Rat (M) oral 500-1000 DuBois and
Raymund, 1963
Rat (F) oral 525 Anon., 1962
DuBois and
Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and
Raymund, 1963
Guinea-pig (M) oral 250 DuBois and
Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly
in a decrease in activity which began several hours after
administration of the compound. With doses around the LD50, death
occurred in one to four days (DuBois and Raymund, 1963).
Short-term studies
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300,
1000, 3000, and 10 000 ppm of dichlofluanid in their diets for four
months. Food consumption, growth, haematology, urinalysis, mortality,
and gross and microscopic pathology were closely comparable in the 30
to 3000 ppm experimental groups and the control groups. The same was
the case with final average body and organ weights except in the 3000
ppm group where the male rats displayed reduction of heart weights and
the female rats an increase in liver weights. In the rats fed 20 000
ppm there was a deterioration of general condition, decreased food
intake, smaller weight gains and higher mortality than the control
group. The male rats showed a decrease in heart weights, while in both
sexes there was a decrease in the weight of adrenal glands and an
increase in liver weights. Histopathological changes were found in the
liver (vacuolization, inflation in size and shrunken nuclei of some
cells), kidneys (increased protein precipitation in the proximal
tubuli) and the spleen (reduction of the lymphatic tissue) (Anon.,
1964).
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and
4500 ppm of dichlofluanid in their diets for four months. Behaviour,
food consumption, body-weight changes, mortality, function tests of
the liver and kidney, urinalysis, gross pathology and final average
body weights and average organ weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Groups of 4 male and 4 female dogs were fed 0, 100, 300, 1000 and
3000 ppm dichlofluanid (97.7% pure) in their diet for 2 years.
Behaviour, body weights, food consumption, mortality, blood and urine
analyses, liver and kidney function tests and absolute and relative
organ weights for the groups fed 100, 300 and 1000 ppm did not differ
from the values of these parameters in the controls. In the groups fed
3000 ppm increased mortality, reduced body weight and food consumption
and decreased absolute and relative testis weight were found.
Microscopic examination of the organs revealed an increase in
interstitial tissue of the testis and vacuolation and degeneration of
the adrenal cortex of the 3000 ppm group as the only dose-dependent
pathological changes (Löser, 1969b; Mawdesley-Thomas et al., 1971).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150,
500, 1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney weights, while both sexes showed elevated
relative liver weights, but normal enzyme function tests (Löser,
1968).
Histological examinations were made on tissues of five male and
five female rats at the end of the study and on animals that died
during the experiment. No significant pathological changes were found
at dietary levels including the 4 500 ppm level. A separate study of
all tumours found at autopsy was performed, and there was no
indication that dichlofluanid produced an increased tumour incidence
(Mawdesley-Thomas, 1969).
Comments
In a 2-year study in dogs a 1 000 ppm level of dichlofluanid in
the diet appears to have no untoward effect, although no data are
given on the individual animals.
A long term study in rats indicated 1 500 ppm in the feed as the
level without apparent toxic effect. There was no indication of
teratogenic effect when female rats were administered a dose as high
as 300 mg/kg bw. Reproduction parameters were not affected in a
3-generation feeding test with levels up to 1500 ppm dichlofluanid.
However, there is a lack of information on absorption, distribution,
excretion and pharmacokinetics on the dichloro-fluoromethylthio moiety
which may produce some carbon-fluoride compounds resistant to
cleavage. The Meeting allocated a temporary acceptable daily intake
for man based on the no-effect level in the dog, the more sensitive
species.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1 500 ppm in diet equivalent to 75 mg/kg bw.
Dog: 1 000 ppm, in diet equivalent to 25 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.3 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Uses (Table 3) are additional to those listed in FAO/WHO, 1970.
They are all recommendations of the Federal Republic of Germany (FRG).
TABLE 3. Pre-harvest treatments with dichlofluanid recommended
in the Federal Republic of Germany
Number of Rate of application
Crop applications (kg a.i./ha)
Pome fruits 4 - 11 1.5
Stone fruits
plums 4 1.5 - 2.0
cherries 3 - 8 2.0
peaches 3 1.5
Soft fruits
strawberries 1 - 6 2.5 - 3.0
red and black
currants 4 - 7 2.0 - 2.4
raspberries 1 -4 2.0 - 2.4
Grapes 2 - 10 1.9 - 2.5
Vegetables
cucumbers 1 - 4 1.5 - 2.0
tomatoes 1 - 3 1.5 - 2.0
lettuce (field grown
and under glass) 2 - 4 0.6 - 1.0
Beans 1 - 3 1.0
Hops 4 - 17 2.0
TABLE 4. Residues of dichlofluanid (I) and DSMA (II) resulting from supervised trials.
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Apples (FRG) 1.1- 4- I 0.7-3.4 0.2-2.8 <0.1-2.2 <0.1 <0.1-0.4 <0.1
1.25 11 II 0.3-1.1 <0.1-1.1 <0.1-1.1 <0.1-0.4 <0.1 <0.1
Beans green incl. 1.0- 1- I 0-1-0.3 0.1-0.2 <0.1-0.2 0.1
pods (FRG; UK) 3.4 3 II 0.1-0.2 0.1-0.2 <0.1 0.1
Cherries (morello) 1.1 6-8 I 0.6-2.5 <0.1-0.4 <0.1 <0.1
(FRG) II 0.2-1.2 <0.1-0.2 <0.1 <0.1
Cucumbers (outdoor); 1.5 3 I <0.1-0.2 <0.1-0.2 <0.1
(FRG) II 0.2-0.5 <0.1-0.3 <0.1
Cucumbers (under 1.5- 1- I 0.3-3.6 0.2-0.3 <0.1 <0.1
glass); (FRG; 6.3 3 II 0.1-0.5 <0.1 <0.1 <0.1
Netherland
Currants, black 2.5/? 2-7 I 2.4-48 2.0-16 2.0-6.3 0.9-5.1 0.5-1.4 0.2
(Denmark2/UK) II 0.5-3.2 1.1-1.5 0.5-1.0 0.4-0.7 0.2-0.3 0.3
Currants, red (FRG; 0.2- 3-5 I 5-39 3-23 2-46 1-40 0.3-3.2
Netherland3) 2.5 II 1.9-6.9 1.1-3.0 0.4-16 0.5-3.0 0.2-0.4
Gooseberries (UK) 5 I 0.3
II 0.2
Wheat, barley
(France) 1.3 1-2 I <0.1
Grapes (FRG4) 0.6- 2- I 0.2-48 0.2-33 0.7-10.6 <0.1-21 <0.1-16 1.3-11 <0.1-12
3.6 10 II <0.1-0.8 <0.1-3.8 <0.1-3.6 <0.1-2.7 <0.1-2.6 0.3-3.4 <0.1-3.8
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Hops (FRG) 2.0 9- I 69 6-140 5 0.3-0.7
17 II 14 3-11
Lettuce (outdoor) 0.6 1-4 I 7.8-30 0.2-2.7 0.1-0.2 <0.1-0.3 <0.1 <0.1
II 2.1-5.8 0.2-2.3 <0.1 <0.1-0.3 0.1-0.2 <0.1
Lettuce
(under glass) 0.6- 1-6 I 4.7-55 0.3-8.3 <0.1-1.9 <0.1-0.3 <0.1-5.6 0.6-2.2
(FRG; UK) 3.4 II 3.0-13 0.2-2.8 <0.1-2.5 <0.1 <0.1-1.6
Onions (UK) 1.1 5 I (100 days)
<0.1
Peaches (FRG) 1.1 3 I 2.2-2.9 0-7-1.2 0.3-1.6
II 0.5-0.8 0.2-1.4 0.5-0.9
Raspberries (FRG; 2.0- 1-4 I 21-32 6-14 <0.1-10.5 <0.1-4.7 1.7
Netherland 2.3 II 1.0-6.2 0.7-6.4 0.6-4.0 0.8-1.0 0.9
Strawberries
(outdoor) 0.8- 1-4 I 0.3-27 0.1-5.0 <0.1-6.3 <0.1-2.4 <0.1-1.6 <0.1-1.7 0.7-1.7
(FRG: Netherland 3.8 II 0.3-8.8 0.5-1.8 <0.1-3.7 0.2-2.4 0.3-1.9 <0.1-0.3 0.1
N.Z.6; Switzerland;
UK)
(Australia7) 1.4- 3 I + II 1.2-15.5 0.4-8.4 <0.1-3.2 <0.1-0.6 0.6 <0.1
2.1 expr. as I
Strawberries
(under glass) 1.2- 3-6 I 4-9 0.5-3.5 0.4-7.1 2.2-4.1 1.7-3.5 1.5-4.7 <0.1-3.6
(Netherland 9.5
(total)
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Tomatoes (FRG) 1-3 I <0.1 <0.1-0.2
II <0.1-0.2 <0.1-0.9
1 Bayer AG (1964-1973) unless otherwise stated
2 Government Plant Pathology Institute, Lyngby; National Pood Institute, Gladsaxe, April 20, 1970
3 Keuringsdienst van Waren Amsterdam (1967a, 1969a)
4 Lemperle et al. (1969, 1970); Bayer (1964-1973)
5 Keuringsdienst van Waren Amsterdam (1965)
6 Brewerton and Gibbs (1968)
7 Tate at al. (1973)
8 Keuringsdienst van Waren Amsterdam (1969b)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 4 shows the residues of dichlofluanid and its metabolite
dimethylphenylsulphamide (DMSA) found in crops after recommended
applications in supervised trials. The analyses were carried out by
Farbenfabriken Bayer AG and various institutes.
FATE OF RESIDUES
In storage and processing
Additional information on the fate of residues during processing,
including wine making, and on the effect of residues on fermentation,
has been received since the previous evaluation.
Analyses of commercial deep-frozen strawberries from two
factories (3 samples from each) showed residues of 0.26 - 0.36 and
0.06 - 0.12 mg/kg dichlofluanid. In canned strawberries residues were
<0.02 mg/kg dichlofluanid and DMSA could not be found (Grevenstuk,
1971).
Strawberries were treated with dichlofluanid at flowering and
analysed with and without caps (Government Plant Pathology Institute,
Lyngby, Denmark, 1969). Most of the dichlofluanid residue was in the
caps (Table 5).
TABLE 5. Residues of dichlofluanid and DMSA in strawberries with and
without caps (Denmark)
Rate of Waiting Residues (mg/kg)
application No. of Period With cap Without cap
(kg.a.i./ha) applications (days) Dichlofluanid DMSA Dichlofluanid DMSA
1.9 3 24 1.6 0.4 0.2 -
(treated 28 1.3 0.3 - -
at
flowering) 35 1.7 - 0.1 0.1
42 0.7 - 0.1 0.1
Apples containing residues of about 0.8 mg/kg dichlofluanid plus
0.2 mg/kg DMSA were cold pressed. The juice contained approx.
0.2 mg/kg dichlofluanid and approx. 0.7 mg/kg DMSA. After heating the
juice for 30 min. at 90°C the residues were 0.1 and 0.7 mg/kg
respectively. Processing the apples to stewed fruit, baby food or
jelly resulted in residues of approx. 0.05 mg/kg dichlofluanid and 0.3
- 0.7 mg/kg DMSA (Maier-Bode, 1972). Residues of 2 mg/kg dichlofluanid
plus 0.7 mg/kg DMSA on apples when stored at -18°C were stable for at
least 6 months (Bayer, 1973).
Several series of experiments were carried out to determine the
effect of wine-making processes on residues in grapes, and the effect
of dichlofluanid residues on fermentation.
At residues of 1.5 - 10.8 mg/kg dichlofluanid and 0.02 - 0.1
mg/kg DMSA on grapes the corresponding residues (mg/kg) in the marc
were <0.05 - 8.0 and 0.1 - 1.4; in must (not clarified) <0.1 - 6.9
and 0.1 - 1.3; in clarified must <0.05 - 0.2 and 0.3 - 0.8 and in
wine <0.05 and 0.1 - 4.0 (Lemperle et al., 1970). There is no direct
correlation between the residues on grapes and those in the processed
products.
In a further study residues up to 48 mg/kg dichlofluanid and 1.6
mg/kg DMSA on grapes resulted in corresponding residues (mg/kg) up to
6.9 and 4.5 mg/kg in must (not clarified), 0.4 and 2.3 mg/kg in
clarified must and <0.05 and 4.0 mg/kg in wine (Lemperle et al.,
1973).
In other experiments (Lemperle and Kerner, 1969; Lemperle et al.,
1970), grapes were treated two or three times at recommended
concentrations. Residues of dichlofluanid and DMSA were determined in
grapes harvested at intervals from 32 to 63 days after the last
application, and in the wine made from them. Residues in the grapes
(19 samples) ranged from 1.5 to 12 mg/kg dichlofluanid and 0.02 to 0.8
mg/kg DMSA. In the wine, dichlofluanid could not be detected in any of
the samples (limit of detection 0.02 - 0.05 mg/kg) and DMSA residues
ranged from 0.1 to 4.0 mg/kg.
Bayer (1967-1973) report trials in FRG in which grapes were
treated at various rates with dichlofluanid. The results (Table 6)
show residues of dichlofluanid from 0.02 - 5.4 mg/kg on the grapes
which have been eliminated during processing to wine. DMSA residues
are again carried through into the wine.
TABLE 6. Residues of dichlofluanid (I) and DMSA (II) on grapes
and in wine produced therefrom
Application Days after Residues, mg/kg*
Rate last Grapes Grapes
kg a.i./ha No. treatment I II I II
- 7 28 - - n.d. -
0.6 4 56 5.4 1.35 n.d. -
1.5 - 2.0 2 57 0.9 0.15 n.d. -
2.0 - 2.5 4 47 0.7 0.25 n.d. 0.2
2.0 - 2.5 5 35 0.4 0.6 n.d. 0.25
2.0 - 2.5 6 22 1.6 0.95 n.d. 0.4
3.0 - 5.0 3 34 3.9 0.2 n.d. n.d.
2.0 - 4.0 4 59 - - n.d. 2.4
3.6 2 60 - - n.d. n.d.
1.8 5 71 1.0 - 0.03 -
0.8 8 54 0.02 - n.d. -
* Limit of detection: dichlofluanid 0.02 mg/kg;
DMSA 0.1 mg/kg.
Fermentation experiments were conducted to determine the
concentration of dichlofluanid required to inhibit yeast growth. At 1
mg/l in must the fermentation was retarded and at > 1.5 mg/l it was
inhibited. The inhibitory effect of dichlofluanid on yeast was
abolished by cysteine, beta-mercapto-ethylamine and other
SH-compounds. Dichlofluanid can therefore be regarded as an
"SH-blocker." The inhibitory effect decreases with increasing pH. The
activity of most enzymes depends on free SH-groups which are part of
cysteine. Therefore the enzymes alcohol-dehydrogenase and
glycerinaldehyde-3-phosphate-dehydrogenase which contain essential
SH-groups were strongly inhibited in vitro and in vivo while maleic
dehydrogenase was not affected. The fluorodichloromethylthio
(-S-CCl2F)-part of the dichlofluanid molecule was shown to be the
inhibiting group (Dittrich and Issinger, 1969).
Retardation of the fermentation process can be overcome by
pre-clarification of the grape juice (must) and addition of about 1%
of a fermenting pure yeast strain preparation, or in the case of red
grape juice, by the addition of 2.5% pure yeast while still in contact
with the skins. In general the fermentation process seems to be less
influenced by dichlofluanid than by folpet. No differences between
wines originating from dichlofluanid-treated and non-treated vines
could be observed either analytically or by tasting (Lemperle et al.,
1970, 1973).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues in 52 samples of red currants examined in the
Netherlands were <0.1 mg/kg (Keuringsdienst van Waren, 1966). Of
276 samples of strawberries, 256 contained <0.1 mg/kg
dichlofluanid, 17 contained 0.1 - 1 mg/kg and 3 contained 1 mg/kg
(Keuringsdienst van Waren, 1966).
Results of food inspection reported in 1973 (Netherlands) are
shown in Table 7.
TABLE 7. Residues of dichlofluanid found during food inspection.
Netherlands, 1973
Number of samples in range
Range, red
mg/kg blackberries raspberries currants strawberries
0 - 0.1 15 1 23 257
>0.1-0.2 2 7 111
>0.2-0.5 7 3 11 215
>0.5-1 5 3 8 168
>1 - 2 4 2 5 134
>2 - 5* 2 4 2 58
>5 - 10 3 3
>10 1 (10.3
mg/kg)
Total 38 13 56 947
* The tolerance was 5 mg/kg in all cases.
