
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DIMETHOATE
Chemical names
S-methylcarbamoyl-methyl-O,O-dimethyl phosphorodithioate;
O,O-dimethyl-S-(N-methylcarbamoylmethyl) phosphorodithioate;
methylamide of O,O-dimethyl-dithiophosphorylacetic acid, methyl
dimethyldithiophosphoryl acetamide.
Synonyms
"Rogor", "Fortion MM"
Empirical formula
C5H12NO3PS2
Structural formula
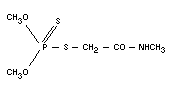 BIOLOGICAL DATA
Biochemical aspects
Dimethoate is a cholinesterase inhibitor. The molar concentration
of the pure compound necessary to produce 50% cholinesterase
inhibition in the rat brain in vitro (I50) is 8.5 × 10-3. It
decomposes to give products which are more toxic than the original
substance (Casida & Sanderson, 1962, Casida & Sanderson, 1963).
Various studies (O'Brien, 1959; O'Brien, 1961; Sanderson & Edson,
1964) carried out with dimethoate labelled with 32P have shown that
there is rapid absorption from the digestive tract. The radioactivity
is concentrated in the liver, bile, kidneys and urine. There is no
accumulation in the fat depots. Elimination is rapid in the rat and in
man, 76-90% of the radioactivity being found in the urine after 24
hours. In the guinea-pig, 25-40% of the radioactivity is recovered in
the faeces. Four dimethoate metabolites with anticholinesterase
activity (molar I50) s in 30 minutes at 37° in rat brain: 4.7 × 10-6;
1.1 × 10-5; approximately 0.2 × 10-5 and approximately 0.1 × 10-5)
have been identified in the rat and in man. One of them seems to be a
product resulting from thiono-oxidation, leading to the formation of
the oxygen homologue of dimethoate and followed by hydrolysis with
production of a thiocarboxyl derivative which constitutes the chief
metabolite of dimethoate in mammals. Although this thiocarboxyl
derivative has not been found in treated plants, the oxygen analogue
of dimethoate has been found in crops (Santi & de Pietri Sonelli,
1959). Similarly, various short-lived intermediate products have been
reported, but have not yet been accurately evaluated from the
toxicological viewpoint (Chilwell & Beechman, 1960; Dauterman et al.,
1960; Sampaolo, 1961; Santi & de Pietri Sonelli, 1959; Santi &
Giacomelli, 1962).
In vitro studies on human liver enzymes indicated that
dimethoate could inhibit the non-specific esterases to a greater
degree than acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
LD50 mg/kg body-weight
Animal Route Laboratory References
Pure grade Technical
Rat, male Oral 500-600 280-350 180-325 Sanderson &
Edson, 1964
Rat, female Oral 570-680 300-356 240-336 "
Rat, male Intraperitoneal - 175-325 - "
Rat, female Intraperitoneal - 350 - "
Rat, male Intravenous - 450 - "
Mouse, female Oral 60 - 60 "
Hamster, male Oral - 200 - "
Guinea-pig Oral 550 600 350-400 "
Rabbit Oral 500 450 approx. 300 "
Hen Oral 50 40 approx. 30 "
Acute oral toxicity was not potentiated by any of 17 other
insecticides (Sanderson & Edson, 1964).
Short-term studies
Rat. Groups of 10 male rats were fed diets containing 1, 5, 25
and 125 ppm of dimethoate for 15 weeks. The animals were killed in
order to determine the cholinesterase activity of the erythrocytes,
plasma and brain as well as to examine and weigh the main organs. At
the highest concentration, i.e., 125 ppm, a slight fall in the rate of
gain of weight was observed as well as mild symptoms of poisoning
(slight muscular fibrillation). In the group fed 25 ppm and higher
concentrations, a significant fall in the cholinesterase activity of
the plasma and erythrocytes was observed, while in the animals fed 5
ppm a fall of 20% in cholinesterase activity was found. At 1 ppm there
was no effect on the cholinesterase activity of the plasma,
erythrocytes or brain (Edson & Noakes, 1960).
Young rats in groups of 20 fed diets containing 2, 8 and 32 ppm
of dimethoate for 90 days and other groups of 20 rats fed 50, 100 and
200 ppm for 35 days showed no haematological abnormalities, nor any
significant histopathological changes. Regarding the cholinesterase
activity of the plasma and the erythrocytes the highest dose which did
not give a significant inhibition was 32 ppm of dimethoate (West et
al., 1961).
