
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
HEPTACHLOR
Additional data on heptachlor have become available since the last
complete monograph was produced (FAO/WHO, 1967). These data, as well
as some pertinent older data, are summarized in this monograph
addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
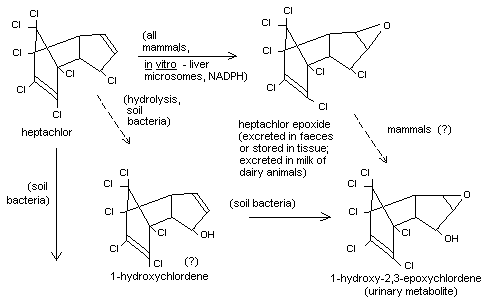
Information on the metabolism of heptachlor in mammals is still
incomplete. The formation of heptachlor epoxide in vivo in several
species of mammals and in vitro using rat and rabbit liver microsomes
in the presence of NADPH has been described (Wong and Terriere, 1965;
Nakotsugawa, 1965; FAO/WHO, 1967). It was suggested early on that
heptachlor epoxide might be further metabolized to a diol (Davidow and
Radomski, 1953),but the occurrence of such a compound has not yet been
demonstrated. However, when 25 µg of 14C-labelled heptachlor was
administered to male and female rats, the radioactivity was largely
encountered in the faeces as heptachlor epoxide, along with a second
metabolite; this second metabolite was also encountered in the urine,
but heptachlor epoxide was not. In both rats and rabbits treated with
heptachlor, heptachlor epoxide was the main metabolite found in
tissues. The urinary metabolite was found to be
1-hydroxy-2,3-epoxychlordene (see Figure 1) (Korte, 1968; Klein et
al., 1968). It is not known if this compound arises via heptachlor
epoxide or via a direct hydrolysis of heptachlor first to form
1-hydroxychlordene. The information available on the mammalian
metabolism of heptachlor has been reviewed (Brooks, 1969). In
experiments with rabbits and pig liver microsomes, the hydration of
heptachlor epoxides to a diol has been demonstrated (Brooks and
Harrison, 1969). See also "Fate of residues, in soil".
Effect on enzymes and other biochemical parameters
Heptachlor and heptachlor epoxide were administered to two or three
male rats at dietary levels of 0 (eight rats) 1.0 or 5.0 ppm for two
weeks. (Aldrin or dieldrin were also fed at the same levels to other
groups). After completion of the feeding, the animals were sacrificed
and microsomal preparations made from their livers. Microsomal
epoxidation, as measured by the epoxidation of aldrin to dieldrin, was
unaffected by 1.0 ppm, but was significantly affected by 5.0 ppm of
heptachlor or its epoxide. The increase in the rate of epoxidation was
correlated to the dietary level and to the concentration of the
cyclodiene compounds in the microsomes. Both heptachlor and aldrin
appeared to be substrates for the same enzyme, which is inhibited by
the epoxide. Microsomal metabolism of the epoxides was not found to
proceed farther (Gillett and Chan, 1968).
 Female rats were fed with heptachlor in the diet at a dose level
corresponding to 5 mg/kg body-weight/day for three months. After this
period 32P-labelled fenitrothion at a dose of 24 mg/kg body-weight
was administered in oil per os, and radioactive measurements were made
(five times during 24 hours) of the total activities of the liver and
of the degradation products in blood and liver. The results were
compared with those of the control group not pre-treated with
heptachlor. Total activity in the liver of the group pre-treated with
heptachlor was 50 percent higher than in the control group, with a
maximum after four hours. In the control group, the values diminished
gradually after the exposure to fenitrothion. The ratio of the oxygen
analogue to fenitrothion in the blood and liver suggests that the
conversion of fenitrothion to its oxygen analogue is enhanced and
accelerated. Results of this experiment demonstrate that pre-treatment
of rats with heptachlor increases the metabolism of fenitrothion
(Mestitzova et al., 1970).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Chicken
Groups comprising four male and twenty female chickens were fed
dietary levels of 0, 0.02, 0.1 or 0.2 ppm of heptachlor epoxide for 25
weeks. Body-weight increase was not affected by heptachlor epoxide.
Mortality was low in all groups, and a slightly higher incidence in
the 0.2 ppm group than in the other groups is of doubtful
significance. No abnormal behaviour was observed. Total weekly egg
production and mean weekly egg-weights were not significantly
different between test and control groups. Hatchability was slightly
decreased in the eggs from the groups fed 0.1 and 0.2 ppm; viability
of hatched chicks was, however, not affected by heptachlor epoxide
(Wolvin et al., 1969).
Chicken egg
Hatchability of hen eggs was not affected by injection of 1.5 mg
heptachlor in the yolk of fertile eggs (Smith et al., 1970).
Quail
Japanese quails were given 10 and 50 ppm heptachlor in the diet. There
was no obvious adverse effect on reproduction when the birds were ten
weeks of age (Shellenberger and Newell, 1965).
Rabbit
Pregnant female rabbits were treated orally with 0 (22 animals) or 5
mg/kg body-weight/day (20 animals) of heptachlor epoxide from days six
to eleven of gestation. Foetuses were recovered on day 28 by caesarean
section. There were no behavioural abnormalities apparent, and
body-weight gain was not affected by heptachlor epoxide. There were no
deaths. No compound-related effects were observed with respect to
numbers of viable and non-viable term foetuses, resorptions, empty
implantation sites, corpora lutea or non-gravid females. A significant
increase in foetus weight was evident in the treated group; this
increase was considered to be compound related. Survival time was not
considered to be affected by heptachlor epoxide. There were no
teratogenic effects attributable to the compound (Wazeter et al.,
1969).
Rat
Male and female rats fed exclusively on diets containing a mixture of
heptachlor and heptachlor epoxide (3.1) in amounts of 0, 0.3, 3 or 7
ppm have been mated through three succeeding generations. The number
of pregnancies in the F0 and F2 generations was slightly reduced in
the 0.3 ppm group, but not at higher dose levels. There was a slight
increase in the mortality of the pups in the second and third week
after birth in the 3 ppm group. This was not consistent with other
data obtained in these experiments. During three successive
generations, the compound exerted no apparent effect upon the
fertility of the progenitors or the ability of the progeny to survive
(Witherup et al., 1967a).
Male and female rats fed exclusively on diets containing 0, 0.3, 3, 6
and 10 ppm heptachlor have been mated through three succeeding
generations. In the second and third week after birth, mortality of
the pups, only in the second generation, was slightly increased in the
10 ppm group. No adverse effects have been reported in the successive
lower dose levels (Witherup et al., 1967b).
Toxicity studies on the metabolites
The acute oral toxicity of four heptachlor metabolites, chlordene,
3-chlorochlordene, 1-hydroxychlordene and chlordene epoxide, was found
to be greater than 4,600 mg/kg body-weight for the LD50 to male or
female rats (Mastri et al., 1969a). In another test in female rats,
the oral LD50 of 1-hydroxy-2, 3-epoxychlordene was calculated to be
between 4,600 and 10,200 mg/kg body-weight and for 2-chlorochlordene,
>10,200 mg/kg (Mastri et al.,1969b).
Groups each comprising 25 male and 25 female rats, were fed 0, 100,
250, 500, 1000 or 2000 ppm of the heptachlor metabolite
1-hydroxychlordene in their diet for up to 224 days. A rat of each sex
was sacrificed at intervals for autopsy. After receiving the test diet
for 110 days, three females from each level were selected and mated
with males from the same level. Growth and food consumption were
normal at all levels, and mortality appeared to be unaffected by the
test compound. At 2000 ppm, the compound may have produced intestinal
irritation. Within the one generation, 1-hydroxychlordene showed no
adverse effects on fertility, litter size, litter weight or survival
and growth of the young at any level. Gross pathology findings were
limited to one hepatoma in a female fed 2000 ppm and one in a male at
500 ppm; one female at 100 ppm had parotid gland tumours. A breast
tumour was seen in a control animal. Histopathology revealed
abnormalities only in the liver which at 1000 and 2000 ppm showed
slight to moderate cytoplasmic margination, which was also evident to
some extent in the controls and lower level groups. Hepatic cell
enlargement which occurred was also doubtfully related to 1-hydroxy-
chlordene (Ingle, 1965).
Special studies on photodecomposition
By irradiation of heptachlor, a caged photoisomerization product
similar to that observed with other cyclodiene insecticides has been
produced. It has been suggested that this compound may result from
exposure of heptachlor to sunlight. It is more toxic to houseflies and
mosquito larvae than heptachlor, but no information is available on
its mammalian toxicity (Rosen, 1969). Present evidence indicates that
neither photo-heptachlor nor photo-heptachlor epoxide contributes
significantly to the terminal residues on plants or soil, even though
films of heptachlor epoxide on glass were readily transformed into the
photo-product (Polen, 1970).
Short-term studies
Rat
Groups, each comprising ten female rats, were fed dietary levels of 0,
5 or 10 ppm of heptachlor or 10 ppm of DDT for eight months.
Examination of the liver cells of the groups fed 10 ppm of heptachlor
or DDT revealed essentially the same pictures, with increase in the
smooth endoplasmic reticulum and the mitochondria, although these
changes occurred to a greater degree with DDT. At 5 ppm, heptachlor
revealed the earlier stages of the development seen in animals fed 10
ppm. Comparison of the described findings with heptoma cells, obtained
from feeding carcinogenic aminoazo compounds, revealed a striking
difference (Stemmer and Hamdi, 1964).
A total of 269 rats of unspecified sex were fed 40, 45 or 60 ppm of
heptachlor or 35, 40 or 45 ppm of heptachlor epoxide or 40, 45 or 60
ppm of a 75 : 25 percent mixture of the two compounds. After feeding
for 140 days, some animals were returned to a basic uncontaminated
diet and others were continued on the test diet. The animals were then
sacrificed after 10, 20, 30, 60, 80 or 120 days from the 140-day
feeding period. Typical liver lesions were shown to regress after
discontinuing feeding, as evidenced by the observations of the rats
which were returned to a normal diet. After 120 days, a significant
number had normal livers. The largest number of recoveries occurred in
the group fed heptachlor, the next with the mixture and the least in
the group fed heptachlor epoxide. Some rats fed a treated diet for 260
days displayed a second type of lesion in the periphery of the liver
lobule, consisting of enlargement of cells with cytoplasm of empty
appearance. The nuclei were small and dense, with distinct cell
boundaries. It was not reported if these lesions regressed after
returning the animals to a normal diet. Evidence of hyperfunction of
the adrenal medulla was also present in rats that had not had
regression of their liver changes. There was indication of depletion
of catecholamine, the cytoplasmic granules were diminished and some
cells showed vacuolations (Stemmer and Jolly, 1964).
Four groups, each comprising 10 male and 20 female rats, were given
daily oral doses of 0, 5, 50 or 100 mg/kg body-weight of pure
heptachlor starting at about four months of age. Administration was
continued for 200 days or until the animals died. By the tenth day,
all the animals in the groups fed 50 or 100 mg/kg had died. At day
200, the surviving animals in the 5 mg/kg group and the controls were
sacrificed for autopsy. Prior to death, the 50 and 100 mg/kg groups
became irritable and had accelerated respiration by the second day.
Convulsions preceded death. In the group given 5 mg/kg, no clinical
abnormalities were seen until the 50th day, when hyper-reflexia, rapid
respirations and chronic convulsions were observed. Two males and two
females in this group died before completion of the experiment,
compared to only one female in the controls. Weight gain was not
affected by 5 mg/kg. Gross pathology revealed changes in liver, kidney
and spleen. Histopathologic examination showed fatty degeneration of
the liver cells and moderate fatty infiltration of the cells of the
urinary tubules, as well as hyperplasia of the smooth endoplasmic
reticulum of the liver and spleen in the group fed 5 mg/kg (Pelikan et
al., 1968).
COMMENT
Since the last evaluation of heptachlor, which was made at the 1966
Joint Meeting, considerable new information on the metabolism of
heptachlor and its epoxide has become available. In acute and
short-term feeding experimental these metabolites appeared to be less
toxic than heptachlor or heptachlor epoxide. It was noted that liver
cellular metabolism was affected by a dietary level of 5 ppm of
heptachlor in the rat, and attention was drawn to its effect on the
metabolism of a compound each as fenithrothion. As is the case with
other organochlorine compounds, the toxicological significance of
these findings could not be determined. In a three-generation
reproduction study in rats, no dose related effects were observed. In
view of the observations from other organochlorine pesticides, an
adequate carcinogenicity study in a second species of animal other
than the rat is needed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
Dog: 2.5 ppm in the diet, equivalent to 0.06 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The approximate distribution of the use of heptachlor outside of
continental United States is as follows:
Area Percentage
Europe 60
Asia 15
South America 15
Africa 5
North America 5
TOTAL 100%
In Europe the dominant use (probably >75%) is for seed treatment in
the culture of sugar beets. Minor significant uses are for seed
dressing of cereal grains and protection of potatoes.
The major uses for heptachlor in Asia are in the control of soil
insects in sugar cane, cereal grains and vegetables. In some areas, to
a small degree, heptachlor is used for the protection of pineapple.
In South America, the major uses of heptachlor are on sugar cane and
maize. The protection of bananas is a minor significant use of
heptachlor.
In Africa and in North America, the most significant uses of
heptachlor are on maize and small grains.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cane berries
No residues were detected (<0.01 ppm) in boysenberries, blackberries
or red or black raspberries maturing in soils treated with 4.0 and 8.0
lb/acre of heptachlor (Velsicol data).
In carrots
(Grown in soils previously subjected to treatment)
Soil treatment at Wooster, Ohio, was made in May 1960 with 6 and 200
pounds of heptachlor per acre. Soil readings three years later (1962)
showed residue of 1.2 to 1.9 ppm (combined H and HE) at 6 pounds per
acre. At the end of four and one half years, the readings at 6 pounds
per acre were 0.52 to 1.25 ppm, and at 200 pounds per acre, 1.8 to
16.5 ppm. Readings in carrots at the 6 pound rate were 0.07 ppm, and
at 200 pounds, 0.5 ppm four and one-half years after treatment.
Carrot/soil residue ratios are respectively: 0.056-0.195 and
0.030-0.278 (Velsicol, soil and carrot data).
Heptachlor was applied at the rate of 5 pounds per acre annually for
five years (1958-1962) to a loam soil. In 1967, combined heptachlor
and heptachlor epoxide reading was 0.78 ppm. Carrots grown in that
soil in 1967 had combined residues at the end of the season of 0.36
ppm. Carrot/soil residue ratio was 0.46 (Lichenstein et al., 1968).
Loam soil treated five consecutive years with abnormally high rates of
heptachlor (5 lb per acre, 25 cumulative) contained 4.6% of the
applied heptachlor five years after cessation of treatment. The mean
residue level (H+HE) in soil was 0.70 ppm; in carrots 0.413;
carrot/soil residue ratio, 0.59. In a parallel test wherein one 25 lb
per acre application was made, ten years after cessation of treatment,
mean residue levels were: in soil 0.719 ppm; in carrots 0.223 ppm;
carrot/soil residue ratio 0.310 (Lichtenstein et al., 1970).
In simulated agricultural experiments, Harris and Sans (unpublished
report, ca. 1969. Absorption of heptachlor epoxide residues by carrots
from three different soil types) demonstrated the strong dependence of
residue transmittal on soil organic content. At a constant level in
soil of 2 ppm of heptachlor epoxide, residues in carrots were
respectively 0.56, 0.03 and 0.01 ppm when grown in soils of 20 and 55
percent organic matter. The respective carrot/soil residue ratios are
0.28, 0.015 and 0.005.
In citrus
Negligible residues were found in oranges and lemons from trees on
soil treated at 3 and 6 lb/acre in California. Soil treatment of 6
lb/acre around grapefruit trees resulted in essentially no residue. A
few samples had readings of 0.01-0.02 ppm for heptachlor and gamma
chlordane, but there was no difference from the check samples
(Velsicol data).
In cottonseed and its products
No residues were found in meal, crude oil, refined oil or soapstock
made from cottonseed grown from heptachlor dressed seed treated at 4
fluid oz /100 lb seed (Velsicol data).
In maize and maize oil
Maize grown on soil into which heptachlor had been incorporated at
rates ranging from 0.75 to 4.0 lb/acre (both row and broadcast
treatment) did not result in measurable residues in natural ear, grain
samples or ensilage prepared from green plants. Stover or mature
stalks contained up to 0.02 ppm of heptachlor, 0.06 ppm of heptachlor
epoxide and 0.04 ppm of gamma chlordane in one experiment only,
however, there did not appear to be any relationship between treatment
level and residue level. Bruce at al. (1966) was able to show a linear
relationship between residue levels of heptachlor epoxide in maize
seed and residue levels in the soil in which the maize was grown using
treatments of 2-20 lb/acre. Maize seed residues (H+HE) did not exceed
0.01 ppm. Maize oil from maize grown in heptachlor treated soil did
not contain measurable residues (Velsicol data).
In peaches
Heptachlor applied at rates of 1.5 and 3.0 lb/acre to soil around
peach trees before petal fall did not result in measurable residues in
the ripe peaches (Velsicol data).
In peppers
Essentially no residues were found in peppers grown in soils treated
with 1-6 lb/acre of heptachlor. Although one sample had an apparent
heptachlor epoxide residue of 0.07 ppm from a 3 lb/acre treatment, no
residue was found in peppers from the 6 lb/acre treatment (Velsicol
data).
In pineapple
Heptachlor was applied to pineapple in Hawaii as a foliar treatment at
1, 2 and 4 lb/acre. Foliage samples collected two months after
treatment showed heptachlor epoxide residues of 0.03 ppm at 1 lb/acre
to 0.20 ppm at 4 lb/acre. Heptachlor and gamma chlordane residues also
occurred in the foliage samples. One heptachlor reading was 2.40 ppm,
while gamma chlordane was 0.94 ppm. Other than these two readings, the
maximum foliage level of heptachlor was 0.65 ppm, and for gamma
chlordane the highest reading was 0.26 ppm. All fruit samples gave
negative readings for heptachlor, heptachlor epoxide and gamma
chlordane. Low levels of residues occurred in samples of fruit shell.
Raw bran samples showed maximum values of 0.05 ppm for heptachlor,
0.11 ppm for heptachlor epoxide and 0.09 ppm for gamma chlordane at
the high treatment rate of 4 lb/acre. Recommended rates gave maximum
levels of 0.01 ppm heptachlor, 0.04 ppm heptachlor epoxide and 0.05
ppm gamma chlordane. Samples analyzed seven months after foliar
treatments gave negative results for fruit and fruit shell and low
values for foliage and raw bran (Velsicol data).
In small grains
Small grains grown in soils treated (preplant) at rates up to 3
lb/acre resulted in no measurable residues of heptachlor or heptachlor
epoxide in the grain for barley, oats, rye and wheat. Gamma chlordane
is reported at levels up to 0.02 ppm but in also observed in the check
sample. Although significant residue appears in the soil from
heptachlor treatment, it is not translocated to the mature grain. Some
levels occur in the straw (Velsicol data).
Seed treatment of small grains with heptachlor does not give residues
in grain of oats, rye or wheat. Rye straw had up to 0.33 ppm of
heptachlor epoxide, which appears to be contamination or an artifact.
Wheat straw gave negative values from one test area and 0.02 ppm to
0.04 ppm for heptachlor and gamma chlordane (Velsicol data).
In sorghum
Seed treatment tests on sorghum at recommended rates gave negative
readings for heptachlor, heptachlor epoxide and gamma chlordane in
samples of grain and straw grown from treated seed (Velsicol data).
In soybeans
Soil treatments were made in September 1966 at 3.0 and 6.0 lb ai/acre.
Soybeans were planted and samples collected in 1967. From both EC and
granular formulations, all green forage samples gave negative
readings. In dry beans, at a 2 lb/acre rate, heptachlor and gamma
chlordane readings were identical with the check samples. The
heptachlor epoxide readings were 0.02 to 0.03 ppm.
Green forage samples grown on soil treated the same year exhibit
negative readings in all samples.
In another experiment, soybeans grown in soil from a heptachlor
treatment made the previous year at 1.0 lb ai/acre show negative
readings for heptachlor expoxide. Heptachlor and gamma chlordane
readings were identical with the check samples.
Soybean processing fractions were obtained from soybeans grown in soil
treated with heptachlor in previous years. Beans grown in 1967 from
treatments made in 1965 show negative readings for heptachlor epoxide
in meal, crude oil, refined oil and soapstock. Some heptachlor
contamination in handling is indicated.
Processing fractions grown in 1967 from a soil treatment in 1966 show
heptachlor epoxide in crude oil at a level of 0.03 ppm. Meal, refined
oil and soapstock contained no heptachlor epoxide.
An extensive study was done at Texas A & M University. At 3.0 lb
heptachlor per acre applied eight days before planting, heptachlor
residues in the plants at time of pod formation were uniformly 0.01
ppm or less. Heptachlor epoxide residue averaged about 0.02 ppm, with
one highest reading of 0.04 ppm. Three pounds per acre soil treatment
is the maximum permitted.
At 3 lb/acre applied eight days before planting, heptachlor in the
bean was uniformly less than 0.01 ppm and heptachlor epoxide averaged
0.044 ppm, with the highest reading being 0.059 ppm. In the crude oil,
these readings were increased about tenfold. For example, heptachlor
readings in the crude oil averaged about 0.03 ppm, with the highest
reading being 0.04 ppm. Heptachlor epoxide in the crude oil averaged
about 0.38 ppm, with the highest reading being 0.52 ppm. These levels
have been shown to be removed during the refining process.
In sugarbeets
Whole sugarbeet sampled five months after preplant broadcast treatment
of soil at 3 lb/acre showed combined H and HE residues of 0.04 ppm,
and at 6 lb/acre showed 0.19 ppm. Laboratory dried pulp showed 0.18
ppm at 3 lb/acre and 0.31 ppm at 6 lb/acre. Pilot plant drying showed
0.14 ppm at 3 lb/acre and 0.34 at 6 lb/acre.
No residues were found in sugarbeets or sugarbeet pulp from furrow and
coated seed treatments at 0.8-1.0 lb/acre (Velsicol data).
Sugarbeets harvested after seed treatment with heptachlor had no
cyclodiene residues (Harris et al., 1966).
In tomatoes
Tomatoes were grown in soil treated prior to planting with 1-3 lb/acre
of granular formulation or 2,3 and 6 lb/acre of emulsifiable
concentrate. No heptachlor epoxide residues were found in the tomatoes
from the granular formulation, and all heptachlor residues were at the
limit of sensitivity (0.01 ppm) except one value of 0.02 ppm. Residues
from the E.C. formulation were mainly <0.01 ppm except one heptachlor
at 0.01 ppm, one at 0.04 ppm (6 lb/acre) and one heptachlor epoxide at
0.02 ppm (6 lb/acre). Maximum combined residue in any one sample at
recommended rates (2-3 lb/acre) was 0.02 ppm.
In a second similar test in soil treated at 2,3 and 6 lb/ acre, no
residues of heptachlor, its epoxide or gamma chlordane were found in
any of the ripened fruit.
Soil samples contained 0.67 ppm heptachlor, 0.08 ppm heptachlor
epoxide and 0.26 ppm gamma chlordane at the 6 lb/acre rate (Velsicol
data).