METHODS OF RESIDUE ANALYSIS
A multiresidue GLC method, with electron-capture detection
(Becker, 1972/74), has been adopted by Deutsche Forschungsgemeinschaft
(DFG, 1974) for the determination of dichlofluanid. The method in
principle is based on the GLC method of Vogeler and Niessen (1967)
already mentioned in FAO/WHO, 1970. For stewed apples, pears, plums,
cherries, peaches, grapes, cabbage, carrots, green beans and lettuce
the recovery at the 0.1 mg/kg level is > 80%. For leeks, parsley and
celery the recovery is below 70%. The method is suitable for
regulatory purposes for dichlofluanid residues in a number of crops,
but further evaluation is desirable before it can be recommended
without reservation. It does not determine DMSA.
A similar analytical method has been elaborated, in which
isopropanol-benzene extracts of the crop are cleaned-up with
Nuchar-Attaclay. The method has been checked for many green
vegetables, tomatoes and fruit. Interferences have sometimes been
found for leek, onion, cabbage and carrots (Rijks Instituut voor de
Volksgesondheid, Bilthoven, 1972).
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances and safety intervals reported to the Meeting
are given in Table 8.
TABLE 8. National Tolerances for dichlofluanid reported
to the Meeting
Safety
interval Tolerance,
Count Crop (days) mg/kg
Australia General 14
Austria General 14
Belgium Fruit, vegetables 5.0
(excl. potatoes)
Strawberries (outdoor),
raspberries, mulberries 7
Strawberries
(under glass) 14
Currants 21
Bulgaria General 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
Denmark Fruit, including
strawberries 14
Finland General 14
Strawberries ( 4.0
Other fruit (proposed) ( 1.0
France General 7
Germany Small fruit (berry dichlofluanid ( 15.0
(FRG) fruit excluding + DMSA (
strawberries), calculated as (
grapes dichlofluanid (
(
Strawberries, (
lettuce, ( 10.0
(
all other fruit ( 5.0
(
Beans, cucumbers, (
tomatoes ( 3.0
(
Onions ( 1.0
Lettuce (field
grown) 21
Lettuce (under
glass) 28
Tomatoes (field
grown and under
glass) 3
Cucumbers (field
grown and under
glass) 3
Pome-fruit 7
Stone-and small
fruit (excluding
strawberries) 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
(Germany) Strawberries 10
Grapes 35
Italy Grapes 40
All other crops 20
Japan Cucumbers, tomatoes,
strawberries (under
glass) 1
Netherlands General 5.0
Tomatoes, peppers,
cucumbers, melons 3
Strawberries (outdoor),
blackberries,
raspberries 7
Strawberries 14
(underglass)
Currants 21
Kohlrabi 14
New Zealand Fruit and vegetables 5.0
General 14
Norway General 7
Poland Vegetables, fruit 14
Field grown crops
(excluding, potatoes,
beets) 14
Grapes 42
Portugal Strawberries 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
South Africa General 5.0
Table grapes 28 - 42
Wine grapes,
peaches, apricots,
plums and prunes 14
Spain Pome fruit, grapes
strawberries,
onions, vegetables 20
Sweden General 7
Switzerland Grapes 21 1.0
Apples, pears 21 0.5
Strawberries 21 7.0
United Apples, blackberries,
Kingdom red and white currants,
black currants, grapes
grown outdoors,
loganberries, autumn-sown
salad onions, outdoor
lettuce, leaf brassicas,
seedlings under glass,
lettuce under glass,
treated with dust
formulation 21
Strawberries, raspberries 14
Tomatoes under
glass, direct
spray on flowering
trusses 3
Yugoslavia Strawberries 14 2.0
+ 2.0 DMSA
APPRAISAL
The 1969 Joint Meeting, after reviewing the information on
dichlofluanid, concluded that further work was necessary, before
tolerances could be recommended. Some of the required information has
been supplied.
Data on the composition of technical dichlofluanid, including its
impurities have been presented. Dimethyl-N-phenylsulphamide (DMSA) is
known to be a metabolite, but detailed information on the pathway of
dichlofluanid degradation, especially on the fate of the
fluorine-containing moiety in plants, is not yet available. Studies
are known to be in progress, and results will presumably be available
to the 1976 Joint Meeting.
The biological activity of dichlofluanid and similar fungicides
(e.g. captan, folpet) is presumed to be localized in the
trihalogeno-methylthio part of the molecule (Kühle et al., 1964). The
inhibitory effect of dichlofluanid on yeast is antagonized by
SH-compounds. The enzymes alcohol dehydrogenase and
glycerinaldehyde-3-phosphate dehydrogenase and the co-factor co-enzyme
A which contain essential SH-groups are strongly inhibited in vitro
and/or in vivo (Lemperle et al., 1973). Dichlofluanid can therefore be
regarded as an "SH-blocker."
Processing of apples containing 0.8 mg/kg dichlofluanid plus 0.2
mg/kg DMSA to stewed fruit, baby food or jelly resulted in residues of
0.05 mg/kg dichlofluanid and 0.3 - 0.7 mg/kg DMSA. Cold pressed juice
contained 0.2 mg/kg and heated juice 0.1 mg/kg dichlofluanid plus 0.7
mg/kg DMSA.
Wine originating from grapes with residues up to 48 mg/kg
dichlofluanid contained up to 4 mg/kg DMSA and always less than 0.05
mg/kg dichlofluanid. During crushing and fermentation much of the
residue seems to be absorbed by the solid particles of the mash.
In addition to the crops mentioned in FAO/WHO, 1970 the
pre-harvest application of dichlofluanid has been extended to
kohlrabi, leaf brassicas, melons, paprika, blackberries, loganberries,
mulberries, raspberries, gooseberries, wheat and barley. In many cases
dichlofluanid can be used as a substitute for other fungicides, e.g.
dithiocarbamates and thiuram disulfides.
Numerous data on rates and frequencies of application,
pre-harvest intervals and the resulting residues of dichlofluanid and
the degradation product DMSA have been supplied from Australia,
Denmark, France, Germany, the Netherlands, Switzerland and the United
Kingdom. The residue data, especially for currants, raspberries,
strawberries and grapes, still show inconsistencies however. More data
from supervised trials and on raw agricultural products moving in
commerce are desirable for re-evaluating residue levels. Nevertheless
the data on maximum residues so far obtained from supervised trials
could be accommodated by the temporary tolerances recommended below,
which seem to be in accordance with the toxicologically permissible
level.
The tolerances should refer only to the parent compound. This
would substantially facilitate the determination of residues, because
a gas-chromatographic multiresidue method could be used. This method
is suitable for regulatory purposes in a range of crops, but further
evaluation is desirable. DMSA can only be determined by a colorimetric
method which is less suitable for regulatory purposes. The
determination of DMSA is not considered necessary because available
data indicate that excessive residues of this compound would be
accompanied by excessive residues of dichlofluanid.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
dichlofluanid only.
TEMPORARY TOLERANCES
dichlofluanid
(mg/kg)
Currants (red, black and white), grapes,
raspberries 15
Lettuce, strawberries 10
Apples, pears, cucumbers, peaches 5
Beans (green, including pods), cherries,
tomatoes 2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Studies on absorption, distribution in various organs, and
excretion of dichlofluanid in the rat.
2. Pharmacokinetics of the dichlorofluoromethylthio moiety.
DESIRABLE
1. Metabolism studies on dichlofluanid.
2. Results from current studies on the pathway of degradation,
especially the fate of the fluorine-containing moiety of the
molecule, in and on plants. These data are expected to be
available during 1975.
3. Further residue data from supervised trials, to resolve certain
inconsistencies in the existing data or to provide new
information, on blackberries, gooseberries, loganberries,
mulberries, raspberries, currants, hops, kohlrabi, leaf
brassicas, melons, onions, paprika and eventually wheat and
barley.
4. Further residue data for raw agricultural products moving in
commerce.
5. Further evaluation of the analytical method of Becker for
regulatory purposes.
REFERENCES
Bayer AG (1962). Product KU 13-032-C. Report prepared and submitted by
the Institute of Toxicology, Bayer AG. (Unpublished)
Bayer AG (1964). Report of 4-months feeding study on rats with active
ingredient 47531. Report prepared and submitted by the Institute of
Toxicology. Bayer AG. (Unpublished)
Bayer AG (1969). Dichlofluanid. Summary Report prepared and submitted
by Bayer AG. (Unpublished)
Bayer AG (1964, 1973). Unpublished results.
Becker (1972, 1974). Deutsche Forschungsgemeinschaft: Methodensammlung
zur Rückstandsanalytik von Pflanzenschutzmitteln; Dichlofluanid 203-1
and S 8 - 1; Verlag Chemie GmbH, Weinheim/Bergstr.; 1972 and 1974.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
residues on strawberries. N.Z. J. Agric. Res., 11:784-788.
Dittrich, H.H. and Issinger, O.G. (1969) Zum Wirkungsmechanismus von
Dichlofluanid (N,N-Dimethyl-N'-phenyl-[N'-Fluordichlormethylthio]
-Sulfamid). A. Pflanzenkr. Pflanzenschutz, 76 (11/12):651-663.
Dubois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Report submitted by Bayer AG. (Unpublished).
Dubois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Report submitted by Bayer AG.
(Unpublished)
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Report prepared and submitted by the Institute of Toxicology.
Bayer AG. (Unpublished)
FAO/WHO (1970). 1969 Evaluations of some pesticide residues in food.
FAO/PL/1969/M/17/1; WHO/Food Add./70.38.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1969). August 4. Unpublished data.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1970). April 20. Unpublished data.
Grevenstuk, W.B.F. (1971) Afname van residuën van bestrijdingsmiddelen
tijdens industriële verwerking II (aardbeien). Rijks Instituut voor de
Volksgezondheid, Utrecht, Netherlands. 64/71 Tox-Rob
Keuringsdienst van Waren Amsterdam (1965). Nr. 102 Residu's van thiram
en "5114" op aardbeien.
Keuringsdienst van Waren Utrecht (1966). Nr. 37 Residu's van thiram,
dichlofluanid, folpet en captan op zacht fruit.
Keuringsdienst van Waren Amsterdam (1967a). Nr. 118 Residu's van
fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1967). Nr. 122 Residu's van
dichlofluanid, thiram en folpet op aardbeien in 1965.
Keuringsdienst van Waren Amsterdam (1969a). Nr. 130 Ten Broeke,
Fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1969b). Nr. 131 Ten Broeke,
Fungiciden op aardbeien onder glas.
Kühle, E., Klauke, E. and Grewe, F. (1964).
Fluordichlormethylthio-Verbindungen und ihre Verwendung im
Pflanzenschutz. Angew. Chem., 7:807-816.
Lemperle, E. and Kerner, E. (1969). Wirkstoffrückstände und
Gärbeein-flussung nach Anwendung von Basfungin, Euparen und
Ortho-Phaltan im Weinbau. Wein-Wiss., 24 (10):357-371.
Lemperle, E., Kerner, E. and Strecker, H. (1970). Wirkstoffrückstände
und Gärverhalten nach Anwendung von Botryziden im Weinbau. Wein-Wiss.,
25 (9/10):313-325.
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstände und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Dtsch Lebensm. Rundsch, 69 (9):313-322.
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Report prepared and
submitted by the Institute of Toxicology, Bayer AG. (Unpublished)
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Report from the Institute of
Toxicology, Bayer AG. (Unpublished)
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Report from the Institute of Toxicology, Bayer AG. (Unpublished)
Löser, E. (1969a) Generationsversuche an Ratten. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Löser, E. (1969b) Chronische toxikologische Untersuchungen an Hunden.
Report from the Institute of Toxicology, submitted by Bayer AG.
(Unpublished)
Machemer, L. (1974a) Untersuchung auf emryotoxische und teratogene
Wirkung nach oraler Verabreichung an der Ratte. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Machemer, L. (1974b) Dominant-lethal-test an der männlichen Maus zur
Prüfung auf mutagene Wirkung. Report from the Institute of Toxicology,
submitted by Bayer AG. (Unpublished)
Maier-Bode, H. (1966, 1972) Pharmakologisches Institut, Bonn.
(Unpublished)
Mawdesley-Thomas, L.E. (1969) Pathology report of the chronic toxicity
of compound Bay 47531 in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished)
Mawdesley-Thomas, L.E., Newman, A.J. and Spicer, E.J.F. (1971).
Pathology report of Bay 47531 two year dog study. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished)
Nangniot, P., Vervier, R. and Martens, P.H. (1967). The use of
polarography to determine small amounts of fungicides or insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulphamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Rijks Instituut voor de Volksgezondheid, Bilthoven (1972). 109/72
Tox-Rob, Zuivering van plantenextracten met beheelp van actieve kool
voorafgaande aan de gaschromatografische bepaling van een aantal
bestrijdingsmiddelen.
Tate, K.G., van der Mespel, G.J., Levin, P.B., Brewerton, H.V., Gibbs,
M.M. and McGrath, H.J.W. (1973). Dichlofluanid ('Euparen') residues on
strawberries. N.Z. J. Exp. Agric., 1(1):100-104.
Vince, A.A. and Spicer, E.J.F. (1971). Pathology report of Bay 47531.
Generation experiment in rats. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished)
Vogeler, K. and Niessen, H. (1967). Kolorimetrische und
gaschromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz-Nach. Bayer, 20:534-549.
N-methyl-N'-(4-hydroxyphenyl)-sulphamide (IV)
All the metabolites are excreted in the free form and the
metabolites III and IV are also excreted as glucuronides. Metabolites
I and II can also be found in serum. The metabolites found in urine
and serum after oral administration of dichlofluanid are the same as
those found after oral administration of dimethylphenylsulphamide.
From these studies it is evident that dichlofluanid is absorbed only
very slightly from the G.I. tract (Eben and Kimmerle, 1968; Anon.,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield
N,N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
TOXICOLOGICAL STUDIES
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and
10 000 ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis gross and
microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ weights were comparable in the different
groups, except in the 10 000 ppm group where the female rats displayed
a decrease in the adrenal weight, and in both sexes an increase in the
liver weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported, such changes might
be camouflaged by the histological changes due to infections (Lorke,
1965).
Special studies on mutagenicity
Mouse
A dominant-lethal-test was carried out by administering 500 mg
dichlofluanid/kg bodyweight to twenty male mice. Untreated female mice
mated with three males did not show any difference in the early and
late resorptions, the number of corpora lutea or living and dead
foetuses when compared to those mated with control males. This study
at one relatively high dose level, did not reveal any dominant lethal
mutagenic effect of dichlofluanid (Machemer, 1974b).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 males and 20 females received
dietary levels of 0, 150, 500, 1500 and 4500 ppm of dichlofluanid
(90.2% pure; 8% inert material; 0.3% DMSA) for a period extending over
three generations. Two litters per generation were studied. No
information is available on the selection of animals from one
generation to the next. None of the dosed groups indicated any
abnormal effects on fertility throughout all three generations. The
groups fed 150, 500 and 1500 ppm displayed no abnormal effects
compared to controls with respect to group averages of birth weight,
litter-size and percentage of survival to weaning. Data for still
births and resorptions are not given in the report of this study. Only
in the group given 4500 ppm, the body weights of the young animals
were lower both at birth and at weaning in all generations except the
F1a at birth. The lactation index was also slightly reduced in one of
the six matings at this level. Although the experiment was not
designed to study teratological effects it may be noted that no
malformations were seen at any dose level (Löser, 1969a).
An additional study of the histology of organs of some animals of
the F3b generation showed no dose related effects of dichlofluanid
(Vince and Spicer, 1971).
Groups of 20-23 pregnant female rats were administered
dichlofluanid by stomach tube, on days 6 to 15 of gestation, at doses
of 30, 100, and 300 mg/kg/day. Symptoms of toxicity were observed in
all test groups and body-weight gains were decreased compared to the
controls both during the ten days treatment and during the total
gestation period. The effect was similar in all test groups and not
dose-dependent. The number of implantations, resorptions and foetuses
were within normal limits in all groups, whereas the average weight of
the placenta and the foetuses were slightly decreased in the groups
administered 300 mg/kg, possibly owing to the general toxic effect on
the maternal animals. In this study comprising only doses with
maternal toxicity no indication of teratogenic potential of
dichlofluanid was found (Machemer, 1974a).
Acute toxicity
TABLE 2. Acute toxicity of dichlofluanid
LD50mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and
Raymund, 1963
Mouse (M) i.p. 6.0 Dubois and
Raymund, 1963
Chicken oral >1000 Dubois, 1963
Rat (M) i.p. 15 Dubois and
Raymund, 1963
Rat (F) i.p. 15 Anon., 1962
DuBois and
Raymund, 1963
Rat (M) oral 500-1000 DuBois and
Raymund, 1963
Rat (F) oral 525 Anon., 1962
DuBois and
Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and
Raymund, 1963
Guinea-pig (M) oral 250 DuBois and
Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly
in a decrease in activity which began several hours after
administration of the compound. With doses around the LD50, death
occurred in one to four days (DuBois and Raymund, 1963).
