
DISULFOTON
First draft prepared by Dr. S. Caroldi,
University of Padua,
Padua, Italy
EXPLANATION
Disulfoton was previously reviewed by the Joint Meeting in 1973
and 1975 (Annex I, 20 and 24). In 1975 an ADI of 0.002 mg/kg bw was
allocated. Since then, a number of additional studies have been
generated, which were evaluated by the 1991 FAO/WHO Joint Meeting.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Rats
Sprague-Dawley rats (3/sex/treatment) were given single oral
doses of either 0.2 or 1 mg 1-ethylen-14C-disulfoton/kg bw. Another
3 rats/sex received 14 daily oral doses of 0.2 mg unlabelled
disulfoton/kg bw followed by one dose of 0.2 mg labelled disulfoton/kg
bw. The primary excretory pathway in both sexes was via urine.
Approximately 90% of radioactivity was recovered in urine within 24
hours of dosing and excretion was practically completed at 72 hours.
Less than 2% of radioactivity was found in faeces and less than 1% was
exhaled as 14CO2. The present study indicates that disulfoton is
rapidly absorbed by oral route and rapidly excreted in rats. Both the
single dose and multiple dose testing regimens indicate kinetics and
route of disulfoton excretion are similar in males and females (Lee
et al., 1985).
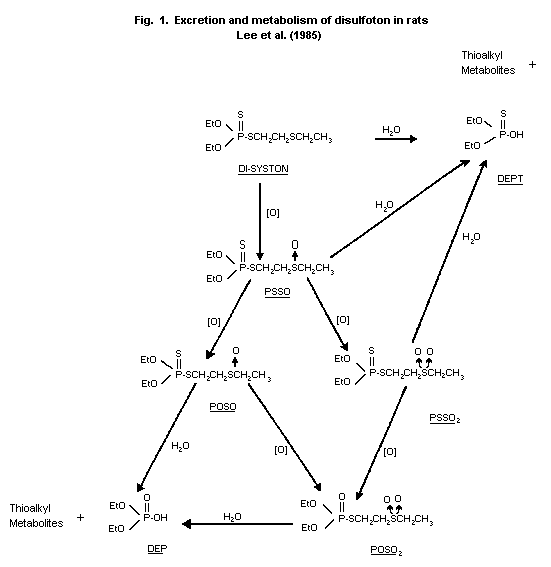
Biotransformation
Rats
Urine samples from the rats subjected to the single-dose (0.2 or
1 mg/kg) and multiple-dose (0.2 mg/kg/day) testing regimens described
above were analyzed for disulfoton metabolites by thin layer
chromatography. The primary metabolite was an unidentified polar
compound that probably was a product of disulfoton hydrolysis.
Oxidative metabolites identified in the urine were disulfoton sulfone
(PSSO2), disulfoton oxygen analogue sulfoxide (POSO), and disulfoton
oxygen analogue sulfone (POSO2). No consistent differences between
sexes were observed after either single or multiple doses (Lee
et al., 1985). The proposed metabolic pathway of disulfoton in rats
is shown in Figure 1.
Chickens
Fourteen adult White Leghorn hens were administered 10 mg/kg/day
1-ethylene-14C-disulfoton p.o. for 3 days. Birds were sacrificed 4
hours after the last disulfoton dose. Three sulfonic acid metabolites
(2-ethyl sulfinyl ethane, 2-ethyl sulfonyl ethane, and 2-ethyl
thioethane) comprised approximately 58% of the disulfoton residue in
eggs, heart, breast and thigh muscle, skin, kidney and liver. Fat and
gizzard contained 87% and 90% unmetabolized disulfoton, respectively.
Minor metabolites found in the tissues included disulfoton oxygen
analogue, disulfoton sulfone, and disulfoton oxygen analogue sulfone
(Krautter et al., 1987)
Goat
A lactating goat was administered 1.1 mg/kg/day
1-ethylene-14C-disulfoton p.o. for 3 days. The animal was killed 2
hours after the last disulfoton dose. Three sulfonic acid metabolites
(2-ethyl sulfinyl ethane, 2-ethyl sulfonyl ethane, and 2-ethyl
thioethane) comprised approximately 66% of the disulfoton residues in
muscle, kidney, liver and fat. Liver and muscle also contained 34%
and 18% unmetabolized disulfoton, respectively (Krautter et al.,
1988)
Short term studies
Rats
Ten male and 10 female Wistar II albino rats (Winkelmann,
Borchen) were exposed in dynamic inhalation chambers to 0, 0.5, 1.8,
or 9.8 mg/m3 of disulfoton (purity 94.4%, dissolved in a 1:1 mixture
of ethanol and Lutrol and aerosolized into chambers) for 5 daily
4-hour exposures. The rats were kept under observation for 14 days
after the last exposure. Animals were weighed weekly and both plasma
and erythrocyte cholinesterase activities were measured before
starting exposure and after the first, third and fifth exposures and
72 hours after the last one. No deaths were observed, except in
female rats at 9.8 mg/m3 disulfoton (9 out of 10 animals died between
1-8 days from the beginning of the study). Symptoms typical for
cholinergic toxicity were observed in all animals of both sexes from
1.8 mg/m3. Body weight and gross pathology were not different among
groups. Dose-related inhibition of plasma cholinesterase activities
(80-90% at the highest dose level in surviving rats) and slight
inhibition of erythrocyte cholinesterase activities (20-30% at the
highest dose level in surviving rats) were measured in both sexes from
1.8 mg/m3 (Thyssen 1978).
 Acute toxicity studies
Table 1. Acute toxicity of Disulfoton
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Mice M oral 7.0 Mihail (1978)a
F 8.2
M&F oral 27 Iyatomi (1980)b
M&F i.p. 14 Iyatomi (1980)b
M s.c. 20 Iyatomi (1980)b
F 28
M&F dermal 35 Iyatomi (1980)b
Rats M oral 6.2 Mihail (1978)a
F 1.9
M oral 9.6 Iyatomi (1980b)b
F 4.2
M inhalation 290 Thyssen (1978)c
F (1 hr exp) 63
M inhalation approx 60 Thyssen (1978)c
F (4 hr exp) approx 15
M i.p. 7.5 Iyatomi (1980)b
F 3.1
M s.c. 7.7 Iyatomi (1980)b
F 4.0
M dermal 15.9 Mihail (1978)a
F (24 hr exp) 3.6
M dermal 22.6 Iyatomi (1980)b
F 7.3
Table 1 (contd).
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Dogs F oral approx .5 Mihail (1978)a
a Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Pulmonary
oedemas were observed in necropsied animals.
b Test substance; disulfoton pure grade 98.6%. Only LD50s are reported. Details about
symptoms and pathology are lacking.
c Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Gross pathology
negative.
Two subacute inhalation studies were conducted on Wistar TNO/W 74
albino rats (Winkelmann, Borchen, Germany) (10/sex/dose level) exposed
in dynamic inhalation chambers to aerosolized concentrations of
technical disulfoton (purity 94.4%). In each study exposure was for
6 hours per day, five days per week for three weeks. Test compound
was dissolved to final concentrations in ethanol and polyethylene
glycol 400 (1:1 mixture). Controls were exposed to solvent mixture at
a concentration of 20,000 µl/m3. In study 1, disulfoton
concentrations were 0, 0.1, 0.5 and 3.7 mg/m3 air. Rats were
observed daily for clinical symptoms and weighed weekly. Twenty-four
hours after the last exposure, 5 rats/sex/dose level were subjected to
haematological tests, clinical chemistry tests and urinalysis. Both
plasma and erythrocyte cholinesterase activities were measured after
0, 5, 10 and 15 exposures and brain cholinesterase activity was
determined at the end of the study. Pathology was performed at the end
of the study.
At the highest dose level, all rats showed clinical symptoms
typical for cholinergic toxicity and 5 female rats died within 12
exposures. At lower dose levels, behavioural disturbance was detected
during the last week of exposure soon after the end of each exposure.
Body weight, haematological tests, clinical chemistry tests and
urinalysis did not show dose-related alterations. Plasma
cholinesterase activities were significantly reduced in females at all
dose levels and in males only at the highest dose level. At 3.7 mg
disulfoton/m3, erythrocyte cholinesterase activity was consistently
reduced throughout the duration of the study in both sexes. At the
end of the study brain cholinesterase activities were 48% and 58% of
control values in males and females, respectively. Mottled distended
lungs and ulcer-like foci were observed in animals which died before
the end of the study, but no toxic effects were detectable in
surviving rats. At the highest dose level, increased relative and
absolute adrenal weights were observed in female rats. Histopathology
showed inflammatory changes in the region of the respiratory tract
and concurrent bone marrow changes from 0.5 mg disulfoton/m3 in both
sexes. In study 2, 10 female and 10 male rats were exposed to a
concentration of 0.02 mg disulfoton/m3 air (controls exposed to
solvent/air only). Also 20 additional female rats were exposed to 3.1
mg disulfoton/m3 air to confirm the high mortality rate observed in
females at the highest dose level in study 1. The protocol of study
2 was like that of study 1. Results at the highest concentration in
study 2 confirmed toxicity detected at a similar dose level in study
1. Three out of 20 rats died before the end of the study. All
clinical, biochemical and pathological parameters were unaffected at
0.02 mg disulfoton/m3 air. A concentration of 0.1 mg/m3 was the
NOAEL for inhalation of a disulfoton aerosol with females being more
sensitive to disulfoton toxicity than males (Thyssen, 1980).
Nine or ten male and 10 female Fischer 344 rats were exposed in
dynamic inhalation chambers to aerosolized technical disulfoton
(purity 97.8%) by the nose-only technique for 6 hours per day, 5 days
per week for 3 weeks. The targeted concentrations of disulfoton were
0.005, 0.05 and 0.5 mg/m3 air which corresponded to analytical
concentrations of 0.006, 0.07 and 0.7 mg disulfoton/m3 air.
Disulfoton was dissolved in a mixture 1:1 of ethanol/polyethylene
glycol and two control groups were added, exposed to either
solvent/air or air only. Rats were observed daily for mortality and
signs of toxicity and weighed weekly. No deaths, signs of toxicity
nor differences in body weight were detected throughout the study. No
reduction of brain cholinesterase activity was measured in any group
at the termination of the study. The NOAEL could be set at 0.7 mg/m3
air based on normal brain cholinesterase activity at the end of the
study (Shiotsuka, 1988).
Twelve male and 12 female Fisher 344 rats were exposed in dynamic
inhalation chambers to aerosolized technical disulfoton (purity 97.8%)
by the nose-only technique for 6 hours per day, 5 days a week for 13
weeks at concentrations of 0.015, 0.15 and 1.5 mg disulfoton/m3 air.
Solutions of disulfoton in vehicle (1:1 ethanol, polyethylene glycol
400) were prepared weekly and actual concentrations in the chambers
(checked daily) were 0.018, 0.16 and 1.4 mg disulfoton/m3 air for the
lowest, mid and highest concentration, respectively. Disulfoton
exposure did not produce clinical symptoms nor increase mortality.
Feed consumption and body weight were not different among groups.
Ophthalmology, clinical chemistry tests, haematological tests and
urinalysis did not reveal toxic alterations. Slight inhibition of
plasma, erythrocyte and brain cholinesterase activities were measured
in both sexes at the highest dose level (at termination of the study,
brain cholinesterase inhibition was 29% and 28% in males and in
females, respectively). Gross pathology and organ weights did not
show toxic effects related to disulfoton exposure. A significantly
increased incidence of inflammation in the nasal turbinate of males
exposed to the highest disulfoton concentration was detected and
judged as a topical irritant effect of the test substance. The NOAEL
in this study was 0.16 mg disulfoton/m3 for both sexes, based on
cholinesterase inhibition and histopathological finding detected at
the next higher dose level (Shiotsuka, 1989).
Rabbits
Groups of adult male and female New Zealand rabbits (5/sex/dose
level) received technical disulfoton (97.8% purity grade, formulated
with Cremophor EL in saline) dermally (skin not abraded, uncovered
after application and test substance washed away at the end of each
exposure period) for 6 hours per day, 5 days per week for 3 weeks.
The tested concentrations were 0, 0.4, 1.6, 6.5 mg disulfoton/kg bw.
Rabbits were observed for signs of toxicity twice a day (skin was
evaluated for irritation before the beginning of the study and 24
hours after the end of each treatment). Body weights and feed
consumption were determined weekly. Cholinergic signs such as muscle
spasms, dyspnoea and salivation were observed in both sexes at the
highest dose level and all animals died within 10 days. Cholinergic
symptoms and deaths appeared earlier in female rabbits. Mortality,
appearance (skin included) and behaviour, feed consumption and body
weight were not affected by treatment up to 1.6 mg disulfoton/kg
bw/day. No dose-related effects were observed in clinical chemistry
tests (except cholinesterase activities), haematological tests,
urinalysis, gross pathology, organ weights nor histopathology up to
1.6 mg disulfoton/kg bw/day. Marginal inhibition of both plasma and
erythrocyte cholinesterase activities were determined at 1.6 mg/kg
bw/day but brain cholinesterase activity was not different from that
of controls. The NOAEL can be set at 1.6 mg disulfoton/kg bw/day
based on normal brain cholinesterase activity measured at this dose
level (Flucke, 1986).
Dogs
Four male and 4 female pure-bred Beagle dogs were treated with
disulfoton (95.7% purity grade) at concentrations of 0, 0.5, 1 and 2
ppm (increased to 5 ppm on week 70 and again to 8 ppm on week 73 up to
the end of the study) equal to 0, 0.155, 0.319 and 1.31 mg/animal/day
for 104 weeks. Disulfoton was mixed with the food (50% premix x 2)
and the food ration (pulverized food + twice as much tap water) was
administered to all dogs in the form of a mash once daily. The
mixture was prepared weekly. The dogs were inspected daily for
toxicity and weighed weekly. Body temperature and pupillary reflex,
patellar reflex, flexor reflex and extensor thrust were tested several
times throughout the study. Disulfoton did not affect food and water
intake nor body weight gain. No clinical symptoms related to
disulfoton administration were detectable at any dose level.
Ophthalmoscopic examinations, reflexes and results of haematological
tests, clinical-chemical tests and urinalysis did not show toxicity
due to disulfoton. A single dog dosed with 0.5 ppm of disulfoton
developed interstitial nephritis of both kidneys and was sacrificed on
week 93. No other dogs died before the scheduled termination of the
study. Plasma, erythrocyte and brain cholinesterase activities were
not reduced in dogs up to 1 ppm disulfoton. Marginal inhibition of
plasma and erythrocyte cholinesterases was observed at 2 ppm which was
further increased by increasing the dose of disulfoton in the diet.
At the end of the study plasma and erythrocyte cholinesterase
activities in dogs dosed with 8 ppm were inhibited 50-60%. In this
group, brain cholinesterase was reduced 34% and 18% in males and
females. Neither macroscopic pathology nor histopathology provided any
evidence of tissue alterations attributable to dietary administration
of disulfoton. The NOAEL is 1 ppm disulfoton equal to 0.319
mg/animal/day (Hoffman & Weischer, 1975)
Long term studies
Mice
Fifty male and 50 female CD1 albino mice were treated with
disulfoton (98.2% purity grade) at concentrations of 0, 1, 4, 16 ppm,
equal to 0, 0.137, 0.548, 2.223 mg/kg bw/day for males and 0, 0.18,
0.73 and 2.3 mg/kg bw/day for females (calculated as average daily
intake throughout the duration of the study) for 99 weeks. The mice
were 4 weeks old at the beginning of the study. The diets were
prepared weekly with corn oil as the vehicle (1% by weight) and
acetone as the solvent and kept in a freezer until presented to the
mice at one dietary level on consecutive days. Stability and
homogeneity of disulfoton were acceptable. The actual content of
disulfoton in the formulation was checked monthly and showed 77%, 89%
and 92% of nominal (mean of the 25 determinations) for 1, 4, 16 ppm,
respectively. Observation for toxicological effects were made twice
daily (1X/day on holidays). Weekly observations for abnormalities and
masses were made and feed consumption and body weights were recorded.
Haematology determinations on 10 mice/sex/dose level were performed at
6 months, 12 months and termination of the study. Plasma, erythrocyte
and brain cholinesterase activities were determined at the end of the
study in controls and in mice treated with the highest dose of
disulfoton. Pathology was performed on all animals found dead or
sacrificed at the end of the study.
In both sexes, food intake and body weight were not influenced by
disulfoton administration. Neither sex showed any changes in the
incidence of clinical signs, or mortality rate at any dose level. At
the end of the treatment the mortality rate was 42%, 40%, 34%, 44%
(male) and 54%, 62%, 46%, 54% (female) at 0, 1, 4, 16 ppm disulfoton,
respectively. Disulfoton had no effects on haematological parameters.
At termination of the study inhibition of plasma, erythrocyte and
brain cholinesterase activities in mice at the 16 ppm dose level were
79%, 56%, 44% (male) and 50%, 82%, 46% (female), respectively.
Trivial differences in organ weights were observed between controls
and dosed mice. Both neoplastic and non-neoplastic histopathologic
observations were those of spontaneous or naturally occurring lesions
of aging albino mice. No differences in the incidence of neoplastic
or non-neoplastic lesions were found when treated mice were compared
to control mice. Disulfoton showed no evidence of an oncogenic effect
when added to the diet up to 16 ppm equal to 2.223 and 2.690 mg/kg
bw/day for males and females, respectively. The NOAEL for disulfoton
in the present study was 4 ppm equal to 0.55 and 0.73 mg/kg bw/day in
males and females, respectively (Hayes, 1983).
Rats
Sixty male and 60 female Sprague-Dawley rats were dosed with
disulfoton (purity 95.7%) at concentrations of 0, 0.5, 1, or 2 ppm for
104 weeks. The lowest dose was increased to 5 ppm from week 80 as at
that time no clear adverse effects were detectable at the next highest
dose. The average daily intakes were: at 0.5/5 ppm 0.03 mg/kg/day and
0.02 mg/kg/day (0.009/0.1 and 0.007/0.7 corresponding to the 0.5 and
5 ppm period), at 1 ppm 0.02 mg/kg/day and 0.01 mg/kg/day and at 2 ppm
0.04 mg/kg/day and 0.03 mg/kg/day in males and females, respectively.
Rats were 4-5 weeks old at the beginning of the study. Powdered
standard rat diet and tap water were available ad libitum. The test
compound was formulated 50% in Ultrasil and mixed into the diet
(prepared every two weeks) up to nominal concentrations. Analyses of
concentrations of the test compound in the food were not reported.
Haematological tests, clinical chemistry tests, and urinalysis were
performed several times throughout the study and at the end of the
study (brain cholinesterase included). At the end of the study 10
rats/sex/dose level were necropsied and organ weights were recorded.
Histopathology was performed on tumour-bearing animals which died or
were killed during the study (except some animals which could not be
investigated as a result of the progressed autolytic state) and at
termination of the study on 5 animals per sex of the control group and
the 5 ppm-group. No significant differences in food and water
consumption nor body weight were observed between controls and treated
animals. No overt signs of toxic effect were observed apart from
transient muscle twitches seen in some animals after increasing the
dose to 5 ppm. At the end of the study mortality rate was 55%, 60%,
60% and 75% in males and 45%, 38%, 42% and 32% in females at 0, 0.5/5,
1 and 2 ppm, respectively. Scattered difference of some haematological
tests, clinical chemistry tests and urinalysis of no biological
relevance were noted. Trivial inhibition of plasma and erythrocyte
cholinesterase (20-30%) activities were not consistent throughout the
study in any group except the 0.5/5 ppm group after increasing the
dose when inhibitions of similar magnitude (20-40%) were observed in
both sexes. No differences of brain cholinesterase activities were
detectable between controls and treated rats. Autopsy and
histopathological findings were unremarkable. Brain cholinesterase
activity was not inhibited at the end of the study suggesting a NOAEL
of 2 ppm in the present study, equal to 0.038 and 0.030 mg
disulfoton/kg bw/day in males and females, respectively. No
carcinogenicity was noted at 2 ppm disulfoton but final judgement can
not be drawn from this study as it was not properly designed to
evaluate this effect (Carpy & Klotzsche, 1975).
Fifty male and 50 female Fisher 344 rats were treated with
technical Disulfoton (97.91% purity, containing 29 identified
impurities) at concentrations of 0, 1, 4 and 16 ppm equal to 0, 0.06,
0.22, 0.92 mg/kg bw/day for males and 0, 0.08, 0.26 and 1.33 mg/kg
bw/day for females (calculated as average daily intake throughout the
duration of the study) for two years. The rats were 4 weeks old at
the beginning of the study. The diets were prepared weekly with corn
oil as the vehicle (1% by weight) and acetone as the solvent and kept
in a freezer until presented to the rats at one dietary level on
consecutive days. The actual content of disulfoton in the formulations
was checked monthly, results 87%, 90% and 90% of nominal (mean of 25
determinations) for 1, 4, and 16 ppm, respectively. Homogeneity and
stability of the diets were acceptable.
