
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 20
DIMETHOATE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA
This is a companion volume to Environmental Health Criteria 90:
Dimethoate
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
ISBN 92 4 154342 6
ISSN 0259 - 7268
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Uses
2. SUMMARY AND EVALUATION
2.1. Human exposure
2.2. Evaluation of human health risks
2.3. Evaluation of effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and
protection, first aid
4.1.1. Advice to physicians
4.1.1.1 Symptoms of poisoning
4.1.1.2 Medical treatment
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.3. Storage
4.4. Transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Waste disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
BIBLIOGRAPHY
ANNEX 1. Treatment of organophosphate insecticide poisoning in man
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is an International Chemical Safety Card
which should be readily available, and should be clearly explained, to
all who could come into contact with the chemical. The section on
regulatory information has been extracted from the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC) and from
other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: dimethoate (accepted by BSI, ISO, ANSI, and
JMAF); fosfamid (used in USSR)
Chemical structure:
CH3O S
\ "
P - SCH2CONHCH3
/
CH3O
Molecular formula: C5H12NO3PS2
IUPAC name: O,O-dimethyl S-methyl-carbamoylmethyl
phosphorodithioate
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester (9CI)
CAS registry number: 60-51-5
RTECS RN: TE1750000
Technical dimethoate is about 93-95% pure. The major impurities are
O,O-dimethyl S-methylphosphorodithioate and O,O,S-trimethyl
phosphorodithioate.
1.2 Physical and Chemical Properties
Pure dimethoate is a colourless crystalline solid with an odour of
mercaptan. Technical dimethoate (about 93% pure) varies from
off-white crystals to a grey semi-crystalline material. Some physical
and chemical properties of dimethoate are given in the International
Chemical Safety Card (pages 18-21).
Dimethoate is highly soluble in chloroform, methylene chloride,
benzene, toluene, alcohols, esters, and ketones, slightly soluble in
xylene, carbon tetrachloride, and aliphatic hydrocarbons, and partly
soluble in water.
Dimethoate is fairly stable in water and acid solution at room
temperature, and unstable in alkaline solution. On heating, it is
converted to O,S-dimethyl phosphorodithioate.
1.3 Analytical Methods
The method of choice for the determination of dimethoate is gas
chromatography with flame photometric detection.
1.4 Uses
Dimethoate is an organophosphorus insecticide with contact and
systemic action that is used against a broad range of insects in
agriculture and also for fly control. It was introduced in 1956 and
is produced in many countries.
Demethoxon, an oxygen analogue metabolite of dimethoate, appears to
play a dominant role in the toxicity for insects and mammals.
Dimethoxon is also used as an insecticide known as omethoate.
Formulations of dimethoate include emulsifiable concentrates, wettable
powders, and granules. There is also a formulation for
ultra-low-volume application.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
The general population is not normally exposed to dimethoate from air
or water. Residues in food are mostly below 1 mg/kg. Dimethoate was
detected only infrequently in the latest reported total-diet studies
(1982).
Occupational exposure to dimethoate may occur during manufacture,
formulation, and application. This exposure is mainly through
inhalation and dermal absorption. Higher occupational exposures may
be observed in the case of accident or as a result of incorrect
handling.
2.2 Evaluation of Human Health Risks
Dimethoate is moderately toxic. Most oral LD50s in rats have ranged
from 150 to 400 mg/kg body weight. The dermal LD50 in rats is
greater than 600mg/kg. It is not irritating to the skin, but may be
slightly irritating to the eye.
Dimethoate can be absorbed following ingestion, inhalation, and skin
contact. It is readily metabolized and eliminated and does not
accumulate in the body. Dimethoxon (omethoate), the oxygen analogue
found in plants, insects, and mammals, is about 10 times more toxic
and is a more potent inhibitor of cholinesterase activity than
dimethoate.
Signs of intoxication from exposure to dimethoate are those typical of
organophosphorus pesticides. Inhibition of cholinesterases is the
most sensitive indicator of exposure to dimethoate and may indicate
toxicity. A dietary level of 5mg/kg equivalent to 0.25 mg of
dimethoate per kg body weight is considered to be a no-observed-
adverse-effect level in the rat.
A five-generation reproduction study on mice given drinking-water
containing dimethoate at 60 mg/litre showed decreased mating success
and increased reproduction time in all generations. On the basis of
available data on experimental animals, dimethoate is not considered
to be a teratogen.
Dimethoate is considered mutagenic in a variety of in vitro and in
vivo test systems.a Although long-term studies have been
conducted on rats and mice administered dimethoate orally and rats
administered the compound by i.m. injection, the available data are
considered to be inadequate to assess the carcinogenic potential of
dimethoate.a
In man, several cases of suicidal or accidental poisoning with
dimethoate have been reported. Some cases of occupational poisoning
have been reported, which were the result of accidents or neglect of
safety precautions. The lethal oral dose for human beings has been
estimated to be in the range of 50-500mg/kg body weight.
Skin sensitization due to dimethoate has been reported.
2.3 Evaluation of the Effects on the Environment
Dimethoate does not persist in the environment; hydrolytic degradation
is rapid and is the main inactivating pathway. In moist air, it is
degraded photochemically into hydrolytic and oxidation products. The
half-life of dimethoate in different plants is between 2 and 5 days.
Degradation in soil is dependent on the type of soil, temperature,
moisture, and pH level. The toxicity of dimethoate for aquatic
organisms and birds is moderate to high. It is more toxic for honey
bees.
a New adequate mutagenicity and carcinogenicity studies have
become available since these conclusions were reached. The
FAO/WHO Joint Meeting on Pesticide Residues (JMPR) evaluated the
new data in 1987 and concluded that dimethoate is mutagenic in
bacterial tests, but negative in mammalian cells in in vivo
tests. Moreover, no indications for carcinogenicity were found
in rats and mice.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
(a) Under proper conditions of use, exposure of the general
population to dimethoate is negligible.
(b) When appropriate safety precautions are taken, exposure to
dimethoate during manufacture, formulation, use, and disposal
should not pose an unacceptable human health hazard.
(c) Dimethoate is rapidly degraded and does not persist in the
environment. Care should be taken not to expose honey bees, fish
and aquatic organisms, and birds.
3.2 Recommendations
(a) Workers should be monitored for exposure to dimethoate and for
potential adverse health effects.
(b) Cleaning and disposal of contaminated equipment, clothing, and
containers should be in accordance with recommended procedures.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Dimethoate is an indirect acting organophosphorus insecticide, i.e.,
it is converted in the body into the active metabolite, dimethoxon.
As a result, signs and symptoms of overexposure develop after a latent
period and may continue to increase after exposure has been
discontinued. The acute oral toxicity of dimethoate is moderate and
it can be hazardous for human beings if incorrectly handled. On
overexposure, typical signs and symptoms of organophosphorus poisoning
may occur rapidly. For details, see the International Chemical Safety
Card on pages 18-21.
4.1.1 Advice to Physicians
For a more complete treatise on the effects of organophosphorus
insecticides, especially their short- and long-term effects on the
nervous system, please refer to EHC 63: Organophosphorus insecticides
- a general introduction (WHO, 1986). The section on "Treatment of
organophosphate insecticide poisoning in man" is included in this
publication as Annex 1.
