
FAO/PL:1967/M/11/1
WHO/Food Add./68.30
1967 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Rome, 4 - 11 December,
1967. (FAO/WHO, 1968)
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1968
DIMETHOATE
IDENTITY
Chemical names
0,0-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate;
S-methylcarbamoylmethyl 0,0-dimethyl phosphorodithioate; 0,0-dimethyl
S-alpha-mercapto-N-methylacetamido dithiophosphate; N-monoethylamide
of 0,0-dimethyldithiophosphoryl acetic acid; methyl
dimethyldithiophosphoryl acetamide.
Synonyms
Rogor, Fortion MM
Empirical formula
C5H12NO3PS2
Structural formula
S
"
(CH3O)2 - P - S -CH2-CO - NHCH3
Other relevant chemical properties
Technical dimethoate - 93.3 per cent
EVALUATION FOR ACCEPTABLE DAILY INTAKES
Biochemical aspects
Dimethoate is a cholinesterase inhibitor. The molar concentration of
the pure compound necessary to produce 50 per cent cholinesterase
inhibition in the rat brain in vitro (I50) is 8.5 × 10-3. It
decomposes to give products which are more toxic than the original
substance (Casida & Sanderson, 1963).
Various studies (O'Brien, 1959; O'Brien, 1961); Sanderson & Edson,
1964) carried out with dimethoate labelled with 32P have shown that
there is rapid absorption from the digestive tract. The radioactivity
is concentrated in the liver, bile, kidneys and urine. There is no
accumulation in the fat depots. Elimination is rapid in the rat and in
man, 76-90 per cent of the radioactivity being found in the urine
after 24 hours. In the guinea-pig, 25-40 per cent of the radioactivity
is recovered in the faeces. Four dimethoate metabolites with
anticholinesterase activity (molar I50's in 30 minutes at 37° in rat
brain; 4.7 × 10-6; 1.1 × 10-5; approximately 0.2 × 10-5 and
approximately 0.1 × 10-5) have been identified in the rat and in man.
One of them seems to be a product resulting from thiono-oxidation,
leading to the formation of the oxygen homologue of dimethoate and
followed by hydrolysis with production of a thiocarboxyl derivative
which constitutes the chief metabolite of dimethoate in mammals.
In vitro studies on human liver enzymes indicated that dimethoate
could inhibit the non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
For further information see In animals.
Acute toxicity Dimethoate
LD50
Animal Route (mg/kg body-weight) Reference
Pure Laboratory Technical
grade
Mouse, female Oral 60 - 60 Sanderson &
Edson, 1964
Rat, male Oral 500-600 280-350 180-325 "
Rat, female Oral 570-680 300-356 240-336 "
Rat, male Intraperitoneal - 175-325 - "
Rat, female Intraperitoneal - 350 - "
Rat, male Intravenous - 450 - "
Hamster, male Oral - 200 - "
Guinea-pig Oral 550 600 350-400 "
Rabbit Oral 500 450 approx. 300 "
Hen Oral 50 40 approx. 30 "
The rat oral LD50's (mg/kg body weight) of desmethyl oxy-carboxy
dimethoate and oxycarboxy dimethoate have been determined to be <600
and <800 respectively whereas the hen oral LD50 of oxygen analog of
dimethoate has been determined to be 100 (Levinskas and Shaffer,
1965).
Acute oral toxicity of dimethoate was not potentiated by any of 17
other insecticides (Sanderson & Edson, 1964).
Short-term studies
Mouse. A three generation reproduction study was conducted at
dietary levels of 0, 5, 15 and 50 ppm of dimethoate, with two litters
produced per generation. Second litter animals were used for composing
succeeding generations. No effect of the compound was seen in
fertility, lactation or survival of the pups to weaning, gross
appearance of all pups produced, weights of major organs of F2b
animals and gross and microscopic appearance of tissues of the F3b
animals, autopsied at 21 days of age (Ribelin et al., 1965).
Rat. Groups of 10 male rats were fed diets containing 1, 5, 25 and
125 ppm of dimethoate for 15 weeks. At the highest concentration, a
slight fall in the rate of gain of weight was observed as well as mild
symptoms of poisoning (slight muscular fibrillation). In the group fed
25 ppm and higher concentrations, a significant fall in the
cholinesterase activity of the plasma and erythrocytes was observed,
while in the animals fed 5 ppm a fall of 20 per cent in cholinesterase
activity was found. At 1 ppm there was no effect on the cholinesterase
activity of the plasma, erythrocytes or brain (Edson & Noakes, 1960).
Young rats in groups of 20 fed diets containing 2, 8 and 32 ppm of
dimethoate for 90 days and other groups of 20 rats fed 50, 100 and 200
ppm for 35 days showed no haematological abnormalities, nor any
significant histopathological changes. Regarding the cholinesterase
activity of the plasma and the erythrocytes, the highest dose which
did not give a significant inhibition was 32 ppm of dimethoate (West
et al., 1961).
Groups of 20 male rats were maintained for 6-12 months on diets
containing various concentrations of laboratory grade dimethoate. At
800 ppm severe intoxication developed within a few days; the chemical
was withdrawn after a week and complete recovery occurred in 10-14
days. No toxic effects were seen at 50 ppm or below. Marked inhibition
of erythrocyte cholinesterase activity occurred at 50 ppm but at 10
ppm and below neither erythrocyte nor plasma cholinesterase showed
significant inhibition throughout the test. At the end of the
experiment there were no gross or microscopic changes in any group
attributable to dimethoate. The maximum no-effect level in these
experiments corresponded to 0.5-0.8 mg/kg body-weight per day.
In the same experiment, further groups of 20 weanling male rats were
treated for 5-1/2 months at dose levels of 5, 10 and 20 ppm of
dimethoate. The maximum no-effect level in these experiments was 5 ppm
corresponding to 0.3-0.6 mg/kg body-weight per day.
A further test with the commercial liquid formulation of dimethoate on
similar groups of male and female rats lasted 12 weeks. The maximum
no-effect level in these experiments was again 5 ppm corresponding to
0.4-0.6 mg/kg body-weight per day (Sanderson & Edson, 1964).
Groups of 10-15 males and 10-15 females were fed 0, 330, 1000 and 3000
ppm of oxy-carboxy dimethoate for 33 days. At 3000 ppm, rate of weight
gain, food consumption and food utilization were affected in both
sexes, and kidney weight ratios were slightly elevated in the males.
Erythrocytic cholinesterase activity was inhibited in relation to dose
in both sexes at 1000 and 3000 ppm, and slightly inhibited in the
females at 330 ppm. Brain cholinesterase activity was significantly
affected in the 3000 ppm females. Plasma cholinesterase was unaffected
at all levels. No effect was seen on mortality, behaviour and gross
and microscopic appearance of major organs (Levinskas & Sheffer,
1965).
Initial groups of 25 males and 25 females were fed 0, 0.2, 0.4, 0.8
and 1.6 ppm at the oxygen analog of dimethoate for 28 days. Animals
from each group were selected for determination of cholinesterase
activities at 3, 7, 14 and 28 days. At 8 ppm, there was a marked
effect on erythrocyte, plasma (except in the females) and brain
cholinesterase activities. At 1.6 ppm, erythrocyte cholinesterase
activity was affected in both sexes and brain cholinesterase activity
in the females at 28 days. No distinct effect on cholinesterase was
found at lower levels. No effect at any level was found on food
intake, rate of weight gain, and gross and microscopic appearance of
major organs (Fogleman et al., 1965).
Guinea-pig. Groups of guinea-pigs were fed for 3 weeks on lettuce
and brassica leaves that had been treated with dimethoate and
contained residues of up to 189 ppm. No toxic effects were seen, and
the cholinesterase inhibition observed was in agreement with that in
parallel groups given daily oral doses of the same quantity of
laboratory grade dimethoate (Sanderson & Edson, 1964).
Chicken. In laying hens, dimethoate given over a period of 59 weeks
at a concentration of 30 ppm daily in the drinking-water caused
inhibition of plasma cholinesterase and some reduction in appetite,
but no egg abnormalities (Sherman, et al., 1963).
Groups of 6 hens were fed 0, 65, 130 and 260 ppm of dimethoate for 4
weeks. There was no effect on mortality, but hens at the high level
were unable to maintain their original weights. Groups of 6 hens
received 0 and 130 ppm of dimethoate and 2000 and 4000 ppm of
tri-o-cresyl phosphate for 4 weeks. At the termination of the study,
samples of brain, thoracic cord and sciatic nerve were examined for
effects on axons and myelin sheaths. Tri-o-cresyl phosphate was found
to produce a demonstrable general myelin loss, but dimethoate had no
effect on either sheaths or axons. (Levinskas and Shaffer, 1965).
Groups of 6 hens were fed 0, 60, 120 and 240 ppm of oxygen analog of
dimethoate for 4 weeks. At the termination of the study, samples of
brain, thoracic cord and sciatic nerve were examined for effects on
axons and myelin sheaths. No effect was found on nerve tissue or on
survival, but it was noted that the test birds were generally unable
to maintain body weight satisfactorily (Levinskas and Shaffer, 1965).
Dog. Three groups each of 4 dogs, 2 males and 2 females, were fed
diets containing 2, 10 and 50 ppm for 13 weeks. No significant harmful
effect was noted. The cholinesterase activity of the erythrocytes was
only slightly decreased at the highest concentration of 50 ppm while
that of the plasma was unaffected at any of the concentrations
employed (West et al., 1961).
Man. Twenty subjects ingested 2.5 mg of dimethoate in aqueous
solution, corresponding to about 0.04 mg/kg body-weight daily for 4
weeks. No toxic effect was observed, nor any significant change in the
blood cholinesterase activity. The same results were found in 2
subjects who ingested daily during 21 days, 9 mg (0.13 mg/kg) and 18
mg (0.26 mg/kg body-weight) dimethoate respectively (Sanderson and
Edson, 1964).
Thirty-six male and female volunteers were given daily oral doses of
dimethoate of 5, 15, 30, 45 and 60 mg for periods of 14 to 57 days.
There was no effect on the blood cholinesterase levels with intakes of
5 and 15 mg daily, but there was at 30 mg and above (Edson et al.,
1967).
Long-term studies
No data available.
Comments
The findings now available from man provide a satisfactory basis for
assessment. The reproduction studies now completed in the mouse meet
the needs of the request previously made for such experiments in the
rat.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Man 0.2 mg/kg body-weight/day.
Estimate of acceptable daily intake for man
0.02 mg/kg body-weight.
EVALUATION FOR TOLERANCES
USE PATTERN
Pre-harvest treatments
Dimethoate is a systemic insecticide used on a number of fruit and
vegetable crops to combat aphids, leafhoppers, leafminers, lygus bugs,
pear psylla, various mites (except rust mites), olive flies, various
citrus pests and fruit eating larvae. Typical maximum recommended
usages are presented in the following Table I.
