
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 90
DIMETHOATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154290 X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHOATE
1. SUMMARY
1.1. Identity, uses and analytical methods
1.2. Environmental concentrations and exposure
1.3. Effects on the environment
1.4. Kinetics and metabolism
1.5. Effects on experimental animals
1.6. Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Industrial production
3.2.2. World production figures
3.2.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Plants
4.1.5. Disposal of wastes
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air, water, and soil
5.1.2. Food
5.2. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption and distribution
6.2. Metabolic transformation
6.3. Elimination and excretion
6.3.1. Animal studies
6.3.2. Human studies
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.3. Terrestrial organisms
7.3.1. Honey-bees
7.3.2. Birds
7.3.3. Farm animals
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Skin and eye irritation
8.3. Repeated exposures
8.4. Reproduction studies
8.5. Teratogenicity
8.6. Mutagenicity
8.7. Carcinogenicity
8.8. Special studies
8.9. Factors modifying toxicity
8.10. Mechanisms of toxicity; mode of action
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Poisoning incidents
9.1.2. Controlled human studies
9.2. Occupational exposure
9.2.1. Poisoning incidents
9.3. Early symptoms and treatment of poisoning
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Toxicity of dimethoate
10.2. Human exposure
10.3. Evaluation of effects on the environment
10.4. Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX I
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHOATE
Members
Dr L. Badaeva, All Union Scientific Research Institute of
Hygiene and Toxicology of Pesticides, Polymers and Plastics,
Kiev, USSR
Dr J. Huff, National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India (Chairman)
Dr J. Liesivuori, Institute of Occupational Health, Kuopio
Regional Institute of Occupational Health, Kuopio, Finland
Dr I. Ritter, Pesticides Division, Environmental Health
Directorate, Department of National Health and Welfare,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr A. Takanaka, Division of Pharmacology, National Institute of
Hygienic Sciences, Tokyo, Japan (Vice-Chairman)
Dr M. Tasheva, Institute of Hygiene & Occupational Health,
Medical Academy, Sofia, Bulgaria (Rapporteur)
Dr E.M. den Tonkelaar, National Institute of Public Health and
Environment, Bilthoven, Netherlands (Rapporteur)
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Ms F. Ouane, United Nations Environment Programme, International
Register of Potentially Toxic Chemicals, Palais des Nations,
Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to
the Manager of the International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland, in order that
they may be included in corrigenda, which will appear in
subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained
from the International Register of Potentially Toxic Chemicals,
Palais des Nations, 1211 Geneva 10, Switzerland (Telephone No.
7988400 - 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHOATE
A WHO Task Group on Environmental Health Criteria for
Dimethoate met in Geneva from 11-15 May 1987. Dr K.W. Jager
opened the meeting and welcomed the participants on behalf of
the Manager of the IPCS and the heads of the three IPCS co-
operating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft criteria document and made an evaluation of
the risks for human health and the environment from exposure to
dimethoate.
The first draft of this document and the second draft
incorporating comments received from the IPCS contact points
for Environmental Health Criteria Documents were prepared by
Dr M. TASHEVA, Institute of Hygiene and Occupational Health,
Medical Academy, Sofia, Bulgaria.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO Collab-
orating Centre for Environmental Health Effects. The United
Kingdom Department of Health and Social Security generously
supported the cost of printing.
1. SUMMARY
1.1. Identity, Uses and Analytical Methods
Dimethoate is an organophosphorus insecticide with a contact
and systemic action. It was introduced in 1956 and is produced
in many countries for use against a broad range of insects in
agriculture and also for the control of the housefly.
Dimethoxon, an oxygen analogue metabolite of dimethoate,
appears to play a dominant role in its toxicity for insects and
mammals. Dimethoxon itself is also used as an insecticide,
known as omethoate.
Dimethoate is fairly soluble in water and highly soluble in
most organic solvents. It is fairly stable in water and acid
solution, and unstable in alkaline solution.
The analytical method of choice for its determination is gas
chromatography with flame photometric detection.
1.2. Environmental Concentrations and Exposure
Hydrolytic degradation is the main inactivating pathway of
dimethoate in the environment. In moist air, it is degraded
photochemically to hydrolytic and oxidation products. The half-
life of dimethoate in different plants is between 2 and 5 days.
Degradation in soil is dependent on the type of soil, tempera-
ture, moisture, and pH level.
The general population is not normally exposed to dimethoate
from air or water. Levels of residues in food are mainly below
1 mg/kg. Dimethoate was only detected infrequently in the
latest reported total-diet studies (1982).
Occupational exposure to dimethoate, which may occur during
manufacture, formulation, and use, is mainly through inhalation
and dermal absorption. Higher occupational exposure may be
observed in case of accident or as a result of incorrect
handling.
1.3. Effects on the Environment
Dimethoate is not persistent in the environment. Its
toxicity for aquatic organisms and birds has been reported to be
moderate to high. However, it is very toxic for honey-bees.
1.4. Kinetics and Metabolism
Dimethoate is absorbed following ingestion, inhalation, and
skin contact. It has been detected in the blood 30 min after
oral administration. Accumulation in the tissues is not likely.
The main metabolic pathways of dimethoate are oxidative desulfu-
ration and hydrolysis. Hydrolytic metabolism predominates over
oxidation in mammals, whereas the opposite is true in insects.
Dimethoxon (omethoate), which has been demonstrated in plants,
insects, and mammals, seems to be the metabolite responsible for
the toxic action of dimethoate. Dimethoate is degraded rapidly
in the rat liver, but very little degradation occurs in other
tissues. It is eliminated predominantly in the form of hydro-
lytic urinary products.
1.5. Effects on Experimental Animals
Dimethoate is moderately toxic; most oral LD50s in rats
ranged from 150 to 400 mg/kg body weight. Signs of intoxication
in the rat were observed 0.5-2 h after administration, and were
typical of exposure to organophosphorus pesticides. Rat and dog
erythrocyte-acetyl cholinesterase activity (AChE) is more
susceptible to inhibition than plasma-cholinesterase (ChE).
When rats were exposed to dimethoate at a concentration of
10 mg/m3 for 4 h, 40% inhibition of ChE activity was reported.
The acute dermal LD50 for dimethoate in rats is greater than
600 mg/kg. It is not irritating to the skin and only slightly
irritating to the eye. No dermal sensitization data are
available on dimethoate.
A dietary level of 5 mg dimethoate/kg is considered to be a
no-observed-adverse-effect level in the rat on the basis of
erythrocyte-cholinesterase depression. No effects were reported
in rats exposed through inhalation to 0.01 mg dimethoate/m3 for
14 h/day for 3 months.
Dimethoate administered at 60 mg/litre drinking-water
affected mating in 5 generations of mice tested.
Dimethoate did not appear to be teratogenic in experimental
animals.
However, dimethoate was found to be mutagenic in a variety
of in vitro and in vivo test systems.a
Long-term studies have been conducted on dimethoate admin-
istered orally to rats and mice and by intramuscular injection
in rats. However, the available data are considered to be inad-
equate to assess the carcinogenic potential of the compound.b
----------------------------------------------------------------
a On the basis of the results of new and published studies,
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) have
concluded that dimethoate is mutagenic in bacterial tests,
but negative in mammalian cells and in vivo tests.
b Since the Task Group met, the results of new long-term
carcinogenicity studies on rats and mice have been submitted
to the FAO/WHO Joint Meeting on Pesticide Residues. No
indication of carcinogenicity was found.
1.6. Effects on Man
Several cases of suicidal and accidental poisoning by
dimethoate have been reported. Some cases of occupational
poisoning that have been reported have been the result of
accidents or neglect of safety precautions. The lethal oral
dose for human beings has been estimated to be in the range of
50-500 mg/kg body weight.
In human volunteers, an oral dose of 0.2 mg/kg body weight
per day, for 39 days, did not produce any effects on whole-blood
cholinesterase values. No skin irritation or ChE inhibition was
observed after a 2-h dermal exposure to 2.5 ml of a 32% liquid
formulation of dimethoate. There have been rare reports of skin
sensitization to dimethoate.
Minimum safe re-entry periods after the application of
dimethoate have been reported.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
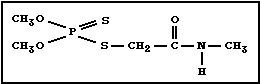 Molecular formula: C5H12NO3PS2
Common name: dimethoate (accepted by BSI, ISO,
ANSI, and JMAF); fosfamid (used in
USSR)
Common trade names: Bi 58; Cygon; Dimethoate; Fosfamid;
Fostion MM, Rogor; Perfekthion;
Roxion
IUPAC name: O,O-dimethyl S-methyl-carbamoyl-
methyl phosphorodithioate
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester
(9CI)
CAS registry number: 60-51-5
RTECS registry number: TE1750000
Technical dimethoate is about 93-95% pure. The major
impurities are O,O-dimethyl S-methylphosphorodithioate and
O-O-S-trimethyl phosphorodithioate.
2.2. Physical and Chemical Properties
Pure dimethoate is a colourless crystalline solid with an
odour of mercaptan. Technical dimethoate (about 93% pure)
varies from off-white crystals to a grey semi-crystalline
material. Some physical and chemical properties of dimethoate
are given in Table 1.
Dimethoate is highly soluble in chloroform, methylene
chloride, benzene, toluene, alcohols, esters, and ketones,
slightly soluble in xylene, carbon tetrachloride, and aliphatic
hydrocarbons, and fairly soluble in water.
Dimethoate is fairly stable in water and acid solution, at
room temperature, and unstable in alkaline solution (Table 1).
Heating converts it to the O,S-dimethyl phosphorodithioate.
Table 1. Some physical and chemical properties of dimethoate
-------------------------------------------------------------
Relative molecular mass 229.2
Odour threshold 0.010 mg/m3
Melting point 45-52.5 °C
Boiling point 107 °C at 0.05 mmHg
86 °C at 0.01 mmHg
Vapour pressure (25 °C) 8.5 x 10-6 mmHg
Volatility 1.107 mg/m3
Specific gravity 1.281
(compared to water)
Partition coefficient n-octanol/water 5.959
Solubility in water (21 °C) up to 39 g/litre
Half-life: in aqueous media at pH 2-7, relatively stable
at pH 9, 50% loss in l2 days
-------------------------------------------------------------
2.3. Analytical Methods
A review of the detection methods for dimethoate in treated
crop plants has been presented by De Pietri-Tonelli et al.
(1965). The procedures reported are based on colorimetry,
column, paper, and thin-layer chromatography, paper electro-
phoresis, gas chromatography, and radiometry. Bioassay tech-
niques and autoradiographic procedures can also be applied.
High-performance thin-layer chromatography has been proposed by
Hauck & Amadori (1980) as a new potential for the determination
of dimethoate.
The Codex Committee on Pesticide Residues has listed
recommended methods for the determination of dimethoate residues
(FAO/WHO 1986) and various methods used in the determination of
dimethoate are summarized in Table 2.
A personal air sampler to measure vapours and aerosols of
dimethoate at low concentrations has been described by Hill &
Arnold (1979).
Table 2. Methods for the determination of dimethoate
---------------------------------------------------------------------------------------------------------
Sample type Method of detection Comments Detection limit Reference
---------------------------------------------------------------------------------------------------------
Soil gas-liquid chromato- 1-20 ng Getzin & Rosefield
graphy/phosphorus (1968)
detection
Soil thin-layer chromato- 50-mg soil samples; ex- 0.1 mg/kg; Akoronko & Girenko
graphy; gas-liquid traction with chloroform 0.05 mg/kg (1977)
chromatography/therm-
ionic detection
Water thin-layer chromato- 200-ml sample; extraction 0.5 µg; Girenko et al.
graphy; gas-liquid with chloroform 5 ng (1978)
chromatography/therm-
ionic detection or
electron capture
detection
Wheat plants gas chromatography/ suitable for determina- 0.02 mg/kg Lee & Westcott
flame photometric tion of dimethoate and (1981)
detection dimethoxon (omethoate)
residues in field wheat
plants
Plants colorimetry enzymatic pig liver 1 µg dimethoate Nanda Kumar &
powder used as Udaya Bhaskar
ChE source; more sensi- (1980)
tive by converting into 50 ng omethoate Udaya Bhaskar &
oxidation product Nanda Kumar (1980)
Fruits and gas-liquid chromato- extraction with methyl 0.02 mg/kg Girenko & Klisenko
vegetables graphy/thermionic chloride (1977)
detection
Fruits and colorimetry 250 g of sample; extrac- 5 µg (0.1 mg/kg) Chilwell & Beecham
vegetables tion with chloroform (1960)
Asparagus gas chromatography extraction with 0.002 mg/kg Szeto et al.
nitrogen/phosphorus/ ethyl acetate (fresh weight) (1985)
detection
---------------------------------------------------------------------------------------------------------
Table 2. (contd).
---------------------------------------------------------------------------------------------------------
Sample type Method of detection Comments Detection limit Reference
---------------------------------------------------------------------------------------------------------
Vegetables gas chromatography/ 25 g of sample; extrac- 0.008 mg/kg Van Middelem &
(snap bean) electron affinity tion with methylene Waites (1964)
detection chloride; oxygen analogue
could not be detected
at a 1:1 ratio; detectable
at 10 parts oxygen to 1
part dimethoate
Food stuffs high-performance suitable for pesticide 0.3 mg/kg Cabras et al.,
liquid mixtures; the method can (1979)
chromatography also be used for air
samples
Honey thin-layer chromato- extraction with hexane 0.1 mg/kg Petukhov (1975)
graphy and chloroform
Honey, nectar, gas chromatography/ extraction with benzene 0.1 ng in nectar; Barker et al.
and pollen flame photometric 0.5 ng in pollen (1980)
samples detection
Milk gas chromatography/ samples heated at 60 °C 0.001 mg/kg Beck et al.
flame photometric in water bath for 20 min (1968)
detection to facilitate precipi-
tation; extraction with
methylene chloride
Animal tissues thin-layer chromato- dimethoate and dimethoxon Sidimanov (1971)
graphy (omethoate) can be detected
25 days after death of the
animal
Skin and respir- gas chromatography/ suitable for field 0.01 µg/sample Copplestone
ator pads flame photometric studies; pads placed in et al. (1976)
detection individual 30-ml glass
bottles, each contain-
ing 10 ml benzene
Technical gas-liquid chromato- - - WHO (1986c)
material and graphy/flame ionization
formalizations detection
---------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Dimethoate does not occur as a natural product.
3.2. Man-made Sources
3.2.1. Industrial production
Dimethoate was first described by Hoegberg & Cassaday (1951)
and was introduced on the market in 1956.
3.2.2. World production figures
Dimethoate is manufactured in many countries, but data on
the world production of dimethoate are not available.
3.2.3. Uses
Dimethoate formulations are widely used as contact and
systemic insecticides against a broad range of insects and mites
and is applied at 0.3-0.7 kg active ingredient/ha on numerous
crops: fruits (apples, citrus, bananas, mangoes), vegetables
(beans, broccoli, cabbage, cauliflower, pepper, potatoes,
spinach, tomatoes), wheat, alfalfa, cotton, tobacco,
ornamentals, olives, sunflower, and others (Worthing & Walker,
1983).
Dimethoate is also used for the indoor control of house-
flies. For residual treatment, 10-25 g/litre formulations are
used (0.046-0.5 g active ingredient/m2) (WHO, 1984). The dose
of dimethoate for outdoor fly control is 224 g active
ingredient/ha (WHO, 1984).
Dimethoate is also applied as a systemic insecticide for
control of cattle grubs (Worthing & Walker, 1983).
The oxygen analogue of dimethoate, dimethoxon, is also used
as insecticide and is known under the common name of omethoate.
Formulations of dimethoate include emulsifiable concen-
trates, wettable powders, and granules. There is also a formu-
lation for ultra low volume application.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
4.1.1. Air
Air concentrations of dimethoate were measured under hot
climatic conditions at a distance of 300 m from a sprayed
area. Concentrations on the day of spraying ranged from
0.061 to 0.142 mg/m3, but decreased during the next 4 days to
0.004-0.014 mg/m3. At a distance of 1500 m, dimethoate was not
detected on either the day of treatment or during the days that
followed (Madzhidov, 1970).
Dimethoate is an intermediate product in the hydrolysis of
the pesticide formothion (Melnikov et al., 1977; Bolotnyi et
al., 1978). After use, formothion is found in the air the day
of spraying and dimethoate during the following days up to the
10th day.
In moist air, dimethoate is degraded photochemically to
hydrolytic and oxidation products (Melnikov et al., 1977).
4.1.2. Water
Aqueous solutions of dimethoate are fairly stable. The
compound is rapidly hydrolysed in alkali (pH 11): about 50-57%
of dimethoate degrades to water-soluble material in ´ h, 68% in
1 h, and 87% in 2 h. The predominant degradation product is
desmethyl dimethoate (49.3%) (Brady & Arthur, 1963). Hydrolysis
is catalyzed by heavy metal ions, such as Cu++, Fe+++, and Mn++
(Sanderson & Edson, 1964).
The degradation pathways of dimethoate in air and water
under environmental conditions are presented in Fig. 1.
4.1.3. Soil
The half-life of dimethoate after application at approxi-
mately 1 kg/ha in sandy loam soil, was approximately 4 days
during drought conditions and 2.5 days after moderate rainfall
(Bohn, 1964). Following 3 applications, dimethoate did not
leach more than 7.5 cm below the surface of the soil.
Getzin & Rosefield (1968) studied the persistence of
dimethoate in non-sterile, autoclaved, and gamma-radiation-
sterilized Orissa soils. Two weeks after application, the
degradation of dimethoate was 18% in the autoclaved soil, 20% in
irradiated soil, and 77% in non-sterile soil. The half-life of
dimethoate ranged from approximately 9 to 11 days under non-
sterile conditions and from 16 to 18 days under sterile
conditions (Sahu & Pattanaik, 1980).
Molecular formula: C5H12NO3PS2
Common name: dimethoate (accepted by BSI, ISO,
ANSI, and JMAF); fosfamid (used in
USSR)
Common trade names: Bi 58; Cygon; Dimethoate; Fosfamid;
Fostion MM, Rogor; Perfekthion;
Roxion
IUPAC name: O,O-dimethyl S-methyl-carbamoyl-
methyl phosphorodithioate
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester
(9CI)
CAS registry number: 60-51-5
RTECS registry number: TE1750000
Technical dimethoate is about 93-95% pure. The major
impurities are O,O-dimethyl S-methylphosphorodithioate and
O-O-S-trimethyl phosphorodithioate.
2.2. Physical and Chemical Properties
Pure dimethoate is a colourless crystalline solid with an
odour of mercaptan. Technical dimethoate (about 93% pure)
varies from off-white crystals to a grey semi-crystalline
material. Some physical and chemical properties of dimethoate
are given in Table 1.
Dimethoate is highly soluble in chloroform, methylene
chloride, benzene, toluene, alcohols, esters, and ketones,
slightly soluble in xylene, carbon tetrachloride, and aliphatic
hydrocarbons, and fairly soluble in water.
Dimethoate is fairly stable in water and acid solution, at
room temperature, and unstable in alkaline solution (Table 1).
Heating converts it to the O,S-dimethyl phosphorodithioate.
Table 1. Some physical and chemical properties of dimethoate
-------------------------------------------------------------
Relative molecular mass 229.2
Odour threshold 0.010 mg/m3
Melting point 45-52.5 °C
Boiling point 107 °C at 0.05 mmHg
86 °C at 0.01 mmHg
Vapour pressure (25 °C) 8.5 x 10-6 mmHg
Volatility 1.107 mg/m3
Specific gravity 1.281
(compared to water)
Partition coefficient n-octanol/water 5.959
Solubility in water (21 °C) up to 39 g/litre
Half-life: in aqueous media at pH 2-7, relatively stable
at pH 9, 50% loss in l2 days
-------------------------------------------------------------
2.3. Analytical Methods
A review of the detection methods for dimethoate in treated
crop plants has been presented by De Pietri-Tonelli et al.
(1965). The procedures reported are based on colorimetry,
column, paper, and thin-layer chromatography, paper electro-
phoresis, gas chromatography, and radiometry. Bioassay tech-
niques and autoradiographic procedures can also be applied.
High-performance thin-layer chromatography has been proposed by
Hauck & Amadori (1980) as a new potential for the determination
of dimethoate.
The Codex Committee on Pesticide Residues has listed
recommended methods for the determination of dimethoate residues
(FAO/WHO 1986) and various methods used in the determination of
dimethoate are summarized in Table 2.
A personal air sampler to measure vapours and aerosols of
dimethoate at low concentrations has been described by Hill &
Arnold (1979).
Table 2. Methods for the determination of dimethoate
---------------------------------------------------------------------------------------------------------
Sample type Method of detection Comments Detection limit Reference
---------------------------------------------------------------------------------------------------------
Soil gas-liquid chromato- 1-20 ng Getzin & Rosefield
graphy/phosphorus (1968)
detection
Soil thin-layer chromato- 50-mg soil samples; ex- 0.1 mg/kg; Akoronko & Girenko
graphy; gas-liquid traction with chloroform 0.05 mg/kg (1977)
chromatography/therm-
ionic detection
Water thin-layer chromato- 200-ml sample; extraction 0.5 µg; Girenko et al.
graphy; gas-liquid with chloroform 5 ng (1978)
chromatography/therm-
ionic detection or
electron capture
detection
Wheat plants gas chromatography/ suitable for determina- 0.02 mg/kg Lee & Westcott
flame photometric tion of dimethoate and (1981)
detection dimethoxon (omethoate)
residues in field wheat
plants
Plants colorimetry enzymatic pig liver 1 µg dimethoate Nanda Kumar &
powder used as Udaya Bhaskar
ChE source; more sensi- (1980)
tive by converting into 50 ng omethoate Udaya Bhaskar &
oxidation product Nanda Kumar (1980)
Fruits and gas-liquid chromato- extraction with methyl 0.02 mg/kg Girenko & Klisenko
vegetables graphy/thermionic chloride (1977)
detection
Fruits and colorimetry 250 g of sample; extrac- 5 µg (0.1 mg/kg) Chilwell & Beecham
vegetables tion with chloroform (1960)
Asparagus gas chromatography extraction with 0.002 mg/kg Szeto et al.
nitrogen/phosphorus/ ethyl acetate (fresh weight) (1985)
detection
---------------------------------------------------------------------------------------------------------
Table 2. (contd).
---------------------------------------------------------------------------------------------------------
Sample type Method of detection Comments Detection limit Reference
---------------------------------------------------------------------------------------------------------
Vegetables gas chromatography/ 25 g of sample; extrac- 0.008 mg/kg Van Middelem &
(snap bean) electron affinity tion with methylene Waites (1964)
detection chloride; oxygen analogue
could not be detected
at a 1:1 ratio; detectable
at 10 parts oxygen to 1
part dimethoate
Food stuffs high-performance suitable for pesticide 0.3 mg/kg Cabras et al.,
liquid mixtures; the method can (1979)
chromatography also be used for air
samples
Honey thin-layer chromato- extraction with hexane 0.1 mg/kg Petukhov (1975)
graphy and chloroform
Honey, nectar, gas chromatography/ extraction with benzene 0.1 ng in nectar; Barker et al.
and pollen flame photometric 0.5 ng in pollen (1980)
samples detection
Milk gas chromatography/ samples heated at 60 °C 0.001 mg/kg Beck et al.
flame photometric in water bath for 20 min (1968)
detection to facilitate precipi-
tation; extraction with
methylene chloride
Animal tissues thin-layer chromato- dimethoate and dimethoxon Sidimanov (1971)
graphy (omethoate) can be detected
25 days after death of the
animal
Skin and respir- gas chromatography/ suitable for field 0.01 µg/sample Copplestone
ator pads flame photometric studies; pads placed in et al. (1976)
detection individual 30-ml glass
bottles, each contain-
ing 10 ml benzene
Technical gas-liquid chromato- - - WHO (1986c)
material and graphy/flame ionization
formalizations detection
---------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Dimethoate does not occur as a natural product.
3.2. Man-made Sources
3.2.1. Industrial production
Dimethoate was first described by Hoegberg & Cassaday (1951)
and was introduced on the market in 1956.
3.2.2. World production figures
Dimethoate is manufactured in many countries, but data on
the world production of dimethoate are not available.
3.2.3. Uses
Dimethoate formulations are widely used as contact and
systemic insecticides against a broad range of insects and mites
and is applied at 0.3-0.7 kg active ingredient/ha on numerous
crops: fruits (apples, citrus, bananas, mangoes), vegetables
(beans, broccoli, cabbage, cauliflower, pepper, potatoes,
spinach, tomatoes), wheat, alfalfa, cotton, tobacco,
ornamentals, olives, sunflower, and others (Worthing & Walker,
1983).
Dimethoate is also used for the indoor control of house-
flies. For residual treatment, 10-25 g/litre formulations are
used (0.046-0.5 g active ingredient/m2) (WHO, 1984). The dose
of dimethoate for outdoor fly control is 224 g active
ingredient/ha (WHO, 1984).
Dimethoate is also applied as a systemic insecticide for
control of cattle grubs (Worthing & Walker, 1983).
The oxygen analogue of dimethoate, dimethoxon, is also used
as insecticide and is known under the common name of omethoate.
Formulations of dimethoate include emulsifiable concen-
trates, wettable powders, and granules. There is also a formu-
lation for ultra low volume application.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
4.1.1. Air
Air concentrations of dimethoate were measured under hot
climatic conditions at a distance of 300 m from a sprayed
area. Concentrations on the day of spraying ranged from
0.061 to 0.142 mg/m3, but decreased during the next 4 days to
0.004-0.014 mg/m3. At a distance of 1500 m, dimethoate was not
detected on either the day of treatment or during the days that
followed (Madzhidov, 1970).
Dimethoate is an intermediate product in the hydrolysis of
the pesticide formothion (Melnikov et al., 1977; Bolotnyi et
al., 1978). After use, formothion is found in the air the day
of spraying and dimethoate during the following days up to the
10th day.
In moist air, dimethoate is degraded photochemically to
hydrolytic and oxidation products (Melnikov et al., 1977).
4.1.2. Water
Aqueous solutions of dimethoate are fairly stable. The
compound is rapidly hydrolysed in alkali (pH 11): about 50-57%
of dimethoate degrades to water-soluble material in ´ h, 68% in
1 h, and 87% in 2 h. The predominant degradation product is
desmethyl dimethoate (49.3%) (Brady & Arthur, 1963). Hydrolysis
is catalyzed by heavy metal ions, such as Cu++, Fe+++, and Mn++
(Sanderson & Edson, 1964).
The degradation pathways of dimethoate in air and water
under environmental conditions are presented in Fig. 1.
4.1.3. Soil
The half-life of dimethoate after application at approxi-
mately 1 kg/ha in sandy loam soil, was approximately 4 days
during drought conditions and 2.5 days after moderate rainfall
(Bohn, 1964). Following 3 applications, dimethoate did not
leach more than 7.5 cm below the surface of the soil.
Getzin & Rosefield (1968) studied the persistence of
dimethoate in non-sterile, autoclaved, and gamma-radiation-
sterilized Orissa soils. Two weeks after application, the
degradation of dimethoate was 18% in the autoclaved soil, 20% in
irradiated soil, and 77% in non-sterile soil. The half-life of
dimethoate ranged from approximately 9 to 11 days under non-
sterile conditions and from 16 to 18 days under sterile
conditions (Sahu & Pattanaik, 1980).
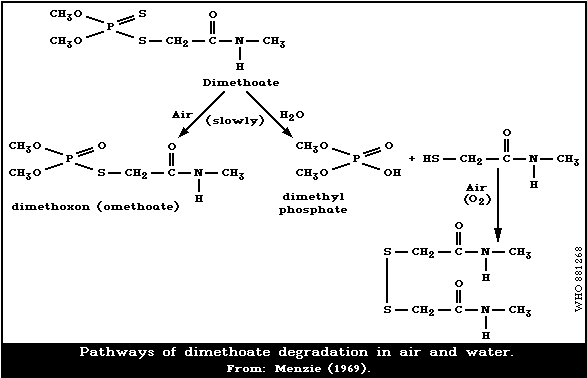 Different factors may affect the accumulation and degra-
dation of dimethoate in soil, such as the soil type, the numbers
and type of microorganisms present in soil, the environmental
temperature, the pH level, the amount of pesticide applied, and
the degree of evaporation (El Beit et al., 1977a,b, 1978). The
persistence of dimethoate was greater in heavy than in light
soil. At pH 4.2, the pesticide was stable for nearly 19 days;
at pH 11, it degraded within 20 h. The amount of dimethoate in
soil increased when higher concentrations were applied. El Beit
et al. (1977a) reported that soil microorganisms played little
part in the degradation of dimethoate.
4.1.4. Plants
When applied to plants, dimethoate was rapidly absorbed and
decomposed, both on the surface and within the plant, by
hydrolysis and oxidation (Menzie, 1969; Melnikov et al., 1977).
The half-life of dimethoate in the different plants varied
between 2-5 days (Melnikov et al., 1977). Dimethoate completely
disappeared after 15-30 days, depending on the plant species and
the climatic conditions. Decomposition in plants and the
hydrolysis of dimethoate increased with temperature (Atabaev,
1972).
The dissipation of dislodgeable residues of dimethoate is
best characterized by two first-order kinetic processes. The
half-life values were 2.2 days in the 1 to 10-day period and 7.0
days in the 10 to 49-day period (Hadjidemetriou et al., 1985).
4.1.5. Disposal of wastes
Hydrolytic decomposition is the main way to inactivate
dimethoate. By adding lime (1-2 kg calcium oxide/m3 water) to
waste waters from agricultural centres, dimethoate was fully
inactivated in 45 min (Winkler & Muller, 1979).
During pyrolysis, approximately 50% decomposition of di-
methoate occurred at 500 °C with the formation of O,O-dimethyl-
S,S-dithionpyrophosphate; decomposition was complete at 1100 °C
(Rosvaga, 1983).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air, water, and soil
No studies have been reported on levels in air, water, or
soil under actual conditions of use and various environmental
conditions.
5.1.2. Food
When a combination of dimethoate and omethoate (dimethoxon)
was given to cows in dosages of 1 and 0.1 mg/kg body weight,
respectively, for 14 days, only residues of the metabolite
omethoate were observed in the milk (0.004-0.125 mg/kg). Three
days after the application, neither compound could be detected.
When dosages of 0.5 mg dimethoate/kg and 0.05 mg omethoate/kg
were given for 14 days or when corn silage containing 1-7 mg
dimethoate/kg (resulting in dosages of 0.06-0.36 mg/kg body
weight) was given for 28 or 42 days, no residues were detected
in the milk (Beck et al., 1968).
Harvest residues found in many crops 1-3 weeks after
spraying with dimethoate, during the first years of application
in the United Kingdom (1957-58), were below 2 mg/kg (Chilwell &
Beecham, 1960).
The dimethoate content of apples was 0.03-0.07 mg/kg, 75
days after an application of 0.72 kg active ingredient/ha
(Atabaev & Stepovaya, 1966).
The pulp of lemon and orange fruit treated with dimethoate
did not show any residues at a detection level of 0.01 mg/kg,
60 days after application (Iwata et al., 1979).
Residues of dimethoate do not concentrate in wine. Analysis
of seven different Californian wines indicated levels of less
than 0.03 mg/litre (Kawar et al., 1979).
No residues were found in grapes, 29 days after treatment
with 0.1-0.15% dimethoate applied at the rate of 1400 litre/2 ha
(Grigorashvili & Dzhibladze, 1965).
A number of studies on residues of dimethoate found through-
out the world have been reported in a review by De Pietri-
Tonelli et al. (1965). The residues were most frequently below
1 mg/kg. Dimethoate residues found in various agricultural
products in India are reported in Khan & Bhaskar Dev (1982).
Dimethoate was not found in total-diet samples studied in
England and Wales during 1966-67 (Abbott et al., 1970).
According to other authors, dimethoate was also not found in the
total diets of adults and infants during 1975-79 (Johnson et
al., 1981a,b, 1984a,b; Podrebarac, 1984a,b; Gartrell et al.,
1985a,b,c). Market-basket surveys carried out in 1976-78 in the
Netherlands showed only omethoate residues in a small number of
fruits (De Vos et al., 1984). In the USA, dimethoate and
omethoate have been identified in about 5% of samples of fruits
and vegetables (Duggan et al., 1983).
The use of additional procedures for the determination of
organophosphates resulted in the identification of dimethoate in
adult and infant total-diet samples in 1980-82 (Gartrell et al.,
1985d, 1986a,b). However, the intakes (0.001 µg/kg body weight
per day) were far below the FAO/WHO acceptable daily intake
(ADI) (see section 12).
5.2. Occupational Exposure
Immediately after the use of dimethoate in greenhouses,
Stroy (1983) determined levels of 0.01-0.42 mg/m3 in the air,
3.6-9.3 mg/kg in the plants, and 0.98-1.75 mg/kg in the soil.
Dimethoate content in greenhouse air has been analysed at
different times after spraying; at 0 time the measured amount
was 0.66 mg/m3, at 2.5 h it was 0.38 mg/m3, at 5 h dimethoate
content was 0.21 mg/m3, at 10 h it was 0.07 mg/m3 and at 20 h
it was 0.01/m3 (Zolotnikova & Zotov, 1978).
The exposure to dimethoate of tractor drivers using airblast
units during treatment of citrus trees was investigated by
Carman et al. (1982). Dermal exposure was measured by placing
ethyleneglycol-treated gauze patches on the shoulders, upper
arms, and knees of the drivers. An emulsifiable concentration
(EC) formulation of dimethoate containing 0.009 kg/litre was
applied at the rate of 16 822 litre/ha using an open tractor, a
cab unit with both side windows open, and a cab unit with the
windows closed. Under these conditions, the patches attached
to the driver absorbed mean deposits of 2.5, 1.5, and
< 0.01 µg dimethoate/cm2 per h, respectively. The correspond-
ing average air concentrations were 10(sic), 48, and 2 µg of
dimethoate/m3.
Procedures for determining foliar residues and to establish
the safe re-entry times for some insecticides were reported by
Knaak (1980) and Knaak et al. (1980). A safe level of
dimethoate on foliage of 53 µg/cm2 was calculated using the
results of dermal-dose ChE-response studies in male rats.
Minimum safe re-entry periods for dimethoate were estimated
to be 3 days in greenhouses, and 7 days in tobacco fields, after
application by tractor, or 5 days after application by plane
(Kaloyanova-Simeonova & Izmirova-Mosheva, 1983). No ChE
inhibition in serum or subjective complaints of workers picking
dimethoate-treated tobacco leaves were established at a concen-
tration of 1 mg/kg on the surface of the plant.
Copplestone et al. (1976) studied the exposure to dimethoate
of 8 spraymen, 1 mixer, and 2 supervisors in the Sudan. The
percentage of toxic dosea received per day, calculated on the
basis of a 4-h working day, varied from 0.02% to less than
0.001% for individual spraymen. No ChE depression was found in
any of the men.
The highest concentration of dimethoate, measured in the
work-place air of a dimethoate-producing factory in Italy, was
0.050 mg/m3 (Armeli et al., 1967).
The respiratory and dermal exposure to dimethoate of
applicators was determined for greenhouse workers. The
respiratory and dermal exposures were 0.034 mg/h and 30 mg/h,
respectively. The hands of the operators were the most affected
parts of the body, accounting for 63-92% of the total exposure
(Adamis et al., 1985).
----------------------------------------------------------------
a Percentage toxic dose per day or h (WHO, 1982). This is
calculated from these indices adapted from the method of
Durham & Wolfe (1962) using the formula:
Dermal exposure (mg/day or h) + respiratory
exposure (mg/day or h) x 10 if measured
-------------------------------------------- x 100
Dermal LD50 mg/kg (rat) x 70
6. KINETICS AND METABOLISM
6.1. Absorption and Distribution
Panshina & Klisenko (1962) checked the blood levels of
dimethoate in cats and rats after single oral doses of 50, 75,
or 200 mg/kg in the cat and 300 mg/kg in the rat. The deter-
minations were carried out 15, 30, 60, 90, 120, and 180 min
after dosing. Dimethoate was detected in the blood of cats and
rats after 30 min, and reached a maximum level after 60-90 min.
Nearly 80% of the dimethoate in the blood was found in the
erythrocytes; only 15-20% was found in the serum. With repeated
daily oral intake of dimethoate at doses of 20 mg/kg or
10 mg/kg, the maximum blood level occurred on the 5-10th day of
the study.
The same pattern in blood levels was observed with repeated
inhalation of dimethoate for 4 h/day over 3 months, at a mean
concentration of 5 mg/m3 air. Dimethoate was detected in blood
from the second day and reached its maximum by the 7-10th day.
Daily application of 50 mg dimethoate/kg on the skin of
rabbits resulted in a maximum concentration in the blood at
about the third day (Kundiev, 1979).
When dimethoate was applied to the skin of rats for 1, 2, 4,
12, or 24 h in a single dose of 560 mg/kg, the maximum concen-
tration in the skin was reached after 12 h of exposure and was
correlated with the maximum inhibition of ChE activity in the
serum and liver. The concentrations of dimethoate in the blood,
liver, and kidney were maximal after 2 h of exposure (Baranova
et al., 1986).
6.2. Metabolic Transformation
The ester and amide groups of dimethoate are cleaved in
reactions that vary with the organism and that contribute to the
selective toxicity of the compound.
The results of in vitro and in vivo studies showed that the
main metabolic pathways of dimethoate were hydrolysis and
oxidation (Hassan et al., 1969; Lucier & Menzer, 1970; North &
Menzer, 1972).
Santi & Giacomelli (1962) studied the metabolic fate of
dimethoate in olives. P=O derivative and degradation products,
such as phosphoric and/or methylphosphoric acid, were found.
The presence of the oxygen analogue dimethoxon (omethoate)
has been demonstrated in insects, plants, and mammals; it
appears to be the metabolite responsible for the toxic action of
dimethoate (Brady & Arthur, 1963; Hassan et al., 1969; Lucier &
Menzer, 1970). The highest levels of this metabolite were found
in insects, particularly in those highly susceptible to
dimethoate. The oxygen analogue was produced in larger
quantities in insects than in rats. The enzymes mediating the
hydrolysis of the carboxyamide bond are much less effective in
insects than in mammals (Mikhailov & Shterbak, 1983).
It has been shown that cleavage of dimethoate by rats and
cows occurs initially at the C-N bond to produce the carboxy
derivative (Dauterman et al., 1959; Hassan et al., 1969). A
second hydrolytic pathway involves an esterase action on the S-C
bond (Hassan et al., 1969).
Oxidative metabolism of dimethoate predominated over
hydrolytic metabolism in the cell culture system. In the whole
rat, the opposite was true. Metabolism of dimethoate in human
embryonic lung cells was much the same as metabolism in rats
(North & Menzer, 1972). In vitro and in vivo studies showed
that dimethoate is biotransformed to the P=O analogue via the
liver cytochrome P-450 system (Kaloyanova et al., 1984).
Concentrations of 0.1-10 mmol dimethoate/litre led to a linear
decrease in the rates of N-demethylation and P-hydroxylation.
Similarly, in microsomes from rats treated with dimethoate in
vivo, increased activity of desulfuration (140%, P < 0.01), and
decreased activity of hydroxylation and demethylation were seen
(Mitova et al., 1986).
In vivo studies on mice showed dimethoate toxicity to be
markedly increased by phenobarbital pre-treatment, as a result
of induction of hepatic microsomal enzymes including the mixed
function oxidases responsible for the conversion of P=S to P=O
(Menzer & Best, 1968).
It has been found that, while dimethoate undergoes rapid
degradation in the rat liver, very little occurs in other
tissues (lung, muscle, pancreas, brain, spleen, blood). The
ability of the liver to degrade dimethoate in various species
decreased in the order: rabbit > sheep > dog > rat > cattle >
hen > guinea-pig > mouse > pig. For the hen, cattle, mouse,
sheep, and rat there was a reasonably good straight-line
relationship between the LD50 values and the degradation ability
of the liver (Uchida et al., 1964).
After administration of 32P-dimethoate to rats, dimethoate,
dimethoxon, dimethoate carboxylic acid, dimethylphosphoro-
dithioate, dimethylphosphorothioate, dimethylphosphate, mono-
methylphosphate, phosphorothioate, formate, and N-methyl 2-
glucuronate acetamide were found in the urine (Hassan et al.,
1969).
Data on the metabolism of dimethoate in plants and animals
have been reviewed by Menzie (1969, 1974, 1978, 1980).
6.3. Elimination and Excretion
6.3.1. Animal studies
About 45% of the 32P-dimethoate administered orally at
50 mg/kg to rats was excreted in the urine, while only 5.8% was
eliminated in the faeces, 72 h after treatment (Brady & Arthur,
1963). The values in rats after dermal application were 30.6%
and 6.5%, respectively. More than 95% of the 32P materials in
the urine and faeces after oral or dermal administration in rats
were hydrolytic products, as determined by chloroform/water
partition coefficients.
Twenty-four h after ip and oral administration of dimethoate
to rats at doses of 0.25, 2.5, or 25 mg/kg, dimethylphosphoro-
dithioate, dimethylphosphorothioate, and dimethyl phosphate
were detected in the urine at concentrations of 12-14%, 11-15%,
and 12-13%, respectively (Riemer et al., 1985). Neither the
route of exposure nor the dose had any influence on the types of
metabolite formed.
About 87-90% of an oral dose of 10 mg dimethoate/kg was
eliminated in the urine of cattle at the end of 24 h. The same
percentage of an intramuscular dose of 10 mg/kg was excreted
after 9 h. Only 3.7-5% of the oral dose and about 1.1% of the
intramuscular dose were eliminated in the faeces after 72 h and
24 h, respectively (Kaplanis et al., 1959).
6.3.2. Human studies
In human beings, 76-100% of radioactivity was reported
to be excreted in the urine, 24 h after oral dosing with 32P-
dimethoate (Sanderson & Edson, 1964).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The addition of dimethoate to soil at 10 or 100 mg/kg did
not result in significant differences in the number of bacteria
solubilizing tricalcium phosphate or in the number of bacteria
mineralizing calcium glycerophosphate, but an increase in the
population of phospholipase-producing organisms solubilizing
lecithin occurred (Congregado et al., 1979). At 10 mg/kg, an
increase in carbon dioxide production occurred for 2 weeks after
treatment, followed by a decrease to control levels. At
100 mg/kg, the increase in carbon dioxide output was slower and
longer.
7.2. Aquatic Organisms
A number of LC50s have been determined for various aquatic
organisms (Table 3).
The median tolerance limit of the fresh-water teleost,
Channa punctatus for dimethoate is 20.5 mg/litre (Anees, 1975).
Exposure for 24 h, 96 h, or 14 days to dimethoate concentrations
of 10.8, 8.0, or 5.0 mg/litre, respectively, produced moderate
vacuolation of the liver and a high degree of cytoplasmic
granulation, which developed for up to 96 h of exposure. The
14-day exposure added little in the way of vacuolation or
granulation (Anees, 1978a). The haematological response to
dimethoate included reduced erythrocyte counts and haemoglobin
concentration, and an elevated mean corpuscular haemoglobin and
colour index indicating that the insecticide exerted an effect
similar to the production of anaemia (Anees, 1978b).
The signs of the toxicity of dimethoate in fish
(Channa punctatus) included jumping, erratic movement,
imbalance, and death (Dikshith & Raizada, 1981a; Dikshith,
1986).
Verma et al. (1978) determined the TLm values of dimethoate
for Channa gachua for 24, 48, 72, or 96 h to be 5.2, 5.0, 4.6,
or 4.5 mg/litre, respectively. The safe concentration of
dimethoate calculated on the basis of TLm values was approxi-
mately 1.4 mg/litre.
Dimethoate inhibited AChE activity in the brain, liver, and
muscle of some fresh-water teleosts (Channa gachua and Cirrhina
mrigala), exposed to sublethal concentrations of 35% EC
formulation (0.9-2.4 and 0.6-1.6 mg/litre, respectively) (Verma
et al., 1979).
Table 3. Summary of acute toxicity values for aquatic organisms
---------------------------------------------------------------------------------------------------------
Species LC50 (mg/litre) Reference
24-h 48-h 72-h 96-h
---------------------------------------------------------------------------------------------------------
Rainbow Trout 20 - - 8.5 Melnikov et al. (1977)
(Salmo gairdneri)
Rainbow Trout - - - 6.2 Johnson & Finley (1980)
(Salmo gairdneri)
Long-nosed killifish 1.0 - - Melnikov et al. (1977)
(Fundulus similis)
Saccobranchus fossilis 5.14 4.80 4.67 4.57 Verma et al. (1982)
Channa punctatus 68 54 - 47 Dikshith & Raizada (1981a)
Dikshith (1986)
Scud 0.9 0.4 - - Menzie (1969)
(Gammarus lacustris)
Scud - - - 0.20 Johnson & Finley (1980)
(Gammarus lacustris)
Red Crayfish - 1.0 - - Muncy & Oliver (1963)
(Procambarus clarkii)
Stonefly - 0.14 - - Menzie (1969)
(Pteronarcys california)
Stonefly - - - 0.043 Johnson & Finley (1980)
(Pteronarcys california)
Unspecified insect 0.51 - - - Sanders & Cope (1968)
Bluegill - - - - Johnson & Finley (1980)
---------------------------------------------------------------------------------------------------------
Dalela et al. (1979) reported that acute (5-h) and short-
term (up to 32 days) exposure of the fish, Channa gachua, to
dimethoate at 6.2 mg/litre and 1.5 mg/litre, respectively,
produced histological changes in the gills. On acute exposure,
there was erosion at the distal end of the gill filaments and
loss of cell membrane. With exposure to a concentration of
1.5 mg/litre, the basement membrane started separating, and the
damage to the gill was found to be more significant with
increasing exposure time, with vacuolization occurring after 32
days.
The exposure of the fish Heteropneustes fossilis to a
dimethoate concentration of 10 mg/litre led to an increased
level of glycogen by the end of the second week in both the
liver and the kidney, and to a slight decrease in the protein
contents at the end of the eighth day (Awasthi et al., 1984). A
sharp rise in the activity of succinate dehydrogenase in both
organs was noted during the first two weeks of this study.
The estimated 48- and 72-hour TLm values for zebrafish
Brachydanio rerio embryos, exposed to dimethoate, were
940 mg/litre and 259 mg/litre, respectively (Roales &
Perlmutter, 1974). Dimethoate retarded the development of
embryos as expressed by lack of heartbeat and little movement at
24 h.
Dimethoate at a concentration of 0.05 mg/litre produced
morphological changes in the melanophores of Bufo melano-
stictus tadpoles and an increase in pigmented areas of the skin
(Pandey & Tomar, 1985).
Dimethoate had a very low toxicity for some aquatic
organisms in Sudan, such as Oreochromis niloticus, Gambusia
affinis, Pseudagrion spp., Crocothemis erythraea, and Lanistes
carinatus. Under laboratory conditions, it did not kill any
animal at concentrations lower than 80 mg/litre (Karim et al.,
1985).
The toxicity of dimethoate for 11 freshwater species was
studied by Slooff & Canton (1983). The results are summarized
in Table 4. The relative susceptibility tests indicated that
Daphnia magna was the organism most sensitive to dimethoate,
while the microorganisms P.fluorescens, M.aeruginoso, and
S.pannonicus were generally less sensitive indicators of
toxicity. The susceptibility of aquatic species to a chemical
may vary by more than two-three orders of magnitude. The data
demonstrate that the sublethal criteria studied were not
necessarily the most sensitive toxicological criteria.
7.3. Terrestrial Organisms
7.3.1. Honey-bees
The oral LD50 for the honey-bee (Apis mellifera L) ranges
from 93 to 150 ng per bee (Jaycox, 1964; Lord et al., 1968;
Stevenson, 1968; Barker et al., 1980). The contact LD50 is
98-120 ng per bee (Stevenson, 1968).
Table 4. Fresh-water species susceptibility to dimethoate
---------------------------------------------------------------------------------------------------------
Type of Test species Lifestage Exposure Test condition Toxicological No-effect
organism time Temper- Test parameter level
(days) ature methods (mg/litre)
---------------------------------------------------------------------------------------------------------
Bacteria (Pseudomonas fluorescens) log-phase 0.3 22 ± 2 Static Specific 320
growth rate
Cyano Bluegreen bacteria log-phase 4 23 ± 2 Static Specific 32
bacteria (Microcystis aeruginosa) growth rate
Algae Green Algae log-phase 4 23 ± 2 Static Growth 100
(Scenedesmus pannonicus) (biomass)
Plant Lemna minor - 7 25 ± 1 Static Specific 32
growth
rate
Crustacean Water flea (Daphnia magna) 1 day 21 19 ± 1 Semi- Mortality 0.032
static reproduction 0.1
Insect Mosquito (Culex pipiens) 1st 25 27 ± 1 Semi- Mortality 0.32
instar static development 0.32
Coelente- Hydrozoan (Hydra oligactis) budless 21 18 ± 1 Semi- Specific 100
rate static growth rate
Mollusc Giant Pond Snail 5 months 40 20 ± 1 Semi- Mortality 32
(Lymnaea stagnalis) static Reproduction 10
egg 7 20 ± 1 Semi- Hatching 32
static
Fish Guppy (Poecilia Reticulata) 3-4 weeks 28 23 ± 1 Semi- Mortality 32
Viviparous static Behaviour + 0.1
Mortality
Fish Japanese Ricefish eggs 40 23 ± 2 Semi- Mortality 0.32
(Oryzias latipes) oviviparous static Behaviour 0.32
Hatching 100
Growth
Amphibian Xenopus laevis 2 days 100 20 ± 1 Semi- Mortality 1
static Development 32
Growth 32
---------------------------------------------------------------------------------------------------------
From: Slooff & Canton (1983).
Dimethoate was only slightly repellent to foraging honey-
bees. The self-limiting dose for foraging was 20-25 times the
lethal oral dose (2.9-3.9 µg/bee vs 150 ng/bee). This can be
interpreted on the basis of 5% absorption by foraging bees,
while 95% is passed on to the colony. Thus, systemic insecti-
cide in nectar may also pose a threat to the rest of the colony
when brought back to the hive (Waller et al., 1979).
Residual toxicity has been supported by several obser-
vations. Nectar from plants sprayed with 0.1% dimethoate was
lethal for honey-bees for at least 2-3 days (Jaycox, 1964) or 10
days (Barker et al., 1980). Waller et al. (1984) also showed
the possible toxic levels of residues in the nectar for up to
10 days after treatment of lemon trees with dimethoate at a rate
of 1.12 kg of ai per ha. The high bee mortality observed,
immediately after treatment, was attributed to dimethoate
residues on the plant surface.
7.3.2. Birds
The acute toxicity studies of dimethoate for birds are
summarized in Table 5.
Table 5. Acute oral LD50 of dimethoate for birds (mg/kg)a
----------------------------------------------------------------
Species Sex Pure Laboratory Technical Liquid
grade grade formulation
----------------------------------------------------------------
Hen F 50 40 30 25
Pheasant M 15 - 20 15 20 25
Duck F - > 40 - -
Sparrow M/F - - - 22
Blackbird M/F - - - 26
----------------------------------------------------------------
a From: Sanderson & Edson (1964).
Hens did not show any evidence of delayed neurotoxicity
(Sanderson & Edson, 1964; Gaines, 1969; Francis et al., 1985).
The effect of dimethoate on esterase levels following the
oral dosing of pheasants and following long-term feeding to
pheasants and pigeons was investigated by Bunyan et al. (1968,
1969). Dimethoate inhibited brain-alpha-naphthyl acetate
esterase more than brain-cholinesterase and triacetin esterase
in acute studies.
A characteristic of dimethoate was the elevation of phenyl
benzoate esterase levels, showing that after initial liver
damage, dimethoate is able to induce certain enzymes.
7.3.3. Farm animals
The acute oral LD50s for several farm animals are
summarized in Table 6.
No visible signs of intoxication were seen in horses
receiving dimethoate orally at doses of 25 or 50 mg/kg. Single
doses of dimethoate at 40 mg/kg were effective in removing
Gasterophilus spp. from infected horses, but toxic signs
appeared in animals treated with higher levels of 60-80 mg/kg
(Jackson et al., 1960).
Table 6. Acute oral LD50 for farm animals
-------------------------------------------------
Species LD50 (mg/kg Reference
body weight)
-------------------------------------------------
Horse > 50 Jackson et al. (1960)
Sheep 80 Hewitt et al. (1958a,b)
Cattle 70 Hewitt et al. (1958a,b)
-------------------------------------------------
Mild signs of intoxication occurred in sheep at 75 mg/kg,
including slight salivation, lachrymation, transitory diarrhoea,
rhinitis, and anorexia. Doses lower than 15 mg/kg were essen-
tially asymptomatic in calves. The data with dimethoate
indicate an appreciable margin of safety between the lowest
dose that kills first instar Hypoderma lineatum (5 mg/kg), and
the doses that produce mild toxicity (15-20 mg/kg), or severe,
reversible toxicity (40 mg/kg) (Hewitt et al., 1985b).
Fetcher (1984) described cases of suspected dimethoate
intoxication in cattle grazing on pasture that had been sprayed
6 weeks earlier. There was a predominance of nicotinic signs
and a poor response to atropine treatment. Chemical analysis of
liver, kidney, and brain tissue did not reveal any organo-
phosphorus compounds or metabolites. Whole blood-ChE was
depressed in 3 out of 14 animals.
After spraying barns (for calves) and pigsties with
dimethoate, only 16-29% of the initial concentration still
persisted after 8 weeks. Nevertheless, the animals showed a
decrease in ChE (Müller & Reinhold, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides, especially their short- and long-term effects on
the nervous systems, can be found in the WHO Environmental
Health Criteria document entitled EHC 63: Organophosphorus
Insecticides, a General Introduction (WHO, 1986a).
8.1. Single Exposures
The acute oral and dermal LD50s of dimethoate for several
animal species are summarized in Tables 7 and 8 (all LD50s are
expressed as active ingredient).
Signs, characteristic of organophosphorus intoxication,
were observed in the rat 0.5-2 h after oral administration of
dimethoate (Sanderson & Edson, 1964). They included muscular
fibrillation, salivation, lachrymation, urinary incontinence,
diarrhoea, respiratory distress, prostration, gasping, coma, and
death (WHO, 1986a).
Oral LD50 values for rats, which were measured in 13
studies, ranged from 150 to 680 mg/kg body weight. The purity
and formulation of the compounds used were not stated in most
of the reports. Oral LD50s were determined of 60-140 mg/kg
body weight for mice, 200 mg/kg body weight for hamsters,
350-600 mg/kg body weight for guinea-pigs, 280-500 mg/kg body
weight for rabbits, and 100 mg/kg body weight for cats.
Administration of 100 mg/kg body weight to dogs did not result
in mortality. The World Health Organization based its classi-
fication of dimethoate as moderately hazardous on an acute oral
LD50 in the rat of 150 mg/kg body weight (WHO, 1986b).
The dermal LD50s for rats were found to range between 500
and 1150 mg/kg body weight, and were of about the same order of
magnitude as the oral LD50s or slightly higher (Table 8).
Data on LD50s after parenteral administration were given by
Sanderson & Edson (1964). The values were comparable with those
for ip, sc, and iv administration. In the rat, the values for
ip administration varied between 175 and 325 mg/kg body weight
and were also of the same order of magnitude as the oral LD50s.
The inhalation LC50 has not been estimated, but, in a 4-h
inhalation study on rats, Panshina (1963b) did not find any
signs of intoxication with exposure to dimethoate at 20 mg/m3
air, 40% cholinesterase inhibition at 10 mg/m3, and no effects
at 2 mg/m3. Visible signs of intoxication were observed in cats
at concentrations of 50-80 mg/m3. At 20 mg/m3, cholinesterase
inhibition was found to be 10-66%, while at 2-8 mg/m3, it was
7-56%. No effects were seen at 1.5 mg/m3 (Panshina, 1963b).
Table 7. Acute oral LD50 of dimethoate in experimental animals
-------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
-------------------------------------------------------------------------
Rat M 32% emulsifiable 247 Edson & Noakes (1960)
solution
Rat M/F technical 185 - 245 West et al. (1961)
Rat 230 Panshina (1963a)
Rat technical 172 Panshina (1963a)
Rat M/F pure 500 - 680 Sanderson & Edson
(1964)
Rat M/F laboratory 280 - 356 Sanderson & Edson
grade (1964)
Rat M/F technical 180 - 336 Sanderson & Edson
(32-40% w/v) (1964)
Rat M/F liquid formul- 150 - 400 Sanderson & Edson
ation (20% ai) (1964)
Rat M/F wettable powder 280 - 300 Sanderson & Edson
(1964)
Rat pure 250 Atabaev & Stepovaya
(1966)
Rat M/F produced 1962 215 - 245 Gaines (1969)a
(43.5%)
Rat pure 200 - 300 Ben-Dyke et al.
(1970)
Rat 250 - 265 Melnikov (1974)
Mouse 99% pure 140 Hewitt et al. (1958b)
Mouse pure 135 Panshina (1963a)
Mouse technical 125 Panshina (1963a)
Mouse F pure 60 Sanderson & Edson
(1964)
----------------------------------------------------------------------------
a Lower figures (20-30 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
Table 7. (contd).
--------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
--------------------------------------------------------------------------
Mouse F technical 60 Sanderson & Edson
(1964)
Hamster M laboratory grade 200 Sanderson & Edson
(1964)
Guinea- M/F pure 550 Sanderson & Edson
pig (1964)
Guinea- M/F laboratory grade 600 Sanderson & Edson
pig (1964)
Guinea- M/F technical 350 - 400 Sanderson & Edson
pig (1964)
Guinea- M/F liquid formul- 350 - 370 Sanderson & Edson
pig ation (1964)
Rabbit M/F pure 500 Sanderson & Edson
(1964)
Rabbit M/F laboratory grade 450 Sanderson & Edson
(1964)
Rabbit M/F technical 300 Sanderson & Edson
(1964)
Rabbit M/F liquid formul- 283 Sanderson & Edson
ation (1964)
Cat technical 100 Panshina (1963a)
---------------------------------------------------------------------------
8.2. Skin and Eye Irritation
A single dose of 300 mg technical dimethoate/kg body weight
did not cause skin irritation in male and female rabbits
(Dikshith & Raizada, 1981b).
In a study by West et al. (1961), dimethoate did not have
any irritant effect on the rabbit eye after introduction of
10 mg of dry material into the conjunctival sac. However, in a
personal communication (1986), the US EPA suggested that
dimethoate had a slight irritant effect on the eye.
8.3. Repeated Exposures
The effects on experimental animals of repeated oral or
inhalation exposure to dimethoate are summarized in Tables 9 and
10.
In the various studies, which ranged from 5 1/2-12 months
in duration, inhibition of cholinesterase (ChE) in the erythro-
cytes was a more sensitive indicator of exposure to dimethoate
than ChE inhibition in plasma. ChE activity in the brain was
measured in one study only.
Table 8. Acute dermal LD50 of dimethoate in experimental animals
------------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight
------------------------------------------------------------------------------
Rat M 32% emulsifiable 1120 Edson & Noakes (1960)
solution
Rat liquid formula- 700 - 1150 Sanderson & Edson
tion (24-h) (1964)
Rat wettable powder 500 (24-h) Sanderson & Edson
(1964)
Rat M/F produced 1962 610 Gaines (1969)a
Rat M 32% w/v emulsifi- 770 - 1090 Noakes & Sanderson
able solution (24-h) (1969)
Rat M 32% w/v emulsi- > 1100 (4-h) Noakes & Sanderson
fiable solution (1969)
Guinea-pig liquid formula- 965 West et al. (1961)
tion (46%)
Guinea-pig wettable powder 995 West et al. (1961)
(25%)
Rabbit not specified 600 Melnikov (1974)
------------------------------------------------------------------------------
a Lower figures (55-61 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
In a study by West et al. (1961), no effects were observed
on ChE inhibition in rats administered dimethoate in the diet at
32 mg/kg. In their first study (12 months) on rats, Sanderson
& Edson (1964) observed inhibition of ChE in erythrocytes at
50 mg/kg diet, but not at 10 mg/kg. In the second study (5 1/2
months), inhibition of ChE in erythrocytes was found at both 20
and 10 mg/kg, but not at 5 mg/kg. The studies of Atabaev (1972)
did not show any inhibition of blood-ChE in rats administered a
40% formulation of dimethoate at 0.5-1 mg/kg body weight, corre-
sponding to 0.2-0.4 mg dimethoate/kg body weight. From all
available data on the rat, a dietary level of dimethoate of 5
mg/kg, corresponding to 0.25 mg/kg body weight, can be con-
sidered as the no-observed-adverse-effect level.
From limited studies on the dog (West et al., 1961), it can
be concluded that a level of 10 mg dimethoate/kg diet, corre-
sponding to 0.25 mg/kg body weight, does not result in ChE
depression in erythrocytes.
ChE inhibition was not observed in an inhalation study in
which rats were exposed for 14 h/day, over 3 months, to 0.01 mg
dimethoate/m3 (measured concentration) (Kaloyanova et al.,
1968).
Table 9. The effects on experimental animals of repeated exposure to dimethoate
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat technical 50, 100, or 200 35 days no mortality; no signs West et al. (1961)
(95%) mg/kg diet of intoxication
Rat technical 2, 8,or 32 mg/kg 3 months no mortality; no ChE West et al. (1961)
(95%) diet inhibition
Rat technical 15 mg/kg (oral) 6 months 100% inhibition of ChE Panshina (1963a)
in serum and erythro-
cytes; approximately
85% inhibition in brain
Rat technical 30 mg/kg (oral) 6 months death of 3 out of 5 Panshina (1963a)
animals
Rat technical 60 mg/kg (oral) 6 months death of all animals Panshina (1963a)
Rat (M) laboratory 10 mg/kg diet 12 months no inhibition of ChE in Sanderson & Edson
grade erythrocytes or plasma (1964)
Rat (M) laboratory 50 mg/kg diet 12 months marked inhibition of Sanderson & Edson
grade ChE in erythrocytes (1964)
Rat (M) laboratory 200 mg/kg diet 12 months marked toxic effects; Sanderson & Edson
grade reduced rate of weight (1964)
gain; inhibition of ap-
proximately 70% and 100%
ChE in plasma and erythro-
cytes, respectively
Rat laboratory 800 mg/kg diet 12 months severe toxic effects Sanderson & Edson
grade (1 week) (cholinergic effect: (1964)
weakness, weight loss
after a few days); the
pesticide was withdrawn
after one week; complete
recovery in 10 - 14 days
Rat (M) technical 20 or 10 mg/kg 5 1/2 months 50 and 40% inhibition Sanderson & Edson
(weanling) diet of ChE in erythrocytes, (1964)
respectively
---------------------------------------------------------------------------------------------------------
Table 9. (contd).
