
DISULFOTON JMPR 1973
Disulfoton is a member of the demeton family of insecticides.
Demeton was reviewed by the 1965 Joint Meeting. The relationship of
disulfoton to other compounds comprising the demeton family can be
seen in Table 1 (Table 1 is reproduced in WHO Monograph FAD/RES/73.5a,
page 4).
Chemical name
O,O-diethyl 2-ethylthioethyl phosphorodithioate
Synonyms
Thiodemeton
DisystonR
S 276
BAYER 19 639
M-74 (common name in USSR)
Structural formula
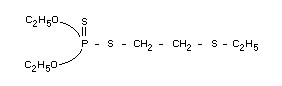 Empirical formula
C8H19O2PS3
Other information on properties
Appearance: colourless, oily liquid
Molecular weight: 274.4
Boiling point: 62°C at 0.01 mm Hg
82°C at 0.05 mm Hg
128°C at 1.0 mm Hg
Vapour pressure: 0.6 x 10-4 mm Hg at 10°C
1.8 x 10-4 mm Hg at 20°C
5.2 x 10-4 mm Hg at 30°C
14.0 x 10-4 mm Hg at 40°C
Volatility: 0.9 mg/m3 at 10°C
2.7 mg/m3 at 20°C
7.5 mg/m3 at 30°C
19.7 mg/m3 at 40°C
Specific gravity: 1.14 at 20°C C
4°
Solubility: approximately 1:40 000 in water at room
temperature: soluble in most organic solvents
Minimum purity: 94%
Impurities: 2-ethylthioethylchloride max. 0.2%
2-ethylthioethanethiol max. 0.2%
bis(2-ethylthioethyl)
disulfide max. 0.6%
O,O S-triethyl
phosphorodithioate max. 2.0%
O,O,O S-triethyl
phosphorothioate max. 0.5%
sulfotepp max. 0.5%
3 oligomeric alkyl(thio)
phosphates max. 1.5%
water max. 0.5%
Disulfoton is a member of the demeton family of insecticides.
Demeton was reviewed by the 1965 Joint Meeting (FAO/WHO, 19G6) and an
ADI was estimated for man to be 0.0025 mg/kg/day. The relationship of
disulfoton to the other compounds comprising the demeton family can be
seen in the monograph of demeton-methyl.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
There are essentially no data available on the absorption of
disulfoton in mammals. Studies on the metabolism and distribution in
mammals have been limited to mouse and intraperitoneal administration.
Following intraperitoneal administration of radio-labelled
disyston to mice, 30-60% of the radio-activity (depending on the dose
administered, 5-15 mg/kg) was recovered in the urine over a 96-hour
period. Approximately 2-3% of the radio-activity was recovered in the
faeces (March et al., 1957).
Metabolism
Two studies on the metabolism of disulfoton were consistent in
observations that disulfoton is rapidly metabolized by three major
biochemical reactions. The first reaction is the oxidation of the
thioether to produce sulfoxides and sulfones; the second reaction is
the oxidation of the thiono-sulfur moiety to produce the thiol
analogue; and the third reaction concerns the hydrolytic cleavage of
the P-S-C linkage of the phosphorothiolate moiety and the P-O-C
linkage of the ethyl ester moieties. The first two reactions act
independently but concurrently and are presumably responsible for
production of toxic metabolites. These oxidations produce increasingly
effective inhibitors of cholinesterase with the thiol analogue of the
sulfone being the most active. The rate of conversion of S to S = O is
considerably more rapid than the oxidation of P = S to P = O which is
about equal to the conversion of S = O to O = S = O (Bull, 1965; March
et al., 1957).
In comparative studies on various species of organisms, it was
found that the routes of metabolism in insects, plants, and mammals
are similar. The main differences are in the rates of reaction with
the reactions being fastest in the animal next in the insects, and
slowest in the plants. In mammals, the reaction to P = S to P = O
takes place at an exceptionally fast rate. In addition, no biological
conversion is known to occur with P(S)O converting rapidly to P(O)S
esters (Fukuto and Metcalf, 1954).
The following scheme depicts the metabolism of disulfoton in all
systems thus far studied:
Empirical formula
C8H19O2PS3
Other information on properties
Appearance: colourless, oily liquid
Molecular weight: 274.4
Boiling point: 62°C at 0.01 mm Hg
82°C at 0.05 mm Hg
128°C at 1.0 mm Hg
Vapour pressure: 0.6 x 10-4 mm Hg at 10°C
1.8 x 10-4 mm Hg at 20°C
5.2 x 10-4 mm Hg at 30°C
14.0 x 10-4 mm Hg at 40°C
Volatility: 0.9 mg/m3 at 10°C
2.7 mg/m3 at 20°C
7.5 mg/m3 at 30°C
19.7 mg/m3 at 40°C
Specific gravity: 1.14 at 20°C C
4°
Solubility: approximately 1:40 000 in water at room
temperature: soluble in most organic solvents
Minimum purity: 94%
Impurities: 2-ethylthioethylchloride max. 0.2%
2-ethylthioethanethiol max. 0.2%
bis(2-ethylthioethyl)
disulfide max. 0.6%
O,O S-triethyl
phosphorodithioate max. 2.0%
O,O,O S-triethyl
phosphorothioate max. 0.5%
sulfotepp max. 0.5%
3 oligomeric alkyl(thio)
phosphates max. 1.5%
water max. 0.5%
Disulfoton is a member of the demeton family of insecticides.
Demeton was reviewed by the 1965 Joint Meeting (FAO/WHO, 19G6) and an
ADI was estimated for man to be 0.0025 mg/kg/day. The relationship of
disulfoton to the other compounds comprising the demeton family can be
seen in the monograph of demeton-methyl.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
There are essentially no data available on the absorption of
disulfoton in mammals. Studies on the metabolism and distribution in
mammals have been limited to mouse and intraperitoneal administration.
Following intraperitoneal administration of radio-labelled
disyston to mice, 30-60% of the radio-activity (depending on the dose
administered, 5-15 mg/kg) was recovered in the urine over a 96-hour
period. Approximately 2-3% of the radio-activity was recovered in the
faeces (March et al., 1957).
Metabolism
Two studies on the metabolism of disulfoton were consistent in
observations that disulfoton is rapidly metabolized by three major
biochemical reactions. The first reaction is the oxidation of the
thioether to produce sulfoxides and sulfones; the second reaction is
the oxidation of the thiono-sulfur moiety to produce the thiol
analogue; and the third reaction concerns the hydrolytic cleavage of
the P-S-C linkage of the phosphorothiolate moiety and the P-O-C
linkage of the ethyl ester moieties. The first two reactions act
independently but concurrently and are presumably responsible for
production of toxic metabolites. These oxidations produce increasingly
effective inhibitors of cholinesterase with the thiol analogue of the
sulfone being the most active. The rate of conversion of S to S = O is
considerably more rapid than the oxidation of P = S to P = O which is
about equal to the conversion of S = O to O = S = O (Bull, 1965; March
et al., 1957).
In comparative studies on various species of organisms, it was
found that the routes of metabolism in insects, plants, and mammals
are similar. The main differences are in the rates of reaction with
the reactions being fastest in the animal next in the insects, and
slowest in the plants. In mammals, the reaction to P = S to P = O
takes place at an exceptionally fast rate. In addition, no biological
conversion is known to occur with P(S)O converting rapidly to P(O)S
esters (Fukuto and Metcalf, 1954).
The following scheme depicts the metabolism of disulfoton in all
systems thus far studied:
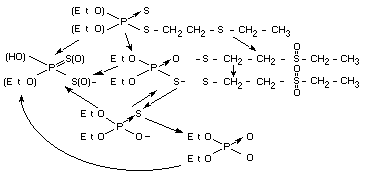 Effects on enzymes and other biochemical parameters
Disulfoton itself has been shown to have little if any effect on
cholinesterase or any other biochemical parameter in the body.
However, as noted above, it is rapidly oxidized to highly active in
vivo and in vitro cholinesterase inhibitors. In the demeton group
of cholinesterase inhibitors demeton-S is the most active compound to
mammalian cholinesterase activity. This can be noted in the pI50
values for rat brain. In contrast demeton-S-methyl sulfone is more
active than other isomers to purified insect cholinesterase. The
sulfoxide and sulfone of demeton-S and its sulfoxide have about the
same cholinesterase inhibiting property (rat brain) while the sulfone
of demeton-O has considerably less activity (FAO/WHO, 1965). In
mammals, demeton-S loses part,of its cholinesterase inhibiting
properties as the molecule undergoes this ether oxidation to the
sulfoxide and sulfone. This is also partly true with demeton-O
(although a less active inhibitor than demeton-S) where oxidation to
the sulfoxide does not change the pI50 value, but the sulfone has a
significantly reduced pI50 Disulfoton as with other
organophosphorodithioates is a poor inhibitor of cholinesterase but
is rapidly converted to an active inhibitor. This can be seen In Table
1 which shows the pI50 values of disulfoton and its oxygen analogue
demeton-S.
In rat brain, disulfoton sulfoxide is about 10 times more active
than disulfoton, whereas disulfoton sulfone has about the same
inhibitory power as disulfoton. Demeton-S however shows a tenfold
stronger inhibitory power than its sulfoxide and its sulfone. In
insects, whole weevil and fly head cholinesterases behave differently.
The whole weevil ChE becomes practically unaffected by disulfoton
sulfone. Disulfoton sulfoxide is only about three times as active as
disulfoton. Demeton-S, its sulfoxide and sulfone show about the same
anticholinesterase activity. In fly head cholinesterase there are no
great differences in the inhibition by disulfoton compared with
disulfoton sulfoxide, but disulfoton sulfone is about tenfold more
active as an inhibitor. Within the demeton group, the inhibitory power
increases with the oxidation to the sulfoxide and the sulfone
respectively, although there exist quantitative discrepancies between
different authors. In general, the demeton-S compounds are better
inhibitors than the compounds of the disulfoton group.
As with other cholinesterase-inhibiting organophosphate esters,
the effects on other biochemical parameters or enzymes were not
reported.
Following acute i.p. administration, 5/8 of the LD50 level,
cholinesterase activity of brain serum and submaxillary gland was
maximally depressed within a very short period of time (less than six
hours) to approximately the same level (15-257 of normal) after which
recovery was initiated but not complete within 72 hours. An induced
tolerance to the cholinergic stimulation has been observed with
disulfoton especially with chronic administration (Bombinski and
DuBois, 1958).
TABLE 1. pI50 - VALUES OF DISULFUTON AND DEMETON-S AND THEIR METABOLITES ON CHOLINESTERASES OF
INSECTS AND RAT BRAIN
Insectb Insectc Insectd Insecte Insecte Ratf,g
Compounda (whole weevil) (fly head) (fly head) (fly head) (fly head) brain
Disulfoton 3.62 - - 4.00 -- 3.85
Disulfoton-S (O) 4.08 - - 4.15 4.30 4.77
Disulfoton-S (O)(O) 2.00 - 5.90 5.46 4.92 4.00
Demeton-S 4.89 5.46 - 5.46 - 6.68
Demeton-S (O) 5.08 5.82 - 5.82 5.70 5.66
Demeton-S (O)(O) 4.66 6.22 6.49 6.22 7.70 5.70
a S (O) = Sulfoxide; S (O)(O) = Sulfone.
b Bull, 1965.
c March et al., 1955.
d Metcalf et al., 1957.
e March et al., 1957.
f FAO/WHO, 1965.
g Bombinski and DuBois, 1958.
Groups of rats (five female Sprague-Dawley strain per group) were
administered disulfoton intraperitoneally at dose levels of 0, 0.25,
0.5, 1.0, 1.2, and 1.5 mg/kg/day for 60 days. Mortality occurred at
the three highest dose levels. No mortality was evident at 0.5
mg/kg/day or below, although inhibition of growth was observed. At 1
mg/kg, typical signs of cholinergic stimulation were evident during
the first days of testing but within 10 days the animals recovered and
were symptomless thereon. Furthermore, these animals began to gain
weight and appeared to adapt to the continuous administration of
disulfoton (Bombinski and DuBois, 1958). A further series of rats was
treated with disulfoton at levels of 0, 0.25, 0.5, and 1.0 mg/kg daily
by intraperitoneal administration for 30 days. Cholinesterase data,
monitored over the course of this experiment, showed that there was an
initial rapid decrease of brain cholinesterase which was dependent
upon the daily dose of disulfoton. With the two highest doses, a
permanent decrease in enzyme activity occurred only during the first
seven days after which time further treatment resulted in maintenance
of the enzyme level at a constant subnormal level. The lowest dose
however induced a permanent decrease of brain cholinesterase activity.
The serum-ChE showed nearly the same time course and degree of
depression as the brain cholinesterase, but the cumulative effect of
the lowest dose between days 7-30 was not marked.
Clinical cholinergic signs of poisoning, including weight loss,
evident over the first seven days of treatment at the highest dose
level disappeared with the result being a symptomless cholinesterase
depression (Bombinski and DuBois, 1958). Further studies to determine
the mechanism of this acquired tolerance were reported (Brodeur and
DuBois, 1964).
It was observed that the development of tolerance to subacute
administration of disulfoton was not paralleled by changes in the
acetylcholine-cholinesterase system. The free acetylcholine level in
the brain was elevated to approximately the same extent by each
successive dose throughout the period of treatment. In addition, it
was believed that the development of tolerance did not involve the
metabolic conversion of disulfoton to its oxidative anticholinesterase
analogues (Stavinoha et al., 1972). These authors postulated that the
development of the tolerance resulting from high repeated
administration of disulfoton was due to the development of a
refractoriness of the cholinergic receptors to prolong exposure to
high levels of acetylcholine. It was further observed that strain
differences exist in the acquired tolerance to cholinergic
stimulation.
Groups of Charles River and Holtzmann rats received a daily
intraperitoneal injection of 1 mg disulfoton/kg for three, 10, and 24
days respectively. In each of the three groups the signs of poisoning
were most severe on the third day. The Holtzmann rats treated for 10
and 24 days exhibited early signs of adaptation while the Charles
River rats took longer to adapt. Measurements of cholinesterase
activity in the brain showed that acetyl cholinesterase activity as
expected decreased, the amount of depression being dependent on the
duration of the application while the acetylcholine concentration
initially increased. In the Holtzmann rat acetylcholine concentration
returned to the control level after 10 days of injections and was
still at the control level when measured after 24 days of treatment.
In the Charles River rats, the acetylcholine concentration of the
brain was still elevated at the end of the 24-day injection period. In
an experiment in which disulfoton was added to the diet at
concentrations of 0, 10, 25, and 50 ppm, it was observed that
adaptation progressed more slowly than in the intraperitoneal
injection experiment. The time required for adaptation was longer as
the amount of disulfoton in the diet was increased, e.g., 1 to 1-1/2
months at 10 ppm; 2 to 3 months at 25 ppm; and very little adaptation
was observed at 50 ppm. There was pronounced suppression of weight
gain at 50 ppm. Acetyl cholinesterase was reduced in relation to the
dietary concentration although only in one strain at the highest dose.
The acetylcholine concentration was not different from the control
level. Choline acetyltransferase activity was not affected (Stavinoha
et al., 1969).
Groups of weanling rats (six rats per group) were fed disulfoton
in the diet at levels of 0, 1, 5, and 25 ppm for seven days. At the
end of one week the animals were sacrificed for the measurement of the
hydrolysis of tributyrin and diethylsuccinate by liver and serum and
for the measurement of cholinesterase in serum, liver and brain.
Dietary levels of disulfoton producing 50% inhibition of aliesterases
and cholinesterase over this one week feeding period were obtained by
analysis of a plot of the logarithm of dietary concentration and
inhibition of the respective enzymes. There was no significant
difference in this experiment between the levels of inhibition caused
by disulfoton and demeton. The liver hydrolysis of tributyrin was the
most sensitive parameter followed by diethylsuccinate hydrolysis.
Depression of cholinesterase activity was caused in the brain by 5.2
ppm disulfoton in the diet; in the liver by 14.5 ppm and the serum by
6.0 ppm. Depression of liver aliesterase hydrolyzing DES was caused by
3.5 ppm while in the serum it was calculated to be 8.4 ppm.
Depression of the enzymes hydrolyzing tributyrin was caused in
the liver by 0.6 ppm and in the serum by 9.2 ppm disulfoton in the
diet. Although these authors point out that there is a possible
relationship of aliesterase inhibition with potentiation, studies (see
Potentiation Section) show that with a selected series of
organophosphorus compounds, potentiation was not demonstrated (Su et
al., 1971).
Results of a recent study of the inhibited portions of rat brain
following disulfoton administration is show in Table 2.
This experiment reveals a severe inhibition of the
cholinesterases in the hippocampus and caudate nucleus, compared with
the hypothalamus and medulla. The recovery of cholinesterase activity
occurred more rapidly in the first two mentioned parts of the brain
than in the others although at the end of the seven day recovery
period, activity in the hypothalamus and caudate nucleus is as still
low (Modek et al., 1971).
TABLE 2. CHOLINESTERASE ACTIVITYa
Tissue Control Treatmentb Recoveryc
Hypothalmus 4.62 1.87 3.27
Medulla 5.26 1.95 4.00
Hippocampus 3.19 0.62 1.79
Caudate nucleus 13.56 2.42 8.65
Ileum 2.90 2.38 2.80
Gastrocnemius 0.48 0.28 0.40
a mM substrate hydrolyzed/gm protein/h (Modek et al., 1971).
b Intraperitoneal administration for 10 days at 1.5 mg/kg/day to
rats.
c Recovery time = seven days.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of male mice (12 mice/group) were administered
disulfoton by intraperitoneal injection at doses of 0, 0.25, and 0.5
mg/kg. Each male was mated with three virgin females each week for six
weeks in the standard dominant lethal mutation test. There were no
abnormalities noted in the data on implantation resorption or on the
embryo itself. In this study, disulfoton did not exhibit any mutagenic
effect on male mice (Arnold et al., 1971).
Disulfoton inhibited growth of three human haematopoietic cell
lives but had no effect on chromosomes (Huang, 1973).
Special studies on reproduction
Rat. Groups of rats (20 females and 10 males/group) were fed dietary
levels of disulfoton at 0, 2, 5, and 10 ppm over the course of a
two-litter per generation, three generation reproduction study. There
did not appear to be a significant effect of disulfoton in the diet on
reproduction in the rat. Levels up to and including 10 ppm did not
significantly affect reproduction parameters. At 10 ppm in the F-1-A
there was a greater than normal mortality of rats at weaning time.
There was no significant difference in any of the groups in the number
of pregnancies or in the number of young per treatment groups.
Histological examination of the F-2-B rats indicated a cloudy swelling
of the liver cells with a fatty metamorphosis especially in male rats
on 10 ppm which was not observed in the controls. This was not
observed in similar F-2-B rats. RBC-cholinesterase depression was
obvious in all treatment groups examined. No gross differences were
observed between dale and female rats. The reduction was dose
dependent and was more significant in females than males. While
disulfoton does not appear to have a definitive effect upon
reproduction parameters, high levels of disulfoton in the diet (10
ppm) have shown somatic effects and levels of 2 ppm in the diet have
shown reduction in cholinesterase (Taylor, 1966).
Special studies on teratogenicity
Groups of 15 pregnant rabbits were administered disulfoton orally
in gelatin capsules at doses of 0, 0.1, and 0.2 mg/kg daily from days
six through 18 of gestation. On day 29 of gestation, the young were
removed by caesarean section. There were no deaths or unusual
reactions among the females in any of the groups and the incidence of
fetal mortality as indicated by resorption sites or abortion was not
affected by disulfoton. There was no indication of fetal external or
internal abnormalities and the weights of the fetuses were similar to
those of the controls. A positive treatment of this test was obtained
with thalidomide. Disulfoton does not appear to cause any teratogenic
effects in rabbits (Ladd et al., 1971).
Demeton while being embryotoxic as a single dose of 5 mg/kg for
three days on days 7-13 of gestation or as a single dose of 7 or 10
mg/kg during the same interval. Only a mild teratogenic potential was
noted (Budreau, 1972; Budreau and Singh, 1973).
Special studies on neurotoxicity
Adult hens were orally administered disulfoton at a dose
estimated to be the LD50 (26 mg/kg) twice at 21 day intervals and
maintained for a further 21 days. Growth and histological examination
of the animals indicated there were no signs of delayed neurotoxicity
while a positive control (TOCP) showed definite signs of poisoning.
Disulfoton does not induce delayed toxicity or demyelination (Fletcher
et al., 1971). Hens, protected from acute cholinergic stimulation and
organophosphorus poisoning by atropine and PAM were orally
administered disulfoton at levels of up to 0.1 ml/kg. Delayed
neurotoxic effects were not noted during the six weeks post-treatment
observation period (Kimmerle, 1961).
Special studies on the neurotoxicity of metabolite
Disulfoton sulfoxide was administered intraperitoneally at levels
up to 0.5 g/kg to hens previously administered PAM and atropine.
Although mortality was evident at high dose levels there was no
evidence of delayed neurotoxicity as observed normally with TOCP
(Hecht and Kimmerle, 1965).
Special studies on potentiation
Simultaneous administration of an LD50 dose with eight other
organophosphate insecticides resulted in a slight additive acute
toxicity with five compounds and less than additive acute toxicities
with the other three. None of the combinations resulted in a
potentiation of the acute toxicity Dubois, 1957 a & b). Further
studies with organophosphate and a carbamate ester were negative
(Dubois, 1960). Equitoxic mixtures of disulfoton and phenamiphos (the
active ingredient of NemacurR, ethyl 4-(methylthio)-m-tolyl
isopropyl phosphoroamidate) resulted in less than additive toxicity
(Kimmerle, 1972). A combination of disulfoton and phosphamidon did not
cause potentiation (Sachsse and Voss, 1971).
The signs of poisoning caused by disulfoton are typical of those
produced by anticholinesterase compounds. The signs of poisoning
consist of excitability, salivation, lacrimation, urination,
defaecation, and muscular fasciculations. The signs were followed by
convulsive seizures, prostration, and respiratory failure. As with
other organophosphorus compounds the occurrence of signs of poisoning
are indicative of both nicotinic and muscarinic actions of
acetylcholine indicating that the compound or its active metabolite
has gained access to both the central and peripheral nervous system.
The time of onset and duration of signs of poisoning are dependent
upon the dose. With lethal doses death usually occurred within the
first 48 hours but upon sublethal administration death was delayed for
several days. A comparison of the toxicity by disulfoton by various
routes i.p. and oral) indicate that the compound is well absorbed from
the GI tract. A considerable sex difference in susceptibility was
noted in rats in some studies (i.e. Bombinski and DuBois, 1958) with
the male being five times less sensitive than the females. This was
not noted with other species. Similar sex differences in
susceptibility of rats have been noted with other thiophosphates. It
has been suggested that because the oxygen analogues did not exhibit
this difference in sex susceptibility that the differences in the rate
or extent of conversion of thiophosphates to their toxic oxygen
analogues is presumably responsible for the observed sex difference.
Special studies on antidotes
Several studies on the antidotal properties of atropine and PAM
administered intramuscularly before and after oral administration of
disulfoton have the distinct therapeutic effects with these materials.
Atropine in combination with other oximes administered
intraperitoneally after the appearance of signs of poisoning also
produced a therapeutic effect (Kimmerle, 1961; Lorke and Kimmerle,
1968). Atropine, when injected intraperitoneally (100 mg/kg) prior to
a dose of disulfoton, protected rats from the acute oral effects of
poisoning of an LD50 dose. Acute administration of two times the LD50
proceeded by administration of atropine was lethal (Bombinski and
Dubois, 1958).
Studies on the antidotal effects of atropine and PAM with
disulfoton sulfoxide were similar to those reported with disulfoton
where a significant reduction in acute toxicity was noted with both
atropine and oximes alone. A more significant protective effect was
noted with a combination of both atropine and oxime (Hecht and
Kimmerle, 1965).
Special studies on inhalation
Female rats were exposed to concentrations of 0, 0.14, 0.35 and
0.70 microgram/litre in the air one hour a day for five or 10 days.
There was no mortality nor any significant decrease in cholinesterase
activity in brain, serum or submaxillary gland (DuBois and Kinoshita,
1971).
Acute Toxicity
(a) Original compound
Species Route LD50 Reference
(mg/kg)
Rat M & F oral 2.3-12.5 Ben-Dykeet al., 1970
DuBois, 1957; Gaines, 1969
Kimmerle, 1961, 1962, 1966, 1972
M oral 12.5 Bombinski and DuBois, 1958
F oral 2.6 Bombinski and DuBois, 1958
Guinea Pig M oral 10.8 Bodbinski and DuBois, 1958
M i.p. 7.0 Bombinski and DuBois, 1958
Rat M i.p. 10.5 Bombinski and DuBois, 1958
F i.p. 2.0 Bombinski and DuBois, 1958
Mouse M i.p. 5.5 Bombinski and DuBois, 1958
F i.p. 6.5 Bombinski and DuBois, 1958
Rat M dermal 25-50 Ben-Dyke et al., 1970
Gaines, 1969; Kimmerle, 1962
0.285
(4 hr exposure) Well et al., 1971
Species Route LD50 Reference
(mg/kg)
Rat M inhalations 200 g/m3
(1 hr exposure) Doull, 1957
Mouse F inhalation 58 mg/m3 Doull, 1957
(1 hr exposure
(b) Metabolites
Material Species Route LD50 Reference
(mg/kg)
Disulfoton Rat oral 1.7-6.5 Hecht and Kimmerle, 1965;
Sulfoxide Kimmerle, 1962;
Schrader, 1963; Wirth, 1958
Mouse oral 5.4 Bombinski and DuBois, 1958
Guinea Pig oral >3.6 Hecht and Kimmerle, 1965
Rabbit oral 2.5-3.6 Hecht and Kimmerle, 1965
Cat oral 1.0-2.5 Hecht and Kimmerle, 1965
Rat i.p. 5.0 Hecht and Kimmerle, 1965
Rat dermal 0.195 ml/kg Hecht and Kimmerle, 1965
4 hr
0.075 ml/kg Hecht and Kimmerle, 1965
7 day
0.192 ml/kg Kimmerle, 1962
Rat inhalation 140 g/m3 Hecht and Kimmerle, 1965
(1 hr exposure)
Disulfoton Rat oral 5.0-7.5 Schrader, 1963; Wirth, 1958
Sulfone
Mouse oral 5.6 Bombinski and Dubois, 1958
Demeton-S Rat oral 1.5-3.5 Klimmer and Pfaff, 1955;
(isosystox) Wirth, 1958
Material Species Route LD50 Reference
(mg/kg)
Mouse i.p. 5.6-7.0 Muhlmann and Tietz, 1956;
FAO/WHO, 1965
Guinea Pig i.p. 5.5 FAO/WHO, 1965
Demeton-S Rat oral 1.5-2.0 Schrader, 1963; Wirth, 1958
Sulfoxide
Demeton-S Rat oral 1.5-2 Schrader, 1963; Wirth,1958
Sulfone
In almost all instances, female rats were more susceptible than males.
Short-term studies
(a) Original compound
Mouse. Groups of mice (12 male and 12 female CF-LP strain per group)
were fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5.0 ppm
for 13 weeks. Food consumption and growth were measured weekly and
behaviour and mortality were observed daily. At 12 weeks, urinalysis
haematological examination and blood chemistry including BBC, plasma
and cholinesterase assays were performed. At the conclusion of the
study, gross and microscopic examination of tissues was performed. No
treatment-related changes were observed in growth, urinalysis,
haematology, or blood chemistry with the exception of cholinesterase
activity. Cholinesterase activity was reduced in all tissues at 5 ppm,
especially in females. Gross histological examination indicated a
slight increase in the liver weight in females at 5 ppm. There were no
other abnormalities noted on gross or microscopic examination of
tissues and organs. The no-effect level based upon this study in mice
is 1 ppm in the diet (Rivett et al., 1972).
Rat. Groups of rats (13 males and 13 females per group) were fed
disulfoton in the diet at levels of 0, 1, 2, 5 and 10 ppm for 16
weeks.
Food consumption and growth were measured daily for the first two
weeks every two days for the next month and then weekly until 16
weeks. At the end of the feeding period, three males and three females
were sacrificed and tissues examined for gross and microscopic
changes. Cholinesterase activity in serum, erythrocyte brain and
submaxillary gland was examined at eight and 16 weeks of feeding.
There were no effects of disulfoton in the diet at any dose level on
growth, food consumption, behaviour or mortality over the 16-week
period. Gross and microscopic examination of all tissues from both
male and female animals revealed no differences. Cholinesterase
depression was observed in erythrocyte and brain at levels of 2 ppm
and above at both eight and 16 weeks and was more marked in female
than male. Submaxillary gland and serum was less sensitive. A
no-effect level in this study based upon cholinesterase inhibition is
1 ppm in the diet (Doull and Vaughn, 1958).
Groups of rats (25 male and 25 female Wistar strain rats) were
fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5 ppm for 90
days. Body weight and food consumption data were recorded weekly and
behaviour and mortality was observed daily. Urinalysis, clinical
chemistry and haematological examinations including RBC and plasma
cholinesterase activity were made periodically. At the conclusion of
the study, brain cholinesterase activity was analysed and all animals
were sacrificed for gross and microscopic examination of tissues and
organs. There were no significant differences between the controls and
the treated animals with regard to growth, behaviour, mortality,
urinalysis, and clinical chemistry. There appear to be no effects of
the feeding of disulfoton on gross or histological examination of the
tissues at the conclusion of the study. Cholinesterase was
significantly depressed (primarily in females) in plasm and red blood
cells at 5 ppm. In this study, 1 ppm is a no-effect level based upon
cholinesterase inhibition Motzsche, 1972).
Dog. Groups of adult mongrel dogs (one male and one female per
group) were fed disulfoton in the diet at levels of 0, 1, 2, and 10
ppm for 12 weeks. At all feeding levels, no weight loss, signs of
poisoning or adverse behaviour were noted. Plasma and RBC
cholinesterase values were significantly decreased at 2 and 10 ppm
while 1 ppm caused do significant inhibition (Vaughn et al., 1958).
After returning to a control diet, the plasma cholinesterase
inhibition rapidly returned to normal. The RBC cholinesterase remained
inhibited for over four weeks.