At 16 ppm dose level, feed consumption and body weight were
reduced. Increased incidences of clinical signs such as rough coat,
urine stain, loose stool, tail rash and skin lesions were observed in
both sexes. These parameters were not consistently affected in rats
treated with 1 nor 4 ppm disulfoton. At the end of the treatment the
mortality rate was 24%, 24%, 24% and 12% (male) and 12%, 22%, 30% and
40% (female) at 0, 1, 4 and 16 ppm, respectively. The historical
mortality range of female control rats in previous studies was 18-34%
which suggests that a marginal effect on mortality could have occurred
in females at the highest dose level. A trend towards increased total
white cell counts in 16 ppm female rats was present at 6, 12, 18, and
24 months. At termination of the study, decreased serum total
protein, albumin and cholesterol were observed at 16 ppm in both
sexes. Significant differences of other haematological or biochemical
parameters were observed but considered to be of no biological
relevance. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were observed throughout the duration of the
study ranging between a borderline inhibition in rats treated with 1
ppm of disulfoton or about 90% inhibition in rats dosed with 16 ppm
disulfoton. At termination of the study acetylcholinesterase
inhibition levels in brain were 15%, 53% and 79% in males and 21%, 53%
and 82% in females at 1, 4, and 16 ppm, respectively. Gross pathology
did not reveal increases in masses between control rats and those
receiving disulfoton. Several non-neoplastic changes observed at
necroscopy were increased in females fed at 16 ppm. These included an
increase in overall number of external observations, particularly
those of the skin which histologically appeared as inflammation,
ulceration, acanthosis, hyperkeratosis and epithelial inclusion cyst,
and those of the eye (increased vascularization). Reduction of muscle
size of the rear limb was confirmed microscopically as skeletal muscle
atrophy. An increased incidence of lung lesions was mainly
granulomatous or suppurative inflammation. There was an increase in
relative organ weight of heart, liver, kidneys and lung in female rats
and brain in both sexes at 16 ppm. The forestomach papillomas
occurred slightly more often in the high-dose groups than in other
groups and was usually associated with mucosal hyperplasia and
hyperkeratosis. An increased incidence of cystic degeneration of the
Harderian gland was seen in 16 ppm males and in 4 and 16 ppm females
(a similar increase at the 1 ppm level was not confirmed after
re-evaluation of specimens by other pathologists). There were no
statistically significant differences in the incidence of neoplasms
between groups. Disulfoton was not carcingenic for male nor female
rats consuming up to 16 ppm disulfoton. The NOEL for disulfoton in
this study was 1 ppm corresponding to 0.059 mg/kg bw/day for males
and 0.075 mg/kg bw/day for females (Hayes, 1985).
Reproduction studies
Rats
In a 2-litter, 2-generation study, groups of 26 (F0) male and
female rats (Sprague-Dawley strain) approximately 6 weeks old
received Disulfoton (purity 97.8%) admixed in the diet at 0, 1, 3, 9
ppm. Homogeneity and stability of disulfoton in the diet were checked
and found acceptable. Actual concentrations of disulfoton in the
diet were measured monthly and corresponded to 87%, 86% and 88% of
nominal at 1, 3 and 9 ppm (average percentage throughout the study),
respectively. Rats of F0 generation were maintained on their
respective diets for 15 weeks prior to mating. The F1b generation
received the compound in the diet for at least 13 weeks prior to
mating to produce F2 generations. F1b rats were maintained on
treated feed continuously throughout production of F2 generations.
Parent rats were observed daily for clinical symptoms. Weight and
food consump-tion were measured weekly.
Sialodacryoadenitis virus infection developed in F0 rats
(clinical symptoms confirmed by serum analysis and histopathology) but
did not interfere with the study as it was present in all groups and
because mating and reproductive parameters and postnatal indices were
not affected during the time of infection. Body weight and feed
consumption were not affected in any group by treatment during
pre-mating period. In the 9 ppm females, tremors were occasionally
observed during production of F1 generation. Fertility index was
decreased during production of both F1a and F1b litters and reduction
of body weight gain and feed consumption was observed during
lactation. The gestation length and gestation index were not
different for control and treated groups. Litter count, litter weight,
and viability and lactation indices were not affected up to the 3 ppm
group but growth and survival of 9 ppm group offspring were
significantly decreased as compared to those of control in F1a and
F1b generations. Acetylcholinesterase activity in brain of F1a pups
was reduced 0, 24 and 50% in males and 0, 32 and 59% in females at 1,
3 and 9 ppm, respectively. No treatment-related clinical signs of
toxicity were observed in either sex of any dose group during the
premating period of F1b animals. Body weight and feed consumption
were not affected up to 3 ppm but they were both decreased at 9 ppm in
females, as was feed consumption only for males. Fertility index was
reduced at 9 ppm during production of both F2a and F2b litters and
reduction of body weight and feed consumption were occasionally
observed during gestation and lactation. The gestation length and
gestation index were not different for control and treated groups.
Litter count, litter weight, viability, and lactation indices were not
affected up to the 3 ppm group (F2a generation) and up to the 1 ppm
group (F2b generation) but growth and survival of 9 ppm group
offspring were significantly decreased as compared to those of control
in both generations. At 3 ppm F2b litters showed a reduction of
gestation index, viability index (day 4) and mean weight (day 0).
Gross pathology and histopathology did not show compound-related
lesions in examined adults nor pups. The significant reduction in
overall reproductive performance was found at 9 ppm in the presence of
overt parental intoxication. At 3 ppm reduced reproduction was found
in only one set of litters (F2b). This effect was assumed to be
related to cholinesterase inhibition which was observed in similarly
treated F1a litters. The parental NOAEL for toxicity was 3 ppm. The
NOAEL for reproductive effects was 1 ppm (Hixson & Hathaway, 1986).
Special studies on delayed neuropathy
Hens
Twenty adult White Leghorn hens were treated orally with 30 mg/kg
bw of technical disulfoton (97.8% purity) on two separate occasions 22
days apart. In preliminary studies 30 mg/kg bw of disulfoton was
lethal to hens so that in the present study birds were protected with
atropine (0.5 mg/kg i.m.) and 2-PAM (12.5 mg/kg i.m.). Controls were
dosed either with atropine/2-PAM (5 birds) or 500 mg/kg of
tri-o-cresyl phosphate (TOCP, 10 birds) or not treated (5 birds).
Animals were observed daily for toxicity for 42 days. Body weights
and feed consumption were recorded twice a week. Untreated control
and antidote control birds were normal throughout the study.
Fourteen out of 20 birds dosed with disulfoton showed loss of
equilibrium, decreased activity, diarrhoea and locomotor ataxia
typical of cholinergic symptoms, starting soon after the first dosing
which disappeared within 5 days. Eight out of 10 TOCP-dosed birds
showed locomotor ataxia starting between days 12-24 which disappeared
in 5 hens before termination of the study. Birds dosed with
disulfoton did not show histopathological changes suggestive of
delayed neurotoxicity in peripheral nerves nor in spinal cord.
TOCP-dosed hens showed axonal degenerations with macrophage
accumulation in brain and spinal cord. Disulfoton does not induce
acute delayed neuropathy after 2 oral 30 mg/kg bw doses (Hixson,
1983).
Special studies on embryotoxicity and teratogencity
Rats
Twenty-five female CD rats were treated with technical Disulfoton
(98.2% purity) at concentrations of 0, 0.1, 0.3 and 1.0 mg/kg/day
orally by gavage (dissolved in polyethylene glycol 400) on days 6
through 15 of gestation. A positive control group (25 females)
received hydroxyurea at 350 mg/kg in distilled water on days 9, 10 and
11 of gestation only. Recovery of disulfoton from dosing solutions
ranged from 74 to 106% of nominal. Neither clinical signs nor
mortality were observed in treated or control groups. There were no
significant differences in body weight or feed consumption between the
groups. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were measured on day 15 of gestation. The
inhibition was trivial at 0.1 mg/kg, about 40% at 0.3 mg/kg and 80-90%
at 1.0 mg/kg (both activities similarly inhibited). Gross pathology
did not show lesions related to disulfoton administration. There were
no significant differences in the number of implantations per litter,
live, dead and resorbed fetuses per litter or in the average weight of
live fetuses between groups treated with disulfoton and control. There
were no statistically significant differences in the incidence of soft
tissue abnormalities. Increased incidence of incomplete ossification
of the sternebrae in fetuses of dams treated with disulfoton at 1.0
mg/kg was observed. Significant increases were found in several gross,
soft tissue and skeletal abnormalities in fetuses in the positive
control group. Disulfoton is not teratogenic at dosages that result
in significant inhibition of cholinesterase activity. The NOAEL for
embryotoxicity was 0.3 mg/kg; the NOAEL for maternal toxicity 0.1
mg/kg (Lamb & Hixson, 1983).
Rabbits
Fourteen (22 for the highest dose level only) pregnant New
Zealand White rabbits were treated by gavage with disulfoton (purity
97.3%) during gestation days 6-18 at doses of 0, 0.3, 1 or 3 mg/kg
bw/day. The highest dose level was reduced to 2 and 1.5 mg/kg bw/day
in some but not all of the high dose animals due to severe toxic
response and mortality. On day 29 of gestation, animals were
sacrificed for examination of their uterine content. Internal,
external and skeletal exams were performed on the fetuses. Maternal
toxicity observed at the highest dose group included muscular tremor,
increased respiratory rate, unsteadiness/incoordination and mortality
(59% at the end of the study). Body weight gain was reduced in animals
dosed with 3/2/1.5 mg/kg and 1 mg/kg disulfoton but only during
treatment. Body weight gain was similar to that of controls for all
treated groups during post-treatment period. Number of implantations
and viability, the extent of pre- and post-implantation losses and
fetal and placental weights were unaffected by treatment. Three
malformed fetuses were observed in the 0.3 mg/kg group but no
malformations were detectable in offspring of animals treated at
higher doses of disulfoton, therefore fetal survival, development and
growth in utero were considered unaffected by treatment. Disulfoton
at dosages of 1.5 mg/kg/day produced marked toxicity but survival,
growth and development of fetuses were unaffected (Tesh, 1982).
Four New Zealand White rabbits were treated with disulfoton as
above at concentrations of 0, 0.1, 0.3 and 1 mg/kg bw/day in a
preliminary study. No clear signs of toxicity occurred in females up
to the highest dose (transient reduction of body weight gain during
treatment). There was no evidence of any adverse effect of treatment
upon fetal morphogenesis or growth (Tesh, 1981).
Special studies on genotoxicity
Table 2. Results of genotoxicity assays on disulfoton
Test system Test object Concentration of Purity Results Reference
test substance
Reversion assay(1) S. typhimurium 0.1-1000 µg/plate 94.1% Negative (2) Inukai & Iyatomi (1976)
TA98, TA100,
TA1535, TA1537
Reversion assay(1) E. coli WP2 hcr 50-20 000 µg/plate 96.5% Positive (3) Shirasu et al. (1979)
S. typhimurium
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation Saccharomyces 1.5-200 µl/well 97.3% Negative (4) Jaganath (1981)
test(1) cerevisiae
S138, S211
CHO/HGPRT Chinese hamster 0.03-10 µg/ml 97.0% Equivocal (5) Yang (1988)
mutation assay(1) ovary cell
Mitotic non- Saccharomyces 20-200 µl/ml 97.3% Negative (6) Brusick (1981)
disjunction(1) cerevisiae D6
Pol test(1) E. coli p3478, 625-10 000 µg/plate 97.3% Negative (7) Herbold (1983)
w3110
Rec-assay Bacillus subtilis 3-300 µg/disc 94.1% Negative (8) Inukai & Iyatomi (1976)
NIG17 Rec+
NIG45 Rec-
Table 2 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Rec-assay Bacillus subtilis 1-100% v/v 96.5% Negative (9) Shirasu et al., (1979)
H17 Rec+ dissolved in DMSO
M45 Rec-
Sister chromatide Chinese hamster 0.004-0.1 µl/ml 97.9% Positive (10) Putman (1987)
exchange ovary cells (nonact)
0.002-0.2 µl/ml Negative (10)
(act)
Dominant lethal Male NMRI/ORIG 1 x 5 mg/kg bw 94.9% Negative (11) Herbold (1980)
test Kissleg strain mice
Micro nucleus test Male/female Bor: 2 x 6 50% Negative (12) Herbold (1981
NMRI-mice 2 x 12 mg/kg bw (pre-mix)
(1) both with and without metabolic activation
(2) Positive control without activation: N-methyl-N-nitro-
N-nitrosoguanidina 10 µg/plate (TA1535) dexon; 50 µg/plate
(TA1537 and TA98); N-fluoren-2-yl-acetamide 50 µg/plate (TA98);
dimethylnitrosamine 1000 µg/plate (TA1535 and TA100;
furylfuramide 0.02 µg/plate (TA100) gave expected positive
response. Positive control with activation: N-fluoren-
2-yl-acetamide 50 µg/plate (TA98); dimethylnitrosamine 1000
µg/plate (TA1535 and TA100) gave expected positive response.
(3) Positive control without activation: 2-aminoanthracene 10
µg/plate (all strains); furylfuramide, 0.05 µg/plate (TA100),
0.25 µg/plate (WP 2 hcr), 0.1 µg/plate (TA 98); ß-propiolactone,
50 µg/plate (TA1538) gave expected positive response. Positive
control with activation: 2-aminoanthracene, 10 µg/plate (all
strains) gave expected positive response. Test compound gave
positive response with and without metabolic activation on
TA1535 strain.
(4) Positive control without activation: Quinacrine mustard, 50
µl/well (S138); ethyl methanesulfonate, 10 µl/well (S211) gave
expected positive response. Positive control with activation:
Cyclophosphamide 50 µg/well (S211) gave expected positive
response. 2-acetylaminofluorene, 10 µg/well (S138) did not give
positive response.
(5) Positive control without activation: Ethyl methanesulfonate, 0.2
µg/l gave expected positive response. Positive control with
activation: Benzo(a)pyrene, 4 µg/ml gave expected positive
response. Disulfoton was mutagen at concentrations that were
either insoluble or partially soluble in the medium. At
soluble concentrations disulfoton was not mutagenic in this
assay.
(6) Positive control without activation: Ethyl methanesulfonate (20
µl/ml) did not induce chromosome aneuploidy and did induce
mitotic recombination and chromosome deletions.
(7) Positive control with and without activation: methyl
methanesulfonate, 10 µl/plate gave expected positive response.
(8) Positive control: Mitimycyn C 0.3 µg/disc gave the expected
positive response.
(9) Positive control: Mitimycyn C 0.1 µg/disc gave the expected
positive response.
(10) Positive control without activation: Triethylenemelamine, 0.025
µg/ml gave the expected positive response. Positive control with
activation: Cyclophosphamide, 2.5 µg/ml gave the expected
positive response. Test material gave positive response at 0.1
µl/ml in the absence of metabolic activation.
(11) Concurrent positive control not conducted. Test material
administered once to mice by oral gavage.
(12) Positive control (TrenimonR 2 x 0.125 mg/kg bw) gave expected
positive response. Test material administered twice (24 hours
apart) to mice by oral gavage.
Special studies on metabolites
Rats
The sulfone metabolite of disulfoton was administered in a pilot
study to Fischer 344 rats (10/sex/level) by diet at concentrations of
0, 0.5, 0.75 or 1 ppm for six weeks to determine its possible effect
on cholinesterase activity. There were neither biologically
significant depression of brain cholinesterase activity at termination
of the study nor depressions of plasma and erythrocyte cholinesterase
activities (performed weekly) (Stuart, 1986a).
Dogs
Two identical studies were conducted on Beagle dogs to determine
the effect of disulfon metabolites on cholinesterases. The oxygen
analog sulfone metabolite of disulfoton was administered in the diet
to Beagle dogs (2/sex/level in each study) for 6 weeks at
concentrations of 0, 0.5, 0.75, and 1 ppm. Trivial differences were
observed on plasma and erythrocyte cholinesterase activities
(determined weekly throughout the study) and on brain cholinesterase
at termination of the study (Stuart, 1986b, 1986c).
Cows
Angus cattle (3/sex/level) were fed diets of alfalfa pellets
containing a mixture of the disulfoton metabolites sulfoxide, sulfone
and their oxygen analogs at a ratio of 1:2:1:1:, respectively. The
study was conducted for 31 days to determine the effect levels for
cholinesterase inhibition in whole blood. Diet concentrations tested
were 0 (4/sex), 3.6, 7.2, 10.8, and 18 ppm. Dose-related inhibition
of cholinesterase activity was observed at concentrations of 7.2 ppm
and greater. The NOEL for cholinesterase inhibition was 3.6 ppm
(Horton et al., 1975)
Holstein dairy cows (3/level) were fed alfalfa pellets containing
a mixture of disulfoton metabolites sulfoxide, sulfone, oxygen analog
sulfoxide and oxygen analog sulfone at a ratio of 1:2:1:1,
respectively. Animals received dietary concentrations of 3.6, 7.2, or
18 ppm for 28 days. A single untreated cow served as control. Blood
cholinesterase activity was determined before starting the study and
weekly throughout the duration of the study. Feed consumption, body
weight and milk production were also recorded. All measured
parameters were affected at the high- and medium-dose levels. A
trivial reduction of blood cholinesterase activity was noted at the
low-dose (30% decrease compared to 19% decrease in control). The NOEL
for cholinesterase inhibition was 3.6 ppm (Thornton, 1976).
Special studies on skin sensitization
Forty male guinea pigs (Pirbright White W 58) were treated with
technical disulfoton (purity 98.6%) to investigate sensitizing effect
on skin. The Magnusson and Kligman maximization test was used.
Evaluation showed that there were 2 positively reacting animals in the
test compound group as against one in the control group. There were
no indications of a skin sensitizing effect on guinea pigs for
disulfoton (Flucke, 1983).
COMMENTS
Disulfoton was previously evaluated by the JMPR in 1973 and 1975
and an ADI of 0-0.002 mg/kg bw was allocated. Disulfoton is rapidly
absorbed in rats after oral dosing and approximately 90% is excreted
via the urine within 24 hours. The biotransformation pathway consists
of hydrolysis and oxidation to metabolites such as disulfoton sulfone,
disulfoton oxon sulfoxide and disulfoton oxon sulfone.
Disulfoton has high acute oral toxicity to mice, rats and dogs.
It is classified by WHO as "extremely hazardous".
Cholinesterase inhibition and related clinical effects were the
only significant findings in long-term bioassays in mice and rats. In
a 99-week study in mice at dietary concentrations of 0, 1, 4, or 16
ppm, the NOAEL was 4 ppm, equal to 0.55 mg/kg bw/day. At the 16 ppm
concentration brain acetylcholinesterase inhibition was reported.
There was no evidence of carcinogenicity.
In a long-term study in rats at dietary concentrations of 0, 1,
4, or 16 ppm the NOAEL was 1 ppm, equal to 0.06 mg/kg bw/day. At
higher concentrations clinical signs of toxicity and inhibition of
plasma, erythrocyte and brain cholinesterase activities were observed.
No carcinogenic effect was detected.
In a 2-year study in dogs at dietary concentrations of 0, 0.5, 1,
or 2/5/8 ppm, the NOAEL was 1 ppm, equal to 0.03 mg/kg bw/day. At the
next highest dose inhibition of brain acetylcholinesterase was
observed. Treatment-related histo-pathological changes were not
found.
Disulfoton did not cause delayed neuropathy in adult hens.
Disulfoton was not teratogenic in rats nor rabbits. In rats
given 0, 0.1, 0.3 or 1 mg/kg bw/day, the NOAELs for embryotoxicity and
maternal toxicity were 0.1 and 0.3 mg/kg bw/day respectively. In
rabbits given 0, 0.3, 1 or 3/2/1.5 mg/kg bw/day, the NOAELs for
embryotoxicity and maternal toxicity were 1.5 and 0.3 mg/kg bw/day,
respectively.
In a 2-litter 2 generation reproduction study in rats at dietary
concentrations of 0, 1, 3 or 9 ppm, the NOAEL for toxicity was 3 ppm
(equivalent to 0.15 mg/kg bw/day), based on signs of maternal toxicity
at 9 ppm. The NOAEL for reproductive effects was 1 ppm (equivalent to
0.05 mg/kg bw/day) based on decreased brain acetylcholinesterase,
body-weight gain and survival of pups at 3 ppm.
Although there was one positive reverse mutation assay it was
concluded, after review of all available in vivo and in vitro
genotoxicity data, that there was no evidence of genotoxicity.
The human volunteer study reviewed by the 1975 JMPR was reported
in summary form only and was considered inadequate for the estimation
of an ADI.
The ADI was based on the 2-year study in dogs, using a 100-fold
safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 4 ppm in the diet, equal to 0.55 mg/kg bw
Rat: 1 ppm in the diet, equal to 0.06 mg/kg bw
Dog: 1 ppm in the diet, equal to 0.03 mg/kg bw
Man: 0.75 mg/man/day, equivalent to 0.01 mg/kg bw
Estimate of acceptable daily intake for humans
0-0.0003 mg/kg bw.
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans.
REFERENCES
Brusick, D.J. (1981) Mutagenicity evaluation of S276 in the mitotic
non-disjunction in Saccharomices cerevisiae strain D6. Unpublished
Report R 2086 from Litton Bionetics Inc., MD, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Carpy, S. & Klotzsche, C. (1975) Disulfoton 2-year feeding study in
rats. Unpublished Report AGRO DOK CBK 1854/75 from Sandoz Ltd.,
Basel, Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1983) S 276 (Disulfoton, Disyston active ingredient)
Study for skin-sensitising effect with guinea pigs. Unpublished
Report 12121 from Bayer AG, Institute of toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Flucke, W. (1986) S 276 technical - Study of subacute dermal toxicity
to rabbits. Unpublished Report 14747 from Bayer AG, Institute of
toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1983) Oncogenicity study of Disulfoton technical on
mice. Unpublished Report 413 from Mobay Chemical Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985) Chronic feeding/oncogenicity study of technical
Disulfoton (Di-Syston) with rats. Unpublished Report 638 from Mobay
Chemical Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1980) S 276, Disulfoton, Disyston active ingredient.
Dominant lethal test on male mouse to evaluate S 276 for mutagenic
potential. Unpublished Report 9440 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) S 276, Disulfoton, Disyston active ingredient.
Micronucleus test on the mouse to evaluate S 276 for mutagenic effect.
Unpublished Report 10451 from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) S 276, Disulfoton, Disyston active ingredient. Pol
test on E. coli to evaluate for potential DNA damage. Unpublished
Report 12139 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Hixson, E.J. (1983) Acute delayed neurotoxicity study on Disulfoton.