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat (M) technical 5 mg/kg diet 5 1/2 months no inhibition of ChE Sanderson & Edson
(weanling) (1964)
Rat 40% formula- 13 mg/kg body 4 months one rat died on the Atabaev (1972)
tion weight (oral) 35th day
Rat 40% formula- 50 mg/kg body 4 months 3 rats died on the 7th Atabaev (1972)
tion weight (oral) day, 2 died on the 8th
day, and one died on the
70th day
Rat 40% formula- 0.5 - 1 mg/kg 6 months no effect on ChE Atabaev (1972)
tion (oral)
Rat 40% formula- 5 mg/kg body 6 months AChE inhibition in Atabaev (1972)
tion weight (oral) blood in the first 2
months (approximately
50%)
Dog technical 2 or 10 mg/kg 13 weeks no inhibition of ChE West et al. (1961)
diet
Dog technical 50 mg/kg diet 13 weeks slight depression of West et al. (1961)
ChE in erythrocytes
Cat technical 10 mg/kg body 3 months death in 2 out of 4 Panshina & Klisenko
weight (oral) animals (1962)
Cat technical 20 mg/kg body 3 months death in 2 out of 3 Panshina & Klisenko
weight (oral) animals (1962)
Cat 40% formula- 0.5 - 1 mg/kg 3 months no effect on ChE Atabaev (1972)
tion body weight
(oral)
Cat 40% formula- 2 mg/kg body 3 months reduced body weight in Atabaev (1972)
tion weight (oral) the first 2 months
(16 - 32%); recovery at
the third month
---------------------------------------------------------------------------------------------------------
Table 10. Inhalation toxicity - repeated exposure
---------------------------------------------------------------------------------------------------------
Species Concentration Duration Effects Reference
(mg/m3) Daily exposure Number of
(h) months
--------------------------------------------------------------------------------------------------------
Rat 2 ? 2 no visible signs of intox- Panshina (1963b)
ication; 26% inhibition of
AChE in blood at the end of
the study
Cat 1.5 ? 1.5 no visible signs of intox- Panshina (1963b)
cation; 40-72% inhibition
of AChE in blood at the end
of the study
Rat in a miniature greenhouse (0.6 m3; temperature no blood-ChE inhibition Sanderson & Edson
21-30 °C); plants sprayed with 5 ml of 0.5% (1964)
aqueous formulation in the course of 28 days
Rat and 4.50 8 3 inhibition of ChE; leuk- Kaloyanova et al.
guinea-pig ocytosis (1968)
Rat 0.05 14 3 inhibition of ChE in blood Kaloyanova et al.
only in the first month; (1968)
inhibition of brain- and
liver-AChE; morphological
alterations in the neurons
Rat 0.01 14 3 no changes observed Kaloyanova et al.
(1968)
Rat 0.495 24 3 inhibition of ChE and Ubaidullaev &
changes Madzhidov (1976)
Rat 0.049 24 3 inhibition of ChE Ubaidullaev &
Madzhidov (1978)
Rat 0.003 24 3 no detectable changes Ubaidullaev &
Madzhidov (1978)
---------------------------------------------------------------------------------------------------------
8.4. Reproduction Studies
One reproduction study on mice has been reported in the
literature (Budreau & Singh, 1973). In this 5-generation study,
initial groups of 14 female and 10 male mice received dimethoate
in the drinking-water at 60 mg/litre for a period of one month,
prior to mating. A comparable control group was included in
the study. Animals receiving dimethoate showed a significant
( P < 0.05) reduction in mating performance (expressed as pro-
portion of females with deliveries to females mated), which
ranged from 33 to 61%, varying with litter and generation.
Similarly, reproduction time (measured as number of days elaps-
ing from first day of mating to day of delivery) was signifi-
cantly ( P < 0.01) increased in all first litters of all 5
generations examined, but unaffected in all second litters. The
biological relevance of this observation is unclear. Litter
size and average weight at birth were not affected by treatment.
Although the mean weights of treated pups were not significantly
lower, the growth rate was consistently lower in treated pups
compared with that in the controls.
A 3-generation reproduction study using technical
dimethoate (98.3%) was carried out on mice. Information sup-
plied by the US EPA indicated that there were no effects on re-
production and teratogenicity at a dimethoate level of 50 mg/kg
(US EPA, personal communication, 1986). Details of this study
were not available.
8.5. Teratogenicitya
Intraperitoneal administration of 40 mg dimethoate/kg body
weight, given as a single dose on the day of mating or on the
9th day of gestation, or given for the first 14 days of
gestation in mice, caused a high incidence of embryonal loss
(Scheufler, 1975).
Cygon 4E (containing 47.3% dimethoate) was given to female
rats by intubation from the 6th to the 15th day of gestation at
dose levels of 3, 6, 12, or 24 mg/kg body weight. The 24 mg/kg
dose was toxic for the dams (8 out of 20 dams manifested clonic
spasms and muscular tremors during the treatment period, 7 re-
covered, and one died on the 16th day of pregnancy). Doses of
12 and 24 mg/kg were associated with an increase ( P < 0.05)
in the numbers of anomalous litters (each having at least one
anomalous fetus) and wavy-ribbed fetuses. The 3 and 6 mg/kg
doses (equal to 1.42-2.84 mg dimethoate/kg) did not produce any
evidence of teratogenicity or embryotoxicity in the rats (Khera
et al., 1979).
----------------------------------------------------------------
a A further teratogenicity study on the rat (Edwards et al.,
1984) was submitted to the FAO/WHO Joint Meeting on Pesti-
cide Residues (JMPR) in 1987. Although fetotoxicity was ob-
served, there were no teratogenic effects (FAO/WHO, 1987).
Cygon 4E (47.3% dimethoate) was given to cats in gelatin
capsules at doses of 3, 6, or 12 mg/kg on the 14th-22nd days of
pregnancy. At the levels tested, the compound did not produce
any effects on the incidence of pregnancy. In the 12 mg/kg
group, forepaw polydactily was observed in 8 out of 39 fetuses.
Cygon 4E at 3 or 6 mg/kg (1.42-2.84 mg dimethoate/kg) did not
produce any effects (Khera, 1979). (The effect observed in
Khera's investigations may be due to the other components in the
formulation).
Courtney et al. (1985) reported that dimethoate adminis-
tered orally was not teratogenic in CD-1 mice at dose levels of
10 or 20 mg/kg body weight, and that these levels were not
lethal to the dams. The two highest dose levels of 40 and
80 mg/kg produced maternal toxicity.
8.6. Mutagenicity
A variety of in vivo and in vitro mutagenicity tests have
been carried out with dimethoate, the results of which are given
in Table 11.
Negative mutagenicity results were reported by Degraeve et
al. (1984) for commercial mixtures of insecticides containing
dimethoate. Two formulations were tested: dimethoate + fenitro-
thion (dose 60 mg/kg body weight, corresponding to 9 mg/kg of
each) and dimethoate + malathion + methoxychlor (dose 100 mg/kg
body weight corresponding to 9.5 mg dimethoate/kg). A single
ip injection did not induce chromosome aberrations in bone
marrow cells, spermatogonia, or primary spermatocytes of the
mouse. No significant increases in pre- or post-implantation
fetal lethality were observed in a dominant lethal mutation
assay.
Alkylation by dimethoate at the N-7 position of guanine in
DNA has been investigated (Dedek et al., 1984). Male mice
(strain AB Jena/Halle, random bred) were injected ip with 14C-
methyl-labelled dimethoate at a dose of 0.35 mmol/kg. The
extent of methylation was in the range of 1-10 µmol N-
7 methylguanine/mol guanine; the values in the kidneys were
higher than those in the liver. The excretion half-life of N-
7 methylguanine was 23-160 h.
In summary, dimethoate has been found to be mutagenic in
both in vitro and in vivo assays.a
----------------------------------------------------------------
a After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
Table 11. Mutagenicity tests
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Escherichia coli 1 - 6.10-3 mmol 5-methyltryptophane resistance Mohn (1973)
K-12/gal Rs mutation -positive
Escherichia coli Up to 5000 µg/plate positive Moriya et al.
WP2 hcr (1983)
Escherichia coli Minimum mutagenic positive Probst et al.
WP2 & WP2uvrA- concentration (1981)
47 nmoles/ml
Salmonella typhimurium negative Probst et al.
TA 98, 100, 1535, 1537, (1981)
1538
Salmonella typhimurium Up to 5000 µg/plate positive with and without Moriya et al.
TA 100 activation (1983)
Salmonella typhimurium Up to 5000 µg/plate negative Moriya et al.
TA 98, 1535, 1537, 1538 (1983)
Salmonella typhimurium 5 - 200 µg/plate positive at all doses, mutagenicity Vishwanath &
TA 100 (base pair subst.) increased with liver microsomes Jamil (1986)
Saccharomyces cerevisiae 7 doses, 40 - 100 mmol induction of mitotic gene Fahrig (1974)
conversions
Schizosaccharomyces pombe 1.3 - 131 mmol negative Gilot-Delhalle
ade 6 (LD50 50 mmol) (1983)
Chinese hamster ovary 20,40 & 80 µg/ml sister chromatid exchange increase Chen et al.
cells V79 10 µg/ml negative (1981)
Rat hepatocytes culture 47 nmol/ml negative unscheduled Probst et
al. (1981)
SV-40 transformed human 100 & 1000 umol positive unscheduled DNA Ahmed et
synthesis al. (1977)
Drosophila melanogaster 1 mg/kg (feeding) negative Woodruff et
al. (1983)
---------------------------------------------------------------------------------------------------------
Table 11. (contd).
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Drosphila melanogaster adult inj. ip 0, 10, increase in sex-linked Velazquez et
20 mg/kg body weight recessive lethals at 10 mg/kg al. (1986)
not at 20 mg/kg
Drosophila melanogaster adult feeding 0, negative Velazquez et
10 mg/kg; larval negative al. (1986)
feeding 0, 20 mg/kg
Host-mediated assay mouse 3 equal oral doses positive - mutation factor of Usha Rani et
Salmonella typhimurium of 155 mg/kg 3.44 ( P < 0.05) al. (1980)
Dominant lethal mutation acute 10 mg/kg ip negative Degraeve &
assay mice strain Q 7 weeks 0.6 mg/litre Moutschen
drinking-water, equiv- (1983)
alent to 0.093 mg/kg
body weight
Dominant lethal test 30 & 60 mg/kg body negative Fischer &
- AB Jena Halle mice 5 x 6 mg/kg body weight Scheufler
ip (1981)
DBA mice 3 x 18 mg/kg body weight
ip
Micronucleus test - 2 equal doses of a significant increase in Usha Rani et
mice - bone marrow 51.7 mg/litre drink- frequency of polychromatic al. (1980)
ing-water at 24-h erythrocytes with micronuclei
intervals -0.85% (controls 0.28%)
Chromosome abnormalities 50 and 100 mg/kg body positive Bhunya & Behera
mice - bone marrow weight (1975)
Mice - bone marrow 20 mg/kg body weight no abnormalities Nehés et al.
ip (1982)
Mice - bone marrow 60 mg/kg body weight increase in number of mitosis, Nehéz et al.
ip aberrations (1983)
Syrian golden hamsters 16, 32, 80, or increase in number of chromatid Dzwonkowska &
Mesocricetus auratus 160 mg/kg body weight and chromosomal breaks Hübner (1986)
ip single (no dose-response relationship)
---------------------------------------------------------------------------------------------------------
8.7. Carcinogenicity
Two carcinogenicity studies, reported by Gibel et al.
(1973), are summarized below.
Rat: Groups of 40, 10-week-old Wistar rats were intubated
with 0 (control), 5, 10, or 30 mg dimethoate/kg body weight
(twice weekly) for the life span. The mean life spans were 743,
518, 511, and 627 days at 0, 5, 15, and 30 mg/kg body weight,
respectively. No malignant tumours were observed in the 36
control animals. In test groups, malignant tumours occurred
in 2/26, 3/25, and 4/20 rats, respectively, at the 3 dose
levels. At 5 mg/kg body weight, a reticulosarcoma of the spleen
with metastases and a malignant reticuloma were observed. At
15 mg/kg body weight, a sarcoma of the colon, a reticulosarcoma
of the spleen (with pancreatic metastases) and a hepatocellular
carcinoma occurred. At 30 mg/kg body weight, a liver fibro-
sarcoma, a malignant reticuloma and two reticulosarcomas of the
spleen were noted. Three, 7, 5, and 2 benign tumours were
noted, respectively, in the controls and at 5, 15, and 30 mg/kg
body weight (Gibel et al., 1973).
Rat: Groups of 40, ten-week-old Wistar rats were injected
im with 15 mg dimethoate/kg body weight (twice weekly) or with
isotonic saline until spontaneous death occurred. The mean life
spans were 711 and 570 days in the saline and dimethoate-treated
animals, respectively. No malignant tumours occurred in the
saline-treated group, but 4/35 animals developed benign tumours.
In the dimethoate group, 6/30 rats had malignant tumours (a
spleen reticulosarcoma, a spleen fibrosarcoma, an ovarian
alveolar sarcoma, a liver hepatocellular carcinoma, a malignant
reticuloma, and a soft tissue spindle-cell sarcoma) and 5/30 had
benign tumours. The first malignant tumour (a splenic
fibrosarcoma) was noted after 410 days (Gibel et al., 1973).
Dimethoate (technical grade, 90-100% pure) was given in the
feed to Osborne Mendel rats and to B6C3F1 mice. Groups of 50
male and 50 female rats (10 animals in each control group)
received "time-weighted average doses" of 155 or 310 mg/kg for
male rats and 192 or 384 mg/kg for female rats. After 80 weeks,
all groups received control diets, and the studies were con-
cluded at week 114.
Groups of 50 male and 50 female B6C3F1 mice were given
dimethoate in the diet at concentrations of 0 (10 "matched"
controls), 250, or 500 mg/kg for 60-69 weeks (males) or for 80
weeks (females). The studies were ended after 94 weeks.
Tremors and hyperexcitability were observed in exposed animals;
rats and mice that survived to termination were generally in
poor condition. Survival was reduced in the high-dose groups of
rats. Several non-neoplastic lesions occurred more frequently
in dimethoate-exposed animals. No increases in neoplasia were
reported to be associated with dimethoate administration, for
any of the organs or tissues examined histologically (NCI-1977).
These studies are not considered adequate to determine properly
the presence or absence of a carcinogenic response, largely
because of the shorter than usual duration of exposures, the
poor condition of the animals, and changes in exposure concen-
trations.a
8.8. Special Studies
The action of dimethoate on the immune system was studied
in mice and rats by Tiefenbach & Lange (1980). A single dose of
75 mg dimethoate/kg (route not specified) decreased the
lymphocyte count to 50% of the pre-exposure value and increased
the neutrophile granulocytes. After 72 h, these changes
returned to normal. A reduction in the thymus cortex with
disrupted lymphocytes, and a reduction in the number of rosette-
forming cells were observed.
When administered to rats at 5-30 mg/kg body weight orally
or 15 mg/kg im, twice a week, until death, dimethoate caused
hyperplasia in the bone marrow, mainly in granulocytopoiesis
(Stieglitz et al., 1974). The authors interpreted the changes
in the haematopoietic system of the rats as a direct haemato-
logical effect of dimethoate.
The effects of dimethoate on the heart were investigated
in rabbits (Mahkambaeva, 1971), and guinea-pigs and rats
(Nadmaiteni & Marosi, 1983). After oral administration of
150 mg dimethoate/kg to rabbits, the effects observed included
bradycardia and increased atrio-ventricular and intraventricu-
lar conductance, with complete recovery after 4-7 days. In rats
and guinea-pigs, a dose-effect relationship was established for
heart rate disturbances, and atrio-ventricular block. An
electron-microscopic study of the myocard did not reveal any
changes. The ip doses that were tested ranged from 500 to
1500 mg/kg body weight.
----------------------------------------------------------------
a After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious ECG disturbances
developed in the early phase of intoxication. The toxic cardiac
phenomena appeared to be unrelated to the degree of cholin-
esterase inhibition, but were correlated with the myocardial
dimethoate concentration. Cardiac failure and mortality were
first observed at a level of about 110 µg/g, while a level of
221 µg/g resulted in death in all cases. The present investi-
gation refers to the direct effect of dimethoate on the
myocardium, independent of its anticholinesterase action (Marosi
et al., 1985a,b).
8.9. Factors Modifying Toxicity
The acute toxicity of dimethoate was not affected by the
dietary content of protein (Boyd & Muis, 1970). The oral LD50
was 147 mg/kg for male albino rats fed for 28 days from weaning
on a diet containing 3.5% protein as casein and, in controls
maintained on a diet containing 26% casein at 152 mg/kg. Other
groups maintained on a diet that contained 24% protein from
various plant and animal sources were apparently less suscep-
tible to dimethoate (LD50, 358 mg/kg). Dimethoate was given to
rats orally for 10 weeks at a dose of 10 mg/kg in diets contain-
ing 90, 160, or 240 g protein/kg (Gontzea & Gorcea, 1977). The
high-protein (240 g) diet diminished the adverse effects of
dimethoate on the growth of the rats and lessened its antichol-
inesterase activity in plasma, total blood, brain, and liver.
A marked increase in the toxicity of dimethoate was noted
in male and female mice after pre-treatment with phenobarbital
and with chlorinated hydrocarbons (DDT and dieldrin) (Menzer &
Best, 1968; Menzer, 1970). The toxicity of dimethoate was
increased from an ip LD50 of 198 mg/kg body weight to 58.5 mg/kg
by pre-treatment of mice for 3 days with sodium phenobarbital.
Liquid formulations of technical dimethoate in the
solvents, 2-methoxy- and 2-ethoxyethanol, showed increased tox-
icity after storage. After storage for 7 months in England and
9 months under tropical conditions, the oral LD50 for the rat
decreased to 30-40 mg/kg and 15 mg/kg, respectively (from an
initial 150-250 mg/kg). The most toxic conversion product was
O,O-dialkyl S-(N-methylcarbamoylmethyl) phosphorothioate with
an oral LD50 for the rat of 1 mg/kg (Casida & Sanderson, 1961).
8.10 Mechanisms of Toxicity; Mode of Action
The mode of action of organophosphorus insecticides is
decribed in WHO (1986a). They act principally by inhibition of
acetyl cholinesterase (AChE) at the cholinergic synapses.
Dimethoxon (omethoate), a metabolite of dimethoate, is
75-100 times more potent than dimethoate in inhibiting AChE,
suggesting that this metabolite plays a dominant role in
mammalian toxicity (Hassan et al., 1969). The LD50 of omethoate
in rats is 25-28 mg/kg body weight (FAO/WHO, 1979); it is about
10 times more toxic than dimethoate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
Cases of both accidental and suicidal poisoning have been
reported with dimethoate. Hayes (1982) has reviewed the human
toxicity and poisoning cases.
Nagler et al. (1980) described a case of attempted suicide
of a 34-year-old female who ingested 10 g dimethoate. Half an
hour after admission to the hospital, the serum-dimethoate level
was 2340 mg/litre. Combined haemoperfusion and haemodialysis
were applied and, after 18 h, dimethoate was no longer detected
in the serum.
A severe case of poisoning after ingestion of approximately
20 g dimethoate was reported by Köppel et al. (1986). On
admission, the 52-year-old man was comatose with unmeasurable
pseudo-cholinesterase (< 200 U/litre). He had been admitted 2 h
after ingestion and received, every 20 min, an injection of
20 mg atropine. Two haemoperfusions with activated charcoal and
amberlite were performed, and atropine was given by infusion up
to day 12. Twenty-five days after admission, he was discharged,
fully recovered.
De Reuck et al. (1979) presented a case of a patient who
died on the 9th day after dimethoate poisoning with an atypical
central neurological disorder. The neuropathological findings,
which were similar to those observed in severe forms of
Wernicke's encephalopathy, included severe haemorrhagic necrosis
of the walls of the ventricles. The authors suggested that the
increased level of acetylcholine in the brain had led to
thiamine depletion in the regions of predilection of Wernicke's
encephalopathy.
Many other cases of human dimethoate poisoning, some of
which were fatal, have been reported, but the information given
was insufficient (Molphy & Rathus, 1964; Masiak & Olajossy,
1973; François & Verbraeken, 1977; Demeter & Heyndrickx, 1978;
Ebel & Karyofilis, 1978; Areekul et al., 1981; Wehran & Hammer,
1984; Bolgar et al., 1985; Le Blanc et al., 1986; Senanayake &
Karalliedde, 1987).
On the basis of a number of cases, the oral lethal dose for
human beings was estimated to be of the order of 50-500 mg/kg
body weight (Gosselin et al., 1984).
Trinh van Bao et al. (1974) reported an increase in the
frequency of breaks and stable chromosome aberrations in 2
patients who died after dimethoate intoxication.
Thirty firemen, exposed to dimethoate in the air as a
result of an accident in a dimethoate-manufacturing plant,
developed symptoms of intoxication (Larripa et al., 1979, 1983).
Peripheral blood lymphocytes from 20 of these workers were
examined for the frequency of sister chromatid exchanges, 2
months after the accident. The frequencies were 9.2 ± 0.2 for
the exposed and 8.5 ± 0.2 for unexposed persons ( P < 0.05).
Dicentric chromosomes and a low frequency of chromatid breaks
were found in 2 exposed workers. It is not certain to what the
firemen were exposed besides dimethoate.
9.1.2 Controlled human studies
The results of a number of studies in which human volun-
teers, without occupational exposure to organophosphates, were
given dimethoate, are summarized in Table 12. From these
studies it can be concluded that repeated doses of up to
0.2 mg/kg body weight did not inhibit cholinesterase activity in
the blood.
9.2 Occupational Exposure
9.2.1 Poisoning incidents
One of the first cases of poisoning with dimethoate (Rogor)
in agriculture was described by Muratore et al. (1960). A 28-
year-old-farmer, who reportedly had worn protective rubber
clothing and equipment, had sprayed olive trees with dimethoate
the day before he was hospitalized. The man began to experience
profound weakness, faintness, and somnolence, followed by
attempts to vomit, chills, and profound prostration on the day
he was hospitalized. His general condition was found to be
serious; his pulse was weak, he exhibited pronounced myosis,
vomited and sweated profusely, and had pronounced inhibition of
cholinesterase activity. Treated with large doses of atropine
(20 mg on day 2, 12 mg on day 3, and 5 mg until day 9),
prednisone, analgesics, and penicillin, he recovered.
A case of poisoning in a woman working in agriculture and
exposed to dimethoate in the field, 2 days after spraying, was
reported by Asatryan (1971). Within 3-3.5 h after beginning
work, the woman noted an unpleasant odour recognized as
dimethoate. She developed headache, dry cough, dyspnoea,
nausea, vomiting and was admitted to hospital in a somnolent
state with muscular fibrillations and an asthmatic component.
After treatment with saline solution, glucose, caffeine,
atropine, and insulin, the state of acute intoxication was
overcome. Allergic symptoms were treated with dimedrol.
Contact allergy due to dimethoate was reported in a 53-
year-old female. She had a positive skin test (Pambor & Bloch,
1985).
Table 12. Controlled human studies
---------------------------------------------------------------------------------------------------------
Number Sex Route of Dose/day Duration of Results Reference
of Admini- exposure
subjects stration
---------------------------------------------------------------------------------------------------------
20 oral 0.04 mg/kg 4 weeks absence of toxic effects Sanderson & Edson
or blood-ChE inhibition (1964)
2 oral 0.13 mg/kg; 21 days absence of blood-ChE Sanderson & Edson
0.26 mg/kg inhibition (1964)
5 M oral 0.25 mg/kg single dose absence of toxic effects Sanderson & Edson
or ChE inhibition (1964)
50 dermal 32% liquid form- 2-h patch absence of skin irritation Sanderson & Edson
ulation, up to test and ChE inhibition (1964)
2.5 ml
12 M/F oral 5 mg (0.068 28 days no significant change in Edson et al. (1967)
mg/kg body weight)
9 M/F oral 15 mg (0.202 39 days no significant change in Edson et al. (1967)
mg/kg body weight)
8 M/F oral 30 mg (0.434 57 days inhibition of ChE by the Edson et al. (1967)
mg/kg body weight)
6 oral 45 mg (0.587 45 days inhibition of ChE (35%) Edson et al. (1967)
mg/kg body weight)
6 M/F oral 60 mg (1.02 14 days inhibition of ChE (21%) Edson et al. (1967)
mg/kg body weight)
---------------------------------------------------------------------------------------------------------
Female greenhouse workers working with dimethoate were
reported to have a high percentage of specific leukocyte
agglomeration, a raised index of lymphoblastic transformations,
and antibodies against dimethoate (Zolotnikova & Somov, 1978;
Zolotnikova, 1980). With increasing duration of work, progress-
ive sensitization towards the pesticide was observed.
On the basis of epidemiological observations and of dermal
testing of workers with a 1-2% solution of dimethoate, Jung
(1979) concluded that the index of sensitization was very low
for dimethoate and that general intoxication might occur, but
rarely contact eczema.
9.3 Early Symptoms and Treatment of Poisoning
Initial symptoms of poisoning may include sweating,
headache, weakness, giddiness, nausea, vomiting, stomach pains,
blurred vision, slurred speech, and muscle twitching (WHO,
1986a). Arrhythmias and cardiac failure have been reported
(Kiss & Fazekas, 1983; Marosi et al., 1985a,b; Duval et al.,
1986). The most important diagnostic finding is inhibition of
blood-cholinesterase activity. For a full discussion of the
clinical picture and treatment of organophosphate insecticide
poisoning, see WHO (1986a). The section on treatment is
presented in the annex to this publication.
In all cases of clinical poisoning with dimethoate and
other organophosphorus insecticides, it is essential to maintain
general surveillance and cholinesterase and cardiac monitoring
for at least 4 days, and longer if necessary, and to adapt
general supportive and specific therapy in accordance with the
findings.
Data on the effects of oxime reactivators in dimethoate
poisoning are contradictory, some indicating that they may have
a negative effect on cholinesterase inhibition (Sanderson &
Edson, 1959; Durham & Hayes, 1962; Molphy & Rathus, 1964;
Erdmann et al., 1966; Zech et al., 1966; Ebel & Karyofilis,
1978; Sterri et al., 1979). It is therefore suggested, that if
oxime reactivators are indicated, these should be used with
caution and under close supervision.
Haemoperfusion may be effective in the early stages of
dimethoate poisoning (Okonek 1976, 1977; Okonek et al., 1976;
Pach et al., 1977; Nagler et al., 1980).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Toxicity of Dimethoate
Dimethoate is moderately toxic. Most oral LD50s in the rat
ranged from 150-400 mg/kg body weight. The dermal LD50 in the
rat was greater than 600 mg/kg body weight. Dimethoate is not
irritating to the skin, but may be slightly irritating to the
eye.
It can be absorbed following ingestion, inhalation, and
skin contact. It is readily metabolized and excreted and does
not accumulate in the body. Dimethoxon (omethoate), the oxygen
analogue found in plants, insects, and mammals, is about 10
times more toxic and is a more potent inhibitor of cholin-
esterase activity than dimethoate.
Signs of intoxication from exposure to dimethoate are those
typical of organophosphorus pesticides. Inhibition of erythro-
cyte-cholinesterase is the most sensitive indicator of exposure
to dimethoate and may indicate toxicity. A dietary level of
5 mg dimethoate/kg, equivalent to 0.25 mg/kg body weight, is
considered to be a no-observed-adverse-effect level in the
rat.a
A 5-generation reproduction study in mice given drinking-
water containing dimethoate at 60 mg/litre resulted in decreased
mating success and increased reproduction time in all gener-
ations. On the basis of available data on experimental animals,
dimethoate is not considered to be a teratogen.
Dimethoate is considered mutagenic in a variety of in vitro
and in vivo test systems.b Long-term studies have been
conducted involving oral administration in rats and mice and im
injection in rats. However, the available data are considered
---------------------------------------------------------------
a FAO/WHO (1987) concluded that a dietary level of 1 mg/kg,
equivalent to 0.05 mg/kg body weight is the no-observed-
adverse-effect level in the rat.
b After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
to be inadequate to assess the carcinogenic potential of
dimethoate.c
10.2. Human Exposure
Air and water are negligible sources of exposure to
dimethoate for the general population. Residues found in food
are generally below the acceptable daily intake (ADI) set by the
FAO/WHO Joint Meeting on Pesticide Residues (JMPR) (See section
12).
Whole blood-cholinesterase was not inhibited in human
volunteers given oral doses of 0.2 mg dimethoate/kg body weight
for 39 days.
Several cases of suicidal or accidental poisoning due to
ingestion of dimethoate have been reported.
Occupational exposure to dimethoate, principally through
inhalation and the skin, may occur during its manufacture,
formulation, and use, and cases of poisoning as a result of
accident or neglect of safety precautions have been reported.
The oral lethal dose for human beings has been estimated to be
in the range of 50-500 mg/kg body weight.
Skin sensitization due to dimethoate has been observed in
some cases.
10.3. Evaluation of the Effects on the Environment
Dimethoate is rapidly hydrolysed and does not persist in
the environment. The toxicity of dimethoate for aquatic
organisms and birds is moderate to high. It is very toxic for
honey-bees.
---------------------------------------------------------------
c After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
10.4. Conclusions
1. Under proper conditions of use, exposure of the general
population to dimethoate is negligible.
2. When appropriate safety precautions are observed, exposure
to dimethoate during manufacture, formulation, use, and
disposal should not pose an unacceptable human health
hazard.
3. Dimethoate is rapidly degraded and not persistent in the
environment. Care must be taken not to expose honey-bees,
fish and aquatic organisms, and birds.
11. RECOMMENDATIONS
1. Figures relating to the current production and use of
dimethoate should be made available.
2. Studies are necessary on dermal sensitization by
dimethoate.
3 Data are required on the absorption and disposition of
dimethoate from different routes of exposure.
4. Long-term toxicology and carcinogenicity studies should be
carried out on laboratory animals.a
5. More research is required to investigate the in vivo
mutagenic effects of dimethoate.a
6. Reproduction and teratology studies should be carried
out.a
7. Epidemiological studies on persons engaged in the
manufacture and professional use of dimethoate should be
considered, and workers should be monitored for exposure to
dimethoate and for potential adverse health effects.
8. Cleaning and disposal of contaminated equipment, clothing,
and containers should be in accordance with recommended
procedures.
9. Further work is necessary to establish safe re-entry
periods under different conditions of use.
10. Acute toxicity studies should be carried out on
formulations in which dimethoate is mixed with other active
ingredients.
11. The role of oxime reactivators in the treatment of human
poisoning by dimethoate, should be clarified.
12. Information should be obtained concerning the changes in
toxicity, due to impurities, that can arise in pesticides
as a consequence of different manufacturing processes, the
use of formulating ingredients, and improper storage.
---------------------------------------------------------------
a Several of these studies were later submitted to the
FAO/WHO Joint Meeting on Pesticide Residues in 1987. See
previous footnotes for sections 8.5, 8.6 and 8.7.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dimethoate was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1963, 1965, 1966, 1967, 1970, 1973
(evaluation of the related compound formothion), 1977, 1978,
1984, and 1987 (FAO/WHO, 1964, 1965, 1967, 1968, 1971, 1974,
1978, 1979, 1985, 1987).
The estimate of an acceptable daily intake (ADI) for man
for dimethoate is 0-0.01 mg/kg body weight, based on the no-
observed-adverse-effect level in man of 0.2 mg/kg body weight
per day, and in the rat of 1 mg/kg diet, equivalent to
0.05 mg/kg body weight (FAO/WHO, 1987).
A data sheet on dimethoate has been issued by WHO in the
series "Data Sheets on Pesticides" (WHO/FAO, 1980).
In the WHO Recommended Classification of Pesticides by
Hazard, technical dimethoate is classified as moderately
hazardous, when handled in accordance with instructions (WHO,
1986b).
The IRPTC (1982) has issued a review on dimethoate, in its
series "Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals".
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-
67. III. Organophosphorus pesticide residues in the total diet.
Pestic. Sci., 1: 10-13.
ABRAMOVA, J.I. & BOLTUSHKINA, L.A. (1968) [Safety action of
several vitamins in poisoning with organophosphorus insecti-
cides.] In: [Hygiene and toxicology of pesticides and the
clinical picture of intoxications,] Vol. 6, Kiev, Zdorovye
Publishers, pp. 448-452 (in Russian).
ADAMIS, Z., ANTAL, A., FUZESI, I., MOLNAR, J., NAGY, L., &
SUSAN, M. (1985) Occupational exposure to organophosphorus
insecticides and synthetic pyrethroids. Int. Arch. occup.
environ. Health, 56: 299-305.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AKORONKO, S.L. & GIRENKO, D.B. (1977) [Methods of estimating
fosfamid in soil using chromatographic methods.] In: [ Methods
of determination of micro-quantities of pesticides in foods,
feed, and the environment,] Moscow, Kolos, pp. 118-120 (in
Russian).
ANEES, M.A. (1975) Acute toxicity of four organophosphorus
insecticides for a fresh-water teleost Channa punctatus
(Bloch). Pak. J. Zool., 7(2): 135-141.
ANEES, M.A. (1978a) Hepatic pathology in a fresh-water teleost
Channa punctatus (Bloch) exposed to sub-lethal and chronic
levels of three organophosphorus insecticides. Bull. environ.
Contam. Toxicol., 19(5): 524-527.
ANEES, M.A. (1978b) Haematological abnormalities in a fresh-
water teleost Channa punctatus (Bloch) exposed to sublethal and
chronic levels of three organophosphorus insecticides. Int. J.
Ecol. environ. Sci., 4: 53-60.
AREEKUL, S., SRICHAIRAT, S., & KIRDUDOM, P. (1981) Serum and
red cell cholinesterase activity in people exposed to organo-
phosphorus insecticides. Southeast Asian J. trop. Med. pub.
Health, 12(2): 94-98.
ARMELI, G., BARTALINI, E., & PISANI, F. (1967) [Occupational
pathology and prevention in the industrial production of rogor].
Lav. Um., 20(8): 355-356 (in Italian).
ASATRYAN, R.S. (1971) [Some peculiarities of acute pesticide
poisonings in application in Ararat land.] In: [ Hygiene and
toxicology of pesticides and the clinical picture of intoxica-
tions,] Kiev, Zdorovye Publishers, pp. 315-318 (in Russian).
ATABAEV, SH.T. (1972) [Rogor (phosphamide).] In: [ Pesticides
and environmental hygiene in hot climate conditions,] Tashkent,
Medizina, pp. 77-93 (in Russian).
ATABAEV, SH.T. & STEPOVAYA, N.E. (1966) [Allowance of phospha-
mide in apples.] In: [ Hygiene and toxicology of pesticides and
the clinical picture of intoxications,] Kiev, Zdorovye Publish-
ers, pp. 280-285 (in Russian).
AWASTHI, M., SHAH, P., DUBALE, M.S., & GADHIA, P. (1984) Meta-
bolic changes induced by organophosphates in the piscine organs.
Environ. Res., 35(I): 320-325.
BARANOVA, N.P., ALEXANDROVA, L.G., & KOVALENKO, V.F. (1986)
[Tissue distribution and biochemical effects of dimethoate
entering the body via the skin.] Gig. tr. prof. Zabol., 3: 20-
23 (in Russian).
BARKER, R.J., LEHNER, Y., & KUNZMANN, M.R. (1980) Pesticides
and honey bees: nectar and pollen contamination in alfalfa
treated with dimethoate. Arch. environ. Toxicol., 9: 125-133.
BECK, E.W., JOHNSON, J.C., Jr, GETZ, M.E., SKINNER, F.B.,
DAWSEY, L.H., WOODHAM, D.W., & DERBYSHIRE, J.C. (1968) Effects
of feeding dimethoate, its oxygen analog, and dimethoate-treated
silage to cattle. J. econ. Entomol., 61(3): 605-610.
BECKER, H. (1985) Dominant lethal study with dimethoate
technical in the mouse. (Unpublished report no. 039003, dated 12
September 1985 from Research & Consulting Company AG, 4452
Itingen/Switzerland).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Contr., 9(3):
119-127.
BHUNYA, S.P. & BEHERA, J. (1975) Centromeric sensitivity of
mouse chromosomes to the systemic insecticide dimethoate
(Rogor). Curr. Sci., 44(23): 859-860.
BOHN, W.R. (1964) The disappearance of dimethoate from soil.
J. econ. Entomol., 57(6): 798-799.
BOLGAR, G., JOJART, G., & TURI, J. (1985) [Spontaneous delivery
of a pregnant woman after dimethoate (Bi 58 EC) poisoning.]
Orvosi Hetilap, 126(52): 3213-3214 (in Hungarian).
BOLOTNYI, A.V., PISMENNAYA, M.V., & AKORONKO, S.L. (1978)
[Transformation of phosphororganic pesticides antio and chloro-
phose in the environment.] Gig. i Sanit., 43(5): 28-31 (in
Russian).
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol-water
partitioning coefficient (Kow) of 61 organophosphorus and carba-
mate insecticides and their relationship to respective water
solubility (S) values. J. environ. Sci. Health, BI8(6): 667-
683.
BOYD, E.M. & MUIS, L.F. (1970) Acute oral toxicity of dimetho-
ate in albino rats fed a protein deficient diet. J. pharm.
Sci., 59: 1098-1102.
BRADY, U.E., Jr & ARTHUR, B.W. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. econ.
Entomol., 56(4): 477-482.
BUDREAU, C.H. & SINGH, R.P. (1973) Effect of fenthion and
dimethoate on reproduction in the mouse. Toxicol. appl. Pharma-
col., 26: 29-38.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1968) Organo-
phosphorus poisoning, Diagnosis of poisoning in pheasants owing
to a number of common pesticides, J. agric. food Chem., 16(2):
332-339.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1969) Organo-
phosphorus poisoning, Chronic feeding of some common pesticides
to pheasants and pigeons, J. agric. food Chem., 17(5): 1027-
1032.
CABRAS, P., MELONI, M., & PIRISI, F.M. (1979) Separation of
insecticides by reversed-phase high-performance liquid chroma-
tography. J. Chromatogr., 176: 473-477.
CARMAN, G.E., IWATA, Y., PAPPAS, J.L., O'NEAL, J.R., & GUNTHER,
F.A. (1982) Pesticide applicator exposure to insecticides
during treatment of citrus trees with oscillating boom and
airblast units. Arch. environ. Contam. Toxicol., 11: 651-659.
CASIDA, J.E. & SANDERSON, D.M. (1961) Toxic hazard from formu-
lating the insecticide dimethoate in methyl "Cellosolve".
Nature (Lond.), 189(4763): 507-508.
CHEN, H.H., HSUEH, J.L, SIRIANNI, S.R., & HUANG, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res., 88(3): 307-316.
CHILWELL, E.D. & BEECHAM, P.T. (1960) Residues of O,O-
dimethyl- S-(N-methylcarbamoylmethyl) phosphorothiolothionate
(dimethoate) in sprayed crops. J. Sci. Food Agric., 11: 400-
407.
CONGREGADO, F., SIMON-PUJOL, D., & JUAREZ, A. (1979) Effect of
two organophosphorus insecticides on the phosphate-dissolving
soil bacteria. Appl. environ. Microbiol., 37(1): 169-171.
COPPLESTONE, J.F., FAKHRI, Z.I., MILES, J.W., MITCHELL, C.A.,
OSMAN, Y., & WOLFE, H.R. (1976) Exposure to pesticides in
agriculture: a survey of spraymen using dimethoate in the Sudan.
Bull. World Health Organ., 54(2): 217-223
COURTNEY, K.D., ANDREWS, J.E., SPRINGER, J., & DALLEY, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. environ. Sci. Health, B20(4): 373-406.
DALELA, R.C., BHATNAGAR, M.C., TYAGI, A.K., & VERMA, S.R.
(1979) Histological damage of gills in Channa gachua after
acute and subacute exposure to endosulfan and Rogor. Mikroskopie
(Vienna), 35: 301-307.
DAUTERMANN, W.C., CASIDA, J.E., KNAAK, J.B., & KOWALCZYK, T.
(1959) Bovine metabolism of organophosphorus insecticides.
Metabolism and residues associated with oral administration of
dimethoate to rats and three lactating cows. J. agric. food
Chem., 7(3): 188-193.
DEDEK, W., GRAHL, R., & SCHMIDT, R. (1984) A comparative study
of guanine N7-alkylation in mice in vivo by the organophosphorus
insecticides trichlorphon, dimethoate, phosmet and bromophos.
Acta pharmacol. toxicol., 55: 104-109.
DEGRAEVE, N. & MOUTSCHEN, J. (1983) Genotoxicity of an organo-
phosphorus insecticide, dimethoate, in the mouse. Mutat. Res.,
119: 331-337.
DEGRAEVE, N., CHOLLET, M.-C., & MOUTSCHEN, J. (1984) Evaluation
of the mutagenic potential of four commercial mixtures of insec-
ticides. Food chem. Toxicol., 22(8): 683-687.
DEMETER, J. & HEYNDRICKX, A. (1978) Two lethal endosulfan
poisonings in man. J. anal. Toxicol., 2(2): 68-74.
DE PIETRI-TONELLI, P., BAZZI, B., & SANTI, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Rev., 11: 60-99.
DE REUCK, J., COLARDYN, F., & WILLEMS, J. (1979) Fatal
encephalopathy in acute poisoning with organophosphorus
insecticides. A clinicopathologic study of two cases. Clin.
Neurol. Neurosurg., 81(4): 247-254.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K.,
MUYS, T., & VAN DER POLL, J.M. (1984) Pesticides and other
chemical residues in Dutch total diet samples (June 1976 - July
1978). Food chem. Toxicol., 22(1): 11-21.
DIKSHITH, T.S.S. (1986) Pesticides. In: Toxicology of
pesticides in animals, Boca Raton, Florida, CRC Press, Vol. I,
Chapter 2.
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981a) Toxicity evaluation of
dimethoate technical in fish (Report to Shaw Wallace & Co.,
India).
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981b) Toxicity evaluation
of dimethoate technical in animals: Primary skin irritation and
irritation of mucous membrane in rabbits (Report to Shaw Wallace
& Co., India).
DUGGAN, R.E., CORNELIUSSEN, P.E., DUGGAN, M.B., MCMAHON, B.M., &
MARTIN, R.J. (1983) Pesticide residue levels in foods in the
United States from July 1, 1969 to June 30, 1976, Washington,
DC, US Food and Drug Administration/Association of Official
Analytical Chemists (published jointly), pp. 13-24.
DURHAM, W.F. & HAYES, W.J. (1962) Organic phosphorus poisoning
and its therapy. Arch. environ. Health, 5: 21-47.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the exposure
to pesticides. Bull. World Health Organ., 26: 75-91.
DUVAL, G., CORBIN, J.C., & MIMRAN, C. (1986) Etude
hémodynamique des intoxications aiguës graves par insecticides
organophosphorés (7 cas). Coeur et Toxiques: XXIVèmes Journées
du Groupement francais de CAP, Strasbourg, 24-25 septembre
1986.
DZWONKOWSKA, A. & HÜBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in
vivo. Arch. Toxicol., 58: 152-156.
EBEL, J. & KARYOFILIS, A. (1978) [Unusual course of poisoning
with dimethoate, an organic phosphorus compound.] Ther. Ggw.,
117(2): 226-236 (in German).
EDSON, E.F. & NOAKES, D.N. (1960) The comparative toxicity of
six organophosphorus insecticides in the rat. Toxicol. appl.
Pharmacol., 2: 523-539.
EDSON, E.F., JONES, K.H., & WATSON, W.A. (1967) Safety of
dimethoate insecticide. Br. med. J., 4(5578): 554-555.
EDWARDS, J.A., LEEMING, N.M. & CLARK, R. (1984) Effect of
dimethoate on pregnancy of the rat (Unpublished report No. DTF
3/84245 dated 13 April 1984 from Huntingdom Research Centre).
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977a) Fac-
tors affecting the fate of dimethoate in soils. Intern. J.
environ. Stud., 11: 113-124.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977b) Fac-
tors affecting the accumulation of dimethoate in soil. Int. J.
environ. Stud., 11: 187-196.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1978) Factors
influencing the degradation of dimethoate in soils and solu-
tions. Int. J. environ. Stud., 11: 253-260.
ERDMANN, W.D., ZECH, R., FRANKE, P., & BOSS, I. (1966) [The
question of the therapeutic efficacy of esterase reactivators in
dimethoate poisoning.] Arzneimittelforschung, 16: 492-494 (in
German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesti-
cides. In: Montesano, R., Tomatis, L., & Davies, W., ed.
Chemical carcinogenesis: essays, Lyons, International Agency
for Research on Cancer, pp. 161-181 (IARC Scientific
Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Dimethoate. In: Evaluation of toxicity of
pesticide residues in food, Rome, Food and Agricultural
Organization of the United Nations, pp. 97-101 (FAO Meeting
Report No. PL/1965/10/1. WHO/Food Add./27.65)
FAO/WHO (1967) Dimethoate. In: Evaluation of some pesticide
residues in food, Rome, Food and Agricultural Organization of
the United Nations, p. 232.
FAO/WHO (1968) Dimethoate. In: 1967 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 117-132 (FAO/PL: 1967/M/11/1.
WHO/Food Add./68.30).
FAO/WHO (1971) Dimethoate. In: 1970 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 239-244 (AGP: 1870/M/12/1. WHO/Food
Add./71.42).
FAO/WHO (1974) Formothion. In: 1973 Evaluations of some pesti-
cide residues in food, Geneva, World Health Organization, pp.
281-290 (WHO Pesticide Residues Series, No. 3).
FAO/WHO (1978) Dimethoate. In: 1977 Evaluations of some pesti-
cide residues in food, Rome, Food and Agriculture Organization
of the United Nations, pp. 155-156 (FAO Plant Production and
Protection Paper No. 10 Sup.).
FAO/WHO (1979) Formothion, dimethoate, omethoate. In: 1978
Evaluations of some pesticide residues in food, Geneva, World
Health Organization, p. 6.
FAO/WHO (1984) Maximum residue limits for pesticides, Codex
Alimentarius Commission, Rome, Food and Agricultural
Organization of the United Nations, Vol. 13.
FAO/WHO (1985) Pesticide residues in food. Report of the 1984
Joint Meeting on Pesticide Residues, Rome, Food and Agricultural
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 62).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FAO/WHO (1987) Dimethoate. In: Pesticide residues in food.
Report of the 1987 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Rome, Food and Agricultural Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 84).
FARM CHEMICALS HANDBOOK (1984) Willoughby, Ohio Meister
Publishing Company.
FETCHER, A. (1984) Suspected dimethoate toxicity in cattle.
Mod. vet. Pract., 65(4): 283-285.
FISCHER, G.W. & SCHEUFLER, H. (1981) [Effects of dimethoate
and O-desmethyldimethoate on dominant lethal test in mice.]
Wiss. Z. Univ. Halle, 30(6): 21-29 (in German).
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity
of organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Health Sci., B20(1): 73-95.
FRANCOIS, J. & VERBRAEKEN, H. (1977) Glaucome aigu après
intoxication par un ester organo-phosphatique Bull. Soc. Belge
Ophthalmol., 176: 19-22.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14(3): 515-534.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1978 - September
1979. J. Assoc. Off. Anal. Chem., 68(5): 842-861.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1978 - September 1979. J.
Assoc. Off. Anal. Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985c) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1979 - September
1980. J. Assoc. Off. Anal. Chem., 68(6): 1163-1183.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985d) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1979 - September 1980. J.
Assoc. Off. Anal. Chem., 68(6): 1184-1197.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1980 - March
1982. J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1980 - March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 146-161.
GETZIN, L.W. & ROSEFIELD, I. (1968) Organophosphorus insecti-
cide degradation by heat-labile substances in soil. J. agric.
food Chem., 16(4): 598-601.
GIBEL, W., LOHS, KH., WILDNER, G.P., ZIEBARTH, D., & STIEGLITZ,
R. (1973) [Experimental study on cancerogenic, haematotoxic,
and hepatotoxic activity of phosphor-organic pesticides.] Arch.
Geschwulstforsch., 41(4): 311-328 (in German).
GILOT - DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN -
DAHMEN, M. (1983) Mutagenicity of some organophosphorus com-
pounds at the ade6 locus of Schizosaccharomyces pombe. Mutat.
Res., 117: 139-148.
GIRENKO, D.B. & KLISENKO, M.A. (1977) [Methods of estimating
antio and fosfamid in fruits by gas-liquid chromatography.] In:
[ Methods of determination of microquantities of pesticides in
foods, feed, and the environment,] Moscow, Kolos, pp. 117-118
(in Russian).
GIRENKO, D.B., KLISENKO, M.A., & PISMENNAYA, M.B. (1978)
[Methods of estimating organophosphorus pesticides in water by
chromatography methods.] In: [ Methods of determination of
microquantities of pesticides in foods, feed, and the
environment,] Moscow, Kolos, pp. 57-62 (in Russian).
GONTZEA, I. & GORCEA, V. (1977) The influence of protein and
fat intake on the body's resistance to dimethoate. Med. Lav.,
68(1): 13-21.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E.
(1984) Clinical toxicology of commercial products, 5th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
GRIGORASHVILI, G.S. & DZHIBLADZE, V.E. (1965) [Hygienic
evaluation of some food products after treatment with
phosphamide.] In: [ Hygiene and toxicology of pesticides, and
clinical aspects of pesticide poisoning,] Kiev, Zdorovye
Publishers, pp. 161-164 (in Russian).
HADJIDEMETRIOU, D.G., IWATA, Y., & GUNTHER, F.A. (1985)
Analysis and dissipation of dislodgable residues of acephate,
dimethoate and formetanate hydrochloride on citrus foliage.
Pestic. Sci., 16: 302-310.
HANNA, P.Y. & DYER, K.F. (1975) Mutagenicity of organo-
phosphorus compounds in bacteria and Drosophila. Mutat. Res.,
28: 405-420.
HASSAN, A., ZAYED, S.M.A.D., & BAHIG, M.R.E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimetho-
ate in the rat. Biochem. Pharmacol., 18: 2429-2438.
HAUCK, H.E. & AMADORI, E. (1980) Recent developments in high-
performance thin-layer chromatography and application to pesti-
cide analysis, New York, American Chemical Society, pp. 159-176
(ACS Symposium Series 136; CH09).
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, pp. 362-365.
HELLWIG, J. (1968a) Report on the study of the toxicity of
dimethoate in mice after 78-week administration in the diet,
(Unpublished report no. 75CO326/8242 from BASF, Department of
toxicology, Ludwigshafen/Rhein, Federal Republic of Germany).
HELLWIG, J. (1968b) Report on the study of the toxicity of
dimethoate in rats after 24 month administration in the diet,
(Project no. 70C0326/8241 dated 9 October 1986 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, Federal Republic
of Germany).
HEWITT, R., BREBBIA, A., & WALETZKY, E. (1958a) Carbamoyl
alkyl phosphorodithioates as chemotherapeutic agents: screening
by aedicidal properties in laboratory mammals, lambs and calves.
J. econ. Entomol., 51(2): 126-131.
HEWITT, R., EMRO, J., ENTWISTLE, J., PANKAVICH, J., THORSON, R.,
WALLACE, W., & WALETZKY, E. (1958b) Carbamoyl alkyl phosphoro-
dithioates as chemotherapeutic agents: effects of dimethoate
against grubs in cattle. J. econ. Entomol., 51(4): 445-450.
HILL, R.H. & ARNOLD, J.E. (1979) A personal air sampler for
pesticides. Arch. environ. Contam. Toxicol., 8: 621-628.
HOEGBERG, E.I. & CASSADAY, J.T. (1951) The reaction of O,O-
dialkyl thiophosphoric acid salts with some alpha-haloacyl deriva-
tives. J. Am. Chem. Soc., 73: 557-559.
IRPTC (1982) Scientific reviews of Soviet literature on toxic-
ity and hazards of chemicals: dimethoate, Moscow, Centre of
International Projects, GKNT, 12 pp (No. 5).
IRPTC (1987) Legal file Vol. 2, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
IWATA, Y., DUSCH, M.E., CARMAN, G.E., & GUNTHER, F.A. (1979)
Worker environment research: residues from carbaryl, chloroben-
zilate, dimethoate, and trichlorfon applied to citrus trees. J.
agric. food Chem., 27(6): 1141-1145.
IZMIROVA, N., BENCHEV, I., VELICHKOVA, V., KALOYANOVA, F.,
LAMBREVA, E., RUSEVA, P., KOSTOVA, S., ANGELOVA, R., INKJOVA,
M., BOGINOVA, L., MAKSIMOVA, F., & TZVETKOVA, P. (1973)
[Determination of minimal safety periods after treatment of
tobacco and cotton with organophosphorus pesticides.] In:
[ Hygiene, use and toxicology of pesticides, and clinical
aspects of pesticide poisoning,] Vol. 10, Moscow, Medizina
Publishers, pp. 120-124 (in Russian).
JACKSON, J.B., DRUMMOND, R.O., BUCK, W.B., & HUNT, L.M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. econ.
Entomol., 53(4): 602-604.
JAYCOX, E. (1964) Effect on Honey bees of nectar from systemic
insecticide-treated plants. J. econ. Entomol., 57(1): 31-35.
JOHNSON, E., CATERSON, C.R., & ALLEN, J.S. (1985) Mutagenicity
testing of dimethoate (AC 12880) in the in vitro CHO/HGPRT
mutation assay. (Unpublished report project no. 0423 dated 30
January 1985 from American Cyanamid Company, Princeton, New
Jersey).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. II. August 1975 - July
1976. Pestic. monit. J., 15(1): 39-50.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Pesti-
cide, metal, and other chemical residues in adult total diet
samples. XII. August 1975 - July 1976. Pestic. monit. J., 15(1):
54-59.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. III. August 1976 - Sep-
tember 1977. J. Assoc. Off. Anal. Chem., 67(1): 145-154.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984b) Pesticide, metal, and other chemical residues in adult
total diet samples. XIII. August 1976 - September 1977. J.
Assoc. Off. Anal. Chem., 67(1): 154-166.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity
of chemicals to fish and aquatic invertebrates, Washington, DC,
USA, US Department. of the Interior, Fish and Wildlife Service
(Resource Publication 137).
JUNG, H.D. (1979) [Occupational dermatoses due to pesticides.]
Dtsch. Gesundheitswes., 34(24): 1144-1147 (in German).
KAGAN, YU.S. (1981) [ General toxicology of pesticides,]
Kiev, Zdorovye Publishers, 176 pp (in Russian).
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1967)
Establishing hygienic norms of Bi 58 (Rogor, Phosphamide) in the
atmospheric air. In: Works of the Scientific Research Institute
of Labour Protection and Occupational Diseases, Vol. 15, pp. 1-
8, Sofia, Bulgaria, Medicina i Fizkultura.
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1968)
[Experimental substantiation of the maximum permissible concen-
tration of Bi 58 (Rogor, Phosphamide) in the atmosphere.] Gig.
i Sanit., 6: 6-10 (in Russian).
KALOYANOVA, F., DOBREVA, V., & MITOVA, S.V. (1984) [Effet du
dimethoate sur certaines activités du système de monoxygénase
hépatique] In: Hommage au Professeur René Truhaut, Paris,
Queray S.A., pp. 552-555.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J., ed.
IUPAC pesticide chemistry - Human welfare and the environment,
Oxford, New York, Pergamon Press, pp. 237-238.
KAPLANIS, J.N., ROBBINS, W.E., DARROW, D.I., HOPKINS, D.E.,
MONROE, R.E., & TREIBER, G. (1959) The metabolism of dimetho-
ate in cattle. J. econ. Entomol., 52(6): 1190-1194.
KARIM, A.A., HARIDI, A.A., & EL RAYAH, E.A. (1985) The
environmental impact of four insecticides on non-target
organisms in the Gezira Irrigation Scheme canals of Sudan. J.
trop. Med. Hyg., 88(2): 161-168.
KAWAR, N.S., IWATA, Y., DUSCH, M.E., & GUNTHER, F.A. (1979)
Behavior of dialifor, dimethoate, and methidothion in artifi-
cially fortified grape juice processed into wine. J. environ.
Sci. Health, B14(5): 505-513.
KHAN, R.R. & BHASKER DEV (1982) Toxicology data sheets on
chemicals: dimethoate, Lucknow, India, Lucknow Industrial
Toxicology Research Centre (No. 6).
KHERA, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for
teratogenic activity in the cat. J. environ. Pathol. Toxicol.,
2: 1283-1288.
KHERA, K.S., WHALEN, C., TRIVETT, G., & ANGERS (1979) Terato-
genicity studies on pesticidal formulations of dimethoate,
diuron, and lindane in rats. Bull. environ. Contam. Toxicol.,
22: 522-529.
KISS, Z. & FAZEKAS, T. (1983) Organophosphates and torsade de
pointes ventricular tachycardia. J. R. Soc. Med., 76: 984-985.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides. Techniques for establishing safe levels of foliar
residues. Residue Rev., 75: 81-96.
KNAAK, J.B., SCHLOCKER, P., ACKERMAN, C.R., & SEIBER, J.N.
(1980) Reentry research: Establishment of safe pesticide levels
on foliage. Bull. environ. Contam. Toxicol., 24: 796-804.
KÖPPEL, C., FORYCKI, Z., & IBE, K. (1986) Hemoperfusion in
severe dimethoate poisoning. Intensive Care Med., 12(2): 110-
112.
KRUEGER, H.R., O'BRIEN, R.D., & DAUTERMAN, W.C. (1960) Rela-
tionship between metabolism and differential toxicity in insects
and mice of dimethoate, parathion, and acethion. J. econ.
Entomol., 53(1): 25-31.
KUNDIEV, YU.I. (1979) [ Absorption of pesticides through the
skin and prophylaxis of poisonings,] Kiev, Zdorovye Publishers,
200 pp (in Russian).
LARRIPA, I., MATOS, E., & DE SALUM, S.B. (1979) [Cytogenetic
study of human population accidentally exposed to an organophos-
phate pesticide.] Medicina (Buenos Aires), 39(6): 773-774 (in
Spanish).
LARRIPA, I., MATOS, E., DE VINUESA, M.L., & DE SALUM, S.B.
(1983) Sister chromatid exchanges in a human population
accidentally exposed to an organophosphorus pesticide. Rev.
Braz. Genet., 4: 719-727.
LEBLANC, F.N., BENSON, B.E., & GILG, A.D. (1986) A severe
organophosphate poisoning requiring the use of an atropine
drip. Clin. Toxicol., 24(1): 69-76.
LEE, Y.W. & WESTCOTT, N.D. (1981) Gas chromatographic quanti-
tative analysis and persistence of dimethoate and dimethoxon
residues on and in wheat plants. J. agric. food Chem., 29(4):
860-862.
LORD, K.A., MAY, M.A., & STEVENSON, J.H. (1968) The secretion
of the systemic insecticides dimethoate and phorate into nectar.
Ann. appl. Biol., 61: 19-22.
LUCIER, G.W. & MENZER, R.E. (1970) Nature of oxidative metabo-
lites of dimethoate formed in rats, liver microsomes, and bean
plants. J. agric. food Chem., 18(4): 698-704.
MADZHIDOV, U.A. (1970) [Atmospheric pollution with fosfamid in
agricultural application.] Med. J. Uzb., 9: 40-42 (in Russian).
MAHKAMBAEVA, Z.G. (1971) [Influence of organophosphorus pesti-
cides (kilval, methylmercaptophos, fosfamid) on bioelectric
activity of heart in experimental animals.] In: [ Hygiene, use
and toxicology of pesticides, and clinical aspects of pesticide
poisoning,] Kiev, Zdorovye Publishers pp. 175-180 (in Russian).
MAROSI, G., IVAN, J., NAGYMAJTENYI, L., CSATLOS, J., & TOSZEGI,
A. (1985a) Dimethoate-induced toxic cardiac failure in the
guinea-pig. Arch. Toxicol., 57: 142-143.
MAROSI, G., IVAN, J., & NAGYMAJTENYI, L. (1985b) Cardiodepres-
sion in organophosphate poisonings. Arch. Toxicol., Suppl. 8:
289-291.
MASIAK, M. & OLAJOSSY, M. (1973) [Depressive reaction
following intoxication with an organic phosphorus preparation,
Rogor (Bi 58).] Wiadom. Lek., 26(20): 1933-1936 (in Polish).
MELNIKOV, N.N. (1974) [ Chemistry and technology of pesti-
cides,] Moscow, Khimiya Publishers, 768 pp (in Russian).
MELNIKOV, N.N., VOLKOV, A.I., & KOROTKOVA, O.A. (1977)
[ Pesticides and the environment,] Moscow, Khimiya Publishers,
pp. 240 (in Russian).
MENZER, R.E. (1970) Effect of chlorinated hydrocarbons in the
diet on the toxicity of several organophosphorus insecticides.
Toxicol. appl. Pharmacol., 16: 446-452.
MENZER, R.E. & BEST, N.H. (1968) Effect of phenobarbital on
the toxicity of several organophosphorus insecticides. Toxicol.
appl. Pharmacol., 13: 37-42.
MENZIE, C.M. (1969) Metabolism of pesticides, Washington, DC,
US Department of the Interior, Fish and Wildlife Service, pp.
160-166.
MENZIE, C.M. (1974) Metabolism of pesticides: an update,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 166-167, 485.
MENZIE, C.M. (1978) Metabolism of pesticides: update II,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 118.
MENZIE, C.M. (1980) Metabolism of pesticides: update III,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 242.
MIKHAILOV, S.S. & SHTERBAC, I.G. (1983) [Metabolism of organo-
phosphorus compounds containing a carboxyamin bond.] In:
[ Metabolism of organophosphorus poisons,] Moscow, Medizina,
pp. 96-101 (in Russian).