(b) Metabolites (disulfoton sulfoxide)
Rat. Groups of rats (10 male rats per group) were administered
disulfoton sulfoxide orally, five doses per week, for one month at
dosage levels of 0, 0.215, 0.43, 0.9 mg/kg. Cholinesterase activity
was significantly depressed at the highest dose level and marginally
depressed at the middle dose level. At seven days after the initiation
of the study, cholinesterase depression reached a maximum level and
thereafter recovered slightly maintaining a constant depressed level
to the end of the experiment. Seven days after the conclusion of the
experiment, the cholinesterase values were essentially normal (Hecht
and Kimmerle, 1965).
Groups of rats (10 female rats per group) were administered
disulfoton sulfoxide daily five days per week for nine weeks at dosage
levels of 0, 0.046, 0.093, 0.186, 0.388 and 0.775 mg/kg/day. Mortality
was obvious at the highest dose level tested although growth was not
affected in any of the groups. Haematological and urinalysis were
normal as was gross pathology. Slight changes in liver epithelial
cells were noted in several animals at the highest dose level (Hecht
and Kimmerle, 1965). On the basis of these studies with disulfoton
sulfoxide with the most sensitive parameter being acetyl
cholinesterase depression, a level of 0.43 mg/kg administered orally
five days per week would be considered to be the marginal effect
level.
Long-term studies
Long-term feeding studies on rats and dogs have been initiated
but data are not available.
Observations in man
Five volunteer subjects each received a daily oral dose of 0.75
mg of disulfoton for 30 days; two persons served as controls. Plasma
and erythrocyte cholinesterase levels were measured twice weekly
during the pre-test control period and during the 30-day test period.
No depression of cholinesterase activity was noted (Rider, 1972).
Comments
Disulfoton, a phosphorodithioate insecticide structurally similar
to demeton, is acutely toxic and produces its primary effect through
inhibition of cholinesterase activity. Disulfoton.is metabolized by
thionate oxidation, thioether oxidation, and hydrolysis or oxidative
cleavage. Thionate oxidation would result in demeton which is further
degraded. As disulfoton is a fast acting organophosphate, the
oxidation of the thionate to demeton is apparently very rapid. Demeton
was evaluated by the Joint Meeting in 1965 and the ADI for man was
estimated to be 0.0025 mg/kg.
Toxicological studies showed disulfoton to have no effect on
reproduction and tests for teratogenicity and mutagenicity gave
negative results. Disulfoton did not produce delayed neurotoxicity in
hens nor potentiate the toxicity of several organophosphorus compounds
although it is an inhibitor of aliesterase activity. In short-term
studies in rats and dogs a no-effect level was estimated to be 1 ppm
based upon inhibition of cholinesterase. At higher levels liver damage
was observed. Studies in man showed that levels of 0.75 mg for 30 days
was without effect on cholinesterase activity.
Long-term studies have been reported to be in progress and on the
basis of short-term studies a temporary ADI was established.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect in animals
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw
Dog: 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man: 0.75 mg/man/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Disulfoton possesses systemic activity and is used to control
aphids, leafhoppers, thrips, beet flies (mangold fly, spinach leaf
miner), coffee leaf miner and spider mites. it is formulated as
granules and liquid concentrate and seed dressing powder (used only on
cotton). Disulfoton formulations are used on cotton, vegetables,
potatoes, cereals (chiefly sorghum and rice), coffee, etc. Products
based on disulfoton are registered in a total of 33 countries, 807
being used on vegetables including potatoes and 20% on field crops.
Pre-harvest treatments
Disulfoton is chiefly applied at sowing or as a top dressing. The
recommended application rates range from 1 to 4 kg/ha on most crops,
with pre-harvest intervals of from 30 to 100 days. These recommended
pre-harvest intervals are not necessarily identical in all countries.
Post-harvest treatments
No uses.
Other uses
Applied to ornamentals.
Residues resulting from supervised trials
A large amount of data are available on residues resulting from
the application of disulfoton to various crops. Most of them are from
the United States of America. Results are presented in Table 1.
TABLE 1
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Alfalfa 11 1-1.5 1 7-28 n.d.-0.7
(Forage) 4 1 3 7-28 6-30
(Hay) 8 1.5 1 3-7 5-15
2 1.5 3 7 14, 19
Beans 16 1-2 1 80-160 n.d.-0.3
Broccoli 7 1-4 1 7-80 n.d.-0.6
Brussels sprouts 4 1-2 1 7-100 n.d.-0.2
Barley (grain) 3 1 1 33-104 n.d.
Cabbage 8 1.7 oz/ 1 35-106 0.01-0.1
1000 ft row
Cabbage (in furrow)
5% granules) 6 1 1 28-87 0.1-1.5
Cotton 3 1.2 1 28 < 0.3
Cottonseed 16 1-4 1 30-180 n.d.-0.6
4 1-1.5 2 30-120 n.d.-0.2
Celery 5 1.2-2.5 1 55-130 n.d.-3
Coffee 6 1-4 oz/ 1 24-180 n.d.
plant
Clover 8 1.5-2 1 3-28 1-7
Clever (hay) 2 1.5 1 3 8, 17
Lettuce head or
leaf 5 1-2 1 16-62 <0.1
Maize 17 1-2 1 30-120 n.d-0.5
11 1-2 20-30 0.2-7
Oats (grain) 2 1 1 77, 98 n.d.
Potatoes 8 1-1.5 1 60-168 n.d-0.3
66 2-3 1 50-170 n.d,-0.4
8 3-3.5 1 86-180 0.2-2
11 3-4.5 2 60-180 0.05-0.3
TABLE 1 (Cont'd.)
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Peanuts 3 1-2 1 116-148 n.d
Peanuts (shells) 11 9-14 1 116-168 0.01-0.4
Peanuts (kernels) 3 16+32 2 70 0.1
Peas (including pods) 13 1-2 1 28-70 n.d.-0.3
2 2 1 28-43 4,9
Pineapple 14 1-5 1 7-70 n.d.
Rice 12 1-4 1 50-200 n.d,-0.5
Spinach 4 1 1 7-21 2-4
10 1 1 40-90 n.d.-0.5
Sorghum 17 1-1.5 1 30-80 n.d.-0.1
Sugar beets 11 1 1 98 0.1-0.3
11 1 1 51 n.d.-6
5 1-2.5 1 160-180 n.d.-0.1
Soybeans 2 1,2 1 132 n.d.
Tomatoes 10 0.5-6 1 or 2 30-108 n.d.-0.5
Wheat 24 0.75-4 1 30-300 n.d.-0.2
5 0.25-1 4 30-52 0.01-0.02
Pecans 5 1-20 1 88-240 n.d.
Soil persistence 1 0.5 1 0-366 n.d.
1 0.5 1 1-424 6-0.4
1 0.5 1 1-181 4-1
FATE OF RESIDUES
In plants
From knowledge of the ready biological oxidation of thioether
groups and in view of the known conversion of phosphothionates to
their P=O analogues, the expected metabolites of disulfoton (I) are
the compounds (II) to (VI):
Effects on enzymes and other biochemical parameters
Disulfoton itself has been shown to have little if any effect on
cholinesterase or any other biochemical parameter in the body.
However, as noted above, it is rapidly oxidized to highly active in
vivo and in vitro cholinesterase inhibitors. In the demeton group
of cholinesterase inhibitors demeton-S is the most active compound to
mammalian cholinesterase activity. This can be noted in the pI50
values for rat brain. In contrast demeton-S-methyl sulfone is more
active than other isomers to purified insect cholinesterase. The
sulfoxide and sulfone of demeton-S and its sulfoxide have about the
same cholinesterase inhibiting property (rat brain) while the sulfone
of demeton-O has considerably less activity (FAO/WHO, 1965). In
mammals, demeton-S loses part,of its cholinesterase inhibiting
properties as the molecule undergoes this ether oxidation to the
sulfoxide and sulfone. This is also partly true with demeton-O
(although a less active inhibitor than demeton-S) where oxidation to
the sulfoxide does not change the pI50 value, but the sulfone has a
significantly reduced pI50 Disulfoton as with other
organophosphorodithioates is a poor inhibitor of cholinesterase but
is rapidly converted to an active inhibitor. This can be seen In Table
1 which shows the pI50 values of disulfoton and its oxygen analogue
demeton-S.
In rat brain, disulfoton sulfoxide is about 10 times more active
than disulfoton, whereas disulfoton sulfone has about the same
inhibitory power as disulfoton. Demeton-S however shows a tenfold
stronger inhibitory power than its sulfoxide and its sulfone. In
insects, whole weevil and fly head cholinesterases behave differently.
The whole weevil ChE becomes practically unaffected by disulfoton
sulfone. Disulfoton sulfoxide is only about three times as active as
disulfoton. Demeton-S, its sulfoxide and sulfone show about the same
anticholinesterase activity. In fly head cholinesterase there are no
great differences in the inhibition by disulfoton compared with
disulfoton sulfoxide, but disulfoton sulfone is about tenfold more
active as an inhibitor. Within the demeton group, the inhibitory power
increases with the oxidation to the sulfoxide and the sulfone
respectively, although there exist quantitative discrepancies between
different authors. In general, the demeton-S compounds are better
inhibitors than the compounds of the disulfoton group.
As with other cholinesterase-inhibiting organophosphate esters,
the effects on other biochemical parameters or enzymes were not
reported.
Following acute i.p. administration, 5/8 of the LD50 level,
cholinesterase activity of brain serum and submaxillary gland was
maximally depressed within a very short period of time (less than six
hours) to approximately the same level (15-257 of normal) after which
recovery was initiated but not complete within 72 hours. An induced
tolerance to the cholinergic stimulation has been observed with
disulfoton especially with chronic administration (Bombinski and
DuBois, 1958).
TABLE 1. pI50 - VALUES OF DISULFUTON AND DEMETON-S AND THEIR METABOLITES ON CHOLINESTERASES OF
INSECTS AND RAT BRAIN
Insectb Insectc Insectd Insecte Insecte Ratf,g
Compounda (whole weevil) (fly head) (fly head) (fly head) (fly head) brain
Disulfoton 3.62 - - 4.00 -- 3.85
Disulfoton-S (O) 4.08 - - 4.15 4.30 4.77
Disulfoton-S (O)(O) 2.00 - 5.90 5.46 4.92 4.00
Demeton-S 4.89 5.46 - 5.46 - 6.68
Demeton-S (O) 5.08 5.82 - 5.82 5.70 5.66
Demeton-S (O)(O) 4.66 6.22 6.49 6.22 7.70 5.70
a S (O) = Sulfoxide; S (O)(O) = Sulfone.
b Bull, 1965.
c March et al., 1955.
d Metcalf et al., 1957.
e March et al., 1957.
f FAO/WHO, 1965.
g Bombinski and DuBois, 1958.
Groups of rats (five female Sprague-Dawley strain per group) were
administered disulfoton intraperitoneally at dose levels of 0, 0.25,
0.5, 1.0, 1.2, and 1.5 mg/kg/day for 60 days. Mortality occurred at
the three highest dose levels. No mortality was evident at 0.5
mg/kg/day or below, although inhibition of growth was observed. At 1
mg/kg, typical signs of cholinergic stimulation were evident during
the first days of testing but within 10 days the animals recovered and
were symptomless thereon. Furthermore, these animals began to gain
weight and appeared to adapt to the continuous administration of
disulfoton (Bombinski and DuBois, 1958). A further series of rats was
treated with disulfoton at levels of 0, 0.25, 0.5, and 1.0 mg/kg daily
by intraperitoneal administration for 30 days. Cholinesterase data,
monitored over the course of this experiment, showed that there was an
initial rapid decrease of brain cholinesterase which was dependent
upon the daily dose of disulfoton. With the two highest doses, a
permanent decrease in enzyme activity occurred only during the first
seven days after which time further treatment resulted in maintenance
of the enzyme level at a constant subnormal level. The lowest dose
however induced a permanent decrease of brain cholinesterase activity.
The serum-ChE showed nearly the same time course and degree of
depression as the brain cholinesterase, but the cumulative effect of
the lowest dose between days 7-30 was not marked.
Clinical cholinergic signs of poisoning, including weight loss,
evident over the first seven days of treatment at the highest dose
level disappeared with the result being a symptomless cholinesterase
depression (Bombinski and DuBois, 1958). Further studies to determine
the mechanism of this acquired tolerance were reported (Brodeur and
DuBois, 1964).
It was observed that the development of tolerance to subacute
administration of disulfoton was not paralleled by changes in the
acetylcholine-cholinesterase system. The free acetylcholine level in
the brain was elevated to approximately the same extent by each
successive dose throughout the period of treatment. In addition, it
was believed that the development of tolerance did not involve the
metabolic conversion of disulfoton to its oxidative anticholinesterase
analogues (Stavinoha et al., 1972). These authors postulated that the
development of the tolerance resulting from high repeated
administration of disulfoton was due to the development of a
refractoriness of the cholinergic receptors to prolong exposure to
high levels of acetylcholine. It was further observed that strain
differences exist in the acquired tolerance to cholinergic
stimulation.
Groups of Charles River and Holtzmann rats received a daily
intraperitoneal injection of 1 mg disulfoton/kg for three, 10, and 24
days respectively. In each of the three groups the signs of poisoning
were most severe on the third day. The Holtzmann rats treated for 10
and 24 days exhibited early signs of adaptation while the Charles
River rats took longer to adapt. Measurements of cholinesterase
activity in the brain showed that acetyl cholinesterase activity as
expected decreased, the amount of depression being dependent on the
duration of the application while the acetylcholine concentration
initially increased. In the Holtzmann rat acetylcholine concentration
returned to the control level after 10 days of injections and was
still at the control level when measured after 24 days of treatment.
In the Charles River rats, the acetylcholine concentration of the
brain was still elevated at the end of the 24-day injection period. In
an experiment in which disulfoton was added to the diet at
concentrations of 0, 10, 25, and 50 ppm, it was observed that
adaptation progressed more slowly than in the intraperitoneal
injection experiment. The time required for adaptation was longer as
the amount of disulfoton in the diet was increased, e.g., 1 to 1-1/2
months at 10 ppm; 2 to 3 months at 25 ppm; and very little adaptation
was observed at 50 ppm. There was pronounced suppression of weight
gain at 50 ppm. Acetyl cholinesterase was reduced in relation to the
dietary concentration although only in one strain at the highest dose.
The acetylcholine concentration was not different from the control
level. Choline acetyltransferase activity was not affected (Stavinoha
et al., 1969).
Groups of weanling rats (six rats per group) were fed disulfoton
in the diet at levels of 0, 1, 5, and 25 ppm for seven days. At the
end of one week the animals were sacrificed for the measurement of the
hydrolysis of tributyrin and diethylsuccinate by liver and serum and
for the measurement of cholinesterase in serum, liver and brain.
Dietary levels of disulfoton producing 50% inhibition of aliesterases
and cholinesterase over this one week feeding period were obtained by
analysis of a plot of the logarithm of dietary concentration and
inhibition of the respective enzymes. There was no significant
difference in this experiment between the levels of inhibition caused
by disulfoton and demeton. The liver hydrolysis of tributyrin was the
most sensitive parameter followed by diethylsuccinate hydrolysis.
Depression of cholinesterase activity was caused in the brain by 5.2
ppm disulfoton in the diet; in the liver by 14.5 ppm and the serum by
6.0 ppm. Depression of liver aliesterase hydrolyzing DES was caused by
3.5 ppm while in the serum it was calculated to be 8.4 ppm.
Depression of the enzymes hydrolyzing tributyrin was caused in
the liver by 0.6 ppm and in the serum by 9.2 ppm disulfoton in the
diet. Although these authors point out that there is a possible
relationship of aliesterase inhibition with potentiation, studies (see
Potentiation Section) show that with a selected series of
organophosphorus compounds, potentiation was not demonstrated (Su et
al., 1971).
Results of a recent study of the inhibited portions of rat brain
following disulfoton administration is show in Table 2.
This experiment reveals a severe inhibition of the
cholinesterases in the hippocampus and caudate nucleus, compared with
the hypothalamus and medulla. The recovery of cholinesterase activity
occurred more rapidly in the first two mentioned parts of the brain
than in the others although at the end of the seven day recovery
period, activity in the hypothalamus and caudate nucleus is as still
low (Modek et al., 1971).
TABLE 2. CHOLINESTERASE ACTIVITYa
Tissue Control Treatmentb Recoveryc
Hypothalmus 4.62 1.87 3.27
Medulla 5.26 1.95 4.00
Hippocampus 3.19 0.62 1.79
Caudate nucleus 13.56 2.42 8.65
Ileum 2.90 2.38 2.80
Gastrocnemius 0.48 0.28 0.40
a mM substrate hydrolyzed/gm protein/h (Modek et al., 1971).
b Intraperitoneal administration for 10 days at 1.5 mg/kg/day to
rats.
c Recovery time = seven days.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of male mice (12 mice/group) were administered
disulfoton by intraperitoneal injection at doses of 0, 0.25, and 0.5
mg/kg. Each male was mated with three virgin females each week for six
weeks in the standard dominant lethal mutation test. There were no
abnormalities noted in the data on implantation resorption or on the
embryo itself. In this study, disulfoton did not exhibit any mutagenic
effect on male mice (Arnold et al., 1971).
Disulfoton inhibited growth of three human haematopoietic cell
lives but had no effect on chromosomes (Huang, 1973).
Special studies on reproduction
Rat. Groups of rats (20 females and 10 males/group) were fed dietary
levels of disulfoton at 0, 2, 5, and 10 ppm over the course of a
two-litter per generation, three generation reproduction study. There
did not appear to be a significant effect of disulfoton in the diet on
reproduction in the rat. Levels up to and including 10 ppm did not
significantly affect reproduction parameters. At 10 ppm in the F-1-A
there was a greater than normal mortality of rats at weaning time.
There was no significant difference in any of the groups in the number
of pregnancies or in the number of young per treatment groups.
Histological examination of the F-2-B rats indicated a cloudy swelling
of the liver cells with a fatty metamorphosis especially in male rats
on 10 ppm which was not observed in the controls. This was not
observed in similar F-2-B rats. RBC-cholinesterase depression was
obvious in all treatment groups examined. No gross differences were
observed between dale and female rats. The reduction was dose
dependent and was more significant in females than males. While
disulfoton does not appear to have a definitive effect upon
reproduction parameters, high levels of disulfoton in the diet (10
ppm) have shown somatic effects and levels of 2 ppm in the diet have
shown reduction in cholinesterase (Taylor, 1966).
Special studies on teratogenicity
Groups of 15 pregnant rabbits were administered disulfoton orally
in gelatin capsules at doses of 0, 0.1, and 0.2 mg/kg daily from days
six through 18 of gestation. On day 29 of gestation, the young were
removed by caesarean section. There were no deaths or unusual
reactions among the females in any of the groups and the incidence of
fetal mortality as indicated by resorption sites or abortion was not
affected by disulfoton. There was no indication of fetal external or
internal abnormalities and the weights of the fetuses were similar to
those of the controls. A positive treatment of this test was obtained
with thalidomide. Disulfoton does not appear to cause any teratogenic
effects in rabbits (Ladd et al., 1971).
Demeton while being embryotoxic as a single dose of 5 mg/kg for
three days on days 7-13 of gestation or as a single dose of 7 or 10
mg/kg during the same interval. Only a mild teratogenic potential was
noted (Budreau, 1972; Budreau and Singh, 1973).
Special studies on neurotoxicity
Adult hens were orally administered disulfoton at a dose
estimated to be the LD50 (26 mg/kg) twice at 21 day intervals and
maintained for a further 21 days. Growth and histological examination
of the animals indicated there were no signs of delayed neurotoxicity
while a positive control (TOCP) showed definite signs of poisoning.
Disulfoton does not induce delayed toxicity or demyelination (Fletcher
et al., 1971). Hens, protected from acute cholinergic stimulation and
organophosphorus poisoning by atropine and PAM were orally
administered disulfoton at levels of up to 0.1 ml/kg. Delayed
neurotoxic effects were not noted during the six weeks post-treatment
observation period (Kimmerle, 1961).
Special studies on the neurotoxicity of metabolite
Disulfoton sulfoxide was administered intraperitoneally at levels
up to 0.5 g/kg to hens previously administered PAM and atropine.
Although mortality was evident at high dose levels there was no
evidence of delayed neurotoxicity as observed normally with TOCP
(Hecht and Kimmerle, 1965).
Special studies on potentiation
Simultaneous administration of an LD50 dose with eight other
organophosphate insecticides resulted in a slight additive acute
toxicity with five compounds and less than additive acute toxicities
with the other three. None of the combinations resulted in a
potentiation of the acute toxicity Dubois, 1957 a & b). Further
studies with organophosphate and a carbamate ester were negative
(Dubois, 1960). Equitoxic mixtures of disulfoton and phenamiphos (the
active ingredient of NemacurR, ethyl 4-(methylthio)-m-tolyl
isopropyl phosphoroamidate) resulted in less than additive toxicity
(Kimmerle, 1972). A combination of disulfoton and phosphamidon did not
cause potentiation (Sachsse and Voss, 1971).
The signs of poisoning caused by disulfoton are typical of those
produced by anticholinesterase compounds. The signs of poisoning
consist of excitability, salivation, lacrimation, urination,
defaecation, and muscular fasciculations. The signs were followed by
convulsive seizures, prostration, and respiratory failure. As with
other organophosphorus compounds the occurrence of signs of poisoning
are indicative of both nicotinic and muscarinic actions of
acetylcholine indicating that the compound or its active metabolite
has gained access to both the central and peripheral nervous system.
The time of onset and duration of signs of poisoning are dependent
upon the dose. With lethal doses death usually occurred within the
first 48 hours but upon sublethal administration death was delayed for
several days. A comparison of the toxicity by disulfoton by various
routes i.p. and oral) indicate that the compound is well absorbed from
the GI tract. A considerable sex difference in susceptibility was
noted in rats in some studies (i.e. Bombinski and DuBois, 1958) with
the male being five times less sensitive than the females. This was
not noted with other species. Similar sex differences in
susceptibility of rats have been noted with other thiophosphates. It
has been suggested that because the oxygen analogues did not exhibit
this difference in sex susceptibility that the differences in the rate
or extent of conversion of thiophosphates to their toxic oxygen
analogues is presumably responsible for the observed sex difference.
Special studies on antidotes
Several studies on the antidotal properties of atropine and PAM
administered intramuscularly before and after oral administration of
disulfoton have the distinct therapeutic effects with these materials.
Atropine in combination with other oximes administered
intraperitoneally after the appearance of signs of poisoning also
produced a therapeutic effect (Kimmerle, 1961; Lorke and Kimmerle,
1968). Atropine, when injected intraperitoneally (100 mg/kg) prior to
a dose of disulfoton, protected rats from the acute oral effects of
poisoning of an LD50 dose. Acute administration of two times the LD50
proceeded by administration of atropine was lethal (Bombinski and
Dubois, 1958).
Studies on the antidotal effects of atropine and PAM with
disulfoton sulfoxide were similar to those reported with disulfoton
where a significant reduction in acute toxicity was noted with both
atropine and oximes alone. A more significant protective effect was
noted with a combination of both atropine and oxime (Hecht and
Kimmerle, 1965).
Special studies on inhalation
Female rats were exposed to concentrations of 0, 0.14, 0.35 and
0.70 microgram/litre in the air one hour a day for five or 10 days.
There was no mortality nor any significant decrease in cholinesterase
activity in brain, serum or submaxillary gland (DuBois and Kinoshita,
1971).
Acute Toxicity
(a) Original compound
Species Route LD50 Reference
(mg/kg)
Rat M & F oral 2.3-12.5 Ben-Dykeet al., 1970
DuBois, 1957; Gaines, 1969
Kimmerle, 1961, 1962, 1966, 1972
M oral 12.5 Bombinski and DuBois, 1958
F oral 2.6 Bombinski and DuBois, 1958
Guinea Pig M oral 10.8 Bodbinski and DuBois, 1958
M i.p. 7.0 Bombinski and DuBois, 1958
Rat M i.p. 10.5 Bombinski and DuBois, 1958
F i.p. 2.0 Bombinski and DuBois, 1958
Mouse M i.p. 5.5 Bombinski and DuBois, 1958
F i.p. 6.5 Bombinski and DuBois, 1958
Rat M dermal 25-50 Ben-Dyke et al., 1970
Gaines, 1969; Kimmerle, 1962
0.285
(4 hr exposure) Well et al., 1971
Species Route LD50 Reference
(mg/kg)
Rat M inhalations 200 g/m3
(1 hr exposure) Doull, 1957
Mouse F inhalation 58 mg/m3 Doull, 1957
(1 hr exposure
(b) Metabolites
Material Species Route LD50 Reference
(mg/kg)
Disulfoton Rat oral 1.7-6.5 Hecht and Kimmerle, 1965;
Sulfoxide Kimmerle, 1962;
Schrader, 1963; Wirth, 1958
Mouse oral 5.4 Bombinski and DuBois, 1958
Guinea Pig oral >3.6 Hecht and Kimmerle, 1965
Rabbit oral 2.5-3.6 Hecht and Kimmerle, 1965
Cat oral 1.0-2.5 Hecht and Kimmerle, 1965
Rat i.p. 5.0 Hecht and Kimmerle, 1965
Rat dermal 0.195 ml/kg Hecht and Kimmerle, 1965
4 hr
0.075 ml/kg Hecht and Kimmerle, 1965
7 day
0.192 ml/kg Kimmerle, 1962
Rat inhalation 140 g/m3 Hecht and Kimmerle, 1965
(1 hr exposure)
Disulfoton Rat oral 5.0-7.5 Schrader, 1963; Wirth, 1958
Sulfone
Mouse oral 5.6 Bombinski and Dubois, 1958
Demeton-S Rat oral 1.5-3.5 Klimmer and Pfaff, 1955;
(isosystox) Wirth, 1958
Material Species Route LD50 Reference
(mg/kg)
Mouse i.p. 5.6-7.0 Muhlmann and Tietz, 1956;
FAO/WHO, 1965
Guinea Pig i.p. 5.5 FAO/WHO, 1965
Demeton-S Rat oral 1.5-2.0 Schrader, 1963; Wirth, 1958
Sulfoxide
Demeton-S Rat oral 1.5-2 Schrader, 1963; Wirth,1958
Sulfone
In almost all instances, female rats were more susceptible than males.
Short-term studies
(a) Original compound
Mouse. Groups of mice (12 male and 12 female CF-LP strain per group)
were fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5.0 ppm
for 13 weeks. Food consumption and growth were measured weekly and
behaviour and mortality were observed daily. At 12 weeks, urinalysis
haematological examination and blood chemistry including BBC, plasma
and cholinesterase assays were performed. At the conclusion of the
study, gross and microscopic examination of tissues was performed. No
treatment-related changes were observed in growth, urinalysis,
haematology, or blood chemistry with the exception of cholinesterase
activity. Cholinesterase activity was reduced in all tissues at 5 ppm,
especially in females. Gross histological examination indicated a
slight increase in the liver weight in females at 5 ppm. There were no
other abnormalities noted on gross or microscopic examination of
tissues and organs. The no-effect level based upon this study in mice
is 1 ppm in the diet (Rivett et al., 1972).
Rat. Groups of rats (13 males and 13 females per group) were fed
disulfoton in the diet at levels of 0, 1, 2, 5 and 10 ppm for 16
weeks.
Food consumption and growth were measured daily for the first two
weeks every two days for the next month and then weekly until 16
weeks. At the end of the feeding period, three males and three females
were sacrificed and tissues examined for gross and microscopic
changes. Cholinesterase activity in serum, erythrocyte brain and
submaxillary gland was examined at eight and 16 weeks of feeding.
There were no effects of disulfoton in the diet at any dose level on
growth, food consumption, behaviour or mortality over the 16-week
period. Gross and microscopic examination of all tissues from both
male and female animals revealed no differences. Cholinesterase
depression was observed in erythrocyte and brain at levels of 2 ppm
and above at both eight and 16 weeks and was more marked in female
than male. Submaxillary gland and serum was less sensitive. A
no-effect level in this study based upon cholinesterase inhibition is
1 ppm in the diet (Doull and Vaughn, 1958).
Groups of rats (25 male and 25 female Wistar strain rats) were
fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5 ppm for 90
days. Body weight and food consumption data were recorded weekly and
behaviour and mortality was observed daily. Urinalysis, clinical
chemistry and haematological examinations including RBC and plasma
cholinesterase activity were made periodically. At the conclusion of
the study, brain cholinesterase activity was analysed and all animals
were sacrificed for gross and microscopic examination of tissues and
organs. There were no significant differences between the controls and
the treated animals with regard to growth, behaviour, mortality,
urinalysis, and clinical chemistry. There appear to be no effects of
the feeding of disulfoton on gross or histological examination of the
tissues at the conclusion of the study. Cholinesterase was
significantly depressed (primarily in females) in plasm and red blood
cells at 5 ppm. In this study, 1 ppm is a no-effect level based upon
cholinesterase inhibition Motzsche, 1972).
Dog. Groups of adult mongrel dogs (one male and one female per
group) were fed disulfoton in the diet at levels of 0, 1, 2, and 10
ppm for 12 weeks. At all feeding levels, no weight loss, signs of
poisoning or adverse behaviour were noted. Plasma and RBC
cholinesterase values were significantly decreased at 2 and 10 ppm
while 1 ppm caused do significant inhibition (Vaughn et al., 1958).
After returning to a control diet, the plasma cholinesterase
inhibition rapidly returned to normal. The RBC cholinesterase remained
inhibited for over four weeks.
(b) Metabolites (disulfoton sulfoxide)
Rat. Groups of rats (10 male rats per group) were administered
disulfoton sulfoxide orally, five doses per week, for one month at
dosage levels of 0, 0.215, 0.43, 0.9 mg/kg. Cholinesterase activity
was significantly depressed at the highest dose level and marginally
depressed at the middle dose level. At seven days after the initiation
of the study, cholinesterase depression reached a maximum level and
thereafter recovered slightly maintaining a constant depressed level
to the end of the experiment. Seven days after the conclusion of the
experiment, the cholinesterase values were essentially normal (Hecht
and Kimmerle, 1965).
Groups of rats (10 female rats per group) were administered
disulfoton sulfoxide daily five days per week for nine weeks at dosage
levels of 0, 0.046, 0.093, 0.186, 0.388 and 0.775 mg/kg/day. Mortality
was obvious at the highest dose level tested although growth was not
affected in any of the groups. Haematological and urinalysis were
normal as was gross pathology. Slight changes in liver epithelial
cells were noted in several animals at the highest dose level (Hecht
and Kimmerle, 1965). On the basis of these studies with disulfoton
sulfoxide with the most sensitive parameter being acetyl
cholinesterase depression, a level of 0.43 mg/kg administered orally
five days per week would be considered to be the marginal effect
level.