Guinea-pig. Groups of guinea-pigs were fed for 3 weeks on
lettuce and brassica leaves that had been treated with dimethoate and
contained residues of up to 189 ppm. No toxic effects were seen and
the cholinesterase inhibition observed was in agreement with that in
parallel groups given daily oral doses of the same quantity of
laboratory grade dimethoate (Sanderson & Edson, 1964).
Chicken. In laying hens dimethoate given over a period of 59
weeks at a concentration of 30 ppm daily in the drinking-water caused
inhibition of plasm cholinesterase and some reduction in appetite, but
no egg abnormalities (Sherman, et al., 1963).
Dog. Three groups each of 4 dogs, 2 males and 2 females, were
fed diets containing 2, 10 and 50 ppm for 13 weeks. No significant
harmful effect was noted. The cholinesterase activity of the
erythrocytes was only slightly decreased at the highest concentration
of 50 ppm while that of the plasma was unaffected at any of the
concentrations employed (West et al., 1961).
Man. Twenty subjects ingested daily for 4 weeks 2.5 mg of
dimethoate in aqueous solution, corresponding to about 0.04 mg/kg
body-weight. No toxic effect was observed nor any significant change
in the blood cholinesterase activity. The same results were found in 2
subjects who ingested daily during 21 days, 9 mg (0.13 mg/kg) and 18
mg (0.26 mg/kg body-weight) dimethoate respectively (Sanderson &
Edson, 1964).
Long-term studies
Rat. Groups of 20 male rats were maintained for 6-12 months on
diets containing various concentrations of laboratory grade
dimethoate. At 800 ppm severe toxic effects developed within a few
days; the chemical was withdrawn after a week and complete recovery
occurred in 10-14 days. No toxic effects were seen at 50 ppm or below.
Marked inhibition of erythrocyte cholinesterase activity occurred at
50 ppm but at 10 ppm and below neither erythrocyte nor plasma
cholinesterase showed significant inhibition throughout the test. At
the end of the experiment there were no macroscopical or microscopical
changes in any group attributable to dimethoate. The maximum no-effect
level in these experiments corresponded to 0.5-0.8 mg/kg body-weight
per day.
In the same experiment further groups of 20 weanling male rats
were treated for 5-1/2 months at dose levels of 5, 10 and 20 ppm of
dimethoate. The maximum no-effect level in these experiments was 5 ppm
corresponding to 0.3-0.6 mg/kg body-weight per day.
A further test with the commercial liquid formulation of
dimethoate on similar groups of male and female rats lasted 12 weeks.
The maximum no-effect level was again 5 ppm corresponding to 0.4-0.6
mg/kg body-weight per day (Sanderson & Edson, 1964).
Comments on the experimental studies reported
Short-term studies have been made on a number of species, in the
rat for as long as one year. Some observations have also been made in
man, and if adequate studies were carried out to establish a level
causing minimal blood cholinesterase inhibition it might be possible
to increase the acceptable daily intake.
It was noted that in the experiment on guinea-pigs fed with
treated vegetation the inhibition of cholinesterase activity produced
by the residue in the plant was equivalent to that produced by
laboratory grade dimethoate.
EVALUATION
Level causing no significant toxicological effect
Rat. 5 ppm in the diet according to the weight of the animals
used in the experiment is approximately equivalent to 0.4 mg/kg
body-weight.
Man. 0.04 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Casida, J. E. & Sanderson, D. M. (1962) Nature, 189, 507
Casida, J. E. & Sanderson, D. M. (1963) J. Agr. Food Chem., 2, 91
Chilwell, E. D. & Beechman, P. T. (1960) J. Sci. Food Agric., 2,
400
Dauterman, W. C. et al. (1960) J. Agr. Food Chem., 8, 115
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
O'Brien, R. D. (1959) Nature, 183, 121
O'Brien, R. D. (1961) Biochem. J., 79, 229
Sampaolo, A. (1961) C.R. Ist Super. Sanità, 24, 936
Sanderson, D. M. & Edson, E. F. (1964) Brit. J. industr. Med., 21,
52
Santi, R. & de Pietri Sonelli, P. (1959) Nature, 183, 398
Santi, R. & Giacomelli, R. (1962) J. Agr. Food Chem., 10, 3
Sherman, M., Ross, E., Sanchet, F. F. & Chang, M. T. Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone. L. B. & Shaffer, C. B. (1961) Toxicol. Appl.
