
DIMETHOATE
First draft prepared by
M. Watson
Pesticides Safety Directorate,
Ministry of Agriculture, Fisheries and Food,
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Immunotoxicity
Effects on the heart
Studies on metabolites
Absorption, distribution, and excretion of omethoate
Biotransformation of omethoate
Effects of omethoate on enzymes and other biochemical
parameters
Acute toxicity of omethoate
Short-term toxicity of omethoate
Long-term toxicity and carcinogenicity of omethoate
Reproductive toxicity of omethoate
Developmental toxicity of omethoate
Genotoxicity of omethoate
Neurotoxicity of omethoate
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Dimethoate was evaluated for toxicological effects by the Joint
Meeting in 1963, 1965, 1967, 1984, and 1987 (Annex 1, references 2, 3,
8, 42, and 50). In 1987, an ADI of 0-0.01 mg/kg bw was established on
the basis of a NOAEL of 0.2 mg/kg bw per day for inhibition of
erythrocyte acetylcholinesterase in volunteers. The compound was
reviewed at the present Meeting within the CCPR periodic review
programme.
Omethoate is the oxygen analogue of dimethoate. It was used
previously as a pesticide in its own right. Information was available
to the Meeting to indicate that omethoate will no longer be used in
this fashion; however, since use of dimethoate on agricultural crops
can lead to residues of omethoate in treated produce, it is important
to consider the toxicity of omethoate when evaluating potential use of
dimethoate. Information on the absorption, distribution, excretion,
metabolism, and toxicity of omethoate was therefore also considered by
the Meeting and summarized in this monograph. These data were taken
from published sources, such as previous JMPR evaluations (Annex 1,
references 17, 25, 31, 33, 37, and 46) and national regulatory
documents, as the original reports were not available.
Formothion is an aldehyde derivative of dimethoate, which was
also previously used as a pesticide in its own right. Information was
available to the Meeting to indicate that formothion will no longer
be used in this fashion. Since use of dimethoate does not lead to
residues of formothion in treated produce, the toxicity of formothion
was not considered at the Meeting.
Data on both dimethoate and omethoate are summarized, including
data not previously reviewed and relevant data from previous
monographs and monograph addenda on dimethoate (Annex 1, references
4, 9, 43, and 52). All of the summaries on omethoate are based on
previous monographs and monograph addenda on this pesticide (Annex 1,
references 17, 25, 31, 33, 37, and 46).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
The 1963 JMPR (Annex 1, reference 2) concluded that the various
studies carried out with dimethoate labelled with 32P showed that it
is rapidly absorbed from the gastrointestinal tract. The radiolabel
is concentrated in the liver, bile, kidneys, and urine, with no
accumulation in fat depots. Elimination is rapid in rats and in
humans, 76-90% of the radiolabel being found in the urine after 24 h.
In guinea-pigs, 25-40% of the radiolabel is recovered in the faeces
(Fenwick et al., 1957; O'Brien, 1959, 1961; Sanderson & Edson,
1964).
The absorption, distribution, metabolism, and excretion of
dimethoate have been investigated with three differently labelled
forms of dimethoate, shown in Figure 1, where the asterisk represents
the position of the radiolabel. In each experiment, male and female
albino rats (strain and number unspecified) received dimethoate at
30 mg/kg bw by intraperitoneal injection. With 32P-labelled material,
'hydrolytic products' recovered from the urine during the first 24 h
after dosing accounted for 55-63% of the administered activity, while
unchanged dimethoate constituted 4-7%. After administration of (3) in
Figure 1, 14C-carbon dioxide (15-18% of the administered activity)
was detected in the expired air over 24 h after dosing, and 39-45% of
the administered activity was detected in the urine. Thus, about 60%
of the administered radiolabel was eliminated in the urine and expired
air during the 24 h after treatment (Hassan et al., 1969).
The blood levels of dimethoate were measured in cats and rats 15,
30, 60, 90, 120, and 180 min after single oral doses of 50, 75, or
200 mg/kg bw in the cats and 300 mg/kg bw in the rats. Dimethoate was
detected in the blood of both species after 30 min and reached a
maximum after 60-90 min. Nearly 80% of the dimethoate in the blood was
found in the erythrocytes of both species, and only 15-20% was found
in the serum. With repeated daily oral doses of dimethoate at doses of
10 or 20 mg/kg bw, the maximal blood level occurred on day 5-10 of the
study (Panshina & Klisenko, 1962).
Figure 1. Labelled forms of dimethoate tested
1. CH3O S O
\" "
*P-S-CH2-C-NHCH3
/
CH3O
2. *CH3O S O
\" "
P-S-CH2-C-NHCH3
/
*CH3O * position of the radiolabel
3. CH3O S O
\" " *
P-S-CH2-C-NHCH3
/
CH3O
About 45% of a dose of 32P-dimethoate administered orally at
50 mg/kg bw to rats was excreted in the urine and only 5.8% in the
faeces 72 h after treatment. The equivalent values in rats after
dermal application were 31 and 6.5%, respectively. More than 95%
of the materials in the urine and faeces after oral or dermal
administration to rats were hydrolytic products, as determined by
chloroform:water partition coefficients (Brady & Arthur, 1963).
About 87-90% of an oral dose of 10 mg/kg bw dimethoate was
eliminated in the urine of cattle within 24 h. The same percentage of
an intramuscular dose of 10 mg/kg bw was excreted within 9 h. Only
3.7-5% of the oral dose was eliminated in the faeces within 72 h and
about 1.1% of the intramuscular dose within 24 h (Kaplanis et al.,
1959).
In humans, 76-100% of an administered oral dose of radiolabel
was reported to be excreted in the urine 24 h after dosing with
32P-dimethoate (Sanderson & Edson, 1964).
A 40% commercial formulation of dimethoate was administered to 16
pregnant rats at a dose of 0 or 21.5 mg/kg bw on day 18 of gestation.
Blood, brain and liver samples were taken from groups of four dams and
fetuses 1, 6, 12, and 24 h after treatment and were examined for
cholinesterase activity. The activity was clearly depressed in
maternal and fetal blood, brain, and liver from 1 h after dosing with
dimethoate, by up to 50% relative to controls. The effect was still
evident 24 h after dosing, although somewhat reduced from the peak
effect seen 6 or 12 h after administration. The inhibition in the
fetus was generally comparable to that seen in the maternal tissues
but was sometimes slightly greater. These results clearly indicate
that dimethoate or active metabolites cross the placenta and have
significant effects in the fetus (El-Elaimy, 1986).
(b) Biotransformation
The 1963 JMPR (Annex 1, reference 2) reported that four dimethoate
metabolites with anticholinesterase activity (molar IC50s within 30
min at 37°C in rat brain: 4.7 × 10-6, 1.1 × 10-5, about 0.2 × 10-5,
and about 0.1 × 10-5) have been identified in rats and humans. One
appeared to be a product resulting from thiono-oxidation, leading to
the formation of the oxygen homologue of dimethoate, followed by
hydrolysis with production of a thiocarboxyl derivative, which
constitutes the chief metabolite of dimethoate in mammals. Although
this thiocarboxyl derivative has not been found in treated plants,
the oxygen analogue has been found in crops (Santi & de Pietri Sonelli,
1959).
The metabolic pathway was similar in rats given 32P-dimethoate
orally at a dose of 100 mg/kg bw and in lactating cows given
10-40 mg/kg bw (Dauterman et al., 1959). Similar results were
obtained for sheep (Chamberlain et al., 1961).
In the paper by Hassan et al. (1969), reviewed above, urinary
metabolites were identified by paper chromatography. Experiments were
also conducted in vitro in which radiolabelled materials (1) and (2)
(Figure 1) were incubated with a rat liver homogenate for 5 h. The
oxygen analogue (omethoate) was proposed as one metabolite, and
cleavage of the C-N bond to produce the carboxy derivative was said to
be a major pathway, along with hydrolysis of the S-C bond to produce
O,O-dimethylphosphorodithioic acid (Figure 2). The oxygen analogue
omethoate may produce equivalent metabolites, although the results did
not clearly confirm this hypothesis. Dimethyl- and monomethyl-
phosphoric acid and thiophosphoric acid may also be produced. Most of
the non-phosphorus part of the molecule was reported to become
conjugated with glucuronic acid.
Figure 2. Structures of dimethoate carboxylic acid and
O,O-dimethylphosphorodithioic acid
CH3O S O CH3O S
\" " \"
P-S-CH2-C-OH P-SH
/ /
CH3O CH3O
dimethoate carboxylic acid O,O-dimethylphosphorodithioic acid
Dimethylphosphorodithioate, dimethylphosphorothioate, and
dimethylphosphate were detected in the urine at concentrations of
12-14, 11-15, and 12-13%, respectively, after intraperitoneal and
oral administration of dimethoate to rats at doses of 0.25, 2.5, or
25 mg/kg bw (Riemer et al., 1985).
Dimethoate undergoes rapid degradation in rat liver, but little
occurs in other tissues (lung, muscle, pancreas, brain, spleen,
blood). The ability of the livers of various species to degrade
dimethoate decreased in the order: rabbit > sheep > dog > rat >
cattle > hen > guinea-pig > mouse > pig. For hens, cattle, mice,
sheep, and rats, there was a reasonably linear relationship between
the LD50 values and the degradation ability of the liver (Uchida
et al., 1964).
The proposed metabolic pathway for dimethoate in rats is shown in
Figure 3.
(c) Effects on enzymes and other biochemical parameters
Dimethoate inhibits cholinesterase activity. The concentration of
pure dimethoate required to inhibit cholinesterase activity in rat
brain in vitro by 50% is 8.5 × 10-3 mol/litre. Dimethoate decomposes
to material(s) that are more toxic than the original substance (Casida
& Sanderson, 1962).
Dimethoate significantly inhibited the active transport of glucose
though the isolated intestine of the mouse (Guthrie et al., 1980).
In studies of human liver enzymes in vitro, it was shown that
dimethoate can inhibit non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of dimethoate are
summarized in Table 1. Clinical signs of toxicity seen 0.5-2 h after
dosing dosing with dimethoate were generally those characteristic
of organophosphate intoxication. The signs included muscular
fibrillation, salivation, lacrimation, urinary incontinence,
diarrhoea, respiratory distress, prostration, gasping, coma, and
death. Macroscopic pathological examination revealed no consistent
target organ.
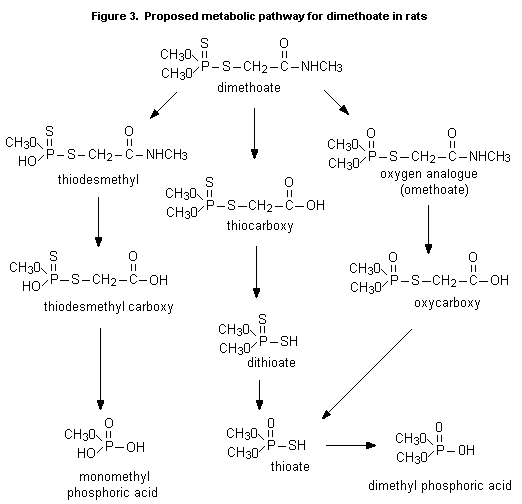 Table 1. Acute toxicity of dimethoate in experimental animals
Species Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Mouse Female Oral NR 60 Sanderson & Edson (1964)
Mouse Male, female Oral NR 160 Ullman et al. (1985)
Rat Male, female Oral NR 314 Ministry of Agriculture,
Fisheries and Food (1993a)
Rat Male, female Oral 97.6-99 540-600 Dal Re & Vola Gera (1980)
Rat Male, female Dermal 97.6-99 >7000 Dal Re & Vola Gera (1976)
Rat Male Intravenous NR 450 Sanderson & Edson (1964)
Rat Male, female Intraperitoneal NR 175-350 Sanderson & Edson (1964)
Hamster Male Oral NR 200 Sanderson & Edson (1964)
Guinea-pig Male, female Oral NR 350-600 Sanderson & Edson (1964)
Rabbit Male, female Oral NR 300-500 Sanderson & Edson (1964)
Hen Male, female Oral NR 30-50 Sanderson & Edson (1964)
NR not reported
(b) Short-term toxicity
Rats
Three studies in rats were briefly summarized in the report of
the 1963 JMPR (Annex 1, reference 2). In a 15-week study, groups of 10
male rats were fed diets containing 1, 5, 25, or 125 ppm dimethoate,
equivalent to 0.1, 0.5, 2.5 and 12 mg/kg bw per day. At the high
dietary level, slight muscular fibrillation and depressed weight gain
were observed. At 5, 25, and 125 ppm, depressed cholinesterase
activity was observed. In another study, groups of 20 rats were fed
diets providing 2, 8, or 32 ppm for 90 days or 50, 100, or 200 ppm
for 35 days. No haematological abnormalities were reported nor any
significant pathological change. The highest dose that did not inhibit
cholinesterase activity was reported to be 32 ppm. In a one-year study
with groups of 20 male rats, the highest dose that did not inhibit
cholinesterase activity was reported to be 10 ppm (Edson & Noakes,
1960; West et al., 1961; Sanderson & Edson, 1964).
The report of a 13-week study in Wistar rats was available only
in an incomplete translation and was neither dated nor signed. Groups
of 24 male and 24 female rats received doses of 0.02, 0.2, 2, or
20 mg/kg bw per day for 18 weeks; a group of 32 males and 32 females
acted as controls. Animals were housed eight per cage, and the
doses were administered orally on five clays a week as aqueous
solutions, which were prepared weekly and refrigerated until use. The
formulations were not analysed for content or for stability in the
vehicle. Body weights were recorded weekly, and food consumption was
recorded, but at unspecified intervals. Blood samples were obtained
from eight males and eight females in each treated group and from 16
controls of each sex on four occasions; a normal range of parameters,
including cholinesterase activity, was measured. Urine samples were
obtained in weeks 12 and 18. Brain cholinesterase activity and a renal
function test (phenol red test) were carried out after 18 weeks.
Histological investigations were performed on six animals of each
sex per group, and the list of tissues chosen for weighing was
satisfactory; however, the list of those chosen for histopathological
examination was short and did not include epididymides.
Slightly reduced body-weight gain was seen in animals treated at
20 mg/kg bw throughout the test, and food consumption was slightly
lower in males of this group than in the controls during the latter
half of the study. There were no treatment-related deaths. During
weeks 6-11, animals in all groups, including the controls, had
diarrhoea, but this was more pronounced in animals treated at 2 or
20 mg/kg bw. Minimally lower haematocrit and erythrocyte count were
noted in animals at the high dose after seven weeks only. There were
no other toxicologically significant changes in haematological
parameters. Plasma cholinesterase activity was 45-75% lower in
animals at 20 mg/kg bw than in the controls. Erythrocyte and brain
acetylcholinesterase activity was 70-90% of that in controls for
animals treated at 2 mg/kg bw and 54-77% in animals at 20 mg/kg bw.
Renal function was unimpaired by treatment, but no data were
presented. There were no treatment-related effects on the urine and
there was no faecal occult blood. Necropsy indicated no effects of
treatment. Organ weight analysis indicated a number of intergroup
differences, none of which was clearly of toxicological significance;
however, the absolute and relative weights of the livers of treated
animals tended to be lower than those of the controls, and this is
likely to represent a treatment-related change, as similar effects
have been seen in other studies. There were no microscopic findings
related to treatment, but no data were presented. It was concluded
that the toxicity expressed was minimal and that 0.2 mg/kg bw was the
NOAEL for inhibition of brain and erythrocyte acetylcholinesterase
activity (Ministry of Agriculture, Fisheries and Food, 1993a).
Dimethoate was administered orally to rats on five days a week
for six weeks at a dose of 10 mg/kg bw per day. The investigations
were confined to an electroencephalogram and cholinesterase
determinations. Treatment was associated with increased frequency and
decreased amplitude on the electroencephalogram, and, as expected,
inhibition of cholinesterase activity in the tissues examined
(Nagymajtenyi, 1988). The relevance of these data to the safety
evaluation of dimethoate is equivocal, and they are not considered
further in this review.
Ten male albino rats received dimethoate by intraperitoneal
injection at 150 mg/kg bw in 0.5 ml saline on alternate days for 30
days; a similar group was treated for 15 days. Ten controls received
saline only, but the duration of their treatment was not specified. On
completion of the treatment, the rats were decapitated and blood
samples were collected for haematological and biochemical examination.
The results were presented as means and standard errors for five
animals per observation. Haemoglobin concentration, haematocrit, and
erythrocyte and leukocyte counts in treated animals were clearly lower
than in controls, the greatest effect being seen after 30 days. The
concentrations of serum urea were greater than in the controls at both
sacrifices; a higher cholesterol concentration was seen only after 30
days. The serum activities of aspartate and alanine aminotransferases
and amylase were higher than in the controls on both occasions. There
was also a small increase in alkaline phosphatase activity; however,
as the timing of the collection of control blood samples was not
given, the significance of this minor change cannot be assessed. The
activity of acid phosphatase was lower than in controls at both blood
sample collections. Cholinesterase determinations in serum indicated
inhibition of 30-50% relative to controls. The above effects were all
more marked after 30 than after 15 days. These results indicate that
dimethoate may affect liver and kidney function, although the changes
were not of pathological significance. Organs were not weighed and no
microscopic examinations were undertaken in order to correlate the
changes seen. Although the changes in amylase activity might indicate
pancreatic effects, confirmatory isoenzyme studies were not carried
out. The route and frequency of administration in this study and the
exiguous nature of the examinations performed render this work of
equivocal use in the safety evaluation of dimethoate (Reena et al.,
1989).
Rabbits
In a five-month study by oral administration in male rabbits, the
doses used were said to be one-tenth and one-hundredth of the LD50
value given once a week, but the actual doses were not specified.
There were no indications of clinical signs, and most of the data were
presented graphically on the basis of monthly sacrifice of four
animals out of a total of 20 animals per group. An initial increase in
cholinesterase activity was noted at the one-month sacrifice, but a
subsequent 40% reduction was reported. Although changes in organ
weights were reported, the body weights of the animals were not, and
the changes were reported only as percentages of absolute weight of
controls. The significance of the various findings cannot be assessed,
and the report is not considered further (Shaker, 1988).
The dermal toxicity of technical-grade dimethoate was investigated
in groups of six male and six female New Zealand white rabbits which
received doses of 100, 300, or 1000 mg/kg bw per day for 21 days; two
control groups, one untreated and one receiving the vehicle (paraffin
oil), were similarly constituted. The doses were selected on the basis
of the results of a five-day range-finding study in two groups of one
male and one female given doses of 1000 or 2000 mg/kg bw per day. In
the range-finding study, slight erythema was seen in one animal at
1000 mg/kg bw per day and in both at 2000 mg/kg bw per day on days 3,
4, and 5; fissuring of the skin was seen in one animal treated at
2000 mg/kg bw per day. Application was made to the abraded skin of
three animals per group or to non-abraded skin on the back of each
animal on five days per week at a volume of 2 ml/kg bw. The test site
was occluded for 6 h with a gauze bandage held in place by tape and
wrapped in occlusive plaster. Observations and food consumption were
recorded daily, and body weight was measured weekly. Blood samples were
taken before treatment and at termination after about 16 h of fasting.
All animals were subjected to a complete necropsy, and a range of
tissues was retained. Microscopic examination was restricted to the
controls and animals at the highest dose.
There were no significant differences between treated and control
animals or between those with intact or abraded skin. Pustules were
seen at the treatment sites of the majority of animals, including
vehicle controls, during the study. Body-weight gain and food
consumption were unaffected by treatment, and there were no changes in
the blood, including cholinesterase activity, that could be ascribed
to treatment. There were no significant treatment-related intergroup
differences in organ weights or in macroscopic or microscopic
pathology. The absence of an effect on cholinesterase activity
indicates that the test material was not absorbed, even across broken
(abraded) skin, at doses up to 1000 mg/kg bw per day. It was concluded
that dimethoate does not irritate rabbit skin (Madison et al., 1986).
Dogs
A study in dogs was briefly summarized in the report of the 1963
JMPR (Annex 1, reference 2), in which groups of two males and two
females were fed diets providing 2, 10, or 50 ppm, equivalent to 0.05,
0.25 and 1.25 mg/kg bw per day dimethoate for 90 days. No significant
effects were noted, and erythrocyte cholinesterase activity was only
slightly depressed in animals at 50 ppm (West et al., 1961).
Groups of six male and six female beagle dogs received diets
containing dimethoate at concentrations of 0, 5, 20, or 125 ppm
for one year. The doses were chosen on the basis of the results
of a preliminary study of 28 days' duration at concentrations
< 1250 ppm, at which dose-related changes were observed at
> 50 ppm, necessitating early sacrifice at 1250 ppm, while changes
at 50 and 250 ppm were confined to dose-dependent reductions in
cholinesterase activity. In the main study, clinical signs of reaction
to treatment and food consumption were recorded daily; body weight was
recorded weekly. The eyes of each animal were examined before
treatment and during weeks 26 and 52. Blood samples were taken on two
occasions before treatment and during weeks 13, 26, and 52. Urine
samples were collected at similar intervals. The samples were examined
for a normal range of haematological and biochemical characteristics,
including plasma and erythrocyte cholinesterase activity; brain
acetylcholinesterase activity was measured at termination. All animals
were necropsied after bone-marrow samples had been taken. A range of
organs was weighed and the tissues preserved for histopathological
processing and examination.
There were no deaths or clinical signs of reaction to treatment
and no effect on body weight, food consumption, or the eyes. The
achieved intakes of test material were calculated to be 0.19, 0.73,
and 4.25 mg/kg bw per day for animals at 0, 5, 20, or 125 ppm. Plasma
cholinesterase activity was reduced by > 20% relative to controls in
animals of each sex at 125 ppm in weeks 13 and 26 and in males only in
week 52. These reductions did not exceed 22%, except in females at
week 13 which had an activity 36% lower than controls. Erythrocyte
acetylcholinesterase activity was reduced in weeks 13 and 26 by 20-27%
in animals at 20 ppm and by 63-76% in those at 125 ppm. In week
52, males at 5 or 20 ppm had marginally, nonsignificantly lower
erythrocyte acetylcholinesterase activities than the controls; females
in these groups were unaffected. A clear reduction (about 65%) in
erythrocyte acetylcholinesterase activity was also seen in animals at
125 ppm. Statistically significant reductions in brain acetylcholin-
esterase activity were seen at all doses after 52 weeks, which were
slight at 5 ppm (about 90% of the control level) and 20 ppm (about 83%
of control) but clear at 125 ppm (45% of control). There were no other
biochemical differences in the blood attributable to treatment. The
urine was unaffected, and there were no findings at necropsy that were
attributable to treatment. The liver weights of animals at 125 ppm
were lower than those of the controls. There was a marginally greater
incidence of pigment, presumed to be haemosiderin, in the livers of
treated animals in all groups, but there was no clear relationship to
dose. In the absence of other effects of treatment, notably on blood,
this effect was considered to be of no toxicological significance. The
only significant evidence of toxicity attributable to dimethoate was
the reduction in cholinesterase activity; the effect on erythrocyte
and brain acetylcholinesterase activity was clearly significant at 20
and 125 ppm, whereas the effects at 5 ppm were confined to minimal
reductions in brain acetylcholinesterase (10% lower than controls) and
a minimal reduction in male plasma cholinesterase activity after 51
weeks. The NOAEL was 5 ppm, equal to 0.19 mg/kg bw per day (Burford
et al., 1991).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade dimethoate was administered to groups of 50 male
and 50 female individually housed B6C3F1 mice for 18 months. Dietary
concentrations of 0, 25, 100, and 200 ppm (equal to 3.2, 12.3 and
25.3 mg/kg bw per day) were selected on the basis of the results of
previous studies, including a study from the US National Cancer
Institute (1977) in this strain. Blood samples were taken for
investigations of haematology and cholinesterase activity after 51
weeks of treatment from an additional 10 males and 10 females
allocated to each group, which were then killed and necropsied.
Haematological investigations only were conducted after 78 weeks on 10
animals of each sex per group. The test diets were mixed weekly, and
formulated diet and the test material were analysed at approximately
three-month intervals; the results of these analyses were satisfactory.
All animals were necropsied; appropriate organs only from animals at
the terminal kill were weighed. A wide range of tissues from all
animals, including satellite animals that died before 51 weeks, were
examined microscopically.
There were no clinical findings that were considered by the
authors to be related to treatment. Survival was > 90% in all groups,
and there was no effect of treatment on mortality. There were no
differences in group mean food consumption that could be related to
treatment. The body-weight gain of treated males was lower than that
of controls during the first few weeks of the study; females receiving
200 ppm were transiently affected during the first two weeks.
Subsequently, the body weights of treated females in all groups were
greater than those of the controls; a similar but less marked
difference was evident in treated males from about 14 months. Female
animals at 25 ppm gained notably less weight than those at the two
higher doses but still gained more than the controls. The overall
weight gains of females were 16.1 ± 5.9 (SD) for the controls,
20.4 ± 6.4 for those at 25 ppm, 27.9 ± 7.3 for those at 100 ppm, and
26.0 ± 5.6 for those at 200 ppm. Haematological analyses after 78
weeks indicated higher nonspecific leukocyte counts in males at 100 or
200 ppm and in females at 200 ppm; no similar difference was seen in
samples taken from satellite animals after 51 weeks of treatment. The
cholinesterase activity in plasma and erythrocytes from treated
animals was lower than That in the controls in a dose-related manner
at all dietary concentrations. No other examination of cholinesterase
activity and no analyses of brain were undertaken. Organ weight
analysis indicated greater absolute liver weights in animals at 100 or
200 ppm; however, the relative liver weights of females were lower
than those of controls as a result of the increased body weights of
these animals. The absolute weight of the ovaries of treated females
was lower than that of the controls after 78 weeks of treatment, but
no similar difference was seen in animals killed after 52 weeks of
treatment. Microscopic examination indicated a greater incidence of
extramedullary haematopoiesis in the spleens of males and females at
100 or 200 ppm, which was dose-related. A greater incidence of
hepatocytic vacuolation was seen in males and female at 100 or 200 ppm
and to a lesser extent in females at 25 ppm; the effect was attributed
to fat and the nutritional status of the affected groups. There were
no differences in the incidences of any neoplastic finding that could
be related unequivocally to treatment. There was no NOAEL, as effects
were seen at all doses (Hellwig, 1986a)
Fifty male B6C3F1 mice received a dietary concentration of
250 ppm dimethoate for 69 weeks or 500 ppm for 60 weeks, and 50
females received the two doses for 80 weeks. All animals were then
observed without treatment until about 94 weeks. A control group of 10
males and 10 females was supplemented by similar groups of animals
from concurrent studies on other pesticides; this gave a pooled
control group of 50-60 animals, although the studies were not
precisely concurrent. The body-weight gain of treated mice, except
females at the low dose, was lower than that of controls during the
first 52 weeks of treatment. Occasional generalized tremor was noted
in treated animals at each dose. During the second half of the study,
alopecia, abdominal distension, and tumours were seen in treated
animals but predominantly in those receiving the lower dose. The
condition of animals at termination was said to be poor. Oncogenic
potential was not assessed (US National Cancer Institute, 1976, 1977).
Rats
Groups of 65 male and 65 female individually housed Wistar rats
received dimethoate in the diet at concentrations providing 0, 5, 25,
or 100 ppm for two years. Fifteen animals of each sex in each group
were allocated for clinical pathology; an additional group of 20 males
and 20 females received a dietary concentration of 1 ppm and were used
to establish or confirm a no-effect level. The dietary concentrations
were selected on the basis of the results of a preliminary study and a
study by the US National Cancer Institute (1977) on Osborne-Mendel
rats. Feed was prepared weekly; both the test material and the
formulated diets were analysed regularly and found to be satisfactory.
Food consumption was recorded weekly; body weight was recorded
weekly for 13 weeks and at fortnightly intervals thereafter. Daily
observations and weekly palpations were recorded; the eyes of all
animals of the main groups (50 of each sex) were examined before
treatment and at six-month intervals during the treatment period for
changes to the refracting media. The ocular fundus of 10 males and 10
females in the control and high-dose groups were examined after 620
days of treatment. Blood samples were obtained before treatment and on
six occasions during the study, and a normal range of chemical and
haematological parameters, including cholinesterase activity, was
measured. Brain acetylcholinesterase activity was measured at the end
of the study. Urine samples were collected twice during the study;
although basic, qualitative 'stick' tests were conducted, volume and
specific gravity were not measured. All animals were necropsied, and a
range of organs was weighed and the tissues retained. Tissues from all
animals in the main study and from satellite animals that died were
examined microscopically.
Females at 100 ppm had a slightly higher mortality rate than
controls from week 65. There were no clinical signs that were
considered by the authors to be related to treatment. Animals at doses
> 25 ppm showed a trend to increased food consumption, especially
during the second year of treatment. The body-weight gain of animals
receiving 100 ppm was slightly lower than that of the controls
during the first half of the study. Examination of the eyes in vivo
revealed no treatment-related effect. Reductions in plasma
cholinesterase activity (generally, 50% of control) were seen in the
group at 100 ppm at all examinations; in females at 25 ppm, the
activities in plasma were minimally lower than in the controls after
four weeks. Reductions in erythrocyte acetylcholinesterase activity
were clear in animals at 25 or 100 ppm (60-75 and 20-40% of control
values, respectively); smaller decreases were seen in females at 5 ppm
during the first 12 months and in males of this group after 24 months.
Clear dose-related reductions in brain acetylcholinesterase activity
were seen at termination in animals at 25 or 100 ppm and slightly
lower values in males at 5 ppm. In animals at this low dose, the
reductions in erythrocyte and brain acetylcholinesterase activity were
not consistent between times or sexes; the variations from control
values are thus of questionable biological significance but probably
represent an intermittent effect of treatment. There were no
statistically significant differences in cholinesterase activity in
the animals at 1 ppm.
The authors reported minimal anaemia throughout the study in
males at 100 ppm; however, this effect was also present before
treatment and was absent in females. Minor variations were also noted
in leukocyte count, potassium and total protein concentrations, and
aspartate aminotransferase activity. Although these effects were
reported to extend in part to the animals at 25 ppm, they were minor;
they may be related to treatment but are considered not to be of clear
toxicological significance. There were no treatment-related changes in
the urine. Animals at 100 ppm had slightly larger spleens and slightly
smaller ovaries than the controls, but no gross pathological findings
could be related to treatment. There were no non-neoplastic findings
that were considered to be related to treatment, and there was no
statistically significant difference in the distribution of individual
tumour types. Treated males had a greater number of malignant tumours
than the controls, but there was no relationship to dose. In a number
of tissues, however, a greater incidence of tumours was seen in
treated animals than in controls. These included exocrine and
islet-cell adenomas in the pancreas of males, haemangiosarcoma in
the spleen of males, and mammary gland fibroadenoma and carcinoma
in females. When the combined incidences of haemangioma and
haemangiosarcoma at any site in treated animals were compared with
those in controls, the difference was statistically significant for
all groups of treated males, but there was no relationship to dose and
females were clearly unaffected. The apparent difference may be due to
a lower than expected incidence in the controls. A large proportion of
these tumours were located in the mesenteric lymph node. The incidence
of these vascular tumours was said to be similar to that of historical
controls from a number of sources. The authors concluded that there
was no evidence that dimethoate has oncogenic potential. The NOAEL for
inhibition of brain and erythrocyte acetylcholinesterase activity was
1 ppm, equivalent to 0.05 mg/kg bw per day (Hellwig, 1986b).
The vascular and proliferative lesions from the above study were
evaluated by a second group of pathologists (Squire, 1988), who were
unaware of the previous interpretation of each slide. This review
supported the conclusion of the original pathologist and indicated
that the Wistar rat is susceptible to these tumours. It also suggested
that the chemicals that induce vascular neoplasms are genotoxic;
however, it asserted that dimethoate is not genotoxic and that by
implication these tumours were not related to treatment.
Groups of 50 male and 50 female Wistar rats received technical-
grade dimethoate in the diet at concentrations of 0, 2, 20, or 200 ppm
for two years, equivalent to 0.1, 1 and 10 mg/kg bw per day. The report
was available only as an English translation of a nearly illegible
German summary with tables of individual data. As no other details of
procedures or results and no group means were presented, the study
could not be reviewed satisfactorily (Ministry of Agriculture,
Fisheries and Food, 1993a).
Groups of 30 male and 30 female Wistar rats received diets
designed to provide technical-grade dimethoate at concentrations of 0,
0.1, 1, 10, or 75 ppm for two years, equivalent to 0.005, 0.05, 0.5
and 3.8 mg/kg bw per day. The feed was prepared four times a week.
Body weight and food intake were recorded weekly for the first four
weeks and every four weeks thereafter. Haematological examinations
were performed four times between weeks 32 and 100. Cholinesterase
activity was determined after 1, 3, 12, 50, 75, and 100 weeks on six
rats of each sex. Brain acetylcholinesterase activity was determined
after 52 and 104 weeks. Other, limited biochemical assays were
performed during the study on blood and urine, mostly after two years.
Six animals of each sex were killed after one year and the surviving
animals after two years; all were examined macroscopically and their
organs weighed. Histological examination was performed on six males
and six females at each sacrifice. There was no indication of how dead
animals were handled or examined, although the report refers to their
necropsy.
Early signs of reaction to treatment at 75 ppm (slight
piloerection, exophthalmia, and fine tremor) disappeared during the
fourth week of treatment, and no other clinical signs were attributed
to treatment. A high mortality rate resulted from an infection after
78 weeks, with the deaths of 55 males and 37 females, evenly
distributed among the groups. The infection was treated with
oxytetracycline as three oral doses of 40 mg/kg bw over three weeks.
There was no treatment-related mortality. Body-weight gain was reduced
in animals treated at 75 ppm, up to week 20 for females and throughout
the study for males. Food consumption was unaffected, but conversion
was reduced in animals treated at 75 ppm, in line with body weight.
The achieved mean doses were 0.02, 0.2, 2, and about 20 mg/kg bw per
day. Haematological investigations indicated no effects of treatment.
Cholinesterase activity was clearly reduced in the plasma,
erythrocytes, and brain of animals receiving 10 or 75 ppm. The brain
acetylcholinesterase activity of animals receiving 1 ppm was 20% lower
than that of controls after one year. There were no other
toxicologically significant intergroup differences in the composition
of the plasma or urine. Organ weight analysis after one year indicated
lower liver, spleen, adrenal, and testis weights in males at 75 ppm
than in the controls; after two years, the adrenal and liver weights
of males and females at 75 ppm were lower than those of controls.
Necropsy of animals that died during the study and of animals killed
at the scheduled sacrifices indicated no treatment-related changes.
The distribution of macroscopically observed rumours was unaffected by
treatment. No microscopic changes were found that were attributed to
treatment. The incomplete data presentation and the infection that
occurred in the middle of the study significantly compromise its
validity (Ministry of Agriculture, Fisheries and Food, 1993a).
Groups of 50 male Osborne-Mendel rats were fed diets providing
technical-grade dimethoate at concentrations of 250 or 500 ppm
(equivalent to 25 and 50 mg/kg bw per day) for 19 weeks; the doses
were then reduced to 125 and 250 ppm (equivalent to 6.3 and 13 mg/kg
bw per day),and treatment continued for 61 weeks. Necropsy was
performed after a further 33-35 weeks without treatment. The same
dietary concentrations were fed to similar groups of females, except
that they were reduced only after 43 weeks; the animals were then
treated at concentrations of 125 or 250 ppm for a further 37 weeks.
The total treatment period for all animals was 80 weeks, followed by
up to 35 weeks without treatment. A control group of 10 males and 10
females was supplemented by similar groups of animals from concurrent
studies on other pesticides, giving a pooled control group of 50-60
animals, although the studies were not precisely concurrent. All
animals were observed daily, and body weights were recorded 'at
regular intervals until 110 weeks'. The animals were then necropsied
and, when feasible, tissues were retained for histopathological
examination. Treatment at 500 ppm was associated with lower
body-weight gains in males and females during the first 20 weeks of
treatment. After the reduction to 250 ppm at 19 weeks, the body-weight
gain of males increased but remained lower than in controls until
treatment ceased. The body-weight gain of males at the low dose and
females at the high dose remained lower than that of controls until
week 80. Clinical signs of inhibition of cholinesterase activity were
seen in animals at the high dose, particularly during the first week
of treatment. Conjunctivitis of vital origin was diagnosed in the
animals at week 38. Animals that survived to termination were said to
be 'generally in poor physical condition'. More animals at the high
dose died than matched or pooled controls, although the number of
matched control males that died during the study (7 of 10) was
reported to be unusually large. There was no difference in the
distribution of non-neoplastic or neoplastic changes among the treated
groups that could clearly be ascribed to treatment. The pathological
assessment indicated no oncogenic potential (US National Cancer
Institute, 1976, 1977).
A review by Reuver (1984) covered several carcinogenicity studies
in rats and mice, including that of the US National Cancer Institute.
It was concluded from the studies of Gibel et al. (1973) and
Stieglitz et al. (1974) that dimethoate is highly carcinogenic to
rats, but insufficient details of these experiments were given,
precluding assessment. The review of the US National Cancer Institute
study involved re-reading of the sections; it was again concluded
that dimethoate is carcinogenic. The basis for the review was not
described, and the numbers of animals given in the tables shown in the
review differ from those in the original report. Reuber quoted text
from the report in reverse order to that in which it was originally
published. This review did not satisfactorily explain the methods used
or discuss the discrepancies in the reported incidences and in the
conclusions from those of the original report. In view of these
deficiencies, no weight is placed on this publication.
(d) Reproductive toxicity
Mice
A multigeneration study was undertaken in CF-1 mice fed diets
containing concentrations of 0, 5, 15, or 50 ppm dimethoate,
equivalent to 1.4, 4.3 and 14.5 mg/kg bw per day, throughout the
study. The study was conducted before Good Laboratory Practice came to
be enforced but was designed according to the recommendations of the
Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics of
the Association of Food and Drug Officials of the United States. Each
generation was mated twice, the first set of litters being discarded
and the next generation (F1b, F2b, and F3b) being produced from the
second litters. For each generation, eight males were mated with 16
females in each group, and males were rotated within their group
during the mating period. The observations were limited in comparison
with current practice. After weaning of the F3b generation, the
parents (F2b) were killed and necropsied; liver, kidneys, and gonads
were weighed, but no microscopy was performed. All animals of the F3b
generation were necropsied at death or at weaning, and a wide range of
tissues from one male and one female from each litter was examined
microscopically. Organ weighing was restricted. All fetuses that were
not examined microscopically were retained in 80% alcohol for skeletal
staining in alizarin red.
There were no clinical reactions to treatment. Tremors were seen
in four dams of the F2b generation treated with 50 ppm, three of
which lost their litters, on one occasion after a weekly change of
diets; the diets were replaced, as a formulation error was suspected,
and the condition was not seen again. No treatment-related differences
in group mean body weights were recorded at any mating. Measurement of
the food consumption of the F0 generation before mating indicated
no effect of treatment, but no data were presented and no other
measurements were carried out. The fertility, gestation, viability,
and lactation indices were unaffected by treatment, and there was
no effect on the weights of the pups at weaning. No intergroup
differences in organ weight or pathological findings were seen that
were related to treatment. The NOAEL for reproductive toxicity was
50 ppm (Ribelin et al., 1965).
In five generations of CD-1 mice given 60 ppm of dimethoate in
drinking-water, reproductive performance was significantly altered, as
indicated by reduced mating success and longer gestation. Litter size
and weight were not reduced at birth, but pup mortality was increased
significantly by treatment. The growth rate of the pups was generally
lower than that of controls. Dimethoate did not show teratogenic
potential or adverse effects on organ weights or histological
appearance (Budreau & Singh, 1973).
Rats
In a multigeneration study, dimethoate (purity, 96.4%) was
administered in the diet at fixed concentrations of 0, 1, 15, or
65 ppm (equal to about 0.08, 1.25 and 5 mg/kg bw per day) to groups of
28 male and 28 female Sprague-Dawley rats for 10 weeks before the
first of two matings to produce F1a and F1b animals. The F1
generation, selected from the F1a litters, was first mated at about
16 weeks of age to produce F2 pups, which were killed and examined at
21 days of age. A second mating of the F1a generation was conducted,
followed by a partial third mating involving animals that had not been
successful at either of their first two pairings. Administration of
dimethoate was continued at the same dietary levels throughout
premating, mating, gestation, and lactation. Treatment at 65 ppm was
associated in the parent animals with marked reductions in plasma,
erythrocyte, and brain cholinesterase activities in animals of both
generations, slightly reduced body-weight gain, increased food intake,
and reduced water intake. In the F1a pups at 65 ppm at four days of
age, a reduction in brain acetylcholinesterase activity was seen in
males but not in females. The parental animals at 15 ppm had a
significant reduction in brain and erythrocyte acetylcholinesterase
activity in both generations, but no effect on cholinesterase activity
was seen in the offspring. Mating performance (as assessed by median
precoital time and duration of pregnancy) was not affected by
treatment; however, an effect was seen on the pregnancy rate (Table
2). These data are clearly indicative of substandard performance at
65 ppm and also show a possible effect at 15 ppm on the second mating
of the F1 generation. The Meeting concluded that this information did
not clarify the possible effect at the intermediate dose. Treatment at
65 ppm was also associated with a reduction in litter size at birth,
as shown in Table 3.
Table 2. Pregnancy rates of animals treated with dimethoate with
live pups at birth
Generation Mating Pregnancy rate (%)
Control 1 ppm 15 ppm 65 ppm
F0 First 93 96 86 89
Second 89 93 89 71
(100) (100) (100) (96)
F1 First 96 71 71 63
Second 73 67 58 50
(100) (79) (92) (75)
In parentheses, the percentages of animals with implantations
confirmed at autopsy by Salewski staining of the uteri of
apparently non-pregnant females
Table 3. Litter size at birth of animals treated with dimethoate
Generation Mating Group mean litter size at birth
Control 1 ppm 15 ppm 65 ppm
F0 First 16.4 15.3* 15.3* 14.2**
Second 14.9 14.9 14.2 14.3
F1 First 12.3 11.9 14.6 12.0
Second 14.1 13.3 13.1 10.0*
* p < 0.05
** p < 0.01
There was also a slight increase in pup mortality during
lactation in animals at 65 ppm. Changes in litter weight reflected the
changes in litter size. The mean pup weight at birth was unaffected by
treatment, but pup body-weight gain was adversely affected by the high
dietary level. There was a slight delay in attainment of the startle
reflex in pups born after the first mating of both generations at
65 ppm, but the mean delay was less than one day and was not apparent
across all four matings; it was thus probably not related to
treatment. There was no other effect on pre- or post-weaning
development. Histopathological examination of tissues associated with
the reproductive tract did not reveal any treatment-related changes.
The NOAEL for toxicity was 1 ppm, equivalent to 0.08 mg/kg bw per day,
on the basis of inhibition of cholinesterase activity at 15 ppm
(Brooker et al., 1992). The Meeting discounted the possible adverse
effect on pregnancy rate at 15 ppm, and this was the NOAEL for
reproductive performance, equivalent to 1.2 mg/kg bw per day.
Rabbits
Three groups of three male rabbits received gelatin capsules
containing individually calculated doses of a dimethoate formulation
(composition unspecified) calculated to be one-tenth and one-hundredth
of the LD50, which was not specified, at an unspecified frequency. A
six-week preliminary period was followed by six weeks of treatment and
then by six weeks of respite. Body weights were recorded weekly, and
semen was collected twice weekly from all animals throughout the
experimental period. Ejaculate volume was recorded after removal of
the gel mass. Seminal initial fructose was determined immediately
after collection, and methylene blue reduction time was recorded. The
numbers of live, dead, and abnormal sperm were assessed, and sperm
concentration was determined with a haemocytometer. Data were
presented in graphs rather than as individual values; there was no
indication of variation between individual animals. Clinical signs
were not reported. Body-weight gain was reduced in treated animals,
and there were indications of reduced libido. The ejaculate volume and
sperm concentration of treated animals, expressed as a percentage of
that of controls, decreased during the treatment period, and the
effect on sperm concentration persisted into the recovery period.
Treatment increased the numbers of abnormal sperm in a dose-related
manner; these were greatest at the end of the treatment period but
declined thereafter. A significant increase in the methylene blue
reduction time and a decrease in the initial fructose concentration
were also seen. There was some evidence of recovery from these effects
during the latter part of the recovery period (Salem et al., 1988).
In view of the deficiencies, it was difficult to assess the
significance of the changes seen, but they should be explained in view
of the reported reproductive effects of dimethoate.
(e) Developmental toxicity
Mice
Intraperitoneal administration of dimethoate at 40 mg/kg bw as a
single dose to mice on the day of mating or on day 9 of gestation or
for the first 14 days of gestation caused a high incidence of
embryonal loss (Scheufler, 1975).
Dimethoate administered orally at doses of 10 or 20 mg/kg bw was
not teratogenic to CD-1 mice, and these levels were not lethal to the
dams. Doses of 40 and 80 mg/kg bw induced maternal toxicity (Courtney
et al., 1985).
Rats
Groups of pregnant rats were given 0, 3, 6, 12, or 24 mg/kg bw of
a dimethoate formulation by gavage daily on days 6-15 of gestation.
The dams were killed on day 22 of gestation; the uterine content was
removed, the carcass weighed, the number of corpora lutea was
determined, and the animals were necropsied. The fetuses were weighed
and examined for viability and external malformations; live fetuses
were studied for skeletal and visceral anomalies. Maternal weight was
decreased significantly in the group receiving 24 mg/kg bw, and clonic
spasms and muscular tremors were seen. The mean fetal weight was not
affected by treatment. Treatment with 12 or 24 mg/kg bw was associated
with an increased number of litters with abnormal fetuses and fetuses
with wavy ribs. The NOAEL was 6 mg/kg bw of formulated product, equal
to 2.84 mg/kg bw dimethoate (Khera et al., 1979).
Three groups of 25 time-mated CrL:COBS CD (SD) BR rats received
technical-grade dimethoate (purity, 97.3%) in 1% aqueous methyl
cellulose (10 ml/kg) at doses of 3, 6, or 18 mg/kg bw per day by
gavage daily on days 6-15 of gestation; a control group received the
vehicle alone. Clinical observations were made at regular intervals,
and food consumption and body weight were recorded. All animals were
killed on day 20 of gestation and the uterine contents examined for a
normal range of parameters. One-half of the pups were preserved in
Bouin's solution for free-hand sectioning and the remainder in
industrial methylated spirits for subsequent alizarin staining and
skeletal examination. The doses were chosen on the basis of the
results of a preliminary study, which was not presented or discussed.
The signs seen in animals at 18 mg/kg bw per day included salivation,
hypersensitivity, ataxia, tremor, fur staining, and small, rounded
faecal pellets. The signs at 3 mg/kg bw per day were confined to
salivation; in animals at 6 mg/kg bw per day, this was accompanied by
a low incidence of small, rounded faecal pellets. Food consumption and
body-weight gain were lower in animals at 18 mg/ kg bw per day than in
the controls during treatment; similar effects were not seen at the
two lower doses. Neither litter parameters nor fetal development (as
indicated by the incidences of visceral or skeletal abnormalities) was
affected by treatment. The NOAEL for toxic signs depends on the
interpretation of the significance of the salivation seen in the
animals at 3 and 6 mg/kg bw per day and on the abnormal faecal pellets
in the latter group. Although salivation is an expected effect of
organophosphate pesticides, there was no clear evidence that the
effect was unduly prolonged (It was described as occurring
'immediately' after treatment.) and may have been incidental. The
presence of abnormal faecal pellets may be related to the action of
this class of compound on the gastrointestinal tract and is unlikely
to be of toxicological significance (Edwards et al., 1984a).The
Meeting concluded that the NOAEL was 6 mg/kg bw per day.
Rabbits
Groups of 16 female New Zealand white rabbits (obtained from
several breeders) were mated with males of proven fertility and
received technical-grade dimethoate (purity, 97.3%) in 1% aqueous
methylcellulose (5 ml/kg) at doses of 10, 20, or 40 mg/kg bw per day
by gavage daily on days 7-19 of gestation; a control group received
the vehicle alone. The doses were chosen from a preliminary study, the
results of which were not summarized. After coitus, each animal
received an injection of luteinizing hormone. Clinical observations
were made at regular intervals, and food consumption and body weight
were recorded. All animals were killed on day 29 of gestation, and the
uterine contents were inspected; a range of parameters for this type
of study was assessed. After examination in vivo, the pups were
killed and dissected. The skinned, eviscerated pups were fixed in
industrial methylated spirits, and the brain was examined for
abnormalities by longitudinal sectioning; carcasses were cleared and
stained by a modified Dawson's technique for skeletal examination.
There were no clinical signs of reaction to treatment with doses of 10
or 20 mg/kg bw per day; at 40 mg/kg bw per day, muscle tremors and
ataxia were seen during the latter part of the treatment period. Food
consumption was reduced between days 15 and 23 of gestation in animals
treated at 40 mg/kg bw per day, and the body-weight gain of these
rabbits was lower than that of controls throughout the treatment and
particularly between days 15 and 20 of gestation. A slight reduction
in body-weight gain was seen in animals at 20 mg/kg bw per day. An
initial reduction in weight gain was seen at the beginning of the
treatment period in animals at the low dose, but these animals
subsequently gained more weight than the controls. Treatment had no
effect on fetal development; litter size and weight were unaffected,
and pups had no abnormalities that could be ascribed to treatment.
Although there was a transient reduction in body-weight gain at the
start of treatment in animals at 10 mg/kg bw per day, the deficit was
quickly corrected. This dose was therefore the NOAEL (Edwards et al.,
1984b).
Cats
Four groups of 17 cats were mated and treated with Cygon-4E, a
commercial insecticide containing 47.3% dimethoate, as single daily
doses of 0, 3, 6, or 12 mg/kg bw on days 14-22 of gestation. The cats
were necropsied on day 43 of gestation, and the fetuses were removed,
weighed, and examined for external malformations. The total number of
anomalous fetuses in cats at 12 mg/kg bw per day was not significantly
higher than that in controls. The only treatment-related malformation
was observed at this dose and consisted of forepaw polydactyly in
eight of 39 fetuses. A dose-response relationship was not established
owing to the limited response and the common occurrence of this
anomaly in cats. The NOAEL was 6 mg/kg bw per day of Cygon-4E, equal
to 2.8 mg/kg bw per day of dimethoate (Khera, 1979).
(f) Genotoxicity
The regulatory reports evaluated by the Meeting concluded that
dimethoate does not induce reverse mutation or gene mutation in
vitro, nor did it induce micronucleus formation, dominant lethal
mutation, or chromosomal aberration in mice in vivo. Dimethoate
induced unscheduled DNA synthesis in vitro in two assays using
different methods of assessing the uptake of tritiated thymidine into
DNA but not in an assay in vivo/in vitro. A review of the literature
on the mutagenic potential of dimethoate revealed a number of positive
results, notably for reverse mutation in Salmonella typhimurium
TA100 and for sister chromatid exchange in mammalian cells in vitro.
It was concluded that although dimethoate has mutagenic potential in
vitro, mutagenicity does not appear to be expressed in vivo. The
results of assays for genotoxicity with dimethoate are summarized in
Table 4.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The dermal irritation potential of a 400-g/litre emulsifiable
concentrate formulation of dimethoate was investigated in rabbits.
Only slight erythema was observed 4 h after application, and the
effect had resolved by 24 h after treatment (Ministry of Agriculture,
Fisheries and Food, 1993a).
The ocular irritation potential of the same formulation of
dimethoate was also investigated in rabbits. Redness and swelling of
the conjunctiva were observed, with slight corneal opacity, 1-72 h
after application. All of the effects had resolved by eight days after
treatment (Ministry of Agriculture, Fisheries and Food, 1993a).
Studies to investigate the ocular irritation potential in
rabbits of 40 and 38.3% dimethoate formulations concluded that the
formulations were irritating to the rabbit eye. The 'in use' dilution
of the 40% formulation (0.84%) was not irritating (Ministry of
Agriculture, Fisheries and Food, 1993a).
Technical-grade dimethoate (purity, 97.3%) did not sensitize the
skin of guinea-pigs when tested by the Buehler method (Madison et al.,
1984).
Table 4. Results of tests for the genotoxicity of dimethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
5-Methyltryptophan E. coli 1.6 × 10-3 mol/litre NR Positivea Mohn (1973)
resistance mutation
Reverse mutation S. typhimurium < 5000 mg/plate NR Positive Moriya et al. (1983)
TA98, TA100, in TA100a
TA1535, TA1537,
TA1538
E. coli WP2hcr < 5000 mg/plate Positivea
Reverse mutation S. typhimurium < 5000 mg/plate NR Negativea Probst et al. (1981)
TA98, TA100,
TA1535, TA1537,
TA1538
E. coli WP2uvrA- 47 nmol/ml Initially
positive,
negative
quantitativelya
Reverse mutation S. typhimurium 2-200 mg/plate NR Positivea Vishwanath & Jamil
TA100 (1986)
Mitotic gene conversion S. cerevisiae 7 doses, 40-100 mmol NR Positive Fahrig (1974)
Mitotic gene conversion S. pombe (ade 6) 1.3-131 mmol NR Negative Gilot-Delhalle et al.
