
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
PYRETHRINS
IDENTITY
Explanatory note
In this report the term "pyrethrins" refers generally to the mixed
active ingredients as present in commercially available extracts of
pyrethrum. Such extracts contain about 75 per cent of pyrethrin I and
pyrethrin II, together with at least four other active ingredients
(i.e. Cinerin I, Cinerin II, Jasmolin I and Jasmolin II).
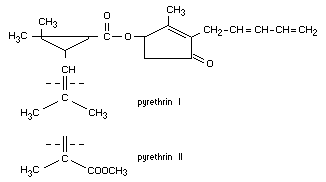
Chemical names
pyrethrin I
4-hydroxy-3-methyl-2-(2,4-pentadienyl)-2-cyclopenten-1-one
2,2-dimethyl-3-(2-methyl-propenyl)cyclopropanecarboxylate or
pyrethrolone ester of chrysanthemummonocarboxylic acid
pyrethrin II
4-hydroxy-3-methyl-2-(2,4-pentadienyl)-2-cyclopenten-1-one
1-methyl-3-carboxy- ,2,2-trimethylcyclopropaneacrylate ester or
pyrethrelolone ester of chrysanthemumdicarboxylic acid
monomethyl ester
Formula
 Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are soluble in
many organic solvents, e.g. kerosene, carbon tetrachloride, alcohol,
petroleum, ether, etc. They are decomposed by exposure to light with
loss of insecticidal activity. They are also rapidly oxidized and
inactivated in air. Antioxidants used to protect insecticidal residues
of pyrethrins include pyrocatechol, pyrogallol, hydroquinone;
benzene-320-napthol is used to protect against effects of sunlight.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Following ingestion, the pyrethrins are hydrolyzed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidal compounds or their derivatives are
absorbed as shown by their toxicity and their effect on the liver. The
pyrethrins or their metabolites are not known to be stored in the body
or to be excreted in the milk, but no modern methods have been
employed in the study of this aspect. Absorption does result in
urinary excretion of chrysanthemummonocarboxylic acid (Audiffren,
1934). It is believed that the diarrhoea produced by pyrethrin results
from central vagal stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum
oil
Rat Oral 200 Lehman, 1951
Rat Oral >2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1950
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be noted.
The very marked difference in the oral and intravenous toxicities
indicates a low rate of absorption from the gastro-intestinal tract,
very efficient destruction by the liver, or a combination of the two.
The acute effects resemble veratrine intoxication, proceeding from
excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally been in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
Man. Injury to man from pyrethrum has most frequently resulted from
the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in man.
Contact dermatitis is by far the most common. The usual picture is a
mild erythematous, vesicular dermatitis with papules in moist areas,
and intense pruritis (McCord et al., 1921; Sequeira, 1936). In a few
cases bullae appear (McCord et al., 1921; Sequeira, 1936; Tonking,
1936). Oedema and cracking develop in severe cases (Sequeira, 1936;
Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may be
made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity similar
to those seen in pollinosis, including sneezing, porous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).1 Thus,
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45 per cent
of persons who are sensitive to ragweed (Feinberg, 1954) and was
1 Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be adsorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941; Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
produced by repeated application of pyrethrum ointment in 10 per cent
and 26 per cent of unselected test populations (Lord & Johnson, 1947).
On the other hand, the insecticide has been considered so innocuous
that an ointment containing 0.75 per cent of pyrethrin was recommended
for treatment of scabies, and such use led to only a few cases of
dermatitis, some of doubtful relation to the treatment (Sweitzer &
Tedder, 1935; Sweitzer, 1936). Pyrethrins have been used extensively
for the control of human body lice. The formulation used early during
World War II was called MYL powder; its use was discontinued only
after the more effective and long-lasting DDT louse powder became
available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal worms.
It is possible to use pyrethrins for short periods in this way because
a considerable period, even two or three years, may be required for
susceptibility to appear (Sequeira, 1936; Tonking, 1936; Martin &
Hester, 1941, Schwartz, 1934). Onset may be delayed even when exposure
is to a purified ointment (Lord & Johnson, 1947; Sweitzer, 1936). This
argues against the unsupported contention (Sequeira, 1936) that the
dermatitis is usually the result of irritation rather than allergy. It
is generally recognized that susceptibility is increased during summer
months or periods of excessive perspiration (McCord et al., 1921;
Sequeira, 1936; Tonking, 1936; Martin & Hester, 1941).
Recent work confirmed that the degree of purity influences the
allergenic activity. In a study of 106 patients, all of whom had shown
positive reactions to ragweed or unrefined pyrethrin, no definite
allergic reactions to purified pyrethrins were observed (Zucker,
1965).
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (U.S. Food and Drug Administration,
1951).
Comments
Because of the long experience in using pyrethrum without observed
injury, except allergy in those with occupational and therapeutic
contact, there is little reason to question the customary uses of the
material. The fact that pyrethrum insecticides can be tolerated for
brief periods is, however, not a justification for recommending
frequent, repeated exposure to them over a period of years. Although
the rapid metabolism and apparent lack of storage are reassuring no
adequate study has been made of the synergized formulations now in
current use.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 200 ppm in the diet equivalent to 10 mg/kg/day
Estimate of temporary acceptable daily intake for man
0-0.4 mg/kg body-weight
Further work required
Short-term toxicity studies in several more species, including the dog
(at least a one-year study), with special emphasis on the effect on
the liver, and including the metabolism of pyrethrins in detail. These
studies should include chemically identified pyrethrin concentrates
alone and combined with major synergists, especially piperonyl
butoxide.
Result of the above work should be made available not later than 3
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments.
Low acute oral toxicity to mammals and rapid decomposition when
exposed to light and air allow pyrethrins to be used for controlling
insects on growling plants just before harvest and on dairy and meat
animals. They have been used in many countries on growing bush and
vine fruits, deciduous fruits and nuts, forage crops and vegetables,
and or, dairy and meat animals. Pyrethrins are usually used in
combination with a synergist. Piperonyl butoxide is the most common
synergist: piperonyl cyclonone, MGK-264(R), and sulfoxide have also
been used. For pre-harvest use the proportions of pyrethrins and
synergist may vary from 1 to 2, to 1 to 10, respectively. For certain
specific purposes pyrethrins sometimes are used without a synergist.
Because of the rapid decomposition of the insecticide, no limitations
have been set on the pre-harvest use of pyrethrins out doors.
(b) Post-harvest treatments
Pyrethrins have also been used in a spray or dust formulation on
freshly picked fruits and vegetables while in the field, in storage,
or in processing plants for the control of drosophila and other
insects. It is also used directly on dried fruit, tree-nuts, grains,
and oil seeds as a protective treatment against insect infestation
during storage. The pyrethrins are formulated in a fixed 1 to 10 ratio
with piperonyl butoxide for application as sprays or dusts directly on
the commodities as they are placed in containers or as they move on a
conveyor into a storage box, bin, or warehouse. Water emulsion or
wettable powder formulations of pyrethrins (in a 1 to 10 ratio with
piperonyl butoxide in the US and certain other countries) are used as
surface sprays on stacks of bagged peanuts and other oil seeds, and on
animal feeds. Aerosol formulations of pyrethrins are commonly used as
space treatments in food handling, processing, and storage facilities,
The use of pyrethrins for the above purposes has diminished
considerably in the past 10 years, being replaced by malathion in many
of the treatments.
(c) Other uses
Pyrethrins in combination with piperonyl butoxide, applied as a
repellent to the outside surface of multiwall paper bags, have been
found to be effective in protecting packaged cereal products against
outside insects. In the USA, such bags are quite widely used for
cereal products destined for storage or overseas shipment.