Unpublished Report 365 from Mobay Chemical Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Hixson, E.J. & Hathaway, T.R. (1986) Effect of Disulfoton (Di-syston)
on reproduction in rats. Unpublished Report 711 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Weischer, C.H. (1975) S 276 (disulfoton) Chronic
toxicity study on dogs (two-year feeding experiment). Unpublished
Report 5618 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Horton, J.R., Thornton J.S. & Lichtenstein, H.C. (1975) The subacute
oral toxicity of a Di-syston metabolite mixture administered in the
feed to cattle. Unpublished Report 45288 from Chemagro Corporation,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Inukai, H. & Iyatomi, Y. (1976) Disulfoton. Mutagenicity test on
bacterial systems. Unpublished Report 29 from Nitokuno Agricultural
Chemicals Institute, Toyoda, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Iyatomi (1980) Report of acute toxicity. Disulfoton. Unpublished
Report A-29 from Nitokuno, Agricultural Chemicals Institute, Tokyo,
Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jagannath, D.R., (1981) Mutagenicity evaluation of S 276 in
Saccharomyces cerevisae reverse mutation induction assay.
Unpublished Report R 2087 from Litton Bionetics, Inc. MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Wells N., Lawrence L.J. (1987)
Quantitative characterization of residues in tissues and eggs of
laying hens treated orally for three consecutive days with (14C)
Di-syston-ethylene. Unpublished Report MR 98435 from Pharmacology
and Toxicology Research Laboratory, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Lawrence L.J. (1988)
Metabolism of (14C) Di-syston in the lactating goat. Unpublished
Report MR97499 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Hixson, E.J. (1983) Embryotoxic and teratogenic effects
of disulfoton. Unpublished Report 376 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K., Hanna L.A., Johnston K. & Ose, K. (1985) Excretion and
metabolism of Di-syston in rats. Unpublished Report MR90946 from
Mobay Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1978) Acute toxicity studies. Unpublished Report 7602
from Bayer AG, Institute of toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. Unpublished Report 969 from
Microbiological Associates, Inc., MD, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988) Pilot study to assess cholinesterase activity
in rats exposed by inhalation to technical grade Disulfoton.
Unpublished Report 1073 from Mobay Corporation, USA. Submitted to WHO
by Bayer, Leverkusen, Germany.
Shiotsuka, R.N. (1989) Subchronic inhalation toxicity study of
technical grade Disulfoton (Di-syston) in rats. Unpublished Report
1131 from Mobay Corporation, USA. Submitted to WHO by Bayer,
Leverkusen, Germany.
Shirasu, Y. et al. (1979) Ethylthiometon - Mutagenicity test on
bacterial systems. Unpublished Report from Institute of Environmental
Toxicology, Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986) Pilot study on DI-SYSTON sulfone with rats.
Unpublished Report 85-971-02 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986a) Pilot study on DI-SYSTON oxygen analog sulfone
with dogs. Unpublished Report 85-974-02 from Mobay Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart B.P. (1986b) Pilot study on DI-SYSTON sulfone with dogs.
Unpublished Report 85-974-01 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. (1982) S 276-Effects of oral administration upon pregnancy
in the rabbit. Unpublished Report 2351 from Life Science Research,
England. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. & Ross, F.W. (1981) S 276-Effects of oral administration
upon pregnancy in the rabbit. 1. Preliminary study. Unpublished LSR
Report BAG010 from Life Science Research, England. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thornton, J.S. (1976) Effect of feeding DI-SYSTON metabolites to
dairy cattle. Unpublished Report MR49100 from Chemagro Agriculture
Division, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1978) S 276 (Disyston active ingredient) Acute
inhalational toxicity studies. Unpublished Report 7827 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen J. (1980) Disulfoton (S 276) The active ingredient of
Di-syston Subacute inhalation study on rats. Unpublished Report 9065
from Bayer AG, Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Yang, Li L. (1988) Disyston technical-CHO/HGPRT assay. Unpublished
Report 994 from Microbiological Associates, Inc. MD, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Acute toxicity studies
Table 1. Acute toxicity of Disulfoton
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Mice M oral 7.0 Mihail (1978)a
F 8.2
M&F oral 27 Iyatomi (1980)b
M&F i.p. 14 Iyatomi (1980)b
M s.c. 20 Iyatomi (1980)b
F 28
M&F dermal 35 Iyatomi (1980)b
Rats M oral 6.2 Mihail (1978)a
F 1.9
M oral 9.6 Iyatomi (1980b)b
F 4.2
M inhalation 290 Thyssen (1978)c
F (1 hr exp) 63
M inhalation approx 60 Thyssen (1978)c
F (4 hr exp) approx 15
M i.p. 7.5 Iyatomi (1980)b
F 3.1
M s.c. 7.7 Iyatomi (1980)b
F 4.0
M dermal 15.9 Mihail (1978)a
F (24 hr exp) 3.6
M dermal 22.6 Iyatomi (1980)b
F 7.3
Table 1 (contd).
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Dogs F oral approx .5 Mihail (1978)a
a Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Pulmonary
oedemas were observed in necropsied animals.
b Test substance; disulfoton pure grade 98.6%. Only LD50s are reported. Details about
symptoms and pathology are lacking.
c Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Gross pathology
negative.
Two subacute inhalation studies were conducted on Wistar TNO/W 74
albino rats (Winkelmann, Borchen, Germany) (10/sex/dose level) exposed
in dynamic inhalation chambers to aerosolized concentrations of
technical disulfoton (purity 94.4%). In each study exposure was for
6 hours per day, five days per week for three weeks. Test compound
was dissolved to final concentrations in ethanol and polyethylene
glycol 400 (1:1 mixture). Controls were exposed to solvent mixture at
a concentration of 20,000 µl/m3. In study 1, disulfoton
concentrations were 0, 0.1, 0.5 and 3.7 mg/m3 air. Rats were
observed daily for clinical symptoms and weighed weekly. Twenty-four
hours after the last exposure, 5 rats/sex/dose level were subjected to
haematological tests, clinical chemistry tests and urinalysis. Both
plasma and erythrocyte cholinesterase activities were measured after
0, 5, 10 and 15 exposures and brain cholinesterase activity was
determined at the end of the study. Pathology was performed at the end
of the study.
At the highest dose level, all rats showed clinical symptoms
typical for cholinergic toxicity and 5 female rats died within 12
exposures. At lower dose levels, behavioural disturbance was detected
during the last week of exposure soon after the end of each exposure.
Body weight, haematological tests, clinical chemistry tests and
urinalysis did not show dose-related alterations. Plasma
cholinesterase activities were significantly reduced in females at all
dose levels and in males only at the highest dose level. At 3.7 mg
disulfoton/m3, erythrocyte cholinesterase activity was consistently
reduced throughout the duration of the study in both sexes. At the
end of the study brain cholinesterase activities were 48% and 58% of
control values in males and females, respectively. Mottled distended
lungs and ulcer-like foci were observed in animals which died before
the end of the study, but no toxic effects were detectable in
surviving rats. At the highest dose level, increased relative and
absolute adrenal weights were observed in female rats. Histopathology
showed inflammatory changes in the region of the respiratory tract
and concurrent bone marrow changes from 0.5 mg disulfoton/m3 in both
sexes. In study 2, 10 female and 10 male rats were exposed to a
concentration of 0.02 mg disulfoton/m3 air (controls exposed to
solvent/air only). Also 20 additional female rats were exposed to 3.1
mg disulfoton/m3 air to confirm the high mortality rate observed in
females at the highest dose level in study 1. The protocol of study
2 was like that of study 1. Results at the highest concentration in
study 2 confirmed toxicity detected at a similar dose level in study
1. Three out of 20 rats died before the end of the study. All
clinical, biochemical and pathological parameters were unaffected at
0.02 mg disulfoton/m3 air. A concentration of 0.1 mg/m3 was the
NOAEL for inhalation of a disulfoton aerosol with females being more
sensitive to disulfoton toxicity than males (Thyssen, 1980).
Nine or ten male and 10 female Fischer 344 rats were exposed in
dynamic inhalation chambers to aerosolized technical disulfoton
(purity 97.8%) by the nose-only technique for 6 hours per day, 5 days
per week for 3 weeks. The targeted concentrations of disulfoton were
0.005, 0.05 and 0.5 mg/m3 air which corresponded to analytical
concentrations of 0.006, 0.07 and 0.7 mg disulfoton/m3 air.
Disulfoton was dissolved in a mixture 1:1 of ethanol/polyethylene
glycol and two control groups were added, exposed to either
solvent/air or air only. Rats were observed daily for mortality and
signs of toxicity and weighed weekly. No deaths, signs of toxicity
nor differences in body weight were detected throughout the study. No
reduction of brain cholinesterase activity was measured in any group
at the termination of the study. The NOAEL could be set at 0.7 mg/m3
air based on normal brain cholinesterase activity at the end of the
study (Shiotsuka, 1988).
Twelve male and 12 female Fisher 344 rats were exposed in dynamic
inhalation chambers to aerosolized technical disulfoton (purity 97.8%)
by the nose-only technique for 6 hours per day, 5 days a week for 13
weeks at concentrations of 0.015, 0.15 and 1.5 mg disulfoton/m3 air.
Solutions of disulfoton in vehicle (1:1 ethanol, polyethylene glycol
400) were prepared weekly and actual concentrations in the chambers
(checked daily) were 0.018, 0.16 and 1.4 mg disulfoton/m3 air for the
lowest, mid and highest concentration, respectively. Disulfoton
exposure did not produce clinical symptoms nor increase mortality.
Feed consumption and body weight were not different among groups.
Ophthalmology, clinical chemistry tests, haematological tests and
urinalysis did not reveal toxic alterations. Slight inhibition of
plasma, erythrocyte and brain cholinesterase activities were measured
in both sexes at the highest dose level (at termination of the study,
brain cholinesterase inhibition was 29% and 28% in males and in
females, respectively). Gross pathology and organ weights did not
show toxic effects related to disulfoton exposure. A significantly
increased incidence of inflammation in the nasal turbinate of males
exposed to the highest disulfoton concentration was detected and
judged as a topical irritant effect of the test substance. The NOAEL
in this study was 0.16 mg disulfoton/m3 for both sexes, based on
cholinesterase inhibition and histopathological finding detected at
the next higher dose level (Shiotsuka, 1989).
Rabbits
Groups of adult male and female New Zealand rabbits (5/sex/dose
level) received technical disulfoton (97.8% purity grade, formulated
with Cremophor EL in saline) dermally (skin not abraded, uncovered
after application and test substance washed away at the end of each
exposure period) for 6 hours per day, 5 days per week for 3 weeks.
The tested concentrations were 0, 0.4, 1.6, 6.5 mg disulfoton/kg bw.
Rabbits were observed for signs of toxicity twice a day (skin was
evaluated for irritation before the beginning of the study and 24
hours after the end of each treatment). Body weights and feed
consumption were determined weekly. Cholinergic signs such as muscle
spasms, dyspnoea and salivation were observed in both sexes at the
highest dose level and all animals died within 10 days. Cholinergic
symptoms and deaths appeared earlier in female rabbits. Mortality,
appearance (skin included) and behaviour, feed consumption and body
weight were not affected by treatment up to 1.6 mg disulfoton/kg
bw/day. No dose-related effects were observed in clinical chemistry
tests (except cholinesterase activities), haematological tests,
urinalysis, gross pathology, organ weights nor histopathology up to
1.6 mg disulfoton/kg bw/day. Marginal inhibition of both plasma and
erythrocyte cholinesterase activities were determined at 1.6 mg/kg
bw/day but brain cholinesterase activity was not different from that
of controls. The NOAEL can be set at 1.6 mg disulfoton/kg bw/day
based on normal brain cholinesterase activity measured at this dose
level (Flucke, 1986).
Dogs
Four male and 4 female pure-bred Beagle dogs were treated with
disulfoton (95.7% purity grade) at concentrations of 0, 0.5, 1 and 2
ppm (increased to 5 ppm on week 70 and again to 8 ppm on week 73 up to
the end of the study) equal to 0, 0.155, 0.319 and 1.31 mg/animal/day
for 104 weeks. Disulfoton was mixed with the food (50% premix x 2)
and the food ration (pulverized food + twice as much tap water) was
administered to all dogs in the form of a mash once daily. The
mixture was prepared weekly. The dogs were inspected daily for
toxicity and weighed weekly. Body temperature and pupillary reflex,
patellar reflex, flexor reflex and extensor thrust were tested several
times throughout the study. Disulfoton did not affect food and water
intake nor body weight gain. No clinical symptoms related to
disulfoton administration were detectable at any dose level.
Ophthalmoscopic examinations, reflexes and results of haematological
tests, clinical-chemical tests and urinalysis did not show toxicity
due to disulfoton. A single dog dosed with 0.5 ppm of disulfoton
developed interstitial nephritis of both kidneys and was sacrificed on
week 93. No other dogs died before the scheduled termination of the
study. Plasma, erythrocyte and brain cholinesterase activities were
not reduced in dogs up to 1 ppm disulfoton. Marginal inhibition of
plasma and erythrocyte cholinesterases was observed at 2 ppm which was
further increased by increasing the dose of disulfoton in the diet.
At the end of the study plasma and erythrocyte cholinesterase
activities in dogs dosed with 8 ppm were inhibited 50-60%. In this
group, brain cholinesterase was reduced 34% and 18% in males and
females. Neither macroscopic pathology nor histopathology provided any
evidence of tissue alterations attributable to dietary administration
of disulfoton. The NOAEL is 1 ppm disulfoton equal to 0.319
mg/animal/day (Hoffman & Weischer, 1975)
Long term studies
Mice
Fifty male and 50 female CD1 albino mice were treated with
disulfoton (98.2% purity grade) at concentrations of 0, 1, 4, 16 ppm,
equal to 0, 0.137, 0.548, 2.223 mg/kg bw/day for males and 0, 0.18,
0.73 and 2.3 mg/kg bw/day for females (calculated as average daily
intake throughout the duration of the study) for 99 weeks. The mice
were 4 weeks old at the beginning of the study. The diets were
prepared weekly with corn oil as the vehicle (1% by weight) and
acetone as the solvent and kept in a freezer until presented to the
mice at one dietary level on consecutive days. Stability and
homogeneity of disulfoton were acceptable. The actual content of
disulfoton in the formulation was checked monthly and showed 77%, 89%
and 92% of nominal (mean of the 25 determinations) for 1, 4, 16 ppm,
respectively. Observation for toxicological effects were made twice
daily (1X/day on holidays). Weekly observations for abnormalities and
masses were made and feed consumption and body weights were recorded.
Haematology determinations on 10 mice/sex/dose level were performed at
6 months, 12 months and termination of the study. Plasma, erythrocyte
and brain cholinesterase activities were determined at the end of the
study in controls and in mice treated with the highest dose of
disulfoton. Pathology was performed on all animals found dead or
sacrificed at the end of the study.
In both sexes, food intake and body weight were not influenced by
disulfoton administration. Neither sex showed any changes in the
incidence of clinical signs, or mortality rate at any dose level. At
the end of the treatment the mortality rate was 42%, 40%, 34%, 44%
(male) and 54%, 62%, 46%, 54% (female) at 0, 1, 4, 16 ppm disulfoton,
respectively. Disulfoton had no effects on haematological parameters.
At termination of the study inhibition of plasma, erythrocyte and
brain cholinesterase activities in mice at the 16 ppm dose level were
79%, 56%, 44% (male) and 50%, 82%, 46% (female), respectively.
Trivial differences in organ weights were observed between controls
and dosed mice. Both neoplastic and non-neoplastic histopathologic
observations were those of spontaneous or naturally occurring lesions
of aging albino mice. No differences in the incidence of neoplastic
or non-neoplastic lesions were found when treated mice were compared
to control mice. Disulfoton showed no evidence of an oncogenic effect
when added to the diet up to 16 ppm equal to 2.223 and 2.690 mg/kg
bw/day for males and females, respectively. The NOAEL for disulfoton
in the present study was 4 ppm equal to 0.55 and 0.73 mg/kg bw/day in
males and females, respectively (Hayes, 1983).
Rats
Sixty male and 60 female Sprague-Dawley rats were dosed with
disulfoton (purity 95.7%) at concentrations of 0, 0.5, 1, or 2 ppm for
104 weeks. The lowest dose was increased to 5 ppm from week 80 as at
that time no clear adverse effects were detectable at the next highest
dose. The average daily intakes were: at 0.5/5 ppm 0.03 mg/kg/day and
0.02 mg/kg/day (0.009/0.1 and 0.007/0.7 corresponding to the 0.5 and
5 ppm period), at 1 ppm 0.02 mg/kg/day and 0.01 mg/kg/day and at 2 ppm
0.04 mg/kg/day and 0.03 mg/kg/day in males and females, respectively.
Rats were 4-5 weeks old at the beginning of the study. Powdered
standard rat diet and tap water were available ad libitum. The test
compound was formulated 50% in Ultrasil and mixed into the diet
(prepared every two weeks) up to nominal concentrations. Analyses of
concentrations of the test compound in the food were not reported.
Haematological tests, clinical chemistry tests, and urinalysis were
performed several times throughout the study and at the end of the
study (brain cholinesterase included). At the end of the study 10
rats/sex/dose level were necropsied and organ weights were recorded.
Histopathology was performed on tumour-bearing animals which died or
were killed during the study (except some animals which could not be
investigated as a result of the progressed autolytic state) and at
termination of the study on 5 animals per sex of the control group and
the 5 ppm-group. No significant differences in food and water
consumption nor body weight were observed between controls and treated
animals. No overt signs of toxic effect were observed apart from
transient muscle twitches seen in some animals after increasing the
dose to 5 ppm. At the end of the study mortality rate was 55%, 60%,
60% and 75% in males and 45%, 38%, 42% and 32% in females at 0, 0.5/5,
1 and 2 ppm, respectively. Scattered difference of some haematological
tests, clinical chemistry tests and urinalysis of no biological
relevance were noted. Trivial inhibition of plasma and erythrocyte
cholinesterase (20-30%) activities were not consistent throughout the
study in any group except the 0.5/5 ppm group after increasing the
dose when inhibitions of similar magnitude (20-40%) were observed in
both sexes. No differences of brain cholinesterase activities were
detectable between controls and treated rats. Autopsy and
histopathological findings were unremarkable. Brain cholinesterase
activity was not inhibited at the end of the study suggesting a NOAEL
of 2 ppm in the present study, equal to 0.038 and 0.030 mg
disulfoton/kg bw/day in males and females, respectively. No
carcinogenicity was noted at 2 ppm disulfoton but final judgement can
not be drawn from this study as it was not properly designed to
evaluate this effect (Carpy & Klotzsche, 1975).
Fifty male and 50 female Fisher 344 rats were treated with
technical Disulfoton (97.91% purity, containing 29 identified
impurities) at concentrations of 0, 1, 4 and 16 ppm equal to 0, 0.06,
0.22, 0.92 mg/kg bw/day for males and 0, 0.08, 0.26 and 1.33 mg/kg
bw/day for females (calculated as average daily intake throughout the
duration of the study) for two years. The rats were 4 weeks old at
the beginning of the study. The diets were prepared weekly with corn
oil as the vehicle (1% by weight) and acetone as the solvent and kept
in a freezer until presented to the rats at one dietary level on
consecutive days. The actual content of disulfoton in the formulations
was checked monthly, results 87%, 90% and 90% of nominal (mean of 25
determinations) for 1, 4, and 16 ppm, respectively. Homogeneity and
stability of the diets were acceptable.
At 16 ppm dose level, feed consumption and body weight were
reduced. Increased incidences of clinical signs such as rough coat,
urine stain, loose stool, tail rash and skin lesions were observed in
both sexes. These parameters were not consistently affected in rats
treated with 1 nor 4 ppm disulfoton. At the end of the treatment the
mortality rate was 24%, 24%, 24% and 12% (male) and 12%, 22%, 30% and
40% (female) at 0, 1, 4 and 16 ppm, respectively. The historical
mortality range of female control rats in previous studies was 18-34%
which suggests that a marginal effect on mortality could have occurred
in females at the highest dose level. A trend towards increased total
white cell counts in 16 ppm female rats was present at 6, 12, 18, and
24 months. At termination of the study, decreased serum total
protein, albumin and cholesterol were observed at 16 ppm in both
sexes. Significant differences of other haematological or biochemical
parameters were observed but considered to be of no biological
relevance. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were observed throughout the duration of the
study ranging between a borderline inhibition in rats treated with 1
ppm of disulfoton or about 90% inhibition in rats dosed with 16 ppm
disulfoton. At termination of the study acetylcholinesterase
inhibition levels in brain were 15%, 53% and 79% in males and 21%, 53%
and 82% in females at 1, 4, and 16 ppm, respectively. Gross pathology
did not reveal increases in masses between control rats and those
receiving disulfoton. Several non-neoplastic changes observed at
necroscopy were increased in females fed at 16 ppm. These included an
increase in overall number of external observations, particularly
those of the skin which histologically appeared as inflammation,
ulceration, acanthosis, hyperkeratosis and epithelial inclusion cyst,
and those of the eye (increased vascularization). Reduction of muscle
size of the rear limb was confirmed microscopically as skeletal muscle
atrophy. An increased incidence of lung lesions was mainly
granulomatous or suppurative inflammation. There was an increase in
relative organ weight of heart, liver, kidneys and lung in female rats
and brain in both sexes at 16 ppm. The forestomach papillomas
occurred slightly more often in the high-dose groups than in other
groups and was usually associated with mucosal hyperplasia and
hyperkeratosis. An increased incidence of cystic degeneration of the
Harderian gland was seen in 16 ppm males and in 4 and 16 ppm females
(a similar increase at the 1 ppm level was not confirmed after
re-evaluation of specimens by other pathologists). There were no
statistically significant differences in the incidence of neoplasms
between groups. Disulfoton was not carcingenic for male nor female
rats consuming up to 16 ppm disulfoton. The NOEL for disulfoton in
this study was 1 ppm corresponding to 0.059 mg/kg bw/day for males
and 0.075 mg/kg bw/day for females (Hayes, 1985).