TABLE I
Crop Dosage, lbs Pre-harvest
actual period, days
apples - pears 0.5 lbs/100 gal 28
beans 0.25 - 0.5 lbs/A 0*
peas 0.2 lbs/A 0
brassica 0.25 - 0.5 lbs/A 7
cabbage 0.25 - 0.5 lbs/A 3
leafy vegetable 0.25 lbs/A 14
* Cattle should not be allowed to graze on vines
Dimethoate is also used on alfalfa, wheat, safflower and pea forage
against aphids, leafhoppers, lygus bugs, grasshoppers and thrips with
applications of 0.25 - 0.5 lb/100 gal and a pre-grazing and
pre-harvest period of 21 - 28 days.
Post-harvest treatments
No post harvest use of dimethoate is known.
Other uses
Dimethoate is used in formulations on a large number of ornamental
plants, shrubs and trees. It has been recommended for control of
houseflies and maggots and is used for this purpose around the home,
barn, and commercial establishments with a caution to avoid
contamination of human and animal food supplies.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Numerous supervised field trials have been made on a large number of
crops under varying cultural and climatic conditions with different
rates of application and harvest times. Much of this data has been
submitted to the U.S. Food and Drug Administration in the form of
petitions. Table II summarizes data representative of residues found
when the maximum recommended dosage of pesticide (see Table I) was
used.
TABLE II
Crop Typical initial Pre-harvest Residue at end Estimated
residues, ppm period, days of pre-harvest half-life
period, ppm. days
Fruits
Apples 4.0 - 6.0 28 1.0 - 1.5 15
Pears 1.0 - 2.0 28 0.4 10
Cole crops
Cauliflower 5.0 - 6.0 7 0.6 - 0.9 3
Broccoli 9.0 - 23.0 7 2.0 2
Cabbage 3.0 - 8.0 3 2.0 1-4
Head lettuce 5.0 7 1.2 4
Leafy vegetables
Spinach 4.0 - 12.0 14 0 - 0.3 2-3
Kale 5.0 14 0.1 1
Turnip and
mustard
greens 7.0 - 9.0 14 0.3 2
Leaf lettuce 0.2 - 0.9 14 0.1 - 0.3 4
Collards 1.0 - 10.0 14 0.1 - 0.5 3
Endive 2.0 - 3.0 14 0.1 2
TABLE II (cont'd)
Crop Typical initial Pre-harvest Residue at end Estimated
residues, ppm period, days of pre-harvest half-life
period, ppm. days
Escarole and
chard 1.0 - 2.0 14 0.1 3
Legumes
(fruit and pod only)
Snap beans 1.5 0 1.5 4
Green beans 0.5 - 1.0 0 0.5 - 1.0 7
Lima beans 0.4 0 0.4 1
Peas 1.0 0 1.0 7
Other vegetables
Peppers 0.1 - 0.3 0 0.1 - 0.3 7
Tomatoes 0.2 - 0.8 7 0.2 4
TABLE III
Crop Dosage Typical Pre-harvest Residue at and Estimated
lbs actual initial period of pre-harvest half-life
per 100 gal residues ppm days period, ppm days
Peaches 0.25 lb 3.0-7.0 14 1.0-1.5 7
Apricots 0.12 lb 8.0-10.0 14 <1.0 5-7
Cherries 0.2 lb 2.0-12.0 14 1.0-1.5 3-5
Grapes 0.5 lb 7.0-10.0 28 <1.0 8
Strawberries 0.4 lb 7 0.3
Grapefruit 0.8 lb 2.0 90 1.2 120
Oranges (peeled) 0.8 lb <1.0 90 <0.5 100
Lemons 1.0 lb 21 0.1
Tangerines 0.5 lb 48 0.1
Artichokes 0.25-4.0 lb 7 1.1
Brussels sprouts 1.5 lb 12 1.1
Potatoes 0.4-1.0 lb 10-126 0.1
Sugar beats 0.4-2.0 lb 48-100 <0.1-0.2
Wheat 0.3 lb 58 0.2
Corn 0.5-1.0 lb 21 0.1
TABLE III (cont'd)
Crop Dosage Typical Pre-harvest Residue at and Estimated
lbs actual initial period of pre-harvest half-life
per 100 gal residues ppm days period, ppm days
Olives (eating) 0.16 lb 2.0-4.0 30 0.6 12
Olive oil 0.5 lb 2.0-3.0 14 <1.0 10
A number of studies made throughout the world are also reported in a
review by de Pietri-Tonelli et al., (1965). Table III summarizes the
results of these studies on crops not listed in Table II.
FATE OF RESIDUES
General considerations
Extensive studies have been made on the metabolism of dimethoate in
both plants and mammals. The major route in mammals appears to be
through the thiocarboxy derivative to the corresponding dimethyl
esters of phosphoric, thiophosphoric or dithiophosphoric acids. A side
pathway may also occur through the thiodesmethyl carboxy derivative.
The oxygen analog of dimethoate forms in animals and although this
route may be considered minor with respect to the principle route, it
is an important one to consider because of the toxicity of the oxygen
analog. Evidence indicates that in plants the formation of the oxygen
analog is a major route. However, various investigators are not in
agreement regarding the quantitative measure of the material degraded
by this oxidation route, nor are they in agreement on the other
pathways of metabolism involving hydrolysis and demethylation which
occur to a substantial degree. See Figure I for the proposed schemes.
In soils
The fate of dimethoate in soils is pertinent to this presentation
since the compound is systemic from soils; that is, it is absorbed by
plants from the soil and transported to the aerial part of the plant
in sufficient quantity to make the whole plant insecticidal to certain
insects.
The persistency of dimethoate in sandy loam soil was determined by
Bohn (1964). The biological half-life was found to be 4 days under
drought conditions and shortened to 2-1/2 days with moderate rainfall.
Parker and Dewey (1965) applied a dosage 5 times that used by Bohn to
gravelly silt loam. Bioassay with vinegar flies showed a rapid decline
of dimethoate in the first few days with 40 per cent remaining on the
5th day. The rate of dissipation then slowed down considerably with
approximately 20 per cent remaining after 54 days.
In plants
Santi and de Pietri-Tonelli (1959b) showed the systemic nature of
dimethoate. Application of the insecticide and its oxygen analog to
roots, stems and leaves of bean plants resulted in translocation to
other parts of the plant. Foliar spray on trees resulted in residues
in the fruit. Both compounds penetrate from the outside to the inside
of the fruit and kill fruit-eating larvae. They showed that
transformation to the oxygen analog occurs in broad bean plants after
root absorption of the insecticide and in cherries picked from sprayed
trees.
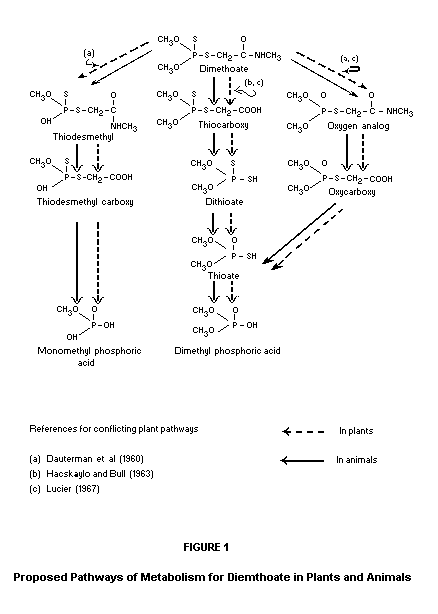 Dauterman et al. (1960) studied the metabolism of dimethoate-32P on
the surface and inside cotton, corn, pea and potato leaves following
foliar application. Substantial quantities of oxygen analog were found
in all samples. Although the quantity decreased with time, there was a
proportionate increase in the oxygen analog relative to dimethoate.
Internally this is attributed to enzymatic oxidation; however, since
this same oxidation results on non-biological surfaces exposed to the
atmosphere, non-enzymatic oxidation may occur on the leaf surface.
Based on the quantities of water-soluble metabolites on the surface
and inside leaves, Dauterman postulates that different mechanisms of
degradation occur. There also appears to be a marked difference in
crop species. Plant-surface water-soluble metabolites were primarily
the oxycarboxy derivative; whereas the desmethyl derivatives were the
major internal metabolites. Pea plants did not follow the pattern of
cotton, corn and potato plants, but had about 50 per cent residue of
phosphoric acid both on the surface and inside leaves. No
dithiophosphoric acids or thiocarboxy derivative were found in any
samples. Apparently oxidation occurs more rapidly than hydrolysis.
Hacskaylo and Bull (1963) found that metabolism of dimethoate in
excised cotton leaves resembles the pathway proposed for mammals and
is different from that occurring from foliar treatments. They found
the level of oxygen analog fairly constant and always less than 6 per
cent of the total metabolites found.
Recently Lucier (1967) studied the metabolism of 32P and 14C-carbonyl
labeled dimethoate in bean plants using four modes of application:
foliar treatment, stem injection, root absorption and excised leaves.
Conversion of dimethoate to the oxygen analog was the major route of
degradation and was directly correlated with the ease of translocation
of dimethoate to the leaf tissue. Thus the amount of oxygen analog was
greatest for the excised leaves and less for the foliar application.
In root absorption studies, the oxygen analog content was 4-1/2 times
the amount of dimethoate and represented 10 per cent of the
administered dose 10 days after application. The major hydrolysis
products were the thiocarboxy derivative, dimethyl phosphorothioate
and dimethyl phosphorodithioate. Only trace quantities of the
thiodesmethyl carboxy compound were found.
A new compound, des-N-methyl dimethoate was detected in trace
quantities in all samples. Foliar application gave rise to large
quantities of two unknown metabolites which were postulated to be the
N-hydroxy methyl derivatives of dimethoate and its oxygen analog.
These unknowns are rapidly degraded and not detectable after four
days.
Chillwell and Beecham (1960) found that climatic conditions had no
substantial effect on residue level of dimethoate if similar
applications were made on crops at the same growth stage. Recent
preliminary investigations by Watts and Storherr (1967) show that
residues on field-sprayed kale may contain five times as much oxygen
analog as the parent compound 14 days after treatment.
The oxygen analog seems to be the only metabolite of toxicological
importance. Therefore, its residue level in food products must be
considered. Data submitted to the U.S. Food and Drug Administration
and data reported in the literature by Santi* (1961), Santi and
Giacomelli (1962) and Dauterman et al. (1960) indicate that the amount
of oxygen analog is generally not above 10-20 per cent of the total
toxic residue. Results in olives are an exception where the oxygen
analog was found as high as 67 per cent of the total residue after 30
days.