Pyrethrins are also used as a premises treatment for the control or
prevention of insect infestations in agricultural buildings and food
processing, handling, shipping, storage and marketing facilities. For
this purpose the pyrethrins are applied usually in combination with a
synergist as surface sprays to the floors, walls, working areas, and
machinery; as an aerosol space treatment in the buildings, or both.
Usually the insecticide is not applied directly to exposed foods and
food is not placed directly on freshly treated surfaces. Malathion has
replaced pyrethrins in many of the above uses.
Pyrethrins are also a common insecticide used for the control of
house-flies and other insects of public health and in the household.
Tolerances (established or considered)
Country Product Parts per million
Brazil Cereals 3
Canada Oats, Sorghum 1
Other grains 3
Czechoslovakia Grain -
Cereals 2
Finland Cereals exempt
Germany Grain 3
Tolerances (cont'd)
Country Product Parts per million
India Cereals exempt
Italy Cereals 2
Kenya Cereals exempt
Netherlands Cereals 3
Switzerland Cereals exempt
Turkey Cereals 3
USA Bush and vine fruits exempt
Cotton seed (post-harvest) 1
Deciduous fruits and nuts exempt
Flax-seed (post-harvest) 1
Forage crops exempt
Fruits and nuts (post-harvest) 1
Grains (post-harvest) 3
Mushrooms exempt
Peanuts (post-harvest) 1
Vegetables exempt
Vegetables (post-harvest) 1
Residues resulting from supervised trials
On growing crops pyrethrins deteriorate so rapidly that no attention
has been given to measuring residues resulting from such use. In most
countries they have been exempt from use regulations because of their
low acute oral toxicity and rapid deterioration. Most of the
information on residues has been obtained from applications to
harvested commodities. Pyrethrins, usually in combination with
piperonyl butoxide at about 1:10 w/w, are applied to grain as a
protective treatment at a dosage rate of pyrethrins of 1.42 ppm on
wheat, 1.52 ppm on shelled corn, 2.67 ppm on oats, 1.78 ppm on barley,
1.52 ppm on rye, and 1.9 ppm on rough rice. Some of the insecticide is
lost during application, and the deterioration is rapid during the
first few months after storage.
Flour exposed to two space treatments of synergized pyrethrins applied
at the rate of two and four ounces of 0.4 per cent pyrethrins per 1000
cubic feet produced less than 1 ppm residue in the top 1-1/2 inches
(Unpublished document supplied by US Department of Agriculture).
Bagged dried citrus pulp animal feed was exposed to weekly treatments
over a three-month period to aerosol formulations containing 0.2 per
cent of pyrethrins applied at the rate of 54 mg/m2. The maximum
pyrethrins residue during the entire period was less than 1 ppm.
Following a 15-month storage period in multiwall paper bags with
special insect-tight closures and pyrethrins and piperonyl butoxide,
the residues of pyrethrins in rice, non-fat dried milk, dried beans
and flour were considerably less than 1 ppm (Unpublished information
from US Department of Agriculture). A similar test involving cornmeal
stored for six months in treated bags showed maximum pyrethrins
residues of 1.07 ppm in 50 lb bags and 0.33 ppm in 100 lb bags
(Laudani et al., 1966).
Residues in food moving in commerce
1.7 ppm of pyrethrins were found on a shipment of grain known to have
been treated before shipment to Britain. 0.1 ppm was the highest
amongst 99 samples taken in Rotterdam and Amsterdam.
Residues at the time of consumption
At the time of preparation of this report no data were available on
the fate of residues of pyrethrins on or in fresh fruit, dried fruit,
tree-nuts, fresh and dried vegetables, and oils. Because of the rapid
deterioration of this insecticide when exposed to light and air, very
little attention has been given to its fate when applied before
harvesting. Some information is available however on the fate of
pyrethrins when applied to grain. Controlled studies showed that a
theoretical deposit on wheat of 1.32 ppm was down to 0.56 ppm in two
months and 0.36 ppm in four months after treatment. A theoretical 1.74
ppm of pyrethrins applied in combination with piperonyl butoxide (1:10
ratio) was down to 0.3 ppm after two months of storage. Other studies
showed that about 50 per cent of the pyrethrins residue on wheat had
disappeared with four to five months, most of the remaining
insecticide went into the screenings and scourings when the treated
grain was milled and only a very small percentage of the pyrethrins
passed into the flour. (Information direct from US Department of
Agriculture).
Methods of residue analysis
Pyrethrins, being a group of insecticidally active esters, are
difficult to detect in their entirety by any single analytical method.
New chromatographic methods look promising and may be perfected if
adequate clean-up methods can be developed. Further research is needed
to perfect methods suitable for detecting down to 0.01 ppm of the
principal esters of pyrethrins. In the meantime, the method of Jones,
Ackerman & Webster (1952) as proposed for residues of piperonyl
butoxide, with the appropriate clean-up procedures for the various
foods, can be used as an index of pyrethrin levels when the exact
ratio of the pyrethrins and the piperonyl butoxide is known; piperonyl
butoxide residues are more stable than those of pyrethrins. This
method has a sensitivity of 0.1 ppm of piperonyl butoxide.
RECOMMENDATIONS FOR TOLERANCES
The available data indicate that 50 per cent or more of the pyrethrins
applied to stored grain disappear during the first three or four
months of storage. At least 80 per cent of the remaining residue
normally is removed by handling, processing and cooking. However,
sufficient information is not available on the fate of pyrethrins
residues obtained from good agricultural practices on fresh fruit,
dried fruit, tree-nuts, dried vegetables and oil seeds. Therefore,
it is recommended that the following temporary tolerances be
established.
Cereals - 3 ppm
Cereal products - 1 ppm
Fresh fruit - 1 ppm
Dried fruit - 1 ppm
Oilseeds - 1 ppm
The maximum residues resulting from the above levels in the daily food
intake will be considerably lower than the acceptable daily intake.
Further work
Further work is needed on methods of detecting and measuring residues
down to 0.01 ppm of pyrethrins. It would be useful to have the results
of using such methods on a range of foods obtained in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smith, H. F., jr
(1950) Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier. J. & Ripert (1927) Compt. rend. Acad. d. sc., 184, 776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Leonard, C. S. (1942) J. econ. Ent., 22, 261
Lord, K. A. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P., Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 48, 473
Shimkin, M. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
U.S. Food and Drug Administration (1951) (unpublished data)
Zucker, A. (1965) Ann. Allergy, 23, 335
REFERENCES PERTINENT TO AGRICULTURAL DATA
Jones, H. A., Ackerman, Webster, M. E. (1952) Colimetric determination
of piperonyl butoxide. J. Assoc. Off. Agr. Chem., 35 (3): 771-780
Laundani, H., Highland, H. A. & Jay, E. G. (1966) Treated bags keep
corn-meal insect free during overseas shipment. Am. Miller and
Processor. Chicago (February 1966)
Walkden, H. H. & Nelson, H. D. (1959) Evaluation of synergised
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Ag., Marketing Research Rept. 322
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are soluble in
many organic solvents, e.g. kerosene, carbon tetrachloride, alcohol,
petroleum, ether, etc. They are decomposed by exposure to light with
loss of insecticidal activity. They are also rapidly oxidized and
inactivated in air. Antioxidants used to protect insecticidal residues
of pyrethrins include pyrocatechol, pyrogallol, hydroquinone;
benzene-320-napthol is used to protect against effects of sunlight.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Following ingestion, the pyrethrins are hydrolyzed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidal compounds or their derivatives are
absorbed as shown by their toxicity and their effect on the liver. The
pyrethrins or their metabolites are not known to be stored in the body
or to be excreted in the milk, but no modern methods have been
employed in the study of this aspect. Absorption does result in
urinary excretion of chrysanthemummonocarboxylic acid (Audiffren,
1934). It is believed that the diarrhoea produced by pyrethrin results
from central vagal stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum
oil
Rat Oral 200 Lehman, 1951
Rat Oral >2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1950
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be noted.
