First draft prepared by
D.B. McGregor,
International Agency for Research on Cancer, Lyon, France
Deltamethrin [(S)-alpha-cyano-3-phenoxybenzyl(1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclo-propanecarboxylate] was first reviewed by the 1980 JMPR, when it was determined that there was insufficient information to establish an ADI (Annex 1, reference 34). Additional data were received and reviewed by the 1981 JMPR (from a 2-year feeding study in dogs, a 2-year study of carcinogenicity in mice, studies of teratogenicity in mice and rats, additional information on mutagenicity, and human data), but again no ADI was established (Annex 1, reference 36). The 1982 JMPR reviewed the results of studies that helped to clarify previous concerns, particularly with regard to embryotoxicity, and an ADI of 0–0.01 mg/kg bw was established (Annex 1, reference 38). Deltamethrin was reviewed by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
(a) Absorption, distribution, and excretion
Male rats (strain not reported) were given single oral doses of deltamethrin labelled at the dibromovinyl carbon (Cv) at 0.90 mg/kg bw (three rats), the benzylic carbon (14Calpha) at 1.6 mg/kg bw (two rats), or the cyano carbon (14CN) at 0.64 mg/kg bw (three rats). No rationale was given for the use of different doses. In a separate set of experiments, rats were dosed orally or intraperitoneally with 0.1 mg/kg bw of [14CN]deltamethrin or 0.1 mg/kg bw of K14CN. The rats were held for 8 days after treatment for collection of urine and faeces, during which time the 14CO2 (if any) was collected for 2 days. Residual radiolabel was determined in 14 tissues and blood after 8 days. The total recovery of 14C in excreta, isolated tissues, and carcass represented 97 ± 5.8% of the administered dose. Radiolabel from [14Cv]- and [14Calpha]deltamethrin was almost completely eliminated from the body within 2–4 days, with very little tissue retention except in fat (0.59 and 0.18 µg/kg from the two labels, respectively). The radiolabel from the 14CN label was excreted more slowly, and the concentrations of residues in tissues were higher, especially in the skin and stomach, due, in the latter case, to temporary retention of thiocyanate formed from released cyanide. No 14CO2 was found, indicating that there was no extensive fragmentation of the acid or alcohol moieties.
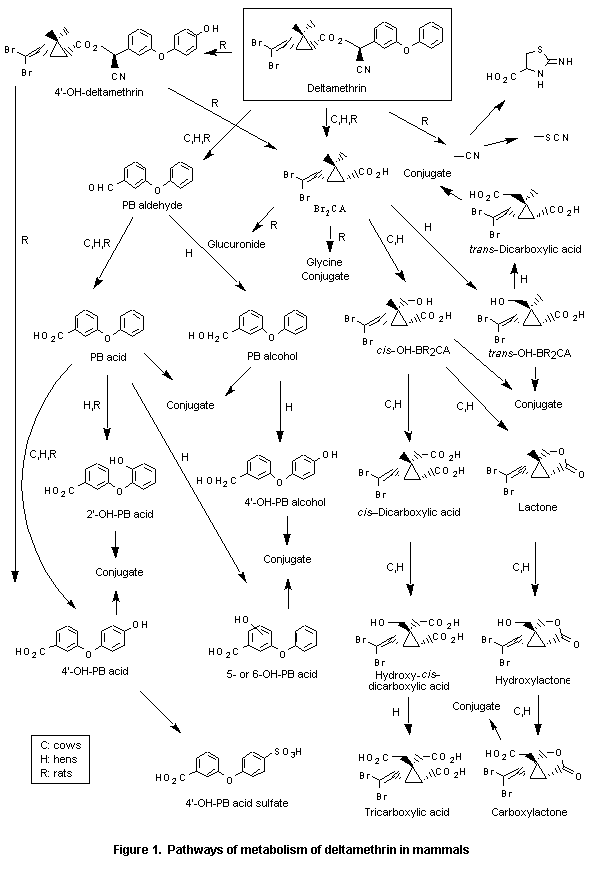
The identified metabolites excreted within 8 days accounted for 94% and 98%, respectively, of the administered [14Cv]- and [14Calpha]deltamethrin but only 64% of the administered [14CN]deltamethrin, partly because of its slower metabolism ([14CN]deltamethrin metabolites were still being excreted after 8 days) and partly because of difficulties in extracting the metabolites from faeces. The principal mechanisms of metabolism are ester cleavage and oxidation at the 4 position of the phenoxy ring of the alcohol moiety. The major compounds (> 10%) were: unchanged deltamethrin (13–20%) in faeces after oral administration, 3-phenoxybenzoic acid (PB acid) as the glucuronide conjugate (13%), and 4’-hydroxy-3-phenoxybenzoic acid sulfate (4’-OH-PB acid sulfate; 49%) from the alcohol moiety and 3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylic acid (Br2CA; 10%) and its glucuronide conjugate (50%) from the acid moiety. The major metabolite of [14CN]deltamethrin administered orally or intraperitoneally was thiocyanate (38% after oral administration). Cleavage of the deltamethrin ester group also led to a small amount of 2-iminothiazolidine-4-carboxylic acid (4.7%). The trans isomer of deltamethrin was also rapidly metabolized in rats. Many metabolites that contributed less than 10% of the total were also identified by the authors (Ruzo et al., 1978).
The pathways of metabolism of deltamethrin in mammals are shown in Figure 1.

The absorption, distribution, elimination, and biotransformation of [14C]deltamethrin were studied in groups of five male and five female Crl:CD(SD)BR rats dosed orally according to the protocol of the Environmental Protection Agency with deltamethrin labelled in one of two positions. Two males and two females were used as controls. The dosing schedule is shown in Table 1. The rats were killed 7 days after treatment, and selected tissue samples were collected, weighed, and saved for determination of total radiolabel. The labelled residues in selected urine and faecal samples collected during the first 24 h after dosing were characterized by thin-layer and high-performance liquid chromatography. The mean amount of radiolabel recovered per group represented 88–95% of the total administered. CO2 was not collected in this study, because no 14CO2 had been detected by Ruzo et al. (1978). Most of the radiolabel was excreted in the urine (31–56%) and faeces (36–59%). during the first 24 h after treatment. The percentage amounts of identified metabolites recovered in urine and faeces are shown in Table 2.
Table 1. Dose regimens of labelled deltamethrin in Crl:CD(SD)BR rats
|
Group no. |
Labelled material |
Dose |
Daily oral dose regimen |
No. of rats |
|
|
Male |
Female |
||||
|
1 |
|
0 |
– |
1 |
1 |
|
2 |
[14C]Benzyl |
0.55 mg/kg bw |
Single |
5 |
5 |
|
3 |
[14C]Benzyl |
0.55 mg/kg bw per day |
14 doses of unlabelled, 1 dose of labelled |
5 |
5 |
|
4 |
[14C]Benzyl |
5.5 mg/kg bw |
Single |
5 |
5 |
|
5 |
|
0 |
– |
1 |
1 |
|
6 |
[14C]Dimethyl |
0.55 mg/kg bw |
Single |
5 |
5 |
|
7 |
[14C]Dimethyl |
0.55 mg/kg bw per day |
14 doses of unlabelled, 1 dose of labelled |
5 |
5 |
|
8 |
[14C]Dimethyl |
5.5 mg/kg bw |
Single |
5 |
5 |
Table 2. 14C-labelled compounds identified in excreta of rats after oral administration of deltamethrin, expressed as per cent of administered dose
|
Label |
Excreta |
Compound |
Per cent of dose |
|||||
|
Males |
Females |
|||||||
|
Group 2 |
Group 3 |
Group 4 |
Group 2 |
Group 3 |
Group 4 |
|||
|
14 C-Benzyl |
Faeces |
Deltamethrin |
17 |
21 |
34 |
34 |
20 |
46 |
|
4´-OH deltamethrin |
3 |
5 |
6 |
3 |
4 |
3 |
||
|
Urine |
4´-OH-PB acid sulfate |
49 |
49 |
35 |
44 |
40 |
30 |
|
|
PB acid |
3 |
2 |
2 |
2 |
4 |
2 |
||
|
Total |
|
72 |
77 |
77 |
83 |
68 |
81 |
|
|
|
Group 6 |
Group 7 |
Group 8 |
Group 6 |
Group 7 |
Group 8 |
||
|
14 C-Dimethyl |
Faeces |
Deltamethrin |
27 |
23 |
35 |
28 |
21 |
32 |
|
4´-OH deltamethrin or Br2CA |
NQ |
NQ |
NQ |
NQ |
NQ |
NQ |
||
|
Urine |
Br2CA glucuronide |
31 |
30 |
22 |
36 |
38 |
33 |
|
|
Br2CA |
5 |
7 |
7 |
9 |
10 |
4 |
||
|
Total |
|
63 |
60 |
64 |
73 |
69 |
69 |
|
NQ, not quantifiable; 3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylic acid (Br2CA) and 4-hydroxydeltamethrin (4-OH deltamethrin) were not separated by thin-layer chromatography, but high-performance liquid chromatography showed there was no Br2CA. Thus, in animals given [14C-benzyl]deltamethrin, the 14C-labelled residues that represented more than 10% of the radiolabel in the sample were deltamethrin in faeces and 4’-OH-PB acid sulfate with a small amount of 3-phenoxybenzoic acid in urine.
When expressed as a percentage of dose, the deltamethrin recovered constituted 17, 21, and 34% of the dose for males and 34, 20, and 46% of the dose for females in groups 2, 3, and 4, respectively. 4’-OH-PB acid sulfate represented averages of 49, 49, and 35% of the dose for males and 44, 40, and 30% of the dose for females in these groups, respectively. When the results for urine and faeces were combined, 60–78% of the dose was identified as either deltamethrin or 4’-OH-PB acid sulfate. The presence of this metabolite was confirmed by acid hydrolysis and co-chromatography with 4’-OH-PB acid.
For animals dosed with [dimethyl-14C]deltamethrin, the 14C-labelled residues that represented more than 10% of the radiolabel in the sample were identified as deltamethrin in faeces and as Br2CA glucuronide with a small amount of the unconjugated compound in urine. When expressed as a percentage of the dose, deltamethrin constituted 27, 23, and 35% of the dose for males and 28, 21, and 32% of that for females in groups 6, 7, and 8, respectively. The glucuronide made up an average of 31, 30, and 22% of the dose for males and 36, 38, and 33% of the dose for females in thse groups, respectively. When the results for urine and faeces analysis were combined, 53–65% of the dose was identified as either deltamethrin or the glucuronide.
Deltamethrin was present in the faeces but not in the urine, indicating cleavage of the ester bond of deltamethrin before excretion in the urine. This degradation pattern was observed with both 14C labels. No other metabolites comprising more than 10% of the dose were present in urine or faecal samples. Females generally excreted more of the dimethyl radiolabel in urine and less in faeces than did males, but the opposite was observed with the benzyl label.
The amounts of radiolabel retained in tissues and carcass 7 days after dosing were generally low, representing only 0.59–1.9% of the total dose administered. Fat contained the highest concentration of residues labelled with either label, the deltamethrin-equivalent concentrations being 0.052–0.093 mg/kg at the low doses (groups 2, 3, 6, and 7) and 0.50–0.84 mg/kg at the high doses (groups 4 and 8) (Bosch, 1990).
Female rats were given a single intravenous dose of [benzyl-14C]deltamethrin (60 µCi/mg or 2.2 MBq/mg) at an average dose of 2.4 mg/kg bw. The concentrations of radiolabel in urine, faeces, and selected organs and tissues were measured at 1, 4, 24, and 120 h. After 1 h, the equivalent concentration in blood was 3.0 µg/g; higher concentrations were found in the liver (6.0 µg/g), kidney (3.1 µg/g), fat (2.2 µg/g), and ovaries (4.0 µg/g) and lower values in brain, the cervical, thoracic, and lumbar areas of the spinal cord, and the sciatic nerve, ranging from 0.22 to 0.33 µg/g. Radiolabel was rapidly eliminated from the blood between 1 and 120 h, with a half-life of 5.5 h. The half-lives were > 24 h in fat, 28 h in sciatic nerve, and 15 h in skin; in all other selected organs and tissues, they were 4.8–7.1 h. After 72 h, urinary excretion was complete and accounted for an average of 55% of the dose, about 10% having been excreted within 0–4 h and 48–50% within the first 24 h. After 120 h, faecal excretion accounted for 27% of the dose, 24% having been excreted within the first 48 h. When the cage wash (average, 4.7 %) was taken into account, the total excreted radiolabel accounted for 87% of the dose. After 120 h, the concentrations of radiolabel in tissues were < 0.08 µg/g, except in fat (1.4 µg/g) and ovaries (0.13 µg/g). The concentrations in brain and spinal cord were at or below the limit of quantification The results indicate that intravenously administered deltamethrin is most persistent in fat. In general, deltamethrin was rapidly eliminated and excreted in the urine in fourfold higher amounts than in the bile and faeces (Van Dijk & Burri, 1993a).
Lactating cows
The fate and residues of [14C-gem-dimethyl]- or [14C-benzyl]deltamethrin were determined in lactating dairy cows after oral administration of10 mg/kg bw per day for 3 consecutive days, the animals being slaughtered 24 h after the last dose. Deltamethrin appeared to be poorly absorbed from the gastrointestinal tract, as the concentrations in blood were low and little radiolabel was recovered in urine, but the deltamethrin that was absorbed was extensively metabolzed and excreted, ostensibly in the bile and urine with little accumulation in the major edible tissues. About 36–43% of the total administered radiolabel was eliminated in faeces within 24 h of the last dose. Most (78–82%) of the radiolabel in faeces was associated with deltamethrin. Only 4–6% of the administered dose was eliminated in urine, and 0.42–1.6% was secreted in the milk. The capacity to metabolize deltamethrin was high, resulting in a very low concentration of parent compound in the milk. Radiolabel was secreted into milk, and the concentration was higher with the gem-dimethyl label (0.69 µg/g) than with the benzyl label (0.36 µg/g). Deltamethrin was the major product in milk (0.10–0.14 µg/g). The radiolabel content of various tissues was higher with the 14C-benzyl label but was generally very low (0.1 µg/g), except in liver (3.2 µg/g), kidney (2.2 µg/g), udder (0.62 µg/g), and abdominal and subcutaneous fat (0.55 µg/g) (Akhtar et al., 1986).