In soil
In Kansas one year after heptachlor soil treatments, soil residues
were: 2 lb/acre rate - 0.26 to 0.47 ppm (combined H and HE), 3 lb/acre
rate - 0.18 to 0.32 ppm and 6 lb/acre rate - 0.63 to 2.24 ppm
(Velsicol, soybean data)
In Illinois, row treatments of 1.0 and 1.6 pounds heptachlor per acre
showed no residues in the soil fifteen months later (Velsicol, soybean
data).
In Kansas, four months after treatment at three lb/acre soil showed
0.21 to 0.40 ppm (combined H and HE), and 6 pounds per acre showed
0.49 to 3.61 ppm (Velsicol, soybean data).
In Mississippi, seed treatment at the rate of 0.07 pound heptachlor
per acre showed no residues in soil five months after application
(Velsicol, cotton data).
In a survey of the soil of 31 farms in Ontario, Harris, Sans and Miles
(1966) found heptachlor and/or epoxide residues (maximum 0.2 ppm) in
three samples of four which had a previous history of treatment with
heptachlor; one of the three had no recorded history of heptachlor
treatment, but 27 others with history of no heptachlor treatment had
no heptachlor residues. Two soils which had seed treatments (maize and
sugarbeets) had no detectable heptachlor residues. Those samples which
contained heptachlor or epoxide residues also contained residues of
gamma chlordane.
Studies on the mobility of organochlorine pesticides, including
heptachlor and epoxide, in soil demonstrated that these residues do
not move by leaching (Harris, 1969), but may be locally redistributed
by mechanical cultivation (Harris and Sans, 1970).
Under South African conditions, Wiese and Bossen (1966) found that
rapidity of degradation of five chlorinated insecticides " ... was
greater than reflected in most published literature ..." and that "
... heptachlor epoxide was the least persistent ..." In a survey of
agricultural soils of the Atlantic Provinces of Canada, heptachlor and
heptachlor epoxide were found in 9 percent of the soils in
concentrations between 0.06 and 0.86 ppm (Duffy and Wong, 1967).
In animal products
A great deal of work has been carried out to elucidate the propensity
of heptachlor and heptachlor epoxide residues, in or on feeds, to
transmit to milk or store in meat. Much of this information is
summarized by Saha (1969).
Meat
A one-year study on twenty beef cows and their calves was carried out
by Virginia Polytechnic Institute and the U.S. Department of
Agriculture (1968). Ten treated cows were fed a conventional ration of
alfalfa hay containing 0.4 ppm heptachlor and heptachlor epoxide
residues. In animals on contaminated feed continuously, the maximum
residue accumulating in the fat was 1.53 ppm (about four times the
residue level in the feed).
In a similar study over a two-year period involving twenty yearling
steers, the same investigators concluded the results were in good
agreement with that reported previously for pregnant and lactating
beef cows. The study showed clearly that with continued feeding of
contaminated ration the residues stabilize around 1.00 ppm, after 18
months on a residue-free regime, the combined H+HE residues in the
cattle ranged from 0.19 to 0.94 ppm (Bovard et al., 1968).
A 98-day feeding experiment, conducted with 56 beef steers in which
half were fed alfalfa hay containing 0.16 ppm H+HE, resulted in 0.017
to 0.020 ppm HE in the fat of treated animals and 0.004 to 0.007 ppm
in the fat of control steers (Hall et al., 1965).
Milk
In a study at the University of Tennessee, alfalfa hay containing
residues of 0.08 or 0.29 ppm heptachlor and heptachlor epoxide were
fed to dairy animals. Supplemental grain was fed according to milk
production. At the end of the 35-day feeding period, the milk fat from
the high and the low level intake groups contained 0.34 and 0.22 ppm
heptachlor epoxide, respectively. Converting to 4% butter fat milk,
this would be 0.014 ppm residue for the high group and 0.009 ppm for
the low group (Demott et al., 1967).
In an extensive study, lactating cows were fed alfalfa hay containing
residues of heptachlor and heptachlor epoxide resulting from treating
alfalfa at three different levels with heptachlor. The hay, fed for 30
days, contained average residues of heptachlor and heptachlor epoxide
of 0.045 ppm, 0.086 ppm and 0.160 ppm from the 0.25, 0.5 and 1.0
lb/acre treatments, respectively. The highest concentration of residue
in milk occurred between the 18th and 24th days of the feeding period
and averaged 0.013, 0.026 and 0.049 ppm for the test animals in each
group. Removal of treated hay from the diet resulted in a sharp
decline of residues in the milk, followed by a gradual disappearance
of residue over 13 weeks (Waldron at al., 1968).
Another study involved feeding lactating animals with measured
quantities of heptachlor epoxide added to the feed. Heptachlor epoxide
(in grain) was fed at the levels of 0.005 ppm and 0.020 ppm based on
total roughage ingested (hay plus silage) assumed at 50 pounds per
day. At the end of the feeding interval of 28 days, the residue of
heptachlor epoxide in milk was 0.0027 and 0.0043 ppm, respectively,
for the low and higher level fed. At 25 days the respective milk
residue levels for heptachlor epoxide were 0.0029 and 0.0044 ppm
(Hardee et al., 1964).
In a practical test (W.A.R.F. report, 1967), cows were fed maize
silage (50 pounds/day) and ground maize and cob meal (12 pounds/day),
all grown on soil treated with heptachlor for at least eight
consecutive years, at the recommended rate of one half pound per acre.
This feed was supplemented with 10 pounds of alfalfa hay, grown, in
rotation with maize in soil treated for two years, nonconsecutive, at
one half pound/acre, with the most recent application being two years
prior to the growing of the hay crop. The bedding for these cows,
consisting of shredded maize stalks, was also obtained from soil
treated with heptachlor for at least eight years. Milk samples gave
equivalent heptachlor epoxide readings after 10, 20 and 30 days of
feeding, varying between 0.004 and 0.007 ppm. There was no evidence of
increased milk residues during the course of the study and there was
no difference from the checks. All readings for heptachlor, in milk,
were reported as less than 0.001 ppm, and gamma-chlordane was not
detected in milk.
A survey of milk collected in Iowa was made in 1965 to determine what,
if any, residues could be detected. Heptachlor epoxide could be
characterized as being virtually absent from milk samples. All
readings averaged less than 0.002 ppm heptachlor epoxide, and only one
of 20 readings was as high as 0.007 ppm (W.A.R.F. report, 1965).
Poultry and eggs
In a chart provided by the U.S. Food and Drug Administration to the
Committee on Pesticides, Agricultural Research Institute (NAS/NRC)
Meeting, May 14, 1969, it is estimated that the contribution to the
diet of heptachlor epoxide from eggs is nil. Poultry is not shown by
itself, but a correlation between poultry and eggs can be assumed.
In a chicken feeding study (Velsicol data), chickens received
heptachlor epoxide in their entire diet at levels up to more than ten
times that which could occur in chicken feed from registered uses of
heptachlor. The feeding continued for 21 weeks, and white meat, dark
meat, fat and eggs were analyzed. White meat was negative as to
residues except for negligible values in the low hundreths of a part
per million range. Residues in the fat, from chickens fed at the level
which would reasonably reflect a tolerance in commodities used as feed
for chickens, were less than the 0.3 ppm non-actionable level in fat.
Similarly, residues in eggs from chickens which had received
heptachlor epoxide in their total diet for 21 weeks at a level
appropriate for a tolerance, but certainly higher than is encountered
in total feed, averaged 0.03 ppm, which is the non-actionable level in
eggs.
Heptachlor epoxide (in combination with other organochlorine
insecticides) were fed to 60 laying hens for 20 weeks at levels of
0.05, 0.15 and 0.45 ppm to determine residue levels in eggs. The
plateau levels in eggs approximate the feeding levels of heptachlor
epoxide; yolk/white concentration ratio is 99/1 (Cummings et al.,
1966).
FATE OF RESIDUES
In soil
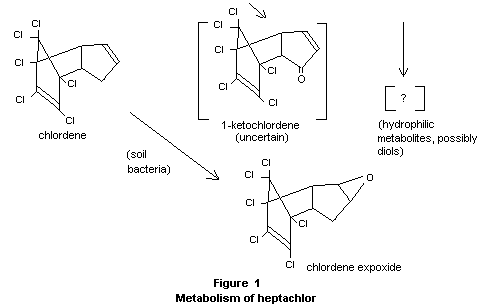
1-Hydroxy chlordene, a conversion product, was found in a small number
of soil samples treated with heptachlor (Duffy and Wong, 1967).
Information on routes of metabolism of heptachlor by soil
micro-organisms has recently become available. As is the case with
mammals, 1-hydroxy 2,3-epoxychlordene was formed and by soil bacteria,
and it was demonstrated that it was derived from 1-hydroxychlordene,
but not from heptachlor epoxide. An unknown metabolite has also been
produced from 1-hydroxychlordene; the structure 1-keto chlordene has
been postulated for this compound but not proven. In addition, other
routes of metabolism involving a reductive stop to chlordene have also
been shown to occur in soil (Miles et al., 1969), but it is not known
if this pathway occurs in mammals. The significance of these chlordene
derivatives has been evaluated toxicologically (see "Toxicity studies
on the metabolites").
Effects of food processing on residues
Evidence continues to accrue that residues of heptachlor epoxide and
associated gamma chlordane are concentrated in the peels of sub-soil
crops, such as rutabagas, carrots and potatoes (Saha and Stewart,
1967). The residue distribution pattern discerned by older analytical
methods (Fox et al., 1964) has now been confirmed by the more
sensitive gas-liquid gas chromatography method (Stewart et al., 1965).
Concentration of heptachlor-derived residues at the outer surfaces of
crops with small surface to volume ratios simplifies effective residue
reduction by several normal food preparation steps. Washing removes
loosely held surface residues such as that contained in adherent soil
(Farrow et al., 1969). Washing plus peeling is highly effective in
residue removal and in frequently used for potatoes and root crops in
home cooking, and always is used in commercial canning of these crops
and tomatoes. Abrasive peeling is also effective; it is normally
used in home cooking and in commercial canning.
Commercial processing of edible vegetable oils frees the finished oil
from residues of heptachlor and heptachlor epoxide, as well as other
organochlorine pesticides (IUPAC Commission on Terminal Pesticide
Residues, 1970; Smith et al., 1968).
In the preparation of animal products, several normal food processing
steps reduce residues. Heptachlor and epoxide, being lipophilic, tend
to remain with fat when it is separated from meat by heating or
mechanical trimming. Reduction of heptachlor and heptachlor epoxide
residues has been reported in milk processing. Production of
condensed, sterilized, spray dried and drum dried milks results in
lower levels (fat basis) of heptachlor epoxide residues than the raw
milk from which they were made (Liska and Stadelman, 1969).
No evidence is available regarding the effect of normal storage
procedures on heptachlor residues.
Minimal evidence (Del Monte Corp., private communication, 1969)
indicates that short, simple cooking of leafy vegetables, such as
spinach, would not affect levels of residue from heptachlor treatment.
Evidence of residues in food in commerce or at consumption
In a series of papers, Duggan and others have assessed the dietary
intake of pesticide chemicals in the United States and determined the
pesticide residue levels in various food groups, domestic and
imported.
For heptachlor and heptachlor epoxide,a three year average (1964 to
1967) was 0.000031 milligrams/kilogram body-weight/day for heptachlor
and heptachlor epoxide combined. This assessment was based on the food
intake of almost twice the "average" intake of the "average"
individual. The value is 6 percent of the Acceptable Daily Intake for
heptachlor and its epoxide (0.0005 mg/kg).
The average levels of heptachlor and heptachlor epoxide residues in
food and feed - domestic, imported and ready to eat - are summarized
in Table I.
Total diet studies in England and Wales (Abbott et al., 1969) detected
heptachlor expoxide in three samples from the cereal group (less than
0.01 ppm), in ten samples from the meat group (up to 0.006 ppm) and
seven samples from the fats group (up to 0.008 ppm). In terms of total
diet, one out of 66 composites contains 0.001 ppm of heptachlor
epoxide; in all other cases the calculated concentration in the diet
was less than 0.0005 ppm heptachlor epoxide. No heptachlor was
detected at limit of detection of 0.0005 ppm.
METHODS OF RESIDUE ANALYSIS
Multidetection analytical techniques, such as those adopted (1969) by
the IUPAC Commission on Pesticide Residue Analysis, can be used to
determine residues of heptachlor, heptachlor epoxide, gamma-chlordane
and nonachlor in crops, soils and animal products down to a lower
sensitivity limit of 0.01 ppm. Procedures based on those given in the
Pesticide Analytical Manual, Vol. I, USHEW, FDA, 1968, with slight
modifications, and designated as AM 0237, AM 0486 and AM 0481, have
been developed by Velsicol Co. for the analysis of residues in fruits,
vegetables, grains, meat and poultry. The method of Cummings et al.
(1966) is suitable for determining heptachlor and heptachlor epoxide
in eggs. A method for the analysis of 1-hydroxychlordene in plant
tissues is under development (Nash and Beall, 1970 pre-print).
APPRAISAL
The data on the absorption of heptachlor and heptachlor epoxide by
carrots grown in soils containing residues, either from current or
previous annual treatments at normal or high rates, indicates that the
temporary practical residue limit should be increased from 0.1 ppm to
0.2 ppm.
New data on residues in maize grain grown in heptachlor treated soil
shows that this commodity is within the practical residue limit of
0.02 ppm established for raw cereals. This continues to be true for
the grain of barley, oats, rye and wheat.
Data from a total of eight studies on the relationship between the
levels of heptachlor and heptachlor epoxide in feed to the levels in
meat and milk show that the maximum residue level in animal feed, that
can be permitted without exceeding the practical residue limits for
these animal products, is 0.04 ppm of combined residues. If dried
sugarbeet pulp is fed in the diet of beef or dairy animals and
constitutes up to 20 percent of the feed, then the maximum permissible
residues in the pulp would be 0.20 ppm and in the whole sugarbeet
would be 0.04 ppm. Similar considerations would apply to maize stover,
soybean forage and pineapple bran used in animal feeds.
TABLE I
Heptachlor epoxide (or heptachlor) residues in food and feed1
RAW AGRICULTURAL PRODUCT READY-TO-EAT FOOD
Food Category Domestic Imported
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large Fruit 0.7 < 0.005 0.7 < 0.005 - -
Small Fruit 1.0 < 0.005 0.7 < 0.005 - -
Grain & Cereals
for human use 0.2 < 0.005 - - 4.1 < 0.001
for animal use 2.5 < 0.005 - - - -
Vegetables
Leaf and Stem 3.0 < 0.005 3.3 < 0.005 1.4 < 0.001
Vine and Ear 2.4 < 0.005 2.3 < 0.005 5.4 < 0.001
Root 3.8 < 0.005 5.7 < 0.005 - -
0.82 < 0.005 4.62 < 0.005 - -
Potatoes 5.4 < 0.001
Beans 0.4 < 0.005 1.2 < 0.005 1.4 < 0.001
Eggs 3.1 < 0.005 3.4 < 0.005
Nuts 0.5 < 0.005 - -
Processed
(canned, frozen)
Domestic 0.8 < 0.001
Imported 0.7 < 0.001
Fluid Milk 23.0 3.03
(fat basis) 0.82 < 0.005
Dairy Products 21.3 0.02 3.0 < 0.005
(fat basis) 0.82 < 0.005 0.1 < 0.005
1/ extracted from USA-FDA surveillance programme report (Duggan, 1968).
2/ Heptachlor residues
The committee received information on residues in pineapple fruit
resulting from foliar treatment at 1,2 and 4 lb/acre. No residues of
heptachlor, heptachlor epoxide or gamma chlordane were found in the
fruit at the detection limit of 0.01 ppm for the method of analysis,
thus supporting a negligible residue tolerance of 0.01 ppm.
With the exception of carrots, the new data available did not indicate
a need to change the previous recommendations for practical residue
limits for the items listed. The previous practical residue limit of
0.125 ppm for milk products was changed to 0.15 to allow for the
limitations and variabilities of residue analytical procedures. Data
was presented which suggested the addition of practical residue levels
for tomatoes, cottonseed, soybeans, crude soybean oil, refined soybean
oil, poultry fat and eggs (on a shell-free basis).
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
TOLERANCES (These are additional to those in previous recommendations)
ppm
Pineapple (edible portions) 0.01
PRACTICAL RESIDUE LIMITS
ppm
Milk and milk products (determined on the
extracted fat) 0.15
Fat of meat and poultry 0.2
Raw cereals, tomatoes, cotton seed, soybeans,
refined soybean oil 0.02
Vegetables (except where otherwise specified),
eggs (shell-free basis) 0.05
Carrots 0.2
Crude soybean oil 0.5
Citrus fruit 0.01
Remarks
Residues of heptachlor and its epoxide should each be determined and
the sum expressed as heptachlor. Tolerances apply to residues from
application to seed and soil only.
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate carcinogenicity study in a second species of animal.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric., 20: 245
Bovard, K.P., Fontenot, J.P. and Priode, B.M. (1968) 1967-1968
Livestock Research Report, Research Div., Virginia Polytechnic
Institute
Bovard, K.P., Fontenot, J.P., Watson, D.F. and Priode, B.M. (1968)
Abstract in J. Animal Science, 27(4): 1131
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Brooks, G.T. and Harrison, A. (1969) Hydration of HEOD (dieldrin and
the heptachlor epoxides by microsomes from the livers of pigs and
rabbits). Bull. environ. Cont. Toxicol., 4, (6): 352-361
Comptes Rendus, (1970) XXV Conference, IUPAC, July 1-5, 1969 Cortina
d'Ampezzo, Italy, 187-195
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide
residues in total diet samples (IV). Pest. Mon., J. 2(4): 140-152
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low-level feeding of five organochlorine insecticides to
hens. J. Assoc. Off. Anal. Chem., 49: 354-364
Davidow, B. and Radomski, J.L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol. exp.
Therap., 107: 259-265
Demott, D.J., Miles, J.T., Hinton, S.A. and Hardin. (1967) L.J.J.
Dairy Science, 49(12): 1495
Dorough, H.W. (1968) Residues in soybeans sprayed with chlordane or
grown in soils treated with heptachlor or chlordane. Report to
Velsicol Chemical Corp., 10 September
Duffy, J.R. and Wong, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic provinces
of Canada. J. Agr. Food Chem., 15: 457-464
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed, Pesticide residues in total diet samples (II). Pest. Mon.
J., 1(2): 2-12
Duggan, R.E. (1968a) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pest. Mon. J., 1(4): 2-7
Duggan, R.E. (1968b) Residues in food and feed. Pesticide residue
levels in foods in the United States from July 1, 1963 to June 30,
1967. Pest. Mon. J., 2(1): 2-46
Duggan, R.E. (1969) Chart provided by U.S. FDA to Committee on
Pesticides, Agricultural Research Institute (NAS/NRC) Meeting, May 14
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest. Mon.
J., 2(4): 153-162
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Calculated daily consumption of pesticides with foods are
discussed and compared with currently accepted values. Science, 157:
1006-1010
FAO/WHO (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO/Food Add./67.32
Farrow, R.P., Elkins, G.R., Rose W.W., Lamb, F.C., Rulls, J.W. and
Mercer W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews,
29:73-87
Fox, C.J.S., Chisholm, D. and Stewart, D.K.R. (1964) Can. J. Plant.
Sci.,44: 149
Gillett, J.W. and Chan, J.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Fd. Chem., 16: 590-593
Hall, O.G., Hardin, L.J., Fryer, M.E. and Glover, J.A. (1965)
Tennessee Farm and Home Science Progress Report No: 56
Hardee, D.D. Gutenmann, W.H., Keenan, G.I. Gyrisco, G.G. Lisk, D.J.,
Fox F.H. Trimburger, G.W. and Holland, R.F. (1964) Residues of
heptachlor epoxide and telodrin in milk from cows fed at part per
billion insecticide levels. J. Econ. Entomol., 57: 404-407
Harris, C.R., Sans, W.W. and Miles, J.R.W. (1966) Exploratory studies
on occurrence of organochlorine insecticide residues in agricultural
soils in southwestern Ontario. J. Agr. Fd. Chem., 14: 398-403
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Fd. Chem.,
17: 80-82
Harris, C.R. and Sans, W.W. (1969) Unpublished report, Absorption of
heptachlor epoxide residues by carrots from three different soil types
Harris, C.R. and Sans, W.W. (1970) Pesticide Progress, Canada
Department of Agriculture, Ottawa, 8(1)
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rate for 224 days. Unpublished report from the University
of Illinois, submitted by Velsicol Chemical Corporation
Klein, W., Korte, F., Weisgerber, I., Kaul, R., Mueller, W. and
Djirsarai, A. (1968) Über den Metabolismas von Endrin, Heptachlor und
Telodrin. Qdal. Pldt. Mater. veg. (Den Haag), 15: 225-238
Korte, F. (1968) Metabolism of chlorinated insecticides.(2)
Heptachlor. Paper submitted to the IUPAC Commission on Terminal
Pesticide Residues, Vienna, 1967. Appendix VI. IUPAC Information
Bulletin No: 32, p. 110
Korte, F. and Porter, P.E. (1970) Minutes of the Fifth Meeting of the
IUPAC Commission on Terminal Pesticide Residues, September 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Lichtenstein, E.P. Fuhremann, T.W. and Schulz, K.R. (1968) Use of
carbon to reduce the uptake of insecticides as soil residues by crop
plants. Effects of carbon on insecticide absorption and toxicity in
soils. J. Agr. Fd. Chem. 16(2): 348-355
Liska, B.J. and Stadelmann, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews, 29: 61-72
Martin, RJ. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III). Pest. Mon. J., 1(4): 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, submitted to Velsicol Chemical
Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories, submitted to Velsicol
Chemical Corporation
Mestitzova, M., Kovac, J. and Durcek, K. (1970) Heptachlor induced
changes in fenitrothion metabolism. Bull. environ. Contam. Toxicol.,
5(3): 195-201
Miles, J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
econ. Entomol., 62: 1334-1338
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Nash, R.G. and Beall, M.L., Jr. (1970) Preprint of unpublished work
Pelikan, Z., Halacka, K., Polster, M. and Cerny, E. (1968)
Intoxication à long terse chez les rats par l'heptachlore à petit
doses. Arch. belges Méd. Soc., 7: 529-538
Polen, P.B. (1970) Terminal residues of chlordane. Paper submitted to
the IUPAC Commission on Terminal P Pesticide Residues, Erbach
(Rheingan) Federal Republic of Germany. 14-18 September
Rosen, J.D. (1969) Personal comminication to the participants of the
IUPAC Commission on Terminal Pesticide Residues, Cortina d'Ampezzo,
July 1969
Saha, J.G. (1966) Significance of organochlorine insecticide residues
in fresh plants as possible contaminants of milk and beef products.
Residue Reviews, 26: 89-126
Saha, J.G. and Stewart, W.W.A. (1967) Can. J. Plant Sci.:47, 79
Seal, W.L., Dawsey, L.H. and Cavin, G.E. (1967) Pesticides in soil.
Monitoring for chlorinated hydrocarbon pesticides in soil and root
crops in the Eastern States in 1965. Pest. Mon. J., 1(3): 22-25
Shellenberger, T.E. and Newell G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail. Lab.Anim.Care., 15:
119
Shellenberger, T.S., Lei, J., Udale B. and Newell, G.W. (1966)
Comparitive toxicity of DDT, dieldrin and heptachlor to Japanese and
bobwhite quail. Toxicol. appl. Pharmacol., 8: 353
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) J.
