
DISULFOTON JMPR 1975
Explanation
Disulfoton was evaluated by the Joint Meeting in 1973 (FAO/WHO,
1974). In the light of data then available tolerances for vegetables,
cereals (except rice) and some other food and feed crops were
recommended. Further work or information was required on the
occurrence of residues in meat, milk and eggs after feeding animals
with disulfoton in order to recommend residue limits in food of animal
origin. In addition, information on residues in food moving in
commerce was mentioned as desirable. None of this information has been
received.
Revised national tolerances and pre-harvest intervals as well as
studies an the fate of residues in rats have become available and are
summarized in the following monograph addendum.
Disulfoton is a member of the demeton family of insecticides.
Disulfoton was reviewed by the 1973 Joint Meeting. the basis of
studies available at the time primarily short-term studies, a
temporary Acceptable Daily Intake for man was estimated to be 0-0.001
mg/kg (FAO/WHO, 1974). At that time it was understood that long-term
feeding studies in rats and dogs had been initiated but were not
complete. Further, it was required that kinetic studies on absorption,
distribution metabolism and excretion be performed in mammals and an
evaluation be made of liver damage observed in short-term studies with
disulfoton sulfoxide. In addition, data was requested on residues in
meat, milk and eggs after feeding animals on crops or foodstuffs
treated with disulfoton in order to determine residue limits in foods
of animal origin. Portions of these requirements have been met and the
new work has been summarized in the following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Disulfoton, 14C-labelled in the O-ethyl position was orally
administered to 2 male rats at a dose of 1.2 mg/kg and to 2 female
rats a dose of 0.2 mg/kg. Urine, faeces and expired air were monitored
over a 10 day interval. The major quantity of 14C-activity was
observed in urine with males excreting 14C faster than females. In
males and females respectively, after 10 days; 14C radioactivity was
found in urine at 84 and 79%, faeces at 6 and 8% and as 14CO2 at 9
and 9%. Total recovery of the administered dose was noted within 96
hours of dosing. The excretion rate was different in males and females
with males excreting one-half the dose in 4-6 hours while females
excreted one-half the dose in 30-32 hours. Peak levels in tissues and
blood were reached at about 6 hours. The liver of females contained
higher (4-fold) relative tissue level than males. These differences
might be related to the differences in dosage levels or to the greater
susceptibility of females to acute toxicity of disulfoton.
Urinary metabolites were characterized predominantly as water
soluble component diethyl phosphate and diethyl thiophosphate. Organic
soluble components (<3% of the dose) was characterized as the oxygen
analogue sulfoxide, disulfoton sulfoxide, disulfoton oxygen analogue
sulfone and an unknown in the following proportions (56:3:31:10).
Similar metabolites were observed as small residues in tissue,
predominantly liver. There was no sex differentiation with respect to
metabolism. A distinct sex difference with respect to excretion was,
however, observed (Puhl and Fredrickson, 1975).
Effects on enzymes and other biochemical parameters
Using cell culture techniques to examine cytotoxicity and
susceptibility or changes in cells to infections by virus or to
destruction by toxin, Gablicks and Friedman (1969) reviewed and
reported on the increased susceptibility or sensitivity of cell
cultures exposed to disulfoton. Cytotoxicity was observed with
disulfoton in both change liver cells and in Hela cells. The effect of
RNA, DNA and protein in Hela cells was examined (Litterst, et al.,
1969). At 60 ppm growth was inhibited 50%. At 10 ppm nucleic acid
synthesis was unaffected while protein systhesis was increased. Other
studies have demonstrated the dose related inhibitory effect of
disulfoton on growth of cells in culture. Huang (1973) observed that
chromosomes were not damaged by disulfoton and the inhibited cell
growth was readily resumed on removal of disulfoton. Cells in culture
exposed to disulfoton showed greater sensitivity to polio virus but
not to diphtheria toxin. Polio virus-treated cells exposed to
disulfoton were more susceptible than the virus treated cells not
exposed (Gablicks and Friedman, 1969). Following short-term
administration of disulfoton to mice at higher levels (1/2 LD50) for
10 days, a reduced barbiturate sleeping time and increased oxidation
rate was noted in liver preparations and disulfoton was found to
induce hepatic enzymes (Stevens, et al., 1972). Clark and Stavinoha
(1969; 1971) also suggested changes in permeabilities of nervous
tissue following 60 days dietary treatment in the rat (50 ppm) and
mice (150 ppm). McPhillips et al. (1969) examined the effect of
disulfoton on the physiological response of rats and confirmed
previous findings on the reduction of sensitivity to cholinergic drugs
by disulfoton. In addition the atrium of disulfoton-treated rats also
developed reduced sensitivity to carbacol. These workers showed that
subsensitivity induced by disulfoton did not occur in all tissues and
cholinesterase inhibition did not relate to subsensitivity (Foley and
McPhillips, 1972, 1973; McPhillips, 1969; McPhillips and Dar, 1967).
The full significance of these interactions has not been defined.
Interactions
Adult rats and mice pretreated with phenobarbital to induce liver
microsomal oxidizing enzymes were found to tolerate higher acute doses
than untreated animals.
LD50 (mg/kg)
Species PB-Treated Untreated
Rats 17.0 2.1
Mice 16.3 6.7
(DuBois and Kinoshita, 1968)
Administration of disulfoton at high levels (up to 1/2 LD50
values) resulted in increased elimination of catecholamines rat urine.
Increased adrenaline and noradrenaline levels in urine were observed
soon after treatment and the levels returned to normal more rapidly
than cholinesterase activity (Brzezinski and Ludwicki, 1973). In
addition, a metabolite of adrenaline and noradrenaline (4-hydroxy,
3-methoxymandelic acid) was found to have increased in rats poisoned
with disulfoton (Wysocka-Paruszewska, 1970). It was suggested that the
excretion of the mandelic acid derivative might be related to the
toxic action of disulfoton (Wysocka-Paruszewska, 1971).
TOXICOLOGICAL STUDIES
Special studies on behaviour
In an extensive series of papers Clark and coworkers (Clark, et
al, 1971; Clark and Pearson, 1973) examined the effect of disulfoton
on rodent behaviour. After 8 weeks of exposure to disulfoton in the
diet at 150-200 ppm, mice were examined for behaviour changes. Treated
mice were different from control mice with respect to a head insertion
test. With rats, again a significant difference was observed in
behaviour testing when disulfoton was administered in the diet,
Treated rats made fewer errors and had shorter running times than
controls. No differences in 3 treated groups (10, 25 and 50 ppm) were
noted with respect to learning but each was greater than controls.
Special studies on the metabolites
Rat
Groups of rats (5 males and 5 females/group) were exposed to
cigarette smoke for 1 hour/day 5 days/week for 3 weeks. The cigarette
tobacco had been treated with disulfoton sulfoxide, demeton-S sulfone
and MocapR in two different mixture concentrations. The first
mixture contained 2.25 mg disulfoton sulfoxide, 1.75 mg demeton-S
sulfone and 0.02 mg MocapR/kg tobacco. The second mixture contained
11.25 mg, 8.75 mg and 0.1 mg of each respectively. Utilizing a
reverse-puff smoking machine, cigarettes made from the treated tobacco
were burned and the smoke introduced to an exposure chamber. No
analyses of the chamber were made to confirm the presence of residues
although smoke concentration was determined. No adverse effects of
this programme were noted with respect to behaviour, growth,
hematology, clinical chemistry and urinalysis. Gross and microscopic
analysis of tissues and organs indicated no unusual pathology.
