
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
PYRETHRINS
Chemical name
Pyrethrin I: Pyrethrolone ester of chrysanthemummonocarboxylic
acid.
Pyrethrin II: Pyrethrolone ester of chrysanthemumdicarboxylic
acid monomethyl ester.
Cinerin I: 3-(2-Butenyl)-4-methyl-2-oxo-3-cyclopenten-1-yl
ester of chrysanthemummonocarboxylic acid.
Cinerin II: 3-(2-Butenyl)-4-methyl-2-oxo-3-cyclopenten-1-yl
ester of chrysanthemumdicarboxylic acid monomethyl ester.
Synonyms
The active ingredients in pyrethrum extract consist of a
mixture of four compounds in approximately the following percentage:
pyrethrin I, 40%; pyrethrin II, 36%; cinerin I, 12%; and cinerin II,
12%. The current commercial product is a more or less purified form of
the mixture. Highly purified separate isomers although available for
research purposes are too expensive for practical use.
Empirical formula
Pyrethrin I C21H28O3 (mol. wt. 328.43)
Pyrethrin II C22H28O5 (mol. wt. 372.44)
Cinerin I C20H28O3 (mol. wt. 316.42)
Cinerin II C21H28O5 (mol. wt. 360.43)
Structural formula
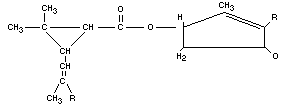 Compound R R'
Pyrethrin I - CH3 - CH2 - CH===CH - CH===CH2
Pyrethrin II - COOCH3 Same
Cinerin I - CH3 - CH2 - CH===CH - CH3
Cinerin II - COOCH3 Same
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are
soluble in many organic solvents, e.g. kerosene, carbon tetrachloride,
alcohol, petroleum, ether, etc. They are decomposed by exposure to
light with loss of insecticidal activity. They are also rapidly
oxidized and inactivated in air. Antioxidants used to protect
insecticidal residues of pyrethrins include pyrocatechol, pyrogallol,
hydroquinone, benzene-320-napthol is used to protect against effects
of sunlight. A more complete description of their physical and
chemical properties is contained in Negherbon (1959).
Uses
Very few uses remain for formulations of the pyrethrins alone.
Some "pyrethrins dusts" are still in use for control of a variety of
insects damaging horticultural crops. These dusts usually contain from
0.3% to 0.5% pyrethrins and are used at the rate of 25 to 50 pounds
per acre. No restrictions have been placed on frequency or timing of
use. Poultry are treated for flea and lice control with 0.5% dusts.
Household and stored product uses of pyrethrin solutions alone (at
0.25%) are for space sprays or direct contact spray. Since these
formulations have little residual effectiveness they are being
superceded by synergized formulations.
Residues
Since there have been no restrictions on use of pyrethrin
formulations alone, very little data are available on resulting
residues. With tolerance established in some countries for residues of
synergized formulations, residues of these formulations are currently
being studied anew.
Effect on treated crop
There are no known recent studies on the effect of pyrethrins
on the treated crop.
BIOLOGICAL DATA
Biochemical aspects
Following ingestion, the pyrethrins are hydrolysed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidally active compounds or their derivatives
are absorbed as shown by their toxicity and their effect on the liver.
The pyrethrins or their metabolites are not known to be stored in the
body or to be excreted in the milk, but no study of the matter has
employed modern methods. Absorption does result in urinary excretion
of chrysanthemummonocarboxylic acid (Audiffren, 1934). It is said that
the diarrhoea produced by pyrethrin results from central vagal
stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum oil
Rat Oral 200 Lehman, 1951
Rat Oral > 2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1930
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be
noted. The very marked difference in the oral and intravenous
toxicities indicates a low rate of absorption from the
gastro-intestinal tract, very efficient destruction by the liver, or a
combination of the two.
The acute effects resemble veratrine intoxication, proceeding
from excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally seen in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
No data available.
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (Lehman, 1965).
