
HEPTACHLOR
First draft prepared by Dr. W. Phang,
US Environmental Protection Agency,
Washington, D.C., United States
EXPLANATION
The JMPR evaluated heptachlor in 1970 (Annex I, 14) and concluded
that an adequate carcinogenicity study in a second species of animal
other than the rat was needed. An estimated ADI of 0.0005 mg/kg/bw
was allocated. Since the last Meeting, several toxicology,
metabolism, and epidemiology studies, along with many case reports,
have become available, which are summarized in this monograph. Some
of the previously published reports reviewed in FAO/WHO monographs of
1967 and 1970 (Annex I, 7 and 15) are also included.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical Aspects
Absorption, distribution, and excretion
Rat
Although quantitative data on the absorption of heptachlor are
not available, heptachlor is readily absorbed from the
gastrointestinal tract as demonstrated by the presence of the parent
compound and/or its metabolites in the urine, faeces, and tissues of
rats which had been fed 14C-labelled heptachlor and in the serum of
humans who had consumed undiluted raw milk products known to be
contamin-ated with residues of heptachlor.
Two male rats were given a single oral dose of 14C-heptachlor
(0.464 µCi) in corn oil. During a 10 day monitoring period, 6% of the
administered radioactivity was found in the urine while 60% was found
in the faeces. Approximately 26% of the total radioactivity
recovered from the faeces was unchanged heptachlor and the rest was in
the form of metabolites (Tashiro & Matsumura, 1978). Heptachlor has
been shown to be readily metabolized to heptachlor epoxide which was
found to be distributed in adipose tissue of dogs and rats (Radomski
& Davidow, 1953; Davidow & Radomski, 1953). When rats were fed a diet
containing 35 ppm heptachlor for 2 months or more, the highest
concentration of heptachlor epoxide was found in fat with
substantially lower levels in liver, kidneys, and muscles. However,
no heptachlor or heptachlor epoxide was found in the brain (Radomski
& Davidow, 1953).
The rate of heptachlor epoxide accumulation and elimination from
the body fat was examined by placing male and female rats on a diet
containing heptachlor for 12 weeks and then on the untreated diet for
12 more weeks. In males, at approximately 2 to 8 weeks of dosing the
heptachlor epoxide level in fat reached a plateau; by 6 weeks after
dosing the level was below the detection limit. In females, the
heptachlor epoxide level in fat was much higher than that in males by
2 weeks and throughout the remaining duration of the study; by the 8th
week after dosing the level was below the limit of detection (Radomski
& Davidow, 1953).
Biotransformation
Rat
In a metabolism study on heptachlor reported by Tashiro and
Matsumura (1978), two male rats were administered a single oral dose
of 14C-heptachlor (0.464 µCi) in 250 µl of corn oil; most of the
radioactivity (approximately 60% of the administered dose) was
recovered in the faeces. The major metabolites in the faeces were
heptachlor epoxide, 1-hydroxychlordene, 1-hydroxychlordene epoxide,
and 1,2-dihydroxydihydrochlordane, as well as two unindentified
products. Similar metabolites were found in an in vitro comparative
and qualitative study using human or rat liver microsomes. Based upon
these findings, the metabolic scheme for heptachlor shown in Figure 1
was proposed.
Heptachlor epoxide was also isolated from fat of dogs which had
been administered heptachlor at dose levels of 1 to 3 mg/kg and rats
fed 30 mg/kg heptachlor for 3 months (Davidow & Radomski, 1953;
Radomski & Davidow, 1953).
Effect on enzymes and other biochemical parameters
From the information published in the 1971 FAO/WHO Monograph,
heptachlor and aldrin appear to be substrates for the same enzyme,
which is inhibited by the epoxide. In vitro microsomal metabolism
of heptachlor does not proceed beyond formation of the epoxide. The
treatment of rats with heptachlor increases the metabolism of
fenitrothion (Annex 1, 15).
Acute toxicity
Some of the acute toxicity data of heptachlor and heptachlor
epoxide were considered by the 1966 Joint Meeting and published in the
1967 FAO/WHO Monograph. Additional data have become available in the
open literature; these data have been evaluated and are summarized
with previously published data in Table 1.
Clinical signs of acute toxicity of heptachlor include
hypoactivity, tremor, convulsion, ataxia, and changes in EEG patterns.
Histopathologically, severe liver damage was reported.
Acute toxicity of metabolites
The oral LD50 for four other metabolites of heptachlor
(chlordene, 3-chlorochlordene, 1-hydroxychlordene, and chlordene
epoxide) was reported to be greater than 4600 mg/kg body weight in
male and female rats (Annex 1, 15).
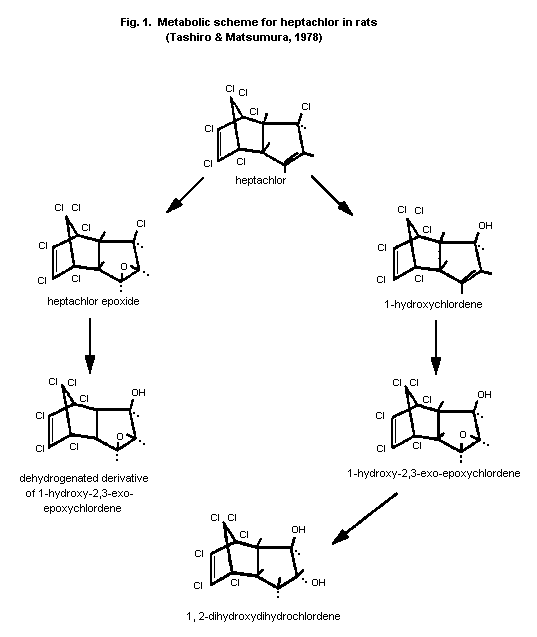 Table 1. Acute toxicity data of heptachlor and heptachlor epoxide
Species Sex Route LD50 Reference
(mg/kg bw)
A. Heptachlor
Rat M oral 71 Podowski et al. (1979)
M&F oral 60-142a Velsicol Corp. (1959)b
Chick d oral 63 Sherman & Ross (1961)b
Mouse d oral 70 Gak et al. (1976)
Hamster d oral 100 Gak et al. (1976)
Rat M dermal 195c Gaines (1969)
F dermal 250c Gaines (1969)
B. Heptachlor epoxide
Rat M&F oral 34-48a Velsicol Corp. (1959)b
Mouse M oral 32-48 Velsicol Corp. (1959)b
a Sex differences
b Data excerpted from the 1966 JMPR monograph (Annex 1, 7)
c Xylene was the solvent.
d Not stated
Short-term studies
Mouse
Groups of 10 Charles River CD-1 mice/sex/dose were fed a mixture
of heptachlor-heptachlor epoxide (25:75) at dietary concentrations of
1, 5, 10, 25, and 50 mg/kg for 30 days. Following 30 days of
treatment, the animals were sacrificed and necropsied. Microscopic
examinations were conducted on liver tissue.
No deaths occurred in the 1, 5, nor 10 mg/kg groups. One female
died in the 25 mg/kg group, and 9 males and 8 females died in the 50
mg/kg group. No marked differences were seen between the treated
animals and the controls with respect to body weights and food
consumption. Mice which were sacrificed at the end of the study from
the 10, 25 and 50 mg/kg groups showed liver enlargement associated
with accentuated lobulation. The liver weight of the only surviving
male mouse from the 50 mg/kg group was markedly increased (control
mean: 1.69 g; 50 mg/kg; 4.00 gm), and increased mean liver weight was
also found in the 25 ppm male mice (control, 1.69 g; 25 ppm, 4.35 gm).
Histopathology findings indicated that livers of male mice in the 5,
10, 25, and 50 ppm and of female mice in the 10, 25, and 50 ppm groups
had enlargement of centrilobular and midzonal hepatocytes with
replacement of the normal coarse cytoplasmic granularity by finely
granular and homogeneous cytoplasm. The severity of this lesion was
dose-related. The incidences of the microscopic findings were not
reported (Wazeter et al., 1971a). Based upon the histopathology
findings, the NOAEL was 1 mg/kg.
Rat
Two short-term studies on heptachlor in rats were summarized in
the 1971 monograph. The dietary levels tested in these two studies
ranged from 5 to 45 ppm. At 10 ppm and above, rat liver cells showed
an increase in smooth endoplasmic reticulum and mitochondria. The
liver effects regressed after the treated rats were placed on a
control diet for 120 days (Annex 1, 15).
Long-term studies
Some of the unpublished long-term studies on heptachlor were
evaluated by the 1966 Joint Meeting and published in the 1967
Monograph. To facilitate consideration of the toxicity of this
chemical the older toxicity studies are also summarized here.
Rat
Groups of Carworth Farms (CF) rats (20/sex/dose) were fed diets
containing heptachlor (purity not specified) at a concentration of 0,
1.5, 3.0, 5.0, 7.0, or 10 ppm for 110 weeks. The treatment diet was
prepared by spraying standard rat diet with an ethanol solution of
heptachlor. After 7 weeks on the test diet, 5 females and 5 males
from each dose group were mated to determine whether or not heptachlor
would affect the reproductive capacity of the treated animals or the
survival and subsequent growth of the offspring. Following similar
procedures, another mating was conducted using different animals from
each dose group after 22 weeks of treatment. The offspring were kept
with their mothers until weaning at 3 weeks of age. After weaning,
the offspring were placed on the control diet for 8 weeks for further
observations. After 110 weeks of treatment, the surviving animals
were sacrificed, certain haematological parameters were examined.
Histopathology was conducted on the heart, liver, lungs, brain,
spleen, kidneys, thyroids, and the adrenal glands.
Heptachlor treatment did not affect mortality, body weight, or
food consumption. The limited reproductive parameters measured did
not indicate any effect on the body weights at birth or at weaning.
Heptachlor administration did not appear to significantly affect the
mortality of the offspring. Organ weights of the heptachlor-treated
rats were not markedly different from those of controls. Haematology
parameters (erythrocyte counts, haemoglobin, leukocyte counts, and
differential white cell counts) did not show any compound-related
effect. The histopathology data indicated 6/16 males and 3/18 females
at 7.0 ppm and 2/12 males and 9/16 females at 10 ppm had slight
hepatic cellular alterations characterized by swelling, homogeneity of
the cytoplasm and peripheral arrangement of the cytoplasmic granules
in the hepatic cell in the central zone of the lobules. Under the
conditions of this study, heptachlor at doses up to 10 ppm did not
produce an increase in the tumour incidence in CF rats (Witherup
et al., 1955).
Groups of CFN (Carworth Farms, Nelson) rats (25/sex/dose) were
fed heptachlor epoxide at dietary concentrations of 0, 0.5, 2.5, 5.0,
7.5, and 10.0 ppm for 108 weeks. The test diet was prepared by
spraying an ethanol solution of heptachlor epoxide on Purina
Laboratory Chow.
The survival rate was greater than 47% in the treated and control
groups. There was an increase in the liver weights of treated females
and a slight increase in treated males. The test chemical was not
reported to cause any additional effects. This study has
methodological deficiencies which include uncertain concentrations in
the feed and insufficient number of rats in each dose level. Also the
report does not contain histopathological data and is therefore not
adequate for evaluating the long-term toxicity of heptachlor (Witherup
et al., 1959). However, a panel of independent pathologists
convened by the US National Academy of Sciences (1977) reviewed the
histopathology and concluded that there was no increase in liver
tumours in treated rats.
Four groups of Charles River CD female rats (25/dose) were fed a
3:1 mixture of heptachlor:heptachlor epoxide at dietary concentrations
of 5, 7.5, 10, and 12.5 ppm. A control group of 54 females was fed a
diet containing the vehicle, corn oil. Interim sacrifices were
conducted at 5 and 19 months to determine the nature and degree of the
histological changes in the liver. At 5 months, 3 controls and 5 rats
from the 7.5 ppm group were sacrificed. At 19 months, 5 controls, 2
rats from 5 ppm, 2 rats from 10 ppm, and 1 rat from 12.5 ppm were
sacrificed. After 24 months of treatment, the surviving rats were
sacrificed, and gross and histological examinations were conducted.
There was an increase in mortality at 12.5 ppm relative to that
of controls (control, 21%; 12.5 ppm, 50%). The test article did not
affect body weight gain. Liver changes which were described as fatty
liver and enlarged cells in the central zone of the hepatic lobules
were reported. However, no histopathology data were included in the
report (Jolley, 1966).
Groups of Osborne-Mendel rats (50/sex/dose) were fed technical
grade heptachlor (73% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at time-weighted doses of 38.9 and 77.9
ppm for males and 25.7 and 51.3 ppm for females. The matched controls
consisted of 10 males and 10 females, and pooled controls were 60
rats/sex. The treated animals received the chemical for 80 weeks and
were observed for 30 weeks. During the 80 weeks of treatment, the
dietary concentrations changed three times for each treatment group
based on changes in body weight.
There was a decrease in the mean body weight of high dose males
which almost returned to the control level after the treatment was
terminated. There was also a slight increase in mortality in high
dose male and female rats. Histopathology data revealed numerous
inflammatory, degenerative, and proliferative lesions with
approximately equal frequencies in treated and control animals. There
was an increased incidence of thyroid follicular cell neoplasms,
including combined adenomas and carcinomas in the high dose female
rats (high dose, 14/38; low dose, 3/43; matched controls, 1/9;
p<0.001) while, in male rats, a non-significant increase was observed
only at the low dose (high dose, 3/38; low dose, 9/38; matched
control, 1/8). At the same time, the incidences of the thyroid C-cell
neoplasms decreased in high dose female rats (high dose, 3/38; low
dose, 7/43; matched controls, 3/9; p<0.05). The results of analyses
of the thyroid lesions were ambiguous and further complicated by
variability in different experiments conducted at about the same time
with this strain of rats (NCI, 1977).
Dog
Groups of Beagle dogs (4/sex/dose) were fed heptachlor epoxide
(purity not specified) at concentrations of 0, 1, 3, 5, 7, and 10 ppm
for two years. After 2 years on test, 2 dogs/sex/dose were sacrificed
and necropsied while the other two dogs/sex/dose were maintained on
the control diet for an additional 6 months (recovery period). In
addition, the test animals in this study were also mated during the
study and employed as the P1 parental animals for a 2-generation
reproduction and teratology study.
No death nor compound-related behavioural change was seen during
the study. The test article did not affect the body weights or food
consumptions of the treated animals. The parameters for haematology
and urinalysis were comparable between treated and control dogs.
There were increases in alkaline phosphatase activities in males and
females at 3 ppm and above; these increases, in some dogs, were more
marked towards the end of the treatment period and tended to persist
throughout the recovery period. The serum albumin and total protein
levels were slightly decreased in 10 ppm male and female dogs during
the treatment and the recovery periods. An increase in the SGPT level
was also seen in 10 ppm animals during the treatment, extending into
the recovery period. After one year of treatment, the animals in 7
ppm group also showed an increase in the SGPT level which lasted into
the recovery period.
There was an increase in liver weights in 10 ppm male and female
dogs relative to those of the controls, and this increase persisted
with a slight attenuation during the recovery period. Histopathologic
examination on dogs (2 dogs/sex/dose) sacrificed at the end of the
treatment period showed an increase in the incidence of liver changes
in animals at 3.0 ppm or above. The changes were described as
enlargement and vacuolation of groups of centrilobular hepatocytes or
scattered individual hepatocytes, presence of finely granular, and a
"ground glass" appearance of the cytoplasm of large numbers of
hepatocytes. These histopathological changes were also seen in dogs
at 3 ppm and above after 6 months of recovery period. No
compound-related histopathology changes were seen in 1 ppm dogs.
Based upon the changes in biochemical parameters and the histological
changes in the liver, the NOAEL was 1 ppm (Wazeter et al., 1971b).
Although this study has certain deficiencies, including an
insufficient number of experimental animals, the results provide
useful information on the toxicity of the test compound in dogs.
Groups of 23 to 27 week old beagle dogs (2 males or 3
females/dose) were fed heptachlor epoxide at dietary concentrations of
0, 0.5, 2.5, 5.5, and 7.5 ppm for sixty weeks. The test diet was
prepared by spraying an ethyl alcohol solution of heptachlor epoxide
on the food. The experimental results were limited to clinical
observation, mortality, body weight changes, food consumption, and
organ weights. The limited results indicated that there was an
increase in liver weights of all the heptachlor treated male and
female dogs relative to that of the controls. For 7.5 ppm males, the
increase was approximately twice that of the controls. However, the
pathology data were not included in the report (Witherup et al.,
1958).
Mouse
Groups of Charles River CD-1 mice (100/sex/dose) received a
mixture of heptachlor/heptachlor epoxide (25%/75%) at dietary
concentrations of 0, 1.0, 5.0, and 10.0 ppm for 18 months. A positive
control group (100 mice/sex) received 2-acetaminofluorene at a dietary
concentration of 250 ppm. An interim sacrifice was performed on 10
mice/sex/dose including the positive control group. Gross pathology
and histology examinations were conducted on animals which died during
the study or were sacrificed on schedule.
There was a decrease in the number of survivors in 10 ppm male
and female mice, in 5 ppm females, and in positive control males
relative to those of the negative controls as shown in Table 2. Mean
body weights and food consumptions of the treated mice were not
affected. There were increases in the mean liver weights of males and
females in 5 and 10 ppm groups.
An increase in the incidence of hepatocytomegaly was seen in all
heptachlor-heptachlor epoxide treated mice relative to that of the
controls. The incidence of hepatic nodular hyperplasia was markedly
increased in 5 and 10 ppm males and females relative to that of the
controls. For heptachlor-treated animals, hepatoma was observed in
only one male mouse at 1 ppm (Wazeter, 1973). Based on the incidence
of hepatocytomegaly, a NOAEL was not established (LOAEL <1ppm).
The histological slides of this study were later evaluated by a
panel of pathologists convened by the Pesticide Information Review and
Evaluation Committee of the National Academy of Sciences, USA. The
results of the evaluation are presented in Table 2.
Levels of 1, 5, and 10 ppm of heptachlor-heptachlor epoxide did
not produce a statistically significant increase in the incidence of
hepatocellular carcinoma. However, at 10 ppm there was a statistically
significant increase in the incidence of combined hepatocellular
carcinomas and nodular changes (NAS, 1977).
Epstein (1976) reported a previously unpublished study conducted
by the US-FDA. Groups of C3H mice (100/sex/dose) were fed 0 or 10 ppm
of either heptachlor or heptachlor epoxide (purity unspecified) for 24
months. The liver histopathology was re-evaluated by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences. Their
results indicated a significant increase in hepatocellular carcinomas
in females given heptachlor and in both males and females given
heptachlor expoxide.
Table 2. The incidence of hepatocellular carcinomas and nodular
changes in CD-1 mice fed heptachlor-epoxide
Male Female
Group HC HC+N HC HC+N
Control 1/59 2/59 1/74 1/74
1 ppm H-HE 2/66 4/66 1/65 3/65
5 ppm H-HE 2/66 4/66 1/65 3/65
10 ppm H-HE 1/73 27/73* 4/52 16/52*
AAF 5/58 9/58 5/75 13/75
HC hepatocellular carcinomas
HC+N hepatocellular carcinomas and nodular changes
* (p<.001)
Groups of B6C3F1 mice (50/sex/dose) received technical-grade
heptachlor (72% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at initial concentrations of 10 and 20
ppm for males and 20 and 40 ppm for females. The initial
concentrations were reduced due to adverse toxic effects, with
time-weighted average concentrations of 6 and 14 ppm for males and 9
and 18 ppm for females. The mice were treated for 80 weeks and placed
on the control diet for another 10 weeks. Matched-controls consisted
of 20 males or 10 females, and the pooled controls were 100 males and
80 females. Under the conditions of the study, heptachlor had no
significant effects on the body weights. Survival at 90 weeks was
high: 70% of treated and control males and 60% of treated and control
females. Survival of female mice showed a significant trend towards
lower survival in treated groups compared to that in the control
group. The incidence of hepatocellular carcinomas was significantly
increased in the high-dose males and females as shown in Table 3
(NCI,1977).
A review of liver histopathology from this study by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences concluded
that there was a low incidence of induced hepatocellular carcinomas
(Table 3). According to this review, the incidence of hepatocellular
carcinomas was statistically increased (p<0.001) in groups of males
and females receiving heptachlor at the high dose only when combined
with the diagnosis of nodular changes (NAS, 1977).
Table 3. Incidence of proliferative lesions of the liver in B6C3F1
mice fed technical grade heptachlor
Lesion Pooled Matched Low Dose High Dose
Control Control
Males
Hepatocellular
carcinomas 17/92 5/19 11/46 34/47*
Nodular hyperplasia 3/92 1/19 9/46 6/47
Diffuse hyperplasia 3/92 0/19 1/46 3/47
Hepatocytomegaly 0/92 0/19 1/46 0/47
Females
Hepatocellular carcinomas 3/78 1/10 3/47 30/42*
Nodular hyperplasia 2/78 0/10 3/47 0/42
Diffuse hyperplasia 1/78 0/10 0/47 0/42
Hepatocytomegaly 1/78 0/10 0/47 1/42
The denominator represents the number of tissues examined microscopically.
* p<0.001 (Fisher exact test)
Reproduction studies
Dogs
Groups of 6-9 month old Beagle dogs (4/sex/dose) were fed
heptachlor epoxide at dietary concentrations of 0, 1, 3, 5, 7, and 10
ppm. When the female dogs attained an age of 14 months, they were
mated twice with male dogs from the same dose group. The females were
allowed to deliver and to nurse their pups.