Cholinesterase activity of plasma, RBC and brain measured at 21 days
was not significantly depressed. A slightly depressed cholinesterase
value of RBC in both males and females was not significantly different
from controls but was dose dependent suggesting that an enzyme
inhibitor or its precursor inhibitor did actually get to the animals
in the smoke stream (Brewer, 1975).
Cattle
Groups of Angus steers and heifers were orally treated with a
mixture of metabolites of disulfoton for periods up to 42 days. The
mixture ratio was 60:40 disulfoton metabolites to demeton-S
metabolites with the ratio of each Isomer being 1:2. Five groups of
equal numbers of males and females were administered doses of 0,
0.045, 0.09 0.18 and 0.36 mg/kg daily. The 0.36 mg/kg group (2 of each
sex) were treated for 28 days; the 0.18 mg/kg group (2 of each sex)
the 0.09 mg/kg group (3 of each sex) and the other two groups (3 of
each sex) were treated for 42 days.
In the two highest groups (0.36 and 0.18 mg/kg), signs of
poisoning were evident and two animals of the high level died.
Cholinesterase (whole blood) activity was measured weekly over the 7
week treatment for 12 weeks after the treatment ended. Depression was
noted in cholinesterase activity at levels of 0.09 mg/kg and above.
Cholinesterase activity slowly recovered to normal in the surviving
animals (generally within 3 weeks of the end of treatment). A
no-effect level for this mixture in cattle was 0.045 mg/kg (Crawford
and Anderson, 1974b).
Sheep
Mixed breed male and female sheep were orally treated with a
mixture of metabolites of disulfoton as described above under
"Cattle". Groups of sheep (2 males and 2 females per group) were
administered the mixture at dose levels of 0, 0.045 and 0.09 mg/kg
daily. One female of the 0.09 mg/kg dosed group died. Behaviour and
body weight were not affected. Slight cholinesterase depression was
noted in the upper level treatment group over the course of the study
(Crawford and Anderson, 1974 b).
Short-term studies
Dogs
Groups of beagle dogs (4 males and 4 females/group) were fed
disulfoton in the diet for two years at dosage levels of 0, 5, 1.0
ppm. A fourth group was fed 2.0 ppm from weeks 1 to 69; 5.0 ppm from
weeks 70 to 72; 8.0 ppm from 72 to 104. There was no compound related
mortality noted during the course of the study. Behaviour and growth
were normal at all dose levels.
Ophthalmological examinations, clinical chemistry, haematology
and urinalyses were normal over this time period. Plasma and RBC
cholinesterase were examined periodically over the course of the study
and at the conclusion brain cholinesterase activity was determined. No
depression was noted in plasma, RBC or brain cholinesterase activity
in the 1.0 ppm group of males and females. At 2.0 ppm and above
depression was noted in plasma and RBC. This depression was
accentuated at the time of increased dosing and was evident at the
conclusion of the study in brain cholinesterase depression (at 8.0
ppm). At the conclusion of the study gross and microscopic examination
of tissues and organs revealed no abnormalities. A no-effect level in
this study is 1.0 ppm in the diet (0.0266 mg/kg body weight) (Hoffmann
and Weischer, 1975).
ACUTE TOXICITY
Species Sex Route LD50 (mg/kg) References
Disulfoton
Rat F Oral 2.0 Crawford &
M Oral >2.0 Anderson, 1974a
Disulfoton Sulfoxide
Rat F Oral 1.7 "
M Oral >1.7 "
Disulfoton Sulfone
Rat F Oral 1.24 "
M Oral >1.24 "
Demeton-S (isosystox)
Rat F Oral 1.17 "
M Oral >1.17 "
Demeton-S Sulfoxide
Rat F Oral 1.24 "
M Oral >1.24 "
Demeton-Sulfone
Rat F Oral 1.10 "
M Oral >1.10 "
Long-term studies
Rat
Groups of rats (60 males and 60 females/group) were fed
disulfoton in the diet for two years at dose levels of 0, 0.5 (from
weeks 0-80), 1.0, 2.0 and 5.0 (from weeks 80 to 104) ppm. Food
consumption behaviour and growth were normal. Haematology, clinical
chemistry and urinalysis parameter were unaffected. Plasma, RBC and
brain cholinesterase inhibition were evident at the higher dose
levels. In males, a slight blood cholinesterase depression (15%) was
noted at 2 ppm while in females this slight depression (12%) was seen
at 1 ppm. The reduction in enzyme activity was dose dependent and
significant (>20%) above the 2 ppm level. Brain cholinesterase was
depressed in a dose dependent manner with significant depression
(>20%) observed at 5 ppm in males and at 2 ppm and above in females.
Liver esterase activity was slightly affected at the highest level
only. Gross and microscopic analysis showed no differences from
controls. No changes in liver epithelial cells were mentioned. A no
effect level in this study was 1.0 ppm (Klotzsche, 1975).
COMMENTS
Disulfoton is rapidly absorbed and excreted primarily in urine.
The metabolic scheme reviewed and reported by the 1973 Meeting has
been further supported by new studies. Toxicological studies of
combinations of metabolites administered to animals directly, as with
cattle and sheep or via inhalation as with a rat cigarette smoke
study, confirm approximately the no-effect level in rat and dog.
These studies suggest that toxicological studies of the
combinations are similar to studies of the parent material confirming
the conclusion of rapid metabolism in animals of disulfoton to active
antesterase metabolites. In cell culture, protein synthesis is
reversibly inhibited at 10 ppm and disulfoton is cytotoxic at 60 ppm.
Results of two year studies, particularly the long-term toxicity
studies and the metabolic data, confirm the previously suggested ADI
for man.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect in animals
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw
Dog: 1 ppm in the diet equivalent to 0.025 mg/kg bw
Estimate of acceptable daily intake for man
0-0.002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
FATE OF RESIDUES
In animals
Feeding studies were carried out in rats using
diethyl-1-14C-labelled disulfoton. A single dose of 1.2 mg/kg (male)
and of 0.2 mg/kg (female) was administered directly into the stomach
(Puhl and Fredrickson, 1975). Each of the doses corresponded to 10% of
the LD50 value (FAO/WHO, 1974). The animals were allowed food and
water ad libitum throughout the experiments. 14C was distributed as
shown in Table 1.
TABLE 1. Recovery (%) of total 14C from rats dosed with disulfoton
% of administered 14C found in
Urine Faeces Expired CO2
Period after
treatment
(hrs) male female male female male female
0 - 4 41.6 6.2 - Na
4 - 8 19.3 5.0 1.6 Na
8 - 12 6.0 12.4 1.3 Na
12 - 24 6.9 19.9 1.6 2.6 6.9b 3.2b
24 - 48 5.6 17.8 0.9 2.5 0.8 1.6
48 - 72 2.2 7.0 0.4 1.4 0.6 1.2
72 - 144 2.0 7.7 0.3 1.2 0.7 1.9
144 - 240 0.7 2.9 0.2 0.2 1.3
Totals 84.3 78.9 6.1 7.8 9.2 9.2
Combined recovery: Urine + faeces + CO2, male = 99.6%
female = 95.9%
a No faeces excreted.
b The first sample was for the period 0-24 hrs.
Tissues and blood were analysed for 14C (disulfoton equivalents)
at various time intervals. Peak levels occurred after about six hours
in both male and female rats. Residues in liver were highest, reaching
a peak of about 3.6 mg/kg for male and 2.3 mg/kg for female rate. The
relative magnitude of peak residues in other samples (male-female)
fell in the order kidney (1.4-0.8), plasma (0.8-0.15), fat
(0.45-0.08), whole blood (0.39-0.01), skin (0.3-0.05), muscle
(0.13-0.01) and brain (0.08-0.01). Ten days after administration, the
residue ranges were as follows. Liver (0.154-0.119), kidney
(0.051-0.026), heart (0.016-0.004), fat (0.090-0.009), muscle
(0.012-0.002), brain (0.015-0.006), skin (0.037-0.006) and blood
(0.007-0.002). It should be borne in mind that the dose for female
rats was only one-sixth of the dose for male rats.