Long-term studies
Long-term feeding studies on rats and dogs have been initiated
but data are not available.
Observations in man
Five volunteer subjects each received a daily oral dose of 0.75
mg of disulfoton for 30 days; two persons served as controls. Plasma
and erythrocyte cholinesterase levels were measured twice weekly
during the pre-test control period and during the 30-day test period.
No depression of cholinesterase activity was noted (Rider, 1972).
Comments
Disulfoton, a phosphorodithioate insecticide structurally similar
to demeton, is acutely toxic and produces its primary effect through
inhibition of cholinesterase activity. Disulfoton.is metabolized by
thionate oxidation, thioether oxidation, and hydrolysis or oxidative
cleavage. Thionate oxidation would result in demeton which is further
degraded. As disulfoton is a fast acting organophosphate, the
oxidation of the thionate to demeton is apparently very rapid. Demeton
was evaluated by the Joint Meeting in 1965 and the ADI for man was
estimated to be 0.0025 mg/kg.
Toxicological studies showed disulfoton to have no effect on
reproduction and tests for teratogenicity and mutagenicity gave
negative results. Disulfoton did not produce delayed neurotoxicity in
hens nor potentiate the toxicity of several organophosphorus compounds
although it is an inhibitor of aliesterase activity. In short-term
studies in rats and dogs a no-effect level was estimated to be 1 ppm
based upon inhibition of cholinesterase. At higher levels liver damage
was observed. Studies in man showed that levels of 0.75 mg for 30 days
was without effect on cholinesterase activity.
Long-term studies have been reported to be in progress and on the
basis of short-term studies a temporary ADI was established.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect in animals
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw
Dog: 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man: 0.75 mg/man/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Disulfoton possesses systemic activity and is used to control
aphids, leafhoppers, thrips, beet flies (mangold fly, spinach leaf
miner), coffee leaf miner and spider mites. it is formulated as
granules and liquid concentrate and seed dressing powder (used only on
cotton). Disulfoton formulations are used on cotton, vegetables,
potatoes, cereals (chiefly sorghum and rice), coffee, etc. Products
based on disulfoton are registered in a total of 33 countries, 807
being used on vegetables including potatoes and 20% on field crops.
Pre-harvest treatments
Disulfoton is chiefly applied at sowing or as a top dressing. The
recommended application rates range from 1 to 4 kg/ha on most crops,
with pre-harvest intervals of from 30 to 100 days. These recommended
pre-harvest intervals are not necessarily identical in all countries.
Post-harvest treatments
No uses.
Other uses
Applied to ornamentals.
Residues resulting from supervised trials
A large amount of data are available on residues resulting from
the application of disulfoton to various crops. Most of them are from
the United States of America. Results are presented in Table 1.
TABLE 1
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Alfalfa 11 1-1.5 1 7-28 n.d.-0.7
(Forage) 4 1 3 7-28 6-30
(Hay) 8 1.5 1 3-7 5-15
2 1.5 3 7 14, 19
Beans 16 1-2 1 80-160 n.d.-0.3
Broccoli 7 1-4 1 7-80 n.d.-0.6
Brussels sprouts 4 1-2 1 7-100 n.d.-0.2
Barley (grain) 3 1 1 33-104 n.d.
Cabbage 8 1.7 oz/ 1 35-106 0.01-0.1
1000 ft row
Cabbage (in furrow)
5% granules) 6 1 1 28-87 0.1-1.5
Cotton 3 1.2 1 28 < 0.3
Cottonseed 16 1-4 1 30-180 n.d.-0.6
4 1-1.5 2 30-120 n.d.-0.2
Celery 5 1.2-2.5 1 55-130 n.d.-3
Coffee 6 1-4 oz/ 1 24-180 n.d.
plant
Clover 8 1.5-2 1 3-28 1-7
Clever (hay) 2 1.5 1 3 8, 17
Lettuce head or
leaf 5 1-2 1 16-62 <0.1
Maize 17 1-2 1 30-120 n.d-0.5
11 1-2 20-30 0.2-7
Oats (grain) 2 1 1 77, 98 n.d.
Potatoes 8 1-1.5 1 60-168 n.d-0.3
66 2-3 1 50-170 n.d,-0.4
8 3-3.5 1 86-180 0.2-2
11 3-4.5 2 60-180 0.05-0.3
TABLE 1 (Cont'd.)
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Peanuts 3 1-2 1 116-148 n.d
Peanuts (shells) 11 9-14 1 116-168 0.01-0.4
Peanuts (kernels) 3 16+32 2 70 0.1
Peas (including pods) 13 1-2 1 28-70 n.d.-0.3
2 2 1 28-43 4,9
Pineapple 14 1-5 1 7-70 n.d.
Rice 12 1-4 1 50-200 n.d,-0.5
Spinach 4 1 1 7-21 2-4
10 1 1 40-90 n.d.-0.5
Sorghum 17 1-1.5 1 30-80 n.d.-0.1
Sugar beets 11 1 1 98 0.1-0.3
11 1 1 51 n.d.-6
5 1-2.5 1 160-180 n.d.-0.1
Soybeans 2 1,2 1 132 n.d.
Tomatoes 10 0.5-6 1 or 2 30-108 n.d.-0.5
Wheat 24 0.75-4 1 30-300 n.d.-0.2
5 0.25-1 4 30-52 0.01-0.02
Pecans 5 1-20 1 88-240 n.d.
Soil persistence 1 0.5 1 0-366 n.d.
1 0.5 1 1-424 6-0.4
1 0.5 1 1-181 4-1
FATE OF RESIDUES
In plants
From knowledge of the ready biological oxidation of thioether
groups and in view of the known conversion of phosphothionates to
their P=O analogues, the expected metabolites of disulfoton (I) are
the compounds (II) to (VI):
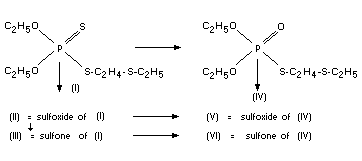 Compound (IV) is demeton-S, one of the active ingredients of the
well-known systemic insecticide SystoxR. Thus the metabolism of
disulfoton dovetails into the metabolism of demeton-S. However, it
should be noted that owing to the rapid formation of the sulfoxides,
the occurrence of (IV) as a metabolite is hardly to be expected.
Following the formation of these metabolites, further degradation can
only be by hydrolysis. The plant metabolism of disulfoton was studied
by the use of 32-p labelled compound (Metcalf et al., 1957, 1959) in
cotton, lemon, bean and alfalfa plants. Disulfoton was rapidly
oxidized to produce the sulfoxide (II) and slowly to produce sulfone
(III). Both those compounds were also oxidized at the thiono-sulfur to
produce V and VI. These same compounds were identified by Bull (1965)
working with other plants, e.g., avocado, brussels sprouts, cabbage,
corn, tomato. The same results were obtained but the proportions of
various compound differed. These studies we re confirmed by later
investigations.
Loeffler (1970b) found II and VI as major metabolites in tobacco.
Gentry et al., (1970) found that in tobacco the order was: Ill, II,
VI, V (see also Bowman et al., 1969).
In soil and water
Generally, the half-life of disulfoton residues in different
soils is between 30 and 100 days (Olson, 1964; Loeffler, 1969). Soil
type and microbial activity seem to have a greater influence on the
rate of decomposition than the temperature (Henzer et al., 1970).
Both sulfones were detected as metabolites in the soil (Henzer et
al., 1970), but the sulfoxides occurred in only minute amounts. Takase
et al., (1971, 1972) however, found chiefly disyston sulfoxide and
sulfone as metabolites in different types of soil.
Disulfoton does not display a strong tendency to leach into the
soil since approximately 1670, 1970 and 4400 m of theoretical rainfall
were required to leach the compound 30 cm into sandy loam, silt loam
and high organic silt loam soils, respectively (Flint et al., 1970).
No effect on soil micro-organisms was observed (Houseworth and
Tweedy, 1972), though some reduction of fungal population was observed
with the high level of 250 ppm in the soil.
The half-life of disulfoton in water under simulated field
conditions was 2.9 days (Flint et al., 1970).
Fate of residues in storage, processing and cooking
In frozen storage, residues remain unchanged for long periods,
sometimes for more than two years (Chemagro Rep. 8857).
The thermal destruction of disulfoton during processing of
apricots (100°C/2 min) and spinach (120°C/55 min) was investigated by
Thornburg and reported (Anderson 1959a, b). Loss of residues was 37%
and 80% respectively.
The fate of disulfoton in potatoes during processing was
investigated by Kleinschmidt (1971). Total residues (1.33 ppm) were
reduced by 35% with lye peeling. Lye peeling plus a single water
blanching reduced the total residue by 38, 74 and 61% for french
fries, dehydrated cubes and dehydrated mashed potatoes, respectively.
On a dry weight basis, overall reduction in residues due to processing
potatoes into french fries, dehydrated cubes, dehydrated mashed, and
chips were 77, 81, 89 and 97% respectively. Lye peeling and cooking
decreased residues of disulfoton by 30% (Zwolinska and Trojanowski,
1968a).
Total diet studies and residues in food moving in commerce
Abbott et al., 1970, found residues of disulfoton only on one
sample of green vegetables. In 1968, 0.1 ppm of disulfoton was found
in only one sample of citrus fruit in New Zealand (N.Z. Min. of Agr.
information, 1973).
Methods of residue analysis
Prior to the advent of GLC methods employing phosphorus sensitive
detectors, residues of disulfoton and its metabolites were determined
by total phosphorus procedures. GLC methods for the determination of
disulfoton residues are now available (Thornton and Anderson, 1968;
Thornton, 1967a, 1967c, 1969; Bowman et al., 1969; Bowman and Beroza,
1969). The principle of most GLC procedures is oxidation of the
residues to disulfoton-sulfone (Ill) and/or demeton-S-sulfone (VI). If
permanganate is used for oxidation, there is usually no transformation
of P = S to P = O so that it is possible to distinguish between P =
S-sulfones and P = O-sulfones. This permits conclusions to be drawn as
to whether the residues present result from application of demeton-S
or disulfoton. It is possible to distinguish clearly between these and
related sulfone pairs by using a 1.1 m column packed with 10% DC-200 +
1% QF -1 on 80/100 mesh Gaschrom Q; at 195°C. The following retention
times are reported (Wagner, 1973):
demeton-S-methyl sulfone 3.75 min
thiometon sulfone 4.75 min
demeton-S-sulfone 5.0 min
disulfoton-sulfone 6.15 min
Confirmatory GLC procedures, using different columns, are also
available (Olson, 1969; Loeffler, 1970). An interference study for
disulfoton residue determinations on alfalfa, clover and potatoes was
carried out by Olson (1970). A great number of organophosphorus
compounds were mixed with the various sulfone compounds.
Chromatographic conditions were modified and/or a confirmatory column
was employed and it was possible to eliminate all interference.
Numerous multi-residue methods capable of measuring disulfoton and its
metabolites are reported (Abbott et al., 1970; Storherr et al., 1971;
Watts, 1969; McCaulley, 1965). The available GLC procedures appear to
be satisfactory, specific and suitable for regulatory purposes.
Appraisal
Disulfoton is an organophosphorus insecticide, possesses systemic
activity and is used to control aphids, leafhoppers, thrips, beet
flies, coffee leaf miner and spider mites. It is formulated
predominantly as granules and for some special uses as a liquid
concentrate. A seed dressing powder is exclusively used in cotton.
Disulfoton is used on a great variety of crops, including vegetables,
potatoes, sugar beets, cotton and cereals. Products based on
disulfoton are registered in a total of 33 countries. The percentage
breakdown of the amounts used in the different crop areas is roughly
80% in vegetables (including potatoes) and 207 in field crops.
Disulfoton is chiefly applied at sowing or as a side dressing.
Recommended application rates are from 1 to 4 kg/ha, pre-harvest
intervals ranging mostly from 30 to 100 days. The minimum purity of
the technical product is 94%. The impurities have been identified and
quantified.
NATIONAL TOLERANCES
Pre-harvest intervals and tolerances
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Australia Potatoes 70 0.5
Vegetables 40 0.5
Cereals 70 0.5
Deciduous fruit 70 0.5
Belgium Deciduous fruit 0.01
Potatoes 0.01
Vegetables 0.01
Bulgaria 60
Canada Beans 0.5
Broccoli 0.5
Brussels sprouts 0.5
Cauliflower 0.5
Lettuce 0.5
Peas 0.5
Potatoes 0.2
Spinach 0.5
Tomatoes 0.5
Germany Beets Do not feed tops before
Hops harvest. Application by
sprinkling method must be
made only up to 1 June at
the latest.
Potatoes Only for seed 0.2
production
Netherlands Vegetables, 0.01
deciduous fruit
Potatoes Only at planting 0.01
New Zealand Barley 56
Beans 56
Broccoli 42
Brussels sprouts 42
Cabbage 42
Carrots 56
Cauliflower 42
Oats 50
Peas 56
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Potatoes 91
Turnips 56
Wheat 56
Poland Hops Apply only at the time
of earthing up.
Potatoes Only for seed production
Beets Apply up to the six-leaf
stage.
For fodder 60
USSR Cereals, cotton 0.35
seed oil
Fodder No residues
South Africa Cabbage 42 0.5
Onions (for aerial 90 0.5
plant parts)
Potatoes 90 0.5
Switzerland Field crops 42
United
Kingdom 42
United States Alfalfa
of America (fresh forage) 5.0
(hay) 12.0
Barley (grain) 60 0.75
(forage or straw) 5.0
Beans (green, Lima, Application at 0.75
snap) time of planting
(on vines) 5.0
Beans (dry) 60 0.75
(on vines) 5.0
Broccoli 14 0.75
Brussels sprouts 30 0.75
Cabbage 42 0.75
Cauliflower 40 0.75
Clover (fresh) 7 5.0
(clover hay) 12.0
Coffee 90 0.3
Cotton
(non-irrigated, seed) 28 0.75
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Cotton (irrigated, 28,90 0.75
seed)
Hops 0.5
Lettuce 60 0.75
Maize (field corn,
sweet corn, 40,100 0.3
popcorn)(grain)
(fodder) 5.0
Oats (grain) 60 0.75
(forage or straw) 5.0
Peanuts Application 0.75
(peanut hay) at time of 5.0
planting
Peas 50 0.75
(vines) 5.0
Pecan 80 0.75
Pineapples 60 0.75
(foliage) 5.0
Potatoes 75 0.75
Rice 100 0.75
(straw) 5.0
Sorghum (grain) 7 0.75
(fodder and 28 5.0
forage)
Soybeans Do not pasture or use treated
crop for feed food or forage
Spinach Application at 0.75
time of planting
Strawberries Do not use fruit from
treated plantg for food
Sugar beets 30 0.5
(tops) 2.0
Sugar cane 28 0.3
Tomatoes 30 0.75
resp. application at time
of planting
Wheat (grain) 45 0.3
(green fodder Do not graze 5.0
and straw) treated fields
Metabolism studies on plants and soil are available, indicating
the formation of sulfoxides and sulfones of disulfoton and the oxygen
analogue (demeton-S). The ratio of these metabolites can vary and
depends on plant variety, soil type and climatic conditions.
A large number of residue data are available from supervised
trials, predominantly from the United States of America, but also from
some European countries and New Zealand.
Evidence on the fate of residues during storage, processing and
cooking indicates that residues are stable under deep freeze
conditions; losses of residues occur during cooking, heating, or
peeling in the case of potatoes.
Information on residues in food moving in commerce or from total
diet studies is scanty. No data are available on the eventual
carry-over of residues from forage crops into animal tissues, milk or
eggs. Methods of analysis for disulfoton residues based on GLC with
phosphorus specific detectors are available and appear to be suitable
for regulatory purposes, the limit of detection being in the order of
0.05-0.1 ppm depending on the crop.
Residues are best determined, following oxidation to
disulfoton-sulfone and/or demeton-S sulfone, as the sum of parent
compound and all of its oxydative metabolites, expressed as parent
disulfoton. By applying-suitable oxidation procedures, a quantitative
differentiation can be made between the two sulfones. Absence of
disulfoton-sulfone would indicate that prevailing residues have arisen
from an application of demeton, in which case residues should be
expressed as parent demeton.
RECOMMENDATIONS
The following tolerances are recommended, the residues being
determined as disulfoton-sulfone and demeton-S-sulfone and expressed
as disulfoton.
Crop Tolerance (ppm)
Vegetables, including beans, broccoli,
brussels sprouts, cabbage, cauliflower, 0.5
lettuce, potatoes, peas, spinach,
tomatoes, rice (in husk), sugar beets
Cereals (except rice) sugar beets, cottonseed 0.2
Coffee beans, peanuts (kernels),
pecans, pineapple, soybeans 0.1a
Forage crops (green) 5.0
a At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required before June 1975
1. Results of the long-term studies now in progress.
2. Kinetic studies on absorption, distribution, metabolism, and
excretion in mammals.
3. Evaluation of liver damage observed in short-term studies.
4. Data on residues in meat, milk, and eggs after feeding animals on
crops or feedstuffs treated with disulfoton, in order to determine
residue limits in foods of animal origin.
Desirable
1. Information on residues in food moving in commerce.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adams, J.M. (1960) A specific method for the detection of residues of
DI-SYSTON and its metabolites in the presence of other cholinesterase
inhibiting pesticides. 1. Application to cottonseed. Chemagro-Report
No. 5928
Anderson, C.A. (1959a) Thermal destruction of DI-SYSTON during
processing of spinach. Chemagro-Report No. 4882d
Anderson, C.A. (1959b) Thermal destruction of DI-SYSTON during the
processing of apricots. Chemagro-Report No. 4882e
Anderson, C.A. (1960) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. III. Application to potatoes, sugar
beets, sugar beet tops, cabbage, broccoli, pineapple and alfalfa.
Chemagro-Report No. 5511
Anderson, C.A. (1961a) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. I. Application to cottonseed.
Chemagro-Report No. 5339
Anderson, C.A. (1961b) Colorimetric determination of DI-SYSTON
residues in plant material. III. Application to Brussels sprouts,
cauliflower, green beans, lettuce, lilies, peas, pineapple and
tomatoes. Chemagro-Report No. 6684
Anderson, C.A. (1962) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. Chemagro-Report No. 8544
Anderson, C.A. (1963) Colorimetric determination of DI-SYSTON residues
in green coffee beans. Chemagro-Report No. 10 919
Arnold, D., Keplinger, M.L. and Fancher, O.E. (1971) Mutagenic Study
with DI-SYSTON in Albino mice. IBT No. E 8920. Unpublished report from
Industrial Bio-TeSt Laboratoricos Inc.
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) "Acute Toxicity
Data for Pesticides (1970)". World Review of Pest Control, 9:
119-127
Bombinski, I.J. and DuBois, K.P. Chicago. (1958) "Toxicity and
Mechanism of Action of Di-Syston". A.M.A Archives of Industrial
Health, Vol. 17: 192-199
Bowman, M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton, and phorate in corn, milk, grass,
and faeces. J.A.O.A.C., 52: 1231-1237
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetomethyl, and their metabolites in
tobacco plants. J.A.O.A.C., 52: 157-162
Brewerton, H.V. and Close, R.C. (1967) Disulfoton residues in potato
tubers. N.Z. J1. agric. Res., 10: 272-277
Brodeur, J. and DuBois, K.P., Chicago. (1964) "Studies on the
Mechanism of Acquired Tolerance by Rats to 0,0-Diethyl S-2-(ethylthio)
ethyl phosphorodithioate (Di-Syston)", Arch. Int. Pharmacodyn., 149:
560-570
Budreau, C.H. (1972) Teratogenicity and chromotoxicity of three
organophosphorus insecticides in CF1 mice. Diss. Abs. Int., 33:
1174B
Budreau, C.H. and Singh, P.P. (1973) Teratogenicity and Embryo
toxicity of Demeton and Penthion in CF1 mouse embryos. Toxicol. Appl.
Pharmacol., 24: 324-323
Bull, D.L. (1965) Metabolism of Di-Syston by Insects, Isolated Cotton
Leaves, and Rats". J. Econ. Entomol., 58: 249-254
Chemagro Corporation, Kansas City, United States of America (1962)
Report No. 8857
Chisholm, D. and Specht, H.B. (1967) Effect of application rates of
disulfoton and phorate, and of irrigation on aphid control and
residues in canning peas. Can. J. Plant Sci., 47: 175-180
Chisholm, D., Specht, H.B. and Leefe, J.S. (1965) Di-Syston residues
and control of pea aphid, Acyrthosiphon pisum, with in-furrow
treatments of canning peas in Nova Scotia. J. Econ. Entomol., 58:
763-765
Cook W. C., Butler, L., Walker, K.C. and Featherston, P.E (1963)
Granular in-furrow treatments with phorate and Di-Syston against the
pea aphid on peas. J. Econ. Entomol., 56: 95-98
Doull, J. (1957) The acute inhalation toxicity of Di-Syston to rats
and mice. Unpublished report from the University of Chicago
Doull, J. and Vaughn, G. (1958) The effects of diets containing
Di-Syston on rats. Unpublished report from the University of Chicago
Dubois, K.P. (1957a) The acute toxicity of Di-Syston in combination
with other organic phosphates to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1957b) The acute oral toxicity of Di-Syston given
simultaneously with Phosdrin to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1960) The acute toxicity of Di-Syston in combination
with delnav ethion and serum to rats. Unpublished report from the
University of Chicago
DuBois, K.P. and Kinoshita, F.K. (1971) Effect of repeated inhalation
exposure of female rats to Di-Syston. Unpublished report from the
University of Chicago
Fletcher, D., Jenkins, D.H. and Keplinger, M.L. (1971) Neurotoxicity
study with Di-Syston technical in chickens. IBT No. J 471. Unpublished
report from Industrial Bio-Test Laboratories, Inc.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour II, J. (1970) Soil
runoff, leaching and adsorption, and water stability studies with
DI-SYSTON. Chemagro-Report No. 28 939
Fukuto, T.R. and March, R.L. (1954) Isomerization of
B-ethyl-mercaptoethyl Diethyl Thionophosphate (Systox). J. Amer.
Chem. Soc. 76: 45103-06
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharmacology, 14: 515-534
Gentry, C.R., Kincaid, R.R. and Bowman, M.C. (1970) Soil treatment
with disulfoton against certain insect pests of cigar-wrapper tobacco.
J. Econ Entomol., 63: 1139-1142.
Getz, M.E. (1962) Degradation of esters of Systox, Di-Syston and
Thimet on field-sprayed kale. J.A.O.A.C., 45: 397-401
Graham-Bryce, I.J. (1967) Adsorption of disulfoton by soil. J. Sci.
Food Agric., 18: 72-77
Graham-Bryce, I.J. (1969) Diffusion of organophosphorus insecticides
in soils. J. Sci. Food Agric., 20: 489-494
Gronberg, R.R. and Olson, T.J. (1966) Colorimetric determination of
Di-Syston residues in sugarcane and sugarcane products.
Chemagro-Report No. 17 565
Guérout, R., Barbier, M. and Gicquiaux, Y. (1968) Recherches sur
Vutilisation du disulfoton dans la lutte centre la cochenille
farineuse do Vananas, Dysmicoccus brevipes Ckl. Fruits, 23,2: 67-78
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Food
Chem., 17: 80-82
Hecht, J. and Kimmerle, G. (1965) Toxikologische untersuchungen mit
dem wirkstoff, E 23 323. Unpublished report submitted by Bayer A.G.
Houseworth, L.D. and Tweedy, B.G. Effect of DI-SYSTON on 1972
microbial populations. Chemagro-Report No. 35 127
Ibrahim, F.B., Gilbert, J.M., Evans, R.T. and Cavagnol, J.C. (1969)
Decomposition of Di-Syston (0,0-Diethyl S-[2 (ethylthio)-ethyl]
phosphorodithioate) on fertilizers by infrared, gas-liquid
chromatography, and thin-layer chromatography. J. Agr. Food Chem.,
17: 300-305
Kawamori, I., Saito, T. and Iyatomi, K. (1971) Fate of
organophosphorus insecticides in soils. 1. The retention of
32P-labeled disulfoton and dimethoate in the three soils. Botyu-
Kagaku, 36: 7-12. II. The changes of the retention and the
metabolism of 32P-labeled disulfoton and dimethoate in the soils.
Botyu-Kagaku, 36: 12-17
Kimmerle, G. (1961) Di-Syston. Unpublished report submitted by Bayer
A.G.
Kimmerle, G. (1962) S 309 and S 276. Unpublished report submitted by
Bayer A.G.
Kimmerle, G. (1966) Di-Syston (S 276)/Anticlotwirkung und
Potenzierung. Unpublished report submitted by Bayer A.G.
Kimmerle, G. (1972) Acute toxicity of SRA 3886 in combination with S
276 and with E 154 to rats. Report No. 3438. Unpublished report
submitted by Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kleinschmidt, M.G. (1971) Rate of Di-Syston (O,O-Diethyl S-[2
(ethylthio)ethyl] phosphorodiothate) in potatoes during processing. J.
Agr. Food Chem., 19: 1196-1197
Klimmer, O.R. and Pfaff, W. (1955) Untersuchungen uber die Toxicitat
des neuen Kontaktinsektizides
O,O-Dimethyl-thiophosphorsaure-O-(O-S.athyl)-athylester
("Metasystox"), Arzneimittelforschung, 5 584-587
Klotzsche, C. (1972) Disulfoton, 90 day feeding study in rats.
Unpublished report from Sandox Agroforschung
Ladd, R., Jonkins, D.H, Keplinger, M.L. and Fancher, O.E. (1971)
Teratogenic study with DI-Syston technical in albino rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.
Loeffler, W.W. (1963) Colorimetric determination of DI-SYSTON residues
in green peanuts. Chemagro-Report No. 11 746
Loeffler, W.W. (1969) A summary of DASANIT and DI-SYSTON soil
persistence data. Chemagro-Report No. 25 122
Loeffler, W.W. (1970a) A confirmatory gas chromatographic procedure
for DI-SYSTON residue analysis for tobacco. Chemagro-Report No. 27 714
Loeffler, W.W. (1970b) Identification of DI-SYSTON metabolites in
field grown tobacco. Chemagro-Report No. 27 746
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
Phosphoric-acid-ester poisoning. Sonderdruck aus Naunyn-Schmiedebergs
Arch. Pharmak. exp. Path. 263: 237
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1957) Metabolism of
p32-dithio-systox in the white mouse and american cockroach.
Unpublished report, University of California, Riverside, Citrus
Experiment Station
March, R.B., Metcalf, R.L., Fukuto, T.R. and Maxon, M.G. (1955)
Metabolism of Systox in the white mouse and american cockroach. J.
Econ. Entomol., 48: 355-363
McCaulley, D.F. (1965) An approach to the separation, identification
and determination of at least ten organophosphate pesticide residues
in raw agricultural products. J.A.O.A,.C., 48: 659-665
Menzer, R.E. and Ditman, L.P. (1968) Residues in spinach grown in
disulfoton-and pnorate-treated soil. J. Econ. Entomol., 61: 225-229
Menzer, R.E., Fontanilla, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Environmental Contam. Toxicol., 5: 1-5
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
dithio-Systax and Thimet. J. Econ. Entomol., 50: 338-345
Metcalf, R.L., Reynolds, H.T., Winton, M. and Fukuto, T.R. (1959)
Effects of temperature and plant species upon the rates of metabolism
of systemically applied Di-Syston. J. Econ. Entomol., 52: 435-439
Modak, A., Barley, L., Weintraub, S. and Stavinoha, W.B. (1971) The
effects of chronic disulfoton treatment on tile cholinesterase
activity of the rat. Toxicol and Appl. Pharm., 19: 367
Mülhmann, R. and Tietz, H. (1956) Das chemische verhalten von
Methylisosystox in der lebenden pflanze und das sich daraus ergebende
rückstandsproblem. Höfehen Briefs, 9: 116-140
Olson, T. (1964) A summary of DI-SYSTON soil persistence data.
Chemagro-Report No. 13 172
Olson, T.J. (1969) A confirmatory gas chromatographic procedure for
DI-SYSTON-SYSTOX residue analysis. Chemagro Report No. 26 335
Olson, T.J. (1970) An interference study for DI-SYSTON residue
determinations on alfalfa, clover and potatoes Chemagro-Report No. 27
312
Rider, J.A., Swadery J.I. and Puletti. E.J, (1972) Anticholinesterase
toxicity studies with Guthion, Phoadrin, Di-Syston and Trithion in
human subjects. Fed. Proc., Fed. Amer. Soc. Exp. Biol., 31(2): 520
Ridgway, R.L., Lindquist, D.A. and Bull, D.L. (1965) Effect of method
of application on uptake of Di-Syston by the cotton plant. J. Econ.
Entomol., 58: 349-352
Rivett, K.F., Bhatt. A., Street, A.E. and Newman, A.J. (1972)
Thio-Demeton/cral toxicity to mice/dietary administration for three
months. Unpublished report from Huntingdon Research Centre, England
Sachsse, K.R. and Voss, G. (1971) Toxicology of phospharidon, Residue
Reviews, 37: 61-88
Schrader, G. (1963) Die Entwicklung neuer insektizider
phosphorsaureester. Chemie-Verlag
Schumann, S. and Olson, T. (1964) Colorimetric determination of
DI-SYSTON residues in soil. Chemagro-Report No. 13 059
Stavinoha, W.B., Ryan, L.C. and Smith, P.W. (1969) Biochemical effects
of an organophosphorus cholinesterase inhibitor on the rat brain.
Annals of the New York Academy of Sciences, 160: 378-382
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticide residues in nonfatty foods. J.A.O.A.C.,
54: 513-516
Su, M. Qu., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. and Appl. Pharm.
20: 241-249
Takase, I., Tsuda, H. and Yoshimoto, Y. (1971) The fate of disulfoton
in soil. Japanese J. Appl. Entomol. Zool., 15, 2: 63-69. Revised
version in: Pflanzenschutz-Nachr. Bayer, 25: 43-63 (1972)
Taylor, R.E. (1966) Di-Syston/three generation breeding study on
rats. Unpublished report from Harris Laboratories
Thornton, J.S. (1967a) Determination of DI-SYSTON residues in corn,
soybeans, sugarcane and sugarcane products by thermionic emission gas
chromatography. Chemagro Report No. 20 109
Thornton, J.S. (1967b) Check on loss of DI-SYSTON oxygen analog
metabolites during alkali refining (simulated). Chemagro-Report No, 20
473
Thornton, J.S. (1967c) Determination of DI-SYSTON residues in various
crops and products. Chemagro-Report No. 21 319
Thornton, J.S. (1969) A study of the possible interference of other
pesticides with the analytical method for DI-SYSTON SYSTOX on crops.