For the F2 generation, 4 female and 2 male pups of the F1
generation were selected from each dose level to be the parental
animals (P2) of the F2 generation. At an approximate age of 14
months, the animals were mated, and the pregnant females were allowed
to deliver and to nurse their pups to 6 weeks of age. At this time,
the females and their pups were sacrificed.
No clinical signs or behaviour changes were seen in treated P1,
P2, or their offspring. There were no indications that body weight
or food consumption were affected by the test chemical. Whether or
not the test chemical produced any reproductive effects could not be
determined with the very limited data presented in this report. There
was a significant increase in the mortality rate of F1 pups in the 10
ppm group (control, 9/20; 10 ppm, 17/18). Only 1 male pup survived to
the scheduled sacrifice, and no female was available to serve as a P2
parental animal. There were slight increases in death rates of F2
pups at 3 and 7 ppm relative to the controls (Control, 0/4; 3 ppm,
3/8; 7 ppm, 3/8). The 5 ppm group had no pups. Haematology and
urinalysis results did not indicate any compound-related effects. Very
limited clinical biochemistry data showed that alkaline phosphatase
and/or SGPT activities in certain dogs were increased. However, the
changes in the clinical chemistry parameters could not be correlated
with pathology findings because the necropsy data on animals showing
clinical biochemistry changes were not presented in the report. The
limited necropsy data showed that, in F1 pups, 1/4 females at 7 ppm
and 3/10 males and 3/7 females at 10 ppm had pale or greasy liver.
This observation was compound-related. Based on the increase in the
mortality rate, the NOAEL was 1 ppm (Wazeter et al., 1971c).
Rat
Three reproduction studies in rats were evaluated by the JMPR in
1966 and 1970. In a 3-generation reproduction study, a group of 80
rats were given 6.9 mg/kg of heptachlor daily for 3 months prior to
mating. Cataracts, which became obvious between the 19th and 26th
days, were found in 6.8% of the young. A similar lesion was present
in 15.2% of the parental animals after 9 months. The only effect on
reproduction was a decrease in the litter size (Annex 1, 7). In two
other reproduction studies in rats, dietary levels of heptachlor
ranging from 0.3 to 10 ppm were tested; no cataracts nor adverse
effects on fertility and reproduction were found. A slight increase
in post-natal mortality of pups was seen at 10 ppm (Annex 1, 15).
Special studies for teratology and embryotoxicity
Rabbit
A rabbit teratology study was previously evaluated by the 1970
Joint Meeting, and it was concluded that no teratogenic effects could
be attributed to the compound (Annex 1, 15).
Special studies on genotoxicity
In a large number of genotoxicity assays (Table 4), heptachlor
did not induce genetic damage. Exceptions were the induction of small
numbers of mutations in bacteria in the presence of plant extracts,
mutations and a chromosomal aberrations in plants and in a single
study, mutations at the highly sensitive tk locus in mouse lymphoma
cells. Gap-junctional intercellular communication was inhibited in
several in vitro assays.
In both a sister chromatid exchange assay and a chromosome
aberration assay in Chinese hamster ovary cells, there were "positive"
results in the presence of rat S9 metabolic activation. In the absence
of metabolic activation the chromosome aberration assay was negative
(NTP, 1985). However, the tests were not repeated to confirm the
positive results.
The mutagenic potential of heptachlor epoxide was also tested.
The results are presented in the following table.
Table 4. Results of genotoxicity assays on heptachlor
Test system Test object Concentration Resultsa Reference
Ames test S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
TA1535, TA1536
TA1537, TA1538
Ames test S. typhimurium 2500 µg/ml -/0 Simmon et al. (1977)
TA98, TA100,
TA1535,
TA1537, TA1538
Ames test S. typhimurium not stated -/- Probst et al. (1981)
TA98, TA100, TA1535,
TA1537, TA1538, G46,
C3076, D3052
E. coli WP2 & WP2 uvr A-
Ames test S. typhimurium 0.1 µg/plate -/- NTP (1983)
TA98, TA100, TA1535, to 10 mg/plate
TA1537
Ames test S. typhimurium 5-10 µg/ml +/-b Gentile et al. (1982)
TA98, TA100, TA1535
Ames test S. typhimurium 2500 µg/ml -/- Moriya et al. (1983)
TA98, TA100, TA1535,
TA1537, TA1538
E. coli WP2 hcr
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Rec assay B. subtilis 356 µg/ml -/- Matsui et al. (1989)
Differential E. coli WP 2 2000 µg/ml 0/- Rashid & Mumma (1986)
toxicity S. typhimurium
TA1538
Ames test S. typhimurium 1 mg/ml -/- Mersch-Sundermann (1988)
TA97, TA98, TA100,
TA102
Ames test S. typhimurium 167 µg/ml -/- Zeiger et al. (1987)
TA98, TA100,
TA1535, TA1537
Gene conversion S. cerevisia f -/- Gentile et al. (1982)
Mutation Zea mays 1.12 kg/ha 0/+ Gentile et al. (1982)
Chromosomal Lens sp. 0.1-0.3% 0/+ Jain (1988)
aberrations Pisum sp.
Micronuclei Tradescantia 1.88 ppm 0/+ Sandhu et al.
(1989)
SLRSc mutation D. melanogaster 5 µg/ml (feeding 0/- Benes & Sram
solution) (1969)
tk locus Mouse lymphoma 25 µg/ml 0/- McGregor et al.
L5178Y mutation cells (1988
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
ARL-HGPRT Adult rat liver 10-6 to 10-4 M -/NAd Telang et al.
epithelial cell line (1982)
Unscheduled DNA Mouse, rat and hamster 10-5 - Maslansky & Williams (1981)
synthesis assay primary hepatocytes
Primary liver cell
explants from rats 10 nmole/ml - Probst et al. (1981)
SV-40 transformed human
fibroblasts 100 & 1000 µM ?/- Ahmed et al. (1977)
(VA-4)
Dominant Swiss mice 4.8 & 24 mg/kg (IP) - Epstein et al. (1972)
lethal 5 & 10 mg/kg (PO)
CD-1 mce 7.5 & 15 mg/kg - Arnold et al. (1977)
25:75e
Inhibition of Mouse testicular cells 40 mg/kg 0/- Seiler (1977)
DNA synthesis in vivo
Inhibition of Chinese hamster V79 10 µg/ml 0/+ Kurata et al. (1982)
metabolic cells
cooperation
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Inhibition of Rat hepatocytes 20 µg/ml 0/+ Ruch et al. (1990)
metabolic Mouse hepatocytes
cooperation
a with S9 activation/without S9 activation; phi indicates that the test was not performed
b with plant S9
c sex-linked recessive lethal
d NA = not applicable
e 25:75 blend w/w of heptachlor:heptachlor-epoxide
f concentration in original paper cited as "an appropriate amount".
Table 5. Results of genotoxicity assays on heptachlor epoxide
Test system Test object Concentration Resultsa Reference
Ames assay S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
Unscheduled DNA SV-40 transformed 100 & 1000 µM +/- Ahmed et al. (1977)
synthesis assay human fibroblasts
(VA-4)
Dominant lethal Swiss mice 6 & 30 mg/kg (IP) - Epstein et al. (1972)
8 mg/kg (PO)
a with S9 activation/without S9 activation
Observations in humans
In 1986, a study was conducted in the Midwest United States on a
group of 45 dairy farm family members who had consumed undiluted raw
milk products known to have been contaminated with heptachlor at
concentrations as high as 89.2 ppm (fat basis). The serum levels of
heptachlor or its metabolites in the exposed individuals were compared
to those from an unexposed group of 94 persons from the same
geographical area and to results from the Second National Health and
Nutrition Examination Survey (2nd NHANES). The exposed group had
significantly higher mean levels of primary metabolites such as
heptachlor expoxide (0.84±1.0 vs 0.50±0.9 ppb) and oxychlordane
(0.71±0.8 vs 0.49±1.1 ppb) than the unexposed group. In addition, the
percentage of individuals in exposed group with elevated serum
concentrations of these same metabolites (21.2%) was higher than that
for the unexposed group (heptachlor expoxide, 3.8%; oxychlordane,
6.3%) and the second National Health and Nutrition Survey (2.5% for
both metabolites) (Stehr-Green et al., 1988).
There is evidence of transplacental transfer of heptachlor
epoxide. The following heptachlor epoxide levels were detected in
various tissues of mother and newborn infants (Polishuk, 1977a):
adipose tissue (maternal): 0.2856 ± 0.3950 ppm
maternal blood: 0.2798 ± 0.4626 ppm
uterine muscle: 0.4895 ± 0.5086 ppm
fetal blood: 0.9959 ± 0.9458 ppm
placenta: 0.5000 ± 0.3950 ppm
amniotic fluid: 0.6730 ± 1.1645 ppm
Several investigators detected heptachlor epoxide levels ranging
from non-detectable to 0.46 ppm in human milk (Kroger, 1972; Polishuk
et al., 1977b; Strassman & Kutz, 1977; Savage et al., 1981;
Takahashi et al., 1981; Takei et al., 1983).
No adverse effects on human fetal development were reported
following ingestion of milk containing heptachlor for 27 to 29 months
among women of child bearing age in Oahu, Hawaii, USA. The levels of
heptachlor in milk fat ranged from 0.12 to 5.00 ppm (Le Marchand
et al., 1986).
A retrospective mortality study was conducted on workers employed
in the manufacture of heptachlor and chlordane between 1946 and 1976.
The study group consisted of 1403 white males who were employed for
more than 3 months at either of the two plants which produced
heptachlor and chlordane in the United States. The mortality
information was obtained from the Social Security Administration files
and supplemented by information collected by another investigator
through follow-up. The results indicated an excess of death from
cerebrovascular disease (17 observed, 9.3 expected) (Wang & MacMahon,
1979a).
The mortality of 16 126 males employed as pesticide applicators
was evaluated. The cohort was employed between 1967 and 1976 by any
of the nationwide (US) pest control companies for 3 months or more.
The information on mortality was traced in Social Security
Administration files. The results did not demonstrate a significant
difference in mortality from the norm in the cohort (Wang & MacMahon,
1979b). An update of the above study group was conducted by following
the same cohort to the end of 1984. The analysis showed an excess of
lung cancer among the pesticide applicators. However, these
applicators were exposed to a broad spectrum of pesticides, and the
increased lung cancer risk among the pesticide applicators might not
relate to their exposure to heptachlor alone (MacMahon et al.,
1987).
A retrospective mortality study of 2141 workers from 4
manufacturing plants of chlorinated hydrocarbon pesticides, which
included chlordane, heptachlor, DDT, aldrin, dieldrin, and endrin was
reported by Ditraglia et al., 1981. The workers in each plant were
considered as a separate cohort, each had worked at least 6 months
prior to 31 December 1964. The results showed too few deaths to allow
any meaningful conclusions (Ditraglia et al., 1981).
Another retrospective study was conducted by Shindell and
Associates (1981) on a cohort of 1115 employees who worked 3 months or
more at the Memphis, Tennessee, USA, plant of Velsicol Chemical Corp.,
from 1 January 1952 to 31 December 1979. The plant began to
manufacture heptachlor in 1952. The data on morbidity and mortality
were obtained for 93.1% of the male and 90% of the female cohort. The
workers were classified according to their job or product exposure.
Mortality data on the cohorts were compared to the overall mortality
experience of like segments (by age and sex) of the United States
population at large to determine whether Memphis plant exposure caused
any discernible variations from the expected mortality from all causes
and from selected significant causes. No significant difference was
found.
COMMENTS
Heptachlor is structurally similar to chlordane. An ADI of
0.0005 mg/kg bw was estimated for heptachlor by JMPR in 1970.
Heptachlor is well absorbed through the GI tract and metabolized to a
large extent to heptachlor epoxide and minor metabolites. Heptachlor
epoxide has been shown to accumulate in adipose tissue, and to cross
the placenta, but it was not found in the brain. In rats, the major
route of elimination is via the faeces.
In both short- and long-term studies in dogs, rats, and mice, the
liver was found to be the target organ. In a 30-day feeding study in
mice at dietary concentrations of 0, 1, 5, 10, 25 or 50 ppm,
histopathology findings indicated that males at 5 ppm or above and
females at 10 ppm or above had enlargement of centrilobular and
midzonal hepatocytes. The severity was dose-related. Based upon
these results, the NOAEL was 1 ppm, equivalent to 0.15 mg/kg bw/day.
Several long-term/carcinogenicity studies in rats were reviewed,
but the majority of them had severe methodological limitations. In
one study, rats received time-weighted dietary concentrations of 39 or
78 ppm (males) and 26 or 51 ppm (females) of heptachlor for 80 weeks.
On the basis of decreased body-weight in high-dose males and increases
in mortality in high-dose males and females, the NOAEL was 26 ppm,
equivalent to 1.3 mg/kg bw/day. Deficiencies in this study precluded
proper evaluation of the carcinogenic potential of heptachlor in rats.
In a 2-year feeding study, dogs fed heptachlor epoxide at dietary
concentrations of 0, 1, 3, 5, 7 or 10 ppm, exhibited an increase in
liver weight at 10 ppm and an increase in the incidence of liver
histopathological changes at 3 ppm and above. The histopathological
changes were enlargement and vacuolation of centrilobular or scattered
hepatocytes. Similar histological changes persisted through six
months of the recovery period. The NOAEL based upon these findings
was 1 ppm, equivalent to 0.025 mg/kg bw/day.
Heptachlor/heptachlor epoxide is carcinogenic in mice; two
studies demonstrated an increase in liver tumour incidence in male and
female Charles River CD-1 and B6C3F1 mice.
Heptachlor/heptachlor epoxide also caused a marginal increase in
the incidence of hepatocytomegaly in all treated CD-1 mice; a NOAEL
was not established in this study.
In a 2-generation reproduction study in dogs at dietary
concentrations of 1, 3, 5, 7, or 10 ppm of heptachlor epoxide, there
was an increase in mortality of F2 pups at 3 ppm and above. The
NOAEL based on this finding was 1 ppm.
The available epidemiology studies have not shown a clear
relationship between any effects in humans and exposure to heptachlor.
After reviewing the available in vitro and in vivo short-term
test data, it was concluded that, although it can interfere with
intercellular communication, heptachlor is not genotoxic.
Many studies available for evaluation were conducted more than 20
years ago and have severe deficiencies. Because of these
deficiencies, its carcinogenicity in mice and its ability to
bioaccumulate, the Meeting recommended that heptachlor should not be
used directly on food crops and its use in the production of food
commodities should be phased out. Because of its environmental
persistence it is found as a contaminant in food commodities. The
Meeting therefore maintained an ADI, basing it on the NOAELs derived
from studies in dogs. However, recognizing the inadequacy of the data
base, the Meeting increased the safety factor to 200-fold. This ADI
will provide a guideline for assessing the significance of dietary
exposure to heptachlor residues.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 26 ppm, equivalent to 1.3 mg/kg bw/day
Dog: 1 ppm, equivalent to 0.025 mg/kg bw/day
(reproduction study)
1 ppm, equivalent to 0.025 mg/kg bw/day (2-year study)
Estimate of acceptable daily intake for humans
0-0.0001 mg/kg bw
REFERENCES
Ahmed, F.E., Hart, R.W., & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res. 42:
161-174.
Arnold, D.W., Kennedy, G.L., Jr., Keplinger, M.L., Calandra, J.C., &
Calo, C.J. (1977) Dominant lethal studies with technical chlordane,
HCS-3260, and heptachlor:heptachlor epoxide. J. Toxicol. Environ.
Health., 2: 547-555.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 442-444.
Davidow, B. & Radomski, J.D. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265.
Ditraglia, D., Brown, D.P., Namekata, T., & Iverson, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health. 7
(Supplemental 4): 140-146.
Epstein, S.S. (1976) Carcinogenicity of heptachlor and chlordane.
Sci. Total Environ., 6(2): 103-154.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14:514-534
Gak, J.C., Grillot, C., & Truhaut, R. (1976) Use of the golden
hamster in toxicology. Lab. Animal Sci. 26(2): 274-280.
Gentile, J.M., Gentile, G.J., Bultman, J., Sechriest, R., Wagoner,
E.D. & Plewa, M.J. (1982) An evaluation of the genotoxic properties
of insecticides following plant and animal activation. Mutat. Res.,
101: 19-29.
Jain, A.K. (1988) Cytogenic effects of heptachlor on Lens and
Pisum species. Geobios, 15, 61-69.
Jolley, W.P., Stemmer, K. L., & Jolley, L. (1966) The effects of
feeding diets containing a mixture of heptachlor and heptachlor
epoxide to female rats for two years. Unpublished report by The
Kettering Laboratory, University of Cincinnati. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Kroger, M. (1972) Insecticide residues in human milk. J. Pediatr.
80: 401-405.
Kurata, M., Hirose, K. & Umeda, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73, 217-227.
Le Marchand, L., Kolonel, L.N., Siegel, B.Z., & Dendle, W.H., III
(1986) Trends in birth defects for a Hawaiian population exposed to
heptachlor and for the United States. Arch. Environ. Health, 41(3):
145-148.
MacMahon, B., Monson, R.R., Wand, H.H., & Zheng, T. (1987) A second
follow-up of mortality in a cohort of pesticide applicators. A report
to Velsicol Chemical Corp., dated June 5, 1987. Submitted to WHO by
Velsico Chemical Corp., Rosemont, Illinois, USA.
Marshall, T.C., Dorough, H.W., & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agri. Food Chem., 24(3): 560-563.
Maslansky, C.J., & Williams, G.M. (1981) Evidence for an epigenetic
mode of action in organochlorine pesticide hepatocarcinogenicity: A
lack of genotoxicity in rats, mouse, and hamster hepatocytes.
J. Toxicol. Environ. Health, 8: 121-130.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Response of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 coded chemicals.
Environ. Mol. Mutagenesis, 12, 85-154.
Mersch-Sundermann, V., Dickgiesser, N., Hablizel, U. & Gruber, B.
(1988) Examination of the mutagenicity of selected herbicides and
insecticides with the Salmonella-microsome test (Ames-Test) in
consideration of the pathogenetic potency of contaminated ground and
drinking water (Ger.). Zbl. Bakt. Hyg. B., 186, 247-260.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
U. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
NAS (National Academy of Science) (1977) An Evaluation of the
carcinogenicity of chlordane and heptachlor. Report to the Chief
Administrative Law Judge of the EPA, dated October, 1977. Submitted to
WHO by Velsicol Chemical Corp., Rosemont, Illinois, USA.
NCI (National Cancer Institute) (1977) Bioassay of heptachlor for
possible carcinogenicity. CAS.No. 76-44-8. Technical Report Series 9.
Bethesda, MD: Carcinogen Bioassay and Program Resources Branch,
Carcinogenicity Program, Division of Cancer Cause and Prevention,
NCI, National Institute of Health. DHEW Publication (NIH) 77-809.
NTP (National Toxicology Program) (1983) National Toxicology Program
Annual Plan for Fiscal Year 1983. NTP-82-119. Chemical Test Results
for Mutagenicity in Salmonella Assays in FY 1982. US Department of
Health and Human Services.
NTP (National Toxicology Program) (1985) National Toxicology Program
Annual Plan for Fiscal Year 1985. NTP-85-055. Chemical Test Results
for Cytogenetic Effects in Chinese Hamster Ovary Cells in FY 1984. US
Department of Health and Human Services.
Podowske, A.A., Banerjee, C.C., Feroz, M., Dudek, M.A., Willey, &
R.L., Khan, M.A.Q. (1979) Photolysis of heptachlor and
cis-chlordane and toxicity of their photoisomers to animals. Arch.
Environ. Contam. Toxicol. 8: 509-518.
Polishuk, Z.W., Ron, M, Wassermann, M., Cucos, S., Wassermann, D., &
Lemesch, C. (1977a) Pesticides in people. Organochlorine compounds in
human blood plasma and milk. Pestic. Nonit. J. 10(4): 121-129.
Polishuk, Z.W., Wassermann, D., Wassermann, D., Cucos, S. & Ron, M.
(1977b) Organochlorine compounds in mother and fetus during labor.
Environ. Res. 13: 278-294.
Probst, G.S., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K., &
Neal, S.B. (1981) Chemical-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ Mutat. 3: 11-32.
Radomski, J.L. & Davidow, B. (1953) The metabolite of heptachlor, its
estimation, storage and toxicity. J. Pharmacol. Exp. Therap. 107:
266-272.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21,
319-334.
Ruch, R.J., Fransson, R., Flodstrom, S., Warngard, L. & Klaunig, J.E.
(1990) Inhibition of hepatocyte gap junctional intercellular
communication by endosulfan, chlordane and heptachlor.
Carcinogenesis, 11, 1097-1101.
Sandhu, S.S., Ma, T-H., Peng, Y., & Zhou, X. (1989) Clastogenicity
evaluation of seven chemical commonly found at hazardous industrial
waste sites. Mutat. Res., 224, 437-445.
Savage, E.P., Keefe, T.J., Tessari, J.D., et al. (1981). National
study of chlorinated hydrocarbon insecticide residues in human milk.
USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor
epoxide, chlordane, oxychlordane, and mirex. Am. J. Epidemiol.
113(4): 413-422.
Seiler, J.P. (1977) Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short-term test. Mutat. Res., 46, 305-310.
Sherman, M. & Ross, E. (1961) Toxicol. App. Pharmacol., 3: 521.
Shindell and Associates (1981) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant Memphis, Tennessee,
January 1955-December 1979. Unpublished Report by Shendell and
Associates, Milwaukee, Wisconsin. Submitted to WHO by Velsicol
Chemical Corp., Rosemont, Illinois, USA.
Simmon, V.F., Kauhanen, K. & Tardiff, R.G. (1977) Mutagenic activity
of chemicals identified in drinking water. Dev. Toxicol. Environ.
