
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
HEPTACHLOR
IDENTITY
Synonyms
Velsicol 104(R), E-3314
Explanation
Residues from the use of heptachlor mostly occur as heptachlor
epoxide. As conversion to the latter rapidly occurs in the animal
body. It is considered here alongside the parent compound.
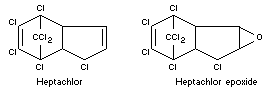
Chemical names
Heptachlor: 1,4,5,6,7,10,10-heptachloro-4,7,8,9,-tetrahydro-4,7-
endomethyleneindene (British interpretation of IUPAC rules of
nomenclature) and
1,4,5,6,7,8,8,-heptachloro-3a,4,7,7a-tetrahydro-4,7-endomethanoindene
(USA Interpretation).
Heptachlor epoxide:
2,3-epoxy-1,4,5,6,7,8,8,-heptachloro-2,3,3a,4,7,7a-hexahydro-4,7-
endomethanoindane.
Formulas
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In plants and soil, heptachlor is converted to its epoxide which is
more persistent on plants than the parent heptachlor (Gannon & Bigger,
1958; Gannon & Decker, 1958).
Rat liver microsomes have been proved to epoxidize heptachlor in
vitro. The extent of epoxidation in vivo was about 10 times
greater in males than in females (Wong & Terriere, 1965).
Heptachlor epoxide is stored in the fat of dogs add rats. Some storage
occurs in the liver, kidney and muscles but none in the brain. The
metabolite was found in dogs after feeding heptachlor in a
concentration of 1-3 mg/kg body-weight. When heptachlor was fed at
high dosage levels to dogs, small amounts of unmetabolized heptachlor
were also found in the fat, but this was not the case in the rat
regardless of the level fed (Davidow & Radomski, 1953).
In the rat, the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm,
storage occurred in females but not in males, and at 0.1 ppm no
storage occurred in either sex. Female rats accumulate about 6 times
as much epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days, the epoxide level in the milk rose to a maximum of 1.8 ppm,
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at 5 ppm and 2 at 10 ppm daily). Three days after feeding
commenced, the minimum levels of heptachlor epoxide in the milk were
0.26 and 0.34 ppm in the 2 cows fed 5 ppm, and 0.23 and 0.65 ppm in
the 2 cows fed 10 ppm; the maximum levels after 15 days' feeding were
0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively (Storherr et
al., 1960).
Investigations on heptachlor storage in human fat in several parts of
the world have recently been reported. The average ranged between 0
and 0.24 ppm; it tended to be lower in those countries in which a high
storage of DDT was recorded (Dale et al., 1965; Hayes et al., 1965;
Robinson et al., 1965; Zavon et al., 1965).
Heptachlor and its epoxide are included among the chlorinated
hydrocarbon insecticides that stimulate the metabolism of drugs by
microsomal enzymes (Remmer, 1964).
Disturbance of the acid-base balance seems to be of great significance
in the mechanism of action of heptachlor (Spynu & Osetrov, 1957).
Acute toxicity
Heptachlor
Animal Route LD50 References
mg/kg body-weight
Rat Oral 60-142* Velsicol Corporation, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 References
mg/kg body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide in the
mouse are given as 40 and 10 mg/kg body-weight respectively (Radomski
& Davidow, 1953). In the rabbit, the lethal dose of epoxide is 5-10
mg/kg body-weight. The acute effects are neurotoxic disturbances like
those observed with other chlorinated hydrocarbons (Velsicol, 1959).
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide (up to
60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleoli, cytoplasmic fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats, it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla. Electron microscopic studies
demonstrated an increase of rough and smooth endoplasmic reticulum
(Stemmer & Hamdi, 1963).
In a reproduction study covering 3 generations, a group of 80 rats was
given 6.9 mg/kg body-weight of heptachlor daily for 3 months before
mating. Cataracts were found in 6.8 per cent of the young and became
obvious between the 19th and 26th day. Among the parents, 15.2 per
cent of the animals were affected and the lesions appeared after 4-9
months. The only effect on reproduction was decrease of litter size
(Mestitzova, 1966).
The continuous exposure of rats to doses of over 7 ppm of either
heptachlor or its epoxide increased the mortality of the pups during
the suckling period. 10 ppm fed to 3 generations of rate showed no
adverse effects on reproductive capacity, growth or survival (Witherup
et al., 1955; Kettering Laboratory, 1959).
Dog. When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Three dogs given heptachlor epoxide orally in dosages of 2, 4 and 8
mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively. Daily oral doses of 0.25 and 0.5 mg/kg body-weight did
not produce any sign of illness during 52 weeks, but 0.25 mg/kg,
estimated to be equivalent to 6 ppm in the diet, was reported as the
minimal dose producing a pathological effect (Velsicol, 1959).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor epoxide were
given to groups of 5 dogs (2 males and 3 females, 23 to 27 weeks of
age) for 60 weeks. No deaths attributable to heptachlor epoxide
occurred. The weights of the male dogs tended to be inversely
proportional to the concentration of the compound in the diet. The
female dogs had normal weights. The liver weights were increased at 5
ppm and above. Degenerative liver changes were seen in only 1 dog at
7.5 ppm (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed diets
containing 5, 10, 20, 40, 80, 160 and 300 ppm of heptachlor epoxide
for 2 years. Concentrations of 80 ppm or higher resulted in 100 per
cent mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females) (Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10, 20 and
40 ppm for 2 years showed significant increases in mortality only in
females at 40 ppm. Liver weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
CFN rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5,
7.5 and 10 ppm. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34 per cent) and 13/24
(54 per cent) in the control males and females respectively; it was
65/111 (58 per cent) and 92/114 (80 per cent) in the experimental
males and females respectively. Again, many tumours were located in
endocrine organs. Liver tumours were observed in 7 males and 12
females in the experimental groups only (over-all incidence 19/225
(8.4 per cent), but only two of them were malignant (Kettering
Laboratory, 1959).
Heptachlor dissolved in ethanol was added to the diet of CF rats as
1.5, 3, 5, 7 and 10 ppm for 110 weeks. Each group, as well as an
untreated control, included 40 animals (20 of each sex). Mortality was
comparable in all groups. The number of tumour-bearing animals was
16/40 at 0 ppm, 9/40 at 1.5 ppm, 13/40 at 3 ppm, 12/40 at 5 ppm, 15/40
at 7 ppm and 12/40 at 10 ppm. Most tumours were found in the pituitary
and other endocrine organs. No liver tumours were recorded. No
preferential tumour site in any particular group was observed but all
the 4 thyroid tumours observed were in the 7 and 10 ppm groups
(Witherup et al., 1955).
In a recent experiment carried out on a total of 154 female rats, a
mixture of heptachlor/heptachlor epoxide 3:1 was added to the diet at
0, 5, 7.5, 10 and 12.5 ppm for two years. Pituitary and mammary
tumours were seen at all dose levels, including the controls; their
incidence varied from group to group but not in relation to the dose
of heptachlor. At the end of the two years, all groups including the
controls showed liver histological changes, i.e. cell enlargement in
centrolobular parts, loss of cytoplasmic granules and appearance of
lipid vacuoles. No quantitative differences could be detected between
rats given 0 and 5 ppm, whereas in the other groups the severity of
the changes was related to the dose. At 12.5 ppm, regenerative liver
changes were present (Kettering Laboratory, 1966).
Comments
It is well established that heptachlor and its epoxide accumulate in
body fat and persist there for long periods. The formation of epoxide
is relatively rapid in the animal, plants and in the soil.
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the two
substances.
A sex difference in toxicity has been observed, particularly in the
rat, females accumulating heptachlor and its epoxide more than males.
The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to explain. A
possible relation with tumour formation has not been demonstrated,
while evidence has been presented that the change is reversible.
Some suspicion of carcinogenicity could arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 5 ppm in the diet, equivalent to 0.25 mg/kg/day.
Dog. 2.5 ppm in the diet, equivalent to 0.0625 mg/kg/day
Estimate of acceptable daily intake for man
0-0.0005 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that heptachlor and its
epoxide are compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of these compounds, with a view to removing doubts
which may remain as to their safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
The pattern of use for heptachlor has been evolving since it was
introduced to agriculture in the 1950's. Up until 1960, it had a broad
scale of use on forage, cereal, oil seed, vegetable, sugar beet and
some nut crops, such as peanuts. There has been a gradual reduction of
scale and pattern of use of this insecticide for various reasons
including a desire to reduce the unintentional contamination of milk
and animal products.
The present trend in Canada, the USA and Western Europe is towards
restricting its use to vegetable and cereal seed and to applications
to the soil against insects therein. In certain countries it is also
applied to young pasture plants that will not be grazed or fed in the
season of use, to transplant water for seedlings and to ground cover
under a restricted number of orchard trees. In each case there are
restrictions concerning the access of livestock. In Japan, up until at
least 1963, large quantities of heptachlor dust have been used on
upland wheat, barley and rice.
Dosages vary between countries. The average recommended dose is 2
kilos of actual heptachlor per hectare for use on soil prior to or at
planting on cereals and legumes, including barley, green beans, lima
beans, corn, oats, rice, soybeans and wheat. To cereal seeds it is
sometimes used as a slurry at 30-45 grams per 100 kilos applied prior
to planting.
(b) Post-harvest treatments
No post-harvest use that has been reported.
* Sum of heptachlor and heptachlor epoxide by weight.
Tolerances
Canada 0.1 ppm (combined heptachlor and heptachlor epoxide) on beans,
cabbage, lettuce, rutabagas (yellow turnips). (Heptachlor is scheduled
under the Feeds Act as a compound that should not be present in
commercial feed. In 1965 the tolerance for cereals was eliminated.)
USA 0.1 in cabbage, lettuce, rutabagas and snap beans; zero for many
other foods.
Netherlands 0.1 ppm.
In Canada and USA administrative "actionable levels" have also been
established for residues of heptachlor and heptachlor epoxide in milk
and other animal products. (It was the impression of the FAO Working
Party that this practice was also in vogue in other countries, but
reliable information was not available.)
Residues resulting from supervised trials
There is a large amount of information but much of it needs reviewing
in the light of developments in analytical methods since about 1961.
Although some of the earlier work has recently been reviewed in N.
America, reliable recent results are scarce. The published literature
in languages other than English is difficult to obtain. For example,
Ishikura (personal communication, 1965) reports a large scale of use
on cereals in Japan, but information does not appear to be available
on the resulting residues.
Lichtenstein & Polvika (1959), Young & Rawlins (1958) and many other
authors publishing prior to 1961, suggested that no residues of
heptachlor would persist in soil, and that none would be translocated
from soil to plants, especially in forage crops. More reliable
analytical methods have since showed that heptachlor and its epoxide
persist in soil and can result in residues in some species of plants.
The early investigations suggested that heptachlor could be used
effectively on a wide range of vegetables, root crops, potatoes,
forage and peanuts, with residues that would in many instances not
exceed 0.1. ppm in the harvested product. Dawsey, Woodham & Lofgren
(1961) stated that no residues were detected in 14 of 16 crops tested,
but radishes and onions showed significant amounts when grown in plots
treated with 0.25 of 0.5 lb of heptachlor per acre. However,
Lichtenstein & Schulz (1965) found some build up, over a three-year
period in soil from annual application of heptachlor at useful rates.
Residues found in various crops grown in such soil were carrots up to
0.69 ppm, potatoes up to 0.27, radishes up to 0.21 and beets up to
0.29.
Information from Burrage & Saha (1976) however suggests that the use
of heptachlor for seed and soil treatments for certain cereals may be
without fear of residues occurring on harvested grain, but the
possible need for restrictions on the use of straw for animal feed and
the significance of soil residues in crop rotations require further
evaluation. Investigations on the relationships between residues in
oil seeds and certain forage crops to their oil contents, recently
reported by Bruce & Decker (1966), have been less reassuring.
It would be most useful to have the results of similar investigations,
with reliable analytical procedures from other countries. Information
pertaining to residues in successive crops grown in rotation over a
period of years after the initial treatment would be of particular
interest.