(1983)
Gene mutation Chinese hamster 1000-3500 mg/ml 97.3 Negative Johnson et al. (1985)
ovary (hprt)
Sister chromatid exchange Cultured human < 120 ppm NR Positive Gomez-Arroyo et al.
lymphocytes (1987)
Sister chromatid exchange Chinese hamster 10, 20, 40, 80 mg/ml 94 Positive Chen et al. (1981)
and cell cycle delay ovary V79 cells + 10 pg/ml BUdR
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Cytotoxicity Chang liver and 50-500 mg/ml 99.8 Positive Gabliks &
HeLa cells Friedman (1965)
Cytotoxicity HeLa cells 2-300 mg/ml 99.8 Positive Gabliks (1965a)
Susceptibility to HeLa cells 2-300 mg/ml for 99.8 Positive Gabliks (1965b)
poliovirus infection < 108 days
Unscheduled DNA SV-40 transformed 100, 1000 mmol NR Positive Ahmed et al. (1977)
synthesis human fibroblast
cell line VA-4
Unscheduled DNA Rat hepatocytes 47 nmol/ml NR Negative Probst et al. (1981)
synthesis
Unscheduled DNA Rat hepatocytes < 2290 mg/ml NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
Unscheduled DNA Rat hepatocytes NR NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
In vivo
Micronucleus formation Mouse 2 equal oral doses NR Positive Rani et al. (1980)
bone marrow of 51.7 mg/kg bw at
24-h interval
Host-mediated Mouse; 3 equal oral doses NR Positive Rani et al. (1980)
mutagenicity S. typhimurium of 155 mg/kg bw
Dominant lethal CFLP mice 30, 60 mg/kg bw ip NR Negative Fisher & Scheufler
mutation AB Jena mice 5 × 6 mg/kg bw ip Negative (1981)
DBA mice 3 × 18 mg/kg bw ip Negative
Dominant lethal NMRI mice 5, 10, 20 mg/kg 96.9 Negative Becker (1985)
mutation orally, 5 days
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Dominant lethal Strain Q mice 10 mg/kg ip + 0.6 NR Negative Degraeve & Moutschen
mutation mg/l drinking-water (1983)
Chromosomal CFLP mice 20-60 mg/kg bw ip NR Positive (gaps Nehéz (1983)
aberration and numerical
changes)
Chromosomal aberration Rats 15, 75, 150 mg/kg bw ip NR Negative Ministry of Agriculture,
Fisheries and Food
(1993a)
Chromosomal aberration Mice 50, 100 mg/kg bw NR Positive Bhunya & Behera
(1975)
Chromosomal aberration Hamster 16-160 mg/kg bw ip NR Weakly Dzwonkowska & Hubner
positive (1986)
Sex-linked recessive Drosophila 1 mg/kg feeding NR Negative Woodruff et al. (1983)
lethal mutation
Sex-linked recessive Drosophila 10 or 20 ppm; adult NR Positive at Velásquez et al. (1986)
lethal mutation feeding low dose
0 or 10 ppm larval Negative
feeding
Sex-linked recessive Drosophila LD50 and half LD50, NR Positive Tripathy (1988)
lethal mutation larval feeding
Unscheduled DNA Rats 50, 100, 200 mg/kg NR Negative Ministry of Agriculture,
synthesis bw orally Fisheries and Food
(1993a)
Micronucleus formation CD-1 mice 55 mg/kg bw ip once 97.3 Negative Sorg (1985)
or twice 24 h apart
Metaphase alteration Rats 15, 75, 150 mg/kg 97.3 Negative San Sebastian (1985)
bw ip
ip intraperitoneally
a With and without metabolic activation
Nineteen cases of allergic, occupational contact eczema and one
of contact dermatitis have been reported (von Jung, 1989). Exposure
to dimethoate was cited in four cases: in two male and one female
gardener and in one female agrochemical technician, 22-69 years of
age. The results of patch tests with dimethoate in these individuals
were positive.
(ii) Neurotoxicity
In a preliminary study, the LD50 of dimethoate in hens was
determined in groups of 10 birds given single oral doses of 0, 30, 45,
68, 100, or 150 mg/kg bw in water. These doses were selected on the
basis of the results of a preliminary study in groups of two birds at
doses between 12.5 and 200 mg/kg bw; both birds at 100 or 200 mg/kg bw
died. In each of these studies, dosing was followed by a 14-day
observation period. Body weights were recorded weekly. Surviving birds
were killed but not necropsied. Neurotoxicity was assessed after a
single subcutaneous dose of 55 mg/kg bw to 16 birds or 55 mg/kg bw
orally to 30 birds; a control group of 14 birds was dosed orally, and
a positive control group of six birds received 500 mg/kg bw of
tri- ortho-cresyl phosphate (TOCP) as a single oral dose in corn oil.
All birds were starved overnight before treatment. In an experiment
conducted before the main study to evaluate the protective
effectiveness of atropine, it was shown to have no protective value at
twice the LD50 value. Birds dosed subcutaneously with dimethoate were
included for comparative biochemical assays. Three birds in the
treated and negative control groups were used to determine
cholinesterase and neuropathy target esterase activity 4 and 48 h
after dosing; these assays were performed for three TOCP-dosed birds
only 48 h after dosing. Analyses of the formulation used indicated
satisfactory content and stability over 24 h. Dosing was followed by
an observation period of 21 days. Birds were assessed daily for ataxia
by observing their ability to walk and to jump onto and off an
obstacle. Body weights were recorded weekly. Nervous tissue from three
TOCP-dosed birds, six negative controls, and six birds dosed orally
with dimethoate was examined histologically.
In the study to determine the LD50, toxicity was seen in a
dose-related manner. Deaths occurred at doses > 45 mg/kg bw, and
all birds at doses > 100 mg/kg died. Deaths occurred up to three
days after dosing, and the surviving birds were normal by day 5. The
LD50 for dimethoate was calculated to be 55 mg/kg bw, with a 95%
confidence interval of 45-67 mg/kg bw. The body weights of survivors
were decreased during the week after treatment but subsequently
increased. The three birds dosed with TOCP that were not sacrificed
for enzyme assays at 48 h developed signs of delayed neurotoxicity
after 13 days, although no clinical signs of cholinesterase inhibition
were seen. All of the birds treated subcutaneously with dimethoate
died within 48 h, after showing clinical signs of cholinesterase
inhibition. After oral administration of 55 mg/kg bw dimethoate, all
birds showed clinical evidence of cholinesterase inhibition. Twelve of
these birds that survived to termination had recovered by day 6 of the
observation period, and none showed signs of delayed neurotoxicity; 12
birds in this group died within 48 h of dosing. Body-weight losses
of up to 10% were seen in treated birds during the first week of
observation, but these losses were subsequently recovered. Microscopic
examination indicated no difference between controls and birds treated
with dimethoate. Brain acetylcholinesterase activity was markedly
reduced in both groups treated with dimethoate; this was more marked
(90% inhibition relative to controls) 4 h after dosing than after 48 h
(61 and 75% inhibition after subcutaneous and oral administration,
respectively). Brain neuropathy target esterase activity was slightly
lower than that in controls in birds treated with dimethoate, but was
markedly lower in TOCP-treated birds. Spinal cord neuropathy target
esterase activity was reduced in TOCP-treated birds but was unaffected
in those that received dimethoate (Redgrave et al., 1991).
Groups of nine or 10 white Leghorn hybrid chickens were given
graded doses of technical-grade dimethoate up to 33 mg/kg bw for three
days, based on the oral LD50 in Japanese quail, in a study designed
to accord with the guidelines then current in eastern Germany and
Poland. Negative and positive controls (TOCP) were used. Antidotes
(atropine sulfate and obidoxime chloride) were administered to treated
birds but not to controls. The route of administration was not stated
but is assumed to have been oral. The precise study design was not
clear from the translation of the original document but indicated
administration of a further series of three doses at intervals
determined by the condition of the birds. Deaths occurred despite use
of the antidotes. Signs of reaction indicative of cholinesterase
inhibition were seen from about 60 min after dosing. Although the
tests indicated that dimethoate has high acute toxicity, there was no
evidence of delayed neurotoxicity, except in the positive controls
(Ministry of Agriculture, Fisheries and Food, 1993a).
(iii) Immunotoxicity
A single dose of dimethoate at 75 mg/kg (route unspecified) to
mice and rats decreased the lymphocyte count to 50% of the value
before exposure and increased the number of neutrophil granulocytes.
After 72 h, these parameters had returned to normal. A reduction in
the thymus cortex, with disrupted lymphocytes, and a reduction in the
number of rosette-forming cells were observed (Tiefenbach & Lange,
1980).
Dimethoate administered to rats at 5-30 mg/kg bw orally or
15 mg/kg bw intramuscularly, twice a week until death, caused
hyperplasia in the bone marrow, resulting mainly in granulocyto-
poiesis. The authors considered the changes to be a direct effect of
dimethoate (Stieglitz et al., 1974).
(iv) Effects on the heart
The effects of dimethoate on the heart have been investigated
in rabbits (Mahkambaeva, 1971), guinea-pigs, and rats (Nadmaiteni &
Marosi, 1983). After oral administration of 150 mg/kg bw to rabbits,
the effects observed included bradycardia and increased atrio-
ventricular and intraventricular conductance, with complete recovery
after four to seven days. In rats and guinea-pigs, a dose-effect
relationship was established for heart rate disturbances and
atrio-ventricular block. An electron microscopic study of the
myocardium showed no changes.
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious electrocardiographic
disturbances developed during the early phase of intoxication. The
toxic cardiac phenomena appeared to be unrelated to the degree of
cholinesterase inhibition but were correlated with the myocardial
concentration of dimethoate. Cardiac failure and death were first
observed at a dose of about 110 mg/kg bw, while a dose of 221 mg/kg bw
resulted in death in all cases. This investigation addressed the
direct effect of dimethoate on the myocardium, independently of its
anticholinesterase action (Marosi et al., 1985a,b).
(v) Studies on metabolites
Omethoate is the oxygen analogue of dimethoate. Information on
the absorption, distribution, excretion, metabolism, and toxicity of
this compound is summarized below, although the original reports were
not available for detailed evaluation.
Absorption, distribution, and excretion of omethoate: After oral
administration of 14C-omethoate at doses of 0.3, 5, or 10 mg/kg bw
to rats, 96-97% of the radiolabel was eliminated in urine, 1-2% in
faeces, and 1% in expired carbon dioxide within 48 h. Intravenous
injection of 0.3 mg/kg bw resulted in a similar, rapid elimination
pattern. Maximal tissue residues were reached 1 h after
administration. After 8 h, about 18% of the residual radiolabel was
found in the body. After two days, < 0.55% of the administered dose
was found. Quantitative analysis and whole-body autoradiography
indicated a relatively homogeneous distribution of 14C activity,
except that a 10-20-fold higher concentration was found in the thyroid
(Weber et al., 1978).
Five male Wistar rats were given 10 mg/kg bw 14C-omethoate
orally in order to obtain preliminary information on pulmonary
excretion. In the main study, groups of five males and five females
received a single intravenous dose of 0.5 mg/kg bw 14C-omethoate, a
single oral dose of 0.5 mg/kg bw 14C-omethoate, 14 daily oral doses
of 0.5 mg/kg bw unlabelled omethoate and a single oral dose of
0.5 mg/kg bw 14C-omethoate on day 15, or a single oral dose of
10 mg/kg bw 14C-omethoate. Urine and faeces were collected over
periods up to 48 h after treatment, and blood samples were collected
until sacrifice 48 h after treatment. Only 0.14% of the administered
radiolabel was detected in expired air. Comparison of the results
obtained with intravenous and oral treatment indicated that > 98%
omethoate had been absorbed from the gastrointestinal tract. Within
48 h, 88-98% of the administered radiolabel was recovered in the
excreta, with 95-98% in the urine and 2-5% in the faeces. Excretion of
the low and the high oral doses was not different in females, but
males at the high dose group tended to excrete more radiolabel in the
faeces than those at the low dose. The maximal plasma concentration
was seen 40-60 min after oral dosing, with an initial half-life of
about 2 h and terminal half-lives of 13-28 h. Less than 0.05% of the
administered dose was found in tissues after 48 h (Ministry of
Agriculture, Fisheries and Food, 1993b).
Biotransformation of omethoate: Urine was collected from two
male rats 12, 24, and 48 h after an oral dose of 50 mg/kg bw
radiolabelled omethoate. The cumulative percentages of administered
radiolabel excreted over the indicated times were 16, 19, and 30%. The
metabolites found in a 24-h composite urine sample by ion-exchange
chromatography were: O,O-dimethylphosphoric acid (34%), unknown A
(52%), O,O-dimethylphosphorothioic acid (9.5%), and unknown B
(4.5%). After treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine within 24 h, while after
treatment with omethoate only 19% was excreted (Dauterman et al.,
1959).
In the experiment conducted by the Ministry of Agriculture,
Fisheries and Food (1993b), the predominant form of excreted
radiolabel was unchanged parent compound (26-62%), with N-methyl-
2-(methylsulfinyl)acetamide accounting for 16-36% and an O-demethylated
omethoate for 4-9%. Pretreatment of animals for 14 days with unlabelled
omethoate followed by a single labelled dose resulted in no significant
difference from the results obtained after a single administration.
Effects of omethoate on enzymes and other biochemical parameters:
Dealkylation of omethoate was proposed to be a significant detoxification
mechanism on the basis of information from assays in fly heads (Aharoni &
O'Brien, 1968). Oxidative metabolism of omethoate results in the
de- N-methyl derivative, which is as toxic as the parent compound
although less active as a cholinesterase inhibitor (Lucier &
Menzer, 1970). Kinetic studies indicated that the reaction between
acetylcholinesterase and omethoate was irreversible and bimolecular.
Omethoate was 75-100 times more potent than dimethoate in inhibiting
rat brain acetylcholinesterase activity.
Acute toxicity of omethoate: The signs of poisoning after a
single dose of omethoate are typical of cholinergic stimulation, as
elicited by other organophosphorus esters. The signs appear 5-60 min
after dosing and include salivation, lacrimation, and tremors. They
may persist for one to three days (Kimmerle, 1968). The LD50 values
are summarized in Table 5.
Short-term toxicity of omethoate: Groups of 50 male and 50
female BOR:NMRI mice were fed diets containing omethoate (purity,
97.1%) providing doses of 0, 1, 3, or 10 ppm for four weeks and were
killed at intervals to investigate brain acetylcholinesterase
activity. There were no clinical signs of reaction to treatment, and
food and water intake, mortality, and body-weight gain were also
unaffected. The cholinesterase activity in plasma was clearly lower
than that in controls in mice receiving 10 ppm, but the differences
were smaller and much less consistent in erythrocytes. Plasma and
erythrocyte cholinesterase activity in mice at 1 and 3 ppm showed no
consistent differences from controls. Brain acetylcholinesterase
activity was clearly depressed in animals at 10 ppm. Inhibition of
brain acetylcholinesterase activity at 3 ppm was inconsistent, but the
level was up to 30% lower than that in contemporary controls. In
animals at 1 ppm, brain acetylcholinesterase activity was biologically
significantly lower than in controls only on day 3 in males (Ministry
of Agriculture, Fisheries and Food, 1993).
Groups of 15 male and 15 female rats (30 of each sex as controls)
were fed diets containing omethoate providing doses of 0, 2.5, 5, 15,
50, or 150 ppm for four months. Signs of cholinergic stimulation was
seen at doses > 15 ppm. Cholinesterase activity was depressed in
females at 50 and 150 ppm and in males at doses > 5 ppm. No effects
were noted on growth, organ weights, blood parameters, or urinary
parameters at levels < 50 ppm. Animals at 150 ppm died or had
depressed body weights and food consumption, and the relative liver
weight in males was increased (Löser & Lorke, 1967).
Groups of 15 male and 15 female rats were fed diets containing
omethoate to provide doses of 0, 0.5, 1.0, 2.0, or 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident in
animals at 4 ppm. Erythrocyte acetylcholinesterase activity was
depressed in animals at 2 ppm, but the effect was only slight in
females. In rats at 4 ppm, the inhibition was 30-50%. No effects were
noted on growth, food consumption, blood parameters, liver and kidney
function tests, organ weights, or histological appearance of tissues
(Löser, 1968).
Table 5. Acute toxicity of omethoate in experimental animals
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse Male Oral 36 Kimmerle (1968)
Mouse Male Oral 27 Santi & de Pietri Tonelli (1960)
Mouse Male Intraperitoneal 13 Lucier & Menzer (1970)
Mouse Male Intravenous 23 Kimmerle (1962)
Rat Male, female Oral 28-65 Kimmerle & Lorke (1967)
Rat Male, female Oral 50 Ben-Dyke et al. (1970)
Rat Male, female Oral 22-28 Ministry of Agriculture,
Fisheries and Food (1993b)
Rat Male Intraperitoneal 14 Kimmerle (1968)
Rat Male Intraperitoneal 38 Kimmerle (1962)
Rat Male, female Dermal 145-232 Ministry of Agriculture,
Fisheries and Food (1993b)
Rabbit Male Oral 50 Kimmerle (1962)
Guinea-pig Male Oral 100 Kimmerle (1962)
Cat Male Oral 50 Kimmerle (1962)
Chicken Male Oral 125 Kimmerle (1962)
Chicken Male Oral 100 Levinskas & Shaffer (1965)
Groups of six male and six female beagle dogs received omethoate
(purity, 97.1%; dissolved in acidulated water) daily for 12 months
by stomach tube at doses of 0, 0.02, 0.125, or 0.625 mg/kg bw.
Administration by gavage was chosen due to the reported instability of
the test material in dietary admixture. The appearance and behaviour
of the animals were normal, and no clinical signs attributable to
treatment were observed. All of the animals survived the treatment.
No significant differences were seen between the control and treated
groups with respect to reflexes, ophthalmoscopic parameters, body
temperatures, pulse rate, food and water consumption, mean
body weight, or haematological, clinical chemical (except for
cholinesterase activity), or urinary parameters. Clear depression
of plasma cholinesterase activity was observed only in rats at
0.625 mg/kg bw, amounting to 25-32% of the control value in males
and 16-29% in females. The depression remained essentially
constant throughout the study. A marked depression of erythrocyte
acetylcholestinerase activity was measured in males (17-40%) and
females (22-40%) at 0.625 mg/kg bw, which varied only slightly during
the study. At 0.125 mg/kg bw, only males showed slight (< 28%)
depression of erythrocyte acetylcholinesterase activity during the
first third of the study. Brain acetylcholinesterase activity was
depressed in males at 0.125 mg/kg bw (by 20%) and 0.625 mg/kg bw (39%)
and in females at 0.625 mg/kg bw (30%). The absolute and relative
organ weights were not significantly different between the control and
treated groups. Gross pathological and histopathological examination
showed no dose-related findings. The NOAEL was 0.62 mg/kg bw for
somatic effects and 0.02 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity (Hoffmann & Schilde, 1984).
Long-term toxicity and carcinogenicity of omethoate: Groups of
50 male and 50 female BOR:CWF1 mice were fed diets containing
omethoate (purity, 94%) providing levels of 0, 1, 3, or 10 ppm for 24
months. Appearance, behaviour, and activity were not significantly
different between the control and treated groups, and total and mean
daily food consumption were essentially the same in all the animals.
The body weights of treated males were generally higher than those of
the controls throughout the experiment, whereas those of the females
were no different from controls. Mortality and the frequency
distribution of mortality were comparable in all the groups. The
mortality rate at 18 months was 12-27% for males and 14-31% for
females. The absolute and relative organ weights of control and
treated groups showed no dose-related, significant differences. Gross
anatomical and histopathological examination revealed a range of
non-neoplastic changes commonly observed in old mice. Comparison of
these changes by type, site, and frequency distribution by sex and
dose gave no indication of treatment-related toxic effects. Neoplastic
changes were found primarily in the lungs, liver, adrenal cortex,
and haematopoietic system. Neither the type, site, or frequency
distribution of tumours by sex and dose level nor the numbers of
tumour-bearing mice, mice with benign tumours, mice with malignant
tumours, or mice with both benign and malignant tumours indicated
effects of treatment. The NOAEL for somatic effects was 10 ppm, equal
to 2.1 mg/kg bw per day for male mice and 3.1 mg/kg bw per day for
female mice (Kroetlinger & Löser, 1982).
Four groups of 50 male and 50 female Wistar rats were maintained
for 24 months on a diet containing omethoate providing concentrations
of 0, 0.3, 1,3, or 10 ppm. The control group consisted of 100 males
and 100 females. Omethoate did not clearly affect behaviour, body
weight, survival, food intake, or haematological, clinical chemical,
or urinary parameters. Plasma and erythrocyte cholinesterase
activities, measured in five males and five females from each group
at 1, 2, 4, 8, 13, 26, 52, and 78 weeks and at the end of the study,
were significantly depressed in all animals at 10 ppm. Erythrocyte
acetylcholinesterase activity was also inhibited in animals of each
sex at 3 ppm. The suppression of brain acetylcholinesterase activity,
measured in 10 males and 10 females per group, was dose-related in
animals at 3 and 10 ppm; it was also significantly affected in females
at 1 ppm, which can be considered the marginal no-effect level. Gross
and microscopic examination revealed no diverse effect of omethoate.
The tumour incidence was not clearly affected by treatment (Bomhard
et al., 1979).
Reproductive toxicity of omethoate: Groups of 10 male and 20
female FB 30 Long-Evans rats were fed diets containing omethoate
(purity, 94%) to give concentrations of 0, 1, 3, or 10 ppm for about
10 weeks, after which they were mated to initiate a three-generation
study of reproductive toxicity with two litters per generation.
Immediately after birth, the litters were examined for malformations.
Four days after birth, the litters were reduced to 10. When the
offspring were three weeks old, they were killed and subjected to
gross examination. Ten males and 10 female rats of the F3b generation
at all doses were examined histopathologically four weeks after birth.
There were no clear effects either on mating performance, pregnancy
rate, mortality, or the type and distribution of abnormalities. The
size of the litters of the second generation at 3 and 10 ppm was
reduced, and in the F2b generation, litter size was reduced at both 3
and 10 ppm after four days and at 10 ppm only after 28 days. Since
this effect was observed in only one progeny generation, 3 ppm was the
NOAEL (Löser, 1981).
In a two-generation study, omethoate was administered to groups
of 25 male and 25 female Wistar rats in the drinking-water at levels
of 0, 0.5, 3, or 18 ppm throughout a 70-day premating period and
throughout pairing, gestation, and lactation during breeding of a
single litter in each of the F1 and F2 generations. Reproductive
performance was adversely affected at 18 ppm, with a reduced
implantation rate, increased postnatal loss, and retarded pup weight
gain in both generations and increased precoital time, an increased
number of non-pregnant females, and increased postimplantation loss in
the F1 generation. Histopathological examination revealed an
increased incidence of epithelial vacuolation in the epididymides of
males treated with 18 ppm. The NOAEL for reproductive effects was
3 ppm, equivalent to 0.2 mg/kg bw per day. There was no NOAEL
for general toxicological effects, since erythrocyte and brain
acetyl-cholinesterase activities were inhibited at the lowest dose
(Ministry of Agriculture, Fisheries and Food, 1993).
Developmental toxicity of omethoate: Groups of 20-24 pregnant
rats were given omethoate orally at doses of 0, 0.3, 1, or 3 mg/kg bw
on days 6-15 of gestation. The animals were killed on day 20 of
gestation, and the fetuses were examined for skeletal and tissue
abnormalities. The fetuses and placentas of the animals at 3 mg/kg bw
weighed less than those of the controls. Other reproductive parameters
were unaffected. No teratogenic effect was observed (Machemer, 1975).
Groups of 14 pregnant New Zealand white rabbits were treated
daily by gavage with omethoate (purity, 96.8%) dissolved in distilled
water at doses of 0, 0.1, 0.3, or 1 mg/kg bw on days 6-18 of
gestation. On day 29 of gestation, the animals were killed and their
uterine contents examined. Whole-blood cholinesterase activity was
determined before treatment on day 6 of gestation and 2 h after
treatment on day 18 of gestation. The general condition of control and
treated females was comparable throughout the study. Maternal mean
body weights and corrected day-29 body weights were unaffected by the
treatment. Mortality, the incidence of abortions and total litter
losses, and the number of pregnant females with viable young on day 29
were not altered by treatment. Whole-blood cholinesterase activity was
significantly depressed only among females at 1 mg/kg bw in comparison
with both the pretreatment level and the control level after
treatment. There were no treatment-related differences between the
control and treated groups with respect to corpora lutea count,
implantations, male and female viable young, early and late
resorptions, pre- and postimplantation losses, or fetal and placental
weights. Examination of fetuses at necroscopy on day 29 of gestation
or after skeletal investigation revealed a number of non-dose-related
findings of types and incidences previously recorded in this strain of
rabbit and in the laboratory that performed the study. The NOAEL for
developmental toxicity was 1 mg/kg bw (Tesh et al., 1982).
Genotoxicity of omethoate: Omethoate has been extensively
tested in assays for mutagenicity in vitro. Positive results were
obtained in Salmonella, in one assay for gene mutation in mammalian
cells, and in assays for clastogenicity. Omethoate has also been
extensively tested in vivo. Negative results were obtained for
end-points in the bone marrow, liver, and germ cells, but a positive
result was obtained in a mouse spot test. The results of assays for
the genotoxicity of omethoate are summarized in Table 6.
Neurotoxicity of omethoate: Groups of 10 hens were given
omethoate orally at the LD50 (92 mg/kg bw) with atropine, and and
five positive controls were given TOCP at 350 mg/kg bw. Although
several hens treated with omethoate died, none showed clinical signs
of delayed neurotoxicity. Clinical signs were observed in those
treated with TOCP (Kimmerle, 1972). Histological examination of
nervous tissue with haematoxylin and eosin staining showed
degeneration in the hens treated with TOCP but not in those given
omethoate (Newman et al., 1972).
Groups of two to four hens were treated orally with omethoate
dissolved in corn oil at doses of 20-300 mg/kg bw, which were four
to eight times the unprotected LD50, under eserine and atropine
protection. The omethoate used was a sample that had caused a fatal
human poisoning accident. The acetylcholinesterase and neurotoxic
esterase activities of brain homogenates were assayed for 24 h after
dosing, and pair-dosed birds that survived were observed for signs of
ataxia for three to four weeks. Hens treated at four times the LD50
showed no inhibition of neurotoxic esterase at 24 h and no signs of
ataxia. Those treated at eight times the LD50 did not survive,
despite treatment with high doses of atropine; however, the neurotoxic
esterase activity in the brains of the animals that died within 36 h,
measured immediately after death, was found to be normal. Acute
cholinergic symptoms in all the birds were correlated with strong
inhibition of brain acetylcholinesterase activity, but 70% inhibition
in a bird treated with 20 mg/kg bw of omethoate still did not produce
detectable signs of acute poisoning.
The capacity of pure omethoate and of the incriminated sample to
inhibit neurotoxic esterase and acetylcholinesterase activities were
measured in hen and human brain tissue in vitro. As the IC50 for
acetylcholinesterase in both tissues was 0.08-0.15 mmol/litre, it
would be virtually completely inhibited at 5 mmol/litre, the
concentration that caused no detectable inhibition of neurotoxic
esterase. The activities of both enzymes were also measured in
cortical tissue samples taken 24 h post mortem from a 30-year-old
male farmer who had been acutely poisoned by a commercial formulation
of omethoate. The neurotoxic esterase activity was within the normal
range, while acetylcholinesterase activity was strongly inhibited. It
was concluded that omethoate is extremely unlikely to cause delayed
neuropathy in humans (Lotti et al., 1981).
Table 6. Results of tests for the genotoxicity of omethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0-12 500 µg/plate 95.1 Weakly positive Herbold (1980)
TA98, TA100, in TA98, TA100, and
TA1535, TA1537 TA1535a Negative in
TA1537a
DNA repair E. coli (pol) 0-10 000 µg/plate 96 Negative Herbold (1983)
Gene mutation Chinese hamster 0-6 mg/ml 97.4 Positivea Ministry of Agriculture,
ovary cells (hprt) Fisheries and Food
(1993)
Cell mutation L5178Y mouse 0-5000 µg/ml 96.9 Negativea Bootman & Rees (1982)
lymphoma cells
Sister chromatid Chinese hamster 0-1000 µg/ml 96 Positive at > 250 µg/ml Ministry of Agriculture,
exchange ovary cells Fisheries and Food
(1993)
Gene conversion; S. cerevisiae 0-66.7 µl/ml 96.9 Negative Hoorn (1982, 1983)
reverse mutation Positive
In vivo
Micronucleus Mouse 2 × 6 or 12 97.1 Negative Herbold (1981)
formation mg/kg bw
Dominant lethal Mouse 0.5 mg/kg bw 95.4 Negative Machemer (1974)
mutation
Unscheduled DNA Wistar rat 0-30 mg/kg bw 96.6 Negative Ministry of Agriculture,
synthesis Fisheries and Food
(1993)
Spot test C57Bl/6J × T 0-16 mg/kg bw 96.7-97% Positive Ministry of Agriculture,
mice Fisheries and Food
(1993)
a With and without metabolic activation
3. Observations in humans
The results of two studies of dimethoate in humans were
summarized briefly in the report of the 1963 JMPR (Annex 1, reference
2). In the first study, 20 volunteers were given daily doses of 2.5 mg
dimethoate in aqueous solution (corresponding to approximately
0.04 mg/kg bw) for four weeks. No toxic effect was observed, and there
were no changes in blood cholinesterase activity. Similar results were
reported in single subjects who ingested 9 mg (0.13 mg/kg bw) or 18 mg
(0.26 mg/kg bw) for 21 days (Sanderson & Edson, 1964).
The results of a number of studies in which human volunteers with
no occupational exposure to organophosphate pesticides were given
dimethoate were summarized in Environmental Health Criteria monograph
No. 90 (WHO, 1989) and are presented in Table 7. The studies of
volunteers indicate that repeated doses of up to 0.2 mg/kg bw
dimethoate do not inhibit cholinesterase activity in the blood.
Table 7. Results of controlled human trials with dimethoate
No. of Sex Route Daily dose Duration of Results Reference
subjects exposure
20 NR Oral 0.04 mg/kg 4 weeks No toxic effects or inhibition of Sanderson & Edson
blood ChE (1964)
2 NR Oral 0.13 mg/kg 21 days No toxic effects or inhibition of Sanderson & Edson
0.26 mg/kg blood ChE (1964)
5 M Oral 0.25 mg/kg Single No toxic effects or inhibition of Sanderson & Edson
dose blood ChE (1964)
50 NR Dermala 2.5 ml 2 h No irritation or inhibition of blood Sanderson & Edson
ChE (1964)
12 M+F Oral 5 mg (0.068 mg/kg bw) 28 days No significant change in whole-blood Edson et al. (1967)
ChE
9 M+F Oral 15 mg (0.202 mg/kg bw) 39 days No significant change in whole-blood Edson et al. (1967)
ChE
8 M+F Oral 30 mg (0.434 mg/kg bw) 57 days Inhibition of ChE by day 20 (24%) Edson et al. (1967)
6 NR Oral 45 mg (0.587 mg/kg bw) 45 days Inhibition of ChE (35%) Edson et al. (1967)
6 M+F Oral 60 mg (1.02 mg/kg bw) 14 days Inhibition of ChE (21%) Edson et al. (1967)
NR, not reported; ChE, cholinesterase
a Patch test with 32% liquid formulation
Comments
Dimethoate
Dimethoate is rapidly and extensively absorbed from the gut and
rapidly excreted. There was no accumulation in fat tissue. In rats and
humans, up to 90% of radiolabel was found in the urine within 24 h.
The report of a study with methylcarbamoyl-labelled dimethoate
indicated that up to 18% of the administered label was excreted in
expired air. Four metabolites with anticholinesterase activity have
been identified in rats and humans. One seems to result from thiono
oxidation, leading to the formation of the oxygen analogue of
dimethoate, i.e. omethoate; this step was followed by hydrolysis to
a thiocarboxyl product, said to be the main metabolite in rats and
humans.
Data on the acute oral toxicity of dimethoate gave LD50 values
of about 310 mg/kg bw in rats, 150 mg/kg bw in mice, and 55 mg/kg bw
in hens. The signs of toxicity were those typical of cholinesterase
inhibition. WHO has classified dimethoate as 'moderately hazardous'
(WHO, 1996).
In short-term and long-term studies at dietary concentrations
> 75 ppm, there were minor reductions in body-weight gain and food
consumption. Apart from inhibition of cholinesterase activity,
dimethoate had no effect on the composition of the blood or urine. The
liver weights of animals treated at the higher doses tended to be
lower than those of the control groups: there were, however, no
microscopic changes, and the effect is unlikely to be of toxicological
significance. Investigations of toxicity at higher doses were limited
by effects due to cholinesterase inhibition. The NOAELs were thus
generally based on reductions in acetylcholinesterase activity in
the brain or erythrocytes. On the basis of minimal reductions in
acetylcholinesterase activity of 10-20%, the NOAEL in a 12-month study
in dogs at doses of 0, 5, 20, or 125 ppm was 5 ppm, equal to 0.2 mg/kg
bw per day; in rats, the NOAEL in a life-span study at doses of 0, 1,
5, 25, or 100 ppm was 1 ppm, equal to 0.04 mg/kg bw per day. In mice,
an NOAEL was not identified, as cholinesterase activity was depressed
at all doses after 52 weeks of treatment in a life-span study at doses
of 0, 25, 100, or 200 ppm.
The results of long-term studies of toxicity and carcinogenicity
in mice (at 0, 25, 100, or 200 ppm) and rats (at 0, 5, 25, or 100 ppm)
reported in 1986 and studies reported in 1977 indicate that dimethoate
is not carcinogenic to rodents.
In a multigeneration study of reproductive toxicity conducted in
1989-90 with doses of 0, 1, 15, or 65 ppm, reproductive performance
of rats was impaired at the high dose. The NOAEL for reproductive
toxicity appeared to be 15 ppm (equal to 1.2 mg/kg bw per day) and
that for parental toxicity was 1 ppm (equal to 0.08 mg/kg bw per day)
on the basis of cholinesterase inhibition, but the Meeting noted that
there was some indication that reproductive performance may have been
affected at lower doses. In a multigeneration study conducted in mice
in 1965 at doses of 0, 5, 15 or 50 ppm, there was no overt effect on
reproductive capacity, even in the presence of cholinergic toxicity.
In a poorly reported study in rabbits, sperm numbers and quality were
adversely affected at doses equivalent to one-tenth and one-hundredth
of the LD50.
Studies of developmental toxicity in rats (at 0, 3, 6, or
18 mg/kg bw per day on days 6-15 of gestation) and rabbits (at 0, 10,
20, or 40 mg/kg bw per day on days 7-19 of gestation) provided no
evidence of a teratogenic effect, although maternal toxicity was
observed at the high dose in rats and at the high and middle doses in
rabbits.
After reviewing the data available on mutagenicity, the Meeting
concluded that although in-vitro studies indicate that dimethoate has
mutagenic potential, this potential does not appear to be expressed
in vivo.
Undiluted dimethoate formulations were irritating to the eye in
rabbits. Skin irritation was minimal and confined to slight, transient
erythema. Dimethoate was not a skin sensitizer in guinea-pigs, but a
32.7% emulsifiable concentrate formulation induced sensitization in
one of 10 guinea-pigs. In a published paper, dimethoate was cited
in four human cases of contact dermatitis, and sensitization was
confirmed in these individuals by patch testing.
In hens given a single dose of 55 mg/kg bw by subcutaneous
injection or orally, dimethoate did not induce delayed neurotoxicity.
In a 39-day study in nine male and female volunteers, the NOAEL
for cholinesterase inhibition was 0.2 mg/kg bw per day. This NOAEL was
supported in seven other studies each involving 6-20 volunteers who
received doses ranging from 0.04 to 1.0 mg/kg bw per day for up to 57
days.
Omethoate
The oral LD50 of omethoate was approximately 25 mg/kg bw
in rats. Signs of reaction to treatment with omethoate were those
consistent with cholinesterase inhibition.
In short-term and long-term studies, the potential toxicity of
omethoate was limited by the onset of cholinesterase inhibition. In a
12-month study of toxicity in dogs at doses of 0, 0.025, 0.12, or
0.62 mg/kg bw per day by gavage, the NOAEL was 0.02 mg/kg bw per day
on the basis of inhibition of acetylcholinesterase activity. In
life-span studies in rats (at 0, 0.3, 1, 3, or 10 ppm) anti mice (at
0, 1, 3, or 10 ppm), there was no evidence of oncogenic potential. The
study in mice was unsuitable for deriving an NOAEL because acetyl-
cholinesterase activity was not investigated; the NOAEL in rats was
0.3 ppm (0.015 mg/kg bw per day) on the basis of inhibition of
acetylcholinesterase activity.
In multigeneration studies in rats at 0, 1, 3, or 10 ppm, a
dietary concentration of 10 ppm was associated with reduced viability
of pups; there was evidence that this effect extended to animals
treated at 3 ppm. The NOAEL was 1 ppm (equivalent to 0.05 mg/kg bw per
day). In a further multigeneration study in rats at doses of 0, 0.5,
3, or 18 ppm in the drinking-water, there was evidence of epididymal
vacuolation and fewer pups per dam at the high dose; these pups had
lower weight gains and were less viable. The precoital time was
increased and the number of non-pregnant females was greater than
among controls. The NOAEL for reproductive performance was 3 ppm
(equivalent to 0.2 mg/kg bw per day), but cholinesterase inhibition
was detected at the lowest dose of 0.5 ppm. In studies of
developmental toxicity, there was no evidence of teratogenicity in
rats given 0, 0.3, 1, or 3 mg/kg bw omethoate per day on days 6-15 of
gestation or in rabbits given 0, 0.1, 0.3, or 1 mg/kg bw omethoate per
day on days 6-18 of gestation.
Omethoate has been extensively investigated for mutagenicity in
vitro and in vivo. The Meeting concluded that it has clear mutagenic
potential but that the weight of the evidence observed in vivo was
negative; however, the positive result obtained in the mouse spot test
could not be completely disregarded.
In studies in hens given single oral doses of 20-300 mg/kg bw,
omethoate did not induce delayed neurotoxicity.
Conclusions
An ADI of 0-0.002 mg/kg bw was established for dimethoate on the
basis of the apparent NOAEL of 1.2 mg/kg bw per day for reproductive
performance in the study of reproductive toxicity in rats and applying
a safety factor of 500. Although a safety factor of 100 would normally
be used in deriving an ADI from a study of this type, the Meeting was
concerned about the possibility that reproductive performance may have
been affected at 1.2 mg/kg bw per day in this study and therefore used
a higher-than-normal safety factor. No data are available to assess
whether the effects on reproductive performance were secondary to
inhibition of cholinesterase. The Meeting concluded that it was
not appropriate to base the ADI on the results of the studies of
volunteers since the crucial end-point (reproductive performance) has
not been assessed in humans.
This ADI would usually be used only when assessing the intake of
dimethoate itself. As the use of dimethoate on crops can give rise to
residues of omethoate, and omethoate has been used as a pesticide in
its own right, previous Joint Meetings have allocated an ADI to
omethoate; however, the primary manufacturer is no longer producing
omethoate. The Meeting noted that omethoate is considerably more toxic
than dimethoate; however, the levels of residues of omethoate
resulting from use of dimethoate on crops are likely to be low. The
Meeting therefore recommended that residues of dimethoate and
omethoate resulting from the use of dimethoate be expressed as
dimethoate and be assessed in comparison with the ADI for dimethoate.
As the primary manufacturer is no longer producing either
omethoate or formothion, toxicological data on these compounds were
not made available to the Meeting. The previous ADIs of 0-0.0003 mg/kg
bw for omethoate and 0-0.02 mg/kg bw for formothion were therefore
withdrawn.
There may be a need to re-evaluate the toxicity of dimethoate
after the periodic review of the residue and analytical aspects of
dimethoate has been completed if it is determined that omethoate is a
major residue.
Toxicological evaluation
Levels that cause no toxic effect (dimethoate)
Rat: 1 ppm, equal to 0.04 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
15 ppm, equal to 1.2 mg/kg bw per day (reproductive
performance in a study of reproductive toxicity)
1 ppm, equal to 0.08 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
6 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.2 mg/kg bw per day (52-week study of
toxicity)
Human: 0.2 mg/kg bw per day (39-day study of cholinesterase
inhibition)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw (sum of dimethoate and omethoate expressed as
dimethoate)
Studies that would provide information useful for continued evaluation
of the compound
1. Further multigeneration study in rats using dimethoate
2. Mouse spot test using dimethoate
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to dimethoate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 310 mg/kg bw
Dermal, toxicity, rat LD50 > 7000 mg/kg bw
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, human Sensitizing
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL > 1000 mg/kg bw per day (highest dose tested)
Repeated oral, reproductive toxicity, rat NOAEL = 1.2 mg/kg bw per day, reproductive toxicity
NOAEL = 0.05 mg/kg bw per day, parental toxicity
Repeated oral, developmental toxicity, rat NOAEL = 6 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 18 mg/kg bw per day (highest dose tested)
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 40 mg/kg bw per day (highest dose tested)
Long term (> 1 year) Repeated oral, toxicity and carcinogenicity, rat NOAEL = 0.04 mg/kg bw per day, cholinesterase
inhibition
References
Aharoni, A.H. & O'Brien, R.D. (1968) The inhibition of acetyl-
cholinesterases by anionic organophosphorus compounds. Biochemistry,
7, 1538.
Ahmed, F.E., Hart, R.W. & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res., 42, 161-174.
Becker, H. (1985) Dominant lethal study with dimethoate technical in
the mouse. Unpublished report No. 039003 from Research & Consulting
Company AG, Itingen, Switzerland. Unpublished report submitted to WHO
by the Dimethoate Task Force, Ingelheim, Germany.
Ben-Dyke, R., Sanderson, D.M. & Noakes, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9, 119.
Bhunya, S.P. & Behera, J. (1975) Centromeric sensitivity of mouse
chromosomes to the systemic insecticide dimethoate (Rogor). Curr. Sci.,
44, 859-860.
Bomhard, E., Loser, E. & Kaliner, G. (1979) S-6876: Chronic toxicity
study on rats (two-year feeding experiment). Unpublished report from
the Bayer AG Institut für Toxikologie, originally submitted to WHO by
Bayer AG, summary from WHO.
Bootman, J. & Rees, R. (1982) S-6876: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report from Life Science Research, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
Brady, U.E., Jr & Arthur, B.U. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56, 477-482.
Brooker, A.J., Homan, B.A., Parker, C.A., Offer, J.M., Anderson, A. &
Dawe, I.S. (1992) The effect of dimethoate on reproductive function
of two generations in the rat. Unpublished report from Huntingdon
Research Centre, United Kingdom. Submitted to WHO by the Dimethoate
Task Force, Milan, Italy.
Budreau C.H. & Singh, R.P. (1973) Effect of fenthion and dimethoate on
reproduction in the mouse. Toxicol. Appl. Pharmacol., 26, 29-38.
Burford, P., McLean, T.A., Buist, D.P., Crook, D., Gregson, R.L. &
Gopinath, C. (1991) Dimethoate: 12 month dietary toxicity study in
beagle dogs. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Casida, J.E. & Sanderson, D.M. (1962) Toxic hazards from formulating
the insecticide dimethoate in methyl 'cellosolve'. Nature, 189,
507-508.
Chamberlain, W.R., Gatterdam, P.E. & Hopkins, D.E. ( 1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54, 733-740.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorous pesticides.
Mutat. Res., 88, 307-316.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health, B20, 373-406.
Dal Re, V. & Vola Gera, F. (1976) Determination of the acute dermal
toxicity of technical Rogor. Unpublished report from Centro Ricerche
Antiparassitari, Montedison, Italy. Submitted to WHO by Farmoplant,
Milan, Italy.
Dal Re, V. & Vola Gera, F. (1980) Acute oral toxicity of technical
Rogor samples recently produced in albino rats. Unpublished report
from Centro Ricerche Antiparassitari Montedison, Italy. Submitted to
WHO by Farmoplant, Italy.
Dauterman, W.C., Casida, J.E., Knaak, J.B. & Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agric. Food Chem., 7, 188-193.
Degraeve, N. & Moutschen, J. (1983) Genotoxicity of an organophosphorus
insecticide, dimethoate, in the mouse. Mutat. Res., 119, 331-337.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo.
Arch. Toxícol., 58, 152-156.
Ecobichon, D.J. & Kalow, W. (1963) Action of organo phosphorus
compounds upon esterases in human liver. Can. J. Biochem., 41, 1537.
Edson, E.F. & Noakes, D.N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. Appl. Pharmacol.,
2, 523.
Edson, E.F., Jones, K.H. & Watson, W.A. (1967) Safety of dimethoate
insecticide. Br. Med. J., iv, 554.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984a) Effect of dimethoate
on pregnancy of the rat. Unpublished report from Huntington Research
Centre, Huntington, Cambs, United Kingdom. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984b) Effect of dimethoate
on pregnancy of the New Zealand white rabbit. Unpublished report from
Huntington Research Centre, Huntington, Cambs, United Kingdom.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
El-Elaimy, I. (1986) Comparative studies on the effect of carbamate
and organophosphorus insecticides on acetylcholinesterase activity in
maternal and foetal tissue of rat. Proc. Zool. Soc. A.R. Egypt, 10,
41-49.
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomatis, L., eds, Chemical Carcinogenesis Essays
(IARC Scientific Publications No. 10), Lyon, International Agency for
Research on Cancer, pp. 161-181.
Fenwick, M.L., Barron, J.R. & Watson, W.A. (1957) Biochem. J., 65, 58.
Fischer, G.W. & Scheufler, H. (1981) Effects of dimethoate and
O-desmethyldimethoate in dominant lethal test in mice. Wiss. Z.
Univ. Halle, 30, 21-29 (in German).
Gabliks, J. (1965a) Responses of cell cultures to insecticides II.
Chronic toxicity and induced resistance. Proc. Soc. Exp. Biol. Med.,
120, 168-171.
Gabliks, J. (1965b) Responses of cell cultures to insecticides. III.
Altered susceptibility to poliovirus and diphtheria toxin. Proc. Soc.
Exp. Biol. Med., 120, 172-175.
Gabliks, J. & Friedman, L. (1965) Responses of cell cultures to
insecticides. I. Acute toxicity to human cells. Proc. Soc. Exp. Biol.
Med., 120, 163-168.
Gibel, U., Lohs, KH., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) Experimental study on cancerogenic, haematotoxic, and
hepatotoxic activity of phosphororganic pesticides. Arch.
Geschwulstforsch., 41, 311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade6
locus of Schizosaccharomyces pombe. Mutat. Res., 117, 139-148.
Gomez-Arroyo, S., Noriega-Aldana, N., Juarez-Rodriguez, D. &
Villalobos-Pietrinir, J. (1987) Sister chromatid exchanges induced by
the organophosphorus insecticides methyl parathion, dimethoate, phoxim
and methyl azinfos in cultured human lymphocytes. Contam. Amb.,
3, 63-70.
Guthrie, F.E., Shah, P.V. & Moreland, D.E. (1980) Effects of pesticides
on active transport of glucose through the isolated intestine of the
mouse. J. Agric. Food Chem., 22, 3.
Hassan, A., Zayed, S.M.A.D. & Bahig, M.R.E. (1969) Metabolic behaviour
of organophosphorus insecticides. XI: Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18, 2429-2438.
Hellwig, J. (1986a) Report on the study of the toxicity of dimethoate
in mice after 78-week administration in the diet. Unpublished report
No. 75C0326/8242 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Hellwig, J. (1986b) Report on the study of the toxicity of dimethoate
in rats after 24-month administration in the diet. Unpublished report
No. 70C0326/8241 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Herbold, B. (1980) S-6876 Salmonella/Mikrosomen Test zur Untersuchung
auf punktmutagene Wirking. Unpublished report from the Bayer AG
Institut für Toxikologie, originally submitted to WHO by Bayer AG,
summary from WHO.
Herbold, B. (1981) S-6876 (Omethoate, Folimat's active ingredient):
Micronucleus test on mouse to evaluate S 6876 for mutagenic potential.
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Herbold, B. (1983) S-6876 (Omethoate, Folimat's active ingredient):
Pol test on E. coli to evaluate for DNA damage. Unpublished report
from Institute of Toxicology, Bayer AG, originally submitted to WHO by
Bayer AG, summary from WHO.
Hoffman, K. & Schilde, B. (1984) S-6876 (Omethoate): Chronic toxicity
to dogs on oral administration (twelve month stomach tube study).
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG. summary from WHO.
Hoorn, A.J.W. (1982) Mutagenic evaluation of S-6876 (omethoate) in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138/S211-alfa. Unpublished report from Litton Bionetics, Netherlands,
originally submitted to WHO by Bayer AG, summary from WHO.
Hoorn, A.J.W. (1983) Evaluation of S-6876 (omethoate) in the induced
mitotic crossing over and gene conversion assay in Saccharomyces
cerevisiae strain D7. Unpublished report from Litton Bionetics,
Netherlands, originally submitted to WHO by Bayer AG, summary from
WHO.
Johnson, E., Caterson, C.R. & Allen, J.S. (1985) Mutagenicity testing
of dimethoate in the in vitro CHO/HGPRT mutation assay. Unpublished
report No. 0423 from American Cyanamid Co., Princeton, NJ, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
von Jung, H.-D. (1989) Kontaktekzem durch pestzide in der DDR.
Dermatol. Monaschr., 175, 203-214 (in German with English summary).
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
R.E. & Treiber, G. (1959) The metabolism of dimethoate in cattle.
J. Econ. Entomol., 52, 1190-1194.
Khera, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for teratogenic
activity in the cat. J. Environ. Pathol. Toxicol., 2, 1283-1288.
Khera, K.S., Whalen, C., Trivett, G. & Angers, G. (1979) Teratogenicity
studies on pesticidal formulations of dimethoate, diuron and lindane in
rats. Bull. Environ. Contam. Toxicol., 22, 522-529.
Kimmerle, G. (1962) Toxicological studies on active ingredient S-6876.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1968) Bayer 45432-Toxikologische Untersuchungen.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1972) Folimat/Akute Neurotoxizitatsuntersuchungen an
Huhnern. Unpublished report from the Institut für Toxikologie,
Wuppertal-Elberfeld, Germany, originally submitted to WHO by Bayer AG,
summary from WHO.
Kimmerle, G. & Lorke, D. (1967) S-6876. Antidotal effect and
potentiation. Unpublished report originally submitted to WHO by Bayer
AG, summary from WHO.
Kroetlinger, F. & Löser, E. (1982) S-6876 (omethoate, the active
ingredient of Folimat): Chronic toxicity study on mice. (2-year
feeding experiment). Unpublished report from Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Levinskas, G.J. & Shaffer, C.B. (1965) Oxygen analogue of dimethoate:
Demyelination in white Leghorn hens. Unpublished report originally
submitted to WHO by American Cyanamid Co., summary from WHO.
Löser, E. (1968) Bayer 45432: Subakute Toxikologische Untersuchungen
an Ratten. Unpublished report originally submitted to WHO by Bayer AG,
summary from WHO.
Löser, E. (1981) S-6876 Folimat-Wirkstoff Multigenerationeversuche an
Ratten. Unpublished report from Bayer AG, originally submitted to WHO
by Bayer AG. Addendum: Histopathology, Consultox Laboratories Ltd,
London, United Kingdom. Summary from WHO.
Löser, E. & Lorke, D. (1967) Bayer 45432: Subchronische Toxikologische
Untersuchungen an Ratten. Unpublished report originally submitted to
WHO by Bayer AG, summary from WHO.
Lotti, M., Ferrara, S.D., Caroldi, S. & Sinigaglia, F. (1981) Enzyme
studies with human and hen autopsy tissue suggest omethoate does not
cause delayed neuropathy in man. Arch. Toxicol., 48, 265-270.
Lucier, G.W. & Menzer, R.E. (1970) Nature of oxidative metabolites of
dimethoate formed in rats, liver microsomes, and bean plants. J. Agric.
Food Chem., 18, 698.
Machemer, L. (1974) Praparat S-6876 to Dominant Letal Test an der
mannlichen Maus zur Prufung auf mutagene Wirkung. Unpublished report
from the Institut für Toxikologie, Wuppertal-Elberfeld, Germany,
originally submitted to WHO by Bayer AG, summary from WHO.
Machemer, L. (1975) Praparat S-6876 to Untersuchung auf Exbryotoxische
and Teratogene Wirkung nach Oraler Verabreichung an der Ratte.
Unpublished report from the Institut für Toxikologie, Wuppertal-
Elberfeld, Germany, originally submitted to WHO by Bayer AG, summary
from WHO.
Madison, W.A., MacKenzie, K.M. & Fields, W.E. (1984) 21 Day dermal
study with dimethoate in rabbits. Unpublished report No. 6123-125
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by the Dimethoate Task Force, Milan, Italy.
Ministry of Agriculture, Fisheries and Food (1993a) Evaluation on
Dimethoate, Pesticides Safety Directorate, United Kingdom.
Ministry of Agriculture, Fisheries and Food (1993b) Evaluation on
Omethoate, Pesticides Safety Directorate, United Kingdom.