MITOVA, S.V., VASILEVA, L., DOBREVA, V., & KALOYANOVA, F. (1986)
Experimental studies on dimethoate oxidative desulfuration.
Toxic interfaces of neurones, smoke and genes. Arch. Toxicol.,
Suppl.9: 377.
MOHN, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides.
Mutat. Res., 20: 7-15.
MOLPHY, R. & RATHUS, E.M. (1964) Organic phosphorus poisoning
and therapy. Med. J. Aust., 29: 337-340.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides
in bacterial reversion assay systems. Mutat. Res., 116: 185-
216.
MULLER, P. & REINHOLD, R. (1973) [Experience with dimethoate
drugs in stable fly control.] Angew. Parasitol., 14(3): 158-169
(in German).
MUNCY, R.J. & OLIVER, A.D. (1963) Toxicity of ten insecticides
to the red crawfish, Procambarus clarki (Girard). Trans. Am.
Fish. Soc., 92: 428-431.
MURATORE, F., FAIOLO, A., & PONZETTA, G. (1960) [Four cases of
intoxication with organophosphorus esters with regard to liver
function.] Minerva Med., 51(80): 3342-3345 (in Italian).
NADMAITENI, L. & MAROSI, G. (1983) [Cardiotoxic action of
organophosphorus pesticides.] G ig. i Sanit., 11: 72-73 (in
Russian).
NAGLER, J., BRAECKMAN, R.A., WILLEMS, J.L., & DE BROE, M.E.
(1980) Combined hemoperfusion hemodialysis in organic phosphate
poisoning. Kidney Int., 18: 139 (Abstract).
NANDA KUMAR, N.V. & UDAYA BHASKAR, S. (1980) Colorimetric
determination of dimethoate by quantification of cholinesterase
inhibition. J. food Sci. Technol., 17: 153-155.
NCI (1977) Bioassay of dimethoate for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (Technical Report
Series No. 4).
NEHES, M., SELYPES, A., & SCHEUFLER, H. (1982) [Mutagenic
effect of trichlorfon and dimethoate on bone marrow cells of
mouse in vivo.] Wiss. Z. Univ. Halle, 31M(2): 57-62 (in
German).
NEHEZ, M., SELYPES, A., SCHEUFLER, H., & FISCHER, G.W. (1983)
Effect of dimethoate and O- demethyldimethoate on bone marrow
cells of CFLP mice. Regul. Toxicol. Pharmacol., 317(3): 349-
354.
NOAKES, D.N. & SANDERSON, D.M. (1969) A method for determining
the dermal toxicity of pesticides. Br. J. ind. Med., 26: 59-64.
NORTH, H.H. & MENZER, R.E. (1972) Biotransformation of
dimethoate by cell culture systems. Pestic. Biochem. Physiol.,
2: 278-285.
OKONEK, S. (1976) Probable progress in the therapy of
organophosphate poisoning: Extracorporeal hemodialysis and
hemoperfusion. Arch. Toxicol., 35(3): 221-227.
OKONEK, S. (1977) [Hemoperfusion with coated activated
charcoal for treating acute poisoning by remedies, plant
protectants, and fungi.] Med. Klin., 72(19): 862-866 (in
German).
OKONEK, S., HOLLMANN, H., & BOELCKE, G. (1976) [The
alkylphosphate poisoning: elimination of the poison by the
hemodialysis or blood exchange transfusion?]. Med. Welt, 27(8):
351-356 (in German).
PACH, J., GROSZEK, B., & BOGUSZ, M. (1977) Haemoperfusion in
the treatment of acute organophosphate poisoning. Acta
pharmacol. Toxicol., Vol 41, Suppl. II): 44.
PAMBOR, M. & BLOCH, Y. (1985) [Dimethoate and dithiocarbamate
as occupational contact allergens in a female agrotechnician.]
Dermatol. Monatsschr., 171(6): 401-405 (in German).
PANDEY, A.K. & TOMAR, V. (1985) Melanophores in Bufo melanos-
tictus (Schneider) tadpoles following exposure to the insecti-
cide dimethoate. Bull. environ. Contam. Toxicol., 35: 796-801.
PANSHINA, T.N. (1963a) [Experimental data on toxicology of new
organophosphorus insecticide phosphamide (Rogor, dimethoate).]
Pharmacol. Toksicol., 4: 476-484 (in Russian).
PANSHINA, T.N. (1963b) [Hygienic background for determining
the maximum permissible concentration of phosphamide in the air
of a working area.] Gig. i Sanit., 3: 21-28 (in Russian).
PANSHINA, T.N. (1966) [Experimental data on therapy of phos-
phamide intoxications.] In: [ Hygiene and toxicology of
pesticides, and clinical aspects of pesticide poisoning,] Kiev,
Zdorovye Publishers, Vol. 4, pp. 137-142 (in Russian).
PANSHINA, T.N. & KLISENKO, M.A. (1962) [Content of fosfamid
and changes in blood cholinesterase activity in different routes
of treatment of the mammalian organism.] In: [ Industrial
toxicology and clinical picture of occupational diseases due to
chemicals,] Moscow, Medgiz, pp. 181-184 (in Russian).
PETUKHOV, R.D. (1975) [Methodics of estimating of antio and
fosfamid in honey by thin-layer chromatography.] In: [ Methods
of determination of microquantities of pesticides in food, feed,
and the environment,] Moscow, Kolos, pp. 63-64 (in Russian).
PODREBARAC, D.S. (1984a) Pesticide, heavy metal residues, and
other chemical residues in infant and tod dler total diet sam-
ples. IV. October 1977 - September 1978. J. Assoc. Off. Anal.
Chem., 67(1): 166-175.
PODREBARAC, D.S. (1984b) Pesticide, metal, and other chemical
residues in adult total diet samples. XIV. October 1977 - Sep-
tember 1978. J. Assoc. Off. Anal. Chem., 67(1): 176-185.
PROBST, G.H., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEAL, S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures: A comparison with
bacterial mutagenicity using 218 compounds. Environ. Mutagen.,
3: 11-32.
RIEMER, F., DAHLENBURG, R., & GRISK, A. (1985) [Renal excretion
of dimethylphosphate and its thio derivatives after application
of dimethoate, bromophos, naled or trichlorphon to rats.]
Nahrung, 29(3): 299-302 (in German).
ROALES, R.R. & PERLMUTTER, A. (1974) Toxicity of zinc and
Cygon, applied singly and jointly, to zebrafish embryos. Bull.
environ. Contam. Toxicol., 12(4): 475-480.
ROSVAGA, R.I. (1983) [Methodology of investigation of pesti-
cide pyrolysis in their chemical-thermal harmlessness.] In:
[ Hygiene of application, toxicology of pesticides and polymeric
materials,] Kiev, Zdorovja, Vol. 13, pp. 73-77 (in Russian).
SAHU, D. & PATTANAIK, M. (1980) Persistence of rogor in some
Orissa soils. Indian J. agric. Sci., 50(6): 505-507.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDERSON, D.M. & EDSON, E.F. (1959) Oxime therapy in poison-
ing by six organophosphorus insecticides in the rat. J. Pharm.
Pharmacol., 11: 721-728.
SANDERSON, D.M. & EDSON, E.F. (1964) Toxicological properties
of the organophosphorus insecticide dimethoate. Br. J. ind.
Med., 21: 52-64.
SANTI, R. & GIACOMELLI, R. (1962) Metabolic fate of P32-
labelled dimethoate in olive fruits and some toxicological
implications. J. agric. food Chem., 10(3): 257-261.
SAN SEBASTIAN, J.R. (1985) In vivo bone marrow cytogenetics
rat metaphase analysis. PH 315-AC-001-84, Dimethoate CL 12880.
(Unpublished report dated 29 August 1985 from Pharmakon
Research International, Inc. Waverly, Pennsylvania 18471).
SCHEUFLER, H. (1975) [Effects of relatively high doses of
dimethoate and trichlorfon on the embryogenesis of laboratory
mice.] Biol. Rundsch., 13: 238-240 (in German).
SENANAYAKE, N. & KARALLIEDE, W. (1987) Neurotoxic effects of
organophosphorus insecticides. An intermediate syndrome. New
Engl. J. Med., 316(13): 761-763.
SHAMSHURIN, A.A. & KRIMER, M.Z. (1976) [ Physicochemical proper-
ties of pesticides: reference book,] Moscow, Khimya Publishers,
pp. 76-77 (in Russian).
SIDIMANOV, B.B. (1971) [Determination of fosfamid in post-
mortem material by thin-layer chromatography.] In: [ Hygiene of
application, toxicology of pesticides, and clinical picture of
poisoning,] Kiev, Zdorovja, Vol. 9, pp. 187-188 (in Russian).
SLOOFF, W. & CANTON, J.H. (1983) Comparison of the suscepti-
bility of 11 freshwater species to 8 chemical compounds. II.
(Semi)chronic toxicity test. Aquat. Toxicol., 4: 271-282.
SORG, R.M. (1985) Micronucleus test (MNT) PH 309A-AC-004-84,
Dimethoate CL 12880, (Unpublished report dated 1 March 1985
from Pharmakon Research International, Inc. Waverly,
Pennsylvania 18471).
STERRI, S.H., ROGNERUD, B., FISKUM, S.E., & LYNGAAS, S. (1979)
Effect of toxogonin and P2S on the toxicity of carbamates and
organphosphorus compounds. Acta pharmacol. toxicol., 45: 9-15.
STEVENSON, J.H. (1968) Laboratory studies on the acute contact
and oral toxicities of insecticides to honey bees. Ann. appl.
Biol., 61: 467-472.
STIEGLITZ, R., GIBEL, W., WERNER, W., & STOBBE, H. (1974)
Experimental study on haematotoxic and leukaemogenic effects of
trichlorfon and dimethoate. Acta haematol., 52: 70-76.
STROY, A.N. (1983) [Hygienic reglementation of fosfamid in
greenhouse conditions.] In: [Hygiene of application, toxicology
of pesticides and polymeric materials,] Kiev, Zdorovja, Vol.
13, pp. 90-91 (in Russian).
SZETO, S.Y., VERNON, R.S., & BROWN, M.J. (1985) Degradation of
dimethoate and pirimicarb in asparagus. J. agric. food Chem.,
33: 763-767.
TIEFENBACH, B. & LANGE, P. (1980) Studies on the action of
dimethoate on the immune system. Arch. Toxicol., Suppl. 4: 167-
170.
TRINH VAN BAO, SZABO, I., RUZICSKA, P., & CZEIZEL, A. (1974)
Chromosome aberrations in patients suffering acute organic
phosphate insecticide intoxication. Humangenetik, 24: 33-57.
UBAIDULLAEV, R.U. & MADZHIDOV, U.A. (1976) [Hygienic charac-
teristics of phosphamide as an atmospheric pollutant.] Gig. i
Sanit., 9: 7-9 (in Russian).
UCHIDA, T., DAUTERMANN, W.C., & O'BRIEN, R.D. (1964) The
metabolism of dimethoate by vertebrate tissues. J. agric. food
Chem., 12(1): 48-52.
UDAYA BHASKAR, S. & NANDA KUMAR, N.V. (1980) Colorimetric
determination of dimethoate or omethoate. Talanta, 27(9): 757-
758.
USHA RANI, M.V., REDDI, O.S., & REDDY, P.P. (1980) Mutageni-
city studies involving aldrin, endosulfan, dimethoate, phospham-
idon, carbaryl, and ceresan. Bull. environ. Contam. Toxicol.,
25(2): 277-282.
VAN MIDDELEM, C.H. & WAITES, R.E. (1964) Gas chromatographic
and colorimetric measurement of dimethoate residues. J. agric.
food Chem., 12(2): 178-182.
VELAZQUEZ, A., XAMENA, N., CREUS, A., & MARCOS, R. (1986)
Indication for weak mutagenicity of the organophosphorus
insecticide dimethoate in Drosophila melanogaster. Mutat. Res.,
172: 237-243.
VERMA, S.R., BHATNAGAR, M.C., & DALELA, R.C. (1978) Biocides
in relation to water pollution. II. Bioassay studies of a few
biocides to a fresh-water fish Channa gachua. Acta hydrochim.
hydrobiol., 6(2): 137-144.
VERMA, S.R., TYAGI, A.K., BHATNAGAR, M.C., & DALELA, R.C.
(1979) Organophosphate poisoning to some fresh-water teleosts -
acetylcholinesterase inhibition. Bull. environ. Contam. Toxi-
col., 21: 502-506.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982) Bioassay
trials with twenty-three pesticides to a fresh-water teleost
Saccobranchus fossilis. Water Res., 16: 525-529.
VISHWANATH, R. & JAMIL, K. (1986) Mutagenic and genotoxic
activities of certain organophosphorus compounds using Ames
Salmonella assay with and without microsomal induction. Ind.
J. exp. Biol., 24: 305- 308.
WALLER, G.D. & BARKER, R.J. (1979) Effects of dimethoate on
honey bee colonies. J. econ. Entomol., 72(4): 549-551.
WALLER, G.D., BARKER, R.J., & MARTIN, J.H. (1979) Effects of
dimethoate on honey bee foraging. Chemosphere, 8(7): 461-463.
WEHRAN, H.-J. & HAMMER, H.-J. (1984) [Lethal poisoning with
the pesticide dimethoate (Bi 58 EC).] Kriminal. forens. Wiss.,
54: 181-183 (in German).
WEST, B., VIDONE, L.B., & SHAFFER, C.B. (1961) Acute and
subacute toxicity of dimethoate. Toxicol. appl. Pharmacol., 3:
210-223.
WHO (1982) Field surveys of exposure to pesticides - Standard
Protocol, Geneva, World Health Organization (Unpublished
document VBC/82.1).
WHO (1984) Chemical methods for the control of arthropod
vectors and pests of public health importance, Geneva, World
Health Organization, pp. 34, 37.
WHO (1986a) Environmental health criteria 63: Organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WHO (1986b) The WHO recommended classification of pesticides
by hazard and guidelines to classification 1986-1987 World
Health Organization, Geneva, (Unpublished document VBC/86.1).
WHO (1986c) Interim specifications for dimethoate technical
and emulsifiable concentrate, Geneva, World Health Organization
(Unpublished report, WHO/IS/86.12).
WHO/FAO (1980) Dimethoate: data sheet on pesticides, Geneva,
World Health Organization (Unpublished document VBC/DS/80.42).
WINKLER, R. & MULLER, R. (1979) [Elimination and inactivation
of waters containing pesticides from agrochemical plants.]
Wasserwirtsch. Wassertech., 29(11): 369-371 (in German).
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-
induced complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ.
Mutagen., 5: 835-846.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th
ed., Lavenham United Kingdom, British Crop Protection Council.
ZECH, R., ENGELHARD, H., & ERDMANN, W.-D. (1966) Reactions of
pyridinium oximes with the alkyl phosphate dimethoate and its
derivatives. Effects on cholinesterases. Biochim. biophys.
Acta, 128: 363-371.
ZOLOTNIKOVA, G.P. (1980) [Disturbance of the immunoreactivity
of the organism under the effect of pesticides in hothouses.]
Gig. tr. prof. Zabol., 3: 38-40 (in Russian).
ZOLOTNIKOVA, G.P. & SOMOV, B.A. (1978) [On the role of pesti-
cides in the occurrence of occupational dermatitis in workers of
hothouses.] Vestnik Dermatol. Venerol., 4: 76-79 (in Russian).
ZOLOTNIKOVA, G.P. & ZOTOV, V.M. (1978) [Prognosis for resi-
dues of pesticides in the air of hothouses.] Gig. i Sanit.,
43(1): 108-109 (in Russian).
ANNEX I. TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus Insecticides - A General Introduction)
All cases of organophosphorus poisoning should be dealt
with as an emergency and the patient sent to hospital as quickly
as possible. Although symptoms may develop rapidly, delay in
onset or a steady increase in severity may be seen up to 48 h
after ingestion of some formulated organophosphorus insecti-
cides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major
references (Kagan, 1977; Taylor, 1980; UK DHSS, 1983; Plestina,
1984) and will also be included in the IPCS Health and Safety
Guides to be prepared for selected organophosphorus insecti-
cides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures
include removal of contaminated clothes and washing of the skin
with alkaline soap or with a sodium bicarbonate solution.
Particular care should be taken in cleaning the skin area where
venupuncture is performed. Blood might be contaminated with
direct-acting organophosphorus esters, and, therefore,
inaccurate measurements of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced,
if the patient is conscious, by the administration of
ipecacuanha syrup (10-30 ml) followed by 200 ml water. This
treatment is, however, contraindicated in the case of pesticides
dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also
be performed, particularly in unconscious patients, taking care
to prevent aspiration of fluids into the lungs (i.e., only after
a tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the
pesticide involved is available, it should also be stored for
further analysis (i.e., detection of toxicologically relevant
impurities). A purge to remove the ingested compound can be
administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be
started at the first sign of respiratory failure and maintained
for as long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given
at 15 to 30-min intervals. The dose and the frequency of
atropine treatment varies from case to case, but should maintain
the patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be
necessary in extreme cases and total daily doses up to several
hundred mg may be necessary during the first few days of
treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophos-
phates. This is not the case with enzymes inhibited by
carbamates. The treatment should begin as soon as possible,
because oximes are not effective on "aged" phosphorylated ChEs.
However, if absorption, distribution, and metabolism are thought
to be delayed for any reasons, oximes can be administered for
several days after intoxication. Effective treatment with
oximes reduces the required dose of atropine. Pralidoxime is
the most widely available oxime. A dose of 1 g pralidoxime can
be given either im or iv and repeated 2-3 times per day or, in
extreme cases, more often. If possible, blood samples should be
taken for AChE determinations before and during treatment. Skin
should be carefully cleansed before sampling. Results of the
assays should influence the decision whether to continue oxime
therapy after the first 2 days.
There are indications that oxime therapy may possibly have
beneficial effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to
counteract some aspects of CNS-derived symptoms, which are not
affected by atropine. Doses of 10 mg sc or iv are appropriate
and may be repeated as required (Vale & Scott, 1974). Other
centrally acting drugs and drugs that may depress respiration
are not recommended in the absence of artificial respiration
procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinization. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and
may be taken into, and then released from, fat depots over a
period of many days. It is therefore quite incorrect to abandon
oxime treatment after 1-2 days on the supposition that all
inhibited enzyme will be aged. Ecobichon et al. (1977) noted
prompt improvement in both condition and blood-ChEs in response
to pralidoxime given on the 11th-15th days after major symptoms
of poisoning appeared due to extended exposure to fenitrothion
(a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually
for an occupational setting, but, in the case of very severe
exposure or massive ingestion (accidental or deliberate), the
therapeutic doses may be extended considerably. Warriner et al.
(1977) reported the case of a patient who drank a large quantity
of dicrotophos, in error, while drunk. Therapeutic dosages were
progressively increased up to 6 mg atropine iv every 15 min
together with continuous iv infusion of pralidoxime chloride at
0.5 g/h for 72 h, from days 3 to 6 after intoxication. After
considerable improvement, the patient relapsed and further
aggressive therapy was given at a declining rate from days 10 to
16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given
and the patient was discharged on the thirty-third day with no
apparent sequelae.
REFERENCES TO ANNEX I
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-
379.
KAGAN, JU.S. (1977) [ Toxicology of organophosphorus pesti-
cides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in
Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (Unpub-
lished document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S.
& Gilman, A., ed. The pharmacological basis of therapeutics, 6th
ed., New York, Macmillan Publishing Company, pp. 100-119.
UK DHSS (1983) Pesticide poisoning: notes for the guidance of
medical practitioners, London, United Kingdom Department of
Health and Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus
poisoning. Guy's Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
Different factors may affect the accumulation and degra-
dation of dimethoate in soil, such as the soil type, the numbers
and type of microorganisms present in soil, the environmental
temperature, the pH level, the amount of pesticide applied, and
the degree of evaporation (El Beit et al., 1977a,b, 1978). The
persistence of dimethoate was greater in heavy than in light
soil. At pH 4.2, the pesticide was stable for nearly 19 days;
at pH 11, it degraded within 20 h. The amount of dimethoate in
soil increased when higher concentrations were applied. El Beit
et al. (1977a) reported that soil microorganisms played little
part in the degradation of dimethoate.
4.1.4. Plants
When applied to plants, dimethoate was rapidly absorbed and
decomposed, both on the surface and within the plant, by
hydrolysis and oxidation (Menzie, 1969; Melnikov et al., 1977).
The half-life of dimethoate in the different plants varied
between 2-5 days (Melnikov et al., 1977). Dimethoate completely
disappeared after 15-30 days, depending on the plant species and
the climatic conditions. Decomposition in plants and the
hydrolysis of dimethoate increased with temperature (Atabaev,
1972).
The dissipation of dislodgeable residues of dimethoate is
best characterized by two first-order kinetic processes. The
half-life values were 2.2 days in the 1 to 10-day period and 7.0
days in the 10 to 49-day period (Hadjidemetriou et al., 1985).
4.1.5. Disposal of wastes
Hydrolytic decomposition is the main way to inactivate
dimethoate. By adding lime (1-2 kg calcium oxide/m3 water) to
waste waters from agricultural centres, dimethoate was fully
inactivated in 45 min (Winkler & Muller, 1979).
During pyrolysis, approximately 50% decomposition of di-
methoate occurred at 500 °C with the formation of O,O-dimethyl-
S,S-dithionpyrophosphate; decomposition was complete at 1100 °C
(Rosvaga, 1983).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air, water, and soil
No studies have been reported on levels in air, water, or
soil under actual conditions of use and various environmental
conditions.
5.1.2. Food
When a combination of dimethoate and omethoate (dimethoxon)
was given to cows in dosages of 1 and 0.1 mg/kg body weight,
respectively, for 14 days, only residues of the metabolite
omethoate were observed in the milk (0.004-0.125 mg/kg). Three
days after the application, neither compound could be detected.
When dosages of 0.5 mg dimethoate/kg and 0.05 mg omethoate/kg
were given for 14 days or when corn silage containing 1-7 mg
dimethoate/kg (resulting in dosages of 0.06-0.36 mg/kg body
weight) was given for 28 or 42 days, no residues were detected
in the milk (Beck et al., 1968).
Harvest residues found in many crops 1-3 weeks after
spraying with dimethoate, during the first years of application
in the United Kingdom (1957-58), were below 2 mg/kg (Chilwell &
Beecham, 1960).
The dimethoate content of apples was 0.03-0.07 mg/kg, 75
days after an application of 0.72 kg active ingredient/ha
(Atabaev & Stepovaya, 1966).
The pulp of lemon and orange fruit treated with dimethoate
did not show any residues at a detection level of 0.01 mg/kg,
60 days after application (Iwata et al., 1979).
Residues of dimethoate do not concentrate in wine. Analysis
of seven different Californian wines indicated levels of less
than 0.03 mg/litre (Kawar et al., 1979).
No residues were found in grapes, 29 days after treatment
with 0.1-0.15% dimethoate applied at the rate of 1400 litre/2 ha
(Grigorashvili & Dzhibladze, 1965).
A number of studies on residues of dimethoate found through-
out the world have been reported in a review by De Pietri-
Tonelli et al. (1965). The residues were most frequently below
1 mg/kg. Dimethoate residues found in various agricultural
products in India are reported in Khan & Bhaskar Dev (1982).
Dimethoate was not found in total-diet samples studied in
England and Wales during 1966-67 (Abbott et al., 1970).
According to other authors, dimethoate was also not found in the
total diets of adults and infants during 1975-79 (Johnson et
al., 1981a,b, 1984a,b; Podrebarac, 1984a,b; Gartrell et al.,
1985a,b,c). Market-basket surveys carried out in 1976-78 in the
Netherlands showed only omethoate residues in a small number of
fruits (De Vos et al., 1984). In the USA, dimethoate and
omethoate have been identified in about 5% of samples of fruits
and vegetables (Duggan et al., 1983).
The use of additional procedures for the determination of
organophosphates resulted in the identification of dimethoate in
adult and infant total-diet samples in 1980-82 (Gartrell et al.,
1985d, 1986a,b). However, the intakes (0.001 µg/kg body weight
per day) were far below the FAO/WHO acceptable daily intake
(ADI) (see section 12).
5.2. Occupational Exposure
Immediately after the use of dimethoate in greenhouses,
Stroy (1983) determined levels of 0.01-0.42 mg/m3 in the air,
3.6-9.3 mg/kg in the plants, and 0.98-1.75 mg/kg in the soil.
Dimethoate content in greenhouse air has been analysed at
different times after spraying; at 0 time the measured amount
was 0.66 mg/m3, at 2.5 h it was 0.38 mg/m3, at 5 h dimethoate
content was 0.21 mg/m3, at 10 h it was 0.07 mg/m3 and at 20 h
it was 0.01/m3 (Zolotnikova & Zotov, 1978).
The exposure to dimethoate of tractor drivers using airblast
units during treatment of citrus trees was investigated by
Carman et al. (1982). Dermal exposure was measured by placing
ethyleneglycol-treated gauze patches on the shoulders, upper
arms, and knees of the drivers. An emulsifiable concentration
(EC) formulation of dimethoate containing 0.009 kg/litre was
applied at the rate of 16 822 litre/ha using an open tractor, a
cab unit with both side windows open, and a cab unit with the
windows closed. Under these conditions, the patches attached
to the driver absorbed mean deposits of 2.5, 1.5, and
< 0.01 µg dimethoate/cm2 per h, respectively. The correspond-
ing average air concentrations were 10(sic), 48, and 2 µg of
dimethoate/m3.
Procedures for determining foliar residues and to establish
the safe re-entry times for some insecticides were reported by
Knaak (1980) and Knaak et al. (1980). A safe level of
dimethoate on foliage of 53 µg/cm2 was calculated using the
results of dermal-dose ChE-response studies in male rats.
Minimum safe re-entry periods for dimethoate were estimated
to be 3 days in greenhouses, and 7 days in tobacco fields, after
application by tractor, or 5 days after application by plane
(Kaloyanova-Simeonova & Izmirova-Mosheva, 1983). No ChE
inhibition in serum or subjective complaints of workers picking
dimethoate-treated tobacco leaves were established at a concen-
tration of 1 mg/kg on the surface of the plant.
Copplestone et al. (1976) studied the exposure to dimethoate
of 8 spraymen, 1 mixer, and 2 supervisors in the Sudan. The
percentage of toxic dosea received per day, calculated on the
basis of a 4-h working day, varied from 0.02% to less than
0.001% for individual spraymen. No ChE depression was found in
any of the men.
The highest concentration of dimethoate, measured in the
work-place air of a dimethoate-producing factory in Italy, was
0.050 mg/m3 (Armeli et al., 1967).
The respiratory and dermal exposure to dimethoate of
applicators was determined for greenhouse workers. The
respiratory and dermal exposures were 0.034 mg/h and 30 mg/h,
respectively. The hands of the operators were the most affected
parts of the body, accounting for 63-92% of the total exposure
(Adamis et al., 1985).
----------------------------------------------------------------
a Percentage toxic dose per day or h (WHO, 1982). This is
calculated from these indices adapted from the method of
Durham & Wolfe (1962) using the formula:
Dermal exposure (mg/day or h) + respiratory
exposure (mg/day or h) x 10 if measured
-------------------------------------------- x 100
Dermal LD50 mg/kg (rat) x 70
6. KINETICS AND METABOLISM
6.1. Absorption and Distribution
Panshina & Klisenko (1962) checked the blood levels of
dimethoate in cats and rats after single oral doses of 50, 75,
or 200 mg/kg in the cat and 300 mg/kg in the rat. The deter-
minations were carried out 15, 30, 60, 90, 120, and 180 min
after dosing. Dimethoate was detected in the blood of cats and
rats after 30 min, and reached a maximum level after 60-90 min.
Nearly 80% of the dimethoate in the blood was found in the
erythrocytes; only 15-20% was found in the serum. With repeated
daily oral intake of dimethoate at doses of 20 mg/kg or
10 mg/kg, the maximum blood level occurred on the 5-10th day of
the study.
The same pattern in blood levels was observed with repeated
inhalation of dimethoate for 4 h/day over 3 months, at a mean
concentration of 5 mg/m3 air. Dimethoate was detected in blood
from the second day and reached its maximum by the 7-10th day.
Daily application of 50 mg dimethoate/kg on the skin of
rabbits resulted in a maximum concentration in the blood at
about the third day (Kundiev, 1979).
When dimethoate was applied to the skin of rats for 1, 2, 4,
12, or 24 h in a single dose of 560 mg/kg, the maximum concen-
tration in the skin was reached after 12 h of exposure and was
correlated with the maximum inhibition of ChE activity in the
serum and liver. The concentrations of dimethoate in the blood,
liver, and kidney were maximal after 2 h of exposure (Baranova
et al., 1986).
6.2. Metabolic Transformation
The ester and amide groups of dimethoate are cleaved in
reactions that vary with the organism and that contribute to the
selective toxicity of the compound.
The results of in vitro and in vivo studies showed that the
main metabolic pathways of dimethoate were hydrolysis and
oxidation (Hassan et al., 1969; Lucier & Menzer, 1970; North &
Menzer, 1972).
Santi & Giacomelli (1962) studied the metabolic fate of
dimethoate in olives. P=O derivative and degradation products,
such as phosphoric and/or methylphosphoric acid, were found.
The presence of the oxygen analogue dimethoxon (omethoate)
has been demonstrated in insects, plants, and mammals; it
appears to be the metabolite responsible for the toxic action of
dimethoate (Brady & Arthur, 1963; Hassan et al., 1969; Lucier &
Menzer, 1970). The highest levels of this metabolite were found
in insects, particularly in those highly susceptible to
dimethoate. The oxygen analogue was produced in larger
quantities in insects than in rats. The enzymes mediating the
hydrolysis of the carboxyamide bond are much less effective in
insects than in mammals (Mikhailov & Shterbak, 1983).
It has been shown that cleavage of dimethoate by rats and
cows occurs initially at the C-N bond to produce the carboxy
derivative (Dauterman et al., 1959; Hassan et al., 1969). A
second hydrolytic pathway involves an esterase action on the S-C
bond (Hassan et al., 1969).
Oxidative metabolism of dimethoate predominated over
hydrolytic metabolism in the cell culture system. In the whole
rat, the opposite was true. Metabolism of dimethoate in human
embryonic lung cells was much the same as metabolism in rats
(North & Menzer, 1972). In vitro and in vivo studies showed
that dimethoate is biotransformed to the P=O analogue via the
liver cytochrome P-450 system (Kaloyanova et al., 1984).
Concentrations of 0.1-10 mmol dimethoate/litre led to a linear
decrease in the rates of N-demethylation and P-hydroxylation.
Similarly, in microsomes from rats treated with dimethoate in
vivo, increased activity of desulfuration (140%, P < 0.01), and
decreased activity of hydroxylation and demethylation were seen
(Mitova et al., 1986).
In vivo studies on mice showed dimethoate toxicity to be
markedly increased by phenobarbital pre-treatment, as a result
of induction of hepatic microsomal enzymes including the mixed
function oxidases responsible for the conversion of P=S to P=O
(Menzer & Best, 1968).
It has been found that, while dimethoate undergoes rapid
degradation in the rat liver, very little occurs in other
tissues (lung, muscle, pancreas, brain, spleen, blood). The
ability of the liver to degrade dimethoate in various species
decreased in the order: rabbit > sheep > dog > rat > cattle >
hen > guinea-pig > mouse > pig. For the hen, cattle, mouse,
sheep, and rat there was a reasonably good straight-line
relationship between the LD50 values and the degradation ability
of the liver (Uchida et al., 1964).
After administration of 32P-dimethoate to rats, dimethoate,
dimethoxon, dimethoate carboxylic acid, dimethylphosphoro-
dithioate, dimethylphosphorothioate, dimethylphosphate, mono-
methylphosphate, phosphorothioate, formate, and N-methyl 2-
glucuronate acetamide were found in the urine (Hassan et al.,
1969).
Data on the metabolism of dimethoate in plants and animals
have been reviewed by Menzie (1969, 1974, 1978, 1980).
6.3. Elimination and Excretion
6.3.1. Animal studies
About 45% of the 32P-dimethoate administered orally at
50 mg/kg to rats was excreted in the urine, while only 5.8% was
eliminated in the faeces, 72 h after treatment (Brady & Arthur,
1963). The values in rats after dermal application were 30.6%
and 6.5%, respectively. More than 95% of the 32P materials in
the urine and faeces after oral or dermal administration in rats
were hydrolytic products, as determined by chloroform/water
partition coefficients.
Twenty-four h after ip and oral administration of dimethoate
to rats at doses of 0.25, 2.5, or 25 mg/kg, dimethylphosphoro-
dithioate, dimethylphosphorothioate, and dimethyl phosphate
were detected in the urine at concentrations of 12-14%, 11-15%,
and 12-13%, respectively (Riemer et al., 1985). Neither the
route of exposure nor the dose had any influence on the types of
metabolite formed.
About 87-90% of an oral dose of 10 mg dimethoate/kg was
eliminated in the urine of cattle at the end of 24 h. The same
percentage of an intramuscular dose of 10 mg/kg was excreted
after 9 h. Only 3.7-5% of the oral dose and about 1.1% of the
intramuscular dose were eliminated in the faeces after 72 h and
24 h, respectively (Kaplanis et al., 1959).
6.3.2. Human studies
In human beings, 76-100% of radioactivity was reported
to be excreted in the urine, 24 h after oral dosing with 32P-
dimethoate (Sanderson & Edson, 1964).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The addition of dimethoate to soil at 10 or 100 mg/kg did
not result in significant differences in the number of bacteria
solubilizing tricalcium phosphate or in the number of bacteria
mineralizing calcium glycerophosphate, but an increase in the
population of phospholipase-producing organisms solubilizing
lecithin occurred (Congregado et al., 1979). At 10 mg/kg, an
increase in carbon dioxide production occurred for 2 weeks after
treatment, followed by a decrease to control levels. At
100 mg/kg, the increase in carbon dioxide output was slower and
longer.
7.2. Aquatic Organisms
A number of LC50s have been determined for various aquatic
organisms (Table 3).
The median tolerance limit of the fresh-water teleost,
Channa punctatus for dimethoate is 20.5 mg/litre (Anees, 1975).
Exposure for 24 h, 96 h, or 14 days to dimethoate concentrations
of 10.8, 8.0, or 5.0 mg/litre, respectively, produced moderate
vacuolation of the liver and a high degree of cytoplasmic
granulation, which developed for up to 96 h of exposure. The
14-day exposure added little in the way of vacuolation or
granulation (Anees, 1978a). The haematological response to
dimethoate included reduced erythrocyte counts and haemoglobin
concentration, and an elevated mean corpuscular haemoglobin and
colour index indicating that the insecticide exerted an effect
similar to the production of anaemia (Anees, 1978b).
The signs of the toxicity of dimethoate in fish
(Channa punctatus) included jumping, erratic movement,
imbalance, and death (Dikshith & Raizada, 1981a; Dikshith,
1986).
Verma et al. (1978) determined the TLm values of dimethoate
for Channa gachua for 24, 48, 72, or 96 h to be 5.2, 5.0, 4.6,
or 4.5 mg/litre, respectively. The safe concentration of
dimethoate calculated on the basis of TLm values was approxi-
mately 1.4 mg/litre.
Dimethoate inhibited AChE activity in the brain, liver, and
muscle of some fresh-water teleosts (Channa gachua and Cirrhina
mrigala), exposed to sublethal concentrations of 35% EC
formulation (0.9-2.4 and 0.6-1.6 mg/litre, respectively) (Verma
et al., 1979).
Table 3. Summary of acute toxicity values for aquatic organisms
---------------------------------------------------------------------------------------------------------
Species LC50 (mg/litre) Reference
24-h 48-h 72-h 96-h
---------------------------------------------------------------------------------------------------------
Rainbow Trout 20 - - 8.5 Melnikov et al. (1977)
(Salmo gairdneri)
Rainbow Trout - - - 6.2 Johnson & Finley (1980)
(Salmo gairdneri)
Long-nosed killifish 1.0 - - Melnikov et al. (1977)
(Fundulus similis)
Saccobranchus fossilis 5.14 4.80 4.67 4.57 Verma et al. (1982)
Channa punctatus 68 54 - 47 Dikshith & Raizada (1981a)
Dikshith (1986)
Scud 0.9 0.4 - - Menzie (1969)
(Gammarus lacustris)
Scud - - - 0.20 Johnson & Finley (1980)
(Gammarus lacustris)
Red Crayfish - 1.0 - - Muncy & Oliver (1963)
(Procambarus clarkii)
Stonefly - 0.14 - - Menzie (1969)
(Pteronarcys california)
Stonefly - - - 0.043 Johnson & Finley (1980)
(Pteronarcys california)
Unspecified insect 0.51 - - - Sanders & Cope (1968)
Bluegill - - - - Johnson & Finley (1980)
---------------------------------------------------------------------------------------------------------
Dalela et al. (1979) reported that acute (5-h) and short-
term (up to 32 days) exposure of the fish, Channa gachua, to
dimethoate at 6.2 mg/litre and 1.5 mg/litre, respectively,
produced histological changes in the gills. On acute exposure,
there was erosion at the distal end of the gill filaments and
loss of cell membrane. With exposure to a concentration of
1.5 mg/litre, the basement membrane started separating, and the
damage to the gill was found to be more significant with
increasing exposure time, with vacuolization occurring after 32
days.
The exposure of the fish Heteropneustes fossilis to a
dimethoate concentration of 10 mg/litre led to an increased
level of glycogen by the end of the second week in both the
liver and the kidney, and to a slight decrease in the protein
contents at the end of the eighth day (Awasthi et al., 1984). A
sharp rise in the activity of succinate dehydrogenase in both
organs was noted during the first two weeks of this study.
The estimated 48- and 72-hour TLm values for zebrafish
Brachydanio rerio embryos, exposed to dimethoate, were
940 mg/litre and 259 mg/litre, respectively (Roales &
Perlmutter, 1974). Dimethoate retarded the development of
embryos as expressed by lack of heartbeat and little movement at
24 h.
Dimethoate at a concentration of 0.05 mg/litre produced
morphological changes in the melanophores of Bufo melano-
stictus tadpoles and an increase in pigmented areas of the skin
(Pandey & Tomar, 1985).
Dimethoate had a very low toxicity for some aquatic
organisms in Sudan, such as Oreochromis niloticus, Gambusia
affinis, Pseudagrion spp., Crocothemis erythraea, and Lanistes
carinatus. Under laboratory conditions, it did not kill any
animal at concentrations lower than 80 mg/litre (Karim et al.,
1985).
The toxicity of dimethoate for 11 freshwater species was
studied by Slooff & Canton (1983). The results are summarized
in Table 4. The relative susceptibility tests indicated that
Daphnia magna was the organism most sensitive to dimethoate,
while the microorganisms P.fluorescens, M.aeruginoso, and
S.pannonicus were generally less sensitive indicators of
toxicity. The susceptibility of aquatic species to a chemical
may vary by more than two-three orders of magnitude. The data
demonstrate that the sublethal criteria studied were not
necessarily the most sensitive toxicological criteria.
7.3. Terrestrial Organisms
7.3.1. Honey-bees
The oral LD50 for the honey-bee (Apis mellifera L) ranges
from 93 to 150 ng per bee (Jaycox, 1964; Lord et al., 1968;
Stevenson, 1968; Barker et al., 1980). The contact LD50 is
98-120 ng per bee (Stevenson, 1968).
Table 4. Fresh-water species susceptibility to dimethoate
---------------------------------------------------------------------------------------------------------
Type of Test species Lifestage Exposure Test condition Toxicological No-effect
organism time Temper- Test parameter level
(days) ature methods (mg/litre)
---------------------------------------------------------------------------------------------------------
Bacteria (Pseudomonas fluorescens) log-phase 0.3 22 ± 2 Static Specific 320
growth rate
Cyano Bluegreen bacteria log-phase 4 23 ± 2 Static Specific 32
bacteria (Microcystis aeruginosa) growth rate
Algae Green Algae log-phase 4 23 ± 2 Static Growth 100
(Scenedesmus pannonicus) (biomass)
Plant Lemna minor - 7 25 ± 1 Static Specific 32
growth
rate
Crustacean Water flea (Daphnia magna) 1 day 21 19 ± 1 Semi- Mortality 0.032
static reproduction 0.1
Insect Mosquito (Culex pipiens) 1st 25 27 ± 1 Semi- Mortality 0.32
instar static development 0.32
Coelente- Hydrozoan (Hydra oligactis) budless 21 18 ± 1 Semi- Specific 100
rate static growth rate
Mollusc Giant Pond Snail 5 months 40 20 ± 1 Semi- Mortality 32
(Lymnaea stagnalis) static Reproduction 10
egg 7 20 ± 1 Semi- Hatching 32
static
Fish Guppy (Poecilia Reticulata) 3-4 weeks 28 23 ± 1 Semi- Mortality 32
Viviparous static Behaviour + 0.1
Mortality
Fish Japanese Ricefish eggs 40 23 ± 2 Semi- Mortality 0.32
(Oryzias latipes) oviviparous static Behaviour 0.32
Hatching 100
Growth
Amphibian Xenopus laevis 2 days 100 20 ± 1 Semi- Mortality 1
static Development 32
Growth 32
---------------------------------------------------------------------------------------------------------
From: Slooff & Canton (1983).
Dimethoate was only slightly repellent to foraging honey-
bees. The self-limiting dose for foraging was 20-25 times the
lethal oral dose (2.9-3.9 µg/bee vs 150 ng/bee). This can be
interpreted on the basis of 5% absorption by foraging bees,
while 95% is passed on to the colony. Thus, systemic insecti-
cide in nectar may also pose a threat to the rest of the colony
when brought back to the hive (Waller et al., 1979).
Residual toxicity has been supported by several obser-
vations. Nectar from plants sprayed with 0.1% dimethoate was
lethal for honey-bees for at least 2-3 days (Jaycox, 1964) or 10
days (Barker et al., 1980). Waller et al. (1984) also showed
the possible toxic levels of residues in the nectar for up to
10 days after treatment of lemon trees with dimethoate at a rate
of 1.12 kg of ai per ha. The high bee mortality observed,
immediately after treatment, was attributed to dimethoate
residues on the plant surface.
7.3.2. Birds
The acute toxicity studies of dimethoate for birds are
summarized in Table 5.
Table 5. Acute oral LD50 of dimethoate for birds (mg/kg)a
----------------------------------------------------------------
Species Sex Pure Laboratory Technical Liquid
grade grade formulation
----------------------------------------------------------------
Hen F 50 40 30 25
Pheasant M 15 - 20 15 20 25
Duck F - > 40 - -
Sparrow M/F - - - 22
Blackbird M/F - - - 26
----------------------------------------------------------------
a From: Sanderson & Edson (1964).
Hens did not show any evidence of delayed neurotoxicity
(Sanderson & Edson, 1964; Gaines, 1969; Francis et al., 1985).
The effect of dimethoate on esterase levels following the
oral dosing of pheasants and following long-term feeding to
pheasants and pigeons was investigated by Bunyan et al. (1968,
1969). Dimethoate inhibited brain-alpha-naphthyl acetate
esterase more than brain-cholinesterase and triacetin esterase
in acute studies.
A characteristic of dimethoate was the elevation of phenyl
benzoate esterase levels, showing that after initial liver
damage, dimethoate is able to induce certain enzymes.
7.3.3. Farm animals
The acute oral LD50s for several farm animals are
summarized in Table 6.
No visible signs of intoxication were seen in horses
receiving dimethoate orally at doses of 25 or 50 mg/kg. Single
doses of dimethoate at 40 mg/kg were effective in removing
Gasterophilus spp. from infected horses, but toxic signs
appeared in animals treated with higher levels of 60-80 mg/kg
(Jackson et al., 1960).
Table 6. Acute oral LD50 for farm animals
-------------------------------------------------
Species LD50 (mg/kg Reference
body weight)
-------------------------------------------------
Horse > 50 Jackson et al. (1960)
Sheep 80 Hewitt et al. (1958a,b)
Cattle 70 Hewitt et al. (1958a,b)
-------------------------------------------------
Mild signs of intoxication occurred in sheep at 75 mg/kg,
including slight salivation, lachrymation, transitory diarrhoea,
rhinitis, and anorexia. Doses lower than 15 mg/kg were essen-
tially asymptomatic in calves. The data with dimethoate
indicate an appreciable margin of safety between the lowest
dose that kills first instar Hypoderma lineatum (5 mg/kg), and
the doses that produce mild toxicity (15-20 mg/kg), or severe,
reversible toxicity (40 mg/kg) (Hewitt et al., 1985b).
Fetcher (1984) described cases of suspected dimethoate
intoxication in cattle grazing on pasture that had been sprayed
6 weeks earlier. There was a predominance of nicotinic signs
and a poor response to atropine treatment. Chemical analysis of
liver, kidney, and brain tissue did not reveal any organo-
phosphorus compounds or metabolites. Whole blood-ChE was
depressed in 3 out of 14 animals.
After spraying barns (for calves) and pigsties with
dimethoate, only 16-29% of the initial concentration still
persisted after 8 weeks. Nevertheless, the animals showed a
decrease in ChE (Müller & Reinhold, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides, especially their short- and long-term effects on
the nervous systems, can be found in the WHO Environmental
Health Criteria document entitled EHC 63: Organophosphorus
Insecticides, a General Introduction (WHO, 1986a).
8.1. Single Exposures
The acute oral and dermal LD50s of dimethoate for several
animal species are summarized in Tables 7 and 8 (all LD50s are
expressed as active ingredient).
Signs, characteristic of organophosphorus intoxication,
were observed in the rat 0.5-2 h after oral administration of
dimethoate (Sanderson & Edson, 1964). They included muscular
fibrillation, salivation, lachrymation, urinary incontinence,
diarrhoea, respiratory distress, prostration, gasping, coma, and
death (WHO, 1986a).
Oral LD50 values for rats, which were measured in 13
studies, ranged from 150 to 680 mg/kg body weight. The purity
and formulation of the compounds used were not stated in most
of the reports. Oral LD50s were determined of 60-140 mg/kg
body weight for mice, 200 mg/kg body weight for hamsters,
350-600 mg/kg body weight for guinea-pigs, 280-500 mg/kg body
weight for rabbits, and 100 mg/kg body weight for cats.
Administration of 100 mg/kg body weight to dogs did not result
in mortality. The World Health Organization based its classi-
fication of dimethoate as moderately hazardous on an acute oral
LD50 in the rat of 150 mg/kg body weight (WHO, 1986b).
The dermal LD50s for rats were found to range between 500
and 1150 mg/kg body weight, and were of about the same order of
magnitude as the oral LD50s or slightly higher (Table 8).
Data on LD50s after parenteral administration were given by
Sanderson & Edson (1964). The values were comparable with those
for ip, sc, and iv administration. In the rat, the values for
ip administration varied between 175 and 325 mg/kg body weight
and were also of the same order of magnitude as the oral LD50s.
The inhalation LC50 has not been estimated, but, in a 4-h
inhalation study on rats, Panshina (1963b) did not find any
signs of intoxication with exposure to dimethoate at 20 mg/m3
air, 40% cholinesterase inhibition at 10 mg/m3, and no effects
at 2 mg/m3. Visible signs of intoxication were observed in cats
at concentrations of 50-80 mg/m3. At 20 mg/m3, cholinesterase
inhibition was found to be 10-66%, while at 2-8 mg/m3, it was
7-56%. No effects were seen at 1.5 mg/m3 (Panshina, 1963b).
Table 7. Acute oral LD50 of dimethoate in experimental animals
-------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
-------------------------------------------------------------------------
Rat M 32% emulsifiable 247 Edson & Noakes (1960)
solution
Rat M/F technical 185 - 245 West et al. (1961)
Rat 230 Panshina (1963a)
Rat technical 172 Panshina (1963a)
Rat M/F pure 500 - 680 Sanderson & Edson
(1964)
Rat M/F laboratory 280 - 356 Sanderson & Edson
grade (1964)
Rat M/F technical 180 - 336 Sanderson & Edson
(32-40% w/v) (1964)
Rat M/F liquid formul- 150 - 400 Sanderson & Edson
ation (20% ai) (1964)
Rat M/F wettable powder 280 - 300 Sanderson & Edson
(1964)
Rat pure 250 Atabaev & Stepovaya
(1966)
Rat M/F produced 1962 215 - 245 Gaines (1969)a
(43.5%)
Rat pure 200 - 300 Ben-Dyke et al.
(1970)
Rat 250 - 265 Melnikov (1974)
Mouse 99% pure 140 Hewitt et al. (1958b)
Mouse pure 135 Panshina (1963a)
Mouse technical 125 Panshina (1963a)
Mouse F pure 60 Sanderson & Edson
(1964)
----------------------------------------------------------------------------
a Lower figures (20-30 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
Table 7. (contd).
--------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
--------------------------------------------------------------------------
Mouse F technical 60 Sanderson & Edson
(1964)
Hamster M laboratory grade 200 Sanderson & Edson
(1964)
Guinea- M/F pure 550 Sanderson & Edson
pig (1964)
Guinea- M/F laboratory grade 600 Sanderson & Edson
pig (1964)
Guinea- M/F technical 350 - 400 Sanderson & Edson
pig (1964)
Guinea- M/F liquid formul- 350 - 370 Sanderson & Edson
pig ation (1964)
Rabbit M/F pure 500 Sanderson & Edson
(1964)
Rabbit M/F laboratory grade 450 Sanderson & Edson
(1964)
Rabbit M/F technical 300 Sanderson & Edson
(1964)
Rabbit M/F liquid formul- 283 Sanderson & Edson
ation (1964)
Cat technical 100 Panshina (1963a)
---------------------------------------------------------------------------
8.2. Skin and Eye Irritation
A single dose of 300 mg technical dimethoate/kg body weight
did not cause skin irritation in male and female rabbits
(Dikshith & Raizada, 1981b).
In a study by West et al. (1961), dimethoate did not have
any irritant effect on the rabbit eye after introduction of
10 mg of dry material into the conjunctival sac. However, in a
personal communication (1986), the US EPA suggested that
dimethoate had a slight irritant effect on the eye.
8.3. Repeated Exposures
The effects on experimental animals of repeated oral or
inhalation exposure to dimethoate are summarized in Tables 9 and
10.
In the various studies, which ranged from 5 1/2-12 months
in duration, inhibition of cholinesterase (ChE) in the erythro-
cytes was a more sensitive indicator of exposure to dimethoate
than ChE inhibition in plasma. ChE activity in the brain was
measured in one study only.
Table 8. Acute dermal LD50 of dimethoate in experimental animals
------------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight
------------------------------------------------------------------------------
Rat M 32% emulsifiable 1120 Edson & Noakes (1960)
solution
Rat liquid formula- 700 - 1150 Sanderson & Edson
tion (24-h) (1964)
Rat wettable powder 500 (24-h) Sanderson & Edson
(1964)
Rat M/F produced 1962 610 Gaines (1969)a
Rat M 32% w/v emulsifi- 770 - 1090 Noakes & Sanderson
able solution (24-h) (1969)
Rat M 32% w/v emulsi- > 1100 (4-h) Noakes & Sanderson
fiable solution (1969)
Guinea-pig liquid formula- 965 West et al. (1961)
tion (46%)
Guinea-pig wettable powder 995 West et al. (1961)
(25%)
Rabbit not specified 600 Melnikov (1974)
------------------------------------------------------------------------------
a Lower figures (55-61 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
In a study by West et al. (1961), no effects were observed
on ChE inhibition in rats administered dimethoate in the diet at
32 mg/kg. In their first study (12 months) on rats, Sanderson
& Edson (1964) observed inhibition of ChE in erythrocytes at
50 mg/kg diet, but not at 10 mg/kg. In the second study (5 1/2
months), inhibition of ChE in erythrocytes was found at both 20
and 10 mg/kg, but not at 5 mg/kg. The studies of Atabaev (1972)
did not show any inhibition of blood-ChE in rats administered a
40% formulation of dimethoate at 0.5-1 mg/kg body weight, corre-
sponding to 0.2-0.4 mg dimethoate/kg body weight. From all
available data on the rat, a dietary level of dimethoate of 5
mg/kg, corresponding to 0.25 mg/kg body weight, can be con-
sidered as the no-observed-adverse-effect level.
From limited studies on the dog (West et al., 1961), it can
be concluded that a level of 10 mg dimethoate/kg diet, corre-
sponding to 0.25 mg/kg body weight, does not result in ChE
depression in erythrocytes.
ChE inhibition was not observed in an inhalation study in
which rats were exposed for 14 h/day, over 3 months, to 0.01 mg
dimethoate/m3 (measured concentration) (Kaloyanova et al.,
1968).
Table 9. The effects on experimental animals of repeated exposure to dimethoate
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat technical 50, 100, or 200 35 days no mortality; no signs West et al. (1961)
(95%) mg/kg diet of intoxication
Rat technical 2, 8,or 32 mg/kg 3 months no mortality; no ChE West et al. (1961)
(95%) diet inhibition
Rat technical 15 mg/kg (oral) 6 months 100% inhibition of ChE Panshina (1963a)
in serum and erythro-
cytes; approximately
85% inhibition in brain
Rat technical 30 mg/kg (oral) 6 months death of 3 out of 5 Panshina (1963a)
animals
Rat technical 60 mg/kg (oral) 6 months death of all animals Panshina (1963a)
Rat (M) laboratory 10 mg/kg diet 12 months no inhibition of ChE in Sanderson & Edson
grade erythrocytes or plasma (1964)
Rat (M) laboratory 50 mg/kg diet 12 months marked inhibition of Sanderson & Edson
grade ChE in erythrocytes (1964)
Rat (M) laboratory 200 mg/kg diet 12 months marked toxic effects; Sanderson & Edson
grade reduced rate of weight (1964)
gain; inhibition of ap-
proximately 70% and 100%
ChE in plasma and erythro-
cytes, respectively
Rat laboratory 800 mg/kg diet 12 months severe toxic effects Sanderson & Edson
grade (1 week) (cholinergic effect: (1964)
weakness, weight loss
after a few days); the
pesticide was withdrawn
after one week; complete
recovery in 10 - 14 days
Rat (M) technical 20 or 10 mg/kg 5 1/2 months 50 and 40% inhibition Sanderson & Edson
(weanling) diet of ChE in erythrocytes, (1964)
respectively
---------------------------------------------------------------------------------------------------------
Table 9. (contd).
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat (M) technical 5 mg/kg diet 5 1/2 months no inhibition of ChE Sanderson & Edson
(weanling) (1964)
Rat 40% formula- 13 mg/kg body 4 months one rat died on the Atabaev (1972)
tion weight (oral) 35th day
Rat 40% formula- 50 mg/kg body 4 months 3 rats died on the 7th Atabaev (1972)
tion weight (oral) day, 2 died on the 8th
day, and one died on the
70th day
Rat 40% formula- 0.5 - 1 mg/kg 6 months no effect on ChE Atabaev (1972)
tion (oral)
Rat 40% formula- 5 mg/kg body 6 months AChE inhibition in Atabaev (1972)
tion weight (oral) blood in the first 2
months (approximately
50%)
Dog technical 2 or 10 mg/kg 13 weeks no inhibition of ChE West et al. (1961)
diet
Dog technical 50 mg/kg diet 13 weeks slight depression of West et al. (1961)
ChE in erythrocytes
Cat technical 10 mg/kg body 3 months death in 2 out of 4 Panshina & Klisenko
weight (oral) animals (1962)
Cat technical 20 mg/kg body 3 months death in 2 out of 3 Panshina & Klisenko
weight (oral) animals (1962)
Cat 40% formula- 0.5 - 1 mg/kg 3 months no effect on ChE Atabaev (1972)
tion body weight
(oral)
Cat 40% formula- 2 mg/kg body 3 months reduced body weight in Atabaev (1972)
tion weight (oral) the first 2 months
(16 - 32%); recovery at
the third month
---------------------------------------------------------------------------------------------------------
Table 10. Inhalation toxicity - repeated exposure
---------------------------------------------------------------------------------------------------------
Species Concentration Duration Effects Reference
(mg/m3) Daily exposure Number of
(h) months
--------------------------------------------------------------------------------------------------------
Rat 2 ? 2 no visible signs of intox- Panshina (1963b)
ication; 26% inhibition of
AChE in blood at the end of
the study
Cat 1.5 ? 1.5 no visible signs of intox- Panshina (1963b)
cation; 40-72% inhibition
of AChE in blood at the end
of the study
Rat in a miniature greenhouse (0.6 m3; temperature no blood-ChE inhibition Sanderson & Edson
21-30 °C); plants sprayed with 5 ml of 0.5% (1964)
aqueous formulation in the course of 28 days
Rat and 4.50 8 3 inhibition of ChE; leuk- Kaloyanova et al.
guinea-pig ocytosis (1968)
Rat 0.05 14 3 inhibition of ChE in blood Kaloyanova et al.
only in the first month; (1968)
inhibition of brain- and
liver-AChE; morphological
alterations in the neurons
Rat 0.01 14 3 no changes observed Kaloyanova et al.
(1968)
Rat 0.495 24 3 inhibition of ChE and Ubaidullaev &
changes Madzhidov (1976)
Rat 0.049 24 3 inhibition of ChE Ubaidullaev &
Madzhidov (1978)
Rat 0.003 24 3 no detectable changes Ubaidullaev &
Madzhidov (1978)
---------------------------------------------------------------------------------------------------------
8.4. Reproduction Studies
One reproduction study on mice has been reported in the
literature (Budreau & Singh, 1973). In this 5-generation study,
initial groups of 14 female and 10 male mice received dimethoate
in the drinking-water at 60 mg/litre for a period of one month,
prior to mating. A comparable control group was included in
the study. Animals receiving dimethoate showed a significant
( P < 0.05) reduction in mating performance (expressed as pro-
portion of females with deliveries to females mated), which
ranged from 33 to 61%, varying with litter and generation.
Similarly, reproduction time (measured as number of days elaps-
ing from first day of mating to day of delivery) was signifi-
cantly ( P < 0.01) increased in all first litters of all 5
generations examined, but unaffected in all second litters. The
biological relevance of this observation is unclear. Litter
size and average weight at birth were not affected by treatment.
Although the mean weights of treated pups were not significantly
lower, the growth rate was consistently lower in treated pups
compared with that in the controls.
A 3-generation reproduction study using technical
dimethoate (98.3%) was carried out on mice. Information sup-
plied by the US EPA indicated that there were no effects on re-
production and teratogenicity at a dimethoate level of 50 mg/kg
(US EPA, personal communication, 1986). Details of this study
were not available.
8.5. Teratogenicitya
Intraperitoneal administration of 40 mg dimethoate/kg body
weight, given as a single dose on the day of mating or on the
9th day of gestation, or given for the first 14 days of
gestation in mice, caused a high incidence of embryonal loss
(Scheufler, 1975).
Cygon 4E (containing 47.3% dimethoate) was given to female
rats by intubation from the 6th to the 15th day of gestation at
dose levels of 3, 6, 12, or 24 mg/kg body weight. The 24 mg/kg
dose was toxic for the dams (8 out of 20 dams manifested clonic
spasms and muscular tremors during the treatment period, 7 re-
covered, and one died on the 16th day of pregnancy). Doses of
12 and 24 mg/kg were associated with an increase ( P < 0.05)
in the numbers of anomalous litters (each having at least one
anomalous fetus) and wavy-ribbed fetuses. The 3 and 6 mg/kg
doses (equal to 1.42-2.84 mg dimethoate/kg) did not produce any
evidence of teratogenicity or embryotoxicity in the rats (Khera
et al., 1979).
----------------------------------------------------------------
a A further teratogenicity study on the rat (Edwards et al.,
1984) was submitted to the FAO/WHO Joint Meeting on Pesti-
cide Residues (JMPR) in 1987. Although fetotoxicity was ob-
served, there were no teratogenic effects (FAO/WHO, 1987).
Cygon 4E (47.3% dimethoate) was given to cats in gelatin
capsules at doses of 3, 6, or 12 mg/kg on the 14th-22nd days of
pregnancy. At the levels tested, the compound did not produce
any effects on the incidence of pregnancy. In the 12 mg/kg
group, forepaw polydactily was observed in 8 out of 39 fetuses.
Cygon 4E at 3 or 6 mg/kg (1.42-2.84 mg dimethoate/kg) did not
produce any effects (Khera, 1979). (The effect observed in
Khera's investigations may be due to the other components in the
formulation).
Courtney et al. (1985) reported that dimethoate adminis-
tered orally was not teratogenic in CD-1 mice at dose levels of
10 or 20 mg/kg body weight, and that these levels were not
lethal to the dams. The two highest dose levels of 40 and
80 mg/kg produced maternal toxicity.
8.6. Mutagenicity
A variety of in vivo and in vitro mutagenicity tests have
been carried out with dimethoate, the results of which are given
in Table 11.
Negative mutagenicity results were reported by Degraeve et
al. (1984) for commercial mixtures of insecticides containing
dimethoate. Two formulations were tested: dimethoate + fenitro-
thion (dose 60 mg/kg body weight, corresponding to 9 mg/kg of
each) and dimethoate + malathion + methoxychlor (dose 100 mg/kg
body weight corresponding to 9.5 mg dimethoate/kg). A single
ip injection did not induce chromosome aberrations in bone
marrow cells, spermatogonia, or primary spermatocytes of the
mouse. No significant increases in pre- or post-implantation
fetal lethality were observed in a dominant lethal mutation
assay.
Alkylation by dimethoate at the N-7 position of guanine in
DNA has been investigated (Dedek et al., 1984). Male mice
(strain AB Jena/Halle, random bred) were injected ip with 14C-
methyl-labelled dimethoate at a dose of 0.35 mmol/kg. The
extent of methylation was in the range of 1-10 µmol N-
7 methylguanine/mol guanine; the values in the kidneys were
higher than those in the liver. The excretion half-life of N-
7 methylguanine was 23-160 h.
In summary, dimethoate has been found to be mutagenic in
both in vitro and in vivo assays.a
----------------------------------------------------------------
a After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
Table 11. Mutagenicity tests
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Escherichia coli 1 - 6.10-3 mmol 5-methyltryptophane resistance Mohn (1973)
K-12/gal Rs mutation -positive
Escherichia coli Up to 5000 µg/plate positive Moriya et al.