4.1.1.1 Symptoms of poisoning
Signs and symptoms may include a feeling of exhaustion, headache,
blurred vision, weakness, and confusion. Vomiting, abdominal pain,
excessive sweating, and salivation may develop. The pupils are
constricted. Difficulty in breathing may be experienced, owing to
congestion of the lungs and weakness of the respiratory muscles.
Arrhythmia and cardiac failure have been reported. In severe
poisoning, there will be muscle spasms, unconsciousness, and
convulsions. Breathing may stop, followed by death.
4.1.1.2 Medical treatment
Induce vomiting if dimethoate has been ingested. It should be noted
that dimethoate may be dissolved in one or more solvents (e.g., a
petroleum distillate) and that inducing vomiting then involves a risk
of aspiration pneumonitis. Alternatively, perform rapid gastric lavage
using 5% sodium bicarbonate. Persons without signs of respiratory
inefficiency, but with manifest peripheral symptoms, should be treated
with 2-4 mg of atropine sulfate by intravenous injection. More
atropine may be given as needed. Severely intoxicated persons
suffering from respiratory difficulties or convulsions, or becoming
unconscious, should be given atropine and a reactivator immediately.
In such severe cases, 4-6 mg of atropine sulfate should be given
initially followed by repeated doses of 2 mg at 5-10-min intervals.
In all cases of clinical poisoning with dimethoate and other
organophosphorus insecticides, it is essential to maintain general
surveillance and cholinesterase and cardiac monitoring for at least 4
days, or more if necessary, and to adapt general supportive and
specific therapy in accordance with the findings.
If the patient is cyanotic, artificial respiration should be given at
the same time as atropine sulfate. Haemoperfusion may be effective in
the early stages of poisoning. The airway should be kept free and
artificial respiration should be applied. If necessary, intubation
should be performed.
The following drugs are contraindicated: morphine, barbiturates,
phenothiazine, tranquillizers, and central stimulants of all kinds.
Data on the effects of oxime reactivators in dimethoate poisoning are
contradictory, some showing that they may have a negative effect on
cholinesterase inhibition. It is therefore suggested that, if oxime
reactivators are indicated, they should be used with caution and under
close supervision.
4.1.2 Health Surveillance Advice
Human beings potentially exposed to dimethoate should undergo periodic
health monitoring and monitoring of cholinesterase activity in the
blood.
Measurement of whole blood-AChE is the most widely adopted method for
monitoring occupational exposure to organophosphorus insecticides.
However, physiological variations in blood-ChE levels occur in healthy
persons.
A 20-25% depression of AChE or ChE is considered diagnostic of
exposure, but not necessarily indicative of hazard. A depression of
30-50% or more is considered an indication that an exposed individual
should be removed from further contact with pesticides until levels
return to normal. Work procedures and hygiene should also be checked.
4.2 Explosion and Fire Hazards
Liquid formulations may be flammable. With sufficient burning or
external heat, dimethoate will decompose emitting toxic fumes.
Fire-fighters should be equipped with protective clothing and
self-contained breathing apparatus.
Fires should be extinguished with alcohol-resistant foam or powder.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding polluted run-off from the site.
4.3 Storage
Technical dimethoate and its formulations must be stored in locked,
well-ventilated buildings, preferably dedicated to insecticides. They
should not be exposed to direct sunlight. Products must be kept out
of reach of children and unauthorized personnel. They should not be
stored near feed or foodstuffs.
4.4 Transport
Any local regulations regarding movement of hazardous goods must be
complied with. Products must not be transported with feed or
foodstuffs. Containers should be checked before despatch to ensure
that they are in good condition and that the labels are undamaged.
4.5 Spillage and Disposal
4.5.1 Spillage
Skin contamination and inhalation of vapour should be avoided.
Spilled liquid should be absorbed and contaminated areas covered
with a 1:3 mixture of sodium carbonate crystals and damp sawdust,
lime, sand, or earth. After sweeping up, it should be transferred to
a safe place for disposal in a tightly closed and labelled container.
It is important to prevent liquid from spreading and contaminating
other cargo, vegetation, or waterways by making a barrier of the most
suitable available material, e.g., earth or sand.
Any of the product remaining in the damaged/leaking container should
be emptied into a clean empty container, which should then be tightly
closed and suitably labelled. The emptied leaking containers can be
decontaminated with a 10% sodium carbonate (washing soda) solution,
added at a rate of at least 1litre per 20-litre drum, and swirled
round to rinse the walls. The rinsings should be added to sawdust.
Containers should be punctured so that they cannot be re-used.
4.5.2 Waste disposal
Large amounts should be incinerated at high temperatures in a unit
with effluent gas scrubbing, or adsorbed on vermiculite and placed in
a landfill if incineration is impossible (IRPTC, 1985). The waste
should be buried in an approved dump, or in an area where there is no
risk of contamination of surface or ground water. Before burying,
liberal mixing with sodium carbonate (washing soda) crystals and soil
rich in organic matter will to help neutralize the product. Local
legislation must be complied with.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Dimethoate is rapidly hydrolysed and does not persist in the
environment. The toxicity of dimethoate for aquatic organisms and
birds is moderate to high. It is more toxic for honey bees.
Avoid contamination of soil, water, and the atmosphere by proper
methods of use, storage, transport, handling, and waste disposal. In
case of spillage, use the methods advised in section 4.5.1.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, dimethoate. It should be displayed at, or near,
entrances to areas where there is potential exposure to dimethoate,
and on processing equipment and containers. The card should be
translated into the appropriate language(s). All persons potentially
exposed to the chemical should also have the instructions on the
chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
DIMETHOATE
CAS chemical name: Phosphorodithioic acid, O,O-dimethyl
S-[2-(methylamino)-2-oxoethyl] ester (9CI)
RTECS RN: TE1750000; CAS No. 60-51-5
Molecular formula: C5H12NO3PS2
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 229.2 Pure dimethoate is a colourless crystalline solid
Odour threshold (mg/m3) 0.010 with an odour of mercaptan; technical dimethoate varies
Melting point (°C) 45-52.5 from off-white crystals to a grey semi-crystalline
Boiling point (°C) 107 at 0.05 mmHg material; dimethoate is soluble in organic
86 at 0.01 mmHg solvents; it is fairly stable in water and acid
Vapour pressure (25°C) 8.5 x 10-6 mmHg solutions at room temperature, and unstable
Water solubility (21°C) up to 39 g/litre in alkaline solutions; it is readily absorbed via
Half-life in aqueous media: the skin, ingestion, and inhalation.
at pH 2- 7 relatively stable
at pH 9 50% loss in 12 days
n-octanol/water partition 5.9
coefficient
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
GENERAL: Cholinesterase inhibition Avoid exposure
SKIN: Contamination may cause Wear PVC or neoprene gloves, apron Remove and wash contaminated clothing;
organophosphorus poisoning: weakness, and rubber boots wash contaminated skin with water and
headache, vomiting, excessive soap
sweating and salivation, pin-point
pupils; in severe cases: convulsions,
unconsciousness, and death due to
respiratory paralysis.