The very marked difference in the oral and intravenous toxicities
indicates a low rate of absorption from the gastro-intestinal tract,
very efficient destruction by the liver, or a combination of the two.
The acute effects resemble veratrine intoxication, proceeding from
excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally been in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
Man. Injury to man from pyrethrum has most frequently resulted from
the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in man.
Contact dermatitis is by far the most common. The usual picture is a
mild erythematous, vesicular dermatitis with papules in moist areas,
and intense pruritis (McCord et al., 1921; Sequeira, 1936). In a few
cases bullae appear (McCord et al., 1921; Sequeira, 1936; Tonking,
1936). Oedema and cracking develop in severe cases (Sequeira, 1936;
Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may be
made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity similar
to those seen in pollinosis, including sneezing, porous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).1 Thus,
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45 per cent
of persons who are sensitive to ragweed (Feinberg, 1954) and was
1 Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be adsorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941; Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
produced by repeated application of pyrethrum ointment in 10 per cent
and 26 per cent of unselected test populations (Lord & Johnson, 1947).
On the other hand, the insecticide has been considered so innocuous
that an ointment containing 0.75 per cent of pyrethrin was recommended
for treatment of scabies, and such use led to only a few cases of
dermatitis, some of doubtful relation to the treatment (Sweitzer &
Tedder, 1935; Sweitzer, 1936). Pyrethrins have been used extensively
for the control of human body lice. The formulation used early during
World War II was called MYL powder; its use was discontinued only
after the more effective and long-lasting DDT louse powder became
available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal worms.
It is possible to use pyrethrins for short periods in this way because
a considerable period, even two or three years, may be required for
susceptibility to appear (Sequeira, 1936; Tonking, 1936; Martin &
Hester, 1941, Schwartz, 1934). Onset may be delayed even when exposure
is to a purified ointment (Lord & Johnson, 1947; Sweitzer, 1936). This
argues against the unsupported contention (Sequeira, 1936) that the
dermatitis is usually the result of irritation rather than allergy. It
is generally recognized that susceptibility is increased during summer
months or periods of excessive perspiration (McCord et al., 1921;
Sequeira, 1936; Tonking, 1936; Martin & Hester, 1941).
Recent work confirmed that the degree of purity influences the
allergenic activity. In a study of 106 patients, all of whom had shown
positive reactions to ragweed or unrefined pyrethrin, no definite
allergic reactions to purified pyrethrins were observed (Zucker,
1965).
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (U.S. Food and Drug Administration,
1951).
Comments
Because of the long experience in using pyrethrum without observed
injury, except allergy in those with occupational and therapeutic
contact, there is little reason to question the customary uses of the
material. The fact that pyrethrum insecticides can be tolerated for
brief periods is, however, not a justification for recommending
frequent, repeated exposure to them over a period of years. Although
the rapid metabolism and apparent lack of storage are reassuring no
adequate study has been made of the synergized formulations now in
current use.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 200 ppm in the diet equivalent to 10 mg/kg/day
Estimate of temporary acceptable daily intake for man
0-0.4 mg/kg body-weight
Further work required
Short-term toxicity studies in several more species, including the dog
(at least a one-year study), with special emphasis on the effect on
the liver, and including the metabolism of pyrethrins in detail. These
studies should include chemically identified pyrethrin concentrates
alone and combined with major synergists, especially piperonyl
butoxide.
Result of the above work should be made available not later than 3
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments.
Low acute oral toxicity to mammals and rapid decomposition when
exposed to light and air allow pyrethrins to be used for controlling
insects on growling plants just before harvest and on dairy and meat
animals. They have been used in many countries on growing bush and
vine fruits, deciduous fruits and nuts, forage crops and vegetables,
and or, dairy and meat animals. Pyrethrins are usually used in
combination with a synergist. Piperonyl butoxide is the most common
synergist: piperonyl cyclonone, MGK-264(R), and sulfoxide have also
been used. For pre-harvest use the proportions of pyrethrins and
synergist may vary from 1 to 2, to 1 to 10, respectively. For certain
specific purposes pyrethrins sometimes are used without a synergist.
Because of the rapid decomposition of the insecticide, no limitations
have been set on the pre-harvest use of pyrethrins out doors.
(b) Post-harvest treatments
Pyrethrins have also been used in a spray or dust formulation on
freshly picked fruits and vegetables while in the field, in storage,
or in processing plants for the control of drosophila and other
insects. It is also used directly on dried fruit, tree-nuts, grains,
and oil seeds as a protective treatment against insect infestation
during storage. The pyrethrins are formulated in a fixed 1 to 10 ratio
with piperonyl butoxide for application as sprays or dusts directly on
the commodities as they are placed in containers or as they move on a
conveyor into a storage box, bin, or warehouse. Water emulsion or
wettable powder formulations of pyrethrins (in a 1 to 10 ratio with
piperonyl butoxide in the US and certain other countries) are used as
surface sprays on stacks of bagged peanuts and other oil seeds, and on
animal feeds. Aerosol formulations of pyrethrins are commonly used as
space treatments in food handling, processing, and storage facilities,
The use of pyrethrins for the above purposes has diminished
considerably in the past 10 years, being replaced by malathion in many
of the treatments.
(c) Other uses
Pyrethrins in combination with piperonyl butoxide, applied as a
repellent to the outside surface of multiwall paper bags, have been
found to be effective in protecting packaged cereal products against
outside insects. In the USA, such bags are quite widely used for
cereal products destined for storage or overseas shipment.
Pyrethrins are also used as a premises treatment for the control or
prevention of insect infestations in agricultural buildings and food
processing, handling, shipping, storage and marketing facilities. For
this purpose the pyrethrins are applied usually in combination with a
synergist as surface sprays to the floors, walls, working areas, and
machinery; as an aerosol space treatment in the buildings, or both.
Usually the insecticide is not applied directly to exposed foods and
food is not placed directly on freshly treated surfaces. Malathion has
replaced pyrethrins in many of the above uses.
Pyrethrins are also a common insecticide used for the control of
house-flies and other insects of public health and in the household.
Tolerances (established or considered)
Country Product Parts per million
Brazil Cereals 3
Canada Oats, Sorghum 1
Other grains 3
Czechoslovakia Grain -
Cereals 2
Finland Cereals exempt
Germany Grain 3
Tolerances (cont'd)
Country Product Parts per million
India Cereals exempt
Italy Cereals 2
Kenya Cereals exempt
Netherlands Cereals 3
Switzerland Cereals exempt
Turkey Cereals 3
USA Bush and vine fruits exempt
Cotton seed (post-harvest) 1
Deciduous fruits and nuts exempt
Flax-seed (post-harvest) 1
Forage crops exempt
Fruits and nuts (post-harvest) 1
Grains (post-harvest) 3
Mushrooms exempt
Peanuts (post-harvest) 1
Vegetables exempt
Vegetables (post-harvest) 1
Residues resulting from supervised trials
On growing crops pyrethrins deteriorate so rapidly that no attention
has been given to measuring residues resulting from such use. In most
countries they have been exempt from use regulations because of their
low acute oral toxicity and rapid deterioration. Most of the
information on residues has been obtained from applications to
harvested commodities. Pyrethrins, usually in combination with
piperonyl butoxide at about 1:10 w/w, are applied to grain as a
protective treatment at a dosage rate of pyrethrins of 1.42 ppm on
wheat, 1.52 ppm on shelled corn, 2.67 ppm on oats, 1.78 ppm on barley,
1.52 ppm on rye, and 1.9 ppm on rough rice. Some of the insecticide is
lost during application, and the deterioration is rapid during the
first few months after storage.