Mahkambaeva, Z.G. (1971) Influence of organophosphorus pesticides
(kilval, methylmercaptophos, fosfamid) on bioelectric activity of
heart in experimental animals. In: Hygiene, Use and Toxicology of
Pesticides, and Clinical Aspects of Pesticide Poisoning, Kiev, Zdorovye
Publishers, pp. 175-180 (in Russian).
Marosi, G., Ivan, J., Nagymajtenyi, L., Csatlos, J. & Toszegi, A.
(1985a) Dimethoate induced toxic cardiac failure in the guinea-pig.
Arch. Toxicol., 57, 142-143.
Marosi, G., Ivan, J. & Nagymajtenyi, L. (1985b) Cardiodepression in
organophosphate poisonings. Arch. Toxicol., Suppl. 8, 289-291.
Mohn, G. (1973) 5-Methyl tryptophan resistance mutations in
Escherichia coli K-12. Mutat. Res., 20, 7-15.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nadmaiteni, L. & Marosi, G. (1983) Cardiotoxic action of organo-
phosphorus pesticides. Gig. Sanit., 11, 72-73 (in Russian).
Nagymajtenyi, L. (1988) Neurophysiological markers as early signs of
organophosphate neurotoxicity. Neurotoxicol. Teratol., 10, 429-434.
Nehéz, M., Selypes, A., Scheufler, H. & Fisher, G.U. (1983) Effect of
dimethoate and O-demethyldimethoate on bone marrow cells of CFLP
mice. Regul. Toxicol. Pharmacol., 317, 349-354.
Newman, A.J., Cherry, C. & Urwin, C. (1972) Pathology report of
folimat active substances in hens. Unpublished report from the
Huntingdon Research Centre, Huntingdon, Cambs, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
O'Brien, R.D (1959) Activation of thionophosphates by liver
microsomes. Nature, 183, 121.
O'Brien, R.D (1961) The effect of SKF 525A (2-diethylaminoethyl
2',2-diphenyl valerate hydrocloride) on organophosphate metabolism in
insect and mammals. Biochem. J., 79, 229.
Panshina, T.N. & Klisenko, M.A. (1962) Content of fosfamid and changes
in blood cholinesterase activity in different routes of treatment of
the mammalian organism. In: Industrial Toxicology and Clinical Picture
of Occupational Diseases Due to Chemicals, Moscow, Medgiz, pp. 181-184
(in Russian).
Probst, G.H., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K. &
Neal, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3, 11-32.
Rani, M.V.U., Reddi, O.S. & Reddy, P.P. (1980) Mutagenicity studies
involving aldrin, endosulfan, dimethoate, phosphamidon, carbaryl and
ceresan. Bull. Environ. Contam. Toxicol., 25, 277-282.
Redgrave, V.A., Johnson, A.A., Gopinath, C., Hadley, J.C., Anderson,
A. & Dawe, I.S. (1991) Dimethoate: Acute delayed neurotoxicity in the
domestic hen. Unpublished report from the Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Reena, et al. (1989) Haematological changes induced by dimethoate in
rat. Arh. Hig. Rada Toksikol., 40, 23-27.
Reuber, M.D. (1984) Carcinogenicity of dimethoate. Environ. Res.,
34, 193-211.
Ribelin, W.E., Levinskas, G.J. & Shaffer, C.B. (1965) A 3-generation
reproduction study with mice. Unpublished report. Submitted to WHO by
the American Cyanamid Company.
Riemer, F., Dahlenburg, R. & Grisk, A. (1985) Renal excretion of
dimethylphosphate and its thio derivatives after application of
dimethoate, bromophos, naled or trichlorphon to rats. Nahrung, 29,
299-302 (in German).
Salem, M.H., Abo-Elezz, Z., Abd-Allah, G.A., Hassan, G.A. & Shaker, N.
(1988) Effect of organophosphorus (dimethoate) and pyrethroid
(deltamethrin) pesticides on semen characteristics in rabbits.
J. Environ. Sci. Health, B23, 279-290.
Sanderson, D.M. & Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Br. J. Ind. Med., 21, 52.
San Sebastian, J.R. (1985) In vivo bone marrow cytogenetics rat
metaphase analysis. PH 315-AC-00-84, Dimethoate CL 12880. Unpublished
report from Pharakon Research International, Inc., Waverly, PA, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
Santi, R. & de Pietri-Sonelli, P. (1959) Mode of action and biological
properties of the S-(methylcarbamyl)methyl O,O-dimethyl-
diophosphate. Nature, 183, 398.
Santi, R. & de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido O,O-dimetiliditio-
fosforilacetico. Ist. Ric. Agr. Soc. Montecatini, 3, 3 (in Italian).
Scheufler, H. (1975) Effects of relatively high doses of dimethoate
and trichlorfon on the embryogenesis of laboratory mice. Biol. Rundsch.,
13, 238-240 (in German).
Shaker, N. (1988) In vivo chronic effect of dimethoate and deltamethrin
on rabbits. J. Environ. Sci. Health, B23, 387-399.
Sorg, R.M., (1985) Micronucleus test (MNT). PM 309A-AC-004-84,
Dimethoate CL 12880. Unpublished report from Pharakon Research
International, Inc., Waverly, PA, USA. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Squire, R.A. (1988) An evaluation of vascular proliferative lesions in
male Wistar rats from project 70C0326/8241. Unpublished report from
Robert A. Squire Associates, Ruxton, MD, USA. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorfon and
dimethoate. Acta Haematol., 52, 70-76.
Tesh, J.M., Ross, F.W., Wightman, T.J. & Wilby, O.K. (1982) S-6876:
Effect of oral administration upon pregnancy in the rabbit. Unpublished
report from Life Science Research, United Kingdom, originally submitted
to WHO by Bayer AG, summary from WHO.
Tiefenbach, B. & Lange, P. (1980) Studies on the action of dimethoate
on the immune system. Arch. Toxicol., Suppl. 4, 167-170.
Tripathy, N.K., Majhi, B., Dey, L. & Das, C.C. (1988) Genotoxicity of
Rogor studied in the sex-linked recessive lethal test and wing eye and
female germ-line mosaic assays in Drosophila melanogaster. Mutat. Res.,
206, 351-360.
Uchida, T., Dautermann, W.C. & O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agric. Food Chem., 12, 48-52.
Ullman, L., Sacher, R. & Chevalier, J. (1985) Acute oral toxicity
study with dimethoate in mice. Unpublished report from Research and
Consulting Co., AG, Itingen, Switzerland. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
US National Cancer Institute (1976) Bioassay of Dimethoate for Possible
Carcinogenicity (NCI-CG-TR-4 DHEW Publication No. (NIH) 77-80),
Bethesda, MD, USA.
US National Cancer Institute (1977) Bioassay of Dimethoate for Possible
Carcinogenicity (Technical Report Series No. 4), Bethesda, MD, USA.
Velázquez, A., Xamena, N., Creus A. & Marcos, R. (1986) Indication for
weak mutagenicity of the organophosphorus insecticide dimethoate in
Drosophila melanogaster. Mutat. Res., 172, 237-243.
Vishwanath, R. & Jamil, K. (1986) Mutagenic and genotoxic activities
of certain organophosphorus compounds using Ames Salmonella assay
with and without microsomal induction. Ind. J. Exp. Biol., 24, 305-308.
Weber, H., Patzschke, K. & Wegner, L.A. (1978) Omethoat-C14-Folimat-
Wirkstoff: Biokinetische Untershungen an Ratten. Unpublished report
originally submitted to WHO by Bayer AG, summary from WHO.
West, B., Vidone, L.B. & Shaffer, C.B. (1961) Acute and subacute
toxicity of dimethoate. Toxicol. Appl. Pharmacol., 3, 210.
WHO (1989) Dimethoate (Environmental Health Criteria 90), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutag., 5, 835-846.
Table 1. Acute toxicity of dimethoate in experimental animals
Species Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Mouse Female Oral NR 60 Sanderson & Edson (1964)
Mouse Male, female Oral NR 160 Ullman et al. (1985)
Rat Male, female Oral NR 314 Ministry of Agriculture,
Fisheries and Food (1993a)
Rat Male, female Oral 97.6-99 540-600 Dal Re & Vola Gera (1980)
Rat Male, female Dermal 97.6-99 >7000 Dal Re & Vola Gera (1976)
Rat Male Intravenous NR 450 Sanderson & Edson (1964)
Rat Male, female Intraperitoneal NR 175-350 Sanderson & Edson (1964)
Hamster Male Oral NR 200 Sanderson & Edson (1964)
Guinea-pig Male, female Oral NR 350-600 Sanderson & Edson (1964)
Rabbit Male, female Oral NR 300-500 Sanderson & Edson (1964)
Hen Male, female Oral NR 30-50 Sanderson & Edson (1964)
NR not reported
(b) Short-term toxicity
Rats
Three studies in rats were briefly summarized in the report of
the 1963 JMPR (Annex 1, reference 2). In a 15-week study, groups of 10
male rats were fed diets containing 1, 5, 25, or 125 ppm dimethoate,
equivalent to 0.1, 0.5, 2.5 and 12 mg/kg bw per day. At the high
dietary level, slight muscular fibrillation and depressed weight gain
were observed. At 5, 25, and 125 ppm, depressed cholinesterase
activity was observed. In another study, groups of 20 rats were fed
diets providing 2, 8, or 32 ppm for 90 days or 50, 100, or 200 ppm
for 35 days. No haematological abnormalities were reported nor any
significant pathological change. The highest dose that did not inhibit
cholinesterase activity was reported to be 32 ppm. In a one-year study
with groups of 20 male rats, the highest dose that did not inhibit
cholinesterase activity was reported to be 10 ppm (Edson & Noakes,
1960; West et al., 1961; Sanderson & Edson, 1964).
The report of a 13-week study in Wistar rats was available only
in an incomplete translation and was neither dated nor signed. Groups
of 24 male and 24 female rats received doses of 0.02, 0.2, 2, or
20 mg/kg bw per day for 18 weeks; a group of 32 males and 32 females
acted as controls. Animals were housed eight per cage, and the
doses were administered orally on five clays a week as aqueous
solutions, which were prepared weekly and refrigerated until use. The
formulations were not analysed for content or for stability in the
vehicle. Body weights were recorded weekly, and food consumption was
recorded, but at unspecified intervals. Blood samples were obtained
from eight males and eight females in each treated group and from 16
controls of each sex on four occasions; a normal range of parameters,
including cholinesterase activity, was measured. Urine samples were
obtained in weeks 12 and 18. Brain cholinesterase activity and a renal
function test (phenol red test) were carried out after 18 weeks.
Histological investigations were performed on six animals of each
sex per group, and the list of tissues chosen for weighing was
satisfactory; however, the list of those chosen for histopathological
examination was short and did not include epididymides.
Slightly reduced body-weight gain was seen in animals treated at
20 mg/kg bw throughout the test, and food consumption was slightly
lower in males of this group than in the controls during the latter
half of the study. There were no treatment-related deaths. During
weeks 6-11, animals in all groups, including the controls, had
diarrhoea, but this was more pronounced in animals treated at 2 or
20 mg/kg bw. Minimally lower haematocrit and erythrocyte count were
noted in animals at the high dose after seven weeks only. There were
no other toxicologically significant changes in haematological
parameters. Plasma cholinesterase activity was 45-75% lower in
animals at 20 mg/kg bw than in the controls. Erythrocyte and brain
acetylcholinesterase activity was 70-90% of that in controls for
animals treated at 2 mg/kg bw and 54-77% in animals at 20 mg/kg bw.
Renal function was unimpaired by treatment, but no data were
presented. There were no treatment-related effects on the urine and
there was no faecal occult blood. Necropsy indicated no effects of
treatment. Organ weight analysis indicated a number of intergroup
differences, none of which was clearly of toxicological significance;
however, the absolute and relative weights of the livers of treated
animals tended to be lower than those of the controls, and this is
likely to represent a treatment-related change, as similar effects
have been seen in other studies. There were no microscopic findings
related to treatment, but no data were presented. It was concluded
that the toxicity expressed was minimal and that 0.2 mg/kg bw was the
NOAEL for inhibition of brain and erythrocyte acetylcholinesterase
activity (Ministry of Agriculture, Fisheries and Food, 1993a).
Dimethoate was administered orally to rats on five days a week
for six weeks at a dose of 10 mg/kg bw per day. The investigations
were confined to an electroencephalogram and cholinesterase
determinations. Treatment was associated with increased frequency and
decreased amplitude on the electroencephalogram, and, as expected,
inhibition of cholinesterase activity in the tissues examined
(Nagymajtenyi, 1988). The relevance of these data to the safety
evaluation of dimethoate is equivocal, and they are not considered
further in this review.
Ten male albino rats received dimethoate by intraperitoneal
injection at 150 mg/kg bw in 0.5 ml saline on alternate days for 30
days; a similar group was treated for 15 days. Ten controls received
saline only, but the duration of their treatment was not specified. On
completion of the treatment, the rats were decapitated and blood
samples were collected for haematological and biochemical examination.
The results were presented as means and standard errors for five
animals per observation. Haemoglobin concentration, haematocrit, and
erythrocyte and leukocyte counts in treated animals were clearly lower
than in controls, the greatest effect being seen after 30 days. The
concentrations of serum urea were greater than in the controls at both
sacrifices; a higher cholesterol concentration was seen only after 30
days. The serum activities of aspartate and alanine aminotransferases
and amylase were higher than in the controls on both occasions. There
was also a small increase in alkaline phosphatase activity; however,
as the timing of the collection of control blood samples was not
given, the significance of this minor change cannot be assessed. The
activity of acid phosphatase was lower than in controls at both blood
sample collections. Cholinesterase determinations in serum indicated
inhibition of 30-50% relative to controls. The above effects were all
more marked after 30 than after 15 days. These results indicate that
dimethoate may affect liver and kidney function, although the changes
were not of pathological significance. Organs were not weighed and no
microscopic examinations were undertaken in order to correlate the
changes seen. Although the changes in amylase activity might indicate
pancreatic effects, confirmatory isoenzyme studies were not carried
out. The route and frequency of administration in this study and the
exiguous nature of the examinations performed render this work of
equivocal use in the safety evaluation of dimethoate (Reena et al.,
1989).
Rabbits
In a five-month study by oral administration in male rabbits, the
doses used were said to be one-tenth and one-hundredth of the LD50
value given once a week, but the actual doses were not specified.
There were no indications of clinical signs, and most of the data were
presented graphically on the basis of monthly sacrifice of four
animals out of a total of 20 animals per group. An initial increase in
cholinesterase activity was noted at the one-month sacrifice, but a
subsequent 40% reduction was reported. Although changes in organ
weights were reported, the body weights of the animals were not, and
the changes were reported only as percentages of absolute weight of
controls. The significance of the various findings cannot be assessed,
and the report is not considered further (Shaker, 1988).
The dermal toxicity of technical-grade dimethoate was investigated
in groups of six male and six female New Zealand white rabbits which
received doses of 100, 300, or 1000 mg/kg bw per day for 21 days; two
control groups, one untreated and one receiving the vehicle (paraffin
oil), were similarly constituted. The doses were selected on the basis
of the results of a five-day range-finding study in two groups of one
male and one female given doses of 1000 or 2000 mg/kg bw per day. In
the range-finding study, slight erythema was seen in one animal at
1000 mg/kg bw per day and in both at 2000 mg/kg bw per day on days 3,
4, and 5; fissuring of the skin was seen in one animal treated at
2000 mg/kg bw per day. Application was made to the abraded skin of
three animals per group or to non-abraded skin on the back of each
animal on five days per week at a volume of 2 ml/kg bw. The test site
was occluded for 6 h with a gauze bandage held in place by tape and
wrapped in occlusive plaster. Observations and food consumption were
recorded daily, and body weight was measured weekly. Blood samples were
taken before treatment and at termination after about 16 h of fasting.
All animals were subjected to a complete necropsy, and a range of
tissues was retained. Microscopic examination was restricted to the
controls and animals at the highest dose.
There were no significant differences between treated and control
animals or between those with intact or abraded skin. Pustules were
seen at the treatment sites of the majority of animals, including
vehicle controls, during the study. Body-weight gain and food
consumption were unaffected by treatment, and there were no changes in
the blood, including cholinesterase activity, that could be ascribed
to treatment. There were no significant treatment-related intergroup
differences in organ weights or in macroscopic or microscopic
pathology. The absence of an effect on cholinesterase activity
indicates that the test material was not absorbed, even across broken
(abraded) skin, at doses up to 1000 mg/kg bw per day. It was concluded
that dimethoate does not irritate rabbit skin (Madison et al., 1986).
Dogs
A study in dogs was briefly summarized in the report of the 1963
JMPR (Annex 1, reference 2), in which groups of two males and two
females were fed diets providing 2, 10, or 50 ppm, equivalent to 0.05,
0.25 and 1.25 mg/kg bw per day dimethoate for 90 days. No significant
effects were noted, and erythrocyte cholinesterase activity was only
slightly depressed in animals at 50 ppm (West et al., 1961).
Groups of six male and six female beagle dogs received diets
containing dimethoate at concentrations of 0, 5, 20, or 125 ppm
for one year. The doses were chosen on the basis of the results
of a preliminary study of 28 days' duration at concentrations
< 1250 ppm, at which dose-related changes were observed at
> 50 ppm, necessitating early sacrifice at 1250 ppm, while changes
at 50 and 250 ppm were confined to dose-dependent reductions in
cholinesterase activity. In the main study, clinical signs of reaction
to treatment and food consumption were recorded daily; body weight was
recorded weekly. The eyes of each animal were examined before
treatment and during weeks 26 and 52. Blood samples were taken on two
occasions before treatment and during weeks 13, 26, and 52. Urine
samples were collected at similar intervals. The samples were examined
for a normal range of haematological and biochemical characteristics,
including plasma and erythrocyte cholinesterase activity; brain
acetylcholinesterase activity was measured at termination. All animals
were necropsied after bone-marrow samples had been taken. A range of
organs was weighed and the tissues preserved for histopathological
processing and examination.
There were no deaths or clinical signs of reaction to treatment
and no effect on body weight, food consumption, or the eyes. The
achieved intakes of test material were calculated to be 0.19, 0.73,
and 4.25 mg/kg bw per day for animals at 0, 5, 20, or 125 ppm. Plasma
cholinesterase activity was reduced by > 20% relative to controls in
animals of each sex at 125 ppm in weeks 13 and 26 and in males only in
week 52. These reductions did not exceed 22%, except in females at
week 13 which had an activity 36% lower than controls. Erythrocyte
acetylcholinesterase activity was reduced in weeks 13 and 26 by 20-27%
in animals at 20 ppm and by 63-76% in those at 125 ppm. In week
52, males at 5 or 20 ppm had marginally, nonsignificantly lower
erythrocyte acetylcholinesterase activities than the controls; females
in these groups were unaffected. A clear reduction (about 65%) in
erythrocyte acetylcholinesterase activity was also seen in animals at
125 ppm. Statistically significant reductions in brain acetylcholin-
esterase activity were seen at all doses after 52 weeks, which were
slight at 5 ppm (about 90% of the control level) and 20 ppm (about 83%
of control) but clear at 125 ppm (45% of control). There were no other
biochemical differences in the blood attributable to treatment. The
urine was unaffected, and there were no findings at necropsy that were
attributable to treatment. The liver weights of animals at 125 ppm
were lower than those of the controls. There was a marginally greater
incidence of pigment, presumed to be haemosiderin, in the livers of
treated animals in all groups, but there was no clear relationship to
dose. In the absence of other effects of treatment, notably on blood,
this effect was considered to be of no toxicological significance. The
only significant evidence of toxicity attributable to dimethoate was
the reduction in cholinesterase activity; the effect on erythrocyte
and brain acetylcholinesterase activity was clearly significant at 20
and 125 ppm, whereas the effects at 5 ppm were confined to minimal
reductions in brain acetylcholinesterase (10% lower than controls) and
a minimal reduction in male plasma cholinesterase activity after 51
weeks. The NOAEL was 5 ppm, equal to 0.19 mg/kg bw per day (Burford
et al., 1991).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade dimethoate was administered to groups of 50 male
and 50 female individually housed B6C3F1 mice for 18 months. Dietary
concentrations of 0, 25, 100, and 200 ppm (equal to 3.2, 12.3 and
25.3 mg/kg bw per day) were selected on the basis of the results of
previous studies, including a study from the US National Cancer
Institute (1977) in this strain. Blood samples were taken for
investigations of haematology and cholinesterase activity after 51
weeks of treatment from an additional 10 males and 10 females
allocated to each group, which were then killed and necropsied.
Haematological investigations only were conducted after 78 weeks on 10
animals of each sex per group. The test diets were mixed weekly, and
formulated diet and the test material were analysed at approximately
three-month intervals; the results of these analyses were satisfactory.
All animals were necropsied; appropriate organs only from animals at
the terminal kill were weighed. A wide range of tissues from all
animals, including satellite animals that died before 51 weeks, were
examined microscopically.
There were no clinical findings that were considered by the
authors to be related to treatment. Survival was > 90% in all groups,
and there was no effect of treatment on mortality. There were no
differences in group mean food consumption that could be related to
treatment. The body-weight gain of treated males was lower than that
of controls during the first few weeks of the study; females receiving
200 ppm were transiently affected during the first two weeks.
Subsequently, the body weights of treated females in all groups were
greater than those of the controls; a similar but less marked
difference was evident in treated males from about 14 months. Female
animals at 25 ppm gained notably less weight than those at the two
higher doses but still gained more than the controls. The overall
weight gains of females were 16.1 ± 5.9 (SD) for the controls,
20.4 ± 6.4 for those at 25 ppm, 27.9 ± 7.3 for those at 100 ppm, and
26.0 ± 5.6 for those at 200 ppm. Haematological analyses after 78
weeks indicated higher nonspecific leukocyte counts in males at 100 or
200 ppm and in females at 200 ppm; no similar difference was seen in
samples taken from satellite animals after 51 weeks of treatment. The
cholinesterase activity in plasma and erythrocytes from treated
animals was lower than That in the controls in a dose-related manner
at all dietary concentrations. No other examination of cholinesterase
activity and no analyses of brain were undertaken. Organ weight
analysis indicated greater absolute liver weights in animals at 100 or
200 ppm; however, the relative liver weights of females were lower
than those of controls as a result of the increased body weights of
these animals. The absolute weight of the ovaries of treated females
was lower than that of the controls after 78 weeks of treatment, but
no similar difference was seen in animals killed after 52 weeks of
treatment. Microscopic examination indicated a greater incidence of
extramedullary haematopoiesis in the spleens of males and females at
100 or 200 ppm, which was dose-related. A greater incidence of
hepatocytic vacuolation was seen in males and female at 100 or 200 ppm
and to a lesser extent in females at 25 ppm; the effect was attributed
to fat and the nutritional status of the affected groups. There were
no differences in the incidences of any neoplastic finding that could
be related unequivocally to treatment. There was no NOAEL, as effects
were seen at all doses (Hellwig, 1986a)
Fifty male B6C3F1 mice received a dietary concentration of
250 ppm dimethoate for 69 weeks or 500 ppm for 60 weeks, and 50
females received the two doses for 80 weeks. All animals were then
observed without treatment until about 94 weeks. A control group of 10
males and 10 females was supplemented by similar groups of animals
from concurrent studies on other pesticides; this gave a pooled
control group of 50-60 animals, although the studies were not
precisely concurrent. The body-weight gain of treated mice, except
females at the low dose, was lower than that of controls during the
first 52 weeks of treatment. Occasional generalized tremor was noted
in treated animals at each dose. During the second half of the study,
alopecia, abdominal distension, and tumours were seen in treated
animals but predominantly in those receiving the lower dose. The
condition of animals at termination was said to be poor. Oncogenic
potential was not assessed (US National Cancer Institute, 1976, 1977).
Rats
Groups of 65 male and 65 female individually housed Wistar rats
received dimethoate in the diet at concentrations providing 0, 5, 25,
or 100 ppm for two years. Fifteen animals of each sex in each group
were allocated for clinical pathology; an additional group of 20 males
and 20 females received a dietary concentration of 1 ppm and were used
to establish or confirm a no-effect level. The dietary concentrations
were selected on the basis of the results of a preliminary study and a
study by the US National Cancer Institute (1977) on Osborne-Mendel
rats. Feed was prepared weekly; both the test material and the
formulated diets were analysed regularly and found to be satisfactory.
Food consumption was recorded weekly; body weight was recorded
weekly for 13 weeks and at fortnightly intervals thereafter. Daily
observations and weekly palpations were recorded; the eyes of all
animals of the main groups (50 of each sex) were examined before
treatment and at six-month intervals during the treatment period for
changes to the refracting media. The ocular fundus of 10 males and 10
females in the control and high-dose groups were examined after 620
days of treatment. Blood samples were obtained before treatment and on
six occasions during the study, and a normal range of chemical and
haematological parameters, including cholinesterase activity, was
measured. Brain acetylcholinesterase activity was measured at the end
of the study. Urine samples were collected twice during the study;
although basic, qualitative 'stick' tests were conducted, volume and
specific gravity were not measured. All animals were necropsied, and a
range of organs was weighed and the tissues retained. Tissues from all
animals in the main study and from satellite animals that died were
examined microscopically.
Females at 100 ppm had a slightly higher mortality rate than
controls from week 65. There were no clinical signs that were
considered by the authors to be related to treatment. Animals at doses
> 25 ppm showed a trend to increased food consumption, especially
during the second year of treatment. The body-weight gain of animals
receiving 100 ppm was slightly lower than that of the controls
during the first half of the study. Examination of the eyes in vivo
revealed no treatment-related effect. Reductions in plasma
cholinesterase activity (generally, 50% of control) were seen in the
group at 100 ppm at all examinations; in females at 25 ppm, the
activities in plasma were minimally lower than in the controls after
four weeks. Reductions in erythrocyte acetylcholinesterase activity
were clear in animals at 25 or 100 ppm (60-75 and 20-40% of control
values, respectively); smaller decreases were seen in females at 5 ppm
during the first 12 months and in males of this group after 24 months.
Clear dose-related reductions in brain acetylcholinesterase activity
were seen at termination in animals at 25 or 100 ppm and slightly
lower values in males at 5 ppm. In animals at this low dose, the
reductions in erythrocyte and brain acetylcholinesterase activity were
not consistent between times or sexes; the variations from control
values are thus of questionable biological significance but probably
represent an intermittent effect of treatment. There were no
statistically significant differences in cholinesterase activity in
the animals at 1 ppm.
The authors reported minimal anaemia throughout the study in
males at 100 ppm; however, this effect was also present before
treatment and was absent in females. Minor variations were also noted
in leukocyte count, potassium and total protein concentrations, and
aspartate aminotransferase activity. Although these effects were
reported to extend in part to the animals at 25 ppm, they were minor;
they may be related to treatment but are considered not to be of clear
toxicological significance. There were no treatment-related changes in
the urine. Animals at 100 ppm had slightly larger spleens and slightly
smaller ovaries than the controls, but no gross pathological findings
could be related to treatment. There were no non-neoplastic findings
that were considered to be related to treatment, and there was no
statistically significant difference in the distribution of individual
tumour types. Treated males had a greater number of malignant tumours
than the controls, but there was no relationship to dose. In a number
of tissues, however, a greater incidence of tumours was seen in
treated animals than in controls. These included exocrine and
islet-cell adenomas in the pancreas of males, haemangiosarcoma in
the spleen of males, and mammary gland fibroadenoma and carcinoma
in females. When the combined incidences of haemangioma and
haemangiosarcoma at any site in treated animals were compared with
those in controls, the difference was statistically significant for
all groups of treated males, but there was no relationship to dose and
females were clearly unaffected. The apparent difference may be due to
a lower than expected incidence in the controls. A large proportion of
these tumours were located in the mesenteric lymph node. The incidence
of these vascular tumours was said to be similar to that of historical
controls from a number of sources. The authors concluded that there
was no evidence that dimethoate has oncogenic potential. The NOAEL for
inhibition of brain and erythrocyte acetylcholinesterase activity was
1 ppm, equivalent to 0.05 mg/kg bw per day (Hellwig, 1986b).
The vascular and proliferative lesions from the above study were
evaluated by a second group of pathologists (Squire, 1988), who were
unaware of the previous interpretation of each slide. This review
supported the conclusion of the original pathologist and indicated
that the Wistar rat is susceptible to these tumours. It also suggested
that the chemicals that induce vascular neoplasms are genotoxic;
however, it asserted that dimethoate is not genotoxic and that by
implication these tumours were not related to treatment.
Groups of 50 male and 50 female Wistar rats received technical-
grade dimethoate in the diet at concentrations of 0, 2, 20, or 200 ppm
for two years, equivalent to 0.1, 1 and 10 mg/kg bw per day. The report
was available only as an English translation of a nearly illegible
German summary with tables of individual data. As no other details of
procedures or results and no group means were presented, the study
could not be reviewed satisfactorily (Ministry of Agriculture,
Fisheries and Food, 1993a).
Groups of 30 male and 30 female Wistar rats received diets
designed to provide technical-grade dimethoate at concentrations of 0,
0.1, 1, 10, or 75 ppm for two years, equivalent to 0.005, 0.05, 0.5
and 3.8 mg/kg bw per day. The feed was prepared four times a week.
Body weight and food intake were recorded weekly for the first four
weeks and every four weeks thereafter. Haematological examinations
were performed four times between weeks 32 and 100. Cholinesterase
activity was determined after 1, 3, 12, 50, 75, and 100 weeks on six
rats of each sex. Brain acetylcholinesterase activity was determined
after 52 and 104 weeks. Other, limited biochemical assays were
performed during the study on blood and urine, mostly after two years.
Six animals of each sex were killed after one year and the surviving
animals after two years; all were examined macroscopically and their
organs weighed. Histological examination was performed on six males
and six females at each sacrifice. There was no indication of how dead
animals were handled or examined, although the report refers to their
necropsy.
Early signs of reaction to treatment at 75 ppm (slight
piloerection, exophthalmia, and fine tremor) disappeared during the
fourth week of treatment, and no other clinical signs were attributed
to treatment. A high mortality rate resulted from an infection after
78 weeks, with the deaths of 55 males and 37 females, evenly
distributed among the groups. The infection was treated with
oxytetracycline as three oral doses of 40 mg/kg bw over three weeks.
There was no treatment-related mortality. Body-weight gain was reduced
in animals treated at 75 ppm, up to week 20 for females and throughout
the study for males. Food consumption was unaffected, but conversion
was reduced in animals treated at 75 ppm, in line with body weight.
The achieved mean doses were 0.02, 0.2, 2, and about 20 mg/kg bw per
day. Haematological investigations indicated no effects of treatment.
Cholinesterase activity was clearly reduced in the plasma,
erythrocytes, and brain of animals receiving 10 or 75 ppm. The brain
acetylcholinesterase activity of animals receiving 1 ppm was 20% lower
than that of controls after one year. There were no other
toxicologically significant intergroup differences in the composition
of the plasma or urine. Organ weight analysis after one year indicated
lower liver, spleen, adrenal, and testis weights in males at 75 ppm
than in the controls; after two years, the adrenal and liver weights
of males and females at 75 ppm were lower than those of controls.
Necropsy of animals that died during the study and of animals killed
at the scheduled sacrifices indicated no treatment-related changes.
The distribution of macroscopically observed rumours was unaffected by
treatment. No microscopic changes were found that were attributed to
treatment. The incomplete data presentation and the infection that
occurred in the middle of the study significantly compromise its
validity (Ministry of Agriculture, Fisheries and Food, 1993a).
Groups of 50 male Osborne-Mendel rats were fed diets providing
technical-grade dimethoate at concentrations of 250 or 500 ppm
(equivalent to 25 and 50 mg/kg bw per day) for 19 weeks; the doses
were then reduced to 125 and 250 ppm (equivalent to 6.3 and 13 mg/kg
bw per day),and treatment continued for 61 weeks. Necropsy was
performed after a further 33-35 weeks without treatment. The same
dietary concentrations were fed to similar groups of females, except
that they were reduced only after 43 weeks; the animals were then
treated at concentrations of 125 or 250 ppm for a further 37 weeks.
The total treatment period for all animals was 80 weeks, followed by
up to 35 weeks without treatment. A control group of 10 males and 10
females was supplemented by similar groups of animals from concurrent
studies on other pesticides, giving a pooled control group of 50-60
animals, although the studies were not precisely concurrent. All
animals were observed daily, and body weights were recorded 'at
regular intervals until 110 weeks'. The animals were then necropsied
and, when feasible, tissues were retained for histopathological
examination. Treatment at 500 ppm was associated with lower
body-weight gains in males and females during the first 20 weeks of
treatment. After the reduction to 250 ppm at 19 weeks, the body-weight
gain of males increased but remained lower than in controls until
treatment ceased. The body-weight gain of males at the low dose and
females at the high dose remained lower than that of controls until
week 80. Clinical signs of inhibition of cholinesterase activity were
seen in animals at the high dose, particularly during the first week
of treatment. Conjunctivitis of vital origin was diagnosed in the
animals at week 38. Animals that survived to termination were said to
be 'generally in poor physical condition'. More animals at the high
dose died than matched or pooled controls, although the number of
matched control males that died during the study (7 of 10) was
reported to be unusually large. There was no difference in the
distribution of non-neoplastic or neoplastic changes among the treated
groups that could clearly be ascribed to treatment. The pathological
assessment indicated no oncogenic potential (US National Cancer
Institute, 1976, 1977).
A review by Reuver (1984) covered several carcinogenicity studies
in rats and mice, including that of the US National Cancer Institute.
It was concluded from the studies of Gibel et al. (1973) and
Stieglitz et al. (1974) that dimethoate is highly carcinogenic to
rats, but insufficient details of these experiments were given,
precluding assessment. The review of the US National Cancer Institute
study involved re-reading of the sections; it was again concluded
that dimethoate is carcinogenic. The basis for the review was not
described, and the numbers of animals given in the tables shown in the
review differ from those in the original report. Reuber quoted text
from the report in reverse order to that in which it was originally
published. This review did not satisfactorily explain the methods used
or discuss the discrepancies in the reported incidences and in the
conclusions from those of the original report. In view of these
deficiencies, no weight is placed on this publication.
(d) Reproductive toxicity
Mice
A multigeneration study was undertaken in CF-1 mice fed diets
containing concentrations of 0, 5, 15, or 50 ppm dimethoate,
equivalent to 1.4, 4.3 and 14.5 mg/kg bw per day, throughout the
study. The study was conducted before Good Laboratory Practice came to
be enforced but was designed according to the recommendations of the
Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics of
the Association of Food and Drug Officials of the United States. Each
generation was mated twice, the first set of litters being discarded
and the next generation (F1b, F2b, and F3b) being produced from the
second litters. For each generation, eight males were mated with 16
females in each group, and males were rotated within their group
during the mating period. The observations were limited in comparison
with current practice. After weaning of the F3b generation, the
parents (F2b) were killed and necropsied; liver, kidneys, and gonads
were weighed, but no microscopy was performed. All animals of the F3b
generation were necropsied at death or at weaning, and a wide range of
tissues from one male and one female from each litter was examined
microscopically. Organ weighing was restricted. All fetuses that were
not examined microscopically were retained in 80% alcohol for skeletal
staining in alizarin red.
There were no clinical reactions to treatment. Tremors were seen
in four dams of the F2b generation treated with 50 ppm, three of
which lost their litters, on one occasion after a weekly change of
diets; the diets were replaced, as a formulation error was suspected,
and the condition was not seen again. No treatment-related differences
in group mean body weights were recorded at any mating. Measurement of
the food consumption of the F0 generation before mating indicated
no effect of treatment, but no data were presented and no other
measurements were carried out. The fertility, gestation, viability,
and lactation indices were unaffected by treatment, and there was
no effect on the weights of the pups at weaning. No intergroup
differences in organ weight or pathological findings were seen that
were related to treatment. The NOAEL for reproductive toxicity was
50 ppm (Ribelin et al., 1965).
In five generations of CD-1 mice given 60 ppm of dimethoate in
drinking-water, reproductive performance was significantly altered, as
indicated by reduced mating success and longer gestation. Litter size
and weight were not reduced at birth, but pup mortality was increased
significantly by treatment. The growth rate of the pups was generally
lower than that of controls. Dimethoate did not show teratogenic
potential or adverse effects on organ weights or histological
appearance (Budreau & Singh, 1973).
Rats
In a multigeneration study, dimethoate (purity, 96.4%) was
administered in the diet at fixed concentrations of 0, 1, 15, or
65 ppm (equal to about 0.08, 1.25 and 5 mg/kg bw per day) to groups of
28 male and 28 female Sprague-Dawley rats for 10 weeks before the
first of two matings to produce F1a and F1b animals. The F1
generation, selected from the F1a litters, was first mated at about
16 weeks of age to produce F2 pups, which were killed and examined at
21 days of age. A second mating of the F1a generation was conducted,
followed by a partial third mating involving animals that had not been
successful at either of their first two pairings. Administration of
dimethoate was continued at the same dietary levels throughout
premating, mating, gestation, and lactation. Treatment at 65 ppm was
associated in the parent animals with marked reductions in plasma,
erythrocyte, and brain cholinesterase activities in animals of both
generations, slightly reduced body-weight gain, increased food intake,
and reduced water intake. In the F1a pups at 65 ppm at four days of
age, a reduction in brain acetylcholinesterase activity was seen in
males but not in females. The parental animals at 15 ppm had a
significant reduction in brain and erythrocyte acetylcholinesterase
activity in both generations, but no effect on cholinesterase activity
was seen in the offspring. Mating performance (as assessed by median
precoital time and duration of pregnancy) was not affected by
treatment; however, an effect was seen on the pregnancy rate (Table
2). These data are clearly indicative of substandard performance at
65 ppm and also show a possible effect at 15 ppm on the second mating
of the F1 generation. The Meeting concluded that this information did
not clarify the possible effect at the intermediate dose. Treatment at
65 ppm was also associated with a reduction in litter size at birth,
as shown in Table 3.
Table 2. Pregnancy rates of animals treated with dimethoate with
live pups at birth
Generation Mating Pregnancy rate (%)
Control 1 ppm 15 ppm 65 ppm
F0 First 93 96 86 89
Second 89 93 89 71
(100) (100) (100) (96)
F1 First 96 71 71 63
Second 73 67 58 50
(100) (79) (92) (75)
In parentheses, the percentages of animals with implantations
confirmed at autopsy by Salewski staining of the uteri of
apparently non-pregnant females
Table 3. Litter size at birth of animals treated with dimethoate
Generation Mating Group mean litter size at birth
Control 1 ppm 15 ppm 65 ppm
F0 First 16.4 15.3* 15.3* 14.2**
Second 14.9 14.9 14.2 14.3
F1 First 12.3 11.9 14.6 12.0
Second 14.1 13.3 13.1 10.0*
* p < 0.05
** p < 0.01
There was also a slight increase in pup mortality during
lactation in animals at 65 ppm. Changes in litter weight reflected the
changes in litter size. The mean pup weight at birth was unaffected by
treatment, but pup body-weight gain was adversely affected by the high
dietary level. There was a slight delay in attainment of the startle
reflex in pups born after the first mating of both generations at
65 ppm, but the mean delay was less than one day and was not apparent
across all four matings; it was thus probably not related to
treatment. There was no other effect on pre- or post-weaning
development. Histopathological examination of tissues associated with
the reproductive tract did not reveal any treatment-related changes.
The NOAEL for toxicity was 1 ppm, equivalent to 0.08 mg/kg bw per day,
on the basis of inhibition of cholinesterase activity at 15 ppm
(Brooker et al., 1992). The Meeting discounted the possible adverse
effect on pregnancy rate at 15 ppm, and this was the NOAEL for
reproductive performance, equivalent to 1.2 mg/kg bw per day.
Rabbits
Three groups of three male rabbits received gelatin capsules
containing individually calculated doses of a dimethoate formulation
(composition unspecified) calculated to be one-tenth and one-hundredth
of the LD50, which was not specified, at an unspecified frequency. A
six-week preliminary period was followed by six weeks of treatment and
then by six weeks of respite. Body weights were recorded weekly, and
semen was collected twice weekly from all animals throughout the
experimental period. Ejaculate volume was recorded after removal of
the gel mass. Seminal initial fructose was determined immediately
after collection, and methylene blue reduction time was recorded. The
numbers of live, dead, and abnormal sperm were assessed, and sperm
concentration was determined with a haemocytometer. Data were
presented in graphs rather than as individual values; there was no
indication of variation between individual animals. Clinical signs
were not reported. Body-weight gain was reduced in treated animals,
and there were indications of reduced libido. The ejaculate volume and
sperm concentration of treated animals, expressed as a percentage of
that of controls, decreased during the treatment period, and the
effect on sperm concentration persisted into the recovery period.
Treatment increased the numbers of abnormal sperm in a dose-related
manner; these were greatest at the end of the treatment period but
declined thereafter. A significant increase in the methylene blue
reduction time and a decrease in the initial fructose concentration
were also seen. There was some evidence of recovery from these effects
during the latter part of the recovery period (Salem et al., 1988).
In view of the deficiencies, it was difficult to assess the
significance of the changes seen, but they should be explained in view
of the reported reproductive effects of dimethoate.
(e) Developmental toxicity
Mice
Intraperitoneal administration of dimethoate at 40 mg/kg bw as a
single dose to mice on the day of mating or on day 9 of gestation or
for the first 14 days of gestation caused a high incidence of
embryonal loss (Scheufler, 1975).
Dimethoate administered orally at doses of 10 or 20 mg/kg bw was
not teratogenic to CD-1 mice, and these levels were not lethal to the
dams. Doses of 40 and 80 mg/kg bw induced maternal toxicity (Courtney
et al., 1985).
Rats
Groups of pregnant rats were given 0, 3, 6, 12, or 24 mg/kg bw of
a dimethoate formulation by gavage daily on days 6-15 of gestation.
The dams were killed on day 22 of gestation; the uterine content was
removed, the carcass weighed, the number of corpora lutea was
determined, and the animals were necropsied. The fetuses were weighed
and examined for viability and external malformations; live fetuses
were studied for skeletal and visceral anomalies. Maternal weight was
decreased significantly in the group receiving 24 mg/kg bw, and clonic
spasms and muscular tremors were seen. The mean fetal weight was not
affected by treatment. Treatment with 12 or 24 mg/kg bw was associated
with an increased number of litters with abnormal fetuses and fetuses
with wavy ribs. The NOAEL was 6 mg/kg bw of formulated product, equal
to 2.84 mg/kg bw dimethoate (Khera et al., 1979).
Three groups of 25 time-mated CrL:COBS CD (SD) BR rats received
technical-grade dimethoate (purity, 97.3%) in 1% aqueous methyl
cellulose (10 ml/kg) at doses of 3, 6, or 18 mg/kg bw per day by
gavage daily on days 6-15 of gestation; a control group received the
vehicle alone. Clinical observations were made at regular intervals,
and food consumption and body weight were recorded. All animals were
killed on day 20 of gestation and the uterine contents examined for a
normal range of parameters. One-half of the pups were preserved in
Bouin's solution for free-hand sectioning and the remainder in
industrial methylated spirits for subsequent alizarin staining and
skeletal examination. The doses were chosen on the basis of the
results of a preliminary study, which was not presented or discussed.
The signs seen in animals at 18 mg/kg bw per day included salivation,
hypersensitivity, ataxia, tremor, fur staining, and small, rounded
faecal pellets. The signs at 3 mg/kg bw per day were confined to
salivation; in animals at 6 mg/kg bw per day, this was accompanied by
a low incidence of small, rounded faecal pellets. Food consumption and
body-weight gain were lower in animals at 18 mg/ kg bw per day than in
the controls during treatment; similar effects were not seen at the
two lower doses. Neither litter parameters nor fetal development (as
indicated by the incidences of visceral or skeletal abnormalities) was
affected by treatment. The NOAEL for toxic signs depends on the
interpretation of the significance of the salivation seen in the
animals at 3 and 6 mg/kg bw per day and on the abnormal faecal pellets
in the latter group. Although salivation is an expected effect of
organophosphate pesticides, there was no clear evidence that the
effect was unduly prolonged (It was described as occurring
'immediately' after treatment.) and may have been incidental. The
presence of abnormal faecal pellets may be related to the action of
this class of compound on the gastrointestinal tract and is unlikely
to be of toxicological significance (Edwards et al., 1984a).The
Meeting concluded that the NOAEL was 6 mg/kg bw per day.
Rabbits
Groups of 16 female New Zealand white rabbits (obtained from
several breeders) were mated with males of proven fertility and
received technical-grade dimethoate (purity, 97.3%) in 1% aqueous
methylcellulose (5 ml/kg) at doses of 10, 20, or 40 mg/kg bw per day
by gavage daily on days 7-19 of gestation; a control group received
the vehicle alone. The doses were chosen from a preliminary study, the
results of which were not summarized. After coitus, each animal
received an injection of luteinizing hormone. Clinical observations
were made at regular intervals, and food consumption and body weight
were recorded. All animals were killed on day 29 of gestation, and the
uterine contents were inspected; a range of parameters for this type
of study was assessed. After examination in vivo, the pups were
killed and dissected. The skinned, eviscerated pups were fixed in
industrial methylated spirits, and the brain was examined for
abnormalities by longitudinal sectioning; carcasses were cleared and
stained by a modified Dawson's technique for skeletal examination.
There were no clinical signs of reaction to treatment with doses of 10
or 20 mg/kg bw per day; at 40 mg/kg bw per day, muscle tremors and
ataxia were seen during the latter part of the treatment period. Food
consumption was reduced between days 15 and 23 of gestation in animals
treated at 40 mg/kg bw per day, and the body-weight gain of these
rabbits was lower than that of controls throughout the treatment and
particularly between days 15 and 20 of gestation. A slight reduction
in body-weight gain was seen in animals at 20 mg/kg bw per day. An
initial reduction in weight gain was seen at the beginning of the
treatment period in animals at the low dose, but these animals
subsequently gained more weight than the controls. Treatment had no
effect on fetal development; litter size and weight were unaffected,
and pups had no abnormalities that could be ascribed to treatment.
Although there was a transient reduction in body-weight gain at the
start of treatment in animals at 10 mg/kg bw per day, the deficit was
quickly corrected. This dose was therefore the NOAEL (Edwards et al.,
1984b).
Cats
Four groups of 17 cats were mated and treated with Cygon-4E, a
commercial insecticide containing 47.3% dimethoate, as single daily
doses of 0, 3, 6, or 12 mg/kg bw on days 14-22 of gestation. The cats
were necropsied on day 43 of gestation, and the fetuses were removed,
weighed, and examined for external malformations. The total number of
anomalous fetuses in cats at 12 mg/kg bw per day was not significantly
higher than that in controls. The only treatment-related malformation
was observed at this dose and consisted of forepaw polydactyly in
eight of 39 fetuses. A dose-response relationship was not established
owing to the limited response and the common occurrence of this
anomaly in cats. The NOAEL was 6 mg/kg bw per day of Cygon-4E, equal
to 2.8 mg/kg bw per day of dimethoate (Khera, 1979).
(f) Genotoxicity
The regulatory reports evaluated by the Meeting concluded that
dimethoate does not induce reverse mutation or gene mutation in
vitro, nor did it induce micronucleus formation, dominant lethal
mutation, or chromosomal aberration in mice in vivo. Dimethoate
induced unscheduled DNA synthesis in vitro in two assays using
different methods of assessing the uptake of tritiated thymidine into
DNA but not in an assay in vivo/in vitro. A review of the literature
on the mutagenic potential of dimethoate revealed a number of positive
results, notably for reverse mutation in Salmonella typhimurium
TA100 and for sister chromatid exchange in mammalian cells in vitro.
It was concluded that although dimethoate has mutagenic potential in
vitro, mutagenicity does not appear to be expressed in vivo. The
results of assays for genotoxicity with dimethoate are summarized in
Table 4.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The dermal irritation potential of a 400-g/litre emulsifiable
concentrate formulation of dimethoate was investigated in rabbits.
Only slight erythema was observed 4 h after application, and the
effect had resolved by 24 h after treatment (Ministry of Agriculture,
Fisheries and Food, 1993a).
The ocular irritation potential of the same formulation of
dimethoate was also investigated in rabbits. Redness and swelling of
the conjunctiva were observed, with slight corneal opacity, 1-72 h
after application. All of the effects had resolved by eight days after
treatment (Ministry of Agriculture, Fisheries and Food, 1993a).
Studies to investigate the ocular irritation potential in
rabbits of 40 and 38.3% dimethoate formulations concluded that the
formulations were irritating to the rabbit eye. The 'in use' dilution
of the 40% formulation (0.84%) was not irritating (Ministry of
Agriculture, Fisheries and Food, 1993a).
Technical-grade dimethoate (purity, 97.3%) did not sensitize the
skin of guinea-pigs when tested by the Buehler method (Madison et al.,
1984).
Table 4. Results of tests for the genotoxicity of dimethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
5-Methyltryptophan E. coli 1.6 × 10-3 mol/litre NR Positivea Mohn (1973)
resistance mutation
Reverse mutation S. typhimurium < 5000 mg/plate NR Positive Moriya et al. (1983)
TA98, TA100, in TA100a
TA1535, TA1537,
TA1538
E. coli WP2hcr < 5000 mg/plate Positivea
Reverse mutation S. typhimurium < 5000 mg/plate NR Negativea Probst et al. (1981)
TA98, TA100,
TA1535, TA1537,
TA1538
E. coli WP2uvrA- 47 nmol/ml Initially
positive,
negative
quantitativelya
Reverse mutation S. typhimurium 2-200 mg/plate NR Positivea Vishwanath & Jamil
TA100 (1986)
Mitotic gene conversion S. cerevisiae 7 doses, 40-100 mmol NR Positive Fahrig (1974)
Mitotic gene conversion S. pombe (ade 6) 1.3-131 mmol NR Negative Gilot-Delhalle et al.
(1983)
Gene mutation Chinese hamster 1000-3500 mg/ml 97.3 Negative Johnson et al. (1985)
ovary (hprt)
Sister chromatid exchange Cultured human < 120 ppm NR Positive Gomez-Arroyo et al.
lymphocytes (1987)
Sister chromatid exchange Chinese hamster 10, 20, 40, 80 mg/ml 94 Positive Chen et al. (1981)
and cell cycle delay ovary V79 cells + 10 pg/ml BUdR
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Cytotoxicity Chang liver and 50-500 mg/ml 99.8 Positive Gabliks &
HeLa cells Friedman (1965)
Cytotoxicity HeLa cells 2-300 mg/ml 99.8 Positive Gabliks (1965a)
Susceptibility to HeLa cells 2-300 mg/ml for 99.8 Positive Gabliks (1965b)
poliovirus infection < 108 days
Unscheduled DNA SV-40 transformed 100, 1000 mmol NR Positive Ahmed et al. (1977)
synthesis human fibroblast
cell line VA-4
Unscheduled DNA Rat hepatocytes 47 nmol/ml NR Negative Probst et al. (1981)
synthesis
Unscheduled DNA Rat hepatocytes < 2290 mg/ml NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
Unscheduled DNA Rat hepatocytes NR NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
In vivo
Micronucleus formation Mouse 2 equal oral doses NR Positive Rani et al. (1980)
bone marrow of 51.7 mg/kg bw at
24-h interval
Host-mediated Mouse; 3 equal oral doses NR Positive Rani et al. (1980)
mutagenicity S. typhimurium of 155 mg/kg bw
Dominant lethal CFLP mice 30, 60 mg/kg bw ip NR Negative Fisher & Scheufler
mutation AB Jena mice 5 × 6 mg/kg bw ip Negative (1981)
DBA mice 3 × 18 mg/kg bw ip Negative
Dominant lethal NMRI mice 5, 10, 20 mg/kg 96.9 Negative Becker (1985)
mutation orally, 5 days
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Dominant lethal Strain Q mice 10 mg/kg ip + 0.6 NR Negative Degraeve & Moutschen
mutation mg/l drinking-water (1983)
Chromosomal CFLP mice 20-60 mg/kg bw ip NR Positive (gaps Nehéz (1983)
aberration and numerical
changes)
Chromosomal aberration Rats 15, 75, 150 mg/kg bw ip NR Negative Ministry of Agriculture,
Fisheries and Food
(1993a)
Chromosomal aberration Mice 50, 100 mg/kg bw NR Positive Bhunya & Behera
(1975)
Chromosomal aberration Hamster 16-160 mg/kg bw ip NR Weakly Dzwonkowska & Hubner
positive (1986)
Sex-linked recessive Drosophila 1 mg/kg feeding NR Negative Woodruff et al. (1983)
lethal mutation
Sex-linked recessive Drosophila 10 or 20 ppm; adult NR Positive at Velásquez et al. (1986)
lethal mutation feeding low dose
0 or 10 ppm larval Negative
feeding
Sex-linked recessive Drosophila LD50 and half LD50, NR Positive Tripathy (1988)
lethal mutation larval feeding
Unscheduled DNA Rats 50, 100, 200 mg/kg NR Negative Ministry of Agriculture,
synthesis bw orally Fisheries and Food
(1993a)
Micronucleus formation CD-1 mice 55 mg/kg bw ip once 97.3 Negative Sorg (1985)
or twice 24 h apart
Metaphase alteration Rats 15, 75, 150 mg/kg 97.3 Negative San Sebastian (1985)
bw ip
ip intraperitoneally
a With and without metabolic activation
Nineteen cases of allergic, occupational contact eczema and one
of contact dermatitis have been reported (von Jung, 1989). Exposure
to dimethoate was cited in four cases: in two male and one female
gardener and in one female agrochemical technician, 22-69 years of
age. The results of patch tests with dimethoate in these individuals
were positive.