Pharmacol., 3, 210
BIOLOGICAL DATA
Biochemical aspects
Dimethoate is a cholinesterase inhibitor. The molar concentration
of the pure compound necessary to produce 50% cholinesterase
inhibition in the rat brain in vitro (I50) is 8.5 × 10-3. It
decomposes to give products which are more toxic than the original
substance (Casida & Sanderson, 1962, Casida & Sanderson, 1963).
Various studies (O'Brien, 1959; O'Brien, 1961; Sanderson & Edson,
1964) carried out with dimethoate labelled with 32P have shown that
there is rapid absorption from the digestive tract. The radioactivity
is concentrated in the liver, bile, kidneys and urine. There is no
accumulation in the fat depots. Elimination is rapid in the rat and in
man, 76-90% of the radioactivity being found in the urine after 24
hours. In the guinea-pig, 25-40% of the radioactivity is recovered in
the faeces. Four dimethoate metabolites with anticholinesterase
activity (molar I50) s in 30 minutes at 37° in rat brain: 4.7 × 10-6;
1.1 × 10-5; approximately 0.2 × 10-5 and approximately 0.1 × 10-5)
have been identified in the rat and in man. One of them seems to be a
product resulting from thiono-oxidation, leading to the formation of
the oxygen homologue of dimethoate and followed by hydrolysis with
production of a thiocarboxyl derivative which constitutes the chief
metabolite of dimethoate in mammals. Although this thiocarboxyl
derivative has not been found in treated plants, the oxygen analogue
of dimethoate has been found in crops (Santi & de Pietri Sonelli,
1959). Similarly, various short-lived intermediate products have been
reported, but have not yet been accurately evaluated from the
toxicological viewpoint (Chilwell & Beechman, 1960; Dauterman et al.,
1960; Sampaolo, 1961; Santi & de Pietri Sonelli, 1959; Santi &
Giacomelli, 1962).
In vitro studies on human liver enzymes indicated that
dimethoate could inhibit the non-specific esterases to a greater
degree than acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
LD50 mg/kg body-weight
Animal Route Laboratory References
Pure grade Technical
Rat, male Oral 500-600 280-350 180-325 Sanderson &
Edson, 1964
Rat, female Oral 570-680 300-356 240-336 "
Rat, male Intraperitoneal - 175-325 - "
Rat, female Intraperitoneal - 350 - "
Rat, male Intravenous - 450 - "
Mouse, female Oral 60 - 60 "
Hamster, male Oral - 200 - "
Guinea-pig Oral 550 600 350-400 "
Rabbit Oral 500 450 approx. 300 "
Hen Oral 50 40 approx. 30 "
Acute oral toxicity was not potentiated by any of 17 other
insecticides (Sanderson & Edson, 1964).
Short-term studies
Rat. Groups of 10 male rats were fed diets containing 1, 5, 25
and 125 ppm of dimethoate for 15 weeks. The animals were killed in
order to determine the cholinesterase activity of the erythrocytes,
plasma and brain as well as to examine and weigh the main organs. At
the highest concentration, i.e., 125 ppm, a slight fall in the rate of
gain of weight was observed as well as mild symptoms of poisoning
(slight muscular fibrillation). In the group fed 25 ppm and higher
concentrations, a significant fall in the cholinesterase activity of
the plasma and erythrocytes was observed, while in the animals fed 5
ppm a fall of 20% in cholinesterase activity was found. At 1 ppm there
was no effect on the cholinesterase activity of the plasma,
erythrocytes or brain (Edson & Noakes, 1960).
Young rats in groups of 20 fed diets containing 2, 8 and 32 ppm
of dimethoate for 90 days and other groups of 20 rats fed 50, 100 and
200 ppm for 35 days showed no haematological abnormalities, nor any
significant histopathological changes. Regarding the cholinesterase
activity of the plasma and the erythrocytes the highest dose which did
not give a significant inhibition was 32 ppm of dimethoate (West et
al., 1961).