Amer. Oil Chem. Soc.,45(12): 866
Smith, S.J., Weber C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocardon pesticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Stemmer, K.L. and Hamdi, E. (1964) Electron microscopic changes in the
liver cells after prolonged feeding of DDT and heptachlor. Unpublished
report from the Kettering Laboratory, University of Cincinnati,
submitted by Velsicol Chemical Corporation
Stemmer, K.L. and Jolley, W.P. (1964) Regression of hepatic lesion of
heptachlor and its epoxide. Unpublished report of the Kettering
Laboratory, University of Cincinnati, submitted by Velsicol Chemical
Corporation
Stewart, D.K.R., Chisholm, D. and Fox, C.J.S. (1965) Can. J. Plant
Sci.,45: 72
Velsicol Chemical Co., (1966-1968) Reports of residue analyses
Waldron, A.C., Kaeser, H.E., Golemam, D.L., Staubus, J.R. and
Niemczyk, H.D. (1968) Heptachlor and heptachlor epoxide residues in
salt-treated alfalfa and in milk and cow tissues. J. Agr. Fd. Chem.,
16: 627-631
Wiese, I.H. and Basson, N.C.J. (1966) S. Afr. J. Agric. Sci.,9: 945
Wisconsin Alumni Research Foundation, (1965) Report to Velsicol
Chemical Co.
Wisconsin Alumni Research Foundation, (1967) Report to Velsicol
Chemical Co.
Wazeter, F.X., Butler, R.M., Geil, R.G. and Rehkemper, J.A. (1969)
Heptachlor epoxide. Teratology study in the Dutch rabbit. Unpublished
report from the International Research and Development Corporation
submitted to Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967a) The
effects exerted on the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor and its epoxide into
their daily diet. Unpublished report from the Kettering Laboratory,
Cincinnati, submitted by Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967b) The
effects exerted upon the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor into their daily
diets. Unpublished report from the Kettering Laboratory, Cincinnati,
submitted by Velsicol Chemical Corporation
Wolvin, A.R., Jenkins, D.H. and Fancher, O.E. (1969) Toxicity residue
and reproduction study on heptachlor epoxide chickens. Unpublished
report by Industrial Bio-test Laboratories, submitted to Velsicol
Chemical Corporation
Wong, D.T. and Terriere, L.C. (1965) Epoxidation of aldrin, isodrin
and heptachlor by rat liver microsomes. Biochem. Pharmacol., 14:
375-377
Female rats were fed with heptachlor in the diet at a dose level
corresponding to 5 mg/kg body-weight/day for three months. After this
period 32P-labelled fenitrothion at a dose of 24 mg/kg body-weight
was administered in oil per os, and radioactive measurements were made
(five times during 24 hours) of the total activities of the liver and
of the degradation products in blood and liver. The results were
compared with those of the control group not pre-treated with
heptachlor. Total activity in the liver of the group pre-treated with
heptachlor was 50 percent higher than in the control group, with a
maximum after four hours. In the control group, the values diminished
gradually after the exposure to fenitrothion. The ratio of the oxygen
analogue to fenitrothion in the blood and liver suggests that the
conversion of fenitrothion to its oxygen analogue is enhanced and
accelerated. Results of this experiment demonstrate that pre-treatment
of rats with heptachlor increases the metabolism of fenitrothion
(Mestitzova et al., 1970).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Chicken
Groups comprising four male and twenty female chickens were fed
dietary levels of 0, 0.02, 0.1 or 0.2 ppm of heptachlor epoxide for 25
weeks. Body-weight increase was not affected by heptachlor epoxide.
Mortality was low in all groups, and a slightly higher incidence in
the 0.2 ppm group than in the other groups is of doubtful
significance. No abnormal behaviour was observed. Total weekly egg
production and mean weekly egg-weights were not significantly
different between test and control groups. Hatchability was slightly
decreased in the eggs from the groups fed 0.1 and 0.2 ppm; viability
of hatched chicks was, however, not affected by heptachlor epoxide
(Wolvin et al., 1969).
Chicken egg
Hatchability of hen eggs was not affected by injection of 1.5 mg
heptachlor in the yolk of fertile eggs (Smith et al., 1970).
Quail
Japanese quails were given 10 and 50 ppm heptachlor in the diet. There
was no obvious adverse effect on reproduction when the birds were ten
weeks of age (Shellenberger and Newell, 1965).
Rabbit
Pregnant female rabbits were treated orally with 0 (22 animals) or 5
mg/kg body-weight/day (20 animals) of heptachlor epoxide from days six
to eleven of gestation. Foetuses were recovered on day 28 by caesarean
section. There were no behavioural abnormalities apparent, and
body-weight gain was not affected by heptachlor epoxide. There were no
deaths. No compound-related effects were observed with respect to
numbers of viable and non-viable term foetuses, resorptions, empty
implantation sites, corpora lutea or non-gravid females. A significant
increase in foetus weight was evident in the treated group; this
increase was considered to be compound related. Survival time was not
considered to be affected by heptachlor epoxide. There were no
teratogenic effects attributable to the compound (Wazeter et al.,
1969).
Rat
Male and female rats fed exclusively on diets containing a mixture of
heptachlor and heptachlor epoxide (3.1) in amounts of 0, 0.3, 3 or 7
ppm have been mated through three succeeding generations. The number
of pregnancies in the F0 and F2 generations was slightly reduced in
the 0.3 ppm group, but not at higher dose levels. There was a slight
increase in the mortality of the pups in the second and third week
after birth in the 3 ppm group. This was not consistent with other
data obtained in these experiments. During three successive
generations, the compound exerted no apparent effect upon the
fertility of the progenitors or the ability of the progeny to survive
(Witherup et al., 1967a).
Male and female rats fed exclusively on diets containing 0, 0.3, 3, 6
and 10 ppm heptachlor have been mated through three succeeding
generations. In the second and third week after birth, mortality of
the pups, only in the second generation, was slightly increased in the
10 ppm group. No adverse effects have been reported in the successive
lower dose levels (Witherup et al., 1967b).
Toxicity studies on the metabolites
The acute oral toxicity of four heptachlor metabolites, chlordene,
3-chlorochlordene, 1-hydroxychlordene and chlordene epoxide, was found
to be greater than 4,600 mg/kg body-weight for the LD50 to male or
female rats (Mastri et al., 1969a). In another test in female rats,
the oral LD50 of 1-hydroxy-2, 3-epoxychlordene was calculated to be
between 4,600 and 10,200 mg/kg body-weight and for 2-chlorochlordene,
>10,200 mg/kg (Mastri et al.,1969b).
Groups each comprising 25 male and 25 female rats, were fed 0, 100,
250, 500, 1000 or 2000 ppm of the heptachlor metabolite
1-hydroxychlordene in their diet for up to 224 days. A rat of each sex
was sacrificed at intervals for autopsy. After receiving the test diet
for 110 days, three females from each level were selected and mated
with males from the same level. Growth and food consumption were
normal at all levels, and mortality appeared to be unaffected by the
test compound. At 2000 ppm, the compound may have produced intestinal
irritation. Within the one generation, 1-hydroxychlordene showed no
adverse effects on fertility, litter size, litter weight or survival
and growth of the young at any level. Gross pathology findings were
limited to one hepatoma in a female fed 2000 ppm and one in a male at
500 ppm; one female at 100 ppm had parotid gland tumours. A breast
tumour was seen in a control animal. Histopathology revealed
abnormalities only in the liver which at 1000 and 2000 ppm showed
slight to moderate cytoplasmic margination, which was also evident to
some extent in the controls and lower level groups. Hepatic cell
enlargement which occurred was also doubtfully related to 1-hydroxy-
chlordene (Ingle, 1965).
Special studies on photodecomposition
By irradiation of heptachlor, a caged photoisomerization product
similar to that observed with other cyclodiene insecticides has been
produced. It has been suggested that this compound may result from
exposure of heptachlor to sunlight. It is more toxic to houseflies and
mosquito larvae than heptachlor, but no information is available on
its mammalian toxicity (Rosen, 1969). Present evidence indicates that
neither photo-heptachlor nor photo-heptachlor epoxide contributes
significantly to the terminal residues on plants or soil, even though
films of heptachlor epoxide on glass were readily transformed into the
photo-product (Polen, 1970).
Short-term studies
Rat
Groups, each comprising ten female rats, were fed dietary levels of 0,
5 or 10 ppm of heptachlor or 10 ppm of DDT for eight months.
Examination of the liver cells of the groups fed 10 ppm of heptachlor
or DDT revealed essentially the same pictures, with increase in the
smooth endoplasmic reticulum and the mitochondria, although these
changes occurred to a greater degree with DDT. At 5 ppm, heptachlor
revealed the earlier stages of the development seen in animals fed 10
ppm. Comparison of the described findings with heptoma cells, obtained
from feeding carcinogenic aminoazo compounds, revealed a striking
difference (Stemmer and Hamdi, 1964).
A total of 269 rats of unspecified sex were fed 40, 45 or 60 ppm of
heptachlor or 35, 40 or 45 ppm of heptachlor epoxide or 40, 45 or 60
ppm of a 75 : 25 percent mixture of the two compounds. After feeding
for 140 days, some animals were returned to a basic uncontaminated
diet and others were continued on the test diet. The animals were then
sacrificed after 10, 20, 30, 60, 80 or 120 days from the 140-day
feeding period. Typical liver lesions were shown to regress after
discontinuing feeding, as evidenced by the observations of the rats
which were returned to a normal diet. After 120 days, a significant
number had normal livers. The largest number of recoveries occurred in
the group fed heptachlor, the next with the mixture and the least in
the group fed heptachlor epoxide. Some rats fed a treated diet for 260
days displayed a second type of lesion in the periphery of the liver
lobule, consisting of enlargement of cells with cytoplasm of empty
appearance. The nuclei were small and dense, with distinct cell
boundaries. It was not reported if these lesions regressed after
returning the animals to a normal diet. Evidence of hyperfunction of
the adrenal medulla was also present in rats that had not had
regression of their liver changes. There was indication of depletion
of catecholamine, the cytoplasmic granules were diminished and some
cells showed vacuolations (Stemmer and Jolly, 1964).
Four groups, each comprising 10 male and 20 female rats, were given
daily oral doses of 0, 5, 50 or 100 mg/kg body-weight of pure
heptachlor starting at about four months of age. Administration was
continued for 200 days or until the animals died. By the tenth day,
all the animals in the groups fed 50 or 100 mg/kg had died. At day
200, the surviving animals in the 5 mg/kg group and the controls were
sacrificed for autopsy. Prior to death, the 50 and 100 mg/kg groups
became irritable and had accelerated respiration by the second day.
Convulsions preceded death. In the group given 5 mg/kg, no clinical
abnormalities were seen until the 50th day, when hyper-reflexia, rapid
respirations and chronic convulsions were observed. Two males and two
females in this group died before completion of the experiment,
compared to only one female in the controls. Weight gain was not
affected by 5 mg/kg. Gross pathology revealed changes in liver, kidney
and spleen. Histopathologic examination showed fatty degeneration of
the liver cells and moderate fatty infiltration of the cells of the
urinary tubules, as well as hyperplasia of the smooth endoplasmic
reticulum of the liver and spleen in the group fed 5 mg/kg (Pelikan et
al., 1968).
COMMENT
Since the last evaluation of heptachlor, which was made at the 1966
Joint Meeting, considerable new information on the metabolism of
heptachlor and its epoxide has become available. In acute and
short-term feeding experimental these metabolites appeared to be less
toxic than heptachlor or heptachlor epoxide. It was noted that liver
cellular metabolism was affected by a dietary level of 5 ppm of
heptachlor in the rat, and attention was drawn to its effect on the
metabolism of a compound each as fenithrothion. As is the case with
other organochlorine compounds, the toxicological significance of
these findings could not be determined. In a three-generation
reproduction study in rats, no dose related effects were observed. In
view of the observations from other organochlorine pesticides, an
adequate carcinogenicity study in a second species of animal other
than the rat is needed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
Dog: 2.5 ppm in the diet, equivalent to 0.06 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The approximate distribution of the use of heptachlor outside of
continental United States is as follows:
Area Percentage
Europe 60
Asia 15
South America 15
Africa 5
North America 5
TOTAL 100%
In Europe the dominant use (probably >75%) is for seed treatment in
the culture of sugar beets. Minor significant uses are for seed
dressing of cereal grains and protection of potatoes.
The major uses for heptachlor in Asia are in the control of soil
insects in sugar cane, cereal grains and vegetables. In some areas, to
a small degree, heptachlor is used for the protection of pineapple.
In South America, the major uses of heptachlor are on sugar cane and
maize. The protection of bananas is a minor significant use of
heptachlor.
In Africa and in North America, the most significant uses of
heptachlor are on maize and small grains.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cane berries
No residues were detected (<0.01 ppm) in boysenberries, blackberries
or red or black raspberries maturing in soils treated with 4.0 and 8.0
lb/acre of heptachlor (Velsicol data).
In carrots
(Grown in soils previously subjected to treatment)
Soil treatment at Wooster, Ohio, was made in May 1960 with 6 and 200
pounds of heptachlor per acre. Soil readings three years later (1962)
showed residue of 1.2 to 1.9 ppm (combined H and HE) at 6 pounds per
acre. At the end of four and one half years, the readings at 6 pounds
per acre were 0.52 to 1.25 ppm, and at 200 pounds per acre, 1.8 to
16.5 ppm. Readings in carrots at the 6 pound rate were 0.07 ppm, and
at 200 pounds, 0.5 ppm four and one-half years after treatment.
Carrot/soil residue ratios are respectively: 0.056-0.195 and
0.030-0.278 (Velsicol, soil and carrot data).
Heptachlor was applied at the rate of 5 pounds per acre annually for
five years (1958-1962) to a loam soil. In 1967, combined heptachlor
and heptachlor epoxide reading was 0.78 ppm. Carrots grown in that
soil in 1967 had combined residues at the end of the season of 0.36
ppm. Carrot/soil residue ratio was 0.46 (Lichenstein et al., 1968).
Loam soil treated five consecutive years with abnormally high rates of
heptachlor (5 lb per acre, 25 cumulative) contained 4.6% of the
applied heptachlor five years after cessation of treatment. The mean
residue level (H+HE) in soil was 0.70 ppm; in carrots 0.413;
carrot/soil residue ratio, 0.59. In a parallel test wherein one 25 lb
per acre application was made, ten years after cessation of treatment,
mean residue levels were: in soil 0.719 ppm; in carrots 0.223 ppm;
carrot/soil residue ratio 0.310 (Lichtenstein et al., 1970).
In simulated agricultural experiments, Harris and Sans (unpublished
report, ca. 1969. Absorption of heptachlor epoxide residues by carrots
from three different soil types) demonstrated the strong dependence of
residue transmittal on soil organic content. At a constant level in
soil of 2 ppm of heptachlor epoxide, residues in carrots were
respectively 0.56, 0.03 and 0.01 ppm when grown in soils of 20 and 55
percent organic matter. The respective carrot/soil residue ratios are
0.28, 0.015 and 0.005.
In citrus
Negligible residues were found in oranges and lemons from trees on
soil treated at 3 and 6 lb/acre in California. Soil treatment of 6
lb/acre around grapefruit trees resulted in essentially no residue. A
few samples had readings of 0.01-0.02 ppm for heptachlor and gamma
chlordane, but there was no difference from the check samples
(Velsicol data).
In cottonseed and its products
No residues were found in meal, crude oil, refined oil or soapstock
made from cottonseed grown from heptachlor dressed seed treated at 4
fluid oz /100 lb seed (Velsicol data).
In maize and maize oil
Maize grown on soil into which heptachlor had been incorporated at
rates ranging from 0.75 to 4.0 lb/acre (both row and broadcast
treatment) did not result in measurable residues in natural ear, grain
samples or ensilage prepared from green plants. Stover or mature
stalks contained up to 0.02 ppm of heptachlor, 0.06 ppm of heptachlor
epoxide and 0.04 ppm of gamma chlordane in one experiment only,
however, there did not appear to be any relationship between treatment
level and residue level. Bruce at al. (1966) was able to show a linear
relationship between residue levels of heptachlor epoxide in maize
seed and residue levels in the soil in which the maize was grown using
treatments of 2-20 lb/acre. Maize seed residues (H+HE) did not exceed
0.01 ppm. Maize oil from maize grown in heptachlor treated soil did
not contain measurable residues (Velsicol data).
In peaches
Heptachlor applied at rates of 1.5 and 3.0 lb/acre to soil around
peach trees before petal fall did not result in measurable residues in
the ripe peaches (Velsicol data).
In peppers
Essentially no residues were found in peppers grown in soils treated
with 1-6 lb/acre of heptachlor. Although one sample had an apparent
heptachlor epoxide residue of 0.07 ppm from a 3 lb/acre treatment, no
residue was found in peppers from the 6 lb/acre treatment (Velsicol
data).
In pineapple
Heptachlor was applied to pineapple in Hawaii as a foliar treatment at
1, 2 and 4 lb/acre. Foliage samples collected two months after
treatment showed heptachlor epoxide residues of 0.03 ppm at 1 lb/acre
to 0.20 ppm at 4 lb/acre. Heptachlor and gamma chlordane residues also
occurred in the foliage samples. One heptachlor reading was 2.40 ppm,
while gamma chlordane was 0.94 ppm. Other than these two readings, the
maximum foliage level of heptachlor was 0.65 ppm, and for gamma
chlordane the highest reading was 0.26 ppm. All fruit samples gave
negative readings for heptachlor, heptachlor epoxide and gamma
chlordane. Low levels of residues occurred in samples of fruit shell.
Raw bran samples showed maximum values of 0.05 ppm for heptachlor,
0.11 ppm for heptachlor epoxide and 0.09 ppm for gamma chlordane at
the high treatment rate of 4 lb/acre. Recommended rates gave maximum
levels of 0.01 ppm heptachlor, 0.04 ppm heptachlor epoxide and 0.05
ppm gamma chlordane. Samples analyzed seven months after foliar
treatments gave negative results for fruit and fruit shell and low
values for foliage and raw bran (Velsicol data).
In small grains
Small grains grown in soils treated (preplant) at rates up to 3
lb/acre resulted in no measurable residues of heptachlor or heptachlor
epoxide in the grain for barley, oats, rye and wheat. Gamma chlordane
is reported at levels up to 0.02 ppm but in also observed in the check
sample. Although significant residue appears in the soil from
heptachlor treatment, it is not translocated to the mature grain. Some
levels occur in the straw (Velsicol data).
Seed treatment of small grains with heptachlor does not give residues
in grain of oats, rye or wheat. Rye straw had up to 0.33 ppm of
heptachlor epoxide, which appears to be contamination or an artifact.
Wheat straw gave negative values from one test area and 0.02 ppm to
0.04 ppm for heptachlor and gamma chlordane (Velsicol data).
In sorghum
Seed treatment tests on sorghum at recommended rates gave negative
readings for heptachlor, heptachlor epoxide and gamma chlordane in
samples of grain and straw grown from treated seed (Velsicol data).
In soybeans
Soil treatments were made in September 1966 at 3.0 and 6.0 lb ai/acre.
Soybeans were planted and samples collected in 1967. From both EC and
granular formulations, all green forage samples gave negative
readings. In dry beans, at a 2 lb/acre rate, heptachlor and gamma
chlordane readings were identical with the check samples. The
heptachlor epoxide readings were 0.02 to 0.03 ppm.
Green forage samples grown on soil treated the same year exhibit
negative readings in all samples.
In another experiment, soybeans grown in soil from a heptachlor
treatment made the previous year at 1.0 lb ai/acre show negative
readings for heptachlor expoxide. Heptachlor and gamma chlordane
readings were identical with the check samples.
Soybean processing fractions were obtained from soybeans grown in soil
treated with heptachlor in previous years. Beans grown in 1967 from
treatments made in 1965 show negative readings for heptachlor epoxide
in meal, crude oil, refined oil and soapstock. Some heptachlor
contamination in handling is indicated.
Processing fractions grown in 1967 from a soil treatment in 1966 show
heptachlor epoxide in crude oil at a level of 0.03 ppm. Meal, refined
oil and soapstock contained no heptachlor epoxide.
An extensive study was done at Texas A & M University. At 3.0 lb
heptachlor per acre applied eight days before planting, heptachlor
residues in the plants at time of pod formation were uniformly 0.01
ppm or less. Heptachlor epoxide residue averaged about 0.02 ppm, with
one highest reading of 0.04 ppm. Three pounds per acre soil treatment
is the maximum permitted.
At 3 lb/acre applied eight days before planting, heptachlor in the
bean was uniformly less than 0.01 ppm and heptachlor epoxide averaged
0.044 ppm, with the highest reading being 0.059 ppm. In the crude oil,
these readings were increased about tenfold. For example, heptachlor
readings in the crude oil averaged about 0.03 ppm, with the highest
reading being 0.04 ppm. Heptachlor epoxide in the crude oil averaged
about 0.38 ppm, with the highest reading being 0.52 ppm. These levels
have been shown to be removed during the refining process.
In sugarbeets
Whole sugarbeet sampled five months after preplant broadcast treatment
of soil at 3 lb/acre showed combined H and HE residues of 0.04 ppm,
and at 6 lb/acre showed 0.19 ppm. Laboratory dried pulp showed 0.18
ppm at 3 lb/acre and 0.31 ppm at 6 lb/acre. Pilot plant drying showed
0.14 ppm at 3 lb/acre and 0.34 at 6 lb/acre.
No residues were found in sugarbeets or sugarbeet pulp from furrow and
coated seed treatments at 0.8-1.0 lb/acre (Velsicol data).
Sugarbeets harvested after seed treatment with heptachlor had no
cyclodiene residues (Harris et al., 1966).
In tomatoes
Tomatoes were grown in soil treated prior to planting with 1-3 lb/acre
of granular formulation or 2,3 and 6 lb/acre of emulsifiable
concentrate. No heptachlor epoxide residues were found in the tomatoes
from the granular formulation, and all heptachlor residues were at the
limit of sensitivity (0.01 ppm) except one value of 0.02 ppm. Residues
from the E.C. formulation were mainly <0.01 ppm except one heptachlor
at 0.01 ppm, one at 0.04 ppm (6 lb/acre) and one heptachlor epoxide at
0.02 ppm (6 lb/acre). Maximum combined residue in any one sample at
recommended rates (2-3 lb/acre) was 0.02 ppm.
In a second similar test in soil treated at 2,3 and 6 lb/ acre, no
residues of heptachlor, its epoxide or gamma chlordane were found in
any of the ripened fruit.
Soil samples contained 0.67 ppm heptachlor, 0.08 ppm heptachlor
epoxide and 0.26 ppm gamma chlordane at the 6 lb/acre rate (Velsicol
data).
In soil
In Kansas one year after heptachlor soil treatments, soil residues
were: 2 lb/acre rate - 0.26 to 0.47 ppm (combined H and HE), 3 lb/acre
rate - 0.18 to 0.32 ppm and 6 lb/acre rate - 0.63 to 2.24 ppm
(Velsicol, soybean data)
In Illinois, row treatments of 1.0 and 1.6 pounds heptachlor per acre
showed no residues in the soil fifteen months later (Velsicol, soybean
data).
In Kansas, four months after treatment at three lb/acre soil showed
0.21 to 0.40 ppm (combined H and HE), and 6 pounds per acre showed
0.49 to 3.61 ppm (Velsicol, soybean data).
In Mississippi, seed treatment at the rate of 0.07 pound heptachlor
per acre showed no residues in soil five months after application
(Velsicol, cotton data).