Flour exposed to two space treatments of synergized pyrethrins applied
at the rate of two and four ounces of 0.4 per cent pyrethrins per 1000
cubic feet produced less than 1 ppm residue in the top 1-1/2 inches
(Unpublished document supplied by US Department of Agriculture).
Bagged dried citrus pulp animal feed was exposed to weekly treatments
over a three-month period to aerosol formulations containing 0.2 per
cent of pyrethrins applied at the rate of 54 mg/m2. The maximum
pyrethrins residue during the entire period was less than 1 ppm.
Following a 15-month storage period in multiwall paper bags with
special insect-tight closures and pyrethrins and piperonyl butoxide,
the residues of pyrethrins in rice, non-fat dried milk, dried beans
and flour were considerably less than 1 ppm (Unpublished information
from US Department of Agriculture). A similar test involving cornmeal
stored for six months in treated bags showed maximum pyrethrins
residues of 1.07 ppm in 50 lb bags and 0.33 ppm in 100 lb bags
(Laudani et al., 1966).
Residues in food moving in commerce
1.7 ppm of pyrethrins were found on a shipment of grain known to have
been treated before shipment to Britain. 0.1 ppm was the highest
amongst 99 samples taken in Rotterdam and Amsterdam.
Residues at the time of consumption
At the time of preparation of this report no data were available on
the fate of residues of pyrethrins on or in fresh fruit, dried fruit,
tree-nuts, fresh and dried vegetables, and oils. Because of the rapid
deterioration of this insecticide when exposed to light and air, very
little attention has been given to its fate when applied before
harvesting. Some information is available however on the fate of
pyrethrins when applied to grain. Controlled studies showed that a
theoretical deposit on wheat of 1.32 ppm was down to 0.56 ppm in two
months and 0.36 ppm in four months after treatment. A theoretical 1.74
ppm of pyrethrins applied in combination with piperonyl butoxide (1:10
ratio) was down to 0.3 ppm after two months of storage. Other studies
showed that about 50 per cent of the pyrethrins residue on wheat had
disappeared with four to five months, most of the remaining
insecticide went into the screenings and scourings when the treated
grain was milled and only a very small percentage of the pyrethrins
passed into the flour. (Information direct from US Department of
Agriculture).
Methods of residue analysis
Pyrethrins, being a group of insecticidally active esters, are
difficult to detect in their entirety by any single analytical method.
New chromatographic methods look promising and may be perfected if
adequate clean-up methods can be developed. Further research is needed
to perfect methods suitable for detecting down to 0.01 ppm of the
principal esters of pyrethrins. In the meantime, the method of Jones,
Ackerman & Webster (1952) as proposed for residues of piperonyl
butoxide, with the appropriate clean-up procedures for the various
foods, can be used as an index of pyrethrin levels when the exact
ratio of the pyrethrins and the piperonyl butoxide is known; piperonyl
butoxide residues are more stable than those of pyrethrins. This
method has a sensitivity of 0.1 ppm of piperonyl butoxide.
RECOMMENDATIONS FOR TOLERANCES
The available data indicate that 50 per cent or more of the pyrethrins
applied to stored grain disappear during the first three or four
months of storage. At least 80 per cent of the remaining residue
normally is removed by handling, processing and cooking. However,
sufficient information is not available on the fate of pyrethrins
residues obtained from good agricultural practices on fresh fruit,
dried fruit, tree-nuts, dried vegetables and oil seeds. Therefore,
it is recommended that the following temporary tolerances be
established.
Cereals - 3 ppm
Cereal products - 1 ppm
Fresh fruit - 1 ppm
Dried fruit - 1 ppm
Oilseeds - 1 ppm
The maximum residues resulting from the above levels in the daily food
intake will be considerably lower than the acceptable daily intake.
Further work
Further work is needed on methods of detecting and measuring residues
down to 0.01 ppm of pyrethrins. It would be useful to have the results
of using such methods on a range of foods obtained in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smith, H. F., jr
(1950) Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier. J. & Ripert (1927) Compt. rend. Acad. d. sc., 184, 776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Leonard, C. S. (1942) J. econ. Ent., 22, 261
Lord, K. A. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P., Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 48, 473
Shimkin, M. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
U.S. Food and Drug Administration (1951) (unpublished data)
Zucker, A. (1965) Ann. Allergy, 23, 335
REFERENCES PERTINENT TO AGRICULTURAL DATA
Jones, H. A., Ackerman, Webster, M. E. (1952) Colimetric determination
of piperonyl butoxide. J. Assoc. Off. Agr. Chem., 35 (3): 771-780
Laundani, H., Highland, H. A. & Jay, E. G. (1966) Treated bags keep
corn-meal insect free during overseas shipment. Am. Miller and
Processor. Chicago (February 1966)
Walkden, H. H. & Nelson, H. D. (1959) Evaluation of synergised
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Ag., Marketing Research Rept. 322
 Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are soluble in
many organic solvents, e.g. kerosene, carbon tetrachloride, alcohol,
petroleum, ether, etc. They are decomposed by exposure to light with
loss of insecticidal activity. They are also rapidly oxidized and
inactivated in air. Antioxidants used to protect insecticidal residues
of pyrethrins include pyrocatechol, pyrogallol, hydroquinone;
benzene-320-napthol is used to protect against effects of sunlight.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Following ingestion, the pyrethrins are hydrolyzed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidal compounds or their derivatives are
absorbed as shown by their toxicity and their effect on the liver. The
pyrethrins or their metabolites are not known to be stored in the body
or to be excreted in the milk, but no modern methods have been
employed in the study of this aspect. Absorption does result in
urinary excretion of chrysanthemummonocarboxylic acid (Audiffren,
1934). It is believed that the diarrhoea produced by pyrethrin results
from central vagal stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum
oil
Rat Oral 200 Lehman, 1951
Rat Oral >2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1950
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be noted.
The very marked difference in the oral and intravenous toxicities
indicates a low rate of absorption from the gastro-intestinal tract,
very efficient destruction by the liver, or a combination of the two.
The acute effects resemble veratrine intoxication, proceeding from
excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally been in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
Man. Injury to man from pyrethrum has most frequently resulted from
the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in man.
Contact dermatitis is by far the most common. The usual picture is a
mild erythematous, vesicular dermatitis with papules in moist areas,
and intense pruritis (McCord et al., 1921; Sequeira, 1936). In a few
cases bullae appear (McCord et al., 1921; Sequeira, 1936; Tonking,
1936). Oedema and cracking develop in severe cases (Sequeira, 1936;
Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may be
made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity similar
to those seen in pollinosis, including sneezing, porous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).1 Thus,
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45 per cent
of persons who are sensitive to ragweed (Feinberg, 1954) and was
1 Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be adsorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941; Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
produced by repeated application of pyrethrum ointment in 10 per cent
and 26 per cent of unselected test populations (Lord & Johnson, 1947).