Laying hens
Leghorn hens were given 7.5 mg of [14C-gem-dimethyl]- or [14C-benzyl]deltamethrin per day orally for 3 consecutive days. About 83% and 90% of the administered 14C was eliminated during the first 24 and 48 h after dosing, respectively. The concentrations in tissues were generally low, except in the liver (Ł 4.0 µg/g) and kidney (Ł 6.9 µg/g), and were very low in muscle (traces to 0.021 µg/g). Very low concentrations of residues were found in eggs obtained within the first 24 h after dosing, but they then increased, reaching a peak within 48 h of the last dose. The concentrations were higher in the yolk (Ł 0.6 mg/kg) than in the albumen (Ł 0.2 mg/kg) but dissipated much more quickly from albumen than from yolk. The metabolic routes consisted of hydrolysis of the ester linkage followed by hydroxylation of one or both gem-dimethyl groups (not observed in rodents) and hydroxylation of the 2-, 4-, 5-, or 6-carbon positions of the phenoxybenzyl moiety. The acid moiety was excreted with its oxidized products as both free compounds and their glucuronides. The major radiolabelled material identified in liver, kidneys, and eggs was unchanged deltamethrin (Akhtar et al., 1985).
In hens given a mixture of pyrethroids (cypermethrin, deltamethrin, and fenvalerate), the substances were still detectable 15 days later in the tissues by conventional analytical methods, and the highest concentrations were invariably found in the brain. It was also reported that the active substances had accumulated in the brain after being eliminated from other organs (Saleh et al., 1986). These results were not supported by the results of studies of the distribution and excretion of deltamethrin in female rats and laying hens after intravenous injection (thereby avoiding any absorption problems), as described above. Nevertheless, the study of the combination was repeated in laying hens after oral administration but with radiolabelled substances to ensure greater sensitivity of detection. Thus, a mixture of [14C]cypermethrin, [benzyl-14C]deltamethrin, and fenvalerate was administered as a single oral dose to laying hens at a total dose of 30 mg/kg bw, representing 10 mg/kg bw of each substance. Radiolabel was eliminated rapidly from the blood with a half-life of 8.1 h and was excreted mainly (80%) within the first 24 h. After 168 h, 89 ± 12% of the dose had been excreted. The highest concentrations of radiolabel were found in liver (7.7 µg/g), kidney (16 µg/g), and blood (3.4 µg/g), followed by fat (1.3 µg/g) and lung (1.2 µg/g). The lowest concentrations were found in sciatic nerve, brain, and spinal cord (0.16–0.38 µg/g). Short half-lives were found in liver, kidney, blood, brain, spinal cord, and sciatic nerve (4.9–8.1 h) but not in fat. By 24 h after oral administration, the concentrations in the brain and spinal cord were below the limits of detection. The mixturethus behaves qualitatively similarly to each pyrethroid separately, and pyrethroid does not persist in laying hens, especially in nervous tissues (Van Dijk, 1994).
In order to obtain information on the possible persistence of deltamethrin residues in organs and tissues, [14C-benzyl]deltamethrin was administered intravenously at an average dose of 0.4 mg/kg bw, and radiolabel was determined in excreta and selected organs and tissues after 1, 4, 24, and 120 h. Excretion, as a percentage of the administered dose, accounted for averages of 40, 47–60, 54, and 64% after 4, 24, 48, and 120 h, respectively. When the cage wash (average, 1.7%) was taken into account, the average total excreted radiolabel represented 66% of the dose. One hour after administration, the concentration of radiolabel in blood was 0.40 µg/g, and the highest concentrations were found in the liver (1.1 µg/g) and kidney (0.96 µg/g); the concentrations in heart, muscle, fat, ovaries, and back skin were 0.10–0.18 µg/g. The lowest values were found in the brain, the cervical, thoracic, and lumbar regions of the spinal cord, and the sciatic nerve, ranging from 0.021 to 0.066 µg/g. Between 1 and 120 h, radiolabel was rapidly eliminated from the blood with a half-life of 5.5 h. The half-lives in the selected organs and tissues were relatively short, 5.6–9.8 h, except in fat (> 24 h), ovaries (53 h), and back skin (17 h). After 120 h, all the values were < 0.040 µg/g except in fat (0.091 µg/g) and ovaries (0.060 µg/g). The concentrations in heart, muscle, brain, and spinal cord were at or below the limit of quantification (Van Dijk & Burri, 1993b).
As the metabolites observed in studies in plants (the only differences being in the conjugated forms) are no different from those observed in animals, the toxicological data derived from experiments with deltamethrin cover them.
These studies are summarized in Table 3.
Table 3. Acute oral toxicity of deltamethrin in rats
|
Strain |
Sex |
Vehicle |
LD50 (range) (mg/kg bw) |
Reference |
|
Sprague-Dawley |
Male |
PEG 200 |
67 (53–84) |
Glomot & Chevalier (1976) |
|
Sprague-Dawley |
Female |
PEG 200 |
86 (71–106) |
Glomot & Chevalier (1976) |
|
Sprague-Dawley |
Male |
Sesame oil |
129 (105–156) |
Glomot & Chevalier (1976) |
|
Sprague-Dawley |
Female |
Sesame oil |
139 (114–168) |
Glomot & Chevalier (1976) |
|
Sprague-Dawley |
Male, |
Aqueous suspension |
> 5000 (no deaths) |
Myer (1989a) |
|
|
Female |
1% methylcellulose |
|
|
|
Sherman |
Male |
Peanut oil |
52 |
Kavlock et al. (1979) |
|
Sherman |
Female |
Peanut oil |
31 |
Kavlock et al. (1979) |
|
Sherman |
Female weanling |
Peanut oil |
50 |
Kavlock et al. (1979) |
|
Sprague-Dawley |
Male |
Corn oil |
95 (74–122) |
Varsho (1995) |
|
Female |
Corn oil |
87 (77–97) |
Varsho (1995) |
Rats given deltamethrin in PEG 200 or sesame oil by gavage showed motor incoordination, convulsions, respiratory defects, and hypomotility shortly afterwards. The surviving animals behaved normally 3 days after dosing but died within the first 4 days (Glomot & Chevalier, 1976).
Deltamethrin administered in aqueous suspension in 1% w/v methylcellulose was not toxic to rats, since all animals at 5000 mg/kg bw survived. There were no changes in body weight, and no visible abnormalities were found either during the study or post mortem (Myer, 1989a).
When deltamethrin was administered to Sherman rats in solution in peanut oil, moderate to severe salivation and convulsions were seen at doses from 10 mg/kg bw in males, and mild salivation was seen at doses from 10 mg/kg bw in females and from 15 mg/kg bw in weanling females. The respective LD50 values were 52, 31, and 50 mg/kg bw (Kavlock et al., 1979).
Technical-grade deltamethrin was micronized so that the mass median aerodynamic diameter of the dust was 3 µm (± 1.9). Three groups of five male and five female rats were exposed for 4 h to actual concentrations of 1, 1.8, and 2.3 mg/L. Deaths occurred on the day of exposure and up to 14 days afterwards. All animals that survived the first 24 h had impaired hindlimb function, mainly on days 2–3 after exposure, which persisted for about 7 days. All animals lost significant body weight during the first week after exposure. Pulmonary congestion was the most significant abnormality at necropsy, observed in two rats at the high dose and three at the intermediate dose. The 4-h LC50 for males and females combined was calculated to be 2.2 mg/L (1.5–3.3 mg/L) (Ulrich, 1990).
Ten adult Sprague-Dawley rats of each sex were exposed to an aerosol generated from 10% dimethyl sulfoxide solutions of the technical substance. The chamber concentration was evaluated by gas chromatography. The LC50 was 940 mg/m3 for males and 780 mg/m3 for females (Kavlock et al., 1979).
When given intravenously, technical-grade deltamethrin in acetone was highly toxic. The LD50 was 4 mg/kg bw in adult female Sherman rats, moderate to severe salivation and convulsions occurring at doses from 1.6 mg/kg bw. Weanling females appeared to be even more sensitive, with an LD50 of 1.8 mg/kg bw, and some clinical signs appeared at doses from 0.78 mg/kg bw (Kavlock et al., 1979). When polyethylene glycol (PEG) 300 was the vehicle, the LD50 in female HanIbm:WIST rats was 24 mg/kg bw (Hoff, 1992).
Deltamethrin did not induce acute toxic effects after percutaneous application. There were no deaths or signs of poisoning in rats treated percutaneously at a dose of 2900 mg/kg bw with deltamethrin in an aqueous suspension with 1% w/v methylcellulose, and no observable dermal reactions at the site of application. The body-weight gains of four of five treated females were depressed during the first week of observation, but they returned to normal during the second week. The findings at autopsy were within normal limits (Kynoch et al., 1979). No signs of toxicity were found after dermal application of deltamethrin at 800 mg/kg bw in xylene to adult female Sherman rats, a dose that was 80 times the minimal oral dose that had effects (Kavlock et al., 1979).
A single dose of 0.5 g of deltamethrin (purity, 98%) was applied to the intact or abraded skin of male New Zealand rabbits (12 per group) for 23 h. No erythema or oedema was observed 24 or 72 h after application (Coquet, 1976a). When a single dose of 0.5 g of deltamethrin (purity, 99.2%) slightly moistened with 1% aqueous methylcellulose was applied to the shaven intact skin of three male and three female New Zealand white rabbits for 4 h, there were no signs of dermal irritation, and the primary irritation index, calculated by the Draize method, was 0.0, indicating no irritation (Myer, 1989b).
Deltamethrin (purity, 100%) was tested for sensitizing potential in a maximization test (modified from the Magnusson-Kligman method) in guinea-pigs. It was applied during the induction phase at 0.5 g per animal to the skin of 10 male and 10 female Hartley albino guinea-pigs three times a week at 2-day intervals for 3 weeks and once at the start of the fourth week (for 10 applications). The compound was kept on the skin under occlusive patches for 48 h. On days 1 and 10, the guinea-pigs were injected intradermally with 0.1 ml of Freund adjuvant. They were challenged 12 days after the last application with 0.5 g of undiluted deltamethrin. The compound did not induce dermal reactions. A preliminary study showed that 0.5 g of deltamethrin did not cause primary skin irritation (Guillot & Guilane, 1977).
Deltamethrin (purity, 99.2%) was also tested in groups of 10 Hartley guinea-pigs of each sex according to the Buehler method. In the induction phase, 0.4 g of deltamethrin moistened slightly with 1% aqueous methylcellulose was applied to the skin for 6 h on days 1, 7, and 14. A positive control group of five male and five female guinea-pigs was treated similarly with 2,4-dinitrochlorobenzene, and another group of five male and five females remained untreated. All animals were challenged for 6 h on day 28 with either deltamethrin (treatment and control groups) or 2,4-dinitrochlorobenzene. All animals survived. No remarkable changes or differences in body weight were observed. None of the guinea-pigs treated with deltamethrin had an erythema score ł 1, whereas seven animals given 2,4-dinitrochlorobenzene had a score ł 1 at 24 or 48 h. Deltamethrin was considered not to be a sensitizing agent in Hartley guinea-pigs treated by the Buehler method (Myer, 1989d).
Deltamethrin (purity, 98%) was administered at 0.1 g into the conjunctival sac of the eye of six male New Zealand white rabbits, with and without rinsing 60 s after instillation. Transient irritation was seen both with and without rinsing, after 1 h and 24 h but not 72 h after instillation (Coquet, 1976b).
Deltamethrin (purity, 99.2%) was applied as a powder at a dose of 87 mg into the conjunctival sac of the right eye of three male and three female New Zealand white rabbits. Marked conjunctival redness, slight to marked discharge, and slight swelling were observed after 1 h. No irritation was seen in any of the rabbits by 72 h. Deltamethrin was considered not to be irritating to the eye (Myer, 1989c).
(b) Short-term studies of toxicity
Mice
Deltamethrin (purity not stated) was administered in the diet to groups of 35 male OFA (Swiss-derived) mice at a concentration of 0 or 200 ppm, equivalent to 0 or 30 mg/kg bw per day, for 10 days and then 0 or 400 ppm, equivalent to 0 or 60 mg/kg bw per day, for the following 17 days. The treated mice had lower food consumption than the controls during the entire 27-day period; however, because of wide interindividual variation, the differences due to treatment were rarely statistically significant. The body weights of treated mice were reduced, with an actual loss of weight in those given the 400 ppm diet; five mice in this group died. The absolute weight of the liver and that relative to body weight and the absolute weights of the kidneys were reduced in the survivors of the treated groups. Histological examination of these organs revealed no lesions (Rouaud & Marzin, 1977b).
Deltamethrin (purity 99.7%) was administered in the diet to groups of 10 CD1 Cr1 mice of each sex at a concentration of 0, 30, 300, 3000, or 6000 ppm, equal to 0, 6, 62, 600, and 1300 mg/kg bw per day for males and 0, 8, 77, 740, and 1400 mg/kg bw per day for females, for 12 weeks. Satellite groups of five animals of each sex per group were given a diet containing 30 or 6000 ppm and were used for determination of plasma concentrations at the end of the study if the toxicity was excessive. The groups were checked for deaths twice daily, and clinical signs were recorded at least once daily. Body weight and food consumption were measured weekly, and blood was taken for haematology at week 12 and blood chemistry at week 13. At the end of the experiment, the mice were killed, organ weights were recorded, the organs were submitted to a full macroscopic examination, and the tissues were examined microscopically.
Treatment-related clinical signs were most frequently observed during the day(s) immediately preceding death. Clonic convulsions were observed in 14/15 males and 10/15 females given 6000 ppm (numbers include the satellite group) and in 1/10 males at 3000 ppm. Convulsions were observed in 1/15 males and 2/15 females at 6000 ppm. Piloerection, dyspnoea, and arched back were seen among the mice given 3000 or 6000 ppm, indicating their poor general condition. Death, most probably resulting from treatment, occurred in 14/15 males and 14/15 females at the highest dose and in 3/10 males and 1/10 females at 3000 ppm. One male and one female in the control group died. The body-weight gain of males was slightly decreased at the end of the study in comparison with the control group, by 8% in those given 30 or 300 ppm and by 14% in those given 3000 ppm. Females at 3000 ppm showed a marked decrease in body weight at the beginning of the treatment period, which was maintained throughout the study, so that the terminal deficit was 11%. No changes were found in haematological or blood chemical parameters, organ weights, or the macroscopic appearance of organs. Histology showed thymic involution (lymphoid depletion) and lipid depletion of the adrenals in mice at 3000 and 6000 ppm, which were considered to be indicative of stress secondary to the poor physical condition of the mice before death. A direct effect of treatment appeared unlikely. The NOAEL was 300 ppm, equal to 62 mg/kg bw per day, on the basis of clonic contractions and clinical signs of poor condition at 3000 ppm, equal to 600 mg/kg bw per day (Fabreguettes, 1991).
Rats
Deltamethrin (purity, 98%) was administered in the diet to groups of 10 male OF1 (Sprague-Dawley derived) weanling rats at a concentration of 0 or 200 ppm, equivalent to 20 mg/kg bw per day, for 28 days. The food consumption of the treated rats was lower than that of the control group during the first week, resulting in reduced body-weight gain over the same period; subsequently, the evolution of body weight remained similar in the two groups (Rouaud & Marzin, 1977a).