Short-term studies
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300,
1000, 3000, and 10 000 ppm of dichlofluanid in their diets for four
months. Food consumption, growth, haematology, urinalysis, mortality,
and gross and microscopic pathology were closely comparable in the 30
to 3000 ppm experimental groups and the control groups. The same was
the case with final average body and organ weights except in the 3000
ppm group where the male rats displayed reduction of heart weights and
the female rats an increase in liver weights. In the rats fed 20 000
ppm there was a deterioration of general condition, decreased food
intake, smaller weight gains and higher mortality than the control
group. The male rats showed a decrease in heart weights, while in both
sexes there was a decrease in the weight of adrenal glands and an
increase in liver weights. Histopathological changes were found in the
liver (vacuolization, inflation in size and shrunken nuclei of some
cells), kidneys (increased protein precipitation in the proximal
tubuli) and the spleen (reduction of the lymphatic tissue) (Anon.,
1964).
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and
4500 ppm of dichlofluanid in their diets for four months. Behaviour,
food consumption, body-weight changes, mortality, function tests of
the liver and kidney, urinalysis, gross pathology and final average
body weights and average organ weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Groups of 4 male and 4 female dogs were fed 0, 100, 300, 1000 and
3000 ppm dichlofluanid (97.7% pure) in their diet for 2 years.
Behaviour, body weights, food consumption, mortality, blood and urine
analyses, liver and kidney function tests and absolute and relative
organ weights for the groups fed 100, 300 and 1000 ppm did not differ
from the values of these parameters in the controls. In the groups fed
3000 ppm increased mortality, reduced body weight and food consumption
and decreased absolute and relative testis weight were found.
Microscopic examination of the organs revealed an increase in
interstitial tissue of the testis and vacuolation and degeneration of
the adrenal cortex of the 3000 ppm group as the only dose-dependent
pathological changes (Löser, 1969b; Mawdesley-Thomas et al., 1971).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150,
500, 1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney weights, while both sexes showed elevated
relative liver weights, but normal enzyme function tests (Löser,
1968).
Histological examinations were made on tissues of five male and
five female rats at the end of the study and on animals that died
during the experiment. No significant pathological changes were found
at dietary levels including the 4 500 ppm level. A separate study of
all tumours found at autopsy was performed, and there was no
indication that dichlofluanid produced an increased tumour incidence
(Mawdesley-Thomas, 1969).
Comments
In a 2-year study in dogs a 1 000 ppm level of dichlofluanid in
the diet appears to have no untoward effect, although no data are
given on the individual animals.
A long term study in rats indicated 1 500 ppm in the feed as the
level without apparent toxic effect. There was no indication of
teratogenic effect when female rats were administered a dose as high
as 300 mg/kg bw. Reproduction parameters were not affected in a
3-generation feeding test with levels up to 1500 ppm dichlofluanid.
However, there is a lack of information on absorption, distribution,
excretion and pharmacokinetics on the dichloro-fluoromethylthio moiety
which may produce some carbon-fluoride compounds resistant to
cleavage. The Meeting allocated a temporary acceptable daily intake
for man based on the no-effect level in the dog, the more sensitive
species.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1 500 ppm in diet equivalent to 75 mg/kg bw.
Dog: 1 000 ppm, in diet equivalent to 25 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.3 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Uses (Table 3) are additional to those listed in FAO/WHO, 1970.
They are all recommendations of the Federal Republic of Germany (FRG).
TABLE 3. Pre-harvest treatments with dichlofluanid recommended
in the Federal Republic of Germany
Number of Rate of application
Crop applications (kg a.i./ha)
Pome fruits 4 - 11 1.5
Stone fruits
plums 4 1.5 - 2.0
cherries 3 - 8 2.0
peaches 3 1.5
Soft fruits
strawberries 1 - 6 2.5 - 3.0
red and black
currants 4 - 7 2.0 - 2.4
raspberries 1 -4 2.0 - 2.4
Grapes 2 - 10 1.9 - 2.5
Vegetables
cucumbers 1 - 4 1.5 - 2.0
tomatoes 1 - 3 1.5 - 2.0
lettuce (field grown
and under glass) 2 - 4 0.6 - 1.0
Beans 1 - 3 1.0
Hops 4 - 17 2.0
TABLE 4. Residues of dichlofluanid (I) and DSMA (II) resulting from supervised trials.
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Apples (FRG) 1.1- 4- I 0.7-3.4 0.2-2.8 <0.1-2.2 <0.1 <0.1-0.4 <0.1
1.25 11 II 0.3-1.1 <0.1-1.1 <0.1-1.1 <0.1-0.4 <0.1 <0.1
Beans green incl. 1.0- 1- I 0-1-0.3 0.1-0.2 <0.1-0.2 0.1
pods (FRG; UK) 3.4 3 II 0.1-0.2 0.1-0.2 <0.1 0.1
Cherries (morello) 1.1 6-8 I 0.6-2.5 <0.1-0.4 <0.1 <0.1
(FRG) II 0.2-1.2 <0.1-0.2 <0.1 <0.1
Cucumbers (outdoor); 1.5 3 I <0.1-0.2 <0.1-0.2 <0.1
(FRG) II 0.2-0.5 <0.1-0.3 <0.1
Cucumbers (under 1.5- 1- I 0.3-3.6 0.2-0.3 <0.1 <0.1
glass); (FRG; 6.3 3 II 0.1-0.5 <0.1 <0.1 <0.1
Netherland
Currants, black 2.5/? 2-7 I 2.4-48 2.0-16 2.0-6.3 0.9-5.1 0.5-1.4 0.2
(Denmark2/UK) II 0.5-3.2 1.1-1.5 0.5-1.0 0.4-0.7 0.2-0.3 0.3
Currants, red (FRG; 0.2- 3-5 I 5-39 3-23 2-46 1-40 0.3-3.2
Netherland3) 2.5 II 1.9-6.9 1.1-3.0 0.4-16 0.5-3.0 0.2-0.4
Gooseberries (UK) 5 I 0.3
II 0.2
Wheat, barley
(France) 1.3 1-2 I <0.1
Grapes (FRG4) 0.6- 2- I 0.2-48 0.2-33 0.7-10.6 <0.1-21 <0.1-16 1.3-11 <0.1-12
3.6 10 II <0.1-0.8 <0.1-3.8 <0.1-3.6 <0.1-2.7 <0.1-2.6 0.3-3.4 <0.1-3.8
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Hops (FRG) 2.0 9- I 69 6-140 5 0.3-0.7
17 II 14 3-11
Lettuce (outdoor) 0.6 1-4 I 7.8-30 0.2-2.7 0.1-0.2 <0.1-0.3 <0.1 <0.1
II 2.1-5.8 0.2-2.3 <0.1 <0.1-0.3 0.1-0.2 <0.1
Lettuce
(under glass) 0.6- 1-6 I 4.7-55 0.3-8.3 <0.1-1.9 <0.1-0.3 <0.1-5.6 0.6-2.2
(FRG; UK) 3.4 II 3.0-13 0.2-2.8 <0.1-2.5 <0.1 <0.1-1.6
Onions (UK) 1.1 5 I (100 days)
<0.1
Peaches (FRG) 1.1 3 I 2.2-2.9 0-7-1.2 0.3-1.6
II 0.5-0.8 0.2-1.4 0.5-0.9
Raspberries (FRG; 2.0- 1-4 I 21-32 6-14 <0.1-10.5 <0.1-4.7 1.7
Netherland 2.3 II 1.0-6.2 0.7-6.4 0.6-4.0 0.8-1.0 0.9
Strawberries
(outdoor) 0.8- 1-4 I 0.3-27 0.1-5.0 <0.1-6.3 <0.1-2.4 <0.1-1.6 <0.1-1.7 0.7-1.7
(FRG: Netherland 3.8 II 0.3-8.8 0.5-1.8 <0.1-3.7 0.2-2.4 0.3-1.9 <0.1-0.3 0.1
N.Z.6; Switzerland;
UK)
(Australia7) 1.4- 3 I + II 1.2-15.5 0.4-8.4 <0.1-3.2 <0.1-0.6 0.6 <0.1
2.1 expr. as I
Strawberries
(under glass) 1.2- 3-6 I 4-9 0.5-3.5 0.4-7.1 2.2-4.1 1.7-3.5 1.5-4.7 <0.1-3.6
(Netherland 9.5
(total)
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Tomatoes (FRG) 1-3 I <0.1 <0.1-0.2
II <0.1-0.2 <0.1-0.9
1 Bayer AG (1964-1973) unless otherwise stated
2 Government Plant Pathology Institute, Lyngby; National Pood Institute, Gladsaxe, April 20, 1970
3 Keuringsdienst van Waren Amsterdam (1967a, 1969a)
4 Lemperle et al. (1969, 1970); Bayer (1964-1973)
5 Keuringsdienst van Waren Amsterdam (1965)
6 Brewerton and Gibbs (1968)
7 Tate at al. (1973)
8 Keuringsdienst van Waren Amsterdam (1969b)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 4 shows the residues of dichlofluanid and its metabolite
dimethylphenylsulphamide (DMSA) found in crops after recommended
applications in supervised trials. The analyses were carried out by
Farbenfabriken Bayer AG and various institutes.
FATE OF RESIDUES
In storage and processing
Additional information on the fate of residues during processing,
including wine making, and on the effect of residues on fermentation,
has been received since the previous evaluation.
Analyses of commercial deep-frozen strawberries from two
factories (3 samples from each) showed residues of 0.26 - 0.36 and
0.06 - 0.12 mg/kg dichlofluanid. In canned strawberries residues were
<0.02 mg/kg dichlofluanid and DMSA could not be found (Grevenstuk,
1971).
Strawberries were treated with dichlofluanid at flowering and
analysed with and without caps (Government Plant Pathology Institute,
Lyngby, Denmark, 1969). Most of the dichlofluanid residue was in the
caps (Table 5).
TABLE 5. Residues of dichlofluanid and DMSA in strawberries with and
without caps (Denmark)
Rate of Waiting Residues (mg/kg)
application No. of Period With cap Without cap
(kg.a.i./ha) applications (days) Dichlofluanid DMSA Dichlofluanid DMSA
1.9 3 24 1.6 0.4 0.2 -
(treated 28 1.3 0.3 - -
at
flowering) 35 1.7 - 0.1 0.1
42 0.7 - 0.1 0.1
Apples containing residues of about 0.8 mg/kg dichlofluanid plus
0.2 mg/kg DMSA were cold pressed. The juice contained approx.
0.2 mg/kg dichlofluanid and approx. 0.7 mg/kg DMSA. After heating the
juice for 30 min. at 90°C the residues were 0.1 and 0.7 mg/kg
respectively. Processing the apples to stewed fruit, baby food or
jelly resulted in residues of approx. 0.05 mg/kg dichlofluanid and 0.3
- 0.7 mg/kg DMSA (Maier-Bode, 1972). Residues of 2 mg/kg dichlofluanid
plus 0.7 mg/kg DMSA on apples when stored at -18°C were stable for at
least 6 months (Bayer, 1973).
Several series of experiments were carried out to determine the
effect of wine-making processes on residues in grapes, and the effect
of dichlofluanid residues on fermentation.
At residues of 1.5 - 10.8 mg/kg dichlofluanid and 0.02 - 0.1
mg/kg DMSA on grapes the corresponding residues (mg/kg) in the marc
were <0.05 - 8.0 and 0.1 - 1.4; in must (not clarified) <0.1 - 6.9
and 0.1 - 1.3; in clarified must <0.05 - 0.2 and 0.3 - 0.8 and in
wine <0.05 and 0.1 - 4.0 (Lemperle et al., 1970). There is no direct
correlation between the residues on grapes and those in the processed
products.
In a further study residues up to 48 mg/kg dichlofluanid and 1.6
mg/kg DMSA on grapes resulted in corresponding residues (mg/kg) up to
6.9 and 4.5 mg/kg in must (not clarified), 0.4 and 2.3 mg/kg in
clarified must and <0.05 and 4.0 mg/kg in wine (Lemperle et al.,
1973).
In other experiments (Lemperle and Kerner, 1969; Lemperle et al.,
1970), grapes were treated two or three times at recommended
concentrations. Residues of dichlofluanid and DMSA were determined in
grapes harvested at intervals from 32 to 63 days after the last
application, and in the wine made from them. Residues in the grapes
(19 samples) ranged from 1.5 to 12 mg/kg dichlofluanid and 0.02 to 0.8
mg/kg DMSA. In the wine, dichlofluanid could not be detected in any of
the samples (limit of detection 0.02 - 0.05 mg/kg) and DMSA residues
ranged from 0.1 to 4.0 mg/kg.
Bayer (1967-1973) report trials in FRG in which grapes were
treated at various rates with dichlofluanid. The results (Table 6)
show residues of dichlofluanid from 0.02 - 5.4 mg/kg on the grapes
which have been eliminated during processing to wine. DMSA residues
are again carried through into the wine.
TABLE 6. Residues of dichlofluanid (I) and DMSA (II) on grapes
and in wine produced therefrom
Application Days after Residues, mg/kg*
Rate last Grapes Grapes
kg a.i./ha No. treatment I II I II
- 7 28 - - n.d. -
0.6 4 56 5.4 1.35 n.d. -
1.5 - 2.0 2 57 0.9 0.15 n.d. -
2.0 - 2.5 4 47 0.7 0.25 n.d. 0.2
2.0 - 2.5 5 35 0.4 0.6 n.d. 0.25
2.0 - 2.5 6 22 1.6 0.95 n.d. 0.4
3.0 - 5.0 3 34 3.9 0.2 n.d. n.d.
2.0 - 4.0 4 59 - - n.d. 2.4
3.6 2 60 - - n.d. n.d.
1.8 5 71 1.0 - 0.03 -
0.8 8 54 0.02 - n.d. -
* Limit of detection: dichlofluanid 0.02 mg/kg;
DMSA 0.1 mg/kg.
Fermentation experiments were conducted to determine the
concentration of dichlofluanid required to inhibit yeast growth. At 1
mg/l in must the fermentation was retarded and at > 1.5 mg/l it was
inhibited. The inhibitory effect of dichlofluanid on yeast was
abolished by cysteine, beta-mercapto-ethylamine and other
SH-compounds. Dichlofluanid can therefore be regarded as an
"SH-blocker." The inhibitory effect decreases with increasing pH. The
activity of most enzymes depends on free SH-groups which are part of
cysteine. Therefore the enzymes alcohol-dehydrogenase and
glycerinaldehyde-3-phosphate-dehydrogenase which contain essential
SH-groups were strongly inhibited in vitro and in vivo while maleic
dehydrogenase was not affected. The fluorodichloromethylthio
(-S-CCl2F)-part of the dichlofluanid molecule was shown to be the
inhibiting group (Dittrich and Issinger, 1969).
Retardation of the fermentation process can be overcome by
pre-clarification of the grape juice (must) and addition of about 1%
of a fermenting pure yeast strain preparation, or in the case of red
grape juice, by the addition of 2.5% pure yeast while still in contact
with the skins. In general the fermentation process seems to be less
influenced by dichlofluanid than by folpet. No differences between
wines originating from dichlofluanid-treated and non-treated vines
could be observed either analytically or by tasting (Lemperle et al.,
1970, 1973).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues in 52 samples of red currants examined in the
Netherlands were <0.1 mg/kg (Keuringsdienst van Waren, 1966). Of
276 samples of strawberries, 256 contained <0.1 mg/kg
dichlofluanid, 17 contained 0.1 - 1 mg/kg and 3 contained 1 mg/kg
(Keuringsdienst van Waren, 1966).
Results of food inspection reported in 1973 (Netherlands) are
shown in Table 7.
TABLE 7. Residues of dichlofluanid found during food inspection.
Netherlands, 1973
Number of samples in range
Range, red
mg/kg blackberries raspberries currants strawberries
0 - 0.1 15 1 23 257
>0.1-0.2 2 7 111
>0.2-0.5 7 3 11 215
>0.5-1 5 3 8 168
>1 - 2 4 2 5 134
>2 - 5* 2 4 2 58
>5 - 10 3 3
>10 1 (10.3
mg/kg)
Total 38 13 56 947
* The tolerance was 5 mg/kg in all cases.
METHODS OF RESIDUE ANALYSIS
A multiresidue GLC method, with electron-capture detection
(Becker, 1972/74), has been adopted by Deutsche Forschungsgemeinschaft
(DFG, 1974) for the determination of dichlofluanid. The method in
principle is based on the GLC method of Vogeler and Niessen (1967)
already mentioned in FAO/WHO, 1970. For stewed apples, pears, plums,
cherries, peaches, grapes, cabbage, carrots, green beans and lettuce
the recovery at the 0.1 mg/kg level is > 80%. For leeks, parsley and
celery the recovery is below 70%. The method is suitable for
regulatory purposes for dichlofluanid residues in a number of crops,
but further evaluation is desirable before it can be recommended
without reservation. It does not determine DMSA.