Excretory pathways were similar for males and females but the
rate of excretion appeared to be slower for females. Diethyl phosphate
(DEP) and diethyl phosphorothionate (DEPT) comprised 93% of the 14C
while minor urinary metabolites included demeton-S sulfone (POSO2)
and disulfoton sulfoxide (PSSO). Depending upon the sex of the animals
and the time after administration 19-48% of the total 14C in the
livers was water-soluble and 0.3-3.4% was soluble in chloroform. The
respective data for kidneys were 39-78% and 0.5-5.6%.
Chloroform-soluble oxidation products in liver and urine were POSO,
PSSO and POSO2. Non-extractable residues in liver may be
diethylphosphorylated protein.
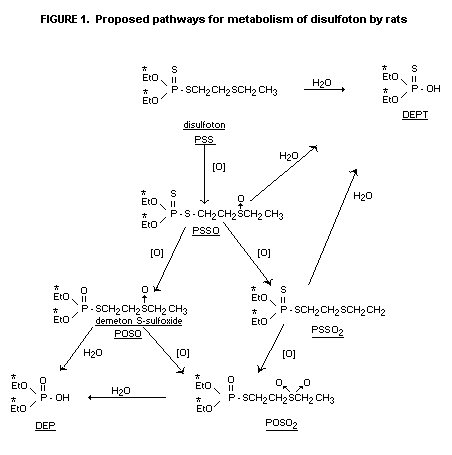 TABLE 2. National tolerances reported to the Meeting
(Changes and additions since last evaluation)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Argentina Lettuce, peanuts, potatoes, 0.75
cabbage, cauliflower, spinach,
tomatoes, pecans, barley,
rice, maize (fodder),
cottonseed
Sugarcane, coffee, maize 0.3
Sugar beet (roots) 0.5
Wheat 0.2
Sugar beet (leaves) 2.0
Alfalfa and clover (hay) 12.0
Carrots 0
Alfalfa (fresh), rye, wheat, 5.0
oats, barley (fodder), clover
(fresh) peanuts (fodder)
Canada Broccoli, cabbage 0.75
Beans (dry, lima, snap), 0.5
Brussels sprouts, cauliflower,
lettuce, peas, spinach,
tomatoes
Potatoes 0.2
Barley (grain), coffee, corn N.R.
(grain) (incl. field corn,
sweet corn [kernels plus cob
with husk removed] & popcorn),
cottonseed, eggplant, oats
(grain), peanuts, peppers,
pineapple, rice (grain), sugar
beets, wheat (grain)
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Federal Potatoes 0.2
Republic of
Germany Cereals 0.1
(disulfoton,
disulfoton-sulfoxide,
disulfoton-sulfone, demeton,
demeton-sulfoxide,
demeton-sulfone: calculated
together as disulfoton)
Hungary General 0.2 56
Japan Rice, fruit, vegetables 0.1
beans, potatoes
Rice 50
Citrus fruit 30
Eggplants, tomatoes 21
Soybeans, azukibeans, garden 60
pea, kidney bean, broad bean
Netherlands Potatoes 0.01
All other crops 0
New Zealand Potatoes 91
Switzerland Lettuce 0.2
Vegetables, field crops 42
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
United States Alfalfa (hay), clover (hay) 12.0
of America
Alfalfa (fresh), barley (green 5.0
fodder, straw), beans (vines),
clover (fresh), corn forage &
fodder (incl. field corn,
sweet corn and popcorn), oats
(green fodder, straw), peanuts
(hay), peas (vines), pineapple
(foliage), rice (straw),
sorghum fodder & forage, sugar
beets (pulp, feed additive),
wheat (green fodder, straw)
Sugar beets (tops) 2.0
Barley (grain), beans (lima 0.75
dry, snap), broccoli,
Brussels sprouts, cabbage,
cauliflower, cottonseed,
lettuce, oats (grain),
peanuts, pecans, peas,
pineapple, potatoes, rice
(grain), sorghum (grain),
spinach, tomatoes
Hops, sugar beets 0.5
Coffee corn grain (incl. field 0.3
corn, sweet corn [kernels plus
cob with husk removed] & popcorn),
sugar-cane (raw), wheat
(grain)
Soybean forage & hay 0.25
Peppers, soybeans 0.1
Yugoslavia General 0
Field crops 60
APPRAISAL
Disulfoton was evaluated at the 1973 Joint Meeting (FAO/WHO
1974). Reviewing the data then available the Meeting required
information on the occurrence of residues in meat, milk and eggs after
feeding animals with disulfoton in order to recommend maximum residue
limits in food of animal origin. In addition, information on residues
in food moving in commerce was mentioned as desirable. None of this
information has been received.
Revised national tolerances and pre-harvest intervals as well as
studies on the fate of residues in rats have become available.
Fourty-eight and 72 hours after oral application of a single dose of
diethyl-l-14C-labelled disulfoton (0.2 mg/kg female rat and 1.2 mg/kg
male rat) 71% and 81% respectively of the administered 14C was
excreted in the urine and faeces and expired as carbon dioxide by the
females. The corresponding figures for males were 93% and 96%. Ten
days after dosing, 96% (female) and 100% (male) of the 14C had been
excreted. Diethyl phosphate and diethyl phosphorothionate were
identified as major metabolites. Peak levels of 14C in tissues
occurred about 6 hours after dosing, reaching maximum levels
(disulfoton equivalents) of 3.6 mg/kg in liver, 1.4 mg/kg in kidney,
0.45 mg/kg in fat, 0.3 mg/kg in skin, 0.13 mg/kg in muscle and 0.08
mg/kg in brain. Ten days after administration maximum residues in
tissues and blood ranged from <0.01 to 0.15 mg/kg. A maximum of 3.4%
of the residues in liver were chloroform-soluble metabolites
(demeton-S sulfoxide, disulfoton sulfoxide and demeton-S sulfone).
Since demeton-S sulfoxide and demeton-S sulfone are metabolites
of disulfoton as well as of demeton, it is difficult or impossible to
determine which parent compound is responsible for residues of them.
Further explanation is given in the Appraisal of demeton (see this
Meeting Report).
For residue analysis, oxidation of the residues and determination
of demeton sulfone and disulfoton sulfone by the GLC method previously
described (FAO/WHO, 1974) should be suitable for regulatory purposes.
RECOMMENDATIONS
New recommendations (alfalfa [hay], clover [hay], maize) together
with previous recommendations as set out-below refer to the total
residue of disulfoton, disulfoton sulfoxide, disulfoton sulfone,
demeton, demeton sulfoxide and demeton sulfone calculated and
expressed as disulfoton.
Maximum
Commodity Residue Limit
(mg/kg)
Alfalfa (hay), clover (hay) 10
Forage crops (green) 5
Vegetables including beans, broccoli,
Brussels sprouts, cabbage, cauliflower,
celery, lettuce, maize, potatoes, peanut
shells, peas (inc. pods), rice (in husk),
spinach, sugar beets (roots), tomatoes 0.5
Raw grain (except rice and maize) 0.2
Coffee beans, pecans, peanuts (kernels),
pineapple, soybeans 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Residue data from supervised trials for commodities not
mentioned above, but included in national tolerance lists.