Deltamethrin (purity not stated) was administered by gavage in PEG 200 to groups of 20 weanling Sprague-Dawley rats of each sex at a dose of 0, 0.1, 1, 2.5, or 10 mg/kg bw per day for 13 weeks. After treatment, five males and five females per group were allowed to recover for 4 weeks. No treatment-related effects were observed on food or water consumption, mortality rate, or urinary or haematological parameters. Extensive neurological examinations and ophthalmoscopy revealed no abnormalities. Some rats at the highest dose showed slight neurological hypersensitivity (not explained) in week 6, but not after 13 weeks of treatment. Statistically significantly reduced body-weight gain was noted in males at 2.5 or 10 mg/kg bw per day (p < 0.05). No clear treatment-related effects were seen in the results of laboratory investigations of urine and blood, the eyes, or the weights of organs. Gross and microscopic examination of various tissues and organs showed no treatment-related changes. The NOAEL was 1 mg/kg bw per day on the basis of lowered body weight in males at 2.5 mg/kg bw per day (Hunter et al., 1977).
Deltamethrin (purity, 98.9%) was administered in the diet to groups of 10 Sprague-Dawley rats of each sex at a concentration of 0, 30, 300, 3000, or 6000 ppm, equal to 0, 2.4, 24, 240, and 420 mg/kg bw per day, for males and 0, 2.7, 31, 270, and 440 mg/kg bw per day for females, for 13 weeks. Additional groups were given a diet containing 0 or 1000 ppm, equal to 72 mg/kg bw for males and 84 mg/kg bw for females for 13 weeks and then maintained for a further 4 weeks on the diet without deltamethrin. Animals were checked for deaths twice daily, and clinical signs were recorded at least once a day. Body weights and food consumption were measured weekly, and blood samples were taken from all surviving rats for haematological examinations and blood chemistry in week 13. Urine was collected at the same time. At the end of the experiment, the rats were killed, the organ weights were recorded, the organs were submitted to a full macroscopic examination, and the tissues of all rats in the main groups and of survivors of the group given 1000 ppm were examined microscopically.
All rats receiving 3000 or 6000 ppm were either found dead or killed in extremis during the first 3 weeks of the study owing to severe reaction to treatment. In addition, three rats receiving 1000 ppm were killed in extremis during the early part of the study due to severe, persistent neurological signs and deteriorating condition. The principal clinical signs in rats receiving 3000 or 6000 ppm included uncoordinated movements, hunched posture, unsteady gait, body tremors, increased sensitivity to sound, ‘wet dog shakes’, spasmodic convulsions, piloerection, semi-closed eyes, poor grooming, wet urogenital fur, and emaciation. Rats at 1000 ppm showed uncoordinated movements, unsteady gait, hunched posture, increased sensitivity to sound, piloerection, dark extremities, and emaciated appearance. The signs were first evident from week 1 or 2 of treatment, but they declined in incidence and severity from week 3 and, on the whole, were no longer apparent after 8 weeks. Food and water consumption were reduced in rats receiving diets containing 1000 ppm and higher. Before the early termination of animals receiving the two higher doses, a marked impairment in body-weight gain was recorded, including dose-related weight loss in week 1. The body-weight gain of rats receiving 1000 ppm was statistically significantly reduced for the first 2 weeks of treatment, but there was clear evidence of recovery thereafter, although absolute parity with controls was not achieved. The body-weight gain of females was slightly (about 10%) but statistically significantly reduced at 30 and 300 ppm, but these changes were not consistent throughout treatment and showed no clear dose–response relationship; they were therefore considered to be of equivocal toxicological importance. There were no treatment-related changes in ophthalmoscopic, haematological, or blood chemical parameters, in organ weights, or in the incidence or type of pathological findings at autopsy or on microscopic examination. The NOAEL was 300 ppm, equal to 24 mg/kg bw per day, on the basis of reductions in food and water consumption and body weight and clinical signs of neurotoxicity at 1000 ppm, equal to 72 mg/kg bw per day (Ryle et al., 1991a).
Deltamethrin (technical-grade; purity, 98%) was aerosolized in a Wright dust generator and distributed into chambers for the exposure of groups of eight CD rats of each sex for 6 h per day, 5 days a week, for 2 weeks, and for 4 days during a third week. The mean aerosol concentrations were 3, 9.6, and 56 mg/m3, and about 87% of the particles had an aerodynamic diameter < 5.5 µm. None of the rats died as a result of exposure. Signs of irritation (agitated grooming and sialorrhoea) due to treatment were noted in all groups during exposure, the toxic signs (ataxia and walking with arched backs) being more pronounced in the group receiving the highest concentration. Treated males also showed reduced body-weight gain, which was statistically significant in the group at the high dose. Although an increase in the serum Na+ content was noted at the two higher doses, it could not be clearly linked to renal dysfunction, as there were no treatment-related microscopic renal lesions. No lesion was found at higher incidence in the high-dose group than in the controls. Most of the alpha-cyano-3-phenoxybenzyl pyrethroids evoke a distinct sequence of symptoms, called the ‘CS syndrome’, in which initial pawing and burrowing behaviour is rapidly followed by profuse salivation and whole-body tremors, progressing to sinuous writhing (choreoathetosis), which gradually becomes more intense. In the final stage, clonic seizures may occur. As in the case of non-cyano pyrethroids, the symptoms may be evoked or enhanced by sensory stimuli. As irritation and weight loss were only slight at 3 mg/m3, the NOAEC, on the basis of symptoms of the ‘CS syndrome’ at the highest dose, was 9.6 mg/m3, corresponding to 2.6 mg/kg bw per exposure (9.6 mg/m3 ´ 0.045 m3/h ´ 6 h) (Coombs et al., 1978).
Deltamethrin (purity, 99.6%) in PEG 400 was administered dermally to groups of five Sprague-Dawley rats of each sex for 21 successive days at a dose of 0, 100, 300, or 1000 mg/kg bw per day. The compound was left on the shaven skin under an occlusive dressing for 6 h each day. All rats survived treatment, and no signs of toxicity were observed. Four males at 100 mg/kg bw per day had low incidences of eschar formation and subsequent exfoliation. All rats at the two higher doses showed slight erythema and desquamation, eschar formation, and thickening of the skin. There were no significant differences in body weights, body-weight gain, food consumption, or haematological or blood chemical parameters between the treated and control groups. Histopathology revealed no dose–response relationship in the incidence or severity of the dermal changes. One-half or more of the treated rats had normal skin, and no changes were found in the liver or kidneys of rats at the highest dose. The NOAEL for systemic toxicity was 1000 mg/kg bw per day, the highest dose tested (Siglin, 1993).
Dogs
Deltamethrin (purity, 100%) dissolved in PEG 200 was administered orally in gelatine capsules to groups of two 25-week-old beagle dogs of each sex at a dose of 0 or 0.1 mg/kg bw per day and to groups of five animals of each sex at a dose of 1, 2.5, or 10 mg/kg bw per day for 13 weeks. There were no deaths. At the highest dose, unsteadiness, body tremors, and jerking movements were seen, particularly in males, in weeks 2, 3, and 4, but these signs diminished markedly in both incidence and severity during weeks 5–9 and were then seen in only one dog during week 13. Vomiting was observed in all groups, including the controls, but was significantly more frequent in animals at the highest dose during the first week of treatment. This group also showed excessive salivation at the beginning of the experiment, which diminished during treatment. Liquid faeces were found in all treated groups throughout treatment but were much more frequent at the two higher doses. Dilatation of the pupils was seen 4–7 h after treatment at these doses, which persisted throughout the day. During the first 2 weeks of treatment, both males and females receiving 10 mg/kg bw per day gained significantly less weight than the controls (males: controls, 830 g, high dose, –20 g, p < 0.01; females: controls, 730 g, high dose, 60 g, p < 0.05). All treated groups showed reduced body-weight gain throughout the study, but this was not strictly dose-related. Thus, the mean body-weight gains of animals in the five groups were: males, 3.6 kg, 1.5 kg (p < 0.05), 2.0 kg, 2.2 kg, and 2.4 kg; females, 3.7 kg, 2.1 kg, 0.58 kg (p < 0.001), 1.5 kg (p < 0.01), and 1.4 kg (p < 0.01). After 5 and 12 weeks, depression of the gag reflex was noted in most treated dogs, but in only one female in the control group. Although this was apparently a treatment-related effect, the possibility that it was associated with administration of the liquid in a capsule could not be excluded. Exaggeration or depression of the patellar reflex was observed in some animals in all treated groups after 5 and 12 weeks, but mainly at the two higher doses. Some treated dogs showed depression of the flexor reflex. The two higher doses of deltamethrin modified the electroencephalographic pattern in some dogs 12 weeks after administration. Histopathological evaluation of tissues and organs, including the nervous system and muscle, revealed no abnormalities that could be related to treatment. During recovery, exaggeration of the patellar reflex was still seen in the two animals at 1 mg/kg bw per day and one at 2.5 mg/kg bw per day but in none at the highest dose. The reflex patterns were not clearly treatment-related and were not observed in the 3- and 12-month studies described below, in which the compound was administered as a powder in gelatin capsules. Consequently, they are considered to be of no toxicological significance. The NOAEL was 1 mg/kg bw per day on the basis of signs of gastrointestinal disturbance (vomiting, liquid faeces), dilatation of the pupils, unsteadiness, body tremors, jerking movements, and abnormal electroencephalographic patterns, all of which were probably linked to stimulation of the nervous system (Chesterman et al., 1977). The Meeting noted that only three dogs of each sex were given 0 or 0.1 mg/kg bw per day.
Deltamethrin powder (purity, 98.9%) was administered orally in gelatine capsules to groups of beagles aged about 26 weeks at a dose of 0, 2, 10, or 50 mg/kg bw per day for 13 weeks. Six animals of each sex received 0 or 50 mg/kg bw per day, and three of each sex were kept for a 4-week recovery period; three animals of each sex received 2 or 10 mg/kg bw per day. There were no deaths. Most animals at the highest dose showed evidence of neurological disturbance, with intermittent unsteady gait (mainly hind gait), which in some cases prevented the animals from standing. These signs were associated with body tremors. During weeks 1–6, animals at the highest dose showed an increased frequency of vomiting and, in a few dogs, salivation, shaking of the head, chewing of the extremities, and hunched posture. Reduced body-weight gain, mainly in males, and reduced food intake were seen, indicating that 50 mg/kg bw per day was close to the maximum tolerated dose. However, no treatment-related clinical signs were observed during the recovery period, indicating their reversibility after cessation of dosing, and food intake returned to normal during that period. The lack of any clear dose–response relationship in weight gain among females indicates that these changes had an uncertain association with treatment, and it was concluded that a clear effect of treatment on weight gain was confined to males at 50 mg/kg bw per day. No treatment-related clinical signs were observed at 2 or 10 mg/kg bw per day. No treatment-related changes were found at any dose by ophthalmoscopy, extensive neurological examinations (cranial nerves, segmental and postural reflexes, behaviour, and muscle tonicity), haematology, blood chemistry, or terminal studies. The NOAEL was 10 mg/kg bw per day on the basis of neurological disturbances and reduced food intake and body-weight gain at 50 mg/kg bw per day (Ryle et al., 1991b).
Deltamethrin powder (purity, 98.9%) was administered orally in gelatine capsules to groups of four male and four female 23–24-week-old beagles at a dose of 0, 1, 10, or 50 mg/kg bw per day for 1 year. There were no deaths. The clinical signs, seen only at the two higher doses, were chewing and scratching of the extremities, abnormal gait, tremors, and liquid faeces. These signs were accompanied by locomotor impairment, and at the highest dose the scratching resulted in lesions on the tail, paws, and scapular regions which necessitated veterinary treatment. Chewing and scratching of the extremities was also noted in two dogs at the lowest dose but also in a few control animals; consequently, this behaviour was not attributed to treatment at that dose. The locomotor effects, clearly observed at the two higher doses, consisted of unsteadiness, incoordination of hindlimb gait, and, sometimes, splaying of the limbs and/or digits. Such effects were also seen in association with body tremors and abnormal movements of the head. None of the animals at 1 mg/kg bw per day showed these neurological signs.
A dose-related reduction in body-weight gain was found in all treated males. However, in seven other studies performed during 1990–91 with males of the same strain, source, and age, the range of weight gain over weeks 0–26 was 2.8–3.3 kg, whereas the male controls in the current experiment gained an average of 4.8 kg over the same period. Furthermore, at the start of dosing, the mean body weight of control males (10 kg) was slightly greater than that of males in the treated groups (9.8–10 kg).
No effects were seen on food intake of animals at the two lower doses, whereas it was reduced in all males at 50 mg/kg bw per day from the time of first dosing until week 4 and intermittently thereafter, sometimes coinciding with the occurrence of significant neurological signs. Extensive neurological examinations performed on animals at the highest dose and the control groups showed some signs (trembling, high-stepping, unsteady gait, and splayed digits) at weeks 26 and 52 in a few animals at the highest dose. These findings were consistent with routine clinical observations made in corresponding dogs and indicated that there was no need to perform specific neurological examinations on animals in the two other treated groups. At week 52, a dose-related reduction in red blood cell parameters (packed cell volume, haemoglobin) was seen in males at the two higher doses. The values for one male at 50 mg/kg bw per day were well reduced, whereas those for the other animals were within the range of historical controls. Decreases in serum albumin and calcium concentrations were seen at the two higher doses, which may have been associated with the increased incidence of liquid faeces. A decreased Na+ concentration was also noted in some males at the highest dose. No changes in blood chemistry were seen in treated females. Inter-group differences in urinary pH and protein content at week 52 were considered to be of no toxicological importance in the absence of any clinical or pathological findings.
The absolute increase in brain weight and decrease in heart weight in some males at the highest dose were not associated with macroscopic or microscopic findings and were considered to be of no toxicological importance. No significant intergroup differences were observed in organ weights in females. There were no macroscopic or microscopic findings that could be attributed to treatment. The NOAEL was 1 mg/kg bw per day on the basis of behavioural changes and liquid faeces at 10 mg/kg bw per day (Ryle et al., 1993).