Sci., 2, 249-258.
Stehr-Green, P.A., Wohlleb, J.C., Royce, W., & Head, S.L.(1988) An
evaluation of serum pesticide residue levels and liver function in
persons exposed to dairy products contaminated with heptachlor.
J. Am. Med. Assoc. 259(3): 374-377.
Strassman, S.C. & Kutz, F.W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi 1973-1974. Pesticide Monit. J.
10(4):130-133.
Takahashi, W., Saidin, D., Takei, G., & Wong, L. (1981)
Organochloride pesticide residues in human milk in Hawaii 1979-1980.
Bull. Environ. Contam. Toxicol. 27: 506-511.
Takei, G.H., Kauahikaua, S.M., & Leong, G.H. (1983) Analyses of human
milk samples collected in Hawaii for residues of organochlorine
pesticides and polychlorobiphenyls. Bull. Environ. Contam. Toxicol.
30: 606-613.
Tashiro, S. & Matsumura, F. (1978) Metabolism of trans-nonachlor
and related chlordane components in rat and man. Arch. Environ.
Contam. Toxicol. 7: 113-127.
Telang, S., Tong, C., & Williams, G.M. (1982) Epigenetic membrane
effects of possible tumour-promoting type on cultured liver cells by
the nongenotoxic organochlorine pesticide chlordane and heptachlor.
Carcinogenesis, 3(10): 1175-1178.
Velsicol Corporation (1959) Unpublished report.
Wang, H.H. & MacMahon, B. (1979a) Mortality of workers employed in
the manufacture of chlordane and heptachlor. J. Occup. Med. 21(11):
746-748.
Wang, H.H. and MacMahon, B. (1979b). Mortality of pesticide
applicators. J. Occup. Med. 21(11): 741-744; (11):746-748.
Wazeter, F.X., Goldenthal, E.I., Geil, R.G., Howell, D.G., & Dean,
W.P. (1971a) 30-Day range-finding study in mice. Unpublished Report
from International Research and Development Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., Cookson, K.M. & Howell,
D.G. (1971b) Two-year oral study in beagle dogs. Unpublished Report
No. 163-048 from International Research and Development Corp.
Submitted to WHO by Velsicol Chemical Co., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., & Howell, D.G. (1971c)
Two-generation reproduction and teratology study in beagle dogs.
Unpublished Report No.163-048 from International Research and
Development Corp. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Wazeter, F.X., Goldenthal, E.I., & Geil, R.G. (1973) Eighteen-month
oral carcinogenic study in mice. Unpublished Report No. 163-084 from
International Research and Developmental Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA
Witherup, S., Cleveland, F.P., Shaffer, F.E., Schlecht, H., & Musen,
L. (1955) The physiological effects of the introduction of heptachlor
into the diet of experimental animals in varying levels of
concentration: Report of experiment No.2. Unpublished report from The
Kettering Laboratory, College of Medicine, University of Cincinnati,
Ohio. Submitted to WHO by Velsicol Chemical Corp., Rosemont, Illinois,
USA.
Witherup, S., Cleveland, F.P., Stemmer, K.L., & Schlecht, H. (1958)
The physiological responses of beagle dogs to the consumption of diets
containing various concentrations of heptachlor epoxide over a period
of sixty weeks. Unpublished report from The Kettering Laboratory,
College of Medicine, University of Cincinnati, Ohio. Submitted to WHO
by Velsicol Chemical Corp., Rosemont, Illinois, USA.
Witherup, S., Cleveland, F.P., & Stemmer, K. (1959) The physiological
effects of the introduction of hepatochlor epoxide in varying levels
of concentration into the diet of CFN rats. Unpublished report from
the Kettering Laboratory, College of Medicine, University of
Cincinnati, Ohio. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III. Results from
the testing of 255 chemicals. Environ. Mutagenesis, 9(Suppl. 9):
1-11.
Table 1. Acute toxicity data of heptachlor and heptachlor epoxide
Species Sex Route LD50 Reference
(mg/kg bw)
A. Heptachlor
Rat M oral 71 Podowski et al. (1979)
M&F oral 60-142a Velsicol Corp. (1959)b
Chick d oral 63 Sherman & Ross (1961)b
Mouse d oral 70 Gak et al. (1976)
Hamster d oral 100 Gak et al. (1976)
Rat M dermal 195c Gaines (1969)
F dermal 250c Gaines (1969)
B. Heptachlor epoxide
Rat M&F oral 34-48a Velsicol Corp. (1959)b
Mouse M oral 32-48 Velsicol Corp. (1959)b
a Sex differences
b Data excerpted from the 1966 JMPR monograph (Annex 1, 7)
c Xylene was the solvent.
d Not stated
Short-term studies
Mouse
Groups of 10 Charles River CD-1 mice/sex/dose were fed a mixture
of heptachlor-heptachlor epoxide (25:75) at dietary concentrations of
1, 5, 10, 25, and 50 mg/kg for 30 days. Following 30 days of
treatment, the animals were sacrificed and necropsied. Microscopic
examinations were conducted on liver tissue.
No deaths occurred in the 1, 5, nor 10 mg/kg groups. One female
died in the 25 mg/kg group, and 9 males and 8 females died in the 50
mg/kg group. No marked differences were seen between the treated
animals and the controls with respect to body weights and food
consumption. Mice which were sacrificed at the end of the study from
the 10, 25 and 50 mg/kg groups showed liver enlargement associated
with accentuated lobulation. The liver weight of the only surviving
male mouse from the 50 mg/kg group was markedly increased (control
mean: 1.69 g; 50 mg/kg; 4.00 gm), and increased mean liver weight was
also found in the 25 ppm male mice (control, 1.69 g; 25 ppm, 4.35 gm).
Histopathology findings indicated that livers of male mice in the 5,
10, 25, and 50 ppm and of female mice in the 10, 25, and 50 ppm groups
had enlargement of centrilobular and midzonal hepatocytes with
replacement of the normal coarse cytoplasmic granularity by finely
granular and homogeneous cytoplasm. The severity of this lesion was
dose-related. The incidences of the microscopic findings were not
reported (Wazeter et al., 1971a). Based upon the histopathology
findings, the NOAEL was 1 mg/kg.
Rat
Two short-term studies on heptachlor in rats were summarized in
the 1971 monograph. The dietary levels tested in these two studies
ranged from 5 to 45 ppm. At 10 ppm and above, rat liver cells showed
an increase in smooth endoplasmic reticulum and mitochondria. The
liver effects regressed after the treated rats were placed on a
control diet for 120 days (Annex 1, 15).
Long-term studies
Some of the unpublished long-term studies on heptachlor were
evaluated by the 1966 Joint Meeting and published in the 1967
Monograph. To facilitate consideration of the toxicity of this
chemical the older toxicity studies are also summarized here.
Rat
Groups of Carworth Farms (CF) rats (20/sex/dose) were fed diets
containing heptachlor (purity not specified) at a concentration of 0,
1.5, 3.0, 5.0, 7.0, or 10 ppm for 110 weeks. The treatment diet was
prepared by spraying standard rat diet with an ethanol solution of
heptachlor. After 7 weeks on the test diet, 5 females and 5 males
from each dose group were mated to determine whether or not heptachlor
would affect the reproductive capacity of the treated animals or the
survival and subsequent growth of the offspring. Following similar
procedures, another mating was conducted using different animals from
each dose group after 22 weeks of treatment. The offspring were kept
with their mothers until weaning at 3 weeks of age. After weaning,
the offspring were placed on the control diet for 8 weeks for further
observations. After 110 weeks of treatment, the surviving animals
were sacrificed, certain haematological parameters were examined.
Histopathology was conducted on the heart, liver, lungs, brain,
spleen, kidneys, thyroids, and the adrenal glands.
Heptachlor treatment did not affect mortality, body weight, or
food consumption. The limited reproductive parameters measured did
not indicate any effect on the body weights at birth or at weaning.
Heptachlor administration did not appear to significantly affect the
mortality of the offspring. Organ weights of the heptachlor-treated
rats were not markedly different from those of controls. Haematology
parameters (erythrocyte counts, haemoglobin, leukocyte counts, and
differential white cell counts) did not show any compound-related
effect. The histopathology data indicated 6/16 males and 3/18 females
at 7.0 ppm and 2/12 males and 9/16 females at 10 ppm had slight
hepatic cellular alterations characterized by swelling, homogeneity of
the cytoplasm and peripheral arrangement of the cytoplasmic granules
in the hepatic cell in the central zone of the lobules. Under the
conditions of this study, heptachlor at doses up to 10 ppm did not
produce an increase in the tumour incidence in CF rats (Witherup
et al., 1955).
Groups of CFN (Carworth Farms, Nelson) rats (25/sex/dose) were
fed heptachlor epoxide at dietary concentrations of 0, 0.5, 2.5, 5.0,
7.5, and 10.0 ppm for 108 weeks. The test diet was prepared by
spraying an ethanol solution of heptachlor epoxide on Purina
Laboratory Chow.
The survival rate was greater than 47% in the treated and control
groups. There was an increase in the liver weights of treated females
and a slight increase in treated males. The test chemical was not
reported to cause any additional effects. This study has
methodological deficiencies which include uncertain concentrations in
the feed and insufficient number of rats in each dose level. Also the
report does not contain histopathological data and is therefore not
adequate for evaluating the long-term toxicity of heptachlor (Witherup
et al., 1959). However, a panel of independent pathologists
convened by the US National Academy of Sciences (1977) reviewed the
histopathology and concluded that there was no increase in liver
tumours in treated rats.
Four groups of Charles River CD female rats (25/dose) were fed a
3:1 mixture of heptachlor:heptachlor epoxide at dietary concentrations
of 5, 7.5, 10, and 12.5 ppm. A control group of 54 females was fed a
diet containing the vehicle, corn oil. Interim sacrifices were
conducted at 5 and 19 months to determine the nature and degree of the
histological changes in the liver. At 5 months, 3 controls and 5 rats
from the 7.5 ppm group were sacrificed. At 19 months, 5 controls, 2
rats from 5 ppm, 2 rats from 10 ppm, and 1 rat from 12.5 ppm were
sacrificed. After 24 months of treatment, the surviving rats were
sacrificed, and gross and histological examinations were conducted.
There was an increase in mortality at 12.5 ppm relative to that
of controls (control, 21%; 12.5 ppm, 50%). The test article did not
affect body weight gain. Liver changes which were described as fatty
liver and enlarged cells in the central zone of the hepatic lobules
were reported. However, no histopathology data were included in the
report (Jolley, 1966).
Groups of Osborne-Mendel rats (50/sex/dose) were fed technical
grade heptachlor (73% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at time-weighted doses of 38.9 and 77.9
ppm for males and 25.7 and 51.3 ppm for females. The matched controls
consisted of 10 males and 10 females, and pooled controls were 60
rats/sex. The treated animals received the chemical for 80 weeks and
were observed for 30 weeks. During the 80 weeks of treatment, the
dietary concentrations changed three times for each treatment group
based on changes in body weight.
There was a decrease in the mean body weight of high dose males
which almost returned to the control level after the treatment was
terminated. There was also a slight increase in mortality in high
dose male and female rats. Histopathology data revealed numerous
inflammatory, degenerative, and proliferative lesions with
approximately equal frequencies in treated and control animals. There
was an increased incidence of thyroid follicular cell neoplasms,
including combined adenomas and carcinomas in the high dose female
rats (high dose, 14/38; low dose, 3/43; matched controls, 1/9;
p<0.001) while, in male rats, a non-significant increase was observed
only at the low dose (high dose, 3/38; low dose, 9/38; matched
control, 1/8). At the same time, the incidences of the thyroid C-cell
neoplasms decreased in high dose female rats (high dose, 3/38; low
dose, 7/43; matched controls, 3/9; p<0.05). The results of analyses
of the thyroid lesions were ambiguous and further complicated by
variability in different experiments conducted at about the same time
with this strain of rats (NCI, 1977).
Dog
Groups of Beagle dogs (4/sex/dose) were fed heptachlor epoxide
(purity not specified) at concentrations of 0, 1, 3, 5, 7, and 10 ppm
for two years. After 2 years on test, 2 dogs/sex/dose were sacrificed
and necropsied while the other two dogs/sex/dose were maintained on
the control diet for an additional 6 months (recovery period). In
addition, the test animals in this study were also mated during the
study and employed as the P1 parental animals for a 2-generation
reproduction and teratology study.
No death nor compound-related behavioural change was seen during
the study. The test article did not affect the body weights or food
consumptions of the treated animals. The parameters for haematology
and urinalysis were comparable between treated and control dogs.
There were increases in alkaline phosphatase activities in males and
females at 3 ppm and above; these increases, in some dogs, were more
marked towards the end of the treatment period and tended to persist
throughout the recovery period. The serum albumin and total protein
levels were slightly decreased in 10 ppm male and female dogs during
the treatment and the recovery periods. An increase in the SGPT level
was also seen in 10 ppm animals during the treatment, extending into
the recovery period. After one year of treatment, the animals in 7
ppm group also showed an increase in the SGPT level which lasted into
the recovery period.
There was an increase in liver weights in 10 ppm male and female
dogs relative to those of the controls, and this increase persisted
with a slight attenuation during the recovery period. Histopathologic
examination on dogs (2 dogs/sex/dose) sacrificed at the end of the
treatment period showed an increase in the incidence of liver changes
in animals at 3.0 ppm or above. The changes were described as
enlargement and vacuolation of groups of centrilobular hepatocytes or
scattered individual hepatocytes, presence of finely granular, and a
"ground glass" appearance of the cytoplasm of large numbers of
hepatocytes. These histopathological changes were also seen in dogs
at 3 ppm and above after 6 months of recovery period. No
compound-related histopathology changes were seen in 1 ppm dogs.
Based upon the changes in biochemical parameters and the histological
changes in the liver, the NOAEL was 1 ppm (Wazeter et al., 1971b).
Although this study has certain deficiencies, including an
insufficient number of experimental animals, the results provide
useful information on the toxicity of the test compound in dogs.
Groups of 23 to 27 week old beagle dogs (2 males or 3
females/dose) were fed heptachlor epoxide at dietary concentrations of
0, 0.5, 2.5, 5.5, and 7.5 ppm for sixty weeks. The test diet was
prepared by spraying an ethyl alcohol solution of heptachlor epoxide
on the food. The experimental results were limited to clinical
observation, mortality, body weight changes, food consumption, and
organ weights. The limited results indicated that there was an
increase in liver weights of all the heptachlor treated male and
female dogs relative to that of the controls. For 7.5 ppm males, the
increase was approximately twice that of the controls. However, the
pathology data were not included in the report (Witherup et al.,
1958).
Mouse
Groups of Charles River CD-1 mice (100/sex/dose) received a
mixture of heptachlor/heptachlor epoxide (25%/75%) at dietary
concentrations of 0, 1.0, 5.0, and 10.0 ppm for 18 months. A positive
control group (100 mice/sex) received 2-acetaminofluorene at a dietary
concentration of 250 ppm. An interim sacrifice was performed on 10
mice/sex/dose including the positive control group. Gross pathology
and histology examinations were conducted on animals which died during
the study or were sacrificed on schedule.
There was a decrease in the number of survivors in 10 ppm male
and female mice, in 5 ppm females, and in positive control males
relative to those of the negative controls as shown in Table 2. Mean
body weights and food consumptions of the treated mice were not
affected. There were increases in the mean liver weights of males and
females in 5 and 10 ppm groups.
An increase in the incidence of hepatocytomegaly was seen in all
heptachlor-heptachlor epoxide treated mice relative to that of the
controls. The incidence of hepatic nodular hyperplasia was markedly
increased in 5 and 10 ppm males and females relative to that of the
controls. For heptachlor-treated animals, hepatoma was observed in
only one male mouse at 1 ppm (Wazeter, 1973). Based on the incidence
of hepatocytomegaly, a NOAEL was not established (LOAEL <1ppm).
The histological slides of this study were later evaluated by a
panel of pathologists convened by the Pesticide Information Review and
Evaluation Committee of the National Academy of Sciences, USA. The
results of the evaluation are presented in Table 2.
Levels of 1, 5, and 10 ppm of heptachlor-heptachlor epoxide did
not produce a statistically significant increase in the incidence of
hepatocellular carcinoma. However, at 10 ppm there was a statistically
significant increase in the incidence of combined hepatocellular
carcinomas and nodular changes (NAS, 1977).
Epstein (1976) reported a previously unpublished study conducted
by the US-FDA. Groups of C3H mice (100/sex/dose) were fed 0 or 10 ppm
of either heptachlor or heptachlor epoxide (purity unspecified) for 24
months. The liver histopathology was re-evaluated by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences. Their
results indicated a significant increase in hepatocellular carcinomas
in females given heptachlor and in both males and females given
heptachlor expoxide.
Table 2. The incidence of hepatocellular carcinomas and nodular
changes in CD-1 mice fed heptachlor-epoxide
Male Female
Group HC HC+N HC HC+N
Control 1/59 2/59 1/74 1/74
1 ppm H-HE 2/66 4/66 1/65 3/65
5 ppm H-HE 2/66 4/66 1/65 3/65
10 ppm H-HE 1/73 27/73* 4/52 16/52*
AAF 5/58 9/58 5/75 13/75
HC hepatocellular carcinomas
HC+N hepatocellular carcinomas and nodular changes
* (p<.001)
Groups of B6C3F1 mice (50/sex/dose) received technical-grade
heptachlor (72% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at initial concentrations of 10 and 20
ppm for males and 20 and 40 ppm for females. The initial
concentrations were reduced due to adverse toxic effects, with
time-weighted average concentrations of 6 and 14 ppm for males and 9
and 18 ppm for females. The mice were treated for 80 weeks and placed
on the control diet for another 10 weeks. Matched-controls consisted
of 20 males or 10 females, and the pooled controls were 100 males and
80 females. Under the conditions of the study, heptachlor had no
significant effects on the body weights. Survival at 90 weeks was
high: 70% of treated and control males and 60% of treated and control
females. Survival of female mice showed a significant trend towards
lower survival in treated groups compared to that in the control
group. The incidence of hepatocellular carcinomas was significantly
increased in the high-dose males and females as shown in Table 3
(NCI,1977).
A review of liver histopathology from this study by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences concluded
that there was a low incidence of induced hepatocellular carcinomas
(Table 3). According to this review, the incidence of hepatocellular
carcinomas was statistically increased (p<0.001) in groups of males
and females receiving heptachlor at the high dose only when combined
with the diagnosis of nodular changes (NAS, 1977).
Table 3. Incidence of proliferative lesions of the liver in B6C3F1
mice fed technical grade heptachlor
Lesion Pooled Matched Low Dose High Dose
Control Control
Males
Hepatocellular
carcinomas 17/92 5/19 11/46 34/47*
Nodular hyperplasia 3/92 1/19 9/46 6/47
Diffuse hyperplasia 3/92 0/19 1/46 3/47
Hepatocytomegaly 0/92 0/19 1/46 0/47
Females
Hepatocellular carcinomas 3/78 1/10 3/47 30/42*
Nodular hyperplasia 2/78 0/10 3/47 0/42
Diffuse hyperplasia 1/78 0/10 0/47 0/42
Hepatocytomegaly 1/78 0/10 0/47 1/42
The denominator represents the number of tissues examined microscopically.
* p<0.001 (Fisher exact test)
Reproduction studies
Dogs
Groups of 6-9 month old Beagle dogs (4/sex/dose) were fed
heptachlor epoxide at dietary concentrations of 0, 1, 3, 5, 7, and 10
ppm. When the female dogs attained an age of 14 months, they were
mated twice with male dogs from the same dose group. The females were
allowed to deliver and to nurse their pups.
For the F2 generation, 4 female and 2 male pups of the F1
generation were selected from each dose level to be the parental
animals (P2) of the F2 generation. At an approximate age of 14
months, the animals were mated, and the pregnant females were allowed
to deliver and to nurse their pups to 6 weeks of age. At this time,
the females and their pups were sacrificed.
No clinical signs or behaviour changes were seen in treated P1,
P2, or their offspring. There were no indications that body weight
or food consumption were affected by the test chemical. Whether or
not the test chemical produced any reproductive effects could not be
determined with the very limited data presented in this report. There
was a significant increase in the mortality rate of F1 pups in the 10
ppm group (control, 9/20; 10 ppm, 17/18). Only 1 male pup survived to
the scheduled sacrifice, and no female was available to serve as a P2
parental animal. There were slight increases in death rates of F2
pups at 3 and 7 ppm relative to the controls (Control, 0/4; 3 ppm,
3/8; 7 ppm, 3/8). The 5 ppm group had no pups. Haematology and
urinalysis results did not indicate any compound-related effects. Very
limited clinical biochemistry data showed that alkaline phosphatase
and/or SGPT activities in certain dogs were increased. However, the
changes in the clinical chemistry parameters could not be correlated
with pathology findings because the necropsy data on animals showing
clinical biochemistry changes were not presented in the report. The
limited necropsy data showed that, in F1 pups, 1/4 females at 7 ppm
and 3/10 males and 3/7 females at 10 ppm had pale or greasy liver.
This observation was compound-related. Based on the increase in the
mortality rate, the NOAEL was 1 ppm (Wazeter et al., 1971c).
Rat
Three reproduction studies in rats were evaluated by the JMPR in
1966 and 1970. In a 3-generation reproduction study, a group of 80
rats were given 6.9 mg/kg of heptachlor daily for 3 months prior to
mating. Cataracts, which became obvious between the 19th and 26th
days, were found in 6.8% of the young. A similar lesion was present
in 15.2% of the parental animals after 9 months. The only effect on
reproduction was a decrease in the litter size (Annex 1, 7). In two
other reproduction studies in rats, dietary levels of heptachlor
ranging from 0.3 to 10 ppm were tested; no cataracts nor adverse
effects on fertility and reproduction were found. A slight increase
in post-natal mortality of pups was seen at 10 ppm (Annex 1, 15).