Reproduction studies
Rats
In a 2-litter, 2-generation study, groups of 26 (F0) male and
female rats (Sprague-Dawley strain) approximately 6 weeks old
received Disulfoton (purity 97.8%) admixed in the diet at 0, 1, 3, 9
ppm. Homogeneity and stability of disulfoton in the diet were checked
and found acceptable. Actual concentrations of disulfoton in the
diet were measured monthly and corresponded to 87%, 86% and 88% of
nominal at 1, 3 and 9 ppm (average percentage throughout the study),
respectively. Rats of F0 generation were maintained on their
respective diets for 15 weeks prior to mating. The F1b generation
received the compound in the diet for at least 13 weeks prior to
mating to produce F2 generations. F1b rats were maintained on
treated feed continuously throughout production of F2 generations.
Parent rats were observed daily for clinical symptoms. Weight and
food consump-tion were measured weekly.
Sialodacryoadenitis virus infection developed in F0 rats
(clinical symptoms confirmed by serum analysis and histopathology) but
did not interfere with the study as it was present in all groups and
because mating and reproductive parameters and postnatal indices were
not affected during the time of infection. Body weight and feed
consumption were not affected in any group by treatment during
pre-mating period. In the 9 ppm females, tremors were occasionally
observed during production of F1 generation. Fertility index was
decreased during production of both F1a and F1b litters and reduction
of body weight gain and feed consumption was observed during
lactation. The gestation length and gestation index were not
different for control and treated groups. Litter count, litter weight,
and viability and lactation indices were not affected up to the 3 ppm
group but growth and survival of 9 ppm group offspring were
significantly decreased as compared to those of control in F1a and
F1b generations. Acetylcholinesterase activity in brain of F1a pups
was reduced 0, 24 and 50% in males and 0, 32 and 59% in females at 1,
3 and 9 ppm, respectively. No treatment-related clinical signs of
toxicity were observed in either sex of any dose group during the
premating period of F1b animals. Body weight and feed consumption
were not affected up to 3 ppm but they were both decreased at 9 ppm in
females, as was feed consumption only for males. Fertility index was
reduced at 9 ppm during production of both F2a and F2b litters and
reduction of body weight and feed consumption were occasionally
observed during gestation and lactation. The gestation length and
gestation index were not different for control and treated groups.
Litter count, litter weight, viability, and lactation indices were not
affected up to the 3 ppm group (F2a generation) and up to the 1 ppm
group (F2b generation) but growth and survival of 9 ppm group
offspring were significantly decreased as compared to those of control
in both generations. At 3 ppm F2b litters showed a reduction of
gestation index, viability index (day 4) and mean weight (day 0).
Gross pathology and histopathology did not show compound-related
lesions in examined adults nor pups. The significant reduction in
overall reproductive performance was found at 9 ppm in the presence of
overt parental intoxication. At 3 ppm reduced reproduction was found
in only one set of litters (F2b). This effect was assumed to be
related to cholinesterase inhibition which was observed in similarly
treated F1a litters. The parental NOAEL for toxicity was 3 ppm. The
NOAEL for reproductive effects was 1 ppm (Hixson & Hathaway, 1986).
Special studies on delayed neuropathy
Hens
Twenty adult White Leghorn hens were treated orally with 30 mg/kg
bw of technical disulfoton (97.8% purity) on two separate occasions 22
days apart. In preliminary studies 30 mg/kg bw of disulfoton was
lethal to hens so that in the present study birds were protected with
atropine (0.5 mg/kg i.m.) and 2-PAM (12.5 mg/kg i.m.). Controls were
dosed either with atropine/2-PAM (5 birds) or 500 mg/kg of
tri-o-cresyl phosphate (TOCP, 10 birds) or not treated (5 birds).
Animals were observed daily for toxicity for 42 days. Body weights
and feed consumption were recorded twice a week. Untreated control
and antidote control birds were normal throughout the study.
Fourteen out of 20 birds dosed with disulfoton showed loss of
equilibrium, decreased activity, diarrhoea and locomotor ataxia
typical of cholinergic symptoms, starting soon after the first dosing
which disappeared within 5 days. Eight out of 10 TOCP-dosed birds
showed locomotor ataxia starting between days 12-24 which disappeared
in 5 hens before termination of the study. Birds dosed with
disulfoton did not show histopathological changes suggestive of
delayed neurotoxicity in peripheral nerves nor in spinal cord.
TOCP-dosed hens showed axonal degenerations with macrophage
accumulation in brain and spinal cord. Disulfoton does not induce
acute delayed neuropathy after 2 oral 30 mg/kg bw doses (Hixson,
1983).
Special studies on embryotoxicity and teratogencity
Rats
Twenty-five female CD rats were treated with technical Disulfoton
(98.2% purity) at concentrations of 0, 0.1, 0.3 and 1.0 mg/kg/day
orally by gavage (dissolved in polyethylene glycol 400) on days 6
through 15 of gestation. A positive control group (25 females)
received hydroxyurea at 350 mg/kg in distilled water on days 9, 10 and
11 of gestation only. Recovery of disulfoton from dosing solutions
ranged from 74 to 106% of nominal. Neither clinical signs nor
mortality were observed in treated or control groups. There were no
significant differences in body weight or feed consumption between the
groups. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were measured on day 15 of gestation. The
inhibition was trivial at 0.1 mg/kg, about 40% at 0.3 mg/kg and 80-90%
at 1.0 mg/kg (both activities similarly inhibited). Gross pathology
did not show lesions related to disulfoton administration. There were
no significant differences in the number of implantations per litter,
live, dead and resorbed fetuses per litter or in the average weight of
live fetuses between groups treated with disulfoton and control. There
were no statistically significant differences in the incidence of soft
tissue abnormalities. Increased incidence of incomplete ossification
of the sternebrae in fetuses of dams treated with disulfoton at 1.0
mg/kg was observed. Significant increases were found in several gross,
soft tissue and skeletal abnormalities in fetuses in the positive
control group. Disulfoton is not teratogenic at dosages that result
in significant inhibition of cholinesterase activity. The NOAEL for
embryotoxicity was 0.3 mg/kg; the NOAEL for maternal toxicity 0.1
mg/kg (Lamb & Hixson, 1983).
Rabbits
Fourteen (22 for the highest dose level only) pregnant New
Zealand White rabbits were treated by gavage with disulfoton (purity
97.3%) during gestation days 6-18 at doses of 0, 0.3, 1 or 3 mg/kg
bw/day. The highest dose level was reduced to 2 and 1.5 mg/kg bw/day
in some but not all of the high dose animals due to severe toxic
response and mortality. On day 29 of gestation, animals were
sacrificed for examination of their uterine content. Internal,
external and skeletal exams were performed on the fetuses. Maternal
toxicity observed at the highest dose group included muscular tremor,
increased respiratory rate, unsteadiness/incoordination and mortality
(59% at the end of the study). Body weight gain was reduced in animals
dosed with 3/2/1.5 mg/kg and 1 mg/kg disulfoton but only during
treatment. Body weight gain was similar to that of controls for all
treated groups during post-treatment period. Number of implantations
and viability, the extent of pre- and post-implantation losses and
fetal and placental weights were unaffected by treatment. Three
malformed fetuses were observed in the 0.3 mg/kg group but no
malformations were detectable in offspring of animals treated at
higher doses of disulfoton, therefore fetal survival, development and
growth in utero were considered unaffected by treatment. Disulfoton
at dosages of 1.5 mg/kg/day produced marked toxicity but survival,
growth and development of fetuses were unaffected (Tesh, 1982).
Four New Zealand White rabbits were treated with disulfoton as
above at concentrations of 0, 0.1, 0.3 and 1 mg/kg bw/day in a
preliminary study. No clear signs of toxicity occurred in females up
to the highest dose (transient reduction of body weight gain during
treatment). There was no evidence of any adverse effect of treatment
upon fetal morphogenesis or growth (Tesh, 1981).
Special studies on genotoxicity
Table 2. Results of genotoxicity assays on disulfoton
Test system Test object Concentration of Purity Results Reference
test substance
Reversion assay(1) S. typhimurium 0.1-1000 µg/plate 94.1% Negative (2) Inukai & Iyatomi (1976)
TA98, TA100,
TA1535, TA1537
Reversion assay(1) E. coli WP2 hcr 50-20 000 µg/plate 96.5% Positive (3) Shirasu et al. (1979)
S. typhimurium
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation Saccharomyces 1.5-200 µl/well 97.3% Negative (4) Jaganath (1981)
test(1) cerevisiae
S138, S211
CHO/HGPRT Chinese hamster 0.03-10 µg/ml 97.0% Equivocal (5) Yang (1988)
mutation assay(1) ovary cell
Mitotic non- Saccharomyces 20-200 µl/ml 97.3% Negative (6) Brusick (1981)
disjunction(1) cerevisiae D6
Pol test(1) E. coli p3478, 625-10 000 µg/plate 97.3% Negative (7) Herbold (1983)
w3110
Rec-assay Bacillus subtilis 3-300 µg/disc 94.1% Negative (8) Inukai & Iyatomi (1976)
NIG17 Rec+
NIG45 Rec-
Table 2 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Rec-assay Bacillus subtilis 1-100% v/v 96.5% Negative (9) Shirasu et al., (1979)
H17 Rec+ dissolved in DMSO
M45 Rec-
Sister chromatide Chinese hamster 0.004-0.1 µl/ml 97.9% Positive (10) Putman (1987)
exchange ovary cells (nonact)
0.002-0.2 µl/ml Negative (10)
(act)
Dominant lethal Male NMRI/ORIG 1 x 5 mg/kg bw 94.9% Negative (11) Herbold (1980)
test Kissleg strain mice
Micro nucleus test Male/female Bor: 2 x 6 50% Negative (12) Herbold (1981
NMRI-mice 2 x 12 mg/kg bw (pre-mix)
(1) both with and without metabolic activation
(2) Positive control without activation: N-methyl-N-nitro-
N-nitrosoguanidina 10 µg/plate (TA1535) dexon; 50 µg/plate
(TA1537 and TA98); N-fluoren-2-yl-acetamide 50 µg/plate (TA98);
dimethylnitrosamine 1000 µg/plate (TA1535 and TA100;
furylfuramide 0.02 µg/plate (TA100) gave expected positive
response. Positive control with activation: N-fluoren-
2-yl-acetamide 50 µg/plate (TA98); dimethylnitrosamine 1000
µg/plate (TA1535 and TA100) gave expected positive response.
(3) Positive control without activation: 2-aminoanthracene 10
µg/plate (all strains); furylfuramide, 0.05 µg/plate (TA100),
0.25 µg/plate (WP 2 hcr), 0.1 µg/plate (TA 98); ß-propiolactone,
50 µg/plate (TA1538) gave expected positive response. Positive
control with activation: 2-aminoanthracene, 10 µg/plate (all
strains) gave expected positive response. Test compound gave
positive response with and without metabolic activation on
TA1535 strain.
(4) Positive control without activation: Quinacrine mustard, 50
µl/well (S138); ethyl methanesulfonate, 10 µl/well (S211) gave
expected positive response. Positive control with activation:
Cyclophosphamide 50 µg/well (S211) gave expected positive
response. 2-acetylaminofluorene, 10 µg/well (S138) did not give
positive response.
(5) Positive control without activation: Ethyl methanesulfonate, 0.2
µg/l gave expected positive response. Positive control with
activation: Benzo(a)pyrene, 4 µg/ml gave expected positive
response. Disulfoton was mutagen at concentrations that were
either insoluble or partially soluble in the medium. At
soluble concentrations disulfoton was not mutagenic in this
assay.
(6) Positive control without activation: Ethyl methanesulfonate (20
µl/ml) did not induce chromosome aneuploidy and did induce
mitotic recombination and chromosome deletions.
(7) Positive control with and without activation: methyl
methanesulfonate, 10 µl/plate gave expected positive response.
(8) Positive control: Mitimycyn C 0.3 µg/disc gave the expected
positive response.
(9) Positive control: Mitimycyn C 0.1 µg/disc gave the expected
positive response.
(10) Positive control without activation: Triethylenemelamine, 0.025
µg/ml gave the expected positive response. Positive control with
activation: Cyclophosphamide, 2.5 µg/ml gave the expected
positive response. Test material gave positive response at 0.1
µl/ml in the absence of metabolic activation.
(11) Concurrent positive control not conducted. Test material
administered once to mice by oral gavage.
(12) Positive control (TrenimonR 2 x 0.125 mg/kg bw) gave expected
positive response. Test material administered twice (24 hours
apart) to mice by oral gavage.
Special studies on metabolites
Rats
The sulfone metabolite of disulfoton was administered in a pilot
study to Fischer 344 rats (10/sex/level) by diet at concentrations of
0, 0.5, 0.75 or 1 ppm for six weeks to determine its possible effect
on cholinesterase activity. There were neither biologically
significant depression of brain cholinesterase activity at termination
of the study nor depressions of plasma and erythrocyte cholinesterase
activities (performed weekly) (Stuart, 1986a).
Dogs
Two identical studies were conducted on Beagle dogs to determine
the effect of disulfon metabolites on cholinesterases. The oxygen
analog sulfone metabolite of disulfoton was administered in the diet
to Beagle dogs (2/sex/level in each study) for 6 weeks at
concentrations of 0, 0.5, 0.75, and 1 ppm. Trivial differences were
observed on plasma and erythrocyte cholinesterase activities
(determined weekly throughout the study) and on brain cholinesterase
at termination of the study (Stuart, 1986b, 1986c).
Cows
Angus cattle (3/sex/level) were fed diets of alfalfa pellets
containing a mixture of the disulfoton metabolites sulfoxide, sulfone
and their oxygen analogs at a ratio of 1:2:1:1:, respectively. The
study was conducted for 31 days to determine the effect levels for
cholinesterase inhibition in whole blood. Diet concentrations tested
were 0 (4/sex), 3.6, 7.2, 10.8, and 18 ppm. Dose-related inhibition
of cholinesterase activity was observed at concentrations of 7.2 ppm
and greater. The NOEL for cholinesterase inhibition was 3.6 ppm
(Horton et al., 1975)
Holstein dairy cows (3/level) were fed alfalfa pellets containing
a mixture of disulfoton metabolites sulfoxide, sulfone, oxygen analog
sulfoxide and oxygen analog sulfone at a ratio of 1:2:1:1,
respectively. Animals received dietary concentrations of 3.6, 7.2, or
18 ppm for 28 days. A single untreated cow served as control. Blood
cholinesterase activity was determined before starting the study and
weekly throughout the duration of the study. Feed consumption, body
weight and milk production were also recorded. All measured
parameters were affected at the high- and medium-dose levels. A
trivial reduction of blood cholinesterase activity was noted at the
low-dose (30% decrease compared to 19% decrease in control). The NOEL
for cholinesterase inhibition was 3.6 ppm (Thornton, 1976).
Special studies on skin sensitization
Forty male guinea pigs (Pirbright White W 58) were treated with
technical disulfoton (purity 98.6%) to investigate sensitizing effect
on skin. The Magnusson and Kligman maximization test was used.
Evaluation showed that there were 2 positively reacting animals in the
test compound group as against one in the control group. There were
no indications of a skin sensitizing effect on guinea pigs for
disulfoton (Flucke, 1983).
COMMENTS
Disulfoton was previously evaluated by the JMPR in 1973 and 1975
and an ADI of 0-0.002 mg/kg bw was allocated. Disulfoton is rapidly
absorbed in rats after oral dosing and approximately 90% is excreted
via the urine within 24 hours. The biotransformation pathway consists
of hydrolysis and oxidation to metabolites such as disulfoton sulfone,
disulfoton oxon sulfoxide and disulfoton oxon sulfone.
Disulfoton has high acute oral toxicity to mice, rats and dogs.
It is classified by WHO as "extremely hazardous".
Cholinesterase inhibition and related clinical effects were the
only significant findings in long-term bioassays in mice and rats. In
a 99-week study in mice at dietary concentrations of 0, 1, 4, or 16
ppm, the NOAEL was 4 ppm, equal to 0.55 mg/kg bw/day. At the 16 ppm
concentration brain acetylcholinesterase inhibition was reported.
There was no evidence of carcinogenicity.
In a long-term study in rats at dietary concentrations of 0, 1,
4, or 16 ppm the NOAEL was 1 ppm, equal to 0.06 mg/kg bw/day. At
higher concentrations clinical signs of toxicity and inhibition of
plasma, erythrocyte and brain cholinesterase activities were observed.
No carcinogenic effect was detected.
In a 2-year study in dogs at dietary concentrations of 0, 0.5, 1,
or 2/5/8 ppm, the NOAEL was 1 ppm, equal to 0.03 mg/kg bw/day. At the
next highest dose inhibition of brain acetylcholinesterase was
observed. Treatment-related histo-pathological changes were not
found.
Disulfoton did not cause delayed neuropathy in adult hens.
Disulfoton was not teratogenic in rats nor rabbits. In rats
given 0, 0.1, 0.3 or 1 mg/kg bw/day, the NOAELs for embryotoxicity and
maternal toxicity were 0.1 and 0.3 mg/kg bw/day respectively. In
rabbits given 0, 0.3, 1 or 3/2/1.5 mg/kg bw/day, the NOAELs for
embryotoxicity and maternal toxicity were 1.5 and 0.3 mg/kg bw/day,
respectively.
In a 2-litter 2 generation reproduction study in rats at dietary
concentrations of 0, 1, 3 or 9 ppm, the NOAEL for toxicity was 3 ppm
(equivalent to 0.15 mg/kg bw/day), based on signs of maternal toxicity
at 9 ppm. The NOAEL for reproductive effects was 1 ppm (equivalent to
0.05 mg/kg bw/day) based on decreased brain acetylcholinesterase,
body-weight gain and survival of pups at 3 ppm.
Although there was one positive reverse mutation assay it was
concluded, after review of all available in vivo and in vitro
genotoxicity data, that there was no evidence of genotoxicity.
The human volunteer study reviewed by the 1975 JMPR was reported
in summary form only and was considered inadequate for the estimation
of an ADI.
The ADI was based on the 2-year study in dogs, using a 100-fold
safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 4 ppm in the diet, equal to 0.55 mg/kg bw
Rat: 1 ppm in the diet, equal to 0.06 mg/kg bw
Dog: 1 ppm in the diet, equal to 0.03 mg/kg bw
Man: 0.75 mg/man/day, equivalent to 0.01 mg/kg bw
Estimate of acceptable daily intake for humans
0-0.0003 mg/kg bw.
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans.
REFERENCES
Brusick, D.J. (1981) Mutagenicity evaluation of S276 in the mitotic
non-disjunction in Saccharomices cerevisiae strain D6. Unpublished
Report R 2086 from Litton Bionetics Inc., MD, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Carpy, S. & Klotzsche, C. (1975) Disulfoton 2-year feeding study in
rats. Unpublished Report AGRO DOK CBK 1854/75 from Sandoz Ltd.,
Basel, Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1983) S 276 (Disulfoton, Disyston active ingredient)
Study for skin-sensitising effect with guinea pigs. Unpublished
Report 12121 from Bayer AG, Institute of toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Flucke, W. (1986) S 276 technical - Study of subacute dermal toxicity
to rabbits. Unpublished Report 14747 from Bayer AG, Institute of
toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1983) Oncogenicity study of Disulfoton technical on
mice. Unpublished Report 413 from Mobay Chemical Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985) Chronic feeding/oncogenicity study of technical
Disulfoton (Di-Syston) with rats. Unpublished Report 638 from Mobay
Chemical Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1980) S 276, Disulfoton, Disyston active ingredient.
Dominant lethal test on male mouse to evaluate S 276 for mutagenic
potential. Unpublished Report 9440 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) S 276, Disulfoton, Disyston active ingredient.
Micronucleus test on the mouse to evaluate S 276 for mutagenic effect.
Unpublished Report 10451 from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) S 276, Disulfoton, Disyston active ingredient. Pol
test on E. coli to evaluate for potential DNA damage. Unpublished
Report 12139 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Hixson, E.J. (1983) Acute delayed neurotoxicity study on Disulfoton.
Unpublished Report 365 from Mobay Chemical Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Hixson, E.J. & Hathaway, T.R. (1986) Effect of Disulfoton (Di-syston)
on reproduction in rats. Unpublished Report 711 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Weischer, C.H. (1975) S 276 (disulfoton) Chronic
toxicity study on dogs (two-year feeding experiment). Unpublished
Report 5618 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Horton, J.R., Thornton J.S. & Lichtenstein, H.C. (1975) The subacute
oral toxicity of a Di-syston metabolite mixture administered in the
feed to cattle. Unpublished Report 45288 from Chemagro Corporation,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Inukai, H. & Iyatomi, Y. (1976) Disulfoton. Mutagenicity test on
bacterial systems. Unpublished Report 29 from Nitokuno Agricultural
Chemicals Institute, Toyoda, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Iyatomi (1980) Report of acute toxicity. Disulfoton. Unpublished
Report A-29 from Nitokuno, Agricultural Chemicals Institute, Tokyo,
Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jagannath, D.R., (1981) Mutagenicity evaluation of S 276 in
Saccharomyces cerevisae reverse mutation induction assay.