Residues in food moving in commerce
Information is slowly becoming available on residues in food moving in
commerce. Some indirect information also is available from total diet
studies, particularly in Canada and the USA. The occurrence of
residues is broadly related to the scale of use in the respective
countries. Residues have varied from traces to 1.0 ppm in up to 10
per cent of the sample diets studied. A six-month average in one USA
study indicated highest residues in these <10 per cent of diets as
meat 0.05 ppm, dairy products (fat basis) 0.025 ppm and other food
0.005 ppm, after processing for consumption.
Canadian investigations of residues in commercial lots of sugar beet
pulp suggest that heptachlor and heptachlor epoxide residues can occur
in this animal feed, which if fed in too large a quantity can result
in undesirable levels of residues occurring in animal products.
Consequently, the feeding of contaminated sugar beet pulp is
restricted to stated levels in the animals' diet. No heptachlor
residues were found in a recent survey of meat and dairy products in
Britain (Lewis, 1965).
The Members of the FAO Working Party on Pesticide Residues, were aware
that information is being obtained on residues in milk and animal
products, commercial feeds, cannery wastes and other agricultural
by-products moving in commerce but these data were not available for
evaluation. Information of this kind is important in the evaluations
of this compound and the co-operation of Member countries of FAO and
WHO is requested in making it available for review.
Fate of residue
(a) In soil
A body of information is becoming available in Canada and the USA.
The persistence of residues in soil appears to be related to soil
type, climate, soil management and cropping practices (Harris, 1966;
Duffy & Wong, 1966; Lichtenstein & Schulz, 1960, 1965a; Harris &
Lichtenstein, 1961; Wilkinson et al., 1964; Saha & Stewart, 1966).
Similar information in other countries is relatively scarce (Weise,
1964). Recent work suggests that low level soil residues may be
contributing directly to residues in animal products, particularly in
arid and semi-arid zones. In these areas, livestock are known to
ingest some soil in foraging, resulting in residues in animal products
that cannot be accounted for on the basis of residues in plants alone.
It is possible that heptachlor and heptachlor epoxide residues can be
dissipated from soil more rapidly than dieldrin or DDT (Harris &
Lichtenstein, 1961).
Bowman et al. (1965) suggested from laboratory evidence that
heptachlor was changed to 1-hydroxychlordene in dry soils with low
organic content and that no conversion of heptachlor to heptachlor
epoxide was detected in these soils. In another study under field
conditions, the same authors detected a small amount of
1-hydroxychlordene in fine sandy loam. Duffy & Wong (1966) have
reported the presence of 1-hydroxychlordene in some Canadian Atlantic
provinces soils, but Harris (personal communication) has not been able
to confirm its presence in Ontario soils. This degradation product has
not been reported as a component of residues in food. (Its presence
would be of interest since the LD50 to rats is only of the order of
2400 mg/kg.) Gamma-chlordane is also a component of residues in soil.
(b) In plants
The conversion of heptachlor to heptachlor epoxide was first reported
by Gannon & Decker (1958a, 1958b). The parameters that control the
rate of epoxidation are still obscure, but several reports suggest
that living tissue, even if only that of micro-organisms, is required
(Davidow & Radomski, 1953). The exact mechanisms by which plants
acquire residues of these compounds are are not understood either.
Evidence for translocation by plants has been produced by Gannon &
Decker (1958), Lichtenstein & Schulz (1960), Dawsey (1961),
Lichtenstein et al. (1965), etc. Special attention has been given
recently to the mechanisms of contamination and the location of these
residues within plants and their stability (Byrne & Steinhauer, 1966;
Burrage & Saha, 1966; King, Clark & Hemken, 1966; Saha & Stewart,
1966). These data are all from Canada and USA. It would be useful to
have comparable information from other sources.
Some information is also becoming available on the relationship
between the residues in soil, the oil content of plants and the
residues that can be expected in them (Bruce & Decker, 1966). This
type of information will have a special bearing on future evaluations
of residues in oil seed crops or fibre crops (soybeans, cotton,
peanuts, rape, etc.) whose by-products become animal food and may thus
lead to residues in human food.
(c) In animals
Residues in animal products have attracted special interest. Low level
residues have been detected and measured recently, in milk and dairy
products using analytical methods that were not previously available
(USA six-month average in up to 10 per cent of diets 0.025 ppm on a
fat basis, in dairy products) and in meat (USA six-month average in 10
per cent diets, 0.05 ppm). It has been suggested that loads of DDT
already stored in the body fat of animals are partially responsible
for the prevention of storage of heptachlor and heptachlor epoxide
(Street, 1966, in press) and their degradation without storage, which
could account for the observation that the loads of heptachlor epoxide
in human body fat are lower in those parts of the world in which a
higher rate of storage of DDT is recorded (Dale et al., 1965; Hayes et
al., 1965; Robinson et al., 1965; Zavon et al., 1965).
(d) In storage and processing
If tolerances are to be based on amounts ingested by consumers,
information on the behaviour of residues during storage and processing
is needed. Although residues from heptachlor have often been regarded
as stable, various data indicate that some disappearance takes place
in storage and processing. This was found in work relating to the
stability of residues in silage (unpublished information from USDA).
Work of Saha & Stewart (1966) indicates that cooking peeled turnips
results in complete disappearance of residues of heptachlor and
heptachlor epoxide in turnip flesh, using an analytical method
sensitive to 0.2 ppm. Similar results have been reported by
Lichtenstein et al. (1965) for carrots. Gooding (1966) suggested that
the normal processes of alkali-refining, bleaching, dehydrogenation
and deodorization will remove all traces from edible vegetable oils,
but definite proof has not been published. Residues do not appear in
sugar or molasses when residues occur in sugar beets, but remain in
the pulp. More information is needed on the fate of residues during
processing.
Methods of analysis
A number of multidetection systems are available for the detection and
determination of residues together with residues of a number of other
compounds. An example is the AOAC system (1966) in which acetonitrile
partition and Florisil column clean-up are used and the residues
identified and measured by gas chromatography coupled with thin layer
or paper chromatography. Alternative clean-up systems, e.g. that of de
Faubert Maunder et al. (1964) using dimethylformamide, and other
methods of confirmation of identity, e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.002 ppm of heptachlor or heptachlor epoxide in milk and
0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The potential hazard resulting from the use of heptachlor in food
production has recently been reduced. The pesticide is now only being
used on seeds and in soil employed in cereal and vegetable production.
Present evidence indicates that soil and seed treatments can be
continued without residues resulting in harvested grain. (The present
evidence pertains to wheat. It may well extend to a wider range of
cereals.)
Since the Temporary ADI is very small (0.0005 mg/kg/day), it is
recommended that a Temporary Tolerance of 0.1 ppm be established, from
uses of the insecticide in the soil and on to seed, only for raw root
vegetables (other than potatoes), cole crops, head lettuce, spinach
and other leafy vegetables. In this recommendation residues of both
heptachlor and heptachlor epoxide are to be combined within the total
of 0.1 ppm. This recommendation is based on the assumption that there
will be no losses of residue in storage and processing, including
cooking.
In addition "practical residue limits" are recommended for potatoes at
0.05 ppm; whole milk 0.002 ppm, dairy products 0.025 ppm on a fat
basis; meat 0.05 ppm (fat basis).
Additional data pertaining to these recommendations should be reviewed
at an early date.
Further information
1. Many of the measurements undertaken prior to about 1961 of residues
after treatments of crops or of soil need to be repeated with the
aid of the more sensitive analytical techniques that have since
become available.
2. More evidence is needed on the extent to which heptachlor is used
in various countries and on the residues which occur in foods in
international commerce; also on the fate of such residues during
the normal course of storage and processing, including cooking,
to which such foods are subjected before consumption.
3. Investigations are desirable on the fate of low levels of
heptachlor in feeds consumed by animals. This is because
information on commercial samples suggest that animal feeds may be
the sources of some of the albeit fairly low residues found in
products from animals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely, R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
Hayes, W. J., jr, Dale, W. E. & Burse, V. W. (1965) Life Sci., 4,
1611
Kettering Laboratory (1959) Unpublished report submitted by Velsicol
Corporation
Kettering Laboratory (1966) Unpublished report submitted by Velsicol
Corporation
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Mestitzova, M. (1966) International Congress on Occupational Health,
Proceedings, 109 (7), 455
Radomski, J. L. & Davidow, B. (1953) J. Pharmacol. exp. Ther.,
107, 266
Remmer, H. (1964) Proceedings of the European Society for the Study
of Drug Toxicity, Vol. 9
Robinson, J., Richardson, J., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Sherman, M. & Ross, E. (1961) Toxicol. appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1957) Nauch. Trudy Inst. Ent.
Fitopat. Akad. Nauk Ukr. SSR, 7, 63
Stemmer, K. L. & Hamdi, E. (1963) Unpublished report of the Kettering
Laboratory, University of Cincinnati
Stemmer, K. L. & Jolley, W. P. (1963) Unpublished report of the
Kettering Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S., Cleveland, F. F., Shaffer, F. E., Schlecht, H. & Musen,
L. (1955) Unpublished report of the Kettering Laboratory, University
of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49: 222-30
Bowman, M. C., Schechter, M. S. & Carter, R. L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. Agric.
Food Chem., 13: 360-365
Bruce, W. N. & Decker, G. C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin, dieldrin,
heptachlor, and heptachlor epoxide. J. Agr. Food Chem., 14: 395-403
Burrage, R. H. & Saha, J. G. (1965) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin or heptachlor.
Can. J. Plant Sci. (In press)
Byrne, H. D. & Steinhauer, A. L. (1966) Mechanisms of contamination of
alfalfa with heptachlor and heptachlor epoxide. J. Econ. Ent., 59:
338-341
Davidow, B. & Radomski, J. L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265
Dale, W. E., Copeland, M. L. & Hayes, W. J. (1965) Chlorinated
insecticides in the body fat of people in India. Bull. Wld Hlth Org.,
33: 477
Dawsey, L. H., Woodham, D. W. & Lofgren, C. S. (1961) Heptachlor and
heptachlor epoxide residues in truck crops. J. Econ. Ent., 54: 6,
1264-5
Duffy, J. R. & Wong, N. (1966) Insecticide residues and their
metabolites in Atlantic soils. In press. J. Agr. Food Chem.
de Faubert Maunder, M. J., Egan, H. & Godly, E. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. Analyst,
89: 168-174
Gannon, N. & Decker, G. C. (1958a) The conversion of heptachlor to its
epoxide on plants. J. Econ. Ent., 51: 3-7
Gannon, N. & Decker, G. C. (1958b) The conversion of aldrin to
dieldrin in plants. J. Econ. Ent., 51: 8-11
Gooding, C. M. B. (1966) Fate of Chlorinated Organic Pesticide
Residues in the Production of Edible Vegetable Oils. Chem. and
Industry, 8: 344
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent.,
54: 1038-1045
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in south western Ontario. Agric. & Food Chem.,
14: 398-403
Hayes, W. J., Dale, W. E. & Burse, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life Sci.,
4: 1611-1615
King, R. L., Clark, N. A. & Hemken, R. W. (1966) Distribution,
movement and persistence of heptachlor and its epoxide in alfalfa
plants and soil. Agric. & Food Chem., 14; 62-65
Lewis, D. T. (1965) Report of the Government Chemist. H.M.S.O. London.
153 pp.
Lichtenstein, E. P. & Polvika, J. B. (1959) Persistence of some
chlorinated hydrocarbon insecticides in turf soils. J. Econ. Ent.,
52: 289-293
Lichtenstein, E. P. & Schulz, K. R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture, and soil
types. J. Econ. Ent., 53: 192-197
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13: 57-62
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1965) Absorption
of insecticidal residues from contaminated soils into five carrot
varieties. J. Agr. Food Chem., 13: 126-131
Robinson, J., Richardson, A., Hunter, G. C., Crabtree, A. N., Rees,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in south eastern England. Brit. J. Ind. Med., 22: 220-229
Saha, J. G. & Stewart, W. W. A. (1966) Vertical and lateral
distribution of heptachlor, heptachlor epoxide, and gamma-chlordane in
rutabagas, grown in heptachlor-treated soil Can. J. Plant Sci. (In
press)
Street, J. C. (1966) Data obtained after feeding heptachlor and DDT to
rats. (In press). J. Agric. Food Chem.