Chemagro-Report.No. 21 606
Thornton, J.S. and Anderson, C.A. (1968) Determination of residues of
Di-Syston and metabolites by thermionic emission flame gas
chromatography. J. Agr. Food Chem., 16: 895-898
Trojanowski, H. and Zwolinska-Sniatalowa, Z. (1967) (Preliminary
studies on residue of Solvirex in potato tubers) Prace Naukowe Inst.
Ochrony Roslin, 9, 2: 257-261
Vaughn, G., Deininger, E. and Doull, J. (1958) Determination of a safe
dietary level of Di-Syston for dogs. Unpublished report from the
University of Chicago
Wagner, K. (1973) Unpublished. Bayer A.G., Pflanzonschutz AT, Biol.
Forschung, Institut für Rückstandsanalytik
Ward, C.R., Owens, J.C., Huddleston, E.W., Ashdown D. and Bailey, C.F.
(1972) Phytotoxic and residual properties of disulfoton used on wheat.
J. Econ. Entomol., 65: 561-563
Watts, R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969) Charcoal
column cleanup method for many organophosphorus pesticide residues in
crop extracts. J.A.O.A.C., 52: 522-526
Weil, C.S., Condra, N.J. and Carpenter, C.P. (1971) Correlation of
four hour vs. 24 hour contact skin penetration toxicity in the rat and
rabbit and use of the former for prediction of relative hazard of
pesticide formulations. Toxicol. and Appl. Pharm. 18: 734-742
Wirth, W. (1958) Zur Wirkung System-Insektizider Phosphorsaure-Ester
im Warmbluter-Stoffwechsel. Naunyn-Schmiedeberg's Arch. Exp. Path. u.
Pharm., 234: 352-363
Zwolinska-Sniatalowa, Z. (1967) (The residues of Disyston in potato
tubers). Biul. Inst. Ochrony Roslin, 36: 139-148
Zwolinska-Sniatalowa, Z. (1968) (Studies upon the residues of Disyston
in potato tubers and some vegetables). Biul. Inst. Ochrony Roslin,
41: 121-128
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1969)
Investigations upon the disulfoton residues when applied as Solvirex
preparation on cabbage variety "Slawa", Biul. Inst. Ochrony Roslin,
45: 293-298
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1971) (The residues
of some organo-phosphorus insecticides, applied into the soil, in
vegetables). Biul. Inst. Ochrony Roslin, 48: 439-448
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968a) (The
investigation on Disyston residue in potato tubers). Prace Naukowe
Inst. Ochrony Roslin, 10, 1: 113-124
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968b) (The breakdown
dynamics of disulfoton in potatoes). Biul. Inst. Ochrony Roslin, 40:
203-210
Compound (IV) is demeton-S, one of the active ingredients of the
well-known systemic insecticide SystoxR. Thus the metabolism of
disulfoton dovetails into the metabolism of demeton-S. However, it
should be noted that owing to the rapid formation of the sulfoxides,
the occurrence of (IV) as a metabolite is hardly to be expected.
Following the formation of these metabolites, further degradation can
only be by hydrolysis. The plant metabolism of disulfoton was studied
by the use of 32-p labelled compound (Metcalf et al., 1957, 1959) in
cotton, lemon, bean and alfalfa plants. Disulfoton was rapidly
oxidized to produce the sulfoxide (II) and slowly to produce sulfone
(III). Both those compounds were also oxidized at the thiono-sulfur to
produce V and VI. These same compounds were identified by Bull (1965)
working with other plants, e.g., avocado, brussels sprouts, cabbage,
corn, tomato. The same results were obtained but the proportions of
various compound differed. These studies we re confirmed by later
investigations.
Loeffler (1970b) found II and VI as major metabolites in tobacco.
Gentry et al., (1970) found that in tobacco the order was: Ill, II,
VI, V (see also Bowman et al., 1969).
In soil and water
Generally, the half-life of disulfoton residues in different
soils is between 30 and 100 days (Olson, 1964; Loeffler, 1969). Soil
type and microbial activity seem to have a greater influence on the
rate of decomposition than the temperature (Henzer et al., 1970).
Both sulfones were detected as metabolites in the soil (Henzer et
al., 1970), but the sulfoxides occurred in only minute amounts. Takase
et al., (1971, 1972) however, found chiefly disyston sulfoxide and
sulfone as metabolites in different types of soil.
Disulfoton does not display a strong tendency to leach into the
soil since approximately 1670, 1970 and 4400 m of theoretical rainfall
were required to leach the compound 30 cm into sandy loam, silt loam
and high organic silt loam soils, respectively (Flint et al., 1970).
No effect on soil micro-organisms was observed (Houseworth and
Tweedy, 1972), though some reduction of fungal population was observed
with the high level of 250 ppm in the soil.
The half-life of disulfoton in water under simulated field
conditions was 2.9 days (Flint et al., 1970).
Fate of residues in storage, processing and cooking
In frozen storage, residues remain unchanged for long periods,
sometimes for more than two years (Chemagro Rep. 8857).
The thermal destruction of disulfoton during processing of
apricots (100°C/2 min) and spinach (120°C/55 min) was investigated by
Thornburg and reported (Anderson 1959a, b). Loss of residues was 37%
and 80% respectively.
The fate of disulfoton in potatoes during processing was
investigated by Kleinschmidt (1971). Total residues (1.33 ppm) were
reduced by 35% with lye peeling. Lye peeling plus a single water
blanching reduced the total residue by 38, 74 and 61% for french
fries, dehydrated cubes and dehydrated mashed potatoes, respectively.
On a dry weight basis, overall reduction in residues due to processing
potatoes into french fries, dehydrated cubes, dehydrated mashed, and
chips were 77, 81, 89 and 97% respectively. Lye peeling and cooking
decreased residues of disulfoton by 30% (Zwolinska and Trojanowski,
1968a).
Total diet studies and residues in food moving in commerce
Abbott et al., 1970, found residues of disulfoton only on one
sample of green vegetables. In 1968, 0.1 ppm of disulfoton was found
in only one sample of citrus fruit in New Zealand (N.Z. Min. of Agr.
information, 1973).
Methods of residue analysis
Prior to the advent of GLC methods employing phosphorus sensitive
detectors, residues of disulfoton and its metabolites were determined
by total phosphorus procedures. GLC methods for the determination of
disulfoton residues are now available (Thornton and Anderson, 1968;
Thornton, 1967a, 1967c, 1969; Bowman et al., 1969; Bowman and Beroza,
1969). The principle of most GLC procedures is oxidation of the
residues to disulfoton-sulfone (Ill) and/or demeton-S-sulfone (VI). If
permanganate is used for oxidation, there is usually no transformation
of P = S to P = O so that it is possible to distinguish between P =
S-sulfones and P = O-sulfones. This permits conclusions to be drawn as
to whether the residues present result from application of demeton-S
or disulfoton. It is possible to distinguish clearly between these and
related sulfone pairs by using a 1.1 m column packed with 10% DC-200 +
1% QF -1 on 80/100 mesh Gaschrom Q; at 195°C. The following retention
times are reported (Wagner, 1973):
demeton-S-methyl sulfone 3.75 min
thiometon sulfone 4.75 min
demeton-S-sulfone 5.0 min
disulfoton-sulfone 6.15 min
Confirmatory GLC procedures, using different columns, are also
available (Olson, 1969; Loeffler, 1970). An interference study for
disulfoton residue determinations on alfalfa, clover and potatoes was
carried out by Olson (1970). A great number of organophosphorus
compounds were mixed with the various sulfone compounds.
Chromatographic conditions were modified and/or a confirmatory column
was employed and it was possible to eliminate all interference.
Numerous multi-residue methods capable of measuring disulfoton and its
metabolites are reported (Abbott et al., 1970; Storherr et al., 1971;
Watts, 1969; McCaulley, 1965). The available GLC procedures appear to
be satisfactory, specific and suitable for regulatory purposes.
Appraisal
Disulfoton is an organophosphorus insecticide, possesses systemic
activity and is used to control aphids, leafhoppers, thrips, beet
flies, coffee leaf miner and spider mites. It is formulated
predominantly as granules and for some special uses as a liquid
concentrate. A seed dressing powder is exclusively used in cotton.
Disulfoton is used on a great variety of crops, including vegetables,
potatoes, sugar beets, cotton and cereals. Products based on
disulfoton are registered in a total of 33 countries. The percentage
breakdown of the amounts used in the different crop areas is roughly
80% in vegetables (including potatoes) and 207 in field crops.
Disulfoton is chiefly applied at sowing or as a side dressing.
Recommended application rates are from 1 to 4 kg/ha, pre-harvest
intervals ranging mostly from 30 to 100 days. The minimum purity of
the technical product is 94%. The impurities have been identified and
quantified.
NATIONAL TOLERANCES
Pre-harvest intervals and tolerances
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Australia Potatoes 70 0.5
Vegetables 40 0.5
Cereals 70 0.5
Deciduous fruit 70 0.5
Belgium Deciduous fruit 0.01
Potatoes 0.01
Vegetables 0.01
Bulgaria 60
Canada Beans 0.5
Broccoli 0.5
Brussels sprouts 0.5
Cauliflower 0.5
Lettuce 0.5
Peas 0.5
Potatoes 0.2
Spinach 0.5
Tomatoes 0.5
Germany Beets Do not feed tops before
Hops harvest. Application by
sprinkling method must be
made only up to 1 June at
the latest.
Potatoes Only for seed 0.2
production
Netherlands Vegetables, 0.01
deciduous fruit
Potatoes Only at planting 0.01
New Zealand Barley 56
Beans 56
Broccoli 42
Brussels sprouts 42
Cabbage 42
Carrots 56
Cauliflower 42
Oats 50
Peas 56
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Potatoes 91
Turnips 56
Wheat 56
Poland Hops Apply only at the time
of earthing up.
Potatoes Only for seed production
Beets Apply up to the six-leaf
stage.
For fodder 60
USSR Cereals, cotton 0.35
seed oil
Fodder No residues
South Africa Cabbage 42 0.5
Onions (for aerial 90 0.5
plant parts)
Potatoes 90 0.5
Switzerland Field crops 42
United
Kingdom 42
United States Alfalfa
of America (fresh forage) 5.0
(hay) 12.0
Barley (grain) 60 0.75
(forage or straw) 5.0
Beans (green, Lima, Application at 0.75
snap) time of planting
(on vines) 5.0
Beans (dry) 60 0.75
(on vines) 5.0
Broccoli 14 0.75
Brussels sprouts 30 0.75
Cabbage 42 0.75
Cauliflower 40 0.75
Clover (fresh) 7 5.0
(clover hay) 12.0
Coffee 90 0.3
Cotton
(non-irrigated, seed) 28 0.75
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Cotton (irrigated, 28,90 0.75
seed)
Hops 0.5
Lettuce 60 0.75
Maize (field corn,
sweet corn, 40,100 0.3
popcorn)(grain)
(fodder) 5.0
Oats (grain) 60 0.75
(forage or straw) 5.0
Peanuts Application 0.75
(peanut hay) at time of 5.0
planting
Peas 50 0.75
(vines) 5.0
Pecan 80 0.75
Pineapples 60 0.75
(foliage) 5.0
Potatoes 75 0.75
Rice 100 0.75
(straw) 5.0
Sorghum (grain) 7 0.75
(fodder and 28 5.0
forage)
Soybeans Do not pasture or use treated
crop for feed food or forage
Spinach Application at 0.75
time of planting
Strawberries Do not use fruit from
treated plantg for food
Sugar beets 30 0.5
(tops) 2.0
Sugar cane 28 0.3
Tomatoes 30 0.75
resp. application at time
of planting
Wheat (grain) 45 0.3
(green fodder Do not graze 5.0
and straw) treated fields
Metabolism studies on plants and soil are available, indicating
the formation of sulfoxides and sulfones of disulfoton and the oxygen
analogue (demeton-S). The ratio of these metabolites can vary and
depends on plant variety, soil type and climatic conditions.
A large number of residue data are available from supervised
trials, predominantly from the United States of America, but also from
some European countries and New Zealand.
Evidence on the fate of residues during storage, processing and
cooking indicates that residues are stable under deep freeze
conditions; losses of residues occur during cooking, heating, or
peeling in the case of potatoes.
Information on residues in food moving in commerce or from total
diet studies is scanty. No data are available on the eventual
carry-over of residues from forage crops into animal tissues, milk or
eggs. Methods of analysis for disulfoton residues based on GLC with
phosphorus specific detectors are available and appear to be suitable
for regulatory purposes, the limit of detection being in the order of
0.05-0.1 ppm depending on the crop.
Residues are best determined, following oxidation to
disulfoton-sulfone and/or demeton-S sulfone, as the sum of parent
compound and all of its oxydative metabolites, expressed as parent
disulfoton. By applying-suitable oxidation procedures, a quantitative
differentiation can be made between the two sulfones. Absence of
disulfoton-sulfone would indicate that prevailing residues have arisen
from an application of demeton, in which case residues should be
expressed as parent demeton.
RECOMMENDATIONS
The following tolerances are recommended, the residues being
determined as disulfoton-sulfone and demeton-S-sulfone and expressed
as disulfoton.
Crop Tolerance (ppm)
Vegetables, including beans, broccoli,
brussels sprouts, cabbage, cauliflower, 0.5
lettuce, potatoes, peas, spinach,
tomatoes, rice (in husk), sugar beets
Cereals (except rice) sugar beets, cottonseed 0.2
Coffee beans, peanuts (kernels),
pecans, pineapple, soybeans 0.1a
Forage crops (green) 5.0
a At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required before June 1975
1. Results of the long-term studies now in progress.
2. Kinetic studies on absorption, distribution, metabolism, and
excretion in mammals.
3. Evaluation of liver damage observed in short-term studies.
4. Data on residues in meat, milk, and eggs after feeding animals on
crops or feedstuffs treated with disulfoton, in order to determine
residue limits in foods of animal origin.
Desirable
1. Information on residues in food moving in commerce.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adams, J.M. (1960) A specific method for the detection of residues of
DI-SYSTON and its metabolites in the presence of other cholinesterase
inhibiting pesticides. 1. Application to cottonseed. Chemagro-Report
No. 5928
Anderson, C.A. (1959a) Thermal destruction of DI-SYSTON during
processing of spinach. Chemagro-Report No. 4882d
Anderson, C.A. (1959b) Thermal destruction of DI-SYSTON during the
processing of apricots. Chemagro-Report No. 4882e
Anderson, C.A. (1960) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. III. Application to potatoes, sugar
beets, sugar beet tops, cabbage, broccoli, pineapple and alfalfa.
Chemagro-Report No. 5511
Anderson, C.A. (1961a) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. I. Application to cottonseed.
Chemagro-Report No. 5339
Anderson, C.A. (1961b) Colorimetric determination of DI-SYSTON
residues in plant material. III. Application to Brussels sprouts,
cauliflower, green beans, lettuce, lilies, peas, pineapple and
tomatoes. Chemagro-Report No. 6684
Anderson, C.A. (1962) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. Chemagro-Report No. 8544
Anderson, C.A. (1963) Colorimetric determination of DI-SYSTON residues
in green coffee beans. Chemagro-Report No. 10 919
Arnold, D., Keplinger, M.L. and Fancher, O.E. (1971) Mutagenic Study
with DI-SYSTON in Albino mice. IBT No. E 8920. Unpublished report from
Industrial Bio-TeSt Laboratoricos Inc.
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) "Acute Toxicity
Data for Pesticides (1970)". World Review of Pest Control, 9:
119-127
Bombinski, I.J. and DuBois, K.P. Chicago. (1958) "Toxicity and
Mechanism of Action of Di-Syston". A.M.A Archives of Industrial
Health, Vol. 17: 192-199
Bowman, M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton, and phorate in corn, milk, grass,
and faeces. J.A.O.A.C., 52: 1231-1237
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetomethyl, and their metabolites in
tobacco plants. J.A.O.A.C., 52: 157-162
Brewerton, H.V. and Close, R.C. (1967) Disulfoton residues in potato
tubers. N.Z. J1. agric. Res., 10: 272-277
Brodeur, J. and DuBois, K.P., Chicago. (1964) "Studies on the
Mechanism of Acquired Tolerance by Rats to 0,0-Diethyl S-2-(ethylthio)
ethyl phosphorodithioate (Di-Syston)", Arch. Int. Pharmacodyn., 149:
560-570
Budreau, C.H. (1972) Teratogenicity and chromotoxicity of three
organophosphorus insecticides in CF1 mice. Diss. Abs. Int., 33:
1174B
Budreau, C.H. and Singh, P.P. (1973) Teratogenicity and Embryo
toxicity of Demeton and Penthion in CF1 mouse embryos. Toxicol. Appl.
Pharmacol., 24: 324-323
Bull, D.L. (1965) Metabolism of Di-Syston by Insects, Isolated Cotton
Leaves, and Rats". J. Econ. Entomol., 58: 249-254
Chemagro Corporation, Kansas City, United States of America (1962)
Report No. 8857
Chisholm, D. and Specht, H.B. (1967) Effect of application rates of
disulfoton and phorate, and of irrigation on aphid control and
residues in canning peas. Can. J. Plant Sci., 47: 175-180
Chisholm, D., Specht, H.B. and Leefe, J.S. (1965) Di-Syston residues
and control of pea aphid, Acyrthosiphon pisum, with in-furrow
treatments of canning peas in Nova Scotia. J. Econ. Entomol., 58:
763-765
Cook W. C., Butler, L., Walker, K.C. and Featherston, P.E (1963)
Granular in-furrow treatments with phorate and Di-Syston against the
pea aphid on peas. J. Econ. Entomol., 56: 95-98
Doull, J. (1957) The acute inhalation toxicity of Di-Syston to rats
and mice. Unpublished report from the University of Chicago
Doull, J. and Vaughn, G. (1958) The effects of diets containing
Di-Syston on rats. Unpublished report from the University of Chicago
Dubois, K.P. (1957a) The acute toxicity of Di-Syston in combination
with other organic phosphates to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1957b) The acute oral toxicity of Di-Syston given
simultaneously with Phosdrin to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1960) The acute toxicity of Di-Syston in combination
with delnav ethion and serum to rats. Unpublished report from the
University of Chicago
DuBois, K.P. and Kinoshita, F.K. (1971) Effect of repeated inhalation
exposure of female rats to Di-Syston. Unpublished report from the
University of Chicago
Fletcher, D., Jenkins, D.H. and Keplinger, M.L. (1971) Neurotoxicity
study with Di-Syston technical in chickens. IBT No. J 471. Unpublished
report from Industrial Bio-Test Laboratories, Inc.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour II, J. (1970) Soil
runoff, leaching and adsorption, and water stability studies with
DI-SYSTON. Chemagro-Report No. 28 939
Fukuto, T.R. and March, R.L. (1954) Isomerization of
B-ethyl-mercaptoethyl Diethyl Thionophosphate (Systox). J. Amer.
Chem. Soc. 76: 45103-06
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharmacology, 14: 515-534
Gentry, C.R., Kincaid, R.R. and Bowman, M.C. (1970) Soil treatment
with disulfoton against certain insect pests of cigar-wrapper tobacco.
J. Econ Entomol., 63: 1139-1142.
Getz, M.E. (1962) Degradation of esters of Systox, Di-Syston and
Thimet on field-sprayed kale. J.A.O.A.C., 45: 397-401
Graham-Bryce, I.J. (1967) Adsorption of disulfoton by soil. J. Sci.
Food Agric., 18: 72-77
Graham-Bryce, I.J. (1969) Diffusion of organophosphorus insecticides
in soils. J. Sci. Food Agric., 20: 489-494
Gronberg, R.R. and Olson, T.J. (1966) Colorimetric determination of
Di-Syston residues in sugarcane and sugarcane products.
Chemagro-Report No. 17 565
Guérout, R., Barbier, M. and Gicquiaux, Y. (1968) Recherches sur
Vutilisation du disulfoton dans la lutte centre la cochenille
farineuse do Vananas, Dysmicoccus brevipes Ckl. Fruits, 23,2: 67-78
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Food
Chem., 17: 80-82
Hecht, J. and Kimmerle, G. (1965) Toxikologische untersuchungen mit
dem wirkstoff, E 23 323. Unpublished report submitted by Bayer A.G.
Houseworth, L.D. and Tweedy, B.G. Effect of DI-SYSTON on 1972
microbial populations. Chemagro-Report No. 35 127
Ibrahim, F.B., Gilbert, J.M., Evans, R.T. and Cavagnol, J.C. (1969)
Decomposition of Di-Syston (0,0-Diethyl S-[2 (ethylthio)-ethyl]
phosphorodithioate) on fertilizers by infrared, gas-liquid
chromatography, and thin-layer chromatography. J. Agr. Food Chem.,
17: 300-305
Kawamori, I., Saito, T. and Iyatomi, K. (1971) Fate of
organophosphorus insecticides in soils. 1. The retention of
32P-labeled disulfoton and dimethoate in the three soils. Botyu-
Kagaku, 36: 7-12. II. The changes of the retention and the
metabolism of 32P-labeled disulfoton and dimethoate in the soils.
Botyu-Kagaku, 36: 12-17
Kimmerle, G. (1961) Di-Syston. Unpublished report submitted by Bayer
A.G.
Kimmerle, G. (1962) S 309 and S 276. Unpublished report submitted by
Bayer A.G.
Kimmerle, G. (1966) Di-Syston (S 276)/Anticlotwirkung und
Potenzierung. Unpublished report submitted by Bayer A.G.
Kimmerle, G. (1972) Acute toxicity of SRA 3886 in combination with S
276 and with E 154 to rats. Report No. 3438. Unpublished report
submitted by Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kleinschmidt, M.G. (1971) Rate of Di-Syston (O,O-Diethyl S-[2
(ethylthio)ethyl] phosphorodiothate) in potatoes during processing. J.
Agr. Food Chem., 19: 1196-1197
Klimmer, O.R. and Pfaff, W. (1955) Untersuchungen uber die Toxicitat
des neuen Kontaktinsektizides
O,O-Dimethyl-thiophosphorsaure-O-(O-S.athyl)-athylester
("Metasystox"), Arzneimittelforschung, 5 584-587
Klotzsche, C. (1972) Disulfoton, 90 day feeding study in rats.
Unpublished report from Sandox Agroforschung
Ladd, R., Jonkins, D.H, Keplinger, M.L. and Fancher, O.E. (1971)
Teratogenic study with DI-Syston technical in albino rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.
Loeffler, W.W. (1963) Colorimetric determination of DI-SYSTON residues
in green peanuts. Chemagro-Report No. 11 746
Loeffler, W.W. (1969) A summary of DASANIT and DI-SYSTON soil
persistence data. Chemagro-Report No. 25 122
Loeffler, W.W. (1970a) A confirmatory gas chromatographic procedure
for DI-SYSTON residue analysis for tobacco. Chemagro-Report No. 27 714
Loeffler, W.W. (1970b) Identification of DI-SYSTON metabolites in
field grown tobacco. Chemagro-Report No. 27 746
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
Phosphoric-acid-ester poisoning. Sonderdruck aus Naunyn-Schmiedebergs
Arch. Pharmak. exp. Path. 263: 237
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1957) Metabolism of
p32-dithio-systox in the white mouse and american cockroach.
Unpublished report, University of California, Riverside, Citrus
Experiment Station
March, R.B., Metcalf, R.L., Fukuto, T.R. and Maxon, M.G. (1955)
Metabolism of Systox in the white mouse and american cockroach. J.
Econ. Entomol., 48: 355-363
McCaulley, D.F. (1965) An approach to the separation, identification
and determination of at least ten organophosphate pesticide residues
in raw agricultural products. J.A.O.A,.C., 48: 659-665
Menzer, R.E. and Ditman, L.P. (1968) Residues in spinach grown in
disulfoton-and pnorate-treated soil. J. Econ. Entomol., 61: 225-229
Menzer, R.E., Fontanilla, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Environmental Contam. Toxicol., 5: 1-5
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
dithio-Systax and Thimet. J. Econ. Entomol., 50: 338-345
Metcalf, R.L., Reynolds, H.T., Winton, M. and Fukuto, T.R. (1959)
Effects of temperature and plant species upon the rates of metabolism
of systemically applied Di-Syston. J. Econ. Entomol., 52: 435-439
Modak, A., Barley, L., Weintraub, S. and Stavinoha, W.B. (1971) The
effects of chronic disulfoton treatment on tile cholinesterase
activity of the rat. Toxicol and Appl. Pharm., 19: 367
Mülhmann, R. and Tietz, H. (1956) Das chemische verhalten von
Methylisosystox in der lebenden pflanze und das sich daraus ergebende
rückstandsproblem. Höfehen Briefs, 9: 116-140
Olson, T. (1964) A summary of DI-SYSTON soil persistence data.
Chemagro-Report No. 13 172
Olson, T.J. (1969) A confirmatory gas chromatographic procedure for
DI-SYSTON-SYSTOX residue analysis. Chemagro Report No. 26 335
Olson, T.J. (1970) An interference study for DI-SYSTON residue
determinations on alfalfa, clover and potatoes Chemagro-Report No. 27
312
Rider, J.A., Swadery J.I. and Puletti. E.J, (1972) Anticholinesterase
toxicity studies with Guthion, Phoadrin, Di-Syston and Trithion in
human subjects. Fed. Proc., Fed. Amer. Soc. Exp. Biol., 31(2): 520
Ridgway, R.L., Lindquist, D.A. and Bull, D.L. (1965) Effect of method
of application on uptake of Di-Syston by the cotton plant. J. Econ.
Entomol., 58: 349-352
Rivett, K.F., Bhatt. A., Street, A.E. and Newman, A.J. (1972)
Thio-Demeton/cral toxicity to mice/dietary administration for three
months. Unpublished report from Huntingdon Research Centre, England
Sachsse, K.R. and Voss, G. (1971) Toxicology of phospharidon, Residue
Reviews, 37: 61-88
Schrader, G. (1963) Die Entwicklung neuer insektizider
phosphorsaureester. Chemie-Verlag
Schumann, S. and Olson, T. (1964) Colorimetric determination of
DI-SYSTON residues in soil. Chemagro-Report No. 13 059
Stavinoha, W.B., Ryan, L.C. and Smith, P.W. (1969) Biochemical effects
of an organophosphorus cholinesterase inhibitor on the rat brain.
Annals of the New York Academy of Sciences, 160: 378-382
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticide residues in nonfatty foods. J.A.O.A.C.,
54: 513-516
Su, M. Qu., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. and Appl. Pharm.
20: 241-249
Takase, I., Tsuda, H. and Yoshimoto, Y. (1971) The fate of disulfoton
in soil. Japanese J. Appl. Entomol. Zool., 15, 2: 63-69. Revised
version in: Pflanzenschutz-Nachr. Bayer, 25: 43-63 (1972)
Taylor, R.E. (1966) Di-Syston/three generation breeding study on
rats. Unpublished report from Harris Laboratories
Thornton, J.S. (1967a) Determination of DI-SYSTON residues in corn,
soybeans, sugarcane and sugarcane products by thermionic emission gas
chromatography. Chemagro Report No. 20 109
Thornton, J.S. (1967b) Check on loss of DI-SYSTON oxygen analog
metabolites during alkali refining (simulated). Chemagro-Report No, 20
473
Thornton, J.S. (1967c) Determination of DI-SYSTON residues in various
crops and products. Chemagro-Report No. 21 319
Thornton, J.S. (1969) A study of the possible interference of other
pesticides with the analytical method for DI-SYSTON SYSTOX on crops.
Chemagro-Report.No. 21 606
Thornton, J.S. and Anderson, C.A. (1968) Determination of residues of
Di-Syston and metabolites by thermionic emission flame gas
chromatography. J. Agr. Food Chem., 16: 895-898
Trojanowski, H. and Zwolinska-Sniatalowa, Z. (1967) (Preliminary
studies on residue of Solvirex in potato tubers) Prace Naukowe Inst.
Ochrony Roslin, 9, 2: 257-261
Vaughn, G., Deininger, E. and Doull, J. (1958) Determination of a safe
dietary level of Di-Syston for dogs. Unpublished report from the
University of Chicago
Wagner, K. (1973) Unpublished. Bayer A.G., Pflanzonschutz AT, Biol.
Forschung, Institut für Rückstandsanalytik
Ward, C.R., Owens, J.C., Huddleston, E.W., Ashdown D. and Bailey, C.F.
(1972) Phytotoxic and residual properties of disulfoton used on wheat.
J. Econ. Entomol., 65: 561-563
Watts, R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969) Charcoal
column cleanup method for many organophosphorus pesticide residues in
crop extracts. J.A.O.A.C., 52: 522-526
Weil, C.S., Condra, N.J. and Carpenter, C.P. (1971) Correlation of
four hour vs. 24 hour contact skin penetration toxicity in the rat and
rabbit and use of the former for prediction of relative hazard of
pesticide formulations. Toxicol. and Appl. Pharm. 18: 734-742
Wirth, W. (1958) Zur Wirkung System-Insektizider Phosphorsaure-Ester
im Warmbluter-Stoffwechsel. Naunyn-Schmiedeberg's Arch. Exp. Path. u.