EYES: Irritation, redness Wear safety goggles or face shield Flush with clean water for a least 15
minutes; if irritation persists, obtain
medical attention immediately
INHALATION: Excessive inhalation Avoid breathing dust; use proper In case of signs and symptoms, remove
may cause poisoning (exhaust) ventilation or suitable from contaminated area and obtain medical
mask attention immediately
INGESTION: Unlikely occupational Wash hands before eating, drinking,
hazard using the toilet, and after work;
do not keep food in areas with
potential exposure
Accidental or intentional Obtain medical attention immediately;a
ingestion may rapidly lead to induce vomiting if subject is conscious;
severe poisoning (see skin) if breathing has stopped, apply artificial
respiration
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
REPEATED EXPOSURE As above In case of poisoning, same as abovea
BY INGESTION, INHALATION,
OR THROUGH SKIN may
gradually lead to signs, symptoms,-
and poisoning; sensitization
may occur
ENVIRONMENT: Toxic for fish, Avoid contamination of soil,
birds, and bees water, and atmosphere
SPILLAGE STORAGE FIRE AND EXPLOSION
Absorb spilled liquid and cover Store in locked, well-ventilated Use alcohol-resistant foam or powder;
contaminated area with 1:3 mixture of storeroom, away from feed and cool unaffected stock; wear protective
of sodium carbonate crystals and damp foodstuffs, children, and unauthorized clothing and self-contained breathing
sawdust, lime, sand, or earth; sweep personnel apparatus
up and place in closed container
WASTE DISPOSAL NATIONAL INFORMATION
Burn at high temperature in National occupational exposure limit: UN: 2783, 2784, 3017, 3018
incinerator with effluent scrubbing;
comply with local legislation; National Poison Control Centre:
when allowed, treat with washing
soda mixed with soil rich in organic Local Trade Names:
matter and bury in approved dump
 a Caution: if dimethoate is dissolved in solvents, e.g., petroleum solvents, vomiting may cause aspiration pneumonitis.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Dimethoate was evaluated by the Joint Meeting on Pesticide Residues
(JMPR) in 1963, 1965, 1966, 1967, 1970, 1973 (evaluation of the
related compound formathion), 1977, 1978, 1984, and 1987.
An acceptable daily intake (ADI) for man for dimethoate was estimated
at 0-0.01 mg/kg body weight per day in 1987.
A data sheet on dimethoate (No. 42) is available from the WHO Division
of Vector Biology and Control in their series "Data sheets on
pesticides" (WHO/FAO 1975-87).
In the WHO Recommended Classification of Pesticides by Hazard,
technical dimethoate has been classified as moderately hazardous, when
handled in accordance with instructions.
IRPTC has issued a review on dimethoate (No. 5) in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 24-26.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
Dimethoate is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents. Since 1986, the use of dimethoate in greenhouses and on
cherries and cabbage has been forbidden in the USSR.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified dimethoate (solid >73%; liquid >29%) in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport
The symbol on the label should be as follows:
a Caution: if dimethoate is dissolved in solvents, e.g., petroleum solvents, vomiting may cause aspiration pneumonitis.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Dimethoate was evaluated by the Joint Meeting on Pesticide Residues
(JMPR) in 1963, 1965, 1966, 1967, 1970, 1973 (evaluation of the
related compound formathion), 1977, 1978, 1984, and 1987.
An acceptable daily intake (ADI) for man for dimethoate was estimated
at 0-0.01 mg/kg body weight per day in 1987.
A data sheet on dimethoate (No. 42) is available from the WHO Division
of Vector Biology and Control in their series "Data sheets on
pesticides" (WHO/FAO 1975-87).
In the WHO Recommended Classification of Pesticides by Hazard,
technical dimethoate has been classified as moderately hazardous, when
handled in accordance with instructions.
IRPTC has issued a review on dimethoate (No. 5) in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 24-26.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
Dimethoate is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents. Since 1986, the use of dimethoate in greenhouses and on
cherries and cabbage has been forbidden in the USSR.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified dimethoate (solid >73%; liquid >29%) in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport
The symbol on the label should be as follows:
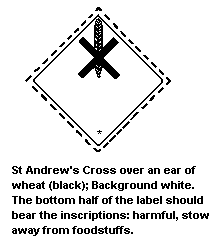 The International Maritime Organization lists dimethoate as a
pesticide of high hazard and as a poisonous substance at
concentrations above 5% (Hazard Class 6.1).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Bulgaria Maximum permissible concentration (MPC) 1971
- Time-weighted average (TWA) 1 mg/m3
Romania Maximum permissible concentration (MPC)
- Time-weighted average (TWA) 7 mg/m3
- Ceiling value 10 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.5 mg/m3 1977
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- 1 time per day 0.003 mg/m3
- Average per day 0.003 mg/m3
Preliminary safety limit
- 1 time per day 0.003 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.03 mg/litre
SOIL USSR Permissible limit 0.3 mg/kg
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.01 mg/kg 1987
body weight
USSR Acceptable daily intake (ADI) 0.02 mg/kg
body weight
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Brazil Acceptable limit (safety 0.02-2 mg/kg
interval: 1 - 28 days)
Czechoslovakia Maximum residue limit (MRL) 1-2 mg/kg 1978
European Maximum residue limit (MRL) 1 mg/kg 1982
Community
India Maximum tolerable concentration 2 mg/kg 1976
Japan Acceptable residue limit (ARL) 1 mg/kg
Kenya Maximum limit 1-2 mg/kg
Sweden Maximum tolerable concentration 0.22 mg/kg 1983
USSR Maximum residue limit (MRL) 0.1-0.4 mg/kg 1983
FOOD Plant Exports and Maximum residue limit (MRL) 0.5 mg/kg
imports by
CMEA
countries
FOOD Raw USA Acceptable residue limit (ARL) 0.002-2 mg/kg
agricultural
products
FOOD Animal USA Acceptable residue limit (ARL) 5 mg/kg
USSR Maximum residue limit (MRL) 2 mg/kg 1981
The WHO specifications for pesticides used in public health, in an
interim specification on dimethoate technical and emulsifiable
concentrate, require that the technical material shall consist of
dimethoate together with related manufacturing compounds and shall be
in the form of white/grey crystals free from extraneous impurities or
added modifying agents.
The emulsifiable concentrate shall consist of technical dimethoate
dissolved in suitable solvents with other necessary formulants added.
It shall be in the form of a stable liquid, free from suspended matter
and sediment. The technical dimethoate used in the manufacture of the
concentrate shall comply with the requirements of specification
WHO/IS/1.0094-1.
Chemical and physical requirements and the methods for checking them
are specified.
The material shall be packed in suitable, clean containers, as
specified in the order.
All packages shall bear, durably and legibly marked on the container,
the following:
Manufacturer's name
Technical dimethoate to Interim Specification WHO/IS/1.0094-1
Batch or reference number, and date of test
Net weight of contents
Date of manufacture
and, in the case of the emulsifiable concentrate:
Manufacturer's name
Dimethoate emulsifiable concentrate to Interim Specification
WHO/IS/3.0094-1
Dimethoate ... g/kg
Batch or reference number, and date of test
Net weight of contents
Instructions for dilution
Date of formulation
and the following minimum cautionary notice:
Dimethoate is an organophosphorus compound that inhibits
cholinesterase. It is poisonous if swallowed or inhaled. It
may be absorbed through the skin. Avoid skin contact: wear
protective gloves, clean protective clothing, and a respirator
when handling the material. Wash thoroughly with soap and water
after using.