2. Results from studies now in progress (expected in the spring
of 1976) on residues in meat, milk and eggs after feeding
animals on fodder treated with disulfoton in order to
determine residue limits in food of animal origin.
3. Information on residues in food moving in commerce.
REFERENCES
Brewer, W. E. (1975) 21-day subacute inhalation toxicity study with
di-syston sulfoxide, di-syston oxygen Analog, sulfone and mocap in
rat. Unpublished report from Industrial Biotest Laboratories submitted
to the World Health Organization by Bayer AG.
Brzezinski, J. and Ludwicki, K. (1973) The interrelationship of the
changes of acetylcholinesterase and catecholamines, blood and urine
levels in rats poisoned with di-syston. Pol. Pharmacol Pharm. 25:
313-316
Clark, G. and Stavinoha, W. B. (1969) Alterations in liver RNA induced
by atropine and disulfoton. Tox. Appl. Pharmacol. 14: 376-79.
Clark, G. and Stavinoha, W. B. (1971) A permeability change in CNS
tissue in chronic poisoning with Disulfoton. Life Sci. 10: 421-23.
Clark, G., Koester, A.G. and Pearson, D. W. (1971) Exploratory
behaviour in chronic Disulfoton poisoning in mice. Psychapharmacologia
(Berl.) 20: 169-71.
Clark, G. and Pearson, D. W. (1973) Learning in chronic disulfoton
poisoning. J. Gen. Psychol. 89: 305-11.
Crawford, C. R. and Anderson, R. H. (1974a) The acute oral toxicity of
several Di-Syston metabolites to female and male rats. Unpublished
Report from Mobay Chemical Corporation submitted to the World Health
Organization by Bayer AG.
Crawford, C. R. and Anderson, R. H. (1974b) Subacute oral toxicity of
a mixture of four Di-Syston metabolites to cattle and sheep.
Unpublished Report from Mobay Chemical Corporation. Submitted to the
World Health Organization by Bayer AG.
DuBois, K. P. and Kinoshita, F. K. (1968) Influence of induction of
hepatic microsomal enzymes by phenobarbital on toxicity of organic
phosphate insecticides. Proc. Soc. Exper. Biol. Med. 129: 699-702.
Echobichon, D. J. and Kalow, W. (1963) Action of organophosphorus
compounds upon esterases of human liver. Can. J. Biochem. Physiol. 41:
1537-46.
Foley, D. J. and McPhillips, J. J. (1972) Relationship between
cholinesterase activity and sensitivity to cholinergic drugs in the
rat ileum. Tox. Appl. Pharmacol. 22: 286-87.
Foley, D. J. and McPhillips, J. J. (1973) Response of the rat ileum
uterus and vas deferens to carbacol and acetyl choline following
repeated daily administration of a cholinesterase inhibitor. Brit. J.
Pharmacol. 48: 418-25.
Gablicks, J. and Friedman, L. (1969) Effects of insecticides on
mammalian cells and virus infections. Ann. N.Y. Acad. Sci. 160: Art.
1: 254-71.
Hoffmann, K. and Weischer, C. H. (1975) S-276. (Disulfoton) Chronic
toxicity study on dogs (two year feeding experiments). Unpublished
report from the Institute for Toxicology submitted to the World Health
Organization by Bayer AG.
Huang, C.C. (1973) Effect on growth but not chromosomes of the
mammalian cells treatment with three organophosphorus insecticides.
Proc. Soc. Exp. Biol. Med. 142: 36-40.
Klotzsche, C. (1975) Disulfoton. 2-year Feeding Study in Rats.
Unpublished report from Agrochemical Research Department. Submitted to
the World Health Organization by Sandoz Ltd.
Litterst, C. L., Lichtenstein, E. P. and Kajiwara, K. (1969) Effects
of insecticides on growth of Hela cells. J. Agr. Food Chem. 17:
1199-1203.
McPhillips, J. J. (1969) Altered sensitivity to drugs following
repeated injections of a cholinesterase inhibitor to rats. Toxicol.
Appl. Pharmacol. 14: 67-73.
McPhillips, J. J. and Dar, M. S. (1967) Resistance to the effect of
Carbacol on the cardiovascular system and on the isolated ileum of
rats after subacute administration of an organophosphorus
cholinesterase inhibitor. J. Pharmacol. Exp. Therap. 156: 507-13.
Puhl, R. J. and Fredrickson, D. R. (1975) The metabolism and excretion
of Di-Syston by rats. Unpublished report from Mobay Chemical
Corporation. Submitted to the World Health Organization by Bayer AG.
Stevens, J. T., Stitzel, R. E. and McPhillips, J. J. (1972) The
effects of subacute administration of anticholinesterase insecticides
on hepatic microsomal metabolism. Life Sci. II (part II): 423-31.
Wysocka-Paruszewska, B. (1970) The urine level of 4-hydroxy-3-methoxy
mandellic acid in urine of rats poisoned with phosphorus organic
insecticides. Dissert. Pharm. Pharmaco. 22: 485-89.
Wysocka-Paruszewska, B. (1971) The changes in urine level of
4-hydroxy-3-methoxy mandelic acid in rats related to the degree of
intoxication with Di-syston. Dissert. Pharm. Parmocol. 22: 271-74.
TABLE 2. National tolerances reported to the Meeting
(Changes and additions since last evaluation)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Argentina Lettuce, peanuts, potatoes, 0.75
cabbage, cauliflower, spinach,
tomatoes, pecans, barley,
rice, maize (fodder),
cottonseed
Sugarcane, coffee, maize 0.3
Sugar beet (roots) 0.5
Wheat 0.2
Sugar beet (leaves) 2.0
Alfalfa and clover (hay) 12.0
Carrots 0
Alfalfa (fresh), rye, wheat, 5.0
oats, barley (fodder), clover
(fresh) peanuts (fodder)
Canada Broccoli, cabbage 0.75
Beans (dry, lima, snap), 0.5
Brussels sprouts, cauliflower,
lettuce, peas, spinach,
tomatoes
Potatoes 0.2
Barley (grain), coffee, corn N.R.
(grain) (incl. field corn,
sweet corn [kernels plus cob
with husk removed] & popcorn),
cottonseed, eggplant, oats
(grain), peanuts, peppers,
pineapple, rice (grain), sugar
beets, wheat (grain)
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Federal Potatoes 0.2
Republic of
Germany Cereals 0.1
(disulfoton,
disulfoton-sulfoxide,
disulfoton-sulfone, demeton,
demeton-sulfoxide,
demeton-sulfone: calculated
together as disulfoton)
Hungary General 0.2 56
Japan Rice, fruit, vegetables 0.1
beans, potatoes
Rice 50
Citrus fruit 30
Eggplants, tomatoes 21
Soybeans, azukibeans, garden 60
pea, kidney bean, broad bean
Netherlands Potatoes 0.01
All other crops 0
New Zealand Potatoes 91
Switzerland Lettuce 0.2
Vegetables, field crops 42
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
United States Alfalfa (hay), clover (hay) 12.0
of America
Alfalfa (fresh), barley (green 5.0
fodder, straw), beans (vines),
clover (fresh), corn forage &
fodder (incl. field corn,
sweet corn and popcorn), oats
(green fodder, straw), peanuts
(hay), peas (vines), pineapple
(foliage), rice (straw),
sorghum fodder & forage, sugar
beets (pulp, feed additive),
wheat (green fodder, straw)
Sugar beets (tops) 2.0
Barley (grain), beans (lima 0.75
dry, snap), broccoli,
Brussels sprouts, cabbage,
cauliflower, cottonseed,
lettuce, oats (grain),
peanuts, pecans, peas,
pineapple, potatoes, rice
(grain), sorghum (grain),
spinach, tomatoes
Hops, sugar beets 0.5
Coffee corn grain (incl. field 0.3
corn, sweet corn [kernels plus
cob with husk removed] & popcorn),
sugar-cane (raw), wheat
(grain)
Soybean forage & hay 0.25
Peppers, soybeans 0.1
Yugoslavia General 0
Field crops 60
APPRAISAL
Disulfoton was evaluated at the 1973 Joint Meeting (FAO/WHO
1974). Reviewing the data then available the Meeting required
information on the occurrence of residues in meat, milk and eggs after
feeding animals with disulfoton in order to recommend maximum residue
limits in food of animal origin. In addition, information on residues
in food moving in commerce was mentioned as desirable. None of this
information has been received.