In a survey of the soil of 31 farms in Ontario, Harris, Sans and Miles
(1966) found heptachlor and/or epoxide residues (maximum 0.2 ppm) in
three samples of four which had a previous history of treatment with
heptachlor; one of the three had no recorded history of heptachlor
treatment, but 27 others with history of no heptachlor treatment had
no heptachlor residues. Two soils which had seed treatments (maize and
sugarbeets) had no detectable heptachlor residues. Those samples which
contained heptachlor or epoxide residues also contained residues of
gamma chlordane.
Studies on the mobility of organochlorine pesticides, including
heptachlor and epoxide, in soil demonstrated that these residues do
not move by leaching (Harris, 1969), but may be locally redistributed
by mechanical cultivation (Harris and Sans, 1970).
Under South African conditions, Wiese and Bossen (1966) found that
rapidity of degradation of five chlorinated insecticides " ... was
greater than reflected in most published literature ..." and that "
... heptachlor epoxide was the least persistent ..." In a survey of
agricultural soils of the Atlantic Provinces of Canada, heptachlor and
heptachlor epoxide were found in 9 percent of the soils in
concentrations between 0.06 and 0.86 ppm (Duffy and Wong, 1967).
In animal products
A great deal of work has been carried out to elucidate the propensity
of heptachlor and heptachlor epoxide residues, in or on feeds, to
transmit to milk or store in meat. Much of this information is
summarized by Saha (1969).
Meat
A one-year study on twenty beef cows and their calves was carried out
by Virginia Polytechnic Institute and the U.S. Department of
Agriculture (1968). Ten treated cows were fed a conventional ration of
alfalfa hay containing 0.4 ppm heptachlor and heptachlor epoxide
residues. In animals on contaminated feed continuously, the maximum
residue accumulating in the fat was 1.53 ppm (about four times the
residue level in the feed).
In a similar study over a two-year period involving twenty yearling
steers, the same investigators concluded the results were in good
agreement with that reported previously for pregnant and lactating
beef cows. The study showed clearly that with continued feeding of
contaminated ration the residues stabilize around 1.00 ppm, after 18
months on a residue-free regime, the combined H+HE residues in the
cattle ranged from 0.19 to 0.94 ppm (Bovard et al., 1968).
A 98-day feeding experiment, conducted with 56 beef steers in which
half were fed alfalfa hay containing 0.16 ppm H+HE, resulted in 0.017
to 0.020 ppm HE in the fat of treated animals and 0.004 to 0.007 ppm
in the fat of control steers (Hall et al., 1965).
Milk
In a study at the University of Tennessee, alfalfa hay containing
residues of 0.08 or 0.29 ppm heptachlor and heptachlor epoxide were
fed to dairy animals. Supplemental grain was fed according to milk
production. At the end of the 35-day feeding period, the milk fat from
the high and the low level intake groups contained 0.34 and 0.22 ppm
heptachlor epoxide, respectively. Converting to 4% butter fat milk,
this would be 0.014 ppm residue for the high group and 0.009 ppm for
the low group (Demott et al., 1967).
In an extensive study, lactating cows were fed alfalfa hay containing
residues of heptachlor and heptachlor epoxide resulting from treating
alfalfa at three different levels with heptachlor. The hay, fed for 30
days, contained average residues of heptachlor and heptachlor epoxide
of 0.045 ppm, 0.086 ppm and 0.160 ppm from the 0.25, 0.5 and 1.0
lb/acre treatments, respectively. The highest concentration of residue
in milk occurred between the 18th and 24th days of the feeding period
and averaged 0.013, 0.026 and 0.049 ppm for the test animals in each
group. Removal of treated hay from the diet resulted in a sharp
decline of residues in the milk, followed by a gradual disappearance
of residue over 13 weeks (Waldron at al., 1968).
Another study involved feeding lactating animals with measured
quantities of heptachlor epoxide added to the feed. Heptachlor epoxide
(in grain) was fed at the levels of 0.005 ppm and 0.020 ppm based on
total roughage ingested (hay plus silage) assumed at 50 pounds per
day. At the end of the feeding interval of 28 days, the residue of
heptachlor epoxide in milk was 0.0027 and 0.0043 ppm, respectively,
for the low and higher level fed. At 25 days the respective milk
residue levels for heptachlor epoxide were 0.0029 and 0.0044 ppm
(Hardee et al., 1964).
In a practical test (W.A.R.F. report, 1967), cows were fed maize
silage (50 pounds/day) and ground maize and cob meal (12 pounds/day),
all grown on soil treated with heptachlor for at least eight
consecutive years, at the recommended rate of one half pound per acre.
This feed was supplemented with 10 pounds of alfalfa hay, grown, in
rotation with maize in soil treated for two years, nonconsecutive, at
one half pound/acre, with the most recent application being two years
prior to the growing of the hay crop. The bedding for these cows,
consisting of shredded maize stalks, was also obtained from soil
treated with heptachlor for at least eight years. Milk samples gave
equivalent heptachlor epoxide readings after 10, 20 and 30 days of
feeding, varying between 0.004 and 0.007 ppm. There was no evidence of
increased milk residues during the course of the study and there was
no difference from the checks. All readings for heptachlor, in milk,
were reported as less than 0.001 ppm, and gamma-chlordane was not
detected in milk.
A survey of milk collected in Iowa was made in 1965 to determine what,
if any, residues could be detected. Heptachlor epoxide could be
characterized as being virtually absent from milk samples. All
readings averaged less than 0.002 ppm heptachlor epoxide, and only one
of 20 readings was as high as 0.007 ppm (W.A.R.F. report, 1965).
Poultry and eggs
In a chart provided by the U.S. Food and Drug Administration to the
Committee on Pesticides, Agricultural Research Institute (NAS/NRC)
Meeting, May 14, 1969, it is estimated that the contribution to the
diet of heptachlor epoxide from eggs is nil. Poultry is not shown by
itself, but a correlation between poultry and eggs can be assumed.
In a chicken feeding study (Velsicol data), chickens received
heptachlor epoxide in their entire diet at levels up to more than ten
times that which could occur in chicken feed from registered uses of
heptachlor. The feeding continued for 21 weeks, and white meat, dark
meat, fat and eggs were analyzed. White meat was negative as to
residues except for negligible values in the low hundreths of a part
per million range. Residues in the fat, from chickens fed at the level
which would reasonably reflect a tolerance in commodities used as feed
for chickens, were less than the 0.3 ppm non-actionable level in fat.
Similarly, residues in eggs from chickens which had received
heptachlor epoxide in their total diet for 21 weeks at a level
appropriate for a tolerance, but certainly higher than is encountered
in total feed, averaged 0.03 ppm, which is the non-actionable level in
eggs.
Heptachlor epoxide (in combination with other organochlorine
insecticides) were fed to 60 laying hens for 20 weeks at levels of
0.05, 0.15 and 0.45 ppm to determine residue levels in eggs. The
plateau levels in eggs approximate the feeding levels of heptachlor
epoxide; yolk/white concentration ratio is 99/1 (Cummings et al.,
1966).
FATE OF RESIDUES
In soil
1-Hydroxy chlordene, a conversion product, was found in a small number
of soil samples treated with heptachlor (Duffy and Wong, 1967).
Information on routes of metabolism of heptachlor by soil
micro-organisms has recently become available. As is the case with
mammals, 1-hydroxy 2,3-epoxychlordene was formed and by soil bacteria,
and it was demonstrated that it was derived from 1-hydroxychlordene,
but not from heptachlor epoxide. An unknown metabolite has also been
produced from 1-hydroxychlordene; the structure 1-keto chlordene has
been postulated for this compound but not proven. In addition, other
routes of metabolism involving a reductive stop to chlordene have also
been shown to occur in soil (Miles et al., 1969), but it is not known
if this pathway occurs in mammals. The significance of these chlordene
derivatives has been evaluated toxicologically (see "Toxicity studies
on the metabolites").
Effects of food processing on residues
Evidence continues to accrue that residues of heptachlor epoxide and
associated gamma chlordane are concentrated in the peels of sub-soil
crops, such as rutabagas, carrots and potatoes (Saha and Stewart,
1967). The residue distribution pattern discerned by older analytical
methods (Fox et al., 1964) has now been confirmed by the more
sensitive gas-liquid gas chromatography method (Stewart et al., 1965).
Concentration of heptachlor-derived residues at the outer surfaces of
crops with small surface to volume ratios simplifies effective residue
reduction by several normal food preparation steps. Washing removes
loosely held surface residues such as that contained in adherent soil
(Farrow et al., 1969). Washing plus peeling is highly effective in
residue removal and in frequently used for potatoes and root crops in
home cooking, and always is used in commercial canning of these crops
and tomatoes. Abrasive peeling is also effective; it is normally
used in home cooking and in commercial canning.
Commercial processing of edible vegetable oils frees the finished oil
from residues of heptachlor and heptachlor epoxide, as well as other
organochlorine pesticides (IUPAC Commission on Terminal Pesticide
Residues, 1970; Smith et al., 1968).
In the preparation of animal products, several normal food processing
steps reduce residues. Heptachlor and epoxide, being lipophilic, tend
to remain with fat when it is separated from meat by heating or
mechanical trimming. Reduction of heptachlor and heptachlor epoxide
residues has been reported in milk processing. Production of
condensed, sterilized, spray dried and drum dried milks results in
lower levels (fat basis) of heptachlor epoxide residues than the raw
milk from which they were made (Liska and Stadelman, 1969).
No evidence is available regarding the effect of normal storage
procedures on heptachlor residues.
Minimal evidence (Del Monte Corp., private communication, 1969)
indicates that short, simple cooking of leafy vegetables, such as
spinach, would not affect levels of residue from heptachlor treatment.
Evidence of residues in food in commerce or at consumption
In a series of papers, Duggan and others have assessed the dietary
intake of pesticide chemicals in the United States and determined the
pesticide residue levels in various food groups, domestic and
imported.
For heptachlor and heptachlor epoxide,a three year average (1964 to
1967) was 0.000031 milligrams/kilogram body-weight/day for heptachlor
and heptachlor epoxide combined. This assessment was based on the food
intake of almost twice the "average" intake of the "average"
individual. The value is 6 percent of the Acceptable Daily Intake for
heptachlor and its epoxide (0.0005 mg/kg).
The average levels of heptachlor and heptachlor epoxide residues in
food and feed - domestic, imported and ready to eat - are summarized
in Table I.
Total diet studies in England and Wales (Abbott et al., 1969) detected
heptachlor expoxide in three samples from the cereal group (less than
0.01 ppm), in ten samples from the meat group (up to 0.006 ppm) and
seven samples from the fats group (up to 0.008 ppm). In terms of total
diet, one out of 66 composites contains 0.001 ppm of heptachlor
epoxide; in all other cases the calculated concentration in the diet
was less than 0.0005 ppm heptachlor epoxide. No heptachlor was
detected at limit of detection of 0.0005 ppm.
METHODS OF RESIDUE ANALYSIS
Multidetection analytical techniques, such as those adopted (1969) by
the IUPAC Commission on Pesticide Residue Analysis, can be used to
determine residues of heptachlor, heptachlor epoxide, gamma-chlordane
and nonachlor in crops, soils and animal products down to a lower
sensitivity limit of 0.01 ppm. Procedures based on those given in the
Pesticide Analytical Manual, Vol. I, USHEW, FDA, 1968, with slight
modifications, and designated as AM 0237, AM 0486 and AM 0481, have
been developed by Velsicol Co. for the analysis of residues in fruits,
vegetables, grains, meat and poultry. The method of Cummings et al.
(1966) is suitable for determining heptachlor and heptachlor epoxide
in eggs. A method for the analysis of 1-hydroxychlordene in plant
tissues is under development (Nash and Beall, 1970 pre-print).
APPRAISAL
The data on the absorption of heptachlor and heptachlor epoxide by
carrots grown in soils containing residues, either from current or
previous annual treatments at normal or high rates, indicates that the
temporary practical residue limit should be increased from 0.1 ppm to
0.2 ppm.
New data on residues in maize grain grown in heptachlor treated soil
shows that this commodity is within the practical residue limit of
0.02 ppm established for raw cereals. This continues to be true for
the grain of barley, oats, rye and wheat.
Data from a total of eight studies on the relationship between the
levels of heptachlor and heptachlor epoxide in feed to the levels in
meat and milk show that the maximum residue level in animal feed, that
can be permitted without exceeding the practical residue limits for
these animal products, is 0.04 ppm of combined residues. If dried
sugarbeet pulp is fed in the diet of beef or dairy animals and
constitutes up to 20 percent of the feed, then the maximum permissible
residues in the pulp would be 0.20 ppm and in the whole sugarbeet
would be 0.04 ppm. Similar considerations would apply to maize stover,
soybean forage and pineapple bran used in animal feeds.
TABLE I
Heptachlor epoxide (or heptachlor) residues in food and feed1
RAW AGRICULTURAL PRODUCT READY-TO-EAT FOOD
Food Category Domestic Imported
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large Fruit 0.7 < 0.005 0.7 < 0.005 - -
Small Fruit 1.0 < 0.005 0.7 < 0.005 - -
Grain & Cereals
for human use 0.2 < 0.005 - - 4.1 < 0.001
for animal use 2.5 < 0.005 - - - -
Vegetables
Leaf and Stem 3.0 < 0.005 3.3 < 0.005 1.4 < 0.001
Vine and Ear 2.4 < 0.005 2.3 < 0.005 5.4 < 0.001
Root 3.8 < 0.005 5.7 < 0.005 - -
0.82 < 0.005 4.62 < 0.005 - -
Potatoes 5.4 < 0.001
Beans 0.4 < 0.005 1.2 < 0.005 1.4 < 0.001
Eggs 3.1 < 0.005 3.4 < 0.005
Nuts 0.5 < 0.005 - -
Processed
(canned, frozen)
Domestic 0.8 < 0.001
Imported 0.7 < 0.001
Fluid Milk 23.0 3.03
(fat basis) 0.82 < 0.005
Dairy Products 21.3 0.02 3.0 < 0.005
(fat basis) 0.82 < 0.005 0.1 < 0.005
1/ extracted from USA-FDA surveillance programme report (Duggan, 1968).
2/ Heptachlor residues
The committee received information on residues in pineapple fruit
resulting from foliar treatment at 1,2 and 4 lb/acre. No residues of
heptachlor, heptachlor epoxide or gamma chlordane were found in the
fruit at the detection limit of 0.01 ppm for the method of analysis,
thus supporting a negligible residue tolerance of 0.01 ppm.
With the exception of carrots, the new data available did not indicate
a need to change the previous recommendations for practical residue
limits for the items listed. The previous practical residue limit of
0.125 ppm for milk products was changed to 0.15 to allow for the
limitations and variabilities of residue analytical procedures. Data
was presented which suggested the addition of practical residue levels
for tomatoes, cottonseed, soybeans, crude soybean oil, refined soybean
oil, poultry fat and eggs (on a shell-free basis).
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
TOLERANCES (These are additional to those in previous recommendations)
ppm
Pineapple (edible portions) 0.01
PRACTICAL RESIDUE LIMITS
ppm
Milk and milk products (determined on the
extracted fat) 0.15
Fat of meat and poultry 0.2
Raw cereals, tomatoes, cotton seed, soybeans,
refined soybean oil 0.02
Vegetables (except where otherwise specified),
eggs (shell-free basis) 0.05
Carrots 0.2
Crude soybean oil 0.5
Citrus fruit 0.01
Remarks
Residues of heptachlor and its epoxide should each be determined and
the sum expressed as heptachlor. Tolerances apply to residues from
application to seed and soil only.
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate carcinogenicity study in a second species of animal.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric., 20: 245
Bovard, K.P., Fontenot, J.P. and Priode, B.M. (1968) 1967-1968
Livestock Research Report, Research Div., Virginia Polytechnic
Institute
Bovard, K.P., Fontenot, J.P., Watson, D.F. and Priode, B.M. (1968)
Abstract in J. Animal Science, 27(4): 1131
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Brooks, G.T. and Harrison, A. (1969) Hydration of HEOD (dieldrin and
the heptachlor epoxides by microsomes from the livers of pigs and
rabbits). Bull. environ. Cont. Toxicol., 4, (6): 352-361
Comptes Rendus, (1970) XXV Conference, IUPAC, July 1-5, 1969 Cortina
d'Ampezzo, Italy, 187-195
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide
residues in total diet samples (IV). Pest. Mon., J. 2(4): 140-152
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low-level feeding of five organochlorine insecticides to
hens. J. Assoc. Off. Anal. Chem., 49: 354-364
Davidow, B. and Radomski, J.L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol. exp.
Therap., 107: 259-265
Demott, D.J., Miles, J.T., Hinton, S.A. and Hardin. (1967) L.J.J.
Dairy Science, 49(12): 1495
Dorough, H.W. (1968) Residues in soybeans sprayed with chlordane or
grown in soils treated with heptachlor or chlordane. Report to
Velsicol Chemical Corp., 10 September
Duffy, J.R. and Wong, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic provinces
of Canada. J. Agr. Food Chem., 15: 457-464
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed, Pesticide residues in total diet samples (II). Pest. Mon.
J., 1(2): 2-12
Duggan, R.E. (1968a) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pest. Mon. J., 1(4): 2-7
Duggan, R.E. (1968b) Residues in food and feed. Pesticide residue
levels in foods in the United States from July 1, 1963 to June 30,
1967. Pest. Mon. J., 2(1): 2-46
Duggan, R.E. (1969) Chart provided by U.S. FDA to Committee on
Pesticides, Agricultural Research Institute (NAS/NRC) Meeting, May 14
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest. Mon.
J., 2(4): 153-162
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Calculated daily consumption of pesticides with foods are
discussed and compared with currently accepted values. Science, 157:
1006-1010
FAO/WHO (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO/Food Add./67.32
Farrow, R.P., Elkins, G.R., Rose W.W., Lamb, F.C., Rulls, J.W. and
Mercer W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews,
29:73-87
Fox, C.J.S., Chisholm, D. and Stewart, D.K.R. (1964) Can. J. Plant.
Sci.,44: 149
Gillett, J.W. and Chan, J.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Fd. Chem., 16: 590-593
Hall, O.G., Hardin, L.J., Fryer, M.E. and Glover, J.A. (1965)
Tennessee Farm and Home Science Progress Report No: 56
Hardee, D.D. Gutenmann, W.H., Keenan, G.I. Gyrisco, G.G. Lisk, D.J.,
Fox F.H. Trimburger, G.W. and Holland, R.F. (1964) Residues of
heptachlor epoxide and telodrin in milk from cows fed at part per
billion insecticide levels. J. Econ. Entomol., 57: 404-407
Harris, C.R., Sans, W.W. and Miles, J.R.W. (1966) Exploratory studies
on occurrence of organochlorine insecticide residues in agricultural
soils in southwestern Ontario. J. Agr. Fd. Chem., 14: 398-403
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Fd. Chem.,
17: 80-82
Harris, C.R. and Sans, W.W. (1969) Unpublished report, Absorption of
heptachlor epoxide residues by carrots from three different soil types
Harris, C.R. and Sans, W.W. (1970) Pesticide Progress, Canada
Department of Agriculture, Ottawa, 8(1)
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rate for 224 days. Unpublished report from the University
of Illinois, submitted by Velsicol Chemical Corporation
Klein, W., Korte, F., Weisgerber, I., Kaul, R., Mueller, W. and
Djirsarai, A. (1968) Über den Metabolismas von Endrin, Heptachlor und
Telodrin. Qdal. Pldt. Mater. veg. (Den Haag), 15: 225-238
Korte, F. (1968) Metabolism of chlorinated insecticides.(2)
Heptachlor. Paper submitted to the IUPAC Commission on Terminal
Pesticide Residues, Vienna, 1967. Appendix VI. IUPAC Information
Bulletin No: 32, p. 110
Korte, F. and Porter, P.E. (1970) Minutes of the Fifth Meeting of the
IUPAC Commission on Terminal Pesticide Residues, September 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Lichtenstein, E.P. Fuhremann, T.W. and Schulz, K.R. (1968) Use of
carbon to reduce the uptake of insecticides as soil residues by crop
plants. Effects of carbon on insecticide absorption and toxicity in
soils. J. Agr. Fd. Chem. 16(2): 348-355
Liska, B.J. and Stadelmann, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews, 29: 61-72
Martin, RJ. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III). Pest. Mon. J., 1(4): 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, submitted to Velsicol Chemical
Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories, submitted to Velsicol
Chemical Corporation
Mestitzova, M., Kovac, J. and Durcek, K. (1970) Heptachlor induced
changes in fenitrothion metabolism. Bull. environ. Contam. Toxicol.,
5(3): 195-201
Miles, J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
econ. Entomol., 62: 1334-1338
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Nash, R.G. and Beall, M.L., Jr. (1970) Preprint of unpublished work
Pelikan, Z., Halacka, K., Polster, M. and Cerny, E. (1968)
Intoxication à long terse chez les rats par l'heptachlore à petit
doses. Arch. belges Méd. Soc., 7: 529-538
Polen, P.B. (1970) Terminal residues of chlordane. Paper submitted to
the IUPAC Commission on Terminal P Pesticide Residues, Erbach
(Rheingan) Federal Republic of Germany. 14-18 September
Rosen, J.D. (1969) Personal comminication to the participants of the
IUPAC Commission on Terminal Pesticide Residues, Cortina d'Ampezzo,
July 1969
Saha, J.G. (1966) Significance of organochlorine insecticide residues
in fresh plants as possible contaminants of milk and beef products.
Residue Reviews, 26: 89-126
Saha, J.G. and Stewart, W.W.A. (1967) Can. J. Plant Sci.:47, 79
Seal, W.L., Dawsey, L.H. and Cavin, G.E. (1967) Pesticides in soil.
Monitoring for chlorinated hydrocarbon pesticides in soil and root
crops in the Eastern States in 1965. Pest. Mon. J., 1(3): 22-25
Shellenberger, T.E. and Newell G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail. Lab.Anim.Care., 15:
119
Shellenberger, T.S., Lei, J., Udale B. and Newell, G.W. (1966)
Comparitive toxicity of DDT, dieldrin and heptachlor to Japanese and
bobwhite quail. Toxicol. appl. Pharmacol., 8: 353
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) J.
Amer. Oil Chem. Soc.,45(12): 866
Smith, S.J., Weber C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocardon pesticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Stemmer, K.L. and Hamdi, E. (1964) Electron microscopic changes in the
liver cells after prolonged feeding of DDT and heptachlor. Unpublished
report from the Kettering Laboratory, University of Cincinnati,
submitted by Velsicol Chemical Corporation
Stemmer, K.L. and Jolley, W.P. (1964) Regression of hepatic lesion of
heptachlor and its epoxide. Unpublished report of the Kettering
Laboratory, University of Cincinnati, submitted by Velsicol Chemical
Corporation
Stewart, D.K.R., Chisholm, D. and Fox, C.J.S. (1965) Can. J. Plant
Sci.,45: 72
Velsicol Chemical Co., (1966-1968) Reports of residue analyses
Waldron, A.C., Kaeser, H.E., Golemam, D.L., Staubus, J.R. and
Niemczyk, H.D. (1968) Heptachlor and heptachlor epoxide residues in
salt-treated alfalfa and in milk and cow tissues. J. Agr. Fd. Chem.,
16: 627-631
Wiese, I.H. and Basson, N.C.J. (1966) S. Afr. J. Agric. Sci.,9: 945
Wisconsin Alumni Research Foundation, (1965) Report to Velsicol
Chemical Co.
Wisconsin Alumni Research Foundation, (1967) Report to Velsicol
Chemical Co.