In animals
Early studies by Roberts at al (1958) and Kaplanis et al (1959)
revealed that dimethoate was absorbed rapidly into the blood of cattle
and was converted to a metabolite several times more toxic than the
parent compound as determined by enzymatic analysis and bioassay. This
compound and many of the major urinary metabolites were not
identified. Santi and de Pietri-Tonelli (1959a) showed that dimethoate
converted chemically and in vitro by incubation in rat liver slices
to the oxygen analog. An unusually high rate of degradation of
dimethoate in sheep liver to the thiocarboxy derivative was found by
Uchida et al, (1964). This was attributed to amidase activity.
Sanderson and Edson (1964) found that dimethoate behaved as a typical
indirect anticholinesterase agent by conversion in the liver to at
least four short lived metabolites whose hydrolysis products are
rapidly excreted mainly in the urine.
Oral administration of dimethoate-32P at dosages of 100 mg/kg to rats
and 10 to 40 mg/kg to lactating cows led Dauterman and co-workers
(1959) to conclude that the metabolic pathway was similar for both
species, and that dimethoate was attacked hydrolytically at five sites
on the molecule. Only small quantities of labelled material were found
in the feces. Dimethoate was rapidly absorbed into the blood and
rapidly excreted in the urine with 75 per cent of the administered
dose eliminated within 24 hours primarily as the thiocarboxy
derivative. As the quantity of the thiocarboxy derivative and
dimethylthiophosphoric acid decreased, the level of dimethylphosphoric
acid increased and appeared relatively stable since conversion to
simpler phosphoric acids was very slow. When the desmethyl derivative
was fed to rats, recovery of better the 85 per cent of unchanged
chemical in the urine occurred suggesting that this is not the major
metabolic route in animals.
Analysis of blood samples indicated that a maximum of chloroform
solubles was reached at two hours with 1.5 ppm dimethoate equivalents
present in cows which were, treated with 10 g/kg. The level of
cholinesterase was severely depressed for the animal treated at
40 mg/kg but no depression occurred at the lower feeding level.
* As quoted by de Pietri-Tonelli et al (1965)
A composite sample representing total milk secreted over a 288 hour
period showed that 0.0068 per cent of the administered dose was
eliminated in this manner. The amount of chloroform solubles decreased
rapidly with less than 0.02 ppm dimethoate equivalents present after
48 hours from the oral dose of 10 mg/kg and 0.04 ppm from the 40 mg/kg
dose.
Chloroform solubles in the fat were less than 0.1 ppm after eight
hours. The only significant quantity of residue in bovine tissues was
found in the liver with a maximum of 0.23 ppm of chloroform solubles
at the lower feeding level.
Chamberlain et al. (1961) investigated the metabolism of dimethoate
by intramuscular administration and oral feeding of sheep at 10 and 40
mg/kg. He too found that only a small per cent of the labeled dose was
excreted in the faeces and that over 80 per cent of the radioactive
material was eliminated in the urine by 72 hours with the thiocarboxy
derivative being the major component. As the thiocarboxy content
decreased there was an increase in dimethyl phosphoric acid and an
unknown which he tentatively identified as the desmethyl carboxy
derivative and suggested that it formed from the thiocarboxy compound.
Trace quantities of dimethoate and its oxygen analog were present in
all sheep urine samples.
Analysis of the blood samples showed that the amount of
organo-solubles decreased very rapidly with less than 0.1 per cent
remaining after 12 hours. Ninety per cent of the organosoluble residue
vas dimethoate or its oxygen analog. The proportion of oxygen analog
to dimethoate increased as the quantity of dimethoate decreased.
In storage and processing
No commercial use of dimethoate for storage products is known. Rowland
(1966) studied the metabolism in stored wheat and sorghum grains
fortified at 2 and 10 parts per million. Trace amounts of dimethoate
and its oxygen analog were found after 4 and 7 days' storage. No
active anti-cholinesterase components were found at subsequent
samplings. No hydrolytic products of the oxygen analog were found, but
significant quantities of dimethoate hydrolysis products were detected
by paper chromatography. These included the thiocarboxy derivative,
the thiodesmethyl and thiodesmethyl carboxy derivative, and the
dimethyl esters of thio and dithiophosphoric acid.
METHODS OF RESIDUE ANALYSIS
The major metabolic pathway in plants indicates that the oxygen analog
of dimethoate is a significant metabolite. A minor pathway in animals
yields the same compound. Any adequate method for residue analysis
must take this compound into account.
Most of the data presented in Table II was obtained from one of two
methods of analysis.
The first method of Waldron (1962) (adapted for apples from a method
described by George (1962) for dimethoate in alfalfa) determines only
the parent compound, dimethoate. The pesticide is extracted with
methylene chloride, followed by alkaline hydrolysis, and colorimetric
determination of the resultant methylamine by reaction with
1-chloro-2,4-dinitrobenzene. Numerous tests were made to show that the
method in specific for dimethoate and that some 28 commonly used
pesticides do not interfere.
Steller and Curry developed a method incorporating a thin layer
chromatographic isolation of dimethoate and its oxygen analog and a
total phosphorous determination of the separate fractions measuring a
molybdenum blue complex. This method is specific for dimethoate and
its oxygen analog. Although recoveries were lower than for the Waldron
method, they were consistent and the authors claim a sensitivity of
0.06 ppm.
The data in Table II were obtained by a variety of methods. Chillwell
and Beecham (1960) macerated plant tissues with water acidified with
acetic acid and extracted the aqueous phase with chloroform.
Microdistillation was used to purify the extracted insecticide and
phosphorous was determined colorimetrically after an acid digestion.
Santi and de Pietri-Tonelli (1959), referred to by de Pietri-Tonelli
et al (1965), used a chloroform extraction with isolation by paper
chromatography and determination of phosphorous with a molybdic
reagent and 2, 6-dibromo-N-chloro-p-quinoneimine and subsequent
spraying with propylene glycol.
Van Middlem and Waites (1964) compared a dinitrochlorobenzene
colorimetric procedure with an electron capture gas chromatography
procedure for dimethoate. Recoveries were 71 per cent for the
colorimetric procedure and about 85 per cent for the gas
chromatography procedure. Correcting for recoveries the results of the
two procedures were in agreement. In both procedures methylene
chloride was used to extract the pesticide. The organic phase was
further cleaned with activated carbon and the aqueous phase was passed
through a polyethylene-coated alumina column prior to colorimetric
determination. For the gas chromatographic analysis benzene was added
to the extract before evaporation of the methylene chloride. These
investigators reported that the oxygen analog is infinitely more
soluble in water than in organic solvents. Unlike dimethoate which has
a partition coefficient of 20 for chloroform and water, the oxygen
analog coefficient is only 0.7.
Watts and Storherr (1967) found a much higher ratio of oxygen analog
in residues using an ethyl acetate extraction with a nuchar carbon
cleanup and a gas chromatography thermionic detection.
Since the oxygen analog in so highly water-soluble and favors the
aqueous phase in many partitioning systems, care must be exercised in
choosing any method of analysis to ensure that the more polar oxygen
analog is not lost. It is difficult to evaluate fully the quantitative
data published on metabolism without knowledge of the exact conditions
of the experiments. It is hoped that improved methodology will assist
in clarifying some apparent anomalies.
NATIONAL TOLERANCES
Country Tolerances, ppm Product
United States of America 2 apples, pears
2 18 vegetables
Canada 2 apples, pears
Benelux 0.5 fruits and
vegetables
Federal Republic of
Germany 0.5 fruits and
vegetables
Australia 2.0
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances until 3 December 1970
When dimethoate is utilized in accordance with good agricultural
practice to protect food products, when necessary against insect
infestation, the treated product may have residues as high as those
shown below (total of dimethoate and oxygen analog):
Tree fruits (including citrus) 2 ppm
Vegetables (excluding tomatoes and 2 ppm
peppers)
Tomatoes and peppers 1 ppm
By no means will all samples of fruits and vegetables contain this
amount of residue; in fact, only a small, yet unknown portion of each
product in these categories is likely to be treated. There are some
data available on the disappearance of this compound during storage of
treated wheat. The nature of this disappearance simulates that which
occurs in living plants. The compound is systemic; it can be assumed
that residues will not be reduced materially during washing and
peeling.
Since the compound is not recommended, generally, for use on a large
variety of food products, it is recommended that the above levels be
adopted on a temporary basis until further data are made available on
losses during cooking and data are acquired from total diet studies.
The proposed temporary tolerance is for a combination of dimethoate
and its oxygen analog.
FURTHER WORK
Further work required before 30 June 1970
1. Studies on the fate of the compound during processing and
preparation for consumption.
2. Amount of residue appearing in total diet studies.
Further work desirable
Worldwide use data.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Casida, J.E. and Sanderson, D.M. (1962) Nature, 189, 507.
Casida, J.E. and Sanderson, D.M. (1963) J. Agr. Food Chem., 2, 91.
Ecobichon, D.J. and Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E.F. and Noakes, D.N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E.F., Jones, K.H. and Watson, W.A. (1967) Brit. med. J., ii,
554
Fogleman, R.W., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished
reports submitted by American Cyanamid Company.
Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished reports submitted
by American Cyanamid Company.
O'Brien, R.D. (1959) Nature, 183, 121
O'Brien, R.D. (1961) Biochem. J., 79, 229
Ribelin, W.B., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublisbed
reports submitted by American Cyanamid Company
Sanderson, D.M. and Edson, E.F. (1964) Brit. J. industr. Med., 21,
52
Sherman, M., Ross, E., Sanchet, F.F. and Chang, M.T.Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone, L.B. and Shaffer, C.B. (1961) Toxicol. appl.
Pharmacol., 3, 210
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
American Cyanamid Co. (1962-7) Pesticide petitions submitted to the
U.S. Food and Drug Administration.
Bohn, W.R. (1964) The disappearance of dimethoate from soil. J. Econ.
Ent. 57: 798-9.
Chamberlain, W.R., Gatterdam, P.E., and Hopkins, D.E. (1961) The
metabolism of p32-labelled dimethoate in sheep. J. Econ. Ent. 54 (4)
: 733-40.
Chillwell, E.D. and Beecham, P.T. (1960) Residues of 0,0-dimethyl
S-(N-methyl-carbamoyl-methyl) Phosphorothiolothionate (dimethoate) in
sprayed crops. J. Sci. Fd. Agric. 11 (7) : 400-7.
Dauterman, W.C., Casida, J.E., Knaak, J.B. and Kowalczyk, T. J. (1959)
Agr. Food Chem. 7 (3) : 188-93.
Dauterman, W.C., Biado, G.B., Casida, J.E. and O'Brien, R.D. J. Agr.
Food Chem. 1960 8 (2) a 115-19.
de Pietri-Tonelli, F. and Barontini, A. (1959) Analisi per via
biologica dei residue di Rogor in campioni di carciofi ed indivia.
Internal report of Montecatini.
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Reviews. Vol 11. New
York : Springer-Verlag.
George, D.A., Walker, K.C., Giang, P.A. and Murphy, R.T. (1962)
Colorimetric method for the determination of dimethoate residues.