Pharm., 234: 352-363
Zwolinska-Sniatalowa, Z. (1967) (The residues of Disyston in potato
tubers). Biul. Inst. Ochrony Roslin, 36: 139-148
Zwolinska-Sniatalowa, Z. (1968) (Studies upon the residues of Disyston
in potato tubers and some vegetables). Biul. Inst. Ochrony Roslin,
41: 121-128
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1969)
Investigations upon the disulfoton residues when applied as Solvirex
preparation on cabbage variety "Slawa", Biul. Inst. Ochrony Roslin,
45: 293-298
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1971) (The residues
of some organo-phosphorus insecticides, applied into the soil, in
vegetables). Biul. Inst. Ochrony Roslin, 48: 439-448
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968a) (The
investigation on Disyston residue in potato tubers). Prace Naukowe
Inst. Ochrony Roslin, 10, 1: 113-124
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968b) (The breakdown
dynamics of disulfoton in potatoes). Biul. Inst. Ochrony Roslin, 40:
203-210
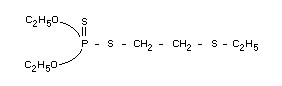 Empirical formula
C8H19O2PS3
Other information on properties
Appearance: colourless, oily liquid
Molecular weight: 274.4
Boiling point: 62°C at 0.01 mm Hg
82°C at 0.05 mm Hg
128°C at 1.0 mm Hg
Vapour pressure: 0.6 x 10-4 mm Hg at 10°C
1.8 x 10-4 mm Hg at 20°C
5.2 x 10-4 mm Hg at 30°C
14.0 x 10-4 mm Hg at 40°C
Volatility: 0.9 mg/m3 at 10°C
2.7 mg/m3 at 20°C
7.5 mg/m3 at 30°C
19.7 mg/m3 at 40°C
Specific gravity: 1.14 at 20°C C
4°
Solubility: approximately 1:40 000 in water at room
temperature: soluble in most organic solvents
Minimum purity: 94%
Impurities: 2-ethylthioethylchloride max. 0.2%
2-ethylthioethanethiol max. 0.2%
bis(2-ethylthioethyl)
disulfide max. 0.6%
O,O S-triethyl
phosphorodithioate max. 2.0%
O,O,O S-triethyl
phosphorothioate max. 0.5%
sulfotepp max. 0.5%
3 oligomeric alkyl(thio)
phosphates max. 1.5%
water max. 0.5%
Disulfoton is a member of the demeton family of insecticides.
Demeton was reviewed by the 1965 Joint Meeting (FAO/WHO, 19G6) and an
ADI was estimated for man to be 0.0025 mg/kg/day. The relationship of
disulfoton to the other compounds comprising the demeton family can be
seen in the monograph of demeton-methyl.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
There are essentially no data available on the absorption of
disulfoton in mammals. Studies on the metabolism and distribution in
mammals have been limited to mouse and intraperitoneal administration.
Following intraperitoneal administration of radio-labelled
disyston to mice, 30-60% of the radio-activity (depending on the dose
administered, 5-15 mg/kg) was recovered in the urine over a 96-hour
period. Approximately 2-3% of the radio-activity was recovered in the
faeces (March et al., 1957).
Metabolism
Two studies on the metabolism of disulfoton were consistent in
observations that disulfoton is rapidly metabolized by three major
biochemical reactions. The first reaction is the oxidation of the
thioether to produce sulfoxides and sulfones; the second reaction is
the oxidation of the thiono-sulfur moiety to produce the thiol
analogue; and the third reaction concerns the hydrolytic cleavage of
the P-S-C linkage of the phosphorothiolate moiety and the P-O-C
linkage of the ethyl ester moieties. The first two reactions act
independently but concurrently and are presumably responsible for
production of toxic metabolites. These oxidations produce increasingly
effective inhibitors of cholinesterase with the thiol analogue of the
sulfone being the most active. The rate of conversion of S to S = O is
considerably more rapid than the oxidation of P = S to P = O which is
about equal to the conversion of S = O to O = S = O (Bull, 1965; March
et al., 1957).
In comparative studies on various species of organisms, it was
found that the routes of metabolism in insects, plants, and mammals
are similar. The main differences are in the rates of reaction with
the reactions being fastest in the animal next in the insects, and
slowest in the plants. In mammals, the reaction to P = S to P = O
takes place at an exceptionally fast rate. In addition, no biological
conversion is known to occur with P(S)O converting rapidly to P(O)S
esters (Fukuto and Metcalf, 1954).
The following scheme depicts the metabolism of disulfoton in all
systems thus far studied:
Empirical formula
C8H19O2PS3
Other information on properties
Appearance: colourless, oily liquid
Molecular weight: 274.4
Boiling point: 62°C at 0.01 mm Hg
82°C at 0.05 mm Hg
128°C at 1.0 mm Hg
Vapour pressure: 0.6 x 10-4 mm Hg at 10°C
1.8 x 10-4 mm Hg at 20°C
5.2 x 10-4 mm Hg at 30°C
14.0 x 10-4 mm Hg at 40°C
Volatility: 0.9 mg/m3 at 10°C
2.7 mg/m3 at 20°C
7.5 mg/m3 at 30°C
19.7 mg/m3 at 40°C
Specific gravity: 1.14 at 20°C C
4°
Solubility: approximately 1:40 000 in water at room
temperature: soluble in most organic solvents
Minimum purity: 94%
Impurities: 2-ethylthioethylchloride max. 0.2%
2-ethylthioethanethiol max. 0.2%
bis(2-ethylthioethyl)
disulfide max. 0.6%
O,O S-triethyl
phosphorodithioate max. 2.0%
O,O,O S-triethyl
phosphorothioate max. 0.5%
sulfotepp max. 0.5%
3 oligomeric alkyl(thio)
phosphates max. 1.5%
water max. 0.5%
Disulfoton is a member of the demeton family of insecticides.
Demeton was reviewed by the 1965 Joint Meeting (FAO/WHO, 19G6) and an
ADI was estimated for man to be 0.0025 mg/kg/day. The relationship of
disulfoton to the other compounds comprising the demeton family can be
seen in the monograph of demeton-methyl.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
There are essentially no data available on the absorption of
disulfoton in mammals. Studies on the metabolism and distribution in
mammals have been limited to mouse and intraperitoneal administration.
Following intraperitoneal administration of radio-labelled
disyston to mice, 30-60% of the radio-activity (depending on the dose
administered, 5-15 mg/kg) was recovered in the urine over a 96-hour
period. Approximately 2-3% of the radio-activity was recovered in the
faeces (March et al., 1957).
Metabolism
Two studies on the metabolism of disulfoton were consistent in
observations that disulfoton is rapidly metabolized by three major
biochemical reactions. The first reaction is the oxidation of the
thioether to produce sulfoxides and sulfones; the second reaction is
the oxidation of the thiono-sulfur moiety to produce the thiol
analogue; and the third reaction concerns the hydrolytic cleavage of
the P-S-C linkage of the phosphorothiolate moiety and the P-O-C
linkage of the ethyl ester moieties. The first two reactions act
independently but concurrently and are presumably responsible for
production of toxic metabolites. These oxidations produce increasingly
effective inhibitors of cholinesterase with the thiol analogue of the
sulfone being the most active. The rate of conversion of S to S = O is
considerably more rapid than the oxidation of P = S to P = O which is
about equal to the conversion of S = O to O = S = O (Bull, 1965; March
et al., 1957).
In comparative studies on various species of organisms, it was
found that the routes of metabolism in insects, plants, and mammals
are similar. The main differences are in the rates of reaction with
the reactions being fastest in the animal next in the insects, and
slowest in the plants. In mammals, the reaction to P = S to P = O
takes place at an exceptionally fast rate. In addition, no biological
conversion is known to occur with P(S)O converting rapidly to P(O)S
esters (Fukuto and Metcalf, 1954).
The following scheme depicts the metabolism of disulfoton in all
systems thus far studied:
 Effects on enzymes and other biochemical parameters
Disulfoton itself has been shown to have little if any effect on
cholinesterase or any other biochemical parameter in the body.
However, as noted above, it is rapidly oxidized to highly active in
vivo and in vitro cholinesterase inhibitors. In the demeton group
of cholinesterase inhibitors demeton-S is the most active compound to
mammalian cholinesterase activity. This can be noted in the pI50
values for rat brain. In contrast demeton-S-methyl sulfone is more
active than other isomers to purified insect cholinesterase. The
sulfoxide and sulfone of demeton-S and its sulfoxide have about the
same cholinesterase inhibiting property (rat brain) while the sulfone
of demeton-O has considerably less activity (FAO/WHO, 1965). In
mammals, demeton-S loses part,of its cholinesterase inhibiting
properties as the molecule undergoes this ether oxidation to the
sulfoxide and sulfone. This is also partly true with demeton-O
(although a less active inhibitor than demeton-S) where oxidation to
the sulfoxide does not change the pI50 value, but the sulfone has a
significantly reduced pI50 Disulfoton as with other
organophosphorodithioates is a poor inhibitor of cholinesterase but
is rapidly converted to an active inhibitor. This can be seen In Table
1 which shows the pI50 values of disulfoton and its oxygen analogue
demeton-S.
In rat brain, disulfoton sulfoxide is about 10 times more active
than disulfoton, whereas disulfoton sulfone has about the same
inhibitory power as disulfoton. Demeton-S however shows a tenfold
stronger inhibitory power than its sulfoxide and its sulfone. In
insects, whole weevil and fly head cholinesterases behave differently.
The whole weevil ChE becomes practically unaffected by disulfoton
sulfone. Disulfoton sulfoxide is only about three times as active as
disulfoton. Demeton-S, its sulfoxide and sulfone show about the same
anticholinesterase activity. In fly head cholinesterase there are no
great differences in the inhibition by disulfoton compared with
disulfoton sulfoxide, but disulfoton sulfone is about tenfold more
active as an inhibitor. Within the demeton group, the inhibitory power
increases with the oxidation to the sulfoxide and the sulfone
respectively, although there exist quantitative discrepancies between
different authors. In general, the demeton-S compounds are better
inhibitors than the compounds of the disulfoton group.
As with other cholinesterase-inhibiting organophosphate esters,
the effects on other biochemical parameters or enzymes were not
reported.
Following acute i.p. administration, 5/8 of the LD50 level,
cholinesterase activity of brain serum and submaxillary gland was
maximally depressed within a very short period of time (less than six
hours) to approximately the same level (15-257 of normal) after which
recovery was initiated but not complete within 72 hours. An induced
tolerance to the cholinergic stimulation has been observed with
disulfoton especially with chronic administration (Bombinski and
DuBois, 1958).
TABLE 1. pI50 - VALUES OF DISULFUTON AND DEMETON-S AND THEIR METABOLITES ON CHOLINESTERASES OF
INSECTS AND RAT BRAIN
Insectb Insectc Insectd Insecte Insecte Ratf,g
Compounda (whole weevil) (fly head) (fly head) (fly head) (fly head) brain
Disulfoton 3.62 - - 4.00 -- 3.85
Disulfoton-S (O) 4.08 - - 4.15 4.30 4.77
Disulfoton-S (O)(O) 2.00 - 5.90 5.46 4.92 4.00
Demeton-S 4.89 5.46 - 5.46 - 6.68
Demeton-S (O) 5.08 5.82 - 5.82 5.70 5.66
Demeton-S (O)(O) 4.66 6.22 6.49 6.22 7.70 5.70
a S (O) = Sulfoxide; S (O)(O) = Sulfone.
b Bull, 1965.
c March et al., 1955.
d Metcalf et al., 1957.
e March et al., 1957.
f FAO/WHO, 1965.
g Bombinski and DuBois, 1958.
Groups of rats (five female Sprague-Dawley strain per group) were
administered disulfoton intraperitoneally at dose levels of 0, 0.25,
0.5, 1.0, 1.2, and 1.5 mg/kg/day for 60 days. Mortality occurred at
the three highest dose levels. No mortality was evident at 0.5
mg/kg/day or below, although inhibition of growth was observed. At 1
mg/kg, typical signs of cholinergic stimulation were evident during
the first days of testing but within 10 days the animals recovered and
were symptomless thereon. Furthermore, these animals began to gain
weight and appeared to adapt to the continuous administration of
disulfoton (Bombinski and DuBois, 1958). A further series of rats was
treated with disulfoton at levels of 0, 0.25, 0.5, and 1.0 mg/kg daily
by intraperitoneal administration for 30 days. Cholinesterase data,
monitored over the course of this experiment, showed that there was an
initial rapid decrease of brain cholinesterase which was dependent
upon the daily dose of disulfoton. With the two highest doses, a
permanent decrease in enzyme activity occurred only during the first
seven days after which time further treatment resulted in maintenance
of the enzyme level at a constant subnormal level. The lowest dose
however induced a permanent decrease of brain cholinesterase activity.
The serum-ChE showed nearly the same time course and degree of
depression as the brain cholinesterase, but the cumulative effect of
the lowest dose between days 7-30 was not marked.
Clinical cholinergic signs of poisoning, including weight loss,
evident over the first seven days of treatment at the highest dose
level disappeared with the result being a symptomless cholinesterase
depression (Bombinski and DuBois, 1958). Further studies to determine
the mechanism of this acquired tolerance were reported (Brodeur and
DuBois, 1964).
It was observed that the development of tolerance to subacute
administration of disulfoton was not paralleled by changes in the
acetylcholine-cholinesterase system. The free acetylcholine level in
the brain was elevated to approximately the same extent by each
successive dose throughout the period of treatment. In addition, it
was believed that the development of tolerance did not involve the
metabolic conversion of disulfoton to its oxidative anticholinesterase
analogues (Stavinoha et al., 1972). These authors postulated that the
development of the tolerance resulting from high repeated
administration of disulfoton was due to the development of a
refractoriness of the cholinergic receptors to prolong exposure to
high levels of acetylcholine. It was further observed that strain
differences exist in the acquired tolerance to cholinergic
stimulation.
Groups of Charles River and Holtzmann rats received a daily
intraperitoneal injection of 1 mg disulfoton/kg for three, 10, and 24
days respectively. In each of the three groups the signs of poisoning
were most severe on the third day. The Holtzmann rats treated for 10
and 24 days exhibited early signs of adaptation while the Charles
River rats took longer to adapt. Measurements of cholinesterase
activity in the brain showed that acetyl cholinesterase activity as
expected decreased, the amount of depression being dependent on the
duration of the application while the acetylcholine concentration
initially increased. In the Holtzmann rat acetylcholine concentration
returned to the control level after 10 days of injections and was
still at the control level when measured after 24 days of treatment.
In the Charles River rats, the acetylcholine concentration of the
brain was still elevated at the end of the 24-day injection period. In
an experiment in which disulfoton was added to the diet at
concentrations of 0, 10, 25, and 50 ppm, it was observed that
adaptation progressed more slowly than in the intraperitoneal
injection experiment. The time required for adaptation was longer as
the amount of disulfoton in the diet was increased, e.g., 1 to 1-1/2
months at 10 ppm; 2 to 3 months at 25 ppm; and very little adaptation
was observed at 50 ppm. There was pronounced suppression of weight
gain at 50 ppm. Acetyl cholinesterase was reduced in relation to the
dietary concentration although only in one strain at the highest dose.
The acetylcholine concentration was not different from the control
level. Choline acetyltransferase activity was not affected (Stavinoha
et al., 1969).
Groups of weanling rats (six rats per group) were fed disulfoton
in the diet at levels of 0, 1, 5, and 25 ppm for seven days. At the
end of one week the animals were sacrificed for the measurement of the
hydrolysis of tributyrin and diethylsuccinate by liver and serum and
for the measurement of cholinesterase in serum, liver and brain.
Dietary levels of disulfoton producing 50% inhibition of aliesterases
and cholinesterase over this one week feeding period were obtained by
analysis of a plot of the logarithm of dietary concentration and
inhibition of the respective enzymes. There was no significant
difference in this experiment between the levels of inhibition caused
by disulfoton and demeton. The liver hydrolysis of tributyrin was the
most sensitive parameter followed by diethylsuccinate hydrolysis.
Depression of cholinesterase activity was caused in the brain by 5.2
ppm disulfoton in the diet; in the liver by 14.5 ppm and the serum by
6.0 ppm. Depression of liver aliesterase hydrolyzing DES was caused by
3.5 ppm while in the serum it was calculated to be 8.4 ppm.
Depression of the enzymes hydrolyzing tributyrin was caused in
the liver by 0.6 ppm and in the serum by 9.2 ppm disulfoton in the
diet. Although these authors point out that there is a possible
relationship of aliesterase inhibition with potentiation, studies (see
Potentiation Section) show that with a selected series of
organophosphorus compounds, potentiation was not demonstrated (Su et
al., 1971).
Results of a recent study of the inhibited portions of rat brain
following disulfoton administration is show in Table 2.
This experiment reveals a severe inhibition of the
cholinesterases in the hippocampus and caudate nucleus, compared with
the hypothalamus and medulla. The recovery of cholinesterase activity
occurred more rapidly in the first two mentioned parts of the brain
than in the others although at the end of the seven day recovery
period, activity in the hypothalamus and caudate nucleus is as still
low (Modek et al., 1971).
TABLE 2. CHOLINESTERASE ACTIVITYa
Tissue Control Treatmentb Recoveryc
Hypothalmus 4.62 1.87 3.27
Medulla 5.26 1.95 4.00
Hippocampus 3.19 0.62 1.79
Caudate nucleus 13.56 2.42 8.65
Ileum 2.90 2.38 2.80
Gastrocnemius 0.48 0.28 0.40
a mM substrate hydrolyzed/gm protein/h (Modek et al., 1971).
b Intraperitoneal administration for 10 days at 1.5 mg/kg/day to
rats.
c Recovery time = seven days.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of male mice (12 mice/group) were administered
disulfoton by intraperitoneal injection at doses of 0, 0.25, and 0.5
mg/kg. Each male was mated with three virgin females each week for six
weeks in the standard dominant lethal mutation test. There were no
abnormalities noted in the data on implantation resorption or on the
embryo itself. In this study, disulfoton did not exhibit any mutagenic
effect on male mice (Arnold et al., 1971).
Disulfoton inhibited growth of three human haematopoietic cell
lives but had no effect on chromosomes (Huang, 1973).
Special studies on reproduction
Rat. Groups of rats (20 females and 10 males/group) were fed dietary
levels of disulfoton at 0, 2, 5, and 10 ppm over the course of a
two-litter per generation, three generation reproduction study. There
did not appear to be a significant effect of disulfoton in the diet on
reproduction in the rat. Levels up to and including 10 ppm did not
significantly affect reproduction parameters. At 10 ppm in the F-1-A
there was a greater than normal mortality of rats at weaning time.
There was no significant difference in any of the groups in the number
of pregnancies or in the number of young per treatment groups.
Histological examination of the F-2-B rats indicated a cloudy swelling
of the liver cells with a fatty metamorphosis especially in male rats
on 10 ppm which was not observed in the controls. This was not
observed in similar F-2-B rats. RBC-cholinesterase depression was
obvious in all treatment groups examined. No gross differences were
observed between dale and female rats. The reduction was dose
dependent and was more significant in females than males. While
disulfoton does not appear to have a definitive effect upon
reproduction parameters, high levels of disulfoton in the diet (10
ppm) have shown somatic effects and levels of 2 ppm in the diet have
shown reduction in cholinesterase (Taylor, 1966).
Special studies on teratogenicity
Groups of 15 pregnant rabbits were administered disulfoton orally
in gelatin capsules at doses of 0, 0.1, and 0.2 mg/kg daily from days
six through 18 of gestation. On day 29 of gestation, the young were
removed by caesarean section. There were no deaths or unusual
reactions among the females in any of the groups and the incidence of
fetal mortality as indicated by resorption sites or abortion was not
affected by disulfoton. There was no indication of fetal external or
internal abnormalities and the weights of the fetuses were similar to
those of the controls. A positive treatment of this test was obtained
with thalidomide. Disulfoton does not appear to cause any teratogenic
effects in rabbits (Ladd et al., 1971).
Demeton while being embryotoxic as a single dose of 5 mg/kg for
three days on days 7-13 of gestation or as a single dose of 7 or 10
mg/kg during the same interval. Only a mild teratogenic potential was
noted (Budreau, 1972; Budreau and Singh, 1973).
Special studies on neurotoxicity
Adult hens were orally administered disulfoton at a dose
estimated to be the LD50 (26 mg/kg) twice at 21 day intervals and
maintained for a further 21 days. Growth and histological examination
of the animals indicated there were no signs of delayed neurotoxicity
while a positive control (TOCP) showed definite signs of poisoning.
Disulfoton does not induce delayed toxicity or demyelination (Fletcher
et al., 1971). Hens, protected from acute cholinergic stimulation and
organophosphorus poisoning by atropine and PAM were orally
administered disulfoton at levels of up to 0.1 ml/kg. Delayed
neurotoxic effects were not noted during the six weeks post-treatment
observation period (Kimmerle, 1961).
Special studies on the neurotoxicity of metabolite
Disulfoton sulfoxide was administered intraperitoneally at levels
up to 0.5 g/kg to hens previously administered PAM and atropine.
Although mortality was evident at high dose levels there was no
evidence of delayed neurotoxicity as observed normally with TOCP
(Hecht and Kimmerle, 1965).
Special studies on potentiation
Simultaneous administration of an LD50 dose with eight other
organophosphate insecticides resulted in a slight additive acute
toxicity with five compounds and less than additive acute toxicities
with the other three. None of the combinations resulted in a
potentiation of the acute toxicity Dubois, 1957 a & b). Further
studies with organophosphate and a carbamate ester were negative
(Dubois, 1960). Equitoxic mixtures of disulfoton and phenamiphos (the
active ingredient of NemacurR, ethyl 4-(methylthio)-m-tolyl
isopropyl phosphoroamidate) resulted in less than additive toxicity
(Kimmerle, 1972). A combination of disulfoton and phosphamidon did not
cause potentiation (Sachsse and Voss, 1971).
The signs of poisoning caused by disulfoton are typical of those
produced by anticholinesterase compounds. The signs of poisoning
consist of excitability, salivation, lacrimation, urination,
defaecation, and muscular fasciculations. The signs were followed by
convulsive seizures, prostration, and respiratory failure. As with
other organophosphorus compounds the occurrence of signs of poisoning
are indicative of both nicotinic and muscarinic actions of
acetylcholine indicating that the compound or its active metabolite
has gained access to both the central and peripheral nervous system.
The time of onset and duration of signs of poisoning are dependent
upon the dose. With lethal doses death usually occurred within the
first 48 hours but upon sublethal administration death was delayed for
several days. A comparison of the toxicity by disulfoton by various
routes i.p. and oral) indicate that the compound is well absorbed from
the GI tract. A considerable sex difference in susceptibility was
noted in rats in some studies (i.e. Bombinski and DuBois, 1958) with
the male being five times less sensitive than the females. This was
not noted with other species. Similar sex differences in
susceptibility of rats have been noted with other thiophosphates. It
has been suggested that because the oxygen analogues did not exhibit
this difference in sex susceptibility that the differences in the rate
or extent of conversion of thiophosphates to their toxic oxygen
analogues is presumably responsible for the observed sex difference.
Special studies on antidotes
Several studies on the antidotal properties of atropine and PAM
administered intramuscularly before and after oral administration of
disulfoton have the distinct therapeutic effects with these materials.
Atropine in combination with other oximes administered
intraperitoneally after the appearance of signs of poisoning also
produced a therapeutic effect (Kimmerle, 1961; Lorke and Kimmerle,
1968). Atropine, when injected intraperitoneally (100 mg/kg) prior to
a dose of disulfoton, protected rats from the acute oral effects of
poisoning of an LD50 dose. Acute administration of two times the LD50
proceeded by administration of atropine was lethal (Bombinski and
Dubois, 1958).
Studies on the antidotal effects of atropine and PAM with
disulfoton sulfoxide were similar to those reported with disulfoton
where a significant reduction in acute toxicity was noted with both
atropine and oximes alone. A more significant protective effect was
noted with a combination of both atropine and oxime (Hecht and
Kimmerle, 1965).
Special studies on inhalation
Female rats were exposed to concentrations of 0, 0.14, 0.35 and
0.70 microgram/litre in the air one hour a day for five or 10 days.
There was no mortality nor any significant decrease in cholinesterase
activity in brain, serum or submaxillary gland (DuBois and Kinoshita,
1971).
Acute Toxicity
(a) Original compound
Species Route LD50 Reference
(mg/kg)
Rat M & F oral 2.3-12.5 Ben-Dykeet al., 1970
DuBois, 1957; Gaines, 1969
Kimmerle, 1961, 1962, 1966, 1972
M oral 12.5 Bombinski and DuBois, 1958
F oral 2.6 Bombinski and DuBois, 1958
Guinea Pig M oral 10.8 Bodbinski and DuBois, 1958
M i.p. 7.0 Bombinski and DuBois, 1958
Rat M i.p. 10.5 Bombinski and DuBois, 1958
F i.p. 2.0 Bombinski and DuBois, 1958
Mouse M i.p. 5.5 Bombinski and DuBois, 1958
F i.p. 6.5 Bombinski and DuBois, 1958
Rat M dermal 25-50 Ben-Dyke et al., 1970
Gaines, 1969; Kimmerle, 1962
0.285
(4 hr exposure) Well et al., 1971
Species Route LD50 Reference
(mg/kg)
Rat M inhalations 200 g/m3
(1 hr exposure) Doull, 1957
Mouse F inhalation 58 mg/m3 Doull, 1957
(1 hr exposure
(b) Metabolites
Material Species Route LD50 Reference
(mg/kg)
Disulfoton Rat oral 1.7-6.5 Hecht and Kimmerle, 1965;
Sulfoxide Kimmerle, 1962;
Schrader, 1963; Wirth, 1958
Mouse oral 5.4 Bombinski and DuBois, 1958
Guinea Pig oral >3.6 Hecht and Kimmerle, 1965
Rabbit oral 2.5-3.6 Hecht and Kimmerle, 1965
Cat oral 1.0-2.5 Hecht and Kimmerle, 1965
Rat i.p. 5.0 Hecht and Kimmerle, 1965
Rat dermal 0.195 ml/kg Hecht and Kimmerle, 1965
4 hr
0.075 ml/kg Hecht and Kimmerle, 1965
7 day
0.192 ml/kg Kimmerle, 1962
Rat inhalation 140 g/m3 Hecht and Kimmerle, 1965
(1 hr exposure)
Disulfoton Rat oral 5.0-7.5 Schrader, 1963; Wirth, 1958
Sulfone
Mouse oral 5.6 Bombinski and Dubois, 1958
Demeton-S Rat oral 1.5-3.5 Klimmer and Pfaff, 1955;
(isosystox) Wirth, 1958
Material Species Route LD50 Reference
(mg/kg)
Mouse i.p. 5.6-7.0 Muhlmann and Tietz, 1956;
FAO/WHO, 1965
Guinea Pig i.p. 5.5 FAO/WHO, 1965
Demeton-S Rat oral 1.5-2.0 Schrader, 1963; Wirth, 1958
Sulfoxide
Demeton-S Rat oral 1.5-2 Schrader, 1963; Wirth,1958
Sulfone
In almost all instances, female rats were more susceptible than males.
Short-term studies
(a) Original compound
Mouse. Groups of mice (12 male and 12 female CF-LP strain per group)
were fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5.0 ppm
for 13 weeks. Food consumption and growth were measured weekly and
behaviour and mortality were observed daily. At 12 weeks, urinalysis
haematological examination and blood chemistry including BBC, plasma
and cholinesterase assays were performed. At the conclusion of the
study, gross and microscopic examination of tissues was performed. No
treatment-related changes were observed in growth, urinalysis,
haematology, or blood chemistry with the exception of cholinesterase
activity. Cholinesterase activity was reduced in all tissues at 5 ppm,
especially in females. Gross histological examination indicated a
slight increase in the liver weight in females at 5 ppm. There were no
other abnormalities noted on gross or microscopic examination of
tissues and organs. The no-effect level based upon this study in mice
is 1 ppm in the diet (Rivett et al., 1972).
Rat. Groups of rats (13 males and 13 females per group) were fed
disulfoton in the diet at levels of 0, 1, 2, 5 and 10 ppm for 16
weeks.
Food consumption and growth were measured daily for the first two
weeks every two days for the next month and then weekly until 16
weeks. At the end of the feeding period, three males and three females
were sacrificed and tissues examined for gross and microscopic
changes. Cholinesterase activity in serum, erythrocyte brain and
submaxillary gland was examined at eight and 16 weeks of feeding.
There were no effects of disulfoton in the diet at any dose level on
growth, food consumption, behaviour or mortality over the 16-week
period. Gross and microscopic examination of all tissues from both
male and female animals revealed no differences. Cholinesterase
depression was observed in erythrocyte and brain at levels of 2 ppm
and above at both eight and 16 weeks and was more marked in female
than male. Submaxillary gland and serum was less sensitive. A
no-effect level in this study based upon cholinesterase inhibition is
1 ppm in the diet (Doull and Vaughn, 1958).
Groups of rats (25 male and 25 female Wistar strain rats) were
fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5 ppm for 90
days. Body weight and food consumption data were recorded weekly and
behaviour and mortality was observed daily. Urinalysis, clinical
chemistry and haematological examinations including RBC and plasma
cholinesterase activity were made periodically. At the conclusion of
the study, brain cholinesterase activity was analysed and all animals
were sacrificed for gross and microscopic examination of tissues and
organs. There were no significant differences between the controls and
the treated animals with regard to growth, behaviour, mortality,
urinalysis, and clinical chemistry. There appear to be no effects of
the feeding of disulfoton on gross or histological examination of the
tissues at the conclusion of the study. Cholinesterase was
significantly depressed (primarily in females) in plasm and red blood
cells at 5 ppm. In this study, 1 ppm is a no-effect level based upon
cholinesterase inhibition Motzsche, 1972).
Dog. Groups of adult mongrel dogs (one male and one female per
group) were fed disulfoton in the diet at levels of 0, 1, 2, and 10
ppm for 12 weeks. At all feeding levels, no weight loss, signs of
poisoning or adverse behaviour were noted. Plasma and RBC
cholinesterase values were significantly decreased at 2 and 10 ppm
while 1 ppm caused do significant inhibition (Vaughn et al., 1958).
After returning to a control diet, the plasma cholinesterase
inhibition rapidly returned to normal. The RBC cholinesterase remained
inhibited for over four weeks.
(b) Metabolites (disulfoton sulfoxide)
Rat. Groups of rats (10 male rats per group) were administered
disulfoton sulfoxide orally, five doses per week, for one month at
dosage levels of 0, 0.215, 0.43, 0.9 mg/kg. Cholinesterase activity
was significantly depressed at the highest dose level and marginally
depressed at the middle dose level. At seven days after the initiation
of the study, cholinesterase depression reached a maximum level and
thereafter recovered slightly maintaining a constant depressed level
to the end of the experiment. Seven days after the conclusion of the
experiment, the cholinesterase values were essentially normal (Hecht
and Kimmerle, 1965).
Groups of rats (10 female rats per group) were administered
disulfoton sulfoxide daily five days per week for nine weeks at dosage
levels of 0, 0.046, 0.093, 0.186, 0.388 and 0.775 mg/kg/day. Mortality
was obvious at the highest dose level tested although growth was not
affected in any of the groups. Haematological and urinalysis were
normal as was gross pathology. Slight changes in liver epithelial
cells were noted in several animals at the highest dose level (Hecht
and Kimmerle, 1965). On the basis of these studies with disulfoton
sulfoxide with the most sensitive parameter being acetyl
cholinesterase depression, a level of 0.43 mg/kg administered orally
five days per week would be considered to be the marginal effect
level.