WP2 hcr (1983)
Escherichia coli Minimum mutagenic positive Probst et al.
WP2 & WP2uvrA- concentration (1981)
47 nmoles/ml
Salmonella typhimurium negative Probst et al.
TA 98, 100, 1535, 1537, (1981)
1538
Salmonella typhimurium Up to 5000 µg/plate positive with and without Moriya et al.
TA 100 activation (1983)
Salmonella typhimurium Up to 5000 µg/plate negative Moriya et al.
TA 98, 1535, 1537, 1538 (1983)
Salmonella typhimurium 5 - 200 µg/plate positive at all doses, mutagenicity Vishwanath &
TA 100 (base pair subst.) increased with liver microsomes Jamil (1986)
Saccharomyces cerevisiae 7 doses, 40 - 100 mmol induction of mitotic gene Fahrig (1974)
conversions
Schizosaccharomyces pombe 1.3 - 131 mmol negative Gilot-Delhalle
ade 6 (LD50 50 mmol) (1983)
Chinese hamster ovary 20,40 & 80 µg/ml sister chromatid exchange increase Chen et al.
cells V79 10 µg/ml negative (1981)
Rat hepatocytes culture 47 nmol/ml negative unscheduled Probst et
al. (1981)
SV-40 transformed human 100 & 1000 umol positive unscheduled DNA Ahmed et
synthesis al. (1977)
Drosophila melanogaster 1 mg/kg (feeding) negative Woodruff et
al. (1983)
---------------------------------------------------------------------------------------------------------
Table 11. (contd).
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Drosphila melanogaster adult inj. ip 0, 10, increase in sex-linked Velazquez et
20 mg/kg body weight recessive lethals at 10 mg/kg al. (1986)
not at 20 mg/kg
Drosophila melanogaster adult feeding 0, negative Velazquez et
10 mg/kg; larval negative al. (1986)
feeding 0, 20 mg/kg
Host-mediated assay mouse 3 equal oral doses positive - mutation factor of Usha Rani et
Salmonella typhimurium of 155 mg/kg 3.44 ( P < 0.05) al. (1980)
Dominant lethal mutation acute 10 mg/kg ip negative Degraeve &
assay mice strain Q 7 weeks 0.6 mg/litre Moutschen
drinking-water, equiv- (1983)
alent to 0.093 mg/kg
body weight
Dominant lethal test 30 & 60 mg/kg body negative Fischer &
- AB Jena Halle mice 5 x 6 mg/kg body weight Scheufler
ip (1981)
DBA mice 3 x 18 mg/kg body weight
ip
Micronucleus test - 2 equal doses of a significant increase in Usha Rani et
mice - bone marrow 51.7 mg/litre drink- frequency of polychromatic al. (1980)
ing-water at 24-h erythrocytes with micronuclei
intervals -0.85% (controls 0.28%)
Chromosome abnormalities 50 and 100 mg/kg body positive Bhunya & Behera
mice - bone marrow weight (1975)
Mice - bone marrow 20 mg/kg body weight no abnormalities Nehés et al.
ip (1982)
Mice - bone marrow 60 mg/kg body weight increase in number of mitosis, Nehéz et al.
ip aberrations (1983)
Syrian golden hamsters 16, 32, 80, or increase in number of chromatid Dzwonkowska &
Mesocricetus auratus 160 mg/kg body weight and chromosomal breaks Hübner (1986)
ip single (no dose-response relationship)
---------------------------------------------------------------------------------------------------------
8.7. Carcinogenicity
Two carcinogenicity studies, reported by Gibel et al.
(1973), are summarized below.
Rat: Groups of 40, 10-week-old Wistar rats were intubated
with 0 (control), 5, 10, or 30 mg dimethoate/kg body weight
(twice weekly) for the life span. The mean life spans were 743,
518, 511, and 627 days at 0, 5, 15, and 30 mg/kg body weight,
respectively. No malignant tumours were observed in the 36
control animals. In test groups, malignant tumours occurred
in 2/26, 3/25, and 4/20 rats, respectively, at the 3 dose
levels. At 5 mg/kg body weight, a reticulosarcoma of the spleen
with metastases and a malignant reticuloma were observed. At
15 mg/kg body weight, a sarcoma of the colon, a reticulosarcoma
of the spleen (with pancreatic metastases) and a hepatocellular
carcinoma occurred. At 30 mg/kg body weight, a liver fibro-
sarcoma, a malignant reticuloma and two reticulosarcomas of the
spleen were noted. Three, 7, 5, and 2 benign tumours were
noted, respectively, in the controls and at 5, 15, and 30 mg/kg
body weight (Gibel et al., 1973).
Rat: Groups of 40, ten-week-old Wistar rats were injected
im with 15 mg dimethoate/kg body weight (twice weekly) or with
isotonic saline until spontaneous death occurred. The mean life
spans were 711 and 570 days in the saline and dimethoate-treated
animals, respectively. No malignant tumours occurred in the
saline-treated group, but 4/35 animals developed benign tumours.
In the dimethoate group, 6/30 rats had malignant tumours (a
spleen reticulosarcoma, a spleen fibrosarcoma, an ovarian
alveolar sarcoma, a liver hepatocellular carcinoma, a malignant
reticuloma, and a soft tissue spindle-cell sarcoma) and 5/30 had
benign tumours. The first malignant tumour (a splenic
fibrosarcoma) was noted after 410 days (Gibel et al., 1973).
Dimethoate (technical grade, 90-100% pure) was given in the
feed to Osborne Mendel rats and to B6C3F1 mice. Groups of 50
male and 50 female rats (10 animals in each control group)
received "time-weighted average doses" of 155 or 310 mg/kg for
male rats and 192 or 384 mg/kg for female rats. After 80 weeks,
all groups received control diets, and the studies were con-
cluded at week 114.
Groups of 50 male and 50 female B6C3F1 mice were given
dimethoate in the diet at concentrations of 0 (10 "matched"
controls), 250, or 500 mg/kg for 60-69 weeks (males) or for 80
weeks (females). The studies were ended after 94 weeks.
Tremors and hyperexcitability were observed in exposed animals;
rats and mice that survived to termination were generally in
poor condition. Survival was reduced in the high-dose groups of
rats. Several non-neoplastic lesions occurred more frequently
in dimethoate-exposed animals. No increases in neoplasia were
reported to be associated with dimethoate administration, for
any of the organs or tissues examined histologically (NCI-1977).
These studies are not considered adequate to determine properly
the presence or absence of a carcinogenic response, largely
because of the shorter than usual duration of exposures, the
poor condition of the animals, and changes in exposure concen-
trations.a
8.8. Special Studies
The action of dimethoate on the immune system was studied
in mice and rats by Tiefenbach & Lange (1980). A single dose of
75 mg dimethoate/kg (route not specified) decreased the
lymphocyte count to 50% of the pre-exposure value and increased
the neutrophile granulocytes. After 72 h, these changes
returned to normal. A reduction in the thymus cortex with
disrupted lymphocytes, and a reduction in the number of rosette-
forming cells were observed.
When administered to rats at 5-30 mg/kg body weight orally
or 15 mg/kg im, twice a week, until death, dimethoate caused
hyperplasia in the bone marrow, mainly in granulocytopoiesis
(Stieglitz et al., 1974). The authors interpreted the changes
in the haematopoietic system of the rats as a direct haemato-
logical effect of dimethoate.
The effects of dimethoate on the heart were investigated
in rabbits (Mahkambaeva, 1971), and guinea-pigs and rats
(Nadmaiteni & Marosi, 1983). After oral administration of
150 mg dimethoate/kg to rabbits, the effects observed included
bradycardia and increased atrio-ventricular and intraventricu-
lar conductance, with complete recovery after 4-7 days. In rats
and guinea-pigs, a dose-effect relationship was established for
heart rate disturbances, and atrio-ventricular block. An
electron-microscopic study of the myocard did not reveal any
changes. The ip doses that were tested ranged from 500 to
1500 mg/kg body weight.
----------------------------------------------------------------
a After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious ECG disturbances
developed in the early phase of intoxication. The toxic cardiac
phenomena appeared to be unrelated to the degree of cholin-
esterase inhibition, but were correlated with the myocardial
dimethoate concentration. Cardiac failure and mortality were
first observed at a level of about 110 µg/g, while a level of
221 µg/g resulted in death in all cases. The present investi-
gation refers to the direct effect of dimethoate on the
myocardium, independent of its anticholinesterase action (Marosi
et al., 1985a,b).
8.9. Factors Modifying Toxicity
The acute toxicity of dimethoate was not affected by the
dietary content of protein (Boyd & Muis, 1970). The oral LD50
was 147 mg/kg for male albino rats fed for 28 days from weaning
on a diet containing 3.5% protein as casein and, in controls
maintained on a diet containing 26% casein at 152 mg/kg. Other
groups maintained on a diet that contained 24% protein from
various plant and animal sources were apparently less suscep-
tible to dimethoate (LD50, 358 mg/kg). Dimethoate was given to
rats orally for 10 weeks at a dose of 10 mg/kg in diets contain-
ing 90, 160, or 240 g protein/kg (Gontzea & Gorcea, 1977). The
high-protein (240 g) diet diminished the adverse effects of
dimethoate on the growth of the rats and lessened its antichol-
inesterase activity in plasma, total blood, brain, and liver.
A marked increase in the toxicity of dimethoate was noted
in male and female mice after pre-treatment with phenobarbital
and with chlorinated hydrocarbons (DDT and dieldrin) (Menzer &
Best, 1968; Menzer, 1970). The toxicity of dimethoate was
increased from an ip LD50 of 198 mg/kg body weight to 58.5 mg/kg
by pre-treatment of mice for 3 days with sodium phenobarbital.
Liquid formulations of technical dimethoate in the
solvents, 2-methoxy- and 2-ethoxyethanol, showed increased tox-
icity after storage. After storage for 7 months in England and
9 months under tropical conditions, the oral LD50 for the rat
decreased to 30-40 mg/kg and 15 mg/kg, respectively (from an
initial 150-250 mg/kg). The most toxic conversion product was
O,O-dialkyl S-(N-methylcarbamoylmethyl) phosphorothioate with
an oral LD50 for the rat of 1 mg/kg (Casida & Sanderson, 1961).
8.10 Mechanisms of Toxicity; Mode of Action
The mode of action of organophosphorus insecticides is
decribed in WHO (1986a). They act principally by inhibition of
acetyl cholinesterase (AChE) at the cholinergic synapses.
Dimethoxon (omethoate), a metabolite of dimethoate, is
75-100 times more potent than dimethoate in inhibiting AChE,
suggesting that this metabolite plays a dominant role in
mammalian toxicity (Hassan et al., 1969). The LD50 of omethoate
in rats is 25-28 mg/kg body weight (FAO/WHO, 1979); it is about
10 times more toxic than dimethoate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
Cases of both accidental and suicidal poisoning have been
reported with dimethoate. Hayes (1982) has reviewed the human
toxicity and poisoning cases.
Nagler et al. (1980) described a case of attempted suicide
of a 34-year-old female who ingested 10 g dimethoate. Half an
hour after admission to the hospital, the serum-dimethoate level
was 2340 mg/litre. Combined haemoperfusion and haemodialysis
were applied and, after 18 h, dimethoate was no longer detected
in the serum.
A severe case of poisoning after ingestion of approximately
20 g dimethoate was reported by Köppel et al. (1986). On
admission, the 52-year-old man was comatose with unmeasurable
pseudo-cholinesterase (< 200 U/litre). He had been admitted 2 h
after ingestion and received, every 20 min, an injection of
20 mg atropine. Two haemoperfusions with activated charcoal and
amberlite were performed, and atropine was given by infusion up
to day 12. Twenty-five days after admission, he was discharged,
fully recovered.
De Reuck et al. (1979) presented a case of a patient who
died on the 9th day after dimethoate poisoning with an atypical
central neurological disorder. The neuropathological findings,
which were similar to those observed in severe forms of
Wernicke's encephalopathy, included severe haemorrhagic necrosis
of the walls of the ventricles. The authors suggested that the
increased level of acetylcholine in the brain had led to
thiamine depletion in the regions of predilection of Wernicke's
encephalopathy.
Many other cases of human dimethoate poisoning, some of
which were fatal, have been reported, but the information given
was insufficient (Molphy & Rathus, 1964; Masiak & Olajossy,
1973; François & Verbraeken, 1977; Demeter & Heyndrickx, 1978;
Ebel & Karyofilis, 1978; Areekul et al., 1981; Wehran & Hammer,
1984; Bolgar et al., 1985; Le Blanc et al., 1986; Senanayake &
Karalliedde, 1987).
On the basis of a number of cases, the oral lethal dose for
human beings was estimated to be of the order of 50-500 mg/kg
body weight (Gosselin et al., 1984).
Trinh van Bao et al. (1974) reported an increase in the
frequency of breaks and stable chromosome aberrations in 2
patients who died after dimethoate intoxication.
Thirty firemen, exposed to dimethoate in the air as a
result of an accident in a dimethoate-manufacturing plant,
developed symptoms of intoxication (Larripa et al., 1979, 1983).
Peripheral blood lymphocytes from 20 of these workers were
examined for the frequency of sister chromatid exchanges, 2
months after the accident. The frequencies were 9.2 ± 0.2 for
the exposed and 8.5 ± 0.2 for unexposed persons ( P < 0.05).
Dicentric chromosomes and a low frequency of chromatid breaks
were found in 2 exposed workers. It is not certain to what the
firemen were exposed besides dimethoate.
9.1.2 Controlled human studies
The results of a number of studies in which human volun-
teers, without occupational exposure to organophosphates, were
given dimethoate, are summarized in Table 12. From these
studies it can be concluded that repeated doses of up to
0.2 mg/kg body weight did not inhibit cholinesterase activity in
the blood.
9.2 Occupational Exposure
9.2.1 Poisoning incidents
One of the first cases of poisoning with dimethoate (Rogor)
in agriculture was described by Muratore et al. (1960). A 28-
year-old-farmer, who reportedly had worn protective rubber
clothing and equipment, had sprayed olive trees with dimethoate
the day before he was hospitalized. The man began to experience
profound weakness, faintness, and somnolence, followed by
attempts to vomit, chills, and profound prostration on the day
he was hospitalized. His general condition was found to be
serious; his pulse was weak, he exhibited pronounced myosis,
vomited and sweated profusely, and had pronounced inhibition of
cholinesterase activity. Treated with large doses of atropine
(20 mg on day 2, 12 mg on day 3, and 5 mg until day 9),
prednisone, analgesics, and penicillin, he recovered.
A case of poisoning in a woman working in agriculture and
exposed to dimethoate in the field, 2 days after spraying, was
reported by Asatryan (1971). Within 3-3.5 h after beginning
work, the woman noted an unpleasant odour recognized as
dimethoate. She developed headache, dry cough, dyspnoea,
nausea, vomiting and was admitted to hospital in a somnolent
state with muscular fibrillations and an asthmatic component.
After treatment with saline solution, glucose, caffeine,
atropine, and insulin, the state of acute intoxication was
overcome. Allergic symptoms were treated with dimedrol.
Contact allergy due to dimethoate was reported in a 53-
year-old female. She had a positive skin test (Pambor & Bloch,
1985).
Table 12. Controlled human studies
---------------------------------------------------------------------------------------------------------
Number Sex Route of Dose/day Duration of Results Reference
of Admini- exposure
subjects stration
---------------------------------------------------------------------------------------------------------
20 oral 0.04 mg/kg 4 weeks absence of toxic effects Sanderson & Edson
or blood-ChE inhibition (1964)
2 oral 0.13 mg/kg; 21 days absence of blood-ChE Sanderson & Edson
0.26 mg/kg inhibition (1964)
5 M oral 0.25 mg/kg single dose absence of toxic effects Sanderson & Edson
or ChE inhibition (1964)
50 dermal 32% liquid form- 2-h patch absence of skin irritation Sanderson & Edson
ulation, up to test and ChE inhibition (1964)
2.5 ml
12 M/F oral 5 mg (0.068 28 days no significant change in Edson et al. (1967)
mg/kg body weight)
9 M/F oral 15 mg (0.202 39 days no significant change in Edson et al. (1967)
mg/kg body weight)
8 M/F oral 30 mg (0.434 57 days inhibition of ChE by the Edson et al. (1967)
mg/kg body weight)
6 oral 45 mg (0.587 45 days inhibition of ChE (35%) Edson et al. (1967)
mg/kg body weight)
6 M/F oral 60 mg (1.02 14 days inhibition of ChE (21%) Edson et al. (1967)
mg/kg body weight)
---------------------------------------------------------------------------------------------------------
Female greenhouse workers working with dimethoate were
reported to have a high percentage of specific leukocyte
agglomeration, a raised index of lymphoblastic transformations,
and antibodies against dimethoate (Zolotnikova & Somov, 1978;
Zolotnikova, 1980). With increasing duration of work, progress-
ive sensitization towards the pesticide was observed.
On the basis of epidemiological observations and of dermal
testing of workers with a 1-2% solution of dimethoate, Jung
(1979) concluded that the index of sensitization was very low
for dimethoate and that general intoxication might occur, but
rarely contact eczema.
9.3 Early Symptoms and Treatment of Poisoning
Initial symptoms of poisoning may include sweating,
headache, weakness, giddiness, nausea, vomiting, stomach pains,
blurred vision, slurred speech, and muscle twitching (WHO,
1986a). Arrhythmias and cardiac failure have been reported
(Kiss & Fazekas, 1983; Marosi et al., 1985a,b; Duval et al.,
1986). The most important diagnostic finding is inhibition of
blood-cholinesterase activity. For a full discussion of the
clinical picture and treatment of organophosphate insecticide
poisoning, see WHO (1986a). The section on treatment is
presented in the annex to this publication.
In all cases of clinical poisoning with dimethoate and
other organophosphorus insecticides, it is essential to maintain
general surveillance and cholinesterase and cardiac monitoring
for at least 4 days, and longer if necessary, and to adapt
general supportive and specific therapy in accordance with the
findings.
Data on the effects of oxime reactivators in dimethoate
poisoning are contradictory, some indicating that they may have
a negative effect on cholinesterase inhibition (Sanderson &
Edson, 1959; Durham & Hayes, 1962; Molphy & Rathus, 1964;
Erdmann et al., 1966; Zech et al., 1966; Ebel & Karyofilis,
1978; Sterri et al., 1979). It is therefore suggested, that if
oxime reactivators are indicated, these should be used with
caution and under close supervision.
Haemoperfusion may be effective in the early stages of
dimethoate poisoning (Okonek 1976, 1977; Okonek et al., 1976;
Pach et al., 1977; Nagler et al., 1980).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Toxicity of Dimethoate
Dimethoate is moderately toxic. Most oral LD50s in the rat
ranged from 150-400 mg/kg body weight. The dermal LD50 in the
rat was greater than 600 mg/kg body weight. Dimethoate is not
irritating to the skin, but may be slightly irritating to the
eye.
It can be absorbed following ingestion, inhalation, and
skin contact. It is readily metabolized and excreted and does
not accumulate in the body. Dimethoxon (omethoate), the oxygen
analogue found in plants, insects, and mammals, is about 10
times more toxic and is a more potent inhibitor of cholin-
esterase activity than dimethoate.
Signs of intoxication from exposure to dimethoate are those
typical of organophosphorus pesticides. Inhibition of erythro-
cyte-cholinesterase is the most sensitive indicator of exposure
to dimethoate and may indicate toxicity. A dietary level of
5 mg dimethoate/kg, equivalent to 0.25 mg/kg body weight, is
considered to be a no-observed-adverse-effect level in the
rat.a
A 5-generation reproduction study in mice given drinking-
water containing dimethoate at 60 mg/litre resulted in decreased
mating success and increased reproduction time in all gener-
ations. On the basis of available data on experimental animals,
dimethoate is not considered to be a teratogen.
Dimethoate is considered mutagenic in a variety of in vitro
and in vivo test systems.b Long-term studies have been
conducted involving oral administration in rats and mice and im
injection in rats. However, the available data are considered
---------------------------------------------------------------
a FAO/WHO (1987) concluded that a dietary level of 1 mg/kg,
equivalent to 0.05 mg/kg body weight is the no-observed-
adverse-effect level in the rat.
b After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
to be inadequate to assess the carcinogenic potential of
dimethoate.c
10.2. Human Exposure
Air and water are negligible sources of exposure to
dimethoate for the general population. Residues found in food
are generally below the acceptable daily intake (ADI) set by the
FAO/WHO Joint Meeting on Pesticide Residues (JMPR) (See section
12).
Whole blood-cholinesterase was not inhibited in human
volunteers given oral doses of 0.2 mg dimethoate/kg body weight
for 39 days.
Several cases of suicidal or accidental poisoning due to
ingestion of dimethoate have been reported.
Occupational exposure to dimethoate, principally through
inhalation and the skin, may occur during its manufacture,
formulation, and use, and cases of poisoning as a result of
accident or neglect of safety precautions have been reported.
The oral lethal dose for human beings has been estimated to be
in the range of 50-500 mg/kg body weight.
Skin sensitization due to dimethoate has been observed in
some cases.
10.3. Evaluation of the Effects on the Environment
Dimethoate is rapidly hydrolysed and does not persist in
the environment. The toxicity of dimethoate for aquatic
organisms and birds is moderate to high. It is very toxic for
honey-bees.
---------------------------------------------------------------
c After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
10.4. Conclusions
1. Under proper conditions of use, exposure of the general
population to dimethoate is negligible.
2. When appropriate safety precautions are observed, exposure
to dimethoate during manufacture, formulation, use, and
disposal should not pose an unacceptable human health
hazard.
3. Dimethoate is rapidly degraded and not persistent in the
environment. Care must be taken not to expose honey-bees,
fish and aquatic organisms, and birds.
11. RECOMMENDATIONS
1. Figures relating to the current production and use of
dimethoate should be made available.
2. Studies are necessary on dermal sensitization by
dimethoate.
3 Data are required on the absorption and disposition of
dimethoate from different routes of exposure.
4. Long-term toxicology and carcinogenicity studies should be
carried out on laboratory animals.a
5. More research is required to investigate the in vivo
mutagenic effects of dimethoate.a
6. Reproduction and teratology studies should be carried
out.a
7. Epidemiological studies on persons engaged in the
manufacture and professional use of dimethoate should be
considered, and workers should be monitored for exposure to
dimethoate and for potential adverse health effects.
8. Cleaning and disposal of contaminated equipment, clothing,
and containers should be in accordance with recommended
procedures.
9. Further work is necessary to establish safe re-entry
periods under different conditions of use.
10. Acute toxicity studies should be carried out on
formulations in which dimethoate is mixed with other active
ingredients.
11. The role of oxime reactivators in the treatment of human
poisoning by dimethoate, should be clarified.
12. Information should be obtained concerning the changes in
toxicity, due to impurities, that can arise in pesticides
as a consequence of different manufacturing processes, the
use of formulating ingredients, and improper storage.
---------------------------------------------------------------
a Several of these studies were later submitted to the
FAO/WHO Joint Meeting on Pesticide Residues in 1987. See
previous footnotes for sections 8.5, 8.6 and 8.7.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dimethoate was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1963, 1965, 1966, 1967, 1970, 1973
(evaluation of the related compound formothion), 1977, 1978,
1984, and 1987 (FAO/WHO, 1964, 1965, 1967, 1968, 1971, 1974,
1978, 1979, 1985, 1987).
The estimate of an acceptable daily intake (ADI) for man
for dimethoate is 0-0.01 mg/kg body weight, based on the no-
observed-adverse-effect level in man of 0.2 mg/kg body weight
per day, and in the rat of 1 mg/kg diet, equivalent to
0.05 mg/kg body weight (FAO/WHO, 1987).
A data sheet on dimethoate has been issued by WHO in the
series "Data Sheets on Pesticides" (WHO/FAO, 1980).
In the WHO Recommended Classification of Pesticides by
Hazard, technical dimethoate is classified as moderately
hazardous, when handled in accordance with instructions (WHO,
1986b).
The IRPTC (1982) has issued a review on dimethoate, in its
series "Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals".
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-
67. III. Organophosphorus pesticide residues in the total diet.
Pestic. Sci., 1: 10-13.
ABRAMOVA, J.I. & BOLTUSHKINA, L.A. (1968) [Safety action of
several vitamins in poisoning with organophosphorus insecti-
cides.] In: [Hygiene and toxicology of pesticides and the
clinical picture of intoxications,] Vol. 6, Kiev, Zdorovye
Publishers, pp. 448-452 (in Russian).
ADAMIS, Z., ANTAL, A., FUZESI, I., MOLNAR, J., NAGY, L., &
SUSAN, M. (1985) Occupational exposure to organophosphorus
insecticides and synthetic pyrethroids. Int. Arch. occup.
environ. Health, 56: 299-305.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AKORONKO, S.L. & GIRENKO, D.B. (1977) [Methods of estimating
fosfamid in soil using chromatographic methods.] In: [ Methods
of determination of micro-quantities of pesticides in foods,
feed, and the environment,] Moscow, Kolos, pp. 118-120 (in
Russian).
ANEES, M.A. (1975) Acute toxicity of four organophosphorus
insecticides for a fresh-water teleost Channa punctatus
(Bloch). Pak. J. Zool., 7(2): 135-141.
ANEES, M.A. (1978a) Hepatic pathology in a fresh-water teleost
Channa punctatus (Bloch) exposed to sub-lethal and chronic
levels of three organophosphorus insecticides. Bull. environ.
Contam. Toxicol., 19(5): 524-527.
ANEES, M.A. (1978b) Haematological abnormalities in a fresh-
water teleost Channa punctatus (Bloch) exposed to sublethal and
chronic levels of three organophosphorus insecticides. Int. J.
Ecol. environ. Sci., 4: 53-60.
AREEKUL, S., SRICHAIRAT, S., & KIRDUDOM, P. (1981) Serum and
red cell cholinesterase activity in people exposed to organo-
phosphorus insecticides. Southeast Asian J. trop. Med. pub.
Health, 12(2): 94-98.
ARMELI, G., BARTALINI, E., & PISANI, F. (1967) [Occupational
pathology and prevention in the industrial production of rogor].
Lav. Um., 20(8): 355-356 (in Italian).
ASATRYAN, R.S. (1971) [Some peculiarities of acute pesticide
poisonings in application in Ararat land.] In: [ Hygiene and
toxicology of pesticides and the clinical picture of intoxica-
tions,] Kiev, Zdorovye Publishers, pp. 315-318 (in Russian).
ATABAEV, SH.T. (1972) [Rogor (phosphamide).] In: [ Pesticides
and environmental hygiene in hot climate conditions,] Tashkent,
Medizina, pp. 77-93 (in Russian).
ATABAEV, SH.T. & STEPOVAYA, N.E. (1966) [Allowance of phospha-
mide in apples.] In: [ Hygiene and toxicology of pesticides and
the clinical picture of intoxications,] Kiev, Zdorovye Publish-
ers, pp. 280-285 (in Russian).
AWASTHI, M., SHAH, P., DUBALE, M.S., & GADHIA, P. (1984) Meta-
bolic changes induced by organophosphates in the piscine organs.
Environ. Res., 35(I): 320-325.
BARANOVA, N.P., ALEXANDROVA, L.G., & KOVALENKO, V.F. (1986)
[Tissue distribution and biochemical effects of dimethoate
entering the body via the skin.] Gig. tr. prof. Zabol., 3: 20-
23 (in Russian).
BARKER, R.J., LEHNER, Y., & KUNZMANN, M.R. (1980) Pesticides
and honey bees: nectar and pollen contamination in alfalfa
treated with dimethoate. Arch. environ. Toxicol., 9: 125-133.
BECK, E.W., JOHNSON, J.C., Jr, GETZ, M.E., SKINNER, F.B.,
DAWSEY, L.H., WOODHAM, D.W., & DERBYSHIRE, J.C. (1968) Effects
of feeding dimethoate, its oxygen analog, and dimethoate-treated
silage to cattle. J. econ. Entomol., 61(3): 605-610.
BECKER, H. (1985) Dominant lethal study with dimethoate
technical in the mouse. (Unpublished report no. 039003, dated 12
September 1985 from Research & Consulting Company AG, 4452
Itingen/Switzerland).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Contr., 9(3):
119-127.
BHUNYA, S.P. & BEHERA, J. (1975) Centromeric sensitivity of
mouse chromosomes to the systemic insecticide dimethoate
(Rogor). Curr. Sci., 44(23): 859-860.
BOHN, W.R. (1964) The disappearance of dimethoate from soil.
J. econ. Entomol., 57(6): 798-799.
BOLGAR, G., JOJART, G., & TURI, J. (1985) [Spontaneous delivery
of a pregnant woman after dimethoate (Bi 58 EC) poisoning.]
Orvosi Hetilap, 126(52): 3213-3214 (in Hungarian).
BOLOTNYI, A.V., PISMENNAYA, M.V., & AKORONKO, S.L. (1978)
[Transformation of phosphororganic pesticides antio and chloro-
phose in the environment.] Gig. i Sanit., 43(5): 28-31 (in
Russian).
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol-water
partitioning coefficient (Kow) of 61 organophosphorus and carba-
mate insecticides and their relationship to respective water
solubility (S) values. J. environ. Sci. Health, BI8(6): 667-
683.
BOYD, E.M. & MUIS, L.F. (1970) Acute oral toxicity of dimetho-
ate in albino rats fed a protein deficient diet. J. pharm.
Sci., 59: 1098-1102.
BRADY, U.E., Jr & ARTHUR, B.W. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. econ.
Entomol., 56(4): 477-482.
BUDREAU, C.H. & SINGH, R.P. (1973) Effect of fenthion and
dimethoate on reproduction in the mouse. Toxicol. appl. Pharma-
col., 26: 29-38.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1968) Organo-
phosphorus poisoning, Diagnosis of poisoning in pheasants owing
to a number of common pesticides, J. agric. food Chem., 16(2):
332-339.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1969) Organo-
phosphorus poisoning, Chronic feeding of some common pesticides
to pheasants and pigeons, J. agric. food Chem., 17(5): 1027-
1032.
CABRAS, P., MELONI, M., & PIRISI, F.M. (1979) Separation of
insecticides by reversed-phase high-performance liquid chroma-
tography. J. Chromatogr., 176: 473-477.
CARMAN, G.E., IWATA, Y., PAPPAS, J.L., O'NEAL, J.R., & GUNTHER,
F.A. (1982) Pesticide applicator exposure to insecticides
during treatment of citrus trees with oscillating boom and
airblast units. Arch. environ. Contam. Toxicol., 11: 651-659.
CASIDA, J.E. & SANDERSON, D.M. (1961) Toxic hazard from formu-
lating the insecticide dimethoate in methyl "Cellosolve".
Nature (Lond.), 189(4763): 507-508.
CHEN, H.H., HSUEH, J.L, SIRIANNI, S.R., & HUANG, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res., 88(3): 307-316.
CHILWELL, E.D. & BEECHAM, P.T. (1960) Residues of O,O-
dimethyl- S-(N-methylcarbamoylmethyl) phosphorothiolothionate
(dimethoate) in sprayed crops. J. Sci. Food Agric., 11: 400-
407.
CONGREGADO, F., SIMON-PUJOL, D., & JUAREZ, A. (1979) Effect of
two organophosphorus insecticides on the phosphate-dissolving
soil bacteria. Appl. environ. Microbiol., 37(1): 169-171.
COPPLESTONE, J.F., FAKHRI, Z.I., MILES, J.W., MITCHELL, C.A.,
OSMAN, Y., & WOLFE, H.R. (1976) Exposure to pesticides in
agriculture: a survey of spraymen using dimethoate in the Sudan.
Bull. World Health Organ., 54(2): 217-223
COURTNEY, K.D., ANDREWS, J.E., SPRINGER, J., & DALLEY, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. environ. Sci. Health, B20(4): 373-406.
DALELA, R.C., BHATNAGAR, M.C., TYAGI, A.K., & VERMA, S.R.
(1979) Histological damage of gills in Channa gachua after
acute and subacute exposure to endosulfan and Rogor. Mikroskopie
(Vienna), 35: 301-307.
DAUTERMANN, W.C., CASIDA, J.E., KNAAK, J.B., & KOWALCZYK, T.
(1959) Bovine metabolism of organophosphorus insecticides.
Metabolism and residues associated with oral administration of
dimethoate to rats and three lactating cows. J. agric. food
Chem., 7(3): 188-193.
DEDEK, W., GRAHL, R., & SCHMIDT, R. (1984) A comparative study
of guanine N7-alkylation in mice in vivo by the organophosphorus
insecticides trichlorphon, dimethoate, phosmet and bromophos.
Acta pharmacol. toxicol., 55: 104-109.
DEGRAEVE, N. & MOUTSCHEN, J. (1983) Genotoxicity of an organo-
phosphorus insecticide, dimethoate, in the mouse. Mutat. Res.,
119: 331-337.
DEGRAEVE, N., CHOLLET, M.-C., & MOUTSCHEN, J. (1984) Evaluation
of the mutagenic potential of four commercial mixtures of insec-
ticides. Food chem. Toxicol., 22(8): 683-687.
DEMETER, J. & HEYNDRICKX, A. (1978) Two lethal endosulfan
poisonings in man. J. anal. Toxicol., 2(2): 68-74.
DE PIETRI-TONELLI, P., BAZZI, B., & SANTI, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Rev., 11: 60-99.
DE REUCK, J., COLARDYN, F., & WILLEMS, J. (1979) Fatal
encephalopathy in acute poisoning with organophosphorus
insecticides. A clinicopathologic study of two cases. Clin.
Neurol. Neurosurg., 81(4): 247-254.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K.,
MUYS, T., & VAN DER POLL, J.M. (1984) Pesticides and other
chemical residues in Dutch total diet samples (June 1976 - July
1978). Food chem. Toxicol., 22(1): 11-21.
DIKSHITH, T.S.S. (1986) Pesticides. In: Toxicology of
pesticides in animals, Boca Raton, Florida, CRC Press, Vol. I,
Chapter 2.
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981a) Toxicity evaluation of
dimethoate technical in fish (Report to Shaw Wallace & Co.,
India).
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981b) Toxicity evaluation
of dimethoate technical in animals: Primary skin irritation and
irritation of mucous membrane in rabbits (Report to Shaw Wallace
& Co., India).
DUGGAN, R.E., CORNELIUSSEN, P.E., DUGGAN, M.B., MCMAHON, B.M., &
MARTIN, R.J. (1983) Pesticide residue levels in foods in the
United States from July 1, 1969 to June 30, 1976, Washington,
DC, US Food and Drug Administration/Association of Official
Analytical Chemists (published jointly), pp. 13-24.
DURHAM, W.F. & HAYES, W.J. (1962) Organic phosphorus poisoning
and its therapy. Arch. environ. Health, 5: 21-47.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the exposure
to pesticides. Bull. World Health Organ., 26: 75-91.
DUVAL, G., CORBIN, J.C., & MIMRAN, C. (1986) Etude
hémodynamique des intoxications aiguës graves par insecticides
organophosphorés (7 cas). Coeur et Toxiques: XXIVèmes Journées
du Groupement francais de CAP, Strasbourg, 24-25 septembre
1986.
DZWONKOWSKA, A. & HÜBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in
vivo. Arch. Toxicol., 58: 152-156.
EBEL, J. & KARYOFILIS, A. (1978) [Unusual course of poisoning
with dimethoate, an organic phosphorus compound.] Ther. Ggw.,
117(2): 226-236 (in German).
EDSON, E.F. & NOAKES, D.N. (1960) The comparative toxicity of
six organophosphorus insecticides in the rat. Toxicol. appl.
Pharmacol., 2: 523-539.
EDSON, E.F., JONES, K.H., & WATSON, W.A. (1967) Safety of
dimethoate insecticide. Br. med. J., 4(5578): 554-555.
EDWARDS, J.A., LEEMING, N.M. & CLARK, R. (1984) Effect of
dimethoate on pregnancy of the rat (Unpublished report No. DTF
3/84245 dated 13 April 1984 from Huntingdom Research Centre).
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977a) Fac-
tors affecting the fate of dimethoate in soils. Intern. J.
environ. Stud., 11: 113-124.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977b) Fac-
tors affecting the accumulation of dimethoate in soil. Int. J.
environ. Stud., 11: 187-196.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1978) Factors
influencing the degradation of dimethoate in soils and solu-
tions. Int. J. environ. Stud., 11: 253-260.
ERDMANN, W.D., ZECH, R., FRANKE, P., & BOSS, I. (1966) [The
question of the therapeutic efficacy of esterase reactivators in
dimethoate poisoning.] Arzneimittelforschung, 16: 492-494 (in
German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesti-
cides. In: Montesano, R., Tomatis, L., & Davies, W., ed.
Chemical carcinogenesis: essays, Lyons, International Agency
for Research on Cancer, pp. 161-181 (IARC Scientific
Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Dimethoate. In: Evaluation of toxicity of
pesticide residues in food, Rome, Food and Agricultural
Organization of the United Nations, pp. 97-101 (FAO Meeting
Report No. PL/1965/10/1. WHO/Food Add./27.65)
FAO/WHO (1967) Dimethoate. In: Evaluation of some pesticide
residues in food, Rome, Food and Agricultural Organization of
the United Nations, p. 232.
FAO/WHO (1968) Dimethoate. In: 1967 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 117-132 (FAO/PL: 1967/M/11/1.
WHO/Food Add./68.30).
FAO/WHO (1971) Dimethoate. In: 1970 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 239-244 (AGP: 1870/M/12/1. WHO/Food
Add./71.42).
FAO/WHO (1974) Formothion. In: 1973 Evaluations of some pesti-
cide residues in food, Geneva, World Health Organization, pp.
281-290 (WHO Pesticide Residues Series, No. 3).
FAO/WHO (1978) Dimethoate. In: 1977 Evaluations of some pesti-
cide residues in food, Rome, Food and Agriculture Organization
of the United Nations, pp. 155-156 (FAO Plant Production and
Protection Paper No. 10 Sup.).
FAO/WHO (1979) Formothion, dimethoate, omethoate. In: 1978
Evaluations of some pesticide residues in food, Geneva, World
Health Organization, p. 6.
FAO/WHO (1984) Maximum residue limits for pesticides, Codex
Alimentarius Commission, Rome, Food and Agricultural
Organization of the United Nations, Vol. 13.
FAO/WHO (1985) Pesticide residues in food. Report of the 1984
Joint Meeting on Pesticide Residues, Rome, Food and Agricultural
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 62).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FAO/WHO (1987) Dimethoate. In: Pesticide residues in food.
Report of the 1987 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Rome, Food and Agricultural Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 84).
FARM CHEMICALS HANDBOOK (1984) Willoughby, Ohio Meister
Publishing Company.
FETCHER, A. (1984) Suspected dimethoate toxicity in cattle.
Mod. vet. Pract., 65(4): 283-285.
FISCHER, G.W. & SCHEUFLER, H. (1981) [Effects of dimethoate
and O-desmethyldimethoate on dominant lethal test in mice.]
Wiss. Z. Univ. Halle, 30(6): 21-29 (in German).
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity
of organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Health Sci., B20(1): 73-95.
FRANCOIS, J. & VERBRAEKEN, H. (1977) Glaucome aigu après
intoxication par un ester organo-phosphatique Bull. Soc. Belge
Ophthalmol., 176: 19-22.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14(3): 515-534.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1978 - September
1979. J. Assoc. Off. Anal. Chem., 68(5): 842-861.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1978 - September 1979. J.
Assoc. Off. Anal. Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985c) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1979 - September
1980. J. Assoc. Off. Anal. Chem., 68(6): 1163-1183.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985d) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1979 - September 1980. J.
Assoc. Off. Anal. Chem., 68(6): 1184-1197.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1980 - March
1982. J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1980 - March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 146-161.
GETZIN, L.W. & ROSEFIELD, I. (1968) Organophosphorus insecti-
cide degradation by heat-labile substances in soil. J. agric.
food Chem., 16(4): 598-601.
GIBEL, W., LOHS, KH., WILDNER, G.P., ZIEBARTH, D., & STIEGLITZ,
R. (1973) [Experimental study on cancerogenic, haematotoxic,
and hepatotoxic activity of phosphor-organic pesticides.] Arch.
Geschwulstforsch., 41(4): 311-328 (in German).
GILOT - DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN -
DAHMEN, M. (1983) Mutagenicity of some organophosphorus com-
pounds at the ade6 locus of Schizosaccharomyces pombe. Mutat.
Res., 117: 139-148.
GIRENKO, D.B. & KLISENKO, M.A. (1977) [Methods of estimating
antio and fosfamid in fruits by gas-liquid chromatography.] In:
[ Methods of determination of microquantities of pesticides in
foods, feed, and the environment,] Moscow, Kolos, pp. 117-118
(in Russian).
GIRENKO, D.B., KLISENKO, M.A., & PISMENNAYA, M.B. (1978)
[Methods of estimating organophosphorus pesticides in water by
chromatography methods.] In: [ Methods of determination of
microquantities of pesticides in foods, feed, and the
environment,] Moscow, Kolos, pp. 57-62 (in Russian).
GONTZEA, I. & GORCEA, V. (1977) The influence of protein and
fat intake on the body's resistance to dimethoate. Med. Lav.,
68(1): 13-21.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E.
(1984) Clinical toxicology of commercial products, 5th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
GRIGORASHVILI, G.S. & DZHIBLADZE, V.E. (1965) [Hygienic
evaluation of some food products after treatment with
phosphamide.] In: [ Hygiene and toxicology of pesticides, and
clinical aspects of pesticide poisoning,] Kiev, Zdorovye
Publishers, pp. 161-164 (in Russian).
HADJIDEMETRIOU, D.G., IWATA, Y., & GUNTHER, F.A. (1985)
Analysis and dissipation of dislodgable residues of acephate,
dimethoate and formetanate hydrochloride on citrus foliage.
Pestic. Sci., 16: 302-310.
HANNA, P.Y. & DYER, K.F. (1975) Mutagenicity of organo-
phosphorus compounds in bacteria and Drosophila. Mutat. Res.,
28: 405-420.
HASSAN, A., ZAYED, S.M.A.D., & BAHIG, M.R.E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimetho-
ate in the rat. Biochem. Pharmacol., 18: 2429-2438.
HAUCK, H.E. & AMADORI, E. (1980) Recent developments in high-
performance thin-layer chromatography and application to pesti-
cide analysis, New York, American Chemical Society, pp. 159-176
(ACS Symposium Series 136; CH09).
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, pp. 362-365.
HELLWIG, J. (1968a) Report on the study of the toxicity of
dimethoate in mice after 78-week administration in the diet,
(Unpublished report no. 75CO326/8242 from BASF, Department of
toxicology, Ludwigshafen/Rhein, Federal Republic of Germany).
HELLWIG, J. (1968b) Report on the study of the toxicity of
dimethoate in rats after 24 month administration in the diet,
(Project no. 70C0326/8241 dated 9 October 1986 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, Federal Republic
of Germany).
HEWITT, R., BREBBIA, A., & WALETZKY, E. (1958a) Carbamoyl
alkyl phosphorodithioates as chemotherapeutic agents: screening
by aedicidal properties in laboratory mammals, lambs and calves.
J. econ. Entomol., 51(2): 126-131.
HEWITT, R., EMRO, J., ENTWISTLE, J., PANKAVICH, J., THORSON, R.,
WALLACE, W., & WALETZKY, E. (1958b) Carbamoyl alkyl phosphoro-
dithioates as chemotherapeutic agents: effects of dimethoate
against grubs in cattle. J. econ. Entomol., 51(4): 445-450.
HILL, R.H. & ARNOLD, J.E. (1979) A personal air sampler for
pesticides. Arch. environ. Contam. Toxicol., 8: 621-628.
HOEGBERG, E.I. & CASSADAY, J.T. (1951) The reaction of O,O-
dialkyl thiophosphoric acid salts with some alpha-haloacyl deriva-
tives. J. Am. Chem. Soc., 73: 557-559.
IRPTC (1982) Scientific reviews of Soviet literature on toxic-
ity and hazards of chemicals: dimethoate, Moscow, Centre of
International Projects, GKNT, 12 pp (No. 5).
IRPTC (1987) Legal file Vol. 2, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
IWATA, Y., DUSCH, M.E., CARMAN, G.E., & GUNTHER, F.A. (1979)
Worker environment research: residues from carbaryl, chloroben-
zilate, dimethoate, and trichlorfon applied to citrus trees. J.
agric. food Chem., 27(6): 1141-1145.
IZMIROVA, N., BENCHEV, I., VELICHKOVA, V., KALOYANOVA, F.,
LAMBREVA, E., RUSEVA, P., KOSTOVA, S., ANGELOVA, R., INKJOVA,
M., BOGINOVA, L., MAKSIMOVA, F., & TZVETKOVA, P. (1973)
[Determination of minimal safety periods after treatment of
tobacco and cotton with organophosphorus pesticides.] In:
[ Hygiene, use and toxicology of pesticides, and clinical
aspects of pesticide poisoning,] Vol. 10, Moscow, Medizina
Publishers, pp. 120-124 (in Russian).
JACKSON, J.B., DRUMMOND, R.O., BUCK, W.B., & HUNT, L.M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. econ.
Entomol., 53(4): 602-604.
JAYCOX, E. (1964) Effect on Honey bees of nectar from systemic
insecticide-treated plants. J. econ. Entomol., 57(1): 31-35.
JOHNSON, E., CATERSON, C.R., & ALLEN, J.S. (1985) Mutagenicity
testing of dimethoate (AC 12880) in the in vitro CHO/HGPRT
mutation assay. (Unpublished report project no. 0423 dated 30
January 1985 from American Cyanamid Company, Princeton, New
Jersey).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. II. August 1975 - July
1976. Pestic. monit. J., 15(1): 39-50.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Pesti-
cide, metal, and other chemical residues in adult total diet
samples. XII. August 1975 - July 1976. Pestic. monit. J., 15(1):
54-59.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. III. August 1976 - Sep-
tember 1977. J. Assoc. Off. Anal. Chem., 67(1): 145-154.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984b) Pesticide, metal, and other chemical residues in adult
total diet samples. XIII. August 1976 - September 1977. J.
Assoc. Off. Anal. Chem., 67(1): 154-166.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity
of chemicals to fish and aquatic invertebrates, Washington, DC,
USA, US Department. of the Interior, Fish and Wildlife Service
(Resource Publication 137).
JUNG, H.D. (1979) [Occupational dermatoses due to pesticides.]
Dtsch. Gesundheitswes., 34(24): 1144-1147 (in German).
KAGAN, YU.S. (1981) [ General toxicology of pesticides,]
Kiev, Zdorovye Publishers, 176 pp (in Russian).
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1967)
Establishing hygienic norms of Bi 58 (Rogor, Phosphamide) in the
atmospheric air. In: Works of the Scientific Research Institute
of Labour Protection and Occupational Diseases, Vol. 15, pp. 1-
8, Sofia, Bulgaria, Medicina i Fizkultura.
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1968)
[Experimental substantiation of the maximum permissible concen-
tration of Bi 58 (Rogor, Phosphamide) in the atmosphere.] Gig.
i Sanit., 6: 6-10 (in Russian).
KALOYANOVA, F., DOBREVA, V., & MITOVA, S.V. (1984) [Effet du
dimethoate sur certaines activités du système de monoxygénase
hépatique] In: Hommage au Professeur René Truhaut, Paris,
Queray S.A., pp. 552-555.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J., ed.
IUPAC pesticide chemistry - Human welfare and the environment,
Oxford, New York, Pergamon Press, pp. 237-238.
KAPLANIS, J.N., ROBBINS, W.E., DARROW, D.I., HOPKINS, D.E.,
MONROE, R.E., & TREIBER, G. (1959) The metabolism of dimetho-
ate in cattle. J. econ. Entomol., 52(6): 1190-1194.
KARIM, A.A., HARIDI, A.A., & EL RAYAH, E.A. (1985) The
environmental impact of four insecticides on non-target
organisms in the Gezira Irrigation Scheme canals of Sudan. J.
trop. Med. Hyg., 88(2): 161-168.
KAWAR, N.S., IWATA, Y., DUSCH, M.E., & GUNTHER, F.A. (1979)
Behavior of dialifor, dimethoate, and methidothion in artifi-
cially fortified grape juice processed into wine. J. environ.
Sci. Health, B14(5): 505-513.
KHAN, R.R. & BHASKER DEV (1982) Toxicology data sheets on
chemicals: dimethoate, Lucknow, India, Lucknow Industrial
Toxicology Research Centre (No. 6).
KHERA, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for
teratogenic activity in the cat. J. environ. Pathol. Toxicol.,
2: 1283-1288.
KHERA, K.S., WHALEN, C., TRIVETT, G., & ANGERS (1979) Terato-
genicity studies on pesticidal formulations of dimethoate,
diuron, and lindane in rats. Bull. environ. Contam. Toxicol.,
22: 522-529.
KISS, Z. & FAZEKAS, T. (1983) Organophosphates and torsade de
pointes ventricular tachycardia. J. R. Soc. Med., 76: 984-985.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides. Techniques for establishing safe levels of foliar
residues. Residue Rev., 75: 81-96.
KNAAK, J.B., SCHLOCKER, P., ACKERMAN, C.R., & SEIBER, J.N.
(1980) Reentry research: Establishment of safe pesticide levels
on foliage. Bull. environ. Contam. Toxicol., 24: 796-804.
KÖPPEL, C., FORYCKI, Z., & IBE, K. (1986) Hemoperfusion in
severe dimethoate poisoning. Intensive Care Med., 12(2): 110-
112.
KRUEGER, H.R., O'BRIEN, R.D., & DAUTERMAN, W.C. (1960) Rela-
tionship between metabolism and differential toxicity in insects
and mice of dimethoate, parathion, and acethion. J. econ.
Entomol., 53(1): 25-31.
KUNDIEV, YU.I. (1979) [ Absorption of pesticides through the
skin and prophylaxis of poisonings,] Kiev, Zdorovye Publishers,
200 pp (in Russian).
LARRIPA, I., MATOS, E., & DE SALUM, S.B. (1979) [Cytogenetic
study of human population accidentally exposed to an organophos-
phate pesticide.] Medicina (Buenos Aires), 39(6): 773-774 (in
Spanish).
LARRIPA, I., MATOS, E., DE VINUESA, M.L., & DE SALUM, S.B.
(1983) Sister chromatid exchanges in a human population
accidentally exposed to an organophosphorus pesticide. Rev.
Braz. Genet., 4: 719-727.
LEBLANC, F.N., BENSON, B.E., & GILG, A.D. (1986) A severe
organophosphate poisoning requiring the use of an atropine
drip. Clin. Toxicol., 24(1): 69-76.
LEE, Y.W. & WESTCOTT, N.D. (1981) Gas chromatographic quanti-
tative analysis and persistence of dimethoate and dimethoxon
residues on and in wheat plants. J. agric. food Chem., 29(4):
860-862.
LORD, K.A., MAY, M.A., & STEVENSON, J.H. (1968) The secretion
of the systemic insecticides dimethoate and phorate into nectar.
Ann. appl. Biol., 61: 19-22.
LUCIER, G.W. & MENZER, R.E. (1970) Nature of oxidative metabo-
lites of dimethoate formed in rats, liver microsomes, and bean
plants. J. agric. food Chem., 18(4): 698-704.
MADZHIDOV, U.A. (1970) [Atmospheric pollution with fosfamid in
agricultural application.] Med. J. Uzb., 9: 40-42 (in Russian).
MAHKAMBAEVA, Z.G. (1971) [Influence of organophosphorus pesti-
cides (kilval, methylmercaptophos, fosfamid) on bioelectric
activity of heart in experimental animals.] In: [ Hygiene, use
and toxicology of pesticides, and clinical aspects of pesticide
poisoning,] Kiev, Zdorovye Publishers pp. 175-180 (in Russian).
MAROSI, G., IVAN, J., NAGYMAJTENYI, L., CSATLOS, J., & TOSZEGI,
A. (1985a) Dimethoate-induced toxic cardiac failure in the
guinea-pig. Arch. Toxicol., 57: 142-143.
MAROSI, G., IVAN, J., & NAGYMAJTENYI, L. (1985b) Cardiodepres-
sion in organophosphate poisonings. Arch. Toxicol., Suppl. 8:
289-291.
MASIAK, M. & OLAJOSSY, M. (1973) [Depressive reaction
following intoxication with an organic phosphorus preparation,
Rogor (Bi 58).] Wiadom. Lek., 26(20): 1933-1936 (in Polish).
MELNIKOV, N.N. (1974) [ Chemistry and technology of pesti-
cides,] Moscow, Khimiya Publishers, 768 pp (in Russian).
MELNIKOV, N.N., VOLKOV, A.I., & KOROTKOVA, O.A. (1977)
[ Pesticides and the environment,] Moscow, Khimiya Publishers,
pp. 240 (in Russian).
MENZER, R.E. (1970) Effect of chlorinated hydrocarbons in the
diet on the toxicity of several organophosphorus insecticides.
Toxicol. appl. Pharmacol., 16: 446-452.
MENZER, R.E. & BEST, N.H. (1968) Effect of phenobarbital on
the toxicity of several organophosphorus insecticides. Toxicol.
appl. Pharmacol., 13: 37-42.
MENZIE, C.M. (1969) Metabolism of pesticides, Washington, DC,
US Department of the Interior, Fish and Wildlife Service, pp.
160-166.
MENZIE, C.M. (1974) Metabolism of pesticides: an update,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 166-167, 485.
MENZIE, C.M. (1978) Metabolism of pesticides: update II,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 118.
MENZIE, C.M. (1980) Metabolism of pesticides: update III,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 242.
MIKHAILOV, S.S. & SHTERBAC, I.G. (1983) [Metabolism of organo-
phosphorus compounds containing a carboxyamin bond.] In:
[ Metabolism of organophosphorus poisons,] Moscow, Medizina,
pp. 96-101 (in Russian).
MITOVA, S.V., VASILEVA, L., DOBREVA, V., & KALOYANOVA, F. (1986)
Experimental studies on dimethoate oxidative desulfuration.
Toxic interfaces of neurones, smoke and genes. Arch. Toxicol.,
Suppl.9: 377.
MOHN, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides.
Mutat. Res., 20: 7-15.
MOLPHY, R. & RATHUS, E.M. (1964) Organic phosphorus poisoning
and therapy. Med. J. Aust., 29: 337-340.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides
in bacterial reversion assay systems. Mutat. Res., 116: 185-
216.
MULLER, P. & REINHOLD, R. (1973) [Experience with dimethoate
drugs in stable fly control.] Angew. Parasitol., 14(3): 158-169
(in German).
MUNCY, R.J. & OLIVER, A.D. (1963) Toxicity of ten insecticides
to the red crawfish, Procambarus clarki (Girard). Trans. Am.
Fish. Soc., 92: 428-431.
MURATORE, F., FAIOLO, A., & PONZETTA, G. (1960) [Four cases of
intoxication with organophosphorus esters with regard to liver
function.] Minerva Med., 51(80): 3342-3345 (in Italian).
NADMAITENI, L. & MAROSI, G. (1983) [Cardiotoxic action of
organophosphorus pesticides.] G ig. i Sanit., 11: 72-73 (in
Russian).
NAGLER, J., BRAECKMAN, R.A., WILLEMS, J.L., & DE BROE, M.E.
(1980) Combined hemoperfusion hemodialysis in organic phosphate
poisoning. Kidney Int., 18: 139 (Abstract).
NANDA KUMAR, N.V. & UDAYA BHASKAR, S. (1980) Colorimetric
determination of dimethoate by quantification of cholinesterase
inhibition. J. food Sci. Technol., 17: 153-155.
NCI (1977) Bioassay of dimethoate for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (Technical Report
Series No. 4).
NEHES, M., SELYPES, A., & SCHEUFLER, H. (1982) [Mutagenic
effect of trichlorfon and dimethoate on bone marrow cells of
mouse in vivo.] Wiss. Z. Univ. Halle, 31M(2): 57-62 (in
German).
NEHEZ, M., SELYPES, A., SCHEUFLER, H., & FISCHER, G.W. (1983)
Effect of dimethoate and O- demethyldimethoate on bone marrow
cells of CFLP mice. Regul. Toxicol. Pharmacol., 317(3): 349-
354.
NOAKES, D.N. & SANDERSON, D.M. (1969) A method for determining
the dermal toxicity of pesticides. Br. J. ind. Med., 26: 59-64.
NORTH, H.H. & MENZER, R.E. (1972) Biotransformation of
dimethoate by cell culture systems. Pestic. Biochem. Physiol.,
2: 278-285.
OKONEK, S. (1976) Probable progress in the therapy of
organophosphate poisoning: Extracorporeal hemodialysis and
hemoperfusion. Arch. Toxicol., 35(3): 221-227.
OKONEK, S. (1977) [Hemoperfusion with coated activated
charcoal for treating acute poisoning by remedies, plant
protectants, and fungi.] Med. Klin., 72(19): 862-866 (in
German).
OKONEK, S., HOLLMANN, H., & BOELCKE, G. (1976) [The
alkylphosphate poisoning: elimination of the poison by the
hemodialysis or blood exchange transfusion?]. Med. Welt, 27(8):
351-356 (in German).
PACH, J., GROSZEK, B., & BOGUSZ, M. (1977) Haemoperfusion in
the treatment of acute organophosphate poisoning. Acta
pharmacol. Toxicol., Vol 41, Suppl. II): 44.
PAMBOR, M. & BLOCH, Y. (1985) [Dimethoate and dithiocarbamate
as occupational contact allergens in a female agrotechnician.]
Dermatol. Monatsschr., 171(6): 401-405 (in German).
PANDEY, A.K. & TOMAR, V. (1985) Melanophores in Bufo melanos-
tictus (Schneider) tadpoles following exposure to the insecti-
cide dimethoate. Bull. environ. Contam. Toxicol., 35: 796-801.
PANSHINA, T.N. (1963a) [Experimental data on toxicology of new
organophosphorus insecticide phosphamide (Rogor, dimethoate).]
Pharmacol. Toksicol., 4: 476-484 (in Russian).
PANSHINA, T.N. (1963b) [Hygienic background for determining
the maximum permissible concentration of phosphamide in the air
of a working area.] Gig. i Sanit., 3: 21-28 (in Russian).
PANSHINA, T.N. (1966) [Experimental data on therapy of phos-
phamide intoxications.] In: [ Hygiene and toxicology of
pesticides, and clinical aspects of pesticide poisoning,] Kiev,
Zdorovye Publishers, Vol. 4, pp. 137-142 (in Russian).
PANSHINA, T.N. & KLISENKO, M.A. (1962) [Content of fosfamid
and changes in blood cholinesterase activity in different routes
of treatment of the mammalian organism.] In: [ Industrial
toxicology and clinical picture of occupational diseases due to
chemicals,] Moscow, Medgiz, pp. 181-184 (in Russian).
PETUKHOV, R.D. (1975) [Methodics of estimating of antio and
fosfamid in honey by thin-layer chromatography.] In: [ Methods
of determination of microquantities of pesticides in food, feed,
and the environment,] Moscow, Kolos, pp. 63-64 (in Russian).
PODREBARAC, D.S. (1984a) Pesticide, heavy metal residues, and
other chemical residues in infant and tod dler total diet sam-
ples. IV. October 1977 - September 1978. J. Assoc. Off. Anal.
Chem., 67(1): 166-175.
PODREBARAC, D.S. (1984b) Pesticide, metal, and other chemical
residues in adult total diet samples. XIV. October 1977 - Sep-
tember 1978. J. Assoc. Off. Anal. Chem., 67(1): 176-185.
PROBST, G.H., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEAL, S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures: A comparison with
bacterial mutagenicity using 218 compounds. Environ. Mutagen.,
3: 11-32.
RIEMER, F., DAHLENBURG, R., & GRISK, A. (1985) [Renal excretion
of dimethylphosphate and its thio derivatives after application
of dimethoate, bromophos, naled or trichlorphon to rats.]
Nahrung, 29(3): 299-302 (in German).
ROALES, R.R. & PERLMUTTER, A. (1974) Toxicity of zinc and
Cygon, applied singly and jointly, to zebrafish embryos. Bull.
environ. Contam. Toxicol., 12(4): 475-480.
ROSVAGA, R.I. (1983) [Methodology of investigation of pesti-
cide pyrolysis in their chemical-thermal harmlessness.] In:
[ Hygiene of application, toxicology of pesticides and polymeric
materials,] Kiev, Zdorovja, Vol. 13, pp. 73-77 (in Russian).
SAHU, D. & PATTANAIK, M. (1980) Persistence of rogor in some
Orissa soils. Indian J. agric. Sci., 50(6): 505-507.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDERSON, D.M. & EDSON, E.F. (1959) Oxime therapy in poison-
ing by six organophosphorus insecticides in the rat. J. Pharm.
Pharmacol., 11: 721-728.
SANDERSON, D.M. & EDSON, E.F. (1964) Toxicological properties
of the organophosphorus insecticide dimethoate. Br. J. ind.
Med., 21: 52-64.
SANTI, R. & GIACOMELLI, R. (1962) Metabolic fate of P32-
labelled dimethoate in olive fruits and some toxicological
implications. J. agric. food Chem., 10(3): 257-261.
SAN SEBASTIAN, J.R. (1985) In vivo bone marrow cytogenetics
rat metaphase analysis. PH 315-AC-001-84, Dimethoate CL 12880.
(Unpublished report dated 29 August 1985 from Pharmakon
Research International, Inc. Waverly, Pennsylvania 18471).
SCHEUFLER, H. (1975) [Effects of relatively high doses of
dimethoate and trichlorfon on the embryogenesis of laboratory
mice.] Biol. Rundsch., 13: 238-240 (in German).
SENANAYAKE, N. & KARALLIEDE, W. (1987) Neurotoxic effects of
organophosphorus insecticides. An intermediate syndrome. New
Engl. J. Med., 316(13): 761-763.
SHAMSHURIN, A.A. & KRIMER, M.Z. (1976) [ Physicochemical proper-
ties of pesticides: reference book,] Moscow, Khimya Publishers,
pp. 76-77 (in Russian).
SIDIMANOV, B.B. (1971) [Determination of fosfamid in post-
mortem material by thin-layer chromatography.] In: [ Hygiene of
application, toxicology of pesticides, and clinical picture of
poisoning,] Kiev, Zdorovja, Vol. 9, pp. 187-188 (in Russian).