Wiese, I. H. (1964) Some biological studies on the inactivation of
insecticides by various soil types. S. Afr. J. Agric. Sci., 7:
823-835
Wilkinson, A. T. S., Finlayson, D. G. & Morley, H. V. (1964) Toxic
residues in soil 9 years after treatment with aldrin and heptachlor.
Science, 143. 681-2
Young, W, R. & Rawlins, W. A. (1958) The persistence of heptachlor in
soils. J. Econ. Ent., 51: 11-18
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) Chlorinated
hydrocarbon insecticides in the US. J.A.M.A., 193: 837-9
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In plants and soil, heptachlor is converted to its epoxide which is
more persistent on plants than the parent heptachlor (Gannon & Bigger,
1958; Gannon & Decker, 1958).
Rat liver microsomes have been proved to epoxidize heptachlor in
vitro. The extent of epoxidation in vivo was about 10 times
greater in males than in females (Wong & Terriere, 1965).
Heptachlor epoxide is stored in the fat of dogs add rats. Some storage
occurs in the liver, kidney and muscles but none in the brain. The
metabolite was found in dogs after feeding heptachlor in a
concentration of 1-3 mg/kg body-weight. When heptachlor was fed at
high dosage levels to dogs, small amounts of unmetabolized heptachlor
were also found in the fat, but this was not the case in the rat
regardless of the level fed (Davidow & Radomski, 1953).
In the rat, the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm,
storage occurred in females but not in males, and at 0.1 ppm no
storage occurred in either sex. Female rats accumulate about 6 times
as much epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days, the epoxide level in the milk rose to a maximum of 1.8 ppm,
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at 5 ppm and 2 at 10 ppm daily). Three days after feeding
commenced, the minimum levels of heptachlor epoxide in the milk were
0.26 and 0.34 ppm in the 2 cows fed 5 ppm, and 0.23 and 0.65 ppm in
the 2 cows fed 10 ppm; the maximum levels after 15 days' feeding were
0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively (Storherr et
al., 1960).
Investigations on heptachlor storage in human fat in several parts of
the world have recently been reported. The average ranged between 0
and 0.24 ppm; it tended to be lower in those countries in which a high
storage of DDT was recorded (Dale et al., 1965; Hayes et al., 1965;
Robinson et al., 1965; Zavon et al., 1965).
Heptachlor and its epoxide are included among the chlorinated
hydrocarbon insecticides that stimulate the metabolism of drugs by
microsomal enzymes (Remmer, 1964).
Disturbance of the acid-base balance seems to be of great significance
in the mechanism of action of heptachlor (Spynu & Osetrov, 1957).
Acute toxicity
Heptachlor
Animal Route LD50 References
mg/kg body-weight
Rat Oral 60-142* Velsicol Corporation, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 References
mg/kg body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide in the
mouse are given as 40 and 10 mg/kg body-weight respectively (Radomski
& Davidow, 1953). In the rabbit, the lethal dose of epoxide is 5-10
mg/kg body-weight. The acute effects are neurotoxic disturbances like
those observed with other chlorinated hydrocarbons (Velsicol, 1959).
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide (up to
60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleoli, cytoplasmic fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats, it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla. Electron microscopic studies
demonstrated an increase of rough and smooth endoplasmic reticulum
(Stemmer & Hamdi, 1963).
In a reproduction study covering 3 generations, a group of 80 rats was
given 6.9 mg/kg body-weight of heptachlor daily for 3 months before
mating. Cataracts were found in 6.8 per cent of the young and became
obvious between the 19th and 26th day. Among the parents, 15.2 per
cent of the animals were affected and the lesions appeared after 4-9
months. The only effect on reproduction was decrease of litter size
(Mestitzova, 1966).
The continuous exposure of rats to doses of over 7 ppm of either
heptachlor or its epoxide increased the mortality of the pups during
the suckling period. 10 ppm fed to 3 generations of rate showed no
adverse effects on reproductive capacity, growth or survival (Witherup
et al., 1955; Kettering Laboratory, 1959).
Dog. When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Three dogs given heptachlor epoxide orally in dosages of 2, 4 and 8
mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively. Daily oral doses of 0.25 and 0.5 mg/kg body-weight did
not produce any sign of illness during 52 weeks, but 0.25 mg/kg,
estimated to be equivalent to 6 ppm in the diet, was reported as the
minimal dose producing a pathological effect (Velsicol, 1959).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor epoxide were
given to groups of 5 dogs (2 males and 3 females, 23 to 27 weeks of
age) for 60 weeks. No deaths attributable to heptachlor epoxide
occurred. The weights of the male dogs tended to be inversely
proportional to the concentration of the compound in the diet. The
female dogs had normal weights. The liver weights were increased at 5
ppm and above. Degenerative liver changes were seen in only 1 dog at
7.5 ppm (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed diets
containing 5, 10, 20, 40, 80, 160 and 300 ppm of heptachlor epoxide
for 2 years. Concentrations of 80 ppm or higher resulted in 100 per
cent mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females) (Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10, 20 and
40 ppm for 2 years showed significant increases in mortality only in
females at 40 ppm. Liver weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
CFN rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5,
7.5 and 10 ppm. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34 per cent) and 13/24
(54 per cent) in the control males and females respectively; it was
65/111 (58 per cent) and 92/114 (80 per cent) in the experimental
males and females respectively. Again, many tumours were located in
endocrine organs. Liver tumours were observed in 7 males and 12
females in the experimental groups only (over-all incidence 19/225
(8.4 per cent), but only two of them were malignant (Kettering
Laboratory, 1959).
Heptachlor dissolved in ethanol was added to the diet of CF rats as
1.5, 3, 5, 7 and 10 ppm for 110 weeks. Each group, as well as an
untreated control, included 40 animals (20 of each sex). Mortality was
comparable in all groups. The number of tumour-bearing animals was
16/40 at 0 ppm, 9/40 at 1.5 ppm, 13/40 at 3 ppm, 12/40 at 5 ppm, 15/40
at 7 ppm and 12/40 at 10 ppm. Most tumours were found in the pituitary
and other endocrine organs. No liver tumours were recorded. No
preferential tumour site in any particular group was observed but all
the 4 thyroid tumours observed were in the 7 and 10 ppm groups
(Witherup et al., 1955).
In a recent experiment carried out on a total of 154 female rats, a
mixture of heptachlor/heptachlor epoxide 3:1 was added to the diet at
0, 5, 7.5, 10 and 12.5 ppm for two years. Pituitary and mammary
tumours were seen at all dose levels, including the controls; their
incidence varied from group to group but not in relation to the dose
of heptachlor. At the end of the two years, all groups including the
controls showed liver histological changes, i.e. cell enlargement in
centrolobular parts, loss of cytoplasmic granules and appearance of
lipid vacuoles. No quantitative differences could be detected between
rats given 0 and 5 ppm, whereas in the other groups the severity of
the changes was related to the dose. At 12.5 ppm, regenerative liver
changes were present (Kettering Laboratory, 1966).
Comments
It is well established that heptachlor and its epoxide accumulate in
body fat and persist there for long periods. The formation of epoxide
is relatively rapid in the animal, plants and in the soil.
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the two
substances.
A sex difference in toxicity has been observed, particularly in the
rat, females accumulating heptachlor and its epoxide more than males.
The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to explain. A
possible relation with tumour formation has not been demonstrated,
while evidence has been presented that the change is reversible.
Some suspicion of carcinogenicity could arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 5 ppm in the diet, equivalent to 0.25 mg/kg/day.
Dog. 2.5 ppm in the diet, equivalent to 0.0625 mg/kg/day
Estimate of acceptable daily intake for man
0-0.0005 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that heptachlor and its
epoxide are compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of these compounds, with a view to removing doubts
which may remain as to their safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
The pattern of use for heptachlor has been evolving since it was
introduced to agriculture in the 1950's. Up until 1960, it had a broad
scale of use on forage, cereal, oil seed, vegetable, sugar beet and
some nut crops, such as peanuts. There has been a gradual reduction of
scale and pattern of use of this insecticide for various reasons
including a desire to reduce the unintentional contamination of milk
and animal products.
The present trend in Canada, the USA and Western Europe is towards
restricting its use to vegetable and cereal seed and to applications
to the soil against insects therein. In certain countries it is also
applied to young pasture plants that will not be grazed or fed in the
season of use, to transplant water for seedlings and to ground cover
under a restricted number of orchard trees. In each case there are
restrictions concerning the access of livestock. In Japan, up until at
least 1963, large quantities of heptachlor dust have been used on
upland wheat, barley and rice.
Dosages vary between countries. The average recommended dose is 2
kilos of actual heptachlor per hectare for use on soil prior to or at
planting on cereals and legumes, including barley, green beans, lima
beans, corn, oats, rice, soybeans and wheat. To cereal seeds it is
sometimes used as a slurry at 30-45 grams per 100 kilos applied prior
to planting.
(b) Post-harvest treatments
No post-harvest use that has been reported.
* Sum of heptachlor and heptachlor epoxide by weight.
Tolerances
Canada 0.1 ppm (combined heptachlor and heptachlor epoxide) on beans,
cabbage, lettuce, rutabagas (yellow turnips). (Heptachlor is scheduled
under the Feeds Act as a compound that should not be present in
commercial feed. In 1965 the tolerance for cereals was eliminated.)
USA 0.1 in cabbage, lettuce, rutabagas and snap beans; zero for many
other foods.
Netherlands 0.1 ppm.
In Canada and USA administrative "actionable levels" have also been
established for residues of heptachlor and heptachlor epoxide in milk
and other animal products. (It was the impression of the FAO Working
Party that this practice was also in vogue in other countries, but
reliable information was not available.)
Residues resulting from supervised trials
There is a large amount of information but much of it needs reviewing
in the light of developments in analytical methods since about 1961.
Although some of the earlier work has recently been reviewed in N.
America, reliable recent results are scarce. The published literature
in languages other than English is difficult to obtain. For example,
Ishikura (personal communication, 1965) reports a large scale of use
on cereals in Japan, but information does not appear to be available
on the resulting residues.
Lichtenstein & Polvika (1959), Young & Rawlins (1958) and many other
authors publishing prior to 1961, suggested that no residues of
heptachlor would persist in soil, and that none would be translocated
from soil to plants, especially in forage crops. More reliable
analytical methods have since showed that heptachlor and its epoxide
persist in soil and can result in residues in some species of plants.
The early investigations suggested that heptachlor could be used
effectively on a wide range of vegetables, root crops, potatoes,
forage and peanuts, with residues that would in many instances not
exceed 0.1. ppm in the harvested product. Dawsey, Woodham & Lofgren
(1961) stated that no residues were detected in 14 of 16 crops tested,
but radishes and onions showed significant amounts when grown in plots
treated with 0.25 of 0.5 lb of heptachlor per acre. However,
Lichtenstein & Schulz (1965) found some build up, over a three-year
period in soil from annual application of heptachlor at useful rates.
Residues found in various crops grown in such soil were carrots up to
0.69 ppm, potatoes up to 0.27, radishes up to 0.21 and beets up to
0.29.
Information from Burrage & Saha (1976) however suggests that the use
of heptachlor for seed and soil treatments for certain cereals may be
without fear of residues occurring on harvested grain, but the
possible need for restrictions on the use of straw for animal feed and
the significance of soil residues in crop rotations require further
evaluation. Investigations on the relationships between residues in
oil seeds and certain forage crops to their oil contents, recently
reported by Bruce & Decker (1966), have been less reassuring.
It would be most useful to have the results of similar investigations,
with reliable analytical procedures from other countries. Information
pertaining to residues in successive crops grown in rotation over a
period of years after the initial treatment would be of particular
interest.
Residues in food moving in commerce
Information is slowly becoming available on residues in food moving in
commerce. Some indirect information also is available from total diet
studies, particularly in Canada and the USA. The occurrence of
residues is broadly related to the scale of use in the respective
countries. Residues have varied from traces to 1.0 ppm in up to 10
per cent of the sample diets studied. A six-month average in one USA
study indicated highest residues in these <10 per cent of diets as
meat 0.05 ppm, dairy products (fat basis) 0.025 ppm and other food
0.005 ppm, after processing for consumption.
Canadian investigations of residues in commercial lots of sugar beet
pulp suggest that heptachlor and heptachlor epoxide residues can occur
in this animal feed, which if fed in too large a quantity can result
in undesirable levels of residues occurring in animal products.