Special studies for teratology and embryotoxicity
Rabbit
A rabbit teratology study was previously evaluated by the 1970
Joint Meeting, and it was concluded that no teratogenic effects could
be attributed to the compound (Annex 1, 15).
Special studies on genotoxicity
In a large number of genotoxicity assays (Table 4), heptachlor
did not induce genetic damage. Exceptions were the induction of small
numbers of mutations in bacteria in the presence of plant extracts,
mutations and a chromosomal aberrations in plants and in a single
study, mutations at the highly sensitive tk locus in mouse lymphoma
cells. Gap-junctional intercellular communication was inhibited in
several in vitro assays.
In both a sister chromatid exchange assay and a chromosome
aberration assay in Chinese hamster ovary cells, there were "positive"
results in the presence of rat S9 metabolic activation. In the absence
of metabolic activation the chromosome aberration assay was negative
(NTP, 1985). However, the tests were not repeated to confirm the
positive results.
The mutagenic potential of heptachlor epoxide was also tested.
The results are presented in the following table.
Table 4. Results of genotoxicity assays on heptachlor
Test system Test object Concentration Resultsa Reference
Ames test S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
TA1535, TA1536
TA1537, TA1538
Ames test S. typhimurium 2500 µg/ml -/0 Simmon et al. (1977)
TA98, TA100,
TA1535,
TA1537, TA1538
Ames test S. typhimurium not stated -/- Probst et al. (1981)
TA98, TA100, TA1535,
TA1537, TA1538, G46,
C3076, D3052
E. coli WP2 & WP2 uvr A-
Ames test S. typhimurium 0.1 µg/plate -/- NTP (1983)
TA98, TA100, TA1535, to 10 mg/plate
TA1537
Ames test S. typhimurium 5-10 µg/ml +/-b Gentile et al. (1982)
TA98, TA100, TA1535
Ames test S. typhimurium 2500 µg/ml -/- Moriya et al. (1983)
TA98, TA100, TA1535,
TA1537, TA1538
E. coli WP2 hcr
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Rec assay B. subtilis 356 µg/ml -/- Matsui et al. (1989)
Differential E. coli WP 2 2000 µg/ml 0/- Rashid & Mumma (1986)
toxicity S. typhimurium
TA1538
Ames test S. typhimurium 1 mg/ml -/- Mersch-Sundermann (1988)
TA97, TA98, TA100,
TA102
Ames test S. typhimurium 167 µg/ml -/- Zeiger et al. (1987)
TA98, TA100,
TA1535, TA1537
Gene conversion S. cerevisia f -/- Gentile et al. (1982)
Mutation Zea mays 1.12 kg/ha 0/+ Gentile et al. (1982)
Chromosomal Lens sp. 0.1-0.3% 0/+ Jain (1988)
aberrations Pisum sp.
Micronuclei Tradescantia 1.88 ppm 0/+ Sandhu et al.
(1989)
SLRSc mutation D. melanogaster 5 µg/ml (feeding 0/- Benes & Sram
solution) (1969)
tk locus Mouse lymphoma 25 µg/ml 0/- McGregor et al.
L5178Y mutation cells (1988
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
ARL-HGPRT Adult rat liver 10-6 to 10-4 M -/NAd Telang et al.
epithelial cell line (1982)
Unscheduled DNA Mouse, rat and hamster 10-5 - Maslansky & Williams (1981)
synthesis assay primary hepatocytes
Primary liver cell
explants from rats 10 nmole/ml - Probst et al. (1981)
SV-40 transformed human
fibroblasts 100 & 1000 µM ?/- Ahmed et al. (1977)
(VA-4)
Dominant Swiss mice 4.8 & 24 mg/kg (IP) - Epstein et al. (1972)
lethal 5 & 10 mg/kg (PO)
CD-1 mce 7.5 & 15 mg/kg - Arnold et al. (1977)
25:75e
Inhibition of Mouse testicular cells 40 mg/kg 0/- Seiler (1977)
DNA synthesis in vivo
Inhibition of Chinese hamster V79 10 µg/ml 0/+ Kurata et al. (1982)
metabolic cells
cooperation
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Inhibition of Rat hepatocytes 20 µg/ml 0/+ Ruch et al. (1990)
metabolic Mouse hepatocytes
cooperation
a with S9 activation/without S9 activation; phi indicates that the test was not performed
b with plant S9
c sex-linked recessive lethal
d NA = not applicable
e 25:75 blend w/w of heptachlor:heptachlor-epoxide
f concentration in original paper cited as "an appropriate amount".
Table 5. Results of genotoxicity assays on heptachlor epoxide
Test system Test object Concentration Resultsa Reference
Ames assay S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
Unscheduled DNA SV-40 transformed 100 & 1000 µM +/- Ahmed et al. (1977)
synthesis assay human fibroblasts
(VA-4)
Dominant lethal Swiss mice 6 & 30 mg/kg (IP) - Epstein et al. (1972)
8 mg/kg (PO)
a with S9 activation/without S9 activation
Observations in humans
In 1986, a study was conducted in the Midwest United States on a
group of 45 dairy farm family members who had consumed undiluted raw
milk products known to have been contaminated with heptachlor at
concentrations as high as 89.2 ppm (fat basis). The serum levels of
heptachlor or its metabolites in the exposed individuals were compared
to those from an unexposed group of 94 persons from the same
geographical area and to results from the Second National Health and
Nutrition Examination Survey (2nd NHANES). The exposed group had
significantly higher mean levels of primary metabolites such as
heptachlor expoxide (0.84±1.0 vs 0.50±0.9 ppb) and oxychlordane
(0.71±0.8 vs 0.49±1.1 ppb) than the unexposed group. In addition, the
percentage of individuals in exposed group with elevated serum
concentrations of these same metabolites (21.2%) was higher than that
for the unexposed group (heptachlor expoxide, 3.8%; oxychlordane,
6.3%) and the second National Health and Nutrition Survey (2.5% for
both metabolites) (Stehr-Green et al., 1988).
There is evidence of transplacental transfer of heptachlor
epoxide. The following heptachlor epoxide levels were detected in
various tissues of mother and newborn infants (Polishuk, 1977a):
adipose tissue (maternal): 0.2856 ± 0.3950 ppm
maternal blood: 0.2798 ± 0.4626 ppm
uterine muscle: 0.4895 ± 0.5086 ppm
fetal blood: 0.9959 ± 0.9458 ppm
placenta: 0.5000 ± 0.3950 ppm
amniotic fluid: 0.6730 ± 1.1645 ppm
Several investigators detected heptachlor epoxide levels ranging
from non-detectable to 0.46 ppm in human milk (Kroger, 1972; Polishuk
et al., 1977b; Strassman & Kutz, 1977; Savage et al., 1981;
Takahashi et al., 1981; Takei et al., 1983).
No adverse effects on human fetal development were reported
following ingestion of milk containing heptachlor for 27 to 29 months
among women of child bearing age in Oahu, Hawaii, USA. The levels of
heptachlor in milk fat ranged from 0.12 to 5.00 ppm (Le Marchand
et al., 1986).
A retrospective mortality study was conducted on workers employed
in the manufacture of heptachlor and chlordane between 1946 and 1976.
The study group consisted of 1403 white males who were employed for
more than 3 months at either of the two plants which produced
heptachlor and chlordane in the United States. The mortality
information was obtained from the Social Security Administration files
and supplemented by information collected by another investigator
through follow-up. The results indicated an excess of death from
cerebrovascular disease (17 observed, 9.3 expected) (Wang & MacMahon,
1979a).
The mortality of 16 126 males employed as pesticide applicators
was evaluated. The cohort was employed between 1967 and 1976 by any
of the nationwide (US) pest control companies for 3 months or more.
The information on mortality was traced in Social Security
Administration files. The results did not demonstrate a significant
difference in mortality from the norm in the cohort (Wang & MacMahon,
1979b). An update of the above study group was conducted by following
the same cohort to the end of 1984. The analysis showed an excess of
lung cancer among the pesticide applicators. However, these
applicators were exposed to a broad spectrum of pesticides, and the
increased lung cancer risk among the pesticide applicators might not
relate to their exposure to heptachlor alone (MacMahon et al.,
1987).
A retrospective mortality study of 2141 workers from 4
manufacturing plants of chlorinated hydrocarbon pesticides, which
included chlordane, heptachlor, DDT, aldrin, dieldrin, and endrin was
reported by Ditraglia et al., 1981. The workers in each plant were
considered as a separate cohort, each had worked at least 6 months
prior to 31 December 1964. The results showed too few deaths to allow
any meaningful conclusions (Ditraglia et al., 1981).
Another retrospective study was conducted by Shindell and
Associates (1981) on a cohort of 1115 employees who worked 3 months or
more at the Memphis, Tennessee, USA, plant of Velsicol Chemical Corp.,
from 1 January 1952 to 31 December 1979. The plant began to
manufacture heptachlor in 1952. The data on morbidity and mortality
were obtained for 93.1% of the male and 90% of the female cohort. The
workers were classified according to their job or product exposure.
Mortality data on the cohorts were compared to the overall mortality
experience of like segments (by age and sex) of the United States
population at large to determine whether Memphis plant exposure caused
any discernible variations from the expected mortality from all causes
and from selected significant causes. No significant difference was
found.
COMMENTS
Heptachlor is structurally similar to chlordane. An ADI of
0.0005 mg/kg bw was estimated for heptachlor by JMPR in 1970.
Heptachlor is well absorbed through the GI tract and metabolized to a
large extent to heptachlor epoxide and minor metabolites. Heptachlor
epoxide has been shown to accumulate in adipose tissue, and to cross
the placenta, but it was not found in the brain. In rats, the major
route of elimination is via the faeces.
In both short- and long-term studies in dogs, rats, and mice, the
liver was found to be the target organ. In a 30-day feeding study in
mice at dietary concentrations of 0, 1, 5, 10, 25 or 50 ppm,
histopathology findings indicated that males at 5 ppm or above and
females at 10 ppm or above had enlargement of centrilobular and
midzonal hepatocytes. The severity was dose-related. Based upon
these results, the NOAEL was 1 ppm, equivalent to 0.15 mg/kg bw/day.
Several long-term/carcinogenicity studies in rats were reviewed,
but the majority of them had severe methodological limitations. In
one study, rats received time-weighted dietary concentrations of 39 or
78 ppm (males) and 26 or 51 ppm (females) of heptachlor for 80 weeks.
On the basis of decreased body-weight in high-dose males and increases
in mortality in high-dose males and females, the NOAEL was 26 ppm,
equivalent to 1.3 mg/kg bw/day. Deficiencies in this study precluded
proper evaluation of the carcinogenic potential of heptachlor in rats.
In a 2-year feeding study, dogs fed heptachlor epoxide at dietary
concentrations of 0, 1, 3, 5, 7 or 10 ppm, exhibited an increase in
liver weight at 10 ppm and an increase in the incidence of liver
histopathological changes at 3 ppm and above. The histopathological
changes were enlargement and vacuolation of centrilobular or scattered
hepatocytes. Similar histological changes persisted through six
months of the recovery period. The NOAEL based upon these findings
was 1 ppm, equivalent to 0.025 mg/kg bw/day.
Heptachlor/heptachlor epoxide is carcinogenic in mice; two
studies demonstrated an increase in liver tumour incidence in male and
female Charles River CD-1 and B6C3F1 mice.
Heptachlor/heptachlor epoxide also caused a marginal increase in
the incidence of hepatocytomegaly in all treated CD-1 mice; a NOAEL
was not established in this study.
In a 2-generation reproduction study in dogs at dietary
concentrations of 1, 3, 5, 7, or 10 ppm of heptachlor epoxide, there
was an increase in mortality of F2 pups at 3 ppm and above. The
NOAEL based on this finding was 1 ppm.
The available epidemiology studies have not shown a clear
relationship between any effects in humans and exposure to heptachlor.
After reviewing the available in vitro and in vivo short-term
test data, it was concluded that, although it can interfere with
intercellular communication, heptachlor is not genotoxic.
Many studies available for evaluation were conducted more than 20
years ago and have severe deficiencies. Because of these
deficiencies, its carcinogenicity in mice and its ability to
bioaccumulate, the Meeting recommended that heptachlor should not be
used directly on food crops and its use in the production of food
commodities should be phased out. Because of its environmental
persistence it is found as a contaminant in food commodities. The
Meeting therefore maintained an ADI, basing it on the NOAELs derived
from studies in dogs. However, recognizing the inadequacy of the data
base, the Meeting increased the safety factor to 200-fold. This ADI
will provide a guideline for assessing the significance of dietary
exposure to heptachlor residues.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 26 ppm, equivalent to 1.3 mg/kg bw/day
Dog: 1 ppm, equivalent to 0.025 mg/kg bw/day
(reproduction study)
1 ppm, equivalent to 0.025 mg/kg bw/day (2-year study)
Estimate of acceptable daily intake for humans
0-0.0001 mg/kg bw
REFERENCES
Ahmed, F.E., Hart, R.W., & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res. 42:
161-174.
Arnold, D.W., Kennedy, G.L., Jr., Keplinger, M.L., Calandra, J.C., &
Calo, C.J. (1977) Dominant lethal studies with technical chlordane,
HCS-3260, and heptachlor:heptachlor epoxide. J. Toxicol. Environ.
Health., 2: 547-555.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 442-444.
Davidow, B. & Radomski, J.D. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265.
Ditraglia, D., Brown, D.P., Namekata, T., & Iverson, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health. 7
(Supplemental 4): 140-146.
Epstein, S.S. (1976) Carcinogenicity of heptachlor and chlordane.
Sci. Total Environ., 6(2): 103-154.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14:514-534
Gak, J.C., Grillot, C., & Truhaut, R. (1976) Use of the golden
hamster in toxicology. Lab. Animal Sci. 26(2): 274-280.
Gentile, J.M., Gentile, G.J., Bultman, J., Sechriest, R., Wagoner,
E.D. & Plewa, M.J. (1982) An evaluation of the genotoxic properties
of insecticides following plant and animal activation. Mutat. Res.,
101: 19-29.
Jain, A.K. (1988) Cytogenic effects of heptachlor on Lens and
Pisum species. Geobios, 15, 61-69.
Jolley, W.P., Stemmer, K. L., & Jolley, L. (1966) The effects of
feeding diets containing a mixture of heptachlor and heptachlor
epoxide to female rats for two years. Unpublished report by The
Kettering Laboratory, University of Cincinnati. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Kroger, M. (1972) Insecticide residues in human milk. J. Pediatr.
80: 401-405.
Kurata, M., Hirose, K. & Umeda, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73, 217-227.
Le Marchand, L., Kolonel, L.N., Siegel, B.Z., & Dendle, W.H., III
(1986) Trends in birth defects for a Hawaiian population exposed to
heptachlor and for the United States. Arch. Environ. Health, 41(3):
145-148.
MacMahon, B., Monson, R.R., Wand, H.H., & Zheng, T. (1987) A second
follow-up of mortality in a cohort of pesticide applicators. A report
to Velsicol Chemical Corp., dated June 5, 1987. Submitted to WHO by
Velsico Chemical Corp., Rosemont, Illinois, USA.
Marshall, T.C., Dorough, H.W., & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agri. Food Chem., 24(3): 560-563.
Maslansky, C.J., & Williams, G.M. (1981) Evidence for an epigenetic
mode of action in organochlorine pesticide hepatocarcinogenicity: A
lack of genotoxicity in rats, mouse, and hamster hepatocytes.
J. Toxicol. Environ. Health, 8: 121-130.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Response of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 coded chemicals.
Environ. Mol. Mutagenesis, 12, 85-154.
Mersch-Sundermann, V., Dickgiesser, N., Hablizel, U. & Gruber, B.
(1988) Examination of the mutagenicity of selected herbicides and
insecticides with the Salmonella-microsome test (Ames-Test) in
consideration of the pathogenetic potency of contaminated ground and
drinking water (Ger.). Zbl. Bakt. Hyg. B., 186, 247-260.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
U. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
NAS (National Academy of Science) (1977) An Evaluation of the
carcinogenicity of chlordane and heptachlor. Report to the Chief
Administrative Law Judge of the EPA, dated October, 1977. Submitted to
WHO by Velsicol Chemical Corp., Rosemont, Illinois, USA.
NCI (National Cancer Institute) (1977) Bioassay of heptachlor for
possible carcinogenicity. CAS.No. 76-44-8. Technical Report Series 9.
Bethesda, MD: Carcinogen Bioassay and Program Resources Branch,
Carcinogenicity Program, Division of Cancer Cause and Prevention,
NCI, National Institute of Health. DHEW Publication (NIH) 77-809.
NTP (National Toxicology Program) (1983) National Toxicology Program
Annual Plan for Fiscal Year 1983. NTP-82-119. Chemical Test Results
for Mutagenicity in Salmonella Assays in FY 1982. US Department of
Health and Human Services.
NTP (National Toxicology Program) (1985) National Toxicology Program
Annual Plan for Fiscal Year 1985. NTP-85-055. Chemical Test Results
for Cytogenetic Effects in Chinese Hamster Ovary Cells in FY 1984. US
Department of Health and Human Services.
Podowske, A.A., Banerjee, C.C., Feroz, M., Dudek, M.A., Willey, &
R.L., Khan, M.A.Q. (1979) Photolysis of heptachlor and
cis-chlordane and toxicity of their photoisomers to animals. Arch.
Environ. Contam. Toxicol. 8: 509-518.
Polishuk, Z.W., Ron, M, Wassermann, M., Cucos, S., Wassermann, D., &
Lemesch, C. (1977a) Pesticides in people. Organochlorine compounds in
human blood plasma and milk. Pestic. Nonit. J. 10(4): 121-129.
Polishuk, Z.W., Wassermann, D., Wassermann, D., Cucos, S. & Ron, M.
(1977b) Organochlorine compounds in mother and fetus during labor.
Environ. Res. 13: 278-294.
Probst, G.S., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K., &
Neal, S.B. (1981) Chemical-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ Mutat. 3: 11-32.
Radomski, J.L. & Davidow, B. (1953) The metabolite of heptachlor, its
estimation, storage and toxicity. J. Pharmacol. Exp. Therap. 107:
266-272.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21,
319-334.
Ruch, R.J., Fransson, R., Flodstrom, S., Warngard, L. & Klaunig, J.E.
(1990) Inhibition of hepatocyte gap junctional intercellular
communication by endosulfan, chlordane and heptachlor.
Carcinogenesis, 11, 1097-1101.
Sandhu, S.S., Ma, T-H., Peng, Y., & Zhou, X. (1989) Clastogenicity
evaluation of seven chemical commonly found at hazardous industrial
waste sites. Mutat. Res., 224, 437-445.
Savage, E.P., Keefe, T.J., Tessari, J.D., et al. (1981). National
study of chlorinated hydrocarbon insecticide residues in human milk.
USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor
epoxide, chlordane, oxychlordane, and mirex. Am. J. Epidemiol.
113(4): 413-422.
Seiler, J.P. (1977) Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short-term test. Mutat. Res., 46, 305-310.
Sherman, M. & Ross, E. (1961) Toxicol. App. Pharmacol., 3: 521.
Shindell and Associates (1981) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant Memphis, Tennessee,
January 1955-December 1979. Unpublished Report by Shendell and
Associates, Milwaukee, Wisconsin. Submitted to WHO by Velsicol
Chemical Corp., Rosemont, Illinois, USA.
Simmon, V.F., Kauhanen, K. & Tardiff, R.G. (1977) Mutagenic activity
of chemicals identified in drinking water. Dev. Toxicol. Environ.
Sci., 2, 249-258.
Stehr-Green, P.A., Wohlleb, J.C., Royce, W., & Head, S.L.(1988) An
evaluation of serum pesticide residue levels and liver function in
persons exposed to dairy products contaminated with heptachlor.
J. Am. Med. Assoc. 259(3): 374-377.
Strassman, S.C. & Kutz, F.W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi 1973-1974. Pesticide Monit. J.
10(4):130-133.
Takahashi, W., Saidin, D., Takei, G., & Wong, L. (1981)
Organochloride pesticide residues in human milk in Hawaii 1979-1980.
Bull. Environ. Contam. Toxicol. 27: 506-511.
Takei, G.H., Kauahikaua, S.M., & Leong, G.H. (1983) Analyses of human
milk samples collected in Hawaii for residues of organochlorine
pesticides and polychlorobiphenyls. Bull. Environ. Contam. Toxicol.
30: 606-613.
Tashiro, S. & Matsumura, F. (1978) Metabolism of trans-nonachlor
and related chlordane components in rat and man. Arch. Environ.
Contam. Toxicol. 7: 113-127.
Telang, S., Tong, C., & Williams, G.M. (1982) Epigenetic membrane
effects of possible tumour-promoting type on cultured liver cells by
the nongenotoxic organochlorine pesticide chlordane and heptachlor.
Carcinogenesis, 3(10): 1175-1178.
Velsicol Corporation (1959) Unpublished report.
Wang, H.H. & MacMahon, B. (1979a) Mortality of workers employed in
the manufacture of chlordane and heptachlor. J. Occup. Med. 21(11):
746-748.
Wang, H.H. and MacMahon, B. (1979b). Mortality of pesticide
applicators. J. Occup. Med. 21(11): 741-744; (11):746-748.