Deltamethrin (purity, 98%) dissolved in corn oil was administered in the diet to groups of eight beagles of each sex at a concentration of 0, 1, 10, or 40 ppm, equivalent to 0, 0.025, 0.25, and 1 mg/kg bw per day, for 24 months. Individual body weights and food consumption were measured weekly. Ophthalmoscopic, haematological, blood chemical, and urinary examinations were conducted before treatment and at 6, 12, 18, and 24 months of the study. Extensive neurological examinations were conducted at approximately 1 year and once more just before termination. No signs of toxicity were observed in any dogs. Sporadic soft stools or diarrhoea was seen in all groups, which may have been related to use of corn oil as the vehicle, with no significant consequences on body-weight gain. Two control and two treated dogs died during the study, but the deaths were not treatment-related. All 16 dogs at the high dose survived. Body weights and food consumption were similar for control and treated dogs. No compound-related effects were observed in the ophthalmoscopic and physical examinations. Although there were some statistically significant differences between the control and treated groups in haematological and biochemical test results, no physiologically significant changes were observed at any time in the study. No compound-related gross or microscopic changes were found in the surviving dogs, which were killed and autopsied. Common inflammatory lesions included lymphocytic thyroiditis, interstitial pneumonia, and lymphocytic and/or mononuclear cell infiltrates in the interstitium of the salivary gland, pancreas, kidneys, lungs, and portal areas of the liver. Degenerative lesions involving axons, testes, and myocardium were observed. The hyperplastic or proliferative changes included parafollicular-cell hyperplasia of the thyroid, hyperplasia of the ducts or acini of the mammary gland, and mucosal hyperplasia of the urinary bladder. Lobular hyperplasia of the mammary gland, observed in a number of bitches, was considered to be related to estrus and was accompanied by endometrial hyperplasia and multiple, large corpora lutea. The prevalence of such changes was generally similar in control and treated groups. The NOAEL was 40 mg/kg of diet, equivalent to 1 mg/kg bw per day, the highest dose tested (Goldenthal, 1980a, 1981).
(c) Long term toxicity and carcinogenicity
Mice
Deltamethrin (purity, 98%) suspended in corn oil was administered in the diet to groups of 80 Charles River CD-1 mice of each sex at a concentration of 0, 1, 5, 25, or 100 ppm, equivalent to 0, 0.12, 0.6, 3.0, and 12 mg/kg bw per day for males and 0, 0.15, 0.75, 3.8, and 15 mg/kg bw per day for females, for 24 months. There were no clear treatment-related effects on general behaviour, mortality rate, body weight, or food consumption. Blood chemistry, haematology, and urine analysis revealed normal values after 12, 18, and 24 months (at the times of interim and terminal sacrifices). Microscopic examination of tissues revealed no lesions indicative of a compound-related effect. Proliferative lesions that occurred at moderate to high incidence included A-cell proliferation in the adrenal cortex, nodular hyperplasia of hepatocytes, focal hyperplasia of pulmonary alveolar cells, proliferation of lymphoid tissue around the airways and vessels of the lungs, hyperplasia of haematopoietic tissues of the spleen, hyperplasia with cystic dilatation of the glands of the uterine mucosa, and mucosal hyperplasia of the stomach. These were considered to be spontaneous lesions, and the incidences were generally similar in control and treated groups. Low incidences of benign or malignant neoplasms were seen in a number of tissues in both control and treated groups. Benign neoplasms predominated, the commonest being alveolar-cell adenomas, but their incidence in treated groups were not higher than that in the control groups. Malignant neoplasms were most common in haematopoietic tissue; lymphosarcomas predominated but were not commoner in the treated than in the control group. Of particular significance, in view of the result obtained by Cabral et al. (1990; see below), is the observation of a single thyroid tumour (a follicular-cell adenoma) in a control female mouse; no other thyroid tumours were found. No systemic effects were noted. The NOAEL was 100 ppm, equivalent to 12 mg/kg bw per day, the highest dose tested. No oncogenic effects were found at this dose (Goldenthal, 1980b, 1982).
Deltamethrin (purity, 99%) dissolved in arachis oil was administered by gavage to groups of 6-week-old C57BL/6 mice of each sex at a dose of 0 (untreated), 0 (arachis oil), 1, 4, or 8 mg/kg bw per day on 5 days/week for 104 weeks. The groups consisted of 50 mice for the two control and the high-dose groups, and 30 for the other two treated groups. The animals were inspected two or three times daily, and body weights were recorded monthly. Survivors were killed when they were 120 weeks old, when they underwent gross and microscopic examination. The survival rates at this time were 64, 56, 40, 60, and 50% in males, and 40, 44, 60, 53, and 53% in females in the five groups, respectively. Clinical signs of toxicity, including salivation, ataxia, and choreoathetosis (writhing) with convulsions, were observed in animals at 8 mg/kg bw per day. The numbers (and percentages) of tumour-bearing mice in the five groups were 25 (52%), 25 (52%), 10 (48%), 11 (46%) and 11 (24%) among males (p = 0.005, Fisher’s exact test) and 28 (62%), 22 (45%), 14 (50%), 20 (71%), and 17 (43%) among females. Thus, there was a decrease in the proportion of tumour-bearing mice among males at the high dose. The tumours found most frequently in all groups were lymphomas, pulmonary adenomas, and liver-cell tumours, but the incidences were no significantly increased in comparison with the control groups. No NOAEL could be established, owing to lack of detail (Cabral et al., 1990).
Deltamethrin (purity, 99.4% and 99.7%) was administered to groups of 50 CD-1 mice of each sex in the diet at a concentration of 0, 10, 100, 1000, or 2000 ppm, equal to 0, 1.5, 16, 160, and 310 mg/kg bw per day for males and 0, 2.0, 20, 190, and 400 mg/kg bw per day for females, for at least 97 weeks. Throughout the study, all mice were observed for signs of ill health or reaction to treatment; body weights and food intake were measured and a differential leukocyte count was determined for each animal during weeks 52, 78, and 97 of the study. Masses were palpated every 2 weeks from the sixth month of the study onwards. At the end of the treatment period, all surviving animals were killed. The weights of designated organs were recorded for the first 10 surviving animals of each sex and group. All animals were then examined macroscopically. Designated organs and any masses or lesions were sampled and examined microscopically. The rates and causes of mortality or moribundity were similar in the control group and in males and females at10 ppm, males at 100 ppm, males at 1000 ppm, and males at 2000 ppm. In females at 100, 1000, or 2000 ppm, the cumulative mortality rate was similar to that of the control group until week 78 of treatment but increased from approximately week 88, so that by week 97 the rates were 54, 52, and 56%, respectively, of the value in controls, 44%. As this finding was not dose-related and the values were close to those for other controls in the laboratory (58% mortality at week 104), the relationship to treatment was considered to be equivocal. In addition, the slightly higher mortality rate (corresponding to four to six mice) did not obviate evaluation of the carcinogenic potential of the test substance.
The food consumption of the treated animals was similar to that of controls, as was the body-weight gain, except for males at the highest dose which had a 5% lower rate, mainly during the first year of treatment. Although the difference was slight, it was statistically significant between weeks 26 and 46. The incidence and nature of the clinical signs in mice at 10, l00, and 1000 ppm was not related to treatment or dose. In animals at the highest dose, signs of altered clinical condition (emaciation and dyspnoea) in moribund and killed animals were found at a higher incidence than in the control group. In the same group, the incidence of cutaneous lesions in various parts of the body group (described at autopsy as scars, sores, or scabs) was higher than in controls. This finding was considered to be related to the known properties of the test substance on the pain sensors, which can result in excessive scratching. Histological examination revealed a higher incidence of skin ulceration and cellulitis in males at 1000 ppm and in males and females at 2000 ppm. The frequency, time of onset, location, size, and multiplicity of palpable masses was not related to treatment or dose. The differential leukocyte counts showed no relevant difference from those of controls. At autopsy, no changes in organ weights or lesions that might have been directly induced by treatment were found. No increases in tumour incidence were found, and there was no reduction in the latency of tumour appearance in the treated mice in comparison with controls. The NOAEL was 100 ppm of diet, equal to 16 mg/kg bw per day, on the basis of skin ulceration secondary to scratching and irritation at 1000 ppm, equal to 160 mg/kg bw per day (Richard, 1995).
Rats
Deltamethrin (purity, 98%) suspended in corn oil was administered in the diet to groups of 90 Charles River CD rats of each sex at a concentration of 0, 2, 20, or 50 mg/kg, equivalent to 0, 0.1, 1, or 2.5 mg/kg bw per day, for 24 months.. A second control group of 60 mice of each sex was included. Ten rats of each sex per group, except from the second control group, were killed after 6, 12, and 18 months. The remaining rats were killed after 24 months of treatment. No treatment-related changes in general behaviour or appearance were observed. The rate of survival (50–67%) was similar for control and treated rats. Rats at 50 ppm gained slightly less weight than control rats, but their food consumption was essentially the same. Body-weight gain was reduced in males from week 26 until the end of the experiment (p < 0.05 or p < 0.01) and in females during weeks 26, 65, and 78 (p < 0.05). The ophthalmoscopic findings were generally the same for control and treated rats. None of the measured haematological or biochemical parameters were changed in a biologically significant way in relation to treatment at any time, except for a decrease in serum alanine aminotransferase activity at 6 months in the groups at the intermediate and high doses. Organ weights were not affected. The macroscopic and microscopic findings were those common for the species and strain, except for a slightly increased incidence of axonal degeneration in sciatic, tibial, and/or plantar nerves in animals at 20 and 50 mg kg bw per day at 18 months, but not at termination. This observation was therefore considered to be unrelated to treatment. A statistically significantly higher incidence of benign testicular tumours (interstitial-cell adenomas) was found at the end of this study in rats at the high dose (7/90, 7.8%; p < 0.05) when compared with the first control group (0/88) but not when compared with the second control group (4/60, 6.7%). When the slides were read again, the incidence in the second control group was found to be 5/60 (8.3%). Thus, the incidence at the high dose was considered to be spontaneous because it was not significantly higher than that in the second control group or in 16 other control groups in the laboratory (mean, 7.6%; range, 0–22%). The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per day, on the basis of decreased body weight (particularly in males). No carcinogenic effects were noted up to 50 ppm, equivalent to 2.5 mg/kg bw per day, the highest dose tested (Goldenthal, 1980c; Richter & Goldenthal, 1983).
Deltamethrin (purity, 99.%) dissolved in arachis oil was administered by gavage to groups of 50 6-week-old BDVI rats of each sex at a dose of 0, 3, or 6 mg/kg bw per day on 5 days/week for 104 weeks. The animals were inspected two to three times daily, and body weights were recorded every month. Survivors were killed when they were 120 weeks old and were examined grossly and microscopically. The survival rates at 120 week were 72, 80, and 64% for males and 66, 60, and 66% for females in the three groups, respectively. Clinical signs of toxicity, including salivation, ataxia, and choreoathetosis (writhing) with convulsions, were observed at the highest dose. The numbers of tumour-bearing rats were not increased in the treated groups. Most of the tumours found were of endocrine origin. The incidences of pituitary and mammary tumours were essentially the same in all groups, but the incidences of thyroid adenomas (tissue of origin not stated) were increased, the numbers (and percentages) of tumour bearing rats being 6 (13%), 19 (38%) (p = 0.003, Fisher’s exact test), and 10 (23%) in males and 4 (9%), 4 (8%), and 14 (29%) (p = 0.011, Fisher’s exact test) in females. The incidence of thyroid tumours in male rats was not clearly dose-related. Furthermore, the incidence of thyroid tumours was not increased in either earlier (Goldenthal, 1980c) or later (Ryle, 1995) studies. The Meeting concluded, therefore, that no reproducible evidence of a carcinogenic response was found in this study. Although the clinical signs of toxicity appeared to have been restricted to the group at the highest dose, the description of the study was not sufficiently detailed to establish a NOAEL (Cabral et al., 1990).
Deltamethrin (purity, 98.9%) was administered to groups of 70 Crl:CD(SD)BR rats of each sex at a dietary concentration of 0, 25, 125, 500, or 800 ppm, equal to 0, 1.1, 5.4, 22, and 36 mg/kg bw per day for males and 0, 1.5, 7.3, 30, and 47 mg/kg bw per day for females, for 104 weeks. Throughout the study, all animals were observed for signs of ill health or reaction to treatment; body weights, food intake, and water consumption were measured, and ophthalmoscopic and laboratory investigations were performed at intervals during the study. Ten rats of each sex per group were killed after 52 weeks of treatment for an interim assessment of toxicity. At termination, all rats underwent a full necropsy and their organs were weighed. About 40 tissues were analysed histologically. A possible treatment-related increase in the survival rate was found for males given 500 or 800 ppm, and a statistically significant trend was found among treated male groups. Uncoordinated movements of the limbs or abnormal gait characterized by splayed limbs was seen in most males and one female at 800 ppm and in a single male at 500 ppm during week 1. Unsteady gait was seen in most males and two females at 800 ppm and in a few males at 500 ppm, also during week 1. Thereafter, their incidence and severity declined gradually, so that by week 8 these effects were no longer apparent. The signs coincided with a period of the study during which the intake of deltamethrin (on a body-weight basis) was higher than that in later periods. There were no other treatment-related clinical signs. The group mean body-weight gain of males at 500 or 800 ppm and of females at 800 ppm was significantly lower than that of controls, largely due to the particularly small weight gain (and low food intake) in week 1. Thereafter, some recovery was noted in these groups, although the overall weight gain of males at 500 or 800 ppm remained slightly reduced throughout much of the treatment period.
Ophthalmoscopy revealed no changes of toxicological significance. Lowered lymphocyte counts were found in blood samples taken at week 13 from males treated with 800 ppm and females at 125, 500, or 800 ppm, and at week 26 from males treated with 125, 500, or 800 ppm. A lower total leukocyte count was seen at week 13 in samples from males at 800 ppm and females at 125, 500, or 800 ppm. In the absence of any corroborative pathological findings and because they were found in weeks 14 and 26 but not later, these minor fluctuations in haematological values were considered to be of no toxicological importance. Slight changes in plasma electrolyte concentrations were noted for all treated groups in blood samples taken at weeks 26, 52, 78, and 104, and a reduction in plasma cholesterol concentration was found among the treated groups at weeks 26, 52, and 78 and in plasma protein concentrations in treated female groups at weeks 26, 52, 78, and 104. An increased plasma glucose concentration was found in treated groups at weeks 52, 78, and 104. A number of these changes were not dose-related, and in the absence of corroborative pathological findings they were considered to be of no major toxicological importance. Analysis of urine did not reveal any changes considered to be related to treatment. At autopsy, no changes in organ weights or lesions were found that might have been induced by treatment. Histology revealed a dose-related, statistically significant increase in the incidence and degree of eosinophilic hepatocytes in male rats at 500 and 800 ppm, and the livers of males at 125 and 800 ppm showed an increased incidence of ballooned cells. There was no evidence of a carcinogenic effect at any dose. The NOAEL was 25 ppm, equivalent to 1.1 mg/kg bw per day, on the basis of minor hepatotoxicity in treated males at 125 ppm, equivalent to 5.4 mg/kg bw per day (Ryle, 1995).
