
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
DICHLOFLUANID
Explanation
Dichlofluanid was evaluated at the meetings in 1969, 1974 and 1977.
In 1977 when the temporary ADI was extended, further data were
required on (1) studies to elucidate the accumulation seen in the
thyroid and (2) results from current studies on the pathways of
degradation, especially the fate of the fluorine-containing moiety of
the molecule in or on plants. In addition, further information
concerning residues, especially residues on melons and leaf brassicas
were considered to be desirable.
At the 1979 Session of the CCPR, a number of questions were raised
concerning the MRLs proposed for blackberries, gooseberries, eggplant
and onions.
The monograph addendum reviews the additional information received.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical Aspects
Rats were dosed with 5 and 10 mg 14C-dichlofluanid/kg/body weight by
oral and intravenous routes. Forty to fifty percent of the dose was
excreted in the urine within 8 hours after application. The main
radioactive metabolite in urine was identified as
thiazolidin-2-thio-carbonic acid (TTC). Eight hours after intravenous
administration of 5 mg 14C-dichlofluanid/kg body weight, 74 to 92% of
the urinary radioactivity was identified at TTC. After oral
administration, TTC was noted to range from 57 to 64% of the
radioactivity in urine. The remaining radioactivity was distributed
among several other minor metabolites. The parent compound was not
identified in urine (Ecker, 1978).
TOXICOLOGICAL STUDIES
Signs of poisoning included apathy and dyspnea. Sedation was observed
in mice at high doses. Post-mortem examination revealed pale and
marble-like kidneys, pale and spotted liver, empty and reddish
stomach, and intestine with erosion and decomposition of the gastric,
glandular mucosa (Flucke, 1978).
The acute oral LD50 for male rats of thiazolidin-2-thio-carbonic acid
(TTC) the main urinary metabolite of dichlofluanid, is greater than
1000 mg/kg body weight (Thyssen, 1978).
Table 1. Acute Toxicity of Dichlofuanid to Various Animals
LD50
Animal Sex Route (mg/kg/b.w.) Reference
Rat M Oral >5000 Flucke, 1978
Rat M&F Oral >5000 Flucke, 1978
Mouse M Oral 5464 Flucke, 1978
Mouse F Oral 5597 Flucke, 1978
Guinea pig F Oral 945 Flucke, 1978
Rabbit F oral approx. 3500 Flucke, 1978
Cat F Oral >1000 Flucke, 1978
Rat M&F Dermal (24
hr exposure) >5000 Flucke, 1978
Special Studies on Mutagenesis
The Micronucleus test was carried out in male and female mice for
possible mutagenic action on chromosomes of erythroblasts in bone
marrow. Two oral doses were given within 24 hours (2 × 1000 and 2 ×
2000 mg dichlofluanid/kg/body weight). Endoxan (cyclophosphamide) (2
× 100 mg/kg body weight) was administered as positive control. This
test did not indicate any mutagenic action of dichlofluanid (Herbold,
1978).
An "Ames test" was performed to evaluate a possible point mutation
activity of dichlofluanid. Salmonella typhimurium TA-1535, TA 1537,
TA-98 and TA100 strains were used in the tests at doses of 4, 20, 100,
500 and 2500 µg/plate. Endoxan (500 µg cyclophosphamid/plate) and
trypoflavin (250 µg/plate) were used as positive controls. An S-9
microsomal activation system was used with all test doses. A
mutagenic effect of dichlofluanid was observed only with Salmonella
typhimurium TA-100 at the 200 µg/plate dose. Higher doses were
toxic to the bacteria. These experiments were repeated with the
following test doses: 0, 50, 100, 200 and 400 µg/plate. A mutagenic
effect was observed in the 100 and 200 µg/plate doses with TA-100.
The 400 µg/plate dose was again toxic. No mutagenic effects were
observed with the TA-1535, TA 1537 or TA-98 strains. Dichlofluanid
was mutagenic in the "Ames test" only with the TA-100 strain (Herbold,
1979a).
Cytogenetic studies in the Chinese hamster spermatogonia were carried
out with dichlofluanid given orally to male animals in two daily doses
(2 × 250 and 2 × 500 mg/kg body weight). The animals presented no
clinical signs of poisoning, although one animal at the high dose
died. The positive control group received Adriblastin (doxorubicin) 2
× 5 mg/kg body weight by intraperitoneal injection. No differences
were observed between the negative control and the
dichlofluanid-treated animals. Differences between the negative and
positive control groups were highly significant (Herbold, 1979b).
A dominant lethal study was carried out in mice. Groups of 20 male
(NMRI) mice received a single oral dose of 0 or 500 mg dichlofluanid
per kg body weight. After administration, each male was mated with 3
untreated females, every week, for eight consecutive weeks. The
females were sacrificed on day 14 of gestation. Pre-implantation and
post-implantation losses were calculated from the number of corpora
lutea, the number of implantations and the number of living and dead
embryos. Dichlofluanid treatment did not have any harmful effects on
the males, did not impair their mating performance, and did not affect
fertility. Post-implantation data did not suggest any mutagenic
action of dichlofluanid. A significant pre-implantation loss was,
however, observed in the second mating week (Machemer, 1974a).
Special Studies on Embryotoxic and Teratogenic Effects
Embryotoxicity and teratogenicity studies were carried out in rats
following oral administration of dichlofluanid at daily doses of 0,
30, 100 and 300 mg/kg body weight from day 6 to day 15 of gestation.
On day 20, the foetuses were removed from the dams by Caesarian
section and examined for visceral and skeletal malformations. All
test doses were slightly toxic to the dams. Several animals showed
acute poisoning signs (diarrhea, ruffled coat, dyspnea, inactivity,
and loss of body weight). There was no overt mortality in the study.
This study revealed: (1) dichlofluanid was toxic by the oral route to
pregnant dams at 30 mg/kg body weight/day and above; (2) a significant
increase in the number of foetuses with malformations (P <0.05) was
observed at the dose level of 100 mg/kg/day (the highest dose (300
mg/kg/day) did not reveal an equivalent increase); and (3) a no effect
level for foetal development was 100 mg/kg/day (Machemer, 1974b).
Short-Term Studies
The threshold dose of dichlofluanid was stated to be 5.4 mg/kg
observed in a 10-month experiment reported to the meeting in abstract
only (Belonozhkol, et al., 1978). A review of the original data was
not made and was considered to be necessary before the data can be
fully evaluated.
COMMENTS
Dichlofluanid was evaluated for an acceptable daily intake by the
meeting in 1969, 1974 and 1977. A temporary ADI was established in
1974 JMPR and extended at the same level in 1977.
Several tests for mutagenicity (micronucleus, cytogenetic, dominant
lethal, and microbial tests) were essentially negative. However, a
positive response was obtained in one bacterial strain in the "Ames
assay".
The studies required by the 1977 JMPR on the effects of the possible
accumulation in the thyroid are known to be in progress. The meeting
extended the temporary ADI to allow these studies to be completed.