Keep the material out of the reach of children and well away
from foodstuffs and animal feed and their containers.
If poisoning occurs, call a physician. Atropine and pralidoxime
are specific antidotes, and artificial respiration may be
needed.
The FAO specifications for plant protection products give similar
specifications and the methods for checking them.
The dimethoate content shall be declared (minimum 95% for the
technical product) and shall not differ from the declared percentage
by more than 2% for the technical product and 5-10% for its
formulations.
Containers shall be suitable, clean, dry, and as specified in the
order, and shall not adversely affect, or be affected by, the product,
but shall adequately protect it from external conditions. They shall
comply with pertinent national and international transport and safety
regulations.
Specifications for storage stability are given.
The European Community legislation requires labelling as a dangerous
substance using the symbol:
The International Maritime Organization lists dimethoate as a
pesticide of high hazard and as a poisonous substance at
concentrations above 5% (Hazard Class 6.1).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Bulgaria Maximum permissible concentration (MPC) 1971
- Time-weighted average (TWA) 1 mg/m3
Romania Maximum permissible concentration (MPC)
- Time-weighted average (TWA) 7 mg/m3
- Ceiling value 10 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.5 mg/m3 1977
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- 1 time per day 0.003 mg/m3
- Average per day 0.003 mg/m3
Preliminary safety limit
- 1 time per day 0.003 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.03 mg/litre
SOIL USSR Permissible limit 0.3 mg/kg
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.01 mg/kg 1987
body weight
USSR Acceptable daily intake (ADI) 0.02 mg/kg
body weight
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Brazil Acceptable limit (safety 0.02-2 mg/kg
interval: 1 - 28 days)
Czechoslovakia Maximum residue limit (MRL) 1-2 mg/kg 1978
European Maximum residue limit (MRL) 1 mg/kg 1982
Community
India Maximum tolerable concentration 2 mg/kg 1976
Japan Acceptable residue limit (ARL) 1 mg/kg
Kenya Maximum limit 1-2 mg/kg
Sweden Maximum tolerable concentration 0.22 mg/kg 1983
USSR Maximum residue limit (MRL) 0.1-0.4 mg/kg 1983
FOOD Plant Exports and Maximum residue limit (MRL) 0.5 mg/kg
imports by
CMEA
countries
FOOD Raw USA Acceptable residue limit (ARL) 0.002-2 mg/kg
agricultural
products
FOOD Animal USA Acceptable residue limit (ARL) 5 mg/kg
USSR Maximum residue limit (MRL) 2 mg/kg 1981
The WHO specifications for pesticides used in public health, in an
interim specification on dimethoate technical and emulsifiable
concentrate, require that the technical material shall consist of
dimethoate together with related manufacturing compounds and shall be
in the form of white/grey crystals free from extraneous impurities or
added modifying agents.
The emulsifiable concentrate shall consist of technical dimethoate
dissolved in suitable solvents with other necessary formulants added.
It shall be in the form of a stable liquid, free from suspended matter
and sediment. The technical dimethoate used in the manufacture of the
concentrate shall comply with the requirements of specification
WHO/IS/1.0094-1.
Chemical and physical requirements and the methods for checking them
are specified.
The material shall be packed in suitable, clean containers, as
specified in the order.
All packages shall bear, durably and legibly marked on the container,
the following:
Manufacturer's name
Technical dimethoate to Interim Specification WHO/IS/1.0094-1
Batch or reference number, and date of test
Net weight of contents
Date of manufacture
and, in the case of the emulsifiable concentrate:
Manufacturer's name
Dimethoate emulsifiable concentrate to Interim Specification
WHO/IS/3.0094-1
Dimethoate ... g/kg
Batch or reference number, and date of test
Net weight of contents
Instructions for dilution
Date of formulation
and the following minimum cautionary notice:
Dimethoate is an organophosphorus compound that inhibits
cholinesterase. It is poisonous if swallowed or inhaled. It
may be absorbed through the skin. Avoid skin contact: wear
protective gloves, clean protective clothing, and a respirator
when handling the material. Wash thoroughly with soap and water
after using.
Keep the material out of the reach of children and well away
from foodstuffs and animal feed and their containers.
If poisoning occurs, call a physician. Atropine and pralidoxime
are specific antidotes, and artificial respiration may be
needed.
The FAO specifications for plant protection products give similar
specifications and the methods for checking them.
The dimethoate content shall be declared (minimum 95% for the
technical product) and shall not differ from the declared percentage
by more than 2% for the technical product and 5-10% for its
formulations.
Containers shall be suitable, clean, dry, and as specified in the
order, and shall not adversely affect, or be affected by, the product,
but shall adequately protect it from external conditions. They shall
comply with pertinent national and international transport and safety
regulations.
Specifications for storage stability are given.
The European Community legislation requires labelling as a dangerous
substance using the symbol:
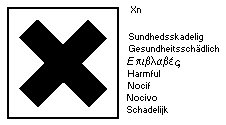 The label must read:
harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuffs.
The European Community legislation on labelling of pesticide
preparations classifies dimethoate in Class II/b for the purpose of
determining the label for preparations containing dimethoate and other
active ingredients.
7.5 Waste Disposal
In the USA, any non-domestic waste containing dimethoate is considered
a hazardous waste and permits are required for its handling,
transport, treatment, storage, or disposal. Waste incinerators must
achieve 99.99% destruction and removal of this substance.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986a) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986b) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 3rd ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention and diagnosis of pesticide poisoning.
Geneva, World Health Organization (Unpublished report No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 14th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO (In press) EHC No. 90: Dimethoate. Geneva, World Health
Organization.
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX 1
TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus insecticides: a general introduction)
All cases of organophosphorus poisoning should be dealt with as an
emergency and the patient sent to hospital as quickly as possible.
Although symptoms may develop rapidly, delay in onset or a steady
increase in severity may be seen up to 48 h after ingestion of some
formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by organophosphorus
insecticides are given in several major references (Kagan, 1977;
Taylor, 1980; UK DHSS, 1983; Plestina, 1984) and will also be included
in the IPCS Health and Safety Guides to be prepared for selected
organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with alkaline
soap or with a sodium bicarbonate solution. Particular care should be
taken in cleaning the skin area where venepuncture is performed.
Blood might be contaminated with direct-acting organophosphorus
esters, and, therefore, inaccurate measures of ChE inhibition might
result. Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced, if
the patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. This treatment is, however,
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with addition of bicarbonate solution or
activated charcoal) can also be performed, particularly in unconscious
patients, taking care to prevent aspiration of fluids into the lungs
(i.e., only after a tracheal tube has been put in place).
The volume of fluid introduced into the stomach should be recorded and
samples of gastric lavage frozen and stored for subsequent chemical
analysis. If the formulation of the pesticide involved is available,
it should also be stored for further analysis (i.e., detection of
toxicologically relevant impurities). A purge to remove the ingested
compound can be administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be started at the
first sign of respiratory failure and maintained for as long as
necessary.
Cautious administration of fluids is advised, as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg i.v. and given at
15-30-min intervals. The dose and the frequency of atropine treatment
varies from case to case, but should maintain the patient fully
atropinized (dilated pupils, dry mouth, skin flushing, etc.).