Man. Injury to man from pyrethrum has most frequently resulted
from the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in
man. Contact dermatitis is by far the most common. The usual picture
is a mild erythematous, vesicular dermatitis with papules in moist
areas, and intense pruritis (McCord et al., 1921; Sequeira, 1936). In
a few cases bullae appear (McCord et al., 1921, Sequeira, 1936;
Tonking, 1936). Oedema and cracking develop in severe cases (Sequeira,
1936; Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may
be made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity
similar to those seen in pollinosis, including sneezing, serous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).* Thus
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45% of
persons who are sensitive to ragweed (Feinberg, 1934) and was produced
by repeated application of pyrethrum ointment in 10% and 26% of
unselected test populations (Lord & Johnson, 1947). On the other hand,
the insecticide has been considered so innocuous that an ointment
containing 0.75% of pyrethrin was recommended for treatment of
scabies, and such use led to only a few cases of dermatitis, some of
doubtful relation to the treatment (Sweitzer & Tedder, 1935; Sweitzer,
1936). Pyrethrins have been used extensively for the control of human
body lice. The formulation used early during World War II was called
MYL powder; its use was discontinued only after the more effective and
long-lasting DDT louse powder became available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal
worms. It is possible to use pyrethrins for short periods in this way
because a considerable period, even two or three years, may be
required for susceptibility to appear (Sequeira, 1936; Tonking, 1936;
Martin & Hester, 1941; Schwartz, 1934). Onset may be delayed even when
exposure is to a purified ointment (Lord & Johnson, 1947; Sweitzer,
1936). This argues against the unsupported contention (Sequeira, 1936)
that the dermatitis is usually the result of irritation rather than
allergy. It is generally recognized that susceptibility is increased
during summer months or periods of excessive perspiration (McCord et
al., 1921; Sequeira, 1956; Tonking, 1936; Martin & Hester, 1941). The
fact that pyrethrum insecticides can be tolerated for brief periods is
not a justification for recommending frequent, repeated exposure to
them over a period of years.
* Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be absorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941, Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
Comment on experimental work reported and evaluation
Because of the long experience in using pyrethrum without
observed injury, except allergy in those with occupational and
therapeutic contact, there is no reason to question the customary uses
of the material. The rapid metabolism and apparent lack of storage are
also reassuring. On the other hand, no long-term study has been made
of the synergized formulations now in current use. At the present time
an acceptable daily intake for man cannot be suggested.
Further work required
Long-term studies should be made in at least one more species
with special emphasis on the effect on the liver. Effects on the rat
liver should be re-evaluated. The tests should include chemically
identified commercial pyrethrin concentrates alone and combined with
major synergists. The metabolism of pyrethrins should be explored in
greater detail.
REFERENCES
Ambrose, A. M. & Robbins, D. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P. Weil, C. S., Pozzani, U. C. & Smith, H. F. (1950)
Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier, J. & Ripert, J. (1927) Compt. rend. Accad. d. sc., 184,
776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1965) Summaries of pesticide toxicity (in press)
Leonard, C. S. (1942) J. econ. Ent., 35, 261
Lord, J. T. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P. Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Negherbon, W. (1959) Insecticides, Handbook of Toxicology W. B.
Saunders, Philadelphia, Vol. 3, pp. 1-854
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 43, 473
Shimkin, N. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Simmons, S. W. (1959) DDT, the insecticide dichlorodiphenyl-
trichloroethane and its significance, Paul Mull, Ed., Birkhauser
Verlag Basel, Vol. II, pp. 251-502
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
Compound R R'
Pyrethrin I - CH3 - CH2 - CH===CH - CH===CH2
Pyrethrin II - COOCH3 Same
Cinerin I - CH3 - CH2 - CH===CH - CH3
Cinerin II - COOCH3 Same
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are
soluble in many organic solvents, e.g. kerosene, carbon tetrachloride,
alcohol, petroleum, ether, etc. They are decomposed by exposure to
light with loss of insecticidal activity. They are also rapidly
oxidized and inactivated in air. Antioxidants used to protect
insecticidal residues of pyrethrins include pyrocatechol, pyrogallol,
hydroquinone, benzene-320-napthol is used to protect against effects
of sunlight. A more complete description of their physical and
chemical properties is contained in Negherbon (1959).
Uses
Very few uses remain for formulations of the pyrethrins alone.
Some "pyrethrins dusts" are still in use for control of a variety of
insects damaging horticultural crops. These dusts usually contain from
0.3% to 0.5% pyrethrins and are used at the rate of 25 to 50 pounds
per acre. No restrictions have been placed on frequency or timing of
use. Poultry are treated for flea and lice control with 0.5% dusts.