Wazeter, F.X., Goldenthal, E.I., Geil, R.G., Howell, D.G., & Dean,
W.P. (1971a) 30-Day range-finding study in mice. Unpublished Report
from International Research and Development Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., Cookson, K.M. & Howell,
D.G. (1971b) Two-year oral study in beagle dogs. Unpublished Report
No. 163-048 from International Research and Development Corp.
Submitted to WHO by Velsicol Chemical Co., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., & Howell, D.G. (1971c)
Two-generation reproduction and teratology study in beagle dogs.
Unpublished Report No.163-048 from International Research and
Development Corp. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Wazeter, F.X., Goldenthal, E.I., & Geil, R.G. (1973) Eighteen-month
oral carcinogenic study in mice. Unpublished Report No. 163-084 from
International Research and Developmental Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA
Witherup, S., Cleveland, F.P., Shaffer, F.E., Schlecht, H., & Musen,
L. (1955) The physiological effects of the introduction of heptachlor
into the diet of experimental animals in varying levels of
concentration: Report of experiment No.2. Unpublished report from The
Kettering Laboratory, College of Medicine, University of Cincinnati,
Ohio. Submitted to WHO by Velsicol Chemical Corp., Rosemont, Illinois,
USA.
Witherup, S., Cleveland, F.P., Stemmer, K.L., & Schlecht, H. (1958)
The physiological responses of beagle dogs to the consumption of diets
containing various concentrations of heptachlor epoxide over a period
of sixty weeks. Unpublished report from The Kettering Laboratory,
College of Medicine, University of Cincinnati, Ohio. Submitted to WHO
by Velsicol Chemical Corp., Rosemont, Illinois, USA.
Witherup, S., Cleveland, F.P., & Stemmer, K. (1959) The physiological
effects of the introduction of hepatochlor epoxide in varying levels
of concentration into the diet of CFN rats. Unpublished report from
the Kettering Laboratory, College of Medicine, University of
Cincinnati, Ohio. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III. Results from
the testing of 255 chemicals. Environ. Mutagenesis, 9(Suppl. 9):
1-11.
 Table 1. Acute toxicity data of heptachlor and heptachlor epoxide
Species Sex Route LD50 Reference
(mg/kg bw)
A. Heptachlor
Rat M oral 71 Podowski et al. (1979)
M&F oral 60-142a Velsicol Corp. (1959)b
Chick d oral 63 Sherman & Ross (1961)b
Mouse d oral 70 Gak et al. (1976)
Hamster d oral 100 Gak et al. (1976)
Rat M dermal 195c Gaines (1969)
F dermal 250c Gaines (1969)
B. Heptachlor epoxide
Rat M&F oral 34-48a Velsicol Corp. (1959)b
Mouse M oral 32-48 Velsicol Corp. (1959)b
a Sex differences
b Data excerpted from the 1966 JMPR monograph (Annex 1, 7)
c Xylene was the solvent.
d Not stated
Short-term studies
Mouse
Groups of 10 Charles River CD-1 mice/sex/dose were fed a mixture
of heptachlor-heptachlor epoxide (25:75) at dietary concentrations of
1, 5, 10, 25, and 50 mg/kg for 30 days. Following 30 days of
treatment, the animals were sacrificed and necropsied. Microscopic
examinations were conducted on liver tissue.
No deaths occurred in the 1, 5, nor 10 mg/kg groups. One female
died in the 25 mg/kg group, and 9 males and 8 females died in the 50
mg/kg group. No marked differences were seen between the treated
animals and the controls with respect to body weights and food
consumption. Mice which were sacrificed at the end of the study from
the 10, 25 and 50 mg/kg groups showed liver enlargement associated
with accentuated lobulation. The liver weight of the only surviving
male mouse from the 50 mg/kg group was markedly increased (control
mean: 1.69 g; 50 mg/kg; 4.00 gm), and increased mean liver weight was
also found in the 25 ppm male mice (control, 1.69 g; 25 ppm, 4.35 gm).
Histopathology findings indicated that livers of male mice in the 5,
10, 25, and 50 ppm and of female mice in the 10, 25, and 50 ppm groups
had enlargement of centrilobular and midzonal hepatocytes with
replacement of the normal coarse cytoplasmic granularity by finely
granular and homogeneous cytoplasm. The severity of this lesion was
dose-related. The incidences of the microscopic findings were not
reported (Wazeter et al., 1971a). Based upon the histopathology
findings, the NOAEL was 1 mg/kg.
Rat
Two short-term studies on heptachlor in rats were summarized in
the 1971 monograph. The dietary levels tested in these two studies
ranged from 5 to 45 ppm. At 10 ppm and above, rat liver cells showed
an increase in smooth endoplasmic reticulum and mitochondria. The
liver effects regressed after the treated rats were placed on a
control diet for 120 days (Annex 1, 15).
Long-term studies
Some of the unpublished long-term studies on heptachlor were
evaluated by the 1966 Joint Meeting and published in the 1967
Monograph. To facilitate consideration of the toxicity of this
chemical the older toxicity studies are also summarized here.
Rat
Groups of Carworth Farms (CF) rats (20/sex/dose) were fed diets
containing heptachlor (purity not specified) at a concentration of 0,
1.5, 3.0, 5.0, 7.0, or 10 ppm for 110 weeks. The treatment diet was
prepared by spraying standard rat diet with an ethanol solution of
heptachlor. After 7 weeks on the test diet, 5 females and 5 males
from each dose group were mated to determine whether or not heptachlor
would affect the reproductive capacity of the treated animals or the
survival and subsequent growth of the offspring. Following similar
procedures, another mating was conducted using different animals from
each dose group after 22 weeks of treatment. The offspring were kept
with their mothers until weaning at 3 weeks of age. After weaning,
the offspring were placed on the control diet for 8 weeks for further
observations. After 110 weeks of treatment, the surviving animals
were sacrificed, certain haematological parameters were examined.
Histopathology was conducted on the heart, liver, lungs, brain,
spleen, kidneys, thyroids, and the adrenal glands.
Heptachlor treatment did not affect mortality, body weight, or
food consumption. The limited reproductive parameters measured did
not indicate any effect on the body weights at birth or at weaning.
Heptachlor administration did not appear to significantly affect the
mortality of the offspring. Organ weights of the heptachlor-treated
rats were not markedly different from those of controls. Haematology
parameters (erythrocyte counts, haemoglobin, leukocyte counts, and
differential white cell counts) did not show any compound-related
effect. The histopathology data indicated 6/16 males and 3/18 females
at 7.0 ppm and 2/12 males and 9/16 females at 10 ppm had slight
hepatic cellular alterations characterized by swelling, homogeneity of
the cytoplasm and peripheral arrangement of the cytoplasmic granules
in the hepatic cell in the central zone of the lobules. Under the
conditions of this study, heptachlor at doses up to 10 ppm did not
produce an increase in the tumour incidence in CF rats (Witherup
et al., 1955).
Groups of CFN (Carworth Farms, Nelson) rats (25/sex/dose) were
fed heptachlor epoxide at dietary concentrations of 0, 0.5, 2.5, 5.0,
7.5, and 10.0 ppm for 108 weeks. The test diet was prepared by
spraying an ethanol solution of heptachlor epoxide on Purina
Laboratory Chow.
The survival rate was greater than 47% in the treated and control
groups. There was an increase in the liver weights of treated females
and a slight increase in treated males. The test chemical was not
reported to cause any additional effects. This study has
methodological deficiencies which include uncertain concentrations in
the feed and insufficient number of rats in each dose level. Also the
report does not contain histopathological data and is therefore not
adequate for evaluating the long-term toxicity of heptachlor (Witherup
et al., 1959). However, a panel of independent pathologists
convened by the US National Academy of Sciences (1977) reviewed the
histopathology and concluded that there was no increase in liver
tumours in treated rats.
Four groups of Charles River CD female rats (25/dose) were fed a
3:1 mixture of heptachlor:heptachlor epoxide at dietary concentrations
of 5, 7.5, 10, and 12.5 ppm. A control group of 54 females was fed a
diet containing the vehicle, corn oil. Interim sacrifices were
conducted at 5 and 19 months to determine the nature and degree of the
histological changes in the liver. At 5 months, 3 controls and 5 rats
from the 7.5 ppm group were sacrificed. At 19 months, 5 controls, 2
rats from 5 ppm, 2 rats from 10 ppm, and 1 rat from 12.5 ppm were
sacrificed. After 24 months of treatment, the surviving rats were
sacrificed, and gross and histological examinations were conducted.
There was an increase in mortality at 12.5 ppm relative to that
of controls (control, 21%; 12.5 ppm, 50%). The test article did not
affect body weight gain. Liver changes which were described as fatty
liver and enlarged cells in the central zone of the hepatic lobules
were reported. However, no histopathology data were included in the
report (Jolley, 1966).
Groups of Osborne-Mendel rats (50/sex/dose) were fed technical
grade heptachlor (73% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at time-weighted doses of 38.9 and 77.9
ppm for males and 25.7 and 51.3 ppm for females. The matched controls
consisted of 10 males and 10 females, and pooled controls were 60
rats/sex. The treated animals received the chemical for 80 weeks and
were observed for 30 weeks. During the 80 weeks of treatment, the
dietary concentrations changed three times for each treatment group
based on changes in body weight.
There was a decrease in the mean body weight of high dose males
which almost returned to the control level after the treatment was
terminated. There was also a slight increase in mortality in high
dose male and female rats. Histopathology data revealed numerous
inflammatory, degenerative, and proliferative lesions with
approximately equal frequencies in treated and control animals. There
was an increased incidence of thyroid follicular cell neoplasms,
including combined adenomas and carcinomas in the high dose female
rats (high dose, 14/38; low dose, 3/43; matched controls, 1/9;
p<0.001) while, in male rats, a non-significant increase was observed
only at the low dose (high dose, 3/38; low dose, 9/38; matched
control, 1/8). At the same time, the incidences of the thyroid C-cell
neoplasms decreased in high dose female rats (high dose, 3/38; low
dose, 7/43; matched controls, 3/9; p<0.05). The results of analyses
of the thyroid lesions were ambiguous and further complicated by
variability in different experiments conducted at about the same time
with this strain of rats (NCI, 1977).
Dog
Groups of Beagle dogs (4/sex/dose) were fed heptachlor epoxide
(purity not specified) at concentrations of 0, 1, 3, 5, 7, and 10 ppm
for two years. After 2 years on test, 2 dogs/sex/dose were sacrificed
and necropsied while the other two dogs/sex/dose were maintained on
the control diet for an additional 6 months (recovery period). In
addition, the test animals in this study were also mated during the
study and employed as the P1 parental animals for a 2-generation
reproduction and teratology study.
No death nor compound-related behavioural change was seen during
the study. The test article did not affect the body weights or food
consumptions of the treated animals. The parameters for haematology
and urinalysis were comparable between treated and control dogs.
There were increases in alkaline phosphatase activities in males and
females at 3 ppm and above; these increases, in some dogs, were more
marked towards the end of the treatment period and tended to persist
throughout the recovery period. The serum albumin and total protein
levels were slightly decreased in 10 ppm male and female dogs during
the treatment and the recovery periods. An increase in the SGPT level
was also seen in 10 ppm animals during the treatment, extending into
the recovery period. After one year of treatment, the animals in 7
ppm group also showed an increase in the SGPT level which lasted into
the recovery period.
There was an increase in liver weights in 10 ppm male and female
dogs relative to those of the controls, and this increase persisted
with a slight attenuation during the recovery period. Histopathologic
examination on dogs (2 dogs/sex/dose) sacrificed at the end of the
treatment period showed an increase in the incidence of liver changes
in animals at 3.0 ppm or above. The changes were described as
enlargement and vacuolation of groups of centrilobular hepatocytes or
scattered individual hepatocytes, presence of finely granular, and a
"ground glass" appearance of the cytoplasm of large numbers of
hepatocytes. These histopathological changes were also seen in dogs
at 3 ppm and above after 6 months of recovery period. No
compound-related histopathology changes were seen in 1 ppm dogs.
Based upon the changes in biochemical parameters and the histological
changes in the liver, the NOAEL was 1 ppm (Wazeter et al., 1971b).
Although this study has certain deficiencies, including an
insufficient number of experimental animals, the results provide
useful information on the toxicity of the test compound in dogs.
Groups of 23 to 27 week old beagle dogs (2 males or 3
females/dose) were fed heptachlor epoxide at dietary concentrations of
0, 0.5, 2.5, 5.5, and 7.5 ppm for sixty weeks. The test diet was
prepared by spraying an ethyl alcohol solution of heptachlor epoxide
on the food. The experimental results were limited to clinical
observation, mortality, body weight changes, food consumption, and
organ weights. The limited results indicated that there was an
increase in liver weights of all the heptachlor treated male and
female dogs relative to that of the controls. For 7.5 ppm males, the
increase was approximately twice that of the controls. However, the
pathology data were not included in the report (Witherup et al.,
1958).
Mouse
Groups of Charles River CD-1 mice (100/sex/dose) received a
mixture of heptachlor/heptachlor epoxide (25%/75%) at dietary
concentrations of 0, 1.0, 5.0, and 10.0 ppm for 18 months. A positive
control group (100 mice/sex) received 2-acetaminofluorene at a dietary
concentration of 250 ppm. An interim sacrifice was performed on 10
mice/sex/dose including the positive control group. Gross pathology
and histology examinations were conducted on animals which died during
the study or were sacrificed on schedule.
There was a decrease in the number of survivors in 10 ppm male
and female mice, in 5 ppm females, and in positive control males
relative to those of the negative controls as shown in Table 2. Mean
body weights and food consumptions of the treated mice were not
affected. There were increases in the mean liver weights of males and
females in 5 and 10 ppm groups.
An increase in the incidence of hepatocytomegaly was seen in all
heptachlor-heptachlor epoxide treated mice relative to that of the
controls. The incidence of hepatic nodular hyperplasia was markedly
increased in 5 and 10 ppm males and females relative to that of the
controls. For heptachlor-treated animals, hepatoma was observed in
only one male mouse at 1 ppm (Wazeter, 1973). Based on the incidence
of hepatocytomegaly, a NOAEL was not established (LOAEL <1ppm).
The histological slides of this study were later evaluated by a
panel of pathologists convened by the Pesticide Information Review and
Evaluation Committee of the National Academy of Sciences, USA. The
results of the evaluation are presented in Table 2.
Levels of 1, 5, and 10 ppm of heptachlor-heptachlor epoxide did
not produce a statistically significant increase in the incidence of
hepatocellular carcinoma. However, at 10 ppm there was a statistically
significant increase in the incidence of combined hepatocellular
carcinomas and nodular changes (NAS, 1977).
Epstein (1976) reported a previously unpublished study conducted
by the US-FDA. Groups of C3H mice (100/sex/dose) were fed 0 or 10 ppm
of either heptachlor or heptachlor epoxide (purity unspecified) for 24
months. The liver histopathology was re-evaluated by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences. Their
results indicated a significant increase in hepatocellular carcinomas
in females given heptachlor and in both males and females given
heptachlor expoxide.
Table 2. The incidence of hepatocellular carcinomas and nodular
changes in CD-1 mice fed heptachlor-epoxide
Male Female
Group HC HC+N HC HC+N
Control 1/59 2/59 1/74 1/74
1 ppm H-HE 2/66 4/66 1/65 3/65
5 ppm H-HE 2/66 4/66 1/65 3/65
10 ppm H-HE 1/73 27/73* 4/52 16/52*
AAF 5/58 9/58 5/75 13/75
HC hepatocellular carcinomas
HC+N hepatocellular carcinomas and nodular changes
* (p<.001)
Groups of B6C3F1 mice (50/sex/dose) received technical-grade
heptachlor (72% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at initial concentrations of 10 and 20
ppm for males and 20 and 40 ppm for females. The initial
concentrations were reduced due to adverse toxic effects, with
time-weighted average concentrations of 6 and 14 ppm for males and 9
and 18 ppm for females. The mice were treated for 80 weeks and placed
on the control diet for another 10 weeks. Matched-controls consisted
of 20 males or 10 females, and the pooled controls were 100 males and
80 females. Under the conditions of the study, heptachlor had no
significant effects on the body weights. Survival at 90 weeks was
high: 70% of treated and control males and 60% of treated and control
females. Survival of female mice showed a significant trend towards
lower survival in treated groups compared to that in the control
group. The incidence of hepatocellular carcinomas was significantly
increased in the high-dose males and females as shown in Table 3
(NCI,1977).
A review of liver histopathology from this study by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences concluded
that there was a low incidence of induced hepatocellular carcinomas
(Table 3). According to this review, the incidence of hepatocellular
carcinomas was statistically increased (p<0.001) in groups of males
and females receiving heptachlor at the high dose only when combined
with the diagnosis of nodular changes (NAS, 1977).
Table 3. Incidence of proliferative lesions of the liver in B6C3F1
mice fed technical grade heptachlor
Lesion Pooled Matched Low Dose High Dose
Control Control
Males
Hepatocellular
carcinomas 17/92 5/19 11/46 34/47*
Nodular hyperplasia 3/92 1/19 9/46 6/47
Diffuse hyperplasia 3/92 0/19 1/46 3/47
Hepatocytomegaly 0/92 0/19 1/46 0/47
Females
Hepatocellular carcinomas 3/78 1/10 3/47 30/42*
Nodular hyperplasia 2/78 0/10 3/47 0/42
Diffuse hyperplasia 1/78 0/10 0/47 0/42
Hepatocytomegaly 1/78 0/10 0/47 1/42
The denominator represents the number of tissues examined microscopically.
* p<0.001 (Fisher exact test)
Reproduction studies
Dogs
Groups of 6-9 month old Beagle dogs (4/sex/dose) were fed
heptachlor epoxide at dietary concentrations of 0, 1, 3, 5, 7, and 10
ppm. When the female dogs attained an age of 14 months, they were
mated twice with male dogs from the same dose group. The females were
allowed to deliver and to nurse their pups.
For the F2 generation, 4 female and 2 male pups of the F1
generation were selected from each dose level to be the parental
animals (P2) of the F2 generation. At an approximate age of 14
months, the animals were mated, and the pregnant females were allowed
to deliver and to nurse their pups to 6 weeks of age. At this time,
the females and their pups were sacrificed.
No clinical signs or behaviour changes were seen in treated P1,
P2, or their offspring. There were no indications that body weight
or food consumption were affected by the test chemical. Whether or
not the test chemical produced any reproductive effects could not be
determined with the very limited data presented in this report. There
was a significant increase in the mortality rate of F1 pups in the 10
ppm group (control, 9/20; 10 ppm, 17/18). Only 1 male pup survived to
the scheduled sacrifice, and no female was available to serve as a P2
parental animal. There were slight increases in death rates of F2
pups at 3 and 7 ppm relative to the controls (Control, 0/4; 3 ppm,
3/8; 7 ppm, 3/8). The 5 ppm group had no pups. Haematology and
urinalysis results did not indicate any compound-related effects. Very
limited clinical biochemistry data showed that alkaline phosphatase
and/or SGPT activities in certain dogs were increased. However, the
changes in the clinical chemistry parameters could not be correlated
with pathology findings because the necropsy data on animals showing
clinical biochemistry changes were not presented in the report. The
limited necropsy data showed that, in F1 pups, 1/4 females at 7 ppm
and 3/10 males and 3/7 females at 10 ppm had pale or greasy liver.
This observation was compound-related. Based on the increase in the
mortality rate, the NOAEL was 1 ppm (Wazeter et al., 1971c).
Rat
Three reproduction studies in rats were evaluated by the JMPR in
1966 and 1970. In a 3-generation reproduction study, a group of 80
rats were given 6.9 mg/kg of heptachlor daily for 3 months prior to
mating. Cataracts, which became obvious between the 19th and 26th
days, were found in 6.8% of the young. A similar lesion was present
in 15.2% of the parental animals after 9 months. The only effect on
reproduction was a decrease in the litter size (Annex 1, 7). In two
other reproduction studies in rats, dietary levels of heptachlor
ranging from 0.3 to 10 ppm were tested; no cataracts nor adverse
effects on fertility and reproduction were found. A slight increase
in post-natal mortality of pups was seen at 10 ppm (Annex 1, 15).
Special studies for teratology and embryotoxicity
Rabbit
A rabbit teratology study was previously evaluated by the 1970
Joint Meeting, and it was concluded that no teratogenic effects could
be attributed to the compound (Annex 1, 15).
Special studies on genotoxicity
In a large number of genotoxicity assays (Table 4), heptachlor
did not induce genetic damage. Exceptions were the induction of small
numbers of mutations in bacteria in the presence of plant extracts,
mutations and a chromosomal aberrations in plants and in a single
study, mutations at the highly sensitive tk locus in mouse lymphoma
cells. Gap-junctional intercellular communication was inhibited in
several in vitro assays.
In both a sister chromatid exchange assay and a chromosome
aberration assay in Chinese hamster ovary cells, there were "positive"
results in the presence of rat S9 metabolic activation. In the absence
of metabolic activation the chromosome aberration assay was negative
(NTP, 1985). However, the tests were not repeated to confirm the
positive results.
The mutagenic potential of heptachlor epoxide was also tested.
The results are presented in the following table.