TOXICOLOGICAL EVALUATION
Level Causing No Toxicological Effect
Rat: 1500 ppm in the diet, equivalent to 75 mg/kg body weight
Dog: 1000 ppm in the diet, equivalent to 25 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.3 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatment
Information from the manufacturer (Bayer, 1979a) stated that they are
not recommending the use of dichlofluanid on melons and leaf brassicas
and therefore would not be supplying data for these crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Information supplied by Bayer AG (Bayer, 1979a) reported that until
1978 when a new ordinance was issued requiring the determination of
the parent only, legislation in the Federal Republic of Germany had
required the determination of the sum of dichlofluanid plus the
metabolite dimethyl aminosulfanilide (DMSA). Therefore, the great
majority of their existing residue data was expressed as the combined
residue with no possibility of differentiation between the two
components. As a result, the residues data compiled in the Table on
pages 127 and 128 of the 1977 Evaluations and referring to Bayer
Reports (coded country D) are invalid for reflecting the true residue
situation and should be deleted, except for the potato results which
were below detectable limits anyway. The Nitokuno values in 1977 from
Japan (coded country J) are correct and should be retained. These
include cucumber, eggplant, hops, lettuce, onion bulbs, strawberries
under glass, and tomatoes. In view of the widespread use of
dichlofluanid and the large number of national MRLs already
established (based mainly on parent compound alone), it is proposed to
retain the other MRLs proposed in 1977 on a temporary basis and until
such time as acceptable data from new field trials becomes available,
including data supplied to this meeting.
Additional residue data were received from supervised trials conducted
in Japan and Finland and are summarized in Table 2.
Table 2. Residues of dichlofluanid in fruits and vegetables from supervised trials
Dose, No. of Days after Residues Reference
Crop Country kg a.i./ha applications last application mg/kg
Apple Finland 0.004 kg/ 3 62 <0.005 peeled State Inst. Agri.
tree <0.005 washed Chem., 1967
0.05 unwashed
4 14 1.5 peeled
4.0 washed
3.2 unwashed
Finland 5 g/tree 1 14 6.9 unwashed State Inst.
Agri. Chem., 1973
Black Finland 3.75 g/bush 2 56 0.5 unwashed State Inst.
currant 3 38 1.0 unwashed Agri. Chem., 1972
Finland 3.75 g/bush 2 57 0.4 unwashed State Inst.
3 49 3.0 unwashed Agri. Chem., 1973
Cucumber Japan 0.08g/m3 0 - )
(steam fog) 1 1 )
3 )
7 ) all <0.006 Nitokuno, 1972
2 1 )
3 )
7 )
Cucumber Japan 2 0 - <0.003 Nitokuno, 1973
(greenhouse) (drench) 3 3 0.007
7 0.004
14 0.005-0.007
5 3 0.003-0.011
7 0.005-0.009
14 0.005-0.009
Table 2. Continued...
Dose, No. of Days after Residues Reference
Crop Country kg a.i./ha applications last application mg/kg
Cucumber Japan 0.08g/m3 0 - )
(greenhouse) (steam fog) 2 1 )
3 )
7 ) All <0.01 Nitokuno, 19761
3 1 )
3 )
7 )
Cucumber Japan 30 0 - <0.01, 0.01 Nitokuno, 19761
(drench) 1 3 <0.01, 0.01
7 0.14, 0.19
3 3 0.04, 0.11
7 0.03, 0.05
Gooseberry Finland 3.75 g/bush 2 41 2.6 unwashed State Inst. Agri.
3 29 2.8 unwashed Chem., 1972
Hop Japan 31.25 2 46 0.27 Nitokuno, 1977
(spray) 3 37 0.28
3 34-36 0.10
Strawberry Japan 0.04 g/m3 0 - <0.004, <0.004 Nitokuno, 19731
(greenhouse) steam fog) 1 1 0.023, 0.021
3 0.012, 0.010
7 0.006, 0.012
2 1 0.040, 0.020
3 0.011, 0.014
7 0.008, 0.010
Table 2. Continued...
Dose, No. of Days after Residues Reference
Crop Country kg a.i./ha applications last application mg/kg
Strawberry Japan 24.9 0 - <0.01, <0.005 Nitokuno, 1975
(drench) 6 1 0.24, 0.166
plus 3 0.10, 0.246
2 7 0.06, 0.082
spray 8 1 0.12, 0.178
3 0.08, 0.278
7 0.03, 0.071
Strawberry Japan 24.9 0 - <0.01, 0.006 Nitokuno,19751
(greenhouse) (drench) 6 1 0.27, 0.292
plus 4 0.08, 0.114
7 7 0.18, 0.234
(spray) 8 1 0.10, 0.266
4 0.10, 0.146
7 0.20, 0.409
Strawberry Finland 0.25 kg/ 3 19 0.2 washed State Inst. Agri.
line meter 0.6 unwashed Chem., 1967
11 g/a 3 19 0.15 washed
0.5 unwashed
0.25 kg/ 2 26 0.02 washed
line meter 0.4 unwashed
11 g/a 2 26 0.01 washed
0.01 unwashed
Finland 0.25 kg/ 3 14 0.03 washed State Inst. Agri.
line meter 0.07 unwashed Chem., 1968
2 26 0.03 washed
<0.03 unwashed
Finland 25 g/a 3 8 18 unwashed State Inst. Agri.
2 15 1.5 unwashed Chem., 1971
Table 2. Continued...
Dose, No. of Days after Residues Reference
Crop Country kg a.i./ha applications last application mg/kg
Tomato Japan 0.08 g/m3 0 - <0.004 Nitokuno, 1972
(greenhouse) (steam fog) 1 1 0.005
3 <0.004
7 <0.004
2 1 0.014
3 <0.004
7 <0.004
Japan 0.08 g/m3 0 - <0.01, <0.01 Nitokuno, 19761
steam fog 2 1 0.02, <0.01
3 <0.01, <0.01
7 <0.01, <0.01
3 1 <0.01, <0.01
3 0.01, <0.01
7 <0.01, <0.01
Watermelon Japan 30 0 - <0.01, <0.01 Nitokuno, 19761
(drench) 5 7 <0.01, <0.01
6 7 <0.01, <0.01
1 Samples analysed by two different institutes.
FATE OF RESIDUES
In plants
Dichlofluanid labelled with 14C in the dichlorofluoromethyl position
was sprayed on wine grapes 35 days before harvest time (Voleler et
al., 1979). At harvest only 29% of the applied radioactivity could
be detected while 71% had vaporised. Of the remaining radioactivity
in the grapes, 91% was in the form of unchanged starting material. A
methanol extract of the grapes contained small quantities (2.2% and
5.3%) of two unidentified metabolites. During wine making, 23% of the
radioactivity in the grapes went over into the wine, 70% remained in
the residue (must), and 6% was evolved with the CO2 given off. Of
the radioactivity in the wine, 70-80% was identified at the metabolite
thiazolidine-2-thione-4-carboxylic acid (TTCA) arising from the
reaction between thiophosgene and cystein. The content of TTCA in
wine was around 0.1 mg/l.