Presented 142nd Meeting Amer. Chem. Soc. 9 -14 September 1962.
Hacskaylo, J. and Bull D.L. (1963) Metabolism of dimethoate in cotton
leaves. J. Agr. Food Chem. 11 (6) : 464-6.
Kaplanis, J.N., Robbins, W.E., Darrow, D.E. et al. (1959) The
metabolism of dimethoate in cattle. J. Econ. Entomol. 52 (6) :
1190-4.
Lucier, G.W. (1967) Thesis entitled : Metabolism of dimethoate in bean
plants in relation to its mode of application. Dept. of Entomol.
University of Maryland, College Park, Md.
Parker, B.L. and Dewey J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based on toxicity to Drosophila
melanogaster. J. Econ. Ent. 58: 106-11.
Roberts, R.H., Radeleff, R.D. and Kaplanis, J.N. (1958) Bioassay of
the blood from cattle treated with Am. Cyanamid 12,880. J. Econ.
Entomol. 51 (6): 861-4.
Rowlands, D.G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Fd. Agric.
17: 90-3.
Sanderson, D.M. and Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Brit. J. Ind. Med. 21, 52.
Santi, R. (1961) Ricerche sulla penetrazione e sul destino metabolico
del Rogor-P32 nelle ciliege e nelle pesche. Internal report of
Montecatini.
Santi, R. and de Pietri-Tonelli, P. (1959a) Research on the mechanism
of action of N-monomethyl-0,0-dimethyl dithiophosphorylacetamide. Is.
Ric. Agr. Soc. Montecatini, Milano, Italy. 2 : 3-28.
Santi, R. and de Pietri-Tonelli, P. (1959b) Mode of action and
biological properties of the S-(Methylcarbanyl) methyl
0,0-dimethyldithiophosphate. Nature 183 : 398-9.
Santi, R. and Giacomelli, R. (1962) Metabolic fate of P32-labeled
dimethoate in olive fruits and some toxicological implications.
J. Agr. Food Chem. 10 (3) : 257-61.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analog by total phosphorus determination
after isolation by thin-layer chromatography. J.A.0.A.C. 47 (4) :
645-51.
Uchida, T., Dauterman, W.C. and O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agr. Food Chem. 12 (1) : 48-52.
Van Middelem, C.H. and Waites, R.E. (1964) Gas chromatographic and
colorimetric measurement of dimethoate residues. J. Agr. Food Chem.
12 (2) : 178-182.
Waldron, A.C. (1962) Colorimetric method for the determination of
dimethoate residues in plant tissues. Unpublished but in dimethoate
petition submitted to U.S. Food and Drug Administration by American
Cyanamid Co.
Watts, R. and Storherr, R. (1967) Personal communication. U.S. Food
and Drug. Administration.
Dauterman et al. (1960) studied the metabolism of dimethoate-32P on
the surface and inside cotton, corn, pea and potato leaves following
foliar application. Substantial quantities of oxygen analog were found
in all samples. Although the quantity decreased with time, there was a
proportionate increase in the oxygen analog relative to dimethoate.
Internally this is attributed to enzymatic oxidation; however, since
this same oxidation results on non-biological surfaces exposed to the
atmosphere, non-enzymatic oxidation may occur on the leaf surface.
Based on the quantities of water-soluble metabolites on the surface
and inside leaves, Dauterman postulates that different mechanisms of
degradation occur. There also appears to be a marked difference in
crop species. Plant-surface water-soluble metabolites were primarily
the oxycarboxy derivative; whereas the desmethyl derivatives were the
major internal metabolites. Pea plants did not follow the pattern of
cotton, corn and potato plants, but had about 50 per cent residue of
phosphoric acid both on the surface and inside leaves. No
dithiophosphoric acids or thiocarboxy derivative were found in any
samples. Apparently oxidation occurs more rapidly than hydrolysis.
Hacskaylo and Bull (1963) found that metabolism of dimethoate in
excised cotton leaves resembles the pathway proposed for mammals and
is different from that occurring from foliar treatments. They found
the level of oxygen analog fairly constant and always less than 6 per
cent of the total metabolites found.
Recently Lucier (1967) studied the metabolism of 32P and 14C-carbonyl
labeled dimethoate in bean plants using four modes of application:
foliar treatment, stem injection, root absorption and excised leaves.
Conversion of dimethoate to the oxygen analog was the major route of
degradation and was directly correlated with the ease of translocation
of dimethoate to the leaf tissue. Thus the amount of oxygen analog was
greatest for the excised leaves and less for the foliar application.
In root absorption studies, the oxygen analog content was 4-1/2 times
the amount of dimethoate and represented 10 per cent of the
administered dose 10 days after application. The major hydrolysis
products were the thiocarboxy derivative, dimethyl phosphorothioate
and dimethyl phosphorodithioate. Only trace quantities of the
thiodesmethyl carboxy compound were found.
A new compound, des-N-methyl dimethoate was detected in trace
quantities in all samples. Foliar application gave rise to large
quantities of two unknown metabolites which were postulated to be the
N-hydroxy methyl derivatives of dimethoate and its oxygen analog.
These unknowns are rapidly degraded and not detectable after four
days.
Chillwell and Beecham (1960) found that climatic conditions had no
substantial effect on residue level of dimethoate if similar
applications were made on crops at the same growth stage. Recent
preliminary investigations by Watts and Storherr (1967) show that
residues on field-sprayed kale may contain five times as much oxygen
analog as the parent compound 14 days after treatment.
The oxygen analog seems to be the only metabolite of toxicological
importance. Therefore, its residue level in food products must be
considered. Data submitted to the U.S. Food and Drug Administration
and data reported in the literature by Santi* (1961), Santi and
Giacomelli (1962) and Dauterman et al. (1960) indicate that the amount
of oxygen analog is generally not above 10-20 per cent of the total
toxic residue. Results in olives are an exception where the oxygen
analog was found as high as 67 per cent of the total residue after 30
days.
In animals
Early studies by Roberts at al (1958) and Kaplanis et al (1959)
revealed that dimethoate was absorbed rapidly into the blood of cattle
and was converted to a metabolite several times more toxic than the
parent compound as determined by enzymatic analysis and bioassay. This
compound and many of the major urinary metabolites were not
identified. Santi and de Pietri-Tonelli (1959a) showed that dimethoate
converted chemically and in vitro by incubation in rat liver slices
to the oxygen analog. An unusually high rate of degradation of
dimethoate in sheep liver to the thiocarboxy derivative was found by
Uchida et al, (1964). This was attributed to amidase activity.
Sanderson and Edson (1964) found that dimethoate behaved as a typical
indirect anticholinesterase agent by conversion in the liver to at
least four short lived metabolites whose hydrolysis products are
rapidly excreted mainly in the urine.
Oral administration of dimethoate-32P at dosages of 100 mg/kg to rats
and 10 to 40 mg/kg to lactating cows led Dauterman and co-workers
(1959) to conclude that the metabolic pathway was similar for both
species, and that dimethoate was attacked hydrolytically at five sites
on the molecule. Only small quantities of labelled material were found
in the feces. Dimethoate was rapidly absorbed into the blood and
rapidly excreted in the urine with 75 per cent of the administered
dose eliminated within 24 hours primarily as the thiocarboxy
derivative. As the quantity of the thiocarboxy derivative and
dimethylthiophosphoric acid decreased, the level of dimethylphosphoric
acid increased and appeared relatively stable since conversion to
simpler phosphoric acids was very slow. When the desmethyl derivative
was fed to rats, recovery of better the 85 per cent of unchanged
chemical in the urine occurred suggesting that this is not the major
metabolic route in animals.
Analysis of blood samples indicated that a maximum of chloroform
solubles was reached at two hours with 1.5 ppm dimethoate equivalents
present in cows which were, treated with 10 g/kg. The level of
cholinesterase was severely depressed for the animal treated at
40 mg/kg but no depression occurred at the lower feeding level.
* As quoted by de Pietri-Tonelli et al (1965)
A composite sample representing total milk secreted over a 288 hour
period showed that 0.0068 per cent of the administered dose was
eliminated in this manner. The amount of chloroform solubles decreased
rapidly with less than 0.02 ppm dimethoate equivalents present after
48 hours from the oral dose of 10 mg/kg and 0.04 ppm from the 40 mg/kg
dose.
Chloroform solubles in the fat were less than 0.1 ppm after eight
hours. The only significant quantity of residue in bovine tissues was
found in the liver with a maximum of 0.23 ppm of chloroform solubles
at the lower feeding level.
Chamberlain et al. (1961) investigated the metabolism of dimethoate
by intramuscular administration and oral feeding of sheep at 10 and 40
mg/kg. He too found that only a small per cent of the labeled dose was
excreted in the faeces and that over 80 per cent of the radioactive
material was eliminated in the urine by 72 hours with the thiocarboxy
derivative being the major component. As the thiocarboxy content
decreased there was an increase in dimethyl phosphoric acid and an
unknown which he tentatively identified as the desmethyl carboxy
derivative and suggested that it formed from the thiocarboxy compound.
Trace quantities of dimethoate and its oxygen analog were present in
all sheep urine samples.
Analysis of the blood samples showed that the amount of
organo-solubles decreased very rapidly with less than 0.1 per cent
remaining after 12 hours. Ninety per cent of the organosoluble residue
vas dimethoate or its oxygen analog. The proportion of oxygen analog
to dimethoate increased as the quantity of dimethoate decreased.
In storage and processing
No commercial use of dimethoate for storage products is known. Rowland
(1966) studied the metabolism in stored wheat and sorghum grains
fortified at 2 and 10 parts per million. Trace amounts of dimethoate
and its oxygen analog were found after 4 and 7 days' storage. No
active anti-cholinesterase components were found at subsequent
samplings. No hydrolytic products of the oxygen analog were found, but
significant quantities of dimethoate hydrolysis products were detected
by paper chromatography. These included the thiocarboxy derivative,
the thiodesmethyl and thiodesmethyl carboxy derivative, and the
dimethyl esters of thio and dithiophosphoric acid.
METHODS OF RESIDUE ANALYSIS
The major metabolic pathway in plants indicates that the oxygen analog
of dimethoate is a significant metabolite. A minor pathway in animals
yields the same compound. Any adequate method for residue analysis
must take this compound into account.
Most of the data presented in Table II was obtained from one of two
methods of analysis.
The first method of Waldron (1962) (adapted for apples from a method
described by George (1962) for dimethoate in alfalfa) determines only
the parent compound, dimethoate. The pesticide is extracted with
methylene chloride, followed by alkaline hydrolysis, and colorimetric
determination of the resultant methylamine by reaction with
1-chloro-2,4-dinitrobenzene. Numerous tests were made to show that the
method in specific for dimethoate and that some 28 commonly used
pesticides do not interfere.