Revised national tolerances and pre-harvest intervals as well as
studies on the fate of residues in rats have become available.
Fourty-eight and 72 hours after oral application of a single dose of
diethyl-l-14C-labelled disulfoton (0.2 mg/kg female rat and 1.2 mg/kg
male rat) 71% and 81% respectively of the administered 14C was
excreted in the urine and faeces and expired as carbon dioxide by the
females. The corresponding figures for males were 93% and 96%. Ten
days after dosing, 96% (female) and 100% (male) of the 14C had been
excreted. Diethyl phosphate and diethyl phosphorothionate were
identified as major metabolites. Peak levels of 14C in tissues
occurred about 6 hours after dosing, reaching maximum levels
(disulfoton equivalents) of 3.6 mg/kg in liver, 1.4 mg/kg in kidney,
0.45 mg/kg in fat, 0.3 mg/kg in skin, 0.13 mg/kg in muscle and 0.08
mg/kg in brain. Ten days after administration maximum residues in
tissues and blood ranged from <0.01 to 0.15 mg/kg. A maximum of 3.4%
of the residues in liver were chloroform-soluble metabolites
(demeton-S sulfoxide, disulfoton sulfoxide and demeton-S sulfone).
Since demeton-S sulfoxide and demeton-S sulfone are metabolites
of disulfoton as well as of demeton, it is difficult or impossible to
determine which parent compound is responsible for residues of them.
Further explanation is given in the Appraisal of demeton (see this
Meeting Report).
For residue analysis, oxidation of the residues and determination
of demeton sulfone and disulfoton sulfone by the GLC method previously
described (FAO/WHO, 1974) should be suitable for regulatory purposes.
RECOMMENDATIONS
New recommendations (alfalfa [hay], clover [hay], maize) together
with previous recommendations as set out-below refer to the total
residue of disulfoton, disulfoton sulfoxide, disulfoton sulfone,
demeton, demeton sulfoxide and demeton sulfone calculated and
expressed as disulfoton.
Maximum
Commodity Residue Limit
(mg/kg)
Alfalfa (hay), clover (hay) 10
Forage crops (green) 5
Vegetables including beans, broccoli,
Brussels sprouts, cabbage, cauliflower,
celery, lettuce, maize, potatoes, peanut
shells, peas (inc. pods), rice (in husk),
spinach, sugar beets (roots), tomatoes 0.5
Raw grain (except rice and maize) 0.2
Coffee beans, pecans, peanuts (kernels),
pineapple, soybeans 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Residue data from supervised trials for commodities not
mentioned above, but included in national tolerance lists.
2. Results from studies now in progress (expected in the spring
of 1976) on residues in meat, milk and eggs after feeding
animals on fodder treated with disulfoton in order to
determine residue limits in food of animal origin.
3. Information on residues in food moving in commerce.
REFERENCES
Brewer, W. E. (1975) 21-day subacute inhalation toxicity study with
di-syston sulfoxide, di-syston oxygen Analog, sulfone and mocap in
rat. Unpublished report from Industrial Biotest Laboratories submitted
to the World Health Organization by Bayer AG.
Brzezinski, J. and Ludwicki, K. (1973) The interrelationship of the
changes of acetylcholinesterase and catecholamines, blood and urine
levels in rats poisoned with di-syston. Pol. Pharmacol Pharm. 25:
313-316
Clark, G. and Stavinoha, W. B. (1969) Alterations in liver RNA induced
by atropine and disulfoton. Tox. Appl. Pharmacol. 14: 376-79.
Clark, G. and Stavinoha, W. B. (1971) A permeability change in CNS
tissue in chronic poisoning with Disulfoton. Life Sci. 10: 421-23.
Clark, G., Koester, A.G. and Pearson, D. W. (1971) Exploratory
behaviour in chronic Disulfoton poisoning in mice. Psychapharmacologia
(Berl.) 20: 169-71.
Clark, G. and Pearson, D. W. (1973) Learning in chronic disulfoton
poisoning. J. Gen. Psychol. 89: 305-11.
Crawford, C. R. and Anderson, R. H. (1974a) The acute oral toxicity of
several Di-Syston metabolites to female and male rats. Unpublished
Report from Mobay Chemical Corporation submitted to the World Health
Organization by Bayer AG.
Crawford, C. R. and Anderson, R. H. (1974b) Subacute oral toxicity of
a mixture of four Di-Syston metabolites to cattle and sheep.
Unpublished Report from Mobay Chemical Corporation. Submitted to the
World Health Organization by Bayer AG.
DuBois, K. P. and Kinoshita, F. K. (1968) Influence of induction of
hepatic microsomal enzymes by phenobarbital on toxicity of organic
phosphate insecticides. Proc. Soc. Exper. Biol. Med. 129: 699-702.
Echobichon, D. J. and Kalow, W. (1963) Action of organophosphorus
compounds upon esterases of human liver. Can. J. Biochem. Physiol. 41:
1537-46.
Foley, D. J. and McPhillips, J. J. (1972) Relationship between
cholinesterase activity and sensitivity to cholinergic drugs in the
rat ileum. Tox. Appl. Pharmacol. 22: 286-87.
Foley, D. J. and McPhillips, J. J. (1973) Response of the rat ileum
uterus and vas deferens to carbacol and acetyl choline following
repeated daily administration of a cholinesterase inhibitor. Brit. J.
Pharmacol. 48: 418-25.
Gablicks, J. and Friedman, L. (1969) Effects of insecticides on
mammalian cells and virus infections. Ann. N.Y. Acad. Sci. 160: Art.
1: 254-71.
Hoffmann, K. and Weischer, C. H. (1975) S-276. (Disulfoton) Chronic
toxicity study on dogs (two year feeding experiments). Unpublished
report from the Institute for Toxicology submitted to the World Health
Organization by Bayer AG.
Huang, C.C. (1973) Effect on growth but not chromosomes of the
mammalian cells treatment with three organophosphorus insecticides.
Proc. Soc. Exp. Biol. Med. 142: 36-40.
Klotzsche, C. (1975) Disulfoton. 2-year Feeding Study in Rats.
Unpublished report from Agrochemical Research Department. Submitted to
the World Health Organization by Sandoz Ltd.
Litterst, C. L., Lichtenstein, E. P. and Kajiwara, K. (1969) Effects
of insecticides on growth of Hela cells. J. Agr. Food Chem. 17:
1199-1203.
McPhillips, J. J. (1969) Altered sensitivity to drugs following
repeated injections of a cholinesterase inhibitor to rats. Toxicol.
Appl. Pharmacol. 14: 67-73.
McPhillips, J. J. and Dar, M. S. (1967) Resistance to the effect of
Carbacol on the cardiovascular system and on the isolated ileum of
rats after subacute administration of an organophosphorus
cholinesterase inhibitor. J. Pharmacol. Exp. Therap. 156: 507-13.