(ii) Neurotoxicity
In a preliminary study, the LD50 of dimethoate in hens was
determined in groups of 10 birds given single oral doses of 0, 30, 45,
68, 100, or 150 mg/kg bw in water. These doses were selected on the
basis of the results of a preliminary study in groups of two birds at
doses between 12.5 and 200 mg/kg bw; both birds at 100 or 200 mg/kg bw
died. In each of these studies, dosing was followed by a 14-day
observation period. Body weights were recorded weekly. Surviving birds
were killed but not necropsied. Neurotoxicity was assessed after a
single subcutaneous dose of 55 mg/kg bw to 16 birds or 55 mg/kg bw
orally to 30 birds; a control group of 14 birds was dosed orally, and
a positive control group of six birds received 500 mg/kg bw of
tri- ortho-cresyl phosphate (TOCP) as a single oral dose in corn oil.
All birds were starved overnight before treatment. In an experiment
conducted before the main study to evaluate the protective
effectiveness of atropine, it was shown to have no protective value at
twice the LD50 value. Birds dosed subcutaneously with dimethoate were
included for comparative biochemical assays. Three birds in the
treated and negative control groups were used to determine
cholinesterase and neuropathy target esterase activity 4 and 48 h
after dosing; these assays were performed for three TOCP-dosed birds
only 48 h after dosing. Analyses of the formulation used indicated
satisfactory content and stability over 24 h. Dosing was followed by
an observation period of 21 days. Birds were assessed daily for ataxia
by observing their ability to walk and to jump onto and off an
obstacle. Body weights were recorded weekly. Nervous tissue from three
TOCP-dosed birds, six negative controls, and six birds dosed orally
with dimethoate was examined histologically.
In the study to determine the LD50, toxicity was seen in a
dose-related manner. Deaths occurred at doses > 45 mg/kg bw, and
all birds at doses > 100 mg/kg died. Deaths occurred up to three
days after dosing, and the surviving birds were normal by day 5. The
LD50 for dimethoate was calculated to be 55 mg/kg bw, with a 95%
confidence interval of 45-67 mg/kg bw. The body weights of survivors
were decreased during the week after treatment but subsequently
increased. The three birds dosed with TOCP that were not sacrificed
for enzyme assays at 48 h developed signs of delayed neurotoxicity
after 13 days, although no clinical signs of cholinesterase inhibition
were seen. All of the birds treated subcutaneously with dimethoate
died within 48 h, after showing clinical signs of cholinesterase
inhibition. After oral administration of 55 mg/kg bw dimethoate, all
birds showed clinical evidence of cholinesterase inhibition. Twelve of
these birds that survived to termination had recovered by day 6 of the
observation period, and none showed signs of delayed neurotoxicity; 12
birds in this group died within 48 h of dosing. Body-weight losses
of up to 10% were seen in treated birds during the first week of
observation, but these losses were subsequently recovered. Microscopic
examination indicated no difference between controls and birds treated
with dimethoate. Brain acetylcholinesterase activity was markedly
reduced in both groups treated with dimethoate; this was more marked
(90% inhibition relative to controls) 4 h after dosing than after 48 h
(61 and 75% inhibition after subcutaneous and oral administration,
respectively). Brain neuropathy target esterase activity was slightly
lower than that in controls in birds treated with dimethoate, but was
markedly lower in TOCP-treated birds. Spinal cord neuropathy target
esterase activity was reduced in TOCP-treated birds but was unaffected
in those that received dimethoate (Redgrave et al., 1991).
Groups of nine or 10 white Leghorn hybrid chickens were given
graded doses of technical-grade dimethoate up to 33 mg/kg bw for three
days, based on the oral LD50 in Japanese quail, in a study designed
to accord with the guidelines then current in eastern Germany and
Poland. Negative and positive controls (TOCP) were used. Antidotes
(atropine sulfate and obidoxime chloride) were administered to treated
birds but not to controls. The route of administration was not stated
but is assumed to have been oral. The precise study design was not
clear from the translation of the original document but indicated
administration of a further series of three doses at intervals
determined by the condition of the birds. Deaths occurred despite use
of the antidotes. Signs of reaction indicative of cholinesterase
inhibition were seen from about 60 min after dosing. Although the
tests indicated that dimethoate has high acute toxicity, there was no
evidence of delayed neurotoxicity, except in the positive controls
(Ministry of Agriculture, Fisheries and Food, 1993a).
(iii) Immunotoxicity
A single dose of dimethoate at 75 mg/kg (route unspecified) to
mice and rats decreased the lymphocyte count to 50% of the value
before exposure and increased the number of neutrophil granulocytes.
After 72 h, these parameters had returned to normal. A reduction in
the thymus cortex, with disrupted lymphocytes, and a reduction in the
number of rosette-forming cells were observed (Tiefenbach & Lange,
1980).
Dimethoate administered to rats at 5-30 mg/kg bw orally or
15 mg/kg bw intramuscularly, twice a week until death, caused
hyperplasia in the bone marrow, resulting mainly in granulocyto-
poiesis. The authors considered the changes to be a direct effect of
dimethoate (Stieglitz et al., 1974).
(iv) Effects on the heart
The effects of dimethoate on the heart have been investigated
in rabbits (Mahkambaeva, 1971), guinea-pigs, and rats (Nadmaiteni &
Marosi, 1983). After oral administration of 150 mg/kg bw to rabbits,
the effects observed included bradycardia and increased atrio-
ventricular and intraventricular conductance, with complete recovery
after four to seven days. In rats and guinea-pigs, a dose-effect
relationship was established for heart rate disturbances and
atrio-ventricular block. An electron microscopic study of the
myocardium showed no changes.
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious electrocardiographic
disturbances developed during the early phase of intoxication. The
toxic cardiac phenomena appeared to be unrelated to the degree of
cholinesterase inhibition but were correlated with the myocardial
concentration of dimethoate. Cardiac failure and death were first
observed at a dose of about 110 mg/kg bw, while a dose of 221 mg/kg bw
resulted in death in all cases. This investigation addressed the
direct effect of dimethoate on the myocardium, independently of its
anticholinesterase action (Marosi et al., 1985a,b).
(v) Studies on metabolites
Omethoate is the oxygen analogue of dimethoate. Information on
the absorption, distribution, excretion, metabolism, and toxicity of
this compound is summarized below, although the original reports were
not available for detailed evaluation.
Absorption, distribution, and excretion of omethoate: After oral
administration of 14C-omethoate at doses of 0.3, 5, or 10 mg/kg bw
to rats, 96-97% of the radiolabel was eliminated in urine, 1-2% in
faeces, and 1% in expired carbon dioxide within 48 h. Intravenous
injection of 0.3 mg/kg bw resulted in a similar, rapid elimination
pattern. Maximal tissue residues were reached 1 h after
administration. After 8 h, about 18% of the residual radiolabel was
found in the body. After two days, < 0.55% of the administered dose
was found. Quantitative analysis and whole-body autoradiography
indicated a relatively homogeneous distribution of 14C activity,
except that a 10-20-fold higher concentration was found in the thyroid
(Weber et al., 1978).
Five male Wistar rats were given 10 mg/kg bw 14C-omethoate
orally in order to obtain preliminary information on pulmonary
excretion. In the main study, groups of five males and five females
received a single intravenous dose of 0.5 mg/kg bw 14C-omethoate, a
single oral dose of 0.5 mg/kg bw 14C-omethoate, 14 daily oral doses
of 0.5 mg/kg bw unlabelled omethoate and a single oral dose of
0.5 mg/kg bw 14C-omethoate on day 15, or a single oral dose of
10 mg/kg bw 14C-omethoate. Urine and faeces were collected over
periods up to 48 h after treatment, and blood samples were collected
until sacrifice 48 h after treatment. Only 0.14% of the administered
radiolabel was detected in expired air. Comparison of the results
obtained with intravenous and oral treatment indicated that > 98%
omethoate had been absorbed from the gastrointestinal tract. Within
48 h, 88-98% of the administered radiolabel was recovered in the
excreta, with 95-98% in the urine and 2-5% in the faeces. Excretion of
the low and the high oral doses was not different in females, but
males at the high dose group tended to excrete more radiolabel in the
faeces than those at the low dose. The maximal plasma concentration
was seen 40-60 min after oral dosing, with an initial half-life of
about 2 h and terminal half-lives of 13-28 h. Less than 0.05% of the
administered dose was found in tissues after 48 h (Ministry of
Agriculture, Fisheries and Food, 1993b).
Biotransformation of omethoate: Urine was collected from two
male rats 12, 24, and 48 h after an oral dose of 50 mg/kg bw
radiolabelled omethoate. The cumulative percentages of administered
radiolabel excreted over the indicated times were 16, 19, and 30%. The
metabolites found in a 24-h composite urine sample by ion-exchange
chromatography were: O,O-dimethylphosphoric acid (34%), unknown A
(52%), O,O-dimethylphosphorothioic acid (9.5%), and unknown B
(4.5%). After treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine within 24 h, while after
treatment with omethoate only 19% was excreted (Dauterman et al.,
1959).
In the experiment conducted by the Ministry of Agriculture,
Fisheries and Food (1993b), the predominant form of excreted
radiolabel was unchanged parent compound (26-62%), with N-methyl-
2-(methylsulfinyl)acetamide accounting for 16-36% and an O-demethylated
omethoate for 4-9%. Pretreatment of animals for 14 days with unlabelled
omethoate followed by a single labelled dose resulted in no significant
difference from the results obtained after a single administration.
Effects of omethoate on enzymes and other biochemical parameters:
Dealkylation of omethoate was proposed to be a significant detoxification
mechanism on the basis of information from assays in fly heads (Aharoni &
O'Brien, 1968). Oxidative metabolism of omethoate results in the
de- N-methyl derivative, which is as toxic as the parent compound
although less active as a cholinesterase inhibitor (Lucier &
Menzer, 1970). Kinetic studies indicated that the reaction between
acetylcholinesterase and omethoate was irreversible and bimolecular.
Omethoate was 75-100 times more potent than dimethoate in inhibiting
rat brain acetylcholinesterase activity.
Acute toxicity of omethoate: The signs of poisoning after a
single dose of omethoate are typical of cholinergic stimulation, as
elicited by other organophosphorus esters. The signs appear 5-60 min
after dosing and include salivation, lacrimation, and tremors. They
may persist for one to three days (Kimmerle, 1968). The LD50 values
are summarized in Table 5.
Short-term toxicity of omethoate: Groups of 50 male and 50
female BOR:NMRI mice were fed diets containing omethoate (purity,
97.1%) providing doses of 0, 1, 3, or 10 ppm for four weeks and were
killed at intervals to investigate brain acetylcholinesterase
activity. There were no clinical signs of reaction to treatment, and
food and water intake, mortality, and body-weight gain were also
unaffected. The cholinesterase activity in plasma was clearly lower
than that in controls in mice receiving 10 ppm, but the differences
were smaller and much less consistent in erythrocytes. Plasma and
erythrocyte cholinesterase activity in mice at 1 and 3 ppm showed no
consistent differences from controls. Brain acetylcholinesterase
activity was clearly depressed in animals at 10 ppm. Inhibition of
brain acetylcholinesterase activity at 3 ppm was inconsistent, but the
level was up to 30% lower than that in contemporary controls. In
animals at 1 ppm, brain acetylcholinesterase activity was biologically
significantly lower than in controls only on day 3 in males (Ministry
of Agriculture, Fisheries and Food, 1993).
Groups of 15 male and 15 female rats (30 of each sex as controls)
were fed diets containing omethoate providing doses of 0, 2.5, 5, 15,
50, or 150 ppm for four months. Signs of cholinergic stimulation was
seen at doses > 15 ppm. Cholinesterase activity was depressed in
females at 50 and 150 ppm and in males at doses > 5 ppm. No effects
were noted on growth, organ weights, blood parameters, or urinary
parameters at levels < 50 ppm. Animals at 150 ppm died or had
depressed body weights and food consumption, and the relative liver
weight in males was increased (Löser & Lorke, 1967).
Groups of 15 male and 15 female rats were fed diets containing
omethoate to provide doses of 0, 0.5, 1.0, 2.0, or 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident in
animals at 4 ppm. Erythrocyte acetylcholinesterase activity was
depressed in animals at 2 ppm, but the effect was only slight in
females. In rats at 4 ppm, the inhibition was 30-50%. No effects were
noted on growth, food consumption, blood parameters, liver and kidney
function tests, organ weights, or histological appearance of tissues
(Löser, 1968).
Table 5. Acute toxicity of omethoate in experimental animals
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse Male Oral 36 Kimmerle (1968)
Mouse Male Oral 27 Santi & de Pietri Tonelli (1960)
Mouse Male Intraperitoneal 13 Lucier & Menzer (1970)
Mouse Male Intravenous 23 Kimmerle (1962)
Rat Male, female Oral 28-65 Kimmerle & Lorke (1967)
Rat Male, female Oral 50 Ben-Dyke et al. (1970)
Rat Male, female Oral 22-28 Ministry of Agriculture,
Fisheries and Food (1993b)
Rat Male Intraperitoneal 14 Kimmerle (1968)
Rat Male Intraperitoneal 38 Kimmerle (1962)
Rat Male, female Dermal 145-232 Ministry of Agriculture,
Fisheries and Food (1993b)
Rabbit Male Oral 50 Kimmerle (1962)
Guinea-pig Male Oral 100 Kimmerle (1962)
Cat Male Oral 50 Kimmerle (1962)
Chicken Male Oral 125 Kimmerle (1962)
Chicken Male Oral 100 Levinskas & Shaffer (1965)
Groups of six male and six female beagle dogs received omethoate
(purity, 97.1%; dissolved in acidulated water) daily for 12 months
by stomach tube at doses of 0, 0.02, 0.125, or 0.625 mg/kg bw.
Administration by gavage was chosen due to the reported instability of
the test material in dietary admixture. The appearance and behaviour
of the animals were normal, and no clinical signs attributable to
treatment were observed. All of the animals survived the treatment.
No significant differences were seen between the control and treated
groups with respect to reflexes, ophthalmoscopic parameters, body
temperatures, pulse rate, food and water consumption, mean
body weight, or haematological, clinical chemical (except for
cholinesterase activity), or urinary parameters. Clear depression
of plasma cholinesterase activity was observed only in rats at
0.625 mg/kg bw, amounting to 25-32% of the control value in males
and 16-29% in females. The depression remained essentially
constant throughout the study. A marked depression of erythrocyte
acetylcholestinerase activity was measured in males (17-40%) and
females (22-40%) at 0.625 mg/kg bw, which varied only slightly during
the study. At 0.125 mg/kg bw, only males showed slight (< 28%)
depression of erythrocyte acetylcholinesterase activity during the
first third of the study. Brain acetylcholinesterase activity was
depressed in males at 0.125 mg/kg bw (by 20%) and 0.625 mg/kg bw (39%)
and in females at 0.625 mg/kg bw (30%). The absolute and relative
organ weights were not significantly different between the control and
treated groups. Gross pathological and histopathological examination
showed no dose-related findings. The NOAEL was 0.62 mg/kg bw for
somatic effects and 0.02 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity (Hoffmann & Schilde, 1984).
Long-term toxicity and carcinogenicity of omethoate: Groups of
50 male and 50 female BOR:CWF1 mice were fed diets containing
omethoate (purity, 94%) providing levels of 0, 1, 3, or 10 ppm for 24
months. Appearance, behaviour, and activity were not significantly
different between the control and treated groups, and total and mean
daily food consumption were essentially the same in all the animals.
The body weights of treated males were generally higher than those of
the controls throughout the experiment, whereas those of the females
were no different from controls. Mortality and the frequency
distribution of mortality were comparable in all the groups. The
mortality rate at 18 months was 12-27% for males and 14-31% for
females. The absolute and relative organ weights of control and
treated groups showed no dose-related, significant differences. Gross
anatomical and histopathological examination revealed a range of
non-neoplastic changes commonly observed in old mice. Comparison of
these changes by type, site, and frequency distribution by sex and
dose gave no indication of treatment-related toxic effects. Neoplastic
changes were found primarily in the lungs, liver, adrenal cortex,
and haematopoietic system. Neither the type, site, or frequency
distribution of tumours by sex and dose level nor the numbers of
tumour-bearing mice, mice with benign tumours, mice with malignant
tumours, or mice with both benign and malignant tumours indicated
effects of treatment. The NOAEL for somatic effects was 10 ppm, equal
to 2.1 mg/kg bw per day for male mice and 3.1 mg/kg bw per day for
female mice (Kroetlinger & Löser, 1982).
Four groups of 50 male and 50 female Wistar rats were maintained
for 24 months on a diet containing omethoate providing concentrations
of 0, 0.3, 1,3, or 10 ppm. The control group consisted of 100 males
and 100 females. Omethoate did not clearly affect behaviour, body
weight, survival, food intake, or haematological, clinical chemical,
or urinary parameters. Plasma and erythrocyte cholinesterase
activities, measured in five males and five females from each group
at 1, 2, 4, 8, 13, 26, 52, and 78 weeks and at the end of the study,
were significantly depressed in all animals at 10 ppm. Erythrocyte
acetylcholinesterase activity was also inhibited in animals of each
sex at 3 ppm. The suppression of brain acetylcholinesterase activity,
measured in 10 males and 10 females per group, was dose-related in
animals at 3 and 10 ppm; it was also significantly affected in females
at 1 ppm, which can be considered the marginal no-effect level. Gross
and microscopic examination revealed no diverse effect of omethoate.
The tumour incidence was not clearly affected by treatment (Bomhard
et al., 1979).
Reproductive toxicity of omethoate: Groups of 10 male and 20
female FB 30 Long-Evans rats were fed diets containing omethoate
(purity, 94%) to give concentrations of 0, 1, 3, or 10 ppm for about
10 weeks, after which they were mated to initiate a three-generation
study of reproductive toxicity with two litters per generation.
Immediately after birth, the litters were examined for malformations.
Four days after birth, the litters were reduced to 10. When the
offspring were three weeks old, they were killed and subjected to
gross examination. Ten males and 10 female rats of the F3b generation
at all doses were examined histopathologically four weeks after birth.
There were no clear effects either on mating performance, pregnancy
rate, mortality, or the type and distribution of abnormalities. The
size of the litters of the second generation at 3 and 10 ppm was
reduced, and in the F2b generation, litter size was reduced at both 3
and 10 ppm after four days and at 10 ppm only after 28 days. Since
this effect was observed in only one progeny generation, 3 ppm was the
NOAEL (Löser, 1981).
In a two-generation study, omethoate was administered to groups
of 25 male and 25 female Wistar rats in the drinking-water at levels
of 0, 0.5, 3, or 18 ppm throughout a 70-day premating period and
throughout pairing, gestation, and lactation during breeding of a
single litter in each of the F1 and F2 generations. Reproductive
performance was adversely affected at 18 ppm, with a reduced
implantation rate, increased postnatal loss, and retarded pup weight
gain in both generations and increased precoital time, an increased
number of non-pregnant females, and increased postimplantation loss in
the F1 generation. Histopathological examination revealed an
increased incidence of epithelial vacuolation in the epididymides of
males treated with 18 ppm. The NOAEL for reproductive effects was
3 ppm, equivalent to 0.2 mg/kg bw per day. There was no NOAEL
for general toxicological effects, since erythrocyte and brain
acetyl-cholinesterase activities were inhibited at the lowest dose
(Ministry of Agriculture, Fisheries and Food, 1993).
Developmental toxicity of omethoate: Groups of 20-24 pregnant
rats were given omethoate orally at doses of 0, 0.3, 1, or 3 mg/kg bw
on days 6-15 of gestation. The animals were killed on day 20 of
gestation, and the fetuses were examined for skeletal and tissue
abnormalities. The fetuses and placentas of the animals at 3 mg/kg bw
weighed less than those of the controls. Other reproductive parameters
were unaffected. No teratogenic effect was observed (Machemer, 1975).
Groups of 14 pregnant New Zealand white rabbits were treated
daily by gavage with omethoate (purity, 96.8%) dissolved in distilled
water at doses of 0, 0.1, 0.3, or 1 mg/kg bw on days 6-18 of
gestation. On day 29 of gestation, the animals were killed and their
uterine contents examined. Whole-blood cholinesterase activity was
determined before treatment on day 6 of gestation and 2 h after
treatment on day 18 of gestation. The general condition of control and
treated females was comparable throughout the study. Maternal mean
body weights and corrected day-29 body weights were unaffected by the
treatment. Mortality, the incidence of abortions and total litter
losses, and the number of pregnant females with viable young on day 29
were not altered by treatment. Whole-blood cholinesterase activity was
significantly depressed only among females at 1 mg/kg bw in comparison
with both the pretreatment level and the control level after
treatment. There were no treatment-related differences between the
control and treated groups with respect to corpora lutea count,
implantations, male and female viable young, early and late
resorptions, pre- and postimplantation losses, or fetal and placental
weights. Examination of fetuses at necroscopy on day 29 of gestation
or after skeletal investigation revealed a number of non-dose-related
findings of types and incidences previously recorded in this strain of
rabbit and in the laboratory that performed the study. The NOAEL for
developmental toxicity was 1 mg/kg bw (Tesh et al., 1982).
Genotoxicity of omethoate: Omethoate has been extensively
tested in assays for mutagenicity in vitro. Positive results were
obtained in Salmonella, in one assay for gene mutation in mammalian
cells, and in assays for clastogenicity. Omethoate has also been
extensively tested in vivo. Negative results were obtained for
end-points in the bone marrow, liver, and germ cells, but a positive
result was obtained in a mouse spot test. The results of assays for
the genotoxicity of omethoate are summarized in Table 6.
Neurotoxicity of omethoate: Groups of 10 hens were given
omethoate orally at the LD50 (92 mg/kg bw) with atropine, and and
five positive controls were given TOCP at 350 mg/kg bw. Although
several hens treated with omethoate died, none showed clinical signs
of delayed neurotoxicity. Clinical signs were observed in those
treated with TOCP (Kimmerle, 1972). Histological examination of
nervous tissue with haematoxylin and eosin staining showed
degeneration in the hens treated with TOCP but not in those given
omethoate (Newman et al., 1972).
Groups of two to four hens were treated orally with omethoate
dissolved in corn oil at doses of 20-300 mg/kg bw, which were four
to eight times the unprotected LD50, under eserine and atropine
protection. The omethoate used was a sample that had caused a fatal
human poisoning accident. The acetylcholinesterase and neurotoxic
esterase activities of brain homogenates were assayed for 24 h after
dosing, and pair-dosed birds that survived were observed for signs of
ataxia for three to four weeks. Hens treated at four times the LD50
showed no inhibition of neurotoxic esterase at 24 h and no signs of
ataxia. Those treated at eight times the LD50 did not survive,
despite treatment with high doses of atropine; however, the neurotoxic
esterase activity in the brains of the animals that died within 36 h,
measured immediately after death, was found to be normal. Acute
cholinergic symptoms in all the birds were correlated with strong
inhibition of brain acetylcholinesterase activity, but 70% inhibition
in a bird treated with 20 mg/kg bw of omethoate still did not produce
detectable signs of acute poisoning.
The capacity of pure omethoate and of the incriminated sample to
inhibit neurotoxic esterase and acetylcholinesterase activities were
measured in hen and human brain tissue in vitro. As the IC50 for
acetylcholinesterase in both tissues was 0.08-0.15 mmol/litre, it
would be virtually completely inhibited at 5 mmol/litre, the
concentration that caused no detectable inhibition of neurotoxic
esterase. The activities of both enzymes were also measured in
cortical tissue samples taken 24 h post mortem from a 30-year-old
male farmer who had been acutely poisoned by a commercial formulation
of omethoate. The neurotoxic esterase activity was within the normal
range, while acetylcholinesterase activity was strongly inhibited. It
was concluded that omethoate is extremely unlikely to cause delayed
neuropathy in humans (Lotti et al., 1981).
Table 6. Results of tests for the genotoxicity of omethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0-12 500 µg/plate 95.1 Weakly positive Herbold (1980)
TA98, TA100, in TA98, TA100, and
TA1535, TA1537 TA1535a Negative in
TA1537a
DNA repair E. coli (pol) 0-10 000 µg/plate 96 Negative Herbold (1983)
Gene mutation Chinese hamster 0-6 mg/ml 97.4 Positivea Ministry of Agriculture,
ovary cells (hprt) Fisheries and Food
(1993)
Cell mutation L5178Y mouse 0-5000 µg/ml 96.9 Negativea Bootman & Rees (1982)
lymphoma cells
Sister chromatid Chinese hamster 0-1000 µg/ml 96 Positive at > 250 µg/ml Ministry of Agriculture,
exchange ovary cells Fisheries and Food
(1993)
Gene conversion; S. cerevisiae 0-66.7 µl/ml 96.9 Negative Hoorn (1982, 1983)
reverse mutation Positive
In vivo
Micronucleus Mouse 2 × 6 or 12 97.1 Negative Herbold (1981)
formation mg/kg bw
Dominant lethal Mouse 0.5 mg/kg bw 95.4 Negative Machemer (1974)
mutation
Unscheduled DNA Wistar rat 0-30 mg/kg bw 96.6 Negative Ministry of Agriculture,
synthesis Fisheries and Food
(1993)
Spot test C57Bl/6J × T 0-16 mg/kg bw 96.7-97% Positive Ministry of Agriculture,
mice Fisheries and Food
(1993)
a With and without metabolic activation
3. Observations in humans
The results of two studies of dimethoate in humans were
summarized briefly in the report of the 1963 JMPR (Annex 1, reference
2). In the first study, 20 volunteers were given daily doses of 2.5 mg
dimethoate in aqueous solution (corresponding to approximately
0.04 mg/kg bw) for four weeks. No toxic effect was observed, and there
were no changes in blood cholinesterase activity. Similar results were
reported in single subjects who ingested 9 mg (0.13 mg/kg bw) or 18 mg
(0.26 mg/kg bw) for 21 days (Sanderson & Edson, 1964).
The results of a number of studies in which human volunteers with
no occupational exposure to organophosphate pesticides were given
dimethoate were summarized in Environmental Health Criteria monograph
No. 90 (WHO, 1989) and are presented in Table 7. The studies of
volunteers indicate that repeated doses of up to 0.2 mg/kg bw
dimethoate do not inhibit cholinesterase activity in the blood.
Table 7. Results of controlled human trials with dimethoate
No. of Sex Route Daily dose Duration of Results Reference
subjects exposure
20 NR Oral 0.04 mg/kg 4 weeks No toxic effects or inhibition of Sanderson & Edson
blood ChE (1964)
2 NR Oral 0.13 mg/kg 21 days No toxic effects or inhibition of Sanderson & Edson
0.26 mg/kg blood ChE (1964)
5 M Oral 0.25 mg/kg Single No toxic effects or inhibition of Sanderson & Edson
dose blood ChE (1964)
50 NR Dermala 2.5 ml 2 h No irritation or inhibition of blood Sanderson & Edson
ChE (1964)
12 M+F Oral 5 mg (0.068 mg/kg bw) 28 days No significant change in whole-blood Edson et al. (1967)
ChE
9 M+F Oral 15 mg (0.202 mg/kg bw) 39 days No significant change in whole-blood Edson et al. (1967)
ChE
8 M+F Oral 30 mg (0.434 mg/kg bw) 57 days Inhibition of ChE by day 20 (24%) Edson et al. (1967)
6 NR Oral 45 mg (0.587 mg/kg bw) 45 days Inhibition of ChE (35%) Edson et al. (1967)
6 M+F Oral 60 mg (1.02 mg/kg bw) 14 days Inhibition of ChE (21%) Edson et al. (1967)
NR, not reported; ChE, cholinesterase
a Patch test with 32% liquid formulation
Comments
Dimethoate
Dimethoate is rapidly and extensively absorbed from the gut and
rapidly excreted. There was no accumulation in fat tissue. In rats and
humans, up to 90% of radiolabel was found in the urine within 24 h.
The report of a study with methylcarbamoyl-labelled dimethoate
indicated that up to 18% of the administered label was excreted in
expired air. Four metabolites with anticholinesterase activity have
been identified in rats and humans. One seems to result from thiono
oxidation, leading to the formation of the oxygen analogue of
dimethoate, i.e. omethoate; this step was followed by hydrolysis to
a thiocarboxyl product, said to be the main metabolite in rats and
humans.
Data on the acute oral toxicity of dimethoate gave LD50 values
of about 310 mg/kg bw in rats, 150 mg/kg bw in mice, and 55 mg/kg bw
in hens. The signs of toxicity were those typical of cholinesterase
inhibition. WHO has classified dimethoate as 'moderately hazardous'
(WHO, 1996).
In short-term and long-term studies at dietary concentrations
> 75 ppm, there were minor reductions in body-weight gain and food
consumption. Apart from inhibition of cholinesterase activity,
dimethoate had no effect on the composition of the blood or urine. The
liver weights of animals treated at the higher doses tended to be
lower than those of the control groups: there were, however, no
microscopic changes, and the effect is unlikely to be of toxicological
significance. Investigations of toxicity at higher doses were limited
by effects due to cholinesterase inhibition. The NOAELs were thus
generally based on reductions in acetylcholinesterase activity in
the brain or erythrocytes. On the basis of minimal reductions in
acetylcholinesterase activity of 10-20%, the NOAEL in a 12-month study
in dogs at doses of 0, 5, 20, or 125 ppm was 5 ppm, equal to 0.2 mg/kg
bw per day; in rats, the NOAEL in a life-span study at doses of 0, 1,
5, 25, or 100 ppm was 1 ppm, equal to 0.04 mg/kg bw per day. In mice,
an NOAEL was not identified, as cholinesterase activity was depressed
at all doses after 52 weeks of treatment in a life-span study at doses
of 0, 25, 100, or 200 ppm.
The results of long-term studies of toxicity and carcinogenicity
in mice (at 0, 25, 100, or 200 ppm) and rats (at 0, 5, 25, or 100 ppm)
reported in 1986 and studies reported in 1977 indicate that dimethoate
is not carcinogenic to rodents.
In a multigeneration study of reproductive toxicity conducted in
1989-90 with doses of 0, 1, 15, or 65 ppm, reproductive performance
of rats was impaired at the high dose. The NOAEL for reproductive
toxicity appeared to be 15 ppm (equal to 1.2 mg/kg bw per day) and
that for parental toxicity was 1 ppm (equal to 0.08 mg/kg bw per day)
on the basis of cholinesterase inhibition, but the Meeting noted that
there was some indication that reproductive performance may have been
affected at lower doses. In a multigeneration study conducted in mice
in 1965 at doses of 0, 5, 15 or 50 ppm, there was no overt effect on
reproductive capacity, even in the presence of cholinergic toxicity.
In a poorly reported study in rabbits, sperm numbers and quality were
adversely affected at doses equivalent to one-tenth and one-hundredth
of the LD50.
Studies of developmental toxicity in rats (at 0, 3, 6, or
18 mg/kg bw per day on days 6-15 of gestation) and rabbits (at 0, 10,
20, or 40 mg/kg bw per day on days 7-19 of gestation) provided no
evidence of a teratogenic effect, although maternal toxicity was
observed at the high dose in rats and at the high and middle doses in
rabbits.
After reviewing the data available on mutagenicity, the Meeting
concluded that although in-vitro studies indicate that dimethoate has
mutagenic potential, this potential does not appear to be expressed
in vivo.
Undiluted dimethoate formulations were irritating to the eye in
rabbits. Skin irritation was minimal and confined to slight, transient
erythema. Dimethoate was not a skin sensitizer in guinea-pigs, but a
32.7% emulsifiable concentrate formulation induced sensitization in
one of 10 guinea-pigs. In a published paper, dimethoate was cited
in four human cases of contact dermatitis, and sensitization was
confirmed in these individuals by patch testing.
In hens given a single dose of 55 mg/kg bw by subcutaneous
injection or orally, dimethoate did not induce delayed neurotoxicity.
In a 39-day study in nine male and female volunteers, the NOAEL
for cholinesterase inhibition was 0.2 mg/kg bw per day. This NOAEL was
supported in seven other studies each involving 6-20 volunteers who
received doses ranging from 0.04 to 1.0 mg/kg bw per day for up to 57
days.
Omethoate
The oral LD50 of omethoate was approximately 25 mg/kg bw
in rats. Signs of reaction to treatment with omethoate were those
consistent with cholinesterase inhibition.
In short-term and long-term studies, the potential toxicity of
omethoate was limited by the onset of cholinesterase inhibition. In a
12-month study of toxicity in dogs at doses of 0, 0.025, 0.12, or
0.62 mg/kg bw per day by gavage, the NOAEL was 0.02 mg/kg bw per day
on the basis of inhibition of acetylcholinesterase activity. In
life-span studies in rats (at 0, 0.3, 1, 3, or 10 ppm) anti mice (at
0, 1, 3, or 10 ppm), there was no evidence of oncogenic potential. The
study in mice was unsuitable for deriving an NOAEL because acetyl-
cholinesterase activity was not investigated; the NOAEL in rats was
0.3 ppm (0.015 mg/kg bw per day) on the basis of inhibition of
acetylcholinesterase activity.
In multigeneration studies in rats at 0, 1, 3, or 10 ppm, a
dietary concentration of 10 ppm was associated with reduced viability
of pups; there was evidence that this effect extended to animals
treated at 3 ppm. The NOAEL was 1 ppm (equivalent to 0.05 mg/kg bw per
day). In a further multigeneration study in rats at doses of 0, 0.5,
3, or 18 ppm in the drinking-water, there was evidence of epididymal
vacuolation and fewer pups per dam at the high dose; these pups had
lower weight gains and were less viable. The precoital time was
increased and the number of non-pregnant females was greater than
among controls. The NOAEL for reproductive performance was 3 ppm
(equivalent to 0.2 mg/kg bw per day), but cholinesterase inhibition
was detected at the lowest dose of 0.5 ppm. In studies of
developmental toxicity, there was no evidence of teratogenicity in
rats given 0, 0.3, 1, or 3 mg/kg bw omethoate per day on days 6-15 of
gestation or in rabbits given 0, 0.1, 0.3, or 1 mg/kg bw omethoate per
day on days 6-18 of gestation.
Omethoate has been extensively investigated for mutagenicity in
vitro and in vivo. The Meeting concluded that it has clear mutagenic
potential but that the weight of the evidence observed in vivo was
negative; however, the positive result obtained in the mouse spot test
could not be completely disregarded.
In studies in hens given single oral doses of 20-300 mg/kg bw,
omethoate did not induce delayed neurotoxicity.
Conclusions
An ADI of 0-0.002 mg/kg bw was established for dimethoate on the
basis of the apparent NOAEL of 1.2 mg/kg bw per day for reproductive
performance in the study of reproductive toxicity in rats and applying
a safety factor of 500. Although a safety factor of 100 would normally
be used in deriving an ADI from a study of this type, the Meeting was
concerned about the possibility that reproductive performance may have
been affected at 1.2 mg/kg bw per day in this study and therefore used
a higher-than-normal safety factor. No data are available to assess
whether the effects on reproductive performance were secondary to
inhibition of cholinesterase. The Meeting concluded that it was
not appropriate to base the ADI on the results of the studies of
volunteers since the crucial end-point (reproductive performance) has
not been assessed in humans.
This ADI would usually be used only when assessing the intake of
dimethoate itself. As the use of dimethoate on crops can give rise to
residues of omethoate, and omethoate has been used as a pesticide in
its own right, previous Joint Meetings have allocated an ADI to
omethoate; however, the primary manufacturer is no longer producing
omethoate. The Meeting noted that omethoate is considerably more toxic
than dimethoate; however, the levels of residues of omethoate
resulting from use of dimethoate on crops are likely to be low. The
Meeting therefore recommended that residues of dimethoate and
omethoate resulting from the use of dimethoate be expressed as
dimethoate and be assessed in comparison with the ADI for dimethoate.
As the primary manufacturer is no longer producing either
omethoate or formothion, toxicological data on these compounds were
not made available to the Meeting. The previous ADIs of 0-0.0003 mg/kg
bw for omethoate and 0-0.02 mg/kg bw for formothion were therefore
withdrawn.
There may be a need to re-evaluate the toxicity of dimethoate
after the periodic review of the residue and analytical aspects of
dimethoate has been completed if it is determined that omethoate is a
major residue.
Toxicological evaluation
Levels that cause no toxic effect (dimethoate)
Rat: 1 ppm, equal to 0.04 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
15 ppm, equal to 1.2 mg/kg bw per day (reproductive
performance in a study of reproductive toxicity)
1 ppm, equal to 0.08 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
6 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.2 mg/kg bw per day (52-week study of
toxicity)
Human: 0.2 mg/kg bw per day (39-day study of cholinesterase
inhibition)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw (sum of dimethoate and omethoate expressed as
dimethoate)
Studies that would provide information useful for continued evaluation
of the compound
1. Further multigeneration study in rats using dimethoate
2. Mouse spot test using dimethoate
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to dimethoate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 310 mg/kg bw
Dermal, toxicity, rat LD50 > 7000 mg/kg bw
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, human Sensitizing
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL > 1000 mg/kg bw per day (highest dose tested)
Repeated oral, reproductive toxicity, rat NOAEL = 1.2 mg/kg bw per day, reproductive toxicity
NOAEL = 0.05 mg/kg bw per day, parental toxicity
Repeated oral, developmental toxicity, rat NOAEL = 6 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 18 mg/kg bw per day (highest dose tested)
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 40 mg/kg bw per day (highest dose tested)
Long term (> 1 year) Repeated oral, toxicity and carcinogenicity, rat NOAEL = 0.04 mg/kg bw per day, cholinesterase
inhibition
References
Aharoni, A.H. & O'Brien, R.D. (1968) The inhibition of acetyl-
cholinesterases by anionic organophosphorus compounds. Biochemistry,
7, 1538.
Ahmed, F.E., Hart, R.W. & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res., 42, 161-174.
Becker, H. (1985) Dominant lethal study with dimethoate technical in
the mouse. Unpublished report No. 039003 from Research & Consulting
Company AG, Itingen, Switzerland. Unpublished report submitted to WHO
by the Dimethoate Task Force, Ingelheim, Germany.
Ben-Dyke, R., Sanderson, D.M. & Noakes, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9, 119.
Bhunya, S.P. & Behera, J. (1975) Centromeric sensitivity of mouse
chromosomes to the systemic insecticide dimethoate (Rogor). Curr. Sci.,
44, 859-860.
Bomhard, E., Loser, E. & Kaliner, G. (1979) S-6876: Chronic toxicity
study on rats (two-year feeding experiment). Unpublished report from
the Bayer AG Institut für Toxikologie, originally submitted to WHO by
Bayer AG, summary from WHO.
Bootman, J. & Rees, R. (1982) S-6876: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report from Life Science Research, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
Brady, U.E., Jr & Arthur, B.U. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56, 477-482.
Brooker, A.J., Homan, B.A., Parker, C.A., Offer, J.M., Anderson, A. &
Dawe, I.S. (1992) The effect of dimethoate on reproductive function
of two generations in the rat. Unpublished report from Huntingdon
Research Centre, United Kingdom. Submitted to WHO by the Dimethoate
Task Force, Milan, Italy.
Budreau C.H. & Singh, R.P. (1973) Effect of fenthion and dimethoate on
reproduction in the mouse. Toxicol. Appl. Pharmacol., 26, 29-38.
Burford, P., McLean, T.A., Buist, D.P., Crook, D., Gregson, R.L. &
Gopinath, C. (1991) Dimethoate: 12 month dietary toxicity study in
beagle dogs. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Casida, J.E. & Sanderson, D.M. (1962) Toxic hazards from formulating
the insecticide dimethoate in methyl 'cellosolve'. Nature, 189,
507-508.
Chamberlain, W.R., Gatterdam, P.E. & Hopkins, D.E. ( 1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54, 733-740.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorous pesticides.
Mutat. Res., 88, 307-316.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health, B20, 373-406.
Dal Re, V. & Vola Gera, F. (1976) Determination of the acute dermal
toxicity of technical Rogor. Unpublished report from Centro Ricerche
Antiparassitari, Montedison, Italy. Submitted to WHO by Farmoplant,
Milan, Italy.
Dal Re, V. & Vola Gera, F. (1980) Acute oral toxicity of technical
Rogor samples recently produced in albino rats. Unpublished report
from Centro Ricerche Antiparassitari Montedison, Italy. Submitted to
WHO by Farmoplant, Italy.
Dauterman, W.C., Casida, J.E., Knaak, J.B. & Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agric. Food Chem., 7, 188-193.
Degraeve, N. & Moutschen, J. (1983) Genotoxicity of an organophosphorus
insecticide, dimethoate, in the mouse. Mutat. Res., 119, 331-337.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo.
Arch. Toxícol., 58, 152-156.
Ecobichon, D.J. & Kalow, W. (1963) Action of organo phosphorus
compounds upon esterases in human liver. Can. J. Biochem., 41, 1537.
Edson, E.F. & Noakes, D.N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. Appl. Pharmacol.,
2, 523.
Edson, E.F., Jones, K.H. & Watson, W.A. (1967) Safety of dimethoate
insecticide. Br. Med. J., iv, 554.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984a) Effect of dimethoate
on pregnancy of the rat. Unpublished report from Huntington Research
Centre, Huntington, Cambs, United Kingdom. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984b) Effect of dimethoate
on pregnancy of the New Zealand white rabbit. Unpublished report from
Huntington Research Centre, Huntington, Cambs, United Kingdom.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
El-Elaimy, I. (1986) Comparative studies on the effect of carbamate
and organophosphorus insecticides on acetylcholinesterase activity in
maternal and foetal tissue of rat. Proc. Zool. Soc. A.R. Egypt, 10,
41-49.
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomatis, L., eds, Chemical Carcinogenesis Essays
(IARC Scientific Publications No. 10), Lyon, International Agency for
Research on Cancer, pp. 161-181.
Fenwick, M.L., Barron, J.R. & Watson, W.A. (1957) Biochem. J., 65, 58.
Fischer, G.W. & Scheufler, H. (1981) Effects of dimethoate and
O-desmethyldimethoate in dominant lethal test in mice. Wiss. Z.
Univ. Halle, 30, 21-29 (in German).
Gabliks, J. (1965a) Responses of cell cultures to insecticides II.
Chronic toxicity and induced resistance. Proc. Soc. Exp. Biol. Med.,
120, 168-171.
Gabliks, J. (1965b) Responses of cell cultures to insecticides. III.
Altered susceptibility to poliovirus and diphtheria toxin. Proc. Soc.
Exp. Biol. Med., 120, 172-175.
Gabliks, J. & Friedman, L. (1965) Responses of cell cultures to
insecticides. I. Acute toxicity to human cells. Proc. Soc. Exp. Biol.
Med., 120, 163-168.
Gibel, U., Lohs, KH., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) Experimental study on cancerogenic, haematotoxic, and
hepatotoxic activity of phosphororganic pesticides. Arch.
Geschwulstforsch., 41, 311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade6
locus of Schizosaccharomyces pombe. Mutat. Res., 117, 139-148.
Gomez-Arroyo, S., Noriega-Aldana, N., Juarez-Rodriguez, D. &
Villalobos-Pietrinir, J. (1987) Sister chromatid exchanges induced by
the organophosphorus insecticides methyl parathion, dimethoate, phoxim
and methyl azinfos in cultured human lymphocytes. Contam. Amb.,
3, 63-70.
Guthrie, F.E., Shah, P.V. & Moreland, D.E. (1980) Effects of pesticides
on active transport of glucose through the isolated intestine of the
mouse. J. Agric. Food Chem., 22, 3.
Hassan, A., Zayed, S.M.A.D. & Bahig, M.R.E. (1969) Metabolic behaviour
of organophosphorus insecticides. XI: Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18, 2429-2438.
Hellwig, J. (1986a) Report on the study of the toxicity of dimethoate
in mice after 78-week administration in the diet. Unpublished report
No. 75C0326/8242 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Hellwig, J. (1986b) Report on the study of the toxicity of dimethoate
in rats after 24-month administration in the diet. Unpublished report
No. 70C0326/8241 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Herbold, B. (1980) S-6876 Salmonella/Mikrosomen Test zur Untersuchung
auf punktmutagene Wirking. Unpublished report from the Bayer AG
Institut für Toxikologie, originally submitted to WHO by Bayer AG,
summary from WHO.
Herbold, B. (1981) S-6876 (Omethoate, Folimat's active ingredient):
Micronucleus test on mouse to evaluate S 6876 for mutagenic potential.
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Herbold, B. (1983) S-6876 (Omethoate, Folimat's active ingredient):
Pol test on E. coli to evaluate for DNA damage. Unpublished report
from Institute of Toxicology, Bayer AG, originally submitted to WHO by
Bayer AG, summary from WHO.
Hoffman, K. & Schilde, B. (1984) S-6876 (Omethoate): Chronic toxicity
to dogs on oral administration (twelve month stomach tube study).
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG. summary from WHO.
Hoorn, A.J.W. (1982) Mutagenic evaluation of S-6876 (omethoate) in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138/S211-alfa. Unpublished report from Litton Bionetics, Netherlands,
originally submitted to WHO by Bayer AG, summary from WHO.
Hoorn, A.J.W. (1983) Evaluation of S-6876 (omethoate) in the induced
mitotic crossing over and gene conversion assay in Saccharomyces
cerevisiae strain D7. Unpublished report from Litton Bionetics,
Netherlands, originally submitted to WHO by Bayer AG, summary from
WHO.
Johnson, E., Caterson, C.R. & Allen, J.S. (1985) Mutagenicity testing
of dimethoate in the in vitro CHO/HGPRT mutation assay. Unpublished
report No. 0423 from American Cyanamid Co., Princeton, NJ, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
von Jung, H.-D. (1989) Kontaktekzem durch pestzide in der DDR.
Dermatol. Monaschr., 175, 203-214 (in German with English summary).
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
R.E. & Treiber, G. (1959) The metabolism of dimethoate in cattle.
J. Econ. Entomol., 52, 1190-1194.
Khera, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for teratogenic
activity in the cat. J. Environ. Pathol. Toxicol., 2, 1283-1288.
Khera, K.S., Whalen, C., Trivett, G. & Angers, G. (1979) Teratogenicity
studies on pesticidal formulations of dimethoate, diuron and lindane in
rats. Bull. Environ. Contam. Toxicol., 22, 522-529.
Kimmerle, G. (1962) Toxicological studies on active ingredient S-6876.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1968) Bayer 45432-Toxikologische Untersuchungen.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1972) Folimat/Akute Neurotoxizitatsuntersuchungen an
Huhnern. Unpublished report from the Institut für Toxikologie,
Wuppertal-Elberfeld, Germany, originally submitted to WHO by Bayer AG,
summary from WHO.
Kimmerle, G. & Lorke, D. (1967) S-6876. Antidotal effect and
potentiation. Unpublished report originally submitted to WHO by Bayer
AG, summary from WHO.
Kroetlinger, F. & Löser, E. (1982) S-6876 (omethoate, the active
ingredient of Folimat): Chronic toxicity study on mice. (2-year
feeding experiment). Unpublished report from Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Levinskas, G.J. & Shaffer, C.B. (1965) Oxygen analogue of dimethoate:
Demyelination in white Leghorn hens. Unpublished report originally
submitted to WHO by American Cyanamid Co., summary from WHO.
Löser, E. (1968) Bayer 45432: Subakute Toxikologische Untersuchungen
an Ratten. Unpublished report originally submitted to WHO by Bayer AG,
summary from WHO.
Löser, E. (1981) S-6876 Folimat-Wirkstoff Multigenerationeversuche an
Ratten. Unpublished report from Bayer AG, originally submitted to WHO
by Bayer AG. Addendum: Histopathology, Consultox Laboratories Ltd,
London, United Kingdom. Summary from WHO.
Löser, E. & Lorke, D. (1967) Bayer 45432: Subchronische Toxikologische
Untersuchungen an Ratten. Unpublished report originally submitted to
WHO by Bayer AG, summary from WHO.
Lotti, M., Ferrara, S.D., Caroldi, S. & Sinigaglia, F. (1981) Enzyme
studies with human and hen autopsy tissue suggest omethoate does not
cause delayed neuropathy in man. Arch. Toxicol., 48, 265-270.
Lucier, G.W. & Menzer, R.E. (1970) Nature of oxidative metabolites of
dimethoate formed in rats, liver microsomes, and bean plants. J. Agric.
Food Chem., 18, 698.
Machemer, L. (1974) Praparat S-6876 to Dominant Letal Test an der
mannlichen Maus zur Prufung auf mutagene Wirkung. Unpublished report
from the Institut für Toxikologie, Wuppertal-Elberfeld, Germany,
originally submitted to WHO by Bayer AG, summary from WHO.
Machemer, L. (1975) Praparat S-6876 to Untersuchung auf Exbryotoxische
and Teratogene Wirkung nach Oraler Verabreichung an der Ratte.
Unpublished report from the Institut für Toxikologie, Wuppertal-
Elberfeld, Germany, originally submitted to WHO by Bayer AG, summary
from WHO.
Madison, W.A., MacKenzie, K.M. & Fields, W.E. (1984) 21 Day dermal
study with dimethoate in rabbits. Unpublished report No. 6123-125
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by the Dimethoate Task Force, Milan, Italy.
Ministry of Agriculture, Fisheries and Food (1993a) Evaluation on
Dimethoate, Pesticides Safety Directorate, United Kingdom.
Ministry of Agriculture, Fisheries and Food (1993b) Evaluation on
Omethoate, Pesticides Safety Directorate, United Kingdom.
Mahkambaeva, Z.G. (1971) Influence of organophosphorus pesticides
(kilval, methylmercaptophos, fosfamid) on bioelectric activity of
heart in experimental animals. In: Hygiene, Use and Toxicology of
Pesticides, and Clinical Aspects of Pesticide Poisoning, Kiev, Zdorovye
Publishers, pp. 175-180 (in Russian).
Marosi, G., Ivan, J., Nagymajtenyi, L., Csatlos, J. & Toszegi, A.
(1985a) Dimethoate induced toxic cardiac failure in the guinea-pig.
Arch. Toxicol., 57, 142-143.
Marosi, G., Ivan, J. & Nagymajtenyi, L. (1985b) Cardiodepression in
organophosphate poisonings. Arch. Toxicol., Suppl. 8, 289-291.
Mohn, G. (1973) 5-Methyl tryptophan resistance mutations in
Escherichia coli K-12. Mutat. Res., 20, 7-15.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nadmaiteni, L. & Marosi, G. (1983) Cardiotoxic action of organo-
phosphorus pesticides. Gig. Sanit., 11, 72-73 (in Russian).
Nagymajtenyi, L. (1988) Neurophysiological markers as early signs of
organophosphate neurotoxicity. Neurotoxicol. Teratol., 10, 429-434.
Nehéz, M., Selypes, A., Scheufler, H. & Fisher, G.U. (1983) Effect of
dimethoate and O-demethyldimethoate on bone marrow cells of CFLP
mice. Regul. Toxicol. Pharmacol., 317, 349-354.
Newman, A.J., Cherry, C. & Urwin, C. (1972) Pathology report of
folimat active substances in hens. Unpublished report from the
Huntingdon Research Centre, Huntingdon, Cambs, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
O'Brien, R.D (1959) Activation of thionophosphates by liver
microsomes. Nature, 183, 121.
O'Brien, R.D (1961) The effect of SKF 525A (2-diethylaminoethyl
2',2-diphenyl valerate hydrocloride) on organophosphate metabolism in
insect and mammals. Biochem. J., 79, 229.
Panshina, T.N. & Klisenko, M.A. (1962) Content of fosfamid and changes
in blood cholinesterase activity in different routes of treatment of
the mammalian organism. In: Industrial Toxicology and Clinical Picture
of Occupational Diseases Due to Chemicals, Moscow, Medgiz, pp. 181-184
(in Russian).
Probst, G.H., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K. &
Neal, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3, 11-32.
Rani, M.V.U., Reddi, O.S. & Reddy, P.P. (1980) Mutagenicity studies
involving aldrin, endosulfan, dimethoate, phosphamidon, carbaryl and
ceresan. Bull. Environ. Contam. Toxicol., 25, 277-282.
Redgrave, V.A., Johnson, A.A., Gopinath, C., Hadley, J.C., Anderson,
A. & Dawe, I.S. (1991) Dimethoate: Acute delayed neurotoxicity in the
domestic hen. Unpublished report from the Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Reena, et al. (1989) Haematological changes induced by dimethoate in
rat. Arh. Hig. Rada Toksikol., 40, 23-27.
Reuber, M.D. (1984) Carcinogenicity of dimethoate. Environ. Res.,
34, 193-211.
Ribelin, W.E., Levinskas, G.J. & Shaffer, C.B. (1965) A 3-generation
reproduction study with mice. Unpublished report. Submitted to WHO by
the American Cyanamid Company.