Table 4. Results of genotoxicity assays on heptachlor
Test system Test object Concentration Resultsa Reference
Ames test S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
TA1535, TA1536
TA1537, TA1538
Ames test S. typhimurium 2500 µg/ml -/0 Simmon et al. (1977)
TA98, TA100,
TA1535,
TA1537, TA1538
Ames test S. typhimurium not stated -/- Probst et al. (1981)
TA98, TA100, TA1535,
TA1537, TA1538, G46,
C3076, D3052
E. coli WP2 & WP2 uvr A-
Ames test S. typhimurium 0.1 µg/plate -/- NTP (1983)
TA98, TA100, TA1535, to 10 mg/plate
TA1537
Ames test S. typhimurium 5-10 µg/ml +/-b Gentile et al. (1982)
TA98, TA100, TA1535
Ames test S. typhimurium 2500 µg/ml -/- Moriya et al. (1983)
TA98, TA100, TA1535,
TA1537, TA1538
E. coli WP2 hcr
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Rec assay B. subtilis 356 µg/ml -/- Matsui et al. (1989)
Differential E. coli WP 2 2000 µg/ml 0/- Rashid & Mumma (1986)
toxicity S. typhimurium
TA1538
Ames test S. typhimurium 1 mg/ml -/- Mersch-Sundermann (1988)
TA97, TA98, TA100,
TA102
Ames test S. typhimurium 167 µg/ml -/- Zeiger et al. (1987)
TA98, TA100,
TA1535, TA1537
Gene conversion S. cerevisia f -/- Gentile et al. (1982)
Mutation Zea mays 1.12 kg/ha 0/+ Gentile et al. (1982)
Chromosomal Lens sp. 0.1-0.3% 0/+ Jain (1988)
aberrations Pisum sp.
Micronuclei Tradescantia 1.88 ppm 0/+ Sandhu et al.
(1989)
SLRSc mutation D. melanogaster 5 µg/ml (feeding 0/- Benes & Sram
solution) (1969)
tk locus Mouse lymphoma 25 µg/ml 0/- McGregor et al.
L5178Y mutation cells (1988
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
ARL-HGPRT Adult rat liver 10-6 to 10-4 M -/NAd Telang et al.
epithelial cell line (1982)
Unscheduled DNA Mouse, rat and hamster 10-5 - Maslansky & Williams (1981)
synthesis assay primary hepatocytes
Primary liver cell
explants from rats 10 nmole/ml - Probst et al. (1981)
SV-40 transformed human
fibroblasts 100 & 1000 µM ?/- Ahmed et al. (1977)
(VA-4)
Dominant Swiss mice 4.8 & 24 mg/kg (IP) - Epstein et al. (1972)
lethal 5 & 10 mg/kg (PO)
CD-1 mce 7.5 & 15 mg/kg - Arnold et al. (1977)
25:75e
Inhibition of Mouse testicular cells 40 mg/kg 0/- Seiler (1977)
DNA synthesis in vivo
Inhibition of Chinese hamster V79 10 µg/ml 0/+ Kurata et al. (1982)
metabolic cells
cooperation
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Inhibition of Rat hepatocytes 20 µg/ml 0/+ Ruch et al. (1990)
metabolic Mouse hepatocytes
cooperation
a with S9 activation/without S9 activation; phi indicates that the test was not performed
b with plant S9
c sex-linked recessive lethal
d NA = not applicable
e 25:75 blend w/w of heptachlor:heptachlor-epoxide
f concentration in original paper cited as "an appropriate amount".
Table 5. Results of genotoxicity assays on heptachlor epoxide
Test system Test object Concentration Resultsa Reference
Ames assay S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
Unscheduled DNA SV-40 transformed 100 & 1000 µM +/- Ahmed et al. (1977)
synthesis assay human fibroblasts
(VA-4)
Dominant lethal Swiss mice 6 & 30 mg/kg (IP) - Epstein et al. (1972)
8 mg/kg (PO)
a with S9 activation/without S9 activation
Observations in humans
In 1986, a study was conducted in the Midwest United States on a
group of 45 dairy farm family members who had consumed undiluted raw
milk products known to have been contaminated with heptachlor at
concentrations as high as 89.2 ppm (fat basis). The serum levels of
heptachlor or its metabolites in the exposed individuals were compared
to those from an unexposed group of 94 persons from the same
geographical area and to results from the Second National Health and
Nutrition Examination Survey (2nd NHANES). The exposed group had
significantly higher mean levels of primary metabolites such as
heptachlor expoxide (0.84±1.0 vs 0.50±0.9 ppb) and oxychlordane
(0.71±0.8 vs 0.49±1.1 ppb) than the unexposed group. In addition, the
percentage of individuals in exposed group with elevated serum
concentrations of these same metabolites (21.2%) was higher than that
for the unexposed group (heptachlor expoxide, 3.8%; oxychlordane,
6.3%) and the second National Health and Nutrition Survey (2.5% for
both metabolites) (Stehr-Green et al., 1988).
There is evidence of transplacental transfer of heptachlor
epoxide. The following heptachlor epoxide levels were detected in
various tissues of mother and newborn infants (Polishuk, 1977a):
adipose tissue (maternal): 0.2856 ± 0.3950 ppm
maternal blood: 0.2798 ± 0.4626 ppm
uterine muscle: 0.4895 ± 0.5086 ppm
fetal blood: 0.9959 ± 0.9458 ppm
placenta: 0.5000 ± 0.3950 ppm
amniotic fluid: 0.6730 ± 1.1645 ppm
Several investigators detected heptachlor epoxide levels ranging
from non-detectable to 0.46 ppm in human milk (Kroger, 1972; Polishuk
et al., 1977b; Strassman & Kutz, 1977; Savage et al., 1981;
Takahashi et al., 1981; Takei et al., 1983).
No adverse effects on human fetal development were reported
following ingestion of milk containing heptachlor for 27 to 29 months
among women of child bearing age in Oahu, Hawaii, USA. The levels of
heptachlor in milk fat ranged from 0.12 to 5.00 ppm (Le Marchand
et al., 1986).
A retrospective mortality study was conducted on workers employed
in the manufacture of heptachlor and chlordane between 1946 and 1976.
The study group consisted of 1403 white males who were employed for
more than 3 months at either of the two plants which produced
heptachlor and chlordane in the United States. The mortality
information was obtained from the Social Security Administration files
and supplemented by information collected by another investigator
through follow-up. The results indicated an excess of death from
cerebrovascular disease (17 observed, 9.3 expected) (Wang & MacMahon,
1979a).
The mortality of 16 126 males employed as pesticide applicators
was evaluated. The cohort was employed between 1967 and 1976 by any
of the nationwide (US) pest control companies for 3 months or more.
The information on mortality was traced in Social Security
Administration files. The results did not demonstrate a significant
difference in mortality from the norm in the cohort (Wang & MacMahon,
1979b). An update of the above study group was conducted by following
the same cohort to the end of 1984. The analysis showed an excess of
lung cancer among the pesticide applicators. However, these
applicators were exposed to a broad spectrum of pesticides, and the
increased lung cancer risk among the pesticide applicators might not
relate to their exposure to heptachlor alone (MacMahon et al.,
1987).
A retrospective mortality study of 2141 workers from 4
manufacturing plants of chlorinated hydrocarbon pesticides, which
included chlordane, heptachlor, DDT, aldrin, dieldrin, and endrin was
reported by Ditraglia et al., 1981. The workers in each plant were
considered as a separate cohort, each had worked at least 6 months
prior to 31 December 1964. The results showed too few deaths to allow
any meaningful conclusions (Ditraglia et al., 1981).
Another retrospective study was conducted by Shindell and
Associates (1981) on a cohort of 1115 employees who worked 3 months or
more at the Memphis, Tennessee, USA, plant of Velsicol Chemical Corp.,
from 1 January 1952 to 31 December 1979. The plant began to
manufacture heptachlor in 1952. The data on morbidity and mortality
were obtained for 93.1% of the male and 90% of the female cohort. The
workers were classified according to their job or product exposure.
Mortality data on the cohorts were compared to the overall mortality
experience of like segments (by age and sex) of the United States
population at large to determine whether Memphis plant exposure caused
any discernible variations from the expected mortality from all causes
and from selected significant causes. No significant difference was
found.
COMMENTS
Heptachlor is structurally similar to chlordane. An ADI of
0.0005 mg/kg bw was estimated for heptachlor by JMPR in 1970.
Heptachlor is well absorbed through the GI tract and metabolized to a
large extent to heptachlor epoxide and minor metabolites. Heptachlor
epoxide has been shown to accumulate in adipose tissue, and to cross
the placenta, but it was not found in the brain. In rats, the major
route of elimination is via the faeces.
In both short- and long-term studies in dogs, rats, and mice, the
liver was found to be the target organ. In a 30-day feeding study in
mice at dietary concentrations of 0, 1, 5, 10, 25 or 50 ppm,
histopathology findings indicated that males at 5 ppm or above and
females at 10 ppm or above had enlargement of centrilobular and
midzonal hepatocytes. The severity was dose-related. Based upon
these results, the NOAEL was 1 ppm, equivalent to 0.15 mg/kg bw/day.
Several long-term/carcinogenicity studies in rats were reviewed,
but the majority of them had severe methodological limitations. In
one study, rats received time-weighted dietary concentrations of 39 or
78 ppm (males) and 26 or 51 ppm (females) of heptachlor for 80 weeks.
On the basis of decreased body-weight in high-dose males and increases
in mortality in high-dose males and females, the NOAEL was 26 ppm,
equivalent to 1.3 mg/kg bw/day. Deficiencies in this study precluded
proper evaluation of the carcinogenic potential of heptachlor in rats.
In a 2-year feeding study, dogs fed heptachlor epoxide at dietary
concentrations of 0, 1, 3, 5, 7 or 10 ppm, exhibited an increase in
liver weight at 10 ppm and an increase in the incidence of liver
histopathological changes at 3 ppm and above. The histopathological
changes were enlargement and vacuolation of centrilobular or scattered
hepatocytes. Similar histological changes persisted through six
months of the recovery period. The NOAEL based upon these findings
was 1 ppm, equivalent to 0.025 mg/kg bw/day.
Heptachlor/heptachlor epoxide is carcinogenic in mice; two
studies demonstrated an increase in liver tumour incidence in male and
female Charles River CD-1 and B6C3F1 mice.
Heptachlor/heptachlor epoxide also caused a marginal increase in
the incidence of hepatocytomegaly in all treated CD-1 mice; a NOAEL
was not established in this study.
In a 2-generation reproduction study in dogs at dietary
concentrations of 1, 3, 5, 7, or 10 ppm of heptachlor epoxide, there
was an increase in mortality of F2 pups at 3 ppm and above. The
NOAEL based on this finding was 1 ppm.
The available epidemiology studies have not shown a clear
relationship between any effects in humans and exposure to heptachlor.
After reviewing the available in vitro and in vivo short-term
test data, it was concluded that, although it can interfere with
intercellular communication, heptachlor is not genotoxic.
Many studies available for evaluation were conducted more than 20
years ago and have severe deficiencies. Because of these
deficiencies, its carcinogenicity in mice and its ability to
bioaccumulate, the Meeting recommended that heptachlor should not be
used directly on food crops and its use in the production of food
commodities should be phased out. Because of its environmental
persistence it is found as a contaminant in food commodities. The
Meeting therefore maintained an ADI, basing it on the NOAELs derived
from studies in dogs. However, recognizing the inadequacy of the data
base, the Meeting increased the safety factor to 200-fold. This ADI
will provide a guideline for assessing the significance of dietary
exposure to heptachlor residues.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 26 ppm, equivalent to 1.3 mg/kg bw/day
Dog: 1 ppm, equivalent to 0.025 mg/kg bw/day
(reproduction study)
1 ppm, equivalent to 0.025 mg/kg bw/day (2-year study)
Estimate of acceptable daily intake for humans
0-0.0001 mg/kg bw
REFERENCES
Ahmed, F.E., Hart, R.W., & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res. 42:
161-174.
Arnold, D.W., Kennedy, G.L., Jr., Keplinger, M.L., Calandra, J.C., &
Calo, C.J. (1977) Dominant lethal studies with technical chlordane,
HCS-3260, and heptachlor:heptachlor epoxide. J. Toxicol. Environ.
Health., 2: 547-555.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 442-444.
Davidow, B. & Radomski, J.D. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265.
Ditraglia, D., Brown, D.P., Namekata, T., & Iverson, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health. 7
(Supplemental 4): 140-146.
Epstein, S.S. (1976) Carcinogenicity of heptachlor and chlordane.
Sci. Total Environ., 6(2): 103-154.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14:514-534
Gak, J.C., Grillot, C., & Truhaut, R. (1976) Use of the golden
hamster in toxicology. Lab. Animal Sci. 26(2): 274-280.
Gentile, J.M., Gentile, G.J., Bultman, J., Sechriest, R., Wagoner,
E.D. & Plewa, M.J. (1982) An evaluation of the genotoxic properties
of insecticides following plant and animal activation. Mutat. Res.,
101: 19-29.
Jain, A.K. (1988) Cytogenic effects of heptachlor on Lens and
Pisum species. Geobios, 15, 61-69.
Jolley, W.P., Stemmer, K. L., & Jolley, L. (1966) The effects of
feeding diets containing a mixture of heptachlor and heptachlor
epoxide to female rats for two years. Unpublished report by The
Kettering Laboratory, University of Cincinnati. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Kroger, M. (1972) Insecticide residues in human milk. J. Pediatr.
80: 401-405.
Kurata, M., Hirose, K. & Umeda, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73, 217-227.
Le Marchand, L., Kolonel, L.N., Siegel, B.Z., & Dendle, W.H., III
(1986) Trends in birth defects for a Hawaiian population exposed to
heptachlor and for the United States. Arch. Environ. Health, 41(3):
145-148.
MacMahon, B., Monson, R.R., Wand, H.H., & Zheng, T. (1987) A second
follow-up of mortality in a cohort of pesticide applicators. A report
to Velsicol Chemical Corp., dated June 5, 1987. Submitted to WHO by
Velsico Chemical Corp., Rosemont, Illinois, USA.
Marshall, T.C., Dorough, H.W., & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agri. Food Chem., 24(3): 560-563.
Maslansky, C.J., & Williams, G.M. (1981) Evidence for an epigenetic
mode of action in organochlorine pesticide hepatocarcinogenicity: A
lack of genotoxicity in rats, mouse, and hamster hepatocytes.
J. Toxicol. Environ. Health, 8: 121-130.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Response of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 coded chemicals.
Environ. Mol. Mutagenesis, 12, 85-154.
Mersch-Sundermann, V., Dickgiesser, N., Hablizel, U. & Gruber, B.
(1988) Examination of the mutagenicity of selected herbicides and
insecticides with the Salmonella-microsome test (Ames-Test) in
consideration of the pathogenetic potency of contaminated ground and
drinking water (Ger.). Zbl. Bakt. Hyg. B., 186, 247-260.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
U. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
NAS (National Academy of Science) (1977) An Evaluation of the
carcinogenicity of chlordane and heptachlor. Report to the Chief
Administrative Law Judge of the EPA, dated October, 1977. Submitted to
WHO by Velsicol Chemical Corp., Rosemont, Illinois, USA.
NCI (National Cancer Institute) (1977) Bioassay of heptachlor for
possible carcinogenicity. CAS.No. 76-44-8. Technical Report Series 9.
Bethesda, MD: Carcinogen Bioassay and Program Resources Branch,
Carcinogenicity Program, Division of Cancer Cause and Prevention,
NCI, National Institute of Health. DHEW Publication (NIH) 77-809.
NTP (National Toxicology Program) (1983) National Toxicology Program
Annual Plan for Fiscal Year 1983. NTP-82-119. Chemical Test Results
for Mutagenicity in Salmonella Assays in FY 1982. US Department of
Health and Human Services.
NTP (National Toxicology Program) (1985) National Toxicology Program
Annual Plan for Fiscal Year 1985. NTP-85-055. Chemical Test Results
for Cytogenetic Effects in Chinese Hamster Ovary Cells in FY 1984. US
Department of Health and Human Services.
Podowske, A.A., Banerjee, C.C., Feroz, M., Dudek, M.A., Willey, &
R.L., Khan, M.A.Q. (1979) Photolysis of heptachlor and
cis-chlordane and toxicity of their photoisomers to animals. Arch.
Environ. Contam. Toxicol. 8: 509-518.
Polishuk, Z.W., Ron, M, Wassermann, M., Cucos, S., Wassermann, D., &
Lemesch, C. (1977a) Pesticides in people. Organochlorine compounds in
human blood plasma and milk. Pestic. Nonit. J. 10(4): 121-129.
Polishuk, Z.W., Wassermann, D., Wassermann, D., Cucos, S. & Ron, M.
(1977b) Organochlorine compounds in mother and fetus during labor.
Environ. Res. 13: 278-294.
Probst, G.S., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K., &
Neal, S.B. (1981) Chemical-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ Mutat. 3: 11-32.
Radomski, J.L. & Davidow, B. (1953) The metabolite of heptachlor, its
estimation, storage and toxicity. J. Pharmacol. Exp. Therap. 107:
266-272.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21,
319-334.
Ruch, R.J., Fransson, R., Flodstrom, S., Warngard, L. & Klaunig, J.E.
(1990) Inhibition of hepatocyte gap junctional intercellular
communication by endosulfan, chlordane and heptachlor.
Carcinogenesis, 11, 1097-1101.
Sandhu, S.S., Ma, T-H., Peng, Y., & Zhou, X. (1989) Clastogenicity
evaluation of seven chemical commonly found at hazardous industrial
waste sites. Mutat. Res., 224, 437-445.
Savage, E.P., Keefe, T.J., Tessari, J.D., et al. (1981). National
study of chlorinated hydrocarbon insecticide residues in human milk.
USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor
epoxide, chlordane, oxychlordane, and mirex. Am. J. Epidemiol.
113(4): 413-422.
Seiler, J.P. (1977) Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short-term test. Mutat. Res., 46, 305-310.
Sherman, M. & Ross, E. (1961) Toxicol. App. Pharmacol., 3: 521.
Shindell and Associates (1981) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant Memphis, Tennessee,
January 1955-December 1979. Unpublished Report by Shendell and
Associates, Milwaukee, Wisconsin. Submitted to WHO by Velsicol
Chemical Corp., Rosemont, Illinois, USA.
Simmon, V.F., Kauhanen, K. & Tardiff, R.G. (1977) Mutagenic activity
of chemicals identified in drinking water. Dev. Toxicol. Environ.
Sci., 2, 249-258.
Stehr-Green, P.A., Wohlleb, J.C., Royce, W., & Head, S.L.(1988) An
evaluation of serum pesticide residue levels and liver function in
persons exposed to dairy products contaminated with heptachlor.
J. Am. Med. Assoc. 259(3): 374-377.
Strassman, S.C. & Kutz, F.W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi 1973-1974. Pesticide Monit. J.
10(4):130-133.
Takahashi, W., Saidin, D., Takei, G., & Wong, L. (1981)
Organochloride pesticide residues in human milk in Hawaii 1979-1980.
Bull. Environ. Contam. Toxicol. 27: 506-511.
Takei, G.H., Kauahikaua, S.M., & Leong, G.H. (1983) Analyses of human
milk samples collected in Hawaii for residues of organochlorine
pesticides and polychlorobiphenyls. Bull. Environ. Contam. Toxicol.
30: 606-613.
Tashiro, S. & Matsumura, F. (1978) Metabolism of trans-nonachlor
and related chlordane components in rat and man. Arch. Environ.
Contam. Toxicol. 7: 113-127.
Telang, S., Tong, C., & Williams, G.M. (1982) Epigenetic membrane
effects of possible tumour-promoting type on cultured liver cells by
the nongenotoxic organochlorine pesticide chlordane and heptachlor.
Carcinogenesis, 3(10): 1175-1178.
Velsicol Corporation (1959) Unpublished report.
Wang, H.H. & MacMahon, B. (1979a) Mortality of workers employed in
the manufacture of chlordane and heptachlor. J. Occup. Med. 21(11):
746-748.
Wang, H.H. and MacMahon, B. (1979b). Mortality of pesticide
applicators. J. Occup. Med. 21(11): 741-744; (11):746-748.
Wazeter, F.X., Goldenthal, E.I., Geil, R.G., Howell, D.G., & Dean,
W.P. (1971a) 30-Day range-finding study in mice. Unpublished Report
from International Research and Development Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., Cookson, K.M. & Howell,
D.G. (1971b) Two-year oral study in beagle dogs. Unpublished Report
No. 163-048 from International Research and Development Corp.
Submitted to WHO by Velsicol Chemical Co., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., & Howell, D.G. (1971c)
Two-generation reproduction and teratology study in beagle dogs.
Unpublished Report No.163-048 from International Research and
Development Corp. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Wazeter, F.X., Goldenthal, E.I., & Geil, R.G. (1973) Eighteen-month
oral carcinogenic study in mice. Unpublished Report No. 163-084 from
International Research and Developmental Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA
Witherup, S., Cleveland, F.P., Shaffer, F.E., Schlecht, H., & Musen,
L. (1955) The physiological effects of the introduction of heptachlor
into the diet of experimental animals in varying levels of
concentration: Report of experiment No.2. Unpublished report from The
Kettering Laboratory, College of Medicine, University of Cincinnati,
Ohio. Submitted to WHO by Velsicol Chemical Corp., Rosemont, Illinois,
USA.
Witherup, S., Cleveland, F.P., Stemmer, K.L., & Schlecht, H. (1958)
The physiological responses of beagle dogs to the consumption of diets
containing various concentrations of heptachlor epoxide over a period
of sixty weeks. Unpublished report from The Kettering Laboratory,
College of Medicine, University of Cincinnati, Ohio. Submitted to WHO
by Velsicol Chemical Corp., Rosemont, Illinois, USA.
Witherup, S., Cleveland, F.P., & Stemmer, K. (1959) The physiological
effects of the introduction of hepatochlor epoxide in varying levels
of concentration into the diet of CFN rats. Unpublished report from
the Kettering Laboratory, College of Medicine, University of
Cincinnati, Ohio. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III. Results from
the testing of 255 chemicals. Environ. Mutagenesis, 9(Suppl. 9):
1-11.