Deltamethrin was tested for genotoxicity in a range of assays, both in vitro and in vivo (Table 4). There was no evidence of genotoxicity in any of these assays, which included tests for DNA damage and repair in bacteria and primary cultures of rat hepatocytes, mitotic recombination in yeast, gene mutation in bacteria and Chinese hamster lung V79 cell cultures, chromosomal aberrations in Chinese hamster ovary cell cultures, and, in vivo, chromosomal aberrations and micronuclei in mouse bone-marrow cells and dominant lethal effects in male mice.
Table 4. Genetic effects of deltamethrin
|
End-point |
Test object |
Dosea |
Result |
Reference |
|
In vitro |
||||
|
Differential toxicity |
E. coli W3110 and p3478 |
5000 µg/ml |
Negativea |
Peyre et al. (1980) |
|
Differential toxicity |
E. coli WP2 and CM611 |
5000 µg/ml |
Negativea |
Peyre et al. (1980) |
|
Gene mutation |
S. typhimurium TA100, TA1535, TA1537, TA1538, TA98; E. coli WP2 |
1000 µg/plate |
Negativeb |
Kavlock et al. (1979) |
|
Gene mutation |
S. typhimurium TA100, TA1535, TA1537, TA1538, TA98 |
5000 µg/plate |
Negativeb |
Peyre et al. (1980) |
|
Gene mutation |
S. typhimurium TA100, TA98 |
600 µg/plate and 10 µg/ml |
Negativeb |
Pluijmen et al. (1984) |
|
Mitotic recombination |
Saccharomyces cerevisiae D3 |
50 000 µg/ml |
Negativeb |
Kavlock et al. (1979) |
|
Unscheduled DNA synthesis |
Male Fischer 344 rat primary hepatocytes |
4200 µg/ml |
Negativea |
Curren (1989) |
|
Gene mutation |
Chinese hamster lung V79 cells, hprt locus and ouabain resistance |
40 µg/ml |
Negativeb |
Pluijmen et al. (1984) |
|
Chromosomal aberration |
Chinese hamster ovary cells |
150 µg/ml |
Negativeb |
Putman & Morris (1989) |
|
Micronucleus formation |
Male and female Swiss CD1 mouse bone-marrow cells |
16 mg/kg bw once orally |
Negative |
Vannier & Fournex (1983) |
|
In vivo |
||||
|
Chromosomal aberration |
Female Swiss mouse bone- marrow cells |
6.8 mg/kg bw once and five times orally |
Negative |
Poláková & Vargová (1983) |
|
Dominant lethal mutation |
Male Swiss CD1 mice |
15 mg/kg bw once orally |
Negative |
Vannier & Glomot (1977) |
LED, lowest effective dose; HID, highest ineffective dose
a
In the absence of exogenous metabolic activation; not tested in the presence of exogenous metabolic activationb
In the absence and presence of exogenous metabolic activationDeltamethrin (purity, 99.7%) was administered in the diet to groups of 30 Charles River Crl:CD BR VAF/Plus rats of each sex at a concentration of 0, 5, 20, 80, or 320 ppm throughout the experiment. The F0 rats were exposed for 12 weeks before a 3-week mating period and then throughout gestation and lactation; they were killed on day 21 after birth. The resulting F1 litters were randomly culled to four of each sex after weighing on day 4 of lactation. Whenever possible, at least one pup of each sex was selected on day 21 after birth for continuation in the study, treated as were the F0 rats, and killed after production of the F2 litters. Clinical signs, body weights, feed consumption, mortality, mating and fertility, rearing capacity, natural delivery, litter observations, and observations at necropsy were recorded.
The death of one F0 rat at 320 ppm was attributed to treatment, as the animal showed gastric erosions similar to those found in a dose range-finding study. Significant numbers of animals in this group showed clinical signs attributable to deltamethrin. In particular, ataxia and hyperactivity, vocalization, and excessive salivation occurred in the females during lactation. The terminal body weights of animals at this dose were reduced, resulting in higher organ:body weight ratios, whereas the organ:brain weight ratios were unaffected. Neither the mating performance nor the fertility of the F0 generation was affected, but the pups of F0 parents at 320 ppm weighed less at birth and significantly less (p 0.01) on days 4, 7, 14, and 21 post partum; the mortality rate among these pups was significantly increased (p 0.01) on days 8 and 14 post partum, and the lactation index was consequently reduced.
In the F1 generation of rats at 320 ppm, the deaths of 17 males (p 0.01) and 19 females (p 0.01) were attributable to deltamethrin. Most of the deaths occurred within 8 days of weaning. Before death, many of these animals showed ataxia, impaired righting reflex, urine-stained abdominal fur, dark material in the stomach and/or intestines, and blood clots in their brains. Many of the remaining surviving animals at this dose showed similar clinical signs One of the male rats that died had gastric erosions. No clinical signs occurred in significant numbers of rats in other group. The body weights of rats at the highest dose were significantly reduced (p 0.05 to p 0.01) throughout the period before mating and, in female rats, during gestation and lactation. Neither the mating performance nor the fertility of F1 rats was affected, but the pups of F1 rats at 320 ppm weighed significantly less than controls (p 0.05 to p 0.01) on days 7, 14, and 21 post partum. No other biologically important differences were found among the groups in terms of pup viability, sex ratio, or clinical or autopsy observations. The NOAEL for parental toxicity was 80 ppm, equal to 4.2 mg/kg bw per day, on the basis of clinical signs (ataxia, hypersensitivity) in females during gestation and lactation, reduced body-weight gain and feed consumption, and increased mortality rates at 320 ppm, equal to 18 mg/kg bw per day. The NOAEL for toxicity in offspring was also 80 ppm, equal to 11 mg/kg bw per day, on the basis of reduced body weight, clinical signs (ataxia, impaired righting reflex, hyperactivity, splayed limbs), reduced viability, and increased mortality rates before and after weaning up to 18 days. The NOAEL for reproductive toxicity was 320 ppm, equal to 18 mg/kg bw per day, the highest dose tested (Hoberman, 1992).
Mice
Deltamethrin (purity not stated) dissolved in corn oil was administered by gavage to groups of 30 inseminated CD-1 mice at a daily dose of 0, 3, 6, or 12 mg/kg bw on days 7–16 of gestation. The mice were killed on day 18 of gestation. A dose-related reduction in maternal weight gain during gestation was observed, animals at the high dose gaining 42% of the weight of controls. The mortality rate was not dose-related, but convulsions were observed in dams at the two higher doses. Treatment did not affect the number of implantation sites, fetal weights, or the number of sternal and caudal ossification centres. A significant (p < 0.01), dose-related increase in the occurrence of supernumerary ribs was observed, from 13% in the controls to 23, 47, and 28% at the three doses, respectively. No other treatment-related skeletal or visceral anomalies were observed. The NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of reduced body weight and convulsions at 6 mg/kg bw per day, and the NOAEL for developmental toxicity was 12 mg/kg bw per day, the highest dose tested (Kavlock et al., 1979).
Rats
Deltamethrin (purity not stated) was dissolved in corn oil and administered by gavage to groups of 29–37 inseminated Sprague-Dawley rats at a daily dose of 0, 1.2, 2.5, or 5.0 mg/kg bw on days 7–20 of gestation. The rats were killed on day 21 of gestation. The body-weight gain of animals at the highest dose was reduced to 80% of that of controls. No effects were observed on the number of implantation sites, fetal mortality, fetal weight gain, or the number of sternal and caudal ossification centres. The NOAEL for maternal toxicity was 2.5 mg/kg bw per day on the basis of reduced body weight and mild salivation at 5.0 mg/kg bw per day. The NOAEL for developmental toxicity was 5.0 mg/kg bw per day in the absence of malformations and developmental variations in the fetuses at the highest dose tested (Kavlock et al., 1979).
Deltamethrin (purity, 99.2%) dissolved in corn oil was administered by gavage to groups of 25 gravid Charles River Crl:CD VAF/Plus rats on days 6–15 of gestation at initial doses of 0, 1, 3.3, and 11 mg/kg bw per day. Owing to excessive toxicity, a group receiving 7 mg/kg bw per day was added; subseqently, because of unacceptable concentration analyses, additional groups given 0, 1, and 3.3 mg/kg bw per day were added. The fetuses were removed from all surviving females on day 20 of gestation and examined. The mortality and moribundity rates of dams at 7 and 11 mg/kg bw per day were significant and excessive. No maternal effects were observed in the two groups given 1 or 3.3 mg/kg bw per day. No reductions were found at any dose in the numbers of pregnant females, viable fetuses per pregnancy, or total implants per pregnancy. Fetal body weights were not affected by treatment, and the male:female ratio was unchanged. In comparisons with the original control group, the incidence of soft tissue or skeletal malformations was not increased in rats at 1, 7, or 11 mg/kg bw per day, but abnormalities were found in those at 3.3 mg/kg bw per day, consisting mainly of folded retina, vertebral malformations, and fused sternebrae. Similar malformations were not observed in the additional group at 3.3 mg/kg bw per day, although they did occur in the additional control group. The NOAEL for maternal toxicity was 3.3 mg/kg bw per day on the bais of clinical signs (moribundity, convulsions, increased salivation, hypersensitivity, staining), reduced body weight (throughout treatment at 7 and 11 mg/kg bw per day and during gestation at 11 mg/kg bw per day), and deaths. The NOAEL was 11 mg/kg bw per day for developmental toxicity in the absence of malformations and developmental variations in fetuses at the highest dose tested (Schardein, 1990a).
Deltamethrin (purity not stated) was dissolved in corn oil and administered by gavage to groups of 12–14 inseminated Sprague-Dawley rats at a daily dose of 0, 2.5, or 5.0 mg/kg bw from day 7 of gestation until day 15 of lactation. The litters were reduced at birth to four of each sex. No effects of treatment were observed on parturition, litter size, or pup viability. The weights of pups at birth were similar in all groups, but a dose-related depression in growth was observed which was maximal on day 15 post partum and disappeared rapidly upon cessation of dosing. No effects were observed on the age at which startle or righting reflexes developed or the age of eye opening. Female pups tested in an open field at 6 weeks of age showed no differences in activity or exploratory measurements. The NOAEL for perinatal development was 2.5 mg/kg bw per day on the basis of reduced pup weight at 5.0 mg/kg bw per day (Kavlock et al., 1979).
Rabbits
Deltamethrin (purity, 99.4%) suspended in Tween 80 and diluted in 0.5% carboxymethylcellulose, was administered by gavage to groups of 16 gravid New Zealand white rabbits at a daily dose of 0, 10, 25, or 100 mg/kg bw on days 7–19 of gestation. Fetuses were removed from all surviving females on day 29 of gestation and observed morphologically. The death of one rabbit at 100 mg/kg bw per day was attributed by the author to treatment, but there were no other indications of maternal toxicity. Resorptions of entire litters were observed at each dose, the proportions per gravid doe being 0/14, 3/14, 2/13, and 1/15 in the four groups, respectively. The lack of a dose–response relationship suggests that this effect is of no toxicological significance. Other observations were similar in the control and treated groups. The occurrence of unossified pubic bones and tail vertebrae in fetuses at 100 mg/kg bw per day is indicative of some growth retardation. The NOAEL for maternal toxicity was 25 mg/kg bw per day on the basis of the death of one female. The NOAEL for developmental toxicity was also 25 mg/kg bw per day, on the basis of retardation of ossification at 100 mg/kg bw per day. No evidence of teratogenic potential was found (Schardein, 1990b).
Deltamethrin (purity unstated) suspended in corn oil or dissolved in sesame oil was administered once by gavage to groups of 10 adult hens at a dose of 0, 500, 1250, or 5000 mg/kg bw in corn oil or 0 or 100 mg/kg bw in sesame oil. A positive control group received tri-ortho-cresyl phosphate at 500 mg/kg bw. The birds were observed for deaths, health effects, signs of neurotoxicity, and body weight. No clinical, macroscopic, or histological signs of delayed neurotoxicity were found (Ross et al., 1978).
Deltamethrin (purity unstated) in corn oil was administered by gavage to groups of five male and five female Wistar rats at a dose of 0 or 25 mg/kg bw per day on 2 consecutive days. The animals were given a tilting plane test every second day from day 4 to day 16 of the study. Two treated males died after the second treatment. No neurological effect was found on the slip angle (Davies et al., 1983).
Deltamethrin (purity, 99.2%) in corn oil was administered once by gavage to groups of 12 male and 12 female Sprague-Dawley rats at a dose of 0, 5, 15, or 50 mg/kg bw, and the animals were tested in a "functional observation battery" before treatment and 3 h, 7 days, and 14 days later. The rats were killed on day 15. All rats were examined for viability twice a day and for clinical signs once a day. The battery of tests consisted of observations (posture, convulsions, tremours, biting, eyelid closure, and faecal consistency) in the home cage; observations of ease of handling, lachrymation, chromodacryorrhoea, piloerection, eyelid closure, red or rusty deposits, eye prominence, salivation, fur appearance, respiration rate and character, colour of mucous membranes, eyes, and skin, and muscular tone; observations (mobility, rearing, convulsions, tremours, grooming, bizarre or stereotypical behaviour, time to first step, gait, arousal, urination, defaecation, gait score, backing) in the open field; sensory observations (approach, startle, touch, tail pinch, hindlimb, eye blink and pupillary responses, olfactory orientation, forelimb extension, and air righting reflex); neuromuscular observations (hindlimb extensor strength, hindlimb foot splay, grip-strength of forelimb and hindlimb, rotarod performance); and physiological observations (catalepsy, body temperature, body weight). In addition, locomotor activity was assessed in all rats before treatment and 3 h, 7 days, and 14 days after treatment. All surviving rats were killed with carbon dioxide, and five rats of each sex per group were then perfused in situ for neuropathological examination of selected central and peripheral neural system tissues.
Animals at 50 mg/kg bw showed altered posture, convulsions (clonic and tonic), tremors, and alterations in biting and eyelid closure in the home cage; alterations in ease of removal from the cage, ease of handling, lachrymation, salivation, and fur appearance; increased time to first step; impaired mobility and gait, convulsions (clonic and tonic), tremors, decreased arousal, bizarre or stereotypic behaviour (writhing), and decreased rearing, grooming, and urination in the open field; in the sensory observations, altered approach, touch startle, and tail-pinch responses, olfactory orientation, forelimb and hindlimb extension, and air righting reflex; during the neuromuscular observations, reduced forelimb and hindlimb grip strength, impaired rotorad performance, and altered hindlimb footsplay (males only); and during physiological observations, increased group mean values for catalepsy and decreased group mean values for body temperature. In general, the responses occurred approximately 3 h after dosing and were transient. Single males and females at 15 mg/kg bw showed potentially treatment-related signs when tested on day 0, including slight salivation in one male and slightly soiled fur on one female during handling and slightly impaired mobility of one male during the open field observations. No treatment-related effects were found on brain weight or dimensions. Microscopic examination of perfused tissues (including sciatic, tibial, and peroneal nerves) from animals at 50 mg/kg bw revealed no treatment-related neuropathological lesions. All six of the functional domains described by Moser (1991), i.e. sensorimotor, autonomic, neuromuscular, physiological, activity, and excitability, were thus affected by deltamethrin at 50 mg/kg bw, and potentially treatment-related effects were observed in one male and one female at 15 mg/kg bw. The NOAEL for neurotoxicity was 5 mg/kg bw on the basis of the functional observation battery and evaluations of locomotor activity at 15 mg/kg bw (Nemec, 1998a).