Riemer, F., Dahlenburg, R. & Grisk, A. (1985) Renal excretion of
dimethylphosphate and its thio derivatives after application of
dimethoate, bromophos, naled or trichlorphon to rats. Nahrung, 29,
299-302 (in German).
Salem, M.H., Abo-Elezz, Z., Abd-Allah, G.A., Hassan, G.A. & Shaker, N.
(1988) Effect of organophosphorus (dimethoate) and pyrethroid
(deltamethrin) pesticides on semen characteristics in rabbits.
J. Environ. Sci. Health, B23, 279-290.
Sanderson, D.M. & Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Br. J. Ind. Med., 21, 52.
San Sebastian, J.R. (1985) In vivo bone marrow cytogenetics rat
metaphase analysis. PH 315-AC-00-84, Dimethoate CL 12880. Unpublished
report from Pharakon Research International, Inc., Waverly, PA, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
Santi, R. & de Pietri-Sonelli, P. (1959) Mode of action and biological
properties of the S-(methylcarbamyl)methyl O,O-dimethyl-
diophosphate. Nature, 183, 398.
Santi, R. & de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido O,O-dimetiliditio-
fosforilacetico. Ist. Ric. Agr. Soc. Montecatini, 3, 3 (in Italian).
Scheufler, H. (1975) Effects of relatively high doses of dimethoate
and trichlorfon on the embryogenesis of laboratory mice. Biol. Rundsch.,
13, 238-240 (in German).
Shaker, N. (1988) In vivo chronic effect of dimethoate and deltamethrin
on rabbits. J. Environ. Sci. Health, B23, 387-399.
Sorg, R.M., (1985) Micronucleus test (MNT). PM 309A-AC-004-84,
Dimethoate CL 12880. Unpublished report from Pharakon Research
International, Inc., Waverly, PA, USA. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Squire, R.A. (1988) An evaluation of vascular proliferative lesions in
male Wistar rats from project 70C0326/8241. Unpublished report from
Robert A. Squire Associates, Ruxton, MD, USA. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorfon and
dimethoate. Acta Haematol., 52, 70-76.
Tesh, J.M., Ross, F.W., Wightman, T.J. & Wilby, O.K. (1982) S-6876:
Effect of oral administration upon pregnancy in the rabbit. Unpublished
report from Life Science Research, United Kingdom, originally submitted
to WHO by Bayer AG, summary from WHO.
Tiefenbach, B. & Lange, P. (1980) Studies on the action of dimethoate
on the immune system. Arch. Toxicol., Suppl. 4, 167-170.
Tripathy, N.K., Majhi, B., Dey, L. & Das, C.C. (1988) Genotoxicity of
Rogor studied in the sex-linked recessive lethal test and wing eye and
female germ-line mosaic assays in Drosophila melanogaster. Mutat. Res.,
206, 351-360.
Uchida, T., Dautermann, W.C. & O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agric. Food Chem., 12, 48-52.
Ullman, L., Sacher, R. & Chevalier, J. (1985) Acute oral toxicity
study with dimethoate in mice. Unpublished report from Research and
Consulting Co., AG, Itingen, Switzerland. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
US National Cancer Institute (1976) Bioassay of Dimethoate for Possible
Carcinogenicity (NCI-CG-TR-4 DHEW Publication No. (NIH) 77-80),
Bethesda, MD, USA.
US National Cancer Institute (1977) Bioassay of Dimethoate for Possible
Carcinogenicity (Technical Report Series No. 4), Bethesda, MD, USA.
Velázquez, A., Xamena, N., Creus A. & Marcos, R. (1986) Indication for
weak mutagenicity of the organophosphorus insecticide dimethoate in
Drosophila melanogaster. Mutat. Res., 172, 237-243.
Vishwanath, R. & Jamil, K. (1986) Mutagenic and genotoxic activities
of certain organophosphorus compounds using Ames Salmonella assay
with and without microsomal induction. Ind. J. Exp. Biol., 24, 305-308.
Weber, H., Patzschke, K. & Wegner, L.A. (1978) Omethoat-C14-Folimat-
Wirkstoff: Biokinetische Untershungen an Ratten. Unpublished report
originally submitted to WHO by Bayer AG, summary from WHO.
West, B., Vidone, L.B. & Shaffer, C.B. (1961) Acute and subacute
toxicity of dimethoate. Toxicol. Appl. Pharmacol., 3, 210.
WHO (1989) Dimethoate (Environmental Health Criteria 90), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutag., 5, 835-846.
 Table 1. Acute toxicity of dimethoate in experimental animals
Species Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Mouse Female Oral NR 60 Sanderson & Edson (1964)
Mouse Male, female Oral NR 160 Ullman et al. (1985)
Rat Male, female Oral NR 314 Ministry of Agriculture,
Fisheries and Food (1993a)
Rat Male, female Oral 97.6-99 540-600 Dal Re & Vola Gera (1980)
Rat Male, female Dermal 97.6-99 >7000 Dal Re & Vola Gera (1976)
Rat Male Intravenous NR 450 Sanderson & Edson (1964)
Rat Male, female Intraperitoneal NR 175-350 Sanderson & Edson (1964)
Hamster Male Oral NR 200 Sanderson & Edson (1964)
Guinea-pig Male, female Oral NR 350-600 Sanderson & Edson (1964)
Rabbit Male, female Oral NR 300-500 Sanderson & Edson (1964)
Hen Male, female Oral NR 30-50 Sanderson & Edson (1964)
NR not reported
(b) Short-term toxicity
Rats
Three studies in rats were briefly summarized in the report of
the 1963 JMPR (Annex 1, reference 2). In a 15-week study, groups of 10
male rats were fed diets containing 1, 5, 25, or 125 ppm dimethoate,
equivalent to 0.1, 0.5, 2.5 and 12 mg/kg bw per day. At the high
dietary level, slight muscular fibrillation and depressed weight gain
were observed. At 5, 25, and 125 ppm, depressed cholinesterase
activity was observed. In another study, groups of 20 rats were fed
diets providing 2, 8, or 32 ppm for 90 days or 50, 100, or 200 ppm
for 35 days. No haematological abnormalities were reported nor any
significant pathological change. The highest dose that did not inhibit
cholinesterase activity was reported to be 32 ppm. In a one-year study
with groups of 20 male rats, the highest dose that did not inhibit
cholinesterase activity was reported to be 10 ppm (Edson & Noakes,
1960; West et al., 1961; Sanderson & Edson, 1964).
The report of a 13-week study in Wistar rats was available only
in an incomplete translation and was neither dated nor signed. Groups
of 24 male and 24 female rats received doses of 0.02, 0.2, 2, or
20 mg/kg bw per day for 18 weeks; a group of 32 males and 32 females
acted as controls. Animals were housed eight per cage, and the
doses were administered orally on five clays a week as aqueous
solutions, which were prepared weekly and refrigerated until use. The
formulations were not analysed for content or for stability in the
vehicle. Body weights were recorded weekly, and food consumption was
recorded, but at unspecified intervals. Blood samples were obtained
from eight males and eight females in each treated group and from 16
controls of each sex on four occasions; a normal range of parameters,
including cholinesterase activity, was measured. Urine samples were
obtained in weeks 12 and 18. Brain cholinesterase activity and a renal
function test (phenol red test) were carried out after 18 weeks.
Histological investigations were performed on six animals of each
sex per group, and the list of tissues chosen for weighing was
satisfactory; however, the list of those chosen for histopathological
examination was short and did not include epididymides.
Slightly reduced body-weight gain was seen in animals treated at
20 mg/kg bw throughout the test, and food consumption was slightly
lower in males of this group than in the controls during the latter
half of the study. There were no treatment-related deaths. During
weeks 6-11, animals in all groups, including the controls, had
diarrhoea, but this was more pronounced in animals treated at 2 or
20 mg/kg bw. Minimally lower haematocrit and erythrocyte count were
noted in animals at the high dose after seven weeks only. There were
no other toxicologically significant changes in haematological
parameters. Plasma cholinesterase activity was 45-75% lower in
animals at 20 mg/kg bw than in the controls. Erythrocyte and brain
acetylcholinesterase activity was 70-90% of that in controls for
animals treated at 2 mg/kg bw and 54-77% in animals at 20 mg/kg bw.
Renal function was unimpaired by treatment, but no data were
presented. There were no treatment-related effects on the urine and
there was no faecal occult blood. Necropsy indicated no effects of
treatment. Organ weight analysis indicated a number of intergroup
differences, none of which was clearly of toxicological significance;
however, the absolute and relative weights of the livers of treated
animals tended to be lower than those of the controls, and this is
likely to represent a treatment-related change, as similar effects
have been seen in other studies. There were no microscopic findings
related to treatment, but no data were presented. It was concluded
that the toxicity expressed was minimal and that 0.2 mg/kg bw was the
NOAEL for inhibition of brain and erythrocyte acetylcholinesterase
activity (Ministry of Agriculture, Fisheries and Food, 1993a).
Dimethoate was administered orally to rats on five days a week
for six weeks at a dose of 10 mg/kg bw per day. The investigations
were confined to an electroencephalogram and cholinesterase
determinations. Treatment was associated with increased frequency and
decreased amplitude on the electroencephalogram, and, as expected,
inhibition of cholinesterase activity in the tissues examined
(Nagymajtenyi, 1988). The relevance of these data to the safety
evaluation of dimethoate is equivocal, and they are not considered
further in this review.
Ten male albino rats received dimethoate by intraperitoneal
injection at 150 mg/kg bw in 0.5 ml saline on alternate days for 30
days; a similar group was treated for 15 days. Ten controls received
saline only, but the duration of their treatment was not specified. On
completion of the treatment, the rats were decapitated and blood
samples were collected for haematological and biochemical examination.
The results were presented as means and standard errors for five
animals per observation. Haemoglobin concentration, haematocrit, and
erythrocyte and leukocyte counts in treated animals were clearly lower
than in controls, the greatest effect being seen after 30 days. The
concentrations of serum urea were greater than in the controls at both
sacrifices; a higher cholesterol concentration was seen only after 30
days. The serum activities of aspartate and alanine aminotransferases
and amylase were higher than in the controls on both occasions. There
was also a small increase in alkaline phosphatase activity; however,
as the timing of the collection of control blood samples was not
given, the significance of this minor change cannot be assessed. The
activity of acid phosphatase was lower than in controls at both blood
sample collections. Cholinesterase determinations in serum indicated
inhibition of 30-50% relative to controls. The above effects were all
more marked after 30 than after 15 days. These results indicate that
dimethoate may affect liver and kidney function, although the changes
were not of pathological significance. Organs were not weighed and no
microscopic examinations were undertaken in order to correlate the
changes seen. Although the changes in amylase activity might indicate
pancreatic effects, confirmatory isoenzyme studies were not carried
out. The route and frequency of administration in this study and the
exiguous nature of the examinations performed render this work of
equivocal use in the safety evaluation of dimethoate (Reena et al.,
1989).
Rabbits
In a five-month study by oral administration in male rabbits, the
doses used were said to be one-tenth and one-hundredth of the LD50
value given once a week, but the actual doses were not specified.
There were no indications of clinical signs, and most of the data were
presented graphically on the basis of monthly sacrifice of four
animals out of a total of 20 animals per group. An initial increase in
cholinesterase activity was noted at the one-month sacrifice, but a
subsequent 40% reduction was reported. Although changes in organ
weights were reported, the body weights of the animals were not, and
the changes were reported only as percentages of absolute weight of
controls. The significance of the various findings cannot be assessed,
and the report is not considered further (Shaker, 1988).
The dermal toxicity of technical-grade dimethoate was investigated
in groups of six male and six female New Zealand white rabbits which
received doses of 100, 300, or 1000 mg/kg bw per day for 21 days; two
control groups, one untreated and one receiving the vehicle (paraffin
oil), were similarly constituted. The doses were selected on the basis
of the results of a five-day range-finding study in two groups of one
male and one female given doses of 1000 or 2000 mg/kg bw per day. In
the range-finding study, slight erythema was seen in one animal at
1000 mg/kg bw per day and in both at 2000 mg/kg bw per day on days 3,
4, and 5; fissuring of the skin was seen in one animal treated at
2000 mg/kg bw per day. Application was made to the abraded skin of
three animals per group or to non-abraded skin on the back of each
animal on five days per week at a volume of 2 ml/kg bw. The test site
was occluded for 6 h with a gauze bandage held in place by tape and
wrapped in occlusive plaster. Observations and food consumption were
recorded daily, and body weight was measured weekly. Blood samples were
taken before treatment and at termination after about 16 h of fasting.
All animals were subjected to a complete necropsy, and a range of
tissues was retained. Microscopic examination was restricted to the
controls and animals at the highest dose.
There were no significant differences between treated and control
animals or between those with intact or abraded skin. Pustules were
seen at the treatment sites of the majority of animals, including
vehicle controls, during the study. Body-weight gain and food
consumption were unaffected by treatment, and there were no changes in
the blood, including cholinesterase activity, that could be ascribed
to treatment. There were no significant treatment-related intergroup
differences in organ weights or in macroscopic or microscopic
pathology. The absence of an effect on cholinesterase activity
indicates that the test material was not absorbed, even across broken
(abraded) skin, at doses up to 1000 mg/kg bw per day. It was concluded
that dimethoate does not irritate rabbit skin (Madison et al., 1986).
Dogs
A study in dogs was briefly summarized in the report of the 1963
JMPR (Annex 1, reference 2), in which groups of two males and two
females were fed diets providing 2, 10, or 50 ppm, equivalent to 0.05,
0.25 and 1.25 mg/kg bw per day dimethoate for 90 days. No significant
effects were noted, and erythrocyte cholinesterase activity was only
slightly depressed in animals at 50 ppm (West et al., 1961).
Groups of six male and six female beagle dogs received diets
containing dimethoate at concentrations of 0, 5, 20, or 125 ppm
for one year. The doses were chosen on the basis of the results
of a preliminary study of 28 days' duration at concentrations
< 1250 ppm, at which dose-related changes were observed at
> 50 ppm, necessitating early sacrifice at 1250 ppm, while changes
at 50 and 250 ppm were confined to dose-dependent reductions in
cholinesterase activity. In the main study, clinical signs of reaction
to treatment and food consumption were recorded daily; body weight was
recorded weekly. The eyes of each animal were examined before
treatment and during weeks 26 and 52. Blood samples were taken on two
occasions before treatment and during weeks 13, 26, and 52. Urine
samples were collected at similar intervals. The samples were examined
for a normal range of haematological and biochemical characteristics,
including plasma and erythrocyte cholinesterase activity; brain
acetylcholinesterase activity was measured at termination. All animals
were necropsied after bone-marrow samples had been taken. A range of
organs was weighed and the tissues preserved for histopathological
processing and examination.
There were no deaths or clinical signs of reaction to treatment
and no effect on body weight, food consumption, or the eyes. The
achieved intakes of test material were calculated to be 0.19, 0.73,
and 4.25 mg/kg bw per day for animals at 0, 5, 20, or 125 ppm. Plasma
cholinesterase activity was reduced by > 20% relative to controls in
animals of each sex at 125 ppm in weeks 13 and 26 and in males only in
week 52. These reductions did not exceed 22%, except in females at
week 13 which had an activity 36% lower than controls. Erythrocyte
acetylcholinesterase activity was reduced in weeks 13 and 26 by 20-27%
in animals at 20 ppm and by 63-76% in those at 125 ppm. In week
52, males at 5 or 20 ppm had marginally, nonsignificantly lower
erythrocyte acetylcholinesterase activities than the controls; females
in these groups were unaffected. A clear reduction (about 65%) in
erythrocyte acetylcholinesterase activity was also seen in animals at
125 ppm. Statistically significant reductions in brain acetylcholin-
esterase activity were seen at all doses after 52 weeks, which were
slight at 5 ppm (about 90% of the control level) and 20 ppm (about 83%
of control) but clear at 125 ppm (45% of control). There were no other
biochemical differences in the blood attributable to treatment. The
urine was unaffected, and there were no findings at necropsy that were
attributable to treatment. The liver weights of animals at 125 ppm
were lower than those of the controls. There was a marginally greater
incidence of pigment, presumed to be haemosiderin, in the livers of
treated animals in all groups, but there was no clear relationship to
dose. In the absence of other effects of treatment, notably on blood,
this effect was considered to be of no toxicological significance. The
only significant evidence of toxicity attributable to dimethoate was
the reduction in cholinesterase activity; the effect on erythrocyte
and brain acetylcholinesterase activity was clearly significant at 20
and 125 ppm, whereas the effects at 5 ppm were confined to minimal
reductions in brain acetylcholinesterase (10% lower than controls) and
a minimal reduction in male plasma cholinesterase activity after 51
weeks. The NOAEL was 5 ppm, equal to 0.19 mg/kg bw per day (Burford
et al., 1991).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade dimethoate was administered to groups of 50 male
and 50 female individually housed B6C3F1 mice for 18 months. Dietary
concentrations of 0, 25, 100, and 200 ppm (equal to 3.2, 12.3 and
25.3 mg/kg bw per day) were selected on the basis of the results of
previous studies, including a study from the US National Cancer
Institute (1977) in this strain. Blood samples were taken for
investigations of haematology and cholinesterase activity after 51
weeks of treatment from an additional 10 males and 10 females
allocated to each group, which were then killed and necropsied.
Haematological investigations only were conducted after 78 weeks on 10
animals of each sex per group. The test diets were mixed weekly, and
formulated diet and the test material were analysed at approximately
three-month intervals; the results of these analyses were satisfactory.
All animals were necropsied; appropriate organs only from animals at
the terminal kill were weighed. A wide range of tissues from all
animals, including satellite animals that died before 51 weeks, were
examined microscopically.
There were no clinical findings that were considered by the
authors to be related to treatment. Survival was > 90% in all groups,
and there was no effect of treatment on mortality. There were no
differences in group mean food consumption that could be related to
treatment. The body-weight gain of treated males was lower than that
of controls during the first few weeks of the study; females receiving
200 ppm were transiently affected during the first two weeks.
Subsequently, the body weights of treated females in all groups were
greater than those of the controls; a similar but less marked
difference was evident in treated males from about 14 months. Female
animals at 25 ppm gained notably less weight than those at the two
higher doses but still gained more than the controls. The overall
weight gains of females were 16.1 ± 5.9 (SD) for the controls,
20.4 ± 6.4 for those at 25 ppm, 27.9 ± 7.3 for those at 100 ppm, and
26.0 ± 5.6 for those at 200 ppm. Haematological analyses after 78
weeks indicated higher nonspecific leukocyte counts in males at 100 or
200 ppm and in females at 200 ppm; no similar difference was seen in
samples taken from satellite animals after 51 weeks of treatment. The
cholinesterase activity in plasma and erythrocytes from treated
animals was lower than That in the controls in a dose-related manner
at all dietary concentrations. No other examination of cholinesterase
activity and no analyses of brain were undertaken. Organ weight
analysis indicated greater absolute liver weights in animals at 100 or
200 ppm; however, the relative liver weights of females were lower
than those of controls as a result of the increased body weights of
these animals. The absolute weight of the ovaries of treated females
was lower than that of the controls after 78 weeks of treatment, but
no similar difference was seen in animals killed after 52 weeks of
treatment. Microscopic examination indicated a greater incidence of
extramedullary haematopoiesis in the spleens of males and females at
100 or 200 ppm, which was dose-related. A greater incidence of
hepatocytic vacuolation was seen in males and female at 100 or 200 ppm
and to a lesser extent in females at 25 ppm; the effect was attributed
to fat and the nutritional status of the affected groups. There were
no differences in the incidences of any neoplastic finding that could
be related unequivocally to treatment. There was no NOAEL, as effects
were seen at all doses (Hellwig, 1986a)
Fifty male B6C3F1 mice received a dietary concentration of
250 ppm dimethoate for 69 weeks or 500 ppm for 60 weeks, and 50
females received the two doses for 80 weeks. All animals were then
observed without treatment until about 94 weeks. A control group of 10
males and 10 females was supplemented by similar groups of animals
from concurrent studies on other pesticides; this gave a pooled
control group of 50-60 animals, although the studies were not
precisely concurrent. The body-weight gain of treated mice, except
females at the low dose, was lower than that of controls during the
first 52 weeks of treatment. Occasional generalized tremor was noted
in treated animals at each dose. During the second half of the study,
alopecia, abdominal distension, and tumours were seen in treated
animals but predominantly in those receiving the lower dose. The
condition of animals at termination was said to be poor. Oncogenic
potential was not assessed (US National Cancer Institute, 1976, 1977).
Rats
Groups of 65 male and 65 female individually housed Wistar rats
received dimethoate in the diet at concentrations providing 0, 5, 25,
or 100 ppm for two years. Fifteen animals of each sex in each group
were allocated for clinical pathology; an additional group of 20 males
and 20 females received a dietary concentration of 1 ppm and were used
to establish or confirm a no-effect level. The dietary concentrations
were selected on the basis of the results of a preliminary study and a
study by the US National Cancer Institute (1977) on Osborne-Mendel
rats. Feed was prepared weekly; both the test material and the
formulated diets were analysed regularly and found to be satisfactory.
Food consumption was recorded weekly; body weight was recorded
weekly for 13 weeks and at fortnightly intervals thereafter. Daily
observations and weekly palpations were recorded; the eyes of all
animals of the main groups (50 of each sex) were examined before
treatment and at six-month intervals during the treatment period for
changes to the refracting media. The ocular fundus of 10 males and 10
females in the control and high-dose groups were examined after 620
days of treatment. Blood samples were obtained before treatment and on
six occasions during the study, and a normal range of chemical and
haematological parameters, including cholinesterase activity, was
measured. Brain acetylcholinesterase activity was measured at the end
of the study. Urine samples were collected twice during the study;
although basic, qualitative 'stick' tests were conducted, volume and
specific gravity were not measured. All animals were necropsied, and a
range of organs was weighed and the tissues retained. Tissues from all
animals in the main study and from satellite animals that died were
examined microscopically.
Females at 100 ppm had a slightly higher mortality rate than
controls from week 65. There were no clinical signs that were
considered by the authors to be related to treatment. Animals at doses
> 25 ppm showed a trend to increased food consumption, especially
during the second year of treatment. The body-weight gain of animals
receiving 100 ppm was slightly lower than that of the controls
during the first half of the study. Examination of the eyes in vivo
revealed no treatment-related effect. Reductions in plasma
cholinesterase activity (generally, 50% of control) were seen in the
group at 100 ppm at all examinations; in females at 25 ppm, the
activities in plasma were minimally lower than in the controls after
four weeks. Reductions in erythrocyte acetylcholinesterase activity
were clear in animals at 25 or 100 ppm (60-75 and 20-40% of control
values, respectively); smaller decreases were seen in females at 5 ppm
during the first 12 months and in males of this group after 24 months.
Clear dose-related reductions in brain acetylcholinesterase activity
were seen at termination in animals at 25 or 100 ppm and slightly
lower values in males at 5 ppm. In animals at this low dose, the
reductions in erythrocyte and brain acetylcholinesterase activity were
not consistent between times or sexes; the variations from control
values are thus of questionable biological significance but probably
represent an intermittent effect of treatment. There were no
statistically significant differences in cholinesterase activity in
the animals at 1 ppm.
The authors reported minimal anaemia throughout the study in
males at 100 ppm; however, this effect was also present before
treatment and was absent in females. Minor variations were also noted
in leukocyte count, potassium and total protein concentrations, and
aspartate aminotransferase activity. Although these effects were
reported to extend in part to the animals at 25 ppm, they were minor;
they may be related to treatment but are considered not to be of clear
toxicological significance. There were no treatment-related changes in
the urine. Animals at 100 ppm had slightly larger spleens and slightly
smaller ovaries than the controls, but no gross pathological findings
could be related to treatment. There were no non-neoplastic findings
that were considered to be related to treatment, and there was no
statistically significant difference in the distribution of individual
tumour types. Treated males had a greater number of malignant tumours
than the controls, but there was no relationship to dose. In a number
of tissues, however, a greater incidence of tumours was seen in
treated animals than in controls. These included exocrine and
islet-cell adenomas in the pancreas of males, haemangiosarcoma in
the spleen of males, and mammary gland fibroadenoma and carcinoma
in females. When the combined incidences of haemangioma and
haemangiosarcoma at any site in treated animals were compared with
those in controls, the difference was statistically significant for
all groups of treated males, but there was no relationship to dose and
females were clearly unaffected. The apparent difference may be due to
a lower than expected incidence in the controls. A large proportion of
these tumours were located in the mesenteric lymph node. The incidence
of these vascular tumours was said to be similar to that of historical
controls from a number of sources. The authors concluded that there
was no evidence that dimethoate has oncogenic potential. The NOAEL for
inhibition of brain and erythrocyte acetylcholinesterase activity was
1 ppm, equivalent to 0.05 mg/kg bw per day (Hellwig, 1986b).
The vascular and proliferative lesions from the above study were
evaluated by a second group of pathologists (Squire, 1988), who were
unaware of the previous interpretation of each slide. This review
supported the conclusion of the original pathologist and indicated
that the Wistar rat is susceptible to these tumours. It also suggested
that the chemicals that induce vascular neoplasms are genotoxic;
however, it asserted that dimethoate is not genotoxic and that by
implication these tumours were not related to treatment.
Groups of 50 male and 50 female Wistar rats received technical-
grade dimethoate in the diet at concentrations of 0, 2, 20, or 200 ppm
for two years, equivalent to 0.1, 1 and 10 mg/kg bw per day. The report
was available only as an English translation of a nearly illegible
German summary with tables of individual data. As no other details of
procedures or results and no group means were presented, the study
could not be reviewed satisfactorily (Ministry of Agriculture,
Fisheries and Food, 1993a).
Groups of 30 male and 30 female Wistar rats received diets
designed to provide technical-grade dimethoate at concentrations of 0,
0.1, 1, 10, or 75 ppm for two years, equivalent to 0.005, 0.05, 0.5
and 3.8 mg/kg bw per day. The feed was prepared four times a week.
Body weight and food intake were recorded weekly for the first four
weeks and every four weeks thereafter. Haematological examinations
were performed four times between weeks 32 and 100. Cholinesterase
activity was determined after 1, 3, 12, 50, 75, and 100 weeks on six
rats of each sex. Brain acetylcholinesterase activity was determined
after 52 and 104 weeks. Other, limited biochemical assays were
performed during the study on blood and urine, mostly after two years.
Six animals of each sex were killed after one year and the surviving
animals after two years; all were examined macroscopically and their
organs weighed. Histological examination was performed on six males
and six females at each sacrifice. There was no indication of how dead
animals were handled or examined, although the report refers to their
necropsy.
Early signs of reaction to treatment at 75 ppm (slight
piloerection, exophthalmia, and fine tremor) disappeared during the
fourth week of treatment, and no other clinical signs were attributed
to treatment. A high mortality rate resulted from an infection after
78 weeks, with the deaths of 55 males and 37 females, evenly
distributed among the groups. The infection was treated with
oxytetracycline as three oral doses of 40 mg/kg bw over three weeks.
There was no treatment-related mortality. Body-weight gain was reduced
in animals treated at 75 ppm, up to week 20 for females and throughout
the study for males. Food consumption was unaffected, but conversion
was reduced in animals treated at 75 ppm, in line with body weight.
The achieved mean doses were 0.02, 0.2, 2, and about 20 mg/kg bw per
day. Haematological investigations indicated no effects of treatment.
Cholinesterase activity was clearly reduced in the plasma,
erythrocytes, and brain of animals receiving 10 or 75 ppm. The brain
acetylcholinesterase activity of animals receiving 1 ppm was 20% lower
than that of controls after one year. There were no other
toxicologically significant intergroup differences in the composition
of the plasma or urine. Organ weight analysis after one year indicated
lower liver, spleen, adrenal, and testis weights in males at 75 ppm
than in the controls; after two years, the adrenal and liver weights
of males and females at 75 ppm were lower than those of controls.
Necropsy of animals that died during the study and of animals killed
at the scheduled sacrifices indicated no treatment-related changes.
The distribution of macroscopically observed rumours was unaffected by
treatment. No microscopic changes were found that were attributed to
treatment. The incomplete data presentation and the infection that
occurred in the middle of the study significantly compromise its
validity (Ministry of Agriculture, Fisheries and Food, 1993a).
Groups of 50 male Osborne-Mendel rats were fed diets providing
technical-grade dimethoate at concentrations of 250 or 500 ppm
(equivalent to 25 and 50 mg/kg bw per day) for 19 weeks; the doses
were then reduced to 125 and 250 ppm (equivalent to 6.3 and 13 mg/kg
bw per day),and treatment continued for 61 weeks. Necropsy was
performed after a further 33-35 weeks without treatment. The same
dietary concentrations were fed to similar groups of females, except
that they were reduced only after 43 weeks; the animals were then
treated at concentrations of 125 or 250 ppm for a further 37 weeks.
The total treatment period for all animals was 80 weeks, followed by
up to 35 weeks without treatment. A control group of 10 males and 10
females was supplemented by similar groups of animals from concurrent
studies on other pesticides, giving a pooled control group of 50-60
animals, although the studies were not precisely concurrent. All
animals were observed daily, and body weights were recorded 'at
regular intervals until 110 weeks'. The animals were then necropsied
and, when feasible, tissues were retained for histopathological
examination. Treatment at 500 ppm was associated with lower
body-weight gains in males and females during the first 20 weeks of
treatment. After the reduction to 250 ppm at 19 weeks, the body-weight
gain of males increased but remained lower than in controls until
treatment ceased. The body-weight gain of males at the low dose and
females at the high dose remained lower than that of controls until
week 80. Clinical signs of inhibition of cholinesterase activity were
seen in animals at the high dose, particularly during the first week
of treatment. Conjunctivitis of vital origin was diagnosed in the
animals at week 38. Animals that survived to termination were said to
be 'generally in poor physical condition'. More animals at the high
dose died than matched or pooled controls, although the number of
matched control males that died during the study (7 of 10) was
reported to be unusually large. There was no difference in the
distribution of non-neoplastic or neoplastic changes among the treated
groups that could clearly be ascribed to treatment. The pathological
assessment indicated no oncogenic potential (US National Cancer
Institute, 1976, 1977).
A review by Reuver (1984) covered several carcinogenicity studies
in rats and mice, including that of the US National Cancer Institute.
It was concluded from the studies of Gibel et al. (1973) and
Stieglitz et al. (1974) that dimethoate is highly carcinogenic to
rats, but insufficient details of these experiments were given,
precluding assessment. The review of the US National Cancer Institute
study involved re-reading of the sections; it was again concluded
that dimethoate is carcinogenic. The basis for the review was not
described, and the numbers of animals given in the tables shown in the
review differ from those in the original report. Reuber quoted text
from the report in reverse order to that in which it was originally
published. This review did not satisfactorily explain the methods used
or discuss the discrepancies in the reported incidences and in the
conclusions from those of the original report. In view of these
deficiencies, no weight is placed on this publication.
(d) Reproductive toxicity
Mice
A multigeneration study was undertaken in CF-1 mice fed diets
containing concentrations of 0, 5, 15, or 50 ppm dimethoate,
equivalent to 1.4, 4.3 and 14.5 mg/kg bw per day, throughout the
study. The study was conducted before Good Laboratory Practice came to
be enforced but was designed according to the recommendations of the
Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics of
the Association of Food and Drug Officials of the United States. Each
generation was mated twice, the first set of litters being discarded
and the next generation (F1b, F2b, and F3b) being produced from the
second litters. For each generation, eight males were mated with 16
females in each group, and males were rotated within their group
during the mating period. The observations were limited in comparison
with current practice. After weaning of the F3b generation, the
parents (F2b) were killed and necropsied; liver, kidneys, and gonads
were weighed, but no microscopy was performed. All animals of the F3b
generation were necropsied at death or at weaning, and a wide range of
tissues from one male and one female from each litter was examined
microscopically. Organ weighing was restricted. All fetuses that were
not examined microscopically were retained in 80% alcohol for skeletal
staining in alizarin red.
There were no clinical reactions to treatment. Tremors were seen
in four dams of the F2b generation treated with 50 ppm, three of
which lost their litters, on one occasion after a weekly change of
diets; the diets were replaced, as a formulation error was suspected,
and the condition was not seen again. No treatment-related differences
in group mean body weights were recorded at any mating. Measurement of
the food consumption of the F0 generation before mating indicated
no effect of treatment, but no data were presented and no other
measurements were carried out. The fertility, gestation, viability,
and lactation indices were unaffected by treatment, and there was
no effect on the weights of the pups at weaning. No intergroup
differences in organ weight or pathological findings were seen that
were related to treatment. The NOAEL for reproductive toxicity was
50 ppm (Ribelin et al., 1965).
In five generations of CD-1 mice given 60 ppm of dimethoate in
drinking-water, reproductive performance was significantly altered, as
indicated by reduced mating success and longer gestation. Litter size
and weight were not reduced at birth, but pup mortality was increased
significantly by treatment. The growth rate of the pups was generally
lower than that of controls. Dimethoate did not show teratogenic
potential or adverse effects on organ weights or histological
appearance (Budreau & Singh, 1973).
Rats
In a multigeneration study, dimethoate (purity, 96.4%) was
administered in the diet at fixed concentrations of 0, 1, 15, or
65 ppm (equal to about 0.08, 1.25 and 5 mg/kg bw per day) to groups of
28 male and 28 female Sprague-Dawley rats for 10 weeks before the
first of two matings to produce F1a and F1b animals. The F1
generation, selected from the F1a litters, was first mated at about
16 weeks of age to produce F2 pups, which were killed and examined at
21 days of age. A second mating of the F1a generation was conducted,
followed by a partial third mating involving animals that had not been
successful at either of their first two pairings. Administration of
dimethoate was continued at the same dietary levels throughout
premating, mating, gestation, and lactation. Treatment at 65 ppm was
associated in the parent animals with marked reductions in plasma,
erythrocyte, and brain cholinesterase activities in animals of both
generations, slightly reduced body-weight gain, increased food intake,
and reduced water intake. In the F1a pups at 65 ppm at four days of
age, a reduction in brain acetylcholinesterase activity was seen in
males but not in females. The parental animals at 15 ppm had a
significant reduction in brain and erythrocyte acetylcholinesterase
activity in both generations, but no effect on cholinesterase activity
was seen in the offspring. Mating performance (as assessed by median
precoital time and duration of pregnancy) was not affected by
treatment; however, an effect was seen on the pregnancy rate (Table
2). These data are clearly indicative of substandard performance at
65 ppm and also show a possible effect at 15 ppm on the second mating
of the F1 generation. The Meeting concluded that this information did
not clarify the possible effect at the intermediate dose. Treatment at
65 ppm was also associated with a reduction in litter size at birth,
as shown in Table 3.
Table 2. Pregnancy rates of animals treated with dimethoate with
live pups at birth
Generation Mating Pregnancy rate (%)
Control 1 ppm 15 ppm 65 ppm
F0 First 93 96 86 89
Second 89 93 89 71
(100) (100) (100) (96)
F1 First 96 71 71 63
Second 73 67 58 50
(100) (79) (92) (75)
In parentheses, the percentages of animals with implantations
confirmed at autopsy by Salewski staining of the uteri of
apparently non-pregnant females
Table 3. Litter size at birth of animals treated with dimethoate
Generation Mating Group mean litter size at birth
Control 1 ppm 15 ppm 65 ppm
F0 First 16.4 15.3* 15.3* 14.2**
Second 14.9 14.9 14.2 14.3
F1 First 12.3 11.9 14.6 12.0
Second 14.1 13.3 13.1 10.0*
* p < 0.05
** p < 0.01
There was also a slight increase in pup mortality during
lactation in animals at 65 ppm. Changes in litter weight reflected the
changes in litter size. The mean pup weight at birth was unaffected by
treatment, but pup body-weight gain was adversely affected by the high
dietary level. There was a slight delay in attainment of the startle
reflex in pups born after the first mating of both generations at
65 ppm, but the mean delay was less than one day and was not apparent
across all four matings; it was thus probably not related to
treatment. There was no other effect on pre- or post-weaning
development. Histopathological examination of tissues associated with
the reproductive tract did not reveal any treatment-related changes.
The NOAEL for toxicity was 1 ppm, equivalent to 0.08 mg/kg bw per day,
on the basis of inhibition of cholinesterase activity at 15 ppm
(Brooker et al., 1992). The Meeting discounted the possible adverse
effect on pregnancy rate at 15 ppm, and this was the NOAEL for
reproductive performance, equivalent to 1.2 mg/kg bw per day.
Rabbits
Three groups of three male rabbits received gelatin capsules
containing individually calculated doses of a dimethoate formulation
(composition unspecified) calculated to be one-tenth and one-hundredth
of the LD50, which was not specified, at an unspecified frequency. A
six-week preliminary period was followed by six weeks of treatment and
then by six weeks of respite. Body weights were recorded weekly, and
semen was collected twice weekly from all animals throughout the
experimental period. Ejaculate volume was recorded after removal of
the gel mass. Seminal initial fructose was determined immediately
after collection, and methylene blue reduction time was recorded. The
numbers of live, dead, and abnormal sperm were assessed, and sperm
concentration was determined with a haemocytometer. Data were
presented in graphs rather than as individual values; there was no
indication of variation between individual animals. Clinical signs
were not reported. Body-weight gain was reduced in treated animals,
and there were indications of reduced libido. The ejaculate volume and
sperm concentration of treated animals, expressed as a percentage of
that of controls, decreased during the treatment period, and the
effect on sperm concentration persisted into the recovery period.
Treatment increased the numbers of abnormal sperm in a dose-related
manner; these were greatest at the end of the treatment period but
declined thereafter. A significant increase in the methylene blue
reduction time and a decrease in the initial fructose concentration
were also seen. There was some evidence of recovery from these effects
during the latter part of the recovery period (Salem et al., 1988).
In view of the deficiencies, it was difficult to assess the
significance of the changes seen, but they should be explained in view
of the reported reproductive effects of dimethoate.
(e) Developmental toxicity
Mice
Intraperitoneal administration of dimethoate at 40 mg/kg bw as a
single dose to mice on the day of mating or on day 9 of gestation or
for the first 14 days of gestation caused a high incidence of
embryonal loss (Scheufler, 1975).
Dimethoate administered orally at doses of 10 or 20 mg/kg bw was
not teratogenic to CD-1 mice, and these levels were not lethal to the
dams. Doses of 40 and 80 mg/kg bw induced maternal toxicity (Courtney
et al., 1985).
Rats
Groups of pregnant rats were given 0, 3, 6, 12, or 24 mg/kg bw of
a dimethoate formulation by gavage daily on days 6-15 of gestation.
The dams were killed on day 22 of gestation; the uterine content was
removed, the carcass weighed, the number of corpora lutea was
determined, and the animals were necropsied. The fetuses were weighed
and examined for viability and external malformations; live fetuses
were studied for skeletal and visceral anomalies. Maternal weight was
decreased significantly in the group receiving 24 mg/kg bw, and clonic
spasms and muscular tremors were seen. The mean fetal weight was not
affected by treatment. Treatment with 12 or 24 mg/kg bw was associated
with an increased number of litters with abnormal fetuses and fetuses
with wavy ribs. The NOAEL was 6 mg/kg bw of formulated product, equal
to 2.84 mg/kg bw dimethoate (Khera et al., 1979).
Three groups of 25 time-mated CrL:COBS CD (SD) BR rats received
technical-grade dimethoate (purity, 97.3%) in 1% aqueous methyl
cellulose (10 ml/kg) at doses of 3, 6, or 18 mg/kg bw per day by
gavage daily on days 6-15 of gestation; a control group received the
vehicle alone. Clinical observations were made at regular intervals,
and food consumption and body weight were recorded. All animals were
killed on day 20 of gestation and the uterine contents examined for a
normal range of parameters. One-half of the pups were preserved in
Bouin's solution for free-hand sectioning and the remainder in
industrial methylated spirits for subsequent alizarin staining and
skeletal examination. The doses were chosen on the basis of the
results of a preliminary study, which was not presented or discussed.
The signs seen in animals at 18 mg/kg bw per day included salivation,
hypersensitivity, ataxia, tremor, fur staining, and small, rounded
faecal pellets. The signs at 3 mg/kg bw per day were confined to
salivation; in animals at 6 mg/kg bw per day, this was accompanied by
a low incidence of small, rounded faecal pellets. Food consumption and
body-weight gain were lower in animals at 18 mg/ kg bw per day than in
the controls during treatment; similar effects were not seen at the
two lower doses. Neither litter parameters nor fetal development (as
indicated by the incidences of visceral or skeletal abnormalities) was
affected by treatment. The NOAEL for toxic signs depends on the
interpretation of the significance of the salivation seen in the
animals at 3 and 6 mg/kg bw per day and on the abnormal faecal pellets
in the latter group. Although salivation is an expected effect of
organophosphate pesticides, there was no clear evidence that the
effect was unduly prolonged (It was described as occurring
'immediately' after treatment.) and may have been incidental. The
presence of abnormal faecal pellets may be related to the action of
this class of compound on the gastrointestinal tract and is unlikely
to be of toxicological significance (Edwards et al., 1984a).The
Meeting concluded that the NOAEL was 6 mg/kg bw per day.
Rabbits
Groups of 16 female New Zealand white rabbits (obtained from
several breeders) were mated with males of proven fertility and
received technical-grade dimethoate (purity, 97.3%) in 1% aqueous
methylcellulose (5 ml/kg) at doses of 10, 20, or 40 mg/kg bw per day
by gavage daily on days 7-19 of gestation; a control group received
the vehicle alone. The doses were chosen from a preliminary study, the
results of which were not summarized. After coitus, each animal
received an injection of luteinizing hormone. Clinical observations
were made at regular intervals, and food consumption and body weight
were recorded. All animals were killed on day 29 of gestation, and the
uterine contents were inspected; a range of parameters for this type
of study was assessed. After examination in vivo, the pups were
killed and dissected. The skinned, eviscerated pups were fixed in
industrial methylated spirits, and the brain was examined for
abnormalities by longitudinal sectioning; carcasses were cleared and
stained by a modified Dawson's technique for skeletal examination.
There were no clinical signs of reaction to treatment with doses of 10
or 20 mg/kg bw per day; at 40 mg/kg bw per day, muscle tremors and
ataxia were seen during the latter part of the treatment period. Food
consumption was reduced between days 15 and 23 of gestation in animals
treated at 40 mg/kg bw per day, and the body-weight gain of these
rabbits was lower than that of controls throughout the treatment and
particularly between days 15 and 20 of gestation. A slight reduction
in body-weight gain was seen in animals at 20 mg/kg bw per day. An
initial reduction in weight gain was seen at the beginning of the
treatment period in animals at the low dose, but these animals
subsequently gained more weight than the controls. Treatment had no
effect on fetal development; litter size and weight were unaffected,
and pups had no abnormalities that could be ascribed to treatment.
Although there was a transient reduction in body-weight gain at the
start of treatment in animals at 10 mg/kg bw per day, the deficit was
quickly corrected. This dose was therefore the NOAEL (Edwards et al.,
1984b).
Cats
Four groups of 17 cats were mated and treated with Cygon-4E, a
commercial insecticide containing 47.3% dimethoate, as single daily
doses of 0, 3, 6, or 12 mg/kg bw on days 14-22 of gestation. The cats
were necropsied on day 43 of gestation, and the fetuses were removed,
weighed, and examined for external malformations. The total number of
anomalous fetuses in cats at 12 mg/kg bw per day was not significantly
higher than that in controls. The only treatment-related malformation
was observed at this dose and consisted of forepaw polydactyly in
eight of 39 fetuses. A dose-response relationship was not established
owing to the limited response and the common occurrence of this
anomaly in cats. The NOAEL was 6 mg/kg bw per day of Cygon-4E, equal
to 2.8 mg/kg bw per day of dimethoate (Khera, 1979).
(f) Genotoxicity
The regulatory reports evaluated by the Meeting concluded that
dimethoate does not induce reverse mutation or gene mutation in
vitro, nor did it induce micronucleus formation, dominant lethal
mutation, or chromosomal aberration in mice in vivo. Dimethoate
induced unscheduled DNA synthesis in vitro in two assays using
different methods of assessing the uptake of tritiated thymidine into
DNA but not in an assay in vivo/in vitro. A review of the literature
on the mutagenic potential of dimethoate revealed a number of positive
results, notably for reverse mutation in Salmonella typhimurium
TA100 and for sister chromatid exchange in mammalian cells in vitro.
It was concluded that although dimethoate has mutagenic potential in
vitro, mutagenicity does not appear to be expressed in vivo. The
results of assays for genotoxicity with dimethoate are summarized in
Table 4.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The dermal irritation potential of a 400-g/litre emulsifiable
concentrate formulation of dimethoate was investigated in rabbits.
Only slight erythema was observed 4 h after application, and the
effect had resolved by 24 h after treatment (Ministry of Agriculture,
Fisheries and Food, 1993a).
The ocular irritation potential of the same formulation of
dimethoate was also investigated in rabbits. Redness and swelling of
the conjunctiva were observed, with slight corneal opacity, 1-72 h
after application. All of the effects had resolved by eight days after
treatment (Ministry of Agriculture, Fisheries and Food, 1993a).
Studies to investigate the ocular irritation potential in
rabbits of 40 and 38.3% dimethoate formulations concluded that the
formulations were irritating to the rabbit eye. The 'in use' dilution
of the 40% formulation (0.84%) was not irritating (Ministry of
Agriculture, Fisheries and Food, 1993a).
Technical-grade dimethoate (purity, 97.3%) did not sensitize the
skin of guinea-pigs when tested by the Buehler method (Madison et al.,
1984).
Table 4. Results of tests for the genotoxicity of dimethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
5-Methyltryptophan E. coli 1.6 × 10-3 mol/litre NR Positivea Mohn (1973)
resistance mutation
Reverse mutation S. typhimurium < 5000 mg/plate NR Positive Moriya et al. (1983)
TA98, TA100, in TA100a
TA1535, TA1537,
TA1538
E. coli WP2hcr < 5000 mg/plate Positivea
Reverse mutation S. typhimurium < 5000 mg/plate NR Negativea Probst et al. (1981)
TA98, TA100,
TA1535, TA1537,
TA1538
E. coli WP2uvrA- 47 nmol/ml Initially
positive,
negative
quantitativelya
Reverse mutation S. typhimurium 2-200 mg/plate NR Positivea Vishwanath & Jamil
TA100 (1986)
Mitotic gene conversion S. cerevisiae 7 doses, 40-100 mmol NR Positive Fahrig (1974)
Mitotic gene conversion S. pombe (ade 6) 1.3-131 mmol NR Negative Gilot-Delhalle et al.
(1983)
Gene mutation Chinese hamster 1000-3500 mg/ml 97.3 Negative Johnson et al. (1985)
ovary (hprt)
Sister chromatid exchange Cultured human < 120 ppm NR Positive Gomez-Arroyo et al.
lymphocytes (1987)
Sister chromatid exchange Chinese hamster 10, 20, 40, 80 mg/ml 94 Positive Chen et al. (1981)
and cell cycle delay ovary V79 cells + 10 pg/ml BUdR
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Cytotoxicity Chang liver and 50-500 mg/ml 99.8 Positive Gabliks &
HeLa cells Friedman (1965)
Cytotoxicity HeLa cells 2-300 mg/ml 99.8 Positive Gabliks (1965a)
Susceptibility to HeLa cells 2-300 mg/ml for 99.8 Positive Gabliks (1965b)
poliovirus infection < 108 days
Unscheduled DNA SV-40 transformed 100, 1000 mmol NR Positive Ahmed et al. (1977)
synthesis human fibroblast
cell line VA-4
Unscheduled DNA Rat hepatocytes 47 nmol/ml NR Negative Probst et al. (1981)
synthesis
Unscheduled DNA Rat hepatocytes < 2290 mg/ml NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
Unscheduled DNA Rat hepatocytes NR NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
In vivo
Micronucleus formation Mouse 2 equal oral doses NR Positive Rani et al. (1980)
bone marrow of 51.7 mg/kg bw at
24-h interval
Host-mediated Mouse; 3 equal oral doses NR Positive Rani et al. (1980)
mutagenicity S. typhimurium of 155 mg/kg bw
Dominant lethal CFLP mice 30, 60 mg/kg bw ip NR Negative Fisher & Scheufler
mutation AB Jena mice 5 × 6 mg/kg bw ip Negative (1981)
DBA mice 3 × 18 mg/kg bw ip Negative
Dominant lethal NMRI mice 5, 10, 20 mg/kg 96.9 Negative Becker (1985)
mutation orally, 5 days
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Dominant lethal Strain Q mice 10 mg/kg ip + 0.6 NR Negative Degraeve & Moutschen
mutation mg/l drinking-water (1983)
Chromosomal CFLP mice 20-60 mg/kg bw ip NR Positive (gaps Nehéz (1983)
aberration and numerical
changes)
Chromosomal aberration Rats 15, 75, 150 mg/kg bw ip NR Negative Ministry of Agriculture,
Fisheries and Food
(1993a)
Chromosomal aberration Mice 50, 100 mg/kg bw NR Positive Bhunya & Behera
(1975)
Chromosomal aberration Hamster 16-160 mg/kg bw ip NR Weakly Dzwonkowska & Hubner
positive (1986)
Sex-linked recessive Drosophila 1 mg/kg feeding NR Negative Woodruff et al. (1983)
lethal mutation
Sex-linked recessive Drosophila 10 or 20 ppm; adult NR Positive at Velásquez et al. (1986)
lethal mutation feeding low dose
0 or 10 ppm larval Negative
feeding
Sex-linked recessive Drosophila LD50 and half LD50, NR Positive Tripathy (1988)
lethal mutation larval feeding
Unscheduled DNA Rats 50, 100, 200 mg/kg NR Negative Ministry of Agriculture,
synthesis bw orally Fisheries and Food
(1993a)
Micronucleus formation CD-1 mice 55 mg/kg bw ip once 97.3 Negative Sorg (1985)
or twice 24 h apart
Metaphase alteration Rats 15, 75, 150 mg/kg 97.3 Negative San Sebastian (1985)
bw ip
ip intraperitoneally
a With and without metabolic activation
Nineteen cases of allergic, occupational contact eczema and one
of contact dermatitis have been reported (von Jung, 1989). Exposure
to dimethoate was cited in four cases: in two male and one female
gardener and in one female agrochemical technician, 22-69 years of
age. The results of patch tests with dimethoate in these individuals
were positive.
(ii) Neurotoxicity
In a preliminary study, the LD50 of dimethoate in hens was
determined in groups of 10 birds given single oral doses of 0, 30, 45,
68, 100, or 150 mg/kg bw in water. These doses were selected on the
basis of the results of a preliminary study in groups of two birds at
doses between 12.5 and 200 mg/kg bw; both birds at 100 or 200 mg/kg bw
died. In each of these studies, dosing was followed by a 14-day
observation period. Body weights were recorded weekly. Surviving birds
were killed but not necropsied. Neurotoxicity was assessed after a
single subcutaneous dose of 55 mg/kg bw to 16 birds or 55 mg/kg bw
orally to 30 birds; a control group of 14 birds was dosed orally, and
a positive control group of six birds received 500 mg/kg bw of
tri- ortho-cresyl phosphate (TOCP) as a single oral dose in corn oil.
All birds were starved overnight before treatment. In an experiment
conducted before the main study to evaluate the protective
effectiveness of atropine, it was shown to have no protective value at
twice the LD50 value. Birds dosed subcutaneously with dimethoate were
included for comparative biochemical assays. Three birds in the
treated and negative control groups were used to determine
cholinesterase and neuropathy target esterase activity 4 and 48 h
after dosing; these assays were performed for three TOCP-dosed birds
only 48 h after dosing. Analyses of the formulation used indicated
satisfactory content and stability over 24 h. Dosing was followed by
an observation period of 21 days. Birds were assessed daily for ataxia
by observing their ability to walk and to jump onto and off an
obstacle. Body weights were recorded weekly. Nervous tissue from three
TOCP-dosed birds, six negative controls, and six birds dosed orally
with dimethoate was examined histologically.
In the study to determine the LD50, toxicity was seen in a
dose-related manner. Deaths occurred at doses > 45 mg/kg bw, and
all birds at doses > 100 mg/kg died. Deaths occurred up to three
days after dosing, and the surviving birds were normal by day 5. The
LD50 for dimethoate was calculated to be 55 mg/kg bw, with a 95%
confidence interval of 45-67 mg/kg bw. The body weights of survivors
were decreased during the week after treatment but subsequently
increased. The three birds dosed with TOCP that were not sacrificed
for enzyme assays at 48 h developed signs of delayed neurotoxicity
after 13 days, although no clinical signs of cholinesterase inhibition
were seen. All of the birds treated subcutaneously with dimethoate
died within 48 h, after showing clinical signs of cholinesterase
inhibition. After oral administration of 55 mg/kg bw dimethoate, all
birds showed clinical evidence of cholinesterase inhibition. Twelve of
these birds that survived to termination had recovered by day 6 of the
observation period, and none showed signs of delayed neurotoxicity; 12
birds in this group died within 48 h of dosing. Body-weight losses
of up to 10% were seen in treated birds during the first week of
observation, but these losses were subsequently recovered. Microscopic
examination indicated no difference between controls and birds treated
with dimethoate. Brain acetylcholinesterase activity was markedly
reduced in both groups treated with dimethoate; this was more marked
(90% inhibition relative to controls) 4 h after dosing than after 48 h
(61 and 75% inhibition after subcutaneous and oral administration,
respectively). Brain neuropathy target esterase activity was slightly
lower than that in controls in birds treated with dimethoate, but was
markedly lower in TOCP-treated birds. Spinal cord neuropathy target
esterase activity was reduced in TOCP-treated birds but was unaffected
in those that received dimethoate (Redgrave et al., 1991).