Table 1. Acute toxicity data of heptachlor and heptachlor epoxide
Species Sex Route LD50 Reference
(mg/kg bw)
A. Heptachlor
Rat M oral 71 Podowski et al. (1979)
M&F oral 60-142a Velsicol Corp. (1959)b
Chick d oral 63 Sherman & Ross (1961)b
Mouse d oral 70 Gak et al. (1976)
Hamster d oral 100 Gak et al. (1976)
Rat M dermal 195c Gaines (1969)
F dermal 250c Gaines (1969)
B. Heptachlor epoxide
Rat M&F oral 34-48a Velsicol Corp. (1959)b
Mouse M oral 32-48 Velsicol Corp. (1959)b
a Sex differences
b Data excerpted from the 1966 JMPR monograph (Annex 1, 7)
c Xylene was the solvent.
d Not stated
Short-term studies
Mouse
Groups of 10 Charles River CD-1 mice/sex/dose were fed a mixture
of heptachlor-heptachlor epoxide (25:75) at dietary concentrations of
1, 5, 10, 25, and 50 mg/kg for 30 days. Following 30 days of
treatment, the animals were sacrificed and necropsied. Microscopic
examinations were conducted on liver tissue.
No deaths occurred in the 1, 5, nor 10 mg/kg groups. One female
died in the 25 mg/kg group, and 9 males and 8 females died in the 50
mg/kg group. No marked differences were seen between the treated
animals and the controls with respect to body weights and food
consumption. Mice which were sacrificed at the end of the study from
the 10, 25 and 50 mg/kg groups showed liver enlargement associated
with accentuated lobulation. The liver weight of the only surviving
male mouse from the 50 mg/kg group was markedly increased (control
mean: 1.69 g; 50 mg/kg; 4.00 gm), and increased mean liver weight was
also found in the 25 ppm male mice (control, 1.69 g; 25 ppm, 4.35 gm).
Histopathology findings indicated that livers of male mice in the 5,
10, 25, and 50 ppm and of female mice in the 10, 25, and 50 ppm groups
had enlargement of centrilobular and midzonal hepatocytes with
replacement of the normal coarse cytoplasmic granularity by finely
granular and homogeneous cytoplasm. The severity of this lesion was
dose-related. The incidences of the microscopic findings were not
reported (Wazeter et al., 1971a). Based upon the histopathology
findings, the NOAEL was 1 mg/kg.
Rat
Two short-term studies on heptachlor in rats were summarized in
the 1971 monograph. The dietary levels tested in these two studies
ranged from 5 to 45 ppm. At 10 ppm and above, rat liver cells showed
an increase in smooth endoplasmic reticulum and mitochondria. The
liver effects regressed after the treated rats were placed on a
control diet for 120 days (Annex 1, 15).
Long-term studies
Some of the unpublished long-term studies on heptachlor were
evaluated by the 1966 Joint Meeting and published in the 1967
Monograph. To facilitate consideration of the toxicity of this
chemical the older toxicity studies are also summarized here.
Rat
Groups of Carworth Farms (CF) rats (20/sex/dose) were fed diets
containing heptachlor (purity not specified) at a concentration of 0,
1.5, 3.0, 5.0, 7.0, or 10 ppm for 110 weeks. The treatment diet was
prepared by spraying standard rat diet with an ethanol solution of
heptachlor. After 7 weeks on the test diet, 5 females and 5 males
from each dose group were mated to determine whether or not heptachlor
would affect the reproductive capacity of the treated animals or the
survival and subsequent growth of the offspring. Following similar
procedures, another mating was conducted using different animals from
each dose group after 22 weeks of treatment. The offspring were kept
with their mothers until weaning at 3 weeks of age. After weaning,
the offspring were placed on the control diet for 8 weeks for further
observations. After 110 weeks of treatment, the surviving animals
were sacrificed, certain haematological parameters were examined.
Histopathology was conducted on the heart, liver, lungs, brain,
spleen, kidneys, thyroids, and the adrenal glands.
Heptachlor treatment did not affect mortality, body weight, or
food consumption. The limited reproductive parameters measured did
not indicate any effect on the body weights at birth or at weaning.
Heptachlor administration did not appear to significantly affect the
mortality of the offspring. Organ weights of the heptachlor-treated
rats were not markedly different from those of controls. Haematology
parameters (erythrocyte counts, haemoglobin, leukocyte counts, and
differential white cell counts) did not show any compound-related
effect. The histopathology data indicated 6/16 males and 3/18 females
at 7.0 ppm and 2/12 males and 9/16 females at 10 ppm had slight
hepatic cellular alterations characterized by swelling, homogeneity of
the cytoplasm and peripheral arrangement of the cytoplasmic granules
in the hepatic cell in the central zone of the lobules. Under the
conditions of this study, heptachlor at doses up to 10 ppm did not
produce an increase in the tumour incidence in CF rats (Witherup
et al., 1955).
Groups of CFN (Carworth Farms, Nelson) rats (25/sex/dose) were
fed heptachlor epoxide at dietary concentrations of 0, 0.5, 2.5, 5.0,
7.5, and 10.0 ppm for 108 weeks. The test diet was prepared by
spraying an ethanol solution of heptachlor epoxide on Purina
Laboratory Chow.
The survival rate was greater than 47% in the treated and control
groups. There was an increase in the liver weights of treated females
and a slight increase in treated males. The test chemical was not
reported to cause any additional effects. This study has
methodological deficiencies which include uncertain concentrations in
the feed and insufficient number of rats in each dose level. Also the
report does not contain histopathological data and is therefore not
adequate for evaluating the long-term toxicity of heptachlor (Witherup
et al., 1959). However, a panel of independent pathologists
convened by the US National Academy of Sciences (1977) reviewed the
histopathology and concluded that there was no increase in liver
tumours in treated rats.
Four groups of Charles River CD female rats (25/dose) were fed a
3:1 mixture of heptachlor:heptachlor epoxide at dietary concentrations
of 5, 7.5, 10, and 12.5 ppm. A control group of 54 females was fed a
diet containing the vehicle, corn oil. Interim sacrifices were
conducted at 5 and 19 months to determine the nature and degree of the
histological changes in the liver. At 5 months, 3 controls and 5 rats
from the 7.5 ppm group were sacrificed. At 19 months, 5 controls, 2
rats from 5 ppm, 2 rats from 10 ppm, and 1 rat from 12.5 ppm were
sacrificed. After 24 months of treatment, the surviving rats were
sacrificed, and gross and histological examinations were conducted.
There was an increase in mortality at 12.5 ppm relative to that
of controls (control, 21%; 12.5 ppm, 50%). The test article did not
affect body weight gain. Liver changes which were described as fatty
liver and enlarged cells in the central zone of the hepatic lobules
were reported. However, no histopathology data were included in the
report (Jolley, 1966).
Groups of Osborne-Mendel rats (50/sex/dose) were fed technical
grade heptachlor (73% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at time-weighted doses of 38.9 and 77.9
ppm for males and 25.7 and 51.3 ppm for females. The matched controls
consisted of 10 males and 10 females, and pooled controls were 60
rats/sex. The treated animals received the chemical for 80 weeks and
were observed for 30 weeks. During the 80 weeks of treatment, the
dietary concentrations changed three times for each treatment group
based on changes in body weight.
There was a decrease in the mean body weight of high dose males
which almost returned to the control level after the treatment was
terminated. There was also a slight increase in mortality in high
dose male and female rats. Histopathology data revealed numerous
inflammatory, degenerative, and proliferative lesions with
approximately equal frequencies in treated and control animals. There
was an increased incidence of thyroid follicular cell neoplasms,
including combined adenomas and carcinomas in the high dose female
rats (high dose, 14/38; low dose, 3/43; matched controls, 1/9;
p<0.001) while, in male rats, a non-significant increase was observed
only at the low dose (high dose, 3/38; low dose, 9/38; matched
control, 1/8). At the same time, the incidences of the thyroid C-cell
neoplasms decreased in high dose female rats (high dose, 3/38; low
dose, 7/43; matched controls, 3/9; p<0.05). The results of analyses
of the thyroid lesions were ambiguous and further complicated by
variability in different experiments conducted at about the same time
with this strain of rats (NCI, 1977).
Dog
Groups of Beagle dogs (4/sex/dose) were fed heptachlor epoxide
(purity not specified) at concentrations of 0, 1, 3, 5, 7, and 10 ppm
for two years. After 2 years on test, 2 dogs/sex/dose were sacrificed
and necropsied while the other two dogs/sex/dose were maintained on
the control diet for an additional 6 months (recovery period). In
addition, the test animals in this study were also mated during the
study and employed as the P1 parental animals for a 2-generation
reproduction and teratology study.
No death nor compound-related behavioural change was seen during
the study. The test article did not affect the body weights or food
consumptions of the treated animals. The parameters for haematology
and urinalysis were comparable between treated and control dogs.
There were increases in alkaline phosphatase activities in males and
females at 3 ppm and above; these increases, in some dogs, were more
marked towards the end of the treatment period and tended to persist
throughout the recovery period. The serum albumin and total protein
levels were slightly decreased in 10 ppm male and female dogs during
the treatment and the recovery periods. An increase in the SGPT level
was also seen in 10 ppm animals during the treatment, extending into
the recovery period. After one year of treatment, the animals in 7
ppm group also showed an increase in the SGPT level which lasted into
the recovery period.
There was an increase in liver weights in 10 ppm male and female
dogs relative to those of the controls, and this increase persisted
with a slight attenuation during the recovery period. Histopathologic
examination on dogs (2 dogs/sex/dose) sacrificed at the end of the
treatment period showed an increase in the incidence of liver changes
in animals at 3.0 ppm or above. The changes were described as
enlargement and vacuolation of groups of centrilobular hepatocytes or
scattered individual hepatocytes, presence of finely granular, and a
"ground glass" appearance of the cytoplasm of large numbers of
hepatocytes. These histopathological changes were also seen in dogs
at 3 ppm and above after 6 months of recovery period. No
compound-related histopathology changes were seen in 1 ppm dogs.
Based upon the changes in biochemical parameters and the histological
changes in the liver, the NOAEL was 1 ppm (Wazeter et al., 1971b).
Although this study has certain deficiencies, including an
insufficient number of experimental animals, the results provide
useful information on the toxicity of the test compound in dogs.
Groups of 23 to 27 week old beagle dogs (2 males or 3
females/dose) were fed heptachlor epoxide at dietary concentrations of
0, 0.5, 2.5, 5.5, and 7.5 ppm for sixty weeks. The test diet was
prepared by spraying an ethyl alcohol solution of heptachlor epoxide
on the food. The experimental results were limited to clinical
observation, mortality, body weight changes, food consumption, and
organ weights. The limited results indicated that there was an
increase in liver weights of all the heptachlor treated male and
female dogs relative to that of the controls. For 7.5 ppm males, the
increase was approximately twice that of the controls. However, the
pathology data were not included in the report (Witherup et al.,
1958).
Mouse
Groups of Charles River CD-1 mice (100/sex/dose) received a
mixture of heptachlor/heptachlor epoxide (25%/75%) at dietary
concentrations of 0, 1.0, 5.0, and 10.0 ppm for 18 months. A positive
control group (100 mice/sex) received 2-acetaminofluorene at a dietary
concentration of 250 ppm. An interim sacrifice was performed on 10
mice/sex/dose including the positive control group. Gross pathology
and histology examinations were conducted on animals which died during
the study or were sacrificed on schedule.
There was a decrease in the number of survivors in 10 ppm male
and female mice, in 5 ppm females, and in positive control males
relative to those of the negative controls as shown in Table 2. Mean
body weights and food consumptions of the treated mice were not
affected. There were increases in the mean liver weights of males and
females in 5 and 10 ppm groups.
An increase in the incidence of hepatocytomegaly was seen in all
heptachlor-heptachlor epoxide treated mice relative to that of the
controls. The incidence of hepatic nodular hyperplasia was markedly
increased in 5 and 10 ppm males and females relative to that of the
controls. For heptachlor-treated animals, hepatoma was observed in
only one male mouse at 1 ppm (Wazeter, 1973). Based on the incidence
of hepatocytomegaly, a NOAEL was not established (LOAEL <1ppm).
The histological slides of this study were later evaluated by a
panel of pathologists convened by the Pesticide Information Review and
Evaluation Committee of the National Academy of Sciences, USA. The
results of the evaluation are presented in Table 2.
Levels of 1, 5, and 10 ppm of heptachlor-heptachlor epoxide did
not produce a statistically significant increase in the incidence of
hepatocellular carcinoma. However, at 10 ppm there was a statistically
significant increase in the incidence of combined hepatocellular
carcinomas and nodular changes (NAS, 1977).
Epstein (1976) reported a previously unpublished study conducted
by the US-FDA. Groups of C3H mice (100/sex/dose) were fed 0 or 10 ppm
of either heptachlor or heptachlor epoxide (purity unspecified) for 24
months. The liver histopathology was re-evaluated by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences. Their
results indicated a significant increase in hepatocellular carcinomas
in females given heptachlor and in both males and females given
heptachlor expoxide.
Table 2. The incidence of hepatocellular carcinomas and nodular
changes in CD-1 mice fed heptachlor-epoxide
Male Female
Group HC HC+N HC HC+N
Control 1/59 2/59 1/74 1/74
1 ppm H-HE 2/66 4/66 1/65 3/65
5 ppm H-HE 2/66 4/66 1/65 3/65
10 ppm H-HE 1/73 27/73* 4/52 16/52*
AAF 5/58 9/58 5/75 13/75
HC hepatocellular carcinomas
HC+N hepatocellular carcinomas and nodular changes
* (p<.001)
Groups of B6C3F1 mice (50/sex/dose) received technical-grade
heptachlor (72% heptachlor, 18% trans-chlordane, and 2%
cis-chlordane) in the diet at initial concentrations of 10 and 20
ppm for males and 20 and 40 ppm for females. The initial
concentrations were reduced due to adverse toxic effects, with
time-weighted average concentrations of 6 and 14 ppm for males and 9
and 18 ppm for females. The mice were treated for 80 weeks and placed
on the control diet for another 10 weeks. Matched-controls consisted
of 20 males or 10 females, and the pooled controls were 100 males and
80 females. Under the conditions of the study, heptachlor had no
significant effects on the body weights. Survival at 90 weeks was
high: 70% of treated and control males and 60% of treated and control
females. Survival of female mice showed a significant trend towards
lower survival in treated groups compared to that in the control
group. The incidence of hepatocellular carcinomas was significantly
increased in the high-dose males and females as shown in Table 3
(NCI,1977).
A review of liver histopathology from this study by a panel of
pathologists convened by the Pesticide Information Review and
Evaluation Committee of the US National Academy of Sciences concluded
that there was a low incidence of induced hepatocellular carcinomas
(Table 3). According to this review, the incidence of hepatocellular
carcinomas was statistically increased (p<0.001) in groups of males
and females receiving heptachlor at the high dose only when combined
with the diagnosis of nodular changes (NAS, 1977).
Table 3. Incidence of proliferative lesions of the liver in B6C3F1
mice fed technical grade heptachlor
Lesion Pooled Matched Low Dose High Dose
Control Control
Males
Hepatocellular
carcinomas 17/92 5/19 11/46 34/47*
Nodular hyperplasia 3/92 1/19 9/46 6/47
Diffuse hyperplasia 3/92 0/19 1/46 3/47
Hepatocytomegaly 0/92 0/19 1/46 0/47
Females
Hepatocellular carcinomas 3/78 1/10 3/47 30/42*
Nodular hyperplasia 2/78 0/10 3/47 0/42
Diffuse hyperplasia 1/78 0/10 0/47 0/42
Hepatocytomegaly 1/78 0/10 0/47 1/42
The denominator represents the number of tissues examined microscopically.
* p<0.001 (Fisher exact test)
Reproduction studies
Dogs
Groups of 6-9 month old Beagle dogs (4/sex/dose) were fed
heptachlor epoxide at dietary concentrations of 0, 1, 3, 5, 7, and 10
ppm. When the female dogs attained an age of 14 months, they were
mated twice with male dogs from the same dose group. The females were
allowed to deliver and to nurse their pups.
For the F2 generation, 4 female and 2 male pups of the F1
generation were selected from each dose level to be the parental
animals (P2) of the F2 generation. At an approximate age of 14
months, the animals were mated, and the pregnant females were allowed
to deliver and to nurse their pups to 6 weeks of age. At this time,
the females and their pups were sacrificed.
No clinical signs or behaviour changes were seen in treated P1,
P2, or their offspring. There were no indications that body weight
or food consumption were affected by the test chemical. Whether or
not the test chemical produced any reproductive effects could not be
determined with the very limited data presented in this report. There
was a significant increase in the mortality rate of F1 pups in the 10
ppm group (control, 9/20; 10 ppm, 17/18). Only 1 male pup survived to
the scheduled sacrifice, and no female was available to serve as a P2
parental animal. There were slight increases in death rates of F2
pups at 3 and 7 ppm relative to the controls (Control, 0/4; 3 ppm,
3/8; 7 ppm, 3/8). The 5 ppm group had no pups. Haematology and
urinalysis results did not indicate any compound-related effects. Very
limited clinical biochemistry data showed that alkaline phosphatase
and/or SGPT activities in certain dogs were increased. However, the
changes in the clinical chemistry parameters could not be correlated
with pathology findings because the necropsy data on animals showing
clinical biochemistry changes were not presented in the report. The
limited necropsy data showed that, in F1 pups, 1/4 females at 7 ppm
and 3/10 males and 3/7 females at 10 ppm had pale or greasy liver.
This observation was compound-related. Based on the increase in the
mortality rate, the NOAEL was 1 ppm (Wazeter et al., 1971c).
Rat
Three reproduction studies in rats were evaluated by the JMPR in
1966 and 1970. In a 3-generation reproduction study, a group of 80
rats were given 6.9 mg/kg of heptachlor daily for 3 months prior to
mating. Cataracts, which became obvious between the 19th and 26th
days, were found in 6.8% of the young. A similar lesion was present
in 15.2% of the parental animals after 9 months. The only effect on
reproduction was a decrease in the litter size (Annex 1, 7). In two
other reproduction studies in rats, dietary levels of heptachlor
ranging from 0.3 to 10 ppm were tested; no cataracts nor adverse
effects on fertility and reproduction were found. A slight increase
in post-natal mortality of pups was seen at 10 ppm (Annex 1, 15).
Special studies for teratology and embryotoxicity
Rabbit
A rabbit teratology study was previously evaluated by the 1970
Joint Meeting, and it was concluded that no teratogenic effects could
be attributed to the compound (Annex 1, 15).
Special studies on genotoxicity
In a large number of genotoxicity assays (Table 4), heptachlor
did not induce genetic damage. Exceptions were the induction of small
numbers of mutations in bacteria in the presence of plant extracts,
mutations and a chromosomal aberrations in plants and in a single
study, mutations at the highly sensitive tk locus in mouse lymphoma
cells. Gap-junctional intercellular communication was inhibited in
several in vitro assays.
In both a sister chromatid exchange assay and a chromosome
aberration assay in Chinese hamster ovary cells, there were "positive"
results in the presence of rat S9 metabolic activation. In the absence
of metabolic activation the chromosome aberration assay was negative
(NTP, 1985). However, the tests were not repeated to confirm the
positive results.
The mutagenic potential of heptachlor epoxide was also tested.
The results are presented in the following table.
Table 4. Results of genotoxicity assays on heptachlor
Test system Test object Concentration Resultsa Reference
Ames test S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
TA1535, TA1536
TA1537, TA1538
Ames test S. typhimurium 2500 µg/ml -/0 Simmon et al. (1977)
TA98, TA100,
TA1535,
TA1537, TA1538
Ames test S. typhimurium not stated -/- Probst et al. (1981)
TA98, TA100, TA1535,
TA1537, TA1538, G46,
C3076, D3052
E. coli WP2 & WP2 uvr A-
Ames test S. typhimurium 0.1 µg/plate -/- NTP (1983)
TA98, TA100, TA1535, to 10 mg/plate
TA1537
Ames test S. typhimurium 5-10 µg/ml +/-b Gentile et al. (1982)
TA98, TA100, TA1535
Ames test S. typhimurium 2500 µg/ml -/- Moriya et al. (1983)
TA98, TA100, TA1535,
TA1537, TA1538
E. coli WP2 hcr
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Rec assay B. subtilis 356 µg/ml -/- Matsui et al. (1989)
Differential E. coli WP 2 2000 µg/ml 0/- Rashid & Mumma (1986)
toxicity S. typhimurium
TA1538
Ames test S. typhimurium 1 mg/ml -/- Mersch-Sundermann (1988)
TA97, TA98, TA100,
TA102
Ames test S. typhimurium 167 µg/ml -/- Zeiger et al. (1987)
TA98, TA100,
TA1535, TA1537
Gene conversion S. cerevisia f -/- Gentile et al. (1982)
Mutation Zea mays 1.12 kg/ha 0/+ Gentile et al. (1982)
Chromosomal Lens sp. 0.1-0.3% 0/+ Jain (1988)
aberrations Pisum sp.
Micronuclei Tradescantia 1.88 ppm 0/+ Sandhu et al.
(1989)
SLRSc mutation D. melanogaster 5 µg/ml (feeding 0/- Benes & Sram
solution) (1969)
tk locus Mouse lymphoma 25 µg/ml 0/- McGregor et al.