Guinea-pig. Groups of guinea-pigs were fed for 3 weeks on
lettuce and brassica leaves that had been treated with dimethoate and
contained residues of up to 189 ppm. No toxic effects were seen and
the cholinesterase inhibition observed was in agreement with that in
parallel groups given daily oral doses of the same quantity of
laboratory grade dimethoate (Sanderson & Edson, 1964).
Chicken. In laying hens dimethoate given over a period of 59
weeks at a concentration of 30 ppm daily in the drinking-water caused
inhibition of plasm cholinesterase and some reduction in appetite, but
no egg abnormalities (Sherman, et al., 1963).
Dog. Three groups each of 4 dogs, 2 males and 2 females, were
fed diets containing 2, 10 and 50 ppm for 13 weeks. No significant
harmful effect was noted. The cholinesterase activity of the
erythrocytes was only slightly decreased at the highest concentration
of 50 ppm while that of the plasma was unaffected at any of the
concentrations employed (West et al., 1961).
Man. Twenty subjects ingested daily for 4 weeks 2.5 mg of
dimethoate in aqueous solution, corresponding to about 0.04 mg/kg
body-weight. No toxic effect was observed nor any significant change
in the blood cholinesterase activity. The same results were found in 2
subjects who ingested daily during 21 days, 9 mg (0.13 mg/kg) and 18
mg (0.26 mg/kg body-weight) dimethoate respectively (Sanderson &
Edson, 1964).
Long-term studies
Rat. Groups of 20 male rats were maintained for 6-12 months on
diets containing various concentrations of laboratory grade
dimethoate. At 800 ppm severe toxic effects developed within a few
days; the chemical was withdrawn after a week and complete recovery
occurred in 10-14 days. No toxic effects were seen at 50 ppm or below.
Marked inhibition of erythrocyte cholinesterase activity occurred at
50 ppm but at 10 ppm and below neither erythrocyte nor plasma
cholinesterase showed significant inhibition throughout the test. At
the end of the experiment there were no macroscopical or microscopical
changes in any group attributable to dimethoate. The maximum no-effect
level in these experiments corresponded to 0.5-0.8 mg/kg body-weight
per day.
In the same experiment further groups of 20 weanling male rats
were treated for 5-1/2 months at dose levels of 5, 10 and 20 ppm of
dimethoate. The maximum no-effect level in these experiments was 5 ppm
corresponding to 0.3-0.6 mg/kg body-weight per day.
A further test with the commercial liquid formulation of
dimethoate on similar groups of male and female rats lasted 12 weeks.
The maximum no-effect level was again 5 ppm corresponding to 0.4-0.6
mg/kg body-weight per day (Sanderson & Edson, 1964).
Comments on the experimental studies reported
Short-term studies have been made on a number of species, in the
rat for as long as one year. Some observations have also been made in
man, and if adequate studies were carried out to establish a level
causing minimal blood cholinesterase inhibition it might be possible
to increase the acceptable daily intake.
It was noted that in the experiment on guinea-pigs fed with
treated vegetation the inhibition of cholinesterase activity produced
by the residue in the plant was equivalent to that produced by
laboratory grade dimethoate.
EVALUATION
Level causing no significant toxicological effect
Rat. 5 ppm in the diet according to the weight of the animals
used in the experiment is approximately equivalent to 0.4 mg/kg
body-weight.
Man. 0.04 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Casida, J. E. & Sanderson, D. M. (1962) Nature, 189, 507
Casida, J. E. & Sanderson, D. M. (1963) J. Agr. Food Chem., 2, 91
Chilwell, E. D. & Beechman, P. T. (1960) J. Sci. Food Agric., 2,
400
Dauterman, W. C. et al. (1960) J. Agr. Food Chem., 8, 115
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
O'Brien, R. D. (1959) Nature, 183, 121
O'Brien, R. D. (1961) Biochem. J., 79, 229
Sampaolo, A. (1961) C.R. Ist Super. Sanità, 24, 936
Sanderson, D. M. & Edson, E. F. (1964) Brit. J. industr. Med., 21,
52
Santi, R. & de Pietri Sonelli, P. (1959) Nature, 183, 398
Santi, R. & Giacomelli, R. (1962) J. Agr. Food Chem., 10, 3
Sherman, M., Ross, E., Sanchet, F. F. & Chang, M. T. Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone. L. B. & Shaffer, C. B. (1961) Toxicol. Appl.