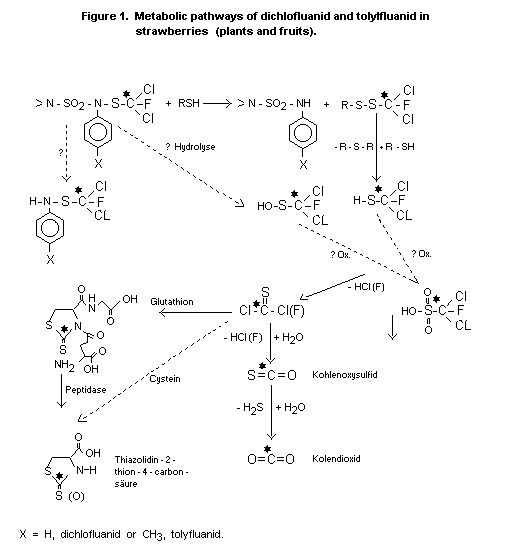
The degradation and metabolism of 14C-dichlofluanid
(dichlorofluoromethyl position labelled) in strawberries was studied
by Westphal, et al. (Westphall et al, 1979). Strawberry plants
grown in a closed, air flow controlled glass cultivating system were
sprayed with an aqueous preparation of the 14C-50% wettable powder at
the end of the flowering period and cultivated in the closed system
until harvest (36 days). Strawberries, leaves, roots and soil
contained 8%, 71%, 3% and 5% respectively, of the originally-applied
radioactivity, while 6% 14C-CO2 was released along with traced of
carbonyl sulphide. Small amounts of radioactivity were found in the
condensed and percolated water of the system. Results are tabulated
as follows:
Table 3. Distribution of 14C-dichlofluanid metabolites in strawberry culture system
Extractable radioactivity, %
Less polar Polar bound
Dichlofluanid TTCA metabolite (5) metabolite (5) radioactivity
Strawberries - 1.3 6.3 37.3 55.1
leaves 38.6 7.0 - 24.6 29.8
soil - - 2.7 12.3 85.0
(upper 5 cm)
From this experiment, it is concluded that the
dichlorofluororomethylmercapto moiety of the parent compound is easily
cleaved off, leading to unstable intermediates such as sulfinic acid
and thiphosgene or its fluoro analogue. This mechanism is supported
by the detection and identification of carbonyl sulphide, a hydrolysis
product of thiophosgene and TTCA, a reaction product of thiophosgene
and naturally-occurring cysteine. Eventually the chlorine and
fluorine atoms are released as inorganic ions. Similar results and
conclusions were obtained for an experiment using 14C-tolylfluanid
although more undegraded parent compound was found in strawberries in
this case (Haque, et al., 1979). The metabolic pathway for either
dichlofluanid or tolylfluanid is shown in Figure 1.
The metabolism of the tolyl analogue of dichlofluanid, tolylfluanid,
was determined on apples growing on small trees growing in a
greenhouse (Vonk, et al., 1977). After 28 days post-treatment,
approximately 42% of the originally applied-radioactivity (as
dichlorofluoromethyl-14C, tolylfluanid spray) had disappeared
probably as volatile compounds, ca. 41% was due to unchanged starting
material, and ca. 17% was unidentified material.
In soil
Information on the rate and extent of the degradation of
14C-dichlofluanid (labelled in the dichlofluoromethylthio carbon) in
soil was provided in a report on an experiment utilizing two soil
types, a high humus sandy loam (soil I) and a medium humus sandy loam
(soil II), incubated in biometric flasks fitted with side-arm chambers
containing 0.1 N KOH (Schuphan, et al., 1979a). The results are
summarised in Table 4.
Similar results were obtained for 14C-tolylfluanid (Schuphan, et
al., 1979b). Additional information on soil degradation was provided
in Table 2.
Table 4. Degradation of 14C-dichlofluanid in soils
Time after Total 14CO2 Extractable 14C-
start of evolved, % remaining, %
experiment, days soil I Soil II Soil I Soil II
3 45.0 36.4 4.7 57.0
7 61.5 57.2 1.3 (5.3)
14 69.5 77.1 0.92 20.4
31 74.0 92.7 0.52 1.7
63 77.5 98.5 0.36 0.80
 In storage and processing
Strawberries treated in a normal way with non-radioactive
dichlofluanid to yield an analytically determined residue of 5.9 mg/kg
parent compound (I) and 1.6 mg/kg of the metabolite
dimethylaminosulfanilid (II) when harvested two days after treatment,
were subjected to various processing steps to give unwashed raw
berries, washed raw berries, canned fruits, pasteurized juice, and jam
which were analysed to evaluate the effect on residues (Hartman, et
al., 1979). Substantial reductions in residues were achieved by
processing as shown in Table 5.
Table 5. Concentration of active ingredient and metabolite on
strawberries and processed products.
Sample I, mg/kg II, mg/kg
Berries, unwashed 5.9 1.6
Berries, washed 0.35 0.85
Wash water 1.95 0.2
Juice, raw 0.02 0.85
Juice, pasteurized N.D.1 0.9
Pomace 0.35 1.4
Canned fruits 0.02 0.75
Jam N.D. 1 0.4
1 N.D. = not detectable.
Photodecomposition
In studies carried out in England, the degradation of dichlofluanid by
ultraviolet light was measured in methanol, benzene, and acetone
solution (Clark et al., 1978). Solutions in methanol or benzene
deposited a brown solid on the UV lamp surface (Hanovia, 100 W.)
stopping the reaction. In acetone solution darkening occurred, but no
solid separated, permitting a reaction time of one hour. Products
from the acetone solution included N,N-dimethyl-N'-phenylsulfamide,
phenyl isocyante, phenyl isothiocyanate, and dimethylamidosulfonyl
chloride. The presence of bis (dichlorofluoromethyl) disulfide,
1-(dichlorofluoromethylthio) propan-2-one and
1-(dichlorofluoromethyl-sulfonyl) propan-2-one. In vivo tests
against Botrytis einerea showed that irradiation decreased the
activity of dichlofluanid and that synergism did not occur.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Information was supplied by Sweden on the results of analysing twenty
samples of Swedish strawberries during 1979. No samples contained
detectable levels of dichlofluanid (<0.03 mg/kg, Sweden, 1979). The
results of monitoring surveys on several lots of produce imported into
Finland during 1978 are shown in Table 6 (Finland, 1979).
Table 6. Occurrence of residues of dichlofluanid in produce imported
into Finland in 1978
Commodity No. of positive results Found (mg/kg)
Cucumbers 6 0.01-0.28
Apples 1 0.06
Strawberries 2 <0.01, 0.11
Sweet peppers 1 0.10
Finland also supplied information on levels of dichlofluanid found in
Finnish strawberries over the period 1975-1978.
Table 7. Occurrence of residues in strawberries grown in Finland
Number of samples containing
Year Number of samples dichlofluanid levels within
analysed given range, mg/kg
<0.1 0.11-1.0 1.1-2.0
1975 98 34 7 -
1976 138 50 14 -
1977 139 48 20 -
1978 119 34 13 2
There was no explanation for the discrepancies in samples numbers.
METHODS OF RESIDUE ANALYSIS
The method of analysis in Sweden (1979) was reported to be that of
Johansson (1978). Finland reported using a method of Johansson (1974)
and Becker (1971).
NATIONAL MRLs REPORTED TO THE MEETING
The Swedish maximum acceptable level for dichlofluanid is 5 mg/kg for
fruits and vegetables except potatoes. Sweden (1979).
APPRAISAL
Data supplied in 1977 from residue trials in Germany (F.R.) were based
on legislation requiring analysis for total residues of dichlofluanid
and metabolite which has since been changed, invalidating that data
source. However, new data were received in 1979 from Japan and
Finland which support the 1974 and 1977 recommendations for MRLs for
apples, cucumbers, gooseberries, hops, strawberries and tomatoes.
Sufficient information on national limits for blackberries, eggplants,
peppers cereal grains and wheat straw was available to make it
possible to retain the 1977 MRLs on a temporary basis until such time
as valid data from supervised trials becomes available.