Groups of nine or 10 white Leghorn hybrid chickens were given
graded doses of technical-grade dimethoate up to 33 mg/kg bw for three
days, based on the oral LD50 in Japanese quail, in a study designed
to accord with the guidelines then current in eastern Germany and
Poland. Negative and positive controls (TOCP) were used. Antidotes
(atropine sulfate and obidoxime chloride) were administered to treated
birds but not to controls. The route of administration was not stated
but is assumed to have been oral. The precise study design was not
clear from the translation of the original document but indicated
administration of a further series of three doses at intervals
determined by the condition of the birds. Deaths occurred despite use
of the antidotes. Signs of reaction indicative of cholinesterase
inhibition were seen from about 60 min after dosing. Although the
tests indicated that dimethoate has high acute toxicity, there was no
evidence of delayed neurotoxicity, except in the positive controls
(Ministry of Agriculture, Fisheries and Food, 1993a).
(iii) Immunotoxicity
A single dose of dimethoate at 75 mg/kg (route unspecified) to
mice and rats decreased the lymphocyte count to 50% of the value
before exposure and increased the number of neutrophil granulocytes.
After 72 h, these parameters had returned to normal. A reduction in
the thymus cortex, with disrupted lymphocytes, and a reduction in the
number of rosette-forming cells were observed (Tiefenbach & Lange,
1980).
Dimethoate administered to rats at 5-30 mg/kg bw orally or
15 mg/kg bw intramuscularly, twice a week until death, caused
hyperplasia in the bone marrow, resulting mainly in granulocyto-
poiesis. The authors considered the changes to be a direct effect of
dimethoate (Stieglitz et al., 1974).
(iv) Effects on the heart
The effects of dimethoate on the heart have been investigated
in rabbits (Mahkambaeva, 1971), guinea-pigs, and rats (Nadmaiteni &
Marosi, 1983). After oral administration of 150 mg/kg bw to rabbits,
the effects observed included bradycardia and increased atrio-
ventricular and intraventricular conductance, with complete recovery
after four to seven days. In rats and guinea-pigs, a dose-effect
relationship was established for heart rate disturbances and
atrio-ventricular block. An electron microscopic study of the
myocardium showed no changes.
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious electrocardiographic
disturbances developed during the early phase of intoxication. The
toxic cardiac phenomena appeared to be unrelated to the degree of
cholinesterase inhibition but were correlated with the myocardial
concentration of dimethoate. Cardiac failure and death were first
observed at a dose of about 110 mg/kg bw, while a dose of 221 mg/kg bw
resulted in death in all cases. This investigation addressed the
direct effect of dimethoate on the myocardium, independently of its
anticholinesterase action (Marosi et al., 1985a,b).
(v) Studies on metabolites
Omethoate is the oxygen analogue of dimethoate. Information on
the absorption, distribution, excretion, metabolism, and toxicity of
this compound is summarized below, although the original reports were
not available for detailed evaluation.
Absorption, distribution, and excretion of omethoate: After oral
administration of 14C-omethoate at doses of 0.3, 5, or 10 mg/kg bw
to rats, 96-97% of the radiolabel was eliminated in urine, 1-2% in
faeces, and 1% in expired carbon dioxide within 48 h. Intravenous
injection of 0.3 mg/kg bw resulted in a similar, rapid elimination
pattern. Maximal tissue residues were reached 1 h after
administration. After 8 h, about 18% of the residual radiolabel was
found in the body. After two days, < 0.55% of the administered dose
was found. Quantitative analysis and whole-body autoradiography
indicated a relatively homogeneous distribution of 14C activity,
except that a 10-20-fold higher concentration was found in the thyroid
(Weber et al., 1978).
Five male Wistar rats were given 10 mg/kg bw 14C-omethoate
orally in order to obtain preliminary information on pulmonary
excretion. In the main study, groups of five males and five females
received a single intravenous dose of 0.5 mg/kg bw 14C-omethoate, a
single oral dose of 0.5 mg/kg bw 14C-omethoate, 14 daily oral doses
of 0.5 mg/kg bw unlabelled omethoate and a single oral dose of
0.5 mg/kg bw 14C-omethoate on day 15, or a single oral dose of
10 mg/kg bw 14C-omethoate. Urine and faeces were collected over
periods up to 48 h after treatment, and blood samples were collected
until sacrifice 48 h after treatment. Only 0.14% of the administered
radiolabel was detected in expired air. Comparison of the results
obtained with intravenous and oral treatment indicated that > 98%
omethoate had been absorbed from the gastrointestinal tract. Within
48 h, 88-98% of the administered radiolabel was recovered in the
excreta, with 95-98% in the urine and 2-5% in the faeces. Excretion of
the low and the high oral doses was not different in females, but
males at the high dose group tended to excrete more radiolabel in the
faeces than those at the low dose. The maximal plasma concentration
was seen 40-60 min after oral dosing, with an initial half-life of
about 2 h and terminal half-lives of 13-28 h. Less than 0.05% of the
administered dose was found in tissues after 48 h (Ministry of
Agriculture, Fisheries and Food, 1993b).
Biotransformation of omethoate: Urine was collected from two
male rats 12, 24, and 48 h after an oral dose of 50 mg/kg bw
radiolabelled omethoate. The cumulative percentages of administered
radiolabel excreted over the indicated times were 16, 19, and 30%. The
metabolites found in a 24-h composite urine sample by ion-exchange
chromatography were: O,O-dimethylphosphoric acid (34%), unknown A
(52%), O,O-dimethylphosphorothioic acid (9.5%), and unknown B
(4.5%). After treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine within 24 h, while after
treatment with omethoate only 19% was excreted (Dauterman et al.,
1959).
In the experiment conducted by the Ministry of Agriculture,
Fisheries and Food (1993b), the predominant form of excreted
radiolabel was unchanged parent compound (26-62%), with N-methyl-
2-(methylsulfinyl)acetamide accounting for 16-36% and an O-demethylated
omethoate for 4-9%. Pretreatment of animals for 14 days with unlabelled
omethoate followed by a single labelled dose resulted in no significant
difference from the results obtained after a single administration.
Effects of omethoate on enzymes and other biochemical parameters:
Dealkylation of omethoate was proposed to be a significant detoxification
mechanism on the basis of information from assays in fly heads (Aharoni &
O'Brien, 1968). Oxidative metabolism of omethoate results in the
de- N-methyl derivative, which is as toxic as the parent compound
although less active as a cholinesterase inhibitor (Lucier &
Menzer, 1970). Kinetic studies indicated that the reaction between
acetylcholinesterase and omethoate was irreversible and bimolecular.
Omethoate was 75-100 times more potent than dimethoate in inhibiting
rat brain acetylcholinesterase activity.
Acute toxicity of omethoate: The signs of poisoning after a
single dose of omethoate are typical of cholinergic stimulation, as
elicited by other organophosphorus esters. The signs appear 5-60 min
after dosing and include salivation, lacrimation, and tremors. They
may persist for one to three days (Kimmerle, 1968). The LD50 values
are summarized in Table 5.
Short-term toxicity of omethoate: Groups of 50 male and 50
female BOR:NMRI mice were fed diets containing omethoate (purity,
97.1%) providing doses of 0, 1, 3, or 10 ppm for four weeks and were
killed at intervals to investigate brain acetylcholinesterase
activity. There were no clinical signs of reaction to treatment, and
food and water intake, mortality, and body-weight gain were also
unaffected. The cholinesterase activity in plasma was clearly lower
than that in controls in mice receiving 10 ppm, but the differences
were smaller and much less consistent in erythrocytes. Plasma and
erythrocyte cholinesterase activity in mice at 1 and 3 ppm showed no
consistent differences from controls. Brain acetylcholinesterase
activity was clearly depressed in animals at 10 ppm. Inhibition of
brain acetylcholinesterase activity at 3 ppm was inconsistent, but the
level was up to 30% lower than that in contemporary controls. In
animals at 1 ppm, brain acetylcholinesterase activity was biologically
significantly lower than in controls only on day 3 in males (Ministry
of Agriculture, Fisheries and Food, 1993).
Groups of 15 male and 15 female rats (30 of each sex as controls)
were fed diets containing omethoate providing doses of 0, 2.5, 5, 15,
50, or 150 ppm for four months. Signs of cholinergic stimulation was
seen at doses > 15 ppm. Cholinesterase activity was depressed in
females at 50 and 150 ppm and in males at doses > 5 ppm. No effects
were noted on growth, organ weights, blood parameters, or urinary
parameters at levels < 50 ppm. Animals at 150 ppm died or had
depressed body weights and food consumption, and the relative liver
weight in males was increased (Löser & Lorke, 1967).
Groups of 15 male and 15 female rats were fed diets containing
omethoate to provide doses of 0, 0.5, 1.0, 2.0, or 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident in
animals at 4 ppm. Erythrocyte acetylcholinesterase activity was
depressed in animals at 2 ppm, but the effect was only slight in
females. In rats at 4 ppm, the inhibition was 30-50%. No effects were
noted on growth, food consumption, blood parameters, liver and kidney
function tests, organ weights, or histological appearance of tissues
(Löser, 1968).
Table 5. Acute toxicity of omethoate in experimental animals
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse Male Oral 36 Kimmerle (1968)
Mouse Male Oral 27 Santi & de Pietri Tonelli (1960)
Mouse Male Intraperitoneal 13 Lucier & Menzer (1970)
Mouse Male Intravenous 23 Kimmerle (1962)
Rat Male, female Oral 28-65 Kimmerle & Lorke (1967)
Rat Male, female Oral 50 Ben-Dyke et al. (1970)
Rat Male, female Oral 22-28 Ministry of Agriculture,
Fisheries and Food (1993b)
Rat Male Intraperitoneal 14 Kimmerle (1968)
Rat Male Intraperitoneal 38 Kimmerle (1962)
Rat Male, female Dermal 145-232 Ministry of Agriculture,
Fisheries and Food (1993b)
Rabbit Male Oral 50 Kimmerle (1962)
Guinea-pig Male Oral 100 Kimmerle (1962)
Cat Male Oral 50 Kimmerle (1962)
Chicken Male Oral 125 Kimmerle (1962)
Chicken Male Oral 100 Levinskas & Shaffer (1965)
Groups of six male and six female beagle dogs received omethoate
(purity, 97.1%; dissolved in acidulated water) daily for 12 months
by stomach tube at doses of 0, 0.02, 0.125, or 0.625 mg/kg bw.
Administration by gavage was chosen due to the reported instability of
the test material in dietary admixture. The appearance and behaviour
of the animals were normal, and no clinical signs attributable to
treatment were observed. All of the animals survived the treatment.
No significant differences were seen between the control and treated
groups with respect to reflexes, ophthalmoscopic parameters, body
temperatures, pulse rate, food and water consumption, mean
body weight, or haematological, clinical chemical (except for
cholinesterase activity), or urinary parameters. Clear depression
of plasma cholinesterase activity was observed only in rats at
0.625 mg/kg bw, amounting to 25-32% of the control value in males
and 16-29% in females. The depression remained essentially
constant throughout the study. A marked depression of erythrocyte
acetylcholestinerase activity was measured in males (17-40%) and
females (22-40%) at 0.625 mg/kg bw, which varied only slightly during
the study. At 0.125 mg/kg bw, only males showed slight (< 28%)
depression of erythrocyte acetylcholinesterase activity during the
first third of the study. Brain acetylcholinesterase activity was
depressed in males at 0.125 mg/kg bw (by 20%) and 0.625 mg/kg bw (39%)
and in females at 0.625 mg/kg bw (30%). The absolute and relative
organ weights were not significantly different between the control and
treated groups. Gross pathological and histopathological examination
showed no dose-related findings. The NOAEL was 0.62 mg/kg bw for
somatic effects and 0.02 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity (Hoffmann & Schilde, 1984).
Long-term toxicity and carcinogenicity of omethoate: Groups of
50 male and 50 female BOR:CWF1 mice were fed diets containing
omethoate (purity, 94%) providing levels of 0, 1, 3, or 10 ppm for 24
months. Appearance, behaviour, and activity were not significantly
different between the control and treated groups, and total and mean
daily food consumption were essentially the same in all the animals.
The body weights of treated males were generally higher than those of
the controls throughout the experiment, whereas those of the females
were no different from controls. Mortality and the frequency
distribution of mortality were comparable in all the groups. The
mortality rate at 18 months was 12-27% for males and 14-31% for
females. The absolute and relative organ weights of control and
treated groups showed no dose-related, significant differences. Gross
anatomical and histopathological examination revealed a range of
non-neoplastic changes commonly observed in old mice. Comparison of
these changes by type, site, and frequency distribution by sex and
dose gave no indication of treatment-related toxic effects. Neoplastic
changes were found primarily in the lungs, liver, adrenal cortex,
and haematopoietic system. Neither the type, site, or frequency
distribution of tumours by sex and dose level nor the numbers of
tumour-bearing mice, mice with benign tumours, mice with malignant
tumours, or mice with both benign and malignant tumours indicated
effects of treatment. The NOAEL for somatic effects was 10 ppm, equal
to 2.1 mg/kg bw per day for male mice and 3.1 mg/kg bw per day for
female mice (Kroetlinger & Löser, 1982).
Four groups of 50 male and 50 female Wistar rats were maintained
for 24 months on a diet containing omethoate providing concentrations
of 0, 0.3, 1,3, or 10 ppm. The control group consisted of 100 males
and 100 females. Omethoate did not clearly affect behaviour, body
weight, survival, food intake, or haematological, clinical chemical,
or urinary parameters. Plasma and erythrocyte cholinesterase
activities, measured in five males and five females from each group
at 1, 2, 4, 8, 13, 26, 52, and 78 weeks and at the end of the study,
were significantly depressed in all animals at 10 ppm. Erythrocyte
acetylcholinesterase activity was also inhibited in animals of each
sex at 3 ppm. The suppression of brain acetylcholinesterase activity,
measured in 10 males and 10 females per group, was dose-related in
animals at 3 and 10 ppm; it was also significantly affected in females
at 1 ppm, which can be considered the marginal no-effect level. Gross
and microscopic examination revealed no diverse effect of omethoate.
The tumour incidence was not clearly affected by treatment (Bomhard
et al., 1979).
Reproductive toxicity of omethoate: Groups of 10 male and 20
female FB 30 Long-Evans rats were fed diets containing omethoate
(purity, 94%) to give concentrations of 0, 1, 3, or 10 ppm for about
10 weeks, after which they were mated to initiate a three-generation
study of reproductive toxicity with two litters per generation.
Immediately after birth, the litters were examined for malformations.
Four days after birth, the litters were reduced to 10. When the
offspring were three weeks old, they were killed and subjected to
gross examination. Ten males and 10 female rats of the F3b generation
at all doses were examined histopathologically four weeks after birth.
There were no clear effects either on mating performance, pregnancy
rate, mortality, or the type and distribution of abnormalities. The
size of the litters of the second generation at 3 and 10 ppm was
reduced, and in the F2b generation, litter size was reduced at both 3
and 10 ppm after four days and at 10 ppm only after 28 days. Since
this effect was observed in only one progeny generation, 3 ppm was the
NOAEL (Löser, 1981).
In a two-generation study, omethoate was administered to groups
of 25 male and 25 female Wistar rats in the drinking-water at levels
of 0, 0.5, 3, or 18 ppm throughout a 70-day premating period and
throughout pairing, gestation, and lactation during breeding of a
single litter in each of the F1 and F2 generations. Reproductive
performance was adversely affected at 18 ppm, with a reduced
implantation rate, increased postnatal loss, and retarded pup weight
gain in both generations and increased precoital time, an increased
number of non-pregnant females, and increased postimplantation loss in
the F1 generation. Histopathological examination revealed an
increased incidence of epithelial vacuolation in the epididymides of
males treated with 18 ppm. The NOAEL for reproductive effects was
3 ppm, equivalent to 0.2 mg/kg bw per day. There was no NOAEL
for general toxicological effects, since erythrocyte and brain
acetyl-cholinesterase activities were inhibited at the lowest dose
(Ministry of Agriculture, Fisheries and Food, 1993).
Developmental toxicity of omethoate: Groups of 20-24 pregnant
rats were given omethoate orally at doses of 0, 0.3, 1, or 3 mg/kg bw
on days 6-15 of gestation. The animals were killed on day 20 of
gestation, and the fetuses were examined for skeletal and tissue
abnormalities. The fetuses and placentas of the animals at 3 mg/kg bw
weighed less than those of the controls. Other reproductive parameters
were unaffected. No teratogenic effect was observed (Machemer, 1975).
Groups of 14 pregnant New Zealand white rabbits were treated
daily by gavage with omethoate (purity, 96.8%) dissolved in distilled
water at doses of 0, 0.1, 0.3, or 1 mg/kg bw on days 6-18 of
gestation. On day 29 of gestation, the animals were killed and their
uterine contents examined. Whole-blood cholinesterase activity was
determined before treatment on day 6 of gestation and 2 h after
treatment on day 18 of gestation. The general condition of control and
treated females was comparable throughout the study. Maternal mean
body weights and corrected day-29 body weights were unaffected by the
treatment. Mortality, the incidence of abortions and total litter
losses, and the number of pregnant females with viable young on day 29
were not altered by treatment. Whole-blood cholinesterase activity was
significantly depressed only among females at 1 mg/kg bw in comparison
with both the pretreatment level and the control level after
treatment. There were no treatment-related differences between the
control and treated groups with respect to corpora lutea count,
implantations, male and female viable young, early and late
resorptions, pre- and postimplantation losses, or fetal and placental
weights. Examination of fetuses at necroscopy on day 29 of gestation
or after skeletal investigation revealed a number of non-dose-related
findings of types and incidences previously recorded in this strain of
rabbit and in the laboratory that performed the study. The NOAEL for
developmental toxicity was 1 mg/kg bw (Tesh et al., 1982).
Genotoxicity of omethoate: Omethoate has been extensively
tested in assays for mutagenicity in vitro. Positive results were
obtained in Salmonella, in one assay for gene mutation in mammalian
cells, and in assays for clastogenicity. Omethoate has also been
extensively tested in vivo. Negative results were obtained for
end-points in the bone marrow, liver, and germ cells, but a positive
result was obtained in a mouse spot test. The results of assays for
the genotoxicity of omethoate are summarized in Table 6.
Neurotoxicity of omethoate: Groups of 10 hens were given
omethoate orally at the LD50 (92 mg/kg bw) with atropine, and and
five positive controls were given TOCP at 350 mg/kg bw. Although
several hens treated with omethoate died, none showed clinical signs
of delayed neurotoxicity. Clinical signs were observed in those
treated with TOCP (Kimmerle, 1972). Histological examination of
nervous tissue with haematoxylin and eosin staining showed
degeneration in the hens treated with TOCP but not in those given
omethoate (Newman et al., 1972).
Groups of two to four hens were treated orally with omethoate
dissolved in corn oil at doses of 20-300 mg/kg bw, which were four
to eight times the unprotected LD50, under eserine and atropine
protection. The omethoate used was a sample that had caused a fatal
human poisoning accident. The acetylcholinesterase and neurotoxic
esterase activities of brain homogenates were assayed for 24 h after
dosing, and pair-dosed birds that survived were observed for signs of
ataxia for three to four weeks. Hens treated at four times the LD50
showed no inhibition of neurotoxic esterase at 24 h and no signs of
ataxia. Those treated at eight times the LD50 did not survive,
despite treatment with high doses of atropine; however, the neurotoxic
esterase activity in the brains of the animals that died within 36 h,
measured immediately after death, was found to be normal. Acute
cholinergic symptoms in all the birds were correlated with strong
inhibition of brain acetylcholinesterase activity, but 70% inhibition
in a bird treated with 20 mg/kg bw of omethoate still did not produce
detectable signs of acute poisoning.
The capacity of pure omethoate and of the incriminated sample to
inhibit neurotoxic esterase and acetylcholinesterase activities were
measured in hen and human brain tissue in vitro. As the IC50 for
acetylcholinesterase in both tissues was 0.08-0.15 mmol/litre, it
would be virtually completely inhibited at 5 mmol/litre, the
concentration that caused no detectable inhibition of neurotoxic
esterase. The activities of both enzymes were also measured in
cortical tissue samples taken 24 h post mortem from a 30-year-old
male farmer who had been acutely poisoned by a commercial formulation
of omethoate. The neurotoxic esterase activity was within the normal
range, while acetylcholinesterase activity was strongly inhibited. It
was concluded that omethoate is extremely unlikely to cause delayed
neuropathy in humans (Lotti et al., 1981).
Table 6. Results of tests for the genotoxicity of omethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0-12 500 µg/plate 95.1 Weakly positive Herbold (1980)
TA98, TA100, in TA98, TA100, and
TA1535, TA1537 TA1535a Negative in
TA1537a
DNA repair E. coli (pol) 0-10 000 µg/plate 96 Negative Herbold (1983)
Gene mutation Chinese hamster 0-6 mg/ml 97.4 Positivea Ministry of Agriculture,
ovary cells (hprt) Fisheries and Food
(1993)
Cell mutation L5178Y mouse 0-5000 µg/ml 96.9 Negativea Bootman & Rees (1982)
lymphoma cells
Sister chromatid Chinese hamster 0-1000 µg/ml 96 Positive at > 250 µg/ml Ministry of Agriculture,
exchange ovary cells Fisheries and Food
(1993)
Gene conversion; S. cerevisiae 0-66.7 µl/ml 96.9 Negative Hoorn (1982, 1983)
reverse mutation Positive
In vivo
Micronucleus Mouse 2 × 6 or 12 97.1 Negative Herbold (1981)
formation mg/kg bw
Dominant lethal Mouse 0.5 mg/kg bw 95.4 Negative Machemer (1974)
mutation
Unscheduled DNA Wistar rat 0-30 mg/kg bw 96.6 Negative Ministry of Agriculture,
synthesis Fisheries and Food
(1993)
Spot test C57Bl/6J × T 0-16 mg/kg bw 96.7-97% Positive Ministry of Agriculture,
mice Fisheries and Food
(1993)
a With and without metabolic activation
3. Observations in humans
The results of two studies of dimethoate in humans were
summarized briefly in the report of the 1963 JMPR (Annex 1, reference
2). In the first study, 20 volunteers were given daily doses of 2.5 mg
dimethoate in aqueous solution (corresponding to approximately
0.04 mg/kg bw) for four weeks. No toxic effect was observed, and there
were no changes in blood cholinesterase activity. Similar results were
reported in single subjects who ingested 9 mg (0.13 mg/kg bw) or 18 mg
(0.26 mg/kg bw) for 21 days (Sanderson & Edson, 1964).
The results of a number of studies in which human volunteers with
no occupational exposure to organophosphate pesticides were given
dimethoate were summarized in Environmental Health Criteria monograph
No. 90 (WHO, 1989) and are presented in Table 7. The studies of
volunteers indicate that repeated doses of up to 0.2 mg/kg bw
dimethoate do not inhibit cholinesterase activity in the blood.
Table 7. Results of controlled human trials with dimethoate
No. of Sex Route Daily dose Duration of Results Reference
subjects exposure
20 NR Oral 0.04 mg/kg 4 weeks No toxic effects or inhibition of Sanderson & Edson
blood ChE (1964)
2 NR Oral 0.13 mg/kg 21 days No toxic effects or inhibition of Sanderson & Edson
0.26 mg/kg blood ChE (1964)
5 M Oral 0.25 mg/kg Single No toxic effects or inhibition of Sanderson & Edson
dose blood ChE (1964)
50 NR Dermala 2.5 ml 2 h No irritation or inhibition of blood Sanderson & Edson
ChE (1964)
12 M+F Oral 5 mg (0.068 mg/kg bw) 28 days No significant change in whole-blood Edson et al. (1967)
ChE
9 M+F Oral 15 mg (0.202 mg/kg bw) 39 days No significant change in whole-blood Edson et al. (1967)
ChE
8 M+F Oral 30 mg (0.434 mg/kg bw) 57 days Inhibition of ChE by day 20 (24%) Edson et al. (1967)
6 NR Oral 45 mg (0.587 mg/kg bw) 45 days Inhibition of ChE (35%) Edson et al. (1967)
6 M+F Oral 60 mg (1.02 mg/kg bw) 14 days Inhibition of ChE (21%) Edson et al. (1967)
NR, not reported; ChE, cholinesterase
a Patch test with 32% liquid formulation
Comments
Dimethoate
Dimethoate is rapidly and extensively absorbed from the gut and
rapidly excreted. There was no accumulation in fat tissue. In rats and
humans, up to 90% of radiolabel was found in the urine within 24 h.
The report of a study with methylcarbamoyl-labelled dimethoate
indicated that up to 18% of the administered label was excreted in
expired air. Four metabolites with anticholinesterase activity have
been identified in rats and humans. One seems to result from thiono
oxidation, leading to the formation of the oxygen analogue of
dimethoate, i.e. omethoate; this step was followed by hydrolysis to
a thiocarboxyl product, said to be the main metabolite in rats and
humans.
Data on the acute oral toxicity of dimethoate gave LD50 values
of about 310 mg/kg bw in rats, 150 mg/kg bw in mice, and 55 mg/kg bw
in hens. The signs of toxicity were those typical of cholinesterase
inhibition. WHO has classified dimethoate as 'moderately hazardous'
(WHO, 1996).
In short-term and long-term studies at dietary concentrations
> 75 ppm, there were minor reductions in body-weight gain and food
consumption. Apart from inhibition of cholinesterase activity,
dimethoate had no effect on the composition of the blood or urine. The
liver weights of animals treated at the higher doses tended to be
lower than those of the control groups: there were, however, no
microscopic changes, and the effect is unlikely to be of toxicological
significance. Investigations of toxicity at higher doses were limited
by effects due to cholinesterase inhibition. The NOAELs were thus
generally based on reductions in acetylcholinesterase activity in
the brain or erythrocytes. On the basis of minimal reductions in
acetylcholinesterase activity of 10-20%, the NOAEL in a 12-month study
in dogs at doses of 0, 5, 20, or 125 ppm was 5 ppm, equal to 0.2 mg/kg
bw per day; in rats, the NOAEL in a life-span study at doses of 0, 1,
5, 25, or 100 ppm was 1 ppm, equal to 0.04 mg/kg bw per day. In mice,
an NOAEL was not identified, as cholinesterase activity was depressed
at all doses after 52 weeks of treatment in a life-span study at doses
of 0, 25, 100, or 200 ppm.
The results of long-term studies of toxicity and carcinogenicity
in mice (at 0, 25, 100, or 200 ppm) and rats (at 0, 5, 25, or 100 ppm)
reported in 1986 and studies reported in 1977 indicate that dimethoate
is not carcinogenic to rodents.
In a multigeneration study of reproductive toxicity conducted in
1989-90 with doses of 0, 1, 15, or 65 ppm, reproductive performance
of rats was impaired at the high dose. The NOAEL for reproductive
toxicity appeared to be 15 ppm (equal to 1.2 mg/kg bw per day) and
that for parental toxicity was 1 ppm (equal to 0.08 mg/kg bw per day)
on the basis of cholinesterase inhibition, but the Meeting noted that
there was some indication that reproductive performance may have been
affected at lower doses. In a multigeneration study conducted in mice
in 1965 at doses of 0, 5, 15 or 50 ppm, there was no overt effect on
reproductive capacity, even in the presence of cholinergic toxicity.
In a poorly reported study in rabbits, sperm numbers and quality were
adversely affected at doses equivalent to one-tenth and one-hundredth
of the LD50.
Studies of developmental toxicity in rats (at 0, 3, 6, or
18 mg/kg bw per day on days 6-15 of gestation) and rabbits (at 0, 10,
20, or 40 mg/kg bw per day on days 7-19 of gestation) provided no
evidence of a teratogenic effect, although maternal toxicity was
observed at the high dose in rats and at the high and middle doses in
rabbits.
After reviewing the data available on mutagenicity, the Meeting
concluded that although in-vitro studies indicate that dimethoate has
mutagenic potential, this potential does not appear to be expressed
in vivo.
Undiluted dimethoate formulations were irritating to the eye in
rabbits. Skin irritation was minimal and confined to slight, transient
erythema. Dimethoate was not a skin sensitizer in guinea-pigs, but a
32.7% emulsifiable concentrate formulation induced sensitization in
one of 10 guinea-pigs. In a published paper, dimethoate was cited
in four human cases of contact dermatitis, and sensitization was
confirmed in these individuals by patch testing.
In hens given a single dose of 55 mg/kg bw by subcutaneous
injection or orally, dimethoate did not induce delayed neurotoxicity.
In a 39-day study in nine male and female volunteers, the NOAEL
for cholinesterase inhibition was 0.2 mg/kg bw per day. This NOAEL was
supported in seven other studies each involving 6-20 volunteers who
received doses ranging from 0.04 to 1.0 mg/kg bw per day for up to 57
days.
Omethoate
The oral LD50 of omethoate was approximately 25 mg/kg bw
in rats. Signs of reaction to treatment with omethoate were those
consistent with cholinesterase inhibition.
In short-term and long-term studies, the potential toxicity of
omethoate was limited by the onset of cholinesterase inhibition. In a
12-month study of toxicity in dogs at doses of 0, 0.025, 0.12, or
0.62 mg/kg bw per day by gavage, the NOAEL was 0.02 mg/kg bw per day
on the basis of inhibition of acetylcholinesterase activity. In
life-span studies in rats (at 0, 0.3, 1, 3, or 10 ppm) anti mice (at
0, 1, 3, or 10 ppm), there was no evidence of oncogenic potential. The
study in mice was unsuitable for deriving an NOAEL because acetyl-
cholinesterase activity was not investigated; the NOAEL in rats was
0.3 ppm (0.015 mg/kg bw per day) on the basis of inhibition of
acetylcholinesterase activity.
In multigeneration studies in rats at 0, 1, 3, or 10 ppm, a
dietary concentration of 10 ppm was associated with reduced viability
of pups; there was evidence that this effect extended to animals
treated at 3 ppm. The NOAEL was 1 ppm (equivalent to 0.05 mg/kg bw per
day). In a further multigeneration study in rats at doses of 0, 0.5,
3, or 18 ppm in the drinking-water, there was evidence of epididymal
vacuolation and fewer pups per dam at the high dose; these pups had
lower weight gains and were less viable. The precoital time was
increased and the number of non-pregnant females was greater than
among controls. The NOAEL for reproductive performance was 3 ppm
(equivalent to 0.2 mg/kg bw per day), but cholinesterase inhibition
was detected at the lowest dose of 0.5 ppm. In studies of
developmental toxicity, there was no evidence of teratogenicity in
rats given 0, 0.3, 1, or 3 mg/kg bw omethoate per day on days 6-15 of
gestation or in rabbits given 0, 0.1, 0.3, or 1 mg/kg bw omethoate per
day on days 6-18 of gestation.
Omethoate has been extensively investigated for mutagenicity in
vitro and in vivo. The Meeting concluded that it has clear mutagenic
potential but that the weight of the evidence observed in vivo was
negative; however, the positive result obtained in the mouse spot test
could not be completely disregarded.
In studies in hens given single oral doses of 20-300 mg/kg bw,
omethoate did not induce delayed neurotoxicity.
Conclusions
An ADI of 0-0.002 mg/kg bw was established for dimethoate on the
basis of the apparent NOAEL of 1.2 mg/kg bw per day for reproductive
performance in the study of reproductive toxicity in rats and applying
a safety factor of 500. Although a safety factor of 100 would normally
be used in deriving an ADI from a study of this type, the Meeting was
concerned about the possibility that reproductive performance may have
been affected at 1.2 mg/kg bw per day in this study and therefore used
a higher-than-normal safety factor. No data are available to assess
whether the effects on reproductive performance were secondary to
inhibition of cholinesterase. The Meeting concluded that it was
not appropriate to base the ADI on the results of the studies of
volunteers since the crucial end-point (reproductive performance) has
not been assessed in humans.
This ADI would usually be used only when assessing the intake of
dimethoate itself. As the use of dimethoate on crops can give rise to
residues of omethoate, and omethoate has been used as a pesticide in
its own right, previous Joint Meetings have allocated an ADI to
omethoate; however, the primary manufacturer is no longer producing
omethoate. The Meeting noted that omethoate is considerably more toxic
than dimethoate; however, the levels of residues of omethoate
resulting from use of dimethoate on crops are likely to be low. The
Meeting therefore recommended that residues of dimethoate and
omethoate resulting from the use of dimethoate be expressed as
dimethoate and be assessed in comparison with the ADI for dimethoate.
As the primary manufacturer is no longer producing either
omethoate or formothion, toxicological data on these compounds were
not made available to the Meeting. The previous ADIs of 0-0.0003 mg/kg
bw for omethoate and 0-0.02 mg/kg bw for formothion were therefore
withdrawn.
There may be a need to re-evaluate the toxicity of dimethoate
after the periodic review of the residue and analytical aspects of
dimethoate has been completed if it is determined that omethoate is a
major residue.
Toxicological evaluation
Levels that cause no toxic effect (dimethoate)
Rat: 1 ppm, equal to 0.04 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
15 ppm, equal to 1.2 mg/kg bw per day (reproductive
performance in a study of reproductive toxicity)
1 ppm, equal to 0.08 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
6 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.2 mg/kg bw per day (52-week study of
toxicity)
Human: 0.2 mg/kg bw per day (39-day study of cholinesterase
inhibition)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw (sum of dimethoate and omethoate expressed as
dimethoate)
Studies that would provide information useful for continued evaluation
of the compound
1. Further multigeneration study in rats using dimethoate
2. Mouse spot test using dimethoate
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to dimethoate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 310 mg/kg bw
Dermal, toxicity, rat LD50 > 7000 mg/kg bw
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, human Sensitizing
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL > 1000 mg/kg bw per day (highest dose tested)
Repeated oral, reproductive toxicity, rat NOAEL = 1.2 mg/kg bw per day, reproductive toxicity
NOAEL = 0.05 mg/kg bw per day, parental toxicity
Repeated oral, developmental toxicity, rat NOAEL = 6 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 18 mg/kg bw per day (highest dose tested)
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 40 mg/kg bw per day (highest dose tested)
Long term (> 1 year) Repeated oral, toxicity and carcinogenicity, rat NOAEL = 0.04 mg/kg bw per day, cholinesterase
inhibition
References
Aharoni, A.H. & O'Brien, R.D. (1968) The inhibition of acetyl-
cholinesterases by anionic organophosphorus compounds. Biochemistry,
7, 1538.
Ahmed, F.E., Hart, R.W. & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res., 42, 161-174.
Becker, H. (1985) Dominant lethal study with dimethoate technical in
the mouse. Unpublished report No. 039003 from Research & Consulting
Company AG, Itingen, Switzerland. Unpublished report submitted to WHO
by the Dimethoate Task Force, Ingelheim, Germany.
Ben-Dyke, R., Sanderson, D.M. & Noakes, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9, 119.
Bhunya, S.P. & Behera, J. (1975) Centromeric sensitivity of mouse
chromosomes to the systemic insecticide dimethoate (Rogor). Curr. Sci.,
44, 859-860.
Bomhard, E., Loser, E. & Kaliner, G. (1979) S-6876: Chronic toxicity
study on rats (two-year feeding experiment). Unpublished report from
the Bayer AG Institut für Toxikologie, originally submitted to WHO by
Bayer AG, summary from WHO.
Bootman, J. & Rees, R. (1982) S-6876: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report from Life Science Research, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
Brady, U.E., Jr & Arthur, B.U. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56, 477-482.
Brooker, A.J., Homan, B.A., Parker, C.A., Offer, J.M., Anderson, A. &
Dawe, I.S. (1992) The effect of dimethoate on reproductive function
of two generations in the rat. Unpublished report from Huntingdon
Research Centre, United Kingdom. Submitted to WHO by the Dimethoate
Task Force, Milan, Italy.
Budreau C.H. & Singh, R.P. (1973) Effect of fenthion and dimethoate on
reproduction in the mouse. Toxicol. Appl. Pharmacol., 26, 29-38.
Burford, P., McLean, T.A., Buist, D.P., Crook, D., Gregson, R.L. &
Gopinath, C. (1991) Dimethoate: 12 month dietary toxicity study in
beagle dogs. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Casida, J.E. & Sanderson, D.M. (1962) Toxic hazards from formulating
the insecticide dimethoate in methyl 'cellosolve'. Nature, 189,
507-508.
Chamberlain, W.R., Gatterdam, P.E. & Hopkins, D.E. ( 1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54, 733-740.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorous pesticides.
Mutat. Res., 88, 307-316.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health, B20, 373-406.
Dal Re, V. & Vola Gera, F. (1976) Determination of the acute dermal
toxicity of technical Rogor. Unpublished report from Centro Ricerche
Antiparassitari, Montedison, Italy. Submitted to WHO by Farmoplant,
Milan, Italy.
Dal Re, V. & Vola Gera, F. (1980) Acute oral toxicity of technical
Rogor samples recently produced in albino rats. Unpublished report
from Centro Ricerche Antiparassitari Montedison, Italy. Submitted to
WHO by Farmoplant, Italy.
Dauterman, W.C., Casida, J.E., Knaak, J.B. & Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agric. Food Chem., 7, 188-193.
Degraeve, N. & Moutschen, J. (1983) Genotoxicity of an organophosphorus
insecticide, dimethoate, in the mouse. Mutat. Res., 119, 331-337.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo.
Arch. Toxícol., 58, 152-156.
Ecobichon, D.J. & Kalow, W. (1963) Action of organo phosphorus
compounds upon esterases in human liver. Can. J. Biochem., 41, 1537.
Edson, E.F. & Noakes, D.N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. Appl. Pharmacol.,
2, 523.
Edson, E.F., Jones, K.H. & Watson, W.A. (1967) Safety of dimethoate
insecticide. Br. Med. J., iv, 554.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984a) Effect of dimethoate
on pregnancy of the rat. Unpublished report from Huntington Research
Centre, Huntington, Cambs, United Kingdom. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984b) Effect of dimethoate
on pregnancy of the New Zealand white rabbit. Unpublished report from
Huntington Research Centre, Huntington, Cambs, United Kingdom.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
El-Elaimy, I. (1986) Comparative studies on the effect of carbamate
and organophosphorus insecticides on acetylcholinesterase activity in
maternal and foetal tissue of rat. Proc. Zool. Soc. A.R. Egypt, 10,
41-49.
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomatis, L., eds, Chemical Carcinogenesis Essays
(IARC Scientific Publications No. 10), Lyon, International Agency for
Research on Cancer, pp. 161-181.
Fenwick, M.L., Barron, J.R. & Watson, W.A. (1957) Biochem. J., 65, 58.
Fischer, G.W. & Scheufler, H. (1981) Effects of dimethoate and
O-desmethyldimethoate in dominant lethal test in mice. Wiss. Z.
Univ. Halle, 30, 21-29 (in German).
Gabliks, J. (1965a) Responses of cell cultures to insecticides II.
Chronic toxicity and induced resistance. Proc. Soc. Exp. Biol. Med.,
120, 168-171.
Gabliks, J. (1965b) Responses of cell cultures to insecticides. III.
Altered susceptibility to poliovirus and diphtheria toxin. Proc. Soc.
Exp. Biol. Med., 120, 172-175.
Gabliks, J. & Friedman, L. (1965) Responses of cell cultures to
insecticides. I. Acute toxicity to human cells. Proc. Soc. Exp. Biol.
Med., 120, 163-168.
Gibel, U., Lohs, KH., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) Experimental study on cancerogenic, haematotoxic, and
hepatotoxic activity of phosphororganic pesticides. Arch.
Geschwulstforsch., 41, 311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade6
locus of Schizosaccharomyces pombe. Mutat. Res., 117, 139-148.
Gomez-Arroyo, S., Noriega-Aldana, N., Juarez-Rodriguez, D. &
Villalobos-Pietrinir, J. (1987) Sister chromatid exchanges induced by
the organophosphorus insecticides methyl parathion, dimethoate, phoxim
and methyl azinfos in cultured human lymphocytes. Contam. Amb.,
3, 63-70.
Guthrie, F.E., Shah, P.V. & Moreland, D.E. (1980) Effects of pesticides
on active transport of glucose through the isolated intestine of the
mouse. J. Agric. Food Chem., 22, 3.
Hassan, A., Zayed, S.M.A.D. & Bahig, M.R.E. (1969) Metabolic behaviour
of organophosphorus insecticides. XI: Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18, 2429-2438.
Hellwig, J. (1986a) Report on the study of the toxicity of dimethoate
in mice after 78-week administration in the diet. Unpublished report
No. 75C0326/8242 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Hellwig, J. (1986b) Report on the study of the toxicity of dimethoate
in rats after 24-month administration in the diet. Unpublished report
No. 70C0326/8241 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Herbold, B. (1980) S-6876 Salmonella/Mikrosomen Test zur Untersuchung
auf punktmutagene Wirking. Unpublished report from the Bayer AG
Institut für Toxikologie, originally submitted to WHO by Bayer AG,
summary from WHO.
Herbold, B. (1981) S-6876 (Omethoate, Folimat's active ingredient):
Micronucleus test on mouse to evaluate S 6876 for mutagenic potential.
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Herbold, B. (1983) S-6876 (Omethoate, Folimat's active ingredient):
Pol test on E. coli to evaluate for DNA damage. Unpublished report
from Institute of Toxicology, Bayer AG, originally submitted to WHO by
Bayer AG, summary from WHO.
Hoffman, K. & Schilde, B. (1984) S-6876 (Omethoate): Chronic toxicity
to dogs on oral administration (twelve month stomach tube study).
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG. summary from WHO.
Hoorn, A.J.W. (1982) Mutagenic evaluation of S-6876 (omethoate) in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138/S211-alfa. Unpublished report from Litton Bionetics, Netherlands,
originally submitted to WHO by Bayer AG, summary from WHO.
Hoorn, A.J.W. (1983) Evaluation of S-6876 (omethoate) in the induced
mitotic crossing over and gene conversion assay in Saccharomyces
cerevisiae strain D7. Unpublished report from Litton Bionetics,
Netherlands, originally submitted to WHO by Bayer AG, summary from
WHO.
Johnson, E., Caterson, C.R. & Allen, J.S. (1985) Mutagenicity testing
of dimethoate in the in vitro CHO/HGPRT mutation assay. Unpublished
report No. 0423 from American Cyanamid Co., Princeton, NJ, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
von Jung, H.-D. (1989) Kontaktekzem durch pestzide in der DDR.
Dermatol. Monaschr., 175, 203-214 (in German with English summary).
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
R.E. & Treiber, G. (1959) The metabolism of dimethoate in cattle.
J. Econ. Entomol., 52, 1190-1194.
Khera, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for teratogenic
activity in the cat. J. Environ. Pathol. Toxicol., 2, 1283-1288.
Khera, K.S., Whalen, C., Trivett, G. & Angers, G. (1979) Teratogenicity
studies on pesticidal formulations of dimethoate, diuron and lindane in
rats. Bull. Environ. Contam. Toxicol., 22, 522-529.
Kimmerle, G. (1962) Toxicological studies on active ingredient S-6876.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1968) Bayer 45432-Toxikologische Untersuchungen.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1972) Folimat/Akute Neurotoxizitatsuntersuchungen an
Huhnern. Unpublished report from the Institut für Toxikologie,
Wuppertal-Elberfeld, Germany, originally submitted to WHO by Bayer AG,
summary from WHO.
Kimmerle, G. & Lorke, D. (1967) S-6876. Antidotal effect and
potentiation. Unpublished report originally submitted to WHO by Bayer
AG, summary from WHO.
Kroetlinger, F. & Löser, E. (1982) S-6876 (omethoate, the active
ingredient of Folimat): Chronic toxicity study on mice. (2-year
feeding experiment). Unpublished report from Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Levinskas, G.J. & Shaffer, C.B. (1965) Oxygen analogue of dimethoate:
Demyelination in white Leghorn hens. Unpublished report originally
submitted to WHO by American Cyanamid Co., summary from WHO.
Löser, E. (1968) Bayer 45432: Subakute Toxikologische Untersuchungen
an Ratten. Unpublished report originally submitted to WHO by Bayer AG,
summary from WHO.
Löser, E. (1981) S-6876 Folimat-Wirkstoff Multigenerationeversuche an
Ratten. Unpublished report from Bayer AG, originally submitted to WHO
by Bayer AG. Addendum: Histopathology, Consultox Laboratories Ltd,
London, United Kingdom. Summary from WHO.
Löser, E. & Lorke, D. (1967) Bayer 45432: Subchronische Toxikologische
Untersuchungen an Ratten. Unpublished report originally submitted to
WHO by Bayer AG, summary from WHO.
Lotti, M., Ferrara, S.D., Caroldi, S. & Sinigaglia, F. (1981) Enzyme
studies with human and hen autopsy tissue suggest omethoate does not
cause delayed neuropathy in man. Arch. Toxicol., 48, 265-270.
Lucier, G.W. & Menzer, R.E. (1970) Nature of oxidative metabolites of
dimethoate formed in rats, liver microsomes, and bean plants. J. Agric.
Food Chem., 18, 698.
Machemer, L. (1974) Praparat S-6876 to Dominant Letal Test an der
mannlichen Maus zur Prufung auf mutagene Wirkung. Unpublished report
from the Institut für Toxikologie, Wuppertal-Elberfeld, Germany,
originally submitted to WHO by Bayer AG, summary from WHO.
Machemer, L. (1975) Praparat S-6876 to Untersuchung auf Exbryotoxische
and Teratogene Wirkung nach Oraler Verabreichung an der Ratte.
Unpublished report from the Institut für Toxikologie, Wuppertal-
Elberfeld, Germany, originally submitted to WHO by Bayer AG, summary
from WHO.
Madison, W.A., MacKenzie, K.M. & Fields, W.E. (1984) 21 Day dermal
study with dimethoate in rabbits. Unpublished report No. 6123-125
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by the Dimethoate Task Force, Milan, Italy.
Ministry of Agriculture, Fisheries and Food (1993a) Evaluation on
Dimethoate, Pesticides Safety Directorate, United Kingdom.
Ministry of Agriculture, Fisheries and Food (1993b) Evaluation on
Omethoate, Pesticides Safety Directorate, United Kingdom.
Mahkambaeva, Z.G. (1971) Influence of organophosphorus pesticides
(kilval, methylmercaptophos, fosfamid) on bioelectric activity of
heart in experimental animals. In: Hygiene, Use and Toxicology of
Pesticides, and Clinical Aspects of Pesticide Poisoning, Kiev, Zdorovye
Publishers, pp. 175-180 (in Russian).
Marosi, G., Ivan, J., Nagymajtenyi, L., Csatlos, J. & Toszegi, A.
(1985a) Dimethoate induced toxic cardiac failure in the guinea-pig.
Arch. Toxicol., 57, 142-143.
Marosi, G., Ivan, J. & Nagymajtenyi, L. (1985b) Cardiodepression in
organophosphate poisonings. Arch. Toxicol., Suppl. 8, 289-291.
Mohn, G. (1973) 5-Methyl tryptophan resistance mutations in
Escherichia coli K-12. Mutat. Res., 20, 7-15.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nadmaiteni, L. & Marosi, G. (1983) Cardiotoxic action of organo-
phosphorus pesticides. Gig. Sanit., 11, 72-73 (in Russian).
Nagymajtenyi, L. (1988) Neurophysiological markers as early signs of
organophosphate neurotoxicity. Neurotoxicol. Teratol., 10, 429-434.
Nehéz, M., Selypes, A., Scheufler, H. & Fisher, G.U. (1983) Effect of
dimethoate and O-demethyldimethoate on bone marrow cells of CFLP
mice. Regul. Toxicol. Pharmacol., 317, 349-354.
Newman, A.J., Cherry, C. & Urwin, C. (1972) Pathology report of
folimat active substances in hens. Unpublished report from the
Huntingdon Research Centre, Huntingdon, Cambs, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
O'Brien, R.D (1959) Activation of thionophosphates by liver
microsomes. Nature, 183, 121.
O'Brien, R.D (1961) The effect of SKF 525A (2-diethylaminoethyl
2',2-diphenyl valerate hydrocloride) on organophosphate metabolism in
insect and mammals. Biochem. J., 79, 229.
Panshina, T.N. & Klisenko, M.A. (1962) Content of fosfamid and changes
in blood cholinesterase activity in different routes of treatment of
the mammalian organism. In: Industrial Toxicology and Clinical Picture
of Occupational Diseases Due to Chemicals, Moscow, Medgiz, pp. 181-184
(in Russian).
Probst, G.H., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K. &
Neal, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3, 11-32.
Rani, M.V.U., Reddi, O.S. & Reddy, P.P. (1980) Mutagenicity studies
involving aldrin, endosulfan, dimethoate, phosphamidon, carbaryl and
ceresan. Bull. Environ. Contam. Toxicol., 25, 277-282.
Redgrave, V.A., Johnson, A.A., Gopinath, C., Hadley, J.C., Anderson,
A. & Dawe, I.S. (1991) Dimethoate: Acute delayed neurotoxicity in the
domestic hen. Unpublished report from the Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Reena, et al. (1989) Haematological changes induced by dimethoate in
rat. Arh. Hig. Rada Toksikol., 40, 23-27.
Reuber, M.D. (1984) Carcinogenicity of dimethoate. Environ. Res.,
34, 193-211.
Ribelin, W.E., Levinskas, G.J. & Shaffer, C.B. (1965) A 3-generation
reproduction study with mice. Unpublished report. Submitted to WHO by
the American Cyanamid Company.
Riemer, F., Dahlenburg, R. & Grisk, A. (1985) Renal excretion of
dimethylphosphate and its thio derivatives after application of
dimethoate, bromophos, naled or trichlorphon to rats. Nahrung, 29,
299-302 (in German).
Salem, M.H., Abo-Elezz, Z., Abd-Allah, G.A., Hassan, G.A. & Shaker, N.
(1988) Effect of organophosphorus (dimethoate) and pyrethroid
(deltamethrin) pesticides on semen characteristics in rabbits.
J. Environ. Sci. Health, B23, 279-290.
Sanderson, D.M. & Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Br. J. Ind. Med., 21, 52.
San Sebastian, J.R. (1985) In vivo bone marrow cytogenetics rat
metaphase analysis. PH 315-AC-00-84, Dimethoate CL 12880. Unpublished
report from Pharakon Research International, Inc., Waverly, PA, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
Santi, R. & de Pietri-Sonelli, P. (1959) Mode of action and biological
properties of the S-(methylcarbamyl)methyl O,O-dimethyl-
diophosphate. Nature, 183, 398.
Santi, R. & de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido O,O-dimetiliditio-
fosforilacetico. Ist. Ric. Agr. Soc. Montecatini, 3, 3 (in Italian).
Scheufler, H. (1975) Effects of relatively high doses of dimethoate
and trichlorfon on the embryogenesis of laboratory mice. Biol. Rundsch.,
13, 238-240 (in German).
Shaker, N. (1988) In vivo chronic effect of dimethoate and deltamethrin
on rabbits. J. Environ. Sci. Health, B23, 387-399.
Sorg, R.M., (1985) Micronucleus test (MNT). PM 309A-AC-004-84,
Dimethoate CL 12880. Unpublished report from Pharakon Research
International, Inc., Waverly, PA, USA. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Squire, R.A. (1988) An evaluation of vascular proliferative lesions in
male Wistar rats from project 70C0326/8241. Unpublished report from
Robert A. Squire Associates, Ruxton, MD, USA. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorfon and
dimethoate. Acta Haematol., 52, 70-76.
Tesh, J.M., Ross, F.W., Wightman, T.J. & Wilby, O.K. (1982) S-6876:
Effect of oral administration upon pregnancy in the rabbit. Unpublished
report from Life Science Research, United Kingdom, originally submitted
to WHO by Bayer AG, summary from WHO.
Tiefenbach, B. & Lange, P. (1980) Studies on the action of dimethoate
on the immune system. Arch. Toxicol., Suppl. 4, 167-170.
Tripathy, N.K., Majhi, B., Dey, L. & Das, C.C. (1988) Genotoxicity of
Rogor studied in the sex-linked recessive lethal test and wing eye and
female germ-line mosaic assays in Drosophila melanogaster. Mutat. Res.,
206, 351-360.
Uchida, T., Dautermann, W.C. & O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agric. Food Chem., 12, 48-52.
Ullman, L., Sacher, R. & Chevalier, J. (1985) Acute oral toxicity
study with dimethoate in mice. Unpublished report from Research and
Consulting Co., AG, Itingen, Switzerland. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
US National Cancer Institute (1976) Bioassay of Dimethoate for Possible
Carcinogenicity (NCI-CG-TR-4 DHEW Publication No. (NIH) 77-80),
Bethesda, MD, USA.
US National Cancer Institute (1977) Bioassay of Dimethoate for Possible
Carcinogenicity (Technical Report Series No. 4), Bethesda, MD, USA.
Velázquez, A., Xamena, N., Creus A. & Marcos, R. (1986) Indication for
weak mutagenicity of the organophosphorus insecticide dimethoate in
Drosophila melanogaster. Mutat. Res., 172, 237-243.