Long-term studies
Long-term feeding studies on rats and dogs have been initiated
but data are not available.
Observations in man
Five volunteer subjects each received a daily oral dose of 0.75
mg of disulfoton for 30 days; two persons served as controls. Plasma
and erythrocyte cholinesterase levels were measured twice weekly
during the pre-test control period and during the 30-day test period.
No depression of cholinesterase activity was noted (Rider, 1972).
Comments
Disulfoton, a phosphorodithioate insecticide structurally similar
to demeton, is acutely toxic and produces its primary effect through
inhibition of cholinesterase activity. Disulfoton.is metabolized by
thionate oxidation, thioether oxidation, and hydrolysis or oxidative
cleavage. Thionate oxidation would result in demeton which is further
degraded. As disulfoton is a fast acting organophosphate, the
oxidation of the thionate to demeton is apparently very rapid. Demeton
was evaluated by the Joint Meeting in 1965 and the ADI for man was
estimated to be 0.0025 mg/kg.
Toxicological studies showed disulfoton to have no effect on
reproduction and tests for teratogenicity and mutagenicity gave
negative results. Disulfoton did not produce delayed neurotoxicity in
hens nor potentiate the toxicity of several organophosphorus compounds
although it is an inhibitor of aliesterase activity. In short-term
studies in rats and dogs a no-effect level was estimated to be 1 ppm
based upon inhibition of cholinesterase. At higher levels liver damage
was observed. Studies in man showed that levels of 0.75 mg for 30 days
was without effect on cholinesterase activity.
Long-term studies have been reported to be in progress and on the
basis of short-term studies a temporary ADI was established.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect in animals
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw
Dog: 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man: 0.75 mg/man/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Disulfoton possesses systemic activity and is used to control
aphids, leafhoppers, thrips, beet flies (mangold fly, spinach leaf
miner), coffee leaf miner and spider mites. it is formulated as
granules and liquid concentrate and seed dressing powder (used only on
cotton). Disulfoton formulations are used on cotton, vegetables,
potatoes, cereals (chiefly sorghum and rice), coffee, etc. Products
based on disulfoton are registered in a total of 33 countries, 807
being used on vegetables including potatoes and 20% on field crops.
Pre-harvest treatments
Disulfoton is chiefly applied at sowing or as a top dressing. The
recommended application rates range from 1 to 4 kg/ha on most crops,
with pre-harvest intervals of from 30 to 100 days. These recommended
pre-harvest intervals are not necessarily identical in all countries.
Post-harvest treatments
No uses.
Other uses
Applied to ornamentals.
Residues resulting from supervised trials
A large amount of data are available on residues resulting from
the application of disulfoton to various crops. Most of them are from
the United States of America. Results are presented in Table 1.
TABLE 1
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Alfalfa 11 1-1.5 1 7-28 n.d.-0.7
(Forage) 4 1 3 7-28 6-30
(Hay) 8 1.5 1 3-7 5-15
2 1.5 3 7 14, 19
Beans 16 1-2 1 80-160 n.d.-0.3
Broccoli 7 1-4 1 7-80 n.d.-0.6
Brussels sprouts 4 1-2 1 7-100 n.d.-0.2
Barley (grain) 3 1 1 33-104 n.d.
Cabbage 8 1.7 oz/ 1 35-106 0.01-0.1
1000 ft row
Cabbage (in furrow)
5% granules) 6 1 1 28-87 0.1-1.5
Cotton 3 1.2 1 28 < 0.3
Cottonseed 16 1-4 1 30-180 n.d.-0.6
4 1-1.5 2 30-120 n.d.-0.2
Celery 5 1.2-2.5 1 55-130 n.d.-3
Coffee 6 1-4 oz/ 1 24-180 n.d.
plant
Clover 8 1.5-2 1 3-28 1-7
Clever (hay) 2 1.5 1 3 8, 17
Lettuce head or
leaf 5 1-2 1 16-62 <0.1
Maize 17 1-2 1 30-120 n.d-0.5
11 1-2 20-30 0.2-7
Oats (grain) 2 1 1 77, 98 n.d.
Potatoes 8 1-1.5 1 60-168 n.d-0.3
66 2-3 1 50-170 n.d,-0.4
8 3-3.5 1 86-180 0.2-2
11 3-4.5 2 60-180 0.05-0.3
TABLE 1 (Cont'd.)
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Peanuts 3 1-2 1 116-148 n.d
Peanuts (shells) 11 9-14 1 116-168 0.01-0.4
Peanuts (kernels) 3 16+32 2 70 0.1
Peas (including pods) 13 1-2 1 28-70 n.d.-0.3
2 2 1 28-43 4,9
Pineapple 14 1-5 1 7-70 n.d.
Rice 12 1-4 1 50-200 n.d,-0.5
Spinach 4 1 1 7-21 2-4
10 1 1 40-90 n.d.-0.5
Sorghum 17 1-1.5 1 30-80 n.d.-0.1
Sugar beets 11 1 1 98 0.1-0.3
11 1 1 51 n.d.-6
5 1-2.5 1 160-180 n.d.-0.1
Soybeans 2 1,2 1 132 n.d.
Tomatoes 10 0.5-6 1 or 2 30-108 n.d.-0.5
Wheat 24 0.75-4 1 30-300 n.d.-0.2
5 0.25-1 4 30-52 0.01-0.02
Pecans 5 1-20 1 88-240 n.d.
Soil persistence 1 0.5 1 0-366 n.d.
1 0.5 1 1-424 6-0.4
1 0.5 1 1-181 4-1
FATE OF RESIDUES
In plants
From knowledge of the ready biological oxidation of thioether
groups and in view of the known conversion of phosphothionates to
their P=O analogues, the expected metabolites of disulfoton (I) are
the compounds (II) to (VI):
Effects on enzymes and other biochemical parameters
Disulfoton itself has been shown to have little if any effect on
cholinesterase or any other biochemical parameter in the body.
However, as noted above, it is rapidly oxidized to highly active in
vivo and in vitro cholinesterase inhibitors. In the demeton group
of cholinesterase inhibitors demeton-S is the most active compound to
mammalian cholinesterase activity. This can be noted in the pI50
values for rat brain. In contrast demeton-S-methyl sulfone is more
active than other isomers to purified insect cholinesterase. The
sulfoxide and sulfone of demeton-S and its sulfoxide have about the
same cholinesterase inhibiting property (rat brain) while the sulfone
of demeton-O has considerably less activity (FAO/WHO, 1965). In
mammals, demeton-S loses part,of its cholinesterase inhibiting
properties as the molecule undergoes this ether oxidation to the
sulfoxide and sulfone. This is also partly true with demeton-O
(although a less active inhibitor than demeton-S) where oxidation to
the sulfoxide does not change the pI50 value, but the sulfone has a
significantly reduced pI50 Disulfoton as with other
organophosphorodithioates is a poor inhibitor of cholinesterase but
is rapidly converted to an active inhibitor. This can be seen In Table
1 which shows the pI50 values of disulfoton and its oxygen analogue
demeton-S.
In rat brain, disulfoton sulfoxide is about 10 times more active
than disulfoton, whereas disulfoton sulfone has about the same
inhibitory power as disulfoton. Demeton-S however shows a tenfold
stronger inhibitory power than its sulfoxide and its sulfone. In
insects, whole weevil and fly head cholinesterases behave differently.
The whole weevil ChE becomes practically unaffected by disulfoton
sulfone. Disulfoton sulfoxide is only about three times as active as
disulfoton. Demeton-S, its sulfoxide and sulfone show about the same
anticholinesterase activity. In fly head cholinesterase there are no
great differences in the inhibition by disulfoton compared with
disulfoton sulfoxide, but disulfoton sulfone is about tenfold more
active as an inhibitor. Within the demeton group, the inhibitory power
increases with the oxidation to the sulfoxide and the sulfone
respectively, although there exist quantitative discrepancies between
different authors. In general, the demeton-S compounds are better
inhibitors than the compounds of the disulfoton group.
As with other cholinesterase-inhibiting organophosphate esters,
the effects on other biochemical parameters or enzymes were not
reported.
Following acute i.p. administration, 5/8 of the LD50 level,
cholinesterase activity of brain serum and submaxillary gland was
maximally depressed within a very short period of time (less than six
hours) to approximately the same level (15-257 of normal) after which
recovery was initiated but not complete within 72 hours. An induced
tolerance to the cholinergic stimulation has been observed with
disulfoton especially with chronic administration (Bombinski and
DuBois, 1958).
TABLE 1. pI50 - VALUES OF DISULFUTON AND DEMETON-S AND THEIR METABOLITES ON CHOLINESTERASES OF
INSECTS AND RAT BRAIN
Insectb Insectc Insectd Insecte Insecte Ratf,g
Compounda (whole weevil) (fly head) (fly head) (fly head) (fly head) brain
Disulfoton 3.62 - - 4.00 -- 3.85
Disulfoton-S (O) 4.08 - - 4.15 4.30 4.77
Disulfoton-S (O)(O) 2.00 - 5.90 5.46 4.92 4.00
Demeton-S 4.89 5.46 - 5.46 - 6.68
Demeton-S (O) 5.08 5.82 - 5.82 5.70 5.66
Demeton-S (O)(O) 4.66 6.22 6.49 6.22 7.70 5.70
a S (O) = Sulfoxide; S (O)(O) = Sulfone.
b Bull, 1965.
c March et al., 1955.
d Metcalf et al., 1957.
e March et al., 1957.
f FAO/WHO, 1965.
g Bombinski and DuBois, 1958.
Groups of rats (five female Sprague-Dawley strain per group) were
administered disulfoton intraperitoneally at dose levels of 0, 0.25,
0.5, 1.0, 1.2, and 1.5 mg/kg/day for 60 days. Mortality occurred at
the three highest dose levels. No mortality was evident at 0.5
mg/kg/day or below, although inhibition of growth was observed. At 1
mg/kg, typical signs of cholinergic stimulation were evident during
the first days of testing but within 10 days the animals recovered and
were symptomless thereon. Furthermore, these animals began to gain
weight and appeared to adapt to the continuous administration of
disulfoton (Bombinski and DuBois, 1958). A further series of rats was
treated with disulfoton at levels of 0, 0.25, 0.5, and 1.0 mg/kg daily
by intraperitoneal administration for 30 days. Cholinesterase data,
monitored over the course of this experiment, showed that there was an
initial rapid decrease of brain cholinesterase which was dependent
upon the daily dose of disulfoton. With the two highest doses, a
permanent decrease in enzyme activity occurred only during the first
seven days after which time further treatment resulted in maintenance
of the enzyme level at a constant subnormal level. The lowest dose
however induced a permanent decrease of brain cholinesterase activity.
The serum-ChE showed nearly the same time course and degree of
depression as the brain cholinesterase, but the cumulative effect of
the lowest dose between days 7-30 was not marked.
Clinical cholinergic signs of poisoning, including weight loss,
evident over the first seven days of treatment at the highest dose
level disappeared with the result being a symptomless cholinesterase
depression (Bombinski and DuBois, 1958). Further studies to determine
the mechanism of this acquired tolerance were reported (Brodeur and
DuBois, 1964).
It was observed that the development of tolerance to subacute
administration of disulfoton was not paralleled by changes in the
acetylcholine-cholinesterase system. The free acetylcholine level in
the brain was elevated to approximately the same extent by each
successive dose throughout the period of treatment. In addition, it
was believed that the development of tolerance did not involve the
metabolic conversion of disulfoton to its oxidative anticholinesterase
analogues (Stavinoha et al., 1972). These authors postulated that the
development of the tolerance resulting from high repeated
administration of disulfoton was due to the development of a
refractoriness of the cholinergic receptors to prolong exposure to
high levels of acetylcholine. It was further observed that strain
differences exist in the acquired tolerance to cholinergic
stimulation.
Groups of Charles River and Holtzmann rats received a daily
intraperitoneal injection of 1 mg disulfoton/kg for three, 10, and 24
days respectively. In each of the three groups the signs of poisoning
were most severe on the third day. The Holtzmann rats treated for 10
and 24 days exhibited early signs of adaptation while the Charles
River rats took longer to adapt. Measurements of cholinesterase
activity in the brain showed that acetyl cholinesterase activity as
expected decreased, the amount of depression being dependent on the
duration of the application while the acetylcholine concentration
initially increased. In the Holtzmann rat acetylcholine concentration
returned to the control level after 10 days of injections and was
still at the control level when measured after 24 days of treatment.
In the Charles River rats, the acetylcholine concentration of the
brain was still elevated at the end of the 24-day injection period. In
an experiment in which disulfoton was added to the diet at
concentrations of 0, 10, 25, and 50 ppm, it was observed that
adaptation progressed more slowly than in the intraperitoneal
injection experiment. The time required for adaptation was longer as
the amount of disulfoton in the diet was increased, e.g., 1 to 1-1/2
months at 10 ppm; 2 to 3 months at 25 ppm; and very little adaptation
was observed at 50 ppm. There was pronounced suppression of weight
gain at 50 ppm. Acetyl cholinesterase was reduced in relation to the
dietary concentration although only in one strain at the highest dose.
The acetylcholine concentration was not different from the control
level. Choline acetyltransferase activity was not affected (Stavinoha
et al., 1969).
Groups of weanling rats (six rats per group) were fed disulfoton
in the diet at levels of 0, 1, 5, and 25 ppm for seven days. At the
end of one week the animals were sacrificed for the measurement of the
hydrolysis of tributyrin and diethylsuccinate by liver and serum and
for the measurement of cholinesterase in serum, liver and brain.
Dietary levels of disulfoton producing 50% inhibition of aliesterases
and cholinesterase over this one week feeding period were obtained by
analysis of a plot of the logarithm of dietary concentration and
inhibition of the respective enzymes. There was no significant
difference in this experiment between the levels of inhibition caused
by disulfoton and demeton. The liver hydrolysis of tributyrin was the
most sensitive parameter followed by diethylsuccinate hydrolysis.
Depression of cholinesterase activity was caused in the brain by 5.2
ppm disulfoton in the diet; in the liver by 14.5 ppm and the serum by
6.0 ppm. Depression of liver aliesterase hydrolyzing DES was caused by
3.5 ppm while in the serum it was calculated to be 8.4 ppm.
Depression of the enzymes hydrolyzing tributyrin was caused in
the liver by 0.6 ppm and in the serum by 9.2 ppm disulfoton in the
diet. Although these authors point out that there is a possible
relationship of aliesterase inhibition with potentiation, studies (see
Potentiation Section) show that with a selected series of
organophosphorus compounds, potentiation was not demonstrated (Su et
al., 1971).
Results of a recent study of the inhibited portions of rat brain
following disulfoton administration is show in Table 2.
This experiment reveals a severe inhibition of the
cholinesterases in the hippocampus and caudate nucleus, compared with
the hypothalamus and medulla. The recovery of cholinesterase activity
occurred more rapidly in the first two mentioned parts of the brain
than in the others although at the end of the seven day recovery
period, activity in the hypothalamus and caudate nucleus is as still
low (Modek et al., 1971).
TABLE 2. CHOLINESTERASE ACTIVITYa
Tissue Control Treatmentb Recoveryc
Hypothalmus 4.62 1.87 3.27
Medulla 5.26 1.95 4.00
Hippocampus 3.19 0.62 1.79
Caudate nucleus 13.56 2.42 8.65
Ileum 2.90 2.38 2.80
Gastrocnemius 0.48 0.28 0.40
a mM substrate hydrolyzed/gm protein/h (Modek et al., 1971).
b Intraperitoneal administration for 10 days at 1.5 mg/kg/day to
rats.
c Recovery time = seven days.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of male mice (12 mice/group) were administered
disulfoton by intraperitoneal injection at doses of 0, 0.25, and 0.5
mg/kg. Each male was mated with three virgin females each week for six
weeks in the standard dominant lethal mutation test. There were no
abnormalities noted in the data on implantation resorption or on the
embryo itself. In this study, disulfoton did not exhibit any mutagenic
effect on male mice (Arnold et al., 1971).
Disulfoton inhibited growth of three human haematopoietic cell
lives but had no effect on chromosomes (Huang, 1973).
Special studies on reproduction
Rat. Groups of rats (20 females and 10 males/group) were fed dietary
levels of disulfoton at 0, 2, 5, and 10 ppm over the course of a
two-litter per generation, three generation reproduction study. There
did not appear to be a significant effect of disulfoton in the diet on
reproduction in the rat. Levels up to and including 10 ppm did not
significantly affect reproduction parameters. At 10 ppm in the F-1-A
there was a greater than normal mortality of rats at weaning time.
There was no significant difference in any of the groups in the number
of pregnancies or in the number of young per treatment groups.
Histological examination of the F-2-B rats indicated a cloudy swelling
of the liver cells with a fatty metamorphosis especially in male rats
on 10 ppm which was not observed in the controls. This was not
observed in similar F-2-B rats. RBC-cholinesterase depression was
obvious in all treatment groups examined. No gross differences were
observed between dale and female rats. The reduction was dose
dependent and was more significant in females than males. While
disulfoton does not appear to have a definitive effect upon
reproduction parameters, high levels of disulfoton in the diet (10
ppm) have shown somatic effects and levels of 2 ppm in the diet have
shown reduction in cholinesterase (Taylor, 1966).
Special studies on teratogenicity
Groups of 15 pregnant rabbits were administered disulfoton orally
in gelatin capsules at doses of 0, 0.1, and 0.2 mg/kg daily from days
six through 18 of gestation. On day 29 of gestation, the young were
removed by caesarean section. There were no deaths or unusual
reactions among the females in any of the groups and the incidence of
fetal mortality as indicated by resorption sites or abortion was not
affected by disulfoton. There was no indication of fetal external or
internal abnormalities and the weights of the fetuses were similar to
those of the controls. A positive treatment of this test was obtained
with thalidomide. Disulfoton does not appear to cause any teratogenic
effects in rabbits (Ladd et al., 1971).
Demeton while being embryotoxic as a single dose of 5 mg/kg for
three days on days 7-13 of gestation or as a single dose of 7 or 10
mg/kg during the same interval. Only a mild teratogenic potential was
noted (Budreau, 1972; Budreau and Singh, 1973).
Special studies on neurotoxicity
Adult hens were orally administered disulfoton at a dose
estimated to be the LD50 (26 mg/kg) twice at 21 day intervals and
maintained for a further 21 days. Growth and histological examination
of the animals indicated there were no signs of delayed neurotoxicity
while a positive control (TOCP) showed definite signs of poisoning.
Disulfoton does not induce delayed toxicity or demyelination (Fletcher
et al., 1971). Hens, protected from acute cholinergic stimulation and
organophosphorus poisoning by atropine and PAM were orally
administered disulfoton at levels of up to 0.1 ml/kg. Delayed
neurotoxic effects were not noted during the six weeks post-treatment
observation period (Kimmerle, 1961).
Special studies on the neurotoxicity of metabolite
Disulfoton sulfoxide was administered intraperitoneally at levels
up to 0.5 g/kg to hens previously administered PAM and atropine.
Although mortality was evident at high dose levels there was no
evidence of delayed neurotoxicity as observed normally with TOCP
(Hecht and Kimmerle, 1965).
Special studies on potentiation
Simultaneous administration of an LD50 dose with eight other
organophosphate insecticides resulted in a slight additive acute
toxicity with five compounds and less than additive acute toxicities
with the other three. None of the combinations resulted in a
potentiation of the acute toxicity Dubois, 1957 a & b). Further
studies with organophosphate and a carbamate ester were negative
(Dubois, 1960). Equitoxic mixtures of disulfoton and phenamiphos (the
active ingredient of NemacurR, ethyl 4-(methylthio)-m-tolyl
isopropyl phosphoroamidate) resulted in less than additive toxicity
(Kimmerle, 1972). A combination of disulfoton and phosphamidon did not
cause potentiation (Sachsse and Voss, 1971).
The signs of poisoning caused by disulfoton are typical of those
produced by anticholinesterase compounds. The signs of poisoning
consist of excitability, salivation, lacrimation, urination,
defaecation, and muscular fasciculations. The signs were followed by
convulsive seizures, prostration, and respiratory failure. As with
other organophosphorus compounds the occurrence of signs of poisoning
are indicative of both nicotinic and muscarinic actions of
acetylcholine indicating that the compound or its active metabolite
has gained access to both the central and peripheral nervous system.
The time of onset and duration of signs of poisoning are dependent
upon the dose. With lethal doses death usually occurred within the
first 48 hours but upon sublethal administration death was delayed for
several days. A comparison of the toxicity by disulfoton by various
routes i.p. and oral) indicate that the compound is well absorbed from
the GI tract. A considerable sex difference in susceptibility was
noted in rats in some studies (i.e. Bombinski and DuBois, 1958) with
the male being five times less sensitive than the females. This was
not noted with other species. Similar sex differences in
susceptibility of rats have been noted with other thiophosphates. It
has been suggested that because the oxygen analogues did not exhibit
this difference in sex susceptibility that the differences in the rate
or extent of conversion of thiophosphates to their toxic oxygen
analogues is presumably responsible for the observed sex difference.
Special studies on antidotes
Several studies on the antidotal properties of atropine and PAM
administered intramuscularly before and after oral administration of
disulfoton have the distinct therapeutic effects with these materials.
Atropine in combination with other oximes administered
intraperitoneally after the appearance of signs of poisoning also
produced a therapeutic effect (Kimmerle, 1961; Lorke and Kimmerle,
1968). Atropine, when injected intraperitoneally (100 mg/kg) prior to
a dose of disulfoton, protected rats from the acute oral effects of
poisoning of an LD50 dose. Acute administration of two times the LD50
proceeded by administration of atropine was lethal (Bombinski and
Dubois, 1958).
Studies on the antidotal effects of atropine and PAM with
disulfoton sulfoxide were similar to those reported with disulfoton
where a significant reduction in acute toxicity was noted with both
atropine and oximes alone. A more significant protective effect was
noted with a combination of both atropine and oxime (Hecht and
Kimmerle, 1965).
Special studies on inhalation
Female rats were exposed to concentrations of 0, 0.14, 0.35 and
0.70 microgram/litre in the air one hour a day for five or 10 days.
There was no mortality nor any significant decrease in cholinesterase
activity in brain, serum or submaxillary gland (DuBois and Kinoshita,
1971).
Acute Toxicity
(a) Original compound
Species Route LD50 Reference
(mg/kg)
Rat M & F oral 2.3-12.5 Ben-Dykeet al., 1970
DuBois, 1957; Gaines, 1969
Kimmerle, 1961, 1962, 1966, 1972
M oral 12.5 Bombinski and DuBois, 1958
F oral 2.6 Bombinski and DuBois, 1958
Guinea Pig M oral 10.8 Bodbinski and DuBois, 1958
M i.p. 7.0 Bombinski and DuBois, 1958
Rat M i.p. 10.5 Bombinski and DuBois, 1958
F i.p. 2.0 Bombinski and DuBois, 1958
Mouse M i.p. 5.5 Bombinski and DuBois, 1958
F i.p. 6.5 Bombinski and DuBois, 1958
Rat M dermal 25-50 Ben-Dyke et al., 1970
Gaines, 1969; Kimmerle, 1962
0.285
(4 hr exposure) Well et al., 1971
Species Route LD50 Reference
(mg/kg)
Rat M inhalations 200 g/m3
(1 hr exposure) Doull, 1957
Mouse F inhalation 58 mg/m3 Doull, 1957
(1 hr exposure
(b) Metabolites
Material Species Route LD50 Reference
(mg/kg)
Disulfoton Rat oral 1.7-6.5 Hecht and Kimmerle, 1965;
Sulfoxide Kimmerle, 1962;
Schrader, 1963; Wirth, 1958
Mouse oral 5.4 Bombinski and DuBois, 1958
Guinea Pig oral >3.6 Hecht and Kimmerle, 1965
Rabbit oral 2.5-3.6 Hecht and Kimmerle, 1965
Cat oral 1.0-2.5 Hecht and Kimmerle, 1965
Rat i.p. 5.0 Hecht and Kimmerle, 1965
Rat dermal 0.195 ml/kg Hecht and Kimmerle, 1965
4 hr
0.075 ml/kg Hecht and Kimmerle, 1965
7 day
0.192 ml/kg Kimmerle, 1962
Rat inhalation 140 g/m3 Hecht and Kimmerle, 1965
(1 hr exposure)
Disulfoton Rat oral 5.0-7.5 Schrader, 1963; Wirth, 1958
Sulfone
Mouse oral 5.6 Bombinski and Dubois, 1958
Demeton-S Rat oral 1.5-3.5 Klimmer and Pfaff, 1955;
(isosystox) Wirth, 1958
Material Species Route LD50 Reference
(mg/kg)
Mouse i.p. 5.6-7.0 Muhlmann and Tietz, 1956;
FAO/WHO, 1965
Guinea Pig i.p. 5.5 FAO/WHO, 1965
Demeton-S Rat oral 1.5-2.0 Schrader, 1963; Wirth, 1958
Sulfoxide
Demeton-S Rat oral 1.5-2 Schrader, 1963; Wirth,1958
Sulfone
In almost all instances, female rats were more susceptible than males.
Short-term studies
(a) Original compound
Mouse. Groups of mice (12 male and 12 female CF-LP strain per group)
were fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5.0 ppm
for 13 weeks. Food consumption and growth were measured weekly and
behaviour and mortality were observed daily. At 12 weeks, urinalysis
haematological examination and blood chemistry including BBC, plasma
and cholinesterase assays were performed. At the conclusion of the
study, gross and microscopic examination of tissues was performed. No
treatment-related changes were observed in growth, urinalysis,
haematology, or blood chemistry with the exception of cholinesterase
activity. Cholinesterase activity was reduced in all tissues at 5 ppm,
especially in females. Gross histological examination indicated a
slight increase in the liver weight in females at 5 ppm. There were no
other abnormalities noted on gross or microscopic examination of
tissues and organs. The no-effect level based upon this study in mice
is 1 ppm in the diet (Rivett et al., 1972).
Rat. Groups of rats (13 males and 13 females per group) were fed
disulfoton in the diet at levels of 0, 1, 2, 5 and 10 ppm for 16
weeks.
Food consumption and growth were measured daily for the first two
weeks every two days for the next month and then weekly until 16
weeks. At the end of the feeding period, three males and three females
were sacrificed and tissues examined for gross and microscopic
changes. Cholinesterase activity in serum, erythrocyte brain and
submaxillary gland was examined at eight and 16 weeks of feeding.
There were no effects of disulfoton in the diet at any dose level on
growth, food consumption, behaviour or mortality over the 16-week
period. Gross and microscopic examination of all tissues from both
male and female animals revealed no differences. Cholinesterase
depression was observed in erythrocyte and brain at levels of 2 ppm
and above at both eight and 16 weeks and was more marked in female
than male. Submaxillary gland and serum was less sensitive. A
no-effect level in this study based upon cholinesterase inhibition is
1 ppm in the diet (Doull and Vaughn, 1958).
Groups of rats (25 male and 25 female Wistar strain rats) were
fed disulfoton in the diet at levels of 0, 0.2, 1.0 and 5 ppm for 90
days. Body weight and food consumption data were recorded weekly and
behaviour and mortality was observed daily. Urinalysis, clinical
chemistry and haematological examinations including RBC and plasma
cholinesterase activity were made periodically. At the conclusion of
the study, brain cholinesterase activity was analysed and all animals
were sacrificed for gross and microscopic examination of tissues and
organs. There were no significant differences between the controls and
the treated animals with regard to growth, behaviour, mortality,
urinalysis, and clinical chemistry. There appear to be no effects of
the feeding of disulfoton on gross or histological examination of the
tissues at the conclusion of the study. Cholinesterase was
significantly depressed (primarily in females) in plasm and red blood
cells at 5 ppm. In this study, 1 ppm is a no-effect level based upon
cholinesterase inhibition Motzsche, 1972).
Dog. Groups of adult mongrel dogs (one male and one female per
group) were fed disulfoton in the diet at levels of 0, 1, 2, and 10
ppm for 12 weeks. At all feeding levels, no weight loss, signs of
poisoning or adverse behaviour were noted. Plasma and RBC
cholinesterase values were significantly decreased at 2 and 10 ppm
while 1 ppm caused do significant inhibition (Vaughn et al., 1958).
After returning to a control diet, the plasma cholinesterase
inhibition rapidly returned to normal. The RBC cholinesterase remained
inhibited for over four weeks.
(b) Metabolites (disulfoton sulfoxide)
Rat. Groups of rats (10 male rats per group) were administered
disulfoton sulfoxide orally, five doses per week, for one month at
dosage levels of 0, 0.215, 0.43, 0.9 mg/kg. Cholinesterase activity
was significantly depressed at the highest dose level and marginally
depressed at the middle dose level. At seven days after the initiation
of the study, cholinesterase depression reached a maximum level and
thereafter recovered slightly maintaining a constant depressed level
to the end of the experiment. Seven days after the conclusion of the
experiment, the cholinesterase values were essentially normal (Hecht
and Kimmerle, 1965).
Groups of rats (10 female rats per group) were administered
disulfoton sulfoxide daily five days per week for nine weeks at dosage
levels of 0, 0.046, 0.093, 0.186, 0.388 and 0.775 mg/kg/day. Mortality
was obvious at the highest dose level tested although growth was not
affected in any of the groups. Haematological and urinalysis were
normal as was gross pathology. Slight changes in liver epithelial
cells were noted in several animals at the highest dose level (Hecht
and Kimmerle, 1965). On the basis of these studies with disulfoton
sulfoxide with the most sensitive parameter being acetyl
cholinesterase depression, a level of 0.43 mg/kg administered orally
five days per week would be considered to be the marginal effect
level.
Long-term studies
Long-term feeding studies on rats and dogs have been initiated
but data are not available.
Observations in man
Five volunteer subjects each received a daily oral dose of 0.75
mg of disulfoton for 30 days; two persons served as controls. Plasma
and erythrocyte cholinesterase levels were measured twice weekly
during the pre-test control period and during the 30-day test period.
No depression of cholinesterase activity was noted (Rider, 1972).
Comments
Disulfoton, a phosphorodithioate insecticide structurally similar
to demeton, is acutely toxic and produces its primary effect through
inhibition of cholinesterase activity. Disulfoton.is metabolized by
thionate oxidation, thioether oxidation, and hydrolysis or oxidative
cleavage. Thionate oxidation would result in demeton which is further
degraded. As disulfoton is a fast acting organophosphate, the
oxidation of the thionate to demeton is apparently very rapid. Demeton
was evaluated by the Joint Meeting in 1965 and the ADI for man was
estimated to be 0.0025 mg/kg.