On the other hand, the insecticide has been considered so innocuous
that an ointment containing 0.75 per cent of pyrethrin was recommended
for treatment of scabies, and such use led to only a few cases of
dermatitis, some of doubtful relation to the treatment (Sweitzer &
Tedder, 1935; Sweitzer, 1936). Pyrethrins have been used extensively
for the control of human body lice. The formulation used early during
World War II was called MYL powder; its use was discontinued only
after the more effective and long-lasting DDT louse powder became
available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal worms.
It is possible to use pyrethrins for short periods in this way because
a considerable period, even two or three years, may be required for
susceptibility to appear (Sequeira, 1936; Tonking, 1936; Martin &
Hester, 1941, Schwartz, 1934). Onset may be delayed even when exposure
is to a purified ointment (Lord & Johnson, 1947; Sweitzer, 1936). This
argues against the unsupported contention (Sequeira, 1936) that the
dermatitis is usually the result of irritation rather than allergy. It
is generally recognized that susceptibility is increased during summer
months or periods of excessive perspiration (McCord et al., 1921;
Sequeira, 1936; Tonking, 1936; Martin & Hester, 1941).
Recent work confirmed that the degree of purity influences the
allergenic activity. In a study of 106 patients, all of whom had shown
positive reactions to ragweed or unrefined pyrethrin, no definite
allergic reactions to purified pyrethrins were observed (Zucker,
1965).
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (U.S. Food and Drug Administration,
1951).
Comments
Because of the long experience in using pyrethrum without observed
injury, except allergy in those with occupational and therapeutic
contact, there is little reason to question the customary uses of the
material. The fact that pyrethrum insecticides can be tolerated for
brief periods is, however, not a justification for recommending
frequent, repeated exposure to them over a period of years. Although
the rapid metabolism and apparent lack of storage are reassuring no
adequate study has been made of the synergized formulations now in
current use.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 200 ppm in the diet equivalent to 10 mg/kg/day
Estimate of temporary acceptable daily intake for man
0-0.4 mg/kg body-weight
Further work required
Short-term toxicity studies in several more species, including the dog
(at least a one-year study), with special emphasis on the effect on
the liver, and including the metabolism of pyrethrins in detail. These
studies should include chemically identified pyrethrin concentrates
alone and combined with major synergists, especially piperonyl
butoxide.
Result of the above work should be made available not later than 3
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments.
Low acute oral toxicity to mammals and rapid decomposition when
exposed to light and air allow pyrethrins to be used for controlling
insects on growling plants just before harvest and on dairy and meat
animals. They have been used in many countries on growing bush and
vine fruits, deciduous fruits and nuts, forage crops and vegetables,
and or, dairy and meat animals. Pyrethrins are usually used in
combination with a synergist. Piperonyl butoxide is the most common
synergist: piperonyl cyclonone, MGK-264(R), and sulfoxide have also
been used. For pre-harvest use the proportions of pyrethrins and
synergist may vary from 1 to 2, to 1 to 10, respectively. For certain
specific purposes pyrethrins sometimes are used without a synergist.
Because of the rapid decomposition of the insecticide, no limitations
have been set on the pre-harvest use of pyrethrins out doors.
(b) Post-harvest treatments
Pyrethrins have also been used in a spray or dust formulation on
freshly picked fruits and vegetables while in the field, in storage,
or in processing plants for the control of drosophila and other
insects. It is also used directly on dried fruit, tree-nuts, grains,
and oil seeds as a protective treatment against insect infestation
during storage. The pyrethrins are formulated in a fixed 1 to 10 ratio
with piperonyl butoxide for application as sprays or dusts directly on
the commodities as they are placed in containers or as they move on a
conveyor into a storage box, bin, or warehouse. Water emulsion or
wettable powder formulations of pyrethrins (in a 1 to 10 ratio with
piperonyl butoxide in the US and certain other countries) are used as
surface sprays on stacks of bagged peanuts and other oil seeds, and on
animal feeds. Aerosol formulations of pyrethrins are commonly used as
space treatments in food handling, processing, and storage facilities,
The use of pyrethrins for the above purposes has diminished
considerably in the past 10 years, being replaced by malathion in many
of the treatments.
(c) Other uses
Pyrethrins in combination with piperonyl butoxide, applied as a
repellent to the outside surface of multiwall paper bags, have been
found to be effective in protecting packaged cereal products against
outside insects. In the USA, such bags are quite widely used for
cereal products destined for storage or overseas shipment.
Pyrethrins are also used as a premises treatment for the control or
prevention of insect infestations in agricultural buildings and food
processing, handling, shipping, storage and marketing facilities. For
this purpose the pyrethrins are applied usually in combination with a
synergist as surface sprays to the floors, walls, working areas, and
machinery; as an aerosol space treatment in the buildings, or both.
Usually the insecticide is not applied directly to exposed foods and
food is not placed directly on freshly treated surfaces. Malathion has
replaced pyrethrins in many of the above uses.
Pyrethrins are also a common insecticide used for the control of
house-flies and other insects of public health and in the household.
Tolerances (established or considered)
Country Product Parts per million
Brazil Cereals 3
Canada Oats, Sorghum 1
Other grains 3
Czechoslovakia Grain -
Cereals 2
Finland Cereals exempt
Germany Grain 3
Tolerances (cont'd)
Country Product Parts per million
India Cereals exempt
Italy Cereals 2
Kenya Cereals exempt
Netherlands Cereals 3
Switzerland Cereals exempt
Turkey Cereals 3
USA Bush and vine fruits exempt
Cotton seed (post-harvest) 1
Deciduous fruits and nuts exempt
Flax-seed (post-harvest) 1
Forage crops exempt
Fruits and nuts (post-harvest) 1
Grains (post-harvest) 3
Mushrooms exempt
Peanuts (post-harvest) 1
Vegetables exempt
Vegetables (post-harvest) 1
Residues resulting from supervised trials
On growing crops pyrethrins deteriorate so rapidly that no attention
has been given to measuring residues resulting from such use. In most
countries they have been exempt from use regulations because of their
low acute oral toxicity and rapid deterioration. Most of the
information on residues has been obtained from applications to
harvested commodities. Pyrethrins, usually in combination with
piperonyl butoxide at about 1:10 w/w, are applied to grain as a
protective treatment at a dosage rate of pyrethrins of 1.42 ppm on
wheat, 1.52 ppm on shelled corn, 2.67 ppm on oats, 1.78 ppm on barley,
1.52 ppm on rye, and 1.9 ppm on rough rice. Some of the insecticide is
lost during application, and the deterioration is rapid during the
first few months after storage.
Flour exposed to two space treatments of synergized pyrethrins applied
at the rate of two and four ounces of 0.4 per cent pyrethrins per 1000
cubic feet produced less than 1 ppm residue in the top 1-1/2 inches
(Unpublished document supplied by US Department of Agriculture).
Bagged dried citrus pulp animal feed was exposed to weekly treatments
over a three-month period to aerosol formulations containing 0.2 per
cent of pyrethrins applied at the rate of 54 mg/m2. The maximum
pyrethrins residue during the entire period was less than 1 ppm.
Following a 15-month storage period in multiwall paper bags with
special insect-tight closures and pyrethrins and piperonyl butoxide,
the residues of pyrethrins in rice, non-fat dried milk, dried beans
and flour were considerably less than 1 ppm (Unpublished information
from US Department of Agriculture). A similar test involving cornmeal
stored for six months in treated bags showed maximum pyrethrins
residues of 1.07 ppm in 50 lb bags and 0.33 ppm in 100 lb bags
(Laudani et al., 1966).