Wazeter, F.X., Butler, R.M., Geil, R.G. and Rehkemper, J.A. (1969)
Heptachlor epoxide. Teratology study in the Dutch rabbit. Unpublished
report from the International Research and Development Corporation
submitted to Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967a) The
effects exerted on the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor and its epoxide into
their daily diet. Unpublished report from the Kettering Laboratory,
Cincinnati, submitted by Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967b) The
effects exerted upon the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor into their daily
diets. Unpublished report from the Kettering Laboratory, Cincinnati,
submitted by Velsicol Chemical Corporation
Wolvin, A.R., Jenkins, D.H. and Fancher, O.E. (1969) Toxicity residue
and reproduction study on heptachlor epoxide chickens. Unpublished
report by Industrial Bio-test Laboratories, submitted to Velsicol
Chemical Corporation
Wong, D.T. and Terriere, L.C. (1965) Epoxidation of aldrin, isodrin
and heptachlor by rat liver microsomes. Biochem. Pharmacol., 14:
375-377

 Female rats were fed with heptachlor in the diet at a dose level
corresponding to 5 mg/kg body-weight/day for three months. After this
period 32P-labelled fenitrothion at a dose of 24 mg/kg body-weight
was administered in oil per os, and radioactive measurements were made
(five times during 24 hours) of the total activities of the liver and
of the degradation products in blood and liver. The results were
compared with those of the control group not pre-treated with
heptachlor. Total activity in the liver of the group pre-treated with
heptachlor was 50 percent higher than in the control group, with a
maximum after four hours. In the control group, the values diminished
gradually after the exposure to fenitrothion. The ratio of the oxygen
analogue to fenitrothion in the blood and liver suggests that the
conversion of fenitrothion to its oxygen analogue is enhanced and
accelerated. Results of this experiment demonstrate that pre-treatment
of rats with heptachlor increases the metabolism of fenitrothion
(Mestitzova et al., 1970).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Chicken
Groups comprising four male and twenty female chickens were fed
dietary levels of 0, 0.02, 0.1 or 0.2 ppm of heptachlor epoxide for 25
weeks. Body-weight increase was not affected by heptachlor epoxide.
Mortality was low in all groups, and a slightly higher incidence in
the 0.2 ppm group than in the other groups is of doubtful
significance. No abnormal behaviour was observed. Total weekly egg
production and mean weekly egg-weights were not significantly
different between test and control groups. Hatchability was slightly
decreased in the eggs from the groups fed 0.1 and 0.2 ppm; viability
of hatched chicks was, however, not affected by heptachlor epoxide
(Wolvin et al., 1969).
Chicken egg
Hatchability of hen eggs was not affected by injection of 1.5 mg
heptachlor in the yolk of fertile eggs (Smith et al., 1970).
Quail
Japanese quails were given 10 and 50 ppm heptachlor in the diet. There
was no obvious adverse effect on reproduction when the birds were ten
weeks of age (Shellenberger and Newell, 1965).
Rabbit
Pregnant female rabbits were treated orally with 0 (22 animals) or 5
mg/kg body-weight/day (20 animals) of heptachlor epoxide from days six
to eleven of gestation. Foetuses were recovered on day 28 by caesarean
section. There were no behavioural abnormalities apparent, and
body-weight gain was not affected by heptachlor epoxide. There were no
deaths. No compound-related effects were observed with respect to
numbers of viable and non-viable term foetuses, resorptions, empty
implantation sites, corpora lutea or non-gravid females. A significant
increase in foetus weight was evident in the treated group; this
increase was considered to be compound related. Survival time was not
considered to be affected by heptachlor epoxide. There were no
teratogenic effects attributable to the compound (Wazeter et al.,
1969).
Rat
Male and female rats fed exclusively on diets containing a mixture of
heptachlor and heptachlor epoxide (3.1) in amounts of 0, 0.3, 3 or 7
ppm have been mated through three succeeding generations. The number
of pregnancies in the F0 and F2 generations was slightly reduced in
the 0.3 ppm group, but not at higher dose levels. There was a slight
increase in the mortality of the pups in the second and third week
after birth in the 3 ppm group. This was not consistent with other
data obtained in these experiments. During three successive
generations, the compound exerted no apparent effect upon the
fertility of the progenitors or the ability of the progeny to survive
(Witherup et al., 1967a).
Male and female rats fed exclusively on diets containing 0, 0.3, 3, 6
and 10 ppm heptachlor have been mated through three succeeding
generations. In the second and third week after birth, mortality of
the pups, only in the second generation, was slightly increased in the
10 ppm group. No adverse effects have been reported in the successive
lower dose levels (Witherup et al., 1967b).
Toxicity studies on the metabolites
The acute oral toxicity of four heptachlor metabolites, chlordene,
3-chlorochlordene, 1-hydroxychlordene and chlordene epoxide, was found
to be greater than 4,600 mg/kg body-weight for the LD50 to male or
female rats (Mastri et al., 1969a). In another test in female rats,
the oral LD50 of 1-hydroxy-2, 3-epoxychlordene was calculated to be
between 4,600 and 10,200 mg/kg body-weight and for 2-chlorochlordene,
>10,200 mg/kg (Mastri et al.,1969b).
Groups each comprising 25 male and 25 female rats, were fed 0, 100,
250, 500, 1000 or 2000 ppm of the heptachlor metabolite
1-hydroxychlordene in their diet for up to 224 days. A rat of each sex
was sacrificed at intervals for autopsy. After receiving the test diet
for 110 days, three females from each level were selected and mated
with males from the same level. Growth and food consumption were
normal at all levels, and mortality appeared to be unaffected by the
test compound. At 2000 ppm, the compound may have produced intestinal
irritation. Within the one generation, 1-hydroxychlordene showed no
adverse effects on fertility, litter size, litter weight or survival
and growth of the young at any level. Gross pathology findings were
limited to one hepatoma in a female fed 2000 ppm and one in a male at
500 ppm; one female at 100 ppm had parotid gland tumours. A breast
tumour was seen in a control animal. Histopathology revealed
abnormalities only in the liver which at 1000 and 2000 ppm showed
slight to moderate cytoplasmic margination, which was also evident to
some extent in the controls and lower level groups. Hepatic cell
enlargement which occurred was also doubtfully related to 1-hydroxy-
chlordene (Ingle, 1965).
Special studies on photodecomposition
By irradiation of heptachlor, a caged photoisomerization product
similar to that observed with other cyclodiene insecticides has been
produced. It has been suggested that this compound may result from
exposure of heptachlor to sunlight. It is more toxic to houseflies and
mosquito larvae than heptachlor, but no information is available on
its mammalian toxicity (Rosen, 1969). Present evidence indicates that
neither photo-heptachlor nor photo-heptachlor epoxide contributes
significantly to the terminal residues on plants or soil, even though
films of heptachlor epoxide on glass were readily transformed into the
photo-product (Polen, 1970).
Short-term studies
Rat
Groups, each comprising ten female rats, were fed dietary levels of 0,
5 or 10 ppm of heptachlor or 10 ppm of DDT for eight months.
Examination of the liver cells of the groups fed 10 ppm of heptachlor
or DDT revealed essentially the same pictures, with increase in the
smooth endoplasmic reticulum and the mitochondria, although these
changes occurred to a greater degree with DDT. At 5 ppm, heptachlor
revealed the earlier stages of the development seen in animals fed 10
ppm. Comparison of the described findings with heptoma cells, obtained
from feeding carcinogenic aminoazo compounds, revealed a striking
difference (Stemmer and Hamdi, 1964).
A total of 269 rats of unspecified sex were fed 40, 45 or 60 ppm of
heptachlor or 35, 40 or 45 ppm of heptachlor epoxide or 40, 45 or 60
ppm of a 75 : 25 percent mixture of the two compounds. After feeding
for 140 days, some animals were returned to a basic uncontaminated
diet and others were continued on the test diet. The animals were then
sacrificed after 10, 20, 30, 60, 80 or 120 days from the 140-day
feeding period. Typical liver lesions were shown to regress after
discontinuing feeding, as evidenced by the observations of the rats
which were returned to a normal diet. After 120 days, a significant
number had normal livers. The largest number of recoveries occurred in
the group fed heptachlor, the next with the mixture and the least in
the group fed heptachlor epoxide. Some rats fed a treated diet for 260
days displayed a second type of lesion in the periphery of the liver
lobule, consisting of enlargement of cells with cytoplasm of empty
appearance. The nuclei were small and dense, with distinct cell
boundaries. It was not reported if these lesions regressed after
returning the animals to a normal diet. Evidence of hyperfunction of
the adrenal medulla was also present in rats that had not had
regression of their liver changes. There was indication of depletion
of catecholamine, the cytoplasmic granules were diminished and some
cells showed vacuolations (Stemmer and Jolly, 1964).
Four groups, each comprising 10 male and 20 female rats, were given
daily oral doses of 0, 5, 50 or 100 mg/kg body-weight of pure
heptachlor starting at about four months of age. Administration was
continued for 200 days or until the animals died. By the tenth day,
all the animals in the groups fed 50 or 100 mg/kg had died. At day
200, the surviving animals in the 5 mg/kg group and the controls were
sacrificed for autopsy. Prior to death, the 50 and 100 mg/kg groups
became irritable and had accelerated respiration by the second day.
Convulsions preceded death. In the group given 5 mg/kg, no clinical
abnormalities were seen until the 50th day, when hyper-reflexia, rapid
respirations and chronic convulsions were observed. Two males and two
females in this group died before completion of the experiment,
compared to only one female in the controls. Weight gain was not
affected by 5 mg/kg. Gross pathology revealed changes in liver, kidney
and spleen. Histopathologic examination showed fatty degeneration of
the liver cells and moderate fatty infiltration of the cells of the
urinary tubules, as well as hyperplasia of the smooth endoplasmic
reticulum of the liver and spleen in the group fed 5 mg/kg (Pelikan et
al., 1968).
COMMENT
Since the last evaluation of heptachlor, which was made at the 1966
Joint Meeting, considerable new information on the metabolism of
heptachlor and its epoxide has become available. In acute and
short-term feeding experimental these metabolites appeared to be less
toxic than heptachlor or heptachlor epoxide. It was noted that liver
cellular metabolism was affected by a dietary level of 5 ppm of
heptachlor in the rat, and attention was drawn to its effect on the
metabolism of a compound each as fenithrothion. As is the case with
other organochlorine compounds, the toxicological significance of
these findings could not be determined. In a three-generation
reproduction study in rats, no dose related effects were observed. In
view of the observations from other organochlorine pesticides, an
adequate carcinogenicity study in a second species of animal other
than the rat is needed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
Dog: 2.5 ppm in the diet, equivalent to 0.06 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The approximate distribution of the use of heptachlor outside of
continental United States is as follows:
Area Percentage
Europe 60
Asia 15
South America 15
Africa 5
North America 5
TOTAL 100%
In Europe the dominant use (probably >75%) is for seed treatment in
the culture of sugar beets. Minor significant uses are for seed
dressing of cereal grains and protection of potatoes.
The major uses for heptachlor in Asia are in the control of soil
insects in sugar cane, cereal grains and vegetables. In some areas, to
a small degree, heptachlor is used for the protection of pineapple.
In South America, the major uses of heptachlor are on sugar cane and
maize. The protection of bananas is a minor significant use of
heptachlor.
In Africa and in North America, the most significant uses of
heptachlor are on maize and small grains.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cane berries
No residues were detected (<0.01 ppm) in boysenberries, blackberries
or red or black raspberries maturing in soils treated with 4.0 and 8.0
lb/acre of heptachlor (Velsicol data).
In carrots
(Grown in soils previously subjected to treatment)
Soil treatment at Wooster, Ohio, was made in May 1960 with 6 and 200
pounds of heptachlor per acre. Soil readings three years later (1962)
showed residue of 1.2 to 1.9 ppm (combined H and HE) at 6 pounds per
acre. At the end of four and one half years, the readings at 6 pounds
per acre were 0.52 to 1.25 ppm, and at 200 pounds per acre, 1.8 to
16.5 ppm. Readings in carrots at the 6 pound rate were 0.07 ppm, and
at 200 pounds, 0.5 ppm four and one-half years after treatment.
Carrot/soil residue ratios are respectively: 0.056-0.195 and
0.030-0.278 (Velsicol, soil and carrot data).
Heptachlor was applied at the rate of 5 pounds per acre annually for
five years (1958-1962) to a loam soil. In 1967, combined heptachlor
and heptachlor epoxide reading was 0.78 ppm. Carrots grown in that
soil in 1967 had combined residues at the end of the season of 0.36
ppm. Carrot/soil residue ratio was 0.46 (Lichenstein et al., 1968).
Loam soil treated five consecutive years with abnormally high rates of
heptachlor (5 lb per acre, 25 cumulative) contained 4.6% of the
applied heptachlor five years after cessation of treatment. The mean
residue level (H+HE) in soil was 0.70 ppm; in carrots 0.413;
carrot/soil residue ratio, 0.59. In a parallel test wherein one 25 lb
per acre application was made, ten years after cessation of treatment,
mean residue levels were: in soil 0.719 ppm; in carrots 0.223 ppm;
carrot/soil residue ratio 0.310 (Lichtenstein et al., 1970).
In simulated agricultural experiments, Harris and Sans (unpublished
report, ca. 1969. Absorption of heptachlor epoxide residues by carrots
from three different soil types) demonstrated the strong dependence of
residue transmittal on soil organic content. At a constant level in
soil of 2 ppm of heptachlor epoxide, residues in carrots were
respectively 0.56, 0.03 and 0.01 ppm when grown in soils of 20 and 55
percent organic matter. The respective carrot/soil residue ratios are
0.28, 0.015 and 0.005.
In citrus
Negligible residues were found in oranges and lemons from trees on
soil treated at 3 and 6 lb/acre in California. Soil treatment of 6
lb/acre around grapefruit trees resulted in essentially no residue. A
few samples had readings of 0.01-0.02 ppm for heptachlor and gamma
chlordane, but there was no difference from the check samples
(Velsicol data).
In cottonseed and its products
No residues were found in meal, crude oil, refined oil or soapstock
made from cottonseed grown from heptachlor dressed seed treated at 4
fluid oz /100 lb seed (Velsicol data).
In maize and maize oil
Maize grown on soil into which heptachlor had been incorporated at
rates ranging from 0.75 to 4.0 lb/acre (both row and broadcast
treatment) did not result in measurable residues in natural ear, grain
samples or ensilage prepared from green plants. Stover or mature
stalks contained up to 0.02 ppm of heptachlor, 0.06 ppm of heptachlor
epoxide and 0.04 ppm of gamma chlordane in one experiment only,
however, there did not appear to be any relationship between treatment
level and residue level. Bruce at al. (1966) was able to show a linear
relationship between residue levels of heptachlor epoxide in maize
seed and residue levels in the soil in which the maize was grown using
treatments of 2-20 lb/acre. Maize seed residues (H+HE) did not exceed
0.01 ppm. Maize oil from maize grown in heptachlor treated soil did
not contain measurable residues (Velsicol data).
In peaches
Heptachlor applied at rates of 1.5 and 3.0 lb/acre to soil around
peach trees before petal fall did not result in measurable residues in
the ripe peaches (Velsicol data).
In peppers
Essentially no residues were found in peppers grown in soils treated
with 1-6 lb/acre of heptachlor. Although one sample had an apparent
heptachlor epoxide residue of 0.07 ppm from a 3 lb/acre treatment, no
residue was found in peppers from the 6 lb/acre treatment (Velsicol
data).
In pineapple
Heptachlor was applied to pineapple in Hawaii as a foliar treatment at
1, 2 and 4 lb/acre. Foliage samples collected two months after
treatment showed heptachlor epoxide residues of 0.03 ppm at 1 lb/acre
to 0.20 ppm at 4 lb/acre. Heptachlor and gamma chlordane residues also
occurred in the foliage samples. One heptachlor reading was 2.40 ppm,
while gamma chlordane was 0.94 ppm. Other than these two readings, the
maximum foliage level of heptachlor was 0.65 ppm, and for gamma
chlordane the highest reading was 0.26 ppm. All fruit samples gave
negative readings for heptachlor, heptachlor epoxide and gamma
chlordane. Low levels of residues occurred in samples of fruit shell.
Raw bran samples showed maximum values of 0.05 ppm for heptachlor,
0.11 ppm for heptachlor epoxide and 0.09 ppm for gamma chlordane at
the high treatment rate of 4 lb/acre. Recommended rates gave maximum
levels of 0.01 ppm heptachlor, 0.04 ppm heptachlor epoxide and 0.05
ppm gamma chlordane. Samples analyzed seven months after foliar
treatments gave negative results for fruit and fruit shell and low
values for foliage and raw bran (Velsicol data).
In small grains
Small grains grown in soils treated (preplant) at rates up to 3
lb/acre resulted in no measurable residues of heptachlor or heptachlor
epoxide in the grain for barley, oats, rye and wheat. Gamma chlordane
is reported at levels up to 0.02 ppm but in also observed in the check
sample. Although significant residue appears in the soil from
heptachlor treatment, it is not translocated to the mature grain. Some
levels occur in the straw (Velsicol data).
Seed treatment of small grains with heptachlor does not give residues
in grain of oats, rye or wheat. Rye straw had up to 0.33 ppm of
heptachlor epoxide, which appears to be contamination or an artifact.
Wheat straw gave negative values from one test area and 0.02 ppm to
0.04 ppm for heptachlor and gamma chlordane (Velsicol data).
In sorghum
Seed treatment tests on sorghum at recommended rates gave negative
readings for heptachlor, heptachlor epoxide and gamma chlordane in
samples of grain and straw grown from treated seed (Velsicol data).
In soybeans
Soil treatments were made in September 1966 at 3.0 and 6.0 lb ai/acre.
Soybeans were planted and samples collected in 1967. From both EC and
granular formulations, all green forage samples gave negative
readings. In dry beans, at a 2 lb/acre rate, heptachlor and gamma
chlordane readings were identical with the check samples. The
heptachlor epoxide readings were 0.02 to 0.03 ppm.
Green forage samples grown on soil treated the same year exhibit
negative readings in all samples.
In another experiment, soybeans grown in soil from a heptachlor
treatment made the previous year at 1.0 lb ai/acre show negative
readings for heptachlor expoxide. Heptachlor and gamma chlordane
readings were identical with the check samples.
Soybean processing fractions were obtained from soybeans grown in soil
treated with heptachlor in previous years. Beans grown in 1967 from
treatments made in 1965 show negative readings for heptachlor epoxide
in meal, crude oil, refined oil and soapstock. Some heptachlor
contamination in handling is indicated.
Processing fractions grown in 1967 from a soil treatment in 1966 show
heptachlor epoxide in crude oil at a level of 0.03 ppm. Meal, refined
oil and soapstock contained no heptachlor epoxide.
An extensive study was done at Texas A & M University. At 3.0 lb
heptachlor per acre applied eight days before planting, heptachlor
residues in the plants at time of pod formation were uniformly 0.01
ppm or less. Heptachlor epoxide residue averaged about 0.02 ppm, with
one highest reading of 0.04 ppm. Three pounds per acre soil treatment
is the maximum permitted.
At 3 lb/acre applied eight days before planting, heptachlor in the
bean was uniformly less than 0.01 ppm and heptachlor epoxide averaged
0.044 ppm, with the highest reading being 0.059 ppm. In the crude oil,
these readings were increased about tenfold. For example, heptachlor
readings in the crude oil averaged about 0.03 ppm, with the highest
reading being 0.04 ppm. Heptachlor epoxide in the crude oil averaged
about 0.38 ppm, with the highest reading being 0.52 ppm. These levels
have been shown to be removed during the refining process.
In sugarbeets
Whole sugarbeet sampled five months after preplant broadcast treatment
of soil at 3 lb/acre showed combined H and HE residues of 0.04 ppm,
and at 6 lb/acre showed 0.19 ppm. Laboratory dried pulp showed 0.18
ppm at 3 lb/acre and 0.31 ppm at 6 lb/acre. Pilot plant drying showed
0.14 ppm at 3 lb/acre and 0.34 at 6 lb/acre.
No residues were found in sugarbeets or sugarbeet pulp from furrow and
coated seed treatments at 0.8-1.0 lb/acre (Velsicol data).
Sugarbeets harvested after seed treatment with heptachlor had no
cyclodiene residues (Harris et al., 1966).
In tomatoes
Tomatoes were grown in soil treated prior to planting with 1-3 lb/acre
of granular formulation or 2,3 and 6 lb/acre of emulsifiable
concentrate. No heptachlor epoxide residues were found in the tomatoes
from the granular formulation, and all heptachlor residues were at the
limit of sensitivity (0.01 ppm) except one value of 0.02 ppm. Residues
from the E.C. formulation were mainly <0.01 ppm except one heptachlor
at 0.01 ppm, one at 0.04 ppm (6 lb/acre) and one heptachlor epoxide at
0.02 ppm (6 lb/acre). Maximum combined residue in any one sample at
recommended rates (2-3 lb/acre) was 0.02 ppm.
In a second similar test in soil treated at 2,3 and 6 lb/ acre, no
residues of heptachlor, its epoxide or gamma chlordane were found in
any of the ripened fruit.
Soil samples contained 0.67 ppm heptachlor, 0.08 ppm heptachlor
epoxide and 0.26 ppm gamma chlordane at the 6 lb/acre rate (Velsicol
data).
In soil
In Kansas one year after heptachlor soil treatments, soil residues
were: 2 lb/acre rate - 0.26 to 0.47 ppm (combined H and HE), 3 lb/acre
rate - 0.18 to 0.32 ppm and 6 lb/acre rate - 0.63 to 2.24 ppm
(Velsicol, soybean data)
In Illinois, row treatments of 1.0 and 1.6 pounds heptachlor per acre
showed no residues in the soil fifteen months later (Velsicol, soybean
data).
In Kansas, four months after treatment at three lb/acre soil showed
0.21 to 0.40 ppm (combined H and HE), and 6 pounds per acre showed
0.49 to 3.61 ppm (Velsicol, soybean data).
In Mississippi, seed treatment at the rate of 0.07 pound heptachlor
per acre showed no residues in soil five months after application
(Velsicol, cotton data).
In a survey of the soil of 31 farms in Ontario, Harris, Sans and Miles
(1966) found heptachlor and/or epoxide residues (maximum 0.2 ppm) in
three samples of four which had a previous history of treatment with
heptachlor; one of the three had no recorded history of heptachlor
treatment, but 27 others with history of no heptachlor treatment had
no heptachlor residues. Two soils which had seed treatments (maize and
sugarbeets) had no detectable heptachlor residues. Those samples which
contained heptachlor or epoxide residues also contained residues of
gamma chlordane.
Studies on the mobility of organochlorine pesticides, including
heptachlor and epoxide, in soil demonstrated that these residues do
not move by leaching (Harris, 1969), but may be locally redistributed
by mechanical cultivation (Harris and Sans, 1970).
Under South African conditions, Wiese and Bossen (1966) found that
rapidity of degradation of five chlorinated insecticides " ... was
greater than reflected in most published literature ..." and that "
... heptachlor epoxide was the least persistent ..." In a survey of
agricultural soils of the Atlantic Provinces of Canada, heptachlor and
heptachlor epoxide were found in 9 percent of the soils in
concentrations between 0.06 and 0.86 ppm (Duffy and Wong, 1967).
In animal products
A great deal of work has been carried out to elucidate the propensity
of heptachlor and heptachlor epoxide residues, in or on feeds, to
transmit to milk or store in meat. Much of this information is
summarized by Saha (1969).