Continuous infusion of atropine may be necessary in extreme cases and
total daily doses up to several hundred mg may be necessary during the
first few days of treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs. However, if absorption,
distribution, and metabolism are thought to be delayed for any
reasons, oximes can be administered for several days after
intoxication. Effective treatment with oximes reduces the required
dose of atropine. Pralidoxime is the most widely available oxime. A
dose of 1 g pralidoxime can be given either i.m. or i.v. and repeated
2-3 times per day or, in extreme cases, more often. If possible,
blood samples should be taken for AChE determinations before and
during treatment. Skin should be carefully cleansed before sampling.
Results of the assays should influence the decision whether to
continue oxime therapy after the first 2 days.
There are indications that oxime therapy may possibly have beneficial
effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the mildest
cases. Besides relieving anxiety, it appears to counteract some
aspects of CNS-derived symptoms, which are not affected by atropine.
Doses of 10 mg s.c. or i.v. are appropriate and may be repeated as
required (Vale & Scott, 1974). Other centrally acting drugs and drugs
that may depress respiration are not recommended in the absence of
artificial respiration procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of secretions
are the best guide to the effectiveness of atropinization. Although
repeated dosing may well be necessary, excessive doses at any one time
may cause toxic side-effects. Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be taken
into, and then released from, fat depots over a period of many days.
It is therefore quite incorrect to abandon oxime treatment after 1-2
days on the supposition that all inhibited enzyme will be aged.
Ecobichon et al. (1977) noted prompt improvement in both condition and
blood-ChEs in response to pralidoxime given on the 11th-15th days
after major symptoms of poisoning appeared due to extended exposure to
fenitrothion (a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in error,
while drunk. Therapeutic dosages were progressively increased up to
6 mg atropine i.v. every 15 min together with continuous i.v. infusion
of pralidoxime chloride at 0.5 g/h for 72 h, from days 3 to 6 after
intoxication. After considerable improvement, the patient relapsed
and further aggressive therapy was given at a declining rate from days
10 to 16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given and the
patient was discharged on the thirty-third day with no apparent
sequelae.
References to Annex 1
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-379.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus pesticides]
Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished report No. VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics, 6th ed.,
New York, Macmillan Publishing Company, pp. 100-119.
DHSS (1983) Pesticide poisoning: notes for the guidance of medical
practitioners. London, United Kingdom Department of Health and
Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning. Guy's
Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
The label must read:
harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuffs.
The European Community legislation on labelling of pesticide
preparations classifies dimethoate in Class II/b for the purpose of
determining the label for preparations containing dimethoate and other
active ingredients.
7.5 Waste Disposal
In the USA, any non-domestic waste containing dimethoate is considered
a hazardous waste and permits are required for its handling,
transport, treatment, storage, or disposal. Waste incinerators must
achieve 99.99% destruction and removal of this substance.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986a) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986b) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 3rd ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention and diagnosis of pesticide poisoning.
Geneva, World Health Organization (Unpublished report No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 14th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO (In press) EHC No. 90: Dimethoate. Geneva, World Health
Organization.
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX 1
TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus insecticides: a general introduction)
All cases of organophosphorus poisoning should be dealt with as an
emergency and the patient sent to hospital as quickly as possible.
Although symptoms may develop rapidly, delay in onset or a steady
increase in severity may be seen up to 48 h after ingestion of some
formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by organophosphorus
insecticides are given in several major references (Kagan, 1977;
Taylor, 1980; UK DHSS, 1983; Plestina, 1984) and will also be included
in the IPCS Health and Safety Guides to be prepared for selected
organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with alkaline
soap or with a sodium bicarbonate solution. Particular care should be
taken in cleaning the skin area where venepuncture is performed.
Blood might be contaminated with direct-acting organophosphorus
esters, and, therefore, inaccurate measures of ChE inhibition might
result. Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced, if
the patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. This treatment is, however,
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with addition of bicarbonate solution or
activated charcoal) can also be performed, particularly in unconscious
patients, taking care to prevent aspiration of fluids into the lungs
(i.e., only after a tracheal tube has been put in place).
The volume of fluid introduced into the stomach should be recorded and
samples of gastric lavage frozen and stored for subsequent chemical
analysis. If the formulation of the pesticide involved is available,
it should also be stored for further analysis (i.e., detection of
toxicologically relevant impurities). A purge to remove the ingested
compound can be administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be started at the
first sign of respiratory failure and maintained for as long as
necessary.
Cautious administration of fluids is advised, as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg i.v. and given at
15-30-min intervals. The dose and the frequency of atropine treatment
varies from case to case, but should maintain the patient fully
atropinized (dilated pupils, dry mouth, skin flushing, etc.).
Continuous infusion of atropine may be necessary in extreme cases and
total daily doses up to several hundred mg may be necessary during the
first few days of treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs. However, if absorption,
distribution, and metabolism are thought to be delayed for any
reasons, oximes can be administered for several days after
intoxication. Effective treatment with oximes reduces the required
dose of atropine. Pralidoxime is the most widely available oxime. A
dose of 1 g pralidoxime can be given either i.m. or i.v. and repeated
2-3 times per day or, in extreme cases, more often. If possible,
blood samples should be taken for AChE determinations before and
during treatment. Skin should be carefully cleansed before sampling.
Results of the assays should influence the decision whether to
continue oxime therapy after the first 2 days.
There are indications that oxime therapy may possibly have beneficial
effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the mildest
cases. Besides relieving anxiety, it appears to counteract some
aspects of CNS-derived symptoms, which are not affected by atropine.
Doses of 10 mg s.c. or i.v. are appropriate and may be repeated as
required (Vale & Scott, 1974). Other centrally acting drugs and drugs
that may depress respiration are not recommended in the absence of
artificial respiration procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of secretions
are the best guide to the effectiveness of atropinization. Although
repeated dosing may well be necessary, excessive doses at any one time
may cause toxic side-effects. Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be taken
into, and then released from, fat depots over a period of many days.
It is therefore quite incorrect to abandon oxime treatment after 1-2
days on the supposition that all inhibited enzyme will be aged.
Ecobichon et al. (1977) noted prompt improvement in both condition and
blood-ChEs in response to pralidoxime given on the 11th-15th days
after major symptoms of poisoning appeared due to extended exposure to
fenitrothion (a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in error,
while drunk. Therapeutic dosages were progressively increased up to
6 mg atropine i.v. every 15 min together with continuous i.v. infusion
of pralidoxime chloride at 0.5 g/h for 72 h, from days 3 to 6 after
intoxication. After considerable improvement, the patient relapsed
and further aggressive therapy was given at a declining rate from days
10 to 16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given and the
patient was discharged on the thirty-third day with no apparent
sequelae.
References to Annex 1
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-379.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus pesticides]
Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished report No. VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics, 6th ed.,
New York, Macmillan Publishing Company, pp. 100-119.
DHSS (1983) Pesticide poisoning: notes for the guidance of medical
practitioners. London, United Kingdom Department of Health and
Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning. Guy's
Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
 a Caution: if dimethoate is dissolved in solvents, e.g., petroleum solvents, vomiting may cause aspiration pneumonitis.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Dimethoate was evaluated by the Joint Meeting on Pesticide Residues
(JMPR) in 1963, 1965, 1966, 1967, 1970, 1973 (evaluation of the
related compound formathion), 1977, 1978, 1984, and 1987.