Residues in food moving in commerce
1.7 ppm of pyrethrins were found on a shipment of grain known to have
been treated before shipment to Britain. 0.1 ppm was the highest
amongst 99 samples taken in Rotterdam and Amsterdam.
Residues at the time of consumption
At the time of preparation of this report no data were available on
the fate of residues of pyrethrins on or in fresh fruit, dried fruit,
tree-nuts, fresh and dried vegetables, and oils. Because of the rapid
deterioration of this insecticide when exposed to light and air, very
little attention has been given to its fate when applied before
harvesting. Some information is available however on the fate of
pyrethrins when applied to grain. Controlled studies showed that a
theoretical deposit on wheat of 1.32 ppm was down to 0.56 ppm in two
months and 0.36 ppm in four months after treatment. A theoretical 1.74
ppm of pyrethrins applied in combination with piperonyl butoxide (1:10
ratio) was down to 0.3 ppm after two months of storage. Other studies
showed that about 50 per cent of the pyrethrins residue on wheat had
disappeared with four to five months, most of the remaining
insecticide went into the screenings and scourings when the treated
grain was milled and only a very small percentage of the pyrethrins
passed into the flour. (Information direct from US Department of
Agriculture).
Methods of residue analysis
Pyrethrins, being a group of insecticidally active esters, are
difficult to detect in their entirety by any single analytical method.
New chromatographic methods look promising and may be perfected if
adequate clean-up methods can be developed. Further research is needed
to perfect methods suitable for detecting down to 0.01 ppm of the
principal esters of pyrethrins. In the meantime, the method of Jones,
Ackerman & Webster (1952) as proposed for residues of piperonyl
butoxide, with the appropriate clean-up procedures for the various
foods, can be used as an index of pyrethrin levels when the exact
ratio of the pyrethrins and the piperonyl butoxide is known; piperonyl
butoxide residues are more stable than those of pyrethrins. This
method has a sensitivity of 0.1 ppm of piperonyl butoxide.
RECOMMENDATIONS FOR TOLERANCES
The available data indicate that 50 per cent or more of the pyrethrins
applied to stored grain disappear during the first three or four
months of storage. At least 80 per cent of the remaining residue
normally is removed by handling, processing and cooking. However,
sufficient information is not available on the fate of pyrethrins
residues obtained from good agricultural practices on fresh fruit,
dried fruit, tree-nuts, dried vegetables and oil seeds. Therefore,
it is recommended that the following temporary tolerances be
established.
Cereals - 3 ppm
Cereal products - 1 ppm
Fresh fruit - 1 ppm
Dried fruit - 1 ppm
Oilseeds - 1 ppm
The maximum residues resulting from the above levels in the daily food
intake will be considerably lower than the acceptable daily intake.
Further work
Further work is needed on methods of detecting and measuring residues
down to 0.01 ppm of pyrethrins. It would be useful to have the results
of using such methods on a range of foods obtained in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smith, H. F., jr
(1950) Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier. J. & Ripert (1927) Compt. rend. Acad. d. sc., 184, 776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Leonard, C. S. (1942) J. econ. Ent., 22, 261
Lord, K. A. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P., Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 48, 473
Shimkin, M. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
U.S. Food and Drug Administration (1951) (unpublished data)
Zucker, A. (1965) Ann. Allergy, 23, 335
REFERENCES PERTINENT TO AGRICULTURAL DATA
Jones, H. A., Ackerman, Webster, M. E. (1952) Colimetric determination
of piperonyl butoxide. J. Assoc. Off. Agr. Chem., 35 (3): 771-780
Laundani, H., Highland, H. A. & Jay, E. G. (1966) Treated bags keep
corn-meal insect free during overseas shipment. Am. Miller and
Processor. Chicago (February 1966)
Walkden, H. H. & Nelson, H. D. (1959) Evaluation of synergised
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Ag., Marketing Research Rept. 322
Relevant physical and chemical properties
These compounds are virtually insoluble in water, but are soluble in
many organic solvents, e.g. kerosene, carbon tetrachloride, alcohol,
petroleum, ether, etc. They are decomposed by exposure to light with
loss of insecticidal activity. They are also rapidly oxidized and
inactivated in air. Antioxidants used to protect insecticidal residues
of pyrethrins include pyrocatechol, pyrogallol, hydroquinone;
benzene-320-napthol is used to protect against effects of sunlight.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Following ingestion, the pyrethrins are hydrolyzed by various
digestive enzymes in the gastro-intestinal tract. However, a small
portion of the insecticidal compounds or their derivatives are
absorbed as shown by their toxicity and their effect on the liver. The
pyrethrins or their metabolites are not known to be stored in the body
or to be excreted in the milk, but no modern methods have been
employed in the study of this aspect. Absorption does result in
urinary excretion of chrysanthemummonocarboxylic acid (Audiffren,
1934). It is believed that the diarrhoea produced by pyrethrin results
from central vagal stimulation (Leonard, 1942).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Rat Oral 820 Carpenter et al., 1950
pyrethrum oleoresin
purified
Rat Oral 1870 Carpenter et al., 1950
purified pyrethrum
extract in petroleum
oil
Rat Oral 200 Lehman, 1951
Rat Oral >2600 Ambrose & Robbins, 1951
Guinea-pig Oral 1500 Shimkin & Anderson, 1936
Dog i.v. 6-8* Chevalier, 1950
* "Lethal dose"
The relatively high inherent toxicity of pyrethrum should be noted.
The very marked difference in the oral and intravenous toxicities
indicates a low rate of absorption from the gastro-intestinal tract,
very efficient destruction by the liver, or a combination of the two.
The acute effects resemble veratrine intoxication, proceeding from
excitation to convulsions to tetanic convulsions, except that
pyrethrins also cause muscular fibrillation. Death is caused by
respiratory failure (Leonard, 1942; Chevalier & Ripert, 1927).
Persistent tremor is occasionally been in animals that recover from a
single large dose (Leonard, 1942).
Short-term studies
Man. Injury to man from pyrethrum has most frequently resulted from
the allergenic properties of the material rather than its direct
toxicity. Although the allergy has been associated with occupational
or therapeutic contact, it is impossible to exclude any importance of
it in connexion with food residues.
Pyrethrum sensitivity may manifest itself in several forms in man.
Contact dermatitis is by far the most common. The usual picture is a
mild erythematous, vesicular dermatitis with papules in moist areas,
and intense pruritis (McCord et al., 1921; Sequeira, 1936). In a few
cases bullae appear (McCord et al., 1921; Sequeira, 1936; Tonking,
1936). Oedema and cracking develop in severe cases (Sequeira, 1936;
Tonking, 1936; Martin & Hester, 1941). Pyrethrum dermatitis may be
made worse by exposure to the sun (Tonking, 1936).
Some individuals show manifestations of pyrethrum sensitivity similar
to those seen in pollinosis, including sneezing, porous nasal
discharge and nasal "stuffiness" (Feinberg, 1934; Ramirez, 1930). A
few cases of extrinsic asthma due to pyrethrum mixtures have been
reported (Ramirez, 1930; Garratt & Bigger, 1923). Some of the
individuals involved had a previous history of asthma with a very
broad allergic background. Several cases have shown what McCord et
al., 1921, called "dermal anaphylaxis" characterized by dermatitis and
sudden severe swelling of the face and lips (McCord et al., 1921;
Ramirez, 1930). A mild form was produced in the course of experiments
(Martin & Hester, 1941). A severe anaphylactic reaction, including
peripheral vascular collapse is rare but can occur (Bosredon, 1897).