L5178Y mutation cells (1988
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
ARL-HGPRT Adult rat liver 10-6 to 10-4 M -/NAd Telang et al.
epithelial cell line (1982)
Unscheduled DNA Mouse, rat and hamster 10-5 - Maslansky & Williams (1981)
synthesis assay primary hepatocytes
Primary liver cell
explants from rats 10 nmole/ml - Probst et al. (1981)
SV-40 transformed human
fibroblasts 100 & 1000 µM ?/- Ahmed et al. (1977)
(VA-4)
Dominant Swiss mice 4.8 & 24 mg/kg (IP) - Epstein et al. (1972)
lethal 5 & 10 mg/kg (PO)
CD-1 mce 7.5 & 15 mg/kg - Arnold et al. (1977)
25:75e
Inhibition of Mouse testicular cells 40 mg/kg 0/- Seiler (1977)
DNA synthesis in vivo
Inhibition of Chinese hamster V79 10 µg/ml 0/+ Kurata et al. (1982)
metabolic cells
cooperation
Table 4 (contd).
Test system Test object Concentration Resultsa Reference
Inhibition of Rat hepatocytes 20 µg/ml 0/+ Ruch et al. (1990)
metabolic Mouse hepatocytes
cooperation
a with S9 activation/without S9 activation; phi indicates that the test was not performed
b with plant S9
c sex-linked recessive lethal
d NA = not applicable
e 25:75 blend w/w of heptachlor:heptachlor-epoxide
f concentration in original paper cited as "an appropriate amount".
Table 5. Results of genotoxicity assays on heptachlor epoxide
Test system Test object Concentration Resultsa Reference
Ames assay S. typhimurium 1000 µg/plate -/- Marshall et al. (1976)
Unscheduled DNA SV-40 transformed 100 & 1000 µM +/- Ahmed et al. (1977)
synthesis assay human fibroblasts
(VA-4)
Dominant lethal Swiss mice 6 & 30 mg/kg (IP) - Epstein et al. (1972)
8 mg/kg (PO)
a with S9 activation/without S9 activation
Observations in humans
In 1986, a study was conducted in the Midwest United States on a
group of 45 dairy farm family members who had consumed undiluted raw
milk products known to have been contaminated with heptachlor at
concentrations as high as 89.2 ppm (fat basis). The serum levels of
heptachlor or its metabolites in the exposed individuals were compared
to those from an unexposed group of 94 persons from the same
geographical area and to results from the Second National Health and
Nutrition Examination Survey (2nd NHANES). The exposed group had
significantly higher mean levels of primary metabolites such as
heptachlor expoxide (0.84±1.0 vs 0.50±0.9 ppb) and oxychlordane
(0.71±0.8 vs 0.49±1.1 ppb) than the unexposed group. In addition, the
percentage of individuals in exposed group with elevated serum
concentrations of these same metabolites (21.2%) was higher than that
for the unexposed group (heptachlor expoxide, 3.8%; oxychlordane,
6.3%) and the second National Health and Nutrition Survey (2.5% for
both metabolites) (Stehr-Green et al., 1988).
There is evidence of transplacental transfer of heptachlor
epoxide. The following heptachlor epoxide levels were detected in
various tissues of mother and newborn infants (Polishuk, 1977a):
adipose tissue (maternal): 0.2856 ± 0.3950 ppm
maternal blood: 0.2798 ± 0.4626 ppm
uterine muscle: 0.4895 ± 0.5086 ppm
fetal blood: 0.9959 ± 0.9458 ppm
placenta: 0.5000 ± 0.3950 ppm
amniotic fluid: 0.6730 ± 1.1645 ppm
Several investigators detected heptachlor epoxide levels ranging
from non-detectable to 0.46 ppm in human milk (Kroger, 1972; Polishuk
et al., 1977b; Strassman & Kutz, 1977; Savage et al., 1981;
Takahashi et al., 1981; Takei et al., 1983).
No adverse effects on human fetal development were reported
following ingestion of milk containing heptachlor for 27 to 29 months
among women of child bearing age in Oahu, Hawaii, USA. The levels of
heptachlor in milk fat ranged from 0.12 to 5.00 ppm (Le Marchand
et al., 1986).
A retrospective mortality study was conducted on workers employed
in the manufacture of heptachlor and chlordane between 1946 and 1976.
The study group consisted of 1403 white males who were employed for
more than 3 months at either of the two plants which produced
heptachlor and chlordane in the United States. The mortality
information was obtained from the Social Security Administration files
and supplemented by information collected by another investigator
through follow-up. The results indicated an excess of death from
cerebrovascular disease (17 observed, 9.3 expected) (Wang & MacMahon,
1979a).
The mortality of 16 126 males employed as pesticide applicators
was evaluated. The cohort was employed between 1967 and 1976 by any
of the nationwide (US) pest control companies for 3 months or more.
The information on mortality was traced in Social Security
Administration files. The results did not demonstrate a significant
difference in mortality from the norm in the cohort (Wang & MacMahon,
1979b). An update of the above study group was conducted by following
the same cohort to the end of 1984. The analysis showed an excess of
lung cancer among the pesticide applicators. However, these
applicators were exposed to a broad spectrum of pesticides, and the
increased lung cancer risk among the pesticide applicators might not
relate to their exposure to heptachlor alone (MacMahon et al.,
1987).
A retrospective mortality study of 2141 workers from 4
manufacturing plants of chlorinated hydrocarbon pesticides, which
included chlordane, heptachlor, DDT, aldrin, dieldrin, and endrin was
reported by Ditraglia et al., 1981. The workers in each plant were
considered as a separate cohort, each had worked at least 6 months
prior to 31 December 1964. The results showed too few deaths to allow
any meaningful conclusions (Ditraglia et al., 1981).
Another retrospective study was conducted by Shindell and
Associates (1981) on a cohort of 1115 employees who worked 3 months or
more at the Memphis, Tennessee, USA, plant of Velsicol Chemical Corp.,
from 1 January 1952 to 31 December 1979. The plant began to
manufacture heptachlor in 1952. The data on morbidity and mortality
were obtained for 93.1% of the male and 90% of the female cohort. The
workers were classified according to their job or product exposure.
Mortality data on the cohorts were compared to the overall mortality
experience of like segments (by age and sex) of the United States
population at large to determine whether Memphis plant exposure caused
any discernible variations from the expected mortality from all causes
and from selected significant causes. No significant difference was
found.
COMMENTS
Heptachlor is structurally similar to chlordane. An ADI of
0.0005 mg/kg bw was estimated for heptachlor by JMPR in 1970.
Heptachlor is well absorbed through the GI tract and metabolized to a
large extent to heptachlor epoxide and minor metabolites. Heptachlor
epoxide has been shown to accumulate in adipose tissue, and to cross
the placenta, but it was not found in the brain. In rats, the major
route of elimination is via the faeces.
In both short- and long-term studies in dogs, rats, and mice, the
liver was found to be the target organ. In a 30-day feeding study in
mice at dietary concentrations of 0, 1, 5, 10, 25 or 50 ppm,
histopathology findings indicated that males at 5 ppm or above and
females at 10 ppm or above had enlargement of centrilobular and
midzonal hepatocytes. The severity was dose-related. Based upon
these results, the NOAEL was 1 ppm, equivalent to 0.15 mg/kg bw/day.
Several long-term/carcinogenicity studies in rats were reviewed,
but the majority of them had severe methodological limitations. In
one study, rats received time-weighted dietary concentrations of 39 or
78 ppm (males) and 26 or 51 ppm (females) of heptachlor for 80 weeks.
On the basis of decreased body-weight in high-dose males and increases
in mortality in high-dose males and females, the NOAEL was 26 ppm,
equivalent to 1.3 mg/kg bw/day. Deficiencies in this study precluded
proper evaluation of the carcinogenic potential of heptachlor in rats.
In a 2-year feeding study, dogs fed heptachlor epoxide at dietary
concentrations of 0, 1, 3, 5, 7 or 10 ppm, exhibited an increase in
liver weight at 10 ppm and an increase in the incidence of liver
histopathological changes at 3 ppm and above. The histopathological
changes were enlargement and vacuolation of centrilobular or scattered
hepatocytes. Similar histological changes persisted through six
months of the recovery period. The NOAEL based upon these findings
was 1 ppm, equivalent to 0.025 mg/kg bw/day.
Heptachlor/heptachlor epoxide is carcinogenic in mice; two
studies demonstrated an increase in liver tumour incidence in male and
female Charles River CD-1 and B6C3F1 mice.
Heptachlor/heptachlor epoxide also caused a marginal increase in
the incidence of hepatocytomegaly in all treated CD-1 mice; a NOAEL
was not established in this study.
In a 2-generation reproduction study in dogs at dietary
concentrations of 1, 3, 5, 7, or 10 ppm of heptachlor epoxide, there
was an increase in mortality of F2 pups at 3 ppm and above. The
NOAEL based on this finding was 1 ppm.
The available epidemiology studies have not shown a clear
relationship between any effects in humans and exposure to heptachlor.
After reviewing the available in vitro and in vivo short-term
test data, it was concluded that, although it can interfere with
intercellular communication, heptachlor is not genotoxic.
Many studies available for evaluation were conducted more than 20
years ago and have severe deficiencies. Because of these
deficiencies, its carcinogenicity in mice and its ability to
bioaccumulate, the Meeting recommended that heptachlor should not be
used directly on food crops and its use in the production of food
commodities should be phased out. Because of its environmental
persistence it is found as a contaminant in food commodities. The
Meeting therefore maintained an ADI, basing it on the NOAELs derived
from studies in dogs. However, recognizing the inadequacy of the data
base, the Meeting increased the safety factor to 200-fold. This ADI
will provide a guideline for assessing the significance of dietary
exposure to heptachlor residues.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 26 ppm, equivalent to 1.3 mg/kg bw/day
Dog: 1 ppm, equivalent to 0.025 mg/kg bw/day
(reproduction study)
1 ppm, equivalent to 0.025 mg/kg bw/day (2-year study)
Estimate of acceptable daily intake for humans
0-0.0001 mg/kg bw
REFERENCES
Ahmed, F.E., Hart, R.W., & Lewis, N.J. (1977) Pesticide induced DNA
damage and its repair in cultured human cells. Mutat. Res. 42:
161-174.
Arnold, D.W., Kennedy, G.L., Jr., Keplinger, M.L., Calandra, J.C., &
Calo, C.J. (1977) Dominant lethal studies with technical chlordane,
HCS-3260, and heptachlor:heptachlor epoxide. J. Toxicol. Environ.
Health., 2: 547-555.
Benes, V. & Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind. Med., 38, 442-444.
Davidow, B. & Radomski, J.D. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265.
Ditraglia, D., Brown, D.P., Namekata, T., & Iverson, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health. 7
(Supplemental 4): 140-146.
Epstein, S.S. (1976) Carcinogenicity of heptachlor and chlordane.
Sci. Total Environ., 6(2): 103-154.
Epstein, S.S., Arnold, E., Andrea, J., Bass, W., & Bishop, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. Appl. Pharmacol. 23: 288-325
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14:514-534
Gak, J.C., Grillot, C., & Truhaut, R. (1976) Use of the golden
hamster in toxicology. Lab. Animal Sci. 26(2): 274-280.
Gentile, J.M., Gentile, G.J., Bultman, J., Sechriest, R., Wagoner,
E.D. & Plewa, M.J. (1982) An evaluation of the genotoxic properties
of insecticides following plant and animal activation. Mutat. Res.,
101: 19-29.
Jain, A.K. (1988) Cytogenic effects of heptachlor on Lens and
Pisum species. Geobios, 15, 61-69.
Jolley, W.P., Stemmer, K. L., & Jolley, L. (1966) The effects of
feeding diets containing a mixture of heptachlor and heptachlor
epoxide to female rats for two years. Unpublished report by The
Kettering Laboratory, University of Cincinnati. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Kroger, M. (1972) Insecticide residues in human milk. J. Pediatr.
80: 401-405.
Kurata, M., Hirose, K. & Umeda, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73, 217-227.
Le Marchand, L., Kolonel, L.N., Siegel, B.Z., & Dendle, W.H., III
(1986) Trends in birth defects for a Hawaiian population exposed to
heptachlor and for the United States. Arch. Environ. Health, 41(3):
145-148.
MacMahon, B., Monson, R.R., Wand, H.H., & Zheng, T. (1987) A second
follow-up of mortality in a cohort of pesticide applicators. A report
to Velsicol Chemical Corp., dated June 5, 1987. Submitted to WHO by
Velsico Chemical Corp., Rosemont, Illinois, USA.
Marshall, T.C., Dorough, H.W., & Swim, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. Agri. Food Chem., 24(3): 560-563.
Maslansky, C.J., & Williams, G.M. (1981) Evidence for an epigenetic
mode of action in organochlorine pesticide hepatocarcinogenicity: A
lack of genotoxicity in rats, mouse, and hamster hepatocytes.
J. Toxicol. Environ. Health, 8: 121-130.
Matsui, S., Yamamoto, R. & Yamada, H. (1989) The Bacillus
subtilis/microsome rec assay for the detection of DNA damaging
substances which may occur in chlorinated and ozonated waters. Water
Sci. Technol., 21, 875-887.
McGregor, D.B., Brown, A., Cattanach, P., Edwards, I., McBride, D.,
Riach, C. & Caspary, W.J. (1988) Response of the L5178Y tk+/tk-
mouse lymphoma cell forward mutation assay: III. 72 coded chemicals.
Environ. Mol. Mutagenesis, 12, 85-154.
Mersch-Sundermann, V., Dickgiesser, N., Hablizel, U. & Gruber, B.
(1988) Examination of the mutagenicity of selected herbicides and
insecticides with the Salmonella-microsome test (Ames-Test) in
consideration of the pathogenetic potency of contaminated ground and
drinking water (Ger.). Zbl. Bakt. Hyg. B., 186, 247-260.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
U. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
NAS (National Academy of Science) (1977) An Evaluation of the
carcinogenicity of chlordane and heptachlor. Report to the Chief
Administrative Law Judge of the EPA, dated October, 1977. Submitted to
WHO by Velsicol Chemical Corp., Rosemont, Illinois, USA.
NCI (National Cancer Institute) (1977) Bioassay of heptachlor for
possible carcinogenicity. CAS.No. 76-44-8. Technical Report Series 9.
Bethesda, MD: Carcinogen Bioassay and Program Resources Branch,
Carcinogenicity Program, Division of Cancer Cause and Prevention,
NCI, National Institute of Health. DHEW Publication (NIH) 77-809.
NTP (National Toxicology Program) (1983) National Toxicology Program
Annual Plan for Fiscal Year 1983. NTP-82-119. Chemical Test Results
for Mutagenicity in Salmonella Assays in FY 1982. US Department of
Health and Human Services.
NTP (National Toxicology Program) (1985) National Toxicology Program
Annual Plan for Fiscal Year 1985. NTP-85-055. Chemical Test Results
for Cytogenetic Effects in Chinese Hamster Ovary Cells in FY 1984. US
Department of Health and Human Services.
Podowske, A.A., Banerjee, C.C., Feroz, M., Dudek, M.A., Willey, &
R.L., Khan, M.A.Q. (1979) Photolysis of heptachlor and
cis-chlordane and toxicity of their photoisomers to animals. Arch.
Environ. Contam. Toxicol. 8: 509-518.
Polishuk, Z.W., Ron, M, Wassermann, M., Cucos, S., Wassermann, D., &
Lemesch, C. (1977a) Pesticides in people. Organochlorine compounds in
human blood plasma and milk. Pestic. Nonit. J. 10(4): 121-129.
Polishuk, Z.W., Wassermann, D., Wassermann, D., Cucos, S. & Ron, M.
(1977b) Organochlorine compounds in mother and fetus during labor.
Environ. Res. 13: 278-294.
Probst, G.S., McMahon, R.E., Hill, L.E., Thompson, C.Z., Epp, J.K., &
Neal, S.B. (1981) Chemical-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: A comparison with bacterial
mutagenicity using 218 compounds. Environ Mutat. 3: 11-32.
Radomski, J.L. & Davidow, B. (1953) The metabolite of heptachlor, its
estimation, storage and toxicity. J. Pharmacol. Exp. Therap. 107:
266-272.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21,
319-334.
Ruch, R.J., Fransson, R., Flodstrom, S., Warngard, L. & Klaunig, J.E.
(1990) Inhibition of hepatocyte gap junctional intercellular
communication by endosulfan, chlordane and heptachlor.
Carcinogenesis, 11, 1097-1101.
Sandhu, S.S., Ma, T-H., Peng, Y., & Zhou, X. (1989) Clastogenicity
evaluation of seven chemical commonly found at hazardous industrial
waste sites. Mutat. Res., 224, 437-445.
Savage, E.P., Keefe, T.J., Tessari, J.D., et al. (1981). National
study of chlorinated hydrocarbon insecticide residues in human milk.
USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor
epoxide, chlordane, oxychlordane, and mirex. Am. J. Epidemiol.
113(4): 413-422.
Seiler, J.P. (1977) Inhibition of testicular DNA synthesis by
chemical mutagens and carcinogens. Preliminary results in the
validation of a novel short-term test. Mutat. Res., 46, 305-310.
Sherman, M. & Ross, E. (1961) Toxicol. App. Pharmacol., 3: 521.
Shindell and Associates (1981) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant Memphis, Tennessee,
January 1955-December 1979. Unpublished Report by Shendell and
Associates, Milwaukee, Wisconsin. Submitted to WHO by Velsicol
Chemical Corp., Rosemont, Illinois, USA.
Simmon, V.F., Kauhanen, K. & Tardiff, R.G. (1977) Mutagenic activity
of chemicals identified in drinking water. Dev. Toxicol. Environ.
Sci., 2, 249-258.
Stehr-Green, P.A., Wohlleb, J.C., Royce, W., & Head, S.L.(1988) An
evaluation of serum pesticide residue levels and liver function in
persons exposed to dairy products contaminated with heptachlor.
J. Am. Med. Assoc. 259(3): 374-377.
Strassman, S.C. & Kutz, F.W. (1977) Insecticide residues in human
milk from Arkansas and Mississippi 1973-1974. Pesticide Monit. J.
10(4):130-133.
Takahashi, W., Saidin, D., Takei, G., & Wong, L. (1981)
Organochloride pesticide residues in human milk in Hawaii 1979-1980.
Bull. Environ. Contam. Toxicol. 27: 506-511.
Takei, G.H., Kauahikaua, S.M., & Leong, G.H. (1983) Analyses of human
milk samples collected in Hawaii for residues of organochlorine
pesticides and polychlorobiphenyls. Bull. Environ. Contam. Toxicol.
30: 606-613.
Tashiro, S. & Matsumura, F. (1978) Metabolism of trans-nonachlor
and related chlordane components in rat and man. Arch. Environ.
Contam. Toxicol. 7: 113-127.
Telang, S., Tong, C., & Williams, G.M. (1982) Epigenetic membrane
effects of possible tumour-promoting type on cultured liver cells by
the nongenotoxic organochlorine pesticide chlordane and heptachlor.
Carcinogenesis, 3(10): 1175-1178.
Velsicol Corporation (1959) Unpublished report.
Wang, H.H. & MacMahon, B. (1979a) Mortality of workers employed in
the manufacture of chlordane and heptachlor. J. Occup. Med. 21(11):
746-748.
Wang, H.H. and MacMahon, B. (1979b). Mortality of pesticide
applicators. J. Occup. Med. 21(11): 741-744; (11):746-748.
Wazeter, F.X., Goldenthal, E.I., Geil, R.G., Howell, D.G., & Dean,
W.P. (1971a) 30-Day range-finding study in mice. Unpublished Report
from International Research and Development Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., Cookson, K.M. & Howell,
D.G. (1971b) Two-year oral study in beagle dogs. Unpublished Report
No. 163-048 from International Research and Development Corp.
Submitted to WHO by Velsicol Chemical Co., Rosemont, Illinois, USA.
Wazeter, F.X., Geil, R.G., Goldenthal, E.I., & Howell, D.G. (1971c)
Two-generation reproduction and teratology study in beagle dogs.
Unpublished Report No.163-048 from International Research and
Development Corp. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Wazeter, F.X., Goldenthal, E.I., & Geil, R.G. (1973) Eighteen-month
oral carcinogenic study in mice. Unpublished Report No. 163-084 from
International Research and Developmental Corp. Submitted to WHO by
Velsicol Chemical Corp., Rosemont, Illinois, USA
Witherup, S., Cleveland, F.P., Shaffer, F.E., Schlecht, H., & Musen,
L. (1955) The physiological effects of the introduction of heptachlor
into the diet of experimental animals in varying levels of
concentration: Report of experiment No.2. Unpublished report from The
Kettering Laboratory, College of Medicine, University of Cincinnati,
Ohio. Submitted to WHO by Velsicol Chemical Corp., Rosemont, Illinois,
USA.
Witherup, S., Cleveland, F.P., Stemmer, K.L., & Schlecht, H. (1958)
The physiological responses of beagle dogs to the consumption of diets
containing various concentrations of heptachlor epoxide over a period
of sixty weeks. Unpublished report from The Kettering Laboratory,
College of Medicine, University of Cincinnati, Ohio. Submitted to WHO
by Velsicol Chemical Corp., Rosemont, Illinois, USA.
Witherup, S., Cleveland, F.P., & Stemmer, K. (1959) The physiological
effects of the introduction of hepatochlor epoxide in varying levels
of concentration into the diet of CFN rats. Unpublished report from
the Kettering Laboratory, College of Medicine, University of
Cincinnati, Ohio. Submitted to WHO by Velsicol Chemical Corp.,
Rosemont, Illinois, USA.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity tests: III. Results from
the testing of 255 chemicals. Environ. Mutagenesis, 9(Suppl. 9):
1-11.