Meat
A one-year study on twenty beef cows and their calves was carried out
by Virginia Polytechnic Institute and the U.S. Department of
Agriculture (1968). Ten treated cows were fed a conventional ration of
alfalfa hay containing 0.4 ppm heptachlor and heptachlor epoxide
residues. In animals on contaminated feed continuously, the maximum
residue accumulating in the fat was 1.53 ppm (about four times the
residue level in the feed).
In a similar study over a two-year period involving twenty yearling
steers, the same investigators concluded the results were in good
agreement with that reported previously for pregnant and lactating
beef cows. The study showed clearly that with continued feeding of
contaminated ration the residues stabilize around 1.00 ppm, after 18
months on a residue-free regime, the combined H+HE residues in the
cattle ranged from 0.19 to 0.94 ppm (Bovard et al., 1968).
A 98-day feeding experiment, conducted with 56 beef steers in which
half were fed alfalfa hay containing 0.16 ppm H+HE, resulted in 0.017
to 0.020 ppm HE in the fat of treated animals and 0.004 to 0.007 ppm
in the fat of control steers (Hall et al., 1965).
Milk
In a study at the University of Tennessee, alfalfa hay containing
residues of 0.08 or 0.29 ppm heptachlor and heptachlor epoxide were
fed to dairy animals. Supplemental grain was fed according to milk
production. At the end of the 35-day feeding period, the milk fat from
the high and the low level intake groups contained 0.34 and 0.22 ppm
heptachlor epoxide, respectively. Converting to 4% butter fat milk,
this would be 0.014 ppm residue for the high group and 0.009 ppm for
the low group (Demott et al., 1967).
In an extensive study, lactating cows were fed alfalfa hay containing
residues of heptachlor and heptachlor epoxide resulting from treating
alfalfa at three different levels with heptachlor. The hay, fed for 30
days, contained average residues of heptachlor and heptachlor epoxide
of 0.045 ppm, 0.086 ppm and 0.160 ppm from the 0.25, 0.5 and 1.0
lb/acre treatments, respectively. The highest concentration of residue
in milk occurred between the 18th and 24th days of the feeding period
and averaged 0.013, 0.026 and 0.049 ppm for the test animals in each
group. Removal of treated hay from the diet resulted in a sharp
decline of residues in the milk, followed by a gradual disappearance
of residue over 13 weeks (Waldron at al., 1968).
Another study involved feeding lactating animals with measured
quantities of heptachlor epoxide added to the feed. Heptachlor epoxide
(in grain) was fed at the levels of 0.005 ppm and 0.020 ppm based on
total roughage ingested (hay plus silage) assumed at 50 pounds per
day. At the end of the feeding interval of 28 days, the residue of
heptachlor epoxide in milk was 0.0027 and 0.0043 ppm, respectively,
for the low and higher level fed. At 25 days the respective milk
residue levels for heptachlor epoxide were 0.0029 and 0.0044 ppm
(Hardee et al., 1964).
In a practical test (W.A.R.F. report, 1967), cows were fed maize
silage (50 pounds/day) and ground maize and cob meal (12 pounds/day),
all grown on soil treated with heptachlor for at least eight
consecutive years, at the recommended rate of one half pound per acre.
This feed was supplemented with 10 pounds of alfalfa hay, grown, in
rotation with maize in soil treated for two years, nonconsecutive, at
one half pound/acre, with the most recent application being two years
prior to the growing of the hay crop. The bedding for these cows,
consisting of shredded maize stalks, was also obtained from soil
treated with heptachlor for at least eight years. Milk samples gave
equivalent heptachlor epoxide readings after 10, 20 and 30 days of
feeding, varying between 0.004 and 0.007 ppm. There was no evidence of
increased milk residues during the course of the study and there was
no difference from the checks. All readings for heptachlor, in milk,
were reported as less than 0.001 ppm, and gamma-chlordane was not
detected in milk.
A survey of milk collected in Iowa was made in 1965 to determine what,
if any, residues could be detected. Heptachlor epoxide could be
characterized as being virtually absent from milk samples. All
readings averaged less than 0.002 ppm heptachlor epoxide, and only one
of 20 readings was as high as 0.007 ppm (W.A.R.F. report, 1965).
Poultry and eggs
In a chart provided by the U.S. Food and Drug Administration to the
Committee on Pesticides, Agricultural Research Institute (NAS/NRC)
Meeting, May 14, 1969, it is estimated that the contribution to the
diet of heptachlor epoxide from eggs is nil. Poultry is not shown by
itself, but a correlation between poultry and eggs can be assumed.
In a chicken feeding study (Velsicol data), chickens received
heptachlor epoxide in their entire diet at levels up to more than ten
times that which could occur in chicken feed from registered uses of
heptachlor. The feeding continued for 21 weeks, and white meat, dark
meat, fat and eggs were analyzed. White meat was negative as to
residues except for negligible values in the low hundreths of a part
per million range. Residues in the fat, from chickens fed at the level
which would reasonably reflect a tolerance in commodities used as feed
for chickens, were less than the 0.3 ppm non-actionable level in fat.
Similarly, residues in eggs from chickens which had received
heptachlor epoxide in their total diet for 21 weeks at a level
appropriate for a tolerance, but certainly higher than is encountered
in total feed, averaged 0.03 ppm, which is the non-actionable level in
eggs.
Heptachlor epoxide (in combination with other organochlorine
insecticides) were fed to 60 laying hens for 20 weeks at levels of
0.05, 0.15 and 0.45 ppm to determine residue levels in eggs. The
plateau levels in eggs approximate the feeding levels of heptachlor
epoxide; yolk/white concentration ratio is 99/1 (Cummings et al.,
1966).
FATE OF RESIDUES
In soil
1-Hydroxy chlordene, a conversion product, was found in a small number
of soil samples treated with heptachlor (Duffy and Wong, 1967).
Information on routes of metabolism of heptachlor by soil
micro-organisms has recently become available. As is the case with
mammals, 1-hydroxy 2,3-epoxychlordene was formed and by soil bacteria,
and it was demonstrated that it was derived from 1-hydroxychlordene,
but not from heptachlor epoxide. An unknown metabolite has also been
produced from 1-hydroxychlordene; the structure 1-keto chlordene has
been postulated for this compound but not proven. In addition, other
routes of metabolism involving a reductive stop to chlordene have also
been shown to occur in soil (Miles et al., 1969), but it is not known
if this pathway occurs in mammals. The significance of these chlordene
derivatives has been evaluated toxicologically (see "Toxicity studies
on the metabolites").
Effects of food processing on residues
Evidence continues to accrue that residues of heptachlor epoxide and
associated gamma chlordane are concentrated in the peels of sub-soil
crops, such as rutabagas, carrots and potatoes (Saha and Stewart,
1967). The residue distribution pattern discerned by older analytical
methods (Fox et al., 1964) has now been confirmed by the more
sensitive gas-liquid gas chromatography method (Stewart et al., 1965).
Concentration of heptachlor-derived residues at the outer surfaces of
crops with small surface to volume ratios simplifies effective residue
reduction by several normal food preparation steps. Washing removes
loosely held surface residues such as that contained in adherent soil
(Farrow et al., 1969). Washing plus peeling is highly effective in
residue removal and in frequently used for potatoes and root crops in
home cooking, and always is used in commercial canning of these crops
and tomatoes. Abrasive peeling is also effective; it is normally
used in home cooking and in commercial canning.
Commercial processing of edible vegetable oils frees the finished oil
from residues of heptachlor and heptachlor epoxide, as well as other
organochlorine pesticides (IUPAC Commission on Terminal Pesticide
Residues, 1970; Smith et al., 1968).
In the preparation of animal products, several normal food processing
steps reduce residues. Heptachlor and epoxide, being lipophilic, tend
to remain with fat when it is separated from meat by heating or
mechanical trimming. Reduction of heptachlor and heptachlor epoxide
residues has been reported in milk processing. Production of
condensed, sterilized, spray dried and drum dried milks results in
lower levels (fat basis) of heptachlor epoxide residues than the raw
milk from which they were made (Liska and Stadelman, 1969).
No evidence is available regarding the effect of normal storage
procedures on heptachlor residues.
Minimal evidence (Del Monte Corp., private communication, 1969)
indicates that short, simple cooking of leafy vegetables, such as
spinach, would not affect levels of residue from heptachlor treatment.
Evidence of residues in food in commerce or at consumption
In a series of papers, Duggan and others have assessed the dietary
intake of pesticide chemicals in the United States and determined the
pesticide residue levels in various food groups, domestic and
imported.
For heptachlor and heptachlor epoxide,a three year average (1964 to
1967) was 0.000031 milligrams/kilogram body-weight/day for heptachlor
and heptachlor epoxide combined. This assessment was based on the food
intake of almost twice the "average" intake of the "average"
individual. The value is 6 percent of the Acceptable Daily Intake for
heptachlor and its epoxide (0.0005 mg/kg).
The average levels of heptachlor and heptachlor epoxide residues in
food and feed - domestic, imported and ready to eat - are summarized
in Table I.
Total diet studies in England and Wales (Abbott et al., 1969) detected
heptachlor expoxide in three samples from the cereal group (less than
0.01 ppm), in ten samples from the meat group (up to 0.006 ppm) and
seven samples from the fats group (up to 0.008 ppm). In terms of total
diet, one out of 66 composites contains 0.001 ppm of heptachlor
epoxide; in all other cases the calculated concentration in the diet
was less than 0.0005 ppm heptachlor epoxide. No heptachlor was
detected at limit of detection of 0.0005 ppm.
METHODS OF RESIDUE ANALYSIS
Multidetection analytical techniques, such as those adopted (1969) by
the IUPAC Commission on Pesticide Residue Analysis, can be used to
determine residues of heptachlor, heptachlor epoxide, gamma-chlordane
and nonachlor in crops, soils and animal products down to a lower
sensitivity limit of 0.01 ppm. Procedures based on those given in the
Pesticide Analytical Manual, Vol. I, USHEW, FDA, 1968, with slight
modifications, and designated as AM 0237, AM 0486 and AM 0481, have
been developed by Velsicol Co. for the analysis of residues in fruits,
vegetables, grains, meat and poultry. The method of Cummings et al.
(1966) is suitable for determining heptachlor and heptachlor epoxide
in eggs. A method for the analysis of 1-hydroxychlordene in plant
tissues is under development (Nash and Beall, 1970 pre-print).
APPRAISAL
The data on the absorption of heptachlor and heptachlor epoxide by
carrots grown in soils containing residues, either from current or
previous annual treatments at normal or high rates, indicates that the
temporary practical residue limit should be increased from 0.1 ppm to
0.2 ppm.
New data on residues in maize grain grown in heptachlor treated soil
shows that this commodity is within the practical residue limit of
0.02 ppm established for raw cereals. This continues to be true for
the grain of barley, oats, rye and wheat.
Data from a total of eight studies on the relationship between the
levels of heptachlor and heptachlor epoxide in feed to the levels in
meat and milk show that the maximum residue level in animal feed, that
can be permitted without exceeding the practical residue limits for
these animal products, is 0.04 ppm of combined residues. If dried
sugarbeet pulp is fed in the diet of beef or dairy animals and
constitutes up to 20 percent of the feed, then the maximum permissible
residues in the pulp would be 0.20 ppm and in the whole sugarbeet
would be 0.04 ppm. Similar considerations would apply to maize stover,
soybean forage and pineapple bran used in animal feeds.
TABLE I
Heptachlor epoxide (or heptachlor) residues in food and feed1
RAW AGRICULTURAL PRODUCT READY-TO-EAT FOOD
Food Category Domestic Imported
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large Fruit 0.7 < 0.005 0.7 < 0.005 - -
Small Fruit 1.0 < 0.005 0.7 < 0.005 - -
Grain & Cereals
for human use 0.2 < 0.005 - - 4.1 < 0.001
for animal use 2.5 < 0.005 - - - -
Vegetables
Leaf and Stem 3.0 < 0.005 3.3 < 0.005 1.4 < 0.001
Vine and Ear 2.4 < 0.005 2.3 < 0.005 5.4 < 0.001
Root 3.8 < 0.005 5.7 < 0.005 - -
0.82 < 0.005 4.62 < 0.005 - -
Potatoes 5.4 < 0.001
Beans 0.4 < 0.005 1.2 < 0.005 1.4 < 0.001
Eggs 3.1 < 0.005 3.4 < 0.005
Nuts 0.5 < 0.005 - -
Processed
(canned, frozen)
Domestic 0.8 < 0.001
Imported 0.7 < 0.001
Fluid Milk 23.0 3.03
(fat basis) 0.82 < 0.005
Dairy Products 21.3 0.02 3.0 < 0.005
(fat basis) 0.82 < 0.005 0.1 < 0.005
1/ extracted from USA-FDA surveillance programme report (Duggan, 1968).
2/ Heptachlor residues
The committee received information on residues in pineapple fruit
resulting from foliar treatment at 1,2 and 4 lb/acre. No residues of
heptachlor, heptachlor epoxide or gamma chlordane were found in the
fruit at the detection limit of 0.01 ppm for the method of analysis,
thus supporting a negligible residue tolerance of 0.01 ppm.
With the exception of carrots, the new data available did not indicate
a need to change the previous recommendations for practical residue
limits for the items listed. The previous practical residue limit of
0.125 ppm for milk products was changed to 0.15 to allow for the
limitations and variabilities of residue analytical procedures. Data
was presented which suggested the addition of practical residue levels
for tomatoes, cottonseed, soybeans, crude soybean oil, refined soybean
oil, poultry fat and eggs (on a shell-free basis).
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
TOLERANCES (These are additional to those in previous recommendations)
ppm
Pineapple (edible portions) 0.01
PRACTICAL RESIDUE LIMITS
ppm
Milk and milk products (determined on the
extracted fat) 0.15
Fat of meat and poultry 0.2
Raw cereals, tomatoes, cotton seed, soybeans,
refined soybean oil 0.02
Vegetables (except where otherwise specified),
eggs (shell-free basis) 0.05
Carrots 0.2
Crude soybean oil 0.5
Citrus fruit 0.01
Remarks
Residues of heptachlor and its epoxide should each be determined and
the sum expressed as heptachlor. Tolerances apply to residues from
application to seed and soil only.
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate carcinogenicity study in a second species of animal.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric., 20: 245
Bovard, K.P., Fontenot, J.P. and Priode, B.M. (1968) 1967-1968
Livestock Research Report, Research Div., Virginia Polytechnic
Institute
Bovard, K.P., Fontenot, J.P., Watson, D.F. and Priode, B.M. (1968)
Abstract in J. Animal Science, 27(4): 1131
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Brooks, G.T. and Harrison, A. (1969) Hydration of HEOD (dieldrin and
the heptachlor epoxides by microsomes from the livers of pigs and
rabbits). Bull. environ. Cont. Toxicol., 4, (6): 352-361
Comptes Rendus, (1970) XXV Conference, IUPAC, July 1-5, 1969 Cortina
d'Ampezzo, Italy, 187-195
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide
residues in total diet samples (IV). Pest. Mon., J. 2(4): 140-152
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low-level feeding of five organochlorine insecticides to
hens. J. Assoc. Off. Anal. Chem., 49: 354-364
Davidow, B. and Radomski, J.L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol. exp.
Therap., 107: 259-265
Demott, D.J., Miles, J.T., Hinton, S.A. and Hardin. (1967) L.J.J.
Dairy Science, 49(12): 1495
Dorough, H.W. (1968) Residues in soybeans sprayed with chlordane or
grown in soils treated with heptachlor or chlordane. Report to
Velsicol Chemical Corp., 10 September
Duffy, J.R. and Wong, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic provinces
of Canada. J. Agr. Food Chem., 15: 457-464
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed, Pesticide residues in total diet samples (II). Pest. Mon.
J., 1(2): 2-12
Duggan, R.E. (1968a) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pest. Mon. J., 1(4): 2-7
Duggan, R.E. (1968b) Residues in food and feed. Pesticide residue
levels in foods in the United States from July 1, 1963 to June 30,
1967. Pest. Mon. J., 2(1): 2-46
Duggan, R.E. (1969) Chart provided by U.S. FDA to Committee on
Pesticides, Agricultural Research Institute (NAS/NRC) Meeting, May 14
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest. Mon.
J., 2(4): 153-162
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Calculated daily consumption of pesticides with foods are
discussed and compared with currently accepted values. Science, 157:
1006-1010
FAO/WHO (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO/Food Add./67.32
Farrow, R.P., Elkins, G.R., Rose W.W., Lamb, F.C., Rulls, J.W. and
Mercer W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews,
29:73-87
Fox, C.J.S., Chisholm, D. and Stewart, D.K.R. (1964) Can. J. Plant.
Sci.,44: 149
Gillett, J.W. and Chan, J.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Fd. Chem., 16: 590-593
Hall, O.G., Hardin, L.J., Fryer, M.E. and Glover, J.A. (1965)
Tennessee Farm and Home Science Progress Report No: 56
Hardee, D.D. Gutenmann, W.H., Keenan, G.I. Gyrisco, G.G. Lisk, D.J.,
Fox F.H. Trimburger, G.W. and Holland, R.F. (1964) Residues of
heptachlor epoxide and telodrin in milk from cows fed at part per
billion insecticide levels. J. Econ. Entomol., 57: 404-407
Harris, C.R., Sans, W.W. and Miles, J.R.W. (1966) Exploratory studies
on occurrence of organochlorine insecticide residues in agricultural
soils in southwestern Ontario. J. Agr. Fd. Chem., 14: 398-403
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Fd. Chem.,
17: 80-82
Harris, C.R. and Sans, W.W. (1969) Unpublished report, Absorption of
heptachlor epoxide residues by carrots from three different soil types
Harris, C.R. and Sans, W.W. (1970) Pesticide Progress, Canada
Department of Agriculture, Ottawa, 8(1)
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rate for 224 days. Unpublished report from the University
of Illinois, submitted by Velsicol Chemical Corporation
Klein, W., Korte, F., Weisgerber, I., Kaul, R., Mueller, W. and
Djirsarai, A. (1968) Über den Metabolismas von Endrin, Heptachlor und
Telodrin. Qdal. Pldt. Mater. veg. (Den Haag), 15: 225-238
Korte, F. (1968) Metabolism of chlorinated insecticides.(2)
Heptachlor. Paper submitted to the IUPAC Commission on Terminal
Pesticide Residues, Vienna, 1967. Appendix VI. IUPAC Information
Bulletin No: 32, p. 110
Korte, F. and Porter, P.E. (1970) Minutes of the Fifth Meeting of the
IUPAC Commission on Terminal Pesticide Residues, September 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Lichtenstein, E.P. Fuhremann, T.W. and Schulz, K.R. (1968) Use of
carbon to reduce the uptake of insecticides as soil residues by crop
plants. Effects of carbon on insecticide absorption and toxicity in
soils. J. Agr. Fd. Chem. 16(2): 348-355
Liska, B.J. and Stadelmann, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews, 29: 61-72
Martin, RJ. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III). Pest. Mon. J., 1(4): 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, submitted to Velsicol Chemical
Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories, submitted to Velsicol
Chemical Corporation
Mestitzova, M., Kovac, J. and Durcek, K. (1970) Heptachlor induced
changes in fenitrothion metabolism. Bull. environ. Contam. Toxicol.,
5(3): 195-201
Miles, J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
econ. Entomol., 62: 1334-1338
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Nash, R.G. and Beall, M.L., Jr. (1970) Preprint of unpublished work
Pelikan, Z., Halacka, K., Polster, M. and Cerny, E. (1968)
Intoxication à long terse chez les rats par l'heptachlore à petit
doses. Arch. belges Méd. Soc., 7: 529-538
Polen, P.B. (1970) Terminal residues of chlordane. Paper submitted to
the IUPAC Commission on Terminal P Pesticide Residues, Erbach
(Rheingan) Federal Republic of Germany. 14-18 September
Rosen, J.D. (1969) Personal comminication to the participants of the
IUPAC Commission on Terminal Pesticide Residues, Cortina d'Ampezzo,
July 1969
Saha, J.G. (1966) Significance of organochlorine insecticide residues
in fresh plants as possible contaminants of milk and beef products.
Residue Reviews, 26: 89-126
Saha, J.G. and Stewart, W.W.A. (1967) Can. J. Plant Sci.:47, 79
Seal, W.L., Dawsey, L.H. and Cavin, G.E. (1967) Pesticides in soil.
Monitoring for chlorinated hydrocarbon pesticides in soil and root
crops in the Eastern States in 1965. Pest. Mon. J., 1(3): 22-25
Shellenberger, T.E. and Newell G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail. Lab.Anim.Care., 15:
119
Shellenberger, T.S., Lei, J., Udale B. and Newell, G.W. (1966)
Comparitive toxicity of DDT, dieldrin and heptachlor to Japanese and
bobwhite quail. Toxicol. appl. Pharmacol., 8: 353
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) J.
Amer. Oil Chem. Soc.,45(12): 866
Smith, S.J., Weber C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocardon pesticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Stemmer, K.L. and Hamdi, E. (1964) Electron microscopic changes in the
liver cells after prolonged feeding of DDT and heptachlor. Unpublished
report from the Kettering Laboratory, University of Cincinnati,
submitted by Velsicol Chemical Corporation
Stemmer, K.L. and Jolley, W.P. (1964) Regression of hepatic lesion of
heptachlor and its epoxide. Unpublished report of the Kettering
Laboratory, University of Cincinnati, submitted by Velsicol Chemical
Corporation
Stewart, D.K.R., Chisholm, D. and Fox, C.J.S. (1965) Can. J. Plant
Sci.,45: 72
Velsicol Chemical Co., (1966-1968) Reports of residue analyses
Waldron, A.C., Kaeser, H.E., Golemam, D.L., Staubus, J.R. and
Niemczyk, H.D. (1968) Heptachlor and heptachlor epoxide residues in
salt-treated alfalfa and in milk and cow tissues. J. Agr. Fd. Chem.,
16: 627-631
Wiese, I.H. and Basson, N.C.J. (1966) S. Afr. J. Agric. Sci.,9: 945
Wisconsin Alumni Research Foundation, (1965) Report to Velsicol
Chemical Co.
Wisconsin Alumni Research Foundation, (1967) Report to Velsicol
Chemical Co.
Wazeter, F.X., Butler, R.M., Geil, R.G. and Rehkemper, J.A. (1969)
Heptachlor epoxide. Teratology study in the Dutch rabbit. Unpublished
report from the International Research and Development Corporation
submitted to Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967a) The
effects exerted on the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor and its epoxide into
their daily diet. Unpublished report from the Kettering Laboratory,
Cincinnati, submitted by Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967b) The
effects exerted upon the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor into their daily
diets. Unpublished report from the Kettering Laboratory, Cincinnati,
submitted by Velsicol Chemical Corporation
Wolvin, A.R., Jenkins, D.H. and Fancher, O.E. (1969) Toxicity residue
and reproduction study on heptachlor epoxide chickens. Unpublished
report by Industrial Bio-test Laboratories, submitted to Velsicol
Chemical Corporation
Wong, D.T. and Terriere, L.C. (1965) Epoxidation of aldrin, isodrin
and heptachlor by rat liver microsomes. Biochem. Pharmacol., 14:
375-377
Female rats were fed with heptachlor in the diet at a dose level
corresponding to 5 mg/kg body-weight/day for three months. After this
period 32P-labelled fenitrothion at a dose of 24 mg/kg body-weight
was administered in oil per os, and radioactive measurements were made
(five times during 24 hours) of the total activities of the liver and
of the degradation products in blood and liver. The results were
compared with those of the control group not pre-treated with
heptachlor. Total activity in the liver of the group pre-treated with
heptachlor was 50 percent higher than in the control group, with a
maximum after four hours. In the control group, the values diminished
gradually after the exposure to fenitrothion. The ratio of the oxygen
analogue to fenitrothion in the blood and liver suggests that the
conversion of fenitrothion to its oxygen analogue is enhanced and
accelerated. Results of this experiment demonstrate that pre-treatment
of rats with heptachlor increases the metabolism of fenitrothion
(Mestitzova et al., 1970).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Chicken
Groups comprising four male and twenty female chickens were fed
dietary levels of 0, 0.02, 0.1 or 0.2 ppm of heptachlor epoxide for 25
weeks. Body-weight increase was not affected by heptachlor epoxide.