Steller and Curry developed a method incorporating a thin layer
chromatographic isolation of dimethoate and its oxygen analog and a
total phosphorous determination of the separate fractions measuring a
molybdenum blue complex. This method is specific for dimethoate and
its oxygen analog. Although recoveries were lower than for the Waldron
method, they were consistent and the authors claim a sensitivity of
0.06 ppm.
The data in Table II were obtained by a variety of methods. Chillwell
and Beecham (1960) macerated plant tissues with water acidified with
acetic acid and extracted the aqueous phase with chloroform.
Microdistillation was used to purify the extracted insecticide and
phosphorous was determined colorimetrically after an acid digestion.
Santi and de Pietri-Tonelli (1959), referred to by de Pietri-Tonelli
et al (1965), used a chloroform extraction with isolation by paper
chromatography and determination of phosphorous with a molybdic
reagent and 2, 6-dibromo-N-chloro-p-quinoneimine and subsequent
spraying with propylene glycol.
Van Middlem and Waites (1964) compared a dinitrochlorobenzene
colorimetric procedure with an electron capture gas chromatography
procedure for dimethoate. Recoveries were 71 per cent for the
colorimetric procedure and about 85 per cent for the gas
chromatography procedure. Correcting for recoveries the results of the
two procedures were in agreement. In both procedures methylene
chloride was used to extract the pesticide. The organic phase was
further cleaned with activated carbon and the aqueous phase was passed
through a polyethylene-coated alumina column prior to colorimetric
determination. For the gas chromatographic analysis benzene was added
to the extract before evaporation of the methylene chloride. These
investigators reported that the oxygen analog is infinitely more
soluble in water than in organic solvents. Unlike dimethoate which has
a partition coefficient of 20 for chloroform and water, the oxygen
analog coefficient is only 0.7.
Watts and Storherr (1967) found a much higher ratio of oxygen analog
in residues using an ethyl acetate extraction with a nuchar carbon
cleanup and a gas chromatography thermionic detection.
Since the oxygen analog in so highly water-soluble and favors the
aqueous phase in many partitioning systems, care must be exercised in
choosing any method of analysis to ensure that the more polar oxygen
analog is not lost. It is difficult to evaluate fully the quantitative
data published on metabolism without knowledge of the exact conditions
of the experiments. It is hoped that improved methodology will assist
in clarifying some apparent anomalies.
NATIONAL TOLERANCES
Country Tolerances, ppm Product
United States of America 2 apples, pears
2 18 vegetables
Canada 2 apples, pears
Benelux 0.5 fruits and
vegetables
Federal Republic of
Germany 0.5 fruits and
vegetables
Australia 2.0
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances until 3 December 1970
When dimethoate is utilized in accordance with good agricultural
practice to protect food products, when necessary against insect
infestation, the treated product may have residues as high as those
shown below (total of dimethoate and oxygen analog):
Tree fruits (including citrus) 2 ppm
Vegetables (excluding tomatoes and 2 ppm
peppers)
Tomatoes and peppers 1 ppm
By no means will all samples of fruits and vegetables contain this
amount of residue; in fact, only a small, yet unknown portion of each
product in these categories is likely to be treated. There are some
data available on the disappearance of this compound during storage of
treated wheat. The nature of this disappearance simulates that which
occurs in living plants. The compound is systemic; it can be assumed
that residues will not be reduced materially during washing and
peeling.
Since the compound is not recommended, generally, for use on a large
variety of food products, it is recommended that the above levels be
adopted on a temporary basis until further data are made available on
losses during cooking and data are acquired from total diet studies.
The proposed temporary tolerance is for a combination of dimethoate
and its oxygen analog.
FURTHER WORK
Further work required before 30 June 1970
1. Studies on the fate of the compound during processing and
preparation for consumption.
2. Amount of residue appearing in total diet studies.
Further work desirable
Worldwide use data.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Casida, J.E. and Sanderson, D.M. (1962) Nature, 189, 507.
Casida, J.E. and Sanderson, D.M. (1963) J. Agr. Food Chem., 2, 91.
Ecobichon, D.J. and Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E.F. and Noakes, D.N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E.F., Jones, K.H. and Watson, W.A. (1967) Brit. med. J., ii,
554
Fogleman, R.W., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished
reports submitted by American Cyanamid Company.
Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished reports submitted
by American Cyanamid Company.
O'Brien, R.D. (1959) Nature, 183, 121
O'Brien, R.D. (1961) Biochem. J., 79, 229
Ribelin, W.B., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublisbed
reports submitted by American Cyanamid Company
Sanderson, D.M. and Edson, E.F. (1964) Brit. J. industr. Med., 21,
52
Sherman, M., Ross, E., Sanchet, F.F. and Chang, M.T.Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone, L.B. and Shaffer, C.B. (1961) Toxicol. appl.
Pharmacol., 3, 210
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
American Cyanamid Co. (1962-7) Pesticide petitions submitted to the
U.S. Food and Drug Administration.
Bohn, W.R. (1964) The disappearance of dimethoate from soil. J. Econ.
Ent. 57: 798-9.
Chamberlain, W.R., Gatterdam, P.E., and Hopkins, D.E. (1961) The
metabolism of p32-labelled dimethoate in sheep. J. Econ. Ent. 54 (4)
: 733-40.
Chillwell, E.D. and Beecham, P.T. (1960) Residues of 0,0-dimethyl
S-(N-methyl-carbamoyl-methyl) Phosphorothiolothionate (dimethoate) in
sprayed crops. J. Sci. Fd. Agric. 11 (7) : 400-7.
Dauterman, W.C., Casida, J.E., Knaak, J.B. and Kowalczyk, T. J. (1959)
Agr. Food Chem. 7 (3) : 188-93.
Dauterman, W.C., Biado, G.B., Casida, J.E. and O'Brien, R.D. J. Agr.
Food Chem. 1960 8 (2) a 115-19.
de Pietri-Tonelli, F. and Barontini, A. (1959) Analisi per via
biologica dei residue di Rogor in campioni di carciofi ed indivia.
Internal report of Montecatini.
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Reviews. Vol 11. New
York : Springer-Verlag.
George, D.A., Walker, K.C., Giang, P.A. and Murphy, R.T. (1962)
Colorimetric method for the determination of dimethoate residues.
Presented 142nd Meeting Amer. Chem. Soc. 9 -14 September 1962.
Hacskaylo, J. and Bull D.L. (1963) Metabolism of dimethoate in cotton
leaves. J. Agr. Food Chem. 11 (6) : 464-6.
Kaplanis, J.N., Robbins, W.E., Darrow, D.E. et al. (1959) The
metabolism of dimethoate in cattle. J. Econ. Entomol. 52 (6) :
1190-4.
Lucier, G.W. (1967) Thesis entitled : Metabolism of dimethoate in bean
plants in relation to its mode of application. Dept. of Entomol.
University of Maryland, College Park, Md.
Parker, B.L. and Dewey J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based on toxicity to Drosophila
melanogaster. J. Econ. Ent. 58: 106-11.
Roberts, R.H., Radeleff, R.D. and Kaplanis, J.N. (1958) Bioassay of
the blood from cattle treated with Am. Cyanamid 12,880. J. Econ.
Entomol. 51 (6): 861-4.
Rowlands, D.G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Fd. Agric.
17: 90-3.
Sanderson, D.M. and Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Brit. J. Ind. Med. 21, 52.
Santi, R. (1961) Ricerche sulla penetrazione e sul destino metabolico
del Rogor-P32 nelle ciliege e nelle pesche. Internal report of
Montecatini.
Santi, R. and de Pietri-Tonelli, P. (1959a) Research on the mechanism
of action of N-monomethyl-0,0-dimethyl dithiophosphorylacetamide. Is.
Ric. Agr. Soc. Montecatini, Milano, Italy. 2 : 3-28.
Santi, R. and de Pietri-Tonelli, P. (1959b) Mode of action and
biological properties of the S-(Methylcarbanyl) methyl
0,0-dimethyldithiophosphate. Nature 183 : 398-9.
Santi, R. and Giacomelli, R. (1962) Metabolic fate of P32-labeled
dimethoate in olive fruits and some toxicological implications.
J. Agr. Food Chem. 10 (3) : 257-61.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analog by total phosphorus determination
after isolation by thin-layer chromatography. J.A.0.A.C. 47 (4) :
645-51.
Uchida, T., Dauterman, W.C. and O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agr. Food Chem. 12 (1) : 48-52.
Van Middelem, C.H. and Waites, R.E. (1964) Gas chromatographic and
colorimetric measurement of dimethoate residues. J. Agr. Food Chem.
12 (2) : 178-182.
Waldron, A.C. (1962) Colorimetric method for the determination of
dimethoate residues in plant tissues. Unpublished but in dimethoate
petition submitted to U.S. Food and Drug Administration by American
Cyanamid Co.
Watts, R. and Storherr, R. (1967) Personal communication. U.S. Food
and Drug. Administration.
 Dauterman et al. (1960) studied the metabolism of dimethoate-32P on
the surface and inside cotton, corn, pea and potato leaves following
foliar application. Substantial quantities of oxygen analog were found
in all samples. Although the quantity decreased with time, there was a
proportionate increase in the oxygen analog relative to dimethoate.
Internally this is attributed to enzymatic oxidation; however, since
this same oxidation results on non-biological surfaces exposed to the
atmosphere, non-enzymatic oxidation may occur on the leaf surface.
Based on the quantities of water-soluble metabolites on the surface
and inside leaves, Dauterman postulates that different mechanisms of
degradation occur. There also appears to be a marked difference in
crop species. Plant-surface water-soluble metabolites were primarily
the oxycarboxy derivative; whereas the desmethyl derivatives were the
major internal metabolites. Pea plants did not follow the pattern of
cotton, corn and potato plants, but had about 50 per cent residue of
phosphoric acid both on the surface and inside leaves. No
dithiophosphoric acids or thiocarboxy derivative were found in any
samples. Apparently oxidation occurs more rapidly than hydrolysis.
Hacskaylo and Bull (1963) found that metabolism of dimethoate in
excised cotton leaves resembles the pathway proposed for mammals and
is different from that occurring from foliar treatments. They found
the level of oxygen analog fairly constant and always less than 6 per
cent of the total metabolites found.
Recently Lucier (1967) studied the metabolism of 32P and 14C-carbonyl
labeled dimethoate in bean plants using four modes of application:
foliar treatment, stem injection, root absorption and excised leaves.
Conversion of dimethoate to the oxygen analog was the major route of
degradation and was directly correlated with the ease of translocation
of dimethoate to the leaf tissue. Thus the amount of oxygen analog was
greatest for the excised leaves and less for the foliar application.
In root absorption studies, the oxygen analog content was 4-1/2 times
the amount of dimethoate and represented 10 per cent of the
administered dose 10 days after application. The major hydrolysis
products were the thiocarboxy derivative, dimethyl phosphorothioate
and dimethyl phosphorodithioate. Only trace quantities of the
thiodesmethyl carboxy compound were found.