Puhl, R. J. and Fredrickson, D. R. (1975) The metabolism and excretion
of Di-Syston by rats. Unpublished report from Mobay Chemical
Corporation. Submitted to the World Health Organization by Bayer AG.
Stevens, J. T., Stitzel, R. E. and McPhillips, J. J. (1972) The
effects of subacute administration of anticholinesterase insecticides
on hepatic microsomal metabolism. Life Sci. II (part II): 423-31.
Wysocka-Paruszewska, B. (1970) The urine level of 4-hydroxy-3-methoxy
mandellic acid in urine of rats poisoned with phosphorus organic
insecticides. Dissert. Pharm. Pharmaco. 22: 485-89.
Wysocka-Paruszewska, B. (1971) The changes in urine level of
4-hydroxy-3-methoxy mandelic acid in rats related to the degree of
intoxication with Di-syston. Dissert. Pharm. Parmocol. 22: 271-74.
 TABLE 2. National tolerances reported to the Meeting
(Changes and additions since last evaluation)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Argentina Lettuce, peanuts, potatoes, 0.75
cabbage, cauliflower, spinach,
tomatoes, pecans, barley,
rice, maize (fodder),
cottonseed
Sugarcane, coffee, maize 0.3
Sugar beet (roots) 0.5
Wheat 0.2
Sugar beet (leaves) 2.0
Alfalfa and clover (hay) 12.0
Carrots 0
Alfalfa (fresh), rye, wheat, 5.0
oats, barley (fodder), clover
(fresh) peanuts (fodder)
Canada Broccoli, cabbage 0.75
Beans (dry, lima, snap), 0.5
Brussels sprouts, cauliflower,
lettuce, peas, spinach,
tomatoes
Potatoes 0.2
Barley (grain), coffee, corn N.R.
(grain) (incl. field corn,
sweet corn [kernels plus cob
with husk removed] & popcorn),
cottonseed, eggplant, oats
(grain), peanuts, peppers,
pineapple, rice (grain), sugar
beets, wheat (grain)
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Federal Potatoes 0.2
Republic of
Germany Cereals 0.1
(disulfoton,
disulfoton-sulfoxide,
disulfoton-sulfone, demeton,
demeton-sulfoxide,
demeton-sulfone: calculated
together as disulfoton)
Hungary General 0.2 56
Japan Rice, fruit, vegetables 0.1
beans, potatoes
Rice 50
Citrus fruit 30
Eggplants, tomatoes 21
Soybeans, azukibeans, garden 60
pea, kidney bean, broad bean
Netherlands Potatoes 0.01
All other crops 0
New Zealand Potatoes 91
Switzerland Lettuce 0.2
Vegetables, field crops 42
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
United States Alfalfa (hay), clover (hay) 12.0
of America
Alfalfa (fresh), barley (green 5.0
fodder, straw), beans (vines),
clover (fresh), corn forage &
fodder (incl. field corn,
sweet corn and popcorn), oats
(green fodder, straw), peanuts
(hay), peas (vines), pineapple
(foliage), rice (straw),
sorghum fodder & forage, sugar
beets (pulp, feed additive),
wheat (green fodder, straw)
Sugar beets (tops) 2.0
Barley (grain), beans (lima 0.75
dry, snap), broccoli,
Brussels sprouts, cabbage,
cauliflower, cottonseed,
lettuce, oats (grain),
peanuts, pecans, peas,
pineapple, potatoes, rice
(grain), sorghum (grain),
spinach, tomatoes
Hops, sugar beets 0.5
Coffee corn grain (incl. field 0.3
corn, sweet corn [kernels plus
cob with husk removed] & popcorn),
sugar-cane (raw), wheat
(grain)
Soybean forage & hay 0.25
Peppers, soybeans 0.1
Yugoslavia General 0
Field crops 60
APPRAISAL
Disulfoton was evaluated at the 1973 Joint Meeting (FAO/WHO
1974). Reviewing the data then available the Meeting required
information on the occurrence of residues in meat, milk and eggs after
feeding animals with disulfoton in order to recommend maximum residue
limits in food of animal origin. In addition, information on residues
in food moving in commerce was mentioned as desirable. None of this
information has been received.
Revised national tolerances and pre-harvest intervals as well as
studies on the fate of residues in rats have become available.
Fourty-eight and 72 hours after oral application of a single dose of
diethyl-l-14C-labelled disulfoton (0.2 mg/kg female rat and 1.2 mg/kg
male rat) 71% and 81% respectively of the administered 14C was
excreted in the urine and faeces and expired as carbon dioxide by the
females. The corresponding figures for males were 93% and 96%. Ten
days after dosing, 96% (female) and 100% (male) of the 14C had been
excreted. Diethyl phosphate and diethyl phosphorothionate were
identified as major metabolites. Peak levels of 14C in tissues
occurred about 6 hours after dosing, reaching maximum levels
(disulfoton equivalents) of 3.6 mg/kg in liver, 1.4 mg/kg in kidney,
0.45 mg/kg in fat, 0.3 mg/kg in skin, 0.13 mg/kg in muscle and 0.08
mg/kg in brain. Ten days after administration maximum residues in
tissues and blood ranged from <0.01 to 0.15 mg/kg. A maximum of 3.4%
of the residues in liver were chloroform-soluble metabolites
(demeton-S sulfoxide, disulfoton sulfoxide and demeton-S sulfone).
Since demeton-S sulfoxide and demeton-S sulfone are metabolites
of disulfoton as well as of demeton, it is difficult or impossible to
determine which parent compound is responsible for residues of them.
Further explanation is given in the Appraisal of demeton (see this
Meeting Report).
For residue analysis, oxidation of the residues and determination
of demeton sulfone and disulfoton sulfone by the GLC method previously
described (FAO/WHO, 1974) should be suitable for regulatory purposes.
RECOMMENDATIONS
New recommendations (alfalfa [hay], clover [hay], maize) together
with previous recommendations as set out-below refer to the total
residue of disulfoton, disulfoton sulfoxide, disulfoton sulfone,
demeton, demeton sulfoxide and demeton sulfone calculated and
expressed as disulfoton.
Maximum
Commodity Residue Limit
(mg/kg)
Alfalfa (hay), clover (hay) 10
Forage crops (green) 5
Vegetables including beans, broccoli,
Brussels sprouts, cabbage, cauliflower,
celery, lettuce, maize, potatoes, peanut
shells, peas (inc. pods), rice (in husk),
spinach, sugar beets (roots), tomatoes 0.5
Raw grain (except rice and maize) 0.2
Coffee beans, pecans, peanuts (kernels),
pineapple, soybeans 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Residue data from supervised trials for commodities not
mentioned above, but included in national tolerance lists.
2. Results from studies now in progress (expected in the spring
of 1976) on residues in meat, milk and eggs after feeding
animals on fodder treated with disulfoton in order to
determine residue limits in food of animal origin.
3. Information on residues in food moving in commerce.
REFERENCES
Brewer, W. E. (1975) 21-day subacute inhalation toxicity study with
di-syston sulfoxide, di-syston oxygen Analog, sulfone and mocap in
rat. Unpublished report from Industrial Biotest Laboratories submitted
to the World Health Organization by Bayer AG.
Brzezinski, J. and Ludwicki, K. (1973) The interrelationship of the
changes of acetylcholinesterase and catecholamines, blood and urine
levels in rats poisoned with di-syston. Pol. Pharmacol Pharm. 25:
313-316
Clark, G. and Stavinoha, W. B. (1969) Alterations in liver RNA induced
by atropine and disulfoton. Tox. Appl. Pharmacol. 14: 376-79.
Clark, G. and Stavinoha, W. B. (1971) A permeability change in CNS
tissue in chronic poisoning with Disulfoton. Life Sci. 10: 421-23.