A similar analytical method has been elaborated, in which
isopropanol-benzene extracts of the crop are cleaned-up with
Nuchar-Attaclay. The method has been checked for many green
vegetables, tomatoes and fruit. Interferences have sometimes been
found for leek, onion, cabbage and carrots (Rijks Instituut voor de
Volksgesondheid, Bilthoven, 1972).
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances and safety intervals reported to the Meeting
are given in Table 8.
TABLE 8. National Tolerances for dichlofluanid reported
to the Meeting
Safety
interval Tolerance,
Count Crop (days) mg/kg
Australia General 14
Austria General 14
Belgium Fruit, vegetables 5.0
(excl. potatoes)
Strawberries (outdoor),
raspberries, mulberries 7
Strawberries
(under glass) 14
Currants 21
Bulgaria General 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
Denmark Fruit, including
strawberries 14
Finland General 14
Strawberries ( 4.0
Other fruit (proposed) ( 1.0
France General 7
Germany Small fruit (berry dichlofluanid ( 15.0
(FRG) fruit excluding + DMSA (
strawberries), calculated as (
grapes dichlofluanid (
(
Strawberries, (
lettuce, ( 10.0
(
all other fruit ( 5.0
(
Beans, cucumbers, (
tomatoes ( 3.0
(
Onions ( 1.0
Lettuce (field
grown) 21
Lettuce (under
glass) 28
Tomatoes (field
grown and under
glass) 3
Cucumbers (field
grown and under
glass) 3
Pome-fruit 7
Stone-and small
fruit (excluding
strawberries) 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
(Germany) Strawberries 10
Grapes 35
Italy Grapes 40
All other crops 20
Japan Cucumbers, tomatoes,
strawberries (under
glass) 1
Netherlands General 5.0
Tomatoes, peppers,
cucumbers, melons 3
Strawberries (outdoor),
blackberries,
raspberries 7
Strawberries 14
(underglass)
Currants 21
Kohlrabi 14
New Zealand Fruit and vegetables 5.0
General 14
Norway General 7
Poland Vegetables, fruit 14
Field grown crops
(excluding, potatoes,
beets) 14
Grapes 42
Portugal Strawberries 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
South Africa General 5.0
Table grapes 28 - 42
Wine grapes,
peaches, apricots,
plums and prunes 14
Spain Pome fruit, grapes
strawberries,
onions, vegetables 20
Sweden General 7
Switzerland Grapes 21 1.0
Apples, pears 21 0.5
Strawberries 21 7.0
United Apples, blackberries,
Kingdom red and white currants,
black currants, grapes
grown outdoors,
loganberries, autumn-sown
salad onions, outdoor
lettuce, leaf brassicas,
seedlings under glass,
lettuce under glass,
treated with dust
formulation 21
Strawberries, raspberries 14
Tomatoes under
glass, direct
spray on flowering
trusses 3
Yugoslavia Strawberries 14 2.0
+ 2.0 DMSA
APPRAISAL
The 1969 Joint Meeting, after reviewing the information on
dichlofluanid, concluded that further work was necessary, before
tolerances could be recommended. Some of the required information has
been supplied.
Data on the composition of technical dichlofluanid, including its
impurities have been presented. Dimethyl-N-phenylsulphamide (DMSA) is
known to be a metabolite, but detailed information on the pathway of
dichlofluanid degradation, especially on the fate of the
fluorine-containing moiety in plants, is not yet available. Studies
are known to be in progress, and results will presumably be available
to the 1976 Joint Meeting.
The biological activity of dichlofluanid and similar fungicides
(e.g. captan, folpet) is presumed to be localized in the
trihalogeno-methylthio part of the molecule (Kühle et al., 1964). The
inhibitory effect of dichlofluanid on yeast is antagonized by
SH-compounds. The enzymes alcohol dehydrogenase and
glycerinaldehyde-3-phosphate dehydrogenase and the co-factor co-enzyme
A which contain essential SH-groups are strongly inhibited in vitro
and/or in vivo (Lemperle et al., 1973). Dichlofluanid can therefore be
regarded as an "SH-blocker."
Processing of apples containing 0.8 mg/kg dichlofluanid plus 0.2
mg/kg DMSA to stewed fruit, baby food or jelly resulted in residues of
0.05 mg/kg dichlofluanid and 0.3 - 0.7 mg/kg DMSA. Cold pressed juice
contained 0.2 mg/kg and heated juice 0.1 mg/kg dichlofluanid plus 0.7
mg/kg DMSA.
Wine originating from grapes with residues up to 48 mg/kg
dichlofluanid contained up to 4 mg/kg DMSA and always less than 0.05
mg/kg dichlofluanid. During crushing and fermentation much of the
residue seems to be absorbed by the solid particles of the mash.
In addition to the crops mentioned in FAO/WHO, 1970 the
pre-harvest application of dichlofluanid has been extended to
kohlrabi, leaf brassicas, melons, paprika, blackberries, loganberries,
mulberries, raspberries, gooseberries, wheat and barley. In many cases
dichlofluanid can be used as a substitute for other fungicides, e.g.
dithiocarbamates and thiuram disulfides.
Numerous data on rates and frequencies of application,
pre-harvest intervals and the resulting residues of dichlofluanid and
the degradation product DMSA have been supplied from Australia,
Denmark, France, Germany, the Netherlands, Switzerland and the United
Kingdom. The residue data, especially for currants, raspberries,
strawberries and grapes, still show inconsistencies however. More data
from supervised trials and on raw agricultural products moving in
commerce are desirable for re-evaluating residue levels. Nevertheless
the data on maximum residues so far obtained from supervised trials
could be accommodated by the temporary tolerances recommended below,
which seem to be in accordance with the toxicologically permissible
level.
The tolerances should refer only to the parent compound. This
would substantially facilitate the determination of residues, because
a gas-chromatographic multiresidue method could be used. This method
is suitable for regulatory purposes in a range of crops, but further
evaluation is desirable. DMSA can only be determined by a colorimetric
method which is less suitable for regulatory purposes. The
determination of DMSA is not considered necessary because available
data indicate that excessive residues of this compound would be
accompanied by excessive residues of dichlofluanid.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
dichlofluanid only.
TEMPORARY TOLERANCES
dichlofluanid
(mg/kg)
Currants (red, black and white), grapes,
raspberries 15
Lettuce, strawberries 10
Apples, pears, cucumbers, peaches 5
Beans (green, including pods), cherries,
tomatoes 2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Studies on absorption, distribution in various organs, and
excretion of dichlofluanid in the rat.
2. Pharmacokinetics of the dichlorofluoromethylthio moiety.
DESIRABLE
1. Metabolism studies on dichlofluanid.
2. Results from current studies on the pathway of degradation,
especially the fate of the fluorine-containing moiety of the
molecule, in and on plants. These data are expected to be
available during 1975.
3. Further residue data from supervised trials, to resolve certain
inconsistencies in the existing data or to provide new
information, on blackberries, gooseberries, loganberries,
mulberries, raspberries, currants, hops, kohlrabi, leaf
brassicas, melons, onions, paprika and eventually wheat and
barley.
4. Further residue data for raw agricultural products moving in
commerce.
5. Further evaluation of the analytical method of Becker for
regulatory purposes.
REFERENCES
Bayer AG (1962). Product KU 13-032-C. Report prepared and submitted by
the Institute of Toxicology, Bayer AG. (Unpublished)
Bayer AG (1964). Report of 4-months feeding study on rats with active
ingredient 47531. Report prepared and submitted by the Institute of
Toxicology. Bayer AG. (Unpublished)
Bayer AG (1969). Dichlofluanid. Summary Report prepared and submitted
by Bayer AG. (Unpublished)
Bayer AG (1964, 1973). Unpublished results.
Becker (1972, 1974). Deutsche Forschungsgemeinschaft: Methodensammlung
zur Rückstandsanalytik von Pflanzenschutzmitteln; Dichlofluanid 203-1
and S 8 - 1; Verlag Chemie GmbH, Weinheim/Bergstr.; 1972 and 1974.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
residues on strawberries. N.Z. J. Agric. Res., 11:784-788.
Dittrich, H.H. and Issinger, O.G. (1969) Zum Wirkungsmechanismus von
Dichlofluanid (N,N-Dimethyl-N'-phenyl-[N'-Fluordichlormethylthio]
-Sulfamid). A. Pflanzenkr. Pflanzenschutz, 76 (11/12):651-663.
Dubois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Report submitted by Bayer AG. (Unpublished).
Dubois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Report submitted by Bayer AG.
(Unpublished)
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Report prepared and submitted by the Institute of Toxicology.
Bayer AG. (Unpublished)
FAO/WHO (1970). 1969 Evaluations of some pesticide residues in food.
FAO/PL/1969/M/17/1; WHO/Food Add./70.38.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1969). August 4. Unpublished data.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1970). April 20. Unpublished data.
Grevenstuk, W.B.F. (1971) Afname van residuën van bestrijdingsmiddelen
tijdens industriële verwerking II (aardbeien). Rijks Instituut voor de
Volksgezondheid, Utrecht, Netherlands. 64/71 Tox-Rob
Keuringsdienst van Waren Amsterdam (1965). Nr. 102 Residu's van thiram
en "5114" op aardbeien.
Keuringsdienst van Waren Utrecht (1966). Nr. 37 Residu's van thiram,
dichlofluanid, folpet en captan op zacht fruit.
Keuringsdienst van Waren Amsterdam (1967a). Nr. 118 Residu's van
fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1967). Nr. 122 Residu's van
dichlofluanid, thiram en folpet op aardbeien in 1965.
Keuringsdienst van Waren Amsterdam (1969a). Nr. 130 Ten Broeke,
Fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1969b). Nr. 131 Ten Broeke,
Fungiciden op aardbeien onder glas.
Kühle, E., Klauke, E. and Grewe, F. (1964).
Fluordichlormethylthio-Verbindungen und ihre Verwendung im
Pflanzenschutz. Angew. Chem., 7:807-816.
Lemperle, E. and Kerner, E. (1969). Wirkstoffrückstände und
Gärbeein-flussung nach Anwendung von Basfungin, Euparen und
Ortho-Phaltan im Weinbau. Wein-Wiss., 24 (10):357-371.
Lemperle, E., Kerner, E. and Strecker, H. (1970). Wirkstoffrückstände
und Gärverhalten nach Anwendung von Botryziden im Weinbau. Wein-Wiss.,
25 (9/10):313-325.
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstände und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Dtsch Lebensm. Rundsch, 69 (9):313-322.
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Report prepared and
submitted by the Institute of Toxicology, Bayer AG. (Unpublished)
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Report from the Institute of
Toxicology, Bayer AG. (Unpublished)
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Report from the Institute of Toxicology, Bayer AG. (Unpublished)
Löser, E. (1969a) Generationsversuche an Ratten. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Löser, E. (1969b) Chronische toxikologische Untersuchungen an Hunden.
Report from the Institute of Toxicology, submitted by Bayer AG.
(Unpublished)
Machemer, L. (1974a) Untersuchung auf emryotoxische und teratogene
Wirkung nach oraler Verabreichung an der Ratte. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Machemer, L. (1974b) Dominant-lethal-test an der männlichen Maus zur
Prüfung auf mutagene Wirkung. Report from the Institute of Toxicology,
submitted by Bayer AG. (Unpublished)
Maier-Bode, H. (1966, 1972) Pharmakologisches Institut, Bonn.
(Unpublished)
Mawdesley-Thomas, L.E. (1969) Pathology report of the chronic toxicity
of compound Bay 47531 in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished)
Mawdesley-Thomas, L.E., Newman, A.J. and Spicer, E.J.F. (1971).
Pathology report of Bay 47531 two year dog study. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished)
Nangniot, P., Vervier, R. and Martens, P.H. (1967). The use of
polarography to determine small amounts of fungicides or insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulphamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Rijks Instituut voor de Volksgezondheid, Bilthoven (1972). 109/72
Tox-Rob, Zuivering van plantenextracten met beheelp van actieve kool
voorafgaande aan de gaschromatografische bepaling van een aantal
bestrijdingsmiddelen.
Tate, K.G., van der Mespel, G.J., Levin, P.B., Brewerton, H.V., Gibbs,
M.M. and McGrath, H.J.W. (1973). Dichlofluanid ('Euparen') residues on
strawberries. N.Z. J. Exp. Agric., 1(1):100-104.
Vince, A.A. and Spicer, E.J.F. (1971). Pathology report of Bay 47531.
Generation experiment in rats. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished)
Vogeler, K. and Niessen, H. (1967). Kolorimetrische und
gaschromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz-Nach. Bayer, 20:534-549.
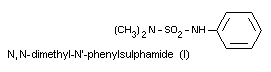 N,N-dimethyl-N'-phenylsulphamide (I)
N,N-dimethyl-N'-phenylsulphamide (I)
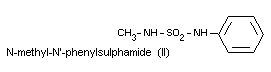 N-methyl-N'-phenylsulphamide (II)
N-methyl-N'-phenylsulphamide (II)
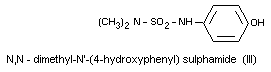 N,N-dimethyl-N'-(4-hydroxyphenyl) sulphamide (III)
N,N-dimethyl-N'-(4-hydroxyphenyl) sulphamide (III)
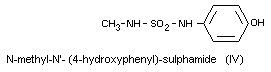 N-methyl-N'-(4-hydroxyphenyl)-sulphamide (IV)
All the metabolites are excreted in the free form and the
metabolites III and IV are also excreted as glucuronides. Metabolites
I and II can also be found in serum. The metabolites found in urine
and serum after oral administration of dichlofluanid are the same as
those found after oral administration of dimethylphenylsulphamide.
From these studies it is evident that dichlofluanid is absorbed only
very slightly from the G.I. tract (Eben and Kimmerle, 1968; Anon.,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield
N,N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
TOXICOLOGICAL STUDIES
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and
10 000 ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis gross and
microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ weights were comparable in the different
groups, except in the 10 000 ppm group where the female rats displayed
a decrease in the adrenal weight, and in both sexes an increase in the
liver weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported, such changes might
be camouflaged by the histological changes due to infections (Lorke,
1965).
Special studies on mutagenicity
Mouse
A dominant-lethal-test was carried out by administering 500 mg
dichlofluanid/kg bodyweight to twenty male mice. Untreated female mice
mated with three males did not show any difference in the early and
late resorptions, the number of corpora lutea or living and dead
foetuses when compared to those mated with control males. This study
at one relatively high dose level, did not reveal any dominant lethal
mutagenic effect of dichlofluanid (Machemer, 1974b).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 males and 20 females received
dietary levels of 0, 150, 500, 1500 and 4500 ppm of dichlofluanid
(90.2% pure; 8% inert material; 0.3% DMSA) for a period extending over
three generations. Two litters per generation were studied. No
information is available on the selection of animals from one
generation to the next. None of the dosed groups indicated any
abnormal effects on fertility throughout all three generations. The
groups fed 150, 500 and 1500 ppm displayed no abnormal effects
compared to controls with respect to group averages of birth weight,
litter-size and percentage of survival to weaning. Data for still
births and resorptions are not given in the report of this study. Only
in the group given 4500 ppm, the body weights of the young animals
were lower both at birth and at weaning in all generations except the
F1a at birth. The lactation index was also slightly reduced in one of
the six matings at this level. Although the experiment was not
designed to study teratological effects it may be noted that no
malformations were seen at any dose level (Löser, 1969a).
An additional study of the histology of organs of some animals of
the F3b generation showed no dose related effects of dichlofluanid
(Vince and Spicer, 1971).
Groups of 20-23 pregnant female rats were administered
dichlofluanid by stomach tube, on days 6 to 15 of gestation, at doses
of 30, 100, and 300 mg/kg/day. Symptoms of toxicity were observed in
all test groups and body-weight gains were decreased compared to the
controls both during the ten days treatment and during the total
gestation period. The effect was similar in all test groups and not
dose-dependent. The number of implantations, resorptions and foetuses
were within normal limits in all groups, whereas the average weight of
the placenta and the foetuses were slightly decreased in the groups
administered 300 mg/kg, possibly owing to the general toxic effect on
the maternal animals. In this study comprising only doses with
maternal toxicity no indication of teratogenic potential of
dichlofluanid was found (Machemer, 1974a).
Acute toxicity
TABLE 2. Acute toxicity of dichlofluanid
LD50mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and
Raymund, 1963
Mouse (M) i.p. 6.0 Dubois and
Raymund, 1963
Chicken oral >1000 Dubois, 1963
Rat (M) i.p. 15 Dubois and
Raymund, 1963
Rat (F) i.p. 15 Anon., 1962
DuBois and
Raymund, 1963
Rat (M) oral 500-1000 DuBois and
Raymund, 1963
Rat (F) oral 525 Anon., 1962
DuBois and
Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and
Raymund, 1963
Guinea-pig (M) oral 250 DuBois and
Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly
in a decrease in activity which began several hours after
administration of the compound. With doses around the LD50, death
occurred in one to four days (DuBois and Raymund, 1963).