An acceptable daily intake (ADI) for man for dimethoate was estimated
at 0-0.01 mg/kg body weight per day in 1987.
A data sheet on dimethoate (No. 42) is available from the WHO Division
of Vector Biology and Control in their series "Data sheets on
pesticides" (WHO/FAO 1975-87).
In the WHO Recommended Classification of Pesticides by Hazard,
technical dimethoate has been classified as moderately hazardous, when
handled in accordance with instructions.
IRPTC has issued a review on dimethoate (No. 5) in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 24-26.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
Dimethoate is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents. Since 1986, the use of dimethoate in greenhouses and on
cherries and cabbage has been forbidden in the USSR.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified dimethoate (solid >73%; liquid >29%) in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport
The symbol on the label should be as follows:
a Caution: if dimethoate is dissolved in solvents, e.g., petroleum solvents, vomiting may cause aspiration pneumonitis.
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Dimethoate was evaluated by the Joint Meeting on Pesticide Residues
(JMPR) in 1963, 1965, 1966, 1967, 1970, 1973 (evaluation of the
related compound formathion), 1977, 1978, 1984, and 1987.
An acceptable daily intake (ADI) for man for dimethoate was estimated
at 0-0.01 mg/kg body weight per day in 1987.
A data sheet on dimethoate (No. 42) is available from the WHO Division
of Vector Biology and Control in their series "Data sheets on
pesticides" (WHO/FAO 1975-87).
In the WHO Recommended Classification of Pesticides by Hazard,
technical dimethoate has been classified as moderately hazardous, when
handled in accordance with instructions.
IRPTC has issued a review on dimethoate (No. 5) in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 24-26.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
7.3 Specific Restrictions
Dimethoate is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents. Since 1986, the use of dimethoate in greenhouses and on
cherries and cabbage has been forbidden in the USSR.
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classified dimethoate (solid >73%; liquid >29%) in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low
risk of poisoning in transport
The symbol on the label should be as follows:
 The International Maritime Organization lists dimethoate as a
pesticide of high hazard and as a poisonous substance at
concentrations above 5% (Hazard Class 6.1).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Bulgaria Maximum permissible concentration (MPC) 1971
- Time-weighted average (TWA) 1 mg/m3
Romania Maximum permissible concentration (MPC)
- Time-weighted average (TWA) 7 mg/m3
- Ceiling value 10 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.5 mg/m3 1977
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- 1 time per day 0.003 mg/m3
- Average per day 0.003 mg/m3
Preliminary safety limit
- 1 time per day 0.003 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.03 mg/litre
SOIL USSR Permissible limit 0.3 mg/kg
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.01 mg/kg 1987
body weight
USSR Acceptable daily intake (ADI) 0.02 mg/kg
body weight
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Brazil Acceptable limit (safety 0.02-2 mg/kg
interval: 1 - 28 days)
Czechoslovakia Maximum residue limit (MRL) 1-2 mg/kg 1978
European Maximum residue limit (MRL) 1 mg/kg 1982
Community
India Maximum tolerable concentration 2 mg/kg 1976
Japan Acceptable residue limit (ARL) 1 mg/kg
Kenya Maximum limit 1-2 mg/kg
Sweden Maximum tolerable concentration 0.22 mg/kg 1983
USSR Maximum residue limit (MRL) 0.1-0.4 mg/kg 1983
FOOD Plant Exports and Maximum residue limit (MRL) 0.5 mg/kg
imports by
CMEA
countries
FOOD Raw USA Acceptable residue limit (ARL) 0.002-2 mg/kg
agricultural
products
FOOD Animal USA Acceptable residue limit (ARL) 5 mg/kg
USSR Maximum residue limit (MRL) 2 mg/kg 1981
The WHO specifications for pesticides used in public health, in an
interim specification on dimethoate technical and emulsifiable
concentrate, require that the technical material shall consist of
dimethoate together with related manufacturing compounds and shall be
in the form of white/grey crystals free from extraneous impurities or
added modifying agents.
The emulsifiable concentrate shall consist of technical dimethoate
dissolved in suitable solvents with other necessary formulants added.
It shall be in the form of a stable liquid, free from suspended matter
and sediment. The technical dimethoate used in the manufacture of the
concentrate shall comply with the requirements of specification
WHO/IS/1.0094-1.
Chemical and physical requirements and the methods for checking them
are specified.
The material shall be packed in suitable, clean containers, as
specified in the order.
All packages shall bear, durably and legibly marked on the container,
the following:
Manufacturer's name
Technical dimethoate to Interim Specification WHO/IS/1.0094-1
Batch or reference number, and date of test
Net weight of contents
Date of manufacture
and, in the case of the emulsifiable concentrate:
Manufacturer's name
Dimethoate emulsifiable concentrate to Interim Specification
WHO/IS/3.0094-1
Dimethoate ... g/kg
Batch or reference number, and date of test
Net weight of contents
Instructions for dilution
Date of formulation
and the following minimum cautionary notice:
Dimethoate is an organophosphorus compound that inhibits
cholinesterase. It is poisonous if swallowed or inhaled. It
may be absorbed through the skin. Avoid skin contact: wear
protective gloves, clean protective clothing, and a respirator
when handling the material. Wash thoroughly with soap and water
after using.
Keep the material out of the reach of children and well away
from foodstuffs and animal feed and their containers.
If poisoning occurs, call a physician. Atropine and pralidoxime
are specific antidotes, and artificial respiration may be
needed.
The FAO specifications for plant protection products give similar
specifications and the methods for checking them.
The dimethoate content shall be declared (minimum 95% for the
technical product) and shall not differ from the declared percentage
by more than 2% for the technical product and 5-10% for its
formulations.
Containers shall be suitable, clean, dry, and as specified in the
order, and shall not adversely affect, or be affected by, the product,
but shall adequately protect it from external conditions. They shall
comply with pertinent national and international transport and safety
regulations.
Specifications for storage stability are given.
The European Community legislation requires labelling as a dangerous
substance using the symbol:
The International Maritime Organization lists dimethoate as a
pesticide of high hazard and as a poisonous substance at
concentrations above 5% (Hazard Class 6.1).
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Bulgaria Maximum permissible concentration (MPC) 1971
- Time-weighted average (TWA) 1 mg/m3
Romania Maximum permissible concentration (MPC)
- Time-weighted average (TWA) 7 mg/m3
- Ceiling value 10 mg/m3
USSR Maximum allowable concentration (MAC)
- Ceiling value 0.5 mg/m3 1977
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC) 1984
- 1 time per day 0.003 mg/m3
- Average per day 0.003 mg/m3
Preliminary safety limit
- 1 time per day 0.003 mg/m3
WATER Surface USSR Maximum allowable concentration (MAC) 0.03 mg/litre
SOIL USSR Permissible limit 0.3 mg/kg
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.01 mg/kg 1987
body weight
USSR Acceptable daily intake (ADI) 0.02 mg/kg
body weight
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Brazil Acceptable limit (safety 0.02-2 mg/kg
interval: 1 - 28 days)
Czechoslovakia Maximum residue limit (MRL) 1-2 mg/kg 1978
European Maximum residue limit (MRL) 1 mg/kg 1982
Community
India Maximum tolerable concentration 2 mg/kg 1976
Japan Acceptable residue limit (ARL) 1 mg/kg
Kenya Maximum limit 1-2 mg/kg
Sweden Maximum tolerable concentration 0.22 mg/kg 1983
USSR Maximum residue limit (MRL) 0.1-0.4 mg/kg 1983
FOOD Plant Exports and Maximum residue limit (MRL) 0.5 mg/kg
imports by
CMEA
countries
FOOD Raw USA Acceptable residue limit (ARL) 0.002-2 mg/kg
agricultural
products
FOOD Animal USA Acceptable residue limit (ARL) 5 mg/kg
USSR Maximum residue limit (MRL) 2 mg/kg 1981
The WHO specifications for pesticides used in public health, in an
interim specification on dimethoate technical and emulsifiable
concentrate, require that the technical material shall consist of
dimethoate together with related manufacturing compounds and shall be
in the form of white/grey crystals free from extraneous impurities or
added modifying agents.