Unpublished Report R 2087 from Litton Bionetics, Inc. MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Wells N., Lawrence L.J. (1987)
Quantitative characterization of residues in tissues and eggs of
laying hens treated orally for three consecutive days with (14C)
Di-syston-ethylene. Unpublished Report MR 98435 from Pharmacology
and Toxicology Research Laboratory, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Lawrence L.J. (1988)
Metabolism of (14C) Di-syston in the lactating goat. Unpublished
Report MR97499 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Hixson, E.J. (1983) Embryotoxic and teratogenic effects
of disulfoton. Unpublished Report 376 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K., Hanna L.A., Johnston K. & Ose, K. (1985) Excretion and
metabolism of Di-syston in rats. Unpublished Report MR90946 from
Mobay Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1978) Acute toxicity studies. Unpublished Report 7602
from Bayer AG, Institute of toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. Unpublished Report 969 from
Microbiological Associates, Inc., MD, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988) Pilot study to assess cholinesterase activity
in rats exposed by inhalation to technical grade Disulfoton.
Unpublished Report 1073 from Mobay Corporation, USA. Submitted to WHO
by Bayer, Leverkusen, Germany.
Shiotsuka, R.N. (1989) Subchronic inhalation toxicity study of
technical grade Disulfoton (Di-syston) in rats. Unpublished Report
1131 from Mobay Corporation, USA. Submitted to WHO by Bayer,
Leverkusen, Germany.
Shirasu, Y. et al. (1979) Ethylthiometon - Mutagenicity test on
bacterial systems. Unpublished Report from Institute of Environmental
Toxicology, Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986) Pilot study on DI-SYSTON sulfone with rats.
Unpublished Report 85-971-02 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986a) Pilot study on DI-SYSTON oxygen analog sulfone
with dogs. Unpublished Report 85-974-02 from Mobay Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart B.P. (1986b) Pilot study on DI-SYSTON sulfone with dogs.
Unpublished Report 85-974-01 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. (1982) S 276-Effects of oral administration upon pregnancy
in the rabbit. Unpublished Report 2351 from Life Science Research,
England. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. & Ross, F.W. (1981) S 276-Effects of oral administration
upon pregnancy in the rabbit. 1. Preliminary study. Unpublished LSR
Report BAG010 from Life Science Research, England. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thornton, J.S. (1976) Effect of feeding DI-SYSTON metabolites to
dairy cattle. Unpublished Report MR49100 from Chemagro Agriculture
Division, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1978) S 276 (Disyston active ingredient) Acute
inhalational toxicity studies. Unpublished Report 7827 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen J. (1980) Disulfoton (S 276) The active ingredient of
Di-syston Subacute inhalation study on rats. Unpublished Report 9065
from Bayer AG, Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Yang, Li L. (1988) Disyston technical-CHO/HGPRT assay. Unpublished
Report 994 from Microbiological Associates, Inc. MD, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
 Acute toxicity studies
Table 1. Acute toxicity of Disulfoton
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Mice M oral 7.0 Mihail (1978)a
F 8.2
M&F oral 27 Iyatomi (1980)b
M&F i.p. 14 Iyatomi (1980)b
M s.c. 20 Iyatomi (1980)b
F 28
M&F dermal 35 Iyatomi (1980)b
Rats M oral 6.2 Mihail (1978)a
F 1.9
M oral 9.6 Iyatomi (1980b)b
F 4.2
M inhalation 290 Thyssen (1978)c
F (1 hr exp) 63
M inhalation approx 60 Thyssen (1978)c
F (4 hr exp) approx 15
M i.p. 7.5 Iyatomi (1980)b
F 3.1
M s.c. 7.7 Iyatomi (1980)b
F 4.0
M dermal 15.9 Mihail (1978)a
F (24 hr exp) 3.6
M dermal 22.6 Iyatomi (1980)b
F 7.3
Table 1 (contd).
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Dogs F oral approx .5 Mihail (1978)a
a Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Pulmonary
oedemas were observed in necropsied animals.
b Test substance; disulfoton pure grade 98.6%. Only LD50s are reported. Details about
symptoms and pathology are lacking.
c Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Gross pathology
negative.
Two subacute inhalation studies were conducted on Wistar TNO/W 74
albino rats (Winkelmann, Borchen, Germany) (10/sex/dose level) exposed
in dynamic inhalation chambers to aerosolized concentrations of
technical disulfoton (purity 94.4%). In each study exposure was for
6 hours per day, five days per week for three weeks. Test compound
was dissolved to final concentrations in ethanol and polyethylene
glycol 400 (1:1 mixture). Controls were exposed to solvent mixture at
a concentration of 20,000 µl/m3. In study 1, disulfoton
concentrations were 0, 0.1, 0.5 and 3.7 mg/m3 air. Rats were
observed daily for clinical symptoms and weighed weekly. Twenty-four
hours after the last exposure, 5 rats/sex/dose level were subjected to
haematological tests, clinical chemistry tests and urinalysis. Both
plasma and erythrocyte cholinesterase activities were measured after
0, 5, 10 and 15 exposures and brain cholinesterase activity was
determined at the end of the study. Pathology was performed at the end
of the study.
At the highest dose level, all rats showed clinical symptoms
typical for cholinergic toxicity and 5 female rats died within 12
exposures. At lower dose levels, behavioural disturbance was detected
during the last week of exposure soon after the end of each exposure.
Body weight, haematological tests, clinical chemistry tests and
urinalysis did not show dose-related alterations. Plasma
cholinesterase activities were significantly reduced in females at all
dose levels and in males only at the highest dose level. At 3.7 mg
disulfoton/m3, erythrocyte cholinesterase activity was consistently
reduced throughout the duration of the study in both sexes. At the
end of the study brain cholinesterase activities were 48% and 58% of
control values in males and females, respectively. Mottled distended
lungs and ulcer-like foci were observed in animals which died before
the end of the study, but no toxic effects were detectable in
surviving rats. At the highest dose level, increased relative and
absolute adrenal weights were observed in female rats. Histopathology
showed inflammatory changes in the region of the respiratory tract
and concurrent bone marrow changes from 0.5 mg disulfoton/m3 in both
sexes. In study 2, 10 female and 10 male rats were exposed to a
concentration of 0.02 mg disulfoton/m3 air (controls exposed to
solvent/air only). Also 20 additional female rats were exposed to 3.1
mg disulfoton/m3 air to confirm the high mortality rate observed in
females at the highest dose level in study 1. The protocol of study
2 was like that of study 1. Results at the highest concentration in
study 2 confirmed toxicity detected at a similar dose level in study
1. Three out of 20 rats died before the end of the study. All
clinical, biochemical and pathological parameters were unaffected at
0.02 mg disulfoton/m3 air. A concentration of 0.1 mg/m3 was the
NOAEL for inhalation of a disulfoton aerosol with females being more
sensitive to disulfoton toxicity than males (Thyssen, 1980).
Nine or ten male and 10 female Fischer 344 rats were exposed in
dynamic inhalation chambers to aerosolized technical disulfoton
(purity 97.8%) by the nose-only technique for 6 hours per day, 5 days
per week for 3 weeks. The targeted concentrations of disulfoton were
0.005, 0.05 and 0.5 mg/m3 air which corresponded to analytical
concentrations of 0.006, 0.07 and 0.7 mg disulfoton/m3 air.
Disulfoton was dissolved in a mixture 1:1 of ethanol/polyethylene
glycol and two control groups were added, exposed to either
solvent/air or air only. Rats were observed daily for mortality and
signs of toxicity and weighed weekly. No deaths, signs of toxicity
nor differences in body weight were detected throughout the study. No
reduction of brain cholinesterase activity was measured in any group
at the termination of the study. The NOAEL could be set at 0.7 mg/m3
air based on normal brain cholinesterase activity at the end of the
study (Shiotsuka, 1988).
Twelve male and 12 female Fisher 344 rats were exposed in dynamic
inhalation chambers to aerosolized technical disulfoton (purity 97.8%)
by the nose-only technique for 6 hours per day, 5 days a week for 13
weeks at concentrations of 0.015, 0.15 and 1.5 mg disulfoton/m3 air.
Solutions of disulfoton in vehicle (1:1 ethanol, polyethylene glycol
400) were prepared weekly and actual concentrations in the chambers
(checked daily) were 0.018, 0.16 and 1.4 mg disulfoton/m3 air for the
lowest, mid and highest concentration, respectively. Disulfoton
exposure did not produce clinical symptoms nor increase mortality.
Feed consumption and body weight were not different among groups.
Ophthalmology, clinical chemistry tests, haematological tests and
urinalysis did not reveal toxic alterations. Slight inhibition of
plasma, erythrocyte and brain cholinesterase activities were measured
in both sexes at the highest dose level (at termination of the study,
brain cholinesterase inhibition was 29% and 28% in males and in
females, respectively). Gross pathology and organ weights did not
show toxic effects related to disulfoton exposure. A significantly
increased incidence of inflammation in the nasal turbinate of males
exposed to the highest disulfoton concentration was detected and
judged as a topical irritant effect of the test substance. The NOAEL
in this study was 0.16 mg disulfoton/m3 for both sexes, based on
cholinesterase inhibition and histopathological finding detected at
the next higher dose level (Shiotsuka, 1989).
Rabbits
Groups of adult male and female New Zealand rabbits (5/sex/dose
level) received technical disulfoton (97.8% purity grade, formulated
with Cremophor EL in saline) dermally (skin not abraded, uncovered
after application and test substance washed away at the end of each
exposure period) for 6 hours per day, 5 days per week for 3 weeks.
The tested concentrations were 0, 0.4, 1.6, 6.5 mg disulfoton/kg bw.
Rabbits were observed for signs of toxicity twice a day (skin was
evaluated for irritation before the beginning of the study and 24
hours after the end of each treatment). Body weights and feed
consumption were determined weekly. Cholinergic signs such as muscle
spasms, dyspnoea and salivation were observed in both sexes at the
highest dose level and all animals died within 10 days. Cholinergic
symptoms and deaths appeared earlier in female rabbits. Mortality,
appearance (skin included) and behaviour, feed consumption and body
weight were not affected by treatment up to 1.6 mg disulfoton/kg
bw/day. No dose-related effects were observed in clinical chemistry
tests (except cholinesterase activities), haematological tests,
urinalysis, gross pathology, organ weights nor histopathology up to
1.6 mg disulfoton/kg bw/day. Marginal inhibition of both plasma and
erythrocyte cholinesterase activities were determined at 1.6 mg/kg
bw/day but brain cholinesterase activity was not different from that
of controls. The NOAEL can be set at 1.6 mg disulfoton/kg bw/day
based on normal brain cholinesterase activity measured at this dose
level (Flucke, 1986).
Dogs
Four male and 4 female pure-bred Beagle dogs were treated with
disulfoton (95.7% purity grade) at concentrations of 0, 0.5, 1 and 2
ppm (increased to 5 ppm on week 70 and again to 8 ppm on week 73 up to
the end of the study) equal to 0, 0.155, 0.319 and 1.31 mg/animal/day
for 104 weeks. Disulfoton was mixed with the food (50% premix x 2)
and the food ration (pulverized food + twice as much tap water) was
administered to all dogs in the form of a mash once daily. The
mixture was prepared weekly. The dogs were inspected daily for
toxicity and weighed weekly. Body temperature and pupillary reflex,
patellar reflex, flexor reflex and extensor thrust were tested several
times throughout the study. Disulfoton did not affect food and water
intake nor body weight gain. No clinical symptoms related to
disulfoton administration were detectable at any dose level.
Ophthalmoscopic examinations, reflexes and results of haematological
tests, clinical-chemical tests and urinalysis did not show toxicity
due to disulfoton. A single dog dosed with 0.5 ppm of disulfoton
developed interstitial nephritis of both kidneys and was sacrificed on
week 93. No other dogs died before the scheduled termination of the
study. Plasma, erythrocyte and brain cholinesterase activities were
not reduced in dogs up to 1 ppm disulfoton. Marginal inhibition of
plasma and erythrocyte cholinesterases was observed at 2 ppm which was
further increased by increasing the dose of disulfoton in the diet.
At the end of the study plasma and erythrocyte cholinesterase
activities in dogs dosed with 8 ppm were inhibited 50-60%. In this
group, brain cholinesterase was reduced 34% and 18% in males and
females. Neither macroscopic pathology nor histopathology provided any
evidence of tissue alterations attributable to dietary administration
of disulfoton. The NOAEL is 1 ppm disulfoton equal to 0.319
mg/animal/day (Hoffman & Weischer, 1975)
Long term studies
Mice
Fifty male and 50 female CD1 albino mice were treated with
disulfoton (98.2% purity grade) at concentrations of 0, 1, 4, 16 ppm,
equal to 0, 0.137, 0.548, 2.223 mg/kg bw/day for males and 0, 0.18,
0.73 and 2.3 mg/kg bw/day for females (calculated as average daily
intake throughout the duration of the study) for 99 weeks. The mice
were 4 weeks old at the beginning of the study. The diets were
prepared weekly with corn oil as the vehicle (1% by weight) and
acetone as the solvent and kept in a freezer until presented to the
mice at one dietary level on consecutive days. Stability and
homogeneity of disulfoton were acceptable. The actual content of
disulfoton in the formulation was checked monthly and showed 77%, 89%
and 92% of nominal (mean of the 25 determinations) for 1, 4, 16 ppm,
respectively. Observation for toxicological effects were made twice
daily (1X/day on holidays). Weekly observations for abnormalities and
masses were made and feed consumption and body weights were recorded.
Haematology determinations on 10 mice/sex/dose level were performed at
6 months, 12 months and termination of the study. Plasma, erythrocyte
and brain cholinesterase activities were determined at the end of the
study in controls and in mice treated with the highest dose of
disulfoton. Pathology was performed on all animals found dead or
sacrificed at the end of the study.
In both sexes, food intake and body weight were not influenced by
disulfoton administration. Neither sex showed any changes in the
incidence of clinical signs, or mortality rate at any dose level. At
the end of the treatment the mortality rate was 42%, 40%, 34%, 44%
(male) and 54%, 62%, 46%, 54% (female) at 0, 1, 4, 16 ppm disulfoton,
respectively. Disulfoton had no effects on haematological parameters.
At termination of the study inhibition of plasma, erythrocyte and
brain cholinesterase activities in mice at the 16 ppm dose level were
79%, 56%, 44% (male) and 50%, 82%, 46% (female), respectively.
Trivial differences in organ weights were observed between controls
and dosed mice. Both neoplastic and non-neoplastic histopathologic
observations were those of spontaneous or naturally occurring lesions
of aging albino mice. No differences in the incidence of neoplastic
or non-neoplastic lesions were found when treated mice were compared
to control mice. Disulfoton showed no evidence of an oncogenic effect
when added to the diet up to 16 ppm equal to 2.223 and 2.690 mg/kg
bw/day for males and females, respectively. The NOAEL for disulfoton
in the present study was 4 ppm equal to 0.55 and 0.73 mg/kg bw/day in
males and females, respectively (Hayes, 1983).
Rats
Sixty male and 60 female Sprague-Dawley rats were dosed with
disulfoton (purity 95.7%) at concentrations of 0, 0.5, 1, or 2 ppm for
104 weeks. The lowest dose was increased to 5 ppm from week 80 as at
that time no clear adverse effects were detectable at the next highest
dose. The average daily intakes were: at 0.5/5 ppm 0.03 mg/kg/day and
0.02 mg/kg/day (0.009/0.1 and 0.007/0.7 corresponding to the 0.5 and
5 ppm period), at 1 ppm 0.02 mg/kg/day and 0.01 mg/kg/day and at 2 ppm
0.04 mg/kg/day and 0.03 mg/kg/day in males and females, respectively.
Rats were 4-5 weeks old at the beginning of the study. Powdered
standard rat diet and tap water were available ad libitum. The test
compound was formulated 50% in Ultrasil and mixed into the diet
(prepared every two weeks) up to nominal concentrations. Analyses of
concentrations of the test compound in the food were not reported.
Haematological tests, clinical chemistry tests, and urinalysis were
performed several times throughout the study and at the end of the
study (brain cholinesterase included). At the end of the study 10
rats/sex/dose level were necropsied and organ weights were recorded.
Histopathology was performed on tumour-bearing animals which died or
were killed during the study (except some animals which could not be
investigated as a result of the progressed autolytic state) and at
termination of the study on 5 animals per sex of the control group and
the 5 ppm-group. No significant differences in food and water
consumption nor body weight were observed between controls and treated
animals. No overt signs of toxic effect were observed apart from
transient muscle twitches seen in some animals after increasing the
dose to 5 ppm. At the end of the study mortality rate was 55%, 60%,
60% and 75% in males and 45%, 38%, 42% and 32% in females at 0, 0.5/5,
1 and 2 ppm, respectively. Scattered difference of some haematological
tests, clinical chemistry tests and urinalysis of no biological
relevance were noted. Trivial inhibition of plasma and erythrocyte
cholinesterase (20-30%) activities were not consistent throughout the
study in any group except the 0.5/5 ppm group after increasing the
dose when inhibitions of similar magnitude (20-40%) were observed in
both sexes. No differences of brain cholinesterase activities were
detectable between controls and treated rats. Autopsy and
histopathological findings were unremarkable. Brain cholinesterase
activity was not inhibited at the end of the study suggesting a NOAEL
of 2 ppm in the present study, equal to 0.038 and 0.030 mg
disulfoton/kg bw/day in males and females, respectively. No
carcinogenicity was noted at 2 ppm disulfoton but final judgement can
not be drawn from this study as it was not properly designed to
evaluate this effect (Carpy & Klotzsche, 1975).
Fifty male and 50 female Fisher 344 rats were treated with
technical Disulfoton (97.91% purity, containing 29 identified
impurities) at concentrations of 0, 1, 4 and 16 ppm equal to 0, 0.06,
0.22, 0.92 mg/kg bw/day for males and 0, 0.08, 0.26 and 1.33 mg/kg
bw/day for females (calculated as average daily intake throughout the
duration of the study) for two years. The rats were 4 weeks old at
the beginning of the study. The diets were prepared weekly with corn
oil as the vehicle (1% by weight) and acetone as the solvent and kept
in a freezer until presented to the rats at one dietary level on
consecutive days. The actual content of disulfoton in the formulations
was checked monthly, results 87%, 90% and 90% of nominal (mean of 25
determinations) for 1, 4, and 16 ppm, respectively. Homogeneity and
stability of the diets were acceptable.
At 16 ppm dose level, feed consumption and body weight were
reduced. Increased incidences of clinical signs such as rough coat,
urine stain, loose stool, tail rash and skin lesions were observed in
both sexes. These parameters were not consistently affected in rats
treated with 1 nor 4 ppm disulfoton. At the end of the treatment the
mortality rate was 24%, 24%, 24% and 12% (male) and 12%, 22%, 30% and
40% (female) at 0, 1, 4 and 16 ppm, respectively. The historical
mortality range of female control rats in previous studies was 18-34%
which suggests that a marginal effect on mortality could have occurred
in females at the highest dose level. A trend towards increased total
white cell counts in 16 ppm female rats was present at 6, 12, 18, and
24 months. At termination of the study, decreased serum total
protein, albumin and cholesterol were observed at 16 ppm in both
sexes. Significant differences of other haematological or biochemical
parameters were observed but considered to be of no biological
relevance. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were observed throughout the duration of the
study ranging between a borderline inhibition in rats treated with 1
ppm of disulfoton or about 90% inhibition in rats dosed with 16 ppm
disulfoton. At termination of the study acetylcholinesterase
inhibition levels in brain were 15%, 53% and 79% in males and 21%, 53%
and 82% in females at 1, 4, and 16 ppm, respectively. Gross pathology
did not reveal increases in masses between control rats and those
receiving disulfoton. Several non-neoplastic changes observed at
necroscopy were increased in females fed at 16 ppm. These included an
increase in overall number of external observations, particularly
those of the skin which histologically appeared as inflammation,
ulceration, acanthosis, hyperkeratosis and epithelial inclusion cyst,
and those of the eye (increased vascularization). Reduction of muscle
size of the rear limb was confirmed microscopically as skeletal muscle
atrophy. An increased incidence of lung lesions was mainly
granulomatous or suppurative inflammation. There was an increase in
relative organ weight of heart, liver, kidneys and lung in female rats
and brain in both sexes at 16 ppm. The forestomach papillomas
occurred slightly more often in the high-dose groups than in other
groups and was usually associated with mucosal hyperplasia and
hyperkeratosis. An increased incidence of cystic degeneration of the
Harderian gland was seen in 16 ppm males and in 4 and 16 ppm females
(a similar increase at the 1 ppm level was not confirmed after
re-evaluation of specimens by other pathologists). There were no
statistically significant differences in the incidence of neoplasms
between groups. Disulfoton was not carcingenic for male nor female
rats consuming up to 16 ppm disulfoton. The NOEL for disulfoton in
this study was 1 ppm corresponding to 0.059 mg/kg bw/day for males
and 0.075 mg/kg bw/day for females (Hayes, 1985).
Reproduction studies
Rats
In a 2-litter, 2-generation study, groups of 26 (F0) male and
female rats (Sprague-Dawley strain) approximately 6 weeks old
received Disulfoton (purity 97.8%) admixed in the diet at 0, 1, 3, 9
ppm. Homogeneity and stability of disulfoton in the diet were checked
and found acceptable. Actual concentrations of disulfoton in the
diet were measured monthly and corresponded to 87%, 86% and 88% of
nominal at 1, 3 and 9 ppm (average percentage throughout the study),
respectively. Rats of F0 generation were maintained on their
respective diets for 15 weeks prior to mating. The F1b generation
received the compound in the diet for at least 13 weeks prior to
mating to produce F2 generations. F1b rats were maintained on
treated feed continuously throughout production of F2 generations.
Parent rats were observed daily for clinical symptoms. Weight and
food consump-tion were measured weekly.