Consequently, the feeding of contaminated sugar beet pulp is
restricted to stated levels in the animals' diet. No heptachlor
residues were found in a recent survey of meat and dairy products in
Britain (Lewis, 1965).
The Members of the FAO Working Party on Pesticide Residues, were aware
that information is being obtained on residues in milk and animal
products, commercial feeds, cannery wastes and other agricultural
by-products moving in commerce but these data were not available for
evaluation. Information of this kind is important in the evaluations
of this compound and the co-operation of Member countries of FAO and
WHO is requested in making it available for review.
Fate of residue
(a) In soil
A body of information is becoming available in Canada and the USA.
The persistence of residues in soil appears to be related to soil
type, climate, soil management and cropping practices (Harris, 1966;
Duffy & Wong, 1966; Lichtenstein & Schulz, 1960, 1965a; Harris &
Lichtenstein, 1961; Wilkinson et al., 1964; Saha & Stewart, 1966).
Similar information in other countries is relatively scarce (Weise,
1964). Recent work suggests that low level soil residues may be
contributing directly to residues in animal products, particularly in
arid and semi-arid zones. In these areas, livestock are known to
ingest some soil in foraging, resulting in residues in animal products
that cannot be accounted for on the basis of residues in plants alone.
It is possible that heptachlor and heptachlor epoxide residues can be
dissipated from soil more rapidly than dieldrin or DDT (Harris &
Lichtenstein, 1961).
Bowman et al. (1965) suggested from laboratory evidence that
heptachlor was changed to 1-hydroxychlordene in dry soils with low
organic content and that no conversion of heptachlor to heptachlor
epoxide was detected in these soils. In another study under field
conditions, the same authors detected a small amount of
1-hydroxychlordene in fine sandy loam. Duffy & Wong (1966) have
reported the presence of 1-hydroxychlordene in some Canadian Atlantic
provinces soils, but Harris (personal communication) has not been able
to confirm its presence in Ontario soils. This degradation product has
not been reported as a component of residues in food. (Its presence
would be of interest since the LD50 to rats is only of the order of
2400 mg/kg.) Gamma-chlordane is also a component of residues in soil.
(b) In plants
The conversion of heptachlor to heptachlor epoxide was first reported
by Gannon & Decker (1958a, 1958b). The parameters that control the
rate of epoxidation are still obscure, but several reports suggest
that living tissue, even if only that of micro-organisms, is required
(Davidow & Radomski, 1953). The exact mechanisms by which plants
acquire residues of these compounds are are not understood either.
Evidence for translocation by plants has been produced by Gannon &
Decker (1958), Lichtenstein & Schulz (1960), Dawsey (1961),
Lichtenstein et al. (1965), etc. Special attention has been given
recently to the mechanisms of contamination and the location of these
residues within plants and their stability (Byrne & Steinhauer, 1966;
Burrage & Saha, 1966; King, Clark & Hemken, 1966; Saha & Stewart,
1966). These data are all from Canada and USA. It would be useful to
have comparable information from other sources.
Some information is also becoming available on the relationship
between the residues in soil, the oil content of plants and the
residues that can be expected in them (Bruce & Decker, 1966). This
type of information will have a special bearing on future evaluations
of residues in oil seed crops or fibre crops (soybeans, cotton,
peanuts, rape, etc.) whose by-products become animal food and may thus
lead to residues in human food.
(c) In animals
Residues in animal products have attracted special interest. Low level
residues have been detected and measured recently, in milk and dairy
products using analytical methods that were not previously available
(USA six-month average in up to 10 per cent of diets 0.025 ppm on a
fat basis, in dairy products) and in meat (USA six-month average in 10
per cent diets, 0.05 ppm). It has been suggested that loads of DDT
already stored in the body fat of animals are partially responsible
for the prevention of storage of heptachlor and heptachlor epoxide
(Street, 1966, in press) and their degradation without storage, which
could account for the observation that the loads of heptachlor epoxide
in human body fat are lower in those parts of the world in which a
higher rate of storage of DDT is recorded (Dale et al., 1965; Hayes et
al., 1965; Robinson et al., 1965; Zavon et al., 1965).
(d) In storage and processing
If tolerances are to be based on amounts ingested by consumers,
information on the behaviour of residues during storage and processing
is needed. Although residues from heptachlor have often been regarded
as stable, various data indicate that some disappearance takes place
in storage and processing. This was found in work relating to the
stability of residues in silage (unpublished information from USDA).
Work of Saha & Stewart (1966) indicates that cooking peeled turnips
results in complete disappearance of residues of heptachlor and
heptachlor epoxide in turnip flesh, using an analytical method
sensitive to 0.2 ppm. Similar results have been reported by
Lichtenstein et al. (1965) for carrots. Gooding (1966) suggested that
the normal processes of alkali-refining, bleaching, dehydrogenation
and deodorization will remove all traces from edible vegetable oils,
but definite proof has not been published. Residues do not appear in
sugar or molasses when residues occur in sugar beets, but remain in
the pulp. More information is needed on the fate of residues during
processing.
Methods of analysis
A number of multidetection systems are available for the detection and
determination of residues together with residues of a number of other
compounds. An example is the AOAC system (1966) in which acetonitrile
partition and Florisil column clean-up are used and the residues
identified and measured by gas chromatography coupled with thin layer
or paper chromatography. Alternative clean-up systems, e.g. that of de
Faubert Maunder et al. (1964) using dimethylformamide, and other
methods of confirmation of identity, e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.002 ppm of heptachlor or heptachlor epoxide in milk and
0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The potential hazard resulting from the use of heptachlor in food
production has recently been reduced. The pesticide is now only being
used on seeds and in soil employed in cereal and vegetable production.
Present evidence indicates that soil and seed treatments can be
continued without residues resulting in harvested grain. (The present
evidence pertains to wheat. It may well extend to a wider range of
cereals.)
Since the Temporary ADI is very small (0.0005 mg/kg/day), it is
recommended that a Temporary Tolerance of 0.1 ppm be established, from
uses of the insecticide in the soil and on to seed, only for raw root
vegetables (other than potatoes), cole crops, head lettuce, spinach
and other leafy vegetables. In this recommendation residues of both
heptachlor and heptachlor epoxide are to be combined within the total
of 0.1 ppm. This recommendation is based on the assumption that there
will be no losses of residue in storage and processing, including
cooking.
In addition "practical residue limits" are recommended for potatoes at
0.05 ppm; whole milk 0.002 ppm, dairy products 0.025 ppm on a fat
basis; meat 0.05 ppm (fat basis).
Additional data pertaining to these recommendations should be reviewed
at an early date.
Further information
1. Many of the measurements undertaken prior to about 1961 of residues
after treatments of crops or of soil need to be repeated with the
aid of the more sensitive analytical techniques that have since
become available.
2. More evidence is needed on the extent to which heptachlor is used
in various countries and on the residues which occur in foods in
international commerce; also on the fate of such residues during
the normal course of storage and processing, including cooking,
to which such foods are subjected before consumption.
3. Investigations are desirable on the fate of low levels of
heptachlor in feeds consumed by animals. This is because
information on commercial samples suggest that animal feeds may be
the sources of some of the albeit fairly low residues found in
products from animals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely, R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
Hayes, W. J., jr, Dale, W. E. & Burse, V. W. (1965) Life Sci., 4,
1611
Kettering Laboratory (1959) Unpublished report submitted by Velsicol
Corporation
Kettering Laboratory (1966) Unpublished report submitted by Velsicol
Corporation
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Mestitzova, M. (1966) International Congress on Occupational Health,
Proceedings, 109 (7), 455
Radomski, J. L. & Davidow, B. (1953) J. Pharmacol. exp. Ther.,
107, 266
Remmer, H. (1964) Proceedings of the European Society for the Study
of Drug Toxicity, Vol. 9
Robinson, J., Richardson, J., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Sherman, M. & Ross, E. (1961) Toxicol. appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1957) Nauch. Trudy Inst. Ent.
Fitopat. Akad. Nauk Ukr. SSR, 7, 63
Stemmer, K. L. & Hamdi, E. (1963) Unpublished report of the Kettering
Laboratory, University of Cincinnati
Stemmer, K. L. & Jolley, W. P. (1963) Unpublished report of the
Kettering Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S., Cleveland, F. F., Shaffer, F. E., Schlecht, H. & Musen,
L. (1955) Unpublished report of the Kettering Laboratory, University
of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49: 222-30
Bowman, M. C., Schechter, M. S. & Carter, R. L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. Agric.
Food Chem., 13: 360-365
Bruce, W. N. & Decker, G. C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin, dieldrin,
heptachlor, and heptachlor epoxide. J. Agr. Food Chem., 14: 395-403
Burrage, R. H. & Saha, J. G. (1965) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin or heptachlor.
Can. J. Plant Sci. (In press)
Byrne, H. D. & Steinhauer, A. L. (1966) Mechanisms of contamination of
alfalfa with heptachlor and heptachlor epoxide. J. Econ. Ent., 59:
338-341
Davidow, B. & Radomski, J. L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265
Dale, W. E., Copeland, M. L. & Hayes, W. J. (1965) Chlorinated
insecticides in the body fat of people in India. Bull. Wld Hlth Org.,
33: 477
Dawsey, L. H., Woodham, D. W. & Lofgren, C. S. (1961) Heptachlor and
heptachlor epoxide residues in truck crops. J. Econ. Ent., 54: 6,
1264-5
Duffy, J. R. & Wong, N. (1966) Insecticide residues and their
metabolites in Atlantic soils. In press. J. Agr. Food Chem.
de Faubert Maunder, M. J., Egan, H. & Godly, E. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. Analyst,
89: 168-174
Gannon, N. & Decker, G. C. (1958a) The conversion of heptachlor to its
epoxide on plants. J. Econ. Ent., 51: 3-7
Gannon, N. & Decker, G. C. (1958b) The conversion of aldrin to
dieldrin in plants. J. Econ. Ent., 51: 8-11
Gooding, C. M. B. (1966) Fate of Chlorinated Organic Pesticide
Residues in the Production of Edible Vegetable Oils. Chem. and
Industry, 8: 344
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent.,
54: 1038-1045
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in south western Ontario. Agric. & Food Chem.,
14: 398-403
Hayes, W. J., Dale, W. E. & Burse, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life Sci.,
4: 1611-1615
King, R. L., Clark, N. A. & Hemken, R. W. (1966) Distribution,
movement and persistence of heptachlor and its epoxide in alfalfa
plants and soil. Agric. & Food Chem., 14; 62-65
Lewis, D. T. (1965) Report of the Government Chemist. H.M.S.O. London.
153 pp.
Lichtenstein, E. P. & Polvika, J. B. (1959) Persistence of some
chlorinated hydrocarbon insecticides in turf soils. J. Econ. Ent.,
52: 289-293
Lichtenstein, E. P. & Schulz, K. R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture, and soil
types. J. Econ. Ent., 53: 192-197
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13: 57-62
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1965) Absorption
of insecticidal residues from contaminated soils into five carrot
varieties. J. Agr. Food Chem., 13: 126-131
Robinson, J., Richardson, A., Hunter, G. C., Crabtree, A. N., Rees,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in south eastern England. Brit. J. Ind. Med., 22: 220-229
Saha, J. G. & Stewart, W. W. A. (1966) Vertical and lateral
distribution of heptachlor, heptachlor epoxide, and gamma-chlordane in
rutabagas, grown in heptachlor-treated soil Can. J. Plant Sci. (In
press)
Street, J. C. (1966) Data obtained after feeding heptachlor and DDT to
rats. (In press). J. Agric. Food Chem.
Wiese, I. H. (1964) Some biological studies on the inactivation of
insecticides by various soil types. S. Afr. J. Agric. Sci., 7:
823-835
Wilkinson, A. T. S., Finlayson, D. G. & Morley, H. V. (1964) Toxic
residues in soil 9 years after treatment with aldrin and heptachlor.
Science, 143. 681-2
Young, W, R. & Rawlins, W. A. (1958) The persistence of heptachlor in
soils. J. Econ. Ent., 51: 11-18
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) Chlorinated
hydrocarbon insecticides in the US. J.A.M.A., 193: 837-9
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In plants and soil, heptachlor is converted to its epoxide which is
more persistent on plants than the parent heptachlor (Gannon & Bigger,
1958; Gannon & Decker, 1958).