The emulsifiable concentrate shall consist of technical dimethoate
dissolved in suitable solvents with other necessary formulants added.
It shall be in the form of a stable liquid, free from suspended matter
and sediment. The technical dimethoate used in the manufacture of the
concentrate shall comply with the requirements of specification
WHO/IS/1.0094-1.
Chemical and physical requirements and the methods for checking them
are specified.
The material shall be packed in suitable, clean containers, as
specified in the order.
All packages shall bear, durably and legibly marked on the container,
the following:
Manufacturer's name
Technical dimethoate to Interim Specification WHO/IS/1.0094-1
Batch or reference number, and date of test
Net weight of contents
Date of manufacture
and, in the case of the emulsifiable concentrate:
Manufacturer's name
Dimethoate emulsifiable concentrate to Interim Specification
WHO/IS/3.0094-1
Dimethoate ... g/kg
Batch or reference number, and date of test
Net weight of contents
Instructions for dilution
Date of formulation
and the following minimum cautionary notice:
Dimethoate is an organophosphorus compound that inhibits
cholinesterase. It is poisonous if swallowed or inhaled. It
may be absorbed through the skin. Avoid skin contact: wear
protective gloves, clean protective clothing, and a respirator
when handling the material. Wash thoroughly with soap and water
after using.
Keep the material out of the reach of children and well away
from foodstuffs and animal feed and their containers.
If poisoning occurs, call a physician. Atropine and pralidoxime
are specific antidotes, and artificial respiration may be
needed.
The FAO specifications for plant protection products give similar
specifications and the methods for checking them.
The dimethoate content shall be declared (minimum 95% for the
technical product) and shall not differ from the declared percentage
by more than 2% for the technical product and 5-10% for its
formulations.
Containers shall be suitable, clean, dry, and as specified in the
order, and shall not adversely affect, or be affected by, the product,
but shall adequately protect it from external conditions. They shall
comply with pertinent national and international transport and safety
regulations.
Specifications for storage stability are given.
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 The label must read:
harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuffs.
The European Community legislation on labelling of pesticide
preparations classifies dimethoate in Class II/b for the purpose of
determining the label for preparations containing dimethoate and other
active ingredients.
7.5 Waste Disposal
In the USA, any non-domestic waste containing dimethoate is considered
a hazardous waste and permits are required for its handling,
transport, treatment, storage, or disposal. Waste incinerators must
achieve 99.99% destruction and removal of this substance.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986a) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986b) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 3rd ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention and diagnosis of pesticide poisoning.
Geneva, World Health Organization (Unpublished report No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 14th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO (In press) EHC No. 90: Dimethoate. Geneva, World Health
Organization.
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX 1
TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus insecticides: a general introduction)
All cases of organophosphorus poisoning should be dealt with as an
emergency and the patient sent to hospital as quickly as possible.
Although symptoms may develop rapidly, delay in onset or a steady
increase in severity may be seen up to 48 h after ingestion of some
formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by organophosphorus
insecticides are given in several major references (Kagan, 1977;
Taylor, 1980; UK DHSS, 1983; Plestina, 1984) and will also be included
in the IPCS Health and Safety Guides to be prepared for selected
organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with alkaline
soap or with a sodium bicarbonate solution. Particular care should be
taken in cleaning the skin area where venepuncture is performed.
Blood might be contaminated with direct-acting organophosphorus
esters, and, therefore, inaccurate measures of ChE inhibition might
result. Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced, if
the patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. This treatment is, however,
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with addition of bicarbonate solution or
activated charcoal) can also be performed, particularly in unconscious
patients, taking care to prevent aspiration of fluids into the lungs
(i.e., only after a tracheal tube has been put in place).
The volume of fluid introduced into the stomach should be recorded and
samples of gastric lavage frozen and stored for subsequent chemical
analysis. If the formulation of the pesticide involved is available,
it should also be stored for further analysis (i.e., detection of
toxicologically relevant impurities). A purge to remove the ingested
compound can be administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be started at the
first sign of respiratory failure and maintained for as long as
necessary.
Cautious administration of fluids is advised, as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg i.v. and given at
15-30-min intervals. The dose and the frequency of atropine treatment
varies from case to case, but should maintain the patient fully
atropinized (dilated pupils, dry mouth, skin flushing, etc.).
Continuous infusion of atropine may be necessary in extreme cases and
total daily doses up to several hundred mg may be necessary during the
first few days of treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs. However, if absorption,
distribution, and metabolism are thought to be delayed for any
reasons, oximes can be administered for several days after
intoxication. Effective treatment with oximes reduces the required
dose of atropine. Pralidoxime is the most widely available oxime. A
dose of 1 g pralidoxime can be given either i.m. or i.v. and repeated
2-3 times per day or, in extreme cases, more often. If possible,
blood samples should be taken for AChE determinations before and
during treatment. Skin should be carefully cleansed before sampling.
Results of the assays should influence the decision whether to
continue oxime therapy after the first 2 days.
There are indications that oxime therapy may possibly have beneficial
effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the mildest
cases. Besides relieving anxiety, it appears to counteract some
aspects of CNS-derived symptoms, which are not affected by atropine.
Doses of 10 mg s.c. or i.v. are appropriate and may be repeated as
required (Vale & Scott, 1974). Other centrally acting drugs and drugs
that may depress respiration are not recommended in the absence of
artificial respiration procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of secretions
are the best guide to the effectiveness of atropinization. Although
repeated dosing may well be necessary, excessive doses at any one time
may cause toxic side-effects. Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be taken
into, and then released from, fat depots over a period of many days.
It is therefore quite incorrect to abandon oxime treatment after 1-2
days on the supposition that all inhibited enzyme will be aged.
Ecobichon et al. (1977) noted prompt improvement in both condition and
blood-ChEs in response to pralidoxime given on the 11th-15th days
after major symptoms of poisoning appeared due to extended exposure to
fenitrothion (a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in error,
while drunk. Therapeutic dosages were progressively increased up to
6 mg atropine i.v. every 15 min together with continuous i.v. infusion
of pralidoxime chloride at 0.5 g/h for 72 h, from days 3 to 6 after
intoxication. After considerable improvement, the patient relapsed
and further aggressive therapy was given at a declining rate from days
10 to 16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given and the
patient was discharged on the thirty-third day with no apparent
sequelae.