Sialodacryoadenitis virus infection developed in F0 rats
(clinical symptoms confirmed by serum analysis and histopathology) but
did not interfere with the study as it was present in all groups and
because mating and reproductive parameters and postnatal indices were
not affected during the time of infection. Body weight and feed
consumption were not affected in any group by treatment during
pre-mating period. In the 9 ppm females, tremors were occasionally
observed during production of F1 generation. Fertility index was
decreased during production of both F1a and F1b litters and reduction
of body weight gain and feed consumption was observed during
lactation. The gestation length and gestation index were not
different for control and treated groups. Litter count, litter weight,
and viability and lactation indices were not affected up to the 3 ppm
group but growth and survival of 9 ppm group offspring were
significantly decreased as compared to those of control in F1a and
F1b generations. Acetylcholinesterase activity in brain of F1a pups
was reduced 0, 24 and 50% in males and 0, 32 and 59% in females at 1,
3 and 9 ppm, respectively. No treatment-related clinical signs of
toxicity were observed in either sex of any dose group during the
premating period of F1b animals. Body weight and feed consumption
were not affected up to 3 ppm but they were both decreased at 9 ppm in
females, as was feed consumption only for males. Fertility index was
reduced at 9 ppm during production of both F2a and F2b litters and
reduction of body weight and feed consumption were occasionally
observed during gestation and lactation. The gestation length and
gestation index were not different for control and treated groups.
Litter count, litter weight, viability, and lactation indices were not
affected up to the 3 ppm group (F2a generation) and up to the 1 ppm
group (F2b generation) but growth and survival of 9 ppm group
offspring were significantly decreased as compared to those of control
in both generations. At 3 ppm F2b litters showed a reduction of
gestation index, viability index (day 4) and mean weight (day 0).
Gross pathology and histopathology did not show compound-related
lesions in examined adults nor pups. The significant reduction in
overall reproductive performance was found at 9 ppm in the presence of
overt parental intoxication. At 3 ppm reduced reproduction was found
in only one set of litters (F2b). This effect was assumed to be
related to cholinesterase inhibition which was observed in similarly
treated F1a litters. The parental NOAEL for toxicity was 3 ppm. The
NOAEL for reproductive effects was 1 ppm (Hixson & Hathaway, 1986).
Special studies on delayed neuropathy
Hens
Twenty adult White Leghorn hens were treated orally with 30 mg/kg
bw of technical disulfoton (97.8% purity) on two separate occasions 22
days apart. In preliminary studies 30 mg/kg bw of disulfoton was
lethal to hens so that in the present study birds were protected with
atropine (0.5 mg/kg i.m.) and 2-PAM (12.5 mg/kg i.m.). Controls were
dosed either with atropine/2-PAM (5 birds) or 500 mg/kg of
tri-o-cresyl phosphate (TOCP, 10 birds) or not treated (5 birds).
Animals were observed daily for toxicity for 42 days. Body weights
and feed consumption were recorded twice a week. Untreated control
and antidote control birds were normal throughout the study.
Fourteen out of 20 birds dosed with disulfoton showed loss of
equilibrium, decreased activity, diarrhoea and locomotor ataxia
typical of cholinergic symptoms, starting soon after the first dosing
which disappeared within 5 days. Eight out of 10 TOCP-dosed birds
showed locomotor ataxia starting between days 12-24 which disappeared
in 5 hens before termination of the study. Birds dosed with
disulfoton did not show histopathological changes suggestive of
delayed neurotoxicity in peripheral nerves nor in spinal cord.
TOCP-dosed hens showed axonal degenerations with macrophage
accumulation in brain and spinal cord. Disulfoton does not induce
acute delayed neuropathy after 2 oral 30 mg/kg bw doses (Hixson,
1983).
Special studies on embryotoxicity and teratogencity
Rats
Twenty-five female CD rats were treated with technical Disulfoton
(98.2% purity) at concentrations of 0, 0.1, 0.3 and 1.0 mg/kg/day
orally by gavage (dissolved in polyethylene glycol 400) on days 6
through 15 of gestation. A positive control group (25 females)
received hydroxyurea at 350 mg/kg in distilled water on days 9, 10 and
11 of gestation only. Recovery of disulfoton from dosing solutions
ranged from 74 to 106% of nominal. Neither clinical signs nor
mortality were observed in treated or control groups. There were no
significant differences in body weight or feed consumption between the
groups. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were measured on day 15 of gestation. The
inhibition was trivial at 0.1 mg/kg, about 40% at 0.3 mg/kg and 80-90%
at 1.0 mg/kg (both activities similarly inhibited). Gross pathology
did not show lesions related to disulfoton administration. There were
no significant differences in the number of implantations per litter,
live, dead and resorbed fetuses per litter or in the average weight of
live fetuses between groups treated with disulfoton and control. There
were no statistically significant differences in the incidence of soft
tissue abnormalities. Increased incidence of incomplete ossification
of the sternebrae in fetuses of dams treated with disulfoton at 1.0
mg/kg was observed. Significant increases were found in several gross,
soft tissue and skeletal abnormalities in fetuses in the positive
control group. Disulfoton is not teratogenic at dosages that result
in significant inhibition of cholinesterase activity. The NOAEL for
embryotoxicity was 0.3 mg/kg; the NOAEL for maternal toxicity 0.1
mg/kg (Lamb & Hixson, 1983).
Rabbits
Fourteen (22 for the highest dose level only) pregnant New
Zealand White rabbits were treated by gavage with disulfoton (purity
97.3%) during gestation days 6-18 at doses of 0, 0.3, 1 or 3 mg/kg
bw/day. The highest dose level was reduced to 2 and 1.5 mg/kg bw/day
in some but not all of the high dose animals due to severe toxic
response and mortality. On day 29 of gestation, animals were
sacrificed for examination of their uterine content. Internal,
external and skeletal exams were performed on the fetuses. Maternal
toxicity observed at the highest dose group included muscular tremor,
increased respiratory rate, unsteadiness/incoordination and mortality
(59% at the end of the study). Body weight gain was reduced in animals
dosed with 3/2/1.5 mg/kg and 1 mg/kg disulfoton but only during
treatment. Body weight gain was similar to that of controls for all
treated groups during post-treatment period. Number of implantations
and viability, the extent of pre- and post-implantation losses and
fetal and placental weights were unaffected by treatment. Three
malformed fetuses were observed in the 0.3 mg/kg group but no
malformations were detectable in offspring of animals treated at
higher doses of disulfoton, therefore fetal survival, development and
growth in utero were considered unaffected by treatment. Disulfoton
at dosages of 1.5 mg/kg/day produced marked toxicity but survival,
growth and development of fetuses were unaffected (Tesh, 1982).
Four New Zealand White rabbits were treated with disulfoton as
above at concentrations of 0, 0.1, 0.3 and 1 mg/kg bw/day in a
preliminary study. No clear signs of toxicity occurred in females up
to the highest dose (transient reduction of body weight gain during
treatment). There was no evidence of any adverse effect of treatment
upon fetal morphogenesis or growth (Tesh, 1981).
Special studies on genotoxicity
Table 2. Results of genotoxicity assays on disulfoton
Test system Test object Concentration of Purity Results Reference
test substance
Reversion assay(1) S. typhimurium 0.1-1000 µg/plate 94.1% Negative (2) Inukai & Iyatomi (1976)
TA98, TA100,
TA1535, TA1537
Reversion assay(1) E. coli WP2 hcr 50-20 000 µg/plate 96.5% Positive (3) Shirasu et al. (1979)
S. typhimurium
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation Saccharomyces 1.5-200 µl/well 97.3% Negative (4) Jaganath (1981)
test(1) cerevisiae
S138, S211
CHO/HGPRT Chinese hamster 0.03-10 µg/ml 97.0% Equivocal (5) Yang (1988)
mutation assay(1) ovary cell
Mitotic non- Saccharomyces 20-200 µl/ml 97.3% Negative (6) Brusick (1981)
disjunction(1) cerevisiae D6
Pol test(1) E. coli p3478, 625-10 000 µg/plate 97.3% Negative (7) Herbold (1983)
w3110
Rec-assay Bacillus subtilis 3-300 µg/disc 94.1% Negative (8) Inukai & Iyatomi (1976)
NIG17 Rec+
NIG45 Rec-
Table 2 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Rec-assay Bacillus subtilis 1-100% v/v 96.5% Negative (9) Shirasu et al., (1979)
H17 Rec+ dissolved in DMSO
M45 Rec-
Sister chromatide Chinese hamster 0.004-0.1 µl/ml 97.9% Positive (10) Putman (1987)
exchange ovary cells (nonact)
0.002-0.2 µl/ml Negative (10)
(act)
Dominant lethal Male NMRI/ORIG 1 x 5 mg/kg bw 94.9% Negative (11) Herbold (1980)
test Kissleg strain mice
Micro nucleus test Male/female Bor: 2 x 6 50% Negative (12) Herbold (1981
NMRI-mice 2 x 12 mg/kg bw (pre-mix)
(1) both with and without metabolic activation
(2) Positive control without activation: N-methyl-N-nitro-
N-nitrosoguanidina 10 µg/plate (TA1535) dexon; 50 µg/plate
(TA1537 and TA98); N-fluoren-2-yl-acetamide 50 µg/plate (TA98);
dimethylnitrosamine 1000 µg/plate (TA1535 and TA100;
furylfuramide 0.02 µg/plate (TA100) gave expected positive
response. Positive control with activation: N-fluoren-
2-yl-acetamide 50 µg/plate (TA98); dimethylnitrosamine 1000
µg/plate (TA1535 and TA100) gave expected positive response.
(3) Positive control without activation: 2-aminoanthracene 10
µg/plate (all strains); furylfuramide, 0.05 µg/plate (TA100),
0.25 µg/plate (WP 2 hcr), 0.1 µg/plate (TA 98); ß-propiolactone,
50 µg/plate (TA1538) gave expected positive response. Positive
control with activation: 2-aminoanthracene, 10 µg/plate (all
strains) gave expected positive response. Test compound gave
positive response with and without metabolic activation on
TA1535 strain.
(4) Positive control without activation: Quinacrine mustard, 50
µl/well (S138); ethyl methanesulfonate, 10 µl/well (S211) gave
expected positive response. Positive control with activation:
Cyclophosphamide 50 µg/well (S211) gave expected positive
response. 2-acetylaminofluorene, 10 µg/well (S138) did not give
positive response.
(5) Positive control without activation: Ethyl methanesulfonate, 0.2
µg/l gave expected positive response. Positive control with
activation: Benzo(a)pyrene, 4 µg/ml gave expected positive
response. Disulfoton was mutagen at concentrations that were
either insoluble or partially soluble in the medium. At
soluble concentrations disulfoton was not mutagenic in this
assay.
(6) Positive control without activation: Ethyl methanesulfonate (20
µl/ml) did not induce chromosome aneuploidy and did induce
mitotic recombination and chromosome deletions.
(7) Positive control with and without activation: methyl
methanesulfonate, 10 µl/plate gave expected positive response.
(8) Positive control: Mitimycyn C 0.3 µg/disc gave the expected
positive response.
(9) Positive control: Mitimycyn C 0.1 µg/disc gave the expected
positive response.
(10) Positive control without activation: Triethylenemelamine, 0.025
µg/ml gave the expected positive response. Positive control with
activation: Cyclophosphamide, 2.5 µg/ml gave the expected
positive response. Test material gave positive response at 0.1
µl/ml in the absence of metabolic activation.
(11) Concurrent positive control not conducted. Test material
administered once to mice by oral gavage.
(12) Positive control (TrenimonR 2 x 0.125 mg/kg bw) gave expected
positive response. Test material administered twice (24 hours
apart) to mice by oral gavage.
Special studies on metabolites
Rats
The sulfone metabolite of disulfoton was administered in a pilot
study to Fischer 344 rats (10/sex/level) by diet at concentrations of
0, 0.5, 0.75 or 1 ppm for six weeks to determine its possible effect
on cholinesterase activity. There were neither biologically
significant depression of brain cholinesterase activity at termination
of the study nor depressions of plasma and erythrocyte cholinesterase
activities (performed weekly) (Stuart, 1986a).
Dogs
Two identical studies were conducted on Beagle dogs to determine
the effect of disulfon metabolites on cholinesterases. The oxygen
analog sulfone metabolite of disulfoton was administered in the diet
to Beagle dogs (2/sex/level in each study) for 6 weeks at
concentrations of 0, 0.5, 0.75, and 1 ppm. Trivial differences were
observed on plasma and erythrocyte cholinesterase activities
(determined weekly throughout the study) and on brain cholinesterase
at termination of the study (Stuart, 1986b, 1986c).
Cows
Angus cattle (3/sex/level) were fed diets of alfalfa pellets
containing a mixture of the disulfoton metabolites sulfoxide, sulfone
and their oxygen analogs at a ratio of 1:2:1:1:, respectively. The
study was conducted for 31 days to determine the effect levels for
cholinesterase inhibition in whole blood. Diet concentrations tested
were 0 (4/sex), 3.6, 7.2, 10.8, and 18 ppm. Dose-related inhibition
of cholinesterase activity was observed at concentrations of 7.2 ppm
and greater. The NOEL for cholinesterase inhibition was 3.6 ppm
(Horton et al., 1975)
Holstein dairy cows (3/level) were fed alfalfa pellets containing
a mixture of disulfoton metabolites sulfoxide, sulfone, oxygen analog
sulfoxide and oxygen analog sulfone at a ratio of 1:2:1:1,
respectively. Animals received dietary concentrations of 3.6, 7.2, or
18 ppm for 28 days. A single untreated cow served as control. Blood
cholinesterase activity was determined before starting the study and
weekly throughout the duration of the study. Feed consumption, body
weight and milk production were also recorded. All measured
parameters were affected at the high- and medium-dose levels. A
trivial reduction of blood cholinesterase activity was noted at the
low-dose (30% decrease compared to 19% decrease in control). The NOEL
for cholinesterase inhibition was 3.6 ppm (Thornton, 1976).
Special studies on skin sensitization
Forty male guinea pigs (Pirbright White W 58) were treated with
technical disulfoton (purity 98.6%) to investigate sensitizing effect
on skin. The Magnusson and Kligman maximization test was used.
Evaluation showed that there were 2 positively reacting animals in the
test compound group as against one in the control group. There were
no indications of a skin sensitizing effect on guinea pigs for
disulfoton (Flucke, 1983).
COMMENTS
Disulfoton was previously evaluated by the JMPR in 1973 and 1975
and an ADI of 0-0.002 mg/kg bw was allocated. Disulfoton is rapidly
absorbed in rats after oral dosing and approximately 90% is excreted
via the urine within 24 hours. The biotransformation pathway consists
of hydrolysis and oxidation to metabolites such as disulfoton sulfone,
disulfoton oxon sulfoxide and disulfoton oxon sulfone.
Disulfoton has high acute oral toxicity to mice, rats and dogs.
It is classified by WHO as "extremely hazardous".
Cholinesterase inhibition and related clinical effects were the
only significant findings in long-term bioassays in mice and rats. In
a 99-week study in mice at dietary concentrations of 0, 1, 4, or 16
ppm, the NOAEL was 4 ppm, equal to 0.55 mg/kg bw/day. At the 16 ppm
concentration brain acetylcholinesterase inhibition was reported.
There was no evidence of carcinogenicity.
In a long-term study in rats at dietary concentrations of 0, 1,
4, or 16 ppm the NOAEL was 1 ppm, equal to 0.06 mg/kg bw/day. At
higher concentrations clinical signs of toxicity and inhibition of
plasma, erythrocyte and brain cholinesterase activities were observed.
No carcinogenic effect was detected.
In a 2-year study in dogs at dietary concentrations of 0, 0.5, 1,
or 2/5/8 ppm, the NOAEL was 1 ppm, equal to 0.03 mg/kg bw/day. At the
next highest dose inhibition of brain acetylcholinesterase was
observed. Treatment-related histo-pathological changes were not
found.
Disulfoton did not cause delayed neuropathy in adult hens.
Disulfoton was not teratogenic in rats nor rabbits. In rats
given 0, 0.1, 0.3 or 1 mg/kg bw/day, the NOAELs for embryotoxicity and
maternal toxicity were 0.1 and 0.3 mg/kg bw/day respectively. In
rabbits given 0, 0.3, 1 or 3/2/1.5 mg/kg bw/day, the NOAELs for
embryotoxicity and maternal toxicity were 1.5 and 0.3 mg/kg bw/day,
respectively.
In a 2-litter 2 generation reproduction study in rats at dietary
concentrations of 0, 1, 3 or 9 ppm, the NOAEL for toxicity was 3 ppm
(equivalent to 0.15 mg/kg bw/day), based on signs of maternal toxicity
at 9 ppm. The NOAEL for reproductive effects was 1 ppm (equivalent to
0.05 mg/kg bw/day) based on decreased brain acetylcholinesterase,
body-weight gain and survival of pups at 3 ppm.
Although there was one positive reverse mutation assay it was
concluded, after review of all available in vivo and in vitro
genotoxicity data, that there was no evidence of genotoxicity.
The human volunteer study reviewed by the 1975 JMPR was reported
in summary form only and was considered inadequate for the estimation
of an ADI.
The ADI was based on the 2-year study in dogs, using a 100-fold
safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 4 ppm in the diet, equal to 0.55 mg/kg bw
Rat: 1 ppm in the diet, equal to 0.06 mg/kg bw
Dog: 1 ppm in the diet, equal to 0.03 mg/kg bw
Man: 0.75 mg/man/day, equivalent to 0.01 mg/kg bw
Estimate of acceptable daily intake for humans
0-0.0003 mg/kg bw.
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans.
REFERENCES
Brusick, D.J. (1981) Mutagenicity evaluation of S276 in the mitotic
non-disjunction in Saccharomices cerevisiae strain D6. Unpublished
Report R 2086 from Litton Bionetics Inc., MD, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Carpy, S. & Klotzsche, C. (1975) Disulfoton 2-year feeding study in
rats. Unpublished Report AGRO DOK CBK 1854/75 from Sandoz Ltd.,
Basel, Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1983) S 276 (Disulfoton, Disyston active ingredient)
Study for skin-sensitising effect with guinea pigs. Unpublished
Report 12121 from Bayer AG, Institute of toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Flucke, W. (1986) S 276 technical - Study of subacute dermal toxicity
to rabbits. Unpublished Report 14747 from Bayer AG, Institute of
toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1983) Oncogenicity study of Disulfoton technical on
mice. Unpublished Report 413 from Mobay Chemical Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985) Chronic feeding/oncogenicity study of technical
Disulfoton (Di-Syston) with rats. Unpublished Report 638 from Mobay
Chemical Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1980) S 276, Disulfoton, Disyston active ingredient.
Dominant lethal test on male mouse to evaluate S 276 for mutagenic
potential. Unpublished Report 9440 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) S 276, Disulfoton, Disyston active ingredient.
Micronucleus test on the mouse to evaluate S 276 for mutagenic effect.
Unpublished Report 10451 from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) S 276, Disulfoton, Disyston active ingredient. Pol
test on E. coli to evaluate for potential DNA damage. Unpublished
Report 12139 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Hixson, E.J. (1983) Acute delayed neurotoxicity study on Disulfoton.
Unpublished Report 365 from Mobay Chemical Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Hixson, E.J. & Hathaway, T.R. (1986) Effect of Disulfoton (Di-syston)
on reproduction in rats. Unpublished Report 711 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Weischer, C.H. (1975) S 276 (disulfoton) Chronic
toxicity study on dogs (two-year feeding experiment). Unpublished
Report 5618 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Horton, J.R., Thornton J.S. & Lichtenstein, H.C. (1975) The subacute
oral toxicity of a Di-syston metabolite mixture administered in the
feed to cattle. Unpublished Report 45288 from Chemagro Corporation,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Inukai, H. & Iyatomi, Y. (1976) Disulfoton. Mutagenicity test on
bacterial systems. Unpublished Report 29 from Nitokuno Agricultural
Chemicals Institute, Toyoda, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Iyatomi (1980) Report of acute toxicity. Disulfoton. Unpublished
Report A-29 from Nitokuno, Agricultural Chemicals Institute, Tokyo,
Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jagannath, D.R., (1981) Mutagenicity evaluation of S 276 in
Saccharomyces cerevisae reverse mutation induction assay.
Unpublished Report R 2087 from Litton Bionetics, Inc. MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Wells N., Lawrence L.J. (1987)
Quantitative characterization of residues in tissues and eggs of
laying hens treated orally for three consecutive days with (14C)
Di-syston-ethylene. Unpublished Report MR 98435 from Pharmacology
and Toxicology Research Laboratory, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Lawrence L.J. (1988)
Metabolism of (14C) Di-syston in the lactating goat. Unpublished
Report MR97499 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Hixson, E.J. (1983) Embryotoxic and teratogenic effects
of disulfoton. Unpublished Report 376 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K., Hanna L.A., Johnston K. & Ose, K. (1985) Excretion and
metabolism of Di-syston in rats. Unpublished Report MR90946 from
Mobay Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1978) Acute toxicity studies. Unpublished Report 7602
from Bayer AG, Institute of toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. Unpublished Report 969 from
Microbiological Associates, Inc., MD, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988) Pilot study to assess cholinesterase activity
in rats exposed by inhalation to technical grade Disulfoton.
Unpublished Report 1073 from Mobay Corporation, USA. Submitted to WHO
by Bayer, Leverkusen, Germany.
Shiotsuka, R.N. (1989) Subchronic inhalation toxicity study of
technical grade Disulfoton (Di-syston) in rats. Unpublished Report
1131 from Mobay Corporation, USA. Submitted to WHO by Bayer,
Leverkusen, Germany.
Shirasu, Y. et al. (1979) Ethylthiometon - Mutagenicity test on
bacterial systems. Unpublished Report from Institute of Environmental
Toxicology, Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986) Pilot study on DI-SYSTON sulfone with rats.
Unpublished Report 85-971-02 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986a) Pilot study on DI-SYSTON oxygen analog sulfone
with dogs. Unpublished Report 85-974-02 from Mobay Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart B.P. (1986b) Pilot study on DI-SYSTON sulfone with dogs.