SLOOFF, W. & CANTON, J.H. (1983) Comparison of the suscepti-
bility of 11 freshwater species to 8 chemical compounds. II.
(Semi)chronic toxicity test. Aquat. Toxicol., 4: 271-282.
SORG, R.M. (1985) Micronucleus test (MNT) PH 309A-AC-004-84,
Dimethoate CL 12880, (Unpublished report dated 1 March 1985
from Pharmakon Research International, Inc. Waverly,
Pennsylvania 18471).
STERRI, S.H., ROGNERUD, B., FISKUM, S.E., & LYNGAAS, S. (1979)
Effect of toxogonin and P2S on the toxicity of carbamates and
organphosphorus compounds. Acta pharmacol. toxicol., 45: 9-15.
STEVENSON, J.H. (1968) Laboratory studies on the acute contact
and oral toxicities of insecticides to honey bees. Ann. appl.
Biol., 61: 467-472.
STIEGLITZ, R., GIBEL, W., WERNER, W., & STOBBE, H. (1974)
Experimental study on haematotoxic and leukaemogenic effects of
trichlorfon and dimethoate. Acta haematol., 52: 70-76.
STROY, A.N. (1983) [Hygienic reglementation of fosfamid in
greenhouse conditions.] In: [Hygiene of application, toxicology
of pesticides and polymeric materials,] Kiev, Zdorovja, Vol.
13, pp. 90-91 (in Russian).
SZETO, S.Y., VERNON, R.S., & BROWN, M.J. (1985) Degradation of
dimethoate and pirimicarb in asparagus. J. agric. food Chem.,
33: 763-767.
TIEFENBACH, B. & LANGE, P. (1980) Studies on the action of
dimethoate on the immune system. Arch. Toxicol., Suppl. 4: 167-
170.
TRINH VAN BAO, SZABO, I., RUZICSKA, P., & CZEIZEL, A. (1974)
Chromosome aberrations in patients suffering acute organic
phosphate insecticide intoxication. Humangenetik, 24: 33-57.
UBAIDULLAEV, R.U. & MADZHIDOV, U.A. (1976) [Hygienic charac-
teristics of phosphamide as an atmospheric pollutant.] Gig. i
Sanit., 9: 7-9 (in Russian).
UCHIDA, T., DAUTERMANN, W.C., & O'BRIEN, R.D. (1964) The
metabolism of dimethoate by vertebrate tissues. J. agric. food
Chem., 12(1): 48-52.
UDAYA BHASKAR, S. & NANDA KUMAR, N.V. (1980) Colorimetric
determination of dimethoate or omethoate. Talanta, 27(9): 757-
758.
USHA RANI, M.V., REDDI, O.S., & REDDY, P.P. (1980) Mutageni-
city studies involving aldrin, endosulfan, dimethoate, phospham-
idon, carbaryl, and ceresan. Bull. environ. Contam. Toxicol.,
25(2): 277-282.
VAN MIDDELEM, C.H. & WAITES, R.E. (1964) Gas chromatographic
and colorimetric measurement of dimethoate residues. J. agric.
food Chem., 12(2): 178-182.
VELAZQUEZ, A., XAMENA, N., CREUS, A., & MARCOS, R. (1986)
Indication for weak mutagenicity of the organophosphorus
insecticide dimethoate in Drosophila melanogaster. Mutat. Res.,
172: 237-243.
VERMA, S.R., BHATNAGAR, M.C., & DALELA, R.C. (1978) Biocides
in relation to water pollution. II. Bioassay studies of a few
biocides to a fresh-water fish Channa gachua. Acta hydrochim.
hydrobiol., 6(2): 137-144.
VERMA, S.R., TYAGI, A.K., BHATNAGAR, M.C., & DALELA, R.C.
(1979) Organophosphate poisoning to some fresh-water teleosts -
acetylcholinesterase inhibition. Bull. environ. Contam. Toxi-
col., 21: 502-506.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982) Bioassay
trials with twenty-three pesticides to a fresh-water teleost
Saccobranchus fossilis. Water Res., 16: 525-529.
VISHWANATH, R. & JAMIL, K. (1986) Mutagenic and genotoxic
activities of certain organophosphorus compounds using Ames
Salmonella assay with and without microsomal induction. Ind.
J. exp. Biol., 24: 305- 308.
WALLER, G.D. & BARKER, R.J. (1979) Effects of dimethoate on
honey bee colonies. J. econ. Entomol., 72(4): 549-551.
WALLER, G.D., BARKER, R.J., & MARTIN, J.H. (1979) Effects of
dimethoate on honey bee foraging. Chemosphere, 8(7): 461-463.
WEHRAN, H.-J. & HAMMER, H.-J. (1984) [Lethal poisoning with
the pesticide dimethoate (Bi 58 EC).] Kriminal. forens. Wiss.,
54: 181-183 (in German).
WEST, B., VIDONE, L.B., & SHAFFER, C.B. (1961) Acute and
subacute toxicity of dimethoate. Toxicol. appl. Pharmacol., 3:
210-223.
WHO (1982) Field surveys of exposure to pesticides - Standard
Protocol, Geneva, World Health Organization (Unpublished
document VBC/82.1).
WHO (1984) Chemical methods for the control of arthropod
vectors and pests of public health importance, Geneva, World
Health Organization, pp. 34, 37.
WHO (1986a) Environmental health criteria 63: Organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WHO (1986b) The WHO recommended classification of pesticides
by hazard and guidelines to classification 1986-1987 World
Health Organization, Geneva, (Unpublished document VBC/86.1).
WHO (1986c) Interim specifications for dimethoate technical
and emulsifiable concentrate, Geneva, World Health Organization
(Unpublished report, WHO/IS/86.12).
WHO/FAO (1980) Dimethoate: data sheet on pesticides, Geneva,
World Health Organization (Unpublished document VBC/DS/80.42).
WINKLER, R. & MULLER, R. (1979) [Elimination and inactivation
of waters containing pesticides from agrochemical plants.]
Wasserwirtsch. Wassertech., 29(11): 369-371 (in German).
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-
induced complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ.
Mutagen., 5: 835-846.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th
ed., Lavenham United Kingdom, British Crop Protection Council.
ZECH, R., ENGELHARD, H., & ERDMANN, W.-D. (1966) Reactions of
pyridinium oximes with the alkyl phosphate dimethoate and its
derivatives. Effects on cholinesterases. Biochim. biophys.
Acta, 128: 363-371.
ZOLOTNIKOVA, G.P. (1980) [Disturbance of the immunoreactivity
of the organism under the effect of pesticides in hothouses.]
Gig. tr. prof. Zabol., 3: 38-40 (in Russian).
ZOLOTNIKOVA, G.P. & SOMOV, B.A. (1978) [On the role of pesti-
cides in the occurrence of occupational dermatitis in workers of
hothouses.] Vestnik Dermatol. Venerol., 4: 76-79 (in Russian).
ZOLOTNIKOVA, G.P. & ZOTOV, V.M. (1978) [Prognosis for resi-
dues of pesticides in the air of hothouses.] Gig. i Sanit.,
43(1): 108-109 (in Russian).
ANNEX I. TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus Insecticides - A General Introduction)
All cases of organophosphorus poisoning should be dealt
with as an emergency and the patient sent to hospital as quickly
as possible. Although symptoms may develop rapidly, delay in
onset or a steady increase in severity may be seen up to 48 h
after ingestion of some formulated organophosphorus insecti-
cides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major
references (Kagan, 1977; Taylor, 1980; UK DHSS, 1983; Plestina,
1984) and will also be included in the IPCS Health and Safety
Guides to be prepared for selected organophosphorus insecti-
cides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures
include removal of contaminated clothes and washing of the skin
with alkaline soap or with a sodium bicarbonate solution.
Particular care should be taken in cleaning the skin area where
venupuncture is performed. Blood might be contaminated with
direct-acting organophosphorus esters, and, therefore,
inaccurate measurements of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced,
if the patient is conscious, by the administration of
ipecacuanha syrup (10-30 ml) followed by 200 ml water. This
treatment is, however, contraindicated in the case of pesticides
dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also
be performed, particularly in unconscious patients, taking care
to prevent aspiration of fluids into the lungs (i.e., only after
a tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the
pesticide involved is available, it should also be stored for
further analysis (i.e., detection of toxicologically relevant
impurities). A purge to remove the ingested compound can be
administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be
started at the first sign of respiratory failure and maintained
for as long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given
at 15 to 30-min intervals. The dose and the frequency of
atropine treatment varies from case to case, but should maintain
the patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be
necessary in extreme cases and total daily doses up to several
hundred mg may be necessary during the first few days of
treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophos-
phates. This is not the case with enzymes inhibited by
carbamates. The treatment should begin as soon as possible,
because oximes are not effective on "aged" phosphorylated ChEs.
However, if absorption, distribution, and metabolism are thought
to be delayed for any reasons, oximes can be administered for
several days after intoxication. Effective treatment with
oximes reduces the required dose of atropine. Pralidoxime is
the most widely available oxime. A dose of 1 g pralidoxime can
be given either im or iv and repeated 2-3 times per day or, in
extreme cases, more often. If possible, blood samples should be
taken for AChE determinations before and during treatment. Skin
should be carefully cleansed before sampling. Results of the
assays should influence the decision whether to continue oxime
therapy after the first 2 days.
There are indications that oxime therapy may possibly have
beneficial effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to
counteract some aspects of CNS-derived symptoms, which are not
affected by atropine. Doses of 10 mg sc or iv are appropriate
and may be repeated as required (Vale & Scott, 1974). Other
centrally acting drugs and drugs that may depress respiration
are not recommended in the absence of artificial respiration
procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinization. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and
may be taken into, and then released from, fat depots over a
period of many days. It is therefore quite incorrect to abandon
oxime treatment after 1-2 days on the supposition that all
inhibited enzyme will be aged. Ecobichon et al. (1977) noted
prompt improvement in both condition and blood-ChEs in response
to pralidoxime given on the 11th-15th days after major symptoms
of poisoning appeared due to extended exposure to fenitrothion
(a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually
for an occupational setting, but, in the case of very severe
exposure or massive ingestion (accidental or deliberate), the
therapeutic doses may be extended considerably. Warriner et al.
(1977) reported the case of a patient who drank a large quantity
of dicrotophos, in error, while drunk. Therapeutic dosages were
progressively increased up to 6 mg atropine iv every 15 min
together with continuous iv infusion of pralidoxime chloride at
0.5 g/h for 72 h, from days 3 to 6 after intoxication. After
considerable improvement, the patient relapsed and further
aggressive therapy was given at a declining rate from days 10 to
16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given
and the patient was discharged on the thirty-third day with no
apparent sequelae.
REFERENCES TO ANNEX I
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-
379.
KAGAN, JU.S. (1977) [ Toxicology of organophosphorus pesti-
cides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in
Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (Unpub-
lished document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S.
& Gilman, A., ed. The pharmacological basis of therapeutics, 6th
ed., New York, Macmillan Publishing Company, pp. 100-119.
UK DHSS (1983) Pesticide poisoning: notes for the guidance of
medical practitioners, London, United Kingdom Department of
Health and Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus
poisoning. Guy's Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
See Also:
Toxicological Abbreviations
Dimethoate (HSG 20, 1988)
Dimethoate (ICSC)
Dimethoate (FAO Meeting Report PL/1965/10/1)
Dimethoate (FAO/PL:CP/15)
Dimethoate (FAO/PL:1967/M/11/1)
Dimethoate (JMPR Evaluations 2003 Part II Toxicological)
Dimethoate (AGP:1970/M/12/1)
Dimethoate (Pesticide residues in food: 1983 evaluations)
Dimethoate (Pesticide residues in food: 1984 evaluations)
Dimethoate (Pesticide residues in food: 1984 evaluations)
Dimethoate (Pesticide residues in food: 1987 evaluations Part II Toxicology)
Dimethoate (Pesticide residues in food: 1996 evaluations Part II Toxicological)
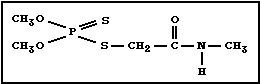 Molecular formula: C5H12NO3PS2
Common name: dimethoate (accepted by BSI, ISO,
ANSI, and JMAF); fosfamid (used in
USSR)
Common trade names: Bi 58; Cygon; Dimethoate; Fosfamid;
Fostion MM, Rogor; Perfekthion;
Roxion
IUPAC name: O,O-dimethyl S-methyl-carbamoyl-
methyl phosphorodithioate
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester
(9CI)
CAS registry number:
Molecular formula: C5H12NO3PS2
Common name: dimethoate (accepted by BSI, ISO,
ANSI, and JMAF); fosfamid (used in
USSR)
Common trade names: Bi 58; Cygon; Dimethoate; Fosfamid;
Fostion MM, Rogor; Perfekthion;
Roxion
IUPAC name: O,O-dimethyl S-methyl-carbamoyl-
methyl phosphorodithioate
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester
(9CI)
CAS registry number:  Different factors may affect the accumulation and degra-
dation of dimethoate in soil, such as the soil type, the numbers
and type of microorganisms present in soil, the environmental
temperature, the pH level, the amount of pesticide applied, and
the degree of evaporation (El Beit et al., 1977a,b, 1978). The
persistence of dimethoate was greater in heavy than in light
soil. At pH 4.2, the pesticide was stable for nearly 19 days;
at pH 11, it degraded within 20 h. The amount of dimethoate in
soil increased when higher concentrations were applied. El Beit
et al. (1977a) reported that soil microorganisms played little
part in the degradation of dimethoate.
4.1.4. Plants
When applied to plants, dimethoate was rapidly absorbed and
decomposed, both on the surface and within the plant, by
hydrolysis and oxidation (Menzie, 1969; Melnikov et al., 1977).
The half-life of dimethoate in the different plants varied
between 2-5 days (Melnikov et al., 1977). Dimethoate completely
disappeared after 15-30 days, depending on the plant species and
the climatic conditions. Decomposition in plants and the
hydrolysis of dimethoate increased with temperature (Atabaev,
1972).
The dissipation of dislodgeable residues of dimethoate is
best characterized by two first-order kinetic processes. The
half-life values were 2.2 days in the 1 to 10-day period and 7.0
days in the 10 to 49-day period (Hadjidemetriou et al., 1985).
4.1.5. Disposal of wastes
Hydrolytic decomposition is the main way to inactivate
dimethoate. By adding lime (1-2 kg calcium oxide/m3 water) to
waste waters from agricultural centres, dimethoate was fully
inactivated in 45 min (Winkler & Muller, 1979).
During pyrolysis, approximately 50% decomposition of di-
methoate occurred at 500 °C with the formation of O,O-dimethyl-
S,S-dithionpyrophosphate; decomposition was complete at 1100 °C
(Rosvaga, 1983).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air, water, and soil
No studies have been reported on levels in air, water, or
soil under actual conditions of use and various environmental
conditions.
5.1.2. Food
When a combination of dimethoate and omethoate (dimethoxon)
was given to cows in dosages of 1 and 0.1 mg/kg body weight,
respectively, for 14 days, only residues of the metabolite
omethoate were observed in the milk (0.004-0.125 mg/kg). Three
days after the application, neither compound could be detected.
When dosages of 0.5 mg dimethoate/kg and 0.05 mg omethoate/kg
were given for 14 days or when corn silage containing 1-7 mg
dimethoate/kg (resulting in dosages of 0.06-0.36 mg/kg body
weight) was given for 28 or 42 days, no residues were detected
in the milk (Beck et al., 1968).
Harvest residues found in many crops 1-3 weeks after
spraying with dimethoate, during the first years of application
in the United Kingdom (1957-58), were below 2 mg/kg (Chilwell &
Beecham, 1960).
The dimethoate content of apples was 0.03-0.07 mg/kg, 75
days after an application of 0.72 kg active ingredient/ha
(Atabaev & Stepovaya, 1966).
The pulp of lemon and orange fruit treated with dimethoate
did not show any residues at a detection level of 0.01 mg/kg,
60 days after application (Iwata et al., 1979).
Residues of dimethoate do not concentrate in wine. Analysis
of seven different Californian wines indicated levels of less
than 0.03 mg/litre (Kawar et al., 1979).
No residues were found in grapes, 29 days after treatment
with 0.1-0.15% dimethoate applied at the rate of 1400 litre/2 ha
(Grigorashvili & Dzhibladze, 1965).
A number of studies on residues of dimethoate found through-
out the world have been reported in a review by De Pietri-
Tonelli et al. (1965). The residues were most frequently below
1 mg/kg. Dimethoate residues found in various agricultural
products in India are reported in Khan & Bhaskar Dev (1982).
Dimethoate was not found in total-diet samples studied in
England and Wales during 1966-67 (Abbott et al., 1970).
According to other authors, dimethoate was also not found in the
total diets of adults and infants during 1975-79 (Johnson et
al., 1981a,b, 1984a,b; Podrebarac, 1984a,b; Gartrell et al.,
1985a,b,c). Market-basket surveys carried out in 1976-78 in the
Netherlands showed only omethoate residues in a small number of
fruits (De Vos et al., 1984). In the USA, dimethoate and
omethoate have been identified in about 5% of samples of fruits
and vegetables (Duggan et al., 1983).
The use of additional procedures for the determination of
organophosphates resulted in the identification of dimethoate in
adult and infant total-diet samples in 1980-82 (Gartrell et al.,
1985d, 1986a,b). However, the intakes (0.001 µg/kg body weight
per day) were far below the FAO/WHO acceptable daily intake
(ADI) (see section 12).
5.2. Occupational Exposure
Immediately after the use of dimethoate in greenhouses,
Stroy (1983) determined levels of 0.01-0.42 mg/m3 in the air,
3.6-9.3 mg/kg in the plants, and 0.98-1.75 mg/kg in the soil.
Dimethoate content in greenhouse air has been analysed at
different times after spraying; at 0 time the measured amount
was 0.66 mg/m3, at 2.5 h it was 0.38 mg/m3, at 5 h dimethoate
content was 0.21 mg/m3, at 10 h it was 0.07 mg/m3 and at 20 h
it was 0.01/m3 (Zolotnikova & Zotov, 1978).
The exposure to dimethoate of tractor drivers using airblast
units during treatment of citrus trees was investigated by
Carman et al. (1982). Dermal exposure was measured by placing
ethyleneglycol-treated gauze patches on the shoulders, upper
arms, and knees of the drivers. An emulsifiable concentration
(EC) formulation of dimethoate containing 0.009 kg/litre was
applied at the rate of 16 822 litre/ha using an open tractor, a
cab unit with both side windows open, and a cab unit with the
windows closed. Under these conditions, the patches attached
to the driver absorbed mean deposits of 2.5, 1.5, and
< 0.01 µg dimethoate/cm2 per h, respectively. The correspond-
ing average air concentrations were 10(sic), 48, and 2 µg of
dimethoate/m3.
Procedures for determining foliar residues and to establish
the safe re-entry times for some insecticides were reported by
Knaak (1980) and Knaak et al. (1980). A safe level of
dimethoate on foliage of 53 µg/cm2 was calculated using the
results of dermal-dose ChE-response studies in male rats.
Minimum safe re-entry periods for dimethoate were estimated
to be 3 days in greenhouses, and 7 days in tobacco fields, after
application by tractor, or 5 days after application by plane
(Kaloyanova-Simeonova & Izmirova-Mosheva, 1983). No ChE
inhibition in serum or subjective complaints of workers picking
dimethoate-treated tobacco leaves were established at a concen-
tration of 1 mg/kg on the surface of the plant.
Copplestone et al. (1976) studied the exposure to dimethoate
of 8 spraymen, 1 mixer, and 2 supervisors in the Sudan. The
percentage of toxic dosea received per day, calculated on the
basis of a 4-h working day, varied from 0.02% to less than
0.001% for individual spraymen. No ChE depression was found in
any of the men.
The highest concentration of dimethoate, measured in the
work-place air of a dimethoate-producing factory in Italy, was
0.050 mg/m3 (Armeli et al., 1967).
The respiratory and dermal exposure to dimethoate of
applicators was determined for greenhouse workers. The
respiratory and dermal exposures were 0.034 mg/h and 30 mg/h,
respectively. The hands of the operators were the most affected
parts of the body, accounting for 63-92% of the total exposure
(Adamis et al., 1985).
----------------------------------------------------------------
a Percentage toxic dose per day or h (WHO, 1982). This is
calculated from these indices adapted from the method of
Durham & Wolfe (1962) using the formula:
Dermal exposure (mg/day or h) + respiratory
exposure (mg/day or h) x 10 if measured
-------------------------------------------- x 100
Dermal LD50 mg/kg (rat) x 70
6. KINETICS AND METABOLISM
6.1. Absorption and Distribution
Panshina & Klisenko (1962) checked the blood levels of
dimethoate in cats and rats after single oral doses of 50, 75,
or 200 mg/kg in the cat and 300 mg/kg in the rat. The deter-
minations were carried out 15, 30, 60, 90, 120, and 180 min
after dosing. Dimethoate was detected in the blood of cats and
rats after 30 min, and reached a maximum level after 60-90 min.
Nearly 80% of the dimethoate in the blood was found in the
erythrocytes; only 15-20% was found in the serum. With repeated
daily oral intake of dimethoate at doses of 20 mg/kg or
10 mg/kg, the maximum blood level occurred on the 5-10th day of
the study.
The same pattern in blood levels was observed with repeated
inhalation of dimethoate for 4 h/day over 3 months, at a mean
concentration of 5 mg/m3 air. Dimethoate was detected in blood
from the second day and reached its maximum by the 7-10th day.
Daily application of 50 mg dimethoate/kg on the skin of
rabbits resulted in a maximum concentration in the blood at
about the third day (Kundiev, 1979).
When dimethoate was applied to the skin of rats for 1, 2, 4,
12, or 24 h in a single dose of 560 mg/kg, the maximum concen-
tration in the skin was reached after 12 h of exposure and was
correlated with the maximum inhibition of ChE activity in the
serum and liver. The concentrations of dimethoate in the blood,
liver, and kidney were maximal after 2 h of exposure (Baranova
et al., 1986).
6.2. Metabolic Transformation
The ester and amide groups of dimethoate are cleaved in
reactions that vary with the organism and that contribute to the
selective toxicity of the compound.
The results of in vitro and in vivo studies showed that the
main metabolic pathways of dimethoate were hydrolysis and
oxidation (Hassan et al., 1969; Lucier & Menzer, 1970; North &
Menzer, 1972).
Santi & Giacomelli (1962) studied the metabolic fate of
dimethoate in olives. P=O derivative and degradation products,
such as phosphoric and/or methylphosphoric acid, were found.
The presence of the oxygen analogue dimethoxon (omethoate)
has been demonstrated in insects, plants, and mammals; it
appears to be the metabolite responsible for the toxic action of
dimethoate (Brady & Arthur, 1963; Hassan et al., 1969; Lucier &
Menzer, 1970). The highest levels of this metabolite were found
in insects, particularly in those highly susceptible to
dimethoate. The oxygen analogue was produced in larger
quantities in insects than in rats. The enzymes mediating the
hydrolysis of the carboxyamide bond are much less effective in
insects than in mammals (Mikhailov & Shterbak, 1983).
It has been shown that cleavage of dimethoate by rats and
cows occurs initially at the C-N bond to produce the carboxy
derivative (Dauterman et al., 1959; Hassan et al., 1969). A
second hydrolytic pathway involves an esterase action on the S-C
bond (Hassan et al., 1969).
Oxidative metabolism of dimethoate predominated over
hydrolytic metabolism in the cell culture system. In the whole
rat, the opposite was true. Metabolism of dimethoate in human
embryonic lung cells was much the same as metabolism in rats
(North & Menzer, 1972). In vitro and in vivo studies showed
that dimethoate is biotransformed to the P=O analogue via the
liver cytochrome P-450 system (Kaloyanova et al., 1984).
Concentrations of 0.1-10 mmol dimethoate/litre led to a linear
decrease in the rates of N-demethylation and P-hydroxylation.
Similarly, in microsomes from rats treated with dimethoate in
vivo, increased activity of desulfuration (140%, P < 0.01), and
decreased activity of hydroxylation and demethylation were seen
(Mitova et al., 1986).
In vivo studies on mice showed dimethoate toxicity to be
markedly increased by phenobarbital pre-treatment, as a result
of induction of hepatic microsomal enzymes including the mixed
function oxidases responsible for the conversion of P=S to P=O
(Menzer & Best, 1968).
It has been found that, while dimethoate undergoes rapid
degradation in the rat liver, very little occurs in other
tissues (lung, muscle, pancreas, brain, spleen, blood). The
ability of the liver to degrade dimethoate in various species
decreased in the order: rabbit > sheep > dog > rat > cattle >
hen > guinea-pig > mouse > pig. For the hen, cattle, mouse,
sheep, and rat there was a reasonably good straight-line
relationship between the LD50 values and the degradation ability
of the liver (Uchida et al., 1964).
After administration of 32P-dimethoate to rats, dimethoate,
dimethoxon, dimethoate carboxylic acid, dimethylphosphoro-
dithioate, dimethylphosphorothioate, dimethylphosphate, mono-
methylphosphate, phosphorothioate, formate, and N-methyl 2-
glucuronate acetamide were found in the urine (Hassan et al.,
1969).
Data on the metabolism of dimethoate in plants and animals
have been reviewed by Menzie (1969, 1974, 1978, 1980).
6.3. Elimination and Excretion
6.3.1. Animal studies
About 45% of the 32P-dimethoate administered orally at
50 mg/kg to rats was excreted in the urine, while only 5.8% was
eliminated in the faeces, 72 h after treatment (Brady & Arthur,
1963). The values in rats after dermal application were 30.6%
and 6.5%, respectively. More than 95% of the 32P materials in
the urine and faeces after oral or dermal administration in rats
were hydrolytic products, as determined by chloroform/water
partition coefficients.
Twenty-four h after ip and oral administration of dimethoate
to rats at doses of 0.25, 2.5, or 25 mg/kg, dimethylphosphoro-
dithioate, dimethylphosphorothioate, and dimethyl phosphate
were detected in the urine at concentrations of 12-14%, 11-15%,
and 12-13%, respectively (Riemer et al., 1985). Neither the
route of exposure nor the dose had any influence on the types of
metabolite formed.
About 87-90% of an oral dose of 10 mg dimethoate/kg was
eliminated in the urine of cattle at the end of 24 h. The same
percentage of an intramuscular dose of 10 mg/kg was excreted
after 9 h. Only 3.7-5% of the oral dose and about 1.1% of the
intramuscular dose were eliminated in the faeces after 72 h and
24 h, respectively (Kaplanis et al., 1959).
6.3.2. Human studies
In human beings, 76-100% of radioactivity was reported
to be excreted in the urine, 24 h after oral dosing with 32P-
dimethoate (Sanderson & Edson, 1964).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The addition of dimethoate to soil at 10 or 100 mg/kg did
not result in significant differences in the number of bacteria
solubilizing tricalcium phosphate or in the number of bacteria
mineralizing calcium glycerophosphate, but an increase in the
population of phospholipase-producing organisms solubilizing
lecithin occurred (Congregado et al., 1979). At 10 mg/kg, an
increase in carbon dioxide production occurred for 2 weeks after
treatment, followed by a decrease to control levels. At
100 mg/kg, the increase in carbon dioxide output was slower and
longer.
7.2. Aquatic Organisms
A number of LC50s have been determined for various aquatic
organisms (Table 3).
The median tolerance limit of the fresh-water teleost,
Channa punctatus for dimethoate is 20.5 mg/litre (Anees, 1975).
Exposure for 24 h, 96 h, or 14 days to dimethoate concentrations
of 10.8, 8.0, or 5.0 mg/litre, respectively, produced moderate
vacuolation of the liver and a high degree of cytoplasmic
granulation, which developed for up to 96 h of exposure. The
14-day exposure added little in the way of vacuolation or
granulation (Anees, 1978a). The haematological response to
dimethoate included reduced erythrocyte counts and haemoglobin
concentration, and an elevated mean corpuscular haemoglobin and
colour index indicating that the insecticide exerted an effect
similar to the production of anaemia (Anees, 1978b).
The signs of the toxicity of dimethoate in fish
(Channa punctatus) included jumping, erratic movement,
imbalance, and death (Dikshith & Raizada, 1981a; Dikshith,
1986).
Verma et al. (1978) determined the TLm values of dimethoate
for Channa gachua for 24, 48, 72, or 96 h to be 5.2, 5.0, 4.6,
or 4.5 mg/litre, respectively. The safe concentration of
dimethoate calculated on the basis of TLm values was approxi-
mately 1.4 mg/litre.
Dimethoate inhibited AChE activity in the brain, liver, and
muscle of some fresh-water teleosts (Channa gachua and Cirrhina
mrigala), exposed to sublethal concentrations of 35% EC
formulation (0.9-2.4 and 0.6-1.6 mg/litre, respectively) (Verma
et al., 1979).
Table 3. Summary of acute toxicity values for aquatic organisms
---------------------------------------------------------------------------------------------------------
Species LC50 (mg/litre) Reference
24-h 48-h 72-h 96-h
---------------------------------------------------------------------------------------------------------
Rainbow Trout 20 - - 8.5 Melnikov et al. (1977)
(Salmo gairdneri)
Rainbow Trout - - - 6.2 Johnson & Finley (1980)
(Salmo gairdneri)
Long-nosed killifish 1.0 - - Melnikov et al. (1977)
(Fundulus similis)
Saccobranchus fossilis 5.14 4.80 4.67 4.57 Verma et al. (1982)
Channa punctatus 68 54 - 47 Dikshith & Raizada (1981a)
Dikshith (1986)
Scud 0.9 0.4 - - Menzie (1969)
(Gammarus lacustris)
Scud - - - 0.20 Johnson & Finley (1980)
(Gammarus lacustris)
Red Crayfish - 1.0 - - Muncy & Oliver (1963)
(Procambarus clarkii)
Stonefly - 0.14 - - Menzie (1969)
(Pteronarcys california)
Stonefly - - - 0.043 Johnson & Finley (1980)
(Pteronarcys california)
Unspecified insect 0.51 - - - Sanders & Cope (1968)
Bluegill - - - - Johnson & Finley (1980)
---------------------------------------------------------------------------------------------------------
Dalela et al. (1979) reported that acute (5-h) and short-
term (up to 32 days) exposure of the fish, Channa gachua, to
dimethoate at 6.2 mg/litre and 1.5 mg/litre, respectively,
produced histological changes in the gills. On acute exposure,
there was erosion at the distal end of the gill filaments and
loss of cell membrane. With exposure to a concentration of
1.5 mg/litre, the basement membrane started separating, and the
damage to the gill was found to be more significant with
increasing exposure time, with vacuolization occurring after 32
days.
The exposure of the fish Heteropneustes fossilis to a
dimethoate concentration of 10 mg/litre led to an increased
level of glycogen by the end of the second week in both the
liver and the kidney, and to a slight decrease in the protein
contents at the end of the eighth day (Awasthi et al., 1984). A
sharp rise in the activity of succinate dehydrogenase in both
organs was noted during the first two weeks of this study.
The estimated 48- and 72-hour TLm values for zebrafish
Brachydanio rerio embryos, exposed to dimethoate, were
940 mg/litre and 259 mg/litre, respectively (Roales &
Perlmutter, 1974). Dimethoate retarded the development of
embryos as expressed by lack of heartbeat and little movement at
24 h.
Dimethoate at a concentration of 0.05 mg/litre produced
morphological changes in the melanophores of Bufo melano-
stictus tadpoles and an increase in pigmented areas of the skin
(Pandey & Tomar, 1985).
Dimethoate had a very low toxicity for some aquatic
organisms in Sudan, such as Oreochromis niloticus, Gambusia
affinis, Pseudagrion spp., Crocothemis erythraea, and Lanistes
carinatus. Under laboratory conditions, it did not kill any
animal at concentrations lower than 80 mg/litre (Karim et al.,
1985).
The toxicity of dimethoate for 11 freshwater species was
studied by Slooff & Canton (1983). The results are summarized
in Table 4. The relative susceptibility tests indicated that
Daphnia magna was the organism most sensitive to dimethoate,
while the microorganisms P.fluorescens, M.aeruginoso, and
S.pannonicus were generally less sensitive indicators of
toxicity. The susceptibility of aquatic species to a chemical
may vary by more than two-three orders of magnitude. The data
demonstrate that the sublethal criteria studied were not
necessarily the most sensitive toxicological criteria.
7.3. Terrestrial Organisms
7.3.1. Honey-bees
The oral LD50 for the honey-bee (Apis mellifera L) ranges
from 93 to 150 ng per bee (Jaycox, 1964; Lord et al., 1968;
Stevenson, 1968; Barker et al., 1980). The contact LD50 is
98-120 ng per bee (Stevenson, 1968).
Table 4. Fresh-water species susceptibility to dimethoate
---------------------------------------------------------------------------------------------------------
Type of Test species Lifestage Exposure Test condition Toxicological No-effect
organism time Temper- Test parameter level
(days) ature methods (mg/litre)
---------------------------------------------------------------------------------------------------------
Bacteria (Pseudomonas fluorescens) log-phase 0.3 22 ± 2 Static Specific 320
growth rate
Cyano Bluegreen bacteria log-phase 4 23 ± 2 Static Specific 32
bacteria (Microcystis aeruginosa) growth rate
Algae Green Algae log-phase 4 23 ± 2 Static Growth 100
(Scenedesmus pannonicus) (biomass)
Plant Lemna minor - 7 25 ± 1 Static Specific 32
growth
rate
Crustacean Water flea (Daphnia magna) 1 day 21 19 ± 1 Semi- Mortality 0.032
static reproduction 0.1
Insect Mosquito (Culex pipiens) 1st 25 27 ± 1 Semi- Mortality 0.32
instar static development 0.32
Coelente- Hydrozoan (Hydra oligactis) budless 21 18 ± 1 Semi- Specific 100
rate static growth rate
Mollusc Giant Pond Snail 5 months 40 20 ± 1 Semi- Mortality 32
(Lymnaea stagnalis) static Reproduction 10
egg 7 20 ± 1 Semi- Hatching 32
static
Fish Guppy (Poecilia Reticulata) 3-4 weeks 28 23 ± 1 Semi- Mortality 32
Viviparous static Behaviour + 0.1
Mortality
Fish Japanese Ricefish eggs 40 23 ± 2 Semi- Mortality 0.32
(Oryzias latipes) oviviparous static Behaviour 0.32
Hatching 100
Growth
Amphibian Xenopus laevis 2 days 100 20 ± 1 Semi- Mortality 1
static Development 32
Growth 32
---------------------------------------------------------------------------------------------------------
From: Slooff & Canton (1983).
Dimethoate was only slightly repellent to foraging honey-
bees. The self-limiting dose for foraging was 20-25 times the
lethal oral dose (2.9-3.9 µg/bee vs 150 ng/bee). This can be
interpreted on the basis of 5% absorption by foraging bees,
while 95% is passed on to the colony. Thus, systemic insecti-
cide in nectar may also pose a threat to the rest of the colony
when brought back to the hive (Waller et al., 1979).
Residual toxicity has been supported by several obser-
vations. Nectar from plants sprayed with 0.1% dimethoate was
lethal for honey-bees for at least 2-3 days (Jaycox, 1964) or 10
days (Barker et al., 1980). Waller et al. (1984) also showed
the possible toxic levels of residues in the nectar for up to
10 days after treatment of lemon trees with dimethoate at a rate
of 1.12 kg of ai per ha. The high bee mortality observed,
immediately after treatment, was attributed to dimethoate
residues on the plant surface.
7.3.2. Birds
The acute toxicity studies of dimethoate for birds are
summarized in Table 5.
Table 5. Acute oral LD50 of dimethoate for birds (mg/kg)a
----------------------------------------------------------------
Species Sex Pure Laboratory Technical Liquid
grade grade formulation
----------------------------------------------------------------
Hen F 50 40 30 25
Pheasant M 15 - 20 15 20 25
Duck F - > 40 - -
Sparrow M/F - - - 22
Blackbird M/F - - - 26
----------------------------------------------------------------
a From: Sanderson & Edson (1964).
Hens did not show any evidence of delayed neurotoxicity
(Sanderson & Edson, 1964; Gaines, 1969; Francis et al., 1985).
The effect of dimethoate on esterase levels following the
oral dosing of pheasants and following long-term feeding to
pheasants and pigeons was investigated by Bunyan et al. (1968,
1969). Dimethoate inhibited brain-alpha-naphthyl acetate
esterase more than brain-cholinesterase and triacetin esterase
in acute studies.
A characteristic of dimethoate was the elevation of phenyl
benzoate esterase levels, showing that after initial liver
damage, dimethoate is able to induce certain enzymes.
7.3.3. Farm animals
The acute oral LD50s for several farm animals are
summarized in Table 6.
No visible signs of intoxication were seen in horses
receiving dimethoate orally at doses of 25 or 50 mg/kg. Single
doses of dimethoate at 40 mg/kg were effective in removing
Gasterophilus spp. from infected horses, but toxic signs
appeared in animals treated with higher levels of 60-80 mg/kg
(Jackson et al., 1960).
Table 6. Acute oral LD50 for farm animals
-------------------------------------------------
Species LD50 (mg/kg Reference
body weight)
-------------------------------------------------
Horse > 50 Jackson et al. (1960)
Sheep 80 Hewitt et al. (1958a,b)
Cattle 70 Hewitt et al. (1958a,b)
-------------------------------------------------
Mild signs of intoxication occurred in sheep at 75 mg/kg,
including slight salivation, lachrymation, transitory diarrhoea,
rhinitis, and anorexia. Doses lower than 15 mg/kg were essen-
tially asymptomatic in calves. The data with dimethoate
indicate an appreciable margin of safety between the lowest
dose that kills first instar Hypoderma lineatum (5 mg/kg), and
the doses that produce mild toxicity (15-20 mg/kg), or severe,
reversible toxicity (40 mg/kg) (Hewitt et al., 1985b).
Fetcher (1984) described cases of suspected dimethoate
intoxication in cattle grazing on pasture that had been sprayed
6 weeks earlier. There was a predominance of nicotinic signs
and a poor response to atropine treatment. Chemical analysis of
liver, kidney, and brain tissue did not reveal any organo-
phosphorus compounds or metabolites. Whole blood-ChE was
depressed in 3 out of 14 animals.
After spraying barns (for calves) and pigsties with
dimethoate, only 16-29% of the initial concentration still
persisted after 8 weeks. Nevertheless, the animals showed a
decrease in ChE (Müller & Reinhold, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides, especially their short- and long-term effects on
the nervous systems, can be found in the WHO Environmental
Health Criteria document entitled EHC 63: Organophosphorus
Insecticides, a General Introduction (WHO, 1986a).
8.1. Single Exposures
The acute oral and dermal LD50s of dimethoate for several
animal species are summarized in Tables 7 and 8 (all LD50s are
expressed as active ingredient).
Signs, characteristic of organophosphorus intoxication,
were observed in the rat 0.5-2 h after oral administration of
dimethoate (Sanderson & Edson, 1964). They included muscular
fibrillation, salivation, lachrymation, urinary incontinence,
diarrhoea, respiratory distress, prostration, gasping, coma, and
death (WHO, 1986a).
Oral LD50 values for rats, which were measured in 13
studies, ranged from 150 to 680 mg/kg body weight. The purity
and formulation of the compounds used were not stated in most
of the reports. Oral LD50s were determined of 60-140 mg/kg
body weight for mice, 200 mg/kg body weight for hamsters,
350-600 mg/kg body weight for guinea-pigs, 280-500 mg/kg body
weight for rabbits, and 100 mg/kg body weight for cats.
Administration of 100 mg/kg body weight to dogs did not result
in mortality. The World Health Organization based its classi-
fication of dimethoate as moderately hazardous on an acute oral
LD50 in the rat of 150 mg/kg body weight (WHO, 1986b).
The dermal LD50s for rats were found to range between 500
and 1150 mg/kg body weight, and were of about the same order of
magnitude as the oral LD50s or slightly higher (Table 8).
Data on LD50s after parenteral administration were given by
Sanderson & Edson (1964). The values were comparable with those
for ip, sc, and iv administration. In the rat, the values for
ip administration varied between 175 and 325 mg/kg body weight
and were also of the same order of magnitude as the oral LD50s.
The inhalation LC50 has not been estimated, but, in a 4-h
inhalation study on rats, Panshina (1963b) did not find any
signs of intoxication with exposure to dimethoate at 20 mg/m3
air, 40% cholinesterase inhibition at 10 mg/m3, and no effects
at 2 mg/m3. Visible signs of intoxication were observed in cats
at concentrations of 50-80 mg/m3. At 20 mg/m3, cholinesterase
inhibition was found to be 10-66%, while at 2-8 mg/m3, it was
7-56%. No effects were seen at 1.5 mg/m3 (Panshina, 1963b).
Table 7. Acute oral LD50 of dimethoate in experimental animals
-------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
-------------------------------------------------------------------------
Rat M 32% emulsifiable 247 Edson & Noakes (1960)
solution
Rat M/F technical 185 - 245 West et al. (1961)
Rat 230 Panshina (1963a)
Rat technical 172 Panshina (1963a)
Rat M/F pure 500 - 680 Sanderson & Edson
(1964)
Rat M/F laboratory 280 - 356 Sanderson & Edson
grade (1964)
Rat M/F technical 180 - 336 Sanderson & Edson
(32-40% w/v) (1964)
Rat M/F liquid formul- 150 - 400 Sanderson & Edson
ation (20% ai) (1964)
Rat M/F wettable powder 280 - 300 Sanderson & Edson
(1964)
Rat pure 250 Atabaev & Stepovaya
(1966)
Rat M/F produced 1962 215 - 245 Gaines (1969)a
(43.5%)
Rat pure 200 - 300 Ben-Dyke et al.
(1970)
Rat 250 - 265 Melnikov (1974)
Mouse 99% pure 140 Hewitt et al. (1958b)
Mouse pure 135 Panshina (1963a)
Mouse technical 125 Panshina (1963a)
Mouse F pure 60 Sanderson & Edson
(1964)
----------------------------------------------------------------------------
a Lower figures (20-30 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
Table 7. (contd).
--------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
--------------------------------------------------------------------------
Mouse F technical 60 Sanderson & Edson
(1964)
Hamster M laboratory grade 200 Sanderson & Edson
(1964)
Guinea- M/F pure 550 Sanderson & Edson
pig (1964)
Guinea- M/F laboratory grade 600 Sanderson & Edson
pig (1964)
Guinea- M/F technical 350 - 400 Sanderson & Edson
pig (1964)
Guinea- M/F liquid formul- 350 - 370 Sanderson & Edson
pig ation (1964)
Rabbit M/F pure 500 Sanderson & Edson
(1964)
Rabbit M/F laboratory grade 450 Sanderson & Edson
(1964)
Rabbit M/F technical 300 Sanderson & Edson
(1964)
Rabbit M/F liquid formul- 283 Sanderson & Edson
ation (1964)
Cat technical 100 Panshina (1963a)
---------------------------------------------------------------------------
8.2. Skin and Eye Irritation
A single dose of 300 mg technical dimethoate/kg body weight
did not cause skin irritation in male and female rabbits
(Dikshith & Raizada, 1981b).
In a study by West et al. (1961), dimethoate did not have
any irritant effect on the rabbit eye after introduction of
10 mg of dry material into the conjunctival sac. However, in a
personal communication (1986), the US EPA suggested that
dimethoate had a slight irritant effect on the eye.
8.3. Repeated Exposures
The effects on experimental animals of repeated oral or
inhalation exposure to dimethoate are summarized in Tables 9 and
10.
In the various studies, which ranged from 5 1/2-12 months
in duration, inhibition of cholinesterase (ChE) in the erythro-
cytes was a more sensitive indicator of exposure to dimethoate
than ChE inhibition in plasma. ChE activity in the brain was
measured in one study only.
Table 8. Acute dermal LD50 of dimethoate in experimental animals
------------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight
------------------------------------------------------------------------------
Rat M 32% emulsifiable 1120 Edson & Noakes (1960)
solution
Rat liquid formula- 700 - 1150 Sanderson & Edson
tion (24-h) (1964)
Rat wettable powder 500 (24-h) Sanderson & Edson
(1964)
Rat M/F produced 1962 610 Gaines (1969)a
Rat M 32% w/v emulsifi- 770 - 1090 Noakes & Sanderson
able solution (24-h) (1969)
Rat M 32% w/v emulsi- > 1100 (4-h) Noakes & Sanderson
fiable solution (1969)
Guinea-pig liquid formula- 965 West et al. (1961)
tion (46%)
Guinea-pig wettable powder 995 West et al. (1961)
(25%)
Rabbit not specified 600 Melnikov (1974)
------------------------------------------------------------------------------
a Lower figures (55-61 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
In a study by West et al. (1961), no effects were observed
on ChE inhibition in rats administered dimethoate in the diet at
32 mg/kg. In their first study (12 months) on rats, Sanderson
& Edson (1964) observed inhibition of ChE in erythrocytes at
50 mg/kg diet, but not at 10 mg/kg. In the second study (5 1/2
months), inhibition of ChE in erythrocytes was found at both 20
and 10 mg/kg, but not at 5 mg/kg. The studies of Atabaev (1972)
did not show any inhibition of blood-ChE in rats administered a
40% formulation of dimethoate at 0.5-1 mg/kg body weight, corre-
sponding to 0.2-0.4 mg dimethoate/kg body weight. From all
available data on the rat, a dietary level of dimethoate of 5
mg/kg, corresponding to 0.25 mg/kg body weight, can be con-
sidered as the no-observed-adverse-effect level.
From limited studies on the dog (West et al., 1961), it can
be concluded that a level of 10 mg dimethoate/kg diet, corre-
sponding to 0.25 mg/kg body weight, does not result in ChE
depression in erythrocytes.
ChE inhibition was not observed in an inhalation study in
which rats were exposed for 14 h/day, over 3 months, to 0.01 mg
dimethoate/m3 (measured concentration) (Kaloyanova et al.,
1968).
Table 9. The effects on experimental animals of repeated exposure to dimethoate
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat technical 50, 100, or 200 35 days no mortality; no signs West et al. (1961)
(95%) mg/kg diet of intoxication
Rat technical 2, 8,or 32 mg/kg 3 months no mortality; no ChE West et al. (1961)
(95%) diet inhibition
Rat technical 15 mg/kg (oral) 6 months 100% inhibition of ChE Panshina (1963a)
in serum and erythro-
cytes; approximately
85% inhibition in brain
Rat technical 30 mg/kg (oral) 6 months death of 3 out of 5 Panshina (1963a)
animals
Rat technical 60 mg/kg (oral) 6 months death of all animals Panshina (1963a)
Rat (M) laboratory 10 mg/kg diet 12 months no inhibition of ChE in Sanderson & Edson
grade erythrocytes or plasma (1964)
Rat (M) laboratory 50 mg/kg diet 12 months marked inhibition of Sanderson & Edson
grade ChE in erythrocytes (1964)
Rat (M) laboratory 200 mg/kg diet 12 months marked toxic effects; Sanderson & Edson
grade reduced rate of weight (1964)
gain; inhibition of ap-
proximately 70% and 100%
ChE in plasma and erythro-
cytes, respectively
Rat laboratory 800 mg/kg diet 12 months severe toxic effects Sanderson & Edson
grade (1 week) (cholinergic effect: (1964)
weakness, weight loss
after a few days); the
pesticide was withdrawn
after one week; complete
recovery in 10 - 14 days
Rat (M) technical 20 or 10 mg/kg 5 1/2 months 50 and 40% inhibition Sanderson & Edson
(weanling) diet of ChE in erythrocytes, (1964)
respectively
---------------------------------------------------------------------------------------------------------
Table 9. (contd).
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat (M) technical 5 mg/kg diet 5 1/2 months no inhibition of ChE Sanderson & Edson
(weanling) (1964)
Rat 40% formula- 13 mg/kg body 4 months one rat died on the Atabaev (1972)
tion weight (oral) 35th day
Rat 40% formula- 50 mg/kg body 4 months 3 rats died on the 7th Atabaev (1972)
tion weight (oral) day, 2 died on the 8th
day, and one died on the
70th day
Rat 40% formula- 0.5 - 1 mg/kg 6 months no effect on ChE Atabaev (1972)
tion (oral)
Rat 40% formula- 5 mg/kg body 6 months AChE inhibition in Atabaev (1972)
tion weight (oral) blood in the first 2
months (approximately
50%)
Dog technical 2 or 10 mg/kg 13 weeks no inhibition of ChE West et al. (1961)
diet
Dog technical 50 mg/kg diet 13 weeks slight depression of West et al. (1961)
ChE in erythrocytes
Cat technical 10 mg/kg body 3 months death in 2 out of 4 Panshina & Klisenko
weight (oral) animals (1962)
Cat technical 20 mg/kg body 3 months death in 2 out of 3 Panshina & Klisenko
weight (oral) animals (1962)
Cat 40% formula- 0.5 - 1 mg/kg 3 months no effect on ChE Atabaev (1972)
tion body weight
(oral)
Cat 40% formula- 2 mg/kg body 3 months reduced body weight in Atabaev (1972)
tion weight (oral) the first 2 months
(16 - 32%); recovery at
the third month
---------------------------------------------------------------------------------------------------------
Table 10. Inhalation toxicity - repeated exposure
---------------------------------------------------------------------------------------------------------
Species Concentration Duration Effects Reference
(mg/m3) Daily exposure Number of
(h) months
--------------------------------------------------------------------------------------------------------
Rat 2 ? 2 no visible signs of intox- Panshina (1963b)
ication; 26% inhibition of
AChE in blood at the end of
the study
Cat 1.5 ? 1.5 no visible signs of intox- Panshina (1963b)
cation; 40-72% inhibition
of AChE in blood at the end
of the study
Rat in a miniature greenhouse (0.6 m3; temperature no blood-ChE inhibition Sanderson & Edson
21-30 °C); plants sprayed with 5 ml of 0.5% (1964)
aqueous formulation in the course of 28 days
Rat and 4.50 8 3 inhibition of ChE; leuk- Kaloyanova et al.
guinea-pig ocytosis (1968)
Rat 0.05 14 3 inhibition of ChE in blood Kaloyanova et al.
only in the first month; (1968)
inhibition of brain- and
liver-AChE; morphological
alterations in the neurons
Rat 0.01 14 3 no changes observed Kaloyanova et al.
(1968)
Rat 0.495 24 3 inhibition of ChE and Ubaidullaev &
changes Madzhidov (1976)
Rat 0.049 24 3 inhibition of ChE Ubaidullaev &
Madzhidov (1978)
Rat 0.003 24 3 no detectable changes Ubaidullaev &
Madzhidov (1978)
---------------------------------------------------------------------------------------------------------
8.4. Reproduction Studies
One reproduction study on mice has been reported in the
literature (Budreau & Singh, 1973). In this 5-generation study,
initial groups of 14 female and 10 male mice received dimethoate
in the drinking-water at 60 mg/litre for a period of one month,
prior to mating. A comparable control group was included in
the study. Animals receiving dimethoate showed a significant
( P < 0.05) reduction in mating performance (expressed as pro-
portion of females with deliveries to females mated), which
ranged from 33 to 61%, varying with litter and generation.
Similarly, reproduction time (measured as number of days elaps-
ing from first day of mating to day of delivery) was signifi-
cantly ( P < 0.01) increased in all first litters of all 5
generations examined, but unaffected in all second litters. The
biological relevance of this observation is unclear. Litter
size and average weight at birth were not affected by treatment.
Although the mean weights of treated pups were not significantly
lower, the growth rate was consistently lower in treated pups
compared with that in the controls.
A 3-generation reproduction study using technical
dimethoate (98.3%) was carried out on mice. Information sup-
plied by the US EPA indicated that there were no effects on re-
production and teratogenicity at a dimethoate level of 50 mg/kg
(US EPA, personal communication, 1986). Details of this study
were not available.
8.5. Teratogenicitya
Intraperitoneal administration of 40 mg dimethoate/kg body
weight, given as a single dose on the day of mating or on the
9th day of gestation, or given for the first 14 days of
gestation in mice, caused a high incidence of embryonal loss
(Scheufler, 1975).
Cygon 4E (containing 47.3% dimethoate) was given to female
rats by intubation from the 6th to the 15th day of gestation at
dose levels of 3, 6, 12, or 24 mg/kg body weight. The 24 mg/kg
dose was toxic for the dams (8 out of 20 dams manifested clonic
spasms and muscular tremors during the treatment period, 7 re-
covered, and one died on the 16th day of pregnancy). Doses of
12 and 24 mg/kg were associated with an increase ( P < 0.05)
in the numbers of anomalous litters (each having at least one
anomalous fetus) and wavy-ribbed fetuses. The 3 and 6 mg/kg
doses (equal to 1.42-2.84 mg dimethoate/kg) did not produce any
evidence of teratogenicity or embryotoxicity in the rats (Khera
et al., 1979).
----------------------------------------------------------------
a A further teratogenicity study on the rat (Edwards et al.,
1984) was submitted to the FAO/WHO Joint Meeting on Pesti-
cide Residues (JMPR) in 1987. Although fetotoxicity was ob-
served, there were no teratogenic effects (FAO/WHO, 1987).
Cygon 4E (47.3% dimethoate) was given to cats in gelatin
capsules at doses of 3, 6, or 12 mg/kg on the 14th-22nd days of
pregnancy. At the levels tested, the compound did not produce
any effects on the incidence of pregnancy. In the 12 mg/kg
group, forepaw polydactily was observed in 8 out of 39 fetuses.
Cygon 4E at 3 or 6 mg/kg (1.42-2.84 mg dimethoate/kg) did not
produce any effects (Khera, 1979). (The effect observed in
Khera's investigations may be due to the other components in the
formulation).
Courtney et al. (1985) reported that dimethoate adminis-
tered orally was not teratogenic in CD-1 mice at dose levels of
10 or 20 mg/kg body weight, and that these levels were not
lethal to the dams. The two highest dose levels of 40 and
80 mg/kg produced maternal toxicity.
8.6. Mutagenicity
A variety of in vivo and in vitro mutagenicity tests have
been carried out with dimethoate, the results of which are given
in Table 11.
Negative mutagenicity results were reported by Degraeve et
al. (1984) for commercial mixtures of insecticides containing
dimethoate. Two formulations were tested: dimethoate + fenitro-
thion (dose 60 mg/kg body weight, corresponding to 9 mg/kg of
each) and dimethoate + malathion + methoxychlor (dose 100 mg/kg
body weight corresponding to 9.5 mg dimethoate/kg). A single
ip injection did not induce chromosome aberrations in bone
marrow cells, spermatogonia, or primary spermatocytes of the
mouse. No significant increases in pre- or post-implantation
fetal lethality were observed in a dominant lethal mutation
assay.
Alkylation by dimethoate at the N-7 position of guanine in
DNA has been investigated (Dedek et al., 1984). Male mice
(strain AB Jena/Halle, random bred) were injected ip with 14C-
methyl-labelled dimethoate at a dose of 0.35 mmol/kg. The
extent of methylation was in the range of 1-10 µmol N-
7 methylguanine/mol guanine; the values in the kidneys were
higher than those in the liver. The excretion half-life of N-
7 methylguanine was 23-160 h.
In summary, dimethoate has been found to be mutagenic in
both in vitro and in vivo assays.a
----------------------------------------------------------------
a After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
Table 11. Mutagenicity tests
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Escherichia coli 1 - 6.10-3 mmol 5-methyltryptophane resistance Mohn (1973)
K-12/gal Rs mutation -positive
Escherichia coli Up to 5000 µg/plate positive Moriya et al.
WP2 hcr (1983)
Escherichia coli Minimum mutagenic positive Probst et al.
WP2 & WP2uvrA- concentration (1981)
47 nmoles/ml
Salmonella typhimurium negative Probst et al.
TA 98, 100, 1535, 1537, (1981)
1538
Salmonella typhimurium Up to 5000 µg/plate positive with and without Moriya et al.
TA 100 activation (1983)
Salmonella typhimurium Up to 5000 µg/plate negative Moriya et al.
TA 98, 1535, 1537, 1538 (1983)
Salmonella typhimurium 5 - 200 µg/plate positive at all doses, mutagenicity Vishwanath &
TA 100 (base pair subst.) increased with liver microsomes Jamil (1986)
Saccharomyces cerevisiae 7 doses, 40 - 100 mmol induction of mitotic gene Fahrig (1974)
conversions
Schizosaccharomyces pombe 1.3 - 131 mmol negative Gilot-Delhalle
ade 6 (LD50 50 mmol) (1983)
Chinese hamster ovary 20,40 & 80 µg/ml sister chromatid exchange increase Chen et al.
cells V79 10 µg/ml negative (1981)
Rat hepatocytes culture 47 nmol/ml negative unscheduled Probst et
al. (1981)
SV-40 transformed human 100 & 1000 umol positive unscheduled DNA Ahmed et
synthesis al. (1977)
Drosophila melanogaster 1 mg/kg (feeding) negative Woodruff et
al. (1983)
---------------------------------------------------------------------------------------------------------
Table 11. (contd).
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Drosphila melanogaster adult inj. ip 0, 10, increase in sex-linked Velazquez et
20 mg/kg body weight recessive lethals at 10 mg/kg al. (1986)
not at 20 mg/kg
Drosophila melanogaster adult feeding 0, negative Velazquez et
10 mg/kg; larval negative al. (1986)
feeding 0, 20 mg/kg
Host-mediated assay mouse 3 equal oral doses positive - mutation factor of Usha Rani et
Salmonella typhimurium of 155 mg/kg 3.44 ( P < 0.05) al. (1980)
Dominant lethal mutation acute 10 mg/kg ip negative Degraeve &
assay mice strain Q 7 weeks 0.6 mg/litre Moutschen
drinking-water, equiv- (1983)
alent to 0.093 mg/kg
body weight
Dominant lethal test 30 & 60 mg/kg body negative Fischer &
- AB Jena Halle mice 5 x 6 mg/kg body weight Scheufler
ip (1981)
DBA mice 3 x 18 mg/kg body weight
ip
Micronucleus test - 2 equal doses of a significant increase in Usha Rani et
mice - bone marrow 51.7 mg/litre drink- frequency of polychromatic al. (1980)
ing-water at 24-h erythrocytes with micronuclei
intervals -0.85% (controls 0.28%)
Chromosome abnormalities 50 and 100 mg/kg body positive Bhunya & Behera
mice - bone marrow weight (1975)
Mice - bone marrow 20 mg/kg body weight no abnormalities Nehés et al.
ip (1982)
Mice - bone marrow 60 mg/kg body weight increase in number of mitosis, Nehéz et al.
ip aberrations (1983)
Syrian golden hamsters 16, 32, 80, or increase in number of chromatid Dzwonkowska &
Mesocricetus auratus 160 mg/kg body weight and chromosomal breaks Hübner (1986)
ip single (no dose-response relationship)
---------------------------------------------------------------------------------------------------------
8.7. Carcinogenicity
Two carcinogenicity studies, reported by Gibel et al.
(1973), are summarized below.
Rat: Groups of 40, 10-week-old Wistar rats were intubated
with 0 (control), 5, 10, or 30 mg dimethoate/kg body weight
(twice weekly) for the life span. The mean life spans were 743,
518, 511, and 627 days at 0, 5, 15, and 30 mg/kg body weight,
respectively. No malignant tumours were observed in the 36
control animals. In test groups, malignant tumours occurred
in 2/26, 3/25, and 4/20 rats, respectively, at the 3 dose
levels. At 5 mg/kg body weight, a reticulosarcoma of the spleen
with metastases and a malignant reticuloma were observed. At
15 mg/kg body weight, a sarcoma of the colon, a reticulosarcoma
of the spleen (with pancreatic metastases) and a hepatocellular
carcinoma occurred. At 30 mg/kg body weight, a liver fibro-
sarcoma, a malignant reticuloma and two reticulosarcomas of the
spleen were noted. Three, 7, 5, and 2 benign tumours were
noted, respectively, in the controls and at 5, 15, and 30 mg/kg
body weight (Gibel et al., 1973).
Rat: Groups of 40, ten-week-old Wistar rats were injected
im with 15 mg dimethoate/kg body weight (twice weekly) or with
isotonic saline until spontaneous death occurred. The mean life
spans were 711 and 570 days in the saline and dimethoate-treated
animals, respectively. No malignant tumours occurred in the
saline-treated group, but 4/35 animals developed benign tumours.
In the dimethoate group, 6/30 rats had malignant tumours (a
spleen reticulosarcoma, a spleen fibrosarcoma, an ovarian
alveolar sarcoma, a liver hepatocellular carcinoma, a malignant
reticuloma, and a soft tissue spindle-cell sarcoma) and 5/30 had
benign tumours. The first malignant tumour (a splenic
fibrosarcoma) was noted after 410 days (Gibel et al., 1973).
Dimethoate (technical grade, 90-100% pure) was given in the
feed to Osborne Mendel rats and to B6C3F1 mice. Groups of 50
male and 50 female rats (10 animals in each control group)
received "time-weighted average doses" of 155 or 310 mg/kg for
male rats and 192 or 384 mg/kg for female rats. After 80 weeks,
all groups received control diets, and the studies were con-
cluded at week 114.
Groups of 50 male and 50 female B6C3F1 mice were given
dimethoate in the diet at concentrations of 0 (10 "matched"
controls), 250, or 500 mg/kg for 60-69 weeks (males) or for 80
weeks (females). The studies were ended after 94 weeks.
Tremors and hyperexcitability were observed in exposed animals;
rats and mice that survived to termination were generally in
poor condition. Survival was reduced in the high-dose groups of
rats. Several non-neoplastic lesions occurred more frequently
in dimethoate-exposed animals. No increases in neoplasia were
reported to be associated with dimethoate administration, for
any of the organs or tissues examined histologically (NCI-1977).
These studies are not considered adequate to determine properly
the presence or absence of a carcinogenic response, largely
because of the shorter than usual duration of exposures, the
poor condition of the animals, and changes in exposure concen-
trations.a
8.8. Special Studies
The action of dimethoate on the immune system was studied
in mice and rats by Tiefenbach & Lange (1980). A single dose of
75 mg dimethoate/kg (route not specified) decreased the
lymphocyte count to 50% of the pre-exposure value and increased
the neutrophile granulocytes. After 72 h, these changes
returned to normal. A reduction in the thymus cortex with
disrupted lymphocytes, and a reduction in the number of rosette-
forming cells were observed.