Vishwanath, R. & Jamil, K. (1986) Mutagenic and genotoxic activities
of certain organophosphorus compounds using Ames Salmonella assay
with and without microsomal induction. Ind. J. Exp. Biol., 24, 305-308.
Weber, H., Patzschke, K. & Wegner, L.A. (1978) Omethoat-C14-Folimat-
Wirkstoff: Biokinetische Untershungen an Ratten. Unpublished report
originally submitted to WHO by Bayer AG, summary from WHO.
West, B., Vidone, L.B. & Shaffer, C.B. (1961) Acute and subacute
toxicity of dimethoate. Toxicol. Appl. Pharmacol., 3, 210.
WHO (1989) Dimethoate (Environmental Health Criteria 90), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutag., 5, 835-846.
Table 1. Acute toxicity of dimethoate in experimental animals
Species Sex Route Purity LD50 Reference
(%) (mg/kg bw)
Mouse Female Oral NR 60 Sanderson & Edson (1964)
Mouse Male, female Oral NR 160 Ullman et al. (1985)
Rat Male, female Oral NR 314 Ministry of Agriculture,
Fisheries and Food (1993a)
Rat Male, female Oral 97.6-99 540-600 Dal Re & Vola Gera (1980)
Rat Male, female Dermal 97.6-99 >7000 Dal Re & Vola Gera (1976)
Rat Male Intravenous NR 450 Sanderson & Edson (1964)
Rat Male, female Intraperitoneal NR 175-350 Sanderson & Edson (1964)
Hamster Male Oral NR 200 Sanderson & Edson (1964)
Guinea-pig Male, female Oral NR 350-600 Sanderson & Edson (1964)
Rabbit Male, female Oral NR 300-500 Sanderson & Edson (1964)
Hen Male, female Oral NR 30-50 Sanderson & Edson (1964)
NR not reported
(b) Short-term toxicity
Rats
Three studies in rats were briefly summarized in the report of
the 1963 JMPR (Annex 1, reference 2). In a 15-week study, groups of 10
male rats were fed diets containing 1, 5, 25, or 125 ppm dimethoate,
equivalent to 0.1, 0.5, 2.5 and 12 mg/kg bw per day. At the high
dietary level, slight muscular fibrillation and depressed weight gain
were observed. At 5, 25, and 125 ppm, depressed cholinesterase
activity was observed. In another study, groups of 20 rats were fed
diets providing 2, 8, or 32 ppm for 90 days or 50, 100, or 200 ppm
for 35 days. No haematological abnormalities were reported nor any
significant pathological change. The highest dose that did not inhibit
cholinesterase activity was reported to be 32 ppm. In a one-year study
with groups of 20 male rats, the highest dose that did not inhibit
cholinesterase activity was reported to be 10 ppm (Edson & Noakes,
1960; West et al., 1961; Sanderson & Edson, 1964).
The report of a 13-week study in Wistar rats was available only
in an incomplete translation and was neither dated nor signed. Groups
of 24 male and 24 female rats received doses of 0.02, 0.2, 2, or
20 mg/kg bw per day for 18 weeks; a group of 32 males and 32 females
acted as controls. Animals were housed eight per cage, and the
doses were administered orally on five clays a week as aqueous
solutions, which were prepared weekly and refrigerated until use. The
formulations were not analysed for content or for stability in the
vehicle. Body weights were recorded weekly, and food consumption was
recorded, but at unspecified intervals. Blood samples were obtained
from eight males and eight females in each treated group and from 16
controls of each sex on four occasions; a normal range of parameters,
including cholinesterase activity, was measured. Urine samples were
obtained in weeks 12 and 18. Brain cholinesterase activity and a renal
function test (phenol red test) were carried out after 18 weeks.
Histological investigations were performed on six animals of each
sex per group, and the list of tissues chosen for weighing was
satisfactory; however, the list of those chosen for histopathological
examination was short and did not include epididymides.
Slightly reduced body-weight gain was seen in animals treated at
20 mg/kg bw throughout the test, and food consumption was slightly
lower in males of this group than in the controls during the latter
half of the study. There were no treatment-related deaths. During
weeks 6-11, animals in all groups, including the controls, had
diarrhoea, but this was more pronounced in animals treated at 2 or
20 mg/kg bw. Minimally lower haematocrit and erythrocyte count were
noted in animals at the high dose after seven weeks only. There were
no other toxicologically significant changes in haematological
parameters. Plasma cholinesterase activity was 45-75% lower in
animals at 20 mg/kg bw than in the controls. Erythrocyte and brain
acetylcholinesterase activity was 70-90% of that in controls for
animals treated at 2 mg/kg bw and 54-77% in animals at 20 mg/kg bw.
Renal function was unimpaired by treatment, but no data were
presented. There were no treatment-related effects on the urine and
there was no faecal occult blood. Necropsy indicated no effects of
treatment. Organ weight analysis indicated a number of intergroup
differences, none of which was clearly of toxicological significance;
however, the absolute and relative weights of the livers of treated
animals tended to be lower than those of the controls, and this is
likely to represent a treatment-related change, as similar effects
have been seen in other studies. There were no microscopic findings
related to treatment, but no data were presented. It was concluded
that the toxicity expressed was minimal and that 0.2 mg/kg bw was the
NOAEL for inhibition of brain and erythrocyte acetylcholinesterase
activity (Ministry of Agriculture, Fisheries and Food, 1993a).
Dimethoate was administered orally to rats on five days a week
for six weeks at a dose of 10 mg/kg bw per day. The investigations
were confined to an electroencephalogram and cholinesterase
determinations. Treatment was associated with increased frequency and
decreased amplitude on the electroencephalogram, and, as expected,
inhibition of cholinesterase activity in the tissues examined
(Nagymajtenyi, 1988). The relevance of these data to the safety
evaluation of dimethoate is equivocal, and they are not considered
further in this review.
Ten male albino rats received dimethoate by intraperitoneal
injection at 150 mg/kg bw in 0.5 ml saline on alternate days for 30
days; a similar group was treated for 15 days. Ten controls received
saline only, but the duration of their treatment was not specified. On
completion of the treatment, the rats were decapitated and blood
samples were collected for haematological and biochemical examination.
The results were presented as means and standard errors for five
animals per observation. Haemoglobin concentration, haematocrit, and
erythrocyte and leukocyte counts in treated animals were clearly lower
than in controls, the greatest effect being seen after 30 days. The
concentrations of serum urea were greater than in the controls at both
sacrifices; a higher cholesterol concentration was seen only after 30
days. The serum activities of aspartate and alanine aminotransferases
and amylase were higher than in the controls on both occasions. There
was also a small increase in alkaline phosphatase activity; however,
as the timing of the collection of control blood samples was not
given, the significance of this minor change cannot be assessed. The
activity of acid phosphatase was lower than in controls at both blood
sample collections. Cholinesterase determinations in serum indicated
inhibition of 30-50% relative to controls. The above effects were all
more marked after 30 than after 15 days. These results indicate that
dimethoate may affect liver and kidney function, although the changes
were not of pathological significance. Organs were not weighed and no
microscopic examinations were undertaken in order to correlate the
changes seen. Although the changes in amylase activity might indicate
pancreatic effects, confirmatory isoenzyme studies were not carried
out. The route and frequency of administration in this study and the
exiguous nature of the examinations performed render this work of
equivocal use in the safety evaluation of dimethoate (Reena et al.,
1989).
Rabbits
In a five-month study by oral administration in male rabbits, the
doses used were said to be one-tenth and one-hundredth of the LD50
value given once a week, but the actual doses were not specified.
There were no indications of clinical signs, and most of the data were
presented graphically on the basis of monthly sacrifice of four
animals out of a total of 20 animals per group. An initial increase in
cholinesterase activity was noted at the one-month sacrifice, but a
subsequent 40% reduction was reported. Although changes in organ
weights were reported, the body weights of the animals were not, and
the changes were reported only as percentages of absolute weight of
controls. The significance of the various findings cannot be assessed,
and the report is not considered further (Shaker, 1988).
The dermal toxicity of technical-grade dimethoate was investigated
in groups of six male and six female New Zealand white rabbits which
received doses of 100, 300, or 1000 mg/kg bw per day for 21 days; two
control groups, one untreated and one receiving the vehicle (paraffin
oil), were similarly constituted. The doses were selected on the basis
of the results of a five-day range-finding study in two groups of one
male and one female given doses of 1000 or 2000 mg/kg bw per day. In
the range-finding study, slight erythema was seen in one animal at
1000 mg/kg bw per day and in both at 2000 mg/kg bw per day on days 3,
4, and 5; fissuring of the skin was seen in one animal treated at
2000 mg/kg bw per day. Application was made to the abraded skin of
three animals per group or to non-abraded skin on the back of each
animal on five days per week at a volume of 2 ml/kg bw. The test site
was occluded for 6 h with a gauze bandage held in place by tape and
wrapped in occlusive plaster. Observations and food consumption were
recorded daily, and body weight was measured weekly. Blood samples were
taken before treatment and at termination after about 16 h of fasting.
All animals were subjected to a complete necropsy, and a range of
tissues was retained. Microscopic examination was restricted to the
controls and animals at the highest dose.
There were no significant differences between treated and control
animals or between those with intact or abraded skin. Pustules were
seen at the treatment sites of the majority of animals, including
vehicle controls, during the study. Body-weight gain and food
consumption were unaffected by treatment, and there were no changes in
the blood, including cholinesterase activity, that could be ascribed
to treatment. There were no significant treatment-related intergroup
differences in organ weights or in macroscopic or microscopic
pathology. The absence of an effect on cholinesterase activity
indicates that the test material was not absorbed, even across broken
(abraded) skin, at doses up to 1000 mg/kg bw per day. It was concluded
that dimethoate does not irritate rabbit skin (Madison et al., 1986).
Dogs
A study in dogs was briefly summarized in the report of the 1963
JMPR (Annex 1, reference 2), in which groups of two males and two
females were fed diets providing 2, 10, or 50 ppm, equivalent to 0.05,
0.25 and 1.25 mg/kg bw per day dimethoate for 90 days. No significant
effects were noted, and erythrocyte cholinesterase activity was only
slightly depressed in animals at 50 ppm (West et al., 1961).
Groups of six male and six female beagle dogs received diets
containing dimethoate at concentrations of 0, 5, 20, or 125 ppm
for one year. The doses were chosen on the basis of the results
of a preliminary study of 28 days' duration at concentrations
< 1250 ppm, at which dose-related changes were observed at
> 50 ppm, necessitating early sacrifice at 1250 ppm, while changes
at 50 and 250 ppm were confined to dose-dependent reductions in
cholinesterase activity. In the main study, clinical signs of reaction
to treatment and food consumption were recorded daily; body weight was
recorded weekly. The eyes of each animal were examined before
treatment and during weeks 26 and 52. Blood samples were taken on two
occasions before treatment and during weeks 13, 26, and 52. Urine
samples were collected at similar intervals. The samples were examined
for a normal range of haematological and biochemical characteristics,
including plasma and erythrocyte cholinesterase activity; brain
acetylcholinesterase activity was measured at termination. All animals
were necropsied after bone-marrow samples had been taken. A range of
organs was weighed and the tissues preserved for histopathological
processing and examination.
There were no deaths or clinical signs of reaction to treatment
and no effect on body weight, food consumption, or the eyes. The
achieved intakes of test material were calculated to be 0.19, 0.73,
and 4.25 mg/kg bw per day for animals at 0, 5, 20, or 125 ppm. Plasma
cholinesterase activity was reduced by > 20% relative to controls in
animals of each sex at 125 ppm in weeks 13 and 26 and in males only in
week 52. These reductions did not exceed 22%, except in females at
week 13 which had an activity 36% lower than controls. Erythrocyte
acetylcholinesterase activity was reduced in weeks 13 and 26 by 20-27%
in animals at 20 ppm and by 63-76% in those at 125 ppm. In week
52, males at 5 or 20 ppm had marginally, nonsignificantly lower
erythrocyte acetylcholinesterase activities than the controls; females
in these groups were unaffected. A clear reduction (about 65%) in
erythrocyte acetylcholinesterase activity was also seen in animals at
125 ppm. Statistically significant reductions in brain acetylcholin-
esterase activity were seen at all doses after 52 weeks, which were
slight at 5 ppm (about 90% of the control level) and 20 ppm (about 83%
of control) but clear at 125 ppm (45% of control). There were no other
biochemical differences in the blood attributable to treatment. The
urine was unaffected, and there were no findings at necropsy that were
attributable to treatment. The liver weights of animals at 125 ppm
were lower than those of the controls. There was a marginally greater
incidence of pigment, presumed to be haemosiderin, in the livers of
treated animals in all groups, but there was no clear relationship to
dose. In the absence of other effects of treatment, notably on blood,
this effect was considered to be of no toxicological significance. The
only significant evidence of toxicity attributable to dimethoate was
the reduction in cholinesterase activity; the effect on erythrocyte
and brain acetylcholinesterase activity was clearly significant at 20
and 125 ppm, whereas the effects at 5 ppm were confined to minimal
reductions in brain acetylcholinesterase (10% lower than controls) and
a minimal reduction in male plasma cholinesterase activity after 51
weeks. The NOAEL was 5 ppm, equal to 0.19 mg/kg bw per day (Burford
et al., 1991).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade dimethoate was administered to groups of 50 male
and 50 female individually housed B6C3F1 mice for 18 months. Dietary
concentrations of 0, 25, 100, and 200 ppm (equal to 3.2, 12.3 and
25.3 mg/kg bw per day) were selected on the basis of the results of
previous studies, including a study from the US National Cancer
Institute (1977) in this strain. Blood samples were taken for
investigations of haematology and cholinesterase activity after 51
weeks of treatment from an additional 10 males and 10 females
allocated to each group, which were then killed and necropsied.
Haematological investigations only were conducted after 78 weeks on 10
animals of each sex per group. The test diets were mixed weekly, and
formulated diet and the test material were analysed at approximately
three-month intervals; the results of these analyses were satisfactory.
All animals were necropsied; appropriate organs only from animals at
the terminal kill were weighed. A wide range of tissues from all
animals, including satellite animals that died before 51 weeks, were
examined microscopically.
There were no clinical findings that were considered by the
authors to be related to treatment. Survival was > 90% in all groups,
and there was no effect of treatment on mortality. There were no
differences in group mean food consumption that could be related to
treatment. The body-weight gain of treated males was lower than that
of controls during the first few weeks of the study; females receiving
200 ppm were transiently affected during the first two weeks.
Subsequently, the body weights of treated females in all groups were
greater than those of the controls; a similar but less marked
difference was evident in treated males from about 14 months. Female
animals at 25 ppm gained notably less weight than those at the two
higher doses but still gained more than the controls. The overall
weight gains of females were 16.1 ± 5.9 (SD) for the controls,
20.4 ± 6.4 for those at 25 ppm, 27.9 ± 7.3 for those at 100 ppm, and
26.0 ± 5.6 for those at 200 ppm. Haematological analyses after 78
weeks indicated higher nonspecific leukocyte counts in males at 100 or
200 ppm and in females at 200 ppm; no similar difference was seen in
samples taken from satellite animals after 51 weeks of treatment. The
cholinesterase activity in plasma and erythrocytes from treated
animals was lower than That in the controls in a dose-related manner
at all dietary concentrations. No other examination of cholinesterase
activity and no analyses of brain were undertaken. Organ weight
analysis indicated greater absolute liver weights in animals at 100 or
200 ppm; however, the relative liver weights of females were lower
than those of controls as a result of the increased body weights of
these animals. The absolute weight of the ovaries of treated females
was lower than that of the controls after 78 weeks of treatment, but
no similar difference was seen in animals killed after 52 weeks of
treatment. Microscopic examination indicated a greater incidence of
extramedullary haematopoiesis in the spleens of males and females at
100 or 200 ppm, which was dose-related. A greater incidence of
hepatocytic vacuolation was seen in males and female at 100 or 200 ppm
and to a lesser extent in females at 25 ppm; the effect was attributed
to fat and the nutritional status of the affected groups. There were
no differences in the incidences of any neoplastic finding that could
be related unequivocally to treatment. There was no NOAEL, as effects
were seen at all doses (Hellwig, 1986a)
Fifty male B6C3F1 mice received a dietary concentration of
250 ppm dimethoate for 69 weeks or 500 ppm for 60 weeks, and 50
females received the two doses for 80 weeks. All animals were then
observed without treatment until about 94 weeks. A control group of 10
males and 10 females was supplemented by similar groups of animals
from concurrent studies on other pesticides; this gave a pooled
control group of 50-60 animals, although the studies were not
precisely concurrent. The body-weight gain of treated mice, except
females at the low dose, was lower than that of controls during the
first 52 weeks of treatment. Occasional generalized tremor was noted
in treated animals at each dose. During the second half of the study,
alopecia, abdominal distension, and tumours were seen in treated
animals but predominantly in those receiving the lower dose. The
condition of animals at termination was said to be poor. Oncogenic
potential was not assessed (US National Cancer Institute, 1976, 1977).
Rats
Groups of 65 male and 65 female individually housed Wistar rats
received dimethoate in the diet at concentrations providing 0, 5, 25,
or 100 ppm for two years. Fifteen animals of each sex in each group
were allocated for clinical pathology; an additional group of 20 males
and 20 females received a dietary concentration of 1 ppm and were used
to establish or confirm a no-effect level. The dietary concentrations
were selected on the basis of the results of a preliminary study and a
study by the US National Cancer Institute (1977) on Osborne-Mendel
rats. Feed was prepared weekly; both the test material and the
formulated diets were analysed regularly and found to be satisfactory.
Food consumption was recorded weekly; body weight was recorded
weekly for 13 weeks and at fortnightly intervals thereafter. Daily
observations and weekly palpations were recorded; the eyes of all
animals of the main groups (50 of each sex) were examined before
treatment and at six-month intervals during the treatment period for
changes to the refracting media. The ocular fundus of 10 males and 10
females in the control and high-dose groups were examined after 620
days of treatment. Blood samples were obtained before treatment and on
six occasions during the study, and a normal range of chemical and
haematological parameters, including cholinesterase activity, was
measured. Brain acetylcholinesterase activity was measured at the end
of the study. Urine samples were collected twice during the study;
although basic, qualitative 'stick' tests were conducted, volume and
specific gravity were not measured. All animals were necropsied, and a
range of organs was weighed and the tissues retained. Tissues from all
animals in the main study and from satellite animals that died were
examined microscopically.
Females at 100 ppm had a slightly higher mortality rate than
controls from week 65. There were no clinical signs that were
considered by the authors to be related to treatment. Animals at doses
> 25 ppm showed a trend to increased food consumption, especially
during the second year of treatment. The body-weight gain of animals
receiving 100 ppm was slightly lower than that of the controls
during the first half of the study. Examination of the eyes in vivo
revealed no treatment-related effect. Reductions in plasma
cholinesterase activity (generally, 50% of control) were seen in the
group at 100 ppm at all examinations; in females at 25 ppm, the
activities in plasma were minimally lower than in the controls after
four weeks. Reductions in erythrocyte acetylcholinesterase activity
were clear in animals at 25 or 100 ppm (60-75 and 20-40% of control
values, respectively); smaller decreases were seen in females at 5 ppm
during the first 12 months and in males of this group after 24 months.
Clear dose-related reductions in brain acetylcholinesterase activity
were seen at termination in animals at 25 or 100 ppm and slightly
lower values in males at 5 ppm. In animals at this low dose, the
reductions in erythrocyte and brain acetylcholinesterase activity were
not consistent between times or sexes; the variations from control
values are thus of questionable biological significance but probably
represent an intermittent effect of treatment. There were no
statistically significant differences in cholinesterase activity in
the animals at 1 ppm.
The authors reported minimal anaemia throughout the study in
males at 100 ppm; however, this effect was also present before
treatment and was absent in females. Minor variations were also noted
in leukocyte count, potassium and total protein concentrations, and
aspartate aminotransferase activity. Although these effects were
reported to extend in part to the animals at 25 ppm, they were minor;
they may be related to treatment but are considered not to be of clear
toxicological significance. There were no treatment-related changes in
the urine. Animals at 100 ppm had slightly larger spleens and slightly
smaller ovaries than the controls, but no gross pathological findings
could be related to treatment. There were no non-neoplastic findings
that were considered to be related to treatment, and there was no
statistically significant difference in the distribution of individual
tumour types. Treated males had a greater number of malignant tumours
than the controls, but there was no relationship to dose. In a number
of tissues, however, a greater incidence of tumours was seen in
treated animals than in controls. These included exocrine and
islet-cell adenomas in the pancreas of males, haemangiosarcoma in
the spleen of males, and mammary gland fibroadenoma and carcinoma
in females. When the combined incidences of haemangioma and
haemangiosarcoma at any site in treated animals were compared with
those in controls, the difference was statistically significant for
all groups of treated males, but there was no relationship to dose and
females were clearly unaffected. The apparent difference may be due to
a lower than expected incidence in the controls. A large proportion of
these tumours were located in the mesenteric lymph node. The incidence
of these vascular tumours was said to be similar to that of historical
controls from a number of sources. The authors concluded that there
was no evidence that dimethoate has oncogenic potential. The NOAEL for
inhibition of brain and erythrocyte acetylcholinesterase activity was
1 ppm, equivalent to 0.05 mg/kg bw per day (Hellwig, 1986b).
The vascular and proliferative lesions from the above study were
evaluated by a second group of pathologists (Squire, 1988), who were
unaware of the previous interpretation of each slide. This review
supported the conclusion of the original pathologist and indicated
that the Wistar rat is susceptible to these tumours. It also suggested
that the chemicals that induce vascular neoplasms are genotoxic;
however, it asserted that dimethoate is not genotoxic and that by
implication these tumours were not related to treatment.
Groups of 50 male and 50 female Wistar rats received technical-
grade dimethoate in the diet at concentrations of 0, 2, 20, or 200 ppm
for two years, equivalent to 0.1, 1 and 10 mg/kg bw per day. The report
was available only as an English translation of a nearly illegible
German summary with tables of individual data. As no other details of
procedures or results and no group means were presented, the study
could not be reviewed satisfactorily (Ministry of Agriculture,
Fisheries and Food, 1993a).
Groups of 30 male and 30 female Wistar rats received diets
designed to provide technical-grade dimethoate at concentrations of 0,
0.1, 1, 10, or 75 ppm for two years, equivalent to 0.005, 0.05, 0.5
and 3.8 mg/kg bw per day. The feed was prepared four times a week.
Body weight and food intake were recorded weekly for the first four
weeks and every four weeks thereafter. Haematological examinations
were performed four times between weeks 32 and 100. Cholinesterase
activity was determined after 1, 3, 12, 50, 75, and 100 weeks on six
rats of each sex. Brain acetylcholinesterase activity was determined
after 52 and 104 weeks. Other, limited biochemical assays were
performed during the study on blood and urine, mostly after two years.
Six animals of each sex were killed after one year and the surviving
animals after two years; all were examined macroscopically and their
organs weighed. Histological examination was performed on six males
and six females at each sacrifice. There was no indication of how dead
animals were handled or examined, although the report refers to their
necropsy.
Early signs of reaction to treatment at 75 ppm (slight
piloerection, exophthalmia, and fine tremor) disappeared during the
fourth week of treatment, and no other clinical signs were attributed
to treatment. A high mortality rate resulted from an infection after
78 weeks, with the deaths of 55 males and 37 females, evenly
distributed among the groups. The infection was treated with
oxytetracycline as three oral doses of 40 mg/kg bw over three weeks.
There was no treatment-related mortality. Body-weight gain was reduced
in animals treated at 75 ppm, up to week 20 for females and throughout
the study for males. Food consumption was unaffected, but conversion
was reduced in animals treated at 75 ppm, in line with body weight.
The achieved mean doses were 0.02, 0.2, 2, and about 20 mg/kg bw per
day. Haematological investigations indicated no effects of treatment.
Cholinesterase activity was clearly reduced in the plasma,
erythrocytes, and brain of animals receiving 10 or 75 ppm. The brain
acetylcholinesterase activity of animals receiving 1 ppm was 20% lower
than that of controls after one year. There were no other
toxicologically significant intergroup differences in the composition
of the plasma or urine. Organ weight analysis after one year indicated
lower liver, spleen, adrenal, and testis weights in males at 75 ppm
than in the controls; after two years, the adrenal and liver weights
of males and females at 75 ppm were lower than those of controls.
Necropsy of animals that died during the study and of animals killed
at the scheduled sacrifices indicated no treatment-related changes.
The distribution of macroscopically observed rumours was unaffected by
treatment. No microscopic changes were found that were attributed to
treatment. The incomplete data presentation and the infection that
occurred in the middle of the study significantly compromise its
validity (Ministry of Agriculture, Fisheries and Food, 1993a).
Groups of 50 male Osborne-Mendel rats were fed diets providing
technical-grade dimethoate at concentrations of 250 or 500 ppm
(equivalent to 25 and 50 mg/kg bw per day) for 19 weeks; the doses
were then reduced to 125 and 250 ppm (equivalent to 6.3 and 13 mg/kg
bw per day),and treatment continued for 61 weeks. Necropsy was
performed after a further 33-35 weeks without treatment. The same
dietary concentrations were fed to similar groups of females, except
that they were reduced only after 43 weeks; the animals were then
treated at concentrations of 125 or 250 ppm for a further 37 weeks.
The total treatment period for all animals was 80 weeks, followed by
up to 35 weeks without treatment. A control group of 10 males and 10
females was supplemented by similar groups of animals from concurrent
studies on other pesticides, giving a pooled control group of 50-60
animals, although the studies were not precisely concurrent. All
animals were observed daily, and body weights were recorded 'at
regular intervals until 110 weeks'. The animals were then necropsied
and, when feasible, tissues were retained for histopathological
examination. Treatment at 500 ppm was associated with lower
body-weight gains in males and females during the first 20 weeks of
treatment. After the reduction to 250 ppm at 19 weeks, the body-weight
gain of males increased but remained lower than in controls until
treatment ceased. The body-weight gain of males at the low dose and
females at the high dose remained lower than that of controls until
week 80. Clinical signs of inhibition of cholinesterase activity were
seen in animals at the high dose, particularly during the first week
of treatment. Conjunctivitis of vital origin was diagnosed in the
animals at week 38. Animals that survived to termination were said to
be 'generally in poor physical condition'. More animals at the high
dose died than matched or pooled controls, although the number of
matched control males that died during the study (7 of 10) was
reported to be unusually large. There was no difference in the
distribution of non-neoplastic or neoplastic changes among the treated
groups that could clearly be ascribed to treatment. The pathological
assessment indicated no oncogenic potential (US National Cancer
Institute, 1976, 1977).
A review by Reuver (1984) covered several carcinogenicity studies
in rats and mice, including that of the US National Cancer Institute.
It was concluded from the studies of Gibel et al. (1973) and
Stieglitz et al. (1974) that dimethoate is highly carcinogenic to
rats, but insufficient details of these experiments were given,
precluding assessment. The review of the US National Cancer Institute
study involved re-reading of the sections; it was again concluded
that dimethoate is carcinogenic. The basis for the review was not
described, and the numbers of animals given in the tables shown in the
review differ from those in the original report. Reuber quoted text
from the report in reverse order to that in which it was originally
published. This review did not satisfactorily explain the methods used
or discuss the discrepancies in the reported incidences and in the
conclusions from those of the original report. In view of these
deficiencies, no weight is placed on this publication.
(d) Reproductive toxicity
Mice
A multigeneration study was undertaken in CF-1 mice fed diets
containing concentrations of 0, 5, 15, or 50 ppm dimethoate,
equivalent to 1.4, 4.3 and 14.5 mg/kg bw per day, throughout the
study. The study was conducted before Good Laboratory Practice came to
be enforced but was designed according to the recommendations of the
Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics of
the Association of Food and Drug Officials of the United States. Each
generation was mated twice, the first set of litters being discarded
and the next generation (F1b, F2b, and F3b) being produced from the
second litters. For each generation, eight males were mated with 16
females in each group, and males were rotated within their group
during the mating period. The observations were limited in comparison
with current practice. After weaning of the F3b generation, the
parents (F2b) were killed and necropsied; liver, kidneys, and gonads
were weighed, but no microscopy was performed. All animals of the F3b
generation were necropsied at death or at weaning, and a wide range of
tissues from one male and one female from each litter was examined
microscopically. Organ weighing was restricted. All fetuses that were
not examined microscopically were retained in 80% alcohol for skeletal
staining in alizarin red.
There were no clinical reactions to treatment. Tremors were seen
in four dams of the F2b generation treated with 50 ppm, three of
which lost their litters, on one occasion after a weekly change of
diets; the diets were replaced, as a formulation error was suspected,
and the condition was not seen again. No treatment-related differences
in group mean body weights were recorded at any mating. Measurement of
the food consumption of the F0 generation before mating indicated
no effect of treatment, but no data were presented and no other
measurements were carried out. The fertility, gestation, viability,
and lactation indices were unaffected by treatment, and there was
no effect on the weights of the pups at weaning. No intergroup
differences in organ weight or pathological findings were seen that
were related to treatment. The NOAEL for reproductive toxicity was
50 ppm (Ribelin et al., 1965).
In five generations of CD-1 mice given 60 ppm of dimethoate in
drinking-water, reproductive performance was significantly altered, as
indicated by reduced mating success and longer gestation. Litter size
and weight were not reduced at birth, but pup mortality was increased
significantly by treatment. The growth rate of the pups was generally
lower than that of controls. Dimethoate did not show teratogenic
potential or adverse effects on organ weights or histological
appearance (Budreau & Singh, 1973).
Rats
In a multigeneration study, dimethoate (purity, 96.4%) was
administered in the diet at fixed concentrations of 0, 1, 15, or
65 ppm (equal to about 0.08, 1.25 and 5 mg/kg bw per day) to groups of
28 male and 28 female Sprague-Dawley rats for 10 weeks before the
first of two matings to produce F1a and F1b animals. The F1
generation, selected from the F1a litters, was first mated at about
16 weeks of age to produce F2 pups, which were killed and examined at
21 days of age. A second mating of the F1a generation was conducted,
followed by a partial third mating involving animals that had not been
successful at either of their first two pairings. Administration of
dimethoate was continued at the same dietary levels throughout
premating, mating, gestation, and lactation. Treatment at 65 ppm was
associated in the parent animals with marked reductions in plasma,
erythrocyte, and brain cholinesterase activities in animals of both
generations, slightly reduced body-weight gain, increased food intake,
and reduced water intake. In the F1a pups at 65 ppm at four days of
age, a reduction in brain acetylcholinesterase activity was seen in
males but not in females. The parental animals at 15 ppm had a
significant reduction in brain and erythrocyte acetylcholinesterase
activity in both generations, but no effect on cholinesterase activity
was seen in the offspring. Mating performance (as assessed by median
precoital time and duration of pregnancy) was not affected by
treatment; however, an effect was seen on the pregnancy rate (Table
2). These data are clearly indicative of substandard performance at
65 ppm and also show a possible effect at 15 ppm on the second mating
of the F1 generation. The Meeting concluded that this information did
not clarify the possible effect at the intermediate dose. Treatment at
65 ppm was also associated with a reduction in litter size at birth,
as shown in Table 3.
Table 2. Pregnancy rates of animals treated with dimethoate with
live pups at birth
Generation Mating Pregnancy rate (%)
Control 1 ppm 15 ppm 65 ppm
F0 First 93 96 86 89
Second 89 93 89 71
(100) (100) (100) (96)
F1 First 96 71 71 63
Second 73 67 58 50
(100) (79) (92) (75)
In parentheses, the percentages of animals with implantations
confirmed at autopsy by Salewski staining of the uteri of
apparently non-pregnant females
Table 3. Litter size at birth of animals treated with dimethoate
Generation Mating Group mean litter size at birth
Control 1 ppm 15 ppm 65 ppm
F0 First 16.4 15.3* 15.3* 14.2**
Second 14.9 14.9 14.2 14.3
F1 First 12.3 11.9 14.6 12.0
Second 14.1 13.3 13.1 10.0*
* p < 0.05
** p < 0.01
There was also a slight increase in pup mortality during
lactation in animals at 65 ppm. Changes in litter weight reflected the
changes in litter size. The mean pup weight at birth was unaffected by
treatment, but pup body-weight gain was adversely affected by the high
dietary level. There was a slight delay in attainment of the startle
reflex in pups born after the first mating of both generations at
65 ppm, but the mean delay was less than one day and was not apparent
across all four matings; it was thus probably not related to
treatment. There was no other effect on pre- or post-weaning
development. Histopathological examination of tissues associated with
the reproductive tract did not reveal any treatment-related changes.
The NOAEL for toxicity was 1 ppm, equivalent to 0.08 mg/kg bw per day,
on the basis of inhibition of cholinesterase activity at 15 ppm
(Brooker et al., 1992). The Meeting discounted the possible adverse
effect on pregnancy rate at 15 ppm, and this was the NOAEL for
reproductive performance, equivalent to 1.2 mg/kg bw per day.
Rabbits
Three groups of three male rabbits received gelatin capsules
containing individually calculated doses of a dimethoate formulation
(composition unspecified) calculated to be one-tenth and one-hundredth
of the LD50, which was not specified, at an unspecified frequency. A
six-week preliminary period was followed by six weeks of treatment and
then by six weeks of respite. Body weights were recorded weekly, and
semen was collected twice weekly from all animals throughout the
experimental period. Ejaculate volume was recorded after removal of
the gel mass. Seminal initial fructose was determined immediately
after collection, and methylene blue reduction time was recorded. The
numbers of live, dead, and abnormal sperm were assessed, and sperm
concentration was determined with a haemocytometer. Data were
presented in graphs rather than as individual values; there was no
indication of variation between individual animals. Clinical signs
were not reported. Body-weight gain was reduced in treated animals,
and there were indications of reduced libido. The ejaculate volume and
sperm concentration of treated animals, expressed as a percentage of
that of controls, decreased during the treatment period, and the
effect on sperm concentration persisted into the recovery period.
Treatment increased the numbers of abnormal sperm in a dose-related
manner; these were greatest at the end of the treatment period but
declined thereafter. A significant increase in the methylene blue
reduction time and a decrease in the initial fructose concentration
were also seen. There was some evidence of recovery from these effects
during the latter part of the recovery period (Salem et al., 1988).
In view of the deficiencies, it was difficult to assess the
significance of the changes seen, but they should be explained in view
of the reported reproductive effects of dimethoate.
(e) Developmental toxicity
Mice
Intraperitoneal administration of dimethoate at 40 mg/kg bw as a
single dose to mice on the day of mating or on day 9 of gestation or
for the first 14 days of gestation caused a high incidence of
embryonal loss (Scheufler, 1975).
Dimethoate administered orally at doses of 10 or 20 mg/kg bw was
not teratogenic to CD-1 mice, and these levels were not lethal to the
dams. Doses of 40 and 80 mg/kg bw induced maternal toxicity (Courtney
et al., 1985).
Rats
Groups of pregnant rats were given 0, 3, 6, 12, or 24 mg/kg bw of
a dimethoate formulation by gavage daily on days 6-15 of gestation.
The dams were killed on day 22 of gestation; the uterine content was
removed, the carcass weighed, the number of corpora lutea was
determined, and the animals were necropsied. The fetuses were weighed
and examined for viability and external malformations; live fetuses
were studied for skeletal and visceral anomalies. Maternal weight was
decreased significantly in the group receiving 24 mg/kg bw, and clonic
spasms and muscular tremors were seen. The mean fetal weight was not
affected by treatment. Treatment with 12 or 24 mg/kg bw was associated
with an increased number of litters with abnormal fetuses and fetuses
with wavy ribs. The NOAEL was 6 mg/kg bw of formulated product, equal
to 2.84 mg/kg bw dimethoate (Khera et al., 1979).
Three groups of 25 time-mated CrL:COBS CD (SD) BR rats received
technical-grade dimethoate (purity, 97.3%) in 1% aqueous methyl
cellulose (10 ml/kg) at doses of 3, 6, or 18 mg/kg bw per day by
gavage daily on days 6-15 of gestation; a control group received the
vehicle alone. Clinical observations were made at regular intervals,
and food consumption and body weight were recorded. All animals were
killed on day 20 of gestation and the uterine contents examined for a
normal range of parameters. One-half of the pups were preserved in
Bouin's solution for free-hand sectioning and the remainder in
industrial methylated spirits for subsequent alizarin staining and
skeletal examination. The doses were chosen on the basis of the
results of a preliminary study, which was not presented or discussed.
The signs seen in animals at 18 mg/kg bw per day included salivation,
hypersensitivity, ataxia, tremor, fur staining, and small, rounded
faecal pellets. The signs at 3 mg/kg bw per day were confined to
salivation; in animals at 6 mg/kg bw per day, this was accompanied by
a low incidence of small, rounded faecal pellets. Food consumption and
body-weight gain were lower in animals at 18 mg/ kg bw per day than in
the controls during treatment; similar effects were not seen at the
two lower doses. Neither litter parameters nor fetal development (as
indicated by the incidences of visceral or skeletal abnormalities) was
affected by treatment. The NOAEL for toxic signs depends on the
interpretation of the significance of the salivation seen in the
animals at 3 and 6 mg/kg bw per day and on the abnormal faecal pellets
in the latter group. Although salivation is an expected effect of
organophosphate pesticides, there was no clear evidence that the
effect was unduly prolonged (It was described as occurring
'immediately' after treatment.) and may have been incidental. The
presence of abnormal faecal pellets may be related to the action of
this class of compound on the gastrointestinal tract and is unlikely
to be of toxicological significance (Edwards et al., 1984a).The
Meeting concluded that the NOAEL was 6 mg/kg bw per day.
Rabbits
Groups of 16 female New Zealand white rabbits (obtained from
several breeders) were mated with males of proven fertility and
received technical-grade dimethoate (purity, 97.3%) in 1% aqueous
methylcellulose (5 ml/kg) at doses of 10, 20, or 40 mg/kg bw per day
by gavage daily on days 7-19 of gestation; a control group received
the vehicle alone. The doses were chosen from a preliminary study, the
results of which were not summarized. After coitus, each animal
received an injection of luteinizing hormone. Clinical observations
were made at regular intervals, and food consumption and body weight
were recorded. All animals were killed on day 29 of gestation, and the
uterine contents were inspected; a range of parameters for this type
of study was assessed. After examination in vivo, the pups were
killed and dissected. The skinned, eviscerated pups were fixed in
industrial methylated spirits, and the brain was examined for
abnormalities by longitudinal sectioning; carcasses were cleared and
stained by a modified Dawson's technique for skeletal examination.
There were no clinical signs of reaction to treatment with doses of 10
or 20 mg/kg bw per day; at 40 mg/kg bw per day, muscle tremors and
ataxia were seen during the latter part of the treatment period. Food
consumption was reduced between days 15 and 23 of gestation in animals
treated at 40 mg/kg bw per day, and the body-weight gain of these
rabbits was lower than that of controls throughout the treatment and
particularly between days 15 and 20 of gestation. A slight reduction
in body-weight gain was seen in animals at 20 mg/kg bw per day. An
initial reduction in weight gain was seen at the beginning of the
treatment period in animals at the low dose, but these animals
subsequently gained more weight than the controls. Treatment had no
effect on fetal development; litter size and weight were unaffected,
and pups had no abnormalities that could be ascribed to treatment.
Although there was a transient reduction in body-weight gain at the
start of treatment in animals at 10 mg/kg bw per day, the deficit was
quickly corrected. This dose was therefore the NOAEL (Edwards et al.,
1984b).
Cats
Four groups of 17 cats were mated and treated with Cygon-4E, a
commercial insecticide containing 47.3% dimethoate, as single daily
doses of 0, 3, 6, or 12 mg/kg bw on days 14-22 of gestation. The cats
were necropsied on day 43 of gestation, and the fetuses were removed,
weighed, and examined for external malformations. The total number of
anomalous fetuses in cats at 12 mg/kg bw per day was not significantly
higher than that in controls. The only treatment-related malformation
was observed at this dose and consisted of forepaw polydactyly in
eight of 39 fetuses. A dose-response relationship was not established
owing to the limited response and the common occurrence of this
anomaly in cats. The NOAEL was 6 mg/kg bw per day of Cygon-4E, equal
to 2.8 mg/kg bw per day of dimethoate (Khera, 1979).
(f) Genotoxicity
The regulatory reports evaluated by the Meeting concluded that
dimethoate does not induce reverse mutation or gene mutation in
vitro, nor did it induce micronucleus formation, dominant lethal
mutation, or chromosomal aberration in mice in vivo. Dimethoate
induced unscheduled DNA synthesis in vitro in two assays using
different methods of assessing the uptake of tritiated thymidine into
DNA but not in an assay in vivo/in vitro. A review of the literature
on the mutagenic potential of dimethoate revealed a number of positive
results, notably for reverse mutation in Salmonella typhimurium
TA100 and for sister chromatid exchange in mammalian cells in vitro.
It was concluded that although dimethoate has mutagenic potential in
vitro, mutagenicity does not appear to be expressed in vivo. The
results of assays for genotoxicity with dimethoate are summarized in
Table 4.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The dermal irritation potential of a 400-g/litre emulsifiable
concentrate formulation of dimethoate was investigated in rabbits.
Only slight erythema was observed 4 h after application, and the
effect had resolved by 24 h after treatment (Ministry of Agriculture,
Fisheries and Food, 1993a).
The ocular irritation potential of the same formulation of
dimethoate was also investigated in rabbits. Redness and swelling of
the conjunctiva were observed, with slight corneal opacity, 1-72 h
after application. All of the effects had resolved by eight days after
treatment (Ministry of Agriculture, Fisheries and Food, 1993a).
Studies to investigate the ocular irritation potential in
rabbits of 40 and 38.3% dimethoate formulations concluded that the
formulations were irritating to the rabbit eye. The 'in use' dilution
of the 40% formulation (0.84%) was not irritating (Ministry of
Agriculture, Fisheries and Food, 1993a).
Technical-grade dimethoate (purity, 97.3%) did not sensitize the
skin of guinea-pigs when tested by the Buehler method (Madison et al.,
1984).
Table 4. Results of tests for the genotoxicity of dimethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
5-Methyltryptophan E. coli 1.6 × 10-3 mol/litre NR Positivea Mohn (1973)
resistance mutation
Reverse mutation S. typhimurium < 5000 mg/plate NR Positive Moriya et al. (1983)
TA98, TA100, in TA100a
TA1535, TA1537,
TA1538
E. coli WP2hcr < 5000 mg/plate Positivea
Reverse mutation S. typhimurium < 5000 mg/plate NR Negativea Probst et al. (1981)
TA98, TA100,
TA1535, TA1537,
TA1538
E. coli WP2uvrA- 47 nmol/ml Initially
positive,
negative
quantitativelya
Reverse mutation S. typhimurium 2-200 mg/plate NR Positivea Vishwanath & Jamil
TA100 (1986)
Mitotic gene conversion S. cerevisiae 7 doses, 40-100 mmol NR Positive Fahrig (1974)
Mitotic gene conversion S. pombe (ade 6) 1.3-131 mmol NR Negative Gilot-Delhalle et al.
(1983)
Gene mutation Chinese hamster 1000-3500 mg/ml 97.3 Negative Johnson et al. (1985)
ovary (hprt)
Sister chromatid exchange Cultured human < 120 ppm NR Positive Gomez-Arroyo et al.
lymphocytes (1987)
Sister chromatid exchange Chinese hamster 10, 20, 40, 80 mg/ml 94 Positive Chen et al. (1981)
and cell cycle delay ovary V79 cells + 10 pg/ml BUdR
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Cytotoxicity Chang liver and 50-500 mg/ml 99.8 Positive Gabliks &
HeLa cells Friedman (1965)
Cytotoxicity HeLa cells 2-300 mg/ml 99.8 Positive Gabliks (1965a)
Susceptibility to HeLa cells 2-300 mg/ml for 99.8 Positive Gabliks (1965b)
poliovirus infection < 108 days
Unscheduled DNA SV-40 transformed 100, 1000 mmol NR Positive Ahmed et al. (1977)
synthesis human fibroblast
cell line VA-4
Unscheduled DNA Rat hepatocytes 47 nmol/ml NR Negative Probst et al. (1981)
synthesis
Unscheduled DNA Rat hepatocytes < 2290 mg/ml NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
Unscheduled DNA Rat hepatocytes NR NR Positive Ministry of Agriculture,
synthesis Fisheries and Food
(1993a)
In vivo
Micronucleus formation Mouse 2 equal oral doses NR Positive Rani et al. (1980)
bone marrow of 51.7 mg/kg bw at
24-h interval
Host-mediated Mouse; 3 equal oral doses NR Positive Rani et al. (1980)
mutagenicity S. typhimurium of 155 mg/kg bw
Dominant lethal CFLP mice 30, 60 mg/kg bw ip NR Negative Fisher & Scheufler
mutation AB Jena mice 5 × 6 mg/kg bw ip Negative (1981)
DBA mice 3 × 18 mg/kg bw ip Negative
Dominant lethal NMRI mice 5, 10, 20 mg/kg 96.9 Negative Becker (1985)
mutation orally, 5 days
Table 4. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Dominant lethal Strain Q mice 10 mg/kg ip + 0.6 NR Negative Degraeve & Moutschen
mutation mg/l drinking-water (1983)
Chromosomal CFLP mice 20-60 mg/kg bw ip NR Positive (gaps Nehéz (1983)
aberration and numerical
changes)
Chromosomal aberration Rats 15, 75, 150 mg/kg bw ip NR Negative Ministry of Agriculture,
Fisheries and Food
(1993a)
Chromosomal aberration Mice 50, 100 mg/kg bw NR Positive Bhunya & Behera
(1975)
Chromosomal aberration Hamster 16-160 mg/kg bw ip NR Weakly Dzwonkowska & Hubner
positive (1986)
Sex-linked recessive Drosophila 1 mg/kg feeding NR Negative Woodruff et al. (1983)
lethal mutation
Sex-linked recessive Drosophila 10 or 20 ppm; adult NR Positive at Velásquez et al. (1986)
lethal mutation feeding low dose
0 or 10 ppm larval Negative
feeding
Sex-linked recessive Drosophila LD50 and half LD50, NR Positive Tripathy (1988)
lethal mutation larval feeding
Unscheduled DNA Rats 50, 100, 200 mg/kg NR Negative Ministry of Agriculture,
synthesis bw orally Fisheries and Food
(1993a)
Micronucleus formation CD-1 mice 55 mg/kg bw ip once 97.3 Negative Sorg (1985)
or twice 24 h apart
Metaphase alteration Rats 15, 75, 150 mg/kg 97.3 Negative San Sebastian (1985)
bw ip
ip intraperitoneally
a With and without metabolic activation
Nineteen cases of allergic, occupational contact eczema and one
of contact dermatitis have been reported (von Jung, 1989). Exposure
to dimethoate was cited in four cases: in two male and one female
gardener and in one female agrochemical technician, 22-69 years of
age. The results of patch tests with dimethoate in these individuals
were positive.
(ii) Neurotoxicity
In a preliminary study, the LD50 of dimethoate in hens was
determined in groups of 10 birds given single oral doses of 0, 30, 45,
68, 100, or 150 mg/kg bw in water. These doses were selected on the
basis of the results of a preliminary study in groups of two birds at
doses between 12.5 and 200 mg/kg bw; both birds at 100 or 200 mg/kg bw
died. In each of these studies, dosing was followed by a 14-day
observation period. Body weights were recorded weekly. Surviving birds
were killed but not necropsied. Neurotoxicity was assessed after a
single subcutaneous dose of 55 mg/kg bw to 16 birds or 55 mg/kg bw
orally to 30 birds; a control group of 14 birds was dosed orally, and
a positive control group of six birds received 500 mg/kg bw of
tri- ortho-cresyl phosphate (TOCP) as a single oral dose in corn oil.
All birds were starved overnight before treatment. In an experiment
conducted before the main study to evaluate the protective
effectiveness of atropine, it was shown to have no protective value at
twice the LD50 value. Birds dosed subcutaneously with dimethoate were
included for comparative biochemical assays. Three birds in the
treated and negative control groups were used to determine
cholinesterase and neuropathy target esterase activity 4 and 48 h
after dosing; these assays were performed for three TOCP-dosed birds
only 48 h after dosing. Analyses of the formulation used indicated
satisfactory content and stability over 24 h. Dosing was followed by
an observation period of 21 days. Birds were assessed daily for ataxia
by observing their ability to walk and to jump onto and off an
obstacle. Body weights were recorded weekly. Nervous tissue from three
TOCP-dosed birds, six negative controls, and six birds dosed orally
with dimethoate was examined histologically.
In the study to determine the LD50, toxicity was seen in a
dose-related manner. Deaths occurred at doses > 45 mg/kg bw, and
all birds at doses > 100 mg/kg died. Deaths occurred up to three
days after dosing, and the surviving birds were normal by day 5. The
LD50 for dimethoate was calculated to be 55 mg/kg bw, with a 95%
confidence interval of 45-67 mg/kg bw. The body weights of survivors
were decreased during the week after treatment but subsequently
increased. The three birds dosed with TOCP that were not sacrificed
for enzyme assays at 48 h developed signs of delayed neurotoxicity
after 13 days, although no clinical signs of cholinesterase inhibition
were seen. All of the birds treated subcutaneously with dimethoate
died within 48 h, after showing clinical signs of cholinesterase
inhibition. After oral administration of 55 mg/kg bw dimethoate, all
birds showed clinical evidence of cholinesterase inhibition. Twelve of
these birds that survived to termination had recovered by day 6 of the
observation period, and none showed signs of delayed neurotoxicity; 12
birds in this group died within 48 h of dosing. Body-weight losses
of up to 10% were seen in treated birds during the first week of
observation, but these losses were subsequently recovered. Microscopic
examination indicated no difference between controls and birds treated
with dimethoate. Brain acetylcholinesterase activity was markedly
reduced in both groups treated with dimethoate; this was more marked
(90% inhibition relative to controls) 4 h after dosing than after 48 h
(61 and 75% inhibition after subcutaneous and oral administration,
respectively). Brain neuropathy target esterase activity was slightly
lower than that in controls in birds treated with dimethoate, but was
markedly lower in TOCP-treated birds. Spinal cord neuropathy target
esterase activity was reduced in TOCP-treated birds but was unaffected
in those that received dimethoate (Redgrave et al., 1991).
Groups of nine or 10 white Leghorn hybrid chickens were given
graded doses of technical-grade dimethoate up to 33 mg/kg bw for three
days, based on the oral LD50 in Japanese quail, in a study designed
to accord with the guidelines then current in eastern Germany and
Poland. Negative and positive controls (TOCP) were used. Antidotes
(atropine sulfate and obidoxime chloride) were administered to treated
birds but not to controls. The route of administration was not stated
but is assumed to have been oral. The precise study design was not
clear from the translation of the original document but indicated
administration of a further series of three doses at intervals
determined by the condition of the birds. Deaths occurred despite use
of the antidotes. Signs of reaction indicative of cholinesterase
inhibition were seen from about 60 min after dosing. Although the
tests indicated that dimethoate has high acute toxicity, there was no
evidence of delayed neurotoxicity, except in the positive controls
(Ministry of Agriculture, Fisheries and Food, 1993a).
(iii) Immunotoxicity
A single dose of dimethoate at 75 mg/kg (route unspecified) to
mice and rats decreased the lymphocyte count to 50% of the value
before exposure and increased the number of neutrophil granulocytes.
After 72 h, these parameters had returned to normal. A reduction in
the thymus cortex, with disrupted lymphocytes, and a reduction in the
number of rosette-forming cells were observed (Tiefenbach & Lange,
1980).
Dimethoate administered to rats at 5-30 mg/kg bw orally or
15 mg/kg bw intramuscularly, twice a week until death, caused
hyperplasia in the bone marrow, resulting mainly in granulocyto-
poiesis. The authors considered the changes to be a direct effect of
dimethoate (Stieglitz et al., 1974).
(iv) Effects on the heart
The effects of dimethoate on the heart have been investigated
in rabbits (Mahkambaeva, 1971), guinea-pigs, and rats (Nadmaiteni &
Marosi, 1983). After oral administration of 150 mg/kg bw to rabbits,
the effects observed included bradycardia and increased atrio-
ventricular and intraventricular conductance, with complete recovery
after four to seven days. In rats and guinea-pigs, a dose-effect
relationship was established for heart rate disturbances and
atrio-ventricular block. An electron microscopic study of the
myocardium showed no changes.
In anaesthetized guinea-pigs treated with lethal doses of
dimethoate, cardiac failure and serious electrocardiographic
disturbances developed during the early phase of intoxication. The
toxic cardiac phenomena appeared to be unrelated to the degree of
cholinesterase inhibition but were correlated with the myocardial
concentration of dimethoate. Cardiac failure and death were first
observed at a dose of about 110 mg/kg bw, while a dose of 221 mg/kg bw
resulted in death in all cases. This investigation addressed the
direct effect of dimethoate on the myocardium, independently of its
anticholinesterase action (Marosi et al., 1985a,b).
(v) Studies on metabolites
Omethoate is the oxygen analogue of dimethoate. Information on
the absorption, distribution, excretion, metabolism, and toxicity of
this compound is summarized below, although the original reports were
not available for detailed evaluation.