Mortality was low in all groups, and a slightly higher incidence in
the 0.2 ppm group than in the other groups is of doubtful
significance. No abnormal behaviour was observed. Total weekly egg
production and mean weekly egg-weights were not significantly
different between test and control groups. Hatchability was slightly
decreased in the eggs from the groups fed 0.1 and 0.2 ppm; viability
of hatched chicks was, however, not affected by heptachlor epoxide
(Wolvin et al., 1969).
Chicken egg
Hatchability of hen eggs was not affected by injection of 1.5 mg
heptachlor in the yolk of fertile eggs (Smith et al., 1970).
Quail
Japanese quails were given 10 and 50 ppm heptachlor in the diet. There
was no obvious adverse effect on reproduction when the birds were ten
weeks of age (Shellenberger and Newell, 1965).
Rabbit
Pregnant female rabbits were treated orally with 0 (22 animals) or 5
mg/kg body-weight/day (20 animals) of heptachlor epoxide from days six
to eleven of gestation. Foetuses were recovered on day 28 by caesarean
section. There were no behavioural abnormalities apparent, and
body-weight gain was not affected by heptachlor epoxide. There were no
deaths. No compound-related effects were observed with respect to
numbers of viable and non-viable term foetuses, resorptions, empty
implantation sites, corpora lutea or non-gravid females. A significant
increase in foetus weight was evident in the treated group; this
increase was considered to be compound related. Survival time was not
considered to be affected by heptachlor epoxide. There were no
teratogenic effects attributable to the compound (Wazeter et al.,
1969).
Rat
Male and female rats fed exclusively on diets containing a mixture of
heptachlor and heptachlor epoxide (3.1) in amounts of 0, 0.3, 3 or 7
ppm have been mated through three succeeding generations. The number
of pregnancies in the F0 and F2 generations was slightly reduced in
the 0.3 ppm group, but not at higher dose levels. There was a slight
increase in the mortality of the pups in the second and third week
after birth in the 3 ppm group. This was not consistent with other
data obtained in these experiments. During three successive
generations, the compound exerted no apparent effect upon the
fertility of the progenitors or the ability of the progeny to survive
(Witherup et al., 1967a).
Male and female rats fed exclusively on diets containing 0, 0.3, 3, 6
and 10 ppm heptachlor have been mated through three succeeding
generations. In the second and third week after birth, mortality of
the pups, only in the second generation, was slightly increased in the
10 ppm group. No adverse effects have been reported in the successive
lower dose levels (Witherup et al., 1967b).
Toxicity studies on the metabolites
The acute oral toxicity of four heptachlor metabolites, chlordene,
3-chlorochlordene, 1-hydroxychlordene and chlordene epoxide, was found
to be greater than 4,600 mg/kg body-weight for the LD50 to male or
female rats (Mastri et al., 1969a). In another test in female rats,
the oral LD50 of 1-hydroxy-2, 3-epoxychlordene was calculated to be
between 4,600 and 10,200 mg/kg body-weight and for 2-chlorochlordene,
>10,200 mg/kg (Mastri et al.,1969b).
Groups each comprising 25 male and 25 female rats, were fed 0, 100,
250, 500, 1000 or 2000 ppm of the heptachlor metabolite
1-hydroxychlordene in their diet for up to 224 days. A rat of each sex
was sacrificed at intervals for autopsy. After receiving the test diet
for 110 days, three females from each level were selected and mated
with males from the same level. Growth and food consumption were
normal at all levels, and mortality appeared to be unaffected by the
test compound. At 2000 ppm, the compound may have produced intestinal
irritation. Within the one generation, 1-hydroxychlordene showed no
adverse effects on fertility, litter size, litter weight or survival
and growth of the young at any level. Gross pathology findings were
limited to one hepatoma in a female fed 2000 ppm and one in a male at
500 ppm; one female at 100 ppm had parotid gland tumours. A breast
tumour was seen in a control animal. Histopathology revealed
abnormalities only in the liver which at 1000 and 2000 ppm showed
slight to moderate cytoplasmic margination, which was also evident to
some extent in the controls and lower level groups. Hepatic cell
enlargement which occurred was also doubtfully related to 1-hydroxy-
chlordene (Ingle, 1965).
Special studies on photodecomposition
By irradiation of heptachlor, a caged photoisomerization product
similar to that observed with other cyclodiene insecticides has been
produced. It has been suggested that this compound may result from
exposure of heptachlor to sunlight. It is more toxic to houseflies and
mosquito larvae than heptachlor, but no information is available on
its mammalian toxicity (Rosen, 1969). Present evidence indicates that
neither photo-heptachlor nor photo-heptachlor epoxide contributes
significantly to the terminal residues on plants or soil, even though
films of heptachlor epoxide on glass were readily transformed into the
photo-product (Polen, 1970).
Short-term studies
Rat
Groups, each comprising ten female rats, were fed dietary levels of 0,
5 or 10 ppm of heptachlor or 10 ppm of DDT for eight months.
Examination of the liver cells of the groups fed 10 ppm of heptachlor
or DDT revealed essentially the same pictures, with increase in the
smooth endoplasmic reticulum and the mitochondria, although these
changes occurred to a greater degree with DDT. At 5 ppm, heptachlor
revealed the earlier stages of the development seen in animals fed 10
ppm. Comparison of the described findings with heptoma cells, obtained
from feeding carcinogenic aminoazo compounds, revealed a striking
difference (Stemmer and Hamdi, 1964).
A total of 269 rats of unspecified sex were fed 40, 45 or 60 ppm of
heptachlor or 35, 40 or 45 ppm of heptachlor epoxide or 40, 45 or 60
ppm of a 75 : 25 percent mixture of the two compounds. After feeding
for 140 days, some animals were returned to a basic uncontaminated
diet and others were continued on the test diet. The animals were then
sacrificed after 10, 20, 30, 60, 80 or 120 days from the 140-day
feeding period. Typical liver lesions were shown to regress after
discontinuing feeding, as evidenced by the observations of the rats
which were returned to a normal diet. After 120 days, a significant
number had normal livers. The largest number of recoveries occurred in
the group fed heptachlor, the next with the mixture and the least in
the group fed heptachlor epoxide. Some rats fed a treated diet for 260
days displayed a second type of lesion in the periphery of the liver
lobule, consisting of enlargement of cells with cytoplasm of empty
appearance. The nuclei were small and dense, with distinct cell
boundaries. It was not reported if these lesions regressed after
returning the animals to a normal diet. Evidence of hyperfunction of
the adrenal medulla was also present in rats that had not had
regression of their liver changes. There was indication of depletion
of catecholamine, the cytoplasmic granules were diminished and some
cells showed vacuolations (Stemmer and Jolly, 1964).
Four groups, each comprising 10 male and 20 female rats, were given
daily oral doses of 0, 5, 50 or 100 mg/kg body-weight of pure
heptachlor starting at about four months of age. Administration was
continued for 200 days or until the animals died. By the tenth day,
all the animals in the groups fed 50 or 100 mg/kg had died. At day
200, the surviving animals in the 5 mg/kg group and the controls were
sacrificed for autopsy. Prior to death, the 50 and 100 mg/kg groups
became irritable and had accelerated respiration by the second day.
Convulsions preceded death. In the group given 5 mg/kg, no clinical
abnormalities were seen until the 50th day, when hyper-reflexia, rapid
respirations and chronic convulsions were observed. Two males and two
females in this group died before completion of the experiment,
compared to only one female in the controls. Weight gain was not
affected by 5 mg/kg. Gross pathology revealed changes in liver, kidney
and spleen. Histopathologic examination showed fatty degeneration of
the liver cells and moderate fatty infiltration of the cells of the
urinary tubules, as well as hyperplasia of the smooth endoplasmic
reticulum of the liver and spleen in the group fed 5 mg/kg (Pelikan et
al., 1968).
COMMENT
Since the last evaluation of heptachlor, which was made at the 1966
Joint Meeting, considerable new information on the metabolism of
heptachlor and its epoxide has become available. In acute and
short-term feeding experimental these metabolites appeared to be less
toxic than heptachlor or heptachlor epoxide. It was noted that liver
cellular metabolism was affected by a dietary level of 5 ppm of
heptachlor in the rat, and attention was drawn to its effect on the
metabolism of a compound each as fenithrothion. As is the case with
other organochlorine compounds, the toxicological significance of
these findings could not be determined. In a three-generation
reproduction study in rats, no dose related effects were observed. In
view of the observations from other organochlorine pesticides, an
adequate carcinogenicity study in a second species of animal other
than the rat is needed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
Dog: 2.5 ppm in the diet, equivalent to 0.06 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The approximate distribution of the use of heptachlor outside of
continental United States is as follows:
Area Percentage
Europe 60
Asia 15
South America 15
Africa 5
North America 5
TOTAL 100%
In Europe the dominant use (probably >75%) is for seed treatment in
the culture of sugar beets. Minor significant uses are for seed
dressing of cereal grains and protection of potatoes.
The major uses for heptachlor in Asia are in the control of soil
insects in sugar cane, cereal grains and vegetables. In some areas, to
a small degree, heptachlor is used for the protection of pineapple.
In South America, the major uses of heptachlor are on sugar cane and
maize. The protection of bananas is a minor significant use of
heptachlor.
In Africa and in North America, the most significant uses of
heptachlor are on maize and small grains.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cane berries
No residues were detected (<0.01 ppm) in boysenberries, blackberries
or red or black raspberries maturing in soils treated with 4.0 and 8.0
lb/acre of heptachlor (Velsicol data).
In carrots
(Grown in soils previously subjected to treatment)
Soil treatment at Wooster, Ohio, was made in May 1960 with 6 and 200
pounds of heptachlor per acre. Soil readings three years later (1962)
showed residue of 1.2 to 1.9 ppm (combined H and HE) at 6 pounds per
acre. At the end of four and one half years, the readings at 6 pounds
per acre were 0.52 to 1.25 ppm, and at 200 pounds per acre, 1.8 to
16.5 ppm. Readings in carrots at the 6 pound rate were 0.07 ppm, and
at 200 pounds, 0.5 ppm four and one-half years after treatment.
Carrot/soil residue ratios are respectively: 0.056-0.195 and
0.030-0.278 (Velsicol, soil and carrot data).
Heptachlor was applied at the rate of 5 pounds per acre annually for
five years (1958-1962) to a loam soil. In 1967, combined heptachlor
and heptachlor epoxide reading was 0.78 ppm. Carrots grown in that
soil in 1967 had combined residues at the end of the season of 0.36
ppm. Carrot/soil residue ratio was 0.46 (Lichenstein et al., 1968).
Loam soil treated five consecutive years with abnormally high rates of
heptachlor (5 lb per acre, 25 cumulative) contained 4.6% of the
applied heptachlor five years after cessation of treatment. The mean
residue level (H+HE) in soil was 0.70 ppm; in carrots 0.413;
carrot/soil residue ratio, 0.59. In a parallel test wherein one 25 lb
per acre application was made, ten years after cessation of treatment,
mean residue levels were: in soil 0.719 ppm; in carrots 0.223 ppm;
carrot/soil residue ratio 0.310 (Lichtenstein et al., 1970).
In simulated agricultural experiments, Harris and Sans (unpublished
report, ca. 1969. Absorption of heptachlor epoxide residues by carrots
from three different soil types) demonstrated the strong dependence of
residue transmittal on soil organic content. At a constant level in
soil of 2 ppm of heptachlor epoxide, residues in carrots were
respectively 0.56, 0.03 and 0.01 ppm when grown in soils of 20 and 55
percent organic matter. The respective carrot/soil residue ratios are
0.28, 0.015 and 0.005.
In citrus
Negligible residues were found in oranges and lemons from trees on
soil treated at 3 and 6 lb/acre in California. Soil treatment of 6
lb/acre around grapefruit trees resulted in essentially no residue. A
few samples had readings of 0.01-0.02 ppm for heptachlor and gamma
chlordane, but there was no difference from the check samples
(Velsicol data).
In cottonseed and its products
No residues were found in meal, crude oil, refined oil or soapstock
made from cottonseed grown from heptachlor dressed seed treated at 4
fluid oz /100 lb seed (Velsicol data).
In maize and maize oil
Maize grown on soil into which heptachlor had been incorporated at
rates ranging from 0.75 to 4.0 lb/acre (both row and broadcast
treatment) did not result in measurable residues in natural ear, grain
samples or ensilage prepared from green plants. Stover or mature
stalks contained up to 0.02 ppm of heptachlor, 0.06 ppm of heptachlor
epoxide and 0.04 ppm of gamma chlordane in one experiment only,
however, there did not appear to be any relationship between treatment
level and residue level. Bruce at al. (1966) was able to show a linear
relationship between residue levels of heptachlor epoxide in maize
seed and residue levels in the soil in which the maize was grown using
treatments of 2-20 lb/acre. Maize seed residues (H+HE) did not exceed
0.01 ppm. Maize oil from maize grown in heptachlor treated soil did
not contain measurable residues (Velsicol data).
In peaches
Heptachlor applied at rates of 1.5 and 3.0 lb/acre to soil around
peach trees before petal fall did not result in measurable residues in
the ripe peaches (Velsicol data).
In peppers
Essentially no residues were found in peppers grown in soils treated
with 1-6 lb/acre of heptachlor. Although one sample had an apparent
heptachlor epoxide residue of 0.07 ppm from a 3 lb/acre treatment, no
residue was found in peppers from the 6 lb/acre treatment (Velsicol
data).
In pineapple
Heptachlor was applied to pineapple in Hawaii as a foliar treatment at
1, 2 and 4 lb/acre. Foliage samples collected two months after
treatment showed heptachlor epoxide residues of 0.03 ppm at 1 lb/acre
to 0.20 ppm at 4 lb/acre. Heptachlor and gamma chlordane residues also
occurred in the foliage samples. One heptachlor reading was 2.40 ppm,
while gamma chlordane was 0.94 ppm. Other than these two readings, the
maximum foliage level of heptachlor was 0.65 ppm, and for gamma
chlordane the highest reading was 0.26 ppm. All fruit samples gave
negative readings for heptachlor, heptachlor epoxide and gamma
chlordane. Low levels of residues occurred in samples of fruit shell.
Raw bran samples showed maximum values of 0.05 ppm for heptachlor,
0.11 ppm for heptachlor epoxide and 0.09 ppm for gamma chlordane at
the high treatment rate of 4 lb/acre. Recommended rates gave maximum
levels of 0.01 ppm heptachlor, 0.04 ppm heptachlor epoxide and 0.05
ppm gamma chlordane. Samples analyzed seven months after foliar
treatments gave negative results for fruit and fruit shell and low
values for foliage and raw bran (Velsicol data).
In small grains
Small grains grown in soils treated (preplant) at rates up to 3
lb/acre resulted in no measurable residues of heptachlor or heptachlor
epoxide in the grain for barley, oats, rye and wheat. Gamma chlordane
is reported at levels up to 0.02 ppm but in also observed in the check
sample. Although significant residue appears in the soil from
heptachlor treatment, it is not translocated to the mature grain. Some
levels occur in the straw (Velsicol data).
Seed treatment of small grains with heptachlor does not give residues
in grain of oats, rye or wheat. Rye straw had up to 0.33 ppm of
heptachlor epoxide, which appears to be contamination or an artifact.
Wheat straw gave negative values from one test area and 0.02 ppm to
0.04 ppm for heptachlor and gamma chlordane (Velsicol data).
In sorghum
Seed treatment tests on sorghum at recommended rates gave negative
readings for heptachlor, heptachlor epoxide and gamma chlordane in
samples of grain and straw grown from treated seed (Velsicol data).
In soybeans
Soil treatments were made in September 1966 at 3.0 and 6.0 lb ai/acre.
Soybeans were planted and samples collected in 1967. From both EC and
granular formulations, all green forage samples gave negative
readings. In dry beans, at a 2 lb/acre rate, heptachlor and gamma
chlordane readings were identical with the check samples. The
heptachlor epoxide readings were 0.02 to 0.03 ppm.
Green forage samples grown on soil treated the same year exhibit
negative readings in all samples.
In another experiment, soybeans grown in soil from a heptachlor
treatment made the previous year at 1.0 lb ai/acre show negative
readings for heptachlor expoxide. Heptachlor and gamma chlordane
readings were identical with the check samples.
Soybean processing fractions were obtained from soybeans grown in soil
treated with heptachlor in previous years. Beans grown in 1967 from
treatments made in 1965 show negative readings for heptachlor epoxide
in meal, crude oil, refined oil and soapstock. Some heptachlor
contamination in handling is indicated.
Processing fractions grown in 1967 from a soil treatment in 1966 show
heptachlor epoxide in crude oil at a level of 0.03 ppm. Meal, refined
oil and soapstock contained no heptachlor epoxide.
An extensive study was done at Texas A & M University. At 3.0 lb
heptachlor per acre applied eight days before planting, heptachlor
residues in the plants at time of pod formation were uniformly 0.01
ppm or less. Heptachlor epoxide residue averaged about 0.02 ppm, with
one highest reading of 0.04 ppm. Three pounds per acre soil treatment
is the maximum permitted.
At 3 lb/acre applied eight days before planting, heptachlor in the
bean was uniformly less than 0.01 ppm and heptachlor epoxide averaged
0.044 ppm, with the highest reading being 0.059 ppm. In the crude oil,
these readings were increased about tenfold. For example, heptachlor
readings in the crude oil averaged about 0.03 ppm, with the highest
reading being 0.04 ppm. Heptachlor epoxide in the crude oil averaged
about 0.38 ppm, with the highest reading being 0.52 ppm. These levels
have been shown to be removed during the refining process.
In sugarbeets
Whole sugarbeet sampled five months after preplant broadcast treatment
of soil at 3 lb/acre showed combined H and HE residues of 0.04 ppm,
and at 6 lb/acre showed 0.19 ppm. Laboratory dried pulp showed 0.18
ppm at 3 lb/acre and 0.31 ppm at 6 lb/acre. Pilot plant drying showed
0.14 ppm at 3 lb/acre and 0.34 at 6 lb/acre.
No residues were found in sugarbeets or sugarbeet pulp from furrow and
coated seed treatments at 0.8-1.0 lb/acre (Velsicol data).
Sugarbeets harvested after seed treatment with heptachlor had no
cyclodiene residues (Harris et al., 1966).
In tomatoes
Tomatoes were grown in soil treated prior to planting with 1-3 lb/acre
of granular formulation or 2,3 and 6 lb/acre of emulsifiable
concentrate. No heptachlor epoxide residues were found in the tomatoes
from the granular formulation, and all heptachlor residues were at the
limit of sensitivity (0.01 ppm) except one value of 0.02 ppm. Residues
from the E.C. formulation were mainly <0.01 ppm except one heptachlor
at 0.01 ppm, one at 0.04 ppm (6 lb/acre) and one heptachlor epoxide at
0.02 ppm (6 lb/acre). Maximum combined residue in any one sample at
recommended rates (2-3 lb/acre) was 0.02 ppm.
In a second similar test in soil treated at 2,3 and 6 lb/ acre, no
residues of heptachlor, its epoxide or gamma chlordane were found in
any of the ripened fruit.
Soil samples contained 0.67 ppm heptachlor, 0.08 ppm heptachlor
epoxide and 0.26 ppm gamma chlordane at the 6 lb/acre rate (Velsicol
data).
In soil
In Kansas one year after heptachlor soil treatments, soil residues
were: 2 lb/acre rate - 0.26 to 0.47 ppm (combined H and HE), 3 lb/acre
rate - 0.18 to 0.32 ppm and 6 lb/acre rate - 0.63 to 2.24 ppm
(Velsicol, soybean data)
In Illinois, row treatments of 1.0 and 1.6 pounds heptachlor per acre
showed no residues in the soil fifteen months later (Velsicol, soybean
data).
In Kansas, four months after treatment at three lb/acre soil showed
0.21 to 0.40 ppm (combined H and HE), and 6 pounds per acre showed
0.49 to 3.61 ppm (Velsicol, soybean data).
In Mississippi, seed treatment at the rate of 0.07 pound heptachlor
per acre showed no residues in soil five months after application
(Velsicol, cotton data).
In a survey of the soil of 31 farms in Ontario, Harris, Sans and Miles
(1966) found heptachlor and/or epoxide residues (maximum 0.2 ppm) in
three samples of four which had a previous history of treatment with
heptachlor; one of the three had no recorded history of heptachlor
treatment, but 27 others with history of no heptachlor treatment had
no heptachlor residues. Two soils which had seed treatments (maize and
sugarbeets) had no detectable heptachlor residues. Those samples which
contained heptachlor or epoxide residues also contained residues of
gamma chlordane.
Studies on the mobility of organochlorine pesticides, including
heptachlor and epoxide, in soil demonstrated that these residues do
not move by leaching (Harris, 1969), but may be locally redistributed
by mechanical cultivation (Harris and Sans, 1970).
Under South African conditions, Wiese and Bossen (1966) found that
rapidity of degradation of five chlorinated insecticides " ... was
greater than reflected in most published literature ..." and that "
... heptachlor epoxide was the least persistent ..." In a survey of
agricultural soils of the Atlantic Provinces of Canada, heptachlor and
heptachlor epoxide were found in 9 percent of the soils in
concentrations between 0.06 and 0.86 ppm (Duffy and Wong, 1967).
In animal products
A great deal of work has been carried out to elucidate the propensity
of heptachlor and heptachlor epoxide residues, in or on feeds, to
transmit to milk or store in meat. Much of this information is
summarized by Saha (1969).
Meat
A one-year study on twenty beef cows and their calves was carried out
by Virginia Polytechnic Institute and the U.S. Department of
Agriculture (1968). Ten treated cows were fed a conventional ration of
alfalfa hay containing 0.4 ppm heptachlor and heptachlor epoxide
residues. In animals on contaminated feed continuously, the maximum
residue accumulating in the fat was 1.53 ppm (about four times the
residue level in the feed).
In a similar study over a two-year period involving twenty yearling
steers, the same investigators concluded the results were in good
agreement with that reported previously for pregnant and lactating
beef cows. The study showed clearly that with continued feeding of
contaminated ration the residues stabilize around 1.00 ppm, after 18
months on a residue-free regime, the combined H+HE residues in the
cattle ranged from 0.19 to 0.94 ppm (Bovard et al., 1968).
A 98-day feeding experiment, conducted with 56 beef steers in which
half were fed alfalfa hay containing 0.16 ppm H+HE, resulted in 0.017
to 0.020 ppm HE in the fat of treated animals and 0.004 to 0.007 ppm
in the fat of control steers (Hall et al., 1965).