Extensive information was received on the pathways of degradation and
metabolism of dichlofluanid, including the fate of the
fluorine-containing moiety of the molecule in plants (especially
strawberries) and soil, satisfying the requirements of the 1977 Joint
Meeting. Dichlofluanid is degraded fairly rapidly as the
dichlofluoromethylmercapto group is easily cleaved off, leading to
unstable intermediates and ultimately to
thiazolidine-2-thione-4-carboxylic acid (TTCA), a reaction product of
thiophosgene and cysteine. Soil residues degraded to CO2 and
extractable products rapidly and extensively. After 63 days, 98.5% of
applied radioactivity had evolved as 14C-CO2, while only 0.80%
remained as extractable products.
Data were received on the effects of processing on residues in
strawberries. Washing reduced dichlofluanid residue from 5.9 mg/kg to
0.35 mg/kg. Further processing into pasteurized juice, canned fruits
and jam reduced the residue to not detectable, 0.02 mg/kg, and not
detectable respectively.
The manufacturer of dichlofluanid has indicated that it is not
recommending uses on leafy brassicas, negating the need for further
residue data on this crop.
RECOMMENDATIONS
The temporary maximum residue limits recommended in 1977 for
blackberries, eggplants, sweet peppers, cereal grain and wheat straw
are retained on a temporary basis until data indicating the level of
residues of the parent compound become available. The limits for all
present and past recommendations refer to dichlofluanid only.
Temporary maximum residue
Commodity Limit, mg/kg
Blackberries 15
Sweet peppers 2
Eggplants 1
Cereal grains 0.1
Wheat straw 0.5
FURTHER WORK OR INFORMATION
Required by 1982
1. Residue data for dichlofluanid only, from supervised trials on
blackberries, eggplants, peppers, cereal grains and wheat straw.
2. Studies to elucidate the accumulation seen in the thyroid.
REFERENCES
Becker, G. Gaschromatographische Simultan-Bestimmung von chlorierten
Kohlenwasserstoffen und säureestern in pflanglichem Material. Dtsch.
Lebensmittel.Resch. 67, 125 (1971).
Belonozhko, G.A., et al. Hygienic Evaluation of Euparen Residues in
Food Products (in Russian) Vop. Pitan. (2): 72-75. (Abstracted in
Pesticide Abstracts 1979).
Clark, T. and Watkins, D.A.M. - Photolysis of dichlofluanid. Pestic.
Sci. 9, 225-228 (1978).
Ecker, W. Biotransformation von (14c) Dichlofluanid in der Ratte.
(1978) Unpublished report from Institut für Pharmakokinetik, submitted
by Bayer AG.
Finland. Submission from national government of reports of State
Institute of Agricultural Chemistry, Helsinki. (1979).
Flucke, W. Untersuchungen zur Acuten Toxizität bei Ratten, Mässen,
Meerschweinchen Kanichen und Katzen. (1978) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Haque, A., Westphal, D., Schuphan, I. and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Tolylfuanid (Euparen M) in Erdbeeren. BBA-Berlin, Unveröffenlichter
Bericht. (1979).
Hartman, V.B., Wrieden, J.and Kirchhoffy J. and Gierschner, K. Daten
und Dokumente zum Umweltschutz Sonderreihe Umweltagung, No. 23, Tagung
Uber Umweltforachung der Universität Hohenheim, Schadstoffe in der
Nahrungskette, Dokumentationestelle der Universität Hohenheim,
January, 1979.
Herbold, B. Mikronucleus-Test an der Maus zur Prüfung auf Mutagene
Wirkung. (1978) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Salmonella/Mikrosomen-Test zur Untersuchung auf Punktmutagene
Wirkung. (1979a) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Zytogenctische Untersuchungen der Spermatogonien beim Chinesisechen
hamster zur Prüfung auf mugatene Wirkung. (1979b) Unpublished report
from the Institut für Toxikologie, submitted by Bayer AG.
Johansson, C.E. Var Zöda 26, 171 (1974)
A multiresidue Analytical Method for Determining Organochlorine,
Organophosphorus Dinitrphenyl and Carbamate Pesticide in Apples
(modified) Pestic. Sci. 9, 313-322 (1978).
Machemer, L. Dominant Lethal Study to Investigate Mutagenic
Potential. (1974a) Unpublished Report from the Institut für
Toxikologie, submitted by Bayer AG.
Studies for Embryotoxic and Teratogenic Effects on Rats following
oral administration. (1974b) Unpublished report from the Institute für
Toxikologie, submitted by Bayer AG.
Nitokuno Institute. (1972/76) Unpublished Analytical test results on
residues ubmitted by Bayer AG. Reports Nos. 56/72, 39/73, 14/76,
15/76 on cucumbers. Report No. 86/78 on Hops. Reports Nos. 60/72,
61/72, 15/75, 16/75 on strawberries. Reports Nos. 55/72 and 13/76 on
tomatoes. Reports No. 11/76 and 12/76 on watermelons.
Schuphan, I., Ebing, W. - Gesamtergebnis der Untersuchungen zum
Verhalten von Euparen WP (Dichlofluanid) im Boden
(Abbau-Laborversuche). BBA-Berlin, unveröffentlichter Bericht. 1979a.
Gesamtergebnis der Untersuchungen zum Verhalten von Eurpanen M WP
(Tolylfluanid) im Boden (Abbau-Laborversuche). BBA-Berlin,
unveröffentlichter Bericht. 1979b.
Thyssen, J. Mitteilung vom 30.1.1978 in Ecker, W. 1978 opus cit.
Vogeler, K., Steffan, H. Ullemeyer, H., Rapp, A. Zum Abbau von
Dichlofluanid in und auf Weintrauben und bei der Weinbercitung. Bayer
AG, Pflanzenschutz Anwendungstechnik, unveröffentlichter Bericht
RA-97, 19.2.1979.
Vonk, J.W., den Daas, H. Metabolism of (dichlofluoromethyl-14C)
Tolylfluanid on apples. TNO Report, October 10, 1977.
Westphal, D., Haque, A., Schuphan, I., and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Dichlofluanid (Euparen) in Erdbeeren. BBA-Berlin, unveroffentlichter
Bericht (1979).
In storage and processing
Strawberries treated in a normal way with non-radioactive
dichlofluanid to yield an analytically determined residue of 5.9 mg/kg
parent compound (I) and 1.6 mg/kg of the metabolite
dimethylaminosulfanilid (II) when harvested two days after treatment,
were subjected to various processing steps to give unwashed raw
berries, washed raw berries, canned fruits, pasteurized juice, and jam
which were analysed to evaluate the effect on residues (Hartman, et
al., 1979). Substantial reductions in residues were achieved by
processing as shown in Table 5.
Table 5. Concentration of active ingredient and metabolite on
strawberries and processed products.