Unpublished Report 85-974-01 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. (1982) S 276-Effects of oral administration upon pregnancy
in the rabbit. Unpublished Report 2351 from Life Science Research,
England. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. & Ross, F.W. (1981) S 276-Effects of oral administration
upon pregnancy in the rabbit. 1. Preliminary study. Unpublished LSR
Report BAG010 from Life Science Research, England. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thornton, J.S. (1976) Effect of feeding DI-SYSTON metabolites to
dairy cattle. Unpublished Report MR49100 from Chemagro Agriculture
Division, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1978) S 276 (Disyston active ingredient) Acute
inhalational toxicity studies. Unpublished Report 7827 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen J. (1980) Disulfoton (S 276) The active ingredient of
Di-syston Subacute inhalation study on rats. Unpublished Report 9065
from Bayer AG, Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Yang, Li L. (1988) Disyston technical-CHO/HGPRT assay. Unpublished
Report 994 from Microbiological Associates, Inc. MD, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Acute toxicity studies
Table 1. Acute toxicity of Disulfoton
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Mice M oral 7.0 Mihail (1978)a
F 8.2
M&F oral 27 Iyatomi (1980)b
M&F i.p. 14 Iyatomi (1980)b
M s.c. 20 Iyatomi (1980)b
F 28
M&F dermal 35 Iyatomi (1980)b
Rats M oral 6.2 Mihail (1978)a
F 1.9
M oral 9.6 Iyatomi (1980b)b
F 4.2
M inhalation 290 Thyssen (1978)c
F (1 hr exp) 63
M inhalation approx 60 Thyssen (1978)c
F (4 hr exp) approx 15
M i.p. 7.5 Iyatomi (1980)b
F 3.1
M s.c. 7.7 Iyatomi (1980)b
F 4.0
M dermal 15.9 Mihail (1978)a
F (24 hr exp) 3.6
M dermal 22.6 Iyatomi (1980)b
F 7.3
Table 1 (contd).
Species Sex Route LD50 LD50 Reference
(mg/kg bw) (mg/m3)
Dogs F oral approx .5 Mihail (1978)a
a Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Pulmonary
oedemas were observed in necropsied animals.
b Test substance; disulfoton pure grade 98.6%. Only LD50s are reported. Details about
symptoms and pathology are lacking.
c Test substance; S 276, pure grade 94.4%. Typical cholinergic symptoms reported. Gross pathology
negative.
Two subacute inhalation studies were conducted on Wistar TNO/W 74
albino rats (Winkelmann, Borchen, Germany) (10/sex/dose level) exposed
in dynamic inhalation chambers to aerosolized concentrations of
technical disulfoton (purity 94.4%). In each study exposure was for
6 hours per day, five days per week for three weeks. Test compound
was dissolved to final concentrations in ethanol and polyethylene
glycol 400 (1:1 mixture). Controls were exposed to solvent mixture at
a concentration of 20,000 µl/m3. In study 1, disulfoton
concentrations were 0, 0.1, 0.5 and 3.7 mg/m3 air. Rats were
observed daily for clinical symptoms and weighed weekly. Twenty-four
hours after the last exposure, 5 rats/sex/dose level were subjected to
haematological tests, clinical chemistry tests and urinalysis. Both
plasma and erythrocyte cholinesterase activities were measured after
0, 5, 10 and 15 exposures and brain cholinesterase activity was
determined at the end of the study. Pathology was performed at the end
of the study.
At the highest dose level, all rats showed clinical symptoms
typical for cholinergic toxicity and 5 female rats died within 12
exposures. At lower dose levels, behavioural disturbance was detected
during the last week of exposure soon after the end of each exposure.
Body weight, haematological tests, clinical chemistry tests and
urinalysis did not show dose-related alterations. Plasma
cholinesterase activities were significantly reduced in females at all
dose levels and in males only at the highest dose level. At 3.7 mg
disulfoton/m3, erythrocyte cholinesterase activity was consistently
reduced throughout the duration of the study in both sexes. At the
end of the study brain cholinesterase activities were 48% and 58% of
control values in males and females, respectively. Mottled distended
lungs and ulcer-like foci were observed in animals which died before
the end of the study, but no toxic effects were detectable in
surviving rats. At the highest dose level, increased relative and
absolute adrenal weights were observed in female rats. Histopathology
showed inflammatory changes in the region of the respiratory tract
and concurrent bone marrow changes from 0.5 mg disulfoton/m3 in both
sexes. In study 2, 10 female and 10 male rats were exposed to a
concentration of 0.02 mg disulfoton/m3 air (controls exposed to
solvent/air only). Also 20 additional female rats were exposed to 3.1
mg disulfoton/m3 air to confirm the high mortality rate observed in
females at the highest dose level in study 1. The protocol of study
2 was like that of study 1. Results at the highest concentration in
study 2 confirmed toxicity detected at a similar dose level in study
1. Three out of 20 rats died before the end of the study. All
clinical, biochemical and pathological parameters were unaffected at
0.02 mg disulfoton/m3 air. A concentration of 0.1 mg/m3 was the
NOAEL for inhalation of a disulfoton aerosol with females being more
sensitive to disulfoton toxicity than males (Thyssen, 1980).
Nine or ten male and 10 female Fischer 344 rats were exposed in
dynamic inhalation chambers to aerosolized technical disulfoton
(purity 97.8%) by the nose-only technique for 6 hours per day, 5 days
per week for 3 weeks. The targeted concentrations of disulfoton were
0.005, 0.05 and 0.5 mg/m3 air which corresponded to analytical
concentrations of 0.006, 0.07 and 0.7 mg disulfoton/m3 air.
Disulfoton was dissolved in a mixture 1:1 of ethanol/polyethylene
glycol and two control groups were added, exposed to either
solvent/air or air only. Rats were observed daily for mortality and
signs of toxicity and weighed weekly. No deaths, signs of toxicity
nor differences in body weight were detected throughout the study. No
reduction of brain cholinesterase activity was measured in any group
at the termination of the study. The NOAEL could be set at 0.7 mg/m3
air based on normal brain cholinesterase activity at the end of the
study (Shiotsuka, 1988).
Twelve male and 12 female Fisher 344 rats were exposed in dynamic
inhalation chambers to aerosolized technical disulfoton (purity 97.8%)
by the nose-only technique for 6 hours per day, 5 days a week for 13
weeks at concentrations of 0.015, 0.15 and 1.5 mg disulfoton/m3 air.
Solutions of disulfoton in vehicle (1:1 ethanol, polyethylene glycol
400) were prepared weekly and actual concentrations in the chambers
(checked daily) were 0.018, 0.16 and 1.4 mg disulfoton/m3 air for the
lowest, mid and highest concentration, respectively. Disulfoton
exposure did not produce clinical symptoms nor increase mortality.
Feed consumption and body weight were not different among groups.
Ophthalmology, clinical chemistry tests, haematological tests and
urinalysis did not reveal toxic alterations. Slight inhibition of
plasma, erythrocyte and brain cholinesterase activities were measured
in both sexes at the highest dose level (at termination of the study,
brain cholinesterase inhibition was 29% and 28% in males and in
females, respectively). Gross pathology and organ weights did not
show toxic effects related to disulfoton exposure. A significantly
increased incidence of inflammation in the nasal turbinate of males
exposed to the highest disulfoton concentration was detected and
judged as a topical irritant effect of the test substance. The NOAEL
in this study was 0.16 mg disulfoton/m3 for both sexes, based on
cholinesterase inhibition and histopathological finding detected at
the next higher dose level (Shiotsuka, 1989).
Rabbits
Groups of adult male and female New Zealand rabbits (5/sex/dose
level) received technical disulfoton (97.8% purity grade, formulated
with Cremophor EL in saline) dermally (skin not abraded, uncovered
after application and test substance washed away at the end of each
exposure period) for 6 hours per day, 5 days per week for 3 weeks.
The tested concentrations were 0, 0.4, 1.6, 6.5 mg disulfoton/kg bw.
Rabbits were observed for signs of toxicity twice a day (skin was
evaluated for irritation before the beginning of the study and 24
hours after the end of each treatment). Body weights and feed
consumption were determined weekly. Cholinergic signs such as muscle
spasms, dyspnoea and salivation were observed in both sexes at the
highest dose level and all animals died within 10 days. Cholinergic
symptoms and deaths appeared earlier in female rabbits. Mortality,
appearance (skin included) and behaviour, feed consumption and body
weight were not affected by treatment up to 1.6 mg disulfoton/kg
bw/day. No dose-related effects were observed in clinical chemistry
tests (except cholinesterase activities), haematological tests,
urinalysis, gross pathology, organ weights nor histopathology up to
1.6 mg disulfoton/kg bw/day. Marginal inhibition of both plasma and
erythrocyte cholinesterase activities were determined at 1.6 mg/kg
bw/day but brain cholinesterase activity was not different from that
of controls. The NOAEL can be set at 1.6 mg disulfoton/kg bw/day
based on normal brain cholinesterase activity measured at this dose
level (Flucke, 1986).
Dogs
Four male and 4 female pure-bred Beagle dogs were treated with
disulfoton (95.7% purity grade) at concentrations of 0, 0.5, 1 and 2
ppm (increased to 5 ppm on week 70 and again to 8 ppm on week 73 up to
the end of the study) equal to 0, 0.155, 0.319 and 1.31 mg/animal/day
for 104 weeks. Disulfoton was mixed with the food (50% premix x 2)
and the food ration (pulverized food + twice as much tap water) was
administered to all dogs in the form of a mash once daily. The
mixture was prepared weekly. The dogs were inspected daily for
toxicity and weighed weekly. Body temperature and pupillary reflex,
patellar reflex, flexor reflex and extensor thrust were tested several
times throughout the study. Disulfoton did not affect food and water
intake nor body weight gain. No clinical symptoms related to
disulfoton administration were detectable at any dose level.
Ophthalmoscopic examinations, reflexes and results of haematological
tests, clinical-chemical tests and urinalysis did not show toxicity
due to disulfoton. A single dog dosed with 0.5 ppm of disulfoton
developed interstitial nephritis of both kidneys and was sacrificed on
week 93. No other dogs died before the scheduled termination of the
study. Plasma, erythrocyte and brain cholinesterase activities were
not reduced in dogs up to 1 ppm disulfoton. Marginal inhibition of
plasma and erythrocyte cholinesterases was observed at 2 ppm which was
further increased by increasing the dose of disulfoton in the diet.
At the end of the study plasma and erythrocyte cholinesterase
activities in dogs dosed with 8 ppm were inhibited 50-60%. In this
group, brain cholinesterase was reduced 34% and 18% in males and
females. Neither macroscopic pathology nor histopathology provided any
evidence of tissue alterations attributable to dietary administration
of disulfoton. The NOAEL is 1 ppm disulfoton equal to 0.319
mg/animal/day (Hoffman & Weischer, 1975)
Long term studies
Mice
Fifty male and 50 female CD1 albino mice were treated with
disulfoton (98.2% purity grade) at concentrations of 0, 1, 4, 16 ppm,
equal to 0, 0.137, 0.548, 2.223 mg/kg bw/day for males and 0, 0.18,
0.73 and 2.3 mg/kg bw/day for females (calculated as average daily
intake throughout the duration of the study) for 99 weeks. The mice
were 4 weeks old at the beginning of the study. The diets were
prepared weekly with corn oil as the vehicle (1% by weight) and
acetone as the solvent and kept in a freezer until presented to the
mice at one dietary level on consecutive days. Stability and
homogeneity of disulfoton were acceptable. The actual content of
disulfoton in the formulation was checked monthly and showed 77%, 89%
and 92% of nominal (mean of the 25 determinations) for 1, 4, 16 ppm,
respectively. Observation for toxicological effects were made twice
daily (1X/day on holidays). Weekly observations for abnormalities and
masses were made and feed consumption and body weights were recorded.
Haematology determinations on 10 mice/sex/dose level were performed at
6 months, 12 months and termination of the study. Plasma, erythrocyte
and brain cholinesterase activities were determined at the end of the
study in controls and in mice treated with the highest dose of
disulfoton. Pathology was performed on all animals found dead or
sacrificed at the end of the study.
In both sexes, food intake and body weight were not influenced by
disulfoton administration. Neither sex showed any changes in the
incidence of clinical signs, or mortality rate at any dose level. At
the end of the treatment the mortality rate was 42%, 40%, 34%, 44%
(male) and 54%, 62%, 46%, 54% (female) at 0, 1, 4, 16 ppm disulfoton,
respectively. Disulfoton had no effects on haematological parameters.
At termination of the study inhibition of plasma, erythrocyte and
brain cholinesterase activities in mice at the 16 ppm dose level were
79%, 56%, 44% (male) and 50%, 82%, 46% (female), respectively.
Trivial differences in organ weights were observed between controls
and dosed mice. Both neoplastic and non-neoplastic histopathologic
observations were those of spontaneous or naturally occurring lesions
of aging albino mice. No differences in the incidence of neoplastic
or non-neoplastic lesions were found when treated mice were compared
to control mice. Disulfoton showed no evidence of an oncogenic effect
when added to the diet up to 16 ppm equal to 2.223 and 2.690 mg/kg
bw/day for males and females, respectively. The NOAEL for disulfoton
in the present study was 4 ppm equal to 0.55 and 0.73 mg/kg bw/day in
males and females, respectively (Hayes, 1983).
Rats
Sixty male and 60 female Sprague-Dawley rats were dosed with
disulfoton (purity 95.7%) at concentrations of 0, 0.5, 1, or 2 ppm for
104 weeks. The lowest dose was increased to 5 ppm from week 80 as at
that time no clear adverse effects were detectable at the next highest
dose. The average daily intakes were: at 0.5/5 ppm 0.03 mg/kg/day and
0.02 mg/kg/day (0.009/0.1 and 0.007/0.7 corresponding to the 0.5 and
5 ppm period), at 1 ppm 0.02 mg/kg/day and 0.01 mg/kg/day and at 2 ppm
0.04 mg/kg/day and 0.03 mg/kg/day in males and females, respectively.
Rats were 4-5 weeks old at the beginning of the study. Powdered
standard rat diet and tap water were available ad libitum. The test
compound was formulated 50% in Ultrasil and mixed into the diet
(prepared every two weeks) up to nominal concentrations. Analyses of
concentrations of the test compound in the food were not reported.
Haematological tests, clinical chemistry tests, and urinalysis were
performed several times throughout the study and at the end of the
study (brain cholinesterase included). At the end of the study 10
rats/sex/dose level were necropsied and organ weights were recorded.
Histopathology was performed on tumour-bearing animals which died or
were killed during the study (except some animals which could not be
investigated as a result of the progressed autolytic state) and at
termination of the study on 5 animals per sex of the control group and
the 5 ppm-group. No significant differences in food and water
consumption nor body weight were observed between controls and treated
animals. No overt signs of toxic effect were observed apart from
transient muscle twitches seen in some animals after increasing the
dose to 5 ppm. At the end of the study mortality rate was 55%, 60%,
60% and 75% in males and 45%, 38%, 42% and 32% in females at 0, 0.5/5,
1 and 2 ppm, respectively. Scattered difference of some haematological
tests, clinical chemistry tests and urinalysis of no biological
relevance were noted. Trivial inhibition of plasma and erythrocyte
cholinesterase (20-30%) activities were not consistent throughout the
study in any group except the 0.5/5 ppm group after increasing the
dose when inhibitions of similar magnitude (20-40%) were observed in
both sexes. No differences of brain cholinesterase activities were
detectable between controls and treated rats. Autopsy and
histopathological findings were unremarkable. Brain cholinesterase
activity was not inhibited at the end of the study suggesting a NOAEL
of 2 ppm in the present study, equal to 0.038 and 0.030 mg
disulfoton/kg bw/day in males and females, respectively. No
carcinogenicity was noted at 2 ppm disulfoton but final judgement can
not be drawn from this study as it was not properly designed to
evaluate this effect (Carpy & Klotzsche, 1975).
Fifty male and 50 female Fisher 344 rats were treated with
technical Disulfoton (97.91% purity, containing 29 identified
impurities) at concentrations of 0, 1, 4 and 16 ppm equal to 0, 0.06,
0.22, 0.92 mg/kg bw/day for males and 0, 0.08, 0.26 and 1.33 mg/kg
bw/day for females (calculated as average daily intake throughout the
duration of the study) for two years. The rats were 4 weeks old at
the beginning of the study. The diets were prepared weekly with corn
oil as the vehicle (1% by weight) and acetone as the solvent and kept
in a freezer until presented to the rats at one dietary level on
consecutive days. The actual content of disulfoton in the formulations
was checked monthly, results 87%, 90% and 90% of nominal (mean of 25
determinations) for 1, 4, and 16 ppm, respectively. Homogeneity and
stability of the diets were acceptable.
At 16 ppm dose level, feed consumption and body weight were
reduced. Increased incidences of clinical signs such as rough coat,
urine stain, loose stool, tail rash and skin lesions were observed in
both sexes. These parameters were not consistently affected in rats
treated with 1 nor 4 ppm disulfoton. At the end of the treatment the
mortality rate was 24%, 24%, 24% and 12% (male) and 12%, 22%, 30% and
40% (female) at 0, 1, 4 and 16 ppm, respectively. The historical
mortality range of female control rats in previous studies was 18-34%
which suggests that a marginal effect on mortality could have occurred
in females at the highest dose level. A trend towards increased total
white cell counts in 16 ppm female rats was present at 6, 12, 18, and
24 months. At termination of the study, decreased serum total
protein, albumin and cholesterol were observed at 16 ppm in both
sexes. Significant differences of other haematological or biochemical
parameters were observed but considered to be of no biological
relevance. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were observed throughout the duration of the
study ranging between a borderline inhibition in rats treated with 1
ppm of disulfoton or about 90% inhibition in rats dosed with 16 ppm
disulfoton. At termination of the study acetylcholinesterase
inhibition levels in brain were 15%, 53% and 79% in males and 21%, 53%
and 82% in females at 1, 4, and 16 ppm, respectively. Gross pathology
did not reveal increases in masses between control rats and those
receiving disulfoton. Several non-neoplastic changes observed at
necroscopy were increased in females fed at 16 ppm. These included an
increase in overall number of external observations, particularly
those of the skin which histologically appeared as inflammation,
ulceration, acanthosis, hyperkeratosis and epithelial inclusion cyst,
and those of the eye (increased vascularization). Reduction of muscle
size of the rear limb was confirmed microscopically as skeletal muscle
atrophy. An increased incidence of lung lesions was mainly
granulomatous or suppurative inflammation. There was an increase in
relative organ weight of heart, liver, kidneys and lung in female rats
and brain in both sexes at 16 ppm. The forestomach papillomas
occurred slightly more often in the high-dose groups than in other
groups and was usually associated with mucosal hyperplasia and
hyperkeratosis. An increased incidence of cystic degeneration of the
Harderian gland was seen in 16 ppm males and in 4 and 16 ppm females
(a similar increase at the 1 ppm level was not confirmed after
re-evaluation of specimens by other pathologists). There were no
statistically significant differences in the incidence of neoplasms
between groups. Disulfoton was not carcingenic for male nor female
rats consuming up to 16 ppm disulfoton. The NOEL for disulfoton in
this study was 1 ppm corresponding to 0.059 mg/kg bw/day for males
and 0.075 mg/kg bw/day for females (Hayes, 1985).
Reproduction studies
Rats
In a 2-litter, 2-generation study, groups of 26 (F0) male and
female rats (Sprague-Dawley strain) approximately 6 weeks old
received Disulfoton (purity 97.8%) admixed in the diet at 0, 1, 3, 9
ppm. Homogeneity and stability of disulfoton in the diet were checked
and found acceptable. Actual concentrations of disulfoton in the
diet were measured monthly and corresponded to 87%, 86% and 88% of
nominal at 1, 3 and 9 ppm (average percentage throughout the study),
respectively. Rats of F0 generation were maintained on their
respective diets for 15 weeks prior to mating. The F1b generation
received the compound in the diet for at least 13 weeks prior to
mating to produce F2 generations. F1b rats were maintained on
treated feed continuously throughout production of F2 generations.
Parent rats were observed daily for clinical symptoms. Weight and
food consump-tion were measured weekly.
Sialodacryoadenitis virus infection developed in F0 rats
(clinical symptoms confirmed by serum analysis and histopathology) but
did not interfere with the study as it was present in all groups and
because mating and reproductive parameters and postnatal indices were
not affected during the time of infection. Body weight and feed
consumption were not affected in any group by treatment during
pre-mating period. In the 9 ppm females, tremors were occasionally
observed during production of F1 generation. Fertility index was
decreased during production of both F1a and F1b litters and reduction
of body weight gain and feed consumption was observed during
lactation. The gestation length and gestation index were not
different for control and treated groups. Litter count, litter weight,
and viability and lactation indices were not affected up to the 3 ppm
group but growth and survival of 9 ppm group offspring were
significantly decreased as compared to those of control in F1a and
F1b generations. Acetylcholinesterase activity in brain of F1a pups
was reduced 0, 24 and 50% in males and 0, 32 and 59% in females at 1,
3 and 9 ppm, respectively. No treatment-related clinical signs of
toxicity were observed in either sex of any dose group during the
premating period of F1b animals. Body weight and feed consumption
were not affected up to 3 ppm but they were both decreased at 9 ppm in
females, as was feed consumption only for males. Fertility index was
reduced at 9 ppm during production of both F2a and F2b litters and
reduction of body weight and feed consumption were occasionally
observed during gestation and lactation. The gestation length and
gestation index were not different for control and treated groups.
Litter count, litter weight, viability, and lactation indices were not
affected up to the 3 ppm group (F2a generation) and up to the 1 ppm
group (F2b generation) but growth and survival of 9 ppm group
offspring were significantly decreased as compared to those of control
in both generations. At 3 ppm F2b litters showed a reduction of
gestation index, viability index (day 4) and mean weight (day 0).