Short-term studies
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300,
1000, 3000, and 10 000 ppm of dichlofluanid in their diets for four
months. Food consumption, growth, haematology, urinalysis, mortality,
and gross and microscopic pathology were closely comparable in the 30
to 3000 ppm experimental groups and the control groups. The same was
the case with final average body and organ weights except in the 3000
ppm group where the male rats displayed reduction of heart weights and
the female rats an increase in liver weights. In the rats fed 20 000
ppm there was a deterioration of general condition, decreased food
intake, smaller weight gains and higher mortality than the control
group. The male rats showed a decrease in heart weights, while in both
sexes there was a decrease in the weight of adrenal glands and an
increase in liver weights. Histopathological changes were found in the
liver (vacuolization, inflation in size and shrunken nuclei of some
cells), kidneys (increased protein precipitation in the proximal
tubuli) and the spleen (reduction of the lymphatic tissue) (Anon.,
1964).
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and
4500 ppm of dichlofluanid in their diets for four months. Behaviour,
food consumption, body-weight changes, mortality, function tests of
the liver and kidney, urinalysis, gross pathology and final average
body weights and average organ weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Groups of 4 male and 4 female dogs were fed 0, 100, 300, 1000 and
3000 ppm dichlofluanid (97.7% pure) in their diet for 2 years.
Behaviour, body weights, food consumption, mortality, blood and urine
analyses, liver and kidney function tests and absolute and relative
organ weights for the groups fed 100, 300 and 1000 ppm did not differ
from the values of these parameters in the controls. In the groups fed
3000 ppm increased mortality, reduced body weight and food consumption
and decreased absolute and relative testis weight were found.
Microscopic examination of the organs revealed an increase in
interstitial tissue of the testis and vacuolation and degeneration of
the adrenal cortex of the 3000 ppm group as the only dose-dependent
pathological changes (Löser, 1969b; Mawdesley-Thomas et al., 1971).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150,
500, 1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney weights, while both sexes showed elevated
relative liver weights, but normal enzyme function tests (Löser,
1968).
Histological examinations were made on tissues of five male and
five female rats at the end of the study and on animals that died
during the experiment. No significant pathological changes were found
at dietary levels including the 4 500 ppm level. A separate study of
all tumours found at autopsy was performed, and there was no
indication that dichlofluanid produced an increased tumour incidence
(Mawdesley-Thomas, 1969).
Comments
In a 2-year study in dogs a 1 000 ppm level of dichlofluanid in
the diet appears to have no untoward effect, although no data are
given on the individual animals.
A long term study in rats indicated 1 500 ppm in the feed as the
level without apparent toxic effect. There was no indication of
teratogenic effect when female rats were administered a dose as high
as 300 mg/kg bw. Reproduction parameters were not affected in a
3-generation feeding test with levels up to 1500 ppm dichlofluanid.
However, there is a lack of information on absorption, distribution,
excretion and pharmacokinetics on the dichloro-fluoromethylthio moiety
which may produce some carbon-fluoride compounds resistant to
cleavage. The Meeting allocated a temporary acceptable daily intake
for man based on the no-effect level in the dog, the more sensitive
species.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1 500 ppm in diet equivalent to 75 mg/kg bw.
Dog: 1 000 ppm, in diet equivalent to 25 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.3 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Uses (Table 3) are additional to those listed in FAO/WHO, 1970.
They are all recommendations of the Federal Republic of Germany (FRG).
TABLE 3. Pre-harvest treatments with dichlofluanid recommended
in the Federal Republic of Germany
Number of Rate of application
Crop applications (kg a.i./ha)
Pome fruits 4 - 11 1.5
Stone fruits
plums 4 1.5 - 2.0
cherries 3 - 8 2.0
peaches 3 1.5
Soft fruits
strawberries 1 - 6 2.5 - 3.0
red and black
currants 4 - 7 2.0 - 2.4
raspberries 1 -4 2.0 - 2.4
Grapes 2 - 10 1.9 - 2.5
Vegetables
cucumbers 1 - 4 1.5 - 2.0
tomatoes 1 - 3 1.5 - 2.0
lettuce (field grown
and under glass) 2 - 4 0.6 - 1.0
Beans 1 - 3 1.0
Hops 4 - 17 2.0
TABLE 4. Residues of dichlofluanid (I) and DSMA (II) resulting from supervised trials.
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Apples (FRG) 1.1- 4- I 0.7-3.4 0.2-2.8 <0.1-2.2 <0.1 <0.1-0.4 <0.1
1.25 11 II 0.3-1.1 <0.1-1.1 <0.1-1.1 <0.1-0.4 <0.1 <0.1
Beans green incl. 1.0- 1- I 0-1-0.3 0.1-0.2 <0.1-0.2 0.1
pods (FRG; UK) 3.4 3 II 0.1-0.2 0.1-0.2 <0.1 0.1
Cherries (morello) 1.1 6-8 I 0.6-2.5 <0.1-0.4 <0.1 <0.1
(FRG) II 0.2-1.2 <0.1-0.2 <0.1 <0.1
Cucumbers (outdoor); 1.5 3 I <0.1-0.2 <0.1-0.2 <0.1
(FRG) II 0.2-0.5 <0.1-0.3 <0.1
Cucumbers (under 1.5- 1- I 0.3-3.6 0.2-0.3 <0.1 <0.1
glass); (FRG; 6.3 3 II 0.1-0.5 <0.1 <0.1 <0.1
Netherland
Currants, black 2.5/? 2-7 I 2.4-48 2.0-16 2.0-6.3 0.9-5.1 0.5-1.4 0.2
(Denmark2/UK) II 0.5-3.2 1.1-1.5 0.5-1.0 0.4-0.7 0.2-0.3 0.3
Currants, red (FRG; 0.2- 3-5 I 5-39 3-23 2-46 1-40 0.3-3.2
Netherland3) 2.5 II 1.9-6.9 1.1-3.0 0.4-16 0.5-3.0 0.2-0.4
Gooseberries (UK) 5 I 0.3
II 0.2
Wheat, barley
(France) 1.3 1-2 I <0.1
Grapes (FRG4) 0.6- 2- I 0.2-48 0.2-33 0.7-10.6 <0.1-21 <0.1-16 1.3-11 <0.1-12
3.6 10 II <0.1-0.8 <0.1-3.8 <0.1-3.6 <0.1-2.7 <0.1-2.6 0.3-3.4 <0.1-3.8
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Hops (FRG) 2.0 9- I 69 6-140 5 0.3-0.7
17 II 14 3-11
Lettuce (outdoor) 0.6 1-4 I 7.8-30 0.2-2.7 0.1-0.2 <0.1-0.3 <0.1 <0.1
II 2.1-5.8 0.2-2.3 <0.1 <0.1-0.3 0.1-0.2 <0.1
Lettuce
(under glass) 0.6- 1-6 I 4.7-55 0.3-8.3 <0.1-1.9 <0.1-0.3 <0.1-5.6 0.6-2.2
(FRG; UK) 3.4 II 3.0-13 0.2-2.8 <0.1-2.5 <0.1 <0.1-1.6
Onions (UK) 1.1 5 I (100 days)
<0.1
Peaches (FRG) 1.1 3 I 2.2-2.9 0-7-1.2 0.3-1.6
II 0.5-0.8 0.2-1.4 0.5-0.9
Raspberries (FRG; 2.0- 1-4 I 21-32 6-14 <0.1-10.5 <0.1-4.7 1.7
Netherland 2.3 II 1.0-6.2 0.7-6.4 0.6-4.0 0.8-1.0 0.9
Strawberries
(outdoor) 0.8- 1-4 I 0.3-27 0.1-5.0 <0.1-6.3 <0.1-2.4 <0.1-1.6 <0.1-1.7 0.7-1.7
(FRG: Netherland 3.8 II 0.3-8.8 0.5-1.8 <0.1-3.7 0.2-2.4 0.3-1.9 <0.1-0.3 0.1
N.Z.6; Switzerland;
UK)
(Australia7) 1.4- 3 I + II 1.2-15.5 0.4-8.4 <0.1-3.2 <0.1-0.6 0.6 <0.1
2.1 expr. as I
Strawberries
(under glass) 1.2- 3-6 I 4-9 0.5-3.5 0.4-7.1 2.2-4.1 1.7-3.5 1.5-4.7 <0.1-3.6
(Netherland 9.5
(total)
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Tomatoes (FRG) 1-3 I <0.1 <0.1-0.2
II <0.1-0.2 <0.1-0.9
1 Bayer AG (1964-1973) unless otherwise stated
2 Government Plant Pathology Institute, Lyngby; National Pood Institute, Gladsaxe, April 20, 1970
3 Keuringsdienst van Waren Amsterdam (1967a, 1969a)
4 Lemperle et al. (1969, 1970); Bayer (1964-1973)
5 Keuringsdienst van Waren Amsterdam (1965)
6 Brewerton and Gibbs (1968)
7 Tate at al. (1973)
8 Keuringsdienst van Waren Amsterdam (1969b)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 4 shows the residues of dichlofluanid and its metabolite
dimethylphenylsulphamide (DMSA) found in crops after recommended
applications in supervised trials. The analyses were carried out by
Farbenfabriken Bayer AG and various institutes.
FATE OF RESIDUES
In storage and processing
Additional information on the fate of residues during processing,
including wine making, and on the effect of residues on fermentation,
has been received since the previous evaluation.
Analyses of commercial deep-frozen strawberries from two
factories (3 samples from each) showed residues of 0.26 - 0.36 and
0.06 - 0.12 mg/kg dichlofluanid. In canned strawberries residues were
<0.02 mg/kg dichlofluanid and DMSA could not be found (Grevenstuk,
1971).
Strawberries were treated with dichlofluanid at flowering and
analysed with and without caps (Government Plant Pathology Institute,
Lyngby, Denmark, 1969). Most of the dichlofluanid residue was in the
caps (Table 5).
TABLE 5. Residues of dichlofluanid and DMSA in strawberries with and
without caps (Denmark)
Rate of Waiting Residues (mg/kg)
application No. of Period With cap Without cap
(kg.a.i./ha) applications (days) Dichlofluanid DMSA Dichlofluanid DMSA
1.9 3 24 1.6 0.4 0.2 -
(treated 28 1.3 0.3 - -
at
flowering) 35 1.7 - 0.1 0.1
42 0.7 - 0.1 0.1
Apples containing residues of about 0.8 mg/kg dichlofluanid plus
0.2 mg/kg DMSA were cold pressed. The juice contained approx.
0.2 mg/kg dichlofluanid and approx. 0.7 mg/kg DMSA. After heating the
juice for 30 min. at 90°C the residues were 0.1 and 0.7 mg/kg
respectively. Processing the apples to stewed fruit, baby food or
jelly resulted in residues of approx. 0.05 mg/kg dichlofluanid and 0.3
- 0.7 mg/kg DMSA (Maier-Bode, 1972). Residues of 2 mg/kg dichlofluanid
plus 0.7 mg/kg DMSA on apples when stored at -18°C were stable for at
least 6 months (Bayer, 1973).
Several series of experiments were carried out to determine the
effect of wine-making processes on residues in grapes, and the effect
of dichlofluanid residues on fermentation.
At residues of 1.5 - 10.8 mg/kg dichlofluanid and 0.02 - 0.1
mg/kg DMSA on grapes the corresponding residues (mg/kg) in the marc
were <0.05 - 8.0 and 0.1 - 1.4; in must (not clarified) <0.1 - 6.9
and 0.1 - 1.3; in clarified must <0.05 - 0.2 and 0.3 - 0.8 and in
wine <0.05 and 0.1 - 4.0 (Lemperle et al., 1970). There is no direct
correlation between the residues on grapes and those in the processed
products.
In a further study residues up to 48 mg/kg dichlofluanid and 1.6
mg/kg DMSA on grapes resulted in corresponding residues (mg/kg) up to
6.9 and 4.5 mg/kg in must (not clarified), 0.4 and 2.3 mg/kg in
clarified must and <0.05 and 4.0 mg/kg in wine (Lemperle et al.,
1973).
In other experiments (Lemperle and Kerner, 1969; Lemperle et al.,
1970), grapes were treated two or three times at recommended
concentrations. Residues of dichlofluanid and DMSA were determined in
grapes harvested at intervals from 32 to 63 days after the last
application, and in the wine made from them. Residues in the grapes
(19 samples) ranged from 1.5 to 12 mg/kg dichlofluanid and 0.02 to 0.8
mg/kg DMSA. In the wine, dichlofluanid could not be detected in any of
the samples (limit of detection 0.02 - 0.05 mg/kg) and DMSA residues
ranged from 0.1 to 4.0 mg/kg.
Bayer (1967-1973) report trials in FRG in which grapes were
treated at various rates with dichlofluanid. The results (Table 6)
show residues of dichlofluanid from 0.02 - 5.4 mg/kg on the grapes
which have been eliminated during processing to wine. DMSA residues
are again carried through into the wine.
TABLE 6. Residues of dichlofluanid (I) and DMSA (II) on grapes
and in wine produced therefrom
Application Days after Residues, mg/kg*
Rate last Grapes Grapes
kg a.i./ha No. treatment I II I II
- 7 28 - - n.d. -
0.6 4 56 5.4 1.35 n.d. -
1.5 - 2.0 2 57 0.9 0.15 n.d. -
2.0 - 2.5 4 47 0.7 0.25 n.d. 0.2
2.0 - 2.5 5 35 0.4 0.6 n.d. 0.25
2.0 - 2.5 6 22 1.6 0.95 n.d. 0.4
3.0 - 5.0 3 34 3.9 0.2 n.d. n.d.
2.0 - 4.0 4 59 - - n.d. 2.4
3.6 2 60 - - n.d. n.d.
1.8 5 71 1.0 - 0.03 -
0.8 8 54 0.02 - n.d. -
* Limit of detection: dichlofluanid 0.02 mg/kg;
DMSA 0.1 mg/kg.
Fermentation experiments were conducted to determine the
concentration of dichlofluanid required to inhibit yeast growth. At 1
mg/l in must the fermentation was retarded and at > 1.5 mg/l it was
inhibited. The inhibitory effect of dichlofluanid on yeast was
abolished by cysteine, beta-mercapto-ethylamine and other
SH-compounds. Dichlofluanid can therefore be regarded as an
"SH-blocker." The inhibitory effect decreases with increasing pH. The
activity of most enzymes depends on free SH-groups which are part of
cysteine. Therefore the enzymes alcohol-dehydrogenase and
glycerinaldehyde-3-phosphate-dehydrogenase which contain essential
SH-groups were strongly inhibited in vitro and in vivo while maleic
dehydrogenase was not affected. The fluorodichloromethylthio
(-S-CCl2F)-part of the dichlofluanid molecule was shown to be the
inhibiting group (Dittrich and Issinger, 1969).
Retardation of the fermentation process can be overcome by
pre-clarification of the grape juice (must) and addition of about 1%
of a fermenting pure yeast strain preparation, or in the case of red
grape juice, by the addition of 2.5% pure yeast while still in contact
with the skins. In general the fermentation process seems to be less
influenced by dichlofluanid than by folpet. No differences between
wines originating from dichlofluanid-treated and non-treated vines
could be observed either analytically or by tasting (Lemperle et al.,
1970, 1973).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues in 52 samples of red currants examined in the
Netherlands were <0.1 mg/kg (Keuringsdienst van Waren, 1966). Of
276 samples of strawberries, 256 contained <0.1 mg/kg
dichlofluanid, 17 contained 0.1 - 1 mg/kg and 3 contained 1 mg/kg
(Keuringsdienst van Waren, 1966).
Results of food inspection reported in 1973 (Netherlands) are
shown in Table 7.
TABLE 7. Residues of dichlofluanid found during food inspection.
Netherlands, 1973
Number of samples in range
Range, red
mg/kg blackberries raspberries currants strawberries
0 - 0.1 15 1 23 257
>0.1-0.2 2 7 111
>0.2-0.5 7 3 11 215
>0.5-1 5 3 8 168
>1 - 2 4 2 5 134
>2 - 5* 2 4 2 58
>5 - 10 3 3
>10 1 (10.3
mg/kg)
Total 38 13 56 947
* The tolerance was 5 mg/kg in all cases.
METHODS OF RESIDUE ANALYSIS
A multiresidue GLC method, with electron-capture detection
(Becker, 1972/74), has been adopted by Deutsche Forschungsgemeinschaft
(DFG, 1974) for the determination of dichlofluanid. The method in
principle is based on the GLC method of Vogeler and Niessen (1967)
already mentioned in FAO/WHO, 1970. For stewed apples, pears, plums,
cherries, peaches, grapes, cabbage, carrots, green beans and lettuce
the recovery at the 0.1 mg/kg level is > 80%. For leeks, parsley and
celery the recovery is below 70%. The method is suitable for
regulatory purposes for dichlofluanid residues in a number of crops,
but further evaluation is desirable before it can be recommended
without reservation. It does not determine DMSA.