When administered to rats at 5-30 mg/kg body weight orally
or 15 mg/kg im, twice a week, until death, dimethoate caused
hyperplasia in the bone marrow, mainly in granulocytopoiesis
(Stieglitz et al., 1974). The authors interpreted the changes
in the haematopoietic system of the rats as a direct haemato-
logical effect of dimethoate.
The effects of dimethoate on the heart were investigated
in rabbits (Mahkambaeva, 1971), and guinea-pigs and rats
(Nadmaiteni & Marosi, 1983). After oral administration of
150 mg dimethoate/kg to rabbits, the effects observed included
bradycardia and increased atrio-ventricular and intraventricu-
lar conductance, with complete recovery after 4-7 days. In rats
and guinea-pigs, a dose-effect relationship was established for
heart rate disturbances, and atrio-ventricular block. An
electron-microscopic study of the myocard did not reveal any
changes. The ip doses that were tested ranged from 500 to
1500 mg/kg body weight.
----------------------------------------------------------------
a After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious ECG disturbances
developed in the early phase of intoxication. The toxic cardiac
phenomena appeared to be unrelated to the degree of cholin-
esterase inhibition, but were correlated with the myocardial
dimethoate concentration. Cardiac failure and mortality were
first observed at a level of about 110 µg/g, while a level of
221 µg/g resulted in death in all cases. The present investi-
gation refers to the direct effect of dimethoate on the
myocardium, independent of its anticholinesterase action (Marosi
et al., 1985a,b).
8.9. Factors Modifying Toxicity
The acute toxicity of dimethoate was not affected by the
dietary content of protein (Boyd & Muis, 1970). The oral LD50
was 147 mg/kg for male albino rats fed for 28 days from weaning
on a diet containing 3.5% protein as casein and, in controls
maintained on a diet containing 26% casein at 152 mg/kg. Other
groups maintained on a diet that contained 24% protein from
various plant and animal sources were apparently less suscep-
tible to dimethoate (LD50, 358 mg/kg). Dimethoate was given to
rats orally for 10 weeks at a dose of 10 mg/kg in diets contain-
ing 90, 160, or 240 g protein/kg (Gontzea & Gorcea, 1977). The
high-protein (240 g) diet diminished the adverse effects of
dimethoate on the growth of the rats and lessened its antichol-
inesterase activity in plasma, total blood, brain, and liver.
A marked increase in the toxicity of dimethoate was noted
in male and female mice after pre-treatment with phenobarbital
and with chlorinated hydrocarbons (DDT and dieldrin) (Menzer &
Best, 1968; Menzer, 1970). The toxicity of dimethoate was
increased from an ip LD50 of 198 mg/kg body weight to 58.5 mg/kg
by pre-treatment of mice for 3 days with sodium phenobarbital.
Liquid formulations of technical dimethoate in the
solvents, 2-methoxy- and 2-ethoxyethanol, showed increased tox-
icity after storage. After storage for 7 months in England and
9 months under tropical conditions, the oral LD50 for the rat
decreased to 30-40 mg/kg and 15 mg/kg, respectively (from an
initial 150-250 mg/kg). The most toxic conversion product was
O,O-dialkyl S-(N-methylcarbamoylmethyl) phosphorothioate with
an oral LD50 for the rat of 1 mg/kg (Casida & Sanderson, 1961).
8.10 Mechanisms of Toxicity; Mode of Action
The mode of action of organophosphorus insecticides is
decribed in WHO (1986a). They act principally by inhibition of
acetyl cholinesterase (AChE) at the cholinergic synapses.
Dimethoxon (omethoate), a metabolite of dimethoate, is
75-100 times more potent than dimethoate in inhibiting AChE,
suggesting that this metabolite plays a dominant role in
mammalian toxicity (Hassan et al., 1969). The LD50 of omethoate
in rats is 25-28 mg/kg body weight (FAO/WHO, 1979); it is about
10 times more toxic than dimethoate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
Cases of both accidental and suicidal poisoning have been
reported with dimethoate. Hayes (1982) has reviewed the human
toxicity and poisoning cases.
Nagler et al. (1980) described a case of attempted suicide
of a 34-year-old female who ingested 10 g dimethoate. Half an
hour after admission to the hospital, the serum-dimethoate level
was 2340 mg/litre. Combined haemoperfusion and haemodialysis
were applied and, after 18 h, dimethoate was no longer detected
in the serum.
A severe case of poisoning after ingestion of approximately
20 g dimethoate was reported by Köppel et al. (1986). On
admission, the 52-year-old man was comatose with unmeasurable
pseudo-cholinesterase (< 200 U/litre). He had been admitted 2 h
after ingestion and received, every 20 min, an injection of
20 mg atropine. Two haemoperfusions with activated charcoal and
amberlite were performed, and atropine was given by infusion up
to day 12. Twenty-five days after admission, he was discharged,
fully recovered.
De Reuck et al. (1979) presented a case of a patient who
died on the 9th day after dimethoate poisoning with an atypical
central neurological disorder. The neuropathological findings,
which were similar to those observed in severe forms of
Wernicke's encephalopathy, included severe haemorrhagic necrosis
of the walls of the ventricles. The authors suggested that the
increased level of acetylcholine in the brain had led to
thiamine depletion in the regions of predilection of Wernicke's
encephalopathy.
Many other cases of human dimethoate poisoning, some of
which were fatal, have been reported, but the information given
was insufficient (Molphy & Rathus, 1964; Masiak & Olajossy,
1973; François & Verbraeken, 1977; Demeter & Heyndrickx, 1978;
Ebel & Karyofilis, 1978; Areekul et al., 1981; Wehran & Hammer,
1984; Bolgar et al., 1985; Le Blanc et al., 1986; Senanayake &
Karalliedde, 1987).
On the basis of a number of cases, the oral lethal dose for
human beings was estimated to be of the order of 50-500 mg/kg
body weight (Gosselin et al., 1984).
Trinh van Bao et al. (1974) reported an increase in the
frequency of breaks and stable chromosome aberrations in 2
patients who died after dimethoate intoxication.
Thirty firemen, exposed to dimethoate in the air as a
result of an accident in a dimethoate-manufacturing plant,
developed symptoms of intoxication (Larripa et al., 1979, 1983).
Peripheral blood lymphocytes from 20 of these workers were
examined for the frequency of sister chromatid exchanges, 2
months after the accident. The frequencies were 9.2 ± 0.2 for
the exposed and 8.5 ± 0.2 for unexposed persons ( P < 0.05).
Dicentric chromosomes and a low frequency of chromatid breaks
were found in 2 exposed workers. It is not certain to what the
firemen were exposed besides dimethoate.
9.1.2 Controlled human studies
The results of a number of studies in which human volun-
teers, without occupational exposure to organophosphates, were
given dimethoate, are summarized in Table 12. From these
studies it can be concluded that repeated doses of up to
0.2 mg/kg body weight did not inhibit cholinesterase activity in
the blood.
9.2 Occupational Exposure
9.2.1 Poisoning incidents
One of the first cases of poisoning with dimethoate (Rogor)
in agriculture was described by Muratore et al. (1960). A 28-
year-old-farmer, who reportedly had worn protective rubber
clothing and equipment, had sprayed olive trees with dimethoate
the day before he was hospitalized. The man began to experience
profound weakness, faintness, and somnolence, followed by
attempts to vomit, chills, and profound prostration on the day
he was hospitalized. His general condition was found to be
serious; his pulse was weak, he exhibited pronounced myosis,
vomited and sweated profusely, and had pronounced inhibition of
cholinesterase activity. Treated with large doses of atropine
(20 mg on day 2, 12 mg on day 3, and 5 mg until day 9),
prednisone, analgesics, and penicillin, he recovered.
A case of poisoning in a woman working in agriculture and
exposed to dimethoate in the field, 2 days after spraying, was
reported by Asatryan (1971). Within 3-3.5 h after beginning
work, the woman noted an unpleasant odour recognized as
dimethoate. She developed headache, dry cough, dyspnoea,
nausea, vomiting and was admitted to hospital in a somnolent
state with muscular fibrillations and an asthmatic component.
After treatment with saline solution, glucose, caffeine,
atropine, and insulin, the state of acute intoxication was
overcome. Allergic symptoms were treated with dimedrol.
Contact allergy due to dimethoate was reported in a 53-
year-old female. She had a positive skin test (Pambor & Bloch,
1985).
Table 12. Controlled human studies
---------------------------------------------------------------------------------------------------------
Number Sex Route of Dose/day Duration of Results Reference
of Admini- exposure
subjects stration
---------------------------------------------------------------------------------------------------------
20 oral 0.04 mg/kg 4 weeks absence of toxic effects Sanderson & Edson
or blood-ChE inhibition (1964)
2 oral 0.13 mg/kg; 21 days absence of blood-ChE Sanderson & Edson
0.26 mg/kg inhibition (1964)
5 M oral 0.25 mg/kg single dose absence of toxic effects Sanderson & Edson
or ChE inhibition (1964)
50 dermal 32% liquid form- 2-h patch absence of skin irritation Sanderson & Edson
ulation, up to test and ChE inhibition (1964)
2.5 ml
12 M/F oral 5 mg (0.068 28 days no significant change in Edson et al. (1967)
mg/kg body weight)
9 M/F oral 15 mg (0.202 39 days no significant change in Edson et al. (1967)
mg/kg body weight)
8 M/F oral 30 mg (0.434 57 days inhibition of ChE by the Edson et al. (1967)
mg/kg body weight)
6 oral 45 mg (0.587 45 days inhibition of ChE (35%) Edson et al. (1967)
mg/kg body weight)
6 M/F oral 60 mg (1.02 14 days inhibition of ChE (21%) Edson et al. (1967)
mg/kg body weight)
---------------------------------------------------------------------------------------------------------
Female greenhouse workers working with dimethoate were
reported to have a high percentage of specific leukocyte
agglomeration, a raised index of lymphoblastic transformations,
and antibodies against dimethoate (Zolotnikova & Somov, 1978;
Zolotnikova, 1980). With increasing duration of work, progress-
ive sensitization towards the pesticide was observed.
On the basis of epidemiological observations and of dermal
testing of workers with a 1-2% solution of dimethoate, Jung
(1979) concluded that the index of sensitization was very low
for dimethoate and that general intoxication might occur, but
rarely contact eczema.
9.3 Early Symptoms and Treatment of Poisoning
Initial symptoms of poisoning may include sweating,
headache, weakness, giddiness, nausea, vomiting, stomach pains,
blurred vision, slurred speech, and muscle twitching (WHO,
1986a). Arrhythmias and cardiac failure have been reported
(Kiss & Fazekas, 1983; Marosi et al., 1985a,b; Duval et al.,
1986). The most important diagnostic finding is inhibition of
blood-cholinesterase activity. For a full discussion of the
clinical picture and treatment of organophosphate insecticide
poisoning, see WHO (1986a). The section on treatment is
presented in the annex to this publication.
In all cases of clinical poisoning with dimethoate and
other organophosphorus insecticides, it is essential to maintain
general surveillance and cholinesterase and cardiac monitoring
for at least 4 days, and longer if necessary, and to adapt
general supportive and specific therapy in accordance with the
findings.
Data on the effects of oxime reactivators in dimethoate
poisoning are contradictory, some indicating that they may have
a negative effect on cholinesterase inhibition (Sanderson &
Edson, 1959; Durham & Hayes, 1962; Molphy & Rathus, 1964;
Erdmann et al., 1966; Zech et al., 1966; Ebel & Karyofilis,
1978; Sterri et al., 1979). It is therefore suggested, that if
oxime reactivators are indicated, these should be used with
caution and under close supervision.
Haemoperfusion may be effective in the early stages of
dimethoate poisoning (Okonek 1976, 1977; Okonek et al., 1976;
Pach et al., 1977; Nagler et al., 1980).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Toxicity of Dimethoate
Dimethoate is moderately toxic. Most oral LD50s in the rat
ranged from 150-400 mg/kg body weight. The dermal LD50 in the
rat was greater than 600 mg/kg body weight. Dimethoate is not
irritating to the skin, but may be slightly irritating to the
eye.
It can be absorbed following ingestion, inhalation, and
skin contact. It is readily metabolized and excreted and does
not accumulate in the body. Dimethoxon (omethoate), the oxygen
analogue found in plants, insects, and mammals, is about 10
times more toxic and is a more potent inhibitor of cholin-
esterase activity than dimethoate.
Signs of intoxication from exposure to dimethoate are those
typical of organophosphorus pesticides. Inhibition of erythro-
cyte-cholinesterase is the most sensitive indicator of exposure
to dimethoate and may indicate toxicity. A dietary level of
5 mg dimethoate/kg, equivalent to 0.25 mg/kg body weight, is
considered to be a no-observed-adverse-effect level in the
rat.a
A 5-generation reproduction study in mice given drinking-
water containing dimethoate at 60 mg/litre resulted in decreased
mating success and increased reproduction time in all gener-
ations. On the basis of available data on experimental animals,
dimethoate is not considered to be a teratogen.
Dimethoate is considered mutagenic in a variety of in vitro
and in vivo test systems.b Long-term studies have been
conducted involving oral administration in rats and mice and im
injection in rats. However, the available data are considered
---------------------------------------------------------------
a FAO/WHO (1987) concluded that a dietary level of 1 mg/kg,
equivalent to 0.05 mg/kg body weight is the no-observed-
adverse-effect level in the rat.
b After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
to be inadequate to assess the carcinogenic potential of
dimethoate.c
10.2. Human Exposure
Air and water are negligible sources of exposure to
dimethoate for the general population. Residues found in food
are generally below the acceptable daily intake (ADI) set by the
FAO/WHO Joint Meeting on Pesticide Residues (JMPR) (See section
12).
Whole blood-cholinesterase was not inhibited in human
volunteers given oral doses of 0.2 mg dimethoate/kg body weight
for 39 days.
Several cases of suicidal or accidental poisoning due to
ingestion of dimethoate have been reported.
Occupational exposure to dimethoate, principally through
inhalation and the skin, may occur during its manufacture,
formulation, and use, and cases of poisoning as a result of
accident or neglect of safety precautions have been reported.
The oral lethal dose for human beings has been estimated to be
in the range of 50-500 mg/kg body weight.
Skin sensitization due to dimethoate has been observed in
some cases.
10.3. Evaluation of the Effects on the Environment
Dimethoate is rapidly hydrolysed and does not persist in
the environment. The toxicity of dimethoate for aquatic
organisms and birds is moderate to high. It is very toxic for
honey-bees.
---------------------------------------------------------------
c After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
10.4. Conclusions
1. Under proper conditions of use, exposure of the general
population to dimethoate is negligible.
2. When appropriate safety precautions are observed, exposure
to dimethoate during manufacture, formulation, use, and
disposal should not pose an unacceptable human health
hazard.
3. Dimethoate is rapidly degraded and not persistent in the
environment. Care must be taken not to expose honey-bees,
fish and aquatic organisms, and birds.
11. RECOMMENDATIONS
1. Figures relating to the current production and use of
dimethoate should be made available.
2. Studies are necessary on dermal sensitization by
dimethoate.
3 Data are required on the absorption and disposition of
dimethoate from different routes of exposure.
4. Long-term toxicology and carcinogenicity studies should be
carried out on laboratory animals.a
5. More research is required to investigate the in vivo
mutagenic effects of dimethoate.a
6. Reproduction and teratology studies should be carried
out.a
7. Epidemiological studies on persons engaged in the
manufacture and professional use of dimethoate should be
considered, and workers should be monitored for exposure to
dimethoate and for potential adverse health effects.
8. Cleaning and disposal of contaminated equipment, clothing,
and containers should be in accordance with recommended
procedures.
9. Further work is necessary to establish safe re-entry
periods under different conditions of use.
10. Acute toxicity studies should be carried out on
formulations in which dimethoate is mixed with other active
ingredients.
11. The role of oxime reactivators in the treatment of human
poisoning by dimethoate, should be clarified.
12. Information should be obtained concerning the changes in
toxicity, due to impurities, that can arise in pesticides
as a consequence of different manufacturing processes, the
use of formulating ingredients, and improper storage.
---------------------------------------------------------------
a Several of these studies were later submitted to the
FAO/WHO Joint Meeting on Pesticide Residues in 1987. See
previous footnotes for sections 8.5, 8.6 and 8.7.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dimethoate was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1963, 1965, 1966, 1967, 1970, 1973
(evaluation of the related compound formothion), 1977, 1978,
1984, and 1987 (FAO/WHO, 1964, 1965, 1967, 1968, 1971, 1974,
1978, 1979, 1985, 1987).
The estimate of an acceptable daily intake (ADI) for man
for dimethoate is 0-0.01 mg/kg body weight, based on the no-
observed-adverse-effect level in man of 0.2 mg/kg body weight
per day, and in the rat of 1 mg/kg diet, equivalent to
0.05 mg/kg body weight (FAO/WHO, 1987).
A data sheet on dimethoate has been issued by WHO in the
series "Data Sheets on Pesticides" (WHO/FAO, 1980).
In the WHO Recommended Classification of Pesticides by
Hazard, technical dimethoate is classified as moderately
hazardous, when handled in accordance with instructions (WHO,
1986b).
The IRPTC (1982) has issued a review on dimethoate, in its
series "Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals".
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-
67. III. Organophosphorus pesticide residues in the total diet.
Pestic. Sci., 1: 10-13.
ABRAMOVA, J.I. & BOLTUSHKINA, L.A. (1968) [Safety action of
several vitamins in poisoning with organophosphorus insecti-
cides.] In: [Hygiene and toxicology of pesticides and the
clinical picture of intoxications,] Vol. 6, Kiev, Zdorovye
Publishers, pp. 448-452 (in Russian).
ADAMIS, Z., ANTAL, A., FUZESI, I., MOLNAR, J., NAGY, L., &
SUSAN, M. (1985) Occupational exposure to organophosphorus
insecticides and synthetic pyrethroids. Int. Arch. occup.
environ. Health, 56: 299-305.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AKORONKO, S.L. & GIRENKO, D.B. (1977) [Methods of estimating
fosfamid in soil using chromatographic methods.] In: [ Methods
of determination of micro-quantities of pesticides in foods,
feed, and the environment,] Moscow, Kolos, pp. 118-120 (in
Russian).
ANEES, M.A. (1975) Acute toxicity of four organophosphorus
insecticides for a fresh-water teleost Channa punctatus
(Bloch). Pak. J. Zool., 7(2): 135-141.
ANEES, M.A. (1978a) Hepatic pathology in a fresh-water teleost
Channa punctatus (Bloch) exposed to sub-lethal and chronic
levels of three organophosphorus insecticides. Bull. environ.
Contam. Toxicol., 19(5): 524-527.
ANEES, M.A. (1978b) Haematological abnormalities in a fresh-
water teleost Channa punctatus (Bloch) exposed to sublethal and
chronic levels of three organophosphorus insecticides. Int. J.
Ecol. environ. Sci., 4: 53-60.
AREEKUL, S., SRICHAIRAT, S., & KIRDUDOM, P. (1981) Serum and
red cell cholinesterase activity in people exposed to organo-
phosphorus insecticides. Southeast Asian J. trop. Med. pub.
Health, 12(2): 94-98.
ARMELI, G., BARTALINI, E., & PISANI, F. (1967) [Occupational
pathology and prevention in the industrial production of rogor].
Lav. Um., 20(8): 355-356 (in Italian).
ASATRYAN, R.S. (1971) [Some peculiarities of acute pesticide
poisonings in application in Ararat land.] In: [ Hygiene and
toxicology of pesticides and the clinical picture of intoxica-
tions,] Kiev, Zdorovye Publishers, pp. 315-318 (in Russian).
ATABAEV, SH.T. (1972) [Rogor (phosphamide).] In: [ Pesticides
and environmental hygiene in hot climate conditions,] Tashkent,
Medizina, pp. 77-93 (in Russian).
ATABAEV, SH.T. & STEPOVAYA, N.E. (1966) [Allowance of phospha-
mide in apples.] In: [ Hygiene and toxicology of pesticides and
the clinical picture of intoxications,] Kiev, Zdorovye Publish-
ers, pp. 280-285 (in Russian).
AWASTHI, M., SHAH, P., DUBALE, M.S., & GADHIA, P. (1984) Meta-
bolic changes induced by organophosphates in the piscine organs.
Environ. Res., 35(I): 320-325.
BARANOVA, N.P., ALEXANDROVA, L.G., & KOVALENKO, V.F. (1986)
[Tissue distribution and biochemical effects of dimethoate
entering the body via the skin.] Gig. tr. prof. Zabol., 3: 20-
23 (in Russian).
BARKER, R.J., LEHNER, Y., & KUNZMANN, M.R. (1980) Pesticides
and honey bees: nectar and pollen contamination in alfalfa
treated with dimethoate. Arch. environ. Toxicol., 9: 125-133.
BECK, E.W., JOHNSON, J.C., Jr, GETZ, M.E., SKINNER, F.B.,
DAWSEY, L.H., WOODHAM, D.W., & DERBYSHIRE, J.C. (1968) Effects
of feeding dimethoate, its oxygen analog, and dimethoate-treated
silage to cattle. J. econ. Entomol., 61(3): 605-610.
BECKER, H. (1985) Dominant lethal study with dimethoate
technical in the mouse. (Unpublished report no. 039003, dated 12
September 1985 from Research & Consulting Company AG, 4452
Itingen/Switzerland).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Contr., 9(3):
119-127.
BHUNYA, S.P. & BEHERA, J. (1975) Centromeric sensitivity of
mouse chromosomes to the systemic insecticide dimethoate
(Rogor). Curr. Sci., 44(23): 859-860.
BOHN, W.R. (1964) The disappearance of dimethoate from soil.
J. econ. Entomol., 57(6): 798-799.
BOLGAR, G., JOJART, G., & TURI, J. (1985) [Spontaneous delivery
of a pregnant woman after dimethoate (Bi 58 EC) poisoning.]
Orvosi Hetilap, 126(52): 3213-3214 (in Hungarian).
BOLOTNYI, A.V., PISMENNAYA, M.V., & AKORONKO, S.L. (1978)
[Transformation of phosphororganic pesticides antio and chloro-
phose in the environment.] Gig. i Sanit., 43(5): 28-31 (in
Russian).
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol-water
partitioning coefficient (Kow) of 61 organophosphorus and carba-
mate insecticides and their relationship to respective water
solubility (S) values. J. environ. Sci. Health, BI8(6): 667-
683.
BOYD, E.M. & MUIS, L.F. (1970) Acute oral toxicity of dimetho-
ate in albino rats fed a protein deficient diet. J. pharm.
Sci., 59: 1098-1102.
BRADY, U.E., Jr & ARTHUR, B.W. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. econ.
Entomol., 56(4): 477-482.
BUDREAU, C.H. & SINGH, R.P. (1973) Effect of fenthion and
dimethoate on reproduction in the mouse. Toxicol. appl. Pharma-
col., 26: 29-38.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1968) Organo-
phosphorus poisoning, Diagnosis of poisoning in pheasants owing
to a number of common pesticides, J. agric. food Chem., 16(2):
332-339.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1969) Organo-
phosphorus poisoning, Chronic feeding of some common pesticides
to pheasants and pigeons, J. agric. food Chem., 17(5): 1027-
1032.
CABRAS, P., MELONI, M., & PIRISI, F.M. (1979) Separation of
insecticides by reversed-phase high-performance liquid chroma-
tography. J. Chromatogr., 176: 473-477.
CARMAN, G.E., IWATA, Y., PAPPAS, J.L., O'NEAL, J.R., & GUNTHER,
F.A. (1982) Pesticide applicator exposure to insecticides
during treatment of citrus trees with oscillating boom and
airblast units. Arch. environ. Contam. Toxicol., 11: 651-659.
CASIDA, J.E. & SANDERSON, D.M. (1961) Toxic hazard from formu-
lating the insecticide dimethoate in methyl "Cellosolve".
Nature (Lond.), 189(4763): 507-508.
CHEN, H.H., HSUEH, J.L, SIRIANNI, S.R., & HUANG, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res., 88(3): 307-316.
CHILWELL, E.D. & BEECHAM, P.T. (1960) Residues of O,O-
dimethyl- S-(N-methylcarbamoylmethyl) phosphorothiolothionate
(dimethoate) in sprayed crops. J. Sci. Food Agric., 11: 400-
407.
CONGREGADO, F., SIMON-PUJOL, D., & JUAREZ, A. (1979) Effect of
two organophosphorus insecticides on the phosphate-dissolving
soil bacteria. Appl. environ. Microbiol., 37(1): 169-171.
COPPLESTONE, J.F., FAKHRI, Z.I., MILES, J.W., MITCHELL, C.A.,
OSMAN, Y., & WOLFE, H.R. (1976) Exposure to pesticides in
agriculture: a survey of spraymen using dimethoate in the Sudan.
Bull. World Health Organ., 54(2): 217-223
COURTNEY, K.D., ANDREWS, J.E., SPRINGER, J., & DALLEY, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. environ. Sci. Health, B20(4): 373-406.
DALELA, R.C., BHATNAGAR, M.C., TYAGI, A.K., & VERMA, S.R.
(1979) Histological damage of gills in Channa gachua after
acute and subacute exposure to endosulfan and Rogor. Mikroskopie
(Vienna), 35: 301-307.
DAUTERMANN, W.C., CASIDA, J.E., KNAAK, J.B., & KOWALCZYK, T.
(1959) Bovine metabolism of organophosphorus insecticides.
Metabolism and residues associated with oral administration of
dimethoate to rats and three lactating cows. J. agric. food
Chem., 7(3): 188-193.
DEDEK, W., GRAHL, R., & SCHMIDT, R. (1984) A comparative study
of guanine N7-alkylation in mice in vivo by the organophosphorus
insecticides trichlorphon, dimethoate, phosmet and bromophos.
Acta pharmacol. toxicol., 55: 104-109.
DEGRAEVE, N. & MOUTSCHEN, J. (1983) Genotoxicity of an organo-
phosphorus insecticide, dimethoate, in the mouse. Mutat. Res.,
119: 331-337.
DEGRAEVE, N., CHOLLET, M.-C., & MOUTSCHEN, J. (1984) Evaluation
of the mutagenic potential of four commercial mixtures of insec-
ticides. Food chem. Toxicol., 22(8): 683-687.
DEMETER, J. & HEYNDRICKX, A. (1978) Two lethal endosulfan
poisonings in man. J. anal. Toxicol., 2(2): 68-74.
DE PIETRI-TONELLI, P., BAZZI, B., & SANTI, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Rev., 11: 60-99.
DE REUCK, J., COLARDYN, F., & WILLEMS, J. (1979) Fatal
encephalopathy in acute poisoning with organophosphorus
insecticides. A clinicopathologic study of two cases. Clin.
Neurol. Neurosurg., 81(4): 247-254.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K.,
MUYS, T., & VAN DER POLL, J.M. (1984) Pesticides and other
chemical residues in Dutch total diet samples (June 1976 - July
1978). Food chem. Toxicol., 22(1): 11-21.
DIKSHITH, T.S.S. (1986) Pesticides. In: Toxicology of
pesticides in animals, Boca Raton, Florida, CRC Press, Vol. I,
Chapter 2.
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981a) Toxicity evaluation of
dimethoate technical in fish (Report to Shaw Wallace & Co.,
India).
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981b) Toxicity evaluation
of dimethoate technical in animals: Primary skin irritation and
irritation of mucous membrane in rabbits (Report to Shaw Wallace
& Co., India).
DUGGAN, R.E., CORNELIUSSEN, P.E., DUGGAN, M.B., MCMAHON, B.M., &
MARTIN, R.J. (1983) Pesticide residue levels in foods in the
United States from July 1, 1969 to June 30, 1976, Washington,
DC, US Food and Drug Administration/Association of Official
Analytical Chemists (published jointly), pp. 13-24.
DURHAM, W.F. & HAYES, W.J. (1962) Organic phosphorus poisoning
and its therapy. Arch. environ. Health, 5: 21-47.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the exposure
to pesticides. Bull. World Health Organ., 26: 75-91.
DUVAL, G., CORBIN, J.C., & MIMRAN, C. (1986) Etude
hémodynamique des intoxications aiguës graves par insecticides
organophosphorés (7 cas). Coeur et Toxiques: XXIVèmes Journées
du Groupement francais de CAP, Strasbourg, 24-25 septembre
1986.
DZWONKOWSKA, A. & HÜBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in
vivo. Arch. Toxicol., 58: 152-156.
EBEL, J. & KARYOFILIS, A. (1978) [Unusual course of poisoning
with dimethoate, an organic phosphorus compound.] Ther. Ggw.,
117(2): 226-236 (in German).
EDSON, E.F. & NOAKES, D.N. (1960) The comparative toxicity of
six organophosphorus insecticides in the rat. Toxicol. appl.
Pharmacol., 2: 523-539.
EDSON, E.F., JONES, K.H., & WATSON, W.A. (1967) Safety of
dimethoate insecticide. Br. med. J., 4(5578): 554-555.
EDWARDS, J.A., LEEMING, N.M. & CLARK, R. (1984) Effect of
dimethoate on pregnancy of the rat (Unpublished report No. DTF
3/84245 dated 13 April 1984 from Huntingdom Research Centre).
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977a) Fac-
tors affecting the fate of dimethoate in soils. Intern. J.
environ. Stud., 11: 113-124.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977b) Fac-
tors affecting the accumulation of dimethoate in soil. Int. J.
environ. Stud., 11: 187-196.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1978) Factors
influencing the degradation of dimethoate in soils and solu-
tions. Int. J. environ. Stud., 11: 253-260.
ERDMANN, W.D., ZECH, R., FRANKE, P., & BOSS, I. (1966) [The
question of the therapeutic efficacy of esterase reactivators in
dimethoate poisoning.] Arzneimittelforschung, 16: 492-494 (in
German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesti-
cides. In: Montesano, R., Tomatis, L., & Davies, W., ed.
Chemical carcinogenesis: essays, Lyons, International Agency
for Research on Cancer, pp. 161-181 (IARC Scientific
Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Dimethoate. In: Evaluation of toxicity of
pesticide residues in food, Rome, Food and Agricultural
Organization of the United Nations, pp. 97-101 (FAO Meeting
Report No. PL/1965/10/1. WHO/Food Add./27.65)
FAO/WHO (1967) Dimethoate. In: Evaluation of some pesticide
residues in food, Rome, Food and Agricultural Organization of
the United Nations, p. 232.
FAO/WHO (1968) Dimethoate. In: 1967 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 117-132 (FAO/PL: 1967/M/11/1.
WHO/Food Add./68.30).
FAO/WHO (1971) Dimethoate. In: 1970 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 239-244 (AGP: 1870/M/12/1. WHO/Food
Add./71.42).
FAO/WHO (1974) Formothion. In: 1973 Evaluations of some pesti-
cide residues in food, Geneva, World Health Organization, pp.
281-290 (WHO Pesticide Residues Series, No. 3).
FAO/WHO (1978) Dimethoate. In: 1977 Evaluations of some pesti-
cide residues in food, Rome, Food and Agriculture Organization
of the United Nations, pp. 155-156 (FAO Plant Production and
Protection Paper No. 10 Sup.).
FAO/WHO (1979) Formothion, dimethoate, omethoate. In: 1978
Evaluations of some pesticide residues in food, Geneva, World
Health Organization, p. 6.
FAO/WHO (1984) Maximum residue limits for pesticides, Codex
Alimentarius Commission, Rome, Food and Agricultural
Organization of the United Nations, Vol. 13.
FAO/WHO (1985) Pesticide residues in food. Report of the 1984
Joint Meeting on Pesticide Residues, Rome, Food and Agricultural
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 62).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FAO/WHO (1987) Dimethoate. In: Pesticide residues in food.
Report of the 1987 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Rome, Food and Agricultural Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 84).
FARM CHEMICALS HANDBOOK (1984) Willoughby, Ohio Meister
Publishing Company.
FETCHER, A. (1984) Suspected dimethoate toxicity in cattle.
Mod. vet. Pract., 65(4): 283-285.
FISCHER, G.W. & SCHEUFLER, H. (1981) [Effects of dimethoate
and O-desmethyldimethoate on dominant lethal test in mice.]
Wiss. Z. Univ. Halle, 30(6): 21-29 (in German).
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity
of organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Health Sci., B20(1): 73-95.
FRANCOIS, J. & VERBRAEKEN, H. (1977) Glaucome aigu après
intoxication par un ester organo-phosphatique Bull. Soc. Belge
Ophthalmol., 176: 19-22.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14(3): 515-534.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1978 - September
1979. J. Assoc. Off. Anal. Chem., 68(5): 842-861.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1978 - September 1979. J.
Assoc. Off. Anal. Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985c) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1979 - September
1980. J. Assoc. Off. Anal. Chem., 68(6): 1163-1183.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985d) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1979 - September 1980. J.
Assoc. Off. Anal. Chem., 68(6): 1184-1197.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1980 - March
1982. J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1980 - March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 146-161.
GETZIN, L.W. & ROSEFIELD, I. (1968) Organophosphorus insecti-
cide degradation by heat-labile substances in soil. J. agric.
food Chem., 16(4): 598-601.
GIBEL, W., LOHS, KH., WILDNER, G.P., ZIEBARTH, D., & STIEGLITZ,
R. (1973) [Experimental study on cancerogenic, haematotoxic,
and hepatotoxic activity of phosphor-organic pesticides.] Arch.
Geschwulstforsch., 41(4): 311-328 (in German).
GILOT - DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN -
DAHMEN, M. (1983) Mutagenicity of some organophosphorus com-
pounds at the ade6 locus of Schizosaccharomyces pombe. Mutat.
Res., 117: 139-148.
GIRENKO, D.B. & KLISENKO, M.A. (1977) [Methods of estimating
antio and fosfamid in fruits by gas-liquid chromatography.] In:
[ Methods of determination of microquantities of pesticides in
foods, feed, and the environment,] Moscow, Kolos, pp. 117-118
(in Russian).
GIRENKO, D.B., KLISENKO, M.A., & PISMENNAYA, M.B. (1978)
[Methods of estimating organophosphorus pesticides in water by
chromatography methods.] In: [ Methods of determination of
microquantities of pesticides in foods, feed, and the
environment,] Moscow, Kolos, pp. 57-62 (in Russian).
GONTZEA, I. & GORCEA, V. (1977) The influence of protein and
fat intake on the body's resistance to dimethoate. Med. Lav.,
68(1): 13-21.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E.
(1984) Clinical toxicology of commercial products, 5th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
GRIGORASHVILI, G.S. & DZHIBLADZE, V.E. (1965) [Hygienic
evaluation of some food products after treatment with
phosphamide.] In: [ Hygiene and toxicology of pesticides, and
clinical aspects of pesticide poisoning,] Kiev, Zdorovye
Publishers, pp. 161-164 (in Russian).
HADJIDEMETRIOU, D.G., IWATA, Y., & GUNTHER, F.A. (1985)
Analysis and dissipation of dislodgable residues of acephate,
dimethoate and formetanate hydrochloride on citrus foliage.
Pestic. Sci., 16: 302-310.
HANNA, P.Y. & DYER, K.F. (1975) Mutagenicity of organo-
phosphorus compounds in bacteria and Drosophila. Mutat. Res.,
28: 405-420.
HASSAN, A., ZAYED, S.M.A.D., & BAHIG, M.R.E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimetho-
ate in the rat. Biochem. Pharmacol., 18: 2429-2438.
HAUCK, H.E. & AMADORI, E. (1980) Recent developments in high-
performance thin-layer chromatography and application to pesti-
cide analysis, New York, American Chemical Society, pp. 159-176
(ACS Symposium Series 136; CH09).
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, pp. 362-365.
HELLWIG, J. (1968a) Report on the study of the toxicity of
dimethoate in mice after 78-week administration in the diet,
(Unpublished report no. 75CO326/8242 from BASF, Department of
toxicology, Ludwigshafen/Rhein, Federal Republic of Germany).
HELLWIG, J. (1968b) Report on the study of the toxicity of
dimethoate in rats after 24 month administration in the diet,
(Project no. 70C0326/8241 dated 9 October 1986 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, Federal Republic
of Germany).
HEWITT, R., BREBBIA, A., & WALETZKY, E. (1958a) Carbamoyl
alkyl phosphorodithioates as chemotherapeutic agents: screening
by aedicidal properties in laboratory mammals, lambs and calves.
J. econ. Entomol., 51(2): 126-131.
HEWITT, R., EMRO, J., ENTWISTLE, J., PANKAVICH, J., THORSON, R.,
WALLACE, W., & WALETZKY, E. (1958b) Carbamoyl alkyl phosphoro-
dithioates as chemotherapeutic agents: effects of dimethoate
against grubs in cattle. J. econ. Entomol., 51(4): 445-450.
HILL, R.H. & ARNOLD, J.E. (1979) A personal air sampler for
pesticides. Arch. environ. Contam. Toxicol., 8: 621-628.
HOEGBERG, E.I. & CASSADAY, J.T. (1951) The reaction of O,O-
dialkyl thiophosphoric acid salts with some alpha-haloacyl deriva-
tives. J. Am. Chem. Soc., 73: 557-559.
IRPTC (1982) Scientific reviews of Soviet literature on toxic-
ity and hazards of chemicals: dimethoate, Moscow, Centre of
International Projects, GKNT, 12 pp (No. 5).
IRPTC (1987) Legal file Vol. 2, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
IWATA, Y., DUSCH, M.E., CARMAN, G.E., & GUNTHER, F.A. (1979)
Worker environment research: residues from carbaryl, chloroben-
zilate, dimethoate, and trichlorfon applied to citrus trees. J.
agric. food Chem., 27(6): 1141-1145.
IZMIROVA, N., BENCHEV, I., VELICHKOVA, V., KALOYANOVA, F.,
LAMBREVA, E., RUSEVA, P., KOSTOVA, S., ANGELOVA, R., INKJOVA,
M., BOGINOVA, L., MAKSIMOVA, F., & TZVETKOVA, P. (1973)
[Determination of minimal safety periods after treatment of
tobacco and cotton with organophosphorus pesticides.] In:
[ Hygiene, use and toxicology of pesticides, and clinical
aspects of pesticide poisoning,] Vol. 10, Moscow, Medizina
Publishers, pp. 120-124 (in Russian).
JACKSON, J.B., DRUMMOND, R.O., BUCK, W.B., & HUNT, L.M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. econ.
Entomol., 53(4): 602-604.
JAYCOX, E. (1964) Effect on Honey bees of nectar from systemic
insecticide-treated plants. J. econ. Entomol., 57(1): 31-35.
JOHNSON, E., CATERSON, C.R., & ALLEN, J.S. (1985) Mutagenicity
testing of dimethoate (AC 12880) in the in vitro CHO/HGPRT
mutation assay. (Unpublished report project no. 0423 dated 30
January 1985 from American Cyanamid Company, Princeton, New
Jersey).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. II. August 1975 - July
1976. Pestic. monit. J., 15(1): 39-50.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Pesti-
cide, metal, and other chemical residues in adult total diet
samples. XII. August 1975 - July 1976. Pestic. monit. J., 15(1):
54-59.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. III. August 1976 - Sep-
tember 1977. J. Assoc. Off. Anal. Chem., 67(1): 145-154.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984b) Pesticide, metal, and other chemical residues in adult
total diet samples. XIII. August 1976 - September 1977. J.
Assoc. Off. Anal. Chem., 67(1): 154-166.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity
of chemicals to fish and aquatic invertebrates, Washington, DC,
USA, US Department. of the Interior, Fish and Wildlife Service
(Resource Publication 137).
JUNG, H.D. (1979) [Occupational dermatoses due to pesticides.]
Dtsch. Gesundheitswes., 34(24): 1144-1147 (in German).
KAGAN, YU.S. (1981) [ General toxicology of pesticides,]
Kiev, Zdorovye Publishers, 176 pp (in Russian).
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1967)
Establishing hygienic norms of Bi 58 (Rogor, Phosphamide) in the
atmospheric air. In: Works of the Scientific Research Institute
of Labour Protection and Occupational Diseases, Vol. 15, pp. 1-
8, Sofia, Bulgaria, Medicina i Fizkultura.
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1968)
[Experimental substantiation of the maximum permissible concen-
tration of Bi 58 (Rogor, Phosphamide) in the atmosphere.] Gig.
i Sanit., 6: 6-10 (in Russian).
KALOYANOVA, F., DOBREVA, V., & MITOVA, S.V. (1984) [Effet du
dimethoate sur certaines activités du système de monoxygénase
hépatique] In: Hommage au Professeur René Truhaut, Paris,
Queray S.A., pp. 552-555.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J., ed.
IUPAC pesticide chemistry - Human welfare and the environment,
Oxford, New York, Pergamon Press, pp. 237-238.
KAPLANIS, J.N., ROBBINS, W.E., DARROW, D.I., HOPKINS, D.E.,
MONROE, R.E., & TREIBER, G. (1959) The metabolism of dimetho-
ate in cattle. J. econ. Entomol., 52(6): 1190-1194.
KARIM, A.A., HARIDI, A.A., & EL RAYAH, E.A. (1985) The
environmental impact of four insecticides on non-target
organisms in the Gezira Irrigation Scheme canals of Sudan. J.
trop. Med. Hyg., 88(2): 161-168.
KAWAR, N.S., IWATA, Y., DUSCH, M.E., & GUNTHER, F.A. (1979)
Behavior of dialifor, dimethoate, and methidothion in artifi-
cially fortified grape juice processed into wine. J. environ.
Sci. Health, B14(5): 505-513.
KHAN, R.R. & BHASKER DEV (1982) Toxicology data sheets on
chemicals: dimethoate, Lucknow, India, Lucknow Industrial
Toxicology Research Centre (No. 6).
KHERA, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for
teratogenic activity in the cat. J. environ. Pathol. Toxicol.,
2: 1283-1288.
KHERA, K.S., WHALEN, C., TRIVETT, G., & ANGERS (1979) Terato-
genicity studies on pesticidal formulations of dimethoate,
diuron, and lindane in rats. Bull. environ. Contam. Toxicol.,
22: 522-529.
KISS, Z. & FAZEKAS, T. (1983) Organophosphates and torsade de
pointes ventricular tachycardia. J. R. Soc. Med., 76: 984-985.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides. Techniques for establishing safe levels of foliar
residues. Residue Rev., 75: 81-96.
KNAAK, J.B., SCHLOCKER, P., ACKERMAN, C.R., & SEIBER, J.N.
(1980) Reentry research: Establishment of safe pesticide levels
on foliage. Bull. environ. Contam. Toxicol., 24: 796-804.
KÖPPEL, C., FORYCKI, Z., & IBE, K. (1986) Hemoperfusion in
severe dimethoate poisoning. Intensive Care Med., 12(2): 110-
112.
KRUEGER, H.R., O'BRIEN, R.D., & DAUTERMAN, W.C. (1960) Rela-
tionship between metabolism and differential toxicity in insects
and mice of dimethoate, parathion, and acethion. J. econ.
Entomol., 53(1): 25-31.
KUNDIEV, YU.I. (1979) [ Absorption of pesticides through the
skin and prophylaxis of poisonings,] Kiev, Zdorovye Publishers,
200 pp (in Russian).
LARRIPA, I., MATOS, E., & DE SALUM, S.B. (1979) [Cytogenetic
study of human population accidentally exposed to an organophos-
phate pesticide.] Medicina (Buenos Aires), 39(6): 773-774 (in
Spanish).
LARRIPA, I., MATOS, E., DE VINUESA, M.L., & DE SALUM, S.B.
(1983) Sister chromatid exchanges in a human population
accidentally exposed to an organophosphorus pesticide. Rev.
Braz. Genet., 4: 719-727.
LEBLANC, F.N., BENSON, B.E., & GILG, A.D. (1986) A severe
organophosphate poisoning requiring the use of an atropine
drip. Clin. Toxicol., 24(1): 69-76.
LEE, Y.W. & WESTCOTT, N.D. (1981) Gas chromatographic quanti-
tative analysis and persistence of dimethoate and dimethoxon
residues on and in wheat plants. J. agric. food Chem., 29(4):
860-862.
LORD, K.A., MAY, M.A., & STEVENSON, J.H. (1968) The secretion
of the systemic insecticides dimethoate and phorate into nectar.
Ann. appl. Biol., 61: 19-22.
LUCIER, G.W. & MENZER, R.E. (1970) Nature of oxidative metabo-
lites of dimethoate formed in rats, liver microsomes, and bean
plants. J. agric. food Chem., 18(4): 698-704.
MADZHIDOV, U.A. (1970) [Atmospheric pollution with fosfamid in
agricultural application.] Med. J. Uzb., 9: 40-42 (in Russian).
MAHKAMBAEVA, Z.G. (1971) [Influence of organophosphorus pesti-
cides (kilval, methylmercaptophos, fosfamid) on bioelectric
activity of heart in experimental animals.] In: [ Hygiene, use
and toxicology of pesticides, and clinical aspects of pesticide
poisoning,] Kiev, Zdorovye Publishers pp. 175-180 (in Russian).
MAROSI, G., IVAN, J., NAGYMAJTENYI, L., CSATLOS, J., & TOSZEGI,
A. (1985a) Dimethoate-induced toxic cardiac failure in the
guinea-pig. Arch. Toxicol., 57: 142-143.
MAROSI, G., IVAN, J., & NAGYMAJTENYI, L. (1985b) Cardiodepres-
sion in organophosphate poisonings. Arch. Toxicol., Suppl. 8:
289-291.
MASIAK, M. & OLAJOSSY, M. (1973) [Depressive reaction
following intoxication with an organic phosphorus preparation,
Rogor (Bi 58).] Wiadom. Lek., 26(20): 1933-1936 (in Polish).
MELNIKOV, N.N. (1974) [ Chemistry and technology of pesti-
cides,] Moscow, Khimiya Publishers, 768 pp (in Russian).
MELNIKOV, N.N., VOLKOV, A.I., & KOROTKOVA, O.A. (1977)
[ Pesticides and the environment,] Moscow, Khimiya Publishers,
pp. 240 (in Russian).
MENZER, R.E. (1970) Effect of chlorinated hydrocarbons in the
diet on the toxicity of several organophosphorus insecticides.
Toxicol. appl. Pharmacol., 16: 446-452.
MENZER, R.E. & BEST, N.H. (1968) Effect of phenobarbital on
the toxicity of several organophosphorus insecticides. Toxicol.
appl. Pharmacol., 13: 37-42.
MENZIE, C.M. (1969) Metabolism of pesticides, Washington, DC,
US Department of the Interior, Fish and Wildlife Service, pp.
160-166.
MENZIE, C.M. (1974) Metabolism of pesticides: an update,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 166-167, 485.
MENZIE, C.M. (1978) Metabolism of pesticides: update II,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 118.
MENZIE, C.M. (1980) Metabolism of pesticides: update III,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 242.
MIKHAILOV, S.S. & SHTERBAC, I.G. (1983) [Metabolism of organo-
phosphorus compounds containing a carboxyamin bond.] In:
[ Metabolism of organophosphorus poisons,] Moscow, Medizina,
pp. 96-101 (in Russian).
MITOVA, S.V., VASILEVA, L., DOBREVA, V., & KALOYANOVA, F. (1986)
Experimental studies on dimethoate oxidative desulfuration.
Toxic interfaces of neurones, smoke and genes. Arch. Toxicol.,
Suppl.9: 377.
MOHN, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides.
Mutat. Res., 20: 7-15.
MOLPHY, R. & RATHUS, E.M. (1964) Organic phosphorus poisoning
and therapy. Med. J. Aust., 29: 337-340.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides
in bacterial reversion assay systems. Mutat. Res., 116: 185-
216.
MULLER, P. & REINHOLD, R. (1973) [Experience with dimethoate
drugs in stable fly control.] Angew. Parasitol., 14(3): 158-169
(in German).
MUNCY, R.J. & OLIVER, A.D. (1963) Toxicity of ten insecticides
to the red crawfish, Procambarus clarki (Girard). Trans. Am.
Fish. Soc., 92: 428-431.
MURATORE, F., FAIOLO, A., & PONZETTA, G. (1960) [Four cases of
intoxication with organophosphorus esters with regard to liver
function.] Minerva Med., 51(80): 3342-3345 (in Italian).
NADMAITENI, L. & MAROSI, G. (1983) [Cardiotoxic action of
organophosphorus pesticides.] G ig. i Sanit., 11: 72-73 (in
Russian).
NAGLER, J., BRAECKMAN, R.A., WILLEMS, J.L., & DE BROE, M.E.
(1980) Combined hemoperfusion hemodialysis in organic phosphate
poisoning. Kidney Int., 18: 139 (Abstract).
NANDA KUMAR, N.V. & UDAYA BHASKAR, S. (1980) Colorimetric
determination of dimethoate by quantification of cholinesterase
inhibition. J. food Sci. Technol., 17: 153-155.
NCI (1977) Bioassay of dimethoate for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (Technical Report
Series No. 4).
NEHES, M., SELYPES, A., & SCHEUFLER, H. (1982) [Mutagenic
effect of trichlorfon and dimethoate on bone marrow cells of
mouse in vivo.] Wiss. Z. Univ. Halle, 31M(2): 57-62 (in
German).
NEHEZ, M., SELYPES, A., SCHEUFLER, H., & FISCHER, G.W. (1983)
Effect of dimethoate and O- demethyldimethoate on bone marrow
cells of CFLP mice. Regul. Toxicol. Pharmacol., 317(3): 349-
354.
NOAKES, D.N. & SANDERSON, D.M. (1969) A method for determining
the dermal toxicity of pesticides. Br. J. ind. Med., 26: 59-64.
NORTH, H.H. & MENZER, R.E. (1972) Biotransformation of
dimethoate by cell culture systems. Pestic. Biochem. Physiol.,
2: 278-285.
OKONEK, S. (1976) Probable progress in the therapy of
organophosphate poisoning: Extracorporeal hemodialysis and
hemoperfusion. Arch. Toxicol., 35(3): 221-227.
OKONEK, S. (1977) [Hemoperfusion with coated activated
charcoal for treating acute poisoning by remedies, plant
protectants, and fungi.] Med. Klin., 72(19): 862-866 (in
German).
OKONEK, S., HOLLMANN, H., & BOELCKE, G. (1976) [The
alkylphosphate poisoning: elimination of the poison by the
hemodialysis or blood exchange transfusion?]. Med. Welt, 27(8):
351-356 (in German).
PACH, J., GROSZEK, B., & BOGUSZ, M. (1977) Haemoperfusion in
the treatment of acute organophosphate poisoning. Acta
pharmacol. Toxicol., Vol 41, Suppl. II): 44.
PAMBOR, M. & BLOCH, Y. (1985) [Dimethoate and dithiocarbamate
as occupational contact allergens in a female agrotechnician.]
Dermatol. Monatsschr., 171(6): 401-405 (in German).
PANDEY, A.K. & TOMAR, V. (1985) Melanophores in Bufo melanos-
tictus (Schneider) tadpoles following exposure to the insecti-
cide dimethoate. Bull. environ. Contam. Toxicol., 35: 796-801.
PANSHINA, T.N. (1963a) [Experimental data on toxicology of new
organophosphorus insecticide phosphamide (Rogor, dimethoate).]
Pharmacol. Toksicol., 4: 476-484 (in Russian).
PANSHINA, T.N. (1963b) [Hygienic background for determining
the maximum permissible concentration of phosphamide in the air
of a working area.] Gig. i Sanit., 3: 21-28 (in Russian).
PANSHINA, T.N. (1966) [Experimental data on therapy of phos-
phamide intoxications.] In: [ Hygiene and toxicology of
pesticides, and clinical aspects of pesticide poisoning,] Kiev,
Zdorovye Publishers, Vol. 4, pp. 137-142 (in Russian).
PANSHINA, T.N. & KLISENKO, M.A. (1962) [Content of fosfamid
and changes in blood cholinesterase activity in different routes
of treatment of the mammalian organism.] In: [ Industrial
toxicology and clinical picture of occupational diseases due to
chemicals,] Moscow, Medgiz, pp. 181-184 (in Russian).
PETUKHOV, R.D. (1975) [Methodics of estimating of antio and
fosfamid in honey by thin-layer chromatography.] In: [ Methods
of determination of microquantities of pesticides in food, feed,
and the environment,] Moscow, Kolos, pp. 63-64 (in Russian).
PODREBARAC, D.S. (1984a) Pesticide, heavy metal residues, and
other chemical residues in infant and tod dler total diet sam-
ples. IV. October 1977 - September 1978. J. Assoc. Off. Anal.
Chem., 67(1): 166-175.
PODREBARAC, D.S. (1984b) Pesticide, metal, and other chemical
residues in adult total diet samples. XIV. October 1977 - Sep-
tember 1978. J. Assoc. Off. Anal. Chem., 67(1): 176-185.
PROBST, G.H., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEAL, S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures: A comparison with
bacterial mutagenicity using 218 compounds. Environ. Mutagen.,
3: 11-32.
RIEMER, F., DAHLENBURG, R., & GRISK, A. (1985) [Renal excretion
of dimethylphosphate and its thio derivatives after application
of dimethoate, bromophos, naled or trichlorphon to rats.]
Nahrung, 29(3): 299-302 (in German).
ROALES, R.R. & PERLMUTTER, A. (1974) Toxicity of zinc and
Cygon, applied singly and jointly, to zebrafish embryos. Bull.
environ. Contam. Toxicol., 12(4): 475-480.
ROSVAGA, R.I. (1983) [Methodology of investigation of pesti-
cide pyrolysis in their chemical-thermal harmlessness.] In:
[ Hygiene of application, toxicology of pesticides and polymeric
materials,] Kiev, Zdorovja, Vol. 13, pp. 73-77 (in Russian).
SAHU, D. & PATTANAIK, M. (1980) Persistence of rogor in some
Orissa soils. Indian J. agric. Sci., 50(6): 505-507.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDERSON, D.M. & EDSON, E.F. (1959) Oxime therapy in poison-
ing by six organophosphorus insecticides in the rat. J. Pharm.
Pharmacol., 11: 721-728.
SANDERSON, D.M. & EDSON, E.F. (1964) Toxicological properties
of the organophosphorus insecticide dimethoate. Br. J. ind.
Med., 21: 52-64.
SANTI, R. & GIACOMELLI, R. (1962) Metabolic fate of P32-
labelled dimethoate in olive fruits and some toxicological
implications. J. agric. food Chem., 10(3): 257-261.
SAN SEBASTIAN, J.R. (1985) In vivo bone marrow cytogenetics
rat metaphase analysis. PH 315-AC-001-84, Dimethoate CL 12880.
(Unpublished report dated 29 August 1985 from Pharmakon
Research International, Inc. Waverly, Pennsylvania 18471).
SCHEUFLER, H. (1975) [Effects of relatively high doses of
dimethoate and trichlorfon on the embryogenesis of laboratory
mice.] Biol. Rundsch., 13: 238-240 (in German).
SENANAYAKE, N. & KARALLIEDE, W. (1987) Neurotoxic effects of
organophosphorus insecticides. An intermediate syndrome. New
Engl. J. Med., 316(13): 761-763.
SHAMSHURIN, A.A. & KRIMER, M.Z. (1976) [ Physicochemical proper-
ties of pesticides: reference book,] Moscow, Khimya Publishers,
pp. 76-77 (in Russian).
SIDIMANOV, B.B. (1971) [Determination of fosfamid in post-
mortem material by thin-layer chromatography.] In: [ Hygiene of
application, toxicology of pesticides, and clinical picture of
poisoning,] Kiev, Zdorovja, Vol. 9, pp. 187-188 (in Russian).
SLOOFF, W. & CANTON, J.H. (1983) Comparison of the suscepti-
bility of 11 freshwater species to 8 chemical compounds. II.
(Semi)chronic toxicity test. Aquat. Toxicol., 4: 271-282.
SORG, R.M. (1985) Micronucleus test (MNT) PH 309A-AC-004-84,
Dimethoate CL 12880, (Unpublished report dated 1 March 1985
from Pharmakon Research International, Inc. Waverly,
Pennsylvania 18471).
STERRI, S.H., ROGNERUD, B., FISKUM, S.E., & LYNGAAS, S. (1979)
Effect of toxogonin and P2S on the toxicity of carbamates and
organphosphorus compounds. Acta pharmacol. toxicol., 45: 9-15.
STEVENSON, J.H. (1968) Laboratory studies on the acute contact
and oral toxicities of insecticides to honey bees. Ann. appl.
Biol., 61: 467-472.
STIEGLITZ, R., GIBEL, W., WERNER, W., & STOBBE, H. (1974)
Experimental study on haematotoxic and leukaemogenic effects of
trichlorfon and dimethoate. Acta haematol., 52: 70-76.
STROY, A.N. (1983) [Hygienic reglementation of fosfamid in
greenhouse conditions.] In: [Hygiene of application, toxicology
of pesticides and polymeric materials,] Kiev, Zdorovja, Vol.
13, pp. 90-91 (in Russian).
SZETO, S.Y., VERNON, R.S., & BROWN, M.J. (1985) Degradation of
dimethoate and pirimicarb in asparagus. J. agric. food Chem.,
33: 763-767.
TIEFENBACH, B. & LANGE, P. (1980) Studies on the action of
dimethoate on the immune system. Arch. Toxicol., Suppl. 4: 167-
170.
TRINH VAN BAO, SZABO, I., RUZICSKA, P., & CZEIZEL, A. (1974)
Chromosome aberrations in patients suffering acute organic
phosphate insecticide intoxication. Humangenetik, 24: 33-57.
UBAIDULLAEV, R.U. & MADZHIDOV, U.A. (1976) [Hygienic charac-
teristics of phosphamide as an atmospheric pollutant.] Gig. i
Sanit., 9: 7-9 (in Russian).
UCHIDA, T., DAUTERMANN, W.C., & O'BRIEN, R.D. (1964) The
metabolism of dimethoate by vertebrate tissues. J. agric. food
Chem., 12(1): 48-52.
UDAYA BHASKAR, S. & NANDA KUMAR, N.V. (1980) Colorimetric
determination of dimethoate or omethoate. Talanta, 27(9): 757-
758.
USHA RANI, M.V., REDDI, O.S., & REDDY, P.P. (1980) Mutageni-
city studies involving aldrin, endosulfan, dimethoate, phospham-
idon, carbaryl, and ceresan. Bull. environ. Contam. Toxicol.,
25(2): 277-282.
VAN MIDDELEM, C.H. & WAITES, R.E. (1964) Gas chromatographic
and colorimetric measurement of dimethoate residues. J. agric.
food Chem., 12(2): 178-182.
VELAZQUEZ, A., XAMENA, N., CREUS, A., & MARCOS, R. (1986)
Indication for weak mutagenicity of the organophosphorus
insecticide dimethoate in Drosophila melanogaster. Mutat. Res.,
172: 237-243.
VERMA, S.R., BHATNAGAR, M.C., & DALELA, R.C. (1978) Biocides
in relation to water pollution. II. Bioassay studies of a few
biocides to a fresh-water fish Channa gachua. Acta hydrochim.
hydrobiol., 6(2): 137-144.
VERMA, S.R., TYAGI, A.K., BHATNAGAR, M.C., & DALELA, R.C.
(1979) Organophosphate poisoning to some fresh-water teleosts -
acetylcholinesterase inhibition. Bull. environ. Contam. Toxi-
col., 21: 502-506.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982) Bioassay
trials with twenty-three pesticides to a fresh-water teleost
Saccobranchus fossilis. Water Res., 16: 525-529.
VISHWANATH, R. & JAMIL, K. (1986) Mutagenic and genotoxic
activities of certain organophosphorus compounds using Ames
Salmonella assay with and without microsomal induction. Ind.
J. exp. Biol., 24: 305- 308.
WALLER, G.D. & BARKER, R.J. (1979) Effects of dimethoate on
honey bee colonies. J. econ. Entomol., 72(4): 549-551.
WALLER, G.D., BARKER, R.J., & MARTIN, J.H. (1979) Effects of
dimethoate on honey bee foraging. Chemosphere, 8(7): 461-463.
WEHRAN, H.-J. & HAMMER, H.-J. (1984) [Lethal poisoning with
the pesticide dimethoate (Bi 58 EC).] Kriminal. forens. Wiss.,
54: 181-183 (in German).
WEST, B., VIDONE, L.B., & SHAFFER, C.B. (1961) Acute and
subacute toxicity of dimethoate. Toxicol. appl. Pharmacol., 3:
210-223.
WHO (1982) Field surveys of exposure to pesticides - Standard
Protocol, Geneva, World Health Organization (Unpublished
document VBC/82.1).
WHO (1984) Chemical methods for the control of arthropod
vectors and pests of public health importance, Geneva, World
Health Organization, pp. 34, 37.
WHO (1986a) Environmental health criteria 63: Organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WHO (1986b) The WHO recommended classification of pesticides
by hazard and guidelines to classification 1986-1987 World
Health Organization, Geneva, (Unpublished document VBC/86.1).
WHO (1986c) Interim specifications for dimethoate technical
and emulsifiable concentrate, Geneva, World Health Organization
(Unpublished report, WHO/IS/86.12).
WHO/FAO (1980) Dimethoate: data sheet on pesticides, Geneva,
World Health Organization (Unpublished document VBC/DS/80.42).
WINKLER, R. & MULLER, R. (1979) [Elimination and inactivation
of waters containing pesticides from agrochemical plants.]
Wasserwirtsch. Wassertech., 29(11): 369-371 (in German).
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-
induced complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ.
Mutagen., 5: 835-846.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th
ed., Lavenham United Kingdom, British Crop Protection Council.
ZECH, R., ENGELHARD, H., & ERDMANN, W.-D. (1966) Reactions of
pyridinium oximes with the alkyl phosphate dimethoate and its
derivatives. Effects on cholinesterases. Biochim. biophys.
Acta, 128: 363-371.
ZOLOTNIKOVA, G.P. (1980) [Disturbance of the immunoreactivity
of the organism under the effect of pesticides in hothouses.]
Gig. tr. prof. Zabol., 3: 38-40 (in Russian).
ZOLOTNIKOVA, G.P. & SOMOV, B.A. (1978) [On the role of pesti-
cides in the occurrence of occupational dermatitis in workers of
hothouses.] Vestnik Dermatol. Venerol., 4: 76-79 (in Russian).