Sample I, mg/kg II, mg/kg
Berries, unwashed 5.9 1.6
Berries, washed 0.35 0.85
Wash water 1.95 0.2
Juice, raw 0.02 0.85
Juice, pasteurized N.D.1 0.9
Pomace 0.35 1.4
Canned fruits 0.02 0.75
Jam N.D. 1 0.4
1 N.D. = not detectable.
Photodecomposition
In studies carried out in England, the degradation of dichlofluanid by
ultraviolet light was measured in methanol, benzene, and acetone
solution (Clark et al., 1978). Solutions in methanol or benzene
deposited a brown solid on the UV lamp surface (Hanovia, 100 W.)
stopping the reaction. In acetone solution darkening occurred, but no
solid separated, permitting a reaction time of one hour. Products
from the acetone solution included N,N-dimethyl-N'-phenylsulfamide,
phenyl isocyante, phenyl isothiocyanate, and dimethylamidosulfonyl
chloride. The presence of bis (dichlorofluoromethyl) disulfide,
1-(dichlorofluoromethylthio) propan-2-one and
1-(dichlorofluoromethyl-sulfonyl) propan-2-one. In vivo tests
against Botrytis einerea showed that irradiation decreased the
activity of dichlofluanid and that synergism did not occur.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Information was supplied by Sweden on the results of analysing twenty
samples of Swedish strawberries during 1979. No samples contained
detectable levels of dichlofluanid (<0.03 mg/kg, Sweden, 1979). The
results of monitoring surveys on several lots of produce imported into
Finland during 1978 are shown in Table 6 (Finland, 1979).
Table 6. Occurrence of residues of dichlofluanid in produce imported
into Finland in 1978
Commodity No. of positive results Found (mg/kg)
Cucumbers 6 0.01-0.28
Apples 1 0.06
Strawberries 2 <0.01, 0.11
Sweet peppers 1 0.10
Finland also supplied information on levels of dichlofluanid found in
Finnish strawberries over the period 1975-1978.
Table 7. Occurrence of residues in strawberries grown in Finland
Number of samples containing
Year Number of samples dichlofluanid levels within
analysed given range, mg/kg
<0.1 0.11-1.0 1.1-2.0
1975 98 34 7 -
1976 138 50 14 -
1977 139 48 20 -
1978 119 34 13 2
There was no explanation for the discrepancies in samples numbers.
METHODS OF RESIDUE ANALYSIS
The method of analysis in Sweden (1979) was reported to be that of
Johansson (1978). Finland reported using a method of Johansson (1974)
and Becker (1971).
NATIONAL MRLs REPORTED TO THE MEETING
The Swedish maximum acceptable level for dichlofluanid is 5 mg/kg for
fruits and vegetables except potatoes. Sweden (1979).
APPRAISAL
Data supplied in 1977 from residue trials in Germany (F.R.) were based
on legislation requiring analysis for total residues of dichlofluanid
and metabolite which has since been changed, invalidating that data
source. However, new data were received in 1979 from Japan and
Finland which support the 1974 and 1977 recommendations for MRLs for
apples, cucumbers, gooseberries, hops, strawberries and tomatoes.
Sufficient information on national limits for blackberries, eggplants,
peppers cereal grains and wheat straw was available to make it
possible to retain the 1977 MRLs on a temporary basis until such time
as valid data from supervised trials becomes available.
Extensive information was received on the pathways of degradation and
metabolism of dichlofluanid, including the fate of the
fluorine-containing moiety of the molecule in plants (especially
strawberries) and soil, satisfying the requirements of the 1977 Joint
Meeting. Dichlofluanid is degraded fairly rapidly as the
dichlofluoromethylmercapto group is easily cleaved off, leading to
unstable intermediates and ultimately to
thiazolidine-2-thione-4-carboxylic acid (TTCA), a reaction product of
thiophosgene and cysteine. Soil residues degraded to CO2 and
extractable products rapidly and extensively. After 63 days, 98.5% of
applied radioactivity had evolved as 14C-CO2, while only 0.80%
remained as extractable products.
Data were received on the effects of processing on residues in
strawberries. Washing reduced dichlofluanid residue from 5.9 mg/kg to
0.35 mg/kg. Further processing into pasteurized juice, canned fruits
and jam reduced the residue to not detectable, 0.02 mg/kg, and not
detectable respectively.
The manufacturer of dichlofluanid has indicated that it is not
recommending uses on leafy brassicas, negating the need for further
residue data on this crop.
RECOMMENDATIONS
The temporary maximum residue limits recommended in 1977 for
blackberries, eggplants, sweet peppers, cereal grain and wheat straw
are retained on a temporary basis until data indicating the level of
residues of the parent compound become available. The limits for all
present and past recommendations refer to dichlofluanid only.
Temporary maximum residue
Commodity Limit, mg/kg
Blackberries 15
Sweet peppers 2
Eggplants 1
Cereal grains 0.1
Wheat straw 0.5
FURTHER WORK OR INFORMATION
Required by 1982
1. Residue data for dichlofluanid only, from supervised trials on
blackberries, eggplants, peppers, cereal grains and wheat straw.
2. Studies to elucidate the accumulation seen in the thyroid.
REFERENCES
Becker, G. Gaschromatographische Simultan-Bestimmung von chlorierten
Kohlenwasserstoffen und säureestern in pflanglichem Material. Dtsch.
Lebensmittel.Resch. 67, 125 (1971).
Belonozhko, G.A., et al. Hygienic Evaluation of Euparen Residues in
Food Products (in Russian) Vop. Pitan. (2): 72-75. (Abstracted in
Pesticide Abstracts 1979).
Clark, T. and Watkins, D.A.M. - Photolysis of dichlofluanid. Pestic.
Sci. 9, 225-228 (1978).
Ecker, W. Biotransformation von (14c) Dichlofluanid in der Ratte.
(1978) Unpublished report from Institut für Pharmakokinetik, submitted
by Bayer AG.
Finland. Submission from national government of reports of State
Institute of Agricultural Chemistry, Helsinki. (1979).
Flucke, W. Untersuchungen zur Acuten Toxizität bei Ratten, Mässen,
Meerschweinchen Kanichen und Katzen. (1978) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Haque, A., Westphal, D., Schuphan, I. and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Tolylfuanid (Euparen M) in Erdbeeren. BBA-Berlin, Unveröffenlichter
Bericht. (1979).
Hartman, V.B., Wrieden, J.and Kirchhoffy J. and Gierschner, K. Daten
und Dokumente zum Umweltschutz Sonderreihe Umweltagung, No. 23, Tagung
Uber Umweltforachung der Universität Hohenheim, Schadstoffe in der
Nahrungskette, Dokumentationestelle der Universität Hohenheim,
January, 1979.
Herbold, B. Mikronucleus-Test an der Maus zur Prüfung auf Mutagene
Wirkung. (1978) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Salmonella/Mikrosomen-Test zur Untersuchung auf Punktmutagene
Wirkung. (1979a) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Zytogenctische Untersuchungen der Spermatogonien beim Chinesisechen
hamster zur Prüfung auf mugatene Wirkung. (1979b) Unpublished report
from the Institut für Toxikologie, submitted by Bayer AG.
Johansson, C.E. Var Zöda 26, 171 (1974)
A multiresidue Analytical Method for Determining Organochlorine,
Organophosphorus Dinitrphenyl and Carbamate Pesticide in Apples
(modified) Pestic. Sci. 9, 313-322 (1978).
Machemer, L. Dominant Lethal Study to Investigate Mutagenic
Potential. (1974a) Unpublished Report from the Institut für
Toxikologie, submitted by Bayer AG.
Studies for Embryotoxic and Teratogenic Effects on Rats following
oral administration. (1974b) Unpublished report from the Institute für
Toxikologie, submitted by Bayer AG.