Pyrethrum flowers and certain extracts from them are much more
allergenic than the more or less purified pyrethrins now marketed as
insecticides (Martin & Hester, 1941; Lord & Johnson, 1947).1 Thus,
many reports of pyrethrum dermatitis involve contact with flowers in
connexion with harvesting, weighing or grinding (McCord et al., 1921;
Tonking, 1936). However, dermatitis (Schwartz, 1934), and especially
allergy of the respiratory tract may result from exposure to pyrethrum
formulations intended for use in the home (Feinberg, 1934; Ramirez,
1930). Sensitivity as judged by skin tests occurs in over 45 per cent
of persons who are sensitive to ragweed (Feinberg, 1954) and was
1 Investigations have shown that the allergenic agent or agents in
pyrethrum are extractable by solvents such as petroleum ether (Martin
& Hester, 1941; Lord & Johnson, 1947); they can be adsorbed on
adsorbents such as fullers earth (Lord & Johnson, 1947) and they are
probably volatile with steam (Martin & Hester, 1941; Frank &
McGeachin, 1949). Acetic acid has been identified as being present in
pyrethrum extracts and is claimed (Frank & McGeachin, 1949) to be a
contributory irritating factor although it is not in itself
allergenic.
produced by repeated application of pyrethrum ointment in 10 per cent
and 26 per cent of unselected test populations (Lord & Johnson, 1947).
On the other hand, the insecticide has been considered so innocuous
that an ointment containing 0.75 per cent of pyrethrin was recommended
for treatment of scabies, and such use led to only a few cases of
dermatitis, some of doubtful relation to the treatment (Sweitzer &
Tedder, 1935; Sweitzer, 1936). Pyrethrins have been used extensively
for the control of human body lice. The formulation used early during
World War II was called MYL powder; its use was discontinued only
after the more effective and long-lasting DDT louse powder became
available (Simmons, 1959).
Pyrethrins have also been given by mouth to combat intestinal worms.
It is possible to use pyrethrins for short periods in this way because
a considerable period, even two or three years, may be required for
susceptibility to appear (Sequeira, 1936; Tonking, 1936; Martin &
Hester, 1941, Schwartz, 1934). Onset may be delayed even when exposure
is to a purified ointment (Lord & Johnson, 1947; Sweitzer, 1936). This
argues against the unsupported contention (Sequeira, 1936) that the
dermatitis is usually the result of irritation rather than allergy. It
is generally recognized that susceptibility is increased during summer
months or periods of excessive perspiration (McCord et al., 1921;
Sequeira, 1936; Tonking, 1936; Martin & Hester, 1941).
Recent work confirmed that the degree of purity influences the
allergenic activity. In a study of 106 patients, all of whom had shown
positive reactions to ragweed or unrefined pyrethrin, no definite
allergic reactions to purified pyrethrins were observed (Zucker,
1965).
Long-term studies
Rat. Groups of 12 male and 12 female rats were fed pyrethrin in
soybean oil at dietary levels of 0, 200, 1000 and 5000 ppm for 2
years. The daily dosage was, therefore, approximately 0, 10, 50 and
250 mg/kg respectively. Even the highest level had no significant
effect on growth or survival. Slight, though definite, liver damage
characterized by bile duct proliferation and focal necrosis was found
at the two highest dosage levels (U.S. Food and Drug Administration,
1951).
Comments
Because of the long experience in using pyrethrum without observed
injury, except allergy in those with occupational and therapeutic
contact, there is little reason to question the customary uses of the
material. The fact that pyrethrum insecticides can be tolerated for
brief periods is, however, not a justification for recommending
frequent, repeated exposure to them over a period of years. Although
the rapid metabolism and apparent lack of storage are reassuring no
adequate study has been made of the synergized formulations now in
current use.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 200 ppm in the diet equivalent to 10 mg/kg/day
Estimate of temporary acceptable daily intake for man
0-0.4 mg/kg body-weight
Further work required
Short-term toxicity studies in several more species, including the dog
(at least a one-year study), with special emphasis on the effect on
the liver, and including the metabolism of pyrethrins in detail. These
studies should include chemically identified pyrethrin concentrates
alone and combined with major synergists, especially piperonyl
butoxide.
Result of the above work should be made available not later than 3
years after the publication of this report, when a re-evaluation of
this compound will be made.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments.
Low acute oral toxicity to mammals and rapid decomposition when
exposed to light and air allow pyrethrins to be used for controlling
insects on growling plants just before harvest and on dairy and meat
animals. They have been used in many countries on growing bush and
vine fruits, deciduous fruits and nuts, forage crops and vegetables,
and or, dairy and meat animals. Pyrethrins are usually used in
combination with a synergist. Piperonyl butoxide is the most common
synergist: piperonyl cyclonone, MGK-264(R), and sulfoxide have also
been used. For pre-harvest use the proportions of pyrethrins and
synergist may vary from 1 to 2, to 1 to 10, respectively. For certain
specific purposes pyrethrins sometimes are used without a synergist.
Because of the rapid decomposition of the insecticide, no limitations
have been set on the pre-harvest use of pyrethrins out doors.
(b) Post-harvest treatments
Pyrethrins have also been used in a spray or dust formulation on
freshly picked fruits and vegetables while in the field, in storage,
or in processing plants for the control of drosophila and other
insects. It is also used directly on dried fruit, tree-nuts, grains,
and oil seeds as a protective treatment against insect infestation
during storage. The pyrethrins are formulated in a fixed 1 to 10 ratio
with piperonyl butoxide for application as sprays or dusts directly on
the commodities as they are placed in containers or as they move on a
conveyor into a storage box, bin, or warehouse. Water emulsion or
wettable powder formulations of pyrethrins (in a 1 to 10 ratio with
piperonyl butoxide in the US and certain other countries) are used as
surface sprays on stacks of bagged peanuts and other oil seeds, and on
animal feeds. Aerosol formulations of pyrethrins are commonly used as
space treatments in food handling, processing, and storage facilities,
The use of pyrethrins for the above purposes has diminished
considerably in the past 10 years, being replaced by malathion in many
of the treatments.
(c) Other uses
Pyrethrins in combination with piperonyl butoxide, applied as a
repellent to the outside surface of multiwall paper bags, have been
found to be effective in protecting packaged cereal products against
outside insects. In the USA, such bags are quite widely used for
cereal products destined for storage or overseas shipment.
Pyrethrins are also used as a premises treatment for the control or
prevention of insect infestations in agricultural buildings and food
processing, handling, shipping, storage and marketing facilities. For
this purpose the pyrethrins are applied usually in combination with a
synergist as surface sprays to the floors, walls, working areas, and
machinery; as an aerosol space treatment in the buildings, or both.
Usually the insecticide is not applied directly to exposed foods and
food is not placed directly on freshly treated surfaces. Malathion has
replaced pyrethrins in many of the above uses.
Pyrethrins are also a common insecticide used for the control of
house-flies and other insects of public health and in the household.