ZOLOTNIKOVA, G.P. & ZOTOV, V.M. (1978) [Prognosis for resi-
dues of pesticides in the air of hothouses.] Gig. i Sanit.,
43(1): 108-109 (in Russian).
ANNEX I. TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus Insecticides - A General Introduction)
All cases of organophosphorus poisoning should be dealt
with as an emergency and the patient sent to hospital as quickly
as possible. Although symptoms may develop rapidly, delay in
onset or a steady increase in severity may be seen up to 48 h
after ingestion of some formulated organophosphorus insecti-
cides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major
references (Kagan, 1977; Taylor, 1980; UK DHSS, 1983; Plestina,
1984) and will also be included in the IPCS Health and Safety
Guides to be prepared for selected organophosphorus insecti-
cides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures
include removal of contaminated clothes and washing of the skin
with alkaline soap or with a sodium bicarbonate solution.
Particular care should be taken in cleaning the skin area where
venupuncture is performed. Blood might be contaminated with
direct-acting organophosphorus esters, and, therefore,
inaccurate measurements of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced,
if the patient is conscious, by the administration of
ipecacuanha syrup (10-30 ml) followed by 200 ml water. This
treatment is, however, contraindicated in the case of pesticides
dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also
be performed, particularly in unconscious patients, taking care
to prevent aspiration of fluids into the lungs (i.e., only after
a tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the
pesticide involved is available, it should also be stored for
further analysis (i.e., detection of toxicologically relevant
impurities). A purge to remove the ingested compound can be
administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be
started at the first sign of respiratory failure and maintained
for as long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given
at 15 to 30-min intervals. The dose and the frequency of
atropine treatment varies from case to case, but should maintain
the patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be
necessary in extreme cases and total daily doses up to several
hundred mg may be necessary during the first few days of
treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophos-
phates. This is not the case with enzymes inhibited by
carbamates. The treatment should begin as soon as possible,
because oximes are not effective on "aged" phosphorylated ChEs.
However, if absorption, distribution, and metabolism are thought
to be delayed for any reasons, oximes can be administered for
several days after intoxication. Effective treatment with
oximes reduces the required dose of atropine. Pralidoxime is
the most widely available oxime. A dose of 1 g pralidoxime can
be given either im or iv and repeated 2-3 times per day or, in
extreme cases, more often. If possible, blood samples should be
taken for AChE determinations before and during treatment. Skin
should be carefully cleansed before sampling. Results of the
assays should influence the decision whether to continue oxime
therapy after the first 2 days.
There are indications that oxime therapy may possibly have
beneficial effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to
counteract some aspects of CNS-derived symptoms, which are not
affected by atropine. Doses of 10 mg sc or iv are appropriate
and may be repeated as required (Vale & Scott, 1974). Other
centrally acting drugs and drugs that may depress respiration
are not recommended in the absence of artificial respiration
procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinization. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and
may be taken into, and then released from, fat depots over a
period of many days. It is therefore quite incorrect to abandon
oxime treatment after 1-2 days on the supposition that all
inhibited enzyme will be aged. Ecobichon et al. (1977) noted
prompt improvement in both condition and blood-ChEs in response
to pralidoxime given on the 11th-15th days after major symptoms
of poisoning appeared due to extended exposure to fenitrothion
(a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually
for an occupational setting, but, in the case of very severe
exposure or massive ingestion (accidental or deliberate), the
therapeutic doses may be extended considerably. Warriner et al.
(1977) reported the case of a patient who drank a large quantity
of dicrotophos, in error, while drunk. Therapeutic dosages were
progressively increased up to 6 mg atropine iv every 15 min
together with continuous iv infusion of pralidoxime chloride at
0.5 g/h for 72 h, from days 3 to 6 after intoxication. After
considerable improvement, the patient relapsed and further
aggressive therapy was given at a declining rate from days 10 to
16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given
and the patient was discharged on the thirty-third day with no
apparent sequelae.
REFERENCES TO ANNEX I
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-
379.
KAGAN, JU.S. (1977) [ Toxicology of organophosphorus pesti-
cides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in
Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (Unpub-
lished document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S.
& Gilman, A., ed. The pharmacological basis of therapeutics, 6th
ed., New York, Macmillan Publishing Company, pp. 100-119.
UK DHSS (1983) Pesticide poisoning: notes for the guidance of
medical practitioners, London, United Kingdom Department of
Health and Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus
poisoning. Guy's Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
Different factors may affect the accumulation and degra-
dation of dimethoate in soil, such as the soil type, the numbers
and type of microorganisms present in soil, the environmental
temperature, the pH level, the amount of pesticide applied, and
the degree of evaporation (El Beit et al., 1977a,b, 1978). The
persistence of dimethoate was greater in heavy than in light
soil. At pH 4.2, the pesticide was stable for nearly 19 days;
at pH 11, it degraded within 20 h. The amount of dimethoate in
soil increased when higher concentrations were applied. El Beit
et al. (1977a) reported that soil microorganisms played little
part in the degradation of dimethoate.
4.1.4. Plants
When applied to plants, dimethoate was rapidly absorbed and
decomposed, both on the surface and within the plant, by
hydrolysis and oxidation (Menzie, 1969; Melnikov et al., 1977).
The half-life of dimethoate in the different plants varied
between 2-5 days (Melnikov et al., 1977). Dimethoate completely
disappeared after 15-30 days, depending on the plant species and
the climatic conditions. Decomposition in plants and the
hydrolysis of dimethoate increased with temperature (Atabaev,
1972).
The dissipation of dislodgeable residues of dimethoate is
best characterized by two first-order kinetic processes. The
half-life values were 2.2 days in the 1 to 10-day period and 7.0
days in the 10 to 49-day period (Hadjidemetriou et al., 1985).
4.1.5. Disposal of wastes
Hydrolytic decomposition is the main way to inactivate
dimethoate. By adding lime (1-2 kg calcium oxide/m3 water) to
waste waters from agricultural centres, dimethoate was fully
inactivated in 45 min (Winkler & Muller, 1979).
During pyrolysis, approximately 50% decomposition of di-
methoate occurred at 500 °C with the formation of O,O-dimethyl-
S,S-dithionpyrophosphate; decomposition was complete at 1100 °C
(Rosvaga, 1983).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air, water, and soil
No studies have been reported on levels in air, water, or
soil under actual conditions of use and various environmental
conditions.
5.1.2. Food
When a combination of dimethoate and omethoate (dimethoxon)
was given to cows in dosages of 1 and 0.1 mg/kg body weight,
respectively, for 14 days, only residues of the metabolite
omethoate were observed in the milk (0.004-0.125 mg/kg). Three
days after the application, neither compound could be detected.
When dosages of 0.5 mg dimethoate/kg and 0.05 mg omethoate/kg
were given for 14 days or when corn silage containing 1-7 mg
dimethoate/kg (resulting in dosages of 0.06-0.36 mg/kg body
weight) was given for 28 or 42 days, no residues were detected
in the milk (Beck et al., 1968).
Harvest residues found in many crops 1-3 weeks after
spraying with dimethoate, during the first years of application
in the United Kingdom (1957-58), were below 2 mg/kg (Chilwell &
Beecham, 1960).
The dimethoate content of apples was 0.03-0.07 mg/kg, 75
days after an application of 0.72 kg active ingredient/ha
(Atabaev & Stepovaya, 1966).
The pulp of lemon and orange fruit treated with dimethoate
did not show any residues at a detection level of 0.01 mg/kg,
60 days after application (Iwata et al., 1979).
Residues of dimethoate do not concentrate in wine. Analysis
of seven different Californian wines indicated levels of less
than 0.03 mg/litre (Kawar et al., 1979).
No residues were found in grapes, 29 days after treatment
with 0.1-0.15% dimethoate applied at the rate of 1400 litre/2 ha
(Grigorashvili & Dzhibladze, 1965).
A number of studies on residues of dimethoate found through-
out the world have been reported in a review by De Pietri-
Tonelli et al. (1965). The residues were most frequently below
1 mg/kg. Dimethoate residues found in various agricultural
products in India are reported in Khan & Bhaskar Dev (1982).
Dimethoate was not found in total-diet samples studied in
England and Wales during 1966-67 (Abbott et al., 1970).
According to other authors, dimethoate was also not found in the
total diets of adults and infants during 1975-79 (Johnson et
al., 1981a,b, 1984a,b; Podrebarac, 1984a,b; Gartrell et al.,
1985a,b,c). Market-basket surveys carried out in 1976-78 in the
Netherlands showed only omethoate residues in a small number of
fruits (De Vos et al., 1984). In the USA, dimethoate and
omethoate have been identified in about 5% of samples of fruits
and vegetables (Duggan et al., 1983).
The use of additional procedures for the determination of
organophosphates resulted in the identification of dimethoate in
adult and infant total-diet samples in 1980-82 (Gartrell et al.,
1985d, 1986a,b). However, the intakes (0.001 µg/kg body weight
per day) were far below the FAO/WHO acceptable daily intake
(ADI) (see section 12).
5.2. Occupational Exposure
Immediately after the use of dimethoate in greenhouses,
Stroy (1983) determined levels of 0.01-0.42 mg/m3 in the air,
3.6-9.3 mg/kg in the plants, and 0.98-1.75 mg/kg in the soil.
Dimethoate content in greenhouse air has been analysed at
different times after spraying; at 0 time the measured amount
was 0.66 mg/m3, at 2.5 h it was 0.38 mg/m3, at 5 h dimethoate
content was 0.21 mg/m3, at 10 h it was 0.07 mg/m3 and at 20 h
it was 0.01/m3 (Zolotnikova & Zotov, 1978).
The exposure to dimethoate of tractor drivers using airblast
units during treatment of citrus trees was investigated by
Carman et al. (1982). Dermal exposure was measured by placing
ethyleneglycol-treated gauze patches on the shoulders, upper
arms, and knees of the drivers. An emulsifiable concentration
(EC) formulation of dimethoate containing 0.009 kg/litre was
applied at the rate of 16 822 litre/ha using an open tractor, a
cab unit with both side windows open, and a cab unit with the
windows closed. Under these conditions, the patches attached
to the driver absorbed mean deposits of 2.5, 1.5, and
< 0.01 µg dimethoate/cm2 per h, respectively. The correspond-
ing average air concentrations were 10(sic), 48, and 2 µg of
dimethoate/m3.
Procedures for determining foliar residues and to establish
the safe re-entry times for some insecticides were reported by
Knaak (1980) and Knaak et al. (1980). A safe level of
dimethoate on foliage of 53 µg/cm2 was calculated using the
results of dermal-dose ChE-response studies in male rats.
Minimum safe re-entry periods for dimethoate were estimated
to be 3 days in greenhouses, and 7 days in tobacco fields, after
application by tractor, or 5 days after application by plane
(Kaloyanova-Simeonova & Izmirova-Mosheva, 1983). No ChE
inhibition in serum or subjective complaints of workers picking
dimethoate-treated tobacco leaves were established at a concen-
tration of 1 mg/kg on the surface of the plant.
Copplestone et al. (1976) studied the exposure to dimethoate
of 8 spraymen, 1 mixer, and 2 supervisors in the Sudan. The
percentage of toxic dosea received per day, calculated on the
basis of a 4-h working day, varied from 0.02% to less than
0.001% for individual spraymen. No ChE depression was found in
any of the men.
The highest concentration of dimethoate, measured in the
work-place air of a dimethoate-producing factory in Italy, was
0.050 mg/m3 (Armeli et al., 1967).
The respiratory and dermal exposure to dimethoate of
applicators was determined for greenhouse workers. The
respiratory and dermal exposures were 0.034 mg/h and 30 mg/h,
respectively. The hands of the operators were the most affected
parts of the body, accounting for 63-92% of the total exposure
(Adamis et al., 1985).
----------------------------------------------------------------
a Percentage toxic dose per day or h (WHO, 1982). This is
calculated from these indices adapted from the method of
Durham & Wolfe (1962) using the formula:
Dermal exposure (mg/day or h) + respiratory
exposure (mg/day or h) x 10 if measured
-------------------------------------------- x 100
Dermal LD50 mg/kg (rat) x 70
6. KINETICS AND METABOLISM
6.1. Absorption and Distribution
Panshina & Klisenko (1962) checked the blood levels of
dimethoate in cats and rats after single oral doses of 50, 75,
or 200 mg/kg in the cat and 300 mg/kg in the rat. The deter-
minations were carried out 15, 30, 60, 90, 120, and 180 min
after dosing. Dimethoate was detected in the blood of cats and
rats after 30 min, and reached a maximum level after 60-90 min.
Nearly 80% of the dimethoate in the blood was found in the
erythrocytes; only 15-20% was found in the serum. With repeated
daily oral intake of dimethoate at doses of 20 mg/kg or
10 mg/kg, the maximum blood level occurred on the 5-10th day of
the study.
The same pattern in blood levels was observed with repeated
inhalation of dimethoate for 4 h/day over 3 months, at a mean
concentration of 5 mg/m3 air. Dimethoate was detected in blood
from the second day and reached its maximum by the 7-10th day.
Daily application of 50 mg dimethoate/kg on the skin of
rabbits resulted in a maximum concentration in the blood at
about the third day (Kundiev, 1979).
When dimethoate was applied to the skin of rats for 1, 2, 4,
12, or 24 h in a single dose of 560 mg/kg, the maximum concen-
tration in the skin was reached after 12 h of exposure and was
correlated with the maximum inhibition of ChE activity in the
serum and liver. The concentrations of dimethoate in the blood,
liver, and kidney were maximal after 2 h of exposure (Baranova
et al., 1986).
6.2. Metabolic Transformation
The ester and amide groups of dimethoate are cleaved in
reactions that vary with the organism and that contribute to the
selective toxicity of the compound.
The results of in vitro and in vivo studies showed that the
main metabolic pathways of dimethoate were hydrolysis and
oxidation (Hassan et al., 1969; Lucier & Menzer, 1970; North &
Menzer, 1972).
Santi & Giacomelli (1962) studied the metabolic fate of
dimethoate in olives. P=O derivative and degradation products,
such as phosphoric and/or methylphosphoric acid, were found.
The presence of the oxygen analogue dimethoxon (omethoate)
has been demonstrated in insects, plants, and mammals; it
appears to be the metabolite responsible for the toxic action of
dimethoate (Brady & Arthur, 1963; Hassan et al., 1969; Lucier &
Menzer, 1970). The highest levels of this metabolite were found
in insects, particularly in those highly susceptible to
dimethoate. The oxygen analogue was produced in larger
quantities in insects than in rats. The enzymes mediating the
hydrolysis of the carboxyamide bond are much less effective in
insects than in mammals (Mikhailov & Shterbak, 1983).
It has been shown that cleavage of dimethoate by rats and
cows occurs initially at the C-N bond to produce the carboxy
derivative (Dauterman et al., 1959; Hassan et al., 1969). A
second hydrolytic pathway involves an esterase action on the S-C
bond (Hassan et al., 1969).
Oxidative metabolism of dimethoate predominated over
hydrolytic metabolism in the cell culture system. In the whole
rat, the opposite was true. Metabolism of dimethoate in human
embryonic lung cells was much the same as metabolism in rats
(North & Menzer, 1972). In vitro and in vivo studies showed
that dimethoate is biotransformed to the P=O analogue via the
liver cytochrome P-450 system (Kaloyanova et al., 1984).
Concentrations of 0.1-10 mmol dimethoate/litre led to a linear
decrease in the rates of N-demethylation and P-hydroxylation.
Similarly, in microsomes from rats treated with dimethoate in
vivo, increased activity of desulfuration (140%, P < 0.01), and
decreased activity of hydroxylation and demethylation were seen
(Mitova et al., 1986).
In vivo studies on mice showed dimethoate toxicity to be
markedly increased by phenobarbital pre-treatment, as a result
of induction of hepatic microsomal enzymes including the mixed
function oxidases responsible for the conversion of P=S to P=O
(Menzer & Best, 1968).
It has been found that, while dimethoate undergoes rapid
degradation in the rat liver, very little occurs in other
tissues (lung, muscle, pancreas, brain, spleen, blood). The
ability of the liver to degrade dimethoate in various species
decreased in the order: rabbit > sheep > dog > rat > cattle >
hen > guinea-pig > mouse > pig. For the hen, cattle, mouse,
sheep, and rat there was a reasonably good straight-line
relationship between the LD50 values and the degradation ability
of the liver (Uchida et al., 1964).
After administration of 32P-dimethoate to rats, dimethoate,
dimethoxon, dimethoate carboxylic acid, dimethylphosphoro-
dithioate, dimethylphosphorothioate, dimethylphosphate, mono-
methylphosphate, phosphorothioate, formate, and N-methyl 2-
glucuronate acetamide were found in the urine (Hassan et al.,
1969).
Data on the metabolism of dimethoate in plants and animals
have been reviewed by Menzie (1969, 1974, 1978, 1980).
6.3. Elimination and Excretion
6.3.1. Animal studies
About 45% of the 32P-dimethoate administered orally at
50 mg/kg to rats was excreted in the urine, while only 5.8% was
eliminated in the faeces, 72 h after treatment (Brady & Arthur,
1963). The values in rats after dermal application were 30.6%
and 6.5%, respectively. More than 95% of the 32P materials in
the urine and faeces after oral or dermal administration in rats
were hydrolytic products, as determined by chloroform/water
partition coefficients.
Twenty-four h after ip and oral administration of dimethoate
to rats at doses of 0.25, 2.5, or 25 mg/kg, dimethylphosphoro-
dithioate, dimethylphosphorothioate, and dimethyl phosphate
were detected in the urine at concentrations of 12-14%, 11-15%,
and 12-13%, respectively (Riemer et al., 1985). Neither the
route of exposure nor the dose had any influence on the types of
metabolite formed.
About 87-90% of an oral dose of 10 mg dimethoate/kg was
eliminated in the urine of cattle at the end of 24 h. The same
percentage of an intramuscular dose of 10 mg/kg was excreted
after 9 h. Only 3.7-5% of the oral dose and about 1.1% of the
intramuscular dose were eliminated in the faeces after 72 h and
24 h, respectively (Kaplanis et al., 1959).
6.3.2. Human studies
In human beings, 76-100% of radioactivity was reported
to be excreted in the urine, 24 h after oral dosing with 32P-
dimethoate (Sanderson & Edson, 1964).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The addition of dimethoate to soil at 10 or 100 mg/kg did
not result in significant differences in the number of bacteria
solubilizing tricalcium phosphate or in the number of bacteria
mineralizing calcium glycerophosphate, but an increase in the
population of phospholipase-producing organisms solubilizing
lecithin occurred (Congregado et al., 1979). At 10 mg/kg, an
increase in carbon dioxide production occurred for 2 weeks after
treatment, followed by a decrease to control levels. At
100 mg/kg, the increase in carbon dioxide output was slower and
longer.
7.2. Aquatic Organisms
A number of LC50s have been determined for various aquatic
organisms (Table 3).
The median tolerance limit of the fresh-water teleost,
Channa punctatus for dimethoate is 20.5 mg/litre (Anees, 1975).
Exposure for 24 h, 96 h, or 14 days to dimethoate concentrations
of 10.8, 8.0, or 5.0 mg/litre, respectively, produced moderate
vacuolation of the liver and a high degree of cytoplasmic
granulation, which developed for up to 96 h of exposure. The
14-day exposure added little in the way of vacuolation or
granulation (Anees, 1978a). The haematological response to
dimethoate included reduced erythrocyte counts and haemoglobin
concentration, and an elevated mean corpuscular haemoglobin and
colour index indicating that the insecticide exerted an effect
similar to the production of anaemia (Anees, 1978b).
The signs of the toxicity of dimethoate in fish
(Channa punctatus) included jumping, erratic movement,
imbalance, and death (Dikshith & Raizada, 1981a; Dikshith,
1986).
Verma et al. (1978) determined the TLm values of dimethoate
for Channa gachua for 24, 48, 72, or 96 h to be 5.2, 5.0, 4.6,
or 4.5 mg/litre, respectively. The safe concentration of
dimethoate calculated on the basis of TLm values was approxi-
mately 1.4 mg/litre.
Dimethoate inhibited AChE activity in the brain, liver, and
muscle of some fresh-water teleosts (Channa gachua and Cirrhina
mrigala), exposed to sublethal concentrations of 35% EC
formulation (0.9-2.4 and 0.6-1.6 mg/litre, respectively) (Verma
et al., 1979).
Table 3. Summary of acute toxicity values for aquatic organisms
---------------------------------------------------------------------------------------------------------
Species LC50 (mg/litre) Reference
24-h 48-h 72-h 96-h
---------------------------------------------------------------------------------------------------------
Rainbow Trout 20 - - 8.5 Melnikov et al. (1977)
(Salmo gairdneri)
Rainbow Trout - - - 6.2 Johnson & Finley (1980)
(Salmo gairdneri)
Long-nosed killifish 1.0 - - Melnikov et al. (1977)
(Fundulus similis)
Saccobranchus fossilis 5.14 4.80 4.67 4.57 Verma et al. (1982)
Channa punctatus 68 54 - 47 Dikshith & Raizada (1981a)
Dikshith (1986)
Scud 0.9 0.4 - - Menzie (1969)
(Gammarus lacustris)
Scud - - - 0.20 Johnson & Finley (1980)
(Gammarus lacustris)
Red Crayfish - 1.0 - - Muncy & Oliver (1963)
(Procambarus clarkii)
Stonefly - 0.14 - - Menzie (1969)
(Pteronarcys california)
Stonefly - - - 0.043 Johnson & Finley (1980)
(Pteronarcys california)
Unspecified insect 0.51 - - - Sanders & Cope (1968)
Bluegill - - - - Johnson & Finley (1980)
---------------------------------------------------------------------------------------------------------
Dalela et al. (1979) reported that acute (5-h) and short-
term (up to 32 days) exposure of the fish, Channa gachua, to
dimethoate at 6.2 mg/litre and 1.5 mg/litre, respectively,
produced histological changes in the gills. On acute exposure,
there was erosion at the distal end of the gill filaments and
loss of cell membrane. With exposure to a concentration of
1.5 mg/litre, the basement membrane started separating, and the
damage to the gill was found to be more significant with
increasing exposure time, with vacuolization occurring after 32
days.
The exposure of the fish Heteropneustes fossilis to a
dimethoate concentration of 10 mg/litre led to an increased
level of glycogen by the end of the second week in both the
liver and the kidney, and to a slight decrease in the protein
contents at the end of the eighth day (Awasthi et al., 1984). A
sharp rise in the activity of succinate dehydrogenase in both
organs was noted during the first two weeks of this study.
The estimated 48- and 72-hour TLm values for zebrafish
Brachydanio rerio embryos, exposed to dimethoate, were
940 mg/litre and 259 mg/litre, respectively (Roales &
Perlmutter, 1974). Dimethoate retarded the development of
embryos as expressed by lack of heartbeat and little movement at
24 h.
Dimethoate at a concentration of 0.05 mg/litre produced
morphological changes in the melanophores of Bufo melano-
stictus tadpoles and an increase in pigmented areas of the skin
(Pandey & Tomar, 1985).
Dimethoate had a very low toxicity for some aquatic
organisms in Sudan, such as Oreochromis niloticus, Gambusia
affinis, Pseudagrion spp., Crocothemis erythraea, and Lanistes
carinatus. Under laboratory conditions, it did not kill any
animal at concentrations lower than 80 mg/litre (Karim et al.,
1985).
The toxicity of dimethoate for 11 freshwater species was
studied by Slooff & Canton (1983). The results are summarized
in Table 4. The relative susceptibility tests indicated that
Daphnia magna was the organism most sensitive to dimethoate,
while the microorganisms P.fluorescens, M.aeruginoso, and
S.pannonicus were generally less sensitive indicators of
toxicity. The susceptibility of aquatic species to a chemical
may vary by more than two-three orders of magnitude. The data
demonstrate that the sublethal criteria studied were not
necessarily the most sensitive toxicological criteria.
7.3. Terrestrial Organisms
7.3.1. Honey-bees
The oral LD50 for the honey-bee (Apis mellifera L) ranges
from 93 to 150 ng per bee (Jaycox, 1964; Lord et al., 1968;
Stevenson, 1968; Barker et al., 1980). The contact LD50 is
98-120 ng per bee (Stevenson, 1968).
Table 4. Fresh-water species susceptibility to dimethoate
---------------------------------------------------------------------------------------------------------
Type of Test species Lifestage Exposure Test condition Toxicological No-effect
organism time Temper- Test parameter level
(days) ature methods (mg/litre)
---------------------------------------------------------------------------------------------------------
Bacteria (Pseudomonas fluorescens) log-phase 0.3 22 ± 2 Static Specific 320
growth rate
Cyano Bluegreen bacteria log-phase 4 23 ± 2 Static Specific 32
bacteria (Microcystis aeruginosa) growth rate
Algae Green Algae log-phase 4 23 ± 2 Static Growth 100
(Scenedesmus pannonicus) (biomass)
Plant Lemna minor - 7 25 ± 1 Static Specific 32
growth
rate
Crustacean Water flea (Daphnia magna) 1 day 21 19 ± 1 Semi- Mortality 0.032
static reproduction 0.1
Insect Mosquito (Culex pipiens) 1st 25 27 ± 1 Semi- Mortality 0.32
instar static development 0.32
Coelente- Hydrozoan (Hydra oligactis) budless 21 18 ± 1 Semi- Specific 100
rate static growth rate
Mollusc Giant Pond Snail 5 months 40 20 ± 1 Semi- Mortality 32
(Lymnaea stagnalis) static Reproduction 10
egg 7 20 ± 1 Semi- Hatching 32
static
Fish Guppy (Poecilia Reticulata) 3-4 weeks 28 23 ± 1 Semi- Mortality 32
Viviparous static Behaviour + 0.1
Mortality
Fish Japanese Ricefish eggs 40 23 ± 2 Semi- Mortality 0.32
(Oryzias latipes) oviviparous static Behaviour 0.32
Hatching 100
Growth
Amphibian Xenopus laevis 2 days 100 20 ± 1 Semi- Mortality 1
static Development 32
Growth 32
---------------------------------------------------------------------------------------------------------
From: Slooff & Canton (1983).
Dimethoate was only slightly repellent to foraging honey-
bees. The self-limiting dose for foraging was 20-25 times the
lethal oral dose (2.9-3.9 µg/bee vs 150 ng/bee). This can be
interpreted on the basis of 5% absorption by foraging bees,
while 95% is passed on to the colony. Thus, systemic insecti-
cide in nectar may also pose a threat to the rest of the colony
when brought back to the hive (Waller et al., 1979).
Residual toxicity has been supported by several obser-
vations. Nectar from plants sprayed with 0.1% dimethoate was
lethal for honey-bees for at least 2-3 days (Jaycox, 1964) or 10
days (Barker et al., 1980). Waller et al. (1984) also showed
the possible toxic levels of residues in the nectar for up to
10 days after treatment of lemon trees with dimethoate at a rate
of 1.12 kg of ai per ha. The high bee mortality observed,
immediately after treatment, was attributed to dimethoate
residues on the plant surface.
7.3.2. Birds
The acute toxicity studies of dimethoate for birds are
summarized in Table 5.
Table 5. Acute oral LD50 of dimethoate for birds (mg/kg)a
----------------------------------------------------------------
Species Sex Pure Laboratory Technical Liquid
grade grade formulation
----------------------------------------------------------------
Hen F 50 40 30 25
Pheasant M 15 - 20 15 20 25
Duck F - > 40 - -
Sparrow M/F - - - 22
Blackbird M/F - - - 26
----------------------------------------------------------------
a From: Sanderson & Edson (1964).
Hens did not show any evidence of delayed neurotoxicity
(Sanderson & Edson, 1964; Gaines, 1969; Francis et al., 1985).
The effect of dimethoate on esterase levels following the
oral dosing of pheasants and following long-term feeding to
pheasants and pigeons was investigated by Bunyan et al. (1968,
1969). Dimethoate inhibited brain-alpha-naphthyl acetate
esterase more than brain-cholinesterase and triacetin esterase
in acute studies.
A characteristic of dimethoate was the elevation of phenyl
benzoate esterase levels, showing that after initial liver
damage, dimethoate is able to induce certain enzymes.
7.3.3. Farm animals
The acute oral LD50s for several farm animals are
summarized in Table 6.
No visible signs of intoxication were seen in horses
receiving dimethoate orally at doses of 25 or 50 mg/kg. Single
doses of dimethoate at 40 mg/kg were effective in removing
Gasterophilus spp. from infected horses, but toxic signs
appeared in animals treated with higher levels of 60-80 mg/kg
(Jackson et al., 1960).
Table 6. Acute oral LD50 for farm animals
-------------------------------------------------
Species LD50 (mg/kg Reference
body weight)
-------------------------------------------------
Horse > 50 Jackson et al. (1960)
Sheep 80 Hewitt et al. (1958a,b)
Cattle 70 Hewitt et al. (1958a,b)
-------------------------------------------------
Mild signs of intoxication occurred in sheep at 75 mg/kg,
including slight salivation, lachrymation, transitory diarrhoea,
rhinitis, and anorexia. Doses lower than 15 mg/kg were essen-
tially asymptomatic in calves. The data with dimethoate
indicate an appreciable margin of safety between the lowest
dose that kills first instar Hypoderma lineatum (5 mg/kg), and
the doses that produce mild toxicity (15-20 mg/kg), or severe,
reversible toxicity (40 mg/kg) (Hewitt et al., 1985b).
Fetcher (1984) described cases of suspected dimethoate
intoxication in cattle grazing on pasture that had been sprayed
6 weeks earlier. There was a predominance of nicotinic signs
and a poor response to atropine treatment. Chemical analysis of
liver, kidney, and brain tissue did not reveal any organo-
phosphorus compounds or metabolites. Whole blood-ChE was
depressed in 3 out of 14 animals.
After spraying barns (for calves) and pigsties with
dimethoate, only 16-29% of the initial concentration still
persisted after 8 weeks. Nevertheless, the animals showed a
decrease in ChE (Müller & Reinhold, 1973).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides, especially their short- and long-term effects on
the nervous systems, can be found in the WHO Environmental
Health Criteria document entitled EHC 63: Organophosphorus
Insecticides, a General Introduction (WHO, 1986a).
8.1. Single Exposures
The acute oral and dermal LD50s of dimethoate for several
animal species are summarized in Tables 7 and 8 (all LD50s are
expressed as active ingredient).
Signs, characteristic of organophosphorus intoxication,
were observed in the rat 0.5-2 h after oral administration of
dimethoate (Sanderson & Edson, 1964). They included muscular
fibrillation, salivation, lachrymation, urinary incontinence,
diarrhoea, respiratory distress, prostration, gasping, coma, and
death (WHO, 1986a).
Oral LD50 values for rats, which were measured in 13
studies, ranged from 150 to 680 mg/kg body weight. The purity
and formulation of the compounds used were not stated in most
of the reports. Oral LD50s were determined of 60-140 mg/kg
body weight for mice, 200 mg/kg body weight for hamsters,
350-600 mg/kg body weight for guinea-pigs, 280-500 mg/kg body
weight for rabbits, and 100 mg/kg body weight for cats.
Administration of 100 mg/kg body weight to dogs did not result
in mortality. The World Health Organization based its classi-
fication of dimethoate as moderately hazardous on an acute oral
LD50 in the rat of 150 mg/kg body weight (WHO, 1986b).
The dermal LD50s for rats were found to range between 500
and 1150 mg/kg body weight, and were of about the same order of
magnitude as the oral LD50s or slightly higher (Table 8).
Data on LD50s after parenteral administration were given by
Sanderson & Edson (1964). The values were comparable with those
for ip, sc, and iv administration. In the rat, the values for
ip administration varied between 175 and 325 mg/kg body weight
and were also of the same order of magnitude as the oral LD50s.
The inhalation LC50 has not been estimated, but, in a 4-h
inhalation study on rats, Panshina (1963b) did not find any
signs of intoxication with exposure to dimethoate at 20 mg/m3
air, 40% cholinesterase inhibition at 10 mg/m3, and no effects
at 2 mg/m3. Visible signs of intoxication were observed in cats
at concentrations of 50-80 mg/m3. At 20 mg/m3, cholinesterase
inhibition was found to be 10-66%, while at 2-8 mg/m3, it was
7-56%. No effects were seen at 1.5 mg/m3 (Panshina, 1963b).
Table 7. Acute oral LD50 of dimethoate in experimental animals
-------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
-------------------------------------------------------------------------
Rat M 32% emulsifiable 247 Edson & Noakes (1960)
solution
Rat M/F technical 185 - 245 West et al. (1961)
Rat 230 Panshina (1963a)
Rat technical 172 Panshina (1963a)
Rat M/F pure 500 - 680 Sanderson & Edson
(1964)
Rat M/F laboratory 280 - 356 Sanderson & Edson
grade (1964)
Rat M/F technical 180 - 336 Sanderson & Edson
(32-40% w/v) (1964)
Rat M/F liquid formul- 150 - 400 Sanderson & Edson
ation (20% ai) (1964)
Rat M/F wettable powder 280 - 300 Sanderson & Edson
(1964)
Rat pure 250 Atabaev & Stepovaya
(1966)
Rat M/F produced 1962 215 - 245 Gaines (1969)a
(43.5%)
Rat pure 200 - 300 Ben-Dyke et al.
(1970)
Rat 250 - 265 Melnikov (1974)
Mouse 99% pure 140 Hewitt et al. (1958b)
Mouse pure 135 Panshina (1963a)
Mouse technical 125 Panshina (1963a)
Mouse F pure 60 Sanderson & Edson
(1964)
----------------------------------------------------------------------------
a Lower figures (20-30 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
Table 7. (contd).
--------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight)
--------------------------------------------------------------------------
Mouse F technical 60 Sanderson & Edson
(1964)
Hamster M laboratory grade 200 Sanderson & Edson
(1964)
Guinea- M/F pure 550 Sanderson & Edson
pig (1964)
Guinea- M/F laboratory grade 600 Sanderson & Edson
pig (1964)
Guinea- M/F technical 350 - 400 Sanderson & Edson
pig (1964)
Guinea- M/F liquid formul- 350 - 370 Sanderson & Edson
pig ation (1964)
Rabbit M/F pure 500 Sanderson & Edson
(1964)
Rabbit M/F laboratory grade 450 Sanderson & Edson
(1964)
Rabbit M/F technical 300 Sanderson & Edson
(1964)
Rabbit M/F liquid formul- 283 Sanderson & Edson
ation (1964)
Cat technical 100 Panshina (1963a)
---------------------------------------------------------------------------
8.2. Skin and Eye Irritation
A single dose of 300 mg technical dimethoate/kg body weight
did not cause skin irritation in male and female rabbits
(Dikshith & Raizada, 1981b).
In a study by West et al. (1961), dimethoate did not have
any irritant effect on the rabbit eye after introduction of
10 mg of dry material into the conjunctival sac. However, in a
personal communication (1986), the US EPA suggested that
dimethoate had a slight irritant effect on the eye.
8.3. Repeated Exposures
The effects on experimental animals of repeated oral or
inhalation exposure to dimethoate are summarized in Tables 9 and
10.
In the various studies, which ranged from 5 1/2-12 months
in duration, inhibition of cholinesterase (ChE) in the erythro-
cytes was a more sensitive indicator of exposure to dimethoate
than ChE inhibition in plasma. ChE activity in the brain was
measured in one study only.
Table 8. Acute dermal LD50 of dimethoate in experimental animals
------------------------------------------------------------------------------
Species Sex Material tested LD50 (mg/kg Reference
body weight
------------------------------------------------------------------------------
Rat M 32% emulsifiable 1120 Edson & Noakes (1960)
solution
Rat liquid formula- 700 - 1150 Sanderson & Edson
tion (24-h) (1964)
Rat wettable powder 500 (24-h) Sanderson & Edson
(1964)
Rat M/F produced 1962 610 Gaines (1969)a
Rat M 32% w/v emulsifi- 770 - 1090 Noakes & Sanderson
able solution (24-h) (1969)
Rat M 32% w/v emulsi- > 1100 (4-h) Noakes & Sanderson
fiable solution (1969)
Guinea-pig liquid formula- 965 West et al. (1961)
tion (46%)
Guinea-pig wettable powder 995 West et al. (1961)
(25%)
Rabbit not specified 600 Melnikov (1974)
------------------------------------------------------------------------------
a Lower figures (55-61 mg/kg body weight) have been reported by the same
author for a material produced in 1959.
In a study by West et al. (1961), no effects were observed
on ChE inhibition in rats administered dimethoate in the diet at
32 mg/kg. In their first study (12 months) on rats, Sanderson
& Edson (1964) observed inhibition of ChE in erythrocytes at
50 mg/kg diet, but not at 10 mg/kg. In the second study (5 1/2
months), inhibition of ChE in erythrocytes was found at both 20
and 10 mg/kg, but not at 5 mg/kg. The studies of Atabaev (1972)
did not show any inhibition of blood-ChE in rats administered a
40% formulation of dimethoate at 0.5-1 mg/kg body weight, corre-
sponding to 0.2-0.4 mg dimethoate/kg body weight. From all
available data on the rat, a dietary level of dimethoate of 5
mg/kg, corresponding to 0.25 mg/kg body weight, can be con-
sidered as the no-observed-adverse-effect level.
From limited studies on the dog (West et al., 1961), it can
be concluded that a level of 10 mg dimethoate/kg diet, corre-
sponding to 0.25 mg/kg body weight, does not result in ChE
depression in erythrocytes.
ChE inhibition was not observed in an inhalation study in
which rats were exposed for 14 h/day, over 3 months, to 0.01 mg
dimethoate/m3 (measured concentration) (Kaloyanova et al.,
1968).
Table 9. The effects on experimental animals of repeated exposure to dimethoate
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat technical 50, 100, or 200 35 days no mortality; no signs West et al. (1961)
(95%) mg/kg diet of intoxication
Rat technical 2, 8,or 32 mg/kg 3 months no mortality; no ChE West et al. (1961)
(95%) diet inhibition
Rat technical 15 mg/kg (oral) 6 months 100% inhibition of ChE Panshina (1963a)
in serum and erythro-
cytes; approximately
85% inhibition in brain
Rat technical 30 mg/kg (oral) 6 months death of 3 out of 5 Panshina (1963a)
animals
Rat technical 60 mg/kg (oral) 6 months death of all animals Panshina (1963a)
Rat (M) laboratory 10 mg/kg diet 12 months no inhibition of ChE in Sanderson & Edson
grade erythrocytes or plasma (1964)
Rat (M) laboratory 50 mg/kg diet 12 months marked inhibition of Sanderson & Edson
grade ChE in erythrocytes (1964)
Rat (M) laboratory 200 mg/kg diet 12 months marked toxic effects; Sanderson & Edson
grade reduced rate of weight (1964)
gain; inhibition of ap-
proximately 70% and 100%
ChE in plasma and erythro-
cytes, respectively
Rat laboratory 800 mg/kg diet 12 months severe toxic effects Sanderson & Edson
grade (1 week) (cholinergic effect: (1964)
weakness, weight loss
after a few days); the
pesticide was withdrawn
after one week; complete
recovery in 10 - 14 days
Rat (M) technical 20 or 10 mg/kg 5 1/2 months 50 and 40% inhibition Sanderson & Edson
(weanling) diet of ChE in erythrocytes, (1964)
respectively
---------------------------------------------------------------------------------------------------------
Table 9. (contd).
---------------------------------------------------------------------------------------------------------
Species Purity Dose Duration Effects Reference
---------------------------------------------------------------------------------------------------------
Rat (M) technical 5 mg/kg diet 5 1/2 months no inhibition of ChE Sanderson & Edson
(weanling) (1964)
Rat 40% formula- 13 mg/kg body 4 months one rat died on the Atabaev (1972)
tion weight (oral) 35th day
Rat 40% formula- 50 mg/kg body 4 months 3 rats died on the 7th Atabaev (1972)
tion weight (oral) day, 2 died on the 8th
day, and one died on the
70th day
Rat 40% formula- 0.5 - 1 mg/kg 6 months no effect on ChE Atabaev (1972)
tion (oral)
Rat 40% formula- 5 mg/kg body 6 months AChE inhibition in Atabaev (1972)
tion weight (oral) blood in the first 2
months (approximately
50%)
Dog technical 2 or 10 mg/kg 13 weeks no inhibition of ChE West et al. (1961)
diet
Dog technical 50 mg/kg diet 13 weeks slight depression of West et al. (1961)
ChE in erythrocytes
Cat technical 10 mg/kg body 3 months death in 2 out of 4 Panshina & Klisenko
weight (oral) animals (1962)
Cat technical 20 mg/kg body 3 months death in 2 out of 3 Panshina & Klisenko
weight (oral) animals (1962)
Cat 40% formula- 0.5 - 1 mg/kg 3 months no effect on ChE Atabaev (1972)
tion body weight
(oral)
Cat 40% formula- 2 mg/kg body 3 months reduced body weight in Atabaev (1972)
tion weight (oral) the first 2 months
(16 - 32%); recovery at
the third month
---------------------------------------------------------------------------------------------------------
Table 10. Inhalation toxicity - repeated exposure
---------------------------------------------------------------------------------------------------------
Species Concentration Duration Effects Reference
(mg/m3) Daily exposure Number of
(h) months
--------------------------------------------------------------------------------------------------------
Rat 2 ? 2 no visible signs of intox- Panshina (1963b)
ication; 26% inhibition of
AChE in blood at the end of
the study
Cat 1.5 ? 1.5 no visible signs of intox- Panshina (1963b)
cation; 40-72% inhibition
of AChE in blood at the end
of the study
Rat in a miniature greenhouse (0.6 m3; temperature no blood-ChE inhibition Sanderson & Edson
21-30 °C); plants sprayed with 5 ml of 0.5% (1964)
aqueous formulation in the course of 28 days
Rat and 4.50 8 3 inhibition of ChE; leuk- Kaloyanova et al.
guinea-pig ocytosis (1968)
Rat 0.05 14 3 inhibition of ChE in blood Kaloyanova et al.
only in the first month; (1968)
inhibition of brain- and
liver-AChE; morphological
alterations in the neurons
Rat 0.01 14 3 no changes observed Kaloyanova et al.
(1968)
Rat 0.495 24 3 inhibition of ChE and Ubaidullaev &
changes Madzhidov (1976)
Rat 0.049 24 3 inhibition of ChE Ubaidullaev &
Madzhidov (1978)
Rat 0.003 24 3 no detectable changes Ubaidullaev &
Madzhidov (1978)
---------------------------------------------------------------------------------------------------------
8.4. Reproduction Studies
One reproduction study on mice has been reported in the
literature (Budreau & Singh, 1973). In this 5-generation study,
initial groups of 14 female and 10 male mice received dimethoate
in the drinking-water at 60 mg/litre for a period of one month,
prior to mating. A comparable control group was included in
the study. Animals receiving dimethoate showed a significant
( P < 0.05) reduction in mating performance (expressed as pro-
portion of females with deliveries to females mated), which
ranged from 33 to 61%, varying with litter and generation.
Similarly, reproduction time (measured as number of days elaps-
ing from first day of mating to day of delivery) was signifi-
cantly ( P < 0.01) increased in all first litters of all 5
generations examined, but unaffected in all second litters. The
biological relevance of this observation is unclear. Litter
size and average weight at birth were not affected by treatment.
Although the mean weights of treated pups were not significantly
lower, the growth rate was consistently lower in treated pups
compared with that in the controls.
A 3-generation reproduction study using technical
dimethoate (98.3%) was carried out on mice. Information sup-
plied by the US EPA indicated that there were no effects on re-
production and teratogenicity at a dimethoate level of 50 mg/kg
(US EPA, personal communication, 1986). Details of this study
were not available.
8.5. Teratogenicitya
Intraperitoneal administration of 40 mg dimethoate/kg body
weight, given as a single dose on the day of mating or on the
9th day of gestation, or given for the first 14 days of
gestation in mice, caused a high incidence of embryonal loss
(Scheufler, 1975).
Cygon 4E (containing 47.3% dimethoate) was given to female
rats by intubation from the 6th to the 15th day of gestation at
dose levels of 3, 6, 12, or 24 mg/kg body weight. The 24 mg/kg
dose was toxic for the dams (8 out of 20 dams manifested clonic
spasms and muscular tremors during the treatment period, 7 re-
covered, and one died on the 16th day of pregnancy). Doses of
12 and 24 mg/kg were associated with an increase ( P < 0.05)
in the numbers of anomalous litters (each having at least one
anomalous fetus) and wavy-ribbed fetuses. The 3 and 6 mg/kg
doses (equal to 1.42-2.84 mg dimethoate/kg) did not produce any
evidence of teratogenicity or embryotoxicity in the rats (Khera
et al., 1979).
----------------------------------------------------------------
a A further teratogenicity study on the rat (Edwards et al.,
1984) was submitted to the FAO/WHO Joint Meeting on Pesti-
cide Residues (JMPR) in 1987. Although fetotoxicity was ob-
served, there were no teratogenic effects (FAO/WHO, 1987).
Cygon 4E (47.3% dimethoate) was given to cats in gelatin
capsules at doses of 3, 6, or 12 mg/kg on the 14th-22nd days of
pregnancy. At the levels tested, the compound did not produce
any effects on the incidence of pregnancy. In the 12 mg/kg
group, forepaw polydactily was observed in 8 out of 39 fetuses.
Cygon 4E at 3 or 6 mg/kg (1.42-2.84 mg dimethoate/kg) did not
produce any effects (Khera, 1979). (The effect observed in
Khera's investigations may be due to the other components in the
formulation).
Courtney et al. (1985) reported that dimethoate adminis-
tered orally was not teratogenic in CD-1 mice at dose levels of
10 or 20 mg/kg body weight, and that these levels were not
lethal to the dams. The two highest dose levels of 40 and
80 mg/kg produced maternal toxicity.
8.6. Mutagenicity
A variety of in vivo and in vitro mutagenicity tests have
been carried out with dimethoate, the results of which are given
in Table 11.
Negative mutagenicity results were reported by Degraeve et
al. (1984) for commercial mixtures of insecticides containing
dimethoate. Two formulations were tested: dimethoate + fenitro-
thion (dose 60 mg/kg body weight, corresponding to 9 mg/kg of
each) and dimethoate + malathion + methoxychlor (dose 100 mg/kg
body weight corresponding to 9.5 mg dimethoate/kg). A single
ip injection did not induce chromosome aberrations in bone
marrow cells, spermatogonia, or primary spermatocytes of the
mouse. No significant increases in pre- or post-implantation
fetal lethality were observed in a dominant lethal mutation
assay.
Alkylation by dimethoate at the N-7 position of guanine in
DNA has been investigated (Dedek et al., 1984). Male mice
(strain AB Jena/Halle, random bred) were injected ip with 14C-
methyl-labelled dimethoate at a dose of 0.35 mmol/kg. The
extent of methylation was in the range of 1-10 µmol N-
7 methylguanine/mol guanine; the values in the kidneys were
higher than those in the liver. The excretion half-life of N-
7 methylguanine was 23-160 h.
In summary, dimethoate has been found to be mutagenic in
both in vitro and in vivo assays.a
----------------------------------------------------------------
a After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
Table 11. Mutagenicity tests
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Escherichia coli 1 - 6.10-3 mmol 5-methyltryptophane resistance Mohn (1973)
K-12/gal Rs mutation -positive
Escherichia coli Up to 5000 µg/plate positive Moriya et al.
WP2 hcr (1983)
Escherichia coli Minimum mutagenic positive Probst et al.
WP2 & WP2uvrA- concentration (1981)
47 nmoles/ml
Salmonella typhimurium negative Probst et al.
TA 98, 100, 1535, 1537, (1981)
1538
Salmonella typhimurium Up to 5000 µg/plate positive with and without Moriya et al.
TA 100 activation (1983)
Salmonella typhimurium Up to 5000 µg/plate negative Moriya et al.
TA 98, 1535, 1537, 1538 (1983)
Salmonella typhimurium 5 - 200 µg/plate positive at all doses, mutagenicity Vishwanath &
TA 100 (base pair subst.) increased with liver microsomes Jamil (1986)
Saccharomyces cerevisiae 7 doses, 40 - 100 mmol induction of mitotic gene Fahrig (1974)
conversions
Schizosaccharomyces pombe 1.3 - 131 mmol negative Gilot-Delhalle
ade 6 (LD50 50 mmol) (1983)
Chinese hamster ovary 20,40 & 80 µg/ml sister chromatid exchange increase Chen et al.
cells V79 10 µg/ml negative (1981)
Rat hepatocytes culture 47 nmol/ml negative unscheduled Probst et
al. (1981)
SV-40 transformed human 100 & 1000 umol positive unscheduled DNA Ahmed et
synthesis al. (1977)
Drosophila melanogaster 1 mg/kg (feeding) negative Woodruff et
al. (1983)
---------------------------------------------------------------------------------------------------------
Table 11. (contd).
---------------------------------------------------------------------------------------------------------
Tests Concentration of Results Reference
dimethoate used
---------------------------------------------------------------------------------------------------------
Drosphila melanogaster adult inj. ip 0, 10, increase in sex-linked Velazquez et
20 mg/kg body weight recessive lethals at 10 mg/kg al. (1986)
not at 20 mg/kg
Drosophila melanogaster adult feeding 0, negative Velazquez et
10 mg/kg; larval negative al. (1986)
feeding 0, 20 mg/kg
Host-mediated assay mouse 3 equal oral doses positive - mutation factor of Usha Rani et
Salmonella typhimurium of 155 mg/kg 3.44 ( P < 0.05) al. (1980)
Dominant lethal mutation acute 10 mg/kg ip negative Degraeve &
assay mice strain Q 7 weeks 0.6 mg/litre Moutschen
drinking-water, equiv- (1983)
alent to 0.093 mg/kg
body weight
Dominant lethal test 30 & 60 mg/kg body negative Fischer &
- AB Jena Halle mice 5 x 6 mg/kg body weight Scheufler
ip (1981)
DBA mice 3 x 18 mg/kg body weight
ip
Micronucleus test - 2 equal doses of a significant increase in Usha Rani et
mice - bone marrow 51.7 mg/litre drink- frequency of polychromatic al. (1980)
ing-water at 24-h erythrocytes with micronuclei
intervals -0.85% (controls 0.28%)
Chromosome abnormalities 50 and 100 mg/kg body positive Bhunya & Behera
mice - bone marrow weight (1975)
Mice - bone marrow 20 mg/kg body weight no abnormalities Nehés et al.
ip (1982)
Mice - bone marrow 60 mg/kg body weight increase in number of mitosis, Nehéz et al.
ip aberrations (1983)
Syrian golden hamsters 16, 32, 80, or increase in number of chromatid Dzwonkowska &
Mesocricetus auratus 160 mg/kg body weight and chromosomal breaks Hübner (1986)
ip single (no dose-response relationship)
---------------------------------------------------------------------------------------------------------
8.7. Carcinogenicity
Two carcinogenicity studies, reported by Gibel et al.
(1973), are summarized below.
Rat: Groups of 40, 10-week-old Wistar rats were intubated
with 0 (control), 5, 10, or 30 mg dimethoate/kg body weight
(twice weekly) for the life span. The mean life spans were 743,
518, 511, and 627 days at 0, 5, 15, and 30 mg/kg body weight,
respectively. No malignant tumours were observed in the 36
control animals. In test groups, malignant tumours occurred
in 2/26, 3/25, and 4/20 rats, respectively, at the 3 dose
levels. At 5 mg/kg body weight, a reticulosarcoma of the spleen
with metastases and a malignant reticuloma were observed. At
15 mg/kg body weight, a sarcoma of the colon, a reticulosarcoma
of the spleen (with pancreatic metastases) and a hepatocellular
carcinoma occurred. At 30 mg/kg body weight, a liver fibro-
sarcoma, a malignant reticuloma and two reticulosarcomas of the
spleen were noted. Three, 7, 5, and 2 benign tumours were
noted, respectively, in the controls and at 5, 15, and 30 mg/kg
body weight (Gibel et al., 1973).
Rat: Groups of 40, ten-week-old Wistar rats were injected
im with 15 mg dimethoate/kg body weight (twice weekly) or with
isotonic saline until spontaneous death occurred. The mean life
spans were 711 and 570 days in the saline and dimethoate-treated
animals, respectively. No malignant tumours occurred in the
saline-treated group, but 4/35 animals developed benign tumours.
In the dimethoate group, 6/30 rats had malignant tumours (a
spleen reticulosarcoma, a spleen fibrosarcoma, an ovarian
alveolar sarcoma, a liver hepatocellular carcinoma, a malignant
reticuloma, and a soft tissue spindle-cell sarcoma) and 5/30 had
benign tumours. The first malignant tumour (a splenic
fibrosarcoma) was noted after 410 days (Gibel et al., 1973).
Dimethoate (technical grade, 90-100% pure) was given in the
feed to Osborne Mendel rats and to B6C3F1 mice. Groups of 50
male and 50 female rats (10 animals in each control group)
received "time-weighted average doses" of 155 or 310 mg/kg for
male rats and 192 or 384 mg/kg for female rats. After 80 weeks,
all groups received control diets, and the studies were con-
cluded at week 114.
Groups of 50 male and 50 female B6C3F1 mice were given
dimethoate in the diet at concentrations of 0 (10 "matched"
controls), 250, or 500 mg/kg for 60-69 weeks (males) or for 80
weeks (females). The studies were ended after 94 weeks.
Tremors and hyperexcitability were observed in exposed animals;
rats and mice that survived to termination were generally in
poor condition. Survival was reduced in the high-dose groups of
rats. Several non-neoplastic lesions occurred more frequently
in dimethoate-exposed animals. No increases in neoplasia were
reported to be associated with dimethoate administration, for
any of the organs or tissues examined histologically (NCI-1977).
These studies are not considered adequate to determine properly
the presence or absence of a carcinogenic response, largely
because of the shorter than usual duration of exposures, the
poor condition of the animals, and changes in exposure concen-
trations.a
8.8. Special Studies
The action of dimethoate on the immune system was studied
in mice and rats by Tiefenbach & Lange (1980). A single dose of
75 mg dimethoate/kg (route not specified) decreased the
lymphocyte count to 50% of the pre-exposure value and increased
the neutrophile granulocytes. After 72 h, these changes
returned to normal. A reduction in the thymus cortex with
disrupted lymphocytes, and a reduction in the number of rosette-
forming cells were observed.
When administered to rats at 5-30 mg/kg body weight orally
or 15 mg/kg im, twice a week, until death, dimethoate caused
hyperplasia in the bone marrow, mainly in granulocytopoiesis
(Stieglitz et al., 1974). The authors interpreted the changes
in the haematopoietic system of the rats as a direct haemato-
logical effect of dimethoate.
The effects of dimethoate on the heart were investigated
in rabbits (Mahkambaeva, 1971), and guinea-pigs and rats
(Nadmaiteni & Marosi, 1983). After oral administration of
150 mg dimethoate/kg to rabbits, the effects observed included
bradycardia and increased atrio-ventricular and intraventricu-
lar conductance, with complete recovery after 4-7 days. In rats
and guinea-pigs, a dose-effect relationship was established for
heart rate disturbances, and atrio-ventricular block. An
electron-microscopic study of the myocard did not reveal any
changes. The ip doses that were tested ranged from 500 to
1500 mg/kg body weight.
----------------------------------------------------------------
a After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious ECG disturbances
developed in the early phase of intoxication. The toxic cardiac
phenomena appeared to be unrelated to the degree of cholin-
esterase inhibition, but were correlated with the myocardial
dimethoate concentration. Cardiac failure and mortality were
first observed at a level of about 110 µg/g, while a level of
221 µg/g resulted in death in all cases. The present investi-
gation refers to the direct effect of dimethoate on the
myocardium, independent of its anticholinesterase action (Marosi
et al., 1985a,b).
8.9. Factors Modifying Toxicity
The acute toxicity of dimethoate was not affected by the
dietary content of protein (Boyd & Muis, 1970). The oral LD50
was 147 mg/kg for male albino rats fed for 28 days from weaning
on a diet containing 3.5% protein as casein and, in controls
maintained on a diet containing 26% casein at 152 mg/kg. Other
groups maintained on a diet that contained 24% protein from
various plant and animal sources were apparently less suscep-
tible to dimethoate (LD50, 358 mg/kg). Dimethoate was given to
rats orally for 10 weeks at a dose of 10 mg/kg in diets contain-
ing 90, 160, or 240 g protein/kg (Gontzea & Gorcea, 1977). The
high-protein (240 g) diet diminished the adverse effects of
dimethoate on the growth of the rats and lessened its antichol-
inesterase activity in plasma, total blood, brain, and liver.
A marked increase in the toxicity of dimethoate was noted
in male and female mice after pre-treatment with phenobarbital
and with chlorinated hydrocarbons (DDT and dieldrin) (Menzer &
Best, 1968; Menzer, 1970). The toxicity of dimethoate was
increased from an ip LD50 of 198 mg/kg body weight to 58.5 mg/kg
by pre-treatment of mice for 3 days with sodium phenobarbital.
Liquid formulations of technical dimethoate in the
solvents, 2-methoxy- and 2-ethoxyethanol, showed increased tox-
icity after storage. After storage for 7 months in England and
9 months under tropical conditions, the oral LD50 for the rat
decreased to 30-40 mg/kg and 15 mg/kg, respectively (from an
initial 150-250 mg/kg). The most toxic conversion product was
O,O-dialkyl S-(N-methylcarbamoylmethyl) phosphorothioate with
an oral LD50 for the rat of 1 mg/kg (Casida & Sanderson, 1961).
8.10 Mechanisms of Toxicity; Mode of Action
The mode of action of organophosphorus insecticides is
decribed in WHO (1986a). They act principally by inhibition of
acetyl cholinesterase (AChE) at the cholinergic synapses.
Dimethoxon (omethoate), a metabolite of dimethoate, is
75-100 times more potent than dimethoate in inhibiting AChE,
suggesting that this metabolite plays a dominant role in
mammalian toxicity (Hassan et al., 1969). The LD50 of omethoate
in rats is 25-28 mg/kg body weight (FAO/WHO, 1979); it is about
10 times more toxic than dimethoate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
Cases of both accidental and suicidal poisoning have been
reported with dimethoate. Hayes (1982) has reviewed the human
toxicity and poisoning cases.
Nagler et al. (1980) described a case of attempted suicide
of a 34-year-old female who ingested 10 g dimethoate. Half an
hour after admission to the hospital, the serum-dimethoate level
was 2340 mg/litre. Combined haemoperfusion and haemodialysis
were applied and, after 18 h, dimethoate was no longer detected
in the serum.
A severe case of poisoning after ingestion of approximately
20 g dimethoate was reported by Köppel et al. (1986). On
admission, the 52-year-old man was comatose with unmeasurable
pseudo-cholinesterase (< 200 U/litre). He had been admitted 2 h
after ingestion and received, every 20 min, an injection of
20 mg atropine. Two haemoperfusions with activated charcoal and
amberlite were performed, and atropine was given by infusion up
to day 12. Twenty-five days after admission, he was discharged,
fully recovered.
De Reuck et al. (1979) presented a case of a patient who
died on the 9th day after dimethoate poisoning with an atypical
central neurological disorder. The neuropathological findings,
which were similar to those observed in severe forms of
Wernicke's encephalopathy, included severe haemorrhagic necrosis
of the walls of the ventricles. The authors suggested that the
increased level of acetylcholine in the brain had led to
thiamine depletion in the regions of predilection of Wernicke's
encephalopathy.
Many other cases of human dimethoate poisoning, some of
which were fatal, have been reported, but the information given
was insufficient (Molphy & Rathus, 1964; Masiak & Olajossy,
1973; François & Verbraeken, 1977; Demeter & Heyndrickx, 1978;
Ebel & Karyofilis, 1978; Areekul et al., 1981; Wehran & Hammer,
1984; Bolgar et al., 1985; Le Blanc et al., 1986; Senanayake &
Karalliedde, 1987).
On the basis of a number of cases, the oral lethal dose for
human beings was estimated to be of the order of 50-500 mg/kg
body weight (Gosselin et al., 1984).
Trinh van Bao et al. (1974) reported an increase in the
frequency of breaks and stable chromosome aberrations in 2
patients who died after dimethoate intoxication.
Thirty firemen, exposed to dimethoate in the air as a
result of an accident in a dimethoate-manufacturing plant,
developed symptoms of intoxication (Larripa et al., 1979, 1983).
Peripheral blood lymphocytes from 20 of these workers were
examined for the frequency of sister chromatid exchanges, 2
months after the accident. The frequencies were 9.2 ± 0.2 for
the exposed and 8.5 ± 0.2 for unexposed persons ( P < 0.05).
Dicentric chromosomes and a low frequency of chromatid breaks
were found in 2 exposed workers. It is not certain to what the
firemen were exposed besides dimethoate.
9.1.2 Controlled human studies
The results of a number of studies in which human volun-
teers, without occupational exposure to organophosphates, were
given dimethoate, are summarized in Table 12. From these
studies it can be concluded that repeated doses of up to
0.2 mg/kg body weight did not inhibit cholinesterase activity in
the blood.
9.2 Occupational Exposure
9.2.1 Poisoning incidents
One of the first cases of poisoning with dimethoate (Rogor)
in agriculture was described by Muratore et al. (1960). A 28-
year-old-farmer, who reportedly had worn protective rubber
clothing and equipment, had sprayed olive trees with dimethoate
the day before he was hospitalized. The man began to experience
profound weakness, faintness, and somnolence, followed by
attempts to vomit, chills, and profound prostration on the day
he was hospitalized. His general condition was found to be
serious; his pulse was weak, he exhibited pronounced myosis,
vomited and sweated profusely, and had pronounced inhibition of
cholinesterase activity. Treated with large doses of atropine
(20 mg on day 2, 12 mg on day 3, and 5 mg until day 9),
prednisone, analgesics, and penicillin, he recovered.
A case of poisoning in a woman working in agriculture and
exposed to dimethoate in the field, 2 days after spraying, was
reported by Asatryan (1971). Within 3-3.5 h after beginning
work, the woman noted an unpleasant odour recognized as
dimethoate. She developed headache, dry cough, dyspnoea,
nausea, vomiting and was admitted to hospital in a somnolent
state with muscular fibrillations and an asthmatic component.