Gross pathology and histopathology did not show compound-related
lesions in examined adults nor pups. The significant reduction in
overall reproductive performance was found at 9 ppm in the presence of
overt parental intoxication. At 3 ppm reduced reproduction was found
in only one set of litters (F2b). This effect was assumed to be
related to cholinesterase inhibition which was observed in similarly
treated F1a litters. The parental NOAEL for toxicity was 3 ppm. The
NOAEL for reproductive effects was 1 ppm (Hixson & Hathaway, 1986).
Special studies on delayed neuropathy
Hens
Twenty adult White Leghorn hens were treated orally with 30 mg/kg
bw of technical disulfoton (97.8% purity) on two separate occasions 22
days apart. In preliminary studies 30 mg/kg bw of disulfoton was
lethal to hens so that in the present study birds were protected with
atropine (0.5 mg/kg i.m.) and 2-PAM (12.5 mg/kg i.m.). Controls were
dosed either with atropine/2-PAM (5 birds) or 500 mg/kg of
tri-o-cresyl phosphate (TOCP, 10 birds) or not treated (5 birds).
Animals were observed daily for toxicity for 42 days. Body weights
and feed consumption were recorded twice a week. Untreated control
and antidote control birds were normal throughout the study.
Fourteen out of 20 birds dosed with disulfoton showed loss of
equilibrium, decreased activity, diarrhoea and locomotor ataxia
typical of cholinergic symptoms, starting soon after the first dosing
which disappeared within 5 days. Eight out of 10 TOCP-dosed birds
showed locomotor ataxia starting between days 12-24 which disappeared
in 5 hens before termination of the study. Birds dosed with
disulfoton did not show histopathological changes suggestive of
delayed neurotoxicity in peripheral nerves nor in spinal cord.
TOCP-dosed hens showed axonal degenerations with macrophage
accumulation in brain and spinal cord. Disulfoton does not induce
acute delayed neuropathy after 2 oral 30 mg/kg bw doses (Hixson,
1983).
Special studies on embryotoxicity and teratogencity
Rats
Twenty-five female CD rats were treated with technical Disulfoton
(98.2% purity) at concentrations of 0, 0.1, 0.3 and 1.0 mg/kg/day
orally by gavage (dissolved in polyethylene glycol 400) on days 6
through 15 of gestation. A positive control group (25 females)
received hydroxyurea at 350 mg/kg in distilled water on days 9, 10 and
11 of gestation only. Recovery of disulfoton from dosing solutions
ranged from 74 to 106% of nominal. Neither clinical signs nor
mortality were observed in treated or control groups. There were no
significant differences in body weight or feed consumption between the
groups. Dose-related inhibition of both plasma and erythrocyte
cholinesterase activities were measured on day 15 of gestation. The
inhibition was trivial at 0.1 mg/kg, about 40% at 0.3 mg/kg and 80-90%
at 1.0 mg/kg (both activities similarly inhibited). Gross pathology
did not show lesions related to disulfoton administration. There were
no significant differences in the number of implantations per litter,
live, dead and resorbed fetuses per litter or in the average weight of
live fetuses between groups treated with disulfoton and control. There
were no statistically significant differences in the incidence of soft
tissue abnormalities. Increased incidence of incomplete ossification
of the sternebrae in fetuses of dams treated with disulfoton at 1.0
mg/kg was observed. Significant increases were found in several gross,
soft tissue and skeletal abnormalities in fetuses in the positive
control group. Disulfoton is not teratogenic at dosages that result
in significant inhibition of cholinesterase activity. The NOAEL for
embryotoxicity was 0.3 mg/kg; the NOAEL for maternal toxicity 0.1
mg/kg (Lamb & Hixson, 1983).
Rabbits
Fourteen (22 for the highest dose level only) pregnant New
Zealand White rabbits were treated by gavage with disulfoton (purity
97.3%) during gestation days 6-18 at doses of 0, 0.3, 1 or 3 mg/kg
bw/day. The highest dose level was reduced to 2 and 1.5 mg/kg bw/day
in some but not all of the high dose animals due to severe toxic
response and mortality. On day 29 of gestation, animals were
sacrificed for examination of their uterine content. Internal,
external and skeletal exams were performed on the fetuses. Maternal
toxicity observed at the highest dose group included muscular tremor,
increased respiratory rate, unsteadiness/incoordination and mortality
(59% at the end of the study). Body weight gain was reduced in animals
dosed with 3/2/1.5 mg/kg and 1 mg/kg disulfoton but only during
treatment. Body weight gain was similar to that of controls for all
treated groups during post-treatment period. Number of implantations
and viability, the extent of pre- and post-implantation losses and
fetal and placental weights were unaffected by treatment. Three
malformed fetuses were observed in the 0.3 mg/kg group but no
malformations were detectable in offspring of animals treated at
higher doses of disulfoton, therefore fetal survival, development and
growth in utero were considered unaffected by treatment. Disulfoton
at dosages of 1.5 mg/kg/day produced marked toxicity but survival,
growth and development of fetuses were unaffected (Tesh, 1982).
Four New Zealand White rabbits were treated with disulfoton as
above at concentrations of 0, 0.1, 0.3 and 1 mg/kg bw/day in a
preliminary study. No clear signs of toxicity occurred in females up
to the highest dose (transient reduction of body weight gain during
treatment). There was no evidence of any adverse effect of treatment
upon fetal morphogenesis or growth (Tesh, 1981).
Special studies on genotoxicity
Table 2. Results of genotoxicity assays on disulfoton
Test system Test object Concentration of Purity Results Reference
test substance
Reversion assay(1) S. typhimurium 0.1-1000 µg/plate 94.1% Negative (2) Inukai & Iyatomi (1976)
TA98, TA100,
TA1535, TA1537
Reversion assay(1) E. coli WP2 hcr 50-20 000 µg/plate 96.5% Positive (3) Shirasu et al. (1979)
S. typhimurium
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation Saccharomyces 1.5-200 µl/well 97.3% Negative (4) Jaganath (1981)
test(1) cerevisiae
S138, S211
CHO/HGPRT Chinese hamster 0.03-10 µg/ml 97.0% Equivocal (5) Yang (1988)
mutation assay(1) ovary cell
Mitotic non- Saccharomyces 20-200 µl/ml 97.3% Negative (6) Brusick (1981)
disjunction(1) cerevisiae D6
Pol test(1) E. coli p3478, 625-10 000 µg/plate 97.3% Negative (7) Herbold (1983)
w3110
Rec-assay Bacillus subtilis 3-300 µg/disc 94.1% Negative (8) Inukai & Iyatomi (1976)
NIG17 Rec+
NIG45 Rec-
Table 2 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Rec-assay Bacillus subtilis 1-100% v/v 96.5% Negative (9) Shirasu et al., (1979)
H17 Rec+ dissolved in DMSO
M45 Rec-
Sister chromatide Chinese hamster 0.004-0.1 µl/ml 97.9% Positive (10) Putman (1987)
exchange ovary cells (nonact)
0.002-0.2 µl/ml Negative (10)
(act)
Dominant lethal Male NMRI/ORIG 1 x 5 mg/kg bw 94.9% Negative (11) Herbold (1980)
test Kissleg strain mice
Micro nucleus test Male/female Bor: 2 x 6 50% Negative (12) Herbold (1981
NMRI-mice 2 x 12 mg/kg bw (pre-mix)
(1) both with and without metabolic activation
(2) Positive control without activation: N-methyl-N-nitro-
N-nitrosoguanidina 10 µg/plate (TA1535) dexon; 50 µg/plate
(TA1537 and TA98); N-fluoren-2-yl-acetamide 50 µg/plate (TA98);
dimethylnitrosamine 1000 µg/plate (TA1535 and TA100;
furylfuramide 0.02 µg/plate (TA100) gave expected positive
response. Positive control with activation: N-fluoren-
2-yl-acetamide 50 µg/plate (TA98); dimethylnitrosamine 1000
µg/plate (TA1535 and TA100) gave expected positive response.
(3) Positive control without activation: 2-aminoanthracene 10
µg/plate (all strains); furylfuramide, 0.05 µg/plate (TA100),
0.25 µg/plate (WP 2 hcr), 0.1 µg/plate (TA 98); ß-propiolactone,
50 µg/plate (TA1538) gave expected positive response. Positive
control with activation: 2-aminoanthracene, 10 µg/plate (all
strains) gave expected positive response. Test compound gave
positive response with and without metabolic activation on
TA1535 strain.
(4) Positive control without activation: Quinacrine mustard, 50
µl/well (S138); ethyl methanesulfonate, 10 µl/well (S211) gave
expected positive response. Positive control with activation:
Cyclophosphamide 50 µg/well (S211) gave expected positive
response. 2-acetylaminofluorene, 10 µg/well (S138) did not give
positive response.
(5) Positive control without activation: Ethyl methanesulfonate, 0.2
µg/l gave expected positive response. Positive control with
activation: Benzo(a)pyrene, 4 µg/ml gave expected positive
response. Disulfoton was mutagen at concentrations that were
either insoluble or partially soluble in the medium. At
soluble concentrations disulfoton was not mutagenic in this
assay.
(6) Positive control without activation: Ethyl methanesulfonate (20
µl/ml) did not induce chromosome aneuploidy and did induce
mitotic recombination and chromosome deletions.
(7) Positive control with and without activation: methyl
methanesulfonate, 10 µl/plate gave expected positive response.
(8) Positive control: Mitimycyn C 0.3 µg/disc gave the expected
positive response.
(9) Positive control: Mitimycyn C 0.1 µg/disc gave the expected
positive response.
(10) Positive control without activation: Triethylenemelamine, 0.025
µg/ml gave the expected positive response. Positive control with
activation: Cyclophosphamide, 2.5 µg/ml gave the expected
positive response. Test material gave positive response at 0.1
µl/ml in the absence of metabolic activation.
(11) Concurrent positive control not conducted. Test material
administered once to mice by oral gavage.
(12) Positive control (TrenimonR 2 x 0.125 mg/kg bw) gave expected
positive response. Test material administered twice (24 hours
apart) to mice by oral gavage.
Special studies on metabolites
Rats
The sulfone metabolite of disulfoton was administered in a pilot
study to Fischer 344 rats (10/sex/level) by diet at concentrations of
0, 0.5, 0.75 or 1 ppm for six weeks to determine its possible effect
on cholinesterase activity. There were neither biologically
significant depression of brain cholinesterase activity at termination
of the study nor depressions of plasma and erythrocyte cholinesterase
activities (performed weekly) (Stuart, 1986a).
Dogs
Two identical studies were conducted on Beagle dogs to determine
the effect of disulfon metabolites on cholinesterases. The oxygen
analog sulfone metabolite of disulfoton was administered in the diet
to Beagle dogs (2/sex/level in each study) for 6 weeks at
concentrations of 0, 0.5, 0.75, and 1 ppm. Trivial differences were
observed on plasma and erythrocyte cholinesterase activities
(determined weekly throughout the study) and on brain cholinesterase
at termination of the study (Stuart, 1986b, 1986c).
Cows
Angus cattle (3/sex/level) were fed diets of alfalfa pellets
containing a mixture of the disulfoton metabolites sulfoxide, sulfone
and their oxygen analogs at a ratio of 1:2:1:1:, respectively. The
study was conducted for 31 days to determine the effect levels for
cholinesterase inhibition in whole blood. Diet concentrations tested
were 0 (4/sex), 3.6, 7.2, 10.8, and 18 ppm. Dose-related inhibition
of cholinesterase activity was observed at concentrations of 7.2 ppm
and greater. The NOEL for cholinesterase inhibition was 3.6 ppm
(Horton et al., 1975)
Holstein dairy cows (3/level) were fed alfalfa pellets containing
a mixture of disulfoton metabolites sulfoxide, sulfone, oxygen analog
sulfoxide and oxygen analog sulfone at a ratio of 1:2:1:1,
respectively. Animals received dietary concentrations of 3.6, 7.2, or
18 ppm for 28 days. A single untreated cow served as control. Blood
cholinesterase activity was determined before starting the study and
weekly throughout the duration of the study. Feed consumption, body
weight and milk production were also recorded. All measured
parameters were affected at the high- and medium-dose levels. A
trivial reduction of blood cholinesterase activity was noted at the
low-dose (30% decrease compared to 19% decrease in control). The NOEL
for cholinesterase inhibition was 3.6 ppm (Thornton, 1976).
Special studies on skin sensitization
Forty male guinea pigs (Pirbright White W 58) were treated with
technical disulfoton (purity 98.6%) to investigate sensitizing effect
on skin. The Magnusson and Kligman maximization test was used.
Evaluation showed that there were 2 positively reacting animals in the
test compound group as against one in the control group. There were
no indications of a skin sensitizing effect on guinea pigs for
disulfoton (Flucke, 1983).
COMMENTS
Disulfoton was previously evaluated by the JMPR in 1973 and 1975
and an ADI of 0-0.002 mg/kg bw was allocated. Disulfoton is rapidly
absorbed in rats after oral dosing and approximately 90% is excreted
via the urine within 24 hours. The biotransformation pathway consists
of hydrolysis and oxidation to metabolites such as disulfoton sulfone,
disulfoton oxon sulfoxide and disulfoton oxon sulfone.
Disulfoton has high acute oral toxicity to mice, rats and dogs.
It is classified by WHO as "extremely hazardous".
Cholinesterase inhibition and related clinical effects were the
only significant findings in long-term bioassays in mice and rats. In
a 99-week study in mice at dietary concentrations of 0, 1, 4, or 16
ppm, the NOAEL was 4 ppm, equal to 0.55 mg/kg bw/day. At the 16 ppm
concentration brain acetylcholinesterase inhibition was reported.
There was no evidence of carcinogenicity.
In a long-term study in rats at dietary concentrations of 0, 1,
4, or 16 ppm the NOAEL was 1 ppm, equal to 0.06 mg/kg bw/day. At
higher concentrations clinical signs of toxicity and inhibition of
plasma, erythrocyte and brain cholinesterase activities were observed.
No carcinogenic effect was detected.
In a 2-year study in dogs at dietary concentrations of 0, 0.5, 1,
or 2/5/8 ppm, the NOAEL was 1 ppm, equal to 0.03 mg/kg bw/day. At the
next highest dose inhibition of brain acetylcholinesterase was
observed. Treatment-related histo-pathological changes were not
found.
Disulfoton did not cause delayed neuropathy in adult hens.
Disulfoton was not teratogenic in rats nor rabbits. In rats
given 0, 0.1, 0.3 or 1 mg/kg bw/day, the NOAELs for embryotoxicity and
maternal toxicity were 0.1 and 0.3 mg/kg bw/day respectively. In
rabbits given 0, 0.3, 1 or 3/2/1.5 mg/kg bw/day, the NOAELs for
embryotoxicity and maternal toxicity were 1.5 and 0.3 mg/kg bw/day,
respectively.
In a 2-litter 2 generation reproduction study in rats at dietary
concentrations of 0, 1, 3 or 9 ppm, the NOAEL for toxicity was 3 ppm
(equivalent to 0.15 mg/kg bw/day), based on signs of maternal toxicity
at 9 ppm. The NOAEL for reproductive effects was 1 ppm (equivalent to
0.05 mg/kg bw/day) based on decreased brain acetylcholinesterase,
body-weight gain and survival of pups at 3 ppm.
Although there was one positive reverse mutation assay it was
concluded, after review of all available in vivo and in vitro
genotoxicity data, that there was no evidence of genotoxicity.
The human volunteer study reviewed by the 1975 JMPR was reported
in summary form only and was considered inadequate for the estimation
of an ADI.
The ADI was based on the 2-year study in dogs, using a 100-fold
safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 4 ppm in the diet, equal to 0.55 mg/kg bw
Rat: 1 ppm in the diet, equal to 0.06 mg/kg bw
Dog: 1 ppm in the diet, equal to 0.03 mg/kg bw
Man: 0.75 mg/man/day, equivalent to 0.01 mg/kg bw
Estimate of acceptable daily intake for humans
0-0.0003 mg/kg bw.
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans.
REFERENCES
Brusick, D.J. (1981) Mutagenicity evaluation of S276 in the mitotic
non-disjunction in Saccharomices cerevisiae strain D6. Unpublished
Report R 2086 from Litton Bionetics Inc., MD, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Carpy, S. & Klotzsche, C. (1975) Disulfoton 2-year feeding study in
rats. Unpublished Report AGRO DOK CBK 1854/75 from Sandoz Ltd.,
Basel, Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1983) S 276 (Disulfoton, Disyston active ingredient)
Study for skin-sensitising effect with guinea pigs. Unpublished
Report 12121 from Bayer AG, Institute of toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Flucke, W. (1986) S 276 technical - Study of subacute dermal toxicity
to rabbits. Unpublished Report 14747 from Bayer AG, Institute of
toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1983) Oncogenicity study of Disulfoton technical on
mice. Unpublished Report 413 from Mobay Chemical Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985) Chronic feeding/oncogenicity study of technical
Disulfoton (Di-Syston) with rats. Unpublished Report 638 from Mobay
Chemical Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1980) S 276, Disulfoton, Disyston active ingredient.
Dominant lethal test on male mouse to evaluate S 276 for mutagenic
potential. Unpublished Report 9440 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1981) S 276, Disulfoton, Disyston active ingredient.
Micronucleus test on the mouse to evaluate S 276 for mutagenic effect.
Unpublished Report 10451 from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) S 276, Disulfoton, Disyston active ingredient. Pol
test on E. coli to evaluate for potential DNA damage. Unpublished
Report 12139 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Hixson, E.J. (1983) Acute delayed neurotoxicity study on Disulfoton.
Unpublished Report 365 from Mobay Chemical Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Hixson, E.J. & Hathaway, T.R. (1986) Effect of Disulfoton (Di-syston)
on reproduction in rats. Unpublished Report 711 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Weischer, C.H. (1975) S 276 (disulfoton) Chronic
toxicity study on dogs (two-year feeding experiment). Unpublished
Report 5618 from Bayer AG, Institute of Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Horton, J.R., Thornton J.S. & Lichtenstein, H.C. (1975) The subacute
oral toxicity of a Di-syston metabolite mixture administered in the
feed to cattle. Unpublished Report 45288 from Chemagro Corporation,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Inukai, H. & Iyatomi, Y. (1976) Disulfoton. Mutagenicity test on
bacterial systems. Unpublished Report 29 from Nitokuno Agricultural
Chemicals Institute, Toyoda, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Iyatomi (1980) Report of acute toxicity. Disulfoton. Unpublished
Report A-29 from Nitokuno, Agricultural Chemicals Institute, Tokyo,
Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Jagannath, D.R., (1981) Mutagenicity evaluation of S 276 in
Saccharomyces cerevisae reverse mutation induction assay.
Unpublished Report R 2087 from Litton Bionetics, Inc. MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Wells N., Lawrence L.J. (1987)
Quantitative characterization of residues in tissues and eggs of
laying hens treated orally for three consecutive days with (14C)
Di-syston-ethylene. Unpublished Report MR 98435 from Pharmacology
and Toxicology Research Laboratory, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Krautter, G.R., Marsh J.D., Downs J., Lawrence L.J. (1988)
Metabolism of (14C) Di-syston in the lactating goat. Unpublished
Report MR97499 from Mobay Corporation, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Lamb, D.W. & Hixson, E.J. (1983) Embryotoxic and teratogenic effects
of disulfoton. Unpublished Report 376 from Mobay Chemical
Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K., Hanna L.A., Johnston K. & Ose, K. (1985) Excretion and
metabolism of Di-syston in rats. Unpublished Report MR90946 from
Mobay Corporation, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Mihail, F. (1978) Acute toxicity studies. Unpublished Report 7602
from Bayer AG, Institute of toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Putman, D.L. (1987) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. Unpublished Report 969 from
Microbiological Associates, Inc., MD, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988) Pilot study to assess cholinesterase activity
in rats exposed by inhalation to technical grade Disulfoton.
Unpublished Report 1073 from Mobay Corporation, USA. Submitted to WHO
by Bayer, Leverkusen, Germany.
Shiotsuka, R.N. (1989) Subchronic inhalation toxicity study of
technical grade Disulfoton (Di-syston) in rats. Unpublished Report
1131 from Mobay Corporation, USA. Submitted to WHO by Bayer,
Leverkusen, Germany.
Shirasu, Y. et al. (1979) Ethylthiometon - Mutagenicity test on
bacterial systems. Unpublished Report from Institute of Environmental
Toxicology, Japan. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986) Pilot study on DI-SYSTON sulfone with rats.
Unpublished Report 85-971-02 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Stuart, B.P. (1986a) Pilot study on DI-SYSTON oxygen analog sulfone
with dogs. Unpublished Report 85-974-02 from Mobay Corporation, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Stuart B.P. (1986b) Pilot study on DI-SYSTON sulfone with dogs.
Unpublished Report 85-974-01 from Mobay Corporation, USA. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. (1982) S 276-Effects of oral administration upon pregnancy
in the rabbit. Unpublished Report 2351 from Life Science Research,
England. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Tesh, J.M. & Ross, F.W. (1981) S 276-Effects of oral administration
upon pregnancy in the rabbit. 1. Preliminary study. Unpublished LSR
Report BAG010 from Life Science Research, England. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Thornton, J.S. (1976) Effect of feeding DI-SYSTON metabolites to
dairy cattle. Unpublished Report MR49100 from Chemagro Agriculture
Division, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. (1978) S 276 (Disyston active ingredient) Acute
inhalational toxicity studies. Unpublished Report 7827 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Thyssen J. (1980) Disulfoton (S 276) The active ingredient of
Di-syston Subacute inhalation study on rats. Unpublished Report 9065
from Bayer AG, Institute of Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Yang, Li L. (1988) Disyston technical-CHO/HGPRT assay. Unpublished
Report 994 from Microbiological Associates, Inc. MD, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