Clark, G., Koester, A.G. and Pearson, D. W. (1971) Exploratory
behaviour in chronic Disulfoton poisoning in mice. Psychapharmacologia
(Berl.) 20: 169-71.
Clark, G. and Pearson, D. W. (1973) Learning in chronic disulfoton
poisoning. J. Gen. Psychol. 89: 305-11.
Crawford, C. R. and Anderson, R. H. (1974a) The acute oral toxicity of
several Di-Syston metabolites to female and male rats. Unpublished
Report from Mobay Chemical Corporation submitted to the World Health
Organization by Bayer AG.
Crawford, C. R. and Anderson, R. H. (1974b) Subacute oral toxicity of
a mixture of four Di-Syston metabolites to cattle and sheep.
Unpublished Report from Mobay Chemical Corporation. Submitted to the
World Health Organization by Bayer AG.
DuBois, K. P. and Kinoshita, F. K. (1968) Influence of induction of
hepatic microsomal enzymes by phenobarbital on toxicity of organic
phosphate insecticides. Proc. Soc. Exper. Biol. Med. 129: 699-702.
Echobichon, D. J. and Kalow, W. (1963) Action of organophosphorus
compounds upon esterases of human liver. Can. J. Biochem. Physiol. 41:
1537-46.
Foley, D. J. and McPhillips, J. J. (1972) Relationship between
cholinesterase activity and sensitivity to cholinergic drugs in the
rat ileum. Tox. Appl. Pharmacol. 22: 286-87.
Foley, D. J. and McPhillips, J. J. (1973) Response of the rat ileum
uterus and vas deferens to carbacol and acetyl choline following
repeated daily administration of a cholinesterase inhibitor. Brit. J.
Pharmacol. 48: 418-25.
Gablicks, J. and Friedman, L. (1969) Effects of insecticides on
mammalian cells and virus infections. Ann. N.Y. Acad. Sci. 160: Art.
1: 254-71.
Hoffmann, K. and Weischer, C. H. (1975) S-276. (Disulfoton) Chronic
toxicity study on dogs (two year feeding experiments). Unpublished
report from the Institute for Toxicology submitted to the World Health
Organization by Bayer AG.
Huang, C.C. (1973) Effect on growth but not chromosomes of the
mammalian cells treatment with three organophosphorus insecticides.
Proc. Soc. Exp. Biol. Med. 142: 36-40.
Klotzsche, C. (1975) Disulfoton. 2-year Feeding Study in Rats.
Unpublished report from Agrochemical Research Department. Submitted to
the World Health Organization by Sandoz Ltd.
Litterst, C. L., Lichtenstein, E. P. and Kajiwara, K. (1969) Effects
of insecticides on growth of Hela cells. J. Agr. Food Chem. 17:
1199-1203.
McPhillips, J. J. (1969) Altered sensitivity to drugs following
repeated injections of a cholinesterase inhibitor to rats. Toxicol.
Appl. Pharmacol. 14: 67-73.
McPhillips, J. J. and Dar, M. S. (1967) Resistance to the effect of
Carbacol on the cardiovascular system and on the isolated ileum of
rats after subacute administration of an organophosphorus
cholinesterase inhibitor. J. Pharmacol. Exp. Therap. 156: 507-13.
Puhl, R. J. and Fredrickson, D. R. (1975) The metabolism and excretion
of Di-Syston by rats. Unpublished report from Mobay Chemical
Corporation. Submitted to the World Health Organization by Bayer AG.
Stevens, J. T., Stitzel, R. E. and McPhillips, J. J. (1972) The
effects of subacute administration of anticholinesterase insecticides
on hepatic microsomal metabolism. Life Sci. II (part II): 423-31.
Wysocka-Paruszewska, B. (1970) The urine level of 4-hydroxy-3-methoxy
mandellic acid in urine of rats poisoned with phosphorus organic
insecticides. Dissert. Pharm. Pharmaco. 22: 485-89.
Wysocka-Paruszewska, B. (1971) The changes in urine level of
4-hydroxy-3-methoxy mandelic acid in rats related to the degree of
intoxication with Di-syston. Dissert. Pharm. Parmocol. 22: 271-74.
TABLE 2. National tolerances reported to the Meeting
(Changes and additions since last evaluation)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Argentina Lettuce, peanuts, potatoes, 0.75
cabbage, cauliflower, spinach,
tomatoes, pecans, barley,
rice, maize (fodder),
cottonseed
Sugarcane, coffee, maize 0.3
Sugar beet (roots) 0.5
Wheat 0.2
Sugar beet (leaves) 2.0
Alfalfa and clover (hay) 12.0
Carrots 0
Alfalfa (fresh), rye, wheat, 5.0
oats, barley (fodder), clover
(fresh) peanuts (fodder)
Canada Broccoli, cabbage 0.75
Beans (dry, lima, snap), 0.5
Brussels sprouts, cauliflower,
lettuce, peas, spinach,
tomatoes
Potatoes 0.2
Barley (grain), coffee, corn N.R.
(grain) (incl. field corn,
sweet corn [kernels plus cob
with husk removed] & popcorn),
cottonseed, eggplant, oats
(grain), peanuts, peppers,
pineapple, rice (grain), sugar
beets, wheat (grain)
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
Federal Potatoes 0.2
Republic of
Germany Cereals 0.1
(disulfoton,
disulfoton-sulfoxide,
disulfoton-sulfone, demeton,
demeton-sulfoxide,
demeton-sulfone: calculated
together as disulfoton)
Hungary General 0.2 56
Japan Rice, fruit, vegetables 0.1
beans, potatoes
Rice 50
Citrus fruit 30
Eggplants, tomatoes 21
Soybeans, azukibeans, garden 60
pea, kidney bean, broad bean
Netherlands Potatoes 0.01
All other crops 0
New Zealand Potatoes 91
Switzerland Lettuce 0.2
Vegetables, field crops 42
TABLE 2. (continued)
Pre-harvest
Tolerance interval
Country Commodity (mg/kg) (days)
United States Alfalfa (hay), clover (hay) 12.0
of America
Alfalfa (fresh), barley (green 5.0
fodder, straw), beans (vines),
clover (fresh), corn forage &
fodder (incl. field corn,
sweet corn and popcorn), oats
(green fodder, straw), peanuts
(hay), peas (vines), pineapple
(foliage), rice (straw),
sorghum fodder & forage, sugar
beets (pulp, feed additive),
wheat (green fodder, straw)
Sugar beets (tops) 2.0
Barley (grain), beans (lima 0.75
dry, snap), broccoli,
Brussels sprouts, cabbage,
cauliflower, cottonseed,
lettuce, oats (grain),
peanuts, pecans, peas,
pineapple, potatoes, rice
(grain), sorghum (grain),
spinach, tomatoes
Hops, sugar beets 0.5
Coffee corn grain (incl. field 0.3
corn, sweet corn [kernels plus
cob with husk removed] & popcorn),
sugar-cane (raw), wheat
(grain)
Soybean forage & hay 0.25
Peppers, soybeans 0.1
Yugoslavia General 0
Field crops 60
APPRAISAL
Disulfoton was evaluated at the 1973 Joint Meeting (FAO/WHO
1974). Reviewing the data then available the Meeting required
information on the occurrence of residues in meat, milk and eggs after
feeding animals with disulfoton in order to recommend maximum residue
limits in food of animal origin. In addition, information on residues
in food moving in commerce was mentioned as desirable. None of this
information has been received.
Revised national tolerances and pre-harvest intervals as well as
studies on the fate of residues in rats have become available.