Rat liver microsomes have been proved to epoxidize heptachlor in
vitro. The extent of epoxidation in vivo was about 10 times
greater in males than in females (Wong & Terriere, 1965).
Heptachlor epoxide is stored in the fat of dogs add rats. Some storage
occurs in the liver, kidney and muscles but none in the brain. The
metabolite was found in dogs after feeding heptachlor in a
concentration of 1-3 mg/kg body-weight. When heptachlor was fed at
high dosage levels to dogs, small amounts of unmetabolized heptachlor
were also found in the fat, but this was not the case in the rat
regardless of the level fed (Davidow & Radomski, 1953).
In the rat, the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm,
storage occurred in females but not in males, and at 0.1 ppm no
storage occurred in either sex. Female rats accumulate about 6 times
as much epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days, the epoxide level in the milk rose to a maximum of 1.8 ppm,
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at 5 ppm and 2 at 10 ppm daily). Three days after feeding
commenced, the minimum levels of heptachlor epoxide in the milk were
0.26 and 0.34 ppm in the 2 cows fed 5 ppm, and 0.23 and 0.65 ppm in
the 2 cows fed 10 ppm; the maximum levels after 15 days' feeding were
0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively (Storherr et
al., 1960).
Investigations on heptachlor storage in human fat in several parts of
the world have recently been reported. The average ranged between 0
and 0.24 ppm; it tended to be lower in those countries in which a high
storage of DDT was recorded (Dale et al., 1965; Hayes et al., 1965;
Robinson et al., 1965; Zavon et al., 1965).
Heptachlor and its epoxide are included among the chlorinated
hydrocarbon insecticides that stimulate the metabolism of drugs by
microsomal enzymes (Remmer, 1964).
Disturbance of the acid-base balance seems to be of great significance
in the mechanism of action of heptachlor (Spynu & Osetrov, 1957).
Acute toxicity
Heptachlor
Animal Route LD50 References
mg/kg body-weight
Rat Oral 60-142* Velsicol Corporation, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 References
mg/kg body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide in the
mouse are given as 40 and 10 mg/kg body-weight respectively (Radomski
& Davidow, 1953). In the rabbit, the lethal dose of epoxide is 5-10
mg/kg body-weight. The acute effects are neurotoxic disturbances like
those observed with other chlorinated hydrocarbons (Velsicol, 1959).
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide (up to
60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleoli, cytoplasmic fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats, it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla. Electron microscopic studies
demonstrated an increase of rough and smooth endoplasmic reticulum
(Stemmer & Hamdi, 1963).
In a reproduction study covering 3 generations, a group of 80 rats was
given 6.9 mg/kg body-weight of heptachlor daily for 3 months before
mating. Cataracts were found in 6.8 per cent of the young and became
obvious between the 19th and 26th day. Among the parents, 15.2 per
cent of the animals were affected and the lesions appeared after 4-9
months. The only effect on reproduction was decrease of litter size
(Mestitzova, 1966).
The continuous exposure of rats to doses of over 7 ppm of either
heptachlor or its epoxide increased the mortality of the pups during
the suckling period. 10 ppm fed to 3 generations of rate showed no
adverse effects on reproductive capacity, growth or survival (Witherup
et al., 1955; Kettering Laboratory, 1959).
Dog. When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Three dogs given heptachlor epoxide orally in dosages of 2, 4 and 8
mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively. Daily oral doses of 0.25 and 0.5 mg/kg body-weight did
not produce any sign of illness during 52 weeks, but 0.25 mg/kg,
estimated to be equivalent to 6 ppm in the diet, was reported as the
minimal dose producing a pathological effect (Velsicol, 1959).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor epoxide were
given to groups of 5 dogs (2 males and 3 females, 23 to 27 weeks of
age) for 60 weeks. No deaths attributable to heptachlor epoxide
occurred. The weights of the male dogs tended to be inversely
proportional to the concentration of the compound in the diet. The
female dogs had normal weights. The liver weights were increased at 5
ppm and above. Degenerative liver changes were seen in only 1 dog at
7.5 ppm (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed diets
containing 5, 10, 20, 40, 80, 160 and 300 ppm of heptachlor epoxide
for 2 years. Concentrations of 80 ppm or higher resulted in 100 per
cent mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females) (Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10, 20 and
40 ppm for 2 years showed significant increases in mortality only in
females at 40 ppm. Liver weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
CFN rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5,
7.5 and 10 ppm. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34 per cent) and 13/24
(54 per cent) in the control males and females respectively; it was
65/111 (58 per cent) and 92/114 (80 per cent) in the experimental
males and females respectively. Again, many tumours were located in
endocrine organs. Liver tumours were observed in 7 males and 12
females in the experimental groups only (over-all incidence 19/225
(8.4 per cent), but only two of them were malignant (Kettering
Laboratory, 1959).
Heptachlor dissolved in ethanol was added to the diet of CF rats as
1.5, 3, 5, 7 and 10 ppm for 110 weeks. Each group, as well as an
untreated control, included 40 animals (20 of each sex). Mortality was
comparable in all groups. The number of tumour-bearing animals was
16/40 at 0 ppm, 9/40 at 1.5 ppm, 13/40 at 3 ppm, 12/40 at 5 ppm, 15/40
at 7 ppm and 12/40 at 10 ppm. Most tumours were found in the pituitary
and other endocrine organs. No liver tumours were recorded. No
preferential tumour site in any particular group was observed but all
the 4 thyroid tumours observed were in the 7 and 10 ppm groups
(Witherup et al., 1955).
In a recent experiment carried out on a total of 154 female rats, a
mixture of heptachlor/heptachlor epoxide 3:1 was added to the diet at
0, 5, 7.5, 10 and 12.5 ppm for two years. Pituitary and mammary
tumours were seen at all dose levels, including the controls; their
incidence varied from group to group but not in relation to the dose
of heptachlor. At the end of the two years, all groups including the
controls showed liver histological changes, i.e. cell enlargement in
centrolobular parts, loss of cytoplasmic granules and appearance of
lipid vacuoles. No quantitative differences could be detected between
rats given 0 and 5 ppm, whereas in the other groups the severity of
the changes was related to the dose. At 12.5 ppm, regenerative liver
changes were present (Kettering Laboratory, 1966).
Comments
It is well established that heptachlor and its epoxide accumulate in
body fat and persist there for long periods. The formation of epoxide
is relatively rapid in the animal, plants and in the soil.
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the two
substances.
A sex difference in toxicity has been observed, particularly in the
rat, females accumulating heptachlor and its epoxide more than males.
The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to explain. A
possible relation with tumour formation has not been demonstrated,
while evidence has been presented that the change is reversible.
Some suspicion of carcinogenicity could arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 5 ppm in the diet, equivalent to 0.25 mg/kg/day.
Dog. 2.5 ppm in the diet, equivalent to 0.0625 mg/kg/day
Estimate of acceptable daily intake for man
0-0.0005 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that heptachlor and its
epoxide are compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of these compounds, with a view to removing doubts
which may remain as to their safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
The pattern of use for heptachlor has been evolving since it was
introduced to agriculture in the 1950's. Up until 1960, it had a broad
scale of use on forage, cereal, oil seed, vegetable, sugar beet and
some nut crops, such as peanuts. There has been a gradual reduction of
scale and pattern of use of this insecticide for various reasons
including a desire to reduce the unintentional contamination of milk
and animal products.
The present trend in Canada, the USA and Western Europe is towards
restricting its use to vegetable and cereal seed and to applications
to the soil against insects therein. In certain countries it is also
applied to young pasture plants that will not be grazed or fed in the
season of use, to transplant water for seedlings and to ground cover
under a restricted number of orchard trees. In each case there are
restrictions concerning the access of livestock. In Japan, up until at
least 1963, large quantities of heptachlor dust have been used on
upland wheat, barley and rice.
Dosages vary between countries. The average recommended dose is 2
kilos of actual heptachlor per hectare for use on soil prior to or at
planting on cereals and legumes, including barley, green beans, lima
beans, corn, oats, rice, soybeans and wheat. To cereal seeds it is
sometimes used as a slurry at 30-45 grams per 100 kilos applied prior
to planting.
(b) Post-harvest treatments
No post-harvest use that has been reported.
* Sum of heptachlor and heptachlor epoxide by weight.
Tolerances
Canada 0.1 ppm (combined heptachlor and heptachlor epoxide) on beans,
cabbage, lettuce, rutabagas (yellow turnips). (Heptachlor is scheduled
under the Feeds Act as a compound that should not be present in
commercial feed. In 1965 the tolerance for cereals was eliminated.)
USA 0.1 in cabbage, lettuce, rutabagas and snap beans; zero for many
other foods.
Netherlands 0.1 ppm.
In Canada and USA administrative "actionable levels" have also been
established for residues of heptachlor and heptachlor epoxide in milk
and other animal products. (It was the impression of the FAO Working
Party that this practice was also in vogue in other countries, but
reliable information was not available.)
Residues resulting from supervised trials
There is a large amount of information but much of it needs reviewing
in the light of developments in analytical methods since about 1961.
Although some of the earlier work has recently been reviewed in N.
America, reliable recent results are scarce. The published literature
in languages other than English is difficult to obtain. For example,
Ishikura (personal communication, 1965) reports a large scale of use
on cereals in Japan, but information does not appear to be available
on the resulting residues.
Lichtenstein & Polvika (1959), Young & Rawlins (1958) and many other
authors publishing prior to 1961, suggested that no residues of
heptachlor would persist in soil, and that none would be translocated
from soil to plants, especially in forage crops. More reliable
analytical methods have since showed that heptachlor and its epoxide
persist in soil and can result in residues in some species of plants.
The early investigations suggested that heptachlor could be used
effectively on a wide range of vegetables, root crops, potatoes,
forage and peanuts, with residues that would in many instances not
exceed 0.1. ppm in the harvested product. Dawsey, Woodham & Lofgren
(1961) stated that no residues were detected in 14 of 16 crops tested,
but radishes and onions showed significant amounts when grown in plots
treated with 0.25 of 0.5 lb of heptachlor per acre. However,
Lichtenstein & Schulz (1965) found some build up, over a three-year
period in soil from annual application of heptachlor at useful rates.
Residues found in various crops grown in such soil were carrots up to
0.69 ppm, potatoes up to 0.27, radishes up to 0.21 and beets up to
0.29.
Information from Burrage & Saha (1976) however suggests that the use
of heptachlor for seed and soil treatments for certain cereals may be
without fear of residues occurring on harvested grain, but the
possible need for restrictions on the use of straw for animal feed and
the significance of soil residues in crop rotations require further
evaluation. Investigations on the relationships between residues in
oil seeds and certain forage crops to their oil contents, recently
reported by Bruce & Decker (1966), have been less reassuring.
It would be most useful to have the results of similar investigations,
with reliable analytical procedures from other countries. Information
pertaining to residues in successive crops grown in rotation over a
period of years after the initial treatment would be of particular
interest.
Residues in food moving in commerce
Information is slowly becoming available on residues in food moving in
commerce. Some indirect information also is available from total diet
studies, particularly in Canada and the USA. The occurrence of
residues is broadly related to the scale of use in the respective
countries. Residues have varied from traces to 1.0 ppm in up to 10
per cent of the sample diets studied. A six-month average in one USA
study indicated highest residues in these <10 per cent of diets as
meat 0.05 ppm, dairy products (fat basis) 0.025 ppm and other food
0.005 ppm, after processing for consumption.
Canadian investigations of residues in commercial lots of sugar beet
pulp suggest that heptachlor and heptachlor epoxide residues can occur
in this animal feed, which if fed in too large a quantity can result
in undesirable levels of residues occurring in animal products.
Consequently, the feeding of contaminated sugar beet pulp is
restricted to stated levels in the animals' diet. No heptachlor
residues were found in a recent survey of meat and dairy products in
Britain (Lewis, 1965).
The Members of the FAO Working Party on Pesticide Residues, were aware
that information is being obtained on residues in milk and animal
products, commercial feeds, cannery wastes and other agricultural
by-products moving in commerce but these data were not available for
evaluation. Information of this kind is important in the evaluations
of this compound and the co-operation of Member countries of FAO and
WHO is requested in making it available for review.