After treatment with saline solution, glucose, caffeine,
atropine, and insulin, the state of acute intoxication was
overcome. Allergic symptoms were treated with dimedrol.
Contact allergy due to dimethoate was reported in a 53-
year-old female. She had a positive skin test (Pambor & Bloch,
1985).
Table 12. Controlled human studies
---------------------------------------------------------------------------------------------------------
Number Sex Route of Dose/day Duration of Results Reference
of Admini- exposure
subjects stration
---------------------------------------------------------------------------------------------------------
20 oral 0.04 mg/kg 4 weeks absence of toxic effects Sanderson & Edson
or blood-ChE inhibition (1964)
2 oral 0.13 mg/kg; 21 days absence of blood-ChE Sanderson & Edson
0.26 mg/kg inhibition (1964)
5 M oral 0.25 mg/kg single dose absence of toxic effects Sanderson & Edson
or ChE inhibition (1964)
50 dermal 32% liquid form- 2-h patch absence of skin irritation Sanderson & Edson
ulation, up to test and ChE inhibition (1964)
2.5 ml
12 M/F oral 5 mg (0.068 28 days no significant change in Edson et al. (1967)
mg/kg body weight)
9 M/F oral 15 mg (0.202 39 days no significant change in Edson et al. (1967)
mg/kg body weight)
8 M/F oral 30 mg (0.434 57 days inhibition of ChE by the Edson et al. (1967)
mg/kg body weight)
6 oral 45 mg (0.587 45 days inhibition of ChE (35%) Edson et al. (1967)
mg/kg body weight)
6 M/F oral 60 mg (1.02 14 days inhibition of ChE (21%) Edson et al. (1967)
mg/kg body weight)
---------------------------------------------------------------------------------------------------------
Female greenhouse workers working with dimethoate were
reported to have a high percentage of specific leukocyte
agglomeration, a raised index of lymphoblastic transformations,
and antibodies against dimethoate (Zolotnikova & Somov, 1978;
Zolotnikova, 1980). With increasing duration of work, progress-
ive sensitization towards the pesticide was observed.
On the basis of epidemiological observations and of dermal
testing of workers with a 1-2% solution of dimethoate, Jung
(1979) concluded that the index of sensitization was very low
for dimethoate and that general intoxication might occur, but
rarely contact eczema.
9.3 Early Symptoms and Treatment of Poisoning
Initial symptoms of poisoning may include sweating,
headache, weakness, giddiness, nausea, vomiting, stomach pains,
blurred vision, slurred speech, and muscle twitching (WHO,
1986a). Arrhythmias and cardiac failure have been reported
(Kiss & Fazekas, 1983; Marosi et al., 1985a,b; Duval et al.,
1986). The most important diagnostic finding is inhibition of
blood-cholinesterase activity. For a full discussion of the
clinical picture and treatment of organophosphate insecticide
poisoning, see WHO (1986a). The section on treatment is
presented in the annex to this publication.
In all cases of clinical poisoning with dimethoate and
other organophosphorus insecticides, it is essential to maintain
general surveillance and cholinesterase and cardiac monitoring
for at least 4 days, and longer if necessary, and to adapt
general supportive and specific therapy in accordance with the
findings.
Data on the effects of oxime reactivators in dimethoate
poisoning are contradictory, some indicating that they may have
a negative effect on cholinesterase inhibition (Sanderson &
Edson, 1959; Durham & Hayes, 1962; Molphy & Rathus, 1964;
Erdmann et al., 1966; Zech et al., 1966; Ebel & Karyofilis,
1978; Sterri et al., 1979). It is therefore suggested, that if
oxime reactivators are indicated, these should be used with
caution and under close supervision.
Haemoperfusion may be effective in the early stages of
dimethoate poisoning (Okonek 1976, 1977; Okonek et al., 1976;
Pach et al., 1977; Nagler et al., 1980).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Toxicity of Dimethoate
Dimethoate is moderately toxic. Most oral LD50s in the rat
ranged from 150-400 mg/kg body weight. The dermal LD50 in the
rat was greater than 600 mg/kg body weight. Dimethoate is not
irritating to the skin, but may be slightly irritating to the
eye.
It can be absorbed following ingestion, inhalation, and
skin contact. It is readily metabolized and excreted and does
not accumulate in the body. Dimethoxon (omethoate), the oxygen
analogue found in plants, insects, and mammals, is about 10
times more toxic and is a more potent inhibitor of cholin-
esterase activity than dimethoate.
Signs of intoxication from exposure to dimethoate are those
typical of organophosphorus pesticides. Inhibition of erythro-
cyte-cholinesterase is the most sensitive indicator of exposure
to dimethoate and may indicate toxicity. A dietary level of
5 mg dimethoate/kg, equivalent to 0.25 mg/kg body weight, is
considered to be a no-observed-adverse-effect level in the
rat.a
A 5-generation reproduction study in mice given drinking-
water containing dimethoate at 60 mg/litre resulted in decreased
mating success and increased reproduction time in all gener-
ations. On the basis of available data on experimental animals,
dimethoate is not considered to be a teratogen.
Dimethoate is considered mutagenic in a variety of in vitro
and in vivo test systems.b Long-term studies have been
conducted involving oral administration in rats and mice and im
injection in rats. However, the available data are considered
---------------------------------------------------------------
a FAO/WHO (1987) concluded that a dietary level of 1 mg/kg,
equivalent to 0.05 mg/kg body weight is the no-observed-
adverse-effect level in the rat.
b After this statement was written, new well-performed
negative mutagenicity studies were submitted to the FAO/WHO
Joint Meeting on Pesticide Residues (JMPR) in 1987 including
an in vitro mutation assay on Chinese hamster ovary cells
(Johnson et al., 1985), a dominant lethal test in mice
(Becker, 1985), a micronucleus test in mice (Sorg, 1985),
and a metaphase analysis assay in rat bone marrow cells (San
Sebastian, 1985). On the basis of these new studies and the
published studies, the JMPR concluded that dimethoate is
mutagenic in bacterial tests, but negative in mammalian
cells and in vivo tests (FAO/WHO, 1987).
to be inadequate to assess the carcinogenic potential of
dimethoate.c
10.2. Human Exposure
Air and water are negligible sources of exposure to
dimethoate for the general population. Residues found in food
are generally below the acceptable daily intake (ADI) set by the
FAO/WHO Joint Meeting on Pesticide Residues (JMPR) (See section
12).
Whole blood-cholinesterase was not inhibited in human
volunteers given oral doses of 0.2 mg dimethoate/kg body weight
for 39 days.
Several cases of suicidal or accidental poisoning due to
ingestion of dimethoate have been reported.
Occupational exposure to dimethoate, principally through
inhalation and the skin, may occur during its manufacture,
formulation, and use, and cases of poisoning as a result of
accident or neglect of safety precautions have been reported.
The oral lethal dose for human beings has been estimated to be
in the range of 50-500 mg/kg body weight.
Skin sensitization due to dimethoate has been observed in
some cases.
10.3. Evaluation of the Effects on the Environment
Dimethoate is rapidly hydrolysed and does not persist in
the environment. The toxicity of dimethoate for aquatic
organisms and birds is moderate to high. It is very toxic for
honey-bees.
---------------------------------------------------------------
c After the Task Group met, new well-performed long-term/
carcinogenicity studies in rats and mice were submitted to
the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in
1987. No indication for carcinogenicity was found. In the
mouse study, the lowest dose of 25 mg/kg produced decreased
body weight, decreased cholinesterase activity in erythro-
cytes, and also slight extramedullary haematopoiesis in the
spleen (Hellwig, 1986a). Administration of dimethoate to
rats for 2 years (Hellwig, 1986b) also resulted in a
decrease in body weight, decreased cholinesterase activity
in erythrocytes and the brain, and slight anaemia. No
effects were observed at 1 mg/kg (0.05 mg/kg body weight)
(FAO/WHO, 1987).
10.4. Conclusions
1. Under proper conditions of use, exposure of the general
population to dimethoate is negligible.
2. When appropriate safety precautions are observed, exposure
to dimethoate during manufacture, formulation, use, and
disposal should not pose an unacceptable human health
hazard.
3. Dimethoate is rapidly degraded and not persistent in the
environment. Care must be taken not to expose honey-bees,
fish and aquatic organisms, and birds.
11. RECOMMENDATIONS
1. Figures relating to the current production and use of
dimethoate should be made available.
2. Studies are necessary on dermal sensitization by
dimethoate.
3 Data are required on the absorption and disposition of
dimethoate from different routes of exposure.
4. Long-term toxicology and carcinogenicity studies should be
carried out on laboratory animals.a
5. More research is required to investigate the in vivo
mutagenic effects of dimethoate.a
6. Reproduction and teratology studies should be carried
out.a
7. Epidemiological studies on persons engaged in the
manufacture and professional use of dimethoate should be
considered, and workers should be monitored for exposure to
dimethoate and for potential adverse health effects.
8. Cleaning and disposal of contaminated equipment, clothing,
and containers should be in accordance with recommended
procedures.
9. Further work is necessary to establish safe re-entry
periods under different conditions of use.
10. Acute toxicity studies should be carried out on
formulations in which dimethoate is mixed with other active
ingredients.
11. The role of oxime reactivators in the treatment of human
poisoning by dimethoate, should be clarified.
12. Information should be obtained concerning the changes in
toxicity, due to impurities, that can arise in pesticides
as a consequence of different manufacturing processes, the
use of formulating ingredients, and improper storage.
---------------------------------------------------------------
a Several of these studies were later submitted to the
FAO/WHO Joint Meeting on Pesticide Residues in 1987. See
previous footnotes for sections 8.5, 8.6 and 8.7.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dimethoate was evaluated by the Joint Meeting on Pesticide
Residues (JMPR) in 1963, 1965, 1966, 1967, 1970, 1973
(evaluation of the related compound formothion), 1977, 1978,
1984, and 1987 (FAO/WHO, 1964, 1965, 1967, 1968, 1971, 1974,
1978, 1979, 1985, 1987).
The estimate of an acceptable daily intake (ADI) for man
for dimethoate is 0-0.01 mg/kg body weight, based on the no-
observed-adverse-effect level in man of 0.2 mg/kg body weight
per day, and in the rat of 1 mg/kg diet, equivalent to
0.05 mg/kg body weight (FAO/WHO, 1987).
A data sheet on dimethoate has been issued by WHO in the
series "Data Sheets on Pesticides" (WHO/FAO, 1980).
In the WHO Recommended Classification of Pesticides by
Hazard, technical dimethoate is classified as moderately
hazardous, when handled in accordance with instructions (WHO,
1986b).
The IRPTC (1982) has issued a review on dimethoate, in its
series "Scientific Reviews of Soviet Literature on Toxicity and
Hazards of Chemicals".
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-
67. III. Organophosphorus pesticide residues in the total diet.
Pestic. Sci., 1: 10-13.
ABRAMOVA, J.I. & BOLTUSHKINA, L.A. (1968) [Safety action of
several vitamins in poisoning with organophosphorus insecti-
cides.] In: [Hygiene and toxicology of pesticides and the
clinical picture of intoxications,] Vol. 6, Kiev, Zdorovye
Publishers, pp. 448-452 (in Russian).
ADAMIS, Z., ANTAL, A., FUZESI, I., MOLNAR, J., NAGY, L., &
SUSAN, M. (1985) Occupational exposure to organophosphorus
insecticides and synthetic pyrethroids. Int. Arch. occup.
environ. Health, 56: 299-305.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res.,
42: 161-174.
AKORONKO, S.L. & GIRENKO, D.B. (1977) [Methods of estimating
fosfamid in soil using chromatographic methods.] In: [ Methods
of determination of micro-quantities of pesticides in foods,
feed, and the environment,] Moscow, Kolos, pp. 118-120 (in
Russian).
ANEES, M.A. (1975) Acute toxicity of four organophosphorus
insecticides for a fresh-water teleost Channa punctatus
(Bloch). Pak. J. Zool., 7(2): 135-141.
ANEES, M.A. (1978a) Hepatic pathology in a fresh-water teleost
Channa punctatus (Bloch) exposed to sub-lethal and chronic
levels of three organophosphorus insecticides. Bull. environ.
Contam. Toxicol., 19(5): 524-527.
ANEES, M.A. (1978b) Haematological abnormalities in a fresh-
water teleost Channa punctatus (Bloch) exposed to sublethal and
chronic levels of three organophosphorus insecticides. Int. J.
Ecol. environ. Sci., 4: 53-60.
AREEKUL, S., SRICHAIRAT, S., & KIRDUDOM, P. (1981) Serum and
red cell cholinesterase activity in people exposed to organo-
phosphorus insecticides. Southeast Asian J. trop. Med. pub.
Health, 12(2): 94-98.
ARMELI, G., BARTALINI, E., & PISANI, F. (1967) [Occupational
pathology and prevention in the industrial production of rogor].
Lav. Um., 20(8): 355-356 (in Italian).
ASATRYAN, R.S. (1971) [Some peculiarities of acute pesticide
poisonings in application in Ararat land.] In: [ Hygiene and
toxicology of pesticides and the clinical picture of intoxica-
tions,] Kiev, Zdorovye Publishers, pp. 315-318 (in Russian).
ATABAEV, SH.T. (1972) [Rogor (phosphamide).] In: [ Pesticides
and environmental hygiene in hot climate conditions,] Tashkent,
Medizina, pp. 77-93 (in Russian).
ATABAEV, SH.T. & STEPOVAYA, N.E. (1966) [Allowance of phospha-
mide in apples.] In: [ Hygiene and toxicology of pesticides and
the clinical picture of intoxications,] Kiev, Zdorovye Publish-
ers, pp. 280-285 (in Russian).
AWASTHI, M., SHAH, P., DUBALE, M.S., & GADHIA, P. (1984) Meta-
bolic changes induced by organophosphates in the piscine organs.
Environ. Res., 35(I): 320-325.
BARANOVA, N.P., ALEXANDROVA, L.G., & KOVALENKO, V.F. (1986)
[Tissue distribution and biochemical effects of dimethoate
entering the body via the skin.] Gig. tr. prof. Zabol., 3: 20-
23 (in Russian).
BARKER, R.J., LEHNER, Y., & KUNZMANN, M.R. (1980) Pesticides
and honey bees: nectar and pollen contamination in alfalfa
treated with dimethoate. Arch. environ. Toxicol., 9: 125-133.
BECK, E.W., JOHNSON, J.C., Jr, GETZ, M.E., SKINNER, F.B.,
DAWSEY, L.H., WOODHAM, D.W., & DERBYSHIRE, J.C. (1968) Effects
of feeding dimethoate, its oxygen analog, and dimethoate-treated
silage to cattle. J. econ. Entomol., 61(3): 605-610.
BECKER, H. (1985) Dominant lethal study with dimethoate
technical in the mouse. (Unpublished report no. 039003, dated 12
September 1985 from Research & Consulting Company AG, 4452
Itingen/Switzerland).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest Contr., 9(3):
119-127.
BHUNYA, S.P. & BEHERA, J. (1975) Centromeric sensitivity of
mouse chromosomes to the systemic insecticide dimethoate
(Rogor). Curr. Sci., 44(23): 859-860.
BOHN, W.R. (1964) The disappearance of dimethoate from soil.
J. econ. Entomol., 57(6): 798-799.
BOLGAR, G., JOJART, G., & TURI, J. (1985) [Spontaneous delivery
of a pregnant woman after dimethoate (Bi 58 EC) poisoning.]
Orvosi Hetilap, 126(52): 3213-3214 (in Hungarian).
BOLOTNYI, A.V., PISMENNAYA, M.V., & AKORONKO, S.L. (1978)
[Transformation of phosphororganic pesticides antio and chloro-
phose in the environment.] Gig. i Sanit., 43(5): 28-31 (in
Russian).
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol-water
partitioning coefficient (Kow) of 61 organophosphorus and carba-
mate insecticides and their relationship to respective water
solubility (S) values. J. environ. Sci. Health, BI8(6): 667-
683.
BOYD, E.M. & MUIS, L.F. (1970) Acute oral toxicity of dimetho-
ate in albino rats fed a protein deficient diet. J. pharm.
Sci., 59: 1098-1102.
BRADY, U.E., Jr & ARTHUR, B.W. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. econ.
Entomol., 56(4): 477-482.
BUDREAU, C.H. & SINGH, R.P. (1973) Effect of fenthion and
dimethoate on reproduction in the mouse. Toxicol. appl. Pharma-
col., 26: 29-38.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1968) Organo-
phosphorus poisoning, Diagnosis of poisoning in pheasants owing
to a number of common pesticides, J. agric. food Chem., 16(2):
332-339.
BUNYAN, P.J., JENNINGS, D.M., & TAYLOR, A. (1969) Organo-
phosphorus poisoning, Chronic feeding of some common pesticides
to pheasants and pigeons, J. agric. food Chem., 17(5): 1027-
1032.
CABRAS, P., MELONI, M., & PIRISI, F.M. (1979) Separation of
insecticides by reversed-phase high-performance liquid chroma-
tography. J. Chromatogr., 176: 473-477.
CARMAN, G.E., IWATA, Y., PAPPAS, J.L., O'NEAL, J.R., & GUNTHER,
F.A. (1982) Pesticide applicator exposure to insecticides
during treatment of citrus trees with oscillating boom and
airblast units. Arch. environ. Contam. Toxicol., 11: 651-659.
CASIDA, J.E. & SANDERSON, D.M. (1961) Toxic hazard from formu-
lating the insecticide dimethoate in methyl "Cellosolve".
Nature (Lond.), 189(4763): 507-508.
CHEN, H.H., HSUEH, J.L, SIRIANNI, S.R., & HUANG, C.C. (1981)
Induction of sister-chromatid exchanges and cell cycle delay in
cultured mammalian cells treated with eight organophosphorus
pesticides. Mutat. Res., 88(3): 307-316.
CHILWELL, E.D. & BEECHAM, P.T. (1960) Residues of O,O-
dimethyl- S-(N-methylcarbamoylmethyl) phosphorothiolothionate
(dimethoate) in sprayed crops. J. Sci. Food Agric., 11: 400-
407.
CONGREGADO, F., SIMON-PUJOL, D., & JUAREZ, A. (1979) Effect of
two organophosphorus insecticides on the phosphate-dissolving
soil bacteria. Appl. environ. Microbiol., 37(1): 169-171.
COPPLESTONE, J.F., FAKHRI, Z.I., MILES, J.W., MITCHELL, C.A.,
OSMAN, Y., & WOLFE, H.R. (1976) Exposure to pesticides in
agriculture: a survey of spraymen using dimethoate in the Sudan.
Bull. World Health Organ., 54(2): 217-223
COURTNEY, K.D., ANDREWS, J.E., SPRINGER, J., & DALLEY, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. environ. Sci. Health, B20(4): 373-406.
DALELA, R.C., BHATNAGAR, M.C., TYAGI, A.K., & VERMA, S.R.
(1979) Histological damage of gills in Channa gachua after
acute and subacute exposure to endosulfan and Rogor. Mikroskopie
(Vienna), 35: 301-307.
DAUTERMANN, W.C., CASIDA, J.E., KNAAK, J.B., & KOWALCZYK, T.
(1959) Bovine metabolism of organophosphorus insecticides.
Metabolism and residues associated with oral administration of
dimethoate to rats and three lactating cows. J. agric. food
Chem., 7(3): 188-193.
DEDEK, W., GRAHL, R., & SCHMIDT, R. (1984) A comparative study
of guanine N7-alkylation in mice in vivo by the organophosphorus
insecticides trichlorphon, dimethoate, phosmet and bromophos.
Acta pharmacol. toxicol., 55: 104-109.
DEGRAEVE, N. & MOUTSCHEN, J. (1983) Genotoxicity of an organo-
phosphorus insecticide, dimethoate, in the mouse. Mutat. Res.,
119: 331-337.
DEGRAEVE, N., CHOLLET, M.-C., & MOUTSCHEN, J. (1984) Evaluation
of the mutagenic potential of four commercial mixtures of insec-
ticides. Food chem. Toxicol., 22(8): 683-687.
DEMETER, J. & HEYNDRICKX, A. (1978) Two lethal endosulfan
poisonings in man. J. anal. Toxicol., 2(2): 68-74.
DE PIETRI-TONELLI, P., BAZZI, B., & SANTI, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Rev., 11: 60-99.
DE REUCK, J., COLARDYN, F., & WILLEMS, J. (1979) Fatal
encephalopathy in acute poisoning with organophosphorus
insecticides. A clinicopathologic study of two cases. Clin.
Neurol. Neurosurg., 81(4): 247-254.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRIJNS, J.K.,
MUYS, T., & VAN DER POLL, J.M. (1984) Pesticides and other
chemical residues in Dutch total diet samples (June 1976 - July
1978). Food chem. Toxicol., 22(1): 11-21.
DIKSHITH, T.S.S. (1986) Pesticides. In: Toxicology of
pesticides in animals, Boca Raton, Florida, CRC Press, Vol. I,
Chapter 2.
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981a) Toxicity evaluation of
dimethoate technical in fish (Report to Shaw Wallace & Co.,
India).
DIKSHITH, T.S.S. & RAIZADA, R.B. (1981b) Toxicity evaluation
of dimethoate technical in animals: Primary skin irritation and
irritation of mucous membrane in rabbits (Report to Shaw Wallace
& Co., India).
DUGGAN, R.E., CORNELIUSSEN, P.E., DUGGAN, M.B., MCMAHON, B.M., &
MARTIN, R.J. (1983) Pesticide residue levels in foods in the
United States from July 1, 1969 to June 30, 1976, Washington,
DC, US Food and Drug Administration/Association of Official
Analytical Chemists (published jointly), pp. 13-24.
DURHAM, W.F. & HAYES, W.J. (1962) Organic phosphorus poisoning
and its therapy. Arch. environ. Health, 5: 21-47.
DURHAM, W.F. & WOLFE, H.R. (1962) Measurement of the exposure
to pesticides. Bull. World Health Organ., 26: 75-91.
DUVAL, G., CORBIN, J.C., & MIMRAN, C. (1986) Etude
hémodynamique des intoxications aiguës graves par insecticides
organophosphorés (7 cas). Coeur et Toxiques: XXIVèmes Journées
du Groupement francais de CAP, Strasbourg, 24-25 septembre
1986.
DZWONKOWSKA, A. & HÜBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in
vivo. Arch. Toxicol., 58: 152-156.
EBEL, J. & KARYOFILIS, A. (1978) [Unusual course of poisoning
with dimethoate, an organic phosphorus compound.] Ther. Ggw.,
117(2): 226-236 (in German).
EDSON, E.F. & NOAKES, D.N. (1960) The comparative toxicity of
six organophosphorus insecticides in the rat. Toxicol. appl.
Pharmacol., 2: 523-539.
EDSON, E.F., JONES, K.H., & WATSON, W.A. (1967) Safety of
dimethoate insecticide. Br. med. J., 4(5578): 554-555.
EDWARDS, J.A., LEEMING, N.M. & CLARK, R. (1984) Effect of
dimethoate on pregnancy of the rat (Unpublished report No. DTF
3/84245 dated 13 April 1984 from Huntingdom Research Centre).
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977a) Fac-
tors affecting the fate of dimethoate in soils. Intern. J.
environ. Stud., 11: 113-124.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1977b) Fac-
tors affecting the accumulation of dimethoate in soil. Int. J.
environ. Stud., 11: 187-196.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1978) Factors
influencing the degradation of dimethoate in soils and solu-
tions. Int. J. environ. Stud., 11: 253-260.
ERDMANN, W.D., ZECH, R., FRANKE, P., & BOSS, I. (1966) [The
question of the therapeutic efficacy of esterase reactivators in
dimethoate poisoning.] Arzneimittelforschung, 16: 492-494 (in
German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesti-
cides. In: Montesano, R., Tomatis, L., & Davies, W., ed.
Chemical carcinogenesis: essays, Lyons, International Agency
for Research on Cancer, pp. 161-181 (IARC Scientific
Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Dimethoate. In: Evaluation of toxicity of
pesticide residues in food, Rome, Food and Agricultural
Organization of the United Nations, pp. 97-101 (FAO Meeting
Report No. PL/1965/10/1. WHO/Food Add./27.65)
FAO/WHO (1967) Dimethoate. In: Evaluation of some pesticide
residues in food, Rome, Food and Agricultural Organization of
the United Nations, p. 232.
FAO/WHO (1968) Dimethoate. In: 1967 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 117-132 (FAO/PL: 1967/M/11/1.
WHO/Food Add./68.30).
FAO/WHO (1971) Dimethoate. In: 1970 Evaluations of some pesti-
cide residues in food, Rome, Food and Agricultural Organization
of the United Nations, pp. 239-244 (AGP: 1870/M/12/1. WHO/Food
Add./71.42).
FAO/WHO (1974) Formothion. In: 1973 Evaluations of some pesti-
cide residues in food, Geneva, World Health Organization, pp.
281-290 (WHO Pesticide Residues Series, No. 3).
FAO/WHO (1978) Dimethoate. In: 1977 Evaluations of some pesti-
cide residues in food, Rome, Food and Agriculture Organization
of the United Nations, pp. 155-156 (FAO Plant Production and
Protection Paper No. 10 Sup.).
FAO/WHO (1979) Formothion, dimethoate, omethoate. In: 1978
Evaluations of some pesticide residues in food, Geneva, World
Health Organization, p. 6.
FAO/WHO (1984) Maximum residue limits for pesticides, Codex
Alimentarius Commission, Rome, Food and Agricultural
Organization of the United Nations, Vol. 13.
FAO/WHO (1985) Pesticide residues in food. Report of the 1984
Joint Meeting on Pesticide Residues, Rome, Food and Agricultural
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 62).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FAO/WHO (1987) Dimethoate. In: Pesticide residues in food.
Report of the 1987 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Rome, Food and Agricultural Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 84).
FARM CHEMICALS HANDBOOK (1984) Willoughby, Ohio Meister
Publishing Company.
FETCHER, A. (1984) Suspected dimethoate toxicity in cattle.
Mod. vet. Pract., 65(4): 283-285.
FISCHER, G.W. & SCHEUFLER, H. (1981) [Effects of dimethoate
and O-desmethyldimethoate on dominant lethal test in mice.]
Wiss. Z. Univ. Halle, 30(6): 21-29 (in German).
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity
of organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Health Sci., B20(1): 73-95.
FRANCOIS, J. & VERBRAEKEN, H. (1977) Glaucome aigu après
intoxication par un ester organo-phosphatique Bull. Soc. Belge
Ophthalmol., 176: 19-22.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14(3): 515-534.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1978 - September
1979. J. Assoc. Off. Anal. Chem., 68(5): 842-861.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1978 - September 1979. J.
Assoc. Off. Anal. Chem., 68(5): 862-875.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985c) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1979 - September
1980. J. Assoc. Off. Anal. Chem., 68(6): 1163-1183.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1985d) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1979 - September 1980. J.
Assoc. Off. Anal. Chem., 68(6): 1184-1197.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total diet samples, October 1980 - March
1982. J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in
adult total diet samples, October 1980 - March 1982. J. Assoc.
Off. Anal. Chem., 69(1): 146-161.
GETZIN, L.W. & ROSEFIELD, I. (1968) Organophosphorus insecti-
cide degradation by heat-labile substances in soil. J. agric.
food Chem., 16(4): 598-601.
GIBEL, W., LOHS, KH., WILDNER, G.P., ZIEBARTH, D., & STIEGLITZ,
R. (1973) [Experimental study on cancerogenic, haematotoxic,
and hepatotoxic activity of phosphor-organic pesticides.] Arch.
Geschwulstforsch., 41(4): 311-328 (in German).
GILOT - DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN -
DAHMEN, M. (1983) Mutagenicity of some organophosphorus com-
pounds at the ade6 locus of Schizosaccharomyces pombe. Mutat.
Res., 117: 139-148.
GIRENKO, D.B. & KLISENKO, M.A. (1977) [Methods of estimating
antio and fosfamid in fruits by gas-liquid chromatography.] In:
[ Methods of determination of microquantities of pesticides in
foods, feed, and the environment,] Moscow, Kolos, pp. 117-118
(in Russian).
GIRENKO, D.B., KLISENKO, M.A., & PISMENNAYA, M.B. (1978)
[Methods of estimating organophosphorus pesticides in water by
chromatography methods.] In: [ Methods of determination of
microquantities of pesticides in foods, feed, and the
environment,] Moscow, Kolos, pp. 57-62 (in Russian).
GONTZEA, I. & GORCEA, V. (1977) The influence of protein and
fat intake on the body's resistance to dimethoate. Med. Lav.,
68(1): 13-21.
GOSSELIN, R.E., SMITH, R.P., HODGE, H.C., & BRADDOCK, J.E.
(1984) Clinical toxicology of commercial products, 5th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
GRIGORASHVILI, G.S. & DZHIBLADZE, V.E. (1965) [Hygienic
evaluation of some food products after treatment with
phosphamide.] In: [ Hygiene and toxicology of pesticides, and
clinical aspects of pesticide poisoning,] Kiev, Zdorovye
Publishers, pp. 161-164 (in Russian).
HADJIDEMETRIOU, D.G., IWATA, Y., & GUNTHER, F.A. (1985)
Analysis and dissipation of dislodgable residues of acephate,
dimethoate and formetanate hydrochloride on citrus foliage.
Pestic. Sci., 16: 302-310.
HANNA, P.Y. & DYER, K.F. (1975) Mutagenicity of organo-
phosphorus compounds in bacteria and Drosophila. Mutat. Res.,
28: 405-420.
HASSAN, A., ZAYED, S.M.A.D., & BAHIG, M.R.E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimetho-
ate in the rat. Biochem. Pharmacol., 18: 2429-2438.
HAUCK, H.E. & AMADORI, E. (1980) Recent developments in high-
performance thin-layer chromatography and application to pesti-
cide analysis, New York, American Chemical Society, pp. 159-176
(ACS Symposium Series 136; CH09).
HAYES, W.J., Jr (1982) Pesticide studies in man, Baltimore,
Maryland, Williams and Wilkins Company, pp. 362-365.
HELLWIG, J. (1968a) Report on the study of the toxicity of
dimethoate in mice after 78-week administration in the diet,
(Unpublished report no. 75CO326/8242 from BASF, Department of
toxicology, Ludwigshafen/Rhein, Federal Republic of Germany).
HELLWIG, J. (1968b) Report on the study of the toxicity of
dimethoate in rats after 24 month administration in the diet,
(Project no. 70C0326/8241 dated 9 October 1986 from BASF,
Department of Toxicology, Ludwigshafen/Rhein, Federal Republic
of Germany).
HEWITT, R., BREBBIA, A., & WALETZKY, E. (1958a) Carbamoyl
alkyl phosphorodithioates as chemotherapeutic agents: screening
by aedicidal properties in laboratory mammals, lambs and calves.
J. econ. Entomol., 51(2): 126-131.
HEWITT, R., EMRO, J., ENTWISTLE, J., PANKAVICH, J., THORSON, R.,
WALLACE, W., & WALETZKY, E. (1958b) Carbamoyl alkyl phosphoro-
dithioates as chemotherapeutic agents: effects of dimethoate
against grubs in cattle. J. econ. Entomol., 51(4): 445-450.
HILL, R.H. & ARNOLD, J.E. (1979) A personal air sampler for
pesticides. Arch. environ. Contam. Toxicol., 8: 621-628.
HOEGBERG, E.I. & CASSADAY, J.T. (1951) The reaction of O,O-
dialkyl thiophosphoric acid salts with some alpha-haloacyl deriva-
tives. J. Am. Chem. Soc., 73: 557-559.
IRPTC (1982) Scientific reviews of Soviet literature on toxic-
ity and hazards of chemicals: dimethoate, Moscow, Centre of
International Projects, GKNT, 12 pp (No. 5).
IRPTC (1987) Legal file Vol. 2, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
IWATA, Y., DUSCH, M.E., CARMAN, G.E., & GUNTHER, F.A. (1979)
Worker environment research: residues from carbaryl, chloroben-
zilate, dimethoate, and trichlorfon applied to citrus trees. J.
agric. food Chem., 27(6): 1141-1145.
IZMIROVA, N., BENCHEV, I., VELICHKOVA, V., KALOYANOVA, F.,
LAMBREVA, E., RUSEVA, P., KOSTOVA, S., ANGELOVA, R., INKJOVA,
M., BOGINOVA, L., MAKSIMOVA, F., & TZVETKOVA, P. (1973)
[Determination of minimal safety periods after treatment of
tobacco and cotton with organophosphorus pesticides.] In:
[ Hygiene, use and toxicology of pesticides, and clinical
aspects of pesticide poisoning,] Vol. 10, Moscow, Medizina
Publishers, pp. 120-124 (in Russian).
JACKSON, J.B., DRUMMOND, R.O., BUCK, W.B., & HUNT, L.M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. econ.
Entomol., 53(4): 602-604.
JAYCOX, E. (1964) Effect on Honey bees of nectar from systemic
insecticide-treated plants. J. econ. Entomol., 57(1): 31-35.
JOHNSON, E., CATERSON, C.R., & ALLEN, J.S. (1985) Mutagenicity
testing of dimethoate (AC 12880) in the in vitro CHO/HGPRT
mutation assay. (Unpublished report project no. 0423 dated 30
January 1985 from American Cyanamid Company, Princeton, New
Jersey).
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. II. August 1975 - July
1976. Pestic. monit. J., 15(1): 39-50.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981b) Pesti-
cide, metal, and other chemical residues in adult total diet
samples. XII. August 1975 - July 1976. Pestic. monit. J., 15(1):
54-59.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984a) Pesticide, heavy metal, and other chemical residues in
infant and toddler total diet samples. III. August 1976 - Sep-
tember 1977. J. Assoc. Off. Anal. Chem., 67(1): 145-154.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1984b) Pesticide, metal, and other chemical residues in adult
total diet samples. XIII. August 1976 - September 1977. J.
Assoc. Off. Anal. Chem., 67(1): 154-166.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity
of chemicals to fish and aquatic invertebrates, Washington, DC,
USA, US Department. of the Interior, Fish and Wildlife Service
(Resource Publication 137).
JUNG, H.D. (1979) [Occupational dermatoses due to pesticides.]
Dtsch. Gesundheitswes., 34(24): 1144-1147 (in German).
KAGAN, YU.S. (1981) [ General toxicology of pesticides,]
Kiev, Zdorovye Publishers, 176 pp (in Russian).
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1967)
Establishing hygienic norms of Bi 58 (Rogor, Phosphamide) in the
atmospheric air. In: Works of the Scientific Research Institute
of Labour Protection and Occupational Diseases, Vol. 15, pp. 1-
8, Sofia, Bulgaria, Medicina i Fizkultura.
KALOYANOVA, F., IVANOVA, L., DIMOV, G., & MUHTAROVA, M. (1968)
[Experimental substantiation of the maximum permissible concen-
tration of Bi 58 (Rogor, Phosphamide) in the atmosphere.] Gig.
i Sanit., 6: 6-10 (in Russian).
KALOYANOVA, F., DOBREVA, V., & MITOVA, S.V. (1984) [Effet du
dimethoate sur certaines activités du système de monoxygénase
hépatique] In: Hommage au Professeur René Truhaut, Paris,
Queray S.A., pp. 552-555.
KALOYANOVA-SIMEONOVA, F. & IZMIROVA-MOSHEVA, N. (1983)
Determination of minimum periods for safe work following
spraying with organophosphate pesticides. In: Miyamoto, J., ed.
IUPAC pesticide chemistry - Human welfare and the environment,
Oxford, New York, Pergamon Press, pp. 237-238.
KAPLANIS, J.N., ROBBINS, W.E., DARROW, D.I., HOPKINS, D.E.,
MONROE, R.E., & TREIBER, G. (1959) The metabolism of dimetho-
ate in cattle. J. econ. Entomol., 52(6): 1190-1194.
KARIM, A.A., HARIDI, A.A., & EL RAYAH, E.A. (1985) The
environmental impact of four insecticides on non-target
organisms in the Gezira Irrigation Scheme canals of Sudan. J.
trop. Med. Hyg., 88(2): 161-168.
KAWAR, N.S., IWATA, Y., DUSCH, M.E., & GUNTHER, F.A. (1979)
Behavior of dialifor, dimethoate, and methidothion in artifi-
cially fortified grape juice processed into wine. J. environ.
Sci. Health, B14(5): 505-513.
KHAN, R.R. & BHASKER DEV (1982) Toxicology data sheets on
chemicals: dimethoate, Lucknow, India, Lucknow Industrial
Toxicology Research Centre (No. 6).
KHERA, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for
teratogenic activity in the cat. J. environ. Pathol. Toxicol.,
2: 1283-1288.
KHERA, K.S., WHALEN, C., TRIVETT, G., & ANGERS (1979) Terato-
genicity studies on pesticidal formulations of dimethoate,
diuron, and lindane in rats. Bull. environ. Contam. Toxicol.,
22: 522-529.
KISS, Z. & FAZEKAS, T. (1983) Organophosphates and torsade de
pointes ventricular tachycardia. J. R. Soc. Med., 76: 984-985.
KNAAK, J.B. (1980) Minimizing occupational exposure to
pesticides. Techniques for establishing safe levels of foliar
residues. Residue Rev., 75: 81-96.
KNAAK, J.B., SCHLOCKER, P., ACKERMAN, C.R., & SEIBER, J.N.
(1980) Reentry research: Establishment of safe pesticide levels
on foliage. Bull. environ. Contam. Toxicol., 24: 796-804.
KÖPPEL, C., FORYCKI, Z., & IBE, K. (1986) Hemoperfusion in
severe dimethoate poisoning. Intensive Care Med., 12(2): 110-
112.
KRUEGER, H.R., O'BRIEN, R.D., & DAUTERMAN, W.C. (1960) Rela-
tionship between metabolism and differential toxicity in insects
and mice of dimethoate, parathion, and acethion. J. econ.
Entomol., 53(1): 25-31.
KUNDIEV, YU.I. (1979) [ Absorption of pesticides through the
skin and prophylaxis of poisonings,] Kiev, Zdorovye Publishers,
200 pp (in Russian).
LARRIPA, I., MATOS, E., & DE SALUM, S.B. (1979) [Cytogenetic
study of human population accidentally exposed to an organophos-
phate pesticide.] Medicina (Buenos Aires), 39(6): 773-774 (in
Spanish).
LARRIPA, I., MATOS, E., DE VINUESA, M.L., & DE SALUM, S.B.
(1983) Sister chromatid exchanges in a human population
accidentally exposed to an organophosphorus pesticide. Rev.
Braz. Genet., 4: 719-727.
LEBLANC, F.N., BENSON, B.E., & GILG, A.D. (1986) A severe
organophosphate poisoning requiring the use of an atropine
drip. Clin. Toxicol., 24(1): 69-76.
LEE, Y.W. & WESTCOTT, N.D. (1981) Gas chromatographic quanti-
tative analysis and persistence of dimethoate and dimethoxon
residues on and in wheat plants. J. agric. food Chem., 29(4):
860-862.
LORD, K.A., MAY, M.A., & STEVENSON, J.H. (1968) The secretion
of the systemic insecticides dimethoate and phorate into nectar.
Ann. appl. Biol., 61: 19-22.
LUCIER, G.W. & MENZER, R.E. (1970) Nature of oxidative metabo-
lites of dimethoate formed in rats, liver microsomes, and bean
plants. J. agric. food Chem., 18(4): 698-704.
MADZHIDOV, U.A. (1970) [Atmospheric pollution with fosfamid in
agricultural application.] Med. J. Uzb., 9: 40-42 (in Russian).
MAHKAMBAEVA, Z.G. (1971) [Influence of organophosphorus pesti-
cides (kilval, methylmercaptophos, fosfamid) on bioelectric
activity of heart in experimental animals.] In: [ Hygiene, use
and toxicology of pesticides, and clinical aspects of pesticide
poisoning,] Kiev, Zdorovye Publishers pp. 175-180 (in Russian).
MAROSI, G., IVAN, J., NAGYMAJTENYI, L., CSATLOS, J., & TOSZEGI,
A. (1985a) Dimethoate-induced toxic cardiac failure in the
guinea-pig. Arch. Toxicol., 57: 142-143.
MAROSI, G., IVAN, J., & NAGYMAJTENYI, L. (1985b) Cardiodepres-
sion in organophosphate poisonings. Arch. Toxicol., Suppl. 8:
289-291.
MASIAK, M. & OLAJOSSY, M. (1973) [Depressive reaction
following intoxication with an organic phosphorus preparation,
Rogor (Bi 58).] Wiadom. Lek., 26(20): 1933-1936 (in Polish).
MELNIKOV, N.N. (1974) [ Chemistry and technology of pesti-
cides,] Moscow, Khimiya Publishers, 768 pp (in Russian).
MELNIKOV, N.N., VOLKOV, A.I., & KOROTKOVA, O.A. (1977)
[ Pesticides and the environment,] Moscow, Khimiya Publishers,
pp. 240 (in Russian).
MENZER, R.E. (1970) Effect of chlorinated hydrocarbons in the
diet on the toxicity of several organophosphorus insecticides.
Toxicol. appl. Pharmacol., 16: 446-452.
MENZER, R.E. & BEST, N.H. (1968) Effect of phenobarbital on
the toxicity of several organophosphorus insecticides. Toxicol.
appl. Pharmacol., 13: 37-42.
MENZIE, C.M. (1969) Metabolism of pesticides, Washington, DC,
US Department of the Interior, Fish and Wildlife Service, pp.
160-166.
MENZIE, C.M. (1974) Metabolism of pesticides: an update,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp. 166-167, 485.
MENZIE, C.M. (1978) Metabolism of pesticides: update II,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 118.
MENZIE, C.M. (1980) Metabolism of pesticides: update III,
Washington, DC, US Department of the Interior, Fish and
Wildlife Service, p. 242.
MIKHAILOV, S.S. & SHTERBAC, I.G. (1983) [Metabolism of organo-
phosphorus compounds containing a carboxyamin bond.] In:
[ Metabolism of organophosphorus poisons,] Moscow, Medizina,
pp. 96-101 (in Russian).
MITOVA, S.V., VASILEVA, L., DOBREVA, V., & KALOYANOVA, F. (1986)
Experimental studies on dimethoate oxidative desulfuration.
Toxic interfaces of neurones, smoke and genes. Arch. Toxicol.,
Suppl.9: 377.
MOHN, G. (1973) 5-Methyltryptophan resistance mutations in
Escherichia coli K-12. Mutagenic activity of monofunctional
alkylating agents including organophosphorus insecticides.
Mutat. Res., 20: 7-15.
MOLPHY, R. & RATHUS, E.M. (1964) Organic phosphorus poisoning
and therapy. Med. J. Aust., 29: 337-340.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides
in bacterial reversion assay systems. Mutat. Res., 116: 185-
216.
MULLER, P. & REINHOLD, R. (1973) [Experience with dimethoate
drugs in stable fly control.] Angew. Parasitol., 14(3): 158-169
(in German).
MUNCY, R.J. & OLIVER, A.D. (1963) Toxicity of ten insecticides
to the red crawfish, Procambarus clarki (Girard). Trans. Am.
Fish. Soc., 92: 428-431.
MURATORE, F., FAIOLO, A., & PONZETTA, G. (1960) [Four cases of
intoxication with organophosphorus esters with regard to liver
function.] Minerva Med., 51(80): 3342-3345 (in Italian).
NADMAITENI, L. & MAROSI, G. (1983) [Cardiotoxic action of
organophosphorus pesticides.] G ig. i Sanit., 11: 72-73 (in
Russian).
NAGLER, J., BRAECKMAN, R.A., WILLEMS, J.L., & DE BROE, M.E.
(1980) Combined hemoperfusion hemodialysis in organic phosphate
poisoning. Kidney Int., 18: 139 (Abstract).
NANDA KUMAR, N.V. & UDAYA BHASKAR, S. (1980) Colorimetric
determination of dimethoate by quantification of cholinesterase
inhibition. J. food Sci. Technol., 17: 153-155.
NCI (1977) Bioassay of dimethoate for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (Technical Report
Series No. 4).
NEHES, M., SELYPES, A., & SCHEUFLER, H. (1982) [Mutagenic
effect of trichlorfon and dimethoate on bone marrow cells of
mouse in vivo.] Wiss. Z. Univ. Halle, 31M(2): 57-62 (in
German).
NEHEZ, M., SELYPES, A., SCHEUFLER, H., & FISCHER, G.W. (1983)
Effect of dimethoate and O- demethyldimethoate on bone marrow
cells of CFLP mice. Regul. Toxicol. Pharmacol., 317(3): 349-
354.
NOAKES, D.N. & SANDERSON, D.M. (1969) A method for determining
the dermal toxicity of pesticides. Br. J. ind. Med., 26: 59-64.
NORTH, H.H. & MENZER, R.E. (1972) Biotransformation of
dimethoate by cell culture systems. Pestic. Biochem. Physiol.,
2: 278-285.
OKONEK, S. (1976) Probable progress in the therapy of
organophosphate poisoning: Extracorporeal hemodialysis and
hemoperfusion. Arch. Toxicol., 35(3): 221-227.
OKONEK, S. (1977) [Hemoperfusion with coated activated
charcoal for treating acute poisoning by remedies, plant
protectants, and fungi.] Med. Klin., 72(19): 862-866 (in
German).
OKONEK, S., HOLLMANN, H., & BOELCKE, G. (1976) [The
alkylphosphate poisoning: elimination of the poison by the
hemodialysis or blood exchange transfusion?]. Med. Welt, 27(8):
351-356 (in German).
PACH, J., GROSZEK, B., & BOGUSZ, M. (1977) Haemoperfusion in
the treatment of acute organophosphate poisoning. Acta
pharmacol. Toxicol., Vol 41, Suppl. II): 44.
PAMBOR, M. & BLOCH, Y. (1985) [Dimethoate and dithiocarbamate
as occupational contact allergens in a female agrotechnician.]
Dermatol. Monatsschr., 171(6): 401-405 (in German).
PANDEY, A.K. & TOMAR, V. (1985) Melanophores in Bufo melanos-
tictus (Schneider) tadpoles following exposure to the insecti-
cide dimethoate. Bull. environ. Contam. Toxicol., 35: 796-801.
PANSHINA, T.N. (1963a) [Experimental data on toxicology of new
organophosphorus insecticide phosphamide (Rogor, dimethoate).]
Pharmacol. Toksicol., 4: 476-484 (in Russian).
PANSHINA, T.N. (1963b) [Hygienic background for determining
the maximum permissible concentration of phosphamide in the air
of a working area.] Gig. i Sanit., 3: 21-28 (in Russian).
PANSHINA, T.N. (1966) [Experimental data on therapy of phos-
phamide intoxications.] In: [ Hygiene and toxicology of
pesticides, and clinical aspects of pesticide poisoning,] Kiev,
Zdorovye Publishers, Vol. 4, pp. 137-142 (in Russian).
PANSHINA, T.N. & KLISENKO, M.A. (1962) [Content of fosfamid
and changes in blood cholinesterase activity in different routes
of treatment of the mammalian organism.] In: [ Industrial
toxicology and clinical picture of occupational diseases due to
chemicals,] Moscow, Medgiz, pp. 181-184 (in Russian).
PETUKHOV, R.D. (1975) [Methodics of estimating of antio and
fosfamid in honey by thin-layer chromatography.] In: [ Methods
of determination of microquantities of pesticides in food, feed,
and the environment,] Moscow, Kolos, pp. 63-64 (in Russian).
PODREBARAC, D.S. (1984a) Pesticide, heavy metal residues, and
other chemical residues in infant and tod dler total diet sam-
ples. IV. October 1977 - September 1978. J. Assoc. Off. Anal.
Chem., 67(1): 166-175.
PODREBARAC, D.S. (1984b) Pesticide, metal, and other chemical
residues in adult total diet samples. XIV. October 1977 - Sep-
tember 1978. J. Assoc. Off. Anal. Chem., 67(1): 176-185.
PROBST, G.H., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEAL, S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures: A comparison with
bacterial mutagenicity using 218 compounds. Environ. Mutagen.,
3: 11-32.
RIEMER, F., DAHLENBURG, R., & GRISK, A. (1985) [Renal excretion
of dimethylphosphate and its thio derivatives after application
of dimethoate, bromophos, naled or trichlorphon to rats.]
Nahrung, 29(3): 299-302 (in German).
ROALES, R.R. & PERLMUTTER, A. (1974) Toxicity of zinc and
Cygon, applied singly and jointly, to zebrafish embryos. Bull.
environ. Contam. Toxicol., 12(4): 475-480.
ROSVAGA, R.I. (1983) [Methodology of investigation of pesti-
cide pyrolysis in their chemical-thermal harmlessness.] In:
[ Hygiene of application, toxicology of pesticides and polymeric
materials,] Kiev, Zdorovja, Vol. 13, pp. 73-77 (in Russian).
SAHU, D. & PATTANAIK, M. (1980) Persistence of rogor in some
Orissa soils. Indian J. agric. Sci., 50(6): 505-507.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDERSON, D.M. & EDSON, E.F. (1959) Oxime therapy in poison-
ing by six organophosphorus insecticides in the rat. J. Pharm.
Pharmacol., 11: 721-728.
SANDERSON, D.M. & EDSON, E.F. (1964) Toxicological properties
of the organophosphorus insecticide dimethoate. Br. J. ind.
Med., 21: 52-64.
SANTI, R. & GIACOMELLI, R. (1962) Metabolic fate of P32-
labelled dimethoate in olive fruits and some toxicological
implications. J. agric. food Chem., 10(3): 257-261.
SAN SEBASTIAN, J.R. (1985) In vivo bone marrow cytogenetics
rat metaphase analysis. PH 315-AC-001-84, Dimethoate CL 12880.
(Unpublished report dated 29 August 1985 from Pharmakon
Research International, Inc. Waverly, Pennsylvania 18471).
SCHEUFLER, H. (1975) [Effects of relatively high doses of
dimethoate and trichlorfon on the embryogenesis of laboratory
mice.] Biol. Rundsch., 13: 238-240 (in German).
SENANAYAKE, N. & KARALLIEDE, W. (1987) Neurotoxic effects of
organophosphorus insecticides. An intermediate syndrome. New
Engl. J. Med., 316(13): 761-763.
SHAMSHURIN, A.A. & KRIMER, M.Z. (1976) [ Physicochemical proper-
ties of pesticides: reference book,] Moscow, Khimya Publishers,
pp. 76-77 (in Russian).
SIDIMANOV, B.B. (1971) [Determination of fosfamid in post-
mortem material by thin-layer chromatography.] In: [ Hygiene of
application, toxicology of pesticides, and clinical picture of
poisoning,] Kiev, Zdorovja, Vol. 9, pp. 187-188 (in Russian).
SLOOFF, W. & CANTON, J.H. (1983) Comparison of the suscepti-
bility of 11 freshwater species to 8 chemical compounds. II.
(Semi)chronic toxicity test. Aquat. Toxicol., 4: 271-282.
SORG, R.M. (1985) Micronucleus test (MNT) PH 309A-AC-004-84,
Dimethoate CL 12880, (Unpublished report dated 1 March 1985
from Pharmakon Research International, Inc. Waverly,
Pennsylvania 18471).
STERRI, S.H., ROGNERUD, B., FISKUM, S.E., & LYNGAAS, S. (1979)
Effect of toxogonin and P2S on the toxicity of carbamates and
organphosphorus compounds. Acta pharmacol. toxicol., 45: 9-15.
STEVENSON, J.H. (1968) Laboratory studies on the acute contact
and oral toxicities of insecticides to honey bees. Ann. appl.
Biol., 61: 467-472.
STIEGLITZ, R., GIBEL, W., WERNER, W., & STOBBE, H. (1974)
Experimental study on haematotoxic and leukaemogenic effects of
trichlorfon and dimethoate. Acta haematol., 52: 70-76.
STROY, A.N. (1983) [Hygienic reglementation of fosfamid in
greenhouse conditions.] In: [Hygiene of application, toxicology
of pesticides and polymeric materials,] Kiev, Zdorovja, Vol.
13, pp. 90-91 (in Russian).
SZETO, S.Y., VERNON, R.S., & BROWN, M.J. (1985) Degradation of
dimethoate and pirimicarb in asparagus. J. agric. food Chem.,
33: 763-767.
TIEFENBACH, B. & LANGE, P. (1980) Studies on the action of
dimethoate on the immune system. Arch. Toxicol., Suppl. 4: 167-
170.
TRINH VAN BAO, SZABO, I., RUZICSKA, P., & CZEIZEL, A. (1974)
Chromosome aberrations in patients suffering acute organic
phosphate insecticide intoxication. Humangenetik, 24: 33-57.
UBAIDULLAEV, R.U. & MADZHIDOV, U.A. (1976) [Hygienic charac-
teristics of phosphamide as an atmospheric pollutant.] Gig. i
Sanit., 9: 7-9 (in Russian).
UCHIDA, T., DAUTERMANN, W.C., & O'BRIEN, R.D. (1964) The
metabolism of dimethoate by vertebrate tissues. J. agric. food
Chem., 12(1): 48-52.
UDAYA BHASKAR, S. & NANDA KUMAR, N.V. (1980) Colorimetric
determination of dimethoate or omethoate. Talanta, 27(9): 757-
758.
USHA RANI, M.V., REDDI, O.S., & REDDY, P.P. (1980) Mutageni-
city studies involving aldrin, endosulfan, dimethoate, phospham-
idon, carbaryl, and ceresan. Bull. environ. Contam. Toxicol.,
25(2): 277-282.
VAN MIDDELEM, C.H. & WAITES, R.E. (1964) Gas chromatographic
and colorimetric measurement of dimethoate residues. J. agric.
food Chem., 12(2): 178-182.
VELAZQUEZ, A., XAMENA, N., CREUS, A., & MARCOS, R. (1986)
Indication for weak mutagenicity of the organophosphorus
insecticide dimethoate in Drosophila melanogaster. Mutat. Res.,
172: 237-243.
VERMA, S.R., BHATNAGAR, M.C., & DALELA, R.C. (1978) Biocides
in relation to water pollution. II. Bioassay studies of a few
biocides to a fresh-water fish Channa gachua. Acta hydrochim.
hydrobiol., 6(2): 137-144.
VERMA, S.R., TYAGI, A.K., BHATNAGAR, M.C., & DALELA, R.C.
(1979) Organophosphate poisoning to some fresh-water teleosts -
acetylcholinesterase inhibition. Bull. environ. Contam. Toxi-
col., 21: 502-506.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982) Bioassay
trials with twenty-three pesticides to a fresh-water teleost
Saccobranchus fossilis. Water Res., 16: 525-529.
VISHWANATH, R. & JAMIL, K. (1986) Mutagenic and genotoxic
activities of certain organophosphorus compounds using Ames
Salmonella assay with and without microsomal induction. Ind.
J. exp. Biol., 24: 305- 308.
WALLER, G.D. & BARKER, R.J. (1979) Effects of dimethoate on
honey bee colonies. J. econ. Entomol., 72(4): 549-551.
WALLER, G.D., BARKER, R.J., & MARTIN, J.H. (1979) Effects of
dimethoate on honey bee foraging. Chemosphere, 8(7): 461-463.
WEHRAN, H.-J. & HAMMER, H.-J. (1984) [Lethal poisoning with
the pesticide dimethoate (Bi 58 EC).] Kriminal. forens. Wiss.,
54: 181-183 (in German).
WEST, B., VIDONE, L.B., & SHAFFER, C.B. (1961) Acute and
subacute toxicity of dimethoate. Toxicol. appl. Pharmacol., 3:
210-223.
WHO (1982) Field surveys of exposure to pesticides - Standard
Protocol, Geneva, World Health Organization (Unpublished
document VBC/82.1).
WHO (1984) Chemical methods for the control of arthropod
vectors and pests of public health importance, Geneva, World
Health Organization, pp. 34, 37.
WHO (1986a) Environmental health criteria 63: Organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WHO (1986b) The WHO recommended classification of pesticides
by hazard and guidelines to classification 1986-1987 World
Health Organization, Geneva, (Unpublished document VBC/86.1).
WHO (1986c) Interim specifications for dimethoate technical
and emulsifiable concentrate, Geneva, World Health Organization
(Unpublished report, WHO/IS/86.12).
WHO/FAO (1980) Dimethoate: data sheet on pesticides, Geneva,
World Health Organization (Unpublished document VBC/DS/80.42).
WINKLER, R. & MULLER, R. (1979) [Elimination and inactivation
of waters containing pesticides from agrochemical plants.]
Wasserwirtsch. Wassertech., 29(11): 369-371 (in German).
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-
induced complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ.
Mutagen., 5: 835-846.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th
ed., Lavenham United Kingdom, British Crop Protection Council.
ZECH, R., ENGELHARD, H., & ERDMANN, W.-D. (1966) Reactions of
pyridinium oximes with the alkyl phosphate dimethoate and its
derivatives. Effects on cholinesterases. Biochim. biophys.
Acta, 128: 363-371.
ZOLOTNIKOVA, G.P. (1980) [Disturbance of the immunoreactivity
of the organism under the effect of pesticides in hothouses.]
Gig. tr. prof. Zabol., 3: 38-40 (in Russian).
ZOLOTNIKOVA, G.P. & SOMOV, B.A. (1978) [On the role of pesti-
cides in the occurrence of occupational dermatitis in workers of
hothouses.] Vestnik Dermatol. Venerol., 4: 76-79 (in Russian).
ZOLOTNIKOVA, G.P. & ZOTOV, V.M. (1978) [Prognosis for resi-
dues of pesticides in the air of hothouses.] Gig. i Sanit.,
43(1): 108-109 (in Russian).
ANNEX I. TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus Insecticides - A General Introduction)
All cases of organophosphorus poisoning should be dealt
with as an emergency and the patient sent to hospital as quickly
as possible. Although symptoms may develop rapidly, delay in
onset or a steady increase in severity may be seen up to 48 h
after ingestion of some formulated organophosphorus insecti-
cides.
Extensive descriptions of treatment of poisoning by
organophosphorus insecticides are given in several major
references (Kagan, 1977; Taylor, 1980; UK DHSS, 1983; Plestina,
1984) and will also be included in the IPCS Health and Safety
Guides to be prepared for selected organophosphorus insecti-
cides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures
include removal of contaminated clothes and washing of the skin
with alkaline soap or with a sodium bicarbonate solution.
Particular care should be taken in cleaning the skin area where
venupuncture is performed. Blood might be contaminated with
direct-acting organophosphorus esters, and, therefore,
inaccurate measurements of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced,
if the patient is conscious, by the administration of
ipecacuanha syrup (10-30 ml) followed by 200 ml water. This
treatment is, however, contraindicated in the case of pesticides
dissolved in hydrocarbon solvents. Gastric lavage (with
addition of bicarbonate solution or activated charcoal) can also
be performed, particularly in unconscious patients, taking care
to prevent aspiration of fluids into the lungs (i.e., only after
a tracheal tube has been placed).
The volume of fluid introduced into the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the
pesticide involved is available, it should also be stored for
further analysis (i.e., detection of toxicologically relevant
impurities). A purge to remove the ingested compound can be
administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be
started at the first sign of respiratory failure and maintained
for as long as necessary.
Cautious administration of fluids is advised, as well as
general supportive and symptomatic pharmacological treatment and
absolute rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv and given
at 15 to 30-min intervals. The dose and the frequency of
atropine treatment varies from case to case, but should maintain
the patient fully atropinized (dilated pupils, dry mouth, skin
flushing, etc.). Continuous infusion of atropine may be
necessary in extreme cases and total daily doses up to several
hundred mg may be necessary during the first few days of
treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophos-
phates. This is not the case with enzymes inhibited by
carbamates. The treatment should begin as soon as possible,
because oximes are not effective on "aged" phosphorylated ChEs.
However, if absorption, distribution, and metabolism are thought
to be delayed for any reasons, oximes can be administered for
several days after intoxication. Effective treatment with
oximes reduces the required dose of atropine. Pralidoxime is
the most widely available oxime. A dose of 1 g pralidoxime can
be given either im or iv and repeated 2-3 times per day or, in
extreme cases, more often. If possible, blood samples should be
taken for AChE determinations before and during treatment. Skin
should be carefully cleansed before sampling. Results of the
assays should influence the decision whether to continue oxime
therapy after the first 2 days.
There are indications that oxime therapy may possibly have
beneficial effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety, it appears to
counteract some aspects of CNS-derived symptoms, which are not
affected by atropine. Doses of 10 mg sc or iv are appropriate
and may be repeated as required (Vale & Scott, 1974). Other
centrally acting drugs and drugs that may depress respiration
are not recommended in the absence of artificial respiration
procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug
singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of
secretions are the best guide to the effectiveness of
atropinization. Although repeated dosing may well be necessary,
excessive doses at any one time may cause toxic side-effects.
Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and
may be taken into, and then released from, fat depots over a
period of many days. It is therefore quite incorrect to abandon
oxime treatment after 1-2 days on the supposition that all
inhibited enzyme will be aged. Ecobichon et al. (1977) noted
prompt improvement in both condition and blood-ChEs in response
to pralidoxime given on the 11th-15th days after major symptoms
of poisoning appeared due to extended exposure to fenitrothion
(a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually
for an occupational setting, but, in the case of very severe
exposure or massive ingestion (accidental or deliberate), the
therapeutic doses may be extended considerably. Warriner et al.
(1977) reported the case of a patient who drank a large quantity
of dicrotophos, in error, while drunk. Therapeutic dosages were
progressively increased up to 6 mg atropine iv every 15 min
together with continuous iv infusion of pralidoxime chloride at
0.5 g/h for 72 h, from days 3 to 6 after intoxication. After
considerable improvement, the patient relapsed and further
aggressive therapy was given at a declining rate from days 10 to
16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given
and the patient was discharged on the thirty-third day with no
apparent sequelae.
REFERENCES TO ANNEX I
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-
379.
KAGAN, JU.S. (1977) [ Toxicology of organophosphorus pesti-
cides,] Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in
Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (Unpub-
lished document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S.
& Gilman, A., ed. The pharmacological basis of therapeutics, 6th
ed., New York, Macmillan Publishing Company, pp. 100-119.
UK DHSS (1983) Pesticide poisoning: notes for the guidance of
medical practitioners, London, United Kingdom Department of
Health and Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus
poisoning. Guy's Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