A similar analytical method has been elaborated, in which
isopropanol-benzene extracts of the crop are cleaned-up with
Nuchar-Attaclay. The method has been checked for many green
vegetables, tomatoes and fruit. Interferences have sometimes been
found for leek, onion, cabbage and carrots (Rijks Instituut voor de
Volksgesondheid, Bilthoven, 1972).
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances and safety intervals reported to the Meeting
are given in Table 8.
TABLE 8. National Tolerances for dichlofluanid reported
to the Meeting
Safety
interval Tolerance,
Count Crop (days) mg/kg
Australia General 14
Austria General 14
Belgium Fruit, vegetables 5.0
(excl. potatoes)
Strawberries (outdoor),
raspberries, mulberries 7
Strawberries
(under glass) 14
Currants 21
Bulgaria General 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
Denmark Fruit, including
strawberries 14
Finland General 14
Strawberries ( 4.0
Other fruit (proposed) ( 1.0
France General 7
Germany Small fruit (berry dichlofluanid ( 15.0
(FRG) fruit excluding + DMSA (
strawberries), calculated as (
grapes dichlofluanid (
(
Strawberries, (
lettuce, ( 10.0
(
all other fruit ( 5.0
(
Beans, cucumbers, (
tomatoes ( 3.0
(
Onions ( 1.0
Lettuce (field
grown) 21
Lettuce (under
glass) 28
Tomatoes (field
grown and under
glass) 3
Cucumbers (field
grown and under
glass) 3
Pome-fruit 7
Stone-and small
fruit (excluding
strawberries) 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
(Germany) Strawberries 10
Grapes 35
Italy Grapes 40
All other crops 20
Japan Cucumbers, tomatoes,
strawberries (under
glass) 1
Netherlands General 5.0
Tomatoes, peppers,
cucumbers, melons 3
Strawberries (outdoor),
blackberries,
raspberries 7
Strawberries 14
(underglass)
Currants 21
Kohlrabi 14
New Zealand Fruit and vegetables 5.0
General 14
Norway General 7
Poland Vegetables, fruit 14
Field grown crops
(excluding, potatoes,
beets) 14
Grapes 42
Portugal Strawberries 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
South Africa General 5.0
Table grapes 28 - 42
Wine grapes,
peaches, apricots,
plums and prunes 14
Spain Pome fruit, grapes
strawberries,
onions, vegetables 20
Sweden General 7
Switzerland Grapes 21 1.0
Apples, pears 21 0.5
Strawberries 21 7.0
United Apples, blackberries,
Kingdom red and white currants,
black currants, grapes
grown outdoors,
loganberries, autumn-sown
salad onions, outdoor
lettuce, leaf brassicas,
seedlings under glass,
lettuce under glass,
treated with dust
formulation 21
Strawberries, raspberries 14
Tomatoes under
glass, direct
spray on flowering
trusses 3
Yugoslavia Strawberries 14 2.0
+ 2.0 DMSA
APPRAISAL
The 1969 Joint Meeting, after reviewing the information on
dichlofluanid, concluded that further work was necessary, before
tolerances could be recommended. Some of the required information has
been supplied.
Data on the composition of technical dichlofluanid, including its
impurities have been presented. Dimethyl-N-phenylsulphamide (DMSA) is
known to be a metabolite, but detailed information on the pathway of
dichlofluanid degradation, especially on the fate of the
fluorine-containing moiety in plants, is not yet available. Studies
are known to be in progress, and results will presumably be available
to the 1976 Joint Meeting.
The biological activity of dichlofluanid and similar fungicides
(e.g. captan, folpet) is presumed to be localized in the
trihalogeno-methylthio part of the molecule (Kühle et al., 1964). The
inhibitory effect of dichlofluanid on yeast is antagonized by
SH-compounds. The enzymes alcohol dehydrogenase and
glycerinaldehyde-3-phosphate dehydrogenase and the co-factor co-enzyme
A which contain essential SH-groups are strongly inhibited in vitro
and/or in vivo (Lemperle et al., 1973). Dichlofluanid can therefore be
regarded as an "SH-blocker."
Processing of apples containing 0.8 mg/kg dichlofluanid plus 0.2
mg/kg DMSA to stewed fruit, baby food or jelly resulted in residues of
0.05 mg/kg dichlofluanid and 0.3 - 0.7 mg/kg DMSA. Cold pressed juice
contained 0.2 mg/kg and heated juice 0.1 mg/kg dichlofluanid plus 0.7
mg/kg DMSA.
Wine originating from grapes with residues up to 48 mg/kg
dichlofluanid contained up to 4 mg/kg DMSA and always less than 0.05
mg/kg dichlofluanid. During crushing and fermentation much of the
residue seems to be absorbed by the solid particles of the mash.
In addition to the crops mentioned in FAO/WHO, 1970 the
pre-harvest application of dichlofluanid has been extended to
kohlrabi, leaf brassicas, melons, paprika, blackberries, loganberries,
mulberries, raspberries, gooseberries, wheat and barley. In many cases
dichlofluanid can be used as a substitute for other fungicides, e.g.
dithiocarbamates and thiuram disulfides.
Numerous data on rates and frequencies of application,
pre-harvest intervals and the resulting residues of dichlofluanid and
the degradation product DMSA have been supplied from Australia,
Denmark, France, Germany, the Netherlands, Switzerland and the United
Kingdom. The residue data, especially for currants, raspberries,
strawberries and grapes, still show inconsistencies however. More data
from supervised trials and on raw agricultural products moving in
commerce are desirable for re-evaluating residue levels. Nevertheless
the data on maximum residues so far obtained from supervised trials
could be accommodated by the temporary tolerances recommended below,
which seem to be in accordance with the toxicologically permissible
level.
The tolerances should refer only to the parent compound. This
would substantially facilitate the determination of residues, because
a gas-chromatographic multiresidue method could be used. This method
is suitable for regulatory purposes in a range of crops, but further
evaluation is desirable. DMSA can only be determined by a colorimetric
method which is less suitable for regulatory purposes. The
determination of DMSA is not considered necessary because available
data indicate that excessive residues of this compound would be
accompanied by excessive residues of dichlofluanid.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
dichlofluanid only.
TEMPORARY TOLERANCES
dichlofluanid
(mg/kg)
Currants (red, black and white), grapes,
raspberries 15
Lettuce, strawberries 10
Apples, pears, cucumbers, peaches 5
Beans (green, including pods), cherries,
tomatoes 2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Studies on absorption, distribution in various organs, and
excretion of dichlofluanid in the rat.
2. Pharmacokinetics of the dichlorofluoromethylthio moiety.
DESIRABLE
1. Metabolism studies on dichlofluanid.
2. Results from current studies on the pathway of degradation,
especially the fate of the fluorine-containing moiety of the
molecule, in and on plants. These data are expected to be
available during 1975.
3. Further residue data from supervised trials, to resolve certain
inconsistencies in the existing data or to provide new
information, on blackberries, gooseberries, loganberries,
mulberries, raspberries, currants, hops, kohlrabi, leaf
brassicas, melons, onions, paprika and eventually wheat and
barley.
4. Further residue data for raw agricultural products moving in
commerce.
5. Further evaluation of the analytical method of Becker for
regulatory purposes.
REFERENCES
Bayer AG (1962). Product KU 13-032-C. Report prepared and submitted by
the Institute of Toxicology, Bayer AG. (Unpublished)
Bayer AG (1964). Report of 4-months feeding study on rats with active
ingredient 47531. Report prepared and submitted by the Institute of
Toxicology. Bayer AG. (Unpublished)
Bayer AG (1969). Dichlofluanid. Summary Report prepared and submitted
by Bayer AG. (Unpublished)
Bayer AG (1964, 1973). Unpublished results.
Becker (1972, 1974). Deutsche Forschungsgemeinschaft: Methodensammlung
zur Rückstandsanalytik von Pflanzenschutzmitteln; Dichlofluanid 203-1
and S 8 - 1; Verlag Chemie GmbH, Weinheim/Bergstr.; 1972 and 1974.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
residues on strawberries. N.Z. J. Agric. Res., 11:784-788.
Dittrich, H.H. and Issinger, O.G. (1969) Zum Wirkungsmechanismus von
Dichlofluanid (N,N-Dimethyl-N'-phenyl-[N'-Fluordichlormethylthio]
-Sulfamid). A. Pflanzenkr. Pflanzenschutz, 76 (11/12):651-663.
Dubois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Report submitted by Bayer AG. (Unpublished).
Dubois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Report submitted by Bayer AG.
(Unpublished)
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Report prepared and submitted by the Institute of Toxicology.
Bayer AG. (Unpublished)
FAO/WHO (1970). 1969 Evaluations of some pesticide residues in food.
FAO/PL/1969/M/17/1; WHO/Food Add./70.38.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1969). August 4. Unpublished data.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1970). April 20. Unpublished data.
Grevenstuk, W.B.F. (1971) Afname van residuën van bestrijdingsmiddelen
tijdens industriële verwerking II (aardbeien). Rijks Instituut voor de
Volksgezondheid, Utrecht, Netherlands. 64/71 Tox-Rob
Keuringsdienst van Waren Amsterdam (1965). Nr. 102 Residu's van thiram
en "5114" op aardbeien.
Keuringsdienst van Waren Utrecht (1966). Nr. 37 Residu's van thiram,
dichlofluanid, folpet en captan op zacht fruit.
Keuringsdienst van Waren Amsterdam (1967a). Nr. 118 Residu's van
fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1967). Nr. 122 Residu's van
dichlofluanid, thiram en folpet op aardbeien in 1965.
Keuringsdienst van Waren Amsterdam (1969a). Nr. 130 Ten Broeke,
Fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1969b). Nr. 131 Ten Broeke,
Fungiciden op aardbeien onder glas.
Kühle, E., Klauke, E. and Grewe, F. (1964).
Fluordichlormethylthio-Verbindungen und ihre Verwendung im
Pflanzenschutz. Angew. Chem., 7:807-816.
Lemperle, E. and Kerner, E. (1969). Wirkstoffrückstände und
Gärbeein-flussung nach Anwendung von Basfungin, Euparen und
Ortho-Phaltan im Weinbau. Wein-Wiss., 24 (10):357-371.
Lemperle, E., Kerner, E. and Strecker, H. (1970). Wirkstoffrückstände
und Gärverhalten nach Anwendung von Botryziden im Weinbau. Wein-Wiss.,
25 (9/10):313-325.
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstände und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Dtsch Lebensm. Rundsch, 69 (9):313-322.
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Report prepared and
submitted by the Institute of Toxicology, Bayer AG. (Unpublished)
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Report from the Institute of
Toxicology, Bayer AG. (Unpublished)
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Report from the Institute of Toxicology, Bayer AG. (Unpublished)
Löser, E. (1969a) Generationsversuche an Ratten. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Löser, E. (1969b) Chronische toxikologische Untersuchungen an Hunden.
Report from the Institute of Toxicology, submitted by Bayer AG.
(Unpublished)
Machemer, L. (1974a) Untersuchung auf emryotoxische und teratogene
Wirkung nach oraler Verabreichung an der Ratte. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Machemer, L. (1974b) Dominant-lethal-test an der männlichen Maus zur
Prüfung auf mutagene Wirkung. Report from the Institute of Toxicology,
submitted by Bayer AG. (Unpublished)
Maier-Bode, H. (1966, 1972) Pharmakologisches Institut, Bonn.
(Unpublished)
Mawdesley-Thomas, L.E. (1969) Pathology report of the chronic toxicity
of compound Bay 47531 in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished)
Mawdesley-Thomas, L.E., Newman, A.J. and Spicer, E.J.F. (1971).
Pathology report of Bay 47531 two year dog study. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished)
Nangniot, P., Vervier, R. and Martens, P.H. (1967). The use of
polarography to determine small amounts of fungicides or insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulphamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Rijks Instituut voor de Volksgezondheid, Bilthoven (1972). 109/72
Tox-Rob, Zuivering van plantenextracten met beheelp van actieve kool
voorafgaande aan de gaschromatografische bepaling van een aantal
bestrijdingsmiddelen.
Tate, K.G., van der Mespel, G.J., Levin, P.B., Brewerton, H.V., Gibbs,
M.M. and McGrath, H.J.W. (1973). Dichlofluanid ('Euparen') residues on
strawberries. N.Z. J. Exp. Agric., 1(1):100-104.
Vince, A.A. and Spicer, E.J.F. (1971). Pathology report of Bay 47531.
Generation experiment in rats. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished)
Vogeler, K. and Niessen, H. (1967). Kolorimetrische und
gaschromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz-Nach. Bayer, 20:534-549.
N-methyl-N'-(4-hydroxyphenyl)-sulphamide (IV)
All the metabolites are excreted in the free form and the
metabolites III and IV are also excreted as glucuronides. Metabolites
I and II can also be found in serum. The metabolites found in urine
and serum after oral administration of dichlofluanid are the same as
those found after oral administration of dimethylphenylsulphamide.
From these studies it is evident that dichlofluanid is absorbed only
very slightly from the G.I. tract (Eben and Kimmerle, 1968; Anon.,
1969).
In the studies on the metabolism of dichlofluanid there is no
information on the fate of the dichlorofluoromethylthio portion of the
molecule, and it is not known if the fluorine atom appears ultimately
as fluoride ion. However, alkaline hydrolysis of dichlofluanid in
methanol solution is reported to yield
N,N-dimethyl-N'-phenylsulphamide and fluorodichloromethanethiol
(Cl2FCSH) which is susceptible to oxidation. No information is given
on the nature of the oxidation products (Nangniot et al., 1967).
TOXICOLOGICAL STUDIES
Special studies on the metabolite, N,N-dimethyl-N'-phenylsulphamide
Rat
Groups of 30 rats (15 of each sex) were fed 0, 1000, 3000, and
10 000 ppm of dimethylphenylsulphamide in their diets for four months.
Mortality, food consumption, growth, haematology, urinalysis gross and
microscopic pathology (10 animals from each group) were closely
comparable in the experimental and the control groups. Also the final
average body and organ weights were comparable in the different
groups, except in the 10 000 ppm group where the female rats displayed
a decrease in the adrenal weight, and in both sexes an increase in the
liver weight was found. Although no histopathological changes in the
liver and the kidney of these rats were reported, such changes might
be camouflaged by the histological changes due to infections (Lorke,
1965).
Special studies on mutagenicity
Mouse
A dominant-lethal-test was carried out by administering 500 mg
dichlofluanid/kg bodyweight to twenty male mice. Untreated female mice
mated with three males did not show any difference in the early and
late resorptions, the number of corpora lutea or living and dead
foetuses when compared to those mated with control males. This study
at one relatively high dose level, did not reveal any dominant lethal
mutagenic effect of dichlofluanid (Machemer, 1974b).
Special studies on reproduction
Rat
Groups of rats, each comprising 10 males and 20 females received
dietary levels of 0, 150, 500, 1500 and 4500 ppm of dichlofluanid
(90.2% pure; 8% inert material; 0.3% DMSA) for a period extending over
three generations. Two litters per generation were studied. No
information is available on the selection of animals from one
generation to the next. None of the dosed groups indicated any
abnormal effects on fertility throughout all three generations. The
groups fed 150, 500 and 1500 ppm displayed no abnormal effects
compared to controls with respect to group averages of birth weight,
litter-size and percentage of survival to weaning. Data for still
births and resorptions are not given in the report of this study. Only
in the group given 4500 ppm, the body weights of the young animals
were lower both at birth and at weaning in all generations except the
F1a at birth. The lactation index was also slightly reduced in one of
the six matings at this level. Although the experiment was not
designed to study teratological effects it may be noted that no
malformations were seen at any dose level (Löser, 1969a).
An additional study of the histology of organs of some animals of
the F3b generation showed no dose related effects of dichlofluanid
(Vince and Spicer, 1971).
Groups of 20-23 pregnant female rats were administered
dichlofluanid by stomach tube, on days 6 to 15 of gestation, at doses
of 30, 100, and 300 mg/kg/day. Symptoms of toxicity were observed in
all test groups and body-weight gains were decreased compared to the
controls both during the ten days treatment and during the total
gestation period. The effect was similar in all test groups and not
dose-dependent. The number of implantations, resorptions and foetuses
were within normal limits in all groups, whereas the average weight of
the placenta and the foetuses were slightly decreased in the groups
administered 300 mg/kg, possibly owing to the general toxic effect on
the maternal animals. In this study comprising only doses with
maternal toxicity no indication of teratogenic potential of
dichlofluanid was found (Machemer, 1974a).