Fate of residue
(a) In soil
A body of information is becoming available in Canada and the USA.
The persistence of residues in soil appears to be related to soil
type, climate, soil management and cropping practices (Harris, 1966;
Duffy & Wong, 1966; Lichtenstein & Schulz, 1960, 1965a; Harris &
Lichtenstein, 1961; Wilkinson et al., 1964; Saha & Stewart, 1966).
Similar information in other countries is relatively scarce (Weise,
1964). Recent work suggests that low level soil residues may be
contributing directly to residues in animal products, particularly in
arid and semi-arid zones. In these areas, livestock are known to
ingest some soil in foraging, resulting in residues in animal products
that cannot be accounted for on the basis of residues in plants alone.
It is possible that heptachlor and heptachlor epoxide residues can be
dissipated from soil more rapidly than dieldrin or DDT (Harris &
Lichtenstein, 1961).
Bowman et al. (1965) suggested from laboratory evidence that
heptachlor was changed to 1-hydroxychlordene in dry soils with low
organic content and that no conversion of heptachlor to heptachlor
epoxide was detected in these soils. In another study under field
conditions, the same authors detected a small amount of
1-hydroxychlordene in fine sandy loam. Duffy & Wong (1966) have
reported the presence of 1-hydroxychlordene in some Canadian Atlantic
provinces soils, but Harris (personal communication) has not been able
to confirm its presence in Ontario soils. This degradation product has
not been reported as a component of residues in food. (Its presence
would be of interest since the LD50 to rats is only of the order of
2400 mg/kg.) Gamma-chlordane is also a component of residues in soil.
(b) In plants
The conversion of heptachlor to heptachlor epoxide was first reported
by Gannon & Decker (1958a, 1958b). The parameters that control the
rate of epoxidation are still obscure, but several reports suggest
that living tissue, even if only that of micro-organisms, is required
(Davidow & Radomski, 1953). The exact mechanisms by which plants
acquire residues of these compounds are are not understood either.
Evidence for translocation by plants has been produced by Gannon &
Decker (1958), Lichtenstein & Schulz (1960), Dawsey (1961),
Lichtenstein et al. (1965), etc. Special attention has been given
recently to the mechanisms of contamination and the location of these
residues within plants and their stability (Byrne & Steinhauer, 1966;
Burrage & Saha, 1966; King, Clark & Hemken, 1966; Saha & Stewart,
1966). These data are all from Canada and USA. It would be useful to
have comparable information from other sources.
Some information is also becoming available on the relationship
between the residues in soil, the oil content of plants and the
residues that can be expected in them (Bruce & Decker, 1966). This
type of information will have a special bearing on future evaluations
of residues in oil seed crops or fibre crops (soybeans, cotton,
peanuts, rape, etc.) whose by-products become animal food and may thus
lead to residues in human food.
(c) In animals
Residues in animal products have attracted special interest. Low level
residues have been detected and measured recently, in milk and dairy
products using analytical methods that were not previously available
(USA six-month average in up to 10 per cent of diets 0.025 ppm on a
fat basis, in dairy products) and in meat (USA six-month average in 10
per cent diets, 0.05 ppm). It has been suggested that loads of DDT
already stored in the body fat of animals are partially responsible
for the prevention of storage of heptachlor and heptachlor epoxide
(Street, 1966, in press) and their degradation without storage, which
could account for the observation that the loads of heptachlor epoxide
in human body fat are lower in those parts of the world in which a
higher rate of storage of DDT is recorded (Dale et al., 1965; Hayes et
al., 1965; Robinson et al., 1965; Zavon et al., 1965).
(d) In storage and processing
If tolerances are to be based on amounts ingested by consumers,
information on the behaviour of residues during storage and processing
is needed. Although residues from heptachlor have often been regarded
as stable, various data indicate that some disappearance takes place
in storage and processing. This was found in work relating to the
stability of residues in silage (unpublished information from USDA).
Work of Saha & Stewart (1966) indicates that cooking peeled turnips
results in complete disappearance of residues of heptachlor and
heptachlor epoxide in turnip flesh, using an analytical method
sensitive to 0.2 ppm. Similar results have been reported by
Lichtenstein et al. (1965) for carrots. Gooding (1966) suggested that
the normal processes of alkali-refining, bleaching, dehydrogenation
and deodorization will remove all traces from edible vegetable oils,
but definite proof has not been published. Residues do not appear in
sugar or molasses when residues occur in sugar beets, but remain in
the pulp. More information is needed on the fate of residues during
processing.
Methods of analysis
A number of multidetection systems are available for the detection and
determination of residues together with residues of a number of other
compounds. An example is the AOAC system (1966) in which acetonitrile
partition and Florisil column clean-up are used and the residues
identified and measured by gas chromatography coupled with thin layer
or paper chromatography. Alternative clean-up systems, e.g. that of de
Faubert Maunder et al. (1964) using dimethylformamide, and other
methods of confirmation of identity, e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.002 ppm of heptachlor or heptachlor epoxide in milk and
0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The potential hazard resulting from the use of heptachlor in food
production has recently been reduced. The pesticide is now only being
used on seeds and in soil employed in cereal and vegetable production.
Present evidence indicates that soil and seed treatments can be
continued without residues resulting in harvested grain. (The present
evidence pertains to wheat. It may well extend to a wider range of
cereals.)
Since the Temporary ADI is very small (0.0005 mg/kg/day), it is
recommended that a Temporary Tolerance of 0.1 ppm be established, from
uses of the insecticide in the soil and on to seed, only for raw root
vegetables (other than potatoes), cole crops, head lettuce, spinach
and other leafy vegetables. In this recommendation residues of both
heptachlor and heptachlor epoxide are to be combined within the total
of 0.1 ppm. This recommendation is based on the assumption that there
will be no losses of residue in storage and processing, including
cooking.
In addition "practical residue limits" are recommended for potatoes at
0.05 ppm; whole milk 0.002 ppm, dairy products 0.025 ppm on a fat
basis; meat 0.05 ppm (fat basis).
Additional data pertaining to these recommendations should be reviewed
at an early date.
Further information
1. Many of the measurements undertaken prior to about 1961 of residues
after treatments of crops or of soil need to be repeated with the
aid of the more sensitive analytical techniques that have since
become available.
2. More evidence is needed on the extent to which heptachlor is used
in various countries and on the residues which occur in foods in
international commerce; also on the fate of such residues during
the normal course of storage and processing, including cooking,
to which such foods are subjected before consumption.
3. Investigations are desirable on the fate of low levels of
heptachlor in feeds consumed by animals. This is because
information on commercial samples suggest that animal feeds may be
the sources of some of the albeit fairly low residues found in
products from animals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely, R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
Hayes, W. J., jr, Dale, W. E. & Burse, V. W. (1965) Life Sci., 4,
1611
Kettering Laboratory (1959) Unpublished report submitted by Velsicol
Corporation
Kettering Laboratory (1966) Unpublished report submitted by Velsicol
Corporation
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Mestitzova, M. (1966) International Congress on Occupational Health,
Proceedings, 109 (7), 455
Radomski, J. L. & Davidow, B. (1953) J. Pharmacol. exp. Ther.,
107, 266
Remmer, H. (1964) Proceedings of the European Society for the Study
of Drug Toxicity, Vol. 9
Robinson, J., Richardson, J., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Sherman, M. & Ross, E. (1961) Toxicol. appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1957) Nauch. Trudy Inst. Ent.
Fitopat. Akad. Nauk Ukr. SSR, 7, 63
Stemmer, K. L. & Hamdi, E. (1963) Unpublished report of the Kettering
Laboratory, University of Cincinnati
Stemmer, K. L. & Jolley, W. P. (1963) Unpublished report of the
Kettering Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S., Cleveland, F. F., Shaffer, F. E., Schlecht, H. & Musen,
L. (1955) Unpublished report of the Kettering Laboratory, University
of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49: 222-30
Bowman, M. C., Schechter, M. S. & Carter, R. L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. Agric.
Food Chem., 13: 360-365
Bruce, W. N. & Decker, G. C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin, dieldrin,
heptachlor, and heptachlor epoxide. J. Agr. Food Chem., 14: 395-403
Burrage, R. H. & Saha, J. G. (1965) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin or heptachlor.
Can. J. Plant Sci. (In press)
Byrne, H. D. & Steinhauer, A. L. (1966) Mechanisms of contamination of
alfalfa with heptachlor and heptachlor epoxide. J. Econ. Ent., 59:
338-341
Davidow, B. & Radomski, J. L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265
Dale, W. E., Copeland, M. L. & Hayes, W. J. (1965) Chlorinated
insecticides in the body fat of people in India. Bull. Wld Hlth Org.,
33: 477
Dawsey, L. H., Woodham, D. W. & Lofgren, C. S. (1961) Heptachlor and
heptachlor epoxide residues in truck crops. J. Econ. Ent., 54: 6,
1264-5
Duffy, J. R. & Wong, N. (1966) Insecticide residues and their
metabolites in Atlantic soils. In press. J. Agr. Food Chem.
de Faubert Maunder, M. J., Egan, H. & Godly, E. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. Analyst,
89: 168-174
Gannon, N. & Decker, G. C. (1958a) The conversion of heptachlor to its
epoxide on plants. J. Econ. Ent., 51: 3-7
Gannon, N. & Decker, G. C. (1958b) The conversion of aldrin to
dieldrin in plants. J. Econ. Ent., 51: 8-11
Gooding, C. M. B. (1966) Fate of Chlorinated Organic Pesticide
Residues in the Production of Edible Vegetable Oils. Chem. and
Industry, 8: 344
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent.,
54: 1038-1045
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in south western Ontario. Agric. & Food Chem.,
14: 398-403
Hayes, W. J., Dale, W. E. & Burse, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life Sci.,
4: 1611-1615
King, R. L., Clark, N. A. & Hemken, R. W. (1966) Distribution,
movement and persistence of heptachlor and its epoxide in alfalfa
plants and soil. Agric. & Food Chem., 14; 62-65
Lewis, D. T. (1965) Report of the Government Chemist. H.M.S.O. London.
153 pp.
Lichtenstein, E. P. & Polvika, J. B. (1959) Persistence of some
chlorinated hydrocarbon insecticides in turf soils. J. Econ. Ent.,
52: 289-293
Lichtenstein, E. P. & Schulz, K. R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture, and soil
types. J. Econ. Ent., 53: 192-197
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13: 57-62
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1965) Absorption
of insecticidal residues from contaminated soils into five carrot
varieties. J. Agr. Food Chem., 13: 126-131
Robinson, J., Richardson, A., Hunter, G. C., Crabtree, A. N., Rees,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in south eastern England. Brit. J. Ind. Med., 22: 220-229
Saha, J. G. & Stewart, W. W. A. (1966) Vertical and lateral
distribution of heptachlor, heptachlor epoxide, and gamma-chlordane in
rutabagas, grown in heptachlor-treated soil Can. J. Plant Sci. (In
press)
Street, J. C. (1966) Data obtained after feeding heptachlor and DDT to
rats. (In press). J. Agric. Food Chem.
Wiese, I. H. (1964) Some biological studies on the inactivation of
insecticides by various soil types. S. Afr. J. Agric. Sci., 7:
823-835
Wilkinson, A. T. S., Finlayson, D. G. & Morley, H. V. (1964) Toxic
residues in soil 9 years after treatment with aldrin and heptachlor.
Science, 143. 681-2
Young, W, R. & Rawlins, W. A. (1958) The persistence of heptachlor in
soils. J. Econ. Ent., 51: 11-18
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) Chlorinated
hydrocarbon insecticides in the US. J.A.M.A., 193: 837-9
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
In plants and soil, heptachlor is converted to its epoxide which is
more persistent on plants than the parent heptachlor (Gannon & Bigger,
1958; Gannon & Decker, 1958).
Rat liver microsomes have been proved to epoxidize heptachlor in
vitro. The extent of epoxidation in vivo was about 10 times
greater in males than in females (Wong & Terriere, 1965).