Nitokuno Institute. (1972/76) Unpublished Analytical test results on
residues ubmitted by Bayer AG. Reports Nos. 56/72, 39/73, 14/76,
15/76 on cucumbers. Report No. 86/78 on Hops. Reports Nos. 60/72,
61/72, 15/75, 16/75 on strawberries. Reports Nos. 55/72 and 13/76 on
tomatoes. Reports No. 11/76 and 12/76 on watermelons.
Schuphan, I., Ebing, W. - Gesamtergebnis der Untersuchungen zum
Verhalten von Euparen WP (Dichlofluanid) im Boden
(Abbau-Laborversuche). BBA-Berlin, unveröffentlichter Bericht. 1979a.
Gesamtergebnis der Untersuchungen zum Verhalten von Eurpanen M WP
(Tolylfluanid) im Boden (Abbau-Laborversuche). BBA-Berlin,
unveröffentlichter Bericht. 1979b.
Thyssen, J. Mitteilung vom 30.1.1978 in Ecker, W. 1978 opus cit.
Vogeler, K., Steffan, H. Ullemeyer, H., Rapp, A. Zum Abbau von
Dichlofluanid in und auf Weintrauben und bei der Weinbercitung. Bayer
AG, Pflanzenschutz Anwendungstechnik, unveröffentlichter Bericht
RA-97, 19.2.1979.
Vonk, J.W., den Daas, H. Metabolism of (dichlofluoromethyl-14C)
Tolylfluanid on apples. TNO Report, October 10, 1977.
Westphal, D., Haque, A., Schuphan, I., and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Dichlofluanid (Euparen) in Erdbeeren. BBA-Berlin, unveroffentlichter
Bericht (1979).
 In storage and processing
Strawberries treated in a normal way with non-radioactive
dichlofluanid to yield an analytically determined residue of 5.9 mg/kg
parent compound (I) and 1.6 mg/kg of the metabolite
dimethylaminosulfanilid (II) when harvested two days after treatment,
were subjected to various processing steps to give unwashed raw
berries, washed raw berries, canned fruits, pasteurized juice, and jam
which were analysed to evaluate the effect on residues (Hartman, et
al., 1979). Substantial reductions in residues were achieved by
processing as shown in Table 5.
Table 5. Concentration of active ingredient and metabolite on
strawberries and processed products.
Sample I, mg/kg II, mg/kg
Berries, unwashed 5.9 1.6
Berries, washed 0.35 0.85
Wash water 1.95 0.2
Juice, raw 0.02 0.85
Juice, pasteurized N.D.1 0.9
Pomace 0.35 1.4
Canned fruits 0.02 0.75
Jam N.D. 1 0.4
1 N.D. = not detectable.
Photodecomposition
In studies carried out in England, the degradation of dichlofluanid by
ultraviolet light was measured in methanol, benzene, and acetone
solution (Clark et al., 1978). Solutions in methanol or benzene
deposited a brown solid on the UV lamp surface (Hanovia, 100 W.)
stopping the reaction. In acetone solution darkening occurred, but no
solid separated, permitting a reaction time of one hour. Products
from the acetone solution included N,N-dimethyl-N'-phenylsulfamide,
phenyl isocyante, phenyl isothiocyanate, and dimethylamidosulfonyl
chloride. The presence of bis (dichlorofluoromethyl) disulfide,
1-(dichlorofluoromethylthio) propan-2-one and
1-(dichlorofluoromethyl-sulfonyl) propan-2-one. In vivo tests
against Botrytis einerea showed that irradiation decreased the
activity of dichlofluanid and that synergism did not occur.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Information was supplied by Sweden on the results of analysing twenty
samples of Swedish strawberries during 1979. No samples contained
detectable levels of dichlofluanid (<0.03 mg/kg, Sweden, 1979). The
results of monitoring surveys on several lots of produce imported into
Finland during 1978 are shown in Table 6 (Finland, 1979).
Table 6. Occurrence of residues of dichlofluanid in produce imported
into Finland in 1978
Commodity No. of positive results Found (mg/kg)
Cucumbers 6 0.01-0.28
Apples 1 0.06
Strawberries 2 <0.01, 0.11
Sweet peppers 1 0.10
Finland also supplied information on levels of dichlofluanid found in
Finnish strawberries over the period 1975-1978.
Table 7. Occurrence of residues in strawberries grown in Finland
Number of samples containing
Year Number of samples dichlofluanid levels within
analysed given range, mg/kg
<0.1 0.11-1.0 1.1-2.0
1975 98 34 7 -
1976 138 50 14 -
1977 139 48 20 -
1978 119 34 13 2
There was no explanation for the discrepancies in samples numbers.
METHODS OF RESIDUE ANALYSIS
The method of analysis in Sweden (1979) was reported to be that of
Johansson (1978). Finland reported using a method of Johansson (1974)
and Becker (1971).
NATIONAL MRLs REPORTED TO THE MEETING
The Swedish maximum acceptable level for dichlofluanid is 5 mg/kg for
fruits and vegetables except potatoes. Sweden (1979).
APPRAISAL
Data supplied in 1977 from residue trials in Germany (F.R.) were based
on legislation requiring analysis for total residues of dichlofluanid
and metabolite which has since been changed, invalidating that data
source. However, new data were received in 1979 from Japan and
Finland which support the 1974 and 1977 recommendations for MRLs for
apples, cucumbers, gooseberries, hops, strawberries and tomatoes.
Sufficient information on national limits for blackberries, eggplants,
peppers cereal grains and wheat straw was available to make it
possible to retain the 1977 MRLs on a temporary basis until such time
as valid data from supervised trials becomes available.
Extensive information was received on the pathways of degradation and
metabolism of dichlofluanid, including the fate of the
fluorine-containing moiety of the molecule in plants (especially
strawberries) and soil, satisfying the requirements of the 1977 Joint
Meeting. Dichlofluanid is degraded fairly rapidly as the
dichlofluoromethylmercapto group is easily cleaved off, leading to
unstable intermediates and ultimately to
thiazolidine-2-thione-4-carboxylic acid (TTCA), a reaction product of
thiophosgene and cysteine. Soil residues degraded to CO2 and
extractable products rapidly and extensively. After 63 days, 98.5% of
applied radioactivity had evolved as 14C-CO2, while only 0.80%
remained as extractable products.
Data were received on the effects of processing on residues in
strawberries. Washing reduced dichlofluanid residue from 5.9 mg/kg to
0.35 mg/kg. Further processing into pasteurized juice, canned fruits
and jam reduced the residue to not detectable, 0.02 mg/kg, and not
detectable respectively.
The manufacturer of dichlofluanid has indicated that it is not
recommending uses on leafy brassicas, negating the need for further
residue data on this crop.
RECOMMENDATIONS
The temporary maximum residue limits recommended in 1977 for
blackberries, eggplants, sweet peppers, cereal grain and wheat straw
are retained on a temporary basis until data indicating the level of
residues of the parent compound become available. The limits for all
present and past recommendations refer to dichlofluanid only.
Temporary maximum residue
Commodity Limit, mg/kg
Blackberries 15
Sweet peppers 2
Eggplants 1
Cereal grains 0.1
Wheat straw 0.5
FURTHER WORK OR INFORMATION
Required by 1982
1. Residue data for dichlofluanid only, from supervised trials on
blackberries, eggplants, peppers, cereal grains and wheat straw.