Absorption, distribution, and excretion of omethoate: After oral
administration of 14C-omethoate at doses of 0.3, 5, or 10 mg/kg bw
to rats, 96-97% of the radiolabel was eliminated in urine, 1-2% in
faeces, and 1% in expired carbon dioxide within 48 h. Intravenous
injection of 0.3 mg/kg bw resulted in a similar, rapid elimination
pattern. Maximal tissue residues were reached 1 h after
administration. After 8 h, about 18% of the residual radiolabel was
found in the body. After two days, < 0.55% of the administered dose
was found. Quantitative analysis and whole-body autoradiography
indicated a relatively homogeneous distribution of 14C activity,
except that a 10-20-fold higher concentration was found in the thyroid
(Weber et al., 1978).
Five male Wistar rats were given 10 mg/kg bw 14C-omethoate
orally in order to obtain preliminary information on pulmonary
excretion. In the main study, groups of five males and five females
received a single intravenous dose of 0.5 mg/kg bw 14C-omethoate, a
single oral dose of 0.5 mg/kg bw 14C-omethoate, 14 daily oral doses
of 0.5 mg/kg bw unlabelled omethoate and a single oral dose of
0.5 mg/kg bw 14C-omethoate on day 15, or a single oral dose of
10 mg/kg bw 14C-omethoate. Urine and faeces were collected over
periods up to 48 h after treatment, and blood samples were collected
until sacrifice 48 h after treatment. Only 0.14% of the administered
radiolabel was detected in expired air. Comparison of the results
obtained with intravenous and oral treatment indicated that > 98%
omethoate had been absorbed from the gastrointestinal tract. Within
48 h, 88-98% of the administered radiolabel was recovered in the
excreta, with 95-98% in the urine and 2-5% in the faeces. Excretion of
the low and the high oral doses was not different in females, but
males at the high dose group tended to excrete more radiolabel in the
faeces than those at the low dose. The maximal plasma concentration
was seen 40-60 min after oral dosing, with an initial half-life of
about 2 h and terminal half-lives of 13-28 h. Less than 0.05% of the
administered dose was found in tissues after 48 h (Ministry of
Agriculture, Fisheries and Food, 1993b).
Biotransformation of omethoate: Urine was collected from two
male rats 12, 24, and 48 h after an oral dose of 50 mg/kg bw
radiolabelled omethoate. The cumulative percentages of administered
radiolabel excreted over the indicated times were 16, 19, and 30%. The
metabolites found in a 24-h composite urine sample by ion-exchange
chromatography were: O,O-dimethylphosphoric acid (34%), unknown A
(52%), O,O-dimethylphosphorothioic acid (9.5%), and unknown B
(4.5%). After treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine within 24 h, while after
treatment with omethoate only 19% was excreted (Dauterman et al.,
1959).
In the experiment conducted by the Ministry of Agriculture,
Fisheries and Food (1993b), the predominant form of excreted
radiolabel was unchanged parent compound (26-62%), with N-methyl-
2-(methylsulfinyl)acetamide accounting for 16-36% and an O-demethylated
omethoate for 4-9%. Pretreatment of animals for 14 days with unlabelled
omethoate followed by a single labelled dose resulted in no significant
difference from the results obtained after a single administration.
Effects of omethoate on enzymes and other biochemical parameters:
Dealkylation of omethoate was proposed to be a significant detoxification
mechanism on the basis of information from assays in fly heads (Aharoni &
O'Brien, 1968). Oxidative metabolism of omethoate results in the
de- N-methyl derivative, which is as toxic as the parent compound
although less active as a cholinesterase inhibitor (Lucier &
Menzer, 1970). Kinetic studies indicated that the reaction between
acetylcholinesterase and omethoate was irreversible and bimolecular.
Omethoate was 75-100 times more potent than dimethoate in inhibiting
rat brain acetylcholinesterase activity.
Acute toxicity of omethoate: The signs of poisoning after a
single dose of omethoate are typical of cholinergic stimulation, as
elicited by other organophosphorus esters. The signs appear 5-60 min
after dosing and include salivation, lacrimation, and tremors. They
may persist for one to three days (Kimmerle, 1968). The LD50 values
are summarized in Table 5.
Short-term toxicity of omethoate: Groups of 50 male and 50
female BOR:NMRI mice were fed diets containing omethoate (purity,
97.1%) providing doses of 0, 1, 3, or 10 ppm for four weeks and were
killed at intervals to investigate brain acetylcholinesterase
activity. There were no clinical signs of reaction to treatment, and
food and water intake, mortality, and body-weight gain were also
unaffected. The cholinesterase activity in plasma was clearly lower
than that in controls in mice receiving 10 ppm, but the differences
were smaller and much less consistent in erythrocytes. Plasma and
erythrocyte cholinesterase activity in mice at 1 and 3 ppm showed no
consistent differences from controls. Brain acetylcholinesterase
activity was clearly depressed in animals at 10 ppm. Inhibition of
brain acetylcholinesterase activity at 3 ppm was inconsistent, but the
level was up to 30% lower than that in contemporary controls. In
animals at 1 ppm, brain acetylcholinesterase activity was biologically
significantly lower than in controls only on day 3 in males (Ministry
of Agriculture, Fisheries and Food, 1993).
Groups of 15 male and 15 female rats (30 of each sex as controls)
were fed diets containing omethoate providing doses of 0, 2.5, 5, 15,
50, or 150 ppm for four months. Signs of cholinergic stimulation was
seen at doses > 15 ppm. Cholinesterase activity was depressed in
females at 50 and 150 ppm and in males at doses > 5 ppm. No effects
were noted on growth, organ weights, blood parameters, or urinary
parameters at levels < 50 ppm. Animals at 150 ppm died or had
depressed body weights and food consumption, and the relative liver
weight in males was increased (Löser & Lorke, 1967).
Groups of 15 male and 15 female rats were fed diets containing
omethoate to provide doses of 0, 0.5, 1.0, 2.0, or 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident in
animals at 4 ppm. Erythrocyte acetylcholinesterase activity was
depressed in animals at 2 ppm, but the effect was only slight in
females. In rats at 4 ppm, the inhibition was 30-50%. No effects were
noted on growth, food consumption, blood parameters, liver and kidney
function tests, organ weights, or histological appearance of tissues
(Löser, 1968).
Table 5. Acute toxicity of omethoate in experimental animals
Species Sex Route LD50 Reference
(mg/kg bw)
Mouse Male Oral 36 Kimmerle (1968)
Mouse Male Oral 27 Santi & de Pietri Tonelli (1960)
Mouse Male Intraperitoneal 13 Lucier & Menzer (1970)
Mouse Male Intravenous 23 Kimmerle (1962)
Rat Male, female Oral 28-65 Kimmerle & Lorke (1967)
Rat Male, female Oral 50 Ben-Dyke et al. (1970)
Rat Male, female Oral 22-28 Ministry of Agriculture,
Fisheries and Food (1993b)
Rat Male Intraperitoneal 14 Kimmerle (1968)
Rat Male Intraperitoneal 38 Kimmerle (1962)
Rat Male, female Dermal 145-232 Ministry of Agriculture,
Fisheries and Food (1993b)
Rabbit Male Oral 50 Kimmerle (1962)
Guinea-pig Male Oral 100 Kimmerle (1962)
Cat Male Oral 50 Kimmerle (1962)
Chicken Male Oral 125 Kimmerle (1962)
Chicken Male Oral 100 Levinskas & Shaffer (1965)
Groups of six male and six female beagle dogs received omethoate
(purity, 97.1%; dissolved in acidulated water) daily for 12 months
by stomach tube at doses of 0, 0.02, 0.125, or 0.625 mg/kg bw.
Administration by gavage was chosen due to the reported instability of
the test material in dietary admixture. The appearance and behaviour
of the animals were normal, and no clinical signs attributable to
treatment were observed. All of the animals survived the treatment.
No significant differences were seen between the control and treated
groups with respect to reflexes, ophthalmoscopic parameters, body
temperatures, pulse rate, food and water consumption, mean
body weight, or haematological, clinical chemical (except for
cholinesterase activity), or urinary parameters. Clear depression
of plasma cholinesterase activity was observed only in rats at
0.625 mg/kg bw, amounting to 25-32% of the control value in males
and 16-29% in females. The depression remained essentially
constant throughout the study. A marked depression of erythrocyte
acetylcholestinerase activity was measured in males (17-40%) and
females (22-40%) at 0.625 mg/kg bw, which varied only slightly during
the study. At 0.125 mg/kg bw, only males showed slight (< 28%)
depression of erythrocyte acetylcholinesterase activity during the
first third of the study. Brain acetylcholinesterase activity was
depressed in males at 0.125 mg/kg bw (by 20%) and 0.625 mg/kg bw (39%)
and in females at 0.625 mg/kg bw (30%). The absolute and relative
organ weights were not significantly different between the control and
treated groups. Gross pathological and histopathological examination
showed no dose-related findings. The NOAEL was 0.62 mg/kg bw for
somatic effects and 0.02 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity (Hoffmann & Schilde, 1984).
Long-term toxicity and carcinogenicity of omethoate: Groups of
50 male and 50 female BOR:CWF1 mice were fed diets containing
omethoate (purity, 94%) providing levels of 0, 1, 3, or 10 ppm for 24
months. Appearance, behaviour, and activity were not significantly
different between the control and treated groups, and total and mean
daily food consumption were essentially the same in all the animals.
The body weights of treated males were generally higher than those of
the controls throughout the experiment, whereas those of the females
were no different from controls. Mortality and the frequency
distribution of mortality were comparable in all the groups. The
mortality rate at 18 months was 12-27% for males and 14-31% for
females. The absolute and relative organ weights of control and
treated groups showed no dose-related, significant differences. Gross
anatomical and histopathological examination revealed a range of
non-neoplastic changes commonly observed in old mice. Comparison of
these changes by type, site, and frequency distribution by sex and
dose gave no indication of treatment-related toxic effects. Neoplastic
changes were found primarily in the lungs, liver, adrenal cortex,
and haematopoietic system. Neither the type, site, or frequency
distribution of tumours by sex and dose level nor the numbers of
tumour-bearing mice, mice with benign tumours, mice with malignant
tumours, or mice with both benign and malignant tumours indicated
effects of treatment. The NOAEL for somatic effects was 10 ppm, equal
to 2.1 mg/kg bw per day for male mice and 3.1 mg/kg bw per day for
female mice (Kroetlinger & Löser, 1982).
Four groups of 50 male and 50 female Wistar rats were maintained
for 24 months on a diet containing omethoate providing concentrations
of 0, 0.3, 1,3, or 10 ppm. The control group consisted of 100 males
and 100 females. Omethoate did not clearly affect behaviour, body
weight, survival, food intake, or haematological, clinical chemical,
or urinary parameters. Plasma and erythrocyte cholinesterase
activities, measured in five males and five females from each group
at 1, 2, 4, 8, 13, 26, 52, and 78 weeks and at the end of the study,
were significantly depressed in all animals at 10 ppm. Erythrocyte
acetylcholinesterase activity was also inhibited in animals of each
sex at 3 ppm. The suppression of brain acetylcholinesterase activity,
measured in 10 males and 10 females per group, was dose-related in
animals at 3 and 10 ppm; it was also significantly affected in females
at 1 ppm, which can be considered the marginal no-effect level. Gross
and microscopic examination revealed no diverse effect of omethoate.
The tumour incidence was not clearly affected by treatment (Bomhard
et al., 1979).
Reproductive toxicity of omethoate: Groups of 10 male and 20
female FB 30 Long-Evans rats were fed diets containing omethoate
(purity, 94%) to give concentrations of 0, 1, 3, or 10 ppm for about
10 weeks, after which they were mated to initiate a three-generation
study of reproductive toxicity with two litters per generation.
Immediately after birth, the litters were examined for malformations.
Four days after birth, the litters were reduced to 10. When the
offspring were three weeks old, they were killed and subjected to
gross examination. Ten males and 10 female rats of the F3b generation
at all doses were examined histopathologically four weeks after birth.
There were no clear effects either on mating performance, pregnancy
rate, mortality, or the type and distribution of abnormalities. The
size of the litters of the second generation at 3 and 10 ppm was
reduced, and in the F2b generation, litter size was reduced at both 3
and 10 ppm after four days and at 10 ppm only after 28 days. Since
this effect was observed in only one progeny generation, 3 ppm was the
NOAEL (Löser, 1981).
In a two-generation study, omethoate was administered to groups
of 25 male and 25 female Wistar rats in the drinking-water at levels
of 0, 0.5, 3, or 18 ppm throughout a 70-day premating period and
throughout pairing, gestation, and lactation during breeding of a
single litter in each of the F1 and F2 generations. Reproductive
performance was adversely affected at 18 ppm, with a reduced
implantation rate, increased postnatal loss, and retarded pup weight
gain in both generations and increased precoital time, an increased
number of non-pregnant females, and increased postimplantation loss in
the F1 generation. Histopathological examination revealed an
increased incidence of epithelial vacuolation in the epididymides of
males treated with 18 ppm. The NOAEL for reproductive effects was
3 ppm, equivalent to 0.2 mg/kg bw per day. There was no NOAEL
for general toxicological effects, since erythrocyte and brain
acetyl-cholinesterase activities were inhibited at the lowest dose
(Ministry of Agriculture, Fisheries and Food, 1993).
Developmental toxicity of omethoate: Groups of 20-24 pregnant
rats were given omethoate orally at doses of 0, 0.3, 1, or 3 mg/kg bw
on days 6-15 of gestation. The animals were killed on day 20 of
gestation, and the fetuses were examined for skeletal and tissue
abnormalities. The fetuses and placentas of the animals at 3 mg/kg bw
weighed less than those of the controls. Other reproductive parameters
were unaffected. No teratogenic effect was observed (Machemer, 1975).
Groups of 14 pregnant New Zealand white rabbits were treated
daily by gavage with omethoate (purity, 96.8%) dissolved in distilled
water at doses of 0, 0.1, 0.3, or 1 mg/kg bw on days 6-18 of
gestation. On day 29 of gestation, the animals were killed and their
uterine contents examined. Whole-blood cholinesterase activity was
determined before treatment on day 6 of gestation and 2 h after
treatment on day 18 of gestation. The general condition of control and
treated females was comparable throughout the study. Maternal mean
body weights and corrected day-29 body weights were unaffected by the
treatment. Mortality, the incidence of abortions and total litter
losses, and the number of pregnant females with viable young on day 29
were not altered by treatment. Whole-blood cholinesterase activity was
significantly depressed only among females at 1 mg/kg bw in comparison
with both the pretreatment level and the control level after
treatment. There were no treatment-related differences between the
control and treated groups with respect to corpora lutea count,
implantations, male and female viable young, early and late
resorptions, pre- and postimplantation losses, or fetal and placental
weights. Examination of fetuses at necroscopy on day 29 of gestation
or after skeletal investigation revealed a number of non-dose-related
findings of types and incidences previously recorded in this strain of
rabbit and in the laboratory that performed the study. The NOAEL for
developmental toxicity was 1 mg/kg bw (Tesh et al., 1982).
Genotoxicity of omethoate: Omethoate has been extensively
tested in assays for mutagenicity in vitro. Positive results were
obtained in Salmonella, in one assay for gene mutation in mammalian
cells, and in assays for clastogenicity. Omethoate has also been
extensively tested in vivo. Negative results were obtained for
end-points in the bone marrow, liver, and germ cells, but a positive
result was obtained in a mouse spot test. The results of assays for
the genotoxicity of omethoate are summarized in Table 6.
Neurotoxicity of omethoate: Groups of 10 hens were given
omethoate orally at the LD50 (92 mg/kg bw) with atropine, and and
five positive controls were given TOCP at 350 mg/kg bw. Although
several hens treated with omethoate died, none showed clinical signs
of delayed neurotoxicity. Clinical signs were observed in those
treated with TOCP (Kimmerle, 1972). Histological examination of
nervous tissue with haematoxylin and eosin staining showed
degeneration in the hens treated with TOCP but not in those given
omethoate (Newman et al., 1972).
Groups of two to four hens were treated orally with omethoate
dissolved in corn oil at doses of 20-300 mg/kg bw, which were four
to eight times the unprotected LD50, under eserine and atropine
protection. The omethoate used was a sample that had caused a fatal
human poisoning accident. The acetylcholinesterase and neurotoxic
esterase activities of brain homogenates were assayed for 24 h after
dosing, and pair-dosed birds that survived were observed for signs of
ataxia for three to four weeks. Hens treated at four times the LD50
showed no inhibition of neurotoxic esterase at 24 h and no signs of
ataxia. Those treated at eight times the LD50 did not survive,
despite treatment with high doses of atropine; however, the neurotoxic
esterase activity in the brains of the animals that died within 36 h,
measured immediately after death, was found to be normal. Acute
cholinergic symptoms in all the birds were correlated with strong
inhibition of brain acetylcholinesterase activity, but 70% inhibition
in a bird treated with 20 mg/kg bw of omethoate still did not produce
detectable signs of acute poisoning.
The capacity of pure omethoate and of the incriminated sample to
inhibit neurotoxic esterase and acetylcholinesterase activities were
measured in hen and human brain tissue in vitro. As the IC50 for
acetylcholinesterase in both tissues was 0.08-0.15 mmol/litre, it
would be virtually completely inhibited at 5 mmol/litre, the
concentration that caused no detectable inhibition of neurotoxic
esterase. The activities of both enzymes were also measured in
cortical tissue samples taken 24 h post mortem from a 30-year-old
male farmer who had been acutely poisoned by a commercial formulation
of omethoate. The neurotoxic esterase activity was within the normal
range, while acetylcholinesterase activity was strongly inhibited. It
was concluded that omethoate is extremely unlikely to cause delayed
neuropathy in humans (Lotti et al., 1981).
Table 6. Results of tests for the genotoxicity of omethoate
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0-12 500 µg/plate 95.1 Weakly positive Herbold (1980)
TA98, TA100, in TA98, TA100, and
TA1535, TA1537 TA1535a Negative in
TA1537a
DNA repair E. coli (pol) 0-10 000 µg/plate 96 Negative Herbold (1983)
Gene mutation Chinese hamster 0-6 mg/ml 97.4 Positivea Ministry of Agriculture,
ovary cells (hprt) Fisheries and Food
(1993)
Cell mutation L5178Y mouse 0-5000 µg/ml 96.9 Negativea Bootman & Rees (1982)
lymphoma cells
Sister chromatid Chinese hamster 0-1000 µg/ml 96 Positive at > 250 µg/ml Ministry of Agriculture,
exchange ovary cells Fisheries and Food
(1993)
Gene conversion; S. cerevisiae 0-66.7 µl/ml 96.9 Negative Hoorn (1982, 1983)
reverse mutation Positive
In vivo
Micronucleus Mouse 2 × 6 or 12 97.1 Negative Herbold (1981)
formation mg/kg bw
Dominant lethal Mouse 0.5 mg/kg bw 95.4 Negative Machemer (1974)
mutation
Unscheduled DNA Wistar rat 0-30 mg/kg bw 96.6 Negative Ministry of Agriculture,
synthesis Fisheries and Food
(1993)
Spot test C57Bl/6J × T 0-16 mg/kg bw 96.7-97% Positive Ministry of Agriculture,
mice Fisheries and Food
(1993)
a With and without metabolic activation
3. Observations in humans
The results of two studies of dimethoate in humans were
summarized briefly in the report of the 1963 JMPR (Annex 1, reference
2). In the first study, 20 volunteers were given daily doses of 2.5 mg
dimethoate in aqueous solution (corresponding to approximately
0.04 mg/kg bw) for four weeks. No toxic effect was observed, and there
were no changes in blood cholinesterase activity. Similar results were
reported in single subjects who ingested 9 mg (0.13 mg/kg bw) or 18 mg
(0.26 mg/kg bw) for 21 days (Sanderson & Edson, 1964).
The results of a number of studies in which human volunteers with
no occupational exposure to organophosphate pesticides were given
dimethoate were summarized in Environmental Health Criteria monograph
No. 90 (WHO, 1989) and are presented in Table 7. The studies of
volunteers indicate that repeated doses of up to 0.2 mg/kg bw
dimethoate do not inhibit cholinesterase activity in the blood.
Table 7. Results of controlled human trials with dimethoate
No. of Sex Route Daily dose Duration of Results Reference
subjects exposure
20 NR Oral 0.04 mg/kg 4 weeks No toxic effects or inhibition of Sanderson & Edson
blood ChE (1964)
2 NR Oral 0.13 mg/kg 21 days No toxic effects or inhibition of Sanderson & Edson
0.26 mg/kg blood ChE (1964)
5 M Oral 0.25 mg/kg Single No toxic effects or inhibition of Sanderson & Edson
dose blood ChE (1964)
50 NR Dermala 2.5 ml 2 h No irritation or inhibition of blood Sanderson & Edson
ChE (1964)
12 M+F Oral 5 mg (0.068 mg/kg bw) 28 days No significant change in whole-blood Edson et al. (1967)
ChE
9 M+F Oral 15 mg (0.202 mg/kg bw) 39 days No significant change in whole-blood Edson et al. (1967)
ChE
8 M+F Oral 30 mg (0.434 mg/kg bw) 57 days Inhibition of ChE by day 20 (24%) Edson et al. (1967)
6 NR Oral 45 mg (0.587 mg/kg bw) 45 days Inhibition of ChE (35%) Edson et al. (1967)
6 M+F Oral 60 mg (1.02 mg/kg bw) 14 days Inhibition of ChE (21%) Edson et al. (1967)
NR, not reported; ChE, cholinesterase
a Patch test with 32% liquid formulation
Comments
Dimethoate
Dimethoate is rapidly and extensively absorbed from the gut and
rapidly excreted. There was no accumulation in fat tissue. In rats and
humans, up to 90% of radiolabel was found in the urine within 24 h.
The report of a study with methylcarbamoyl-labelled dimethoate
indicated that up to 18% of the administered label was excreted in
expired air. Four metabolites with anticholinesterase activity have
been identified in rats and humans. One seems to result from thiono
oxidation, leading to the formation of the oxygen analogue of
dimethoate, i.e. omethoate; this step was followed by hydrolysis to
a thiocarboxyl product, said to be the main metabolite in rats and
humans.
Data on the acute oral toxicity of dimethoate gave LD50 values
of about 310 mg/kg bw in rats, 150 mg/kg bw in mice, and 55 mg/kg bw
in hens. The signs of toxicity were those typical of cholinesterase
inhibition. WHO has classified dimethoate as 'moderately hazardous'
(WHO, 1996).
In short-term and long-term studies at dietary concentrations
> 75 ppm, there were minor reductions in body-weight gain and food
consumption. Apart from inhibition of cholinesterase activity,
dimethoate had no effect on the composition of the blood or urine. The
liver weights of animals treated at the higher doses tended to be
lower than those of the control groups: there were, however, no
microscopic changes, and the effect is unlikely to be of toxicological
significance. Investigations of toxicity at higher doses were limited
by effects due to cholinesterase inhibition. The NOAELs were thus
generally based on reductions in acetylcholinesterase activity in
the brain or erythrocytes. On the basis of minimal reductions in
acetylcholinesterase activity of 10-20%, the NOAEL in a 12-month study
in dogs at doses of 0, 5, 20, or 125 ppm was 5 ppm, equal to 0.2 mg/kg
bw per day; in rats, the NOAEL in a life-span study at doses of 0, 1,
5, 25, or 100 ppm was 1 ppm, equal to 0.04 mg/kg bw per day. In mice,
an NOAEL was not identified, as cholinesterase activity was depressed
at all doses after 52 weeks of treatment in a life-span study at doses
of 0, 25, 100, or 200 ppm.
The results of long-term studies of toxicity and carcinogenicity
in mice (at 0, 25, 100, or 200 ppm) and rats (at 0, 5, 25, or 100 ppm)
reported in 1986 and studies reported in 1977 indicate that dimethoate
is not carcinogenic to rodents.
In a multigeneration study of reproductive toxicity conducted in
1989-90 with doses of 0, 1, 15, or 65 ppm, reproductive performance
of rats was impaired at the high dose. The NOAEL for reproductive
toxicity appeared to be 15 ppm (equal to 1.2 mg/kg bw per day) and
that for parental toxicity was 1 ppm (equal to 0.08 mg/kg bw per day)
on the basis of cholinesterase inhibition, but the Meeting noted that
there was some indication that reproductive performance may have been
affected at lower doses. In a multigeneration study conducted in mice
in 1965 at doses of 0, 5, 15 or 50 ppm, there was no overt effect on
reproductive capacity, even in the presence of cholinergic toxicity.
In a poorly reported study in rabbits, sperm numbers and quality were
adversely affected at doses equivalent to one-tenth and one-hundredth
of the LD50.
Studies of developmental toxicity in rats (at 0, 3, 6, or
18 mg/kg bw per day on days 6-15 of gestation) and rabbits (at 0, 10,
20, or 40 mg/kg bw per day on days 7-19 of gestation) provided no
evidence of a teratogenic effect, although maternal toxicity was
observed at the high dose in rats and at the high and middle doses in
rabbits.
After reviewing the data available on mutagenicity, the Meeting
concluded that although in-vitro studies indicate that dimethoate has
mutagenic potential, this potential does not appear to be expressed
in vivo.
Undiluted dimethoate formulations were irritating to the eye in
rabbits. Skin irritation was minimal and confined to slight, transient
erythema. Dimethoate was not a skin sensitizer in guinea-pigs, but a
32.7% emulsifiable concentrate formulation induced sensitization in
one of 10 guinea-pigs. In a published paper, dimethoate was cited
in four human cases of contact dermatitis, and sensitization was
confirmed in these individuals by patch testing.
In hens given a single dose of 55 mg/kg bw by subcutaneous
injection or orally, dimethoate did not induce delayed neurotoxicity.
In a 39-day study in nine male and female volunteers, the NOAEL
for cholinesterase inhibition was 0.2 mg/kg bw per day. This NOAEL was
supported in seven other studies each involving 6-20 volunteers who
received doses ranging from 0.04 to 1.0 mg/kg bw per day for up to 57
days.
Omethoate
The oral LD50 of omethoate was approximately 25 mg/kg bw
in rats. Signs of reaction to treatment with omethoate were those
consistent with cholinesterase inhibition.
In short-term and long-term studies, the potential toxicity of
omethoate was limited by the onset of cholinesterase inhibition. In a
12-month study of toxicity in dogs at doses of 0, 0.025, 0.12, or
0.62 mg/kg bw per day by gavage, the NOAEL was 0.02 mg/kg bw per day
on the basis of inhibition of acetylcholinesterase activity. In
life-span studies in rats (at 0, 0.3, 1, 3, or 10 ppm) anti mice (at
0, 1, 3, or 10 ppm), there was no evidence of oncogenic potential. The
study in mice was unsuitable for deriving an NOAEL because acetyl-
cholinesterase activity was not investigated; the NOAEL in rats was
0.3 ppm (0.015 mg/kg bw per day) on the basis of inhibition of
acetylcholinesterase activity.
In multigeneration studies in rats at 0, 1, 3, or 10 ppm, a
dietary concentration of 10 ppm was associated with reduced viability
of pups; there was evidence that this effect extended to animals
treated at 3 ppm. The NOAEL was 1 ppm (equivalent to 0.05 mg/kg bw per
day). In a further multigeneration study in rats at doses of 0, 0.5,
3, or 18 ppm in the drinking-water, there was evidence of epididymal
vacuolation and fewer pups per dam at the high dose; these pups had
lower weight gains and were less viable. The precoital time was
increased and the number of non-pregnant females was greater than
among controls. The NOAEL for reproductive performance was 3 ppm
(equivalent to 0.2 mg/kg bw per day), but cholinesterase inhibition
was detected at the lowest dose of 0.5 ppm. In studies of
developmental toxicity, there was no evidence of teratogenicity in
rats given 0, 0.3, 1, or 3 mg/kg bw omethoate per day on days 6-15 of
gestation or in rabbits given 0, 0.1, 0.3, or 1 mg/kg bw omethoate per
day on days 6-18 of gestation.
Omethoate has been extensively investigated for mutagenicity in
vitro and in vivo. The Meeting concluded that it has clear mutagenic
potential but that the weight of the evidence observed in vivo was
negative; however, the positive result obtained in the mouse spot test
could not be completely disregarded.
In studies in hens given single oral doses of 20-300 mg/kg bw,
omethoate did not induce delayed neurotoxicity.
Conclusions
An ADI of 0-0.002 mg/kg bw was established for dimethoate on the
basis of the apparent NOAEL of 1.2 mg/kg bw per day for reproductive
performance in the study of reproductive toxicity in rats and applying
a safety factor of 500. Although a safety factor of 100 would normally
be used in deriving an ADI from a study of this type, the Meeting was
concerned about the possibility that reproductive performance may have
been affected at 1.2 mg/kg bw per day in this study and therefore used
a higher-than-normal safety factor. No data are available to assess
whether the effects on reproductive performance were secondary to
inhibition of cholinesterase. The Meeting concluded that it was
not appropriate to base the ADI on the results of the studies of
volunteers since the crucial end-point (reproductive performance) has
not been assessed in humans.
This ADI would usually be used only when assessing the intake of
dimethoate itself. As the use of dimethoate on crops can give rise to
residues of omethoate, and omethoate has been used as a pesticide in
its own right, previous Joint Meetings have allocated an ADI to
omethoate; however, the primary manufacturer is no longer producing
omethoate. The Meeting noted that omethoate is considerably more toxic
than dimethoate; however, the levels of residues of omethoate
resulting from use of dimethoate on crops are likely to be low. The
Meeting therefore recommended that residues of dimethoate and
omethoate resulting from the use of dimethoate be expressed as
dimethoate and be assessed in comparison with the ADI for dimethoate.
As the primary manufacturer is no longer producing either
omethoate or formothion, toxicological data on these compounds were
not made available to the Meeting. The previous ADIs of 0-0.0003 mg/kg
bw for omethoate and 0-0.02 mg/kg bw for formothion were therefore
withdrawn.
There may be a need to re-evaluate the toxicity of dimethoate
after the periodic review of the residue and analytical aspects of
dimethoate has been completed if it is determined that omethoate is a
major residue.
Toxicological evaluation
Levels that cause no toxic effect (dimethoate)
Rat: 1 ppm, equal to 0.04 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
15 ppm, equal to 1.2 mg/kg bw per day (reproductive
performance in a study of reproductive toxicity)
1 ppm, equal to 0.08 mg/kg bw per day (parental
toxicity in a study of reproductive toxicity)
6 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.2 mg/kg bw per day (52-week study of
toxicity)
Human: 0.2 mg/kg bw per day (39-day study of cholinesterase
inhibition)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw (sum of dimethoate and omethoate expressed as
dimethoate)
Studies that would provide information useful for continued evaluation
of the compound
1. Further multigeneration study in rats using dimethoate
2. Mouse spot test using dimethoate
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to dimethoate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 310 mg/kg bw
Dermal, toxicity, rat LD50 > 7000 mg/kg bw
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, human Sensitizing
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL > 1000 mg/kg bw per day (highest dose tested)
Repeated oral, reproductive toxicity, rat NOAEL = 1.2 mg/kg bw per day, reproductive toxicity
NOAEL = 0.05 mg/kg bw per day, parental toxicity
Repeated oral, developmental toxicity, rat NOAEL = 6 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 18 mg/kg bw per day (highest dose tested)
Repeated oral, developmental toxicity, rabbit NOAEL = 10 mg/kg bw per day, parental toxicity.
No evidence of embryotoxicity or teratogenicity
at 40 mg/kg bw per day (highest dose tested)
Long term (> 1 year) Repeated oral, toxicity and carcinogenicity, rat NOAEL = 0.04 mg/kg bw per day, cholinesterase
inhibition
References
Aharoni, A.H. & O'Brien, R.D. (1968) The inhibition of acetyl-
cholinesterases by anionic organophosphorus compounds. Biochemistry,
7, 1538.
Ahmed, F.E., Hart, R.W. & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res., 42, 161-174.
Becker, H. (1985) Dominant lethal study with dimethoate technical in
the mouse. Unpublished report No. 039003 from Research & Consulting
Company AG, Itingen, Switzerland. Unpublished report submitted to WHO
by the Dimethoate Task Force, Ingelheim, Germany.
Ben-Dyke, R., Sanderson, D.M. & Noakes, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9, 119.
Bhunya, S.P. & Behera, J. (1975) Centromeric sensitivity of mouse
chromosomes to the systemic insecticide dimethoate (Rogor). Curr. Sci.,
44, 859-860.
Bomhard, E., Loser, E. & Kaliner, G. (1979) S-6876: Chronic toxicity
study on rats (two-year feeding experiment). Unpublished report from
the Bayer AG Institut für Toxikologie, originally submitted to WHO by
Bayer AG, summary from WHO.
Bootman, J. & Rees, R. (1982) S-6876: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report from Life Science Research, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
Brady, U.E., Jr & Arthur, B.U. (1963) Biological and clinical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56, 477-482.
Brooker, A.J., Homan, B.A., Parker, C.A., Offer, J.M., Anderson, A. &
Dawe, I.S. (1992) The effect of dimethoate on reproductive function
of two generations in the rat. Unpublished report from Huntingdon
Research Centre, United Kingdom. Submitted to WHO by the Dimethoate
Task Force, Milan, Italy.
Budreau C.H. & Singh, R.P. (1973) Effect of fenthion and dimethoate on
reproduction in the mouse. Toxicol. Appl. Pharmacol., 26, 29-38.
Burford, P., McLean, T.A., Buist, D.P., Crook, D., Gregson, R.L. &
Gopinath, C. (1991) Dimethoate: 12 month dietary toxicity study in
beagle dogs. Unpublished report from Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Casida, J.E. & Sanderson, D.M. (1962) Toxic hazards from formulating
the insecticide dimethoate in methyl 'cellosolve'. Nature, 189,
507-508.
Chamberlain, W.R., Gatterdam, P.E. & Hopkins, D.E. ( 1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54, 733-740.
Chen, H.H., Hsueh, J.L., Sirianni, S.R. & Huang, C.C. (1981) Induction
of sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorous pesticides.
Mutat. Res., 88, 307-316.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health, B20, 373-406.
Dal Re, V. & Vola Gera, F. (1976) Determination of the acute dermal
toxicity of technical Rogor. Unpublished report from Centro Ricerche
Antiparassitari, Montedison, Italy. Submitted to WHO by Farmoplant,
Milan, Italy.
Dal Re, V. & Vola Gera, F. (1980) Acute oral toxicity of technical
Rogor samples recently produced in albino rats. Unpublished report
from Centro Ricerche Antiparassitari Montedison, Italy. Submitted to
WHO by Farmoplant, Italy.
Dauterman, W.C., Casida, J.E., Knaak, J.B. & Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agric. Food Chem., 7, 188-193.
Degraeve, N. & Moutschen, J. (1983) Genotoxicity of an organophosphorus
insecticide, dimethoate, in the mouse. Mutat. Res., 119, 331-337.
Dzwonkowska, A. & Hubner, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo.
Arch. Toxícol., 58, 152-156.
Ecobichon, D.J. & Kalow, W. (1963) Action of organo phosphorus
compounds upon esterases in human liver. Can. J. Biochem., 41, 1537.
Edson, E.F. & Noakes, D.N. (1960) The comparative toxicity of six
organophosphorus insecticides in the rat. Toxicol. Appl. Pharmacol.,
2, 523.
Edson, E.F., Jones, K.H. & Watson, W.A. (1967) Safety of dimethoate
insecticide. Br. Med. J., iv, 554.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984a) Effect of dimethoate
on pregnancy of the rat. Unpublished report from Huntington Research
Centre, Huntington, Cambs, United Kingdom. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Edwards, J.A., Leeming, N.M. & Clark, R. (1984b) Effect of dimethoate
on pregnancy of the New Zealand white rabbit. Unpublished report from
Huntington Research Centre, Huntington, Cambs, United Kingdom.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
El-Elaimy, I. (1986) Comparative studies on the effect of carbamate
and organophosphorus insecticides on acetylcholinesterase activity in
maternal and foetal tissue of rat. Proc. Zool. Soc. A.R. Egypt, 10,
41-49.
Fahrig, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomatis, L., eds, Chemical Carcinogenesis Essays
(IARC Scientific Publications No. 10), Lyon, International Agency for
Research on Cancer, pp. 161-181.
Fenwick, M.L., Barron, J.R. & Watson, W.A. (1957) Biochem. J., 65, 58.
Fischer, G.W. & Scheufler, H. (1981) Effects of dimethoate and
O-desmethyldimethoate in dominant lethal test in mice. Wiss. Z.
Univ. Halle, 30, 21-29 (in German).
Gabliks, J. (1965a) Responses of cell cultures to insecticides II.
Chronic toxicity and induced resistance. Proc. Soc. Exp. Biol. Med.,
120, 168-171.
Gabliks, J. (1965b) Responses of cell cultures to insecticides. III.
Altered susceptibility to poliovirus and diphtheria toxin. Proc. Soc.
Exp. Biol. Med., 120, 172-175.
Gabliks, J. & Friedman, L. (1965) Responses of cell cultures to
insecticides. I. Acute toxicity to human cells. Proc. Soc. Exp. Biol.
Med., 120, 163-168.
Gibel, U., Lohs, KH., Wildner, G.P., Ziebarth, D. & Stieglitz, R.
(1973) Experimental study on cancerogenic, haematotoxic, and
hepatotoxic activity of phosphororganic pesticides. Arch.
Geschwulstforsch., 41, 311-328 (in German).
Gilot-Delhalle, J., Colizzi, A., Moutschen, J. & Moutschen-Dahmen, M.
(1983) Mutagenicity of some organophosphorus compounds at the ade6
locus of Schizosaccharomyces pombe. Mutat. Res., 117, 139-148.
Gomez-Arroyo, S., Noriega-Aldana, N., Juarez-Rodriguez, D. &
Villalobos-Pietrinir, J. (1987) Sister chromatid exchanges induced by
the organophosphorus insecticides methyl parathion, dimethoate, phoxim
and methyl azinfos in cultured human lymphocytes. Contam. Amb.,
3, 63-70.
Guthrie, F.E., Shah, P.V. & Moreland, D.E. (1980) Effects of pesticides
on active transport of glucose through the isolated intestine of the
mouse. J. Agric. Food Chem., 22, 3.
Hassan, A., Zayed, S.M.A.D. & Bahig, M.R.E. (1969) Metabolic behaviour
of organophosphorus insecticides. XI: Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18, 2429-2438.
Hellwig, J. (1986a) Report on the study of the toxicity of dimethoate
in mice after 78-week administration in the diet. Unpublished report
No. 75C0326/8242 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Hellwig, J. (1986b) Report on the study of the toxicity of dimethoate
in rats after 24-month administration in the diet. Unpublished report
No. 70C0326/8241 from BASF, Department of Toxicology, Ludwigshafen/
Rhein, Germany. Submitted to WHO by the Dimethoate Task Force,
Ingelheim, Germany.
Herbold, B. (1980) S-6876 Salmonella/Mikrosomen Test zur Untersuchung
auf punktmutagene Wirking. Unpublished report from the Bayer AG
Institut für Toxikologie, originally submitted to WHO by Bayer AG,
summary from WHO.
Herbold, B. (1981) S-6876 (Omethoate, Folimat's active ingredient):
Micronucleus test on mouse to evaluate S 6876 for mutagenic potential.
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Herbold, B. (1983) S-6876 (Omethoate, Folimat's active ingredient):
Pol test on E. coli to evaluate for DNA damage. Unpublished report
from Institute of Toxicology, Bayer AG, originally submitted to WHO by
Bayer AG, summary from WHO.
Hoffman, K. & Schilde, B. (1984) S-6876 (Omethoate): Chronic toxicity
to dogs on oral administration (twelve month stomach tube study).
Unpublished report from Institute of Toxicology, Bayer AG, originally
submitted to WHO by Bayer AG. summary from WHO.
Hoorn, A.J.W. (1982) Mutagenic evaluation of S-6876 (omethoate) in the
reverse mutation induction assay with Saccharomyces cerevisiae strains
S138/S211-alfa. Unpublished report from Litton Bionetics, Netherlands,
originally submitted to WHO by Bayer AG, summary from WHO.
Hoorn, A.J.W. (1983) Evaluation of S-6876 (omethoate) in the induced
mitotic crossing over and gene conversion assay in Saccharomyces
cerevisiae strain D7. Unpublished report from Litton Bionetics,
Netherlands, originally submitted to WHO by Bayer AG, summary from
WHO.
Johnson, E., Caterson, C.R. & Allen, J.S. (1985) Mutagenicity testing
of dimethoate in the in vitro CHO/HGPRT mutation assay. Unpublished
report No. 0423 from American Cyanamid Co., Princeton, NJ, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
von Jung, H.-D. (1989) Kontaktekzem durch pestzide in der DDR.
Dermatol. Monaschr., 175, 203-214 (in German with English summary).
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
R.E. & Treiber, G. (1959) The metabolism of dimethoate in cattle.
J. Econ. Entomol., 52, 1190-1194.
Khera, K.S. (1979) Evaluation of dimethoate (Cygon 4E) for teratogenic
activity in the cat. J. Environ. Pathol. Toxicol., 2, 1283-1288.
Khera, K.S., Whalen, C., Trivett, G. & Angers, G. (1979) Teratogenicity
studies on pesticidal formulations of dimethoate, diuron and lindane in
rats. Bull. Environ. Contam. Toxicol., 22, 522-529.
Kimmerle, G. (1962) Toxicological studies on active ingredient S-6876.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1968) Bayer 45432-Toxikologische Untersuchungen.
Unpublished report originally submitted to WHO by Bayer AG, summary
from WHO.
Kimmerle, G. (1972) Folimat/Akute Neurotoxizitatsuntersuchungen an
Huhnern. Unpublished report from the Institut für Toxikologie,
Wuppertal-Elberfeld, Germany, originally submitted to WHO by Bayer AG,
summary from WHO.
Kimmerle, G. & Lorke, D. (1967) S-6876. Antidotal effect and
potentiation. Unpublished report originally submitted to WHO by Bayer
AG, summary from WHO.
Kroetlinger, F. & Löser, E. (1982) S-6876 (omethoate, the active
ingredient of Folimat): Chronic toxicity study on mice. (2-year
feeding experiment). Unpublished report from Bayer AG, originally
submitted to WHO by Bayer AG, summary from WHO.
Levinskas, G.J. & Shaffer, C.B. (1965) Oxygen analogue of dimethoate:
Demyelination in white Leghorn hens. Unpublished report originally
submitted to WHO by American Cyanamid Co., summary from WHO.
Löser, E. (1968) Bayer 45432: Subakute Toxikologische Untersuchungen
an Ratten. Unpublished report originally submitted to WHO by Bayer AG,
summary from WHO.
Löser, E. (1981) S-6876 Folimat-Wirkstoff Multigenerationeversuche an
Ratten. Unpublished report from Bayer AG, originally submitted to WHO
by Bayer AG. Addendum: Histopathology, Consultox Laboratories Ltd,
London, United Kingdom. Summary from WHO.
Löser, E. & Lorke, D. (1967) Bayer 45432: Subchronische Toxikologische
Untersuchungen an Ratten. Unpublished report originally submitted to
WHO by Bayer AG, summary from WHO.
Lotti, M., Ferrara, S.D., Caroldi, S. & Sinigaglia, F. (1981) Enzyme
studies with human and hen autopsy tissue suggest omethoate does not
cause delayed neuropathy in man. Arch. Toxicol., 48, 265-270.
Lucier, G.W. & Menzer, R.E. (1970) Nature of oxidative metabolites of
dimethoate formed in rats, liver microsomes, and bean plants. J. Agric.
Food Chem., 18, 698.
Machemer, L. (1974) Praparat S-6876 to Dominant Letal Test an der
mannlichen Maus zur Prufung auf mutagene Wirkung. Unpublished report
from the Institut für Toxikologie, Wuppertal-Elberfeld, Germany,
originally submitted to WHO by Bayer AG, summary from WHO.
Machemer, L. (1975) Praparat S-6876 to Untersuchung auf Exbryotoxische
and Teratogene Wirkung nach Oraler Verabreichung an der Ratte.
Unpublished report from the Institut für Toxikologie, Wuppertal-
Elberfeld, Germany, originally submitted to WHO by Bayer AG, summary
from WHO.
Madison, W.A., MacKenzie, K.M. & Fields, W.E. (1984) 21 Day dermal
study with dimethoate in rabbits. Unpublished report No. 6123-125
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by the Dimethoate Task Force, Milan, Italy.
Ministry of Agriculture, Fisheries and Food (1993a) Evaluation on
Dimethoate, Pesticides Safety Directorate, United Kingdom.
Ministry of Agriculture, Fisheries and Food (1993b) Evaluation on
Omethoate, Pesticides Safety Directorate, United Kingdom.
Mahkambaeva, Z.G. (1971) Influence of organophosphorus pesticides
(kilval, methylmercaptophos, fosfamid) on bioelectric activity of
heart in experimental animals. In: Hygiene, Use and Toxicology of
Pesticides, and Clinical Aspects of Pesticide Poisoning, Kiev, Zdorovye
Publishers, pp. 175-180 (in Russian).
Marosi, G., Ivan, J., Nagymajtenyi, L., Csatlos, J. & Toszegi, A.
(1985a) Dimethoate induced toxic cardiac failure in the guinea-pig.
Arch. Toxicol., 57, 142-143.
Marosi, G., Ivan, J. & Nagymajtenyi, L. (1985b) Cardiodepression in
organophosphate poisonings. Arch. Toxicol., Suppl. 8, 289-291.
Mohn, G. (1973) 5-Methyl tryptophan resistance mutations in
Escherichia coli K-12. Mutat. Res., 20, 7-15.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nadmaiteni, L. & Marosi, G. (1983) Cardiotoxic action of organo-
phosphorus pesticides. Gig. Sanit., 11, 72-73 (in Russian).
Nagymajtenyi, L. (1988) Neurophysiological markers as early signs of
organophosphate neurotoxicity. Neurotoxicol. Teratol., 10, 429-434.
Nehéz, M., Selypes, A., Scheufler, H. & Fisher, G.U. (1983) Effect of
dimethoate and O-demethyldimethoate on bone marrow cells of CFLP
mice. Regul. Toxicol. Pharmacol., 317, 349-354.
Newman, A.J., Cherry, C. & Urwin, C. (1972) Pathology report of
folimat active substances in hens. Unpublished report from the
Huntingdon Research Centre, Huntingdon, Cambs, United Kingdom,
originally submitted to WHO by Bayer AG, summary from WHO.
O'Brien, R.D (1959) Activation of thionophosphates by liver
microsomes. Nature, 183, 121.
O'Brien, R.D (1961) The effect of SKF 525A (2-diethylaminoethyl
2',2-diphenyl valerate hydrocloride) on organophosphate metabolism in
insect and mammals. Biochem. J., 79, 229.
Panshina, T.N. & Klisenko, M.A. (1962) Content of fosfamid and changes
in blood cholinesterase activity in different routes of treatment of
the mammalian organism. In: Industrial Toxicology and Clinical Picture
of Occupational Diseases Due to Chemicals, Moscow, Medgiz, pp. 181-184
(in Russian).
Probst, G.H., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K. &
Neal, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutag., 3, 11-32.
Rani, M.V.U., Reddi, O.S. & Reddy, P.P. (1980) Mutagenicity studies
involving aldrin, endosulfan, dimethoate, phosphamidon, carbaryl and
ceresan. Bull. Environ. Contam. Toxicol., 25, 277-282.
Redgrave, V.A., Johnson, A.A., Gopinath, C., Hadley, J.C., Anderson,
A. & Dawe, I.S. (1991) Dimethoate: Acute delayed neurotoxicity in the
domestic hen. Unpublished report from the Huntingdon Research Centre,
United Kingdom. Submitted to WHO by the Dimethoate Task Force, Milan,
Italy.
Reena, et al. (1989) Haematological changes induced by dimethoate in
rat. Arh. Hig. Rada Toksikol., 40, 23-27.
Reuber, M.D. (1984) Carcinogenicity of dimethoate. Environ. Res.,
34, 193-211.
Ribelin, W.E., Levinskas, G.J. & Shaffer, C.B. (1965) A 3-generation
reproduction study with mice. Unpublished report. Submitted to WHO by
the American Cyanamid Company.
Riemer, F., Dahlenburg, R. & Grisk, A. (1985) Renal excretion of
dimethylphosphate and its thio derivatives after application of
dimethoate, bromophos, naled or trichlorphon to rats. Nahrung, 29,
299-302 (in German).
Salem, M.H., Abo-Elezz, Z., Abd-Allah, G.A., Hassan, G.A. & Shaker, N.
(1988) Effect of organophosphorus (dimethoate) and pyrethroid
(deltamethrin) pesticides on semen characteristics in rabbits.
J. Environ. Sci. Health, B23, 279-290.
Sanderson, D.M. & Edson, E.F. (1964) Toxicological properties of the
organophosphorus insecticide dimethoate. Br. J. Ind. Med., 21, 52.
San Sebastian, J.R. (1985) In vivo bone marrow cytogenetics rat
metaphase analysis. PH 315-AC-00-84, Dimethoate CL 12880. Unpublished
report from Pharakon Research International, Inc., Waverly, PA, USA.
Submitted to WHO by the Dimethoate Task Force, Ingelheim, Germany.
Santi, R. & de Pietri-Sonelli, P. (1959) Mode of action and biological
properties of the S-(methylcarbamyl)methyl O,O-dimethyl-
diophosphate. Nature, 183, 398.
Santi, R. & de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido O,O-dimetiliditio-
fosforilacetico. Ist. Ric. Agr. Soc. Montecatini, 3, 3 (in Italian).
Scheufler, H. (1975) Effects of relatively high doses of dimethoate
and trichlorfon on the embryogenesis of laboratory mice. Biol. Rundsch.,
13, 238-240 (in German).
Shaker, N. (1988) In vivo chronic effect of dimethoate and deltamethrin
on rabbits. J. Environ. Sci. Health, B23, 387-399.
Sorg, R.M., (1985) Micronucleus test (MNT). PM 309A-AC-004-84,
Dimethoate CL 12880. Unpublished report from Pharakon Research
International, Inc., Waverly, PA, USA. Submitted to WHO by the
Dimethoate Task Force, Ingelheim, Germany.
Squire, R.A. (1988) An evaluation of vascular proliferative lesions in
male Wistar rats from project 70C0326/8241. Unpublished report from
Robert A. Squire Associates, Ruxton, MD, USA. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
Stieglitz, R., Gibel, W., Werner, W. & Stobbe, H. (1974) Experimental
study on haematotoxic and leukaemogenic effects of trichlorfon and
dimethoate. Acta Haematol., 52, 70-76.
Tesh, J.M., Ross, F.W., Wightman, T.J. & Wilby, O.K. (1982) S-6876:
Effect of oral administration upon pregnancy in the rabbit. Unpublished
report from Life Science Research, United Kingdom, originally submitted
to WHO by Bayer AG, summary from WHO.
Tiefenbach, B. & Lange, P. (1980) Studies on the action of dimethoate
on the immune system. Arch. Toxicol., Suppl. 4, 167-170.
Tripathy, N.K., Majhi, B., Dey, L. & Das, C.C. (1988) Genotoxicity of
Rogor studied in the sex-linked recessive lethal test and wing eye and
female germ-line mosaic assays in Drosophila melanogaster. Mutat. Res.,
206, 351-360.
Uchida, T., Dautermann, W.C. & O'Brien, R.D. (1964) The metabolism of
dimethoate by vertebrate tissues. J. Agric. Food Chem., 12, 48-52.
Ullman, L., Sacher, R. & Chevalier, J. (1985) Acute oral toxicity
study with dimethoate in mice. Unpublished report from Research and
Consulting Co., AG, Itingen, Switzerland. Submitted to WHO by the
Dimethoate Task Force, Milan, Italy.
US National Cancer Institute (1976) Bioassay of Dimethoate for Possible
Carcinogenicity (NCI-CG-TR-4 DHEW Publication No. (NIH) 77-80),
Bethesda, MD, USA.
US National Cancer Institute (1977) Bioassay of Dimethoate for Possible
Carcinogenicity (Technical Report Series No. 4), Bethesda, MD, USA.
Velázquez, A., Xamena, N., Creus A. & Marcos, R. (1986) Indication for
weak mutagenicity of the organophosphorus insecticide dimethoate in
Drosophila melanogaster. Mutat. Res., 172, 237-243.
Vishwanath, R. & Jamil, K. (1986) Mutagenic and genotoxic activities
of certain organophosphorus compounds using Ames Salmonella assay
with and without microsomal induction. Ind. J. Exp. Biol., 24, 305-308.
Weber, H., Patzschke, K. & Wegner, L.A. (1978) Omethoat-C14-Folimat-
Wirkstoff: Biokinetische Untershungen an Ratten. Unpublished report
originally submitted to WHO by Bayer AG, summary from WHO.
West, B., Vidone, L.B. & Shaffer, C.B. (1961) Acute and subacute
toxicity of dimethoate. Toxicol. Appl. Pharmacol., 3, 210.
WHO (1989) Dimethoate (Environmental Health Criteria 90), International
Programme on Chemical Safety, Geneva.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutag., 5, 835-846.