References to Annex 1
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-379.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus pesticides]
Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished report No. VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics, 6th ed.,
New York, Macmillan Publishing Company, pp. 100-119.
DHSS (1983) Pesticide poisoning: notes for the guidance of medical
practitioners. London, United Kingdom Department of Health and
Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning. Guy's
Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
The label must read:
harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuffs.
The European Community legislation on labelling of pesticide
preparations classifies dimethoate in Class II/b for the purpose of
determining the label for preparations containing dimethoate and other
active ingredients.
7.5 Waste Disposal
In the USA, any non-domestic waste containing dimethoate is considered
a hazardous waste and permits are required for its handling,
transport, treatment, storage, or disposal. Waste incinerators must
achieve 99.99% destruction and removal of this substance.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986a) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986b) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 3rd ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention and diagnosis of pesticide poisoning.
Geneva, World Health Organization (Unpublished report No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 14th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO (In press) EHC No. 90: Dimethoate. Geneva, World Health
Organization.
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX 1
TREATMENT OF ORGANOPHOSPHATE INSECTICIDE POISONING IN MAN
(From EHC 63: Organophosphorus insecticides: a general introduction)
All cases of organophosphorus poisoning should be dealt with as an
emergency and the patient sent to hospital as quickly as possible.
Although symptoms may develop rapidly, delay in onset or a steady
increase in severity may be seen up to 48 h after ingestion of some
formulated organophosphorus insecticides.
Extensive descriptions of treatment of poisoning by organophosphorus
insecticides are given in several major references (Kagan, 1977;
Taylor, 1980; UK DHSS, 1983; Plestina, 1984) and will also be included
in the IPCS Health and Safety Guides to be prepared for selected
organophosphorus insecticides.
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the Absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with alkaline
soap or with a sodium bicarbonate solution. Particular care should be
taken in cleaning the skin area where venepuncture is performed.
Blood might be contaminated with direct-acting organophosphorus
esters, and, therefore, inaccurate measures of ChE inhibition might
result. Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting might be induced, if
the patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. This treatment is, however,
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with addition of bicarbonate solution or
activated charcoal) can also be performed, particularly in unconscious
patients, taking care to prevent aspiration of fluids into the lungs
(i.e., only after a tracheal tube has been put in place).
The volume of fluid introduced into the stomach should be recorded and
samples of gastric lavage frozen and stored for subsequent chemical
analysis. If the formulation of the pesticide involved is available,
it should also be stored for further analysis (i.e., detection of
toxicologically relevant impurities). A purge to remove the ingested
compound can be administered.
I.2 General Supportive Treatment
Artificial respiration (via a tracheal tube) should be started at the
first sign of respiratory failure and maintained for as long as
necessary.
Cautious administration of fluids is advised, as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific Pharmacological Treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg i.v. and given at
15-30-min intervals. The dose and the frequency of atropine treatment
varies from case to case, but should maintain the patient fully
atropinized (dilated pupils, dry mouth, skin flushing, etc.).
Continuous infusion of atropine may be necessary in extreme cases and
total daily doses up to several hundred mg may be necessary during the
first few days of treatment.
I.3.2 Oxime reactivators
Cholinesterase reactivators (e.g., pralidoxime, obidoxime)
specifically restore AChE activity inhibited by organophosphates.
This is not the case with enzymes inhibited by carbamates. The
treatment should begin as soon as possible, because oximes are not
effective on "aged" phosphorylated ChEs. However, if absorption,
distribution, and metabolism are thought to be delayed for any
reasons, oximes can be administered for several days after
intoxication. Effective treatment with oximes reduces the required
dose of atropine. Pralidoxime is the most widely available oxime. A
dose of 1 g pralidoxime can be given either i.m. or i.v. and repeated
2-3 times per day or, in extreme cases, more often. If possible,
blood samples should be taken for AChE determinations before and
during treatment. Skin should be carefully cleansed before sampling.
Results of the assays should influence the decision whether to
continue oxime therapy after the first 2 days.
There are indications that oxime therapy may possibly have beneficial
effects on CNS-derived symptoms.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the mildest
cases. Besides relieving anxiety, it appears to counteract some
aspects of CNS-derived symptoms, which are not affected by atropine.
Doses of 10 mg s.c. or i.v. are appropriate and may be repeated as
required (Vale & Scott, 1974). Other centrally acting drugs and drugs
that may depress respiration are not recommended in the absence of
artificial respiration procedures.
I.3.4 Notes on the recommended treatment
I.3.4.1 Effects of atropine and oxime
The combined effect far exceeds the benefit of either drug singly.
I.3.4.2 Response to atropine
The response of the eye pupil may be unreliable in cases of
organophosphorus poisoning. A flushed skin and drying of secretions
are the best guide to the effectiveness of atropinization. Although
repeated dosing may well be necessary, excessive doses at any one time
may cause toxic side-effects. Pulse-rate should not exceed 120/min.
I.3.4.3 Persistence of treatment
Some organophosphorus pesticides are very lipophilic and may be taken
into, and then released from, fat depots over a period of many days.
It is therefore quite incorrect to abandon oxime treatment after 1-2
days on the supposition that all inhibited enzyme will be aged.
Ecobichon et al. (1977) noted prompt improvement in both condition and
blood-ChEs in response to pralidoxime given on the 11th-15th days
after major symptoms of poisoning appeared due to extended exposure to
fenitrothion (a dimethyl phosphate with a short half-life for aging of
inhibited AChE).
I.3.4.4 Dosage of atropine and oxime
The recommended doses above pertain to exposures, usually for an
occupational setting, but, in the case of very severe exposure or
massive ingestion (accidental or deliberate), the therapeutic doses
may be extended considerably. Warriner et al. (1977) reported the
case of a patient who drank a large quantity of dicrotophos, in error,
while drunk. Therapeutic dosages were progressively increased up to
6 mg atropine i.v. every 15 min together with continuous i.v. infusion
of pralidoxime chloride at 0.5 g/h for 72 h, from days 3 to 6 after
intoxication. After considerable improvement, the patient relapsed
and further aggressive therapy was given at a declining rate from days
10 to 16 (atropine) and to day 23 (oxime), respectively. In total,
92 g of pralidoxime chloride and 3912 mg of atropine were given and the
patient was discharged on the thirty-third day with no apparent
sequelae.
References to Annex 1
ECOBICHON, D.J., OZERE, R.L., REID, E., & CROCKER, J.F.S (1977)
Acute fenitrothion poisoning. Can. Med. Assoc. J., 116: 377-379.
KAGAN, JU.S. (1977) [Toxicology of organophosphorus pesticides]
Moscow, Meditsina, pp. 111-121, 219-233, 260-269 (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished report No. VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics, 6th ed.,
New York, Macmillan Publishing Company, pp. 100-119.
DHSS (1983) Pesticide poisoning: notes for the guidance of medical
practitioners. London, United Kingdom Department of Health and
Social Security, pp. 41-47.
VALE, J.A. & SCOTT, G.W. (1974) Organophosphorus poisoning. Guy's
Hosp. Rep., 123: 13-25.
WARRINER, R.A., III, NIES, A.S., & HAYES, W.J., Jr (1977) Severe
organophosphate poisoning complicated by alcohol and turpentine
ingestion. Arch. environ. Health, 32: 203-205.