A new compound, des-N-methyl dimethoate was detected in trace
quantities in all samples. Foliar application gave rise to large
quantities of two unknown metabolites which were postulated to be the
N-hydroxy methyl derivatives of dimethoate and its oxygen analog.
These unknowns are rapidly degraded and not detectable after four
days.
Chillwell and Beecham (1960) found that climatic conditions had no
substantial effect on residue level of dimethoate if similar
applications were made on crops at the same growth stage. Recent
preliminary investigations by Watts and Storherr (1967) show that
residues on field-sprayed kale may contain five times as much oxygen
analog as the parent compound 14 days after treatment.
The oxygen analog seems to be the only metabolite of toxicological
importance. Therefore, its residue level in food products must be
considered. Data submitted to the U.S. Food and Drug Administration
and data reported in the literature by Santi* (1961), Santi and
Giacomelli (1962) and Dauterman et al. (1960) indicate that the amount
of oxygen analog is generally not above 10-20 per cent of the total
toxic residue. Results in olives are an exception where the oxygen
analog was found as high as 67 per cent of the total residue after 30
days.
In animals
Early studies by Roberts at al (1958) and Kaplanis et al (1959)
revealed that dimethoate was absorbed rapidly into the blood of cattle
and was converted to a metabolite several times more toxic than the
parent compound as determined by enzymatic analysis and bioassay. This
compound and many of the major urinary metabolites were not
identified. Santi and de Pietri-Tonelli (1959a) showed that dimethoate
converted chemically and in vitro by incubation in rat liver slices
to the oxygen analog. An unusually high rate of degradation of
dimethoate in sheep liver to the thiocarboxy derivative was found by
Uchida et al, (1964). This was attributed to amidase activity.
Sanderson and Edson (1964) found that dimethoate behaved as a typical
indirect anticholinesterase agent by conversion in the liver to at
least four short lived metabolites whose hydrolysis products are
rapidly excreted mainly in the urine.
Oral administration of dimethoate-32P at dosages of 100 mg/kg to rats
and 10 to 40 mg/kg to lactating cows led Dauterman and co-workers
(1959) to conclude that the metabolic pathway was similar for both
species, and that dimethoate was attacked hydrolytically at five sites
on the molecule. Only small quantities of labelled material were found
in the feces. Dimethoate was rapidly absorbed into the blood and
rapidly excreted in the urine with 75 per cent of the administered
dose eliminated within 24 hours primarily as the thiocarboxy
derivative. As the quantity of the thiocarboxy derivative and
dimethylthiophosphoric acid decreased, the level of dimethylphosphoric
acid increased and appeared relatively stable since conversion to
simpler phosphoric acids was very slow. When the desmethyl derivative
was fed to rats, recovery of better the 85 per cent of unchanged
chemical in the urine occurred suggesting that this is not the major
metabolic route in animals.
Analysis of blood samples indicated that a maximum of chloroform
solubles was reached at two hours with 1.5 ppm dimethoate equivalents
present in cows which were, treated with 10 g/kg. The level of
cholinesterase was severely depressed for the animal treated at
40 mg/kg but no depression occurred at the lower feeding level.
* As quoted by de Pietri-Tonelli et al (1965)
A composite sample representing total milk secreted over a 288 hour
period showed that 0.0068 per cent of the administered dose was
eliminated in this manner. The amount of chloroform solubles decreased
rapidly with less than 0.02 ppm dimethoate equivalents present after
48 hours from the oral dose of 10 mg/kg and 0.04 ppm from the 40 mg/kg
dose.
Chloroform solubles in the fat were less than 0.1 ppm after eight
hours. The only significant quantity of residue in bovine tissues was
found in the liver with a maximum of 0.23 ppm of chloroform solubles
at the lower feeding level.
Chamberlain et al. (1961) investigated the metabolism of dimethoate
by intramuscular administration and oral feeding of sheep at 10 and 40
mg/kg. He too found that only a small per cent of the labeled dose was
excreted in the faeces and that over 80 per cent of the radioactive
material was eliminated in the urine by 72 hours with the thiocarboxy
derivative being the major component. As the thiocarboxy content
decreased there was an increase in dimethyl phosphoric acid and an
unknown which he tentatively identified as the desmethyl carboxy
derivative and suggested that it formed from the thiocarboxy compound.
Trace quantities of dimethoate and its oxygen analog were present in
all sheep urine samples.
Analysis of the blood samples showed that the amount of
organo-solubles decreased very rapidly with less than 0.1 per cent
remaining after 12 hours. Ninety per cent of the organosoluble residue
vas dimethoate or its oxygen analog. The proportion of oxygen analog
to dimethoate increased as the quantity of dimethoate decreased.
In storage and processing
No commercial use of dimethoate for storage products is known. Rowland
(1966) studied the metabolism in stored wheat and sorghum grains
fortified at 2 and 10 parts per million. Trace amounts of dimethoate
and its oxygen analog were found after 4 and 7 days' storage. No
active anti-cholinesterase components were found at subsequent
samplings. No hydrolytic products of the oxygen analog were found, but
significant quantities of dimethoate hydrolysis products were detected
by paper chromatography. These included the thiocarboxy derivative,
the thiodesmethyl and thiodesmethyl carboxy derivative, and the
dimethyl esters of thio and dithiophosphoric acid.
METHODS OF RESIDUE ANALYSIS
The major metabolic pathway in plants indicates that the oxygen analog
of dimethoate is a significant metabolite. A minor pathway in animals
yields the same compound. Any adequate method for residue analysis
must take this compound into account.
Most of the data presented in Table II was obtained from one of two
methods of analysis.
The first method of Waldron (1962) (adapted for apples from a method
described by George (1962) for dimethoate in alfalfa) determines only
the parent compound, dimethoate. The pesticide is extracted with
methylene chloride, followed by alkaline hydrolysis, and colorimetric
determination of the resultant methylamine by reaction with
1-chloro-2,4-dinitrobenzene. Numerous tests were made to show that the
method in specific for dimethoate and that some 28 commonly used
pesticides do not interfere.
Steller and Curry developed a method incorporating a thin layer
chromatographic isolation of dimethoate and its oxygen analog and a
total phosphorous determination of the separate fractions measuring a
molybdenum blue complex. This method is specific for dimethoate and
its oxygen analog. Although recoveries were lower than for the Waldron
method, they were consistent and the authors claim a sensitivity of
0.06 ppm.
The data in Table II were obtained by a variety of methods. Chillwell
and Beecham (1960) macerated plant tissues with water acidified with
acetic acid and extracted the aqueous phase with chloroform.
Microdistillation was used to purify the extracted insecticide and
phosphorous was determined colorimetrically after an acid digestion.
Santi and de Pietri-Tonelli (1959), referred to by de Pietri-Tonelli
et al (1965), used a chloroform extraction with isolation by paper
chromatography and determination of phosphorous with a molybdic
reagent and 2, 6-dibromo-N-chloro-p-quinoneimine and subsequent
spraying with propylene glycol.
Van Middlem and Waites (1964) compared a dinitrochlorobenzene
colorimetric procedure with an electron capture gas chromatography
procedure for dimethoate. Recoveries were 71 per cent for the
colorimetric procedure and about 85 per cent for the gas
chromatography procedure. Correcting for recoveries the results of the
two procedures were in agreement. In both procedures methylene
chloride was used to extract the pesticide. The organic phase was
further cleaned with activated carbon and the aqueous phase was passed
through a polyethylene-coated alumina column prior to colorimetric
determination. For the gas chromatographic analysis benzene was added
to the extract before evaporation of the methylene chloride. These
investigators reported that the oxygen analog is infinitely more
soluble in water than in organic solvents. Unlike dimethoate which has
a partition coefficient of 20 for chloroform and water, the oxygen
analog coefficient is only 0.7.
Watts and Storherr (1967) found a much higher ratio of oxygen analog
in residues using an ethyl acetate extraction with a nuchar carbon
cleanup and a gas chromatography thermionic detection.
Since the oxygen analog in so highly water-soluble and favors the
aqueous phase in many partitioning systems, care must be exercised in
choosing any method of analysis to ensure that the more polar oxygen
analog is not lost. It is difficult to evaluate fully the quantitative
data published on metabolism without knowledge of the exact conditions
of the experiments. It is hoped that improved methodology will assist
in clarifying some apparent anomalies.
NATIONAL TOLERANCES
Country Tolerances, ppm Product
United States of America 2 apples, pears
2 18 vegetables
Canada 2 apples, pears
Benelux 0.5 fruits and
vegetables
Federal Republic of
Germany 0.5 fruits and
vegetables
Australia 2.0
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances until 3 December 1970
When dimethoate is utilized in accordance with good agricultural
practice to protect food products, when necessary against insect
infestation, the treated product may have residues as high as those
shown below (total of dimethoate and oxygen analog):
Tree fruits (including citrus) 2 ppm
Vegetables (excluding tomatoes and 2 ppm
peppers)
Tomatoes and peppers 1 ppm
By no means will all samples of fruits and vegetables contain this
amount of residue; in fact, only a small, yet unknown portion of each
product in these categories is likely to be treated. There are some
data available on the disappearance of this compound during storage of
treated wheat. The nature of this disappearance simulates that which
occurs in living plants. The compound is systemic; it can be assumed
that residues will not be reduced materially during washing and
peeling.
Since the compound is not recommended, generally, for use on a large
variety of food products, it is recommended that the above levels be
adopted on a temporary basis until further data are made available on
losses during cooking and data are acquired from total diet studies.
The proposed temporary tolerance is for a combination of dimethoate
and its oxygen analog.
FURTHER WORK
Further work required before 30 June 1970
1. Studies on the fate of the compound during processing and
preparation for consumption.
2. Amount of residue appearing in total diet studies.
Further work desirable
Worldwide use data.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Casida, J.E. and Sanderson, D.M. (1962) Nature, 189, 507.
Casida, J.E. and Sanderson, D.M. (1963) J. Agr. Food Chem., 2, 91.
Ecobichon, D.J. and Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E.F. and Noakes, D.N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E.F., Jones, K.H. and Watson, W.A. (1967) Brit. med. J., ii,
554
Fogleman, R.W., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished
reports submitted by American Cyanamid Company.
Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished reports submitted
by American Cyanamid Company.
O'Brien, R.D. (1959) Nature, 183, 121
O'Brien, R.D. (1961) Biochem. J., 79, 229
Ribelin, W.B., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublisbed
reports submitted by American Cyanamid Company
Sanderson, D.M. and Edson, E.F. (1964) Brit. J. industr. Med., 21,
52
Sherman, M., Ross, E., Sanchet, F.F. and Chang, M.T.Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone, L.B. and Shaffer, C.B. (1961) Toxicol. appl.