Household and stored product uses of pyrethrin solutions alone (at
0.25%) are for space sprays or direct contact spray. Since these
formulations have little residual effectiveness they are being
superceded by synergized formulations.
Residues
Since there have been no restrictions on use of pyrethrin
formulations alone, very little data are available on resulting
residues. With tolerance established in some countries for residues of
synergized formulations, residues of these formulations are currently
being studied anew.
Effect on treated crop
There are no known recent studies on the effect of pyrethrins
on the treated crop.
BIOLOGICAL DATA
Biochemical aspects
Following ingestion, the pyrethrins are hydrolysed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidally active compounds or their derivatives
are absorbed as shown by their toxicity and their effect on the liver.
The pyrethrins or their metabolites are not known to be stored in the
body or to be excreted in the milk, but no study of the matter has
employed modern methods. Absorption does result in urinary excretion
of chrysanthemummonocarboxylic acid (Audiffren, 1934). It is said that
the diarrhoea produced by pyrethrin results from central vagal
stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum oil
Rat Oral 200 Lehman, 1951
Rat Oral > 2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1930
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be
noted. The very marked difference in the oral and intravenous
toxicities indicates a low rate of absorption from the
gastro-intestinal tract, very efficient destruction by the liver, or a
combination of the two.
The acute effects resemble veratrine intoxication, proceeding
from excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally seen in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
No data available.
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (Lehman, 1965).
Man. Injury to man from pyrethrum has most frequently resulted
from the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in
man. Contact dermatitis is by far the most common. The usual picture
is a mild erythematous, vesicular dermatitis with papules in moist
areas, and intense pruritis (McCord et al., 1921; Sequeira, 1936). In
a few cases bullae appear (McCord et al., 1921, Sequeira, 1936;
Tonking, 1936). Oedema and cracking develop in severe cases (Sequeira,
1936; Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may
be made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity
similar to those seen in pollinosis, including sneezing, serous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).* Thus
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45% of
persons who are sensitive to ragweed (Feinberg, 1934) and was produced
by repeated application of pyrethrum ointment in 10% and 26% of
unselected test populations (Lord & Johnson, 1947). On the other hand,
the insecticide has been considered so innocuous that an ointment
containing 0.75% of pyrethrin was recommended for treatment of
scabies, and such use led to only a few cases of dermatitis, some of
doubtful relation to the treatment (Sweitzer & Tedder, 1935; Sweitzer,
1936). Pyrethrins have been used extensively for the control of human
body lice. The formulation used early during World War II was called
MYL powder; its use was discontinued only after the more effective and
long-lasting DDT louse powder became available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal
worms. It is possible to use pyrethrins for short periods in this way
because a considerable period, even two or three years, may be
required for susceptibility to appear (Sequeira, 1936; Tonking, 1936;
Martin & Hester, 1941; Schwartz, 1934). Onset may be delayed even when
exposure is to a purified ointment (Lord & Johnson, 1947; Sweitzer,
1936). This argues against the unsupported contention (Sequeira, 1936)
that the dermatitis is usually the result of irritation rather than
allergy. It is generally recognized that susceptibility is increased
during summer months or periods of excessive perspiration (McCord et
al., 1921; Sequeira, 1956; Tonking, 1936; Martin & Hester, 1941). The
fact that pyrethrum insecticides can be tolerated for brief periods is
not a justification for recommending frequent, repeated exposure to
them over a period of years.
* Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be absorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941, Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
Comment on experimental work reported and evaluation
Because of the long experience in using pyrethrum without
observed injury, except allergy in those with occupational and
therapeutic contact, there is no reason to question the customary uses
of the material. The rapid metabolism and apparent lack of storage are
also reassuring. On the other hand, no long-term study has been made
of the synergized formulations now in current use. At the present time
an acceptable daily intake for man cannot be suggested.
Further work required
Long-term studies should be made in at least one more species
with special emphasis on the effect on the liver. Effects on the rat
liver should be re-evaluated. The tests should include chemically
identified commercial pyrethrin concentrates alone and combined with
major synergists. The metabolism of pyrethrins should be explored in
greater detail.