Deltamethrin (purity, 99.2%) was administered in the diet to groups of 10 male and 10 female Sprague-Dawley rats at a concentration of 0, 50, 200, or 800 ppm, equal to 0, 4, 14, or 54 mg/kg bw per day for males and 0, 4, 16, or 58 mg/kg bw per day for females, for 91 days . The animals were subjected to the same functional observation battery of tests as described above and for locomotor activity before treatment and during weeks 4, 8, and 13 after treatment. All rats were examined for viability twice a day and for clinical signs once a day. All surviving rats were killed with carbon dioxide, and five rats of each sex per group at 0 and 800 ppm were then perfused in situ for neuropathological examination of selected central and peripheral neural system tissues.
Three males and two females at 800 ppm died during week 1, 3, 6, or 12. Treatment-related clinical signs were limited to animals at this dose, the most prevalent being gait alterations (rocking, lurching, or swaying, walking with hindlimbs splayed, walking on tiptoes, and/or writhing), hypersensitivity to noise, and impaired righting reflex. Clinical signs that occurred less frequently included piloerection, convulsions, "popcorn seizures", altered posture (flattened with limbs extended), and tan staining of the fur. The mean body weights of males and females at 800 ppm were decreased during weeks 1–13 due primarily to low mean body-weight gains or mean body-weight losses during the first 3 weeks of the study. The food consumption of animals at this dose was also reduced throughout the study. The functional observational battery revealed changes in animals at 800 ppm, often at all three times (weeks 3, 7, and 12), which included piloerection and slightly soiled fur in the home cage; impaired mobility and gait and bizarre or stereotypic behaviour (rocking side-to-side) in the open field; altered air righting reflex; and altered hindlimb extensor strength and reduced forelimb and hindlimb strength. No treatment-related effect was seen on locomotor activity or on brain weight or dimensions. Microscopic examination of perfused tissues (including sciatic, sural, tibial, and peroneal nerves) from animals at 800 ppm revealed no treatment-related neuropathological lesions. The NOAEL for systemic toxicity and neurotoxicity was 200 ppm, equal to 14 mg/kg bw per day, on the basis of the observations in the functional test battery at 800 ppm, equal to 54 mg/kg bw per day (Nemec, 1998b).
The toxicity of deltamethrin and other pyrethroids has been reviewed by Aldridge (1990), Vijverberg and van den Bercken (1990), and Appel and Gericke (1993). Pyrethroids at high doses induced toxic signs in all species tested that are characteristic of a strong excitatory action on the nervous system (Vijverberg & Oortgiessen, 1988). The principal action of pyrethroids on the peripheral nervous system is to induce pronounced repetitive activity. In particular, sensory organs produce trains of nerve impulses instead of single impulses after exposure to pyrethroids, both in vitro and in vivo. Depending on the structure of the pyrethoid, sensory nerves, motor nerve endings, and muscle fibres may also show repetitive activity. Another possible effect is depolarization of membranes, leading to enhanced release of neurotransmitters or even blockage of excitation.
The pyrethroids can be divided into two classes by the pattern of toxic signs found in rats after administration of sublethal or lethal doses. The early synthetic pyrethroids induced signs in rats very similar to those of the natural pyrethrins. Initial tremors, aggressive behaviour, and extreme sensitivity to sensor stimuli were followed by prostration, with whole-body tremors preceding death. This sequence, later designated the ‘T syndrome’, is characteristic of non-cyano pyrethroids and may be incomplete, depending on the molecular structure and on the route of application and dose of the pyrethroid. Most of the alpha-cyano-3-phenoxybenzyl pyrethroids evoke a distinct sequence of symptoms, called the ‘CS syndrome’. Initial pawing and burrowing behaviour is rapidly followed by profuse salivation and whole-body tremors, progressing to sinuous writhing (choreoathetosis), which gradually becomes more intense. In the final stage, clonic seizures may occur. As in the case of non-cyano pyrethroids, the symptoms may be evoked or enhanced by sensory stimuli.
Vijverberg and van den Bercken (1990) summarized the evidence for the hypothesis that the main biological activity of pyrethroids is mediated by effects on sodium channels. The stereoselective pyrethroids fix themselves onto the sodium channel and cause them to stay open much longer than normal, resulting in prolongation of the transient sodium current associated with membrane depolarization. In frog myelinated nerve fibres, this propagation ranges from from 6 ms with phenothrin to 1770 ms with deltamethrin. The time constant is a characteristic of each structure, and those causing the ‘T syndrome’ and those causing the ‘CS syndrome’ in rats can generally be distinguished on this basis. Investigations of the site of action of pyrethroids within the nervous system have not resulted in a clear distinction between central and peripheral effects. Several studies suggest that pyrethroids are lethal when located peripherally, possibly in the cardiovascular or respiratory system, in rats. These effects can be attributed to modification of presynaptic and postsynaptic sodium channels. Postsynaptic neurotransmitter responses are unaffected by concentrations of pyrethroids that cause marked sodium channel modification. At high concentrations, both insecticidal and non-insecticidal pyrethroid isomers cause non-specific suppression of the postsynaptic neurotransmitter response.
The toxicity of many pyrethroids to laboratory mammals treated orally or dermally is very low: when the lethal doses are compared with those needed to kill insects by topical application on a weight basis, the selectivity factors range from 50 to 30 000. Conversely, pyrethroids may be highly toxic to mammals and birds after intravenous or intracerebroventricular administration. The acute toxicity of various pyrethroids differed by several orders of magnitude in rats dosed orally, by intravenous injection, or by dermal application: If it is assumed that a particular dose produces a certain toxic effect (e.g. death) when administered intravenously, the dose would have to be 100 times higher when taken up via the stomach and as much as 1000 times higher when applied to the skin. Intravenous administration of a wide range of pyrethroids has shown that the steric requirements for pyrethroid activity in rats and other vertebrates are similar to those in insects. In a comparative study of the actual toxicity of orally administered pyrethroids, mice appeared to be more sensitive than rats. No significant differences in toxicity to male and female animals were found for five pyrethroids. All of the cis isomers were more toxic than the corresponding trans isomers. The absolute toxicities of the two isomers were higher after intracerebral injection to mice, but the relationship between the two was similar (Miyamoto, 1976). The large interspecies differences in the toxicity of pyrethroids are due to differences in pharmacokinetics and metabolism.
Similar considerations apply to the toxicity of pyrethroids in fish, which may be at the level of nanograms per litre under laboratory conditions. Owing to their lipophilicity, these insecticides readily accumulate in aquatic organisms. The bioconcentration factors obtained in experiments with various aquatic species indicate that the steady-state concentration in the organism exceeds that in the surrounding water by two to four orders of magnitude.
(a) Medical surveillance of manufacturing plant personnel
In a plant at Neuville, France, where deltamethrin has been produced since 1978, no incidents of poisoning were recorded. The only effect noted by occupational physicians was paraesthesia or dysaesthesia (subjective sensations of tingling, itching, or burning). Slight erythema sometimes followed after about 48 h by slight desquamation was seen occasionally (Boggio, 1994).
In a plant at Romainville, France, where deltamethrin and many other pyrethroids were produced, 185 consultations for subjective cutaneous sensations were recorded between 1982 and 1990. Some persons were reported to have presented with this symptom at least 10 times after their first exposure. Up to 90% of the workers in the workshops presented once with acute subjective cutaneous sensations. The onset appeared to be more frequent in summer. The clinical description at this plant was similar to that at the previous one. Thus, the predominant sites were the jaws and periorbital area. In one man, paraesthesia occurred on the external genital organs. No tingling or burning sensations on the hands or forearms were reported. In most cases, non-concomitant cutaneous lesions were reported. Some objective signs were sometimes observed at the same time as the subjective sensations, either as slight erythema followed by discrete desquamation or as slight, transient dermal oedema. It is not clear that these effects were due to deltamethrin, as the organic solvent in which it was dissolved may have been responsible for some local symptoms. Transient tingling of the mouth and some gustative effects were also reported (Boggio, 1994).
A delay generally occurred before the onset of symptoms, which varied from 30 min after the beginning of exposure to 3–4 h after cessation. The sensations were usually increased by sensory stimulation (heat, sun, or water). The paraesthesia was always reported as transient, its duration generally varying from 30 min to 8 h, with a maximum of 24 h (Hermouet, 1994).
No published report of poisoning with technical-grade deltamethrin is available. Most of the observed cases of intoxication were due to a 2.5% emulsifiable concentrate, sometimes in association with organophosphates. The number of cases of poisoning can be assessed from three reports.
The Anti-poison Centre in Marseille received 230 000 telephone calls concerning intoxications during 1973–86, of which 6700 involved agricultural products and 89 (0.04%) referred to deltamethrin (Jouglard & Boulet, 1987).
Eighty-four cases of poisoning with deltamethrin-based products were recorded in the 5 years 1988–92 at the Anti-poison Centre of Paris. In 1992, the proportion of accidents due to deltamethrin accounted for 0.02% of all poisoning cases recorded at this Centre. Sixty-three of the cases involved deltamethrin in petroleum solvents, and eight involved deltamethrin and an organophosphorous compound in petroleum solvents (Chataigner & Garnier, 1995).
A review of 573 cases of acute pyrethroid poisoning reported in the Chinese medical literature during 1983–88 showed that 325 cases were due to a 2.5% deltamethrin emulsifiable concentrate. Of these, 153 were due to occupational exposure and 167 were accidental. Two patients died after convulsions; all the others recovered, after symptomatic and supportive treatment, within 1–6 days (He et al., 1989).
The symptoms vary according to the circumstances and route of exposure. Ingestion is the most frequent route in cases of accidental intoxication and suicide. Adverse effects occur mainly after cutaneous and respiratory exposure. After dermal exposure, symptoms such as tingling or itching of the face develop, similar in nature and duration to the paraesthesia described in plant personnel. They are always transient and are usually not associated with objective cutaneous signs. Chataigner & Garnier (1995) observed no systemic signs after cutaneous exposure. Splashing of a formulation containing deltamethrin and petroleum distillates into the eye induced pain and conjunctival hyperaemia. Recovery was complete within 4 days. Inhalation of deltamethrin-based formulations was followed by nausea, vomiting, headache, and irritation of the upper respiratory tract resulting in rhinorrhoea, cough, and dyspnoea. These signs of irritation were more intense with aerosols than with vapours. Only the paraesthesia could clearly be attributed to exposure to deltamethrin, whereas nausea, headache, and dizziness are known to be induced by organic solvents. The signs reported by He et al. (1988, 1989) were therefore not typical of deltamethrin poisoning but may have been due to the emulsifiable concentrate as a whole.
(c) Exposure of the general population
A field study was carried out in the United Kingdom on three unprotected operators and one operator wearing a hood, gloves, and respirator. All were involved in spraying orchards with deltamethrin according to normal field practice. They were exposed for 3.5 h. No changes were found in blood cell counts, total protein, urea, or the activities of alkaline phosphatase, gamma-glutamyl transferase, and aspartate aminotransferase in serum. Little deltamethrin was found in the respirator pad, and no residues were found in the urine. The nerve conduction velocity was not decreased, and in fact there was a slight tendency in the opposite reaction. None of the operators experienced facial sensations (Hewson & Burgess, 1981).
Four operators were evaluated by the same authors during normal field application of deltamethrin over 1 day. Three of the operators did not wear any protection on their heads or hands, while one wore a hood, gloves, and a respirator. Motor and sensory nerve conduction velocities were determined, and haematological and biochemical parameters were measured. No residues were found in the urine samples, and the nerve conduction velocity did not decrease. Residues were found primarily in the gloves and legs. None of the operators experienced facial sensations (Hewson & Burgess, 1981).
Health surveys were conducted in 1985 in France on farmers or on workers engaged in packaging a 2.5% deltamethrin emulsifiable concentrate in order to assess pesticide use, use of protection, and potential health effects. Of 2836 farmers who used crop protection products, 541 used the 2.5% deltamethrin product, which was the most commonly used insecticide. Despite generally poor use of personal protective equipment, the deltamethrin formulation induced no severe effects. Except for cutaneous sensations, deltamethrin induced fewer effects than other pesticides (Delemotte et al., 1986, 1987).
A study of Chinese farmers exposed to deltamethrin emulsifiable concentrate confirmed its low toxic potential during usual field practice. Cutaneous sensations were experienced, but no other symptom occurred. Clinical examinations, laboratory tests, and electrocardiographic monitoring revealed no abnormality. Determinations of airborne deltamethrin and biomonitoring in the urine showed that the airborne concentration ranged from 0.022 to 24 µg/m3, reflecting the variability of airborne concentrations in the open air. Deltamethrin was not found in urine because of low levels of exposure and rapid metabolism of the substance (Shujie et al., 1988).
Potential side-effects and levels of exposure were assessed in Chinese workers engaged in packaging, who were exposed to pure deltamethrin emulsifiable concentrate. There was no mechanical ventilation in the workshops. The workers wore gauze masks. They experienced cutaneous sensations of irritation and sometimes sneezing or increased nasal secretion. No abnormality was found in laboratory tests. The urinary concentration of deltamethrin was below the limit of detection. The mean airborne concentration was 0.005–0.012 µg/m3. Although the formulation was used in undiluted form, the airborne concentrations were well below those attained in the fields, where workers are exposed to aerosols (He et al., 1988).
Several pyrethroids, including deltamethrin, were applied in patch tests to 230 agricultural and non-agricultural workers with skin disorders. Deltamethrin was not irritating or sensitizing (Lisi, 1992).
Deltamethrin is a synthetic pyrethroid insecticide. Its insecticidal action is due, like all the synthetic pyrethroids, to interaction with ion channels on the axons of the target species.
The metabolism of [14C]deltamethrin was studied in rats, lactating cattle, and laying hens. The compound was rapidly absorbed, distributed, and excreted in rats and laying hens after oral administration, but it appeared to be poorly absorbed from the intestines of cattle. In rats, deltamethrin was readily metabolized and excreted, with a half-time in blood of about 6 h. In urine, only metabolites were detected, whereas in the faeces small amounts of parent compound were also detected.