Pharmacol., 3, 210
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
American Cyanamid Co. (1962-7) Pesticide petitions submitted to the
U.S. Food and Drug Administration.
Bohn, W.R. (1964) The disappearance of dimethoate from soil. J. Econ.
Ent. 57: 798-9.
Chamberlain, W.R., Gatterdam, P.E., and Hopkins, D.E. (1961) The
metabolism of p32-labelled dimethoate in sheep. J. Econ. Ent. 54 (4)
: 733-40.
Chillwell, E.D. and Beecham, P.T. (1960) Residues of 0,0-dimethyl
S-(N-methyl-carbamoyl-methyl) Phosphorothiolothionate (dimethoate) in
sprayed crops. J. Sci. Fd. Agric. 11 (7) : 400-7.
Dauterman, W.C., Casida, J.E., Knaak, J.B. and Kowalczyk, T. J. (1959)
Agr. Food Chem. 7 (3) : 188-93.
Dauterman, W.C., Biado, G.B., Casida, J.E. and O'Brien, R.D. J. Agr.
Food Chem. 1960 8 (2) a 115-19.
de Pietri-Tonelli, F. and Barontini, A. (1959) Analisi per via
biologica dei residue di Rogor in campioni di carciofi ed indivia.
Internal report of Montecatini.
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Reviews. Vol 11. New
York : Springer-Verlag.
George, D.A., Walker, K.C., Giang, P.A. and Murphy, R.T. (1962)
Colorimetric method for the determination of dimethoate residues.
Presented 142nd Meeting Amer. Chem. Soc. 9 -14 September 1962.
Hacskaylo, J. and Bull D.L. (1963) Metabolism of dimethoate in cotton
leaves. J. Agr. Food Chem. 11 (6) : 464-6.
Kaplanis, J.N., Robbins, W.E., Darrow, D.E. et al. (1959) The
metabolism of dimethoate in cattle. J. Econ. Entomol. 52 (6) :
1190-4.
Lucier, G.W. (1967) Thesis entitled : Metabolism of dimethoate in bean
plants in relation to its mode of application. Dept. of Entomol.
University of Maryland, College Park, Md.
Parker, B.L. and Dewey J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based on toxicity to Drosophila
melanogaster. J. Econ. Ent. 58: 106-11.
Roberts, R.H., Radeleff, R.D. and Kaplanis, J.N. (1958) Bioassay of
the blood from cattle treated with Am. Cyanamid 12,880. J. Econ.
Entomol. 51 (6): 861-4.
Rowlands, D.G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Fd. Agric.
17: 90-3.
Sanderson, D.M. and Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Brit. J. Ind. Med. 21, 52.
Santi, R. (1961) Ricerche sulla penetrazione e sul destino metabolico
del Rogor-P32 nelle ciliege e nelle pesche. Internal report of
Montecatini.
Santi, R. and de Pietri-Tonelli, P. (1959a) Research on the mechanism
of action of N-monomethyl-0,0-dimethyl dithiophosphorylacetamide. Is.
Ric. Agr. Soc. Montecatini, Milano, Italy. 2 : 3-28.
Santi, R. and de Pietri-Tonelli, P. (1959b) Mode of action and
biological properties of the S-(Methylcarbanyl) methyl
0,0-dimethyldithiophosphate. Nature 183 : 398-9.
Santi, R. and Giacomelli, R. (1962) Metabolic fate of P32-labeled
dimethoate in olive fruits and some toxicological implications.
J. Agr. Food Chem. 10 (3) : 257-61.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analog by total phosphorus determination
after isolation by thin-layer chromatography. J.A.0.A.C. 47 (4) :
645-51.
Uchida, T., Dauterman, W.C. and O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agr. Food Chem. 12 (1) : 48-52.
Van Middelem, C.H. and Waites, R.E. (1964) Gas chromatographic and
colorimetric measurement of dimethoate residues. J. Agr. Food Chem.
12 (2) : 178-182.
Waldron, A.C. (1962) Colorimetric method for the determination of
dimethoate residues in plant tissues. Unpublished but in dimethoate
petition submitted to U.S. Food and Drug Administration by American
Cyanamid Co.
Watts, R. and Storherr, R. (1967) Personal communication. U.S. Food
and Drug. Administration.
Dauterman et al. (1960) studied the metabolism of dimethoate-32P on
the surface and inside cotton, corn, pea and potato leaves following
foliar application. Substantial quantities of oxygen analog were found
in all samples. Although the quantity decreased with time, there was a
proportionate increase in the oxygen analog relative to dimethoate.
Internally this is attributed to enzymatic oxidation; however, since
this same oxidation results on non-biological surfaces exposed to the
atmosphere, non-enzymatic oxidation may occur on the leaf surface.
Based on the quantities of water-soluble metabolites on the surface
and inside leaves, Dauterman postulates that different mechanisms of
degradation occur. There also appears to be a marked difference in
crop species. Plant-surface water-soluble metabolites were primarily
the oxycarboxy derivative; whereas the desmethyl derivatives were the
major internal metabolites. Pea plants did not follow the pattern of
cotton, corn and potato plants, but had about 50 per cent residue of
phosphoric acid both on the surface and inside leaves. No
dithiophosphoric acids or thiocarboxy derivative were found in any
samples. Apparently oxidation occurs more rapidly than hydrolysis.
Hacskaylo and Bull (1963) found that metabolism of dimethoate in
excised cotton leaves resembles the pathway proposed for mammals and
is different from that occurring from foliar treatments. They found
the level of oxygen analog fairly constant and always less than 6 per
cent of the total metabolites found.
Recently Lucier (1967) studied the metabolism of 32P and 14C-carbonyl
labeled dimethoate in bean plants using four modes of application:
foliar treatment, stem injection, root absorption and excised leaves.
Conversion of dimethoate to the oxygen analog was the major route of
degradation and was directly correlated with the ease of translocation
of dimethoate to the leaf tissue. Thus the amount of oxygen analog was
greatest for the excised leaves and less for the foliar application.
In root absorption studies, the oxygen analog content was 4-1/2 times
the amount of dimethoate and represented 10 per cent of the
administered dose 10 days after application. The major hydrolysis
products were the thiocarboxy derivative, dimethyl phosphorothioate
and dimethyl phosphorodithioate. Only trace quantities of the
thiodesmethyl carboxy compound were found.
A new compound, des-N-methyl dimethoate was detected in trace
quantities in all samples. Foliar application gave rise to large
quantities of two unknown metabolites which were postulated to be the
N-hydroxy methyl derivatives of dimethoate and its oxygen analog.
These unknowns are rapidly degraded and not detectable after four
days.
Chillwell and Beecham (1960) found that climatic conditions had no
substantial effect on residue level of dimethoate if similar
applications were made on crops at the same growth stage. Recent
preliminary investigations by Watts and Storherr (1967) show that
residues on field-sprayed kale may contain five times as much oxygen
analog as the parent compound 14 days after treatment.
The oxygen analog seems to be the only metabolite of toxicological
importance. Therefore, its residue level in food products must be
considered. Data submitted to the U.S. Food and Drug Administration
and data reported in the literature by Santi* (1961), Santi and
Giacomelli (1962) and Dauterman et al. (1960) indicate that the amount
of oxygen analog is generally not above 10-20 per cent of the total
toxic residue. Results in olives are an exception where the oxygen
analog was found as high as 67 per cent of the total residue after 30
days.
In animals
Early studies by Roberts at al (1958) and Kaplanis et al (1959)
revealed that dimethoate was absorbed rapidly into the blood of cattle
and was converted to a metabolite several times more toxic than the
parent compound as determined by enzymatic analysis and bioassay. This
compound and many of the major urinary metabolites were not
identified. Santi and de Pietri-Tonelli (1959a) showed that dimethoate
converted chemically and in vitro by incubation in rat liver slices
to the oxygen analog. An unusually high rate of degradation of
dimethoate in sheep liver to the thiocarboxy derivative was found by
Uchida et al, (1964). This was attributed to amidase activity.
Sanderson and Edson (1964) found that dimethoate behaved as a typical
indirect anticholinesterase agent by conversion in the liver to at
least four short lived metabolites whose hydrolysis products are
rapidly excreted mainly in the urine.
Oral administration of dimethoate-32P at dosages of 100 mg/kg to rats
and 10 to 40 mg/kg to lactating cows led Dauterman and co-workers
(1959) to conclude that the metabolic pathway was similar for both
species, and that dimethoate was attacked hydrolytically at five sites
on the molecule. Only small quantities of labelled material were found
in the feces. Dimethoate was rapidly absorbed into the blood and
rapidly excreted in the urine with 75 per cent of the administered
dose eliminated within 24 hours primarily as the thiocarboxy
derivative. As the quantity of the thiocarboxy derivative and
dimethylthiophosphoric acid decreased, the level of dimethylphosphoric
acid increased and appeared relatively stable since conversion to
simpler phosphoric acids was very slow. When the desmethyl derivative
was fed to rats, recovery of better the 85 per cent of unchanged
chemical in the urine occurred suggesting that this is not the major
metabolic route in animals.
Analysis of blood samples indicated that a maximum of chloroform
solubles was reached at two hours with 1.5 ppm dimethoate equivalents
present in cows which were, treated with 10 g/kg. The level of
cholinesterase was severely depressed for the animal treated at
40 mg/kg but no depression occurred at the lower feeding level.
* As quoted by de Pietri-Tonelli et al (1965)
A composite sample representing total milk secreted over a 288 hour
period showed that 0.0068 per cent of the administered dose was
eliminated in this manner. The amount of chloroform solubles decreased
rapidly with less than 0.02 ppm dimethoate equivalents present after
48 hours from the oral dose of 10 mg/kg and 0.04 ppm from the 40 mg/kg
dose.
Chloroform solubles in the fat were less than 0.1 ppm after eight
hours. The only significant quantity of residue in bovine tissues was
found in the liver with a maximum of 0.23 ppm of chloroform solubles
at the lower feeding level.
Chamberlain et al. (1961) investigated the metabolism of dimethoate
by intramuscular administration and oral feeding of sheep at 10 and 40
mg/kg. He too found that only a small per cent of the labeled dose was
excreted in the faeces and that over 80 per cent of the radioactive
material was eliminated in the urine by 72 hours with the thiocarboxy
derivative being the major component. As the thiocarboxy content
decreased there was an increase in dimethyl phosphoric acid and an
unknown which he tentatively identified as the desmethyl carboxy
derivative and suggested that it formed from the thiocarboxy compound.
Trace quantities of dimethoate and its oxygen analog were present in
all sheep urine samples.
Analysis of the blood samples showed that the amount of
organo-solubles decreased very rapidly with less than 0.1 per cent
remaining after 12 hours. Ninety per cent of the organosoluble residue
vas dimethoate or its oxygen analog. The proportion of oxygen analog
to dimethoate increased as the quantity of dimethoate decreased.