Pharmacol., 3, 210
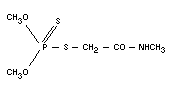 BIOLOGICAL DATA
Biochemical aspects
Dimethoate is a cholinesterase inhibitor. The molar concentration
of the pure compound necessary to produce 50% cholinesterase
inhibition in the rat brain in vitro (I50) is 8.5 × 10-3. It
decomposes to give products which are more toxic than the original
substance (Casida & Sanderson, 1962, Casida & Sanderson, 1963).
Various studies (O'Brien, 1959; O'Brien, 1961; Sanderson & Edson,
1964) carried out with dimethoate labelled with 32P have shown that
there is rapid absorption from the digestive tract. The radioactivity
is concentrated in the liver, bile, kidneys and urine. There is no
accumulation in the fat depots. Elimination is rapid in the rat and in
man, 76-90% of the radioactivity being found in the urine after 24
hours. In the guinea-pig, 25-40% of the radioactivity is recovered in
the faeces. Four dimethoate metabolites with anticholinesterase
activity (molar I50) s in 30 minutes at 37° in rat brain: 4.7 × 10-6;
1.1 × 10-5; approximately 0.2 × 10-5 and approximately 0.1 × 10-5)
have been identified in the rat and in man. One of them seems to be a
product resulting from thiono-oxidation, leading to the formation of
the oxygen homologue of dimethoate and followed by hydrolysis with
production of a thiocarboxyl derivative which constitutes the chief
metabolite of dimethoate in mammals. Although this thiocarboxyl
derivative has not been found in treated plants, the oxygen analogue
of dimethoate has been found in crops (Santi & de Pietri Sonelli,
1959). Similarly, various short-lived intermediate products have been
reported, but have not yet been accurately evaluated from the
toxicological viewpoint (Chilwell & Beechman, 1960; Dauterman et al.,
1960; Sampaolo, 1961; Santi & de Pietri Sonelli, 1959; Santi &
Giacomelli, 1962).
In vitro studies on human liver enzymes indicated that
dimethoate could inhibit the non-specific esterases to a greater
degree than acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
LD50 mg/kg body-weight
Animal Route Laboratory References
Pure grade Technical
Rat, male Oral 500-600 280-350 180-325 Sanderson &
Edson, 1964
Rat, female Oral 570-680 300-356 240-336 "
Rat, male Intraperitoneal - 175-325 - "
Rat, female Intraperitoneal - 350 - "
Rat, male Intravenous - 450 - "
Mouse, female Oral 60 - 60 "
Hamster, male Oral - 200 - "
Guinea-pig Oral 550 600 350-400 "
Rabbit Oral 500 450 approx. 300 "
Hen Oral 50 40 approx. 30 "
Acute oral toxicity was not potentiated by any of 17 other
insecticides (Sanderson & Edson, 1964).
Short-term studies
Rat. Groups of 10 male rats were fed diets containing 1, 5, 25
and 125 ppm of dimethoate for 15 weeks. The animals were killed in
order to determine the cholinesterase activity of the erythrocytes,
plasma and brain as well as to examine and weigh the main organs. At
the highest concentration, i.e., 125 ppm, a slight fall in the rate of
gain of weight was observed as well as mild symptoms of poisoning
(slight muscular fibrillation). In the group fed 25 ppm and higher
concentrations, a significant fall in the cholinesterase activity of
the plasma and erythrocytes was observed, while in the animals fed 5
ppm a fall of 20% in cholinesterase activity was found. At 1 ppm there
was no effect on the cholinesterase activity of the plasma,
erythrocytes or brain (Edson & Noakes, 1960).
Young rats in groups of 20 fed diets containing 2, 8 and 32 ppm
of dimethoate for 90 days and other groups of 20 rats fed 50, 100 and
200 ppm for 35 days showed no haematological abnormalities, nor any
significant histopathological changes. Regarding the cholinesterase
activity of the plasma and the erythrocytes the highest dose which did
not give a significant inhibition was 32 ppm of dimethoate (West et
al., 1961).