Acute toxicity
TABLE 2. Acute toxicity of dichlofluanid
LD50mg/kg
Animal Route body-weight References
Mouse (F) i.p. 7.8 DuBois and
Raymund, 1963
Mouse (M) i.p. 6.0 Dubois and
Raymund, 1963
Chicken oral >1000 Dubois, 1963
Rat (M) i.p. 15 Dubois and
Raymund, 1963
Rat (F) i.p. 15 Anon., 1962
DuBois and
Raymund, 1963
Rat (M) oral 500-1000 DuBois and
Raymund, 1963
Rat (F) oral 525 Anon., 1962
DuBois and
Raymund, 1963
Guinea-pig (M) i.p. 35 DuBois and
Raymund, 1963
Guinea-pig (M) oral 250 DuBois and
Raymund, 1963
The symptoms of poisoning were non-typical and consisted mainly
in a decrease in activity which began several hours after
administration of the compound. With doses around the LD50, death
occurred in one to four days (DuBois and Raymund, 1963).
Short-term studies
Rat
Groups of 30 rats (15 of each sex) were fed 0, 30, 100, 300,
1000, 3000, and 10 000 ppm of dichlofluanid in their diets for four
months. Food consumption, growth, haematology, urinalysis, mortality,
and gross and microscopic pathology were closely comparable in the 30
to 3000 ppm experimental groups and the control groups. The same was
the case with final average body and organ weights except in the 3000
ppm group where the male rats displayed reduction of heart weights and
the female rats an increase in liver weights. In the rats fed 20 000
ppm there was a deterioration of general condition, decreased food
intake, smaller weight gains and higher mortality than the control
group. The male rats showed a decrease in heart weights, while in both
sexes there was a decrease in the weight of adrenal glands and an
increase in liver weights. Histopathological changes were found in the
liver (vacuolization, inflation in size and shrunken nuclei of some
cells), kidneys (increased protein precipitation in the proximal
tubuli) and the spleen (reduction of the lymphatic tissue) (Anon.,
1964).
Dog
Groups of four dogs (two of each sex) were fed 0, 500, 1500, and
4500 ppm of dichlofluanid in their diets for four months. Behaviour,
food consumption, body-weight changes, mortality, function tests of
the liver and kidney, urinalysis, gross pathology and final average
body weights and average organ weights were closely comparable in the
500 ppm and the control group. The same was the case with the male
dogs of the 1500 ppm group while in the females changes in
liver-function tests and decrease in body weight pointed at an
impaired liver-function. Three of the four dogs fed 4500 ppm of
dichlofluanid died. Before death these dogs and also the surviving
animal displayed signs of impaired liver and kidney function (Lorke
and Löser, 1966).
Groups of 4 male and 4 female dogs were fed 0, 100, 300, 1000 and
3000 ppm dichlofluanid (97.7% pure) in their diet for 2 years.
Behaviour, body weights, food consumption, mortality, blood and urine
analyses, liver and kidney function tests and absolute and relative
organ weights for the groups fed 100, 300 and 1000 ppm did not differ
from the values of these parameters in the controls. In the groups fed
3000 ppm increased mortality, reduced body weight and food consumption
and decreased absolute and relative testis weight were found.
Microscopic examination of the organs revealed an increase in
interstitial tissue of the testis and vacuolation and degeneration of
the adrenal cortex of the 3000 ppm group as the only dose-dependent
pathological changes (Löser, 1969b; Mawdesley-Thomas et al., 1971).
Long-term studies
Rat
Groups of 80 rats (40 of each sex) were fed 0 (two groups), 150,
500, 1500 and 4500 ppm of dichlofluanid in their diets for two years.
Behaviour, food consumption and mortality of the test groups did not
differ from the parameters of the control groups. The same was the
case with haematology (at 4 and 24 months) and liver function and
urine examination (at 24 months). Gross pathology of animals which
died during the experiment, and of sacrificed test rats at the end of
the study, did not reveal any changes that might be caused by
dichlofluanid, but no histological examination of the organs is
recorded. Female rats in the 4500 ppm group showed decreased weight
gain and relative kidney weights, while both sexes showed elevated
relative liver weights, but normal enzyme function tests (Löser,
1968).
Histological examinations were made on tissues of five male and
five female rats at the end of the study and on animals that died
during the experiment. No significant pathological changes were found
at dietary levels including the 4 500 ppm level. A separate study of
all tumours found at autopsy was performed, and there was no
indication that dichlofluanid produced an increased tumour incidence
(Mawdesley-Thomas, 1969).
Comments
In a 2-year study in dogs a 1 000 ppm level of dichlofluanid in
the diet appears to have no untoward effect, although no data are
given on the individual animals.
A long term study in rats indicated 1 500 ppm in the feed as the
level without apparent toxic effect. There was no indication of
teratogenic effect when female rats were administered a dose as high
as 300 mg/kg bw. Reproduction parameters were not affected in a
3-generation feeding test with levels up to 1500 ppm dichlofluanid.
However, there is a lack of information on absorption, distribution,
excretion and pharmacokinetics on the dichloro-fluoromethylthio moiety
which may produce some carbon-fluoride compounds resistant to
cleavage. The Meeting allocated a temporary acceptable daily intake
for man based on the no-effect level in the dog, the more sensitive
species.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1 500 ppm in diet equivalent to 75 mg/kg bw.
Dog: 1 000 ppm, in diet equivalent to 25 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.3 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Uses (Table 3) are additional to those listed in FAO/WHO, 1970.
They are all recommendations of the Federal Republic of Germany (FRG).
TABLE 3. Pre-harvest treatments with dichlofluanid recommended
in the Federal Republic of Germany
Number of Rate of application
Crop applications (kg a.i./ha)
Pome fruits 4 - 11 1.5
Stone fruits
plums 4 1.5 - 2.0
cherries 3 - 8 2.0
peaches 3 1.5
Soft fruits
strawberries 1 - 6 2.5 - 3.0
red and black
currants 4 - 7 2.0 - 2.4
raspberries 1 -4 2.0 - 2.4
Grapes 2 - 10 1.9 - 2.5
Vegetables
cucumbers 1 - 4 1.5 - 2.0
tomatoes 1 - 3 1.5 - 2.0
lettuce (field grown
and under glass) 2 - 4 0.6 - 1.0
Beans 1 - 3 1.0
Hops 4 - 17 2.0
TABLE 4. Residues of dichlofluanid (I) and DSMA (II) resulting from supervised trials.
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Apples (FRG) 1.1- 4- I 0.7-3.4 0.2-2.8 <0.1-2.2 <0.1 <0.1-0.4 <0.1
1.25 11 II 0.3-1.1 <0.1-1.1 <0.1-1.1 <0.1-0.4 <0.1 <0.1
Beans green incl. 1.0- 1- I 0-1-0.3 0.1-0.2 <0.1-0.2 0.1
pods (FRG; UK) 3.4 3 II 0.1-0.2 0.1-0.2 <0.1 0.1
Cherries (morello) 1.1 6-8 I 0.6-2.5 <0.1-0.4 <0.1 <0.1
(FRG) II 0.2-1.2 <0.1-0.2 <0.1 <0.1
Cucumbers (outdoor); 1.5 3 I <0.1-0.2 <0.1-0.2 <0.1
(FRG) II 0.2-0.5 <0.1-0.3 <0.1
Cucumbers (under 1.5- 1- I 0.3-3.6 0.2-0.3 <0.1 <0.1
glass); (FRG; 6.3 3 II 0.1-0.5 <0.1 <0.1 <0.1
Netherland
Currants, black 2.5/? 2-7 I 2.4-48 2.0-16 2.0-6.3 0.9-5.1 0.5-1.4 0.2
(Denmark2/UK) II 0.5-3.2 1.1-1.5 0.5-1.0 0.4-0.7 0.2-0.3 0.3
Currants, red (FRG; 0.2- 3-5 I 5-39 3-23 2-46 1-40 0.3-3.2
Netherland3) 2.5 II 1.9-6.9 1.1-3.0 0.4-16 0.5-3.0 0.2-0.4
Gooseberries (UK) 5 I 0.3
II 0.2
Wheat, barley
(France) 1.3 1-2 I <0.1
Grapes (FRG4) 0.6- 2- I 0.2-48 0.2-33 0.7-10.6 <0.1-21 <0.1-16 1.3-11 <0.1-12
3.6 10 II <0.1-0.8 <0.1-3.8 <0.1-3.6 <0.1-2.7 <0.1-2.6 0.3-3.4 <0.1-3.8
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Hops (FRG) 2.0 9- I 69 6-140 5 0.3-0.7
17 II 14 3-11
Lettuce (outdoor) 0.6 1-4 I 7.8-30 0.2-2.7 0.1-0.2 <0.1-0.3 <0.1 <0.1
II 2.1-5.8 0.2-2.3 <0.1 <0.1-0.3 0.1-0.2 <0.1
Lettuce
(under glass) 0.6- 1-6 I 4.7-55 0.3-8.3 <0.1-1.9 <0.1-0.3 <0.1-5.6 0.6-2.2
(FRG; UK) 3.4 II 3.0-13 0.2-2.8 <0.1-2.5 <0.1 <0.1-1.6
Onions (UK) 1.1 5 I (100 days)
<0.1
Peaches (FRG) 1.1 3 I 2.2-2.9 0-7-1.2 0.3-1.6
II 0.5-0.8 0.2-1.4 0.5-0.9
Raspberries (FRG; 2.0- 1-4 I 21-32 6-14 <0.1-10.5 <0.1-4.7 1.7
Netherland 2.3 II 1.0-6.2 0.7-6.4 0.6-4.0 0.8-1.0 0.9
Strawberries
(outdoor) 0.8- 1-4 I 0.3-27 0.1-5.0 <0.1-6.3 <0.1-2.4 <0.1-1.6 <0.1-1.7 0.7-1.7
(FRG: Netherland 3.8 II 0.3-8.8 0.5-1.8 <0.1-3.7 0.2-2.4 0.3-1.9 <0.1-0.3 0.1
N.Z.6; Switzerland;
UK)
(Australia7) 1.4- 3 I + II 1.2-15.5 0.4-8.4 <0.1-3.2 <0.1-0.6 0.6 <0.1
2.1 expr. as I
Strawberries
(under glass) 1.2- 3-6 I 4-9 0.5-3.5 0.4-7.1 2.2-4.1 1.7-3.5 1.5-4.7 <0.1-3.6
(Netherland 9.5
(total)
TABLE 4. (Cont'd.)
Crop Application Residue, mg/kg, after interval (days)
(Country) Rate
Reference1 kg/ha No. Compound 0-3 4-7 8-14 15-21 22-28 29-35 36-42
Tomatoes (FRG) 1-3 I <0.1 <0.1-0.2
II <0.1-0.2 <0.1-0.9
1 Bayer AG (1964-1973) unless otherwise stated
2 Government Plant Pathology Institute, Lyngby; National Pood Institute, Gladsaxe, April 20, 1970
3 Keuringsdienst van Waren Amsterdam (1967a, 1969a)
4 Lemperle et al. (1969, 1970); Bayer (1964-1973)
5 Keuringsdienst van Waren Amsterdam (1965)
6 Brewerton and Gibbs (1968)
7 Tate at al. (1973)
8 Keuringsdienst van Waren Amsterdam (1969b)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Table 4 shows the residues of dichlofluanid and its metabolite
dimethylphenylsulphamide (DMSA) found in crops after recommended
applications in supervised trials. The analyses were carried out by
Farbenfabriken Bayer AG and various institutes.
FATE OF RESIDUES
In storage and processing
Additional information on the fate of residues during processing,
including wine making, and on the effect of residues on fermentation,
has been received since the previous evaluation.
Analyses of commercial deep-frozen strawberries from two
factories (3 samples from each) showed residues of 0.26 - 0.36 and
0.06 - 0.12 mg/kg dichlofluanid. In canned strawberries residues were
<0.02 mg/kg dichlofluanid and DMSA could not be found (Grevenstuk,
1971).
Strawberries were treated with dichlofluanid at flowering and
analysed with and without caps (Government Plant Pathology Institute,
Lyngby, Denmark, 1969). Most of the dichlofluanid residue was in the
caps (Table 5).
TABLE 5. Residues of dichlofluanid and DMSA in strawberries with and
without caps (Denmark)
Rate of Waiting Residues (mg/kg)
application No. of Period With cap Without cap
(kg.a.i./ha) applications (days) Dichlofluanid DMSA Dichlofluanid DMSA
1.9 3 24 1.6 0.4 0.2 -
(treated 28 1.3 0.3 - -
at
flowering) 35 1.7 - 0.1 0.1
42 0.7 - 0.1 0.1
Apples containing residues of about 0.8 mg/kg dichlofluanid plus
0.2 mg/kg DMSA were cold pressed. The juice contained approx.
0.2 mg/kg dichlofluanid and approx. 0.7 mg/kg DMSA. After heating the
juice for 30 min. at 90°C the residues were 0.1 and 0.7 mg/kg
respectively. Processing the apples to stewed fruit, baby food or
jelly resulted in residues of approx. 0.05 mg/kg dichlofluanid and 0.3
- 0.7 mg/kg DMSA (Maier-Bode, 1972). Residues of 2 mg/kg dichlofluanid
plus 0.7 mg/kg DMSA on apples when stored at -18°C were stable for at
least 6 months (Bayer, 1973).
Several series of experiments were carried out to determine the
effect of wine-making processes on residues in grapes, and the effect
of dichlofluanid residues on fermentation.
At residues of 1.5 - 10.8 mg/kg dichlofluanid and 0.02 - 0.1
mg/kg DMSA on grapes the corresponding residues (mg/kg) in the marc
were <0.05 - 8.0 and 0.1 - 1.4; in must (not clarified) <0.1 - 6.9
and 0.1 - 1.3; in clarified must <0.05 - 0.2 and 0.3 - 0.8 and in
wine <0.05 and 0.1 - 4.0 (Lemperle et al., 1970). There is no direct
correlation between the residues on grapes and those in the processed
products.
In a further study residues up to 48 mg/kg dichlofluanid and 1.6
mg/kg DMSA on grapes resulted in corresponding residues (mg/kg) up to
6.9 and 4.5 mg/kg in must (not clarified), 0.4 and 2.3 mg/kg in
clarified must and <0.05 and 4.0 mg/kg in wine (Lemperle et al.,
1973).
In other experiments (Lemperle and Kerner, 1969; Lemperle et al.,
1970), grapes were treated two or three times at recommended
concentrations. Residues of dichlofluanid and DMSA were determined in
grapes harvested at intervals from 32 to 63 days after the last
application, and in the wine made from them. Residues in the grapes
(19 samples) ranged from 1.5 to 12 mg/kg dichlofluanid and 0.02 to 0.8
mg/kg DMSA. In the wine, dichlofluanid could not be detected in any of
the samples (limit of detection 0.02 - 0.05 mg/kg) and DMSA residues
ranged from 0.1 to 4.0 mg/kg.
Bayer (1967-1973) report trials in FRG in which grapes were
treated at various rates with dichlofluanid. The results (Table 6)
show residues of dichlofluanid from 0.02 - 5.4 mg/kg on the grapes
which have been eliminated during processing to wine. DMSA residues
are again carried through into the wine.
TABLE 6. Residues of dichlofluanid (I) and DMSA (II) on grapes
and in wine produced therefrom
Application Days after Residues, mg/kg*
Rate last Grapes Grapes
kg a.i./ha No. treatment I II I II
- 7 28 - - n.d. -
0.6 4 56 5.4 1.35 n.d. -
1.5 - 2.0 2 57 0.9 0.15 n.d. -
2.0 - 2.5 4 47 0.7 0.25 n.d. 0.2
2.0 - 2.5 5 35 0.4 0.6 n.d. 0.25
2.0 - 2.5 6 22 1.6 0.95 n.d. 0.4
3.0 - 5.0 3 34 3.9 0.2 n.d. n.d.
2.0 - 4.0 4 59 - - n.d. 2.4
3.6 2 60 - - n.d. n.d.
1.8 5 71 1.0 - 0.03 -
0.8 8 54 0.02 - n.d. -
* Limit of detection: dichlofluanid 0.02 mg/kg;
DMSA 0.1 mg/kg.
Fermentation experiments were conducted to determine the
concentration of dichlofluanid required to inhibit yeast growth. At 1
mg/l in must the fermentation was retarded and at > 1.5 mg/l it was
inhibited. The inhibitory effect of dichlofluanid on yeast was
abolished by cysteine, beta-mercapto-ethylamine and other
SH-compounds. Dichlofluanid can therefore be regarded as an
"SH-blocker." The inhibitory effect decreases with increasing pH. The
activity of most enzymes depends on free SH-groups which are part of
cysteine. Therefore the enzymes alcohol-dehydrogenase and
glycerinaldehyde-3-phosphate-dehydrogenase which contain essential
SH-groups were strongly inhibited in vitro and in vivo while maleic
dehydrogenase was not affected. The fluorodichloromethylthio
(-S-CCl2F)-part of the dichlofluanid molecule was shown to be the
inhibiting group (Dittrich and Issinger, 1969).