REFERENCES
Ambrose, A. M. & Robbins, D. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P. Weil, C. S., Pozzani, U. C. & Smith, H. F. (1950)
Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier, J. & Ripert, J. (1927) Compt. rend. Accad. d. sc., 184,
776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1965) Summaries of pesticide toxicity (in press)
Leonard, C. S. (1942) J. econ. Ent., 35, 261
Lord, J. T. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P. Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Negherbon, W. (1959) Insecticides, Handbook of Toxicology W. B.
Saunders, Philadelphia, Vol. 3, pp. 1-854
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 43, 473
Shimkin, N. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Simmons, S. W. (1959) DDT, the insecticide dichlorodiphenyl-
trichloroethane and its significance, Paul Mull, Ed., Birkhauser
Verlag Basel, Vol. II, pp. 251-502
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
 Compound R R'
Pyrethrin I - CH3 - CH2 - CH===CH - CH===CH2
Pyrethrin II - COOCH3 Same
Cinerin I - CH3 - CH2 - CH===CH - CH3
Cinerin II - COOCH3 Same
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are
soluble in many organic solvents, e.g. kerosene, carbon tetrachloride,
alcohol, petroleum, ether, etc. They are decomposed by exposure to
light with loss of insecticidal activity. They are also rapidly
oxidized and inactivated in air. Antioxidants used to protect
insecticidal residues of pyrethrins include pyrocatechol, pyrogallol,
hydroquinone, benzene-320-napthol is used to protect against effects
of sunlight. A more complete description of their physical and
chemical properties is contained in Negherbon (1959).
Uses
Very few uses remain for formulations of the pyrethrins alone.
Some "pyrethrins dusts" are still in use for control of a variety of
insects damaging horticultural crops. These dusts usually contain from
0.3% to 0.5% pyrethrins and are used at the rate of 25 to 50 pounds
per acre. No restrictions have been placed on frequency or timing of
use. Poultry are treated for flea and lice control with 0.5% dusts.
Household and stored product uses of pyrethrin solutions alone (at
0.25%) are for space sprays or direct contact spray. Since these
formulations have little residual effectiveness they are being
superceded by synergized formulations.
Residues
Since there have been no restrictions on use of pyrethrin
formulations alone, very little data are available on resulting
residues. With tolerance established in some countries for residues of
synergized formulations, residues of these formulations are currently
being studied anew.
Effect on treated crop
There are no known recent studies on the effect of pyrethrins
on the treated crop.
BIOLOGICAL DATA
Biochemical aspects
Following ingestion, the pyrethrins are hydrolysed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidally active compounds or their derivatives
are absorbed as shown by their toxicity and their effect on the liver.
The pyrethrins or their metabolites are not known to be stored in the
body or to be excreted in the milk, but no study of the matter has
employed modern methods. Absorption does result in urinary excretion
of chrysanthemummonocarboxylic acid (Audiffren, 1934). It is said that
the diarrhoea produced by pyrethrin results from central vagal
stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum oil
Rat Oral 200 Lehman, 1951
Rat Oral > 2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1930
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be
noted. The very marked difference in the oral and intravenous
toxicities indicates a low rate of absorption from the
gastro-intestinal tract, very efficient destruction by the liver, or a
combination of the two.
The acute effects resemble veratrine intoxication, proceeding
from excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally seen in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
No data available.
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (Lehman, 1965).
Man. Injury to man from pyrethrum has most frequently resulted
from the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in
man. Contact dermatitis is by far the most common. The usual picture
is a mild erythematous, vesicular dermatitis with papules in moist
areas, and intense pruritis (McCord et al., 1921; Sequeira, 1936). In
a few cases bullae appear (McCord et al., 1921, Sequeira, 1936;
Tonking, 1936). Oedema and cracking develop in severe cases (Sequeira,
1936; Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may
be made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity
similar to those seen in pollinosis, including sneezing, serous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).* Thus
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45% of
persons who are sensitive to ragweed (Feinberg, 1934) and was produced
by repeated application of pyrethrum ointment in 10% and 26% of
unselected test populations (Lord & Johnson, 1947). On the other hand,
the insecticide has been considered so innocuous that an ointment
containing 0.75% of pyrethrin was recommended for treatment of
scabies, and such use led to only a few cases of dermatitis, some of
doubtful relation to the treatment (Sweitzer & Tedder, 1935; Sweitzer,
1936). Pyrethrins have been used extensively for the control of human
body lice. The formulation used early during World War II was called
MYL powder; its use was discontinued only after the more effective and
long-lasting DDT louse powder became available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal
worms. It is possible to use pyrethrins for short periods in this way
because a considerable period, even two or three years, may be
required for susceptibility to appear (Sequeira, 1936; Tonking, 1936;
Martin & Hester, 1941; Schwartz, 1934). Onset may be delayed even when
exposure is to a purified ointment (Lord & Johnson, 1947; Sweitzer,
1936). This argues against the unsupported contention (Sequeira, 1936)
that the dermatitis is usually the result of irritation rather than
allergy. It is generally recognized that susceptibility is increased
during summer months or periods of excessive perspiration (McCord et
al., 1921; Sequeira, 1956; Tonking, 1936; Martin & Hester, 1941). The
fact that pyrethrum insecticides can be tolerated for brief periods is
not a justification for recommending frequent, repeated exposure to
them over a period of years.
* Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be absorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941, Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
Comment on experimental work reported and evaluation
Because of the long experience in using pyrethrum without
observed injury, except allergy in those with occupational and
therapeutic contact, there is no reason to question the customary uses
of the material. The rapid metabolism and apparent lack of storage are
also reassuring. On the other hand, no long-term study has been made
of the synergized formulations now in current use. At the present time
an acceptable daily intake for man cannot be suggested.
Further work required
Long-term studies should be made in at least one more species
with special emphasis on the effect on the liver. Effects on the rat
liver should be re-evaluated. The tests should include chemically
identified commercial pyrethrin concentrates alone and combined with
major synergists. The metabolism of pyrethrins should be explored in
greater detail.
REFERENCES
Ambrose, A. M. & Robbins, D. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P. Weil, C. S., Pozzani, U. C. & Smith, H. F. (1950)
Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier, J. & Ripert, J. (1927) Compt. rend. Accad. d. sc., 184,
776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1965) Summaries of pesticide toxicity (in press)
Leonard, C. S. (1942) J. econ. Ent., 35, 261
Lord, J. T. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P. Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Negherbon, W. (1959) Insecticides, Handbook of Toxicology W. B.
Saunders, Philadelphia, Vol. 3, pp. 1-854
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 43, 473
Shimkin, N. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Simmons, S. W. (1959) DDT, the insecticide dichlorodiphenyl-
trichloroethane and its significance, Paul Mull, Ed., Birkhauser
Verlag Basel, Vol. II, pp. 251-502
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
Compound R R'
Pyrethrin I - CH3 - CH2 - CH===CH - CH===CH2
Pyrethrin II - COOCH3 Same
Cinerin I - CH3 - CH2 - CH===CH - CH3
Cinerin II - COOCH3 Same
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are
soluble in many organic solvents, e.g. kerosene, carbon tetrachloride,
alcohol, petroleum, ether, etc. They are decomposed by exposure to
light with loss of insecticidal activity. They are also rapidly
oxidized and inactivated in air. Antioxidants used to protect
insecticidal residues of pyrethrins include pyrocatechol, pyrogallol,
hydroquinone, benzene-320-napthol is used to protect against effects
of sunlight. A more complete description of their physical and
chemical properties is contained in Negherbon (1959).
Uses
Very few uses remain for formulations of the pyrethrins alone.
Some "pyrethrins dusts" are still in use for control of a variety of
insects damaging horticultural crops. These dusts usually contain from
0.3% to 0.5% pyrethrins and are used at the rate of 25 to 50 pounds
per acre. No restrictions have been placed on frequency or timing of
use. Poultry are treated for flea and lice control with 0.5% dusts.
Household and stored product uses of pyrethrin solutions alone (at
0.25%) are for space sprays or direct contact spray. Since these
formulations have little residual effectiveness they are being
superceded by synergized formulations.
Residues
Since there have been no restrictions on use of pyrethrin
formulations alone, very little data are available on resulting
residues. With tolerance established in some countries for residues of
synergized formulations, residues of these formulations are currently
being studied anew.
Effect on treated crop
There are no known recent studies on the effect of pyrethrins
on the treated crop.
BIOLOGICAL DATA
Biochemical aspects
Following ingestion, the pyrethrins are hydrolysed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidally active compounds or their derivatives
are absorbed as shown by their toxicity and their effect on the liver.