Guinea-pig. Groups of guinea-pigs were fed for 3 weeks on
lettuce and brassica leaves that had been treated with dimethoate and
contained residues of up to 189 ppm. No toxic effects were seen and
the cholinesterase inhibition observed was in agreement with that in
parallel groups given daily oral doses of the same quantity of
laboratory grade dimethoate (Sanderson & Edson, 1964).
Chicken. In laying hens dimethoate given over a period of 59
weeks at a concentration of 30 ppm daily in the drinking-water caused
inhibition of plasm cholinesterase and some reduction in appetite, but
no egg abnormalities (Sherman, et al., 1963).
Dog. Three groups each of 4 dogs, 2 males and 2 females, were
fed diets containing 2, 10 and 50 ppm for 13 weeks. No significant
harmful effect was noted. The cholinesterase activity of the
erythrocytes was only slightly decreased at the highest concentration
of 50 ppm while that of the plasma was unaffected at any of the
concentrations employed (West et al., 1961).
Man. Twenty subjects ingested daily for 4 weeks 2.5 mg of
dimethoate in aqueous solution, corresponding to about 0.04 mg/kg
body-weight. No toxic effect was observed nor any significant change
in the blood cholinesterase activity. The same results were found in 2
subjects who ingested daily during 21 days, 9 mg (0.13 mg/kg) and 18
mg (0.26 mg/kg body-weight) dimethoate respectively (Sanderson &
Edson, 1964).
Long-term studies
Rat. Groups of 20 male rats were maintained for 6-12 months on
diets containing various concentrations of laboratory grade
dimethoate. At 800 ppm severe toxic effects developed within a few
days; the chemical was withdrawn after a week and complete recovery
occurred in 10-14 days. No toxic effects were seen at 50 ppm or below.
Marked inhibition of erythrocyte cholinesterase activity occurred at
50 ppm but at 10 ppm and below neither erythrocyte nor plasma
cholinesterase showed significant inhibition throughout the test. At
the end of the experiment there were no macroscopical or microscopical
changes in any group attributable to dimethoate. The maximum no-effect
level in these experiments corresponded to 0.5-0.8 mg/kg body-weight
per day.
In the same experiment further groups of 20 weanling male rats
were treated for 5-1/2 months at dose levels of 5, 10 and 20 ppm of
dimethoate. The maximum no-effect level in these experiments was 5 ppm
corresponding to 0.3-0.6 mg/kg body-weight per day.
A further test with the commercial liquid formulation of
dimethoate on similar groups of male and female rats lasted 12 weeks.
The maximum no-effect level was again 5 ppm corresponding to 0.4-0.6
mg/kg body-weight per day (Sanderson & Edson, 1964).
Comments on the experimental studies reported
Short-term studies have been made on a number of species, in the
rat for as long as one year. Some observations have also been made in
man, and if adequate studies were carried out to establish a level
causing minimal blood cholinesterase inhibition it might be possible
to increase the acceptable daily intake.
It was noted that in the experiment on guinea-pigs fed with
treated vegetation the inhibition of cholinesterase activity produced
by the residue in the plant was equivalent to that produced by
laboratory grade dimethoate.
EVALUATION
Level causing no significant toxicological effect
Rat. 5 ppm in the diet according to the weight of the animals
used in the experiment is approximately equivalent to 0.4 mg/kg
body-weight.
Man. 0.04 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Casida, J. E. & Sanderson, D. M. (1962) Nature, 189, 507
Casida, J. E. & Sanderson, D. M. (1963) J. Agr. Food Chem., 2, 91
Chilwell, E. D. & Beechman, P. T. (1960) J. Sci. Food Agric., 2,
400
Dauterman, W. C. et al. (1960) J. Agr. Food Chem., 8, 115
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
O'Brien, R. D. (1959) Nature, 183, 121
O'Brien, R. D. (1961) Biochem. J., 79, 229
Sampaolo, A. (1961) C.R. Ist Super. Sanità, 24, 936
Sanderson, D. M. & Edson, E. F. (1964) Brit. J. industr. Med., 21,
52
Santi, R. & de Pietri Sonelli, P. (1959) Nature, 183, 398
Santi, R. & Giacomelli, R. (1962) J. Agr. Food Chem., 10, 3
Sherman, M., Ross, E., Sanchet, F. F. & Chang, M. T. Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone. L. B. & Shaffer, C. B. (1961) Toxicol. Appl.