Toxicological studies showed disulfoton to have no effect on
reproduction and tests for teratogenicity and mutagenicity gave
negative results. Disulfoton did not produce delayed neurotoxicity in
hens nor potentiate the toxicity of several organophosphorus compounds
although it is an inhibitor of aliesterase activity. In short-term
studies in rats and dogs a no-effect level was estimated to be 1 ppm
based upon inhibition of cholinesterase. At higher levels liver damage
was observed. Studies in man showed that levels of 0.75 mg for 30 days
was without effect on cholinesterase activity.
Long-term studies have been reported to be in progress and on the
basis of short-term studies a temporary ADI was established.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect in animals
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw
Dog: 1 ppm in the diet equivalent to 0.025 mg/kg bw
Man: 0.75 mg/man/day
Estimate of temporary acceptable daily intake for man
0-0.001 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Disulfoton possesses systemic activity and is used to control
aphids, leafhoppers, thrips, beet flies (mangold fly, spinach leaf
miner), coffee leaf miner and spider mites. it is formulated as
granules and liquid concentrate and seed dressing powder (used only on
cotton). Disulfoton formulations are used on cotton, vegetables,
potatoes, cereals (chiefly sorghum and rice), coffee, etc. Products
based on disulfoton are registered in a total of 33 countries, 807
being used on vegetables including potatoes and 20% on field crops.
Pre-harvest treatments
Disulfoton is chiefly applied at sowing or as a top dressing. The
recommended application rates range from 1 to 4 kg/ha on most crops,
with pre-harvest intervals of from 30 to 100 days. These recommended
pre-harvest intervals are not necessarily identical in all countries.
Post-harvest treatments
No uses.
Other uses
Applied to ornamentals.
Residues resulting from supervised trials
A large amount of data are available on residues resulting from
the application of disulfoton to various crops. Most of them are from
the United States of America. Results are presented in Table 1.
TABLE 1
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Alfalfa 11 1-1.5 1 7-28 n.d.-0.7
(Forage) 4 1 3 7-28 6-30
(Hay) 8 1.5 1 3-7 5-15
2 1.5 3 7 14, 19
Beans 16 1-2 1 80-160 n.d.-0.3
Broccoli 7 1-4 1 7-80 n.d.-0.6
Brussels sprouts 4 1-2 1 7-100 n.d.-0.2
Barley (grain) 3 1 1 33-104 n.d.
Cabbage 8 1.7 oz/ 1 35-106 0.01-0.1
1000 ft row
Cabbage (in furrow)
5% granules) 6 1 1 28-87 0.1-1.5
Cotton 3 1.2 1 28 < 0.3
Cottonseed 16 1-4 1 30-180 n.d.-0.6
4 1-1.5 2 30-120 n.d.-0.2
Celery 5 1.2-2.5 1 55-130 n.d.-3
Coffee 6 1-4 oz/ 1 24-180 n.d.
plant
Clover 8 1.5-2 1 3-28 1-7
Clever (hay) 2 1.5 1 3 8, 17
Lettuce head or
leaf 5 1-2 1 16-62 <0.1
Maize 17 1-2 1 30-120 n.d-0.5
11 1-2 20-30 0.2-7
Oats (grain) 2 1 1 77, 98 n.d.
Potatoes 8 1-1.5 1 60-168 n.d-0.3
66 2-3 1 50-170 n.d,-0.4
8 3-3.5 1 86-180 0.2-2
11 3-4.5 2 60-180 0.05-0.3
TABLE 1 (Cont'd.)
Rate of
Crop No. of Application No. of Days after Residue
trials kg/ha treatments application PPM
Peanuts 3 1-2 1 116-148 n.d
Peanuts (shells) 11 9-14 1 116-168 0.01-0.4
Peanuts (kernels) 3 16+32 2 70 0.1
Peas (including pods) 13 1-2 1 28-70 n.d.-0.3
2 2 1 28-43 4,9
Pineapple 14 1-5 1 7-70 n.d.
Rice 12 1-4 1 50-200 n.d,-0.5
Spinach 4 1 1 7-21 2-4
10 1 1 40-90 n.d.-0.5
Sorghum 17 1-1.5 1 30-80 n.d.-0.1
Sugar beets 11 1 1 98 0.1-0.3
11 1 1 51 n.d.-6
5 1-2.5 1 160-180 n.d.-0.1
Soybeans 2 1,2 1 132 n.d.
Tomatoes 10 0.5-6 1 or 2 30-108 n.d.-0.5
Wheat 24 0.75-4 1 30-300 n.d.-0.2
5 0.25-1 4 30-52 0.01-0.02
Pecans 5 1-20 1 88-240 n.d.
Soil persistence 1 0.5 1 0-366 n.d.
1 0.5 1 1-424 6-0.4
1 0.5 1 1-181 4-1
FATE OF RESIDUES
In plants
From knowledge of the ready biological oxidation of thioether
groups and in view of the known conversion of phosphothionates to
their P=O analogues, the expected metabolites of disulfoton (I) are
the compounds (II) to (VI):
 Compound (IV) is demeton-S, one of the active ingredients of the
well-known systemic insecticide SystoxR. Thus the metabolism of
disulfoton dovetails into the metabolism of demeton-S. However, it
should be noted that owing to the rapid formation of the sulfoxides,
the occurrence of (IV) as a metabolite is hardly to be expected.
Following the formation of these metabolites, further degradation can
only be by hydrolysis. The plant metabolism of disulfoton was studied
by the use of 32-p labelled compound (Metcalf et al., 1957, 1959) in
cotton, lemon, bean and alfalfa plants. Disulfoton was rapidly
oxidized to produce the sulfoxide (II) and slowly to produce sulfone
(III). Both those compounds were also oxidized at the thiono-sulfur to
produce V and VI. These same compounds were identified by Bull (1965)
working with other plants, e.g., avocado, brussels sprouts, cabbage,
corn, tomato. The same results were obtained but the proportions of
various compound differed. These studies we re confirmed by later
investigations.
Loeffler (1970b) found II and VI as major metabolites in tobacco.
Gentry et al., (1970) found that in tobacco the order was: Ill, II,
VI, V (see also Bowman et al., 1969).
In soil and water
Generally, the half-life of disulfoton residues in different
soils is between 30 and 100 days (Olson, 1964; Loeffler, 1969). Soil
type and microbial activity seem to have a greater influence on the
rate of decomposition than the temperature (Henzer et al., 1970).
Both sulfones were detected as metabolites in the soil (Henzer et
al., 1970), but the sulfoxides occurred in only minute amounts. Takase
et al., (1971, 1972) however, found chiefly disyston sulfoxide and
sulfone as metabolites in different types of soil.
Disulfoton does not display a strong tendency to leach into the
soil since approximately 1670, 1970 and 4400 m of theoretical rainfall
were required to leach the compound 30 cm into sandy loam, silt loam
and high organic silt loam soils, respectively (Flint et al., 1970).
No effect on soil micro-organisms was observed (Houseworth and
Tweedy, 1972), though some reduction of fungal population was observed
with the high level of 250 ppm in the soil.
The half-life of disulfoton in water under simulated field
conditions was 2.9 days (Flint et al., 1970).
Fate of residues in storage, processing and cooking
In frozen storage, residues remain unchanged for long periods,
sometimes for more than two years (Chemagro Rep. 8857).
The thermal destruction of disulfoton during processing of
apricots (100°C/2 min) and spinach (120°C/55 min) was investigated by
Thornburg and reported (Anderson 1959a, b). Loss of residues was 37%
and 80% respectively.
The fate of disulfoton in potatoes during processing was
investigated by Kleinschmidt (1971). Total residues (1.33 ppm) were
reduced by 35% with lye peeling. Lye peeling plus a single water
blanching reduced the total residue by 38, 74 and 61% for french
fries, dehydrated cubes and dehydrated mashed potatoes, respectively.
On a dry weight basis, overall reduction in residues due to processing
potatoes into french fries, dehydrated cubes, dehydrated mashed, and
chips were 77, 81, 89 and 97% respectively. Lye peeling and cooking
decreased residues of disulfoton by 30% (Zwolinska and Trojanowski,
1968a).
Total diet studies and residues in food moving in commerce
Abbott et al., 1970, found residues of disulfoton only on one
sample of green vegetables. In 1968, 0.1 ppm of disulfoton was found
in only one sample of citrus fruit in New Zealand (N.Z. Min. of Agr.
information, 1973).
Methods of residue analysis
Prior to the advent of GLC methods employing phosphorus sensitive
detectors, residues of disulfoton and its metabolites were determined
by total phosphorus procedures. GLC methods for the determination of
disulfoton residues are now available (Thornton and Anderson, 1968;
Thornton, 1967a, 1967c, 1969; Bowman et al., 1969; Bowman and Beroza,
1969). The principle of most GLC procedures is oxidation of the
residues to disulfoton-sulfone (Ill) and/or demeton-S-sulfone (VI). If
permanganate is used for oxidation, there is usually no transformation
of P = S to P = O so that it is possible to distinguish between P =
S-sulfones and P = O-sulfones. This permits conclusions to be drawn as
to whether the residues present result from application of demeton-S
or disulfoton. It is possible to distinguish clearly between these and
related sulfone pairs by using a 1.1 m column packed with 10% DC-200 +
1% QF -1 on 80/100 mesh Gaschrom Q; at 195°C. The following retention
times are reported (Wagner, 1973):
demeton-S-methyl sulfone 3.75 min
thiometon sulfone 4.75 min
demeton-S-sulfone 5.0 min
disulfoton-sulfone 6.15 min
Confirmatory GLC procedures, using different columns, are also
available (Olson, 1969; Loeffler, 1970). An interference study for
disulfoton residue determinations on alfalfa, clover and potatoes was
carried out by Olson (1970). A great number of organophosphorus
compounds were mixed with the various sulfone compounds.
Chromatographic conditions were modified and/or a confirmatory column
was employed and it was possible to eliminate all interference.
Numerous multi-residue methods capable of measuring disulfoton and its
metabolites are reported (Abbott et al., 1970; Storherr et al., 1971;
Watts, 1969; McCaulley, 1965). The available GLC procedures appear to
be satisfactory, specific and suitable for regulatory purposes.
Appraisal
Disulfoton is an organophosphorus insecticide, possesses systemic
activity and is used to control aphids, leafhoppers, thrips, beet
flies, coffee leaf miner and spider mites. It is formulated
predominantly as granules and for some special uses as a liquid
concentrate. A seed dressing powder is exclusively used in cotton.
Disulfoton is used on a great variety of crops, including vegetables,
potatoes, sugar beets, cotton and cereals. Products based on
disulfoton are registered in a total of 33 countries. The percentage
breakdown of the amounts used in the different crop areas is roughly
80% in vegetables (including potatoes) and 207 in field crops.
Disulfoton is chiefly applied at sowing or as a side dressing.
Recommended application rates are from 1 to 4 kg/ha, pre-harvest
intervals ranging mostly from 30 to 100 days. The minimum purity of
the technical product is 94%. The impurities have been identified and
quantified.
NATIONAL TOLERANCES
Pre-harvest intervals and tolerances
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Australia Potatoes 70 0.5
Vegetables 40 0.5
Cereals 70 0.5
Deciduous fruit 70 0.5
Belgium Deciduous fruit 0.01
Potatoes 0.01
Vegetables 0.01
Bulgaria 60
Canada Beans 0.5
Broccoli 0.5
Brussels sprouts 0.5
Cauliflower 0.5
Lettuce 0.5
Peas 0.5
Potatoes 0.2
Spinach 0.5
Tomatoes 0.5
Germany Beets Do not feed tops before
Hops harvest. Application by
sprinkling method must be
made only up to 1 June at
the latest.
Potatoes Only for seed 0.2
production
Netherlands Vegetables, 0.01
deciduous fruit
Potatoes Only at planting 0.01
New Zealand Barley 56
Beans 56
Broccoli 42
Brussels sprouts 42
Cabbage 42
Carrots 56
Cauliflower 42
Oats 50
Peas 56
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Potatoes 91
Turnips 56
Wheat 56
Poland Hops Apply only at the time
of earthing up.
Potatoes Only for seed production
Beets Apply up to the six-leaf
stage.
For fodder 60
USSR Cereals, cotton 0.35
seed oil
Fodder No residues
South Africa Cabbage 42 0.5
Onions (for aerial 90 0.5
plant parts)
Potatoes 90 0.5
Switzerland Field crops 42
United
Kingdom 42
United States Alfalfa
of America (fresh forage) 5.0
(hay) 12.0
Barley (grain) 60 0.75
(forage or straw) 5.0
Beans (green, Lima, Application at 0.75
snap) time of planting
(on vines) 5.0
Beans (dry) 60 0.75
(on vines) 5.0
Broccoli 14 0.75
Brussels sprouts 30 0.75
Cabbage 42 0.75
Cauliflower 40 0.75
Clover (fresh) 7 5.0
(clover hay) 12.0
Coffee 90 0.3
Cotton
(non-irrigated, seed) 28 0.75
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Cotton (irrigated, 28,90 0.75
seed)
Hops 0.5
Lettuce 60 0.75
Maize (field corn,
sweet corn, 40,100 0.3
popcorn)(grain)
(fodder) 5.0
Oats (grain) 60 0.75
(forage or straw) 5.0
Peanuts Application 0.75
(peanut hay) at time of 5.0
planting
Peas 50 0.75
(vines) 5.0
Pecan 80 0.75
Pineapples 60 0.75
(foliage) 5.0
Potatoes 75 0.75
Rice 100 0.75
(straw) 5.0
Sorghum (grain) 7 0.75
(fodder and 28 5.0
forage)
Soybeans Do not pasture or use treated
crop for feed food or forage
Spinach Application at 0.75
time of planting
Strawberries Do not use fruit from
treated plantg for food
Sugar beets 30 0.5
(tops) 2.0
Sugar cane 28 0.3
Tomatoes 30 0.75
resp. application at time
of planting
Wheat (grain) 45 0.3
(green fodder Do not graze 5.0
and straw) treated fields
Metabolism studies on plants and soil are available, indicating
the formation of sulfoxides and sulfones of disulfoton and the oxygen
analogue (demeton-S). The ratio of these metabolites can vary and
depends on plant variety, soil type and climatic conditions.
A large number of residue data are available from supervised
trials, predominantly from the United States of America, but also from
some European countries and New Zealand.
Evidence on the fate of residues during storage, processing and
cooking indicates that residues are stable under deep freeze
conditions; losses of residues occur during cooking, heating, or
peeling in the case of potatoes.
Information on residues in food moving in commerce or from total
diet studies is scanty. No data are available on the eventual
carry-over of residues from forage crops into animal tissues, milk or
eggs. Methods of analysis for disulfoton residues based on GLC with
phosphorus specific detectors are available and appear to be suitable
for regulatory purposes, the limit of detection being in the order of
0.05-0.1 ppm depending on the crop.
Residues are best determined, following oxidation to
disulfoton-sulfone and/or demeton-S sulfone, as the sum of parent
compound and all of its oxydative metabolites, expressed as parent
disulfoton. By applying-suitable oxidation procedures, a quantitative
differentiation can be made between the two sulfones. Absence of
disulfoton-sulfone would indicate that prevailing residues have arisen
from an application of demeton, in which case residues should be
expressed as parent demeton.
RECOMMENDATIONS
The following tolerances are recommended, the residues being
determined as disulfoton-sulfone and demeton-S-sulfone and expressed
as disulfoton.
Crop Tolerance (ppm)
Vegetables, including beans, broccoli,
brussels sprouts, cabbage, cauliflower, 0.5
lettuce, potatoes, peas, spinach,
tomatoes, rice (in husk), sugar beets
Cereals (except rice) sugar beets, cottonseed 0.2
Coffee beans, peanuts (kernels),
pecans, pineapple, soybeans 0.1a
Forage crops (green) 5.0
a At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required before June 1975
1. Results of the long-term studies now in progress.
2. Kinetic studies on absorption, distribution, metabolism, and
excretion in mammals.
3. Evaluation of liver damage observed in short-term studies.
4. Data on residues in meat, milk, and eggs after feeding animals on
crops or feedstuffs treated with disulfoton, in order to determine
residue limits in foods of animal origin.
Desirable
1. Information on residues in food moving in commerce.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adams, J.M. (1960) A specific method for the detection of residues of
DI-SYSTON and its metabolites in the presence of other cholinesterase
inhibiting pesticides. 1. Application to cottonseed. Chemagro-Report
No. 5928
Anderson, C.A. (1959a) Thermal destruction of DI-SYSTON during
processing of spinach. Chemagro-Report No. 4882d
Anderson, C.A. (1959b) Thermal destruction of DI-SYSTON during the
processing of apricots. Chemagro-Report No. 4882e
Anderson, C.A. (1960) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. III. Application to potatoes, sugar
beets, sugar beet tops, cabbage, broccoli, pineapple and alfalfa.
Chemagro-Report No. 5511
Anderson, C.A. (1961a) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. I. Application to cottonseed.
Chemagro-Report No. 5339
Anderson, C.A. (1961b) Colorimetric determination of DI-SYSTON
residues in plant material. III. Application to Brussels sprouts,
cauliflower, green beans, lettuce, lilies, peas, pineapple and
tomatoes. Chemagro-Report No. 6684
Anderson, C.A. (1962) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. Chemagro-Report No. 8544
Anderson, C.A. (1963) Colorimetric determination of DI-SYSTON residues
in green coffee beans. Chemagro-Report No. 10 919
Arnold, D., Keplinger, M.L. and Fancher, O.E. (1971) Mutagenic Study
with DI-SYSTON in Albino mice. IBT No. E 8920. Unpublished report from
Industrial Bio-TeSt Laboratoricos Inc.
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) "Acute Toxicity
Data for Pesticides (1970)". World Review of Pest Control, 9:
119-127
Bombinski, I.J. and DuBois, K.P. Chicago. (1958) "Toxicity and
Mechanism of Action of Di-Syston". A.M.A Archives of Industrial
Health, Vol. 17: 192-199
Bowman, M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton, and phorate in corn, milk, grass,
and faeces. J.A.O.A.C., 52: 1231-1237
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetomethyl, and their metabolites in
tobacco plants. J.A.O.A.C., 52: 157-162
Brewerton, H.V. and Close, R.C. (1967) Disulfoton residues in potato
tubers. N.Z. J1. agric. Res., 10: 272-277
Brodeur, J. and DuBois, K.P., Chicago. (1964) "Studies on the
Mechanism of Acquired Tolerance by Rats to 0,0-Diethyl S-2-(ethylthio)
ethyl phosphorodithioate (Di-Syston)", Arch. Int. Pharmacodyn., 149:
560-570
Budreau, C.H. (1972) Teratogenicity and chromotoxicity of three
organophosphorus insecticides in CF1 mice. Diss. Abs. Int., 33:
1174B
Budreau, C.H. and Singh, P.P. (1973) Teratogenicity and Embryo
toxicity of Demeton and Penthion in CF1 mouse embryos. Toxicol. Appl.
Pharmacol., 24: 324-323
Bull, D.L. (1965) Metabolism of Di-Syston by Insects, Isolated Cotton
Leaves, and Rats". J. Econ. Entomol., 58: 249-254
Chemagro Corporation, Kansas City, United States of America (1962)
Report No. 8857
Chisholm, D. and Specht, H.B. (1967) Effect of application rates of
disulfoton and phorate, and of irrigation on aphid control and
residues in canning peas. Can. J. Plant Sci., 47: 175-180
Chisholm, D., Specht, H.B. and Leefe, J.S. (1965) Di-Syston residues
and control of pea aphid, Acyrthosiphon pisum, with in-furrow
treatments of canning peas in Nova Scotia. J. Econ. Entomol., 58:
763-765
Cook W. C., Butler, L., Walker, K.C. and Featherston, P.E (1963)
Granular in-furrow treatments with phorate and Di-Syston against the
pea aphid on peas. J. Econ. Entomol., 56: 95-98
Doull, J. (1957) The acute inhalation toxicity of Di-Syston to rats
and mice. Unpublished report from the University of Chicago
Doull, J. and Vaughn, G. (1958) The effects of diets containing
Di-Syston on rats. Unpublished report from the University of Chicago
Dubois, K.P. (1957a) The acute toxicity of Di-Syston in combination
with other organic phosphates to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1957b) The acute oral toxicity of Di-Syston given
simultaneously with Phosdrin to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1960) The acute toxicity of Di-Syston in combination
with delnav ethion and serum to rats. Unpublished report from the
University of Chicago
DuBois, K.P. and Kinoshita, F.K. (1971) Effect of repeated inhalation
exposure of female rats to Di-Syston. Unpublished report from the
University of Chicago
Fletcher, D., Jenkins, D.H. and Keplinger, M.L. (1971) Neurotoxicity
study with Di-Syston technical in chickens. IBT No. J 471. Unpublished
report from Industrial Bio-Test Laboratories, Inc.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour II, J. (1970) Soil
runoff, leaching and adsorption, and water stability studies with
DI-SYSTON. Chemagro-Report No. 28 939
Fukuto, T.R. and March, R.L. (1954) Isomerization of
B-ethyl-mercaptoethyl Diethyl Thionophosphate (Systox). J. Amer.
Chem. Soc. 76: 45103-06
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharmacology, 14: 515-534
Gentry, C.R., Kincaid, R.R. and Bowman, M.C. (1970) Soil treatment
with disulfoton against certain insect pests of cigar-wrapper tobacco.
J. Econ Entomol., 63: 1139-1142.
Getz, M.E. (1962) Degradation of esters of Systox, Di-Syston and
Thimet on field-sprayed kale. J.A.O.A.C., 45: 397-401
Graham-Bryce, I.J. (1967) Adsorption of disulfoton by soil. J. Sci.
Food Agric., 18: 72-77
Graham-Bryce, I.J. (1969) Diffusion of organophosphorus insecticides
in soils. J. Sci. Food Agric., 20: 489-494
Gronberg, R.R. and Olson, T.J. (1966) Colorimetric determination of
Di-Syston residues in sugarcane and sugarcane products.
Chemagro-Report No. 17 565
Guérout, R., Barbier, M. and Gicquiaux, Y. (1968) Recherches sur
Vutilisation du disulfoton dans la lutte centre la cochenille
farineuse do Vananas, Dysmicoccus brevipes Ckl. Fruits, 23,2: 67-78
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Food
Chem., 17: 80-82
Hecht, J. and Kimmerle, G. (1965) Toxikologische untersuchungen mit
dem wirkstoff, E 23 323. Unpublished report submitted by Bayer A.G.
Houseworth, L.D. and Tweedy, B.G. Effect of DI-SYSTON on 1972
microbial populations. Chemagro-Report No. 35 127
Ibrahim, F.B., Gilbert, J.M., Evans, R.T. and Cavagnol, J.C. (1969)
Decomposition of Di-Syston (0,0-Diethyl S-[2 (ethylthio)-ethyl]
phosphorodithioate) on fertilizers by infrared, gas-liquid
chromatography, and thin-layer chromatography. J. Agr. Food Chem.,
17: 300-305
Kawamori, I., Saito, T. and Iyatomi, K. (1971) Fate of
organophosphorus insecticides in soils. 1. The retention of
32P-labeled disulfoton and dimethoate in the three soils. Botyu-
Kagaku, 36: 7-12. II. The changes of the retention and the
metabolism of 32P-labeled disulfoton and dimethoate in the soils.
Botyu-Kagaku, 36: 12-17
Kimmerle, G. (1961) Di-Syston. Unpublished report submitted by Bayer
A.G.
Kimmerle, G. (1962) S 309 and S 276. Unpublished report submitted by
Bayer A.G.
Kimmerle, G. (1966) Di-Syston (S 276)/Anticlotwirkung und
Potenzierung. Unpublished report submitted by Bayer A.G.
Kimmerle, G. (1972) Acute toxicity of SRA 3886 in combination with S
276 and with E 154 to rats. Report No. 3438. Unpublished report
submitted by Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kleinschmidt, M.G. (1971) Rate of Di-Syston (O,O-Diethyl S-[2
(ethylthio)ethyl] phosphorodiothate) in potatoes during processing. J.
Agr. Food Chem., 19: 1196-1197
Klimmer, O.R. and Pfaff, W. (1955) Untersuchungen uber die Toxicitat
des neuen Kontaktinsektizides
O,O-Dimethyl-thiophosphorsaure-O-(O-S.athyl)-athylester
("Metasystox"), Arzneimittelforschung, 5 584-587
Klotzsche, C. (1972) Disulfoton, 90 day feeding study in rats.
Unpublished report from Sandox Agroforschung
Ladd, R., Jonkins, D.H, Keplinger, M.L. and Fancher, O.E. (1971)
Teratogenic study with DI-Syston technical in albino rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.
Loeffler, W.W. (1963) Colorimetric determination of DI-SYSTON residues
in green peanuts. Chemagro-Report No. 11 746
Loeffler, W.W. (1969) A summary of DASANIT and DI-SYSTON soil
persistence data. Chemagro-Report No. 25 122
Loeffler, W.W. (1970a) A confirmatory gas chromatographic procedure
for DI-SYSTON residue analysis for tobacco. Chemagro-Report No. 27 714
Loeffler, W.W. (1970b) Identification of DI-SYSTON metabolites in
field grown tobacco. Chemagro-Report No. 27 746
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
Phosphoric-acid-ester poisoning. Sonderdruck aus Naunyn-Schmiedebergs
Arch. Pharmak. exp. Path. 263: 237
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1957) Metabolism of
p32-dithio-systox in the white mouse and american cockroach.
Unpublished report, University of California, Riverside, Citrus
Experiment Station
March, R.B., Metcalf, R.L., Fukuto, T.R. and Maxon, M.G. (1955)
Metabolism of Systox in the white mouse and american cockroach. J.
Econ. Entomol., 48: 355-363
McCaulley, D.F. (1965) An approach to the separation, identification
and determination of at least ten organophosphate pesticide residues
in raw agricultural products. J.A.O.A,.C., 48: 659-665
Menzer, R.E. and Ditman, L.P. (1968) Residues in spinach grown in
disulfoton-and pnorate-treated soil. J. Econ. Entomol., 61: 225-229
Menzer, R.E., Fontanilla, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Environmental Contam. Toxicol., 5: 1-5
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
dithio-Systax and Thimet. J. Econ. Entomol., 50: 338-345
Metcalf, R.L., Reynolds, H.T., Winton, M. and Fukuto, T.R. (1959)
Effects of temperature and plant species upon the rates of metabolism
of systemically applied Di-Syston. J. Econ. Entomol., 52: 435-439
Modak, A., Barley, L., Weintraub, S. and Stavinoha, W.B. (1971) The
effects of chronic disulfoton treatment on tile cholinesterase
activity of the rat. Toxicol and Appl. Pharm., 19: 367
Mülhmann, R. and Tietz, H. (1956) Das chemische verhalten von
Methylisosystox in der lebenden pflanze und das sich daraus ergebende
rückstandsproblem. Höfehen Briefs, 9: 116-140
Olson, T. (1964) A summary of DI-SYSTON soil persistence data.
Chemagro-Report No. 13 172
Olson, T.J. (1969) A confirmatory gas chromatographic procedure for
DI-SYSTON-SYSTOX residue analysis. Chemagro Report No. 26 335
Olson, T.J. (1970) An interference study for DI-SYSTON residue
determinations on alfalfa, clover and potatoes Chemagro-Report No. 27
312
Rider, J.A., Swadery J.I. and Puletti. E.J, (1972) Anticholinesterase
toxicity studies with Guthion, Phoadrin, Di-Syston and Trithion in
human subjects. Fed. Proc., Fed. Amer. Soc. Exp. Biol., 31(2): 520
Ridgway, R.L., Lindquist, D.A. and Bull, D.L. (1965) Effect of method
of application on uptake of Di-Syston by the cotton plant. J. Econ.
Entomol., 58: 349-352
Rivett, K.F., Bhatt. A., Street, A.E. and Newman, A.J. (1972)
Thio-Demeton/cral toxicity to mice/dietary administration for three
months. Unpublished report from Huntingdon Research Centre, England
Sachsse, K.R. and Voss, G. (1971) Toxicology of phospharidon, Residue
Reviews, 37: 61-88
Schrader, G. (1963) Die Entwicklung neuer insektizider
phosphorsaureester. Chemie-Verlag
Schumann, S. and Olson, T. (1964) Colorimetric determination of
DI-SYSTON residues in soil. Chemagro-Report No. 13 059
Stavinoha, W.B., Ryan, L.C. and Smith, P.W. (1969) Biochemical effects
of an organophosphorus cholinesterase inhibitor on the rat brain.
Annals of the New York Academy of Sciences, 160: 378-382
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticide residues in nonfatty foods. J.A.O.A.C.,
54: 513-516
Su, M. Qu., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. and Appl. Pharm.
20: 241-249
Takase, I., Tsuda, H. and Yoshimoto, Y. (1971) The fate of disulfoton
in soil. Japanese J. Appl. Entomol. Zool., 15, 2: 63-69. Revised
version in: Pflanzenschutz-Nachr. Bayer, 25: 43-63 (1972)
Taylor, R.E. (1966) Di-Syston/three generation breeding study on
rats. Unpublished report from Harris Laboratories
Thornton, J.S. (1967a) Determination of DI-SYSTON residues in corn,
soybeans, sugarcane and sugarcane products by thermionic emission gas
chromatography. Chemagro Report No. 20 109
Thornton, J.S. (1967b) Check on loss of DI-SYSTON oxygen analog
metabolites during alkali refining (simulated). Chemagro-Report No, 20
473
Thornton, J.S. (1967c) Determination of DI-SYSTON residues in various
crops and products. Chemagro-Report No. 21 319
Thornton, J.S. (1969) A study of the possible interference of other
pesticides with the analytical method for DI-SYSTON SYSTOX on crops.
Chemagro-Report.No. 21 606
Thornton, J.S. and Anderson, C.A. (1968) Determination of residues of
Di-Syston and metabolites by thermionic emission flame gas
chromatography. J. Agr. Food Chem., 16: 895-898
Trojanowski, H. and Zwolinska-Sniatalowa, Z. (1967) (Preliminary
studies on residue of Solvirex in potato tubers) Prace Naukowe Inst.
Ochrony Roslin, 9, 2: 257-261
Vaughn, G., Deininger, E. and Doull, J. (1958) Determination of a safe
dietary level of Di-Syston for dogs. Unpublished report from the
University of Chicago
Wagner, K. (1973) Unpublished. Bayer A.G., Pflanzonschutz AT, Biol.