The pyrethrins or their metabolites are not known to be stored in the
body or to be excreted in the milk, but no study of the matter has
employed modern methods. Absorption does result in urinary excretion
of chrysanthemummonocarboxylic acid (Audiffren, 1934). It is said that
the diarrhoea produced by pyrethrin results from central vagal
stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum oil
Rat Oral 200 Lehman, 1951
Rat Oral > 2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1930
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be
noted. The very marked difference in the oral and intravenous
toxicities indicates a low rate of absorption from the
gastro-intestinal tract, very efficient destruction by the liver, or a
combination of the two.
The acute effects resemble veratrine intoxication, proceeding
from excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally seen in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
No data available.
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (Lehman, 1965).
Man. Injury to man from pyrethrum has most frequently resulted
from the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in
man. Contact dermatitis is by far the most common. The usual picture
is a mild erythematous, vesicular dermatitis with papules in moist
areas, and intense pruritis (McCord et al., 1921; Sequeira, 1936). In
a few cases bullae appear (McCord et al., 1921, Sequeira, 1936;
Tonking, 1936). Oedema and cracking develop in severe cases (Sequeira,
1936; Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may
be made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity
similar to those seen in pollinosis, including sneezing, serous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).* Thus
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45% of
persons who are sensitive to ragweed (Feinberg, 1934) and was produced
by repeated application of pyrethrum ointment in 10% and 26% of
unselected test populations (Lord & Johnson, 1947). On the other hand,
the insecticide has been considered so innocuous that an ointment
containing 0.75% of pyrethrin was recommended for treatment of
scabies, and such use led to only a few cases of dermatitis, some of
doubtful relation to the treatment (Sweitzer & Tedder, 1935; Sweitzer,
1936). Pyrethrins have been used extensively for the control of human
body lice. The formulation used early during World War II was called
MYL powder; its use was discontinued only after the more effective and
long-lasting DDT louse powder became available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal
worms. It is possible to use pyrethrins for short periods in this way
because a considerable period, even two or three years, may be
required for susceptibility to appear (Sequeira, 1936; Tonking, 1936;
Martin & Hester, 1941; Schwartz, 1934). Onset may be delayed even when
exposure is to a purified ointment (Lord & Johnson, 1947; Sweitzer,
1936). This argues against the unsupported contention (Sequeira, 1936)
that the dermatitis is usually the result of irritation rather than
allergy. It is generally recognized that susceptibility is increased
during summer months or periods of excessive perspiration (McCord et
al., 1921; Sequeira, 1956; Tonking, 1936; Martin & Hester, 1941). The
fact that pyrethrum insecticides can be tolerated for brief periods is
not a justification for recommending frequent, repeated exposure to
them over a period of years.
* Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be absorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941, Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
Comment on experimental work reported and evaluation
Because of the long experience in using pyrethrum without
observed injury, except allergy in those with occupational and
therapeutic contact, there is no reason to question the customary uses
of the material. The rapid metabolism and apparent lack of storage are
also reassuring. On the other hand, no long-term study has been made
of the synergized formulations now in current use. At the present time
an acceptable daily intake for man cannot be suggested.
Further work required
Long-term studies should be made in at least one more species
with special emphasis on the effect on the liver. Effects on the rat
liver should be re-evaluated. The tests should include chemically
identified commercial pyrethrin concentrates alone and combined with
major synergists. The metabolism of pyrethrins should be explored in
greater detail.
REFERENCES
Ambrose, A. M. & Robbins, D. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P. Weil, C. S., Pozzani, U. C. & Smith, H. F. (1950)
Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier, J. & Ripert, J. (1927) Compt. rend. Accad. d. sc., 184,
776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1965) Summaries of pesticide toxicity (in press)
Leonard, C. S. (1942) J. econ. Ent., 35, 261
Lord, J. T. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P. Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Negherbon, W. (1959) Insecticides, Handbook of Toxicology W. B.
Saunders, Philadelphia, Vol. 3, pp. 1-854
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 43, 473
Shimkin, N. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Simmons, S. W. (1959) DDT, the insecticide dichlorodiphenyl-
trichloroethane and its significance, Paul Mull, Ed., Birkhauser
Verlag Basel, Vol. II, pp. 251-502
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