2. Studies to elucidate the accumulation seen in the thyroid.
REFERENCES
Becker, G. Gaschromatographische Simultan-Bestimmung von chlorierten
Kohlenwasserstoffen und säureestern in pflanglichem Material. Dtsch.
Lebensmittel.Resch. 67, 125 (1971).
Belonozhko, G.A., et al. Hygienic Evaluation of Euparen Residues in
Food Products (in Russian) Vop. Pitan. (2): 72-75. (Abstracted in
Pesticide Abstracts 1979).
Clark, T. and Watkins, D.A.M. - Photolysis of dichlofluanid. Pestic.
Sci. 9, 225-228 (1978).
Ecker, W. Biotransformation von (14c) Dichlofluanid in der Ratte.
(1978) Unpublished report from Institut für Pharmakokinetik, submitted
by Bayer AG.
Finland. Submission from national government of reports of State
Institute of Agricultural Chemistry, Helsinki. (1979).
Flucke, W. Untersuchungen zur Acuten Toxizität bei Ratten, Mässen,
Meerschweinchen Kanichen und Katzen. (1978) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Haque, A., Westphal, D., Schuphan, I. and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Tolylfuanid (Euparen M) in Erdbeeren. BBA-Berlin, Unveröffenlichter
Bericht. (1979).
Hartman, V.B., Wrieden, J.and Kirchhoffy J. and Gierschner, K. Daten
und Dokumente zum Umweltschutz Sonderreihe Umweltagung, No. 23, Tagung
Uber Umweltforachung der Universität Hohenheim, Schadstoffe in der
Nahrungskette, Dokumentationestelle der Universität Hohenheim,
January, 1979.
Herbold, B. Mikronucleus-Test an der Maus zur Prüfung auf Mutagene
Wirkung. (1978) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Salmonella/Mikrosomen-Test zur Untersuchung auf Punktmutagene
Wirkung. (1979a) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Zytogenctische Untersuchungen der Spermatogonien beim Chinesisechen
hamster zur Prüfung auf mugatene Wirkung. (1979b) Unpublished report
from the Institut für Toxikologie, submitted by Bayer AG.
Johansson, C.E. Var Zöda 26, 171 (1974)
A multiresidue Analytical Method for Determining Organochlorine,
Organophosphorus Dinitrphenyl and Carbamate Pesticide in Apples
(modified) Pestic. Sci. 9, 313-322 (1978).
Machemer, L. Dominant Lethal Study to Investigate Mutagenic
Potential. (1974a) Unpublished Report from the Institut für
Toxikologie, submitted by Bayer AG.
Studies for Embryotoxic and Teratogenic Effects on Rats following
oral administration. (1974b) Unpublished report from the Institute für
Toxikologie, submitted by Bayer AG.
Nitokuno Institute. (1972/76) Unpublished Analytical test results on
residues ubmitted by Bayer AG. Reports Nos. 56/72, 39/73, 14/76,
15/76 on cucumbers. Report No. 86/78 on Hops. Reports Nos. 60/72,
61/72, 15/75, 16/75 on strawberries. Reports Nos. 55/72 and 13/76 on
tomatoes. Reports No. 11/76 and 12/76 on watermelons.
Schuphan, I., Ebing, W. - Gesamtergebnis der Untersuchungen zum
Verhalten von Euparen WP (Dichlofluanid) im Boden
(Abbau-Laborversuche). BBA-Berlin, unveröffentlichter Bericht. 1979a.
Gesamtergebnis der Untersuchungen zum Verhalten von Eurpanen M WP
(Tolylfluanid) im Boden (Abbau-Laborversuche). BBA-Berlin,
unveröffentlichter Bericht. 1979b.
Thyssen, J. Mitteilung vom 30.1.1978 in Ecker, W. 1978 opus cit.
Vogeler, K., Steffan, H. Ullemeyer, H., Rapp, A. Zum Abbau von
Dichlofluanid in und auf Weintrauben und bei der Weinbercitung. Bayer
AG, Pflanzenschutz Anwendungstechnik, unveröffentlichter Bericht
RA-97, 19.2.1979.
Vonk, J.W., den Daas, H. Metabolism of (dichlofluoromethyl-14C)
Tolylfluanid on apples. TNO Report, October 10, 1977.
Westphal, D., Haque, A., Schuphan, I., and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Dichlofluanid (Euparen) in Erdbeeren. BBA-Berlin, unveroffentlichter
Bericht (1979).
In storage and processing
Strawberries treated in a normal way with non-radioactive
dichlofluanid to yield an analytically determined residue of 5.9 mg/kg
parent compound (I) and 1.6 mg/kg of the metabolite
dimethylaminosulfanilid (II) when harvested two days after treatment,
were subjected to various processing steps to give unwashed raw
berries, washed raw berries, canned fruits, pasteurized juice, and jam
which were analysed to evaluate the effect on residues (Hartman, et
al., 1979). Substantial reductions in residues were achieved by
processing as shown in Table 5.
Table 5. Concentration of active ingredient and metabolite on
strawberries and processed products.
Sample I, mg/kg II, mg/kg
Berries, unwashed 5.9 1.6
Berries, washed 0.35 0.85
Wash water 1.95 0.2
Juice, raw 0.02 0.85
Juice, pasteurized N.D.1 0.9
Pomace 0.35 1.4
Canned fruits 0.02 0.75
Jam N.D. 1 0.4
1 N.D. = not detectable.
Photodecomposition
In studies carried out in England, the degradation of dichlofluanid by
ultraviolet light was measured in methanol, benzene, and acetone
solution (Clark et al., 1978). Solutions in methanol or benzene
deposited a brown solid on the UV lamp surface (Hanovia, 100 W.)
stopping the reaction. In acetone solution darkening occurred, but no
solid separated, permitting a reaction time of one hour. Products
from the acetone solution included N,N-dimethyl-N'-phenylsulfamide,
phenyl isocyante, phenyl isothiocyanate, and dimethylamidosulfonyl
chloride. The presence of bis (dichlorofluoromethyl) disulfide,
1-(dichlorofluoromethylthio) propan-2-one and
1-(dichlorofluoromethyl-sulfonyl) propan-2-one. In vivo tests
against Botrytis einerea showed that irradiation decreased the
activity of dichlofluanid and that synergism did not occur.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Information was supplied by Sweden on the results of analysing twenty
samples of Swedish strawberries during 1979. No samples contained
detectable levels of dichlofluanid (<0.03 mg/kg, Sweden, 1979). The
results of monitoring surveys on several lots of produce imported into
Finland during 1978 are shown in Table 6 (Finland, 1979).
Table 6. Occurrence of residues of dichlofluanid in produce imported
into Finland in 1978
Commodity No. of positive results Found (mg/kg)
Cucumbers 6 0.01-0.28
Apples 1 0.06
Strawberries 2 <0.01, 0.11
Sweet peppers 1 0.10
Finland also supplied information on levels of dichlofluanid found in
Finnish strawberries over the period 1975-1978.
Table 7. Occurrence of residues in strawberries grown in Finland
Number of samples containing
Year Number of samples dichlofluanid levels within
analysed given range, mg/kg
<0.1 0.11-1.0 1.1-2.0
1975 98 34 7 -
1976 138 50 14 -
1977 139 48 20 -
1978 119 34 13 2
There was no explanation for the discrepancies in samples numbers.