The basic metabolic reactions are cleavage of the ester bond by oxidation and/or hydrolysis, followed by oxidation of the released acid and alcohol moieties. The acid moiety, 3-(2,2-dibromo-vinyl)-2,2-dimethylcyclo-propanecarboxylic acid, is transformed into conjugates, chiefly in the form of glucuronide, and excreted in urine. It can also be hydroxylated at one of the gem-methyl groups, which is in turn conjugated and excreted. The unstable alcohol moiety is transformed via the aldehyde to 3-phenoxybenzoic acid, which undergoes further oxidation by hydroxylation on the aromatic rings. It is then extensively excreted in urine, mainly as the 4’-hydroxy sulfate conjugate. Rapid ester cleavage is the major detoxification step in the metabolism of deltamethrin, suggesting that the parent compound is the only residue of toxicological concern.
As a type II pyrethroid, deltamethrin induces the ‘CS syndrome’, characterized by choreoathetosis (coarse tremors progressing to sinuous writhing), sedation, salivation, dyspnoea, and/or clonic seizures, sometimes with body tremors and prostration. These toxic signs, observed in various animal species given deltamethrin, are characteristic of a strong excitatory action on the nervous system resulting from a specific interaction between deltamethrin and the sodium channels of the nerve membranes. Series of nerve impulses are induced as a result of a change in the permeability of the membranes to sodium (repetitive effect). The nerve endings of sensory organs are particularly sensitive, although other parts of the nervous system are also affected.
The toxicity of deltamethrin is influenced by the vehicle. Thus, the oral LD50 value in rats is 30–140 mg/kg bw when the compound is administered in an oily vehicle, but > 5000 mg/kg bw when it is administered as an aqueous suspension. The dermal LD50 in rats was > 800 mg/kg bw when it was applied in xylene, but this dose produced no signs of toxicity when applied in methylcellulose; the LD50 after administration in this vehicle was > 2940 mg/kg bw. The LC50 value in rats of deltamethrin aerosol was 790 mg/m3. Deltamethrin is not irritating to the skin or eyes, and no sensitizing potential has been demonstrated. WHO (1999) has classified deltamethrin as ‘moderately hazardous’.
Studies of repeated administration by inhalation, orally, and dermally to mice, rats, rabbits, guinea-pigs, and dogs showed that deltamethrin induces mainly agitation, hypersensitivity, impaired locomotor activity, and reduced body-weight gain. The NOAEC was 9.6 mg/m3 (equivalent to 2.6 mg/kg bw) in a 3-week study in rats in which the LOAEC was 56 mg/m3. In dogs, the NOAEL was 1 mg/kg bw per day in a 1-year study of administration in capsules, on the basis of altered behaviour and liquid faeces at 10 mg/kg bw per day. In a 2-year dietary study, the NOAEL was 1 mg/kg bw per day, the highest dose tested. In rabbits treated cutaneously for 21 days with deltamethrin in an aqueous vehicle (PEG 400), the NOAEL was 1000 mg/kg bw per day, the highest dose tested.
In three long-term studies in two strains of mice (CD-1 and C57BL/6) in different laboratories, deltamethrin was not carcinogenic. The NOAEL for long-term toxicity was 100 ppm, equal to 16 mg/kg bw per day, on the basis of skin ulceration secondary to scratching and irritation due to the pharmacological effects of deltamethrin at 1000 ppm, equal to 160 mg/kg bw per day. In rats, the weight of evidence from three studies conducted in different laboratories and in different strains (CD, BDV1, and Crl:CD(SD)) indicated that deltamethrin was not carcinogenic. An increased frequency of thyroid tumours seen in one of these studies was not dose-related, and no increase in incidence was seen in the other two studies. The NOAEL for long-term toxicity in rats was 25 ppm, equal to 1.1 mg/kg bw per day, on the basis of minor hepatoxicity at 125 ppm, equal to 5.4 mg/kg bw per day.
Deltamethrin was tested for genotoxicity in an adequate range of assays, both in vitro and in vivo, and gave no evidence of genotoxicity.
Because of the absence of a carcinogenic effect in long-term experiments in rats and mice, the Meeting concluded that exposure to deltamethrin is unlikely to be a carcinogenic hazard to humans.
In a multigeneration study of reproductive toxicity in rats, the NOAEL for systemic toxicity was 80 ppm, equal to 4.2 mg/kg bw per day, on the basis of clinical signs in females during gestation and lactation, reduced food consumption and body-weight gain, and an increased mortality rate at 320 ppm, equal to 18 mg/kg bw per day; the NOAEL for toxicity in the offspring was also 80 ppm, equal to 11 mg/kg bw per day, on the basis of reduced body-weight gain, clinical signs, and an increased mortality rate before and after weaning up to 18 days. There were no adverse effects on mating or fertility, and the NOAEL for reproductive toxicity was 320 ppm, equal to 18 mg/kg bw per day, the highest dose tested.
In a study of developmental toxicity in mice, the NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of reduced body-weight gain and convulsions at 6 mg/kg bw per day. There were no malformations or developmental variations, and the NOAEL for developmental toxicity was 12 mg/kg bw per day, the highest dose tested. In a study of developmental toxicity in rats, the NOAEL for maternal toxicity was 3.3 mg/kg bw perday on the basis of clinical signs, reduced body-weight gain, and an increased mortality rate. The NOAEL for developmental toxicity was 11 mg/kg bw per day in the absence of malformations and developmental variations in fetuses at the highest dose. In a study of peri- and postnatal toxicity in rats, the NOAEL for perinatal development was 2.5 mg/kg bw per day, on the basis of reduced pup weight gain at 5.0 mg/kg bw per day. In a study of developmental toxicity in rabbits, the NOAEL for maternal toxicity was 25 mg/kg bw per day on the basis of the death of one of 16 females at 100 mg/kg bw per day, although there were no signs of maternal toxicity at any dose. The NOAEL for developmental toxicity was 25 mg/kg bw per day, on the basis of retardation of ossification at 100 mg/kg bw per day.
The results of acute and 90-day studies of neurotoxicity in rats and of acute delayed neurotoxicity in hens showed that deltamethrin does not induce neuropathological changes. The NOAEL for neurotoxicity in a study in rats given a single dose by gavage was 5 mg/kg bw on the basis of effects in a battery of tests for function and locomotor activity at 15 mg/kg bw per day. The NOAEL for systemic toxicity and neurotoxicity in a 90-day study in rats was 200 ppm, equal to 14 mg/kg bw per day, on the basis of effects on function in a battery of tests at 800 ppm, equal to 54 mg/kg bw per day, the highest dose tested.
Paresthaesia has been observed among exposed workers, but the symptoms were reversible upon cessation of exposure.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of deltamethrin to fetuses, infants, and children. Although deltamethrin is known to be neurotoxic to adults, the Meeting did not recommend that a study of developmental neurotoxicity be conducted since there was no evidence that offspring exposed pre- or postnatally are more sensitive than adults in the same experiment.
An ADI of 0–0.01 mg/kg bw was established for deltamethrin on the basis of the NOAEL of 1 mg/kg bw per day in a 1-year study in dogs treated by capsule, a 2-year study in dogs treated in the diet, and two 2-year studies in rats treated in the diet, with a safety factor of 100.
The Meeting established an acute RfD of 0.05 mg/kg bw on the basis of the NOAEL of 5 mg/kg bw in the study of acute neurotoxicity in rats and a safety factor of 100.
Levels relevant for risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
97-week study of toxicity and carcinogenicitya |
Toxicity |
100 ppm, equal to 16 mg/kg bw per day |
1000 ppm, equal to 160 mg/kg bw per day |
|
|
|
Carcinogenicity |
2000 ppm, equal to 320 mg/kg bw per dayb |
– |
|
|
Reproductive toxicityc |
Maternal toxicity |
3 mg/kg bw per day |
6 mg/kg bw per day |
|
|
Developmental toxicity |
|
12 mg/kg bw per dayb |
– |
|
Rat |
104-week study of toxicity and carcinogenicitya |
Toxicity |
20 ppm, equivalent to 1 mg/kg bw per day |
50 ppm, equivalent to 2.5 mg/kg bw per day |
|
|
|
Carcinogenicity |
800 ppm, equal to 36 mg/kg bw per dayb |
– |
|
|
Two-generation reproductive toxicitya |
Dam and pup toxicity |
80 ppm, equal to 4.2 mg/kg bw per day |
320 ppm, equal to 18 mg/kg bw per day |
|
|
|
Reproductive toxicity |
320 ppm, equal to 18 mg/kg bw per dayb |
– |
|
|
Developmental toxicityc |
Dam and pup toxicity |
2.5 mg/kg bw per day |
5 mg/kg bw per day |
|
|
Acute neurotoxicityc |
Neurotoxicity |
5 mg/kg bw |
15 mg/kg bw per day |
|
|
13-week study of neurotoxicitya |
Neurotoxicity |
200 ppm, equal to 14 mg/kg bw per day |
800 ppm, equal to 54 mg/kg bw per day |
|
Rabbit |
Developmental toxicityc |
Maternal, embryo-, and fetotoxicity |
25 mg/kg bw per day |
100 mg/kg bw per day |
|
Dog |
1-yeard and 2-year studies of toxicitya |
Toxicity |
1 mg/kg bw per day |
10 mg/kg bw per day |
a
Dietary administrationb
Highest dose testedc
Gavaged
CapsuleEstimate of acceptable daily intake for humans
0–0.01 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful to the continued evaluation of the compound
• Further studies in humans
Summary of critical end-points
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of absorption |
Rapid |
|
Distribution |
Mainly to liver, ovaries, kidneys, blood, and fat |
|
Potential for accumulation |
Low |
|
Rate and extent of excretion |
Rapid, 87–95% in rats |
|
Metabolism in animals |
Extensive; cleavage of ester by oxidation or hydrolysis, hydroxylation, then oxidation and conjugation |
|
Toxicologically significant compounds |
Parent compound |
|
(animals, plants and environment) |
|
|
Acute toxicity |
|
|
Rat, LD50, oral |
30–130 mg/kg bw in oily vehicle; > 5000 mg/kg bw in aqueous vehicle |
|
Rat, LD50, dermal |
> 800 in xylene solvent |
|
Rat, LC50, inhalation |
790 mg/m3 |
|
Dermal irritation |
Not irritating to rabbit skin |
|
Ocular irritation |
Not irritating to rabbit eyes |
|
Dermal sensitization |
No sensitizing potential in guinea-pigs |
|
Short-term toxicity |
|
|
Target/critical effect |
Nervous system |
|
Lowest relevant oral NOAEL |
1 mg/kg bw per day in dogs |
|
Lowest relevant dermal NOAEL |
1000 mg/kg bw per day in rabbits |
|
Lowest relevant inhalation NOAEC |
9.6 mg/m3 in rats |
|
Genotoxicity |
Not genotoxic |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
No consistently identified target in rats or mice |
|
Lowest relevant NOAEL |
Mice, 16 mg/kg bw; rats, 1 mg/kg bw |
|
Carcinogenicity |
Not carcinogenic to mice and rats |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
None identified in rats |
|
Lowest relevant reproductive NOAEL |
18 mg/kg bw per day in rats, highest dose tested |
|
Developmental target/critical effect |
Rats and mice, none identified; rabbits, delayed ossification |
|
Lowest relevant developmental NOAEL |
Rats, 11 mg/kg bw per day; mice, 12 mg/kg bw per day; rabbits, 25 mg/kg bw per day |
|
Neurotoxicity/Delayed neurotoxicity |
NOAEL, 5 mg/kg bw per day in a single-dose study in rats |
|
Other toxicological studies |
None |
|
Medical data |
Paresthaesia; irritation to skin and upper respiratory tract (perhaps due to solvents) |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.01 mg/kg bw |
Two 2-year dietary studies in rats; |
100 |
|
Acute RfD |
0.05 mg/kg bw |
Study of acute neurotoxicity in rats |
100 |
Akhtar, M.H., Hamilton, R.M.G. & Trenholm, H.L. (1985) Metabolism, distribution, and excretion of deltamethrin by Leghorn hens. J. Agric. Food Chem., 33, 610–617.
Akhtar, M.H., Hartin, K.E. & Trenholm, H.L. (1986) Fate of [14C]deltamethrin in lactating dairy cows. J. Agric. Food Chem., 34, 753–758.
Aldridge, W.N. (1990) An assessment of the toxicological properties of pyrethroids and their neurotoxicity. Crit. Rev. Toxicol., 21, 89–104
Appel, K.E. & Gericke S. (1993) [Neurotoxicity and toxicokinetics of pyrethroids.] Bundesgesundhblatt, 6, 219–228 (in German).
Boggio, M. (1994) [Medical data on deltamethrin.] Unpublished report No. FR224 from Service Medical Roussel Uclaf, Neuville-sur-Saöne, France, 18 November, 1994, AgrEvo document A70883. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (in French).
Bosch, A. (1990) Metabolism of 14C-deltamethrin in rats. Unpublished report No. 187108 from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA, AgrEvo document A70824. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Cabral, J.R., Galendo, D., Laval, M. & Lyandrat, N. (1990) Carcinogenicity studies with deltamethrin in mice and rats. Cancer Lett., 49, 14–152.
Chataignier, D. & Garnier, R. (1995) [Poisoning by deltamethrin. 84 cases notified to the Anti-Poison Centre of Paris between 1988 and 1992.] Unpublished report No. FR950406 from Centre Anti-Poisons, Paris, 1995, AgrEvo document A72704. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (in French).
Chesterman, H., Heywood, R., Perkin, C.J., Beard, D., Street, A.E. & Prentice, D.E. (1977) RU 22974. Oral toxicity study in beagle dogs (repeated dosage for 13 weeks followed by a 20 week observation period). Unpublished report No. GB253 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 9 June 1977, AgrEvo document A70871. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Coquet, B. (1976a) RU 22974. Test to determine primary cutaneous irritation in the rabbit. Unpublished report No. 761157 from Institut Français de Recherches et Essais biologiques, Joinville le Pont, France, 15 November 1976, AgrEvo document A08473. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Coquet, B. (1976b) RU 22974. Test to evaluate occular irritation in the rabbit. Unpublished report No. 761158 from Institut Français de Recherches et Essais biologiques, Joinville le Pont, France, 15 November 1976, AgrEvo document A08475. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Curren, R.D. (1989) Unscheduled DNA synthesis of deltamethrin in rat primary hepatocytes. Unpublished report No. 418380 from Microbiological Associates, Inc., Rockville, Maryland, USA, 13 March, 1989,, AgroEvo document A70853. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Davies, J.E., Munt, P.L. & Goor, K.L. (1983) RU 22974. Investigation of possible neurological effects using the tilting plane test. Unpublished report No. GB603 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 5 May 1983, AgrEvo document A41890. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Delemotte, B., Foulhoux, P., N’Guyen, S.N., Fages, J. & Portos, J.L. (1986) [Specific study of DECIS in the investigation of CCMA. Analysis of secondary effects], 18 November 1986, AgrEvo document A70766. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (in French).