Fourty-eight and 72 hours after oral application of a single dose of
diethyl-l-14C-labelled disulfoton (0.2 mg/kg female rat and 1.2 mg/kg
male rat) 71% and 81% respectively of the administered 14C was
excreted in the urine and faeces and expired as carbon dioxide by the
females. The corresponding figures for males were 93% and 96%. Ten
days after dosing, 96% (female) and 100% (male) of the 14C had been
excreted. Diethyl phosphate and diethyl phosphorothionate were
identified as major metabolites. Peak levels of 14C in tissues
occurred about 6 hours after dosing, reaching maximum levels
(disulfoton equivalents) of 3.6 mg/kg in liver, 1.4 mg/kg in kidney,
0.45 mg/kg in fat, 0.3 mg/kg in skin, 0.13 mg/kg in muscle and 0.08
mg/kg in brain. Ten days after administration maximum residues in
tissues and blood ranged from <0.01 to 0.15 mg/kg. A maximum of 3.4%
of the residues in liver were chloroform-soluble metabolites
(demeton-S sulfoxide, disulfoton sulfoxide and demeton-S sulfone).
Since demeton-S sulfoxide and demeton-S sulfone are metabolites
of disulfoton as well as of demeton, it is difficult or impossible to
determine which parent compound is responsible for residues of them.
Further explanation is given in the Appraisal of demeton (see this
Meeting Report).
For residue analysis, oxidation of the residues and determination
of demeton sulfone and disulfoton sulfone by the GLC method previously
described (FAO/WHO, 1974) should be suitable for regulatory purposes.
RECOMMENDATIONS
New recommendations (alfalfa [hay], clover [hay], maize) together
with previous recommendations as set out-below refer to the total
residue of disulfoton, disulfoton sulfoxide, disulfoton sulfone,
demeton, demeton sulfoxide and demeton sulfone calculated and
expressed as disulfoton.
Maximum
Commodity Residue Limit
(mg/kg)
Alfalfa (hay), clover (hay) 10
Forage crops (green) 5
Vegetables including beans, broccoli,
Brussels sprouts, cabbage, cauliflower,
celery, lettuce, maize, potatoes, peanut
shells, peas (inc. pods), rice (in husk),
spinach, sugar beets (roots), tomatoes 0.5
Raw grain (except rice and maize) 0.2
Coffee beans, pecans, peanuts (kernels),
pineapple, soybeans 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Residue data from supervised trials for commodities not
mentioned above, but included in national tolerance lists.
2. Results from studies now in progress (expected in the spring
of 1976) on residues in meat, milk and eggs after feeding
animals on fodder treated with disulfoton in order to
determine residue limits in food of animal origin.
3. Information on residues in food moving in commerce.
REFERENCES
Brewer, W. E. (1975) 21-day subacute inhalation toxicity study with
di-syston sulfoxide, di-syston oxygen Analog, sulfone and mocap in
rat. Unpublished report from Industrial Biotest Laboratories submitted
to the World Health Organization by Bayer AG.
Brzezinski, J. and Ludwicki, K. (1973) The interrelationship of the
changes of acetylcholinesterase and catecholamines, blood and urine
levels in rats poisoned with di-syston. Pol. Pharmacol Pharm. 25:
313-316
Clark, G. and Stavinoha, W. B. (1969) Alterations in liver RNA induced
by atropine and disulfoton. Tox. Appl. Pharmacol. 14: 376-79.
Clark, G. and Stavinoha, W. B. (1971) A permeability change in CNS
tissue in chronic poisoning with Disulfoton. Life Sci. 10: 421-23.
Clark, G., Koester, A.G. and Pearson, D. W. (1971) Exploratory
behaviour in chronic Disulfoton poisoning in mice. Psychapharmacologia
(Berl.) 20: 169-71.
Clark, G. and Pearson, D. W. (1973) Learning in chronic disulfoton
poisoning. J. Gen. Psychol. 89: 305-11.
Crawford, C. R. and Anderson, R. H. (1974a) The acute oral toxicity of
several Di-Syston metabolites to female and male rats. Unpublished
Report from Mobay Chemical Corporation submitted to the World Health
Organization by Bayer AG.
Crawford, C. R. and Anderson, R. H. (1974b) Subacute oral toxicity of
a mixture of four Di-Syston metabolites to cattle and sheep.
Unpublished Report from Mobay Chemical Corporation. Submitted to the
World Health Organization by Bayer AG.
DuBois, K. P. and Kinoshita, F. K. (1968) Influence of induction of
hepatic microsomal enzymes by phenobarbital on toxicity of organic
phosphate insecticides. Proc. Soc. Exper. Biol. Med. 129: 699-702.
Echobichon, D. J. and Kalow, W. (1963) Action of organophosphorus
compounds upon esterases of human liver. Can. J. Biochem. Physiol. 41:
1537-46.
Foley, D. J. and McPhillips, J. J. (1972) Relationship between
cholinesterase activity and sensitivity to cholinergic drugs in the
rat ileum. Tox. Appl. Pharmacol. 22: 286-87.
Foley, D. J. and McPhillips, J. J. (1973) Response of the rat ileum
uterus and vas deferens to carbacol and acetyl choline following
repeated daily administration of a cholinesterase inhibitor. Brit. J.
Pharmacol. 48: 418-25.
Gablicks, J. and Friedman, L. (1969) Effects of insecticides on
mammalian cells and virus infections. Ann. N.Y. Acad. Sci. 160: Art.
1: 254-71.
Hoffmann, K. and Weischer, C. H. (1975) S-276. (Disulfoton) Chronic
toxicity study on dogs (two year feeding experiments). Unpublished
report from the Institute for Toxicology submitted to the World Health
Organization by Bayer AG.
Huang, C.C. (1973) Effect on growth but not chromosomes of the
mammalian cells treatment with three organophosphorus insecticides.
Proc. Soc. Exp. Biol. Med. 142: 36-40.
Klotzsche, C. (1975) Disulfoton. 2-year Feeding Study in Rats.
Unpublished report from Agrochemical Research Department. Submitted to
the World Health Organization by Sandoz Ltd.
Litterst, C. L., Lichtenstein, E. P. and Kajiwara, K. (1969) Effects
of insecticides on growth of Hela cells. J. Agr. Food Chem. 17:
1199-1203.
McPhillips, J. J. (1969) Altered sensitivity to drugs following
repeated injections of a cholinesterase inhibitor to rats. Toxicol.
Appl. Pharmacol. 14: 67-73.
McPhillips, J. J. and Dar, M. S. (1967) Resistance to the effect of
Carbacol on the cardiovascular system and on the isolated ileum of
rats after subacute administration of an organophosphorus
cholinesterase inhibitor. J. Pharmacol. Exp. Therap. 156: 507-13.
Puhl, R. J. and Fredrickson, D. R. (1975) The metabolism and excretion
of Di-Syston by rats. Unpublished report from Mobay Chemical
Corporation. Submitted to the World Health Organization by Bayer AG.
Stevens, J. T., Stitzel, R. E. and McPhillips, J. J. (1972) The
effects of subacute administration of anticholinesterase insecticides
on hepatic microsomal metabolism. Life Sci. II (part II): 423-31.
Wysocka-Paruszewska, B. (1970) The urine level of 4-hydroxy-3-methoxy
mandellic acid in urine of rats poisoned with phosphorus organic
insecticides. Dissert. Pharm. Pharmaco. 22: 485-89.
Wysocka-Paruszewska, B. (1971) The changes in urine level of
4-hydroxy-3-methoxy mandelic acid in rats related to the degree of
intoxication with Di-syston. Dissert. Pharm. Parmocol. 22: 271-74.