Milk
In a study at the University of Tennessee, alfalfa hay containing
residues of 0.08 or 0.29 ppm heptachlor and heptachlor epoxide were
fed to dairy animals. Supplemental grain was fed according to milk
production. At the end of the 35-day feeding period, the milk fat from
the high and the low level intake groups contained 0.34 and 0.22 ppm
heptachlor epoxide, respectively. Converting to 4% butter fat milk,
this would be 0.014 ppm residue for the high group and 0.009 ppm for
the low group (Demott et al., 1967).
In an extensive study, lactating cows were fed alfalfa hay containing
residues of heptachlor and heptachlor epoxide resulting from treating
alfalfa at three different levels with heptachlor. The hay, fed for 30
days, contained average residues of heptachlor and heptachlor epoxide
of 0.045 ppm, 0.086 ppm and 0.160 ppm from the 0.25, 0.5 and 1.0
lb/acre treatments, respectively. The highest concentration of residue
in milk occurred between the 18th and 24th days of the feeding period
and averaged 0.013, 0.026 and 0.049 ppm for the test animals in each
group. Removal of treated hay from the diet resulted in a sharp
decline of residues in the milk, followed by a gradual disappearance
of residue over 13 weeks (Waldron at al., 1968).
Another study involved feeding lactating animals with measured
quantities of heptachlor epoxide added to the feed. Heptachlor epoxide
(in grain) was fed at the levels of 0.005 ppm and 0.020 ppm based on
total roughage ingested (hay plus silage) assumed at 50 pounds per
day. At the end of the feeding interval of 28 days, the residue of
heptachlor epoxide in milk was 0.0027 and 0.0043 ppm, respectively,
for the low and higher level fed. At 25 days the respective milk
residue levels for heptachlor epoxide were 0.0029 and 0.0044 ppm
(Hardee et al., 1964).
In a practical test (W.A.R.F. report, 1967), cows were fed maize
silage (50 pounds/day) and ground maize and cob meal (12 pounds/day),
all grown on soil treated with heptachlor for at least eight
consecutive years, at the recommended rate of one half pound per acre.
This feed was supplemented with 10 pounds of alfalfa hay, grown, in
rotation with maize in soil treated for two years, nonconsecutive, at
one half pound/acre, with the most recent application being two years
prior to the growing of the hay crop. The bedding for these cows,
consisting of shredded maize stalks, was also obtained from soil
treated with heptachlor for at least eight years. Milk samples gave
equivalent heptachlor epoxide readings after 10, 20 and 30 days of
feeding, varying between 0.004 and 0.007 ppm. There was no evidence of
increased milk residues during the course of the study and there was
no difference from the checks. All readings for heptachlor, in milk,
were reported as less than 0.001 ppm, and gamma-chlordane was not
detected in milk.
A survey of milk collected in Iowa was made in 1965 to determine what,
if any, residues could be detected. Heptachlor epoxide could be
characterized as being virtually absent from milk samples. All
readings averaged less than 0.002 ppm heptachlor epoxide, and only one
of 20 readings was as high as 0.007 ppm (W.A.R.F. report, 1965).
Poultry and eggs
In a chart provided by the U.S. Food and Drug Administration to the
Committee on Pesticides, Agricultural Research Institute (NAS/NRC)
Meeting, May 14, 1969, it is estimated that the contribution to the
diet of heptachlor epoxide from eggs is nil. Poultry is not shown by
itself, but a correlation between poultry and eggs can be assumed.
In a chicken feeding study (Velsicol data), chickens received
heptachlor epoxide in their entire diet at levels up to more than ten
times that which could occur in chicken feed from registered uses of
heptachlor. The feeding continued for 21 weeks, and white meat, dark
meat, fat and eggs were analyzed. White meat was negative as to
residues except for negligible values in the low hundreths of a part
per million range. Residues in the fat, from chickens fed at the level
which would reasonably reflect a tolerance in commodities used as feed
for chickens, were less than the 0.3 ppm non-actionable level in fat.
Similarly, residues in eggs from chickens which had received
heptachlor epoxide in their total diet for 21 weeks at a level
appropriate for a tolerance, but certainly higher than is encountered
in total feed, averaged 0.03 ppm, which is the non-actionable level in
eggs.
Heptachlor epoxide (in combination with other organochlorine
insecticides) were fed to 60 laying hens for 20 weeks at levels of
0.05, 0.15 and 0.45 ppm to determine residue levels in eggs. The
plateau levels in eggs approximate the feeding levels of heptachlor
epoxide; yolk/white concentration ratio is 99/1 (Cummings et al.,
1966).
FATE OF RESIDUES
In soil
1-Hydroxy chlordene, a conversion product, was found in a small number
of soil samples treated with heptachlor (Duffy and Wong, 1967).
Information on routes of metabolism of heptachlor by soil
micro-organisms has recently become available. As is the case with
mammals, 1-hydroxy 2,3-epoxychlordene was formed and by soil bacteria,
and it was demonstrated that it was derived from 1-hydroxychlordene,
but not from heptachlor epoxide. An unknown metabolite has also been
produced from 1-hydroxychlordene; the structure 1-keto chlordene has
been postulated for this compound but not proven. In addition, other
routes of metabolism involving a reductive stop to chlordene have also
been shown to occur in soil (Miles et al., 1969), but it is not known
if this pathway occurs in mammals. The significance of these chlordene
derivatives has been evaluated toxicologically (see "Toxicity studies
on the metabolites").
Effects of food processing on residues
Evidence continues to accrue that residues of heptachlor epoxide and
associated gamma chlordane are concentrated in the peels of sub-soil
crops, such as rutabagas, carrots and potatoes (Saha and Stewart,
1967). The residue distribution pattern discerned by older analytical
methods (Fox et al., 1964) has now been confirmed by the more
sensitive gas-liquid gas chromatography method (Stewart et al., 1965).
Concentration of heptachlor-derived residues at the outer surfaces of
crops with small surface to volume ratios simplifies effective residue
reduction by several normal food preparation steps. Washing removes
loosely held surface residues such as that contained in adherent soil
(Farrow et al., 1969). Washing plus peeling is highly effective in
residue removal and in frequently used for potatoes and root crops in
home cooking, and always is used in commercial canning of these crops
and tomatoes. Abrasive peeling is also effective; it is normally
used in home cooking and in commercial canning.
Commercial processing of edible vegetable oils frees the finished oil
from residues of heptachlor and heptachlor epoxide, as well as other
organochlorine pesticides (IUPAC Commission on Terminal Pesticide
Residues, 1970; Smith et al., 1968).
In the preparation of animal products, several normal food processing
steps reduce residues. Heptachlor and epoxide, being lipophilic, tend
to remain with fat when it is separated from meat by heating or
mechanical trimming. Reduction of heptachlor and heptachlor epoxide
residues has been reported in milk processing. Production of
condensed, sterilized, spray dried and drum dried milks results in
lower levels (fat basis) of heptachlor epoxide residues than the raw
milk from which they were made (Liska and Stadelman, 1969).
No evidence is available regarding the effect of normal storage
procedures on heptachlor residues.
Minimal evidence (Del Monte Corp., private communication, 1969)
indicates that short, simple cooking of leafy vegetables, such as
spinach, would not affect levels of residue from heptachlor treatment.
Evidence of residues in food in commerce or at consumption
In a series of papers, Duggan and others have assessed the dietary
intake of pesticide chemicals in the United States and determined the
pesticide residue levels in various food groups, domestic and
imported.
For heptachlor and heptachlor epoxide,a three year average (1964 to
1967) was 0.000031 milligrams/kilogram body-weight/day for heptachlor
and heptachlor epoxide combined. This assessment was based on the food
intake of almost twice the "average" intake of the "average"
individual. The value is 6 percent of the Acceptable Daily Intake for
heptachlor and its epoxide (0.0005 mg/kg).
The average levels of heptachlor and heptachlor epoxide residues in
food and feed - domestic, imported and ready to eat - are summarized
in Table I.
Total diet studies in England and Wales (Abbott et al., 1969) detected
heptachlor expoxide in three samples from the cereal group (less than
0.01 ppm), in ten samples from the meat group (up to 0.006 ppm) and
seven samples from the fats group (up to 0.008 ppm). In terms of total
diet, one out of 66 composites contains 0.001 ppm of heptachlor
epoxide; in all other cases the calculated concentration in the diet
was less than 0.0005 ppm heptachlor epoxide. No heptachlor was
detected at limit of detection of 0.0005 ppm.
METHODS OF RESIDUE ANALYSIS
Multidetection analytical techniques, such as those adopted (1969) by
the IUPAC Commission on Pesticide Residue Analysis, can be used to
determine residues of heptachlor, heptachlor epoxide, gamma-chlordane
and nonachlor in crops, soils and animal products down to a lower
sensitivity limit of 0.01 ppm. Procedures based on those given in the
Pesticide Analytical Manual, Vol. I, USHEW, FDA, 1968, with slight
modifications, and designated as AM 0237, AM 0486 and AM 0481, have
been developed by Velsicol Co. for the analysis of residues in fruits,
vegetables, grains, meat and poultry. The method of Cummings et al.
(1966) is suitable for determining heptachlor and heptachlor epoxide
in eggs. A method for the analysis of 1-hydroxychlordene in plant
tissues is under development (Nash and Beall, 1970 pre-print).
APPRAISAL
The data on the absorption of heptachlor and heptachlor epoxide by
carrots grown in soils containing residues, either from current or
previous annual treatments at normal or high rates, indicates that the
temporary practical residue limit should be increased from 0.1 ppm to
0.2 ppm.
New data on residues in maize grain grown in heptachlor treated soil
shows that this commodity is within the practical residue limit of
0.02 ppm established for raw cereals. This continues to be true for
the grain of barley, oats, rye and wheat.
Data from a total of eight studies on the relationship between the
levels of heptachlor and heptachlor epoxide in feed to the levels in
meat and milk show that the maximum residue level in animal feed, that
can be permitted without exceeding the practical residue limits for
these animal products, is 0.04 ppm of combined residues. If dried
sugarbeet pulp is fed in the diet of beef or dairy animals and
constitutes up to 20 percent of the feed, then the maximum permissible
residues in the pulp would be 0.20 ppm and in the whole sugarbeet
would be 0.04 ppm. Similar considerations would apply to maize stover,
soybean forage and pineapple bran used in animal feeds.
TABLE I
Heptachlor epoxide (or heptachlor) residues in food and feed1
RAW AGRICULTURAL PRODUCT READY-TO-EAT FOOD
Food Category Domestic Imported
Incidence Average Incidence Average Incidence Average
percent ppm percent ppm percent ppm
Large Fruit 0.7 < 0.005 0.7 < 0.005 - -
Small Fruit 1.0 < 0.005 0.7 < 0.005 - -
Grain & Cereals
for human use 0.2 < 0.005 - - 4.1 < 0.001
for animal use 2.5 < 0.005 - - - -
Vegetables
Leaf and Stem 3.0 < 0.005 3.3 < 0.005 1.4 < 0.001
Vine and Ear 2.4 < 0.005 2.3 < 0.005 5.4 < 0.001
Root 3.8 < 0.005 5.7 < 0.005 - -
0.82 < 0.005 4.62 < 0.005 - -
Potatoes 5.4 < 0.001
Beans 0.4 < 0.005 1.2 < 0.005 1.4 < 0.001
Eggs 3.1 < 0.005 3.4 < 0.005
Nuts 0.5 < 0.005 - -
Processed
(canned, frozen)
Domestic 0.8 < 0.001
Imported 0.7 < 0.001
Fluid Milk 23.0 3.03
(fat basis) 0.82 < 0.005
Dairy Products 21.3 0.02 3.0 < 0.005
(fat basis) 0.82 < 0.005 0.1 < 0.005
1/ extracted from USA-FDA surveillance programme report (Duggan, 1968).
2/ Heptachlor residues
The committee received information on residues in pineapple fruit
resulting from foliar treatment at 1,2 and 4 lb/acre. No residues of
heptachlor, heptachlor epoxide or gamma chlordane were found in the
fruit at the detection limit of 0.01 ppm for the method of analysis,
thus supporting a negligible residue tolerance of 0.01 ppm.
With the exception of carrots, the new data available did not indicate
a need to change the previous recommendations for practical residue
limits for the items listed. The previous practical residue limit of
0.125 ppm for milk products was changed to 0.15 to allow for the
limitations and variabilities of residue analytical procedures. Data
was presented which suggested the addition of practical residue levels
for tomatoes, cottonseed, soybeans, crude soybean oil, refined soybean
oil, poultry fat and eggs (on a shell-free basis).
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
TOLERANCES (These are additional to those in previous recommendations)
ppm
Pineapple (edible portions) 0.01
PRACTICAL RESIDUE LIMITS
ppm
Milk and milk products (determined on the
extracted fat) 0.15
Fat of meat and poultry 0.2
Raw cereals, tomatoes, cotton seed, soybeans,
refined soybean oil 0.02
Vegetables (except where otherwise specified),
eggs (shell-free basis) 0.05
Carrots 0.2
Crude soybean oil 0.5
Citrus fruit 0.01
Remarks
Residues of heptachlor and its epoxide should each be determined and
the sum expressed as heptachlor. Tolerances apply to residues from
application to seed and soil only.
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate carcinogenicity study in a second species of animal.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric., 20: 245
Bovard, K.P., Fontenot, J.P. and Priode, B.M. (1968) 1967-1968
Livestock Research Report, Research Div., Virginia Polytechnic
Institute
Bovard, K.P., Fontenot, J.P., Watson, D.F. and Priode, B.M. (1968)
Abstract in J. Animal Science, 27(4): 1131
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Residue Reviews, 27: 81-138
Brooks, G.T. and Harrison, A. (1969) Hydration of HEOD (dieldrin and
the heptachlor epoxides by microsomes from the livers of pigs and
rabbits). Bull. environ. Cont. Toxicol., 4, (6): 352-361
Comptes Rendus, (1970) XXV Conference, IUPAC, July 1-5, 1969 Cortina
d'Ampezzo, Italy, 187-195
Corneliussen, P.E. (1969) Residues in food and feed. Pesticide
residues in total diet samples (IV). Pest. Mon., J. 2(4): 140-152
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low-level feeding of five organochlorine insecticides to
hens. J. Assoc. Off. Anal. Chem., 49: 354-364
Davidow, B. and Radomski, J.L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol. exp.
Therap., 107: 259-265
Demott, D.J., Miles, J.T., Hinton, S.A. and Hardin. (1967) L.J.J.
Dairy Science, 49(12): 1495
Dorough, H.W. (1968) Residues in soybeans sprayed with chlordane or
grown in soils treated with heptachlor or chlordane. Report to
Velsicol Chemical Corp., 10 September
Duffy, J.R. and Wong, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic provinces
of Canada. J. Agr. Food Chem., 15: 457-464
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966) Pesticide residues
in total-diet samples. Science, 151: 101-104
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1967) Residues in food
and feed, Pesticide residues in total diet samples (II). Pest. Mon.
J., 1(2): 2-12
Duggan, R.E. (1968a) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pest. Mon. J., 1(4): 2-7
Duggan, R.E. (1968b) Residues in food and feed. Pesticide residue
levels in foods in the United States from July 1, 1963 to June 30,
1967. Pest. Mon. J., 2(1): 2-46
Duggan, R.E. (1969) Chart provided by U.S. FDA to Committee on
Pesticides, Agricultural Research Institute (NAS/NRC) Meeting, May 14
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest. Mon.
J., 2(4): 153-162
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Calculated daily consumption of pesticides with foods are
discussed and compared with currently accepted values. Science, 157:
1006-1010
FAO/WHO (1967) Evaluation of some pesticide residues in food. FAO,
PL:CP/15; WHO/Food Add./67.32
Farrow, R.P., Elkins, G.R., Rose W.W., Lamb, F.C., Rulls, J.W. and
Mercer W.A. (1969) Canning operations that reduce insecticide levels
in prepared foods and in solid food wastes. Residue Reviews,
29:73-87
Fox, C.J.S., Chisholm, D. and Stewart, D.K.R. (1964) Can. J. Plant.
Sci.,44: 149
Gillett, J.W. and Chan, J.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Fd. Chem., 16: 590-593
Hall, O.G., Hardin, L.J., Fryer, M.E. and Glover, J.A. (1965)
Tennessee Farm and Home Science Progress Report No: 56
Hardee, D.D. Gutenmann, W.H., Keenan, G.I. Gyrisco, G.G. Lisk, D.J.,
Fox F.H. Trimburger, G.W. and Holland, R.F. (1964) Residues of
heptachlor epoxide and telodrin in milk from cows fed at part per
billion insecticide levels. J. Econ. Entomol., 57: 404-407
Harris, C.R., Sans, W.W. and Miles, J.R.W. (1966) Exploratory studies
on occurrence of organochlorine insecticide residues in agricultural
soils in southwestern Ontario. J. Agr. Fd. Chem., 14: 398-403
Harris, C.I. (1969) Movement of pesticides in soil. J. Agr. Fd. Chem.,
17: 80-82
Harris, C.R. and Sans, W.W. (1969) Unpublished report, Absorption of
heptachlor epoxide residues by carrots from three different soil types
Harris, C.R. and Sans, W.W. (1970) Pesticide Progress, Canada
Department of Agriculture, Ottawa, 8(1)
Ingle, L. (1965) Effects of 1-hydroxychlordene when incorporated into
the diets of rate for 224 days. Unpublished report from the University
of Illinois, submitted by Velsicol Chemical Corporation
Klein, W., Korte, F., Weisgerber, I., Kaul, R., Mueller, W. and
Djirsarai, A. (1968) Über den Metabolismas von Endrin, Heptachlor und
Telodrin. Qdal. Pldt. Mater. veg. (Den Haag), 15: 225-238
Korte, F. (1968) Metabolism of chlorinated insecticides.(2)
Heptachlor. Paper submitted to the IUPAC Commission on Terminal
Pesticide Residues, Vienna, 1967. Appendix VI. IUPAC Information
Bulletin No: 32, p. 110
Korte, F. and Porter, P.E. (1970) Minutes of the Fifth Meeting of the
IUPAC Commission on Terminal Pesticide Residues, September 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Lichtenstein, E.P. Fuhremann, T.W. and Schulz, K.R. (1968) Use of
carbon to reduce the uptake of insecticides as soil residues by crop
plants. Effects of carbon on insecticide absorption and toxicity in
soils. J. Agr. Fd. Chem. 16(2): 348-355
Liska, B.J. and Stadelmann, W.J. (1969) Effects of processing on
pesticides in foods. Residue Reviews, 29: 61-72
Martin, RJ. and Duggan, R.E. (1968) Pesticide residues in total diet
samples (III). Pest. Mon. J., 1(4): 11-20
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969a) Acute oral
toxicity study on four chlordenes in albino rats. Unpublished report
from Industrial Bio-Test Laboratories, submitted to Velsicol Chemical
Corporation
Mastri, C., Keplinger, M.L. and Fancher, O.E. (1969b) Acute oral
toxicity study on two chlordenes in female albino rats. Unpublished
report from Industrial Bio-Test Laboratories, submitted to Velsicol
Chemical Corporation
Mestitzova, M., Kovac, J. and Durcek, K. (1970) Heptachlor induced
changes in fenitrothion metabolism. Bull. environ. Contam. Toxicol.,
5(3): 195-201
Miles, J.R.W., Tu, C.M. and Harris, C.R. (1969) Metabolism of
heptachlor and its degradation products by soil micro-organisms. J.
econ. Entomol., 62: 1334-1338
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Nash, R.G. and Beall, M.L., Jr. (1970) Preprint of unpublished work
Pelikan, Z., Halacka, K., Polster, M. and Cerny, E. (1968)
Intoxication à long terse chez les rats par l'heptachlore à petit
doses. Arch. belges Méd. Soc., 7: 529-538
Polen, P.B. (1970) Terminal residues of chlordane. Paper submitted to
the IUPAC Commission on Terminal P Pesticide Residues, Erbach
(Rheingan) Federal Republic of Germany. 14-18 September
Rosen, J.D. (1969) Personal comminication to the participants of the
IUPAC Commission on Terminal Pesticide Residues, Cortina d'Ampezzo,
July 1969
Saha, J.G. (1966) Significance of organochlorine insecticide residues
in fresh plants as possible contaminants of milk and beef products.
Residue Reviews, 26: 89-126
Saha, J.G. and Stewart, W.W.A. (1967) Can. J. Plant Sci.:47, 79
Seal, W.L., Dawsey, L.H. and Cavin, G.E. (1967) Pesticides in soil.
Monitoring for chlorinated hydrocarbon pesticides in soil and root
crops in the Eastern States in 1965. Pest. Mon. J., 1(3): 22-25
Shellenberger, T.E. and Newell G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail. Lab.Anim.Care., 15:
119
Shellenberger, T.S., Lei, J., Udale B. and Newell, G.W. (1966)
Comparitive toxicity of DDT, dieldrin and heptachlor to Japanese and
bobwhite quail. Toxicol. appl. Pharmacol., 8: 353
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) J.
Amer. Oil Chem. Soc.,45(12): 866
Smith, S.J., Weber C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocardon pesticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Stemmer, K.L. and Hamdi, E. (1964) Electron microscopic changes in the
liver cells after prolonged feeding of DDT and heptachlor. Unpublished
report from the Kettering Laboratory, University of Cincinnati,
submitted by Velsicol Chemical Corporation
Stemmer, K.L. and Jolley, W.P. (1964) Regression of hepatic lesion of
heptachlor and its epoxide. Unpublished report of the Kettering
Laboratory, University of Cincinnati, submitted by Velsicol Chemical
Corporation
Stewart, D.K.R., Chisholm, D. and Fox, C.J.S. (1965) Can. J. Plant
Sci.,45: 72
Velsicol Chemical Co., (1966-1968) Reports of residue analyses
Waldron, A.C., Kaeser, H.E., Golemam, D.L., Staubus, J.R. and
Niemczyk, H.D. (1968) Heptachlor and heptachlor epoxide residues in
salt-treated alfalfa and in milk and cow tissues. J. Agr. Fd. Chem.,
16: 627-631
Wiese, I.H. and Basson, N.C.J. (1966) S. Afr. J. Agric. Sci.,9: 945
Wisconsin Alumni Research Foundation, (1965) Report to Velsicol
Chemical Co.
Wisconsin Alumni Research Foundation, (1967) Report to Velsicol
Chemical Co.
Wazeter, F.X., Butler, R.M., Geil, R.G. and Rehkemper, J.A. (1969)
Heptachlor epoxide. Teratology study in the Dutch rabbit. Unpublished
report from the International Research and Development Corporation
submitted to Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967a) The
effects exerted on the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor and its epoxide into
their daily diet. Unpublished report from the Kettering Laboratory,
Cincinnati, submitted by Velsicol Chemical Corporation
Witherup, S., Stemmer, K.L., Taylor, P. and Hull, L. (1967b) The
effects exerted upon the fertility of rats and upon the viability of
their offspring by the introduction of heptachlor into their daily
diets. Unpublished report from the Kettering Laboratory, Cincinnati,
submitted by Velsicol Chemical Corporation
Wolvin, A.R., Jenkins, D.H. and Fancher, O.E. (1969) Toxicity residue
and reproduction study on heptachlor epoxide chickens. Unpublished
report by Industrial Bio-test Laboratories, submitted to Velsicol
Chemical Corporation
Wong, D.T. and Terriere, L.C. (1965) Epoxidation of aldrin, isodrin
and heptachlor by rat liver microsomes. Biochem. Pharmacol., 14:
375-377