Delemotte, B., Foulhoux, P., N’Guyen, S.N., Fages, J. & Portos, J.L. (1987) [Pesticide risk in agriculture.] Arch. Mal. Prof., 48, 467–475 (in French).
Fabreguettes, C. (1991) Toxicity study for 12 weeks by oral administration to mice: Deltamethrin. Unpublished report No. 5566 TSS from Centre International de Toxicologie, Misery, Evreux, France, 4 May 1990, AgrEvo document A70876. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GPL).
Glomot, R. & Chevalier, B. (1976) RU22974: Acute toxicity study in rat by oral route. Unpublished report No. RU-76.820/A from Roussel UCLAF, Romainville, France, 30 March 1976, AgrEvo document A78773. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Goldenthal, E.I. (1980a) RU 22974—2 year chronic dog study. Unpublished report No. 406004 from International Research and Development Corp., Mattawan, Michigan, USA, 16 September 1980, AgrEvo document A21228. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Goldenthal, E.I. (1980b) RU 22974—Two year oral toxicity and carcinogenicity study in mice. Unpublished report No. 406001 from International Research and Development Corp., Mattawan, Michigan, USA, 6 May 1980, AgrEvo document A20242. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Goldenthal, E.I. (1980c) RU 22974—Two year oral toxicity and carcinogenicity study in rats. Unpublished report No. 406002 from International Research and Development Corp., Mattawan, Michigan, USA, 6 May 1980, AgrEvo document A20243. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Goldenthal, E.I. (1981) RU 22974—2 year chronic dog study. Unpublished report No. 406004 from International Research and Development Corp., Mattawan, Michigan, USA, 10 April, 1981 (amendment). Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Goldenthal, E.I. (1982) RU 22974—Two year oral toxicity and carcinogenicity study in mice. Amendment to the final report. Unpublished report No. 406001 from International Research and Development Corp., Mattawan, Michigan, USA, 28 May 1982, AgrEvo document A20242. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Guillot, J.P. & Guilaine, J. (1977) RU 22974—Decamethrin-Decis technical Roussel Uclaf. Sensitisation test in the guinea pig. Unpublished report No. 709241 from Institut Français de Recherches et Essais Biologiques, L’Arbresle, France, 26 September 1977, AgrEvo document A28978. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
He, F., Sun, J., Han, K., Wu, Y., Yao, P., Wang, S. & Liu, L. (1988) Effects of pyrethroid insecticides on subjects engaged in packaging pyrethroids. Br. J. Ind. Med., 45, 548–551.
He, F., Wang, S., Liu, L., Chen, S., Zhang, Z. & Sun, J. (1989) Clinical manifestations and diagnosis of acute pyrethroid poisoning. Arch. Toxicol., 63, 54–58.
Hermouet, C. (1994) Cutaneous subjective sensations. Pyrethroids handling in the Roussel Uclaf plants in France for 10 years. Unpublished report No. 940908 from the Roussel Uclaf, Romainville, France, AgrEvo document A70823. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Hewson, R.T. & Burgess, J.E. (1981) An operator study with deltamethrin including measurements of nerve conduction times. Unpublished report no. GB1181 from Hoechst UK Ltd and Occupational Health and Hygiene Services Ltd, United Kingdom, November 1981, AgrEvo document A41943. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Hoberman, A.M. (1992) Reproductive effects of deltamethrin administered orally in the diet to Crl:CD® BR VAF/Plus® rats for two generations. Unpublished report No. 818001 from Argus Research Laboratories Inc., Horsham, Pennsylvania, USA, 17 January 1992, AgrEvo document A70863. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Hoff, N. (1992) Acute intravenous toxicity study with deltamethrin (preparation with PEG 300) in rats. Unpublished report No. CH11117 from RCC Itingen, Switzerland, 15 June 1992, AgrEvo document A49669. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Hunter, B., Jordan, J., Heywood, R., Hepworth, P., Street, A.E. & Prentice, D.E. (1977) RU 22974. Assessment of toxicity to rats by oral administration for 13 weeks (followed by a 4 weeks withdrawal period). Unpublished report No. GB254 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 21 March 1977, AgroEvo document A70872. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Jouglard, J. & Boulet, O. (1987) [Records Decis in the activity of the Anti-poison Centre of Marseille.] Unpublished report No. FR870505 from Centre Anti-Poisons de Marseille, France, 1987, AgrEvo document A71237. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (in French).
Kavlock, R., Chernoff, N., Baron, R., Linder, R., Rogers, E. & Carver, B. (1979) Toxicity studies with decamethrin, a synthetic pyrethroid insecticide. J. Environ. Pathol. Toxicol., 2, 751–765.
Kynoch, S.R., Lloyd, G.K. & Andrews, C.D. (1979) Acute percutaneous toxicity to rats of deltamethrin. Unpublished report No. 10098 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 21 February 1979, AgrEvo document A28974. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Lisi, P. (1992) Sensitisation risk of pyrethroid insecticides. Contact Derm., 26, 349–350.
Moser, V.C. (1991) Applications of neurobehavioral screening battery. J. Am.Coll.Toxicol., 10, 661–669.
Miyamoto, J. (1976) Degradation, metabolism and toxicity of synthetic pyrethroids. Environ. Health Perspectives, 14, 15–28.
Myer, J.R. (1989a) Acute oral toxicity study of deltamethrin in rats. Unpublished report No. 327122 from International Research and Development Corp., Mattawan, Michigan, USA, 4 May 1989, AgrEvo document A70785. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Myer, J.R. (1989b) Primary dermal irritation test of deltamethrin in rabbits. Unpublished report No. 327124 from International Research and Development Corp., Mattawan, Michigan, USA, 17 April 1989, AgrEvo document A70797. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Myer, J.R. (1989c) Eye irritation study of deltamethrin in rabbits. Unpublished report No. 327125 from International Research and Development Corp., Mattawan, Michigan, USA, 17 April 1989, AgrEvo document A70799. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Myer, J.R. (1989d) Dermal sensitisation study of deltamethrin in the albino guinea pig (Buehler). Unpublished report No. 327126 from International Research and Development Corp., Mattawan, Michigan, USA, 7 September, 1989, AgrEvo document A70795. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Nemec, M.D. (1998a) An acute neurotoxicity study of deltamethrin in rats. Unpublished report No. A74318 from WIL Research Laboratories, Inc., Ashland, Ohio, USA, 18 March 1998, AgrEvo document C006785. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Nemec, M.D. (1998b) A subchronic (13-week) neurotoxicity study of deltamethrin in rats. Unpublished report No. A74317 from WIL Research Laboratories, Inc., Ashland, Ohio, USA, 19 March 1998, AgrEvo document C006785. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Peyre, M., Chantot, J.F., Glomot, R. & Penasse, L. (1980) Detection of a mutagenic potency of decamethrin (RU 22974). Unpublished report No. 80210a from Centre de Recherches Roussel Uclaf, Romainville, France, 21 January 1980, AgrEvo document A28932. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille, A. & Bartsch, H. (1984) Lack of mutagenicity of synthetic pyrethroids in Salmonella typhimurium strains and in V79 Chinese hamster cells. Mutat. Res., 137, 7–15.
Poláková, H. & Vargová, M. (1983) Evaluation of the mutagenic effects of decamethrin: Cytogenetic analysis of bone marrow. Mutat. Res., 120, 167–171.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberration assay of deltamethrin in Chinese hamster ovary (CHO) cells. Unpublished report No. 337003 from Microbiological Associates, Inc., Rockville, Maryland, USA, 23 February 1989, AgrEvo document A70851. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Richard, J. (1995) 97-week carcinogenicity study by oral route (dietary admixture) in mice: Deltamethrin (technical). Unpublished report No. 7347 from Centre International de Toxicologie, Miserey, Evreux, France, 26 December 1995, AgrEvo document A56270. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Richter, W.R. & Goldenthal, E.I. (1983) RU 22974—Two year oral toxicity and carcinogenicity study in rats. Amendment to the final report. Unpublished report No. 406002 from International Research and Development Corp., Mattawan, Michigan, USA, 6 May 1980, AgrEvo document A20243. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E., Cooke, L. & Gibson, W.A. (1978). RU22974, LD50 determination and assessment of neurotoxicity in the domestic hen. Unpublished report No. GB293 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 19 January 1978, AgroEvo document A20307. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Rouaud, J.L. & Marzin, D. (1977a) Study of the effects of RU 22974 on the food consumption in the rat. Unpublished report No. 770363 from Institut Français de Recherches et Essais Biologiques, Joinville le Pont, France, 22 March 1977, AgroEvo document A70878. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Rouaud, J.L. & Marzin, D. (1977b) Study of the effects of RU 22974 on the food consumption in the mouse. Unpublished report No. 709117 from Institut Français de Recherches et Essais Biologiques, Joinville le Pont, France, 5 October 1977, AgroEvo document A70877. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Ruzo, L.O., Unai, T. & Casida, J.E. (1978) Decamethrin metabolism in rats. J. Agric. Food Chem., 26, 918–925.
Ryle, P.R., Scrivener, R., Gibson, W.A., Crook, D., Hadley, J.C. & Gopinath, C. (1991a) Deltamethrin, toxicity studies in rats by dietary administration for 13 weeks with a 4-week recovery period (according to Japanese MAFF, OECD and EPA guidelines) Unpublished report No. GB920929 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 3 July 1991. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Ryle, P.R., Ashman, K.W., Buist, D.P., Crook, D., Gregson, R.L. & Gopinth, C. (1991b) Deltamethrin—Oral toxicity study in beagle dogs (repeated daily dosage for 13 weeks with by a 4-week recovery period for selected animals). Unpublished report No. GB1755 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 8 July 1991, AgroEvo document A70874. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Ryle, P.R., Buist, D.P., Crook, D., Rao, R.S. & Gopinth, C. (1993) Deltamethrin (technical)—Toxicity to dogs by repeated oral administration for 52 weeks. Unpublished report No. GB920929 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 21 October 1993, AgroEvo document A70808. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Ryle, P.R. (1995) Deltamethrin (technical)—Potential tumorigenic and toxic effects in prolonged dietary administration to rats. Unpublished report No. RSL 814/932275 from Huntingdon Research Centre, Huntingdon, Cambridgeshire, United Kingdom, 11 December 1995, AgroEvo document A56161. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Saleh, M.A., Ibrahim, N.A., Soliman, N.Z. & El Sheimy, M.K. (1986) Persistence and distribution of cypermethrin, deltamethrin and fenvalerate in laying chickens. J. Agric. Food Chem., 34, 895–898.
Schardein, J.L. (1990a) Developmental toxicity study in rats. Unpublished report No. 327120 from International Research and Development Corp., Mattawan, Michigan, USA., 6 July 1990, AgroEvo document A70869. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Schardein, J.L. (1990b) Developmental toxicity study in New Zealand white rabbits. Unpublished report No. 3271112 from International Research and Development Corp., Mattawan, Michigan, USA, 6 May 1990, AgroEvo document A70865. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Shujie, W., Quiglang, Z., Lan, Y., Bohong, X. & Yurui, L. (1988) Health survey among farmers exposed to deltamethrin in the cotton fields. Unpublished report No. CN16P from Section Toxicology, Institute of Occupational Medicine, Beijing, China, AgroEvo document A41945. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Siglin, J.C. (1993) 21-day dermal toxicity study in rats with deltamethrin technical. Unpublished report no. 320728 from Spingborn Laboratories, Inc., Spencerville, Ohio, USA, 9 April 1993, AgroEvo document A50968. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Ulrich, C.E. (1990) Deltamethrin, acute inhalation toxicity evaluation in rats. Unpublished report No. 327121 from International Research and Development Corp., Mattawan, Michigan, USA, 9 July 1990, AgroEvo document A70770. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Van Dijk, A. (1994) Equivalent mixture of 14C-phenyl-cypermethrin, 14C-benzyl-deltamethrin and 14C-phenoxyphenyl-fenvalerate: Distribution, kinetics and excretion after single oral administration to laying hens. Unpublished report No. 346915 from RCC Itingen, Switzerland, 30 March 1994, AgroEvo document A52315. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Van Dijk, A. & Burri, R. (1993a) [14C-Benzyl]-deltamethrin: Distribution, kinetics and excretion after single intravenous administration to female rats. Unpublished report No. 299160 from RCC Itingen, Switzerland, 5 August 1993, AgroEvo document A51513. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Van Dijk, A. & Burri, R. (1993b) [14C-Benzyl]-deltamethrin: Distribution, kinetics and excretion after single intravenous administration to laying hens. Unpublished report No. 299182 from RCC Itingen, Switzerland, 5 August 1993, AgroEvo document A51514. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Vannier, R. & Fournex, R. (1983) Deltamethrin. Detection of a mutagenic potency: Micronucleus test in the mouse. Unpublished report No. 83607 from Centre de Recherches Roussel Uclaf, Romainville, France, 27 July 1983, AgroEvo document A41868. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Vannier, R. & Glomot, R. (1977) RU 22974. Mutagenic study. Dominant lethal assay in the male mouse. Unpublished report No. 76533 from Centre de Recherches Roussel Uclaf, Romainville, France, 17 May 1977, AgroEvo document A20259. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany.
Varsho, B.J. (1995) Acute oral toxicity study of deltamethrin in albino rats. Unpublished report No. 53013A from WIL Research Laboratories, Inc., Ashland, Ohio, USA, AgroEvo document A55812. Submitted to WHO by Hoechst Schering AgrEvo, Frankfurt-am-Main, Germany (GLP).
Vijverberg, H.P.M. & van den Bercken, J. (1990) Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol., 21, 105–126.
Vijverberg, H.P.M. & Oortgiessen, M. (1988) Steric structure and action of pyrethroids. In: Ariens, E.J., van Rensen, J.J.S. & Welling, W., eds, Stereoselectivity of Pesticides; Biological and Chemical Problems, Amsterdam: Elsevier Science Publishers.
WHO (1999) Recommended classification of pesticides by hazard and guidelines to classification 1998–1999 (WHO/PCS/98.21/Rev.1), Geneva, International Programme on Chemical Safety (www.who.int/pcs/pcs-act.htm).
See Also:
Toxicological Abbreviations
Deltamethrin (EHC 97, 1990)
Deltamethrin (HSG 30, 1989)
Deltamethrin (ICSC)
DELTAMETHRIN (JECFA Evaluation)
Deltamethrin (Pesticide residues in food: 1980 evaluations)
Deltamethrin (Pesticide residues in food: 1981 evaluations)
Deltamethrin (Pesticide residues in food: 1982 evaluations)
Deltamethrin (Pesticide residues in food: 1984 evaluations)
Deltamethrin (UKPID)
Deltamethrin (IARC Summary & Evaluation, Volume 53, 1991)