In storage and processing
No commercial use of dimethoate for storage products is known. Rowland
(1966) studied the metabolism in stored wheat and sorghum grains
fortified at 2 and 10 parts per million. Trace amounts of dimethoate
and its oxygen analog were found after 4 and 7 days' storage. No
active anti-cholinesterase components were found at subsequent
samplings. No hydrolytic products of the oxygen analog were found, but
significant quantities of dimethoate hydrolysis products were detected
by paper chromatography. These included the thiocarboxy derivative,
the thiodesmethyl and thiodesmethyl carboxy derivative, and the
dimethyl esters of thio and dithiophosphoric acid.
METHODS OF RESIDUE ANALYSIS
The major metabolic pathway in plants indicates that the oxygen analog
of dimethoate is a significant metabolite. A minor pathway in animals
yields the same compound. Any adequate method for residue analysis
must take this compound into account.
Most of the data presented in Table II was obtained from one of two
methods of analysis.
The first method of Waldron (1962) (adapted for apples from a method
described by George (1962) for dimethoate in alfalfa) determines only
the parent compound, dimethoate. The pesticide is extracted with
methylene chloride, followed by alkaline hydrolysis, and colorimetric
determination of the resultant methylamine by reaction with
1-chloro-2,4-dinitrobenzene. Numerous tests were made to show that the
method in specific for dimethoate and that some 28 commonly used
pesticides do not interfere.
Steller and Curry developed a method incorporating a thin layer
chromatographic isolation of dimethoate and its oxygen analog and a
total phosphorous determination of the separate fractions measuring a
molybdenum blue complex. This method is specific for dimethoate and
its oxygen analog. Although recoveries were lower than for the Waldron
method, they were consistent and the authors claim a sensitivity of
0.06 ppm.
The data in Table II were obtained by a variety of methods. Chillwell
and Beecham (1960) macerated plant tissues with water acidified with
acetic acid and extracted the aqueous phase with chloroform.
Microdistillation was used to purify the extracted insecticide and
phosphorous was determined colorimetrically after an acid digestion.
Santi and de Pietri-Tonelli (1959), referred to by de Pietri-Tonelli
et al (1965), used a chloroform extraction with isolation by paper
chromatography and determination of phosphorous with a molybdic
reagent and 2, 6-dibromo-N-chloro-p-quinoneimine and subsequent
spraying with propylene glycol.
Van Middlem and Waites (1964) compared a dinitrochlorobenzene
colorimetric procedure with an electron capture gas chromatography
procedure for dimethoate. Recoveries were 71 per cent for the
colorimetric procedure and about 85 per cent for the gas
chromatography procedure. Correcting for recoveries the results of the
two procedures were in agreement. In both procedures methylene
chloride was used to extract the pesticide. The organic phase was
further cleaned with activated carbon and the aqueous phase was passed
through a polyethylene-coated alumina column prior to colorimetric
determination. For the gas chromatographic analysis benzene was added
to the extract before evaporation of the methylene chloride. These
investigators reported that the oxygen analog is infinitely more
soluble in water than in organic solvents. Unlike dimethoate which has
a partition coefficient of 20 for chloroform and water, the oxygen
analog coefficient is only 0.7.
Watts and Storherr (1967) found a much higher ratio of oxygen analog
in residues using an ethyl acetate extraction with a nuchar carbon
cleanup and a gas chromatography thermionic detection.
Since the oxygen analog in so highly water-soluble and favors the
aqueous phase in many partitioning systems, care must be exercised in
choosing any method of analysis to ensure that the more polar oxygen
analog is not lost. It is difficult to evaluate fully the quantitative
data published on metabolism without knowledge of the exact conditions
of the experiments. It is hoped that improved methodology will assist
in clarifying some apparent anomalies.
NATIONAL TOLERANCES
Country Tolerances, ppm Product
United States of America 2 apples, pears
2 18 vegetables
Canada 2 apples, pears
Benelux 0.5 fruits and
vegetables
Federal Republic of
Germany 0.5 fruits and
vegetables
Australia 2.0
RECOMMENDATIONS FOR TOLERANCES
Temporary tolerances until 3 December 1970
When dimethoate is utilized in accordance with good agricultural
practice to protect food products, when necessary against insect
infestation, the treated product may have residues as high as those
shown below (total of dimethoate and oxygen analog):
Tree fruits (including citrus) 2 ppm
Vegetables (excluding tomatoes and 2 ppm
peppers)
Tomatoes and peppers 1 ppm
By no means will all samples of fruits and vegetables contain this
amount of residue; in fact, only a small, yet unknown portion of each
product in these categories is likely to be treated. There are some
data available on the disappearance of this compound during storage of
treated wheat. The nature of this disappearance simulates that which
occurs in living plants. The compound is systemic; it can be assumed
that residues will not be reduced materially during washing and
peeling.
Since the compound is not recommended, generally, for use on a large
variety of food products, it is recommended that the above levels be
adopted on a temporary basis until further data are made available on
losses during cooking and data are acquired from total diet studies.
The proposed temporary tolerance is for a combination of dimethoate
and its oxygen analog.
FURTHER WORK
Further work required before 30 June 1970
1. Studies on the fate of the compound during processing and
preparation for consumption.
2. Amount of residue appearing in total diet studies.
Further work desirable
Worldwide use data.
REFERENCES PERTINENT TO EVALUATION FOR ACCEPTABLE DAILY INTAKES
Casida, J.E. and Sanderson, D.M. (1962) Nature, 189, 507.
Casida, J.E. and Sanderson, D.M. (1963) J. Agr. Food Chem., 2, 91.
Ecobichon, D.J. and Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E.F. and Noakes, D.N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E.F., Jones, K.H. and Watson, W.A. (1967) Brit. med. J., ii,
554
Fogleman, R.W., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished
reports submitted by American Cyanamid Company.
Levinskas, G.J. and Shaffer, C.B. (1965) Unpublished reports submitted
by American Cyanamid Company.
O'Brien, R.D. (1959) Nature, 183, 121
O'Brien, R.D. (1961) Biochem. J., 79, 229
Ribelin, W.B., Levinskas, G.J. and Shaffer, C.B. (1965) Unpublisbed
reports submitted by American Cyanamid Company
Sanderson, D.M. and Edson, E.F. (1964) Brit. J. industr. Med., 21,
52
Sherman, M., Ross, E., Sanchet, F.F. and Chang, M.T.Y. (1963)
J. econ. Ent., 56, 10
West, B., Vidone, L.B. and Shaffer, C.B. (1961) Toxicol. appl.
Pharmacol., 3, 210
REFERENCES PERTINENT TO EVALUATION FOR TOLERANCES
American Cyanamid Co. (1962-7) Pesticide petitions submitted to the
U.S. Food and Drug Administration.
Bohn, W.R. (1964) The disappearance of dimethoate from soil. J. Econ.
Ent. 57: 798-9.
Chamberlain, W.R., Gatterdam, P.E., and Hopkins, D.E. (1961) The
metabolism of p32-labelled dimethoate in sheep. J. Econ. Ent. 54 (4)
: 733-40.
Chillwell, E.D. and Beecham, P.T. (1960) Residues of 0,0-dimethyl
S-(N-methyl-carbamoyl-methyl) Phosphorothiolothionate (dimethoate) in
sprayed crops. J. Sci. Fd. Agric. 11 (7) : 400-7.
Dauterman, W.C., Casida, J.E., Knaak, J.B. and Kowalczyk, T. J. (1959)
Agr. Food Chem. 7 (3) : 188-93.
Dauterman, W.C., Biado, G.B., Casida, J.E. and O'Brien, R.D. J. Agr.
Food Chem. 1960 8 (2) a 115-19.
de Pietri-Tonelli, F. and Barontini, A. (1959) Analisi per via
biologica dei residue di Rogor in campioni di carciofi ed indivia.
Internal report of Montecatini.
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. (1965) Rogor
(dimethoate) residues in food crops. Residue Reviews. Vol 11. New
York : Springer-Verlag.
George, D.A., Walker, K.C., Giang, P.A. and Murphy, R.T. (1962)
Colorimetric method for the determination of dimethoate residues.
Presented 142nd Meeting Amer. Chem. Soc. 9 -14 September 1962.
Hacskaylo, J. and Bull D.L. (1963) Metabolism of dimethoate in cotton
leaves. J. Agr. Food Chem. 11 (6) : 464-6.
Kaplanis, J.N., Robbins, W.E., Darrow, D.E. et al. (1959) The
metabolism of dimethoate in cattle. J. Econ. Entomol. 52 (6) :
1190-4.
Lucier, G.W. (1967) Thesis entitled : Metabolism of dimethoate in bean
plants in relation to its mode of application. Dept. of Entomol.
University of Maryland, College Park, Md.
Parker, B.L. and Dewey J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based on toxicity to Drosophila
melanogaster. J. Econ. Ent. 58: 106-11.
Roberts, R.H., Radeleff, R.D. and Kaplanis, J.N. (1958) Bioassay of
the blood from cattle treated with Am. Cyanamid 12,880. J. Econ.
Entomol. 51 (6): 861-4.
Rowlands, D.G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Fd. Agric.
17: 90-3.
Sanderson, D.M. and Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Brit. J. Ind. Med. 21, 52.
Santi, R. (1961) Ricerche sulla penetrazione e sul destino metabolico
del Rogor-P32 nelle ciliege e nelle pesche. Internal report of
Montecatini.
Santi, R. and de Pietri-Tonelli, P. (1959a) Research on the mechanism
of action of N-monomethyl-0,0-dimethyl dithiophosphorylacetamide. Is.
Ric. Agr. Soc. Montecatini, Milano, Italy. 2 : 3-28.
Santi, R. and de Pietri-Tonelli, P. (1959b) Mode of action and
biological properties of the S-(Methylcarbanyl) methyl
0,0-dimethyldithiophosphate. Nature 183 : 398-9.
Santi, R. and Giacomelli, R. (1962) Metabolic fate of P32-labeled
dimethoate in olive fruits and some toxicological implications.
J. Agr. Food Chem. 10 (3) : 257-61.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analog by total phosphorus determination
after isolation by thin-layer chromatography. J.A.0.A.C. 47 (4) :
645-51.
Uchida, T., Dauterman, W.C. and O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agr. Food Chem. 12 (1) : 48-52.
Van Middelem, C.H. and Waites, R.E. (1964) Gas chromatographic and
colorimetric measurement of dimethoate residues. J. Agr. Food Chem.
12 (2) : 178-182.
Waldron, A.C. (1962) Colorimetric method for the determination of
dimethoate residues in plant tissues. Unpublished but in dimethoate
petition submitted to U.S. Food and Drug Administration by American
Cyanamid Co.
Watts, R. and Storherr, R. (1967) Personal communication. U.S. Food
and Drug. Administration.