Pharmacol., 3, 210
BIOLOGICAL DATA
Biochemical aspects
Dimethoate is a cholinesterase inhibitor. The molar concentration
of the pure compound necessary to produce 50% cholinesterase
inhibition in the rat brain in vitro (I50) is 8.5 × 10-3. It
decomposes to give products which are more toxic than the original
substance (Casida & Sanderson, 1962, Casida & Sanderson, 1963).
Various studies (O'Brien, 1959; O'Brien, 1961; Sanderson & Edson,
1964) carried out with dimethoate labelled with 32P have shown that
there is rapid absorption from the digestive tract. The radioactivity
is concentrated in the liver, bile, kidneys and urine. There is no
accumulation in the fat depots. Elimination is rapid in the rat and in
man, 76-90% of the radioactivity being found in the urine after 24
hours. In the guinea-pig, 25-40% of the radioactivity is recovered in
the faeces. Four dimethoate metabolites with anticholinesterase
activity (molar I50) s in 30 minutes at 37° in rat brain: 4.7 × 10-6;
1.1 × 10-5; approximately 0.2 × 10-5 and approximately 0.1 × 10-5)
have been identified in the rat and in man. One of them seems to be a
product resulting from thiono-oxidation, leading to the formation of
the oxygen homologue of dimethoate and followed by hydrolysis with
production of a thiocarboxyl derivative which constitutes the chief
metabolite of dimethoate in mammals. Although this thiocarboxyl
derivative has not been found in treated plants, the oxygen analogue
of dimethoate has been found in crops (Santi & de Pietri Sonelli,
1959). Similarly, various short-lived intermediate products have been
reported, but have not yet been accurately evaluated from the
toxicological viewpoint (Chilwell & Beechman, 1960; Dauterman et al.,
1960; Sampaolo, 1961; Santi & de Pietri Sonelli, 1959; Santi &
Giacomelli, 1962).
In vitro studies on human liver enzymes indicated that
dimethoate could inhibit the non-specific esterases to a greater
degree than acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
LD50 mg/kg body-weight
Animal Route Laboratory References
Pure grade Technical
Rat, male Oral 500-600 280-350 180-325 Sanderson &
Edson, 1964
Rat, female Oral 570-680 300-356 240-336 "
Rat, male Intraperitoneal - 175-325 - "
Rat, female Intraperitoneal - 350 - "
Rat, male Intravenous - 450 - "
Mouse, female Oral 60 - 60 "
Hamster, male Oral - 200 - "
Guinea-pig Oral 550 600 350-400 "
Rabbit Oral 500 450 approx. 300 "
Hen Oral 50 40 approx. 30 "
Acute oral toxicity was not potentiated by any of 17 other
insecticides (Sanderson & Edson, 1964).
Short-term studies
Rat. Groups of 10 male rats were fed diets containing 1, 5, 25
and 125 ppm of dimethoate for 15 weeks. The animals were killed in
order to determine the cholinesterase activity of the erythrocytes,
plasma and brain as well as to examine and weigh the main organs. At
the highest concentration, i.e., 125 ppm, a slight fall in the rate of
gain of weight was observed as well as mild symptoms of poisoning
(slight muscular fibrillation). In the group fed 25 ppm and higher
concentrations, a significant fall in the cholinesterase activity of
the plasma and erythrocytes was observed, while in the animals fed 5
ppm a fall of 20% in cholinesterase activity was found. At 1 ppm there
was no effect on the cholinesterase activity of the plasma,
erythrocytes or brain (Edson & Noakes, 1960).
Young rats in groups of 20 fed diets containing 2, 8 and 32 ppm
of dimethoate for 90 days and other groups of 20 rats fed 50, 100 and
200 ppm for 35 days showed no haematological abnormalities, nor any
significant histopathological changes. Regarding the cholinesterase
activity of the plasma and the erythrocytes the highest dose which did
not give a significant inhibition was 32 ppm of dimethoate (West et
al., 1961).
Guinea-pig. Groups of guinea-pigs were fed for 3 weeks on
lettuce and brassica leaves that had been treated with dimethoate and
contained residues of up to 189 ppm. No toxic effects were seen and
the cholinesterase inhibition observed was in agreement with that in
parallel groups given daily oral doses of the same quantity of
laboratory grade dimethoate (Sanderson & Edson, 1964).