Forschung, Institut für Rückstandsanalytik
Ward, C.R., Owens, J.C., Huddleston, E.W., Ashdown D. and Bailey, C.F.
(1972) Phytotoxic and residual properties of disulfoton used on wheat.
J. Econ. Entomol., 65: 561-563
Watts, R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969) Charcoal
column cleanup method for many organophosphorus pesticide residues in
crop extracts. J.A.O.A.C., 52: 522-526
Weil, C.S., Condra, N.J. and Carpenter, C.P. (1971) Correlation of
four hour vs. 24 hour contact skin penetration toxicity in the rat and
rabbit and use of the former for prediction of relative hazard of
pesticide formulations. Toxicol. and Appl. Pharm. 18: 734-742
Wirth, W. (1958) Zur Wirkung System-Insektizider Phosphorsaure-Ester
im Warmbluter-Stoffwechsel. Naunyn-Schmiedeberg's Arch. Exp. Path. u.
Pharm., 234: 352-363
Zwolinska-Sniatalowa, Z. (1967) (The residues of Disyston in potato
tubers). Biul. Inst. Ochrony Roslin, 36: 139-148
Zwolinska-Sniatalowa, Z. (1968) (Studies upon the residues of Disyston
in potato tubers and some vegetables). Biul. Inst. Ochrony Roslin,
41: 121-128
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1969)
Investigations upon the disulfoton residues when applied as Solvirex
preparation on cabbage variety "Slawa", Biul. Inst. Ochrony Roslin,
45: 293-298
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1971) (The residues
of some organo-phosphorus insecticides, applied into the soil, in
vegetables). Biul. Inst. Ochrony Roslin, 48: 439-448
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968a) (The
investigation on Disyston residue in potato tubers). Prace Naukowe
Inst. Ochrony Roslin, 10, 1: 113-124
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968b) (The breakdown
dynamics of disulfoton in potatoes). Biul. Inst. Ochrony Roslin, 40:
203-210
Compound (IV) is demeton-S, one of the active ingredients of the
well-known systemic insecticide SystoxR. Thus the metabolism of
disulfoton dovetails into the metabolism of demeton-S. However, it
should be noted that owing to the rapid formation of the sulfoxides,
the occurrence of (IV) as a metabolite is hardly to be expected.
Following the formation of these metabolites, further degradation can
only be by hydrolysis. The plant metabolism of disulfoton was studied
by the use of 32-p labelled compound (Metcalf et al., 1957, 1959) in
cotton, lemon, bean and alfalfa plants. Disulfoton was rapidly
oxidized to produce the sulfoxide (II) and slowly to produce sulfone
(III). Both those compounds were also oxidized at the thiono-sulfur to
produce V and VI. These same compounds were identified by Bull (1965)
working with other plants, e.g., avocado, brussels sprouts, cabbage,
corn, tomato. The same results were obtained but the proportions of
various compound differed. These studies we re confirmed by later
investigations.
Loeffler (1970b) found II and VI as major metabolites in tobacco.
Gentry et al., (1970) found that in tobacco the order was: Ill, II,
VI, V (see also Bowman et al., 1969).
In soil and water
Generally, the half-life of disulfoton residues in different
soils is between 30 and 100 days (Olson, 1964; Loeffler, 1969). Soil
type and microbial activity seem to have a greater influence on the
rate of decomposition than the temperature (Henzer et al., 1970).
Both sulfones were detected as metabolites in the soil (Henzer et
al., 1970), but the sulfoxides occurred in only minute amounts. Takase
et al., (1971, 1972) however, found chiefly disyston sulfoxide and
sulfone as metabolites in different types of soil.
Disulfoton does not display a strong tendency to leach into the
soil since approximately 1670, 1970 and 4400 m of theoretical rainfall
were required to leach the compound 30 cm into sandy loam, silt loam
and high organic silt loam soils, respectively (Flint et al., 1970).
No effect on soil micro-organisms was observed (Houseworth and
Tweedy, 1972), though some reduction of fungal population was observed
with the high level of 250 ppm in the soil.
The half-life of disulfoton in water under simulated field
conditions was 2.9 days (Flint et al., 1970).
Fate of residues in storage, processing and cooking
In frozen storage, residues remain unchanged for long periods,
sometimes for more than two years (Chemagro Rep. 8857).
The thermal destruction of disulfoton during processing of
apricots (100°C/2 min) and spinach (120°C/55 min) was investigated by
Thornburg and reported (Anderson 1959a, b). Loss of residues was 37%
and 80% respectively.
The fate of disulfoton in potatoes during processing was
investigated by Kleinschmidt (1971). Total residues (1.33 ppm) were
reduced by 35% with lye peeling. Lye peeling plus a single water
blanching reduced the total residue by 38, 74 and 61% for french
fries, dehydrated cubes and dehydrated mashed potatoes, respectively.
On a dry weight basis, overall reduction in residues due to processing
potatoes into french fries, dehydrated cubes, dehydrated mashed, and
chips were 77, 81, 89 and 97% respectively. Lye peeling and cooking
decreased residues of disulfoton by 30% (Zwolinska and Trojanowski,
1968a).
Total diet studies and residues in food moving in commerce
Abbott et al., 1970, found residues of disulfoton only on one
sample of green vegetables. In 1968, 0.1 ppm of disulfoton was found
in only one sample of citrus fruit in New Zealand (N.Z. Min. of Agr.
information, 1973).
Methods of residue analysis
Prior to the advent of GLC methods employing phosphorus sensitive
detectors, residues of disulfoton and its metabolites were determined
by total phosphorus procedures. GLC methods for the determination of
disulfoton residues are now available (Thornton and Anderson, 1968;
Thornton, 1967a, 1967c, 1969; Bowman et al., 1969; Bowman and Beroza,
1969). The principle of most GLC procedures is oxidation of the
residues to disulfoton-sulfone (Ill) and/or demeton-S-sulfone (VI). If
permanganate is used for oxidation, there is usually no transformation
of P = S to P = O so that it is possible to distinguish between P =
S-sulfones and P = O-sulfones. This permits conclusions to be drawn as
to whether the residues present result from application of demeton-S
or disulfoton. It is possible to distinguish clearly between these and
related sulfone pairs by using a 1.1 m column packed with 10% DC-200 +
1% QF -1 on 80/100 mesh Gaschrom Q; at 195°C. The following retention
times are reported (Wagner, 1973):
demeton-S-methyl sulfone 3.75 min
thiometon sulfone 4.75 min
demeton-S-sulfone 5.0 min
disulfoton-sulfone 6.15 min
Confirmatory GLC procedures, using different columns, are also
available (Olson, 1969; Loeffler, 1970). An interference study for
disulfoton residue determinations on alfalfa, clover and potatoes was
carried out by Olson (1970). A great number of organophosphorus
compounds were mixed with the various sulfone compounds.
Chromatographic conditions were modified and/or a confirmatory column
was employed and it was possible to eliminate all interference.
Numerous multi-residue methods capable of measuring disulfoton and its
metabolites are reported (Abbott et al., 1970; Storherr et al., 1971;
Watts, 1969; McCaulley, 1965). The available GLC procedures appear to
be satisfactory, specific and suitable for regulatory purposes.
Appraisal
Disulfoton is an organophosphorus insecticide, possesses systemic
activity and is used to control aphids, leafhoppers, thrips, beet
flies, coffee leaf miner and spider mites. It is formulated
predominantly as granules and for some special uses as a liquid
concentrate. A seed dressing powder is exclusively used in cotton.
Disulfoton is used on a great variety of crops, including vegetables,
potatoes, sugar beets, cotton and cereals. Products based on
disulfoton are registered in a total of 33 countries. The percentage
breakdown of the amounts used in the different crop areas is roughly
80% in vegetables (including potatoes) and 207 in field crops.
Disulfoton is chiefly applied at sowing or as a side dressing.
Recommended application rates are from 1 to 4 kg/ha, pre-harvest
intervals ranging mostly from 30 to 100 days. The minimum purity of
the technical product is 94%. The impurities have been identified and
quantified.
NATIONAL TOLERANCES
Pre-harvest intervals and tolerances
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Australia Potatoes 70 0.5
Vegetables 40 0.5
Cereals 70 0.5
Deciduous fruit 70 0.5
Belgium Deciduous fruit 0.01
Potatoes 0.01
Vegetables 0.01
Bulgaria 60
Canada Beans 0.5
Broccoli 0.5
Brussels sprouts 0.5
Cauliflower 0.5
Lettuce 0.5
Peas 0.5
Potatoes 0.2
Spinach 0.5
Tomatoes 0.5
Germany Beets Do not feed tops before
Hops harvest. Application by
sprinkling method must be
made only up to 1 June at
the latest.
Potatoes Only for seed 0.2
production
Netherlands Vegetables, 0.01
deciduous fruit
Potatoes Only at planting 0.01
New Zealand Barley 56
Beans 56
Broccoli 42
Brussels sprouts 42
Cabbage 42
Carrots 56
Cauliflower 42
Oats 50
Peas 56
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Potatoes 91
Turnips 56
Wheat 56
Poland Hops Apply only at the time
of earthing up.
Potatoes Only for seed production
Beets Apply up to the six-leaf
stage.
For fodder 60
USSR Cereals, cotton 0.35
seed oil
Fodder No residues
South Africa Cabbage 42 0.5
Onions (for aerial 90 0.5
plant parts)
Potatoes 90 0.5
Switzerland Field crops 42
United
Kingdom 42
United States Alfalfa
of America (fresh forage) 5.0
(hay) 12.0
Barley (grain) 60 0.75
(forage or straw) 5.0
Beans (green, Lima, Application at 0.75
snap) time of planting
(on vines) 5.0
Beans (dry) 60 0.75
(on vines) 5.0
Broccoli 14 0.75
Brussels sprouts 30 0.75
Cabbage 42 0.75
Cauliflower 40 0.75
Clover (fresh) 7 5.0
(clover hay) 12.0
Coffee 90 0.3
Cotton
(non-irrigated, seed) 28 0.75
Pre-harvest intervals and tolerances (cont'd.)
Pre-harvest
Country Crop interval Tolerance
(days) (ppm)
Cotton (irrigated, 28,90 0.75
seed)
Hops 0.5
Lettuce 60 0.75
Maize (field corn,
sweet corn, 40,100 0.3
popcorn)(grain)
(fodder) 5.0
Oats (grain) 60 0.75
(forage or straw) 5.0
Peanuts Application 0.75
(peanut hay) at time of 5.0
planting
Peas 50 0.75
(vines) 5.0
Pecan 80 0.75
Pineapples 60 0.75
(foliage) 5.0
Potatoes 75 0.75
Rice 100 0.75
(straw) 5.0
Sorghum (grain) 7 0.75
(fodder and 28 5.0
forage)
Soybeans Do not pasture or use treated
crop for feed food or forage
Spinach Application at 0.75
time of planting
Strawberries Do not use fruit from
treated plantg for food
Sugar beets 30 0.5
(tops) 2.0
Sugar cane 28 0.3
Tomatoes 30 0.75
resp. application at time
of planting
Wheat (grain) 45 0.3
(green fodder Do not graze 5.0
and straw) treated fields
Metabolism studies on plants and soil are available, indicating
the formation of sulfoxides and sulfones of disulfoton and the oxygen
analogue (demeton-S). The ratio of these metabolites can vary and
depends on plant variety, soil type and climatic conditions.
A large number of residue data are available from supervised
trials, predominantly from the United States of America, but also from
some European countries and New Zealand.
Evidence on the fate of residues during storage, processing and
cooking indicates that residues are stable under deep freeze
conditions; losses of residues occur during cooking, heating, or
peeling in the case of potatoes.
Information on residues in food moving in commerce or from total
diet studies is scanty. No data are available on the eventual
carry-over of residues from forage crops into animal tissues, milk or
eggs. Methods of analysis for disulfoton residues based on GLC with
phosphorus specific detectors are available and appear to be suitable
for regulatory purposes, the limit of detection being in the order of
0.05-0.1 ppm depending on the crop.
Residues are best determined, following oxidation to
disulfoton-sulfone and/or demeton-S sulfone, as the sum of parent
compound and all of its oxydative metabolites, expressed as parent
disulfoton. By applying-suitable oxidation procedures, a quantitative
differentiation can be made between the two sulfones. Absence of
disulfoton-sulfone would indicate that prevailing residues have arisen
from an application of demeton, in which case residues should be
expressed as parent demeton.
RECOMMENDATIONS
The following tolerances are recommended, the residues being
determined as disulfoton-sulfone and demeton-S-sulfone and expressed
as disulfoton.
Crop Tolerance (ppm)
Vegetables, including beans, broccoli,
brussels sprouts, cabbage, cauliflower, 0.5
lettuce, potatoes, peas, spinach,
tomatoes, rice (in husk), sugar beets
Cereals (except rice) sugar beets, cottonseed 0.2
Coffee beans, peanuts (kernels),
pecans, pineapple, soybeans 0.1a
Forage crops (green) 5.0
a At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required before June 1975
1. Results of the long-term studies now in progress.
2. Kinetic studies on absorption, distribution, metabolism, and
excretion in mammals.
3. Evaluation of liver damage observed in short-term studies.
4. Data on residues in meat, milk, and eggs after feeding animals on
crops or feedstuffs treated with disulfoton, in order to determine
residue limits in foods of animal origin.
Desirable
1. Information on residues in food moving in commerce.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adams, J.M. (1960) A specific method for the detection of residues of
DI-SYSTON and its metabolites in the presence of other cholinesterase
inhibiting pesticides. 1. Application to cottonseed. Chemagro-Report
No. 5928
Anderson, C.A. (1959a) Thermal destruction of DI-SYSTON during
processing of spinach. Chemagro-Report No. 4882d
Anderson, C.A. (1959b) Thermal destruction of DI-SYSTON during the
processing of apricots. Chemagro-Report No. 4882e
Anderson, C.A. (1960) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. III. Application to potatoes, sugar
beets, sugar beet tops, cabbage, broccoli, pineapple and alfalfa.
Chemagro-Report No. 5511
Anderson, C.A. (1961a) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. I. Application to cottonseed.
Chemagro-Report No. 5339
Anderson, C.A. (1961b) Colorimetric determination of DI-SYSTON
residues in plant material. III. Application to Brussels sprouts,
cauliflower, green beans, lettuce, lilies, peas, pineapple and
tomatoes. Chemagro-Report No. 6684
Anderson, C.A. (1962) Colorimetric determination of DI-SYSTON and
SYSTOX residues in plant material. Chemagro-Report No. 8544
Anderson, C.A. (1963) Colorimetric determination of DI-SYSTON residues
in green coffee beans. Chemagro-Report No. 10 919
Arnold, D., Keplinger, M.L. and Fancher, O.E. (1971) Mutagenic Study
with DI-SYSTON in Albino mice. IBT No. E 8920. Unpublished report from
Industrial Bio-TeSt Laboratoricos Inc.
Ben-Dyke, R., Sanderson, D.M. and Noakes, D.N. (1970) "Acute Toxicity
Data for Pesticides (1970)". World Review of Pest Control, 9:
119-127
Bombinski, I.J. and DuBois, K.P. Chicago. (1958) "Toxicity and
Mechanism of Action of Di-Syston". A.M.A Archives of Industrial
Health, Vol. 17: 192-199
Bowman, M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton, and phorate in corn, milk, grass,
and faeces. J.A.O.A.C., 52: 1231-1237
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetomethyl, and their metabolites in
tobacco plants. J.A.O.A.C., 52: 157-162
Brewerton, H.V. and Close, R.C. (1967) Disulfoton residues in potato
tubers. N.Z. J1. agric. Res., 10: 272-277
Brodeur, J. and DuBois, K.P., Chicago. (1964) "Studies on the
Mechanism of Acquired Tolerance by Rats to 0,0-Diethyl S-2-(ethylthio)
ethyl phosphorodithioate (Di-Syston)", Arch. Int. Pharmacodyn., 149:
560-570
Budreau, C.H. (1972) Teratogenicity and chromotoxicity of three
organophosphorus insecticides in CF1 mice. Diss. Abs. Int., 33:
1174B
Budreau, C.H. and Singh, P.P. (1973) Teratogenicity and Embryo
toxicity of Demeton and Penthion in CF1 mouse embryos. Toxicol. Appl.
Pharmacol., 24: 324-323
Bull, D.L. (1965) Metabolism of Di-Syston by Insects, Isolated Cotton
Leaves, and Rats". J. Econ. Entomol., 58: 249-254
Chemagro Corporation, Kansas City, United States of America (1962)
Report No. 8857
Chisholm, D. and Specht, H.B. (1967) Effect of application rates of
disulfoton and phorate, and of irrigation on aphid control and
residues in canning peas. Can. J. Plant Sci., 47: 175-180
Chisholm, D., Specht, H.B. and Leefe, J.S. (1965) Di-Syston residues
and control of pea aphid, Acyrthosiphon pisum, with in-furrow
treatments of canning peas in Nova Scotia. J. Econ. Entomol., 58:
763-765
Cook W. C., Butler, L., Walker, K.C. and Featherston, P.E (1963)
Granular in-furrow treatments with phorate and Di-Syston against the
pea aphid on peas. J. Econ. Entomol., 56: 95-98
Doull, J. (1957) The acute inhalation toxicity of Di-Syston to rats
and mice. Unpublished report from the University of Chicago
Doull, J. and Vaughn, G. (1958) The effects of diets containing
Di-Syston on rats. Unpublished report from the University of Chicago
Dubois, K.P. (1957a) The acute toxicity of Di-Syston in combination
with other organic phosphates to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1957b) The acute oral toxicity of Di-Syston given
simultaneously with Phosdrin to rats. Unpublished report from the
University of Chicago
DuBois, K.P. (1960) The acute toxicity of Di-Syston in combination
with delnav ethion and serum to rats. Unpublished report from the
University of Chicago
DuBois, K.P. and Kinoshita, F.K. (1971) Effect of repeated inhalation
exposure of female rats to Di-Syston. Unpublished report from the
University of Chicago
Fletcher, D., Jenkins, D.H. and Keplinger, M.L. (1971) Neurotoxicity
study with Di-Syston technical in chickens. IBT No. J 471. Unpublished
report from Industrial Bio-Test Laboratories, Inc.
Flint, D.R., Church, D.D., Shaw, H.R. and Armour II, J. (1970) Soil
runoff, leaching and adsorption, and water stability studies with
DI-SYSTON. Chemagro-Report No. 28 939
Fukuto, T.R. and March, R.L. (1954) Isomerization of
B-ethyl-mercaptoethyl Diethyl Thionophosphate (Systox). J. Amer.
Chem. Soc. 76: 45103-06
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. and Appl.
Pharmacology, 14: 515-534
Gentry, C.R., Kincaid, R.R. and Bowman, M.C. (1970) Soil treatment
with disulfoton against certain insect pests of cigar-wrapper tobacco.
J. Econ Entomol., 63: 1139-1142.
Getz, M.E. (1962) Degradation of esters of Systox, Di-Syston and
Thimet on field-sprayed kale. J.A.O.A.C., 45: 397-401
Graham-Bryce, I.J. (1967) Adsorption of disulfoton by soil. J. Sci.
Food Agric., 18: 72-77
Graham-Bryce, I.J. (1969) Diffusion of organophosphorus insecticides
in soils. J. Sci. Food Agric., 20: 489-494
Gronberg, R.R. and Olson, T.J. (1966) Colorimetric determination of
Di-Syston residues in sugarcane and sugarcane products.
Chemagro-Report No. 17 565
Guérout, R., Barbier, M. and Gicquiaux, Y. (1968) Recherches sur
Vutilisation du disulfoton dans la lutte centre la cochenille
farineuse do Vananas, Dysmicoccus brevipes Ckl. Fruits, 23,2: 67-78
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Food
Chem., 17: 80-82
Hecht, J. and Kimmerle, G. (1965) Toxikologische untersuchungen mit
dem wirkstoff, E 23 323. Unpublished report submitted by Bayer A.G.
Houseworth, L.D. and Tweedy, B.G. Effect of DI-SYSTON on 1972
microbial populations. Chemagro-Report No. 35 127
Ibrahim, F.B., Gilbert, J.M., Evans, R.T. and Cavagnol, J.C. (1969)
Decomposition of Di-Syston (0,0-Diethyl S-[2 (ethylthio)-ethyl]
phosphorodithioate) on fertilizers by infrared, gas-liquid
chromatography, and thin-layer chromatography. J. Agr. Food Chem.,
17: 300-305
Kawamori, I., Saito, T. and Iyatomi, K. (1971) Fate of
organophosphorus insecticides in soils. 1. The retention of
32P-labeled disulfoton and dimethoate in the three soils. Botyu-
Kagaku, 36: 7-12. II. The changes of the retention and the
metabolism of 32P-labeled disulfoton and dimethoate in the soils.
Botyu-Kagaku, 36: 12-17
Kimmerle, G. (1961) Di-Syston. Unpublished report submitted by Bayer
A.G.
Kimmerle, G. (1962) S 309 and S 276. Unpublished report submitted by
Bayer A.G.
Kimmerle, G. (1966) Di-Syston (S 276)/Anticlotwirkung und
Potenzierung. Unpublished report submitted by Bayer A.G.
Kimmerle, G. (1972) Acute toxicity of SRA 3886 in combination with S
276 and with E 154 to rats. Report No. 3438. Unpublished report
submitted by Bayer A.G.
Kimmerle, G. and Lorke, D. (1968) Toxicology of insecticidal
organophosphates. Pflanzenschutz-Nachrichten Bayer, 21: 111-142
Kleinschmidt, M.G. (1971) Rate of Di-Syston (O,O-Diethyl S-[2
(ethylthio)ethyl] phosphorodiothate) in potatoes during processing. J.
Agr. Food Chem., 19: 1196-1197
Klimmer, O.R. and Pfaff, W. (1955) Untersuchungen uber die Toxicitat
des neuen Kontaktinsektizides
O,O-Dimethyl-thiophosphorsaure-O-(O-S.athyl)-athylester
("Metasystox"), Arzneimittelforschung, 5 584-587
Klotzsche, C. (1972) Disulfoton, 90 day feeding study in rats.
Unpublished report from Sandox Agroforschung
Ladd, R., Jonkins, D.H, Keplinger, M.L. and Fancher, O.E. (1971)
Teratogenic study with DI-Syston technical in albino rabbits.
Unpublished report from Industrial Bio-Test Laboratories, Inc.
Loeffler, W.W. (1963) Colorimetric determination of DI-SYSTON residues
in green peanuts. Chemagro-Report No. 11 746
Loeffler, W.W. (1969) A summary of DASANIT and DI-SYSTON soil
persistence data. Chemagro-Report No. 25 122
Loeffler, W.W. (1970a) A confirmatory gas chromatographic procedure
for DI-SYSTON residue analysis for tobacco. Chemagro-Report No. 27 714
Loeffler, W.W. (1970b) Identification of DI-SYSTON metabolites in
field grown tobacco. Chemagro-Report No. 27 746
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
Phosphoric-acid-ester poisoning. Sonderdruck aus Naunyn-Schmiedebergs
Arch. Pharmak. exp. Path. 263: 237
March, R.B., Fukuto, T.R. and Metcalf, R.L. (1957) Metabolism of
p32-dithio-systox in the white mouse and american cockroach.
Unpublished report, University of California, Riverside, Citrus
Experiment Station
March, R.B., Metcalf, R.L., Fukuto, T.R. and Maxon, M.G. (1955)
Metabolism of Systox in the white mouse and american cockroach. J.
Econ. Entomol., 48: 355-363
McCaulley, D.F. (1965) An approach to the separation, identification
and determination of at least ten organophosphate pesticide residues
in raw agricultural products. J.A.O.A,.C., 48: 659-665
Menzer, R.E. and Ditman, L.P. (1968) Residues in spinach grown in
disulfoton-and pnorate-treated soil. J. Econ. Entomol., 61: 225-229
Menzer, R.E., Fontanilla, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Environmental Contam. Toxicol., 5: 1-5
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
dithio-Systax and Thimet. J. Econ. Entomol., 50: 338-345
Metcalf, R.L., Reynolds, H.T., Winton, M. and Fukuto, T.R. (1959)
Effects of temperature and plant species upon the rates of metabolism
of systemically applied Di-Syston. J. Econ. Entomol., 52: 435-439
Modak, A., Barley, L., Weintraub, S. and Stavinoha, W.B. (1971) The
effects of chronic disulfoton treatment on tile cholinesterase
activity of the rat. Toxicol and Appl. Pharm., 19: 367
Mülhmann, R. and Tietz, H. (1956) Das chemische verhalten von
Methylisosystox in der lebenden pflanze und das sich daraus ergebende
rückstandsproblem. Höfehen Briefs, 9: 116-140
Olson, T. (1964) A summary of DI-SYSTON soil persistence data.
Chemagro-Report No. 13 172
Olson, T.J. (1969) A confirmatory gas chromatographic procedure for
DI-SYSTON-SYSTOX residue analysis. Chemagro Report No. 26 335
Olson, T.J. (1970) An interference study for DI-SYSTON residue
determinations on alfalfa, clover and potatoes Chemagro-Report No. 27
312
Rider, J.A., Swadery J.I. and Puletti. E.J, (1972) Anticholinesterase
toxicity studies with Guthion, Phoadrin, Di-Syston and Trithion in
human subjects. Fed. Proc., Fed. Amer. Soc. Exp. Biol., 31(2): 520
Ridgway, R.L., Lindquist, D.A. and Bull, D.L. (1965) Effect of method
of application on uptake of Di-Syston by the cotton plant. J. Econ.
Entomol., 58: 349-352
Rivett, K.F., Bhatt. A., Street, A.E. and Newman, A.J. (1972)
Thio-Demeton/cral toxicity to mice/dietary administration for three
months. Unpublished report from Huntingdon Research Centre, England
Sachsse, K.R. and Voss, G. (1971) Toxicology of phospharidon, Residue
Reviews, 37: 61-88
Schrader, G. (1963) Die Entwicklung neuer insektizider
phosphorsaureester. Chemie-Verlag
Schumann, S. and Olson, T. (1964) Colorimetric determination of
DI-SYSTON residues in soil. Chemagro-Report No. 13 059
Stavinoha, W.B., Ryan, L.C. and Smith, P.W. (1969) Biochemical effects
of an organophosphorus cholinesterase inhibitor on the rat brain.
Annals of the New York Academy of Sciences, 160: 378-382
Storherr, R.W., Ott, P. and Watts, R.R. (1971) A general method for
organophosphorus pesticide residues in nonfatty foods. J.A.O.A.C.,
54: 513-516
Su, M. Qu., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. and Appl. Pharm.
20: 241-249
Takase, I., Tsuda, H. and Yoshimoto, Y. (1971) The fate of disulfoton
in soil. Japanese J. Appl. Entomol. Zool., 15, 2: 63-69. Revised
version in: Pflanzenschutz-Nachr. Bayer, 25: 43-63 (1972)
Taylor, R.E. (1966) Di-Syston/three generation breeding study on
rats. Unpublished report from Harris Laboratories
Thornton, J.S. (1967a) Determination of DI-SYSTON residues in corn,
soybeans, sugarcane and sugarcane products by thermionic emission gas
chromatography. Chemagro Report No. 20 109
Thornton, J.S. (1967b) Check on loss of DI-SYSTON oxygen analog
metabolites during alkali refining (simulated). Chemagro-Report No, 20
473
Thornton, J.S. (1967c) Determination of DI-SYSTON residues in various
crops and products. Chemagro-Report No. 21 319
Thornton, J.S. (1969) A study of the possible interference of other
pesticides with the analytical method for DI-SYSTON SYSTOX on crops.
Chemagro-Report.No. 21 606
Thornton, J.S. and Anderson, C.A. (1968) Determination of residues of
Di-Syston and metabolites by thermionic emission flame gas
chromatography. J. Agr. Food Chem., 16: 895-898
Trojanowski, H. and Zwolinska-Sniatalowa, Z. (1967) (Preliminary
studies on residue of Solvirex in potato tubers) Prace Naukowe Inst.
Ochrony Roslin, 9, 2: 257-261
Vaughn, G., Deininger, E. and Doull, J. (1958) Determination of a safe
dietary level of Di-Syston for dogs. Unpublished report from the
University of Chicago
Wagner, K. (1973) Unpublished. Bayer A.G., Pflanzonschutz AT, Biol.
Forschung, Institut für Rückstandsanalytik
Ward, C.R., Owens, J.C., Huddleston, E.W., Ashdown D. and Bailey, C.F.
(1972) Phytotoxic and residual properties of disulfoton used on wheat.
J. Econ. Entomol., 65: 561-563
Watts, R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969) Charcoal
column cleanup method for many organophosphorus pesticide residues in
crop extracts. J.A.O.A.C., 52: 522-526
Weil, C.S., Condra, N.J. and Carpenter, C.P. (1971) Correlation of
four hour vs. 24 hour contact skin penetration toxicity in the rat and
rabbit and use of the former for prediction of relative hazard of
pesticide formulations. Toxicol. and Appl. Pharm. 18: 734-742
Wirth, W. (1958) Zur Wirkung System-Insektizider Phosphorsaure-Ester
im Warmbluter-Stoffwechsel. Naunyn-Schmiedeberg's Arch. Exp. Path. u.
Pharm., 234: 352-363
Zwolinska-Sniatalowa, Z. (1967) (The residues of Disyston in potato
tubers). Biul. Inst. Ochrony Roslin, 36: 139-148
Zwolinska-Sniatalowa, Z. (1968) (Studies upon the residues of Disyston
in potato tubers and some vegetables). Biul. Inst. Ochrony Roslin,
41: 121-128
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1969)
Investigations upon the disulfoton residues when applied as Solvirex
preparation on cabbage variety "Slawa", Biul. Inst. Ochrony Roslin,
45: 293-298
Zwolinska-Sniatalowa, Z. and Narkiewiez-Jodko, J. (1971) (The residues
of some organo-phosphorus insecticides, applied into the soil, in
vegetables). Biul. Inst. Ochrony Roslin, 48: 439-448
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968a) (The
investigation on Disyston residue in potato tubers). Prace Naukowe
Inst. Ochrony Roslin, 10, 1: 113-124
Zwolinska-Sniatalowa, Z. and Trojanowski, H. (1968b) (The breakdown
dynamics of disulfoton in potatoes). Biul. Inst. Ochrony Roslin, 40:
203-210