Tolerances (established or considered)
Country Product Parts per million
Brazil Cereals 3
Canada Oats, Sorghum 1
Other grains 3
Czechoslovakia Grain -
Cereals 2
Finland Cereals exempt
Germany Grain 3
Tolerances (cont'd)
Country Product Parts per million
India Cereals exempt
Italy Cereals 2
Kenya Cereals exempt
Netherlands Cereals 3
Switzerland Cereals exempt
Turkey Cereals 3
USA Bush and vine fruits exempt
Cotton seed (post-harvest) 1
Deciduous fruits and nuts exempt
Flax-seed (post-harvest) 1
Forage crops exempt
Fruits and nuts (post-harvest) 1
Grains (post-harvest) 3
Mushrooms exempt
Peanuts (post-harvest) 1
Vegetables exempt
Vegetables (post-harvest) 1
Residues resulting from supervised trials
On growing crops pyrethrins deteriorate so rapidly that no attention
has been given to measuring residues resulting from such use. In most
countries they have been exempt from use regulations because of their
low acute oral toxicity and rapid deterioration. Most of the
information on residues has been obtained from applications to
harvested commodities. Pyrethrins, usually in combination with
piperonyl butoxide at about 1:10 w/w, are applied to grain as a
protective treatment at a dosage rate of pyrethrins of 1.42 ppm on
wheat, 1.52 ppm on shelled corn, 2.67 ppm on oats, 1.78 ppm on barley,
1.52 ppm on rye, and 1.9 ppm on rough rice. Some of the insecticide is
lost during application, and the deterioration is rapid during the
first few months after storage.
Flour exposed to two space treatments of synergized pyrethrins applied
at the rate of two and four ounces of 0.4 per cent pyrethrins per 1000
cubic feet produced less than 1 ppm residue in the top 1-1/2 inches
(Unpublished document supplied by US Department of Agriculture).
Bagged dried citrus pulp animal feed was exposed to weekly treatments
over a three-month period to aerosol formulations containing 0.2 per
cent of pyrethrins applied at the rate of 54 mg/m2. The maximum
pyrethrins residue during the entire period was less than 1 ppm.
Following a 15-month storage period in multiwall paper bags with
special insect-tight closures and pyrethrins and piperonyl butoxide,
the residues of pyrethrins in rice, non-fat dried milk, dried beans
and flour were considerably less than 1 ppm (Unpublished information
from US Department of Agriculture). A similar test involving cornmeal
stored for six months in treated bags showed maximum pyrethrins
residues of 1.07 ppm in 50 lb bags and 0.33 ppm in 100 lb bags
(Laudani et al., 1966).
Residues in food moving in commerce
1.7 ppm of pyrethrins were found on a shipment of grain known to have
been treated before shipment to Britain. 0.1 ppm was the highest
amongst 99 samples taken in Rotterdam and Amsterdam.
Residues at the time of consumption
At the time of preparation of this report no data were available on
the fate of residues of pyrethrins on or in fresh fruit, dried fruit,
tree-nuts, fresh and dried vegetables, and oils. Because of the rapid
deterioration of this insecticide when exposed to light and air, very
little attention has been given to its fate when applied before
harvesting. Some information is available however on the fate of
pyrethrins when applied to grain. Controlled studies showed that a
theoretical deposit on wheat of 1.32 ppm was down to 0.56 ppm in two
months and 0.36 ppm in four months after treatment. A theoretical 1.74
ppm of pyrethrins applied in combination with piperonyl butoxide (1:10
ratio) was down to 0.3 ppm after two months of storage. Other studies
showed that about 50 per cent of the pyrethrins residue on wheat had
disappeared with four to five months, most of the remaining
insecticide went into the screenings and scourings when the treated
grain was milled and only a very small percentage of the pyrethrins
passed into the flour. (Information direct from US Department of
Agriculture).
Methods of residue analysis
Pyrethrins, being a group of insecticidally active esters, are
difficult to detect in their entirety by any single analytical method.
New chromatographic methods look promising and may be perfected if
adequate clean-up methods can be developed. Further research is needed
to perfect methods suitable for detecting down to 0.01 ppm of the
principal esters of pyrethrins. In the meantime, the method of Jones,
Ackerman & Webster (1952) as proposed for residues of piperonyl
butoxide, with the appropriate clean-up procedures for the various
foods, can be used as an index of pyrethrin levels when the exact
ratio of the pyrethrins and the piperonyl butoxide is known; piperonyl
butoxide residues are more stable than those of pyrethrins. This
method has a sensitivity of 0.1 ppm of piperonyl butoxide.
RECOMMENDATIONS FOR TOLERANCES
The available data indicate that 50 per cent or more of the pyrethrins
applied to stored grain disappear during the first three or four
months of storage. At least 80 per cent of the remaining residue
normally is removed by handling, processing and cooking. However,
sufficient information is not available on the fate of pyrethrins
residues obtained from good agricultural practices on fresh fruit,
dried fruit, tree-nuts, dried vegetables and oil seeds. Therefore,
it is recommended that the following temporary tolerances be
established.
Cereals - 3 ppm
Cereal products - 1 ppm
Fresh fruit - 1 ppm
Dried fruit - 1 ppm
Oilseeds - 1 ppm
The maximum residues resulting from the above levels in the daily food
intake will be considerably lower than the acceptable daily intake.
Further work
Further work is needed on methods of detecting and measuring residues
down to 0.01 ppm of pyrethrins. It would be useful to have the results
of using such methods on a range of foods obtained in commerce.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Audiffren, M. (1934) J. Pharm. Chim., 19, 535
Bosredon, Dr (1897) Bull. gen. therapeutique, medicale, chirurgical,
obstetrical et pharmaceutique, 132, 275
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smith, H. F., jr
(1950) Arch. industr. Hyg. Occup. Med., 2, 420
Chevalier, J. (1930) Bull. d. sci. pharmacol., 37, 154
Chevalier. J. & Ripert (1927) Compt. rend. Acad. d. sc., 184, 776
Feinberg, S. M. (1934) J. Amer. med. Ass., 102, 1557
Frank, R. L. & McGeachin, R. L. (1949) J. Amer. Pharm. Ass. sci.
Ed., 38, 297
Garratt, J. R. & Bigger, J. W. (1923) Brit. med. J., 2, 764
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Leonard, C. S. (1942) J. econ. Ent., 22, 261
Lord, K. A. & Johnson, C. G. (1947) Brit. J. Dermatol., 59, 367
Martin, J. T. & Hester, K. H. C. (1941) Brit. J. Dermatol., 53,
127
McCord, C. P., Kilker, C. H. & Minster, D. K. (1921) J. Amer. med.
Ass., 77, 448
Ramirez, M. A. (1930) J. Allergy, 1, 149
Schwartz, L. (1934) Publ. Hlth Bull., 215, 51
Sequeira, J. H. (1936) Brit. J. Dermatol., 48, 473
Shimkin, M. B. & Anderson, H. H. (1936) Proc. Soc. exp. Biol.
(N.Y.), 34, 135
Sweitzer, S. E. & Tedder, J. W. (1935) Minnesota Med., 18, 793
Sweitzer, S. E. (1936) Journal-Lancet, 56, 467
Tonking, H. D. (1936) E. Afr. med. J., 13, 7
U.S. Food and Drug Administration (1951) (unpublished data)
Zucker, A. (1965) Ann. Allergy, 23, 335
REFERENCES PERTINENT TO AGRICULTURAL DATA
Jones, H. A., Ackerman, Webster, M. E. (1952) Colimetric determination
of piperonyl butoxide. J. Assoc. Off. Agr. Chem., 35 (3): 771-780
Laundani, H., Highland, H. A. & Jay, E. G. (1966) Treated bags keep
corn-meal insect free during overseas shipment. Am. Miller and
Processor. Chicago (February 1966)
Walkden, H. H. & Nelson, H. D. (1959) Evaluation of synergised
pyrethrum for the protection of stored wheat and shelled corn from
insect attack. U.S. Dept. Ag., Marketing Research Rept. 322