Heptachlor epoxide is stored in the fat of dogs add rats. Some storage
occurs in the liver, kidney and muscles but none in the brain. The
metabolite was found in dogs after feeding heptachlor in a
concentration of 1-3 mg/kg body-weight. When heptachlor was fed at
high dosage levels to dogs, small amounts of unmetabolized heptachlor
were also found in the fat, but this was not the case in the rat
regardless of the level fed (Davidow & Radomski, 1953).
In the rat, the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm,
storage occurred in females but not in males, and at 0.1 ppm no
storage occurred in either sex. Female rats accumulate about 6 times
as much epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days, the epoxide level in the milk rose to a maximum of 1.8 ppm,
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at 5 ppm and 2 at 10 ppm daily). Three days after feeding
commenced, the minimum levels of heptachlor epoxide in the milk were
0.26 and 0.34 ppm in the 2 cows fed 5 ppm, and 0.23 and 0.65 ppm in
the 2 cows fed 10 ppm; the maximum levels after 15 days' feeding were
0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively (Storherr et
al., 1960).
Investigations on heptachlor storage in human fat in several parts of
the world have recently been reported. The average ranged between 0
and 0.24 ppm; it tended to be lower in those countries in which a high
storage of DDT was recorded (Dale et al., 1965; Hayes et al., 1965;
Robinson et al., 1965; Zavon et al., 1965).
Heptachlor and its epoxide are included among the chlorinated
hydrocarbon insecticides that stimulate the metabolism of drugs by
microsomal enzymes (Remmer, 1964).
Disturbance of the acid-base balance seems to be of great significance
in the mechanism of action of heptachlor (Spynu & Osetrov, 1957).
Acute toxicity
Heptachlor
Animal Route LD50 References
mg/kg body-weight
Rat Oral 60-142* Velsicol Corporation, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 References
mg/kg body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide in the
mouse are given as 40 and 10 mg/kg body-weight respectively (Radomski
& Davidow, 1953). In the rabbit, the lethal dose of epoxide is 5-10
mg/kg body-weight. The acute effects are neurotoxic disturbances like
those observed with other chlorinated hydrocarbons (Velsicol, 1959).
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide (up to
60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleoli, cytoplasmic fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats, it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla. Electron microscopic studies
demonstrated an increase of rough and smooth endoplasmic reticulum
(Stemmer & Hamdi, 1963).
In a reproduction study covering 3 generations, a group of 80 rats was
given 6.9 mg/kg body-weight of heptachlor daily for 3 months before
mating. Cataracts were found in 6.8 per cent of the young and became
obvious between the 19th and 26th day. Among the parents, 15.2 per
cent of the animals were affected and the lesions appeared after 4-9
months. The only effect on reproduction was decrease of litter size
(Mestitzova, 1966).
The continuous exposure of rats to doses of over 7 ppm of either
heptachlor or its epoxide increased the mortality of the pups during
the suckling period. 10 ppm fed to 3 generations of rate showed no
adverse effects on reproductive capacity, growth or survival (Witherup
et al., 1955; Kettering Laboratory, 1959).
Dog. When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Three dogs given heptachlor epoxide orally in dosages of 2, 4 and 8
mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively. Daily oral doses of 0.25 and 0.5 mg/kg body-weight did
not produce any sign of illness during 52 weeks, but 0.25 mg/kg,
estimated to be equivalent to 6 ppm in the diet, was reported as the
minimal dose producing a pathological effect (Velsicol, 1959).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor epoxide were
given to groups of 5 dogs (2 males and 3 females, 23 to 27 weeks of
age) for 60 weeks. No deaths attributable to heptachlor epoxide
occurred. The weights of the male dogs tended to be inversely
proportional to the concentration of the compound in the diet. The
female dogs had normal weights. The liver weights were increased at 5
ppm and above. Degenerative liver changes were seen in only 1 dog at
7.5 ppm (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed diets
containing 5, 10, 20, 40, 80, 160 and 300 ppm of heptachlor epoxide
for 2 years. Concentrations of 80 ppm or higher resulted in 100 per
cent mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females) (Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10, 20 and
40 ppm for 2 years showed significant increases in mortality only in
females at 40 ppm. Liver weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
CFN rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5,
7.5 and 10 ppm. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34 per cent) and 13/24
(54 per cent) in the control males and females respectively; it was
65/111 (58 per cent) and 92/114 (80 per cent) in the experimental
males and females respectively. Again, many tumours were located in
endocrine organs. Liver tumours were observed in 7 males and 12
females in the experimental groups only (over-all incidence 19/225
(8.4 per cent), but only two of them were malignant (Kettering
Laboratory, 1959).
Heptachlor dissolved in ethanol was added to the diet of CF rats as
1.5, 3, 5, 7 and 10 ppm for 110 weeks. Each group, as well as an
untreated control, included 40 animals (20 of each sex). Mortality was
comparable in all groups. The number of tumour-bearing animals was
16/40 at 0 ppm, 9/40 at 1.5 ppm, 13/40 at 3 ppm, 12/40 at 5 ppm, 15/40
at 7 ppm and 12/40 at 10 ppm. Most tumours were found in the pituitary
and other endocrine organs. No liver tumours were recorded. No
preferential tumour site in any particular group was observed but all
the 4 thyroid tumours observed were in the 7 and 10 ppm groups
(Witherup et al., 1955).
In a recent experiment carried out on a total of 154 female rats, a
mixture of heptachlor/heptachlor epoxide 3:1 was added to the diet at
0, 5, 7.5, 10 and 12.5 ppm for two years. Pituitary and mammary
tumours were seen at all dose levels, including the controls; their
incidence varied from group to group but not in relation to the dose
of heptachlor. At the end of the two years, all groups including the
controls showed liver histological changes, i.e. cell enlargement in
centrolobular parts, loss of cytoplasmic granules and appearance of
lipid vacuoles. No quantitative differences could be detected between
rats given 0 and 5 ppm, whereas in the other groups the severity of
the changes was related to the dose. At 12.5 ppm, regenerative liver
changes were present (Kettering Laboratory, 1966).
Comments
It is well established that heptachlor and its epoxide accumulate in
body fat and persist there for long periods. The formation of epoxide
is relatively rapid in the animal, plants and in the soil.
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the two
substances.
A sex difference in toxicity has been observed, particularly in the
rat, females accumulating heptachlor and its epoxide more than males.
The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to explain. A
possible relation with tumour formation has not been demonstrated,
while evidence has been presented that the change is reversible.
Some suspicion of carcinogenicity could arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 5 ppm in the diet, equivalent to 0.25 mg/kg/day.
Dog. 2.5 ppm in the diet, equivalent to 0.0625 mg/kg/day
Estimate of acceptable daily intake for man
0-0.0005 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that heptachlor and its
epoxide are compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of these compounds, with a view to removing doubts
which may remain as to their safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
The pattern of use for heptachlor has been evolving since it was
introduced to agriculture in the 1950's. Up until 1960, it had a broad
scale of use on forage, cereal, oil seed, vegetable, sugar beet and
some nut crops, such as peanuts. There has been a gradual reduction of
scale and pattern of use of this insecticide for various reasons
including a desire to reduce the unintentional contamination of milk
and animal products.
The present trend in Canada, the USA and Western Europe is towards
restricting its use to vegetable and cereal seed and to applications
to the soil against insects therein. In certain countries it is also
applied to young pasture plants that will not be grazed or fed in the
season of use, to transplant water for seedlings and to ground cover
under a restricted number of orchard trees. In each case there are
restrictions concerning the access of livestock. In Japan, up until at
least 1963, large quantities of heptachlor dust have been used on
upland wheat, barley and rice.
Dosages vary between countries. The average recommended dose is 2
kilos of actual heptachlor per hectare for use on soil prior to or at
planting on cereals and legumes, including barley, green beans, lima
beans, corn, oats, rice, soybeans and wheat. To cereal seeds it is
sometimes used as a slurry at 30-45 grams per 100 kilos applied prior
to planting.
(b) Post-harvest treatments
No post-harvest use that has been reported.
* Sum of heptachlor and heptachlor epoxide by weight.
Tolerances
Canada 0.1 ppm (combined heptachlor and heptachlor epoxide) on beans,
cabbage, lettuce, rutabagas (yellow turnips). (Heptachlor is scheduled
under the Feeds Act as a compound that should not be present in
commercial feed. In 1965 the tolerance for cereals was eliminated.)
USA 0.1 in cabbage, lettuce, rutabagas and snap beans; zero for many
other foods.
Netherlands 0.1 ppm.
In Canada and USA administrative "actionable levels" have also been
established for residues of heptachlor and heptachlor epoxide in milk
and other animal products. (It was the impression of the FAO Working
Party that this practice was also in vogue in other countries, but
reliable information was not available.)
Residues resulting from supervised trials
There is a large amount of information but much of it needs reviewing
in the light of developments in analytical methods since about 1961.
Although some of the earlier work has recently been reviewed in N.
America, reliable recent results are scarce. The published literature
in languages other than English is difficult to obtain. For example,
Ishikura (personal communication, 1965) reports a large scale of use
on cereals in Japan, but information does not appear to be available
on the resulting residues.
Lichtenstein & Polvika (1959), Young & Rawlins (1958) and many other
authors publishing prior to 1961, suggested that no residues of
heptachlor would persist in soil, and that none would be translocated
from soil to plants, especially in forage crops. More reliable
analytical methods have since showed that heptachlor and its epoxide
persist in soil and can result in residues in some species of plants.
The early investigations suggested that heptachlor could be used
effectively on a wide range of vegetables, root crops, potatoes,
forage and peanuts, with residues that would in many instances not
exceed 0.1. ppm in the harvested product. Dawsey, Woodham & Lofgren
(1961) stated that no residues were detected in 14 of 16 crops tested,
but radishes and onions showed significant amounts when grown in plots
treated with 0.25 of 0.5 lb of heptachlor per acre. However,
Lichtenstein & Schulz (1965) found some build up, over a three-year
period in soil from annual application of heptachlor at useful rates.
Residues found in various crops grown in such soil were carrots up to
0.69 ppm, potatoes up to 0.27, radishes up to 0.21 and beets up to
0.29.
Information from Burrage & Saha (1976) however suggests that the use
of heptachlor for seed and soil treatments for certain cereals may be
without fear of residues occurring on harvested grain, but the
possible need for restrictions on the use of straw for animal feed and
the significance of soil residues in crop rotations require further
evaluation. Investigations on the relationships between residues in
oil seeds and certain forage crops to their oil contents, recently
reported by Bruce & Decker (1966), have been less reassuring.
It would be most useful to have the results of similar investigations,
with reliable analytical procedures from other countries. Information
pertaining to residues in successive crops grown in rotation over a
period of years after the initial treatment would be of particular
interest.
Residues in food moving in commerce
Information is slowly becoming available on residues in food moving in
commerce. Some indirect information also is available from total diet
studies, particularly in Canada and the USA. The occurrence of
residues is broadly related to the scale of use in the respective
countries. Residues have varied from traces to 1.0 ppm in up to 10
per cent of the sample diets studied. A six-month average in one USA
study indicated highest residues in these <10 per cent of diets as
meat 0.05 ppm, dairy products (fat basis) 0.025 ppm and other food
0.005 ppm, after processing for consumption.
Canadian investigations of residues in commercial lots of sugar beet
pulp suggest that heptachlor and heptachlor epoxide residues can occur
in this animal feed, which if fed in too large a quantity can result
in undesirable levels of residues occurring in animal products.
Consequently, the feeding of contaminated sugar beet pulp is
restricted to stated levels in the animals' diet. No heptachlor
residues were found in a recent survey of meat and dairy products in
Britain (Lewis, 1965).
The Members of the FAO Working Party on Pesticide Residues, were aware
that information is being obtained on residues in milk and animal
products, commercial feeds, cannery wastes and other agricultural
by-products moving in commerce but these data were not available for
evaluation. Information of this kind is important in the evaluations
of this compound and the co-operation of Member countries of FAO and
WHO is requested in making it available for review.
Fate of residue
(a) In soil
A body of information is becoming available in Canada and the USA.
The persistence of residues in soil appears to be related to soil
type, climate, soil management and cropping practices (Harris, 1966;
Duffy & Wong, 1966; Lichtenstein & Schulz, 1960, 1965a; Harris &
Lichtenstein, 1961; Wilkinson et al., 1964; Saha & Stewart, 1966).