METHODS OF RESIDUE ANALYSIS
The method of analysis in Sweden (1979) was reported to be that of
Johansson (1978). Finland reported using a method of Johansson (1974)
and Becker (1971).
NATIONAL MRLs REPORTED TO THE MEETING
The Swedish maximum acceptable level for dichlofluanid is 5 mg/kg for
fruits and vegetables except potatoes. Sweden (1979).
APPRAISAL
Data supplied in 1977 from residue trials in Germany (F.R.) were based
on legislation requiring analysis for total residues of dichlofluanid
and metabolite which has since been changed, invalidating that data
source. However, new data were received in 1979 from Japan and
Finland which support the 1974 and 1977 recommendations for MRLs for
apples, cucumbers, gooseberries, hops, strawberries and tomatoes.
Sufficient information on national limits for blackberries, eggplants,
peppers cereal grains and wheat straw was available to make it
possible to retain the 1977 MRLs on a temporary basis until such time
as valid data from supervised trials becomes available.
Extensive information was received on the pathways of degradation and
metabolism of dichlofluanid, including the fate of the
fluorine-containing moiety of the molecule in plants (especially
strawberries) and soil, satisfying the requirements of the 1977 Joint
Meeting. Dichlofluanid is degraded fairly rapidly as the
dichlofluoromethylmercapto group is easily cleaved off, leading to
unstable intermediates and ultimately to
thiazolidine-2-thione-4-carboxylic acid (TTCA), a reaction product of
thiophosgene and cysteine. Soil residues degraded to CO2 and
extractable products rapidly and extensively. After 63 days, 98.5% of
applied radioactivity had evolved as 14C-CO2, while only 0.80%
remained as extractable products.
Data were received on the effects of processing on residues in
strawberries. Washing reduced dichlofluanid residue from 5.9 mg/kg to
0.35 mg/kg. Further processing into pasteurized juice, canned fruits
and jam reduced the residue to not detectable, 0.02 mg/kg, and not
detectable respectively.
The manufacturer of dichlofluanid has indicated that it is not
recommending uses on leafy brassicas, negating the need for further
residue data on this crop.
RECOMMENDATIONS
The temporary maximum residue limits recommended in 1977 for
blackberries, eggplants, sweet peppers, cereal grain and wheat straw
are retained on a temporary basis until data indicating the level of
residues of the parent compound become available. The limits for all
present and past recommendations refer to dichlofluanid only.
Temporary maximum residue
Commodity Limit, mg/kg
Blackberries 15
Sweet peppers 2
Eggplants 1
Cereal grains 0.1
Wheat straw 0.5
FURTHER WORK OR INFORMATION
Required by 1982
1. Residue data for dichlofluanid only, from supervised trials on
blackberries, eggplants, peppers, cereal grains and wheat straw.
2. Studies to elucidate the accumulation seen in the thyroid.
REFERENCES
Becker, G. Gaschromatographische Simultan-Bestimmung von chlorierten
Kohlenwasserstoffen und säureestern in pflanglichem Material. Dtsch.
Lebensmittel.Resch. 67, 125 (1971).
Belonozhko, G.A., et al. Hygienic Evaluation of Euparen Residues in
Food Products (in Russian) Vop. Pitan. (2): 72-75. (Abstracted in
Pesticide Abstracts 1979).
Clark, T. and Watkins, D.A.M. - Photolysis of dichlofluanid. Pestic.
Sci. 9, 225-228 (1978).
Ecker, W. Biotransformation von (14c) Dichlofluanid in der Ratte.
(1978) Unpublished report from Institut für Pharmakokinetik, submitted
by Bayer AG.
Finland. Submission from national government of reports of State
Institute of Agricultural Chemistry, Helsinki. (1979).
Flucke, W. Untersuchungen zur Acuten Toxizität bei Ratten, Mässen,
Meerschweinchen Kanichen und Katzen. (1978) Unpublished report from
the Institut für Toxikologie, submitted by Bayer AG.
Haque, A., Westphal, D., Schuphan, I. and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Tolylfuanid (Euparen M) in Erdbeeren. BBA-Berlin, Unveröffenlichter
Bericht. (1979).
Hartman, V.B., Wrieden, J.and Kirchhoffy J. and Gierschner, K. Daten
und Dokumente zum Umweltschutz Sonderreihe Umweltagung, No. 23, Tagung
Uber Umweltforachung der Universität Hohenheim, Schadstoffe in der
Nahrungskette, Dokumentationestelle der Universität Hohenheim,
January, 1979.
Herbold, B. Mikronucleus-Test an der Maus zur Prüfung auf Mutagene
Wirkung. (1978) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Salmonella/Mikrosomen-Test zur Untersuchung auf Punktmutagene
Wirkung. (1979a) Unpublished report from the Institut für Toxikologie,
submitted by Bayer AG.
Zytogenctische Untersuchungen der Spermatogonien beim Chinesisechen
hamster zur Prüfung auf mugatene Wirkung. (1979b) Unpublished report
from the Institut für Toxikologie, submitted by Bayer AG.
Johansson, C.E. Var Zöda 26, 171 (1974)
A multiresidue Analytical Method for Determining Organochlorine,
Organophosphorus Dinitrphenyl and Carbamate Pesticide in Apples
(modified) Pestic. Sci. 9, 313-322 (1978).
Machemer, L. Dominant Lethal Study to Investigate Mutagenic
Potential. (1974a) Unpublished Report from the Institut für
Toxikologie, submitted by Bayer AG.
Studies for Embryotoxic and Teratogenic Effects on Rats following
oral administration. (1974b) Unpublished report from the Institute für
Toxikologie, submitted by Bayer AG.
Nitokuno Institute. (1972/76) Unpublished Analytical test results on
residues ubmitted by Bayer AG. Reports Nos. 56/72, 39/73, 14/76,
15/76 on cucumbers. Report No. 86/78 on Hops. Reports Nos. 60/72,
61/72, 15/75, 16/75 on strawberries. Reports Nos. 55/72 and 13/76 on
tomatoes. Reports No. 11/76 and 12/76 on watermelons.
Schuphan, I., Ebing, W. - Gesamtergebnis der Untersuchungen zum
Verhalten von Euparen WP (Dichlofluanid) im Boden
(Abbau-Laborversuche). BBA-Berlin, unveröffentlichter Bericht. 1979a.
Gesamtergebnis der Untersuchungen zum Verhalten von Eurpanen M WP
(Tolylfluanid) im Boden (Abbau-Laborversuche). BBA-Berlin,
unveröffentlichter Bericht. 1979b.
Thyssen, J. Mitteilung vom 30.1.1978 in Ecker, W. 1978 opus cit.
Vogeler, K., Steffan, H. Ullemeyer, H., Rapp, A. Zum Abbau von
Dichlofluanid in und auf Weintrauben und bei der Weinbercitung. Bayer
AG, Pflanzenschutz Anwendungstechnik, unveröffentlichter Bericht
RA-97, 19.2.1979.
Vonk, J.W., den Daas, H. Metabolism of (dichlofluoromethyl-14C)
Tolylfluanid on apples. TNO Report, October 10, 1977.
Westphal, D., Haque, A., Schuphan, I., and Ebing, W. Zusammengefabte
Endergebnisse über das Abbau - und Metabolismus-verhalten von
Dichlofluanid (Euparen) in Erdbeeren. BBA-Berlin, unveroffentlichter
Bericht (1979).