Chicken. In laying hens dimethoate given over a period of 59
weeks at a concentration of 30 ppm daily in the drinking-water caused
inhibition of plasm cholinesterase and some reduction in appetite, but
no egg abnormalities (Sherman, et al., 1963).
Dog. Three groups each of 4 dogs, 2 males and 2 females, were
fed diets containing 2, 10 and 50 ppm for 13 weeks. No significant
harmful effect was noted. The cholinesterase activity of the
erythrocytes was only slightly decreased at the highest concentration
of 50 ppm while that of the plasma was unaffected at any of the
concentrations employed (West et al., 1961).
Man. Twenty subjects ingested daily for 4 weeks 2.5 mg of
dimethoate in aqueous solution, corresponding to about 0.04 mg/kg
body-weight. No toxic effect was observed nor any significant change
in the blood cholinesterase activity. The same results were found in 2
subjects who ingested daily during 21 days, 9 mg (0.13 mg/kg) and 18
mg (0.26 mg/kg body-weight) dimethoate respectively (Sanderson &
Edson, 1964).
Long-term studies
Rat. Groups of 20 male rats were maintained for 6-12 months on
diets containing various concentrations of laboratory grade
dimethoate. At 800 ppm severe toxic effects developed within a few
days; the chemical was withdrawn after a week and complete recovery
occurred in 10-14 days. No toxic effects were seen at 50 ppm or below.
Marked inhibition of erythrocyte cholinesterase activity occurred at
50 ppm but at 10 ppm and below neither erythrocyte nor plasma
cholinesterase showed significant inhibition throughout the test. At
the end of the experiment there were no macroscopical or microscopical
changes in any group attributable to dimethoate. The maximum no-effect
level in these experiments corresponded to 0.5-0.8 mg/kg body-weight
per day.
In the same experiment further groups of 20 weanling male rats
were treated for 5-1/2 months at dose levels of 5, 10 and 20 ppm of
dimethoate. The maximum no-effect level in these experiments was 5 ppm
corresponding to 0.3-0.6 mg/kg body-weight per day.
A further test with the commercial liquid formulation of
dimethoate on similar groups of male and female rats lasted 12 weeks.
The maximum no-effect level was again 5 ppm corresponding to 0.4-0.6
mg/kg body-weight per day (Sanderson & Edson, 1964).
Comments on the experimental studies reported
Short-term studies have been made on a number of species, in the
rat for as long as one year. Some observations have also been made in
man, and if adequate studies were carried out to establish a level
causing minimal blood cholinesterase inhibition it might be possible
to increase the acceptable daily intake.
It was noted that in the experiment on guinea-pigs fed with
treated vegetation the inhibition of cholinesterase activity produced
by the residue in the plant was equivalent to that produced by
laboratory grade dimethoate.
EVALUATION
Level causing no significant toxicological effect
Rat. 5 ppm in the diet according to the weight of the animals
used in the experiment is approximately equivalent to 0.4 mg/kg
body-weight.
Man. 0.04 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.004 mg/kg body-weight.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Casida, J. E. & Sanderson, D. M. (1962) Nature, 189, 507
Casida, J. E. & Sanderson, D. M. (1963) J. Agr. Food Chem., 2, 91
Chilwell, E. D. & Beechman, P. T. (1960) J. Sci. Food Agric., 2,
400
Dauterman, W. C. et al. (1960) J. Agr. Food Chem., 8, 115
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. & Noakes, D. N. (1960) Toxicol. Appl. Pharmacol., 2,
523
O'Brien, R. D. (1959) Nature, 183, 121
O'Brien, R. D. (1961) Biochem. J., 79, 229
Sampaolo, A. (1961) C.R. Ist Super. Sanità, 24, 936
Sanderson, D. M. & Edson, E. F. (1964) Brit. J. industr. Med., 21,
52
Santi, R. & de Pietri Sonelli, P. (1959) Nature, 183, 398
Santi, R. & Giacomelli, R. (1962) J. Agr. Food Chem., 10, 3
Sherman, M., Ross, E., Sanchet, F. F. & Chang, M. T. Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone. L. B. & Shaffer, C. B. (1961) Toxicol. Appl.
Pharmacol., 3, 210