Similar information in other countries is relatively scarce (Weise,
1964). Recent work suggests that low level soil residues may be
contributing directly to residues in animal products, particularly in
arid and semi-arid zones. In these areas, livestock are known to
ingest some soil in foraging, resulting in residues in animal products
that cannot be accounted for on the basis of residues in plants alone.
It is possible that heptachlor and heptachlor epoxide residues can be
dissipated from soil more rapidly than dieldrin or DDT (Harris &
Lichtenstein, 1961).
Bowman et al. (1965) suggested from laboratory evidence that
heptachlor was changed to 1-hydroxychlordene in dry soils with low
organic content and that no conversion of heptachlor to heptachlor
epoxide was detected in these soils. In another study under field
conditions, the same authors detected a small amount of
1-hydroxychlordene in fine sandy loam. Duffy & Wong (1966) have
reported the presence of 1-hydroxychlordene in some Canadian Atlantic
provinces soils, but Harris (personal communication) has not been able
to confirm its presence in Ontario soils. This degradation product has
not been reported as a component of residues in food. (Its presence
would be of interest since the LD50 to rats is only of the order of
2400 mg/kg.) Gamma-chlordane is also a component of residues in soil.
(b) In plants
The conversion of heptachlor to heptachlor epoxide was first reported
by Gannon & Decker (1958a, 1958b). The parameters that control the
rate of epoxidation are still obscure, but several reports suggest
that living tissue, even if only that of micro-organisms, is required
(Davidow & Radomski, 1953). The exact mechanisms by which plants
acquire residues of these compounds are are not understood either.
Evidence for translocation by plants has been produced by Gannon &
Decker (1958), Lichtenstein & Schulz (1960), Dawsey (1961),
Lichtenstein et al. (1965), etc. Special attention has been given
recently to the mechanisms of contamination and the location of these
residues within plants and their stability (Byrne & Steinhauer, 1966;
Burrage & Saha, 1966; King, Clark & Hemken, 1966; Saha & Stewart,
1966). These data are all from Canada and USA. It would be useful to
have comparable information from other sources.
Some information is also becoming available on the relationship
between the residues in soil, the oil content of plants and the
residues that can be expected in them (Bruce & Decker, 1966). This
type of information will have a special bearing on future evaluations
of residues in oil seed crops or fibre crops (soybeans, cotton,
peanuts, rape, etc.) whose by-products become animal food and may thus
lead to residues in human food.
(c) In animals
Residues in animal products have attracted special interest. Low level
residues have been detected and measured recently, in milk and dairy
products using analytical methods that were not previously available
(USA six-month average in up to 10 per cent of diets 0.025 ppm on a
fat basis, in dairy products) and in meat (USA six-month average in 10
per cent diets, 0.05 ppm). It has been suggested that loads of DDT
already stored in the body fat of animals are partially responsible
for the prevention of storage of heptachlor and heptachlor epoxide
(Street, 1966, in press) and their degradation without storage, which
could account for the observation that the loads of heptachlor epoxide
in human body fat are lower in those parts of the world in which a
higher rate of storage of DDT is recorded (Dale et al., 1965; Hayes et
al., 1965; Robinson et al., 1965; Zavon et al., 1965).
(d) In storage and processing
If tolerances are to be based on amounts ingested by consumers,
information on the behaviour of residues during storage and processing
is needed. Although residues from heptachlor have often been regarded
as stable, various data indicate that some disappearance takes place
in storage and processing. This was found in work relating to the
stability of residues in silage (unpublished information from USDA).
Work of Saha & Stewart (1966) indicates that cooking peeled turnips
results in complete disappearance of residues of heptachlor and
heptachlor epoxide in turnip flesh, using an analytical method
sensitive to 0.2 ppm. Similar results have been reported by
Lichtenstein et al. (1965) for carrots. Gooding (1966) suggested that
the normal processes of alkali-refining, bleaching, dehydrogenation
and deodorization will remove all traces from edible vegetable oils,
but definite proof has not been published. Residues do not appear in
sugar or molasses when residues occur in sugar beets, but remain in
the pulp. More information is needed on the fate of residues during
processing.
Methods of analysis
A number of multidetection systems are available for the detection and
determination of residues together with residues of a number of other
compounds. An example is the AOAC system (1966) in which acetonitrile
partition and Florisil column clean-up are used and the residues
identified and measured by gas chromatography coupled with thin layer
or paper chromatography. Alternative clean-up systems, e.g. that of de
Faubert Maunder et al. (1964) using dimethylformamide, and other
methods of confirmation of identity, e.g. using infra-red
spectrophotometry, are also available. The methods are in general
sensitive to 0.002 ppm of heptachlor or heptachlor epoxide in milk and
0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATIONS FOR TOLERANCES
The potential hazard resulting from the use of heptachlor in food
production has recently been reduced. The pesticide is now only being
used on seeds and in soil employed in cereal and vegetable production.
Present evidence indicates that soil and seed treatments can be
continued without residues resulting in harvested grain. (The present
evidence pertains to wheat. It may well extend to a wider range of
cereals.)
Since the Temporary ADI is very small (0.0005 mg/kg/day), it is
recommended that a Temporary Tolerance of 0.1 ppm be established, from
uses of the insecticide in the soil and on to seed, only for raw root
vegetables (other than potatoes), cole crops, head lettuce, spinach
and other leafy vegetables. In this recommendation residues of both
heptachlor and heptachlor epoxide are to be combined within the total
of 0.1 ppm. This recommendation is based on the assumption that there
will be no losses of residue in storage and processing, including
cooking.
In addition "practical residue limits" are recommended for potatoes at
0.05 ppm; whole milk 0.002 ppm, dairy products 0.025 ppm on a fat
basis; meat 0.05 ppm (fat basis).
Additional data pertaining to these recommendations should be reviewed
at an early date.
Further information
1. Many of the measurements undertaken prior to about 1961 of residues
after treatments of crops or of soil need to be repeated with the
aid of the more sensitive analytical techniques that have since
become available.
2. More evidence is needed on the extent to which heptachlor is used
in various countries and on the residues which occur in foods in
international commerce; also on the fate of such residues during
the normal course of storage and processing, including cooking,
to which such foods are subjected before consumption.
3. Investigations are desirable on the fate of low levels of
heptachlor in feeds consumed by animals. This is because
information on commercial samples suggest that animal feeds may be
the sources of some of the albeit fairly low residues found in
products from animals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely, R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
Hayes, W. J., jr, Dale, W. E. & Burse, V. W. (1965) Life Sci., 4,
1611
Kettering Laboratory (1959) Unpublished report submitted by Velsicol
Corporation
Kettering Laboratory (1966) Unpublished report submitted by Velsicol
Corporation
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 126
Mestitzova, M. (1966) International Congress on Occupational Health,
Proceedings, 109 (7), 455
Radomski, J. L. & Davidow, B. (1953) J. Pharmacol. exp. Ther.,
107, 266
Remmer, H. (1964) Proceedings of the European Society for the Study
of Drug Toxicity, Vol. 9
Robinson, J., Richardson, J., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. Ind. Med., 22, 220
Sherman, M. & Ross, E. (1961) Toxicol. appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1957) Nauch. Trudy Inst. Ent.
Fitopat. Akad. Nauk Ukr. SSR, 7, 63
Stemmer, K. L. & Hamdi, E. (1963) Unpublished report of the Kettering
Laboratory, University of Cincinnati
Stemmer, K. L. & Jolley, W. P. (1963) Unpublished report of the
Kettering Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S., Cleveland, F. F., Shaffer, F. E., Schlecht, H. & Musen,
L. (1955) Unpublished report of the Kettering Laboratory, University
of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
AOAC (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49: 222-30
Bowman, M. C., Schechter, M. S. & Carter, R. L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. Agric.
Food Chem., 13: 360-365
Bruce, W. N. & Decker, G. C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin, dieldrin,
heptachlor, and heptachlor epoxide. J. Agr. Food Chem., 14: 395-403
Burrage, R. H. & Saha, J. G. (1965) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin or heptachlor.
Can. J. Plant Sci. (In press)
Byrne, H. D. & Steinhauer, A. L. (1966) Mechanisms of contamination of
alfalfa with heptachlor and heptachlor epoxide. J. Econ. Ent., 59:
338-341
Davidow, B. & Radomski, J. L. (1953) Isolation of an epoxide
metabolite from fat tissues of dogs fed heptachlor. J. Pharmacol.
Expt. Therap., 107: 259-265
Dale, W. E., Copeland, M. L. & Hayes, W. J. (1965) Chlorinated
insecticides in the body fat of people in India. Bull. Wld Hlth Org.,
33: 477
Dawsey, L. H., Woodham, D. W. & Lofgren, C. S. (1961) Heptachlor and
heptachlor epoxide residues in truck crops. J. Econ. Ent., 54: 6,
1264-5
Duffy, J. R. & Wong, N. (1966) Insecticide residues and their
metabolites in Atlantic soils. In press. J. Agr. Food Chem.
de Faubert Maunder, M. J., Egan, H. & Godly, E. W., Hammond, E. W.,
Roburn, J. & Thomson, J. (1964) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. Analyst,
89: 168-174
Gannon, N. & Decker, G. C. (1958a) The conversion of heptachlor to its
epoxide on plants. J. Econ. Ent., 51: 3-7
Gannon, N. & Decker, G. C. (1958b) The conversion of aldrin to
dieldrin in plants. J. Econ. Ent., 51: 8-11
Gooding, C. M. B. (1966) Fate of Chlorinated Organic Pesticide
Residues in the Production of Edible Vegetable Oils. Chem. and
Industry, 8: 344
Harris, C. R. & Lichtenstein, E. P. (1961) Factors affecting the
volatilization of insecticidal residues from soils. J. Econ. Ent.,
54: 1038-1045
Harris, C. R., Sans, W. W. & Miles, J. R. W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in south western Ontario. Agric. & Food Chem.,
14: 398-403
Hayes, W. J., Dale, W. E. & Burse, V. W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life Sci.,
4: 1611-1615
King, R. L., Clark, N. A. & Hemken, R. W. (1966) Distribution,
movement and persistence of heptachlor and its epoxide in alfalfa
plants and soil. Agric. & Food Chem., 14; 62-65
Lewis, D. T. (1965) Report of the Government Chemist. H.M.S.O. London.
153 pp.
Lichtenstein, E. P. & Polvika, J. B. (1959) Persistence of some
chlorinated hydrocarbon insecticides in turf soils. J. Econ. Ent.,
52: 289-293
Lichtenstein, E. P. & Schulz, K. R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture, and soil
types. J. Econ. Ent., 53: 192-197
Lichtenstein, E. P. & Schulz, K. R. (1965) Residues of aldrin and
heptachlor in soils and their translocation into various crops. Agric.
& Food Chem., 13: 57-62
Lichtenstein, E. P., Myrdal, G. R. & Schulz, K. R. (1965) Absorption
of insecticidal residues from contaminated soils into five carrot
varieties. J. Agr. Food Chem., 13: 126-131
Robinson, J., Richardson, A., Hunter, G. C., Crabtree, A. N., Rees,
H. J. (1965) Organochlorine insecticide content of human adipose
tissue in south eastern England. Brit. J. Ind. Med., 22: 220-229
Saha, J. G. & Stewart, W. W. A. (1966) Vertical and lateral
distribution of heptachlor, heptachlor epoxide, and gamma-chlordane in
rutabagas, grown in heptachlor-treated soil Can. J. Plant Sci. (In
press)
Street, J. C. (1966) Data obtained after feeding heptachlor and DDT to
rats. (In press). J. Agric. Food Chem.
Wiese, I. H. (1964) Some biological studies on the inactivation of
insecticides by various soil types. S. Afr. J. Agric. Sci., 7:
823-835
Wilkinson, A. T. S., Finlayson, D. G. & Morley, H. V. (1964) Toxic
residues in soil 9 years after treatment with aldrin and heptachlor.
Science, 143. 681-2
Young, W, R. & Rawlins, W. A. (1958) The persistence of heptachlor in
soils. J. Econ. Ent., 51: 11-18
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) Chlorinated
hydrocarbon insecticides in the US. J.A.M.A., 193: 837-9