Retardation of the fermentation process can be overcome by
pre-clarification of the grape juice (must) and addition of about 1%
of a fermenting pure yeast strain preparation, or in the case of red
grape juice, by the addition of 2.5% pure yeast while still in contact
with the skins. In general the fermentation process seems to be less
influenced by dichlofluanid than by folpet. No differences between
wines originating from dichlofluanid-treated and non-treated vines
could be observed either analytically or by tasting (Lemperle et al.,
1970, 1973).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues in 52 samples of red currants examined in the
Netherlands were <0.1 mg/kg (Keuringsdienst van Waren, 1966). Of
276 samples of strawberries, 256 contained <0.1 mg/kg
dichlofluanid, 17 contained 0.1 - 1 mg/kg and 3 contained 1 mg/kg
(Keuringsdienst van Waren, 1966).
Results of food inspection reported in 1973 (Netherlands) are
shown in Table 7.
TABLE 7. Residues of dichlofluanid found during food inspection.
Netherlands, 1973
Number of samples in range
Range, red
mg/kg blackberries raspberries currants strawberries
0 - 0.1 15 1 23 257
>0.1-0.2 2 7 111
>0.2-0.5 7 3 11 215
>0.5-1 5 3 8 168
>1 - 2 4 2 5 134
>2 - 5* 2 4 2 58
>5 - 10 3 3
>10 1 (10.3
mg/kg)
Total 38 13 56 947
* The tolerance was 5 mg/kg in all cases.
METHODS OF RESIDUE ANALYSIS
A multiresidue GLC method, with electron-capture detection
(Becker, 1972/74), has been adopted by Deutsche Forschungsgemeinschaft
(DFG, 1974) for the determination of dichlofluanid. The method in
principle is based on the GLC method of Vogeler and Niessen (1967)
already mentioned in FAO/WHO, 1970. For stewed apples, pears, plums,
cherries, peaches, grapes, cabbage, carrots, green beans and lettuce
the recovery at the 0.1 mg/kg level is > 80%. For leeks, parsley and
celery the recovery is below 70%. The method is suitable for
regulatory purposes for dichlofluanid residues in a number of crops,
but further evaluation is desirable before it can be recommended
without reservation. It does not determine DMSA.
A similar analytical method has been elaborated, in which
isopropanol-benzene extracts of the crop are cleaned-up with
Nuchar-Attaclay. The method has been checked for many green
vegetables, tomatoes and fruit. Interferences have sometimes been
found for leek, onion, cabbage and carrots (Rijks Instituut voor de
Volksgesondheid, Bilthoven, 1972).
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances and safety intervals reported to the Meeting
are given in Table 8.
TABLE 8. National Tolerances for dichlofluanid reported
to the Meeting
Safety
interval Tolerance,
Count Crop (days) mg/kg
Australia General 14
Austria General 14
Belgium Fruit, vegetables 5.0
(excl. potatoes)
Strawberries (outdoor),
raspberries, mulberries 7
Strawberries
(under glass) 14
Currants 21
Bulgaria General 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
Denmark Fruit, including
strawberries 14
Finland General 14
Strawberries ( 4.0
Other fruit (proposed) ( 1.0
France General 7
Germany Small fruit (berry dichlofluanid ( 15.0
(FRG) fruit excluding + DMSA (
strawberries), calculated as (
grapes dichlofluanid (
(
Strawberries, (
lettuce, ( 10.0
(
all other fruit ( 5.0
(
Beans, cucumbers, (
tomatoes ( 3.0
(
Onions ( 1.0
Lettuce (field
grown) 21
Lettuce (under
glass) 28
Tomatoes (field
grown and under
glass) 3
Cucumbers (field
grown and under
glass) 3
Pome-fruit 7
Stone-and small
fruit (excluding
strawberries) 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
(Germany) Strawberries 10
Grapes 35
Italy Grapes 40
All other crops 20
Japan Cucumbers, tomatoes,
strawberries (under
glass) 1
Netherlands General 5.0
Tomatoes, peppers,
cucumbers, melons 3
Strawberries (outdoor),
blackberries,
raspberries 7
Strawberries 14
(underglass)
Currants 21
Kohlrabi 14
New Zealand Fruit and vegetables 5.0
General 14
Norway General 7
Poland Vegetables, fruit 14
Field grown crops
(excluding, potatoes,
beets) 14
Grapes 42
Portugal Strawberries 14
TABLE 8. (Cont'd.)
Safety
interval Tolerance,
Count Crop (days) mg/kg
South Africa General 5.0
Table grapes 28 - 42
Wine grapes,
peaches, apricots,
plums and prunes 14
Spain Pome fruit, grapes
strawberries,
onions, vegetables 20
Sweden General 7
Switzerland Grapes 21 1.0
Apples, pears 21 0.5
Strawberries 21 7.0
United Apples, blackberries,
Kingdom red and white currants,
black currants, grapes
grown outdoors,
loganberries, autumn-sown
salad onions, outdoor
lettuce, leaf brassicas,
seedlings under glass,
lettuce under glass,
treated with dust
formulation 21
Strawberries, raspberries 14
Tomatoes under
glass, direct
spray on flowering
trusses 3
Yugoslavia Strawberries 14 2.0
+ 2.0 DMSA
APPRAISAL
The 1969 Joint Meeting, after reviewing the information on
dichlofluanid, concluded that further work was necessary, before
tolerances could be recommended. Some of the required information has
been supplied.
Data on the composition of technical dichlofluanid, including its
impurities have been presented. Dimethyl-N-phenylsulphamide (DMSA) is
known to be a metabolite, but detailed information on the pathway of
dichlofluanid degradation, especially on the fate of the
fluorine-containing moiety in plants, is not yet available. Studies
are known to be in progress, and results will presumably be available
to the 1976 Joint Meeting.
The biological activity of dichlofluanid and similar fungicides
(e.g. captan, folpet) is presumed to be localized in the
trihalogeno-methylthio part of the molecule (Kühle et al., 1964). The
inhibitory effect of dichlofluanid on yeast is antagonized by
SH-compounds. The enzymes alcohol dehydrogenase and
glycerinaldehyde-3-phosphate dehydrogenase and the co-factor co-enzyme
A which contain essential SH-groups are strongly inhibited in vitro
and/or in vivo (Lemperle et al., 1973). Dichlofluanid can therefore be
regarded as an "SH-blocker."
Processing of apples containing 0.8 mg/kg dichlofluanid plus 0.2
mg/kg DMSA to stewed fruit, baby food or jelly resulted in residues of
0.05 mg/kg dichlofluanid and 0.3 - 0.7 mg/kg DMSA. Cold pressed juice
contained 0.2 mg/kg and heated juice 0.1 mg/kg dichlofluanid plus 0.7
mg/kg DMSA.
Wine originating from grapes with residues up to 48 mg/kg
dichlofluanid contained up to 4 mg/kg DMSA and always less than 0.05
mg/kg dichlofluanid. During crushing and fermentation much of the
residue seems to be absorbed by the solid particles of the mash.
In addition to the crops mentioned in FAO/WHO, 1970 the
pre-harvest application of dichlofluanid has been extended to
kohlrabi, leaf brassicas, melons, paprika, blackberries, loganberries,
mulberries, raspberries, gooseberries, wheat and barley. In many cases
dichlofluanid can be used as a substitute for other fungicides, e.g.
dithiocarbamates and thiuram disulfides.
Numerous data on rates and frequencies of application,
pre-harvest intervals and the resulting residues of dichlofluanid and
the degradation product DMSA have been supplied from Australia,
Denmark, France, Germany, the Netherlands, Switzerland and the United
Kingdom. The residue data, especially for currants, raspberries,
strawberries and grapes, still show inconsistencies however. More data
from supervised trials and on raw agricultural products moving in
commerce are desirable for re-evaluating residue levels. Nevertheless
the data on maximum residues so far obtained from supervised trials
could be accommodated by the temporary tolerances recommended below,
which seem to be in accordance with the toxicologically permissible
level.
The tolerances should refer only to the parent compound. This
would substantially facilitate the determination of residues, because
a gas-chromatographic multiresidue method could be used. This method
is suitable for regulatory purposes in a range of crops, but further
evaluation is desirable. DMSA can only be determined by a colorimetric
method which is less suitable for regulatory purposes. The
determination of DMSA is not considered necessary because available
data indicate that excessive residues of this compound would be
accompanied by excessive residues of dichlofluanid.
RECOMMENDATIONS
The following temporary tolerances are recommended. They refer to
dichlofluanid only.
TEMPORARY TOLERANCES
dichlofluanid
(mg/kg)
Currants (red, black and white), grapes,
raspberries 15
Lettuce, strawberries 10
Apples, pears, cucumbers, peaches 5
Beans (green, including pods), cherries,
tomatoes 2
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Studies on absorption, distribution in various organs, and
excretion of dichlofluanid in the rat.
2. Pharmacokinetics of the dichlorofluoromethylthio moiety.
DESIRABLE
1. Metabolism studies on dichlofluanid.
2. Results from current studies on the pathway of degradation,
especially the fate of the fluorine-containing moiety of the
molecule, in and on plants. These data are expected to be
available during 1975.
3. Further residue data from supervised trials, to resolve certain
inconsistencies in the existing data or to provide new
information, on blackberries, gooseberries, loganberries,
mulberries, raspberries, currants, hops, kohlrabi, leaf
brassicas, melons, onions, paprika and eventually wheat and
barley.
4. Further residue data for raw agricultural products moving in
commerce.
5. Further evaluation of the analytical method of Becker for
regulatory purposes.
REFERENCES
Bayer AG (1962). Product KU 13-032-C. Report prepared and submitted by
the Institute of Toxicology, Bayer AG. (Unpublished)
Bayer AG (1964). Report of 4-months feeding study on rats with active
ingredient 47531. Report prepared and submitted by the Institute of
Toxicology. Bayer AG. (Unpublished)
Bayer AG (1969). Dichlofluanid. Summary Report prepared and submitted
by Bayer AG. (Unpublished)
Bayer AG (1964, 1973). Unpublished results.
Becker (1972, 1974). Deutsche Forschungsgemeinschaft: Methodensammlung
zur Rückstandsanalytik von Pflanzenschutzmitteln; Dichlofluanid 203-1
and S 8 - 1; Verlag Chemie GmbH, Weinheim/Bergstr.; 1972 and 1974.
Brewerton, H.V. and Gibbs, M.M. (1968) Dichlofluanid ("Euparen")
residues on strawberries. N.Z. J. Agric. Res., 11:784-788.
Dittrich, H.H. and Issinger, O.G. (1969) Zum Wirkungsmechanismus von
Dichlofluanid (N,N-Dimethyl-N'-phenyl-[N'-Fluordichlormethylthio]
-Sulfamid). A. Pflanzenkr. Pflanzenschutz, 76 (11/12):651-663.
Dubois, K.P. (1963) The acute toxicity of Bayer 47531 to chickens.
University of Chicago. Report submitted by Bayer AG. (Unpublished).
Dubois, K.P. and Raymund, A.B. (1963) The acute toxicity of Bayer
47531 to mammals. University of Chicago. Report submitted by Bayer AG.
(Unpublished)
Eben, A. and Kimmerle, G. (1968) Studies on the metabolism of Bayer
47531. Report prepared and submitted by the Institute of Toxicology.
Bayer AG. (Unpublished)
FAO/WHO (1970). 1969 Evaluations of some pesticide residues in food.
FAO/PL/1969/M/17/1; WHO/Food Add./70.38.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1969). August 4. Unpublished data.
Government Plant Pathology Institute, Lyngby; National Food Institute,
Gladsaxe, Denmark (1970). April 20. Unpublished data.
Grevenstuk, W.B.F. (1971) Afname van residuën van bestrijdingsmiddelen
tijdens industriële verwerking II (aardbeien). Rijks Instituut voor de
Volksgezondheid, Utrecht, Netherlands. 64/71 Tox-Rob
Keuringsdienst van Waren Amsterdam (1965). Nr. 102 Residu's van thiram
en "5114" op aardbeien.
Keuringsdienst van Waren Utrecht (1966). Nr. 37 Residu's van thiram,
dichlofluanid, folpet en captan op zacht fruit.
Keuringsdienst van Waren Amsterdam (1967a). Nr. 118 Residu's van
fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1967). Nr. 122 Residu's van
dichlofluanid, thiram en folpet op aardbeien in 1965.
Keuringsdienst van Waren Amsterdam (1969a). Nr. 130 Ten Broeke,
Fungiciden op bessen.
Keuringsdienst van Waren Amsterdam (1969b). Nr. 131 Ten Broeke,
Fungiciden op aardbeien onder glas.
Kühle, E., Klauke, E. and Grewe, F. (1964).
Fluordichlormethylthio-Verbindungen und ihre Verwendung im
Pflanzenschutz. Angew. Chem., 7:807-816.
Lemperle, E. and Kerner, E. (1969). Wirkstoffrückstände und
Gärbeein-flussung nach Anwendung von Basfungin, Euparen und
Ortho-Phaltan im Weinbau. Wein-Wiss., 24 (10):357-371.
Lemperle, E., Kerner, E. and Strecker, H. (1970). Wirkstoffrückstände
und Gärverhalten nach Anwendung von Botryziden im Weinbau. Wein-Wiss.,
25 (9/10):313-325.
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstände und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Dtsch Lebensm. Rundsch, 69 (9):313-322.
Lorke, D. (1965) Bericht über viermonatige Fütterungsversuche an
Ratten mit Dimethylaminosulfanilid. Unpub. Report prepared and
submitted by the Institute of Toxicology, Bayer AG. (Unpublished)
Lorke, D. and Löser, E. (1966) Bayer 47531. Subchronische
toxicologische Untersuchungen an Hunden. Report from the Institute of
Toxicology, Bayer AG. (Unpublished)
Löser, E. (1968) Bayer 47531, Chronic toxicological studies on rats.
Report from the Institute of Toxicology, Bayer AG. (Unpublished)
Löser, E. (1969a) Generationsversuche an Ratten. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Löser, E. (1969b) Chronische toxikologische Untersuchungen an Hunden.
Report from the Institute of Toxicology, submitted by Bayer AG.
(Unpublished)
Machemer, L. (1974a) Untersuchung auf emryotoxische und teratogene
Wirkung nach oraler Verabreichung an der Ratte. Report from the
Institute of Toxicology, submitted by Bayer AG. (Unpublished)
Machemer, L. (1974b) Dominant-lethal-test an der männlichen Maus zur
Prüfung auf mutagene Wirkung. Report from the Institute of Toxicology,
submitted by Bayer AG. (Unpublished)
Maier-Bode, H. (1966, 1972) Pharmakologisches Institut, Bonn.
(Unpublished)
Mawdesley-Thomas, L.E. (1969) Pathology report of the chronic toxicity
of compound Bay 47531 in rats. Report from Huntingdon Research
Centre, submitted by Bayer AG. (Unpublished)
Mawdesley-Thomas, L.E., Newman, A.J. and Spicer, E.J.F. (1971).
Pathology report of Bay 47531 two year dog study. Report from
Huntingdon Research Centre, submitted by Bayer AG. (Unpublished)
Nangniot, P., Vervier, R. and Martens, P.H. (1967). The use of
polarography to determine small amounts of fungicides or insecticides
in deposits or residues on plants. V. Determination of
N,N-dimethyl-N'-phenyl (N'-fluorodichloromethylthio) sulphamide
(Euparen) on fruits (Fr.) Bull. Rech. Agron. Gembloux, 2(2):285-93
[Chem. Abstr. 68:58579r (1968)].
Rijks Instituut voor de Volksgezondheid, Bilthoven (1972). 109/72
Tox-Rob, Zuivering van plantenextracten met beheelp van actieve kool
voorafgaande aan de gaschromatografische bepaling van een aantal
bestrijdingsmiddelen.
Tate, K.G., van der Mespel, G.J., Levin, P.B., Brewerton, H.V., Gibbs,
M.M. and McGrath, H.J.W. (1973). Dichlofluanid ('Euparen') residues on
strawberries. N.Z. J. Exp. Agric., 1(1):100-104.
Vince, A.A. and Spicer, E.J.F. (1971). Pathology report of Bay 47531.
Generation experiment in rats. Report from Huntingdon Research Centre,
submitted by Bayer AG. (Unpublished)
Vogeler, K. and Niessen, H. (1967). Kolorimetrische und
gaschromatographische Bestimmungen von Rückständen in Pflanzen nach
Anwendung von Euparen. Pflanzenschutz-Nach. Bayer, 20:534-549.
